User login
Inside Out or Outside In: Does Atopic Dermatitis Disrupt Barrier Function or Does Disruption of Barrier Function Trigger Atopic Dermatitis?
Atopic dermatitis (AD) is a multifactorial inflammatory disorder with an estimated prevalence of 279,889,120 cases worldwide.1 Most cases of AD begin in early childhood (with almost 85% developing by 5 years of age),2 but recent studies have found that 40% to over 80% of cases persist into adulthood.1,3,4 Although a previous study focused largely on T helper type 1/T helper type 2 (Th2) immune dysregulation as the pathogenesis of the disease,5 disruption of the skin barrier and systemic inflammation are at the center of current AD research. In AD, breakdown of the skin barrier results in increased transepidermal water loss, reduced skin hydration, and increased antigen presentation by Langerhans cells initiating inflammation.6-8 The cascade largely activated is the Th2 and T helper type 22 cascade with resultant cytokine release (ie, IL-4, IL-13, IL-2, IL-8, IL-10, IL-17, IL-22, tumor necrosis factor α, interferon γ).9,10 In active AD, Th2 inflammation and barrier breakdown result in reduced filaggrin and claudin 1 expression, resulting in further exacerbation of the barrier defect and enhancing the risk of development of asthma and hay fever as well as transcutaneous sensitization to a variety of food allergens (eg, peanuts).9,11,12 Although all of these immunologic features are well established in AD, controversy remains as to whether AD is caused by systemic inflammation triggering barrier dysfunction (the “inside-out” hypothesis) or from the epidermal skin barrier disruption triggering immunologic imbalance (the “outside-in” hypothesis).
Inside-Out Hypothesis
While barrier impairment appears to occur in all patients with AD, it still is unclear how AD begins. The inside-out hypothesis suggests that cutaneous inflammation precedes barrier impairment and in fact may result in an impaired skin barrier. It has previously been reported that inflammatory states weaken the barrier by downregulating filaggrin production in the skin.13 Barrier disruption may be accompanied by transcutaneous penetration of allergens and increased Staphylococcus aureus counts. Recently, mutations and polymorphisms of inflammatory genes have been linked to AD (eg, single nucleotide polymorphisms of the IL4RA [interleukin 4 receptor, alpha] and CD14 [cluster of differentiation 14] genes, the serine protease inhibitor SPINK5 [serine peptidase inhibitor, Kazal type 5], RANTES [chemokine (C-C motif) ligand 5], IL-4, IL-13).14 These alterations highlight the role of systemic inflammation in triggering AD.
Outside-In Hypothesis
The outside-in hypothesis suggests that the impaired skin barrier precedes AD and is required for immune dysregulation to occur. This hypothesis was largely advanced by a study demonstrating that deactivating mutations of the filaggrin gene were linked to nearly 20% of AD cases in Northern European populations.15 Filaggrin (chromosome 1q21.3) performs an essential function in the skin barrier through its differential cleavage and the breakdown and release of natural moisturizing factor.16 Filaggrin gene mutations are associated with persistent AD, and it has been posited that environmental factors such as temperature and humidity also can affect filaggrin production as it relates to barrier function.17-19 Skin barrier disruption results in increased cutaneous and systemic Th2 responses (ie, IL-4/13), with thymic stromal lymphopoietin as the potential mechanism of Th2 cell recruitment.10,20 Inflammatory Th2 cells triggered by an impaired skin barrier also may predispose patients to the development of allergic diseases such as asthma, in line with Atopic March, or the progression of AD to other forms of atopy (eg, food allergy, asthma).5,7,21-23
The outside-in hypothesis may only explain the root pathogenesis of AD in a subset of patients, however, as only 1 in 5 cases of AD in Northern European and Asian populations are associated with underlying filaggrin mutations (which are only present in about 10% of those who are unaffected by AD).15 Filaggrin does not appear to account for the basis of AD in all cases. In a study of 762 newborns in Cincinnati, Ohio, 39% of children with at least one parent with atopy developed AD by 3 years of age, about quadruple of what would be projected based on filaggrin defects in general population studies, which are noted in only about 10% of white individuals.24 Furthermore, less than 5% of patients of African descent have mutations of the filaggrin 1 gene.25
Implications for the Prevention and Treatment of Atopic Dermatitis
Preventative strategies for AD currently are in development. Atopic dermatitis may be unpreventable because the in utero environment triggers some of the barrier alterations, which can be noted as early as 2 days following birth and will predict early-onset AD. The putative mechanism is via Th2 cytokines (IL-4, IL-13).26
Certainly, application of over-the-counter and prescription emollients are mainstays of treatment for AD and may suffice as monotherapy in cases of mild disease. In a recent randomized trial in the United States and the United Kingdom, emollients were used in newborns considered at high risk for AD (family history of atopy) until 6 months of age.27 The risk of AD development was reduced by half, irrespective of the emollient used. Unfortunately, 21.8% of children without a family history of atopy will develop AD; therefore, not all cases can be prevented if use of emollients is limited to newborns with a family history of atopy.28 Long-term follow-up is needed to track whether emollient use in newborns will prevent AD indefinitely.
Prevention of AD onset using systemic interventions has also been investigated. Probiotics have been suggested as a means to modify the gut microbiota and reduce systemic and mucosal inflammation. Lactobacillus reuteri taken prenatally by pregnant women and by newborns has shown mild benefit in preventing some forms of AD.29 Although they are not approved by the US Food and Drug Administration for this indication, systemic interventions for moderate-to-severe AD such as methotrexate and cyclosporine certainly have shown benefit in managing ongoing illness and breaking the cycle of disease.30 The efficacy of these agents points to the role of systemic inflammation in ongoing AD activity. Moreover, the inside-out hypothesis recently has led to the proliferation of promising new therapeutic agents in the pipeline to treat the systemic Th2 inflammation that occurs in severe AD (eg, anti–IL-4/13 receptor antibody, anti–IL-13 antibodies, and biologics targeting IL-12/23, IL-22, and IL-31 receptors).31
Final Thoughts
Atopic dermatitis is a multifactorial disease associated with barrier disruption and intense systemic inflammation. It is likely that both the inside-out and outside-in hypotheses hold true in different subsets of AD patients. It is clear that some individuals are born with filaggrin defects that sufficiently trigger systemic inflammation, resulting in AD. On the other hand, there are clearly some individuals with inflammatory dysregulation that results in systemic inflammation and secondary barrier disruption. Until we can determine the genomic triggering or promoting event in each individual patient, large-scale introduction of active prevention and severity reduction strategies may not be realistic. In the meantime, we can approach AD in childhood from the inside out, through appropriate treatment of systemic inflammation of AD, and from the outside in, with treatment and prevention via emollient use in newborns.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
- Margolis JS, Abuabara K, Bilker W, et al. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150:593-600.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Zheng T, Jinho Y, Oh MH, et al. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3:67-73.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care. Clin Dermatol. 2015;33:271-280.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
- Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
- Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Brown SJ, Irvine AD. Atopic eczema and the filaggrin story. Semin Cutan Med Surg. 2008;27:128-137.
- Harding CR, Aho S, Bosko CA. Filaggrin—revisited. Int J Cosmet Sci. 2013;35:412-423.
- Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29-40.
- Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease and increased heart attacks in 3 population-based studies [published online ahead of print July 4, 2015]. Allergy. doi:10.1111/all.12685.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
- Kelleher M, Dunn-Galvin A, Hourihane JO, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135:930-935.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Parazzini F, Cipriani S, Zinetti C, et al. Perinatal factors and the risk of atopic dermatitis: a cohort study. Pediatr Allergy Immunol. 2014;25:43-50.
- Abrahamsson TR, Jakobsson T, Böttcher MF, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174-1180.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Eczema drugs in development. National Eczema Association Web site. https://nationaleczema.org/research/phases-drug-development/. Accessed August 18, 2015.
Atopic dermatitis (AD) is a multifactorial inflammatory disorder with an estimated prevalence of 279,889,120 cases worldwide.1 Most cases of AD begin in early childhood (with almost 85% developing by 5 years of age),2 but recent studies have found that 40% to over 80% of cases persist into adulthood.1,3,4 Although a previous study focused largely on T helper type 1/T helper type 2 (Th2) immune dysregulation as the pathogenesis of the disease,5 disruption of the skin barrier and systemic inflammation are at the center of current AD research. In AD, breakdown of the skin barrier results in increased transepidermal water loss, reduced skin hydration, and increased antigen presentation by Langerhans cells initiating inflammation.6-8 The cascade largely activated is the Th2 and T helper type 22 cascade with resultant cytokine release (ie, IL-4, IL-13, IL-2, IL-8, IL-10, IL-17, IL-22, tumor necrosis factor α, interferon γ).9,10 In active AD, Th2 inflammation and barrier breakdown result in reduced filaggrin and claudin 1 expression, resulting in further exacerbation of the barrier defect and enhancing the risk of development of asthma and hay fever as well as transcutaneous sensitization to a variety of food allergens (eg, peanuts).9,11,12 Although all of these immunologic features are well established in AD, controversy remains as to whether AD is caused by systemic inflammation triggering barrier dysfunction (the “inside-out” hypothesis) or from the epidermal skin barrier disruption triggering immunologic imbalance (the “outside-in” hypothesis).
Inside-Out Hypothesis
While barrier impairment appears to occur in all patients with AD, it still is unclear how AD begins. The inside-out hypothesis suggests that cutaneous inflammation precedes barrier impairment and in fact may result in an impaired skin barrier. It has previously been reported that inflammatory states weaken the barrier by downregulating filaggrin production in the skin.13 Barrier disruption may be accompanied by transcutaneous penetration of allergens and increased Staphylococcus aureus counts. Recently, mutations and polymorphisms of inflammatory genes have been linked to AD (eg, single nucleotide polymorphisms of the IL4RA [interleukin 4 receptor, alpha] and CD14 [cluster of differentiation 14] genes, the serine protease inhibitor SPINK5 [serine peptidase inhibitor, Kazal type 5], RANTES [chemokine (C-C motif) ligand 5], IL-4, IL-13).14 These alterations highlight the role of systemic inflammation in triggering AD.
Outside-In Hypothesis
The outside-in hypothesis suggests that the impaired skin barrier precedes AD and is required for immune dysregulation to occur. This hypothesis was largely advanced by a study demonstrating that deactivating mutations of the filaggrin gene were linked to nearly 20% of AD cases in Northern European populations.15 Filaggrin (chromosome 1q21.3) performs an essential function in the skin barrier through its differential cleavage and the breakdown and release of natural moisturizing factor.16 Filaggrin gene mutations are associated with persistent AD, and it has been posited that environmental factors such as temperature and humidity also can affect filaggrin production as it relates to barrier function.17-19 Skin barrier disruption results in increased cutaneous and systemic Th2 responses (ie, IL-4/13), with thymic stromal lymphopoietin as the potential mechanism of Th2 cell recruitment.10,20 Inflammatory Th2 cells triggered by an impaired skin barrier also may predispose patients to the development of allergic diseases such as asthma, in line with Atopic March, or the progression of AD to other forms of atopy (eg, food allergy, asthma).5,7,21-23
The outside-in hypothesis may only explain the root pathogenesis of AD in a subset of patients, however, as only 1 in 5 cases of AD in Northern European and Asian populations are associated with underlying filaggrin mutations (which are only present in about 10% of those who are unaffected by AD).15 Filaggrin does not appear to account for the basis of AD in all cases. In a study of 762 newborns in Cincinnati, Ohio, 39% of children with at least one parent with atopy developed AD by 3 years of age, about quadruple of what would be projected based on filaggrin defects in general population studies, which are noted in only about 10% of white individuals.24 Furthermore, less than 5% of patients of African descent have mutations of the filaggrin 1 gene.25
Implications for the Prevention and Treatment of Atopic Dermatitis
Preventative strategies for AD currently are in development. Atopic dermatitis may be unpreventable because the in utero environment triggers some of the barrier alterations, which can be noted as early as 2 days following birth and will predict early-onset AD. The putative mechanism is via Th2 cytokines (IL-4, IL-13).26
Certainly, application of over-the-counter and prescription emollients are mainstays of treatment for AD and may suffice as monotherapy in cases of mild disease. In a recent randomized trial in the United States and the United Kingdom, emollients were used in newborns considered at high risk for AD (family history of atopy) until 6 months of age.27 The risk of AD development was reduced by half, irrespective of the emollient used. Unfortunately, 21.8% of children without a family history of atopy will develop AD; therefore, not all cases can be prevented if use of emollients is limited to newborns with a family history of atopy.28 Long-term follow-up is needed to track whether emollient use in newborns will prevent AD indefinitely.
Prevention of AD onset using systemic interventions has also been investigated. Probiotics have been suggested as a means to modify the gut microbiota and reduce systemic and mucosal inflammation. Lactobacillus reuteri taken prenatally by pregnant women and by newborns has shown mild benefit in preventing some forms of AD.29 Although they are not approved by the US Food and Drug Administration for this indication, systemic interventions for moderate-to-severe AD such as methotrexate and cyclosporine certainly have shown benefit in managing ongoing illness and breaking the cycle of disease.30 The efficacy of these agents points to the role of systemic inflammation in ongoing AD activity. Moreover, the inside-out hypothesis recently has led to the proliferation of promising new therapeutic agents in the pipeline to treat the systemic Th2 inflammation that occurs in severe AD (eg, anti–IL-4/13 receptor antibody, anti–IL-13 antibodies, and biologics targeting IL-12/23, IL-22, and IL-31 receptors).31
Final Thoughts
Atopic dermatitis is a multifactorial disease associated with barrier disruption and intense systemic inflammation. It is likely that both the inside-out and outside-in hypotheses hold true in different subsets of AD patients. It is clear that some individuals are born with filaggrin defects that sufficiently trigger systemic inflammation, resulting in AD. On the other hand, there are clearly some individuals with inflammatory dysregulation that results in systemic inflammation and secondary barrier disruption. Until we can determine the genomic triggering or promoting event in each individual patient, large-scale introduction of active prevention and severity reduction strategies may not be realistic. In the meantime, we can approach AD in childhood from the inside out, through appropriate treatment of systemic inflammation of AD, and from the outside in, with treatment and prevention via emollient use in newborns.
Atopic dermatitis (AD) is a multifactorial inflammatory disorder with an estimated prevalence of 279,889,120 cases worldwide.1 Most cases of AD begin in early childhood (with almost 85% developing by 5 years of age),2 but recent studies have found that 40% to over 80% of cases persist into adulthood.1,3,4 Although a previous study focused largely on T helper type 1/T helper type 2 (Th2) immune dysregulation as the pathogenesis of the disease,5 disruption of the skin barrier and systemic inflammation are at the center of current AD research. In AD, breakdown of the skin barrier results in increased transepidermal water loss, reduced skin hydration, and increased antigen presentation by Langerhans cells initiating inflammation.6-8 The cascade largely activated is the Th2 and T helper type 22 cascade with resultant cytokine release (ie, IL-4, IL-13, IL-2, IL-8, IL-10, IL-17, IL-22, tumor necrosis factor α, interferon γ).9,10 In active AD, Th2 inflammation and barrier breakdown result in reduced filaggrin and claudin 1 expression, resulting in further exacerbation of the barrier defect and enhancing the risk of development of asthma and hay fever as well as transcutaneous sensitization to a variety of food allergens (eg, peanuts).9,11,12 Although all of these immunologic features are well established in AD, controversy remains as to whether AD is caused by systemic inflammation triggering barrier dysfunction (the “inside-out” hypothesis) or from the epidermal skin barrier disruption triggering immunologic imbalance (the “outside-in” hypothesis).
Inside-Out Hypothesis
While barrier impairment appears to occur in all patients with AD, it still is unclear how AD begins. The inside-out hypothesis suggests that cutaneous inflammation precedes barrier impairment and in fact may result in an impaired skin barrier. It has previously been reported that inflammatory states weaken the barrier by downregulating filaggrin production in the skin.13 Barrier disruption may be accompanied by transcutaneous penetration of allergens and increased Staphylococcus aureus counts. Recently, mutations and polymorphisms of inflammatory genes have been linked to AD (eg, single nucleotide polymorphisms of the IL4RA [interleukin 4 receptor, alpha] and CD14 [cluster of differentiation 14] genes, the serine protease inhibitor SPINK5 [serine peptidase inhibitor, Kazal type 5], RANTES [chemokine (C-C motif) ligand 5], IL-4, IL-13).14 These alterations highlight the role of systemic inflammation in triggering AD.
Outside-In Hypothesis
The outside-in hypothesis suggests that the impaired skin barrier precedes AD and is required for immune dysregulation to occur. This hypothesis was largely advanced by a study demonstrating that deactivating mutations of the filaggrin gene were linked to nearly 20% of AD cases in Northern European populations.15 Filaggrin (chromosome 1q21.3) performs an essential function in the skin barrier through its differential cleavage and the breakdown and release of natural moisturizing factor.16 Filaggrin gene mutations are associated with persistent AD, and it has been posited that environmental factors such as temperature and humidity also can affect filaggrin production as it relates to barrier function.17-19 Skin barrier disruption results in increased cutaneous and systemic Th2 responses (ie, IL-4/13), with thymic stromal lymphopoietin as the potential mechanism of Th2 cell recruitment.10,20 Inflammatory Th2 cells triggered by an impaired skin barrier also may predispose patients to the development of allergic diseases such as asthma, in line with Atopic March, or the progression of AD to other forms of atopy (eg, food allergy, asthma).5,7,21-23
The outside-in hypothesis may only explain the root pathogenesis of AD in a subset of patients, however, as only 1 in 5 cases of AD in Northern European and Asian populations are associated with underlying filaggrin mutations (which are only present in about 10% of those who are unaffected by AD).15 Filaggrin does not appear to account for the basis of AD in all cases. In a study of 762 newborns in Cincinnati, Ohio, 39% of children with at least one parent with atopy developed AD by 3 years of age, about quadruple of what would be projected based on filaggrin defects in general population studies, which are noted in only about 10% of white individuals.24 Furthermore, less than 5% of patients of African descent have mutations of the filaggrin 1 gene.25
Implications for the Prevention and Treatment of Atopic Dermatitis
Preventative strategies for AD currently are in development. Atopic dermatitis may be unpreventable because the in utero environment triggers some of the barrier alterations, which can be noted as early as 2 days following birth and will predict early-onset AD. The putative mechanism is via Th2 cytokines (IL-4, IL-13).26
Certainly, application of over-the-counter and prescription emollients are mainstays of treatment for AD and may suffice as monotherapy in cases of mild disease. In a recent randomized trial in the United States and the United Kingdom, emollients were used in newborns considered at high risk for AD (family history of atopy) until 6 months of age.27 The risk of AD development was reduced by half, irrespective of the emollient used. Unfortunately, 21.8% of children without a family history of atopy will develop AD; therefore, not all cases can be prevented if use of emollients is limited to newborns with a family history of atopy.28 Long-term follow-up is needed to track whether emollient use in newborns will prevent AD indefinitely.
Prevention of AD onset using systemic interventions has also been investigated. Probiotics have been suggested as a means to modify the gut microbiota and reduce systemic and mucosal inflammation. Lactobacillus reuteri taken prenatally by pregnant women and by newborns has shown mild benefit in preventing some forms of AD.29 Although they are not approved by the US Food and Drug Administration for this indication, systemic interventions for moderate-to-severe AD such as methotrexate and cyclosporine certainly have shown benefit in managing ongoing illness and breaking the cycle of disease.30 The efficacy of these agents points to the role of systemic inflammation in ongoing AD activity. Moreover, the inside-out hypothesis recently has led to the proliferation of promising new therapeutic agents in the pipeline to treat the systemic Th2 inflammation that occurs in severe AD (eg, anti–IL-4/13 receptor antibody, anti–IL-13 antibodies, and biologics targeting IL-12/23, IL-22, and IL-31 receptors).31
Final Thoughts
Atopic dermatitis is a multifactorial disease associated with barrier disruption and intense systemic inflammation. It is likely that both the inside-out and outside-in hypotheses hold true in different subsets of AD patients. It is clear that some individuals are born with filaggrin defects that sufficiently trigger systemic inflammation, resulting in AD. On the other hand, there are clearly some individuals with inflammatory dysregulation that results in systemic inflammation and secondary barrier disruption. Until we can determine the genomic triggering or promoting event in each individual patient, large-scale introduction of active prevention and severity reduction strategies may not be realistic. In the meantime, we can approach AD in childhood from the inside out, through appropriate treatment of systemic inflammation of AD, and from the outside in, with treatment and prevention via emollient use in newborns.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
- Margolis JS, Abuabara K, Bilker W, et al. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150:593-600.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Zheng T, Jinho Y, Oh MH, et al. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3:67-73.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care. Clin Dermatol. 2015;33:271-280.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
- Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
- Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Brown SJ, Irvine AD. Atopic eczema and the filaggrin story. Semin Cutan Med Surg. 2008;27:128-137.
- Harding CR, Aho S, Bosko CA. Filaggrin—revisited. Int J Cosmet Sci. 2013;35:412-423.
- Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29-40.
- Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease and increased heart attacks in 3 population-based studies [published online ahead of print July 4, 2015]. Allergy. doi:10.1111/all.12685.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
- Kelleher M, Dunn-Galvin A, Hourihane JO, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135:930-935.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Parazzini F, Cipriani S, Zinetti C, et al. Perinatal factors and the risk of atopic dermatitis: a cohort study. Pediatr Allergy Immunol. 2014;25:43-50.
- Abrahamsson TR, Jakobsson T, Böttcher MF, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174-1180.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Eczema drugs in development. National Eczema Association Web site. https://nationaleczema.org/research/phases-drug-development/. Accessed August 18, 2015.
- Hay RJ, Johns NE, Williams HC, et al. The global burden of skin disease in 2010: an analysis of the prevalence and impact of skin conditions. J Invest Dermatol. 2014;134:1527-1534.
- Kay J, Gawkrodger DJ, Mortimer MJ, et al. The prevalence of childhood atopic eczema in a general population. J Am Acad Dermatol. 1994;30:35-39.
- Margolis JS, Abuabara K, Bilker W, et al. Persistence of mild to moderate atopic dermatitis. JAMA Dermatol. 2014;150:593-600.
- Shaw TE, Currie GP, Koudelka CW, et al. Eczema prevalence in the United States: data from the 2003 National Survey of Children’s Health. J Invest Dermatol. 2011;131:67-73.
- Zheng T, Jinho Y, Oh MH, et al. The atopic march: progression from atopic dermatitis to allergic rhinitis and asthma. Allergy Asthma Immunol Res. 2011;3:67-73.
- De Benedetto A, Kubo A, Beck LA. Skin barrier disruption: a requirement for allergen sensitization? J Invest Dermatol. 2012;132:949-963.
- Visscher MO, Adam R, Brink S, et al. Newborn infant skin: physiology, development, and care. Clin Dermatol. 2015;33:271-280.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Kondo H, Ichikawa Y, Imokawa G. Percutaneous sensitization with allergens through barrier-disrupted skin elicits a Th2-dominant cytokine response. Eur J Immunol. 1998;28:769-779.
- Correa da Rosa J, Malajian D, Shemer A, et al. Patients with atopic dermatitis have attenuated and distinct contact hypersensitivity responses to common allergens in skin. J Allergy Clin Immunol. 2015;135:712-720.
- Paller AS. Latest approaches to treating atopic dermatitis. Chem Immunol Allergy. 2012;96:132-140.
- Batista DI, Perez L, Orfali RL, et al. Profile of skin barrier proteins (filaggrin, claudins 1 and 4) and Th1/Th2/Th17 cytokines in adults with atopic dermatitis. J Eur Acad Dermatol Venereol. 2015;29:1091-1095.
- Elias PM, Schmuth M. Abnormal skin barrier in the etiopathogenesis of atopic dermatitis. Curr Opin Allergy Clin Immunol. 2009;9:437-446.
- Hanifin JM. Evolving concepts of pathogenesis in atopic dermatitis and other eczemas. J Invest Dermatol. 2009;129:320-322.
- Brown SJ, Irvine AD. Atopic eczema and the filaggrin story. Semin Cutan Med Surg. 2008;27:128-137.
- Harding CR, Aho S, Bosko CA. Filaggrin—revisited. Int J Cosmet Sci. 2013;35:412-423.
- Carson CG, Rasmussen MA, Thyssen JP, et al. Clinical presentation of atopic dermatitis by filaggrin gene mutation status during the first 7 years of life in a prospective cohort study. PLoS One. 2012;7:e48678.
- Silverberg JI, Hanifin J, Simpson EL. Climatic factors are associated with childhood eczema prevalence in the United States. J Invest Dermatol. 2013;133:1752-1759.
- Sargen MR, Hoffstad O, Margolis DJ. Warm, humid, and high sun exposure climates are associated with poorly controlled eczema: PEER (Pediatric Eczema Elective Registry) cohort, 2004-2012. J Invest Dermatol. 2014;134:51-57.
- Hammad H, Lambrecht BN. Barrier epithelial cells and the control of type 2 immunity. Immunity. 2015;43:29-40.
- Silverberg JI. Association between adult atopic dermatitis, cardiovascular disease and increased heart attacks in 3 population-based studies [published online ahead of print July 4, 2015]. Allergy. doi:10.1111/all.12685.
- Amat F, Saint-Pierre P, Bourrat E, et al. Early-onset atopic dermatitis in children: which are the phenotypes at risk of asthma? results from the ORCA cohort. PLoS One. 2015;10:e0131369.
- Demehri S, Morimoto M, Holtzman MJ, et al. Skin-derived TSLP triggers progression from epidermal-barrier defects to asthma. PLoS Biol. 2009;7:e1000067.
- Biagini Myers JM, Wang N, LeMasters GK, et al. Genetic and environmental risk factors for childhood eczema development and allergic sensitization in the CCAAPS cohort. J Invest Dermatol. 2010;130:430-437.
- Margolis DJ, Apter AJ, Gupta J, et al. The persistence of atopic dermatitis and filaggrin (FLG) mutations in a US longitudinal cohort. J Allergy Clin Immunol. 2012;130:912-917.
- Kelleher M, Dunn-Galvin A, Hourihane JO, et al. Skin barrier dysfunction measured by transepidermal water loss at 2 days and 2 months predates and predicts atopic dermatitis at 1 year. J Allergy Clin Immunol. 2015;135:930-935.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Parazzini F, Cipriani S, Zinetti C, et al. Perinatal factors and the risk of atopic dermatitis: a cohort study. Pediatr Allergy Immunol. 2014;25:43-50.
- Abrahamsson TR, Jakobsson T, Böttcher MF, et al. Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:1174-1180.
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. Management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349.
- Eczema drugs in development. National Eczema Association Web site. https://nationaleczema.org/research/phases-drug-development/. Accessed August 18, 2015.
Creating an Action Plan for Eczema Patients
What does your patient need to know at the first visit?
The most essential information to share with patients at the first visit (as well as at all subsequent visits) is your eczema action plan, which should include discussion and potential modification of bathing regimens, use of topical emollients and medications, and exposure to detergents. It also is important to discuss the patient’s disease pattern (eg, triggers, seasonal flares, other forms of atopy), medication history, and past treatment responses. The eczema action plan is extremely vital for a variety of reasons. As a physician, I can’t be present every time a patient has a severe flare, and it would be difficult—both physically and financially—for patients to come in to my office every time a flare occurs. Patients and their caregivers need to develop a sense of empowerment at the first visit so they can address symptoms as they arise and actively prevent severe flares by following a gentle skin care plan that includes topical emollients and gentle cleansing.
I also like to emphasize to my eczema patients that they are not alone. In the United States, almost one-quarter of the population may have eczema. It’s also essential to explain to patients/caregivers that eczema is not caused by food allergies and cannot be cured by food elimination or other dietary modifications. Finally, I like to explain to patients that there is no true cure for eczema and that they will need to follow the action plan throughout their lifetime to help treat and prevent flares. Follow-up visits to review therapeutic response and review the patient’s eczema action plan can reinforce adherence and knowledge about the disease.
What are your go-to treatments? Are there any side effects?
Typically I prescribe 3 to 4 medications, which include an agent for the head and neck areas and/or areas of sensitive or thin skin, an agent for the body, an antihistamine to address sleep disturbances, and a rescue medication, which is a somewhat stronger topical agent for severe areas if present. Elimination of triggers such as fragrance and wool can be discussed. Review of Staphylococcus aureus as a trigger and addressing this trigger with bleach baths or other modifications (eg, topical antibacterials for crusted areas of skin) is needed.
Eczema treatment is a multistep process that varies by individual as well as by cost. For most eczema patients, treatment typically costs hundreds of dollars per year; therefore, I try to be mindful of the financial hardship that can be brought on by the need for many products. The mainstay of eczema therapy includes topical emollients along with gentle cleansers, laundry detergents, and other topical products. Topical corticosteroids are the first-line treatment and have been used for over 60 years with good outcomes in most patients when used judiciously; however, side effects including striae, glaucoma and hypothalamic-pituitary-adrenal axis suppression can occur. Topical corticosteroids should be selected by class and formulation—ointments and some newer base formulations are known to cause the least amount of stinging. In infants, the least potent agent that clears the skin effectively may maximize outcomes and minimize risk for side effects. Topical calcineurin inhibitors may be a good option in patients who do not respond to corticosteroids and are supported by excellent clinical evidence; however, be sure to consider the black box warnings.1-3 Sedating antihistamines can be prescribed for bedtime usage in pruritic patients who experience sleep disturbances.
How do you keep patients compliant with treatment?
Patients can only comply with treatment if they have an adequate supply of the treatment product. It is important to prescribe the right amount of product needed to treat the affected area. Provision of refills for recurrent disease also can ensure long-term treatment compliance.
It also is important to have a conversation with patients about the nature of their disease flares. In my practice, patients typically report having seasonal flares, especially in midsummer temperatures or when the indoor heating kicks on in late fall. Encourage patients to schedule appointments in advance of these seasons; refilling medications beforehand and liberal application of emollients also can mitigate seasonal flares.
Finally, I try to recommend or prescribe treatments that appeal to patients both physically and emotionally. Some patients have a fear of using topical corticosteroids (known as corticophobia or steroid phobia). For these patients, I maximize the use of topical emollients and/or enhanced emollients (eg, agents with lipid additives and ceramides) to reduce the need for topical corticosteroids. I also have found that many preteen boys dislike “sticky” emollients, so light or midweight creams may be more tolerable for nightly use in this population. Another common scenario is the patient who prefers natural products. There are a variety of natural agents available that can aid in the treatment of eczema, including coconut oil, ceramide-based products, and oleodistillates. I try to refer to the literature to encourage the use of natural products that are backed by good science rather than big hype.
What do you do if patients refuse treatment?
As a physician, I can’t force patients or their caregivers to adhere to the therapies I prescribe; however, most patients are genuinely seeking a better quality of life and therefore there usually is at least some aspect of a skin care regimen they will follow to achieve relief when needed. First I make sure that serious issues (eg, bacterial infections) are addressed. I do mention to patients/caregivers that lack of treatment with topical prescription agents may have biological consequences; for example, there is evidence to support the Atopic March (ie, progression of atopic diseases to food allergies, asthma, etc). Consequences also can include discomfort, reduced quality of life, and negative effects on personal relationships; pediatric patients also may be stigmatized by their peers. Exploration of the root cause of treatment refusal usually yields a helpful discussion with the patient/caregiver about their fears as well as alternative treatment agents. Sometimes I engage the pediatrician/primary care physician, an allergist, or a family member in the discussion to enhance compliance and provide patient/caregiver support. At the very least, most patients/caregivers will adhere to trigger avoidance and barrier repair through application of emollients.
What resources do you recommend to patients for more information?
There are many resources available to patients that may enhance the overall management of eczema. I give my patients an educational handout about eczema as well as a hardcopy of their personal eczema action plan. For pediatric patients, I write the child’s first name and the date to help his or her caregivers remember when they received the plan. Examples of eczema action plans can be found in published resources ranging from simple to complex regimens and should be tailored to the physician’s own patient education and treatment patterns.4,5 The National Eczema Association Web site (https://nationaleczema.org/) provides many resources for patients, including educational tools and an online community.
- Luger T, Boguniewicz M, Carr W, et al. Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants [published online ahead of print April 13, 2015]. Pediatr Allergy Immunol. 2015;26:306-315.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Hui RL, Lide W, Chan J, et al. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann Pharmacother. 2009;43:1956-1963.
- Eczema action plan. University of California, San Francisco Office of Continuing Medical Education Web site. http://www.ucsfcme.com/2011/slides/MPD11001/29 Cordoro-ADD1.pdf. Accessed November 17, 2015.
- Tollefson MM, Bruckner AL; Section On Dermatology. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:e1735-e1744.
What does your patient need to know at the first visit?
The most essential information to share with patients at the first visit (as well as at all subsequent visits) is your eczema action plan, which should include discussion and potential modification of bathing regimens, use of topical emollients and medications, and exposure to detergents. It also is important to discuss the patient’s disease pattern (eg, triggers, seasonal flares, other forms of atopy), medication history, and past treatment responses. The eczema action plan is extremely vital for a variety of reasons. As a physician, I can’t be present every time a patient has a severe flare, and it would be difficult—both physically and financially—for patients to come in to my office every time a flare occurs. Patients and their caregivers need to develop a sense of empowerment at the first visit so they can address symptoms as they arise and actively prevent severe flares by following a gentle skin care plan that includes topical emollients and gentle cleansing.
I also like to emphasize to my eczema patients that they are not alone. In the United States, almost one-quarter of the population may have eczema. It’s also essential to explain to patients/caregivers that eczema is not caused by food allergies and cannot be cured by food elimination or other dietary modifications. Finally, I like to explain to patients that there is no true cure for eczema and that they will need to follow the action plan throughout their lifetime to help treat and prevent flares. Follow-up visits to review therapeutic response and review the patient’s eczema action plan can reinforce adherence and knowledge about the disease.
What are your go-to treatments? Are there any side effects?
Typically I prescribe 3 to 4 medications, which include an agent for the head and neck areas and/or areas of sensitive or thin skin, an agent for the body, an antihistamine to address sleep disturbances, and a rescue medication, which is a somewhat stronger topical agent for severe areas if present. Elimination of triggers such as fragrance and wool can be discussed. Review of Staphylococcus aureus as a trigger and addressing this trigger with bleach baths or other modifications (eg, topical antibacterials for crusted areas of skin) is needed.
Eczema treatment is a multistep process that varies by individual as well as by cost. For most eczema patients, treatment typically costs hundreds of dollars per year; therefore, I try to be mindful of the financial hardship that can be brought on by the need for many products. The mainstay of eczema therapy includes topical emollients along with gentle cleansers, laundry detergents, and other topical products. Topical corticosteroids are the first-line treatment and have been used for over 60 years with good outcomes in most patients when used judiciously; however, side effects including striae, glaucoma and hypothalamic-pituitary-adrenal axis suppression can occur. Topical corticosteroids should be selected by class and formulation—ointments and some newer base formulations are known to cause the least amount of stinging. In infants, the least potent agent that clears the skin effectively may maximize outcomes and minimize risk for side effects. Topical calcineurin inhibitors may be a good option in patients who do not respond to corticosteroids and are supported by excellent clinical evidence; however, be sure to consider the black box warnings.1-3 Sedating antihistamines can be prescribed for bedtime usage in pruritic patients who experience sleep disturbances.
How do you keep patients compliant with treatment?
Patients can only comply with treatment if they have an adequate supply of the treatment product. It is important to prescribe the right amount of product needed to treat the affected area. Provision of refills for recurrent disease also can ensure long-term treatment compliance.
It also is important to have a conversation with patients about the nature of their disease flares. In my practice, patients typically report having seasonal flares, especially in midsummer temperatures or when the indoor heating kicks on in late fall. Encourage patients to schedule appointments in advance of these seasons; refilling medications beforehand and liberal application of emollients also can mitigate seasonal flares.
Finally, I try to recommend or prescribe treatments that appeal to patients both physically and emotionally. Some patients have a fear of using topical corticosteroids (known as corticophobia or steroid phobia). For these patients, I maximize the use of topical emollients and/or enhanced emollients (eg, agents with lipid additives and ceramides) to reduce the need for topical corticosteroids. I also have found that many preteen boys dislike “sticky” emollients, so light or midweight creams may be more tolerable for nightly use in this population. Another common scenario is the patient who prefers natural products. There are a variety of natural agents available that can aid in the treatment of eczema, including coconut oil, ceramide-based products, and oleodistillates. I try to refer to the literature to encourage the use of natural products that are backed by good science rather than big hype.
What do you do if patients refuse treatment?
As a physician, I can’t force patients or their caregivers to adhere to the therapies I prescribe; however, most patients are genuinely seeking a better quality of life and therefore there usually is at least some aspect of a skin care regimen they will follow to achieve relief when needed. First I make sure that serious issues (eg, bacterial infections) are addressed. I do mention to patients/caregivers that lack of treatment with topical prescription agents may have biological consequences; for example, there is evidence to support the Atopic March (ie, progression of atopic diseases to food allergies, asthma, etc). Consequences also can include discomfort, reduced quality of life, and negative effects on personal relationships; pediatric patients also may be stigmatized by their peers. Exploration of the root cause of treatment refusal usually yields a helpful discussion with the patient/caregiver about their fears as well as alternative treatment agents. Sometimes I engage the pediatrician/primary care physician, an allergist, or a family member in the discussion to enhance compliance and provide patient/caregiver support. At the very least, most patients/caregivers will adhere to trigger avoidance and barrier repair through application of emollients.
What resources do you recommend to patients for more information?
There are many resources available to patients that may enhance the overall management of eczema. I give my patients an educational handout about eczema as well as a hardcopy of their personal eczema action plan. For pediatric patients, I write the child’s first name and the date to help his or her caregivers remember when they received the plan. Examples of eczema action plans can be found in published resources ranging from simple to complex regimens and should be tailored to the physician’s own patient education and treatment patterns.4,5 The National Eczema Association Web site (https://nationaleczema.org/) provides many resources for patients, including educational tools and an online community.
What does your patient need to know at the first visit?
The most essential information to share with patients at the first visit (as well as at all subsequent visits) is your eczema action plan, which should include discussion and potential modification of bathing regimens, use of topical emollients and medications, and exposure to detergents. It also is important to discuss the patient’s disease pattern (eg, triggers, seasonal flares, other forms of atopy), medication history, and past treatment responses. The eczema action plan is extremely vital for a variety of reasons. As a physician, I can’t be present every time a patient has a severe flare, and it would be difficult—both physically and financially—for patients to come in to my office every time a flare occurs. Patients and their caregivers need to develop a sense of empowerment at the first visit so they can address symptoms as they arise and actively prevent severe flares by following a gentle skin care plan that includes topical emollients and gentle cleansing.
I also like to emphasize to my eczema patients that they are not alone. In the United States, almost one-quarter of the population may have eczema. It’s also essential to explain to patients/caregivers that eczema is not caused by food allergies and cannot be cured by food elimination or other dietary modifications. Finally, I like to explain to patients that there is no true cure for eczema and that they will need to follow the action plan throughout their lifetime to help treat and prevent flares. Follow-up visits to review therapeutic response and review the patient’s eczema action plan can reinforce adherence and knowledge about the disease.
What are your go-to treatments? Are there any side effects?
Typically I prescribe 3 to 4 medications, which include an agent for the head and neck areas and/or areas of sensitive or thin skin, an agent for the body, an antihistamine to address sleep disturbances, and a rescue medication, which is a somewhat stronger topical agent for severe areas if present. Elimination of triggers such as fragrance and wool can be discussed. Review of Staphylococcus aureus as a trigger and addressing this trigger with bleach baths or other modifications (eg, topical antibacterials for crusted areas of skin) is needed.
Eczema treatment is a multistep process that varies by individual as well as by cost. For most eczema patients, treatment typically costs hundreds of dollars per year; therefore, I try to be mindful of the financial hardship that can be brought on by the need for many products. The mainstay of eczema therapy includes topical emollients along with gentle cleansers, laundry detergents, and other topical products. Topical corticosteroids are the first-line treatment and have been used for over 60 years with good outcomes in most patients when used judiciously; however, side effects including striae, glaucoma and hypothalamic-pituitary-adrenal axis suppression can occur. Topical corticosteroids should be selected by class and formulation—ointments and some newer base formulations are known to cause the least amount of stinging. In infants, the least potent agent that clears the skin effectively may maximize outcomes and minimize risk for side effects. Topical calcineurin inhibitors may be a good option in patients who do not respond to corticosteroids and are supported by excellent clinical evidence; however, be sure to consider the black box warnings.1-3 Sedating antihistamines can be prescribed for bedtime usage in pruritic patients who experience sleep disturbances.
How do you keep patients compliant with treatment?
Patients can only comply with treatment if they have an adequate supply of the treatment product. It is important to prescribe the right amount of product needed to treat the affected area. Provision of refills for recurrent disease also can ensure long-term treatment compliance.
It also is important to have a conversation with patients about the nature of their disease flares. In my practice, patients typically report having seasonal flares, especially in midsummer temperatures or when the indoor heating kicks on in late fall. Encourage patients to schedule appointments in advance of these seasons; refilling medications beforehand and liberal application of emollients also can mitigate seasonal flares.
Finally, I try to recommend or prescribe treatments that appeal to patients both physically and emotionally. Some patients have a fear of using topical corticosteroids (known as corticophobia or steroid phobia). For these patients, I maximize the use of topical emollients and/or enhanced emollients (eg, agents with lipid additives and ceramides) to reduce the need for topical corticosteroids. I also have found that many preteen boys dislike “sticky” emollients, so light or midweight creams may be more tolerable for nightly use in this population. Another common scenario is the patient who prefers natural products. There are a variety of natural agents available that can aid in the treatment of eczema, including coconut oil, ceramide-based products, and oleodistillates. I try to refer to the literature to encourage the use of natural products that are backed by good science rather than big hype.
What do you do if patients refuse treatment?
As a physician, I can’t force patients or their caregivers to adhere to the therapies I prescribe; however, most patients are genuinely seeking a better quality of life and therefore there usually is at least some aspect of a skin care regimen they will follow to achieve relief when needed. First I make sure that serious issues (eg, bacterial infections) are addressed. I do mention to patients/caregivers that lack of treatment with topical prescription agents may have biological consequences; for example, there is evidence to support the Atopic March (ie, progression of atopic diseases to food allergies, asthma, etc). Consequences also can include discomfort, reduced quality of life, and negative effects on personal relationships; pediatric patients also may be stigmatized by their peers. Exploration of the root cause of treatment refusal usually yields a helpful discussion with the patient/caregiver about their fears as well as alternative treatment agents. Sometimes I engage the pediatrician/primary care physician, an allergist, or a family member in the discussion to enhance compliance and provide patient/caregiver support. At the very least, most patients/caregivers will adhere to trigger avoidance and barrier repair through application of emollients.
What resources do you recommend to patients for more information?
There are many resources available to patients that may enhance the overall management of eczema. I give my patients an educational handout about eczema as well as a hardcopy of their personal eczema action plan. For pediatric patients, I write the child’s first name and the date to help his or her caregivers remember when they received the plan. Examples of eczema action plans can be found in published resources ranging from simple to complex regimens and should be tailored to the physician’s own patient education and treatment patterns.4,5 The National Eczema Association Web site (https://nationaleczema.org/) provides many resources for patients, including educational tools and an online community.
- Luger T, Boguniewicz M, Carr W, et al. Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants [published online ahead of print April 13, 2015]. Pediatr Allergy Immunol. 2015;26:306-315.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Hui RL, Lide W, Chan J, et al. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann Pharmacother. 2009;43:1956-1963.
- Eczema action plan. University of California, San Francisco Office of Continuing Medical Education Web site. http://www.ucsfcme.com/2011/slides/MPD11001/29 Cordoro-ADD1.pdf. Accessed November 17, 2015.
- Tollefson MM, Bruckner AL; Section On Dermatology. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:e1735-e1744.
- Luger T, Boguniewicz M, Carr W, et al. Pimecrolimus in atopic dermatitis: consensus on safety and the need to allow use in infants [published online ahead of print April 13, 2015]. Pediatr Allergy Immunol. 2015;26:306-315.
- Carr WW. Topical calcineurin inhibitors for atopic dermatitis: review and treatment recommendations. Paediatr Drugs. 2013;15:303-310.
- Hui RL, Lide W, Chan J, et al. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann Pharmacother. 2009;43:1956-1963.
- Eczema action plan. University of California, San Francisco Office of Continuing Medical Education Web site. http://www.ucsfcme.com/2011/slides/MPD11001/29 Cordoro-ADD1.pdf. Accessed November 17, 2015.
- Tollefson MM, Bruckner AL; Section On Dermatology. Atopic dermatitis: skin-directed management. Pediatrics. 2014;134:e1735-e1744.
Status Report From the American Acne & Rosacea Society on Medical Management of Acne in Adult Women, Part 1: Overview, Clinical Characteristics, and Laboratory Evaluation
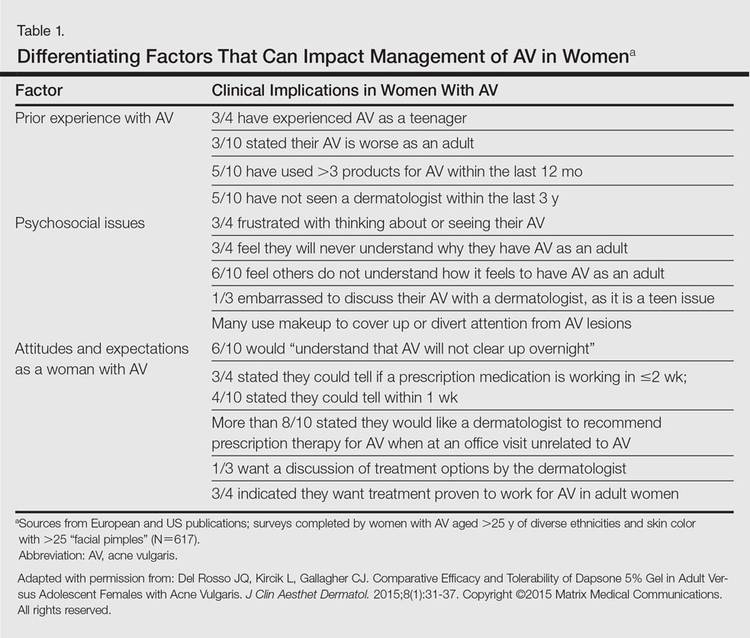
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.
Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
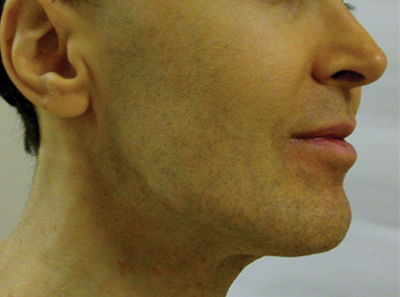
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33
Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.
Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33
Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
It was not long ago that acne vulgaris (AV) was commonly considered to be a skin disease that affected teenagers with little attention given to preadolescent and postadolescent AV. This perspective has changed, with more attention being given to AV across a broad range of affected age groups, including preadolescent, adolescent, and postadolescent subgroups.1-5 Earlier onset of adrenarche has led to earlier development of AV in many young girls, with a higher range of dehydroepiandrosterone sulfate (DHEAS) levels observed overall in those with AV as compared to a normal age-matched population.3,4 At the other end of the age spectrum, AV is a common phenomenon in adult females, with at least half of women estimated to exhibit some form of AV.1,2,5-8 Based on a large survey of females and males (N=1013), the prevalence of AV in adult females has been reported to be 50.9%, 35.2%, 26.3%, and 15.3% among women aged 20 to 29 years, 30 to 39 years, 40 to 49 years, and 50 years and older, respectively.2 Acne vulgaris that persists beyond adolescence into adulthood is termed persistent acne, or early-onset acne, and the development of AV in women 25 years and older who have not previously been affected by AV has been termed late-onset acne.6,8,9 Publications on the management of AV in adult women have focused primarily on systemic hormonal therapies; however, topical therapies more recently have received greater attention in this subpopulation9-12 and will be discussed in part 2 of this series. Because data on AV in women are limited primarily to involvement of the face and neck region, this article does not address truncal AV unless otherwise specified. Table 1 depicts factors that can influence the management of AV in adult women.
Visible Patterns and Considerations for Clinical Evaluation
Clinical Patterns
Although epidemiologic and demographic data are limited in the subpopulation of women with AV, it is reported that females account for up to 82% of adults with AV, with approximately 75% presenting with AV that is clinically similar to their disease course in adolescence.2,5,13 Among those women with persistent AV, some state that their AV is worse compared to adolescence, while others report it is not as severe. The pattern of AV often is similar to that seen in adolescence, presenting as mixed comedonal and inflammatory papular/pustular lesions diffusely distributed on the face; in other cases, a more selectively distributed U-shaped pattern is noted, characterized predominantly by inflammatory papules and/or nodules involving the lower cheeks and jawline margin, with lesions also commonly noted on the anterior and lateral neck.5,8,9,13-16 A U-shaped pattern is believed to be more common in late-onset AV, often with persistence into the mid-40s.1,15,17 It is important to emphasize the need for additional studies on the demographics and clinical characteristics of AV in adult females, especially correlations between onset, age, and clinical patterns of AV.
An international, prospective, observational study assessed the clinical characteristics of AV in adults (aged ≥25 years) at a dermatology visit for acne (N=374).16 Participants who were under management for their AV showed severity grades of mild (clear/almost clear) in 47.3% of cases. Involvement of multiple facial sites—cheeks, forehead, mandibular region, and temples—was noted in 89.8% of women, often with both inflammatory and comedonal lesions, which is a pattern similar to adolescent AV. Inflammatory lesions alone were observed in 6.4% of women, 17.1% had comedonal AV only, and truncal AV was present in 48.4%.16 Additional well-designed studies are needed to determine if this study reflects an accurate qualitative and quantitative depiction of the spectrum of AV in adult females.
Mandibular Pattern
In the observational study of AV in adults, AV localized to the mandibular area was noted in only 11.2% of participants.16 Women with localized mandibular AV were more likely than women without localized AV to be employed, noted greater daily stress levels, and tended to report more psychologically stressful jobs. Interestingly, the subgroup with mandibular acne alone was much less likely to exhibit a global severity grade of moderate or higher (7.1% vs 50.1%), truncal acne (19.0% vs 51.9%), postinflammatory hyperpigmentation (23.8% vs 51.9%), and erythema (19.0% vs 48.4%), suggesting a unique subset of AV presentation.16
Ethnicity/Skin Color
Women of all ethnicities and skin types may be affected by AV.1,18-20 Earlier age of onset of AV has been suggested in white women; however, earlier onset of adrenarche may be more frequent in black girls, which supports an earlier age of onset of AV in this subpopulation.15-17 Women with skin of color usually express greater concern with persistent dyschromia at sites where lesions have resolved, and presence of acne scars is a concern among women regardless of skin color, ethnicity, or race.18,20-22
Scarring
Acne scarring has been noted to affect up to three-fourths of adult women in one report17 and often is stated by patients to be a cause of concern and frustration.1,5,17
Perimenstrual Flaring
Flaring associated with menses is commonly reported in adult females with AV, with 56%, 17%, and 3% of women in one study (n=230) reporting worsening before, during, or after menses, respectively.21
External Factors
Comedogenic products used for skin care, cover-up makeup, or hair care may be important to consider in selected cases as potential etiologic or exacerbating factors in adult females with AV; they also may be used in the management of AV.23-25 Adult females often are perplexed and frustrated by the presence of AV after their teenaged years and anxiously wonder about or search for the potential causes. Many women use cosmetic products to cover up facial AV.5,23-25 Therefore, even if skin care or personal hygiene products or makeup are not believed to be an etiologic factor, many patients appreciate that their dermatologist addressed skin care and cosmetics as a component of AV management and provided appropriate recommendations.5,13
Ingestion of dietary supplements containing whey protein have been associated with precipitation of AV.26,27 Diets with specific content characteristics have been implicated as potential etiologic or exacerbating factors for AV; however, data are limited and specific recommendations remain elusive at present. Individual cases may warrant consideration of dietary factors, especially when treatment resistance is noted.28 Importantly, progestin-only contraceptives (ie, injectables, intrauterine devices) also can exacerbate or induce AV.29
Hyperandrogenism
Although most adult females with AV are reported to have normal serum androgen levels when tested, it is important to explore potential signs and symptoms that are suggestive of underlying hyperandrogenism through both the patient’s history and physical examination.9-11,21,29-33 Some investigators have suggested that underlying peripheral hyperandrogenism is the leading cause of AV in adult females, with or without concurrent polycystic ovarian syndrome (PCOS), though it is believed that most women with AV exhibit normal results when undergoing laboratory testing for androgen excess.10,11,21,29,30 Nevertheless, it is important to consider the possibility of underlying causes of androgen excess (Table 2), the most common being PCOS and late-onset congenital adrenal hyperplasia; an androgen-secreting tumor is less common.11,29-33 It is suggested that screening for underlying endocrinopathy should be conducted in women presenting with (1) AV recalcitrant to conventional treatment, (2) sudden emergence of severe AV, (3) concurrent signs/symptoms of androgen excess, and/or (4) AV relapse shortly after isotretinoin therapy.7,11,16,33
Hirsutism and acanthosis nigricans have been reported to be more reliable predictors of hyperandrogenism than androgenic alopecia.21 Although it may be subtle in some cases, acanthosis nigricans is harder to camouflage, so the clinician can usually detect it if a thorough physical examination is performed. However, a patient may not voluntarily report to the clinician and their staff that she has hair removed, so despite a thorough examination, the clinician may not detect hirsutism. Therefore, it is important to inquire directly about the presence of hairs (pigmented terminal vs “peach fuzz” hairs), their anatomic location, and any hair removal practices the patient has used. The absence of androgenic alopecia does not exclude underlying hyperandrogenism; however, its presence, especially in younger women, may serve as a clinical marker for underlying hyperandrogenism.5 Some women may camouflage more subtle alopecia through hairstyling, but obtaining this history usually is not problematic, as most women are distressed by any degree of hair loss.
Laboratory Evaluation—A relatively straightforward approach to the workup of androgen excess includes assessment of serum DHEAS, free testosterone, and total testosterone levels.10,30 Elevation of serum DHEAS levels indicates an adrenal source of androgen production. Elevation of testosterone is associated with excess androgens produced by the ovaries. Modest elevations of DHEAS are most commonly associated with late-onset congenital adrenal hyperplasia that may not have been previously diagnosed. Modest elevation of testosterone is most commonly associated with PCOS, which also can be accompanied by an elevated luteinizing hormone:follicle-stimulating hormone ratio of 2.5:1 to 3:1.10,30 Marked elevations of DHEAS or testosterone can be indicative of adrenal or ovarian tumors, respectively.30
In some cases, a woman might have elevated DHEAS and testosterone levels. A 17-hydroxyprogesterone test can help discriminate between an adrenal or ovarian source of androgen excess in these cases, as elevated 17-hydroxyprogesterone levels indicate that the androgens are coming from the adrenal gland.10,30
It is important that laboratory evaluation be performed when ovulation is not occurring. Blood tests can be drawn just prior to or during menses. It is important that a woman is not taking an oral contraceptive at the time of testing, which can mask an underlying endocrine abnormality.10,11,29,30 Generally, testing can be performed at least 4 to 6 weeks after stopping the oral contraceptive.
Psychosocial Impact
Facial AV exhibits a broad range of adverse psychological and social effects on many adult females.2,5,13,18 It can be associated with depression, anxiety, psychological stress, and suicidal ideation; therefore, thorough screening for these comorbidities may be warranted in some patients.2,18
Conclusion
The epidemiology, clinical presentation, and clinical and laboratory evaluation of AV in adult females was reviewed in part 1 of this 3-part series. It is important for the clinician to assess the clinical presentation, psychosocial effects, and the possibility of underlying causes of androgen excess. In part 2, skin care and topical management of AV in adult females will be discussed.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
1. Perkins AC, Maglione J, Hillebrand GG, et al. Acne vulgaris in women: prevalence across the life span. J Womens Health (Larchmt). 2012;21: 223-230.
2. Collier CN, Harper JC, Cafardi JA, et al. The prevalence of acne in adults 20 years and older. J Am Acad Dermatol. 2008;58:56-59.
3. Lucky AW, Biro FM, Huster GA, et al. Acne vulgaris in premenarchal girls. an early sign of puberty associated with rising levels of dehydroepiandrosterone. Arch Dermatol. 1994;130:308-314.
4. Mancini AJ, Baldwin HE, Eichenfield LF, et al. Acne life cycle: the spectrum of pediatric disease. Semin Cutan Med Surg. 2011;30(suppl 3):S2-S5.
5. Tanghetti EA, Kawata AK, Daniels SR, et al. Understanding the burden of adult female acne. J Clin Aesthet Dermatol. 2014;7:22-30.
6. Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41: 577-580.
7. Marks R. Acne and its management beyond the age of 35 years. Am J Clin Dermatol. 2004;5:459-462.
8. Preneau S, Dreno B. Female acne—a different subtype of teenager acne? J Eur Acad Dermatol Venereol. 2012;26:277-282.
9. Kim GK, Del Rosso JQ. Oral spironolactone in post-teenage female patients with acne vulgaris: practical considerations for the clinician based on current data and clinical experience. J Clin Aesthet Dermatol. 2012;5:37-50.
10. Thiboutot D, Chen W. Update and future of hormonal therapy in acne. Dermatology. 2003;206:57-67.
11. Villasenor J, Berson D, Kroshinsky D. Treatment guidelines in adult women. In: Shalita AR, Del Rosso JQ, Webster GF, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:198-207.
12. Del Rosso JQ, Zeichner J. What’s new in the medicine cabinet? a panoramic review of clinically relevant information for the busy dermatologist. J Clin Aesthet Dermatol. 2014;7:26-30.
13. Del Rosso JQ, Kircik L, Gallagher CJ. Comparative efficacy and tolerability of dapsone 5% gel in adult versus adolescent females with acne vulgaris. J Clin Aesthet Dermatol. 2015;8:31-37.
14. Dreno B, Layton A, Zouboulis CC, et al. Adult female acne: a new paradigm. J Eur Acad Dermatol Venereol. 2013;27:1063-1070.
15. Choi CW, Lee DH, Kim HS, et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25:454-461.
16. Dréno B, Thiboutot D, Layton AM, et al; Global Alliance to Improve Outcomes in Acne. Large-scale international study enhances understanding of an emerging acne population: adult females. J Eur Acad Dermatol Venereol. 2015;29:1096-1106.
17. Kane A, Niang SO, Diagne AC, et al. Epidemiologic, clinical, and therapeutic features of acne in Dakar, Senegal. Int J Dermatol. 2007;46(suppl 1):36-38.
18. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7:19-31.
19. Davis SA, Narahari S, Feldman SR, et al. Top dermatologic conditions in patients of color: an analysis of nationally representative data. J Drugs Dermatol. 2012;11:466-473.
20. Rendon MI, Rodriguez DA, Kawata AK, et al. Acne treatment patterns, expectations, and satisfaction among adult females of different races/ethnicities. Clin Cosmet Investig Dermatol. 2015;8:231-238.
21. Khunger N, Kumar C. A clinico-epidemiological study of adult acne: is it different from adolescent acne? Indian J Dermatol Venereol Leprol. 2012;78: 335-341.
22. Alexis AF. Acne vulgaris in skin of color: understanding nuances and optimizing treatment outcomes. J Drugs Dermatol. 2014;13(suppl 6):S61-S65.
23. Dall’oglio F, Tedeschi A, Fabbrocini G, et al. Cosmetics for acne: indications and recommendations for an evidence-based approach. G Ital Dermatol Venereol. 2015;150:1-11.
24. Draelos Z. Facial cosmetics for acne patients. In: Draelos Z. Cosmetics in Dermatology. 2nd Ed. New York, NY: Churchill Livingstone Inc; 1995:15-28.
25. Cunliffe WJ. Acne. London, United Kingdom: Martin Dunitz Ltd; 1989.
26. Simonart T. Acne and whey protein supplementation among bodybuilders. Dermatology. 2012;225:256-258.
27. Silverberg NB. Whey protein precipitating moderate to severe acne flares in 5 teenaged athletes. Cutis. 2012;90:70-72.
28. Bronsnick T, Murzaku EC, Rao BK. Diet in dermatology: part I. atopic dermatitis, acne, and nonmelanoma skin cancer. J Am Acad Dermatol. 2014;71:1039.
29. Keri J, Berson DS, Thiboutot DM. Hormonal treatment of acne in women. In: Shalita AR, Del Rosso J, Webster G, eds. Acne Vulgaris. London, United Kingdom: Informa Healthcare; 2011:146-155.
30. Thiboutot D. Hormones and acne: pathophysiology, clinical evaluation and therapies. Sem Cutan Med Surg. 2001;20:144-153.
31. Borgia F, Cannavò S, Guarneri F, et al. Correlation between endocrinological parameters and acne severity in adult women. Acta Derm Venereol. 2004;84:201-204.
32. Clark CM, Rudolph J, Gerber DA, et al. Dermatologic manifestation of hyperandrogenism: a retrospective chart review. Skinmed. 2014;12:84-88.
33. Zeichner JA. Evaluating and treating the adult female patient with acne. J Drugs Dermatol. 2013;12:1416-1427.
Practice Points
- Acne in adult women is common and may persist beyond the adolescent years or may be late in onset with emergence usually during the early to mid-20s.
- Adult women with acne often are frustrated, as they perceive it as a disorder of teenagers and are perplexed by its presence later in life. They often are distressed by unpredictable flares as well as difficulty with covering lesions and associated dyschromia and scarring.
- Clinical patterns of acne in adult women are mixed inflammatory and comedonal facial acne or a U-shaped pattern of inflammatory lesions involving the lower face and neck.
- Laboratory testing is not considered mandatory in all cases. The clinician is encouraged to carefully evaluate each case and determine if further evaluation to detect a cause of androgen excess is warranted.
What Is Your Diagnosis? Onychomadesis Following Hand-foot-and-mouth Disease
The Diagnosis: Onychomadesis Following Hand-foot-and-mouth Disease
In 1846, Joseph Honoré Simon Beau described specific diagnostic signs manifested in the nails during various disease states.1 He suggested that the width of the nail plate depression correlated with the duration of illness. Since then, the correlation of nail changes during times of illness has been confirmed. The term Beau lines currently is used to describe transverse ridging of the nail plate due to transient arrest in nail plate formation.1 Onychomadesis is believed to be an extreme form of Beau lines in which the whole thickness of the nail plate is affected, resulting in its separation from the proximal nail fold and shedding of the nail plate.
Nail plate detachment in onychomadesis is due to a severe insult that results in complete arrest of the nail matrix activity. Onychomadesis has a wide spectrum of clinical presentations, ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.2 Trauma is the leading cause of single-digit onychomadesis, while multiple-digit onychomadesis usually is caused by a systemic disease (eg, blistering illnesses). Cases of multiple-nail onychomadesis have been reported following hand-foot-and-mouth disease (HFMD), though the majority of cases of HFMD do not present with onychomadesis.
Hand-foot-and-mouth disease is most commonly caused by 2 types of intestinal strains of Human enterovirus A: (1) coxsackievirus A6 (CVA6) or A16 (CVA16) and (2) enterovirus 71.3,4 Symptoms of HFMD include fever and sore throat followed by the development of oral ulcerations 1 to 2 days later. A vesicular or maculopapular rash can then develop on the hands, feet, and mouth. Complications following HFMD are rare but can include encephalitis, meningitis, and pneumonia. Symptoms typically resolve after 6 days without any treatment.3
A cluster of onychomadesis cases following HFMD outbreaks have been reported in Europe, Asia, and the United States. In some reports, causative viral strains have been identified. After a national HFMD outbreak in Finland in fall 2008, investigators isolated strains of CVA6 in the shedded nails of sibling patients.4 The CVA6 strain was found to be the primary pathogen causing that particular HFMD outbreak and onychomadesis was a hallmark presentation of this viral epidemic. Previously, HFMD outbreaks were known to be caused by CVA16 or enterovirus 71, with enterovirus 71 strains occurring mostly in Southeast Asia and Australia.4 In a report from Taiwan, the incidence of onychomadesis after CVA6 infection was 37% (48/130) as compared to 5% (7/145) in cases with non-CVA6 causative strains. Among patients with onychomadesis, 69% (33/48) were reported to experience concurrent palmoplantar desquamation before or during presentation of nail changes.5
Another Finnish study investigated an atypical outbreak of HFMD that occurred primarily in adult patients.6 Many of these patients also had onychomadesis several weeks following HFMD. Of 317 cases, human enteroviruses were detected in specimens from 212 cases (67%), including both children and adults. Two human enterovirus types—CVA6 (71% [83/117]) and coxsackievirus A10 (28% [33/117])—were identified as the causative agents of the outbreak. One genetic variant of CVA6 predominated, but 3 other genetically distinct CVA6 strains also were found.6 The 2008 HFMD outbreak in Finland was found to be caused by 2 concomitantly circulating human enteroviruses, which up until now have been infrequently detected together as causative agents of HFMD. Onychomadesis was a common occurrence in the Finnish HFMD outbreak, which has been previously linked to CVA6. The co-circulation of CVA6 and coxsackievirus A10 suggests an endemic emergence of new genetic variants of these enteroviruses.6
There also have been several reports of onychomadesis outbreaks in Spain, 2 of which occurred in nursery settings. One report noted that patients with a history of HFMD were 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).3 There also was a noted difference in prevalence of onychomadesis regarding age: a 55% (18/33) occurrence rate was noted in the youngest age group (9–23 months), 30% (8/27) in the middle age group (24–32 months), and 4% (1/28) in the oldest age group (33–42 months). Occurrence of onychomadesis and nail plate changes was observed on average 40 days after HFMD, and an average of 4 nails were shed per case.3 A report investigating a separate HFMD outbreak in Spain found a high percentage of onychomadesis (96% [298/311]) occurring in children younger than 6 years. This outbreak, which occurred in the metropolitan area of Valencia, was associated with an outbreak of HFMD primarily caused by coxsackievirus A10.7 A third Spanish study uncovered a high occurrence of onychomadesis in a nursery setting as a consequence of HFMD, where 92% (11/12) of onychomadesis cases were preceded by HFMD 2 months prior.8
A case series reported in Chicago, Illinois, in the late 1990s identified 5 pediatric patients with HFMD associated with Beau lines and onychomadesis.1 Only 3 of 5 (60%) patients had a fever; therefore, fever-induced nail matrix arrest was ruled out as the inciting factor of the nail changes seen. All patients were given over-the-counter analgesics and 2 received antibiotics (amoxicillin for the first 48 hours). None of these medications have been implicated as plausible causes of nail matrix arrest. Two patients were siblings and the rest were not related. None of the patients had a history of close physical proximity (eg, attendance at the same day care or school). All 5 patients developed HFMD within 4 weeks of one another, and all were from the suburbs of Chicago (with 4 of 5 [80%] patients living within a 60-mile radius of each other). Although the causative viral strain was not isolated, the authors concluded that all the patients were likely to have been infected by the same virus due to the general vicinity of the patients to each other. Furthermore, the collective case reports likely represented an HFMD epidemic caused by a particular strain that can induce onychomadesis.1
Supportive care for the viral illness paired with protection of the nail bed until new nail growth occurs is ideal, which requires maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails.
Onychomadesis can follow HFMD, especially in cases caused by CVA6. Cases of onychomadesis are mild and self-limited. When onychomadesis is noted, historical review of viral illnesses within 1 to 2 months prior to nail changes often will identify the causative disease.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. China: Elsevier; 2012:1130-1131.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain, associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
The Diagnosis: Onychomadesis Following Hand-foot-and-mouth Disease
In 1846, Joseph Honoré Simon Beau described specific diagnostic signs manifested in the nails during various disease states.1 He suggested that the width of the nail plate depression correlated with the duration of illness. Since then, the correlation of nail changes during times of illness has been confirmed. The term Beau lines currently is used to describe transverse ridging of the nail plate due to transient arrest in nail plate formation.1 Onychomadesis is believed to be an extreme form of Beau lines in which the whole thickness of the nail plate is affected, resulting in its separation from the proximal nail fold and shedding of the nail plate.
Nail plate detachment in onychomadesis is due to a severe insult that results in complete arrest of the nail matrix activity. Onychomadesis has a wide spectrum of clinical presentations, ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.2 Trauma is the leading cause of single-digit onychomadesis, while multiple-digit onychomadesis usually is caused by a systemic disease (eg, blistering illnesses). Cases of multiple-nail onychomadesis have been reported following hand-foot-and-mouth disease (HFMD), though the majority of cases of HFMD do not present with onychomadesis.
Hand-foot-and-mouth disease is most commonly caused by 2 types of intestinal strains of Human enterovirus A: (1) coxsackievirus A6 (CVA6) or A16 (CVA16) and (2) enterovirus 71.3,4 Symptoms of HFMD include fever and sore throat followed by the development of oral ulcerations 1 to 2 days later. A vesicular or maculopapular rash can then develop on the hands, feet, and mouth. Complications following HFMD are rare but can include encephalitis, meningitis, and pneumonia. Symptoms typically resolve after 6 days without any treatment.3
A cluster of onychomadesis cases following HFMD outbreaks have been reported in Europe, Asia, and the United States. In some reports, causative viral strains have been identified. After a national HFMD outbreak in Finland in fall 2008, investigators isolated strains of CVA6 in the shedded nails of sibling patients.4 The CVA6 strain was found to be the primary pathogen causing that particular HFMD outbreak and onychomadesis was a hallmark presentation of this viral epidemic. Previously, HFMD outbreaks were known to be caused by CVA16 or enterovirus 71, with enterovirus 71 strains occurring mostly in Southeast Asia and Australia.4 In a report from Taiwan, the incidence of onychomadesis after CVA6 infection was 37% (48/130) as compared to 5% (7/145) in cases with non-CVA6 causative strains. Among patients with onychomadesis, 69% (33/48) were reported to experience concurrent palmoplantar desquamation before or during presentation of nail changes.5
Another Finnish study investigated an atypical outbreak of HFMD that occurred primarily in adult patients.6 Many of these patients also had onychomadesis several weeks following HFMD. Of 317 cases, human enteroviruses were detected in specimens from 212 cases (67%), including both children and adults. Two human enterovirus types—CVA6 (71% [83/117]) and coxsackievirus A10 (28% [33/117])—were identified as the causative agents of the outbreak. One genetic variant of CVA6 predominated, but 3 other genetically distinct CVA6 strains also were found.6 The 2008 HFMD outbreak in Finland was found to be caused by 2 concomitantly circulating human enteroviruses, which up until now have been infrequently detected together as causative agents of HFMD. Onychomadesis was a common occurrence in the Finnish HFMD outbreak, which has been previously linked to CVA6. The co-circulation of CVA6 and coxsackievirus A10 suggests an endemic emergence of new genetic variants of these enteroviruses.6
There also have been several reports of onychomadesis outbreaks in Spain, 2 of which occurred in nursery settings. One report noted that patients with a history of HFMD were 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).3 There also was a noted difference in prevalence of onychomadesis regarding age: a 55% (18/33) occurrence rate was noted in the youngest age group (9–23 months), 30% (8/27) in the middle age group (24–32 months), and 4% (1/28) in the oldest age group (33–42 months). Occurrence of onychomadesis and nail plate changes was observed on average 40 days after HFMD, and an average of 4 nails were shed per case.3 A report investigating a separate HFMD outbreak in Spain found a high percentage of onychomadesis (96% [298/311]) occurring in children younger than 6 years. This outbreak, which occurred in the metropolitan area of Valencia, was associated with an outbreak of HFMD primarily caused by coxsackievirus A10.7 A third Spanish study uncovered a high occurrence of onychomadesis in a nursery setting as a consequence of HFMD, where 92% (11/12) of onychomadesis cases were preceded by HFMD 2 months prior.8
A case series reported in Chicago, Illinois, in the late 1990s identified 5 pediatric patients with HFMD associated with Beau lines and onychomadesis.1 Only 3 of 5 (60%) patients had a fever; therefore, fever-induced nail matrix arrest was ruled out as the inciting factor of the nail changes seen. All patients were given over-the-counter analgesics and 2 received antibiotics (amoxicillin for the first 48 hours). None of these medications have been implicated as plausible causes of nail matrix arrest. Two patients were siblings and the rest were not related. None of the patients had a history of close physical proximity (eg, attendance at the same day care or school). All 5 patients developed HFMD within 4 weeks of one another, and all were from the suburbs of Chicago (with 4 of 5 [80%] patients living within a 60-mile radius of each other). Although the causative viral strain was not isolated, the authors concluded that all the patients were likely to have been infected by the same virus due to the general vicinity of the patients to each other. Furthermore, the collective case reports likely represented an HFMD epidemic caused by a particular strain that can induce onychomadesis.1
Supportive care for the viral illness paired with protection of the nail bed until new nail growth occurs is ideal, which requires maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails.
Onychomadesis can follow HFMD, especially in cases caused by CVA6. Cases of onychomadesis are mild and self-limited. When onychomadesis is noted, historical review of viral illnesses within 1 to 2 months prior to nail changes often will identify the causative disease.
The Diagnosis: Onychomadesis Following Hand-foot-and-mouth Disease
In 1846, Joseph Honoré Simon Beau described specific diagnostic signs manifested in the nails during various disease states.1 He suggested that the width of the nail plate depression correlated with the duration of illness. Since then, the correlation of nail changes during times of illness has been confirmed. The term Beau lines currently is used to describe transverse ridging of the nail plate due to transient arrest in nail plate formation.1 Onychomadesis is believed to be an extreme form of Beau lines in which the whole thickness of the nail plate is affected, resulting in its separation from the proximal nail fold and shedding of the nail plate.
Nail plate detachment in onychomadesis is due to a severe insult that results in complete arrest of the nail matrix activity. Onychomadesis has a wide spectrum of clinical presentations, ranging from mild transverse ridges of the nail plate (Beau lines) to complete nail shedding.2 Trauma is the leading cause of single-digit onychomadesis, while multiple-digit onychomadesis usually is caused by a systemic disease (eg, blistering illnesses). Cases of multiple-nail onychomadesis have been reported following hand-foot-and-mouth disease (HFMD), though the majority of cases of HFMD do not present with onychomadesis.
Hand-foot-and-mouth disease is most commonly caused by 2 types of intestinal strains of Human enterovirus A: (1) coxsackievirus A6 (CVA6) or A16 (CVA16) and (2) enterovirus 71.3,4 Symptoms of HFMD include fever and sore throat followed by the development of oral ulcerations 1 to 2 days later. A vesicular or maculopapular rash can then develop on the hands, feet, and mouth. Complications following HFMD are rare but can include encephalitis, meningitis, and pneumonia. Symptoms typically resolve after 6 days without any treatment.3
A cluster of onychomadesis cases following HFMD outbreaks have been reported in Europe, Asia, and the United States. In some reports, causative viral strains have been identified. After a national HFMD outbreak in Finland in fall 2008, investigators isolated strains of CVA6 in the shedded nails of sibling patients.4 The CVA6 strain was found to be the primary pathogen causing that particular HFMD outbreak and onychomadesis was a hallmark presentation of this viral epidemic. Previously, HFMD outbreaks were known to be caused by CVA16 or enterovirus 71, with enterovirus 71 strains occurring mostly in Southeast Asia and Australia.4 In a report from Taiwan, the incidence of onychomadesis after CVA6 infection was 37% (48/130) as compared to 5% (7/145) in cases with non-CVA6 causative strains. Among patients with onychomadesis, 69% (33/48) were reported to experience concurrent palmoplantar desquamation before or during presentation of nail changes.5
Another Finnish study investigated an atypical outbreak of HFMD that occurred primarily in adult patients.6 Many of these patients also had onychomadesis several weeks following HFMD. Of 317 cases, human enteroviruses were detected in specimens from 212 cases (67%), including both children and adults. Two human enterovirus types—CVA6 (71% [83/117]) and coxsackievirus A10 (28% [33/117])—were identified as the causative agents of the outbreak. One genetic variant of CVA6 predominated, but 3 other genetically distinct CVA6 strains also were found.6 The 2008 HFMD outbreak in Finland was found to be caused by 2 concomitantly circulating human enteroviruses, which up until now have been infrequently detected together as causative agents of HFMD. Onychomadesis was a common occurrence in the Finnish HFMD outbreak, which has been previously linked to CVA6. The co-circulation of CVA6 and coxsackievirus A10 suggests an endemic emergence of new genetic variants of these enteroviruses.6
There also have been several reports of onychomadesis outbreaks in Spain, 2 of which occurred in nursery settings. One report noted that patients with a history of HFMD were 14 times more likely to develop onychomadesis (relative risk, 14; 95% confidence interval, 4.57-42.86).3 There also was a noted difference in prevalence of onychomadesis regarding age: a 55% (18/33) occurrence rate was noted in the youngest age group (9–23 months), 30% (8/27) in the middle age group (24–32 months), and 4% (1/28) in the oldest age group (33–42 months). Occurrence of onychomadesis and nail plate changes was observed on average 40 days after HFMD, and an average of 4 nails were shed per case.3 A report investigating a separate HFMD outbreak in Spain found a high percentage of onychomadesis (96% [298/311]) occurring in children younger than 6 years. This outbreak, which occurred in the metropolitan area of Valencia, was associated with an outbreak of HFMD primarily caused by coxsackievirus A10.7 A third Spanish study uncovered a high occurrence of onychomadesis in a nursery setting as a consequence of HFMD, where 92% (11/12) of onychomadesis cases were preceded by HFMD 2 months prior.8
A case series reported in Chicago, Illinois, in the late 1990s identified 5 pediatric patients with HFMD associated with Beau lines and onychomadesis.1 Only 3 of 5 (60%) patients had a fever; therefore, fever-induced nail matrix arrest was ruled out as the inciting factor of the nail changes seen. All patients were given over-the-counter analgesics and 2 received antibiotics (amoxicillin for the first 48 hours). None of these medications have been implicated as plausible causes of nail matrix arrest. Two patients were siblings and the rest were not related. None of the patients had a history of close physical proximity (eg, attendance at the same day care or school). All 5 patients developed HFMD within 4 weeks of one another, and all were from the suburbs of Chicago (with 4 of 5 [80%] patients living within a 60-mile radius of each other). Although the causative viral strain was not isolated, the authors concluded that all the patients were likely to have been infected by the same virus due to the general vicinity of the patients to each other. Furthermore, the collective case reports likely represented an HFMD epidemic caused by a particular strain that can induce onychomadesis.1
Supportive care for the viral illness paired with protection of the nail bed until new nail growth occurs is ideal, which requires maintaining short nails and using adhesive bandages over the affected nails to avoid snagging the nail or ripping off the partially attached nails.
Onychomadesis can follow HFMD, especially in cases caused by CVA6. Cases of onychomadesis are mild and self-limited. When onychomadesis is noted, historical review of viral illnesses within 1 to 2 months prior to nail changes often will identify the causative disease.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. China: Elsevier; 2012:1130-1131.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain, associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
- Clementz GC, Mancini AJ. Nail matrix arrest following hand-foot-mouth disease: a report of five children. Pediatr Dermatol. 2000;17:7-11.
- Tosti A, Piraccini BM. Nail disorders. In: Bolognia JL, Jorizzo JL, Rapini RP, eds. Dermatology. China: Elsevier; 2012:1130-1131.
- Guimbao J, Rodrigo P, Alberto MJ, et al. Onychomadesis outbreak linked to hand, foot, and mouth disease, Spain, July 2008. Euro Surveill. 2010;15:19663.
- Osterback R, Vuorinen T, Linna M, et al. Coxsackievirus A6 and hand, foot, and mouth disease, Finland. Emerg Infect Dis. 2009;15:1485-1488.
- Wei SH, Huang YP, Liu MC, et al. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect Dis. 2011;11:346.
- Blomqvist S, Klemola P, Kaijalainen S, et al. Co-circulation of coxsackieviruses A6 and A10 in hand, foot and mouth disease outbreak in Finland. J Clin Virol. 2010;48:49-54.
- Davia JL, Bel PH, Ninet VZ, et al. Onychomadesis outbreak in Valencia, Spain, associated with hand, foot, and mouth disease caused by enteroviruses. Pediatr Dermatol. 2011;28:1-5.
- Cabrerizo M, De Miguel T, Armada A, et al. Onychomadesis after a hand, foot, and mouth disease outbreak in Spain, 2009. Epidemiol Infect. 2010;138:1775-1778.
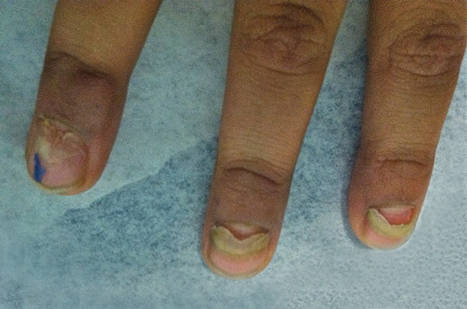
Vitiligo Disease Triggers: Psychological Stressors Preceding the Onset of Disease
Vitiligo is the loss of skin pigmentation caused by autoimmune destruction of melanocytes. Multiple pathogenic factors for vitiligo have been described, including CD8+ T lymphocyte/T helper 1 infiltrates in lesional skin1,2 with increased expression of IFN-γ3 and tumor necrosis factor α,3-6 decreased transforming growth factor β,7 and circulating autoantibodies against tyrosine hydroxylase.8 Additionally, several studies have found a high prevalence of antecedent psychological stressors in vitiligo patients, suggesting that specific stressors may trigger and/or exacerbate vitiligo.9-12
The relationship between antecedent psychological stressors and vitiligo extent has not been well studied. Potential mechanisms for stress-triggered vitiligo include increased catecholamines13 and neuropeptides,14 which have been found in vitiligo patients. However, the complex relationship between stressors and subsequent vitiligo is not well defined. We hypothesized that persistent stressors are associated with increased vitiligo extent.
Vitiligo is classically considered to be a silent pigmentary disorder with few or no symptoms. Prior studies have demonstrated that one-third of vitiligo patients report skin symptoms (eg, pruritus, burning), which may be specifically associated with early-onset disease.15-17 Further, we observed that some vitiligo patients report abdominal cramping associated with their disease. Few studies have described the burden of skin symptoms and other associated symptoms in vitiligo or their determinants.
We conducted a prospective questionnaire-based study of 1541 adult vitiligo patients to identify psychological factors that may precede vitiligo onset. We hypothesized that some types of stressors that occur within 2 years prior to disease onset would have specific associations with vitiligo and/or somatic symptoms.
Methods
Study Population and Questionnaire Distribution
This prospective questionnaire-based study was approved by the institutional review board at St. Luke’s-Roosevelt Hospital Center (now Mount Sinai St. Luke’s-Roosevelt) (New York, New York) for adults (>18 years; male or female) with vitiligo. The survey was validated in paper format at St. Luke’s-Roosevelt Hospital Center and distributed online to members of nonprofit support groups for vitiligo vulgaris, as previously described.15
Questionnaire
The a priori aim of this questionnaire was to identify psychological factors that may precede vitiligo onset. The questionnaire consisted of 77 items (55 closed questions and 22 open questions) pertaining to participant demographics/vitiligo phenotype and psychological stressors preceding vitiligo onset. The questions related to this study and response rates are listed in eTable 1. Responses were verified by screening for noninteger or implausible values (eg, <0 or >100 years of age).
Sample Size
The primary outcome used for sample size calculation was the potential association between vitiligo and the presence of antecedent psychological stressors. Using a 2-tailed test, we determined that a sample size of 1264 participants would have 90% power at α=.05 and a baseline proportion of 0.01 (1% presumed prevalence of vitiligo) to detect an odds ratio (OR) of 2.5 or higher.18
Data and Statistical Analysis
Closed question responses were analyzed using descriptive statistics. Open-ended question responses were analyzed using content analysis. Related comments were coded and grouped, with similarities and differences noted. All data processing and statistics were done with SAS version 9.2. Age at diagnosis (years) and number of anatomic sites affected were divided into tertiles for statistical analysis due to wide skewing.
Logistic regression models were constructed with numbers of reported deaths or stressors per participant within the 2 years prior to vitiligo onset as independent variables (0, 1, or ≥2), and symptoms associated with vitiligo as dependent variables. Adjusted ORs were calculated from multivariate models that included sex, current age (continuous), and comorbid autoimmune disease (binary) as covariates. Linear interaction terms were tested and were included in final models if statistically significant (P<.05).
Ordinal logistic regression was used to analyze the relationship between stressors (and other independent variables) and number of anatomic sites affected with vitiligo (tertiles). Ordinal logistic regression models were constructed to examine the impact of psychological stressors on pruritus secondary to vitiligo (not relevant combined with not at all, a little, a lot, very much) as the dependent variable. The proportional odds assumption was met in both models, as judged by score testing (P>.05). Binary logistic regression was used to analyze laterality, body surface area (BSA) greater than 25%, and involvement of the face and/or body with vitiligo lesions (binary).
Binary logistic regression models were constructed with impact of psychological stressors preceding vitiligo onset on comorbid abdominal cramping and specific etiologies as the dependent variables. There were 20 candidate stressors occurring within the 2 years prior to vitiligo onset. Selection methods for predictors were used to identify significant covariates within the context of the other covariates included in the final models. The results of forward, backward, and stepwise approaches were similar, and the stepwise selection output was presented.
Missing values were encountered because some participants did not respond to all the questionnaire items. A complete case analysis was performed (ie, missing values were ignored throughout the study). Data imputation was considered by multiple imputations; however, there were few or no differences between the estimates from the 2 approaches. Therefore, final models did not involve data imputation.
The statistical significance for all estimates was considered to be P<.05. However, a P value near .05 should be interpreted with caution given the multiple dependent tests performed in this study with increased risk for falsely rejecting the null hypothesis.
Results
Survey Population Characteristics
One thousand seven hundred participants started the survey; 1632 completed the survey (96.0% completion rate) and 1553 had been diagnosed with vitiligo by a physician. Twelve participants were excluded because they were younger than 18 years, leaving 1541 evaluable participants. Five hundred thirty-eight participants (34.9%) had comorbid autoimmune disorders. Demographics and disease phenotypes of the study participants are listed in Table 1.
Stressors Preceding Vitiligo Onset
Eight hundred twenty-one participants (56.6%) experienced at least one death or stressor within 2 years prior to vitiligo onset (Table 2), including death of a loved one (16.6%) and stressful life events (51.0%) within the 2 years prior to the onset of vitiligo, especially work/financial problems (10.8%), end of a long-term relationship (10.2%), and family problems (not otherwise specified)(7.8%). Two hundred (13.5%) participants reported experiencing 1 death and 46 (3.1%) reported multiple deaths. Five hundred participants (33.6%) reported experiencing 1 stressor and 259 (17.4%) reported multiple stressors.
Stressors Not Associated With Vitiligo Extent
The number of deaths or stressors reported per participant within the 2 years prior to vitiligo onset were not associated with BSA, laterality, or distribution of lesions (Table 3 and eTable 2–eTable 4).
Symptoms Associated With Vitiligo
Five hundred twenty-two participants (34.5%) reported intermittent abdominal cramping, including premenstrual and/or menstrual cramping in women (9.7%), food-related abdominal cramping (4.4%), inflammatory bowel syndrome (IBS)(2.6%), anxiety-related abdominal cramping (1.5%), autoimmune gastrointestinal disorders (1.2%), and “other” etiologies (20.4%). Five hundred ten participants reported itching and/or burning associated with vitiligo lesions (35.1%).
Intermittent abdominal cramping overall was associated with a BSA greater than 75% (OR, 1.65; 95% confidence interval (CI), 1.17-2.32; P=.004). However, specific etiologies of abdominal cramping were not significantly associated with BSA (P≥.11). In contrast, itching and/or burning from vitiligo lesions was associated with a BSA greater than 25% (OR, 1.53; 95% CI, 1.23-1.90; P<.0001).
Association Between Number of Stressors and Symptoms in Vitiligo
A history of multiple stressors (≥2) within the 2 years prior to vitiligo onset was associated with intermittent abdominal cramping overall (OR, 1.84; 95% CI, 1.38-2.47; P<.0001), including premenstrual and/or menstrual cramping in women (OR, 1.84; 95% CI, 1.15-2.95; P=.01), IBS (OR, 3.29; 95% CI, 1.34-8.05; P=.01), and autoimmune gastrointestinal disorders (OR, 4.02; 95% CI, 1.27-12.80; P=.02)(eTable 5). These associations remained significant in multivariate models that included age, sex, and BSA as covariates. However, a history of 1 stressor or death or multiple deaths in the 2 years prior to vitiligo onset was not associated with any etiology of abdominal cramping.
Experiencing 1 (OR, 1.43; 95% CI, 1.12-1.82; P=.005) or multiple stressors (OR, 1.51; 95% CI, 1.12-2.04; P=.007) also was associated with itching and/or burning secondary to vitiligo. This association remained significant in a multivariate model that included age, sex, and BSA as covariates. However, a history of 1 or multiple deaths in the 2 years prior to vitiligo onset was not associated with itching and/or burning.
Association Between Specific Stressors and Vitiligo Symptoms
Perimenstrual (premenstrual and/or menstrual) cramping in women was associated with family problems (not otherwise specified) within the 2 years prior to vitiligo onset (Table 4). Food-related abdominal cramping was associated with school- and/or test-related stressors. Diagnosis of IBS was associated with health problems or surgery and being a victim of abuse within the 2 years prior to onset of vitiligo. Autoimmune gastrointestinal disorders were associated with moving to a new home/region, health problems or surgery, and witness to a violent crime or death. Finally, itching and/or burning of vitiligo lesions was associated with work and financial problems.
Comment
The present study found a high frequency of stressful life events and deaths of loved ones occurring within the 2 years preceding vitiligo onset. A history of multiple stressors but not deaths of loved ones was associated with more frequent symptoms in vitiligo patients, including itching and/or burning and intermittent abdominal pain. Specific stressors were associated with intermittent abdominal cramping, which occurred in approximately one-third of vitiligo patients. Abdominal cramping was related to menses in women, anxiety, foods, IBS, autoimmune gastrointestinal disorders, and other etiologies of abdominal cramping, which underscores the complex relationship between stressors, vitiligo, and inflammation. It is possible that stress-related immune abnormalities occur in vitiligo, which may influence the development of other autoimmune disorders. Alternatively, abdominal symptoms may precede and perhaps contribute to psychological stressors and impaired quality of life in vitiligo patients; however, the cross-sectional nature of the study did not allow us to elucidate this temporal relationship.
The present study found that 56.6% of participants experienced 1 or more deaths (17%) and/or stressful life events (51%) within the 2 years prior to vitiligo onset. These results are consistent with prior smaller studies that demonstrated a high frequency of stressful events preceding vitiligo onset. A case-controlled study found stressful events in 12 of 21 (57%) Romanian children with vitiligo, which was higher than controls.19 Another questionnaire-based, case-controlled study compared a heterogeneous group of 32 adolescent and adult Romanian patients with vitiligo and found higher odds of a stressful event in women preceding vitiligo diagnosis compared to controls.10 A retrospective analysis of 65 Croatian patients with vitiligo also reported that 56.9% (37/65) had some associated psychological factors.9 Another retrospective study of 31 adults with vitiligo found increased occurrence of 3 or more uncontrollable events, decreased perceived social support, and increased anxiety in vitiligo patients versus 116 other dermatologic disease controls.12 A questionnaire-based study found increased bereavements, changes in sleeping and eating habits, and personal injuries/illnesses in 73 British adults with vitiligo compared to 73 other age- and sex-matched dermatologic disease controls.11 All of these studies were limited by a small sample size, and the patient populations were localized to a regional dermatology referral center. The present study provided a larger analysis of stressful life events preceding vitiligo onset and included a diverse patient population.
The present study found that stressful life events and deaths of a loved one are not associated with vitiligo extent and distribution. This finding suggests that stressful life events may act as vitiligo triggers in genetically predisposed individuals, but ultimately the disease course and prognosis are driven by other factors, such as increased systemic inflammation or other immunologic abnormalities. Indeed, Silverberg and Silverberg20 and other investigators21,22 reported relative deficiencies of 25-hydroxyvitamin D,23 vitamins B6 and B12, and folic acid,20 as well as elevated serum homocysteine levels in vitiligo patients. Increased serum homocysteine levels were associated with increased BSA of vitiligo lesions.20 Elevated serum homocysteine levels also have been associated with increased inflammation in coronary artery disease,24 psoriasis,25,26 and in vitro.27 These laboratory anomalies likely reflect an underlying predisposition toward vitiligo, which might be triggered by stress responses or secondarily altered immune responses.
The present study had several strengths, including being prospective with a large sample size. The patient population included a large sample of men and women with representation of various adult ages and vitiligo extent. However, this study also had potential limitations. Measures of vitiligo extent were self-reported and were not clinically assessed. To address this limitation, we validated the questionnaire before posting it online.15 Invitation to participate in the survey was distributed by vitiligo support groups, which may have resulted in a selection bias toward participants with greater disease severity or with a poorer quality of life associated with vitiligo. Invitation to participate in this study was sent to members of vitiligo support groups, which allowed for recruitment of a large number of vitiligo patients despite a relatively low prevalence of disease in the general population. However, there are several challenges using this approach for nonvitiligo controls. Using participants with another dermatological disease as a control group may yield spurious results. Ideally, a large randomized sample of healthy participants with minimization of bias should be used for controls, which is an ambitious undertaking that was beyond the scope of this pilot study and will be the subject of future studies. Finally, this analysis found associations between stressors that occurred in the 2 years prior to vitiligo onset with symptomatic disease. We chose a broad interval for stressors because early vitiligo lesions may go unnoticed, making recognition of stressors occurring within days or weeks of onset infeasible. Further, we considered that chronic and prolonged stressors are more likely to have harmful consequences than acute stressors. Thus, stressors occurring within a more narrow interval (eg, 2 months) may not have the same association with vitiligo. Future studies are warranted to precisely identify the type and timing of psychological stressors preceding vitiligo onset.
Conclusion
In conclusion, there is a high prevalence of stressful life events preceding vitiligo, which may play an important role as disease triggers as well as predict the presence of intermittent abdominal cramping and itching or burning of skin. These associations indicate that screening of vitiligo patients for psychological stressors, abdominal cramping, and itching and/or burning of skin should be included in the routine assessment of vitiligo patients.
Appendix
1. Goronzy J, Weyand CM, Waase I. T cell subpopulations in inflammatory bowel disease: evidence for a defective induction of T8+ suppressor/cytotoxic T lymphocytes. Clin Exp Immunol. 1985;61:593-600.
2. Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90-100.
3. Grimes PE, Morris R, Avaniss-Aghajani E, et al. Topical tacrolimus therapy for vitiligo: therapeutic responses and skin messenger RNA expression of proinflammatory cytokines. J Am Acad Dermatol. 2004;51:52-61.
4. Birol A, Kisa U, Kurtipek GS, et al. Increased tumor necrosis factor alpha (TNF-alpha) and interleukin 1 alpha (IL1-alpha) levels in the lesional skin of patients with nonsegmental vitiligo. Int J Dermatol. 2006;45:992-993.
5. Moretti S, Spallanzani A, Amato L, et al. New insights into the pathogenesis of vitiligo: imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res. 2002;15:87-92.
6. Zailaie MZ. Decreased proinflammatory cytokine production by peripheral blood mononuclear cells from vitiligo patients following aspirin treatment. Saudi Med J. 2005;26:799-805.
7. Basak PY, Adiloglu AK, Ceyhan AM, et al. The role of helper and regulatory T cells in the pathogenesis of vitiligo. J Am Acad Dermatol. 2009;60:256-260.
8. Kemp EH, Emhemad S, Akhtar S, et al. Autoantibodies against tyrosine hydroxylase in patients with non-segmental (generalised) vitiligo. Exp Dermatol. 2011;20:35-40.
9. Barisic´-Drusko V, Rucevic I. Trigger factors in childhood psoriasis and vitiligo. Coll Antropol. 2004;28:277-285.
10. Manolache L, Benea V. Stress in patients with alopecia areata and vitiligo. J Eur Acad Dermatol Venereol. 2007;21:921-928.
11. Papadopoulos L, Bor R, Legg C, et al. Impact of life events on the onset of vitiligo in adults: preliminary evidence for a psychological dimension in aetiology. Clin Exp Dermatol. 1998;23:243-248.
12. Picardi A, Pasquini P, Cattaruzza MS, et al. Stressful life events, social support, attachment security and alexithymia in vitiligo. a case-control study. Psychother Psychosom. 2003;72:150-158.
13. Salzer BA, Schallreuter KU. Investigation of the personality structure in patients with vitiligo and a possible association with impaired catecholamine metabolism. Dermatology. 1995;190:109-115.
14. Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptide and neuronal marker studies in vitiligo. Br J Dermatol. 1994;131:160-165.
15. Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
16. Silverberg JI, Silverberg NB. Quality of life impairments in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
17. Kanwar AJ, Mahajan R, Parsad D. Effect of age at onset on disease characteristics in vitiligo. J Cutan Med Surg. 2013;17:253-258.
18. Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998;17:1623-1634.
19. Manolache L, Petrescu-Seceleanu D, Benea V. Correlation of stressful events with onset of vitiligo in children. J Eur Acad Dermatol Venereol. 2009;23:187-188.
20. Silverberg JI, Silverberg NB. Serum homocysteine as a biomarker of vitiligo vulgaris severity: a pilot study. J Am Acad Dermatol. 2011;64:445-447.
21. Shaker OG, El-Tahlawi SM. Is there a relationship between homocysteine and vitiligo? a pilot study. Br J Dermatol. 2008;159:720-724.
22. Balci DD, Yonden Z, Yenin JZ, et al. Serum homocysteine, folic acid and vitamin B12 levels in vitiligo. Eur J Dermatol. 2009;19:382-383.
23. Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941.
24. Jonasson T, Ohlin AK, Gottsater A, et al. Plasma homocysteine and markers for oxidative stress and inflammation in patients with coronary artery disease—a prospective randomized study of vitamin supplementation. Clin Chem Lab Med. 2005;43:628-634.
25. Cakmak SK, Gul U, Kilic C, et al. Homocysteine, vitamin B12 and folic acid levels in psoriasis patients. J Eur Acad Dermatol Venereol. 2009;23:300-303.
26. Malerba M, Gisondi P, Radaeli A, et al. Plasma homocysteine and folate levels in patients with chronic plaque psoriasis. Br J Dermatol. 2006;155:1165-1169.
27. Shastry S, James LR. Homocysteine-induced macrophage inflammatory protein-2 production by glomerular mesangial cells is mediated by PI3 Kinase and p38 MAPK. J Inflamm (Lond). 2009;6:27.
Vitiligo is the loss of skin pigmentation caused by autoimmune destruction of melanocytes. Multiple pathogenic factors for vitiligo have been described, including CD8+ T lymphocyte/T helper 1 infiltrates in lesional skin1,2 with increased expression of IFN-γ3 and tumor necrosis factor α,3-6 decreased transforming growth factor β,7 and circulating autoantibodies against tyrosine hydroxylase.8 Additionally, several studies have found a high prevalence of antecedent psychological stressors in vitiligo patients, suggesting that specific stressors may trigger and/or exacerbate vitiligo.9-12
The relationship between antecedent psychological stressors and vitiligo extent has not been well studied. Potential mechanisms for stress-triggered vitiligo include increased catecholamines13 and neuropeptides,14 which have been found in vitiligo patients. However, the complex relationship between stressors and subsequent vitiligo is not well defined. We hypothesized that persistent stressors are associated with increased vitiligo extent.
Vitiligo is classically considered to be a silent pigmentary disorder with few or no symptoms. Prior studies have demonstrated that one-third of vitiligo patients report skin symptoms (eg, pruritus, burning), which may be specifically associated with early-onset disease.15-17 Further, we observed that some vitiligo patients report abdominal cramping associated with their disease. Few studies have described the burden of skin symptoms and other associated symptoms in vitiligo or their determinants.
We conducted a prospective questionnaire-based study of 1541 adult vitiligo patients to identify psychological factors that may precede vitiligo onset. We hypothesized that some types of stressors that occur within 2 years prior to disease onset would have specific associations with vitiligo and/or somatic symptoms.
Methods
Study Population and Questionnaire Distribution
This prospective questionnaire-based study was approved by the institutional review board at St. Luke’s-Roosevelt Hospital Center (now Mount Sinai St. Luke’s-Roosevelt) (New York, New York) for adults (>18 years; male or female) with vitiligo. The survey was validated in paper format at St. Luke’s-Roosevelt Hospital Center and distributed online to members of nonprofit support groups for vitiligo vulgaris, as previously described.15
Questionnaire
The a priori aim of this questionnaire was to identify psychological factors that may precede vitiligo onset. The questionnaire consisted of 77 items (55 closed questions and 22 open questions) pertaining to participant demographics/vitiligo phenotype and psychological stressors preceding vitiligo onset. The questions related to this study and response rates are listed in eTable 1. Responses were verified by screening for noninteger or implausible values (eg, <0 or >100 years of age).
Sample Size
The primary outcome used for sample size calculation was the potential association between vitiligo and the presence of antecedent psychological stressors. Using a 2-tailed test, we determined that a sample size of 1264 participants would have 90% power at α=.05 and a baseline proportion of 0.01 (1% presumed prevalence of vitiligo) to detect an odds ratio (OR) of 2.5 or higher.18
Data and Statistical Analysis
Closed question responses were analyzed using descriptive statistics. Open-ended question responses were analyzed using content analysis. Related comments were coded and grouped, with similarities and differences noted. All data processing and statistics were done with SAS version 9.2. Age at diagnosis (years) and number of anatomic sites affected were divided into tertiles for statistical analysis due to wide skewing.
Logistic regression models were constructed with numbers of reported deaths or stressors per participant within the 2 years prior to vitiligo onset as independent variables (0, 1, or ≥2), and symptoms associated with vitiligo as dependent variables. Adjusted ORs were calculated from multivariate models that included sex, current age (continuous), and comorbid autoimmune disease (binary) as covariates. Linear interaction terms were tested and were included in final models if statistically significant (P<.05).
Ordinal logistic regression was used to analyze the relationship between stressors (and other independent variables) and number of anatomic sites affected with vitiligo (tertiles). Ordinal logistic regression models were constructed to examine the impact of psychological stressors on pruritus secondary to vitiligo (not relevant combined with not at all, a little, a lot, very much) as the dependent variable. The proportional odds assumption was met in both models, as judged by score testing (P>.05). Binary logistic regression was used to analyze laterality, body surface area (BSA) greater than 25%, and involvement of the face and/or body with vitiligo lesions (binary).
Binary logistic regression models were constructed with impact of psychological stressors preceding vitiligo onset on comorbid abdominal cramping and specific etiologies as the dependent variables. There were 20 candidate stressors occurring within the 2 years prior to vitiligo onset. Selection methods for predictors were used to identify significant covariates within the context of the other covariates included in the final models. The results of forward, backward, and stepwise approaches were similar, and the stepwise selection output was presented.
Missing values were encountered because some participants did not respond to all the questionnaire items. A complete case analysis was performed (ie, missing values were ignored throughout the study). Data imputation was considered by multiple imputations; however, there were few or no differences between the estimates from the 2 approaches. Therefore, final models did not involve data imputation.
The statistical significance for all estimates was considered to be P<.05. However, a P value near .05 should be interpreted with caution given the multiple dependent tests performed in this study with increased risk for falsely rejecting the null hypothesis.
Results
Survey Population Characteristics
One thousand seven hundred participants started the survey; 1632 completed the survey (96.0% completion rate) and 1553 had been diagnosed with vitiligo by a physician. Twelve participants were excluded because they were younger than 18 years, leaving 1541 evaluable participants. Five hundred thirty-eight participants (34.9%) had comorbid autoimmune disorders. Demographics and disease phenotypes of the study participants are listed in Table 1.
Stressors Preceding Vitiligo Onset
Eight hundred twenty-one participants (56.6%) experienced at least one death or stressor within 2 years prior to vitiligo onset (Table 2), including death of a loved one (16.6%) and stressful life events (51.0%) within the 2 years prior to the onset of vitiligo, especially work/financial problems (10.8%), end of a long-term relationship (10.2%), and family problems (not otherwise specified)(7.8%). Two hundred (13.5%) participants reported experiencing 1 death and 46 (3.1%) reported multiple deaths. Five hundred participants (33.6%) reported experiencing 1 stressor and 259 (17.4%) reported multiple stressors.
Stressors Not Associated With Vitiligo Extent
The number of deaths or stressors reported per participant within the 2 years prior to vitiligo onset were not associated with BSA, laterality, or distribution of lesions (Table 3 and eTable 2–eTable 4).
Symptoms Associated With Vitiligo
Five hundred twenty-two participants (34.5%) reported intermittent abdominal cramping, including premenstrual and/or menstrual cramping in women (9.7%), food-related abdominal cramping (4.4%), inflammatory bowel syndrome (IBS)(2.6%), anxiety-related abdominal cramping (1.5%), autoimmune gastrointestinal disorders (1.2%), and “other” etiologies (20.4%). Five hundred ten participants reported itching and/or burning associated with vitiligo lesions (35.1%).
Intermittent abdominal cramping overall was associated with a BSA greater than 75% (OR, 1.65; 95% confidence interval (CI), 1.17-2.32; P=.004). However, specific etiologies of abdominal cramping were not significantly associated with BSA (P≥.11). In contrast, itching and/or burning from vitiligo lesions was associated with a BSA greater than 25% (OR, 1.53; 95% CI, 1.23-1.90; P<.0001).
Association Between Number of Stressors and Symptoms in Vitiligo
A history of multiple stressors (≥2) within the 2 years prior to vitiligo onset was associated with intermittent abdominal cramping overall (OR, 1.84; 95% CI, 1.38-2.47; P<.0001), including premenstrual and/or menstrual cramping in women (OR, 1.84; 95% CI, 1.15-2.95; P=.01), IBS (OR, 3.29; 95% CI, 1.34-8.05; P=.01), and autoimmune gastrointestinal disorders (OR, 4.02; 95% CI, 1.27-12.80; P=.02)(eTable 5). These associations remained significant in multivariate models that included age, sex, and BSA as covariates. However, a history of 1 stressor or death or multiple deaths in the 2 years prior to vitiligo onset was not associated with any etiology of abdominal cramping.
Experiencing 1 (OR, 1.43; 95% CI, 1.12-1.82; P=.005) or multiple stressors (OR, 1.51; 95% CI, 1.12-2.04; P=.007) also was associated with itching and/or burning secondary to vitiligo. This association remained significant in a multivariate model that included age, sex, and BSA as covariates. However, a history of 1 or multiple deaths in the 2 years prior to vitiligo onset was not associated with itching and/or burning.
Association Between Specific Stressors and Vitiligo Symptoms
Perimenstrual (premenstrual and/or menstrual) cramping in women was associated with family problems (not otherwise specified) within the 2 years prior to vitiligo onset (Table 4). Food-related abdominal cramping was associated with school- and/or test-related stressors. Diagnosis of IBS was associated with health problems or surgery and being a victim of abuse within the 2 years prior to onset of vitiligo. Autoimmune gastrointestinal disorders were associated with moving to a new home/region, health problems or surgery, and witness to a violent crime or death. Finally, itching and/or burning of vitiligo lesions was associated with work and financial problems.
Comment
The present study found a high frequency of stressful life events and deaths of loved ones occurring within the 2 years preceding vitiligo onset. A history of multiple stressors but not deaths of loved ones was associated with more frequent symptoms in vitiligo patients, including itching and/or burning and intermittent abdominal pain. Specific stressors were associated with intermittent abdominal cramping, which occurred in approximately one-third of vitiligo patients. Abdominal cramping was related to menses in women, anxiety, foods, IBS, autoimmune gastrointestinal disorders, and other etiologies of abdominal cramping, which underscores the complex relationship between stressors, vitiligo, and inflammation. It is possible that stress-related immune abnormalities occur in vitiligo, which may influence the development of other autoimmune disorders. Alternatively, abdominal symptoms may precede and perhaps contribute to psychological stressors and impaired quality of life in vitiligo patients; however, the cross-sectional nature of the study did not allow us to elucidate this temporal relationship.
The present study found that 56.6% of participants experienced 1 or more deaths (17%) and/or stressful life events (51%) within the 2 years prior to vitiligo onset. These results are consistent with prior smaller studies that demonstrated a high frequency of stressful events preceding vitiligo onset. A case-controlled study found stressful events in 12 of 21 (57%) Romanian children with vitiligo, which was higher than controls.19 Another questionnaire-based, case-controlled study compared a heterogeneous group of 32 adolescent and adult Romanian patients with vitiligo and found higher odds of a stressful event in women preceding vitiligo diagnosis compared to controls.10 A retrospective analysis of 65 Croatian patients with vitiligo also reported that 56.9% (37/65) had some associated psychological factors.9 Another retrospective study of 31 adults with vitiligo found increased occurrence of 3 or more uncontrollable events, decreased perceived social support, and increased anxiety in vitiligo patients versus 116 other dermatologic disease controls.12 A questionnaire-based study found increased bereavements, changes in sleeping and eating habits, and personal injuries/illnesses in 73 British adults with vitiligo compared to 73 other age- and sex-matched dermatologic disease controls.11 All of these studies were limited by a small sample size, and the patient populations were localized to a regional dermatology referral center. The present study provided a larger analysis of stressful life events preceding vitiligo onset and included a diverse patient population.
The present study found that stressful life events and deaths of a loved one are not associated with vitiligo extent and distribution. This finding suggests that stressful life events may act as vitiligo triggers in genetically predisposed individuals, but ultimately the disease course and prognosis are driven by other factors, such as increased systemic inflammation or other immunologic abnormalities. Indeed, Silverberg and Silverberg20 and other investigators21,22 reported relative deficiencies of 25-hydroxyvitamin D,23 vitamins B6 and B12, and folic acid,20 as well as elevated serum homocysteine levels in vitiligo patients. Increased serum homocysteine levels were associated with increased BSA of vitiligo lesions.20 Elevated serum homocysteine levels also have been associated with increased inflammation in coronary artery disease,24 psoriasis,25,26 and in vitro.27 These laboratory anomalies likely reflect an underlying predisposition toward vitiligo, which might be triggered by stress responses or secondarily altered immune responses.
The present study had several strengths, including being prospective with a large sample size. The patient population included a large sample of men and women with representation of various adult ages and vitiligo extent. However, this study also had potential limitations. Measures of vitiligo extent were self-reported and were not clinically assessed. To address this limitation, we validated the questionnaire before posting it online.15 Invitation to participate in the survey was distributed by vitiligo support groups, which may have resulted in a selection bias toward participants with greater disease severity or with a poorer quality of life associated with vitiligo. Invitation to participate in this study was sent to members of vitiligo support groups, which allowed for recruitment of a large number of vitiligo patients despite a relatively low prevalence of disease in the general population. However, there are several challenges using this approach for nonvitiligo controls. Using participants with another dermatological disease as a control group may yield spurious results. Ideally, a large randomized sample of healthy participants with minimization of bias should be used for controls, which is an ambitious undertaking that was beyond the scope of this pilot study and will be the subject of future studies. Finally, this analysis found associations between stressors that occurred in the 2 years prior to vitiligo onset with symptomatic disease. We chose a broad interval for stressors because early vitiligo lesions may go unnoticed, making recognition of stressors occurring within days or weeks of onset infeasible. Further, we considered that chronic and prolonged stressors are more likely to have harmful consequences than acute stressors. Thus, stressors occurring within a more narrow interval (eg, 2 months) may not have the same association with vitiligo. Future studies are warranted to precisely identify the type and timing of psychological stressors preceding vitiligo onset.
Conclusion
In conclusion, there is a high prevalence of stressful life events preceding vitiligo, which may play an important role as disease triggers as well as predict the presence of intermittent abdominal cramping and itching or burning of skin. These associations indicate that screening of vitiligo patients for psychological stressors, abdominal cramping, and itching and/or burning of skin should be included in the routine assessment of vitiligo patients.
Appendix
Vitiligo is the loss of skin pigmentation caused by autoimmune destruction of melanocytes. Multiple pathogenic factors for vitiligo have been described, including CD8+ T lymphocyte/T helper 1 infiltrates in lesional skin1,2 with increased expression of IFN-γ3 and tumor necrosis factor α,3-6 decreased transforming growth factor β,7 and circulating autoantibodies against tyrosine hydroxylase.8 Additionally, several studies have found a high prevalence of antecedent psychological stressors in vitiligo patients, suggesting that specific stressors may trigger and/or exacerbate vitiligo.9-12
The relationship between antecedent psychological stressors and vitiligo extent has not been well studied. Potential mechanisms for stress-triggered vitiligo include increased catecholamines13 and neuropeptides,14 which have been found in vitiligo patients. However, the complex relationship between stressors and subsequent vitiligo is not well defined. We hypothesized that persistent stressors are associated with increased vitiligo extent.
Vitiligo is classically considered to be a silent pigmentary disorder with few or no symptoms. Prior studies have demonstrated that one-third of vitiligo patients report skin symptoms (eg, pruritus, burning), which may be specifically associated with early-onset disease.15-17 Further, we observed that some vitiligo patients report abdominal cramping associated with their disease. Few studies have described the burden of skin symptoms and other associated symptoms in vitiligo or their determinants.
We conducted a prospective questionnaire-based study of 1541 adult vitiligo patients to identify psychological factors that may precede vitiligo onset. We hypothesized that some types of stressors that occur within 2 years prior to disease onset would have specific associations with vitiligo and/or somatic symptoms.
Methods
Study Population and Questionnaire Distribution
This prospective questionnaire-based study was approved by the institutional review board at St. Luke’s-Roosevelt Hospital Center (now Mount Sinai St. Luke’s-Roosevelt) (New York, New York) for adults (>18 years; male or female) with vitiligo. The survey was validated in paper format at St. Luke’s-Roosevelt Hospital Center and distributed online to members of nonprofit support groups for vitiligo vulgaris, as previously described.15
Questionnaire
The a priori aim of this questionnaire was to identify psychological factors that may precede vitiligo onset. The questionnaire consisted of 77 items (55 closed questions and 22 open questions) pertaining to participant demographics/vitiligo phenotype and psychological stressors preceding vitiligo onset. The questions related to this study and response rates are listed in eTable 1. Responses were verified by screening for noninteger or implausible values (eg, <0 or >100 years of age).
Sample Size
The primary outcome used for sample size calculation was the potential association between vitiligo and the presence of antecedent psychological stressors. Using a 2-tailed test, we determined that a sample size of 1264 participants would have 90% power at α=.05 and a baseline proportion of 0.01 (1% presumed prevalence of vitiligo) to detect an odds ratio (OR) of 2.5 or higher.18
Data and Statistical Analysis
Closed question responses were analyzed using descriptive statistics. Open-ended question responses were analyzed using content analysis. Related comments were coded and grouped, with similarities and differences noted. All data processing and statistics were done with SAS version 9.2. Age at diagnosis (years) and number of anatomic sites affected were divided into tertiles for statistical analysis due to wide skewing.
Logistic regression models were constructed with numbers of reported deaths or stressors per participant within the 2 years prior to vitiligo onset as independent variables (0, 1, or ≥2), and symptoms associated with vitiligo as dependent variables. Adjusted ORs were calculated from multivariate models that included sex, current age (continuous), and comorbid autoimmune disease (binary) as covariates. Linear interaction terms were tested and were included in final models if statistically significant (P<.05).
Ordinal logistic regression was used to analyze the relationship between stressors (and other independent variables) and number of anatomic sites affected with vitiligo (tertiles). Ordinal logistic regression models were constructed to examine the impact of psychological stressors on pruritus secondary to vitiligo (not relevant combined with not at all, a little, a lot, very much) as the dependent variable. The proportional odds assumption was met in both models, as judged by score testing (P>.05). Binary logistic regression was used to analyze laterality, body surface area (BSA) greater than 25%, and involvement of the face and/or body with vitiligo lesions (binary).
Binary logistic regression models were constructed with impact of psychological stressors preceding vitiligo onset on comorbid abdominal cramping and specific etiologies as the dependent variables. There were 20 candidate stressors occurring within the 2 years prior to vitiligo onset. Selection methods for predictors were used to identify significant covariates within the context of the other covariates included in the final models. The results of forward, backward, and stepwise approaches were similar, and the stepwise selection output was presented.
Missing values were encountered because some participants did not respond to all the questionnaire items. A complete case analysis was performed (ie, missing values were ignored throughout the study). Data imputation was considered by multiple imputations; however, there were few or no differences between the estimates from the 2 approaches. Therefore, final models did not involve data imputation.
The statistical significance for all estimates was considered to be P<.05. However, a P value near .05 should be interpreted with caution given the multiple dependent tests performed in this study with increased risk for falsely rejecting the null hypothesis.
Results
Survey Population Characteristics
One thousand seven hundred participants started the survey; 1632 completed the survey (96.0% completion rate) and 1553 had been diagnosed with vitiligo by a physician. Twelve participants were excluded because they were younger than 18 years, leaving 1541 evaluable participants. Five hundred thirty-eight participants (34.9%) had comorbid autoimmune disorders. Demographics and disease phenotypes of the study participants are listed in Table 1.
Stressors Preceding Vitiligo Onset
Eight hundred twenty-one participants (56.6%) experienced at least one death or stressor within 2 years prior to vitiligo onset (Table 2), including death of a loved one (16.6%) and stressful life events (51.0%) within the 2 years prior to the onset of vitiligo, especially work/financial problems (10.8%), end of a long-term relationship (10.2%), and family problems (not otherwise specified)(7.8%). Two hundred (13.5%) participants reported experiencing 1 death and 46 (3.1%) reported multiple deaths. Five hundred participants (33.6%) reported experiencing 1 stressor and 259 (17.4%) reported multiple stressors.
Stressors Not Associated With Vitiligo Extent
The number of deaths or stressors reported per participant within the 2 years prior to vitiligo onset were not associated with BSA, laterality, or distribution of lesions (Table 3 and eTable 2–eTable 4).
Symptoms Associated With Vitiligo
Five hundred twenty-two participants (34.5%) reported intermittent abdominal cramping, including premenstrual and/or menstrual cramping in women (9.7%), food-related abdominal cramping (4.4%), inflammatory bowel syndrome (IBS)(2.6%), anxiety-related abdominal cramping (1.5%), autoimmune gastrointestinal disorders (1.2%), and “other” etiologies (20.4%). Five hundred ten participants reported itching and/or burning associated with vitiligo lesions (35.1%).
Intermittent abdominal cramping overall was associated with a BSA greater than 75% (OR, 1.65; 95% confidence interval (CI), 1.17-2.32; P=.004). However, specific etiologies of abdominal cramping were not significantly associated with BSA (P≥.11). In contrast, itching and/or burning from vitiligo lesions was associated with a BSA greater than 25% (OR, 1.53; 95% CI, 1.23-1.90; P<.0001).
Association Between Number of Stressors and Symptoms in Vitiligo
A history of multiple stressors (≥2) within the 2 years prior to vitiligo onset was associated with intermittent abdominal cramping overall (OR, 1.84; 95% CI, 1.38-2.47; P<.0001), including premenstrual and/or menstrual cramping in women (OR, 1.84; 95% CI, 1.15-2.95; P=.01), IBS (OR, 3.29; 95% CI, 1.34-8.05; P=.01), and autoimmune gastrointestinal disorders (OR, 4.02; 95% CI, 1.27-12.80; P=.02)(eTable 5). These associations remained significant in multivariate models that included age, sex, and BSA as covariates. However, a history of 1 stressor or death or multiple deaths in the 2 years prior to vitiligo onset was not associated with any etiology of abdominal cramping.
Experiencing 1 (OR, 1.43; 95% CI, 1.12-1.82; P=.005) or multiple stressors (OR, 1.51; 95% CI, 1.12-2.04; P=.007) also was associated with itching and/or burning secondary to vitiligo. This association remained significant in a multivariate model that included age, sex, and BSA as covariates. However, a history of 1 or multiple deaths in the 2 years prior to vitiligo onset was not associated with itching and/or burning.
Association Between Specific Stressors and Vitiligo Symptoms
Perimenstrual (premenstrual and/or menstrual) cramping in women was associated with family problems (not otherwise specified) within the 2 years prior to vitiligo onset (Table 4). Food-related abdominal cramping was associated with school- and/or test-related stressors. Diagnosis of IBS was associated with health problems or surgery and being a victim of abuse within the 2 years prior to onset of vitiligo. Autoimmune gastrointestinal disorders were associated with moving to a new home/region, health problems or surgery, and witness to a violent crime or death. Finally, itching and/or burning of vitiligo lesions was associated with work and financial problems.
Comment
The present study found a high frequency of stressful life events and deaths of loved ones occurring within the 2 years preceding vitiligo onset. A history of multiple stressors but not deaths of loved ones was associated with more frequent symptoms in vitiligo patients, including itching and/or burning and intermittent abdominal pain. Specific stressors were associated with intermittent abdominal cramping, which occurred in approximately one-third of vitiligo patients. Abdominal cramping was related to menses in women, anxiety, foods, IBS, autoimmune gastrointestinal disorders, and other etiologies of abdominal cramping, which underscores the complex relationship between stressors, vitiligo, and inflammation. It is possible that stress-related immune abnormalities occur in vitiligo, which may influence the development of other autoimmune disorders. Alternatively, abdominal symptoms may precede and perhaps contribute to psychological stressors and impaired quality of life in vitiligo patients; however, the cross-sectional nature of the study did not allow us to elucidate this temporal relationship.
The present study found that 56.6% of participants experienced 1 or more deaths (17%) and/or stressful life events (51%) within the 2 years prior to vitiligo onset. These results are consistent with prior smaller studies that demonstrated a high frequency of stressful events preceding vitiligo onset. A case-controlled study found stressful events in 12 of 21 (57%) Romanian children with vitiligo, which was higher than controls.19 Another questionnaire-based, case-controlled study compared a heterogeneous group of 32 adolescent and adult Romanian patients with vitiligo and found higher odds of a stressful event in women preceding vitiligo diagnosis compared to controls.10 A retrospective analysis of 65 Croatian patients with vitiligo also reported that 56.9% (37/65) had some associated psychological factors.9 Another retrospective study of 31 adults with vitiligo found increased occurrence of 3 or more uncontrollable events, decreased perceived social support, and increased anxiety in vitiligo patients versus 116 other dermatologic disease controls.12 A questionnaire-based study found increased bereavements, changes in sleeping and eating habits, and personal injuries/illnesses in 73 British adults with vitiligo compared to 73 other age- and sex-matched dermatologic disease controls.11 All of these studies were limited by a small sample size, and the patient populations were localized to a regional dermatology referral center. The present study provided a larger analysis of stressful life events preceding vitiligo onset and included a diverse patient population.
The present study found that stressful life events and deaths of a loved one are not associated with vitiligo extent and distribution. This finding suggests that stressful life events may act as vitiligo triggers in genetically predisposed individuals, but ultimately the disease course and prognosis are driven by other factors, such as increased systemic inflammation or other immunologic abnormalities. Indeed, Silverberg and Silverberg20 and other investigators21,22 reported relative deficiencies of 25-hydroxyvitamin D,23 vitamins B6 and B12, and folic acid,20 as well as elevated serum homocysteine levels in vitiligo patients. Increased serum homocysteine levels were associated with increased BSA of vitiligo lesions.20 Elevated serum homocysteine levels also have been associated with increased inflammation in coronary artery disease,24 psoriasis,25,26 and in vitro.27 These laboratory anomalies likely reflect an underlying predisposition toward vitiligo, which might be triggered by stress responses or secondarily altered immune responses.
The present study had several strengths, including being prospective with a large sample size. The patient population included a large sample of men and women with representation of various adult ages and vitiligo extent. However, this study also had potential limitations. Measures of vitiligo extent were self-reported and were not clinically assessed. To address this limitation, we validated the questionnaire before posting it online.15 Invitation to participate in the survey was distributed by vitiligo support groups, which may have resulted in a selection bias toward participants with greater disease severity or with a poorer quality of life associated with vitiligo. Invitation to participate in this study was sent to members of vitiligo support groups, which allowed for recruitment of a large number of vitiligo patients despite a relatively low prevalence of disease in the general population. However, there are several challenges using this approach for nonvitiligo controls. Using participants with another dermatological disease as a control group may yield spurious results. Ideally, a large randomized sample of healthy participants with minimization of bias should be used for controls, which is an ambitious undertaking that was beyond the scope of this pilot study and will be the subject of future studies. Finally, this analysis found associations between stressors that occurred in the 2 years prior to vitiligo onset with symptomatic disease. We chose a broad interval for stressors because early vitiligo lesions may go unnoticed, making recognition of stressors occurring within days or weeks of onset infeasible. Further, we considered that chronic and prolonged stressors are more likely to have harmful consequences than acute stressors. Thus, stressors occurring within a more narrow interval (eg, 2 months) may not have the same association with vitiligo. Future studies are warranted to precisely identify the type and timing of psychological stressors preceding vitiligo onset.
Conclusion
In conclusion, there is a high prevalence of stressful life events preceding vitiligo, which may play an important role as disease triggers as well as predict the presence of intermittent abdominal cramping and itching or burning of skin. These associations indicate that screening of vitiligo patients for psychological stressors, abdominal cramping, and itching and/or burning of skin should be included in the routine assessment of vitiligo patients.
Appendix
1. Goronzy J, Weyand CM, Waase I. T cell subpopulations in inflammatory bowel disease: evidence for a defective induction of T8+ suppressor/cytotoxic T lymphocytes. Clin Exp Immunol. 1985;61:593-600.
2. Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90-100.
3. Grimes PE, Morris R, Avaniss-Aghajani E, et al. Topical tacrolimus therapy for vitiligo: therapeutic responses and skin messenger RNA expression of proinflammatory cytokines. J Am Acad Dermatol. 2004;51:52-61.
4. Birol A, Kisa U, Kurtipek GS, et al. Increased tumor necrosis factor alpha (TNF-alpha) and interleukin 1 alpha (IL1-alpha) levels in the lesional skin of patients with nonsegmental vitiligo. Int J Dermatol. 2006;45:992-993.
5. Moretti S, Spallanzani A, Amato L, et al. New insights into the pathogenesis of vitiligo: imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res. 2002;15:87-92.
6. Zailaie MZ. Decreased proinflammatory cytokine production by peripheral blood mononuclear cells from vitiligo patients following aspirin treatment. Saudi Med J. 2005;26:799-805.
7. Basak PY, Adiloglu AK, Ceyhan AM, et al. The role of helper and regulatory T cells in the pathogenesis of vitiligo. J Am Acad Dermatol. 2009;60:256-260.
8. Kemp EH, Emhemad S, Akhtar S, et al. Autoantibodies against tyrosine hydroxylase in patients with non-segmental (generalised) vitiligo. Exp Dermatol. 2011;20:35-40.
9. Barisic´-Drusko V, Rucevic I. Trigger factors in childhood psoriasis and vitiligo. Coll Antropol. 2004;28:277-285.
10. Manolache L, Benea V. Stress in patients with alopecia areata and vitiligo. J Eur Acad Dermatol Venereol. 2007;21:921-928.
11. Papadopoulos L, Bor R, Legg C, et al. Impact of life events on the onset of vitiligo in adults: preliminary evidence for a psychological dimension in aetiology. Clin Exp Dermatol. 1998;23:243-248.
12. Picardi A, Pasquini P, Cattaruzza MS, et al. Stressful life events, social support, attachment security and alexithymia in vitiligo. a case-control study. Psychother Psychosom. 2003;72:150-158.
13. Salzer BA, Schallreuter KU. Investigation of the personality structure in patients with vitiligo and a possible association with impaired catecholamine metabolism. Dermatology. 1995;190:109-115.
14. Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptide and neuronal marker studies in vitiligo. Br J Dermatol. 1994;131:160-165.
15. Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
16. Silverberg JI, Silverberg NB. Quality of life impairments in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
17. Kanwar AJ, Mahajan R, Parsad D. Effect of age at onset on disease characteristics in vitiligo. J Cutan Med Surg. 2013;17:253-258.
18. Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998;17:1623-1634.
19. Manolache L, Petrescu-Seceleanu D, Benea V. Correlation of stressful events with onset of vitiligo in children. J Eur Acad Dermatol Venereol. 2009;23:187-188.
20. Silverberg JI, Silverberg NB. Serum homocysteine as a biomarker of vitiligo vulgaris severity: a pilot study. J Am Acad Dermatol. 2011;64:445-447.
21. Shaker OG, El-Tahlawi SM. Is there a relationship between homocysteine and vitiligo? a pilot study. Br J Dermatol. 2008;159:720-724.
22. Balci DD, Yonden Z, Yenin JZ, et al. Serum homocysteine, folic acid and vitamin B12 levels in vitiligo. Eur J Dermatol. 2009;19:382-383.
23. Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941.
24. Jonasson T, Ohlin AK, Gottsater A, et al. Plasma homocysteine and markers for oxidative stress and inflammation in patients with coronary artery disease—a prospective randomized study of vitamin supplementation. Clin Chem Lab Med. 2005;43:628-634.
25. Cakmak SK, Gul U, Kilic C, et al. Homocysteine, vitamin B12 and folic acid levels in psoriasis patients. J Eur Acad Dermatol Venereol. 2009;23:300-303.
26. Malerba M, Gisondi P, Radaeli A, et al. Plasma homocysteine and folate levels in patients with chronic plaque psoriasis. Br J Dermatol. 2006;155:1165-1169.
27. Shastry S, James LR. Homocysteine-induced macrophage inflammatory protein-2 production by glomerular mesangial cells is mediated by PI3 Kinase and p38 MAPK. J Inflamm (Lond). 2009;6:27.
1. Goronzy J, Weyand CM, Waase I. T cell subpopulations in inflammatory bowel disease: evidence for a defective induction of T8+ suppressor/cytotoxic T lymphocytes. Clin Exp Immunol. 1985;61:593-600.
2. Ongenae K, Van Geel N, Naeyaert JM. Evidence for an autoimmune pathogenesis of vitiligo. Pigment Cell Res. 2003;16:90-100.
3. Grimes PE, Morris R, Avaniss-Aghajani E, et al. Topical tacrolimus therapy for vitiligo: therapeutic responses and skin messenger RNA expression of proinflammatory cytokines. J Am Acad Dermatol. 2004;51:52-61.
4. Birol A, Kisa U, Kurtipek GS, et al. Increased tumor necrosis factor alpha (TNF-alpha) and interleukin 1 alpha (IL1-alpha) levels in the lesional skin of patients with nonsegmental vitiligo. Int J Dermatol. 2006;45:992-993.
5. Moretti S, Spallanzani A, Amato L, et al. New insights into the pathogenesis of vitiligo: imbalance of epidermal cytokines at sites of lesions. Pigment Cell Res. 2002;15:87-92.
6. Zailaie MZ. Decreased proinflammatory cytokine production by peripheral blood mononuclear cells from vitiligo patients following aspirin treatment. Saudi Med J. 2005;26:799-805.
7. Basak PY, Adiloglu AK, Ceyhan AM, et al. The role of helper and regulatory T cells in the pathogenesis of vitiligo. J Am Acad Dermatol. 2009;60:256-260.
8. Kemp EH, Emhemad S, Akhtar S, et al. Autoantibodies against tyrosine hydroxylase in patients with non-segmental (generalised) vitiligo. Exp Dermatol. 2011;20:35-40.
9. Barisic´-Drusko V, Rucevic I. Trigger factors in childhood psoriasis and vitiligo. Coll Antropol. 2004;28:277-285.
10. Manolache L, Benea V. Stress in patients with alopecia areata and vitiligo. J Eur Acad Dermatol Venereol. 2007;21:921-928.
11. Papadopoulos L, Bor R, Legg C, et al. Impact of life events on the onset of vitiligo in adults: preliminary evidence for a psychological dimension in aetiology. Clin Exp Dermatol. 1998;23:243-248.
12. Picardi A, Pasquini P, Cattaruzza MS, et al. Stressful life events, social support, attachment security and alexithymia in vitiligo. a case-control study. Psychother Psychosom. 2003;72:150-158.
13. Salzer BA, Schallreuter KU. Investigation of the personality structure in patients with vitiligo and a possible association with impaired catecholamine metabolism. Dermatology. 1995;190:109-115.
14. Al’Abadie MS, Senior HJ, Bleehen SS, et al. Neuropeptide and neuronal marker studies in vitiligo. Br J Dermatol. 1994;131:160-165.
15. Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149:159-164.
16. Silverberg JI, Silverberg NB. Quality of life impairments in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
17. Kanwar AJ, Mahajan R, Parsad D. Effect of age at onset on disease characteristics in vitiligo. J Cutan Med Surg. 2013;17:253-258.
18. Hsieh FY, Bloch DA, Larsen MD. A simple method of sample size calculation for linear and logistic regression. Stat Med. 1998;17:1623-1634.
19. Manolache L, Petrescu-Seceleanu D, Benea V. Correlation of stressful events with onset of vitiligo in children. J Eur Acad Dermatol Venereol. 2009;23:187-188.
20. Silverberg JI, Silverberg NB. Serum homocysteine as a biomarker of vitiligo vulgaris severity: a pilot study. J Am Acad Dermatol. 2011;64:445-447.
21. Shaker OG, El-Tahlawi SM. Is there a relationship between homocysteine and vitiligo? a pilot study. Br J Dermatol. 2008;159:720-724.
22. Balci DD, Yonden Z, Yenin JZ, et al. Serum homocysteine, folic acid and vitamin B12 levels in vitiligo. Eur J Dermatol. 2009;19:382-383.
23. Silverberg JI, Silverberg AI, Malka E, et al. A pilot study assessing the role of 25 hydroxy vitamin D levels in patients with vitiligo vulgaris. J Am Acad Dermatol. 2010;62:937-941.
24. Jonasson T, Ohlin AK, Gottsater A, et al. Plasma homocysteine and markers for oxidative stress and inflammation in patients with coronary artery disease—a prospective randomized study of vitamin supplementation. Clin Chem Lab Med. 2005;43:628-634.
25. Cakmak SK, Gul U, Kilic C, et al. Homocysteine, vitamin B12 and folic acid levels in psoriasis patients. J Eur Acad Dermatol Venereol. 2009;23:300-303.
26. Malerba M, Gisondi P, Radaeli A, et al. Plasma homocysteine and folate levels in patients with chronic plaque psoriasis. Br J Dermatol. 2006;155:1165-1169.
27. Shastry S, James LR. Homocysteine-induced macrophage inflammatory protein-2 production by glomerular mesangial cells is mediated by PI3 Kinase and p38 MAPK. J Inflamm (Lond). 2009;6:27.
Practice Points
- Psychological stressors (eg, loss of a loved one) that occurred within 2 years prior to vitiligo onset should be considered as potential disease triggers.
- Psychological stressors have been associated with symptoms of abdominal cramping and itching/burning in vitiligo patients but not disease extent or distribution.
Scalp Hyperkeratosis in Children With Skin of Color: Diagnostic and Therapeutic Considerations
Scalp hyperkeratosis (scaling or flaking) is a common symptom in childhood and is typified by fine to thick hyperkeratosis of the scalp with or without underlying erythema. The causes of scalp hyperkeratosis in childhood vary based on the demographics of the population. In a population where approximately half of the pediatric patients were white, scaling of the scalp was more common in patients with seborrheic dermatitis and/or atopic dermatitis (AD) who were aged 0 to 2 years, and tinea capitis was only noted in children who were black.1 In children with skin of color, scalp hyperkeratosis has been noted as a marker of tinea capitis, especially in patients aged 3 to 11 years,2,3 and the level of suspicion should consistently remain high for this age group. In another study of an all-black population of schoolchildren aged 5 to 13 years (N=224), 3% demonstrated signs and symptoms of tinea capitis and 14% were found to be asymptomatic carriers.4 Although generally benign in nature, scalp hyperkeratosis can be associated with systemic illnesses such as juvenile dermatomyositis and Langerhans cell histiocytosis.5 This article addresses the diagnosis and treatment of scalp hyperkeratosis in children with skin of color, focusing on differences in exposure to contagious cases, hairstyling practices, and biological factors that may impact the disease process.
CAUSES OF SCALP HYPERKERATOSIS IN CHILDHOOD
Scalp hyperkeratosis in childhood usually is caused by common benign conditions, but some level of suspicion should be maintained for more severe etiologic conditions such as Langerhans cell histiocytosis and collagen vascular diseases (eg, juvenile dermatomyositis).6 Langerhans cell histiocytosis of the scalp might be obscured by background pigmentation in black children.
Scalp scaling can be a minor criterion in the diagnosis of AD. Atopic dermatitis should be suspected in Asian children with scalp scaling. Although one study in Bangladesh revealed scalp involvement in only 5.2% of pediatric patients with AD,7 a study in China reported an incidence rate as high as 49.7% (with a similarly high incidence of eyelid dermatitis).8 Children with AD also may have dry hair.9 Atopic dermatitis of the scalp is typified by itching, fine hyperkeratosis, and notably eczematous scalp lesions ranging from excoriated or oozing erythematous plaques to lichenification with hair miniaturization, primarily from scratch-induced breakage.10 The latter finding often is noted in black adolescent girls with long-term moderate to severe AD (personal observation).
Seborrheic dermatitis is a hypersensitivity response to yeast colonization of the scalp with Malassezia species. The infantile form is extremely common (also known as cradle cap). Characteristically, greasy yellow hyperkeratosis in fine to thick sheets is noted on the scalp in children younger than 2 years, especially infants, often with involvement of skin folds. One study noted that seborrheic dermatitis occurs in 6% of school-aged children as opposed to 19% of children younger than 2 years.1 Severe seborrheic dermatitis in infancy may be a prelude to AD, with the incidence being 3 times higher in children with prior seborrheic dermatitis.11 In teenagers, seborrheic dermatitis often accompanies acne onset in the early pubertal years.12
Psoriasis is an autoimmune inflammatory dermatosis that most commonly affects white children. In childhood, pityriasis amiantacea, psoriasiform scalp hyperkeratosis, is more common than in adulthood, with thick, stuck-on scales bound to the hairs. This variant is uncommon in Hispanic and Asian children and is almost never seen in black children but has been reported in cohorts of Turkish children.13 In a series of 85 Egyptian children with pityriasis amiantacea, diagnosis of scalp psoriasis was made in 35.3%, eczematous dermatitis in 34.2%, and tinea capitis in 12.9%.14 Consequently, a high degree of suspicion for tinea capitis should be held if pityriasis amiantacea is found in children with skin of color.15,16
Tinea capitis is a dermatophyte infection of the scalp, hair, and surrounding skin. The presence of tinea capitis on the scalp is associated with environmental exposure to dermatophytes (eg, school, household).4,17 The infection is largely caused by Trichophyton tonsurans in the United States, which causes a seborrheic appearance and less commonly alopecia (black dot or thinning), plaques with scale, or kerion. The presence of cervical lymph nodes and/or alopecia increases the chances of tinea being the diagnosis. Potassium hydroxide preparation and fungal culture can be performed to corroborate the diagnosis.1-3 Other etiologies of scalp hyperkeratosis such as juvenile pityriasis rubra pilaris and lice are extremely uncommon in black children, but lice may be seen in Hispanic and Asian girls with long straight hair who attend school. Discoid lupus is more common in children with skin of color but is rare overall. When noted, accompanying mottled dyspigmentation and scarring alopecia are noted in addition to a high risk for developing systemic lupus erythematosus. Biopsy and screening for systemic lupus are necessary, as the risk for progression from discoid lupus to systemic disease is 26% over 3 years.18
THE BIOLOGY OF HAIR IN CHILDREN WITH SKIN OF COLOR
To some extent, the biology of hair impacts the occurrence, appearance, and treatment of scalp hyperkeratosis in children with skin of color. First, it is important to remember that follicular density is lower in black patients as compared to Asian patients with a consequently lower hair count overall, which results in the easy appearance of hair loss, particularly at the margins of the scalp.19,20 Second, the shape of the hair follicle differs among races and ethnicities. Asian patients have round hair shafts coming from straight follicles, which allows for greater natural hair hydration, resulting in somewhat less aggressive scalp disease. Hispanic patients may have similarly straight hair or may have elliptical or curled shafts, the latter being noted in black patients. Furthermore, a curled hair shaft results in poor flow of sebum across the hair, resulting in greater scalp xerosis, more susceptibility to traction alopecia, and ultimately a greater risk for infections.20-23 Finally, the scalp is continuous with the face and neck, and Asian patients have greater sensitivity to skin care products in these areas, resulting in difficulty of treatment in this patient population and the need for use of gentle products.
HAIR CARE PRACTICES IN CHILDREN WITH SKIN OF COLOR
Hair care in patients with skin of color can be costly, difficult, and potentially damaging, with 99% of black girls reporting pomade or oil usage. Costly and complex hair care practices begin in childhood for patients with skin of color. In a series of 201 surveyed black girls with a mean age of 9.8 years, 80% had used hot combs and 42% used relaxers.24 Traction styles were common with 81% using ponytails, 67% braids, and 49% cornrows in the last 12 months. These styles are thought to affect hair health, particularly through induction of traction-related damage, folliculitis, and alopecia. Furthermore, chemical relaxers, hot combs, blowouts, and hair setting may be introduced during childhood.24 These practices appear to disturb the integrity of the hair follicle, leaving it more susceptible to irritation and infection.
Hair care in the pediatric population often is complicated by the fact that multiple children are being styled in tandem, either at home or in a salon, resulting in shared equipment and fomite spread. Even just proximity to a case of tinea capitis in the household will increase risk for tinea capitis. Furthermore, it is quite commonplace for black patients to use pomades and shampoos that contain antifungals, especially selenium sulfide, which makes it difficult to obtain accurate culture results. In India, use of mustard oil also has been linked to increased risk for tinea capitis.25
Other issues related to hair care include frequent dry scalp in patients with skin of color due to poor sebum distribution along the hair shaft. As a result, frequent washing may exacerbate scalp xerosis and further irritate seborrheic dermatitis and/or AD.
DIAGNOSTIC CONSIDERATIONS FOR SCALP HYPERKERATOSIS IN CHILDHOOD
Dermatologists should have a greater level of suspicion for tinea capitis in black and Hispanic children compared to white children. The index of suspicion should be high given that antifungal shampoos and pomades may minimize the clinical appearance. Although trends in overall incidence in the United States suggest tinea capitis is becoming less common, there still is a stronger representation of the disease in black patients.26 A study of positive fungal cultures from one clinic in Mississippi (N=1220) showed that two-thirds of patients were children younger than 13 years; 87% of patients with positive cultures for dermatophytes were black.27 The endothrix type of tinea capitis caused by T tonsurans often presents with a seborrheic appearance, and fungal culture is warranted in all pediatric patients with skin of color who have scalp hyperkeratosis. Asian children can be regarded with a lower level of suspicion for tinea capitis, similar to white patients in the United States. Variation in incidence of tinea capitis does exist worldwide and the practitioner may need to address these issues in patients who travel or are recent immigrants.
When identifying tinea capitis infections in children with skin of color, physicians should consider the patient’s personal and family history, comorbid skin disorders, dermoscopy, microscopy and fungal staining, and fungal culture (Figure).
A paradigm for the diagnosis of scalp hyperkeratosis in children with skin of color.
Personal and Family History
The first diagnostic consideration is the patient’s personal and family history. A history of AD, asthma, or allergies will support but not confirm the diagnosis of AD. Prior tinea capitis infections and household contacts with tinea infections support the presence of tinea capitis.17 Recent implementation of anti–tumor necrosis factor a inhibitor therapy in a psoriatic child can flare scalp disease, mimicking tinea capitis.28 The patient’s guardians should be queried about potential infectious contacts, whether they themselves have signs of scalp disease or tinea corporis (ringworm) or whether they have a pet with problematic fur. Physicians also should query patients and their guardians about recent use of topical antifungal shampoos, pomades, creams (both over-the-counter [OTC] and prescription), and/or oral antifungals. When these agents are used, there is a possibility that fungal examinations may be negative in the presence of true infection with tinea capitis. Traction alopecia, often preceded by fine scale, is more likely to present in patients who wear their hair in cornrows, while seborrheic dermatitis may be associated with hair extensions, reduced frequency of washing (61% of black girls surveyed wash every 2 weeks), and/or reduced usage of hair oils in black girls.24 Knowledge of the patient’s personal hair care history, such as use of pomades; frequency and method of washing/drying hair; types of hair care products used daily to wash and style hair; use of chemical relaxers; or recent hairstyling with cornrows, braids, or hair extensions, also is essential to the diagnosis of tinea capitis. Usage of traction-related styling practices in patients with chemically relaxed hair can enhance the risk for traction alopecia.29
Comorbid Skin Disorders
The patient also should be examined for comorbid skin disorders, including tinea corporis, alopecia (particularly in the areas of hyperkeratosis), and the presence of nuchal lymphadenopathy. For each extra clinical finding, the chances of a final diagnosis of tinea capitis rises, allowing for empiric diagnosis to be made that can be confirmed by a variety of tests.1-3
Dermoscopy
Next, the patient should undergo dermoscopic evaluation. On dermoscopy, tinea capitis typically presents with broken hairs, black dots on the scalp, comma-shaped hairs, and short corkscrew hairs, all of which should clear with therapy.30-33 Dermoscopic findings of AD would reveal underlying xerosis and prominent vasculature due to inflammation, and alopecia areata would present with yellow dots at the orifices of the hair follicles, exclamation point hairs, and vellus hairs.34,35 Traction alopecia may be noted by retained hairs along the hairline, which is known as the fringe sign.36
Microscopy and Fungal Staining
Microscopic preparations can be performed to identify tinea capitis using fungal stains of slide-based specimens. Breakage of short hairs onto the slide and/or cotton swab is a soft sign corroborating endothrix infection of the hairs. Potassium hydroxide can enhance visualization of the hyperkeratotic scalp, but for most black patients, use of antifungal agents reduces fungal hyphae and spores in the areas of hyperkeratosis and may limit the utility of examining the skin microscopically. Assessment of the broken hairs obtained by gentle friction with one glass slide and catching the scales onto another glass slide may yield the best results in the evaluation of tinea capitis (a technique taught to me by Robin Hornung, MD, Everett, Washington). Hairs obtained in this manner often are fragile and break due to endothrix infection replacing and weakening the shaft of the hairs. In the United States, fungal samples usually are obtained with cotton swabs, but a recent study suggested that brushing is superior to scraping to obtain samples; the combination of sampling techniques may improve the yield of a culture.37 Because topical agents are unable to enter the hair cortex, the hair shaft is the most likely to show fungal spores under the microscope when antifungal shampoos or pomades are used. Other testing methods such as Swartz-Lamkins or calcofluor white staining can be used on similar scrapings. Biopsy and periodic acid–Schiff staining of thick scales or crust can help differentiate tinea capitis from pityriasis amiantacea when the crust is too thick to be softened via potassium hydroxide preparation.38
Fungal Culture
Fungal culture onto media that contains nutrients for dermatophyte growth can be used for 4 purposes in tinea capitis: (1) to confirm infection, (2) to identify species of infection, (3) to confirm mycological cure when difficulty in clearance of disease has been noted, and (4) to obtain a specimen for sensitivity screening regarding antifungals when necessary, an uncommon but occasionally useful test in individuals with disease that has failed treatment with 1 or more antifungals.27
THERAPY FOR SCALP HYPERKERATOSIS IN CHILDREN WITH SKIN OF COLOR
In patients with scalp hyperkeratosis, it is important to address the specific cause of the disease. Therapy for scalp hyperkeratosis in children with skin of color includes altered hair care practices, use of OTC and prescription agents, and containment of fomites in the case of infections. Biopsy of atypical scalp hyperkeratosis cases is needed to diagnose rare etiologies such as discoid lupus or Langerhans cell histiocytosis. For individuals with systemic disease including Langerhans cell histiocytosis, which is generally accompanied by nodes and plaques in the inguinal region or other intertriginous sites, immediate hematology and oncology workup is required.39 For collagen vascular diseases such as lupus or dermatomyositis, appropriate referral to rheumatology and systemic therapy is warranted.
Altered Hair Care Practices
The use of prophylactic ketoconazole 1% shampoo may not reduce the risk for recurrence of tinea capitis over standard good hygiene, removal of fomites, and adherence to prescribed therapy.40 Use of selenium sulfide has been shown to effectively reduce contagion risk.41
Fragrance- and dye-free shampoos can be helpful in providing gentle cleansing of the scalp, which is especially important in Asian patients who have greater facial and eyelid sensitivity. Free-and-clear shampoos can be used alternatively with shampoos containing selenium sulfide or sulfur to eliminate comorbid seborrhea. Black patients should be advised to shampoo and condition their hair once weekly, and Asian and Hispanic patients should shampoo and condition 2 to 3 times weekly to remove scale and potentially reduce risk for tinea acquisition.42 Children with straight hair should shampoo with increased frequency in the summer to manually remove sweat-induced macerated hyperkeratosis. Conditioners also should be used consistently after shampooing to enhance hair health.
Use of OTC and Prescription Agents
Atopic Dermatitis
Topical corticosteroid agents can be used in increasing strengths to treat AD of the scalp in children with skin of color, from OTC scalp products containing hydrocortisone 1% to prescription-based agents. Hydration of the hair also is needed to counteract reduced water content.43 Due to the innate xerosis of the scalp in black patients and atopic patients, the use of oil-based or lotion products may provide the most hydration for patients with scalp disease.44 Alcohol-based agents, either drops or foams, may enhance xerosis and should be used sparingly.
Seborrheic Dermatitis
Alternating treatment with medicated shampoos containing selenium sulfide and ketoconazole can aid in the removal of seborrhea. Pomades including borage seed oil–based agents can be massaged into the scalp,45 particularly for treatment of infantile seborrhea, and should not necessarily be washed off daily in dark-skinned patients. Additional focused application of topical corticosteroids to the scalp also is helpful. Due to innate scalp xerosis in black children, therapy should be similar to AD.
Psoriasis
In the setting of pityriasis amiantacea, albeit rare in children with skin of color, oil-based agents can soften hyperkeratosis for removal. Sterile mineral oil or commercially available scalp preparations of peanut oil with fluocinolone or mineral oil with glycerin can aid in the removal of scales without harming the hair, but usage must be age appropriate. The addition of focused application of age-appropriate topical corticosteroids for areas of severe hyperkeratosis can aid in clearance of the lesions.44 Recently, a stable combination of calcipo-triene 0.005%–betamethasone dipropionate 0.064% has been approved in the United States for the therapy of scalp psoriasis in adolescents.46
Tinea Capitis
Antifungal shampoos including selenium sulfide will reduce contagion risk when used by both the patient and his/her family members. Frequency of shampooing is similar to that described for AD. Between shampooing, pomades with selenium sulfide can be applied to the scalp to enhance overall clearance.
Oral antifungals are the basis of treatment and use of griseofulvin is the gold standard. Terbinafine has been approved by the US Food and Drug Administration for treatment of tinea capitis; for children weighing less than 25 kg the dosage is 125 mg daily, for 25 to 35 kg the dosage is 187.5 mg daily, and for more than 35 kg the dosage is 250 mg daily. Shorter therapeutic courses may be required, making it a good second-line agent. Laboratory screening in children prior to therapy is not always performed but should be done in cases where fatty liver might be suspected.47 Monitoring liver function tests is best when exceeding 3 months of usage or shifting from one antifungal to another.3
Containment of Fomites
There are several procedures that should be followed to contain scalp infection in children with skin of color. First, all objects that come into contact with the scalp (eg, hats, hoods, brushes, pillowcases) should be washed with hot water or replaced weekly. Sharing these objects with friends or family should be strongly discouraged. Patients and their family members also should be instructed to use medicated (eg, selenium sulfide) shampoos and conditioners. Finally, patients are advised to avoid use of shared classroom garments or mats for sleeping.
LONG-TERM SEQUELAE OF SCALP HYPERKERATOSIS
Long-term sequelae of scalp hyperkeratosis often are discounted in children, but the disease can have lasting and damaging effects on the scalp. Sequelae include discomfort from chronicity and psychological distress. In particular, years of scalp pruritus can promote lichenification of the scalp and miniaturization of the hair follicles. Furthermore, itching due to sweating can limit participation in sports. Finally, tinea capitis is thought to be a risk factor for central centrifugal cicatricial alopecia (or can occur comorbidly with central centrifugal cicatricial alopecia causing severe pruritus), a chronic scarring hair loss that is seen primarily in black adult females.48 Erythema nodosum also has been reported as an associated finding in the case of kerion.49 One study reported associated findings that included thyroid cancer in individuals irradiated for tinea capitis in the 1950s.50
Conclusion
Scalp hyperkeratosis in children with skin of color, especially black patients, is more likely to be associated with tinea capitis and is more challenging to treat due to innate scalp xerosis in black patients and increased sensitivity of facial skin in Asian children. Ultimately, institution of therapy when needed and good scalp and hair care may prevent long-term sequelae.
1. Williams JV, Eichenfield LF, Burke BL, et al. Prevalence of scalp scaling in prepubertal children. Pediatrics. 2005;115:e1-e6.
2. Coley MK, Bhanusali DG, Silverberg JI, et al. Scalp hyperkeratosis and alopecia in children of color. J Drugs Dermatol. 2011;10:511-516.
3. Bhanusali D, Coley M, Silverberg JI, et al. Treatment outcomes for tinea capitis in a skin of color population. J Drugs Dermatol. 2012;11:852-856.
4. Williams JV, Honig PJ, McGinley KJ, et al. Semiquantitative study of tinea capitis and the asymptomatic carrier state in inner-city school children. Pediatrics. 1995;96:265-267.
5. McDonald LL, Smith ML. Diagnostic dilemmas in pediatric/adolescent dermatology: scaly scalp. J Pediatr Health Care. 1998;12:80-84.
6. Peloro TM, Miller OF 3rd, Hahn TF, et al. Juvenile dermatomyositis: a retrospective review of a 30-year experience. J Am Acad Dermatol. 2001;45:28-34.
7. Wahab MA, Rahman MH, Khondker L, et al. Minor criteria for atopic dermatitis in children. Mymensingh Med J. 2011;20:419-424.
8. Shi M, Zhang H, Chen X, et al. Clinical features of atopic dermatitis in a hospital-based setting in China. J Eur Acad Dermatol Venereol [published online ahead of print January 9, 2011]. 2011;25:1206-1212.
9. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair. Microsc Res Tech. 2012;75:620-625.
10. Sabin BR, Peters N, Peters AT. Chapter 20: atopic dermatitis. Allergy Asthma Proc. 2012;33:S67-S69.
11. Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis [published online ahead of print November 13, 2013]. Pediatr Dermatol. 2014;31:125-130.
12. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
13. Sarifakioglu E, Yilmaz AE, Gorpelioglu C, et al. Prevalence of scalp disorders and hair loss in children. Cutis. 2012;90:225-229.
14. Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
15. Oostveen AM, Jong EM, Evers AW, et al. Reliability, responsiveness and validity of Scalpdex in children with scalp psoriasis: the Dutch study. Acta Derm Venereol. 2014;94:198-202.
16. Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity: Comparative Dermatologic Atlas of Pediatric Skin of All Colors. New York, NY: Springer; 2012.
17. Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
18. Moises-Alfaro C, Berrón-Pérez R, Carrasco-Daza D, et al. Discoid lupus erythematosus in children: clinical, histopathologic, and follow-up features in 27 cases. Pediatr Dermatol. 2003;20:103-107.
19. Ramos-e-Silva M. Ethnic hair and skin: what is the state of the science? Chicago, Illinois—September 29-30, 2001. Clin Dermatol. 2002;20:321-324.
20. Heath CR, McMichael AJ. Biology of hair follicle. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. New York, NY: McGraw Hill; 2009:105-109.
21. Khumalo NP. African hair morphology: macrostructure to ultrastructure. Int J Dermatol. 2005;44(suppl 1):10-12.
22. Thibaut S, Bernard BA. The biology of hair shape. Int J Dermatol. 2005;44(suppl 1):2-3.
23. Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
24. Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
25. Kumar V, Sharma RC, Chander R. Clinicomycological study of tinea capitis. Indian J Dermatol Venereol Leprol. 1996;62:207-209.
26. Mirmirani P, Tucker LY. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California [published online ahead of print October 2, 2013]. J Am Acad Dermatol. 2013;69:916-921.
27. Chapman JC, Daniel CR 3rd, Daniel JG, et al. Tinea capitis caused by dermatophytes: a 15-year retrospective study from a Mississippi Dermatology Clinic. Cutis. 2011;88:230-233.
28. Perman MJ, Lovell DJ, Denson LA, et al. Five cases of anti-tumor necrosis factor alpha-induced psoriasis presenting with severe scalp involvement in children. Pediatr Dermatol. 2012;29:454-459.
29. Khumalo NP, Jessop S, Gumedze F, et al. Determinants of marginal traction alopecia in African girls and women. J Am Acad Dermatol. 2008;59:432-438.
30. Vazquez-Lopez F, Palacios-Garcia L, Argenziano G. Dermoscopic corkscrew hairs dissolve after successful therapy of Trichophyton violaceum tinea capitis: a case report. Australas J Dermatol. 2012;53:118-119.
31. Pinheiro AM, Lobato LA, Varella TC. Dermoscopy findings in tinea capitis: case report and literature review. An Bras Dermatol. 2012;87:313-314.
32. Mapelli ET, Gualandri L, Cerri A, et al. Comma hairs in tinea capitis: a useful dermatoscopic sign for diagnosis of tinea capitis. Pediatr Dermatol. 2012;29:223-224.
33. Hughes R, Chiaverini C, Bahadoran P, et al. Corkscrew hair: a new dermoscopic sign for diagnosis of tinea capitis in black children. Arch Dermatol. 2011;147:355-356.
34. Ekiz O, Sen BB, Rifaiog˘lu EN, et al. Trichoscopy in paediatric patients with tinea capitis: a useful method to differentiate from alopecia areata [published online ahead of print August 24, 2013]. J Eur Acad Dermatol Venereol. 2014;28:1255-1258.
35. Lencastre A, Tosti A. Role of trichoscopy in children’s scalp and hair disorders [published online ahead of print Aug 13, 2013]. Pediatr Dermatol. 2013;30:674-682.
36. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
37. Nasir S, Ralph N, O’Neill C, et al. Trends in tinea capitis in an Irish pediatric population and a comparison of scalp brushings versus scalp scrapings as methods of investigation [published online ahead of print February 22, 2013]. Pediatr Dermatol. 2014;31:622-623.
38. Alvarez MS, Silverberg NB. Tinea capitis. Cutis. 2006;78:189-196.
39. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996.
40. Bookstaver PB, Watson HJ, Winters SD, et al. Prophylactic ketoconazole shampoo for tinea capitis in a high-risk pediatric population. J Pediatr Pharmacol Ther. 2011;16:199-203.
41. Allen HB, Honig PJ, Leyden JJ, et al. Selenium sulfide: adjunctive therapy for tinea capitis. Pediatrics. 1982;69:81-83.
42. Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
43. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair [published online ahead of print November 7, 2011]. Microsc Res Tech. 2012;75:620-625.
44. Kapila S, Hong E, Fischer G. A comparative study of childhood psoriasis and atopic dermatitis and greater understanding of the overlapping condition, psoriasis-dermatitis. Australas J Dermatol. 2012;53:98-105.
45. Tollesson A, Frithz A. Borage oil, an effective new treatment for infantile seborrhoeic dermatitis. Br J Dermatol. 1993;129:95.
46. Gooderham M, Debarre JM, Keddy-Grant J, et al. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12-17 years of age [published online ahead of print October 22, 2014]. Br J Dermatol. 2014;171:1470-1477.
47. Singer C, Stancu P, Coşoveanu S, et al. Non-alcoholic fatty liver disease in children. Curr Health Sci J. 2014;40:170-176.
48. Chiang C, Price V, Mirmirani P. Central centrifugal cicatricial alopecia: superimposed tinea capitis as the etiology of chronic scalp pruritus. Dermatol Online J. 2008;14:3.
49. Morrone A, Calcaterra R, Valenzano M, et al. Erythema nodosum induced by kerion celsi of the scalp in a woman. Mycoses. 2011;54:e237-e239.
50. Boaventura P, Pereira D, Celestino R, et al. Genetic alterations in thyroid tumors from patients irradiated in childhood for tinea capitis treatment. Eur J Endocrinol. 2013;169:673-679.
Scalp hyperkeratosis (scaling or flaking) is a common symptom in childhood and is typified by fine to thick hyperkeratosis of the scalp with or without underlying erythema. The causes of scalp hyperkeratosis in childhood vary based on the demographics of the population. In a population where approximately half of the pediatric patients were white, scaling of the scalp was more common in patients with seborrheic dermatitis and/or atopic dermatitis (AD) who were aged 0 to 2 years, and tinea capitis was only noted in children who were black.1 In children with skin of color, scalp hyperkeratosis has been noted as a marker of tinea capitis, especially in patients aged 3 to 11 years,2,3 and the level of suspicion should consistently remain high for this age group. In another study of an all-black population of schoolchildren aged 5 to 13 years (N=224), 3% demonstrated signs and symptoms of tinea capitis and 14% were found to be asymptomatic carriers.4 Although generally benign in nature, scalp hyperkeratosis can be associated with systemic illnesses such as juvenile dermatomyositis and Langerhans cell histiocytosis.5 This article addresses the diagnosis and treatment of scalp hyperkeratosis in children with skin of color, focusing on differences in exposure to contagious cases, hairstyling practices, and biological factors that may impact the disease process.
CAUSES OF SCALP HYPERKERATOSIS IN CHILDHOOD
Scalp hyperkeratosis in childhood usually is caused by common benign conditions, but some level of suspicion should be maintained for more severe etiologic conditions such as Langerhans cell histiocytosis and collagen vascular diseases (eg, juvenile dermatomyositis).6 Langerhans cell histiocytosis of the scalp might be obscured by background pigmentation in black children.
Scalp scaling can be a minor criterion in the diagnosis of AD. Atopic dermatitis should be suspected in Asian children with scalp scaling. Although one study in Bangladesh revealed scalp involvement in only 5.2% of pediatric patients with AD,7 a study in China reported an incidence rate as high as 49.7% (with a similarly high incidence of eyelid dermatitis).8 Children with AD also may have dry hair.9 Atopic dermatitis of the scalp is typified by itching, fine hyperkeratosis, and notably eczematous scalp lesions ranging from excoriated or oozing erythematous plaques to lichenification with hair miniaturization, primarily from scratch-induced breakage.10 The latter finding often is noted in black adolescent girls with long-term moderate to severe AD (personal observation).
Seborrheic dermatitis is a hypersensitivity response to yeast colonization of the scalp with Malassezia species. The infantile form is extremely common (also known as cradle cap). Characteristically, greasy yellow hyperkeratosis in fine to thick sheets is noted on the scalp in children younger than 2 years, especially infants, often with involvement of skin folds. One study noted that seborrheic dermatitis occurs in 6% of school-aged children as opposed to 19% of children younger than 2 years.1 Severe seborrheic dermatitis in infancy may be a prelude to AD, with the incidence being 3 times higher in children with prior seborrheic dermatitis.11 In teenagers, seborrheic dermatitis often accompanies acne onset in the early pubertal years.12
Psoriasis is an autoimmune inflammatory dermatosis that most commonly affects white children. In childhood, pityriasis amiantacea, psoriasiform scalp hyperkeratosis, is more common than in adulthood, with thick, stuck-on scales bound to the hairs. This variant is uncommon in Hispanic and Asian children and is almost never seen in black children but has been reported in cohorts of Turkish children.13 In a series of 85 Egyptian children with pityriasis amiantacea, diagnosis of scalp psoriasis was made in 35.3%, eczematous dermatitis in 34.2%, and tinea capitis in 12.9%.14 Consequently, a high degree of suspicion for tinea capitis should be held if pityriasis amiantacea is found in children with skin of color.15,16
Tinea capitis is a dermatophyte infection of the scalp, hair, and surrounding skin. The presence of tinea capitis on the scalp is associated with environmental exposure to dermatophytes (eg, school, household).4,17 The infection is largely caused by Trichophyton tonsurans in the United States, which causes a seborrheic appearance and less commonly alopecia (black dot or thinning), plaques with scale, or kerion. The presence of cervical lymph nodes and/or alopecia increases the chances of tinea being the diagnosis. Potassium hydroxide preparation and fungal culture can be performed to corroborate the diagnosis.1-3 Other etiologies of scalp hyperkeratosis such as juvenile pityriasis rubra pilaris and lice are extremely uncommon in black children, but lice may be seen in Hispanic and Asian girls with long straight hair who attend school. Discoid lupus is more common in children with skin of color but is rare overall. When noted, accompanying mottled dyspigmentation and scarring alopecia are noted in addition to a high risk for developing systemic lupus erythematosus. Biopsy and screening for systemic lupus are necessary, as the risk for progression from discoid lupus to systemic disease is 26% over 3 years.18
THE BIOLOGY OF HAIR IN CHILDREN WITH SKIN OF COLOR
To some extent, the biology of hair impacts the occurrence, appearance, and treatment of scalp hyperkeratosis in children with skin of color. First, it is important to remember that follicular density is lower in black patients as compared to Asian patients with a consequently lower hair count overall, which results in the easy appearance of hair loss, particularly at the margins of the scalp.19,20 Second, the shape of the hair follicle differs among races and ethnicities. Asian patients have round hair shafts coming from straight follicles, which allows for greater natural hair hydration, resulting in somewhat less aggressive scalp disease. Hispanic patients may have similarly straight hair or may have elliptical or curled shafts, the latter being noted in black patients. Furthermore, a curled hair shaft results in poor flow of sebum across the hair, resulting in greater scalp xerosis, more susceptibility to traction alopecia, and ultimately a greater risk for infections.20-23 Finally, the scalp is continuous with the face and neck, and Asian patients have greater sensitivity to skin care products in these areas, resulting in difficulty of treatment in this patient population and the need for use of gentle products.
HAIR CARE PRACTICES IN CHILDREN WITH SKIN OF COLOR
Hair care in patients with skin of color can be costly, difficult, and potentially damaging, with 99% of black girls reporting pomade or oil usage. Costly and complex hair care practices begin in childhood for patients with skin of color. In a series of 201 surveyed black girls with a mean age of 9.8 years, 80% had used hot combs and 42% used relaxers.24 Traction styles were common with 81% using ponytails, 67% braids, and 49% cornrows in the last 12 months. These styles are thought to affect hair health, particularly through induction of traction-related damage, folliculitis, and alopecia. Furthermore, chemical relaxers, hot combs, blowouts, and hair setting may be introduced during childhood.24 These practices appear to disturb the integrity of the hair follicle, leaving it more susceptible to irritation and infection.
Hair care in the pediatric population often is complicated by the fact that multiple children are being styled in tandem, either at home or in a salon, resulting in shared equipment and fomite spread. Even just proximity to a case of tinea capitis in the household will increase risk for tinea capitis. Furthermore, it is quite commonplace for black patients to use pomades and shampoos that contain antifungals, especially selenium sulfide, which makes it difficult to obtain accurate culture results. In India, use of mustard oil also has been linked to increased risk for tinea capitis.25
Other issues related to hair care include frequent dry scalp in patients with skin of color due to poor sebum distribution along the hair shaft. As a result, frequent washing may exacerbate scalp xerosis and further irritate seborrheic dermatitis and/or AD.
DIAGNOSTIC CONSIDERATIONS FOR SCALP HYPERKERATOSIS IN CHILDHOOD
Dermatologists should have a greater level of suspicion for tinea capitis in black and Hispanic children compared to white children. The index of suspicion should be high given that antifungal shampoos and pomades may minimize the clinical appearance. Although trends in overall incidence in the United States suggest tinea capitis is becoming less common, there still is a stronger representation of the disease in black patients.26 A study of positive fungal cultures from one clinic in Mississippi (N=1220) showed that two-thirds of patients were children younger than 13 years; 87% of patients with positive cultures for dermatophytes were black.27 The endothrix type of tinea capitis caused by T tonsurans often presents with a seborrheic appearance, and fungal culture is warranted in all pediatric patients with skin of color who have scalp hyperkeratosis. Asian children can be regarded with a lower level of suspicion for tinea capitis, similar to white patients in the United States. Variation in incidence of tinea capitis does exist worldwide and the practitioner may need to address these issues in patients who travel or are recent immigrants.
When identifying tinea capitis infections in children with skin of color, physicians should consider the patient’s personal and family history, comorbid skin disorders, dermoscopy, microscopy and fungal staining, and fungal culture (Figure).
A paradigm for the diagnosis of scalp hyperkeratosis in children with skin of color.
Personal and Family History
The first diagnostic consideration is the patient’s personal and family history. A history of AD, asthma, or allergies will support but not confirm the diagnosis of AD. Prior tinea capitis infections and household contacts with tinea infections support the presence of tinea capitis.17 Recent implementation of anti–tumor necrosis factor a inhibitor therapy in a psoriatic child can flare scalp disease, mimicking tinea capitis.28 The patient’s guardians should be queried about potential infectious contacts, whether they themselves have signs of scalp disease or tinea corporis (ringworm) or whether they have a pet with problematic fur. Physicians also should query patients and their guardians about recent use of topical antifungal shampoos, pomades, creams (both over-the-counter [OTC] and prescription), and/or oral antifungals. When these agents are used, there is a possibility that fungal examinations may be negative in the presence of true infection with tinea capitis. Traction alopecia, often preceded by fine scale, is more likely to present in patients who wear their hair in cornrows, while seborrheic dermatitis may be associated with hair extensions, reduced frequency of washing (61% of black girls surveyed wash every 2 weeks), and/or reduced usage of hair oils in black girls.24 Knowledge of the patient’s personal hair care history, such as use of pomades; frequency and method of washing/drying hair; types of hair care products used daily to wash and style hair; use of chemical relaxers; or recent hairstyling with cornrows, braids, or hair extensions, also is essential to the diagnosis of tinea capitis. Usage of traction-related styling practices in patients with chemically relaxed hair can enhance the risk for traction alopecia.29
Comorbid Skin Disorders
The patient also should be examined for comorbid skin disorders, including tinea corporis, alopecia (particularly in the areas of hyperkeratosis), and the presence of nuchal lymphadenopathy. For each extra clinical finding, the chances of a final diagnosis of tinea capitis rises, allowing for empiric diagnosis to be made that can be confirmed by a variety of tests.1-3
Dermoscopy
Next, the patient should undergo dermoscopic evaluation. On dermoscopy, tinea capitis typically presents with broken hairs, black dots on the scalp, comma-shaped hairs, and short corkscrew hairs, all of which should clear with therapy.30-33 Dermoscopic findings of AD would reveal underlying xerosis and prominent vasculature due to inflammation, and alopecia areata would present with yellow dots at the orifices of the hair follicles, exclamation point hairs, and vellus hairs.34,35 Traction alopecia may be noted by retained hairs along the hairline, which is known as the fringe sign.36
Microscopy and Fungal Staining
Microscopic preparations can be performed to identify tinea capitis using fungal stains of slide-based specimens. Breakage of short hairs onto the slide and/or cotton swab is a soft sign corroborating endothrix infection of the hairs. Potassium hydroxide can enhance visualization of the hyperkeratotic scalp, but for most black patients, use of antifungal agents reduces fungal hyphae and spores in the areas of hyperkeratosis and may limit the utility of examining the skin microscopically. Assessment of the broken hairs obtained by gentle friction with one glass slide and catching the scales onto another glass slide may yield the best results in the evaluation of tinea capitis (a technique taught to me by Robin Hornung, MD, Everett, Washington). Hairs obtained in this manner often are fragile and break due to endothrix infection replacing and weakening the shaft of the hairs. In the United States, fungal samples usually are obtained with cotton swabs, but a recent study suggested that brushing is superior to scraping to obtain samples; the combination of sampling techniques may improve the yield of a culture.37 Because topical agents are unable to enter the hair cortex, the hair shaft is the most likely to show fungal spores under the microscope when antifungal shampoos or pomades are used. Other testing methods such as Swartz-Lamkins or calcofluor white staining can be used on similar scrapings. Biopsy and periodic acid–Schiff staining of thick scales or crust can help differentiate tinea capitis from pityriasis amiantacea when the crust is too thick to be softened via potassium hydroxide preparation.38
Fungal Culture
Fungal culture onto media that contains nutrients for dermatophyte growth can be used for 4 purposes in tinea capitis: (1) to confirm infection, (2) to identify species of infection, (3) to confirm mycological cure when difficulty in clearance of disease has been noted, and (4) to obtain a specimen for sensitivity screening regarding antifungals when necessary, an uncommon but occasionally useful test in individuals with disease that has failed treatment with 1 or more antifungals.27
THERAPY FOR SCALP HYPERKERATOSIS IN CHILDREN WITH SKIN OF COLOR
In patients with scalp hyperkeratosis, it is important to address the specific cause of the disease. Therapy for scalp hyperkeratosis in children with skin of color includes altered hair care practices, use of OTC and prescription agents, and containment of fomites in the case of infections. Biopsy of atypical scalp hyperkeratosis cases is needed to diagnose rare etiologies such as discoid lupus or Langerhans cell histiocytosis. For individuals with systemic disease including Langerhans cell histiocytosis, which is generally accompanied by nodes and plaques in the inguinal region or other intertriginous sites, immediate hematology and oncology workup is required.39 For collagen vascular diseases such as lupus or dermatomyositis, appropriate referral to rheumatology and systemic therapy is warranted.
Altered Hair Care Practices
The use of prophylactic ketoconazole 1% shampoo may not reduce the risk for recurrence of tinea capitis over standard good hygiene, removal of fomites, and adherence to prescribed therapy.40 Use of selenium sulfide has been shown to effectively reduce contagion risk.41
Fragrance- and dye-free shampoos can be helpful in providing gentle cleansing of the scalp, which is especially important in Asian patients who have greater facial and eyelid sensitivity. Free-and-clear shampoos can be used alternatively with shampoos containing selenium sulfide or sulfur to eliminate comorbid seborrhea. Black patients should be advised to shampoo and condition their hair once weekly, and Asian and Hispanic patients should shampoo and condition 2 to 3 times weekly to remove scale and potentially reduce risk for tinea acquisition.42 Children with straight hair should shampoo with increased frequency in the summer to manually remove sweat-induced macerated hyperkeratosis. Conditioners also should be used consistently after shampooing to enhance hair health.
Use of OTC and Prescription Agents
Atopic Dermatitis
Topical corticosteroid agents can be used in increasing strengths to treat AD of the scalp in children with skin of color, from OTC scalp products containing hydrocortisone 1% to prescription-based agents. Hydration of the hair also is needed to counteract reduced water content.43 Due to the innate xerosis of the scalp in black patients and atopic patients, the use of oil-based or lotion products may provide the most hydration for patients with scalp disease.44 Alcohol-based agents, either drops or foams, may enhance xerosis and should be used sparingly.
Seborrheic Dermatitis
Alternating treatment with medicated shampoos containing selenium sulfide and ketoconazole can aid in the removal of seborrhea. Pomades including borage seed oil–based agents can be massaged into the scalp,45 particularly for treatment of infantile seborrhea, and should not necessarily be washed off daily in dark-skinned patients. Additional focused application of topical corticosteroids to the scalp also is helpful. Due to innate scalp xerosis in black children, therapy should be similar to AD.
Psoriasis
In the setting of pityriasis amiantacea, albeit rare in children with skin of color, oil-based agents can soften hyperkeratosis for removal. Sterile mineral oil or commercially available scalp preparations of peanut oil with fluocinolone or mineral oil with glycerin can aid in the removal of scales without harming the hair, but usage must be age appropriate. The addition of focused application of age-appropriate topical corticosteroids for areas of severe hyperkeratosis can aid in clearance of the lesions.44 Recently, a stable combination of calcipo-triene 0.005%–betamethasone dipropionate 0.064% has been approved in the United States for the therapy of scalp psoriasis in adolescents.46
Tinea Capitis
Antifungal shampoos including selenium sulfide will reduce contagion risk when used by both the patient and his/her family members. Frequency of shampooing is similar to that described for AD. Between shampooing, pomades with selenium sulfide can be applied to the scalp to enhance overall clearance.
Oral antifungals are the basis of treatment and use of griseofulvin is the gold standard. Terbinafine has been approved by the US Food and Drug Administration for treatment of tinea capitis; for children weighing less than 25 kg the dosage is 125 mg daily, for 25 to 35 kg the dosage is 187.5 mg daily, and for more than 35 kg the dosage is 250 mg daily. Shorter therapeutic courses may be required, making it a good second-line agent. Laboratory screening in children prior to therapy is not always performed but should be done in cases where fatty liver might be suspected.47 Monitoring liver function tests is best when exceeding 3 months of usage or shifting from one antifungal to another.3
Containment of Fomites
There are several procedures that should be followed to contain scalp infection in children with skin of color. First, all objects that come into contact with the scalp (eg, hats, hoods, brushes, pillowcases) should be washed with hot water or replaced weekly. Sharing these objects with friends or family should be strongly discouraged. Patients and their family members also should be instructed to use medicated (eg, selenium sulfide) shampoos and conditioners. Finally, patients are advised to avoid use of shared classroom garments or mats for sleeping.
LONG-TERM SEQUELAE OF SCALP HYPERKERATOSIS
Long-term sequelae of scalp hyperkeratosis often are discounted in children, but the disease can have lasting and damaging effects on the scalp. Sequelae include discomfort from chronicity and psychological distress. In particular, years of scalp pruritus can promote lichenification of the scalp and miniaturization of the hair follicles. Furthermore, itching due to sweating can limit participation in sports. Finally, tinea capitis is thought to be a risk factor for central centrifugal cicatricial alopecia (or can occur comorbidly with central centrifugal cicatricial alopecia causing severe pruritus), a chronic scarring hair loss that is seen primarily in black adult females.48 Erythema nodosum also has been reported as an associated finding in the case of kerion.49 One study reported associated findings that included thyroid cancer in individuals irradiated for tinea capitis in the 1950s.50
Conclusion
Scalp hyperkeratosis in children with skin of color, especially black patients, is more likely to be associated with tinea capitis and is more challenging to treat due to innate scalp xerosis in black patients and increased sensitivity of facial skin in Asian children. Ultimately, institution of therapy when needed and good scalp and hair care may prevent long-term sequelae.
Scalp hyperkeratosis (scaling or flaking) is a common symptom in childhood and is typified by fine to thick hyperkeratosis of the scalp with or without underlying erythema. The causes of scalp hyperkeratosis in childhood vary based on the demographics of the population. In a population where approximately half of the pediatric patients were white, scaling of the scalp was more common in patients with seborrheic dermatitis and/or atopic dermatitis (AD) who were aged 0 to 2 years, and tinea capitis was only noted in children who were black.1 In children with skin of color, scalp hyperkeratosis has been noted as a marker of tinea capitis, especially in patients aged 3 to 11 years,2,3 and the level of suspicion should consistently remain high for this age group. In another study of an all-black population of schoolchildren aged 5 to 13 years (N=224), 3% demonstrated signs and symptoms of tinea capitis and 14% were found to be asymptomatic carriers.4 Although generally benign in nature, scalp hyperkeratosis can be associated with systemic illnesses such as juvenile dermatomyositis and Langerhans cell histiocytosis.5 This article addresses the diagnosis and treatment of scalp hyperkeratosis in children with skin of color, focusing on differences in exposure to contagious cases, hairstyling practices, and biological factors that may impact the disease process.
CAUSES OF SCALP HYPERKERATOSIS IN CHILDHOOD
Scalp hyperkeratosis in childhood usually is caused by common benign conditions, but some level of suspicion should be maintained for more severe etiologic conditions such as Langerhans cell histiocytosis and collagen vascular diseases (eg, juvenile dermatomyositis).6 Langerhans cell histiocytosis of the scalp might be obscured by background pigmentation in black children.
Scalp scaling can be a minor criterion in the diagnosis of AD. Atopic dermatitis should be suspected in Asian children with scalp scaling. Although one study in Bangladesh revealed scalp involvement in only 5.2% of pediatric patients with AD,7 a study in China reported an incidence rate as high as 49.7% (with a similarly high incidence of eyelid dermatitis).8 Children with AD also may have dry hair.9 Atopic dermatitis of the scalp is typified by itching, fine hyperkeratosis, and notably eczematous scalp lesions ranging from excoriated or oozing erythematous plaques to lichenification with hair miniaturization, primarily from scratch-induced breakage.10 The latter finding often is noted in black adolescent girls with long-term moderate to severe AD (personal observation).
Seborrheic dermatitis is a hypersensitivity response to yeast colonization of the scalp with Malassezia species. The infantile form is extremely common (also known as cradle cap). Characteristically, greasy yellow hyperkeratosis in fine to thick sheets is noted on the scalp in children younger than 2 years, especially infants, often with involvement of skin folds. One study noted that seborrheic dermatitis occurs in 6% of school-aged children as opposed to 19% of children younger than 2 years.1 Severe seborrheic dermatitis in infancy may be a prelude to AD, with the incidence being 3 times higher in children with prior seborrheic dermatitis.11 In teenagers, seborrheic dermatitis often accompanies acne onset in the early pubertal years.12
Psoriasis is an autoimmune inflammatory dermatosis that most commonly affects white children. In childhood, pityriasis amiantacea, psoriasiform scalp hyperkeratosis, is more common than in adulthood, with thick, stuck-on scales bound to the hairs. This variant is uncommon in Hispanic and Asian children and is almost never seen in black children but has been reported in cohorts of Turkish children.13 In a series of 85 Egyptian children with pityriasis amiantacea, diagnosis of scalp psoriasis was made in 35.3%, eczematous dermatitis in 34.2%, and tinea capitis in 12.9%.14 Consequently, a high degree of suspicion for tinea capitis should be held if pityriasis amiantacea is found in children with skin of color.15,16
Tinea capitis is a dermatophyte infection of the scalp, hair, and surrounding skin. The presence of tinea capitis on the scalp is associated with environmental exposure to dermatophytes (eg, school, household).4,17 The infection is largely caused by Trichophyton tonsurans in the United States, which causes a seborrheic appearance and less commonly alopecia (black dot or thinning), plaques with scale, or kerion. The presence of cervical lymph nodes and/or alopecia increases the chances of tinea being the diagnosis. Potassium hydroxide preparation and fungal culture can be performed to corroborate the diagnosis.1-3 Other etiologies of scalp hyperkeratosis such as juvenile pityriasis rubra pilaris and lice are extremely uncommon in black children, but lice may be seen in Hispanic and Asian girls with long straight hair who attend school. Discoid lupus is more common in children with skin of color but is rare overall. When noted, accompanying mottled dyspigmentation and scarring alopecia are noted in addition to a high risk for developing systemic lupus erythematosus. Biopsy and screening for systemic lupus are necessary, as the risk for progression from discoid lupus to systemic disease is 26% over 3 years.18
THE BIOLOGY OF HAIR IN CHILDREN WITH SKIN OF COLOR
To some extent, the biology of hair impacts the occurrence, appearance, and treatment of scalp hyperkeratosis in children with skin of color. First, it is important to remember that follicular density is lower in black patients as compared to Asian patients with a consequently lower hair count overall, which results in the easy appearance of hair loss, particularly at the margins of the scalp.19,20 Second, the shape of the hair follicle differs among races and ethnicities. Asian patients have round hair shafts coming from straight follicles, which allows for greater natural hair hydration, resulting in somewhat less aggressive scalp disease. Hispanic patients may have similarly straight hair or may have elliptical or curled shafts, the latter being noted in black patients. Furthermore, a curled hair shaft results in poor flow of sebum across the hair, resulting in greater scalp xerosis, more susceptibility to traction alopecia, and ultimately a greater risk for infections.20-23 Finally, the scalp is continuous with the face and neck, and Asian patients have greater sensitivity to skin care products in these areas, resulting in difficulty of treatment in this patient population and the need for use of gentle products.
HAIR CARE PRACTICES IN CHILDREN WITH SKIN OF COLOR
Hair care in patients with skin of color can be costly, difficult, and potentially damaging, with 99% of black girls reporting pomade or oil usage. Costly and complex hair care practices begin in childhood for patients with skin of color. In a series of 201 surveyed black girls with a mean age of 9.8 years, 80% had used hot combs and 42% used relaxers.24 Traction styles were common with 81% using ponytails, 67% braids, and 49% cornrows in the last 12 months. These styles are thought to affect hair health, particularly through induction of traction-related damage, folliculitis, and alopecia. Furthermore, chemical relaxers, hot combs, blowouts, and hair setting may be introduced during childhood.24 These practices appear to disturb the integrity of the hair follicle, leaving it more susceptible to irritation and infection.
Hair care in the pediatric population often is complicated by the fact that multiple children are being styled in tandem, either at home or in a salon, resulting in shared equipment and fomite spread. Even just proximity to a case of tinea capitis in the household will increase risk for tinea capitis. Furthermore, it is quite commonplace for black patients to use pomades and shampoos that contain antifungals, especially selenium sulfide, which makes it difficult to obtain accurate culture results. In India, use of mustard oil also has been linked to increased risk for tinea capitis.25
Other issues related to hair care include frequent dry scalp in patients with skin of color due to poor sebum distribution along the hair shaft. As a result, frequent washing may exacerbate scalp xerosis and further irritate seborrheic dermatitis and/or AD.
DIAGNOSTIC CONSIDERATIONS FOR SCALP HYPERKERATOSIS IN CHILDHOOD
Dermatologists should have a greater level of suspicion for tinea capitis in black and Hispanic children compared to white children. The index of suspicion should be high given that antifungal shampoos and pomades may minimize the clinical appearance. Although trends in overall incidence in the United States suggest tinea capitis is becoming less common, there still is a stronger representation of the disease in black patients.26 A study of positive fungal cultures from one clinic in Mississippi (N=1220) showed that two-thirds of patients were children younger than 13 years; 87% of patients with positive cultures for dermatophytes were black.27 The endothrix type of tinea capitis caused by T tonsurans often presents with a seborrheic appearance, and fungal culture is warranted in all pediatric patients with skin of color who have scalp hyperkeratosis. Asian children can be regarded with a lower level of suspicion for tinea capitis, similar to white patients in the United States. Variation in incidence of tinea capitis does exist worldwide and the practitioner may need to address these issues in patients who travel or are recent immigrants.
When identifying tinea capitis infections in children with skin of color, physicians should consider the patient’s personal and family history, comorbid skin disorders, dermoscopy, microscopy and fungal staining, and fungal culture (Figure).
A paradigm for the diagnosis of scalp hyperkeratosis in children with skin of color.
Personal and Family History
The first diagnostic consideration is the patient’s personal and family history. A history of AD, asthma, or allergies will support but not confirm the diagnosis of AD. Prior tinea capitis infections and household contacts with tinea infections support the presence of tinea capitis.17 Recent implementation of anti–tumor necrosis factor a inhibitor therapy in a psoriatic child can flare scalp disease, mimicking tinea capitis.28 The patient’s guardians should be queried about potential infectious contacts, whether they themselves have signs of scalp disease or tinea corporis (ringworm) or whether they have a pet with problematic fur. Physicians also should query patients and their guardians about recent use of topical antifungal shampoos, pomades, creams (both over-the-counter [OTC] and prescription), and/or oral antifungals. When these agents are used, there is a possibility that fungal examinations may be negative in the presence of true infection with tinea capitis. Traction alopecia, often preceded by fine scale, is more likely to present in patients who wear their hair in cornrows, while seborrheic dermatitis may be associated with hair extensions, reduced frequency of washing (61% of black girls surveyed wash every 2 weeks), and/or reduced usage of hair oils in black girls.24 Knowledge of the patient’s personal hair care history, such as use of pomades; frequency and method of washing/drying hair; types of hair care products used daily to wash and style hair; use of chemical relaxers; or recent hairstyling with cornrows, braids, or hair extensions, also is essential to the diagnosis of tinea capitis. Usage of traction-related styling practices in patients with chemically relaxed hair can enhance the risk for traction alopecia.29
Comorbid Skin Disorders
The patient also should be examined for comorbid skin disorders, including tinea corporis, alopecia (particularly in the areas of hyperkeratosis), and the presence of nuchal lymphadenopathy. For each extra clinical finding, the chances of a final diagnosis of tinea capitis rises, allowing for empiric diagnosis to be made that can be confirmed by a variety of tests.1-3
Dermoscopy
Next, the patient should undergo dermoscopic evaluation. On dermoscopy, tinea capitis typically presents with broken hairs, black dots on the scalp, comma-shaped hairs, and short corkscrew hairs, all of which should clear with therapy.30-33 Dermoscopic findings of AD would reveal underlying xerosis and prominent vasculature due to inflammation, and alopecia areata would present with yellow dots at the orifices of the hair follicles, exclamation point hairs, and vellus hairs.34,35 Traction alopecia may be noted by retained hairs along the hairline, which is known as the fringe sign.36
Microscopy and Fungal Staining
Microscopic preparations can be performed to identify tinea capitis using fungal stains of slide-based specimens. Breakage of short hairs onto the slide and/or cotton swab is a soft sign corroborating endothrix infection of the hairs. Potassium hydroxide can enhance visualization of the hyperkeratotic scalp, but for most black patients, use of antifungal agents reduces fungal hyphae and spores in the areas of hyperkeratosis and may limit the utility of examining the skin microscopically. Assessment of the broken hairs obtained by gentle friction with one glass slide and catching the scales onto another glass slide may yield the best results in the evaluation of tinea capitis (a technique taught to me by Robin Hornung, MD, Everett, Washington). Hairs obtained in this manner often are fragile and break due to endothrix infection replacing and weakening the shaft of the hairs. In the United States, fungal samples usually are obtained with cotton swabs, but a recent study suggested that brushing is superior to scraping to obtain samples; the combination of sampling techniques may improve the yield of a culture.37 Because topical agents are unable to enter the hair cortex, the hair shaft is the most likely to show fungal spores under the microscope when antifungal shampoos or pomades are used. Other testing methods such as Swartz-Lamkins or calcofluor white staining can be used on similar scrapings. Biopsy and periodic acid–Schiff staining of thick scales or crust can help differentiate tinea capitis from pityriasis amiantacea when the crust is too thick to be softened via potassium hydroxide preparation.38
Fungal Culture
Fungal culture onto media that contains nutrients for dermatophyte growth can be used for 4 purposes in tinea capitis: (1) to confirm infection, (2) to identify species of infection, (3) to confirm mycological cure when difficulty in clearance of disease has been noted, and (4) to obtain a specimen for sensitivity screening regarding antifungals when necessary, an uncommon but occasionally useful test in individuals with disease that has failed treatment with 1 or more antifungals.27
THERAPY FOR SCALP HYPERKERATOSIS IN CHILDREN WITH SKIN OF COLOR
In patients with scalp hyperkeratosis, it is important to address the specific cause of the disease. Therapy for scalp hyperkeratosis in children with skin of color includes altered hair care practices, use of OTC and prescription agents, and containment of fomites in the case of infections. Biopsy of atypical scalp hyperkeratosis cases is needed to diagnose rare etiologies such as discoid lupus or Langerhans cell histiocytosis. For individuals with systemic disease including Langerhans cell histiocytosis, which is generally accompanied by nodes and plaques in the inguinal region or other intertriginous sites, immediate hematology and oncology workup is required.39 For collagen vascular diseases such as lupus or dermatomyositis, appropriate referral to rheumatology and systemic therapy is warranted.
Altered Hair Care Practices
The use of prophylactic ketoconazole 1% shampoo may not reduce the risk for recurrence of tinea capitis over standard good hygiene, removal of fomites, and adherence to prescribed therapy.40 Use of selenium sulfide has been shown to effectively reduce contagion risk.41
Fragrance- and dye-free shampoos can be helpful in providing gentle cleansing of the scalp, which is especially important in Asian patients who have greater facial and eyelid sensitivity. Free-and-clear shampoos can be used alternatively with shampoos containing selenium sulfide or sulfur to eliminate comorbid seborrhea. Black patients should be advised to shampoo and condition their hair once weekly, and Asian and Hispanic patients should shampoo and condition 2 to 3 times weekly to remove scale and potentially reduce risk for tinea acquisition.42 Children with straight hair should shampoo with increased frequency in the summer to manually remove sweat-induced macerated hyperkeratosis. Conditioners also should be used consistently after shampooing to enhance hair health.
Use of OTC and Prescription Agents
Atopic Dermatitis
Topical corticosteroid agents can be used in increasing strengths to treat AD of the scalp in children with skin of color, from OTC scalp products containing hydrocortisone 1% to prescription-based agents. Hydration of the hair also is needed to counteract reduced water content.43 Due to the innate xerosis of the scalp in black patients and atopic patients, the use of oil-based or lotion products may provide the most hydration for patients with scalp disease.44 Alcohol-based agents, either drops or foams, may enhance xerosis and should be used sparingly.
Seborrheic Dermatitis
Alternating treatment with medicated shampoos containing selenium sulfide and ketoconazole can aid in the removal of seborrhea. Pomades including borage seed oil–based agents can be massaged into the scalp,45 particularly for treatment of infantile seborrhea, and should not necessarily be washed off daily in dark-skinned patients. Additional focused application of topical corticosteroids to the scalp also is helpful. Due to innate scalp xerosis in black children, therapy should be similar to AD.
Psoriasis
In the setting of pityriasis amiantacea, albeit rare in children with skin of color, oil-based agents can soften hyperkeratosis for removal. Sterile mineral oil or commercially available scalp preparations of peanut oil with fluocinolone or mineral oil with glycerin can aid in the removal of scales without harming the hair, but usage must be age appropriate. The addition of focused application of age-appropriate topical corticosteroids for areas of severe hyperkeratosis can aid in clearance of the lesions.44 Recently, a stable combination of calcipo-triene 0.005%–betamethasone dipropionate 0.064% has been approved in the United States for the therapy of scalp psoriasis in adolescents.46
Tinea Capitis
Antifungal shampoos including selenium sulfide will reduce contagion risk when used by both the patient and his/her family members. Frequency of shampooing is similar to that described for AD. Between shampooing, pomades with selenium sulfide can be applied to the scalp to enhance overall clearance.
Oral antifungals are the basis of treatment and use of griseofulvin is the gold standard. Terbinafine has been approved by the US Food and Drug Administration for treatment of tinea capitis; for children weighing less than 25 kg the dosage is 125 mg daily, for 25 to 35 kg the dosage is 187.5 mg daily, and for more than 35 kg the dosage is 250 mg daily. Shorter therapeutic courses may be required, making it a good second-line agent. Laboratory screening in children prior to therapy is not always performed but should be done in cases where fatty liver might be suspected.47 Monitoring liver function tests is best when exceeding 3 months of usage or shifting from one antifungal to another.3
Containment of Fomites
There are several procedures that should be followed to contain scalp infection in children with skin of color. First, all objects that come into contact with the scalp (eg, hats, hoods, brushes, pillowcases) should be washed with hot water or replaced weekly. Sharing these objects with friends or family should be strongly discouraged. Patients and their family members also should be instructed to use medicated (eg, selenium sulfide) shampoos and conditioners. Finally, patients are advised to avoid use of shared classroom garments or mats for sleeping.
LONG-TERM SEQUELAE OF SCALP HYPERKERATOSIS
Long-term sequelae of scalp hyperkeratosis often are discounted in children, but the disease can have lasting and damaging effects on the scalp. Sequelae include discomfort from chronicity and psychological distress. In particular, years of scalp pruritus can promote lichenification of the scalp and miniaturization of the hair follicles. Furthermore, itching due to sweating can limit participation in sports. Finally, tinea capitis is thought to be a risk factor for central centrifugal cicatricial alopecia (or can occur comorbidly with central centrifugal cicatricial alopecia causing severe pruritus), a chronic scarring hair loss that is seen primarily in black adult females.48 Erythema nodosum also has been reported as an associated finding in the case of kerion.49 One study reported associated findings that included thyroid cancer in individuals irradiated for tinea capitis in the 1950s.50
Conclusion
Scalp hyperkeratosis in children with skin of color, especially black patients, is more likely to be associated with tinea capitis and is more challenging to treat due to innate scalp xerosis in black patients and increased sensitivity of facial skin in Asian children. Ultimately, institution of therapy when needed and good scalp and hair care may prevent long-term sequelae.
1. Williams JV, Eichenfield LF, Burke BL, et al. Prevalence of scalp scaling in prepubertal children. Pediatrics. 2005;115:e1-e6.
2. Coley MK, Bhanusali DG, Silverberg JI, et al. Scalp hyperkeratosis and alopecia in children of color. J Drugs Dermatol. 2011;10:511-516.
3. Bhanusali D, Coley M, Silverberg JI, et al. Treatment outcomes for tinea capitis in a skin of color population. J Drugs Dermatol. 2012;11:852-856.
4. Williams JV, Honig PJ, McGinley KJ, et al. Semiquantitative study of tinea capitis and the asymptomatic carrier state in inner-city school children. Pediatrics. 1995;96:265-267.
5. McDonald LL, Smith ML. Diagnostic dilemmas in pediatric/adolescent dermatology: scaly scalp. J Pediatr Health Care. 1998;12:80-84.
6. Peloro TM, Miller OF 3rd, Hahn TF, et al. Juvenile dermatomyositis: a retrospective review of a 30-year experience. J Am Acad Dermatol. 2001;45:28-34.
7. Wahab MA, Rahman MH, Khondker L, et al. Minor criteria for atopic dermatitis in children. Mymensingh Med J. 2011;20:419-424.
8. Shi M, Zhang H, Chen X, et al. Clinical features of atopic dermatitis in a hospital-based setting in China. J Eur Acad Dermatol Venereol [published online ahead of print January 9, 2011]. 2011;25:1206-1212.
9. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair. Microsc Res Tech. 2012;75:620-625.
10. Sabin BR, Peters N, Peters AT. Chapter 20: atopic dermatitis. Allergy Asthma Proc. 2012;33:S67-S69.
11. Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis [published online ahead of print November 13, 2013]. Pediatr Dermatol. 2014;31:125-130.
12. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
13. Sarifakioglu E, Yilmaz AE, Gorpelioglu C, et al. Prevalence of scalp disorders and hair loss in children. Cutis. 2012;90:225-229.
14. Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
15. Oostveen AM, Jong EM, Evers AW, et al. Reliability, responsiveness and validity of Scalpdex in children with scalp psoriasis: the Dutch study. Acta Derm Venereol. 2014;94:198-202.
16. Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity: Comparative Dermatologic Atlas of Pediatric Skin of All Colors. New York, NY: Springer; 2012.
17. Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
18. Moises-Alfaro C, Berrón-Pérez R, Carrasco-Daza D, et al. Discoid lupus erythematosus in children: clinical, histopathologic, and follow-up features in 27 cases. Pediatr Dermatol. 2003;20:103-107.
19. Ramos-e-Silva M. Ethnic hair and skin: what is the state of the science? Chicago, Illinois—September 29-30, 2001. Clin Dermatol. 2002;20:321-324.
20. Heath CR, McMichael AJ. Biology of hair follicle. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. New York, NY: McGraw Hill; 2009:105-109.
21. Khumalo NP. African hair morphology: macrostructure to ultrastructure. Int J Dermatol. 2005;44(suppl 1):10-12.
22. Thibaut S, Bernard BA. The biology of hair shape. Int J Dermatol. 2005;44(suppl 1):2-3.
23. Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
24. Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
25. Kumar V, Sharma RC, Chander R. Clinicomycological study of tinea capitis. Indian J Dermatol Venereol Leprol. 1996;62:207-209.
26. Mirmirani P, Tucker LY. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California [published online ahead of print October 2, 2013]. J Am Acad Dermatol. 2013;69:916-921.
27. Chapman JC, Daniel CR 3rd, Daniel JG, et al. Tinea capitis caused by dermatophytes: a 15-year retrospective study from a Mississippi Dermatology Clinic. Cutis. 2011;88:230-233.
28. Perman MJ, Lovell DJ, Denson LA, et al. Five cases of anti-tumor necrosis factor alpha-induced psoriasis presenting with severe scalp involvement in children. Pediatr Dermatol. 2012;29:454-459.
29. Khumalo NP, Jessop S, Gumedze F, et al. Determinants of marginal traction alopecia in African girls and women. J Am Acad Dermatol. 2008;59:432-438.
30. Vazquez-Lopez F, Palacios-Garcia L, Argenziano G. Dermoscopic corkscrew hairs dissolve after successful therapy of Trichophyton violaceum tinea capitis: a case report. Australas J Dermatol. 2012;53:118-119.
31. Pinheiro AM, Lobato LA, Varella TC. Dermoscopy findings in tinea capitis: case report and literature review. An Bras Dermatol. 2012;87:313-314.
32. Mapelli ET, Gualandri L, Cerri A, et al. Comma hairs in tinea capitis: a useful dermatoscopic sign for diagnosis of tinea capitis. Pediatr Dermatol. 2012;29:223-224.
33. Hughes R, Chiaverini C, Bahadoran P, et al. Corkscrew hair: a new dermoscopic sign for diagnosis of tinea capitis in black children. Arch Dermatol. 2011;147:355-356.
34. Ekiz O, Sen BB, Rifaiog˘lu EN, et al. Trichoscopy in paediatric patients with tinea capitis: a useful method to differentiate from alopecia areata [published online ahead of print August 24, 2013]. J Eur Acad Dermatol Venereol. 2014;28:1255-1258.
35. Lencastre A, Tosti A. Role of trichoscopy in children’s scalp and hair disorders [published online ahead of print Aug 13, 2013]. Pediatr Dermatol. 2013;30:674-682.
36. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
37. Nasir S, Ralph N, O’Neill C, et al. Trends in tinea capitis in an Irish pediatric population and a comparison of scalp brushings versus scalp scrapings as methods of investigation [published online ahead of print February 22, 2013]. Pediatr Dermatol. 2014;31:622-623.
38. Alvarez MS, Silverberg NB. Tinea capitis. Cutis. 2006;78:189-196.
39. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996.
40. Bookstaver PB, Watson HJ, Winters SD, et al. Prophylactic ketoconazole shampoo for tinea capitis in a high-risk pediatric population. J Pediatr Pharmacol Ther. 2011;16:199-203.
41. Allen HB, Honig PJ, Leyden JJ, et al. Selenium sulfide: adjunctive therapy for tinea capitis. Pediatrics. 1982;69:81-83.
42. Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
43. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair [published online ahead of print November 7, 2011]. Microsc Res Tech. 2012;75:620-625.
44. Kapila S, Hong E, Fischer G. A comparative study of childhood psoriasis and atopic dermatitis and greater understanding of the overlapping condition, psoriasis-dermatitis. Australas J Dermatol. 2012;53:98-105.
45. Tollesson A, Frithz A. Borage oil, an effective new treatment for infantile seborrhoeic dermatitis. Br J Dermatol. 1993;129:95.
46. Gooderham M, Debarre JM, Keddy-Grant J, et al. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12-17 years of age [published online ahead of print October 22, 2014]. Br J Dermatol. 2014;171:1470-1477.
47. Singer C, Stancu P, Coşoveanu S, et al. Non-alcoholic fatty liver disease in children. Curr Health Sci J. 2014;40:170-176.
48. Chiang C, Price V, Mirmirani P. Central centrifugal cicatricial alopecia: superimposed tinea capitis as the etiology of chronic scalp pruritus. Dermatol Online J. 2008;14:3.
49. Morrone A, Calcaterra R, Valenzano M, et al. Erythema nodosum induced by kerion celsi of the scalp in a woman. Mycoses. 2011;54:e237-e239.
50. Boaventura P, Pereira D, Celestino R, et al. Genetic alterations in thyroid tumors from patients irradiated in childhood for tinea capitis treatment. Eur J Endocrinol. 2013;169:673-679.
1. Williams JV, Eichenfield LF, Burke BL, et al. Prevalence of scalp scaling in prepubertal children. Pediatrics. 2005;115:e1-e6.
2. Coley MK, Bhanusali DG, Silverberg JI, et al. Scalp hyperkeratosis and alopecia in children of color. J Drugs Dermatol. 2011;10:511-516.
3. Bhanusali D, Coley M, Silverberg JI, et al. Treatment outcomes for tinea capitis in a skin of color population. J Drugs Dermatol. 2012;11:852-856.
4. Williams JV, Honig PJ, McGinley KJ, et al. Semiquantitative study of tinea capitis and the asymptomatic carrier state in inner-city school children. Pediatrics. 1995;96:265-267.
5. McDonald LL, Smith ML. Diagnostic dilemmas in pediatric/adolescent dermatology: scaly scalp. J Pediatr Health Care. 1998;12:80-84.
6. Peloro TM, Miller OF 3rd, Hahn TF, et al. Juvenile dermatomyositis: a retrospective review of a 30-year experience. J Am Acad Dermatol. 2001;45:28-34.
7. Wahab MA, Rahman MH, Khondker L, et al. Minor criteria for atopic dermatitis in children. Mymensingh Med J. 2011;20:419-424.
8. Shi M, Zhang H, Chen X, et al. Clinical features of atopic dermatitis in a hospital-based setting in China. J Eur Acad Dermatol Venereol [published online ahead of print January 9, 2011]. 2011;25:1206-1212.
9. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair. Microsc Res Tech. 2012;75:620-625.
10. Sabin BR, Peters N, Peters AT. Chapter 20: atopic dermatitis. Allergy Asthma Proc. 2012;33:S67-S69.
11. Alexopoulos A, Kakourou T, Orfanou I, et al. Retrospective analysis of the relationship between infantile seborrheic dermatitis and atopic dermatitis [published online ahead of print November 13, 2013]. Pediatr Dermatol. 2014;31:125-130.
12. Elish D, Silverberg NB. Infantile seborrheic dermatitis. Cutis. 2006;77:297-300.
13. Sarifakioglu E, Yilmaz AE, Gorpelioglu C, et al. Prevalence of scalp disorders and hair loss in children. Cutis. 2012;90:225-229.
14. Abdel-Hamid IA, Agha SA, Moustafa YM, et al. Pityriasis amiantacea: a clinical and etiopathologic study of 85 patients. Int J Dermatol. 2003;42:260-264.
15. Oostveen AM, Jong EM, Evers AW, et al. Reliability, responsiveness and validity of Scalpdex in children with scalp psoriasis: the Dutch study. Acta Derm Venereol. 2014;94:198-202.
16. Silverberg NB. Atlas of Pediatric Cutaneous Biodiversity: Comparative Dermatologic Atlas of Pediatric Skin of All Colors. New York, NY: Springer; 2012.
17. Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
18. Moises-Alfaro C, Berrón-Pérez R, Carrasco-Daza D, et al. Discoid lupus erythematosus in children: clinical, histopathologic, and follow-up features in 27 cases. Pediatr Dermatol. 2003;20:103-107.
19. Ramos-e-Silva M. Ethnic hair and skin: what is the state of the science? Chicago, Illinois—September 29-30, 2001. Clin Dermatol. 2002;20:321-324.
20. Heath CR, McMichael AJ. Biology of hair follicle. In: Kelly AP, Taylor SC, eds. Dermatology for Skin of Color. New York, NY: McGraw Hill; 2009:105-109.
21. Khumalo NP. African hair morphology: macrostructure to ultrastructure. Int J Dermatol. 2005;44(suppl 1):10-12.
22. Thibaut S, Bernard BA. The biology of hair shape. Int J Dermatol. 2005;44(suppl 1):2-3.
23. Taylor SC. Skin of color: biology, structure, function, and implications for dermatologic disease. J Am Acad Dermatol. 2002;46(suppl 2):S41-S62.
24. Rucker Wright D, Gathers R, Kapke A, et al. Hair care practices and their association with scalp and hair disorders in African American girls. J Am Acad Dermatol. 2011;64:253-262.
25. Kumar V, Sharma RC, Chander R. Clinicomycological study of tinea capitis. Indian J Dermatol Venereol Leprol. 1996;62:207-209.
26. Mirmirani P, Tucker LY. Epidemiologic trends in pediatric tinea capitis: a population-based study from Kaiser Permanente Northern California [published online ahead of print October 2, 2013]. J Am Acad Dermatol. 2013;69:916-921.
27. Chapman JC, Daniel CR 3rd, Daniel JG, et al. Tinea capitis caused by dermatophytes: a 15-year retrospective study from a Mississippi Dermatology Clinic. Cutis. 2011;88:230-233.
28. Perman MJ, Lovell DJ, Denson LA, et al. Five cases of anti-tumor necrosis factor alpha-induced psoriasis presenting with severe scalp involvement in children. Pediatr Dermatol. 2012;29:454-459.
29. Khumalo NP, Jessop S, Gumedze F, et al. Determinants of marginal traction alopecia in African girls and women. J Am Acad Dermatol. 2008;59:432-438.
30. Vazquez-Lopez F, Palacios-Garcia L, Argenziano G. Dermoscopic corkscrew hairs dissolve after successful therapy of Trichophyton violaceum tinea capitis: a case report. Australas J Dermatol. 2012;53:118-119.
31. Pinheiro AM, Lobato LA, Varella TC. Dermoscopy findings in tinea capitis: case report and literature review. An Bras Dermatol. 2012;87:313-314.
32. Mapelli ET, Gualandri L, Cerri A, et al. Comma hairs in tinea capitis: a useful dermatoscopic sign for diagnosis of tinea capitis. Pediatr Dermatol. 2012;29:223-224.
33. Hughes R, Chiaverini C, Bahadoran P, et al. Corkscrew hair: a new dermoscopic sign for diagnosis of tinea capitis in black children. Arch Dermatol. 2011;147:355-356.
34. Ekiz O, Sen BB, Rifaiog˘lu EN, et al. Trichoscopy in paediatric patients with tinea capitis: a useful method to differentiate from alopecia areata [published online ahead of print August 24, 2013]. J Eur Acad Dermatol Venereol. 2014;28:1255-1258.
35. Lencastre A, Tosti A. Role of trichoscopy in children’s scalp and hair disorders [published online ahead of print Aug 13, 2013]. Pediatr Dermatol. 2013;30:674-682.
36. Samrao A, Price VH, Zedek D, et al. The “fringe sign”—a useful clinical finding in traction alopecia of the marginal hair line. Dermatol Online J. 2011;17:1.
37. Nasir S, Ralph N, O’Neill C, et al. Trends in tinea capitis in an Irish pediatric population and a comparison of scalp brushings versus scalp scrapings as methods of investigation [published online ahead of print February 22, 2013]. Pediatr Dermatol. 2014;31:622-623.
38. Alvarez MS, Silverberg NB. Tinea capitis. Cutis. 2006;78:189-196.
39. Simko SJ, Garmezy B, Abhyankar H, et al. Differentiating skin-limited and multisystem Langerhans cell histiocytosis. J Pediatr. 2014;165:990-996.
40. Bookstaver PB, Watson HJ, Winters SD, et al. Prophylactic ketoconazole shampoo for tinea capitis in a high-risk pediatric population. J Pediatr Pharmacol Ther. 2011;16:199-203.
41. Allen HB, Honig PJ, Leyden JJ, et al. Selenium sulfide: adjunctive therapy for tinea capitis. Pediatrics. 1982;69:81-83.
42. Crawford K, Hernandez C. A review of hair care products for black individuals. Cutis. 2014;93:289-293.
43. Kim KS, Shin MK, Kim JH, et al. Effects of atopic dermatitis on the morphology and water content of scalp hair [published online ahead of print November 7, 2011]. Microsc Res Tech. 2012;75:620-625.
44. Kapila S, Hong E, Fischer G. A comparative study of childhood psoriasis and atopic dermatitis and greater understanding of the overlapping condition, psoriasis-dermatitis. Australas J Dermatol. 2012;53:98-105.
45. Tollesson A, Frithz A. Borage oil, an effective new treatment for infantile seborrhoeic dermatitis. Br J Dermatol. 1993;129:95.
46. Gooderham M, Debarre JM, Keddy-Grant J, et al. Safety and efficacy of calcipotriol plus betamethasone dipropionate gel in the treatment of scalp psoriasis in adolescents 12-17 years of age [published online ahead of print October 22, 2014]. Br J Dermatol. 2014;171:1470-1477.
47. Singer C, Stancu P, Coşoveanu S, et al. Non-alcoholic fatty liver disease in children. Curr Health Sci J. 2014;40:170-176.
48. Chiang C, Price V, Mirmirani P. Central centrifugal cicatricial alopecia: superimposed tinea capitis as the etiology of chronic scalp pruritus. Dermatol Online J. 2008;14:3.
49. Morrone A, Calcaterra R, Valenzano M, et al. Erythema nodosum induced by kerion celsi of the scalp in a woman. Mycoses. 2011;54:e237-e239.
50. Boaventura P, Pereira D, Celestino R, et al. Genetic alterations in thyroid tumors from patients irradiated in childhood for tinea capitis treatment. Eur J Endocrinol. 2013;169:673-679.
Practice Points
- Scalp hyperkeratosis is a common finding in children, especially those with skin of color.
- Fungal culture may be helpful in the diagnosis of scalp hyperkeratosis in children of any age but should be performed in patients aged 3 to 11 years with skin of color.
- Therapy of scalp disease in children with skin of color should be adjusted based on hair type and disease features.
Update on Pediatric Psoriasis
Psoriasis affects 2% to 4% of the US population, with approximately one-third of cases beginning in childhood. The understanding of pediatric psoriasis has developed at a far slower pace than adult disease, with limitations in care including few medications that are approved by the US Food and Drug Administration for pediatric and adolescent use. Recently, a stable fixed-combination dose of calcipo-triene 0.005%–betamethasone dipropionate 0.064% topical suspension was approved for treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years, which hopefully will lead a trend in psoriasis medication approval for children and teenagers.1 Based on a PubMed search of articles indexed for MEDLINE using the search terms pediatric psoriasis, psoriasis, and strep that were published from April 2012 to April 2014, this article reviews newer data to address the issues that surround pediatric psoriasis and to provide an update on prior review articles on pediatric psoriasis.2-5 This article reviews some of the newer literature on clinical presentation and comorbidities in pediatric psoriasis.5 Based on these recent findings, additional screenings including review of obesity parameters are recommended for pediatric patients with psoriasis (Table 1).
Update on Disease Manifestations, Associations, and Comorbidities
Disease Manifestations
A 2013 multicenter study delineated the clinical features of pediatric psoriasis.6 The study was conducted at 8 geographically diverse dermatology clinics in the United States to delineate the clinical manifestations of pediatric psoriasis. In an assessment of 181 participants aged 5 to 17 years, the investigators sought to determine the frequency of disease sites, severity, and guttate disease. Over a period of approximately 2 years, 43.1% of participants were determined to have mild disease and 56.9% had severe disease. Family history of psoriasis was present in 51.4% of participants, with first-degree relatives affected in 59.8% of cases. Scalp involvement at some time was noted in 79.0% of participants, and nail disease was noted in 55% of boys and 29% of girls. Guttate psoriasis was noted in 30% of participants, with more cases in the severe range (35.9%) versus the mild range (21.8%). Additionally, 22.1% of participants had a precipitating streptococcal infection, with the association being more common in pediatric patients with guttate psoriasis than plaque psoriasis.6 This study highlighted that pediatric psoriasis has a genetic basis, is frequently guttate in nature, commonly affects the nails, shows a trend toward being classified as severe, and may be triggered by streptococcal infections.
Streptococcal Infection
Pediatric psoriasis may be triggered or flared by Streptococcus pyogenes (group A β-hemolytic streptococci) infections, specifically β-hemolytic streptococci groups A, C, and G that have streptococcal M protein,2,3,7 and this tendency can be associated with HLA-Cw6 or guttate psoriasis. Newer data have elucidated the role of streptococcal throat infections in psoriasis. Given that streptococcal throat infections are most common in school-aged children, these studies suggest a putative mechanism in pediatric psoriasis for triggering streptococcal infections, which would need to be confirmed in future studies, specifically in pediatric psoriasis patients.
It has been shown that T cells in psoriasis patients recognize common streptococcal M proteins and keratin determinants.7 Ferran et al8 recently demonstrated activation of circulating cutaneous lymphocyte–associated antigen (CLA)+ T cells but not CLA- memory T cells in 27 psoriasis patients (ages not specified) when mixed with streptococcal throat extracts, causing production of IL-17, IP-10, IL-22, and IFN-γ; activation was not found in 6 healthy control patients. Antistreptolysin O levels were correlated with the messenger RNA up- regulation for IL-17, IP-10, IL-22, and IFN-γ, and also correlated with psoriasis area and severity index score in psoriasis patients. In this same study, injection of the activated culture supernatant into mouse skin caused epidermal hyperkeratosis and activation of nonlesional epidermal cells from psoriatic patients. This study thereby delineated some of the potential pathways of the streptococcal induction of psoriasis and psoriatic flares in childhood8; however, confirmation is needed through further study of pediatric psoriatic lymphocyte activity.
Differential Diagnosis
Additions to the extensive differential list have been cited in the recent literature. The differential diagnosis of pediatric psoriasis now includes sodium valproate–induced psoriasiform drug eruption9 and allergic contact dermatitis to methylchloroisothiazolinone and methylisothiazolinone, which are present in many sanitizing hand and diaper wipes and has been reported to cause psoriasiform dermatitis in a periorificial or perineal distribution.10 Clinicians should inquire about the use of these wipes, as caregivers rarely suspect this agent to be causative of the eruption.
Psoriatic Arthritis
Previously, psoriasis and psoriatic arthritis have been linked to autoimmune thyroid disease in adults.11 A study of the Childhood Arthritis & Rheumatology Research Alliance (CARRA) registry showed that family history of psoriasis, autoimmune thyroiditis, Crohn disease, and ankylosing spondylitis in a first-degree relative has been linked to juvenile idiopathic arthritis, highlighting that pediatric psoriasis can be genetically linked or associated with multiple autoimmune conditions and vice versa.12
Obesity, Metabolic Syndrome, and Cardiovascular Risks
Obesity is associated with pediatric psoriasis as highlighted in a growing body of recent literature.13 Excess adiposity as manifested by body mass index in the 85th percentile or greater (37.9% of 155 pediatric psoriasis patients vs 20.5% of 42 controls) and excess central adiposity as manifested by excess waist circumference and increased waist-to-height ratios are more common in pediatric patients with psoriasis than in controls.14
Obesity may be a trigger or associated with increased disease activity in pediatric psoriasis patients. Excess overall adiposity correlates with more severe disease. Obesity parameters may correlate with the onset of psoriasis and with disease severity. In fact, the odds of obesity may be higher in childhood than in adults.14,15 A 2011 report of pediatric psoriasis patients aged 10 to 17 years (n=12) and wart controls (n=6)(mean age, 13.2 and 13.5 years, respectively) demonstrated that 4 of 12 patients with psoriasis and 0 of 6 patients with warts met criteria for metabolic syndrome as defined by 3 of the following: (1) triglycerides greater than or equal to 100 mg/dL; (2) high-density lipoprotein cholesterol less than 50 mg/dL in females and less than 5 mg/dL in males; (3) fasting blood glucose levels greater than or equal to 110 mg/dL, (4) waist circumference greater than the 75th percentile for age and sex; and (5) systolic or diastolic blood pressure greater than the 90th percentile for age, sex, and height.16 These studies highlight that obesity and metabolic syndrome are of concern in pediatric psoriasis patients; however, the best management approach using diet and weight interventions has yet to be identified.
Adiposity may precede the onset of psoriasis. A recent cohort of 27 pediatric psoriasis patients reported that the average age at onset of psoriasis was 8.7 years and the average age at onset of obesity was 4.1 years.15 In this study, 93% (25/27) of patients had adiposity preceding their psoriasis by 2 or more years. It is unclear if this is nature or nurture, as 48% (13/27) of patients had a family history of obesity, 41% (11/27) had a family history of psoriasis, and 48% (13/27) had a family history of hyperlipidemia.15 Therefore, obesity may be cultivated in some psoriatic families. The issue of household influences on diet and obesity needs to be addressed if successful weight management is to be achieved in future studies of pediatric psoriasis.
Cardiovascular risks in the pediatric psoriasis population are the subject of ongoing assessment but will likely mimic studies of adult psoriasis patients when reviewed longitudinally.16 Weight loss and healthy lifestyle interventions likely are beneficial to long-term health, but there is a lack of published data addressing dietary modification as a disease modifier for long-term care of pediatric psoriasis.
Anxiety and Depression
Anxiety and depression have been noted in adults with chronic skin diseases. A recent study assessed 118 patients and caregivers of pediatric patients with atopic dermatitis (n=50), psoriasis (n=25), or vitiligo (n=43) using the Children’s Dermatology Life Quality Index, the Hamilton Anxiety Scale, and the Beck Depression Inventory.17 Anxiety and depression were found in 36% of caregivers of pediatric psoriasis patients and depression was found in 36% of pediatric psoriasis patients, highlighting the need for interventions on a personal and family level to improve quality of life. As a comparator, anxiety was more prevalent in vitiligo caregivers (42%), but depression was only found in 26% of caregivers in the same group. Extent of disease (25%–75% body surface area affected) correlated with both depression and anxiety in the caregivers of pediatric patients with psoriasis as well as with anxiety in caregivers of pediatric patients with increased visible surface area of vitiligo.17 Parental anxiety has been reported at times to be linked to corticosteroid phobia, or corticophobia, which may interfere with disease therapy, as topical corticosteroids are considered the mainstay of therapy in childhood disease.18 Coordinating care with caregivers and addressing their concerns about the safety of medications should be integral to the pediatric psoriasis visit.
Pustular Psoriasis
Pustular psoriasis can be seen in any age group. Researchers recently have attempted to delineate the features and successful management of this severe subset of pediatric psoriasis patients. Twenty-four pediatric pustular psoriasis cases reviewed by Posso-De Los Rios et al19 revealed that 92% (22/24) had generalized and 8% (2/24) had limited acral disease. The mean (standard deviation) age at onset of pediatric pustular psoriasis was 6.3 (4.9) years. Half of the reported cases required more than one intervention. Treatment with acitretin, cyclosporine, and methotrexate was effective, but the investigators identified that there is a true dearth of evidence-based therapeutics in pediatric pustular psoriasis and much rebound with discontinuation.19 Although the subset of pediatric pustular psoriasis is rare, study of evidence-based intervention is needed.
Therapy
Recent reviews of pediatric and adolescent psoriasis highlight the paucity of therapeutic information for these patient populations. Investigators typically focus on topical therapies as the basis of treatment,20 as well as the addition of phototherapy in mild to moderate plaque or guttate psoriasis and biologic or systemic agents in moderate to severe flares of plaque, erythrodermic, or pustular psoriasis.21 Further studies are needed to identify evidence-based therapeutic paradigms for pediatric psoriasis and to pinpoint therapies associated with the best quality of life in patients and their caregivers.
Tumor Necrosis Factor α Inhibitors
Safety and efficacy of etanercept for juvenile idiopathic arthritis including oligoarthritis, enthesitis-related arthritis, and psoriatic arthritis recently was reviewed by Windschall et al22 using data from the German pediatric Biologika in der Kinderrheumatologie registry. Juvenile Arthritis Disease Activity Score 10 improved from baseline for 127 pediatric patients with psoriatic arthritis in 3 to 24 months (mean [standard deviation], 14.7 [6.4], 5.0 [4.6], 5.3 [6.4] at baseline, 3 months, and 24 months, respectively). Overall side effects were relatively higher in the psoriatic arthritis group; the rate of serious (relative risk, 1.39 [0.95-2.03; P=.08]) and nonserious (relative risk, 1.18 [1.02-1.35; P=.03]) adverse events also was elevated. Uveitis risk was greatest in the psoriatic arthritis group and the number of associated cases of inflammatory bowel disease outnumbered those seen in other forms of arthritis. The investigators concluded that monitoring for extra-articular immunopathies should be conducted in pediatric patients with psoriatic arthritis who are undergoing etanercept therapy.22
Tumor necrosis factor α (TNF-α) inhibitors have been associated with triggering psoriasiform dermatitis in pediatric patients treated for inflammatory bowel disease. A Finnish study of infliximab side effects in pediatric patients with inflammatory bowel disease (n=84; Crohn disease: n=64) demonstrated that almost half (47.6% [40/84]) of the participants presented with chronic skin reactions, 23% of which were severe in nature.23 Psoriasiform lesions of the scalp and ears were most common, followed by the periorificial area, genitals, trunk, and extremities. Rare association with HLA-Cw*0602 genotype was noted. Skin manifestations did not correlate with gut inflammation (as determined by fecal calprotectin levels). Discontinuation of therapy rarely was required.23 Other studies also have highlighted this side effect, suggesting an incidence of 2.7% in adults with colitis treated with TNF-α inhibitors24 and 10.5% in pediatric patients with Crohn disease.25 In a study by Sherlock et al,25 pediatric patients with Crohn disease developing psoriasis following infliximab therapy were more likely to be homozygous for specific polymorphisms in the IL-23R gene (rs10489628, rs10789229, and rs1343151).
Methotrexate
For pediatric patients who are being treated with methotrexate, the polyglutamate assay recently has been reported to be helpful in identifying patients needing a dose escalation.26 Higher numbers on the polyglutamate assay are associated with superior response to methotrexate therapy. Doses can be increased after 12 weeks in patients with low assays.26
IL-23
The safety of IL-23 blockade in pediatric psoriasis patients has not yet been established, but data from adult cases have implicated the IL-17 and IL-23 pathways in psoriasis/psoriatic arthritis, including an association with IL-23R polymorphisms27 and increases in soluble IL-20 and IL-22 associated with disease severity and an association of IL-17 levels with activity on the psoriasis area and severity index scores.28 The data are more limited for pediatric cases. Pediatric patients with inflammatory bowel disease who have an IL-23R polymorphism appear to be susceptible to psoriatic flares while on TNF-α inhibitor therapy,25 which suggests that the IL-23 blockade may be of benefit for some pediatric patients with psoriasis or psoriatic arthritis.
Conclusion
Pediatric psoriasis and psoriatic arthritis have now been identified as being part of the autoimmune spectrum and are associated with metabolic syndrome, including obesity and excess central adiposity, similar to their adult variants. An overview of potential unmet needs in pediatric psoriasis is included in Table 2. These unmet needs include further delineation of diet and weight modification in the care and prevention of psoriasis; expansion of therapeutic trials and US Food and Drug Administration–approved medications for children with psoriasis, especially severe variants such as extensive plaque and pustular disease; and development of guidelines for ongoing monitoring of children with psoriasis. The role of therapeutic interventions and weight management on long-term disease course remains to be shown in extended clinical trials. Despite the great advancements in psoriatic care, knowledge gaps remain in pediatric psoriasis that will need to be addressed in the future.
1. Taclonex Expanded Indication. OptumRx Web site. https://www.optumrx.com/vgnpreview/HCP/Assets/RxNews/Clinical%20Updates_Taclonex_2014-1003.pdf. Published August 29, 2014. Accessed January 28, 2015.
2. Silverberg NB. Update on pediatric psoriasis, part 1: clinical features and demographics. Cutis. 2010;86:118-124.
3. Silverberg NB. Update on pediatric psoriasis, part 2: therapeutic management. Cutis. 2010;86:172-176.
4. Cather JC. Psoriasis in children and women: addressing some special needs. Semin Cutan Med Surg. 2014;33(2 suppl 2):S42-S44.
5. Khorsand K, Sidbury R. Recent advances in pediatric dermatology. Arch Dis Child. 2014;99:944-948.
6. Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30:424-428.
7. Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, et al. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530-534.
8. Ferran M, Galván AB, Rincón C, et al. Streptococcus induces circulating CLA(+) memory T-cell-dependent epidermal cell activation in psoriasis. J Invest Dermatol. 2013;133:999-1007.
9. Gul Mert G, Incecik F, Gunasti S, et al. Psoriasiform drug eruption associated with sodium valproate [published online ahead of print November 13, 2013]. Case Rep Pediatr. 2013;2013:823469.
10. Chang MW, Nakrani R. Six children with allergic contact dermatitis to methylisothiazolinone in wet wipes (baby wipes). Pediatrics. 2014;133:e434-e438.
11. Gul U, Gonul M, Kaya I, et al. Autoimmune thyroid disorders in patients with psoriasis. Eur J Dermatol. 2009;19:221-223.
12. Prahalad S, McCracken C, Ponder L, et al. A120: Familial autoimmunity in the CARRA registry. Arthritis Rheumatol. 2014;66(suppl 11):S157.
13. Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
14. Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149:166-176.
15. Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
16. Volf EM, Levine DE, Michelon MA, et al. Assessor-blinded study of the metabolic syndrome and surrogate markers of increased cardiovascular risk in children with moderate-to-severe psoriasis compared with age-matched population of children with warts. J Drugs Dermatol. 2011;10:900-901.
17. Manzoni AP, Weber MB, Nagatomi AR, et al. Assessing depression and anxiety in the caregivers of pediatric patients with chronic skin disorders. An Bras Dermatol. 2013;88:894-899.
18. Belloni Fortina A, Neri L. Topical steroids and corticophobia. G Ital Dermatol Venereol. 2013;148:651-654.
19. Posso-De Los Rios CJ, Pope E, Lara-Corrales I. A systematic review of systemic medications for pustular psoriasis in pediatrics. Pediatr Dermatol. 2014;31:430-439.
20. Tollefson MM. Diagnosis and management of psoriasis in children. Pediatr Clin North Am. 2014;61:261-277.
21. Fotiadou C, Lazaridou E, Ioannides D. Management of psoriasis in adolescence. Adolesc Health Med Ther. 2014;5:25-34.
22. Windschall D, Müller T, Becker I, et al. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis [published online ahead of print July 18, 2014]. Clin Rheumatol. 2015;34:61-69.
23. Mälkönen T, Wikström A, Heiskanen K, et al. Skin reactions during anti-TNFa therapy for pediatric inflammatory bowel disease: a 2-year prospective study. Inflamm Bowel Dis. 2014;20:1309-1315.
24. Afzali A, Wheat CL, Hu JK, et al. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: a single academic center case series. J Crohns Colitis. 2014;8:480-488.
25. Sherlock ME, Walters T, Tabbers MM, et al. Infliximab-induced psoriasis and psoriasiform skin lesions in pediatric Crohn disease and a potential association with IL-23 receptor polymorphisms. J Pediatr Gastroenterol Nutr. 2013;56:512-518.
26. Rahman SI, Siegfried E, Flanagan KH, et al. The methotrexate polyglutamate assay supports the efficacy of methotrexate for severe inflammatory skin disease in children. J Am Acad Dermatol. 2014;70:252-256.
27. Suzuki E, Mellins ED, Gershwin ME, et al. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496-502.
28. Michalak-Stoma A, Bartosi´nska J, Kowal M, et al. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 2013;35:625-631.
Psoriasis affects 2% to 4% of the US population, with approximately one-third of cases beginning in childhood. The understanding of pediatric psoriasis has developed at a far slower pace than adult disease, with limitations in care including few medications that are approved by the US Food and Drug Administration for pediatric and adolescent use. Recently, a stable fixed-combination dose of calcipo-triene 0.005%–betamethasone dipropionate 0.064% topical suspension was approved for treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years, which hopefully will lead a trend in psoriasis medication approval for children and teenagers.1 Based on a PubMed search of articles indexed for MEDLINE using the search terms pediatric psoriasis, psoriasis, and strep that were published from April 2012 to April 2014, this article reviews newer data to address the issues that surround pediatric psoriasis and to provide an update on prior review articles on pediatric psoriasis.2-5 This article reviews some of the newer literature on clinical presentation and comorbidities in pediatric psoriasis.5 Based on these recent findings, additional screenings including review of obesity parameters are recommended for pediatric patients with psoriasis (Table 1).
Update on Disease Manifestations, Associations, and Comorbidities
Disease Manifestations
A 2013 multicenter study delineated the clinical features of pediatric psoriasis.6 The study was conducted at 8 geographically diverse dermatology clinics in the United States to delineate the clinical manifestations of pediatric psoriasis. In an assessment of 181 participants aged 5 to 17 years, the investigators sought to determine the frequency of disease sites, severity, and guttate disease. Over a period of approximately 2 years, 43.1% of participants were determined to have mild disease and 56.9% had severe disease. Family history of psoriasis was present in 51.4% of participants, with first-degree relatives affected in 59.8% of cases. Scalp involvement at some time was noted in 79.0% of participants, and nail disease was noted in 55% of boys and 29% of girls. Guttate psoriasis was noted in 30% of participants, with more cases in the severe range (35.9%) versus the mild range (21.8%). Additionally, 22.1% of participants had a precipitating streptococcal infection, with the association being more common in pediatric patients with guttate psoriasis than plaque psoriasis.6 This study highlighted that pediatric psoriasis has a genetic basis, is frequently guttate in nature, commonly affects the nails, shows a trend toward being classified as severe, and may be triggered by streptococcal infections.
Streptococcal Infection
Pediatric psoriasis may be triggered or flared by Streptococcus pyogenes (group A β-hemolytic streptococci) infections, specifically β-hemolytic streptococci groups A, C, and G that have streptococcal M protein,2,3,7 and this tendency can be associated with HLA-Cw6 or guttate psoriasis. Newer data have elucidated the role of streptococcal throat infections in psoriasis. Given that streptococcal throat infections are most common in school-aged children, these studies suggest a putative mechanism in pediatric psoriasis for triggering streptococcal infections, which would need to be confirmed in future studies, specifically in pediatric psoriasis patients.
It has been shown that T cells in psoriasis patients recognize common streptococcal M proteins and keratin determinants.7 Ferran et al8 recently demonstrated activation of circulating cutaneous lymphocyte–associated antigen (CLA)+ T cells but not CLA- memory T cells in 27 psoriasis patients (ages not specified) when mixed with streptococcal throat extracts, causing production of IL-17, IP-10, IL-22, and IFN-γ; activation was not found in 6 healthy control patients. Antistreptolysin O levels were correlated with the messenger RNA up- regulation for IL-17, IP-10, IL-22, and IFN-γ, and also correlated with psoriasis area and severity index score in psoriasis patients. In this same study, injection of the activated culture supernatant into mouse skin caused epidermal hyperkeratosis and activation of nonlesional epidermal cells from psoriatic patients. This study thereby delineated some of the potential pathways of the streptococcal induction of psoriasis and psoriatic flares in childhood8; however, confirmation is needed through further study of pediatric psoriatic lymphocyte activity.
Differential Diagnosis
Additions to the extensive differential list have been cited in the recent literature. The differential diagnosis of pediatric psoriasis now includes sodium valproate–induced psoriasiform drug eruption9 and allergic contact dermatitis to methylchloroisothiazolinone and methylisothiazolinone, which are present in many sanitizing hand and diaper wipes and has been reported to cause psoriasiform dermatitis in a periorificial or perineal distribution.10 Clinicians should inquire about the use of these wipes, as caregivers rarely suspect this agent to be causative of the eruption.
Psoriatic Arthritis
Previously, psoriasis and psoriatic arthritis have been linked to autoimmune thyroid disease in adults.11 A study of the Childhood Arthritis & Rheumatology Research Alliance (CARRA) registry showed that family history of psoriasis, autoimmune thyroiditis, Crohn disease, and ankylosing spondylitis in a first-degree relative has been linked to juvenile idiopathic arthritis, highlighting that pediatric psoriasis can be genetically linked or associated with multiple autoimmune conditions and vice versa.12
Obesity, Metabolic Syndrome, and Cardiovascular Risks
Obesity is associated with pediatric psoriasis as highlighted in a growing body of recent literature.13 Excess adiposity as manifested by body mass index in the 85th percentile or greater (37.9% of 155 pediatric psoriasis patients vs 20.5% of 42 controls) and excess central adiposity as manifested by excess waist circumference and increased waist-to-height ratios are more common in pediatric patients with psoriasis than in controls.14
Obesity may be a trigger or associated with increased disease activity in pediatric psoriasis patients. Excess overall adiposity correlates with more severe disease. Obesity parameters may correlate with the onset of psoriasis and with disease severity. In fact, the odds of obesity may be higher in childhood than in adults.14,15 A 2011 report of pediatric psoriasis patients aged 10 to 17 years (n=12) and wart controls (n=6)(mean age, 13.2 and 13.5 years, respectively) demonstrated that 4 of 12 patients with psoriasis and 0 of 6 patients with warts met criteria for metabolic syndrome as defined by 3 of the following: (1) triglycerides greater than or equal to 100 mg/dL; (2) high-density lipoprotein cholesterol less than 50 mg/dL in females and less than 5 mg/dL in males; (3) fasting blood glucose levels greater than or equal to 110 mg/dL, (4) waist circumference greater than the 75th percentile for age and sex; and (5) systolic or diastolic blood pressure greater than the 90th percentile for age, sex, and height.16 These studies highlight that obesity and metabolic syndrome are of concern in pediatric psoriasis patients; however, the best management approach using diet and weight interventions has yet to be identified.
Adiposity may precede the onset of psoriasis. A recent cohort of 27 pediatric psoriasis patients reported that the average age at onset of psoriasis was 8.7 years and the average age at onset of obesity was 4.1 years.15 In this study, 93% (25/27) of patients had adiposity preceding their psoriasis by 2 or more years. It is unclear if this is nature or nurture, as 48% (13/27) of patients had a family history of obesity, 41% (11/27) had a family history of psoriasis, and 48% (13/27) had a family history of hyperlipidemia.15 Therefore, obesity may be cultivated in some psoriatic families. The issue of household influences on diet and obesity needs to be addressed if successful weight management is to be achieved in future studies of pediatric psoriasis.
Cardiovascular risks in the pediatric psoriasis population are the subject of ongoing assessment but will likely mimic studies of adult psoriasis patients when reviewed longitudinally.16 Weight loss and healthy lifestyle interventions likely are beneficial to long-term health, but there is a lack of published data addressing dietary modification as a disease modifier for long-term care of pediatric psoriasis.
Anxiety and Depression
Anxiety and depression have been noted in adults with chronic skin diseases. A recent study assessed 118 patients and caregivers of pediatric patients with atopic dermatitis (n=50), psoriasis (n=25), or vitiligo (n=43) using the Children’s Dermatology Life Quality Index, the Hamilton Anxiety Scale, and the Beck Depression Inventory.17 Anxiety and depression were found in 36% of caregivers of pediatric psoriasis patients and depression was found in 36% of pediatric psoriasis patients, highlighting the need for interventions on a personal and family level to improve quality of life. As a comparator, anxiety was more prevalent in vitiligo caregivers (42%), but depression was only found in 26% of caregivers in the same group. Extent of disease (25%–75% body surface area affected) correlated with both depression and anxiety in the caregivers of pediatric patients with psoriasis as well as with anxiety in caregivers of pediatric patients with increased visible surface area of vitiligo.17 Parental anxiety has been reported at times to be linked to corticosteroid phobia, or corticophobia, which may interfere with disease therapy, as topical corticosteroids are considered the mainstay of therapy in childhood disease.18 Coordinating care with caregivers and addressing their concerns about the safety of medications should be integral to the pediatric psoriasis visit.
Pustular Psoriasis
Pustular psoriasis can be seen in any age group. Researchers recently have attempted to delineate the features and successful management of this severe subset of pediatric psoriasis patients. Twenty-four pediatric pustular psoriasis cases reviewed by Posso-De Los Rios et al19 revealed that 92% (22/24) had generalized and 8% (2/24) had limited acral disease. The mean (standard deviation) age at onset of pediatric pustular psoriasis was 6.3 (4.9) years. Half of the reported cases required more than one intervention. Treatment with acitretin, cyclosporine, and methotrexate was effective, but the investigators identified that there is a true dearth of evidence-based therapeutics in pediatric pustular psoriasis and much rebound with discontinuation.19 Although the subset of pediatric pustular psoriasis is rare, study of evidence-based intervention is needed.
Therapy
Recent reviews of pediatric and adolescent psoriasis highlight the paucity of therapeutic information for these patient populations. Investigators typically focus on topical therapies as the basis of treatment,20 as well as the addition of phototherapy in mild to moderate plaque or guttate psoriasis and biologic or systemic agents in moderate to severe flares of plaque, erythrodermic, or pustular psoriasis.21 Further studies are needed to identify evidence-based therapeutic paradigms for pediatric psoriasis and to pinpoint therapies associated with the best quality of life in patients and their caregivers.
Tumor Necrosis Factor α Inhibitors
Safety and efficacy of etanercept for juvenile idiopathic arthritis including oligoarthritis, enthesitis-related arthritis, and psoriatic arthritis recently was reviewed by Windschall et al22 using data from the German pediatric Biologika in der Kinderrheumatologie registry. Juvenile Arthritis Disease Activity Score 10 improved from baseline for 127 pediatric patients with psoriatic arthritis in 3 to 24 months (mean [standard deviation], 14.7 [6.4], 5.0 [4.6], 5.3 [6.4] at baseline, 3 months, and 24 months, respectively). Overall side effects were relatively higher in the psoriatic arthritis group; the rate of serious (relative risk, 1.39 [0.95-2.03; P=.08]) and nonserious (relative risk, 1.18 [1.02-1.35; P=.03]) adverse events also was elevated. Uveitis risk was greatest in the psoriatic arthritis group and the number of associated cases of inflammatory bowel disease outnumbered those seen in other forms of arthritis. The investigators concluded that monitoring for extra-articular immunopathies should be conducted in pediatric patients with psoriatic arthritis who are undergoing etanercept therapy.22
Tumor necrosis factor α (TNF-α) inhibitors have been associated with triggering psoriasiform dermatitis in pediatric patients treated for inflammatory bowel disease. A Finnish study of infliximab side effects in pediatric patients with inflammatory bowel disease (n=84; Crohn disease: n=64) demonstrated that almost half (47.6% [40/84]) of the participants presented with chronic skin reactions, 23% of which were severe in nature.23 Psoriasiform lesions of the scalp and ears were most common, followed by the periorificial area, genitals, trunk, and extremities. Rare association with HLA-Cw*0602 genotype was noted. Skin manifestations did not correlate with gut inflammation (as determined by fecal calprotectin levels). Discontinuation of therapy rarely was required.23 Other studies also have highlighted this side effect, suggesting an incidence of 2.7% in adults with colitis treated with TNF-α inhibitors24 and 10.5% in pediatric patients with Crohn disease.25 In a study by Sherlock et al,25 pediatric patients with Crohn disease developing psoriasis following infliximab therapy were more likely to be homozygous for specific polymorphisms in the IL-23R gene (rs10489628, rs10789229, and rs1343151).
Methotrexate
For pediatric patients who are being treated with methotrexate, the polyglutamate assay recently has been reported to be helpful in identifying patients needing a dose escalation.26 Higher numbers on the polyglutamate assay are associated with superior response to methotrexate therapy. Doses can be increased after 12 weeks in patients with low assays.26
IL-23
The safety of IL-23 blockade in pediatric psoriasis patients has not yet been established, but data from adult cases have implicated the IL-17 and IL-23 pathways in psoriasis/psoriatic arthritis, including an association with IL-23R polymorphisms27 and increases in soluble IL-20 and IL-22 associated with disease severity and an association of IL-17 levels with activity on the psoriasis area and severity index scores.28 The data are more limited for pediatric cases. Pediatric patients with inflammatory bowel disease who have an IL-23R polymorphism appear to be susceptible to psoriatic flares while on TNF-α inhibitor therapy,25 which suggests that the IL-23 blockade may be of benefit for some pediatric patients with psoriasis or psoriatic arthritis.
Conclusion
Pediatric psoriasis and psoriatic arthritis have now been identified as being part of the autoimmune spectrum and are associated with metabolic syndrome, including obesity and excess central adiposity, similar to their adult variants. An overview of potential unmet needs in pediatric psoriasis is included in Table 2. These unmet needs include further delineation of diet and weight modification in the care and prevention of psoriasis; expansion of therapeutic trials and US Food and Drug Administration–approved medications for children with psoriasis, especially severe variants such as extensive plaque and pustular disease; and development of guidelines for ongoing monitoring of children with psoriasis. The role of therapeutic interventions and weight management on long-term disease course remains to be shown in extended clinical trials. Despite the great advancements in psoriatic care, knowledge gaps remain in pediatric psoriasis that will need to be addressed in the future.
Psoriasis affects 2% to 4% of the US population, with approximately one-third of cases beginning in childhood. The understanding of pediatric psoriasis has developed at a far slower pace than adult disease, with limitations in care including few medications that are approved by the US Food and Drug Administration for pediatric and adolescent use. Recently, a stable fixed-combination dose of calcipo-triene 0.005%–betamethasone dipropionate 0.064% topical suspension was approved for treatment of plaque psoriasis of the scalp in patients aged 12 to 17 years, which hopefully will lead a trend in psoriasis medication approval for children and teenagers.1 Based on a PubMed search of articles indexed for MEDLINE using the search terms pediatric psoriasis, psoriasis, and strep that were published from April 2012 to April 2014, this article reviews newer data to address the issues that surround pediatric psoriasis and to provide an update on prior review articles on pediatric psoriasis.2-5 This article reviews some of the newer literature on clinical presentation and comorbidities in pediatric psoriasis.5 Based on these recent findings, additional screenings including review of obesity parameters are recommended for pediatric patients with psoriasis (Table 1).
Update on Disease Manifestations, Associations, and Comorbidities
Disease Manifestations
A 2013 multicenter study delineated the clinical features of pediatric psoriasis.6 The study was conducted at 8 geographically diverse dermatology clinics in the United States to delineate the clinical manifestations of pediatric psoriasis. In an assessment of 181 participants aged 5 to 17 years, the investigators sought to determine the frequency of disease sites, severity, and guttate disease. Over a period of approximately 2 years, 43.1% of participants were determined to have mild disease and 56.9% had severe disease. Family history of psoriasis was present in 51.4% of participants, with first-degree relatives affected in 59.8% of cases. Scalp involvement at some time was noted in 79.0% of participants, and nail disease was noted in 55% of boys and 29% of girls. Guttate psoriasis was noted in 30% of participants, with more cases in the severe range (35.9%) versus the mild range (21.8%). Additionally, 22.1% of participants had a precipitating streptococcal infection, with the association being more common in pediatric patients with guttate psoriasis than plaque psoriasis.6 This study highlighted that pediatric psoriasis has a genetic basis, is frequently guttate in nature, commonly affects the nails, shows a trend toward being classified as severe, and may be triggered by streptococcal infections.
Streptococcal Infection
Pediatric psoriasis may be triggered or flared by Streptococcus pyogenes (group A β-hemolytic streptococci) infections, specifically β-hemolytic streptococci groups A, C, and G that have streptococcal M protein,2,3,7 and this tendency can be associated with HLA-Cw6 or guttate psoriasis. Newer data have elucidated the role of streptococcal throat infections in psoriasis. Given that streptococcal throat infections are most common in school-aged children, these studies suggest a putative mechanism in pediatric psoriasis for triggering streptococcal infections, which would need to be confirmed in future studies, specifically in pediatric psoriasis patients.
It has been shown that T cells in psoriasis patients recognize common streptococcal M proteins and keratin determinants.7 Ferran et al8 recently demonstrated activation of circulating cutaneous lymphocyte–associated antigen (CLA)+ T cells but not CLA- memory T cells in 27 psoriasis patients (ages not specified) when mixed with streptococcal throat extracts, causing production of IL-17, IP-10, IL-22, and IFN-γ; activation was not found in 6 healthy control patients. Antistreptolysin O levels were correlated with the messenger RNA up- regulation for IL-17, IP-10, IL-22, and IFN-γ, and also correlated with psoriasis area and severity index score in psoriasis patients. In this same study, injection of the activated culture supernatant into mouse skin caused epidermal hyperkeratosis and activation of nonlesional epidermal cells from psoriatic patients. This study thereby delineated some of the potential pathways of the streptococcal induction of psoriasis and psoriatic flares in childhood8; however, confirmation is needed through further study of pediatric psoriatic lymphocyte activity.
Differential Diagnosis
Additions to the extensive differential list have been cited in the recent literature. The differential diagnosis of pediatric psoriasis now includes sodium valproate–induced psoriasiform drug eruption9 and allergic contact dermatitis to methylchloroisothiazolinone and methylisothiazolinone, which are present in many sanitizing hand and diaper wipes and has been reported to cause psoriasiform dermatitis in a periorificial or perineal distribution.10 Clinicians should inquire about the use of these wipes, as caregivers rarely suspect this agent to be causative of the eruption.
Psoriatic Arthritis
Previously, psoriasis and psoriatic arthritis have been linked to autoimmune thyroid disease in adults.11 A study of the Childhood Arthritis & Rheumatology Research Alliance (CARRA) registry showed that family history of psoriasis, autoimmune thyroiditis, Crohn disease, and ankylosing spondylitis in a first-degree relative has been linked to juvenile idiopathic arthritis, highlighting that pediatric psoriasis can be genetically linked or associated with multiple autoimmune conditions and vice versa.12
Obesity, Metabolic Syndrome, and Cardiovascular Risks
Obesity is associated with pediatric psoriasis as highlighted in a growing body of recent literature.13 Excess adiposity as manifested by body mass index in the 85th percentile or greater (37.9% of 155 pediatric psoriasis patients vs 20.5% of 42 controls) and excess central adiposity as manifested by excess waist circumference and increased waist-to-height ratios are more common in pediatric patients with psoriasis than in controls.14
Obesity may be a trigger or associated with increased disease activity in pediatric psoriasis patients. Excess overall adiposity correlates with more severe disease. Obesity parameters may correlate with the onset of psoriasis and with disease severity. In fact, the odds of obesity may be higher in childhood than in adults.14,15 A 2011 report of pediatric psoriasis patients aged 10 to 17 years (n=12) and wart controls (n=6)(mean age, 13.2 and 13.5 years, respectively) demonstrated that 4 of 12 patients with psoriasis and 0 of 6 patients with warts met criteria for metabolic syndrome as defined by 3 of the following: (1) triglycerides greater than or equal to 100 mg/dL; (2) high-density lipoprotein cholesterol less than 50 mg/dL in females and less than 5 mg/dL in males; (3) fasting blood glucose levels greater than or equal to 110 mg/dL, (4) waist circumference greater than the 75th percentile for age and sex; and (5) systolic or diastolic blood pressure greater than the 90th percentile for age, sex, and height.16 These studies highlight that obesity and metabolic syndrome are of concern in pediatric psoriasis patients; however, the best management approach using diet and weight interventions has yet to be identified.
Adiposity may precede the onset of psoriasis. A recent cohort of 27 pediatric psoriasis patients reported that the average age at onset of psoriasis was 8.7 years and the average age at onset of obesity was 4.1 years.15 In this study, 93% (25/27) of patients had adiposity preceding their psoriasis by 2 or more years. It is unclear if this is nature or nurture, as 48% (13/27) of patients had a family history of obesity, 41% (11/27) had a family history of psoriasis, and 48% (13/27) had a family history of hyperlipidemia.15 Therefore, obesity may be cultivated in some psoriatic families. The issue of household influences on diet and obesity needs to be addressed if successful weight management is to be achieved in future studies of pediatric psoriasis.
Cardiovascular risks in the pediatric psoriasis population are the subject of ongoing assessment but will likely mimic studies of adult psoriasis patients when reviewed longitudinally.16 Weight loss and healthy lifestyle interventions likely are beneficial to long-term health, but there is a lack of published data addressing dietary modification as a disease modifier for long-term care of pediatric psoriasis.
Anxiety and Depression
Anxiety and depression have been noted in adults with chronic skin diseases. A recent study assessed 118 patients and caregivers of pediatric patients with atopic dermatitis (n=50), psoriasis (n=25), or vitiligo (n=43) using the Children’s Dermatology Life Quality Index, the Hamilton Anxiety Scale, and the Beck Depression Inventory.17 Anxiety and depression were found in 36% of caregivers of pediatric psoriasis patients and depression was found in 36% of pediatric psoriasis patients, highlighting the need for interventions on a personal and family level to improve quality of life. As a comparator, anxiety was more prevalent in vitiligo caregivers (42%), but depression was only found in 26% of caregivers in the same group. Extent of disease (25%–75% body surface area affected) correlated with both depression and anxiety in the caregivers of pediatric patients with psoriasis as well as with anxiety in caregivers of pediatric patients with increased visible surface area of vitiligo.17 Parental anxiety has been reported at times to be linked to corticosteroid phobia, or corticophobia, which may interfere with disease therapy, as topical corticosteroids are considered the mainstay of therapy in childhood disease.18 Coordinating care with caregivers and addressing their concerns about the safety of medications should be integral to the pediatric psoriasis visit.
Pustular Psoriasis
Pustular psoriasis can be seen in any age group. Researchers recently have attempted to delineate the features and successful management of this severe subset of pediatric psoriasis patients. Twenty-four pediatric pustular psoriasis cases reviewed by Posso-De Los Rios et al19 revealed that 92% (22/24) had generalized and 8% (2/24) had limited acral disease. The mean (standard deviation) age at onset of pediatric pustular psoriasis was 6.3 (4.9) years. Half of the reported cases required more than one intervention. Treatment with acitretin, cyclosporine, and methotrexate was effective, but the investigators identified that there is a true dearth of evidence-based therapeutics in pediatric pustular psoriasis and much rebound with discontinuation.19 Although the subset of pediatric pustular psoriasis is rare, study of evidence-based intervention is needed.
Therapy
Recent reviews of pediatric and adolescent psoriasis highlight the paucity of therapeutic information for these patient populations. Investigators typically focus on topical therapies as the basis of treatment,20 as well as the addition of phototherapy in mild to moderate plaque or guttate psoriasis and biologic or systemic agents in moderate to severe flares of plaque, erythrodermic, or pustular psoriasis.21 Further studies are needed to identify evidence-based therapeutic paradigms for pediatric psoriasis and to pinpoint therapies associated with the best quality of life in patients and their caregivers.
Tumor Necrosis Factor α Inhibitors
Safety and efficacy of etanercept for juvenile idiopathic arthritis including oligoarthritis, enthesitis-related arthritis, and psoriatic arthritis recently was reviewed by Windschall et al22 using data from the German pediatric Biologika in der Kinderrheumatologie registry. Juvenile Arthritis Disease Activity Score 10 improved from baseline for 127 pediatric patients with psoriatic arthritis in 3 to 24 months (mean [standard deviation], 14.7 [6.4], 5.0 [4.6], 5.3 [6.4] at baseline, 3 months, and 24 months, respectively). Overall side effects were relatively higher in the psoriatic arthritis group; the rate of serious (relative risk, 1.39 [0.95-2.03; P=.08]) and nonserious (relative risk, 1.18 [1.02-1.35; P=.03]) adverse events also was elevated. Uveitis risk was greatest in the psoriatic arthritis group and the number of associated cases of inflammatory bowel disease outnumbered those seen in other forms of arthritis. The investigators concluded that monitoring for extra-articular immunopathies should be conducted in pediatric patients with psoriatic arthritis who are undergoing etanercept therapy.22
Tumor necrosis factor α (TNF-α) inhibitors have been associated with triggering psoriasiform dermatitis in pediatric patients treated for inflammatory bowel disease. A Finnish study of infliximab side effects in pediatric patients with inflammatory bowel disease (n=84; Crohn disease: n=64) demonstrated that almost half (47.6% [40/84]) of the participants presented with chronic skin reactions, 23% of which were severe in nature.23 Psoriasiform lesions of the scalp and ears were most common, followed by the periorificial area, genitals, trunk, and extremities. Rare association with HLA-Cw*0602 genotype was noted. Skin manifestations did not correlate with gut inflammation (as determined by fecal calprotectin levels). Discontinuation of therapy rarely was required.23 Other studies also have highlighted this side effect, suggesting an incidence of 2.7% in adults with colitis treated with TNF-α inhibitors24 and 10.5% in pediatric patients with Crohn disease.25 In a study by Sherlock et al,25 pediatric patients with Crohn disease developing psoriasis following infliximab therapy were more likely to be homozygous for specific polymorphisms in the IL-23R gene (rs10489628, rs10789229, and rs1343151).
Methotrexate
For pediatric patients who are being treated with methotrexate, the polyglutamate assay recently has been reported to be helpful in identifying patients needing a dose escalation.26 Higher numbers on the polyglutamate assay are associated with superior response to methotrexate therapy. Doses can be increased after 12 weeks in patients with low assays.26
IL-23
The safety of IL-23 blockade in pediatric psoriasis patients has not yet been established, but data from adult cases have implicated the IL-17 and IL-23 pathways in psoriasis/psoriatic arthritis, including an association with IL-23R polymorphisms27 and increases in soluble IL-20 and IL-22 associated with disease severity and an association of IL-17 levels with activity on the psoriasis area and severity index scores.28 The data are more limited for pediatric cases. Pediatric patients with inflammatory bowel disease who have an IL-23R polymorphism appear to be susceptible to psoriatic flares while on TNF-α inhibitor therapy,25 which suggests that the IL-23 blockade may be of benefit for some pediatric patients with psoriasis or psoriatic arthritis.
Conclusion
Pediatric psoriasis and psoriatic arthritis have now been identified as being part of the autoimmune spectrum and are associated with metabolic syndrome, including obesity and excess central adiposity, similar to their adult variants. An overview of potential unmet needs in pediatric psoriasis is included in Table 2. These unmet needs include further delineation of diet and weight modification in the care and prevention of psoriasis; expansion of therapeutic trials and US Food and Drug Administration–approved medications for children with psoriasis, especially severe variants such as extensive plaque and pustular disease; and development of guidelines for ongoing monitoring of children with psoriasis. The role of therapeutic interventions and weight management on long-term disease course remains to be shown in extended clinical trials. Despite the great advancements in psoriatic care, knowledge gaps remain in pediatric psoriasis that will need to be addressed in the future.
1. Taclonex Expanded Indication. OptumRx Web site. https://www.optumrx.com/vgnpreview/HCP/Assets/RxNews/Clinical%20Updates_Taclonex_2014-1003.pdf. Published August 29, 2014. Accessed January 28, 2015.
2. Silverberg NB. Update on pediatric psoriasis, part 1: clinical features and demographics. Cutis. 2010;86:118-124.
3. Silverberg NB. Update on pediatric psoriasis, part 2: therapeutic management. Cutis. 2010;86:172-176.
4. Cather JC. Psoriasis in children and women: addressing some special needs. Semin Cutan Med Surg. 2014;33(2 suppl 2):S42-S44.
5. Khorsand K, Sidbury R. Recent advances in pediatric dermatology. Arch Dis Child. 2014;99:944-948.
6. Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30:424-428.
7. Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, et al. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530-534.
8. Ferran M, Galván AB, Rincón C, et al. Streptococcus induces circulating CLA(+) memory T-cell-dependent epidermal cell activation in psoriasis. J Invest Dermatol. 2013;133:999-1007.
9. Gul Mert G, Incecik F, Gunasti S, et al. Psoriasiform drug eruption associated with sodium valproate [published online ahead of print November 13, 2013]. Case Rep Pediatr. 2013;2013:823469.
10. Chang MW, Nakrani R. Six children with allergic contact dermatitis to methylisothiazolinone in wet wipes (baby wipes). Pediatrics. 2014;133:e434-e438.
11. Gul U, Gonul M, Kaya I, et al. Autoimmune thyroid disorders in patients with psoriasis. Eur J Dermatol. 2009;19:221-223.
12. Prahalad S, McCracken C, Ponder L, et al. A120: Familial autoimmunity in the CARRA registry. Arthritis Rheumatol. 2014;66(suppl 11):S157.
13. Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
14. Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149:166-176.
15. Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
16. Volf EM, Levine DE, Michelon MA, et al. Assessor-blinded study of the metabolic syndrome and surrogate markers of increased cardiovascular risk in children with moderate-to-severe psoriasis compared with age-matched population of children with warts. J Drugs Dermatol. 2011;10:900-901.
17. Manzoni AP, Weber MB, Nagatomi AR, et al. Assessing depression and anxiety in the caregivers of pediatric patients with chronic skin disorders. An Bras Dermatol. 2013;88:894-899.
18. Belloni Fortina A, Neri L. Topical steroids and corticophobia. G Ital Dermatol Venereol. 2013;148:651-654.
19. Posso-De Los Rios CJ, Pope E, Lara-Corrales I. A systematic review of systemic medications for pustular psoriasis in pediatrics. Pediatr Dermatol. 2014;31:430-439.
20. Tollefson MM. Diagnosis and management of psoriasis in children. Pediatr Clin North Am. 2014;61:261-277.
21. Fotiadou C, Lazaridou E, Ioannides D. Management of psoriasis in adolescence. Adolesc Health Med Ther. 2014;5:25-34.
22. Windschall D, Müller T, Becker I, et al. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis [published online ahead of print July 18, 2014]. Clin Rheumatol. 2015;34:61-69.
23. Mälkönen T, Wikström A, Heiskanen K, et al. Skin reactions during anti-TNFa therapy for pediatric inflammatory bowel disease: a 2-year prospective study. Inflamm Bowel Dis. 2014;20:1309-1315.
24. Afzali A, Wheat CL, Hu JK, et al. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: a single academic center case series. J Crohns Colitis. 2014;8:480-488.
25. Sherlock ME, Walters T, Tabbers MM, et al. Infliximab-induced psoriasis and psoriasiform skin lesions in pediatric Crohn disease and a potential association with IL-23 receptor polymorphisms. J Pediatr Gastroenterol Nutr. 2013;56:512-518.
26. Rahman SI, Siegfried E, Flanagan KH, et al. The methotrexate polyglutamate assay supports the efficacy of methotrexate for severe inflammatory skin disease in children. J Am Acad Dermatol. 2014;70:252-256.
27. Suzuki E, Mellins ED, Gershwin ME, et al. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496-502.
28. Michalak-Stoma A, Bartosi´nska J, Kowal M, et al. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 2013;35:625-631.
1. Taclonex Expanded Indication. OptumRx Web site. https://www.optumrx.com/vgnpreview/HCP/Assets/RxNews/Clinical%20Updates_Taclonex_2014-1003.pdf. Published August 29, 2014. Accessed January 28, 2015.
2. Silverberg NB. Update on pediatric psoriasis, part 1: clinical features and demographics. Cutis. 2010;86:118-124.
3. Silverberg NB. Update on pediatric psoriasis, part 2: therapeutic management. Cutis. 2010;86:172-176.
4. Cather JC. Psoriasis in children and women: addressing some special needs. Semin Cutan Med Surg. 2014;33(2 suppl 2):S42-S44.
5. Khorsand K, Sidbury R. Recent advances in pediatric dermatology. Arch Dis Child. 2014;99:944-948.
6. Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30:424-428.
7. Gudjonsson JE, Thorarinsson AM, Sigurgeirsson B, et al. Streptococcal throat infections and exacerbation of chronic plaque psoriasis: a prospective study. Br J Dermatol. 2003;149:530-534.
8. Ferran M, Galván AB, Rincón C, et al. Streptococcus induces circulating CLA(+) memory T-cell-dependent epidermal cell activation in psoriasis. J Invest Dermatol. 2013;133:999-1007.
9. Gul Mert G, Incecik F, Gunasti S, et al. Psoriasiform drug eruption associated with sodium valproate [published online ahead of print November 13, 2013]. Case Rep Pediatr. 2013;2013:823469.
10. Chang MW, Nakrani R. Six children with allergic contact dermatitis to methylisothiazolinone in wet wipes (baby wipes). Pediatrics. 2014;133:e434-e438.
11. Gul U, Gonul M, Kaya I, et al. Autoimmune thyroid disorders in patients with psoriasis. Eur J Dermatol. 2009;19:221-223.
12. Prahalad S, McCracken C, Ponder L, et al. A120: Familial autoimmunity in the CARRA registry. Arthritis Rheumatol. 2014;66(suppl 11):S157.
13. Mercy KM, Paller AS. The relationship between obesity and psoriasis in the pediatric population: implications and future directions. Cutis. 2013;92:107-109.
14. Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149:166-176.
15. Becker L, Tom WL, Eshagh K, et al. Excess adiposity preceding pediatric psoriasis. JAMA Dermatol. 2014;150:573-574.
16. Volf EM, Levine DE, Michelon MA, et al. Assessor-blinded study of the metabolic syndrome and surrogate markers of increased cardiovascular risk in children with moderate-to-severe psoriasis compared with age-matched population of children with warts. J Drugs Dermatol. 2011;10:900-901.
17. Manzoni AP, Weber MB, Nagatomi AR, et al. Assessing depression and anxiety in the caregivers of pediatric patients with chronic skin disorders. An Bras Dermatol. 2013;88:894-899.
18. Belloni Fortina A, Neri L. Topical steroids and corticophobia. G Ital Dermatol Venereol. 2013;148:651-654.
19. Posso-De Los Rios CJ, Pope E, Lara-Corrales I. A systematic review of systemic medications for pustular psoriasis in pediatrics. Pediatr Dermatol. 2014;31:430-439.
20. Tollefson MM. Diagnosis and management of psoriasis in children. Pediatr Clin North Am. 2014;61:261-277.
21. Fotiadou C, Lazaridou E, Ioannides D. Management of psoriasis in adolescence. Adolesc Health Med Ther. 2014;5:25-34.
22. Windschall D, Müller T, Becker I, et al. Safety and efficacy of etanercept in children with the JIA categories extended oligoarthritis, enthesitis-related arthritis and psoriasis arthritis [published online ahead of print July 18, 2014]. Clin Rheumatol. 2015;34:61-69.
23. Mälkönen T, Wikström A, Heiskanen K, et al. Skin reactions during anti-TNFa therapy for pediatric inflammatory bowel disease: a 2-year prospective study. Inflamm Bowel Dis. 2014;20:1309-1315.
24. Afzali A, Wheat CL, Hu JK, et al. The association of psoriasiform rash with anti-tumor necrosis factor (anti-TNF) therapy in inflammatory bowel disease: a single academic center case series. J Crohns Colitis. 2014;8:480-488.
25. Sherlock ME, Walters T, Tabbers MM, et al. Infliximab-induced psoriasis and psoriasiform skin lesions in pediatric Crohn disease and a potential association with IL-23 receptor polymorphisms. J Pediatr Gastroenterol Nutr. 2013;56:512-518.
26. Rahman SI, Siegfried E, Flanagan KH, et al. The methotrexate polyglutamate assay supports the efficacy of methotrexate for severe inflammatory skin disease in children. J Am Acad Dermatol. 2014;70:252-256.
27. Suzuki E, Mellins ED, Gershwin ME, et al. The IL-23/IL-17 axis in psoriatic arthritis. Autoimmun Rev. 2014;13:496-502.
28. Michalak-Stoma A, Bartosi´nska J, Kowal M, et al. Serum levels of selected Th17 and Th22 cytokines in psoriatic patients. Dis Markers. 2013;35:625-631.
Practice Points
- The majority of children with psoriasis have severe disease, scalp involvement, and a family history.
- Pediatric psoriasis is associated with metabolic syndrome, especially obesity.
- Anxiety and depression may be noted in children with psoriasis as well as their caregivers.
Generalized Yellow Discoloration of the Skin
The Diagnosis: Carotenemia
Laboratory parameters including thyroid function testing as well as total protein and bilirubin levels were within reference range. Testing revealed multiple food allergies to almonds, oranges, cashews, garlic, peanuts, and cantaloupe. The patient was treated with a dietary expansion based on his allergy testing.
ß-Carotene converts to vitamin A in the intestine and acts as a lipochrome. Lack of conversion can be noted as an inborn error of metabolism.1 Many green, yellow, and orange fruits and vegetables contain ß-carotene, including carrots, sweet potatoes, squash, green beans, papayas, and pumpkins.1-3 ß-Carotene also is used as a vitamin supplement4 or therapeutic agent in photosensitive disorders such as genetic porphyrias.5
ß-Carotene can accumulate in the stratum corneum and impart a yellow color to the skin when the circulating levels are high; this coloration is termed carotenemia.1,4 Carotenemia is common in infants and young children who have diets rich in green and orange vegetable purees.6 Carotenemia limited to thick areas of the skin, such as the palms and soles, can be seen in adults who eat large amounts of carrots; generalized carotenemia is rare.1,4
Carotenemia is a benign condition of excess cutaneous buildup of ß-carotene through excessive intake of carotene-rich foods1-4 or nutritional supplements7 or through association with anorexia, liver disease, renal disease, hypothyroidism, or diabetes mellitus.1,4,8,9 Carotene deposits usually are most notable in areas with thick stratum corneum, such as the nasolabial folds, palms, and soles, as opposed to areas such as the conjunctivae and mucosa.1,4
Carotenemia may mimic jaundice and should be differentiated through scleral examination for icterus and bilirubin levels. Carotene levels can be tested but generally are unnecessary. Carotenemia can be seen in liver or renal disease and can exacerbate the yellow coloration seen in jaundiced individuals.1,4,9
Because it is a benign condition, the pathology usually is limited to skin discoloration, as seen in our patient. Although this condition can be reversed with a modified diet, our patient had multiple food allergies that further restricted his vegetarian diet, thereby limiting the modifications that he was willing to make to his diet.
1. Schwartz RA. Carotenemia. Emedicine. http://emedicine.medscape.com/article/1104368-overview. Updated April 8, 2014. Accessed April 30, 2014.
2. Sale TA, Stratman E. Carotenemia associated with green bean ingestion. Pediatr Dermatol. 2004;21:657-659.
3. Costanza DJ. Carotenemia associated with papaya ingestion. Calif Med. 1968;109:319-320.
4. Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
5. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
6. Karthik SV, Campbell-Davidson D, Isherwood D. Carotenemia in infancy and its association with prevalent feeding practices. Pediatr Dermatol. 2006;23:571-573.
7. Takita Y, Ichimiya M, Hamamoto Y, et al. A case of carotenemia associated with ingestion of nutrient supplements. J Dermatol. 2006;2:132-134.
8. Thibault L, Roberge AG. The nutritional status of subjects with nervosa. Int J Vitam Nutr Res. 1987;57:447-452.
9. Matthews-Roth M, Gulbrandsen CL. Transport of beta-carotene in serum of individuals with carotenemia. Clin Chem. 1974;20:1578-1579.
The Diagnosis: Carotenemia
Laboratory parameters including thyroid function testing as well as total protein and bilirubin levels were within reference range. Testing revealed multiple food allergies to almonds, oranges, cashews, garlic, peanuts, and cantaloupe. The patient was treated with a dietary expansion based on his allergy testing.
ß-Carotene converts to vitamin A in the intestine and acts as a lipochrome. Lack of conversion can be noted as an inborn error of metabolism.1 Many green, yellow, and orange fruits and vegetables contain ß-carotene, including carrots, sweet potatoes, squash, green beans, papayas, and pumpkins.1-3 ß-Carotene also is used as a vitamin supplement4 or therapeutic agent in photosensitive disorders such as genetic porphyrias.5
ß-Carotene can accumulate in the stratum corneum and impart a yellow color to the skin when the circulating levels are high; this coloration is termed carotenemia.1,4 Carotenemia is common in infants and young children who have diets rich in green and orange vegetable purees.6 Carotenemia limited to thick areas of the skin, such as the palms and soles, can be seen in adults who eat large amounts of carrots; generalized carotenemia is rare.1,4
Carotenemia is a benign condition of excess cutaneous buildup of ß-carotene through excessive intake of carotene-rich foods1-4 or nutritional supplements7 or through association with anorexia, liver disease, renal disease, hypothyroidism, or diabetes mellitus.1,4,8,9 Carotene deposits usually are most notable in areas with thick stratum corneum, such as the nasolabial folds, palms, and soles, as opposed to areas such as the conjunctivae and mucosa.1,4
Carotenemia may mimic jaundice and should be differentiated through scleral examination for icterus and bilirubin levels. Carotene levels can be tested but generally are unnecessary. Carotenemia can be seen in liver or renal disease and can exacerbate the yellow coloration seen in jaundiced individuals.1,4,9
Because it is a benign condition, the pathology usually is limited to skin discoloration, as seen in our patient. Although this condition can be reversed with a modified diet, our patient had multiple food allergies that further restricted his vegetarian diet, thereby limiting the modifications that he was willing to make to his diet.
The Diagnosis: Carotenemia
Laboratory parameters including thyroid function testing as well as total protein and bilirubin levels were within reference range. Testing revealed multiple food allergies to almonds, oranges, cashews, garlic, peanuts, and cantaloupe. The patient was treated with a dietary expansion based on his allergy testing.
ß-Carotene converts to vitamin A in the intestine and acts as a lipochrome. Lack of conversion can be noted as an inborn error of metabolism.1 Many green, yellow, and orange fruits and vegetables contain ß-carotene, including carrots, sweet potatoes, squash, green beans, papayas, and pumpkins.1-3 ß-Carotene also is used as a vitamin supplement4 or therapeutic agent in photosensitive disorders such as genetic porphyrias.5
ß-Carotene can accumulate in the stratum corneum and impart a yellow color to the skin when the circulating levels are high; this coloration is termed carotenemia.1,4 Carotenemia is common in infants and young children who have diets rich in green and orange vegetable purees.6 Carotenemia limited to thick areas of the skin, such as the palms and soles, can be seen in adults who eat large amounts of carrots; generalized carotenemia is rare.1,4
Carotenemia is a benign condition of excess cutaneous buildup of ß-carotene through excessive intake of carotene-rich foods1-4 or nutritional supplements7 or through association with anorexia, liver disease, renal disease, hypothyroidism, or diabetes mellitus.1,4,8,9 Carotene deposits usually are most notable in areas with thick stratum corneum, such as the nasolabial folds, palms, and soles, as opposed to areas such as the conjunctivae and mucosa.1,4
Carotenemia may mimic jaundice and should be differentiated through scleral examination for icterus and bilirubin levels. Carotene levels can be tested but generally are unnecessary. Carotenemia can be seen in liver or renal disease and can exacerbate the yellow coloration seen in jaundiced individuals.1,4,9
Because it is a benign condition, the pathology usually is limited to skin discoloration, as seen in our patient. Although this condition can be reversed with a modified diet, our patient had multiple food allergies that further restricted his vegetarian diet, thereby limiting the modifications that he was willing to make to his diet.
1. Schwartz RA. Carotenemia. Emedicine. http://emedicine.medscape.com/article/1104368-overview. Updated April 8, 2014. Accessed April 30, 2014.
2. Sale TA, Stratman E. Carotenemia associated with green bean ingestion. Pediatr Dermatol. 2004;21:657-659.
3. Costanza DJ. Carotenemia associated with papaya ingestion. Calif Med. 1968;109:319-320.
4. Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
5. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
6. Karthik SV, Campbell-Davidson D, Isherwood D. Carotenemia in infancy and its association with prevalent feeding practices. Pediatr Dermatol. 2006;23:571-573.
7. Takita Y, Ichimiya M, Hamamoto Y, et al. A case of carotenemia associated with ingestion of nutrient supplements. J Dermatol. 2006;2:132-134.
8. Thibault L, Roberge AG. The nutritional status of subjects with nervosa. Int J Vitam Nutr Res. 1987;57:447-452.
9. Matthews-Roth M, Gulbrandsen CL. Transport of beta-carotene in serum of individuals with carotenemia. Clin Chem. 1974;20:1578-1579.
1. Schwartz RA. Carotenemia. Emedicine. http://emedicine.medscape.com/article/1104368-overview. Updated April 8, 2014. Accessed April 30, 2014.
2. Sale TA, Stratman E. Carotenemia associated with green bean ingestion. Pediatr Dermatol. 2004;21:657-659.
3. Costanza DJ. Carotenemia associated with papaya ingestion. Calif Med. 1968;109:319-320.
4. Lascari AD. Carotenemia. a review. Clin Pediatr (Phila). 1981;20:25-29.
5. Puy H, Gouya L, Deybach JC. Porphyrias. Lancet. 2010;375:924-937.
6. Karthik SV, Campbell-Davidson D, Isherwood D. Carotenemia in infancy and its association with prevalent feeding practices. Pediatr Dermatol. 2006;23:571-573.
7. Takita Y, Ichimiya M, Hamamoto Y, et al. A case of carotenemia associated with ingestion of nutrient supplements. J Dermatol. 2006;2:132-134.
8. Thibault L, Roberge AG. The nutritional status of subjects with nervosa. Int J Vitam Nutr Res. 1987;57:447-452.
9. Matthews-Roth M, Gulbrandsen CL. Transport of beta-carotene in serum of individuals with carotenemia. Clin Chem. 1974;20:1578-1579.
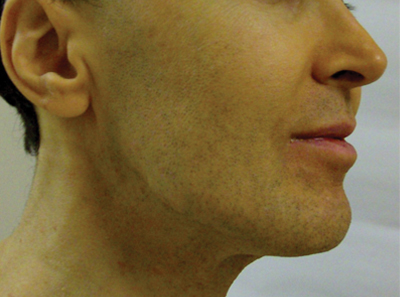
A 50-year-old man presented with yellow, pruritic, xerotic skin and lethargy. The patient also reported nasal congestion and sneezing, especially when eating peanuts. He was fearful of allergic reactions and restricted his diet to “safe foods” such as squash, green beans, and sweet potatoes. On examination the patient had marked generalized yellow discoloration of the skin with pale mucous membranes, nonicteric sclerae, infraocular violaceous and hyperpigmented skin (allergic shiners), and Dennie-Morgan folds.