User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Even those who just test positive at more risk for long COVID: CDC
Long-term symptoms, like those linked with COVID-19, were common in people who had even just a single positive test, new Centers for Disease Control and Prevention data show.
The data show that symptoms in this group – including fatigue, cough, and headache – tended to last for more than a month.
Frequency of symptoms in people with a positive test was 1.5 times higher, compared with people whose tests had always been negative, according to the research published in the CDC’s latest Morbidity and Mortality Weekly Report.
Lead author Valentine Wanga, PhD, with the CDC’s COVID-19 response team, and colleagues conducted a non–probability-based internet panel survey of about 6,000 U.S. adults to assess long-term symptoms often associated with COVID-19 among those who had ever tested positive or always tested negative for COVID-19 between January 2020 and April 2021.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., said in an interview that this research “establishes more securely than before that you don’t have to be hospitalized with COVID in order to develop long COVID symptoms.”
That’s better known among infectious disease experts, he said, but added that “this survey really gives a firm database for that.”
Study results
The study’s results showed that, compared with respondents who had a negative test result, those who received a positive result reported a significantly higher prevalence of any long-term symptom (65.9% vs. 42.9%), fatigue (22.5% vs. 12.0%), change in sense of smell or taste (17.3% vs. 1.7%), shortness of breath (15.5% vs. 5.2%), cough (14.5% vs. 4.9%), and headache (13.8% vs. 9.9%).
More people who had a positive test result (76.2%) reported persistence for more than a month of at least one initially occurring symptom, compared with those whose test results were always negative (69.6%).
The numbers are further proof, Dr. Schaffner said, that COVID not only will be an acute stressor on the health care system but patients with long COVID will need help with managing care for the long term.
“We still don’t know what the COVID virus does that results in these long COVID symptoms,” he said. Vanderbilt and many other institutions have developed “long COVID” centers as a testament to how important the problem is.
Long COVID symptoms are not well understood and most studies have looked at the effects from patients who had been hospitalized with COVID-19.
In this survey, respondents self-reported whether they had ever had a positive SARS-CoV-2 test result (698), always received a negative test result (2,437), or never were tested for SARS-CoV-2 (2,750).
Compared with those who always tested negative, a larger proportion of those who tested positive (28.7% vs. 15.7%) reported believing that receiving a COVID-19 vaccine made their long-term symptoms better. No difference was found in reported beliefs that a vaccine made long-term symptoms worse.
Dr. Schaffner said he found that survey result interesting, but said that is not backed up by current data and would need further study.
“I would treat that with great caution,” he said. “I’m not dismissing it, but you can’t take that at face value. All of us who get sick and those of us who care for people who are sick – if there’s an intervention, we all hope for the best. We’re being optimistic. It’s when you do a randomized, double-blind, placebo-controlled study that you can find out whether your instincts or hopes were correct.”
The authors said that findings can inform public health preparedness, help guide care for people with post-COVID conditions, and help make the case for vaccines.
The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term symptoms, like those linked with COVID-19, were common in people who had even just a single positive test, new Centers for Disease Control and Prevention data show.
The data show that symptoms in this group – including fatigue, cough, and headache – tended to last for more than a month.
Frequency of symptoms in people with a positive test was 1.5 times higher, compared with people whose tests had always been negative, according to the research published in the CDC’s latest Morbidity and Mortality Weekly Report.
Lead author Valentine Wanga, PhD, with the CDC’s COVID-19 response team, and colleagues conducted a non–probability-based internet panel survey of about 6,000 U.S. adults to assess long-term symptoms often associated with COVID-19 among those who had ever tested positive or always tested negative for COVID-19 between January 2020 and April 2021.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., said in an interview that this research “establishes more securely than before that you don’t have to be hospitalized with COVID in order to develop long COVID symptoms.”
That’s better known among infectious disease experts, he said, but added that “this survey really gives a firm database for that.”
Study results
The study’s results showed that, compared with respondents who had a negative test result, those who received a positive result reported a significantly higher prevalence of any long-term symptom (65.9% vs. 42.9%), fatigue (22.5% vs. 12.0%), change in sense of smell or taste (17.3% vs. 1.7%), shortness of breath (15.5% vs. 5.2%), cough (14.5% vs. 4.9%), and headache (13.8% vs. 9.9%).
More people who had a positive test result (76.2%) reported persistence for more than a month of at least one initially occurring symptom, compared with those whose test results were always negative (69.6%).
The numbers are further proof, Dr. Schaffner said, that COVID not only will be an acute stressor on the health care system but patients with long COVID will need help with managing care for the long term.
“We still don’t know what the COVID virus does that results in these long COVID symptoms,” he said. Vanderbilt and many other institutions have developed “long COVID” centers as a testament to how important the problem is.
Long COVID symptoms are not well understood and most studies have looked at the effects from patients who had been hospitalized with COVID-19.
In this survey, respondents self-reported whether they had ever had a positive SARS-CoV-2 test result (698), always received a negative test result (2,437), or never were tested for SARS-CoV-2 (2,750).
Compared with those who always tested negative, a larger proportion of those who tested positive (28.7% vs. 15.7%) reported believing that receiving a COVID-19 vaccine made their long-term symptoms better. No difference was found in reported beliefs that a vaccine made long-term symptoms worse.
Dr. Schaffner said he found that survey result interesting, but said that is not backed up by current data and would need further study.
“I would treat that with great caution,” he said. “I’m not dismissing it, but you can’t take that at face value. All of us who get sick and those of us who care for people who are sick – if there’s an intervention, we all hope for the best. We’re being optimistic. It’s when you do a randomized, double-blind, placebo-controlled study that you can find out whether your instincts or hopes were correct.”
The authors said that findings can inform public health preparedness, help guide care for people with post-COVID conditions, and help make the case for vaccines.
The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Long-term symptoms, like those linked with COVID-19, were common in people who had even just a single positive test, new Centers for Disease Control and Prevention data show.
The data show that symptoms in this group – including fatigue, cough, and headache – tended to last for more than a month.
Frequency of symptoms in people with a positive test was 1.5 times higher, compared with people whose tests had always been negative, according to the research published in the CDC’s latest Morbidity and Mortality Weekly Report.
Lead author Valentine Wanga, PhD, with the CDC’s COVID-19 response team, and colleagues conducted a non–probability-based internet panel survey of about 6,000 U.S. adults to assess long-term symptoms often associated with COVID-19 among those who had ever tested positive or always tested negative for COVID-19 between January 2020 and April 2021.
William Schaffner, MD, an infectious disease expert at Vanderbilt University, Nashville, Tenn., said in an interview that this research “establishes more securely than before that you don’t have to be hospitalized with COVID in order to develop long COVID symptoms.”
That’s better known among infectious disease experts, he said, but added that “this survey really gives a firm database for that.”
Study results
The study’s results showed that, compared with respondents who had a negative test result, those who received a positive result reported a significantly higher prevalence of any long-term symptom (65.9% vs. 42.9%), fatigue (22.5% vs. 12.0%), change in sense of smell or taste (17.3% vs. 1.7%), shortness of breath (15.5% vs. 5.2%), cough (14.5% vs. 4.9%), and headache (13.8% vs. 9.9%).
More people who had a positive test result (76.2%) reported persistence for more than a month of at least one initially occurring symptom, compared with those whose test results were always negative (69.6%).
The numbers are further proof, Dr. Schaffner said, that COVID not only will be an acute stressor on the health care system but patients with long COVID will need help with managing care for the long term.
“We still don’t know what the COVID virus does that results in these long COVID symptoms,” he said. Vanderbilt and many other institutions have developed “long COVID” centers as a testament to how important the problem is.
Long COVID symptoms are not well understood and most studies have looked at the effects from patients who had been hospitalized with COVID-19.
In this survey, respondents self-reported whether they had ever had a positive SARS-CoV-2 test result (698), always received a negative test result (2,437), or never were tested for SARS-CoV-2 (2,750).
Compared with those who always tested negative, a larger proportion of those who tested positive (28.7% vs. 15.7%) reported believing that receiving a COVID-19 vaccine made their long-term symptoms better. No difference was found in reported beliefs that a vaccine made long-term symptoms worse.
Dr. Schaffner said he found that survey result interesting, but said that is not backed up by current data and would need further study.
“I would treat that with great caution,” he said. “I’m not dismissing it, but you can’t take that at face value. All of us who get sick and those of us who care for people who are sick – if there’s an intervention, we all hope for the best. We’re being optimistic. It’s when you do a randomized, double-blind, placebo-controlled study that you can find out whether your instincts or hopes were correct.”
The authors said that findings can inform public health preparedness, help guide care for people with post-COVID conditions, and help make the case for vaccines.
The study authors and Dr. Schaffner disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Seizure a first sign of COVID in kids?
Unlike in adults, seizures, including status epilepticus (SE), may be the first and main manifestation of COVID-19 in children, new research suggests.
Seizures may occur even in children with no history of epilepsy and in the absence of fever or severe COVID-19 illness, necessitating a “high index” of suspicion for the virus to make an early diagnosis and allow for appropriate infection control measures, say the researchers.
“We hope to increase physicians’ awareness of noninfluenza-like presentations of COVID in children. In areas with a high prevalence of COVID, we suggest that children with seizures be tested for COVID,” Tal Gilboa, MD, director of the child neurology unit and codirector of epilepsy, Hadassah Medical Center, Jerusalem, told this news organization.
The study was published online August 27 in the journal Seizure.
Presenting symptom
Among 175 children diagnosed with acute SARS-CoV-2 infection in the emergency department over 10 months in 2020, 11 (6%) presented with seizures. Studies in adults with COVID-19 have reported seizures in 0% to 2% of cases, the investigators note.
The 11 children with seizures (seven boys) ranged in age from 6 months to 17 years (median age, 11.5 years). All of them had seizures as the presenting sign of infection and none had severe COVID-19 requiring ventilatory or hemodynamic support. Six of the 11 children presented with fever.
Seven of the children had a prior history of neurological disorder: Five had epilepsy, one had a single unprovoked seizure 3 years before admission, and one had an intellectual disability. Three of the children had uncontrolled seizures despite appropriate treatment with antiseizure medication.
Nine of the 11 children presented with generalized tonic-clonic seizures. One child with a prior history of uncontrolled epilepsy with multiple seizure types had a focal tonic seizure. The youngest patient, a 5-month-old infant, presented with bilateral asymmetrical tonic-clonic seizure.
Of note, say the investigators, five of the 11 children presented with convulsive SE; none had a history of prior SE, and one had no history of seizures.
Although young age, especially under 12 months, is a known risk factor for SE, four of the five patients with SE were between 5 and 17 years old. All five children with SE responded to treatment with antiseizure medications.
All 11 children made a full recovery while in hospital, although further follow-up is essential to determine long-term outcomes, the researchers report.
“Children with no prior history of epilepsy and those with well-controlled epilepsy who present with breakthrough seizures, regardless of their body temperature, should be considered as potentially infected by SARS-CoV-2,” said Dr. Gilboa.
“It is possible, however unlikely, that a child, especially with prior epilepsy, may have an unprovoked seizure while being asymptomatically infected by SARS-CoV-2; in any case, infection control measures should be taken,” Dr. Gilboa added.
Need for replication
Weighing in on the study, Carl E. Stafstrom, MD, PhD, professor of neurology and pediatrics, Johns Hopkins University, Baltimore, said it’s important to note that “about half of the children had had epilepsy already, and for whatever reason, had a seizure, which required an ED visit, and then they found COVID.”
“Nevertheless, this article is interesting and surprising in what they found because nobody else has found nearly as frequent a seizure presentation,” said Dr. Stafstrom, director of the John M. Freeman Pediatric Epilepsy Center, Johns Hopkins Medicine.
“We would want to see some replication from other institutions and other populations,” he added.
The study had no specific funding. Dr. Gilboa and Dr. Stafstrom have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unlike in adults, seizures, including status epilepticus (SE), may be the first and main manifestation of COVID-19 in children, new research suggests.
Seizures may occur even in children with no history of epilepsy and in the absence of fever or severe COVID-19 illness, necessitating a “high index” of suspicion for the virus to make an early diagnosis and allow for appropriate infection control measures, say the researchers.
“We hope to increase physicians’ awareness of noninfluenza-like presentations of COVID in children. In areas with a high prevalence of COVID, we suggest that children with seizures be tested for COVID,” Tal Gilboa, MD, director of the child neurology unit and codirector of epilepsy, Hadassah Medical Center, Jerusalem, told this news organization.
The study was published online August 27 in the journal Seizure.
Presenting symptom
Among 175 children diagnosed with acute SARS-CoV-2 infection in the emergency department over 10 months in 2020, 11 (6%) presented with seizures. Studies in adults with COVID-19 have reported seizures in 0% to 2% of cases, the investigators note.
The 11 children with seizures (seven boys) ranged in age from 6 months to 17 years (median age, 11.5 years). All of them had seizures as the presenting sign of infection and none had severe COVID-19 requiring ventilatory or hemodynamic support. Six of the 11 children presented with fever.
Seven of the children had a prior history of neurological disorder: Five had epilepsy, one had a single unprovoked seizure 3 years before admission, and one had an intellectual disability. Three of the children had uncontrolled seizures despite appropriate treatment with antiseizure medication.
Nine of the 11 children presented with generalized tonic-clonic seizures. One child with a prior history of uncontrolled epilepsy with multiple seizure types had a focal tonic seizure. The youngest patient, a 5-month-old infant, presented with bilateral asymmetrical tonic-clonic seizure.
Of note, say the investigators, five of the 11 children presented with convulsive SE; none had a history of prior SE, and one had no history of seizures.
Although young age, especially under 12 months, is a known risk factor for SE, four of the five patients with SE were between 5 and 17 years old. All five children with SE responded to treatment with antiseizure medications.
All 11 children made a full recovery while in hospital, although further follow-up is essential to determine long-term outcomes, the researchers report.
“Children with no prior history of epilepsy and those with well-controlled epilepsy who present with breakthrough seizures, regardless of their body temperature, should be considered as potentially infected by SARS-CoV-2,” said Dr. Gilboa.
“It is possible, however unlikely, that a child, especially with prior epilepsy, may have an unprovoked seizure while being asymptomatically infected by SARS-CoV-2; in any case, infection control measures should be taken,” Dr. Gilboa added.
Need for replication
Weighing in on the study, Carl E. Stafstrom, MD, PhD, professor of neurology and pediatrics, Johns Hopkins University, Baltimore, said it’s important to note that “about half of the children had had epilepsy already, and for whatever reason, had a seizure, which required an ED visit, and then they found COVID.”
“Nevertheless, this article is interesting and surprising in what they found because nobody else has found nearly as frequent a seizure presentation,” said Dr. Stafstrom, director of the John M. Freeman Pediatric Epilepsy Center, Johns Hopkins Medicine.
“We would want to see some replication from other institutions and other populations,” he added.
The study had no specific funding. Dr. Gilboa and Dr. Stafstrom have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Unlike in adults, seizures, including status epilepticus (SE), may be the first and main manifestation of COVID-19 in children, new research suggests.
Seizures may occur even in children with no history of epilepsy and in the absence of fever or severe COVID-19 illness, necessitating a “high index” of suspicion for the virus to make an early diagnosis and allow for appropriate infection control measures, say the researchers.
“We hope to increase physicians’ awareness of noninfluenza-like presentations of COVID in children. In areas with a high prevalence of COVID, we suggest that children with seizures be tested for COVID,” Tal Gilboa, MD, director of the child neurology unit and codirector of epilepsy, Hadassah Medical Center, Jerusalem, told this news organization.
The study was published online August 27 in the journal Seizure.
Presenting symptom
Among 175 children diagnosed with acute SARS-CoV-2 infection in the emergency department over 10 months in 2020, 11 (6%) presented with seizures. Studies in adults with COVID-19 have reported seizures in 0% to 2% of cases, the investigators note.
The 11 children with seizures (seven boys) ranged in age from 6 months to 17 years (median age, 11.5 years). All of them had seizures as the presenting sign of infection and none had severe COVID-19 requiring ventilatory or hemodynamic support. Six of the 11 children presented with fever.
Seven of the children had a prior history of neurological disorder: Five had epilepsy, one had a single unprovoked seizure 3 years before admission, and one had an intellectual disability. Three of the children had uncontrolled seizures despite appropriate treatment with antiseizure medication.
Nine of the 11 children presented with generalized tonic-clonic seizures. One child with a prior history of uncontrolled epilepsy with multiple seizure types had a focal tonic seizure. The youngest patient, a 5-month-old infant, presented with bilateral asymmetrical tonic-clonic seizure.
Of note, say the investigators, five of the 11 children presented with convulsive SE; none had a history of prior SE, and one had no history of seizures.
Although young age, especially under 12 months, is a known risk factor for SE, four of the five patients with SE were between 5 and 17 years old. All five children with SE responded to treatment with antiseizure medications.
All 11 children made a full recovery while in hospital, although further follow-up is essential to determine long-term outcomes, the researchers report.
“Children with no prior history of epilepsy and those with well-controlled epilepsy who present with breakthrough seizures, regardless of their body temperature, should be considered as potentially infected by SARS-CoV-2,” said Dr. Gilboa.
“It is possible, however unlikely, that a child, especially with prior epilepsy, may have an unprovoked seizure while being asymptomatically infected by SARS-CoV-2; in any case, infection control measures should be taken,” Dr. Gilboa added.
Need for replication
Weighing in on the study, Carl E. Stafstrom, MD, PhD, professor of neurology and pediatrics, Johns Hopkins University, Baltimore, said it’s important to note that “about half of the children had had epilepsy already, and for whatever reason, had a seizure, which required an ED visit, and then they found COVID.”
“Nevertheless, this article is interesting and surprising in what they found because nobody else has found nearly as frequent a seizure presentation,” said Dr. Stafstrom, director of the John M. Freeman Pediatric Epilepsy Center, Johns Hopkins Medicine.
“We would want to see some replication from other institutions and other populations,” he added.
The study had no specific funding. Dr. Gilboa and Dr. Stafstrom have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Medical boards: Docs who spread COVID misinformation put license at risk
Leaders of the American Board of Family Medicine, the American Board of Internal Medicine, and the American Board of Pediatrics said Sept. 9 that they support FSMB’s position.
“We also want all physicians certified by our boards to know that such unethical or unprofessional conduct may prompt their respective Board to take action that could put their certification at risk,” a statement read.
“Expertise matters, and board-certified physicians have demonstrated that they have stayed current in their field. Spreading misinformation or falsehoods to the public during a time of a public health emergency goes against everything our boards and our community of board-certified physicians stand for,” the leaders wrote.
“The evidence that we have safe, effective, and widely available vaccines against COVID-19 is overwhelming. We are particularly concerned about physicians who use their authority to denigrate vaccination at a time when vaccines continue to demonstrate excellent effectiveness against severe illness, hospitalization, and death.”
Small number spread false information
However, a small number of doctors continue to spread misinformation against the vaccines and communicate other false information surrounding COVID-19.
Some of the misinformation spreaders have had ultra-viral reach.
Among them is Daniel Stock, MD, a family physician in Indiana who has come out against COVID-19 vaccines. At a recent meeting of the Mt. Vernon Community School board in Indiana, he gave a speech urging the board to ignore the prevailing recommendations around COVID-19, such as test-and-trace measures.
Forbes reported in August that versions of the video of Stock›s speech on Facebook “have collected a total of 90 million engagements – a metric encompassing things such as comments, likes and shares – according to data collected by Media Matters for America, a liberal tech-watchdog group.”
This news organization published a story in August asking whether physicians who spread such information should lose their license and the question drew rapid-fire comments.
Commenters who argued with potential disciplinary actions raised questions about where the line will be drawn between misinformation and deeply held beliefs in terms of care.
Several comments centered on ivermectin, which is not approved by the Food and Drug Administration to treat COVID-19 but is enthusiastically supported as a COVID-19 treatment by a group of physicians called the Front Line COVID-19 Critical Care Alliance, whose website includes requests for donations.
Some cited free speech protections.
‘Not consistent with standards’
As for ivermectin, David G. Nichols, MD, president and CEO of the American Board of Pediatrics, gave this news organization an example: “Spreading the notion that one would not need to get vaccinated because if you get sick you could take ivermectin is a very dangerous statement. That is not consistent with the standards of professionalism required for certification or licensure.”
Ivermectin, he noted, is not an approved treatment for COVID-19.
“To say that it is or has any benefit is a false statement. We’re not willing to allow individuals who make false statements to devalue the terrific work of tens of thousands of physicians across the United States doing work under very difficult circumstances,” Dr. Nichols said.
He continued: “To suggest treatments that are known not to be effective in exchange for treatment that is known to be effective is dangerous – and ivermectin falls under that category.”
Asked whether such suggestions could result in suspension or revocation of a physician’s license, Dr. Nichols said, “It’s the kind of thing that would certainly trigger a review.”
He said the standard for separating misinformation from personal beliefs is based on whether there is scientific evidence to support the belief.
The boards are not, with this statement, attempting to referee legitimate scientific debate, he said.
The misinformation the boards are referring to, Dr. Nichols said, is “where the evidence is 100% on one side and zero on another. And the zero is not only that the opinions or beliefs are unsupported or unsubstantiated, they are indeed harmful if followed. That’s the distinction we’re trying to make here.”
As for free-speech arguments, he said, “Free speech is a constitutional right. You can say whatever you want. The issue here is you do not have the right to expect continued professional sanction of a board certificate if you are lying to the public.”
The board statement also said: “We all look to board-certified physicians to provide outstanding care and guidance; providing misinformation about a lethal disease is unethical, unprofessional, and dangerous. In times of medical emergency, the community of expert physicians committed to science and evidence collectively shares a responsibility for giving the public the most accurate and timely health information available, so they can make decisions that work best for themselves and their families.”
In addition to Dr. Nichols, the statement was signed by Warren Newton, MD, MPH, president and CEO of the American Board of Family Medicine, and Richard J. Baron, MD, president and CEO of the American Board of Internal Medicine.
A version of this article first appeared on Medscape.com.
Leaders of the American Board of Family Medicine, the American Board of Internal Medicine, and the American Board of Pediatrics said Sept. 9 that they support FSMB’s position.
“We also want all physicians certified by our boards to know that such unethical or unprofessional conduct may prompt their respective Board to take action that could put their certification at risk,” a statement read.
“Expertise matters, and board-certified physicians have demonstrated that they have stayed current in their field. Spreading misinformation or falsehoods to the public during a time of a public health emergency goes against everything our boards and our community of board-certified physicians stand for,” the leaders wrote.
“The evidence that we have safe, effective, and widely available vaccines against COVID-19 is overwhelming. We are particularly concerned about physicians who use their authority to denigrate vaccination at a time when vaccines continue to demonstrate excellent effectiveness against severe illness, hospitalization, and death.”
Small number spread false information
However, a small number of doctors continue to spread misinformation against the vaccines and communicate other false information surrounding COVID-19.
Some of the misinformation spreaders have had ultra-viral reach.
Among them is Daniel Stock, MD, a family physician in Indiana who has come out against COVID-19 vaccines. At a recent meeting of the Mt. Vernon Community School board in Indiana, he gave a speech urging the board to ignore the prevailing recommendations around COVID-19, such as test-and-trace measures.
Forbes reported in August that versions of the video of Stock›s speech on Facebook “have collected a total of 90 million engagements – a metric encompassing things such as comments, likes and shares – according to data collected by Media Matters for America, a liberal tech-watchdog group.”
This news organization published a story in August asking whether physicians who spread such information should lose their license and the question drew rapid-fire comments.
Commenters who argued with potential disciplinary actions raised questions about where the line will be drawn between misinformation and deeply held beliefs in terms of care.
Several comments centered on ivermectin, which is not approved by the Food and Drug Administration to treat COVID-19 but is enthusiastically supported as a COVID-19 treatment by a group of physicians called the Front Line COVID-19 Critical Care Alliance, whose website includes requests for donations.
Some cited free speech protections.
‘Not consistent with standards’
As for ivermectin, David G. Nichols, MD, president and CEO of the American Board of Pediatrics, gave this news organization an example: “Spreading the notion that one would not need to get vaccinated because if you get sick you could take ivermectin is a very dangerous statement. That is not consistent with the standards of professionalism required for certification or licensure.”
Ivermectin, he noted, is not an approved treatment for COVID-19.
“To say that it is or has any benefit is a false statement. We’re not willing to allow individuals who make false statements to devalue the terrific work of tens of thousands of physicians across the United States doing work under very difficult circumstances,” Dr. Nichols said.
He continued: “To suggest treatments that are known not to be effective in exchange for treatment that is known to be effective is dangerous – and ivermectin falls under that category.”
Asked whether such suggestions could result in suspension or revocation of a physician’s license, Dr. Nichols said, “It’s the kind of thing that would certainly trigger a review.”
He said the standard for separating misinformation from personal beliefs is based on whether there is scientific evidence to support the belief.
The boards are not, with this statement, attempting to referee legitimate scientific debate, he said.
The misinformation the boards are referring to, Dr. Nichols said, is “where the evidence is 100% on one side and zero on another. And the zero is not only that the opinions or beliefs are unsupported or unsubstantiated, they are indeed harmful if followed. That’s the distinction we’re trying to make here.”
As for free-speech arguments, he said, “Free speech is a constitutional right. You can say whatever you want. The issue here is you do not have the right to expect continued professional sanction of a board certificate if you are lying to the public.”
The board statement also said: “We all look to board-certified physicians to provide outstanding care and guidance; providing misinformation about a lethal disease is unethical, unprofessional, and dangerous. In times of medical emergency, the community of expert physicians committed to science and evidence collectively shares a responsibility for giving the public the most accurate and timely health information available, so they can make decisions that work best for themselves and their families.”
In addition to Dr. Nichols, the statement was signed by Warren Newton, MD, MPH, president and CEO of the American Board of Family Medicine, and Richard J. Baron, MD, president and CEO of the American Board of Internal Medicine.
A version of this article first appeared on Medscape.com.
Leaders of the American Board of Family Medicine, the American Board of Internal Medicine, and the American Board of Pediatrics said Sept. 9 that they support FSMB’s position.
“We also want all physicians certified by our boards to know that such unethical or unprofessional conduct may prompt their respective Board to take action that could put their certification at risk,” a statement read.
“Expertise matters, and board-certified physicians have demonstrated that they have stayed current in their field. Spreading misinformation or falsehoods to the public during a time of a public health emergency goes against everything our boards and our community of board-certified physicians stand for,” the leaders wrote.
“The evidence that we have safe, effective, and widely available vaccines against COVID-19 is overwhelming. We are particularly concerned about physicians who use their authority to denigrate vaccination at a time when vaccines continue to demonstrate excellent effectiveness against severe illness, hospitalization, and death.”
Small number spread false information
However, a small number of doctors continue to spread misinformation against the vaccines and communicate other false information surrounding COVID-19.
Some of the misinformation spreaders have had ultra-viral reach.
Among them is Daniel Stock, MD, a family physician in Indiana who has come out against COVID-19 vaccines. At a recent meeting of the Mt. Vernon Community School board in Indiana, he gave a speech urging the board to ignore the prevailing recommendations around COVID-19, such as test-and-trace measures.
Forbes reported in August that versions of the video of Stock›s speech on Facebook “have collected a total of 90 million engagements – a metric encompassing things such as comments, likes and shares – according to data collected by Media Matters for America, a liberal tech-watchdog group.”
This news organization published a story in August asking whether physicians who spread such information should lose their license and the question drew rapid-fire comments.
Commenters who argued with potential disciplinary actions raised questions about where the line will be drawn between misinformation and deeply held beliefs in terms of care.
Several comments centered on ivermectin, which is not approved by the Food and Drug Administration to treat COVID-19 but is enthusiastically supported as a COVID-19 treatment by a group of physicians called the Front Line COVID-19 Critical Care Alliance, whose website includes requests for donations.
Some cited free speech protections.
‘Not consistent with standards’
As for ivermectin, David G. Nichols, MD, president and CEO of the American Board of Pediatrics, gave this news organization an example: “Spreading the notion that one would not need to get vaccinated because if you get sick you could take ivermectin is a very dangerous statement. That is not consistent with the standards of professionalism required for certification or licensure.”
Ivermectin, he noted, is not an approved treatment for COVID-19.
“To say that it is or has any benefit is a false statement. We’re not willing to allow individuals who make false statements to devalue the terrific work of tens of thousands of physicians across the United States doing work under very difficult circumstances,” Dr. Nichols said.
He continued: “To suggest treatments that are known not to be effective in exchange for treatment that is known to be effective is dangerous – and ivermectin falls under that category.”
Asked whether such suggestions could result in suspension or revocation of a physician’s license, Dr. Nichols said, “It’s the kind of thing that would certainly trigger a review.”
He said the standard for separating misinformation from personal beliefs is based on whether there is scientific evidence to support the belief.
The boards are not, with this statement, attempting to referee legitimate scientific debate, he said.
The misinformation the boards are referring to, Dr. Nichols said, is “where the evidence is 100% on one side and zero on another. And the zero is not only that the opinions or beliefs are unsupported or unsubstantiated, they are indeed harmful if followed. That’s the distinction we’re trying to make here.”
As for free-speech arguments, he said, “Free speech is a constitutional right. You can say whatever you want. The issue here is you do not have the right to expect continued professional sanction of a board certificate if you are lying to the public.”
The board statement also said: “We all look to board-certified physicians to provide outstanding care and guidance; providing misinformation about a lethal disease is unethical, unprofessional, and dangerous. In times of medical emergency, the community of expert physicians committed to science and evidence collectively shares a responsibility for giving the public the most accurate and timely health information available, so they can make decisions that work best for themselves and their families.”
In addition to Dr. Nichols, the statement was signed by Warren Newton, MD, MPH, president and CEO of the American Board of Family Medicine, and Richard J. Baron, MD, president and CEO of the American Board of Internal Medicine.
A version of this article first appeared on Medscape.com.
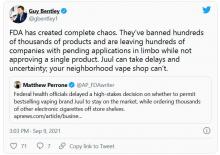
FDA moves to block some vape products, delays action on Juul
The agency had a court-ordered deadline of Sept. 9 to review more than 6.5 million applications for approval of what are considered new tobacco products – the vast majority of which are e-cigarettes and liquids, none of which have gone through FDA review before.
The FDA reviewed 93% of those applications in the past year, acting FDA Commissioner Janet Woodcock, MD, and Mitch Zeller, director of the FDA’s Center for Tobacco Products, said in a statement.
Of those reviewed, the agency rejected more than 946,000 flavored vape products, “because their applications lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health threat posed by the well-documented, alarming levels of youth use of such products,” Dr. Woodcock and Mr. Zeller said.
The pair said more work is needed to finish the reviews to “ensure that we continue taking appropriate action to protect our nation’s youth from the dangers of all tobacco products, including e-cigarettes, which remain the most commonly used tobacco product by youth in the United States.”
No e-cigarette product has been given official FDA approval to be sold, meaning all e-cigarette products technically are on the market illegally, the agency said in 2020, but federal officials decided only to begin enforcing rules against flavored products, which surveys show are more often used by children. Tobacco-flavored and menthol e-cigarette products – which some adults use to quit smoking cigarettes – were exempted.
The American Cancer Society and other advocacy groups slammed the FDA’s decision to withhold action on major e-cigarette manufacturers, including Juul.
“The FDA’s failure today to act on applications by Juul, the manufacturer with the single biggest e-cigarette market share, is extremely disappointing and will allow the industry to further endanger public health and hook more kids on their highly addictive products,” Lisa Lacasse, president of ACS CAN, said in a statement, according to CNN.
“The FDA has had ample time to review the applications and allowing additional delays is unconscionable. There is overwhelming data to demonstrate the negative impact these kinds of flavored products have had on public health and their role in the youth e-cigarette epidemic. The time to act is now,” Ms. Lacasse added.
E-cigarette use among high school students rose from 11.7% in 2017 to 19.6% in 2020, the American Cancer Society said. Nearly 5% of middle schoolers reported using them in 2020.
A version of this article first appeared on WebMD.com.
The agency had a court-ordered deadline of Sept. 9 to review more than 6.5 million applications for approval of what are considered new tobacco products – the vast majority of which are e-cigarettes and liquids, none of which have gone through FDA review before.
The FDA reviewed 93% of those applications in the past year, acting FDA Commissioner Janet Woodcock, MD, and Mitch Zeller, director of the FDA’s Center for Tobacco Products, said in a statement.
Of those reviewed, the agency rejected more than 946,000 flavored vape products, “because their applications lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health threat posed by the well-documented, alarming levels of youth use of such products,” Dr. Woodcock and Mr. Zeller said.
The pair said more work is needed to finish the reviews to “ensure that we continue taking appropriate action to protect our nation’s youth from the dangers of all tobacco products, including e-cigarettes, which remain the most commonly used tobacco product by youth in the United States.”
No e-cigarette product has been given official FDA approval to be sold, meaning all e-cigarette products technically are on the market illegally, the agency said in 2020, but federal officials decided only to begin enforcing rules against flavored products, which surveys show are more often used by children. Tobacco-flavored and menthol e-cigarette products – which some adults use to quit smoking cigarettes – were exempted.
The American Cancer Society and other advocacy groups slammed the FDA’s decision to withhold action on major e-cigarette manufacturers, including Juul.
“The FDA’s failure today to act on applications by Juul, the manufacturer with the single biggest e-cigarette market share, is extremely disappointing and will allow the industry to further endanger public health and hook more kids on their highly addictive products,” Lisa Lacasse, president of ACS CAN, said in a statement, according to CNN.
“The FDA has had ample time to review the applications and allowing additional delays is unconscionable. There is overwhelming data to demonstrate the negative impact these kinds of flavored products have had on public health and their role in the youth e-cigarette epidemic. The time to act is now,” Ms. Lacasse added.
E-cigarette use among high school students rose from 11.7% in 2017 to 19.6% in 2020, the American Cancer Society said. Nearly 5% of middle schoolers reported using them in 2020.
A version of this article first appeared on WebMD.com.
The agency had a court-ordered deadline of Sept. 9 to review more than 6.5 million applications for approval of what are considered new tobacco products – the vast majority of which are e-cigarettes and liquids, none of which have gone through FDA review before.
The FDA reviewed 93% of those applications in the past year, acting FDA Commissioner Janet Woodcock, MD, and Mitch Zeller, director of the FDA’s Center for Tobacco Products, said in a statement.
Of those reviewed, the agency rejected more than 946,000 flavored vape products, “because their applications lacked sufficient evidence that they have a benefit to adult smokers sufficient to overcome the public health threat posed by the well-documented, alarming levels of youth use of such products,” Dr. Woodcock and Mr. Zeller said.
The pair said more work is needed to finish the reviews to “ensure that we continue taking appropriate action to protect our nation’s youth from the dangers of all tobacco products, including e-cigarettes, which remain the most commonly used tobacco product by youth in the United States.”
No e-cigarette product has been given official FDA approval to be sold, meaning all e-cigarette products technically are on the market illegally, the agency said in 2020, but federal officials decided only to begin enforcing rules against flavored products, which surveys show are more often used by children. Tobacco-flavored and menthol e-cigarette products – which some adults use to quit smoking cigarettes – were exempted.
The American Cancer Society and other advocacy groups slammed the FDA’s decision to withhold action on major e-cigarette manufacturers, including Juul.
“The FDA’s failure today to act on applications by Juul, the manufacturer with the single biggest e-cigarette market share, is extremely disappointing and will allow the industry to further endanger public health and hook more kids on their highly addictive products,” Lisa Lacasse, president of ACS CAN, said in a statement, according to CNN.
“The FDA has had ample time to review the applications and allowing additional delays is unconscionable. There is overwhelming data to demonstrate the negative impact these kinds of flavored products have had on public health and their role in the youth e-cigarette epidemic. The time to act is now,” Ms. Lacasse added.
E-cigarette use among high school students rose from 11.7% in 2017 to 19.6% in 2020, the American Cancer Society said. Nearly 5% of middle schoolers reported using them in 2020.
A version of this article first appeared on WebMD.com.
COVID-19 spares lung function in young adults
Here’s some encouraging news for once regarding SARS-CoV-2 infections: A study of young adults for whom prepandemic spirometry data were available showed that COVID-19 did not have a significant impact on lung function, even among patients with asthma.
Among 853 Swedish men and women (mean age, 22 years) who were part of a birth cohort study, there were no significant differences in either forced expiratory volume in 1 second (FEV1) or in the ratio of FEV1 to forced vital capacity, reported Ida Mogensen, MD, PhD, a postdoctoral fellow at the Karolinska Institute in Stockholm.
“We found no effect of COVID-19 on spirometric lung function in generally healthy adults,” she said in an oral abstract presented at the European Respiratory Society 2021 International Congress.
The findings echo those of a small study that involved 73 children and adolescents with COVID-19 and 45 uninfected control persons. The investigators in that study, which was also presented at ERS 2021, found that there were no significant differences in the frequency of abnormal pulmonary function measures between case patients and control patients (abstract OA1303).
“The findings from these two studies provide important reassurance about the impact of COVID infection on lung function in children and young adults,” commented Anita Simonds, MD, an honorary consultant in respiratory and sleep medicine at the Royal Brompton Hospital, London.
“We know already that this group is less likely to suffer severe illness if they contract the virus, and these studies, which importantly include comparator groups without COVID-19, show that they are also less likely to suffer long-term consequences with respect to lung function,” she said. Dr. Simonds was not involved in either study.
Young adult study
Dr. Mogenson and colleagues assessed data on 853 participants in the BAMSE Project, a prospective birth cohort study that included 4,089 children born in Stockholm from 1994 to 1996. Of the participants, 147 had asthma. They have been regularly followed with questionnaires on respiratory symptoms and medications. In addition, at 8 and 16 years’ follow-up, spirometry measures and fractional exhaled nitric oxide (FeNO) levels were assessed, allergic sensitization tests were administered, and blood eosinophil levels were measured.
In 2020 and 2021, during the pandemic, the participants underwent spirometry testing and were assessed for antibodies against SARS-CoV-2, and they self-reported use of inhaled corticosteroids.
The investigators defined asthma as any physician diagnosis and asthma symptoms and/or asthma medication use within the previous year. Participants were determined to be COVID-19 seropositive if they had IgG antibodies to the SARS-CoV-2 spike greater than 25.09 AU/mL, IgM antibodies greater than 14.42 AU/mL, or IgA antibodies greater than 2.61 AU/mL, as measured with enzyme-linked immunosorbent assay.
Participants who had been vaccinated against COVID-19 were excluded.
No significant decreases
A total of 243 participants, including 38 with asthma, were seropositive for SARS-CoV-2 antibodies. The mean change in lung function from before the pandemic to the study end date during the pandemic were not significantly different between seropositive participants and seronegative participants or IgM-positive participants and seronegative participants.
Similarly, there were no significant differences in lung function between seropositive and seronegative participants in an analysis that was adjusted for sex, body mass index, smoking status, or prepandemic lung function.
Although there was a trend toward slightly lower function among seropositive participants with asthma in comparison with seronegative patients with asthma, it was not statistically significant, Dr. Mogenson said.
There were also no significant decreases in lung function from the prepandemic measure to the present in any of the inflammatory parameters, including blood eosinophil levels, FeNO, allergic sensitization, or inhaled corticosteroid use.
Potential misclassification
In the question-and-answer period that followed the presentation, session comoderator Sam Bayat, MD, PhD, from the University of Grenoble (France), who was not involved in the study, noted that “some subjects can have positive serology without any symptoms, while others can have symptomatic disease and a couple of months later they have negative serology.”
He asked Dr. Mogenson whether they had included in their study participants with symptomatic COVID-19 and whether that would change the findings.
“We did not have access to RNA testing, so we only had serology, and of course some participants could be wrongly classified to have disease – probably around 15%,” she acknowledged.
She noted that there were no significant changes in lung function among patients who reported having respiratory symptoms.
The study was funded by the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Research Council for Health, Working Life and Welfare, the Karolinska Institutet, Formas, the European Research Council, and Region Stockholm. Dr. Mogenson, Dr. Simonds, and Dr. Bayat disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Here’s some encouraging news for once regarding SARS-CoV-2 infections: A study of young adults for whom prepandemic spirometry data were available showed that COVID-19 did not have a significant impact on lung function, even among patients with asthma.
Among 853 Swedish men and women (mean age, 22 years) who were part of a birth cohort study, there were no significant differences in either forced expiratory volume in 1 second (FEV1) or in the ratio of FEV1 to forced vital capacity, reported Ida Mogensen, MD, PhD, a postdoctoral fellow at the Karolinska Institute in Stockholm.
“We found no effect of COVID-19 on spirometric lung function in generally healthy adults,” she said in an oral abstract presented at the European Respiratory Society 2021 International Congress.
The findings echo those of a small study that involved 73 children and adolescents with COVID-19 and 45 uninfected control persons. The investigators in that study, which was also presented at ERS 2021, found that there were no significant differences in the frequency of abnormal pulmonary function measures between case patients and control patients (abstract OA1303).
“The findings from these two studies provide important reassurance about the impact of COVID infection on lung function in children and young adults,” commented Anita Simonds, MD, an honorary consultant in respiratory and sleep medicine at the Royal Brompton Hospital, London.
“We know already that this group is less likely to suffer severe illness if they contract the virus, and these studies, which importantly include comparator groups without COVID-19, show that they are also less likely to suffer long-term consequences with respect to lung function,” she said. Dr. Simonds was not involved in either study.
Young adult study
Dr. Mogenson and colleagues assessed data on 853 participants in the BAMSE Project, a prospective birth cohort study that included 4,089 children born in Stockholm from 1994 to 1996. Of the participants, 147 had asthma. They have been regularly followed with questionnaires on respiratory symptoms and medications. In addition, at 8 and 16 years’ follow-up, spirometry measures and fractional exhaled nitric oxide (FeNO) levels were assessed, allergic sensitization tests were administered, and blood eosinophil levels were measured.
In 2020 and 2021, during the pandemic, the participants underwent spirometry testing and were assessed for antibodies against SARS-CoV-2, and they self-reported use of inhaled corticosteroids.
The investigators defined asthma as any physician diagnosis and asthma symptoms and/or asthma medication use within the previous year. Participants were determined to be COVID-19 seropositive if they had IgG antibodies to the SARS-CoV-2 spike greater than 25.09 AU/mL, IgM antibodies greater than 14.42 AU/mL, or IgA antibodies greater than 2.61 AU/mL, as measured with enzyme-linked immunosorbent assay.
Participants who had been vaccinated against COVID-19 were excluded.
No significant decreases
A total of 243 participants, including 38 with asthma, were seropositive for SARS-CoV-2 antibodies. The mean change in lung function from before the pandemic to the study end date during the pandemic were not significantly different between seropositive participants and seronegative participants or IgM-positive participants and seronegative participants.
Similarly, there were no significant differences in lung function between seropositive and seronegative participants in an analysis that was adjusted for sex, body mass index, smoking status, or prepandemic lung function.
Although there was a trend toward slightly lower function among seropositive participants with asthma in comparison with seronegative patients with asthma, it was not statistically significant, Dr. Mogenson said.
There were also no significant decreases in lung function from the prepandemic measure to the present in any of the inflammatory parameters, including blood eosinophil levels, FeNO, allergic sensitization, or inhaled corticosteroid use.
Potential misclassification
In the question-and-answer period that followed the presentation, session comoderator Sam Bayat, MD, PhD, from the University of Grenoble (France), who was not involved in the study, noted that “some subjects can have positive serology without any symptoms, while others can have symptomatic disease and a couple of months later they have negative serology.”
He asked Dr. Mogenson whether they had included in their study participants with symptomatic COVID-19 and whether that would change the findings.
“We did not have access to RNA testing, so we only had serology, and of course some participants could be wrongly classified to have disease – probably around 15%,” she acknowledged.
She noted that there were no significant changes in lung function among patients who reported having respiratory symptoms.
The study was funded by the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Research Council for Health, Working Life and Welfare, the Karolinska Institutet, Formas, the European Research Council, and Region Stockholm. Dr. Mogenson, Dr. Simonds, and Dr. Bayat disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Here’s some encouraging news for once regarding SARS-CoV-2 infections: A study of young adults for whom prepandemic spirometry data were available showed that COVID-19 did not have a significant impact on lung function, even among patients with asthma.
Among 853 Swedish men and women (mean age, 22 years) who were part of a birth cohort study, there were no significant differences in either forced expiratory volume in 1 second (FEV1) or in the ratio of FEV1 to forced vital capacity, reported Ida Mogensen, MD, PhD, a postdoctoral fellow at the Karolinska Institute in Stockholm.
“We found no effect of COVID-19 on spirometric lung function in generally healthy adults,” she said in an oral abstract presented at the European Respiratory Society 2021 International Congress.
The findings echo those of a small study that involved 73 children and adolescents with COVID-19 and 45 uninfected control persons. The investigators in that study, which was also presented at ERS 2021, found that there were no significant differences in the frequency of abnormal pulmonary function measures between case patients and control patients (abstract OA1303).
“The findings from these two studies provide important reassurance about the impact of COVID infection on lung function in children and young adults,” commented Anita Simonds, MD, an honorary consultant in respiratory and sleep medicine at the Royal Brompton Hospital, London.
“We know already that this group is less likely to suffer severe illness if they contract the virus, and these studies, which importantly include comparator groups without COVID-19, show that they are also less likely to suffer long-term consequences with respect to lung function,” she said. Dr. Simonds was not involved in either study.
Young adult study
Dr. Mogenson and colleagues assessed data on 853 participants in the BAMSE Project, a prospective birth cohort study that included 4,089 children born in Stockholm from 1994 to 1996. Of the participants, 147 had asthma. They have been regularly followed with questionnaires on respiratory symptoms and medications. In addition, at 8 and 16 years’ follow-up, spirometry measures and fractional exhaled nitric oxide (FeNO) levels were assessed, allergic sensitization tests were administered, and blood eosinophil levels were measured.
In 2020 and 2021, during the pandemic, the participants underwent spirometry testing and were assessed for antibodies against SARS-CoV-2, and they self-reported use of inhaled corticosteroids.
The investigators defined asthma as any physician diagnosis and asthma symptoms and/or asthma medication use within the previous year. Participants were determined to be COVID-19 seropositive if they had IgG antibodies to the SARS-CoV-2 spike greater than 25.09 AU/mL, IgM antibodies greater than 14.42 AU/mL, or IgA antibodies greater than 2.61 AU/mL, as measured with enzyme-linked immunosorbent assay.
Participants who had been vaccinated against COVID-19 were excluded.
No significant decreases
A total of 243 participants, including 38 with asthma, were seropositive for SARS-CoV-2 antibodies. The mean change in lung function from before the pandemic to the study end date during the pandemic were not significantly different between seropositive participants and seronegative participants or IgM-positive participants and seronegative participants.
Similarly, there were no significant differences in lung function between seropositive and seronegative participants in an analysis that was adjusted for sex, body mass index, smoking status, or prepandemic lung function.
Although there was a trend toward slightly lower function among seropositive participants with asthma in comparison with seronegative patients with asthma, it was not statistically significant, Dr. Mogenson said.
There were also no significant decreases in lung function from the prepandemic measure to the present in any of the inflammatory parameters, including blood eosinophil levels, FeNO, allergic sensitization, or inhaled corticosteroid use.
Potential misclassification
In the question-and-answer period that followed the presentation, session comoderator Sam Bayat, MD, PhD, from the University of Grenoble (France), who was not involved in the study, noted that “some subjects can have positive serology without any symptoms, while others can have symptomatic disease and a couple of months later they have negative serology.”
He asked Dr. Mogenson whether they had included in their study participants with symptomatic COVID-19 and whether that would change the findings.
“We did not have access to RNA testing, so we only had serology, and of course some participants could be wrongly classified to have disease – probably around 15%,” she acknowledged.
She noted that there were no significant changes in lung function among patients who reported having respiratory symptoms.
The study was funded by the Swedish Research Council, the Swedish Heart-Lung Foundation, the Swedish Research Council for Health, Working Life and Welfare, the Karolinska Institutet, Formas, the European Research Council, and Region Stockholm. Dr. Mogenson, Dr. Simonds, and Dr. Bayat disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Atopic dermatitis subtype worsens into midlife, predicting poor health
giving reason to observe patients beyond the pediatric stage, according to a cohort study of more than 30,000 patients.
Early-life environmental factors, such as tobacco smoke exposure, were not reliable predictors of increasing AD into mid-adulthood, suggesting that a patient’s contemporaneous environment may impact disease course throughout life, reported lead author Katrina Abuabara, MD, associate professor of dermatology at the University of California, San Francisco, and colleagues.
“There is a lack of studies that prospectively examine the course of atopic eczema beyond adolescence/early adulthood, and a more comprehensive understanding of disease activity across the life span is needed,” the investigators wrote in JAMA Dermatology. “Data on long-term disease course may offer insight into mechanisms for disease onset and persistence, are important when counseling patients, and would establish baseline trajectories for future studies of whether new treatments can modify disease course and development of comorbidities.”
The present study included 30,905 patients from two population-based birth cohorts: the 1958 National Childhood Development Study (NCDS) and the 1970 British Cohort Study (BCS70). Follow-up data were collected between 1958 and 2016 via nine waves of standardized questionnaires, and subtypes of atopic eczema patterns were identified “based on parent-reported or self-reported atopic eczema period prevalence.”
This measure “was previously shown to coincide with standardized clinical examinations among children in the NCDS, and a similar questionnaire demonstrated high sensitivity and specificity for physician-diagnosed atopic eczema in U.S. populations,” the investigators noted.
Latent class analysis identified four disease subtypes based on probability of reporting prevalent AD into midlife: low (88%-91%), decreasing (4%), increasing (2%-6%), and persistently high (2%-3%) probability.
Next, the investigators looked for associations between these subtypes and established early-life risk factors, such as history of breastfeeding and childhood smoke exposure. None of the childhood environmental factors differentiated between high versus decreasing disease in adulthood, or increasing versus decreasing disease in adulthood. In contrast, female sex predicted the high versus decreasing adult subtype (odds ratio, 1.99; 95% confidence interval, 1.66-2.38), and the increasing versus decreasing adult subtype (OR, 1.99; 95% CI, 1.69-2.35).
These findings suggest that “disease trajectory is modifiable and may be influenced by environmental factors throughout life,” the investigators wrote.
Further analysis uncovered associations between adult AD subtypes and other health outcomes. For example, compared with adults in the low probability group, those in the high probability group were significantly more likely to report rhinitis (OR, 2.70; 95% CI, 2.24-3.26) and asthma (OR, 3.45; 95% CI, 2.82-4.21). Adults with the increasing subtype also had elevated rates of asthma and rhinitis, along with worse self-reported mental health at age 42 (OR, 1.45; 95% CI, 1.23-1.72) and poor general health at age 46/50 (OR, 1.29; 95% CI, 1.09-1.53).
“When extending the window of observation beyond childhood, clear subtypes of atopic eczema based on patterns of disease activity emerged,” the investigators concluded. “In particular, a newly identified subtype with increasing probability of activity in adulthood warrants additional attention given associations with poor self-reported physical and mental health in midlife.”
Commenting on these results, Robert Sidbury, MD, professor of dermatology at the University of Washington, Seattle, said that this is an “important study” because it adds to our understanding of natural disease course over time.
This knowledge, as a pediatric dermatologist, will help Dr. Sidbury answer one of the most common questions he hears from parents: When is it going to stop?
“Trying to put a little bit more evidence-based heft behind the answer ... is really important,” he said in an interview.
Based on available data, up to 10% of children with AD may have disease activity into adulthood, according to Dr. Sidbury, who is also chief of dermatology at Seattle Children’s Hospital.
“I would hazard to guess that most of those adults who have atopic dermatitis – at least the ones who had it in childhood – were told that they would grow out of it,” he said. “And so I think awareness is important – that [resolution with age] does not always happen.”
The findings also support the possibility that AD is a systemic disease, and that underlying immune dysregulation may be linked with serious health consequences later in life, Dr. Sidbury said, noting that “the stakes get higher and higher when you start speculating in that way.”
According to Dr. Sidbury, the reported link between childhood AD and poor midlife health raises questions about how modifiable the disease course may be, particularly in response to earlier intervention with emerging AD medications, which “seem to be much more effective and potent.”
“Will the advent of these medications and their adoption and use in treatment perhaps have a significant impact, not just on the prevention of atopic dermatitis itself, but maybe other comorbidities?” he asked.
For the time being, this question remains unanswered.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Wellcome Trust. Dr. Abuabara received grants from the National Institutes of Health during the study, as well as personal fees from Target RWE and Pfizer outside of this study. One author reported receiving NIH grants during the study, another reported receiving grants from the Wellcome Trust and the Innovative Medicine Initiative Horizon 2020 (BIOMAP project) during the study; there were no other disclosures. Dr. Sidbury disclosed relationships with Galderma, Regeneron, and Pfizer.
giving reason to observe patients beyond the pediatric stage, according to a cohort study of more than 30,000 patients.
Early-life environmental factors, such as tobacco smoke exposure, were not reliable predictors of increasing AD into mid-adulthood, suggesting that a patient’s contemporaneous environment may impact disease course throughout life, reported lead author Katrina Abuabara, MD, associate professor of dermatology at the University of California, San Francisco, and colleagues.
“There is a lack of studies that prospectively examine the course of atopic eczema beyond adolescence/early adulthood, and a more comprehensive understanding of disease activity across the life span is needed,” the investigators wrote in JAMA Dermatology. “Data on long-term disease course may offer insight into mechanisms for disease onset and persistence, are important when counseling patients, and would establish baseline trajectories for future studies of whether new treatments can modify disease course and development of comorbidities.”
The present study included 30,905 patients from two population-based birth cohorts: the 1958 National Childhood Development Study (NCDS) and the 1970 British Cohort Study (BCS70). Follow-up data were collected between 1958 and 2016 via nine waves of standardized questionnaires, and subtypes of atopic eczema patterns were identified “based on parent-reported or self-reported atopic eczema period prevalence.”
This measure “was previously shown to coincide with standardized clinical examinations among children in the NCDS, and a similar questionnaire demonstrated high sensitivity and specificity for physician-diagnosed atopic eczema in U.S. populations,” the investigators noted.
Latent class analysis identified four disease subtypes based on probability of reporting prevalent AD into midlife: low (88%-91%), decreasing (4%), increasing (2%-6%), and persistently high (2%-3%) probability.
Next, the investigators looked for associations between these subtypes and established early-life risk factors, such as history of breastfeeding and childhood smoke exposure. None of the childhood environmental factors differentiated between high versus decreasing disease in adulthood, or increasing versus decreasing disease in adulthood. In contrast, female sex predicted the high versus decreasing adult subtype (odds ratio, 1.99; 95% confidence interval, 1.66-2.38), and the increasing versus decreasing adult subtype (OR, 1.99; 95% CI, 1.69-2.35).
These findings suggest that “disease trajectory is modifiable and may be influenced by environmental factors throughout life,” the investigators wrote.
Further analysis uncovered associations between adult AD subtypes and other health outcomes. For example, compared with adults in the low probability group, those in the high probability group were significantly more likely to report rhinitis (OR, 2.70; 95% CI, 2.24-3.26) and asthma (OR, 3.45; 95% CI, 2.82-4.21). Adults with the increasing subtype also had elevated rates of asthma and rhinitis, along with worse self-reported mental health at age 42 (OR, 1.45; 95% CI, 1.23-1.72) and poor general health at age 46/50 (OR, 1.29; 95% CI, 1.09-1.53).
“When extending the window of observation beyond childhood, clear subtypes of atopic eczema based on patterns of disease activity emerged,” the investigators concluded. “In particular, a newly identified subtype with increasing probability of activity in adulthood warrants additional attention given associations with poor self-reported physical and mental health in midlife.”
Commenting on these results, Robert Sidbury, MD, professor of dermatology at the University of Washington, Seattle, said that this is an “important study” because it adds to our understanding of natural disease course over time.
This knowledge, as a pediatric dermatologist, will help Dr. Sidbury answer one of the most common questions he hears from parents: When is it going to stop?
“Trying to put a little bit more evidence-based heft behind the answer ... is really important,” he said in an interview.
Based on available data, up to 10% of children with AD may have disease activity into adulthood, according to Dr. Sidbury, who is also chief of dermatology at Seattle Children’s Hospital.
“I would hazard to guess that most of those adults who have atopic dermatitis – at least the ones who had it in childhood – were told that they would grow out of it,” he said. “And so I think awareness is important – that [resolution with age] does not always happen.”
The findings also support the possibility that AD is a systemic disease, and that underlying immune dysregulation may be linked with serious health consequences later in life, Dr. Sidbury said, noting that “the stakes get higher and higher when you start speculating in that way.”
According to Dr. Sidbury, the reported link between childhood AD and poor midlife health raises questions about how modifiable the disease course may be, particularly in response to earlier intervention with emerging AD medications, which “seem to be much more effective and potent.”
“Will the advent of these medications and their adoption and use in treatment perhaps have a significant impact, not just on the prevention of atopic dermatitis itself, but maybe other comorbidities?” he asked.
For the time being, this question remains unanswered.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Wellcome Trust. Dr. Abuabara received grants from the National Institutes of Health during the study, as well as personal fees from Target RWE and Pfizer outside of this study. One author reported receiving NIH grants during the study, another reported receiving grants from the Wellcome Trust and the Innovative Medicine Initiative Horizon 2020 (BIOMAP project) during the study; there were no other disclosures. Dr. Sidbury disclosed relationships with Galderma, Regeneron, and Pfizer.
giving reason to observe patients beyond the pediatric stage, according to a cohort study of more than 30,000 patients.
Early-life environmental factors, such as tobacco smoke exposure, were not reliable predictors of increasing AD into mid-adulthood, suggesting that a patient’s contemporaneous environment may impact disease course throughout life, reported lead author Katrina Abuabara, MD, associate professor of dermatology at the University of California, San Francisco, and colleagues.
“There is a lack of studies that prospectively examine the course of atopic eczema beyond adolescence/early adulthood, and a more comprehensive understanding of disease activity across the life span is needed,” the investigators wrote in JAMA Dermatology. “Data on long-term disease course may offer insight into mechanisms for disease onset and persistence, are important when counseling patients, and would establish baseline trajectories for future studies of whether new treatments can modify disease course and development of comorbidities.”
The present study included 30,905 patients from two population-based birth cohorts: the 1958 National Childhood Development Study (NCDS) and the 1970 British Cohort Study (BCS70). Follow-up data were collected between 1958 and 2016 via nine waves of standardized questionnaires, and subtypes of atopic eczema patterns were identified “based on parent-reported or self-reported atopic eczema period prevalence.”
This measure “was previously shown to coincide with standardized clinical examinations among children in the NCDS, and a similar questionnaire demonstrated high sensitivity and specificity for physician-diagnosed atopic eczema in U.S. populations,” the investigators noted.
Latent class analysis identified four disease subtypes based on probability of reporting prevalent AD into midlife: low (88%-91%), decreasing (4%), increasing (2%-6%), and persistently high (2%-3%) probability.
Next, the investigators looked for associations between these subtypes and established early-life risk factors, such as history of breastfeeding and childhood smoke exposure. None of the childhood environmental factors differentiated between high versus decreasing disease in adulthood, or increasing versus decreasing disease in adulthood. In contrast, female sex predicted the high versus decreasing adult subtype (odds ratio, 1.99; 95% confidence interval, 1.66-2.38), and the increasing versus decreasing adult subtype (OR, 1.99; 95% CI, 1.69-2.35).
These findings suggest that “disease trajectory is modifiable and may be influenced by environmental factors throughout life,” the investigators wrote.
Further analysis uncovered associations between adult AD subtypes and other health outcomes. For example, compared with adults in the low probability group, those in the high probability group were significantly more likely to report rhinitis (OR, 2.70; 95% CI, 2.24-3.26) and asthma (OR, 3.45; 95% CI, 2.82-4.21). Adults with the increasing subtype also had elevated rates of asthma and rhinitis, along with worse self-reported mental health at age 42 (OR, 1.45; 95% CI, 1.23-1.72) and poor general health at age 46/50 (OR, 1.29; 95% CI, 1.09-1.53).
“When extending the window of observation beyond childhood, clear subtypes of atopic eczema based on patterns of disease activity emerged,” the investigators concluded. “In particular, a newly identified subtype with increasing probability of activity in adulthood warrants additional attention given associations with poor self-reported physical and mental health in midlife.”
Commenting on these results, Robert Sidbury, MD, professor of dermatology at the University of Washington, Seattle, said that this is an “important study” because it adds to our understanding of natural disease course over time.
This knowledge, as a pediatric dermatologist, will help Dr. Sidbury answer one of the most common questions he hears from parents: When is it going to stop?
“Trying to put a little bit more evidence-based heft behind the answer ... is really important,” he said in an interview.
Based on available data, up to 10% of children with AD may have disease activity into adulthood, according to Dr. Sidbury, who is also chief of dermatology at Seattle Children’s Hospital.
“I would hazard to guess that most of those adults who have atopic dermatitis – at least the ones who had it in childhood – were told that they would grow out of it,” he said. “And so I think awareness is important – that [resolution with age] does not always happen.”
The findings also support the possibility that AD is a systemic disease, and that underlying immune dysregulation may be linked with serious health consequences later in life, Dr. Sidbury said, noting that “the stakes get higher and higher when you start speculating in that way.”
According to Dr. Sidbury, the reported link between childhood AD and poor midlife health raises questions about how modifiable the disease course may be, particularly in response to earlier intervention with emerging AD medications, which “seem to be much more effective and potent.”
“Will the advent of these medications and their adoption and use in treatment perhaps have a significant impact, not just on the prevention of atopic dermatitis itself, but maybe other comorbidities?” he asked.
For the time being, this question remains unanswered.
The study was funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases and the Wellcome Trust. Dr. Abuabara received grants from the National Institutes of Health during the study, as well as personal fees from Target RWE and Pfizer outside of this study. One author reported receiving NIH grants during the study, another reported receiving grants from the Wellcome Trust and the Innovative Medicine Initiative Horizon 2020 (BIOMAP project) during the study; there were no other disclosures. Dr. Sidbury disclosed relationships with Galderma, Regeneron, and Pfizer.
FROM JAMA DERMATOLOGY
Sweeping new vaccine mandates will impact most U.S. workers
, including sweeping vaccine mandates that will affect 100 million American workers, nearly two-thirds of the country’s workforce.
“As your president, I’m announcing tonight a new plan to get more Americans vaccinated to combat those blocking public health,” he said Sept. 9.
As part of a six-part plan unveiled in a speech from the State Dining Room of the White House, President Biden said he would require vaccinations for nearly 4 million federal workers and the employees of companies that contract with the federal government.
He has also directed the Occupational Safety and Health Administration to develop a rule that will require large employers -- those with at least 100 employees -- to ensure their workers are vaccinated or tested weekly.
Nearly 17 million health care workers will face new vaccine mandates as part of the conditions of participation in the Medicare and Medicaid programs.
President Biden said the federal government will require staff at federally funded Head Start programs and schools to be vaccinated. He’s also calling on all states to mandate vaccines for teachers.
“A distinct minority of Americans, supported by a distinct minority of elected officials, are keeping us from turning the corner,” PresidentBiden said. “These pandemic politics, as I refer to them, are making people sick, causing unvaccinated people to die.”
One public health official said he was glad to see the president’s bold action.
“What I saw today was the federal government trying to use its powers to create greater safety in the American population,” said Ashish K. Jha, MD, dean of the school of public health at Brown University, Providence, R.I., in a call with reporters after the speech.
National Nurses United, the largest union of registered nurses in the United States, issued a statement in support of President Biden’s new vaccination requirements, but pushed back on his language.
“…as advocates for public health, registered nurses want to be extremely clear: There is no such thing as a pandemic of only the unvaccinated. The science of epidemiology tells us there is just one deadly, global pandemic that has not yet ended, and we are all in it together. To get out of it, we must act together. All of us,” the statement says.
A host of other professional groups, including the American Medical Association and the Association of State and Territorial Health Officials, also issued statements of support for President Biden’s plan.
But the plan was not well received by all.
“I will pursue every legal option available to the state of Georgia to stop this blatantly unlawful overreach by the Biden Administration,” said Georgia Governor Brian Kemp, a Republican, in a Tweet.
The National Council for Occupational Safety and Health called the plan “a missed opportunity” because it failed to include workplace protections for essential workers such as grocery, postal, and transit workers.
“Social distancing, improved ventilation, shift rotation, and protective equipment to reduce exposure are important components of an overall plan to reduce risk and stop the virus. These tools are missing from the new steps President Biden announced today,” said Jessica Martinez, co-executive director of the group.
In addition to the new vaccination requirements, President Biden said extra doses would be on the way for people who have already been fully vaccinated in order to protect against waning immunity, starting on Sept. 20. But he noted that those plans would be contingent on the Food and Drug Administration’s approval for third doses and the Centers for Disease Control and Prevention’s recommendation of the shots.
President Biden pledged to use the Defense Production Act to ramp up production of at-home tests, which have been selling out across the nation as the Delta variant spreads.
He also announced plans to expand access to COVID-19 testing, including offering testing for free at thousands of pharmacies nationwide and getting major retailers to sell at-home COVID-19 tests at cost.
The BinaxNow test kit, which currently retails for $23.99, will now cost about $15 for two tests at Kroger, Amazon, and Walmart, according to the White House. Food banks and community health centers will get free tests, too.
He called on states to set up COVID-19 testing programs at all schools.
Jha said that in his view, the big, game-changing news out of the president’s speech was the expansion of testing.
“Our country has failed to deploy tests in a way that can really bring this pandemic under control,” Jha said. “There are plenty of reasons, data, experience to indicate that if these were widely available, it would make a dramatic difference in reducing infection numbers across our country.”.
Dr. Jha said the private market had not worked effectively to make testing more widely available, so it was “absolutely a requirement of the federal government to step in and make testing more widely available,” he said.
President Biden also announced new economic stimulus programs, saying he’s expanding loan programs to small businesses and streamlining the loan forgiveness process.
President Biden said he’s boosting help for overburdened hospitals, doubling the number of federal surge response teams sent to hard-hit areas to reduce the strain on local health care workers. He said he would increase the pace of antibody treatments to states by 50%.
“We made so much progress during the past 7 months of this pandemic. Even so, we remain at a critical moment, a critical time,” he said. “We have the tools. Now, we just have to finish the job with truth, with science, with confidence and together as one nation.”
A version of this article first appeared on WebMD.com.
, including sweeping vaccine mandates that will affect 100 million American workers, nearly two-thirds of the country’s workforce.
“As your president, I’m announcing tonight a new plan to get more Americans vaccinated to combat those blocking public health,” he said Sept. 9.
As part of a six-part plan unveiled in a speech from the State Dining Room of the White House, President Biden said he would require vaccinations for nearly 4 million federal workers and the employees of companies that contract with the federal government.
He has also directed the Occupational Safety and Health Administration to develop a rule that will require large employers -- those with at least 100 employees -- to ensure their workers are vaccinated or tested weekly.
Nearly 17 million health care workers will face new vaccine mandates as part of the conditions of participation in the Medicare and Medicaid programs.
President Biden said the federal government will require staff at federally funded Head Start programs and schools to be vaccinated. He’s also calling on all states to mandate vaccines for teachers.
“A distinct minority of Americans, supported by a distinct minority of elected officials, are keeping us from turning the corner,” PresidentBiden said. “These pandemic politics, as I refer to them, are making people sick, causing unvaccinated people to die.”
One public health official said he was glad to see the president’s bold action.
“What I saw today was the federal government trying to use its powers to create greater safety in the American population,” said Ashish K. Jha, MD, dean of the school of public health at Brown University, Providence, R.I., in a call with reporters after the speech.
National Nurses United, the largest union of registered nurses in the United States, issued a statement in support of President Biden’s new vaccination requirements, but pushed back on his language.
“…as advocates for public health, registered nurses want to be extremely clear: There is no such thing as a pandemic of only the unvaccinated. The science of epidemiology tells us there is just one deadly, global pandemic that has not yet ended, and we are all in it together. To get out of it, we must act together. All of us,” the statement says.
A host of other professional groups, including the American Medical Association and the Association of State and Territorial Health Officials, also issued statements of support for President Biden’s plan.
But the plan was not well received by all.
“I will pursue every legal option available to the state of Georgia to stop this blatantly unlawful overreach by the Biden Administration,” said Georgia Governor Brian Kemp, a Republican, in a Tweet.
The National Council for Occupational Safety and Health called the plan “a missed opportunity” because it failed to include workplace protections for essential workers such as grocery, postal, and transit workers.
“Social distancing, improved ventilation, shift rotation, and protective equipment to reduce exposure are important components of an overall plan to reduce risk and stop the virus. These tools are missing from the new steps President Biden announced today,” said Jessica Martinez, co-executive director of the group.
In addition to the new vaccination requirements, President Biden said extra doses would be on the way for people who have already been fully vaccinated in order to protect against waning immunity, starting on Sept. 20. But he noted that those plans would be contingent on the Food and Drug Administration’s approval for third doses and the Centers for Disease Control and Prevention’s recommendation of the shots.
President Biden pledged to use the Defense Production Act to ramp up production of at-home tests, which have been selling out across the nation as the Delta variant spreads.
He also announced plans to expand access to COVID-19 testing, including offering testing for free at thousands of pharmacies nationwide and getting major retailers to sell at-home COVID-19 tests at cost.
The BinaxNow test kit, which currently retails for $23.99, will now cost about $15 for two tests at Kroger, Amazon, and Walmart, according to the White House. Food banks and community health centers will get free tests, too.
He called on states to set up COVID-19 testing programs at all schools.
Jha said that in his view, the big, game-changing news out of the president’s speech was the expansion of testing.
“Our country has failed to deploy tests in a way that can really bring this pandemic under control,” Jha said. “There are plenty of reasons, data, experience to indicate that if these were widely available, it would make a dramatic difference in reducing infection numbers across our country.”.
Dr. Jha said the private market had not worked effectively to make testing more widely available, so it was “absolutely a requirement of the federal government to step in and make testing more widely available,” he said.
President Biden also announced new economic stimulus programs, saying he’s expanding loan programs to small businesses and streamlining the loan forgiveness process.
President Biden said he’s boosting help for overburdened hospitals, doubling the number of federal surge response teams sent to hard-hit areas to reduce the strain on local health care workers. He said he would increase the pace of antibody treatments to states by 50%.
“We made so much progress during the past 7 months of this pandemic. Even so, we remain at a critical moment, a critical time,” he said. “We have the tools. Now, we just have to finish the job with truth, with science, with confidence and together as one nation.”
A version of this article first appeared on WebMD.com.
, including sweeping vaccine mandates that will affect 100 million American workers, nearly two-thirds of the country’s workforce.
“As your president, I’m announcing tonight a new plan to get more Americans vaccinated to combat those blocking public health,” he said Sept. 9.
As part of a six-part plan unveiled in a speech from the State Dining Room of the White House, President Biden said he would require vaccinations for nearly 4 million federal workers and the employees of companies that contract with the federal government.
He has also directed the Occupational Safety and Health Administration to develop a rule that will require large employers -- those with at least 100 employees -- to ensure their workers are vaccinated or tested weekly.
Nearly 17 million health care workers will face new vaccine mandates as part of the conditions of participation in the Medicare and Medicaid programs.
President Biden said the federal government will require staff at federally funded Head Start programs and schools to be vaccinated. He’s also calling on all states to mandate vaccines for teachers.
“A distinct minority of Americans, supported by a distinct minority of elected officials, are keeping us from turning the corner,” PresidentBiden said. “These pandemic politics, as I refer to them, are making people sick, causing unvaccinated people to die.”
One public health official said he was glad to see the president’s bold action.
“What I saw today was the federal government trying to use its powers to create greater safety in the American population,” said Ashish K. Jha, MD, dean of the school of public health at Brown University, Providence, R.I., in a call with reporters after the speech.
National Nurses United, the largest union of registered nurses in the United States, issued a statement in support of President Biden’s new vaccination requirements, but pushed back on his language.
“…as advocates for public health, registered nurses want to be extremely clear: There is no such thing as a pandemic of only the unvaccinated. The science of epidemiology tells us there is just one deadly, global pandemic that has not yet ended, and we are all in it together. To get out of it, we must act together. All of us,” the statement says.
A host of other professional groups, including the American Medical Association and the Association of State and Territorial Health Officials, also issued statements of support for President Biden’s plan.
But the plan was not well received by all.
“I will pursue every legal option available to the state of Georgia to stop this blatantly unlawful overreach by the Biden Administration,” said Georgia Governor Brian Kemp, a Republican, in a Tweet.
The National Council for Occupational Safety and Health called the plan “a missed opportunity” because it failed to include workplace protections for essential workers such as grocery, postal, and transit workers.
“Social distancing, improved ventilation, shift rotation, and protective equipment to reduce exposure are important components of an overall plan to reduce risk and stop the virus. These tools are missing from the new steps President Biden announced today,” said Jessica Martinez, co-executive director of the group.
In addition to the new vaccination requirements, President Biden said extra doses would be on the way for people who have already been fully vaccinated in order to protect against waning immunity, starting on Sept. 20. But he noted that those plans would be contingent on the Food and Drug Administration’s approval for third doses and the Centers for Disease Control and Prevention’s recommendation of the shots.
President Biden pledged to use the Defense Production Act to ramp up production of at-home tests, which have been selling out across the nation as the Delta variant spreads.
He also announced plans to expand access to COVID-19 testing, including offering testing for free at thousands of pharmacies nationwide and getting major retailers to sell at-home COVID-19 tests at cost.
The BinaxNow test kit, which currently retails for $23.99, will now cost about $15 for two tests at Kroger, Amazon, and Walmart, according to the White House. Food banks and community health centers will get free tests, too.
He called on states to set up COVID-19 testing programs at all schools.
Jha said that in his view, the big, game-changing news out of the president’s speech was the expansion of testing.
“Our country has failed to deploy tests in a way that can really bring this pandemic under control,” Jha said. “There are plenty of reasons, data, experience to indicate that if these were widely available, it would make a dramatic difference in reducing infection numbers across our country.”.
Dr. Jha said the private market had not worked effectively to make testing more widely available, so it was “absolutely a requirement of the federal government to step in and make testing more widely available,” he said.
President Biden also announced new economic stimulus programs, saying he’s expanding loan programs to small businesses and streamlining the loan forgiveness process.
President Biden said he’s boosting help for overburdened hospitals, doubling the number of federal surge response teams sent to hard-hit areas to reduce the strain on local health care workers. He said he would increase the pace of antibody treatments to states by 50%.
“We made so much progress during the past 7 months of this pandemic. Even so, we remain at a critical moment, a critical time,” he said. “We have the tools. Now, we just have to finish the job with truth, with science, with confidence and together as one nation.”
A version of this article first appeared on WebMD.com.
Korean siblings face high familial IBD risk
Among Asian-Pacific populations, the first-degree relatives (FDRs) of individuals with inflammatory bowel disease (IBD) have a significantly increased risk for IBD themselves, according to a large analysis of data from South Korea. The greatest risk was found in siblings and for Crohn’s disease (CD).
The analysis of the South Korean Health Insurance Database included a cohort of 21,940,795 individuals from about 12 million families, with data collected between 2002 and 2017.
Previous studies have examined risk of IBD and familial relationships with existing IBD patients, but they have been subject to biases and have been heterogeneous in design, according to the authors, led by co–first authors Hyun Jung Kim, MD, of Korea University in Seoul, South Korea, and Shailja C. Shah, MD, of Vanderbilt University in Nashville, Tenn. There are few true population-based studies that quantify specific risks for family members of IBD patients, and none that were conducted in non-Western populations.
There are concerns about extrapolating familial IBD risk estimates from Western European populations to Asian populations because new data suggest that there are both genetic and nongenetic disease risk factors that reflect geography and ethnicity, the authors noted.
The researchers identified 45,717 individuals with ulcerative colitis (UC) and 17,848 with CD. Mean annual incidence rates were 4.6 cases of UC and 3.2 cases of CD per 100,000 person-years, which was relatively stable across the study period.
In all, 3.8% of UC and 3.1% of CD diagnoses occurred in FDR’s of existing patients. Among those with an FDR with IBD, the incidence of UC and CD was 54.5 and 99.2 per 100,000 person-years, respectively. When compared with individuals who had no FDRs with IBD, subjects who had an FDR with CD were at a more than 20-fold increased risk of CD (incident rate ratio, 22.2; 95% confidence interval, 20.5-24.5), whereas individuals with an FDR with UC were at a little more than a 10-fold risk for UC (IRR, 10.2; 95% CI, 9.39-11.1).
Subjects with an FDR with CD were at higher risk of UC (IRR, 3.56; 95% CI, 2.77-4.50), and those with an FDR with UC were at higher risk of CD (IRR, 2.94; 95% CI, 2.45-3.49). After adjustment for smoking, having an FDR with IBD was associated with an almost eightfold increased risk of UC (IRR, 7.94; 95% CI, 6.98-9.03) and a nearly 20-fold increased risk of CD (IRR, 19.03; 95% CI, 15.58-23.25).
The investigators also performed an analysis based on type of relative, with matching relations with unaffected relatives as the reference for each comparison. The highest risk for incident CD was with twin siblings (IRR, 336.2; 95% CI, 235.0-481.1) followed by nontwin siblings (IRR, 27.6; 95% CI, 24.6-30.9). The risk of CD among offspring of an affected father was 9.40 (95% CI, 6.81-13.0) and 6.54 (95% CI, 4.17-10.3) for offspring of affected mothers. There was a similar pattern for UC, although the magnitude was smaller: 163.7 for twin siblings (95% CI, 105.6-253.9), 13.1 for nontwin siblings (95% CI, 11.4-15.0), 7.11 for offspring of affected fathers (95% CI, 6.10-8.29), and 8.77 for offspring of affected mothers (95% CI, 7.46-10.3).
The researchers found no evidence of a birth cohort effect. Family history and IBD risk is a complicated relationship. Family history includes shared genetics as well as similar environmental exposures, and gene-environment interactions can add another layer of uncertainty. Previous studies have found that asymptomatic family members of IBD patients sometimes have preclinical signs such as changes in intestinal permeability, immune function, the microbiome, and biomarker levels.
IBD has emerged recently among Asian-Pacific populations as a serious health concern, with a recent rapid increase. This may reflect a shift in potentially modifiable environmental triggers. “Precisely quantifying familial risk and patterns might enable more accurate risk counseling and better-targeted clinical surveillance for earlier diagnosis and treatment among FDRs. Moreover, an accurate definition of familial IBD risk across populations also might inform subsequent investigations untangling the various shared environmental and genetic contributions,” the authors wrote.
Although genetic susceptibility is generally accepted as the predominant driver in familial trends for IBD, the authors noted their “study was not designed to determine the contribution of genetic vs. nongenetic determinants to familial IBD risk, and future well-designed dedicated investigations are needed to provide this clarity.”
The study is limited by the relatively short follow-up period, which may not have captured all IBD cases within patients’ families.
The authors have no relevant financial disclosures.
Among Asian-Pacific populations, the first-degree relatives (FDRs) of individuals with inflammatory bowel disease (IBD) have a significantly increased risk for IBD themselves, according to a large analysis of data from South Korea. The greatest risk was found in siblings and for Crohn’s disease (CD).
The analysis of the South Korean Health Insurance Database included a cohort of 21,940,795 individuals from about 12 million families, with data collected between 2002 and 2017.
Previous studies have examined risk of IBD and familial relationships with existing IBD patients, but they have been subject to biases and have been heterogeneous in design, according to the authors, led by co–first authors Hyun Jung Kim, MD, of Korea University in Seoul, South Korea, and Shailja C. Shah, MD, of Vanderbilt University in Nashville, Tenn. There are few true population-based studies that quantify specific risks for family members of IBD patients, and none that were conducted in non-Western populations.
There are concerns about extrapolating familial IBD risk estimates from Western European populations to Asian populations because new data suggest that there are both genetic and nongenetic disease risk factors that reflect geography and ethnicity, the authors noted.
The researchers identified 45,717 individuals with ulcerative colitis (UC) and 17,848 with CD. Mean annual incidence rates were 4.6 cases of UC and 3.2 cases of CD per 100,000 person-years, which was relatively stable across the study period.
In all, 3.8% of UC and 3.1% of CD diagnoses occurred in FDR’s of existing patients. Among those with an FDR with IBD, the incidence of UC and CD was 54.5 and 99.2 per 100,000 person-years, respectively. When compared with individuals who had no FDRs with IBD, subjects who had an FDR with CD were at a more than 20-fold increased risk of CD (incident rate ratio, 22.2; 95% confidence interval, 20.5-24.5), whereas individuals with an FDR with UC were at a little more than a 10-fold risk for UC (IRR, 10.2; 95% CI, 9.39-11.1).
Subjects with an FDR with CD were at higher risk of UC (IRR, 3.56; 95% CI, 2.77-4.50), and those with an FDR with UC were at higher risk of CD (IRR, 2.94; 95% CI, 2.45-3.49). After adjustment for smoking, having an FDR with IBD was associated with an almost eightfold increased risk of UC (IRR, 7.94; 95% CI, 6.98-9.03) and a nearly 20-fold increased risk of CD (IRR, 19.03; 95% CI, 15.58-23.25).
The investigators also performed an analysis based on type of relative, with matching relations with unaffected relatives as the reference for each comparison. The highest risk for incident CD was with twin siblings (IRR, 336.2; 95% CI, 235.0-481.1) followed by nontwin siblings (IRR, 27.6; 95% CI, 24.6-30.9). The risk of CD among offspring of an affected father was 9.40 (95% CI, 6.81-13.0) and 6.54 (95% CI, 4.17-10.3) for offspring of affected mothers. There was a similar pattern for UC, although the magnitude was smaller: 163.7 for twin siblings (95% CI, 105.6-253.9), 13.1 for nontwin siblings (95% CI, 11.4-15.0), 7.11 for offspring of affected fathers (95% CI, 6.10-8.29), and 8.77 for offspring of affected mothers (95% CI, 7.46-10.3).
The researchers found no evidence of a birth cohort effect. Family history and IBD risk is a complicated relationship. Family history includes shared genetics as well as similar environmental exposures, and gene-environment interactions can add another layer of uncertainty. Previous studies have found that asymptomatic family members of IBD patients sometimes have preclinical signs such as changes in intestinal permeability, immune function, the microbiome, and biomarker levels.
IBD has emerged recently among Asian-Pacific populations as a serious health concern, with a recent rapid increase. This may reflect a shift in potentially modifiable environmental triggers. “Precisely quantifying familial risk and patterns might enable more accurate risk counseling and better-targeted clinical surveillance for earlier diagnosis and treatment among FDRs. Moreover, an accurate definition of familial IBD risk across populations also might inform subsequent investigations untangling the various shared environmental and genetic contributions,” the authors wrote.
Although genetic susceptibility is generally accepted as the predominant driver in familial trends for IBD, the authors noted their “study was not designed to determine the contribution of genetic vs. nongenetic determinants to familial IBD risk, and future well-designed dedicated investigations are needed to provide this clarity.”
The study is limited by the relatively short follow-up period, which may not have captured all IBD cases within patients’ families.
The authors have no relevant financial disclosures.
Among Asian-Pacific populations, the first-degree relatives (FDRs) of individuals with inflammatory bowel disease (IBD) have a significantly increased risk for IBD themselves, according to a large analysis of data from South Korea. The greatest risk was found in siblings and for Crohn’s disease (CD).
The analysis of the South Korean Health Insurance Database included a cohort of 21,940,795 individuals from about 12 million families, with data collected between 2002 and 2017.
Previous studies have examined risk of IBD and familial relationships with existing IBD patients, but they have been subject to biases and have been heterogeneous in design, according to the authors, led by co–first authors Hyun Jung Kim, MD, of Korea University in Seoul, South Korea, and Shailja C. Shah, MD, of Vanderbilt University in Nashville, Tenn. There are few true population-based studies that quantify specific risks for family members of IBD patients, and none that were conducted in non-Western populations.
There are concerns about extrapolating familial IBD risk estimates from Western European populations to Asian populations because new data suggest that there are both genetic and nongenetic disease risk factors that reflect geography and ethnicity, the authors noted.
The researchers identified 45,717 individuals with ulcerative colitis (UC) and 17,848 with CD. Mean annual incidence rates were 4.6 cases of UC and 3.2 cases of CD per 100,000 person-years, which was relatively stable across the study period.
In all, 3.8% of UC and 3.1% of CD diagnoses occurred in FDR’s of existing patients. Among those with an FDR with IBD, the incidence of UC and CD was 54.5 and 99.2 per 100,000 person-years, respectively. When compared with individuals who had no FDRs with IBD, subjects who had an FDR with CD were at a more than 20-fold increased risk of CD (incident rate ratio, 22.2; 95% confidence interval, 20.5-24.5), whereas individuals with an FDR with UC were at a little more than a 10-fold risk for UC (IRR, 10.2; 95% CI, 9.39-11.1).
Subjects with an FDR with CD were at higher risk of UC (IRR, 3.56; 95% CI, 2.77-4.50), and those with an FDR with UC were at higher risk of CD (IRR, 2.94; 95% CI, 2.45-3.49). After adjustment for smoking, having an FDR with IBD was associated with an almost eightfold increased risk of UC (IRR, 7.94; 95% CI, 6.98-9.03) and a nearly 20-fold increased risk of CD (IRR, 19.03; 95% CI, 15.58-23.25).
The investigators also performed an analysis based on type of relative, with matching relations with unaffected relatives as the reference for each comparison. The highest risk for incident CD was with twin siblings (IRR, 336.2; 95% CI, 235.0-481.1) followed by nontwin siblings (IRR, 27.6; 95% CI, 24.6-30.9). The risk of CD among offspring of an affected father was 9.40 (95% CI, 6.81-13.0) and 6.54 (95% CI, 4.17-10.3) for offspring of affected mothers. There was a similar pattern for UC, although the magnitude was smaller: 163.7 for twin siblings (95% CI, 105.6-253.9), 13.1 for nontwin siblings (95% CI, 11.4-15.0), 7.11 for offspring of affected fathers (95% CI, 6.10-8.29), and 8.77 for offspring of affected mothers (95% CI, 7.46-10.3).
The researchers found no evidence of a birth cohort effect. Family history and IBD risk is a complicated relationship. Family history includes shared genetics as well as similar environmental exposures, and gene-environment interactions can add another layer of uncertainty. Previous studies have found that asymptomatic family members of IBD patients sometimes have preclinical signs such as changes in intestinal permeability, immune function, the microbiome, and biomarker levels.
IBD has emerged recently among Asian-Pacific populations as a serious health concern, with a recent rapid increase. This may reflect a shift in potentially modifiable environmental triggers. “Precisely quantifying familial risk and patterns might enable more accurate risk counseling and better-targeted clinical surveillance for earlier diagnosis and treatment among FDRs. Moreover, an accurate definition of familial IBD risk across populations also might inform subsequent investigations untangling the various shared environmental and genetic contributions,” the authors wrote.
Although genetic susceptibility is generally accepted as the predominant driver in familial trends for IBD, the authors noted their “study was not designed to determine the contribution of genetic vs. nongenetic determinants to familial IBD risk, and future well-designed dedicated investigations are needed to provide this clarity.”
The study is limited by the relatively short follow-up period, which may not have captured all IBD cases within patients’ families.
The authors have no relevant financial disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Spiral Plaque on the Left Ankle
The Diagnosis: Recurrent Cutaneous T-Cell Lymphoma
The skin biopsy revealed alternating orthokeratosis and parakeratosis with mild to moderate spongiosis and intraepidermal vesiculation as well as individual and nested atypical mononuclear cells with moderately enlarged hyperchromatic nuclei in the epidermis. There was a superficial interstitial lymphocytic infiltrate with occasional enlarged cells (Figure, A and B), and atypical cells in the epidermis and dermis stained with antibodies against CD3 and CD4 (Figure, C and D) but not against CD20 or CD8. These histopathologic findings were consistent with cutaneous T-cell lymphoma (CTCL), mycosis fungoides (MF) type. Additional application of bexarotene gel on days the patient received narrowband UVB was recommended with noted improvement of the skin.
Cutaneous T-cell lymphomas are a heterogenous group of diseases with monoclonal proliferation of T lymphocytes that largely are confined to the skin at the time of diagnosis.1 The incidence of CTCL rose steadily for more than 25 years, with an annual age-adjusted incidence of 6.4 to 9.6 cases per million individuals in the United States from 1973 to 2002.2 Mycosis fungoides is the most common classification of CTCL. It usually is characterized by patches or plaques of scaly erythema or poikiloderma; however, it also can present with annular, arcuate, concentrative, annular and linear morphologies. Mycosis fungoides tumor cells typically express a mature memory T helper cell phenotype of CD3+, CD4+, and CD8−, but there are different variants that have been discovered.3 Mycosis fungoides distributed in a spiral pattern is a distinctly unusual manifestation. Mechanisms of such dynamic morphologies are unknown but may represent an interplay between malignant cell proliferation and lost immune responses in temporospatial relationships.
The presence of keratotic gyrate lesions on acral surfaces should raise the possibility of pagetoid reticulosis. However, our patient had a history of MF involving areas of the body beyond the extremities, making this diagnosis less likely. Pagetoid reticulosis is categorized as an MF variant under the current World Health Organization– European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas.4 Pagetoid reticulosis clinically presents as a solitary psoriasiform or hyperkeratotic patch or plaque that affects the distal extremities. Variable immunophenotypes have been shown in pagetoid reticulosis, such as CD4−/CD8+ and CD4−/CD8−, while classic MF typically shows CD4+/CD8−, as in our case.5
Tinea pedis is a superficial fungal infection usually caused by anthropophilic dermatophytes, with Trichophyton rubrum being the most common organism. Four common clinical presentations of tinea pedis have been identified: interdigital, moccasin, vesicular, and acute ulcerative. Clinical presentation ranges from macerations, ulcerations, and erosions in the toe web spaces to dry hyperkeratotic scaling and fissures on the plantar foot.6 Tinea pedis primarily affects the plantar and interdigital spaces, sparing the dorsal foot and ankle. Treatment is recommended to alleviate symptoms and limit the spread of infection; topical antifungals for 4 weeks is the treatment of choice. However, recurrence is common, and maintenance therapy often is indicated. Oral antifungals or a combination of both topical and oral medications may be needed in certain cases.7
Erythema annulare centrifugum (EAC) is a rare dermatologic disease described as erythematous or urticarial papules that can enlarge centrifugally to form annular lesions that clear centrally. Thought to be a hypersensitivity reaction to an underlying condition, EAC has been associated with fungal infections, various cutaneous diseases, and even internal malignancies. Clinically, EAC can be divided into 2 forms: deep and superficial. Deep gyrate erythema is characterized by a firm indurated border with rare scaling and pruritus that histologically shows perivascular lymphocytic infiltration in the upper and deep dermis. Superficial gyrate erythema has minimally elevated lesions with an indistinct border and trailing scales and pruritus; histopathologic findings present a dense, perivascular, lymphocytic infiltration restricted to the upper dermis.8 Therapy for EAC is directed at relieving symptoms and treating the underlying condition if there is one associated.
Granuloma annulare (GA) is a common skin disorder classically characterized by ringed erythematous plaques, though many variants have been identified. Localized GA is the most common variant and presents with pink-red, nonscaly, annular patches or plaques, typically affecting the hands and feet. Generalized GA is characterized as diffuse annular patches or plaques classically affecting the trunk and extremities. Histology is notable for mucin with a palisading or interstitial pattern of granulomatous inflammation, which was not evident in our patient.9 Topical or intralesional corticosteroids are the first-line treatment of localized GA; however, localized GA generally is self-limited, and treatment often is not necessary. Treatment with cryosurgery, laser therapy, and topical dapsone and tacrolimus also has been described, but evidence of the efficacy of these agents is limited. For generalized GA, phototherapy currently is the most reliable therapy. Systemic therapies include antimalarials, fumaric acid esters, biologics, antimicrobials, and isotretinoin.10
Erythema gyratum repens (EGR) is a rare dermatologic disease described as erythematous concentric bands arranged in parallel rings that can be annular, figurate, or gyrate, with a fine scale trailing the leading edge. Histopathologic features of EGR are nonspecific but are characterized by a perivascular, superficial, mononuclear dermatitis. Diagnosis is based on its characteristic clinical presentation. Although EGR commonly is associated with internal malignancies such as bronchial carcinoma, it also may be associated with benign conditions.11 Improvement often is seen with successful therapy of the underlying associated malignancy.12
Treatment of MF is based on tumor-node-metastasisblood classification, prognostic factors, and clinical stage at the time of diagnosis. Early-stage MF (IA–IIA) commonly is treated with skin-directed therapies such as topical corticosteroids, topical mechlorethamine, topical retinoids, UV phototherapy, and localized radiotherapy. In late stages (IIB–IV), systemic therapy is indicated and includes systemic retinoids, interferon alfa, chemotherapy, monoclonal antibodies, and psoralen plus UVA.13 In many cases, patients may require combination therapy to achieve remission or better control of their condition, as in our patient.
The Diagnosis: Recurrent Cutaneous T-Cell Lymphoma
The skin biopsy revealed alternating orthokeratosis and parakeratosis with mild to moderate spongiosis and intraepidermal vesiculation as well as individual and nested atypical mononuclear cells with moderately enlarged hyperchromatic nuclei in the epidermis. There was a superficial interstitial lymphocytic infiltrate with occasional enlarged cells (Figure, A and B), and atypical cells in the epidermis and dermis stained with antibodies against CD3 and CD4 (Figure, C and D) but not against CD20 or CD8. These histopathologic findings were consistent with cutaneous T-cell lymphoma (CTCL), mycosis fungoides (MF) type. Additional application of bexarotene gel on days the patient received narrowband UVB was recommended with noted improvement of the skin.
Cutaneous T-cell lymphomas are a heterogenous group of diseases with monoclonal proliferation of T lymphocytes that largely are confined to the skin at the time of diagnosis.1 The incidence of CTCL rose steadily for more than 25 years, with an annual age-adjusted incidence of 6.4 to 9.6 cases per million individuals in the United States from 1973 to 2002.2 Mycosis fungoides is the most common classification of CTCL. It usually is characterized by patches or plaques of scaly erythema or poikiloderma; however, it also can present with annular, arcuate, concentrative, annular and linear morphologies. Mycosis fungoides tumor cells typically express a mature memory T helper cell phenotype of CD3+, CD4+, and CD8−, but there are different variants that have been discovered.3 Mycosis fungoides distributed in a spiral pattern is a distinctly unusual manifestation. Mechanisms of such dynamic morphologies are unknown but may represent an interplay between malignant cell proliferation and lost immune responses in temporospatial relationships.
The presence of keratotic gyrate lesions on acral surfaces should raise the possibility of pagetoid reticulosis. However, our patient had a history of MF involving areas of the body beyond the extremities, making this diagnosis less likely. Pagetoid reticulosis is categorized as an MF variant under the current World Health Organization– European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas.4 Pagetoid reticulosis clinically presents as a solitary psoriasiform or hyperkeratotic patch or plaque that affects the distal extremities. Variable immunophenotypes have been shown in pagetoid reticulosis, such as CD4−/CD8+ and CD4−/CD8−, while classic MF typically shows CD4+/CD8−, as in our case.5
Tinea pedis is a superficial fungal infection usually caused by anthropophilic dermatophytes, with Trichophyton rubrum being the most common organism. Four common clinical presentations of tinea pedis have been identified: interdigital, moccasin, vesicular, and acute ulcerative. Clinical presentation ranges from macerations, ulcerations, and erosions in the toe web spaces to dry hyperkeratotic scaling and fissures on the plantar foot.6 Tinea pedis primarily affects the plantar and interdigital spaces, sparing the dorsal foot and ankle. Treatment is recommended to alleviate symptoms and limit the spread of infection; topical antifungals for 4 weeks is the treatment of choice. However, recurrence is common, and maintenance therapy often is indicated. Oral antifungals or a combination of both topical and oral medications may be needed in certain cases.7
Erythema annulare centrifugum (EAC) is a rare dermatologic disease described as erythematous or urticarial papules that can enlarge centrifugally to form annular lesions that clear centrally. Thought to be a hypersensitivity reaction to an underlying condition, EAC has been associated with fungal infections, various cutaneous diseases, and even internal malignancies. Clinically, EAC can be divided into 2 forms: deep and superficial. Deep gyrate erythema is characterized by a firm indurated border with rare scaling and pruritus that histologically shows perivascular lymphocytic infiltration in the upper and deep dermis. Superficial gyrate erythema has minimally elevated lesions with an indistinct border and trailing scales and pruritus; histopathologic findings present a dense, perivascular, lymphocytic infiltration restricted to the upper dermis.8 Therapy for EAC is directed at relieving symptoms and treating the underlying condition if there is one associated.
Granuloma annulare (GA) is a common skin disorder classically characterized by ringed erythematous plaques, though many variants have been identified. Localized GA is the most common variant and presents with pink-red, nonscaly, annular patches or plaques, typically affecting the hands and feet. Generalized GA is characterized as diffuse annular patches or plaques classically affecting the trunk and extremities. Histology is notable for mucin with a palisading or interstitial pattern of granulomatous inflammation, which was not evident in our patient.9 Topical or intralesional corticosteroids are the first-line treatment of localized GA; however, localized GA generally is self-limited, and treatment often is not necessary. Treatment with cryosurgery, laser therapy, and topical dapsone and tacrolimus also has been described, but evidence of the efficacy of these agents is limited. For generalized GA, phototherapy currently is the most reliable therapy. Systemic therapies include antimalarials, fumaric acid esters, biologics, antimicrobials, and isotretinoin.10
Erythema gyratum repens (EGR) is a rare dermatologic disease described as erythematous concentric bands arranged in parallel rings that can be annular, figurate, or gyrate, with a fine scale trailing the leading edge. Histopathologic features of EGR are nonspecific but are characterized by a perivascular, superficial, mononuclear dermatitis. Diagnosis is based on its characteristic clinical presentation. Although EGR commonly is associated with internal malignancies such as bronchial carcinoma, it also may be associated with benign conditions.11 Improvement often is seen with successful therapy of the underlying associated malignancy.12
Treatment of MF is based on tumor-node-metastasisblood classification, prognostic factors, and clinical stage at the time of diagnosis. Early-stage MF (IA–IIA) commonly is treated with skin-directed therapies such as topical corticosteroids, topical mechlorethamine, topical retinoids, UV phototherapy, and localized radiotherapy. In late stages (IIB–IV), systemic therapy is indicated and includes systemic retinoids, interferon alfa, chemotherapy, monoclonal antibodies, and psoralen plus UVA.13 In many cases, patients may require combination therapy to achieve remission or better control of their condition, as in our patient.
The Diagnosis: Recurrent Cutaneous T-Cell Lymphoma
The skin biopsy revealed alternating orthokeratosis and parakeratosis with mild to moderate spongiosis and intraepidermal vesiculation as well as individual and nested atypical mononuclear cells with moderately enlarged hyperchromatic nuclei in the epidermis. There was a superficial interstitial lymphocytic infiltrate with occasional enlarged cells (Figure, A and B), and atypical cells in the epidermis and dermis stained with antibodies against CD3 and CD4 (Figure, C and D) but not against CD20 or CD8. These histopathologic findings were consistent with cutaneous T-cell lymphoma (CTCL), mycosis fungoides (MF) type. Additional application of bexarotene gel on days the patient received narrowband UVB was recommended with noted improvement of the skin.
Cutaneous T-cell lymphomas are a heterogenous group of diseases with monoclonal proliferation of T lymphocytes that largely are confined to the skin at the time of diagnosis.1 The incidence of CTCL rose steadily for more than 25 years, with an annual age-adjusted incidence of 6.4 to 9.6 cases per million individuals in the United States from 1973 to 2002.2 Mycosis fungoides is the most common classification of CTCL. It usually is characterized by patches or plaques of scaly erythema or poikiloderma; however, it also can present with annular, arcuate, concentrative, annular and linear morphologies. Mycosis fungoides tumor cells typically express a mature memory T helper cell phenotype of CD3+, CD4+, and CD8−, but there are different variants that have been discovered.3 Mycosis fungoides distributed in a spiral pattern is a distinctly unusual manifestation. Mechanisms of such dynamic morphologies are unknown but may represent an interplay between malignant cell proliferation and lost immune responses in temporospatial relationships.
The presence of keratotic gyrate lesions on acral surfaces should raise the possibility of pagetoid reticulosis. However, our patient had a history of MF involving areas of the body beyond the extremities, making this diagnosis less likely. Pagetoid reticulosis is categorized as an MF variant under the current World Health Organization– European Organization for Research and Treatment of Cancer classification for cutaneous lymphomas.4 Pagetoid reticulosis clinically presents as a solitary psoriasiform or hyperkeratotic patch or plaque that affects the distal extremities. Variable immunophenotypes have been shown in pagetoid reticulosis, such as CD4−/CD8+ and CD4−/CD8−, while classic MF typically shows CD4+/CD8−, as in our case.5
Tinea pedis is a superficial fungal infection usually caused by anthropophilic dermatophytes, with Trichophyton rubrum being the most common organism. Four common clinical presentations of tinea pedis have been identified: interdigital, moccasin, vesicular, and acute ulcerative. Clinical presentation ranges from macerations, ulcerations, and erosions in the toe web spaces to dry hyperkeratotic scaling and fissures on the plantar foot.6 Tinea pedis primarily affects the plantar and interdigital spaces, sparing the dorsal foot and ankle. Treatment is recommended to alleviate symptoms and limit the spread of infection; topical antifungals for 4 weeks is the treatment of choice. However, recurrence is common, and maintenance therapy often is indicated. Oral antifungals or a combination of both topical and oral medications may be needed in certain cases.7
Erythema annulare centrifugum (EAC) is a rare dermatologic disease described as erythematous or urticarial papules that can enlarge centrifugally to form annular lesions that clear centrally. Thought to be a hypersensitivity reaction to an underlying condition, EAC has been associated with fungal infections, various cutaneous diseases, and even internal malignancies. Clinically, EAC can be divided into 2 forms: deep and superficial. Deep gyrate erythema is characterized by a firm indurated border with rare scaling and pruritus that histologically shows perivascular lymphocytic infiltration in the upper and deep dermis. Superficial gyrate erythema has minimally elevated lesions with an indistinct border and trailing scales and pruritus; histopathologic findings present a dense, perivascular, lymphocytic infiltration restricted to the upper dermis.8 Therapy for EAC is directed at relieving symptoms and treating the underlying condition if there is one associated.
Granuloma annulare (GA) is a common skin disorder classically characterized by ringed erythematous plaques, though many variants have been identified. Localized GA is the most common variant and presents with pink-red, nonscaly, annular patches or plaques, typically affecting the hands and feet. Generalized GA is characterized as diffuse annular patches or plaques classically affecting the trunk and extremities. Histology is notable for mucin with a palisading or interstitial pattern of granulomatous inflammation, which was not evident in our patient.9 Topical or intralesional corticosteroids are the first-line treatment of localized GA; however, localized GA generally is self-limited, and treatment often is not necessary. Treatment with cryosurgery, laser therapy, and topical dapsone and tacrolimus also has been described, but evidence of the efficacy of these agents is limited. For generalized GA, phototherapy currently is the most reliable therapy. Systemic therapies include antimalarials, fumaric acid esters, biologics, antimicrobials, and isotretinoin.10
Erythema gyratum repens (EGR) is a rare dermatologic disease described as erythematous concentric bands arranged in parallel rings that can be annular, figurate, or gyrate, with a fine scale trailing the leading edge. Histopathologic features of EGR are nonspecific but are characterized by a perivascular, superficial, mononuclear dermatitis. Diagnosis is based on its characteristic clinical presentation. Although EGR commonly is associated with internal malignancies such as bronchial carcinoma, it also may be associated with benign conditions.11 Improvement often is seen with successful therapy of the underlying associated malignancy.12
Treatment of MF is based on tumor-node-metastasisblood classification, prognostic factors, and clinical stage at the time of diagnosis. Early-stage MF (IA–IIA) commonly is treated with skin-directed therapies such as topical corticosteroids, topical mechlorethamine, topical retinoids, UV phototherapy, and localized radiotherapy. In late stages (IIB–IV), systemic therapy is indicated and includes systemic retinoids, interferon alfa, chemotherapy, monoclonal antibodies, and psoralen plus UVA.13 In many cases, patients may require combination therapy to achieve remission or better control of their condition, as in our patient.
A 60-year-old man presented with a whorl-like plaque on the left ankle that he had noticed while undergoing treatment with narrowband UVB every other week and nitrogen mustard gel daily for stage IB cutaneous T-cell lymphoma, mycosis fungoides type. He denied pain, pruritus, and any other associated symptoms at the site. He denied recent illness, new medications, or changes in diet. His medical history included multiple sclerosis, vascular disease, and stroke. Physical examination revealed an 8×6-cm, welldemarcated, slightly scaly, erythematous plaque with a spiral appearance and peripheral hyperpigmentation involving the left ankle. The remainder of the examination was notable for well-controlled mycosis fungoides with several hyperpigmented patches at sites of prior involvement on the trunk and upper and lower extremities. No cervical, axillary, or inguinal lymphadenopathy was noted. A 4-mm punch biopsy was performed and sent for histopathologic examination.
COVID-19 linked to rise in suicide-related ED visits among youth
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
After a steep decline in the first months of the COVID-19 pandemic, youth with suicidal thoughts and behaviors visiting an emergency department (ED) resurged with an overrepresentation among girls and children without a prior psychiatric history, according to a cross-sectional study conducted at Kaiser Permanente Northern California.
The latest findings, published in JAMA Psychiatry, are highly consistent with Centers for Disease Control and Prevention data published a few months earlier in the Morbidity and Mortality Weekly Report that looked at these trends among young people aged 12-25 before and during the pandemic. In addition, the new data suggest that prevention efforts might be particularly helpful for these young people and their families.
“As suicide-related encounters have made up more ED volume during the pandemic, increasing ED-based interventions, staff trained in addressing emergency mental health needs, and aftercare resources may also be valuable in addressing the needs of this population,” wrote Kathryn K. Erickson-Ridout, MD, PhD, first author of the more recent study and an adjunct investigator with the Kaiser Permanente Division of Research, Oakland, Calif.
“While these results suggest many of our youth are coping well through the pandemic, we need to be vigilant as health care workers,” she said in an interview.
Dr. Ridout and associates evaluated suicide-related ED visits among children aged 5-17 years presenting to EDs in the Kaiser Permanente Northern California system. Four periods in 2020 were compared with the same four periods in 2019.
In the first period, March through May 2020, the incidence rate ratio (IRR) of suicide-related ED visits among children and adolescents fell more than 40% (IRR, 0.53; P < .001) relative to the same months in 2019. This is consistent with a broader decline in other types of ED visits during the beginning of the COVID-19 pandemic.
In the periods of June through August and September to Dec. 15, suicide-related ED visits increased, reaching prepandemic levels overall but with differences between genders. In girls, an increase in the first period reached significance (IRR, 1.19; P = .04) and then climbed even higher in the second (hazard ratio, 1.22; P < .001).
Youth present with no prior psychiatric history
Among boys, the increase relative to 2019 in the first period was modest and nonsignificant (IRR, 1.05; P = .26) In the second period, suicide-related ED visits fell and the difference relative to the same period in 2019 reached statistical significance (IRR, 0.81; P = .02).
Other characteristics of suicide-related ED visits during the pandemic were also different from those observed in 2019. One was an increase in visits from youth with no prior history of mental health or suicide-related outpatient visits.
“This finding may suggest that a youth’s first presentation to the ED for suicidal thoughts and behaviors differed during the pandemic, compared to prior to the pandemic,” Dr. Erickson-Ridout explained. “Mental health comorbidities first diagnosed at the ED encounter may suggest an increased complexity at first presentation.”
These data are remarkably similar to ED visits for suicide attempts that were reported by the CDC several months earlier. The CDC data were drawn from the National Syndromic Surveillance Program, which captures data from 71% of the EDs in the United States.
Measured from March 29 to April 25, 2020, ED visits for a suicide attempt declined by more than 25% in both girls and boys, but by early May, those visits were already starting to return to prepandemic levels, particularly among girls, according to the CDC report. When evaluated from July 26 through Aug. 22, 2020, the mean weekly ED visits for suspected suicide attempts had increased 26.2% among girls but only 10.8% among boys, compared with the same period in 2019.
‘More severe distress’ found among girls
In the most recent period evaluated, starting Feb. 21, 2021, and extending to March 20, , compared with the same period in 2019. For boys, the increase was 3.7%.
Higher rates of suicide attempts among girls have been reported by others independent of the COVID-19 pandemic, but the CDC investigators led by Ellen Yard, PhD, a researcher in the CDC’s National Center for Environmental Health, Chamblee, Ga., reported that others have also found greater suicide ideation and attempts by girls during the pandemic.
Neither study was designed to isolate the reason or reasons for an increase in suicide-related ED visits overall or among girls specifically, but Dr. Yard and her coinvestigators speculated that social distancing and isolation during the COVID-19 pandemic might be affecting girls more.
The faster increase in suicide attempts among girls compared with boys “speaks to the importance of upstream prevention to prevent this group from becoming suicidal in the first place as well as improving suicide care both during and after ED visits,” said Dr. Yard, who was interviewed about this research.
For clinicians, she recommended “scaling up adoption of evidence-based best practice.” She cited two such resources from the National Action Alliance for Suicide Prevention (NAASP). One resource is the Best Practices in Care Transitions for Individuals with Suicide Risk: Inpatient Care to Outpatient Care; the other is called Recommended Standard Care. Both are available online for free from the NAASP.
Dr. Erickson-Ridout and Dr. Yard reported no potential conflicts of interest.
FROM JAMA PSYCHIATRY