User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Effect of COVID-19 pandemic on respiratory infectious diseases in primary care practice
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.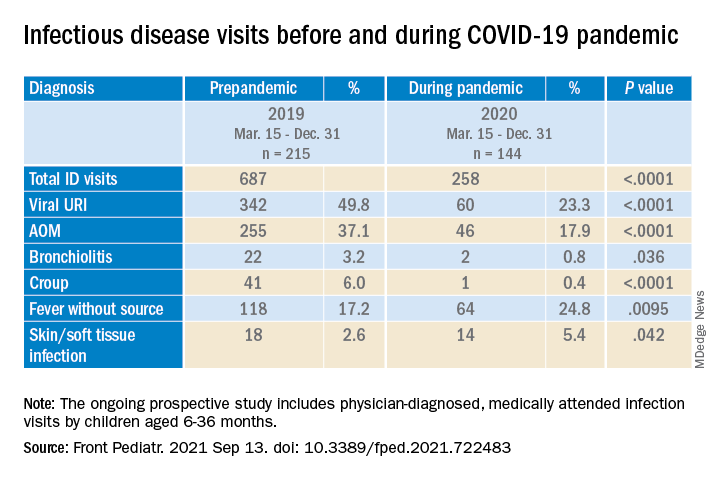
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.
A secondary consequence of public health measures to prevent the spread of SARS-CoV-2 included a concurrent reduction in risk for children to acquire and spread other respiratory viral infectious diseases. In the Rochester, N.Y., area, we had an ongoing prospective study in primary care pediatric practices that afforded an opportunity to assess the effect of the pandemic control measures on all infectious disease illness visits in young children. Specifically, in children aged 6-36 months old, our study was in place when the pandemic began with a primary objective to evaluate the changing epidemiology of acute otitis media (AOM) and nasopharyngeal colonization by potential bacterial respiratory pathogens in community-based primary care pediatric practices. As the public health measures mandated by New York State Department of Health were implemented, we prospectively quantified their effect on physician-diagnosed infectious disease illness visits. The incidence of infectious disease visits by a cohort of young children during the COVID-19 pandemic period March 15, 2020, through Dec. 31, 2020, was compared with the same time frame in the preceding year, 2019.1
Recommendations of the New York State Department of Health for public health, changes in school and day care attendance, and clinical practice during the study time frame
On March 7, 2020, a state of emergency was declared in New York because of the COVID-19 pandemic. All schools were required to close. A mandated order for public use of masks in adults and children more than 2 years of age was enacted. In the Finger Lakes region of Upstate New York, where the two primary care pediatric practices reside, complete lockdown was partially lifted on May 15, 2020, and further lifted on June 26, 2020. Almost all regional school districts opened to at least hybrid learning models for all students starting Sept. 8, 2020. On March 6, 2020, video telehealth and telephone call visits were introduced as routine practice. Well-child visits were limited to those less than 2 years of age, then gradually expanded to all ages by late May 2020. During the “stay at home” phase of the New York State lockdown, day care services were considered an essential business. Day care child density was limited. All children less than 2 years old were required to wear a mask while in the facility. Upon arrival, children with any respiratory symptoms or fever were excluded. For the school year commencing September 2020, almost all regional school districts opened to virtual, hybrid, or in-person learning models. Exclusion occurred similar to that of the day care facilities.
Incidence of respiratory infectious disease illnesses
Clinical diagnoses and healthy visits of 144 children from March 15 to Dec. 31, 2020 (beginning of the pandemic) were compared to 215 children during the same months in 2019 (prepandemic). Pediatric SARS-CoV-2 positivity rates trended up alongside community spread. Pediatric practice positivity rates rose from 1.9% in October 2020 to 19% in December 2020.
The table shows the incidence of significantly different infectious disease illness visits in the two study cohorts.
During the pandemic, 258 infection visits occurred among 144 pandemic cohort children, compared with 687 visits among 215 prepandemic cohort children, a 1.8-fold decrease (P < .0001). The proportion of children with visits for AOM (3.7-fold; P < .0001), bronchiolitis (7.4-fold; P = .036), croup (27.5-fold; P < .0001), and viral upper respiratory infection (3.8-fold; P < .0001) decreased significantly. Fever without a source (1.4-fold decrease; P = .009) and skin/soft tissue infection (2.1-fold decrease; P = .042) represented a higher proportion of visits during the pandemic.
Prescription of antibiotics significantly decreased (P < .001) during the pandemic.
Change in care practices
In the prepandemic period, virtual visits, leading to a diagnosis and treatment and referring children to an urgent care or hospital emergency department during regular office hours were rare. During the pandemic, this changed. Significantly increased use of telemedicine visits (P < .0001) and significantly decreased office and urgent care visits (P < .0001) occurred during the pandemic. Telehealth visits peaked the week of April 12, 2020, at 45% of all pediatric visits. In-person illness visits gradually returned to year-to-year volumes in August-September 2020 with school opening. Early in the pandemic, both pediatric offices limited patient encounters to well-child visits in the first 2 years of life to not miss opportunities for childhood vaccinations. However, some parents were reluctant to bring their children to those visits. There was no significant change in frequency of healthy child visits during the pandemic.
To our knowledge, this was the first study from primary care pediatric practices in the United States to analyze the effect on infectious diseases during the first 9 months of the pandemic, including the 6-month time period after the reopening from the first 3 months of lockdown. One prior study from a primary care network in Massachusetts reported significant decreases in respiratory infectious diseases for children aged 0-17 years during the first months of the pandemic during lockdown.2 A study in Tennessee that included hospital emergency department, urgent care, primary care, and retail health clinics also reported respiratory infection diagnoses as well as antibiotic prescription were reduced in the early months of the pandemic.3
Our study shows an overall reduction in frequency of respiratory illness visits in children 6-36 months old during the first 9 months of the COVID-19 pandemic. We learned the value of using technology in the form of virtual visits to render care. Perhaps as the pandemic subsides, many of the hand-washing and sanitizing practices will remain in place and lead to less frequent illness in children in the future. However, there may be temporary negative consequences from the “immune debt” that has occurred from a prolonged time span when children were not becoming infected with respiratory pathogens.4 We will see what unfolds in the future.
Dr. Pichichero is a specialist in pediatric infectious diseases and director of the Research Institute at Rochester (N.Y.) General Hospital. Dr. Schulz is pediatric medical director at Rochester (N.Y.) Regional Health. Dr. Pichichero and Dr. Schulz have no conflicts of interest to disclose. This study was funded in part by the Centers for Disease Control and Prevention.
References
1. Kaur R et al. Front Pediatr. 2021;(9)722483:1-8.
2. Hatoun J et al. Pediatrics. 2020;146(4):e2020006460.
3. Katz SE et al. J Pediatric Infect Dis Soc. 2021;10(1):62-4.
4. Cohen R et al. Infect. Dis Now. 2021; 51(5)418-23.
New reports help nail down myocarditis risk with COVID-19 vaccine
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Recent literature features new descriptions of myocarditis linked to the two available mRNA vaccines against SARS-CoV-2. They tell a story largely consistent with experience to date, and support what might be its most useful public health message: The associated myocarditis is usually mild and self-limiting, and is far less likely to occur than myocarditis or death in unvaccinated people with COVID-19.
In line with previous research, the new analyses suggest the myocarditis – with onset usually a few days to a week after injection – has an overall incidence that ranges from less than 1 to perhaps 3 per 100,000 people who received at least one of the full mRNA-vaccine regimen’s two injections. Also, as in earlier studies, the incidence climbed higher – sometimes sharply – in certain groups by age and sex, particularly in young men and older male teens.
The new studies “are confirmatory, in terms of the risk being low,” but underscore that clinicians still must be wary of myocarditis as a potential complication of the mRNA vaccines, Biykem Bozkurt, MD, PhD, Baylor College of Medicine, Houston, told this news organization.
Dr. Bozkurt, a leading heart failure specialist and researcher, did not contribute to any of the new reports but does study the myocarditis of COVID-19 and was lead author on a recent review of the potential vaccine complication’s features and possible mechanisms.
In the new myocarditis reports, she observed, more than 90% of the cases were mild and “resolved on their own without a major adverse outcome.” Dr. Bozkurt emphasized the need for perspective regarding the risk. For example, the myocarditis associated with SARS-CoV-2 infection is not only more likely than the vaccine-related myocarditis, but it’s also usually far more severe.
Dr. Bozkurt pointed to a recent study in which the mRNA vaccines, compared with no vaccination, appeared to escalate the myocarditis risk by a factor of 3, whereas the risk for myocarditis in SARS-CoV-2 infection was increased 18 times.
In contrast, she observed, the new myocarditis cases reported this week feature a few that are novel or are at least very rare, including the case of a patient who developed cardiogenic shock and another with fulminant myocarditis who died.
The Centers for Disease Control and Prevention in May publicly described the apparent link between myocarditis and the two available mRNA vaccines against SARS-CoV-2: BNT162b2 (Pfizer-BioNTech) and mRNA-1273 (Moderna). The next month, the Food and Drug Administration added a warning about the risk to the labeling.
Less than 1 case per 100,000
Fifteen confirmed cases of myocarditis were identified among about 2.4 million members of Kaiser Permanente Southern California aged 18 or older who received at least one injection of the Pfizer or Moderna mRNA vaccines between December 2020 and July 2021, in a report published in JAMA Internal Medicine. The study counted cases up to 10 days after the first or second injection, of which there were 2 and 13, respectively.
All eight patients who received the Pfizer BNT162b2 vaccine and the eight given the Moderna mRNA-1273 vaccine were male with a median age of 25 years (interquartile range, 20-32 years).
“The main takeaway messages from our study are that the incidence of myocarditis after COVID-19 mRNA vaccinations is very low, that this condition is primarily observed in young men within a few days after the second dose, and that most patients recover quickly,” senior author Mingsum Lee, MD, PhD, Kaiser Permanente Los Angeles Medical Center, told this news organization.
“The incidence of vaccine-related myocarditis was significantly lower than rates of COVID-19 hospitalization during the same period and population area,” she added.
The group saw a per-million incidence of 0.8 and 5.8 myocarditis cases in the 10 days after first and second injections, respectively. That made for an incidence of 0.58 per 100,000, or 1 case per 172,414 fully vaccinated adults.
The group also considered a cohort of 1,577,741 unvaccinated people with a median age of 39 years (interquartile range, 28-53 years) during the same period. Of the 75 cases of myocarditis, 52% were in men, they reported.
Comparing the vaccinated and unvaccinated cohorts, they saw a 10-day vaccine-associated myocarditis incidence rate ratio of 0.38 (95% confidence interval, 0.05-1.40; P = .15) after the first dose, and 2.7 (95% CI, 1.4-4.8; P = .004) after the second dose.
In a comparison of the vaccinated group with itself using data from a 10-day period in the previous year, the corresponding myocarditis IRRs were 1.0 (P > .99) and 3.3 (P = .03), respectively.
Dr. Lee said none of the 15 patients required admission to an intensive care unit. “All patients with myocarditis responded well to treatment and felt better quickly,” she noted.
Myocarditis after an mRNA vaccine injection is rare and, Dr. Lee said emphatically, and “the benefits of the COVID-19 vaccine greatly outweigh the risks.”
Sex- and age-stratified rates
In a separate analysis of 5,442,696 people given a first dose of the Pfizer BNT162b2 vaccine and 5,125,635 given a second dose, there were 142 cases of myocarditis with onset 21 days after dose 1 and 30 days after dose 2. Of those cases, 136 were documented as “definite or probable” in an Israeli Ministry of Health database that covered up to the end of May 2021.
There were also 40 cases among vaccinated people seen after the 30-day window, which were considered not related to the vaccination, and 101 cases among unvaccinated people; of the latter, 29 had confirmed diagnoses of COVID-19.
Of the 136 people with definite or probable cases, the myocarditis was “generally mild” in 129 and usually resolved on its own, notes the report on the study, published in the New England Journal of Medicine, with lead author Dror Mevorach, MD, Hadassah-Hebrew University Medical Center, Jerusalem.
The estimated myocarditis incidence after a second such vaccine dose across the entire Israeli population, based on the current study, was about one per 26,000 males and one per 218,000 females, the group writes. Those figures compare with one case per 10,857 among “the general unvaccinated population.”
Again, the risk was concentrated among younger men and male adolescents. In an analysis limited to vaccinated people aged 16-19 years, myocarditis in the 21 days after a second mRNA injection was seen in about one of 6,637 males and one of 99,853 females, the group reported.
The standardized incidence ratio of 5.34 (95% CI, 4.48-6.40) after a second injection, across all groups, “was driven mostly by the diagnosis of myocarditis in younger male recipients.” Among that male subgroup, the ratios by age group were 13.60 (95% CI, 9.30-19.20) for 16-19 years, 8.53 (95% CI, 5.57-12.50) for 20-24 years, and 6.96 (95% CI, 4.25-10.75) for 25-29 years.
Among people who received a second injection, compared with unvaccinated people, the 30-day rate ratio was 2.35 (95% CI, 1.10-5.02). Again, the effect was concentrated in males aged 16-19 years. Among them, the myocarditis rate ratios in the 30 days after a second mRNA vaccine injection were 8.96 (95% CI, 4.50-17.83) for the 16-19 years group, 6.13 (95% CI, 3.16-11.88) for the 20-24 group, and 3.58 (95% CI, 1.82-7.01) for 25-29 years.
Most of the patients with myocarditis showed “significant clinical improvement,” with a mean hospitalization time of only 3-4 days, the report notes. Treatment consisted of nonsteroidal anti-inflammatory drugs “with or without colchicine for presumed pericardial inflammation.”
However, seven patients (4.9%) developed important complications, including left-ventricular dysfunction, ventricular arrhythmias, and heart failure. Among them was a 22-year-old patient who died of fulminant myocarditis within 24 hours of diagnosis, the group wrote.
From an Israeli health care organization
Published by the same journal as the study by Dr. Menvorach and associates, an analysis of a separate database showed largely consistent findings among patients in the largest of Israel’s four health care organizations charged by the government to administer health services.
The report, with authors led by Guy Witberg, MD, Rabin Medical Center, Petah Tikva, Israel, focused on members of the health care organization aged 16 years or older who had received at least one Pfizer mRNA vaccine dose by the end of May 2021.
The cohorts from the two separate reports surely overlap substantially, as the Ministry of Health analysis from Dr. Mevorach and colleagues derived from a nationwide database, and – as Dr. Witberg and associates wrote – the health care organization providing their data covers 52% of the Israeli population.
Of 2,558,421 vaccinated people in the analysis, of whom 94% received two doses, 54 developed confirmed myocarditis in the 42 days after the first dose. Their median age was 27 years (interquartile range, 21-35 years) and all but three (94%) were male. Of those 54 cases, 41 were considered mild and 12 intermediate in severity, and one was fulminant with the patient in cardiogenic shock, the group writes. In addition, nonsustained ventricular tachycardia and atrial fibrillation developed in 5% and 3% of cases, respectively.
The estimated myocarditis incidence in the 42 days after administration of at least one mRNA vaccine dose was 2.13 per 100,000 vaccinated people. In that group, Dr. Witberg and colleagues note, the corresponding incidences per 100,000 were 4.12 and 0.23 for males and females, respectively.
Also in the current report, incidences per 100,000 vaccinated people aged 16-29 years, by sex, included 5.49 (95% CI, 3.59-7.39) overall, and 10.69 (95% CI, 6.93-14.46) for males (the highest rate in the report).
There was only one case in a female aged 16-29 years, and two cases in females 30 years or older.
Of note, some authors of the current study are also authors on the high-profile report from Noam Barda, MD, and colleagues published in the New England Journal of Medicine, that used the same database to arrive at an mRNA-vaccine-related incidence of myocarditis of 2.7 per 100,000. Eligibility criteria and follow-up time were different in that report, as were case ascertainment criteria.
The myocarditis risk associated with the two mRNA vaccines is small compared with “the morbidity and mortality of COVID-19 infection, in which up to 28% of hospitalized patients showed signs of myocardial injury,” wrote Vinay Guduguntla, MD, University of California, San Francisco, and Mitchell H. Katz, MD, NYC Health + Hospitals, New York, in an editorial accompanying the report from Dr. Lee and associates.
“Randomized clinical trials show that COVID-19 mRNA vaccines represent a safe and effective method of preventing infection,” they stated. “The identification of rare myocarditis does not change clinical decision-making.”
Dr. Bozkurt, who is immediate past president of the Heart Failure Society of America, has disclosed consulting for Bayer and scPharmaceuticals and serving on a clinical events committee for a trial supported by Abbott Pharmaceuticals and on a data and safety monitoring board for a trial supported by Liva Nova Pharmaceuticals. Dr. Lee and the report’s other authors had no disclosures. Dr. Mevorach discloses consulting for Enlivex Therapeutics; disclosures for the other authors are available at NEJM.org. Dr. Witberg said he has no interests to disclose; disclosures for the other authors are available at NEJM.org. Dr. Guduguntla is an editorial fellow and Dr. Katz a deputy editor at JAMA Internal Medicine; neither had disclosures.
A version of this article first appeared on Medscape.com.
Merck seeks FDA authorization for antiviral COVID-19 pill
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
, an experimental antiviral COVID-19 treatment.
If the FDA grants authorization, the drug would be the first oral antiviral treatment for COVID-19. The capsule, made by Merck and Ridgeback Biotherapeutics, is intended to treat mild to moderate COVID-19 in adults who are at risk of having severe COVID-19 or hospitalization.
“The extraordinary impact of this pandemic demands that we move with unprecedented urgency, and that is what our teams have done by submitting this application for molnupiravir to the FDA within 10 days of receiving the data,” Robert Davis, CEO and president of Merck, said in a statement. On Oct. 1, Merck and Ridgeback released interim data from its phase III clinical trial, which showed that molnupiravir reduced the risk of hospitalization or death by about 50%. About 7% of patients who received the drug were hospitalized within 30 days in the study, as compared with 14% of patients who took a placebo, the company said.
No deaths were reported in the group that received the drug, as compared with eight deaths in the group that received the placebo. None of the trial participants had been vaccinated.
“Medicines and vaccines are both essential to our collective efforts,” Mr. Davis said. “We look forward to working with the FDA on its review of our application, and to working with other regulatory agencies as we do everything we can to bring molnupiravir to patients around the world as quickly as possible.”
Merck has been producing molnupiravir in anticipation of the clinical trial results and FDA authorization. The company expects to produce 10 million courses of treatment by the end of the year, with more expected for 2022.
In June, Merck signed an agreement with the United States to supply 1.7 million courses of molnupiravir once the FDA authorizes the drug. The company has agreed to advance purchase agreements with other countries as well.
Earlier in the year, Merck also announced voluntary licensing agreements with several generics manufacturers in India to provide molnupiravir to more than 100 low- and middle-income countries after approval from local regulatory agencies.
Data from the company’s late-stage clinical trial has not yet been peer-reviewed or published.
Last week, Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, said the clinical trial results were “very encouraging” but noted that the FDA should closely scrutinize the drug, CNN reported.
“It is very important that this now must go through the usual process of careful examination of the data by the Food and Drug Administration, both for effectiveness but also for safety, because whenever you introduce a new compound, safety is very important,” Dr. Fauci said, adding that vaccines remain “our best tools against COVID-19.”
A version of this article firsts appeared on WebMD.com.
High-dose omega-3s tied to higher AFib risk
Taking high-doses of marine omega-3 fatty acids, more than 1 gram daily, may raise the risk for atrial fibrillation (AFib), according to a meta-analysis of relevant research.
However, the risk of developing AFib appears to be “relatively small” for those taking 1 gram or less of fish oil per day, Christine M. Albert, MD, chair of the department of cardiology at the Smidt Heart Institute at Cedars-Sinai, Los Angeles, told this news organization.
The study was published online Oct. 6 in the journal Circulation.
It’s estimated that 7.8% of U.S. adults – almost 19 million in all – take fish oil supplements, often unbeknownst to their health care providers, the researchers noted. Yet, the literature on the effects of omega-3 fatty acid supplementation on cardiovascular outcomes are mixed.
“Some, but not all” large-scale randomized controlled trials investigating the effects of marine omega-3 fatty acid supplements on cardiovascular outcomes have reported increased risks for AFib. The potential reasons for differing findings may be dose related, the authors note in their paper.
The goal of this meta-analysis was to “bring clarity, answers, and actionable information” to doctors and patients, said Dr. Albert. The results suggest, however, that there may not be a “straightforward answer” to whether fish oil is good or bad for AFib. Instead, the answer may depend on the dose, she added.
Pooled data
After screening 4,049 articles and abstracts, the researchers included in their analysis seven large-scale randomized controlled trials reporting cardiovascular outcomes of marine omega-3 fatty acids.
The trials reported results for AFib, either as prespecified outcome, adverse event, or a reason for hospitalization. Each had a minimum of 500 patients and a median follow-up of at least 1 year.
Trials examining the effects of omega-3 fatty acids on recurrent AFib in patients with established AFib or postoperative AFib were excluded.
The seven trials enrolled a total of 81,210 patients (mean age, 65 years; 39% women); 72.6% of participants were enrolled in clinical trials testing ≤1 gram of marine omega-3 fatty acids per day and 27.4% were enrolled in clinical trials testing >1 gram of the supplement per day. The weighted average follow-up was 4.9 years.
Overall, use of omega-3 fatty acids was associated with a 25% increased risk for AFib (hazard ratio, 1.25; 95% confidence interval, 1.07-1.46; P = .013).
In analyses stratified by dose, the risk for AFib was “significantly more pronounced” in trials testing high doses of marine omega-3 fatty acid supplements (>1 gram per day: HR, 1.49; 95% CI, 1.04-2.15; P = .042) compared with those testing lower doses (≤1 gram per day: HR, 1.12; 95% CI, 1.03-1.22; P = .024; P for interaction < .001).
In meta-regression, the HR for AFib increased per 1 gram increase in daily omega-3 fatty acid dose (HR. 1.11; 95% CI, 1.06-1.15; P = .001).
Risk-benefit balance
“This meta-analysis adds new evidence regarding the risk of AFib in patients taking marine omega-3 fatty acid supplements,” wrote Dr. Albert and colleagues.
“Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AFib should be balanced against the benefit on atherosclerotic cardiovascular outcomes,” they suggested.
They cautioned that the meta-analysis pooled aggregate-level trial data, not individual patient data. Therefore, subgroup analyses by age or other patient level characteristics were not possible.
The risk of developing AFib increases with advancing age and is more common in men than in women. Additional risk factors include elevated blood pressure, coronary artery disease, heart failure, heart valve defects, obesity, and diabetes.
The authors said the potential risk of developing AFib with high doses of omega-3 fatty acid supplements should be discussed with patients and they should know the signs and symptoms of the condition.
The study had no specific funding. Dr. Albert has received grants from St. Jude Medical, Abbott, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
Taking high-doses of marine omega-3 fatty acids, more than 1 gram daily, may raise the risk for atrial fibrillation (AFib), according to a meta-analysis of relevant research.
However, the risk of developing AFib appears to be “relatively small” for those taking 1 gram or less of fish oil per day, Christine M. Albert, MD, chair of the department of cardiology at the Smidt Heart Institute at Cedars-Sinai, Los Angeles, told this news organization.
The study was published online Oct. 6 in the journal Circulation.
It’s estimated that 7.8% of U.S. adults – almost 19 million in all – take fish oil supplements, often unbeknownst to their health care providers, the researchers noted. Yet, the literature on the effects of omega-3 fatty acid supplementation on cardiovascular outcomes are mixed.
“Some, but not all” large-scale randomized controlled trials investigating the effects of marine omega-3 fatty acid supplements on cardiovascular outcomes have reported increased risks for AFib. The potential reasons for differing findings may be dose related, the authors note in their paper.
The goal of this meta-analysis was to “bring clarity, answers, and actionable information” to doctors and patients, said Dr. Albert. The results suggest, however, that there may not be a “straightforward answer” to whether fish oil is good or bad for AFib. Instead, the answer may depend on the dose, she added.
Pooled data
After screening 4,049 articles and abstracts, the researchers included in their analysis seven large-scale randomized controlled trials reporting cardiovascular outcomes of marine omega-3 fatty acids.
The trials reported results for AFib, either as prespecified outcome, adverse event, or a reason for hospitalization. Each had a minimum of 500 patients and a median follow-up of at least 1 year.
Trials examining the effects of omega-3 fatty acids on recurrent AFib in patients with established AFib or postoperative AFib were excluded.
The seven trials enrolled a total of 81,210 patients (mean age, 65 years; 39% women); 72.6% of participants were enrolled in clinical trials testing ≤1 gram of marine omega-3 fatty acids per day and 27.4% were enrolled in clinical trials testing >1 gram of the supplement per day. The weighted average follow-up was 4.9 years.
Overall, use of omega-3 fatty acids was associated with a 25% increased risk for AFib (hazard ratio, 1.25; 95% confidence interval, 1.07-1.46; P = .013).
In analyses stratified by dose, the risk for AFib was “significantly more pronounced” in trials testing high doses of marine omega-3 fatty acid supplements (>1 gram per day: HR, 1.49; 95% CI, 1.04-2.15; P = .042) compared with those testing lower doses (≤1 gram per day: HR, 1.12; 95% CI, 1.03-1.22; P = .024; P for interaction < .001).
In meta-regression, the HR for AFib increased per 1 gram increase in daily omega-3 fatty acid dose (HR. 1.11; 95% CI, 1.06-1.15; P = .001).
Risk-benefit balance
“This meta-analysis adds new evidence regarding the risk of AFib in patients taking marine omega-3 fatty acid supplements,” wrote Dr. Albert and colleagues.
“Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AFib should be balanced against the benefit on atherosclerotic cardiovascular outcomes,” they suggested.
They cautioned that the meta-analysis pooled aggregate-level trial data, not individual patient data. Therefore, subgroup analyses by age or other patient level characteristics were not possible.
The risk of developing AFib increases with advancing age and is more common in men than in women. Additional risk factors include elevated blood pressure, coronary artery disease, heart failure, heart valve defects, obesity, and diabetes.
The authors said the potential risk of developing AFib with high doses of omega-3 fatty acid supplements should be discussed with patients and they should know the signs and symptoms of the condition.
The study had no specific funding. Dr. Albert has received grants from St. Jude Medical, Abbott, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
Taking high-doses of marine omega-3 fatty acids, more than 1 gram daily, may raise the risk for atrial fibrillation (AFib), according to a meta-analysis of relevant research.
However, the risk of developing AFib appears to be “relatively small” for those taking 1 gram or less of fish oil per day, Christine M. Albert, MD, chair of the department of cardiology at the Smidt Heart Institute at Cedars-Sinai, Los Angeles, told this news organization.
The study was published online Oct. 6 in the journal Circulation.
It’s estimated that 7.8% of U.S. adults – almost 19 million in all – take fish oil supplements, often unbeknownst to their health care providers, the researchers noted. Yet, the literature on the effects of omega-3 fatty acid supplementation on cardiovascular outcomes are mixed.
“Some, but not all” large-scale randomized controlled trials investigating the effects of marine omega-3 fatty acid supplements on cardiovascular outcomes have reported increased risks for AFib. The potential reasons for differing findings may be dose related, the authors note in their paper.
The goal of this meta-analysis was to “bring clarity, answers, and actionable information” to doctors and patients, said Dr. Albert. The results suggest, however, that there may not be a “straightforward answer” to whether fish oil is good or bad for AFib. Instead, the answer may depend on the dose, she added.
Pooled data
After screening 4,049 articles and abstracts, the researchers included in their analysis seven large-scale randomized controlled trials reporting cardiovascular outcomes of marine omega-3 fatty acids.
The trials reported results for AFib, either as prespecified outcome, adverse event, or a reason for hospitalization. Each had a minimum of 500 patients and a median follow-up of at least 1 year.
Trials examining the effects of omega-3 fatty acids on recurrent AFib in patients with established AFib or postoperative AFib were excluded.
The seven trials enrolled a total of 81,210 patients (mean age, 65 years; 39% women); 72.6% of participants were enrolled in clinical trials testing ≤1 gram of marine omega-3 fatty acids per day and 27.4% were enrolled in clinical trials testing >1 gram of the supplement per day. The weighted average follow-up was 4.9 years.
Overall, use of omega-3 fatty acids was associated with a 25% increased risk for AFib (hazard ratio, 1.25; 95% confidence interval, 1.07-1.46; P = .013).
In analyses stratified by dose, the risk for AFib was “significantly more pronounced” in trials testing high doses of marine omega-3 fatty acid supplements (>1 gram per day: HR, 1.49; 95% CI, 1.04-2.15; P = .042) compared with those testing lower doses (≤1 gram per day: HR, 1.12; 95% CI, 1.03-1.22; P = .024; P for interaction < .001).
In meta-regression, the HR for AFib increased per 1 gram increase in daily omega-3 fatty acid dose (HR. 1.11; 95% CI, 1.06-1.15; P = .001).
Risk-benefit balance
“This meta-analysis adds new evidence regarding the risk of AFib in patients taking marine omega-3 fatty acid supplements,” wrote Dr. Albert and colleagues.
“Since the benefit of omega-3 fatty acids also appears to be dose dependent, the associated risk of AFib should be balanced against the benefit on atherosclerotic cardiovascular outcomes,” they suggested.
They cautioned that the meta-analysis pooled aggregate-level trial data, not individual patient data. Therefore, subgroup analyses by age or other patient level characteristics were not possible.
The risk of developing AFib increases with advancing age and is more common in men than in women. Additional risk factors include elevated blood pressure, coronary artery disease, heart failure, heart valve defects, obesity, and diabetes.
The authors said the potential risk of developing AFib with high doses of omega-3 fatty acid supplements should be discussed with patients and they should know the signs and symptoms of the condition.
The study had no specific funding. Dr. Albert has received grants from St. Jude Medical, Abbott, and Roche Diagnostics.
A version of this article first appeared on Medscape.com.
HEPA filters may clean SARS-CoV-2 from the air: Study
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
, researchers report in the preprint server medRxiv.
The journal Nature reported Oct. 6 that the research, which has not been peer-reviewed, suggests the filters may help reduce the risk of hospital-acquired SARS-CoV-2.
Researchers, led by intensivist Andrew Conway-Morris, MBChB, PhD, with the division of anaesthesia in the school of clinical medicine at University of Cambridge, United Kingdom, write that earlier experiments assessed air filters’ ability to remove inactive particles in carefully controlled environments, but it was unknown how they would work in a real-world setting.
Co-author Vilas Navapurkar, MBChB, an ICU physician at Addenbrooke’s Hospital in Cambridge, United Kingdom, said that hospitals have used portable air filters when their isolation facilities are full, but evidence was needed as to whether such filters are effective or whether they provide a false sense of security.
The researchers installed the filters in two fully occupied COVID-19 wards — a general ward and an ICU. They chose HEPA filters because they can catch extremely small particles.
The team collected air samples from the wards during a week when the air filters were on and 2 weeks when they were turned off, then compared results.
According to the study, “airborne SARS-CoV-2 was detected in the ward on all five days before activation of air/UV filtration, but on none of the five days when the air/UV filter was operational; SARS-CoV-2 was again detected on four out of five days when the filter was off.”
Airborne SARS-CoV-2 was not frequently detected in the ICU, even when the filters were off.
Cheap and easy
According to the Nature article, the authors suggest several potential explanations for this, “including slower viral replication at later stages of the disease.” Therefore, the authors say, filtering the virus from the air might be more important in general wards than in ICUs.
The filters significantly reduced the other microbial bioaerosols in both the ward (48 pathogens detected before filtration, 2 after, P = .05) and the ICU (45 pathogens detected before filtration, 5 after P = .05).
National Institute for Occupational Safety and Health (NIOSH) cyclonic aerosol samplers and PCR tests were used to detect airborne SARS-CoV-2 and other microbial bioaerosol.
David Fisman, MD, an epidemiologist at the University of Toronto, who was not involved in the research, said in the Nature article, “This study suggests that HEPA air cleaners, which remain little-used in Canadian hospitals, are a cheap and easy way to reduce risk from airborne pathogens.”This work was supported by a Wellcome senior research fellowship to co-author Stephen Baker. Conway Morris is supported by a Clinician Scientist Fellowship from the Medical Research Council. Dr. Navapurkar is the founder, director, and shareholder of Cambridge Infection Diagnostics Ltd. Dr. Conway-Morris and several co-authors are members of the Scientific Advisory Board of Cambridge Infection Diagnostics Ltd. Co-author Theodore Gouliouris has received a research grant from Shionogi and co-author R. Andres Floto has received research grants and/or consultancy payments from GSK, AstraZeneca, Chiesi, Shionogi, Insmed, and Thirty Technology.
A version of this article first appeared on Medscape.com.
Lie down for orthostatic hypotension assessment
New research shows that supine orthostatic hypotension is more common and better predicts falls and orthostatic symptoms than seated OH, supporting a supine OH protocol in clinical practice, the researchers say.
“Older adults at risk for falls undergoing assessment for OH should lie supine rather than sitting prior to standing to get the most informative OH assessment,” study author Stephen Juraschek, MD, PhD, Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, said in an interview.
“The findings call for a change in current practice,” Dr. Juraschek said.
He presented the study Sept. 29 at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The seated position for detecting OH is “commonly used for convenience. Since many clinics already perform a seated blood pressure, it saves time for people to stand shortly afterward,” he explained.
“It has also been thought that the two are interchangeable [i.e., the change in blood pressure from seated to standing was just a lower magnitude than the change from supine to standing]. However, we showed that the physiology is on average quite different, questioning prior perspectives on the interchangeability of the two protocols,” he added.
The researchers studied 522 adults (mean age, 76 years; 42% women) at high risk for falls and with vitamin D levels in the insufficient/deficient range participating in the Study to Understand Fall Reduction and Vitamin D (STURDY).
The study showed that vitamin D supplementation was not associated with OH or the main study outcome of falls.
The study used two different OH assessment protocols – seated to standing and supine to standing – and Dr. Juraschek’s team used the data to gauge the impact of supine and seated positions on OH prevalence and its relation with fall risk and orthostatic symptoms.
OH was defined as a drop in systolic BP of at least 20 mm Hg or diastolic BP of at least 10 mm Hg.
At baseline, mean BP was 129/68 mm Hg. Mean BP increased 3.4/2.6 mm Hg after sitting, but decreased 3.7/0.7 mm Hg after lying supine.
Of the 953 OH assessments (supine and seated), OH was detected in 14.8% of the supine measurements but in only 2.2% of the seated measures.
Supine OH better predicted falls (hazard ratio, 1.60; 95% CI, 0.98-2.61; P = .06) than seated OH (HR, 0.70; 95% CI, 0.30-1.60; P = .39).
Although both were nonsignificant, “potentially due to power,” the association with falls was stronger for supine OH than for seated OH, Dr. Juraschek said.
In addition, seated OH was not associated with orthostatic symptoms, whereas supine OH was significantly associated with a greater risk of fainting, blacking out, seeing spots, room spinning, and headache in the previous month (P = .048-.002).
Useful study confirms anecdotal evidence
This is a “useful study” from a “reputable” group, “and the results reveal what I would have expected,” Robert Carey, MD, University of Virginia, Charlottesville, who wasn’t involved in the study, said in an interview.
The findings, Dr. Carey said, show that measuring supine, compared with standing, “actually correlates much better with the untoward effects of orthostatic hypotension which are falls and symptoms such as dizziness and spots before your eyes.”
“Seated BP is mostly used for convenience and a little bit shorter protocol. Most clinical trials do seated orthostatic hypotension measurements. I’ve always taught my medical students and others to use the supine to standing because I’ve just anecdotally felt that this was a much better way of detecting true orthostatic hypotension and that’s how we do it at the University of Virginia Hospital,” Dr. Carey said.
The study had no funding. Dr. Juraschek and Dr. Carey have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research shows that supine orthostatic hypotension is more common and better predicts falls and orthostatic symptoms than seated OH, supporting a supine OH protocol in clinical practice, the researchers say.
“Older adults at risk for falls undergoing assessment for OH should lie supine rather than sitting prior to standing to get the most informative OH assessment,” study author Stephen Juraschek, MD, PhD, Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, said in an interview.
“The findings call for a change in current practice,” Dr. Juraschek said.
He presented the study Sept. 29 at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The seated position for detecting OH is “commonly used for convenience. Since many clinics already perform a seated blood pressure, it saves time for people to stand shortly afterward,” he explained.
“It has also been thought that the two are interchangeable [i.e., the change in blood pressure from seated to standing was just a lower magnitude than the change from supine to standing]. However, we showed that the physiology is on average quite different, questioning prior perspectives on the interchangeability of the two protocols,” he added.
The researchers studied 522 adults (mean age, 76 years; 42% women) at high risk for falls and with vitamin D levels in the insufficient/deficient range participating in the Study to Understand Fall Reduction and Vitamin D (STURDY).
The study showed that vitamin D supplementation was not associated with OH or the main study outcome of falls.
The study used two different OH assessment protocols – seated to standing and supine to standing – and Dr. Juraschek’s team used the data to gauge the impact of supine and seated positions on OH prevalence and its relation with fall risk and orthostatic symptoms.
OH was defined as a drop in systolic BP of at least 20 mm Hg or diastolic BP of at least 10 mm Hg.
At baseline, mean BP was 129/68 mm Hg. Mean BP increased 3.4/2.6 mm Hg after sitting, but decreased 3.7/0.7 mm Hg after lying supine.
Of the 953 OH assessments (supine and seated), OH was detected in 14.8% of the supine measurements but in only 2.2% of the seated measures.
Supine OH better predicted falls (hazard ratio, 1.60; 95% CI, 0.98-2.61; P = .06) than seated OH (HR, 0.70; 95% CI, 0.30-1.60; P = .39).
Although both were nonsignificant, “potentially due to power,” the association with falls was stronger for supine OH than for seated OH, Dr. Juraschek said.
In addition, seated OH was not associated with orthostatic symptoms, whereas supine OH was significantly associated with a greater risk of fainting, blacking out, seeing spots, room spinning, and headache in the previous month (P = .048-.002).
Useful study confirms anecdotal evidence
This is a “useful study” from a “reputable” group, “and the results reveal what I would have expected,” Robert Carey, MD, University of Virginia, Charlottesville, who wasn’t involved in the study, said in an interview.
The findings, Dr. Carey said, show that measuring supine, compared with standing, “actually correlates much better with the untoward effects of orthostatic hypotension which are falls and symptoms such as dizziness and spots before your eyes.”
“Seated BP is mostly used for convenience and a little bit shorter protocol. Most clinical trials do seated orthostatic hypotension measurements. I’ve always taught my medical students and others to use the supine to standing because I’ve just anecdotally felt that this was a much better way of detecting true orthostatic hypotension and that’s how we do it at the University of Virginia Hospital,” Dr. Carey said.
The study had no funding. Dr. Juraschek and Dr. Carey have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
New research shows that supine orthostatic hypotension is more common and better predicts falls and orthostatic symptoms than seated OH, supporting a supine OH protocol in clinical practice, the researchers say.
“Older adults at risk for falls undergoing assessment for OH should lie supine rather than sitting prior to standing to get the most informative OH assessment,” study author Stephen Juraschek, MD, PhD, Beth Israel Deaconess Medical Center/Harvard Medical School, Boston, said in an interview.
“The findings call for a change in current practice,” Dr. Juraschek said.
He presented the study Sept. 29 at the joint scientific sessions of the American Heart Association Council on Hypertension, AHA Council on Kidney in Cardiovascular Disease, and American Society of Hypertension.
The seated position for detecting OH is “commonly used for convenience. Since many clinics already perform a seated blood pressure, it saves time for people to stand shortly afterward,” he explained.
“It has also been thought that the two are interchangeable [i.e., the change in blood pressure from seated to standing was just a lower magnitude than the change from supine to standing]. However, we showed that the physiology is on average quite different, questioning prior perspectives on the interchangeability of the two protocols,” he added.
The researchers studied 522 adults (mean age, 76 years; 42% women) at high risk for falls and with vitamin D levels in the insufficient/deficient range participating in the Study to Understand Fall Reduction and Vitamin D (STURDY).
The study showed that vitamin D supplementation was not associated with OH or the main study outcome of falls.
The study used two different OH assessment protocols – seated to standing and supine to standing – and Dr. Juraschek’s team used the data to gauge the impact of supine and seated positions on OH prevalence and its relation with fall risk and orthostatic symptoms.
OH was defined as a drop in systolic BP of at least 20 mm Hg or diastolic BP of at least 10 mm Hg.
At baseline, mean BP was 129/68 mm Hg. Mean BP increased 3.4/2.6 mm Hg after sitting, but decreased 3.7/0.7 mm Hg after lying supine.
Of the 953 OH assessments (supine and seated), OH was detected in 14.8% of the supine measurements but in only 2.2% of the seated measures.
Supine OH better predicted falls (hazard ratio, 1.60; 95% CI, 0.98-2.61; P = .06) than seated OH (HR, 0.70; 95% CI, 0.30-1.60; P = .39).
Although both were nonsignificant, “potentially due to power,” the association with falls was stronger for supine OH than for seated OH, Dr. Juraschek said.
In addition, seated OH was not associated with orthostatic symptoms, whereas supine OH was significantly associated with a greater risk of fainting, blacking out, seeing spots, room spinning, and headache in the previous month (P = .048-.002).
Useful study confirms anecdotal evidence
This is a “useful study” from a “reputable” group, “and the results reveal what I would have expected,” Robert Carey, MD, University of Virginia, Charlottesville, who wasn’t involved in the study, said in an interview.
The findings, Dr. Carey said, show that measuring supine, compared with standing, “actually correlates much better with the untoward effects of orthostatic hypotension which are falls and symptoms such as dizziness and spots before your eyes.”
“Seated BP is mostly used for convenience and a little bit shorter protocol. Most clinical trials do seated orthostatic hypotension measurements. I’ve always taught my medical students and others to use the supine to standing because I’ve just anecdotally felt that this was a much better way of detecting true orthostatic hypotension and that’s how we do it at the University of Virginia Hospital,” Dr. Carey said.
The study had no funding. Dr. Juraschek and Dr. Carey have no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Depression rates up threefold since start of COVID-19
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
A year into the COVID-19 pandemic, the share of the U.S. adult population reporting symptoms of elevated depression had more than tripled from prepandemic levels and worsened significantly since restrictions went into effect, a study of more than 1,000 adults surveyed at the start of the pandemic and 1 year into it has reported.
The study also found that younger adults, people with lower incomes and savings, unmarried people, and those exposed to multiple stress factors were most vulnerable to elevated levels of depression through the first year of the pandemic.
“The pandemic has been an ongoing exposure,” lead author Catherine K. Ettman, a PhD candidate at Brown University, Providence, R.I., said in an interview. “Mental health is sensitive to economic and social conditions. While living conditions have improved for some people over the last 12 months, the pandemic has been disruptive to life and economic well-being for many,” said Ms. Ettman, who is also chief of staff and director of strategic initiatives in the office of the dean at Boston University. Her study was published in Lancet Regional Health – Americas.
Ms. Ettman and coauthors reported that 32.8% (95% confidence interval, 29.1%-36.8%) of surveyed adults had elevated depressive symptoms in 2021, compared with 27.8% (95% CI, 24.9%-30.9%) in the early months of the pandemic in 2020 (P = .0016). That compares with a rate of 8.5% before the pandemic, a figure based on a prepandemic sample of 5,065 patients from the National Health and Nutrition Examination Survey reported previously by Ms. Ettman and associates.
“The COVID-19 pandemic and its economic consequences have displaced social networks, created ongoing stressors, and reduced access to the resources that protect mental health,” Ms. Ettman said.
Four groups most affected
In this latest research, a longitudinal panel study of a nationally representative group of U.S. adults, the researchers surveyed participants in March and April 2020 (n = 1,414) and the same group again in March and April 2021 (n = 1,161). The participants completed the Patient Health Questionnaire–9 (PHQ-9) and were enrolled in the COVID-19 and Life Stressors Impact on Mental Health and Well-Being study.
The study found that elevated depressive symptoms were most prevalent in four groups:
- Younger patients, with 43.9% of patients aged 18-39 years self-reporting elevated depressive symptoms, compared with 32.4% of those aged 40-59, and 19.1% of patients aged 60 and older.
- People with lower incomes, with 58.1% of people making $19,999 or less reporting elevated symptoms, compared with 41.3% of those making $20,000-$44,999, 31.4% of people making $45,000-$74,999, and 14.1% of those making $75,000 or more.
- People with less than $5,000 in family savings, with a rate of 51.1%, compared with 24.2% of those with more than that.
- People never married, with a rate of 39.8% versus 37.7% of those living with a partner; 31.5% widowed, divorced, or separated; and 18.3% married.
The study also found correlations between the number of self-reported stressors and elevated depression symptoms: a rate of 51.1% in people with four or more stressors; 25.8% in those with two or three stressors; and 17% in people with one or no stressors.
Among the groups reporting the lowest rates of depressive symptoms in 2021 were people making more than $75,000 a year; those with one or no COVID-19 stressors; and non-Hispanic Asian persons.
“Stressors such as difficulties finding childcare, difficulties paying for housing, and job loss were associated with greater depression 12 months into the COVID-19 pandemic,” Ms. Ettman said. “Efforts to address stressors and improve access to childcare, housing, employment, and fair wages can improve mental health.”
The duration of the pandemic is another explanation for the significant rise in depressive symptoms, senior author Sandro Galea, MD, MPH, DrPH, said in an interview. Dr. Galea added. “Unlike acute traumatic events, the COVID-19 pandemic has been ongoing.”
He said clinicians, public health officials, and policy makers need to be aware of the impact COVID-19 has had on mental health. “We can take steps as a society to treat and prevent depression and create conditions that allow all populations to be healthy,” said Dr. Galea, who is dean and a professor of family medicine at Boston University.
Age of sample cited as limitation
The study builds on existing evidence linking depression trends and the COVID-19 pandemic, David Puder, MD, a medical director at Loma Linda (Calif.) University, said in an interview. However, he noted it had some limitations. “The age range is only 18 and older, so we don’t get to see what is happening with a highly impacted group of students who have not been able to go to school and be with their friends during COVID,” said Dr. Puder, who also hosts the podcast “Psychiatry & Psychotherapy.” “Further, the PHQ-9 is often a screening tool for depression and is not best used for changes in mental health over time.”
At the same time, Dr. Puder said, one of the study’s strengths was that it showed how depressive symptoms increased during the COVID lockdown. “It shows certain groups are at higher risk, including those with less financial resources and those with higher amounts of stress,” Dr. Puder said.
Ms. Ettman, Dr. Galea, and Dr. Puder reported no relevant disclosures.
FROM LANCET REGIONAL HEALTH – AMERICAS
The Long-term Effects of Underdiagnosed Conditions in Sleep Disorders
Jessie Wrobel is a board-certified Family Nurse Practitioner. She earned a Bachelor of Science in Nursing from Southern Connecticut State University, concurrently graduating from their Honor’s College in 2009 and spent 9 years at the bedside focusing on heart failure and stroke populations. She then obtained a Master of Science in Nursing from Southern Connecticut State University and holds a certification with the American Academy of Nurse Practitioners (AANP) as a family nurse practitioner. She has since worked at Yale-New Haven’s Sleep Medicine Center where she follows patients for sleep disordered breathing, narcolepsy, parasomnias, restless leg syndrome and insomnia.
Jessie Wrobel is a board-certified Family Nurse Practitioner. She earned a Bachelor of Science in Nursing from Southern Connecticut State University, concurrently graduating from their Honor’s College in 2009 and spent 9 years at the bedside focusing on heart failure and stroke populations. She then obtained a Master of Science in Nursing from Southern Connecticut State University and holds a certification with the American Academy of Nurse Practitioners (AANP) as a family nurse practitioner. She has since worked at Yale-New Haven’s Sleep Medicine Center where she follows patients for sleep disordered breathing, narcolepsy, parasomnias, restless leg syndrome and insomnia.
Jessie Wrobel is a board-certified Family Nurse Practitioner. She earned a Bachelor of Science in Nursing from Southern Connecticut State University, concurrently graduating from their Honor’s College in 2009 and spent 9 years at the bedside focusing on heart failure and stroke populations. She then obtained a Master of Science in Nursing from Southern Connecticut State University and holds a certification with the American Academy of Nurse Practitioners (AANP) as a family nurse practitioner. She has since worked at Yale-New Haven’s Sleep Medicine Center where she follows patients for sleep disordered breathing, narcolepsy, parasomnias, restless leg syndrome and insomnia.
Pfizer asks FDA to authorize COVID vaccine for kids 5-11
The request comes after the drugmaker submitted clinical trial data to the FDA on Sept. 28. Pfizer said the study of 2,268 children showed the vaccine was safe and produced a robust immune response.
Participants in the studies received a lower dose of the vaccine, 10 micrograms. Their response 2 weeks after a second dose was reportedly equal to the immune protection in a control group of 16- to 25-year-olds who received the fully approved 30-microgram doses.
Currently, the Pfizer EUA applies to 12- to 15-year-olds and people eligible for a Pfizer booster shot. The drugmaker received full FDA approval for the vaccine for Americans 16 years and older in August.
The filing for authorization in 5- to 11-year-olds comes as overall cases of COVID-19 in the United States continue to decline. The decrease includes a drop in new cases in children for the fourth consecutive week, according to analysis of data from the American Academy of Pediatrics and the Children’s Hospital Association.
The next step is an FDA decision on whether to expand the current emergency use authorization (EUA) for teenagers to the younger age group.
Timing of any official word from the agency is unknown. But possibly in anticipation of today’s filing, the FDA already scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee for Oct. 25.
A version of this article first appeared on WebMD.com.
The request comes after the drugmaker submitted clinical trial data to the FDA on Sept. 28. Pfizer said the study of 2,268 children showed the vaccine was safe and produced a robust immune response.
Participants in the studies received a lower dose of the vaccine, 10 micrograms. Their response 2 weeks after a second dose was reportedly equal to the immune protection in a control group of 16- to 25-year-olds who received the fully approved 30-microgram doses.
Currently, the Pfizer EUA applies to 12- to 15-year-olds and people eligible for a Pfizer booster shot. The drugmaker received full FDA approval for the vaccine for Americans 16 years and older in August.
The filing for authorization in 5- to 11-year-olds comes as overall cases of COVID-19 in the United States continue to decline. The decrease includes a drop in new cases in children for the fourth consecutive week, according to analysis of data from the American Academy of Pediatrics and the Children’s Hospital Association.
The next step is an FDA decision on whether to expand the current emergency use authorization (EUA) for teenagers to the younger age group.
Timing of any official word from the agency is unknown. But possibly in anticipation of today’s filing, the FDA already scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee for Oct. 25.
A version of this article first appeared on WebMD.com.
The request comes after the drugmaker submitted clinical trial data to the FDA on Sept. 28. Pfizer said the study of 2,268 children showed the vaccine was safe and produced a robust immune response.
Participants in the studies received a lower dose of the vaccine, 10 micrograms. Their response 2 weeks after a second dose was reportedly equal to the immune protection in a control group of 16- to 25-year-olds who received the fully approved 30-microgram doses.
Currently, the Pfizer EUA applies to 12- to 15-year-olds and people eligible for a Pfizer booster shot. The drugmaker received full FDA approval for the vaccine for Americans 16 years and older in August.
The filing for authorization in 5- to 11-year-olds comes as overall cases of COVID-19 in the United States continue to decline. The decrease includes a drop in new cases in children for the fourth consecutive week, according to analysis of data from the American Academy of Pediatrics and the Children’s Hospital Association.
The next step is an FDA decision on whether to expand the current emergency use authorization (EUA) for teenagers to the younger age group.
Timing of any official word from the agency is unknown. But possibly in anticipation of today’s filing, the FDA already scheduled a meeting of its Vaccines and Related Biological Products Advisory Committee for Oct. 25.
A version of this article first appeared on WebMD.com.
Constipation med boosts cognitive performance in mental illness
, new research suggests.
In a randomized controlled trial, 44 healthy individuals were assigned to receive the selective serotonin-4 (5-HT4) receptor agonist prucalopride (Motegrity) or placebo for 1 week.
After 6 days, the active-treatment group performed significantly better on memory tests than the participants who received placebo. In addition, the drug increased activity in brain areas related to cognition.
“What we’re hoping is...these agents may be able to help those with cognitive impairment as part of their mental illness,” lead author Angharad N. de Cates, a clinical DPhil student in the department of psychiatry, University of Oxford, Oxford, United Kingdom, told meeting attendees.
“Currently, we’re looking to see if we can translate our finding a step further and do a similar study in those with depression,” Ms. de Cates added.
The findings were presented at the 34th European College of Neuropsychopharmacology (ECNP) Congress and were simultaneously published in Translational Psychiatry.
“Exciting early evidence”
“Even when the low mood associated with depression is well-treated with conventional antidepressants, many patients continue to experience problems with their memory,” co-investigator Susannah Murphy, PhD, a senior research fellow at the University of Oxford, said in a release.
“Our study provides exciting early evidence in humans of a new approach that might be a helpful way to treat these residual cognitive symptoms,” Dr. Murphy added.
Preclinical and animal studies suggest that the 5-HT4 receptor is a promising treatment target for cognitive impairment in individuals with psychiatric disorders, although studies in humans have been limited by the adverse effects of early agents.
“We’ve had our eye on this receptor for a while,” explained de Cates, inasmuch as the animal data “have been so good.”
However, she said in an interview that “a lack of safe human agents made translation tricky.”
As previously reported, prucalopride, a selective high-affinity 5-HT4 partial agonist, was approved in 2018 by the U.S. Food and Drug Administration for the treatment of chronic idiopathic constipation.
The current researchers note that the drug has “good brain penetration,” which “allowed us to investigate 5-HT4-receptor agonism in humans.”
Having previously shown that a single dose of the drug has “pro-cognitive effects,” the investigators conducted the new trial in 44 healthy participants. All were randomly assigned in a 1:1 ratio to receive either prucalopride 1 mg for 7 days or placebo.
In accordance with enrollment criteria, patients were 18 to 36 years of age, right-handed, and were not pregnant or breastfeeding. Participants’ body mass index was 18 to 30 kg/m2, and they had no contraindications to the study drug. The two treatment groups were well balanced; the participants who received placebo were significantly more likely to be nonnative English speakers (P = .02).
On day 6 of treatment administration, all participants underwent 3T MRI.
Before undergoing imaging, the participants were presented with eight emotionally neutral images of animals or landscapes and were asked to indicate whether or not the images were of animals. The task was then repeated with the eight familiar images and eight novel ones.
During the scan, participants were shown the same images or eight novel images and were again asked whether or not the images contained an animal. They were also instructed to try to remember the images for a subsequent memory task. In that task, the eight original images, 48 novel images, and 27 “distractor” images were presented.
Better memory
In the pre-scan assessment, results showed no significant differences in the ability of members of the prucalopride and placebo groups to identify images as being familiar or different.
However, taking prucalopride was associated with significantly improved memory performance in the post-scan recall task.
Compared to the placebo group, participants in the prucalopride group were more accurate in selecting images as familiar vs distractors (P = .029) and in distinguishing images as familiar, novel, or distractors (P = .035).
Functional MRI revealed increased activity in the left and right hippocampus in response to both novel and familiar images among the participants in the prucalopride group in comparison with those in the placebo group.
There was also increased activity in the right angular gyrus in the prucalopride group in comparison with the placebo group in response to familiar images (P < .005).
“Clinically, angular gyri lesions cause language dysfunction, low mood, and poor memory and can mimic dementia or pseudodementia,” the investigators write. They note that the right angular gyrus “shows significantly decreased activity” in mild cognitive impairment.
“Therefore, the increased activity seen in the right angular gyrus following prucalopride administration in our study is consistent with the pro-cognitive behavioural effects we observed,” they add.
Ms. De Cates noted that the dose used in their study was lower than the 2 mg given for constipation.
“At the low dose, there were no differences in side effects between groups and no withdrawals from the prucalopride group for side effects. We are going to try increasing the dose in our next study actually, as we don’t have PET data to tell us what the optimal dose for binding at the receptor should be,” said Ms. de Cates.
“In safety studies, the dose was trialled in healthy volunteers at 4 mg, which was found to be safe, although perhaps less well tolerated than 2 mg,” she said.
Generalizable findings?
Commenting on the research, Vibe G. Frøkjær, MD, adjunct professor, department of psychology, Copenhagen University, Denmark, said the study “highlights a very interesting and much needed potential for repurposing drugs to help cognitive dysfunction.”
He noted that cognitive dysfunction is often associated with psychiatric disorders -- even in states of remission.
“Importantly, as the authors also state, it will be vital to translate these findings from healthy populations into clinical populations,” said Dr. Frøkjær, who was not involved in the research.
“It will also be important to understand if prucalopride adds to the effects of existing antidepressant treatments or can be used as a stand-alone therapy,” he added.
The study was funded by the NIHR Oxford Health Biomedical Research Center and by the Wellcome Center for Integrative Neuroscience. Ms. De Cates has received a travel grant from the Royal College of Psychiatrists/Gatsby Foundation and support from Wellcome. The other authors have relationships with P1vital Ltd, Janssen Pharmaceuticals, Sage Therapeutics, Pfizer, Zogenix, Compass Pathways, and Lundbeck.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a randomized controlled trial, 44 healthy individuals were assigned to receive the selective serotonin-4 (5-HT4) receptor agonist prucalopride (Motegrity) or placebo for 1 week.
After 6 days, the active-treatment group performed significantly better on memory tests than the participants who received placebo. In addition, the drug increased activity in brain areas related to cognition.
“What we’re hoping is...these agents may be able to help those with cognitive impairment as part of their mental illness,” lead author Angharad N. de Cates, a clinical DPhil student in the department of psychiatry, University of Oxford, Oxford, United Kingdom, told meeting attendees.
“Currently, we’re looking to see if we can translate our finding a step further and do a similar study in those with depression,” Ms. de Cates added.
The findings were presented at the 34th European College of Neuropsychopharmacology (ECNP) Congress and were simultaneously published in Translational Psychiatry.
“Exciting early evidence”
“Even when the low mood associated with depression is well-treated with conventional antidepressants, many patients continue to experience problems with their memory,” co-investigator Susannah Murphy, PhD, a senior research fellow at the University of Oxford, said in a release.
“Our study provides exciting early evidence in humans of a new approach that might be a helpful way to treat these residual cognitive symptoms,” Dr. Murphy added.
Preclinical and animal studies suggest that the 5-HT4 receptor is a promising treatment target for cognitive impairment in individuals with psychiatric disorders, although studies in humans have been limited by the adverse effects of early agents.
“We’ve had our eye on this receptor for a while,” explained de Cates, inasmuch as the animal data “have been so good.”
However, she said in an interview that “a lack of safe human agents made translation tricky.”
As previously reported, prucalopride, a selective high-affinity 5-HT4 partial agonist, was approved in 2018 by the U.S. Food and Drug Administration for the treatment of chronic idiopathic constipation.
The current researchers note that the drug has “good brain penetration,” which “allowed us to investigate 5-HT4-receptor agonism in humans.”
Having previously shown that a single dose of the drug has “pro-cognitive effects,” the investigators conducted the new trial in 44 healthy participants. All were randomly assigned in a 1:1 ratio to receive either prucalopride 1 mg for 7 days or placebo.
In accordance with enrollment criteria, patients were 18 to 36 years of age, right-handed, and were not pregnant or breastfeeding. Participants’ body mass index was 18 to 30 kg/m2, and they had no contraindications to the study drug. The two treatment groups were well balanced; the participants who received placebo were significantly more likely to be nonnative English speakers (P = .02).
On day 6 of treatment administration, all participants underwent 3T MRI.
Before undergoing imaging, the participants were presented with eight emotionally neutral images of animals or landscapes and were asked to indicate whether or not the images were of animals. The task was then repeated with the eight familiar images and eight novel ones.
During the scan, participants were shown the same images or eight novel images and were again asked whether or not the images contained an animal. They were also instructed to try to remember the images for a subsequent memory task. In that task, the eight original images, 48 novel images, and 27 “distractor” images were presented.
Better memory
In the pre-scan assessment, results showed no significant differences in the ability of members of the prucalopride and placebo groups to identify images as being familiar or different.
However, taking prucalopride was associated with significantly improved memory performance in the post-scan recall task.
Compared to the placebo group, participants in the prucalopride group were more accurate in selecting images as familiar vs distractors (P = .029) and in distinguishing images as familiar, novel, or distractors (P = .035).
Functional MRI revealed increased activity in the left and right hippocampus in response to both novel and familiar images among the participants in the prucalopride group in comparison with those in the placebo group.
There was also increased activity in the right angular gyrus in the prucalopride group in comparison with the placebo group in response to familiar images (P < .005).
“Clinically, angular gyri lesions cause language dysfunction, low mood, and poor memory and can mimic dementia or pseudodementia,” the investigators write. They note that the right angular gyrus “shows significantly decreased activity” in mild cognitive impairment.
“Therefore, the increased activity seen in the right angular gyrus following prucalopride administration in our study is consistent with the pro-cognitive behavioural effects we observed,” they add.
Ms. De Cates noted that the dose used in their study was lower than the 2 mg given for constipation.
“At the low dose, there were no differences in side effects between groups and no withdrawals from the prucalopride group for side effects. We are going to try increasing the dose in our next study actually, as we don’t have PET data to tell us what the optimal dose for binding at the receptor should be,” said Ms. de Cates.
“In safety studies, the dose was trialled in healthy volunteers at 4 mg, which was found to be safe, although perhaps less well tolerated than 2 mg,” she said.
Generalizable findings?
Commenting on the research, Vibe G. Frøkjær, MD, adjunct professor, department of psychology, Copenhagen University, Denmark, said the study “highlights a very interesting and much needed potential for repurposing drugs to help cognitive dysfunction.”
He noted that cognitive dysfunction is often associated with psychiatric disorders -- even in states of remission.
“Importantly, as the authors also state, it will be vital to translate these findings from healthy populations into clinical populations,” said Dr. Frøkjær, who was not involved in the research.
“It will also be important to understand if prucalopride adds to the effects of existing antidepressant treatments or can be used as a stand-alone therapy,” he added.
The study was funded by the NIHR Oxford Health Biomedical Research Center and by the Wellcome Center for Integrative Neuroscience. Ms. De Cates has received a travel grant from the Royal College of Psychiatrists/Gatsby Foundation and support from Wellcome. The other authors have relationships with P1vital Ltd, Janssen Pharmaceuticals, Sage Therapeutics, Pfizer, Zogenix, Compass Pathways, and Lundbeck.
A version of this article first appeared on Medscape.com.
, new research suggests.
In a randomized controlled trial, 44 healthy individuals were assigned to receive the selective serotonin-4 (5-HT4) receptor agonist prucalopride (Motegrity) or placebo for 1 week.
After 6 days, the active-treatment group performed significantly better on memory tests than the participants who received placebo. In addition, the drug increased activity in brain areas related to cognition.
“What we’re hoping is...these agents may be able to help those with cognitive impairment as part of their mental illness,” lead author Angharad N. de Cates, a clinical DPhil student in the department of psychiatry, University of Oxford, Oxford, United Kingdom, told meeting attendees.
“Currently, we’re looking to see if we can translate our finding a step further and do a similar study in those with depression,” Ms. de Cates added.
The findings were presented at the 34th European College of Neuropsychopharmacology (ECNP) Congress and were simultaneously published in Translational Psychiatry.
“Exciting early evidence”
“Even when the low mood associated with depression is well-treated with conventional antidepressants, many patients continue to experience problems with their memory,” co-investigator Susannah Murphy, PhD, a senior research fellow at the University of Oxford, said in a release.
“Our study provides exciting early evidence in humans of a new approach that might be a helpful way to treat these residual cognitive symptoms,” Dr. Murphy added.
Preclinical and animal studies suggest that the 5-HT4 receptor is a promising treatment target for cognitive impairment in individuals with psychiatric disorders, although studies in humans have been limited by the adverse effects of early agents.
“We’ve had our eye on this receptor for a while,” explained de Cates, inasmuch as the animal data “have been so good.”
However, she said in an interview that “a lack of safe human agents made translation tricky.”
As previously reported, prucalopride, a selective high-affinity 5-HT4 partial agonist, was approved in 2018 by the U.S. Food and Drug Administration for the treatment of chronic idiopathic constipation.
The current researchers note that the drug has “good brain penetration,” which “allowed us to investigate 5-HT4-receptor agonism in humans.”
Having previously shown that a single dose of the drug has “pro-cognitive effects,” the investigators conducted the new trial in 44 healthy participants. All were randomly assigned in a 1:1 ratio to receive either prucalopride 1 mg for 7 days or placebo.
In accordance with enrollment criteria, patients were 18 to 36 years of age, right-handed, and were not pregnant or breastfeeding. Participants’ body mass index was 18 to 30 kg/m2, and they had no contraindications to the study drug. The two treatment groups were well balanced; the participants who received placebo were significantly more likely to be nonnative English speakers (P = .02).
On day 6 of treatment administration, all participants underwent 3T MRI.
Before undergoing imaging, the participants were presented with eight emotionally neutral images of animals or landscapes and were asked to indicate whether or not the images were of animals. The task was then repeated with the eight familiar images and eight novel ones.
During the scan, participants were shown the same images or eight novel images and were again asked whether or not the images contained an animal. They were also instructed to try to remember the images for a subsequent memory task. In that task, the eight original images, 48 novel images, and 27 “distractor” images were presented.
Better memory
In the pre-scan assessment, results showed no significant differences in the ability of members of the prucalopride and placebo groups to identify images as being familiar or different.
However, taking prucalopride was associated with significantly improved memory performance in the post-scan recall task.
Compared to the placebo group, participants in the prucalopride group were more accurate in selecting images as familiar vs distractors (P = .029) and in distinguishing images as familiar, novel, or distractors (P = .035).
Functional MRI revealed increased activity in the left and right hippocampus in response to both novel and familiar images among the participants in the prucalopride group in comparison with those in the placebo group.
There was also increased activity in the right angular gyrus in the prucalopride group in comparison with the placebo group in response to familiar images (P < .005).
“Clinically, angular gyri lesions cause language dysfunction, low mood, and poor memory and can mimic dementia or pseudodementia,” the investigators write. They note that the right angular gyrus “shows significantly decreased activity” in mild cognitive impairment.
“Therefore, the increased activity seen in the right angular gyrus following prucalopride administration in our study is consistent with the pro-cognitive behavioural effects we observed,” they add.
Ms. De Cates noted that the dose used in their study was lower than the 2 mg given for constipation.
“At the low dose, there were no differences in side effects between groups and no withdrawals from the prucalopride group for side effects. We are going to try increasing the dose in our next study actually, as we don’t have PET data to tell us what the optimal dose for binding at the receptor should be,” said Ms. de Cates.
“In safety studies, the dose was trialled in healthy volunteers at 4 mg, which was found to be safe, although perhaps less well tolerated than 2 mg,” she said.
Generalizable findings?
Commenting on the research, Vibe G. Frøkjær, MD, adjunct professor, department of psychology, Copenhagen University, Denmark, said the study “highlights a very interesting and much needed potential for repurposing drugs to help cognitive dysfunction.”
He noted that cognitive dysfunction is often associated with psychiatric disorders -- even in states of remission.
“Importantly, as the authors also state, it will be vital to translate these findings from healthy populations into clinical populations,” said Dr. Frøkjær, who was not involved in the research.
“It will also be important to understand if prucalopride adds to the effects of existing antidepressant treatments or can be used as a stand-alone therapy,” he added.
The study was funded by the NIHR Oxford Health Biomedical Research Center and by the Wellcome Center for Integrative Neuroscience. Ms. De Cates has received a travel grant from the Royal College of Psychiatrists/Gatsby Foundation and support from Wellcome. The other authors have relationships with P1vital Ltd, Janssen Pharmaceuticals, Sage Therapeutics, Pfizer, Zogenix, Compass Pathways, and Lundbeck.
A version of this article first appeared on Medscape.com.















