User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
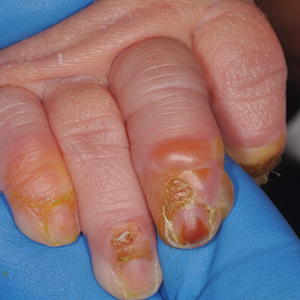
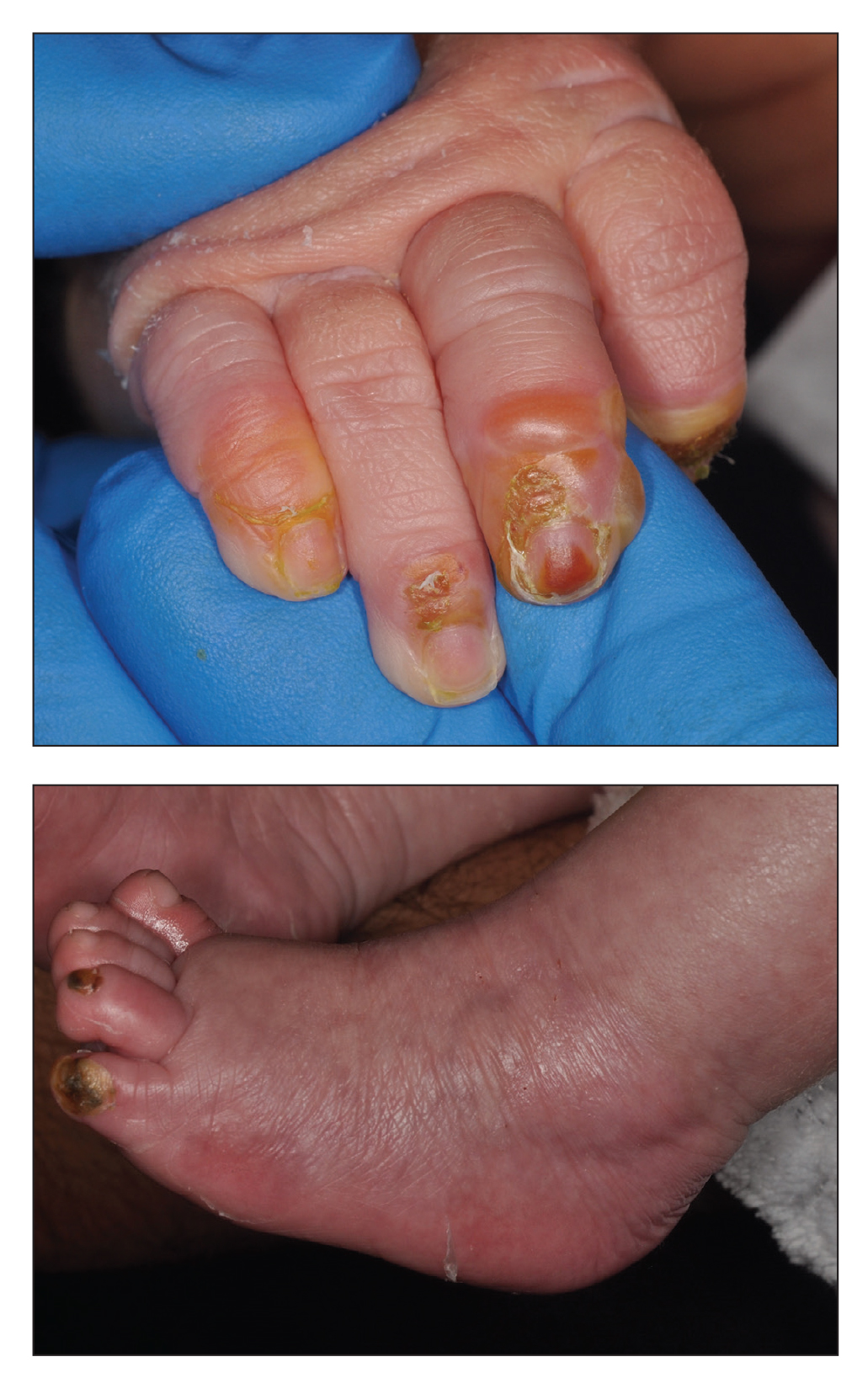
Blistering Lesions in a Newborn
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
The Diagnosis: Epidermolysis Bullosa
Our patient was found to have epidermolysis bullosa (EB), a rare genetic disease in which the superficial layers of the skin separate to form vesicles or bullae due to a mutation in the keratin 14 gene, KRT14. Separation of the skin occurs due to cleavage of various proteins that connect the epidermis to the dermis. A genetic mutation in KRT14, one of the more common genetic mutations associated with EB, results in cleavage at the basal epidermal protein keratin 14. The skin of individuals with EB typically is fragile and cannot tolerate friction or manipulation due to the risk for new bullae formation.1 Epidermolysis bullosa is rare, affecting approximately 20 children per 1 million births in the United States, and is not commonly seen by most general adult dermatologists.2
In our patient, the differential diagnoses included staphylococcal scalded skin syndrome (SSSS), Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN), herpes simplex virus (HSV), and bullous pemphigoid (BP). Symptoms of SSSS can range from mild and localized to full-body exfoliation of the skin. Although SSSS can resemble other bullous disorders, its etiology arises from the Staphylococcus exotoxin targeting desmoglein in the stratum granulosum— the layer of the epidermis between the stratum corneum and stratum spinosum.3 Lesions start on the face, neck, and body folds, which was consistent with our patient’s presentation. However, bullae continued to develop in our patient despite antibiotic therapy, which reduced the likelihood of SSSS. Stevens-Johnson syndrome/toxic epidermal necrolysis develops rapidly and often involves the mucosa, which our patient initially did not have. In children, SJS/TEN can develop secondary to infection, whereas in adults it more commonly is associated with medication administration.4 Although the mother tested negative for HSV, the infant was started on acyclovir, which ultimately was discontinued due to low clinical suspicion. The clinical presentation of HSV (ie, clustered vesicles) was not consistent with our patient’s presentation. Bullous pemphigoid is a subepithelial blistering disease seen in older adults. Tense, fluidfilled blisters primarily are seen on the trunk and flexures. Although infantile BP can occur, it usually does not present in the neonatal period but rather at approximately 3 to 5 months of age.5
High clinical suspicion for EB due to the common characteristics of bullae location and formation following skin manipulation led to genetic testing in our patient. Mild forms of EB simplex typically appear on the upper and lower extremities with sparing of the trunk. In more severe cases of EB simplex, truncal and mucosal involvement may occur.6 In our case, the infant had a classic distribution of arm and leg blisters with truncal sparing. Epidermolysis bullosa may not be diagnosed in the neonatal period because of its similarities to other more common diseases, such as HSV or bullous impetigo, or other genetic blistering diseases, such as epidermolytic ichthyosis and incontinentia pigmenti.6
Epidermolysis bullosa can be inherited in an autosomal-dominant or autosomal-recessive fashion or with de novo mutations and is classified based on the location of cleavage in the skin. The 4 classical subtypes— simplex, junctional, dystrophic, and Kindler—have now been further subclassified. Epidermolysis bullosa simplex (intraepidermal split) is now separated into basal and suprabasal, with further subclassification including the distribution of blisters (generalized or localized) and the severity of cutaneous or extracutaneous involvement.7
In our case, the infant was found to have intraepidermal EB (simplex) due to a KRT14 mutation (missense mutation).6 KRT14 (17q21.2) and KRT5 (12q13.3) are the 2 most common mutations causing cleavage at the basal intraepidermal layer. Thickening of the palms, soles, and nails can be seen; however, blisters heal well without scarring, as seen in our patient. Junctional EB due to cleavage at the intralamina lucida often involves mutations in laminin 332, plectin, and α6β4 integrin. Infants with junctional EB often die from severe infection, dehydration, or malnutrition due to mucosal involvement. Dystrophic EB occurs due to a collagen VII mutation in the dermis, leading to blisters at the sublamina densa and more severe symptoms in the recessive form.7
Newborn management for infants with EB differs from normal newborn care due to increased skin fragility with physical manipulation. Minimal skin manipulation and proper wound care are essential from the first day of life. For new bullae formation, bullae should be ruptured with a needle at the base of the blister and drained. The remaining skin overlying the wound should remain in place as a natural wound barrier. Patients with EB should not have tape or adhesive bandages applied directly to the skin. Instead, nonadhesive dressings can be placed directly on wounds and covered in soft wraps circumferentially. Dressings can be taped together without involving the skin. The cost for supplies for families to manage bullae is expensive. Fortunately, there are resources available for supplies and support for families, including the EB Research Partnership (https://www.ebresearch.org/) and DEBRA of America (https://www.debra.org/).
Currently, there is no cure for EB. Current treatment involves wound care, prevention, and symptomatic relief. Prevention includes avoiding activities that may result in increased friction of the skin and ensuring careful manipulation. Children with EB may have pain or itching from their blisters, which can be treated with oral acetaminophen or ibuprofen and diphenhydramine, respectively. Other complications of EB include anemia, dehydration, constipation, infection, and malnutrition. In more severe forms of EB, complications including eye problems, mucosal strictures, and skin cancer may occur.8 Future treatment directions include gene therapy, bone marrow transplantation, protein replacement therapies, and cell-based therapies. Prognosis for infants with EB due to KRT14 mutation is good, as it is a milder subtype of EB with a full life expectancy and improvement of blistering skin with age. The most at-risk time for early death is during infancy due to increased risk for infection.8 In this case, our patient showed full healing with no scar formation, which suggested a reassuring prognosis.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
- Fine JD, Bruckner-Tuderman L, Eady RAJ, et al. Inherited epidermolysis bullosa: updated recommendations on diagnosis and classification. J Am Acad Dermatol. 2014;70:1103-1126.
- Wolff K, Johnson RA, Saavedra AP, et al. Hereditary epidermolysis bullosa. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 8th ed. McGraw-Hill Education; 2017:94-99.
- Ross A, Shoff HW. Staphylococcus scalded skin syndrome. In: StatPearls. StatPearls Publishing; 2020:1-20.
- Alerhand S, Cassella C, Koyfman A. Steven-Johnson syndrome and toxic epidermal necrolysis in the pediatric population. Pediatr Emerg Care. 2016;32:472-476.
- Schwieger-Briel A, Moellmann C, Mattulat B, et al. Bullous pemphigoid in infants: characteristics, diagnosis and treatment. Orphanet J Rare Dis. 2014;9:185.
- Gonzalez ME. Evaluation and treatment of the newborn with epidermolysis bullosa. Semin Perinatol. 2013;37:32-39.
- Has C, Bauer JW, Bodemer C, et al. Consensus reclassification of inherited epidermolysis bullosa and other disorders with skin fragility. Br J Dermatol. 2020;183:614-627.
- Watkins J. Diagnosis, treatment and management of epidermolysis bullosa. Br J Nurs. 2016;25:428-431.
A 4-day-old infant boy presented with blisters on the skin. He was born at 36 weeks’ gestation by cesarean delivery to a nulliparous mother who received appropriate prenatal care. On day 2 of life, the patient developed bullae with breakdown of the skin on the bilateral heels and on the skin surrounding intravenous injection sites. Similar blisters subsequently developed on the fingers (top), thighs, groin, and toes (bottom), sparing the oral mucosa and trunk. He remained afebrile and stable and was started on ampicillin, gentamicin, and acyclovir with continued development of blisters. Two weeks later he developed painful ulcers on the tongue that bled upon scraping.
Age and ferritin levels may predict MIS-C severity
, according to a Canadian multicenter cohort study.
The adjusted absolute risk for admission to an intensive care unit was 43.6% among children aged 6 years and older and 46.2% in children aged 13 to 17 years, compared with 18.4% in children aged 5 years or younger.
“We do not understand why teens get more severe MIS-C than younger children,” senior author Joan Robinson, MD, of the University of Alberta, Edmonton, told this news organization. “It is possible that more exposures to other coronaviruses in the past result in them having a more robust immune response to SARS-CoV-2, which results in more inflammation.”
The data were published in the Canadian Medical Association Journal.
A multinational study
The study included data on 232 children admitted with probable or confirmed MIS-C at 15 hospitals in Canada, Iran, and Costa Rica between March 1, 2020, and March 7, 2021. The median age of the children was 5.8 years, 56.0% were boys, and 21.6% had comorbidities.
Although cardiac involvement was common (58.6%), and almost one-third of the cohort (31.5%) was admitted to an ICU, “recovery was typically rapid, with 85% of patients discharged within 10 days,” said Dr. Robinson, for the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC).
Older age as a risk
The results suggest that older age is associated with increased risk of severe MIS-C. “However, one would then predict that adults would be at even higher risk than teens, whereas the same syndrome in adults (MIS-A) is very, very rare,” said Dr. Robinson.
The study also found that children admitted with ferritin levels greater than 500 μg/L, signaling greater inflammation, also had an increased risk for ICU admission, compared with those with lower levels (adjusted risk difference, 18.4%; relative risk, 1.69). “This is presumably because the more inflammation that the child has, the more likely they are to have inflammation of the heart, which can lead to low blood pressure,” said Dr. Robinson.
Features of MIS-C
Among all patients with MIS-C, gastrointestinal involvement was common (89.2%), as were mucocutaneous findings (84.5%). Children with MIS-C had fever for a median duration of 6 days. “Clinicians who see children in their practice commonly have to determine why a child is febrile. Our study shows that one mainly has to consider MIS-C if febrile children have a rash and one or more of vomiting, diarrhea, or abdominal pain,” said Dr. Robinson.
The study also found that patients with MIS-C who were admitted to the hospital in the latter part of the study period (Nov. 1, 2020, to March 7, 2021) were slightly more likely to require ICU admission, compared with those admitted between March 1 and Oct. 31, 2020. “We cannot provide a clear explanation [for this],” the authors noted. “The features of severe MIS-C were widely publicized by May 2020, so it seems unlikely that severe cases were missed early in the study period. SARS-CoV-2 variants of concern have replaced the wild-type virus. It is possible that the immune response to circulating variants alters the severity of COVID-19 and MIS-C, when compared with wild-type virus.”
Despite initial concerns that pediatric COVID-19 vaccines might cause MIS-C, Dr. Robinson says data suggest this is rarely, if ever, the case, and that vaccines actually prevent the syndrome. She says further studies will be needed to assess MIS-C risk following reinfection with SARS-CoV-2. “I am an optimistic person, and it is my hope that MIS-C following reinfection is rare,” she said. “If this is the case, perhaps we will see very few cases once almost all children have been immunized and/or had SARS-CoV-2 infection.”
‘Differences across countries’
Adrienne Randolph, MD, a pediatrician at Harvard Medical School, Boston, and senior author of a large case series of patients with MIS-C, said that the Canadian study is valuable because it includes children from three countries. “It’s very interesting that there are differences across countries,” she said. “The patients in Iran had the highest percentage (58.7%) going into the ICU, whereas Costa Rica had the lowest percentage (9.2%), and the percentage going to the ICU in Canada (34.7%) was less than the percentages we see in the U.S. – which is pretty consistently about 60% to 70% of MIS-C patients going into the ICU.” Dr. Randolph was not involved in the current study.
Reasons for differences in the rates of ICU visits will be important to explore in the effort to standardize diagnostic criteria, stratification of severity, and recommendations for treatment of MIS-C, said Dr. Randolph.
“What is consistent is that the younger kids, zero to 5 years, in general are less ill,” she said. “That’s been consistent across multiple countries.” It’s unclear whether the cause of this difference is that parents observe younger patients more closely than they do teenagers, or whether other aspects of adolescence, such as prevalence of obesity and attendant inflammation, are at work, said Dr. Randolph.
What is also unclear is why hospitalized patients with MIS-C had higher percentages of ICU admission in the latter part of the study period, compared with the earlier period. “Did the patients change, or did practice change as we got to understand the disease process?” asked Dr. Randolph. “It could be that they got better at the diagnosis and were weeding out some of the patients who they realized didn’t need to be hospitalized. At the very beginning, we had a very low threshold to admit patients, because we didn’t know, and then, over time, people understood what was going on and felt more comfortable monitoring them as outpatients.”
This study was partially funded by a Janeway Foundation Research Grant to support data collection. Dr. Robinson disclosed no conflicts of interest. Dr. Randolph reported receiving royalties from UpToDate and personal fees from the La Jolla Pharmaceutical Company.
A version of this article first appeared on Medscape.com.
, according to a Canadian multicenter cohort study.
The adjusted absolute risk for admission to an intensive care unit was 43.6% among children aged 6 years and older and 46.2% in children aged 13 to 17 years, compared with 18.4% in children aged 5 years or younger.
“We do not understand why teens get more severe MIS-C than younger children,” senior author Joan Robinson, MD, of the University of Alberta, Edmonton, told this news organization. “It is possible that more exposures to other coronaviruses in the past result in them having a more robust immune response to SARS-CoV-2, which results in more inflammation.”
The data were published in the Canadian Medical Association Journal.
A multinational study
The study included data on 232 children admitted with probable or confirmed MIS-C at 15 hospitals in Canada, Iran, and Costa Rica between March 1, 2020, and March 7, 2021. The median age of the children was 5.8 years, 56.0% were boys, and 21.6% had comorbidities.
Although cardiac involvement was common (58.6%), and almost one-third of the cohort (31.5%) was admitted to an ICU, “recovery was typically rapid, with 85% of patients discharged within 10 days,” said Dr. Robinson, for the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC).
Older age as a risk
The results suggest that older age is associated with increased risk of severe MIS-C. “However, one would then predict that adults would be at even higher risk than teens, whereas the same syndrome in adults (MIS-A) is very, very rare,” said Dr. Robinson.
The study also found that children admitted with ferritin levels greater than 500 μg/L, signaling greater inflammation, also had an increased risk for ICU admission, compared with those with lower levels (adjusted risk difference, 18.4%; relative risk, 1.69). “This is presumably because the more inflammation that the child has, the more likely they are to have inflammation of the heart, which can lead to low blood pressure,” said Dr. Robinson.
Features of MIS-C
Among all patients with MIS-C, gastrointestinal involvement was common (89.2%), as were mucocutaneous findings (84.5%). Children with MIS-C had fever for a median duration of 6 days. “Clinicians who see children in their practice commonly have to determine why a child is febrile. Our study shows that one mainly has to consider MIS-C if febrile children have a rash and one or more of vomiting, diarrhea, or abdominal pain,” said Dr. Robinson.
The study also found that patients with MIS-C who were admitted to the hospital in the latter part of the study period (Nov. 1, 2020, to March 7, 2021) were slightly more likely to require ICU admission, compared with those admitted between March 1 and Oct. 31, 2020. “We cannot provide a clear explanation [for this],” the authors noted. “The features of severe MIS-C were widely publicized by May 2020, so it seems unlikely that severe cases were missed early in the study period. SARS-CoV-2 variants of concern have replaced the wild-type virus. It is possible that the immune response to circulating variants alters the severity of COVID-19 and MIS-C, when compared with wild-type virus.”
Despite initial concerns that pediatric COVID-19 vaccines might cause MIS-C, Dr. Robinson says data suggest this is rarely, if ever, the case, and that vaccines actually prevent the syndrome. She says further studies will be needed to assess MIS-C risk following reinfection with SARS-CoV-2. “I am an optimistic person, and it is my hope that MIS-C following reinfection is rare,” she said. “If this is the case, perhaps we will see very few cases once almost all children have been immunized and/or had SARS-CoV-2 infection.”
‘Differences across countries’
Adrienne Randolph, MD, a pediatrician at Harvard Medical School, Boston, and senior author of a large case series of patients with MIS-C, said that the Canadian study is valuable because it includes children from three countries. “It’s very interesting that there are differences across countries,” she said. “The patients in Iran had the highest percentage (58.7%) going into the ICU, whereas Costa Rica had the lowest percentage (9.2%), and the percentage going to the ICU in Canada (34.7%) was less than the percentages we see in the U.S. – which is pretty consistently about 60% to 70% of MIS-C patients going into the ICU.” Dr. Randolph was not involved in the current study.
Reasons for differences in the rates of ICU visits will be important to explore in the effort to standardize diagnostic criteria, stratification of severity, and recommendations for treatment of MIS-C, said Dr. Randolph.
“What is consistent is that the younger kids, zero to 5 years, in general are less ill,” she said. “That’s been consistent across multiple countries.” It’s unclear whether the cause of this difference is that parents observe younger patients more closely than they do teenagers, or whether other aspects of adolescence, such as prevalence of obesity and attendant inflammation, are at work, said Dr. Randolph.
What is also unclear is why hospitalized patients with MIS-C had higher percentages of ICU admission in the latter part of the study period, compared with the earlier period. “Did the patients change, or did practice change as we got to understand the disease process?” asked Dr. Randolph. “It could be that they got better at the diagnosis and were weeding out some of the patients who they realized didn’t need to be hospitalized. At the very beginning, we had a very low threshold to admit patients, because we didn’t know, and then, over time, people understood what was going on and felt more comfortable monitoring them as outpatients.”
This study was partially funded by a Janeway Foundation Research Grant to support data collection. Dr. Robinson disclosed no conflicts of interest. Dr. Randolph reported receiving royalties from UpToDate and personal fees from the La Jolla Pharmaceutical Company.
A version of this article first appeared on Medscape.com.
, according to a Canadian multicenter cohort study.
The adjusted absolute risk for admission to an intensive care unit was 43.6% among children aged 6 years and older and 46.2% in children aged 13 to 17 years, compared with 18.4% in children aged 5 years or younger.
“We do not understand why teens get more severe MIS-C than younger children,” senior author Joan Robinson, MD, of the University of Alberta, Edmonton, told this news organization. “It is possible that more exposures to other coronaviruses in the past result in them having a more robust immune response to SARS-CoV-2, which results in more inflammation.”
The data were published in the Canadian Medical Association Journal.
A multinational study
The study included data on 232 children admitted with probable or confirmed MIS-C at 15 hospitals in Canada, Iran, and Costa Rica between March 1, 2020, and March 7, 2021. The median age of the children was 5.8 years, 56.0% were boys, and 21.6% had comorbidities.
Although cardiac involvement was common (58.6%), and almost one-third of the cohort (31.5%) was admitted to an ICU, “recovery was typically rapid, with 85% of patients discharged within 10 days,” said Dr. Robinson, for the Pediatric Investigators Collaborative Network on Infections in Canada (PICNIC).
Older age as a risk
The results suggest that older age is associated with increased risk of severe MIS-C. “However, one would then predict that adults would be at even higher risk than teens, whereas the same syndrome in adults (MIS-A) is very, very rare,” said Dr. Robinson.
The study also found that children admitted with ferritin levels greater than 500 μg/L, signaling greater inflammation, also had an increased risk for ICU admission, compared with those with lower levels (adjusted risk difference, 18.4%; relative risk, 1.69). “This is presumably because the more inflammation that the child has, the more likely they are to have inflammation of the heart, which can lead to low blood pressure,” said Dr. Robinson.
Features of MIS-C
Among all patients with MIS-C, gastrointestinal involvement was common (89.2%), as were mucocutaneous findings (84.5%). Children with MIS-C had fever for a median duration of 6 days. “Clinicians who see children in their practice commonly have to determine why a child is febrile. Our study shows that one mainly has to consider MIS-C if febrile children have a rash and one or more of vomiting, diarrhea, or abdominal pain,” said Dr. Robinson.
The study also found that patients with MIS-C who were admitted to the hospital in the latter part of the study period (Nov. 1, 2020, to March 7, 2021) were slightly more likely to require ICU admission, compared with those admitted between March 1 and Oct. 31, 2020. “We cannot provide a clear explanation [for this],” the authors noted. “The features of severe MIS-C were widely publicized by May 2020, so it seems unlikely that severe cases were missed early in the study period. SARS-CoV-2 variants of concern have replaced the wild-type virus. It is possible that the immune response to circulating variants alters the severity of COVID-19 and MIS-C, when compared with wild-type virus.”
Despite initial concerns that pediatric COVID-19 vaccines might cause MIS-C, Dr. Robinson says data suggest this is rarely, if ever, the case, and that vaccines actually prevent the syndrome. She says further studies will be needed to assess MIS-C risk following reinfection with SARS-CoV-2. “I am an optimistic person, and it is my hope that MIS-C following reinfection is rare,” she said. “If this is the case, perhaps we will see very few cases once almost all children have been immunized and/or had SARS-CoV-2 infection.”
‘Differences across countries’
Adrienne Randolph, MD, a pediatrician at Harvard Medical School, Boston, and senior author of a large case series of patients with MIS-C, said that the Canadian study is valuable because it includes children from three countries. “It’s very interesting that there are differences across countries,” she said. “The patients in Iran had the highest percentage (58.7%) going into the ICU, whereas Costa Rica had the lowest percentage (9.2%), and the percentage going to the ICU in Canada (34.7%) was less than the percentages we see in the U.S. – which is pretty consistently about 60% to 70% of MIS-C patients going into the ICU.” Dr. Randolph was not involved in the current study.
Reasons for differences in the rates of ICU visits will be important to explore in the effort to standardize diagnostic criteria, stratification of severity, and recommendations for treatment of MIS-C, said Dr. Randolph.
“What is consistent is that the younger kids, zero to 5 years, in general are less ill,” she said. “That’s been consistent across multiple countries.” It’s unclear whether the cause of this difference is that parents observe younger patients more closely than they do teenagers, or whether other aspects of adolescence, such as prevalence of obesity and attendant inflammation, are at work, said Dr. Randolph.
What is also unclear is why hospitalized patients with MIS-C had higher percentages of ICU admission in the latter part of the study period, compared with the earlier period. “Did the patients change, or did practice change as we got to understand the disease process?” asked Dr. Randolph. “It could be that they got better at the diagnosis and were weeding out some of the patients who they realized didn’t need to be hospitalized. At the very beginning, we had a very low threshold to admit patients, because we didn’t know, and then, over time, people understood what was going on and felt more comfortable monitoring them as outpatients.”
This study was partially funded by a Janeway Foundation Research Grant to support data collection. Dr. Robinson disclosed no conflicts of interest. Dr. Randolph reported receiving royalties from UpToDate and personal fees from the La Jolla Pharmaceutical Company.
A version of this article first appeared on Medscape.com.
Cancer diet studies: Veggies get another rave, while red meat’s busted again
Researchers report that high consumption of vegetables – especially lettuce, legumes, and cruciferous varieties – appears to lower the risk of liver cancer/liver disease. A separate team suggests that high consumption of red meat, organ meats, and processed meats boosts the risk of gastric cancer.
The findings of the latter study “reinforce the idea that avoidance of red meat and processed meat is probably good beyond [the prevention of] colorectal cancer,” said corresponding author and epidemiologist Paolo Boffetta, MD, MPH, of Stony Brook University Cancer Center, New York, in an interview. “The possible carcinogenic effect may extend beyond the colon.”
Both studies were released at the annual meeting of the American Association for Cancer Research.
For the red meat study, researchers examined statistics from the Golestan cohort study, which is prospectively tracking 50,045 people aged 40-75 from northeastern Iran. The study focuses on esophageal cancer due to the region’s high rate of the disease.
Red meat consumption is fairly rare in the region, where residents typically prefer chicken, said study lead author Giulia Collatuzzo, MD, a resident physician in occupational medicine at the University of Bologna, Italy, in an interview. On average, participants reported eating 18.4 grams daily of red meat and 72.1 grams daily of white meat.
The researchers tracked study participants for a median 12-year follow-up, during which 369 developed esophageal cancer and 368 developed gastric cancer. Red meat was only linked to more esophageal cancer in women (hazard ratio, 1.13, 95% confidence interval, 1.00-1.18, for each quintile increase in consumption).
Overall red meat consumption (including red meat, organ meat, and processed meat) was linked to higher rates of gastric cancer (HR, 1.08, 95% CI, 1.00-1.17) for each quartile increase in consumption, as was consumption of the red meat subtype alone (HR, 1.09, 95% CI, 1.00-1.18).
According to Dr. Collatuzzo, the findings suggest that those in the highest quartile of overall red meat consumption may have around a 25% increase in risk, compared with the lowest quartile.
Overall, she said, the study findings aren’t surprising. The lack of a connection between red meat consumption and esophageal cancer may be due to the fact that meat only temporarily transits through the esophagus, she said.
For the liver cancer/liver disease study, researchers examined the medical records of 470,653 subjects in the NIH-AARP Diet and Health Study. They were recruited in 1995-1996 when they were 50-71 years old. Over a median follow-up of 15.5 years, 899 developed liver cancer, and 934 died of chronic liver disease.
The median intakes of vegetables in quintile 5 (highest) and quintile 1 (lowest) were 3.7 cups daily and 1.0 cups daily, respectively, said study lead author Long-Gang Zhao, MS, a graduate student at Harvard University.
After adjusting for possible cofounders, those in the highest quintile of vegetable consumption were a third less likely to develop liver cancer, compared with the lowest quintile (HR, 0.66, 95% CI, 0.53-0.82, P < 0.01). Several types of vegetables appeared to be the strongest cancer fighters: cruciferous (broccoli, cauliflower), lettuce, legumes, and carrots. These kinds of vegetables were also linked to lower rates of chronic liver disease mortality (all P < 0.01), as was total vegetable intake for the top quintile versus the lowest quintile (HR, 0.60, 95% CI, 0.49-0.74, P = < 0.01).
“A one-cup increase (8 oz or 225 g) in vegetable intake was associated with about 20% decreased risk of liver cancer incidence and chronic liver mortality,” Zhao said.
There was no statistically significant link between fruit consumption and liver cancer or chronic liver disease mortality.
The findings provide more insight into diet and liver disease, Zhao said. “Chronic liver disease, which predisposes to liver cancer, is the tenth cause of death worldwide, causing two million deaths each year. It shares some etiological processes with liver cancer. Therefore, examining both chronic liver disease mortality and liver cancer incidence in our study may provide a more general picture for the prevention of liver diseases.”
As for limitations, both studies are based on self-reports about food consumption, which can be unreliable, and the subjects in the fruit/vegetable analysis were mainly of European origin.
The authors of both studies report no relevant disclosures. No funding is reported for either study.
Researchers report that high consumption of vegetables – especially lettuce, legumes, and cruciferous varieties – appears to lower the risk of liver cancer/liver disease. A separate team suggests that high consumption of red meat, organ meats, and processed meats boosts the risk of gastric cancer.
The findings of the latter study “reinforce the idea that avoidance of red meat and processed meat is probably good beyond [the prevention of] colorectal cancer,” said corresponding author and epidemiologist Paolo Boffetta, MD, MPH, of Stony Brook University Cancer Center, New York, in an interview. “The possible carcinogenic effect may extend beyond the colon.”
Both studies were released at the annual meeting of the American Association for Cancer Research.
For the red meat study, researchers examined statistics from the Golestan cohort study, which is prospectively tracking 50,045 people aged 40-75 from northeastern Iran. The study focuses on esophageal cancer due to the region’s high rate of the disease.
Red meat consumption is fairly rare in the region, where residents typically prefer chicken, said study lead author Giulia Collatuzzo, MD, a resident physician in occupational medicine at the University of Bologna, Italy, in an interview. On average, participants reported eating 18.4 grams daily of red meat and 72.1 grams daily of white meat.
The researchers tracked study participants for a median 12-year follow-up, during which 369 developed esophageal cancer and 368 developed gastric cancer. Red meat was only linked to more esophageal cancer in women (hazard ratio, 1.13, 95% confidence interval, 1.00-1.18, for each quintile increase in consumption).
Overall red meat consumption (including red meat, organ meat, and processed meat) was linked to higher rates of gastric cancer (HR, 1.08, 95% CI, 1.00-1.17) for each quartile increase in consumption, as was consumption of the red meat subtype alone (HR, 1.09, 95% CI, 1.00-1.18).
According to Dr. Collatuzzo, the findings suggest that those in the highest quartile of overall red meat consumption may have around a 25% increase in risk, compared with the lowest quartile.
Overall, she said, the study findings aren’t surprising. The lack of a connection between red meat consumption and esophageal cancer may be due to the fact that meat only temporarily transits through the esophagus, she said.
For the liver cancer/liver disease study, researchers examined the medical records of 470,653 subjects in the NIH-AARP Diet and Health Study. They were recruited in 1995-1996 when they were 50-71 years old. Over a median follow-up of 15.5 years, 899 developed liver cancer, and 934 died of chronic liver disease.
The median intakes of vegetables in quintile 5 (highest) and quintile 1 (lowest) were 3.7 cups daily and 1.0 cups daily, respectively, said study lead author Long-Gang Zhao, MS, a graduate student at Harvard University.
After adjusting for possible cofounders, those in the highest quintile of vegetable consumption were a third less likely to develop liver cancer, compared with the lowest quintile (HR, 0.66, 95% CI, 0.53-0.82, P < 0.01). Several types of vegetables appeared to be the strongest cancer fighters: cruciferous (broccoli, cauliflower), lettuce, legumes, and carrots. These kinds of vegetables were also linked to lower rates of chronic liver disease mortality (all P < 0.01), as was total vegetable intake for the top quintile versus the lowest quintile (HR, 0.60, 95% CI, 0.49-0.74, P = < 0.01).
“A one-cup increase (8 oz or 225 g) in vegetable intake was associated with about 20% decreased risk of liver cancer incidence and chronic liver mortality,” Zhao said.
There was no statistically significant link between fruit consumption and liver cancer or chronic liver disease mortality.
The findings provide more insight into diet and liver disease, Zhao said. “Chronic liver disease, which predisposes to liver cancer, is the tenth cause of death worldwide, causing two million deaths each year. It shares some etiological processes with liver cancer. Therefore, examining both chronic liver disease mortality and liver cancer incidence in our study may provide a more general picture for the prevention of liver diseases.”
As for limitations, both studies are based on self-reports about food consumption, which can be unreliable, and the subjects in the fruit/vegetable analysis were mainly of European origin.
The authors of both studies report no relevant disclosures. No funding is reported for either study.
Researchers report that high consumption of vegetables – especially lettuce, legumes, and cruciferous varieties – appears to lower the risk of liver cancer/liver disease. A separate team suggests that high consumption of red meat, organ meats, and processed meats boosts the risk of gastric cancer.
The findings of the latter study “reinforce the idea that avoidance of red meat and processed meat is probably good beyond [the prevention of] colorectal cancer,” said corresponding author and epidemiologist Paolo Boffetta, MD, MPH, of Stony Brook University Cancer Center, New York, in an interview. “The possible carcinogenic effect may extend beyond the colon.”
Both studies were released at the annual meeting of the American Association for Cancer Research.
For the red meat study, researchers examined statistics from the Golestan cohort study, which is prospectively tracking 50,045 people aged 40-75 from northeastern Iran. The study focuses on esophageal cancer due to the region’s high rate of the disease.
Red meat consumption is fairly rare in the region, where residents typically prefer chicken, said study lead author Giulia Collatuzzo, MD, a resident physician in occupational medicine at the University of Bologna, Italy, in an interview. On average, participants reported eating 18.4 grams daily of red meat and 72.1 grams daily of white meat.
The researchers tracked study participants for a median 12-year follow-up, during which 369 developed esophageal cancer and 368 developed gastric cancer. Red meat was only linked to more esophageal cancer in women (hazard ratio, 1.13, 95% confidence interval, 1.00-1.18, for each quintile increase in consumption).
Overall red meat consumption (including red meat, organ meat, and processed meat) was linked to higher rates of gastric cancer (HR, 1.08, 95% CI, 1.00-1.17) for each quartile increase in consumption, as was consumption of the red meat subtype alone (HR, 1.09, 95% CI, 1.00-1.18).
According to Dr. Collatuzzo, the findings suggest that those in the highest quartile of overall red meat consumption may have around a 25% increase in risk, compared with the lowest quartile.
Overall, she said, the study findings aren’t surprising. The lack of a connection between red meat consumption and esophageal cancer may be due to the fact that meat only temporarily transits through the esophagus, she said.
For the liver cancer/liver disease study, researchers examined the medical records of 470,653 subjects in the NIH-AARP Diet and Health Study. They were recruited in 1995-1996 when they were 50-71 years old. Over a median follow-up of 15.5 years, 899 developed liver cancer, and 934 died of chronic liver disease.
The median intakes of vegetables in quintile 5 (highest) and quintile 1 (lowest) were 3.7 cups daily and 1.0 cups daily, respectively, said study lead author Long-Gang Zhao, MS, a graduate student at Harvard University.
After adjusting for possible cofounders, those in the highest quintile of vegetable consumption were a third less likely to develop liver cancer, compared with the lowest quintile (HR, 0.66, 95% CI, 0.53-0.82, P < 0.01). Several types of vegetables appeared to be the strongest cancer fighters: cruciferous (broccoli, cauliflower), lettuce, legumes, and carrots. These kinds of vegetables were also linked to lower rates of chronic liver disease mortality (all P < 0.01), as was total vegetable intake for the top quintile versus the lowest quintile (HR, 0.60, 95% CI, 0.49-0.74, P = < 0.01).
“A one-cup increase (8 oz or 225 g) in vegetable intake was associated with about 20% decreased risk of liver cancer incidence and chronic liver mortality,” Zhao said.
There was no statistically significant link between fruit consumption and liver cancer or chronic liver disease mortality.
The findings provide more insight into diet and liver disease, Zhao said. “Chronic liver disease, which predisposes to liver cancer, is the tenth cause of death worldwide, causing two million deaths each year. It shares some etiological processes with liver cancer. Therefore, examining both chronic liver disease mortality and liver cancer incidence in our study may provide a more general picture for the prevention of liver diseases.”
As for limitations, both studies are based on self-reports about food consumption, which can be unreliable, and the subjects in the fruit/vegetable analysis were mainly of European origin.
The authors of both studies report no relevant disclosures. No funding is reported for either study.
FROM AACR 2022
Judge strikes down Biden mask mandate for planes, transit
The mandate, enacted in February 2021, is unconstitutional because Congress never granted the Centers for Disease Control and Prevention the power to create such a requirement, U.S. District Judge Kathryn Kimball Mizelle said in her order issued April 18.
“Congress addressed whether the CDC may enact preventative measures that condition the interstate travel of an entire population to CDC dictates. It may not,” the order says.
While the government argued that the definition of “sanitation” in federal law allows it to create travel restrictions like the use of masks, Judge Mizelle disagreed.
“A power to improve ‘sanitation’ would easily extend to requiring vaccinations against COVID-19, the seasonal flu, or other diseases. Or to mandatory social distancing, coughing-into-elbows, and daily multivitamins,” she wrote.
The Biden administration has extended the mask mandate several times since it was first announced. Most recently, the mandate was extended last week and was set to end May 3.
The rule has been alternately praised and criticized by airlines, pilots, and flight attendants. Lawsuits have been filed over the mandate, but Judge Mizelle ruled in favor of two people and the Health Freedom Defense Fund, who filed suit in July 2021.
It is not yet clear if the Biden administration will appeal the decision.
A version of this article first appeared on WebMD.com.
The mandate, enacted in February 2021, is unconstitutional because Congress never granted the Centers for Disease Control and Prevention the power to create such a requirement, U.S. District Judge Kathryn Kimball Mizelle said in her order issued April 18.
“Congress addressed whether the CDC may enact preventative measures that condition the interstate travel of an entire population to CDC dictates. It may not,” the order says.
While the government argued that the definition of “sanitation” in federal law allows it to create travel restrictions like the use of masks, Judge Mizelle disagreed.
“A power to improve ‘sanitation’ would easily extend to requiring vaccinations against COVID-19, the seasonal flu, or other diseases. Or to mandatory social distancing, coughing-into-elbows, and daily multivitamins,” she wrote.
The Biden administration has extended the mask mandate several times since it was first announced. Most recently, the mandate was extended last week and was set to end May 3.
The rule has been alternately praised and criticized by airlines, pilots, and flight attendants. Lawsuits have been filed over the mandate, but Judge Mizelle ruled in favor of two people and the Health Freedom Defense Fund, who filed suit in July 2021.
It is not yet clear if the Biden administration will appeal the decision.
A version of this article first appeared on WebMD.com.
The mandate, enacted in February 2021, is unconstitutional because Congress never granted the Centers for Disease Control and Prevention the power to create such a requirement, U.S. District Judge Kathryn Kimball Mizelle said in her order issued April 18.
“Congress addressed whether the CDC may enact preventative measures that condition the interstate travel of an entire population to CDC dictates. It may not,” the order says.
While the government argued that the definition of “sanitation” in federal law allows it to create travel restrictions like the use of masks, Judge Mizelle disagreed.
“A power to improve ‘sanitation’ would easily extend to requiring vaccinations against COVID-19, the seasonal flu, or other diseases. Or to mandatory social distancing, coughing-into-elbows, and daily multivitamins,” she wrote.
The Biden administration has extended the mask mandate several times since it was first announced. Most recently, the mandate was extended last week and was set to end May 3.
The rule has been alternately praised and criticized by airlines, pilots, and flight attendants. Lawsuits have been filed over the mandate, but Judge Mizelle ruled in favor of two people and the Health Freedom Defense Fund, who filed suit in July 2021.
It is not yet clear if the Biden administration will appeal the decision.
A version of this article first appeared on WebMD.com.
Who doesn’t text in 2022? Most state Medicaid programs
West Virginia will use the U.S. Postal Service and an online account in the summer of 2022 to connect with Medicaid enrollees about the expected end of the COVID public health emergency, which will put many recipients at risk of losing their coverage.
What West Virginia won’t do is use a form of communication that’s ubiquitous worldwide: text messaging.
“West Virginia isn’t set up to text its members,” Allison Adler, the state’s Medicaid spokesperson, wrote to KHN in an email.
Indeed, most states’ Medicaid programs won’t text enrollees despite the urgency to reach them about renewing their coverage. A KFF report published in March found just 11 states said they would use texting to alert Medicaid recipients about the end of the COVID public health emergency. In contrast, 33 states plan to use snail mail and at least 20 will reach out with individual or automated phone calls.
“It doesn’t make any sense when texting is how most people communicate today,” said Kinda Serafi, a partner with the consulting firm Manatt Health.
State Medicaid agencies for months have been preparing for the end of the public health emergency. As part of a COVID relief law approved in March 2020, Congress prohibited states from dropping anyone from Medicaid coverage unless they moved out of state during the public health emergency. When the emergency ends, state Medicaid officials must reevaluate each enrollee’s eligibility. Millions of people could lose their coverage if they earn too much or fail to provide the information needed to verify income or residency.
As of November, about 86 million people were enrolled in Medicaid, according to the Centers for Medicare & Medicaid Services. That’s up from 71 million in February 2020, before COVID began to ravage the nation.
West Virginia has more than 600,000 Medicaid enrollees. Adler said about 100,000 of them could lose their eligibility at the end of the public health emergency because either the state has determined they’re ineligible or they’ve failed to respond to requests that they update their income information.
“It’s frustrating that texting is a means to meet people where they are and that this has not been picked up more by states,” said Jennifer Wagner, director of Medicaid eligibility and enrollment for the Center on Budget and Policy Priorities, a Washington-based research group.
The problem with relying on the Postal Service is that a letter can get hidden in “junk” mail or can fail to reach people who have moved or are homeless, Ms. Serafi said. And email, if people have an account, can end up in spam folders.
In contrast, surveys show lower-income Americans are just as likely to have smartphones and cellphones as the general population. And most people regularly use texting.
In Michigan, Medicaid officials started using text messaging to communicate with enrollees in 2020 after building a system with the help of federal COVID relief funding. They said texting is an economical way to reach enrollees.
“It costs us 2 cents per text message, which is incredibly cheap,” said Steph White, an enrollment coordinator for the Michigan Department of Health and Human Services. “It’s a great return on investment.”
CMS officials have told states they should consider texting, along with other communication methods, when trying to reach enrollees when the public health emergency ends. But many states don’t have the technology or information about enrollees to do it.
Efforts to add texting also face legal barriers, including a federal law that bars texting people without their consent. The Federal Communications Commission ruled in 2021 that state agencies are exempt from the law, but whether counties that handle Medicaid duties for some states and Medicaid managed-care organizations that work in more than 40 states are exempt as well is unclear, said Matt Salo, executive director of the National Association of Medicaid Directors.
CMS spokesperson Beth Lynk said the agency is trying to figure out how Medicaid agencies, counties, and health plans can text enrollees within the constraints of federal law.
Several states told KHN that Medicaid health plans will be helping connect with enrollees and that they expect the plans to use text messaging. But the requirement to get consent from enrollees before texting could limit that effort.
That’s the situation in Virginia, where only about 30,000 Medicaid enrollees – out of more than a million – have agreed to receive text messages directly from the state, said spokesperson Christina Nuckols.
In an effort to boost that number, the state plans to ask enrollees if they want to opt out of receiving text messages, rather than ask them to opt in, she said. This way enrollees would contact the state only if they don’t want to be texted. The state is reviewing its legal options to make that happen.
Meanwhile, Ms. Nuckols added, the state expects Medicaid health plans to contact enrollees about updating their contact information. Four of Virginia’s six Medicaid plans, which serve the bulk of the state’s enrollees, have permission to text about 316,000.
Craig Kennedy, CEO of Medicaid Health Plans of America, a trade group, said that most plans are using texting and that Medicaid officials will use multiple strategies to connect with enrollees. “I do not see this as a detriment, that states are not texting information about reenrollment,” he said. “I know we will be helping with that.”
California officials in March directed Medicaid health plans to use a variety of communication methods, including texting, to ensure that members can retain coverage if they remain eligible. The officials told health plans they could ask for consent through an initial text.
California officials say they also plan to ask enrollees for consent to be texted on the enrollment application, although federal approval for the change is not expected until the fall.
A few state Medicaid programs have experimented in recent years with pilot programs that included texting enrollees.
In 2019, Louisiana worked with the nonprofit group Code for America to send text messages that reminded people about renewing coverage and providing income information for verification. Compared with traditional communication methods, the texts led to a 67% increase in enrollees being renewed for coverage and a 56% increase in enrollees verifying their income in response to inquiries, said Medicaid spokesperson Alyson Neel.
Nonetheless, the state isn’t planning to text Medicaid enrollees about the end of the public health emergency because it hasn’t set up a system for that. “Medicaid has not yet been able to implement a text messaging system of its own due to other agency priorities,” Ms. Neel said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
West Virginia will use the U.S. Postal Service and an online account in the summer of 2022 to connect with Medicaid enrollees about the expected end of the COVID public health emergency, which will put many recipients at risk of losing their coverage.
What West Virginia won’t do is use a form of communication that’s ubiquitous worldwide: text messaging.
“West Virginia isn’t set up to text its members,” Allison Adler, the state’s Medicaid spokesperson, wrote to KHN in an email.
Indeed, most states’ Medicaid programs won’t text enrollees despite the urgency to reach them about renewing their coverage. A KFF report published in March found just 11 states said they would use texting to alert Medicaid recipients about the end of the COVID public health emergency. In contrast, 33 states plan to use snail mail and at least 20 will reach out with individual or automated phone calls.
“It doesn’t make any sense when texting is how most people communicate today,” said Kinda Serafi, a partner with the consulting firm Manatt Health.
State Medicaid agencies for months have been preparing for the end of the public health emergency. As part of a COVID relief law approved in March 2020, Congress prohibited states from dropping anyone from Medicaid coverage unless they moved out of state during the public health emergency. When the emergency ends, state Medicaid officials must reevaluate each enrollee’s eligibility. Millions of people could lose their coverage if they earn too much or fail to provide the information needed to verify income or residency.
As of November, about 86 million people were enrolled in Medicaid, according to the Centers for Medicare & Medicaid Services. That’s up from 71 million in February 2020, before COVID began to ravage the nation.
West Virginia has more than 600,000 Medicaid enrollees. Adler said about 100,000 of them could lose their eligibility at the end of the public health emergency because either the state has determined they’re ineligible or they’ve failed to respond to requests that they update their income information.
“It’s frustrating that texting is a means to meet people where they are and that this has not been picked up more by states,” said Jennifer Wagner, director of Medicaid eligibility and enrollment for the Center on Budget and Policy Priorities, a Washington-based research group.
The problem with relying on the Postal Service is that a letter can get hidden in “junk” mail or can fail to reach people who have moved or are homeless, Ms. Serafi said. And email, if people have an account, can end up in spam folders.
In contrast, surveys show lower-income Americans are just as likely to have smartphones and cellphones as the general population. And most people regularly use texting.
In Michigan, Medicaid officials started using text messaging to communicate with enrollees in 2020 after building a system with the help of federal COVID relief funding. They said texting is an economical way to reach enrollees.
“It costs us 2 cents per text message, which is incredibly cheap,” said Steph White, an enrollment coordinator for the Michigan Department of Health and Human Services. “It’s a great return on investment.”
CMS officials have told states they should consider texting, along with other communication methods, when trying to reach enrollees when the public health emergency ends. But many states don’t have the technology or information about enrollees to do it.
Efforts to add texting also face legal barriers, including a federal law that bars texting people without their consent. The Federal Communications Commission ruled in 2021 that state agencies are exempt from the law, but whether counties that handle Medicaid duties for some states and Medicaid managed-care organizations that work in more than 40 states are exempt as well is unclear, said Matt Salo, executive director of the National Association of Medicaid Directors.
CMS spokesperson Beth Lynk said the agency is trying to figure out how Medicaid agencies, counties, and health plans can text enrollees within the constraints of federal law.
Several states told KHN that Medicaid health plans will be helping connect with enrollees and that they expect the plans to use text messaging. But the requirement to get consent from enrollees before texting could limit that effort.
That’s the situation in Virginia, where only about 30,000 Medicaid enrollees – out of more than a million – have agreed to receive text messages directly from the state, said spokesperson Christina Nuckols.
In an effort to boost that number, the state plans to ask enrollees if they want to opt out of receiving text messages, rather than ask them to opt in, she said. This way enrollees would contact the state only if they don’t want to be texted. The state is reviewing its legal options to make that happen.
Meanwhile, Ms. Nuckols added, the state expects Medicaid health plans to contact enrollees about updating their contact information. Four of Virginia’s six Medicaid plans, which serve the bulk of the state’s enrollees, have permission to text about 316,000.
Craig Kennedy, CEO of Medicaid Health Plans of America, a trade group, said that most plans are using texting and that Medicaid officials will use multiple strategies to connect with enrollees. “I do not see this as a detriment, that states are not texting information about reenrollment,” he said. “I know we will be helping with that.”
California officials in March directed Medicaid health plans to use a variety of communication methods, including texting, to ensure that members can retain coverage if they remain eligible. The officials told health plans they could ask for consent through an initial text.
California officials say they also plan to ask enrollees for consent to be texted on the enrollment application, although federal approval for the change is not expected until the fall.
A few state Medicaid programs have experimented in recent years with pilot programs that included texting enrollees.
In 2019, Louisiana worked with the nonprofit group Code for America to send text messages that reminded people about renewing coverage and providing income information for verification. Compared with traditional communication methods, the texts led to a 67% increase in enrollees being renewed for coverage and a 56% increase in enrollees verifying their income in response to inquiries, said Medicaid spokesperson Alyson Neel.
Nonetheless, the state isn’t planning to text Medicaid enrollees about the end of the public health emergency because it hasn’t set up a system for that. “Medicaid has not yet been able to implement a text messaging system of its own due to other agency priorities,” Ms. Neel said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
West Virginia will use the U.S. Postal Service and an online account in the summer of 2022 to connect with Medicaid enrollees about the expected end of the COVID public health emergency, which will put many recipients at risk of losing their coverage.
What West Virginia won’t do is use a form of communication that’s ubiquitous worldwide: text messaging.
“West Virginia isn’t set up to text its members,” Allison Adler, the state’s Medicaid spokesperson, wrote to KHN in an email.
Indeed, most states’ Medicaid programs won’t text enrollees despite the urgency to reach them about renewing their coverage. A KFF report published in March found just 11 states said they would use texting to alert Medicaid recipients about the end of the COVID public health emergency. In contrast, 33 states plan to use snail mail and at least 20 will reach out with individual or automated phone calls.
“It doesn’t make any sense when texting is how most people communicate today,” said Kinda Serafi, a partner with the consulting firm Manatt Health.
State Medicaid agencies for months have been preparing for the end of the public health emergency. As part of a COVID relief law approved in March 2020, Congress prohibited states from dropping anyone from Medicaid coverage unless they moved out of state during the public health emergency. When the emergency ends, state Medicaid officials must reevaluate each enrollee’s eligibility. Millions of people could lose their coverage if they earn too much or fail to provide the information needed to verify income or residency.
As of November, about 86 million people were enrolled in Medicaid, according to the Centers for Medicare & Medicaid Services. That’s up from 71 million in February 2020, before COVID began to ravage the nation.
West Virginia has more than 600,000 Medicaid enrollees. Adler said about 100,000 of them could lose their eligibility at the end of the public health emergency because either the state has determined they’re ineligible or they’ve failed to respond to requests that they update their income information.
“It’s frustrating that texting is a means to meet people where they are and that this has not been picked up more by states,” said Jennifer Wagner, director of Medicaid eligibility and enrollment for the Center on Budget and Policy Priorities, a Washington-based research group.
The problem with relying on the Postal Service is that a letter can get hidden in “junk” mail or can fail to reach people who have moved or are homeless, Ms. Serafi said. And email, if people have an account, can end up in spam folders.
In contrast, surveys show lower-income Americans are just as likely to have smartphones and cellphones as the general population. And most people regularly use texting.
In Michigan, Medicaid officials started using text messaging to communicate with enrollees in 2020 after building a system with the help of federal COVID relief funding. They said texting is an economical way to reach enrollees.
“It costs us 2 cents per text message, which is incredibly cheap,” said Steph White, an enrollment coordinator for the Michigan Department of Health and Human Services. “It’s a great return on investment.”
CMS officials have told states they should consider texting, along with other communication methods, when trying to reach enrollees when the public health emergency ends. But many states don’t have the technology or information about enrollees to do it.
Efforts to add texting also face legal barriers, including a federal law that bars texting people without their consent. The Federal Communications Commission ruled in 2021 that state agencies are exempt from the law, but whether counties that handle Medicaid duties for some states and Medicaid managed-care organizations that work in more than 40 states are exempt as well is unclear, said Matt Salo, executive director of the National Association of Medicaid Directors.
CMS spokesperson Beth Lynk said the agency is trying to figure out how Medicaid agencies, counties, and health plans can text enrollees within the constraints of federal law.
Several states told KHN that Medicaid health plans will be helping connect with enrollees and that they expect the plans to use text messaging. But the requirement to get consent from enrollees before texting could limit that effort.
That’s the situation in Virginia, where only about 30,000 Medicaid enrollees – out of more than a million – have agreed to receive text messages directly from the state, said spokesperson Christina Nuckols.
In an effort to boost that number, the state plans to ask enrollees if they want to opt out of receiving text messages, rather than ask them to opt in, she said. This way enrollees would contact the state only if they don’t want to be texted. The state is reviewing its legal options to make that happen.
Meanwhile, Ms. Nuckols added, the state expects Medicaid health plans to contact enrollees about updating their contact information. Four of Virginia’s six Medicaid plans, which serve the bulk of the state’s enrollees, have permission to text about 316,000.
Craig Kennedy, CEO of Medicaid Health Plans of America, a trade group, said that most plans are using texting and that Medicaid officials will use multiple strategies to connect with enrollees. “I do not see this as a detriment, that states are not texting information about reenrollment,” he said. “I know we will be helping with that.”
California officials in March directed Medicaid health plans to use a variety of communication methods, including texting, to ensure that members can retain coverage if they remain eligible. The officials told health plans they could ask for consent through an initial text.
California officials say they also plan to ask enrollees for consent to be texted on the enrollment application, although federal approval for the change is not expected until the fall.
A few state Medicaid programs have experimented in recent years with pilot programs that included texting enrollees.
In 2019, Louisiana worked with the nonprofit group Code for America to send text messages that reminded people about renewing coverage and providing income information for verification. Compared with traditional communication methods, the texts led to a 67% increase in enrollees being renewed for coverage and a 56% increase in enrollees verifying their income in response to inquiries, said Medicaid spokesperson Alyson Neel.
Nonetheless, the state isn’t planning to text Medicaid enrollees about the end of the public health emergency because it hasn’t set up a system for that. “Medicaid has not yet been able to implement a text messaging system of its own due to other agency priorities,” Ms. Neel said.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
Omicron BA.2: What do we know so far?
Omicron has 30 mutations of the spike protein, compared with the original Wuhan-Hu-1 variant, with 15 mutations of the receptor-binding domain (which are linked to a decrease in antibody binding), mutations at the furin S1/S2 site (which improves furin binding and increases infectiousness), and mutations of the amino terminal domain (which is the main binding site for some of the therapeutic antibodies used to treat COVID-19 infections).
Omicron’s functional characteristics
Non–peer-reviewed studies have shown a replication of Omicron in pulmonary epithelial cells, which was shown to be less efficient, when compared with Delta and Wuhan-Hu-1. The number of viral copies from an Omicron infection in pulmonary epithelial cells was significantly lower, compared with infection with the Delta or Wuhan-Hu-1 variants. The association of these characteristics found an increase in the number of viral copies in human epithelial cells (taken from the nasal airways) infected with Omicron. This supports the understanding that Omicron is more transmissible but results in a less severe manifestation of the disease.
As for the phenotypic expression of the infection, attention has been focused on Omicron’s reduced capacity to cause syncytia in pulmonary tissue cultures, information which is relevant to its clinical significance, if we consider that the formation of syncytia has been associated with a more severe manifestation of the disease. Furthermore, it has emerged that Omicron can use different cellular entry routes, with a preference for endosomal fusion over superficial cellular fusion. This characteristic allows Omicron to significantly increase the number of types of cells it can infect.
Omicron BA.2 evolves
Between November and December 2021, Omicron progressed, evolving into a variant with characteristics similar to those of its predecessors (that is, it underwent a gradual and progressive increase in transmissibility). Early studies on the Omicron variant were mainly based on the BA.1 subvariant. Since the start of January 2022, there has been an unexpected increase in BA.2 in Europe and Asia. Since then, continued surveillance on the evolution of Omicron has shown an increased prevalence of two subvariants: BA.1 with a R346K mutation (BA.1 + R346K) and B.1.1.529.2 (BA.2), with the latter containing eight unique spike mutations and 13 missing spike mutations, compared with those found in BA.1.
From these differences, we cannot presume that their antigenic properties are similar or different, but they seem to be antigenically equidistant from wild-type SARS-CoV-2, likely jeopardizing in equal measures the efficacy of current COVID-19 vaccines. Furthermore, BA.2 shows significant resistance to 17 out of 19 neutralizing monoclonal antibodies tested in this study, demonstrating that current monoclonal antibody therapy may have significant limitations in terms of adequate coverage for all subvariants of the Omicron variant.
Omicron BA.2 and reinfection
BA.2 initially represented only 13% of Omicron sequences at a global level, quickly becoming the dominant form in some countries, such as Denmark. At the end of 2021, BA.2 represented around 20% of all Danish cases of SARS-CoV-2. Halfway through January 2022, this had increased to around 45%, data that indicate that BA.2 carries an advantage over BA.1 within the highly vaccinated population of Denmark.
BA.2 is associated with an increased susceptibility of infection for unvaccinated individuals (odds ratio, 2.19; 95% confidence interval, 1.58-3.04), fully vaccinated individuals (OR, 2.45; 95% CI, 1.77-3.40), and booster-vaccinated individuals (OR, 2.99; 95% CI, 2.11-4.24), compared with BA.1. The pattern of increased transmissibility in BA.2 households was not observed for fully vaccinated and booster-vaccinated primary cases, where the OR of transmission was below 1 for BA.2, compared with BA.1. These data confirm the immune-evasive properties of BA.2 that further reduce the protective effect of vaccination against infection, but do not increase its transmissibility from vaccinated individuals with breakthrough infections.
Omicron, BA.2, and vaccination
The understanding of serum neutralizing activity, in correlation to the efficacy of a vaccine, is a priority of research because of the growing epidemiological significance of BA.2. There is evidence to support the claim that the immune-evasive nature of BA.2 doesn›t seem to be as severe as that of BA.1, and it is possible that there are other viral or host factors that are enabling the rapid diffusion of BA.2. A study published in Science Immunology investigated humoral and cellular immune responses to Omicron and other variants of concern (VOCs), looking to understand how, and to what degree, vaccinated individuals are protected against Omicron. From the results, a very low level of antibody cross-neutralization of Omicron, or a lack thereof, was seen when compared with wild type, Beta, and Delta variants, which could be partially restored by a third booster vaccination. Furthermore, T lymphocytes were shown to recognize Omicron with the same efficacy as seen for the other VOCs, suggesting that vaccinated individuals maintain T lymphocyte immunity, an element that is capable of providing protection in the absence of neutralizing antibodies, limiting the chance of serious disease.
These results are consistent with those available from a study performed in a population from Qatar made up of 2,239,193 people who had received at least two doses of a BNT162b2 or mRNA-1273 vaccine. The efficacy of the booster against a symptomatic Omicron infection, compared with that from the primary series, was 49.4% (95% CI, 47.1-51.6). The efficacy of the booster against hospitalization for COVID-19 and the death rate from Omicron infection, compared with the primary series, was 76.5% (95% CI, 55.9-87.5). The efficacy of the BNT162b2 booster against a symptomatic Delta variant infection (or B.1.617.2), compared with the primary series, was 86.1% (95% CI, 67.3-94.1).
To summarize, the constant increase in the prevalence of BA.2 in more countries over the world has confirmed the growth advantage that this variant has compared with others. BA.2 reduces the protective effect of vaccination against infection. Omicron antibody cross-neutralization can be partially restored by a third booster vaccination, an aspect that becomes problematic in the context of a low vaccination rate, where peaks of Omicron may increase the likelihood of infection in the elderly and in other groups at a higher risk of severe disease. Omicron BA.2 opens up new evolution channels, but what do the experts think will happen?
A version of this article was originally published in Italian on Univadis.
Omicron has 30 mutations of the spike protein, compared with the original Wuhan-Hu-1 variant, with 15 mutations of the receptor-binding domain (which are linked to a decrease in antibody binding), mutations at the furin S1/S2 site (which improves furin binding and increases infectiousness), and mutations of the amino terminal domain (which is the main binding site for some of the therapeutic antibodies used to treat COVID-19 infections).
Omicron’s functional characteristics
Non–peer-reviewed studies have shown a replication of Omicron in pulmonary epithelial cells, which was shown to be less efficient, when compared with Delta and Wuhan-Hu-1. The number of viral copies from an Omicron infection in pulmonary epithelial cells was significantly lower, compared with infection with the Delta or Wuhan-Hu-1 variants. The association of these characteristics found an increase in the number of viral copies in human epithelial cells (taken from the nasal airways) infected with Omicron. This supports the understanding that Omicron is more transmissible but results in a less severe manifestation of the disease.
As for the phenotypic expression of the infection, attention has been focused on Omicron’s reduced capacity to cause syncytia in pulmonary tissue cultures, information which is relevant to its clinical significance, if we consider that the formation of syncytia has been associated with a more severe manifestation of the disease. Furthermore, it has emerged that Omicron can use different cellular entry routes, with a preference for endosomal fusion over superficial cellular fusion. This characteristic allows Omicron to significantly increase the number of types of cells it can infect.
Omicron BA.2 evolves
Between November and December 2021, Omicron progressed, evolving into a variant with characteristics similar to those of its predecessors (that is, it underwent a gradual and progressive increase in transmissibility). Early studies on the Omicron variant were mainly based on the BA.1 subvariant. Since the start of January 2022, there has been an unexpected increase in BA.2 in Europe and Asia. Since then, continued surveillance on the evolution of Omicron has shown an increased prevalence of two subvariants: BA.1 with a R346K mutation (BA.1 + R346K) and B.1.1.529.2 (BA.2), with the latter containing eight unique spike mutations and 13 missing spike mutations, compared with those found in BA.1.
From these differences, we cannot presume that their antigenic properties are similar or different, but they seem to be antigenically equidistant from wild-type SARS-CoV-2, likely jeopardizing in equal measures the efficacy of current COVID-19 vaccines. Furthermore, BA.2 shows significant resistance to 17 out of 19 neutralizing monoclonal antibodies tested in this study, demonstrating that current monoclonal antibody therapy may have significant limitations in terms of adequate coverage for all subvariants of the Omicron variant.
Omicron BA.2 and reinfection
BA.2 initially represented only 13% of Omicron sequences at a global level, quickly becoming the dominant form in some countries, such as Denmark. At the end of 2021, BA.2 represented around 20% of all Danish cases of SARS-CoV-2. Halfway through January 2022, this had increased to around 45%, data that indicate that BA.2 carries an advantage over BA.1 within the highly vaccinated population of Denmark.
BA.2 is associated with an increased susceptibility of infection for unvaccinated individuals (odds ratio, 2.19; 95% confidence interval, 1.58-3.04), fully vaccinated individuals (OR, 2.45; 95% CI, 1.77-3.40), and booster-vaccinated individuals (OR, 2.99; 95% CI, 2.11-4.24), compared with BA.1. The pattern of increased transmissibility in BA.2 households was not observed for fully vaccinated and booster-vaccinated primary cases, where the OR of transmission was below 1 for BA.2, compared with BA.1. These data confirm the immune-evasive properties of BA.2 that further reduce the protective effect of vaccination against infection, but do not increase its transmissibility from vaccinated individuals with breakthrough infections.
Omicron, BA.2, and vaccination
The understanding of serum neutralizing activity, in correlation to the efficacy of a vaccine, is a priority of research because of the growing epidemiological significance of BA.2. There is evidence to support the claim that the immune-evasive nature of BA.2 doesn›t seem to be as severe as that of BA.1, and it is possible that there are other viral or host factors that are enabling the rapid diffusion of BA.2. A study published in Science Immunology investigated humoral and cellular immune responses to Omicron and other variants of concern (VOCs), looking to understand how, and to what degree, vaccinated individuals are protected against Omicron. From the results, a very low level of antibody cross-neutralization of Omicron, or a lack thereof, was seen when compared with wild type, Beta, and Delta variants, which could be partially restored by a third booster vaccination. Furthermore, T lymphocytes were shown to recognize Omicron with the same efficacy as seen for the other VOCs, suggesting that vaccinated individuals maintain T lymphocyte immunity, an element that is capable of providing protection in the absence of neutralizing antibodies, limiting the chance of serious disease.
These results are consistent with those available from a study performed in a population from Qatar made up of 2,239,193 people who had received at least two doses of a BNT162b2 or mRNA-1273 vaccine. The efficacy of the booster against a symptomatic Omicron infection, compared with that from the primary series, was 49.4% (95% CI, 47.1-51.6). The efficacy of the booster against hospitalization for COVID-19 and the death rate from Omicron infection, compared with the primary series, was 76.5% (95% CI, 55.9-87.5). The efficacy of the BNT162b2 booster against a symptomatic Delta variant infection (or B.1.617.2), compared with the primary series, was 86.1% (95% CI, 67.3-94.1).
To summarize, the constant increase in the prevalence of BA.2 in more countries over the world has confirmed the growth advantage that this variant has compared with others. BA.2 reduces the protective effect of vaccination against infection. Omicron antibody cross-neutralization can be partially restored by a third booster vaccination, an aspect that becomes problematic in the context of a low vaccination rate, where peaks of Omicron may increase the likelihood of infection in the elderly and in other groups at a higher risk of severe disease. Omicron BA.2 opens up new evolution channels, but what do the experts think will happen?
A version of this article was originally published in Italian on Univadis.
Omicron has 30 mutations of the spike protein, compared with the original Wuhan-Hu-1 variant, with 15 mutations of the receptor-binding domain (which are linked to a decrease in antibody binding), mutations at the furin S1/S2 site (which improves furin binding and increases infectiousness), and mutations of the amino terminal domain (which is the main binding site for some of the therapeutic antibodies used to treat COVID-19 infections).
Omicron’s functional characteristics
Non–peer-reviewed studies have shown a replication of Omicron in pulmonary epithelial cells, which was shown to be less efficient, when compared with Delta and Wuhan-Hu-1. The number of viral copies from an Omicron infection in pulmonary epithelial cells was significantly lower, compared with infection with the Delta or Wuhan-Hu-1 variants. The association of these characteristics found an increase in the number of viral copies in human epithelial cells (taken from the nasal airways) infected with Omicron. This supports the understanding that Omicron is more transmissible but results in a less severe manifestation of the disease.
As for the phenotypic expression of the infection, attention has been focused on Omicron’s reduced capacity to cause syncytia in pulmonary tissue cultures, information which is relevant to its clinical significance, if we consider that the formation of syncytia has been associated with a more severe manifestation of the disease. Furthermore, it has emerged that Omicron can use different cellular entry routes, with a preference for endosomal fusion over superficial cellular fusion. This characteristic allows Omicron to significantly increase the number of types of cells it can infect.
Omicron BA.2 evolves
Between November and December 2021, Omicron progressed, evolving into a variant with characteristics similar to those of its predecessors (that is, it underwent a gradual and progressive increase in transmissibility). Early studies on the Omicron variant were mainly based on the BA.1 subvariant. Since the start of January 2022, there has been an unexpected increase in BA.2 in Europe and Asia. Since then, continued surveillance on the evolution of Omicron has shown an increased prevalence of two subvariants: BA.1 with a R346K mutation (BA.1 + R346K) and B.1.1.529.2 (BA.2), with the latter containing eight unique spike mutations and 13 missing spike mutations, compared with those found in BA.1.
From these differences, we cannot presume that their antigenic properties are similar or different, but they seem to be antigenically equidistant from wild-type SARS-CoV-2, likely jeopardizing in equal measures the efficacy of current COVID-19 vaccines. Furthermore, BA.2 shows significant resistance to 17 out of 19 neutralizing monoclonal antibodies tested in this study, demonstrating that current monoclonal antibody therapy may have significant limitations in terms of adequate coverage for all subvariants of the Omicron variant.
Omicron BA.2 and reinfection
BA.2 initially represented only 13% of Omicron sequences at a global level, quickly becoming the dominant form in some countries, such as Denmark. At the end of 2021, BA.2 represented around 20% of all Danish cases of SARS-CoV-2. Halfway through January 2022, this had increased to around 45%, data that indicate that BA.2 carries an advantage over BA.1 within the highly vaccinated population of Denmark.
BA.2 is associated with an increased susceptibility of infection for unvaccinated individuals (odds ratio, 2.19; 95% confidence interval, 1.58-3.04), fully vaccinated individuals (OR, 2.45; 95% CI, 1.77-3.40), and booster-vaccinated individuals (OR, 2.99; 95% CI, 2.11-4.24), compared with BA.1. The pattern of increased transmissibility in BA.2 households was not observed for fully vaccinated and booster-vaccinated primary cases, where the OR of transmission was below 1 for BA.2, compared with BA.1. These data confirm the immune-evasive properties of BA.2 that further reduce the protective effect of vaccination against infection, but do not increase its transmissibility from vaccinated individuals with breakthrough infections.
Omicron, BA.2, and vaccination
The understanding of serum neutralizing activity, in correlation to the efficacy of a vaccine, is a priority of research because of the growing epidemiological significance of BA.2. There is evidence to support the claim that the immune-evasive nature of BA.2 doesn›t seem to be as severe as that of BA.1, and it is possible that there are other viral or host factors that are enabling the rapid diffusion of BA.2. A study published in Science Immunology investigated humoral and cellular immune responses to Omicron and other variants of concern (VOCs), looking to understand how, and to what degree, vaccinated individuals are protected against Omicron. From the results, a very low level of antibody cross-neutralization of Omicron, or a lack thereof, was seen when compared with wild type, Beta, and Delta variants, which could be partially restored by a third booster vaccination. Furthermore, T lymphocytes were shown to recognize Omicron with the same efficacy as seen for the other VOCs, suggesting that vaccinated individuals maintain T lymphocyte immunity, an element that is capable of providing protection in the absence of neutralizing antibodies, limiting the chance of serious disease.
These results are consistent with those available from a study performed in a population from Qatar made up of 2,239,193 people who had received at least two doses of a BNT162b2 or mRNA-1273 vaccine. The efficacy of the booster against a symptomatic Omicron infection, compared with that from the primary series, was 49.4% (95% CI, 47.1-51.6). The efficacy of the booster against hospitalization for COVID-19 and the death rate from Omicron infection, compared with the primary series, was 76.5% (95% CI, 55.9-87.5). The efficacy of the BNT162b2 booster against a symptomatic Delta variant infection (or B.1.617.2), compared with the primary series, was 86.1% (95% CI, 67.3-94.1).
To summarize, the constant increase in the prevalence of BA.2 in more countries over the world has confirmed the growth advantage that this variant has compared with others. BA.2 reduces the protective effect of vaccination against infection. Omicron antibody cross-neutralization can be partially restored by a third booster vaccination, an aspect that becomes problematic in the context of a low vaccination rate, where peaks of Omicron may increase the likelihood of infection in the elderly and in other groups at a higher risk of severe disease. Omicron BA.2 opens up new evolution channels, but what do the experts think will happen?
A version of this article was originally published in Italian on Univadis.
New York NPs join half of states with full practice authority
according to leading national nurse organizations.
New York joins 24 other states, the District of Columbia, and two U.S. territories that have adopted FPA legislation, as reported by the American Association of Nurse Practitioners (AANP). Like other states, New York has been under an emergency order during the pandemic that allowed NPs to practice to their full authority because of staffing shortages. That order was extended multiple times and was expected to expire this month, AANP reports.
“This has been in the making for nurse practitioners in New York since 2014, trying to get full practice authority,” Michelle Jones, RN, MSN, ANP-C, director at large for the New York State Nurses Association, said in an interview.
NPs who were allowed to practice independently during the pandemic campaigned for that provision to become permanent once the emergency order expired, she said. Ms. Jones explained that the FPA law expands the scope of practice and “removes unnecessary barriers,” namely an agreement with doctors to oversee NPs’ actions.
FPA gives NPs the authority to evaluate patients; diagnose, order, and interpret diagnostic tests; and initiate and manage treatments – including prescribing medications – without oversight by a doctor or state medical board, according to AANP.
Before the pandemic, New York NPs had “reduced” practice authority with those who had more than 3,600 hours of experience required to maintain a collaborative practice agreement with doctors and those with less experience maintaining a written agreement. The change gives full practice authority to those with more than 3,600 hours of experience, Stephen A. Ferrara, DNP, FNP-BC, AANP regional director, said in an interview.
Ferrara, who practices in New York, said the state is the largest to change to FPA. He said the state and others that have moved to FPA have determined that there “has been no lapse in quality care” during the emergency order period and that the regulatory barriers kept NPs from providing access to care.
Jones said that the law also will allow NPs to open private practices and serve underserved patients in areas that lack access to health care. “This is a step to improve access to health care and health equity of the New York population.”
It’s been a while since another state passed FPA legislation, Massachusetts in January 2021 and Delaware in August 2021, according to AANP.
Earlier this month, AANP released new data showing a 9% increase in NPs licensed to practice in the United States, rising from 325,000 in May 2021 to 355,000.
The New York legislation “will help New York attract and retain nurse practitioners and provide New Yorkers better access to quality care,” AANP President April Kapu, DNP, APRN, said in a statement.
A version of this article first appeared on Medscape.com.
according to leading national nurse organizations.
New York joins 24 other states, the District of Columbia, and two U.S. territories that have adopted FPA legislation, as reported by the American Association of Nurse Practitioners (AANP). Like other states, New York has been under an emergency order during the pandemic that allowed NPs to practice to their full authority because of staffing shortages. That order was extended multiple times and was expected to expire this month, AANP reports.
“This has been in the making for nurse practitioners in New York since 2014, trying to get full practice authority,” Michelle Jones, RN, MSN, ANP-C, director at large for the New York State Nurses Association, said in an interview.
NPs who were allowed to practice independently during the pandemic campaigned for that provision to become permanent once the emergency order expired, she said. Ms. Jones explained that the FPA law expands the scope of practice and “removes unnecessary barriers,” namely an agreement with doctors to oversee NPs’ actions.
FPA gives NPs the authority to evaluate patients; diagnose, order, and interpret diagnostic tests; and initiate and manage treatments – including prescribing medications – without oversight by a doctor or state medical board, according to AANP.
Before the pandemic, New York NPs had “reduced” practice authority with those who had more than 3,600 hours of experience required to maintain a collaborative practice agreement with doctors and those with less experience maintaining a written agreement. The change gives full practice authority to those with more than 3,600 hours of experience, Stephen A. Ferrara, DNP, FNP-BC, AANP regional director, said in an interview.
Ferrara, who practices in New York, said the state is the largest to change to FPA. He said the state and others that have moved to FPA have determined that there “has been no lapse in quality care” during the emergency order period and that the regulatory barriers kept NPs from providing access to care.
Jones said that the law also will allow NPs to open private practices and serve underserved patients in areas that lack access to health care. “This is a step to improve access to health care and health equity of the New York population.”
It’s been a while since another state passed FPA legislation, Massachusetts in January 2021 and Delaware in August 2021, according to AANP.
Earlier this month, AANP released new data showing a 9% increase in NPs licensed to practice in the United States, rising from 325,000 in May 2021 to 355,000.
The New York legislation “will help New York attract and retain nurse practitioners and provide New Yorkers better access to quality care,” AANP President April Kapu, DNP, APRN, said in a statement.
A version of this article first appeared on Medscape.com.
according to leading national nurse organizations.
New York joins 24 other states, the District of Columbia, and two U.S. territories that have adopted FPA legislation, as reported by the American Association of Nurse Practitioners (AANP). Like other states, New York has been under an emergency order during the pandemic that allowed NPs to practice to their full authority because of staffing shortages. That order was extended multiple times and was expected to expire this month, AANP reports.
“This has been in the making for nurse practitioners in New York since 2014, trying to get full practice authority,” Michelle Jones, RN, MSN, ANP-C, director at large for the New York State Nurses Association, said in an interview.
NPs who were allowed to practice independently during the pandemic campaigned for that provision to become permanent once the emergency order expired, she said. Ms. Jones explained that the FPA law expands the scope of practice and “removes unnecessary barriers,” namely an agreement with doctors to oversee NPs’ actions.
FPA gives NPs the authority to evaluate patients; diagnose, order, and interpret diagnostic tests; and initiate and manage treatments – including prescribing medications – without oversight by a doctor or state medical board, according to AANP.
Before the pandemic, New York NPs had “reduced” practice authority with those who had more than 3,600 hours of experience required to maintain a collaborative practice agreement with doctors and those with less experience maintaining a written agreement. The change gives full practice authority to those with more than 3,600 hours of experience, Stephen A. Ferrara, DNP, FNP-BC, AANP regional director, said in an interview.
Ferrara, who practices in New York, said the state is the largest to change to FPA. He said the state and others that have moved to FPA have determined that there “has been no lapse in quality care” during the emergency order period and that the regulatory barriers kept NPs from providing access to care.
Jones said that the law also will allow NPs to open private practices and serve underserved patients in areas that lack access to health care. “This is a step to improve access to health care and health equity of the New York population.”
It’s been a while since another state passed FPA legislation, Massachusetts in January 2021 and Delaware in August 2021, according to AANP.
Earlier this month, AANP released new data showing a 9% increase in NPs licensed to practice in the United States, rising from 325,000 in May 2021 to 355,000.
The New York legislation “will help New York attract and retain nurse practitioners and provide New Yorkers better access to quality care,” AANP President April Kapu, DNP, APRN, said in a statement.
A version of this article first appeared on Medscape.com.
Managing Heavy Menstrual Bleeding Associated with Fibroids
Kelsey Kennedy is a Family Nurse Practitioner (FNP) and has been working as a nurse practitioner in the Women's Health Institute at the Cleveland Clinic in General Gynecology for the past three years. She has her undergraduate degree from Saint Louis University and completed her graduate studies at Walsh University. She works with patients providing annual/wellness care, contraceptive counseling, abnormal bleeding evaluation and addresses many other non-OB gynecologic issues. The best part about her job is connecting with women and empowering the women she serves to be in control of their reproductive health and wellness.
As a nurse practitioner focused on benign gynecological treatment, what is your role as it relates to uterine fibroids, which are benign non-cancerous tumors?
Ms. Kennedy: As Nurse Practitioner (NP) working in GYN, I see both common and complex gynecologic, sexual, reproductive, menopausal issues. We really do it all. The NP collaborates with the entire medical health care team, including physicians, medical assistants, nursing, and administrators.
In the outpatient office practice with the Cleveland Clinic, I provide mostly benign or general GYN care, which means I see a lot of annual wellness exams, infection checks, birth control consults, and abnormal bleeding. I see patients that may have complaints of heavy periods, pelvic pain, pressure, but they might not have a formal diagnosis or know exactly why they have such heavy periods. Sometimes I see patients that have never even heard of fibroids. It’s my job to take a thorough history, perform a physical exam, and order any additional testing indicated to get the workup started to figure out exactly what's going on.
Abnormal uterine bleeding is defined as a change in the frequency, duration, or amount of menstrual bleeding. It's a common GYN complaint that affects anywhere from 10% to 30% of reproductive age women. In fact, abnormal bleeding is the reason for 1/3 of all outpatient GYN visits and a common cause of abnormal uterine bleeding is fibroids.
So just to review, fibroids are those benign, meaning non-cancerous, tumors made of smooth muscle tissue. Fibroids affect up to 40% of women of reproductive age. And by age 50, up to 70% of women have at least one fibroid. Fibroids are very common. It is important to know that only about 25% of fibroids are clinically significant or problematic enough to require intervention. With that being said, we know that fibroids can be asymptomatic, but often, they can cause pain and bleeding.
Some situations that occur that might make me suspect fibroids include a history of periods that are regular, once monthly, but progressively becoming heavier and heavier over time; periods that last longer than seven days; menstrual bleeding so heavy patients soak through overnight pads or their clothes during the day or overnight; menstrual bleeding with large clots; pelvic pain; pressure; urinary frequency, which could be related to fibroids pressing on the bladder; or constipation, which we know has many causes but can sometimes be related to fibroids pressing on the rectum. That’s it.
What impact does Long-Acting Reversible Contraception, or LARC, have on the uterus?
Ms. Kennedy: Generally speaking, Long-Acting Reversible Contraception, or LARC, is a broad category of birth control methods that provide contraception for an extended period of time-- anywhere from 3 to 10 years, depending on the type you choose. The best part about LARC options is they do not require user action. It's 99% effective in preventing unwanted pregnancy. I like to call it set-it-and-forget-it birth control.
The LARC options we have included are all the IUDs, which consists of the Paragard or copper IUD, and the three different hormonal IUDs-- the Mirena, Kyleena, and the Skyla. That also includes the Nexplanon arm implant. Out of all these LARC options, the Mirena IUD is the only one that’s FDA approved to treat heavy menstrual bleeding which can include heavy menstrual bleeding related to fibroids. The Mirena is FDA approved right now for seven years of use, which is a nice long time to get a lot of good benefit.
The Mirena IUD, specifically, is a T-shaped device that's placed in the uterus. It releases a steady local, meaning the hormone is released pretty much just in the uterus, amount of levonorgestrel, which is a second-generation synthetic progesterone. Basically, it's a hormone. The Mirena releases 20 micrograms of levonorgestrel into the uterus every day. What this hormone does is cause a dramatic reduction of blood flow by changing the endometrium, or the lining of the uterus.
The Mirena IUD was found to reduce blood loss by 86% after three months of use and by up to 97% after 12 months of use. This big reduction in bleeding subsequently leads to an increase in iron and hemoglobin levels in women with heavy bleeding and fibroids.
Hysterectomy has long been the definitive solution for abnormal bleeding that doesn't respond to our usual treatments. But since the Mirena was developed in the 1990s, more and more evidence has come to light that the Mirena can be a safe and effective medical alternative to hysterectomy. Many women benefit from and seek other management options for bleeding and heavy bleeding relating to fibroids other than hysterectomy because they might desire future childbearing, or they just might want to retain their uterus.
In addition, we also know that fibroids typically regress in menopause. Using the Mirena IUD is a great solution to control heavy bleeding that a woman can use until they're in menopause and no longer having periods. The Mirena IUD is much less invasive than a hysterectomy and has lower risk for complications.
What steps do you take to identify encounters that might impose challenges as it relates to the uterine structure?
Ms. Kennedy: One thing that may impose a challenge in using the Mirena IUD to manage heavy bleeding related to fibroids is the specific size and location of the fibroids. Submucosal fibroids, also called intracavitary fibroids, grow into the uterus. These submucosal fibroids grow just below the inner lining of the uterus. They often cause more bleeding and problems than other types of fibroids because they crowd the uterine space. If the submucosal fibroid is too big or filling up too much of the uterine cavity, there may not be enough room for us to place a Mirena IUD.
The rates of IUD expulsion are increased in patients with fibroids that distort the uterine cavity. That's one thing we definitely want to consider. I typically refer these patients to one of my GYN surgeon colleagues to determine if the submucosal fibroid should be removed hysteroscopically in the OR. Sometimes after removing these submucosal fibroids via hysteroscopy in the OR, the surgeons will place the Mirena IUD at the end of the case.
I think you touched on this a little bit, but are there methods, in addition to IUDs, that would assist in managing heavy menstrual bleeding associated with uterine fibroids?
Ms. Kennedy: Yes. We have several current methods to manage heavy menstrual bleeding associated with uterine fibroids. One method is simply expectant management or watchful waiting. This means that the amount of bleeding or pain a woman is having related to fibroids is neither severe nor debilitating for her, but we continue to monitor them closely. We usually review what criteria the patient should look out for that may indicate she needs to follow up on, for us to take a closer look at her fibroids. Typically, that would be worsening bleeding or worsening pain.
Some oral medication options are hormonal methods which can include combined oral birth control pills, oral progesterone pills, and sometimes we use injections such as Depo-Provera, which is typically used for birth control. Oral contraceptives can reduce bleeding associated with fibroids by about 40% to 50%.
We also have non-hormonal medications like tranexamic acid, or Lysteda, which is an antifibrinolytic agent that women take only during their monthly periods for up to five days. Women who have a history of clots cannot take this drug. However, for women who can safely use this medication, on average, Lysteda has been shown to reduce the amount of blood loss during monthly periods by about 40%. There are other medication options, including GnRH antagonists that I don't prescribe as often.
The Mirena IUD, as we've discussed, is also such a great option to reduce heavy menstrual bleeding in women which can reduce menstrual bleeding by 86% to 97%. That’s a big jump. For patients that might need procedural interventions or surgical approaches, that may be most appropriate if they are having bulk symptoms associated with their fibroids like pelvic pain, pelvic pressure, urinary, or frequency. These kinds of symptoms are caused by the sheer size of the fibroids or from the fibroids pressing on surrounding structures. Bulk symptoms often do not improve much with medications or the IUDs. Those options address bleeding associated with fibroids much better.
How likely are women to stay on these medications that have contraceptive benefits based on the impact it may have in relation to uterine fibroids?
Ms. Kennedy: In one study that examined the effectiveness of the Mirena IUD versus other medications, including oral progesterone therapy to manage heavy bleeding, 76% of women using the Mirena IUD wished to continue the treatment compared to 22% of women that wished to continue the oral progestin therapy.
In another prospective observational clinical study, 82.5% of women had improvement of heavy menstrual bleeding with the Mirena. They continued to use the Mirena after 12 months. The Mirena IUD is effective in controlling heavy menstrual bleeding related to fibroids in about 77% of cases. The most common side effect is menstrual spotting for a few months after insertion. But overall, we do see a pretty high user satisfaction rate.
American College of Obstetricians and Gynecologists. (2021). Management of symptomatic uterine leiomyomas. (Practice Bulletin 228). https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
Desai, R. M. (2012). Efficacy of levonorgestrel releasing intrauterine system for the treatment of menorrhagia due to benign uterine lesions in perimenopausal women. Journal of Mid-Life Health, 3(1), 20–23. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.98812
Machado, R. B., de Souza, I. M., Beltrame, A., Bernardes, C. R., Morimoto, M. S., & Santana, N. (2013). The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology, 29(5), 492–495. https://doi-org.proxy.library.kent.edu/10.3109/09513590.2013.769517
Osama Shawki, Amr Wahba, & Navneet Magon. (2013). Abnormal uterine bleeding in midlife: The role of levonorgestrel intrauterine system. Journal of Mid-Life Health, 4(1), 36–39. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.109634
Kelsey Kennedy is a Family Nurse Practitioner (FNP) and has been working as a nurse practitioner in the Women's Health Institute at the Cleveland Clinic in General Gynecology for the past three years. She has her undergraduate degree from Saint Louis University and completed her graduate studies at Walsh University. She works with patients providing annual/wellness care, contraceptive counseling, abnormal bleeding evaluation and addresses many other non-OB gynecologic issues. The best part about her job is connecting with women and empowering the women she serves to be in control of their reproductive health and wellness.
As a nurse practitioner focused on benign gynecological treatment, what is your role as it relates to uterine fibroids, which are benign non-cancerous tumors?
Ms. Kennedy: As Nurse Practitioner (NP) working in GYN, I see both common and complex gynecologic, sexual, reproductive, menopausal issues. We really do it all. The NP collaborates with the entire medical health care team, including physicians, medical assistants, nursing, and administrators.
In the outpatient office practice with the Cleveland Clinic, I provide mostly benign or general GYN care, which means I see a lot of annual wellness exams, infection checks, birth control consults, and abnormal bleeding. I see patients that may have complaints of heavy periods, pelvic pain, pressure, but they might not have a formal diagnosis or know exactly why they have such heavy periods. Sometimes I see patients that have never even heard of fibroids. It’s my job to take a thorough history, perform a physical exam, and order any additional testing indicated to get the workup started to figure out exactly what's going on.
Abnormal uterine bleeding is defined as a change in the frequency, duration, or amount of menstrual bleeding. It's a common GYN complaint that affects anywhere from 10% to 30% of reproductive age women. In fact, abnormal bleeding is the reason for 1/3 of all outpatient GYN visits and a common cause of abnormal uterine bleeding is fibroids.
So just to review, fibroids are those benign, meaning non-cancerous, tumors made of smooth muscle tissue. Fibroids affect up to 40% of women of reproductive age. And by age 50, up to 70% of women have at least one fibroid. Fibroids are very common. It is important to know that only about 25% of fibroids are clinically significant or problematic enough to require intervention. With that being said, we know that fibroids can be asymptomatic, but often, they can cause pain and bleeding.
Some situations that occur that might make me suspect fibroids include a history of periods that are regular, once monthly, but progressively becoming heavier and heavier over time; periods that last longer than seven days; menstrual bleeding so heavy patients soak through overnight pads or their clothes during the day or overnight; menstrual bleeding with large clots; pelvic pain; pressure; urinary frequency, which could be related to fibroids pressing on the bladder; or constipation, which we know has many causes but can sometimes be related to fibroids pressing on the rectum. That’s it.
What impact does Long-Acting Reversible Contraception, or LARC, have on the uterus?
Ms. Kennedy: Generally speaking, Long-Acting Reversible Contraception, or LARC, is a broad category of birth control methods that provide contraception for an extended period of time-- anywhere from 3 to 10 years, depending on the type you choose. The best part about LARC options is they do not require user action. It's 99% effective in preventing unwanted pregnancy. I like to call it set-it-and-forget-it birth control.
The LARC options we have included are all the IUDs, which consists of the Paragard or copper IUD, and the three different hormonal IUDs-- the Mirena, Kyleena, and the Skyla. That also includes the Nexplanon arm implant. Out of all these LARC options, the Mirena IUD is the only one that’s FDA approved to treat heavy menstrual bleeding which can include heavy menstrual bleeding related to fibroids. The Mirena is FDA approved right now for seven years of use, which is a nice long time to get a lot of good benefit.
The Mirena IUD, specifically, is a T-shaped device that's placed in the uterus. It releases a steady local, meaning the hormone is released pretty much just in the uterus, amount of levonorgestrel, which is a second-generation synthetic progesterone. Basically, it's a hormone. The Mirena releases 20 micrograms of levonorgestrel into the uterus every day. What this hormone does is cause a dramatic reduction of blood flow by changing the endometrium, or the lining of the uterus.
The Mirena IUD was found to reduce blood loss by 86% after three months of use and by up to 97% after 12 months of use. This big reduction in bleeding subsequently leads to an increase in iron and hemoglobin levels in women with heavy bleeding and fibroids.
Hysterectomy has long been the definitive solution for abnormal bleeding that doesn't respond to our usual treatments. But since the Mirena was developed in the 1990s, more and more evidence has come to light that the Mirena can be a safe and effective medical alternative to hysterectomy. Many women benefit from and seek other management options for bleeding and heavy bleeding relating to fibroids other than hysterectomy because they might desire future childbearing, or they just might want to retain their uterus.
In addition, we also know that fibroids typically regress in menopause. Using the Mirena IUD is a great solution to control heavy bleeding that a woman can use until they're in menopause and no longer having periods. The Mirena IUD is much less invasive than a hysterectomy and has lower risk for complications.
What steps do you take to identify encounters that might impose challenges as it relates to the uterine structure?
Ms. Kennedy: One thing that may impose a challenge in using the Mirena IUD to manage heavy bleeding related to fibroids is the specific size and location of the fibroids. Submucosal fibroids, also called intracavitary fibroids, grow into the uterus. These submucosal fibroids grow just below the inner lining of the uterus. They often cause more bleeding and problems than other types of fibroids because they crowd the uterine space. If the submucosal fibroid is too big or filling up too much of the uterine cavity, there may not be enough room for us to place a Mirena IUD.
The rates of IUD expulsion are increased in patients with fibroids that distort the uterine cavity. That's one thing we definitely want to consider. I typically refer these patients to one of my GYN surgeon colleagues to determine if the submucosal fibroid should be removed hysteroscopically in the OR. Sometimes after removing these submucosal fibroids via hysteroscopy in the OR, the surgeons will place the Mirena IUD at the end of the case.
I think you touched on this a little bit, but are there methods, in addition to IUDs, that would assist in managing heavy menstrual bleeding associated with uterine fibroids?
Ms. Kennedy: Yes. We have several current methods to manage heavy menstrual bleeding associated with uterine fibroids. One method is simply expectant management or watchful waiting. This means that the amount of bleeding or pain a woman is having related to fibroids is neither severe nor debilitating for her, but we continue to monitor them closely. We usually review what criteria the patient should look out for that may indicate she needs to follow up on, for us to take a closer look at her fibroids. Typically, that would be worsening bleeding or worsening pain.
Some oral medication options are hormonal methods which can include combined oral birth control pills, oral progesterone pills, and sometimes we use injections such as Depo-Provera, which is typically used for birth control. Oral contraceptives can reduce bleeding associated with fibroids by about 40% to 50%.
We also have non-hormonal medications like tranexamic acid, or Lysteda, which is an antifibrinolytic agent that women take only during their monthly periods for up to five days. Women who have a history of clots cannot take this drug. However, for women who can safely use this medication, on average, Lysteda has been shown to reduce the amount of blood loss during monthly periods by about 40%. There are other medication options, including GnRH antagonists that I don't prescribe as often.
The Mirena IUD, as we've discussed, is also such a great option to reduce heavy menstrual bleeding in women which can reduce menstrual bleeding by 86% to 97%. That’s a big jump. For patients that might need procedural interventions or surgical approaches, that may be most appropriate if they are having bulk symptoms associated with their fibroids like pelvic pain, pelvic pressure, urinary, or frequency. These kinds of symptoms are caused by the sheer size of the fibroids or from the fibroids pressing on surrounding structures. Bulk symptoms often do not improve much with medications or the IUDs. Those options address bleeding associated with fibroids much better.
How likely are women to stay on these medications that have contraceptive benefits based on the impact it may have in relation to uterine fibroids?
Ms. Kennedy: In one study that examined the effectiveness of the Mirena IUD versus other medications, including oral progesterone therapy to manage heavy bleeding, 76% of women using the Mirena IUD wished to continue the treatment compared to 22% of women that wished to continue the oral progestin therapy.
In another prospective observational clinical study, 82.5% of women had improvement of heavy menstrual bleeding with the Mirena. They continued to use the Mirena after 12 months. The Mirena IUD is effective in controlling heavy menstrual bleeding related to fibroids in about 77% of cases. The most common side effect is menstrual spotting for a few months after insertion. But overall, we do see a pretty high user satisfaction rate.
Kelsey Kennedy is a Family Nurse Practitioner (FNP) and has been working as a nurse practitioner in the Women's Health Institute at the Cleveland Clinic in General Gynecology for the past three years. She has her undergraduate degree from Saint Louis University and completed her graduate studies at Walsh University. She works with patients providing annual/wellness care, contraceptive counseling, abnormal bleeding evaluation and addresses many other non-OB gynecologic issues. The best part about her job is connecting with women and empowering the women she serves to be in control of their reproductive health and wellness.
As a nurse practitioner focused on benign gynecological treatment, what is your role as it relates to uterine fibroids, which are benign non-cancerous tumors?
Ms. Kennedy: As Nurse Practitioner (NP) working in GYN, I see both common and complex gynecologic, sexual, reproductive, menopausal issues. We really do it all. The NP collaborates with the entire medical health care team, including physicians, medical assistants, nursing, and administrators.
In the outpatient office practice with the Cleveland Clinic, I provide mostly benign or general GYN care, which means I see a lot of annual wellness exams, infection checks, birth control consults, and abnormal bleeding. I see patients that may have complaints of heavy periods, pelvic pain, pressure, but they might not have a formal diagnosis or know exactly why they have such heavy periods. Sometimes I see patients that have never even heard of fibroids. It’s my job to take a thorough history, perform a physical exam, and order any additional testing indicated to get the workup started to figure out exactly what's going on.
Abnormal uterine bleeding is defined as a change in the frequency, duration, or amount of menstrual bleeding. It's a common GYN complaint that affects anywhere from 10% to 30% of reproductive age women. In fact, abnormal bleeding is the reason for 1/3 of all outpatient GYN visits and a common cause of abnormal uterine bleeding is fibroids.
So just to review, fibroids are those benign, meaning non-cancerous, tumors made of smooth muscle tissue. Fibroids affect up to 40% of women of reproductive age. And by age 50, up to 70% of women have at least one fibroid. Fibroids are very common. It is important to know that only about 25% of fibroids are clinically significant or problematic enough to require intervention. With that being said, we know that fibroids can be asymptomatic, but often, they can cause pain and bleeding.
Some situations that occur that might make me suspect fibroids include a history of periods that are regular, once monthly, but progressively becoming heavier and heavier over time; periods that last longer than seven days; menstrual bleeding so heavy patients soak through overnight pads or their clothes during the day or overnight; menstrual bleeding with large clots; pelvic pain; pressure; urinary frequency, which could be related to fibroids pressing on the bladder; or constipation, which we know has many causes but can sometimes be related to fibroids pressing on the rectum. That’s it.
What impact does Long-Acting Reversible Contraception, or LARC, have on the uterus?
Ms. Kennedy: Generally speaking, Long-Acting Reversible Contraception, or LARC, is a broad category of birth control methods that provide contraception for an extended period of time-- anywhere from 3 to 10 years, depending on the type you choose. The best part about LARC options is they do not require user action. It's 99% effective in preventing unwanted pregnancy. I like to call it set-it-and-forget-it birth control.
The LARC options we have included are all the IUDs, which consists of the Paragard or copper IUD, and the three different hormonal IUDs-- the Mirena, Kyleena, and the Skyla. That also includes the Nexplanon arm implant. Out of all these LARC options, the Mirena IUD is the only one that’s FDA approved to treat heavy menstrual bleeding which can include heavy menstrual bleeding related to fibroids. The Mirena is FDA approved right now for seven years of use, which is a nice long time to get a lot of good benefit.
The Mirena IUD, specifically, is a T-shaped device that's placed in the uterus. It releases a steady local, meaning the hormone is released pretty much just in the uterus, amount of levonorgestrel, which is a second-generation synthetic progesterone. Basically, it's a hormone. The Mirena releases 20 micrograms of levonorgestrel into the uterus every day. What this hormone does is cause a dramatic reduction of blood flow by changing the endometrium, or the lining of the uterus.
The Mirena IUD was found to reduce blood loss by 86% after three months of use and by up to 97% after 12 months of use. This big reduction in bleeding subsequently leads to an increase in iron and hemoglobin levels in women with heavy bleeding and fibroids.
Hysterectomy has long been the definitive solution for abnormal bleeding that doesn't respond to our usual treatments. But since the Mirena was developed in the 1990s, more and more evidence has come to light that the Mirena can be a safe and effective medical alternative to hysterectomy. Many women benefit from and seek other management options for bleeding and heavy bleeding relating to fibroids other than hysterectomy because they might desire future childbearing, or they just might want to retain their uterus.
In addition, we also know that fibroids typically regress in menopause. Using the Mirena IUD is a great solution to control heavy bleeding that a woman can use until they're in menopause and no longer having periods. The Mirena IUD is much less invasive than a hysterectomy and has lower risk for complications.
What steps do you take to identify encounters that might impose challenges as it relates to the uterine structure?
Ms. Kennedy: One thing that may impose a challenge in using the Mirena IUD to manage heavy bleeding related to fibroids is the specific size and location of the fibroids. Submucosal fibroids, also called intracavitary fibroids, grow into the uterus. These submucosal fibroids grow just below the inner lining of the uterus. They often cause more bleeding and problems than other types of fibroids because they crowd the uterine space. If the submucosal fibroid is too big or filling up too much of the uterine cavity, there may not be enough room for us to place a Mirena IUD.
The rates of IUD expulsion are increased in patients with fibroids that distort the uterine cavity. That's one thing we definitely want to consider. I typically refer these patients to one of my GYN surgeon colleagues to determine if the submucosal fibroid should be removed hysteroscopically in the OR. Sometimes after removing these submucosal fibroids via hysteroscopy in the OR, the surgeons will place the Mirena IUD at the end of the case.
I think you touched on this a little bit, but are there methods, in addition to IUDs, that would assist in managing heavy menstrual bleeding associated with uterine fibroids?
Ms. Kennedy: Yes. We have several current methods to manage heavy menstrual bleeding associated with uterine fibroids. One method is simply expectant management or watchful waiting. This means that the amount of bleeding or pain a woman is having related to fibroids is neither severe nor debilitating for her, but we continue to monitor them closely. We usually review what criteria the patient should look out for that may indicate she needs to follow up on, for us to take a closer look at her fibroids. Typically, that would be worsening bleeding or worsening pain.
Some oral medication options are hormonal methods which can include combined oral birth control pills, oral progesterone pills, and sometimes we use injections such as Depo-Provera, which is typically used for birth control. Oral contraceptives can reduce bleeding associated with fibroids by about 40% to 50%.
We also have non-hormonal medications like tranexamic acid, or Lysteda, which is an antifibrinolytic agent that women take only during their monthly periods for up to five days. Women who have a history of clots cannot take this drug. However, for women who can safely use this medication, on average, Lysteda has been shown to reduce the amount of blood loss during monthly periods by about 40%. There are other medication options, including GnRH antagonists that I don't prescribe as often.
The Mirena IUD, as we've discussed, is also such a great option to reduce heavy menstrual bleeding in women which can reduce menstrual bleeding by 86% to 97%. That’s a big jump. For patients that might need procedural interventions or surgical approaches, that may be most appropriate if they are having bulk symptoms associated with their fibroids like pelvic pain, pelvic pressure, urinary, or frequency. These kinds of symptoms are caused by the sheer size of the fibroids or from the fibroids pressing on surrounding structures. Bulk symptoms often do not improve much with medications or the IUDs. Those options address bleeding associated with fibroids much better.
How likely are women to stay on these medications that have contraceptive benefits based on the impact it may have in relation to uterine fibroids?
Ms. Kennedy: In one study that examined the effectiveness of the Mirena IUD versus other medications, including oral progesterone therapy to manage heavy bleeding, 76% of women using the Mirena IUD wished to continue the treatment compared to 22% of women that wished to continue the oral progestin therapy.
In another prospective observational clinical study, 82.5% of women had improvement of heavy menstrual bleeding with the Mirena. They continued to use the Mirena after 12 months. The Mirena IUD is effective in controlling heavy menstrual bleeding related to fibroids in about 77% of cases. The most common side effect is menstrual spotting for a few months after insertion. But overall, we do see a pretty high user satisfaction rate.
American College of Obstetricians and Gynecologists. (2021). Management of symptomatic uterine leiomyomas. (Practice Bulletin 228). https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
Desai, R. M. (2012). Efficacy of levonorgestrel releasing intrauterine system for the treatment of menorrhagia due to benign uterine lesions in perimenopausal women. Journal of Mid-Life Health, 3(1), 20–23. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.98812
Machado, R. B., de Souza, I. M., Beltrame, A., Bernardes, C. R., Morimoto, M. S., & Santana, N. (2013). The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology, 29(5), 492–495. https://doi-org.proxy.library.kent.edu/10.3109/09513590.2013.769517
Osama Shawki, Amr Wahba, & Navneet Magon. (2013). Abnormal uterine bleeding in midlife: The role of levonorgestrel intrauterine system. Journal of Mid-Life Health, 4(1), 36–39. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.109634
American College of Obstetricians and Gynecologists. (2021). Management of symptomatic uterine leiomyomas. (Practice Bulletin 228). https://www.acog.org/clinical/clinical-guidance/practice-bulletin/articles/2021/06/management-of-symptomatic-uterine-leiomyomas
Desai, R. M. (2012). Efficacy of levonorgestrel releasing intrauterine system for the treatment of menorrhagia due to benign uterine lesions in perimenopausal women. Journal of Mid-Life Health, 3(1), 20–23. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.98812
Machado, R. B., de Souza, I. M., Beltrame, A., Bernardes, C. R., Morimoto, M. S., & Santana, N. (2013). The levonorgestrel-releasing intrauterine system: its effect on the number of hysterectomies performed in perimenopausal women with uterine fibroids. Gynecological Endocrinology : The Official Journal of the International Society of Gynecological Endocrinology, 29(5), 492–495. https://doi-org.proxy.library.kent.edu/10.3109/09513590.2013.769517
Osama Shawki, Amr Wahba, & Navneet Magon. (2013). Abnormal uterine bleeding in midlife: The role of levonorgestrel intrauterine system. Journal of Mid-Life Health, 4(1), 36–39. https://doi-org.proxy.library.kent.edu/10.4103/0976-7800.109634
Combo of SGLT2 inhibitor + GLP-1 RA boosts diabetes survival
WASHINGTON – Patients with type 2 diabetes and established atherosclerotic cardiovascular disease treated with both an sodium-glucose transporter 2 inhibitor and a glucagonlike peptide–1 receptor agonist had a significant 80% cut in their rate of all-cause death during 1-year follow-up, compared with matched patients treated with an agent from either class alone in an observational, retrospective study of more than 15,000 people in the U.S. Veterans Affairs health system.
For the study’s primary endpoint, the combined rate of all-cause death, nonfatal MI, or nonfatal stroke, combined treatment with both an agent from the sodium-glucose transporter 2 (SGLT2) inhibitor class and from the glucagonlike peptide–1 receptor agonist (GLP-1 RA) class linked with a significant, roughly 50% cut in events during 1-year follow-up, compared with patients treated with an agent from just one of these two classes, Persio D. Lopez, MD, reported at the annual scientific sessions of the American College of Cardiology.
This improvement in the combined endpoint outcome resulted entirely from reduced all-cause mortality. Dual treatment showed no significant association with the incidence of nonfatal MIs or strokes, compared with monotherapy, with rates that were nearly identical regardless of whether patients took one of the agents or both, said Dr. Lopez, a cardiologist at Mount Sinai Morningside and the James J. Peters VA Medical Center, both in New York.
Combining classes for hard-to-control diabetes
“We’re not sure what drives combined use” of agents from both drug classes in these types of patients, admitted Dr. Lopez during his talk. “Our hypothesis is that dual treatment is used in patients with harder-to-control diabetes.”
Salim S. Virani, MD, PhD, who practices in the VA system but was not involved with the study, agreed that this is the likely explanation for most instances of high-risk VA patients with diabetes who receive agents from both classes.
“I have a few patients” on both classes, usually “patients with higher starting A1c levels who need greater glycemic control,” said Dr. Virani, professor of medicine at Baylor College of Medicine and a cardiologist at the Michael E. DeBakey VA Medical Center, both in Houston.
U.S. use of either drug class, let alone both, in patients with type 2 diabetes is still struggling to gain traction in U.S. practice and remains limited to a minority of these patients, a prescribing pattern reflected in recent VA data. Analysis of more than half a million patients in the VA system with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) who received treatment at any of 130 VA medical centers throughout 2020 showed that 11% had received an SGLT2 inhibitor, and 8% a GLP-1 RA.
The most frequently used antidiabetes drug classes in these patients were insulin in 36%, biguanides in 47%, and sulfonylureas in 22%.
These data also showed a striking level of variability among the 130 VA centers, with some of the sites prescribing either an SGLT2 inhibitor or a GLP-1 RA to as few as about 3% each of these patients, while other centers had a roughly 10-fold higher prescription rate for each of about 25%-30% of their patients with type 2 diabetes and ASCVD.
Despite the overall modest level of use of both classes in these types of patients as recently as 2020, no barriers exist at the VA to prescribing an agent from one or both classes “if you provide a good reason” for a patient to receive the drugs, Dr. Virani said in an interview. He also predicted that use of both classes in these patients, including combination treatment, will likely soon expand.
‘A lot of interest’ in combining an SGLT2 inhibitor and a GLP-1 RA
“There will be a lot of interest in combing the two classes. It makes intuitive sense [to treat with both classes] because most patients with diabetes need more than one drug” for glycemic control, he noted. “Why not use two classes that each reduce a patient’s risk” for adverse outcomes involving ASCVD, heart failure, and renal dysfunction, added Dr. Virani.
The study run by Dr. Lopez and his associates used data collected in the National VA Database and included 121,156 patients with both type 2 diabetes and established ASCVD. Using propensity-score matching the researchers compiled three subgroups that each included 5,277 matched patients. One subgroup had patients prescribed an SGLT2 inhibitor, a second subgroup included patients on a GLP-1 RA, and a third subgroup had patients on agents from both classes. Patient matching relied on age, sex, left ventricular ejection fraction, hemoglobin A1c level, systolic blood pressure, and the presence of coronary artery disease or peripheral artery disease.
Patients included in the analysis averaged about 67 years of age; 97% were men, their average body mass index was about 34 kg/m2, their average A1c was about 7.9%, their average estimated glomerular filtration rate was about 55-66 mL/min per 1.73 m2, and their average left ventricular ejection fraction was about 55%. The database provided a median follow-up of 902 days (about 2.5 years). The prespecified primary endpoint focused on events that occurred during the first year of follow-up, but the investigators also ran a 3-year follow-up analysis on a post hoc basis.
The most common SGLT2 inhibitor received by these patients was empagliflozin (Jardiance), used on virtually everyone who received an agent from this class. In contrast, the GLP-1 RA drugs that patients received split more widely. The most prescribed agent was liraglutide (Victoza), followed by semaglutide (Ozempic), and dulaglutide (Trulicity), with fewer than 5% receiving exenatide (Bydureon, Byetta).
Regarding other treatments, about 97% of all patients received a statin, about 94% were on a renin-angiotensin system inhibitor, about 90% were on metformin, and roughly 75% were on insulin, aspirin, and a beta-blocker, with smaller numbers on other types of agents.
For the study’s primary endpoint, the 1-year incidence of combined ASCVD events including all-cause death, patients on agents from both classes had a significant 46% reduced rate compared with those on an SGLT2 inhibitor only, and a significant 49% reduced rate, compared with those on a GLP-1 RA only. These between-group separations broadened slightly during 3-year follow-up. Dr. Lopez did not report results of a direct comparison between patients on just an SGLT2 inhibitor and those on just a GLP-1 RA.
For the endpoint of all-cause death, those on combined treatment had a 1-year rate that was 83% below the rate among patients on only an SGLT2 inhibitor, and 81% below the rate among patients who received a GLP-1 RA but not the other class.
Dr. Lopez cautioned that selection bias could have influenced the outcomes of patients who received both classes rather than one or the other, and he also highlighted that the analysis relied on administrative data rather than information gleaned from more detailed medical records or prospectively collected findings and was limited by only including a very small number of women.
“Our results need to be validated in prospective studies,” he declared.
Dr. Lopez and Dr. Virani had no commercial disclosures.
WASHINGTON – Patients with type 2 diabetes and established atherosclerotic cardiovascular disease treated with both an sodium-glucose transporter 2 inhibitor and a glucagonlike peptide–1 receptor agonist had a significant 80% cut in their rate of all-cause death during 1-year follow-up, compared with matched patients treated with an agent from either class alone in an observational, retrospective study of more than 15,000 people in the U.S. Veterans Affairs health system.
For the study’s primary endpoint, the combined rate of all-cause death, nonfatal MI, or nonfatal stroke, combined treatment with both an agent from the sodium-glucose transporter 2 (SGLT2) inhibitor class and from the glucagonlike peptide–1 receptor agonist (GLP-1 RA) class linked with a significant, roughly 50% cut in events during 1-year follow-up, compared with patients treated with an agent from just one of these two classes, Persio D. Lopez, MD, reported at the annual scientific sessions of the American College of Cardiology.
This improvement in the combined endpoint outcome resulted entirely from reduced all-cause mortality. Dual treatment showed no significant association with the incidence of nonfatal MIs or strokes, compared with monotherapy, with rates that were nearly identical regardless of whether patients took one of the agents or both, said Dr. Lopez, a cardiologist at Mount Sinai Morningside and the James J. Peters VA Medical Center, both in New York.
Combining classes for hard-to-control diabetes
“We’re not sure what drives combined use” of agents from both drug classes in these types of patients, admitted Dr. Lopez during his talk. “Our hypothesis is that dual treatment is used in patients with harder-to-control diabetes.”
Salim S. Virani, MD, PhD, who practices in the VA system but was not involved with the study, agreed that this is the likely explanation for most instances of high-risk VA patients with diabetes who receive agents from both classes.
“I have a few patients” on both classes, usually “patients with higher starting A1c levels who need greater glycemic control,” said Dr. Virani, professor of medicine at Baylor College of Medicine and a cardiologist at the Michael E. DeBakey VA Medical Center, both in Houston.
U.S. use of either drug class, let alone both, in patients with type 2 diabetes is still struggling to gain traction in U.S. practice and remains limited to a minority of these patients, a prescribing pattern reflected in recent VA data. Analysis of more than half a million patients in the VA system with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) who received treatment at any of 130 VA medical centers throughout 2020 showed that 11% had received an SGLT2 inhibitor, and 8% a GLP-1 RA.
The most frequently used antidiabetes drug classes in these patients were insulin in 36%, biguanides in 47%, and sulfonylureas in 22%.
These data also showed a striking level of variability among the 130 VA centers, with some of the sites prescribing either an SGLT2 inhibitor or a GLP-1 RA to as few as about 3% each of these patients, while other centers had a roughly 10-fold higher prescription rate for each of about 25%-30% of their patients with type 2 diabetes and ASCVD.
Despite the overall modest level of use of both classes in these types of patients as recently as 2020, no barriers exist at the VA to prescribing an agent from one or both classes “if you provide a good reason” for a patient to receive the drugs, Dr. Virani said in an interview. He also predicted that use of both classes in these patients, including combination treatment, will likely soon expand.
‘A lot of interest’ in combining an SGLT2 inhibitor and a GLP-1 RA
“There will be a lot of interest in combing the two classes. It makes intuitive sense [to treat with both classes] because most patients with diabetes need more than one drug” for glycemic control, he noted. “Why not use two classes that each reduce a patient’s risk” for adverse outcomes involving ASCVD, heart failure, and renal dysfunction, added Dr. Virani.
The study run by Dr. Lopez and his associates used data collected in the National VA Database and included 121,156 patients with both type 2 diabetes and established ASCVD. Using propensity-score matching the researchers compiled three subgroups that each included 5,277 matched patients. One subgroup had patients prescribed an SGLT2 inhibitor, a second subgroup included patients on a GLP-1 RA, and a third subgroup had patients on agents from both classes. Patient matching relied on age, sex, left ventricular ejection fraction, hemoglobin A1c level, systolic blood pressure, and the presence of coronary artery disease or peripheral artery disease.
Patients included in the analysis averaged about 67 years of age; 97% were men, their average body mass index was about 34 kg/m2, their average A1c was about 7.9%, their average estimated glomerular filtration rate was about 55-66 mL/min per 1.73 m2, and their average left ventricular ejection fraction was about 55%. The database provided a median follow-up of 902 days (about 2.5 years). The prespecified primary endpoint focused on events that occurred during the first year of follow-up, but the investigators also ran a 3-year follow-up analysis on a post hoc basis.
The most common SGLT2 inhibitor received by these patients was empagliflozin (Jardiance), used on virtually everyone who received an agent from this class. In contrast, the GLP-1 RA drugs that patients received split more widely. The most prescribed agent was liraglutide (Victoza), followed by semaglutide (Ozempic), and dulaglutide (Trulicity), with fewer than 5% receiving exenatide (Bydureon, Byetta).
Regarding other treatments, about 97% of all patients received a statin, about 94% were on a renin-angiotensin system inhibitor, about 90% were on metformin, and roughly 75% were on insulin, aspirin, and a beta-blocker, with smaller numbers on other types of agents.
For the study’s primary endpoint, the 1-year incidence of combined ASCVD events including all-cause death, patients on agents from both classes had a significant 46% reduced rate compared with those on an SGLT2 inhibitor only, and a significant 49% reduced rate, compared with those on a GLP-1 RA only. These between-group separations broadened slightly during 3-year follow-up. Dr. Lopez did not report results of a direct comparison between patients on just an SGLT2 inhibitor and those on just a GLP-1 RA.
For the endpoint of all-cause death, those on combined treatment had a 1-year rate that was 83% below the rate among patients on only an SGLT2 inhibitor, and 81% below the rate among patients who received a GLP-1 RA but not the other class.
Dr. Lopez cautioned that selection bias could have influenced the outcomes of patients who received both classes rather than one or the other, and he also highlighted that the analysis relied on administrative data rather than information gleaned from more detailed medical records or prospectively collected findings and was limited by only including a very small number of women.
“Our results need to be validated in prospective studies,” he declared.
Dr. Lopez and Dr. Virani had no commercial disclosures.
WASHINGTON – Patients with type 2 diabetes and established atherosclerotic cardiovascular disease treated with both an sodium-glucose transporter 2 inhibitor and a glucagonlike peptide–1 receptor agonist had a significant 80% cut in their rate of all-cause death during 1-year follow-up, compared with matched patients treated with an agent from either class alone in an observational, retrospective study of more than 15,000 people in the U.S. Veterans Affairs health system.
For the study’s primary endpoint, the combined rate of all-cause death, nonfatal MI, or nonfatal stroke, combined treatment with both an agent from the sodium-glucose transporter 2 (SGLT2) inhibitor class and from the glucagonlike peptide–1 receptor agonist (GLP-1 RA) class linked with a significant, roughly 50% cut in events during 1-year follow-up, compared with patients treated with an agent from just one of these two classes, Persio D. Lopez, MD, reported at the annual scientific sessions of the American College of Cardiology.
This improvement in the combined endpoint outcome resulted entirely from reduced all-cause mortality. Dual treatment showed no significant association with the incidence of nonfatal MIs or strokes, compared with monotherapy, with rates that were nearly identical regardless of whether patients took one of the agents or both, said Dr. Lopez, a cardiologist at Mount Sinai Morningside and the James J. Peters VA Medical Center, both in New York.
Combining classes for hard-to-control diabetes
“We’re not sure what drives combined use” of agents from both drug classes in these types of patients, admitted Dr. Lopez during his talk. “Our hypothesis is that dual treatment is used in patients with harder-to-control diabetes.”
Salim S. Virani, MD, PhD, who practices in the VA system but was not involved with the study, agreed that this is the likely explanation for most instances of high-risk VA patients with diabetes who receive agents from both classes.
“I have a few patients” on both classes, usually “patients with higher starting A1c levels who need greater glycemic control,” said Dr. Virani, professor of medicine at Baylor College of Medicine and a cardiologist at the Michael E. DeBakey VA Medical Center, both in Houston.
U.S. use of either drug class, let alone both, in patients with type 2 diabetes is still struggling to gain traction in U.S. practice and remains limited to a minority of these patients, a prescribing pattern reflected in recent VA data. Analysis of more than half a million patients in the VA system with type 2 diabetes and atherosclerotic cardiovascular disease (ASCVD) who received treatment at any of 130 VA medical centers throughout 2020 showed that 11% had received an SGLT2 inhibitor, and 8% a GLP-1 RA.
The most frequently used antidiabetes drug classes in these patients were insulin in 36%, biguanides in 47%, and sulfonylureas in 22%.
These data also showed a striking level of variability among the 130 VA centers, with some of the sites prescribing either an SGLT2 inhibitor or a GLP-1 RA to as few as about 3% each of these patients, while other centers had a roughly 10-fold higher prescription rate for each of about 25%-30% of their patients with type 2 diabetes and ASCVD.
Despite the overall modest level of use of both classes in these types of patients as recently as 2020, no barriers exist at the VA to prescribing an agent from one or both classes “if you provide a good reason” for a patient to receive the drugs, Dr. Virani said in an interview. He also predicted that use of both classes in these patients, including combination treatment, will likely soon expand.
‘A lot of interest’ in combining an SGLT2 inhibitor and a GLP-1 RA
“There will be a lot of interest in combing the two classes. It makes intuitive sense [to treat with both classes] because most patients with diabetes need more than one drug” for glycemic control, he noted. “Why not use two classes that each reduce a patient’s risk” for adverse outcomes involving ASCVD, heart failure, and renal dysfunction, added Dr. Virani.
The study run by Dr. Lopez and his associates used data collected in the National VA Database and included 121,156 patients with both type 2 diabetes and established ASCVD. Using propensity-score matching the researchers compiled three subgroups that each included 5,277 matched patients. One subgroup had patients prescribed an SGLT2 inhibitor, a second subgroup included patients on a GLP-1 RA, and a third subgroup had patients on agents from both classes. Patient matching relied on age, sex, left ventricular ejection fraction, hemoglobin A1c level, systolic blood pressure, and the presence of coronary artery disease or peripheral artery disease.
Patients included in the analysis averaged about 67 years of age; 97% were men, their average body mass index was about 34 kg/m2, their average A1c was about 7.9%, their average estimated glomerular filtration rate was about 55-66 mL/min per 1.73 m2, and their average left ventricular ejection fraction was about 55%. The database provided a median follow-up of 902 days (about 2.5 years). The prespecified primary endpoint focused on events that occurred during the first year of follow-up, but the investigators also ran a 3-year follow-up analysis on a post hoc basis.
The most common SGLT2 inhibitor received by these patients was empagliflozin (Jardiance), used on virtually everyone who received an agent from this class. In contrast, the GLP-1 RA drugs that patients received split more widely. The most prescribed agent was liraglutide (Victoza), followed by semaglutide (Ozempic), and dulaglutide (Trulicity), with fewer than 5% receiving exenatide (Bydureon, Byetta).
Regarding other treatments, about 97% of all patients received a statin, about 94% were on a renin-angiotensin system inhibitor, about 90% were on metformin, and roughly 75% were on insulin, aspirin, and a beta-blocker, with smaller numbers on other types of agents.
For the study’s primary endpoint, the 1-year incidence of combined ASCVD events including all-cause death, patients on agents from both classes had a significant 46% reduced rate compared with those on an SGLT2 inhibitor only, and a significant 49% reduced rate, compared with those on a GLP-1 RA only. These between-group separations broadened slightly during 3-year follow-up. Dr. Lopez did not report results of a direct comparison between patients on just an SGLT2 inhibitor and those on just a GLP-1 RA.
For the endpoint of all-cause death, those on combined treatment had a 1-year rate that was 83% below the rate among patients on only an SGLT2 inhibitor, and 81% below the rate among patients who received a GLP-1 RA but not the other class.
Dr. Lopez cautioned that selection bias could have influenced the outcomes of patients who received both classes rather than one or the other, and he also highlighted that the analysis relied on administrative data rather than information gleaned from more detailed medical records or prospectively collected findings and was limited by only including a very small number of women.
“Our results need to be validated in prospective studies,” he declared.
Dr. Lopez and Dr. Virani had no commercial disclosures.
AT ACC 2022
Cardiac issues after COVID infection and vaccination: New data
The new information comes from the Centers for Disease Control and Prevention’s National Patient-Centered Clinical Research Network (PCORnet) and from a separate large international clinical study published online in Circulation.
CDC data
The CDC study analyzed electronic health record data from 40 U.S. health care systems from Jan. 1, 2021, to Jan. 31, 2022, on more than 15 million people aged 5 years or older.
It reports a rate of myocarditis or pericarditis after mRNA COVID-19 vaccination of 0-35.9 per 100,000 for males and 0-10.9 per 100,000 for females across different age groups and vaccine cohorts.
Rates of myocarditis or pericarditis after SARS-CoV-2 infection ranged from 12.6 to 114 per 100,000 for males and from 5.4 to 61.7 per 100,000 for females across different age groups.
Even among males aged 12-17 years, the group with the highest incidence of cardiac complications after receipt of a second mRNA COVID-19 vaccine dose, the risk was 1.8-5.6 times higher after SARS-CoV-2 infection than after vaccination, the CDC report notes.
“These findings provide important context for balancing risks and benefits of mRNA COVID-19 vaccination among eligible persons greater than or equal to 5 years,” the report states. They also “support the continued use of recommended mRNA vaccines among all eligible persons aged greater than or equal to 5 years,” it concludes.
International study
The international study focused on prevalence, clinical characteristics, and outcomes of clinically manifest acute myocarditis in patients with COVID-19 infection.
The study showed a rate of acute myocarditis of 2.4 per 1,000 patients hospitalized with COVID-19.
“A small study previously indicated acute myocarditis is a rare occurrence in people infected with COVID-19. Our analysis of international data offers better insight to the occurrence of acute myocarditis during COVID-19 hospitalization, particularly before the COVID-19 vaccines were widely available,” coauthor Enrico Ammirati, MD, PhD, Niguarda Hospital, Milan, commented.
“This analysis indicates that, although rare, hospitalized patients with acute myocarditis associated with COVID-19 infection have a much greater need for intensive care unit admission, in up to 70.5% of the cases, despite the average age of the individuals in the study being much younger than expected, at 38 years old,” added coauthor Marco Metra, MD, University of Brescia, Italy.
The researchers report that the use of corticosteroids in patients with acute myocarditis appeared safe, and, in most cases, a rapid increase in the left ventricular ejection fraction was observed. In addition, they say that discharged patients with acute myocarditis had “an excellent short-term prognosis without occurrence of cardiovascular events.”
The authors also point out that these data show much higher frequency and severity of acute myocarditis linked to COVID-19 infection, compared with myocarditis cases linked to the mRNA COVID-19 vaccines.
The international study examined health data on 56,963 patients who were hospitalized with COVID-19 at 23 hospitals across the United States and Europe from February 2020 through April 2021.
Among these patients, 97 with possible acute myocarditis were identified (4.1 per 1,000), of whom 54 (2.4 per 1,000) were classified as having “definite or probable” acute myocarditis supported by endomyocardial biopsy (31.5% of cases) or magnetic resonance imaging (92.6% of cases).
The median age of definite/probable acute myocarditis cases was 38 years, and 39% were female. On admission, chest pain and dyspnea were the most frequent symptoms (55.5% and 53.7%, respectively), and 31 cases (57.4%) occurred in the absence of COVID-19–associated pneumonia. A fulminant presentation requiring inotropic support or temporary mechanical circulatory support occurred in 21 cases (39%).
Overall, 38 patients (70.4%) were admitted to the intensive care unit for a median time of 6 days. Ten patients (18.5%) received temporary mechanical circulatory support for a median time of 5 days. Three patients died (5.5%) during the index hospitalization, all of whom also had pneumonia. At 120 days, estimated mortality was 6.6%. Patients with pneumonia were more likely to develop hemodynamic instability, require mechanical circulatory support, and die, compared with those without pneumonia.
The authors note that their reported prevalence of acute myocarditis associated with COVID-19 is lower, compared with studies that performed universal cardiac MRI screening during the convalescent COVID-19 period.
They say that underestimation of the prevalence of mild or subclinical acute myocarditis is likely in this study because of the retrospective nature of the registry, the lack of systematic cardiac MRI, and the possibility of missing some diagnoses, particularly during the first pandemic wave when cardiac MRI and endomyocardial biopsy were less frequently performed.
The authors also point out that data on myocarditis after COVID-19 vaccination suggest that vaccination-linked myocarditis is milder than that associated with the virus itself.
With regard to the prevalence of acute myocarditis after vaccination, they report that among 2.8 million doses of mRNA COVID-19 vaccine in the armed forces, 23 individuals had evidence of acute myocarditis, suggesting a prevalence of less than 1 case of acute myocarditis per 100,000 mRNA COVID-19 vaccine doses.
They note that the CDC has also reported 399 reports of myocarditis among 129 million fully vaccinated individuals with the mRNA COVID-19 vaccines.
“These figures appear reassuring, compared with the prevalence of clinically manifest acute myocarditis observed in this study among hospitalized patients with COVID-19,” they conclude.
A version of this article first appeared on Medscape.com.
The new information comes from the Centers for Disease Control and Prevention’s National Patient-Centered Clinical Research Network (PCORnet) and from a separate large international clinical study published online in Circulation.
CDC data
The CDC study analyzed electronic health record data from 40 U.S. health care systems from Jan. 1, 2021, to Jan. 31, 2022, on more than 15 million people aged 5 years or older.
It reports a rate of myocarditis or pericarditis after mRNA COVID-19 vaccination of 0-35.9 per 100,000 for males and 0-10.9 per 100,000 for females across different age groups and vaccine cohorts.
Rates of myocarditis or pericarditis after SARS-CoV-2 infection ranged from 12.6 to 114 per 100,000 for males and from 5.4 to 61.7 per 100,000 for females across different age groups.
Even among males aged 12-17 years, the group with the highest incidence of cardiac complications after receipt of a second mRNA COVID-19 vaccine dose, the risk was 1.8-5.6 times higher after SARS-CoV-2 infection than after vaccination, the CDC report notes.
“These findings provide important context for balancing risks and benefits of mRNA COVID-19 vaccination among eligible persons greater than or equal to 5 years,” the report states. They also “support the continued use of recommended mRNA vaccines among all eligible persons aged greater than or equal to 5 years,” it concludes.
International study
The international study focused on prevalence, clinical characteristics, and outcomes of clinically manifest acute myocarditis in patients with COVID-19 infection.
The study showed a rate of acute myocarditis of 2.4 per 1,000 patients hospitalized with COVID-19.
“A small study previously indicated acute myocarditis is a rare occurrence in people infected with COVID-19. Our analysis of international data offers better insight to the occurrence of acute myocarditis during COVID-19 hospitalization, particularly before the COVID-19 vaccines were widely available,” coauthor Enrico Ammirati, MD, PhD, Niguarda Hospital, Milan, commented.
“This analysis indicates that, although rare, hospitalized patients with acute myocarditis associated with COVID-19 infection have a much greater need for intensive care unit admission, in up to 70.5% of the cases, despite the average age of the individuals in the study being much younger than expected, at 38 years old,” added coauthor Marco Metra, MD, University of Brescia, Italy.
The researchers report that the use of corticosteroids in patients with acute myocarditis appeared safe, and, in most cases, a rapid increase in the left ventricular ejection fraction was observed. In addition, they say that discharged patients with acute myocarditis had “an excellent short-term prognosis without occurrence of cardiovascular events.”
The authors also point out that these data show much higher frequency and severity of acute myocarditis linked to COVID-19 infection, compared with myocarditis cases linked to the mRNA COVID-19 vaccines.
The international study examined health data on 56,963 patients who were hospitalized with COVID-19 at 23 hospitals across the United States and Europe from February 2020 through April 2021.
Among these patients, 97 with possible acute myocarditis were identified (4.1 per 1,000), of whom 54 (2.4 per 1,000) were classified as having “definite or probable” acute myocarditis supported by endomyocardial biopsy (31.5% of cases) or magnetic resonance imaging (92.6% of cases).
The median age of definite/probable acute myocarditis cases was 38 years, and 39% were female. On admission, chest pain and dyspnea were the most frequent symptoms (55.5% and 53.7%, respectively), and 31 cases (57.4%) occurred in the absence of COVID-19–associated pneumonia. A fulminant presentation requiring inotropic support or temporary mechanical circulatory support occurred in 21 cases (39%).
Overall, 38 patients (70.4%) were admitted to the intensive care unit for a median time of 6 days. Ten patients (18.5%) received temporary mechanical circulatory support for a median time of 5 days. Three patients died (5.5%) during the index hospitalization, all of whom also had pneumonia. At 120 days, estimated mortality was 6.6%. Patients with pneumonia were more likely to develop hemodynamic instability, require mechanical circulatory support, and die, compared with those without pneumonia.
The authors note that their reported prevalence of acute myocarditis associated with COVID-19 is lower, compared with studies that performed universal cardiac MRI screening during the convalescent COVID-19 period.
They say that underestimation of the prevalence of mild or subclinical acute myocarditis is likely in this study because of the retrospective nature of the registry, the lack of systematic cardiac MRI, and the possibility of missing some diagnoses, particularly during the first pandemic wave when cardiac MRI and endomyocardial biopsy were less frequently performed.
The authors also point out that data on myocarditis after COVID-19 vaccination suggest that vaccination-linked myocarditis is milder than that associated with the virus itself.
With regard to the prevalence of acute myocarditis after vaccination, they report that among 2.8 million doses of mRNA COVID-19 vaccine in the armed forces, 23 individuals had evidence of acute myocarditis, suggesting a prevalence of less than 1 case of acute myocarditis per 100,000 mRNA COVID-19 vaccine doses.
They note that the CDC has also reported 399 reports of myocarditis among 129 million fully vaccinated individuals with the mRNA COVID-19 vaccines.
“These figures appear reassuring, compared with the prevalence of clinically manifest acute myocarditis observed in this study among hospitalized patients with COVID-19,” they conclude.
A version of this article first appeared on Medscape.com.
The new information comes from the Centers for Disease Control and Prevention’s National Patient-Centered Clinical Research Network (PCORnet) and from a separate large international clinical study published online in Circulation.
CDC data
The CDC study analyzed electronic health record data from 40 U.S. health care systems from Jan. 1, 2021, to Jan. 31, 2022, on more than 15 million people aged 5 years or older.
It reports a rate of myocarditis or pericarditis after mRNA COVID-19 vaccination of 0-35.9 per 100,000 for males and 0-10.9 per 100,000 for females across different age groups and vaccine cohorts.
Rates of myocarditis or pericarditis after SARS-CoV-2 infection ranged from 12.6 to 114 per 100,000 for males and from 5.4 to 61.7 per 100,000 for females across different age groups.
Even among males aged 12-17 years, the group with the highest incidence of cardiac complications after receipt of a second mRNA COVID-19 vaccine dose, the risk was 1.8-5.6 times higher after SARS-CoV-2 infection than after vaccination, the CDC report notes.
“These findings provide important context for balancing risks and benefits of mRNA COVID-19 vaccination among eligible persons greater than or equal to 5 years,” the report states. They also “support the continued use of recommended mRNA vaccines among all eligible persons aged greater than or equal to 5 years,” it concludes.
International study
The international study focused on prevalence, clinical characteristics, and outcomes of clinically manifest acute myocarditis in patients with COVID-19 infection.
The study showed a rate of acute myocarditis of 2.4 per 1,000 patients hospitalized with COVID-19.
“A small study previously indicated acute myocarditis is a rare occurrence in people infected with COVID-19. Our analysis of international data offers better insight to the occurrence of acute myocarditis during COVID-19 hospitalization, particularly before the COVID-19 vaccines were widely available,” coauthor Enrico Ammirati, MD, PhD, Niguarda Hospital, Milan, commented.
“This analysis indicates that, although rare, hospitalized patients with acute myocarditis associated with COVID-19 infection have a much greater need for intensive care unit admission, in up to 70.5% of the cases, despite the average age of the individuals in the study being much younger than expected, at 38 years old,” added coauthor Marco Metra, MD, University of Brescia, Italy.
The researchers report that the use of corticosteroids in patients with acute myocarditis appeared safe, and, in most cases, a rapid increase in the left ventricular ejection fraction was observed. In addition, they say that discharged patients with acute myocarditis had “an excellent short-term prognosis without occurrence of cardiovascular events.”
The authors also point out that these data show much higher frequency and severity of acute myocarditis linked to COVID-19 infection, compared with myocarditis cases linked to the mRNA COVID-19 vaccines.
The international study examined health data on 56,963 patients who were hospitalized with COVID-19 at 23 hospitals across the United States and Europe from February 2020 through April 2021.
Among these patients, 97 with possible acute myocarditis were identified (4.1 per 1,000), of whom 54 (2.4 per 1,000) were classified as having “definite or probable” acute myocarditis supported by endomyocardial biopsy (31.5% of cases) or magnetic resonance imaging (92.6% of cases).
The median age of definite/probable acute myocarditis cases was 38 years, and 39% were female. On admission, chest pain and dyspnea were the most frequent symptoms (55.5% and 53.7%, respectively), and 31 cases (57.4%) occurred in the absence of COVID-19–associated pneumonia. A fulminant presentation requiring inotropic support or temporary mechanical circulatory support occurred in 21 cases (39%).
Overall, 38 patients (70.4%) were admitted to the intensive care unit for a median time of 6 days. Ten patients (18.5%) received temporary mechanical circulatory support for a median time of 5 days. Three patients died (5.5%) during the index hospitalization, all of whom also had pneumonia. At 120 days, estimated mortality was 6.6%. Patients with pneumonia were more likely to develop hemodynamic instability, require mechanical circulatory support, and die, compared with those without pneumonia.
The authors note that their reported prevalence of acute myocarditis associated with COVID-19 is lower, compared with studies that performed universal cardiac MRI screening during the convalescent COVID-19 period.
They say that underestimation of the prevalence of mild or subclinical acute myocarditis is likely in this study because of the retrospective nature of the registry, the lack of systematic cardiac MRI, and the possibility of missing some diagnoses, particularly during the first pandemic wave when cardiac MRI and endomyocardial biopsy were less frequently performed.
The authors also point out that data on myocarditis after COVID-19 vaccination suggest that vaccination-linked myocarditis is milder than that associated with the virus itself.
With regard to the prevalence of acute myocarditis after vaccination, they report that among 2.8 million doses of mRNA COVID-19 vaccine in the armed forces, 23 individuals had evidence of acute myocarditis, suggesting a prevalence of less than 1 case of acute myocarditis per 100,000 mRNA COVID-19 vaccine doses.
They note that the CDC has also reported 399 reports of myocarditis among 129 million fully vaccinated individuals with the mRNA COVID-19 vaccines.
“These figures appear reassuring, compared with the prevalence of clinically manifest acute myocarditis observed in this study among hospitalized patients with COVID-19,” they conclude.
A version of this article first appeared on Medscape.com.