User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
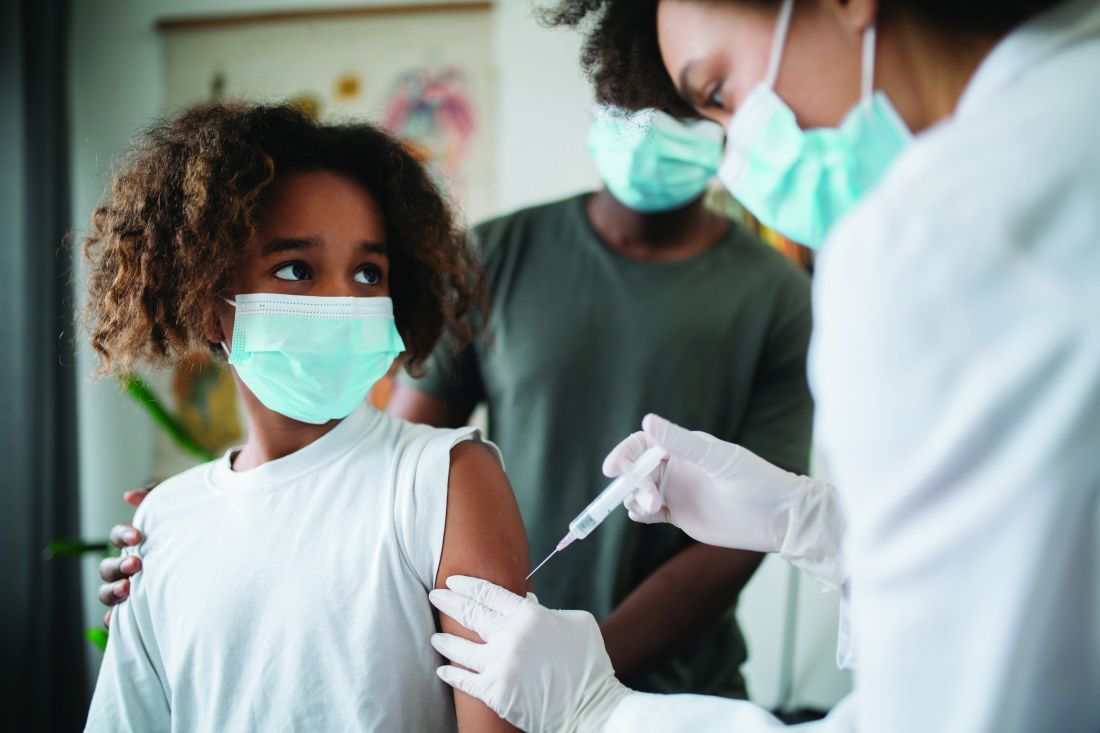
Small myocarditis risk now seen for adenovirus-based COVID-19 vaccine
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
The first large population study to investigate the association between different COVID-19 vaccines types and cardiac effects and adverse events shows a small increase in the risk for acute myocarditis with both the mRNA-based vaccines and – in what may a first in the literature – an adenovirus-vector vaccine.
The excess risk was seen following the first dose of the ChAdOc1 (AstraZeneca/Oxford), the adenovirus-based vaccine, and the mRNA-based BNT162b2 (Pfizer/BioNTech). It was observed after first and second doses of the mRNA-1273 (Moderna) vaccine.
The incidence rate ratios for myocarditis 1-7 days after the first AstraZeneca, Pfizer, and Moderna injections were 1.76, 1.45, and 8.38, respectively, and 23.1 after the second dose of the Moderna vaccine.
“There’s a bit more uncertainty and worry about mRNA vaccines because it’s quite a new vector for vaccination and, therefore, there’s been more focus on the potential side effects,” said Nicholas Mills, MD.
“But it doesn’t surprise me the signal is present for all types of vaccines because they’re designed to generate a systemic immune response and that is, unfortunately, where you can cause small risks for immune-mediated illnesses like myocarditis,” Dr. Mills, from the University of Edinburgh, told this news organization. Dr. Mills is a coauthor on the study, published Dec. 14 in Nature Medicine.
To put the risks in context, the group estimated between 1 and 10 additional myocarditis hospitalizations or deaths per 1 million people vaccinated, but 40 excess myocarditis events per million following a positive SARS-CoV-2 test result.
As reported, rates of excess myocarditis events associated with a first dose were 2 per million injections of the AstraZeneca vaccine, 1 per million for the Pfizer vaccine, and 6 per million with the Moderna vaccine.
Following a second dose, there were 10 additional myocarditis events per million people receiving the Moderna vaccine and none among recipients of the AstraZeneca or Pfizer vaccines.
“It was particularly seen within the first 7 days of the first dose, which is very consistent with what we see in people who have viral myocarditis,” Dr. Mills said. “So it looks like a real signal but it’s very small.”
The results are in line with previous studies of the Pfizer vaccine in Israel and studies of the Moderna vaccine in the United States, Biykem Bozkurt, MD, PhD, professor of medicine at Baylor College of Medicine, Houston, told this news organization.
“What this paper does is confirm that cardiovascular complications – and they are only looking at a small component of those cardiovascular complications – are markedly higher with the COVID-19 infection than with the vaccines,” she said.
It also adds a new twist to the search for the mechanisms of myocarditis, which has focused on the immunogenicity of the RNA in the Pfizer and Moderna vaccines but also hypothesized that molecular mimicry between the SARS-CoV-2 spike glycoprotein and cell antigens, antibody production against cardiac proteins, and testosterone may play a role.
“But now it doesn’t look like the risk is solely confined to the mRNA vaccine platform because it’s also happening with the adenovirus,” Dr. Bozkurt said. “The mechanisms require future experimental and clinical research and we’ll need more granular data with cohorts that are closely followed up as well as subclinical follow-up.”
James de Lemos, MD, professor of medicine at the University of Texas Southwestern Medical Center, Dallas, and cochair of the American Heart Association’s COVID-19 CVD Registry, said he was also not surprised by a myocarditis signal with AstraZeneca’s adenovirus vaccine.
“Looking at relative risks has biological implications, but the clinical and public health implications are that the absolute risk with the adenovirus is trivial. And you see that with their estimations of absolute risk where it’s literally sort of a needle in the haystack of 1 or 2 per million,” he said in an interview.
Large-scale data
The investigators examined the rates of hospital admission or death from myocarditis, pericarditis, and cardiac arrhythmia in the 28 days following SARS-CoV-2 vaccination or infection by linking the English National Immunisation Database of COVID-19 vaccination with a national patient-level health care database of 38.6 million people, aged 16 years or older, vaccinated from Dec.1, 2020, to Aug. 24, 2021.
The number of people admitted to the hospital or who died during the study period was 1,615 for myocarditis, 1,574 for pericarditis, and 385,508 for cardiac arrhythmia.
There was no evidence of an increased risk for pericarditis or cardiac arrhythmia following vaccination, except for arrhythmia in the 28 days following a second dose of the Moderna vaccine (IRR, 1.46).
In contrast, the risk was increased for pericarditis (IRR, 2.79) and cardiac arrhythmia (IRR, 5.35) in the 28 days following a positive SARS-CoV-2 test result.
Although the scale of the analysis allows for more precise estimates than what’s been possible in smaller data sets, there is the challenge of diagnosing COVID-19 from billing codes and the potential for ascertainment bias, noted Dr. de Lemos.
“Having said that, I think it’s a really important study, because it’s the first study to put the incidence in context in the same general population the risks of myocarditis with various vaccines and with COVID-19,” he said.
“That’s really important and provides a lot of reassurance for those who are trying to balance the risks and benefits of vaccination.”
Analyses by sex and age
A subgroup analysis by age showed increased risks for myocarditis with the mRNA vaccines only in those younger than 40, whereas no association was found with the Oxford adenovirus vaccine.
“We’re not seeing any signal here that would make us change the recommendation for vaccination in children as a consequence of this risk,” Dr. Mills said during a press briefing.
Dr. Bozkurt pointed out, however, that the estimated excess in myocarditis events following a second dose of the Moderna vaccine in these younger adults reportedly exceeded that for SARS-CoV-2 infection (15 per million vs. 10 per million).
“For that age group, it’s concerning and needs further clarification. This hasn’t been seen before,” she said.
The average age was 39 years for those receiving two doses of the Moderna vaccine and 55 for recipients of the Pfizer and Oxford vaccines. The Moderna vaccine wasn’t rolled out until April 2021 in the United Kingdom, the authors noted, so the number of patients who received this vaccine is lower.
Although reports have suggested young males are at greater risk for myocarditis after vaccination, an analysis by sex found that women had an increased risk for myocarditis after a first dose of the AstraZeneca (IRR, 1.40) and Pfizer (IRR, 1.54) vaccines and following a positive COVID-19 test result (IRR, 11.00).
“Women being at increased risk is rather a new message,” Dr. Bozkurt said. “But the incidence rate ratios are being compared against the unvaccinated, so when you see the increase in women, it doesn’t mean it’s increased against men. It would be helpful for sex-specific incidence rate ratios to be reported for younger age subgroups, such as ages 16-20 and 20-30, to determine whether there’s an increased risk for males compared to females at younger ages.”
Age and sex differences are huge questions, but “I think we’ll learn a lot about myocarditis in general from what is going to be an explosion of research into the vaccine-associated causes,” Dr. de Lemos said.
“That will help us understand myocarditis more broadly and prepare us for the next generation of vaccines, which inevitably will be mRNA based.”
Dr. Mills reported having no relevant disclosures. Dr. Bozkurt reported consulting for Bayer and scPharmaceuticals and serving on a clinical-events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. De Lemos reported having no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
FROM NATURE MEDICINE
COVID-19 hospital data: New-onset seizures more common than breakthrough seizures
An analysis of hospitalized patients with COVID-19 finds that those with no history of epilepsy had more than 3 times the odds of suffering a new-onset seizure than patients with epilepsy were to have breakthrough seizures (odds radio [OR] 3.15, P < .0001), researchers reported at the annual meeting of the American Epilepsy Society.
study lead author Neeraj Singh, MD, a neurologist at the New York-based Northwell Health system, said in an interview. “That’s new. We don’t normally see that when someone has a bacterial or viral infection. It’s demonstrating that this infection is having direct effect on the brain and brain signals.”
According to Dr. Singh, there’s little data about seizures in patients with COVID-19 because doctors have focused on other symptoms. A 2021 multicenter study found that electrographic seizures were detected in 9.6% of 197 patients with COVID-19 who were referred for cEEG.
For the new study, Dr. Singh and a colleague tracked 917 patients with COVID-19 in the Northwell Health system who were treated from Feb. 14 to June 14, 2020, with antiepileptic medication. Of the patients, 451 had a history of epilepsy, and 466 did not.
According to Dr. Singh, 27.6% of the patients without a history of epilepsy had new-onset seizures, while 10.1% of the patients with history of epilepsy had breakthrough seizures. The difference in odds was more than threefold after adjustment. (Among all COVID-19 patients, he said, perhaps 8%-16% had seizures).
The researchers also found that patients with new-onset seizures stayed in the hospital much longer (average, 26.9 days) than any patients with a known history of epilepsy (12.8 days, P < .0001, for those who had breakthrough seizures and 10.9 days, P < .0001, for those who didn’t).
In addition, the researchers found that having any seizures – new-onset or breakthrough – was linked to higher risk of death (OR 1.41, P = .03).
Antiseizure medications are key treatments for these patients, Dr. Singh said. As for the patients with new-onset seizures who recover from COVID-19, Dr. Singh said, “it’s suspected that these people are going to have a new diagnosis of epilepsy, not just a one-time seizure.”
The findings suggest that some patients with epilepsy are protected against COVID-19-related seizures because they take antiepileptic medications that “protect the brain from getting a trigger for an abnormal signal that leads to a seizure,” he said. “That’s one possibility.”
What can neurologists learn from the study? Dr. Singh recommends a “lower threshold” to recommend or approve EEGs in patients with COVID-19 who are confused/altered when they come in, especially if this is not normal. “They may actually be having silent seizures that no one’s noticing,” he said.
No study funding was reported. The authors reported no relevant disclosures.
An analysis of hospitalized patients with COVID-19 finds that those with no history of epilepsy had more than 3 times the odds of suffering a new-onset seizure than patients with epilepsy were to have breakthrough seizures (odds radio [OR] 3.15, P < .0001), researchers reported at the annual meeting of the American Epilepsy Society.
study lead author Neeraj Singh, MD, a neurologist at the New York-based Northwell Health system, said in an interview. “That’s new. We don’t normally see that when someone has a bacterial or viral infection. It’s demonstrating that this infection is having direct effect on the brain and brain signals.”
According to Dr. Singh, there’s little data about seizures in patients with COVID-19 because doctors have focused on other symptoms. A 2021 multicenter study found that electrographic seizures were detected in 9.6% of 197 patients with COVID-19 who were referred for cEEG.
For the new study, Dr. Singh and a colleague tracked 917 patients with COVID-19 in the Northwell Health system who were treated from Feb. 14 to June 14, 2020, with antiepileptic medication. Of the patients, 451 had a history of epilepsy, and 466 did not.
According to Dr. Singh, 27.6% of the patients without a history of epilepsy had new-onset seizures, while 10.1% of the patients with history of epilepsy had breakthrough seizures. The difference in odds was more than threefold after adjustment. (Among all COVID-19 patients, he said, perhaps 8%-16% had seizures).
The researchers also found that patients with new-onset seizures stayed in the hospital much longer (average, 26.9 days) than any patients with a known history of epilepsy (12.8 days, P < .0001, for those who had breakthrough seizures and 10.9 days, P < .0001, for those who didn’t).
In addition, the researchers found that having any seizures – new-onset or breakthrough – was linked to higher risk of death (OR 1.41, P = .03).
Antiseizure medications are key treatments for these patients, Dr. Singh said. As for the patients with new-onset seizures who recover from COVID-19, Dr. Singh said, “it’s suspected that these people are going to have a new diagnosis of epilepsy, not just a one-time seizure.”
The findings suggest that some patients with epilepsy are protected against COVID-19-related seizures because they take antiepileptic medications that “protect the brain from getting a trigger for an abnormal signal that leads to a seizure,” he said. “That’s one possibility.”
What can neurologists learn from the study? Dr. Singh recommends a “lower threshold” to recommend or approve EEGs in patients with COVID-19 who are confused/altered when they come in, especially if this is not normal. “They may actually be having silent seizures that no one’s noticing,” he said.
No study funding was reported. The authors reported no relevant disclosures.
An analysis of hospitalized patients with COVID-19 finds that those with no history of epilepsy had more than 3 times the odds of suffering a new-onset seizure than patients with epilepsy were to have breakthrough seizures (odds radio [OR] 3.15, P < .0001), researchers reported at the annual meeting of the American Epilepsy Society.
study lead author Neeraj Singh, MD, a neurologist at the New York-based Northwell Health system, said in an interview. “That’s new. We don’t normally see that when someone has a bacterial or viral infection. It’s demonstrating that this infection is having direct effect on the brain and brain signals.”
According to Dr. Singh, there’s little data about seizures in patients with COVID-19 because doctors have focused on other symptoms. A 2021 multicenter study found that electrographic seizures were detected in 9.6% of 197 patients with COVID-19 who were referred for cEEG.
For the new study, Dr. Singh and a colleague tracked 917 patients with COVID-19 in the Northwell Health system who were treated from Feb. 14 to June 14, 2020, with antiepileptic medication. Of the patients, 451 had a history of epilepsy, and 466 did not.
According to Dr. Singh, 27.6% of the patients without a history of epilepsy had new-onset seizures, while 10.1% of the patients with history of epilepsy had breakthrough seizures. The difference in odds was more than threefold after adjustment. (Among all COVID-19 patients, he said, perhaps 8%-16% had seizures).
The researchers also found that patients with new-onset seizures stayed in the hospital much longer (average, 26.9 days) than any patients with a known history of epilepsy (12.8 days, P < .0001, for those who had breakthrough seizures and 10.9 days, P < .0001, for those who didn’t).
In addition, the researchers found that having any seizures – new-onset or breakthrough – was linked to higher risk of death (OR 1.41, P = .03).
Antiseizure medications are key treatments for these patients, Dr. Singh said. As for the patients with new-onset seizures who recover from COVID-19, Dr. Singh said, “it’s suspected that these people are going to have a new diagnosis of epilepsy, not just a one-time seizure.”
The findings suggest that some patients with epilepsy are protected against COVID-19-related seizures because they take antiepileptic medications that “protect the brain from getting a trigger for an abnormal signal that leads to a seizure,” he said. “That’s one possibility.”
What can neurologists learn from the study? Dr. Singh recommends a “lower threshold” to recommend or approve EEGs in patients with COVID-19 who are confused/altered when they come in, especially if this is not normal. “They may actually be having silent seizures that no one’s noticing,” he said.
No study funding was reported. The authors reported no relevant disclosures.
FROM AES 2021
Advisory on youth mental health crisis gets mixed reviews
The advisory on youth mental health from Surgeon General Vivek Murthy, MD, casts a necessary spotlight on the crisis, clinical psychiatrists say. But some think it could have produced more specifics about funding and payment parity for reimbursement.
The 53-page advisory says that about one in five U.S. children and adolescents aged 3-17 suffer from a mental, emotional, developmental, or behavioral disorder. In the decade before COVID, feelings of sadness and hopelessness, as well as suicidal behaviors, were on the rise. The pandemic has exacerbated symptoms of anxiety, depression, and other mental health issues in young people. Compared with 2019, ED visits in early 2021 for suspected suicide attempts rose 51% in adolescent girls and 4% in boys. “Depressive and anxiety symptoms doubled during the pandemic,” the advisory said.
Scope of the advisory
The advisory, released Dec. 7, covers all sectors and considers all social and policy factors that might be contributing to this crisis, said Jessica (Jessi) Gold, MD, MS, an assistant professor in the department of psychiatry at Washington University, St. Louis.
“It is always possible to reimagine health care to be more patient centered and mental health forward.” But changes of this magnitude take time, Dr. Gold, also director of wellness, engagement, and outreach at the university, said in an interview.
She has seen the impact of the pandemic firsthand in her clinic among students and frontline health care workers aged 18-30. People in that age group “feel everything deeply,” Dr. Gold said. Emotions tied to COVID-19 are just a part of it. Confounding factors, such as climate change, racism, and school shootings all contribute to their overall mental health.
Some children and adolescents with social anxiety have fared better during the pandemic, but those who are part of demographic groups such as racial and ethnic minorities, LGBTQ individuals, low-income youth, and those involved in juvenile justice or welfare systems face a higher risk of mental health challenges, the pandemic notwithstanding.
In her work with schools, Denese Shervington, MD, MPH, has witnessed more mental health challenges related to isolation and separation. “There’s an overall worry about the loss of what used to be, the seeming predictability and certainty of prepandemic life,” said Dr. Shervington, clinical professor of psychiatry at Tulane University, and president and CEO of the Institute for Women and Ethnic Studies, both in New Orleans.
A systems of care plan
The advisory lists actionable items for health care and 10 other industry sectors to improve mental health of children and young adults.
Health care organizations and professionals were advised to take the following six steps:
- Implement trauma-informed care principles and other prevention strategies. This may involve referring patients to resources such as economic and legal supports, school enrichment programs, and educating families on healthy child development in the clinic.
- Routinely screen children for mental health challenges and risk factors such as adverse childhood experiences during primary care well-visits or annual physicals, or at schools or EDs. Primary care physicians should use principles of trauma-informed care to conduct these screenings.
- Screen parents, caregivers, and other family members for depression, intimate partner violence, substance use, and other challenges. These can be done in tandem with broader assessments of social determinants of health such as food or housing insecurity.
- Combine efforts of clinical staff with trusted community partners and child welfare and juvenile justice. Hospital-based violence intervention programs, for example, identify patients at risk of repeat violent injury and refer them to hospital- and community-based resources.
- Build multidisciplinary teams, enlisting children and families to develop services that are tailored to their needs for screening and treatment. Such services should reflect cultural diversity and offered in multiple languages.
- Support the well-being of mental health workers and community leaders to foster their ability to help youth and their families.
Dr. Murthy is talking about a “systems of care” approach, in which all sectors that touch children and youth – not just health care – must work together and do their jobs effectively but collaboratively to address this public health crisis, said Aradhana (Bela) Sood MD, MSHA, FAACAP, senior professor of child mental health policy at Virginia Commonwealth University, Richmond. “An investment in infrastructure support of positive mental health in early childhood, be it in schools, communities, or family well-being will lead to a future where illness is not the result of major preventable societal factors, such as a lack of social supports and trauma.”
Changes will ‘take a lot of buy-in’
The recommendations are actionable in the real world – but there are a lot of them, said Dr. Gold. Dr. Murthy doesn’t specify what the plan is to accomplish these metrics or fund them, she added. He “has money and funders like foundations as steps, but foundations have also suffered in the pandemic, so it is not that simple.” Many of these changes are wide in scope and will take a lot of buy-in.
Dr. Shervington would like to have seen more of a focus on educator well-being, given that young people spend a lot of time in educational settings.
“My organization just completed a study in New Orleans that showed teachers having elevated levels of trauma-based conditions since the pandemic,” she said. Schools are indeed a key place to support holistic mental health by focusing on school climate, Dr. Sood added. “If school administrators became uniformly consistent with recognizing the importance of psychological wellness as a prerequisite of good learning, they will create environments where teachers are keenly aware of a child’s mental wellness and make reduction of bullying, wellness check-ins, [and] school-based mental health clinics a priority.
“These are ways nonmedical, community-based supports can enhance student well-being, and reduce depression and other mental health conditions,” Dr. Sood added.
Child psychiatrists stretched ‘even thinner’
Despite mental health parity rules, health plans have not been held accountable. That failure, combined with excessive demands for prior authorization for mental health treatments “have led to dangerous shortages of psychiatrists able to accept insurance,” said Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore.
“This is particularly true for child psychiatrists, who are stretched even thinner than those of us in general practice,” Dr. Nestadt said.
While he doesn’t address it head on, Dr. Murthy uses classic parity language when he states that “mental health is no less important than physical health,” said Dr. Nestadt, who consulted with the surgeon general on developing this advisory. “While many of us would have liked to see parity highlighted more directly, this advisory was designed to be an overview.”
Highlighting social media, gun violence
Dr. Nestadt said he was pleased that the advisory emphasized the importance of restricting access to lethal means in preventing youth suicide.
“With youth suicide rates rising faster than in other age groups, and suicide mortality tied so closely to method availability, the surgeon general made the right choice in highlighting the role of guns in suicide,” he said.
The advisory also discussed the role of media and social media companies in addressing the crisis, which is important, said Dr. Gold.
“I believe very strongly that the way we talk about and portray mental health in the media matters,” she said. “I have seen it matter in the clinic with patients. They’ll wonder if someone will think they are now violent if they are diagnosed with a mental illness. Stories change the narrative.”
While the advisory isn’t perfect, the state of youth mental health “will only get worse if we don’t do something,” noted Dr. Gold. “It is critical that this is validated and discussed at the highest level and messages like Dr. Murthy’s get heard.”
Dr. Gold, Dr. Shervington, and Dr. Sood had no disclosures. Dr. Nestadt disclosed serving as a consultant to the surgeon general advisory.
The advisory on youth mental health from Surgeon General Vivek Murthy, MD, casts a necessary spotlight on the crisis, clinical psychiatrists say. But some think it could have produced more specifics about funding and payment parity for reimbursement.
The 53-page advisory says that about one in five U.S. children and adolescents aged 3-17 suffer from a mental, emotional, developmental, or behavioral disorder. In the decade before COVID, feelings of sadness and hopelessness, as well as suicidal behaviors, were on the rise. The pandemic has exacerbated symptoms of anxiety, depression, and other mental health issues in young people. Compared with 2019, ED visits in early 2021 for suspected suicide attempts rose 51% in adolescent girls and 4% in boys. “Depressive and anxiety symptoms doubled during the pandemic,” the advisory said.
Scope of the advisory
The advisory, released Dec. 7, covers all sectors and considers all social and policy factors that might be contributing to this crisis, said Jessica (Jessi) Gold, MD, MS, an assistant professor in the department of psychiatry at Washington University, St. Louis.
“It is always possible to reimagine health care to be more patient centered and mental health forward.” But changes of this magnitude take time, Dr. Gold, also director of wellness, engagement, and outreach at the university, said in an interview.
She has seen the impact of the pandemic firsthand in her clinic among students and frontline health care workers aged 18-30. People in that age group “feel everything deeply,” Dr. Gold said. Emotions tied to COVID-19 are just a part of it. Confounding factors, such as climate change, racism, and school shootings all contribute to their overall mental health.
Some children and adolescents with social anxiety have fared better during the pandemic, but those who are part of demographic groups such as racial and ethnic minorities, LGBTQ individuals, low-income youth, and those involved in juvenile justice or welfare systems face a higher risk of mental health challenges, the pandemic notwithstanding.
In her work with schools, Denese Shervington, MD, MPH, has witnessed more mental health challenges related to isolation and separation. “There’s an overall worry about the loss of what used to be, the seeming predictability and certainty of prepandemic life,” said Dr. Shervington, clinical professor of psychiatry at Tulane University, and president and CEO of the Institute for Women and Ethnic Studies, both in New Orleans.
A systems of care plan
The advisory lists actionable items for health care and 10 other industry sectors to improve mental health of children and young adults.
Health care organizations and professionals were advised to take the following six steps:
- Implement trauma-informed care principles and other prevention strategies. This may involve referring patients to resources such as economic and legal supports, school enrichment programs, and educating families on healthy child development in the clinic.
- Routinely screen children for mental health challenges and risk factors such as adverse childhood experiences during primary care well-visits or annual physicals, or at schools or EDs. Primary care physicians should use principles of trauma-informed care to conduct these screenings.
- Screen parents, caregivers, and other family members for depression, intimate partner violence, substance use, and other challenges. These can be done in tandem with broader assessments of social determinants of health such as food or housing insecurity.
- Combine efforts of clinical staff with trusted community partners and child welfare and juvenile justice. Hospital-based violence intervention programs, for example, identify patients at risk of repeat violent injury and refer them to hospital- and community-based resources.
- Build multidisciplinary teams, enlisting children and families to develop services that are tailored to their needs for screening and treatment. Such services should reflect cultural diversity and offered in multiple languages.
- Support the well-being of mental health workers and community leaders to foster their ability to help youth and their families.
Dr. Murthy is talking about a “systems of care” approach, in which all sectors that touch children and youth – not just health care – must work together and do their jobs effectively but collaboratively to address this public health crisis, said Aradhana (Bela) Sood MD, MSHA, FAACAP, senior professor of child mental health policy at Virginia Commonwealth University, Richmond. “An investment in infrastructure support of positive mental health in early childhood, be it in schools, communities, or family well-being will lead to a future where illness is not the result of major preventable societal factors, such as a lack of social supports and trauma.”
Changes will ‘take a lot of buy-in’
The recommendations are actionable in the real world – but there are a lot of them, said Dr. Gold. Dr. Murthy doesn’t specify what the plan is to accomplish these metrics or fund them, she added. He “has money and funders like foundations as steps, but foundations have also suffered in the pandemic, so it is not that simple.” Many of these changes are wide in scope and will take a lot of buy-in.
Dr. Shervington would like to have seen more of a focus on educator well-being, given that young people spend a lot of time in educational settings.
“My organization just completed a study in New Orleans that showed teachers having elevated levels of trauma-based conditions since the pandemic,” she said. Schools are indeed a key place to support holistic mental health by focusing on school climate, Dr. Sood added. “If school administrators became uniformly consistent with recognizing the importance of psychological wellness as a prerequisite of good learning, they will create environments where teachers are keenly aware of a child’s mental wellness and make reduction of bullying, wellness check-ins, [and] school-based mental health clinics a priority.
“These are ways nonmedical, community-based supports can enhance student well-being, and reduce depression and other mental health conditions,” Dr. Sood added.
Child psychiatrists stretched ‘even thinner’
Despite mental health parity rules, health plans have not been held accountable. That failure, combined with excessive demands for prior authorization for mental health treatments “have led to dangerous shortages of psychiatrists able to accept insurance,” said Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore.
“This is particularly true for child psychiatrists, who are stretched even thinner than those of us in general practice,” Dr. Nestadt said.
While he doesn’t address it head on, Dr. Murthy uses classic parity language when he states that “mental health is no less important than physical health,” said Dr. Nestadt, who consulted with the surgeon general on developing this advisory. “While many of us would have liked to see parity highlighted more directly, this advisory was designed to be an overview.”
Highlighting social media, gun violence
Dr. Nestadt said he was pleased that the advisory emphasized the importance of restricting access to lethal means in preventing youth suicide.
“With youth suicide rates rising faster than in other age groups, and suicide mortality tied so closely to method availability, the surgeon general made the right choice in highlighting the role of guns in suicide,” he said.
The advisory also discussed the role of media and social media companies in addressing the crisis, which is important, said Dr. Gold.
“I believe very strongly that the way we talk about and portray mental health in the media matters,” she said. “I have seen it matter in the clinic with patients. They’ll wonder if someone will think they are now violent if they are diagnosed with a mental illness. Stories change the narrative.”
While the advisory isn’t perfect, the state of youth mental health “will only get worse if we don’t do something,” noted Dr. Gold. “It is critical that this is validated and discussed at the highest level and messages like Dr. Murthy’s get heard.”
Dr. Gold, Dr. Shervington, and Dr. Sood had no disclosures. Dr. Nestadt disclosed serving as a consultant to the surgeon general advisory.
The advisory on youth mental health from Surgeon General Vivek Murthy, MD, casts a necessary spotlight on the crisis, clinical psychiatrists say. But some think it could have produced more specifics about funding and payment parity for reimbursement.
The 53-page advisory says that about one in five U.S. children and adolescents aged 3-17 suffer from a mental, emotional, developmental, or behavioral disorder. In the decade before COVID, feelings of sadness and hopelessness, as well as suicidal behaviors, were on the rise. The pandemic has exacerbated symptoms of anxiety, depression, and other mental health issues in young people. Compared with 2019, ED visits in early 2021 for suspected suicide attempts rose 51% in adolescent girls and 4% in boys. “Depressive and anxiety symptoms doubled during the pandemic,” the advisory said.
Scope of the advisory
The advisory, released Dec. 7, covers all sectors and considers all social and policy factors that might be contributing to this crisis, said Jessica (Jessi) Gold, MD, MS, an assistant professor in the department of psychiatry at Washington University, St. Louis.
“It is always possible to reimagine health care to be more patient centered and mental health forward.” But changes of this magnitude take time, Dr. Gold, also director of wellness, engagement, and outreach at the university, said in an interview.
She has seen the impact of the pandemic firsthand in her clinic among students and frontline health care workers aged 18-30. People in that age group “feel everything deeply,” Dr. Gold said. Emotions tied to COVID-19 are just a part of it. Confounding factors, such as climate change, racism, and school shootings all contribute to their overall mental health.
Some children and adolescents with social anxiety have fared better during the pandemic, but those who are part of demographic groups such as racial and ethnic minorities, LGBTQ individuals, low-income youth, and those involved in juvenile justice or welfare systems face a higher risk of mental health challenges, the pandemic notwithstanding.
In her work with schools, Denese Shervington, MD, MPH, has witnessed more mental health challenges related to isolation and separation. “There’s an overall worry about the loss of what used to be, the seeming predictability and certainty of prepandemic life,” said Dr. Shervington, clinical professor of psychiatry at Tulane University, and president and CEO of the Institute for Women and Ethnic Studies, both in New Orleans.
A systems of care plan
The advisory lists actionable items for health care and 10 other industry sectors to improve mental health of children and young adults.
Health care organizations and professionals were advised to take the following six steps:
- Implement trauma-informed care principles and other prevention strategies. This may involve referring patients to resources such as economic and legal supports, school enrichment programs, and educating families on healthy child development in the clinic.
- Routinely screen children for mental health challenges and risk factors such as adverse childhood experiences during primary care well-visits or annual physicals, or at schools or EDs. Primary care physicians should use principles of trauma-informed care to conduct these screenings.
- Screen parents, caregivers, and other family members for depression, intimate partner violence, substance use, and other challenges. These can be done in tandem with broader assessments of social determinants of health such as food or housing insecurity.
- Combine efforts of clinical staff with trusted community partners and child welfare and juvenile justice. Hospital-based violence intervention programs, for example, identify patients at risk of repeat violent injury and refer them to hospital- and community-based resources.
- Build multidisciplinary teams, enlisting children and families to develop services that are tailored to their needs for screening and treatment. Such services should reflect cultural diversity and offered in multiple languages.
- Support the well-being of mental health workers and community leaders to foster their ability to help youth and their families.
Dr. Murthy is talking about a “systems of care” approach, in which all sectors that touch children and youth – not just health care – must work together and do their jobs effectively but collaboratively to address this public health crisis, said Aradhana (Bela) Sood MD, MSHA, FAACAP, senior professor of child mental health policy at Virginia Commonwealth University, Richmond. “An investment in infrastructure support of positive mental health in early childhood, be it in schools, communities, or family well-being will lead to a future where illness is not the result of major preventable societal factors, such as a lack of social supports and trauma.”
Changes will ‘take a lot of buy-in’
The recommendations are actionable in the real world – but there are a lot of them, said Dr. Gold. Dr. Murthy doesn’t specify what the plan is to accomplish these metrics or fund them, she added. He “has money and funders like foundations as steps, but foundations have also suffered in the pandemic, so it is not that simple.” Many of these changes are wide in scope and will take a lot of buy-in.
Dr. Shervington would like to have seen more of a focus on educator well-being, given that young people spend a lot of time in educational settings.
“My organization just completed a study in New Orleans that showed teachers having elevated levels of trauma-based conditions since the pandemic,” she said. Schools are indeed a key place to support holistic mental health by focusing on school climate, Dr. Sood added. “If school administrators became uniformly consistent with recognizing the importance of psychological wellness as a prerequisite of good learning, they will create environments where teachers are keenly aware of a child’s mental wellness and make reduction of bullying, wellness check-ins, [and] school-based mental health clinics a priority.
“These are ways nonmedical, community-based supports can enhance student well-being, and reduce depression and other mental health conditions,” Dr. Sood added.
Child psychiatrists stretched ‘even thinner’
Despite mental health parity rules, health plans have not been held accountable. That failure, combined with excessive demands for prior authorization for mental health treatments “have led to dangerous shortages of psychiatrists able to accept insurance,” said Paul S. Nestadt, MD, an assistant professor and public mental health researcher at Johns Hopkins University, Baltimore.
“This is particularly true for child psychiatrists, who are stretched even thinner than those of us in general practice,” Dr. Nestadt said.
While he doesn’t address it head on, Dr. Murthy uses classic parity language when he states that “mental health is no less important than physical health,” said Dr. Nestadt, who consulted with the surgeon general on developing this advisory. “While many of us would have liked to see parity highlighted more directly, this advisory was designed to be an overview.”
Highlighting social media, gun violence
Dr. Nestadt said he was pleased that the advisory emphasized the importance of restricting access to lethal means in preventing youth suicide.
“With youth suicide rates rising faster than in other age groups, and suicide mortality tied so closely to method availability, the surgeon general made the right choice in highlighting the role of guns in suicide,” he said.
The advisory also discussed the role of media and social media companies in addressing the crisis, which is important, said Dr. Gold.
“I believe very strongly that the way we talk about and portray mental health in the media matters,” she said. “I have seen it matter in the clinic with patients. They’ll wonder if someone will think they are now violent if they are diagnosed with a mental illness. Stories change the narrative.”
While the advisory isn’t perfect, the state of youth mental health “will only get worse if we don’t do something,” noted Dr. Gold. “It is critical that this is validated and discussed at the highest level and messages like Dr. Murthy’s get heard.”
Dr. Gold, Dr. Shervington, and Dr. Sood had no disclosures. Dr. Nestadt disclosed serving as a consultant to the surgeon general advisory.
CDC panel backs mRNA COVID vaccines over J&J because of clot risk
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
because the Johnson & Johnson shot carries the risk of a rare but potentially fatal side effect that causes blood clots and bleeding in the brain.
In an emergency meeting on December 16, the CDC’s Advisory Committee on Immunization Practices, or ACIP, voted unanimously (15-0) to state a preference for the mRNA vaccines over the Johnson & Johnson shot. The vote came after the panel heard a safety update on cases of thrombosis with thrombocytopenia syndrome, or TTS, a condition that causes large clots that deplete the blood of platelets, resulting in uncontrolled bleeding.
The move brings the United States in line with other wealthy countries. In May, Denmark dropped the Johnson & Johnson shot from its vaccination program because of this risk. Australia and Greece have limited the use of a similar vaccine, made by AstraZeneca, in younger people because of the TTS risk. Both vaccines use the envelope of a different kind of virus, called an adenovirus, to sneak the vaccine instructions into cells. On Dec. 16, health officials said they had determined that TTS was likely due to a class effect, meaning it happens with all adenovirus vector vaccines.
The risk of dying from TTS after a Johnson & Johnson shot is extremely rare. There is an estimated 1 death for every 2 million doses of the vaccine given in the general population. That risk is higher for women ages 30 to 49, rising to about 2 deaths for every 1 million doses given in this age group. There’s no question that the Johnson & Johnson shot has saved many more lives than it has taken, experts said
Still, the committee previously paused the use of the Johnson & Johnson vaccine in April after the first cases of TTS came to light. That pause was lifted just 10 days later, after a new warning was added to the vaccine’s label to raise awareness of the risk.
In updating the safety information on Johnson & Johnson, the panel noted that the warning label had not sufficiently lowered the risk of death from TTS. Doctors seem to be aware of the condition because none of the patients who had developed TTS had been treated with the blood thinner heparin, which can make the syndrome worse. But patients continued to die even after the label was added, the panel noted, because TTS can progress so quickly that doctors simply don’t have time to treat it.
For that reason, and because there are other, safer vaccines available, the panel decided to make what’s called a preferential statement, saying the Pfizer and Moderna mRNA vaccines should be preferred over Johnson & Johnson.
The statement leaves the J&J vaccine on the market and available to patients who are at risk of a severe allergic reaction to the mRNA vaccines. It also means that people can still choose the J&J vaccine if they still want it after being informed about the risks.
About 17 million first doses and 900,000 second doses of the Johnson & Johnson vaccine have been given in the United States. Through the end of August, 54 cases of thrombosis with thrombocytopenia syndrome (TTS) have occurred after the J&J shots in the United States. Nearly half of those were in women ages 30 to 49. There have been nine deaths from TTS after Johnson & Johnson shots.
A version of this article first appeared on WebMD.com.
FDA updates risks, cautions for clotting-bleeding disorder on Janssen COVID-19 vaccine
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
Updated Janssen/Johnson & Johnson COVID-19 vaccine fact sheets for health care professionals and the general public now include a contraindication to its use in persons with a history of thrombosis with thrombocytopenia after receiving it “or any other adenovirus-vectored COVID-19 vaccine,” the U.S. Food and Drug Administration has announced.
Thrombosis with thrombocytopenia syndrome (TTS) – thrombocytopenia and increased bleeding risk along with documented thrombosis – after administration of the Janssen Ad26.COV2.S vaccine remains rare. But over all age groups, about one in seven cases have been fatal, said the agency.
the provider fact sheet states.
Although TTS associated with the Janssen COVID-19 vaccine has been reported in men and women aged 18 and older, the highest reported rate has been for women aged 30-49, the agency states. The rate in that group has been about 1 case per 100,000 doses administered.
Symptoms of TTS may occur 1-2 weeks after administration of the Janssen COVID-19 vaccine, the FDA says, based on data from the Vaccine Adverse Events Reporting System (VAERS).
Its clinical course shares features with autoimmune heparin-induced thrombocytopenia. In individuals with suspected TTS following receipt of the Janssen COVID-19 vaccine, the agency cautions, the use of heparin “may be harmful and alternative treatments may be needed. Consultation with hematology specialists is strongly recommended.”
The apparent excess risk of TTS remains under investigation, but “the FDA continues to find that the known and potential benefits of the Janssen COVID-19 vaccine outweigh its known and potential risks in individuals 18 years of age and older,” the agency states.
A version of this article first appeared on Medscape.com.
A pandemic silver lining? Dramatic drop in teen drug use
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.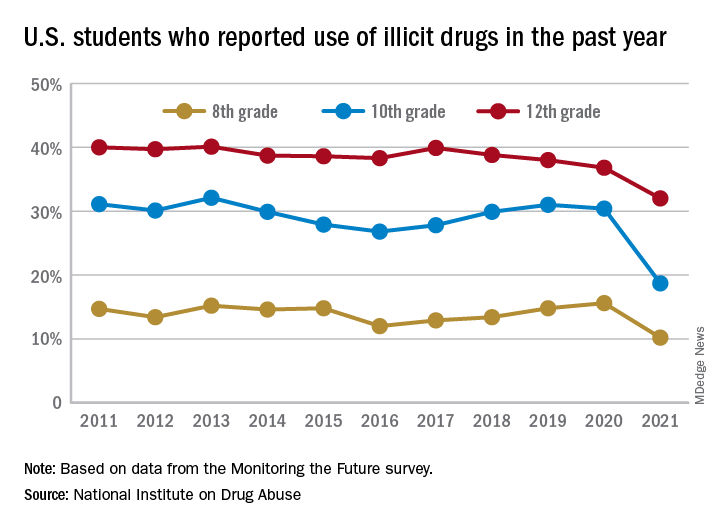
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
Illicit drug use among U.S. teenagers dropped sharply in 2021, likely because of stay-at-home orders and other restrictions on social activities due to the COVID-19 pandemic.
The latest findings, from the Monitoring the Future survey, represent the largest 1-year decrease in overall illicit drug use reported since the survey began in 1975.
“We have never seen such dramatic decreases in drug use among teens in just a 1-year period,” Nora Volkow, MD, director of the National Institute on Drug Abuse (NIDA), said in a news release.
“These data are unprecedented and highlight one unexpected potential consequence of the COVID-19 pandemic, which caused seismic shifts in the day-to-day lives of adolescents,” said Dr. Volkow.
The annual Monitoring the Future survey is conducted by researchers at the University of Michigan, Ann Arbor, and funded by NIDA, to assess drug and alcohol use and related attitudes among adolescent students across the United States.
This year’s self-reported survey included 32,260 students in grades 8, 10, and 12 across 319 public and private schools.
Compared with 2020, the percentage of students reporting any illicit drug use (other than marijuana) in 2021 decreased significantly for 8th graders (down 5.4%), 10th graders (down 11.7%), and 12th graders (down 4.8%).
For alcohol, about 47% of 12th graders and 29% of 10th graders said they drank alcohol in 2021, down significantly from 55% and 41%, respectively, in 2020. The percentage of 8th graders who said they drank alcohol remained stable (17% in 2021 and 20% in 2020).
For teen vaping, about 27% of 12th graders and 20% of 10th graders said they had vaped nicotine in 2021, down significantly from nearly 35% and 31%, respectively, in 2020. Fewer 8th graders also vaped nicotine in 2021 compared with 2020 (12% vs. 17%).
For marijuana, use dropped significantly for all three grades in 2021 compared with 2020. About 31% of 12th graders and 17% of 10th graders said they used marijuana in 2021, down from 35% and 28% in 2020. Among 8th graders, 7% used marijuana in 2021, down from 11% in 2020.
The latest survey also shows significant declines in use of a range of other drugs for many of the age cohorts, including cocaine, hallucinogens, and nonmedical use of amphetamines, tranquilizers, and prescription opioids.
“We knew that this year’s data would illuminate how the COVID-19 pandemic may have impacted substance use among young people, and in the coming years, we will find out whether those impacts are long-lasting as we continue tracking the drug use patterns of these unique cohorts of adolescents,” Richard A. Miech, PhD, who heads the Monitoring the Future study at the University of Michigan, said in the news release.
“Moving forward, it will be crucial to identify the pivotal elements of this past year that contributed to decreased drug use – whether related to drug availability, family involvement, differences in peer pressure, or other factors – and harness them to inform future prevention efforts,” Dr. Volkow added.
In 2021, students across all age groups reported moderate increases in feelings of boredom, anxiety, depression, loneliness, worry, difficulty sleeping, and other negative mental health indicators since the beginning of the pandemic.
A version of this article first appeared on Medscape.com.
A mass on the ear
Pathology indicated a proliferation of basaloid cells with matrical differentiation in transition and “shadow” cells, pointing to a diagnosis of pilomatricoma.
Pilomatricoma, also known as pilomatrixoma, is a benign skin tumor associated with hair follicles. The lesions are most often found on the neck or head area but can occur on the arms, legs, or torso. They are usually slow growing, solitary, and painless. The frequency of occurrence is rare, accounting for less than 1% of all benign skin tumors.1
A mutation in the Catenin beta-1 (CTNNB1) gene is the most common cause of isolated pilomatricoma and is a somatic defect, meaning it is acquired, not inherited. The mutation of the CTNNB1 gene causes disruption of normal function and maturation of the hair follicle. This leads to rapid cell growth and uncontrolled division, resulting in the formation of the pilomatricoma.1
A comprehensive review performed in 2018 noted that only 16% of pilomatricomas were accurately diagnosed on clinical exam.1 Clues that point to the diagnosis of pilomatricoma are the irregular, whitish yellow spots just under the skin. In contrast, epidermoid cysts usually have a central pore and a ballotable feel. The expression of calcification and gritty material from the lesion in this case ruled out a diagnosis of an epidermoid cyst. The most common method of treatment is surgical removal.1
This patient was counseled regarding her diagnosis and given the option of a plastic surgery referral to excise the affected tissue in its entirety. She opted to wait and see if the growth would scar down and not return.
Image courtesy of Edward A. Jackson, MD. Text courtesy of Edward A. Jackson, MD, FAAFP, Advent Health Medical Group Family Medicine at East Orlando, FL, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018;40:631-641. doi: 10.1097/DAD.0000000000001118

Pathology indicated a proliferation of basaloid cells with matrical differentiation in transition and “shadow” cells, pointing to a diagnosis of pilomatricoma.
Pilomatricoma, also known as pilomatrixoma, is a benign skin tumor associated with hair follicles. The lesions are most often found on the neck or head area but can occur on the arms, legs, or torso. They are usually slow growing, solitary, and painless. The frequency of occurrence is rare, accounting for less than 1% of all benign skin tumors.1
A mutation in the Catenin beta-1 (CTNNB1) gene is the most common cause of isolated pilomatricoma and is a somatic defect, meaning it is acquired, not inherited. The mutation of the CTNNB1 gene causes disruption of normal function and maturation of the hair follicle. This leads to rapid cell growth and uncontrolled division, resulting in the formation of the pilomatricoma.1
A comprehensive review performed in 2018 noted that only 16% of pilomatricomas were accurately diagnosed on clinical exam.1 Clues that point to the diagnosis of pilomatricoma are the irregular, whitish yellow spots just under the skin. In contrast, epidermoid cysts usually have a central pore and a ballotable feel. The expression of calcification and gritty material from the lesion in this case ruled out a diagnosis of an epidermoid cyst. The most common method of treatment is surgical removal.1
This patient was counseled regarding her diagnosis and given the option of a plastic surgery referral to excise the affected tissue in its entirety. She opted to wait and see if the growth would scar down and not return.
Image courtesy of Edward A. Jackson, MD. Text courtesy of Edward A. Jackson, MD, FAAFP, Advent Health Medical Group Family Medicine at East Orlando, FL, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

Pathology indicated a proliferation of basaloid cells with matrical differentiation in transition and “shadow” cells, pointing to a diagnosis of pilomatricoma.
Pilomatricoma, also known as pilomatrixoma, is a benign skin tumor associated with hair follicles. The lesions are most often found on the neck or head area but can occur on the arms, legs, or torso. They are usually slow growing, solitary, and painless. The frequency of occurrence is rare, accounting for less than 1% of all benign skin tumors.1
A mutation in the Catenin beta-1 (CTNNB1) gene is the most common cause of isolated pilomatricoma and is a somatic defect, meaning it is acquired, not inherited. The mutation of the CTNNB1 gene causes disruption of normal function and maturation of the hair follicle. This leads to rapid cell growth and uncontrolled division, resulting in the formation of the pilomatricoma.1
A comprehensive review performed in 2018 noted that only 16% of pilomatricomas were accurately diagnosed on clinical exam.1 Clues that point to the diagnosis of pilomatricoma are the irregular, whitish yellow spots just under the skin. In contrast, epidermoid cysts usually have a central pore and a ballotable feel. The expression of calcification and gritty material from the lesion in this case ruled out a diagnosis of an epidermoid cyst. The most common method of treatment is surgical removal.1
This patient was counseled regarding her diagnosis and given the option of a plastic surgery referral to excise the affected tissue in its entirety. She opted to wait and see if the growth would scar down and not return.
Image courtesy of Edward A. Jackson, MD. Text courtesy of Edward A. Jackson, MD, FAAFP, Advent Health Medical Group Family Medicine at East Orlando, FL, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018;40:631-641. doi: 10.1097/DAD.0000000000001118
1. Jones CD, Ho W, Robertson BF, et al. Pilomatrixoma: a comprehensive review of the literature. Am J Dermatopathol. 2018;40:631-641. doi: 10.1097/DAD.0000000000001118
Diabetes tied to Parkinson’s risk, more rapid disease progression
Diabetes mellitus (DM) is associated with Parkinson’s disease (PD) development, as well as more severe symptoms and more rapid disease progression, new research suggests.
In a systematic review, patients with type 2 diabetes were 34% more likely to develop PD than those without comorbid DM. In addition, patients with both conditions had significantly worse scores on the Unified Parkinson’s Disease Rating Scale (UPDRS) and worse cognitive performance.
Together, the results suggest that “DM may be a facilitating factor of neurodegeneration,” wrote the investigators, led by Gennaro Pagano, MD, PhD, expert medical director at Roche Pharma Research and Early Development, in Basel, Switzerland.
The findings were published in a recent issue of the Journal of Parkinson’s Disease.
Unanswered questions
Researchers have long proposed a potential relationship between diabetes and PD. However, case-control studies have yielded conflicting results about this relationship – and previous systematic reviews have failed to clarify the question.
In the current systematic review and meta-analysis, investigators identified relevant studies in databases such as MEDLINE/PubMed, Cochrane CENTRAL, and Scopus.
Eligible studies reported prevalence of DM in patients with PD, reported incidence of PD in those with and those without DM, and analyzed Parkinson’s phenotype and progression in those with and those without DM.
The researchers identified 3,829 articles in their initial search, evaluated 90 articles in detail, and included 43 studies in their analysis. Study quality was judged to be moderate or good, and the investigators did not find significant publication bias.
Twenty-one studies that encompassed 11,396 patients were examined to determine prevalence of DM in PD. This prevalence was calculated to be 10.02%, which is similar to the global prevalence of 9.3% reported in 2019.
The researchers also analyzed 12 cohort studies that included 17,797,221 patients to calculate risk for PD in patients with comorbid diabetes. The pooled summary odds ratio for incident PD among patients with type 2 diabetes was 1.34.
The evaluation of the effect of diabetes on PD severity was based on 10 studies that included 603 patients with both diseases. Because data on motor symptoms were not available for all studies, the researchers considered Hoehn and Yahr stage, UPDRS score, and cognitive impairment.
Patients with both conditions had a worse Hoehn and Yahr stage (standardized mean difference, 0.36; P < .001), and higher UPDRS score (SMD, 0.60; P < .001). In 7 of the 10 studies, diabetes was associated with worse cognitive performance in patients with PD.
Mechanisms uncertain
The mechanisms of the effect of diabetes on risk for and severity of PD are uncertain, but the researchers have developed hypotheses.
“Overlapping mechanisms between insulin resistance, mitochondrial dysfunction, oxidative stress, and alpha-synuclein expression could influence the development of the neurodegeneration process,” they wrote.
Because the current analysis demonstrated a trend toward more pronounced cognitive decline in patients with the comorbidities, clinicians should pay particular attention to the progression of motor and cognitive symptoms in patients with these diseases, the investigators noted.
“Additional studies are needed in order to better define the clinical phenotype of PD-DM patients and explore the role of antidiabetic drugs on PD progression,” they wrote.
They add that future studies also are needed to evaluate whether antidiabetic drugs might reduce risk for PD in these patients.
The investigators noted several limitations of their research. In many of the studies they examined, for example, diagnostic criteria of type 2 diabetes and PD were based only on medical records or self-reported health questionnaires. The diagnoses were rarely confirmed.
In addition, not all studies clearly stated that their populations presented with type 2 diabetes. Finally, patients with diabetes may be at increased risk for cardiovascular death, which could affect follow-up related to the development of PD, the investigators noted.
A version of this article first appeared on Medscape.com.
Diabetes mellitus (DM) is associated with Parkinson’s disease (PD) development, as well as more severe symptoms and more rapid disease progression, new research suggests.
In a systematic review, patients with type 2 diabetes were 34% more likely to develop PD than those without comorbid DM. In addition, patients with both conditions had significantly worse scores on the Unified Parkinson’s Disease Rating Scale (UPDRS) and worse cognitive performance.
Together, the results suggest that “DM may be a facilitating factor of neurodegeneration,” wrote the investigators, led by Gennaro Pagano, MD, PhD, expert medical director at Roche Pharma Research and Early Development, in Basel, Switzerland.
The findings were published in a recent issue of the Journal of Parkinson’s Disease.
Unanswered questions
Researchers have long proposed a potential relationship between diabetes and PD. However, case-control studies have yielded conflicting results about this relationship – and previous systematic reviews have failed to clarify the question.
In the current systematic review and meta-analysis, investigators identified relevant studies in databases such as MEDLINE/PubMed, Cochrane CENTRAL, and Scopus.
Eligible studies reported prevalence of DM in patients with PD, reported incidence of PD in those with and those without DM, and analyzed Parkinson’s phenotype and progression in those with and those without DM.
The researchers identified 3,829 articles in their initial search, evaluated 90 articles in detail, and included 43 studies in their analysis. Study quality was judged to be moderate or good, and the investigators did not find significant publication bias.
Twenty-one studies that encompassed 11,396 patients were examined to determine prevalence of DM in PD. This prevalence was calculated to be 10.02%, which is similar to the global prevalence of 9.3% reported in 2019.
The researchers also analyzed 12 cohort studies that included 17,797,221 patients to calculate risk for PD in patients with comorbid diabetes. The pooled summary odds ratio for incident PD among patients with type 2 diabetes was 1.34.
The evaluation of the effect of diabetes on PD severity was based on 10 studies that included 603 patients with both diseases. Because data on motor symptoms were not available for all studies, the researchers considered Hoehn and Yahr stage, UPDRS score, and cognitive impairment.
Patients with both conditions had a worse Hoehn and Yahr stage (standardized mean difference, 0.36; P < .001), and higher UPDRS score (SMD, 0.60; P < .001). In 7 of the 10 studies, diabetes was associated with worse cognitive performance in patients with PD.
Mechanisms uncertain
The mechanisms of the effect of diabetes on risk for and severity of PD are uncertain, but the researchers have developed hypotheses.
“Overlapping mechanisms between insulin resistance, mitochondrial dysfunction, oxidative stress, and alpha-synuclein expression could influence the development of the neurodegeneration process,” they wrote.
Because the current analysis demonstrated a trend toward more pronounced cognitive decline in patients with the comorbidities, clinicians should pay particular attention to the progression of motor and cognitive symptoms in patients with these diseases, the investigators noted.
“Additional studies are needed in order to better define the clinical phenotype of PD-DM patients and explore the role of antidiabetic drugs on PD progression,” they wrote.
They add that future studies also are needed to evaluate whether antidiabetic drugs might reduce risk for PD in these patients.
The investigators noted several limitations of their research. In many of the studies they examined, for example, diagnostic criteria of type 2 diabetes and PD were based only on medical records or self-reported health questionnaires. The diagnoses were rarely confirmed.
In addition, not all studies clearly stated that their populations presented with type 2 diabetes. Finally, patients with diabetes may be at increased risk for cardiovascular death, which could affect follow-up related to the development of PD, the investigators noted.
A version of this article first appeared on Medscape.com.
Diabetes mellitus (DM) is associated with Parkinson’s disease (PD) development, as well as more severe symptoms and more rapid disease progression, new research suggests.
In a systematic review, patients with type 2 diabetes were 34% more likely to develop PD than those without comorbid DM. In addition, patients with both conditions had significantly worse scores on the Unified Parkinson’s Disease Rating Scale (UPDRS) and worse cognitive performance.
Together, the results suggest that “DM may be a facilitating factor of neurodegeneration,” wrote the investigators, led by Gennaro Pagano, MD, PhD, expert medical director at Roche Pharma Research and Early Development, in Basel, Switzerland.
The findings were published in a recent issue of the Journal of Parkinson’s Disease.
Unanswered questions
Researchers have long proposed a potential relationship between diabetes and PD. However, case-control studies have yielded conflicting results about this relationship – and previous systematic reviews have failed to clarify the question.
In the current systematic review and meta-analysis, investigators identified relevant studies in databases such as MEDLINE/PubMed, Cochrane CENTRAL, and Scopus.
Eligible studies reported prevalence of DM in patients with PD, reported incidence of PD in those with and those without DM, and analyzed Parkinson’s phenotype and progression in those with and those without DM.
The researchers identified 3,829 articles in their initial search, evaluated 90 articles in detail, and included 43 studies in their analysis. Study quality was judged to be moderate or good, and the investigators did not find significant publication bias.
Twenty-one studies that encompassed 11,396 patients were examined to determine prevalence of DM in PD. This prevalence was calculated to be 10.02%, which is similar to the global prevalence of 9.3% reported in 2019.
The researchers also analyzed 12 cohort studies that included 17,797,221 patients to calculate risk for PD in patients with comorbid diabetes. The pooled summary odds ratio for incident PD among patients with type 2 diabetes was 1.34.
The evaluation of the effect of diabetes on PD severity was based on 10 studies that included 603 patients with both diseases. Because data on motor symptoms were not available for all studies, the researchers considered Hoehn and Yahr stage, UPDRS score, and cognitive impairment.
Patients with both conditions had a worse Hoehn and Yahr stage (standardized mean difference, 0.36; P < .001), and higher UPDRS score (SMD, 0.60; P < .001). In 7 of the 10 studies, diabetes was associated with worse cognitive performance in patients with PD.
Mechanisms uncertain
The mechanisms of the effect of diabetes on risk for and severity of PD are uncertain, but the researchers have developed hypotheses.
“Overlapping mechanisms between insulin resistance, mitochondrial dysfunction, oxidative stress, and alpha-synuclein expression could influence the development of the neurodegeneration process,” they wrote.
Because the current analysis demonstrated a trend toward more pronounced cognitive decline in patients with the comorbidities, clinicians should pay particular attention to the progression of motor and cognitive symptoms in patients with these diseases, the investigators noted.
“Additional studies are needed in order to better define the clinical phenotype of PD-DM patients and explore the role of antidiabetic drugs on PD progression,” they wrote.
They add that future studies also are needed to evaluate whether antidiabetic drugs might reduce risk for PD in these patients.
The investigators noted several limitations of their research. In many of the studies they examined, for example, diagnostic criteria of type 2 diabetes and PD were based only on medical records or self-reported health questionnaires. The diagnoses were rarely confirmed.
In addition, not all studies clearly stated that their populations presented with type 2 diabetes. Finally, patients with diabetes may be at increased risk for cardiovascular death, which could affect follow-up related to the development of PD, the investigators noted.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF PARKINSON’S DISEASE
Inadequate routine diabetes screening common in HIV
, research shows.
“Despite known risk in this patient population, most patients were not up to date with routine preventative screenings,” report Maya Hardman, PharmD, and colleagues with Southwest CARE Center, in Santa Fe, New Mexico, in research presented at the United States Conference on HIV/AIDS (USCHA) 2021 Annual Meeting.
“Routine preventative screenings can help identify chronic complications of diabetes early, if performed at the recommended intervals,” they write.
People with HIV are known to be at an increased risk of diabetes and the long-term complications of the disease, making the need for routine screening to prevent such complications all the more pressing due to their higher-risk health status.
Among the key routine diabetes care quality measures recommended by the Healthcare Effectiveness Data and Information Set (HEDIS) for people with HIV are testing for A1c once every 3 months, foot and eye exams every 12 months, urine albumin creatinine ratio (UACR) screenings every 12 months, and two controlled blood pressure readings every 12 months.
To investigate the rates of adherence to the HEDIS screening recommendations and identify predictors of poor compliance among people with HIV, Dr. Hardman and her colleagues evaluated data on 121 adult patients at the Southwest CARE Center who had been diagnosed with diabetes and HIV and were treated between 2019 and 2020.
The patients had a mean age of 57.5, and 9% were female. Their mean duration of being HIV positive was 19.8 years, and they had an intermediate Atherosclerotic Cardiovascular Disease (ASCVD) risk score of 17.08%.
Despite their known diagnoses of having diabetes, as many as 93.4% were found not to be up to date on their routine preventive screenings.
Of the 121 patients, only 30 had received the recommended A1c screenings, 37 had the recommended UACR screenings, and just 18 had received the recommended foot exam screenings.
Only blood pressure screenings, reported in 90 of the 121 patients, were up to date in the majority of patients in the group.
In looking at factors associated with compliance with A1c screening, only age (OR, 0.95; P = .04) was a significant predictor.
The authors pointed out that routine screenings for diabetes complications are relatively easy to implement.
“Screening for these chronic complications is minimally invasive and can be provided by individuals trained in diabetes management during routine clinic appointments.”
The team’s ongoing research is evaluating the potential benefits of clinical pharmacy services in assisting with the screenings for patients with HIV.
Research underscoring the increased risk and poorer treatment outcomes of diabetes in people with HIV include a study comparing 337 people with HIV in 2005 with a cohort of 338 participants in 2015.
The study showed the prevalence of type 2 diabetes had increased to 15.1% in 2015 from 6.8% 10 years earlier, for a relative risk of 2.4 compared with the general population.
“The alarmingly high prevalence of type 2 diabetes in HIV requires improved screening, targeted to older patients and those with a longer duration of exposure to antiretrovirals,” the authors wrote.
“Effective diabetes prevention and management strategies are needed urgently to reduce this risk; such interventions should target both conventional risk factors, such as abdominal obesity and HIV-specific risk factors such as weight gain following initiation of antiretrovirals.”
Of note, the 2015 cohort was significantly older and had higher BMI and higher hypertension than the 2005 cohort.
First author Alastair Duncan, PhD, principal dietitian at Guy’s & St. Thomas’ Hospital and lecturer, King’s College London, noted that since that 2015 study was published, concerns particularly with weight gain in the HIV population have only increased.
“Weight gain appears to be more of an issue [now],” he told this news organization in an interview.
“As in the general population, people living with HIV experienced significant weight gain during COVID-related lockdowns. Added to the high number of people living with HIV being treated with integrase inhibitors, weight gain remains a challenge.”
Meanwhile, “there are not enough studies comparing people living with HIV with the general population,” Dr. Duncan added. “We need to conduct studies where participants are matched.”
Sudipa Sarkar, MD, who co-authored a report on the issue of diabetes and HIV this year but was not involved in the study presented at USCHA, noted that the setting of care could play an important role in the quality of screening for diabetes that people with HIV receive.
“It may depend on factors such as whether a patient is being followed regularly by an HIV care provider and the larger health care system that the patient is in,” Dr. Sarkar, an assistant professor of medicine at Johns Hopkins University School of Medicine, Division of Endocrinology, Diabetes, and Metabolism, told this news organization.
“For example, one might find differences between a patient being seen in a managed care group versus not.”
The issue of how the strikingly high rates of inadequate screening in the current study compare with routine screening in the general diabetes population “is a good question and warrants more research,” she said.
The authors and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, research shows.
“Despite known risk in this patient population, most patients were not up to date with routine preventative screenings,” report Maya Hardman, PharmD, and colleagues with Southwest CARE Center, in Santa Fe, New Mexico, in research presented at the United States Conference on HIV/AIDS (USCHA) 2021 Annual Meeting.
“Routine preventative screenings can help identify chronic complications of diabetes early, if performed at the recommended intervals,” they write.
People with HIV are known to be at an increased risk of diabetes and the long-term complications of the disease, making the need for routine screening to prevent such complications all the more pressing due to their higher-risk health status.
Among the key routine diabetes care quality measures recommended by the Healthcare Effectiveness Data and Information Set (HEDIS) for people with HIV are testing for A1c once every 3 months, foot and eye exams every 12 months, urine albumin creatinine ratio (UACR) screenings every 12 months, and two controlled blood pressure readings every 12 months.
To investigate the rates of adherence to the HEDIS screening recommendations and identify predictors of poor compliance among people with HIV, Dr. Hardman and her colleagues evaluated data on 121 adult patients at the Southwest CARE Center who had been diagnosed with diabetes and HIV and were treated between 2019 and 2020.
The patients had a mean age of 57.5, and 9% were female. Their mean duration of being HIV positive was 19.8 years, and they had an intermediate Atherosclerotic Cardiovascular Disease (ASCVD) risk score of 17.08%.
Despite their known diagnoses of having diabetes, as many as 93.4% were found not to be up to date on their routine preventive screenings.
Of the 121 patients, only 30 had received the recommended A1c screenings, 37 had the recommended UACR screenings, and just 18 had received the recommended foot exam screenings.
Only blood pressure screenings, reported in 90 of the 121 patients, were up to date in the majority of patients in the group.
In looking at factors associated with compliance with A1c screening, only age (OR, 0.95; P = .04) was a significant predictor.
The authors pointed out that routine screenings for diabetes complications are relatively easy to implement.
“Screening for these chronic complications is minimally invasive and can be provided by individuals trained in diabetes management during routine clinic appointments.”
The team’s ongoing research is evaluating the potential benefits of clinical pharmacy services in assisting with the screenings for patients with HIV.
Research underscoring the increased risk and poorer treatment outcomes of diabetes in people with HIV include a study comparing 337 people with HIV in 2005 with a cohort of 338 participants in 2015.
The study showed the prevalence of type 2 diabetes had increased to 15.1% in 2015 from 6.8% 10 years earlier, for a relative risk of 2.4 compared with the general population.
“The alarmingly high prevalence of type 2 diabetes in HIV requires improved screening, targeted to older patients and those with a longer duration of exposure to antiretrovirals,” the authors wrote.
“Effective diabetes prevention and management strategies are needed urgently to reduce this risk; such interventions should target both conventional risk factors, such as abdominal obesity and HIV-specific risk factors such as weight gain following initiation of antiretrovirals.”
Of note, the 2015 cohort was significantly older and had higher BMI and higher hypertension than the 2005 cohort.
First author Alastair Duncan, PhD, principal dietitian at Guy’s & St. Thomas’ Hospital and lecturer, King’s College London, noted that since that 2015 study was published, concerns particularly with weight gain in the HIV population have only increased.
“Weight gain appears to be more of an issue [now],” he told this news organization in an interview.
“As in the general population, people living with HIV experienced significant weight gain during COVID-related lockdowns. Added to the high number of people living with HIV being treated with integrase inhibitors, weight gain remains a challenge.”
Meanwhile, “there are not enough studies comparing people living with HIV with the general population,” Dr. Duncan added. “We need to conduct studies where participants are matched.”
Sudipa Sarkar, MD, who co-authored a report on the issue of diabetes and HIV this year but was not involved in the study presented at USCHA, noted that the setting of care could play an important role in the quality of screening for diabetes that people with HIV receive.
“It may depend on factors such as whether a patient is being followed regularly by an HIV care provider and the larger health care system that the patient is in,” Dr. Sarkar, an assistant professor of medicine at Johns Hopkins University School of Medicine, Division of Endocrinology, Diabetes, and Metabolism, told this news organization.
“For example, one might find differences between a patient being seen in a managed care group versus not.”
The issue of how the strikingly high rates of inadequate screening in the current study compare with routine screening in the general diabetes population “is a good question and warrants more research,” she said.
The authors and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, research shows.
“Despite known risk in this patient population, most patients were not up to date with routine preventative screenings,” report Maya Hardman, PharmD, and colleagues with Southwest CARE Center, in Santa Fe, New Mexico, in research presented at the United States Conference on HIV/AIDS (USCHA) 2021 Annual Meeting.
“Routine preventative screenings can help identify chronic complications of diabetes early, if performed at the recommended intervals,” they write.
People with HIV are known to be at an increased risk of diabetes and the long-term complications of the disease, making the need for routine screening to prevent such complications all the more pressing due to their higher-risk health status.
Among the key routine diabetes care quality measures recommended by the Healthcare Effectiveness Data and Information Set (HEDIS) for people with HIV are testing for A1c once every 3 months, foot and eye exams every 12 months, urine albumin creatinine ratio (UACR) screenings every 12 months, and two controlled blood pressure readings every 12 months.
To investigate the rates of adherence to the HEDIS screening recommendations and identify predictors of poor compliance among people with HIV, Dr. Hardman and her colleagues evaluated data on 121 adult patients at the Southwest CARE Center who had been diagnosed with diabetes and HIV and were treated between 2019 and 2020.
The patients had a mean age of 57.5, and 9% were female. Their mean duration of being HIV positive was 19.8 years, and they had an intermediate Atherosclerotic Cardiovascular Disease (ASCVD) risk score of 17.08%.
Despite their known diagnoses of having diabetes, as many as 93.4% were found not to be up to date on their routine preventive screenings.
Of the 121 patients, only 30 had received the recommended A1c screenings, 37 had the recommended UACR screenings, and just 18 had received the recommended foot exam screenings.
Only blood pressure screenings, reported in 90 of the 121 patients, were up to date in the majority of patients in the group.
In looking at factors associated with compliance with A1c screening, only age (OR, 0.95; P = .04) was a significant predictor.
The authors pointed out that routine screenings for diabetes complications are relatively easy to implement.
“Screening for these chronic complications is minimally invasive and can be provided by individuals trained in diabetes management during routine clinic appointments.”
The team’s ongoing research is evaluating the potential benefits of clinical pharmacy services in assisting with the screenings for patients with HIV.
Research underscoring the increased risk and poorer treatment outcomes of diabetes in people with HIV include a study comparing 337 people with HIV in 2005 with a cohort of 338 participants in 2015.
The study showed the prevalence of type 2 diabetes had increased to 15.1% in 2015 from 6.8% 10 years earlier, for a relative risk of 2.4 compared with the general population.
“The alarmingly high prevalence of type 2 diabetes in HIV requires improved screening, targeted to older patients and those with a longer duration of exposure to antiretrovirals,” the authors wrote.
“Effective diabetes prevention and management strategies are needed urgently to reduce this risk; such interventions should target both conventional risk factors, such as abdominal obesity and HIV-specific risk factors such as weight gain following initiation of antiretrovirals.”
Of note, the 2015 cohort was significantly older and had higher BMI and higher hypertension than the 2005 cohort.
First author Alastair Duncan, PhD, principal dietitian at Guy’s & St. Thomas’ Hospital and lecturer, King’s College London, noted that since that 2015 study was published, concerns particularly with weight gain in the HIV population have only increased.
“Weight gain appears to be more of an issue [now],” he told this news organization in an interview.
“As in the general population, people living with HIV experienced significant weight gain during COVID-related lockdowns. Added to the high number of people living with HIV being treated with integrase inhibitors, weight gain remains a challenge.”
Meanwhile, “there are not enough studies comparing people living with HIV with the general population,” Dr. Duncan added. “We need to conduct studies where participants are matched.”
Sudipa Sarkar, MD, who co-authored a report on the issue of diabetes and HIV this year but was not involved in the study presented at USCHA, noted that the setting of care could play an important role in the quality of screening for diabetes that people with HIV receive.
“It may depend on factors such as whether a patient is being followed regularly by an HIV care provider and the larger health care system that the patient is in,” Dr. Sarkar, an assistant professor of medicine at Johns Hopkins University School of Medicine, Division of Endocrinology, Diabetes, and Metabolism, told this news organization.
“For example, one might find differences between a patient being seen in a managed care group versus not.”
The issue of how the strikingly high rates of inadequate screening in the current study compare with routine screening in the general diabetes population “is a good question and warrants more research,” she said.
The authors and Dr. Sarkar have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
COVID-19 asymptomatic infection rate remains high
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Based on data from a meta-analysis of 95 studies that included nearly 30,000,000 individuals, the pooled percentage of asymptomatic COVID-19 infections was 0.25% in the tested population and 40.5% among confirmed cases.
, wrote Qiuyue Ma, PhD, and colleagues of Peking University, Beijing.
In a study published in JAMA Network Open the researchers identified 44 cross-sectional studies, 41 cohort studies, seven case series, and three case series on transmission studies. A total of 74 studies were conducted in developed countries, including those in Europe, North America, and Asia. Approximately one-third (37) of the studies were conducted among health care workers or in-hospital patients, 17 among nursing home staff or residents, and 14 among community residents. In addition, 13 studies involved pregnant women, eight involved air or cruise ship travelers, and six involved close contacts of individuals with confirmed infections.
The meta-analysis included 29,776,306 tested individuals; 11,516 of them had asymptomatic infections.
Overall, the pooled percentage of asymptomatic infections among the tested population was 0.25%. In an analysis of different study populations, the percentage was higher in nursing home residents or staff (4.52%), air or cruise ship travelers (2.02%), and pregnant women (2.34%), compared against the pooled percentage.
The pooled percentage of asymptomatic infections among the confirmed population was 40.50%, and this percentage was higher in pregnant women (54.11%), air or cruise ship travelers (52.91%), and nursing home residents or staff (47.53%).
The pooled percentage in the tested population was higher than the overall percentage when the mean age of the study population was 60 years or older (3.69%). By contrast, in the confirmed population, the pooled percentage was higher than the overall percentage when the study population was younger than 20 years (60.2%) or aged 20 to 39 years (49.5%).
The researchers noted in their discussion that the varying percentage of asymptomatic individuals according to community prevalence might impact the heterogeneity of the included studies. They also noted the high number of studies conducted in nursing home populations, groups in which asymptomatic individuals were more likely to be tested.
The study findings were limited by several factors, including the potential for missed studies that were not published at the time of the meta-analysis, as well as the exclusion of studies written in Chinese, the researchers noted. Other limitations included lack of follow-up on presymptomatic and covert infections, and the focus on specific populations, factors that may limit the degree to which the results can be generalized.
However, the results highlight the need to screen for asymptomatic infections, especially in countries where COVID-19 has been better controlled, the researchers said. Management strategies for asymptomatic infections, when identified, should include isolation and contact tracing similar to strategies used with confirmed cases, they added.
More testing needed to catch cases early
“During the initial phase of [the] COVID-19 pandemic, testing was not widely available in the United States or the rest of the world,” Setu Patolia, MD, of Saint Louis University School of Medicine, Missouri, said in an interview. Much of the world still lacks access to COVID-19 testing, and early in the pandemic only severely symptomatic patients were tested, he said. “With new variants, particularly the Omicron variant, which may have mild or minimally symptomatic disease, asymptomatic carriers play an important role in propagation of the pandemic,” he explained. “It is important to know the asymptomatic carrier rate among the general population for the future control of [the] pandemic,” he added.
Dr. Patolia said he was surprised by the study finding that one in 400 people in the general population could be asymptomatic carriers of COVID-19.
“Also, nursing home patients are more at risk of complications of COVID, and I expected that they would have a higher rate of symptomatic disease as compared to [the] general population,” said Dr. Patolia. He was also surprised by the high rate of asymptomatic infections in travelers.
“Physicians should be more aware about the asymptomatic carrier rate, particularly in travelers and nursing home patients,” he noted. “Travelers carry high risk of transferring infection from one region to another region of the world, and physicians should advise them to get tested despite the absence of symptoms,” Dr. Patolia emphasized. “Similarly, once any nursing home patient has been diagnosed with COVID-19, physicians should be more careful with the rest of the nursing home patients and test them despite the absence of the symptoms,” he added.
Dr. Patolia also recommended that pregnant women wear masks to help prevent disease transmission when visiting a doctor’s office or labor unit.
Looking ahead, there is a need for cheaper at-home testing kits so that all vulnerable populations can be tested fast and frequently, Dr. Patolia said.
The study was supported by the National Natural Science Foundation of China. The researchers had no financial conflicts to disclose. Dr. Patolia has disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.