User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
What’s right and wrong for doctors on social media
She went by the name “Dr. Roxy” on social media and became something of a sensation on TikTok, where she livestreamed her patients’ operations. Ultimately, however, plastic surgeon Katharine Roxanne Grawe, MD, lost her medical license based partly on her “life-altering, reckless treatment,” heightened by her social media fame. In July, the Ohio state medical board permanently revoked Dr. Grawe’s license after twice reprimanding her for her failure to meet the standard of care. The board also determined that, by livestreaming procedures, she placed her patients in danger of immediate and serious harm.
Although most doctors don’t use social media to the degree that Dr. Grawe did, using the various platforms – from X (formerly Twitter) to Facebook, Instagram, and TikTok – can be a slippery slope. Medscape’s Physician Behavior Report 2023 revealed that doctors have seen their share of unprofessional or offensive social media use from their peers. Nearly 7 in 10 said it is unethical for a doctor to act rudely, offensively, or unprofessionally on social media, even if their medical practice isn’t mentioned. As one physician put it: “Professional is not a 9-to-5 descriptor.”
“There’s still a stigma attached,” said Liudmila Schafer, MD, an oncologist with The Doctor Connect, a career consulting firm. “Physicians face a tougher challenge due to societal expectations of perfection, with greater consequences for mistakes. We’re under constant ‘observation’ from peers, employers, and patients.”
Beverly Hills plastic surgeon Jay Calvert, MD, says he holds firm boundaries with how he uses social media. “I do comedy on the side, but it’s not acceptable for me as a doctor to share that on social media,” he said. “People want doctors who are professional, and I’m always concerned about how I present myself.”
Dr. Calvert said it is fairly easy to spot doctors who cross the line with social media. “You have to hold yourself back when posting. Doing things like dancing in the OR are out of whack with the profession.”
According to Dr. Schafer, a definite line to avoid crossing is offering medical advice or guidance on social media. “You also can’t discuss confidential practice details, respond to unfamiliar contacts, or discuss institutional policies without permission,” she said. “It’s important to add disclaimers if a personal scientific opinion is shared without reference [or] research or with unchecked sources.”
Navigating the many social media sites
Each social media platform has its pros and cons. Doctors need to determine why to use them and what the payback of each might be. Dr. Schafer uses multiple sites, including LinkedIn, Facebook, Instagram, X, Threads, YouTube, and, to a lesser degree, Clubhouse. How and what she posts on each varies. “I use them almost 95% professionally,” she said. “It’s challenging to meet and engage in person, so that is where social media helps.”
Stephen Pribut, MD, a Washington-based podiatrist, likes to use X as an information source. He follows pretty simple rules when it comes to what he tweets and shares on various sites: “I stay away from politics and religion,” he said. “I also avoid controversial topics online, such as vaccines.”
Joseph Daibes, DO, who specializes in cardiovascular medicine at New Jersey Heart and Vein, Clifton, said he has changed how he uses social media. “Initially, I was a passive consumer, but as I recognized the importance of accurate medical information online, I became more active in weighing in responsibly, occasionally sharing studies, debunking myths, and engaging in meaningful conversations,” he said. “Social media can get dangerous, so we have a duty to use it responsibly, and I cannot stress that enough.”
For plastic surgeons like Dr. Calvert, the visual platforms such as Instagram can prove invaluable for marketing purposes. “I’ve been using Instagram since 2012, and it’s been my most positive experience,” he said. “I don’t generate business from it, but I use it to back up my qualifications as a surgeon.”
Potential patients like to scroll through posts by plastic surgeons to learn what their finished product looks like, Dr. Calvert said. In many cases, plastic surgeons hire social media experts to cultivate their content. “I’ve hired and fired social media managers over the years, ultimately deciding I should develop my own content,” he said. “I want people to see the same doctor on social media that they will see in the office. I like an authentic presentation, not glitzy.”
Social media gone wrong
Dr. Calvert said that in the world of plastic surgery, some doctors use social media to present “before and after” compilations that in his opinion aren’t necessarily fully authentic, and this rubs him wrong. “There’s a bit of ‘cheating’ in some of these posts, using filters, making the ‘befores’ particularly bad, and other tricks,” he said.
Dr. Daibes has also seen his share of social media misuse: ”Red flags include oversharing personal indulgences, engaging in online spats, or making unfounded medical claims,” he said. “It’s essential to remember our role as educators and advocates, and to present ourselves in a way that upholds the dignity of our profession.”
At the end of the day, social media can have positive uses for physicians, and it is clearly here to stay. The onus for responsible use ultimately falls to the physicians using it.
Dr. Daibes emphasizes the fact that a doctor’s words carry weight – perhaps more so than those of other professionals. “The added scrutiny is good because it keeps us accountable; it’s crucial that our information is accurate,” he said. “The downside is that the scrutiny can be stifling at times and lead to self-censorship, even on nonmedical matters.”
Physicians have suggested eight guidelines for doctors to follow when using social media:
- Remember that you represent your profession, even if posting on personal accounts.
- Never post from the operating room, the emergency department, or any sort of medical space.
- If you’re employed, before you post, check with your employer to see whether they have any rules or guidance surrounding social media.
- Never use social media to badmouth colleagues, hospitals, or other healthcare organizations.
- Never use social media to dispense medical advice.
- Steer clear of the obvious hot-button issues, like religion and politics.
- Always protect patient privacy when posting.
- Be careful with how and whom you engage on social media.
A version of this article first appeared on Medscape.com.
She went by the name “Dr. Roxy” on social media and became something of a sensation on TikTok, where she livestreamed her patients’ operations. Ultimately, however, plastic surgeon Katharine Roxanne Grawe, MD, lost her medical license based partly on her “life-altering, reckless treatment,” heightened by her social media fame. In July, the Ohio state medical board permanently revoked Dr. Grawe’s license after twice reprimanding her for her failure to meet the standard of care. The board also determined that, by livestreaming procedures, she placed her patients in danger of immediate and serious harm.
Although most doctors don’t use social media to the degree that Dr. Grawe did, using the various platforms – from X (formerly Twitter) to Facebook, Instagram, and TikTok – can be a slippery slope. Medscape’s Physician Behavior Report 2023 revealed that doctors have seen their share of unprofessional or offensive social media use from their peers. Nearly 7 in 10 said it is unethical for a doctor to act rudely, offensively, or unprofessionally on social media, even if their medical practice isn’t mentioned. As one physician put it: “Professional is not a 9-to-5 descriptor.”
“There’s still a stigma attached,” said Liudmila Schafer, MD, an oncologist with The Doctor Connect, a career consulting firm. “Physicians face a tougher challenge due to societal expectations of perfection, with greater consequences for mistakes. We’re under constant ‘observation’ from peers, employers, and patients.”
Beverly Hills plastic surgeon Jay Calvert, MD, says he holds firm boundaries with how he uses social media. “I do comedy on the side, but it’s not acceptable for me as a doctor to share that on social media,” he said. “People want doctors who are professional, and I’m always concerned about how I present myself.”
Dr. Calvert said it is fairly easy to spot doctors who cross the line with social media. “You have to hold yourself back when posting. Doing things like dancing in the OR are out of whack with the profession.”
According to Dr. Schafer, a definite line to avoid crossing is offering medical advice or guidance on social media. “You also can’t discuss confidential practice details, respond to unfamiliar contacts, or discuss institutional policies without permission,” she said. “It’s important to add disclaimers if a personal scientific opinion is shared without reference [or] research or with unchecked sources.”
Navigating the many social media sites
Each social media platform has its pros and cons. Doctors need to determine why to use them and what the payback of each might be. Dr. Schafer uses multiple sites, including LinkedIn, Facebook, Instagram, X, Threads, YouTube, and, to a lesser degree, Clubhouse. How and what she posts on each varies. “I use them almost 95% professionally,” she said. “It’s challenging to meet and engage in person, so that is where social media helps.”
Stephen Pribut, MD, a Washington-based podiatrist, likes to use X as an information source. He follows pretty simple rules when it comes to what he tweets and shares on various sites: “I stay away from politics and religion,” he said. “I also avoid controversial topics online, such as vaccines.”
Joseph Daibes, DO, who specializes in cardiovascular medicine at New Jersey Heart and Vein, Clifton, said he has changed how he uses social media. “Initially, I was a passive consumer, but as I recognized the importance of accurate medical information online, I became more active in weighing in responsibly, occasionally sharing studies, debunking myths, and engaging in meaningful conversations,” he said. “Social media can get dangerous, so we have a duty to use it responsibly, and I cannot stress that enough.”
For plastic surgeons like Dr. Calvert, the visual platforms such as Instagram can prove invaluable for marketing purposes. “I’ve been using Instagram since 2012, and it’s been my most positive experience,” he said. “I don’t generate business from it, but I use it to back up my qualifications as a surgeon.”
Potential patients like to scroll through posts by plastic surgeons to learn what their finished product looks like, Dr. Calvert said. In many cases, plastic surgeons hire social media experts to cultivate their content. “I’ve hired and fired social media managers over the years, ultimately deciding I should develop my own content,” he said. “I want people to see the same doctor on social media that they will see in the office. I like an authentic presentation, not glitzy.”
Social media gone wrong
Dr. Calvert said that in the world of plastic surgery, some doctors use social media to present “before and after” compilations that in his opinion aren’t necessarily fully authentic, and this rubs him wrong. “There’s a bit of ‘cheating’ in some of these posts, using filters, making the ‘befores’ particularly bad, and other tricks,” he said.
Dr. Daibes has also seen his share of social media misuse: ”Red flags include oversharing personal indulgences, engaging in online spats, or making unfounded medical claims,” he said. “It’s essential to remember our role as educators and advocates, and to present ourselves in a way that upholds the dignity of our profession.”
At the end of the day, social media can have positive uses for physicians, and it is clearly here to stay. The onus for responsible use ultimately falls to the physicians using it.
Dr. Daibes emphasizes the fact that a doctor’s words carry weight – perhaps more so than those of other professionals. “The added scrutiny is good because it keeps us accountable; it’s crucial that our information is accurate,” he said. “The downside is that the scrutiny can be stifling at times and lead to self-censorship, even on nonmedical matters.”
Physicians have suggested eight guidelines for doctors to follow when using social media:
- Remember that you represent your profession, even if posting on personal accounts.
- Never post from the operating room, the emergency department, or any sort of medical space.
- If you’re employed, before you post, check with your employer to see whether they have any rules or guidance surrounding social media.
- Never use social media to badmouth colleagues, hospitals, or other healthcare organizations.
- Never use social media to dispense medical advice.
- Steer clear of the obvious hot-button issues, like religion and politics.
- Always protect patient privacy when posting.
- Be careful with how and whom you engage on social media.
A version of this article first appeared on Medscape.com.
She went by the name “Dr. Roxy” on social media and became something of a sensation on TikTok, where she livestreamed her patients’ operations. Ultimately, however, plastic surgeon Katharine Roxanne Grawe, MD, lost her medical license based partly on her “life-altering, reckless treatment,” heightened by her social media fame. In July, the Ohio state medical board permanently revoked Dr. Grawe’s license after twice reprimanding her for her failure to meet the standard of care. The board also determined that, by livestreaming procedures, she placed her patients in danger of immediate and serious harm.
Although most doctors don’t use social media to the degree that Dr. Grawe did, using the various platforms – from X (formerly Twitter) to Facebook, Instagram, and TikTok – can be a slippery slope. Medscape’s Physician Behavior Report 2023 revealed that doctors have seen their share of unprofessional or offensive social media use from their peers. Nearly 7 in 10 said it is unethical for a doctor to act rudely, offensively, or unprofessionally on social media, even if their medical practice isn’t mentioned. As one physician put it: “Professional is not a 9-to-5 descriptor.”
“There’s still a stigma attached,” said Liudmila Schafer, MD, an oncologist with The Doctor Connect, a career consulting firm. “Physicians face a tougher challenge due to societal expectations of perfection, with greater consequences for mistakes. We’re under constant ‘observation’ from peers, employers, and patients.”
Beverly Hills plastic surgeon Jay Calvert, MD, says he holds firm boundaries with how he uses social media. “I do comedy on the side, but it’s not acceptable for me as a doctor to share that on social media,” he said. “People want doctors who are professional, and I’m always concerned about how I present myself.”
Dr. Calvert said it is fairly easy to spot doctors who cross the line with social media. “You have to hold yourself back when posting. Doing things like dancing in the OR are out of whack with the profession.”
According to Dr. Schafer, a definite line to avoid crossing is offering medical advice or guidance on social media. “You also can’t discuss confidential practice details, respond to unfamiliar contacts, or discuss institutional policies without permission,” she said. “It’s important to add disclaimers if a personal scientific opinion is shared without reference [or] research or with unchecked sources.”
Navigating the many social media sites
Each social media platform has its pros and cons. Doctors need to determine why to use them and what the payback of each might be. Dr. Schafer uses multiple sites, including LinkedIn, Facebook, Instagram, X, Threads, YouTube, and, to a lesser degree, Clubhouse. How and what she posts on each varies. “I use them almost 95% professionally,” she said. “It’s challenging to meet and engage in person, so that is where social media helps.”
Stephen Pribut, MD, a Washington-based podiatrist, likes to use X as an information source. He follows pretty simple rules when it comes to what he tweets and shares on various sites: “I stay away from politics and religion,” he said. “I also avoid controversial topics online, such as vaccines.”
Joseph Daibes, DO, who specializes in cardiovascular medicine at New Jersey Heart and Vein, Clifton, said he has changed how he uses social media. “Initially, I was a passive consumer, but as I recognized the importance of accurate medical information online, I became more active in weighing in responsibly, occasionally sharing studies, debunking myths, and engaging in meaningful conversations,” he said. “Social media can get dangerous, so we have a duty to use it responsibly, and I cannot stress that enough.”
For plastic surgeons like Dr. Calvert, the visual platforms such as Instagram can prove invaluable for marketing purposes. “I’ve been using Instagram since 2012, and it’s been my most positive experience,” he said. “I don’t generate business from it, but I use it to back up my qualifications as a surgeon.”
Potential patients like to scroll through posts by plastic surgeons to learn what their finished product looks like, Dr. Calvert said. In many cases, plastic surgeons hire social media experts to cultivate their content. “I’ve hired and fired social media managers over the years, ultimately deciding I should develop my own content,” he said. “I want people to see the same doctor on social media that they will see in the office. I like an authentic presentation, not glitzy.”
Social media gone wrong
Dr. Calvert said that in the world of plastic surgery, some doctors use social media to present “before and after” compilations that in his opinion aren’t necessarily fully authentic, and this rubs him wrong. “There’s a bit of ‘cheating’ in some of these posts, using filters, making the ‘befores’ particularly bad, and other tricks,” he said.
Dr. Daibes has also seen his share of social media misuse: ”Red flags include oversharing personal indulgences, engaging in online spats, or making unfounded medical claims,” he said. “It’s essential to remember our role as educators and advocates, and to present ourselves in a way that upholds the dignity of our profession.”
At the end of the day, social media can have positive uses for physicians, and it is clearly here to stay. The onus for responsible use ultimately falls to the physicians using it.
Dr. Daibes emphasizes the fact that a doctor’s words carry weight – perhaps more so than those of other professionals. “The added scrutiny is good because it keeps us accountable; it’s crucial that our information is accurate,” he said. “The downside is that the scrutiny can be stifling at times and lead to self-censorship, even on nonmedical matters.”
Physicians have suggested eight guidelines for doctors to follow when using social media:
- Remember that you represent your profession, even if posting on personal accounts.
- Never post from the operating room, the emergency department, or any sort of medical space.
- If you’re employed, before you post, check with your employer to see whether they have any rules or guidance surrounding social media.
- Never use social media to badmouth colleagues, hospitals, or other healthcare organizations.
- Never use social media to dispense medical advice.
- Steer clear of the obvious hot-button issues, like religion and politics.
- Always protect patient privacy when posting.
- Be careful with how and whom you engage on social media.
A version of this article first appeared on Medscape.com.
Video-Based Coaching for Dermatology Resident Surgical Education
To the Editor:
Video-based coaching (VBC) involves a surgeon recording a surgery and then reviewing the video with a surgical coach; it is a form of education that is gaining popularity among surgical specialties.1 Video-based education is underutilized in dermatology residency training.2 We conducted a pilot study at our dermatology residency program to evaluate the efficacy and feasibility of VBC.
The University of Texas at Austin Dell Medical School institutional review board approved this study. All 4 first-year dermatology residents were recruited to participate in this study. Participants filled out a prestudy survey assessing their surgical experience, confidence in performing surgery, and attitudes on VBC. Participants used a head-mounted point-of-view camera to record themselves performing a wide local excision on the trunk or extremities of a live human patient. Participants then reviewed the recording on their own and scored themselves using the Objective Structured Assessment of Technical Skills (OSATS) scoring table (scored from 1 to 5, with 5 being the highest possible score for each element), which is a validated tool for assessing surgical skills (eTable 1).3 Given that there were no assistants participating in the surgery, this element of the OSATS scoring table was excluded, making a maximum possible score of 30 and a minimum possible score of 6. After scoring themselves, participants then had a 1-on-1 coaching session with a fellowship-trained dermatologic surgeon (M.F. or T.H.) via online teleconferencing.
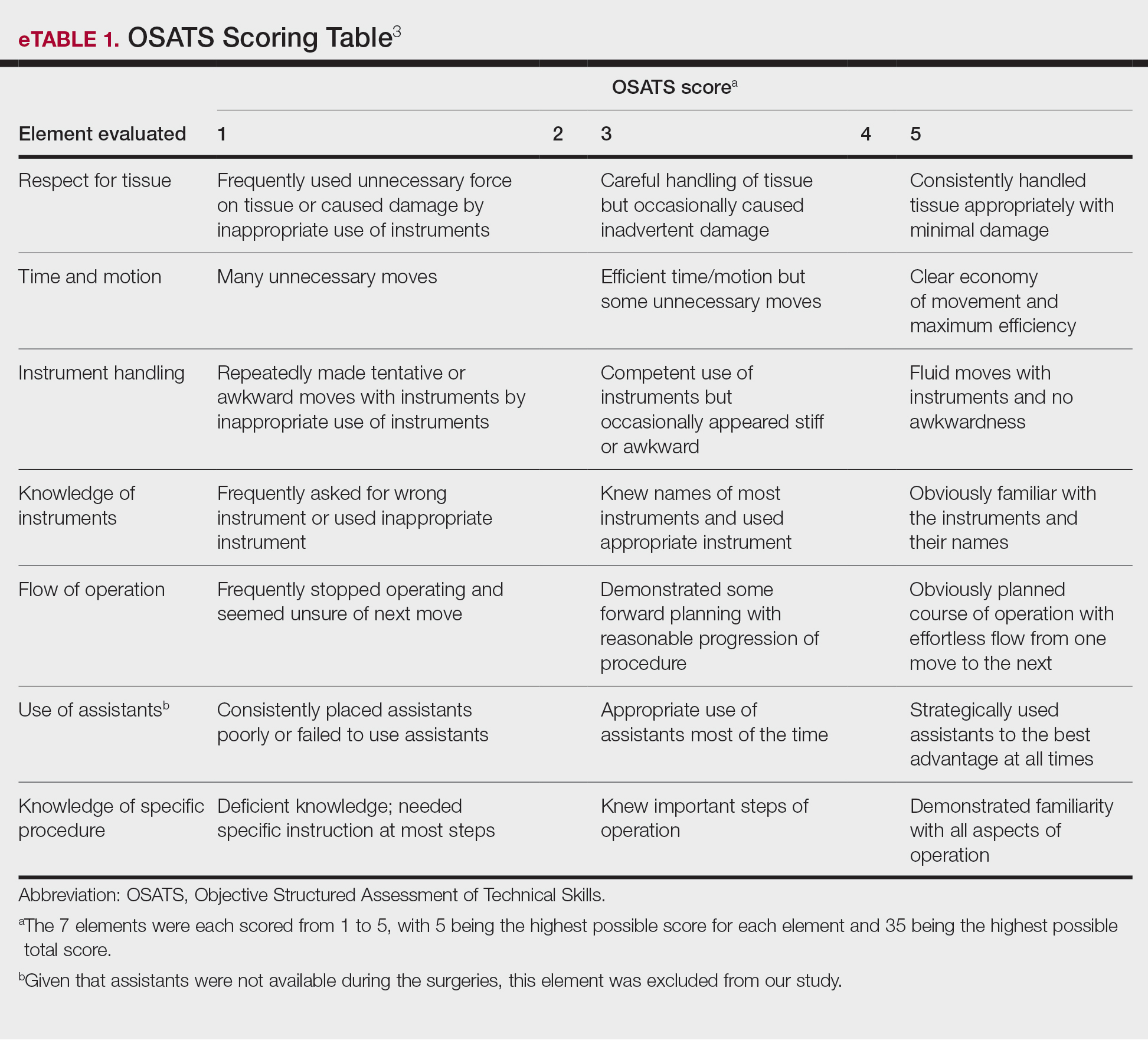
During the coaching session, participants and coaches reviewed the video. The surgical coaches also scored the residents using the OSATS, then residents and coaches discussed how the resident could improve using the OSATS scores as a guide. The residents then completed a poststudy survey assessing their surgical experience, confidence in performing surgery, and attitudes on VBC. Descriptive statistics were reported.
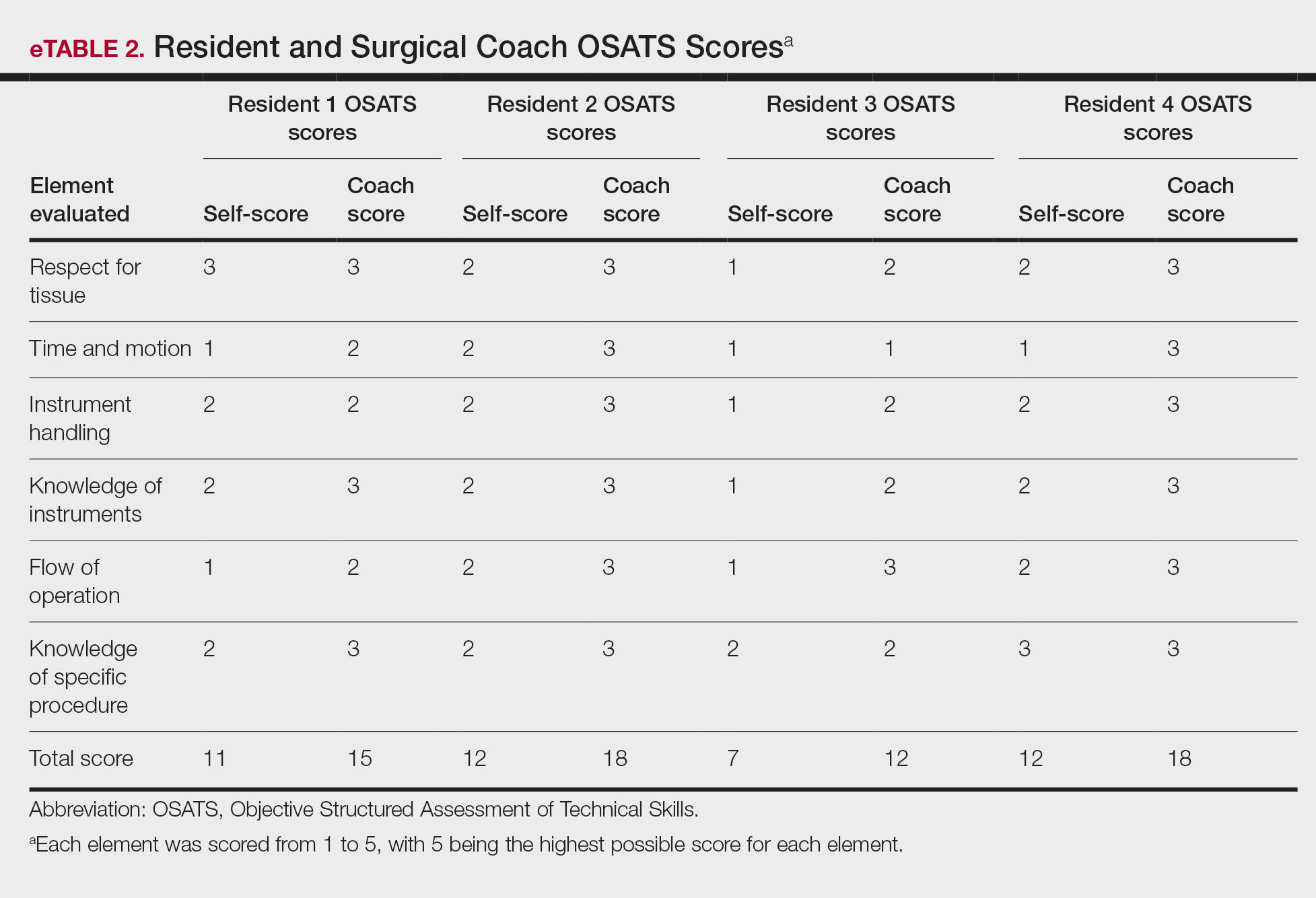
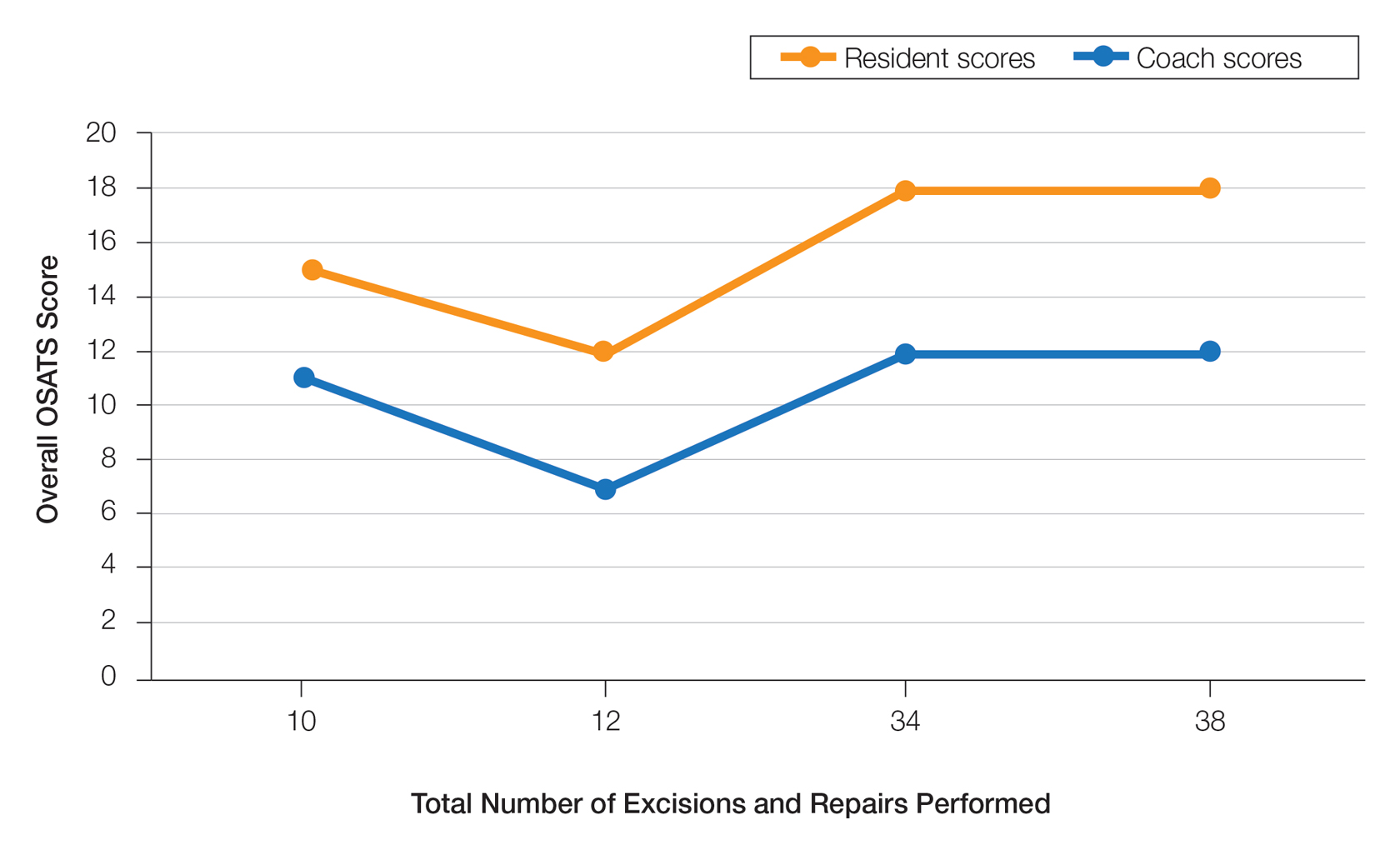
On average, residents spent 31.3 minutes reviewing their own surgeries and scoring themselves. The average time for a coaching session, which included time spent scoring, was 13.8 minutes. Residents scored themselves lower than the surgical coaches did by an average of 5.25 points (eTable 2). Residents gave themselves an average total score of 10.5, while their respective surgical coaches gave the residents an average score of 15.75. There was a trend of residents with greater surgical experience having higher OSATS scores (Figure). After the coaching session, 3 of 4 residents reported that they felt more confident in their surgical skills. All residents felt more confident in assessing their surgical skills and felt that VBC was an effective teaching measure. All residents agreed that VBC should be continued as part of their residency training.
Video-based coaching has the potential to provide several benefits for dermatology trainees. Because receiving feedback intraoperatively often can be distracting and incomplete, video review can instead allow the surgeon to focus on performing the surgery and then later focus on learning while reviewing the video.1,4 Feedback also can be more comprehensive and delivered without concern for time constraints or disturbing clinic flow as well as without the additional concern of the patient overhearing comments and feedback.3 Although independent video review in the absence of coaching can lead to improvement in surgical skills, the addition of VBC provides even greater potential educational benefit.4 During the COVID-19 pandemic, VBC allowed coaches to provide feedback without additional exposures. We utilized dermatologic surgery faculty as coaches, but this format of training also would apply to general dermatology faculty.
Another goal of VBC is to enhance a trainee’s ability to perform self-directed learning, which requires accurate self-assessment.4 Accurately assessing one’s own strengths empowers a trainee to act with appropriate confidence, while understanding one’s own weaknesses allows a trainee to effectively balance confidence and caution in daily practice.5 Interestingly, in our study all residents scored themselves lower than surgical coaches, but with 1 coaching session, the residents subsequently reported greater surgical confidence.
Time constraints can be a potential barrier to surgical coaching.4 Our study demonstrates that VBC requires minimal time investment. Increasing the speed of video playback allowed for efficient evaluation of resident surgeries without compromising the coach’s ability to provide comprehensive feedback. Our feedback sessions were performed virtually, which allowed for ease of scheduling between trainees and coaches.
Our pilot study demonstrated that VBC is relatively easy to implement in a dermatology residency training setting, leveraging relatively low-cost technologies and allowing for a means of learning that residents felt was effective. Video-based coaching requires minimal time investment from both trainees and coaches and has the potential to enhance surgical confidence. Our current study is limited by its small sample size. Future studies should include follow-up recordings and assess the efficacy of VBC in enhancing surgical skills.
- Greenberg CC, Dombrowski J, Dimick JB. Video-based surgical coaching: an emerging approach to performance improvement. JAMA Surg. 2016;151:282-283.
- Dai J, Bordeaux JS, Miller CJ, et al. Assessing surgical training and deliberate practice methods in dermatology residency: a survey of dermatology program directors. Dermatol Surg. 2016;42:977-984.
- Chitgopeker P, Sidey K, Aronson A, et al. Surgical skills video-based assessment tool for dermatology residents: a prospective pilot study. J Am Acad Dermatol. 2020;83:614-616.
- Bull NB, Silverman CD, Bonrath EM. Targeted surgical coaching can improve operative self-assessment ability: a single-blinded nonrandomized trial. Surgery. 2020;167:308-313.
- Eva KW, Regehr G. Self-assessment in the health professions: a reformulation and research agenda. Acad Med. 2005;80(10 suppl):S46-S54.
To the Editor:
Video-based coaching (VBC) involves a surgeon recording a surgery and then reviewing the video with a surgical coach; it is a form of education that is gaining popularity among surgical specialties.1 Video-based education is underutilized in dermatology residency training.2 We conducted a pilot study at our dermatology residency program to evaluate the efficacy and feasibility of VBC.
The University of Texas at Austin Dell Medical School institutional review board approved this study. All 4 first-year dermatology residents were recruited to participate in this study. Participants filled out a prestudy survey assessing their surgical experience, confidence in performing surgery, and attitudes on VBC. Participants used a head-mounted point-of-view camera to record themselves performing a wide local excision on the trunk or extremities of a live human patient. Participants then reviewed the recording on their own and scored themselves using the Objective Structured Assessment of Technical Skills (OSATS) scoring table (scored from 1 to 5, with 5 being the highest possible score for each element), which is a validated tool for assessing surgical skills (eTable 1).3 Given that there were no assistants participating in the surgery, this element of the OSATS scoring table was excluded, making a maximum possible score of 30 and a minimum possible score of 6. After scoring themselves, participants then had a 1-on-1 coaching session with a fellowship-trained dermatologic surgeon (M.F. or T.H.) via online teleconferencing.

During the coaching session, participants and coaches reviewed the video. The surgical coaches also scored the residents using the OSATS, then residents and coaches discussed how the resident could improve using the OSATS scores as a guide. The residents then completed a poststudy survey assessing their surgical experience, confidence in performing surgery, and attitudes on VBC. Descriptive statistics were reported.

On average, residents spent 31.3 minutes reviewing their own surgeries and scoring themselves. The average time for a coaching session, which included time spent scoring, was 13.8 minutes. Residents scored themselves lower than the surgical coaches did by an average of 5.25 points (eTable 2). Residents gave themselves an average total score of 10.5, while their respective surgical coaches gave the residents an average score of 15.75. There was a trend of residents with greater surgical experience having higher OSATS scores (Figure). After the coaching session, 3 of 4 residents reported that they felt more confident in their surgical skills. All residents felt more confident in assessing their surgical skills and felt that VBC was an effective teaching measure. All residents agreed that VBC should be continued as part of their residency training.

Video-based coaching has the potential to provide several benefits for dermatology trainees. Because receiving feedback intraoperatively often can be distracting and incomplete, video review can instead allow the surgeon to focus on performing the surgery and then later focus on learning while reviewing the video.1,4 Feedback also can be more comprehensive and delivered without concern for time constraints or disturbing clinic flow as well as without the additional concern of the patient overhearing comments and feedback.3 Although independent video review in the absence of coaching can lead to improvement in surgical skills, the addition of VBC provides even greater potential educational benefit.4 During the COVID-19 pandemic, VBC allowed coaches to provide feedback without additional exposures. We utilized dermatologic surgery faculty as coaches, but this format of training also would apply to general dermatology faculty.
Another goal of VBC is to enhance a trainee’s ability to perform self-directed learning, which requires accurate self-assessment.4 Accurately assessing one’s own strengths empowers a trainee to act with appropriate confidence, while understanding one’s own weaknesses allows a trainee to effectively balance confidence and caution in daily practice.5 Interestingly, in our study all residents scored themselves lower than surgical coaches, but with 1 coaching session, the residents subsequently reported greater surgical confidence.
Time constraints can be a potential barrier to surgical coaching.4 Our study demonstrates that VBC requires minimal time investment. Increasing the speed of video playback allowed for efficient evaluation of resident surgeries without compromising the coach’s ability to provide comprehensive feedback. Our feedback sessions were performed virtually, which allowed for ease of scheduling between trainees and coaches.
Our pilot study demonstrated that VBC is relatively easy to implement in a dermatology residency training setting, leveraging relatively low-cost technologies and allowing for a means of learning that residents felt was effective. Video-based coaching requires minimal time investment from both trainees and coaches and has the potential to enhance surgical confidence. Our current study is limited by its small sample size. Future studies should include follow-up recordings and assess the efficacy of VBC in enhancing surgical skills.
To the Editor:
Video-based coaching (VBC) involves a surgeon recording a surgery and then reviewing the video with a surgical coach; it is a form of education that is gaining popularity among surgical specialties.1 Video-based education is underutilized in dermatology residency training.2 We conducted a pilot study at our dermatology residency program to evaluate the efficacy and feasibility of VBC.
The University of Texas at Austin Dell Medical School institutional review board approved this study. All 4 first-year dermatology residents were recruited to participate in this study. Participants filled out a prestudy survey assessing their surgical experience, confidence in performing surgery, and attitudes on VBC. Participants used a head-mounted point-of-view camera to record themselves performing a wide local excision on the trunk or extremities of a live human patient. Participants then reviewed the recording on their own and scored themselves using the Objective Structured Assessment of Technical Skills (OSATS) scoring table (scored from 1 to 5, with 5 being the highest possible score for each element), which is a validated tool for assessing surgical skills (eTable 1).3 Given that there were no assistants participating in the surgery, this element of the OSATS scoring table was excluded, making a maximum possible score of 30 and a minimum possible score of 6. After scoring themselves, participants then had a 1-on-1 coaching session with a fellowship-trained dermatologic surgeon (M.F. or T.H.) via online teleconferencing.

During the coaching session, participants and coaches reviewed the video. The surgical coaches also scored the residents using the OSATS, then residents and coaches discussed how the resident could improve using the OSATS scores as a guide. The residents then completed a poststudy survey assessing their surgical experience, confidence in performing surgery, and attitudes on VBC. Descriptive statistics were reported.

On average, residents spent 31.3 minutes reviewing their own surgeries and scoring themselves. The average time for a coaching session, which included time spent scoring, was 13.8 minutes. Residents scored themselves lower than the surgical coaches did by an average of 5.25 points (eTable 2). Residents gave themselves an average total score of 10.5, while their respective surgical coaches gave the residents an average score of 15.75. There was a trend of residents with greater surgical experience having higher OSATS scores (Figure). After the coaching session, 3 of 4 residents reported that they felt more confident in their surgical skills. All residents felt more confident in assessing their surgical skills and felt that VBC was an effective teaching measure. All residents agreed that VBC should be continued as part of their residency training.

Video-based coaching has the potential to provide several benefits for dermatology trainees. Because receiving feedback intraoperatively often can be distracting and incomplete, video review can instead allow the surgeon to focus on performing the surgery and then later focus on learning while reviewing the video.1,4 Feedback also can be more comprehensive and delivered without concern for time constraints or disturbing clinic flow as well as without the additional concern of the patient overhearing comments and feedback.3 Although independent video review in the absence of coaching can lead to improvement in surgical skills, the addition of VBC provides even greater potential educational benefit.4 During the COVID-19 pandemic, VBC allowed coaches to provide feedback without additional exposures. We utilized dermatologic surgery faculty as coaches, but this format of training also would apply to general dermatology faculty.
Another goal of VBC is to enhance a trainee’s ability to perform self-directed learning, which requires accurate self-assessment.4 Accurately assessing one’s own strengths empowers a trainee to act with appropriate confidence, while understanding one’s own weaknesses allows a trainee to effectively balance confidence and caution in daily practice.5 Interestingly, in our study all residents scored themselves lower than surgical coaches, but with 1 coaching session, the residents subsequently reported greater surgical confidence.
Time constraints can be a potential barrier to surgical coaching.4 Our study demonstrates that VBC requires minimal time investment. Increasing the speed of video playback allowed for efficient evaluation of resident surgeries without compromising the coach’s ability to provide comprehensive feedback. Our feedback sessions were performed virtually, which allowed for ease of scheduling between trainees and coaches.
Our pilot study demonstrated that VBC is relatively easy to implement in a dermatology residency training setting, leveraging relatively low-cost technologies and allowing for a means of learning that residents felt was effective. Video-based coaching requires minimal time investment from both trainees and coaches and has the potential to enhance surgical confidence. Our current study is limited by its small sample size. Future studies should include follow-up recordings and assess the efficacy of VBC in enhancing surgical skills.
- Greenberg CC, Dombrowski J, Dimick JB. Video-based surgical coaching: an emerging approach to performance improvement. JAMA Surg. 2016;151:282-283.
- Dai J, Bordeaux JS, Miller CJ, et al. Assessing surgical training and deliberate practice methods in dermatology residency: a survey of dermatology program directors. Dermatol Surg. 2016;42:977-984.
- Chitgopeker P, Sidey K, Aronson A, et al. Surgical skills video-based assessment tool for dermatology residents: a prospective pilot study. J Am Acad Dermatol. 2020;83:614-616.
- Bull NB, Silverman CD, Bonrath EM. Targeted surgical coaching can improve operative self-assessment ability: a single-blinded nonrandomized trial. Surgery. 2020;167:308-313.
- Eva KW, Regehr G. Self-assessment in the health professions: a reformulation and research agenda. Acad Med. 2005;80(10 suppl):S46-S54.
- Greenberg CC, Dombrowski J, Dimick JB. Video-based surgical coaching: an emerging approach to performance improvement. JAMA Surg. 2016;151:282-283.
- Dai J, Bordeaux JS, Miller CJ, et al. Assessing surgical training and deliberate practice methods in dermatology residency: a survey of dermatology program directors. Dermatol Surg. 2016;42:977-984.
- Chitgopeker P, Sidey K, Aronson A, et al. Surgical skills video-based assessment tool for dermatology residents: a prospective pilot study. J Am Acad Dermatol. 2020;83:614-616.
- Bull NB, Silverman CD, Bonrath EM. Targeted surgical coaching can improve operative self-assessment ability: a single-blinded nonrandomized trial. Surgery. 2020;167:308-313.
- Eva KW, Regehr G. Self-assessment in the health professions: a reformulation and research agenda. Acad Med. 2005;80(10 suppl):S46-S54.
PRACTICE POINTS
- Video-based coaching (VBC) for surgical procedures is an up-and-coming form of medical education that allows a “coach” to provide thoughtful and in-depth feedback while reviewing a recording with the surgeon in a private setting. This format has potential utility in teaching dermatology resident surgeons being coached by a dermatology faculty member.
- We performed a pilot study demonstrating that VBC can be performed easily with a minimal time investment for both the surgeon and the coach. Dermatology residents not only felt that VBC was an effective teaching method but also should become a formal part of their education.
Update on Dermatology Reimbursement in 2024
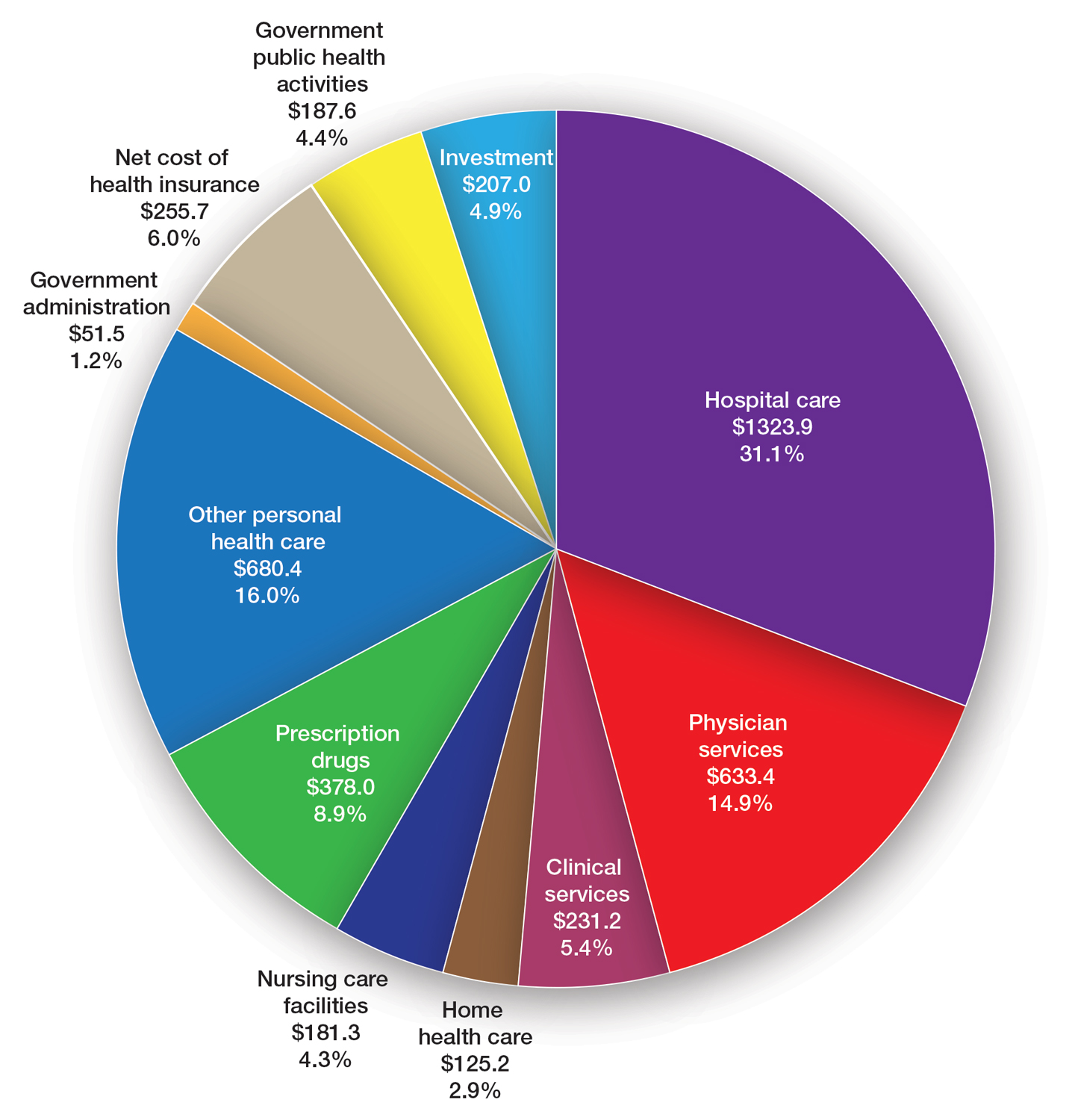
Health care spending in the United States remained relatively flat from 2019 to 2021 and only increased 2.7% in 2021, reaching $4.3 billion or $12,914 per person. Physician services account for 15% of health care spending (Figure). Relative value units (RVUs) signify the time it took a physician to complete a task multiplied by a conversion factor (CF). When RVUs initially were created in 1992 by what is now the Centers for Medicare &Medicaid Services (CMS), the CF was $32.00. Thirty-one years later, the CF is $33.89 in 2023; however, it would be $66.00 if the CF had increased with inflation.1 If the proposed 2024 Medicare physician fee schedule (MPFS) is adopted, the payment formula would decrease by 3.4% ($32.75) relative to the 2023 fee schedule ($33.89), which would be a 9% decrease relative to 2019 ($36.04).2,3 This reduction is due to the budget neutrality adjustment required by changes in RVUs, implementation of the evaluation and management (E/M) add-on code G2211, and proposed increases in primary are services.2,3 Since 2001, Medicare physician payment has declined by 26%.4 Adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index (MEI); (2) an expenditure target performance adjustment; and (3) miscellaneous adjustments, including those for budget neutrality required by law. Despite continued substantial increases in practice expenses, physicians’ reimbursement has remained flat while other service providers, such as those in skilled nursing facilities and hospitals, have received favorable payment increases compared to practice cost inflation and the Consumer Price Index.4
The CMS will not incorporate 2017 MEI cost weights for the RVUs in the MPFS rate setting for 2024 because all key measures of practice expenses in the MEI accelerated in 2022. Instead, the CMS is updating data on practice expense per hour to calculate payment for physician services with a survey for physician practices that launched on July 31, 2023.5 The American Medical Association contracted with Mathematica, an independent research company, to conduct a physician practice information survey that will be used to determine indirect practice expenses. Physicians should be on the lookout for emails regarding completion of these surveys and the appropriate financial expert in their practice should be contacted so the responses are accurate, as these data are key to future updates in the Medicare pay formula used to reimburse physicians.
Impact of Medicare Cuts
The recent congressional debt limit deal set spending caps for the next 2 fiscal years. Dermatology is facing an overall payment reduction of 1.87% (range, 1%–4%).2,3 The impact will depend on the services offered in an individual practice; for example, payment for a punch biopsy (Current Procedural Terminology [CPT] code 11104) would decrease by 3.9%. Payment for benign destruction (CPT code 17110) would decrease by 2.8%, and payment for even simple E/M of an established patient (CPT code 99213) would decrease by 1.6%. Overall, there would be a reduction of 2.75% for dermatopathology services, with a decrease of 2% for CPT code 88305 global and decreases for the technical component of 1% and professional component of 3%.2,3
Medicare cuts have reached a critical level, and physicians cannot continue to absorb the costs to own and operate their practices.4 This has led to health market consolidation, which in turn limits competition and patient access while driving up health care costs and driving down the quality of care. Small independent rural practices as well as those caring for historically marginalized patients will be disproportionately affected.
Proposed Addition of E/M Code G2211
In the calendar year (CY) 2021 final rule, the CMS tried to adopt a new add-on code—G2211—patients with a serious or complex condition that typically require referral and coordination of multispecialty care. Per the CMS, the primary policy goal of G2211 is to increase payments to primary care physicians and to reimburse them more appropriately for the care provided to patients with a serious or complex condition.2,3 It can be reported in conjunction with all office and outpatient E/M visits to better account for additional resources associated with primary care, or similarly ongoing medical care related to a patient’s single, serious condition, or complex condition.3 Typically, G2211 would not be used by dermatologists, as this add-on code requires visit complexity inherent to E/M associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single serious condition or a complex condition.2,3
Initially, the CMS assumed that G2211 would be reported with 90% of all office and outpatient E/M visit claims, which would account for a considerable portion of total MPFS schedule spending; however, the House of Medicine disagreed and believed it would be 75%.2,3 Given the extremely high utilization estimate, G2211 would have had a substantial effect on budget neutrality, accounting for an estimated increase of $3.3 billion and a corresponding 3.0% cut to the CY 2021 MPFS. Because of the potential payment reductions to physicians and a successful advocacy effort by organized medicine, including the American Academy of Dermatology Association (AADA), Congress delayed implementation of G2211 until CY 2024. Modifier -25 cannot be reported with G2211. The CMS revised its utilization assumptions from 90% of all E/M services to an initial utilization of 38% and then 54% when fully adopted. The proposed 2024 payment for G2211 is an additional $16.05.2,3
Advancing Health Equity With Healthcare Common Procedure Coding System G Codes
The CMS is proposing coding and payment for several new services to help underserved populations, including addressing unmet health-related social needs that can potentially interfere with the diagnosis and treatment of medical conditions, which includes paying for certain caregiver training services as well as payment for community health integration services.2,3 These are the first MPFS services designed to include care involving community health workers, who link underserved communities with critical health care and social services in the community. Additionally, the rule also proposes coding and payment for evaluating the risks related to social factors that affect a patient’s health, such as access to affordable quality health care, that can take place during an annual wellness visit or in combination with an E/M visit.2,3 As dermatologists, we should be familiar with this set of G codes, as we will likely use them in practice for patients with transportation needs.
Advocacy Efforts on Medicare Payment Reform
Medicare physician payment reform needs to happen at a national level. Advocacy efforts by the AADA and other groups have been underway to mitigate the proposed 2024 cuts. The Strengthening Medicare for Patients and Providers Act (HR 2474) is a bill that was introduced by a bipartisan coalition of physicians to provide an inflation-based increase in Medicare payments in 2024 and beyond.6
Other Legislative Updates Affecting Dermatology
Modifier -25—Cigna’s policy requiring dermatologists to submit documentation to use modifier -25 when billing with E/M CPT codes 99212 through 99215 has been delayed indefinitely.7 If a payer denies a dermatologist payment, contact the AADA Patient Access and Payer Relations committee ([email protected]) for assistance.
Telehealth and Digital Pathology—Recent legislation authorized extension of many of the Medicare telehealth and digital pathology flexibilities that were put in place during the COVID-19 public health emergency through December 31, 2024.8,9 Seventeen newly approved CPT telemedicine codes for new and established patient audio-visual and audio-only visits recently were surveyed.2,3 The data from the survey will be used as a key element in assigning a specific RVU to the CMS and will be included in the MPFS.
Thirty additional new digital pathology add-on CPT category III codes for 2024 were added to the ones from 2023.2,3 These codes can be used to report additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis. They cannot be used for archival or educational purposes, clinical conferences, training, or validating artificial intelligence algorithms. Category III codes used for emerging technologies have no assigned RVUs or reimbursement.2,3
The Cures Act—The Cures Act aims to ensure that patients have timely access to their health information.10 It requires all physicians to make their office notes, laboratory results, and other diagnostic reports available to patients as soon as the office receives them. The rules went into effect on April 5, 2021, with a limited definition of electronic health information; on October 6, 2022, the Cures Act rule expanded to include all electronic health information. The AADA has urged the Office of the National Coordinator for Health Information Technology to collaborate with stakeholder organizations to re-evaluate federal policies concerning the immediate release of electronic health information and information blocking, particularly in cases with life-altering diagnoses.10 They stressed the importance of prioritizing the well-being and emotional stability of patients and enhancing care by providing patients adequate time and support to process, comprehend, and discuss findings with their physician.
Proposed 2024 Medicare Quality Payment Program Requirements
The CMS proposed to increase the performance threshold in the quality payment program from 75 to 82 points for the 2024 Merit-based Incentive Payment System (MIPS) performance period, impacting the 2026 payment year.2,3,11 As a result of this increase, there could be more MIPS-eligible clinicians receiving penalties, which could be a reduction of up to 9%. The AADA will firmly oppose any increase in the threshold and strongly urge CMS to maintain the 75-point threshold. The performance category weights for the 2024 performance year will remain unchanged from the 2023 performance year.2,3,11
2024 Proposed Quality MIPS Measures Set—The CMS proposed to remove the topped-out MIPS measure 138 (coordination of care for melanoma).2,3,11 Additionally, it proposed to remove MIPS measure 402 (tobacco use and help with quitting among adolescents) as a quality measure from MIPS because the agency believes it is duplicative of measure 226 (preventive care and screening: tobacco use: screening and cessation intervention).2,3,11
MIPS Value Pathways—The CMS consolidated 2 previously established MIPS value pathways (MVPs): the Promoting Wellness MVP and the Optimizing Chronic Disease Management MVP.2,3,11 Proposed new MVPs for 2024 include Focusing on Women’s Health; Quality Care for the Treatment of Ear, Nose, and Throat Disorders; Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV; Quality Care in Mental Health and Substance Use Disorders; and Rehabilitative Support for Musculoskeletal Care. Dermatology is not impacted; however, the CMS plans to sunset traditional MIPS and replace it with MVPs—the future of MIPS.2,3,11 The AADA maintains that traditional MIPS should continue to be an option because MVPs have a limited number of measures for dermatologists.
Update on Reporting Suture Removal
There are 2 new CPT add-on codes—15853 and 15854—for the removal of sutures or staples not requiring anesthesia to be listed separately in addition to an appropriate E/M service. These add-on codes went into effect on January 1, 2023.12 These codes were created with the intent to capture and ensure remuneration for practice expenses that are not included in a stand-alone E/M encounter that occur after a 0-day procedure (eg, services reported with CPT codes 11102–11107 and 11300–11313) for wound check and suture removal where appropriate. These new add-on codes do not have physician work RVUs assigned to them because they are only for practice expenses (eg, clinical staff time, disposable supplies, use of equipment); CPT code 15853 is reported for the removal of sutures or staples, and CPT code 15854 is reported when both sutures and staples are removed. These codes can only be reported if an E/M service also is reported for the patient encounter.12
Final Thoughts
The AADA is working with the House of Medicine and the medical specialty community to develop specific proposals to reform the Medicare payment system.4 The proposed 2024 MPFS was released on July 13, 2023, and final regulations are expected in the late fall of 2023. The AADA will continue to engage with the CMS, but it is important for physicians to learn about and support advocacy priorities and efforts as well as join forces to protect their practices. As health care professionals, we have unique insights into the challenges and needs of our patients and the health care system. Advocacy can take various forms, such as supporting or opposing specific legislations, participating in grassroots campaigns, engaging with policymakers, and/or joining professional organizations that advocate for health care–related issues. Get involved, stay informed, and stay engaged through dermatology medical societies; together we can make a difference.
- Centers for Medicare & Medicaid Services. NHE fact sheet. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Medicare and Medicaid Programs; CY 2024 payment policies under the physician fee schedule and other changes to part B payment and coverage policies; Medicare shared savings program requirements; Medicare advantage; Medicare and Medicaid provider and supplier enrollment policies; and basic health program. Fed Regist. 2023;88:52262-53197. To be codified at 42 CFR §405, §410, §411, §414, §415, §418, §422, §423, §424, §425, §455, §489, §491, §495, §498, and §600. https://www.federalregister.gov/documents/2023/08/07/2023-14624/medicare-and-medicaid-programs-cy-2024-payment-policies-under-the-physician-fee-schedule-and-other
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2024 Medicare physician fee schedule proposed rule. Published July 13, 2023. Accessed September 18, 2023. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-proposed-rule
- American Medical Association. Payment reform. Accessed September 18, 2023. https://www.ama-assn.org/health-care-advocacypayment-reform
- American Medical Association. Physician answers on this survey will shape future Medicare pay. Published July 31, 2023. Accessed September 18, 2023. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future -medicare-pay
- Strengthening Medicare for Patients and Providers Act, HR 2474, 118 Congress (2023-2024). https://www.congress.gov/bill/118th-congress/house-bill/2474
- American Academy of Dermatology Association. Academy advocacy priorities. Accessed September 18, 2023. https://www.aad.org/member/advocacy/priorities
- College of American Pathologists. Remote sign-out of cases with digital pathology FAQs. Accessed September 18, 2023. https://www.cap.org/covid-19/remote-sign-out-faqs
- Centers for Medicare & Medicaid Services. Telehealth. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/medicare/coverage/telehealth
- The Office of the National Coordinator for Health Information Technology. ONC’s Cures Act final rule. Accessed September 18, 2023. https://www.healthit.gov/topic/oncs-cures-act-final-rule
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Notice of Proposed Rule Making Quality Payment Program Policy Overview: Proposals and Requests for Information. Accessed September 12, 2023. https://email.aadresources.org/e3t/Ctc/I6+113/cVKqx04/VVWzj43dDbctW8c23GW1ZLnJHW1xTZ7Q50Y DYN89Qzy5nCVhV3Zsc37CgFV9W5Ck4-D42qs9BW38PtXn4LSlNLW1QKpPL4xT8BMW6Mcwww3FdwCHN3vfGTMXbtF-W2-Zzfy5WHDg6W88tx1F1KgsgxW7zDzT46C2sFXW800vQJ3lLsS_W5D6f1d30-f3cN1njgZ_dX7xkW447ldH2-kgc5VCs7Xg1GY6dsN87pLVJqJG5XW8VWwD-7VxVkJN777f5fJL7jBW8RxkQM1lcSDjVV746T3C-stpN52V_S5xj7q6W3_vldf3p1Yk2Vbd4ZD3cPrHqW5Pwv9m567fkzW1vfDm51H-T7rW1jVrxl8gstXyW5RVTn8863CVFW8g6LgK2YdhpkW34HC4z3_pGYgW8V_qWH3g-tTlW4S3RD-1dKry7W4_rW8d1ssZ1fVwXQjQ9krVMW8Y0bTt8Nr5CNW6vbG0h3wyx59W8WCrNW50p5n6W1r-VBC2rKh93N4W2RyYr7vvm3kxG1
- Centers for Medicare & Medicaid Services. Chapter III surgery: integumentary system CPT codes 10000-19999 for Medicare national correct coding initiative policy manual. Updated January 1, 2023. Accessed September 26, 2023. https://www.cms.gov/files/document/medicare-ncci-policy-manual-2023-chapter-3.pdf
Health care spending in the United States remained relatively flat from 2019 to 2021 and only increased 2.7% in 2021, reaching $4.3 billion or $12,914 per person. Physician services account for 15% of health care spending (Figure). Relative value units (RVUs) signify the time it took a physician to complete a task multiplied by a conversion factor (CF). When RVUs initially were created in 1992 by what is now the Centers for Medicare &Medicaid Services (CMS), the CF was $32.00. Thirty-one years later, the CF is $33.89 in 2023; however, it would be $66.00 if the CF had increased with inflation.1 If the proposed 2024 Medicare physician fee schedule (MPFS) is adopted, the payment formula would decrease by 3.4% ($32.75) relative to the 2023 fee schedule ($33.89), which would be a 9% decrease relative to 2019 ($36.04).2,3 This reduction is due to the budget neutrality adjustment required by changes in RVUs, implementation of the evaluation and management (E/M) add-on code G2211, and proposed increases in primary are services.2,3 Since 2001, Medicare physician payment has declined by 26%.4 Adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index (MEI); (2) an expenditure target performance adjustment; and (3) miscellaneous adjustments, including those for budget neutrality required by law. Despite continued substantial increases in practice expenses, physicians’ reimbursement has remained flat while other service providers, such as those in skilled nursing facilities and hospitals, have received favorable payment increases compared to practice cost inflation and the Consumer Price Index.4

The CMS will not incorporate 2017 MEI cost weights for the RVUs in the MPFS rate setting for 2024 because all key measures of practice expenses in the MEI accelerated in 2022. Instead, the CMS is updating data on practice expense per hour to calculate payment for physician services with a survey for physician practices that launched on July 31, 2023.5 The American Medical Association contracted with Mathematica, an independent research company, to conduct a physician practice information survey that will be used to determine indirect practice expenses. Physicians should be on the lookout for emails regarding completion of these surveys and the appropriate financial expert in their practice should be contacted so the responses are accurate, as these data are key to future updates in the Medicare pay formula used to reimburse physicians.
Impact of Medicare Cuts
The recent congressional debt limit deal set spending caps for the next 2 fiscal years. Dermatology is facing an overall payment reduction of 1.87% (range, 1%–4%).2,3 The impact will depend on the services offered in an individual practice; for example, payment for a punch biopsy (Current Procedural Terminology [CPT] code 11104) would decrease by 3.9%. Payment for benign destruction (CPT code 17110) would decrease by 2.8%, and payment for even simple E/M of an established patient (CPT code 99213) would decrease by 1.6%. Overall, there would be a reduction of 2.75% for dermatopathology services, with a decrease of 2% for CPT code 88305 global and decreases for the technical component of 1% and professional component of 3%.2,3
Medicare cuts have reached a critical level, and physicians cannot continue to absorb the costs to own and operate their practices.4 This has led to health market consolidation, which in turn limits competition and patient access while driving up health care costs and driving down the quality of care. Small independent rural practices as well as those caring for historically marginalized patients will be disproportionately affected.
Proposed Addition of E/M Code G2211
In the calendar year (CY) 2021 final rule, the CMS tried to adopt a new add-on code—G2211—patients with a serious or complex condition that typically require referral and coordination of multispecialty care. Per the CMS, the primary policy goal of G2211 is to increase payments to primary care physicians and to reimburse them more appropriately for the care provided to patients with a serious or complex condition.2,3 It can be reported in conjunction with all office and outpatient E/M visits to better account for additional resources associated with primary care, or similarly ongoing medical care related to a patient’s single, serious condition, or complex condition.3 Typically, G2211 would not be used by dermatologists, as this add-on code requires visit complexity inherent to E/M associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single serious condition or a complex condition.2,3
Initially, the CMS assumed that G2211 would be reported with 90% of all office and outpatient E/M visit claims, which would account for a considerable portion of total MPFS schedule spending; however, the House of Medicine disagreed and believed it would be 75%.2,3 Given the extremely high utilization estimate, G2211 would have had a substantial effect on budget neutrality, accounting for an estimated increase of $3.3 billion and a corresponding 3.0% cut to the CY 2021 MPFS. Because of the potential payment reductions to physicians and a successful advocacy effort by organized medicine, including the American Academy of Dermatology Association (AADA), Congress delayed implementation of G2211 until CY 2024. Modifier -25 cannot be reported with G2211. The CMS revised its utilization assumptions from 90% of all E/M services to an initial utilization of 38% and then 54% when fully adopted. The proposed 2024 payment for G2211 is an additional $16.05.2,3
Advancing Health Equity With Healthcare Common Procedure Coding System G Codes
The CMS is proposing coding and payment for several new services to help underserved populations, including addressing unmet health-related social needs that can potentially interfere with the diagnosis and treatment of medical conditions, which includes paying for certain caregiver training services as well as payment for community health integration services.2,3 These are the first MPFS services designed to include care involving community health workers, who link underserved communities with critical health care and social services in the community. Additionally, the rule also proposes coding and payment for evaluating the risks related to social factors that affect a patient’s health, such as access to affordable quality health care, that can take place during an annual wellness visit or in combination with an E/M visit.2,3 As dermatologists, we should be familiar with this set of G codes, as we will likely use them in practice for patients with transportation needs.
Advocacy Efforts on Medicare Payment Reform
Medicare physician payment reform needs to happen at a national level. Advocacy efforts by the AADA and other groups have been underway to mitigate the proposed 2024 cuts. The Strengthening Medicare for Patients and Providers Act (HR 2474) is a bill that was introduced by a bipartisan coalition of physicians to provide an inflation-based increase in Medicare payments in 2024 and beyond.6
Other Legislative Updates Affecting Dermatology
Modifier -25—Cigna’s policy requiring dermatologists to submit documentation to use modifier -25 when billing with E/M CPT codes 99212 through 99215 has been delayed indefinitely.7 If a payer denies a dermatologist payment, contact the AADA Patient Access and Payer Relations committee ([email protected]) for assistance.
Telehealth and Digital Pathology—Recent legislation authorized extension of many of the Medicare telehealth and digital pathology flexibilities that were put in place during the COVID-19 public health emergency through December 31, 2024.8,9 Seventeen newly approved CPT telemedicine codes for new and established patient audio-visual and audio-only visits recently were surveyed.2,3 The data from the survey will be used as a key element in assigning a specific RVU to the CMS and will be included in the MPFS.
Thirty additional new digital pathology add-on CPT category III codes for 2024 were added to the ones from 2023.2,3 These codes can be used to report additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis. They cannot be used for archival or educational purposes, clinical conferences, training, or validating artificial intelligence algorithms. Category III codes used for emerging technologies have no assigned RVUs or reimbursement.2,3
The Cures Act—The Cures Act aims to ensure that patients have timely access to their health information.10 It requires all physicians to make their office notes, laboratory results, and other diagnostic reports available to patients as soon as the office receives them. The rules went into effect on April 5, 2021, with a limited definition of electronic health information; on October 6, 2022, the Cures Act rule expanded to include all electronic health information. The AADA has urged the Office of the National Coordinator for Health Information Technology to collaborate with stakeholder organizations to re-evaluate federal policies concerning the immediate release of electronic health information and information blocking, particularly in cases with life-altering diagnoses.10 They stressed the importance of prioritizing the well-being and emotional stability of patients and enhancing care by providing patients adequate time and support to process, comprehend, and discuss findings with their physician.
Proposed 2024 Medicare Quality Payment Program Requirements
The CMS proposed to increase the performance threshold in the quality payment program from 75 to 82 points for the 2024 Merit-based Incentive Payment System (MIPS) performance period, impacting the 2026 payment year.2,3,11 As a result of this increase, there could be more MIPS-eligible clinicians receiving penalties, which could be a reduction of up to 9%. The AADA will firmly oppose any increase in the threshold and strongly urge CMS to maintain the 75-point threshold. The performance category weights for the 2024 performance year will remain unchanged from the 2023 performance year.2,3,11
2024 Proposed Quality MIPS Measures Set—The CMS proposed to remove the topped-out MIPS measure 138 (coordination of care for melanoma).2,3,11 Additionally, it proposed to remove MIPS measure 402 (tobacco use and help with quitting among adolescents) as a quality measure from MIPS because the agency believes it is duplicative of measure 226 (preventive care and screening: tobacco use: screening and cessation intervention).2,3,11
MIPS Value Pathways—The CMS consolidated 2 previously established MIPS value pathways (MVPs): the Promoting Wellness MVP and the Optimizing Chronic Disease Management MVP.2,3,11 Proposed new MVPs for 2024 include Focusing on Women’s Health; Quality Care for the Treatment of Ear, Nose, and Throat Disorders; Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV; Quality Care in Mental Health and Substance Use Disorders; and Rehabilitative Support for Musculoskeletal Care. Dermatology is not impacted; however, the CMS plans to sunset traditional MIPS and replace it with MVPs—the future of MIPS.2,3,11 The AADA maintains that traditional MIPS should continue to be an option because MVPs have a limited number of measures for dermatologists.
Update on Reporting Suture Removal
There are 2 new CPT add-on codes—15853 and 15854—for the removal of sutures or staples not requiring anesthesia to be listed separately in addition to an appropriate E/M service. These add-on codes went into effect on January 1, 2023.12 These codes were created with the intent to capture and ensure remuneration for practice expenses that are not included in a stand-alone E/M encounter that occur after a 0-day procedure (eg, services reported with CPT codes 11102–11107 and 11300–11313) for wound check and suture removal where appropriate. These new add-on codes do not have physician work RVUs assigned to them because they are only for practice expenses (eg, clinical staff time, disposable supplies, use of equipment); CPT code 15853 is reported for the removal of sutures or staples, and CPT code 15854 is reported when both sutures and staples are removed. These codes can only be reported if an E/M service also is reported for the patient encounter.12
Final Thoughts
The AADA is working with the House of Medicine and the medical specialty community to develop specific proposals to reform the Medicare payment system.4 The proposed 2024 MPFS was released on July 13, 2023, and final regulations are expected in the late fall of 2023. The AADA will continue to engage with the CMS, but it is important for physicians to learn about and support advocacy priorities and efforts as well as join forces to protect their practices. As health care professionals, we have unique insights into the challenges and needs of our patients and the health care system. Advocacy can take various forms, such as supporting or opposing specific legislations, participating in grassroots campaigns, engaging with policymakers, and/or joining professional organizations that advocate for health care–related issues. Get involved, stay informed, and stay engaged through dermatology medical societies; together we can make a difference.
Health care spending in the United States remained relatively flat from 2019 to 2021 and only increased 2.7% in 2021, reaching $4.3 billion or $12,914 per person. Physician services account for 15% of health care spending (Figure). Relative value units (RVUs) signify the time it took a physician to complete a task multiplied by a conversion factor (CF). When RVUs initially were created in 1992 by what is now the Centers for Medicare &Medicaid Services (CMS), the CF was $32.00. Thirty-one years later, the CF is $33.89 in 2023; however, it would be $66.00 if the CF had increased with inflation.1 If the proposed 2024 Medicare physician fee schedule (MPFS) is adopted, the payment formula would decrease by 3.4% ($32.75) relative to the 2023 fee schedule ($33.89), which would be a 9% decrease relative to 2019 ($36.04).2,3 This reduction is due to the budget neutrality adjustment required by changes in RVUs, implementation of the evaluation and management (E/M) add-on code G2211, and proposed increases in primary are services.2,3 Since 2001, Medicare physician payment has declined by 26%.4 Adjustments to the CF typically are made based on 3 factors: (1) the Medicare Economic Index (MEI); (2) an expenditure target performance adjustment; and (3) miscellaneous adjustments, including those for budget neutrality required by law. Despite continued substantial increases in practice expenses, physicians’ reimbursement has remained flat while other service providers, such as those in skilled nursing facilities and hospitals, have received favorable payment increases compared to practice cost inflation and the Consumer Price Index.4

The CMS will not incorporate 2017 MEI cost weights for the RVUs in the MPFS rate setting for 2024 because all key measures of practice expenses in the MEI accelerated in 2022. Instead, the CMS is updating data on practice expense per hour to calculate payment for physician services with a survey for physician practices that launched on July 31, 2023.5 The American Medical Association contracted with Mathematica, an independent research company, to conduct a physician practice information survey that will be used to determine indirect practice expenses. Physicians should be on the lookout for emails regarding completion of these surveys and the appropriate financial expert in their practice should be contacted so the responses are accurate, as these data are key to future updates in the Medicare pay formula used to reimburse physicians.
Impact of Medicare Cuts
The recent congressional debt limit deal set spending caps for the next 2 fiscal years. Dermatology is facing an overall payment reduction of 1.87% (range, 1%–4%).2,3 The impact will depend on the services offered in an individual practice; for example, payment for a punch biopsy (Current Procedural Terminology [CPT] code 11104) would decrease by 3.9%. Payment for benign destruction (CPT code 17110) would decrease by 2.8%, and payment for even simple E/M of an established patient (CPT code 99213) would decrease by 1.6%. Overall, there would be a reduction of 2.75% for dermatopathology services, with a decrease of 2% for CPT code 88305 global and decreases for the technical component of 1% and professional component of 3%.2,3
Medicare cuts have reached a critical level, and physicians cannot continue to absorb the costs to own and operate their practices.4 This has led to health market consolidation, which in turn limits competition and patient access while driving up health care costs and driving down the quality of care. Small independent rural practices as well as those caring for historically marginalized patients will be disproportionately affected.
Proposed Addition of E/M Code G2211
In the calendar year (CY) 2021 final rule, the CMS tried to adopt a new add-on code—G2211—patients with a serious or complex condition that typically require referral and coordination of multispecialty care. Per the CMS, the primary policy goal of G2211 is to increase payments to primary care physicians and to reimburse them more appropriately for the care provided to patients with a serious or complex condition.2,3 It can be reported in conjunction with all office and outpatient E/M visits to better account for additional resources associated with primary care, or similarly ongoing medical care related to a patient’s single, serious condition, or complex condition.3 Typically, G2211 would not be used by dermatologists, as this add-on code requires visit complexity inherent to E/M associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single serious condition or a complex condition.2,3
Initially, the CMS assumed that G2211 would be reported with 90% of all office and outpatient E/M visit claims, which would account for a considerable portion of total MPFS schedule spending; however, the House of Medicine disagreed and believed it would be 75%.2,3 Given the extremely high utilization estimate, G2211 would have had a substantial effect on budget neutrality, accounting for an estimated increase of $3.3 billion and a corresponding 3.0% cut to the CY 2021 MPFS. Because of the potential payment reductions to physicians and a successful advocacy effort by organized medicine, including the American Academy of Dermatology Association (AADA), Congress delayed implementation of G2211 until CY 2024. Modifier -25 cannot be reported with G2211. The CMS revised its utilization assumptions from 90% of all E/M services to an initial utilization of 38% and then 54% when fully adopted. The proposed 2024 payment for G2211 is an additional $16.05.2,3
Advancing Health Equity With Healthcare Common Procedure Coding System G Codes
The CMS is proposing coding and payment for several new services to help underserved populations, including addressing unmet health-related social needs that can potentially interfere with the diagnosis and treatment of medical conditions, which includes paying for certain caregiver training services as well as payment for community health integration services.2,3 These are the first MPFS services designed to include care involving community health workers, who link underserved communities with critical health care and social services in the community. Additionally, the rule also proposes coding and payment for evaluating the risks related to social factors that affect a patient’s health, such as access to affordable quality health care, that can take place during an annual wellness visit or in combination with an E/M visit.2,3 As dermatologists, we should be familiar with this set of G codes, as we will likely use them in practice for patients with transportation needs.
Advocacy Efforts on Medicare Payment Reform
Medicare physician payment reform needs to happen at a national level. Advocacy efforts by the AADA and other groups have been underway to mitigate the proposed 2024 cuts. The Strengthening Medicare for Patients and Providers Act (HR 2474) is a bill that was introduced by a bipartisan coalition of physicians to provide an inflation-based increase in Medicare payments in 2024 and beyond.6
Other Legislative Updates Affecting Dermatology
Modifier -25—Cigna’s policy requiring dermatologists to submit documentation to use modifier -25 when billing with E/M CPT codes 99212 through 99215 has been delayed indefinitely.7 If a payer denies a dermatologist payment, contact the AADA Patient Access and Payer Relations committee ([email protected]) for assistance.
Telehealth and Digital Pathology—Recent legislation authorized extension of many of the Medicare telehealth and digital pathology flexibilities that were put in place during the COVID-19 public health emergency through December 31, 2024.8,9 Seventeen newly approved CPT telemedicine codes for new and established patient audio-visual and audio-only visits recently were surveyed.2,3 The data from the survey will be used as a key element in assigning a specific RVU to the CMS and will be included in the MPFS.
Thirty additional new digital pathology add-on CPT category III codes for 2024 were added to the ones from 2023.2,3 These codes can be used to report additional clinical staff work and service requirements associated with digitizing glass microscope slides for primary diagnosis. They cannot be used for archival or educational purposes, clinical conferences, training, or validating artificial intelligence algorithms. Category III codes used for emerging technologies have no assigned RVUs or reimbursement.2,3
The Cures Act—The Cures Act aims to ensure that patients have timely access to their health information.10 It requires all physicians to make their office notes, laboratory results, and other diagnostic reports available to patients as soon as the office receives them. The rules went into effect on April 5, 2021, with a limited definition of electronic health information; on October 6, 2022, the Cures Act rule expanded to include all electronic health information. The AADA has urged the Office of the National Coordinator for Health Information Technology to collaborate with stakeholder organizations to re-evaluate federal policies concerning the immediate release of electronic health information and information blocking, particularly in cases with life-altering diagnoses.10 They stressed the importance of prioritizing the well-being and emotional stability of patients and enhancing care by providing patients adequate time and support to process, comprehend, and discuss findings with their physician.
Proposed 2024 Medicare Quality Payment Program Requirements
The CMS proposed to increase the performance threshold in the quality payment program from 75 to 82 points for the 2024 Merit-based Incentive Payment System (MIPS) performance period, impacting the 2026 payment year.2,3,11 As a result of this increase, there could be more MIPS-eligible clinicians receiving penalties, which could be a reduction of up to 9%. The AADA will firmly oppose any increase in the threshold and strongly urge CMS to maintain the 75-point threshold. The performance category weights for the 2024 performance year will remain unchanged from the 2023 performance year.2,3,11
2024 Proposed Quality MIPS Measures Set—The CMS proposed to remove the topped-out MIPS measure 138 (coordination of care for melanoma).2,3,11 Additionally, it proposed to remove MIPS measure 402 (tobacco use and help with quitting among adolescents) as a quality measure from MIPS because the agency believes it is duplicative of measure 226 (preventive care and screening: tobacco use: screening and cessation intervention).2,3,11
MIPS Value Pathways—The CMS consolidated 2 previously established MIPS value pathways (MVPs): the Promoting Wellness MVP and the Optimizing Chronic Disease Management MVP.2,3,11 Proposed new MVPs for 2024 include Focusing on Women’s Health; Quality Care for the Treatment of Ear, Nose, and Throat Disorders; Prevention and Treatment of Infectious Disorders Including Hepatitis C and HIV; Quality Care in Mental Health and Substance Use Disorders; and Rehabilitative Support for Musculoskeletal Care. Dermatology is not impacted; however, the CMS plans to sunset traditional MIPS and replace it with MVPs—the future of MIPS.2,3,11 The AADA maintains that traditional MIPS should continue to be an option because MVPs have a limited number of measures for dermatologists.
Update on Reporting Suture Removal
There are 2 new CPT add-on codes—15853 and 15854—for the removal of sutures or staples not requiring anesthesia to be listed separately in addition to an appropriate E/M service. These add-on codes went into effect on January 1, 2023.12 These codes were created with the intent to capture and ensure remuneration for practice expenses that are not included in a stand-alone E/M encounter that occur after a 0-day procedure (eg, services reported with CPT codes 11102–11107 and 11300–11313) for wound check and suture removal where appropriate. These new add-on codes do not have physician work RVUs assigned to them because they are only for practice expenses (eg, clinical staff time, disposable supplies, use of equipment); CPT code 15853 is reported for the removal of sutures or staples, and CPT code 15854 is reported when both sutures and staples are removed. These codes can only be reported if an E/M service also is reported for the patient encounter.12
Final Thoughts
The AADA is working with the House of Medicine and the medical specialty community to develop specific proposals to reform the Medicare payment system.4 The proposed 2024 MPFS was released on July 13, 2023, and final regulations are expected in the late fall of 2023. The AADA will continue to engage with the CMS, but it is important for physicians to learn about and support advocacy priorities and efforts as well as join forces to protect their practices. As health care professionals, we have unique insights into the challenges and needs of our patients and the health care system. Advocacy can take various forms, such as supporting or opposing specific legislations, participating in grassroots campaigns, engaging with policymakers, and/or joining professional organizations that advocate for health care–related issues. Get involved, stay informed, and stay engaged through dermatology medical societies; together we can make a difference.
- Centers for Medicare & Medicaid Services. NHE fact sheet. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Medicare and Medicaid Programs; CY 2024 payment policies under the physician fee schedule and other changes to part B payment and coverage policies; Medicare shared savings program requirements; Medicare advantage; Medicare and Medicaid provider and supplier enrollment policies; and basic health program. Fed Regist. 2023;88:52262-53197. To be codified at 42 CFR §405, §410, §411, §414, §415, §418, §422, §423, §424, §425, §455, §489, §491, §495, §498, and §600. https://www.federalregister.gov/documents/2023/08/07/2023-14624/medicare-and-medicaid-programs-cy-2024-payment-policies-under-the-physician-fee-schedule-and-other
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2024 Medicare physician fee schedule proposed rule. Published July 13, 2023. Accessed September 18, 2023. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-proposed-rule
- American Medical Association. Payment reform. Accessed September 18, 2023. https://www.ama-assn.org/health-care-advocacypayment-reform
- American Medical Association. Physician answers on this survey will shape future Medicare pay. Published July 31, 2023. Accessed September 18, 2023. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future -medicare-pay
- Strengthening Medicare for Patients and Providers Act, HR 2474, 118 Congress (2023-2024). https://www.congress.gov/bill/118th-congress/house-bill/2474
- American Academy of Dermatology Association. Academy advocacy priorities. Accessed September 18, 2023. https://www.aad.org/member/advocacy/priorities
- College of American Pathologists. Remote sign-out of cases with digital pathology FAQs. Accessed September 18, 2023. https://www.cap.org/covid-19/remote-sign-out-faqs
- Centers for Medicare & Medicaid Services. Telehealth. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/medicare/coverage/telehealth
- The Office of the National Coordinator for Health Information Technology. ONC’s Cures Act final rule. Accessed September 18, 2023. https://www.healthit.gov/topic/oncs-cures-act-final-rule
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Notice of Proposed Rule Making Quality Payment Program Policy Overview: Proposals and Requests for Information. Accessed September 12, 2023. https://email.aadresources.org/e3t/Ctc/I6+113/cVKqx04/VVWzj43dDbctW8c23GW1ZLnJHW1xTZ7Q50Y DYN89Qzy5nCVhV3Zsc37CgFV9W5Ck4-D42qs9BW38PtXn4LSlNLW1QKpPL4xT8BMW6Mcwww3FdwCHN3vfGTMXbtF-W2-Zzfy5WHDg6W88tx1F1KgsgxW7zDzT46C2sFXW800vQJ3lLsS_W5D6f1d30-f3cN1njgZ_dX7xkW447ldH2-kgc5VCs7Xg1GY6dsN87pLVJqJG5XW8VWwD-7VxVkJN777f5fJL7jBW8RxkQM1lcSDjVV746T3C-stpN52V_S5xj7q6W3_vldf3p1Yk2Vbd4ZD3cPrHqW5Pwv9m567fkzW1vfDm51H-T7rW1jVrxl8gstXyW5RVTn8863CVFW8g6LgK2YdhpkW34HC4z3_pGYgW8V_qWH3g-tTlW4S3RD-1dKry7W4_rW8d1ssZ1fVwXQjQ9krVMW8Y0bTt8Nr5CNW6vbG0h3wyx59W8WCrNW50p5n6W1r-VBC2rKh93N4W2RyYr7vvm3kxG1
- Centers for Medicare & Medicaid Services. Chapter III surgery: integumentary system CPT codes 10000-19999 for Medicare national correct coding initiative policy manual. Updated January 1, 2023. Accessed September 26, 2023. https://www.cms.gov/files/document/medicare-ncci-policy-manual-2023-chapter-3.pdf
- Centers for Medicare & Medicaid Services. NHE fact sheet. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet
- Medicare and Medicaid Programs; CY 2024 payment policies under the physician fee schedule and other changes to part B payment and coverage policies; Medicare shared savings program requirements; Medicare advantage; Medicare and Medicaid provider and supplier enrollment policies; and basic health program. Fed Regist. 2023;88:52262-53197. To be codified at 42 CFR §405, §410, §411, §414, §415, §418, §422, §423, §424, §425, §455, §489, §491, §495, §498, and §600. https://www.federalregister.gov/documents/2023/08/07/2023-14624/medicare-and-medicaid-programs-cy-2024-payment-policies-under-the-physician-fee-schedule-and-other
- Centers for Medicare & Medicaid Services. Calendar year (CY) 2024 Medicare physician fee schedule proposed rule. Published July 13, 2023. Accessed September 18, 2023. https://www.cms.gov/newsroom/fact-sheets/calendar-year-cy-2024-medicare-physician-fee-schedule-proposed-rule
- American Medical Association. Payment reform. Accessed September 18, 2023. https://www.ama-assn.org/health-care-advocacypayment-reform
- American Medical Association. Physician answers on this survey will shape future Medicare pay. Published July 31, 2023. Accessed September 18, 2023. https://www.ama-assn.org/practice-management/medicare-medicaid/physician-answers-survey-will-shape-future -medicare-pay
- Strengthening Medicare for Patients and Providers Act, HR 2474, 118 Congress (2023-2024). https://www.congress.gov/bill/118th-congress/house-bill/2474
- American Academy of Dermatology Association. Academy advocacy priorities. Accessed September 18, 2023. https://www.aad.org/member/advocacy/priorities
- College of American Pathologists. Remote sign-out of cases with digital pathology FAQs. Accessed September 18, 2023. https://www.cap.org/covid-19/remote-sign-out-faqs
- Centers for Medicare & Medicaid Services. Telehealth. Updated September 6, 2023. Accessed September 18, 2023. https://www.cms.gov/medicare/coverage/telehealth
- The Office of the National Coordinator for Health Information Technology. ONC’s Cures Act final rule. Accessed September 18, 2023. https://www.healthit.gov/topic/oncs-cures-act-final-rule
- Centers for Medicare & Medicaid Services. Calendar Year (CY) 2024 Medicare Physician Fee Schedule (PFS) Notice of Proposed Rule Making Quality Payment Program Policy Overview: Proposals and Requests for Information. Accessed September 12, 2023. https://email.aadresources.org/e3t/Ctc/I6+113/cVKqx04/VVWzj43dDbctW8c23GW1ZLnJHW1xTZ7Q50Y DYN89Qzy5nCVhV3Zsc37CgFV9W5Ck4-D42qs9BW38PtXn4LSlNLW1QKpPL4xT8BMW6Mcwww3FdwCHN3vfGTMXbtF-W2-Zzfy5WHDg6W88tx1F1KgsgxW7zDzT46C2sFXW800vQJ3lLsS_W5D6f1d30-f3cN1njgZ_dX7xkW447ldH2-kgc5VCs7Xg1GY6dsN87pLVJqJG5XW8VWwD-7VxVkJN777f5fJL7jBW8RxkQM1lcSDjVV746T3C-stpN52V_S5xj7q6W3_vldf3p1Yk2Vbd4ZD3cPrHqW5Pwv9m567fkzW1vfDm51H-T7rW1jVrxl8gstXyW5RVTn8863CVFW8g6LgK2YdhpkW34HC4z3_pGYgW8V_qWH3g-tTlW4S3RD-1dKry7W4_rW8d1ssZ1fVwXQjQ9krVMW8Y0bTt8Nr5CNW6vbG0h3wyx59W8WCrNW50p5n6W1r-VBC2rKh93N4W2RyYr7vvm3kxG1
- Centers for Medicare & Medicaid Services. Chapter III surgery: integumentary system CPT codes 10000-19999 for Medicare national correct coding initiative policy manual. Updated January 1, 2023. Accessed September 26, 2023. https://www.cms.gov/files/document/medicare-ncci-policy-manual-2023-chapter-3.pdf
PRACTICE POINTS
- The proposed 2024 Medicare physician fee schedule published by the Centers for Medicare & Medicaid Services in July 2023 will negatively impact dermatology practices.
- The final regulations are expected in November 2023.
What’s Eating You? Noble False Widow Spider (Steatoda nobilis)
Incidence and Characteristics
The noble false widow spider (Steatoda nobilis) is one of the world’s most invasive spider species, having spread across the globe from Madeira and the Canary Islands into the North Atlantic.1,2 Steatoda comprise multiple species of false widow spiders, named for their resemblance to black widow spiders (Latrodectus). The noble false widow spider is the dominant species in buildings in southern Ireland and Great Britain, with a population surge in 2018 that caused multiple temporary school closures in London, England, for fumigation.3 The noble false widow spider was first documented in the United States in Ventura County, California, in 2011, with numerous specimens found in urban areas (eg, in parks, underneath garbage cans) closer to the coastline as well as farther inland. The species may have been introduced to this area by way of Port Hueneme, a city in California with a US naval base with routes to various other military bases in Western Europe.4 Given its already rapid expansion outside of the United States with a concurrent rise in bite reports, dermatologists should be familiar with these invasive and potentially dangerous arachnids.
The spread of noble false widow spiders is assisted by their wide range of temperature tolerance and ability to survive for months with little food and no water. They can live for several years, with one report of a noble false widow spider living up to 7 years.5 These spiders are found inside homes and buildings year-round, and they prefer to build their webs in an elevated position such as the top corner of a room. Steatoda weave tangle webs with crisscrossing threads that often have a denser middle section.5
Noble false widow spiders are sexually dimorphic, with males typically no larger than 1-cm long and females up to 1.4-cm long. They have a dark brown to black thorax and brown abdomen with red-brown legs. Males have brighter cream-colored abdominal markings than females, who lack markings altogether on their distinctive globular abdomen (Figure). The abdominal markings are known to resemble a skull or house.

Although noble false widow spiders are not exclusively synanthropic, they can be found in any crevice in homes or other structures where there are humans such as office buildings.5-7 Up until the last 20 years, reports of bites from noble false widow spiders worldwide were few and far between. In Great Britain, the spiders were first considered to be common in the 1980s, with recent evidence of an urban population boom in the last 5 to 10 years that has coincided with an increase in bite reports.5,8,9
Clinical Significance
Most bites occur in a defensive manner, such as when humans perform activities that disturb the hiding space, cause vibrations in the web, or compress the body of the arachnid. Most envenomations in Great Britain occur while the individual is in bed, though they also may occur during other activities that disturb the spider, such as moving boxes or putting on a pair of pants.5 Occupational exposure to noble false widow spiders may soon be a concern for those involved in construction, carpentry, cleaning, and decorating given their recent invasive spread into the United States.
The venom from these spiders is neurotoxic and cytotoxic, causing moderate to intense pain that may resemble a wasp sting. The incidence of steatodism—which can include symptoms of pain in addition to fever, hypotension, headache, lethargy, nausea, localized diaphoresis, abdominal pain, paresthesias, and malaise—is unknown but reportedly rare.5,10 There are considerable similarities between Steatoda and true black widow spider venom, which explains the symptom overlap with latrodectism. There are reports of severe debilitation lasting weeks due to pain and decreased affected limb movement after bites from noble false widow spiders.10-12
Nearly all noble false widow spider bite reports describe immediate pain upon bite/envenomation, which is unlike the delayed pain from a black widow spider bite (after 10 minutes or more).6,13,14 Erythema and swelling occur around a pale raised site of envenomation lasting up to 72 hours. The bite site may be highly tender and blister or ulcerate, with reports of cellulitis and local skin necrosis.7,15 Pruritus during this period can be intense, and excoriation increases the risk for complications such as infection. Reports of anaphylaxis following a noble false widow spider bite are rare.5,16 The incidence of bites may be underreported due to the lack of proper identification of the responsible arachnid for those who do not seek care or require hospitalization, though this is not unique to Steatoda.
There are reports of secondary infection after bites and even cases of limb amputation, septicemia, and death.14,17 However, it is unknown if noble false widow spiders are vectors for bacteria transmitted during envenomation, and infection likely is secondary to scratching or inadequate wound care.18,19 Potentially pathogenic bacteria have been isolated from the body surfaces of the noble false widow spider, including Pseudomonas putida, Staphylococcus capitis, and Staphylococcus epidermidis.20 Fortunately, most captured cases (ie, events in which the biting arachnid was properly identified) report symptoms ranging from mild to moderate in severity without the need for hospitalization. A series of 24 reports revealed that all individuals experienced sharp pain upon the initial bite followed by erythema, and 18 of them experienced considerable swelling of the area soon thereafter. One individual experienced temporary paralysis of the affected limb, and 3 individuals experienced hypotension or hypertension in addition to fever, skin necrosis, or cellulitis.14
Treatment
The envenomation site should be washed with antibacterial soap and warm water and should be kept clean to prevent infection. There is no evidence that tight pressure bandaging of these bite sites will restrict venom flow; because it may worsen pain in the area, pressure bandaging is not recommended. When possible, the arachnid should be collected for identification. Supportive care is warranted for symptoms of pain, erythema, and swelling, with the use of cool compresses, oral pain relievers (eg, nonsteroidal anti-inflammatory drugs, acetaminophen), topical anesthetic (eg, lidocaine), or antihistamines as needed.
Urgent care is warranted for patients who experience severe symptoms of steatodism such as hypertension, lymphadenopathy, paresthesia, or limb paralysis. Limited reports show onset of this distress typically within an hour of envenomation. Treatments analogous to those for latrodectism including muscle relaxers and pain medications have demonstrated rapid attenuation of symptoms upon intramuscular administration of antivenom made from Latrodectus species.21-23
Signs of infection warrant bacterial culture with antibiotic susceptibilities to ensure adequate treatment.20 Infections from spider bites can present a few days to a week following envenomation. Symptoms may include spreading redness or an enlarging wound site, pus formation, worsening or unrelenting pain after 24 hours, fevers, flulike symptoms, and muscle cramps.
Final Thoughts
- Kulczycki A, Legittimo C, Simeon E, et al. New records of Steatoda nobilis (Thorell, 1875) (Araneae, Theridiidae), an introduced species on the Italian mainland and in Sardinia. Bull Br Arachnological Soc. 2012;15:269-272.
- Bauer T, Feldmeier S, Krehenwinkel H, et al. Steatoda nobilis, a false widow on the rise: a synthesis of past and current distribution trends. NeoBiota. 2019; 42:19. doi:10.3897/neobiota.42.31582
- Murphy A. Web of cries: false widow spider infestation fears forceeleventh school in London to close as outbreak spreads. The Sun.October 19, 2018. Accessed September 21, 2023. https://www.thesun.co.uk/news/7534016/false-widow-spider-infestation-fears-force-eleventh-londonschool-closing
- Vetter R, Rust M. A large European combfoot spider, Steatoda nobilis (Thorell 1875)(Araneae: Theridiidae), newly established in Ventura County, California. The Pan-Pacific Entomologist. 2012;88:92-97.
- Hambler C. The ‘noble false widow’ spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints. Preprint posted online October 15, 2019. doi:10.31219/osf.io/axbd4
- Dunbar J, Schulte J, Lyons K, et al. New Irish record for Steatoda triangulosa (Walckenaer, 1802), and new county records for Steatoda nobilis (Thorell, 1875), Steatoda bipunctata (Linnaeus, 1758) and Steatoda grossa (C.L. Koch, 1838). Ir Naturalists J. 2018;36:39-43.
- Duon M, Dunbar J, Afoullouss S, et al. Occurrence, reproductive rate and identification of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) in Ireland. Biol Environment: Proc Royal Ir Acad. 2017;117B:77-89. doi:10.3318/bioe.2017.11
- Burrows T. Great bitten: Britain’s spider bite capital revealed as Essex with 450 attacks—find out where your town ranks. The Sun. Published April 3, 2019. Accessed September 14, 2023. https://www.thesun.co.uk/news/8782355/britains-spider-bite-capital-revealed-as-essex-with-450- attacks-find-out-where-your-town-ranks/
- Wathen T. Essex is the UK capital for spider bites—and the amount is terrifying. Essex News. April 4, 2019. Accessed September 21, 2023. https://www.essexlive.news/news/essex-news/essex-uk-capital-spider-bites- 2720935
- Dunbar J, Afoullouss S, Sulpice R, et al. Envenomation by the noble false widow spider Steatoda nobilis (Thorell, 1875)—five new cases of steatodism from Ireland and Great Britain. Clin Toxicol (Phila). 2018;56:433-435. doi:10.1080/15563650.2017.1393084
- Dunbar J, Fort A, Redureau D, et al. Venomics approach reveals a high proportion of Latrodectus-like toxins in the venom of the noble false widow spider Steatoda nobilis. Toxins. 2020;12:402.
- Warrell D, Shaheen J, Hillyard P, et al. Neurotoxic envenoming by an immigrant spider (Steatoda nobilis) in southern England. Toxicon. 1991;29:1263-1265.
- Zhou H, Xu K, Zheng PY, et. al. Clinical characteristics of patients with black widow spider bites: a report of 59 patients and single-center experience. World J Emerg Med. 2021;12:317-320. doi:10.5847/wjem.j.1920-8642.2021.04.011
- Dunbar J, Vitkauskaite A, O’Keeffe D, et. al. Bites by the noble false widow spider Steatoda nobilis can induce Latrodectus-like symptoms and vector-borne bacterial infections with implications for public health: a case series. Clin Toxicol (Phila). 2022;60:59-70. doi:10.1080/15563650.2021.1928165
- Dunbar J, Sulpice R, Dugon M. The kiss of (cell) death: can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin Toxicol. 2019;57:677-685. doi:10.1080/15563650.2019.1578367
- Magee J. Bite ‘nightmare’: close encounter with a false widow. The Bournemouth Echo. September 7, 2009. Accessed September 21, 2023. http://www.bournemouthecho.co.uk/news/4582887.Bite____nightmare_____close_encounter_with_a_false_widow_spider/
- Marsh H. Woman nearly loses hand after bite from false widow. Daily Echo. April 17, 2012. Accessed September 21, 2023. https://www.bournemouthecho.co.uk/news/9652335.woman-nearly-loses-hand-after-bite-from-false-widow-spider/
- Stuber N, Nentwig W. How informative are case studies of spider bites in the medical literature? Toxicon. 2016;114:40-44. doi:10.1016/j.toxicon.2016.02.023
- Vetter R, Swanson D, Weinstein S, et. al. Do spiders vector bacteria during bites? the evidence indicates otherwise. Toxicon. 2015;93:171-174. doi:10.1016/j.toxicon.2014.11.229
- Dunbar J, Khan N, Abberton C, et al. Synanthropic spiders, including the global invasive noble false widow Steatoda nobilis, are reservoirs for medically important and antibiotic resistant bacteria. Sci Rep. 2020;10:20916. doi:10.1038/s41598-020-77839-9
- Atakuziev BU, Wright CE, Graudins A, et al. Efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of clinical envenomation by the cupboard spider Steatoda capensis (Theridiidae). Toxicon. 2014;86:68-78. doi:10.1016/j.toxicon.2014.04.011
- Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775. doi:10.1016/S0041-0101(01)00280-X.
- South M, Wirth P, Winkel KD. Redback spider antivenom used to treat envenomation by a juvenile Steatoda spider. Med J Aust. 1998;169:642-642. doi:10.5694/j.1326-5377.1998.tb123445.x
Incidence and Characteristics
The noble false widow spider (Steatoda nobilis) is one of the world’s most invasive spider species, having spread across the globe from Madeira and the Canary Islands into the North Atlantic.1,2 Steatoda comprise multiple species of false widow spiders, named for their resemblance to black widow spiders (Latrodectus). The noble false widow spider is the dominant species in buildings in southern Ireland and Great Britain, with a population surge in 2018 that caused multiple temporary school closures in London, England, for fumigation.3 The noble false widow spider was first documented in the United States in Ventura County, California, in 2011, with numerous specimens found in urban areas (eg, in parks, underneath garbage cans) closer to the coastline as well as farther inland. The species may have been introduced to this area by way of Port Hueneme, a city in California with a US naval base with routes to various other military bases in Western Europe.4 Given its already rapid expansion outside of the United States with a concurrent rise in bite reports, dermatologists should be familiar with these invasive and potentially dangerous arachnids.
The spread of noble false widow spiders is assisted by their wide range of temperature tolerance and ability to survive for months with little food and no water. They can live for several years, with one report of a noble false widow spider living up to 7 years.5 These spiders are found inside homes and buildings year-round, and they prefer to build their webs in an elevated position such as the top corner of a room. Steatoda weave tangle webs with crisscrossing threads that often have a denser middle section.5
Noble false widow spiders are sexually dimorphic, with males typically no larger than 1-cm long and females up to 1.4-cm long. They have a dark brown to black thorax and brown abdomen with red-brown legs. Males have brighter cream-colored abdominal markings than females, who lack markings altogether on their distinctive globular abdomen (Figure). The abdominal markings are known to resemble a skull or house.

Although noble false widow spiders are not exclusively synanthropic, they can be found in any crevice in homes or other structures where there are humans such as office buildings.5-7 Up until the last 20 years, reports of bites from noble false widow spiders worldwide were few and far between. In Great Britain, the spiders were first considered to be common in the 1980s, with recent evidence of an urban population boom in the last 5 to 10 years that has coincided with an increase in bite reports.5,8,9
Clinical Significance
Most bites occur in a defensive manner, such as when humans perform activities that disturb the hiding space, cause vibrations in the web, or compress the body of the arachnid. Most envenomations in Great Britain occur while the individual is in bed, though they also may occur during other activities that disturb the spider, such as moving boxes or putting on a pair of pants.5 Occupational exposure to noble false widow spiders may soon be a concern for those involved in construction, carpentry, cleaning, and decorating given their recent invasive spread into the United States.
The venom from these spiders is neurotoxic and cytotoxic, causing moderate to intense pain that may resemble a wasp sting. The incidence of steatodism—which can include symptoms of pain in addition to fever, hypotension, headache, lethargy, nausea, localized diaphoresis, abdominal pain, paresthesias, and malaise—is unknown but reportedly rare.5,10 There are considerable similarities between Steatoda and true black widow spider venom, which explains the symptom overlap with latrodectism. There are reports of severe debilitation lasting weeks due to pain and decreased affected limb movement after bites from noble false widow spiders.10-12
Nearly all noble false widow spider bite reports describe immediate pain upon bite/envenomation, which is unlike the delayed pain from a black widow spider bite (after 10 minutes or more).6,13,14 Erythema and swelling occur around a pale raised site of envenomation lasting up to 72 hours. The bite site may be highly tender and blister or ulcerate, with reports of cellulitis and local skin necrosis.7,15 Pruritus during this period can be intense, and excoriation increases the risk for complications such as infection. Reports of anaphylaxis following a noble false widow spider bite are rare.5,16 The incidence of bites may be underreported due to the lack of proper identification of the responsible arachnid for those who do not seek care or require hospitalization, though this is not unique to Steatoda.
There are reports of secondary infection after bites and even cases of limb amputation, septicemia, and death.14,17 However, it is unknown if noble false widow spiders are vectors for bacteria transmitted during envenomation, and infection likely is secondary to scratching or inadequate wound care.18,19 Potentially pathogenic bacteria have been isolated from the body surfaces of the noble false widow spider, including Pseudomonas putida, Staphylococcus capitis, and Staphylococcus epidermidis.20 Fortunately, most captured cases (ie, events in which the biting arachnid was properly identified) report symptoms ranging from mild to moderate in severity without the need for hospitalization. A series of 24 reports revealed that all individuals experienced sharp pain upon the initial bite followed by erythema, and 18 of them experienced considerable swelling of the area soon thereafter. One individual experienced temporary paralysis of the affected limb, and 3 individuals experienced hypotension or hypertension in addition to fever, skin necrosis, or cellulitis.14
Treatment
The envenomation site should be washed with antibacterial soap and warm water and should be kept clean to prevent infection. There is no evidence that tight pressure bandaging of these bite sites will restrict venom flow; because it may worsen pain in the area, pressure bandaging is not recommended. When possible, the arachnid should be collected for identification. Supportive care is warranted for symptoms of pain, erythema, and swelling, with the use of cool compresses, oral pain relievers (eg, nonsteroidal anti-inflammatory drugs, acetaminophen), topical anesthetic (eg, lidocaine), or antihistamines as needed.
Urgent care is warranted for patients who experience severe symptoms of steatodism such as hypertension, lymphadenopathy, paresthesia, or limb paralysis. Limited reports show onset of this distress typically within an hour of envenomation. Treatments analogous to those for latrodectism including muscle relaxers and pain medications have demonstrated rapid attenuation of symptoms upon intramuscular administration of antivenom made from Latrodectus species.21-23
Signs of infection warrant bacterial culture with antibiotic susceptibilities to ensure adequate treatment.20 Infections from spider bites can present a few days to a week following envenomation. Symptoms may include spreading redness or an enlarging wound site, pus formation, worsening or unrelenting pain after 24 hours, fevers, flulike symptoms, and muscle cramps.
Final Thoughts
Incidence and Characteristics
The noble false widow spider (Steatoda nobilis) is one of the world’s most invasive spider species, having spread across the globe from Madeira and the Canary Islands into the North Atlantic.1,2 Steatoda comprise multiple species of false widow spiders, named for their resemblance to black widow spiders (Latrodectus). The noble false widow spider is the dominant species in buildings in southern Ireland and Great Britain, with a population surge in 2018 that caused multiple temporary school closures in London, England, for fumigation.3 The noble false widow spider was first documented in the United States in Ventura County, California, in 2011, with numerous specimens found in urban areas (eg, in parks, underneath garbage cans) closer to the coastline as well as farther inland. The species may have been introduced to this area by way of Port Hueneme, a city in California with a US naval base with routes to various other military bases in Western Europe.4 Given its already rapid expansion outside of the United States with a concurrent rise in bite reports, dermatologists should be familiar with these invasive and potentially dangerous arachnids.
The spread of noble false widow spiders is assisted by their wide range of temperature tolerance and ability to survive for months with little food and no water. They can live for several years, with one report of a noble false widow spider living up to 7 years.5 These spiders are found inside homes and buildings year-round, and they prefer to build their webs in an elevated position such as the top corner of a room. Steatoda weave tangle webs with crisscrossing threads that often have a denser middle section.5
Noble false widow spiders are sexually dimorphic, with males typically no larger than 1-cm long and females up to 1.4-cm long. They have a dark brown to black thorax and brown abdomen with red-brown legs. Males have brighter cream-colored abdominal markings than females, who lack markings altogether on their distinctive globular abdomen (Figure). The abdominal markings are known to resemble a skull or house.

Although noble false widow spiders are not exclusively synanthropic, they can be found in any crevice in homes or other structures where there are humans such as office buildings.5-7 Up until the last 20 years, reports of bites from noble false widow spiders worldwide were few and far between. In Great Britain, the spiders were first considered to be common in the 1980s, with recent evidence of an urban population boom in the last 5 to 10 years that has coincided with an increase in bite reports.5,8,9
Clinical Significance
Most bites occur in a defensive manner, such as when humans perform activities that disturb the hiding space, cause vibrations in the web, or compress the body of the arachnid. Most envenomations in Great Britain occur while the individual is in bed, though they also may occur during other activities that disturb the spider, such as moving boxes or putting on a pair of pants.5 Occupational exposure to noble false widow spiders may soon be a concern for those involved in construction, carpentry, cleaning, and decorating given their recent invasive spread into the United States.
The venom from these spiders is neurotoxic and cytotoxic, causing moderate to intense pain that may resemble a wasp sting. The incidence of steatodism—which can include symptoms of pain in addition to fever, hypotension, headache, lethargy, nausea, localized diaphoresis, abdominal pain, paresthesias, and malaise—is unknown but reportedly rare.5,10 There are considerable similarities between Steatoda and true black widow spider venom, which explains the symptom overlap with latrodectism. There are reports of severe debilitation lasting weeks due to pain and decreased affected limb movement after bites from noble false widow spiders.10-12
Nearly all noble false widow spider bite reports describe immediate pain upon bite/envenomation, which is unlike the delayed pain from a black widow spider bite (after 10 minutes or more).6,13,14 Erythema and swelling occur around a pale raised site of envenomation lasting up to 72 hours. The bite site may be highly tender and blister or ulcerate, with reports of cellulitis and local skin necrosis.7,15 Pruritus during this period can be intense, and excoriation increases the risk for complications such as infection. Reports of anaphylaxis following a noble false widow spider bite are rare.5,16 The incidence of bites may be underreported due to the lack of proper identification of the responsible arachnid for those who do not seek care or require hospitalization, though this is not unique to Steatoda.
There are reports of secondary infection after bites and even cases of limb amputation, septicemia, and death.14,17 However, it is unknown if noble false widow spiders are vectors for bacteria transmitted during envenomation, and infection likely is secondary to scratching or inadequate wound care.18,19 Potentially pathogenic bacteria have been isolated from the body surfaces of the noble false widow spider, including Pseudomonas putida, Staphylococcus capitis, and Staphylococcus epidermidis.20 Fortunately, most captured cases (ie, events in which the biting arachnid was properly identified) report symptoms ranging from mild to moderate in severity without the need for hospitalization. A series of 24 reports revealed that all individuals experienced sharp pain upon the initial bite followed by erythema, and 18 of them experienced considerable swelling of the area soon thereafter. One individual experienced temporary paralysis of the affected limb, and 3 individuals experienced hypotension or hypertension in addition to fever, skin necrosis, or cellulitis.14
Treatment
The envenomation site should be washed with antibacterial soap and warm water and should be kept clean to prevent infection. There is no evidence that tight pressure bandaging of these bite sites will restrict venom flow; because it may worsen pain in the area, pressure bandaging is not recommended. When possible, the arachnid should be collected for identification. Supportive care is warranted for symptoms of pain, erythema, and swelling, with the use of cool compresses, oral pain relievers (eg, nonsteroidal anti-inflammatory drugs, acetaminophen), topical anesthetic (eg, lidocaine), or antihistamines as needed.
Urgent care is warranted for patients who experience severe symptoms of steatodism such as hypertension, lymphadenopathy, paresthesia, or limb paralysis. Limited reports show onset of this distress typically within an hour of envenomation. Treatments analogous to those for latrodectism including muscle relaxers and pain medications have demonstrated rapid attenuation of symptoms upon intramuscular administration of antivenom made from Latrodectus species.21-23
Signs of infection warrant bacterial culture with antibiotic susceptibilities to ensure adequate treatment.20 Infections from spider bites can present a few days to a week following envenomation. Symptoms may include spreading redness or an enlarging wound site, pus formation, worsening or unrelenting pain after 24 hours, fevers, flulike symptoms, and muscle cramps.
Final Thoughts
- Kulczycki A, Legittimo C, Simeon E, et al. New records of Steatoda nobilis (Thorell, 1875) (Araneae, Theridiidae), an introduced species on the Italian mainland and in Sardinia. Bull Br Arachnological Soc. 2012;15:269-272.
- Bauer T, Feldmeier S, Krehenwinkel H, et al. Steatoda nobilis, a false widow on the rise: a synthesis of past and current distribution trends. NeoBiota. 2019; 42:19. doi:10.3897/neobiota.42.31582
- Murphy A. Web of cries: false widow spider infestation fears forceeleventh school in London to close as outbreak spreads. The Sun.October 19, 2018. Accessed September 21, 2023. https://www.thesun.co.uk/news/7534016/false-widow-spider-infestation-fears-force-eleventh-londonschool-closing
- Vetter R, Rust M. A large European combfoot spider, Steatoda nobilis (Thorell 1875)(Araneae: Theridiidae), newly established in Ventura County, California. The Pan-Pacific Entomologist. 2012;88:92-97.
- Hambler C. The ‘noble false widow’ spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints. Preprint posted online October 15, 2019. doi:10.31219/osf.io/axbd4
- Dunbar J, Schulte J, Lyons K, et al. New Irish record for Steatoda triangulosa (Walckenaer, 1802), and new county records for Steatoda nobilis (Thorell, 1875), Steatoda bipunctata (Linnaeus, 1758) and Steatoda grossa (C.L. Koch, 1838). Ir Naturalists J. 2018;36:39-43.
- Duon M, Dunbar J, Afoullouss S, et al. Occurrence, reproductive rate and identification of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) in Ireland. Biol Environment: Proc Royal Ir Acad. 2017;117B:77-89. doi:10.3318/bioe.2017.11
- Burrows T. Great bitten: Britain’s spider bite capital revealed as Essex with 450 attacks—find out where your town ranks. The Sun. Published April 3, 2019. Accessed September 14, 2023. https://www.thesun.co.uk/news/8782355/britains-spider-bite-capital-revealed-as-essex-with-450- attacks-find-out-where-your-town-ranks/
- Wathen T. Essex is the UK capital for spider bites—and the amount is terrifying. Essex News. April 4, 2019. Accessed September 21, 2023. https://www.essexlive.news/news/essex-news/essex-uk-capital-spider-bites- 2720935
- Dunbar J, Afoullouss S, Sulpice R, et al. Envenomation by the noble false widow spider Steatoda nobilis (Thorell, 1875)—five new cases of steatodism from Ireland and Great Britain. Clin Toxicol (Phila). 2018;56:433-435. doi:10.1080/15563650.2017.1393084
- Dunbar J, Fort A, Redureau D, et al. Venomics approach reveals a high proportion of Latrodectus-like toxins in the venom of the noble false widow spider Steatoda nobilis. Toxins. 2020;12:402.
- Warrell D, Shaheen J, Hillyard P, et al. Neurotoxic envenoming by an immigrant spider (Steatoda nobilis) in southern England. Toxicon. 1991;29:1263-1265.
- Zhou H, Xu K, Zheng PY, et. al. Clinical characteristics of patients with black widow spider bites: a report of 59 patients and single-center experience. World J Emerg Med. 2021;12:317-320. doi:10.5847/wjem.j.1920-8642.2021.04.011
- Dunbar J, Vitkauskaite A, O’Keeffe D, et. al. Bites by the noble false widow spider Steatoda nobilis can induce Latrodectus-like symptoms and vector-borne bacterial infections with implications for public health: a case series. Clin Toxicol (Phila). 2022;60:59-70. doi:10.1080/15563650.2021.1928165
- Dunbar J, Sulpice R, Dugon M. The kiss of (cell) death: can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin Toxicol. 2019;57:677-685. doi:10.1080/15563650.2019.1578367
- Magee J. Bite ‘nightmare’: close encounter with a false widow. The Bournemouth Echo. September 7, 2009. Accessed September 21, 2023. http://www.bournemouthecho.co.uk/news/4582887.Bite____nightmare_____close_encounter_with_a_false_widow_spider/
- Marsh H. Woman nearly loses hand after bite from false widow. Daily Echo. April 17, 2012. Accessed September 21, 2023. https://www.bournemouthecho.co.uk/news/9652335.woman-nearly-loses-hand-after-bite-from-false-widow-spider/
- Stuber N, Nentwig W. How informative are case studies of spider bites in the medical literature? Toxicon. 2016;114:40-44. doi:10.1016/j.toxicon.2016.02.023
- Vetter R, Swanson D, Weinstein S, et. al. Do spiders vector bacteria during bites? the evidence indicates otherwise. Toxicon. 2015;93:171-174. doi:10.1016/j.toxicon.2014.11.229
- Dunbar J, Khan N, Abberton C, et al. Synanthropic spiders, including the global invasive noble false widow Steatoda nobilis, are reservoirs for medically important and antibiotic resistant bacteria. Sci Rep. 2020;10:20916. doi:10.1038/s41598-020-77839-9
- Atakuziev BU, Wright CE, Graudins A, et al. Efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of clinical envenomation by the cupboard spider Steatoda capensis (Theridiidae). Toxicon. 2014;86:68-78. doi:10.1016/j.toxicon.2014.04.011
- Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775. doi:10.1016/S0041-0101(01)00280-X.
- South M, Wirth P, Winkel KD. Redback spider antivenom used to treat envenomation by a juvenile Steatoda spider. Med J Aust. 1998;169:642-642. doi:10.5694/j.1326-5377.1998.tb123445.x
- Kulczycki A, Legittimo C, Simeon E, et al. New records of Steatoda nobilis (Thorell, 1875) (Araneae, Theridiidae), an introduced species on the Italian mainland and in Sardinia. Bull Br Arachnological Soc. 2012;15:269-272.
- Bauer T, Feldmeier S, Krehenwinkel H, et al. Steatoda nobilis, a false widow on the rise: a synthesis of past and current distribution trends. NeoBiota. 2019; 42:19. doi:10.3897/neobiota.42.31582
- Murphy A. Web of cries: false widow spider infestation fears forceeleventh school in London to close as outbreak spreads. The Sun.October 19, 2018. Accessed September 21, 2023. https://www.thesun.co.uk/news/7534016/false-widow-spider-infestation-fears-force-eleventh-londonschool-closing
- Vetter R, Rust M. A large European combfoot spider, Steatoda nobilis (Thorell 1875)(Araneae: Theridiidae), newly established in Ventura County, California. The Pan-Pacific Entomologist. 2012;88:92-97.
- Hambler C. The ‘noble false widow’ spider Steatoda nobilis is an emerging public health and ecological threat. OSF Preprints. Preprint posted online October 15, 2019. doi:10.31219/osf.io/axbd4
- Dunbar J, Schulte J, Lyons K, et al. New Irish record for Steatoda triangulosa (Walckenaer, 1802), and new county records for Steatoda nobilis (Thorell, 1875), Steatoda bipunctata (Linnaeus, 1758) and Steatoda grossa (C.L. Koch, 1838). Ir Naturalists J. 2018;36:39-43.
- Duon M, Dunbar J, Afoullouss S, et al. Occurrence, reproductive rate and identification of the non-native noble false widow spider Steatoda nobilis (Thorell, 1875) in Ireland. Biol Environment: Proc Royal Ir Acad. 2017;117B:77-89. doi:10.3318/bioe.2017.11
- Burrows T. Great bitten: Britain’s spider bite capital revealed as Essex with 450 attacks—find out where your town ranks. The Sun. Published April 3, 2019. Accessed September 14, 2023. https://www.thesun.co.uk/news/8782355/britains-spider-bite-capital-revealed-as-essex-with-450- attacks-find-out-where-your-town-ranks/
- Wathen T. Essex is the UK capital for spider bites—and the amount is terrifying. Essex News. April 4, 2019. Accessed September 21, 2023. https://www.essexlive.news/news/essex-news/essex-uk-capital-spider-bites- 2720935
- Dunbar J, Afoullouss S, Sulpice R, et al. Envenomation by the noble false widow spider Steatoda nobilis (Thorell, 1875)—five new cases of steatodism from Ireland and Great Britain. Clin Toxicol (Phila). 2018;56:433-435. doi:10.1080/15563650.2017.1393084
- Dunbar J, Fort A, Redureau D, et al. Venomics approach reveals a high proportion of Latrodectus-like toxins in the venom of the noble false widow spider Steatoda nobilis. Toxins. 2020;12:402.
- Warrell D, Shaheen J, Hillyard P, et al. Neurotoxic envenoming by an immigrant spider (Steatoda nobilis) in southern England. Toxicon. 1991;29:1263-1265.
- Zhou H, Xu K, Zheng PY, et. al. Clinical characteristics of patients with black widow spider bites: a report of 59 patients and single-center experience. World J Emerg Med. 2021;12:317-320. doi:10.5847/wjem.j.1920-8642.2021.04.011
- Dunbar J, Vitkauskaite A, O’Keeffe D, et. al. Bites by the noble false widow spider Steatoda nobilis can induce Latrodectus-like symptoms and vector-borne bacterial infections with implications for public health: a case series. Clin Toxicol (Phila). 2022;60:59-70. doi:10.1080/15563650.2021.1928165
- Dunbar J, Sulpice R, Dugon M. The kiss of (cell) death: can venom-induced immune response contribute to dermal necrosis following arthropod envenomations? Clin Toxicol. 2019;57:677-685. doi:10.1080/15563650.2019.1578367
- Magee J. Bite ‘nightmare’: close encounter with a false widow. The Bournemouth Echo. September 7, 2009. Accessed September 21, 2023. http://www.bournemouthecho.co.uk/news/4582887.Bite____nightmare_____close_encounter_with_a_false_widow_spider/
- Marsh H. Woman nearly loses hand after bite from false widow. Daily Echo. April 17, 2012. Accessed September 21, 2023. https://www.bournemouthecho.co.uk/news/9652335.woman-nearly-loses-hand-after-bite-from-false-widow-spider/
- Stuber N, Nentwig W. How informative are case studies of spider bites in the medical literature? Toxicon. 2016;114:40-44. doi:10.1016/j.toxicon.2016.02.023
- Vetter R, Swanson D, Weinstein S, et. al. Do spiders vector bacteria during bites? the evidence indicates otherwise. Toxicon. 2015;93:171-174. doi:10.1016/j.toxicon.2014.11.229
- Dunbar J, Khan N, Abberton C, et al. Synanthropic spiders, including the global invasive noble false widow Steatoda nobilis, are reservoirs for medically important and antibiotic resistant bacteria. Sci Rep. 2020;10:20916. doi:10.1038/s41598-020-77839-9
- Atakuziev BU, Wright CE, Graudins A, et al. Efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of clinical envenomation by the cupboard spider Steatoda capensis (Theridiidae). Toxicon. 2014;86:68-78. doi:10.1016/j.toxicon.2014.04.011
- Graudins A, Gunja N, Broady KW, et al. Clinical and in vitro evidence for the efficacy of Australian red-back spider (Latrodectus hasselti) antivenom in the treatment of envenomation by a cupboard spider (Steatoda grossa). Toxicon. 2002;40:767-775. doi:10.1016/S0041-0101(01)00280-X.
- South M, Wirth P, Winkel KD. Redback spider antivenom used to treat envenomation by a juvenile Steatoda spider. Med J Aust. 1998;169:642-642. doi:10.5694/j.1326-5377.1998.tb123445.x
PRACTICE POINTS
- With evidence of a recent population boom of noble false widow spiders in Europe and spread to California, dermatologists should be aware of these spiders and their bites.
- Symptoms of Steatoda bites (steatodism) include immediate pain followed by intense pruritus, swelling, erythema, and possibly systemic symptoms such as fever. Secondary infections such as cellulitis and septicemia are risks.
- The envenomation site should be kept clean to prevent secondary infection, and medical care should be sought when there is evidence of ulceration or cellulitis.
Youth Exposure to Spironolactone in TikTok Videos
The short-form video hosting service TikTok has become a mainstream platform for individuals to share their ideas and educate the public regarding dermatologic diseases such as atopic dermatitis, alopecia, and acne. Users can create and post videos, leave comments, and indicate their interest in or approval of certain content by “liking” videos. In 2022, according to a Pew Research Center survey, approximately 67% of American teenagers aged 13 to 17 years reported using TikTok at least once.1 This population, along with the rest of its users, are increasing their use of TikTok to share information on dermatologic topics such as acne and isotretinoin.2,3 Spironolactone is an effective medication for acne but is not as widely known to the public as other acne medications such as retinoids, salicylic acid, and benzoyl peroxide. Being aware of youth exposure to media related to acne and spironolactone can help dermatologists understand gaps in education and refine their interactions with this patient population.
To gain insight into youth exposure to spironolactone, we conducted a search of TikTok on July 26, 2022, using the term #spironolactone to retrieve the top 50 videos identified by TikTok under the “Top” tab on spironolactone. Search results and the top 10 comments for each video were reviewed. The total number of views and likes for the top 50 videos were 6,735,992 and 851,856, respectively.
Videos were subdivided into educational information related to spironolactone and/or skin care (32% [16/50]), discussion of side effects of spironolactone (26% [13/50]), those with noticeable improvement of acne following treatment with spironolactone (20% [10/50]), recommendations to see a physician or dermatologist to treat acne (10% [5/50]), and other (12% [6/50]). Other takeaways from the top 50 videos included the following:
- Common side effects: irregular periods (10% [5/50]), frequent urination (8% [4/50]), dizziness/lightheadedness (8% [4/50]), and breast tenderness (6% [3/50])
- Longest reported use of spironolactone: 4 years, with complete acne resolution
- Average treatment length prior to noticeable results: 4 to 6 months, with the shortest being 1 month
- Reported dosages of spironolactone: ranged from 50 to 200 mg/d. The most common dosage was 100 mg/d (10% [5/50]). The lowest reported dosage was 50 mg/d (4% [2/50]), while the highest reported dosage was 200 mg/d (2% [1/50])
- Self-reported concurrent use of spironolactone with a combined oral contraceptive: drospirenoneTimes New Roman–ethinyl estradiol (4% [2/50]), norethindrone acetateTimes New Roman–ethinyl estradiol/ferrous fumarate (2% [1/50]), and norgestimateTimes New Roman–ethinyl estradiol (2% [1/50])
- Negative experiences with side effects and lack of acne improvement that led to treatment cessation: 8% (4/50).
Even though spironolactone is not as well-known as other treatments for acne, we found many TikTok users posting about, commenting on, and highlighting the relevance of this therapeutic option. There was no suggestion in any of the videos that spironolactone could be obtained without physician care and/or prescription. A prior report discussing youth sentiment of isotretinoin use on TikTok found that popular videos and videos with the most likes focused on the drug’s positive impact on acne improvement, while comments displayed heightened desires to learn more about isotretinoin and its side effects.3 Our analysis showed a similar response to spironolactone. In all videos showcasing the skin before and after treatment, there were noticeable improvements in the poster’s acne. Most of the video comments displayed a desire to learn more about spironolactone and its side effects. There also were many questions about time to noticeable results. In contrast to the study on isotretinoin,3 the most-liked spironolactone videos contained educational information about spironolactone and/or skin care rather than focusing solely on the impact of the drug on acne. Additionally, the study on isotretinoin found no videos mentioning the importance of seeing a dermatologist or other health care professional,3 while our search found multiple videos (10% [5/50]) on spironolactone that advised seeking physician help. In fact, several popular videos (8% [4/50]) were created by board-certified dermatologists who mainly focused on providing educational information. This difference in educational content may be attributed to spironolactone’s lesser-known function in treating acne. Furthermore, the comments suggested a growing interest in learning more about spironolactone as a treatment option for acne, specifically its mechanism of action and side effects.
With nearly 2 billion monthly active users globally and 94.1 million monthly active users in the United States (as of March 2023),4 TikTok is a popular social media platform that allows dermatologists to better understand youth sentiment on acne treatments such as spironolactone and isotretinoin and also provides an opportunity for medical education to reach a larger audience. This increased youth insight from TikTok can be utilized by dermatologists to make more informed decisions in developing patient-centered care that appeals to the adolescent population.
- Vogels EA, Gelles-Watnick R, Massarat N. Teens, social media and technology 2022. Published August 10, 2022. Accessed September 16, 2023. https://www.pewresearch.org/internet/2022/08/10/teens-social-media-and-technology-2022/
- Szeto MD, Mamo A, Afrin A, et al. Social media in dermatology and an overview of popular social media platforms. Curr Dermatol Rep. 2021;10:97-104. doi:10.1007/s13671-021-00343-4
- Galamgam J, Jia JL. “Accutane check”: insights into youth sentiment toward isotretinoin from a TikTok trend. Pediatr Dermatol. 2021;38:980-981. doi:10.1111/pde.14660
- Aslam S. TikTok by the numbers: stats, demographics & fun facts. Omnicore website. February 27, 2023. Accessed September 14, 2023. https://www.omnicoreagency.com/tiktok-statistics/
The short-form video hosting service TikTok has become a mainstream platform for individuals to share their ideas and educate the public regarding dermatologic diseases such as atopic dermatitis, alopecia, and acne. Users can create and post videos, leave comments, and indicate their interest in or approval of certain content by “liking” videos. In 2022, according to a Pew Research Center survey, approximately 67% of American teenagers aged 13 to 17 years reported using TikTok at least once.1 This population, along with the rest of its users, are increasing their use of TikTok to share information on dermatologic topics such as acne and isotretinoin.2,3 Spironolactone is an effective medication for acne but is not as widely known to the public as other acne medications such as retinoids, salicylic acid, and benzoyl peroxide. Being aware of youth exposure to media related to acne and spironolactone can help dermatologists understand gaps in education and refine their interactions with this patient population.
To gain insight into youth exposure to spironolactone, we conducted a search of TikTok on July 26, 2022, using the term #spironolactone to retrieve the top 50 videos identified by TikTok under the “Top” tab on spironolactone. Search results and the top 10 comments for each video were reviewed. The total number of views and likes for the top 50 videos were 6,735,992 and 851,856, respectively.
Videos were subdivided into educational information related to spironolactone and/or skin care (32% [16/50]), discussion of side effects of spironolactone (26% [13/50]), those with noticeable improvement of acne following treatment with spironolactone (20% [10/50]), recommendations to see a physician or dermatologist to treat acne (10% [5/50]), and other (12% [6/50]). Other takeaways from the top 50 videos included the following:
- Common side effects: irregular periods (10% [5/50]), frequent urination (8% [4/50]), dizziness/lightheadedness (8% [4/50]), and breast tenderness (6% [3/50])
- Longest reported use of spironolactone: 4 years, with complete acne resolution
- Average treatment length prior to noticeable results: 4 to 6 months, with the shortest being 1 month
- Reported dosages of spironolactone: ranged from 50 to 200 mg/d. The most common dosage was 100 mg/d (10% [5/50]). The lowest reported dosage was 50 mg/d (4% [2/50]), while the highest reported dosage was 200 mg/d (2% [1/50])
- Self-reported concurrent use of spironolactone with a combined oral contraceptive: drospirenoneTimes New Roman–ethinyl estradiol (4% [2/50]), norethindrone acetateTimes New Roman–ethinyl estradiol/ferrous fumarate (2% [1/50]), and norgestimateTimes New Roman–ethinyl estradiol (2% [1/50])
- Negative experiences with side effects and lack of acne improvement that led to treatment cessation: 8% (4/50).
Even though spironolactone is not as well-known as other treatments for acne, we found many TikTok users posting about, commenting on, and highlighting the relevance of this therapeutic option. There was no suggestion in any of the videos that spironolactone could be obtained without physician care and/or prescription. A prior report discussing youth sentiment of isotretinoin use on TikTok found that popular videos and videos with the most likes focused on the drug’s positive impact on acne improvement, while comments displayed heightened desires to learn more about isotretinoin and its side effects.3 Our analysis showed a similar response to spironolactone. In all videos showcasing the skin before and after treatment, there were noticeable improvements in the poster’s acne. Most of the video comments displayed a desire to learn more about spironolactone and its side effects. There also were many questions about time to noticeable results. In contrast to the study on isotretinoin,3 the most-liked spironolactone videos contained educational information about spironolactone and/or skin care rather than focusing solely on the impact of the drug on acne. Additionally, the study on isotretinoin found no videos mentioning the importance of seeing a dermatologist or other health care professional,3 while our search found multiple videos (10% [5/50]) on spironolactone that advised seeking physician help. In fact, several popular videos (8% [4/50]) were created by board-certified dermatologists who mainly focused on providing educational information. This difference in educational content may be attributed to spironolactone’s lesser-known function in treating acne. Furthermore, the comments suggested a growing interest in learning more about spironolactone as a treatment option for acne, specifically its mechanism of action and side effects.
With nearly 2 billion monthly active users globally and 94.1 million monthly active users in the United States (as of March 2023),4 TikTok is a popular social media platform that allows dermatologists to better understand youth sentiment on acne treatments such as spironolactone and isotretinoin and also provides an opportunity for medical education to reach a larger audience. This increased youth insight from TikTok can be utilized by dermatologists to make more informed decisions in developing patient-centered care that appeals to the adolescent population.
The short-form video hosting service TikTok has become a mainstream platform for individuals to share their ideas and educate the public regarding dermatologic diseases such as atopic dermatitis, alopecia, and acne. Users can create and post videos, leave comments, and indicate their interest in or approval of certain content by “liking” videos. In 2022, according to a Pew Research Center survey, approximately 67% of American teenagers aged 13 to 17 years reported using TikTok at least once.1 This population, along with the rest of its users, are increasing their use of TikTok to share information on dermatologic topics such as acne and isotretinoin.2,3 Spironolactone is an effective medication for acne but is not as widely known to the public as other acne medications such as retinoids, salicylic acid, and benzoyl peroxide. Being aware of youth exposure to media related to acne and spironolactone can help dermatologists understand gaps in education and refine their interactions with this patient population.
To gain insight into youth exposure to spironolactone, we conducted a search of TikTok on July 26, 2022, using the term #spironolactone to retrieve the top 50 videos identified by TikTok under the “Top” tab on spironolactone. Search results and the top 10 comments for each video were reviewed. The total number of views and likes for the top 50 videos were 6,735,992 and 851,856, respectively.
Videos were subdivided into educational information related to spironolactone and/or skin care (32% [16/50]), discussion of side effects of spironolactone (26% [13/50]), those with noticeable improvement of acne following treatment with spironolactone (20% [10/50]), recommendations to see a physician or dermatologist to treat acne (10% [5/50]), and other (12% [6/50]). Other takeaways from the top 50 videos included the following:
- Common side effects: irregular periods (10% [5/50]), frequent urination (8% [4/50]), dizziness/lightheadedness (8% [4/50]), and breast tenderness (6% [3/50])
- Longest reported use of spironolactone: 4 years, with complete acne resolution
- Average treatment length prior to noticeable results: 4 to 6 months, with the shortest being 1 month
- Reported dosages of spironolactone: ranged from 50 to 200 mg/d. The most common dosage was 100 mg/d (10% [5/50]). The lowest reported dosage was 50 mg/d (4% [2/50]), while the highest reported dosage was 200 mg/d (2% [1/50])
- Self-reported concurrent use of spironolactone with a combined oral contraceptive: drospirenoneTimes New Roman–ethinyl estradiol (4% [2/50]), norethindrone acetateTimes New Roman–ethinyl estradiol/ferrous fumarate (2% [1/50]), and norgestimateTimes New Roman–ethinyl estradiol (2% [1/50])
- Negative experiences with side effects and lack of acne improvement that led to treatment cessation: 8% (4/50).
Even though spironolactone is not as well-known as other treatments for acne, we found many TikTok users posting about, commenting on, and highlighting the relevance of this therapeutic option. There was no suggestion in any of the videos that spironolactone could be obtained without physician care and/or prescription. A prior report discussing youth sentiment of isotretinoin use on TikTok found that popular videos and videos with the most likes focused on the drug’s positive impact on acne improvement, while comments displayed heightened desires to learn more about isotretinoin and its side effects.3 Our analysis showed a similar response to spironolactone. In all videos showcasing the skin before and after treatment, there were noticeable improvements in the poster’s acne. Most of the video comments displayed a desire to learn more about spironolactone and its side effects. There also were many questions about time to noticeable results. In contrast to the study on isotretinoin,3 the most-liked spironolactone videos contained educational information about spironolactone and/or skin care rather than focusing solely on the impact of the drug on acne. Additionally, the study on isotretinoin found no videos mentioning the importance of seeing a dermatologist or other health care professional,3 while our search found multiple videos (10% [5/50]) on spironolactone that advised seeking physician help. In fact, several popular videos (8% [4/50]) were created by board-certified dermatologists who mainly focused on providing educational information. This difference in educational content may be attributed to spironolactone’s lesser-known function in treating acne. Furthermore, the comments suggested a growing interest in learning more about spironolactone as a treatment option for acne, specifically its mechanism of action and side effects.
With nearly 2 billion monthly active users globally and 94.1 million monthly active users in the United States (as of March 2023),4 TikTok is a popular social media platform that allows dermatologists to better understand youth sentiment on acne treatments such as spironolactone and isotretinoin and also provides an opportunity for medical education to reach a larger audience. This increased youth insight from TikTok can be utilized by dermatologists to make more informed decisions in developing patient-centered care that appeals to the adolescent population.
- Vogels EA, Gelles-Watnick R, Massarat N. Teens, social media and technology 2022. Published August 10, 2022. Accessed September 16, 2023. https://www.pewresearch.org/internet/2022/08/10/teens-social-media-and-technology-2022/
- Szeto MD, Mamo A, Afrin A, et al. Social media in dermatology and an overview of popular social media platforms. Curr Dermatol Rep. 2021;10:97-104. doi:10.1007/s13671-021-00343-4
- Galamgam J, Jia JL. “Accutane check”: insights into youth sentiment toward isotretinoin from a TikTok trend. Pediatr Dermatol. 2021;38:980-981. doi:10.1111/pde.14660
- Aslam S. TikTok by the numbers: stats, demographics & fun facts. Omnicore website. February 27, 2023. Accessed September 14, 2023. https://www.omnicoreagency.com/tiktok-statistics/
- Vogels EA, Gelles-Watnick R, Massarat N. Teens, social media and technology 2022. Published August 10, 2022. Accessed September 16, 2023. https://www.pewresearch.org/internet/2022/08/10/teens-social-media-and-technology-2022/
- Szeto MD, Mamo A, Afrin A, et al. Social media in dermatology and an overview of popular social media platforms. Curr Dermatol Rep. 2021;10:97-104. doi:10.1007/s13671-021-00343-4
- Galamgam J, Jia JL. “Accutane check”: insights into youth sentiment toward isotretinoin from a TikTok trend. Pediatr Dermatol. 2021;38:980-981. doi:10.1111/pde.14660
- Aslam S. TikTok by the numbers: stats, demographics & fun facts. Omnicore website. February 27, 2023. Accessed September 14, 2023. https://www.omnicoreagency.com/tiktok-statistics/
Knead a Hand? Use of a Portable Massager to Reduce Patient Pain and Anxiety During Nail Surgery
Practice Gap
Pain and anxiety are common in fully conscious patients undergoing dermatologic surgery with local anesthesia. Particularly during nail surgery, pain from anesthetic injection—caused by both needle insertion and fluid infiltration—occurs because the nail unit is highly vascularized and innervated.1 Current methods to improve patient comfort during infiltration include use of a buffered anesthetic solution, warming the anesthetic, slower technique, and direct cold application.2
Perioperative anxiety correlates with increased postoperative pain, analgesic use, and delayed recovery. Furthermore, increased perioperative anxiety reduces the pain threshold and elevates estimates of pain intensity.3 Therefore, reducing procedure-related anxiety and pain may improve quality of care and ease patient discomfort.
Distraction is a common and practical nonpharmacotherapeutic technique for reducing pain and anxiety during medical procedures. The refocusing method of distraction aims to divert attention away from pain to more pleasant stimuli to reduce pain perception.3 Several methods of distraction—using stress balls, engaging in conversation, hand-holding, applying virtual reality, and playing videos—can decrease perioperative anxiety and pain.3-6
Procedural pain and distraction techniques have been evaluated in the pediatric population more than in adults.4 Nail surgery–associated pain and distraction techniques for nail surgery have been inadequately studied.7
We offer a distraction technique utilizing a portable massager to ensure that patients are as comfortable as possible when the local anesthetic is injected prior to the first incision.
The Technique
A portable shiatsu massager that uses heat and deep-tissue kneading is placed on the upper thigh for toenail cases or lower arm for fingernail cases during injection of anesthetic to divert the patient’s attention from the surgical site (Figure). Kneading from the massage helps distract the patient from pain by introducing a competing, more pleasant, vibrating sensation that overrides pain signals; the relaxation component helps to diminish patient anxiety during injection.
Practice Implications
Use of a portable massager may reduce pain through both distraction and vibration. In a randomized clinical trial of 115 patients undergoing hand or facial surgery, patients who viewed a distraction video during the procedure reported a lower pain score compared to the control group (mean [SD] visual analog scale of pain score, 3.4 [2.6] vs 4.5 [2.6][P=.01]).4 In another randomized clinical trial of 25 patients undergoing lip augmentation, 92% of patients (23/25) in the vibration-assisted arm endorsed less pain during procedures compared to the arm without vibration (mean [SD] pain score, 3.82 [1.73] vs 5.6 [1.76][P<.001]).8
Utilization of a portable massager is a safe means of improving the patient experience; the distracting and relaxing effects and intense pulsations simultaneously reduce anxiety and pain during nail surgery. Controlled clinical trials are needed to evaluate its efficacy in diminishing both anxiety and pain during nail procedures compared to other analgesic methods.
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:E231-E232. doi:10.1016/j.jaad.2019.11.032
- Hudson BF, Ogden J, Whiteley MS. Randomized controlled trial to compare the effect of simple distraction interventions on pain and anxiety experienced during conscious surgery. Eur J Pain. 2015;19:1447-1455. doi:10.1002/ejp.675
- Molleman J, Tielemans JF, Braam MJI, et al. Distraction as a simple and effective method to reduce pain during local anesthesia: a randomized controlled trial. J Plast Reconstr Aesthet Surg. 2019;72:1979-1985. doi:10.1016/j.bjps.2019.07.023
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294.
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ricardo JW, Qiu Y, Lipner SR. Longitudinal perioperative pain assessment in nail surgery. J Am Acad Dermatol. 2022;87:874-876. doi:10.1016/j.jaad.2021.11.042
- Guney K, Sezgin B, Yavuzer R. The efficacy of vibration anesthesia on reducing pain levels during lip augmentation: worth the buzz? Aesthet Surg J. 2017;37:1044-1048. doi:10.1093/asj/sjx073
Practice Gap
Pain and anxiety are common in fully conscious patients undergoing dermatologic surgery with local anesthesia. Particularly during nail surgery, pain from anesthetic injection—caused by both needle insertion and fluid infiltration—occurs because the nail unit is highly vascularized and innervated.1 Current methods to improve patient comfort during infiltration include use of a buffered anesthetic solution, warming the anesthetic, slower technique, and direct cold application.2
Perioperative anxiety correlates with increased postoperative pain, analgesic use, and delayed recovery. Furthermore, increased perioperative anxiety reduces the pain threshold and elevates estimates of pain intensity.3 Therefore, reducing procedure-related anxiety and pain may improve quality of care and ease patient discomfort.
Distraction is a common and practical nonpharmacotherapeutic technique for reducing pain and anxiety during medical procedures. The refocusing method of distraction aims to divert attention away from pain to more pleasant stimuli to reduce pain perception.3 Several methods of distraction—using stress balls, engaging in conversation, hand-holding, applying virtual reality, and playing videos—can decrease perioperative anxiety and pain.3-6
Procedural pain and distraction techniques have been evaluated in the pediatric population more than in adults.4 Nail surgery–associated pain and distraction techniques for nail surgery have been inadequately studied.7
We offer a distraction technique utilizing a portable massager to ensure that patients are as comfortable as possible when the local anesthetic is injected prior to the first incision.
The Technique
A portable shiatsu massager that uses heat and deep-tissue kneading is placed on the upper thigh for toenail cases or lower arm for fingernail cases during injection of anesthetic to divert the patient’s attention from the surgical site (Figure). Kneading from the massage helps distract the patient from pain by introducing a competing, more pleasant, vibrating sensation that overrides pain signals; the relaxation component helps to diminish patient anxiety during injection.

Practice Implications
Use of a portable massager may reduce pain through both distraction and vibration. In a randomized clinical trial of 115 patients undergoing hand or facial surgery, patients who viewed a distraction video during the procedure reported a lower pain score compared to the control group (mean [SD] visual analog scale of pain score, 3.4 [2.6] vs 4.5 [2.6][P=.01]).4 In another randomized clinical trial of 25 patients undergoing lip augmentation, 92% of patients (23/25) in the vibration-assisted arm endorsed less pain during procedures compared to the arm without vibration (mean [SD] pain score, 3.82 [1.73] vs 5.6 [1.76][P<.001]).8
Utilization of a portable massager is a safe means of improving the patient experience; the distracting and relaxing effects and intense pulsations simultaneously reduce anxiety and pain during nail surgery. Controlled clinical trials are needed to evaluate its efficacy in diminishing both anxiety and pain during nail procedures compared to other analgesic methods.
Practice Gap
Pain and anxiety are common in fully conscious patients undergoing dermatologic surgery with local anesthesia. Particularly during nail surgery, pain from anesthetic injection—caused by both needle insertion and fluid infiltration—occurs because the nail unit is highly vascularized and innervated.1 Current methods to improve patient comfort during infiltration include use of a buffered anesthetic solution, warming the anesthetic, slower technique, and direct cold application.2
Perioperative anxiety correlates with increased postoperative pain, analgesic use, and delayed recovery. Furthermore, increased perioperative anxiety reduces the pain threshold and elevates estimates of pain intensity.3 Therefore, reducing procedure-related anxiety and pain may improve quality of care and ease patient discomfort.
Distraction is a common and practical nonpharmacotherapeutic technique for reducing pain and anxiety during medical procedures. The refocusing method of distraction aims to divert attention away from pain to more pleasant stimuli to reduce pain perception.3 Several methods of distraction—using stress balls, engaging in conversation, hand-holding, applying virtual reality, and playing videos—can decrease perioperative anxiety and pain.3-6
Procedural pain and distraction techniques have been evaluated in the pediatric population more than in adults.4 Nail surgery–associated pain and distraction techniques for nail surgery have been inadequately studied.7
We offer a distraction technique utilizing a portable massager to ensure that patients are as comfortable as possible when the local anesthetic is injected prior to the first incision.
The Technique
A portable shiatsu massager that uses heat and deep-tissue kneading is placed on the upper thigh for toenail cases or lower arm for fingernail cases during injection of anesthetic to divert the patient’s attention from the surgical site (Figure). Kneading from the massage helps distract the patient from pain by introducing a competing, more pleasant, vibrating sensation that overrides pain signals; the relaxation component helps to diminish patient anxiety during injection.

Practice Implications
Use of a portable massager may reduce pain through both distraction and vibration. In a randomized clinical trial of 115 patients undergoing hand or facial surgery, patients who viewed a distraction video during the procedure reported a lower pain score compared to the control group (mean [SD] visual analog scale of pain score, 3.4 [2.6] vs 4.5 [2.6][P=.01]).4 In another randomized clinical trial of 25 patients undergoing lip augmentation, 92% of patients (23/25) in the vibration-assisted arm endorsed less pain during procedures compared to the arm without vibration (mean [SD] pain score, 3.82 [1.73] vs 5.6 [1.76][P<.001]).8
Utilization of a portable massager is a safe means of improving the patient experience; the distracting and relaxing effects and intense pulsations simultaneously reduce anxiety and pain during nail surgery. Controlled clinical trials are needed to evaluate its efficacy in diminishing both anxiety and pain during nail procedures compared to other analgesic methods.
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:E231-E232. doi:10.1016/j.jaad.2019.11.032
- Hudson BF, Ogden J, Whiteley MS. Randomized controlled trial to compare the effect of simple distraction interventions on pain and anxiety experienced during conscious surgery. Eur J Pain. 2015;19:1447-1455. doi:10.1002/ejp.675
- Molleman J, Tielemans JF, Braam MJI, et al. Distraction as a simple and effective method to reduce pain during local anesthesia: a randomized controlled trial. J Plast Reconstr Aesthet Surg. 2019;72:1979-1985. doi:10.1016/j.bjps.2019.07.023
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294.
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ricardo JW, Qiu Y, Lipner SR. Longitudinal perioperative pain assessment in nail surgery. J Am Acad Dermatol. 2022;87:874-876. doi:10.1016/j.jaad.2021.11.042
- Guney K, Sezgin B, Yavuzer R. The efficacy of vibration anesthesia on reducing pain levels during lip augmentation: worth the buzz? Aesthet Surg J. 2017;37:1044-1048. doi:10.1093/asj/sjx073
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:E231-E232. doi:10.1016/j.jaad.2019.11.032
- Hudson BF, Ogden J, Whiteley MS. Randomized controlled trial to compare the effect of simple distraction interventions on pain and anxiety experienced during conscious surgery. Eur J Pain. 2015;19:1447-1455. doi:10.1002/ejp.675
- Molleman J, Tielemans JF, Braam MJI, et al. Distraction as a simple and effective method to reduce pain during local anesthesia: a randomized controlled trial. J Plast Reconstr Aesthet Surg. 2019;72:1979-1985. doi:10.1016/j.bjps.2019.07.023
- Ricardo JW, Lipner SR. Utilization of a stress ball to diminish anxiety during nail surgery. Cutis. 2020;105:294.
- Ricardo JW, Lipner SR. Utilizing a sleep mask to reduce patient anxiety during nail surgery. Cutis. 2021;108:36. doi:10.12788/cutis.0285
- Ricardo JW, Qiu Y, Lipner SR. Longitudinal perioperative pain assessment in nail surgery. J Am Acad Dermatol. 2022;87:874-876. doi:10.1016/j.jaad.2021.11.042
- Guney K, Sezgin B, Yavuzer R. The efficacy of vibration anesthesia on reducing pain levels during lip augmentation: worth the buzz? Aesthet Surg J. 2017;37:1044-1048. doi:10.1093/asj/sjx073
FLOTCH Syndrome: A Case of Leukonychia Totalis and Multiple Pilar Cysts
FLOTCH (leukonychia totalis-trichilemmal cysts-ciliary dystrophy syndrome) syndrome is a rare genetic cutaneous disorder primarily characterized by multiple recurrent trichilemmal pilar cysts and leukonychia. It may be associated with ciliary dystrophy, koilonychia, and/or less frequently renal calculi and pancreatitis. This disorder often presents in an autosomal-dominant pattern of inheritance. Leukonychia and associated pilar cysts originally were termed Bauer syndrome in 1920 and later described in 1986 as FLOTCH syndrome secondary to the association with ciliary dystrophy. 1,2 The term FLOTCH was coined by Friedel et al 1 to describe a combination of diagnoses experienced by a family in which several members had multiple pilar cysts, leukonychia, and ciliary dystrophy. We present a 25-year-old Black woman with suspected FLOTCH syndrome who was seen in our clinic for enlarging cysts.
Case Report
A 25-year-old Black woman with no notable medical history presented to the clinic for a surgical evaluation of cysts of several years’ duration that were enlarging and tender. Physical examination revealed multiple firm, fixed, tender nodules on the left superior parietal scalp, left inferior frontal scalp (Figure 1A), right inferior parietal scalp, right central postauricular skin, and right inferior occipital scalp. Similar-appearing cysts measuring 1.5 to 2 cm were seen on the left rib cage (Figure 1B) and left lateral forearm. Upon further examination, there was homogeneous, nonblanchable, white discoloration of all 10 fingernails consistent with true leukonychia (Figure 1C). When questioned about the nails, the patient stated they had been this color her whole life. Moreover, the patient confirmed that her brother’s nails had a similar appearance.
The patient subsequently underwent elliptical excision of the cysts located on the left medial forehead and left rib cage, and histopathology revealed trichilemmal pilar cysts with dystrophic calcification, dermal fibrosis, and mild chronic inflammation (Figure 2). The pathology report also noted that the anatomic site was somewhat unusual; however, the features were otherwise typical and diagnostic. Given the presentation of multiple pilar cysts throughout the body, leukonychia totalis, and positive family history, the patient was diagnosed with FLOTCH syndrome. Unfortunately, the patient was lost to follow-up following the excision, and no further management could be provided.
Comment
Leukonychia is an abnormality of the nail that results in a visible distribution of white color across the nail plate. It can be classified as totalis when covering the entire nail or partialis when covering localized areas of the nail. The disease also is categorized as acquired or inherited. Acquired leukonychia may appear after damage to a particular area of the nail or secondary to an underlying systemic disease, clinically appearing as white puncta or transverse striae. Hereditary leukonychia is rare, primarily covering the entire nail (totalis), and often is inherited in an autosomal-dominant pattern.3,4 The appearance of this disease can be an isolated occurrence or may be a component of a condition such as FLOTCH syndrome, as proposed in this case.
Pilar cysts (also known as trichilemmal cysts) are benign, slowly growing, firm, subcutaneous nodules that are similar to epidermoid cysts but arise from the root sheaths of hair follicles. Pilar cysts are inherited in an autosomal-dominant pattern and are caused by a mutation involving a 2-hit mechanism of variants of the phospholipase C delta 1 gene, PLCD1. Patients typically present with multiple cysts,5 as in our case.
This association of leukonychia and multiple pilar cysts previously has been reported in 7 family lines.1-3,6-9 The molecular basis of FLOTCH syndrome is unknown, and these combined diagnoses may be of syndromic nature. Histologic observations of leukonychia and the mechanism of the creation of pilar cysts suggest derivation from similar abnormal keratinization in the nail beds and hair follicles, respectively.6
The first familial association between leukonychia totalis and sebaceous cysts was described by Bauer2 in 1920. In 1975, Bushkell and Gorlin7 reported a similar inherited association with the addition of a history of renal calculi. In 1986, Friedel et al1 coined the term FLOTCH syndrome when reporting a case of an affected family presenting with leukonychia, recurrent cysts, and ciliary dystrophy. Slee et al8 reported 2 cases of pancreatitis experienced by patients presenting with these cysts and leukonychia. The etiology of the pancreatitis was unknown, leading researchers to believe it may be a complication associated with the spectrum of diseases.8 In 2008, Morin et al6 proposed that those with linked leukonychia and trichilemmal cysts may be at risk for neuromas or spinal tumors and suggested systematic screening after observing a family member with an ependymoma and bilateral multiple acoustic tumors. Rodríguez-Lojo et al3 described a 5-generation family with leukonychia totalis and numerous pilar cysts. Mutoh et al9 reported another 5-generation family with associated leukonychia and multiple pilar cysts as well as koilonychia. One family member had a reported history of renal calculus.9
In our case, FLOTCH syndrome was suspected given the patient’s concurrent pilar and follicular infundibular cysts. No specific treatment was indicated; however, as seen in prior cases and in ours, many patients prefer to have the cysts excised. A more comprehensive investigation could have revealed other associations, such as ciliary dystrophy, renal calculi, or pancreatitis. It is possible that in conjunction with the syndrome, patients could develop other such clinical manifestations. Pilar cysts most frequently are found on the scalp, yet in patients with concurrent leukonychia, the cysts have been shown to also develop in other regions of the body, as seen in our patient and in the case reported by Mutoh et al.9 Given the autosomal-dominant nature of this disease and the keratinizing structures affected, we confer with the hypotheses that a general keratin dysfunction is suspected. Further investigation is needed to determine the exact altered genetic mechanism or deficiency that may be causing this abnormal keratinization as well as a more extensive examination of patients to confirm if other described symptoms may be related.
- Friedel J, Heid E, Grosshans E. The FLOTCH syndrome. familial occurrence of total leukonychia, trichilemmal cysts and ciliary dystrophy with dominant autosomal heredity [in French]. Ann Dermatol Venereol. 1986;113:549-553.
- Bauer AW. Beiträge zur klinischen Konstitutionspathologie, V. heredofamiliäre leukonychie und multiple atherombilderung der kopfhaut. Z Menschl Vererb. Konstitutitionslehre. 1920;5:47-48.
- Rodríguez-Lojo R, Del Pozo J, Sacristán F, et al. Leukonychia totalis associated with multiple pilar cysts: report of a five-generation family: FLOTCH syndrome? Eur J Dermatol. 2011;21:484-486.
- Claudel CD, Zic JA, Boyd AS. Idiopathic leukonychia totalis and partialis in a 12-year-old patient. J Am Acad Dermatol. 2001;44:379-380.
- Hörer S, Marrakchi S, Radner FPW, et al. A monoallelic two-hit mechanism in PLCD1 explains the genetic pathogenesis of hereditary trichilemmal cyst formation. J Invest Dermatol. 2019;139:2154-2163.e5.
- Morin G, Desenclos C, Jeanpetit C, et al. Additional familial case of subtotal leukonychia and sebaceous cysts (Bauer syndrome): belong the nervous tumours to the phenotype? Eur J Med Genet. 2008;51:436-443.
- Bushkell LL, Gorlin RJ. Leukonychia totalis, multiple sebaceous cysts, and renal calculi. Arch Dermatol. 1975;111:899-901.
- Slee JJ, Wallman IS, Goldblatt J. A syndrome or leukonychia totalis and multiple sebaceous cysts. Clin Dysmorphol. 1997;6:229-233.
- Mutoh M, Niiyama S, Nishikawa S, et al. A syndrome of leukonychia, koilonychia and multiple pilar cysts. Acta Derm Venereol. 2015;95:249-250. doi:10.2340/00015555-1893
FLOTCH (leukonychia totalis-trichilemmal cysts-ciliary dystrophy syndrome) syndrome is a rare genetic cutaneous disorder primarily characterized by multiple recurrent trichilemmal pilar cysts and leukonychia. It may be associated with ciliary dystrophy, koilonychia, and/or less frequently renal calculi and pancreatitis. This disorder often presents in an autosomal-dominant pattern of inheritance. Leukonychia and associated pilar cysts originally were termed Bauer syndrome in 1920 and later described in 1986 as FLOTCH syndrome secondary to the association with ciliary dystrophy. 1,2 The term FLOTCH was coined by Friedel et al 1 to describe a combination of diagnoses experienced by a family in which several members had multiple pilar cysts, leukonychia, and ciliary dystrophy. We present a 25-year-old Black woman with suspected FLOTCH syndrome who was seen in our clinic for enlarging cysts.
Case Report
A 25-year-old Black woman with no notable medical history presented to the clinic for a surgical evaluation of cysts of several years’ duration that were enlarging and tender. Physical examination revealed multiple firm, fixed, tender nodules on the left superior parietal scalp, left inferior frontal scalp (Figure 1A), right inferior parietal scalp, right central postauricular skin, and right inferior occipital scalp. Similar-appearing cysts measuring 1.5 to 2 cm were seen on the left rib cage (Figure 1B) and left lateral forearm. Upon further examination, there was homogeneous, nonblanchable, white discoloration of all 10 fingernails consistent with true leukonychia (Figure 1C). When questioned about the nails, the patient stated they had been this color her whole life. Moreover, the patient confirmed that her brother’s nails had a similar appearance.

The patient subsequently underwent elliptical excision of the cysts located on the left medial forehead and left rib cage, and histopathology revealed trichilemmal pilar cysts with dystrophic calcification, dermal fibrosis, and mild chronic inflammation (Figure 2). The pathology report also noted that the anatomic site was somewhat unusual; however, the features were otherwise typical and diagnostic. Given the presentation of multiple pilar cysts throughout the body, leukonychia totalis, and positive family history, the patient was diagnosed with FLOTCH syndrome. Unfortunately, the patient was lost to follow-up following the excision, and no further management could be provided.

Comment
Leukonychia is an abnormality of the nail that results in a visible distribution of white color across the nail plate. It can be classified as totalis when covering the entire nail or partialis when covering localized areas of the nail. The disease also is categorized as acquired or inherited. Acquired leukonychia may appear after damage to a particular area of the nail or secondary to an underlying systemic disease, clinically appearing as white puncta or transverse striae. Hereditary leukonychia is rare, primarily covering the entire nail (totalis), and often is inherited in an autosomal-dominant pattern.3,4 The appearance of this disease can be an isolated occurrence or may be a component of a condition such as FLOTCH syndrome, as proposed in this case.
Pilar cysts (also known as trichilemmal cysts) are benign, slowly growing, firm, subcutaneous nodules that are similar to epidermoid cysts but arise from the root sheaths of hair follicles. Pilar cysts are inherited in an autosomal-dominant pattern and are caused by a mutation involving a 2-hit mechanism of variants of the phospholipase C delta 1 gene, PLCD1. Patients typically present with multiple cysts,5 as in our case.
This association of leukonychia and multiple pilar cysts previously has been reported in 7 family lines.1-3,6-9 The molecular basis of FLOTCH syndrome is unknown, and these combined diagnoses may be of syndromic nature. Histologic observations of leukonychia and the mechanism of the creation of pilar cysts suggest derivation from similar abnormal keratinization in the nail beds and hair follicles, respectively.6
The first familial association between leukonychia totalis and sebaceous cysts was described by Bauer2 in 1920. In 1975, Bushkell and Gorlin7 reported a similar inherited association with the addition of a history of renal calculi. In 1986, Friedel et al1 coined the term FLOTCH syndrome when reporting a case of an affected family presenting with leukonychia, recurrent cysts, and ciliary dystrophy. Slee et al8 reported 2 cases of pancreatitis experienced by patients presenting with these cysts and leukonychia. The etiology of the pancreatitis was unknown, leading researchers to believe it may be a complication associated with the spectrum of diseases.8 In 2008, Morin et al6 proposed that those with linked leukonychia and trichilemmal cysts may be at risk for neuromas or spinal tumors and suggested systematic screening after observing a family member with an ependymoma and bilateral multiple acoustic tumors. Rodríguez-Lojo et al3 described a 5-generation family with leukonychia totalis and numerous pilar cysts. Mutoh et al9 reported another 5-generation family with associated leukonychia and multiple pilar cysts as well as koilonychia. One family member had a reported history of renal calculus.9
In our case, FLOTCH syndrome was suspected given the patient’s concurrent pilar and follicular infundibular cysts. No specific treatment was indicated; however, as seen in prior cases and in ours, many patients prefer to have the cysts excised. A more comprehensive investigation could have revealed other associations, such as ciliary dystrophy, renal calculi, or pancreatitis. It is possible that in conjunction with the syndrome, patients could develop other such clinical manifestations. Pilar cysts most frequently are found on the scalp, yet in patients with concurrent leukonychia, the cysts have been shown to also develop in other regions of the body, as seen in our patient and in the case reported by Mutoh et al.9 Given the autosomal-dominant nature of this disease and the keratinizing structures affected, we confer with the hypotheses that a general keratin dysfunction is suspected. Further investigation is needed to determine the exact altered genetic mechanism or deficiency that may be causing this abnormal keratinization as well as a more extensive examination of patients to confirm if other described symptoms may be related.
FLOTCH (leukonychia totalis-trichilemmal cysts-ciliary dystrophy syndrome) syndrome is a rare genetic cutaneous disorder primarily characterized by multiple recurrent trichilemmal pilar cysts and leukonychia. It may be associated with ciliary dystrophy, koilonychia, and/or less frequently renal calculi and pancreatitis. This disorder often presents in an autosomal-dominant pattern of inheritance. Leukonychia and associated pilar cysts originally were termed Bauer syndrome in 1920 and later described in 1986 as FLOTCH syndrome secondary to the association with ciliary dystrophy. 1,2 The term FLOTCH was coined by Friedel et al 1 to describe a combination of diagnoses experienced by a family in which several members had multiple pilar cysts, leukonychia, and ciliary dystrophy. We present a 25-year-old Black woman with suspected FLOTCH syndrome who was seen in our clinic for enlarging cysts.
Case Report
A 25-year-old Black woman with no notable medical history presented to the clinic for a surgical evaluation of cysts of several years’ duration that were enlarging and tender. Physical examination revealed multiple firm, fixed, tender nodules on the left superior parietal scalp, left inferior frontal scalp (Figure 1A), right inferior parietal scalp, right central postauricular skin, and right inferior occipital scalp. Similar-appearing cysts measuring 1.5 to 2 cm were seen on the left rib cage (Figure 1B) and left lateral forearm. Upon further examination, there was homogeneous, nonblanchable, white discoloration of all 10 fingernails consistent with true leukonychia (Figure 1C). When questioned about the nails, the patient stated they had been this color her whole life. Moreover, the patient confirmed that her brother’s nails had a similar appearance.

The patient subsequently underwent elliptical excision of the cysts located on the left medial forehead and left rib cage, and histopathology revealed trichilemmal pilar cysts with dystrophic calcification, dermal fibrosis, and mild chronic inflammation (Figure 2). The pathology report also noted that the anatomic site was somewhat unusual; however, the features were otherwise typical and diagnostic. Given the presentation of multiple pilar cysts throughout the body, leukonychia totalis, and positive family history, the patient was diagnosed with FLOTCH syndrome. Unfortunately, the patient was lost to follow-up following the excision, and no further management could be provided.

Comment
Leukonychia is an abnormality of the nail that results in a visible distribution of white color across the nail plate. It can be classified as totalis when covering the entire nail or partialis when covering localized areas of the nail. The disease also is categorized as acquired or inherited. Acquired leukonychia may appear after damage to a particular area of the nail or secondary to an underlying systemic disease, clinically appearing as white puncta or transverse striae. Hereditary leukonychia is rare, primarily covering the entire nail (totalis), and often is inherited in an autosomal-dominant pattern.3,4 The appearance of this disease can be an isolated occurrence or may be a component of a condition such as FLOTCH syndrome, as proposed in this case.
Pilar cysts (also known as trichilemmal cysts) are benign, slowly growing, firm, subcutaneous nodules that are similar to epidermoid cysts but arise from the root sheaths of hair follicles. Pilar cysts are inherited in an autosomal-dominant pattern and are caused by a mutation involving a 2-hit mechanism of variants of the phospholipase C delta 1 gene, PLCD1. Patients typically present with multiple cysts,5 as in our case.
This association of leukonychia and multiple pilar cysts previously has been reported in 7 family lines.1-3,6-9 The molecular basis of FLOTCH syndrome is unknown, and these combined diagnoses may be of syndromic nature. Histologic observations of leukonychia and the mechanism of the creation of pilar cysts suggest derivation from similar abnormal keratinization in the nail beds and hair follicles, respectively.6
The first familial association between leukonychia totalis and sebaceous cysts was described by Bauer2 in 1920. In 1975, Bushkell and Gorlin7 reported a similar inherited association with the addition of a history of renal calculi. In 1986, Friedel et al1 coined the term FLOTCH syndrome when reporting a case of an affected family presenting with leukonychia, recurrent cysts, and ciliary dystrophy. Slee et al8 reported 2 cases of pancreatitis experienced by patients presenting with these cysts and leukonychia. The etiology of the pancreatitis was unknown, leading researchers to believe it may be a complication associated with the spectrum of diseases.8 In 2008, Morin et al6 proposed that those with linked leukonychia and trichilemmal cysts may be at risk for neuromas or spinal tumors and suggested systematic screening after observing a family member with an ependymoma and bilateral multiple acoustic tumors. Rodríguez-Lojo et al3 described a 5-generation family with leukonychia totalis and numerous pilar cysts. Mutoh et al9 reported another 5-generation family with associated leukonychia and multiple pilar cysts as well as koilonychia. One family member had a reported history of renal calculus.9
In our case, FLOTCH syndrome was suspected given the patient’s concurrent pilar and follicular infundibular cysts. No specific treatment was indicated; however, as seen in prior cases and in ours, many patients prefer to have the cysts excised. A more comprehensive investigation could have revealed other associations, such as ciliary dystrophy, renal calculi, or pancreatitis. It is possible that in conjunction with the syndrome, patients could develop other such clinical manifestations. Pilar cysts most frequently are found on the scalp, yet in patients with concurrent leukonychia, the cysts have been shown to also develop in other regions of the body, as seen in our patient and in the case reported by Mutoh et al.9 Given the autosomal-dominant nature of this disease and the keratinizing structures affected, we confer with the hypotheses that a general keratin dysfunction is suspected. Further investigation is needed to determine the exact altered genetic mechanism or deficiency that may be causing this abnormal keratinization as well as a more extensive examination of patients to confirm if other described symptoms may be related.
- Friedel J, Heid E, Grosshans E. The FLOTCH syndrome. familial occurrence of total leukonychia, trichilemmal cysts and ciliary dystrophy with dominant autosomal heredity [in French]. Ann Dermatol Venereol. 1986;113:549-553.
- Bauer AW. Beiträge zur klinischen Konstitutionspathologie, V. heredofamiliäre leukonychie und multiple atherombilderung der kopfhaut. Z Menschl Vererb. Konstitutitionslehre. 1920;5:47-48.
- Rodríguez-Lojo R, Del Pozo J, Sacristán F, et al. Leukonychia totalis associated with multiple pilar cysts: report of a five-generation family: FLOTCH syndrome? Eur J Dermatol. 2011;21:484-486.
- Claudel CD, Zic JA, Boyd AS. Idiopathic leukonychia totalis and partialis in a 12-year-old patient. J Am Acad Dermatol. 2001;44:379-380.
- Hörer S, Marrakchi S, Radner FPW, et al. A monoallelic two-hit mechanism in PLCD1 explains the genetic pathogenesis of hereditary trichilemmal cyst formation. J Invest Dermatol. 2019;139:2154-2163.e5.
- Morin G, Desenclos C, Jeanpetit C, et al. Additional familial case of subtotal leukonychia and sebaceous cysts (Bauer syndrome): belong the nervous tumours to the phenotype? Eur J Med Genet. 2008;51:436-443.
- Bushkell LL, Gorlin RJ. Leukonychia totalis, multiple sebaceous cysts, and renal calculi. Arch Dermatol. 1975;111:899-901.
- Slee JJ, Wallman IS, Goldblatt J. A syndrome or leukonychia totalis and multiple sebaceous cysts. Clin Dysmorphol. 1997;6:229-233.
- Mutoh M, Niiyama S, Nishikawa S, et al. A syndrome of leukonychia, koilonychia and multiple pilar cysts. Acta Derm Venereol. 2015;95:249-250. doi:10.2340/00015555-1893
- Friedel J, Heid E, Grosshans E. The FLOTCH syndrome. familial occurrence of total leukonychia, trichilemmal cysts and ciliary dystrophy with dominant autosomal heredity [in French]. Ann Dermatol Venereol. 1986;113:549-553.
- Bauer AW. Beiträge zur klinischen Konstitutionspathologie, V. heredofamiliäre leukonychie und multiple atherombilderung der kopfhaut. Z Menschl Vererb. Konstitutitionslehre. 1920;5:47-48.
- Rodríguez-Lojo R, Del Pozo J, Sacristán F, et al. Leukonychia totalis associated with multiple pilar cysts: report of a five-generation family: FLOTCH syndrome? Eur J Dermatol. 2011;21:484-486.
- Claudel CD, Zic JA, Boyd AS. Idiopathic leukonychia totalis and partialis in a 12-year-old patient. J Am Acad Dermatol. 2001;44:379-380.
- Hörer S, Marrakchi S, Radner FPW, et al. A monoallelic two-hit mechanism in PLCD1 explains the genetic pathogenesis of hereditary trichilemmal cyst formation. J Invest Dermatol. 2019;139:2154-2163.e5.
- Morin G, Desenclos C, Jeanpetit C, et al. Additional familial case of subtotal leukonychia and sebaceous cysts (Bauer syndrome): belong the nervous tumours to the phenotype? Eur J Med Genet. 2008;51:436-443.
- Bushkell LL, Gorlin RJ. Leukonychia totalis, multiple sebaceous cysts, and renal calculi. Arch Dermatol. 1975;111:899-901.
- Slee JJ, Wallman IS, Goldblatt J. A syndrome or leukonychia totalis and multiple sebaceous cysts. Clin Dysmorphol. 1997;6:229-233.
- Mutoh M, Niiyama S, Nishikawa S, et al. A syndrome of leukonychia, koilonychia and multiple pilar cysts. Acta Derm Venereol. 2015;95:249-250. doi:10.2340/00015555-1893
PRACTICE POINTS
- FLOTCH (leukonychia totalis-trichilemmal cysts-ciliary dystrophy syndrome) syndrome is an extremely rare condition that presents with multiple pilar cysts and leukonychia totalis. Pilar cysts in unusual locations along with distinct nail changes should prompt clinicians to consider further investigation for conditions such as FLOTCH syndrome.
- Although FLOTCH syndrome has been associated with other conditions such as ciliary dystrophy, renal calculi, pancreatitis, and central nervous system tumors, this does not preclude an extensive workup. Rather, careful family history may be the best predictor of clinical manifestations along the spectrum of this disease.
Perceived Benefits of a Research Fellowship for Dermatology Residency Applicants: Outcomes of a Faculty-Reported Survey
Dermatology residency positions continue to be highly coveted among applicants in the match. In 2019, dermatology proved to be the most competitive specialty, with 36.3% of US medical school seniors and independent applicants going unmatched.1 Prior to the transition to a pass/fail system, the mean US Medical Licensing Examination (USMLE) Step 1 score for matched applicants increased from 247 in 2014 to 251 in 2019. The growing number of scholarly activities reported by applicants has contributed to the competitiveness of the specialty. In 2018, the mean number of abstracts, presentations, and publications reported by matched applicants was 14.71, which was higher than other competitive specialties, including orthopedic surgery and otolaryngology (11.5 and 10.4, respectively). Dermatology applicants who did not match in 2018 reported a mean of 8.6 abstracts, presentations, and publications, which was on par with successful applicants in many other specialties.1 In 2011, Stratman and Ness2 found that publishing manuscripts and listing research experience were factors strongly associated with matching into dermatology for reapplicants. These trends in reported research have added pressure for applicants to increase their publications.
Given that many students do not choose a career in dermatology until later in medical school, some students choose to take a gap year between their third and fourth years of medical school to pursue a research fellowship (RF) and produce publications, in theory to increase the chances of matching in dermatology. A survey of dermatology applicants conducted by Costello et al3 in 2021 found that, of the students who completed a gap year (n=90; 31.25%), 78.7% (n=71) of them completed an RF, and those who completed RFs were more likely to match at top dermatology residency programs (P<.01). The authors also reported that there was no significant difference in overall match rates between gap-year and non–gap-year applicants.3 Another survey of 328 medical students found that the most common reason students take years off for research during medical school is to increase competitiveness for residency application.4 Although it is clear that students completing an RF often find success in the match, there are limited published data on how those involved in selecting dermatology residents view this additional year. We surveyed faculty members participating in the resident selection process to assess their viewpoints on how RFs factored into an applicant’s odds of matching into dermatology residency and performance as a resident.
Materials and Methods
An institutional review board application was submitted through the Geisinger Health System (Danville, Pennsylvania), and an exemption to complete the survey was granted. The survey consisted of 16 questions via REDCap electronic data capture and was sent to a listserve of dermatology program directors who were asked to distribute the survey to program chairs and faculty members within their department. Survey questions evaluated the participants’ involvement in medical student advising and the residency selection process. Questions relating to the respondents’ opinions were based on a 5-point Likert scale on level of agreement (1=strongly agree; 5=strongly disagree) or importance (1=a great deal; 5=not at all). All responses were collected anonymously. Data points were compiled and analyzed using REDCap. Statistical analysis via χ2 tests were conducted when appropriate.
Results
The survey was sent to 142 individuals and distributed to faculty members within those departments between August 16, 2019, and September 24, 2019. The survey elicited a total of 110 respondents. Demographic information is shown in eTable 1. Of these respondents, 35.5% were program directors, 23.6% were program chairs, 3.6% were both program director and program chair, and 37.3% were core faculty members. Although respondents’ roles were varied, 96.4% indicated that they were involved in both advising medical students and in selecting residents.
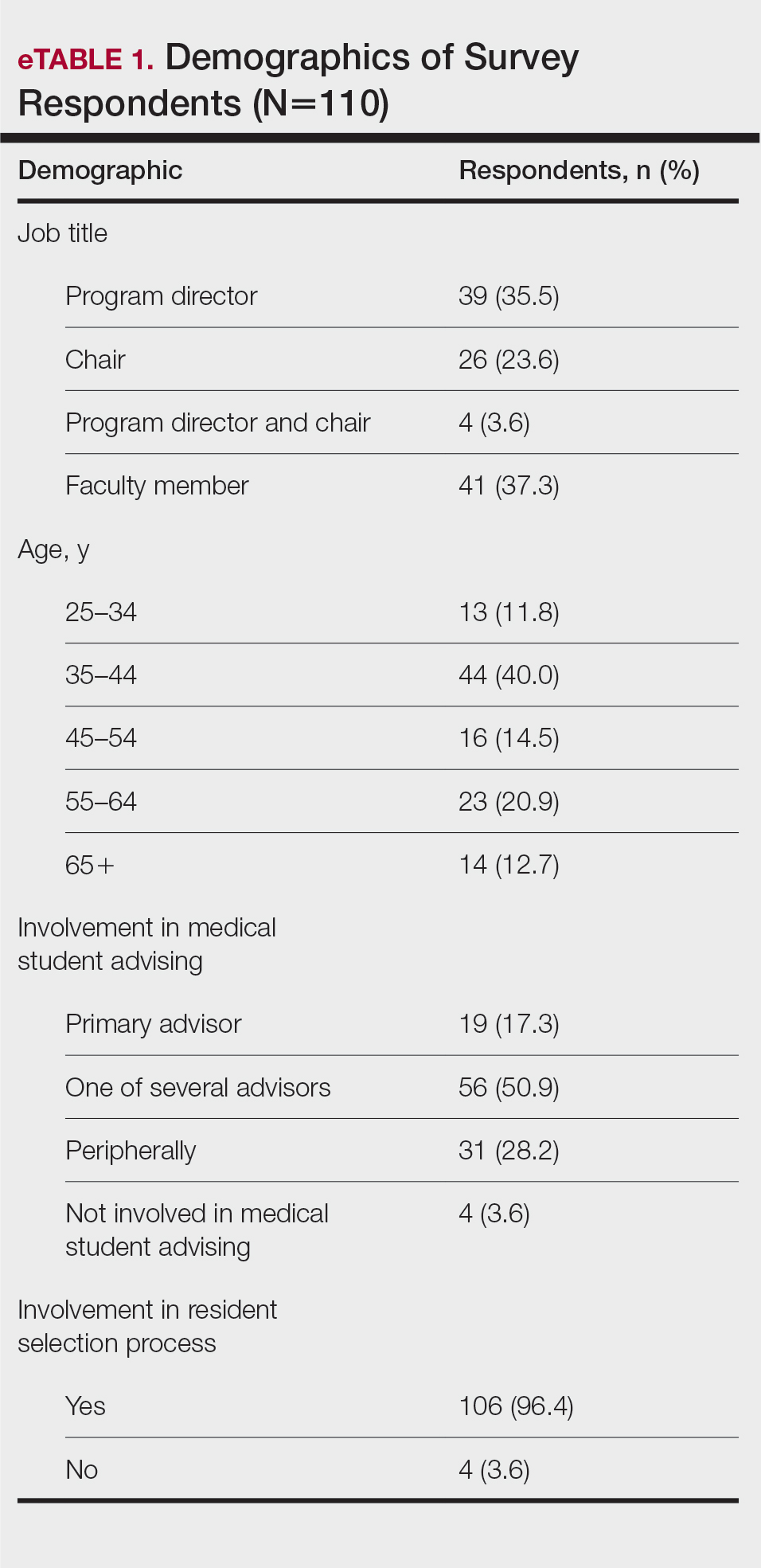
None of the respondents indicated that they always recommend that students complete an RF, and only 4.5% indicated that they usually recommend it; 40% of respondents rarely or never recommend an RF, while 55.5% sometimes recommend it. Although there was a variety of responses to how frequently faculty members recommend an RF, almost all respondents (98.2%) agreed that the reason medical students pursued an RF prior to residency application was to increase the competitiveness of their residency application. However, 20% of respondents believed that students in this cohort were seeking to gain a deeper understanding of the specialty, and 27.3% thought that this cohort had genuine interest in research. Interestingly, despite the medical students’ intentions of choosing an RF, most respondents (67.3%) agreed or strongly agreed that the publications produced by fellows make an impact on the dermatologic scientific community.
Although some respondents indicated that completion of an RF positively impacts resident performance with regard to patient care, most indicated that the impact was a little (26.4%) or not at all (50%). Additionally, a minority of respondents (11.8%) believed that RFs positively impact resident performance on in-service and board examinations at least a moderate amount, with 62.7% indicating no positive impact at all. Only 12.7% of participants agreed or strongly agreed that completion of an RF led to increased applicant involvement in research throughout their career, and most (73.6%) believed there were downsides to completing an RF. Finally, only 20% agreed or strongly agreed that students who completed an RF were more dedicated to the field of dermatology (eTable 2).
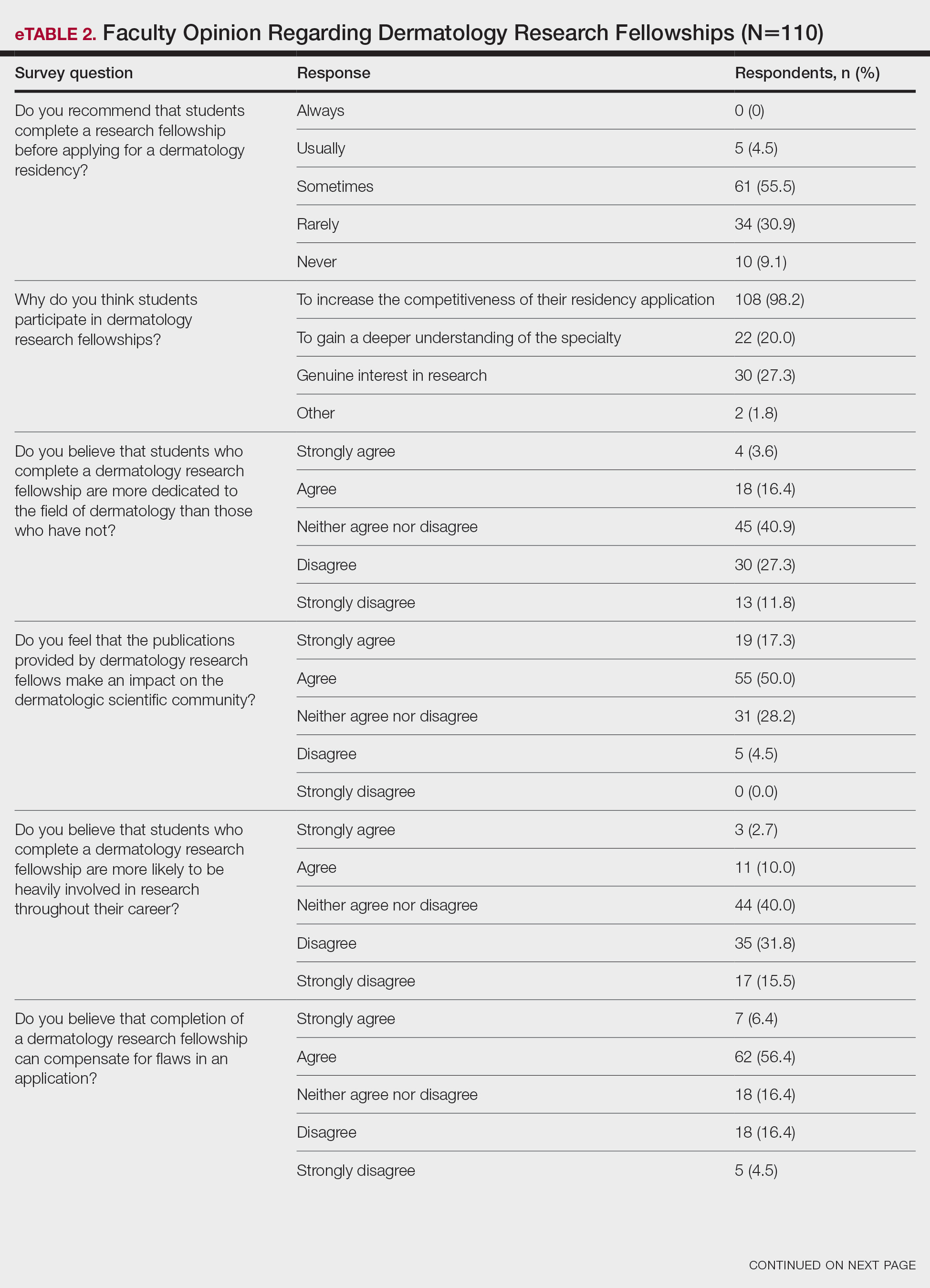
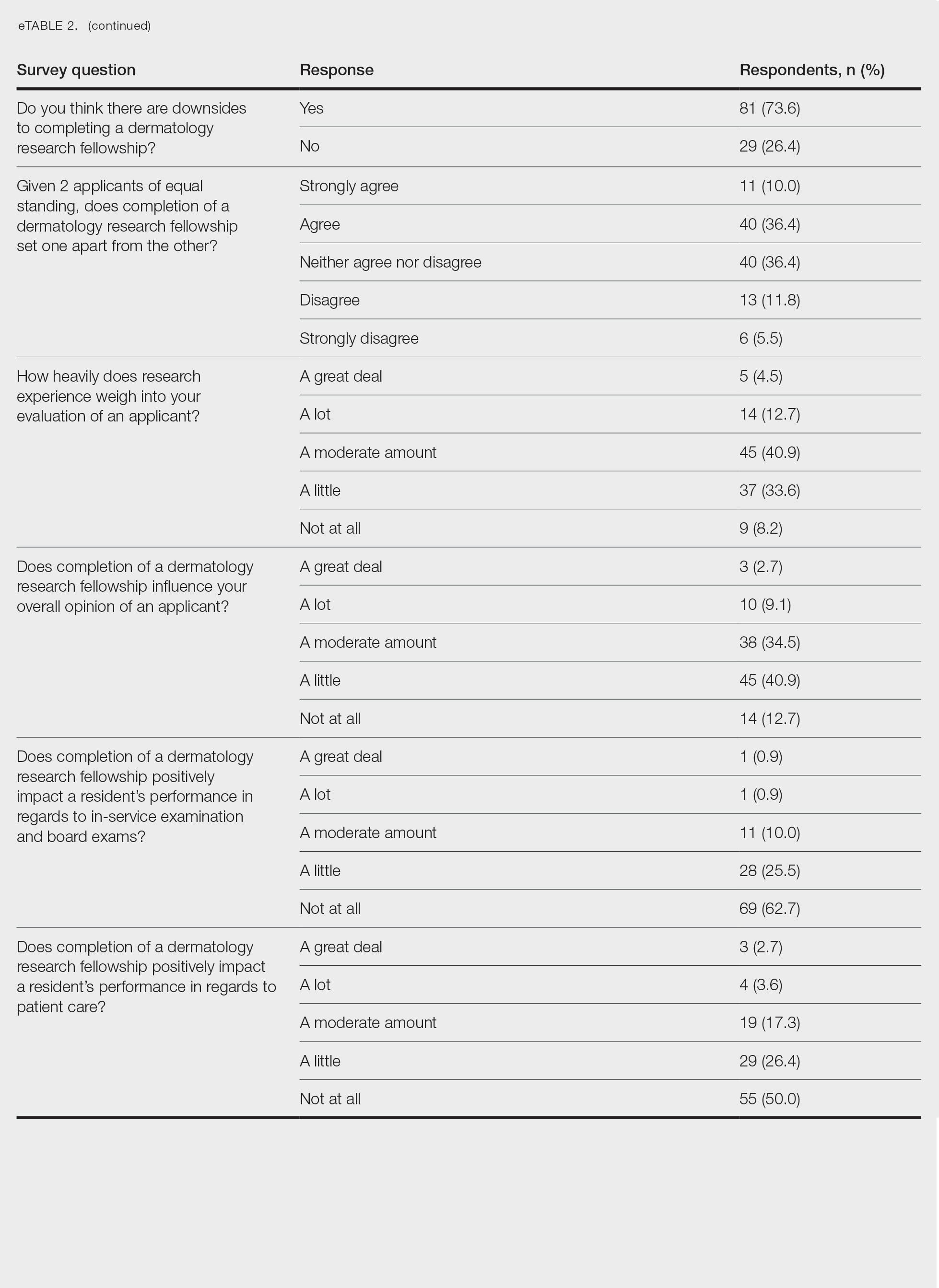
Further evaluation of the data indicated that the perceived utility of RFs did not affect respondents’ recommendation on whether to pursue an RF or not. For example, of the 4.5% of respondents who indicated that they always or usually recommended RFs, only 1 respondent believed that students who completed an RF were more dedicated to the field of dermatology than those who did not. Although 55.5% of respondents answered that they sometimes recommended completion of an RF, less than a quarter of this group believed that students who completed an RF were more likely to be heavily involved in research throughout their career (P=.99).
Overall, 11.8% of respondents indicated that completion of a dermatology RF influenced the evaluation of an applicant a great deal or a lot, while 53.6% of respondents indicated a little or no influence at all. Most respondents (62.8%) agreed or strongly agreed that completion of an RF can compensate for flaws in a residency application. Furthermore, when asked if completion of an RF could set 2 otherwise equivocal applicants apart from one another, 46.4% of respondents agreed or strongly agreed with the statement, while only 17.3% disagreed or strongly disagreed (eTable 2).
Comment
This study characterized how completion of an RF is viewed by those involved in advising medical students and selecting dermatology residents. The growing pressure for applicants to increase the number of publications combined with the competitiveness of applying for a dermatology residency position has led to increased participation in RFs. However, studies have found that students who completed an RF often did so despite a lack of interest.4 Nonetheless, little is known about how this is perceived by those involved in choosing residents.
We found that few respondents always or usually advised applicants to complete an RF, but the majority sometimes recommended them, demonstrating the complexity of this issue. Completion of an RF impacted 11.8% of respondents’ overall opinion of an applicant a lot or a great deal, while most respondents (53.6%) were influenced a little or not at all. However, 46.4% of respondents indicated that completion of a dermatology RF would set apart 2 applicants of otherwise equal standing, and 62.8% agreed or strongly agreed that completion of an RF would compensate for flaws in an application. These responses align with the findings of a study conducted by Kaffenberger et al,5 who surveyed members of the Association of Professors of Dermatology and found that 74.5% (73/98) of mentors almost always or sometimes recommended a research gap year for reasons that included low grades, low USMLE Step scores, and little research. These data suggest that completion of an RF can give a competitive advantage to applicants despite most advisors acknowledging that these applicants are not likely to be involved in research throughout their careers, perform better on standardized examinations, or provide better patient care.
Given the complexity of this issue, respondents may not have been able to accurately answer the question about how much an RF influenced their overall opinion of an applicant because of subconscious bias. Furthermore, respondents likely tailored their recommendations to complete an RF based on individual applicant strengths and weaknesses, and the specific reasons why one may recommend an RF need to be further investigated.
Although there may be other perceived advantages to RFs that were not captured by our survey, completion of a dermatology RF is not without disadvantages. Fellowships often are unfunded and offered in cities with high costs of living. Additionally, students are forced to delay graduation from medical school by a year at minimum and continue to accrue interest on medical school loans during this time. The financial burdens of completing an RF may exclude students of lower socioeconomic status and contribute to a decrease in diversity within the field. Dermatology has been found to be the second least diverse specialty, behind orthopedics.6 Soliman et al7 found that racial minorities and low-income students were more likely to cite socioeconomic barriers as factors involved in their decision not to pursue a career in dermatology. This notion was supported by Rinderknecht et al,8 who found that Black and Latinx dermatology applicants were more likely to come from disadvantaged backgrounds, and Black applicants were more likely to indicate financial concerns as their primary reason for not pursuing an RF. The impact of accumulated student debt and decreased access should be carefully weighed against the potential benefits of an RF. However, as the USMLE transitions their Step 1 score reporting from numerical to a pass/fail system, it also is possible that dermatology programs will place more emphasis on research productivity when evaluating applications for residency. Overall, the decision to recommend an RF represents an extremely complex topic, as indicated by the results of this study.
Limitations—Our survey-based study is limited by response rate and response bias. Despite the large number of responses, the overall response rate cannot be determined because it is unknown how many total faculty members actually received the survey. Moreover, data collected from current dermatology residents who have completed RFs vs those who have not as they pertain to resident performance and preparedness for the rigors of a dermatology residency would be useful.
- National Resident Matching Program. Results and Data: 2019 Main Residency Match. National Resident Matching Program; 2019. Accessed September 13, 2023. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-Results-and-Data-2019_04112019_final.pdf
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap-years play in a successful dermatology match. J Am Acad Dermatol. 2021;85:AB22.
- Pathipati AS, Taleghani N. Research in medical school: a survey evaluating why medical students take research years. Cureus. 2016;8:E741.
- Kaffenberger J, Lee B, Ahmed AM. How to advise medical students interested in dermatology: a survey of academic dermatology mentors. Cutis. 2023;111:124-127.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Rinderknecht FA, Brumfiel CM, Jefferson IS, et al. Differences in underrepresented in medicine applicant backgrounds and outcomes in the 2020-2021 dermatology residency match. Cutis. 2022;110:76-79.
Dermatology residency positions continue to be highly coveted among applicants in the match. In 2019, dermatology proved to be the most competitive specialty, with 36.3% of US medical school seniors and independent applicants going unmatched.1 Prior to the transition to a pass/fail system, the mean US Medical Licensing Examination (USMLE) Step 1 score for matched applicants increased from 247 in 2014 to 251 in 2019. The growing number of scholarly activities reported by applicants has contributed to the competitiveness of the specialty. In 2018, the mean number of abstracts, presentations, and publications reported by matched applicants was 14.71, which was higher than other competitive specialties, including orthopedic surgery and otolaryngology (11.5 and 10.4, respectively). Dermatology applicants who did not match in 2018 reported a mean of 8.6 abstracts, presentations, and publications, which was on par with successful applicants in many other specialties.1 In 2011, Stratman and Ness2 found that publishing manuscripts and listing research experience were factors strongly associated with matching into dermatology for reapplicants. These trends in reported research have added pressure for applicants to increase their publications.
Given that many students do not choose a career in dermatology until later in medical school, some students choose to take a gap year between their third and fourth years of medical school to pursue a research fellowship (RF) and produce publications, in theory to increase the chances of matching in dermatology. A survey of dermatology applicants conducted by Costello et al3 in 2021 found that, of the students who completed a gap year (n=90; 31.25%), 78.7% (n=71) of them completed an RF, and those who completed RFs were more likely to match at top dermatology residency programs (P<.01). The authors also reported that there was no significant difference in overall match rates between gap-year and non–gap-year applicants.3 Another survey of 328 medical students found that the most common reason students take years off for research during medical school is to increase competitiveness for residency application.4 Although it is clear that students completing an RF often find success in the match, there are limited published data on how those involved in selecting dermatology residents view this additional year. We surveyed faculty members participating in the resident selection process to assess their viewpoints on how RFs factored into an applicant’s odds of matching into dermatology residency and performance as a resident.
Materials and Methods
An institutional review board application was submitted through the Geisinger Health System (Danville, Pennsylvania), and an exemption to complete the survey was granted. The survey consisted of 16 questions via REDCap electronic data capture and was sent to a listserve of dermatology program directors who were asked to distribute the survey to program chairs and faculty members within their department. Survey questions evaluated the participants’ involvement in medical student advising and the residency selection process. Questions relating to the respondents’ opinions were based on a 5-point Likert scale on level of agreement (1=strongly agree; 5=strongly disagree) or importance (1=a great deal; 5=not at all). All responses were collected anonymously. Data points were compiled and analyzed using REDCap. Statistical analysis via χ2 tests were conducted when appropriate.
Results
The survey was sent to 142 individuals and distributed to faculty members within those departments between August 16, 2019, and September 24, 2019. The survey elicited a total of 110 respondents. Demographic information is shown in eTable 1. Of these respondents, 35.5% were program directors, 23.6% were program chairs, 3.6% were both program director and program chair, and 37.3% were core faculty members. Although respondents’ roles were varied, 96.4% indicated that they were involved in both advising medical students and in selecting residents.

None of the respondents indicated that they always recommend that students complete an RF, and only 4.5% indicated that they usually recommend it; 40% of respondents rarely or never recommend an RF, while 55.5% sometimes recommend it. Although there was a variety of responses to how frequently faculty members recommend an RF, almost all respondents (98.2%) agreed that the reason medical students pursued an RF prior to residency application was to increase the competitiveness of their residency application. However, 20% of respondents believed that students in this cohort were seeking to gain a deeper understanding of the specialty, and 27.3% thought that this cohort had genuine interest in research. Interestingly, despite the medical students’ intentions of choosing an RF, most respondents (67.3%) agreed or strongly agreed that the publications produced by fellows make an impact on the dermatologic scientific community.
Although some respondents indicated that completion of an RF positively impacts resident performance with regard to patient care, most indicated that the impact was a little (26.4%) or not at all (50%). Additionally, a minority of respondents (11.8%) believed that RFs positively impact resident performance on in-service and board examinations at least a moderate amount, with 62.7% indicating no positive impact at all. Only 12.7% of participants agreed or strongly agreed that completion of an RF led to increased applicant involvement in research throughout their career, and most (73.6%) believed there were downsides to completing an RF. Finally, only 20% agreed or strongly agreed that students who completed an RF were more dedicated to the field of dermatology (eTable 2).


Further evaluation of the data indicated that the perceived utility of RFs did not affect respondents’ recommendation on whether to pursue an RF or not. For example, of the 4.5% of respondents who indicated that they always or usually recommended RFs, only 1 respondent believed that students who completed an RF were more dedicated to the field of dermatology than those who did not. Although 55.5% of respondents answered that they sometimes recommended completion of an RF, less than a quarter of this group believed that students who completed an RF were more likely to be heavily involved in research throughout their career (P=.99).
Overall, 11.8% of respondents indicated that completion of a dermatology RF influenced the evaluation of an applicant a great deal or a lot, while 53.6% of respondents indicated a little or no influence at all. Most respondents (62.8%) agreed or strongly agreed that completion of an RF can compensate for flaws in a residency application. Furthermore, when asked if completion of an RF could set 2 otherwise equivocal applicants apart from one another, 46.4% of respondents agreed or strongly agreed with the statement, while only 17.3% disagreed or strongly disagreed (eTable 2).
Comment
This study characterized how completion of an RF is viewed by those involved in advising medical students and selecting dermatology residents. The growing pressure for applicants to increase the number of publications combined with the competitiveness of applying for a dermatology residency position has led to increased participation in RFs. However, studies have found that students who completed an RF often did so despite a lack of interest.4 Nonetheless, little is known about how this is perceived by those involved in choosing residents.
We found that few respondents always or usually advised applicants to complete an RF, but the majority sometimes recommended them, demonstrating the complexity of this issue. Completion of an RF impacted 11.8% of respondents’ overall opinion of an applicant a lot or a great deal, while most respondents (53.6%) were influenced a little or not at all. However, 46.4% of respondents indicated that completion of a dermatology RF would set apart 2 applicants of otherwise equal standing, and 62.8% agreed or strongly agreed that completion of an RF would compensate for flaws in an application. These responses align with the findings of a study conducted by Kaffenberger et al,5 who surveyed members of the Association of Professors of Dermatology and found that 74.5% (73/98) of mentors almost always or sometimes recommended a research gap year for reasons that included low grades, low USMLE Step scores, and little research. These data suggest that completion of an RF can give a competitive advantage to applicants despite most advisors acknowledging that these applicants are not likely to be involved in research throughout their careers, perform better on standardized examinations, or provide better patient care.
Given the complexity of this issue, respondents may not have been able to accurately answer the question about how much an RF influenced their overall opinion of an applicant because of subconscious bias. Furthermore, respondents likely tailored their recommendations to complete an RF based on individual applicant strengths and weaknesses, and the specific reasons why one may recommend an RF need to be further investigated.
Although there may be other perceived advantages to RFs that were not captured by our survey, completion of a dermatology RF is not without disadvantages. Fellowships often are unfunded and offered in cities with high costs of living. Additionally, students are forced to delay graduation from medical school by a year at minimum and continue to accrue interest on medical school loans during this time. The financial burdens of completing an RF may exclude students of lower socioeconomic status and contribute to a decrease in diversity within the field. Dermatology has been found to be the second least diverse specialty, behind orthopedics.6 Soliman et al7 found that racial minorities and low-income students were more likely to cite socioeconomic barriers as factors involved in their decision not to pursue a career in dermatology. This notion was supported by Rinderknecht et al,8 who found that Black and Latinx dermatology applicants were more likely to come from disadvantaged backgrounds, and Black applicants were more likely to indicate financial concerns as their primary reason for not pursuing an RF. The impact of accumulated student debt and decreased access should be carefully weighed against the potential benefits of an RF. However, as the USMLE transitions their Step 1 score reporting from numerical to a pass/fail system, it also is possible that dermatology programs will place more emphasis on research productivity when evaluating applications for residency. Overall, the decision to recommend an RF represents an extremely complex topic, as indicated by the results of this study.
Limitations—Our survey-based study is limited by response rate and response bias. Despite the large number of responses, the overall response rate cannot be determined because it is unknown how many total faculty members actually received the survey. Moreover, data collected from current dermatology residents who have completed RFs vs those who have not as they pertain to resident performance and preparedness for the rigors of a dermatology residency would be useful.
Dermatology residency positions continue to be highly coveted among applicants in the match. In 2019, dermatology proved to be the most competitive specialty, with 36.3% of US medical school seniors and independent applicants going unmatched.1 Prior to the transition to a pass/fail system, the mean US Medical Licensing Examination (USMLE) Step 1 score for matched applicants increased from 247 in 2014 to 251 in 2019. The growing number of scholarly activities reported by applicants has contributed to the competitiveness of the specialty. In 2018, the mean number of abstracts, presentations, and publications reported by matched applicants was 14.71, which was higher than other competitive specialties, including orthopedic surgery and otolaryngology (11.5 and 10.4, respectively). Dermatology applicants who did not match in 2018 reported a mean of 8.6 abstracts, presentations, and publications, which was on par with successful applicants in many other specialties.1 In 2011, Stratman and Ness2 found that publishing manuscripts and listing research experience were factors strongly associated with matching into dermatology for reapplicants. These trends in reported research have added pressure for applicants to increase their publications.
Given that many students do not choose a career in dermatology until later in medical school, some students choose to take a gap year between their third and fourth years of medical school to pursue a research fellowship (RF) and produce publications, in theory to increase the chances of matching in dermatology. A survey of dermatology applicants conducted by Costello et al3 in 2021 found that, of the students who completed a gap year (n=90; 31.25%), 78.7% (n=71) of them completed an RF, and those who completed RFs were more likely to match at top dermatology residency programs (P<.01). The authors also reported that there was no significant difference in overall match rates between gap-year and non–gap-year applicants.3 Another survey of 328 medical students found that the most common reason students take years off for research during medical school is to increase competitiveness for residency application.4 Although it is clear that students completing an RF often find success in the match, there are limited published data on how those involved in selecting dermatology residents view this additional year. We surveyed faculty members participating in the resident selection process to assess their viewpoints on how RFs factored into an applicant’s odds of matching into dermatology residency and performance as a resident.
Materials and Methods
An institutional review board application was submitted through the Geisinger Health System (Danville, Pennsylvania), and an exemption to complete the survey was granted. The survey consisted of 16 questions via REDCap electronic data capture and was sent to a listserve of dermatology program directors who were asked to distribute the survey to program chairs and faculty members within their department. Survey questions evaluated the participants’ involvement in medical student advising and the residency selection process. Questions relating to the respondents’ opinions were based on a 5-point Likert scale on level of agreement (1=strongly agree; 5=strongly disagree) or importance (1=a great deal; 5=not at all). All responses were collected anonymously. Data points were compiled and analyzed using REDCap. Statistical analysis via χ2 tests were conducted when appropriate.
Results
The survey was sent to 142 individuals and distributed to faculty members within those departments between August 16, 2019, and September 24, 2019. The survey elicited a total of 110 respondents. Demographic information is shown in eTable 1. Of these respondents, 35.5% were program directors, 23.6% were program chairs, 3.6% were both program director and program chair, and 37.3% were core faculty members. Although respondents’ roles were varied, 96.4% indicated that they were involved in both advising medical students and in selecting residents.

None of the respondents indicated that they always recommend that students complete an RF, and only 4.5% indicated that they usually recommend it; 40% of respondents rarely or never recommend an RF, while 55.5% sometimes recommend it. Although there was a variety of responses to how frequently faculty members recommend an RF, almost all respondents (98.2%) agreed that the reason medical students pursued an RF prior to residency application was to increase the competitiveness of their residency application. However, 20% of respondents believed that students in this cohort were seeking to gain a deeper understanding of the specialty, and 27.3% thought that this cohort had genuine interest in research. Interestingly, despite the medical students’ intentions of choosing an RF, most respondents (67.3%) agreed or strongly agreed that the publications produced by fellows make an impact on the dermatologic scientific community.
Although some respondents indicated that completion of an RF positively impacts resident performance with regard to patient care, most indicated that the impact was a little (26.4%) or not at all (50%). Additionally, a minority of respondents (11.8%) believed that RFs positively impact resident performance on in-service and board examinations at least a moderate amount, with 62.7% indicating no positive impact at all. Only 12.7% of participants agreed or strongly agreed that completion of an RF led to increased applicant involvement in research throughout their career, and most (73.6%) believed there were downsides to completing an RF. Finally, only 20% agreed or strongly agreed that students who completed an RF were more dedicated to the field of dermatology (eTable 2).


Further evaluation of the data indicated that the perceived utility of RFs did not affect respondents’ recommendation on whether to pursue an RF or not. For example, of the 4.5% of respondents who indicated that they always or usually recommended RFs, only 1 respondent believed that students who completed an RF were more dedicated to the field of dermatology than those who did not. Although 55.5% of respondents answered that they sometimes recommended completion of an RF, less than a quarter of this group believed that students who completed an RF were more likely to be heavily involved in research throughout their career (P=.99).
Overall, 11.8% of respondents indicated that completion of a dermatology RF influenced the evaluation of an applicant a great deal or a lot, while 53.6% of respondents indicated a little or no influence at all. Most respondents (62.8%) agreed or strongly agreed that completion of an RF can compensate for flaws in a residency application. Furthermore, when asked if completion of an RF could set 2 otherwise equivocal applicants apart from one another, 46.4% of respondents agreed or strongly agreed with the statement, while only 17.3% disagreed or strongly disagreed (eTable 2).
Comment
This study characterized how completion of an RF is viewed by those involved in advising medical students and selecting dermatology residents. The growing pressure for applicants to increase the number of publications combined with the competitiveness of applying for a dermatology residency position has led to increased participation in RFs. However, studies have found that students who completed an RF often did so despite a lack of interest.4 Nonetheless, little is known about how this is perceived by those involved in choosing residents.
We found that few respondents always or usually advised applicants to complete an RF, but the majority sometimes recommended them, demonstrating the complexity of this issue. Completion of an RF impacted 11.8% of respondents’ overall opinion of an applicant a lot or a great deal, while most respondents (53.6%) were influenced a little or not at all. However, 46.4% of respondents indicated that completion of a dermatology RF would set apart 2 applicants of otherwise equal standing, and 62.8% agreed or strongly agreed that completion of an RF would compensate for flaws in an application. These responses align with the findings of a study conducted by Kaffenberger et al,5 who surveyed members of the Association of Professors of Dermatology and found that 74.5% (73/98) of mentors almost always or sometimes recommended a research gap year for reasons that included low grades, low USMLE Step scores, and little research. These data suggest that completion of an RF can give a competitive advantage to applicants despite most advisors acknowledging that these applicants are not likely to be involved in research throughout their careers, perform better on standardized examinations, or provide better patient care.
Given the complexity of this issue, respondents may not have been able to accurately answer the question about how much an RF influenced their overall opinion of an applicant because of subconscious bias. Furthermore, respondents likely tailored their recommendations to complete an RF based on individual applicant strengths and weaknesses, and the specific reasons why one may recommend an RF need to be further investigated.
Although there may be other perceived advantages to RFs that were not captured by our survey, completion of a dermatology RF is not without disadvantages. Fellowships often are unfunded and offered in cities with high costs of living. Additionally, students are forced to delay graduation from medical school by a year at minimum and continue to accrue interest on medical school loans during this time. The financial burdens of completing an RF may exclude students of lower socioeconomic status and contribute to a decrease in diversity within the field. Dermatology has been found to be the second least diverse specialty, behind orthopedics.6 Soliman et al7 found that racial minorities and low-income students were more likely to cite socioeconomic barriers as factors involved in their decision not to pursue a career in dermatology. This notion was supported by Rinderknecht et al,8 who found that Black and Latinx dermatology applicants were more likely to come from disadvantaged backgrounds, and Black applicants were more likely to indicate financial concerns as their primary reason for not pursuing an RF. The impact of accumulated student debt and decreased access should be carefully weighed against the potential benefits of an RF. However, as the USMLE transitions their Step 1 score reporting from numerical to a pass/fail system, it also is possible that dermatology programs will place more emphasis on research productivity when evaluating applications for residency. Overall, the decision to recommend an RF represents an extremely complex topic, as indicated by the results of this study.
Limitations—Our survey-based study is limited by response rate and response bias. Despite the large number of responses, the overall response rate cannot be determined because it is unknown how many total faculty members actually received the survey. Moreover, data collected from current dermatology residents who have completed RFs vs those who have not as they pertain to resident performance and preparedness for the rigors of a dermatology residency would be useful.
- National Resident Matching Program. Results and Data: 2019 Main Residency Match. National Resident Matching Program; 2019. Accessed September 13, 2023. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-Results-and-Data-2019_04112019_final.pdf
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap-years play in a successful dermatology match. J Am Acad Dermatol. 2021;85:AB22.
- Pathipati AS, Taleghani N. Research in medical school: a survey evaluating why medical students take research years. Cureus. 2016;8:E741.
- Kaffenberger J, Lee B, Ahmed AM. How to advise medical students interested in dermatology: a survey of academic dermatology mentors. Cutis. 2023;111:124-127.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Rinderknecht FA, Brumfiel CM, Jefferson IS, et al. Differences in underrepresented in medicine applicant backgrounds and outcomes in the 2020-2021 dermatology residency match. Cutis. 2022;110:76-79.
- National Resident Matching Program. Results and Data: 2019 Main Residency Match. National Resident Matching Program; 2019. Accessed September 13, 2023. https://www.nrmp.org/wp-content/uploads/2021/07/NRMP-Results-and-Data-2019_04112019_final.pdf
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap-years play in a successful dermatology match. J Am Acad Dermatol. 2021;85:AB22.
- Pathipati AS, Taleghani N. Research in medical school: a survey evaluating why medical students take research years. Cureus. 2016;8:E741.
- Kaffenberger J, Lee B, Ahmed AM. How to advise medical students interested in dermatology: a survey of academic dermatology mentors. Cutis. 2023;111:124-127.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587.
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254.
- Rinderknecht FA, Brumfiel CM, Jefferson IS, et al. Differences in underrepresented in medicine applicant backgrounds and outcomes in the 2020-2021 dermatology residency match. Cutis. 2022;110:76-79.
PRACTICE POINTS
- Many medical students seeking to match into a dermatology residency program complete a research fellowship (RF).
- Completion of an RF can give a competitive advantage to applicants even though most advisors acknowledge that these applicants are not likely to be involved in research throughout their career, perform better on standardized examinations, or provide better patient care.
- The decision to recommend an RF represents an extremely complex topic and should be tailored to each individual applicant.
Assessment of the Efficacy of Tranexamic Acid Solution 5% in the Treatment of Melasma in Patients of South Asian Descent
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).
Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19
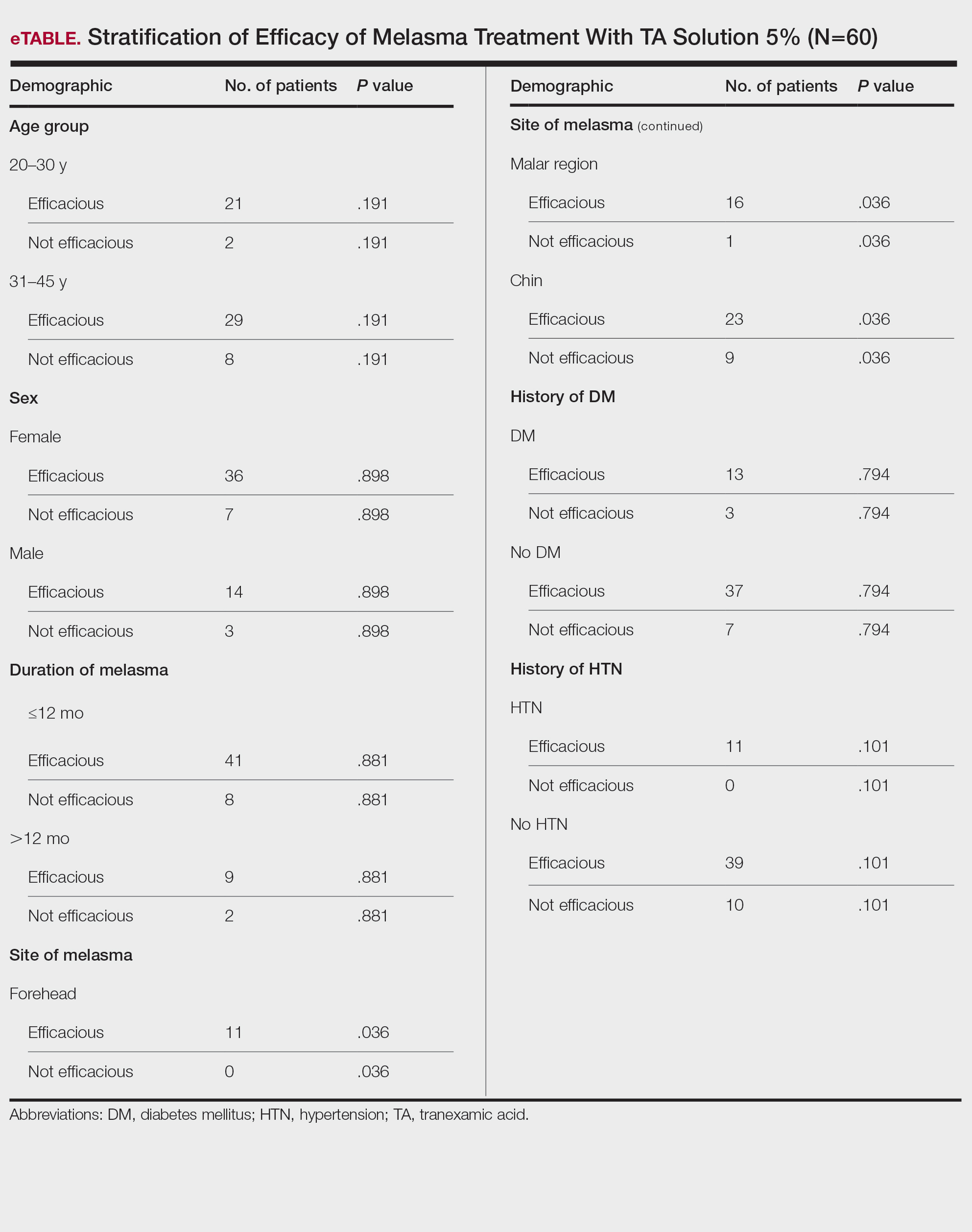
Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).

Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19

Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
Melasma is a complex, long-lasting, acquired dermatologic pigmentation disorder resulting in grey-brown patches that last for more than 3 months. Sun-exposed areas including the nose, cheeks, forehead, and forearms are most likely to be affected.1 In Southeast Asia, 0.25% to 4% of the population affected by melasma is aged 30 to 40 years.2 In particular, melasma is a concern among pregnant women due to increased levels of melanocyte-stimulating hormones (MSHs) and is impacted by genetics, hormonal influence, and exposure to UV light.3,4 In Pakistan, approximately 46% of women are affected by melasma during pregnancy.2,5 Although few studies have focused on the clinical approaches to melasma in darker skin types, it continues to disproportionately affect the skin of color population.4
The areas of hyperpigmentation seen in melasma exhibit increased deposition of melanin in the epidermis and dermis, but melanocytes are not elevated. However, in areas of hyperpigmentation, the melanocytes are larger and more dendritic and demonstrate an increased level of melanogenesis.6 During pregnancy, especially in the third trimester, elevated levels of estrogen, progesterone, and MSH often are found in association with melasma.7 Tyrosinase (TYR) activity increases and cellular proliferation is reduced after treatment of melanocytes in culture with β-estradiol.8 Sex steroids increase transcription of genes encoding melanogenic enzymes in normal human melanocytes, especially TYR.9 These results are consistent with the notable increases in melanin synthesis and TYR activity reported for normal human melanocytes under similar conditions in culture.10 Because melanocytes contain both cytosolic and nuclear estrogen receptors, melanocytes in patients with melasma may be inherently more sensitive to the stimulatory effects of estrogens and possibly other steroid hormones.11
The current treatment options for melasma have varying levels of success and include topical depigmenting agents such as hydroquinone, tretinoin, azelaic acid, kojic acid, and corticosteroids; dermabrasion; and chemical peels.12-14 Chemical peels with glycolic acid, salicylic acid, lactic acid, trichloroacetic acid, and phenol, as well as laser therapy, are reliable management options.13,14 Traditionally, melasma has been treated with a combination of modalities along with photoprotection and trigger avoidance.12
The efficacy and safety of the available therapies for melasma are still controversial and require further exploration. In recent years, off-label tranexamic acid (TA) has emerged as a potential therapy for melasma. Although the mechanism of action remains unclear, TA may inhibit melanin synthesis by blocking the interaction between melanocytes and keratinocytes.15 Tranexamic acid also may reverse the abnormal dermal changes associated with melasma by inhibiting melanogenesis and angiogenesis.16
Although various therapeutic options exist for melasma, the search for a reliable option in patients with darker skin types continues.13 We sought to evaluate the efficacy of TA solution 5% in reducing the severity of melasma in South Asian patients, thereby improving patient outcomes and maximizing patient satisfaction. Topical TA is inexpensive and readily accessible and does not cause systemic side effects. These qualities make it a promising treatment compared to traditional therapies.
Methods
We conducted a randomized controlled trial at Rawalpindi Medical Institute (Punjab, Pakistan). The researchers obtained informed consent for all enrolled patients. Cases were sampled from the original patient population seen at the office using nonprobability consecutive sampling. The sample size was calculated with a 95% CI, margin of error of 9%, and expected percentage of efficacy of 86.1% by using TA solution 5%. South Asian male and female patients aged 20 to 45 years with melasma were included in the analysis. Patients were excluded if they were already taking TA, oral contraceptive pills, or photosensitizing drugs (eg, nonsteroidal anti-inflammatory drugs, tetracyclines, phenytoin, carbamazepine); were pregnant; had chronic kidney disease (creatinine >2.0 mg/dL); had cardiac abnormalities (abnormal electrocardiogram); had hematologic disorders (international normalized ratio >2); or had received another melasma treatment within the last 3 to 6 months.
All enrolled patients underwent a detailed history and physical examination. Patient demographics were subsequently noted, including age, sex, history of diabetes mellitus or hypertension, and duration of melasma. The melasma area and severity index (MASI) score of each patient was calculated at baseline, and a corresponding photograph was taken.
The topical solution was prepared with 5 g of TA dissolved in 10 cc of ethanol at 96 °F, 10 cc of 1,3-butanediol, and distilled water up to 100 cc. The TA solution was applied to the affected areas once daily by the patient for 12 weeks. Each application covered the affected areas completely. Patients were instructed to apply sunscreen with sun protection factor 60 to those same areas for UV protection after 15 minutes of TA application. Biweekly follow-ups were scheduled during the trial, and the MASI score was recorded at these visits. If the mean MASI score was reduced by half after 12 weeks of treatment, then the treatment was considered efficacious with a 95% CI.
The percentage reduction from baseline was calculated as follows: percentage reduction=(baseline score– follow-up score)/baseline score×100.
Statistical Analysis—Data were analyzed in SPSS Statistics 25 (IBM). The quantitative variables of age, duration of melasma, and body mass index were presented as mean (SD). Qualitative variables such as sex, history of diabetes mellitus or hypertension, site of melasma, and efficacy were presented as frequencies and percentages. Mean MASI scores at baseline and 12 weeks posttreatment were compared using a paired t test (P≤.05). Data were stratified for age, sex, history of diabetes mellitus or hypertension, site of melasma, and duration of melasma, and a χ2 test was applied to compare efficacy in stratified groups (P≤.05).
Results
Sixty patients were enrolled in the study. Of them, 17 (28.33%) were male, and 43 (71.67%) were female (2:5 ratio). They ranged in age from 20 to 45 years (mean [SD], 31.93 [6.26] years). Thirty-seven patients (61.67%) were aged 31 to 45 years of age (Table 1). The mean (SD) duration of disease was 10.18 (2.10) months. The response to TA was recorded based on patient distribution according to the site of melasma as well as history of diabetes mellitus and hypertension.

Topical TA was found to be efficacious for melasma in 50 (83.33%) patients. The mean (SD) baseline and week 12 MASI scores were 23.15 (5.02) and 12.71 (4.10)(P<.0001), respectively (Table 2). The stratification of efficacy with respect to age, sex, duration of melasma, site of melasma, and history of diabetes mellitus or hypertension is shown in the eTable. The site of melasma was significant with respect to stratification of efficacy. On the forehead, TA was found to be efficacious in 11 patients and nonefficacious in 0 patients (P=.036). In the malar region, it was efficacious in 16 patients and nonefficacious in 1 patient (P=.036). Finally, on the chin, it was efficacious in 23 patients and nonefficacious in 9 patients (P=.036).

Comment
Melasma Presentation and Development—Melasma is a chronic skin condition that more often affects patients with darker skin types. This condition is characterized by hyperpigmentation of skin that is directly exposed to the sun, such as the cheek, nose, forehead, and above the upper lip.17 Although the mechanism behind how melasma develops is unknown, one theory suggests that UV light can lead to increased plasmin in keratinocytes.18 This increased plasmin will thereby increase the arachidonic acid and α-MSH, leading to the observed uneven hyperpigmentation that is notable in melasma. Melasma is common in patients using oral contraceptives or expired cosmetic drugs; in those who are pregnant; and in those with liver dysfunction.18 Melasma has a negative impact on patients’ quality of life because of substantial psychological and social distress. Thus, finding an accessible treatment is imperative.19

Melasma Management—The most common treatments for melasma have been topical bleaching agents and photoprotection. Combination therapy options include chemical peels, dermabrasion, and laser treatments, though they present with limited efficacy.17,20 Because melasma focuses on pigmentation correction, topical treatments work to disturb melanocyte pigment production at the enzymatic level.21 Tyrosinase is rate limiting in melanin production, as it converts L-tyrosinase to L-3,4-dihydroxyphenylalanine, using copper to interact with L-3,4-dihydroxyphenylalanine as a cofactor in the active site.22 Therefore, tyrosine is a major target for many drugs that have been developed for melasma to decrease melaninization.21
Recently, research has focused on the effects of topical, intradermal, and oral TA for melasma.17 Tranexamic acid most commonly has been used in medicine as a fibrinolytic agent because of its antiplasmin properties. It has been hypothesized that TA can inhibit the release of paracrine melanogenic factors that normally act to stimulate melanocytes.17 Although studies have supported the safety and efficacy of TA, there remains a lack of clinical studies that are sufficiently powered. No definitive consensus on the use of TA for melasma currently exists, which indicates the need for large-scale, randomized, controlled trials.23
One trial (N=25) found that TA solution 5% achieved efficacy (>50% reduction in MASI score from baseline) in 86.1% of patients with melasma.24 In another study (N=18), topical TA 5% achieved efficacy (>50% reduction in MASI score) in 86% of patients with melasma.25
Melasma Comorbidities—To determine if certain comorbidities, such as diabetes mellitus or hypertension, influenced the progression of melasma, we stratified the efficacy results for patients with these 2 comorbidities, which showed no significant difference (P=.794 and P=.101, respectively). Thus, the relatively higher prevalence of diabetes mellitus (16 patients) and hypertension (11 patients) did not contribute to the efficacy of TA in lowering MASI scores over the 12-week period, which supports the findings of Doolan and Gupta,26 who investigated the endocrinologic conditions associated with melasma and found no such association with diabetes mellitus or hypertension.
TA Formulations for Melasma—The efficacy of topical TA has been explored in several studies. Six studies with sample sizes of 13 to 50 patients each showed statistically significant differences in MASI scores between baseline and following TA treatment (P<.001).27-32 Several formulations and regimens were utilized, including TA cream 3% for 12 weeks, TA gel 5% for 12 weeks, TA solution 3% for 12 weeks, TA liposome 5% for 12 weeks, and TA solution 2% for 12 weeks.18 Additionally, these studies found TA to be effective in limiting dyschromia and decreasing MASI scores. There were no statistically significant differences between formulations and method of application. Topical TA has been found to be just as effective as other treatments for melasma, including intradermal TA injections, topical hydroquinone, and a combination of topical hydroquinone and dexamethasone.18
Further study of the efficacy of intradermal TA is necessary because many human trials have lacked statistical significance or a control group. Lee et al32 conducted a trial of 100 female patients who received weekly intradermal TA microinjections for 12 weeks. After 8 and 12 weeks, MASI scores decreased significantly (P<.01).32 Similarly, Badran et al33 observed 60 female patients in 3 trial groups: group A received TA (4 mg/mL) intradermal injections every 2 weeks, group B received TA (10 mg/mL) intradermal injections every 2 weeks, and group C received TA cream 10% twice daily. Although all groups showed improvement in MASI, group B, which had the highest intradermal TA concentration, exhibited the most improvement. Thus, it was determined that intradermal application led to better results, but the cream was still effective.33
Saki et al34 conducted a randomized, split-face trial of 37 patients comparing the efficacy of intradermal TA and topical hydroquinone. Each group was treated with either monthly intradermal TA injections or nightly hydroquinone for 3 months. After 4 weeks of treatment, TA initially had a greater improvement. However, after 20 weeks, the overall changes were not significant between the 2 groups.34 Pazyar et al35 conducted a randomized, split-face trial of 49 patients comparing the efficacy of intradermal TA and hydroquinone cream. After 24 weeks of biweekly TA injections or twice-daily hydroquinone, there were no statistically significant differences in the decreased MASI scores between treatments.35 Additional large, double-blind, controlled trials are needed to thoroughly assess the role of intradermal TA in comparison to its treatment counterpart of hydroquinone.
Ebrahimi and Naeini29 conducted a 12-week, double-blind, split-phase trial of 50 Iranian melasma patients, which showed that 27.3% of patients rated the improvement in melasma as excellent, 42.4% as good, and 30.3% as fair after using TA solution 3%. Wu et al36 also showed a total melasma improvement rate of 80.9% in 256 patients with long-term oral use of TA. In a study by Kim et al31 (N=245), the mean MASI score considerably decreased after topical TA use, with a total response rate of 95.6%. In another study, Atefi et al37 presented significantly increased levels of satisfaction in patients treated with topical TA 5% vs hydroquinone (P=.015).
Melasma in Patients With Darker Skin Types—Special attention must be given to choosing the appropriate medication in melasma patients with darker skin types, as there is an increased risk for postinflammatory hyperpigmentation. Currently, few randomized controlled trials exist that fulfill the criteria of evaluating pharmacologic options for patients with melasma, and even fewer studies solely focus on patients with darker skin types.38 In addition to treatment advances, patients must be educated on the need to avoid sun exposure when possible or to use photoprotection, especially in the South Asian region, where these practices rarely are taught. Our study provided a unique analysis regarding the efficacy of TA solution 5% for the treatment of melasma in patients of South Asian descent. Clinicians can use these findings as a foundation for treating all patients with melasma but particularly those with darker skin types.
Study Limitations—Our study consisted of 60 patients; although our study had more patients than similar trials, larger studies are needed. Additionally, other variables were excluded from our analysis, such as comorbidities beyond diabetes mellitus and hypertension.
Conclusion
This study contributes to the growing field of melasma therapeutics by evaluating the efficacy of using TA solution 5% for the treatment of melasma in South Asian patients with darker skin types. Clinicians may use our study to broaden their treatment options for a common condition while also addressing the lack of clinical options for patients with darker skin types. Further studies investigating the effectiveness of TA in large clinical trials in humans are warranted to understand the efficacy and the risk for any complications.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
- Espósito ACC, Brianezi G, De Souza NP, et al. Exploratory study of epidermis, basement membrane zone, upper dermis alterations and Wnt pathway activation in melasma compared to adjacent and retroauricular skin. Ann Dermatol. 2020;32:101-108.
- Janney MS, Subramaniyan R, Dabas R, et al. A randomized controlled study comparing the efficacy of topical 5% tranexamic acid solution versus 3% hydroquinone cream in melasma. J Cutan Aesthet Surg. 2019;12:63-67.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasmaJ Cosmet Laser Ther. 2018;20:134-139.
- Grimes PE, Ijaz S, Nashawati R, et al. New oral and topical approaches for the treatment of melasma. Int J Womens Dermatol. 2019;5:30-36.
- Handel AC, Miot LDB, Miot HA. Melasma: a clinical and epidemiological review. An Bras Dermatol. 2014;89:771-782.
- Barankin B, Silver SG, Carruthers A. The skin in pregnancy. J Cutan Med Surg. 2002;6:236-240.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Smith AG, Shuster S, Thody AJ, et al. Chloasma, oral contraceptives, and plasma immunoreactive beta-melanocyte-stimulating hormone. J Invest Dermatol. 1977;68:169-170.
- Ranson M, Posen S, Mason RS. Human melanocytes as a target tissue for hormones: in vitro studies with 1 alpha-25, dihydroxyvitamin D3, alpha-melanocyte stimulating hormone, and beta-estradiol. J Invest Dermatol. 1988;91:593-598.
- Kippenberger S, Loitsch S, Solano F, et al. Quantification of tyrosinase, TRP-1, and Trp-2 transcripts in human melanocytes by reverse transcriptase-competitive multiplex PCR—regulation by steroid hormones. J Invest Dermatol. 1998;110:364-367.
- McLeod SD, Ranson M, Mason RS. Effects of estrogens on human melanocytes in vitro. J Steroid Biochem Mol Biol. 1994;49:9-14.
- Chalermchai T, Rummaneethorn P. Effects of a fractional picosecond 1,064 nm laser for the treatment of dermal and mixed type melasma. J Cosmet Laser Ther. 2018;20:134-139.
- Sheu SL. Treatment of melasma using tranexamic acid: what’s known and what’s next. Cutis. 2018;101:E7-E8.
- Tian B. The Asian problem of frequent laser toning for melasma. J Clin Aesthet Dermatol. 2017;10:40-42.
- Zhang L, Tan WQ, Fang QQ, et al. Tranexamic acid for adults with melasma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:1683414.
- Zhu JW, Ni YJ, Tong XY, et al. Tranexamic acid inhibits angiogenesis and melanogenesis in vitro by targeting VEGF receptors. Int J Med Sci. 2020;17:903-911.
- Colferai MMT, Miquelin GM, Steiner D. Evaluation of oral tranexamic acid in the treatment of melasma. J Cosmet Dermatol. 2019;18:1495-1501.
- Taraz M, Niknam S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies. Dermatol Ther. 2017;30:19-26.
- Yalamanchili R, Shastry V, Betkerur J. Clinico-epidemiological study and quality of life assessment in melasma. Indian J Dermatol. 2015;60:519.
- Kim HJ, Moon SH, Cho SH, et al. Efficacy and safety of tranexamic acid in melasma: a meta-analysis and systematic review. Acta Derm Venereol. 2017;97:776-781.
- Kim YJ, Kim MJ, Kweon DK, et al. Quantification of hypopigmentation activity in vitro. J Vis Exp. 2019;145:20-25.
- Cardoso R, Valente R, Souza da Costa CH, et al. Analysis of kojic acid derivatives as competitive inhibitors of tyrosinase: a molecular modeling approach. Molecules. 2021;26:2875.
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Khuraiya S, Kachhawa D, Chouhan B, et al. A comparative study of topical 5% tranexamic acid and triple combination therapy for the treatment of melasma in Indian population. Pigment International. 2019;6:18-23.
- Steiner D, Feola C, Bialeski N, et al. Study evaluating the efficacy of topical and injected tranexamic acid in treatment of melasma. Surg Cosmet Dermatol. 2009;1:174-177.
- Doolan B, Gupta M. Melasma. Aust J Gen Pract. 2021;50:880-885.
- Banihashemi M, Zabolinejad N, Jaafari MR, et al. Comparison of therapeutic effects of liposomal tranexamic acid and conventional hydroquinone on melasma. J Cosmet Dermatol. 2015;14:174-177.
- Chung JY, Lee JH, Lee JH. Topical tranexamic acid as an adjuvant treatment in melasma: side-by-side comparison clinical study. J Dermatolog Treat. 2016;27:373-377.
- Ebrahimi B, Naeini FF. Topical tranexamic acid as a promising treatment for melasma. J Res Med Sci. 2014;19:753-757.
- Kanechorn Na Ayuthaya P, Niumphradit N, Manosroi A, et al. Topical 5% tranexamic acid for the treatment of melasma in Asians: a double-blind randomized controlled clinical trial. J Cosmet Laser Ther. 2012;14:150-154.
- Kim SJ, Park JY, Shibata T, et al. Efficacy and possible mechanisms of topical tranexamic acid in melasma. Clin Exp Dermatol. 2016;41:480-485.
- Lee JH, Park JG, Lim SH, et al. Localized intradermal microinjection of tranexamic acid for treatment of melasma in Asian patients: a preliminary clinical trial. Dermatol Surg. 2006;32:626-631.
- Badran AY, Ali AU, Gomaa AS. Efficacy of topical versus intradermal injection of tranexamic acid in Egyptian melasma patients: a randomised clinical trial. Australas J Dermatol. 2021;62:E373-E379.
- Saki N, Darayesh M, Heiran A. Comparing the efficacy of topical hydroquinone 2% versus intradermal tranexamic acid microinjections in treating melasma: a split-face controlled trial. J Dermatolog Treat. 2018;29:405-410.
- Pazyar N, Yaghoobi R, Zeynalie M, et al. Comparison of the efficacy of intradermal injected tranexamic acid vs hydroquinone cream in the treatment of melasma. Clin Cosmet Investig Dermatol. 2019;12:115-122.
- Wu S, Shi H, Wu H, et al. Treatment of melasma with oral administration of tranexamic acid. Aesthetic Plast Surg. 2012;36:964-970.
- Atefi N, Dalvand B, Ghassemi M, et al. Therapeutic effects of topical tranexamic acid in comparison with hydroquinone in treatment of women with melasma. Dermatol Ther (Heidelb). 2017;7:417-424.
- Cestari T, Arellano I, Hexsel D, et al. Melasma in Latin America: options for therapy and treatment algorithm. J Eur Acad Dermatol Venereol. 2009;23:760-772.
PRATICE POINTS
- Tranexamic acid (TA) solution 5% is an efficacious treatment for skin of color patients with melasma.
- Topical TA is a treatment alternative for patients who may not be able to tolerate oral TA.
- Our study revealed the greatest efficacy for TA solution 5% was seen on the forehead and malar region, with less efficacy on the chin.
Verrucous Plaque on the Foot
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.
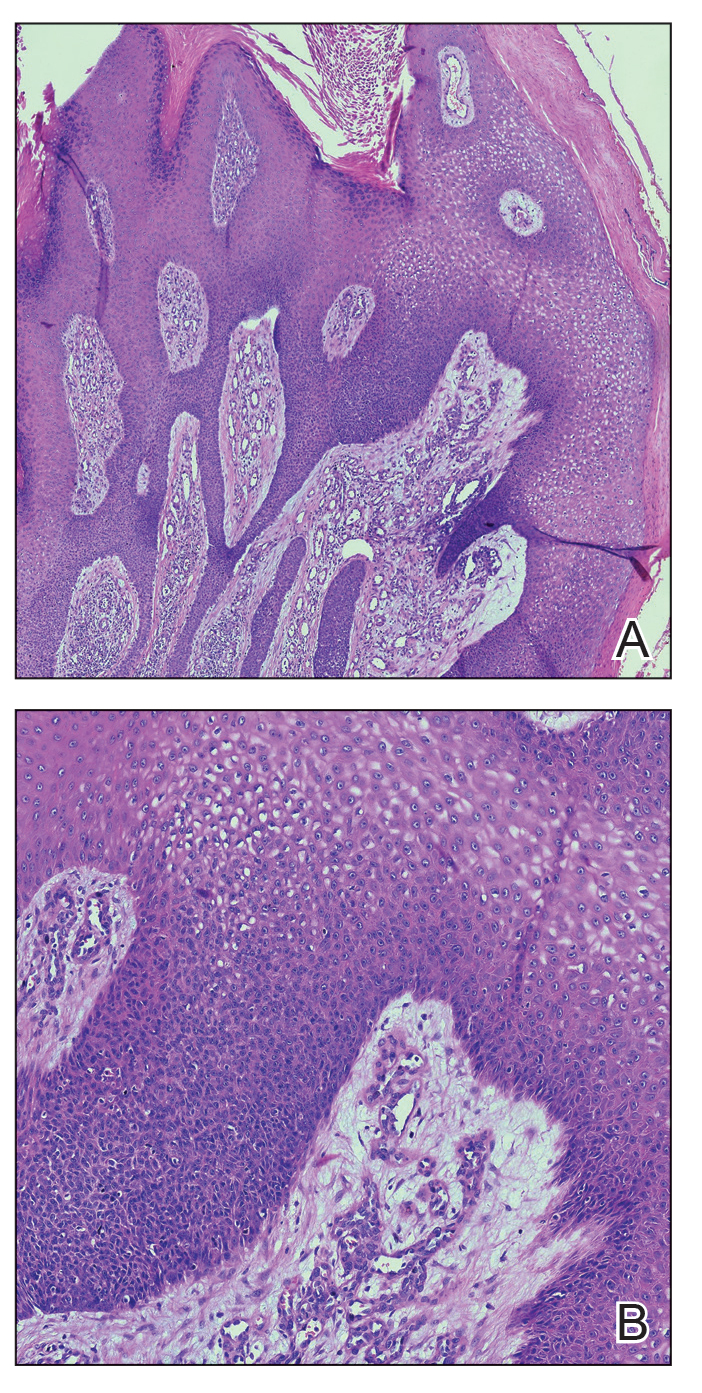
Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.
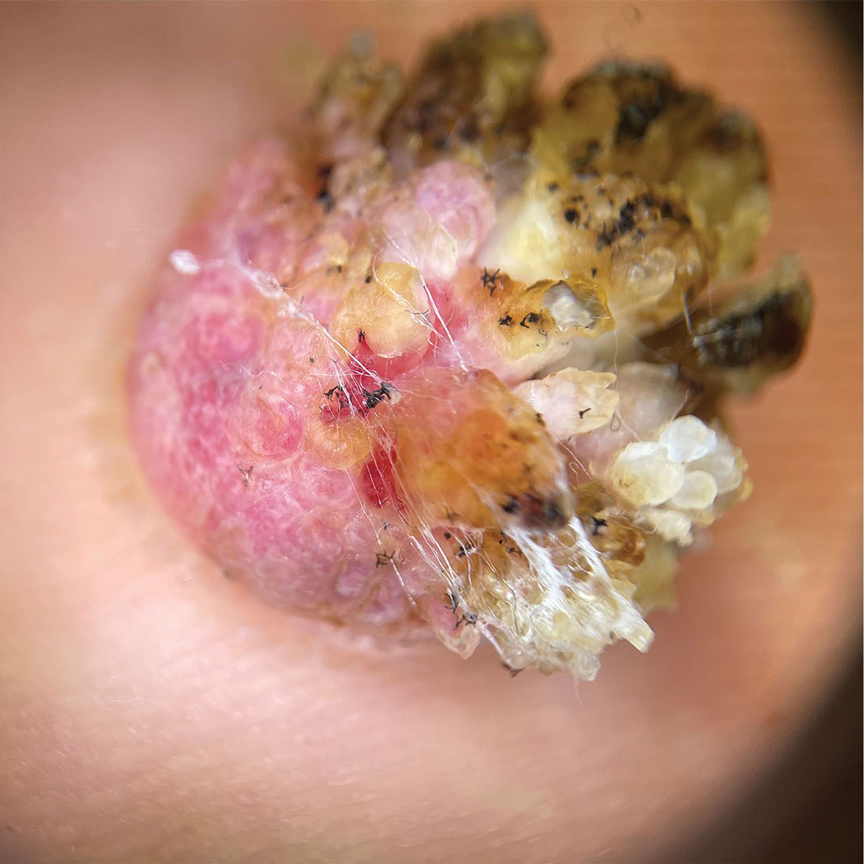
Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9
There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.

Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.

Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9

There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
The Diagnosis: Eccrine Poroma
Histopathology demonstrated epidermal thickening, epidermal protrusions, a well-defined mass of tumor cells that extended from the epidermis down to the dermis, and luminal structures. Poroid cells and ovoid nuclei with basophilic cytoplasm also were evident (Figure 1). Dermoscopy showed papillomatous growth, milky-red areas, and dotted vessels (Figure 2). Reflectance confocal microscopy (RCM) at the spinous layer showed hyporefractile, dark, roundish lumina surrounded by keratinocytes (Figure 3). Based on the histologic, dermoscopic, and RCM findings, our patient was diagnosed with eccrine poroma.

Goldman et al1 first described poroma in 1956. Poromas, which include eccrine poroma, are a group of benign cutaneous neoplasms arising from the terminal eccrine or apocrine sweat gland ducts.2 Histologically, poroid cells appear as cuboidal keratinocytes with monomorphous ovoid nuclei and discrete nucleoli.3 They usually appear as nodules or plaques with colors varying from flesh colored to red, brown, or bluish, and they clinically mimic several benign and malignant skin tumors. The differential diagnosis may include keratoacanthoma, plantar wart, verrucous carcinoma, basal cell carcinoma, and squamous cell carcinoma. Poromas can be of eccrine or apocrine origin.4 They also belong to a broad group of neoplasms, including nodular hidradenomas, clear cell hidradenomas, hidroacanthoma simplex, dermal duct tumors, and hidradenomas.5 Four subtypes—poroma, poroid hidradenoma, hidroacanthoma simplex, and dermal duct tumor—have been documented.6 Because poromas have nonspecific and variable clinical presentations, they often are misdiagnosed as other skin neoplasms, and differentiation may be difficult. For example, some cases of poroma present with follicular, sebaceous, and/or apocrine differentiation, leading to difficulty in diagnosis.

Characteristic features of eccrine poroma seen on dermoscopy and RCM have the potential to aid in the diagnosis compared to histopathology. Marchetti et al7 proposed 4 patterns of characteristic dermoscopic findings. Pattern 1 refers to the classic description with bleeding spots, a structureless yellow appearance, milkyred globules, and branched vessels. Patterns 2 and 3 simulate basal cell carcinoma, dermal nevus, or vascular tumors. Pattern 4 refers to tumors that are large in size and resemble keratinizing neoplasms.7 Brugués et al8 described poromas with the following RCM findings: an atypical honeycomb shape that was well separated from the normal epithelium, hyporefractile nests with atypical cells, lack of palisading, and dark holes. One study described RCM parameters as cords without palisading, dark holes, prominent vascularization, and abundant stroma—findings that were positively associated with poroma in a univariate analysis. These findings assist in distinguishing poromas from other conditions in the differential diagnosis.9

There is a substantial overlap in clinical appearance with malignant conditions, including basal cell carcinoma, squamous cell carcinoma, cutaneous metastases, and Paget disease; therefore, the use of dermoscopy and RCM may be helpful in the diagnosis and recognition of specific features, as well as the corresponding patterns of poroma. Poromas commonly display vascularized features due to the variability of dermoscopic patterns of eccrine poroma, and further studies are required to establish the specificity of vascularized features.
Acral lesions are more likely to show the classic clinical features of erythema and exophytic growth. A case of a collision tumor with the verrucous changes of poroma, seborrheic keratosis, and viral wart has been described.10 The verrucous changes may lead to misdiagnosis as plantar warts or other neoplasms. Clinicians also should consider conditions that are induced by friction or trauma. In our patient, dermoscopy and RCM aided in the diagnosis of eccrine poroma due to the interference of prominent overlying verrucous changes.
Treatment of poroma is optional. Deeper lesions can be treated with surgical excision, and superficial lesions may be treated with electrosurgical destruction. Our patient was treated with surgical excision followed by repair of the surgical defect with a double V-Y flap.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
- Goldman P, Pinkus H, Rogin JR. Eccrine poroma; tumors exhibiting features of the epidermal sweat duct unit. AMA Arch Derm. 1956; 74:511-521.
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma [published online January 28, 2022]. Dermatopathology (Basel). 2022;9:36-47. doi:10.3390/dermatopathology9010007
- Ahmed Jan N, Masood S. Poroma. StatPearls [Internet]. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK560909/
- Casper DJ, Glass LF, Shenefelt PD. An unusually large eccrine poroma: a case report and review of the literature. Cutis. 2011; 88:227-229.
- Sawaya JL, Khachemoune A. Poroma: a review of eccrine, apocrine, and malignant forms. Int J Dermatol. 2014;53:1053-1061.
- Betti R, Bombonato C, Cerri A, et al. Unusual sites for poromas are not very unusual: a survey of 101 cases. Clin Exp Dermatol. 2014; 39:119-122.
- Marchetti MA, Marino ML, Virmani P, et al. Dermoscopic features and patterns of poromas: a multicenter observational case-control study conducted by the International Dermoscopy Society (IDS). J Eur Acad Dermatol Venereol. 2018;32:1263-1271.
- Brugués A, Gamboa M, Alós L, et al. The challenging diagnosis of eccrine poromas. J Am Acad Dermatol. 2016;74:E113-E115.
- Di Tullio F, Mandel VD, Ignazio S, et al. The role of reflectance confocal microscopy in the diagnosis of eccrine poroma: a retrospective casecontrol study. Exp Dermatol. 2022;31:1779-1790.
- Bloom BS, Kamino H, Hale CS, et al. Collision tumor of eccrine poroma, seborrheic keratosis, and a viral wart. Dermatol Online J. 2014;20:13030/qt8tm0r9b9.
A 62-year-old man presented with an enlarging plaque on the foot of 3 years’ duration. He experienced minor pain while walking but reported no other symptoms. His family history was negative for similar anomalies, and his medical history was negative for the presence of malignant tumors. Physical examination revealed a 2-mm erythematous plaque on the plantar aspect of the right foot with prominent overlying verrucous changes and no ulceration or regional lymphadenopathy. Dermoscopy and reflectance confocal microscopy of the lesion were performed along with a histopathologic examination after complete surgical excision.