User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Patients went into the hospital for care. After testing positive there for COVID, some never came out.
They went into hospitals with heart attacks, kidney failure or in a psychiatric crisis.
They left with COVID-19 — if they left at all.
More than 10,000 patients were diagnosed with COVID in a U.S. hospital last year after they were admitted for something else, according to federal and state records analyzed exclusively for KHN. The number is certainly an undercount, since it includes mostly patients 65 and older, plus California and Florida patients of all ages.
Yet in the scheme of things that can go wrong in a hospital, it is catastrophic: About 21% of the patients who contracted COVID in the hospital from April to September last year died, the data shows. In contrast, nearly 8% of other Medicare patients died in the hospital at the time.
Steven Johnson, 66, was expecting to get an infection cut out of his hip flesh and bone at Blake Medical Center in Bradenton, Fla., last November. The retired pharmacist had survived colon cancer and was meticulous to avoid contracting COVID. He could not have known that, from April through September, 8% of that hospital’s Medicare COVID patients were diagnosed with the virus after they were admitted for another concern.
Mr. Johnson had tested negative for COVID two days before he was admitted. After 13 days in the hospital, he tested positive, said his wife, Cindy Johnson, also a retired pharmacist.
Soon he was struggling to clear a glue-like phlegm from his lungs. A medical team could hardly control his pain. They prompted Cindy to share his final wishes. She asked: “Honey, do you want to be intubated?” He responded with an emphatic “no.” He died three days later.
After her husband tested positive, Cindy Johnson, trained in contact tracing, quickly got a COVID test. She tested negative. Then she thought about the large number of hospital staffers flowing into and out of his room — where he was often unmasked — and suspected a staff member had infected him. That the hospital, part of the HCA Healthcare chain, still has not mandated staff vaccinations is “appalling,” she said.
“I’m furious,” she said.
“How can they say on their website,” she asked, “that the safety precautions ‘we’ve put into place make our facilities among the safest possible places to receive healthcare at this time’?”
Blake Medical Center spokesperson Lisa Kirkland said the hospital is “strongly encouraging vaccination” and noted that it follows Centers for Disease Control and Prevention and federal and state guidelines to protect patients. President Joe Biden has called for all hospital employees to be vaccinated, but the requirement could face resistance in a dozen states, including Florida, that have banned vaccine mandates.
Overall, the rate of in-hospital spread among Medicare and other patients was lower than in other countries, including the United Kingdom, which makes such data public and openly discusses it. On average, about 1.7% of U.S. hospitalized COVID patients were diagnosed with the virus in U.S. hospitals, according to an analysis of Medicare records from April 1 to Sept. 30, 2020, provided by Dr. James Kennedy, founder of CDIMD, a Nashville-based consulting and data analytics company.
Yet the rate of infection was far higher in 38 hospitals where 5% or more of the Medicare COVID cases were documented as hospital-acquired. The data is from a challenging stretch last year when protective gear was in short supply and tests were scarce or slow to produce results. The Medicare data for the fourth quarter of 2020 and this year isn’t available yet, and the state data reflects April 1 through Dec. 31, 2020.
A KHN review of work-safety records, medical literature and interviews with staff at high-spread hospitals points to why the virus took hold: Hospital leaders were slow to appreciate its airborne nature, which made coughing patients hazardous to roommates and staff members, who often wore less-protective surgical masks instead of N95s. Hospitals failed to test every admitted patient, enabled by CDC guidance that leaves such testing to the “discretion of the facility.” Management often failed to inform workers when they’d been exposed to COVID and so were at risk of spreading it themselves.
Spread among patients and staffers seemed to go hand in hand. At Beaumont Hospital, Taylor, in Michigan, 139 employee COVID infections were logged between April 6 to Oct. 20 last year, a hospital inspection report shows. Nearly 7% of the Medicare patients with COVID tested positive after they were admitted to that hospital for something else, the federal data shows. A hospital spokesperson said tests were not available to screen all patients last year, resulting in some late diagnoses. He said all incoming patients are tested now.
Tracking COVID inside health facilities is no new task to federal officials, who publicly report new staff and resident cases weekly for each U.S. nursing home. Yet the Department of Health and Human Services reports data on COVID’s spread in hospitals only on a statewide basis, so patients are in the dark about which facilities have cases.
KHN commissioned analyses of hospital billing records, which are also used more broadly to spot various hospital-acquired infections. For COVID, the data has limitations. It can pick up some community-acquired cases that were slow to show up, as it can take two to 14 days from exposure to the virus for symptoms to appear, with the average being four to five days. The records do not account for cases picked up in an emergency room or diagnosed after a hospital patient was discharged.
Linda Moore, 71, tested positive at least 15 days into a hospital stay for spinal surgery, according to her daughter Trisha Tavolazzi. Her mother was at Havasu Regional Medical Center in Lake Havasu City, Ariz., which did not have a higher-than-average rate of internal spread last summer.
The hospital implemented “rigorous health and safety protocols to protect all of our patients” during the pandemic, said hospital spokesperson Corey Santoriello, who would not comment on Ms. Moore’s case, citing privacy laws.
Ms. Moore was airlifted to another hospital, where her condition only declined further, her daughter said. After the ventilator was removed, she clung to life fitfully for 5½ hours, as her daughter prayed for her mother to find her way to heaven.
“I asked her mom and her dad and her family and prayed to God, ‘Please just come show her the way,’” Ms. Tavolazzi said. “I relive it every day.”
When Ms. Tavolazzi sought answers from the hospital about where her mom got the virus, she said, she got none: “No one ever called me back.”
Two negative COVID tests, then ‘patient zero’
As the second surge of COVID subsided last September, doctors from the prestigious Brigham and Women’s Hospital published a reassuringstudy: With careful infection control, only two of 697 COVID patients acquired the virus within the Boston hospital. That is about 0.3% of patients --about six times lower than the overall Medicare rate. Brigham tested every patient it admitted, exceeding CDC recommendations. It was transparent and open about safety concerns.
But the study, published in the high-profile JAMA Network Open journal, conveyed the wrong message, according to Dr. Manoj Jain, an infectious-disease physician and adjunct professor at the Rollins School of Public Health at Emory University. COVID was spreading in hospitals, he said, and the study buried “the problem under the rug.”
Before the virtual ink on the study was dry, the virus began a stealthy streak through the elite hospital. It slipped in with a patient who tested negative twice -- but turned out to be positive. She was “patient zero” in an outbreak affecting 38 staffers and 14 patients, according to a study in Annals of Internal Medicine initially published Feb. 9.
That study’s authors sequenced the genome of the virus to confirm which cases were related and precisely how it traveled through the hospital.
As patients were moved from room to room in the early days of the outbreak, COVID spread among roommates 8 out of 9 times, likely through aerosol transmission, the study says. A survey of staff members revealed that those caring for coughing patients were more likely to get sick.
The virus also appeared to have breached the CDC-OK’d protective gear. Two staff members who had close patient contact while wearing a surgical mask and face shield still wound up infected. The findings suggested that more-protective N95 respirators could help safeguard staff.
Brigham and Women’s now tests every patient upon admission and again soon after. Nurses are encouraged to test again if they see a subtle sign of COVID, said Dr. Erica Shenoy, associate chief of the Infection Control Unit at Massachusetts General Hospital, who helped craft policy at Brigham.
She said nurses and environmental services workers are at the table for policymaking: “I personally make it a point to say, ‘Tell me what you’re thinking,’” Dr. Shenoy said. “‘There’s no retribution because we need to know.’”
CDC guidelines, though, left wide latitude on protective gear and testing. To this day, Dr. Shenoy said, hospitals employ a wide range of policies.
The CDC said in a statement that its guidelines “provide a comprehensive and layered approach to preventing transmission of SARS-CoV-2 in healthcare settings,” and include testing patients with “even mild symptoms” or recent exposure to someone with COVID.
Infection control policies are rarely apparent to patients or visitors, beyond whether they’re asked to wear a mask. But reviews of public records and interviews with more than a dozen people show that at hospitals with high rates of COVID spread, staff members were often alarmed by the lack of safety practices.
Nurses sound the alarm on COVID spread
As COVID crept into Florida in spring 2020, nurse Victoria Holland clashed with managers at Blake Medical Center in Bradenton, where Steven Johnson died.
She said managers suspended her early in the pandemic after taking part in a protest and “having a hissy fit” when she was denied a new N95 respirator before an “aerosol-generating” procedure. The CDC warns that such procedures can spread the virus through the air. Before the pandemic, nurses were trained to dispose of an N95 after each patient encounter.
When the suspension was over, Ms. Holland said, she felt unsafe. “They told us nothing,” she said. “It was all a little whisper between the doctors. You had potential COVIDs and you’d get a little surgical mask because [they didn’t] want to waste” an N95 unless they knew the patient was positive.
Ms. Holland said she quit in mid-April. Her nursing colleagues lodged a complaint with the Occupational Safety and Health Administration in late June alleging that staff “working around possible COVID-19 positive cases” had been denied PPE. Staff members protested outside the hospital in July and filed another OSHA complaint that said the hospital was allowing COVID-exposed employees to keep working.
Ms. Kirkland, the Blake spokesperson, said the hospital responded to OSHA and “no deficiencies were identified.”
The Medicare analysis shows that 22 of 273 patients with COVID, or 8%, were diagnosed with the virus after they were admitted to Blake. That’s about five times as high as the national average.
Ms. Kirkland said “there is no standard way for measuring COVID-19 hospital-associated transmissions” and “there is no evidence to suggest the risk of transmission at Blake Medical Center is different than what you would find at other hospitals.”
In Washington, D.C., 34 Medicare COVID patients contracted the virus at MedStar Washington Hospital Center, or nearly 6% of its total, the analysis shows.
Unhappy with the safety practices — which included gas sterilization and reuse of N95s — National Nurses United members protested on the hospital lawn in July 2020. At the protest, nurse Zoe Bendixen said one nurse had died of the virus and 50 had gotten sick: “[Nurses] can become a source for spreading the disease to other patients, co-workers and family members.”
Nurse Yuhana Gidey said she caught COVID after treating a patient who turned out to be infected. Another nurse, not managers doing contact tracing, told her she’d been exposed, she said.
Nurse Kimberly Walsh said in an interview there was an outbreak in a geriatric unit where she worked in September 2020. She said management blamed nurses for bringing the virus into the unit. But Ms. Walsh pointed to another problem: The hospital wasn’t COVID-testing patients coming in from nursing homes, where spread was rampant last year.
MedStar declined a request for an interview about its infection control practices and did not respond to specific questions.
While hospitals must track and publicly report rates of persistent infections like C. diff, antibiotic-resistant staph and surgical site infections, similar hospital-acquired COVID rates are not reported.
KHN examined a different source of data that Congress required hospitals to document about “hospital-acquired conditions.” The Medicare data, which notes whether each COVID case was “present on admission” or not, becomes available months after a hospitalization in obscure files that require a data-use agreement typically granted to researchers. KHN counted cases, as federal officials do, in some instances in which the documentation is deemed insufficient to categorize a case (see data methodology on the KHN website).
For this data, whether to deem a COVID case hospital-acquired lies with medical coders who review doctors’ notes and discharge summaries and ask doctors questions if the status is unclear, said Sue Bowman, senior director of coding policy and compliance at American Health Information Management Association.
She said medical coders are aware that the data is used for hospital quality measures and would be careful to review the contract tracing or other information in the medical record.
If a case was in the data KHN used, “that would mean it was acquired during the hospital stay either from a health care worker or another patient or maybe if a hospital allowed visitors, from a visitor,” Ms. Bowman said. “That would be a fair interpretation of the data.”
The high death rate for those diagnosed with COVID during a hospital stay — about 21% — mirrors the death rate for other Medicare COVID patients last year, when doctors had few proven methods to help patients. It also highlights the hazard unvaccinated staffers pose to patients, said Dr. Jain, the infectious-disease doctor. The American Hospital Association estimates that about 42% of U.S. hospitals have mandated that all staff members be vaccinated.
“We don’t need [unvaccinated staff] to be a threat to patients,” Dr. Jain said. “[Hospital] administration is too afraid to push the nursing staff, and the general public is clueless at what a threat a non-vaccinated person poses to a vulnerable population.”
Cindy Johnson said the hospital where she believes her husband contracted COVID faced minimal scrutiny in a state inspection, even after she said she reported that he caught COVID there. She explored suing, but an attorney told her it would be nearly impossible to win such a case. A 2021 state law requires proof of “at least gross negligence” to prevail in court.
Ms. Johnson did ask a doctor who sees patients at the hospital for this: Please take down the big “OPEN & SAFE” sign outside.
Within days, the sign was gone.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
They went into hospitals with heart attacks, kidney failure or in a psychiatric crisis.
They left with COVID-19 — if they left at all.
More than 10,000 patients were diagnosed with COVID in a U.S. hospital last year after they were admitted for something else, according to federal and state records analyzed exclusively for KHN. The number is certainly an undercount, since it includes mostly patients 65 and older, plus California and Florida patients of all ages.
Yet in the scheme of things that can go wrong in a hospital, it is catastrophic: About 21% of the patients who contracted COVID in the hospital from April to September last year died, the data shows. In contrast, nearly 8% of other Medicare patients died in the hospital at the time.
Steven Johnson, 66, was expecting to get an infection cut out of his hip flesh and bone at Blake Medical Center in Bradenton, Fla., last November. The retired pharmacist had survived colon cancer and was meticulous to avoid contracting COVID. He could not have known that, from April through September, 8% of that hospital’s Medicare COVID patients were diagnosed with the virus after they were admitted for another concern.
Mr. Johnson had tested negative for COVID two days before he was admitted. After 13 days in the hospital, he tested positive, said his wife, Cindy Johnson, also a retired pharmacist.
Soon he was struggling to clear a glue-like phlegm from his lungs. A medical team could hardly control his pain. They prompted Cindy to share his final wishes. She asked: “Honey, do you want to be intubated?” He responded with an emphatic “no.” He died three days later.
After her husband tested positive, Cindy Johnson, trained in contact tracing, quickly got a COVID test. She tested negative. Then she thought about the large number of hospital staffers flowing into and out of his room — where he was often unmasked — and suspected a staff member had infected him. That the hospital, part of the HCA Healthcare chain, still has not mandated staff vaccinations is “appalling,” she said.
“I’m furious,” she said.
“How can they say on their website,” she asked, “that the safety precautions ‘we’ve put into place make our facilities among the safest possible places to receive healthcare at this time’?”
Blake Medical Center spokesperson Lisa Kirkland said the hospital is “strongly encouraging vaccination” and noted that it follows Centers for Disease Control and Prevention and federal and state guidelines to protect patients. President Joe Biden has called for all hospital employees to be vaccinated, but the requirement could face resistance in a dozen states, including Florida, that have banned vaccine mandates.
Overall, the rate of in-hospital spread among Medicare and other patients was lower than in other countries, including the United Kingdom, which makes such data public and openly discusses it. On average, about 1.7% of U.S. hospitalized COVID patients were diagnosed with the virus in U.S. hospitals, according to an analysis of Medicare records from April 1 to Sept. 30, 2020, provided by Dr. James Kennedy, founder of CDIMD, a Nashville-based consulting and data analytics company.
Yet the rate of infection was far higher in 38 hospitals where 5% or more of the Medicare COVID cases were documented as hospital-acquired. The data is from a challenging stretch last year when protective gear was in short supply and tests were scarce or slow to produce results. The Medicare data for the fourth quarter of 2020 and this year isn’t available yet, and the state data reflects April 1 through Dec. 31, 2020.
A KHN review of work-safety records, medical literature and interviews with staff at high-spread hospitals points to why the virus took hold: Hospital leaders were slow to appreciate its airborne nature, which made coughing patients hazardous to roommates and staff members, who often wore less-protective surgical masks instead of N95s. Hospitals failed to test every admitted patient, enabled by CDC guidance that leaves such testing to the “discretion of the facility.” Management often failed to inform workers when they’d been exposed to COVID and so were at risk of spreading it themselves.
Spread among patients and staffers seemed to go hand in hand. At Beaumont Hospital, Taylor, in Michigan, 139 employee COVID infections were logged between April 6 to Oct. 20 last year, a hospital inspection report shows. Nearly 7% of the Medicare patients with COVID tested positive after they were admitted to that hospital for something else, the federal data shows. A hospital spokesperson said tests were not available to screen all patients last year, resulting in some late diagnoses. He said all incoming patients are tested now.
Tracking COVID inside health facilities is no new task to federal officials, who publicly report new staff and resident cases weekly for each U.S. nursing home. Yet the Department of Health and Human Services reports data on COVID’s spread in hospitals only on a statewide basis, so patients are in the dark about which facilities have cases.
KHN commissioned analyses of hospital billing records, which are also used more broadly to spot various hospital-acquired infections. For COVID, the data has limitations. It can pick up some community-acquired cases that were slow to show up, as it can take two to 14 days from exposure to the virus for symptoms to appear, with the average being four to five days. The records do not account for cases picked up in an emergency room or diagnosed after a hospital patient was discharged.
Linda Moore, 71, tested positive at least 15 days into a hospital stay for spinal surgery, according to her daughter Trisha Tavolazzi. Her mother was at Havasu Regional Medical Center in Lake Havasu City, Ariz., which did not have a higher-than-average rate of internal spread last summer.
The hospital implemented “rigorous health and safety protocols to protect all of our patients” during the pandemic, said hospital spokesperson Corey Santoriello, who would not comment on Ms. Moore’s case, citing privacy laws.
Ms. Moore was airlifted to another hospital, where her condition only declined further, her daughter said. After the ventilator was removed, she clung to life fitfully for 5½ hours, as her daughter prayed for her mother to find her way to heaven.
“I asked her mom and her dad and her family and prayed to God, ‘Please just come show her the way,’” Ms. Tavolazzi said. “I relive it every day.”
When Ms. Tavolazzi sought answers from the hospital about where her mom got the virus, she said, she got none: “No one ever called me back.”
Two negative COVID tests, then ‘patient zero’
As the second surge of COVID subsided last September, doctors from the prestigious Brigham and Women’s Hospital published a reassuringstudy: With careful infection control, only two of 697 COVID patients acquired the virus within the Boston hospital. That is about 0.3% of patients --about six times lower than the overall Medicare rate. Brigham tested every patient it admitted, exceeding CDC recommendations. It was transparent and open about safety concerns.
But the study, published in the high-profile JAMA Network Open journal, conveyed the wrong message, according to Dr. Manoj Jain, an infectious-disease physician and adjunct professor at the Rollins School of Public Health at Emory University. COVID was spreading in hospitals, he said, and the study buried “the problem under the rug.”
Before the virtual ink on the study was dry, the virus began a stealthy streak through the elite hospital. It slipped in with a patient who tested negative twice -- but turned out to be positive. She was “patient zero” in an outbreak affecting 38 staffers and 14 patients, according to a study in Annals of Internal Medicine initially published Feb. 9.
That study’s authors sequenced the genome of the virus to confirm which cases were related and precisely how it traveled through the hospital.
As patients were moved from room to room in the early days of the outbreak, COVID spread among roommates 8 out of 9 times, likely through aerosol transmission, the study says. A survey of staff members revealed that those caring for coughing patients were more likely to get sick.
The virus also appeared to have breached the CDC-OK’d protective gear. Two staff members who had close patient contact while wearing a surgical mask and face shield still wound up infected. The findings suggested that more-protective N95 respirators could help safeguard staff.
Brigham and Women’s now tests every patient upon admission and again soon after. Nurses are encouraged to test again if they see a subtle sign of COVID, said Dr. Erica Shenoy, associate chief of the Infection Control Unit at Massachusetts General Hospital, who helped craft policy at Brigham.
She said nurses and environmental services workers are at the table for policymaking: “I personally make it a point to say, ‘Tell me what you’re thinking,’” Dr. Shenoy said. “‘There’s no retribution because we need to know.’”
CDC guidelines, though, left wide latitude on protective gear and testing. To this day, Dr. Shenoy said, hospitals employ a wide range of policies.
The CDC said in a statement that its guidelines “provide a comprehensive and layered approach to preventing transmission of SARS-CoV-2 in healthcare settings,” and include testing patients with “even mild symptoms” or recent exposure to someone with COVID.
Infection control policies are rarely apparent to patients or visitors, beyond whether they’re asked to wear a mask. But reviews of public records and interviews with more than a dozen people show that at hospitals with high rates of COVID spread, staff members were often alarmed by the lack of safety practices.
Nurses sound the alarm on COVID spread
As COVID crept into Florida in spring 2020, nurse Victoria Holland clashed with managers at Blake Medical Center in Bradenton, where Steven Johnson died.
She said managers suspended her early in the pandemic after taking part in a protest and “having a hissy fit” when she was denied a new N95 respirator before an “aerosol-generating” procedure. The CDC warns that such procedures can spread the virus through the air. Before the pandemic, nurses were trained to dispose of an N95 after each patient encounter.
When the suspension was over, Ms. Holland said, she felt unsafe. “They told us nothing,” she said. “It was all a little whisper between the doctors. You had potential COVIDs and you’d get a little surgical mask because [they didn’t] want to waste” an N95 unless they knew the patient was positive.
Ms. Holland said she quit in mid-April. Her nursing colleagues lodged a complaint with the Occupational Safety and Health Administration in late June alleging that staff “working around possible COVID-19 positive cases” had been denied PPE. Staff members protested outside the hospital in July and filed another OSHA complaint that said the hospital was allowing COVID-exposed employees to keep working.
Ms. Kirkland, the Blake spokesperson, said the hospital responded to OSHA and “no deficiencies were identified.”
The Medicare analysis shows that 22 of 273 patients with COVID, or 8%, were diagnosed with the virus after they were admitted to Blake. That’s about five times as high as the national average.
Ms. Kirkland said “there is no standard way for measuring COVID-19 hospital-associated transmissions” and “there is no evidence to suggest the risk of transmission at Blake Medical Center is different than what you would find at other hospitals.”
In Washington, D.C., 34 Medicare COVID patients contracted the virus at MedStar Washington Hospital Center, or nearly 6% of its total, the analysis shows.
Unhappy with the safety practices — which included gas sterilization and reuse of N95s — National Nurses United members protested on the hospital lawn in July 2020. At the protest, nurse Zoe Bendixen said one nurse had died of the virus and 50 had gotten sick: “[Nurses] can become a source for spreading the disease to other patients, co-workers and family members.”
Nurse Yuhana Gidey said she caught COVID after treating a patient who turned out to be infected. Another nurse, not managers doing contact tracing, told her she’d been exposed, she said.
Nurse Kimberly Walsh said in an interview there was an outbreak in a geriatric unit where she worked in September 2020. She said management blamed nurses for bringing the virus into the unit. But Ms. Walsh pointed to another problem: The hospital wasn’t COVID-testing patients coming in from nursing homes, where spread was rampant last year.
MedStar declined a request for an interview about its infection control practices and did not respond to specific questions.
While hospitals must track and publicly report rates of persistent infections like C. diff, antibiotic-resistant staph and surgical site infections, similar hospital-acquired COVID rates are not reported.
KHN examined a different source of data that Congress required hospitals to document about “hospital-acquired conditions.” The Medicare data, which notes whether each COVID case was “present on admission” or not, becomes available months after a hospitalization in obscure files that require a data-use agreement typically granted to researchers. KHN counted cases, as federal officials do, in some instances in which the documentation is deemed insufficient to categorize a case (see data methodology on the KHN website).
For this data, whether to deem a COVID case hospital-acquired lies with medical coders who review doctors’ notes and discharge summaries and ask doctors questions if the status is unclear, said Sue Bowman, senior director of coding policy and compliance at American Health Information Management Association.
She said medical coders are aware that the data is used for hospital quality measures and would be careful to review the contract tracing or other information in the medical record.
If a case was in the data KHN used, “that would mean it was acquired during the hospital stay either from a health care worker or another patient or maybe if a hospital allowed visitors, from a visitor,” Ms. Bowman said. “That would be a fair interpretation of the data.”
The high death rate for those diagnosed with COVID during a hospital stay — about 21% — mirrors the death rate for other Medicare COVID patients last year, when doctors had few proven methods to help patients. It also highlights the hazard unvaccinated staffers pose to patients, said Dr. Jain, the infectious-disease doctor. The American Hospital Association estimates that about 42% of U.S. hospitals have mandated that all staff members be vaccinated.
“We don’t need [unvaccinated staff] to be a threat to patients,” Dr. Jain said. “[Hospital] administration is too afraid to push the nursing staff, and the general public is clueless at what a threat a non-vaccinated person poses to a vulnerable population.”
Cindy Johnson said the hospital where she believes her husband contracted COVID faced minimal scrutiny in a state inspection, even after she said she reported that he caught COVID there. She explored suing, but an attorney told her it would be nearly impossible to win such a case. A 2021 state law requires proof of “at least gross negligence” to prevail in court.
Ms. Johnson did ask a doctor who sees patients at the hospital for this: Please take down the big “OPEN & SAFE” sign outside.
Within days, the sign was gone.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
They went into hospitals with heart attacks, kidney failure or in a psychiatric crisis.
They left with COVID-19 — if they left at all.
More than 10,000 patients were diagnosed with COVID in a U.S. hospital last year after they were admitted for something else, according to federal and state records analyzed exclusively for KHN. The number is certainly an undercount, since it includes mostly patients 65 and older, plus California and Florida patients of all ages.
Yet in the scheme of things that can go wrong in a hospital, it is catastrophic: About 21% of the patients who contracted COVID in the hospital from April to September last year died, the data shows. In contrast, nearly 8% of other Medicare patients died in the hospital at the time.
Steven Johnson, 66, was expecting to get an infection cut out of his hip flesh and bone at Blake Medical Center in Bradenton, Fla., last November. The retired pharmacist had survived colon cancer and was meticulous to avoid contracting COVID. He could not have known that, from April through September, 8% of that hospital’s Medicare COVID patients were diagnosed with the virus after they were admitted for another concern.
Mr. Johnson had tested negative for COVID two days before he was admitted. After 13 days in the hospital, he tested positive, said his wife, Cindy Johnson, also a retired pharmacist.
Soon he was struggling to clear a glue-like phlegm from his lungs. A medical team could hardly control his pain. They prompted Cindy to share his final wishes. She asked: “Honey, do you want to be intubated?” He responded with an emphatic “no.” He died three days later.
After her husband tested positive, Cindy Johnson, trained in contact tracing, quickly got a COVID test. She tested negative. Then she thought about the large number of hospital staffers flowing into and out of his room — where he was often unmasked — and suspected a staff member had infected him. That the hospital, part of the HCA Healthcare chain, still has not mandated staff vaccinations is “appalling,” she said.
“I’m furious,” she said.
“How can they say on their website,” she asked, “that the safety precautions ‘we’ve put into place make our facilities among the safest possible places to receive healthcare at this time’?”
Blake Medical Center spokesperson Lisa Kirkland said the hospital is “strongly encouraging vaccination” and noted that it follows Centers for Disease Control and Prevention and federal and state guidelines to protect patients. President Joe Biden has called for all hospital employees to be vaccinated, but the requirement could face resistance in a dozen states, including Florida, that have banned vaccine mandates.
Overall, the rate of in-hospital spread among Medicare and other patients was lower than in other countries, including the United Kingdom, which makes such data public and openly discusses it. On average, about 1.7% of U.S. hospitalized COVID patients were diagnosed with the virus in U.S. hospitals, according to an analysis of Medicare records from April 1 to Sept. 30, 2020, provided by Dr. James Kennedy, founder of CDIMD, a Nashville-based consulting and data analytics company.
Yet the rate of infection was far higher in 38 hospitals where 5% or more of the Medicare COVID cases were documented as hospital-acquired. The data is from a challenging stretch last year when protective gear was in short supply and tests were scarce or slow to produce results. The Medicare data for the fourth quarter of 2020 and this year isn’t available yet, and the state data reflects April 1 through Dec. 31, 2020.
A KHN review of work-safety records, medical literature and interviews with staff at high-spread hospitals points to why the virus took hold: Hospital leaders were slow to appreciate its airborne nature, which made coughing patients hazardous to roommates and staff members, who often wore less-protective surgical masks instead of N95s. Hospitals failed to test every admitted patient, enabled by CDC guidance that leaves such testing to the “discretion of the facility.” Management often failed to inform workers when they’d been exposed to COVID and so were at risk of spreading it themselves.
Spread among patients and staffers seemed to go hand in hand. At Beaumont Hospital, Taylor, in Michigan, 139 employee COVID infections were logged between April 6 to Oct. 20 last year, a hospital inspection report shows. Nearly 7% of the Medicare patients with COVID tested positive after they were admitted to that hospital for something else, the federal data shows. A hospital spokesperson said tests were not available to screen all patients last year, resulting in some late diagnoses. He said all incoming patients are tested now.
Tracking COVID inside health facilities is no new task to federal officials, who publicly report new staff and resident cases weekly for each U.S. nursing home. Yet the Department of Health and Human Services reports data on COVID’s spread in hospitals only on a statewide basis, so patients are in the dark about which facilities have cases.
KHN commissioned analyses of hospital billing records, which are also used more broadly to spot various hospital-acquired infections. For COVID, the data has limitations. It can pick up some community-acquired cases that were slow to show up, as it can take two to 14 days from exposure to the virus for symptoms to appear, with the average being four to five days. The records do not account for cases picked up in an emergency room or diagnosed after a hospital patient was discharged.
Linda Moore, 71, tested positive at least 15 days into a hospital stay for spinal surgery, according to her daughter Trisha Tavolazzi. Her mother was at Havasu Regional Medical Center in Lake Havasu City, Ariz., which did not have a higher-than-average rate of internal spread last summer.
The hospital implemented “rigorous health and safety protocols to protect all of our patients” during the pandemic, said hospital spokesperson Corey Santoriello, who would not comment on Ms. Moore’s case, citing privacy laws.
Ms. Moore was airlifted to another hospital, where her condition only declined further, her daughter said. After the ventilator was removed, she clung to life fitfully for 5½ hours, as her daughter prayed for her mother to find her way to heaven.
“I asked her mom and her dad and her family and prayed to God, ‘Please just come show her the way,’” Ms. Tavolazzi said. “I relive it every day.”
When Ms. Tavolazzi sought answers from the hospital about where her mom got the virus, she said, she got none: “No one ever called me back.”
Two negative COVID tests, then ‘patient zero’
As the second surge of COVID subsided last September, doctors from the prestigious Brigham and Women’s Hospital published a reassuringstudy: With careful infection control, only two of 697 COVID patients acquired the virus within the Boston hospital. That is about 0.3% of patients --about six times lower than the overall Medicare rate. Brigham tested every patient it admitted, exceeding CDC recommendations. It was transparent and open about safety concerns.
But the study, published in the high-profile JAMA Network Open journal, conveyed the wrong message, according to Dr. Manoj Jain, an infectious-disease physician and adjunct professor at the Rollins School of Public Health at Emory University. COVID was spreading in hospitals, he said, and the study buried “the problem under the rug.”
Before the virtual ink on the study was dry, the virus began a stealthy streak through the elite hospital. It slipped in with a patient who tested negative twice -- but turned out to be positive. She was “patient zero” in an outbreak affecting 38 staffers and 14 patients, according to a study in Annals of Internal Medicine initially published Feb. 9.
That study’s authors sequenced the genome of the virus to confirm which cases were related and precisely how it traveled through the hospital.
As patients were moved from room to room in the early days of the outbreak, COVID spread among roommates 8 out of 9 times, likely through aerosol transmission, the study says. A survey of staff members revealed that those caring for coughing patients were more likely to get sick.
The virus also appeared to have breached the CDC-OK’d protective gear. Two staff members who had close patient contact while wearing a surgical mask and face shield still wound up infected. The findings suggested that more-protective N95 respirators could help safeguard staff.
Brigham and Women’s now tests every patient upon admission and again soon after. Nurses are encouraged to test again if they see a subtle sign of COVID, said Dr. Erica Shenoy, associate chief of the Infection Control Unit at Massachusetts General Hospital, who helped craft policy at Brigham.
She said nurses and environmental services workers are at the table for policymaking: “I personally make it a point to say, ‘Tell me what you’re thinking,’” Dr. Shenoy said. “‘There’s no retribution because we need to know.’”
CDC guidelines, though, left wide latitude on protective gear and testing. To this day, Dr. Shenoy said, hospitals employ a wide range of policies.
The CDC said in a statement that its guidelines “provide a comprehensive and layered approach to preventing transmission of SARS-CoV-2 in healthcare settings,” and include testing patients with “even mild symptoms” or recent exposure to someone with COVID.
Infection control policies are rarely apparent to patients or visitors, beyond whether they’re asked to wear a mask. But reviews of public records and interviews with more than a dozen people show that at hospitals with high rates of COVID spread, staff members were often alarmed by the lack of safety practices.
Nurses sound the alarm on COVID spread
As COVID crept into Florida in spring 2020, nurse Victoria Holland clashed with managers at Blake Medical Center in Bradenton, where Steven Johnson died.
She said managers suspended her early in the pandemic after taking part in a protest and “having a hissy fit” when she was denied a new N95 respirator before an “aerosol-generating” procedure. The CDC warns that such procedures can spread the virus through the air. Before the pandemic, nurses were trained to dispose of an N95 after each patient encounter.
When the suspension was over, Ms. Holland said, she felt unsafe. “They told us nothing,” she said. “It was all a little whisper between the doctors. You had potential COVIDs and you’d get a little surgical mask because [they didn’t] want to waste” an N95 unless they knew the patient was positive.
Ms. Holland said she quit in mid-April. Her nursing colleagues lodged a complaint with the Occupational Safety and Health Administration in late June alleging that staff “working around possible COVID-19 positive cases” had been denied PPE. Staff members protested outside the hospital in July and filed another OSHA complaint that said the hospital was allowing COVID-exposed employees to keep working.
Ms. Kirkland, the Blake spokesperson, said the hospital responded to OSHA and “no deficiencies were identified.”
The Medicare analysis shows that 22 of 273 patients with COVID, or 8%, were diagnosed with the virus after they were admitted to Blake. That’s about five times as high as the national average.
Ms. Kirkland said “there is no standard way for measuring COVID-19 hospital-associated transmissions” and “there is no evidence to suggest the risk of transmission at Blake Medical Center is different than what you would find at other hospitals.”
In Washington, D.C., 34 Medicare COVID patients contracted the virus at MedStar Washington Hospital Center, or nearly 6% of its total, the analysis shows.
Unhappy with the safety practices — which included gas sterilization and reuse of N95s — National Nurses United members protested on the hospital lawn in July 2020. At the protest, nurse Zoe Bendixen said one nurse had died of the virus and 50 had gotten sick: “[Nurses] can become a source for spreading the disease to other patients, co-workers and family members.”
Nurse Yuhana Gidey said she caught COVID after treating a patient who turned out to be infected. Another nurse, not managers doing contact tracing, told her she’d been exposed, she said.
Nurse Kimberly Walsh said in an interview there was an outbreak in a geriatric unit where she worked in September 2020. She said management blamed nurses for bringing the virus into the unit. But Ms. Walsh pointed to another problem: The hospital wasn’t COVID-testing patients coming in from nursing homes, where spread was rampant last year.
MedStar declined a request for an interview about its infection control practices and did not respond to specific questions.
While hospitals must track and publicly report rates of persistent infections like C. diff, antibiotic-resistant staph and surgical site infections, similar hospital-acquired COVID rates are not reported.
KHN examined a different source of data that Congress required hospitals to document about “hospital-acquired conditions.” The Medicare data, which notes whether each COVID case was “present on admission” or not, becomes available months after a hospitalization in obscure files that require a data-use agreement typically granted to researchers. KHN counted cases, as federal officials do, in some instances in which the documentation is deemed insufficient to categorize a case (see data methodology on the KHN website).
For this data, whether to deem a COVID case hospital-acquired lies with medical coders who review doctors’ notes and discharge summaries and ask doctors questions if the status is unclear, said Sue Bowman, senior director of coding policy and compliance at American Health Information Management Association.
She said medical coders are aware that the data is used for hospital quality measures and would be careful to review the contract tracing or other information in the medical record.
If a case was in the data KHN used, “that would mean it was acquired during the hospital stay either from a health care worker or another patient or maybe if a hospital allowed visitors, from a visitor,” Ms. Bowman said. “That would be a fair interpretation of the data.”
The high death rate for those diagnosed with COVID during a hospital stay — about 21% — mirrors the death rate for other Medicare COVID patients last year, when doctors had few proven methods to help patients. It also highlights the hazard unvaccinated staffers pose to patients, said Dr. Jain, the infectious-disease doctor. The American Hospital Association estimates that about 42% of U.S. hospitals have mandated that all staff members be vaccinated.
“We don’t need [unvaccinated staff] to be a threat to patients,” Dr. Jain said. “[Hospital] administration is too afraid to push the nursing staff, and the general public is clueless at what a threat a non-vaccinated person poses to a vulnerable population.”
Cindy Johnson said the hospital where she believes her husband contracted COVID faced minimal scrutiny in a state inspection, even after she said she reported that he caught COVID there. She explored suing, but an attorney told her it would be nearly impossible to win such a case. A 2021 state law requires proof of “at least gross negligence” to prevail in court.
Ms. Johnson did ask a doctor who sees patients at the hospital for this: Please take down the big “OPEN & SAFE” sign outside.
Within days, the sign was gone.
KHN (Kaiser Health News) is a national newsroom that produces in-depth journalism about health issues. Together with Policy Analysis and Polling, KHN is one of the three major operating programs at KFF (Kaiser Family Foundation). KFF is an endowed nonprofit organization providing information on health issues to the nation.
COVID-19 has brought more complex, longer office visits
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
Evidence of this came from the latest Primary Care Collaborative (PCC) survey, which found that primary care clinicians are seeing more complex patients requiring longer appointments in the wake of COVID-19.
The PCC with the Larry A. Green Center regularly surveys primary care clinicians. This round of questions came August 14-17 and included 1,263 respondents from 49 states, the District of Columbia, and two territories.
More than 7 in 10 (71%) respondents said their patients are more complex and nearly the same percentage said appointments are taking more time.
Ann Greiner, president and CEO of the PCC, said in an interview that 55% of respondents reported that clinicians are struggling to keep up with pent-up demand after patients have delayed or canceled care. Sixty-five percent in the survey said they had seen a rise in children’s mental health issues, and 58% said they were unsure how to help their patients with long COVID.
In addition, primary care clinicians are having repeated conversations with patients on why they should get a vaccine and which one.
“I think that’s adding to the complexity. There is a lot going on here with patient trust,” Ms. Greiner said.
‘We’re going to be playing catch-up’
Jacqueline Fincher, MD, an internist in Thompson, Ga., said in an interview that appointments have gotten longer and more complex in the wake of the pandemic – “no question.”
The immediate past president of the American College of Physicians is seeing patients with chronic disease that has gone untreated for sometimes a year or more, she said.
“Their blood pressure was not under good control, they were under more stress, their sugars were up and weren’t being followed as closely for conditions such as congestive heart failure,” she said.
Dr. Fincher, who works in a rural practice 40 miles from Augusta, Ga., with her physician husband and two other physicians, said patients are ready to come back in, “but I don’t have enough slots for them.”
She said she prioritizes what to help patients with first and schedules the next tier for the next appointment, but added, “honestly, over the next 2 years we’re going to be playing catch-up.”
At the same time, the CDC has estimated that 45% of U.S. adults are at increased risk for complications from COVID-19 because of cardiovascular disease, diabetes, respiratory disease, hypertension, or cancer. Rates ranged from 19.8% for people 18-29 years old to 80.7% for people over 80 years of age.
Long COVID could overwhelm existing health care capacity
Primary care physicians are also having to diagnose sometimes “invisible” symptoms after people have recovered from acute COVID-19 infection. Diagnosing takes intent listening to patients who describe symptoms that tests can’t confirm.
As this news organization has previously reported, half of COVID-19 survivors report postacute sequelae of COVID-19 (PASC) lasting longer than 6 months.
“These long-term PASC effects occur on a scale that could overwhelm existing health care capacity, particularly in low- and middle-income countries,” the authors wrote.
Anxiety, depression ‘have gone off the charts’
Danielle Loeb, MD, MPH, associate professor of internal medicine at the University of Colorado in Denver, who studies complexity in primary care, said in the wake of COVID-19, more patients have developed “new, serious anxiety.”
“That got extremely exacerbated during the pandemic. Anxiety and depression have gone off the charts,” said Dr. Loeb, who prefers the pronoun “they.”
Dr. Loeb cares for a large number of transgender patients. As offices reopen, some patients are having trouble reintegrating into the workplace and resuming social contacts. The primary care doctor says appointments can get longer because of the need to complete tasks, such as filling out forms for Family Medical Leave Act for those not yet ready to return to work.
COVID-19–related fears are keeping many patients from coming into the office, Dr. Loeb said, either from fear of exposure or because they have mental health issues that keep them from feeling safe leaving the house.
“That really affects my ability to care for them,” they said.
Loss of employment in the pandemic or fear of job loss and subsequent changing of insurance has complicated primary care in terms of treatment and administrative tasks, according to Dr. Loeb.
To help treat patients with acute mental health issues and manage other patients, Dr. Loeb’s practice has brought in a social worker and a therapist.
Team-based care is key in the survival of primary care practices, though providing that is difficult in the smaller clinics because of the critical mass of patients needed to make it viable, they said.
“It’s the only answer. It’s the only way you don’t drown,” Dr. Loeb added. “I’m not drowning, and I credit that to my clinic having the help to support the mental health piece of things.”
Rethinking workflow
Tricia McGinnis, MPP, MPH, executive vice president of the nonprofit Center for Health Care Strategies (CHCS) says complexity has forced rethinking workflow.
“A lot of the trends we’re seeing in primary care were there pre-COVID, but COVID has exacerbated those trends,” she said in an interview.
“The good news ... is that it was already becoming clear that primary care needed to provide basic mental health services and integrate with behavioral health. It had also become clear that effective primary care needed to address social issues that keep patients from accessing health care,” she said.
Expanding care teams, as Dr. Loeb mentioned, is a key strategy, according to Ms. McGinnis. Potential teams would include the clinical staff, but also social workers and community health workers – people who come from the community primary care is serving who can help build trust with patients and connect the patient to the primary care team.
“There’s a lot that needs to happen that the clinician doesn’t need to do,” she said.
Telehealth can be a big factor in coordinating the team, Ms. McGinnis added.
“It’s thinking less about who’s doing the work, but more about the work that needs to be done to keep people healthy. Then let’s think about the type of workers best suited to perform those tasks,” she said.
As for reimbursing more complex care, population-based, up-front capitated payments linked to high-quality care and better outcomes will need to replace fee-for-service models, according to Ms. McGinnis.
That will provide reliable incomes for primary care offices, but also flexibility in how each patient with different levels of complexity is managed, she said.
Ms. Greiner, Dr. Fincher, Dr. Loeb, and Ms. McGinnis have no relevant financial relationships.
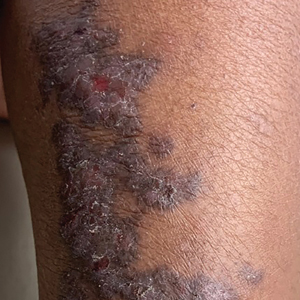
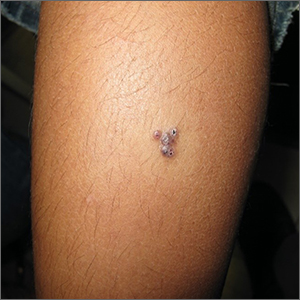
Linear Violaceous Papules in a Child
The Diagnosis: Linear Lichen Planus
The patient was clinically diagnosed with linear lichen planus and was started on betamethasone dipropionate ointment 0.05% applied once daily with improvement in both the pruritus and appearance at 4-month follow-up. A biopsy was deferred based on the parents’ wishes.
Lichen planus is an inflammatory disorder involving the skin and oral mucosa. Cutaneous lichen planus classically presents as flat-topped, violaceous, pruritic, polygonal papules with overlying fine white or grey lines known as Wickham striae.1 Postinflammatory hyperpigmentation is common, especially in patients with darker skin tones. Expected histologic findings include orthokeratosis, apoptotic keratinocytes, and bandlike lymphocytic infiltration at the dermoepidermal junction.1
An estimated 5% of cases of cutaneous lichen planus occur in children.2 A study of 316 children with lichen planus demonstrated that the classic morphology remained the most common presentation, while the linear variant was present in only 6.9% of pediatric cases.3 Linear lichen planus appears to be more common among children than adults. A study of 36 pediatric cases showed a greater representation of lichen planus in Black children (67% affected vs 21% cohort).2
Cutaneous lichen planus often clears spontaneously in approximately 1 year.4 Treatment in children primarily is focused on shortening the time to resolution and relieving pruritus, with topical corticosteroids as firstline therapy.3,4 Oral corticosteroids have a faster clinical response; greater efficacy; and more effectively prevent residual hyperpigmentation, which is especially relevant in individuals with darker skin.3 Nonetheless, oral corticosteroids are considered a second-line treatment due to their unfavorable side-effect profile. Additional treatment options include oral aromatic retinoids (acitretin) and phototherapy.3
Incontinentia pigmenti is characterized by a defect in the inhibitor of nuclear factor–κB kinase regulatory subunit gamma, IKBKG, gene on the X chromosome. Incontinentia pigmenti usually is lethal in males; in females, it leads to ectodermal dysplasia associated with skin findings in a blaschkoid distribution occurring in 4 stages.5 The verrucous stage is preceded by the vesicular stage and expected to occur within the first few months of life, making it unlikely in our 5-year-old patient. Inflammatory linear verrucous epidermal nevus usually occurs in children younger than 5 years and is characterized by psoriasiform papules coalescing into a plaque with substantial scale instead of Wickham striae, as seen in our patient.6 Lichen striatus consists of smaller, pink to flesh-colored papules that rarely are pruritic.7 It is more common among atopic individuals and is associated with postinflammatory hypopigmentation.8 Linear psoriasis presents similarly to inflammatory linear verrucous epidermal nevus, with greater erythema and scale compared to the fine lacy Wickham striae that were seen in our patient.8
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Walton KE, Bowers EV, Drolet BA, et al. Childhood lichen planus: demographics of a U.S. population. Pediatr Dermatol. 2010;27:34-38.
- Pandhi D, Singal A, Bhattacharya SN. Lichen planus in childhood: a series of 316 patients. Pediatr Dermatol. 2014;31:59-67.
- Le Cleach L, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;366:723-732.
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31:E45-E52.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
- Payette MJ, Weston G, Humphrey S, et al. Lichen planus and other lichenoid dermatoses: kids are not just little people. Clin Dermatol. 2015;33:631-643.
- Moss C, Browne F. Mosaicism and linear lesions. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
The Diagnosis: Linear Lichen Planus
The patient was clinically diagnosed with linear lichen planus and was started on betamethasone dipropionate ointment 0.05% applied once daily with improvement in both the pruritus and appearance at 4-month follow-up. A biopsy was deferred based on the parents’ wishes.
Lichen planus is an inflammatory disorder involving the skin and oral mucosa. Cutaneous lichen planus classically presents as flat-topped, violaceous, pruritic, polygonal papules with overlying fine white or grey lines known as Wickham striae.1 Postinflammatory hyperpigmentation is common, especially in patients with darker skin tones. Expected histologic findings include orthokeratosis, apoptotic keratinocytes, and bandlike lymphocytic infiltration at the dermoepidermal junction.1
An estimated 5% of cases of cutaneous lichen planus occur in children.2 A study of 316 children with lichen planus demonstrated that the classic morphology remained the most common presentation, while the linear variant was present in only 6.9% of pediatric cases.3 Linear lichen planus appears to be more common among children than adults. A study of 36 pediatric cases showed a greater representation of lichen planus in Black children (67% affected vs 21% cohort).2
Cutaneous lichen planus often clears spontaneously in approximately 1 year.4 Treatment in children primarily is focused on shortening the time to resolution and relieving pruritus, with topical corticosteroids as firstline therapy.3,4 Oral corticosteroids have a faster clinical response; greater efficacy; and more effectively prevent residual hyperpigmentation, which is especially relevant in individuals with darker skin.3 Nonetheless, oral corticosteroids are considered a second-line treatment due to their unfavorable side-effect profile. Additional treatment options include oral aromatic retinoids (acitretin) and phototherapy.3
Incontinentia pigmenti is characterized by a defect in the inhibitor of nuclear factor–κB kinase regulatory subunit gamma, IKBKG, gene on the X chromosome. Incontinentia pigmenti usually is lethal in males; in females, it leads to ectodermal dysplasia associated with skin findings in a blaschkoid distribution occurring in 4 stages.5 The verrucous stage is preceded by the vesicular stage and expected to occur within the first few months of life, making it unlikely in our 5-year-old patient. Inflammatory linear verrucous epidermal nevus usually occurs in children younger than 5 years and is characterized by psoriasiform papules coalescing into a plaque with substantial scale instead of Wickham striae, as seen in our patient.6 Lichen striatus consists of smaller, pink to flesh-colored papules that rarely are pruritic.7 It is more common among atopic individuals and is associated with postinflammatory hypopigmentation.8 Linear psoriasis presents similarly to inflammatory linear verrucous epidermal nevus, with greater erythema and scale compared to the fine lacy Wickham striae that were seen in our patient.8
The Diagnosis: Linear Lichen Planus
The patient was clinically diagnosed with linear lichen planus and was started on betamethasone dipropionate ointment 0.05% applied once daily with improvement in both the pruritus and appearance at 4-month follow-up. A biopsy was deferred based on the parents’ wishes.
Lichen planus is an inflammatory disorder involving the skin and oral mucosa. Cutaneous lichen planus classically presents as flat-topped, violaceous, pruritic, polygonal papules with overlying fine white or grey lines known as Wickham striae.1 Postinflammatory hyperpigmentation is common, especially in patients with darker skin tones. Expected histologic findings include orthokeratosis, apoptotic keratinocytes, and bandlike lymphocytic infiltration at the dermoepidermal junction.1
An estimated 5% of cases of cutaneous lichen planus occur in children.2 A study of 316 children with lichen planus demonstrated that the classic morphology remained the most common presentation, while the linear variant was present in only 6.9% of pediatric cases.3 Linear lichen planus appears to be more common among children than adults. A study of 36 pediatric cases showed a greater representation of lichen planus in Black children (67% affected vs 21% cohort).2
Cutaneous lichen planus often clears spontaneously in approximately 1 year.4 Treatment in children primarily is focused on shortening the time to resolution and relieving pruritus, with topical corticosteroids as firstline therapy.3,4 Oral corticosteroids have a faster clinical response; greater efficacy; and more effectively prevent residual hyperpigmentation, which is especially relevant in individuals with darker skin.3 Nonetheless, oral corticosteroids are considered a second-line treatment due to their unfavorable side-effect profile. Additional treatment options include oral aromatic retinoids (acitretin) and phototherapy.3
Incontinentia pigmenti is characterized by a defect in the inhibitor of nuclear factor–κB kinase regulatory subunit gamma, IKBKG, gene on the X chromosome. Incontinentia pigmenti usually is lethal in males; in females, it leads to ectodermal dysplasia associated with skin findings in a blaschkoid distribution occurring in 4 stages.5 The verrucous stage is preceded by the vesicular stage and expected to occur within the first few months of life, making it unlikely in our 5-year-old patient. Inflammatory linear verrucous epidermal nevus usually occurs in children younger than 5 years and is characterized by psoriasiform papules coalescing into a plaque with substantial scale instead of Wickham striae, as seen in our patient.6 Lichen striatus consists of smaller, pink to flesh-colored papules that rarely are pruritic.7 It is more common among atopic individuals and is associated with postinflammatory hypopigmentation.8 Linear psoriasis presents similarly to inflammatory linear verrucous epidermal nevus, with greater erythema and scale compared to the fine lacy Wickham striae that were seen in our patient.8
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Walton KE, Bowers EV, Drolet BA, et al. Childhood lichen planus: demographics of a U.S. population. Pediatr Dermatol. 2010;27:34-38.
- Pandhi D, Singal A, Bhattacharya SN. Lichen planus in childhood: a series of 316 patients. Pediatr Dermatol. 2014;31:59-67.
- Le Cleach L, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;366:723-732.
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31:E45-E52.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
- Payette MJ, Weston G, Humphrey S, et al. Lichen planus and other lichenoid dermatoses: kids are not just little people. Clin Dermatol. 2015;33:631-643.
- Moss C, Browne F. Mosaicism and linear lesions. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Walton KE, Bowers EV, Drolet BA, et al. Childhood lichen planus: demographics of a U.S. population. Pediatr Dermatol. 2010;27:34-38.
- Pandhi D, Singal A, Bhattacharya SN. Lichen planus in childhood: a series of 316 patients. Pediatr Dermatol. 2014;31:59-67.
- Le Cleach L, Chosidow O. Clinical practice. lichen planus. N Engl J Med. 2012;366:723-732.
- Greene-Roethke C. Incontinentia pigmenti: a summary review of this rare ectodermal dysplasia with neurologic manifestations, including treatment protocols. J Pediatr Health Care. 2017;31:E45-E52.
- Requena L, Requena C, Cockerell CJ. Benign epidermal tumors and proliferations. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
- Payette MJ, Weston G, Humphrey S, et al. Lichen planus and other lichenoid dermatoses: kids are not just little people. Clin Dermatol. 2015;33:631-643.
- Moss C, Browne F. Mosaicism and linear lesions. In: Bolognia JL, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017:1894-1916.
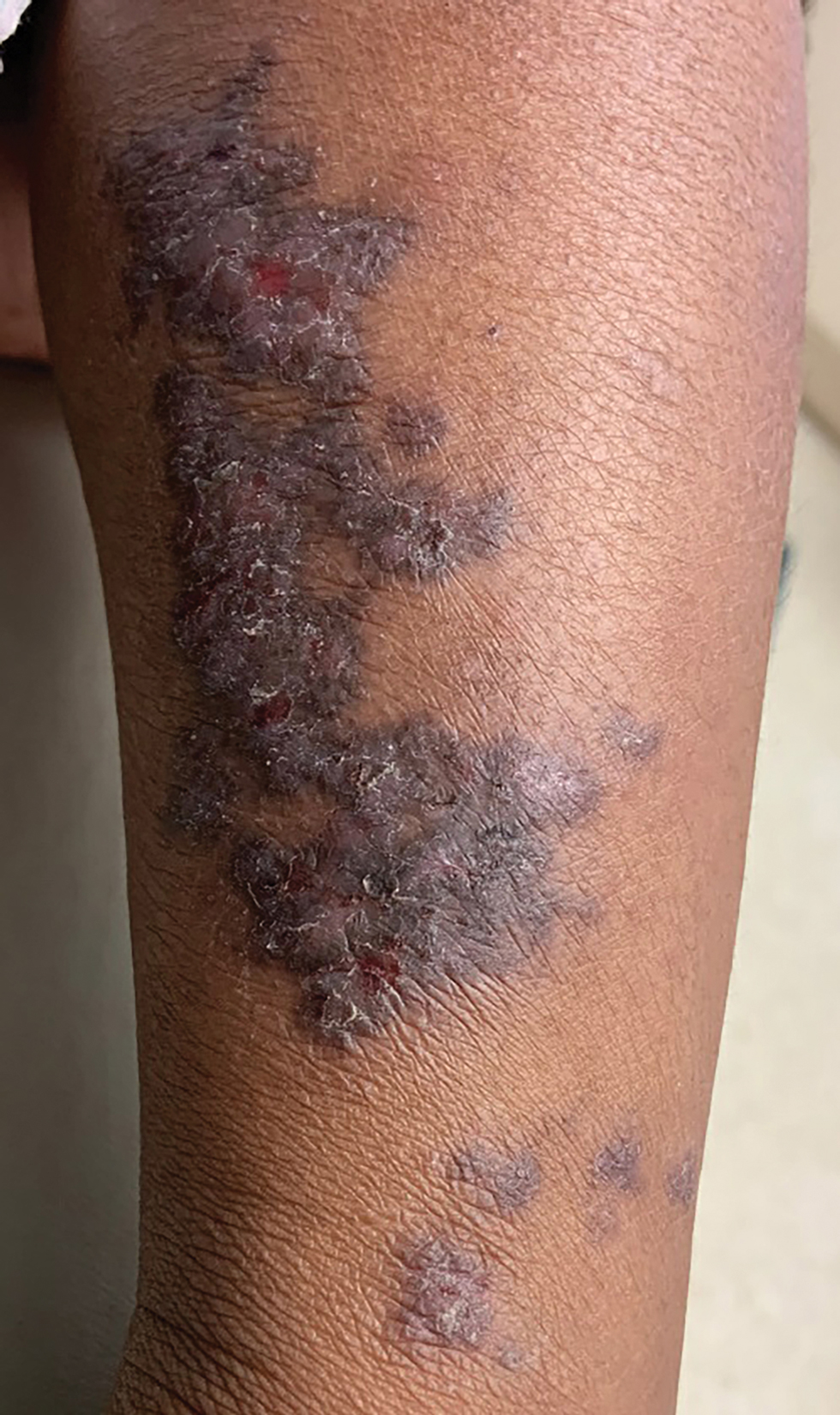
A 5-year-old Black girl presented to the dermatology clinic with a stable pruritic eruption on the right leg of 1 month’s duration. Over-the-counter hydrocortisone cream was applied for 3 days with no response. Physical examination revealed grouped, flat-topped, violaceous papules coalescing into plaques with overlying lacy white striae along the right lower leg, wrapping around to the right dorsal foot in a blaschkoid distribution. The patient was otherwise healthy and up-to-date on immunizations and had an unremarkable birth history.
‘Residents’ Viewpoint’ revisited
We are currently republishing an installment of this column as part of our continuing celebration of Family Practice News’s 50th anniversary.
Bruce A. Bagley, MD, wrote the first batch of these columns, when he was chief resident in family medicine at St. Joseph’s Hospital, Syracuse, N.Y. Joseph E. Scherger, MD, was the second writer for Family Practice News’s monthly “Residents’ Viewpoint.” At the time Dr. Scherger became a columnist, he was a 26-year-old, 2nd-year family practice resident at the Family Medical Center, University Hospital, University of Washington, Seattle.
Dr. Scherger’s first column was published on Feb. 5, 1977. We are republishing his “Residents’ Viewpoint” from June 15, 1977 (see below) and a new column by Victoria Persampiere, DO, who is currently a 2nd-year resident in the family medicine program at Abington Jefferson Health. (See “My experience as a family medicine resident in 2021” after Dr. Scherger’s column.).
We hope you will enjoy comparing and contrasting the experiences of a resident practicing family medicine today to those of a resident practicing family medicine nearly 4½ decades ago.To learn about Dr. Scherger’s current practice and long career, you can read his profile on the cover of the September 2021 issue of Family Practice News or on MDedge.com/FamilyMedicine in our “Family Practice News 50th Anniversary” section.
Art of medicine or deception?
Originally published in Family Practice News on June 15, 1977.
In medical school I learned the science of medicine. There I diligently studied the basic sciences and gained a thorough understanding of the pathophysiology of disease. In the clinical years I learned to apply this knowledge to a wide variety of interesting patients who came to the academic center.
Yet, when I started my family practice residency, I lacked the ability to care for patients. Though I could take a thorough history, perform a complete physical examination, and diagnose and treat specific illnesses, I had little idea how to satisfy patients by meeting their needs.
The art of medicine is the nonscientific part of a successful doctor-patient interaction. For a doctor-patient interaction to be successful, not only must the illness be appropriately addressed, but both patient and physician must be satisfied.
In the university environment, the art of medicine often gets inadequate attention. Indeed, most academic physicians think that only scientific medicine exists and that patients should be satisfied with a sophisticated approach to their problems. Some patients are satisfied, but many are disgruntled. It is not unusual for a patient, after a $1,000 work-up, to go to a family physician or chiropractor for satisfaction.
I was eager to discover the art of medicine at its finest during my rotation away from the university in a rural community. During these 2 months I looked for the pearls of wisdom that allowed community physicians to be so successful. I found that a very explicit technique was used by some physicians to achieve not only satisfaction but adoration from their patients. Unfortunately, this technique is dishonest.
Early in my community experience I was impressed by how often patients told me a doctor had saved them. I heard such statements as “Dr. X saved my leg,” or “Dr. X saved my life.” I know that it does occur, but not as often as I was hearing it.
Investigating these statements I found such stories as, “One day l twisted my ankle very badly, and it became quite swollen. My doctor told me 1 could lose my leg from this but that he would take x-rays, put my leg in an Ace bandage, and give me crutches. In 3 days I was well. I am so thankful he saved my leg.”
And, “One day I had a temperature of 104. All of my muscles ached, my head hurt, and I had a terrible sore throat and cough. My doctor told me l could die from this, but he gave me a medicine and made me stay home. I was sick for about 2 weeks, but I got better. He saved my life.”
Is the art of medicine the art of deception? This horrifying thought actually came to me after hearing several such stories, but I learned that most of the physicians involved in such stories were not well respected by their colleagues.
I learned many honest techniques for successfully caring for patients. The several family physicians with whom I worked, all clinical instructors associated with my residency, were impeccably honest and taught me to combine compassion and efficiency.
Despite learning many positive techniques and having good role models, I left the community experience somewhat saddened by the lack of integrity that can exist in the profession. I was naive in believing that all the nonscientific aspects of medicine that made patients happy must be good.
By experiencing deception, I learned why quackery continues to flourish despite the widespread availability of honest medical care. Most significantly, I learned the importance of a sometimes frustrating humility; my patients with sprained ankles and influenza will not believe I saved their lives.
My experience as a family medicine resident in 2021
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19 era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome, which was strengthened by every “there is nothing else we can offer your loved one at this time” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today; you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician. ■
Dr. Persampiere is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
We are currently republishing an installment of this column as part of our continuing celebration of Family Practice News’s 50th anniversary.
Bruce A. Bagley, MD, wrote the first batch of these columns, when he was chief resident in family medicine at St. Joseph’s Hospital, Syracuse, N.Y. Joseph E. Scherger, MD, was the second writer for Family Practice News’s monthly “Residents’ Viewpoint.” At the time Dr. Scherger became a columnist, he was a 26-year-old, 2nd-year family practice resident at the Family Medical Center, University Hospital, University of Washington, Seattle.
Dr. Scherger’s first column was published on Feb. 5, 1977. We are republishing his “Residents’ Viewpoint” from June 15, 1977 (see below) and a new column by Victoria Persampiere, DO, who is currently a 2nd-year resident in the family medicine program at Abington Jefferson Health. (See “My experience as a family medicine resident in 2021” after Dr. Scherger’s column.).
We hope you will enjoy comparing and contrasting the experiences of a resident practicing family medicine today to those of a resident practicing family medicine nearly 4½ decades ago.To learn about Dr. Scherger’s current practice and long career, you can read his profile on the cover of the September 2021 issue of Family Practice News or on MDedge.com/FamilyMedicine in our “Family Practice News 50th Anniversary” section.
Art of medicine or deception?
Originally published in Family Practice News on June 15, 1977.
In medical school I learned the science of medicine. There I diligently studied the basic sciences and gained a thorough understanding of the pathophysiology of disease. In the clinical years I learned to apply this knowledge to a wide variety of interesting patients who came to the academic center.
Yet, when I started my family practice residency, I lacked the ability to care for patients. Though I could take a thorough history, perform a complete physical examination, and diagnose and treat specific illnesses, I had little idea how to satisfy patients by meeting their needs.
The art of medicine is the nonscientific part of a successful doctor-patient interaction. For a doctor-patient interaction to be successful, not only must the illness be appropriately addressed, but both patient and physician must be satisfied.
In the university environment, the art of medicine often gets inadequate attention. Indeed, most academic physicians think that only scientific medicine exists and that patients should be satisfied with a sophisticated approach to their problems. Some patients are satisfied, but many are disgruntled. It is not unusual for a patient, after a $1,000 work-up, to go to a family physician or chiropractor for satisfaction.
I was eager to discover the art of medicine at its finest during my rotation away from the university in a rural community. During these 2 months I looked for the pearls of wisdom that allowed community physicians to be so successful. I found that a very explicit technique was used by some physicians to achieve not only satisfaction but adoration from their patients. Unfortunately, this technique is dishonest.
Early in my community experience I was impressed by how often patients told me a doctor had saved them. I heard such statements as “Dr. X saved my leg,” or “Dr. X saved my life.” I know that it does occur, but not as often as I was hearing it.
Investigating these statements I found such stories as, “One day l twisted my ankle very badly, and it became quite swollen. My doctor told me 1 could lose my leg from this but that he would take x-rays, put my leg in an Ace bandage, and give me crutches. In 3 days I was well. I am so thankful he saved my leg.”
And, “One day I had a temperature of 104. All of my muscles ached, my head hurt, and I had a terrible sore throat and cough. My doctor told me l could die from this, but he gave me a medicine and made me stay home. I was sick for about 2 weeks, but I got better. He saved my life.”
Is the art of medicine the art of deception? This horrifying thought actually came to me after hearing several such stories, but I learned that most of the physicians involved in such stories were not well respected by their colleagues.
I learned many honest techniques for successfully caring for patients. The several family physicians with whom I worked, all clinical instructors associated with my residency, were impeccably honest and taught me to combine compassion and efficiency.
Despite learning many positive techniques and having good role models, I left the community experience somewhat saddened by the lack of integrity that can exist in the profession. I was naive in believing that all the nonscientific aspects of medicine that made patients happy must be good.
By experiencing deception, I learned why quackery continues to flourish despite the widespread availability of honest medical care. Most significantly, I learned the importance of a sometimes frustrating humility; my patients with sprained ankles and influenza will not believe I saved their lives.
My experience as a family medicine resident in 2021
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19 era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome, which was strengthened by every “there is nothing else we can offer your loved one at this time” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today; you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician. ■
Dr. Persampiere is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
We are currently republishing an installment of this column as part of our continuing celebration of Family Practice News’s 50th anniversary.
Bruce A. Bagley, MD, wrote the first batch of these columns, when he was chief resident in family medicine at St. Joseph’s Hospital, Syracuse, N.Y. Joseph E. Scherger, MD, was the second writer for Family Practice News’s monthly “Residents’ Viewpoint.” At the time Dr. Scherger became a columnist, he was a 26-year-old, 2nd-year family practice resident at the Family Medical Center, University Hospital, University of Washington, Seattle.
Dr. Scherger’s first column was published on Feb. 5, 1977. We are republishing his “Residents’ Viewpoint” from June 15, 1977 (see below) and a new column by Victoria Persampiere, DO, who is currently a 2nd-year resident in the family medicine program at Abington Jefferson Health. (See “My experience as a family medicine resident in 2021” after Dr. Scherger’s column.).
We hope you will enjoy comparing and contrasting the experiences of a resident practicing family medicine today to those of a resident practicing family medicine nearly 4½ decades ago.To learn about Dr. Scherger’s current practice and long career, you can read his profile on the cover of the September 2021 issue of Family Practice News or on MDedge.com/FamilyMedicine in our “Family Practice News 50th Anniversary” section.
Art of medicine or deception?
Originally published in Family Practice News on June 15, 1977.
In medical school I learned the science of medicine. There I diligently studied the basic sciences and gained a thorough understanding of the pathophysiology of disease. In the clinical years I learned to apply this knowledge to a wide variety of interesting patients who came to the academic center.
Yet, when I started my family practice residency, I lacked the ability to care for patients. Though I could take a thorough history, perform a complete physical examination, and diagnose and treat specific illnesses, I had little idea how to satisfy patients by meeting their needs.
The art of medicine is the nonscientific part of a successful doctor-patient interaction. For a doctor-patient interaction to be successful, not only must the illness be appropriately addressed, but both patient and physician must be satisfied.
In the university environment, the art of medicine often gets inadequate attention. Indeed, most academic physicians think that only scientific medicine exists and that patients should be satisfied with a sophisticated approach to their problems. Some patients are satisfied, but many are disgruntled. It is not unusual for a patient, after a $1,000 work-up, to go to a family physician or chiropractor for satisfaction.
I was eager to discover the art of medicine at its finest during my rotation away from the university in a rural community. During these 2 months I looked for the pearls of wisdom that allowed community physicians to be so successful. I found that a very explicit technique was used by some physicians to achieve not only satisfaction but adoration from their patients. Unfortunately, this technique is dishonest.
Early in my community experience I was impressed by how often patients told me a doctor had saved them. I heard such statements as “Dr. X saved my leg,” or “Dr. X saved my life.” I know that it does occur, but not as often as I was hearing it.
Investigating these statements I found such stories as, “One day l twisted my ankle very badly, and it became quite swollen. My doctor told me 1 could lose my leg from this but that he would take x-rays, put my leg in an Ace bandage, and give me crutches. In 3 days I was well. I am so thankful he saved my leg.”
And, “One day I had a temperature of 104. All of my muscles ached, my head hurt, and I had a terrible sore throat and cough. My doctor told me l could die from this, but he gave me a medicine and made me stay home. I was sick for about 2 weeks, but I got better. He saved my life.”
Is the art of medicine the art of deception? This horrifying thought actually came to me after hearing several such stories, but I learned that most of the physicians involved in such stories were not well respected by their colleagues.
I learned many honest techniques for successfully caring for patients. The several family physicians with whom I worked, all clinical instructors associated with my residency, were impeccably honest and taught me to combine compassion and efficiency.
Despite learning many positive techniques and having good role models, I left the community experience somewhat saddened by the lack of integrity that can exist in the profession. I was naive in believing that all the nonscientific aspects of medicine that made patients happy must be good.
By experiencing deception, I learned why quackery continues to flourish despite the widespread availability of honest medical care. Most significantly, I learned the importance of a sometimes frustrating humility; my patients with sprained ankles and influenza will not believe I saved their lives.
My experience as a family medicine resident in 2021
I did not get a medical school graduation; I was one of the many thousands of newly graduated students who simply left their 4th-year rotation sites one chilly day in March 2020 and just never went back. My medical school education didn’t end with me walking triumphantly across the stage – a first-generation college student finally achieving the greatest dream in her life. Instead, it ended with a Zoom “graduation” and a cross-country move from Georgia to Pennsylvania amidst the greatest pandemic in recent memory. To say my impostor syndrome was bad would be an understatement.
Residency in the COVID-19 era
The joy and the draw to family medicine for me has always been the broad scope of conditions that we see and treat. From day 1, however, much of my residency has been devoted to one very small subset of patients – those with COVID-19. At one point, our hospital was so strained that our family medicine program had to run a second inpatient service alongside our usual five-resident service team just to provide care to everybody. Patients were in the hallways. The ER was packed to the gills. We were sleepless, terrified, unvaccinated, and desperate to help our patients survive a disease that was incompletely understood, with very few tools in our toolbox to combat it.
I distinctly remember sitting in the workroom with a coresident of mine, our faces seemingly permanently lined from wearing N95s all shift, and saying to him, “I worry I will be a bad family medicine physician. I worry I haven’t seen enough, other than COVID.” It was midway through my intern year; the days were short, so I was driving to and from the hospital in chilly darkness. My patients, like many around the country, were doing poorly. Vaccines seemed like a promise too good to be true. Worst of all: Those of us who were interns, who had no triumphant podium moment to end our medical school education, were suffering with an intense sense of impostor syndrome, which was strengthened by every “there is nothing else we can offer your loved one at this time” conversation we had. My apprehension about not having seen a wider breadth of medicine during my training is a sentiment still widely shared by COVID-era residents.
Luckily, my coresident was supportive.
“We’re going to be great family medicine physicians,” he said. “We’re learning the hard stuff – the bread and butter of FM – up-front. You’ll see.”
In some ways, I think he was right. Clinical skills, empathy, humility, and forging strong relationships are at the center of every family medicine physician’s heart; my generation has had to learn these skills early and under pressure. Sometimes, there are no answers. Sometimes, the best thing a family doctor can do for a patient is to hear them, understand them, and hold their hand.
‘We watched Cinderella together’
Shortly after that conversation with my coresident, I had a particular case which moved me. This gentleman with intellectual disability and COVID had been declining steadily since his admission to the hospital. He was isolated from everybody he knew and loved, but it did not dampen his spirits. He was cheerful to every person who entered his room, clad in their shrouds of PPE, which more often than not felt more like mourning garb than protective wear. I remember very little about this patient’s clinical picture – the COVID, the superimposed pneumonia, the repeated intubations. What I do remember is he loved the Disney classic Cinderella. I knew this because I developed a very close relationship with his family during the course of his hospitalization. Amidst the torrential onslaught of patients, I made sure to call families every day – not because I wanted to, but because my mentors and attendings and coresidents had all drilled into me from day 1 that we are family medicine, and a large part of our role is to advocate for our patients, and to communicate with their loved ones. So I called. I learned a lot about him; his likes, his dislikes, his close bond with his siblings, and of course his lifelong love for Cinderella. On the last week of my ICU rotation, my patient passed peacefully. His nurse and I were bedside. We held his hand. We told him his family loved him. We watched Cinderella together on an iPad encased in protective plastic.
My next rotation was an outpatient one and it looked more like the “bread and butter” of family medicine. But as I whisked in and out of patient rooms, attending to patients with diabetes, with depression, with pain, I could not stop thinking about my hospitalized patients who my coresidents had assumed care of. Each exam room I entered, I rather morbidly thought “this patient could be next on our hospital service.” Without realizing it, I made more of an effort to get to know each patient holistically. I learned who they were as people. I found myself writing small, medically low-yield details in the chart: “Margaret loves to sing in her church choir;” “Katherine is a self-published author.”
I learned from my attendings. As I sat at the precepting table with them, observing their conversations about patients, their collective decades of experience were apparent.
“I’ve been seeing this patient every few weeks since I was a resident,” said one of my attendings.
“I don’t even see my parents that often,” I thought.
The depth of her relationship with, understanding of, and compassion for this patient struck me deeply. This was why I went into family medicine. My attending knew her patients; they were not faceless unknowns in a hospital gown to her. She would have known to play Cinderella for them in the end.
This is a unique time for trainees. We have been challenged, terrified, overwhelmed, and heartbroken. But at no point have we been isolated. We’ve had the generations of doctors before us to lead the way, to teach us the “hard stuff.” We’ve had senior residents to lean on, who have taken us aside and told us, “I can do the goals-of-care talk today; you need a break.” While the plague seems to have passed over our hospital for now, it has left behind a class of family medicine residents who are proud to carry on our specialty’s long tradition of compassionate, empathetic, lifelong care. “We care for all life stages, from cradle to grave,” says every family medicine physician.
My class, for better or for worse, has cared more often for patients in the twilight of their lives, and while it has been hard, I believe it has made us all better doctors. Now, when I hold a newborn in my arms for a well-child check, I am exceptionally grateful – for the opportunities I have been given, for new beginnings amidst so much sadness, and for the great privilege of being a family medicine physician. ■
Dr. Persampiere is a second-year resident in the family medicine residency program at Abington (Pa.) Jefferson Health. You can contact her directly at [email protected] or via [email protected].
Success of HPV vaccination: ‘Dramatic’ reduction in cervical cancer
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
Among young women who received the HPV vaccine when they were 12-13 years old (before their sexual debut), cervical cancer rates are 87% lower than among previous nonvaccinated generations.
“It’s been incredible to see the impact of HPV vaccination, and now we can prove it prevented hundreds of women from developing cancer in England,” senior author Peter Sasieni, MD, King’s College London, said in a statement. “To see the real-life impact of the vaccine has been truly rewarding.”
“This study provides the first direct evidence of the impact of the UK HPV vaccination campaign on cervical cancer incidence, showing a large reduction in cervical cancer rates in vaccinated cohorts,” Kate Soldan, MD, U.K. Health Security Agency, London, commented in a statement.
Vanessa Saliba, MD, a consultant epidemiologist for the U.K. Health Security Agency, agreed, saying that “these remarkable findings confirm that the HPV vaccine saves lives by dramatically reducing cervical cancer rates among women.
“This reminds us that vaccines are one of the most important tools we have to help us live longer, healthier lives,” she added.
The study was published online Nov. 3, 2021, in The Lancet.
Approached for comment on the new study, Maurice Markman, MD, president, Medicine and Science Cancer Treatment Centers of America, noted that the results of the English study are very similar to those of a Swedish study of the quadrivalent vaccine alone.
“You can put any superlatives you want in here, but these are stunningly positive results,” Dr. Markman said in an interview. He said that, as an oncologist who has been treating cervical cancer for 40 years, particularly patients with advanced cervical cancer, “I can tell you this is one of the most devastating diseases to women, and the ability to eliminate this cancer with something as simple as a vaccine is the goal of cancer therapy, and it’s been remarkably successful.
“I can only emphasize the critical importance of all parents to see that their children who are eligible for the vaccine receive it. This is a cancer prevention strategy that is unbelievably, remarkably effective and safe,” Dr. Markman added.
National vaccination program
The national HPV vaccination program in England began in 2008. Initially, the bivalent Cervarix vaccine against HPV 16 and 18 was used. HPV 16 and 18 are responsible for 70% to 80% of all cervical cancers in England, the researchers note in their article.
In 2012, the program switched to the quadrivalent HPV vaccine (Gardasil), which is effective against two additional HPV types, HPV 6 and 11. Those strains cause genital warts.
The prevention program originally recommended a three-dose regimen in which both HPV vaccines were used. Currently, two doses are given to girls younger than 15 years. In addition, a single dose of the HPV vaccine provides good protection against persistent infection. The efficacy rate of a single dose is similar to that of three doses, the authors comment.
Population-based registry
The new data come from a population-based cancer registry that shows the incidence of cervical cancer and noninvasive cervical carcinoma (CIN3) in England between January 2006 and June 2019.
The study included seven cohorts of women who were aged 20-64 years at the end of 2019. Three of these cohorts composed the vaccinated population.
The team reports that overall, from January 2006 to June 2019, there were 27,946 cases of cervical cancer and 318,058 cases of CIN3.
In the three vaccinated cohorts, there were around 450 fewer cases of cervical cancer and 17,200 fewer cases of CIN3 than would be expected in a nonvaccinated population.
The three vaccinated cohorts had been eligible to receive Cervarix when they were aged 12-13 years. A catch-up scheme aimed at 14- to 16-year-olds and 16- to 18-year-olds. Most of these persons were vaccinated through a school vaccination program.
The team analyzed the data for each of these cohorts.
Among the cohort eligible for vaccination at 12-13 years of age, 89% received at least one dose of the HPV vaccine; 85% received three shots and were fully vaccinated. Among these persons, the rate of cervical cancer was 87% lower than expected in a nonvaccinated population, and the rate of CIN3 was 97% lower than expected.
For the cohort that was eligible to be vaccinated between the ages of 14 and 16 years, the corresponding reductions were 62% for cervical cancer and 75% for CIN3.
For the cohort eligible for vaccination between the ages of 16 and 18 years (of whom 60% had received at least one dose and 45% were fully vaccinated), the corresponding reduction were 34% for cervical cancer and 39% for CIN3.
The authors acknowledge some limitations with the study, principally that cervical cancer is rare in young women, and these vaccinated populations are still young. The youngest would have been vaccinated at age 12 in 2008 and so would be only 23 years old in 2019, when the follow-up in this current study ended. The authors emphasize that because the vaccinated populations are still young, it is too early to assess the full impact of HPV vaccination on cervical cancer rates.
Editorial commentary
“The relative reductions in cervical cancer, expected as a result of the HPV vaccination program, support the anticipated vaccine effectiveness,” commented two authors of an accompanying editorial, Maggie Cruickshank, MD, University of Aberdeen (Scotland), and Mihaela Grigore, MD, University of Medicine and Pharmacy, Lasi, Romania.
“The scale of the HPV vaccination effect reported by this study should also stimulate vaccination programs in low-income and middle-income countries where the problem of cervical cancer is a far greater public health issue than in those with well established systems of vaccination and screening,” they comment.
“The most important issue, besides the availability of the vaccine ... is the education of the population to accept the vaccination because a high rate of immunization is a key element of success,” they emphasize. “Even in a wealthy country, such as England with free access to HPV immunization, uptake has not reached the 90% vaccination target of girls aged 15 years set by WHO [World Health Organization].”
The authors and editorialists disclosed no relevant financial relationships. Dr. Markman is a regular contributor to Medscape Oncology. He has received income of $250 or more from Genentech, AstraZeneca, Celgene, Clovis, and Amgen.
A version of this article first appeared on Medscape.com.
New single-button blood glucose monitor available in U.S.
The POGO Automatic Blood Glucose Monitoring System (Intuity Medical) has been cleared by the U.S. Food and Drug Administration for people with diabetes aged 13 years and older.
It contains a 10-test cartridge, and once loaded and the monitor is turned on, the user only has to press their finger on a button to activate POGO Automatic, which then does all the work of lancing and blood collection, followed by a 4-second countdown and a result. Users only need to carry the monitor and not separate lancets or strips.
An app called Patterns is available for iOS and Android that allows the results from the device to automatically sync via Bluetooth. It visually presents glucose trends and enables data sharing with health care providers.
“We know that people with diabetes are more effective at managing their diabetes when they regularly check their blood glucose and use the information to take action,” said Daniel Einhorn, MD, medical director of Scripps Whittier Diabetes Institute, president of Diabetes and Endocrine Associates, and chairperson of the Intuity Medical Scientific Advisory Board, in a company statement.
“My patients and millions of others with diabetes have struggled for decades with the burden of checking their glucose because it’s complicated, there’s a lot to carry around, and it’s intrusive,” he added. “What they’ve needed is a simple, quick, and truly discreet way to check their blood glucose, so they’ll actually do it.”
How does POGO compare with CGM?
Continuous glucose monitors (CGMs), such as the Abbott FreeStyle Libre, Dexcom G6, and Eversense implant, are increasingly employed by people with type 1 diabetes, and some with type 2 diabetes, to keep a close eye on their blood glucose levels.
Asked how the POGO device compares with CGM systems, Intuity Chief Commercial Officer Dean Zikria said: “While [CGM] is certainly an important option for a subset of people with diabetes, CGM is a very different technology, requiring a user to wear a sensor and transmitter on their body.”
“Patients also need to obtain a prescription in order to use CGM.”
“Conversely, POGO Automatic is available with or without a prescription. POGO Automatic also gives people who do not want to wear a device on their body a new choice other than traditional blood glucose monitoring,” Mr. Zikria added.
The POGO system is available at U.S. pharmacies, including CVS and Walgreens, and can also be purchased online.
The device costs $68 from the company website and a pack of 5 cartridges (each containing 10 tests, with an aim of people performing 1-2 tests per day) costs a further $32 as a one-off, or $32 per month as a subscription.
The product is also eligible for purchase using Flexible Spending Accounts and Health Savings Accounts.
A version of this article first appeared on Medscape.com.
The POGO Automatic Blood Glucose Monitoring System (Intuity Medical) has been cleared by the U.S. Food and Drug Administration for people with diabetes aged 13 years and older.
It contains a 10-test cartridge, and once loaded and the monitor is turned on, the user only has to press their finger on a button to activate POGO Automatic, which then does all the work of lancing and blood collection, followed by a 4-second countdown and a result. Users only need to carry the monitor and not separate lancets or strips.
An app called Patterns is available for iOS and Android that allows the results from the device to automatically sync via Bluetooth. It visually presents glucose trends and enables data sharing with health care providers.
“We know that people with diabetes are more effective at managing their diabetes when they regularly check their blood glucose and use the information to take action,” said Daniel Einhorn, MD, medical director of Scripps Whittier Diabetes Institute, president of Diabetes and Endocrine Associates, and chairperson of the Intuity Medical Scientific Advisory Board, in a company statement.
“My patients and millions of others with diabetes have struggled for decades with the burden of checking their glucose because it’s complicated, there’s a lot to carry around, and it’s intrusive,” he added. “What they’ve needed is a simple, quick, and truly discreet way to check their blood glucose, so they’ll actually do it.”
How does POGO compare with CGM?
Continuous glucose monitors (CGMs), such as the Abbott FreeStyle Libre, Dexcom G6, and Eversense implant, are increasingly employed by people with type 1 diabetes, and some with type 2 diabetes, to keep a close eye on their blood glucose levels.
Asked how the POGO device compares with CGM systems, Intuity Chief Commercial Officer Dean Zikria said: “While [CGM] is certainly an important option for a subset of people with diabetes, CGM is a very different technology, requiring a user to wear a sensor and transmitter on their body.”
“Patients also need to obtain a prescription in order to use CGM.”
“Conversely, POGO Automatic is available with or without a prescription. POGO Automatic also gives people who do not want to wear a device on their body a new choice other than traditional blood glucose monitoring,” Mr. Zikria added.
The POGO system is available at U.S. pharmacies, including CVS and Walgreens, and can also be purchased online.
The device costs $68 from the company website and a pack of 5 cartridges (each containing 10 tests, with an aim of people performing 1-2 tests per day) costs a further $32 as a one-off, or $32 per month as a subscription.
The product is also eligible for purchase using Flexible Spending Accounts and Health Savings Accounts.
A version of this article first appeared on Medscape.com.
The POGO Automatic Blood Glucose Monitoring System (Intuity Medical) has been cleared by the U.S. Food and Drug Administration for people with diabetes aged 13 years and older.
It contains a 10-test cartridge, and once loaded and the monitor is turned on, the user only has to press their finger on a button to activate POGO Automatic, which then does all the work of lancing and blood collection, followed by a 4-second countdown and a result. Users only need to carry the monitor and not separate lancets or strips.
An app called Patterns is available for iOS and Android that allows the results from the device to automatically sync via Bluetooth. It visually presents glucose trends and enables data sharing with health care providers.
“We know that people with diabetes are more effective at managing their diabetes when they regularly check their blood glucose and use the information to take action,” said Daniel Einhorn, MD, medical director of Scripps Whittier Diabetes Institute, president of Diabetes and Endocrine Associates, and chairperson of the Intuity Medical Scientific Advisory Board, in a company statement.
“My patients and millions of others with diabetes have struggled for decades with the burden of checking their glucose because it’s complicated, there’s a lot to carry around, and it’s intrusive,” he added. “What they’ve needed is a simple, quick, and truly discreet way to check their blood glucose, so they’ll actually do it.”
How does POGO compare with CGM?
Continuous glucose monitors (CGMs), such as the Abbott FreeStyle Libre, Dexcom G6, and Eversense implant, are increasingly employed by people with type 1 diabetes, and some with type 2 diabetes, to keep a close eye on their blood glucose levels.
Asked how the POGO device compares with CGM systems, Intuity Chief Commercial Officer Dean Zikria said: “While [CGM] is certainly an important option for a subset of people with diabetes, CGM is a very different technology, requiring a user to wear a sensor and transmitter on their body.”
“Patients also need to obtain a prescription in order to use CGM.”
“Conversely, POGO Automatic is available with or without a prescription. POGO Automatic also gives people who do not want to wear a device on their body a new choice other than traditional blood glucose monitoring,” Mr. Zikria added.
The POGO system is available at U.S. pharmacies, including CVS and Walgreens, and can also be purchased online.
The device costs $68 from the company website and a pack of 5 cartridges (each containing 10 tests, with an aim of people performing 1-2 tests per day) costs a further $32 as a one-off, or $32 per month as a subscription.
The product is also eligible for purchase using Flexible Spending Accounts and Health Savings Accounts.
A version of this article first appeared on Medscape.com.
Ivermectin–COVID-19 study retracted; authors blame file mix-up
The paper, “Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon,” appeared in the journal Viruses in May. According to the abstract: “A randomized controlled trial was conducted in 100 asymptomatic Lebanese subjects that have tested positive for SARS-CoV2. Fifty patients received standard preventive treatment, mainly supplements, and the experimental group received a single dose (according to body weight) of ivermectin, in addition to the same supplements the control group received.”
Results results results … and: “Ivermectin appears to be efficacious in providing clinical benefits in a randomized treatment of asymptomatic SARS-CoV-2-positive subjects, effectively resulting in fewer symptoms, lower viral load and reduced hospital admissions. However, larger-scale trials are warranted for this conclusion to be further cemented.”
However, in early October, the BBC reported — in a larger piece about the concerns about ivermectin-Covid-19 research — that the study “was found to have blocks of details of 11 patients that had been copied and pasted repeatedly – suggesting many of the trial’s apparent patients didn’t really exist.”
The study’s authors told the BBC that the ‘original set of data was rigged, sabotaged or mistakenly entered in the final file’ and that they have submitted a retraction to the scientific journal which published it.
That’s not quite what the retraction notice states: “The journal retracts the article, Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon [ 1 ], cited above. Following publication, the authors contacted the editorial office regarding an error between files used for the statistical analysis. Adhering to our complaints procedure, an investigation was conducted that confirmed the error reported by the authors.
This retraction was approved by the Editor in Chief of the journal. The authors agreed to this retraction.”
Ali Samaha, of Lebanese University in Beirut, and the lead author of the study, told us: “It was brought to our attention that we have used wrong file for our paper. We informed immediately the journal and we have run investigations. After revising the raw data we realised that a file that was used to train a research assistant was sent by mistake for analysis. Re-analysing the original data , the conclusions of the paper remained valid. For our transparency we asked for retraction.”
About that BBC report? Samaha said: “The BBC article was generated before the report of independent reviewers who confirmed an innocent mistake by using wrong file.”
Samaha added that he and his colleagues are now considering whether to resubmit the paper.
The article has been cited four times, according to Clarivate Analytics’ Web of Science — including in this meta-analysis published in June in the American Journal of Therapeutics , which concluded that: “Moderate-certainty evidence finds that large reductions in COVID-19 deaths are possible using ivermectin. Using ivermectin early in the clinical course may reduce numbers progressing to severe disease. The apparent safety and low cost suggest that ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally.”
That article was a social media darling, receiving more than 45,000 tweets and pickups in 90 news outlets, according to Altmetrics, which ranks it No. 7 among all papers published at that time.
A version of this article first appeared on Retraction Watch.
The paper, “Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon,” appeared in the journal Viruses in May. According to the abstract: “A randomized controlled trial was conducted in 100 asymptomatic Lebanese subjects that have tested positive for SARS-CoV2. Fifty patients received standard preventive treatment, mainly supplements, and the experimental group received a single dose (according to body weight) of ivermectin, in addition to the same supplements the control group received.”
Results results results … and: “Ivermectin appears to be efficacious in providing clinical benefits in a randomized treatment of asymptomatic SARS-CoV-2-positive subjects, effectively resulting in fewer symptoms, lower viral load and reduced hospital admissions. However, larger-scale trials are warranted for this conclusion to be further cemented.”
However, in early October, the BBC reported — in a larger piece about the concerns about ivermectin-Covid-19 research — that the study “was found to have blocks of details of 11 patients that had been copied and pasted repeatedly – suggesting many of the trial’s apparent patients didn’t really exist.”
The study’s authors told the BBC that the ‘original set of data was rigged, sabotaged or mistakenly entered in the final file’ and that they have submitted a retraction to the scientific journal which published it.
That’s not quite what the retraction notice states: “The journal retracts the article, Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon [ 1 ], cited above. Following publication, the authors contacted the editorial office regarding an error between files used for the statistical analysis. Adhering to our complaints procedure, an investigation was conducted that confirmed the error reported by the authors.
This retraction was approved by the Editor in Chief of the journal. The authors agreed to this retraction.”
Ali Samaha, of Lebanese University in Beirut, and the lead author of the study, told us: “It was brought to our attention that we have used wrong file for our paper. We informed immediately the journal and we have run investigations. After revising the raw data we realised that a file that was used to train a research assistant was sent by mistake for analysis. Re-analysing the original data , the conclusions of the paper remained valid. For our transparency we asked for retraction.”
About that BBC report? Samaha said: “The BBC article was generated before the report of independent reviewers who confirmed an innocent mistake by using wrong file.”
Samaha added that he and his colleagues are now considering whether to resubmit the paper.
The article has been cited four times, according to Clarivate Analytics’ Web of Science — including in this meta-analysis published in June in the American Journal of Therapeutics , which concluded that: “Moderate-certainty evidence finds that large reductions in COVID-19 deaths are possible using ivermectin. Using ivermectin early in the clinical course may reduce numbers progressing to severe disease. The apparent safety and low cost suggest that ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally.”
That article was a social media darling, receiving more than 45,000 tweets and pickups in 90 news outlets, according to Altmetrics, which ranks it No. 7 among all papers published at that time.
A version of this article first appeared on Retraction Watch.
The paper, “Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon,” appeared in the journal Viruses in May. According to the abstract: “A randomized controlled trial was conducted in 100 asymptomatic Lebanese subjects that have tested positive for SARS-CoV2. Fifty patients received standard preventive treatment, mainly supplements, and the experimental group received a single dose (according to body weight) of ivermectin, in addition to the same supplements the control group received.”
Results results results … and: “Ivermectin appears to be efficacious in providing clinical benefits in a randomized treatment of asymptomatic SARS-CoV-2-positive subjects, effectively resulting in fewer symptoms, lower viral load and reduced hospital admissions. However, larger-scale trials are warranted for this conclusion to be further cemented.”
However, in early October, the BBC reported — in a larger piece about the concerns about ivermectin-Covid-19 research — that the study “was found to have blocks of details of 11 patients that had been copied and pasted repeatedly – suggesting many of the trial’s apparent patients didn’t really exist.”
The study’s authors told the BBC that the ‘original set of data was rigged, sabotaged or mistakenly entered in the final file’ and that they have submitted a retraction to the scientific journal which published it.
That’s not quite what the retraction notice states: “The journal retracts the article, Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon [ 1 ], cited above. Following publication, the authors contacted the editorial office regarding an error between files used for the statistical analysis. Adhering to our complaints procedure, an investigation was conducted that confirmed the error reported by the authors.
This retraction was approved by the Editor in Chief of the journal. The authors agreed to this retraction.”
Ali Samaha, of Lebanese University in Beirut, and the lead author of the study, told us: “It was brought to our attention that we have used wrong file for our paper. We informed immediately the journal and we have run investigations. After revising the raw data we realised that a file that was used to train a research assistant was sent by mistake for analysis. Re-analysing the original data , the conclusions of the paper remained valid. For our transparency we asked for retraction.”
About that BBC report? Samaha said: “The BBC article was generated before the report of independent reviewers who confirmed an innocent mistake by using wrong file.”
Samaha added that he and his colleagues are now considering whether to resubmit the paper.
The article has been cited four times, according to Clarivate Analytics’ Web of Science — including in this meta-analysis published in June in the American Journal of Therapeutics , which concluded that: “Moderate-certainty evidence finds that large reductions in COVID-19 deaths are possible using ivermectin. Using ivermectin early in the clinical course may reduce numbers progressing to severe disease. The apparent safety and low cost suggest that ivermectin is likely to have a significant impact on the SARS-CoV-2 pandemic globally.”
That article was a social media darling, receiving more than 45,000 tweets and pickups in 90 news outlets, according to Altmetrics, which ranks it No. 7 among all papers published at that time.
A version of this article first appeared on Retraction Watch.
Feds launch COVID-19 worker vaccine mandates
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
The Biden administration on Nov. 4 unveiled its rule to require most of the country’s larger employers to mandate workers be fully vaccinated against COVID-19, but set a Jan. 4 deadline, avoiding the busy holiday season.
The White House also shifted the time lines for earlier mandates applying to federal workers and contractors to Jan. 4. And the same deadline applies to a new separate rule for health care workers.
The new rules are meant to preempt “any inconsistent state or local laws,” including bans and limits on employers’ authority to require vaccination, masks, or testing, the White House said in a statement.
The rule on employers from the Occupational Safety and Health Administration will apply to organizations with 100 or more employees. These employers will need to make sure each worker is fully vaccinated or tests for COVID-19 on at least a weekly basis. The OSHA rule will also require that employers provide paid time for employees to get vaccinated and ensure that all unvaccinated workers wear a face mask in the workplace. This rule will cover 84 million employees. The OSHA rule will not apply to workplaces covered by either the Centers for Medicare & Medicaid Services rule or the federal contractor vaccination requirement
“The virus will not go away by itself, or because we wish it away: We have to act,” President Joe Biden said in a statement. “Vaccination is the single best pathway out of this pandemic.”
Mandates were not the preferred route to managing the pandemic, he said.
“Too many people remain unvaccinated for us to get out of this pandemic for good,” he said. “So I instituted requirements – and they are working.”
The White House said 70% percent of U.S. adults are now fully vaccinated – up from less than 1% when Mr. Biden took office in January.
The CMS vaccine rule is meant to cover more than 17 million workers and about 76,000 medical care sites, including hospitals, ambulatory surgery centers, nursing homes, dialysis facilities, home health agencies, and long-term care facilities. The rule will apply to employees whether their positions involve patient care or not.
Unlike the OSHA mandate, the one for health care workers will not offer the option of frequent COVID-19 testing instead of vaccination. There is a “higher bar” for health care workers, given their role in treating patients, so the mandate allows only for vaccination or limited exemptions, a senior administration official said on Nov. 3 during a call with reporters.
The CMS rule includes a “range of remedies,” including penalties and denial of payment for health care facilities that fail to meet the vaccine mandate. CMS could theoretically cut off hospitals and other medical organizations for failure to comply, but that would be a “last resort,” a senior administration official said. CMS will instead work with health care facilities to help them comply with the federal rule on vaccination of medical workers.
The new CMS rules apply only to Medicare- and Medicaid-certified centers and organizations. The rule does not directly apply to other health care entities, such as doctor’s offices, that are not regulated by CMS.
“Most states have separate licensing requirements for health care staff and health care providers that would be applicable to physician office staff and other staff in small health care entities that are not subject to vaccination requirements under this IFC,” CMS said in the rule.
A version of this article first appeared on WebMD.com.
Renal denervation remains only promising, per latest meta-analysis
Questions remain despite efficacy
According to the latest meta-analysis of sham-controlled randomized trials, catheter-based renal sympathetic denervation produces clinically meaningful reductions in blood pressure with acceptable safety, but the strategy is not yet regarded as ready for prime time, according to a summary of the results to be presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
This meta-analysis was based on seven blinded trials, all of which associated denervation with a reduction in systolic ambulatory BP, according to Yousif Ahmad, BMBS, PhD, an interventional cardiologist at Yale University, New Haven, Conn.
Although the BP-lowering advantage in two of these studies did not reach statistical significance, the other five did, and all the data moved in the same direction.
For ambulatory diastolic pressure, the effect was more modest. One of the studies showed essentially a neutral effect. The reductions were statistically significant in only two, but, again, the data moved in the same direction in six of the studies, and a random-effects analysis suggested that the reductions, although modest, were potentially meaningful, according to Dr. Ahmad.
Overall, at a mean follow-up of 4.5 months, the reductions in ambulatory systolic and diastolic BPs were 3.61 and 1.85 mm Hg, respectively. The benefit was about the same whether renal denervation was or was not performed on the background of antihypertensive drugs, which was permitted in five of the seven trials. In the other two, all patients were off hypertensive medication.
Office-based systolic reduction: 6 mm Hg
When the same analysis was performed for office-based BP reductions, which were available for five of the seven trials, the overall reductions based on the meta-analysis were 5.86 and 3.63 mm Hg for the systolic and diastolic pressures, respectively. Again, background antihypertensive therapy was not a factor.
Of the seven trials, three randomized fewer than 100 patients. The largest, SYMPLICITY HTN-3, randomized 491 patients in 2:1 ratio to denervation or sham.
Three of the studies in the meta-analysis were trials of the Symplicity flex device. Another two evaluated the Symplicity Spyral catheter. Both deliver radiofrequency energy to for denervation. The Paradise device, the focus of the remaining two trials, employs energy in the form of ultrasound.
According to Dr. Ahmad, adverse events regardless of device were rare and not more common among those in the active treatment arm than in those treated with a sham procedure. Although one of these trials, RADIANCE-HTN SOLO associated denervation with efficacy and safety out to 12 months , Dr. Ahmad concluded that the mean follow-up of 4.5 months is not sufficient to consider long-term effects.
More than 20 meta-analyses published so far
By one count, there have been more than 20 meta-analyses of renal denervation published previously yet this intervention is still considered “controversial,” according to Dr. Ahmad. Relative to the previous meta-analyses, this included the RADIANCE-HTN TRIO trial, which is the latest such sham-controlled study and added 136 patients to the dataset of high-quality trials.
Basically, the results led Dr. Ahmad to conclude that, although the treatment effect is modest, it could be valuable in specific groups of patients, such as those reluctant or unable to take multiple medications or any medications at all. In addition to generating more data on efficacy and safety, he said longer follow-up is also needed for calculations of cost-effectiveness. Larger-scale observational studies might be one way of collecting these data, he reported.
The results of this study were published online in JACC Cardiovascular Interventions with an accompanying editorial by David E. Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta.
Commenting on the large pile of meta-analyses, sometimes published months apart, Dr. Kandzari explained that their “short half-life” is a product of the continuous updating of data with new trials. For a procedure that remains controversial, he said these constant relooks are inevitable.
“My point is that, with more studies, we can expect to see more meta-analyses. It is just the way this is going to work,” Dr. Kandzari said in an interview.
Individual study data also relevant
Even as the authors of these analyses attempt to cull the best data from the most rigorously performed trials, “we are also going to have to look at the individual studies, because of the differences in the trial designs, particularly the devices used,” according to Dr. Kandzari, who was the principle investigator of the sham-controlled SPYRAL HTN-ON MED trial.
So far, the data, despite some inconsistencies, have supported “clinically meaningful” BP reductions and acceptable safety regardless of the device used, according to Dr. Kandzari. Although he also agrees with the basic premise that more long-term data are needed to better determine how renal denervation should be applied in management of hypertension, he does think it will eventually find a role that is “complimentary to, rather than a replacement for, drugs.”
“The effect is modest, but keep in mind that the effect size is similar to that of a single oral medication, and there are some features, such as an always-on 24-hour effect that could be useful,” he said.
“We have enough of a signal to start thinking of how this will be enveloped into routine care,” he said.
But it is not ready yet. This was the point made by Dr. Ahmad, and it was seconded by Dr. Kandzari. One of the senior authors of the meta-analysis, Deepak Bhatt, MD, executive director of interventional cardiovascular programs, Brigham and Women’s Health, Boston, was also asked to weigh on when it will be ready for prime time.
“At a minimum, I would recommend completion of ongoing sham-controlled randomized trials before considering clinical use of renal denervation. Longer term safety and durability data, as well as data on cost-effectiveness, are all still needed – preferably from randomized trials as opposed to registries,” he said.
“Ideally, larger sham-controlled trials with longer follow-up and clinical endpoints, as opposed to only blood pressure measurements, would be performed, although I am not aware of any plans at present,” he added.
Dr. Ahmad reported no financial relationships relevant to this research. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, including those developing products relevant to hypertension and renal denervation. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
Questions remain despite efficacy
Questions remain despite efficacy
According to the latest meta-analysis of sham-controlled randomized trials, catheter-based renal sympathetic denervation produces clinically meaningful reductions in blood pressure with acceptable safety, but the strategy is not yet regarded as ready for prime time, according to a summary of the results to be presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
This meta-analysis was based on seven blinded trials, all of which associated denervation with a reduction in systolic ambulatory BP, according to Yousif Ahmad, BMBS, PhD, an interventional cardiologist at Yale University, New Haven, Conn.
Although the BP-lowering advantage in two of these studies did not reach statistical significance, the other five did, and all the data moved in the same direction.
For ambulatory diastolic pressure, the effect was more modest. One of the studies showed essentially a neutral effect. The reductions were statistically significant in only two, but, again, the data moved in the same direction in six of the studies, and a random-effects analysis suggested that the reductions, although modest, were potentially meaningful, according to Dr. Ahmad.
Overall, at a mean follow-up of 4.5 months, the reductions in ambulatory systolic and diastolic BPs were 3.61 and 1.85 mm Hg, respectively. The benefit was about the same whether renal denervation was or was not performed on the background of antihypertensive drugs, which was permitted in five of the seven trials. In the other two, all patients were off hypertensive medication.
Office-based systolic reduction: 6 mm Hg
When the same analysis was performed for office-based BP reductions, which were available for five of the seven trials, the overall reductions based on the meta-analysis were 5.86 and 3.63 mm Hg for the systolic and diastolic pressures, respectively. Again, background antihypertensive therapy was not a factor.
Of the seven trials, three randomized fewer than 100 patients. The largest, SYMPLICITY HTN-3, randomized 491 patients in 2:1 ratio to denervation or sham.
Three of the studies in the meta-analysis were trials of the Symplicity flex device. Another two evaluated the Symplicity Spyral catheter. Both deliver radiofrequency energy to for denervation. The Paradise device, the focus of the remaining two trials, employs energy in the form of ultrasound.
According to Dr. Ahmad, adverse events regardless of device were rare and not more common among those in the active treatment arm than in those treated with a sham procedure. Although one of these trials, RADIANCE-HTN SOLO associated denervation with efficacy and safety out to 12 months , Dr. Ahmad concluded that the mean follow-up of 4.5 months is not sufficient to consider long-term effects.
More than 20 meta-analyses published so far
By one count, there have been more than 20 meta-analyses of renal denervation published previously yet this intervention is still considered “controversial,” according to Dr. Ahmad. Relative to the previous meta-analyses, this included the RADIANCE-HTN TRIO trial, which is the latest such sham-controlled study and added 136 patients to the dataset of high-quality trials.
Basically, the results led Dr. Ahmad to conclude that, although the treatment effect is modest, it could be valuable in specific groups of patients, such as those reluctant or unable to take multiple medications or any medications at all. In addition to generating more data on efficacy and safety, he said longer follow-up is also needed for calculations of cost-effectiveness. Larger-scale observational studies might be one way of collecting these data, he reported.
The results of this study were published online in JACC Cardiovascular Interventions with an accompanying editorial by David E. Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta.
Commenting on the large pile of meta-analyses, sometimes published months apart, Dr. Kandzari explained that their “short half-life” is a product of the continuous updating of data with new trials. For a procedure that remains controversial, he said these constant relooks are inevitable.
“My point is that, with more studies, we can expect to see more meta-analyses. It is just the way this is going to work,” Dr. Kandzari said in an interview.
Individual study data also relevant
Even as the authors of these analyses attempt to cull the best data from the most rigorously performed trials, “we are also going to have to look at the individual studies, because of the differences in the trial designs, particularly the devices used,” according to Dr. Kandzari, who was the principle investigator of the sham-controlled SPYRAL HTN-ON MED trial.
So far, the data, despite some inconsistencies, have supported “clinically meaningful” BP reductions and acceptable safety regardless of the device used, according to Dr. Kandzari. Although he also agrees with the basic premise that more long-term data are needed to better determine how renal denervation should be applied in management of hypertension, he does think it will eventually find a role that is “complimentary to, rather than a replacement for, drugs.”
“The effect is modest, but keep in mind that the effect size is similar to that of a single oral medication, and there are some features, such as an always-on 24-hour effect that could be useful,” he said.
“We have enough of a signal to start thinking of how this will be enveloped into routine care,” he said.
But it is not ready yet. This was the point made by Dr. Ahmad, and it was seconded by Dr. Kandzari. One of the senior authors of the meta-analysis, Deepak Bhatt, MD, executive director of interventional cardiovascular programs, Brigham and Women’s Health, Boston, was also asked to weigh on when it will be ready for prime time.
“At a minimum, I would recommend completion of ongoing sham-controlled randomized trials before considering clinical use of renal denervation. Longer term safety and durability data, as well as data on cost-effectiveness, are all still needed – preferably from randomized trials as opposed to registries,” he said.
“Ideally, larger sham-controlled trials with longer follow-up and clinical endpoints, as opposed to only blood pressure measurements, would be performed, although I am not aware of any plans at present,” he added.
Dr. Ahmad reported no financial relationships relevant to this research. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, including those developing products relevant to hypertension and renal denervation. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
According to the latest meta-analysis of sham-controlled randomized trials, catheter-based renal sympathetic denervation produces clinically meaningful reductions in blood pressure with acceptable safety, but the strategy is not yet regarded as ready for prime time, according to a summary of the results to be presented at the Transcatheter Cardiovascular Therapeutics annual meeting.
This meta-analysis was based on seven blinded trials, all of which associated denervation with a reduction in systolic ambulatory BP, according to Yousif Ahmad, BMBS, PhD, an interventional cardiologist at Yale University, New Haven, Conn.
Although the BP-lowering advantage in two of these studies did not reach statistical significance, the other five did, and all the data moved in the same direction.
For ambulatory diastolic pressure, the effect was more modest. One of the studies showed essentially a neutral effect. The reductions were statistically significant in only two, but, again, the data moved in the same direction in six of the studies, and a random-effects analysis suggested that the reductions, although modest, were potentially meaningful, according to Dr. Ahmad.
Overall, at a mean follow-up of 4.5 months, the reductions in ambulatory systolic and diastolic BPs were 3.61 and 1.85 mm Hg, respectively. The benefit was about the same whether renal denervation was or was not performed on the background of antihypertensive drugs, which was permitted in five of the seven trials. In the other two, all patients were off hypertensive medication.
Office-based systolic reduction: 6 mm Hg
When the same analysis was performed for office-based BP reductions, which were available for five of the seven trials, the overall reductions based on the meta-analysis were 5.86 and 3.63 mm Hg for the systolic and diastolic pressures, respectively. Again, background antihypertensive therapy was not a factor.
Of the seven trials, three randomized fewer than 100 patients. The largest, SYMPLICITY HTN-3, randomized 491 patients in 2:1 ratio to denervation or sham.
Three of the studies in the meta-analysis were trials of the Symplicity flex device. Another two evaluated the Symplicity Spyral catheter. Both deliver radiofrequency energy to for denervation. The Paradise device, the focus of the remaining two trials, employs energy in the form of ultrasound.
According to Dr. Ahmad, adverse events regardless of device were rare and not more common among those in the active treatment arm than in those treated with a sham procedure. Although one of these trials, RADIANCE-HTN SOLO associated denervation with efficacy and safety out to 12 months , Dr. Ahmad concluded that the mean follow-up of 4.5 months is not sufficient to consider long-term effects.
More than 20 meta-analyses published so far
By one count, there have been more than 20 meta-analyses of renal denervation published previously yet this intervention is still considered “controversial,” according to Dr. Ahmad. Relative to the previous meta-analyses, this included the RADIANCE-HTN TRIO trial, which is the latest such sham-controlled study and added 136 patients to the dataset of high-quality trials.
Basically, the results led Dr. Ahmad to conclude that, although the treatment effect is modest, it could be valuable in specific groups of patients, such as those reluctant or unable to take multiple medications or any medications at all. In addition to generating more data on efficacy and safety, he said longer follow-up is also needed for calculations of cost-effectiveness. Larger-scale observational studies might be one way of collecting these data, he reported.
The results of this study were published online in JACC Cardiovascular Interventions with an accompanying editorial by David E. Kandzari, MD, director of interventional cardiology, Piedmont Hart Institute, Atlanta.
Commenting on the large pile of meta-analyses, sometimes published months apart, Dr. Kandzari explained that their “short half-life” is a product of the continuous updating of data with new trials. For a procedure that remains controversial, he said these constant relooks are inevitable.
“My point is that, with more studies, we can expect to see more meta-analyses. It is just the way this is going to work,” Dr. Kandzari said in an interview.
Individual study data also relevant
Even as the authors of these analyses attempt to cull the best data from the most rigorously performed trials, “we are also going to have to look at the individual studies, because of the differences in the trial designs, particularly the devices used,” according to Dr. Kandzari, who was the principle investigator of the sham-controlled SPYRAL HTN-ON MED trial.
So far, the data, despite some inconsistencies, have supported “clinically meaningful” BP reductions and acceptable safety regardless of the device used, according to Dr. Kandzari. Although he also agrees with the basic premise that more long-term data are needed to better determine how renal denervation should be applied in management of hypertension, he does think it will eventually find a role that is “complimentary to, rather than a replacement for, drugs.”
“The effect is modest, but keep in mind that the effect size is similar to that of a single oral medication, and there are some features, such as an always-on 24-hour effect that could be useful,” he said.
“We have enough of a signal to start thinking of how this will be enveloped into routine care,” he said.
But it is not ready yet. This was the point made by Dr. Ahmad, and it was seconded by Dr. Kandzari. One of the senior authors of the meta-analysis, Deepak Bhatt, MD, executive director of interventional cardiovascular programs, Brigham and Women’s Health, Boston, was also asked to weigh on when it will be ready for prime time.
“At a minimum, I would recommend completion of ongoing sham-controlled randomized trials before considering clinical use of renal denervation. Longer term safety and durability data, as well as data on cost-effectiveness, are all still needed – preferably from randomized trials as opposed to registries,” he said.
“Ideally, larger sham-controlled trials with longer follow-up and clinical endpoints, as opposed to only blood pressure measurements, would be performed, although I am not aware of any plans at present,” he added.
Dr. Ahmad reported no financial relationships relevant to this research. Dr. Bhatt has financial relationships with more than 30 pharmaceutical companies, including those developing products relevant to hypertension and renal denervation. Dr. Kandzari reported financial relationships with Ablative Solutions and Medtronic.
FROM TCT 2021
Enlarging purple plaque on leg
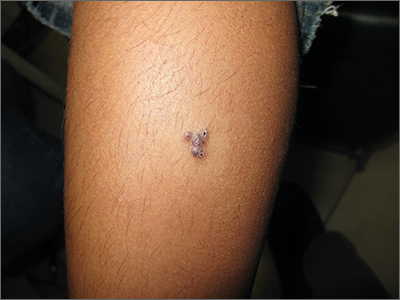
Clinical and dermoscopic features were consistent with a sporadic angiokeratoma, a benign ectasia of vessels associated with keratinization. Diagnosis was confirmed with shave biopsy.
Sporadic angiokeratomas are common and increase with age. They may occur anywhere on the skin, including mucous membranes. Dermoscopy of an angiokeratoma will reveal vascular lacunae—small pools of red or near black blood—as well as keratin scale, which appears as a white veil or shiny white lines.1
Other subtypes of angiokeratomas may be more widespread, including angiokeratoma of Fordyce, which manifests on the vulva and scrotum (usually in adulthood), or angiokeratoma circumscriptum, which occurs congenitally. There are some rare syndromic conditions associated with widespread angiokeratomas, such as Fabry disease.
The differential diagnosis for this solitary, vascular-appearing papule or plaque includes cherry angioma, pyogenic granuloma, and melanoma. Over time, the keratinization may increase, and lesions may look like a wart. A punch or shave biopsy will distinguish an angiokeratoma from other types of lesions. When a lesion is small enough, it may also be curative.
Angiokeratomas do not require treatment. However, because they may occur in cosmetically sensitive areas or bleed when traumatized, remedies are often sought. Excision, cryotherapy, electrocautery, and vascular laser are all possible treatments.
In this case, the patient was treated with curettage and light electrocautery, which left a small scar.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Zaballos P, Daufí C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318-325. doi: 10.1001/archderm.143.3.318

Clinical and dermoscopic features were consistent with a sporadic angiokeratoma, a benign ectasia of vessels associated with keratinization. Diagnosis was confirmed with shave biopsy.
Sporadic angiokeratomas are common and increase with age. They may occur anywhere on the skin, including mucous membranes. Dermoscopy of an angiokeratoma will reveal vascular lacunae—small pools of red or near black blood—as well as keratin scale, which appears as a white veil or shiny white lines.1
Other subtypes of angiokeratomas may be more widespread, including angiokeratoma of Fordyce, which manifests on the vulva and scrotum (usually in adulthood), or angiokeratoma circumscriptum, which occurs congenitally. There are some rare syndromic conditions associated with widespread angiokeratomas, such as Fabry disease.
The differential diagnosis for this solitary, vascular-appearing papule or plaque includes cherry angioma, pyogenic granuloma, and melanoma. Over time, the keratinization may increase, and lesions may look like a wart. A punch or shave biopsy will distinguish an angiokeratoma from other types of lesions. When a lesion is small enough, it may also be curative.
Angiokeratomas do not require treatment. However, because they may occur in cosmetically sensitive areas or bleed when traumatized, remedies are often sought. Excision, cryotherapy, electrocautery, and vascular laser are all possible treatments.
In this case, the patient was treated with curettage and light electrocautery, which left a small scar.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

Clinical and dermoscopic features were consistent with a sporadic angiokeratoma, a benign ectasia of vessels associated with keratinization. Diagnosis was confirmed with shave biopsy.
Sporadic angiokeratomas are common and increase with age. They may occur anywhere on the skin, including mucous membranes. Dermoscopy of an angiokeratoma will reveal vascular lacunae—small pools of red or near black blood—as well as keratin scale, which appears as a white veil or shiny white lines.1
Other subtypes of angiokeratomas may be more widespread, including angiokeratoma of Fordyce, which manifests on the vulva and scrotum (usually in adulthood), or angiokeratoma circumscriptum, which occurs congenitally. There are some rare syndromic conditions associated with widespread angiokeratomas, such as Fabry disease.
The differential diagnosis for this solitary, vascular-appearing papule or plaque includes cherry angioma, pyogenic granuloma, and melanoma. Over time, the keratinization may increase, and lesions may look like a wart. A punch or shave biopsy will distinguish an angiokeratoma from other types of lesions. When a lesion is small enough, it may also be curative.
Angiokeratomas do not require treatment. However, because they may occur in cosmetically sensitive areas or bleed when traumatized, remedies are often sought. Excision, cryotherapy, electrocautery, and vascular laser are all possible treatments.
In this case, the patient was treated with curettage and light electrocautery, which left a small scar.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Zaballos P, Daufí C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318-325. doi: 10.1001/archderm.143.3.318
1. Zaballos P, Daufí C, Puig S, et al. Dermoscopy of solitary angiokeratomas: a morphological study. Arch Dermatol. 2007;143:318-325. doi: 10.1001/archderm.143.3.318