User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Fourth Pfizer dose better for severe than symptomatic COVID: Study
A fourth dose of the Pfizer-BioNTech vaccine is effective in reducing the short-term risk for COVID-19 infection, hospitalization, and death in people who got a third dose at least 4 months before, a large study shows.
However, Paul Offit, MD, author of an editorial accompanying the study, told this news organization, “I would argue, without fear of contradiction, that this is going to have no impact on this pandemic.”
“We are still in the midst of a zero-tolerance policy for this virus. We don’t accept mild illness and if we’re not going to accept mild illness, we think we have to boost it away, which would mean probably about two doses every year. That’s not a reasonable public health strategy,” said Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Booster confusion
Results of the research out of Israel, published in the New England Journal of Medicine, make a case for a fourth booster for people 60 and over.
Researchers, led by Ori Magen, MD, Clalit Research Institute, innovation division, Clalit Health Services, Tel Aviv, analyzed data comparing 182,122 matched pairs recorded by the largest health care organization in Israel from Jan. 3 to Feb. 18, 2022. With more than 4.7 million members, Clalit Health Services covers more than half of the population of Israel.
The researchers compared outcomes in people 60 or older (average age, 72 years) who got a fourth dose with outcomes in those who had only a third dose. They individually matched people from the two groups, considering factors such as age, health status, and ethnicity.
Relative vaccine effectiveness in days 7-30 after the fourth dose was estimated to be 45% (95% confidence interval, 44%-47%) against confirmed SARS-CoV-2 infection, 55% (95% CI, 53%-58%) against symptomatic COVID-19, 68% (95% CI, 59%-74%) against hospitalization, 62% (95% CI, 50%-74%) against severe COVID, and 74% (95% CI, 50%-90%) against COVID-related death.
Several countries, including the United States, have begun offering a fourth vaccine dose for higher-risk populations in light of evidence of waning immunity after the third dose and waves of infection, driven by Omicron and its variants, in some parts of the world. But the recommended age groups differ considerably.
In the United States, for instance, the Food and Drug Administration in late March approved a fourth dose of the Pfizer or Moderna vaccine for anyone over 50 and people over 18 who have gotten a solid organ transplant or have a similar level of immune risk.
Dr. Offit pointed out that Israel offers the fourth vaccine for people 60 and over and the European Medical Association offers it for those over 80. No surprise that confusion over the fourth dose is rampant.
Booster advice
Dr. Offit offered this perspective: People who are immunocompromised could reasonably get a fourth dose, depending on the manner in which they are compromised.
“Someone who has a solid organ transplant is not the same as someone who is getting a monoclonal antibody for their rheumatoid arthritis,” Dr. Offit said, adding that people could also make a reasonable argument for the fourth dose if they are over 65 and have multiple comorbidities.
“I’m over 65,” Dr. Offit said. “I’m generally healthy. I’m not going to get a fourth dose.”
People with multiple comorbidities over age 12 could reasonably get a third dose, he said. “For everybody else – healthy people less than 65 – I would argue this is a two-dose vaccine.”
CHOP, he noted as an example, mandates the vaccine but doesn’t mandate three doses and he says that’s not unusual for hospital systems.
“How many lives are you really saving with that fourth dose? If you really want to have an effect on this pandemic, vaccinate the unvaccinated,” Dr. Offit said.
Focus on the memory cells
Dr. Offit wrote in the editorial: “Arguably, the most disappointing error surrounding the use of COVID-19 vaccines was the labeling of mild illnesses or asymptomatic infections after vaccination as ‘breakthroughs.’ As is true for all mucosal vaccines, the goal is to protect against serious illness – to keep people out of the hospital, intensive care unit, and morgue. The term ‘breakthrough,’ which implies failure, created unrealistic expectations and led to the adoption of a zero-tolerance strategy for this virus.”
Dr. Offit said that the focus should be on the memory cells, not the neutralizing antibodies.
Regarding mRNA vaccines, Dr. Offit said “the surprise of this vaccine – it surprised me and other vaccine researchers – is that with these two doses of mRNA separated by 3-4 weeks, you actually appear to have long-lived memory response.
“That’s not the history of vaccines. If you look at the inactivated polio vaccine or the inactivated hepatitis A vaccine, you really do need a 4- to 6-month interval between doses to get high frequencies of memory cells. That doesn’t appear to be the case here. It looks like two doses given close together do just that. Memory cells last for years if not, sometimes, decades.”
Neutralizing antibodies, on the other hand, protect against mild illness and their effectiveness wanes after months.
“At some point we are going to have to get used to mild illness,” Dr. Offit said.
The Centers for Disease Control and Prevention must now determine who will benefit most from booster dosing and educate the public about the limits of mucosal vaccines, Dr. Offit wrote in the editorial.
“Otherwise, a zero-tolerance strategy for mild or asymptomatic infection, which can be implemented only with frequent booster doses, will continue to mislead the public about what COVID-19 vaccines can and cannot do.”
The work was funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.
A version of this article first appeared on Medscape.com.
A fourth dose of the Pfizer-BioNTech vaccine is effective in reducing the short-term risk for COVID-19 infection, hospitalization, and death in people who got a third dose at least 4 months before, a large study shows.
However, Paul Offit, MD, author of an editorial accompanying the study, told this news organization, “I would argue, without fear of contradiction, that this is going to have no impact on this pandemic.”
“We are still in the midst of a zero-tolerance policy for this virus. We don’t accept mild illness and if we’re not going to accept mild illness, we think we have to boost it away, which would mean probably about two doses every year. That’s not a reasonable public health strategy,” said Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Booster confusion
Results of the research out of Israel, published in the New England Journal of Medicine, make a case for a fourth booster for people 60 and over.
Researchers, led by Ori Magen, MD, Clalit Research Institute, innovation division, Clalit Health Services, Tel Aviv, analyzed data comparing 182,122 matched pairs recorded by the largest health care organization in Israel from Jan. 3 to Feb. 18, 2022. With more than 4.7 million members, Clalit Health Services covers more than half of the population of Israel.
The researchers compared outcomes in people 60 or older (average age, 72 years) who got a fourth dose with outcomes in those who had only a third dose. They individually matched people from the two groups, considering factors such as age, health status, and ethnicity.
Relative vaccine effectiveness in days 7-30 after the fourth dose was estimated to be 45% (95% confidence interval, 44%-47%) against confirmed SARS-CoV-2 infection, 55% (95% CI, 53%-58%) against symptomatic COVID-19, 68% (95% CI, 59%-74%) against hospitalization, 62% (95% CI, 50%-74%) against severe COVID, and 74% (95% CI, 50%-90%) against COVID-related death.
Several countries, including the United States, have begun offering a fourth vaccine dose for higher-risk populations in light of evidence of waning immunity after the third dose and waves of infection, driven by Omicron and its variants, in some parts of the world. But the recommended age groups differ considerably.
In the United States, for instance, the Food and Drug Administration in late March approved a fourth dose of the Pfizer or Moderna vaccine for anyone over 50 and people over 18 who have gotten a solid organ transplant or have a similar level of immune risk.
Dr. Offit pointed out that Israel offers the fourth vaccine for people 60 and over and the European Medical Association offers it for those over 80. No surprise that confusion over the fourth dose is rampant.
Booster advice
Dr. Offit offered this perspective: People who are immunocompromised could reasonably get a fourth dose, depending on the manner in which they are compromised.
“Someone who has a solid organ transplant is not the same as someone who is getting a monoclonal antibody for their rheumatoid arthritis,” Dr. Offit said, adding that people could also make a reasonable argument for the fourth dose if they are over 65 and have multiple comorbidities.
“I’m over 65,” Dr. Offit said. “I’m generally healthy. I’m not going to get a fourth dose.”
People with multiple comorbidities over age 12 could reasonably get a third dose, he said. “For everybody else – healthy people less than 65 – I would argue this is a two-dose vaccine.”
CHOP, he noted as an example, mandates the vaccine but doesn’t mandate three doses and he says that’s not unusual for hospital systems.
“How many lives are you really saving with that fourth dose? If you really want to have an effect on this pandemic, vaccinate the unvaccinated,” Dr. Offit said.
Focus on the memory cells
Dr. Offit wrote in the editorial: “Arguably, the most disappointing error surrounding the use of COVID-19 vaccines was the labeling of mild illnesses or asymptomatic infections after vaccination as ‘breakthroughs.’ As is true for all mucosal vaccines, the goal is to protect against serious illness – to keep people out of the hospital, intensive care unit, and morgue. The term ‘breakthrough,’ which implies failure, created unrealistic expectations and led to the adoption of a zero-tolerance strategy for this virus.”
Dr. Offit said that the focus should be on the memory cells, not the neutralizing antibodies.
Regarding mRNA vaccines, Dr. Offit said “the surprise of this vaccine – it surprised me and other vaccine researchers – is that with these two doses of mRNA separated by 3-4 weeks, you actually appear to have long-lived memory response.
“That’s not the history of vaccines. If you look at the inactivated polio vaccine or the inactivated hepatitis A vaccine, you really do need a 4- to 6-month interval between doses to get high frequencies of memory cells. That doesn’t appear to be the case here. It looks like two doses given close together do just that. Memory cells last for years if not, sometimes, decades.”
Neutralizing antibodies, on the other hand, protect against mild illness and their effectiveness wanes after months.
“At some point we are going to have to get used to mild illness,” Dr. Offit said.
The Centers for Disease Control and Prevention must now determine who will benefit most from booster dosing and educate the public about the limits of mucosal vaccines, Dr. Offit wrote in the editorial.
“Otherwise, a zero-tolerance strategy for mild or asymptomatic infection, which can be implemented only with frequent booster doses, will continue to mislead the public about what COVID-19 vaccines can and cannot do.”
The work was funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.
A version of this article first appeared on Medscape.com.
A fourth dose of the Pfizer-BioNTech vaccine is effective in reducing the short-term risk for COVID-19 infection, hospitalization, and death in people who got a third dose at least 4 months before, a large study shows.
However, Paul Offit, MD, author of an editorial accompanying the study, told this news organization, “I would argue, without fear of contradiction, that this is going to have no impact on this pandemic.”
“We are still in the midst of a zero-tolerance policy for this virus. We don’t accept mild illness and if we’re not going to accept mild illness, we think we have to boost it away, which would mean probably about two doses every year. That’s not a reasonable public health strategy,” said Dr. Offit, director of the Vaccine Education Center at the Children’s Hospital of Philadelphia.
Booster confusion
Results of the research out of Israel, published in the New England Journal of Medicine, make a case for a fourth booster for people 60 and over.
Researchers, led by Ori Magen, MD, Clalit Research Institute, innovation division, Clalit Health Services, Tel Aviv, analyzed data comparing 182,122 matched pairs recorded by the largest health care organization in Israel from Jan. 3 to Feb. 18, 2022. With more than 4.7 million members, Clalit Health Services covers more than half of the population of Israel.
The researchers compared outcomes in people 60 or older (average age, 72 years) who got a fourth dose with outcomes in those who had only a third dose. They individually matched people from the two groups, considering factors such as age, health status, and ethnicity.
Relative vaccine effectiveness in days 7-30 after the fourth dose was estimated to be 45% (95% confidence interval, 44%-47%) against confirmed SARS-CoV-2 infection, 55% (95% CI, 53%-58%) against symptomatic COVID-19, 68% (95% CI, 59%-74%) against hospitalization, 62% (95% CI, 50%-74%) against severe COVID, and 74% (95% CI, 50%-90%) against COVID-related death.
Several countries, including the United States, have begun offering a fourth vaccine dose for higher-risk populations in light of evidence of waning immunity after the third dose and waves of infection, driven by Omicron and its variants, in some parts of the world. But the recommended age groups differ considerably.
In the United States, for instance, the Food and Drug Administration in late March approved a fourth dose of the Pfizer or Moderna vaccine for anyone over 50 and people over 18 who have gotten a solid organ transplant or have a similar level of immune risk.
Dr. Offit pointed out that Israel offers the fourth vaccine for people 60 and over and the European Medical Association offers it for those over 80. No surprise that confusion over the fourth dose is rampant.
Booster advice
Dr. Offit offered this perspective: People who are immunocompromised could reasonably get a fourth dose, depending on the manner in which they are compromised.
“Someone who has a solid organ transplant is not the same as someone who is getting a monoclonal antibody for their rheumatoid arthritis,” Dr. Offit said, adding that people could also make a reasonable argument for the fourth dose if they are over 65 and have multiple comorbidities.
“I’m over 65,” Dr. Offit said. “I’m generally healthy. I’m not going to get a fourth dose.”
People with multiple comorbidities over age 12 could reasonably get a third dose, he said. “For everybody else – healthy people less than 65 – I would argue this is a two-dose vaccine.”
CHOP, he noted as an example, mandates the vaccine but doesn’t mandate three doses and he says that’s not unusual for hospital systems.
“How many lives are you really saving with that fourth dose? If you really want to have an effect on this pandemic, vaccinate the unvaccinated,” Dr. Offit said.
Focus on the memory cells
Dr. Offit wrote in the editorial: “Arguably, the most disappointing error surrounding the use of COVID-19 vaccines was the labeling of mild illnesses or asymptomatic infections after vaccination as ‘breakthroughs.’ As is true for all mucosal vaccines, the goal is to protect against serious illness – to keep people out of the hospital, intensive care unit, and morgue. The term ‘breakthrough,’ which implies failure, created unrealistic expectations and led to the adoption of a zero-tolerance strategy for this virus.”
Dr. Offit said that the focus should be on the memory cells, not the neutralizing antibodies.
Regarding mRNA vaccines, Dr. Offit said “the surprise of this vaccine – it surprised me and other vaccine researchers – is that with these two doses of mRNA separated by 3-4 weeks, you actually appear to have long-lived memory response.
“That’s not the history of vaccines. If you look at the inactivated polio vaccine or the inactivated hepatitis A vaccine, you really do need a 4- to 6-month interval between doses to get high frequencies of memory cells. That doesn’t appear to be the case here. It looks like two doses given close together do just that. Memory cells last for years if not, sometimes, decades.”
Neutralizing antibodies, on the other hand, protect against mild illness and their effectiveness wanes after months.
“At some point we are going to have to get used to mild illness,” Dr. Offit said.
The Centers for Disease Control and Prevention must now determine who will benefit most from booster dosing and educate the public about the limits of mucosal vaccines, Dr. Offit wrote in the editorial.
“Otherwise, a zero-tolerance strategy for mild or asymptomatic infection, which can be implemented only with frequent booster doses, will continue to mislead the public about what COVID-19 vaccines can and cannot do.”
The work was funded by the Ivan and Francesca Berkowitz Family Living Laboratory Collaboration at Harvard Medical School and Clalit Research Institute.
A version of this article first appeared on Medscape.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Monoclonal antibodies for COVID – Give IV infusion or an injection?
New research suggests that the casirivimab-imdevimab monoclonal antibody treatment for COVID-19 could have been delivered via injection instead of intravenously. There was no statistically significant difference in 28-day hospitalization or death in those treated intravenously and via subcutaneous injection.
The findings, published in JAMA Network Open, aren’t directly relevant at the moment, since the casirivimab-imdevimab treatment was abandoned when it failed to work during the Omicron outbreak. However, they point toward the importance of studying multiple routes of administration, said study lead author and pharmacist Erin K. McCreary, PharmD, of the University of Pittsburgh, in an interview.
“It would be beneficial for all future monoclonal antibodies for COVID-19 to be studied subcutaneously or intramuscularly, if possible, since that’s logistically easier than IV in the outpatient setting,” she said.
According to Dr. McCreary, an outpatient casirivimab-imdevimab treatment was used from 2020 to 2022 to treat higher-risk patients with mild to moderate COVID-19. The treatment was typically given intravenously as recommended by the federal government’s Emergency Use Authorization, she said. Clinical trials of the treatment, according to the study, allowed only IV administration.
“However, during the Delta surge, we were faced with so many patient referrals for treatment and staffing shortages that we couldn’t accommodate every patient unless we switched to [the] subcutaneous route,” Dr. McCreary said. This approach shortened appointment times by 30 minutes vs. infusion, she said.
There are many benefits to subcutaneous administration versus IV, Dr. McCreary said. “You don’t need to start an intravenous line, so you avoid the line kit and the nursing time needed for that. You draw up the drug directly into syringes and inject under the skin, so you avoid the need for a fluid bag to mix the drug in and run intravenously,” she said. “The appointment times are shorter, so you can accommodate more patients per day. Pharmacy interns can give subcutaneous injections, so you avoid the need for a nurse trained in placing intravenous lines.”
The researchers prospectively assigned 1,959 matched adults with mild to moderate COVID-19 to subcutaneous or intravenous treatment. Of 969 patients who received the subcutaneous treatment (mean age, 53.8; 56.4% women), the 28-day rate of hospitalization or death was 3.4%. Of 1,216 patients who received intravenous treatment (mean age, 54.3; 54.4% women), the rate was 1.7%. The difference was not statistically significant (P = .16).
Among 1,306 nontreated controls, 7.0% were hospitalized or died within 28 days (risk ratio = 0.48 vs. subcutaneous treatment group; 95% confidence interval, 0.30-0.80; P = .002).
“We did not find any patients where IV is a must,” Dr. McCreary said. “However, our study wasn’t powered to see a difference in certain subgroups.”
In an interview, University of Toronto internal medicine and pharmacology/toxicology physician Peter Wu, MD, said he agrees that the study has value because it emphasizes the importance of testing whether monoclonal antibodies can be administered in ways other than intravenously.
However, in the larger picture, he said, this may be irrelevant since it’s clear that anti-spike treatments are not holding up against COVID-19 variants.
No study funding is reported. Some study authors reported disclosures outside the submitted work. Dr. Wu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research suggests that the casirivimab-imdevimab monoclonal antibody treatment for COVID-19 could have been delivered via injection instead of intravenously. There was no statistically significant difference in 28-day hospitalization or death in those treated intravenously and via subcutaneous injection.
The findings, published in JAMA Network Open, aren’t directly relevant at the moment, since the casirivimab-imdevimab treatment was abandoned when it failed to work during the Omicron outbreak. However, they point toward the importance of studying multiple routes of administration, said study lead author and pharmacist Erin K. McCreary, PharmD, of the University of Pittsburgh, in an interview.
“It would be beneficial for all future monoclonal antibodies for COVID-19 to be studied subcutaneously or intramuscularly, if possible, since that’s logistically easier than IV in the outpatient setting,” she said.
According to Dr. McCreary, an outpatient casirivimab-imdevimab treatment was used from 2020 to 2022 to treat higher-risk patients with mild to moderate COVID-19. The treatment was typically given intravenously as recommended by the federal government’s Emergency Use Authorization, she said. Clinical trials of the treatment, according to the study, allowed only IV administration.
“However, during the Delta surge, we were faced with so many patient referrals for treatment and staffing shortages that we couldn’t accommodate every patient unless we switched to [the] subcutaneous route,” Dr. McCreary said. This approach shortened appointment times by 30 minutes vs. infusion, she said.
There are many benefits to subcutaneous administration versus IV, Dr. McCreary said. “You don’t need to start an intravenous line, so you avoid the line kit and the nursing time needed for that. You draw up the drug directly into syringes and inject under the skin, so you avoid the need for a fluid bag to mix the drug in and run intravenously,” she said. “The appointment times are shorter, so you can accommodate more patients per day. Pharmacy interns can give subcutaneous injections, so you avoid the need for a nurse trained in placing intravenous lines.”
The researchers prospectively assigned 1,959 matched adults with mild to moderate COVID-19 to subcutaneous or intravenous treatment. Of 969 patients who received the subcutaneous treatment (mean age, 53.8; 56.4% women), the 28-day rate of hospitalization or death was 3.4%. Of 1,216 patients who received intravenous treatment (mean age, 54.3; 54.4% women), the rate was 1.7%. The difference was not statistically significant (P = .16).
Among 1,306 nontreated controls, 7.0% were hospitalized or died within 28 days (risk ratio = 0.48 vs. subcutaneous treatment group; 95% confidence interval, 0.30-0.80; P = .002).
“We did not find any patients where IV is a must,” Dr. McCreary said. “However, our study wasn’t powered to see a difference in certain subgroups.”
In an interview, University of Toronto internal medicine and pharmacology/toxicology physician Peter Wu, MD, said he agrees that the study has value because it emphasizes the importance of testing whether monoclonal antibodies can be administered in ways other than intravenously.
However, in the larger picture, he said, this may be irrelevant since it’s clear that anti-spike treatments are not holding up against COVID-19 variants.
No study funding is reported. Some study authors reported disclosures outside the submitted work. Dr. Wu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
New research suggests that the casirivimab-imdevimab monoclonal antibody treatment for COVID-19 could have been delivered via injection instead of intravenously. There was no statistically significant difference in 28-day hospitalization or death in those treated intravenously and via subcutaneous injection.
The findings, published in JAMA Network Open, aren’t directly relevant at the moment, since the casirivimab-imdevimab treatment was abandoned when it failed to work during the Omicron outbreak. However, they point toward the importance of studying multiple routes of administration, said study lead author and pharmacist Erin K. McCreary, PharmD, of the University of Pittsburgh, in an interview.
“It would be beneficial for all future monoclonal antibodies for COVID-19 to be studied subcutaneously or intramuscularly, if possible, since that’s logistically easier than IV in the outpatient setting,” she said.
According to Dr. McCreary, an outpatient casirivimab-imdevimab treatment was used from 2020 to 2022 to treat higher-risk patients with mild to moderate COVID-19. The treatment was typically given intravenously as recommended by the federal government’s Emergency Use Authorization, she said. Clinical trials of the treatment, according to the study, allowed only IV administration.
“However, during the Delta surge, we were faced with so many patient referrals for treatment and staffing shortages that we couldn’t accommodate every patient unless we switched to [the] subcutaneous route,” Dr. McCreary said. This approach shortened appointment times by 30 minutes vs. infusion, she said.
There are many benefits to subcutaneous administration versus IV, Dr. McCreary said. “You don’t need to start an intravenous line, so you avoid the line kit and the nursing time needed for that. You draw up the drug directly into syringes and inject under the skin, so you avoid the need for a fluid bag to mix the drug in and run intravenously,” she said. “The appointment times are shorter, so you can accommodate more patients per day. Pharmacy interns can give subcutaneous injections, so you avoid the need for a nurse trained in placing intravenous lines.”
The researchers prospectively assigned 1,959 matched adults with mild to moderate COVID-19 to subcutaneous or intravenous treatment. Of 969 patients who received the subcutaneous treatment (mean age, 53.8; 56.4% women), the 28-day rate of hospitalization or death was 3.4%. Of 1,216 patients who received intravenous treatment (mean age, 54.3; 54.4% women), the rate was 1.7%. The difference was not statistically significant (P = .16).
Among 1,306 nontreated controls, 7.0% were hospitalized or died within 28 days (risk ratio = 0.48 vs. subcutaneous treatment group; 95% confidence interval, 0.30-0.80; P = .002).
“We did not find any patients where IV is a must,” Dr. McCreary said. “However, our study wasn’t powered to see a difference in certain subgroups.”
In an interview, University of Toronto internal medicine and pharmacology/toxicology physician Peter Wu, MD, said he agrees that the study has value because it emphasizes the importance of testing whether monoclonal antibodies can be administered in ways other than intravenously.
However, in the larger picture, he said, this may be irrelevant since it’s clear that anti-spike treatments are not holding up against COVID-19 variants.
No study funding is reported. Some study authors reported disclosures outside the submitted work. Dr. Wu has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
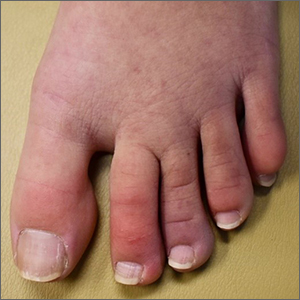
A case of cold, purple toes
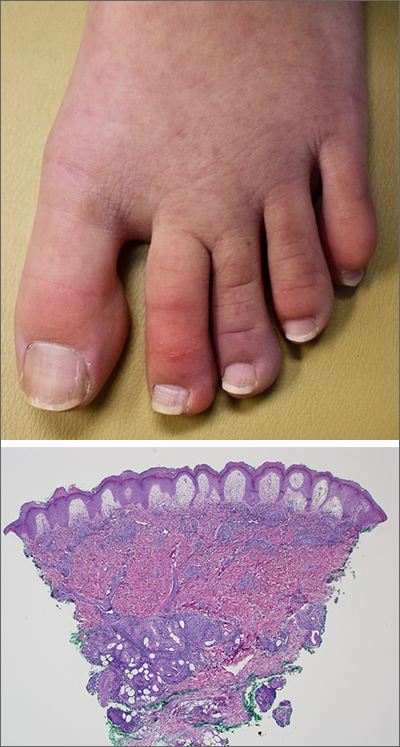
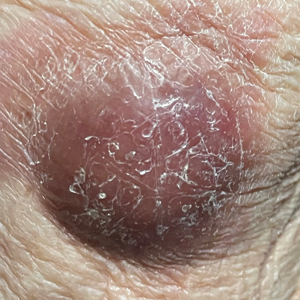
A punch-biopsy was performed on the left second toe where the erythema was the most intense. It demonstrated classic findings for pernio: superficial and deep perivascular lymphocytic inflammation and papillary dermal edema on the acral surface.
Pernio, alternatively known as chilblains, is characterized by erythema, violaceous changes, and swelling at acral sites (especially the toes or fingers). There can also be blistering, pain/tenderness, and itch. Pernio results in an abnormal localized inflammatory response to nonfreezing cold and is more common in damp climates. Pernio may also occur in occupational settings where patients handle frozen food. When a patient presents with the classic findings and consistent history, biopsy is not strictly necessary, but can aid in a definitive diagnosis.
The pathogenesis of pernio is not clearly understood. Inflammation secondary to vasospasm and type I interferon immune response to repeated or chronic cold exposure likely play a significant role. Symptoms can arise within 24 hours of exposure and resolve just as quickly. However, persistent and repeated exposure can also trigger ongoing symptoms that last for weeks.
As with most autoinflammatory conditions, pernio has a proclivity to affect younger women. It also affects children and the elderly. Because it is an inflammatory response to nonfreezing cold temperatures, the disease tends to occur during autumn in patients who live in homes without central heating.
A diagnosis of idiopathic pernio necessitates excluding several other similar, cold-induced entities. These include acrocyanosis (due to erythromelalgia, anorexia, medications), Raynaud phenomenon, cryoglobulinemia, cold urticaria, and chilblain lupus (among others). Pernio tends to lack other clinical findings such as true retiform purpura.
Of note, during the COVID-19 pandemic, physicians identified a spike in the incidence of pernio-like acral eruptions. This phenomenon has been coined “COVID toes.” While the direct temporal and causal relationships between COVID-19 and the observed eruption has not been clearly established, any patient who presents with a new onset pernio-like eruption should receive a COVID-19 test to ensure proper precautions are followed.1
In our patient, the work-up did not show any evidence of other underlying conditions. As her symptoms were minimal, we provided reassurance and counseling on preventive measures such as keeping her hands and feet warm and dry. In cases where treatment is needed, high-potency topical corticosteroids can be utilized judiciously during flares to decrease local inflammation. (There is minimal concern for adverse effects due to the thicker skin on acral surfaces.) Another treatment option is oral nifedipine (20-60 mg/d). One double-blinded trial showed it can improve symptoms in up to 70% of patients.2
Clinical image courtesy of Jiasen Wang, MD; microscopy image courtesy of Shelly Stepenaskie, MD. Text courtesy of Jiasen Wang, MD, Aimee Smidt, MD, Shelly Stepenaskie, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Cappel MA, Cappel JA, Wetter DA. Pernio (Chilblains), SARS-CoV-2, and covid toes unified through cutaneous and systemic mechanisms. Mayo Clin Proc. 2021;96:989-1005. doi: 10.1016/j.mayocp.2021.01.009
2. Simon TD, Soep JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:e472-e475. doi: 10.1542/peds.2004-2681

A punch-biopsy was performed on the left second toe where the erythema was the most intense. It demonstrated classic findings for pernio: superficial and deep perivascular lymphocytic inflammation and papillary dermal edema on the acral surface.
Pernio, alternatively known as chilblains, is characterized by erythema, violaceous changes, and swelling at acral sites (especially the toes or fingers). There can also be blistering, pain/tenderness, and itch. Pernio results in an abnormal localized inflammatory response to nonfreezing cold and is more common in damp climates. Pernio may also occur in occupational settings where patients handle frozen food. When a patient presents with the classic findings and consistent history, biopsy is not strictly necessary, but can aid in a definitive diagnosis.
The pathogenesis of pernio is not clearly understood. Inflammation secondary to vasospasm and type I interferon immune response to repeated or chronic cold exposure likely play a significant role. Symptoms can arise within 24 hours of exposure and resolve just as quickly. However, persistent and repeated exposure can also trigger ongoing symptoms that last for weeks.
As with most autoinflammatory conditions, pernio has a proclivity to affect younger women. It also affects children and the elderly. Because it is an inflammatory response to nonfreezing cold temperatures, the disease tends to occur during autumn in patients who live in homes without central heating.
A diagnosis of idiopathic pernio necessitates excluding several other similar, cold-induced entities. These include acrocyanosis (due to erythromelalgia, anorexia, medications), Raynaud phenomenon, cryoglobulinemia, cold urticaria, and chilblain lupus (among others). Pernio tends to lack other clinical findings such as true retiform purpura.
Of note, during the COVID-19 pandemic, physicians identified a spike in the incidence of pernio-like acral eruptions. This phenomenon has been coined “COVID toes.” While the direct temporal and causal relationships between COVID-19 and the observed eruption has not been clearly established, any patient who presents with a new onset pernio-like eruption should receive a COVID-19 test to ensure proper precautions are followed.1
In our patient, the work-up did not show any evidence of other underlying conditions. As her symptoms were minimal, we provided reassurance and counseling on preventive measures such as keeping her hands and feet warm and dry. In cases where treatment is needed, high-potency topical corticosteroids can be utilized judiciously during flares to decrease local inflammation. (There is minimal concern for adverse effects due to the thicker skin on acral surfaces.) Another treatment option is oral nifedipine (20-60 mg/d). One double-blinded trial showed it can improve symptoms in up to 70% of patients.2
Clinical image courtesy of Jiasen Wang, MD; microscopy image courtesy of Shelly Stepenaskie, MD. Text courtesy of Jiasen Wang, MD, Aimee Smidt, MD, Shelly Stepenaskie, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

A punch-biopsy was performed on the left second toe where the erythema was the most intense. It demonstrated classic findings for pernio: superficial and deep perivascular lymphocytic inflammation and papillary dermal edema on the acral surface.
Pernio, alternatively known as chilblains, is characterized by erythema, violaceous changes, and swelling at acral sites (especially the toes or fingers). There can also be blistering, pain/tenderness, and itch. Pernio results in an abnormal localized inflammatory response to nonfreezing cold and is more common in damp climates. Pernio may also occur in occupational settings where patients handle frozen food. When a patient presents with the classic findings and consistent history, biopsy is not strictly necessary, but can aid in a definitive diagnosis.
The pathogenesis of pernio is not clearly understood. Inflammation secondary to vasospasm and type I interferon immune response to repeated or chronic cold exposure likely play a significant role. Symptoms can arise within 24 hours of exposure and resolve just as quickly. However, persistent and repeated exposure can also trigger ongoing symptoms that last for weeks.
As with most autoinflammatory conditions, pernio has a proclivity to affect younger women. It also affects children and the elderly. Because it is an inflammatory response to nonfreezing cold temperatures, the disease tends to occur during autumn in patients who live in homes without central heating.
A diagnosis of idiopathic pernio necessitates excluding several other similar, cold-induced entities. These include acrocyanosis (due to erythromelalgia, anorexia, medications), Raynaud phenomenon, cryoglobulinemia, cold urticaria, and chilblain lupus (among others). Pernio tends to lack other clinical findings such as true retiform purpura.
Of note, during the COVID-19 pandemic, physicians identified a spike in the incidence of pernio-like acral eruptions. This phenomenon has been coined “COVID toes.” While the direct temporal and causal relationships between COVID-19 and the observed eruption has not been clearly established, any patient who presents with a new onset pernio-like eruption should receive a COVID-19 test to ensure proper precautions are followed.1
In our patient, the work-up did not show any evidence of other underlying conditions. As her symptoms were minimal, we provided reassurance and counseling on preventive measures such as keeping her hands and feet warm and dry. In cases where treatment is needed, high-potency topical corticosteroids can be utilized judiciously during flares to decrease local inflammation. (There is minimal concern for adverse effects due to the thicker skin on acral surfaces.) Another treatment option is oral nifedipine (20-60 mg/d). One double-blinded trial showed it can improve symptoms in up to 70% of patients.2
Clinical image courtesy of Jiasen Wang, MD; microscopy image courtesy of Shelly Stepenaskie, MD. Text courtesy of Jiasen Wang, MD, Aimee Smidt, MD, Shelly Stepenaskie, MD, Department of Dermatology, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Cappel MA, Cappel JA, Wetter DA. Pernio (Chilblains), SARS-CoV-2, and covid toes unified through cutaneous and systemic mechanisms. Mayo Clin Proc. 2021;96:989-1005. doi: 10.1016/j.mayocp.2021.01.009
2. Simon TD, Soep JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:e472-e475. doi: 10.1542/peds.2004-2681
1. Cappel MA, Cappel JA, Wetter DA. Pernio (Chilblains), SARS-CoV-2, and covid toes unified through cutaneous and systemic mechanisms. Mayo Clin Proc. 2021;96:989-1005. doi: 10.1016/j.mayocp.2021.01.009
2. Simon TD, Soep JB, Hollister JR. Pernio in pediatrics. Pediatrics. 2005;116:e472-e475. doi: 10.1542/peds.2004-2681
COVID-19 cardiovascular complications in children: AHA statement
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
Cardiovascular complications are uncommon for children and young adults after COVID-19 disease or SARS-CoV-2 infection, according to a new scientific statement from the American Heart Association.
However, the infection can cause some children and young people to experience arrhythmias, myocarditis, pericarditis, or multisystem inflammatory syndrome (MIS-C), a new condition identified during the pandemic, it notes.
The statement details what has been learned about how to treat, manage, and prevent cardiovascular complications associated with COVID-19 in children and young adults and calls for more research, including studies following the short- and long-term cardiovascular effects.
It also reports that COVID-19 vaccines have been found to prevent severe COVID-19 disease and decrease the risk of developing MIS-C by 91% among children ages 12-18 years.
On returning to sports, it says data suggest it is safe for young people with mild or asymptomatic COVID-19 to resume exercise after recovery from symptoms. For those with more serious infections, it recommends additional tests, including cardiac enzyme levels, electrocardiogram, and echocardiogram, before returning to sports or strenuous physical exercise.
The scientific statement was published online on in Circulation.
“Two years into the pandemic and with vast amounts of research conducted in children with COVID-19, this statement summarizes what we know so far related to COVID-19 in children,” said chair of the statement writing group Pei-Ni Jone, MD, from the Children’s Hospital Colorado, Aurora.
Analysis of the latest research indicates children generally have mild symptoms from SARS-CoV-2 infection. In the U.S., as of Feb. 24, 2022, children under 18 years of age have accounted for 17.6% of total COVID-19 cases and about 0.1% of deaths from the virus, the report states.
In addition, young adults, ages 18-29 years, have accounted for 21.3% of cases and 0.8% of deaths from COVID-19.
Like adults, children with underlying medical conditions such as chronic lung disease or obesity and those who are immunocompromised are more likely to be hospitalized, to be admitted to an intensive care unit, and to die of COVID-19, the statement notes. There are conflicting reports on the risk of severe COVID-19 in children and young adults with congenital heart disease, with some reports suggesting a slightly increased risk of severe COVID-19.
In terms of cardiovascular complications of COVID-19 in children, arrhythmias have included ventricular tachycardia and atrial tachycardia, as well as first-degree atrioventricular block. Although arrhythmias generally self-resolve without the need for treatment, prophylactic antiarrhythmics have been administered in some cases, and death caused by recurrent ventricular tachycardia in an adolescent with hypertrophic cardiomyopathy has been described.
Elevations of troponin, electrocardiographic abnormalities, including ST-segment changes, and delayed gadolinium enhancement on cardiac magnetic resonance imaging have been seen in those with myocardial involvement. Although death is rare, both sudden cardiac death and death after intensive medical and supportive therapies have occurred in children with severe myocardial involvement.
In a large retrospective pediatric case series of SARS-CoV-2–associated deaths in individuals under 21 years of age, the median age at death was 17 years, 63% were male, 28% were Black, and 46% were Hispanic. Of those who died, 86% had a comorbid condition, with obesity (42%) and asthma (29%) being the most common.
But the report concludes that: “Although children with comorbidities are at increased risk for symptomatic SARS-CoV-2 infection, compared with healthy children, cardiovascular complications, severe illness, and death are uncommon.”
MIS-C: Rare but severe
The authors of the statement explain that children and some young adults may develop MIS-C, a relatively rare but severe inflammatory syndrome generally occurring 2-6 weeks after infection with SARS-CoV-2 that can affect the heart and multiple organ systems.
In the first year of the pandemic, more than 2,600 cases of MIS-C were reported to the Centers for Disease Control and Prevention, at an estimated rate of 1 case per 3,164 cases of SARS-CoV-2 infection in children, with MIS-C disproportionately affecting Hispanic and Black children.
As many as 50% of children with MIS-C have myocardial involvement, including decreased left ventricular function, coronary artery dilation or aneurysms, myocarditis, elevated troponin and BNP or NT-proBNP, or pericardial effusion. Acute-phase reactants, including C-reactive protein, D-dimer, ferritin, and fibrinogen, can be significantly elevated in MIS-C, neutrophil/lymphocyte ratio may be higher, and platelet counts lower than those with non–MIS-C febrile illnesses.
Fortunately, the outcome of MIS-C is generally very good, with resolution of inflammation and cardiovascular abnormalities within 1-4 weeks of diagnosis, the report says.
However, there have been reports of progression of coronary artery aneurysms after discharge, highlighting the potential for long-term complications. Death resulting from MIS-C is rare, with a mortality rate of 1.4%-1.9%.
Compared with children and young adults who died of acute SARS-CoV-2 infection, most of the fatalities from MIS-C were in previously healthy individuals without comorbidities.
The authors recommend structured follow-up of patients with MIS-C because of concern about progression of cardiac complications and an unclear long-term prognosis.
The statement notes that the first-line treatment for MIS-C is typically intravenous immunoglobulin (IVIG) and patients with poor ventricular function may need to have IVIG in divided doses to tolerate the fluid load.
Supportive treatment for heart failure and vasoplegic shock often requires aggressive management in an ICU for administration of inotropes and vasoactive medications. Antiplatelet therapy with low-dose aspirin is considered in patients with coronary artery involvement, and anticoagulation is added, depending on the degree of coronary artery dilation.
COVID-19 vaccination
The statement notes that vaccines can prevent patients from getting COVID-19 and decrease the risk of MIS-C by 91% among children 12-18 years of age.
On vaccine-associated myocarditis, it concludes the benefits of getting the vaccines outweigh the risks.
For example, for every 1 million doses of the mRNA COVID-19 vaccines in males ages 12-29 years (the highest risk group for vaccine-associated myocarditis), it is estimated that 11,000 COVID-19 cases, 560 hospitalizations, and six deaths would be prevented, whereas 39-47 cases of myocarditis would be expected.
But it adds that the CDC is continuing to follow myocarditis in children and young adults closely, particularly a possible connection to the mRNA COVID-19 vaccines.
The statement says that more research is needed to better understand the mechanisms and optimal treatment approaches for SARS-CoV-2 infection, vaccine-associated myocarditis, the long-term outcomes of both COVID-19 and MIS-C, and the impact of these various conditions on the heart in children and young adults. In addition, any new antiviral therapies need to be tested in clinical trials focused on children.
“Although much has been learned about how the virus impacts children’s and young adult’s hearts, how to best treat cardiovascular complications, and prevent severe illness, continued clinical research trials are needed to better understand the long-term cardiovascular impacts,” Dr. Jone said. “It is also important to address health disparities that have become more apparent during the pandemic. We must work to ensure all children receive equal access to vaccination and high-quality care.”
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Are all medical errors now crimes? The Nurse Vaught verdict
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome! I’m Dr Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have a distinguished panel joining us to discuss an important legal decision resulting in a criminal conviction, involving a medical error due to administration of the wrong medication by a critical care nurse that led to a patient’s death.
Joining us to discuss this case is Dr. Megan Ranney, professor of emergency medicine and the academic dean at Brown University School of Public Health. Also joining us is Dr. Jane Barnsteiner, emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. Welcome to both of you.
Jane Barnsteiner, PhD, RN: Thank you.
Megan L. Ranney, MD, MPH: Thank you. It’s a joy to be with you.
Dr. Glatter: Let’s discuss this very tragic case involving RaDonda Vaught, who was an ICU nurse who was recently convicted in Tennessee of criminally negligent homicide and gross neglect of an impaired adult. She accidentally administered a paralytic medication, vecuronium, instead of a sedative, Versed, which was ordered to sedate a 75-year-old patient who had a brain bleed and TBI. She was scheduled to have a PET scan. After receiving the wrong medication and not really being monitored in any true way, just being in the care of an MRI tech, she suffered cardiac arrest and subsequently died.
Dr. Ranney, I want to begin with you. I saw on Twitter that you had written something that really stuck with me. I’ll quote you. “A culture of safety is one in which the system that allowed the mistake to happen is changed, not one in which the individual is scapegoated. And a culture of safety correlates with better patient outcomes that we know. This verdict is the opposite.”
I’ll let you explain from here. The system issue is the medication dispensing cabinet, in my mind, and there was a medication override. The question is, how was this override allowed to occur in the first place?
Dr. Ranney: My goodness, overrides happen every single day across this country, dozens of times a day in any particular shift. I would think of the system as being much bigger than just the Pyxis or that kind of automated dispensing cabinet, but around the larger system of the verbal orders, the time pressures that the nurse is under, the fact that the nurses are with a trainee, the fact that they’re being asked to operate outside of their normal environment by going down to MRI. There’s a series of issues.
Just as we thought about the Swiss cheese model for COVID-19, that model originated when we talked about patient safety and medical errors. It is a Swiss cheese of circumstances that allows this type of tragic error to occur.
Many of us have worked for years on trying to change the system from one of punishing people, changing it from that punitive system, to rather a system where we can do root-cause analysis, allow people to disclose errors, and allow us to inquire as to what are those series of Swiss cheese holes that allowed this mistake or any other to happen.
When you punish people, you lead them to hide their mistakes instead of allowing them to disclose them and allowing that important inquiry to happen. That’s why this is just so harmful to that culture of safety that so many of us are trying to create.
Dr. Glatter: It’s a chilling verdict in so many ways. I’m right on the same page with you, having worked for so long in the emergency department and seeing nurses that are overtaxed, overburdened, but also on patient floors. This goes to an ICU-type environment where this woman was having a nonemergent head scan and required some sedation.
The question I want to get to is how the system allowed the nurse to dispense this medication —though she was distracted, she’ll admit that. Jane, I want to get to you on this. How can we avoid this? What are the system checks that can be done in some fashion to make this safer and to avoid this tragic error?
Dr. Barnsteiner: First of all, I would say that you do not put in a major change, as they were doing with their EPIC system, as a big bank where you do the change through the entire organization. You do it in one area where you get the whole system smoothed out and all the errors taken care of so that you’re not having a problem like they had through their entire organization, which required overrides multiple times a day.
One of the things that’s been recommended is that these systems, like the Pyxis system, require the first five letters of a medication to be entered into the system so that when you have multiple medications where the first two letters are the same, the chances of pulling out the wrong medication are much smaller.
There’s a question of whether this medication, vecuronium, should have even been in this machine. You can have high-alert medications like this in baggies that have written on the front of the bag, “This is a high-alert medication. It requires two independent double checks.” These are all the things that will help alert the fatigued or distracted nurse or physician and will make things safer. There are many things that can be put into place.
Dr. Glatter: It’s almost like a hard stop. This is a different class of medication. Even if the nurse had a lapse and didn’t realize that, there should have been a hard stop asking whether you want this class. A sedative and a paralytic are two very different medications.
I’m not trying to assign any blame here. I’m just trying to look at mechanics of what happened and how we can put in place methods to avoid these types of errors where a system clearly is overtaxed and overburdened. Is it an artificial intelligence alert? Is it a pharmacy alert that goes out? Is it a Vocera message that gets triggered? It’s something to stop the nurse from doing something where they know better.
She’s used Versed before, apparently, and knows it’s a liquid and doesn’t have to be reconstituted. In my mind, as a practicing doctor for a long time, I see this and I see how it can happen. There are ways I think we can address it. Megan, I want to bring you into this and get your viewpoint.
Dr. Ranney: We’re working in an environment right now — and obviously, this happened pre-COVID — where medicines are constantly in short supply and we’re constantly dealing with substitutions of one for another. This has worsened during COVID, but it existed in the pre-COVID era as well. We’d have time periods where, like today, we’re out of D50 and we have to use D10, or we have a different formulation of a common antibiotic.
I could totally imagine that this nurse had been exposed to multiple medication substitution and so they were rushing; they thought, well, they just put one thing in instead of another and didn’t make that kind of cognitive connection.
What we know so well from our studies of human factors, engineering, and the way that systems work is that when someone is cognitively overloaded and constantly having to think outside the box and make decisions, particularly when they’re exposed to a new system for ordering medicine, there’s only so much that the brain can do at a time. This person was set up for this type of error.
Again, not to say that they didn’t do something wrong. That’s why we have a civil system. That’s why we have licensing. That’s why we have malpractice. To call this a criminal error when they were working within a system that had all these other problems where they were constantly having to make do for system failures, it’s almost inevitable that at some point something really horrible happened.
I’m sorry that it was this nurse, and how horrible for the patient and the family. I’m not excusing that. You can totally imagine, as a practicing physician, nurse, or anyone else in the healthcare system, how this happened.
Dr. Barnsteiner: The other part of it was that they did not have in place, at this time, the barcoding system in this particular patient area. What nurses are used to doing is when they have to pull a medication, they’re using the barcoding system to coordinate with what’s in the electronic health record, with the medication, and with the person’s ID band.
Those are all well-known safety checks that obviously were used to being used by this nurse in the critical care unit but that weren’t available in this MRI area. That is something that absolutely is a system failure. Those kinds of safety systems have to be available at any place in a health system where medications are being delivered.
Dr. Glatter: I think that’s an important point. Here, we have a technology that can supersede the ability of a human to make a mistake, and to have that in place is very critical. I want to go back to the idea of medical malpractice vs homicide charges.
Megan, you made a point of this. This nurse is now an example of someone who went to trial and was convicted, and it could have a chilling effect on healthcare providers. Pre-COVID, post-COVID, it is just chilling. It makes people want to leave the field. It causes PTSD. The psychiatric downstream effects of such an error are just immense.
I don’t know how the district attorney went for criminal charges here. I’m not an attorney and we don’t have a legal expert with us. For this to have happened is just setting precedent that it’s okay to have the effect of making so many people leave the field.
Dr. Ranney: I’m not a lawyer, but I’ve certainly been on the front lines, not only for the past 2 years during COVID but for almost 20 years prior to that. I will say that these types of errors are never-events that sit with our colleagues and friends for their entire career. No one goes into medicine intending to hurt someone. The system fails us and fails the patient.
There are certainly examples of intentional harm, and those people deserve to be prosecuted. This type of thing where a system let them down, again, should require an inquiry of the system. Don’t punish the individuals to the point of putting them in jail.
I think about my last few months working in the emergency department and what my nurses, in particular, have said to me. They worry that they’re going to lose their license and their ability to practice because of the horrific circumstances that we’ve been working in — the understaffing, the lack of access to standard medications, the long wait times, and on and on. They’re not able to take care of patients the way that they’ve been taught to do.
They’re worried already about the downstream effects on their sense of self, as well as on their ability to maintain their livelihood. When you put something like this on top of it, where again, an unintentional error that was potentiated by a somewhat broken system or by a series of Swiss cheese holes that just happened to line up, what message does that send to my nursing colleagues who have stayed on the front lines and who know that they have not been able to provide the standard of care that they’re used to?
Dr. Barnsteiner: On Friday, I did a program on fair and just culture with three health systems and a university school of nursing. Already, some of the faculty reported that students are talking about transferring to another major outside of the School of Nursing because of their worry about this particular guilty verdict.
The other thing is that we already have a tremendous shortage of nurses. We’ve seen many people leave the profession or retire in the past couple of years, and this is only going to compound it further. It is a sobering message that the public can’t afford to have, actually, because this will impact the quality of care and the safety of care that can be delivered to people and families as a result of not having sufficient numbers of professionals to deliver care.
Dr. Glatter: That’s such an important point. In any high-reliability organization, a culture of safety is key. There are tenets we try to adhere to. When we have people leaving the field after seeing a case like this, it’s chilling. We have to re-educate the public and we need to have a realignment of how errors are handled.
This is just the beginning. Her sentencing is going to be in about a month, and we’ll see what happens on reckless homicide charges and neglect. I think there’s going to be a follow-up to this and we’re going to need to discuss this more.
I just wanted to get a couple of takeaways for our audience to just really sear in the brain what we can learn from such an event.
Dr. Ranney: The big takeaway, to me, is the importance of us both continuing to use our voices and working across professional boundaries to help to create this culture of safety, one in which we all feel safe and supported in advocating for systems that work for us. We cannot ask nurses, respiratory technicians, radiology technicians, physicians, or anyone else within the healthcare system to work unsupported, and we have to recognize the degree to which we are all interdependent. My biggest takeaway is for us to use our voices together.
Dr. Barnsteiner: The takeaway that I would have from this, and what I’m working with a number of health systems on, is to have the chair of the board, the CEO of the hospital, the chief medical officer, and the chief nursing officer together promulgate a statement that is sent out to all employees to discuss this verdict and to say what they’re doing to promote a high-reliability organization and a fair and just culture. They should also ask for open conversation and for employees to let the top leadership know any concerns that they have about vulnerabilities in the system. It’s extremely important right now with this verdict that the leaders in healthcare settings, as well as in education settings, let people know what they’ll be doing to protect their employees.
Dr. Glatter: Jane and Megan, I want to thank you so much for such an important discussion that was very informative. I think there’s going to be a follow-up to this that’ll be very, very important. Thanks again.
Robert D. Glatter, MD, is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Glatter has disclosed no relevant financial relationships.
Megan Ranney, MD, MPH, is professor of emergency medicine and the academic dean at Brown University School of Public Health in Providence, Rhode Island. She is the director and founder of the Brown Emergency Digital Health Innovation (eDHI) program. She is also chief research officer for the American Foundation for Firearm Injury Reduction in Medicine, the country’s only nonprofit committed to reducing firearm injury through the public health approach, and a founding partner of GetUsPPE.org, dedicated to matching donors to health systems in need of protective equipment. Dr. Ranney has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for: Medscape; Merck.
Jane Barnsteiner, PhD, RN, is an emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. In addition to her teaching responsibilities, she was director of translational research at the Hospital of the University of Pennsylvania. Jane was one of the developers of the Quality and Safety in Education for Nurses (QSEN) initiative and is co-editor of Quality and Safety in Nursing: A Competency Based Approach to Improving Outcomes, published by Wiley. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome! I’m Dr Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have a distinguished panel joining us to discuss an important legal decision resulting in a criminal conviction, involving a medical error due to administration of the wrong medication by a critical care nurse that led to a patient’s death.
Joining us to discuss this case is Dr. Megan Ranney, professor of emergency medicine and the academic dean at Brown University School of Public Health. Also joining us is Dr. Jane Barnsteiner, emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. Welcome to both of you.
Jane Barnsteiner, PhD, RN: Thank you.
Megan L. Ranney, MD, MPH: Thank you. It’s a joy to be with you.
Dr. Glatter: Let’s discuss this very tragic case involving RaDonda Vaught, who was an ICU nurse who was recently convicted in Tennessee of criminally negligent homicide and gross neglect of an impaired adult. She accidentally administered a paralytic medication, vecuronium, instead of a sedative, Versed, which was ordered to sedate a 75-year-old patient who had a brain bleed and TBI. She was scheduled to have a PET scan. After receiving the wrong medication and not really being monitored in any true way, just being in the care of an MRI tech, she suffered cardiac arrest and subsequently died.
Dr. Ranney, I want to begin with you. I saw on Twitter that you had written something that really stuck with me. I’ll quote you. “A culture of safety is one in which the system that allowed the mistake to happen is changed, not one in which the individual is scapegoated. And a culture of safety correlates with better patient outcomes that we know. This verdict is the opposite.”
I’ll let you explain from here. The system issue is the medication dispensing cabinet, in my mind, and there was a medication override. The question is, how was this override allowed to occur in the first place?
Dr. Ranney: My goodness, overrides happen every single day across this country, dozens of times a day in any particular shift. I would think of the system as being much bigger than just the Pyxis or that kind of automated dispensing cabinet, but around the larger system of the verbal orders, the time pressures that the nurse is under, the fact that the nurses are with a trainee, the fact that they’re being asked to operate outside of their normal environment by going down to MRI. There’s a series of issues.
Just as we thought about the Swiss cheese model for COVID-19, that model originated when we talked about patient safety and medical errors. It is a Swiss cheese of circumstances that allows this type of tragic error to occur.
Many of us have worked for years on trying to change the system from one of punishing people, changing it from that punitive system, to rather a system where we can do root-cause analysis, allow people to disclose errors, and allow us to inquire as to what are those series of Swiss cheese holes that allowed this mistake or any other to happen.
When you punish people, you lead them to hide their mistakes instead of allowing them to disclose them and allowing that important inquiry to happen. That’s why this is just so harmful to that culture of safety that so many of us are trying to create.
Dr. Glatter: It’s a chilling verdict in so many ways. I’m right on the same page with you, having worked for so long in the emergency department and seeing nurses that are overtaxed, overburdened, but also on patient floors. This goes to an ICU-type environment where this woman was having a nonemergent head scan and required some sedation.
The question I want to get to is how the system allowed the nurse to dispense this medication —though she was distracted, she’ll admit that. Jane, I want to get to you on this. How can we avoid this? What are the system checks that can be done in some fashion to make this safer and to avoid this tragic error?
Dr. Barnsteiner: First of all, I would say that you do not put in a major change, as they were doing with their EPIC system, as a big bank where you do the change through the entire organization. You do it in one area where you get the whole system smoothed out and all the errors taken care of so that you’re not having a problem like they had through their entire organization, which required overrides multiple times a day.
One of the things that’s been recommended is that these systems, like the Pyxis system, require the first five letters of a medication to be entered into the system so that when you have multiple medications where the first two letters are the same, the chances of pulling out the wrong medication are much smaller.
There’s a question of whether this medication, vecuronium, should have even been in this machine. You can have high-alert medications like this in baggies that have written on the front of the bag, “This is a high-alert medication. It requires two independent double checks.” These are all the things that will help alert the fatigued or distracted nurse or physician and will make things safer. There are many things that can be put into place.
Dr. Glatter: It’s almost like a hard stop. This is a different class of medication. Even if the nurse had a lapse and didn’t realize that, there should have been a hard stop asking whether you want this class. A sedative and a paralytic are two very different medications.
I’m not trying to assign any blame here. I’m just trying to look at mechanics of what happened and how we can put in place methods to avoid these types of errors where a system clearly is overtaxed and overburdened. Is it an artificial intelligence alert? Is it a pharmacy alert that goes out? Is it a Vocera message that gets triggered? It’s something to stop the nurse from doing something where they know better.
She’s used Versed before, apparently, and knows it’s a liquid and doesn’t have to be reconstituted. In my mind, as a practicing doctor for a long time, I see this and I see how it can happen. There are ways I think we can address it. Megan, I want to bring you into this and get your viewpoint.
Dr. Ranney: We’re working in an environment right now — and obviously, this happened pre-COVID — where medicines are constantly in short supply and we’re constantly dealing with substitutions of one for another. This has worsened during COVID, but it existed in the pre-COVID era as well. We’d have time periods where, like today, we’re out of D50 and we have to use D10, or we have a different formulation of a common antibiotic.
I could totally imagine that this nurse had been exposed to multiple medication substitution and so they were rushing; they thought, well, they just put one thing in instead of another and didn’t make that kind of cognitive connection.
What we know so well from our studies of human factors, engineering, and the way that systems work is that when someone is cognitively overloaded and constantly having to think outside the box and make decisions, particularly when they’re exposed to a new system for ordering medicine, there’s only so much that the brain can do at a time. This person was set up for this type of error.
Again, not to say that they didn’t do something wrong. That’s why we have a civil system. That’s why we have licensing. That’s why we have malpractice. To call this a criminal error when they were working within a system that had all these other problems where they were constantly having to make do for system failures, it’s almost inevitable that at some point something really horrible happened.
I’m sorry that it was this nurse, and how horrible for the patient and the family. I’m not excusing that. You can totally imagine, as a practicing physician, nurse, or anyone else in the healthcare system, how this happened.
Dr. Barnsteiner: The other part of it was that they did not have in place, at this time, the barcoding system in this particular patient area. What nurses are used to doing is when they have to pull a medication, they’re using the barcoding system to coordinate with what’s in the electronic health record, with the medication, and with the person’s ID band.
Those are all well-known safety checks that obviously were used to being used by this nurse in the critical care unit but that weren’t available in this MRI area. That is something that absolutely is a system failure. Those kinds of safety systems have to be available at any place in a health system where medications are being delivered.
Dr. Glatter: I think that’s an important point. Here, we have a technology that can supersede the ability of a human to make a mistake, and to have that in place is very critical. I want to go back to the idea of medical malpractice vs homicide charges.
Megan, you made a point of this. This nurse is now an example of someone who went to trial and was convicted, and it could have a chilling effect on healthcare providers. Pre-COVID, post-COVID, it is just chilling. It makes people want to leave the field. It causes PTSD. The psychiatric downstream effects of such an error are just immense.
I don’t know how the district attorney went for criminal charges here. I’m not an attorney and we don’t have a legal expert with us. For this to have happened is just setting precedent that it’s okay to have the effect of making so many people leave the field.
Dr. Ranney: I’m not a lawyer, but I’ve certainly been on the front lines, not only for the past 2 years during COVID but for almost 20 years prior to that. I will say that these types of errors are never-events that sit with our colleagues and friends for their entire career. No one goes into medicine intending to hurt someone. The system fails us and fails the patient.
There are certainly examples of intentional harm, and those people deserve to be prosecuted. This type of thing where a system let them down, again, should require an inquiry of the system. Don’t punish the individuals to the point of putting them in jail.
I think about my last few months working in the emergency department and what my nurses, in particular, have said to me. They worry that they’re going to lose their license and their ability to practice because of the horrific circumstances that we’ve been working in — the understaffing, the lack of access to standard medications, the long wait times, and on and on. They’re not able to take care of patients the way that they’ve been taught to do.
They’re worried already about the downstream effects on their sense of self, as well as on their ability to maintain their livelihood. When you put something like this on top of it, where again, an unintentional error that was potentiated by a somewhat broken system or by a series of Swiss cheese holes that just happened to line up, what message does that send to my nursing colleagues who have stayed on the front lines and who know that they have not been able to provide the standard of care that they’re used to?
Dr. Barnsteiner: On Friday, I did a program on fair and just culture with three health systems and a university school of nursing. Already, some of the faculty reported that students are talking about transferring to another major outside of the School of Nursing because of their worry about this particular guilty verdict.
The other thing is that we already have a tremendous shortage of nurses. We’ve seen many people leave the profession or retire in the past couple of years, and this is only going to compound it further. It is a sobering message that the public can’t afford to have, actually, because this will impact the quality of care and the safety of care that can be delivered to people and families as a result of not having sufficient numbers of professionals to deliver care.
Dr. Glatter: That’s such an important point. In any high-reliability organization, a culture of safety is key. There are tenets we try to adhere to. When we have people leaving the field after seeing a case like this, it’s chilling. We have to re-educate the public and we need to have a realignment of how errors are handled.
This is just the beginning. Her sentencing is going to be in about a month, and we’ll see what happens on reckless homicide charges and neglect. I think there’s going to be a follow-up to this and we’re going to need to discuss this more.
I just wanted to get a couple of takeaways for our audience to just really sear in the brain what we can learn from such an event.
Dr. Ranney: The big takeaway, to me, is the importance of us both continuing to use our voices and working across professional boundaries to help to create this culture of safety, one in which we all feel safe and supported in advocating for systems that work for us. We cannot ask nurses, respiratory technicians, radiology technicians, physicians, or anyone else within the healthcare system to work unsupported, and we have to recognize the degree to which we are all interdependent. My biggest takeaway is for us to use our voices together.
Dr. Barnsteiner: The takeaway that I would have from this, and what I’m working with a number of health systems on, is to have the chair of the board, the CEO of the hospital, the chief medical officer, and the chief nursing officer together promulgate a statement that is sent out to all employees to discuss this verdict and to say what they’re doing to promote a high-reliability organization and a fair and just culture. They should also ask for open conversation and for employees to let the top leadership know any concerns that they have about vulnerabilities in the system. It’s extremely important right now with this verdict that the leaders in healthcare settings, as well as in education settings, let people know what they’ll be doing to protect their employees.
Dr. Glatter: Jane and Megan, I want to thank you so much for such an important discussion that was very informative. I think there’s going to be a follow-up to this that’ll be very, very important. Thanks again.
Robert D. Glatter, MD, is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Glatter has disclosed no relevant financial relationships.
Megan Ranney, MD, MPH, is professor of emergency medicine and the academic dean at Brown University School of Public Health in Providence, Rhode Island. She is the director and founder of the Brown Emergency Digital Health Innovation (eDHI) program. She is also chief research officer for the American Foundation for Firearm Injury Reduction in Medicine, the country’s only nonprofit committed to reducing firearm injury through the public health approach, and a founding partner of GetUsPPE.org, dedicated to matching donors to health systems in need of protective equipment. Dr. Ranney has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for: Medscape; Merck.
Jane Barnsteiner, PhD, RN, is an emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. In addition to her teaching responsibilities, she was director of translational research at the Hospital of the University of Pennsylvania. Jane was one of the developers of the Quality and Safety in Education for Nurses (QSEN) initiative and is co-editor of Quality and Safety in Nursing: A Competency Based Approach to Improving Outcomes, published by Wiley. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
This video transcript has been edited for clarity.
Robert D. Glatter, MD: Welcome! I’m Dr Robert Glatter, medical advisor for Medscape Emergency Medicine. Today we have a distinguished panel joining us to discuss an important legal decision resulting in a criminal conviction, involving a medical error due to administration of the wrong medication by a critical care nurse that led to a patient’s death.
Joining us to discuss this case is Dr. Megan Ranney, professor of emergency medicine and the academic dean at Brown University School of Public Health. Also joining us is Dr. Jane Barnsteiner, emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. Welcome to both of you.
Jane Barnsteiner, PhD, RN: Thank you.
Megan L. Ranney, MD, MPH: Thank you. It’s a joy to be with you.
Dr. Glatter: Let’s discuss this very tragic case involving RaDonda Vaught, who was an ICU nurse who was recently convicted in Tennessee of criminally negligent homicide and gross neglect of an impaired adult. She accidentally administered a paralytic medication, vecuronium, instead of a sedative, Versed, which was ordered to sedate a 75-year-old patient who had a brain bleed and TBI. She was scheduled to have a PET scan. After receiving the wrong medication and not really being monitored in any true way, just being in the care of an MRI tech, she suffered cardiac arrest and subsequently died.
Dr. Ranney, I want to begin with you. I saw on Twitter that you had written something that really stuck with me. I’ll quote you. “A culture of safety is one in which the system that allowed the mistake to happen is changed, not one in which the individual is scapegoated. And a culture of safety correlates with better patient outcomes that we know. This verdict is the opposite.”
I’ll let you explain from here. The system issue is the medication dispensing cabinet, in my mind, and there was a medication override. The question is, how was this override allowed to occur in the first place?
Dr. Ranney: My goodness, overrides happen every single day across this country, dozens of times a day in any particular shift. I would think of the system as being much bigger than just the Pyxis or that kind of automated dispensing cabinet, but around the larger system of the verbal orders, the time pressures that the nurse is under, the fact that the nurses are with a trainee, the fact that they’re being asked to operate outside of their normal environment by going down to MRI. There’s a series of issues.
Just as we thought about the Swiss cheese model for COVID-19, that model originated when we talked about patient safety and medical errors. It is a Swiss cheese of circumstances that allows this type of tragic error to occur.
Many of us have worked for years on trying to change the system from one of punishing people, changing it from that punitive system, to rather a system where we can do root-cause analysis, allow people to disclose errors, and allow us to inquire as to what are those series of Swiss cheese holes that allowed this mistake or any other to happen.
When you punish people, you lead them to hide their mistakes instead of allowing them to disclose them and allowing that important inquiry to happen. That’s why this is just so harmful to that culture of safety that so many of us are trying to create.
Dr. Glatter: It’s a chilling verdict in so many ways. I’m right on the same page with you, having worked for so long in the emergency department and seeing nurses that are overtaxed, overburdened, but also on patient floors. This goes to an ICU-type environment where this woman was having a nonemergent head scan and required some sedation.
The question I want to get to is how the system allowed the nurse to dispense this medication —though she was distracted, she’ll admit that. Jane, I want to get to you on this. How can we avoid this? What are the system checks that can be done in some fashion to make this safer and to avoid this tragic error?
Dr. Barnsteiner: First of all, I would say that you do not put in a major change, as they were doing with their EPIC system, as a big bank where you do the change through the entire organization. You do it in one area where you get the whole system smoothed out and all the errors taken care of so that you’re not having a problem like they had through their entire organization, which required overrides multiple times a day.
One of the things that’s been recommended is that these systems, like the Pyxis system, require the first five letters of a medication to be entered into the system so that when you have multiple medications where the first two letters are the same, the chances of pulling out the wrong medication are much smaller.
There’s a question of whether this medication, vecuronium, should have even been in this machine. You can have high-alert medications like this in baggies that have written on the front of the bag, “This is a high-alert medication. It requires two independent double checks.” These are all the things that will help alert the fatigued or distracted nurse or physician and will make things safer. There are many things that can be put into place.
Dr. Glatter: It’s almost like a hard stop. This is a different class of medication. Even if the nurse had a lapse and didn’t realize that, there should have been a hard stop asking whether you want this class. A sedative and a paralytic are two very different medications.
I’m not trying to assign any blame here. I’m just trying to look at mechanics of what happened and how we can put in place methods to avoid these types of errors where a system clearly is overtaxed and overburdened. Is it an artificial intelligence alert? Is it a pharmacy alert that goes out? Is it a Vocera message that gets triggered? It’s something to stop the nurse from doing something where they know better.
She’s used Versed before, apparently, and knows it’s a liquid and doesn’t have to be reconstituted. In my mind, as a practicing doctor for a long time, I see this and I see how it can happen. There are ways I think we can address it. Megan, I want to bring you into this and get your viewpoint.
Dr. Ranney: We’re working in an environment right now — and obviously, this happened pre-COVID — where medicines are constantly in short supply and we’re constantly dealing with substitutions of one for another. This has worsened during COVID, but it existed in the pre-COVID era as well. We’d have time periods where, like today, we’re out of D50 and we have to use D10, or we have a different formulation of a common antibiotic.
I could totally imagine that this nurse had been exposed to multiple medication substitution and so they were rushing; they thought, well, they just put one thing in instead of another and didn’t make that kind of cognitive connection.
What we know so well from our studies of human factors, engineering, and the way that systems work is that when someone is cognitively overloaded and constantly having to think outside the box and make decisions, particularly when they’re exposed to a new system for ordering medicine, there’s only so much that the brain can do at a time. This person was set up for this type of error.
Again, not to say that they didn’t do something wrong. That’s why we have a civil system. That’s why we have licensing. That’s why we have malpractice. To call this a criminal error when they were working within a system that had all these other problems where they were constantly having to make do for system failures, it’s almost inevitable that at some point something really horrible happened.
I’m sorry that it was this nurse, and how horrible for the patient and the family. I’m not excusing that. You can totally imagine, as a practicing physician, nurse, or anyone else in the healthcare system, how this happened.
Dr. Barnsteiner: The other part of it was that they did not have in place, at this time, the barcoding system in this particular patient area. What nurses are used to doing is when they have to pull a medication, they’re using the barcoding system to coordinate with what’s in the electronic health record, with the medication, and with the person’s ID band.
Those are all well-known safety checks that obviously were used to being used by this nurse in the critical care unit but that weren’t available in this MRI area. That is something that absolutely is a system failure. Those kinds of safety systems have to be available at any place in a health system where medications are being delivered.
Dr. Glatter: I think that’s an important point. Here, we have a technology that can supersede the ability of a human to make a mistake, and to have that in place is very critical. I want to go back to the idea of medical malpractice vs homicide charges.
Megan, you made a point of this. This nurse is now an example of someone who went to trial and was convicted, and it could have a chilling effect on healthcare providers. Pre-COVID, post-COVID, it is just chilling. It makes people want to leave the field. It causes PTSD. The psychiatric downstream effects of such an error are just immense.
I don’t know how the district attorney went for criminal charges here. I’m not an attorney and we don’t have a legal expert with us. For this to have happened is just setting precedent that it’s okay to have the effect of making so many people leave the field.
Dr. Ranney: I’m not a lawyer, but I’ve certainly been on the front lines, not only for the past 2 years during COVID but for almost 20 years prior to that. I will say that these types of errors are never-events that sit with our colleagues and friends for their entire career. No one goes into medicine intending to hurt someone. The system fails us and fails the patient.
There are certainly examples of intentional harm, and those people deserve to be prosecuted. This type of thing where a system let them down, again, should require an inquiry of the system. Don’t punish the individuals to the point of putting them in jail.
I think about my last few months working in the emergency department and what my nurses, in particular, have said to me. They worry that they’re going to lose their license and their ability to practice because of the horrific circumstances that we’ve been working in — the understaffing, the lack of access to standard medications, the long wait times, and on and on. They’re not able to take care of patients the way that they’ve been taught to do.
They’re worried already about the downstream effects on their sense of self, as well as on their ability to maintain their livelihood. When you put something like this on top of it, where again, an unintentional error that was potentiated by a somewhat broken system or by a series of Swiss cheese holes that just happened to line up, what message does that send to my nursing colleagues who have stayed on the front lines and who know that they have not been able to provide the standard of care that they’re used to?
Dr. Barnsteiner: On Friday, I did a program on fair and just culture with three health systems and a university school of nursing. Already, some of the faculty reported that students are talking about transferring to another major outside of the School of Nursing because of their worry about this particular guilty verdict.
The other thing is that we already have a tremendous shortage of nurses. We’ve seen many people leave the profession or retire in the past couple of years, and this is only going to compound it further. It is a sobering message that the public can’t afford to have, actually, because this will impact the quality of care and the safety of care that can be delivered to people and families as a result of not having sufficient numbers of professionals to deliver care.
Dr. Glatter: That’s such an important point. In any high-reliability organization, a culture of safety is key. There are tenets we try to adhere to. When we have people leaving the field after seeing a case like this, it’s chilling. We have to re-educate the public and we need to have a realignment of how errors are handled.
This is just the beginning. Her sentencing is going to be in about a month, and we’ll see what happens on reckless homicide charges and neglect. I think there’s going to be a follow-up to this and we’re going to need to discuss this more.
I just wanted to get a couple of takeaways for our audience to just really sear in the brain what we can learn from such an event.
Dr. Ranney: The big takeaway, to me, is the importance of us both continuing to use our voices and working across professional boundaries to help to create this culture of safety, one in which we all feel safe and supported in advocating for systems that work for us. We cannot ask nurses, respiratory technicians, radiology technicians, physicians, or anyone else within the healthcare system to work unsupported, and we have to recognize the degree to which we are all interdependent. My biggest takeaway is for us to use our voices together.
Dr. Barnsteiner: The takeaway that I would have from this, and what I’m working with a number of health systems on, is to have the chair of the board, the CEO of the hospital, the chief medical officer, and the chief nursing officer together promulgate a statement that is sent out to all employees to discuss this verdict and to say what they’re doing to promote a high-reliability organization and a fair and just culture. They should also ask for open conversation and for employees to let the top leadership know any concerns that they have about vulnerabilities in the system. It’s extremely important right now with this verdict that the leaders in healthcare settings, as well as in education settings, let people know what they’ll be doing to protect their employees.
Dr. Glatter: Jane and Megan, I want to thank you so much for such an important discussion that was very informative. I think there’s going to be a follow-up to this that’ll be very, very important. Thanks again.
Robert D. Glatter, MD, is assistant professor of emergency medicine at Lenox Hill Hospital in New York City and at Zucker School of Medicine at Hofstra/Northwell in Hempstead, New York. He is an editorial advisor and hosts the Hot Topics in EM series on Medscape. He is also a medical contributor for Forbes. Dr. Glatter has disclosed no relevant financial relationships.
Megan Ranney, MD, MPH, is professor of emergency medicine and the academic dean at Brown University School of Public Health in Providence, Rhode Island. She is the director and founder of the Brown Emergency Digital Health Innovation (eDHI) program. She is also chief research officer for the American Foundation for Firearm Injury Reduction in Medicine, the country’s only nonprofit committed to reducing firearm injury through the public health approach, and a founding partner of GetUsPPE.org, dedicated to matching donors to health systems in need of protective equipment. Dr. Ranney has disclosed the following relevant financial relationships: Serve(d) as a speaker or a member of a speakers bureau for: Medscape; Merck.
Jane Barnsteiner, PhD, RN, is an emeritus professor at the University of Pennsylvania School of Nursing and an expert on patient safety, quality improvement, and system modeling. In addition to her teaching responsibilities, she was director of translational research at the Hospital of the University of Pennsylvania. Jane was one of the developers of the Quality and Safety in Education for Nurses (QSEN) initiative and is co-editor of Quality and Safety in Nursing: A Competency Based Approach to Improving Outcomes, published by Wiley. She has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Breakthrough COVID dangerous for vaccinated cancer patients
, according to a study published in JAMA Oncology.
The risks were highest among patients who had certain cancers and those who had received cancer treatment within the past year.
“These results emphasize the need for patients with cancer to maintain mitigation practice, especially with the emergence of different virus variants and the waning immunity of vaccines,” the study authors wrote.
Researchers at Case Western Reserve University in Cleveland analyzed electronic health record data for more than 636,000 vaccinated patients, including more than 45,000 vaccinated patients with cancer. They looked for the time trends, risks, and outcomes of breakthrough COVID-19 infections for vaccinated cancer patients in the United States between December 2020 and November 2021.
Overall, the cumulative risk of breakthrough infections in vaccinated cancer patients was 13.6%, with the highest risk for pancreatic (24.7%), liver (22.8%), lung (20.4%), and colorectal (17.5%) cancers and the lowest risk for thyroid (10.3%), endometrial (11.9%), and breast (11.9%) cancers, versus 4.9% in vaccinated patients without cancer.
Patients who had medical encounters for their cancer within the past year had a higher risk for a breakthrough infection, particularly those with breast cancer, blood cancers, colorectal cancer, bladder cancer, and pancreatic cancer.
Among patients with cancer, the overall risk for hospitalization after a breakthrough infection was 31.6%, as compared with 3.9% in those without a breakthrough infection. In addition, the risk of death was 6.7% after a breakthrough infection, as compared with 1.3% in those without a breakthrough infection.
Among patients who didn’t have cancer, the overall hospitalization risk was 25.9% in patients with a breakthrough infection, as compared with 3% in those without a breakthrough infection. The overall risk of death was 2.7% after a breakthrough infection, as compared with 0.5% in those without a breakthrough infection.
In addition, breakthrough infections continuously increased for all patients from December 2020 to November 2021, with the numbers consistently higher among patients with cancer.
“This increasing time trend may reflect waning immunity of vaccines, the emergence of different virus variants, and varied measures taken by individuals and communities over time during the pandemic,” the study authors wrote.
Vaccines are likely less protective against coronavirus infection in cancer patients, and in turn, cancer patients may be more susceptible to COVID-19 infections, the researchers wrote. As breakthrough infections continue to increase for everyone, patients with cancer will face increased risks for severe breakthroughs, hospitalization, and death, they concluded.
A version of this article first appeared on WebMD.com.
, according to a study published in JAMA Oncology.
The risks were highest among patients who had certain cancers and those who had received cancer treatment within the past year.
“These results emphasize the need for patients with cancer to maintain mitigation practice, especially with the emergence of different virus variants and the waning immunity of vaccines,” the study authors wrote.
Researchers at Case Western Reserve University in Cleveland analyzed electronic health record data for more than 636,000 vaccinated patients, including more than 45,000 vaccinated patients with cancer. They looked for the time trends, risks, and outcomes of breakthrough COVID-19 infections for vaccinated cancer patients in the United States between December 2020 and November 2021.
Overall, the cumulative risk of breakthrough infections in vaccinated cancer patients was 13.6%, with the highest risk for pancreatic (24.7%), liver (22.8%), lung (20.4%), and colorectal (17.5%) cancers and the lowest risk for thyroid (10.3%), endometrial (11.9%), and breast (11.9%) cancers, versus 4.9% in vaccinated patients without cancer.
Patients who had medical encounters for their cancer within the past year had a higher risk for a breakthrough infection, particularly those with breast cancer, blood cancers, colorectal cancer, bladder cancer, and pancreatic cancer.
Among patients with cancer, the overall risk for hospitalization after a breakthrough infection was 31.6%, as compared with 3.9% in those without a breakthrough infection. In addition, the risk of death was 6.7% after a breakthrough infection, as compared with 1.3% in those without a breakthrough infection.
Among patients who didn’t have cancer, the overall hospitalization risk was 25.9% in patients with a breakthrough infection, as compared with 3% in those without a breakthrough infection. The overall risk of death was 2.7% after a breakthrough infection, as compared with 0.5% in those without a breakthrough infection.
In addition, breakthrough infections continuously increased for all patients from December 2020 to November 2021, with the numbers consistently higher among patients with cancer.
“This increasing time trend may reflect waning immunity of vaccines, the emergence of different virus variants, and varied measures taken by individuals and communities over time during the pandemic,” the study authors wrote.
Vaccines are likely less protective against coronavirus infection in cancer patients, and in turn, cancer patients may be more susceptible to COVID-19 infections, the researchers wrote. As breakthrough infections continue to increase for everyone, patients with cancer will face increased risks for severe breakthroughs, hospitalization, and death, they concluded.
A version of this article first appeared on WebMD.com.
, according to a study published in JAMA Oncology.
The risks were highest among patients who had certain cancers and those who had received cancer treatment within the past year.
“These results emphasize the need for patients with cancer to maintain mitigation practice, especially with the emergence of different virus variants and the waning immunity of vaccines,” the study authors wrote.
Researchers at Case Western Reserve University in Cleveland analyzed electronic health record data for more than 636,000 vaccinated patients, including more than 45,000 vaccinated patients with cancer. They looked for the time trends, risks, and outcomes of breakthrough COVID-19 infections for vaccinated cancer patients in the United States between December 2020 and November 2021.
Overall, the cumulative risk of breakthrough infections in vaccinated cancer patients was 13.6%, with the highest risk for pancreatic (24.7%), liver (22.8%), lung (20.4%), and colorectal (17.5%) cancers and the lowest risk for thyroid (10.3%), endometrial (11.9%), and breast (11.9%) cancers, versus 4.9% in vaccinated patients without cancer.
Patients who had medical encounters for their cancer within the past year had a higher risk for a breakthrough infection, particularly those with breast cancer, blood cancers, colorectal cancer, bladder cancer, and pancreatic cancer.
Among patients with cancer, the overall risk for hospitalization after a breakthrough infection was 31.6%, as compared with 3.9% in those without a breakthrough infection. In addition, the risk of death was 6.7% after a breakthrough infection, as compared with 1.3% in those without a breakthrough infection.
Among patients who didn’t have cancer, the overall hospitalization risk was 25.9% in patients with a breakthrough infection, as compared with 3% in those without a breakthrough infection. The overall risk of death was 2.7% after a breakthrough infection, as compared with 0.5% in those without a breakthrough infection.
In addition, breakthrough infections continuously increased for all patients from December 2020 to November 2021, with the numbers consistently higher among patients with cancer.
“This increasing time trend may reflect waning immunity of vaccines, the emergence of different virus variants, and varied measures taken by individuals and communities over time during the pandemic,” the study authors wrote.
Vaccines are likely less protective against coronavirus infection in cancer patients, and in turn, cancer patients may be more susceptible to COVID-19 infections, the researchers wrote. As breakthrough infections continue to increase for everyone, patients with cancer will face increased risks for severe breakthroughs, hospitalization, and death, they concluded.
A version of this article first appeared on WebMD.com.
FROM JAMA ONCOLOGY
Long-term smell loss in COVID-19 tied to damage in the brain’s olfactory bulb
Patients with COVID-19, especially those with an altered sense of smell, have significantly more axon and microvasculopathy damage in the brain’s olfactory tissue versus non-COVID patients. These new findings from a postmortem study may explain long-term loss of smell in some patients with the virus.
“The striking axonal pathology in some cases indicates that olfactory dysfunction in COVID-19 may be severe and permanent,” the investigators led by Cheng-Ying Ho, MD, PhD, associate professor, department of pathology, Johns Hopkins University School of Medicine, Baltimore, write.
“The results show the damage caused by COVID can extend beyond the nasal cavity and involve the brain,” Dr. Ho told this news organization.
The study was published online April 11 in JAMA Neurology.
A more thorough investigation
Patients infected with SARS-CoV-2, which causes COVID-19, present with a wide range of symptoms. In addition to respiratory illnesses, they may exhibit various nonrespiratory manifestations of COVID-19.
One of the most prevalent of these is olfactory dysfunction. Research shows such dysfunction, including anosmia (loss of smell), hyposmia (reduced sense of smell), and parosmia (smells that are distorted or unpleasant), affects 30%-60% of patients with COVID-19, said Dr. Ho.
However, these statistics come from research before the advent of the Omicron variant, which evidence suggests causes less smell loss in patients with COVID, she said.
Previous studies in this area mainly focused on the lining of the nasal cavity. “We wanted to go a step beyond to see how the olfactory bulb was affected by COVID infection,” said Dr. Ho.
The study included 23 deceased patients with confirmed COVID-19 ranging in age from 28 to 93 years at death (median 62 years, 60.9% men). It also included 14 controls who tested negative for COVID-19, ranging in age from 20 to 77 years (median 53.5 years, 50% men).
Researchers collected postmortem tissue from the brain, lung, and other organs and reviewed pertinent clinical information.
Most patients with COVID died of COVID pneumonia or related complications, although some died from a different cause. Some had an active COVID infection and others were “post infection, meaning they were in the recovery stage,” said Dr. Ho.
Six patients with COVID-19 and eight controls had significant brain pathology.
Compared with controls, those with COVID-19 showed significantly worse olfactory axonal damage. The mean axon pathology score (range 1-3 with 3 the worst) was 1.921 in patients with COVID-19 and 1.198 in controls (95% confidence interval, 0.444-1.002; P < .001).
The mean axon density in the lateral olfactory tract was significantly less in patients with COVID-19 than in controls (P = .002), indicating a 23% loss of olfactory axons in the COVID group.
Comparing COVID patients with and without reported loss of smell, researchers found those with an altered sense of smell had significantly more severe olfactory axon pathology.
Vascular damage
Patients with COVID also had worse vascular damage. The mean microvasculopathy score (range, 1-3) was 1.907 in patients with COVID-19 and 1.405 in controls (95% CI, 0.259-0.745; P < .001).
There was no evidence of the virus in the olfactory tissue of most patients, suggesting the olfactory pathology was likely caused by vascular damage, said Dr. Ho.
What’s unique about SARS-CoV-2 is that, although it’s a respiratory virus, it’s capable of infecting endothelial cells lining vessels.
“Other respiratory viruses only attack the airways and won’t attack vessels, but vascular damage has been seen in the heart and lung in COVID patients, and our study showed the same findings in the olfactory bulb,” Dr. Ho explained.
The researchers divided patients with COVID by infection severity: mild, moderate, severe, and critical. Interestingly, those with the most severe olfactory pathology were the ones with milder infections, said Dr. Ho.
She noted other studies have reported patients with mild infection are more likely to lose the sense of smell than those with severe infection, but she’s skeptical about this finding.
“Patients with severe COVID are usually hospitalized and intubated, so it’s hard to get them to tell you whether they’ve lost smell or not; they have other more important issues to deal with like respiratory failure,” said Dr. Ho.
Advanced age is associated with neuropathologic changes, such as tau deposits, so the researchers conducted an analysis factoring in age-related brain changes. They found a COVID-19 diagnosis remained associated with increased axonal pathology, reduced axonal density, and increased vascular pathology.
“This means that the COVID patients had more severe olfactory pathology not just because they had more tau pathology,” Dr. Ho added.
New guidance for patients
Commenting for this news organization, Davangere P. Devanand, MD, professor of psychiatry and neurology and director of geriatric psychiatry, Columbia University Irving Medical Center, New York, said the findings indicate the damage from COVID in the olfactory pathway may not be reversible as was previously thought.
“This has been suggested before as a possibility, but the autopsy findings in this case series indicate clearly that there may be permanent damage,” he said.
The results highlight the need to monitor patients with COVID for a smell deficit, said Dr. Devanand.
“Assuring patients of a full recovery in smell and taste may not be sound advice, although recovery does occur in many patients,” he added.
He praised the study design, especially the blinding of raters, but noted a number of weaknesses, including the small sample size and the age and gender discrepancies between the groups.
Another possible limitation was inclusion of patients with Alzheimer’s and Lewy body pathology, said Dr. Devanand.
“These patients typically already have pathology in the olfactory pathways, which means we don’t know if it was COVID or the underlying brain pathology contributing to smell difficulties in these patients,” he said.
He noted that, unlike deceased COVID cases in the study, patients who survive COVID may not experience axonal and microvascular injury in olfactory neurons and pathways and their sense of smell may make a full return.
Dr. Devanand said he would have liked more detailed information on the clinical history and course of study participants and whether these factors affected the pathology findings.
The study was supported by grants from the National Institutes of Health.
Dr. Ho and Dr. Devanand have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Patients with COVID-19, especially those with an altered sense of smell, have significantly more axon and microvasculopathy damage in the brain’s olfactory tissue versus non-COVID patients. These new findings from a postmortem study may explain long-term loss of smell in some patients with the virus.
“The striking axonal pathology in some cases indicates that olfactory dysfunction in COVID-19 may be severe and permanent,” the investigators led by Cheng-Ying Ho, MD, PhD, associate professor, department of pathology, Johns Hopkins University School of Medicine, Baltimore, write.
“The results show the damage caused by COVID can extend beyond the nasal cavity and involve the brain,” Dr. Ho told this news organization.
The study was published online April 11 in JAMA Neurology.
A more thorough investigation
Patients infected with SARS-CoV-2, which causes COVID-19, present with a wide range of symptoms. In addition to respiratory illnesses, they may exhibit various nonrespiratory manifestations of COVID-19.
One of the most prevalent of these is olfactory dysfunction. Research shows such dysfunction, including anosmia (loss of smell), hyposmia (reduced sense of smell), and parosmia (smells that are distorted or unpleasant), affects 30%-60% of patients with COVID-19, said Dr. Ho.
However, these statistics come from research before the advent of the Omicron variant, which evidence suggests causes less smell loss in patients with COVID, she said.
Previous studies in this area mainly focused on the lining of the nasal cavity. “We wanted to go a step beyond to see how the olfactory bulb was affected by COVID infection,” said Dr. Ho.
The study included 23 deceased patients with confirmed COVID-19 ranging in age from 28 to 93 years at death (median 62 years, 60.9% men). It also included 14 controls who tested negative for COVID-19, ranging in age from 20 to 77 years (median 53.5 years, 50% men).
Researchers collected postmortem tissue from the brain, lung, and other organs and reviewed pertinent clinical information.
Most patients with COVID died of COVID pneumonia or related complications, although some died from a different cause. Some had an active COVID infection and others were “post infection, meaning they were in the recovery stage,” said Dr. Ho.
Six patients with COVID-19 and eight controls had significant brain pathology.
Compared with controls, those with COVID-19 showed significantly worse olfactory axonal damage. The mean axon pathology score (range 1-3 with 3 the worst) was 1.921 in patients with COVID-19 and 1.198 in controls (95% confidence interval, 0.444-1.002; P < .001).
The mean axon density in the lateral olfactory tract was significantly less in patients with COVID-19 than in controls (P = .002), indicating a 23% loss of olfactory axons in the COVID group.
Comparing COVID patients with and without reported loss of smell, researchers found those with an altered sense of smell had significantly more severe olfactory axon pathology.
Vascular damage
Patients with COVID also had worse vascular damage. The mean microvasculopathy score (range, 1-3) was 1.907 in patients with COVID-19 and 1.405 in controls (95% CI, 0.259-0.745; P < .001).
There was no evidence of the virus in the olfactory tissue of most patients, suggesting the olfactory pathology was likely caused by vascular damage, said Dr. Ho.
What’s unique about SARS-CoV-2 is that, although it’s a respiratory virus, it’s capable of infecting endothelial cells lining vessels.
“Other respiratory viruses only attack the airways and won’t attack vessels, but vascular damage has been seen in the heart and lung in COVID patients, and our study showed the same findings in the olfactory bulb,” Dr. Ho explained.
The researchers divided patients with COVID by infection severity: mild, moderate, severe, and critical. Interestingly, those with the most severe olfactory pathology were the ones with milder infections, said Dr. Ho.
She noted other studies have reported patients with mild infection are more likely to lose the sense of smell than those with severe infection, but she’s skeptical about this finding.
“Patients with severe COVID are usually hospitalized and intubated, so it’s hard to get them to tell you whether they’ve lost smell or not; they have other more important issues to deal with like respiratory failure,” said Dr. Ho.
Advanced age is associated with neuropathologic changes, such as tau deposits, so the researchers conducted an analysis factoring in age-related brain changes. They found a COVID-19 diagnosis remained associated with increased axonal pathology, reduced axonal density, and increased vascular pathology.
“This means that the COVID patients had more severe olfactory pathology not just because they had more tau pathology,” Dr. Ho added.
New guidance for patients
Commenting for this news organization, Davangere P. Devanand, MD, professor of psychiatry and neurology and director of geriatric psychiatry, Columbia University Irving Medical Center, New York, said the findings indicate the damage from COVID in the olfactory pathway may not be reversible as was previously thought.
“This has been suggested before as a possibility, but the autopsy findings in this case series indicate clearly that there may be permanent damage,” he said.
The results highlight the need to monitor patients with COVID for a smell deficit, said Dr. Devanand.
“Assuring patients of a full recovery in smell and taste may not be sound advice, although recovery does occur in many patients,” he added.
He praised the study design, especially the blinding of raters, but noted a number of weaknesses, including the small sample size and the age and gender discrepancies between the groups.
Another possible limitation was inclusion of patients with Alzheimer’s and Lewy body pathology, said Dr. Devanand.
“These patients typically already have pathology in the olfactory pathways, which means we don’t know if it was COVID or the underlying brain pathology contributing to smell difficulties in these patients,” he said.
He noted that, unlike deceased COVID cases in the study, patients who survive COVID may not experience axonal and microvascular injury in olfactory neurons and pathways and their sense of smell may make a full return.
Dr. Devanand said he would have liked more detailed information on the clinical history and course of study participants and whether these factors affected the pathology findings.
The study was supported by grants from the National Institutes of Health.
Dr. Ho and Dr. Devanand have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
Patients with COVID-19, especially those with an altered sense of smell, have significantly more axon and microvasculopathy damage in the brain’s olfactory tissue versus non-COVID patients. These new findings from a postmortem study may explain long-term loss of smell in some patients with the virus.
“The striking axonal pathology in some cases indicates that olfactory dysfunction in COVID-19 may be severe and permanent,” the investigators led by Cheng-Ying Ho, MD, PhD, associate professor, department of pathology, Johns Hopkins University School of Medicine, Baltimore, write.
“The results show the damage caused by COVID can extend beyond the nasal cavity and involve the brain,” Dr. Ho told this news organization.
The study was published online April 11 in JAMA Neurology.
A more thorough investigation
Patients infected with SARS-CoV-2, which causes COVID-19, present with a wide range of symptoms. In addition to respiratory illnesses, they may exhibit various nonrespiratory manifestations of COVID-19.
One of the most prevalent of these is olfactory dysfunction. Research shows such dysfunction, including anosmia (loss of smell), hyposmia (reduced sense of smell), and parosmia (smells that are distorted or unpleasant), affects 30%-60% of patients with COVID-19, said Dr. Ho.
However, these statistics come from research before the advent of the Omicron variant, which evidence suggests causes less smell loss in patients with COVID, she said.
Previous studies in this area mainly focused on the lining of the nasal cavity. “We wanted to go a step beyond to see how the olfactory bulb was affected by COVID infection,” said Dr. Ho.
The study included 23 deceased patients with confirmed COVID-19 ranging in age from 28 to 93 years at death (median 62 years, 60.9% men). It also included 14 controls who tested negative for COVID-19, ranging in age from 20 to 77 years (median 53.5 years, 50% men).
Researchers collected postmortem tissue from the brain, lung, and other organs and reviewed pertinent clinical information.
Most patients with COVID died of COVID pneumonia or related complications, although some died from a different cause. Some had an active COVID infection and others were “post infection, meaning they were in the recovery stage,” said Dr. Ho.
Six patients with COVID-19 and eight controls had significant brain pathology.
Compared with controls, those with COVID-19 showed significantly worse olfactory axonal damage. The mean axon pathology score (range 1-3 with 3 the worst) was 1.921 in patients with COVID-19 and 1.198 in controls (95% confidence interval, 0.444-1.002; P < .001).
The mean axon density in the lateral olfactory tract was significantly less in patients with COVID-19 than in controls (P = .002), indicating a 23% loss of olfactory axons in the COVID group.
Comparing COVID patients with and without reported loss of smell, researchers found those with an altered sense of smell had significantly more severe olfactory axon pathology.
Vascular damage
Patients with COVID also had worse vascular damage. The mean microvasculopathy score (range, 1-3) was 1.907 in patients with COVID-19 and 1.405 in controls (95% CI, 0.259-0.745; P < .001).
There was no evidence of the virus in the olfactory tissue of most patients, suggesting the olfactory pathology was likely caused by vascular damage, said Dr. Ho.
What’s unique about SARS-CoV-2 is that, although it’s a respiratory virus, it’s capable of infecting endothelial cells lining vessels.
“Other respiratory viruses only attack the airways and won’t attack vessels, but vascular damage has been seen in the heart and lung in COVID patients, and our study showed the same findings in the olfactory bulb,” Dr. Ho explained.
The researchers divided patients with COVID by infection severity: mild, moderate, severe, and critical. Interestingly, those with the most severe olfactory pathology were the ones with milder infections, said Dr. Ho.
She noted other studies have reported patients with mild infection are more likely to lose the sense of smell than those with severe infection, but she’s skeptical about this finding.
“Patients with severe COVID are usually hospitalized and intubated, so it’s hard to get them to tell you whether they’ve lost smell or not; they have other more important issues to deal with like respiratory failure,” said Dr. Ho.
Advanced age is associated with neuropathologic changes, such as tau deposits, so the researchers conducted an analysis factoring in age-related brain changes. They found a COVID-19 diagnosis remained associated with increased axonal pathology, reduced axonal density, and increased vascular pathology.
“This means that the COVID patients had more severe olfactory pathology not just because they had more tau pathology,” Dr. Ho added.
New guidance for patients
Commenting for this news organization, Davangere P. Devanand, MD, professor of psychiatry and neurology and director of geriatric psychiatry, Columbia University Irving Medical Center, New York, said the findings indicate the damage from COVID in the olfactory pathway may not be reversible as was previously thought.
“This has been suggested before as a possibility, but the autopsy findings in this case series indicate clearly that there may be permanent damage,” he said.
The results highlight the need to monitor patients with COVID for a smell deficit, said Dr. Devanand.
“Assuring patients of a full recovery in smell and taste may not be sound advice, although recovery does occur in many patients,” he added.
He praised the study design, especially the blinding of raters, but noted a number of weaknesses, including the small sample size and the age and gender discrepancies between the groups.
Another possible limitation was inclusion of patients with Alzheimer’s and Lewy body pathology, said Dr. Devanand.
“These patients typically already have pathology in the olfactory pathways, which means we don’t know if it was COVID or the underlying brain pathology contributing to smell difficulties in these patients,” he said.
He noted that, unlike deceased COVID cases in the study, patients who survive COVID may not experience axonal and microvascular injury in olfactory neurons and pathways and their sense of smell may make a full return.
Dr. Devanand said he would have liked more detailed information on the clinical history and course of study participants and whether these factors affected the pathology findings.
The study was supported by grants from the National Institutes of Health.
Dr. Ho and Dr. Devanand have reported no relevant financial disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Violaceous Nodules on the Lower Leg
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.
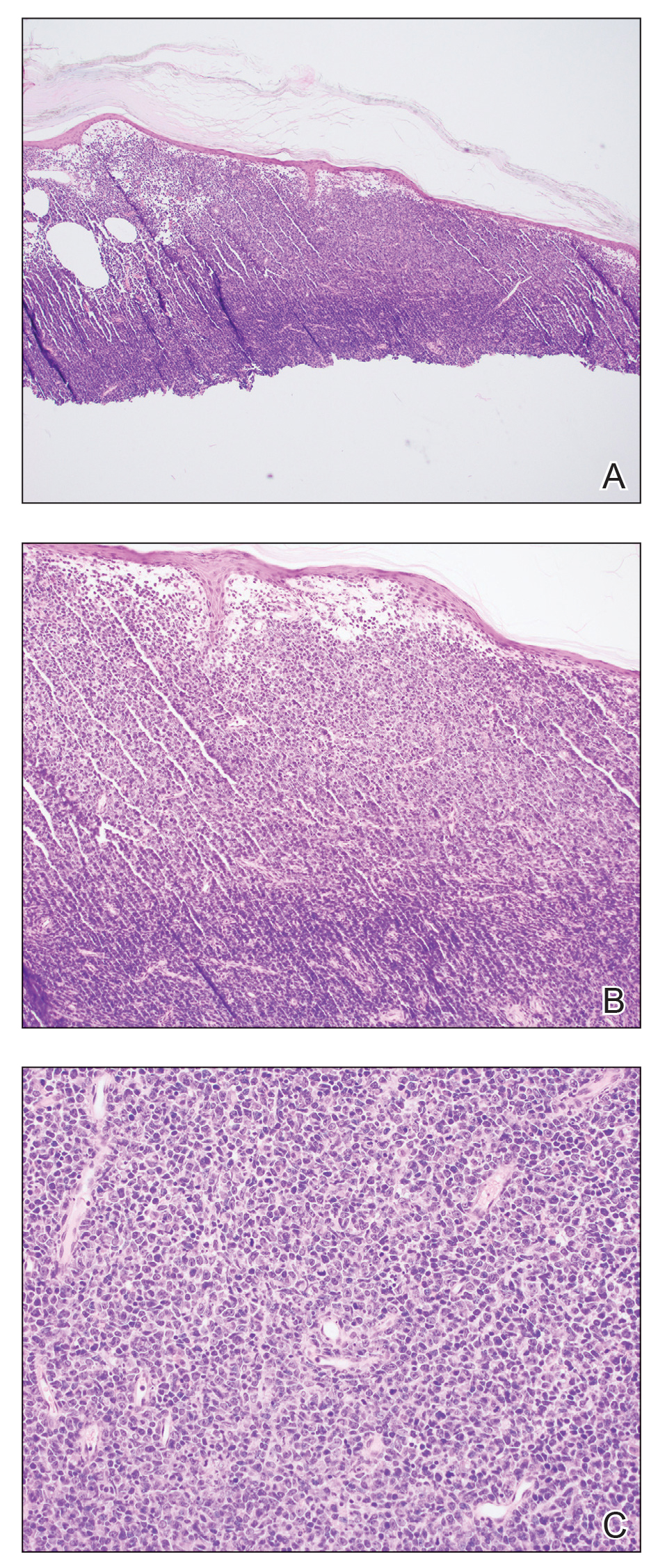
Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
The Diagnosis: Cutaneous B-cell Lymphoma
Shave biopsies of 3 lesions revealed a dense, diffuse, atypical lymphoid infiltrate occupying the entirety of the dermis and obscuring the dermoepidermal junction. The infiltrate consisted predominantly of largesized lymphoid cells with fine chromatin and conspicuous nucleoli (Figure). Immunohistochemistry was positive for CD45 and CD20, indicating B-cell lineage. Bcl-2, multiple myeloma oncogene 1, and forkhead box protein P1 also were expressed in the vast majority of lesional cells, distinguishing the lesion from other forms of cutaneous B-cell lymphomas.1 These findings were consistent with large B-cell lymphoma with a high proliferation index, consistent with primary cutaneous diffuse large B-cell lymphoma, leg type, which often presents on the lower leg.2 The patient had a negative systemic workup including bone marrow biopsy. He was started on the R-CEOP (rituximab, cyclophosphamide, etoposide, vincristine, prednisone) chemotherapy regimen.

Primary cutaneous diffuse large B-cell lymphoma, leg type, is an intermediately aggressive and rare form of B-cell lymphoma with a poor prognosis that primarily affects elderly female patients. Primary cutaneous diffuse large B-cell lymphoma, leg type, accounts for only 1% to 3% of cutaneous lymphomas and approximately 10% to 20% of primary cutaneous B-cell lymphomas.2 It typically presents as multiple red-brown or bluish nodules on the lower extremities or trunk. Presentation as a solitary nodule also is possible.1,2 Histologic analysis of primary cutaneous diffuse large B-cell lymphoma, leg type, reveals large cells with round nuclei (immunoblasts and centroblasts), and the immunohistochemical profile shows strong Bcl-2 expression often accompanied by the multiple myeloma oncogene 1 protein.3 The 5-year survival rate is approximately 50%, which is lower than other types of primary cutaneous B-cell lymphomas, and the progression of disease is characterized by frequent relapses and involvement of extracutaneous regions such as the lymph nodes, bone marrow, and central nervous system.1,2,4 Patients with multiple tumors on the leg have a particularly poor prognosis; in particular, having 1 or more lesions on the leg results in a 43% 3-year survival rate while having multiple lesions has a 36% 3-year survival rate compared with a 77% 3-year survival rate for patients with the non–leg subtype or a single lesion.3 Treatment with rituximab has been shown to be effective in at least short-term control of the disease, and the R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) regimen is the standard of treatment.3,4
Primary cutaneous diffuse large B-cell lymphoma, leg type, can mimic multiple other cutaneous presentations of disease. Myeloid sarcoma (leukemia cutis) is a rare condition that presents as an extramedullary tumor often simultaneously with the onset or relapse of acute myeloid leukemia.5 Our patient had no history of leukemia, but myeloid sarcoma may predate acute myeloid leukemia in about a quarter of cases.5 It most commonly presents histologically as a diffuse dermal infiltrate that splays between collagen bundles and often is associated with an overlying Grenz zone. A nodular, or perivascular and periadnexal, pattern also may be seen. Upon closer inspection, the infiltrate is composed of immature myeloid cells (blasts) with background inflammation occasionally containing eosinophils. The immunohistochemical profile varies depending on the type of differentiation and degree of maturity of the cells. The histologic findings in our patient were inconsistent with myeloid sarcoma.
Erythema elevatum diutinum (EED) usually presents as dark red, brown, or violaceous papules or plaques and often is found on the extensor surfaces. It often is associated with hematologic abnormalities as well as recurrent bacterial or viral infections.6 Histologically, EED initially manifests as leukocytoclastic vasculitis with a mixed inflammatory infiltrate typically featuring an abundance of neutrophils, making this condition unlikely in this case. As the lesion progresses, fibrosis and scarring ensue as inflammation wanes. The fibrosis often is described as having an onion skin–like pattern, which is characteristic of established EED lesions. Our patient had no history of vasculitis, and the histologic findings were inconsistent with EED.
Angiosarcoma can present as a central nodule surrounded by an erythematous plaque. Although potentially clinically similar to primary cutaneous diffuse large B-cell lymphoma, leg type, angiosarcoma was unlikely in this case because of an absence of lymphedema and no history of radiation to the leg, both of which are key historical features of angiosarcoma.7 Additionally, the histology of cutaneous angiosarcoma is marked by vascular proliferation, which was not seen in the lesion biopsied in our patient. The histology of angiosarcoma is that of an atypical vascular proliferation, and a hallmark feature is infiltration between collagen, often referred to as giving the appearance of dissection between collagen bundles. The degree of atypia can vary widely, and epithelioid variants exist, producing a potential diagnostic pitfall. Lesional cells are positive for vascular markers, which can be used for confirmation of the endothelial lineage.
Sarcoidosis is notorious for its mimicry, which can be the case both clinically and histologically. Characteristic pathology of sarcoidosis is that of well-formed epithelioid granulomas with minimal associated inflammation and lack of caseating necrosis. Our patient had no known history of systemic sarcoidosis, and the pathologic features of noncaseating granulomas were not present. As a diagnosis of exclusion, correlation with special stains and culture studies is necessary to exclude an infectious process. The differential diagnosis for sarcoidal granulomatous dermatitis also includes foreign body reaction, inflammatory bowel disease, and granulomatous cheilitis, among others.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
- Athalye L, Nami N, Shitabata P. A rare case of primary cutaneous diffuse large B-cell lymphoma, leg type. Cutis. 2018;102:E31-E34.
- Sokol L, Naghashpour M, Glass LF. Primary cutaneous B-cell lymphomas: recent advances in diagnosis and management. Cancer Control. 2012;19:236-244. doi:10.1177/107327481201900308
- Grange F, Beylot-Barry M, Courville P, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type: clinicopathologic features and prognostic analysis in 60 cases. Arch Dermatol. 2007;143:1144-1150. doi:10.1001/archderm.143.9.1144
- Patsatsi A, Kyriakou A, Karavasilis V, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type, with multiple local relapses: case presentation and brief review of literature. Hippokratia. 2013;17:174-176.
- Avni B, Koren-Michowitz M. Myeloid sarcoma: current approach and therapeutic options. Ther Adv Hematol. 2011;2:309-316.
- Yiannias JA, el-Azhary RA, Gibson LE. Erythema elevatum diutinum: a clinical and histopathologic study of 13 patients. J Am Acad Dermatol. 1992;26:38-44.
- Scholtz J, Mishra MM, Simman R. Cutaneous angiosarcoma of the lower leg. Cutis. 2018;102:E8-E11.
A 79-year-old man presented to the dermatology clinic with 4 enlarging, asymptomatic, violaceous, desquamating nodules on the left pretibial region and calf of 3 months’ duration. He denied any constitutional symptoms such as night sweats or weight loss. His medical history included a malignant melanoma on the left ear that was excised 5 years prior. He also had a history of peripheral edema, hypertension, and rheumatoid arthritis, as well as a 50-pack-year history of smoking. Physical examination revealed 2 large nodules measuring 3.0×3.0 cm each and 2 smaller nodules measuring 1.0×1.0 cm each. There was no appreciable lymphadenopathy.
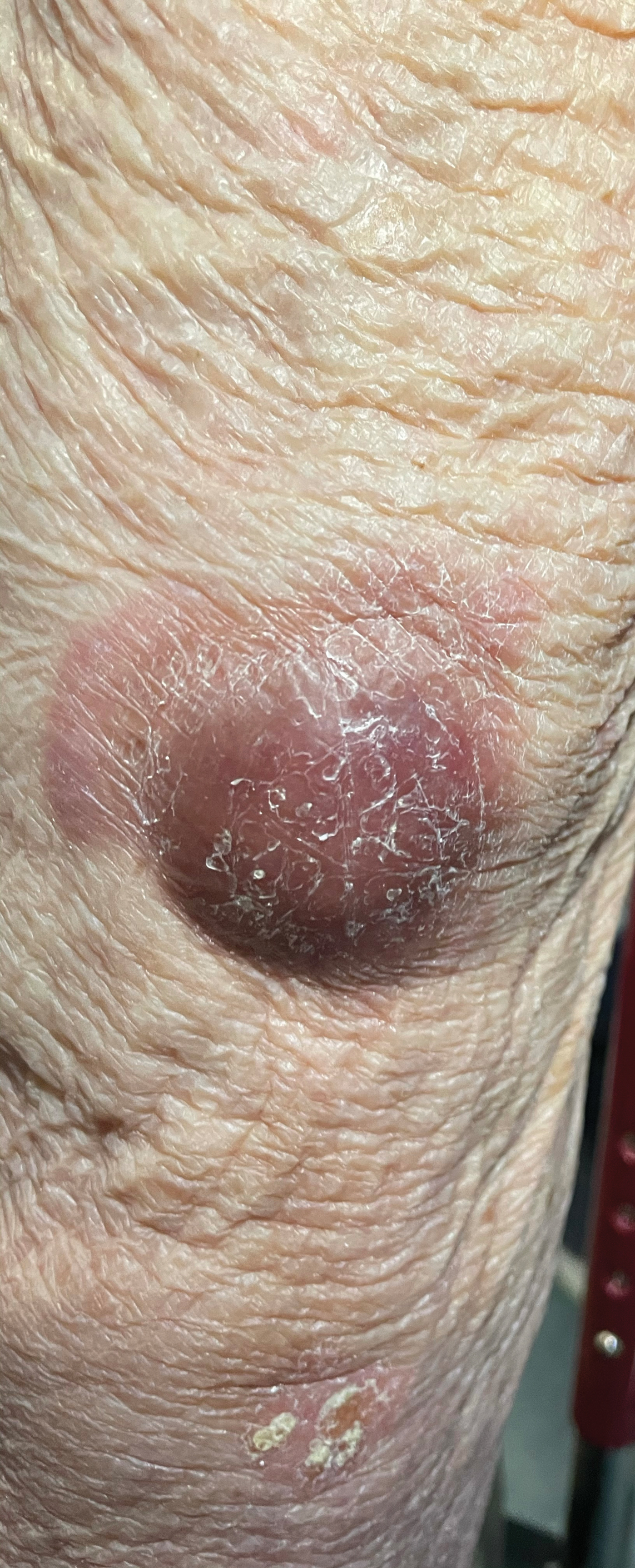
Sex differences in COPD slow to be recognized, treated
When Sigmund Freud claimed that “anatomy is destiny” he was referring to anatomical sex as a determinant of personality traits. Expert consensus statements have previously offered some recommendations for managing these syndromes, but clinical data are scarce, so the present review “is intended to establish a starting point for future research,”
That notion has been widely discredited, but Freud appears to be inadvertently right in one respect: When it comes to chronic obstructive pulmonary disease (COPD), anatomy really is destiny, and sex may be as well, pulmonary researchers say.
There is a growing body of evidence to indicate that COPD affects men and women differently, and that men and women patients with COPD require different clinical management. Yet women are often underdiagnosed or misdiagnosed, partly because of poorly understood sex differences, but also because of cultural biases.
But plunging any farther into the weeds, it’s important to define terms. Although various investigators have used the terms “sex” and “gender” interchangeably, sex is the preferred term when referring to biological attributes of individual patients, while gender refers to personal identity.
These distinctions are important, contended Amik Sodhi, MBBS, MPH, from the division of allergy, pulmonology, and critical care medicine at the University of Wisconsin–Madison.
“Sex is essentially a biologic construct, so it’s got to do with the sex chromosomes, the genetics of that person, and it refers to the anatomic variations that can change susceptibility to different diseases,” she said in an interview.
An example of sex differences or “sexual dimorphism” can be found in a recent meta-analysis of sex-based genetic associations by Megan Hardin, MD, MPH from Brigham & Women’s Hospital in Boston and colleagues.
They reported that CELSR1, a gene involved in fetal lung development, was expressed more among women than among men and that a single nucleotide polymorphism in the gene was associated with COPD among women smokers, but not among men smokers.
The finding points to a potential risk locus for COPD in women, and could help shed light on sexual dimorphism in COPD, Dr. Hardin and colleagues said.
In contrast to sex, “gender is more of a psychosocial construct which can impact how diseases manifest themselves, how they are potentially managed, and what outcomes might occur for that particular disease,” Dr. Sodhi said.
She and her colleagues recently published a review of sex and gender in common lung disorders and sleep in the journal CHEST, where they wrote that the “influence of sex and gender is portrayed in epidemiological data, disease pathogenesis and pathophysiology, clinical manifestations, response to treatment, access to care, and health outcomes. Hence, sex and gender should be considered in all types of research, clinical practice and educational curricula.”
For example, as previously reported at the 2021 annual meeting of the American Thoracic Society, sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with COPD may point to different criteria for diagnosing cardiac comorbidities in women and men.
Those conclusions came from a retrospective analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease.
The investigators looked at the patients’ clinical history, comorbidities, lung function, COPD Assessment Test scores, and modified Medical Research Council (mMRC) dyspnea score, and found significant differences between men and women for most functional parameters and comorbidities, and for CAT items of cough, phlegm, and energy.
In logistic regression analysis, predictors for cardiac disease in men were energy, mMRC score, smoking status, body mass index, age, and spirometric lung function, but in women only age was significantly predictive for cardiac disease.
An example of gender effects on COPD differences in men and women is the increase in cigarette advertising aimed at women in the 1960s and the advent of women-targeted brands such as Virginia Slims, which in turn lead to increased smoking rates among women. In addition, in the developing world, where the sex/gender gap in COPD is narrowing, women tend to have greater exposure to wood smoke and cooking fuels in unventilated or poorly ventilated spaces, compared with men.
Increasing incidence among women
According to the Centers for Disease Control and Prevention, chronic lower respiratory diseases, primarily COPD, were the fourth-leading cause of death in women in the United States in 2018, following only heart disease, cancer, and accidents/injuries.
And as a CDC analysis of data from the 2013 Behavioral Risk Factor Surveillance System showed, women were more likely to report being told by a physician that they had COPD than did men (6.6%, compared with 5.4%).
Dr. Sodhi and colleagues noted that, at all time points examined from 2005 to 2014, women had a higher proportion than men of COPD hospitalizations and in-hospital deaths. They also noted that female sex is associated with a threefold risk for severe early-onset COPD, and that women with COPD have lower diffusion capacity of lungs for carbon monoxide, despite having higher predicted forced expiratory volume in 1 second, compared with men.
“Historically, COPD wasn’t a disease that was so prevalent in women. It’s been in the past 20 years that the trends have changed,” said Patricia Silveyra, MSc, PhD, ATSF, associate professor of environmental and occupational health at Indiana University, Bloomington.
The increasing prevalence of COPD among women cannot be explained by smoking alone, she said in an interview.
“It used to be thought that it was because more women smoked, but actually a lot of women who don’t smoke can develop COPD, so it appears to be probably something environmental, but because it used to be a disease of older men, in the clinic there was also a bias to diagnose men with COPD, and women with asthma, so a lot of women went underdiagnosed,” Dr. Silveyra said.
In their review, Dr. Sodhi and colleagues noted that women with COPD “may be underdiagnosed as a result of having different symptoms from those classically recognized. Reasons for underdiagnosis or a delay in diagnosis may also be due to lack of a formal evaluation with spirometry, women seeking care later in the course of disease, physician bias, or associated fatigue or depression misdirecting diagnostic strategies. Underdiagnosis may be associated with psychological distress and worse health-related quality of life.”
Although the evidence is mixed, women tend to present more frequently with the chronic bronchitis phenotype of COPD, compared with the emphysema phenotype, and women tend to have greater degrees of pulmonary function impairment when exposed to tobacco smoke, even after controlling for differences in height and weight.
“For the same amount of exposure to tobacco smoke, females are likely to develop more severe airflow limitation at an earlier age than males, and have more exacerbation,” Dr. Sodhi and colleagues wrote.
Both Dr. Silveyra and Dr. Sodhi said that reason why men and women differ in their physiological reactions to smoke are still unknown.
Sex differences in drug responses
There is only limited evidence to indicate that women and men respond differently to various therapeutic agents, but what is clear is that more research into this area is needed, Dr. Sodhi and Dr. Silveyra said.
For example, among the few studies that have documented sex differences, one showed no sex differences in the efficacy of salmeterol/fluticasone combination therapy for reducing exacerbations or improving quality of life, whereas another showed that women were more likely than men to experience COPD symptoms or exacerbations after stopping inhaled corticosteroids, Dr. Sodhi and colleagues noted.
Both Dr. Sodhi and Dr. Silveyra emphasized the need for clinical trials that study the effects of sex on treatment outcomes in COPD, which could lead to better, more personalized therapeutic regimens that take sex and gender into account.
Dr. Sodhi and colleagues offered the following advice to clinicians: “Interaction with female patients should take into account that their symptoms may not conform to traditionally accepted presentations. Challenges exist for female patients at all levels of health care interaction and as clinicians we need to acknowledge the bias and willfully work toward recognition and elimination of unconscious and conscious bias. Empowering our patients to have frank discussions with their health care team when they perceive bias is another step to help promote equity.”
The review by Dr. Sodhi and colleagues was supported by grants from the National Institutes of Health. Dr. Sodhi and Dr. Silveyra reported having no conflicts of interest to disclose.
When Sigmund Freud claimed that “anatomy is destiny” he was referring to anatomical sex as a determinant of personality traits. Expert consensus statements have previously offered some recommendations for managing these syndromes, but clinical data are scarce, so the present review “is intended to establish a starting point for future research,”
That notion has been widely discredited, but Freud appears to be inadvertently right in one respect: When it comes to chronic obstructive pulmonary disease (COPD), anatomy really is destiny, and sex may be as well, pulmonary researchers say.
There is a growing body of evidence to indicate that COPD affects men and women differently, and that men and women patients with COPD require different clinical management. Yet women are often underdiagnosed or misdiagnosed, partly because of poorly understood sex differences, but also because of cultural biases.
But plunging any farther into the weeds, it’s important to define terms. Although various investigators have used the terms “sex” and “gender” interchangeably, sex is the preferred term when referring to biological attributes of individual patients, while gender refers to personal identity.
These distinctions are important, contended Amik Sodhi, MBBS, MPH, from the division of allergy, pulmonology, and critical care medicine at the University of Wisconsin–Madison.
“Sex is essentially a biologic construct, so it’s got to do with the sex chromosomes, the genetics of that person, and it refers to the anatomic variations that can change susceptibility to different diseases,” she said in an interview.
An example of sex differences or “sexual dimorphism” can be found in a recent meta-analysis of sex-based genetic associations by Megan Hardin, MD, MPH from Brigham & Women’s Hospital in Boston and colleagues.
They reported that CELSR1, a gene involved in fetal lung development, was expressed more among women than among men and that a single nucleotide polymorphism in the gene was associated with COPD among women smokers, but not among men smokers.
The finding points to a potential risk locus for COPD in women, and could help shed light on sexual dimorphism in COPD, Dr. Hardin and colleagues said.
In contrast to sex, “gender is more of a psychosocial construct which can impact how diseases manifest themselves, how they are potentially managed, and what outcomes might occur for that particular disease,” Dr. Sodhi said.
She and her colleagues recently published a review of sex and gender in common lung disorders and sleep in the journal CHEST, where they wrote that the “influence of sex and gender is portrayed in epidemiological data, disease pathogenesis and pathophysiology, clinical manifestations, response to treatment, access to care, and health outcomes. Hence, sex and gender should be considered in all types of research, clinical practice and educational curricula.”
For example, as previously reported at the 2021 annual meeting of the American Thoracic Society, sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with COPD may point to different criteria for diagnosing cardiac comorbidities in women and men.
Those conclusions came from a retrospective analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease.
The investigators looked at the patients’ clinical history, comorbidities, lung function, COPD Assessment Test scores, and modified Medical Research Council (mMRC) dyspnea score, and found significant differences between men and women for most functional parameters and comorbidities, and for CAT items of cough, phlegm, and energy.
In logistic regression analysis, predictors for cardiac disease in men were energy, mMRC score, smoking status, body mass index, age, and spirometric lung function, but in women only age was significantly predictive for cardiac disease.
An example of gender effects on COPD differences in men and women is the increase in cigarette advertising aimed at women in the 1960s and the advent of women-targeted brands such as Virginia Slims, which in turn lead to increased smoking rates among women. In addition, in the developing world, where the sex/gender gap in COPD is narrowing, women tend to have greater exposure to wood smoke and cooking fuels in unventilated or poorly ventilated spaces, compared with men.
Increasing incidence among women
According to the Centers for Disease Control and Prevention, chronic lower respiratory diseases, primarily COPD, were the fourth-leading cause of death in women in the United States in 2018, following only heart disease, cancer, and accidents/injuries.
And as a CDC analysis of data from the 2013 Behavioral Risk Factor Surveillance System showed, women were more likely to report being told by a physician that they had COPD than did men (6.6%, compared with 5.4%).
Dr. Sodhi and colleagues noted that, at all time points examined from 2005 to 2014, women had a higher proportion than men of COPD hospitalizations and in-hospital deaths. They also noted that female sex is associated with a threefold risk for severe early-onset COPD, and that women with COPD have lower diffusion capacity of lungs for carbon monoxide, despite having higher predicted forced expiratory volume in 1 second, compared with men.
“Historically, COPD wasn’t a disease that was so prevalent in women. It’s been in the past 20 years that the trends have changed,” said Patricia Silveyra, MSc, PhD, ATSF, associate professor of environmental and occupational health at Indiana University, Bloomington.
The increasing prevalence of COPD among women cannot be explained by smoking alone, she said in an interview.
“It used to be thought that it was because more women smoked, but actually a lot of women who don’t smoke can develop COPD, so it appears to be probably something environmental, but because it used to be a disease of older men, in the clinic there was also a bias to diagnose men with COPD, and women with asthma, so a lot of women went underdiagnosed,” Dr. Silveyra said.
In their review, Dr. Sodhi and colleagues noted that women with COPD “may be underdiagnosed as a result of having different symptoms from those classically recognized. Reasons for underdiagnosis or a delay in diagnosis may also be due to lack of a formal evaluation with spirometry, women seeking care later in the course of disease, physician bias, or associated fatigue or depression misdirecting diagnostic strategies. Underdiagnosis may be associated with psychological distress and worse health-related quality of life.”
Although the evidence is mixed, women tend to present more frequently with the chronic bronchitis phenotype of COPD, compared with the emphysema phenotype, and women tend to have greater degrees of pulmonary function impairment when exposed to tobacco smoke, even after controlling for differences in height and weight.
“For the same amount of exposure to tobacco smoke, females are likely to develop more severe airflow limitation at an earlier age than males, and have more exacerbation,” Dr. Sodhi and colleagues wrote.
Both Dr. Silveyra and Dr. Sodhi said that reason why men and women differ in their physiological reactions to smoke are still unknown.
Sex differences in drug responses
There is only limited evidence to indicate that women and men respond differently to various therapeutic agents, but what is clear is that more research into this area is needed, Dr. Sodhi and Dr. Silveyra said.
For example, among the few studies that have documented sex differences, one showed no sex differences in the efficacy of salmeterol/fluticasone combination therapy for reducing exacerbations or improving quality of life, whereas another showed that women were more likely than men to experience COPD symptoms or exacerbations after stopping inhaled corticosteroids, Dr. Sodhi and colleagues noted.
Both Dr. Sodhi and Dr. Silveyra emphasized the need for clinical trials that study the effects of sex on treatment outcomes in COPD, which could lead to better, more personalized therapeutic regimens that take sex and gender into account.
Dr. Sodhi and colleagues offered the following advice to clinicians: “Interaction with female patients should take into account that their symptoms may not conform to traditionally accepted presentations. Challenges exist for female patients at all levels of health care interaction and as clinicians we need to acknowledge the bias and willfully work toward recognition and elimination of unconscious and conscious bias. Empowering our patients to have frank discussions with their health care team when they perceive bias is another step to help promote equity.”
The review by Dr. Sodhi and colleagues was supported by grants from the National Institutes of Health. Dr. Sodhi and Dr. Silveyra reported having no conflicts of interest to disclose.
When Sigmund Freud claimed that “anatomy is destiny” he was referring to anatomical sex as a determinant of personality traits. Expert consensus statements have previously offered some recommendations for managing these syndromes, but clinical data are scarce, so the present review “is intended to establish a starting point for future research,”
That notion has been widely discredited, but Freud appears to be inadvertently right in one respect: When it comes to chronic obstructive pulmonary disease (COPD), anatomy really is destiny, and sex may be as well, pulmonary researchers say.
There is a growing body of evidence to indicate that COPD affects men and women differently, and that men and women patients with COPD require different clinical management. Yet women are often underdiagnosed or misdiagnosed, partly because of poorly understood sex differences, but also because of cultural biases.
But plunging any farther into the weeds, it’s important to define terms. Although various investigators have used the terms “sex” and “gender” interchangeably, sex is the preferred term when referring to biological attributes of individual patients, while gender refers to personal identity.
These distinctions are important, contended Amik Sodhi, MBBS, MPH, from the division of allergy, pulmonology, and critical care medicine at the University of Wisconsin–Madison.
“Sex is essentially a biologic construct, so it’s got to do with the sex chromosomes, the genetics of that person, and it refers to the anatomic variations that can change susceptibility to different diseases,” she said in an interview.
An example of sex differences or “sexual dimorphism” can be found in a recent meta-analysis of sex-based genetic associations by Megan Hardin, MD, MPH from Brigham & Women’s Hospital in Boston and colleagues.
They reported that CELSR1, a gene involved in fetal lung development, was expressed more among women than among men and that a single nucleotide polymorphism in the gene was associated with COPD among women smokers, but not among men smokers.
The finding points to a potential risk locus for COPD in women, and could help shed light on sexual dimorphism in COPD, Dr. Hardin and colleagues said.
In contrast to sex, “gender is more of a psychosocial construct which can impact how diseases manifest themselves, how they are potentially managed, and what outcomes might occur for that particular disease,” Dr. Sodhi said.
She and her colleagues recently published a review of sex and gender in common lung disorders and sleep in the journal CHEST, where they wrote that the “influence of sex and gender is portrayed in epidemiological data, disease pathogenesis and pathophysiology, clinical manifestations, response to treatment, access to care, and health outcomes. Hence, sex and gender should be considered in all types of research, clinical practice and educational curricula.”
For example, as previously reported at the 2021 annual meeting of the American Thoracic Society, sex-specific differences in the severity of symptoms and prevalence of comorbidities in patients with COPD may point to different criteria for diagnosing cardiac comorbidities in women and men.
Those conclusions came from a retrospective analysis of data on 795 women and 1,251 men with GOLD (Global Initiative for Chronic Obstructive Lung Disease) class 1-3 disease.
The investigators looked at the patients’ clinical history, comorbidities, lung function, COPD Assessment Test scores, and modified Medical Research Council (mMRC) dyspnea score, and found significant differences between men and women for most functional parameters and comorbidities, and for CAT items of cough, phlegm, and energy.
In logistic regression analysis, predictors for cardiac disease in men were energy, mMRC score, smoking status, body mass index, age, and spirometric lung function, but in women only age was significantly predictive for cardiac disease.
An example of gender effects on COPD differences in men and women is the increase in cigarette advertising aimed at women in the 1960s and the advent of women-targeted brands such as Virginia Slims, which in turn lead to increased smoking rates among women. In addition, in the developing world, where the sex/gender gap in COPD is narrowing, women tend to have greater exposure to wood smoke and cooking fuels in unventilated or poorly ventilated spaces, compared with men.
Increasing incidence among women
According to the Centers for Disease Control and Prevention, chronic lower respiratory diseases, primarily COPD, were the fourth-leading cause of death in women in the United States in 2018, following only heart disease, cancer, and accidents/injuries.
And as a CDC analysis of data from the 2013 Behavioral Risk Factor Surveillance System showed, women were more likely to report being told by a physician that they had COPD than did men (6.6%, compared with 5.4%).
Dr. Sodhi and colleagues noted that, at all time points examined from 2005 to 2014, women had a higher proportion than men of COPD hospitalizations and in-hospital deaths. They also noted that female sex is associated with a threefold risk for severe early-onset COPD, and that women with COPD have lower diffusion capacity of lungs for carbon monoxide, despite having higher predicted forced expiratory volume in 1 second, compared with men.
“Historically, COPD wasn’t a disease that was so prevalent in women. It’s been in the past 20 years that the trends have changed,” said Patricia Silveyra, MSc, PhD, ATSF, associate professor of environmental and occupational health at Indiana University, Bloomington.
The increasing prevalence of COPD among women cannot be explained by smoking alone, she said in an interview.
“It used to be thought that it was because more women smoked, but actually a lot of women who don’t smoke can develop COPD, so it appears to be probably something environmental, but because it used to be a disease of older men, in the clinic there was also a bias to diagnose men with COPD, and women with asthma, so a lot of women went underdiagnosed,” Dr. Silveyra said.
In their review, Dr. Sodhi and colleagues noted that women with COPD “may be underdiagnosed as a result of having different symptoms from those classically recognized. Reasons for underdiagnosis or a delay in diagnosis may also be due to lack of a formal evaluation with spirometry, women seeking care later in the course of disease, physician bias, or associated fatigue or depression misdirecting diagnostic strategies. Underdiagnosis may be associated with psychological distress and worse health-related quality of life.”
Although the evidence is mixed, women tend to present more frequently with the chronic bronchitis phenotype of COPD, compared with the emphysema phenotype, and women tend to have greater degrees of pulmonary function impairment when exposed to tobacco smoke, even after controlling for differences in height and weight.
“For the same amount of exposure to tobacco smoke, females are likely to develop more severe airflow limitation at an earlier age than males, and have more exacerbation,” Dr. Sodhi and colleagues wrote.
Both Dr. Silveyra and Dr. Sodhi said that reason why men and women differ in their physiological reactions to smoke are still unknown.
Sex differences in drug responses
There is only limited evidence to indicate that women and men respond differently to various therapeutic agents, but what is clear is that more research into this area is needed, Dr. Sodhi and Dr. Silveyra said.
For example, among the few studies that have documented sex differences, one showed no sex differences in the efficacy of salmeterol/fluticasone combination therapy for reducing exacerbations or improving quality of life, whereas another showed that women were more likely than men to experience COPD symptoms or exacerbations after stopping inhaled corticosteroids, Dr. Sodhi and colleagues noted.
Both Dr. Sodhi and Dr. Silveyra emphasized the need for clinical trials that study the effects of sex on treatment outcomes in COPD, which could lead to better, more personalized therapeutic regimens that take sex and gender into account.
Dr. Sodhi and colleagues offered the following advice to clinicians: “Interaction with female patients should take into account that their symptoms may not conform to traditionally accepted presentations. Challenges exist for female patients at all levels of health care interaction and as clinicians we need to acknowledge the bias and willfully work toward recognition and elimination of unconscious and conscious bias. Empowering our patients to have frank discussions with their health care team when they perceive bias is another step to help promote equity.”
The review by Dr. Sodhi and colleagues was supported by grants from the National Institutes of Health. Dr. Sodhi and Dr. Silveyra reported having no conflicts of interest to disclose.
Children and COVID: Cases drop again, admission rate up slightly
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.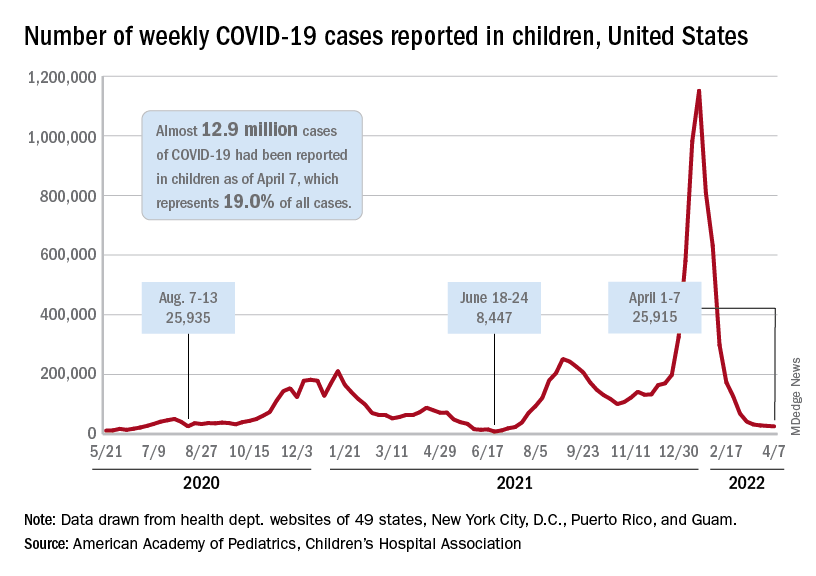
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.
The decline in new cases of child COVID-19 in the last week continued at about the same, somewhat slower pace as the week before, but admissions have moved upward slightly, according to the most recent data.
which, in turn, was 5.2% lower than a week earlier, according to the American Academy of Pediatrics and the Children’s Hospital Association, which have been collecting COVID-related data from state and territorial health departments since the early stages of the pandemic. New case declines in previous weeks had ranged from 9.3% to 46%.
The nearly 26,000 cases reported during the first week of April represent a fall of 97.7% from the peak of the Omicron surge in mid-January, when weekly cases hit 1.15 million, and they represent the lowest weekly count since mid-July of 2021. Cumulative cases in children now number close to 12.9 million over the course of the pandemic, which is 19.0% of cases among all ages, the AAP and CHA said in their weekly COVID report.
Data on new-case rates from the Centers for Disease Control and Prevention show the same continued decline, but the CDC acknowledges the possibility of reporting delays in recent weeks. The numbers for the latest week, April 3-9, maintain the larger overall decline, but there have been a couple of small, temporary increases over the last month, the CDC reported on its COVID Data Tracker.
Daily new admissions of children aged 0-17 years with confirmed COVID were right around 0.14 per 100,000 population for April 3-9, compared with 0.13 per 100,000 during the week ending April 2, the CDC said, with reporting delays making it possible that the 0.14 figure could be revised upward in the near future. The highest admission rate, 1.25 children per 100,000 population, occurred on Jan. 15 and 16.
The latest on vaccination
New vaccinations slipped a bit in the last week, with the drop slightly larger among those aged 12-17 years – from 47,000 for the week of March 24-30 to 43,000 during March 31 to April 6 – than in children aged 5-11, who went from 70,000 initial doses to 69,000 over the same 2-week period, the AAP said in its weekly report on vaccination trends.
Among the states, Vermont has fully vaccinated more children aged 5-11 (58%) than any other state, while Hawaii is the leader in fully vaccinated 12- to 17-year-olds at 86%. The lowest comparable figures for both groups can be found in Alabama, where 10% of children aged 5-11 are fully vaccinated and 34% of those aged 12-17 have received both doses of the Pfizer-BioNTech vaccine, the AAP said.
National figures show equally large COVID vaccination gaps between the two age groups. As of April 11, 68% of all children aged 12-17 years had received at least one dose, compared with 34.6% of those aged 5-11, and 58.5% of the older group was fully vaccinated, versus 28.0% of the 5- to 11-year-olds, the CDC reported.