User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
A Systematic Review of Dermatologic Findings in Adults With Hemophagocytic Lymphohistiocytosis
A Systematic Review of Dermatologic Findings in Adults With Hemophagocytic Lymphohistiocytosis
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening immunologic phenomenon characterized by a systemic inflammatory response syndrome—like clinical picture with additional features, including hepatosplenomegaly, hyperferritinemia, and increased natural killer cell activity. Clinical manifestations of HLH often are nonspecific, making HLH diagnosis challenging. High persistent fever is a key feature of HLH; patients also may report gastrointestinal distress, lethargy, and/or widespread rash.1
Hemophagocytic lymphohistiocytosis is believed to stem from inherited defects in several genes, such as perforin (PRF1), as well as immune dysregulation due to infections, rheumatologic diseases, hematologic malignancies, or drug reactions.2 The primary mechanism of HLH is hypothesized to be driven by aberrant immune activation, interferon gamma released from CD8+ T cells, and uncontrolled phagocytosis by activated macrophages. The cytokine cascade results in tissue injury and multiorgan dysfunction.3,4
Although HLH historically has been categorized as primary (familial) or secondary (acquired), the most recent guidelines suggest the etiology is not always binary.3,5 That said, the concept of secondary causes is useful in understanding risk factors for developing HLH. Both forms of the disease are thought to be elicited by a trigger (eg, infection), even when inherited genetic mutations exist.6 The primary form commonly affects the pediatric population,4,6-8 whereas the secondary form is more common in adults.7
Several sets of diagnostic criteria for HLH have been developed, the most well-known being the HLH-2004 criteria.1,3 The HLH-2009 modified criteria were developed after further evidence provided a refined sense of how the HLH-2004 criteria should be stratified.9 Finally, Fardet et al10 presented the HScore as an estimation of likelihood of diagnosis of HLH. These sets of HLH criteria include clinical and laboratory features that demonstrate inflammation, natrual killer cell activity, hemophagocytosis, end-organ damage, and cell lineage effects. The HScore differs from the other sets of HLH criteria in that it is designed to estimate an individual patient’s risk of having reactive hemophagocytic syndrome, which likely is equivalent to secondary HLH, although the authors do not use this exact terminology.10
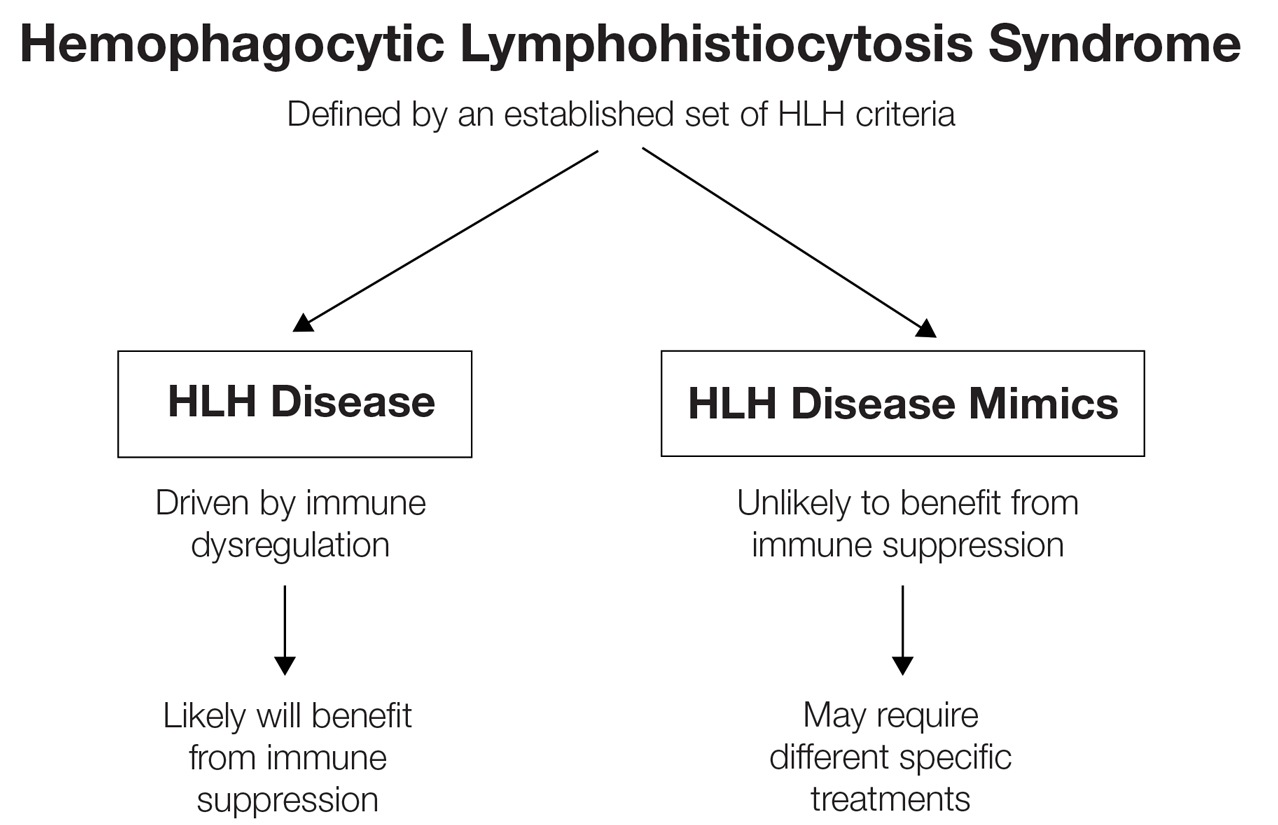
While these criteria provide a framework for diagnosing HLH, they may fail to distinguish between HLH disease and HLH disease mimics, a concept described by the North American Consortium for Histiocytosis that may impact the success of immunosuppressive treatment.3 Individuals with HLH syndrome meet the aforementioned diagnostic criteria; HLH syndrome is further divided into HLH disease and HLH disease mimics (Figure 1). The “disease” label describes the traditional concept of HLH, driven by aberrant immune overactivation, in which patients benefit from immunosuppression. In contrast, HLH mimics include a subset of patients who meet the HLH criteria but are unlikely to benefit from immunosuppression because the primary mechanism driving their condition is not owed to immune overactivation, as is the case with HLH disease. Examples of HLH mimics include certain infections, such as Epstein-Barr virus (EBV), that may demonstrate clinical findings consistent with HLH but would not benefit from immunosuppression. Ironically, infections (including EBV) also are known triggers of HLH disease, making this concept difficult to understand and adopt. In this study, we refer to HLH disease simply as HLH.
Although cutaneous manifestations of HLH are not included in the diagnostic criteria, skin findings are common and may coincide with the severity and progression of the disease.11 Despite the fact that HLH can manifest with rash,1 comprehensive reviews of reported cutaneous findings in adult HLH are lacking. Thus, the goal of this study was to provide an organized characterization of reported cutaneous findings in adults with HLH and context for how the dermatologic examination may support the diagnosis or uncover the underlying etiology of this condition.
Methods
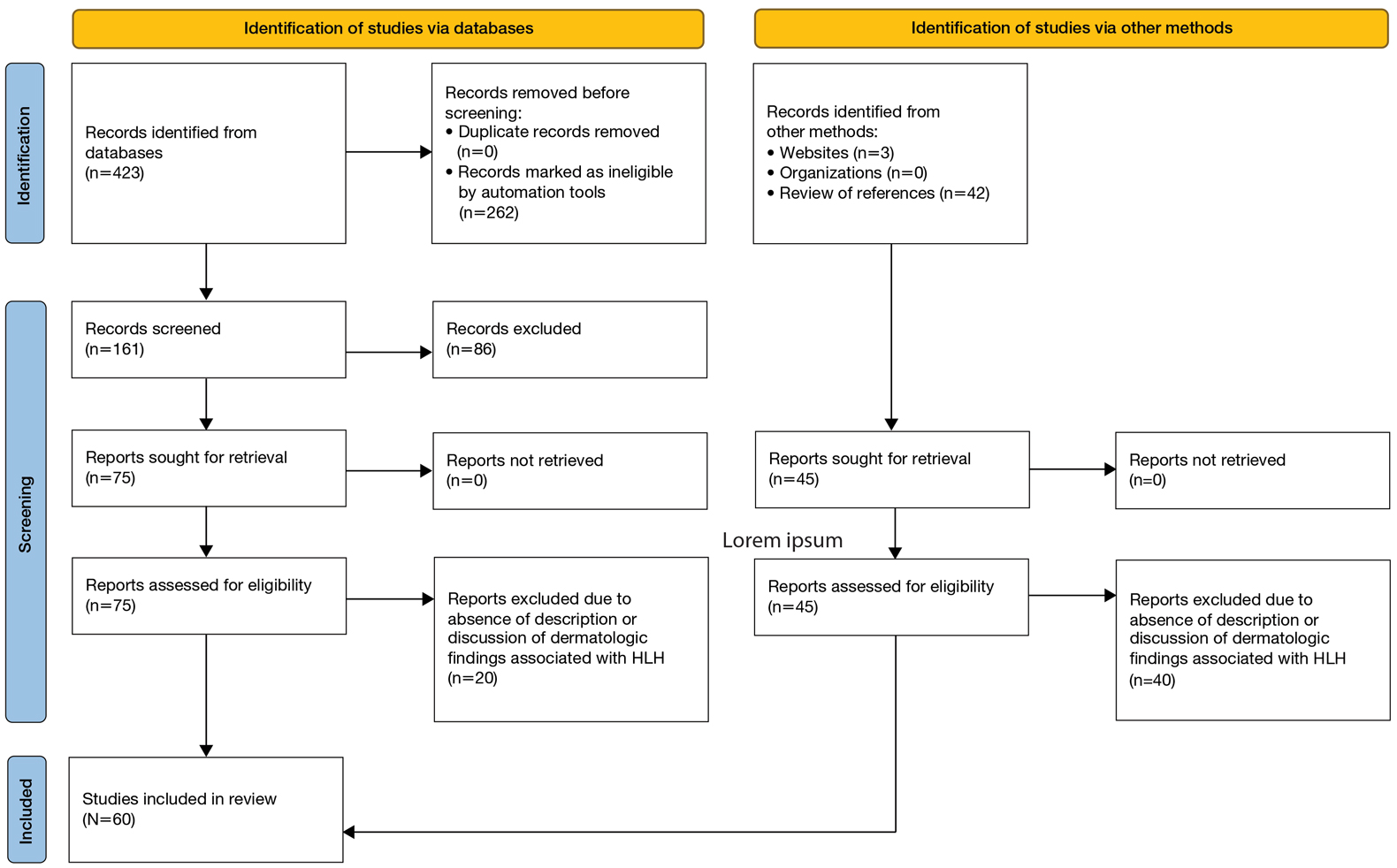
A search of PubMed articles indexed for MEDLINE using the phrase (cutaneous OR dermatologic OR skin) findings) AND hemophagocytic lymphohistiocytosis performed on September 20, 2023, yielded 423 results (Figure 2). Filters to exclude non–English language publications and pediatric populations were applied, resulting in 161 articles. Other exclusion criteria included the absence of a description of dermatologic findings. Seventy-five articles remained after screening titles and abstracts, and full-text review yielded 55 articles that were deemed appropriate for inclusion in the study. Subsequent reference searches and use of the online resource Litmaps revealed 45 additional publications that underwent full-text screening; of these articles, 5 were included in the final review.
Results
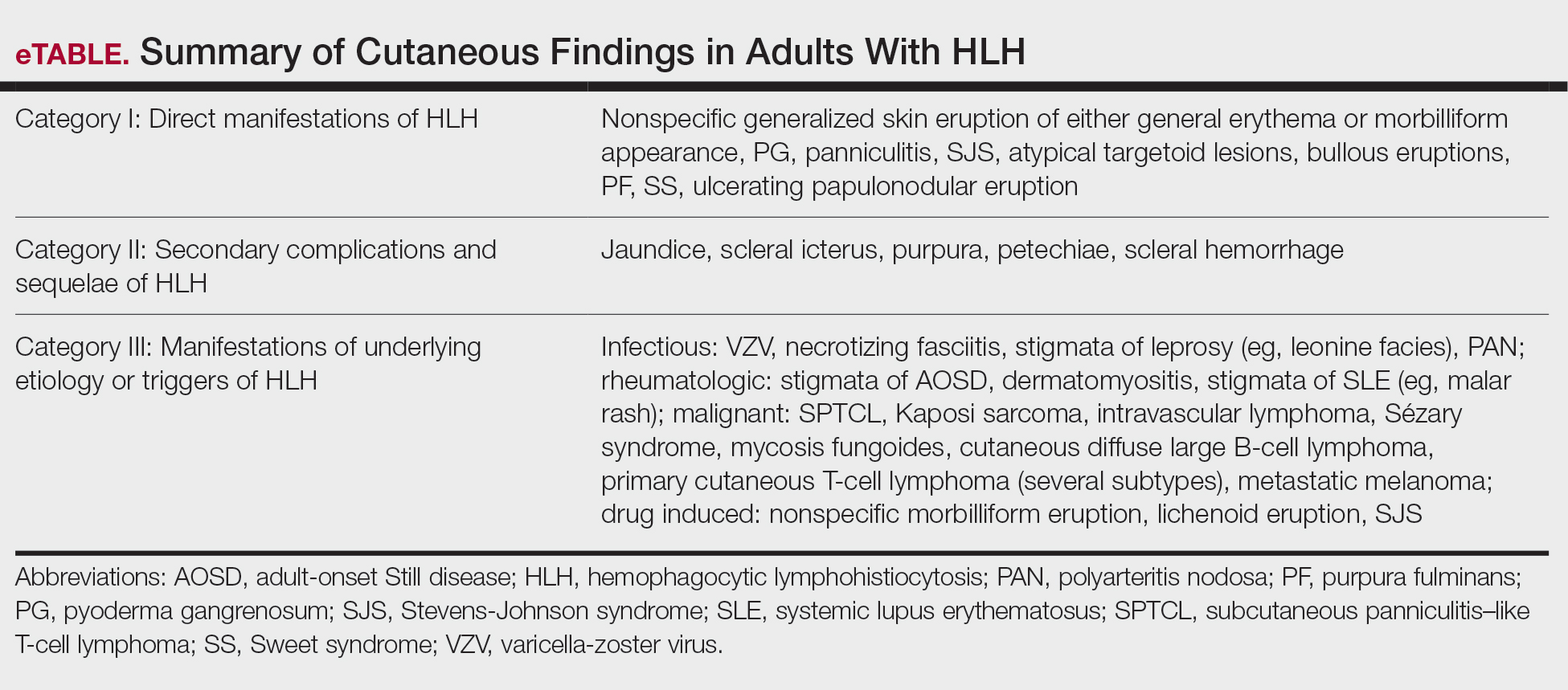
Sixty studies were included in this systematic review.5,7,11-68 The reported prevalence of skin findings among patients with HLH from the included retrospective studies ranged from 15% to 85%.12-15 Several literature reviews reported similarly varied prevalence among adult patients with HLH.7,16 Fardet et al14 categorized cutaneous manifestations of HLH into 3 types: direct manifestations of HLH not explained by systemic features (eg, generalized maculopapular eruption), indirect manifestations of HLH that are explained by systemic features of the disease (eg, purpura due to HLH-induced coagulopathy), and findings specific to the underlying etiology of HLH (eg, malar rash seen in systemic lupus erythematosus [SLE]–associated HLH). This categorization served as the outline for the results below, providing an organized review of cutaneous findings and context for how they may support the diagnosis or uncover the underlying etiology of HLH.
Category I: Direct Manifestations of HLH
Several articles reported cutaneous findings that seemed to be the direct result of HLH and not attributed to an underlying trigger or sequalae of HLH.11,14,16-31 The most common descriptions were a generalized, morbilliform, or nonspecific eruption that encompasses large areas of the skin, commonly the trunk and extremities, sometimes extending to the face and scalp.14,16-23,25,31,32 There were variations in secondary features such as pruritus and tenderness; some studies also described violaceous discoloration in addition to erythema.16,23
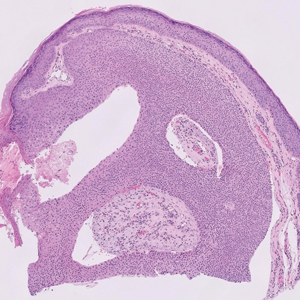
Other skin findings thought to be a direct result of HLH were described in detail by Zerah and DeWitt11 in their retrospective study, including pyoderma gangrenosum, panniculitis, Stevens-Johnson syndrome, atypical targetoid lesions, and bullous eruptions. The authors also analyzed dermatopathologic data that ultimately revealed that pathologic analysis was largely inconsistent and nondescript.11 There was a single case report of purpura fulminans arising alongside signs and symptoms of HLH,26 and several case reports described Sweet syndrome developing around the same time as HLH.27-29 Lastly, Collins et al30 described a case of HLH manifesting with violaceous ulcerating papules and nodules scattered across the legs, abdomen, and arms. Biopsy of this patient’s lesions showed a diffuse dermal infiltrate of histiocytes and hemophagocytosis.
Category II: Secondary Complications and Sequelae of HLH
This was the smallest group among the 3 categories, comprising a few case reports and retrospective cohort studies primarily reporting jaundice/icterus and hemorrhagic lesions such as purpura, petechiae, and scleral hemorrhage.11,21,23,33-35 Several literature reviews described these conditions as nonspecific findings in HLH.16,20 The cause of jaundice in HLH likely can be attributed to its characteristic hepatic dysfunction, whereas hemorrhagic lesions likely are the result of both hepatic and bone marrow dysfunction resulting in coagulopathy.
Category III: Manifestations of Underlying Etiology or Triggers of HLH
Infectious—Infection is known to be one of the most common triggers of HLH, with several retrospective studies reporting infectious triggers in approximately 20% of cases.13,15 Although many pathogens have been implicated, only a few of these infection-induced HLH reports described cutaneous findings, which included a case of varicella zoster virus, Escherichia coli necrotizing fasciitis, leprosy, EBV reactivation, parvovirus B19, and both focal and disseminated herpes simplex virus 2.36-42 Most of these patients presented with classic findings of each disease. The case of varicella zoster virus exhibited pruritic erythematous papules on the face, trunk, and limbs.36 The necrotizing fasciitis case presented with tender erythematous swelling of the lower extremity.37 The patient with leprosy exhibited leonine facies and numerous erythematous nodules, plaques, and superficial ulcerating plaques over the trunk and limbs with palmoplantar involvement,39 and both cases of herpes simplex virus 2 reported small bullae either diffusely over the face, trunk, and extremities or over the genitalia.38,40 Interestingly, the cases of parvovirus B19 and EBV reactivation both exhibited polyarteritis nodosa and occurred in patients with underlying autoimmune conditions, raising the question of whether these cases of HLH had a single trigger or were the result of the overall immunologic dysregulation induced by both infection and autoimmunity.41,42
Rheumatologic—Several articles reported dermatologic findings associated with macrophage activation syndrome, a term that often is used to describe HLH associated with autoimmune conditions. Cases of HLH in adult-onset Still disease, dermatomyositis, polyarteritis nodosa, and SLE described skin findings characteristic of the underlying rheumatologic disease, sometimes with acutely worse dermatologic findings at the time of HLH presentation.35,41-48 With regard to SLE, the acute manifestation of classic findings of the disease with HLH has sometimes been described as acute lupus hemophagocytic syndrome (HPS).48 Lambotte at al48 described common findings of acute lupus hemophagocytic syndrome in their retrospective study as malar rash, weight loss, polyarthralgia, and nephritis in addition to classic HLH findings including fever, lymphadenopathy, and hepatosplenomegaly. Many other rheumatologic conditions have been associated with HLH, including rheumatoid arthritis, mixed connective tissue disease, systemic sclerosis, and Sjögren disease. All these conditions can have dermatologic manifestations; however, no descriptions of dermatologic findings in cases of HLH associated with these diseases were found.13
Malignancy—Several cases of malignancy-induced HLH described cutaneous findings, the majority being cutaneous lymphomas, namely subcutaneous panniculitis-like T-cell lymphoma (SPTCL). Other less commonly reported malignancies in this group included Kaposi sarcoma, intravascular lymphoma, Sézary syndrome, mycosis fungoides, cutaneous diffuse large B-cell lymphoma, and several subtypes of primary cutaneous T-cell lymphoma.2,32,49-60 The most common description of SPTCL included multiple scattered plaques and subcutaneous nodules, some associated with tenderness, induration, drainage, or hemorrhagic features.32,50,52,55,57,60 Cases of mycosis fungoides and Sézary syndrome presented with variations in size and distribution of erythroderma with associated lymphadenopathy.2 A unique case of HLH developing in a patient with intravascular lymphoma described an eruption of multiple telangiectasias and petechial hemorrhages on the trunk,58 while one case associated with primary cutaneous anaplastic large cell lymphoma presented with a rapidly enlarging tumor with central ulceration and eschar.59
Drug Induced—Interestingly, most of the drug-induced cases of HLH identified in our search were secondary to biologic therapies used in the treatment of metastatic melanoma, specifically the immune checkpoint inhibitors (ICIs), which have been reported to have an association with HLH in prior literature reviews.61-65 Choi et al66 described an interesting case of ICI-induced HLH presenting with a concurrent severe lichenoid drug eruption that progressed from a pruritic truncal rash to mucocutaneous bullae, erosions, and desquamation resembling a Stevens-Johnson syndrome–type picture. This patient had treatment-refractory, HIV-negative Kaposi sarcoma, where the underlying immunologic dysregulation may explain the more severe cutaneous presentation not observed in other reported cases of ICI-induced HLH.
Yang et al’s67 review of 23 cases with concurrent diagnoses of HLH and DIHS found that 61% (14/23) of cases were diagnosed initially as DIHS before failing treatment and receiving a diagnosis of HLH several weeks later. Additionally, the authors found that several cases met criteria for one diagnosis while clinically presenting strongly for the other.67 This overlap in clinical presentation also was demonstrated in Zerah and DeWitt’s11 retrospective study regarding cutaneous findings in HLH, in which several of the morbilliform eruptions thought to be contributed to HLH ultimately were decided to be drug reactions.
Comment
Regarding direct (or primary) cutaneous findings in HLH (category I), there seem to be 2 groups of features associated with the onset of HLH that are not related to its characteristic hepatic dysfunction (category II) nor its underlying triggers (category III): a nonspecific, generalized, erythematous eruption; and dermatologic conditions separate from HLH itself (eg, Sweet syndrome, pyoderma gangrenosum). Whether the latter group truly is a direct manifestation of HLH is difficult to discern with the evidence available. Nevertheless, we can conclude that there is some type of association between these dermatologic diseases and HLH, and this association can serve as both a diagnostic tool for clinicians and a point of interest for further clinical research.
The relatively low number of articles identified through our systematic review that specifically reported secondary findings, such as jaundice or coagulopathy-associated hemorrhagic lesions, may lead one to believe that these are not common findings in HLH; however, it is possible that these are not regularly reported in the literature simply because these findings are nonspecific and can be considered expected results of the characteristic organ dysfunction in HLH.
As suspected, the skin findings in category III were the most broad given the variety of underlying etiologies that have been associated with HLH. Like the other 2 categories, these skin findings generally are nonspecific to HLH; however, the ones in category III are specific to underlying etiology of HLH and may aid in identifying and treating the underlying cause of a patient’s HLH when indicated.
Most of the rheumatologic diseases seem to have been known at the time of HLH development and diagnosis, which may highlight the importance of considering a diagnosis of HLH early on in patients with known autoimmune disease and systemic signs of illness or acutely worsening signs and symptoms of their underlying autoimmune disease.
Interestingly, several cases of malignancy-associated HLH reported signs and symptoms of HLH at initial presentation of the malignant disease.32,50,59 This situation seems to be somewhat common, as Go and Wester’s68 systematic analysis of 156 patients with SPTCL found HLH was the presenting feature in 37% of patients included in their study. This may call attention to the importance of considering cutaneous lymphomas as the cause of skin lesions in patients with signs and symptoms of HLH, where it may be easy to assume that skin findings are a result of their systemic disease.
In highlighting cases of HLH related to medication use, we found it pertinent to include and discuss the complex relationship between drug-induced hypersensitivity syndrome (DIHS [formerly known as drug rash with eosinophilia and systemic symptoms [DRESS] syndrome) and HLH. The results of this study suggest that DIHS may have considerable clinical overlap with HLH11 and may even lead to development of HLH,67 creating difficulty in distinguishing between these conditions where there may be similar findings, such as cutaneous eruptions, fever, and hepatic or other internal organ involvement. We agree with Yang et al67 that there can be large overlap in symptomology between these two conditions and that more investigation is necessary to explore the relationship between them.
Conclusion
Diagnosis of HLH in adults continues to be challenging, with several diagnostic tools but no true gold standard. In addition to the nonspecific symptomology, there is a myriad of cutaneous findings that can be present in adults with HLH (eTable), all of which are also nonspecific. Even so, awareness of which dermatologic findings have been associated with HLH may provide a cue to consider HLH in the systemically ill patient with a notable dermatologic examination. Furthermore, there are several avenues for further investigation that can be drawn, including further dermatologic analysis among nonspecific eruptions attributed to HLH, clinical and pathologic differentiation between DIHS/DRESS and HLH, and correlation between severity of skin manifestations and severity of HLH disease.
Limitations of this study included a lack of clarity in diagnosis of HLH in patients described in the included articles, as some reports use variable terminology (HLH vs hemophagocytic syndrome vs macrophage activation syndrome, etc), and it is impossible to know if all authors used the same diagnostic criteria—or any validated diagnostic criteria—unless specifically stated. Additionally, including case reports in our study limited the amount and quality of information described in each report. Despite its limitations, this systematic review outlines the cutaneous manifestations associated with HLH. These data will promote clinical awareness of this complex condition and allow for consideration of HLH in patients meeting criteria for HLH syndrome. More studies ultimately are needed to differentiate HLH from its mimics.
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. doi:10.1002/pbc.21039
- Blom A, Beylot-Barry M, D’Incan M, et al. Lymphoma-associated hemophagocytic syndrome (LAHS) in advanced-stage mycosis fungoides/ Sézary syndrome cutaneous T-cell lymphoma. J Am Acad Dermatol. 2011;65:404-410. doi:10.1016/j.jaad.2010.05.029
- Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66:e27929. doi:10.1002/pbc.27929
- Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34:101515. doi:10.1016/j.berh.2020.101515
- Tomasini D, Berti E. Subcutaneous panniculitis-like T-cell lymphoma. G Ital Dermatol Venereol. 2013;148:395-411.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. doi:10.1016/s0140-6736(13)61048-x
- Sieni E, Cetica V, Piccin A, et al. Familial hemophagocytic lymphohistiocytosis may present during adulthood: clinical and genetic features of a small series. PLoS One. 2012;7:e44649. doi:10.1371/journal.pone.0044649
- Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology. 2009:127-131. doi:10.1182 /asheducation-2009.1.127
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613-2620. doi:10.1002/art.38690
- Zerah ML, DeWitt CA. Cutaneous findings in hemophagocytic lymphohistiocytosis. Dermatology. 2015;230:234-243. doi:10.1159/000368552
- Fardet L, Galicier L, Vignon-Pennamen MD, et al. Frequency, clinical features and prognosis of cutaneous manifestations in adult patients with reactive haemophagocytic syndrome. Br J Dermatol. 2010;162:547-553. doi:10.1111/j.1365-2133.2009.09549.x
- Dhote R, Simon J, Papo T, et al. Reactive hemophagocytic syndrome in adult systemic disease: report of twenty-six cases and literature review. Arthritis Rheum. 2003;49:633-639. doi:10.1002/art.11368
- Li J, Wang Q, Zheng W, et al. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93:100-105. doi:10.1097/md.0000000000000022
- Tudesq JJ, Valade S, Galicier L, et al. Diagnostic strategy for trigger identification in severe reactive hemophagocytic lymphohistiocytosis: a diagnostic accuracy study. Hematol Oncol. 2021;39:114-122. doi:10.1002 /hon.2819
- Sakai H, Otsubo S, Miura T, et al. Hemophagocytic syndrome presenting with a facial erythema in a patient with systemic lupus erythematosus. J Am Acad Dermatol. 2007;57(5 Suppl):S111-S114. doi:10.1016/j .jaad.2006.11.024
- Chung SM, Song JY, Kim W, et al. Dengue-associated hemophagocytic lymphohistiocytosis in an adult: a case report and literature review. Medicine (Baltimore). 2017;96:e6159. doi:10.1097/md.0000000000006159
- Esmaili H, Rahmani O, Fouladi RF. Hemophagocytic syndrome in patients with unexplained cytopenia: report of 15 cases. Turk Patoloji Derg. 2013;29:15-18. doi:10.5146/tjpath.2013.01142
- Jiwnani S, Karimundackal G, Kulkarni A, et al. Hemophagocytic syndrome complicating lung resection. Asian Cardiovasc Thorac Ann. 2012;20:341-343. doi:10.1177/0218492311435686
- Lee WJ, Lee DW, Kim CH, et al. Dermatopathic lymphadenitis with generalized erythroderma in a patient with Epstein-Barr virusassociated hemophagocytic lymphohistiocytosis. Am J Dermatopathol. 2010;32:357-361. doi:10.1097/DAD.0b013e3181b2a50f
- Lovisari F, Terzi V, Lippi MG, et al. Hemophagocytic lymphohistiocytosis complicated by multiorgan failure: a case report. Medicine (Baltimore). 2017;96:e9198. doi:10.1097/md.0000000000009198
- Miechowiecki J, Stainer W, Wallner G, et al. Severe complication during remission of Crohn’s disease: hemophagocytic lymphohistiocytosis due to acute cytomegalovirus infection. Z Gastroenterol. 2018;56:259-263. doi:10.1055/s-0043-123999
- Ochoa S, Cheng K, Fleury CM, et al. A 28-year-old woman with fever, rash, and pancytopenia. Allergy Asthma Proc. 2017;38:322-327. doi:10.2500/aap.2017.38.4042
- Tokoro S, Namiki T, Miura K, et al. Chronic active Epstein-Barr virus infection with cutaneous lymphoproliferation: haemophagocytosis in the skin and haemophagocytic syndrome. J Eur Acad Dermatol Venereol. 2018;32:e116-e117. doi:10.1111/jdv.14640
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitislike T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106 (2 Suppl):S55-S59. doi:10.1016/s0929-6646(09)60354-5
- Honjo O, Kubo T, Sugaya F, et al. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer. 2019;7:97. doi:10.1186/s40425- 019-0582-4
- Kao RL, Jacobsen AA, Billington CJ Jr, et al. A case of VEXAS syndrome associated with EBV-associated hemophagocytic lymphohistiocytosis. Blood Cells Mol Dis. 2022;93:102636. doi:10.1016/j .bcmd.2021.102636
- Koga T, Takano K, Horai Y, et al. Sweet’s syndrome complicated by Kikuchi’s disease and hemophagocytic syndrome which presented with retinoic acid-inducible gene-I in both the skin epidermal basal layer and the cervical lymph nodes. Intern Med. 2013;52:1839-1843. doi:10.2169 /internalmedicine.52.9542
- Lin WL, Lin WC, Chiu CS, et al. Paraneoplastic Sweet’s syndrome in a patient with hemophagocytic syndrome. Int J Dermatol. 2008;3:305-307.
- Collins MK, Ho J, Akilov OE. Case 52. A unique presentation of hemophagocytic lymphohistiocytosis with ulcerating papulonodules. In: Akilov OE, ed. Cutaneous Lymphomas: Unusual Cases 3. Springer International Publishing; 2021:126-127.
- Chakrapani A, Avery A, Warnke R. Primary cutaneous gamma delta T-cell lymphoma with brain involvement and hemophagocytic syndrome. Am J Dermatopathol. 2013;35:270-272. doi:10.1097 /DAD.0b013e3182624e98
- Sullivan C, Loghmani A, Thomas K, et al. Hemophagocytic lymphohistiocytosis as the initial presentation of subcutaneous panniculitis-like T-cell lymphoma: a rare case responding to cyclosporine A and steroids. J Investig Med High Impact Case Rep. 2020;8:2324709620981531. doi:10.1177/2324709620981531
- Darmawan G, Salido EO, Concepcion ML, et al. Hemophagocytic lymphohistiocytosis: “a dreadful mimic.” Int J Rheum Dis. 2015; 18:810-812. doi:10.1111/1756-185x.12506
- Maus MV, Leick MB, Cornejo KM, et al. Case 35-2019: a 66-year-old man with pancytopenia and rash. N Engl J Med. 2019;381:1951-1960. doi:10.1056/NEJMcpc1909627
- Chamseddin B, Marks E, Dominguez A, et al. Refractory macrophage activation syndrome in the setting of adult-onset Still disease with hemophagocytic lymphohistiocytosis detected on skin biopsy treated with canakinumab and tacrolimus. J Cutan Pathol. 2019;46:528-531. doi:10.1111/cup.13466
- Bérar A, Ardois S, Walter-Moraux P, et al. Primary varicella-zoster virus infection of the immunocompromised associated with acute pancreatitis and hemophagocytic lymphohistiocytosis: a case report. Medicine (Baltimore). 2021;100:e25351. doi:10.1097 /md.0000000000025351
- Chang CC, Hsiao PJ, Chiu CC, et al. Catastrophic hemophagocytic lymphohistiocytosis in a young man with nephrotic syndrome. Clin Chim Acta. 2015;439:168-171. doi:10.1016/j.cca.2014.10.025
- Kurosawa S, Sekiya N, Fukushima K, et al. Unusual manifestation of disseminated herpes simplex virus type 2 infection associated with pharyngotonsilitis, esophagitis, and hemophagocytic lymphohisitocytosis without genital involvement. BMC Infect Dis. 2019;19:65. doi:10.1186/s12879-019-3721-0
- Saidi W, Gammoudi R, Korbi M, et al. Hemophagocytic lymphohistiocytosis: an unusual complication of leprosy. Int J Dermatol. 2015;54: 1054-1059. doi:10.1111/ijd.12792
- Yamaguchi K, Yamamoto A, Hisano M, et al. Herpes simplex virus 2-associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet Gynecol. 2005;105(5 Pt 2):1241-1244. doi:10.1097 /01.AOG.0000157757.54948.9b
- Hayakawa I, Shirasaki F, Ikeda H, et al. Reactive hemophagocytic syndrome in a patient with polyarteritis nodosa associated with Epstein- Barr virus reactivation. Rheumatol Int. 2006;26:573-576. doi:10.1007 /s00296-005-0024-0
- Jeong JY, Park JY, Ham JY, et al. Molecular evidence of parvovirus B19 in the cutaneous polyarteritis nodosa tissue from a patient with parvovirus-associated hemophagocytic syndrome: case report. Medicine (Baltimore). 2020;99:e22079. doi:10.1097 /md.0000000000022079
- Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med. 2018;57:3473-3478. doi:10.2169 /internalmedicine.1121-18
- Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune- associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244-246. doi:10 .1080/03009742.2019.1653493
- Jung SY. Hemophagocytic syndrome diagnosed by liver biopsy in a female patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19:449-451. doi:10.1097/rhu.0000000000000040
- Kerl K, Wolf IH, Cerroni L, et al. Hemophagocytosis in cutaneous autoimmune disease. Am J Dermatopathol. 2015;37:539-543. doi:10.1097 /dad.0000000000000166
- Komiya Y, Saito T, Mizoguchi F, et al. Hemophagocytic syndrome complicated with dermatomyositis controlled successfully with infliximab and conventional therapies. Intern Med. 2017;56:3237-3241. doi:10.2169 /internalmedicine.7966-16
- Lambotte O, Khellaf M, Harmouche H, et al. Characteristics and long-term outcome of 15 episodes of systemic lupus erythematosusassociated hemophagocytic syndrome. Medicine (Baltimore). 2006;85: 169-182. doi:10.1097/01.md.0000224708.62510.d1
- Guitart J, Mangold AR, Martinez-Escala ME, et al. Clinical and pathological characteristics and outcomes among patients with subcutaneous panniculitis-like T-cell lymphoma and related adipotropic lymphoproliferative disorders. JAMA Dermatol. 2022;158:1167-1174. doi:10.1001/jamadermatol.2022.3347
- Hung GD, Chen YH, Chen DY, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with hemophagocytic lymphohistiocytosis and skin lesions with characteristic high-resolution ultrasonographic findings. Clin Rheumatol. 2007;26:775-778. doi:10.1007/s10067 -005-0193-y
- Jamil A, Nadzri N, Harun N, et al. Primary cutaneous diffuse large B-cell lymphoma leg type presenting with hemophagocytic syndrome. J Am Acad Dermatol. 2012;67:e222-3. doi:10.1016/j.jaad.2012.04.021
- LeBlanc RE, Lansigan F. Unraveling subcutaneous panniculitis-like T-cell lymphoma: an association between subcutaneous panniculitislike T-cell lymphoma, autoimmune lymphoproliferative syndrome, and familial hemophagocytic lymphohistiocytosis. J Cutan Pathol. 2021;48:572-577. doi:10.1111/cup.13863
- Lee DE, Martinez-Escala ME, Serrano LM, et al. Hemophagocytic lymphohistiocytosis in cutaneous T-cell lymphoma. JAMA Dermatol. 2018;154:828-831. doi:10.1001/jamadermatol.2018.1264
- Maejima H, Tanei R, Morioka T, et al. Haemophagocytosis-related intravascular large B-cell lymphoma associated with skin eruption. Acta Derm Venereol. 2011;91:339-340. doi:10.2340/00015555-0981
- Mody A, Cherry D, Georgescu G, et al. A rare case of subcutaneous panniculitis-like T cell lymphoma with hemophagocytic lymphohistiocytosis mimicking cellulitis. Am J Case Rep. 2021;22:E927142. doi:10.12659/ajcr.927142
- Park YJ, Bae HJ, Chang JY, et al. Development of Kaposi sarcoma and hemophagocytic lymphohistiocytosis associated with human herpesvirus 8 in a renal transplant recipient. Korean J Intern Med. 2017;4:750-752.
- Phatak S, Gupta L, Aggarwal A. A young woman with panniculitis and cytopenia who later developed coagulopathy. J Assoc Physicians India. 2016;64:65-67.
- Pongpairoj K, Rerknimitr P, Wititsuwannakul J, et al. Eruptive telangiectasia in a patient with fever and haemophagocytic syndrome. Clin Exp Dermatol. 2016;41:696-698. doi:10.1111/ced.12859
- Shimizu Y, Tanae K, Takahashi N, et al. Primary cutaneous anaplastic large-cell lymphoma presenting with hemophagocytic syndrome: a case report and review of the literature. Leuk Res. 2010;34:263-266. doi:10.1016/j.leukres.2009.07.001
- Sirka CS, Pradhan S, Patra S, et al. Hemophagocytic lymphohistiocytosis: a rare, potentially fatal complication in subcutaneous panniculitis like T cell lymphoma. Indian J Dermatol Venereol Leprol. 2019;5:481-485.
- Chin CK, Hall S, Green C, et al. Secondary haemophagocytic lymphohistiocytosis due to checkpoint inhibitor therapy. Eur J Cancer. 2019;115: 84-87. doi:10.1016/j.ejca.2019.04.026
- Dudda M, Mann C, Heinz J, et al. Hemophagocytic lymphohistiocytosis of a melanoma patient under BRAF/MEK-inhibitor therapy following anti-PD1 inhibitor treatment: a case report and review to the literature. Melanoma Res. 2021;31:81-84. doi:10.1097 /cmr.0000000000000703
- Mizuta H, Nakano E, Takahashi A, et al. Hemophagocytic lymphohistiocytosis with advanced malignant melanoma accompanied by ipilimumab and nivolumab: a case report and literature review. Dermatol Ther. 2020;33:e13321. doi:10.1111/dth.13321
- Satzger I, Ivanyi P, Länger F, et al. Treatment-related hemophagocytic lymphohistiocytosis secondary to checkpoint inhibition with nivolumab plus ipilimumab. Eur J Cancer. 2018;93:150-153. doi:10.1016/j.ejca.2018.01.063
- Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72-90. doi:10.1016/J.EJCA.2019.07.014
- Choi S, Zhou M, Bahrani E, et al. Rare and fatal complication of immune checkpoint inhibition: a case report of haemophagocytic lymphohistiocytosis with severe lichenoid dermatitis. Br J Haematol. 2021;193:e44-e47. doi:10.1111/BJH.17442
- Yang JJ, Lei DK, Ravi V, et al. Overlap between hemophagocytic lymphohistiocytosis and drug reaction and eosinophilia with systemic symptoms: a review. Int J Dermatol. 2021;60:925-932. doi:10.1111 /ijd.15196
- Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404-1413. doi:10.1002/cncr.20502
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening immunologic phenomenon characterized by a systemic inflammatory response syndrome—like clinical picture with additional features, including hepatosplenomegaly, hyperferritinemia, and increased natural killer cell activity. Clinical manifestations of HLH often are nonspecific, making HLH diagnosis challenging. High persistent fever is a key feature of HLH; patients also may report gastrointestinal distress, lethargy, and/or widespread rash.1
Hemophagocytic lymphohistiocytosis is believed to stem from inherited defects in several genes, such as perforin (PRF1), as well as immune dysregulation due to infections, rheumatologic diseases, hematologic malignancies, or drug reactions.2 The primary mechanism of HLH is hypothesized to be driven by aberrant immune activation, interferon gamma released from CD8+ T cells, and uncontrolled phagocytosis by activated macrophages. The cytokine cascade results in tissue injury and multiorgan dysfunction.3,4
Although HLH historically has been categorized as primary (familial) or secondary (acquired), the most recent guidelines suggest the etiology is not always binary.3,5 That said, the concept of secondary causes is useful in understanding risk factors for developing HLH. Both forms of the disease are thought to be elicited by a trigger (eg, infection), even when inherited genetic mutations exist.6 The primary form commonly affects the pediatric population,4,6-8 whereas the secondary form is more common in adults.7
Several sets of diagnostic criteria for HLH have been developed, the most well-known being the HLH-2004 criteria.1,3 The HLH-2009 modified criteria were developed after further evidence provided a refined sense of how the HLH-2004 criteria should be stratified.9 Finally, Fardet et al10 presented the HScore as an estimation of likelihood of diagnosis of HLH. These sets of HLH criteria include clinical and laboratory features that demonstrate inflammation, natrual killer cell activity, hemophagocytosis, end-organ damage, and cell lineage effects. The HScore differs from the other sets of HLH criteria in that it is designed to estimate an individual patient’s risk of having reactive hemophagocytic syndrome, which likely is equivalent to secondary HLH, although the authors do not use this exact terminology.10
While these criteria provide a framework for diagnosing HLH, they may fail to distinguish between HLH disease and HLH disease mimics, a concept described by the North American Consortium for Histiocytosis that may impact the success of immunosuppressive treatment.3 Individuals with HLH syndrome meet the aforementioned diagnostic criteria; HLH syndrome is further divided into HLH disease and HLH disease mimics (Figure 1). The “disease” label describes the traditional concept of HLH, driven by aberrant immune overactivation, in which patients benefit from immunosuppression. In contrast, HLH mimics include a subset of patients who meet the HLH criteria but are unlikely to benefit from immunosuppression because the primary mechanism driving their condition is not owed to immune overactivation, as is the case with HLH disease. Examples of HLH mimics include certain infections, such as Epstein-Barr virus (EBV), that may demonstrate clinical findings consistent with HLH but would not benefit from immunosuppression. Ironically, infections (including EBV) also are known triggers of HLH disease, making this concept difficult to understand and adopt. In this study, we refer to HLH disease simply as HLH.

Although cutaneous manifestations of HLH are not included in the diagnostic criteria, skin findings are common and may coincide with the severity and progression of the disease.11 Despite the fact that HLH can manifest with rash,1 comprehensive reviews of reported cutaneous findings in adult HLH are lacking. Thus, the goal of this study was to provide an organized characterization of reported cutaneous findings in adults with HLH and context for how the dermatologic examination may support the diagnosis or uncover the underlying etiology of this condition.
Methods
A search of PubMed articles indexed for MEDLINE using the phrase (cutaneous OR dermatologic OR skin) findings) AND hemophagocytic lymphohistiocytosis performed on September 20, 2023, yielded 423 results (Figure 2). Filters to exclude non–English language publications and pediatric populations were applied, resulting in 161 articles. Other exclusion criteria included the absence of a description of dermatologic findings. Seventy-five articles remained after screening titles and abstracts, and full-text review yielded 55 articles that were deemed appropriate for inclusion in the study. Subsequent reference searches and use of the online resource Litmaps revealed 45 additional publications that underwent full-text screening; of these articles, 5 were included in the final review.

Results
Sixty studies were included in this systematic review.5,7,11-68 The reported prevalence of skin findings among patients with HLH from the included retrospective studies ranged from 15% to 85%.12-15 Several literature reviews reported similarly varied prevalence among adult patients with HLH.7,16 Fardet et al14 categorized cutaneous manifestations of HLH into 3 types: direct manifestations of HLH not explained by systemic features (eg, generalized maculopapular eruption), indirect manifestations of HLH that are explained by systemic features of the disease (eg, purpura due to HLH-induced coagulopathy), and findings specific to the underlying etiology of HLH (eg, malar rash seen in systemic lupus erythematosus [SLE]–associated HLH). This categorization served as the outline for the results below, providing an organized review of cutaneous findings and context for how they may support the diagnosis or uncover the underlying etiology of HLH.
Category I: Direct Manifestations of HLH
Several articles reported cutaneous findings that seemed to be the direct result of HLH and not attributed to an underlying trigger or sequalae of HLH.11,14,16-31 The most common descriptions were a generalized, morbilliform, or nonspecific eruption that encompasses large areas of the skin, commonly the trunk and extremities, sometimes extending to the face and scalp.14,16-23,25,31,32 There were variations in secondary features such as pruritus and tenderness; some studies also described violaceous discoloration in addition to erythema.16,23
Other skin findings thought to be a direct result of HLH were described in detail by Zerah and DeWitt11 in their retrospective study, including pyoderma gangrenosum, panniculitis, Stevens-Johnson syndrome, atypical targetoid lesions, and bullous eruptions. The authors also analyzed dermatopathologic data that ultimately revealed that pathologic analysis was largely inconsistent and nondescript.11 There was a single case report of purpura fulminans arising alongside signs and symptoms of HLH,26 and several case reports described Sweet syndrome developing around the same time as HLH.27-29 Lastly, Collins et al30 described a case of HLH manifesting with violaceous ulcerating papules and nodules scattered across the legs, abdomen, and arms. Biopsy of this patient’s lesions showed a diffuse dermal infiltrate of histiocytes and hemophagocytosis.
Category II: Secondary Complications and Sequelae of HLH
This was the smallest group among the 3 categories, comprising a few case reports and retrospective cohort studies primarily reporting jaundice/icterus and hemorrhagic lesions such as purpura, petechiae, and scleral hemorrhage.11,21,23,33-35 Several literature reviews described these conditions as nonspecific findings in HLH.16,20 The cause of jaundice in HLH likely can be attributed to its characteristic hepatic dysfunction, whereas hemorrhagic lesions likely are the result of both hepatic and bone marrow dysfunction resulting in coagulopathy.
Category III: Manifestations of Underlying Etiology or Triggers of HLH
Infectious—Infection is known to be one of the most common triggers of HLH, with several retrospective studies reporting infectious triggers in approximately 20% of cases.13,15 Although many pathogens have been implicated, only a few of these infection-induced HLH reports described cutaneous findings, which included a case of varicella zoster virus, Escherichia coli necrotizing fasciitis, leprosy, EBV reactivation, parvovirus B19, and both focal and disseminated herpes simplex virus 2.36-42 Most of these patients presented with classic findings of each disease. The case of varicella zoster virus exhibited pruritic erythematous papules on the face, trunk, and limbs.36 The necrotizing fasciitis case presented with tender erythematous swelling of the lower extremity.37 The patient with leprosy exhibited leonine facies and numerous erythematous nodules, plaques, and superficial ulcerating plaques over the trunk and limbs with palmoplantar involvement,39 and both cases of herpes simplex virus 2 reported small bullae either diffusely over the face, trunk, and extremities or over the genitalia.38,40 Interestingly, the cases of parvovirus B19 and EBV reactivation both exhibited polyarteritis nodosa and occurred in patients with underlying autoimmune conditions, raising the question of whether these cases of HLH had a single trigger or were the result of the overall immunologic dysregulation induced by both infection and autoimmunity.41,42
Rheumatologic—Several articles reported dermatologic findings associated with macrophage activation syndrome, a term that often is used to describe HLH associated with autoimmune conditions. Cases of HLH in adult-onset Still disease, dermatomyositis, polyarteritis nodosa, and SLE described skin findings characteristic of the underlying rheumatologic disease, sometimes with acutely worse dermatologic findings at the time of HLH presentation.35,41-48 With regard to SLE, the acute manifestation of classic findings of the disease with HLH has sometimes been described as acute lupus hemophagocytic syndrome (HPS).48 Lambotte at al48 described common findings of acute lupus hemophagocytic syndrome in their retrospective study as malar rash, weight loss, polyarthralgia, and nephritis in addition to classic HLH findings including fever, lymphadenopathy, and hepatosplenomegaly. Many other rheumatologic conditions have been associated with HLH, including rheumatoid arthritis, mixed connective tissue disease, systemic sclerosis, and Sjögren disease. All these conditions can have dermatologic manifestations; however, no descriptions of dermatologic findings in cases of HLH associated with these diseases were found.13
Malignancy—Several cases of malignancy-induced HLH described cutaneous findings, the majority being cutaneous lymphomas, namely subcutaneous panniculitis-like T-cell lymphoma (SPTCL). Other less commonly reported malignancies in this group included Kaposi sarcoma, intravascular lymphoma, Sézary syndrome, mycosis fungoides, cutaneous diffuse large B-cell lymphoma, and several subtypes of primary cutaneous T-cell lymphoma.2,32,49-60 The most common description of SPTCL included multiple scattered plaques and subcutaneous nodules, some associated with tenderness, induration, drainage, or hemorrhagic features.32,50,52,55,57,60 Cases of mycosis fungoides and Sézary syndrome presented with variations in size and distribution of erythroderma with associated lymphadenopathy.2 A unique case of HLH developing in a patient with intravascular lymphoma described an eruption of multiple telangiectasias and petechial hemorrhages on the trunk,58 while one case associated with primary cutaneous anaplastic large cell lymphoma presented with a rapidly enlarging tumor with central ulceration and eschar.59
Drug Induced—Interestingly, most of the drug-induced cases of HLH identified in our search were secondary to biologic therapies used in the treatment of metastatic melanoma, specifically the immune checkpoint inhibitors (ICIs), which have been reported to have an association with HLH in prior literature reviews.61-65 Choi et al66 described an interesting case of ICI-induced HLH presenting with a concurrent severe lichenoid drug eruption that progressed from a pruritic truncal rash to mucocutaneous bullae, erosions, and desquamation resembling a Stevens-Johnson syndrome–type picture. This patient had treatment-refractory, HIV-negative Kaposi sarcoma, where the underlying immunologic dysregulation may explain the more severe cutaneous presentation not observed in other reported cases of ICI-induced HLH.
Yang et al’s67 review of 23 cases with concurrent diagnoses of HLH and DIHS found that 61% (14/23) of cases were diagnosed initially as DIHS before failing treatment and receiving a diagnosis of HLH several weeks later. Additionally, the authors found that several cases met criteria for one diagnosis while clinically presenting strongly for the other.67 This overlap in clinical presentation also was demonstrated in Zerah and DeWitt’s11 retrospective study regarding cutaneous findings in HLH, in which several of the morbilliform eruptions thought to be contributed to HLH ultimately were decided to be drug reactions.
Comment
Regarding direct (or primary) cutaneous findings in HLH (category I), there seem to be 2 groups of features associated with the onset of HLH that are not related to its characteristic hepatic dysfunction (category II) nor its underlying triggers (category III): a nonspecific, generalized, erythematous eruption; and dermatologic conditions separate from HLH itself (eg, Sweet syndrome, pyoderma gangrenosum). Whether the latter group truly is a direct manifestation of HLH is difficult to discern with the evidence available. Nevertheless, we can conclude that there is some type of association between these dermatologic diseases and HLH, and this association can serve as both a diagnostic tool for clinicians and a point of interest for further clinical research.
The relatively low number of articles identified through our systematic review that specifically reported secondary findings, such as jaundice or coagulopathy-associated hemorrhagic lesions, may lead one to believe that these are not common findings in HLH; however, it is possible that these are not regularly reported in the literature simply because these findings are nonspecific and can be considered expected results of the characteristic organ dysfunction in HLH.
As suspected, the skin findings in category III were the most broad given the variety of underlying etiologies that have been associated with HLH. Like the other 2 categories, these skin findings generally are nonspecific to HLH; however, the ones in category III are specific to underlying etiology of HLH and may aid in identifying and treating the underlying cause of a patient’s HLH when indicated.
Most of the rheumatologic diseases seem to have been known at the time of HLH development and diagnosis, which may highlight the importance of considering a diagnosis of HLH early on in patients with known autoimmune disease and systemic signs of illness or acutely worsening signs and symptoms of their underlying autoimmune disease.
Interestingly, several cases of malignancy-associated HLH reported signs and symptoms of HLH at initial presentation of the malignant disease.32,50,59 This situation seems to be somewhat common, as Go and Wester’s68 systematic analysis of 156 patients with SPTCL found HLH was the presenting feature in 37% of patients included in their study. This may call attention to the importance of considering cutaneous lymphomas as the cause of skin lesions in patients with signs and symptoms of HLH, where it may be easy to assume that skin findings are a result of their systemic disease.
In highlighting cases of HLH related to medication use, we found it pertinent to include and discuss the complex relationship between drug-induced hypersensitivity syndrome (DIHS [formerly known as drug rash with eosinophilia and systemic symptoms [DRESS] syndrome) and HLH. The results of this study suggest that DIHS may have considerable clinical overlap with HLH11 and may even lead to development of HLH,67 creating difficulty in distinguishing between these conditions where there may be similar findings, such as cutaneous eruptions, fever, and hepatic or other internal organ involvement. We agree with Yang et al67 that there can be large overlap in symptomology between these two conditions and that more investigation is necessary to explore the relationship between them.
Conclusion
Diagnosis of HLH in adults continues to be challenging, with several diagnostic tools but no true gold standard. In addition to the nonspecific symptomology, there is a myriad of cutaneous findings that can be present in adults with HLH (eTable), all of which are also nonspecific. Even so, awareness of which dermatologic findings have been associated with HLH may provide a cue to consider HLH in the systemically ill patient with a notable dermatologic examination. Furthermore, there are several avenues for further investigation that can be drawn, including further dermatologic analysis among nonspecific eruptions attributed to HLH, clinical and pathologic differentiation between DIHS/DRESS and HLH, and correlation between severity of skin manifestations and severity of HLH disease.

Limitations of this study included a lack of clarity in diagnosis of HLH in patients described in the included articles, as some reports use variable terminology (HLH vs hemophagocytic syndrome vs macrophage activation syndrome, etc), and it is impossible to know if all authors used the same diagnostic criteria—or any validated diagnostic criteria—unless specifically stated. Additionally, including case reports in our study limited the amount and quality of information described in each report. Despite its limitations, this systematic review outlines the cutaneous manifestations associated with HLH. These data will promote clinical awareness of this complex condition and allow for consideration of HLH in patients meeting criteria for HLH syndrome. More studies ultimately are needed to differentiate HLH from its mimics.
Hemophagocytic lymphohistiocytosis (HLH) is a life-threatening immunologic phenomenon characterized by a systemic inflammatory response syndrome—like clinical picture with additional features, including hepatosplenomegaly, hyperferritinemia, and increased natural killer cell activity. Clinical manifestations of HLH often are nonspecific, making HLH diagnosis challenging. High persistent fever is a key feature of HLH; patients also may report gastrointestinal distress, lethargy, and/or widespread rash.1
Hemophagocytic lymphohistiocytosis is believed to stem from inherited defects in several genes, such as perforin (PRF1), as well as immune dysregulation due to infections, rheumatologic diseases, hematologic malignancies, or drug reactions.2 The primary mechanism of HLH is hypothesized to be driven by aberrant immune activation, interferon gamma released from CD8+ T cells, and uncontrolled phagocytosis by activated macrophages. The cytokine cascade results in tissue injury and multiorgan dysfunction.3,4
Although HLH historically has been categorized as primary (familial) or secondary (acquired), the most recent guidelines suggest the etiology is not always binary.3,5 That said, the concept of secondary causes is useful in understanding risk factors for developing HLH. Both forms of the disease are thought to be elicited by a trigger (eg, infection), even when inherited genetic mutations exist.6 The primary form commonly affects the pediatric population,4,6-8 whereas the secondary form is more common in adults.7
Several sets of diagnostic criteria for HLH have been developed, the most well-known being the HLH-2004 criteria.1,3 The HLH-2009 modified criteria were developed after further evidence provided a refined sense of how the HLH-2004 criteria should be stratified.9 Finally, Fardet et al10 presented the HScore as an estimation of likelihood of diagnosis of HLH. These sets of HLH criteria include clinical and laboratory features that demonstrate inflammation, natrual killer cell activity, hemophagocytosis, end-organ damage, and cell lineage effects. The HScore differs from the other sets of HLH criteria in that it is designed to estimate an individual patient’s risk of having reactive hemophagocytic syndrome, which likely is equivalent to secondary HLH, although the authors do not use this exact terminology.10
While these criteria provide a framework for diagnosing HLH, they may fail to distinguish between HLH disease and HLH disease mimics, a concept described by the North American Consortium for Histiocytosis that may impact the success of immunosuppressive treatment.3 Individuals with HLH syndrome meet the aforementioned diagnostic criteria; HLH syndrome is further divided into HLH disease and HLH disease mimics (Figure 1). The “disease” label describes the traditional concept of HLH, driven by aberrant immune overactivation, in which patients benefit from immunosuppression. In contrast, HLH mimics include a subset of patients who meet the HLH criteria but are unlikely to benefit from immunosuppression because the primary mechanism driving their condition is not owed to immune overactivation, as is the case with HLH disease. Examples of HLH mimics include certain infections, such as Epstein-Barr virus (EBV), that may demonstrate clinical findings consistent with HLH but would not benefit from immunosuppression. Ironically, infections (including EBV) also are known triggers of HLH disease, making this concept difficult to understand and adopt. In this study, we refer to HLH disease simply as HLH.

Although cutaneous manifestations of HLH are not included in the diagnostic criteria, skin findings are common and may coincide with the severity and progression of the disease.11 Despite the fact that HLH can manifest with rash,1 comprehensive reviews of reported cutaneous findings in adult HLH are lacking. Thus, the goal of this study was to provide an organized characterization of reported cutaneous findings in adults with HLH and context for how the dermatologic examination may support the diagnosis or uncover the underlying etiology of this condition.
Methods
A search of PubMed articles indexed for MEDLINE using the phrase (cutaneous OR dermatologic OR skin) findings) AND hemophagocytic lymphohistiocytosis performed on September 20, 2023, yielded 423 results (Figure 2). Filters to exclude non–English language publications and pediatric populations were applied, resulting in 161 articles. Other exclusion criteria included the absence of a description of dermatologic findings. Seventy-five articles remained after screening titles and abstracts, and full-text review yielded 55 articles that were deemed appropriate for inclusion in the study. Subsequent reference searches and use of the online resource Litmaps revealed 45 additional publications that underwent full-text screening; of these articles, 5 were included in the final review.

Results
Sixty studies were included in this systematic review.5,7,11-68 The reported prevalence of skin findings among patients with HLH from the included retrospective studies ranged from 15% to 85%.12-15 Several literature reviews reported similarly varied prevalence among adult patients with HLH.7,16 Fardet et al14 categorized cutaneous manifestations of HLH into 3 types: direct manifestations of HLH not explained by systemic features (eg, generalized maculopapular eruption), indirect manifestations of HLH that are explained by systemic features of the disease (eg, purpura due to HLH-induced coagulopathy), and findings specific to the underlying etiology of HLH (eg, malar rash seen in systemic lupus erythematosus [SLE]–associated HLH). This categorization served as the outline for the results below, providing an organized review of cutaneous findings and context for how they may support the diagnosis or uncover the underlying etiology of HLH.
Category I: Direct Manifestations of HLH
Several articles reported cutaneous findings that seemed to be the direct result of HLH and not attributed to an underlying trigger or sequalae of HLH.11,14,16-31 The most common descriptions were a generalized, morbilliform, or nonspecific eruption that encompasses large areas of the skin, commonly the trunk and extremities, sometimes extending to the face and scalp.14,16-23,25,31,32 There were variations in secondary features such as pruritus and tenderness; some studies also described violaceous discoloration in addition to erythema.16,23
Other skin findings thought to be a direct result of HLH were described in detail by Zerah and DeWitt11 in their retrospective study, including pyoderma gangrenosum, panniculitis, Stevens-Johnson syndrome, atypical targetoid lesions, and bullous eruptions. The authors also analyzed dermatopathologic data that ultimately revealed that pathologic analysis was largely inconsistent and nondescript.11 There was a single case report of purpura fulminans arising alongside signs and symptoms of HLH,26 and several case reports described Sweet syndrome developing around the same time as HLH.27-29 Lastly, Collins et al30 described a case of HLH manifesting with violaceous ulcerating papules and nodules scattered across the legs, abdomen, and arms. Biopsy of this patient’s lesions showed a diffuse dermal infiltrate of histiocytes and hemophagocytosis.
Category II: Secondary Complications and Sequelae of HLH
This was the smallest group among the 3 categories, comprising a few case reports and retrospective cohort studies primarily reporting jaundice/icterus and hemorrhagic lesions such as purpura, petechiae, and scleral hemorrhage.11,21,23,33-35 Several literature reviews described these conditions as nonspecific findings in HLH.16,20 The cause of jaundice in HLH likely can be attributed to its characteristic hepatic dysfunction, whereas hemorrhagic lesions likely are the result of both hepatic and bone marrow dysfunction resulting in coagulopathy.
Category III: Manifestations of Underlying Etiology or Triggers of HLH
Infectious—Infection is known to be one of the most common triggers of HLH, with several retrospective studies reporting infectious triggers in approximately 20% of cases.13,15 Although many pathogens have been implicated, only a few of these infection-induced HLH reports described cutaneous findings, which included a case of varicella zoster virus, Escherichia coli necrotizing fasciitis, leprosy, EBV reactivation, parvovirus B19, and both focal and disseminated herpes simplex virus 2.36-42 Most of these patients presented with classic findings of each disease. The case of varicella zoster virus exhibited pruritic erythematous papules on the face, trunk, and limbs.36 The necrotizing fasciitis case presented with tender erythematous swelling of the lower extremity.37 The patient with leprosy exhibited leonine facies and numerous erythematous nodules, plaques, and superficial ulcerating plaques over the trunk and limbs with palmoplantar involvement,39 and both cases of herpes simplex virus 2 reported small bullae either diffusely over the face, trunk, and extremities or over the genitalia.38,40 Interestingly, the cases of parvovirus B19 and EBV reactivation both exhibited polyarteritis nodosa and occurred in patients with underlying autoimmune conditions, raising the question of whether these cases of HLH had a single trigger or were the result of the overall immunologic dysregulation induced by both infection and autoimmunity.41,42
Rheumatologic—Several articles reported dermatologic findings associated with macrophage activation syndrome, a term that often is used to describe HLH associated with autoimmune conditions. Cases of HLH in adult-onset Still disease, dermatomyositis, polyarteritis nodosa, and SLE described skin findings characteristic of the underlying rheumatologic disease, sometimes with acutely worse dermatologic findings at the time of HLH presentation.35,41-48 With regard to SLE, the acute manifestation of classic findings of the disease with HLH has sometimes been described as acute lupus hemophagocytic syndrome (HPS).48 Lambotte at al48 described common findings of acute lupus hemophagocytic syndrome in their retrospective study as malar rash, weight loss, polyarthralgia, and nephritis in addition to classic HLH findings including fever, lymphadenopathy, and hepatosplenomegaly. Many other rheumatologic conditions have been associated with HLH, including rheumatoid arthritis, mixed connective tissue disease, systemic sclerosis, and Sjögren disease. All these conditions can have dermatologic manifestations; however, no descriptions of dermatologic findings in cases of HLH associated with these diseases were found.13
Malignancy—Several cases of malignancy-induced HLH described cutaneous findings, the majority being cutaneous lymphomas, namely subcutaneous panniculitis-like T-cell lymphoma (SPTCL). Other less commonly reported malignancies in this group included Kaposi sarcoma, intravascular lymphoma, Sézary syndrome, mycosis fungoides, cutaneous diffuse large B-cell lymphoma, and several subtypes of primary cutaneous T-cell lymphoma.2,32,49-60 The most common description of SPTCL included multiple scattered plaques and subcutaneous nodules, some associated with tenderness, induration, drainage, or hemorrhagic features.32,50,52,55,57,60 Cases of mycosis fungoides and Sézary syndrome presented with variations in size and distribution of erythroderma with associated lymphadenopathy.2 A unique case of HLH developing in a patient with intravascular lymphoma described an eruption of multiple telangiectasias and petechial hemorrhages on the trunk,58 while one case associated with primary cutaneous anaplastic large cell lymphoma presented with a rapidly enlarging tumor with central ulceration and eschar.59
Drug Induced—Interestingly, most of the drug-induced cases of HLH identified in our search were secondary to biologic therapies used in the treatment of metastatic melanoma, specifically the immune checkpoint inhibitors (ICIs), which have been reported to have an association with HLH in prior literature reviews.61-65 Choi et al66 described an interesting case of ICI-induced HLH presenting with a concurrent severe lichenoid drug eruption that progressed from a pruritic truncal rash to mucocutaneous bullae, erosions, and desquamation resembling a Stevens-Johnson syndrome–type picture. This patient had treatment-refractory, HIV-negative Kaposi sarcoma, where the underlying immunologic dysregulation may explain the more severe cutaneous presentation not observed in other reported cases of ICI-induced HLH.
Yang et al’s67 review of 23 cases with concurrent diagnoses of HLH and DIHS found that 61% (14/23) of cases were diagnosed initially as DIHS before failing treatment and receiving a diagnosis of HLH several weeks later. Additionally, the authors found that several cases met criteria for one diagnosis while clinically presenting strongly for the other.67 This overlap in clinical presentation also was demonstrated in Zerah and DeWitt’s11 retrospective study regarding cutaneous findings in HLH, in which several of the morbilliform eruptions thought to be contributed to HLH ultimately were decided to be drug reactions.
Comment
Regarding direct (or primary) cutaneous findings in HLH (category I), there seem to be 2 groups of features associated with the onset of HLH that are not related to its characteristic hepatic dysfunction (category II) nor its underlying triggers (category III): a nonspecific, generalized, erythematous eruption; and dermatologic conditions separate from HLH itself (eg, Sweet syndrome, pyoderma gangrenosum). Whether the latter group truly is a direct manifestation of HLH is difficult to discern with the evidence available. Nevertheless, we can conclude that there is some type of association between these dermatologic diseases and HLH, and this association can serve as both a diagnostic tool for clinicians and a point of interest for further clinical research.
The relatively low number of articles identified through our systematic review that specifically reported secondary findings, such as jaundice or coagulopathy-associated hemorrhagic lesions, may lead one to believe that these are not common findings in HLH; however, it is possible that these are not regularly reported in the literature simply because these findings are nonspecific and can be considered expected results of the characteristic organ dysfunction in HLH.
As suspected, the skin findings in category III were the most broad given the variety of underlying etiologies that have been associated with HLH. Like the other 2 categories, these skin findings generally are nonspecific to HLH; however, the ones in category III are specific to underlying etiology of HLH and may aid in identifying and treating the underlying cause of a patient’s HLH when indicated.
Most of the rheumatologic diseases seem to have been known at the time of HLH development and diagnosis, which may highlight the importance of considering a diagnosis of HLH early on in patients with known autoimmune disease and systemic signs of illness or acutely worsening signs and symptoms of their underlying autoimmune disease.
Interestingly, several cases of malignancy-associated HLH reported signs and symptoms of HLH at initial presentation of the malignant disease.32,50,59 This situation seems to be somewhat common, as Go and Wester’s68 systematic analysis of 156 patients with SPTCL found HLH was the presenting feature in 37% of patients included in their study. This may call attention to the importance of considering cutaneous lymphomas as the cause of skin lesions in patients with signs and symptoms of HLH, where it may be easy to assume that skin findings are a result of their systemic disease.
In highlighting cases of HLH related to medication use, we found it pertinent to include and discuss the complex relationship between drug-induced hypersensitivity syndrome (DIHS [formerly known as drug rash with eosinophilia and systemic symptoms [DRESS] syndrome) and HLH. The results of this study suggest that DIHS may have considerable clinical overlap with HLH11 and may even lead to development of HLH,67 creating difficulty in distinguishing between these conditions where there may be similar findings, such as cutaneous eruptions, fever, and hepatic or other internal organ involvement. We agree with Yang et al67 that there can be large overlap in symptomology between these two conditions and that more investigation is necessary to explore the relationship between them.
Conclusion
Diagnosis of HLH in adults continues to be challenging, with several diagnostic tools but no true gold standard. In addition to the nonspecific symptomology, there is a myriad of cutaneous findings that can be present in adults with HLH (eTable), all of which are also nonspecific. Even so, awareness of which dermatologic findings have been associated with HLH may provide a cue to consider HLH in the systemically ill patient with a notable dermatologic examination. Furthermore, there are several avenues for further investigation that can be drawn, including further dermatologic analysis among nonspecific eruptions attributed to HLH, clinical and pathologic differentiation between DIHS/DRESS and HLH, and correlation between severity of skin manifestations and severity of HLH disease.

Limitations of this study included a lack of clarity in diagnosis of HLH in patients described in the included articles, as some reports use variable terminology (HLH vs hemophagocytic syndrome vs macrophage activation syndrome, etc), and it is impossible to know if all authors used the same diagnostic criteria—or any validated diagnostic criteria—unless specifically stated. Additionally, including case reports in our study limited the amount and quality of information described in each report. Despite its limitations, this systematic review outlines the cutaneous manifestations associated with HLH. These data will promote clinical awareness of this complex condition and allow for consideration of HLH in patients meeting criteria for HLH syndrome. More studies ultimately are needed to differentiate HLH from its mimics.
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. doi:10.1002/pbc.21039
- Blom A, Beylot-Barry M, D’Incan M, et al. Lymphoma-associated hemophagocytic syndrome (LAHS) in advanced-stage mycosis fungoides/ Sézary syndrome cutaneous T-cell lymphoma. J Am Acad Dermatol. 2011;65:404-410. doi:10.1016/j.jaad.2010.05.029
- Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66:e27929. doi:10.1002/pbc.27929
- Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34:101515. doi:10.1016/j.berh.2020.101515
- Tomasini D, Berti E. Subcutaneous panniculitis-like T-cell lymphoma. G Ital Dermatol Venereol. 2013;148:395-411.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. doi:10.1016/s0140-6736(13)61048-x
- Sieni E, Cetica V, Piccin A, et al. Familial hemophagocytic lymphohistiocytosis may present during adulthood: clinical and genetic features of a small series. PLoS One. 2012;7:e44649. doi:10.1371/journal.pone.0044649
- Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology. 2009:127-131. doi:10.1182 /asheducation-2009.1.127
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613-2620. doi:10.1002/art.38690
- Zerah ML, DeWitt CA. Cutaneous findings in hemophagocytic lymphohistiocytosis. Dermatology. 2015;230:234-243. doi:10.1159/000368552
- Fardet L, Galicier L, Vignon-Pennamen MD, et al. Frequency, clinical features and prognosis of cutaneous manifestations in adult patients with reactive haemophagocytic syndrome. Br J Dermatol. 2010;162:547-553. doi:10.1111/j.1365-2133.2009.09549.x
- Dhote R, Simon J, Papo T, et al. Reactive hemophagocytic syndrome in adult systemic disease: report of twenty-six cases and literature review. Arthritis Rheum. 2003;49:633-639. doi:10.1002/art.11368
- Li J, Wang Q, Zheng W, et al. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93:100-105. doi:10.1097/md.0000000000000022
- Tudesq JJ, Valade S, Galicier L, et al. Diagnostic strategy for trigger identification in severe reactive hemophagocytic lymphohistiocytosis: a diagnostic accuracy study. Hematol Oncol. 2021;39:114-122. doi:10.1002 /hon.2819
- Sakai H, Otsubo S, Miura T, et al. Hemophagocytic syndrome presenting with a facial erythema in a patient with systemic lupus erythematosus. J Am Acad Dermatol. 2007;57(5 Suppl):S111-S114. doi:10.1016/j .jaad.2006.11.024
- Chung SM, Song JY, Kim W, et al. Dengue-associated hemophagocytic lymphohistiocytosis in an adult: a case report and literature review. Medicine (Baltimore). 2017;96:e6159. doi:10.1097/md.0000000000006159
- Esmaili H, Rahmani O, Fouladi RF. Hemophagocytic syndrome in patients with unexplained cytopenia: report of 15 cases. Turk Patoloji Derg. 2013;29:15-18. doi:10.5146/tjpath.2013.01142
- Jiwnani S, Karimundackal G, Kulkarni A, et al. Hemophagocytic syndrome complicating lung resection. Asian Cardiovasc Thorac Ann. 2012;20:341-343. doi:10.1177/0218492311435686
- Lee WJ, Lee DW, Kim CH, et al. Dermatopathic lymphadenitis with generalized erythroderma in a patient with Epstein-Barr virusassociated hemophagocytic lymphohistiocytosis. Am J Dermatopathol. 2010;32:357-361. doi:10.1097/DAD.0b013e3181b2a50f
- Lovisari F, Terzi V, Lippi MG, et al. Hemophagocytic lymphohistiocytosis complicated by multiorgan failure: a case report. Medicine (Baltimore). 2017;96:e9198. doi:10.1097/md.0000000000009198
- Miechowiecki J, Stainer W, Wallner G, et al. Severe complication during remission of Crohn’s disease: hemophagocytic lymphohistiocytosis due to acute cytomegalovirus infection. Z Gastroenterol. 2018;56:259-263. doi:10.1055/s-0043-123999
- Ochoa S, Cheng K, Fleury CM, et al. A 28-year-old woman with fever, rash, and pancytopenia. Allergy Asthma Proc. 2017;38:322-327. doi:10.2500/aap.2017.38.4042
- Tokoro S, Namiki T, Miura K, et al. Chronic active Epstein-Barr virus infection with cutaneous lymphoproliferation: haemophagocytosis in the skin and haemophagocytic syndrome. J Eur Acad Dermatol Venereol. 2018;32:e116-e117. doi:10.1111/jdv.14640
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitislike T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106 (2 Suppl):S55-S59. doi:10.1016/s0929-6646(09)60354-5
- Honjo O, Kubo T, Sugaya F, et al. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer. 2019;7:97. doi:10.1186/s40425- 019-0582-4
- Kao RL, Jacobsen AA, Billington CJ Jr, et al. A case of VEXAS syndrome associated with EBV-associated hemophagocytic lymphohistiocytosis. Blood Cells Mol Dis. 2022;93:102636. doi:10.1016/j .bcmd.2021.102636
- Koga T, Takano K, Horai Y, et al. Sweet’s syndrome complicated by Kikuchi’s disease and hemophagocytic syndrome which presented with retinoic acid-inducible gene-I in both the skin epidermal basal layer and the cervical lymph nodes. Intern Med. 2013;52:1839-1843. doi:10.2169 /internalmedicine.52.9542
- Lin WL, Lin WC, Chiu CS, et al. Paraneoplastic Sweet’s syndrome in a patient with hemophagocytic syndrome. Int J Dermatol. 2008;3:305-307.
- Collins MK, Ho J, Akilov OE. Case 52. A unique presentation of hemophagocytic lymphohistiocytosis with ulcerating papulonodules. In: Akilov OE, ed. Cutaneous Lymphomas: Unusual Cases 3. Springer International Publishing; 2021:126-127.
- Chakrapani A, Avery A, Warnke R. Primary cutaneous gamma delta T-cell lymphoma with brain involvement and hemophagocytic syndrome. Am J Dermatopathol. 2013;35:270-272. doi:10.1097 /DAD.0b013e3182624e98
- Sullivan C, Loghmani A, Thomas K, et al. Hemophagocytic lymphohistiocytosis as the initial presentation of subcutaneous panniculitis-like T-cell lymphoma: a rare case responding to cyclosporine A and steroids. J Investig Med High Impact Case Rep. 2020;8:2324709620981531. doi:10.1177/2324709620981531
- Darmawan G, Salido EO, Concepcion ML, et al. Hemophagocytic lymphohistiocytosis: “a dreadful mimic.” Int J Rheum Dis. 2015; 18:810-812. doi:10.1111/1756-185x.12506
- Maus MV, Leick MB, Cornejo KM, et al. Case 35-2019: a 66-year-old man with pancytopenia and rash. N Engl J Med. 2019;381:1951-1960. doi:10.1056/NEJMcpc1909627
- Chamseddin B, Marks E, Dominguez A, et al. Refractory macrophage activation syndrome in the setting of adult-onset Still disease with hemophagocytic lymphohistiocytosis detected on skin biopsy treated with canakinumab and tacrolimus. J Cutan Pathol. 2019;46:528-531. doi:10.1111/cup.13466
- Bérar A, Ardois S, Walter-Moraux P, et al. Primary varicella-zoster virus infection of the immunocompromised associated with acute pancreatitis and hemophagocytic lymphohistiocytosis: a case report. Medicine (Baltimore). 2021;100:e25351. doi:10.1097 /md.0000000000025351
- Chang CC, Hsiao PJ, Chiu CC, et al. Catastrophic hemophagocytic lymphohistiocytosis in a young man with nephrotic syndrome. Clin Chim Acta. 2015;439:168-171. doi:10.1016/j.cca.2014.10.025
- Kurosawa S, Sekiya N, Fukushima K, et al. Unusual manifestation of disseminated herpes simplex virus type 2 infection associated with pharyngotonsilitis, esophagitis, and hemophagocytic lymphohisitocytosis without genital involvement. BMC Infect Dis. 2019;19:65. doi:10.1186/s12879-019-3721-0
- Saidi W, Gammoudi R, Korbi M, et al. Hemophagocytic lymphohistiocytosis: an unusual complication of leprosy. Int J Dermatol. 2015;54: 1054-1059. doi:10.1111/ijd.12792
- Yamaguchi K, Yamamoto A, Hisano M, et al. Herpes simplex virus 2-associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet Gynecol. 2005;105(5 Pt 2):1241-1244. doi:10.1097 /01.AOG.0000157757.54948.9b
- Hayakawa I, Shirasaki F, Ikeda H, et al. Reactive hemophagocytic syndrome in a patient with polyarteritis nodosa associated with Epstein- Barr virus reactivation. Rheumatol Int. 2006;26:573-576. doi:10.1007 /s00296-005-0024-0
- Jeong JY, Park JY, Ham JY, et al. Molecular evidence of parvovirus B19 in the cutaneous polyarteritis nodosa tissue from a patient with parvovirus-associated hemophagocytic syndrome: case report. Medicine (Baltimore). 2020;99:e22079. doi:10.1097 /md.0000000000022079
- Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med. 2018;57:3473-3478. doi:10.2169 /internalmedicine.1121-18
- Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune- associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244-246. doi:10 .1080/03009742.2019.1653493
- Jung SY. Hemophagocytic syndrome diagnosed by liver biopsy in a female patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19:449-451. doi:10.1097/rhu.0000000000000040
- Kerl K, Wolf IH, Cerroni L, et al. Hemophagocytosis in cutaneous autoimmune disease. Am J Dermatopathol. 2015;37:539-543. doi:10.1097 /dad.0000000000000166
- Komiya Y, Saito T, Mizoguchi F, et al. Hemophagocytic syndrome complicated with dermatomyositis controlled successfully with infliximab and conventional therapies. Intern Med. 2017;56:3237-3241. doi:10.2169 /internalmedicine.7966-16
- Lambotte O, Khellaf M, Harmouche H, et al. Characteristics and long-term outcome of 15 episodes of systemic lupus erythematosusassociated hemophagocytic syndrome. Medicine (Baltimore). 2006;85: 169-182. doi:10.1097/01.md.0000224708.62510.d1
- Guitart J, Mangold AR, Martinez-Escala ME, et al. Clinical and pathological characteristics and outcomes among patients with subcutaneous panniculitis-like T-cell lymphoma and related adipotropic lymphoproliferative disorders. JAMA Dermatol. 2022;158:1167-1174. doi:10.1001/jamadermatol.2022.3347
- Hung GD, Chen YH, Chen DY, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with hemophagocytic lymphohistiocytosis and skin lesions with characteristic high-resolution ultrasonographic findings. Clin Rheumatol. 2007;26:775-778. doi:10.1007/s10067 -005-0193-y
- Jamil A, Nadzri N, Harun N, et al. Primary cutaneous diffuse large B-cell lymphoma leg type presenting with hemophagocytic syndrome. J Am Acad Dermatol. 2012;67:e222-3. doi:10.1016/j.jaad.2012.04.021
- LeBlanc RE, Lansigan F. Unraveling subcutaneous panniculitis-like T-cell lymphoma: an association between subcutaneous panniculitislike T-cell lymphoma, autoimmune lymphoproliferative syndrome, and familial hemophagocytic lymphohistiocytosis. J Cutan Pathol. 2021;48:572-577. doi:10.1111/cup.13863
- Lee DE, Martinez-Escala ME, Serrano LM, et al. Hemophagocytic lymphohistiocytosis in cutaneous T-cell lymphoma. JAMA Dermatol. 2018;154:828-831. doi:10.1001/jamadermatol.2018.1264
- Maejima H, Tanei R, Morioka T, et al. Haemophagocytosis-related intravascular large B-cell lymphoma associated with skin eruption. Acta Derm Venereol. 2011;91:339-340. doi:10.2340/00015555-0981
- Mody A, Cherry D, Georgescu G, et al. A rare case of subcutaneous panniculitis-like T cell lymphoma with hemophagocytic lymphohistiocytosis mimicking cellulitis. Am J Case Rep. 2021;22:E927142. doi:10.12659/ajcr.927142
- Park YJ, Bae HJ, Chang JY, et al. Development of Kaposi sarcoma and hemophagocytic lymphohistiocytosis associated with human herpesvirus 8 in a renal transplant recipient. Korean J Intern Med. 2017;4:750-752.
- Phatak S, Gupta L, Aggarwal A. A young woman with panniculitis and cytopenia who later developed coagulopathy. J Assoc Physicians India. 2016;64:65-67.
- Pongpairoj K, Rerknimitr P, Wititsuwannakul J, et al. Eruptive telangiectasia in a patient with fever and haemophagocytic syndrome. Clin Exp Dermatol. 2016;41:696-698. doi:10.1111/ced.12859
- Shimizu Y, Tanae K, Takahashi N, et al. Primary cutaneous anaplastic large-cell lymphoma presenting with hemophagocytic syndrome: a case report and review of the literature. Leuk Res. 2010;34:263-266. doi:10.1016/j.leukres.2009.07.001
- Sirka CS, Pradhan S, Patra S, et al. Hemophagocytic lymphohistiocytosis: a rare, potentially fatal complication in subcutaneous panniculitis like T cell lymphoma. Indian J Dermatol Venereol Leprol. 2019;5:481-485.
- Chin CK, Hall S, Green C, et al. Secondary haemophagocytic lymphohistiocytosis due to checkpoint inhibitor therapy. Eur J Cancer. 2019;115: 84-87. doi:10.1016/j.ejca.2019.04.026
- Dudda M, Mann C, Heinz J, et al. Hemophagocytic lymphohistiocytosis of a melanoma patient under BRAF/MEK-inhibitor therapy following anti-PD1 inhibitor treatment: a case report and review to the literature. Melanoma Res. 2021;31:81-84. doi:10.1097 /cmr.0000000000000703
- Mizuta H, Nakano E, Takahashi A, et al. Hemophagocytic lymphohistiocytosis with advanced malignant melanoma accompanied by ipilimumab and nivolumab: a case report and literature review. Dermatol Ther. 2020;33:e13321. doi:10.1111/dth.13321
- Satzger I, Ivanyi P, Länger F, et al. Treatment-related hemophagocytic lymphohistiocytosis secondary to checkpoint inhibition with nivolumab plus ipilimumab. Eur J Cancer. 2018;93:150-153. doi:10.1016/j.ejca.2018.01.063
- Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72-90. doi:10.1016/J.EJCA.2019.07.014
- Choi S, Zhou M, Bahrani E, et al. Rare and fatal complication of immune checkpoint inhibition: a case report of haemophagocytic lymphohistiocytosis with severe lichenoid dermatitis. Br J Haematol. 2021;193:e44-e47. doi:10.1111/BJH.17442
- Yang JJ, Lei DK, Ravi V, et al. Overlap between hemophagocytic lymphohistiocytosis and drug reaction and eosinophilia with systemic symptoms: a review. Int J Dermatol. 2021;60:925-932. doi:10.1111 /ijd.15196
- Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404-1413. doi:10.1002/cncr.20502
- Henter JI, Horne A, Aricó M, et al. HLH-2004: diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. doi:10.1002/pbc.21039
- Blom A, Beylot-Barry M, D’Incan M, et al. Lymphoma-associated hemophagocytic syndrome (LAHS) in advanced-stage mycosis fungoides/ Sézary syndrome cutaneous T-cell lymphoma. J Am Acad Dermatol. 2011;65:404-410. doi:10.1016/j.jaad.2010.05.029
- Jordan MB, Allen CE, Greenberg J, et al. Challenges in the diagnosis of hemophagocytic lymphohistiocytosis: recommendations from the North American Consortium for Histiocytosis (NACHO). Pediatr Blood Cancer. 2019;66:e27929. doi:10.1002/pbc.27929
- Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: an update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34:101515. doi:10.1016/j.berh.2020.101515
- Tomasini D, Berti E. Subcutaneous panniculitis-like T-cell lymphoma. G Ital Dermatol Venereol. 2013;148:395-411.
- Emile JF, Abla O, Fraitag S, et al. Revised classification of histiocytoses and neoplasms of the macrophage-dendritic cell lineages. Blood. 2016;127:2672-2681. doi:10.1182/blood-2016-01-690636
- Ramos-Casals M, Brito-Zerón P, López-Guillermo A, et al. Adult haemophagocytic syndrome. Lancet. 2014;383:1503-1516. doi:10.1016/s0140-6736(13)61048-x
- Sieni E, Cetica V, Piccin A, et al. Familial hemophagocytic lymphohistiocytosis may present during adulthood: clinical and genetic features of a small series. PLoS One. 2012;7:e44649. doi:10.1371/journal.pone.0044649
- Filipovich AH. Hemophagocytic lymphohistiocytosis (HLH) and related disorders. Hematology. 2009:127-131. doi:10.1182 /asheducation-2009.1.127
- Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66:2613-2620. doi:10.1002/art.38690
- Zerah ML, DeWitt CA. Cutaneous findings in hemophagocytic lymphohistiocytosis. Dermatology. 2015;230:234-243. doi:10.1159/000368552
- Fardet L, Galicier L, Vignon-Pennamen MD, et al. Frequency, clinical features and prognosis of cutaneous manifestations in adult patients with reactive haemophagocytic syndrome. Br J Dermatol. 2010;162:547-553. doi:10.1111/j.1365-2133.2009.09549.x
- Dhote R, Simon J, Papo T, et al. Reactive hemophagocytic syndrome in adult systemic disease: report of twenty-six cases and literature review. Arthritis Rheum. 2003;49:633-639. doi:10.1002/art.11368
- Li J, Wang Q, Zheng W, et al. Hemophagocytic lymphohistiocytosis: clinical analysis of 103 adult patients. Medicine (Baltimore). 2014;93:100-105. doi:10.1097/md.0000000000000022
- Tudesq JJ, Valade S, Galicier L, et al. Diagnostic strategy for trigger identification in severe reactive hemophagocytic lymphohistiocytosis: a diagnostic accuracy study. Hematol Oncol. 2021;39:114-122. doi:10.1002 /hon.2819
- Sakai H, Otsubo S, Miura T, et al. Hemophagocytic syndrome presenting with a facial erythema in a patient with systemic lupus erythematosus. J Am Acad Dermatol. 2007;57(5 Suppl):S111-S114. doi:10.1016/j .jaad.2006.11.024
- Chung SM, Song JY, Kim W, et al. Dengue-associated hemophagocytic lymphohistiocytosis in an adult: a case report and literature review. Medicine (Baltimore). 2017;96:e6159. doi:10.1097/md.0000000000006159
- Esmaili H, Rahmani O, Fouladi RF. Hemophagocytic syndrome in patients with unexplained cytopenia: report of 15 cases. Turk Patoloji Derg. 2013;29:15-18. doi:10.5146/tjpath.2013.01142
- Jiwnani S, Karimundackal G, Kulkarni A, et al. Hemophagocytic syndrome complicating lung resection. Asian Cardiovasc Thorac Ann. 2012;20:341-343. doi:10.1177/0218492311435686
- Lee WJ, Lee DW, Kim CH, et al. Dermatopathic lymphadenitis with generalized erythroderma in a patient with Epstein-Barr virusassociated hemophagocytic lymphohistiocytosis. Am J Dermatopathol. 2010;32:357-361. doi:10.1097/DAD.0b013e3181b2a50f
- Lovisari F, Terzi V, Lippi MG, et al. Hemophagocytic lymphohistiocytosis complicated by multiorgan failure: a case report. Medicine (Baltimore). 2017;96:e9198. doi:10.1097/md.0000000000009198
- Miechowiecki J, Stainer W, Wallner G, et al. Severe complication during remission of Crohn’s disease: hemophagocytic lymphohistiocytosis due to acute cytomegalovirus infection. Z Gastroenterol. 2018;56:259-263. doi:10.1055/s-0043-123999
- Ochoa S, Cheng K, Fleury CM, et al. A 28-year-old woman with fever, rash, and pancytopenia. Allergy Asthma Proc. 2017;38:322-327. doi:10.2500/aap.2017.38.4042
- Tokoro S, Namiki T, Miura K, et al. Chronic active Epstein-Barr virus infection with cutaneous lymphoproliferation: haemophagocytosis in the skin and haemophagocytic syndrome. J Eur Acad Dermatol Venereol. 2018;32:e116-e117. doi:10.1111/jdv.14640
- Tzeng HE, Teng CL, Yang Y, et al. Occult subcutaneous panniculitislike T-cell lymphoma with initial presentations of cellulitis-like skin lesion and fulminant hemophagocytosis. J Formos Med Assoc. 2007;106 (2 Suppl):S55-S59. doi:10.1016/s0929-6646(09)60354-5
- Honjo O, Kubo T, Sugaya F, et al. Severe cytokine release syndrome resulting in purpura fulminans despite successful response to nivolumab therapy in a patient with pleomorphic carcinoma of the lung: a case report. J Immunother Cancer. 2019;7:97. doi:10.1186/s40425- 019-0582-4
- Kao RL, Jacobsen AA, Billington CJ Jr, et al. A case of VEXAS syndrome associated with EBV-associated hemophagocytic lymphohistiocytosis. Blood Cells Mol Dis. 2022;93:102636. doi:10.1016/j .bcmd.2021.102636
- Koga T, Takano K, Horai Y, et al. Sweet’s syndrome complicated by Kikuchi’s disease and hemophagocytic syndrome which presented with retinoic acid-inducible gene-I in both the skin epidermal basal layer and the cervical lymph nodes. Intern Med. 2013;52:1839-1843. doi:10.2169 /internalmedicine.52.9542
- Lin WL, Lin WC, Chiu CS, et al. Paraneoplastic Sweet’s syndrome in a patient with hemophagocytic syndrome. Int J Dermatol. 2008;3:305-307.
- Collins MK, Ho J, Akilov OE. Case 52. A unique presentation of hemophagocytic lymphohistiocytosis with ulcerating papulonodules. In: Akilov OE, ed. Cutaneous Lymphomas: Unusual Cases 3. Springer International Publishing; 2021:126-127.
- Chakrapani A, Avery A, Warnke R. Primary cutaneous gamma delta T-cell lymphoma with brain involvement and hemophagocytic syndrome. Am J Dermatopathol. 2013;35:270-272. doi:10.1097 /DAD.0b013e3182624e98
- Sullivan C, Loghmani A, Thomas K, et al. Hemophagocytic lymphohistiocytosis as the initial presentation of subcutaneous panniculitis-like T-cell lymphoma: a rare case responding to cyclosporine A and steroids. J Investig Med High Impact Case Rep. 2020;8:2324709620981531. doi:10.1177/2324709620981531
- Darmawan G, Salido EO, Concepcion ML, et al. Hemophagocytic lymphohistiocytosis: “a dreadful mimic.” Int J Rheum Dis. 2015; 18:810-812. doi:10.1111/1756-185x.12506
- Maus MV, Leick MB, Cornejo KM, et al. Case 35-2019: a 66-year-old man with pancytopenia and rash. N Engl J Med. 2019;381:1951-1960. doi:10.1056/NEJMcpc1909627
- Chamseddin B, Marks E, Dominguez A, et al. Refractory macrophage activation syndrome in the setting of adult-onset Still disease with hemophagocytic lymphohistiocytosis detected on skin biopsy treated with canakinumab and tacrolimus. J Cutan Pathol. 2019;46:528-531. doi:10.1111/cup.13466
- Bérar A, Ardois S, Walter-Moraux P, et al. Primary varicella-zoster virus infection of the immunocompromised associated with acute pancreatitis and hemophagocytic lymphohistiocytosis: a case report. Medicine (Baltimore). 2021;100:e25351. doi:10.1097 /md.0000000000025351
- Chang CC, Hsiao PJ, Chiu CC, et al. Catastrophic hemophagocytic lymphohistiocytosis in a young man with nephrotic syndrome. Clin Chim Acta. 2015;439:168-171. doi:10.1016/j.cca.2014.10.025
- Kurosawa S, Sekiya N, Fukushima K, et al. Unusual manifestation of disseminated herpes simplex virus type 2 infection associated with pharyngotonsilitis, esophagitis, and hemophagocytic lymphohisitocytosis without genital involvement. BMC Infect Dis. 2019;19:65. doi:10.1186/s12879-019-3721-0
- Saidi W, Gammoudi R, Korbi M, et al. Hemophagocytic lymphohistiocytosis: an unusual complication of leprosy. Int J Dermatol. 2015;54: 1054-1059. doi:10.1111/ijd.12792
- Yamaguchi K, Yamamoto A, Hisano M, et al. Herpes simplex virus 2-associated hemophagocytic lymphohistiocytosis in a pregnant patient. Obstet Gynecol. 2005;105(5 Pt 2):1241-1244. doi:10.1097 /01.AOG.0000157757.54948.9b
- Hayakawa I, Shirasaki F, Ikeda H, et al. Reactive hemophagocytic syndrome in a patient with polyarteritis nodosa associated with Epstein- Barr virus reactivation. Rheumatol Int. 2006;26:573-576. doi:10.1007 /s00296-005-0024-0
- Jeong JY, Park JY, Ham JY, et al. Molecular evidence of parvovirus B19 in the cutaneous polyarteritis nodosa tissue from a patient with parvovirus-associated hemophagocytic syndrome: case report. Medicine (Baltimore). 2020;99:e22079. doi:10.1097 /md.0000000000022079
- Fujita Y, Fukui S, Suzuki T, et al. Anti-MDA5 antibody-positive dermatomyositis complicated by autoimmune-associated hemophagocytic syndrome that was successfully treated with immunosuppressive therapy and plasmapheresis. Intern Med. 2018;57:3473-3478. doi:10.2169 /internalmedicine.1121-18
- Honda M, Moriyama M, Kondo M, et al. Three cases of autoimmune- associated haemophagocytic syndrome in dermatomyositis with anti-MDA5 autoantibody. Scand J Rheumatol. 2020;49:244-246. doi:10 .1080/03009742.2019.1653493
- Jung SY. Hemophagocytic syndrome diagnosed by liver biopsy in a female patient with systemic lupus erythematosus. J Clin Rheumatol. 2013;19:449-451. doi:10.1097/rhu.0000000000000040
- Kerl K, Wolf IH, Cerroni L, et al. Hemophagocytosis in cutaneous autoimmune disease. Am J Dermatopathol. 2015;37:539-543. doi:10.1097 /dad.0000000000000166
- Komiya Y, Saito T, Mizoguchi F, et al. Hemophagocytic syndrome complicated with dermatomyositis controlled successfully with infliximab and conventional therapies. Intern Med. 2017;56:3237-3241. doi:10.2169 /internalmedicine.7966-16
- Lambotte O, Khellaf M, Harmouche H, et al. Characteristics and long-term outcome of 15 episodes of systemic lupus erythematosusassociated hemophagocytic syndrome. Medicine (Baltimore). 2006;85: 169-182. doi:10.1097/01.md.0000224708.62510.d1
- Guitart J, Mangold AR, Martinez-Escala ME, et al. Clinical and pathological characteristics and outcomes among patients with subcutaneous panniculitis-like T-cell lymphoma and related adipotropic lymphoproliferative disorders. JAMA Dermatol. 2022;158:1167-1174. doi:10.1001/jamadermatol.2022.3347
- Hung GD, Chen YH, Chen DY, et al. Subcutaneous panniculitis-like T-cell lymphoma presenting with hemophagocytic lymphohistiocytosis and skin lesions with characteristic high-resolution ultrasonographic findings. Clin Rheumatol. 2007;26:775-778. doi:10.1007/s10067 -005-0193-y
- Jamil A, Nadzri N, Harun N, et al. Primary cutaneous diffuse large B-cell lymphoma leg type presenting with hemophagocytic syndrome. J Am Acad Dermatol. 2012;67:e222-3. doi:10.1016/j.jaad.2012.04.021
- LeBlanc RE, Lansigan F. Unraveling subcutaneous panniculitis-like T-cell lymphoma: an association between subcutaneous panniculitislike T-cell lymphoma, autoimmune lymphoproliferative syndrome, and familial hemophagocytic lymphohistiocytosis. J Cutan Pathol. 2021;48:572-577. doi:10.1111/cup.13863
- Lee DE, Martinez-Escala ME, Serrano LM, et al. Hemophagocytic lymphohistiocytosis in cutaneous T-cell lymphoma. JAMA Dermatol. 2018;154:828-831. doi:10.1001/jamadermatol.2018.1264
- Maejima H, Tanei R, Morioka T, et al. Haemophagocytosis-related intravascular large B-cell lymphoma associated with skin eruption. Acta Derm Venereol. 2011;91:339-340. doi:10.2340/00015555-0981
- Mody A, Cherry D, Georgescu G, et al. A rare case of subcutaneous panniculitis-like T cell lymphoma with hemophagocytic lymphohistiocytosis mimicking cellulitis. Am J Case Rep. 2021;22:E927142. doi:10.12659/ajcr.927142
- Park YJ, Bae HJ, Chang JY, et al. Development of Kaposi sarcoma and hemophagocytic lymphohistiocytosis associated with human herpesvirus 8 in a renal transplant recipient. Korean J Intern Med. 2017;4:750-752.
- Phatak S, Gupta L, Aggarwal A. A young woman with panniculitis and cytopenia who later developed coagulopathy. J Assoc Physicians India. 2016;64:65-67.
- Pongpairoj K, Rerknimitr P, Wititsuwannakul J, et al. Eruptive telangiectasia in a patient with fever and haemophagocytic syndrome. Clin Exp Dermatol. 2016;41:696-698. doi:10.1111/ced.12859
- Shimizu Y, Tanae K, Takahashi N, et al. Primary cutaneous anaplastic large-cell lymphoma presenting with hemophagocytic syndrome: a case report and review of the literature. Leuk Res. 2010;34:263-266. doi:10.1016/j.leukres.2009.07.001
- Sirka CS, Pradhan S, Patra S, et al. Hemophagocytic lymphohistiocytosis: a rare, potentially fatal complication in subcutaneous panniculitis like T cell lymphoma. Indian J Dermatol Venereol Leprol. 2019;5:481-485.
- Chin CK, Hall S, Green C, et al. Secondary haemophagocytic lymphohistiocytosis due to checkpoint inhibitor therapy. Eur J Cancer. 2019;115: 84-87. doi:10.1016/j.ejca.2019.04.026
- Dudda M, Mann C, Heinz J, et al. Hemophagocytic lymphohistiocytosis of a melanoma patient under BRAF/MEK-inhibitor therapy following anti-PD1 inhibitor treatment: a case report and review to the literature. Melanoma Res. 2021;31:81-84. doi:10.1097 /cmr.0000000000000703
- Mizuta H, Nakano E, Takahashi A, et al. Hemophagocytic lymphohistiocytosis with advanced malignant melanoma accompanied by ipilimumab and nivolumab: a case report and literature review. Dermatol Ther. 2020;33:e13321. doi:10.1111/dth.13321
- Satzger I, Ivanyi P, Länger F, et al. Treatment-related hemophagocytic lymphohistiocytosis secondary to checkpoint inhibition with nivolumab plus ipilimumab. Eur J Cancer. 2018;93:150-153. doi:10.1016/j.ejca.2018.01.063
- Michot JM, Lazarovici J, Tieu A, et al. Haematological immune-related adverse events with immune checkpoint inhibitors, how to manage? Eur J Cancer. 2019;122:72-90. doi:10.1016/J.EJCA.2019.07.014
- Choi S, Zhou M, Bahrani E, et al. Rare and fatal complication of immune checkpoint inhibition: a case report of haemophagocytic lymphohistiocytosis with severe lichenoid dermatitis. Br J Haematol. 2021;193:e44-e47. doi:10.1111/BJH.17442
- Yang JJ, Lei DK, Ravi V, et al. Overlap between hemophagocytic lymphohistiocytosis and drug reaction and eosinophilia with systemic symptoms: a review. Int J Dermatol. 2021;60:925-932. doi:10.1111 /ijd.15196
- Go RS, Wester SM. Immunophenotypic and molecular features, clinical outcomes, treatments, and prognostic factors associated with subcutaneous panniculitis-like T-cell lymphoma: a systematic analysis of 156 patients reported in the literature. Cancer. 2004;101:1404-1413. doi:10.1002/cncr.20502
A Systematic Review of Dermatologic Findings in Adults With Hemophagocytic Lymphohistiocytosis
A Systematic Review of Dermatologic Findings in Adults With Hemophagocytic Lymphohistiocytosis
PRACTICE POINTS
- Hemophagocytic lymphohistiocytosis (HLH) is a complex, life-threatening immunologic condition that is associated with various diagnostic tools.
- Physicians who care for patients with HLH should know that skin findings are not uncommon but are largely nonspecific and can be a direct result of HLH itself, systemic complications, or the underlying etiology of the condition.
- There is a myriad of cutaneous findings that can manifest in adult patients with HLH. Awareness of HLH-associated dermatologic conditions and available diagnostic tools among multidisciplinary teams will aid in diagnosis.
Cyclically Bleeding Umbilical Papules
Cyclically Bleeding Umbilical Papules
THE DIAGNOSIS: Cutaneous Endometriosis
On histopathology, a biopsy specimen of an umbilical papule showed a dermal lymphohistiocyticrich infiltrate, hemorrhage, and ectopic endometrial glands consistent with cutaneous endometriosis (CE)(Figure). Cutaneous endometriosis is a rare condition that typically affects females of reproductive potential and is characterized by endometrial glands and stroma within the dermis and hypodermis. Cutaneous endometriosis is classified as primary or secondary. There is no surgical history of the abdomen or pelvis in primary CE. In contrast, a history of abdominopelvic surgery is the defining characteristic of secondary CE, which is more common than primary CE and typically manifests as painful red, brown, or purple papules along preexisting surgical scars of the umbilicus, lower abdomen, or pelvic region.1 Our patient may have developed secondary CE related to the laparoscopic cholecystectomy performed 10 years prior. Surgical excision is considered the definitive treatment for CE, and hormonal therapy with danazol or leuprolide may help ameliorate symptoms.1 Our patient deferred any hormonal or surgical interventions to undergo fertility treatments for pregnancy.
Cyclical bleeding and pain that coincides with menstruation is consistent with CE; however, cyclical symptoms are not always present, which can lead to delayed or incorrect diagnosis. Biopsy and histopathologic analysis are required for definitive diagnosis and are critical for distinguishing CE from other conditions. The differential diagnosis in our patient included pyogenic granuloma, dermatofibrosarcoma protuberans, keloid, and cutaneous metastasis of a primary malignancy. Vascular lesions such as pyogenic granuloma can manifest with bleeding but have a characteristic histopathologic lobular capillary arrangement that was not present in our patient.
Dermatofibrosarcoma protuberans is a rare, slow-growing, malignant soft-tissue sarcoma that most commonly manifests on the trunk, arms, and legs.2 It is characterized by a slow-growing, indurated plaque that often is present for years and may suddenly progress into a smooth, red-brown, multinodular mass. Histopathology typically shows spindle cells infiltrating the dermis and subcutaneous tissue in storiform or whorled pattern with variations based on the tumor stage, as well as diffuse CD34 immunoreactivity.2
Keloids are dense, raised, hyperpigmented, fibrous nodules—sometimes with accompanying telangiectasias—that typically grow secondary to trauma and project past the boundaries of the initial trauma site.1 Keloids are more commonly seen in individuals with darker skin types and tend to grow larger in this population. Histopathology reveals thickened hyalinized collagen bundles, which were not seen in our patient.1
Metastatic skin lesions of the umbilicus are rare but can arise from internal malignancies including cancers of the lung, colon, and breast.3 We considered Sister Mary Joseph nodule, which is caused most commonly by metastasis of a primary gastrointestinal cancer and signifies poor prognosis. The histopathology of metastatic lesions would reveal the presence of atypical cells with cancer-specific markers. Histopathology along with the patient’s personal and family history, a comprehensive review of symptoms, and cancer screening may help with reaching the correct diagnosis.
The average duration between abdominopelvic surgery and onset of secondary CE symptoms is 3.7 to 5.3 years.4 Our patient presented 10 years post surgery and after cessation of oral contraception, which may suggest a potential role of hormonal contraception in delayed CE onset. Diagnosis of CE can be challenging due to atypical signs or symptoms, delayed onset, and lack of awareness among health care professionals. Patients with delayed diagnosis may endure multiple procedures, prolonged physical pain, and emotional distress. Furthermore, 30% to 50% of females with endometriosis experience infertility. Delayed diagnosis of CE compounded with associated age-related increase in oocyte atresia could potentially worsen fecundity as patients age.5 It is important to consider CE in the differential diagnosis of females of reproductive age who present with cyclical bleeding and abdominal or umbilical nodules.
- James WD, Elston D, Treat JR, et al. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2019. Accessed March 19, 2024. https://search.worldcat.org/title/1084979207
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Komurcugil I, Arslan Z, Bal ZI, et al. Cutaneous metastases different clinical presentations: case series and review of the literature. Dermatol Reports. 2022;15:9553.
- Marras S, Pluchino N, Petignat P, et al. Abdominal wall endometriosis: an 11-year retrospective observational cohort study. Published online September 16, 2019. Eur J Obstet Gynecol Reprod Biol X.
- Missmer SA, Hankinson SE, Spiegelman D, et al. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
THE DIAGNOSIS: Cutaneous Endometriosis
On histopathology, a biopsy specimen of an umbilical papule showed a dermal lymphohistiocyticrich infiltrate, hemorrhage, and ectopic endometrial glands consistent with cutaneous endometriosis (CE)(Figure). Cutaneous endometriosis is a rare condition that typically affects females of reproductive potential and is characterized by endometrial glands and stroma within the dermis and hypodermis. Cutaneous endometriosis is classified as primary or secondary. There is no surgical history of the abdomen or pelvis in primary CE. In contrast, a history of abdominopelvic surgery is the defining characteristic of secondary CE, which is more common than primary CE and typically manifests as painful red, brown, or purple papules along preexisting surgical scars of the umbilicus, lower abdomen, or pelvic region.1 Our patient may have developed secondary CE related to the laparoscopic cholecystectomy performed 10 years prior. Surgical excision is considered the definitive treatment for CE, and hormonal therapy with danazol or leuprolide may help ameliorate symptoms.1 Our patient deferred any hormonal or surgical interventions to undergo fertility treatments for pregnancy.

Cyclical bleeding and pain that coincides with menstruation is consistent with CE; however, cyclical symptoms are not always present, which can lead to delayed or incorrect diagnosis. Biopsy and histopathologic analysis are required for definitive diagnosis and are critical for distinguishing CE from other conditions. The differential diagnosis in our patient included pyogenic granuloma, dermatofibrosarcoma protuberans, keloid, and cutaneous metastasis of a primary malignancy. Vascular lesions such as pyogenic granuloma can manifest with bleeding but have a characteristic histopathologic lobular capillary arrangement that was not present in our patient.
Dermatofibrosarcoma protuberans is a rare, slow-growing, malignant soft-tissue sarcoma that most commonly manifests on the trunk, arms, and legs.2 It is characterized by a slow-growing, indurated plaque that often is present for years and may suddenly progress into a smooth, red-brown, multinodular mass. Histopathology typically shows spindle cells infiltrating the dermis and subcutaneous tissue in storiform or whorled pattern with variations based on the tumor stage, as well as diffuse CD34 immunoreactivity.2
Keloids are dense, raised, hyperpigmented, fibrous nodules—sometimes with accompanying telangiectasias—that typically grow secondary to trauma and project past the boundaries of the initial trauma site.1 Keloids are more commonly seen in individuals with darker skin types and tend to grow larger in this population. Histopathology reveals thickened hyalinized collagen bundles, which were not seen in our patient.1
Metastatic skin lesions of the umbilicus are rare but can arise from internal malignancies including cancers of the lung, colon, and breast.3 We considered Sister Mary Joseph nodule, which is caused most commonly by metastasis of a primary gastrointestinal cancer and signifies poor prognosis. The histopathology of metastatic lesions would reveal the presence of atypical cells with cancer-specific markers. Histopathology along with the patient’s personal and family history, a comprehensive review of symptoms, and cancer screening may help with reaching the correct diagnosis.
The average duration between abdominopelvic surgery and onset of secondary CE symptoms is 3.7 to 5.3 years.4 Our patient presented 10 years post surgery and after cessation of oral contraception, which may suggest a potential role of hormonal contraception in delayed CE onset. Diagnosis of CE can be challenging due to atypical signs or symptoms, delayed onset, and lack of awareness among health care professionals. Patients with delayed diagnosis may endure multiple procedures, prolonged physical pain, and emotional distress. Furthermore, 30% to 50% of females with endometriosis experience infertility. Delayed diagnosis of CE compounded with associated age-related increase in oocyte atresia could potentially worsen fecundity as patients age.5 It is important to consider CE in the differential diagnosis of females of reproductive age who present with cyclical bleeding and abdominal or umbilical nodules.
THE DIAGNOSIS: Cutaneous Endometriosis
On histopathology, a biopsy specimen of an umbilical papule showed a dermal lymphohistiocyticrich infiltrate, hemorrhage, and ectopic endometrial glands consistent with cutaneous endometriosis (CE)(Figure). Cutaneous endometriosis is a rare condition that typically affects females of reproductive potential and is characterized by endometrial glands and stroma within the dermis and hypodermis. Cutaneous endometriosis is classified as primary or secondary. There is no surgical history of the abdomen or pelvis in primary CE. In contrast, a history of abdominopelvic surgery is the defining characteristic of secondary CE, which is more common than primary CE and typically manifests as painful red, brown, or purple papules along preexisting surgical scars of the umbilicus, lower abdomen, or pelvic region.1 Our patient may have developed secondary CE related to the laparoscopic cholecystectomy performed 10 years prior. Surgical excision is considered the definitive treatment for CE, and hormonal therapy with danazol or leuprolide may help ameliorate symptoms.1 Our patient deferred any hormonal or surgical interventions to undergo fertility treatments for pregnancy.

Cyclical bleeding and pain that coincides with menstruation is consistent with CE; however, cyclical symptoms are not always present, which can lead to delayed or incorrect diagnosis. Biopsy and histopathologic analysis are required for definitive diagnosis and are critical for distinguishing CE from other conditions. The differential diagnosis in our patient included pyogenic granuloma, dermatofibrosarcoma protuberans, keloid, and cutaneous metastasis of a primary malignancy. Vascular lesions such as pyogenic granuloma can manifest with bleeding but have a characteristic histopathologic lobular capillary arrangement that was not present in our patient.
Dermatofibrosarcoma protuberans is a rare, slow-growing, malignant soft-tissue sarcoma that most commonly manifests on the trunk, arms, and legs.2 It is characterized by a slow-growing, indurated plaque that often is present for years and may suddenly progress into a smooth, red-brown, multinodular mass. Histopathology typically shows spindle cells infiltrating the dermis and subcutaneous tissue in storiform or whorled pattern with variations based on the tumor stage, as well as diffuse CD34 immunoreactivity.2
Keloids are dense, raised, hyperpigmented, fibrous nodules—sometimes with accompanying telangiectasias—that typically grow secondary to trauma and project past the boundaries of the initial trauma site.1 Keloids are more commonly seen in individuals with darker skin types and tend to grow larger in this population. Histopathology reveals thickened hyalinized collagen bundles, which were not seen in our patient.1
Metastatic skin lesions of the umbilicus are rare but can arise from internal malignancies including cancers of the lung, colon, and breast.3 We considered Sister Mary Joseph nodule, which is caused most commonly by metastasis of a primary gastrointestinal cancer and signifies poor prognosis. The histopathology of metastatic lesions would reveal the presence of atypical cells with cancer-specific markers. Histopathology along with the patient’s personal and family history, a comprehensive review of symptoms, and cancer screening may help with reaching the correct diagnosis.
The average duration between abdominopelvic surgery and onset of secondary CE symptoms is 3.7 to 5.3 years.4 Our patient presented 10 years post surgery and after cessation of oral contraception, which may suggest a potential role of hormonal contraception in delayed CE onset. Diagnosis of CE can be challenging due to atypical signs or symptoms, delayed onset, and lack of awareness among health care professionals. Patients with delayed diagnosis may endure multiple procedures, prolonged physical pain, and emotional distress. Furthermore, 30% to 50% of females with endometriosis experience infertility. Delayed diagnosis of CE compounded with associated age-related increase in oocyte atresia could potentially worsen fecundity as patients age.5 It is important to consider CE in the differential diagnosis of females of reproductive age who present with cyclical bleeding and abdominal or umbilical nodules.
- James WD, Elston D, Treat JR, et al. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2019. Accessed March 19, 2024. https://search.worldcat.org/title/1084979207
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Komurcugil I, Arslan Z, Bal ZI, et al. Cutaneous metastases different clinical presentations: case series and review of the literature. Dermatol Reports. 2022;15:9553.
- Marras S, Pluchino N, Petignat P, et al. Abdominal wall endometriosis: an 11-year retrospective observational cohort study. Published online September 16, 2019. Eur J Obstet Gynecol Reprod Biol X.
- Missmer SA, Hankinson SE, Spiegelman D, et al. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
- James WD, Elston D, Treat JR, et al. Andrews Diseases of the Skin: Clinical Dermatology. 13th ed. Elsevier; 2019. Accessed March 19, 2024. https://search.worldcat.org/title/1084979207
- Hao X, Billings SD, Wu F, et al. Dermatofibrosarcoma protuberans: update on the diagnosis and treatment. J Clin Med. 2020;9:1752.
- Komurcugil I, Arslan Z, Bal ZI, et al. Cutaneous metastases different clinical presentations: case series and review of the literature. Dermatol Reports. 2022;15:9553.
- Marras S, Pluchino N, Petignat P, et al. Abdominal wall endometriosis: an 11-year retrospective observational cohort study. Published online September 16, 2019. Eur J Obstet Gynecol Reprod Biol X.
- Missmer SA, Hankinson SE, Spiegelman D, et al. Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors. Am J Epidemiol. 2004;160:784-796.
Cyclically Bleeding Umbilical Papules
Cyclically Bleeding Umbilical Papules
A 38-year-old nulligravid female with menorrhagia and dysmenorrhea presented with cyclical umbilical bleeding of 1 year’s duration. Shortly before the onset of symptoms, the patient had discontinued oral contraceptive therapy with the intent to become pregnant. She had an uncomplicated laparoscopic cholecystectomy 10 years prior, but her medical history was otherwise unremarkable. At the current presentation, physical examination revealed multilobular brown papules with serosanguineous crusting in the umbilicus.
Dupilumab in the Treatment of Pemphigoid Gestationis
Dupilumab in the Treatment of Pemphigoid Gestationis
Pemphigoid gestationis (PG), which manifests in the second or third trimester of pregnancy, is thought to result from an excessive type 2 inflammatory response that leads to the formation of antibodies primarily targeting BP180 antigens with resultant damage to the skin basement membrane.1 Maternal antibodies can be transferred to the fetus, resulting in neonatal pemphigoid with the development of widespread vesicles and bullae.2 Maternal morbidity from placental insufficiency, intrauterine growth restriction, and premature labor are common comorbidities of PG, underscoring the critical need for safe and effective treatments for this condition.3
Systemic corticosteroids currently are the first-line treatment for moderate to severe PG but carry considerable risks to both the mother and fetus, including preterm labor and intrauterine growth restriction.4,5 Dupilumab is approved by the US Food and Drug Administration for moderate to severe atopic dermatitis in children aged 6 months and older. Dupilumab inhibits downstream signaling of IL-4Rα, reducing IL-4 and IL-13. Use of dupilumab to target the type 2 inflammatory response has shown significant promise in the treatment of BP, where it met primary and secondary endpoints in adults with moderate to severe disease, but studies in PG are limited.6-8 There are multiple reports in the literature demonstrating the safety of dupilumab in pregnancy and postpartum,9-27 including a pharmacovigilance report that found no adverse drug reactions from dupilumab reported during pregnancy.9 There also are 4 reports of pregnant patients who were diagnosed with PG and treated with dupilumab, all of whom were initially started on prednisone prior to treatment initiation.9-12 In this article, we report 2 additional cases of dupilumab treatment in patients with PG.
Case Reports
Patient 1—A 39-year-old G5P1 woman presented to the dermatology department at 27.5 weeks’ gestation with a widespread eruption of erythematous, annular, urticarial, edematous papules and plaques on the abdomen of 4 weeks’ duration (Figure 1A). Direct immunofluorescence was positive, indirect immunofluorescence confirmed an IgG-positive epidermal pattern, and serum BP180 levels were elevated, supporting a diagnosis of PG. The patient was prescribed prednisone (60 mg/d) but developed type 1 diabetes mellitus after 1 week of treatment. Following insurance approval, dupilumab therapy was initiated 3 weeks later at a dose of 300 mg subcutaneously every 2 weeks. Rapid and complete resolution of papules and plaques as well as symptomatic relief from pruritus was noted within 2 weeks of treatment (Figure 1B). The prednisone dose was tapered to 2.5 mg every other day at 6 weeks prior to induction of labor; the diabetes resolved 7 weeks after initiation of dupilumab.
At the recommendation of the patient’s high-risk maternal-fetal medicine team, 100 mg of stress-dose hydrocortisone was administered intravenously just prior to delivery to prevent flaring of PG. She delivered a healthy infant at 37 weeks and 3 days’ gestation without bullous disease and was discharged from the hospital the day after delivery on a prednisone dose of 2.5 mg every other day.
The patient subsequently developed localized pruritic papules on the hands and feet at 2 weeks postpartum. Based on shared decision-making and the patient’s concern for the severity of the previous pruritic eruption, prednisone was increased to 10 mg daily for 5 days and then was tapered over 2 weeks without flaring. Dupilumab was continued until 12 weeks postpartum with complete resolution of PG and no further sequelae.
Patient 2—A 30-year-old G1P0 woman presented to the dermatology department at 25 weeks’ gestation with a widespread eruption of 1 week’s duration on the abdomen, hands, thighs, legs, buttocks, and feet that was clinically consistent with PG (Figure 2A). Direct immunofluorescence was positive, indirect immunofluorescence showed an IgG-positive epidermal pattern, and an enzyme-linked immunosorbent assay for BP180 was elevated, confirming a diagnosis of PG. The patient was started on 40 mg of prednisone and topical steroids daily, with improvement of the pruritus but persistence of the eruption after 3 to 4 days. Five days after the initial presentation following expedited insurance approval, dupilumab 300 mg was initiated subcutaneously every 2 weeks along with a slow taper of prednisone to 5 mg, with complete clearance of the eruption within 4 weeks (Figure 2B). She delivered a healthy infant at 38 weeks’ gestation without bullous disease.
In contrast to patient 1, this patient did not receive corticosteroids at the time of delivery and did not experience flaring of her disease. The patient remained on dupilumab 5 weeks postpartum without subsequent recurrence after treatment discontinuation.
Comment
Although a myriad of effective treatments exist for bullous pemphigoid, there are very few options for PG due to the need for treatment during pregnancy. Systemic corticosteroids—the treatment of choice in severe PG disease—are not without risk in pregnancy and complicate assessment of morbidity, as both PG and chronic steroid exposure are associated with preterm labor and intrauterine growth restriction.3
Dupilumab currently is undergoing phase III trials (Clinicaltrials.gov identifiers NCT02277743 and NCT02277769) for the treatment of bullous pemphigoid, with interim reports suggesting efficacy across all primary and key secondary endpoints in moderate to severe disease, including notable steroid-sparing effects.8 In our patients, treatment with dupilumab resulted in resolution of cutaneous disease and was well tolerated, facilitating the tapering of corticosteroids and resolution of type 1 diabetes in patient 1. Although the response to dupilumab in both cases may have been confounded by concomitant steroid administration, which was started due to the severity of symptoms and uncertainty regarding insurance approval, the dose was tapered in both patients after initiation of dupilumab. Patient 1 was given a stress dose of hydrocortisone during delivery and developed a mild flare following delivery, consistent with previous literature.28, 29 Because the flare was localized to the hands and feet, she might have responded to clobetasol in addition to dupilumab, but given the severity of disease at presentation and her concern that it might worsen, low-dose prednisone was added with resolution of the flare within 2 weeks.
Dupilumab dosing regimens have not been studied in a controlled prospective manner for PG. We acknowledge that dupilumab (at least using the conventional atopic dermatitis dosing regimen) may be insufficient as monotherapy to control PG, as both patients received steroids prior to initiation of dupilumab, in part due to concern that the insurance might delay or deny approval. Previous World Health Organization vigilance reporting has suggested that dupilumab appears safe during pregnancy although it lacks pregnancy categorization in the United States due to limited studies in this population.9-28 This observation supports the conclusion that, like bullous pemphigoid, PG also is driven by Th2–mediated inflammation. Treatment with dupilumab may be safe and effective in pregnancy, reducing maternal complications from long-term corticosteroids. Additional studies are needed to confirm these hypotheses.
- Vičić M, MarinoviĆ B. Autoimmune bullous diseases in pregnancy: an overview of pathogenesis, clinical presentations, diagnostics and available therapies. Ital J Dermatol Venerol. 2023;158:99-109. doi:10.23736/ S2784-8671.23.07553-9
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly follow-up of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168- 1172. doi:10.1001/archderm.143.9.1168
- Patsatsi A, Marinovic B, Murrell D. Autoimmune bullous diseases during pregnancy: solving common and uncommon issues. Int J Womens Dermatol. 2019;5:166-170. doi:10.1016/j.ijwd.2019.01.003
- Genovese G, Derlino F, Cerri A, et al. A systematic review of treatment options and clinical outcomes in pemphigoid gestationis. Front Med (Lausanne). 2020;7:604945. doi:10.3389/fmed.2020.604945
- Tavakolpour S, Mirsafaei HS, Delshad S. Management of pemphigus disease in pregnancy. Am J Reprod Immunol. 2017;77. doi:10.1111/aji.12601
- Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol. 2022;13:928621. doi:10.3389/fimmu.2022.928621
- Zhang Y, Xu Q, Chen L, et al. Efficacy and safety of dupilumab in moderate- to-severe bullous pemphigoid. Front Immunol. 2021;12: 738907. doi:10.3389/fimmu.2021.738907
- Dupixent is the first and only biologic to achieve significant improvements in disease remission and symptoms in bullous pemphigoid positive pivotal study. News release. Sanofi. September 11, 2024. Accessed February 17, 2025. https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-11-05-00-00-2944237
- Khamisy-Farah R, Damiani G, Kong JD, et al. Safety profile of dupilumab during pregnancy: a data mining and disproportionality analysis of over 37,000 reports from the WHO individual case safety reporting database (VigiBase™). Eur Rev Med Pharmacol Sci. 2021;25:5448-5451. doi:10.26355/eurrev_202109_26652
- Avallone G, Cavallo F, Tancredi A, et al. Association between maternal dupilumab exposure and pregnancy outcomes in patients with moderate-to-severe atopic dermatitis: a nationwide retrospective cohort study. J Eur Acad Dermatol Venereol. 2024;38:1799 -1808. doi:10.1111/jdv.19794
- Chen RE, Yokoyama CC, Anadkat MJ. Pemphigoid gestationis treated with dupilumab. JAAD Case Rep. 2023;41:10-12. doi:10.1016/ j.jdcr.2023.08.013
- Liu Y, Yuan J, Xia Y, et al. A case of pemphigoid gestationis successfully treated with dupilumab. J Eur Acad Dermatol Venereol. 2023;37:E1164-E1165. doi:10.1111/jdv.19171
- Alvarez Martinez D, Russo G, Fontao L, et al. Successful therapy of pemphigoid gestationis with dupilumab—a new case. J Eur Acad Dermatol Venereol. 2023;37:E752-E753. doi:10.1111/jdv.18911
- Riquelme-Mc Loughlin C, Mascaró JM Jr. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021;46:1578-1579. doi:10.1111/ced.14765
- Adam DN, Gooderham MJ, Beecker JR, et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J Eur Acad Dermatol Venereol. 2023;37:1135-1148. doi:10.1111/jdv.18922
- Akhtar NH, Khosravi-Hafshejani T, Akhtar D, et al. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol. 2022;18:9. doi:10.1186 /s13223-022-00650-w
- Bosma AL, Gerbens LAA, Middelkamp-Hup MA, et al. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol. 2021;46:1089-1092. doi:10.1111 /ced.14725
- Chan TC, Wu NL, Wong LS, et al. Taiwanese dermatological association consensus for the management of atopic dermatitis: a 2020 update. J Formos Med Assoc. 2021;120:429-442. doi:10.101 6/j.jfma.2020.06.008
- Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. 2022;47:960-961. doi:10.1111/ced.15049
- Gracia-Darder I, Pons De Ves J, Reyero Cortina M, et al. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. 2022;35:E15237. doi:10.1111/dth.15237
- Heilskov S, Deleuran MS, Vestergaard C. Immunosuppressive and immunomodulating therapy for atopic dermatitis in pregnancy: an appraisal of the literature. Dermatol Ther (Heidelb). 2020;10:1215-1228. doi:10.1007/s13555-020-00457-w
- Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol. 2020;34:E256-E257. doi:10.1111/jdv.16235
- Kage P, Simon JC, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. 2021;48:E484-E485. doi:10.1111/1346-8138.16033
- Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021;13:248-256. doi:10.1159/000515246
- Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. 2020;6:1051-1052. doi:10.1016/j.jdcr.2020.08.001
- Napolitano M, Ruggiero A, Fontanella G, et al. New emergent therapies for atopic dermatitis: a review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol Ther. 2021;34:E14475. doi:10.1111/dth.14475
- Vestergaard C, Wollenberg A, Barbarot S, et al. European task force on atopic dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol. 2019;33:1644-1659. doi:10.1111/jdv.15709
- Minakawa S, Kaneko T, Rokunohe D, et al. Pemphigoid gestationis with prepartum flare. J Dermatol. 2014;41:850-851. doi:10.1111 /1346-8138.12576
- Baxi LV, Kovilam OP, Collins MH, et al. Recurrent herpes gestationis with postpartum flare: a case report. Am J Obstet Gynecol. 1991;164: 778-780. doi:10.1016/0002-9378(91)90514-r
Pemphigoid gestationis (PG), which manifests in the second or third trimester of pregnancy, is thought to result from an excessive type 2 inflammatory response that leads to the formation of antibodies primarily targeting BP180 antigens with resultant damage to the skin basement membrane.1 Maternal antibodies can be transferred to the fetus, resulting in neonatal pemphigoid with the development of widespread vesicles and bullae.2 Maternal morbidity from placental insufficiency, intrauterine growth restriction, and premature labor are common comorbidities of PG, underscoring the critical need for safe and effective treatments for this condition.3
Systemic corticosteroids currently are the first-line treatment for moderate to severe PG but carry considerable risks to both the mother and fetus, including preterm labor and intrauterine growth restriction.4,5 Dupilumab is approved by the US Food and Drug Administration for moderate to severe atopic dermatitis in children aged 6 months and older. Dupilumab inhibits downstream signaling of IL-4Rα, reducing IL-4 and IL-13. Use of dupilumab to target the type 2 inflammatory response has shown significant promise in the treatment of BP, where it met primary and secondary endpoints in adults with moderate to severe disease, but studies in PG are limited.6-8 There are multiple reports in the literature demonstrating the safety of dupilumab in pregnancy and postpartum,9-27 including a pharmacovigilance report that found no adverse drug reactions from dupilumab reported during pregnancy.9 There also are 4 reports of pregnant patients who were diagnosed with PG and treated with dupilumab, all of whom were initially started on prednisone prior to treatment initiation.9-12 In this article, we report 2 additional cases of dupilumab treatment in patients with PG.
Case Reports
Patient 1—A 39-year-old G5P1 woman presented to the dermatology department at 27.5 weeks’ gestation with a widespread eruption of erythematous, annular, urticarial, edematous papules and plaques on the abdomen of 4 weeks’ duration (Figure 1A). Direct immunofluorescence was positive, indirect immunofluorescence confirmed an IgG-positive epidermal pattern, and serum BP180 levels were elevated, supporting a diagnosis of PG. The patient was prescribed prednisone (60 mg/d) but developed type 1 diabetes mellitus after 1 week of treatment. Following insurance approval, dupilumab therapy was initiated 3 weeks later at a dose of 300 mg subcutaneously every 2 weeks. Rapid and complete resolution of papules and plaques as well as symptomatic relief from pruritus was noted within 2 weeks of treatment (Figure 1B). The prednisone dose was tapered to 2.5 mg every other day at 6 weeks prior to induction of labor; the diabetes resolved 7 weeks after initiation of dupilumab.

At the recommendation of the patient’s high-risk maternal-fetal medicine team, 100 mg of stress-dose hydrocortisone was administered intravenously just prior to delivery to prevent flaring of PG. She delivered a healthy infant at 37 weeks and 3 days’ gestation without bullous disease and was discharged from the hospital the day after delivery on a prednisone dose of 2.5 mg every other day.
The patient subsequently developed localized pruritic papules on the hands and feet at 2 weeks postpartum. Based on shared decision-making and the patient’s concern for the severity of the previous pruritic eruption, prednisone was increased to 10 mg daily for 5 days and then was tapered over 2 weeks without flaring. Dupilumab was continued until 12 weeks postpartum with complete resolution of PG and no further sequelae.
Patient 2—A 30-year-old G1P0 woman presented to the dermatology department at 25 weeks’ gestation with a widespread eruption of 1 week’s duration on the abdomen, hands, thighs, legs, buttocks, and feet that was clinically consistent with PG (Figure 2A). Direct immunofluorescence was positive, indirect immunofluorescence showed an IgG-positive epidermal pattern, and an enzyme-linked immunosorbent assay for BP180 was elevated, confirming a diagnosis of PG. The patient was started on 40 mg of prednisone and topical steroids daily, with improvement of the pruritus but persistence of the eruption after 3 to 4 days. Five days after the initial presentation following expedited insurance approval, dupilumab 300 mg was initiated subcutaneously every 2 weeks along with a slow taper of prednisone to 5 mg, with complete clearance of the eruption within 4 weeks (Figure 2B). She delivered a healthy infant at 38 weeks’ gestation without bullous disease.

In contrast to patient 1, this patient did not receive corticosteroids at the time of delivery and did not experience flaring of her disease. The patient remained on dupilumab 5 weeks postpartum without subsequent recurrence after treatment discontinuation.
Comment
Although a myriad of effective treatments exist for bullous pemphigoid, there are very few options for PG due to the need for treatment during pregnancy. Systemic corticosteroids—the treatment of choice in severe PG disease—are not without risk in pregnancy and complicate assessment of morbidity, as both PG and chronic steroid exposure are associated with preterm labor and intrauterine growth restriction.3
Dupilumab currently is undergoing phase III trials (Clinicaltrials.gov identifiers NCT02277743 and NCT02277769) for the treatment of bullous pemphigoid, with interim reports suggesting efficacy across all primary and key secondary endpoints in moderate to severe disease, including notable steroid-sparing effects.8 In our patients, treatment with dupilumab resulted in resolution of cutaneous disease and was well tolerated, facilitating the tapering of corticosteroids and resolution of type 1 diabetes in patient 1. Although the response to dupilumab in both cases may have been confounded by concomitant steroid administration, which was started due to the severity of symptoms and uncertainty regarding insurance approval, the dose was tapered in both patients after initiation of dupilumab. Patient 1 was given a stress dose of hydrocortisone during delivery and developed a mild flare following delivery, consistent with previous literature.28, 29 Because the flare was localized to the hands and feet, she might have responded to clobetasol in addition to dupilumab, but given the severity of disease at presentation and her concern that it might worsen, low-dose prednisone was added with resolution of the flare within 2 weeks.
Dupilumab dosing regimens have not been studied in a controlled prospective manner for PG. We acknowledge that dupilumab (at least using the conventional atopic dermatitis dosing regimen) may be insufficient as monotherapy to control PG, as both patients received steroids prior to initiation of dupilumab, in part due to concern that the insurance might delay or deny approval. Previous World Health Organization vigilance reporting has suggested that dupilumab appears safe during pregnancy although it lacks pregnancy categorization in the United States due to limited studies in this population.9-28 This observation supports the conclusion that, like bullous pemphigoid, PG also is driven by Th2–mediated inflammation. Treatment with dupilumab may be safe and effective in pregnancy, reducing maternal complications from long-term corticosteroids. Additional studies are needed to confirm these hypotheses.
Pemphigoid gestationis (PG), which manifests in the second or third trimester of pregnancy, is thought to result from an excessive type 2 inflammatory response that leads to the formation of antibodies primarily targeting BP180 antigens with resultant damage to the skin basement membrane.1 Maternal antibodies can be transferred to the fetus, resulting in neonatal pemphigoid with the development of widespread vesicles and bullae.2 Maternal morbidity from placental insufficiency, intrauterine growth restriction, and premature labor are common comorbidities of PG, underscoring the critical need for safe and effective treatments for this condition.3
Systemic corticosteroids currently are the first-line treatment for moderate to severe PG but carry considerable risks to both the mother and fetus, including preterm labor and intrauterine growth restriction.4,5 Dupilumab is approved by the US Food and Drug Administration for moderate to severe atopic dermatitis in children aged 6 months and older. Dupilumab inhibits downstream signaling of IL-4Rα, reducing IL-4 and IL-13. Use of dupilumab to target the type 2 inflammatory response has shown significant promise in the treatment of BP, where it met primary and secondary endpoints in adults with moderate to severe disease, but studies in PG are limited.6-8 There are multiple reports in the literature demonstrating the safety of dupilumab in pregnancy and postpartum,9-27 including a pharmacovigilance report that found no adverse drug reactions from dupilumab reported during pregnancy.9 There also are 4 reports of pregnant patients who were diagnosed with PG and treated with dupilumab, all of whom were initially started on prednisone prior to treatment initiation.9-12 In this article, we report 2 additional cases of dupilumab treatment in patients with PG.
Case Reports
Patient 1—A 39-year-old G5P1 woman presented to the dermatology department at 27.5 weeks’ gestation with a widespread eruption of erythematous, annular, urticarial, edematous papules and plaques on the abdomen of 4 weeks’ duration (Figure 1A). Direct immunofluorescence was positive, indirect immunofluorescence confirmed an IgG-positive epidermal pattern, and serum BP180 levels were elevated, supporting a diagnosis of PG. The patient was prescribed prednisone (60 mg/d) but developed type 1 diabetes mellitus after 1 week of treatment. Following insurance approval, dupilumab therapy was initiated 3 weeks later at a dose of 300 mg subcutaneously every 2 weeks. Rapid and complete resolution of papules and plaques as well as symptomatic relief from pruritus was noted within 2 weeks of treatment (Figure 1B). The prednisone dose was tapered to 2.5 mg every other day at 6 weeks prior to induction of labor; the diabetes resolved 7 weeks after initiation of dupilumab.

At the recommendation of the patient’s high-risk maternal-fetal medicine team, 100 mg of stress-dose hydrocortisone was administered intravenously just prior to delivery to prevent flaring of PG. She delivered a healthy infant at 37 weeks and 3 days’ gestation without bullous disease and was discharged from the hospital the day after delivery on a prednisone dose of 2.5 mg every other day.
The patient subsequently developed localized pruritic papules on the hands and feet at 2 weeks postpartum. Based on shared decision-making and the patient’s concern for the severity of the previous pruritic eruption, prednisone was increased to 10 mg daily for 5 days and then was tapered over 2 weeks without flaring. Dupilumab was continued until 12 weeks postpartum with complete resolution of PG and no further sequelae.
Patient 2—A 30-year-old G1P0 woman presented to the dermatology department at 25 weeks’ gestation with a widespread eruption of 1 week’s duration on the abdomen, hands, thighs, legs, buttocks, and feet that was clinically consistent with PG (Figure 2A). Direct immunofluorescence was positive, indirect immunofluorescence showed an IgG-positive epidermal pattern, and an enzyme-linked immunosorbent assay for BP180 was elevated, confirming a diagnosis of PG. The patient was started on 40 mg of prednisone and topical steroids daily, with improvement of the pruritus but persistence of the eruption after 3 to 4 days. Five days after the initial presentation following expedited insurance approval, dupilumab 300 mg was initiated subcutaneously every 2 weeks along with a slow taper of prednisone to 5 mg, with complete clearance of the eruption within 4 weeks (Figure 2B). She delivered a healthy infant at 38 weeks’ gestation without bullous disease.

In contrast to patient 1, this patient did not receive corticosteroids at the time of delivery and did not experience flaring of her disease. The patient remained on dupilumab 5 weeks postpartum without subsequent recurrence after treatment discontinuation.
Comment
Although a myriad of effective treatments exist for bullous pemphigoid, there are very few options for PG due to the need for treatment during pregnancy. Systemic corticosteroids—the treatment of choice in severe PG disease—are not without risk in pregnancy and complicate assessment of morbidity, as both PG and chronic steroid exposure are associated with preterm labor and intrauterine growth restriction.3
Dupilumab currently is undergoing phase III trials (Clinicaltrials.gov identifiers NCT02277743 and NCT02277769) for the treatment of bullous pemphigoid, with interim reports suggesting efficacy across all primary and key secondary endpoints in moderate to severe disease, including notable steroid-sparing effects.8 In our patients, treatment with dupilumab resulted in resolution of cutaneous disease and was well tolerated, facilitating the tapering of corticosteroids and resolution of type 1 diabetes in patient 1. Although the response to dupilumab in both cases may have been confounded by concomitant steroid administration, which was started due to the severity of symptoms and uncertainty regarding insurance approval, the dose was tapered in both patients after initiation of dupilumab. Patient 1 was given a stress dose of hydrocortisone during delivery and developed a mild flare following delivery, consistent with previous literature.28, 29 Because the flare was localized to the hands and feet, she might have responded to clobetasol in addition to dupilumab, but given the severity of disease at presentation and her concern that it might worsen, low-dose prednisone was added with resolution of the flare within 2 weeks.
Dupilumab dosing regimens have not been studied in a controlled prospective manner for PG. We acknowledge that dupilumab (at least using the conventional atopic dermatitis dosing regimen) may be insufficient as monotherapy to control PG, as both patients received steroids prior to initiation of dupilumab, in part due to concern that the insurance might delay or deny approval. Previous World Health Organization vigilance reporting has suggested that dupilumab appears safe during pregnancy although it lacks pregnancy categorization in the United States due to limited studies in this population.9-28 This observation supports the conclusion that, like bullous pemphigoid, PG also is driven by Th2–mediated inflammation. Treatment with dupilumab may be safe and effective in pregnancy, reducing maternal complications from long-term corticosteroids. Additional studies are needed to confirm these hypotheses.
- Vičić M, MarinoviĆ B. Autoimmune bullous diseases in pregnancy: an overview of pathogenesis, clinical presentations, diagnostics and available therapies. Ital J Dermatol Venerol. 2023;158:99-109. doi:10.23736/ S2784-8671.23.07553-9
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly follow-up of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168- 1172. doi:10.1001/archderm.143.9.1168
- Patsatsi A, Marinovic B, Murrell D. Autoimmune bullous diseases during pregnancy: solving common and uncommon issues. Int J Womens Dermatol. 2019;5:166-170. doi:10.1016/j.ijwd.2019.01.003
- Genovese G, Derlino F, Cerri A, et al. A systematic review of treatment options and clinical outcomes in pemphigoid gestationis. Front Med (Lausanne). 2020;7:604945. doi:10.3389/fmed.2020.604945
- Tavakolpour S, Mirsafaei HS, Delshad S. Management of pemphigus disease in pregnancy. Am J Reprod Immunol. 2017;77. doi:10.1111/aji.12601
- Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol. 2022;13:928621. doi:10.3389/fimmu.2022.928621
- Zhang Y, Xu Q, Chen L, et al. Efficacy and safety of dupilumab in moderate- to-severe bullous pemphigoid. Front Immunol. 2021;12: 738907. doi:10.3389/fimmu.2021.738907
- Dupixent is the first and only biologic to achieve significant improvements in disease remission and symptoms in bullous pemphigoid positive pivotal study. News release. Sanofi. September 11, 2024. Accessed February 17, 2025. https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-11-05-00-00-2944237
- Khamisy-Farah R, Damiani G, Kong JD, et al. Safety profile of dupilumab during pregnancy: a data mining and disproportionality analysis of over 37,000 reports from the WHO individual case safety reporting database (VigiBase™). Eur Rev Med Pharmacol Sci. 2021;25:5448-5451. doi:10.26355/eurrev_202109_26652
- Avallone G, Cavallo F, Tancredi A, et al. Association between maternal dupilumab exposure and pregnancy outcomes in patients with moderate-to-severe atopic dermatitis: a nationwide retrospective cohort study. J Eur Acad Dermatol Venereol. 2024;38:1799 -1808. doi:10.1111/jdv.19794
- Chen RE, Yokoyama CC, Anadkat MJ. Pemphigoid gestationis treated with dupilumab. JAAD Case Rep. 2023;41:10-12. doi:10.1016/ j.jdcr.2023.08.013
- Liu Y, Yuan J, Xia Y, et al. A case of pemphigoid gestationis successfully treated with dupilumab. J Eur Acad Dermatol Venereol. 2023;37:E1164-E1165. doi:10.1111/jdv.19171
- Alvarez Martinez D, Russo G, Fontao L, et al. Successful therapy of pemphigoid gestationis with dupilumab—a new case. J Eur Acad Dermatol Venereol. 2023;37:E752-E753. doi:10.1111/jdv.18911
- Riquelme-Mc Loughlin C, Mascaró JM Jr. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021;46:1578-1579. doi:10.1111/ced.14765
- Adam DN, Gooderham MJ, Beecker JR, et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J Eur Acad Dermatol Venereol. 2023;37:1135-1148. doi:10.1111/jdv.18922
- Akhtar NH, Khosravi-Hafshejani T, Akhtar D, et al. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol. 2022;18:9. doi:10.1186 /s13223-022-00650-w
- Bosma AL, Gerbens LAA, Middelkamp-Hup MA, et al. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol. 2021;46:1089-1092. doi:10.1111 /ced.14725
- Chan TC, Wu NL, Wong LS, et al. Taiwanese dermatological association consensus for the management of atopic dermatitis: a 2020 update. J Formos Med Assoc. 2021;120:429-442. doi:10.101 6/j.jfma.2020.06.008
- Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. 2022;47:960-961. doi:10.1111/ced.15049
- Gracia-Darder I, Pons De Ves J, Reyero Cortina M, et al. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. 2022;35:E15237. doi:10.1111/dth.15237
- Heilskov S, Deleuran MS, Vestergaard C. Immunosuppressive and immunomodulating therapy for atopic dermatitis in pregnancy: an appraisal of the literature. Dermatol Ther (Heidelb). 2020;10:1215-1228. doi:10.1007/s13555-020-00457-w
- Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol. 2020;34:E256-E257. doi:10.1111/jdv.16235
- Kage P, Simon JC, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. 2021;48:E484-E485. doi:10.1111/1346-8138.16033
- Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021;13:248-256. doi:10.1159/000515246
- Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. 2020;6:1051-1052. doi:10.1016/j.jdcr.2020.08.001
- Napolitano M, Ruggiero A, Fontanella G, et al. New emergent therapies for atopic dermatitis: a review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol Ther. 2021;34:E14475. doi:10.1111/dth.14475
- Vestergaard C, Wollenberg A, Barbarot S, et al. European task force on atopic dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol. 2019;33:1644-1659. doi:10.1111/jdv.15709
- Minakawa S, Kaneko T, Rokunohe D, et al. Pemphigoid gestationis with prepartum flare. J Dermatol. 2014;41:850-851. doi:10.1111 /1346-8138.12576
- Baxi LV, Kovilam OP, Collins MH, et al. Recurrent herpes gestationis with postpartum flare: a case report. Am J Obstet Gynecol. 1991;164: 778-780. doi:10.1016/0002-9378(91)90514-r
- Vičić M, MarinoviĆ B. Autoimmune bullous diseases in pregnancy: an overview of pathogenesis, clinical presentations, diagnostics and available therapies. Ital J Dermatol Venerol. 2023;158:99-109. doi:10.23736/ S2784-8671.23.07553-9
- Aoyama Y, Asai K, Hioki K, et al. Herpes gestationis in a mother and newborn: immunoclinical perspectives based on a weekly follow-up of the enzyme-linked immunosorbent assay index of a bullous pemphigoid antigen noncollagenous domain. Arch Dermatol. 2007;143:1168- 1172. doi:10.1001/archderm.143.9.1168
- Patsatsi A, Marinovic B, Murrell D. Autoimmune bullous diseases during pregnancy: solving common and uncommon issues. Int J Womens Dermatol. 2019;5:166-170. doi:10.1016/j.ijwd.2019.01.003
- Genovese G, Derlino F, Cerri A, et al. A systematic review of treatment options and clinical outcomes in pemphigoid gestationis. Front Med (Lausanne). 2020;7:604945. doi:10.3389/fmed.2020.604945
- Tavakolpour S, Mirsafaei HS, Delshad S. Management of pemphigus disease in pregnancy. Am J Reprod Immunol. 2017;77. doi:10.1111/aji.12601
- Cao P, Xu W, Zhang L. Rituximab, omalizumab, and dupilumab treatment outcomes in bullous pemphigoid: a systematic review. Front Immunol. 2022;13:928621. doi:10.3389/fimmu.2022.928621
- Zhang Y, Xu Q, Chen L, et al. Efficacy and safety of dupilumab in moderate- to-severe bullous pemphigoid. Front Immunol. 2021;12: 738907. doi:10.3389/fimmu.2021.738907
- Dupixent is the first and only biologic to achieve significant improvements in disease remission and symptoms in bullous pemphigoid positive pivotal study. News release. Sanofi. September 11, 2024. Accessed February 17, 2025. https://www.sanofi.com/en/media-room/press-releases/2024/2024-09-11-05-00-00-2944237
- Khamisy-Farah R, Damiani G, Kong JD, et al. Safety profile of dupilumab during pregnancy: a data mining and disproportionality analysis of over 37,000 reports from the WHO individual case safety reporting database (VigiBase™). Eur Rev Med Pharmacol Sci. 2021;25:5448-5451. doi:10.26355/eurrev_202109_26652
- Avallone G, Cavallo F, Tancredi A, et al. Association between maternal dupilumab exposure and pregnancy outcomes in patients with moderate-to-severe atopic dermatitis: a nationwide retrospective cohort study. J Eur Acad Dermatol Venereol. 2024;38:1799 -1808. doi:10.1111/jdv.19794
- Chen RE, Yokoyama CC, Anadkat MJ. Pemphigoid gestationis treated with dupilumab. JAAD Case Rep. 2023;41:10-12. doi:10.1016/ j.jdcr.2023.08.013
- Liu Y, Yuan J, Xia Y, et al. A case of pemphigoid gestationis successfully treated with dupilumab. J Eur Acad Dermatol Venereol. 2023;37:E1164-E1165. doi:10.1111/jdv.19171
- Alvarez Martinez D, Russo G, Fontao L, et al. Successful therapy of pemphigoid gestationis with dupilumab—a new case. J Eur Acad Dermatol Venereol. 2023;37:E752-E753. doi:10.1111/jdv.18911
- Riquelme-Mc Loughlin C, Mascaró JM Jr. Treatment of pemphigoid gestationis with dupilumab. Clin Exp Dermatol. 2021;46:1578-1579. doi:10.1111/ced.14765
- Adam DN, Gooderham MJ, Beecker JR, et al. Expert consensus on the systemic treatment of atopic dermatitis in special populations. J Eur Acad Dermatol Venereol. 2023;37:1135-1148. doi:10.1111/jdv.18922
- Akhtar NH, Khosravi-Hafshejani T, Akhtar D, et al. The use of dupilumab in severe atopic dermatitis during pregnancy: a case report. Allergy Asthma Clin Immunol. 2022;18:9. doi:10.1186 /s13223-022-00650-w
- Bosma AL, Gerbens LAA, Middelkamp-Hup MA, et al. Paternal and maternal use of dupilumab in patients with atopic dermatitis: a case series. Clin Exp Dermatol. 2021;46:1089-1092. doi:10.1111 /ced.14725
- Chan TC, Wu NL, Wong LS, et al. Taiwanese dermatological association consensus for the management of atopic dermatitis: a 2020 update. J Formos Med Assoc. 2021;120:429-442. doi:10.101 6/j.jfma.2020.06.008
- Costley M, Murphy B. Severe atopic dermatitis treated successfully with dupilumab throughout pregnancy. Clin Exp Dermatol. 2022;47:960-961. doi:10.1111/ced.15049
- Gracia-Darder I, Pons De Ves J, Reyero Cortina M, et al. Patient with atopic dermatitis, hyper IgE syndrome and ulcerative colitis, treated successfully with dupilumab during pregnancy. Dermatol Ther. 2022;35:E15237. doi:10.1111/dth.15237
- Heilskov S, Deleuran MS, Vestergaard C. Immunosuppressive and immunomodulating therapy for atopic dermatitis in pregnancy: an appraisal of the literature. Dermatol Ther (Heidelb). 2020;10:1215-1228. doi:10.1007/s13555-020-00457-w
- Kage P, Simon JC, Treudler R. A case of atopic eczema treated safely with dupilumab during pregnancy and lactation. J Eur Acad Dermatol Venereol. 2020;34:E256-E257. doi:10.1111/jdv.16235
- Kage P, Simon JC, Treudler R. Case of atopic eczema treated with dupilumab throughout conception, pregnancy, and lactation. J Dermatol. 2021;48:E484-E485. doi:10.1111/1346-8138.16033
- Lobo Y, Lee RC, Spelman L. Atopic dermatitis treated safely with dupilumab during pregnancy: a case report and review of the literature. Case Rep Dermatol. 2021;13:248-256. doi:10.1159/000515246
- Mian M, Dunlap R, Simpson E. Dupilumab for the treatment of severe atopic dermatitis in a pregnant patient: a case report. JAAD Case Rep. 2020;6:1051-1052. doi:10.1016/j.jdcr.2020.08.001
- Napolitano M, Ruggiero A, Fontanella G, et al. New emergent therapies for atopic dermatitis: a review of safety profile with respect to female fertility, pregnancy, and breastfeeding. Dermatol Ther. 2021;34:E14475. doi:10.1111/dth.14475
- Vestergaard C, Wollenberg A, Barbarot S, et al. European task force on atopic dermatitis position paper: treatment of parental atopic dermatitis during preconception, pregnancy and lactation period. J Eur Acad Dermatol Venereol. 2019;33:1644-1659. doi:10.1111/jdv.15709
- Minakawa S, Kaneko T, Rokunohe D, et al. Pemphigoid gestationis with prepartum flare. J Dermatol. 2014;41:850-851. doi:10.1111 /1346-8138.12576
- Baxi LV, Kovilam OP, Collins MH, et al. Recurrent herpes gestationis with postpartum flare: a case report. Am J Obstet Gynecol. 1991;164: 778-780. doi:10.1016/0002-9378(91)90514-r
Dupilumab in the Treatment of Pemphigoid Gestationis
Dupilumab in the Treatment of Pemphigoid Gestationis
PRACTICE POINTS
- Dupilumab inhibits the IL-4Rα subunit, which is bound by IL‐4 and IL‐13, thereby reducing type 2 inflammation associated with pemphigoid gestationis (PG).
- Dupilumab may reduce the dose and duration of systemic corticosteroid therapy for PG, and its use in the second and third trimesters of pregnancy has been supported by emerging safety data.
Managing Contact Dermatitis Related to Amputee Care
Managing Contact Dermatitis Related to Amputee Care
Amputees who use prosthetic devices are particularly susceptible to contact dermatitis due to moisture, irritation, and prolonged contact with components of the device. Contact dermatitis accounts for approximately one-third of the dermatoses encountered by amputees who wear a prosthesis.1 Diagnosing allergic contact dermatitis (ACD) and irritant contact dermatitis (ICD) is challenging due to errors of omission from the differential and the substantial clinical overlap with other eczematous dermatoses. Diagnosis relies on patient history, clinical examination, exposure assessment, diagnostic testing, and a high index of suspicion. Conventionally, ACD comprises approximately 20% of all contact dermatitis cases, whereas ICD accounts for 80%.2 Symptoms vary between the 2 conditions, with pruritus more common in ACD and burning and soreness more common in ICD.3 Onset of dermatitis relative to exposure is crucial, with ICD often manifesting more quickly and ACD requiring an initial sensitization phase.4 Additionally, the complexity of ICD as a condition with variable features adds to the diagnostic difficulty, especially when allergens also have irritant effects.
Understanding these 2 primary types of contact dermatitis is crucial for effective management and prevention strategies in amputees who use prosthetics. In this article, we describe common causes of ACD and ICD related to amputee prosthetics and propose a tailored patch testing panel in order to better diagnose ACD in this patient population.
Allergic Contact Dermatitis
Allergic contact dermatitis occurs when the skin comes into contact with a substance to which the individual is sensitized. In amputees who use prosthetics, the socket and sock liner materials are frequent culprits for triggering allergic reactions. Components such as rubber, metals (eg, nickel), adhesives, and various plastic monomers can induce ACD in susceptible individuals. Additionally, chronic friction and sweat augment hapten penetration, increasing the risk of developing ACD.5
Contact allergens (typically small molecules under 500 Da) penetrate the skin, engage dendritic cells, activate T lymphocytes, and trigger the immune response and memory.6 The skin contains a substantial population of memory T cells, with CD8+ T cells in the epidermis and CD4+ T cells in the dermis, expressing markers that facilitate skin reactivity. The balance between effector and regulatory T cells, which can produce suppressive cytokines such as IL-10, promotes clinical tolerance to allergens such as nickel.
Textile-driven ACD presents with a distinct clinical pattern, often manifesting as patchy generalized dermatitis that coincides with sites where garments fit most snugly. This presentation can mimic other forms of dermatitis, such as nummular or asteatotic dermatitis. The skin beneath undergarments such as underwear or prosthetic socks may be spared, as these act as shields from contact allergens. Notably, the face and hands typically are spared unless the patient has a cross-reaction to formaldehyde-based preservatives found in personal care products.4
Allergy to Components of the Prosthetic Socket and Sock Liner
A prosthesis consists of several key components, including a socket, sleeve, liner, and stump shrinker (eFigure 1). The prosthetic socket, custom-made to fit the residual limb, is the upper part of the prosthesis, while the lower part consists of prosthetic components such as joints and terminal devices ordered to meet individual needs. Prosthetic sleeves provide suspension by securely holding the prosthetic limb in place, while liners offer cushioning and protection to the residual limb, enhancing comfort and reducing friction. Stump shrinkers aid in reducing swelling and shaping the residual limb, facilitating a better fit for the prosthetic socket. Together, these components work in harmony to optimize stability, comfort, and functionality for the user, enabling them to navigate daily activities with greater ease and confidence. Common allergens found in components of the socket and sock liner include rubbers and other elastomers, metals, plastics, adhesives, and textiles.
Rubbers and Other Elastomers—Consumables, including liners, knee sleeves, and socks, are tailored to each client and utilize materials such as silicone and natural and synthetic rubbers for comfort and secure fit. Allergic reactions to natural rubber latex, more commonly used in earlier prosthetics, are associated with both type I and type IV hypersensitivity reactions.4 Proteins inherent to natural rubber are overwhelmingly associated with an immediate urticarial eruption, whereas chemical additives used to produce latex are mostly linked to delayed hypersensitivity reactions, manifesting as allergic reactions ranging from mild itching to severe skin blistering.4
Vulcanization is the process of using heat and other accelerators to manufacture rubber. Common rubber accelerators include thiurams (the most common allergen associated with rubbers and other elastomers), carbamates/carba mix, 1,3-diphenylguanidine, and mercaptobenzothiazole.4 Thiourea is an implicated cause of ACD to neoprene rubber.7 These sensitizing chemicals are all included in the North American 80 Comprehensive Series; only thiuram mix, carba mix, and mercaptobenzothiazole are available in the T.R.U.E. TEST (SmartPractice). Sensitization often occurs due to repeated exposure, particularly in individuals who have undergone multiple prosthetic fittings. Many modern prospective liners utilize a medical-grade silicone as an elastomer for its high flexibility; silicone is considered biologically nonreactive and generally is considered a rare cause of ACD.8
Metals—Nickel, a ubiquitous allergen found in metal alloys used in prosthetic hardware, can cause localized itching, redness, and even blistering upon contact with the skin. Other metals, such as cobalt and chromium, also may trigger allergic reactions in susceptible individuals. Though many elastic fitting prosthetic socks contain silver fibers to reduce odors and friction-causing blisters, pure silver used in clothing or jewelry rarely causes dermatitis.4
Plastics and Adhesives—Leg prosthesis sockets typically are finished with the application of varnish, plastics, and/or resins—all potential allergens—to improve the appearance of the device and protect it from external agents.9 Polyester plastics themselves can cause ICD, only rarely leading to ACD.4 Incomplete curing during their manufacture may result in inadvertent exposure to epoxy resins or other phenol- formaldehyde resins such as 4-tert-butylcatechol and 4-tert-butylphenol formaldehyde, demonstrated causes of ACD in amputees.10 Adhesives used in sock liners or tapes to secure prosthetic devices can contain ingredients such as acrylates (a well-known cause of nail allergens) and other formaldehyde resins.4 Additionally, benzophenone commonly is added to paints and rubbers as a UV light absorber, reducing UV degradation and enhancing the material’s durability under light exposure.11
Textiles—Cotton, a common component in prosthetic sock liners, is almost 100% cellulose and typically does not cause ACD; however, synthetic fibers such as polypropylene and elastane (spandex) can elicit allergic reactions.4 Allergy to textiles often is driven by the chemicals used in the manufacturing process, particularly textile finishes, dyes, and formaldehyde resins, which are commonly used as fabric treatments. Disperse dyes are another common cause of allergic reactions. Para-phenylenediamine, a dye found in permanent hair dye and other darkly colored fabrics, is a potent sensitizer that may cross-react with other compounds that also contain similar amine groups, such as ester anesthetics, sunscreens containing para-aminobenzoic acid, other para dyes, and sulfonamides.12 Sweat can exacerbate these reactions by causing allergens to leach out of textiles, increasing skin exposure. Additionally, prosthetics containing leather may trigger allergies to potassium dichromate and other chromium compounds used in the leather-tanning process.12
Allergy to Personal Care Products
Skin protectants and prosthetic cleansers are crucial in dermatologic care for amputees, working together to safeguard the skin and maintain prosthetic hygiene. Skin protectants form a barrier against irritation, friction, and moisture, protecting the residual limb from damage and enhancing comfort and mobility. Meanwhile, prosthetic cleansers remove sweat, oils, and bacteria from the prosthetic socket, reducing the risk of infections and odors and ensuring the longevity and optimal function of the prosthetic device. Together, they support skin health, comfort, and overall quality of life for amputees.
The socket should be cleaned with warm water prior to use, but more importantly, immediately after removing the prosthesis. If cleaning products are used at night, residual haptens may remain on the device, increasing the risk of sensitization. Common contact irritants found in personal care products utilized in amputee care include sulfates, surfactants, preservatives, and fragrances (eTable 1).4 Additionally, common household cleaners and disinfectants can damage the prosthesis, leading to breakdown and the release of the monomers, precipitating ACD.
Patch Testing to Identify Causative Allergens
Patch testing is a valuable tool for identifying specific allergens responsible for ACD in amputees. This procedure involves applying small amounts of suspected allergens to the patient’s skin under occlusion and leaving the patches in place for 48 hours. After removal, the skin is assessed for reactions at 48 hours, with additional assessments conducted according to International Contact Dermatitis Research Group guidelines, typically at 72 and 96 hours, to identify delayed responses. This diagnostic approach helps pinpoint the substances to which the individual is allergic, enabling targeted avoidance strategies and treatment recommendations. Two widely used patch tests—the T.R.U.E. TEST, a preassembled patch test encompassing 35 allergens, and the North American 80 Comprehensive Series, which includes 80 allergens—demonstrate a sensitivity range between 70% and 80%.13,14 eTable 2 shows a recommended custom contact dermatitis panel to assess the most common causes of ACD related to amputee care.
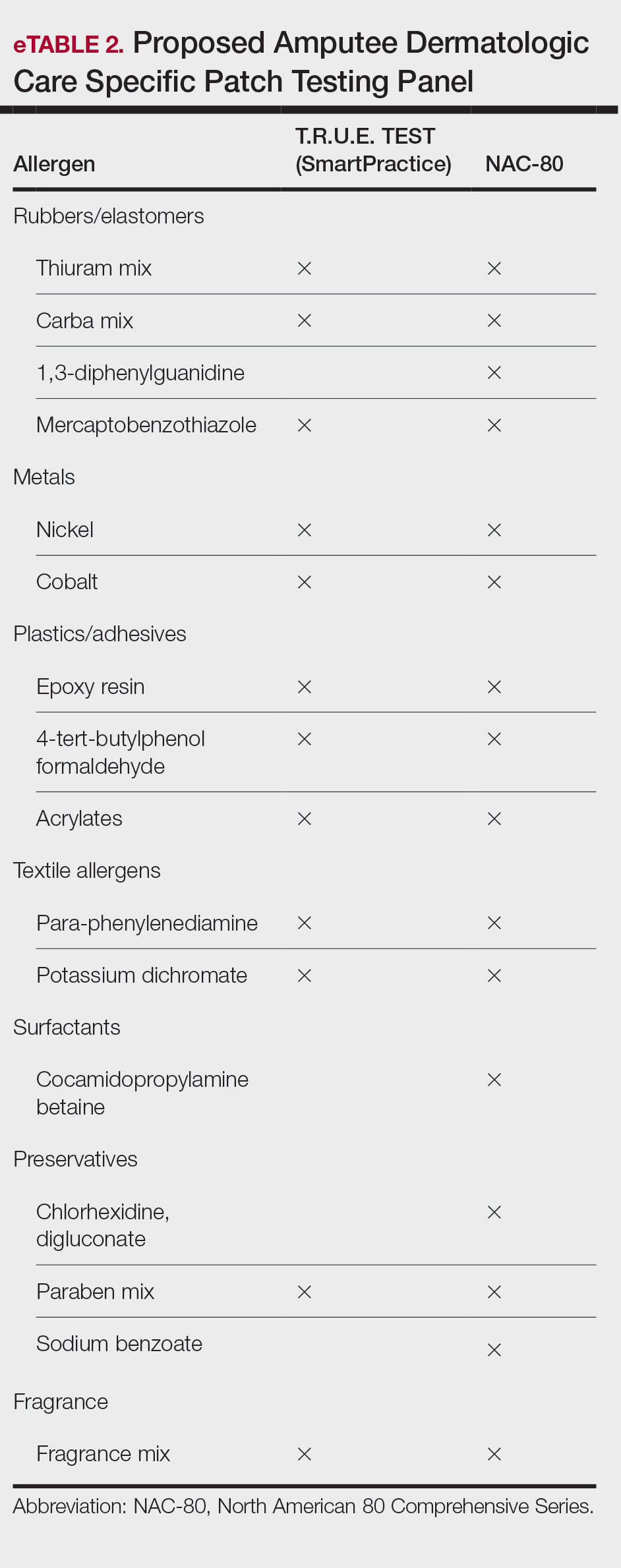
Irritant Contact Dermatitis
Irritant contact dermatitis occurs when the skin’s protective barrier is damaged by repeated exposure to a particular irritant. In amputees, perspiration, friction, and pressure from prosthetic devices can exacerbate irritant reactions, leading to skin maceration, breakdown, and increased transepidermal penetration. Sweat accumulation within the prosthetic socket creates a moist environment conducive to ICD. The combination of sweat and friction can strip the skin of its natural oils, leading to dryness, chafing, and maceration. Continuous exposure to moisture also can exacerbate existing dermatitis and compromise skin integrity.4 Additionally, chronic irritation may increase transepidermal penetration of haptens, potentiating the development of ACD.15
Management of ICD in amputees involves a combination of treatments aimed at reducing friction, reducing sweating, and restoring barrier protection. Strategies to minimize mechanical trauma to the skin include ensuring proper socket fit, managing moisture, and protecting the skin. Using moisture-wicking sock liners and breathable prosthetic materials can help keep the skin dry. Topical antiperspirants containing aluminum chloride or similar compounds that help to block sweat glands often are the first line of treatment. Oral anticholinergics may be prescribed to reduce overall sweating, though they can have systemic side effects. Iontophoresis, a procedure where the affected area is exposed to a mild electrical current, can also be effective, especially for sweating of the hands and feet, though its application in amputees might be more limited.14
Recently, 2 treatments have emerged as options for managing excessive sweating (hyperhidrosis) in amputees: botulinum toxin injections and laser hair removal. By inhibiting the release of acetylcholine from sweat glands, botulinum toxin effectively reduces sweat production, thereby alleviating perspiration-induced skin irritation. Approximately 2 to 3 units of botulinum toxin at a dilution of 100 units in 1 mL of bacteriostatic saline 0.9% are injected transdermally at 1-cm intervals in a circumferential pattern on the skin covered by the prosthesis socket (typically a total of 300-500 units are utilized in the procedure)(eFigure 2).16 Laser hair removal can assist amputees with hyperhidrosis by reducing hair in the residual limb area, which decreases sweat retention and the potential for skin irritation due to friction.
Final Thoughts
In amputee dermatologic care, individuals with limb loss are particularly prone to contact dermatitis due to moisture, friction, and prolonged contact with prosthetic components. Diagnosing ACD and ICD is challenging due to overlapping symptoms and the potential for simultaneous occurrence. Distinguishing between these conditions is crucial for effective management. Understanding their causes, particularly in relation to prosthetic use, is essential for developing targeted prevention and treatment strategies, including the use of tailored patch testing panels to better diagnose ACD in amputees.
- Lyon CC, Kulkarni J, Zimersonc E, et al. Skin disorders in amputees. J Am Acad Dermatol. 2000;42:501-507.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2018;56:99-109.
- Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis: A Practical Approach. Springer; 2021:57-92.
- Fisher AA, Rietschel RL, Fowler JF. Fisher’s Contact Dermatitis. BC Decker Inc; 2008.
- Johansen JD, Frosch PJ, Lepoittevin JP. Contact Dermatitis. Springer; 2010:43-90.
- Eisen HN, Orris L, Belman S. Elicitation of delayed allergic skin reactions with haptens: the dependence of elicitation on hapten combination with protein. J Exp Med. 1952;95:473-487.
- Johnson R. Wrist dermatitis: contact allergy to neoprene in a keyboard wrist rest. Am J Contact Dermat. 1997;8:172-174.
- Adams RM. Occupational Skin Disease. WB Saunders; 1999:501-551.
- Requena L, Vázquez F, Requena C, et al. Epoxy dermatitis of an amputation stump. Contact Dermatitis. 1986;14:320.
- Freeman S. Contact dermatitis of a limb stump caused by p-tertiary butyl catechol in the artificial limb. Contact Dermatitis. 1986;14:68-69.
- Heurung AR, Raju SI, Warshaw EM. Benzophenones. Dermatitis. 2014;25:3-10.
- Manneschi V, Palmerio B, Pauluzzi P, et al. Contact dermatitis from myoelectric prostheses. Contact Dermatitis. 1989;21:116-117.
- Heinrich D, Altmeyer P, Brasch J. “New” techniques for more sensitive patch testing? J Dtsch Dermatol Ges. 2011;9:889-896.
- James WD. Contact dermatitis update. Presented at: Walter Reed National Military Medical Center; April 18, 2024.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486.
Amputees who use prosthetic devices are particularly susceptible to contact dermatitis due to moisture, irritation, and prolonged contact with components of the device. Contact dermatitis accounts for approximately one-third of the dermatoses encountered by amputees who wear a prosthesis.1 Diagnosing allergic contact dermatitis (ACD) and irritant contact dermatitis (ICD) is challenging due to errors of omission from the differential and the substantial clinical overlap with other eczematous dermatoses. Diagnosis relies on patient history, clinical examination, exposure assessment, diagnostic testing, and a high index of suspicion. Conventionally, ACD comprises approximately 20% of all contact dermatitis cases, whereas ICD accounts for 80%.2 Symptoms vary between the 2 conditions, with pruritus more common in ACD and burning and soreness more common in ICD.3 Onset of dermatitis relative to exposure is crucial, with ICD often manifesting more quickly and ACD requiring an initial sensitization phase.4 Additionally, the complexity of ICD as a condition with variable features adds to the diagnostic difficulty, especially when allergens also have irritant effects.
Understanding these 2 primary types of contact dermatitis is crucial for effective management and prevention strategies in amputees who use prosthetics. In this article, we describe common causes of ACD and ICD related to amputee prosthetics and propose a tailored patch testing panel in order to better diagnose ACD in this patient population.
Allergic Contact Dermatitis
Allergic contact dermatitis occurs when the skin comes into contact with a substance to which the individual is sensitized. In amputees who use prosthetics, the socket and sock liner materials are frequent culprits for triggering allergic reactions. Components such as rubber, metals (eg, nickel), adhesives, and various plastic monomers can induce ACD in susceptible individuals. Additionally, chronic friction and sweat augment hapten penetration, increasing the risk of developing ACD.5
Contact allergens (typically small molecules under 500 Da) penetrate the skin, engage dendritic cells, activate T lymphocytes, and trigger the immune response and memory.6 The skin contains a substantial population of memory T cells, with CD8+ T cells in the epidermis and CD4+ T cells in the dermis, expressing markers that facilitate skin reactivity. The balance between effector and regulatory T cells, which can produce suppressive cytokines such as IL-10, promotes clinical tolerance to allergens such as nickel.
Textile-driven ACD presents with a distinct clinical pattern, often manifesting as patchy generalized dermatitis that coincides with sites where garments fit most snugly. This presentation can mimic other forms of dermatitis, such as nummular or asteatotic dermatitis. The skin beneath undergarments such as underwear or prosthetic socks may be spared, as these act as shields from contact allergens. Notably, the face and hands typically are spared unless the patient has a cross-reaction to formaldehyde-based preservatives found in personal care products.4
Allergy to Components of the Prosthetic Socket and Sock Liner
A prosthesis consists of several key components, including a socket, sleeve, liner, and stump shrinker (eFigure 1). The prosthetic socket, custom-made to fit the residual limb, is the upper part of the prosthesis, while the lower part consists of prosthetic components such as joints and terminal devices ordered to meet individual needs. Prosthetic sleeves provide suspension by securely holding the prosthetic limb in place, while liners offer cushioning and protection to the residual limb, enhancing comfort and reducing friction. Stump shrinkers aid in reducing swelling and shaping the residual limb, facilitating a better fit for the prosthetic socket. Together, these components work in harmony to optimize stability, comfort, and functionality for the user, enabling them to navigate daily activities with greater ease and confidence. Common allergens found in components of the socket and sock liner include rubbers and other elastomers, metals, plastics, adhesives, and textiles.

Rubbers and Other Elastomers—Consumables, including liners, knee sleeves, and socks, are tailored to each client and utilize materials such as silicone and natural and synthetic rubbers for comfort and secure fit. Allergic reactions to natural rubber latex, more commonly used in earlier prosthetics, are associated with both type I and type IV hypersensitivity reactions.4 Proteins inherent to natural rubber are overwhelmingly associated with an immediate urticarial eruption, whereas chemical additives used to produce latex are mostly linked to delayed hypersensitivity reactions, manifesting as allergic reactions ranging from mild itching to severe skin blistering.4
Vulcanization is the process of using heat and other accelerators to manufacture rubber. Common rubber accelerators include thiurams (the most common allergen associated with rubbers and other elastomers), carbamates/carba mix, 1,3-diphenylguanidine, and mercaptobenzothiazole.4 Thiourea is an implicated cause of ACD to neoprene rubber.7 These sensitizing chemicals are all included in the North American 80 Comprehensive Series; only thiuram mix, carba mix, and mercaptobenzothiazole are available in the T.R.U.E. TEST (SmartPractice). Sensitization often occurs due to repeated exposure, particularly in individuals who have undergone multiple prosthetic fittings. Many modern prospective liners utilize a medical-grade silicone as an elastomer for its high flexibility; silicone is considered biologically nonreactive and generally is considered a rare cause of ACD.8
Metals—Nickel, a ubiquitous allergen found in metal alloys used in prosthetic hardware, can cause localized itching, redness, and even blistering upon contact with the skin. Other metals, such as cobalt and chromium, also may trigger allergic reactions in susceptible individuals. Though many elastic fitting prosthetic socks contain silver fibers to reduce odors and friction-causing blisters, pure silver used in clothing or jewelry rarely causes dermatitis.4
Plastics and Adhesives—Leg prosthesis sockets typically are finished with the application of varnish, plastics, and/or resins—all potential allergens—to improve the appearance of the device and protect it from external agents.9 Polyester plastics themselves can cause ICD, only rarely leading to ACD.4 Incomplete curing during their manufacture may result in inadvertent exposure to epoxy resins or other phenol- formaldehyde resins such as 4-tert-butylcatechol and 4-tert-butylphenol formaldehyde, demonstrated causes of ACD in amputees.10 Adhesives used in sock liners or tapes to secure prosthetic devices can contain ingredients such as acrylates (a well-known cause of nail allergens) and other formaldehyde resins.4 Additionally, benzophenone commonly is added to paints and rubbers as a UV light absorber, reducing UV degradation and enhancing the material’s durability under light exposure.11
Textiles—Cotton, a common component in prosthetic sock liners, is almost 100% cellulose and typically does not cause ACD; however, synthetic fibers such as polypropylene and elastane (spandex) can elicit allergic reactions.4 Allergy to textiles often is driven by the chemicals used in the manufacturing process, particularly textile finishes, dyes, and formaldehyde resins, which are commonly used as fabric treatments. Disperse dyes are another common cause of allergic reactions. Para-phenylenediamine, a dye found in permanent hair dye and other darkly colored fabrics, is a potent sensitizer that may cross-react with other compounds that also contain similar amine groups, such as ester anesthetics, sunscreens containing para-aminobenzoic acid, other para dyes, and sulfonamides.12 Sweat can exacerbate these reactions by causing allergens to leach out of textiles, increasing skin exposure. Additionally, prosthetics containing leather may trigger allergies to potassium dichromate and other chromium compounds used in the leather-tanning process.12
Allergy to Personal Care Products
Skin protectants and prosthetic cleansers are crucial in dermatologic care for amputees, working together to safeguard the skin and maintain prosthetic hygiene. Skin protectants form a barrier against irritation, friction, and moisture, protecting the residual limb from damage and enhancing comfort and mobility. Meanwhile, prosthetic cleansers remove sweat, oils, and bacteria from the prosthetic socket, reducing the risk of infections and odors and ensuring the longevity and optimal function of the prosthetic device. Together, they support skin health, comfort, and overall quality of life for amputees.
The socket should be cleaned with warm water prior to use, but more importantly, immediately after removing the prosthesis. If cleaning products are used at night, residual haptens may remain on the device, increasing the risk of sensitization. Common contact irritants found in personal care products utilized in amputee care include sulfates, surfactants, preservatives, and fragrances (eTable 1).4 Additionally, common household cleaners and disinfectants can damage the prosthesis, leading to breakdown and the release of the monomers, precipitating ACD.

Patch Testing to Identify Causative Allergens
Patch testing is a valuable tool for identifying specific allergens responsible for ACD in amputees. This procedure involves applying small amounts of suspected allergens to the patient’s skin under occlusion and leaving the patches in place for 48 hours. After removal, the skin is assessed for reactions at 48 hours, with additional assessments conducted according to International Contact Dermatitis Research Group guidelines, typically at 72 and 96 hours, to identify delayed responses. This diagnostic approach helps pinpoint the substances to which the individual is allergic, enabling targeted avoidance strategies and treatment recommendations. Two widely used patch tests—the T.R.U.E. TEST, a preassembled patch test encompassing 35 allergens, and the North American 80 Comprehensive Series, which includes 80 allergens—demonstrate a sensitivity range between 70% and 80%.13,14 eTable 2 shows a recommended custom contact dermatitis panel to assess the most common causes of ACD related to amputee care.

Irritant Contact Dermatitis
Irritant contact dermatitis occurs when the skin’s protective barrier is damaged by repeated exposure to a particular irritant. In amputees, perspiration, friction, and pressure from prosthetic devices can exacerbate irritant reactions, leading to skin maceration, breakdown, and increased transepidermal penetration. Sweat accumulation within the prosthetic socket creates a moist environment conducive to ICD. The combination of sweat and friction can strip the skin of its natural oils, leading to dryness, chafing, and maceration. Continuous exposure to moisture also can exacerbate existing dermatitis and compromise skin integrity.4 Additionally, chronic irritation may increase transepidermal penetration of haptens, potentiating the development of ACD.15
Management of ICD in amputees involves a combination of treatments aimed at reducing friction, reducing sweating, and restoring barrier protection. Strategies to minimize mechanical trauma to the skin include ensuring proper socket fit, managing moisture, and protecting the skin. Using moisture-wicking sock liners and breathable prosthetic materials can help keep the skin dry. Topical antiperspirants containing aluminum chloride or similar compounds that help to block sweat glands often are the first line of treatment. Oral anticholinergics may be prescribed to reduce overall sweating, though they can have systemic side effects. Iontophoresis, a procedure where the affected area is exposed to a mild electrical current, can also be effective, especially for sweating of the hands and feet, though its application in amputees might be more limited.14
Recently, 2 treatments have emerged as options for managing excessive sweating (hyperhidrosis) in amputees: botulinum toxin injections and laser hair removal. By inhibiting the release of acetylcholine from sweat glands, botulinum toxin effectively reduces sweat production, thereby alleviating perspiration-induced skin irritation. Approximately 2 to 3 units of botulinum toxin at a dilution of 100 units in 1 mL of bacteriostatic saline 0.9% are injected transdermally at 1-cm intervals in a circumferential pattern on the skin covered by the prosthesis socket (typically a total of 300-500 units are utilized in the procedure)(eFigure 2).16 Laser hair removal can assist amputees with hyperhidrosis by reducing hair in the residual limb area, which decreases sweat retention and the potential for skin irritation due to friction.

Final Thoughts
In amputee dermatologic care, individuals with limb loss are particularly prone to contact dermatitis due to moisture, friction, and prolonged contact with prosthetic components. Diagnosing ACD and ICD is challenging due to overlapping symptoms and the potential for simultaneous occurrence. Distinguishing between these conditions is crucial for effective management. Understanding their causes, particularly in relation to prosthetic use, is essential for developing targeted prevention and treatment strategies, including the use of tailored patch testing panels to better diagnose ACD in amputees.
Amputees who use prosthetic devices are particularly susceptible to contact dermatitis due to moisture, irritation, and prolonged contact with components of the device. Contact dermatitis accounts for approximately one-third of the dermatoses encountered by amputees who wear a prosthesis.1 Diagnosing allergic contact dermatitis (ACD) and irritant contact dermatitis (ICD) is challenging due to errors of omission from the differential and the substantial clinical overlap with other eczematous dermatoses. Diagnosis relies on patient history, clinical examination, exposure assessment, diagnostic testing, and a high index of suspicion. Conventionally, ACD comprises approximately 20% of all contact dermatitis cases, whereas ICD accounts for 80%.2 Symptoms vary between the 2 conditions, with pruritus more common in ACD and burning and soreness more common in ICD.3 Onset of dermatitis relative to exposure is crucial, with ICD often manifesting more quickly and ACD requiring an initial sensitization phase.4 Additionally, the complexity of ICD as a condition with variable features adds to the diagnostic difficulty, especially when allergens also have irritant effects.
Understanding these 2 primary types of contact dermatitis is crucial for effective management and prevention strategies in amputees who use prosthetics. In this article, we describe common causes of ACD and ICD related to amputee prosthetics and propose a tailored patch testing panel in order to better diagnose ACD in this patient population.
Allergic Contact Dermatitis
Allergic contact dermatitis occurs when the skin comes into contact with a substance to which the individual is sensitized. In amputees who use prosthetics, the socket and sock liner materials are frequent culprits for triggering allergic reactions. Components such as rubber, metals (eg, nickel), adhesives, and various plastic monomers can induce ACD in susceptible individuals. Additionally, chronic friction and sweat augment hapten penetration, increasing the risk of developing ACD.5
Contact allergens (typically small molecules under 500 Da) penetrate the skin, engage dendritic cells, activate T lymphocytes, and trigger the immune response and memory.6 The skin contains a substantial population of memory T cells, with CD8+ T cells in the epidermis and CD4+ T cells in the dermis, expressing markers that facilitate skin reactivity. The balance between effector and regulatory T cells, which can produce suppressive cytokines such as IL-10, promotes clinical tolerance to allergens such as nickel.
Textile-driven ACD presents with a distinct clinical pattern, often manifesting as patchy generalized dermatitis that coincides with sites where garments fit most snugly. This presentation can mimic other forms of dermatitis, such as nummular or asteatotic dermatitis. The skin beneath undergarments such as underwear or prosthetic socks may be spared, as these act as shields from contact allergens. Notably, the face and hands typically are spared unless the patient has a cross-reaction to formaldehyde-based preservatives found in personal care products.4
Allergy to Components of the Prosthetic Socket and Sock Liner
A prosthesis consists of several key components, including a socket, sleeve, liner, and stump shrinker (eFigure 1). The prosthetic socket, custom-made to fit the residual limb, is the upper part of the prosthesis, while the lower part consists of prosthetic components such as joints and terminal devices ordered to meet individual needs. Prosthetic sleeves provide suspension by securely holding the prosthetic limb in place, while liners offer cushioning and protection to the residual limb, enhancing comfort and reducing friction. Stump shrinkers aid in reducing swelling and shaping the residual limb, facilitating a better fit for the prosthetic socket. Together, these components work in harmony to optimize stability, comfort, and functionality for the user, enabling them to navigate daily activities with greater ease and confidence. Common allergens found in components of the socket and sock liner include rubbers and other elastomers, metals, plastics, adhesives, and textiles.

Rubbers and Other Elastomers—Consumables, including liners, knee sleeves, and socks, are tailored to each client and utilize materials such as silicone and natural and synthetic rubbers for comfort and secure fit. Allergic reactions to natural rubber latex, more commonly used in earlier prosthetics, are associated with both type I and type IV hypersensitivity reactions.4 Proteins inherent to natural rubber are overwhelmingly associated with an immediate urticarial eruption, whereas chemical additives used to produce latex are mostly linked to delayed hypersensitivity reactions, manifesting as allergic reactions ranging from mild itching to severe skin blistering.4
Vulcanization is the process of using heat and other accelerators to manufacture rubber. Common rubber accelerators include thiurams (the most common allergen associated with rubbers and other elastomers), carbamates/carba mix, 1,3-diphenylguanidine, and mercaptobenzothiazole.4 Thiourea is an implicated cause of ACD to neoprene rubber.7 These sensitizing chemicals are all included in the North American 80 Comprehensive Series; only thiuram mix, carba mix, and mercaptobenzothiazole are available in the T.R.U.E. TEST (SmartPractice). Sensitization often occurs due to repeated exposure, particularly in individuals who have undergone multiple prosthetic fittings. Many modern prospective liners utilize a medical-grade silicone as an elastomer for its high flexibility; silicone is considered biologically nonreactive and generally is considered a rare cause of ACD.8
Metals—Nickel, a ubiquitous allergen found in metal alloys used in prosthetic hardware, can cause localized itching, redness, and even blistering upon contact with the skin. Other metals, such as cobalt and chromium, also may trigger allergic reactions in susceptible individuals. Though many elastic fitting prosthetic socks contain silver fibers to reduce odors and friction-causing blisters, pure silver used in clothing or jewelry rarely causes dermatitis.4
Plastics and Adhesives—Leg prosthesis sockets typically are finished with the application of varnish, plastics, and/or resins—all potential allergens—to improve the appearance of the device and protect it from external agents.9 Polyester plastics themselves can cause ICD, only rarely leading to ACD.4 Incomplete curing during their manufacture may result in inadvertent exposure to epoxy resins or other phenol- formaldehyde resins such as 4-tert-butylcatechol and 4-tert-butylphenol formaldehyde, demonstrated causes of ACD in amputees.10 Adhesives used in sock liners or tapes to secure prosthetic devices can contain ingredients such as acrylates (a well-known cause of nail allergens) and other formaldehyde resins.4 Additionally, benzophenone commonly is added to paints and rubbers as a UV light absorber, reducing UV degradation and enhancing the material’s durability under light exposure.11
Textiles—Cotton, a common component in prosthetic sock liners, is almost 100% cellulose and typically does not cause ACD; however, synthetic fibers such as polypropylene and elastane (spandex) can elicit allergic reactions.4 Allergy to textiles often is driven by the chemicals used in the manufacturing process, particularly textile finishes, dyes, and formaldehyde resins, which are commonly used as fabric treatments. Disperse dyes are another common cause of allergic reactions. Para-phenylenediamine, a dye found in permanent hair dye and other darkly colored fabrics, is a potent sensitizer that may cross-react with other compounds that also contain similar amine groups, such as ester anesthetics, sunscreens containing para-aminobenzoic acid, other para dyes, and sulfonamides.12 Sweat can exacerbate these reactions by causing allergens to leach out of textiles, increasing skin exposure. Additionally, prosthetics containing leather may trigger allergies to potassium dichromate and other chromium compounds used in the leather-tanning process.12
Allergy to Personal Care Products
Skin protectants and prosthetic cleansers are crucial in dermatologic care for amputees, working together to safeguard the skin and maintain prosthetic hygiene. Skin protectants form a barrier against irritation, friction, and moisture, protecting the residual limb from damage and enhancing comfort and mobility. Meanwhile, prosthetic cleansers remove sweat, oils, and bacteria from the prosthetic socket, reducing the risk of infections and odors and ensuring the longevity and optimal function of the prosthetic device. Together, they support skin health, comfort, and overall quality of life for amputees.
The socket should be cleaned with warm water prior to use, but more importantly, immediately after removing the prosthesis. If cleaning products are used at night, residual haptens may remain on the device, increasing the risk of sensitization. Common contact irritants found in personal care products utilized in amputee care include sulfates, surfactants, preservatives, and fragrances (eTable 1).4 Additionally, common household cleaners and disinfectants can damage the prosthesis, leading to breakdown and the release of the monomers, precipitating ACD.

Patch Testing to Identify Causative Allergens
Patch testing is a valuable tool for identifying specific allergens responsible for ACD in amputees. This procedure involves applying small amounts of suspected allergens to the patient’s skin under occlusion and leaving the patches in place for 48 hours. After removal, the skin is assessed for reactions at 48 hours, with additional assessments conducted according to International Contact Dermatitis Research Group guidelines, typically at 72 and 96 hours, to identify delayed responses. This diagnostic approach helps pinpoint the substances to which the individual is allergic, enabling targeted avoidance strategies and treatment recommendations. Two widely used patch tests—the T.R.U.E. TEST, a preassembled patch test encompassing 35 allergens, and the North American 80 Comprehensive Series, which includes 80 allergens—demonstrate a sensitivity range between 70% and 80%.13,14 eTable 2 shows a recommended custom contact dermatitis panel to assess the most common causes of ACD related to amputee care.

Irritant Contact Dermatitis
Irritant contact dermatitis occurs when the skin’s protective barrier is damaged by repeated exposure to a particular irritant. In amputees, perspiration, friction, and pressure from prosthetic devices can exacerbate irritant reactions, leading to skin maceration, breakdown, and increased transepidermal penetration. Sweat accumulation within the prosthetic socket creates a moist environment conducive to ICD. The combination of sweat and friction can strip the skin of its natural oils, leading to dryness, chafing, and maceration. Continuous exposure to moisture also can exacerbate existing dermatitis and compromise skin integrity.4 Additionally, chronic irritation may increase transepidermal penetration of haptens, potentiating the development of ACD.15
Management of ICD in amputees involves a combination of treatments aimed at reducing friction, reducing sweating, and restoring barrier protection. Strategies to minimize mechanical trauma to the skin include ensuring proper socket fit, managing moisture, and protecting the skin. Using moisture-wicking sock liners and breathable prosthetic materials can help keep the skin dry. Topical antiperspirants containing aluminum chloride or similar compounds that help to block sweat glands often are the first line of treatment. Oral anticholinergics may be prescribed to reduce overall sweating, though they can have systemic side effects. Iontophoresis, a procedure where the affected area is exposed to a mild electrical current, can also be effective, especially for sweating of the hands and feet, though its application in amputees might be more limited.14
Recently, 2 treatments have emerged as options for managing excessive sweating (hyperhidrosis) in amputees: botulinum toxin injections and laser hair removal. By inhibiting the release of acetylcholine from sweat glands, botulinum toxin effectively reduces sweat production, thereby alleviating perspiration-induced skin irritation. Approximately 2 to 3 units of botulinum toxin at a dilution of 100 units in 1 mL of bacteriostatic saline 0.9% are injected transdermally at 1-cm intervals in a circumferential pattern on the skin covered by the prosthesis socket (typically a total of 300-500 units are utilized in the procedure)(eFigure 2).16 Laser hair removal can assist amputees with hyperhidrosis by reducing hair in the residual limb area, which decreases sweat retention and the potential for skin irritation due to friction.

Final Thoughts
In amputee dermatologic care, individuals with limb loss are particularly prone to contact dermatitis due to moisture, friction, and prolonged contact with prosthetic components. Diagnosing ACD and ICD is challenging due to overlapping symptoms and the potential for simultaneous occurrence. Distinguishing between these conditions is crucial for effective management. Understanding their causes, particularly in relation to prosthetic use, is essential for developing targeted prevention and treatment strategies, including the use of tailored patch testing panels to better diagnose ACD in amputees.
- Lyon CC, Kulkarni J, Zimersonc E, et al. Skin disorders in amputees. J Am Acad Dermatol. 2000;42:501-507.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2018;56:99-109.
- Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis: A Practical Approach. Springer; 2021:57-92.
- Fisher AA, Rietschel RL, Fowler JF. Fisher’s Contact Dermatitis. BC Decker Inc; 2008.
- Johansen JD, Frosch PJ, Lepoittevin JP. Contact Dermatitis. Springer; 2010:43-90.
- Eisen HN, Orris L, Belman S. Elicitation of delayed allergic skin reactions with haptens: the dependence of elicitation on hapten combination with protein. J Exp Med. 1952;95:473-487.
- Johnson R. Wrist dermatitis: contact allergy to neoprene in a keyboard wrist rest. Am J Contact Dermat. 1997;8:172-174.
- Adams RM. Occupational Skin Disease. WB Saunders; 1999:501-551.
- Requena L, Vázquez F, Requena C, et al. Epoxy dermatitis of an amputation stump. Contact Dermatitis. 1986;14:320.
- Freeman S. Contact dermatitis of a limb stump caused by p-tertiary butyl catechol in the artificial limb. Contact Dermatitis. 1986;14:68-69.
- Heurung AR, Raju SI, Warshaw EM. Benzophenones. Dermatitis. 2014;25:3-10.
- Manneschi V, Palmerio B, Pauluzzi P, et al. Contact dermatitis from myoelectric prostheses. Contact Dermatitis. 1989;21:116-117.
- Heinrich D, Altmeyer P, Brasch J. “New” techniques for more sensitive patch testing? J Dtsch Dermatol Ges. 2011;9:889-896.
- James WD. Contact dermatitis update. Presented at: Walter Reed National Military Medical Center; April 18, 2024.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486.
- Lyon CC, Kulkarni J, Zimersonc E, et al. Skin disorders in amputees. J Am Acad Dermatol. 2000;42:501-507.
- Bains SN, Nash P, Fonacier L. Irritant contact dermatitis. Clin Rev Allergy Immunol. 2018;56:99-109.
- Angelini G, Bonamonte D, Foti C, eds. Clinical Contact Dermatitis: A Practical Approach. Springer; 2021:57-92.
- Fisher AA, Rietschel RL, Fowler JF. Fisher’s Contact Dermatitis. BC Decker Inc; 2008.
- Johansen JD, Frosch PJ, Lepoittevin JP. Contact Dermatitis. Springer; 2010:43-90.
- Eisen HN, Orris L, Belman S. Elicitation of delayed allergic skin reactions with haptens: the dependence of elicitation on hapten combination with protein. J Exp Med. 1952;95:473-487.
- Johnson R. Wrist dermatitis: contact allergy to neoprene in a keyboard wrist rest. Am J Contact Dermat. 1997;8:172-174.
- Adams RM. Occupational Skin Disease. WB Saunders; 1999:501-551.
- Requena L, Vázquez F, Requena C, et al. Epoxy dermatitis of an amputation stump. Contact Dermatitis. 1986;14:320.
- Freeman S. Contact dermatitis of a limb stump caused by p-tertiary butyl catechol in the artificial limb. Contact Dermatitis. 1986;14:68-69.
- Heurung AR, Raju SI, Warshaw EM. Benzophenones. Dermatitis. 2014;25:3-10.
- Manneschi V, Palmerio B, Pauluzzi P, et al. Contact dermatitis from myoelectric prostheses. Contact Dermatitis. 1989;21:116-117.
- Heinrich D, Altmeyer P, Brasch J. “New” techniques for more sensitive patch testing? J Dtsch Dermatol Ges. 2011;9:889-896.
- James WD. Contact dermatitis update. Presented at: Walter Reed National Military Medical Center; April 18, 2024.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Lannan FM, Powell J, Kim GM, et al. Hyperhidrosis of the residual limb: a narrative review of the measurement and treatment of excess perspiration affecting individuals with amputation. Prosthet Orthot Int. 2021;45:477-486.
Managing Contact Dermatitis Related to Amputee Care
Managing Contact Dermatitis Related to Amputee Care
PRACTICE POINTS
- Incorporating a tailored patch testing panel that includes common prosthetic-related allergens (eg, rubber, metals, adhesives) can greatly improve the diagnosis and treatment of allergic vs irritant contact dermatitis in amputees.
- Effective management of irritant contact dermatitis in amputees involves reducing moisture and friction in the prosthetic socket with moisture-wicking liners, ensuring proper fit, and utilizing treatments such as topical antiperspirants and botulinum toxin injections.
Comorbidities and Lifestyle Risk Factors Associated With Scabies Infestation
Comorbidities and Lifestyle Risk Factors Associated With Scabies Infestation
To the Editor:
Scabies infestation, which has been recognized as a neglected tropical disease by the World Health Organization since 2017, is caused by the human itch mite (Sarcoptes scabiei var hominis).1 Infected individuals experience a pruritic papular rash when the mite burrows into the epidermis, where it lives and lays eggs.2,3 Infected individuals also may develop bacterial superinfections if the skin barrier becomes compromised, leading to systemic complications and considerable morbidity.3
In countries with high human development indices, scabies outbreaks are linked to densely populated living conditions, such as those found in nursing homes or prisons.3,4 Scabies also is transmitted via sexual contact in adults. Beyond immunosuppression, little is known about other comorbid conditions or lifestyle risk factors associated with scabies infestation.2 Because scabies can mimic a range of other dermatologic conditions such as folliculitis, atopic dermatitis, and arthropod bites, misdiagnosis is common and can lead to delayed treatment and increased transmission risk.4 In this study, we sought to examine comorbid conditions and/or lifestyle risk factors associated with scabies infestation.
A matched case-control study was performed using the Registered Tier dataset of the National Institutes of Health All of Us Research Program Curated Data Repository version 7, which includes more than 400,000 unique participants aged 18 years or older from across the United States. The All of Us Research Program excludes adults who are unable to consent independently as well as incarcerated populations and children younger than 18 years. Participants diagnosed with scabies were identified using SNOMED code 62752005 and compared to a control group matched 1:4 based on age, sex, and selfidentified race. SNOMED codes also were used to identify various comorbidities and lifestyle risk factors, including depression, bipolar disorder, anxiety, schizophrenia, peripheral vascular disease (PVD), HIV, type 2 diabetes mellitus (T2DM), unsheltered status, tobacco use, difficulty with activities of daily living, insurance status, and any recent travel history. Logistic regression models were used to calculate odds ratios (ORs) and estimate effect sizes, with statistical significance set at P<.05.
We identified 691 cases of scabies infestation and 2073 controls. The average age of the patients diagnosed with scabies was 55.1 years. Seventy percent (481/691) identified as female and 32.4% (224/491) identified as Black or African American. Matched controls were similar for all analyzed demographic characteristics (P=1.0)(eTable 1). Patients diagnosed with scabies were more likely to be unsheltered (OR, 2.33 [95% CI, 1.91-2.85]), use tobacco (OR 1.77 [95% CI, 1.48-2.11]) and have a comorbid diagnosis of HIV (OR, 3.08 [95% CI, 2.03-4.66]), T2DM (OR, 2.05 [95% CI, 1.57- 2.66]) or PVD (OR, 2.06 [95% CI, 1.43-2.97]) compared with controls (P<.001). Psychiatric comorbidities were more common in the patients diagnosed with scabies, including depression (OR, 3.07 [95% CI, 2.54-3.72]), anxiety (OR, 2.48 [95% CI, 2.06-2.98]), bipolar disorder (OR, 3.08 [95% CI, 2.34-4.05]), and schizophrenia (OR, 4.68 [95% CI, 2.93-7.49])(P<.001). Difficulties with activities of daily living, including running errands alone (OR, 2.32 [95% CI, 1.43-3.76]) and concentrating (OR, 5.78; 95% CI, 3.86-8.64), were more prevalent in the scabies group compared to controls (both P<.05). In a multivariate logistic regression model including unsheltered status as a covariate, all associations remained statistically significant (P<.05)(eTable 2).
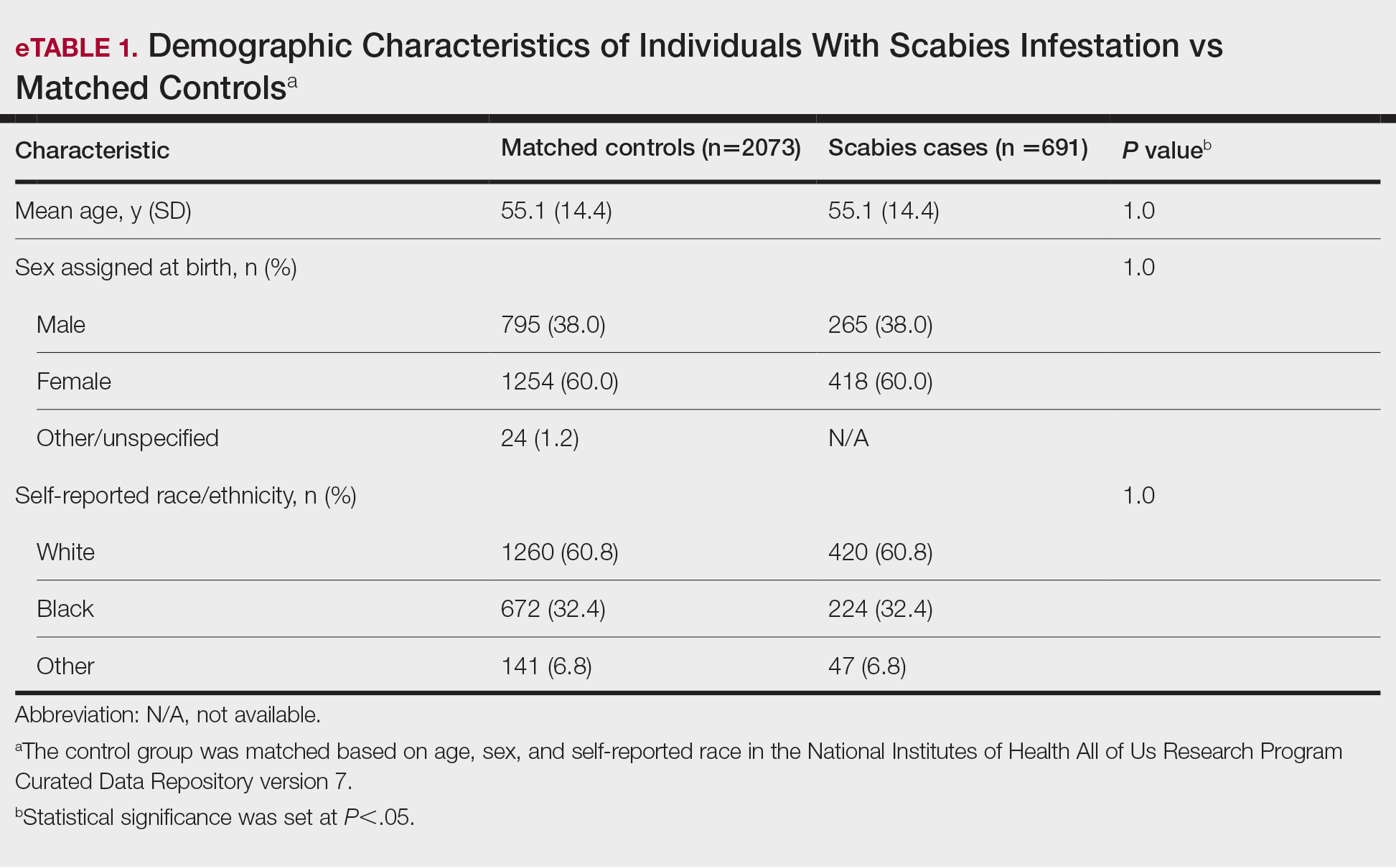
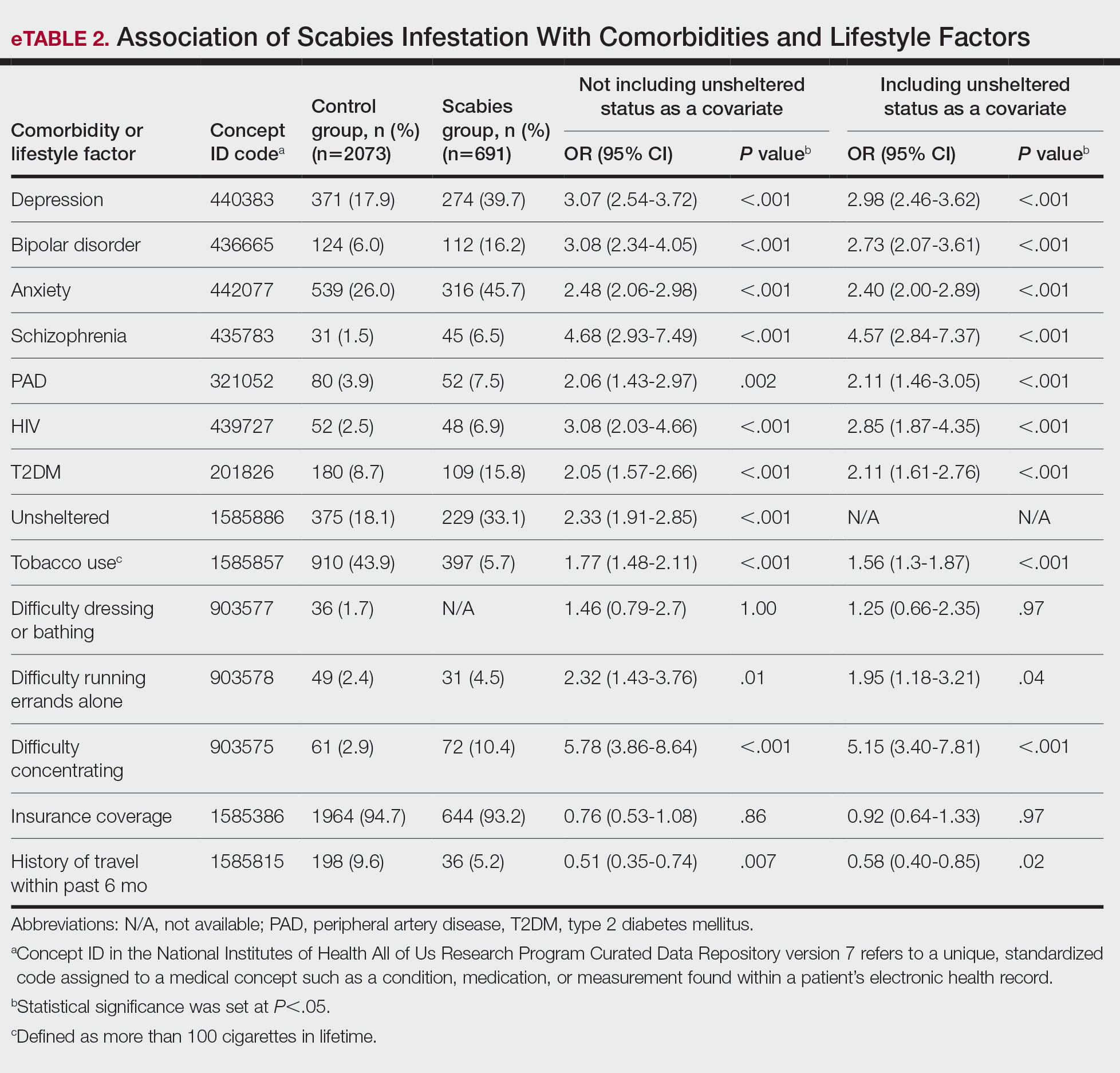
This large diverse study demonstrated an association between scabies infestation and unsheltered status. Previous studies have shown that unsheltered populations are at increased risk for many dermatologic conditions, perhaps due to decreased access to health care and social support, lack of access to hygiene facilities (eg, public showers), and increased prevalence of substance use and psychiatric disorders among this population.5 In a cross-sectional analysis of hospitalized patients, 8.6% of unsheltered patients (n=197) had an ectoparasitic disease (including scabies) compared with 1.0% of patients with stable housing (n=1018), with a 9.43-fold increased risk for ectoparasitic infestation among unsheltered patients (95% CI, 3.79-23.47; P<.001).6 Increased attention to public health initiatives among unsheltered populations— including access to hygiene facilities and increased dermatologic services—are needed, as ectoparasitic infections are both preventable and treatable, and these initiatives could reduce morbidity associated with superimposed bacterial infections for which unsheltered patients are at increased risk.6
Our results also showed that individuals diagnosed with scabies were more likely than the controls to have been diagnosed with HIV, T2DM, and PVD. Our findings are similar to those of a systematic review of immunosuppressive factors associated with crusted scabies (a severe form of scabies infestation) in which 10.2% and 15.7% of patients (n=683) had comorbid HIV and T2DM, respectively.7 A functioning cell-mediated response to scabies mite antigens limits proliferation of the human itch mite; thus, infection with HIV/AIDS, which induces the destruction of CD4+ T cells, limits the immune system’s ability to mount an effective response against these antigens. The association of scabies with T2DM likely is multifactorial; for example, chronic hyperglycemia may lead to immune system impairment, and peripheral neuropathy may reduce the itch sensation, allowing scabies mites to proliferate without removal by scratching.7 In a descriptive epidemiologic study in Japan, 11.7% of patients with scabies (N=857) had comorbid PVD.8 Peripheral vascular disease can lead to the development of ulcers, gangrene, and stasis dermatitis, all of which compromise the skin barrier and increase susceptibility to infection.9 Notably, these associations remained even when unsheltered status was considered as a confounding variable. Because individuals with HIV, T2DM, and PVD may be at higher risk for serious complications of scabies infestation (eg, secondary bacterial infections, invasive group A streptococcal infections), prompt detection and treatment of scabies are crucial in curbing morbidity in these at-risk populations.
Our study also demonstrated that psychiatric comorbidities including depression, anxiety, bipolar disorder, and schizophrenia were associated with scabies infestation, even when controlling for unsheltered status, which may have a bidirectional relationship with mental health disorders.10 In a cross-sectional study of 83 adult patients diagnosed with scabies, 72.2% (60/83) reported moderate to extremely large effect of scabies infestation on quality of life using the Dermatology Life Quality Index, and these scores positively correlated with increased Beck Depression Scale and Beck Anxiety Scale scores (rs=0.448 and rs=0.456 0.456, respectively; both P=.000). The results of this study suggest that scabies negatively impacts quality of life, which might increase symptoms of depression and anxiety.11
Studies are needed to assess whether patients with pre-existing depression and anxiety face increased risk for scabies infestation. In a retrospective case-control study using data from the National Health Insurance Research Database of Taiwan, 0.8% (58/7096) of patients with scabies (n=7096) and 0.4% of controls (n=28,375) were newly diagnosed with bipolar disorder over a 7-year period, indicating a 1.55-fold increased risk for bipolar disorder in patients with scabies compared to those without (95% CI, 1.12-2.09; P<.05).12 Future studies are needed to determine whether the relationship between bipolar disorder and scabies is bidirectional, with pre-existing bipolar disorder evaluated as a risk factor for subsequent scabies infestation. Increased difficulties with activities of daily living, including running errands independently and concentrating, were associated with scabies. These difficulties may reflect sequelae of psychiatric illness or pruritus associated with scabies affecting daily living.
Physician awareness of comorbidities and lifestyle risk factors associated with scabies infestation may improve diagnosis and prevent treatment delays. In a retrospective study at a single dermatology outpatient clinic, 45.3% of patients with scabies (n=428) had previously been misdiagnosed with another dermatologic condition, and the most common erroneous diagnosis was atopic dermatitis.13 Our study provides a framework of comorbidities and lifestyle risk factors associated with scabies infestation that dermatologists can use to stratify patients who may be at greater risk for this condition, allowing dermatologists to select appropriate treatment when clinical signs are ambiguous.
Limitations of our study included the potential for miscoding in the database, lack of information about treatment regimens employed (if any), and lack of information about the temporal relationship between associations.
In summary, it is recommended that patients with pruritus and other characteristic clinical findings of scabies receive appropriate workup for scabies regardless of risk factors; however, the medical and psychiatric comorbidities and lifestyle risk factors identified in this study may help to identify at-risk patients. Our study showed that unsheltered patients are at increased risk for scabies, potentially due to unique dermatologic challenges and lack of access to health care and hygiene facilities. Positive correlations between scabies and HIV, T2DM, and PVD suggest that patients with chronic immunocompromising illnesses who live in group homes or other crowded quarters and present with symptoms could be evaluated for scabies infestation to prevent widespread and difficult- to-control outbreaks in these communities. Based on our findings, scabies also should be included in the differential diagnosis for patients with psychiatric illness and suggestive symptoms. Early identification and treatment of scabies infestation could prevent misdiagnosis and treatment delays.
- World Health Organization. Scabies fact sheet. May 31, 2023. Accessed February 13, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Schneider S, Wu J, Tizek L, et al. Prevalence of scabies worldwidean updated systematic literature review in 2022. J Eur Acad Dermatol Venereol. 2023;37:1749-1757. doi:10.1111/jdv.19167
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: Scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Henry T, Khachemoune A. Dermatologic conditions and risk factors in people experiencing homelessness (PEH): systematic review. Arch Dermatol Res. 2023;315:2795-2803. doi:10.1007/s00403-023-02722-2
- Zakaria A, Amerson EH, Kim-Lim P, et al. Characterization of dermatological diagnoses among hospitalized patients experiencing homelessness. Clin Exp Dermatol. 2022;47:117-120. doi:10.1111/ced.14828
- Bergamin G, Hudson J, Currie BJ, et al. A systematic review of immunosuppressive risk factors and comorbidities associated with the development of crusted scabies. Int J Infect Dis. 2024;143:107036. doi:10.1016/j.ijid.2024.107036
- Yamaguchi Y, Murata F, Maeda M, et al. Investigating the epidemiology and outbreaks of scabies in Japanese households, residential care facilities, and hospitals using claims data: the Longevity Improvement & Fair Evidence (LIFE) study. IJID Reg. 2024;11:100353. doi:10.1016 /j.ijregi.2024.03.008
- Raja A, Karch J, Shih AF, et al. Part II: Cutaneous manifestations of peripheral vascular disease. J Am Acad Dermatol. 2023;89:211-226. doi:10.1016/j.jaad.2021.05.077
- Barry R, Anderson J, Tran L, et al. Prevalence of mental health disorders among individuals experiencing homelessness: a systematic review and meta-analysis. JAMA Psychiatry. 2024;81:691-699. doi:10.1001 /jamapsychiatry.2024.0426
- Koc Y.ld.r.m S, Demirel Og. ut N, Erbag. c. E, et al. Scabies affects quality of life in correlation with depression and anxiety. Dermatol Pract Concept. 2023;13:E2023144. doi:10.5826/dpc.1302a144
- Lin CY, Chang FW, Yang JJ, et al. Increased risk of bipolar disorder in patients with scabies: a nationwide population-based matched-cohort study. Psychiatry Res. 2017;257:14-20. doi:10.1016 /j.psychres.2017.07.013
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017;30:78-84. doi:10.3122/jabfm.2017.01.160190
To the Editor:
Scabies infestation, which has been recognized as a neglected tropical disease by the World Health Organization since 2017, is caused by the human itch mite (Sarcoptes scabiei var hominis).1 Infected individuals experience a pruritic papular rash when the mite burrows into the epidermis, where it lives and lays eggs.2,3 Infected individuals also may develop bacterial superinfections if the skin barrier becomes compromised, leading to systemic complications and considerable morbidity.3
In countries with high human development indices, scabies outbreaks are linked to densely populated living conditions, such as those found in nursing homes or prisons.3,4 Scabies also is transmitted via sexual contact in adults. Beyond immunosuppression, little is known about other comorbid conditions or lifestyle risk factors associated with scabies infestation.2 Because scabies can mimic a range of other dermatologic conditions such as folliculitis, atopic dermatitis, and arthropod bites, misdiagnosis is common and can lead to delayed treatment and increased transmission risk.4 In this study, we sought to examine comorbid conditions and/or lifestyle risk factors associated with scabies infestation.
A matched case-control study was performed using the Registered Tier dataset of the National Institutes of Health All of Us Research Program Curated Data Repository version 7, which includes more than 400,000 unique participants aged 18 years or older from across the United States. The All of Us Research Program excludes adults who are unable to consent independently as well as incarcerated populations and children younger than 18 years. Participants diagnosed with scabies were identified using SNOMED code 62752005 and compared to a control group matched 1:4 based on age, sex, and selfidentified race. SNOMED codes also were used to identify various comorbidities and lifestyle risk factors, including depression, bipolar disorder, anxiety, schizophrenia, peripheral vascular disease (PVD), HIV, type 2 diabetes mellitus (T2DM), unsheltered status, tobacco use, difficulty with activities of daily living, insurance status, and any recent travel history. Logistic regression models were used to calculate odds ratios (ORs) and estimate effect sizes, with statistical significance set at P<.05.
We identified 691 cases of scabies infestation and 2073 controls. The average age of the patients diagnosed with scabies was 55.1 years. Seventy percent (481/691) identified as female and 32.4% (224/491) identified as Black or African American. Matched controls were similar for all analyzed demographic characteristics (P=1.0)(eTable 1). Patients diagnosed with scabies were more likely to be unsheltered (OR, 2.33 [95% CI, 1.91-2.85]), use tobacco (OR 1.77 [95% CI, 1.48-2.11]) and have a comorbid diagnosis of HIV (OR, 3.08 [95% CI, 2.03-4.66]), T2DM (OR, 2.05 [95% CI, 1.57- 2.66]) or PVD (OR, 2.06 [95% CI, 1.43-2.97]) compared with controls (P<.001). Psychiatric comorbidities were more common in the patients diagnosed with scabies, including depression (OR, 3.07 [95% CI, 2.54-3.72]), anxiety (OR, 2.48 [95% CI, 2.06-2.98]), bipolar disorder (OR, 3.08 [95% CI, 2.34-4.05]), and schizophrenia (OR, 4.68 [95% CI, 2.93-7.49])(P<.001). Difficulties with activities of daily living, including running errands alone (OR, 2.32 [95% CI, 1.43-3.76]) and concentrating (OR, 5.78; 95% CI, 3.86-8.64), were more prevalent in the scabies group compared to controls (both P<.05). In a multivariate logistic regression model including unsheltered status as a covariate, all associations remained statistically significant (P<.05)(eTable 2).


This large diverse study demonstrated an association between scabies infestation and unsheltered status. Previous studies have shown that unsheltered populations are at increased risk for many dermatologic conditions, perhaps due to decreased access to health care and social support, lack of access to hygiene facilities (eg, public showers), and increased prevalence of substance use and psychiatric disorders among this population.5 In a cross-sectional analysis of hospitalized patients, 8.6% of unsheltered patients (n=197) had an ectoparasitic disease (including scabies) compared with 1.0% of patients with stable housing (n=1018), with a 9.43-fold increased risk for ectoparasitic infestation among unsheltered patients (95% CI, 3.79-23.47; P<.001).6 Increased attention to public health initiatives among unsheltered populations— including access to hygiene facilities and increased dermatologic services—are needed, as ectoparasitic infections are both preventable and treatable, and these initiatives could reduce morbidity associated with superimposed bacterial infections for which unsheltered patients are at increased risk.6
Our results also showed that individuals diagnosed with scabies were more likely than the controls to have been diagnosed with HIV, T2DM, and PVD. Our findings are similar to those of a systematic review of immunosuppressive factors associated with crusted scabies (a severe form of scabies infestation) in which 10.2% and 15.7% of patients (n=683) had comorbid HIV and T2DM, respectively.7 A functioning cell-mediated response to scabies mite antigens limits proliferation of the human itch mite; thus, infection with HIV/AIDS, which induces the destruction of CD4+ T cells, limits the immune system’s ability to mount an effective response against these antigens. The association of scabies with T2DM likely is multifactorial; for example, chronic hyperglycemia may lead to immune system impairment, and peripheral neuropathy may reduce the itch sensation, allowing scabies mites to proliferate without removal by scratching.7 In a descriptive epidemiologic study in Japan, 11.7% of patients with scabies (N=857) had comorbid PVD.8 Peripheral vascular disease can lead to the development of ulcers, gangrene, and stasis dermatitis, all of which compromise the skin barrier and increase susceptibility to infection.9 Notably, these associations remained even when unsheltered status was considered as a confounding variable. Because individuals with HIV, T2DM, and PVD may be at higher risk for serious complications of scabies infestation (eg, secondary bacterial infections, invasive group A streptococcal infections), prompt detection and treatment of scabies are crucial in curbing morbidity in these at-risk populations.
Our study also demonstrated that psychiatric comorbidities including depression, anxiety, bipolar disorder, and schizophrenia were associated with scabies infestation, even when controlling for unsheltered status, which may have a bidirectional relationship with mental health disorders.10 In a cross-sectional study of 83 adult patients diagnosed with scabies, 72.2% (60/83) reported moderate to extremely large effect of scabies infestation on quality of life using the Dermatology Life Quality Index, and these scores positively correlated with increased Beck Depression Scale and Beck Anxiety Scale scores (rs=0.448 and rs=0.456 0.456, respectively; both P=.000). The results of this study suggest that scabies negatively impacts quality of life, which might increase symptoms of depression and anxiety.11
Studies are needed to assess whether patients with pre-existing depression and anxiety face increased risk for scabies infestation. In a retrospective case-control study using data from the National Health Insurance Research Database of Taiwan, 0.8% (58/7096) of patients with scabies (n=7096) and 0.4% of controls (n=28,375) were newly diagnosed with bipolar disorder over a 7-year period, indicating a 1.55-fold increased risk for bipolar disorder in patients with scabies compared to those without (95% CI, 1.12-2.09; P<.05).12 Future studies are needed to determine whether the relationship between bipolar disorder and scabies is bidirectional, with pre-existing bipolar disorder evaluated as a risk factor for subsequent scabies infestation. Increased difficulties with activities of daily living, including running errands independently and concentrating, were associated with scabies. These difficulties may reflect sequelae of psychiatric illness or pruritus associated with scabies affecting daily living.
Physician awareness of comorbidities and lifestyle risk factors associated with scabies infestation may improve diagnosis and prevent treatment delays. In a retrospective study at a single dermatology outpatient clinic, 45.3% of patients with scabies (n=428) had previously been misdiagnosed with another dermatologic condition, and the most common erroneous diagnosis was atopic dermatitis.13 Our study provides a framework of comorbidities and lifestyle risk factors associated with scabies infestation that dermatologists can use to stratify patients who may be at greater risk for this condition, allowing dermatologists to select appropriate treatment when clinical signs are ambiguous.
Limitations of our study included the potential for miscoding in the database, lack of information about treatment regimens employed (if any), and lack of information about the temporal relationship between associations.
In summary, it is recommended that patients with pruritus and other characteristic clinical findings of scabies receive appropriate workup for scabies regardless of risk factors; however, the medical and psychiatric comorbidities and lifestyle risk factors identified in this study may help to identify at-risk patients. Our study showed that unsheltered patients are at increased risk for scabies, potentially due to unique dermatologic challenges and lack of access to health care and hygiene facilities. Positive correlations between scabies and HIV, T2DM, and PVD suggest that patients with chronic immunocompromising illnesses who live in group homes or other crowded quarters and present with symptoms could be evaluated for scabies infestation to prevent widespread and difficult- to-control outbreaks in these communities. Based on our findings, scabies also should be included in the differential diagnosis for patients with psychiatric illness and suggestive symptoms. Early identification and treatment of scabies infestation could prevent misdiagnosis and treatment delays.
To the Editor:
Scabies infestation, which has been recognized as a neglected tropical disease by the World Health Organization since 2017, is caused by the human itch mite (Sarcoptes scabiei var hominis).1 Infected individuals experience a pruritic papular rash when the mite burrows into the epidermis, where it lives and lays eggs.2,3 Infected individuals also may develop bacterial superinfections if the skin barrier becomes compromised, leading to systemic complications and considerable morbidity.3
In countries with high human development indices, scabies outbreaks are linked to densely populated living conditions, such as those found in nursing homes or prisons.3,4 Scabies also is transmitted via sexual contact in adults. Beyond immunosuppression, little is known about other comorbid conditions or lifestyle risk factors associated with scabies infestation.2 Because scabies can mimic a range of other dermatologic conditions such as folliculitis, atopic dermatitis, and arthropod bites, misdiagnosis is common and can lead to delayed treatment and increased transmission risk.4 In this study, we sought to examine comorbid conditions and/or lifestyle risk factors associated with scabies infestation.
A matched case-control study was performed using the Registered Tier dataset of the National Institutes of Health All of Us Research Program Curated Data Repository version 7, which includes more than 400,000 unique participants aged 18 years or older from across the United States. The All of Us Research Program excludes adults who are unable to consent independently as well as incarcerated populations and children younger than 18 years. Participants diagnosed with scabies were identified using SNOMED code 62752005 and compared to a control group matched 1:4 based on age, sex, and selfidentified race. SNOMED codes also were used to identify various comorbidities and lifestyle risk factors, including depression, bipolar disorder, anxiety, schizophrenia, peripheral vascular disease (PVD), HIV, type 2 diabetes mellitus (T2DM), unsheltered status, tobacco use, difficulty with activities of daily living, insurance status, and any recent travel history. Logistic regression models were used to calculate odds ratios (ORs) and estimate effect sizes, with statistical significance set at P<.05.
We identified 691 cases of scabies infestation and 2073 controls. The average age of the patients diagnosed with scabies was 55.1 years. Seventy percent (481/691) identified as female and 32.4% (224/491) identified as Black or African American. Matched controls were similar for all analyzed demographic characteristics (P=1.0)(eTable 1). Patients diagnosed with scabies were more likely to be unsheltered (OR, 2.33 [95% CI, 1.91-2.85]), use tobacco (OR 1.77 [95% CI, 1.48-2.11]) and have a comorbid diagnosis of HIV (OR, 3.08 [95% CI, 2.03-4.66]), T2DM (OR, 2.05 [95% CI, 1.57- 2.66]) or PVD (OR, 2.06 [95% CI, 1.43-2.97]) compared with controls (P<.001). Psychiatric comorbidities were more common in the patients diagnosed with scabies, including depression (OR, 3.07 [95% CI, 2.54-3.72]), anxiety (OR, 2.48 [95% CI, 2.06-2.98]), bipolar disorder (OR, 3.08 [95% CI, 2.34-4.05]), and schizophrenia (OR, 4.68 [95% CI, 2.93-7.49])(P<.001). Difficulties with activities of daily living, including running errands alone (OR, 2.32 [95% CI, 1.43-3.76]) and concentrating (OR, 5.78; 95% CI, 3.86-8.64), were more prevalent in the scabies group compared to controls (both P<.05). In a multivariate logistic regression model including unsheltered status as a covariate, all associations remained statistically significant (P<.05)(eTable 2).


This large diverse study demonstrated an association between scabies infestation and unsheltered status. Previous studies have shown that unsheltered populations are at increased risk for many dermatologic conditions, perhaps due to decreased access to health care and social support, lack of access to hygiene facilities (eg, public showers), and increased prevalence of substance use and psychiatric disorders among this population.5 In a cross-sectional analysis of hospitalized patients, 8.6% of unsheltered patients (n=197) had an ectoparasitic disease (including scabies) compared with 1.0% of patients with stable housing (n=1018), with a 9.43-fold increased risk for ectoparasitic infestation among unsheltered patients (95% CI, 3.79-23.47; P<.001).6 Increased attention to public health initiatives among unsheltered populations— including access to hygiene facilities and increased dermatologic services—are needed, as ectoparasitic infections are both preventable and treatable, and these initiatives could reduce morbidity associated with superimposed bacterial infections for which unsheltered patients are at increased risk.6
Our results also showed that individuals diagnosed with scabies were more likely than the controls to have been diagnosed with HIV, T2DM, and PVD. Our findings are similar to those of a systematic review of immunosuppressive factors associated with crusted scabies (a severe form of scabies infestation) in which 10.2% and 15.7% of patients (n=683) had comorbid HIV and T2DM, respectively.7 A functioning cell-mediated response to scabies mite antigens limits proliferation of the human itch mite; thus, infection with HIV/AIDS, which induces the destruction of CD4+ T cells, limits the immune system’s ability to mount an effective response against these antigens. The association of scabies with T2DM likely is multifactorial; for example, chronic hyperglycemia may lead to immune system impairment, and peripheral neuropathy may reduce the itch sensation, allowing scabies mites to proliferate without removal by scratching.7 In a descriptive epidemiologic study in Japan, 11.7% of patients with scabies (N=857) had comorbid PVD.8 Peripheral vascular disease can lead to the development of ulcers, gangrene, and stasis dermatitis, all of which compromise the skin barrier and increase susceptibility to infection.9 Notably, these associations remained even when unsheltered status was considered as a confounding variable. Because individuals with HIV, T2DM, and PVD may be at higher risk for serious complications of scabies infestation (eg, secondary bacterial infections, invasive group A streptococcal infections), prompt detection and treatment of scabies are crucial in curbing morbidity in these at-risk populations.
Our study also demonstrated that psychiatric comorbidities including depression, anxiety, bipolar disorder, and schizophrenia were associated with scabies infestation, even when controlling for unsheltered status, which may have a bidirectional relationship with mental health disorders.10 In a cross-sectional study of 83 adult patients diagnosed with scabies, 72.2% (60/83) reported moderate to extremely large effect of scabies infestation on quality of life using the Dermatology Life Quality Index, and these scores positively correlated with increased Beck Depression Scale and Beck Anxiety Scale scores (rs=0.448 and rs=0.456 0.456, respectively; both P=.000). The results of this study suggest that scabies negatively impacts quality of life, which might increase symptoms of depression and anxiety.11
Studies are needed to assess whether patients with pre-existing depression and anxiety face increased risk for scabies infestation. In a retrospective case-control study using data from the National Health Insurance Research Database of Taiwan, 0.8% (58/7096) of patients with scabies (n=7096) and 0.4% of controls (n=28,375) were newly diagnosed with bipolar disorder over a 7-year period, indicating a 1.55-fold increased risk for bipolar disorder in patients with scabies compared to those without (95% CI, 1.12-2.09; P<.05).12 Future studies are needed to determine whether the relationship between bipolar disorder and scabies is bidirectional, with pre-existing bipolar disorder evaluated as a risk factor for subsequent scabies infestation. Increased difficulties with activities of daily living, including running errands independently and concentrating, were associated with scabies. These difficulties may reflect sequelae of psychiatric illness or pruritus associated with scabies affecting daily living.
Physician awareness of comorbidities and lifestyle risk factors associated with scabies infestation may improve diagnosis and prevent treatment delays. In a retrospective study at a single dermatology outpatient clinic, 45.3% of patients with scabies (n=428) had previously been misdiagnosed with another dermatologic condition, and the most common erroneous diagnosis was atopic dermatitis.13 Our study provides a framework of comorbidities and lifestyle risk factors associated with scabies infestation that dermatologists can use to stratify patients who may be at greater risk for this condition, allowing dermatologists to select appropriate treatment when clinical signs are ambiguous.
Limitations of our study included the potential for miscoding in the database, lack of information about treatment regimens employed (if any), and lack of information about the temporal relationship between associations.
In summary, it is recommended that patients with pruritus and other characteristic clinical findings of scabies receive appropriate workup for scabies regardless of risk factors; however, the medical and psychiatric comorbidities and lifestyle risk factors identified in this study may help to identify at-risk patients. Our study showed that unsheltered patients are at increased risk for scabies, potentially due to unique dermatologic challenges and lack of access to health care and hygiene facilities. Positive correlations between scabies and HIV, T2DM, and PVD suggest that patients with chronic immunocompromising illnesses who live in group homes or other crowded quarters and present with symptoms could be evaluated for scabies infestation to prevent widespread and difficult- to-control outbreaks in these communities. Based on our findings, scabies also should be included in the differential diagnosis for patients with psychiatric illness and suggestive symptoms. Early identification and treatment of scabies infestation could prevent misdiagnosis and treatment delays.
- World Health Organization. Scabies fact sheet. May 31, 2023. Accessed February 13, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Schneider S, Wu J, Tizek L, et al. Prevalence of scabies worldwidean updated systematic literature review in 2022. J Eur Acad Dermatol Venereol. 2023;37:1749-1757. doi:10.1111/jdv.19167
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: Scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Henry T, Khachemoune A. Dermatologic conditions and risk factors in people experiencing homelessness (PEH): systematic review. Arch Dermatol Res. 2023;315:2795-2803. doi:10.1007/s00403-023-02722-2
- Zakaria A, Amerson EH, Kim-Lim P, et al. Characterization of dermatological diagnoses among hospitalized patients experiencing homelessness. Clin Exp Dermatol. 2022;47:117-120. doi:10.1111/ced.14828
- Bergamin G, Hudson J, Currie BJ, et al. A systematic review of immunosuppressive risk factors and comorbidities associated with the development of crusted scabies. Int J Infect Dis. 2024;143:107036. doi:10.1016/j.ijid.2024.107036
- Yamaguchi Y, Murata F, Maeda M, et al. Investigating the epidemiology and outbreaks of scabies in Japanese households, residential care facilities, and hospitals using claims data: the Longevity Improvement & Fair Evidence (LIFE) study. IJID Reg. 2024;11:100353. doi:10.1016 /j.ijregi.2024.03.008
- Raja A, Karch J, Shih AF, et al. Part II: Cutaneous manifestations of peripheral vascular disease. J Am Acad Dermatol. 2023;89:211-226. doi:10.1016/j.jaad.2021.05.077
- Barry R, Anderson J, Tran L, et al. Prevalence of mental health disorders among individuals experiencing homelessness: a systematic review and meta-analysis. JAMA Psychiatry. 2024;81:691-699. doi:10.1001 /jamapsychiatry.2024.0426
- Koc Y.ld.r.m S, Demirel Og. ut N, Erbag. c. E, et al. Scabies affects quality of life in correlation with depression and anxiety. Dermatol Pract Concept. 2023;13:E2023144. doi:10.5826/dpc.1302a144
- Lin CY, Chang FW, Yang JJ, et al. Increased risk of bipolar disorder in patients with scabies: a nationwide population-based matched-cohort study. Psychiatry Res. 2017;257:14-20. doi:10.1016 /j.psychres.2017.07.013
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017;30:78-84. doi:10.3122/jabfm.2017.01.160190
- World Health Organization. Scabies fact sheet. May 31, 2023. Accessed February 13, 2025. https://www.who.int/news-room/fact-sheets/detail/scabies
- Chandler DJ, Fuller LC. A review of scabies: an infestation more than skin deep. Dermatology. 2019;235:79-90. doi:10.1159/000495290
- Schneider S, Wu J, Tizek L, et al. Prevalence of scabies worldwidean updated systematic literature review in 2022. J Eur Acad Dermatol Venereol. 2023;37:1749-1757. doi:10.1111/jdv.19167
- Thomas C, Coates SJ, Engelman D, et al. Ectoparasites: Scabies. J Am Acad Dermatol. 2020;82:533-548. doi:10.1016/j.jaad.2019.05.109
- Henry T, Khachemoune A. Dermatologic conditions and risk factors in people experiencing homelessness (PEH): systematic review. Arch Dermatol Res. 2023;315:2795-2803. doi:10.1007/s00403-023-02722-2
- Zakaria A, Amerson EH, Kim-Lim P, et al. Characterization of dermatological diagnoses among hospitalized patients experiencing homelessness. Clin Exp Dermatol. 2022;47:117-120. doi:10.1111/ced.14828
- Bergamin G, Hudson J, Currie BJ, et al. A systematic review of immunosuppressive risk factors and comorbidities associated with the development of crusted scabies. Int J Infect Dis. 2024;143:107036. doi:10.1016/j.ijid.2024.107036
- Yamaguchi Y, Murata F, Maeda M, et al. Investigating the epidemiology and outbreaks of scabies in Japanese households, residential care facilities, and hospitals using claims data: the Longevity Improvement & Fair Evidence (LIFE) study. IJID Reg. 2024;11:100353. doi:10.1016 /j.ijregi.2024.03.008
- Raja A, Karch J, Shih AF, et al. Part II: Cutaneous manifestations of peripheral vascular disease. J Am Acad Dermatol. 2023;89:211-226. doi:10.1016/j.jaad.2021.05.077
- Barry R, Anderson J, Tran L, et al. Prevalence of mental health disorders among individuals experiencing homelessness: a systematic review and meta-analysis. JAMA Psychiatry. 2024;81:691-699. doi:10.1001 /jamapsychiatry.2024.0426
- Koc Y.ld.r.m S, Demirel Og. ut N, Erbag. c. E, et al. Scabies affects quality of life in correlation with depression and anxiety. Dermatol Pract Concept. 2023;13:E2023144. doi:10.5826/dpc.1302a144
- Lin CY, Chang FW, Yang JJ, et al. Increased risk of bipolar disorder in patients with scabies: a nationwide population-based matched-cohort study. Psychiatry Res. 2017;257:14-20. doi:10.1016 /j.psychres.2017.07.013
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Fam Med. 2017;30:78-84. doi:10.3122/jabfm.2017.01.160190
Comorbidities and Lifestyle Risk Factors Associated With Scabies Infestation
Comorbidities and Lifestyle Risk Factors Associated With Scabies Infestation
PRACTICE POINTS
- Scabies infestation is caused by the human itch mite (Sarcoptes scabiei var hominis) and can be spread via sexual contact in adults.
- Crowded living conditions are associated with scabies infestation in countries with high human development indices, such as the United States.
- Patients with certain comorbid conditions or lifestyle risk factors should be screened for scabies infestation when presenting with pruritus and other characteristic clinical findings.
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Vitiligo is a common autoimmune disorder characterized by cutaneous depigmentation that has a substantial impact on patient quality of life.1 Vitiligo affects approximately 28.5 million individuals globally, with the highest lifetime prevalence occurring in Central Europe and South Asia.2 In the United States, Asian American and Hispanic/Latine populations most commonly are affected.3 The accompanying psychosocial burdens of vitiligo are particularly substantial among individuals with darker skin types, as evidenced by higher rates of concomitant anxiety and depression in these patients.4 Despite this, patients with skin of color are underrepresented in vitiligo research.2
Treatment algorithms developed based on worldwide expert consensus recommendations provide valuable insights into the management of segmental and nonsegmental vitiligo.5 The mainstay therapeutics include topical and oral corticosteroids, topical calcineurin inhibitors, and phototherapy. While vitiligo pathogenesis is not completely understood, recent advances have focused on the role of the Janus kinase (JAK)/signal transducer and activator of transcription pathway. Interferon gamma drives vitiligo pathogenesis through this pathway, upregulating C-X-C motif chemokine ligand 10 and promoting CD8+ T-cell recruitment, resulting in targeted melanocyte destruction.6 The emergence of targeted therapeutics may address equity and inclusion gaps. Herein, we highlight innovations in vitiligo treatment with a focus on oral and topical JAK inhibitors.
Oral JAK Inhibitors for Vitiligo
The therapeutic potential of JAK inhibitors for vitiligo was first reported when patients with alopecia areata and comorbid vitiligo experienced repigmentation of the skin following administration of oral ruxolitinib.7 Since this discovery, other oral JAK inhibitors have been investigated for vitiligo treatment. A phase 2b randomized clinical trial (RCT) of 364 patients examined oral ritlecitinib, a JAK3 inhibitor, and found it to be effective in treating active nonsegmental vitiligo.8 Patients aged 18 to 65 years with active nonsegmental vitiligo that had been present for 3 months or more as well as 4% to 50% body surface area (BSA) affected excluding acral surfaces and at least 0.25% facial involvement were included. Treatment groups received 50 mg (with or without a 100- or 200- mg loading dose), 30 mg, or 10 mg daily for 24 weeks. The primary endpoint measured the percentage change in Facial Vitiligo Area Scoring Index (F-VASI) score. Significant differences in F-VASI percentage change compared with placebo occurred for those in the 50-mg group who received a loading dose (-21.2 vs 2.1 [P<.001]) and those who did not receive a loading dose (–18.5 vs 2.1 [P<.001]) as well as the 30-mg group (-14.6 vs 2.1 [P=.01]). Continued repigmentation of the skin was observed in the 24-week extension period, indicating that longer treatment periods may be necessary for optimal repigmentation results. Ritlecitinib generally was well tolerated, and the most common treatment-emergent adverse events were nasopharyngitis (15.9%), upper respiratory tract infection (11.5%), and headache (8.8%). Most patients identified as White (67.6%), with 23.6% identifying as Asian and 2.7% identifying as Black. The authors stated that continued improvement was observed in the extension period across all skin types; however, the data were not reported.8
Upadacitnib, an oral selective JAK1 inhibitor, also has demonstrated efficacy in nonsegmental vitiligo in a phase 2 RCT.9 Adult patients (N=185) with nonsegmental vitiligo were randomized to receive upadacitinib 6 mg, 11 mg, or 22 mg or placebo (the placebo group subsequently was switched to upadacitinib 11 mg or 22 mg after 24 weeks). The primary endpoint measured the percentage change in F-VASI score at 24 weeks. The higher doses of upadacitinib resulted in significant changes in F-VASI scored compared with placebo (6 mg: -7.60 [95% CI, -22.18 to 6.97][P=.30]; 11 mg: -21.27 [95% CI, -36.02 to -6.52][P=.01]; 22 mg: -19.60 [95% CI, -35.04 to –4.16][P=.01]). As with ritlecitinib, continued repigmentation was observed beyond the initial 24-week period. Of the 185 participants, 5.9% identified as Black and 13.5% identified as Asian. The investigators reported that the percentage change in F-VASI score was consistent across skin types.9 The results of these phase 2 RCTs are encouraging, and we anticipate the findings of 2 phase 3 RCTs for ritlecitinib and upadacitinib that currently are underway (Clinicaltrials.gov identifiers NCT05583526 and NCT06118411).
Topical JAK Inhibitors for Vitiligo
Tofacitinib cream 2%, a selective JAK3 inhibitor, has shown therapeutic potential for treatment of vitiligo. One of the earliest pilot studies on topical tofacitinib examined the efficacy of tofacitinib cream 2% applied twice daily combined with narrowband UVB therapy 3 times weekly for facial vitiligo. The investigators reported repigmentation of the skin in all 11 patients (which included 4 Asian patients and 1 Hispanic patient), with a mean improvement of 70% in F-VASI score (range, 50%-87%).10 In a nonrandomized cohort study of 16 patients later that year, twice-daily application of tofacitinib cream 2% on facial and nonfacial vitiligo lesions resulted in partial repigmentation in 81.3% of patients: 4 (25%) achieved greater than 90% improvement, 5 (31.3%) achieved improvement of 25% to 75%, and 4 (25%) achieved 5% to 15% improvement.11 The researchers also found that tofacitinib cream 2% was significantly more effective in facial than nonfacial lesions (P=.02).
While tofacitinib has shown promise in early studies, recent advancements have led to US Food and Drug Administration approval of ruxolitinib cream 1.5%, another topical JAK inhibitor that has undergone robust clinical testing for vitiligo.12-14 Ruxolitinib, a JAK1, JAK2, and JAK3 inhibitor, is the first and only US Food and Drug Administration–approved topical JAK inhibitor for vitiligo.14,15 Two phase 3, double-blind, vehicle-controlled trials of identical design conducted across 101 centers in North America and Europe (TRuE-V1 and TRuE-V2) assessed the efficacy of ruxolitinib cream 1.5% in 674 patients aged 12 years and older with nonsegmental vitiligo covering 10% or lower total BSA.13 In both trials, twice-daily application of topical ruxolitinib resulted in greater facial repigmentation and improvement in F-VASI75 score (ie, a reduction of at least 75% from baseline) at 24 weeks in 29.9% (66/221) and 30.1% (69/222) of patients in TRuE-V1 and TRuE-V2, respectively. Continued application through 52 weeks resulted in F-VASI75 response in 52.6% (91/173) and 48.0% (85/177) of patients in TRuE-V1 and TRuE-V2, respectively. The most frequently reported adverse events were acne (6.3% [14/221] and 6.6% [15/228]), nasopharyngitis (5.4% [12/221] and 6.1% [14/228]), and pruritus (5.4% [12/221] and 5.3% [12/228]). These findings align with prior subgroup analyses of an earlier phase 2 double- blind RCT of ruxolitinib cream 1.5% that indicated similar improvement in vitiligo among patients with differing skin tones.17
There are no additional large-scale RCTs examining topical JAK inhibitors with intentional subanalysis of diverse skin tones.16,17,18 Studies examining topical JAK inhibitors have expanded to be more inclusive, providing hope for the future of topical vitiligo therapeutics for all patients.
Final Thoughts
It is imperative to increase racial/ethnic and skin type diversity in research on JAK inhibitors for vitiligo. While the studies mentioned here are inclusive of an array of races and skin tones, it is crucial that future research continue to expand the number of diverse participants, especially given the increased psychosocial burdens of vitiligo in patients with darker skin types.4 Intentional subgroup analyses across skin tones are vital to characterize and unmask potential differences between lighter and darker skin types. This point was exemplified by a 2024 RCT that investigated ritlecitinib efficacy with biomarker analysis across skin types.19 For patients receiving ritlecitinib 50 mg, IL-9 and IL-22 expression were decreased in darker vs lighter skin tones (P<.05). This intentional and inclusive analysis revealed a potential immunologic mechanism for why darker skin tones respond to JAK inhibitor therapy earlier than lighter skin tones.19
In the expanding landscape of oral and topical JAK inhibitors for vitiligo, continued efforts to assess these therapies across a range of skin tones and racial/ ethnic groups are critical. The efficacy of JAK inhibitors in other populations, including pediatric patients and patients with refractory segmental disease, have been reported.20,21 As larger studies are developed based on the success of individual cases, researchers should investigate the efficacy of JAK inhibitors for various vitiligo subtypes (eg, segmental, nonsegmental) and recalcitrant disease and conduct direct comparisons with traditional treatments across diverse skin tones and racial/ethnic subgroup analyses to ensure broad therapeutic applicability.
- Alikhan Ali, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview. part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016 /j.jaad.2010.11.061
- Akl J, Lee S, Ju HJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. 2024;9:E386-E396. doi:10.1016/S2468-2667(24)00026-4
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159:986-990. doi:10.1001/jama dermatol.2023.2162
- Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159:1124-1128. doi:10.1001/jamadermatol.2023.2787
- van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the International Vitiligo Task Force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. 2023; 37:2173-2184. doi:10.1111/jdv.19451
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi:10.1126 /scitranslmed.3007811
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370-371. doi:10.1016/ j.jaad.2015.09.073
- Ezzedine K, Peeva E, Yamguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403. doi:10.1016/j.jaad.2022.11.005
- Passeron T, Ezzedine K, Hamzavi I, et al. Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study. EClinicalMedicine. 2024;73:102655. doi:10.1016 /j.eclinm.2024.102655
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-648. doi:10.1016/j.jaad.2019.04.032
- Mobasher P, Guerra R, Li SJ, et al. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Brit J Dermatol. 2020;182:1047-1049. doi:10.1111/bjd.18606
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-120. doi:10.1016/S0140-6736(20)30609-7
- Rosmarin D, Passeron T, Pandya AG, et al; TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455. doi:10.1056/NEJMoa2118828
- FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. Published July 19, 2022. Accessed January 30, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109-3117. doi:10.1182/blood-2009-04-214957
- Seneschal J, Wolkerstorfer A, Desai SR, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: week 52 pooled subgroup analysis from two randomized phase 3 studies. Brit J Dermatol. 2023;188 (suppl 1):ljac106.006. doi:10.1093/bjd/ljac106.006
- Hamzavi I, Rosmarin D, Harris JE, et al. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86:1398-1401. doi:10.1016/j.jaad.2021.05.047
- Inoue S, Suzuki T, Sano S, et al. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci. 2024;113:86-92. doi:10.1016/j.jdermsci.2023.12.008
- Peeva E, Yamaguchi Y, Ye Z, et al. Efficacy and safety of ritlecitinib in vitiligo patients across Fitzpatrick skin types with biomarker analyses. Exp Dermatol. 2024;33:E15177. doi:10.1111/exd.15177
- Mu Y, Pan T, Chen L. Treatment of refractory segmental vitiligo and alopecia areata in a child with upadacitinib and NB-UVB: a case report. Clin Cosmet Investig Dermatol. 2024;17:1789-1792. doi:10.2147 /CCID.S467026
- Shah RR, McMichael A. Resistant vitiligo treated with tofacitinib and sustained repigmentation after discontinuation. Skinmed. 2024;22:384-385.
Vitiligo is a common autoimmune disorder characterized by cutaneous depigmentation that has a substantial impact on patient quality of life.1 Vitiligo affects approximately 28.5 million individuals globally, with the highest lifetime prevalence occurring in Central Europe and South Asia.2 In the United States, Asian American and Hispanic/Latine populations most commonly are affected.3 The accompanying psychosocial burdens of vitiligo are particularly substantial among individuals with darker skin types, as evidenced by higher rates of concomitant anxiety and depression in these patients.4 Despite this, patients with skin of color are underrepresented in vitiligo research.2
Treatment algorithms developed based on worldwide expert consensus recommendations provide valuable insights into the management of segmental and nonsegmental vitiligo.5 The mainstay therapeutics include topical and oral corticosteroids, topical calcineurin inhibitors, and phototherapy. While vitiligo pathogenesis is not completely understood, recent advances have focused on the role of the Janus kinase (JAK)/signal transducer and activator of transcription pathway. Interferon gamma drives vitiligo pathogenesis through this pathway, upregulating C-X-C motif chemokine ligand 10 and promoting CD8+ T-cell recruitment, resulting in targeted melanocyte destruction.6 The emergence of targeted therapeutics may address equity and inclusion gaps. Herein, we highlight innovations in vitiligo treatment with a focus on oral and topical JAK inhibitors.
Oral JAK Inhibitors for Vitiligo
The therapeutic potential of JAK inhibitors for vitiligo was first reported when patients with alopecia areata and comorbid vitiligo experienced repigmentation of the skin following administration of oral ruxolitinib.7 Since this discovery, other oral JAK inhibitors have been investigated for vitiligo treatment. A phase 2b randomized clinical trial (RCT) of 364 patients examined oral ritlecitinib, a JAK3 inhibitor, and found it to be effective in treating active nonsegmental vitiligo.8 Patients aged 18 to 65 years with active nonsegmental vitiligo that had been present for 3 months or more as well as 4% to 50% body surface area (BSA) affected excluding acral surfaces and at least 0.25% facial involvement were included. Treatment groups received 50 mg (with or without a 100- or 200- mg loading dose), 30 mg, or 10 mg daily for 24 weeks. The primary endpoint measured the percentage change in Facial Vitiligo Area Scoring Index (F-VASI) score. Significant differences in F-VASI percentage change compared with placebo occurred for those in the 50-mg group who received a loading dose (-21.2 vs 2.1 [P<.001]) and those who did not receive a loading dose (–18.5 vs 2.1 [P<.001]) as well as the 30-mg group (-14.6 vs 2.1 [P=.01]). Continued repigmentation of the skin was observed in the 24-week extension period, indicating that longer treatment periods may be necessary for optimal repigmentation results. Ritlecitinib generally was well tolerated, and the most common treatment-emergent adverse events were nasopharyngitis (15.9%), upper respiratory tract infection (11.5%), and headache (8.8%). Most patients identified as White (67.6%), with 23.6% identifying as Asian and 2.7% identifying as Black. The authors stated that continued improvement was observed in the extension period across all skin types; however, the data were not reported.8
Upadacitnib, an oral selective JAK1 inhibitor, also has demonstrated efficacy in nonsegmental vitiligo in a phase 2 RCT.9 Adult patients (N=185) with nonsegmental vitiligo were randomized to receive upadacitinib 6 mg, 11 mg, or 22 mg or placebo (the placebo group subsequently was switched to upadacitinib 11 mg or 22 mg after 24 weeks). The primary endpoint measured the percentage change in F-VASI score at 24 weeks. The higher doses of upadacitinib resulted in significant changes in F-VASI scored compared with placebo (6 mg: -7.60 [95% CI, -22.18 to 6.97][P=.30]; 11 mg: -21.27 [95% CI, -36.02 to -6.52][P=.01]; 22 mg: -19.60 [95% CI, -35.04 to –4.16][P=.01]). As with ritlecitinib, continued repigmentation was observed beyond the initial 24-week period. Of the 185 participants, 5.9% identified as Black and 13.5% identified as Asian. The investigators reported that the percentage change in F-VASI score was consistent across skin types.9 The results of these phase 2 RCTs are encouraging, and we anticipate the findings of 2 phase 3 RCTs for ritlecitinib and upadacitinib that currently are underway (Clinicaltrials.gov identifiers NCT05583526 and NCT06118411).
Topical JAK Inhibitors for Vitiligo
Tofacitinib cream 2%, a selective JAK3 inhibitor, has shown therapeutic potential for treatment of vitiligo. One of the earliest pilot studies on topical tofacitinib examined the efficacy of tofacitinib cream 2% applied twice daily combined with narrowband UVB therapy 3 times weekly for facial vitiligo. The investigators reported repigmentation of the skin in all 11 patients (which included 4 Asian patients and 1 Hispanic patient), with a mean improvement of 70% in F-VASI score (range, 50%-87%).10 In a nonrandomized cohort study of 16 patients later that year, twice-daily application of tofacitinib cream 2% on facial and nonfacial vitiligo lesions resulted in partial repigmentation in 81.3% of patients: 4 (25%) achieved greater than 90% improvement, 5 (31.3%) achieved improvement of 25% to 75%, and 4 (25%) achieved 5% to 15% improvement.11 The researchers also found that tofacitinib cream 2% was significantly more effective in facial than nonfacial lesions (P=.02).
While tofacitinib has shown promise in early studies, recent advancements have led to US Food and Drug Administration approval of ruxolitinib cream 1.5%, another topical JAK inhibitor that has undergone robust clinical testing for vitiligo.12-14 Ruxolitinib, a JAK1, JAK2, and JAK3 inhibitor, is the first and only US Food and Drug Administration–approved topical JAK inhibitor for vitiligo.14,15 Two phase 3, double-blind, vehicle-controlled trials of identical design conducted across 101 centers in North America and Europe (TRuE-V1 and TRuE-V2) assessed the efficacy of ruxolitinib cream 1.5% in 674 patients aged 12 years and older with nonsegmental vitiligo covering 10% or lower total BSA.13 In both trials, twice-daily application of topical ruxolitinib resulted in greater facial repigmentation and improvement in F-VASI75 score (ie, a reduction of at least 75% from baseline) at 24 weeks in 29.9% (66/221) and 30.1% (69/222) of patients in TRuE-V1 and TRuE-V2, respectively. Continued application through 52 weeks resulted in F-VASI75 response in 52.6% (91/173) and 48.0% (85/177) of patients in TRuE-V1 and TRuE-V2, respectively. The most frequently reported adverse events were acne (6.3% [14/221] and 6.6% [15/228]), nasopharyngitis (5.4% [12/221] and 6.1% [14/228]), and pruritus (5.4% [12/221] and 5.3% [12/228]). These findings align with prior subgroup analyses of an earlier phase 2 double- blind RCT of ruxolitinib cream 1.5% that indicated similar improvement in vitiligo among patients with differing skin tones.17
There are no additional large-scale RCTs examining topical JAK inhibitors with intentional subanalysis of diverse skin tones.16,17,18 Studies examining topical JAK inhibitors have expanded to be more inclusive, providing hope for the future of topical vitiligo therapeutics for all patients.
Final Thoughts
It is imperative to increase racial/ethnic and skin type diversity in research on JAK inhibitors for vitiligo. While the studies mentioned here are inclusive of an array of races and skin tones, it is crucial that future research continue to expand the number of diverse participants, especially given the increased psychosocial burdens of vitiligo in patients with darker skin types.4 Intentional subgroup analyses across skin tones are vital to characterize and unmask potential differences between lighter and darker skin types. This point was exemplified by a 2024 RCT that investigated ritlecitinib efficacy with biomarker analysis across skin types.19 For patients receiving ritlecitinib 50 mg, IL-9 and IL-22 expression were decreased in darker vs lighter skin tones (P<.05). This intentional and inclusive analysis revealed a potential immunologic mechanism for why darker skin tones respond to JAK inhibitor therapy earlier than lighter skin tones.19
In the expanding landscape of oral and topical JAK inhibitors for vitiligo, continued efforts to assess these therapies across a range of skin tones and racial/ ethnic groups are critical. The efficacy of JAK inhibitors in other populations, including pediatric patients and patients with refractory segmental disease, have been reported.20,21 As larger studies are developed based on the success of individual cases, researchers should investigate the efficacy of JAK inhibitors for various vitiligo subtypes (eg, segmental, nonsegmental) and recalcitrant disease and conduct direct comparisons with traditional treatments across diverse skin tones and racial/ethnic subgroup analyses to ensure broad therapeutic applicability.
Vitiligo is a common autoimmune disorder characterized by cutaneous depigmentation that has a substantial impact on patient quality of life.1 Vitiligo affects approximately 28.5 million individuals globally, with the highest lifetime prevalence occurring in Central Europe and South Asia.2 In the United States, Asian American and Hispanic/Latine populations most commonly are affected.3 The accompanying psychosocial burdens of vitiligo are particularly substantial among individuals with darker skin types, as evidenced by higher rates of concomitant anxiety and depression in these patients.4 Despite this, patients with skin of color are underrepresented in vitiligo research.2
Treatment algorithms developed based on worldwide expert consensus recommendations provide valuable insights into the management of segmental and nonsegmental vitiligo.5 The mainstay therapeutics include topical and oral corticosteroids, topical calcineurin inhibitors, and phototherapy. While vitiligo pathogenesis is not completely understood, recent advances have focused on the role of the Janus kinase (JAK)/signal transducer and activator of transcription pathway. Interferon gamma drives vitiligo pathogenesis through this pathway, upregulating C-X-C motif chemokine ligand 10 and promoting CD8+ T-cell recruitment, resulting in targeted melanocyte destruction.6 The emergence of targeted therapeutics may address equity and inclusion gaps. Herein, we highlight innovations in vitiligo treatment with a focus on oral and topical JAK inhibitors.
Oral JAK Inhibitors for Vitiligo
The therapeutic potential of JAK inhibitors for vitiligo was first reported when patients with alopecia areata and comorbid vitiligo experienced repigmentation of the skin following administration of oral ruxolitinib.7 Since this discovery, other oral JAK inhibitors have been investigated for vitiligo treatment. A phase 2b randomized clinical trial (RCT) of 364 patients examined oral ritlecitinib, a JAK3 inhibitor, and found it to be effective in treating active nonsegmental vitiligo.8 Patients aged 18 to 65 years with active nonsegmental vitiligo that had been present for 3 months or more as well as 4% to 50% body surface area (BSA) affected excluding acral surfaces and at least 0.25% facial involvement were included. Treatment groups received 50 mg (with or without a 100- or 200- mg loading dose), 30 mg, or 10 mg daily for 24 weeks. The primary endpoint measured the percentage change in Facial Vitiligo Area Scoring Index (F-VASI) score. Significant differences in F-VASI percentage change compared with placebo occurred for those in the 50-mg group who received a loading dose (-21.2 vs 2.1 [P<.001]) and those who did not receive a loading dose (–18.5 vs 2.1 [P<.001]) as well as the 30-mg group (-14.6 vs 2.1 [P=.01]). Continued repigmentation of the skin was observed in the 24-week extension period, indicating that longer treatment periods may be necessary for optimal repigmentation results. Ritlecitinib generally was well tolerated, and the most common treatment-emergent adverse events were nasopharyngitis (15.9%), upper respiratory tract infection (11.5%), and headache (8.8%). Most patients identified as White (67.6%), with 23.6% identifying as Asian and 2.7% identifying as Black. The authors stated that continued improvement was observed in the extension period across all skin types; however, the data were not reported.8
Upadacitnib, an oral selective JAK1 inhibitor, also has demonstrated efficacy in nonsegmental vitiligo in a phase 2 RCT.9 Adult patients (N=185) with nonsegmental vitiligo were randomized to receive upadacitinib 6 mg, 11 mg, or 22 mg or placebo (the placebo group subsequently was switched to upadacitinib 11 mg or 22 mg after 24 weeks). The primary endpoint measured the percentage change in F-VASI score at 24 weeks. The higher doses of upadacitinib resulted in significant changes in F-VASI scored compared with placebo (6 mg: -7.60 [95% CI, -22.18 to 6.97][P=.30]; 11 mg: -21.27 [95% CI, -36.02 to -6.52][P=.01]; 22 mg: -19.60 [95% CI, -35.04 to –4.16][P=.01]). As with ritlecitinib, continued repigmentation was observed beyond the initial 24-week period. Of the 185 participants, 5.9% identified as Black and 13.5% identified as Asian. The investigators reported that the percentage change in F-VASI score was consistent across skin types.9 The results of these phase 2 RCTs are encouraging, and we anticipate the findings of 2 phase 3 RCTs for ritlecitinib and upadacitinib that currently are underway (Clinicaltrials.gov identifiers NCT05583526 and NCT06118411).
Topical JAK Inhibitors for Vitiligo
Tofacitinib cream 2%, a selective JAK3 inhibitor, has shown therapeutic potential for treatment of vitiligo. One of the earliest pilot studies on topical tofacitinib examined the efficacy of tofacitinib cream 2% applied twice daily combined with narrowband UVB therapy 3 times weekly for facial vitiligo. The investigators reported repigmentation of the skin in all 11 patients (which included 4 Asian patients and 1 Hispanic patient), with a mean improvement of 70% in F-VASI score (range, 50%-87%).10 In a nonrandomized cohort study of 16 patients later that year, twice-daily application of tofacitinib cream 2% on facial and nonfacial vitiligo lesions resulted in partial repigmentation in 81.3% of patients: 4 (25%) achieved greater than 90% improvement, 5 (31.3%) achieved improvement of 25% to 75%, and 4 (25%) achieved 5% to 15% improvement.11 The researchers also found that tofacitinib cream 2% was significantly more effective in facial than nonfacial lesions (P=.02).
While tofacitinib has shown promise in early studies, recent advancements have led to US Food and Drug Administration approval of ruxolitinib cream 1.5%, another topical JAK inhibitor that has undergone robust clinical testing for vitiligo.12-14 Ruxolitinib, a JAK1, JAK2, and JAK3 inhibitor, is the first and only US Food and Drug Administration–approved topical JAK inhibitor for vitiligo.14,15 Two phase 3, double-blind, vehicle-controlled trials of identical design conducted across 101 centers in North America and Europe (TRuE-V1 and TRuE-V2) assessed the efficacy of ruxolitinib cream 1.5% in 674 patients aged 12 years and older with nonsegmental vitiligo covering 10% or lower total BSA.13 In both trials, twice-daily application of topical ruxolitinib resulted in greater facial repigmentation and improvement in F-VASI75 score (ie, a reduction of at least 75% from baseline) at 24 weeks in 29.9% (66/221) and 30.1% (69/222) of patients in TRuE-V1 and TRuE-V2, respectively. Continued application through 52 weeks resulted in F-VASI75 response in 52.6% (91/173) and 48.0% (85/177) of patients in TRuE-V1 and TRuE-V2, respectively. The most frequently reported adverse events were acne (6.3% [14/221] and 6.6% [15/228]), nasopharyngitis (5.4% [12/221] and 6.1% [14/228]), and pruritus (5.4% [12/221] and 5.3% [12/228]). These findings align with prior subgroup analyses of an earlier phase 2 double- blind RCT of ruxolitinib cream 1.5% that indicated similar improvement in vitiligo among patients with differing skin tones.17
There are no additional large-scale RCTs examining topical JAK inhibitors with intentional subanalysis of diverse skin tones.16,17,18 Studies examining topical JAK inhibitors have expanded to be more inclusive, providing hope for the future of topical vitiligo therapeutics for all patients.
Final Thoughts
It is imperative to increase racial/ethnic and skin type diversity in research on JAK inhibitors for vitiligo. While the studies mentioned here are inclusive of an array of races and skin tones, it is crucial that future research continue to expand the number of diverse participants, especially given the increased psychosocial burdens of vitiligo in patients with darker skin types.4 Intentional subgroup analyses across skin tones are vital to characterize and unmask potential differences between lighter and darker skin types. This point was exemplified by a 2024 RCT that investigated ritlecitinib efficacy with biomarker analysis across skin types.19 For patients receiving ritlecitinib 50 mg, IL-9 and IL-22 expression were decreased in darker vs lighter skin tones (P<.05). This intentional and inclusive analysis revealed a potential immunologic mechanism for why darker skin tones respond to JAK inhibitor therapy earlier than lighter skin tones.19
In the expanding landscape of oral and topical JAK inhibitors for vitiligo, continued efforts to assess these therapies across a range of skin tones and racial/ ethnic groups are critical. The efficacy of JAK inhibitors in other populations, including pediatric patients and patients with refractory segmental disease, have been reported.20,21 As larger studies are developed based on the success of individual cases, researchers should investigate the efficacy of JAK inhibitors for various vitiligo subtypes (eg, segmental, nonsegmental) and recalcitrant disease and conduct direct comparisons with traditional treatments across diverse skin tones and racial/ethnic subgroup analyses to ensure broad therapeutic applicability.
- Alikhan Ali, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview. part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016 /j.jaad.2010.11.061
- Akl J, Lee S, Ju HJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. 2024;9:E386-E396. doi:10.1016/S2468-2667(24)00026-4
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159:986-990. doi:10.1001/jama dermatol.2023.2162
- Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159:1124-1128. doi:10.1001/jamadermatol.2023.2787
- van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the International Vitiligo Task Force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. 2023; 37:2173-2184. doi:10.1111/jdv.19451
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi:10.1126 /scitranslmed.3007811
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370-371. doi:10.1016/ j.jaad.2015.09.073
- Ezzedine K, Peeva E, Yamguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403. doi:10.1016/j.jaad.2022.11.005
- Passeron T, Ezzedine K, Hamzavi I, et al. Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study. EClinicalMedicine. 2024;73:102655. doi:10.1016 /j.eclinm.2024.102655
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-648. doi:10.1016/j.jaad.2019.04.032
- Mobasher P, Guerra R, Li SJ, et al. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Brit J Dermatol. 2020;182:1047-1049. doi:10.1111/bjd.18606
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-120. doi:10.1016/S0140-6736(20)30609-7
- Rosmarin D, Passeron T, Pandya AG, et al; TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455. doi:10.1056/NEJMoa2118828
- FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. Published July 19, 2022. Accessed January 30, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109-3117. doi:10.1182/blood-2009-04-214957
- Seneschal J, Wolkerstorfer A, Desai SR, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: week 52 pooled subgroup analysis from two randomized phase 3 studies. Brit J Dermatol. 2023;188 (suppl 1):ljac106.006. doi:10.1093/bjd/ljac106.006
- Hamzavi I, Rosmarin D, Harris JE, et al. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86:1398-1401. doi:10.1016/j.jaad.2021.05.047
- Inoue S, Suzuki T, Sano S, et al. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci. 2024;113:86-92. doi:10.1016/j.jdermsci.2023.12.008
- Peeva E, Yamaguchi Y, Ye Z, et al. Efficacy and safety of ritlecitinib in vitiligo patients across Fitzpatrick skin types with biomarker analyses. Exp Dermatol. 2024;33:E15177. doi:10.1111/exd.15177
- Mu Y, Pan T, Chen L. Treatment of refractory segmental vitiligo and alopecia areata in a child with upadacitinib and NB-UVB: a case report. Clin Cosmet Investig Dermatol. 2024;17:1789-1792. doi:10.2147 /CCID.S467026
- Shah RR, McMichael A. Resistant vitiligo treated with tofacitinib and sustained repigmentation after discontinuation. Skinmed. 2024;22:384-385.
- Alikhan Ali, Felsten LM, Daly M, et al. Vitiligo: a comprehensive overview. part I. introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473-491. doi:10.1016 /j.jaad.2010.11.061
- Akl J, Lee S, Ju HJ, et al. Estimating the burden of vitiligo: a systematic review and modelling study. Lancet Public Health. 2024;9:E386-E396. doi:10.1016/S2468-2667(24)00026-4
- Mastacouris N, Strunk A, Garg A. Incidence and prevalence of diagnosed vitiligo according to race and ethnicity, age, and sex in the US. JAMA Dermatol. 2023;159:986-990. doi:10.1001/jama dermatol.2023.2162
- Bibeau K, Ezzedine K, Harris JE, et al. Mental health and psychosocial quality-of-life burden among patients with vitiligo: findings from the global VALIANT study. JAMA Dermatol. 2023;159:1124-1128. doi:10.1001/jamadermatol.2023.2787
- van Geel N, Speeckaert R, Taïeb A, et al. Worldwide expert recommendations for the diagnosis and management of vitiligo: position statement from the International Vitiligo Task Force part 1: towards a new management algorithm. J Eur Acad Dermatol Venereol. 2023; 37:2173-2184. doi:10.1111/jdv.19451
- Rashighi M, Agarwal P, Richmond JM, et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci Transl Med. 2014;6:223ra23. doi:10.1126 /scitranslmed.3007811
- Harris JE, Rashighi M, Nguyen N, et al. Rapid skin repigmentation on oral ruxolitinib in a patient with coexistent vitiligo and alopecia areata (AA). J Am Acad Dermatol. 2016;74:370-371. doi:10.1016/ j.jaad.2015.09.073
- Ezzedine K, Peeva E, Yamguchi Y, et al. Efficacy and safety of oral ritlecitinib for the treatment of active nonsegmental vitiligo: a randomized phase 2b clinical trial. J Am Acad Dermatol. 2023;88:395-403. doi:10.1016/j.jaad.2022.11.005
- Passeron T, Ezzedine K, Hamzavi I, et al. Once-daily upadacitinib versus placebo in adults with extensive non-segmental vitiligo: a phase 2, multicentre, randomised, double-blind, placebo-controlled, dose-ranging study. EClinicalMedicine. 2024;73:102655. doi:10.1016 /j.eclinm.2024.102655
- McKesey J, Pandya AG. A pilot study of 2% tofacitinib cream with narrowband ultraviolet B for the treatment of facial vitiligo. J Am Acad Dermatol. 2019;81:646-648. doi:10.1016/j.jaad.2019.04.032
- Mobasher P, Guerra R, Li SJ, et al. Open-label pilot study of tofacitinib 2% for the treatment of refractory vitiligo. Brit J Dermatol. 2020;182:1047-1049. doi:10.1111/bjd.18606
- Rosmarin D, Pandya AG, Lebwohl M, et al. Ruxolitinib cream for treatment of vitiligo: a randomised, controlled, phase 2 trial. Lancet. 2020;396:110-120. doi:10.1016/S0140-6736(20)30609-7
- Rosmarin D, Passeron T, Pandya AG, et al; TRuE-V Study Group. Two phase 3, randomized, controlled trials of ruxolitinib cream for vitiligo. N Engl J Med. 2022;387:1445-1455. doi:10.1056/NEJMoa2118828
- FDA. FDA approves topical treatment addressing repigmentation in vitiligo in patients aged 12 and older. Published July 19, 2022. Accessed January 30, 2025. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-topical-treatment-addressing-repigmentation-vitiligo-patients-aged-12-and-older
- Quintás-Cardama A, Vaddi K, Liu P, et al. Preclinical characterization of the selective JAK1/2 inhibitor INCB018424: therapeutic implications for the treatment of myeloproliferative neoplasms. Blood. 2010;115:3109-3117. doi:10.1182/blood-2009-04-214957
- Seneschal J, Wolkerstorfer A, Desai SR, et al. Efficacy and safety of ruxolitinib cream for the treatment of vitiligo by patient demographics and baseline clinical characteristics: week 52 pooled subgroup analysis from two randomized phase 3 studies. Brit J Dermatol. 2023;188 (suppl 1):ljac106.006. doi:10.1093/bjd/ljac106.006
- Hamzavi I, Rosmarin D, Harris JE, et al. Efficacy of ruxolitinib cream in vitiligo by patient characteristics and affected body areas: descriptive subgroup analyses from a phase 2, randomized, double-blind trial. J Am Acad Dermatol. 2022;86:1398-1401. doi:10.1016/j.jaad.2021.05.047
- Inoue S, Suzuki T, Sano S, et al. JAK inhibitors for the treatment of vitiligo. J Dermatol Sci. 2024;113:86-92. doi:10.1016/j.jdermsci.2023.12.008
- Peeva E, Yamaguchi Y, Ye Z, et al. Efficacy and safety of ritlecitinib in vitiligo patients across Fitzpatrick skin types with biomarker analyses. Exp Dermatol. 2024;33:E15177. doi:10.1111/exd.15177
- Mu Y, Pan T, Chen L. Treatment of refractory segmental vitiligo and alopecia areata in a child with upadacitinib and NB-UVB: a case report. Clin Cosmet Investig Dermatol. 2024;17:1789-1792. doi:10.2147 /CCID.S467026
- Shah RR, McMichael A. Resistant vitiligo treated with tofacitinib and sustained repigmentation after discontinuation. Skinmed. 2024;22:384-385.
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Emerging Insights in Vitiligo Therapeutics: A Focus on Oral and Topical JAK Inhibitors
Pink Papule on the Lower Eyelid
Pink Papule on the Lower Eyelid
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11
Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5
Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13
Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4
Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11

Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5

Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13

Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4

Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
THE DIAGNOSIS: Poroma
Poromas are benign adnexal neoplasms that often are classified into the broader category of acrospiromas. They most commonly affect areas with a high density of eccrine sweat glands, such as the palms and soles, but also can appear in any area of the body with sweat glands.1 Poromas may have cuboidal eccrine cells with ovoid nuclei and a delicate vascularized stroma on histology or may show apocrinelike features with sebaceous cells.2,3 Immunohistochemically, poromas stain positively for carcinoembryonic antigen, epithelial membrane antigen, and periodic acid–Schiff (PAS) with diastase sensitivity.1,4 Cytokeratin (CK) 1 and CK-10 are expressed in the tumor nests.1
Poromas are the benign counterpart of porocarcinomas, which can recur and may become invasive and metastasize. Porocarcinomas have been shown to undergo malignant transformation from poromas as well as develop de novo.5 Histologic differentiation between the 2 conditions is key in determining excisional margins for treatment and follow-up. Poromas are histologically similar to porocarcinomas, but the latter show invasion into the dermis, nuclear and cytoplasmic pleomorphism, nuclear hyperchromatism, and increased mitotic activity.6 S-100 protein can be positive in porocarcinoma.7 Both poromas and porocarcinomas are associated with Yes-associated protein 1 (YAP1), Mastermind-like protein 2 (MAML2), and NUT midline carcinoma family member 1 (NUTM1) gene fusions.5
Basal cell carcinoma (BCC) is the most common cutaneous malignancy. It rarely metastasizes but can be locally destructive.8 Basal cell carcinomas typically occur on sun-exposed skin in middle-aged and elderly patients and classically manifest as pink or flesh-colored pearly papules with rolled borders and overlying telangiectasia.9 Risk factors for BCC include a chronic sun exposure, lighter skin phenotypes, immunosuppression, and a family history of skin cancer. The 2 most common subtypes of BCC are nodular and superficial, which comprise around 85% of BCCs.10 Histologically, nodular BCCs demonstrate nests of malignant basaloid cells with central disorganization, peripheral palisading, tumor-stroma clefting, and a mucoid stroma with spindle cells (Figure 1). Superficial BCC manifests with small islands of malignant basaloid cells with peripheral palisading that connect with the epidermis, often with a lichenoid inflammatory infiltrate.9 Basal cell carcinomas stain positively for Ber-EP4 and are associated with patched 1 (PTCH1), patched 2 (PTCH2), and tumor protein 53 (TP53) gene mutations.9,11

Spiradenomas are benign adnexal tumors manifesting as painful, usually singular, 1- to 3-cm nodules in younger adults.12 Histologically, spiradenomas have large clusters of small irregularly shaped aggregations of small basaloid and large polygonal cells with surrounding hyalinized basement membrane material and intratumoral lymphocytes (Figure 2).4 Spiradenomas stain positive for p63, D2-40, and CK7 and are associated with cylindromatosis lysine 63 deubiquitinase (CYLD) and alpha-protein kinase 1 (ALPK1) gene mutations.5

Squamous cell carcinoma (SCC) is the second most common nonmelanoma skin cancer worldwide.13 Lesions typically develop on sun-exposed skin and manifest as red, hyperkeratotic, and sometimes ulcerated plaques or nodules.14 Risk factors for SCC include chronic sun exposure, lighter skin phenotypes, increased age, and immunosuppression. Histologically, there are several variants of SCC: low-risk variants include keratoacanthomas, verrucous carcinomas, and clear cell SCC, and high-risk variants include acantholytic SCC, spindle cell SCC, and adenosquamous carcinoma.14 Generally, low-grade SCC will have well-differentiated or moderately differentiated intercellular bridges or keratin pearls with tumor cells in a solid or sheetlike pattern (Figure 3). High-grade SCC will be poorly differentiated with the presence of infiltrating individual tumor cells.15 Immunohistochemically, SCC stains positive for p63, p40, AE1/AE3, CK5/6, and MNF116 while Ber-Ep4 is negative.14,15 Poorly differentiated SCCs have high rates of mutation, commonly in the tumor protein 53 (TP53), Cyclin-dependent kinase inhibitor 2A (CDKN2A), Ras pathway, and notch receptor 1 (NOTCH-1) genes.13

Syringomas are benign adnexal tumors that manifest as multiple soft, yellow to flesh-colored, 1- to 2-mm papules typically located on the lower eyelids, most commonly in women of reproductive age.16 Syringomas are described on histology as small comma-shaped nests with cords of eosinophilic to clear cells with central ducts surrounded by a sclerotic stroma (Figure 4). They stain positively for carcinoembryonic antigen, epithelial membrane antigen, and CK-5 and are associated with genetic mutations in phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA) and AKT serine/threonine kinase 1 (ATK1).4

Due to its regular exposure to sunlight, the eyelid accounts for 5% to 10% of all skin malignancies. Common eyelid lesions include squamous papilloma, seborrheic keratosis, epidermal inclusion cyst, hidrocystoma, intradermal nevus, BCC, SCC, and sebaceous carcinoma.17 Aside from syringomas, benign sweat gland tumors like poromas, hidradenomas, and spiradenomas usually do not manifest on the eyelids but should be included in the differential diagnosis of an unidentifiable lesion due to the small risk for malignant transformation. Eyelid poromas manifest polymorphically, most commonly being clinically diagnosed as BCC, making the histologic examination key for proper diagnosis and management.18
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
- Patterson J. Weedon’s Skin Pathology. 5th ed. Elsevier Limited; 2021.
- Aoki K, Baba S, Nohara T, et al. Eccrine poroma. J Dermatol. 1980; 7:263-269. doi:10.1111/j.1346-8138.1980.tb01967.x
- Harvell JD, Kerschmann RL, LeBoit PE. Eccrine or apocrine poroma? six poromas with divergent adnexal differentiation. Am J Dermatopathol. 1996;18:1-9. doi:10.1097/00000372-199602000-00001
- Miller AC, Adjei S, Temiz LA, et al. Dermal duct tumor: a diagnostic dilemma. Dermatopathology. 2022;9:36-47. doi:10.3390
- Macagno N, Sohier P, Kervarrec T, et al. Recent advances on immunohistochemistry and molecular biology for the diagnosis of adnexal sweat gland tumors. Cancers. 2022;14:476. doi:10.3390/cancers14030476
- Robson A, Greene J, Ansari N, et al. Eccrine porocarcinoma (malignant eccrine poroma): a clinicopathologic study of 69 cases. Am J Surg Pathol. 2001;25:710-720. doi:10.1097/00000478-200106000-00002 /dermatopathology9010007
- Kurisu Y, Tsuji M, Yasuda E, et al. A case of eccrine porocarcinoma: usefulness of immunostain for S-100 protein in the diagnoses of recurrent and metastatic dedifferentiated lesions. Ann Dermatol. 2013;25:348-351. doi:10.5021/ad.2013.25.3.348
- Stanoszek LM, Wang GY, Harms PW. Histologic mimics of basal cell carcinoma. Arch Pathol Lab Med. 2017;141:1490-1502. doi:10.5858 /arpa.2017-0222-RA
- Niculet E, Craescu M, Rebegea L, et al. Basal cell carcinoma: comprehensive clinical and histopathological aspects, novel imaging tools and therapeutic approaches (review). Exp Ther Med. 2022;23:60. doi:10.3892/etm.2021.10982
- Pelucchi C, Di Landro A, Naldi L, et al. Risk factors for histological types and anatomic sites of cutaneous basal-cell carcinoma: an Italian case-control study. J Invest Dermatol. 2007;127:935-944. doi:10.1038/sj.jid.5700598
- Sunjaya AP, Sunjaya AF, Tan ST. The use of BEREP4 immunohistochemistry staining for detection of basal cell carcinoma. J Skin Cancer. 2017;2017:2692604. doi:10.1155/2017/2692604
- Kim J, Yang HJ, Pyo JS. Eccrine spiradenoma of the scalp. Arch Craniofacial Surg. 2017;18:211-213. doi:10.7181/acfs.2017.18.3.211
- Que SKT, Zwald FO, Schmults CD. Cutaneous squamous cell carcinoma: incidence, risk factors, diagnosis, and staging. J Am Acad Dermatol. 2018;78:237-247. doi:10.1016/j.jaad.2017.08.059
- Waldman A, Schmults C. Cutaneous squamous cell carcinoma. Hematol Oncol Clin North Am. 2019;33:1-12. doi:10.1016/j.hoc.2018.08.001
- Yanofsky VR, Mercer SE, Phelps RG. Histopathological variants of cutaneous squamous cell carcinoma: a review. J Skin Cancer. 2011;2011:210813. doi:10.1155/2011/210813
- Lee JH, Chang JY, Lee KH. Syringoma: a clinicopathologic and immunohistologic study and results of treatment. Yonsei Med J. 2007;48:35-40. doi:10.3349/ymj.2007.48.1.35
- Adamski WZ, Maciejewski J, Adamska K, et al. The prevalence of various eyelid skin lesions in a single-centre observation study. Adv Dermatol Allergol Dermatol Alergol. 2021;38:804-807. doi:10.5114 /ada.2020.95652
- Mencía-Gutiérrez E, Navarro-Perea C, Gutiérrez-Díaz E, et al. Eyelid eccrine poroma: a case report and review of literature. Cureus. 202:12:E8906. doi:10.7759/cureus.8906
Pink Papule on the Lower Eyelid
Pink Papule on the Lower Eyelid
A 57-year-old man with no notable medical history presented to the dermatology clinic for evaluation of an asymptomatic papule on the left lower eyelid. The patient reported that the lesion seemed to wax and wane in size over time. Physical examination revealed a small, pink, verrucous papule on the left lower eyelid. A shave biopsy of the lesion revealed a well-circumscribed collection of small, monomorphic, cuboidal cells with basophilic round nuclei, inconspicuous nucleoli, and compact eosinophilic cytoplasm (top) with focal areas of duct formation (bottom) that was sharply demarcated from normal keratinocytes.
Bilateral Brownish-Red Indurated Facial Plaques in an Adult Man
Bilateral Brownish-Red Indurated Facial Plaques in an Adult Man
THE DIAGNOSIS: Granuloma Faciale
Histology revealed a dense mixed inflammatory cell infiltrate with conspicuous neutrophils and eosinophils in the upper to mid dermis with a narrow uninvolved grenz zone beneath the epidermis (Figures 1 and 2). These findings along with the clinical presentation (Figure 3) were consistent with a diagnosis of granuloma faciale (GF). Most often seen in middle-aged White men, GF is an uncommon localized inflammatory skin condition that often manifests as a single, well-defined, red-to-brown papule, nodule, or plaque on the face or other sun-exposed areas of the skin. Since numerous other skin diseases manifest similarly to GF, biopsy is necessary for definitive diagnosis.1 Histopathology of GF classically shows a mixed inflammatory infiltrate with a narrow band of uninvolved dermis separating it from the epidermis (grenz zone). Dilated follicular plugs and vascular changes frequently are appreciated. Despite its name, GF does not include granulomas and is thought to be similar to leukocytoclastic vasculitis.1 Reports of GF in the literature have shown immunohistochemical staining with the presence of CD4+ lymphocytes that secrete IL-5, a chemotactic agent responsible for attracting eosinophils that contributes to the eosinophilic infiltrate on histology.2
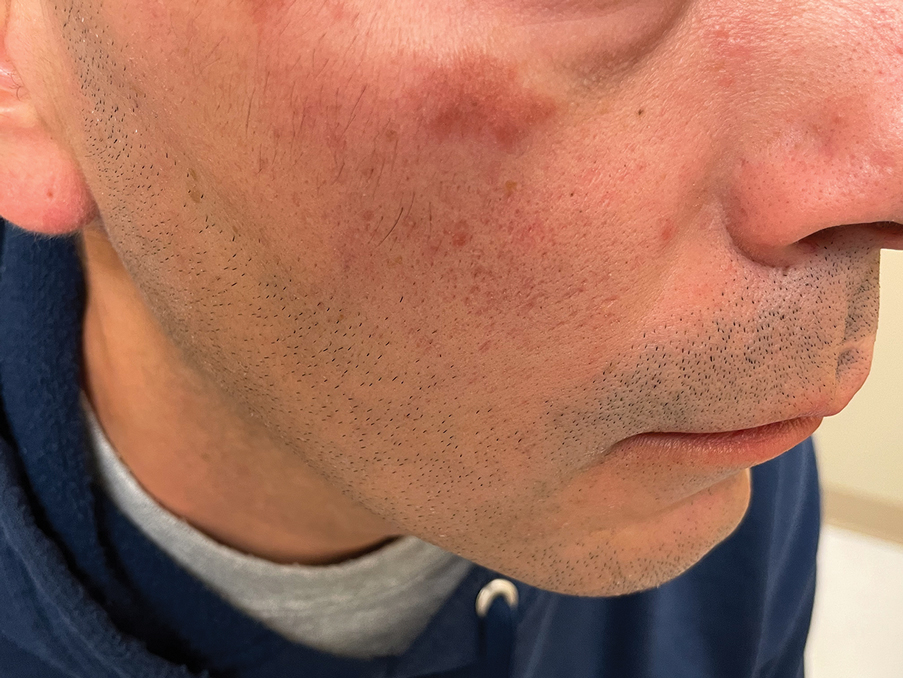
Topical corticosteroids and topical tacrolimus are the first-line treatments for GF. Intralesional corticosteroids also are a treatment option and can be used in combination with cryotherapy.1,3 Additionally, both topical and oral dapsone have been shown to be effective for GF.1 Oral dapsone is given at a dose of 50 mg to 150 mg once daily.1 Clofazimine, typically used as an antileprosy treatment, also has been efficacious in treating GF. Clofazimine has anti-inflammatory and antiproliferative effects on lymphocytes that may attenuate the inflammation underlying GF. It is prescribed at a dose of 300 mg once daily for 3 to 5 months.1
The differential diagnosis for GF is broad and includes tumid lupus erythematosus, Jessner lymphocytic infiltrate (JLI), cutaneous sarcoidosis, and mycosis fungoides. Tumid lupus erythematosus is a subtype of cutaneous lupus erythematosus that rarely is associated with systemic lupus manifestations. Tumid lupus erythematosus manifests as annular, indurated, erythematous plaques, whereas JLI manifests with erythematous papular to nodular lesions without scale on the upper back or face.4 Jessner lymphocytic infiltrate and tumid lupus erythematosus are histopathologically identical, with abundant dermal mucin deposition and a superficial and deep perivascular and periadnexal lymphocytic infiltrate. It is debatable whether JLI is a separate entity or a variant of tumid lupus erythematosus. Sarcoidosis is a granulomatous disease that manifests with a myriad of clinical features. The skin is the second most commonly involved organ.5 The most common morphology is numerous small, firm, nonscaly papules, typically on the face. Histology in cutaneous sarcoidosis will show lymphocyte-poor, noncaseating epithelioid cell granulomas with positive reticulin staining, which were not seen in our patient.6 Lastly, mycosis fungoides is the most common type of cutaneous T-cell lymphoma. It can manifest as patches, plaques, or tumors. The plaque stage may mimic GF as lesions are infiltrative, annular, and raised, with well-defined margins. Histopathology will show intraepidermal lymphocytes out of proportion with spongiosis.7
- Al Dhafiri M, Kaliyadan F. Granuloma faciale. StatPearls Publishing. Updated July 4, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539832/
- Chen A, Harview CL, Rand SE, et al. Refractory granuloma faciale successfully treated with adjunct topical JAK inhibitor. JAAD Case Rep. 2023;33:91-94. doi:10.1016/j.jdcr.2023.01.016
- Dowlati B, Firooz A, Dowlati Y. Granuloma faciale: successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-551. doi:10.1046 /j.1365-4362.1997.00161.x
- Koritala T, Grubbs H, Crane J. Tumid lupus erythematosus. StatPearls Publishing. Updated June 28, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482515/
- Caplan A, Rosenbach M, Imadojemu S. Cutaneous sarcoidosis. Semin Respir Crit Care Med. 2020;41:689-699. doi:10.1055/s-0040-1713130
- Singh P, Jain E, Dhingra H, et al. Clinico-pathological spectrum of cutaneous sarcoidosis: an experience from a government institute in North India. Med Pharm Rep. 2020;93:241-245. doi:10.15386 /mpr-1384
- Vaidya T, Badri T. Mycosis fungoides. StatPearls Publishing. Updated July 31, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519572/
THE DIAGNOSIS: Granuloma Faciale
Histology revealed a dense mixed inflammatory cell infiltrate with conspicuous neutrophils and eosinophils in the upper to mid dermis with a narrow uninvolved grenz zone beneath the epidermis (Figures 1 and 2). These findings along with the clinical presentation (Figure 3) were consistent with a diagnosis of granuloma faciale (GF). Most often seen in middle-aged White men, GF is an uncommon localized inflammatory skin condition that often manifests as a single, well-defined, red-to-brown papule, nodule, or plaque on the face or other sun-exposed areas of the skin. Since numerous other skin diseases manifest similarly to GF, biopsy is necessary for definitive diagnosis.1 Histopathology of GF classically shows a mixed inflammatory infiltrate with a narrow band of uninvolved dermis separating it from the epidermis (grenz zone). Dilated follicular plugs and vascular changes frequently are appreciated. Despite its name, GF does not include granulomas and is thought to be similar to leukocytoclastic vasculitis.1 Reports of GF in the literature have shown immunohistochemical staining with the presence of CD4+ lymphocytes that secrete IL-5, a chemotactic agent responsible for attracting eosinophils that contributes to the eosinophilic infiltrate on histology.2



Topical corticosteroids and topical tacrolimus are the first-line treatments for GF. Intralesional corticosteroids also are a treatment option and can be used in combination with cryotherapy.1,3 Additionally, both topical and oral dapsone have been shown to be effective for GF.1 Oral dapsone is given at a dose of 50 mg to 150 mg once daily.1 Clofazimine, typically used as an antileprosy treatment, also has been efficacious in treating GF. Clofazimine has anti-inflammatory and antiproliferative effects on lymphocytes that may attenuate the inflammation underlying GF. It is prescribed at a dose of 300 mg once daily for 3 to 5 months.1
The differential diagnosis for GF is broad and includes tumid lupus erythematosus, Jessner lymphocytic infiltrate (JLI), cutaneous sarcoidosis, and mycosis fungoides. Tumid lupus erythematosus is a subtype of cutaneous lupus erythematosus that rarely is associated with systemic lupus manifestations. Tumid lupus erythematosus manifests as annular, indurated, erythematous plaques, whereas JLI manifests with erythematous papular to nodular lesions without scale on the upper back or face.4 Jessner lymphocytic infiltrate and tumid lupus erythematosus are histopathologically identical, with abundant dermal mucin deposition and a superficial and deep perivascular and periadnexal lymphocytic infiltrate. It is debatable whether JLI is a separate entity or a variant of tumid lupus erythematosus. Sarcoidosis is a granulomatous disease that manifests with a myriad of clinical features. The skin is the second most commonly involved organ.5 The most common morphology is numerous small, firm, nonscaly papules, typically on the face. Histology in cutaneous sarcoidosis will show lymphocyte-poor, noncaseating epithelioid cell granulomas with positive reticulin staining, which were not seen in our patient.6 Lastly, mycosis fungoides is the most common type of cutaneous T-cell lymphoma. It can manifest as patches, plaques, or tumors. The plaque stage may mimic GF as lesions are infiltrative, annular, and raised, with well-defined margins. Histopathology will show intraepidermal lymphocytes out of proportion with spongiosis.7
THE DIAGNOSIS: Granuloma Faciale
Histology revealed a dense mixed inflammatory cell infiltrate with conspicuous neutrophils and eosinophils in the upper to mid dermis with a narrow uninvolved grenz zone beneath the epidermis (Figures 1 and 2). These findings along with the clinical presentation (Figure 3) were consistent with a diagnosis of granuloma faciale (GF). Most often seen in middle-aged White men, GF is an uncommon localized inflammatory skin condition that often manifests as a single, well-defined, red-to-brown papule, nodule, or plaque on the face or other sun-exposed areas of the skin. Since numerous other skin diseases manifest similarly to GF, biopsy is necessary for definitive diagnosis.1 Histopathology of GF classically shows a mixed inflammatory infiltrate with a narrow band of uninvolved dermis separating it from the epidermis (grenz zone). Dilated follicular plugs and vascular changes frequently are appreciated. Despite its name, GF does not include granulomas and is thought to be similar to leukocytoclastic vasculitis.1 Reports of GF in the literature have shown immunohistochemical staining with the presence of CD4+ lymphocytes that secrete IL-5, a chemotactic agent responsible for attracting eosinophils that contributes to the eosinophilic infiltrate on histology.2



Topical corticosteroids and topical tacrolimus are the first-line treatments for GF. Intralesional corticosteroids also are a treatment option and can be used in combination with cryotherapy.1,3 Additionally, both topical and oral dapsone have been shown to be effective for GF.1 Oral dapsone is given at a dose of 50 mg to 150 mg once daily.1 Clofazimine, typically used as an antileprosy treatment, also has been efficacious in treating GF. Clofazimine has anti-inflammatory and antiproliferative effects on lymphocytes that may attenuate the inflammation underlying GF. It is prescribed at a dose of 300 mg once daily for 3 to 5 months.1
The differential diagnosis for GF is broad and includes tumid lupus erythematosus, Jessner lymphocytic infiltrate (JLI), cutaneous sarcoidosis, and mycosis fungoides. Tumid lupus erythematosus is a subtype of cutaneous lupus erythematosus that rarely is associated with systemic lupus manifestations. Tumid lupus erythematosus manifests as annular, indurated, erythematous plaques, whereas JLI manifests with erythematous papular to nodular lesions without scale on the upper back or face.4 Jessner lymphocytic infiltrate and tumid lupus erythematosus are histopathologically identical, with abundant dermal mucin deposition and a superficial and deep perivascular and periadnexal lymphocytic infiltrate. It is debatable whether JLI is a separate entity or a variant of tumid lupus erythematosus. Sarcoidosis is a granulomatous disease that manifests with a myriad of clinical features. The skin is the second most commonly involved organ.5 The most common morphology is numerous small, firm, nonscaly papules, typically on the face. Histology in cutaneous sarcoidosis will show lymphocyte-poor, noncaseating epithelioid cell granulomas with positive reticulin staining, which were not seen in our patient.6 Lastly, mycosis fungoides is the most common type of cutaneous T-cell lymphoma. It can manifest as patches, plaques, or tumors. The plaque stage may mimic GF as lesions are infiltrative, annular, and raised, with well-defined margins. Histopathology will show intraepidermal lymphocytes out of proportion with spongiosis.7
- Al Dhafiri M, Kaliyadan F. Granuloma faciale. StatPearls Publishing. Updated July 4, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539832/
- Chen A, Harview CL, Rand SE, et al. Refractory granuloma faciale successfully treated with adjunct topical JAK inhibitor. JAAD Case Rep. 2023;33:91-94. doi:10.1016/j.jdcr.2023.01.016
- Dowlati B, Firooz A, Dowlati Y. Granuloma faciale: successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-551. doi:10.1046 /j.1365-4362.1997.00161.x
- Koritala T, Grubbs H, Crane J. Tumid lupus erythematosus. StatPearls Publishing. Updated June 28, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482515/
- Caplan A, Rosenbach M, Imadojemu S. Cutaneous sarcoidosis. Semin Respir Crit Care Med. 2020;41:689-699. doi:10.1055/s-0040-1713130
- Singh P, Jain E, Dhingra H, et al. Clinico-pathological spectrum of cutaneous sarcoidosis: an experience from a government institute in North India. Med Pharm Rep. 2020;93:241-245. doi:10.15386 /mpr-1384
- Vaidya T, Badri T. Mycosis fungoides. StatPearls Publishing. Updated July 31, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519572/
- Al Dhafiri M, Kaliyadan F. Granuloma faciale. StatPearls Publishing. Updated July 4, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK539832/
- Chen A, Harview CL, Rand SE, et al. Refractory granuloma faciale successfully treated with adjunct topical JAK inhibitor. JAAD Case Rep. 2023;33:91-94. doi:10.1016/j.jdcr.2023.01.016
- Dowlati B, Firooz A, Dowlati Y. Granuloma faciale: successful treatment of nine cases with a combination of cryotherapy and intralesional corticosteroid injection. Int J Dermatol. 1997;36:548-551. doi:10.1046 /j.1365-4362.1997.00161.x
- Koritala T, Grubbs H, Crane J. Tumid lupus erythematosus. StatPearls Publishing. Updated June 28, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK482515/
- Caplan A, Rosenbach M, Imadojemu S. Cutaneous sarcoidosis. Semin Respir Crit Care Med. 2020;41:689-699. doi:10.1055/s-0040-1713130
- Singh P, Jain E, Dhingra H, et al. Clinico-pathological spectrum of cutaneous sarcoidosis: an experience from a government institute in North India. Med Pharm Rep. 2020;93:241-245. doi:10.15386 /mpr-1384
- Vaidya T, Badri T. Mycosis fungoides. StatPearls Publishing. Updated July 31, 2023. Accessed February 18, 2025. https://www.ncbi.nlm.nih.gov/books/NBK519572/
Bilateral Brownish-Red Indurated Facial Plaques in an Adult Man
Bilateral Brownish-Red Indurated Facial Plaques in an Adult Man
A 44-year-old man presented to the dermatology clinic with a facial rash of 2 years’ duration. The patient reported associated pruritus but no systemic symptoms. His medical history was relevant for childhood eczema. He had tried various over-the-counter treatments for the facial rash, including topical hydrocortisone, neomycin/bacitracin/polymyxin antibiotic ointment, moisturizers, and antihistamines, with no success. Physical examination demonstrated symmetric, well-circumscribed, circinate, brownish-red, indurated plaques without scaling on the cheeks. A 4-mm punch biopsy was obtained from a plaque on the left cheek.
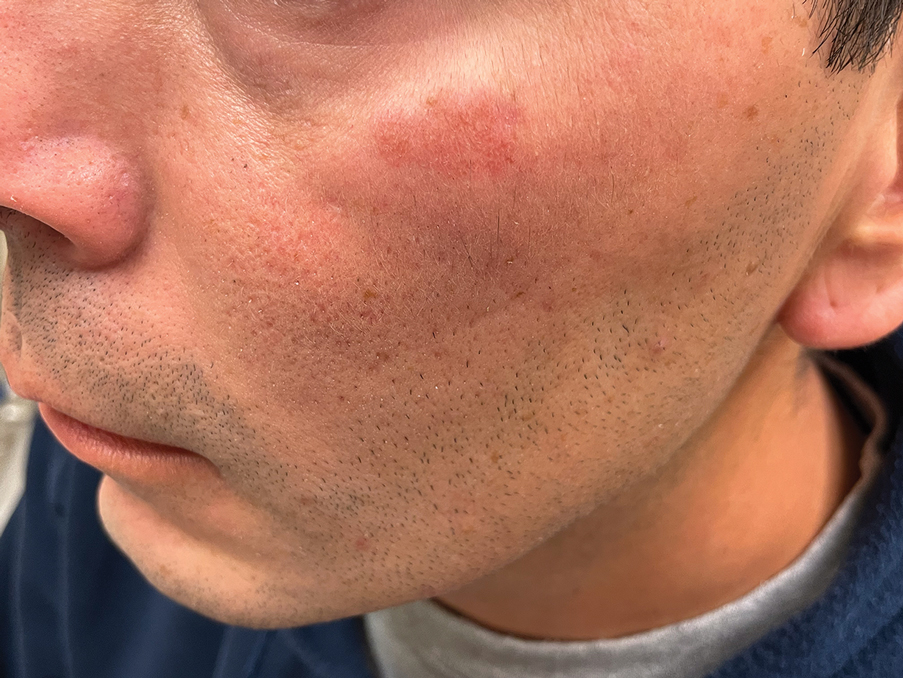
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
THE DIAGNOSIS: Nail-Patella Syndrome
Nail-patella syndrome (NPS) is an autosomaldominant disorder that is present in approximately 1 in 50,000 live births worldwide.1,2 It manifests with a spectrum of clinical findings affecting the nails, skeletal system, kidneys, and eyes.3 Most cases of NPS are caused by loss-of-function mutations in LMX1B,1 a gene encoding the LIM homeobox transcription factor.4 The LMX1B gene plays a critical role in the dorsoventral patterning of developing limbs.5 Mutations of this gene impair the development and function of podocytes and glomerular filtration slits6 and have been found to affect the development of the dopaminergic and mesencephalic serotoninergic neurons.2 Approximately 5% of patients with NPS have an unexplained genetic cause, suggesting an alternate mechanism for disease.1 Loss-of-function mutations also were observed in the Wnt inhibitory factor 1 gene (WIF1) in a family with an NPS-like presentation and could represent a novel cause of the condition.1 Regardless, NPS may be diagnosed clinically based on characteristic medical history, imaging, and physical examination findings.
Nail changes are the most consistent feature of NPS. The nails may be absent, hypoplastic, dystrophic, ridged (horizontally or vertically), or pitted or may demonstrate characteristic triangular lacunae. Nail findings often are congenital, bilateral, and symmetrical. The first digits typically are most severely affected, with progressive improvement appreciated toward the fifth fingers, as seen in our patient. The nail changes can be subtle, sometimes manifesting only as a single triangular lacuna on both thumbnails. Toenail involvement is less common and, when present, tends to be even more discreet. In contrast to the fingernails, the fifth toenails are most commonly affected.7
There are many skeletal manifestations of NPS. Patellae may be absent, hypoplastic, or irregularly shaped on physical examination or imaging, and changes may involve one or both knees. The Figure shows plain radiographs of the knees with bilateral patellar subluxation. Elbow dysplasia or radial head subluxation may result in physical limitations in extension, pronation, or supination of the joint.7 In approximately 70% of patients seen with the disorder, imaging may reveal symmetric posterior and lateral bony projections from the iliac crests, known as iliac horns; when present, these are considered pathognomonic.8
Open-angle glaucoma is the most common ocular finding in NPS. Other less commonly associated eye abnormalities include hyperpigmentation of the pupillary margin (Lester iris).6 Renal involvement occurs in 30% to 50% of patients with NPS and is the main predictor of mortality, with percentages as high as 5% to 14%.7 Defects occur in the glomerular basement membrane and manifest clinically with hematuria and/or proteinuria. The course of proteinuria is unpredictable. Some cases remit spontaneously, while others remain asymptomatic, progress to nephrotic syndrome, or, although rare, advance to renal failure.7,9
Bowel symptoms, neurologic problems, vasomotor concerns, thin dental enamel, attention-deficit disorder or attention-deficit/hyperactivity disorder, and major depressive disorder all have been reported in association with NPS.2,7
Nail psoriasis typically exhibits nail pitting and onycholysis. Other manifestations include subungual hyperkeratosis, oil drop discoloration, and splinter hemorrhages. Topical and intralesional treatments are used to manage symptoms of the disease, as it can become debilitating if left untreated, unlike the nail disease seen in NPS.10 Onychomycosis can have a similar manifestation to psoriasis with sublingual hyperkeratosis of the nail, but it usually is caused by dermatophytes or yeasts such as Candida albicans. Onycholysis and thickening of the subungual region also can be seen. Diagnosis relies on direct microscopy and fungal culture, and a thorough patient history will help distinguish fungal vs nonfungal etiology. New-generation antifungals are used to eradicate the infection.11 Leukonychia manifests with white-appearing nails due to nail-plate or nail-bed abnormalities. Leukonychia can have multisystem involvement, but nails demonstrate a white discoloration rather than the other abnormalities discussed here.12 Hypohidrotic ectodermal dysplasia is a rare hereditary congenital disease that affects ectodermal structures and manifests with a triad of symptoms: hypotrichosis, hypohidrosis, and hypodontia. The condition often manifests in childhood with characteristic features such as light-pigmented sparse and fine hair. Physical growth as well as psychomotor development are within normal limits. Neither bone nor renal involvement is typical for hypohidrotic ectodermal dysplasia.13
Our case highlights the typical manifestation of NPS with multisystem involvement, demonstrating the complexity of the disease. For cases in which a clinical diagnosis of NPS is uncertain, gene-targeted or comprehensive genomic testing is recommended, as well as genetic counseling. Given the broad spectrum of clinical manifestations, it is imperative that patients undergo screening for musculoskeletal, renal, and ophthalmologic involvement. Treatment is targeted at symptom management and prevention of long-term complications, reliant on clinical presentation, and specific to each patient.
- Jones MC, Topol SE, Rueda M, et al. Mutation of WIF1: a potential novel cause of a nail-patella–like disorder. Genet Med. 2017;19:1179-1183.
- López-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in Nail-patella syndrome: potential association with LMX1B loss-of-function. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016; 30:1614-1617.
- Vollrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091-1098. Published correction appears in Hum Mol Genet. 1998;7:1333.
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in LMX1B mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-55.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington; 2003.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey. Orthop Traumatol Surg Res. 2015;101:959-962.
- Harita Y, Urae S, Akashio R, et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet. 2020;28:1414-1421.
- Tan ES, Chong WS, Tey HL. Nail psoriasis. Am J Clin Dermatol. 2012; 13:375-388.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193.
- Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1993-2024.
THE DIAGNOSIS: Nail-Patella Syndrome
Nail-patella syndrome (NPS) is an autosomaldominant disorder that is present in approximately 1 in 50,000 live births worldwide.1,2 It manifests with a spectrum of clinical findings affecting the nails, skeletal system, kidneys, and eyes.3 Most cases of NPS are caused by loss-of-function mutations in LMX1B,1 a gene encoding the LIM homeobox transcription factor.4 The LMX1B gene plays a critical role in the dorsoventral patterning of developing limbs.5 Mutations of this gene impair the development and function of podocytes and glomerular filtration slits6 and have been found to affect the development of the dopaminergic and mesencephalic serotoninergic neurons.2 Approximately 5% of patients with NPS have an unexplained genetic cause, suggesting an alternate mechanism for disease.1 Loss-of-function mutations also were observed in the Wnt inhibitory factor 1 gene (WIF1) in a family with an NPS-like presentation and could represent a novel cause of the condition.1 Regardless, NPS may be diagnosed clinically based on characteristic medical history, imaging, and physical examination findings.
Nail changes are the most consistent feature of NPS. The nails may be absent, hypoplastic, dystrophic, ridged (horizontally or vertically), or pitted or may demonstrate characteristic triangular lacunae. Nail findings often are congenital, bilateral, and symmetrical. The first digits typically are most severely affected, with progressive improvement appreciated toward the fifth fingers, as seen in our patient. The nail changes can be subtle, sometimes manifesting only as a single triangular lacuna on both thumbnails. Toenail involvement is less common and, when present, tends to be even more discreet. In contrast to the fingernails, the fifth toenails are most commonly affected.7
There are many skeletal manifestations of NPS. Patellae may be absent, hypoplastic, or irregularly shaped on physical examination or imaging, and changes may involve one or both knees. The Figure shows plain radiographs of the knees with bilateral patellar subluxation. Elbow dysplasia or radial head subluxation may result in physical limitations in extension, pronation, or supination of the joint.7 In approximately 70% of patients seen with the disorder, imaging may reveal symmetric posterior and lateral bony projections from the iliac crests, known as iliac horns; when present, these are considered pathognomonic.8

Open-angle glaucoma is the most common ocular finding in NPS. Other less commonly associated eye abnormalities include hyperpigmentation of the pupillary margin (Lester iris).6 Renal involvement occurs in 30% to 50% of patients with NPS and is the main predictor of mortality, with percentages as high as 5% to 14%.7 Defects occur in the glomerular basement membrane and manifest clinically with hematuria and/or proteinuria. The course of proteinuria is unpredictable. Some cases remit spontaneously, while others remain asymptomatic, progress to nephrotic syndrome, or, although rare, advance to renal failure.7,9
Bowel symptoms, neurologic problems, vasomotor concerns, thin dental enamel, attention-deficit disorder or attention-deficit/hyperactivity disorder, and major depressive disorder all have been reported in association with NPS.2,7
Nail psoriasis typically exhibits nail pitting and onycholysis. Other manifestations include subungual hyperkeratosis, oil drop discoloration, and splinter hemorrhages. Topical and intralesional treatments are used to manage symptoms of the disease, as it can become debilitating if left untreated, unlike the nail disease seen in NPS.10 Onychomycosis can have a similar manifestation to psoriasis with sublingual hyperkeratosis of the nail, but it usually is caused by dermatophytes or yeasts such as Candida albicans. Onycholysis and thickening of the subungual region also can be seen. Diagnosis relies on direct microscopy and fungal culture, and a thorough patient history will help distinguish fungal vs nonfungal etiology. New-generation antifungals are used to eradicate the infection.11 Leukonychia manifests with white-appearing nails due to nail-plate or nail-bed abnormalities. Leukonychia can have multisystem involvement, but nails demonstrate a white discoloration rather than the other abnormalities discussed here.12 Hypohidrotic ectodermal dysplasia is a rare hereditary congenital disease that affects ectodermal structures and manifests with a triad of symptoms: hypotrichosis, hypohidrosis, and hypodontia. The condition often manifests in childhood with characteristic features such as light-pigmented sparse and fine hair. Physical growth as well as psychomotor development are within normal limits. Neither bone nor renal involvement is typical for hypohidrotic ectodermal dysplasia.13
Our case highlights the typical manifestation of NPS with multisystem involvement, demonstrating the complexity of the disease. For cases in which a clinical diagnosis of NPS is uncertain, gene-targeted or comprehensive genomic testing is recommended, as well as genetic counseling. Given the broad spectrum of clinical manifestations, it is imperative that patients undergo screening for musculoskeletal, renal, and ophthalmologic involvement. Treatment is targeted at symptom management and prevention of long-term complications, reliant on clinical presentation, and specific to each patient.
THE DIAGNOSIS: Nail-Patella Syndrome
Nail-patella syndrome (NPS) is an autosomaldominant disorder that is present in approximately 1 in 50,000 live births worldwide.1,2 It manifests with a spectrum of clinical findings affecting the nails, skeletal system, kidneys, and eyes.3 Most cases of NPS are caused by loss-of-function mutations in LMX1B,1 a gene encoding the LIM homeobox transcription factor.4 The LMX1B gene plays a critical role in the dorsoventral patterning of developing limbs.5 Mutations of this gene impair the development and function of podocytes and glomerular filtration slits6 and have been found to affect the development of the dopaminergic and mesencephalic serotoninergic neurons.2 Approximately 5% of patients with NPS have an unexplained genetic cause, suggesting an alternate mechanism for disease.1 Loss-of-function mutations also were observed in the Wnt inhibitory factor 1 gene (WIF1) in a family with an NPS-like presentation and could represent a novel cause of the condition.1 Regardless, NPS may be diagnosed clinically based on characteristic medical history, imaging, and physical examination findings.
Nail changes are the most consistent feature of NPS. The nails may be absent, hypoplastic, dystrophic, ridged (horizontally or vertically), or pitted or may demonstrate characteristic triangular lacunae. Nail findings often are congenital, bilateral, and symmetrical. The first digits typically are most severely affected, with progressive improvement appreciated toward the fifth fingers, as seen in our patient. The nail changes can be subtle, sometimes manifesting only as a single triangular lacuna on both thumbnails. Toenail involvement is less common and, when present, tends to be even more discreet. In contrast to the fingernails, the fifth toenails are most commonly affected.7
There are many skeletal manifestations of NPS. Patellae may be absent, hypoplastic, or irregularly shaped on physical examination or imaging, and changes may involve one or both knees. The Figure shows plain radiographs of the knees with bilateral patellar subluxation. Elbow dysplasia or radial head subluxation may result in physical limitations in extension, pronation, or supination of the joint.7 In approximately 70% of patients seen with the disorder, imaging may reveal symmetric posterior and lateral bony projections from the iliac crests, known as iliac horns; when present, these are considered pathognomonic.8

Open-angle glaucoma is the most common ocular finding in NPS. Other less commonly associated eye abnormalities include hyperpigmentation of the pupillary margin (Lester iris).6 Renal involvement occurs in 30% to 50% of patients with NPS and is the main predictor of mortality, with percentages as high as 5% to 14%.7 Defects occur in the glomerular basement membrane and manifest clinically with hematuria and/or proteinuria. The course of proteinuria is unpredictable. Some cases remit spontaneously, while others remain asymptomatic, progress to nephrotic syndrome, or, although rare, advance to renal failure.7,9
Bowel symptoms, neurologic problems, vasomotor concerns, thin dental enamel, attention-deficit disorder or attention-deficit/hyperactivity disorder, and major depressive disorder all have been reported in association with NPS.2,7
Nail psoriasis typically exhibits nail pitting and onycholysis. Other manifestations include subungual hyperkeratosis, oil drop discoloration, and splinter hemorrhages. Topical and intralesional treatments are used to manage symptoms of the disease, as it can become debilitating if left untreated, unlike the nail disease seen in NPS.10 Onychomycosis can have a similar manifestation to psoriasis with sublingual hyperkeratosis of the nail, but it usually is caused by dermatophytes or yeasts such as Candida albicans. Onycholysis and thickening of the subungual region also can be seen. Diagnosis relies on direct microscopy and fungal culture, and a thorough patient history will help distinguish fungal vs nonfungal etiology. New-generation antifungals are used to eradicate the infection.11 Leukonychia manifests with white-appearing nails due to nail-plate or nail-bed abnormalities. Leukonychia can have multisystem involvement, but nails demonstrate a white discoloration rather than the other abnormalities discussed here.12 Hypohidrotic ectodermal dysplasia is a rare hereditary congenital disease that affects ectodermal structures and manifests with a triad of symptoms: hypotrichosis, hypohidrosis, and hypodontia. The condition often manifests in childhood with characteristic features such as light-pigmented sparse and fine hair. Physical growth as well as psychomotor development are within normal limits. Neither bone nor renal involvement is typical for hypohidrotic ectodermal dysplasia.13
Our case highlights the typical manifestation of NPS with multisystem involvement, demonstrating the complexity of the disease. For cases in which a clinical diagnosis of NPS is uncertain, gene-targeted or comprehensive genomic testing is recommended, as well as genetic counseling. Given the broad spectrum of clinical manifestations, it is imperative that patients undergo screening for musculoskeletal, renal, and ophthalmologic involvement. Treatment is targeted at symptom management and prevention of long-term complications, reliant on clinical presentation, and specific to each patient.
- Jones MC, Topol SE, Rueda M, et al. Mutation of WIF1: a potential novel cause of a nail-patella–like disorder. Genet Med. 2017;19:1179-1183.
- López-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in Nail-patella syndrome: potential association with LMX1B loss-of-function. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016; 30:1614-1617.
- Vollrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091-1098. Published correction appears in Hum Mol Genet. 1998;7:1333.
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in LMX1B mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-55.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington; 2003.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey. Orthop Traumatol Surg Res. 2015;101:959-962.
- Harita Y, Urae S, Akashio R, et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet. 2020;28:1414-1421.
- Tan ES, Chong WS, Tey HL. Nail psoriasis. Am J Clin Dermatol. 2012; 13:375-388.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193.
- Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1993-2024.
- Jones MC, Topol SE, Rueda M, et al. Mutation of WIF1: a potential novel cause of a nail-patella–like disorder. Genet Med. 2017;19:1179-1183.
- López-Arvizu C, Sparrow EP, Strube MJ, et al. Increased symptoms of attention deficit hyperactivity disorder and major depressive disorder symptoms in Nail-patella syndrome: potential association with LMX1B loss-of-function. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:59-66.
- Figueroa-Silva O, Vicente A, Agudo A, et al. Nail-patella syndrome: report of 11 pediatric cases. J Eur Acad Dermatol Venereol. 2016; 30:1614-1617.
- Vollrath D, Jaramillo-Babb VL, Clough MV, et al. Loss-of-function mutations in the LIM-homeodomain gene, LMX1B, in nail-patella syndrome. Hum Mol Genet. 1998;7:1091-1098. Published correction appears in Hum Mol Genet. 1998;7:1333.
- Chen H, Lun Y, Ovchinnikov D, et al. Limb and kidney defects in LMX1B mutant mice suggest an involvement of LMX1B in human nail patella syndrome. Nat Genet. 1998;19:51-55.
- Witzgall R. Nail-patella syndrome. Pflugers Arch. 2017;469:927-936.
- Sweeney E, Hoover-Fong JE, McIntosh I. Nail-patella syndrome. In: Adam MP, Ardinger HH, Pagon RA, et al, eds. GeneReviews. University of Washington; 2003.
- Tigchelaar S, Lenting A, Bongers EM, et al. Nail patella syndrome: knee symptoms and surgical outcomes. a questionnaire-based survey. Orthop Traumatol Surg Res. 2015;101:959-962.
- Harita Y, Urae S, Akashio R, et al. Clinical and genetic characterization of nephropathy in patients with nail-patella syndrome. Eur J Hum Genet. 2020;28:1414-1421.
- Tan ES, Chong WS, Tey HL. Nail psoriasis. Am J Clin Dermatol. 2012; 13:375-388.
- Elewski BE. Onychomycosis: pathogenesis, diagnosis, and management. Clin Microbiol Rev. 1998;11:415-429.
- Iorizzo M, Starace M, Pasch MC. Leukonychia: what can white nails tell us? Am J Clin Dermatol. 2022;23:177-193.
- Wright JT, Grange DK, Fete M. Hypohidrotic ectodermal dysplasia. In: Adam MP, Feldman J, Mirzaa GM, et al, eds. GeneReviews®. University of Washington, Seattle; 1993-2024.
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
Fingernail Abnormalities in an Adolescent With a History of Toe Walking
A 14-year-old boy with a history of toe walking, attention-deficit/hyperactivity disorder, and mixed receptive expressive language disorder presented to our pediatric dermatology clinic with fingernail abnormalities that had been present since birth. Physical examination revealed narrowing and longitudinal splitting of the nail plates with triangular lacunae and progressive improvement appreciated toward the fifth digits. The nail changes were most prominent on the first digits. A review of the patient’s medical record revealed incidental bilateral iliac horns of the pelvis on radiographs taken at age 18 months. The patient reported waxing and waning knee pain that worsened with prolonged activity and when climbing stairs. Urinalysis demonstrated mild hematuria without proteinuria. The patient was normotensive. There was no evidence of glaucoma, cataracts, or hyperpigmentation of the pupillary margin (Lester iris) on ophthalmologic examination. Genetic testing was performed.
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.
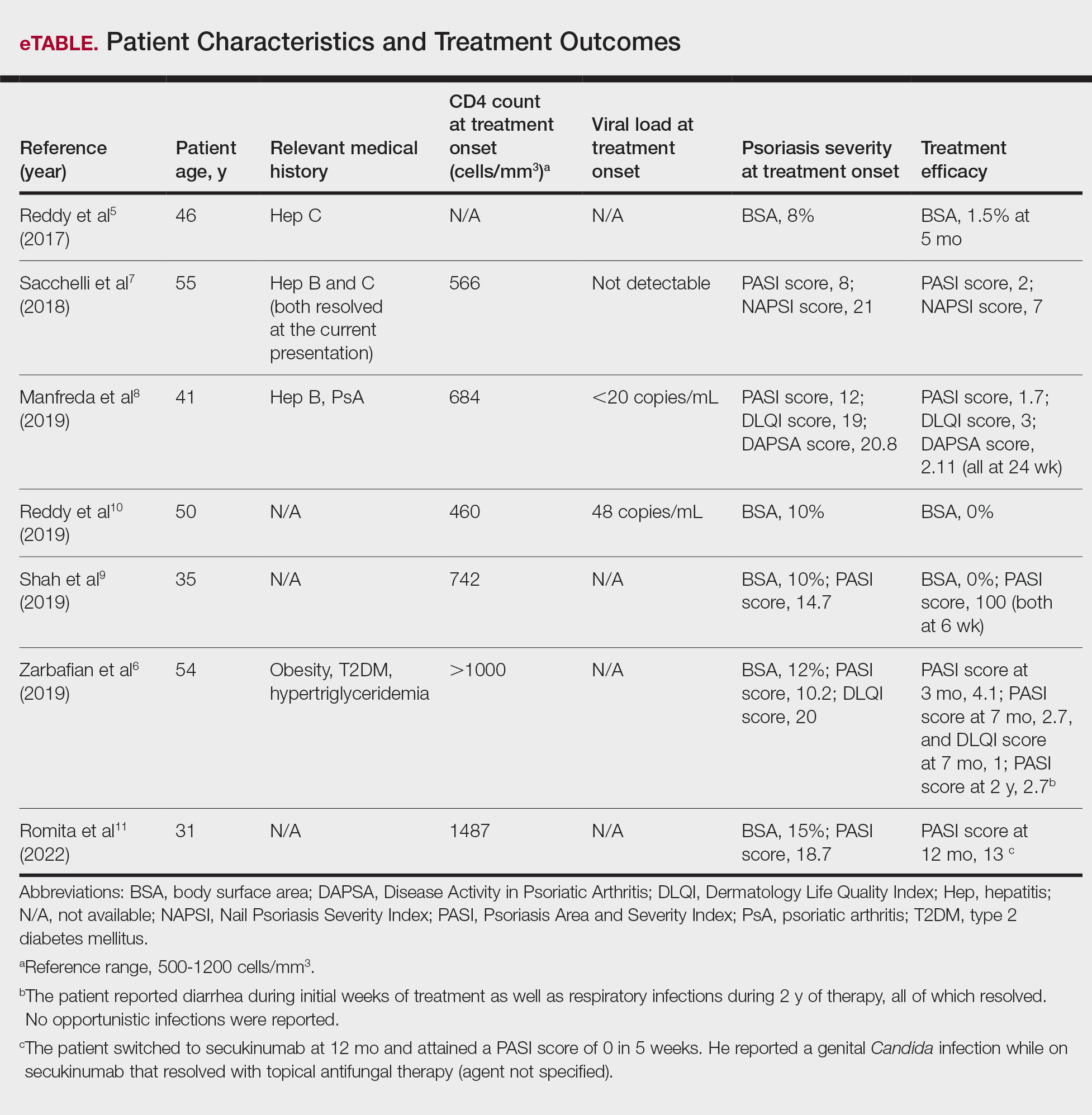
The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. doi:10.1016/j.jaad.2013.11.013
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53. doi:10.1016/j.jaad.2018.06.056
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:E481-E482. doi:10.1111/jdv.14301
- Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193 doi:10.1016/j.jaad.2017.01.052
- Sacchelli L, Patrizi A, Ferrara F, et al. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31:E12719. doi:10.1111/dth.12719
- Manfreda V, Esposito M, Campione E, et al. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131:239-240. doi:10.1080/00325481.2019 .1575613
- Shah BJ, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: a case report. Indian J Dermatol. 2019;64:242- 244. doi:10.4103/ijd.IJD_633_18
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7.
- Romita P, Foti C, Calianno G, et al. Successful treatment with secukinumab in an HIV-positive psoriatic patient after failure of apremilast. Dermatol Ther. 2022;35:E15610. doi:10.1111/dth.15610
- Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34:E14826. doi:10.1111/dth.14826
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. doi:10.1016/j.bcp.2012.01.001
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.

The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.
To the Editor:
Psoriasis is a chronic systemic inflammatory disease that affects 1% to 3% of the global population.1,2 Due to dysregulation of the immune system, patients with HIV who have concurrent moderate to severe psoriasis present a clinical therapeutic challenge for dermatologists. Recent guidelines from the American Academy of Dermatology recommended avoiding certain systemic treatments (eg, methotrexate, cyclosporine) in patients who are HIV positive due to their immunosuppressive effects, as well as cautious use of certain biologics in populations with HIV.3 Traditional therapies for managing psoriasis in patients with HIV have included topical agents, antiretroviral therapy (ART), phototherapy, and acitretin; however, phototherapy can be logistically cumbersome for patients, and in the setting of ART, acitretin has the potential to exacerbate hypertriglyceridemia as well as other undesirable adverse effects.3
Apremilast is a phosphodiesterase 4 inhibitor that has emerged as a promising alternative in patients with HIV who require treatment for psoriasis. It has demonstrated clinical efficacy in psoriasis and has minimal immunosuppressive risk.4 Despite its potential in this population, reports of apremilast used in patients who are HIV positive are rare, and these patients often are excluded from larges studies. In this study, we reviewed the literature to evaluate outcomes and adverse events in patients with HIV who underwent psoriasis treatment with apremilast.
A search of PubMed articles indexed for MEDLINE from the inception of the database through January 2023 was conducted using the terms psoriasis, human immunodeficiency virus, acquired immunodeficiency syndrome, therapy, apremilast, and adverse events. The inclusion criteria were articles that reported patients with HIV and psoriasis undergoing treatment with apremilast with subsequent follow-up to delineate potential outcomes and adverse effects. Non–English language articles were excluded.
Our search of the literature yielded 7 patients with HIV and psoriasis who were treated with apremilast (eTable).5-11 All of the patients were male and ranged in age from 31 to 55 years, and all had pretreatment CD4 cell counts greater than 450 cells/mm3. All but 1 patient were confirmed to have undergone ART prior to treatment with apremilast, and all were treated using the traditional apremilast titration from 10 mg to 30 mg orally twice daily.

The mean pretreatment Psoriasis Area and Severity Index (PASI) score in the patients we evaluated was 12.2, with an average reduction in PASI score of 9.3. This equated to achievement of PASI 75 or greater (ie, representing at least a 75% improvement in psoriasis) in 4 (57.1%) patients, with clinical improvement confirmed in all 7 patients (100.0%)(eTable). The average follow-up time was 9.7 months (range, 6 weeks to 24 months). Only 1 (14.3%) patient experienced any adverse effects, which included self-resolving diarrhea and respiratory infections (nonopportunistic) over a follow-up period of 2 years.6 Of note, gastrointestinal upset is common with apremilast and usually improves over time.12
Apremilast represents a safe and effective alternative systemic therapy for patients with HIV and psoriasis.4 As a phosphodiesterase 4 inhibitor, apremilast leads to increased levels of cyclic adenosine monophosphate, which restores an equilibrium between proinflammatory (eg, tumor necrosis factors, interferons, IL-2, IL-6, IL-12, IL-23) and anti-inflammatory (eg, IL-10) cytokines.13 Unlike most biologics that target and inhibit a specific proinflammatory cytokine, apremilast’s homeostatic mechanism may explain its minimal immunosuppressive adverse effects.
In the majority of patients we evaluated, initiation of apremilast led to documented clinical improvement. It is worth noting that some patients presented with a relevant medical history and/or comorbidities such as hepatitis and metabolic conditions (eg, obesity, type 2 diabetes mellitus, hypertriglyceridemia). Despite these comorbidities, initiation of apremilast therapy in these patients led to clinical improvement of psoriasis overall. Notable cases from our study included a 41-year-old man with concurrent hepatitis B and psoriatic arthritis who achieved PASI 90 after 24 weeks of apremilast therapy8; a 46-year-old man with concurrent hepatitis C who went from 8% to 1.5% body surface area affected after 5 months of treatment with apremilast5; and a 54-year-old man with concurrent obesity, type 2 diabetes mellitus, and hypertriglyceridemia who went from a PASI score of 10.2 to 4.1 after 3 months of apremilast treatment and maintained a PASI score of 2.7 at 2 years’ follow up (eTable).6
Limitations of this study included the small sample size and homogeneous demographic consisting only of adult males, which restrict the external validity of the findings. Despite limitations, apremilast was utilized effectively for patients with both psoriasis and psoriatic arthritis. The observed effectiveness of apremilast in multiple forms of psoriasis provides valuable insights into the drug’s versatility in this patient population.
The use of apremilast for treatment of psoriasis in patients with HIV represents an important therapeutic development. Its effectiveness in reducing psoriasis symptoms in these immunocompromised patients makes it a viable alternative to traditional systemic therapies that might be contraindicated in this population. While larger studies would be ideal, the exclusion of patients with HIV from clinical trials presents an obstacle and therefore makes case series and reviews helpful for clinicians in bridging the gap with respect to treatment options for these patients. Apremilast may be a safe and effective medication for patients with HIV and psoriasis who require systemic therapy to treat their skin disease.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. doi:10.1016/j.jaad.2013.11.013
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53. doi:10.1016/j.jaad.2018.06.056
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:E481-E482. doi:10.1111/jdv.14301
- Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193 doi:10.1016/j.jaad.2017.01.052
- Sacchelli L, Patrizi A, Ferrara F, et al. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31:E12719. doi:10.1111/dth.12719
- Manfreda V, Esposito M, Campione E, et al. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131:239-240. doi:10.1080/00325481.2019 .1575613
- Shah BJ, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: a case report. Indian J Dermatol. 2019;64:242- 244. doi:10.4103/ijd.IJD_633_18
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7.
- Romita P, Foti C, Calianno G, et al. Successful treatment with secukinumab in an HIV-positive psoriatic patient after failure of apremilast. Dermatol Ther. 2022;35:E15610. doi:10.1111/dth.15610
- Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34:E14826. doi:10.1111/dth.14826
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. doi:10.1016/j.bcp.2012.01.001
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516. doi:10.1016/j.jaad.2013.11.013
- Parisi R, Symmons DP, Griffiths CE, et al; Identification and Management of Psoriasis and Associated ComorbidiTy (IMPACT) project team. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol. 2013;133:377-385. doi:10.1038/jid.2012.339
- Kaushik SB, Lebwohl MG. Psoriasis: which therapy for which patient: focus on special populations and chronic infections. J Am Acad Dermatol. 2019;80:43-53. doi:10.1016/j.jaad.2018.06.056
- Crowley J, Thaci D, Joly P, et al. Long-term safety and tolerability of apremilast in patients with psoriasis: pooled safety analysis for >156 weeks from 2 phase 3, randomized, controlled trials (ESTEEM 1 and 2). J Am Acad Dermatol. 2017;77:310-317.e1.
- Reddy SP, Shah VV, Wu JJ. Apremilast for a psoriasis patient with HIV and hepatitis C. J Eur Acad Dermatol Venereol. 2017;31:E481-E482. doi:10.1111/jdv.14301
- Zarbafian M, Cote B, Richer V. Treatment of moderate to severe psoriasis with apremilast over 2 years in the context of long-term treated HIV infection: a case report. SAGE Open Med Case Rep. 2019;7:2050313X19845193. doi:10.1177/2050313X19845193 doi:10.1016/j.jaad.2017.01.052
- Sacchelli L, Patrizi A, Ferrara F, et al. Apremilast as therapeutic option in a HIV positive patient with severe psoriasis. Dermatol Ther. 2018;31:E12719. doi:10.1111/dth.12719
- Manfreda V, Esposito M, Campione E, et al. Apremilast efficacy and safety in a psoriatic arthritis patient affected by HIV and HBV virus infections. Postgrad Med. 2019;131:239-240. doi:10.1080/00325481.2019 .1575613
- Shah BJ, Mistry D, Chaudhary N. Apremilast in people living with HIV with psoriasis vulgaris: a case report. Indian J Dermatol. 2019;64:242- 244. doi:10.4103/ijd.IJD_633_18
- Reddy SP, Lee E, Wu JJ. Apremilast and phototherapy for treatment of psoriasis in a patient with human immunodeficiency virus. Cutis. 2019;103:E6-E7.
- Romita P, Foti C, Calianno G, et al. Successful treatment with secukinumab in an HIV-positive psoriatic patient after failure of apremilast. Dermatol Ther. 2022;35:E15610. doi:10.1111/dth.15610
- Zeb L, Mhaskar R, Lewis S, et al. Real-world drug survival and reasons for treatment discontinuation of biologics and apremilast in patients with psoriasis in an academic center. Dermatol Ther. 2021;34:E14826. doi:10.1111/dth.14826
- Schafer P. Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol. 2012;83:1583-1590. doi:10.1016/j.bcp.2012.01.001
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
Apremilast Treatment Outcomes and Adverse Events in Psoriasis Patients With HIV
PRACTICE POINT
- For patients with HIV who require systemic therapy for psoriasis, apremilast may provide an effective and safe therapeutic option, with minimal immunosuppressive adverse effects.