User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Why 9 is not too young for the HPV vaccine
For Sonja O’Leary, MD, higher rates of vaccination against human papillomavirus came with the flip of a switch.
Dr. O’Leary, the interim director of service for outpatient pediatric services at Denver Health and Hospital Authority, and her colleagues saw rates of HPV and other childhood immunizations drop during the COVID-19 pandemic and decided to act. Their health system, which includes 28 federally qualified health centers, offers vaccines at any inpatient or outpatient visit based on alerts from their electronic health record.
“It was actually really simple; it was really just changing our best-practice alert,” Dr. O’Leary said. Beginning in May 2021, and after notifying clinic staff of the impending change, DHHA dropped the alert for first dose of HPV from age 11 to 9.
The approach worked. Compared with the first 5 months of 2021, the percentage of children aged 9-13 years with an in-person visit who received at least one dose of HPV vaccine between June 2021 and August 2022 rose from 30.3% to 42.8% – a 41% increase. The share who received two doses by age 13 years more than doubled, from 19.3% to 42.7%, Dr. O’Leary said.
Frustrated efforts
Although those figures might seem to make an iron-clad case for earlier vaccinations against HPV – which is responsible for nearly 35,000 cases of cancer annually – factors beyond statistics have frustrated efforts to increase acceptance of the shots.
Data published in 2022 from the U.S. Centers for Disease Control and Prevention found that 89.6% of teens aged 13-17 years received at least one dose of tetanus, diphtheria, and acellular pertussis vaccine, and 89% got one or more doses of meningococcal conjugate vaccine. However, only 76.9% had received one or more doses of HPV vaccine. The rate of receiving both doses needed for full protection was much lower (61.7%).
Both the American Academy of Pediatrics and the American Cancer Society now endorse the strategy of offering HPV vaccine as early as age 9, which avoids the need for multiple shots at a single visit and results in more kids getting both doses. In a recent study that surveyed primary care professionals who see pediatric patients, 21% were already offering HPV vaccine at age 9, and another 48% were willing to try the approach.
What was the most common objection to the earlier age? Nearly three-quarters of clinicians said they felt that parents weren’t ready to talk about HPV vaccination yet.
Noel Brewer, PhD, one of the authors of the survey study, wondered why clinicians feel the need to bring up sex at all. “Providers should never be talking about sex when they are talking about vaccine, because that’s not the point,” said Dr. Brewer, the distinguished professor in public health at the University of North Carolina at Chapel Hill. He pointed out that providers don’t talk about the route of transmission for any other vaccine.
Dr. Brewer led a randomized controlled trial that trained pediatric clinicians in the “announcement” strategy, in which the clinician announces the vaccines that are due at that visit. If the parent hesitates, the clinician then probes further to identify and address their concerns and provides more information. If the parent is still not convinced, the clinician notes the discussion in the chart and tries again at the next visit.
The strategy was effective: Intervention clinics had a 5.4% higher rate of HPV vaccination coverage than control clinics after six months. Dr. Brewer and his colleagues have trained over 1,700 providers in the technique since 2020.
A cancer – not STI – vaccine
Although DHHA hasn’t participated in Dr. Brewer’s training, Dr. O’Leary and her colleagues take a similar approach of simply stating which vaccines the child should receive that day. And they talk about HPV as a cancer vaccine instead of one to prevent a sexually transmitted infection.
In her experience, this emphasis changes the conversation. Dr. O’Leary described a typical comment from parents as, “Oh, of course I would give my child a vaccine that could prevent cancer.”
Ana Rodriguez, MD, MPH, an obstetrician, became interested in raising rates of vaccination against HPV after watching too many women battle a preventable cancer. She worked for several years in the Rio Grande Valley along the U.S. border with Mexico, an impoverished rural area with poor access to health care and high rates of HPV infection.
“I would treat women very young – not even 30 years of age – already fighting advanced precancerous lesions secondary to HPV,” said Dr. Rodriguez, an associate professor of Obstetrics & Gynecology at the University of Texas Medical Branch at Galveston.
In 2016, when Texas ranked 47th in the nation for rates of up-to-date HPV vaccination, Dr. Rodriguez helped launch a community-based educational campaign in four rural counties in the Rio Grande Valley using social media, radio, and in-person meetings with school PTA members and members of school boards to educate staff and parents about the need for vaccination against the infection.
In 2019, the team began offering the vaccine to children ages 9-12 years at back-to-school events, progress report nights, and other school events, pivoting to outdoor events using a mobile vaccine van after COVID-19 struck. They recently published a study showing that 73.6% of students who received their first dose of vaccine at age 11 or younger completed the series, compared with only 45.1% of children who got their first dose at age 12 or older.
Dr. Rodriguez encountered parents who felt 9 or 10 years old was too young because their children were not going to be sexually active anytime soon. Her response was to describe HPV as a tool to prevent cancer, telling parents, “If you vaccinate your kids young enough, they will be protected for life.”
Lifetime protection is another point in favor of giving HPV vaccine prior to Tdap and MenACWY. The response to the two-dose series of HPV in preadolescents is robust and long-lasting, with no downside to giving it a few years earlier. In contrast, immunity to MenACWY wanes after a few years, so the immunization must be given before children enter high school, when their risk for meningitis increases.
The annual toll of deaths in the United States from meningococcus, tetanus, diphtheria, and pertussis typically totals less than 100, whereas cancer deaths attributable to HPV infection number in the thousands each year. And that may be the best reason for attempting new strategies to help HPV vaccination rates catch up to the rest of the preteen vaccines.
Dr. Brewer’s work was supported by the Gillings School of Global Public Health, the Lineberger Comprehensive Cancer Center at the University of North Carolina, and from training grants from the National Cancer Institute. Dr. Brewer has received research funding from Merck, Pfizer, and GSK and served as a paid advisor for Merck. Dr. O’Leary reports no relevant financial relationships. Dr. Rodriguez received a grant from the Cancer Prevention Research Institute of Texas, and the study was supported by the Institute for Translational Sciences at the University of Texas Medical Branch.
A version of this article first appeared on Medscape.com.
For Sonja O’Leary, MD, higher rates of vaccination against human papillomavirus came with the flip of a switch.
Dr. O’Leary, the interim director of service for outpatient pediatric services at Denver Health and Hospital Authority, and her colleagues saw rates of HPV and other childhood immunizations drop during the COVID-19 pandemic and decided to act. Their health system, which includes 28 federally qualified health centers, offers vaccines at any inpatient or outpatient visit based on alerts from their electronic health record.
“It was actually really simple; it was really just changing our best-practice alert,” Dr. O’Leary said. Beginning in May 2021, and after notifying clinic staff of the impending change, DHHA dropped the alert for first dose of HPV from age 11 to 9.
The approach worked. Compared with the first 5 months of 2021, the percentage of children aged 9-13 years with an in-person visit who received at least one dose of HPV vaccine between June 2021 and August 2022 rose from 30.3% to 42.8% – a 41% increase. The share who received two doses by age 13 years more than doubled, from 19.3% to 42.7%, Dr. O’Leary said.
Frustrated efforts
Although those figures might seem to make an iron-clad case for earlier vaccinations against HPV – which is responsible for nearly 35,000 cases of cancer annually – factors beyond statistics have frustrated efforts to increase acceptance of the shots.
Data published in 2022 from the U.S. Centers for Disease Control and Prevention found that 89.6% of teens aged 13-17 years received at least one dose of tetanus, diphtheria, and acellular pertussis vaccine, and 89% got one or more doses of meningococcal conjugate vaccine. However, only 76.9% had received one or more doses of HPV vaccine. The rate of receiving both doses needed for full protection was much lower (61.7%).
Both the American Academy of Pediatrics and the American Cancer Society now endorse the strategy of offering HPV vaccine as early as age 9, which avoids the need for multiple shots at a single visit and results in more kids getting both doses. In a recent study that surveyed primary care professionals who see pediatric patients, 21% were already offering HPV vaccine at age 9, and another 48% were willing to try the approach.
What was the most common objection to the earlier age? Nearly three-quarters of clinicians said they felt that parents weren’t ready to talk about HPV vaccination yet.
Noel Brewer, PhD, one of the authors of the survey study, wondered why clinicians feel the need to bring up sex at all. “Providers should never be talking about sex when they are talking about vaccine, because that’s not the point,” said Dr. Brewer, the distinguished professor in public health at the University of North Carolina at Chapel Hill. He pointed out that providers don’t talk about the route of transmission for any other vaccine.
Dr. Brewer led a randomized controlled trial that trained pediatric clinicians in the “announcement” strategy, in which the clinician announces the vaccines that are due at that visit. If the parent hesitates, the clinician then probes further to identify and address their concerns and provides more information. If the parent is still not convinced, the clinician notes the discussion in the chart and tries again at the next visit.
The strategy was effective: Intervention clinics had a 5.4% higher rate of HPV vaccination coverage than control clinics after six months. Dr. Brewer and his colleagues have trained over 1,700 providers in the technique since 2020.
A cancer – not STI – vaccine
Although DHHA hasn’t participated in Dr. Brewer’s training, Dr. O’Leary and her colleagues take a similar approach of simply stating which vaccines the child should receive that day. And they talk about HPV as a cancer vaccine instead of one to prevent a sexually transmitted infection.
In her experience, this emphasis changes the conversation. Dr. O’Leary described a typical comment from parents as, “Oh, of course I would give my child a vaccine that could prevent cancer.”
Ana Rodriguez, MD, MPH, an obstetrician, became interested in raising rates of vaccination against HPV after watching too many women battle a preventable cancer. She worked for several years in the Rio Grande Valley along the U.S. border with Mexico, an impoverished rural area with poor access to health care and high rates of HPV infection.
“I would treat women very young – not even 30 years of age – already fighting advanced precancerous lesions secondary to HPV,” said Dr. Rodriguez, an associate professor of Obstetrics & Gynecology at the University of Texas Medical Branch at Galveston.
In 2016, when Texas ranked 47th in the nation for rates of up-to-date HPV vaccination, Dr. Rodriguez helped launch a community-based educational campaign in four rural counties in the Rio Grande Valley using social media, radio, and in-person meetings with school PTA members and members of school boards to educate staff and parents about the need for vaccination against the infection.
In 2019, the team began offering the vaccine to children ages 9-12 years at back-to-school events, progress report nights, and other school events, pivoting to outdoor events using a mobile vaccine van after COVID-19 struck. They recently published a study showing that 73.6% of students who received their first dose of vaccine at age 11 or younger completed the series, compared with only 45.1% of children who got their first dose at age 12 or older.
Dr. Rodriguez encountered parents who felt 9 or 10 years old was too young because their children were not going to be sexually active anytime soon. Her response was to describe HPV as a tool to prevent cancer, telling parents, “If you vaccinate your kids young enough, they will be protected for life.”
Lifetime protection is another point in favor of giving HPV vaccine prior to Tdap and MenACWY. The response to the two-dose series of HPV in preadolescents is robust and long-lasting, with no downside to giving it a few years earlier. In contrast, immunity to MenACWY wanes after a few years, so the immunization must be given before children enter high school, when their risk for meningitis increases.
The annual toll of deaths in the United States from meningococcus, tetanus, diphtheria, and pertussis typically totals less than 100, whereas cancer deaths attributable to HPV infection number in the thousands each year. And that may be the best reason for attempting new strategies to help HPV vaccination rates catch up to the rest of the preteen vaccines.
Dr. Brewer’s work was supported by the Gillings School of Global Public Health, the Lineberger Comprehensive Cancer Center at the University of North Carolina, and from training grants from the National Cancer Institute. Dr. Brewer has received research funding from Merck, Pfizer, and GSK and served as a paid advisor for Merck. Dr. O’Leary reports no relevant financial relationships. Dr. Rodriguez received a grant from the Cancer Prevention Research Institute of Texas, and the study was supported by the Institute for Translational Sciences at the University of Texas Medical Branch.
A version of this article first appeared on Medscape.com.
For Sonja O’Leary, MD, higher rates of vaccination against human papillomavirus came with the flip of a switch.
Dr. O’Leary, the interim director of service for outpatient pediatric services at Denver Health and Hospital Authority, and her colleagues saw rates of HPV and other childhood immunizations drop during the COVID-19 pandemic and decided to act. Their health system, which includes 28 federally qualified health centers, offers vaccines at any inpatient or outpatient visit based on alerts from their electronic health record.
“It was actually really simple; it was really just changing our best-practice alert,” Dr. O’Leary said. Beginning in May 2021, and after notifying clinic staff of the impending change, DHHA dropped the alert for first dose of HPV from age 11 to 9.
The approach worked. Compared with the first 5 months of 2021, the percentage of children aged 9-13 years with an in-person visit who received at least one dose of HPV vaccine between June 2021 and August 2022 rose from 30.3% to 42.8% – a 41% increase. The share who received two doses by age 13 years more than doubled, from 19.3% to 42.7%, Dr. O’Leary said.
Frustrated efforts
Although those figures might seem to make an iron-clad case for earlier vaccinations against HPV – which is responsible for nearly 35,000 cases of cancer annually – factors beyond statistics have frustrated efforts to increase acceptance of the shots.
Data published in 2022 from the U.S. Centers for Disease Control and Prevention found that 89.6% of teens aged 13-17 years received at least one dose of tetanus, diphtheria, and acellular pertussis vaccine, and 89% got one or more doses of meningococcal conjugate vaccine. However, only 76.9% had received one or more doses of HPV vaccine. The rate of receiving both doses needed for full protection was much lower (61.7%).
Both the American Academy of Pediatrics and the American Cancer Society now endorse the strategy of offering HPV vaccine as early as age 9, which avoids the need for multiple shots at a single visit and results in more kids getting both doses. In a recent study that surveyed primary care professionals who see pediatric patients, 21% were already offering HPV vaccine at age 9, and another 48% were willing to try the approach.
What was the most common objection to the earlier age? Nearly three-quarters of clinicians said they felt that parents weren’t ready to talk about HPV vaccination yet.
Noel Brewer, PhD, one of the authors of the survey study, wondered why clinicians feel the need to bring up sex at all. “Providers should never be talking about sex when they are talking about vaccine, because that’s not the point,” said Dr. Brewer, the distinguished professor in public health at the University of North Carolina at Chapel Hill. He pointed out that providers don’t talk about the route of transmission for any other vaccine.
Dr. Brewer led a randomized controlled trial that trained pediatric clinicians in the “announcement” strategy, in which the clinician announces the vaccines that are due at that visit. If the parent hesitates, the clinician then probes further to identify and address their concerns and provides more information. If the parent is still not convinced, the clinician notes the discussion in the chart and tries again at the next visit.
The strategy was effective: Intervention clinics had a 5.4% higher rate of HPV vaccination coverage than control clinics after six months. Dr. Brewer and his colleagues have trained over 1,700 providers in the technique since 2020.
A cancer – not STI – vaccine
Although DHHA hasn’t participated in Dr. Brewer’s training, Dr. O’Leary and her colleagues take a similar approach of simply stating which vaccines the child should receive that day. And they talk about HPV as a cancer vaccine instead of one to prevent a sexually transmitted infection.
In her experience, this emphasis changes the conversation. Dr. O’Leary described a typical comment from parents as, “Oh, of course I would give my child a vaccine that could prevent cancer.”
Ana Rodriguez, MD, MPH, an obstetrician, became interested in raising rates of vaccination against HPV after watching too many women battle a preventable cancer. She worked for several years in the Rio Grande Valley along the U.S. border with Mexico, an impoverished rural area with poor access to health care and high rates of HPV infection.
“I would treat women very young – not even 30 years of age – already fighting advanced precancerous lesions secondary to HPV,” said Dr. Rodriguez, an associate professor of Obstetrics & Gynecology at the University of Texas Medical Branch at Galveston.
In 2016, when Texas ranked 47th in the nation for rates of up-to-date HPV vaccination, Dr. Rodriguez helped launch a community-based educational campaign in four rural counties in the Rio Grande Valley using social media, radio, and in-person meetings with school PTA members and members of school boards to educate staff and parents about the need for vaccination against the infection.
In 2019, the team began offering the vaccine to children ages 9-12 years at back-to-school events, progress report nights, and other school events, pivoting to outdoor events using a mobile vaccine van after COVID-19 struck. They recently published a study showing that 73.6% of students who received their first dose of vaccine at age 11 or younger completed the series, compared with only 45.1% of children who got their first dose at age 12 or older.
Dr. Rodriguez encountered parents who felt 9 or 10 years old was too young because their children were not going to be sexually active anytime soon. Her response was to describe HPV as a tool to prevent cancer, telling parents, “If you vaccinate your kids young enough, they will be protected for life.”
Lifetime protection is another point in favor of giving HPV vaccine prior to Tdap and MenACWY. The response to the two-dose series of HPV in preadolescents is robust and long-lasting, with no downside to giving it a few years earlier. In contrast, immunity to MenACWY wanes after a few years, so the immunization must be given before children enter high school, when their risk for meningitis increases.
The annual toll of deaths in the United States from meningococcus, tetanus, diphtheria, and pertussis typically totals less than 100, whereas cancer deaths attributable to HPV infection number in the thousands each year. And that may be the best reason for attempting new strategies to help HPV vaccination rates catch up to the rest of the preteen vaccines.
Dr. Brewer’s work was supported by the Gillings School of Global Public Health, the Lineberger Comprehensive Cancer Center at the University of North Carolina, and from training grants from the National Cancer Institute. Dr. Brewer has received research funding from Merck, Pfizer, and GSK and served as a paid advisor for Merck. Dr. O’Leary reports no relevant financial relationships. Dr. Rodriguez received a grant from the Cancer Prevention Research Institute of Texas, and the study was supported by the Institute for Translational Sciences at the University of Texas Medical Branch.
A version of this article first appeared on Medscape.com.
Antimicrobial resistance requires a manifold response
BUENOS AIRES – Antimicrobial resistance (AMR) has become a global concern. And while one issue to be addressed is the deficit in research and development for new antibiotics, efforts to tackle this public health threat also should be directed toward promoting more rational prescription practices and strengthening the ability to identify the microorganisms responsible for infections, according to the World Health Organization. This was the conclusion reached at the fourth meeting of the WHO AMR Surveillance and Quality Assessment Collaborating Centres Network, which was held in Buenos Aires.
“We have to provide assistance to countries to ensure that the drugs are being used responsibly. We can come up with new antibiotics, but the issue at hand is not simply one of innovation: If nothing is done to correct inappropriate prescription practices and to overcome the lack of diagnostic laboratories at the country level, we’re going to miss out on those drugs as soon as they become available,” Kitty van Weezenbeek, MD, PhD, MPH, director of the AMR Surveillance, Prevention, and Control (AMR/SPC) Department at the WHO’s headquarters in Geneva, told this news organization.
Dr. van Weezenbeek pointed out that although there are currently no shortages of antimicrobials, the development and launch of new drugs that fight multidrug-resistant infections – infections for which there are few therapeutic options – has proceeded slowly. “It takes 10 to 15 years to develop a new antibiotic,” she said, adding that “the majority of pharmaceutical companies that had been engaged in the development of antimicrobials have filed for bankruptcy.”
In 2019, more people died – 1.2 million – from AMR than from malaria, tuberculosis, and HIV combined. Why are there so few market incentives when there is such a great need for those drugs? “One reason is that the pharmaceutical industry makes more money with long-term treatments, such as those for cancer and respiratory diseases. The other problem is that people everywhere are told not to use antibiotics,” said Dr. van Weezenbeek.
“A course of antibiotics lasts a few days, especially because we’re promoting rational use. Therefore, the trend is for the total amount of antimicrobials being used to be lower. So, it’s not as profitable,” added Carmem Lucia Pessoa-Silva, MD, PhD, head of the Surveillance, Evidence, and Laboratory Strengthening Unit of the WHO’s AMR/SPC Department.
On that note, Dr. van Weezenbeek mentioned that member countries are working with pharmaceutical companies and universities to address this problem. The WHO, for its part, has responded by implementing a global mechanism with a public health approach to create a “healthy” and equitable market for these medicines.
AMR is one of the top 10 global threats to human health. But it also has an impact on animal production, agricultural production, and the environment. Strategies to tackle AMR based on the One Health approach should involve all actors, social sectors, and citizens, according to Eva Jané Llopis, PhD, the representative of the Pan American Health Organization/WHO in Argentina.
At the root of the AMR problem is the widespread use of these drugs as growth promoters in animal production – for which several countries have enacted regulations – as well as “misunderstandings” between patients and physicians when there is not sufficient, timely access to laboratory diagnostics, especially in low- and middle-income countries.
“People think that if they’re given broad-spectrum antibiotics, they’re being prescribed the best antibiotics; and doctors, because there are no laboratory services, prescribe broad-spectrum antibiotics because they want to help patients. But that ends up causing more resistance to drugs, and thus, those antibiotics aren’t good for the patients,” said Dr. van Weezenbeek.
The WHO Global AMR and Use Surveillance System (GLASS) was launched in 2015. Its 2022 report, which marked the end of the system’s early implementation period, noted that the reported AMR rates are often lower in countries, territories, and areas with better testing coverage for most pathogen-drug-infection site combinations. However, as Dr. Pessoa-Silva acknowledged, monitoring “has not yet generated representative data,” because in many cases, countries either do not have surveillance systems or have only recently started implementing them.
Even so, the indicators that are available paint an increasingly worrisome picture. “For example, in many countries, resistance rates to first-line antibiotics were around 10%-20% with respect to Escherichia coli urinary tract infections and bloodstream bacteriologically confirmed infections. So, the risk of treatment failure is very high,” explained Dr. Pessoa-Silva.
The latest estimates indicate that every 2 or 3 minutes, somewhere in the world, a child dies from AMR. And the situation is particularly “dramatic” in neonatal intensive care units, where outbreaks of multidrug-resistant infections have a mortality rate of 50%, said Pilar Ramón-Pardo, MD, PhD, lead of the Special Program on AMR at the Pan American Health Organization, the WHO Regional Office for the Americas.
AMR rates also got worse during the pandemic because of the inappropriate prescription of massive amounts of antibiotics to hospitalized patients – something that was not in compliance with guidelines or protocols. Silvia Bertagnolio, MD, is an infectious disease specialist and the head of the Control and Response Strategies Unit in the WHO’s AMR Division. She spoke about the global clinical platform data pertaining to more than 1,500,000 patients who were hospitalized for COVID-19. Since 2020, 85% received antimicrobial treatment, despite the fact that only 5% had a concomitant infection at admission. “It’s easier to give antibiotics than to make a proper diagnosis,” said Dr. Bertagnolio.
This article was translated from Medscape’s Spanish edition and a version appeared on Medscape.com.
BUENOS AIRES – Antimicrobial resistance (AMR) has become a global concern. And while one issue to be addressed is the deficit in research and development for new antibiotics, efforts to tackle this public health threat also should be directed toward promoting more rational prescription practices and strengthening the ability to identify the microorganisms responsible for infections, according to the World Health Organization. This was the conclusion reached at the fourth meeting of the WHO AMR Surveillance and Quality Assessment Collaborating Centres Network, which was held in Buenos Aires.
“We have to provide assistance to countries to ensure that the drugs are being used responsibly. We can come up with new antibiotics, but the issue at hand is not simply one of innovation: If nothing is done to correct inappropriate prescription practices and to overcome the lack of diagnostic laboratories at the country level, we’re going to miss out on those drugs as soon as they become available,” Kitty van Weezenbeek, MD, PhD, MPH, director of the AMR Surveillance, Prevention, and Control (AMR/SPC) Department at the WHO’s headquarters in Geneva, told this news organization.
Dr. van Weezenbeek pointed out that although there are currently no shortages of antimicrobials, the development and launch of new drugs that fight multidrug-resistant infections – infections for which there are few therapeutic options – has proceeded slowly. “It takes 10 to 15 years to develop a new antibiotic,” she said, adding that “the majority of pharmaceutical companies that had been engaged in the development of antimicrobials have filed for bankruptcy.”
In 2019, more people died – 1.2 million – from AMR than from malaria, tuberculosis, and HIV combined. Why are there so few market incentives when there is such a great need for those drugs? “One reason is that the pharmaceutical industry makes more money with long-term treatments, such as those for cancer and respiratory diseases. The other problem is that people everywhere are told not to use antibiotics,” said Dr. van Weezenbeek.
“A course of antibiotics lasts a few days, especially because we’re promoting rational use. Therefore, the trend is for the total amount of antimicrobials being used to be lower. So, it’s not as profitable,” added Carmem Lucia Pessoa-Silva, MD, PhD, head of the Surveillance, Evidence, and Laboratory Strengthening Unit of the WHO’s AMR/SPC Department.
On that note, Dr. van Weezenbeek mentioned that member countries are working with pharmaceutical companies and universities to address this problem. The WHO, for its part, has responded by implementing a global mechanism with a public health approach to create a “healthy” and equitable market for these medicines.
AMR is one of the top 10 global threats to human health. But it also has an impact on animal production, agricultural production, and the environment. Strategies to tackle AMR based on the One Health approach should involve all actors, social sectors, and citizens, according to Eva Jané Llopis, PhD, the representative of the Pan American Health Organization/WHO in Argentina.
At the root of the AMR problem is the widespread use of these drugs as growth promoters in animal production – for which several countries have enacted regulations – as well as “misunderstandings” between patients and physicians when there is not sufficient, timely access to laboratory diagnostics, especially in low- and middle-income countries.
“People think that if they’re given broad-spectrum antibiotics, they’re being prescribed the best antibiotics; and doctors, because there are no laboratory services, prescribe broad-spectrum antibiotics because they want to help patients. But that ends up causing more resistance to drugs, and thus, those antibiotics aren’t good for the patients,” said Dr. van Weezenbeek.
The WHO Global AMR and Use Surveillance System (GLASS) was launched in 2015. Its 2022 report, which marked the end of the system’s early implementation period, noted that the reported AMR rates are often lower in countries, territories, and areas with better testing coverage for most pathogen-drug-infection site combinations. However, as Dr. Pessoa-Silva acknowledged, monitoring “has not yet generated representative data,” because in many cases, countries either do not have surveillance systems or have only recently started implementing them.
Even so, the indicators that are available paint an increasingly worrisome picture. “For example, in many countries, resistance rates to first-line antibiotics were around 10%-20% with respect to Escherichia coli urinary tract infections and bloodstream bacteriologically confirmed infections. So, the risk of treatment failure is very high,” explained Dr. Pessoa-Silva.
The latest estimates indicate that every 2 or 3 minutes, somewhere in the world, a child dies from AMR. And the situation is particularly “dramatic” in neonatal intensive care units, where outbreaks of multidrug-resistant infections have a mortality rate of 50%, said Pilar Ramón-Pardo, MD, PhD, lead of the Special Program on AMR at the Pan American Health Organization, the WHO Regional Office for the Americas.
AMR rates also got worse during the pandemic because of the inappropriate prescription of massive amounts of antibiotics to hospitalized patients – something that was not in compliance with guidelines or protocols. Silvia Bertagnolio, MD, is an infectious disease specialist and the head of the Control and Response Strategies Unit in the WHO’s AMR Division. She spoke about the global clinical platform data pertaining to more than 1,500,000 patients who were hospitalized for COVID-19. Since 2020, 85% received antimicrobial treatment, despite the fact that only 5% had a concomitant infection at admission. “It’s easier to give antibiotics than to make a proper diagnosis,” said Dr. Bertagnolio.
This article was translated from Medscape’s Spanish edition and a version appeared on Medscape.com.
BUENOS AIRES – Antimicrobial resistance (AMR) has become a global concern. And while one issue to be addressed is the deficit in research and development for new antibiotics, efforts to tackle this public health threat also should be directed toward promoting more rational prescription practices and strengthening the ability to identify the microorganisms responsible for infections, according to the World Health Organization. This was the conclusion reached at the fourth meeting of the WHO AMR Surveillance and Quality Assessment Collaborating Centres Network, which was held in Buenos Aires.
“We have to provide assistance to countries to ensure that the drugs are being used responsibly. We can come up with new antibiotics, but the issue at hand is not simply one of innovation: If nothing is done to correct inappropriate prescription practices and to overcome the lack of diagnostic laboratories at the country level, we’re going to miss out on those drugs as soon as they become available,” Kitty van Weezenbeek, MD, PhD, MPH, director of the AMR Surveillance, Prevention, and Control (AMR/SPC) Department at the WHO’s headquarters in Geneva, told this news organization.
Dr. van Weezenbeek pointed out that although there are currently no shortages of antimicrobials, the development and launch of new drugs that fight multidrug-resistant infections – infections for which there are few therapeutic options – has proceeded slowly. “It takes 10 to 15 years to develop a new antibiotic,” she said, adding that “the majority of pharmaceutical companies that had been engaged in the development of antimicrobials have filed for bankruptcy.”
In 2019, more people died – 1.2 million – from AMR than from malaria, tuberculosis, and HIV combined. Why are there so few market incentives when there is such a great need for those drugs? “One reason is that the pharmaceutical industry makes more money with long-term treatments, such as those for cancer and respiratory diseases. The other problem is that people everywhere are told not to use antibiotics,” said Dr. van Weezenbeek.
“A course of antibiotics lasts a few days, especially because we’re promoting rational use. Therefore, the trend is for the total amount of antimicrobials being used to be lower. So, it’s not as profitable,” added Carmem Lucia Pessoa-Silva, MD, PhD, head of the Surveillance, Evidence, and Laboratory Strengthening Unit of the WHO’s AMR/SPC Department.
On that note, Dr. van Weezenbeek mentioned that member countries are working with pharmaceutical companies and universities to address this problem. The WHO, for its part, has responded by implementing a global mechanism with a public health approach to create a “healthy” and equitable market for these medicines.
AMR is one of the top 10 global threats to human health. But it also has an impact on animal production, agricultural production, and the environment. Strategies to tackle AMR based on the One Health approach should involve all actors, social sectors, and citizens, according to Eva Jané Llopis, PhD, the representative of the Pan American Health Organization/WHO in Argentina.
At the root of the AMR problem is the widespread use of these drugs as growth promoters in animal production – for which several countries have enacted regulations – as well as “misunderstandings” between patients and physicians when there is not sufficient, timely access to laboratory diagnostics, especially in low- and middle-income countries.
“People think that if they’re given broad-spectrum antibiotics, they’re being prescribed the best antibiotics; and doctors, because there are no laboratory services, prescribe broad-spectrum antibiotics because they want to help patients. But that ends up causing more resistance to drugs, and thus, those antibiotics aren’t good for the patients,” said Dr. van Weezenbeek.
The WHO Global AMR and Use Surveillance System (GLASS) was launched in 2015. Its 2022 report, which marked the end of the system’s early implementation period, noted that the reported AMR rates are often lower in countries, territories, and areas with better testing coverage for most pathogen-drug-infection site combinations. However, as Dr. Pessoa-Silva acknowledged, monitoring “has not yet generated representative data,” because in many cases, countries either do not have surveillance systems or have only recently started implementing them.
Even so, the indicators that are available paint an increasingly worrisome picture. “For example, in many countries, resistance rates to first-line antibiotics were around 10%-20% with respect to Escherichia coli urinary tract infections and bloodstream bacteriologically confirmed infections. So, the risk of treatment failure is very high,” explained Dr. Pessoa-Silva.
The latest estimates indicate that every 2 or 3 minutes, somewhere in the world, a child dies from AMR. And the situation is particularly “dramatic” in neonatal intensive care units, where outbreaks of multidrug-resistant infections have a mortality rate of 50%, said Pilar Ramón-Pardo, MD, PhD, lead of the Special Program on AMR at the Pan American Health Organization, the WHO Regional Office for the Americas.
AMR rates also got worse during the pandemic because of the inappropriate prescription of massive amounts of antibiotics to hospitalized patients – something that was not in compliance with guidelines or protocols. Silvia Bertagnolio, MD, is an infectious disease specialist and the head of the Control and Response Strategies Unit in the WHO’s AMR Division. She spoke about the global clinical platform data pertaining to more than 1,500,000 patients who were hospitalized for COVID-19. Since 2020, 85% received antimicrobial treatment, despite the fact that only 5% had a concomitant infection at admission. “It’s easier to give antibiotics than to make a proper diagnosis,” said Dr. Bertagnolio.
This article was translated from Medscape’s Spanish edition and a version appeared on Medscape.com.
New Medicare rule streamlines prior authorization in Medicare Advantage plans
A new federal rule seeks to reduce Medicare Advantage insurance plans’ prior authorization burdens on physicians while also ensuring that enrollees have the same access to necessary care that they would receive under traditional fee-for-service Medicare.
The prior authorization changes, announced this week, are part of the Centers for Medicare & Medicaid Services’ 2024 update of policy changes for Medicare Advantage and Part D pharmacy plans
Medicare Advantage plans’ business practices have raised significant concerns in recent years. More than 28 million Americans were enrolled in a Medicare Advantage plan in 2022, which is nearly half of all Medicare enrollees, according to the Kaiser Family Foundation.
Medicare pays a fixed amount per enrollee per year to these privately run managed care plans, in contrast to traditional fee-for-service Medicare. Medicare Advantage plans have been criticized for aggressive marketing, for overbilling the federal government for care, and for using prior authorization to inappropriately deny needed care to patients.
About 13% of prior authorization requests that are denied by Medicare Advantage plans actually met Medicare coverage rules and should have been approved, the Office of the Inspector General at the U.S. Department of Health & Human Services reported in 2022.
The newly finalized rule now requires Medicare Advantage plans to do the following.
- Ensure that a prior authorization approval, once granted, remains valid for as long as medically necessary to avoid disruptions in care.
- Conduct an annual review of utilization management policies.
- Ensure that coverage denials based on medical necessity be reviewed by health care professionals with relevant expertise before a denial can be issued.
Physician groups welcomed the changes. In a statement, the American Medical Association said that an initial reading of the rule suggested CMS had “taken important steps toward right-sizing the prior authorization process.”
The Medical Group Management Association praised CMS in a statement for having limited “dangerous disruptions and delays to necessary patient care” resulting from the cumbersome processes of prior approval. With the new rules, CMS will provide greater consistency across Advantage plans as well as traditional Medicare, said Anders Gilberg, MGMA’s senior vice president of government affairs, in a statement.
Peer consideration
The final rule did disappoint physician groups in one key way. CMS rebuffed requests to have CMS require Advantage plans to use reviewers of the same specialty as treating physicians in handling disputes about prior authorization. CMS said it expects plans to exercise judgment in finding reviewers with “sufficient expertise to make an informed and supportable decision.”
“In some instances, we expect that plans will use a physician or other health care professional of the same specialty or subspecialty as the treating physician,” CMS said. “In other instances, we expect that plans will utilize a reviewer with specialized training, certification, or clinical experience in the applicable field of medicine.”
Medicare Advantage marketing ‘sowing confusion’
With this final rule, CMS also sought to protect consumers from “potentially misleading marketing practices” used in promoting Medicare Advantage and Part D prescription drug plans.
The agency said it had received complaints about people who have received official-looking promotional materials for Medicare that directed them not to government sources of information but to Medicare Advantage and Part D plans or their agents and brokers.
Ads now must mention a specific plan name, and they cannot use the Medicare name, CMS logo, Medicare card, or other government information in a misleading way, CMS said.
“CMS can see no value or purpose in a non-governmental entity’s use of the Medicare logo or HHS logo except for the express purpose of sowing confusion and misrepresenting itself as the government,” the agency said.
A version of this article first appeared on Medscape.com.
A new federal rule seeks to reduce Medicare Advantage insurance plans’ prior authorization burdens on physicians while also ensuring that enrollees have the same access to necessary care that they would receive under traditional fee-for-service Medicare.
The prior authorization changes, announced this week, are part of the Centers for Medicare & Medicaid Services’ 2024 update of policy changes for Medicare Advantage and Part D pharmacy plans
Medicare Advantage plans’ business practices have raised significant concerns in recent years. More than 28 million Americans were enrolled in a Medicare Advantage plan in 2022, which is nearly half of all Medicare enrollees, according to the Kaiser Family Foundation.
Medicare pays a fixed amount per enrollee per year to these privately run managed care plans, in contrast to traditional fee-for-service Medicare. Medicare Advantage plans have been criticized for aggressive marketing, for overbilling the federal government for care, and for using prior authorization to inappropriately deny needed care to patients.
About 13% of prior authorization requests that are denied by Medicare Advantage plans actually met Medicare coverage rules and should have been approved, the Office of the Inspector General at the U.S. Department of Health & Human Services reported in 2022.
The newly finalized rule now requires Medicare Advantage plans to do the following.
- Ensure that a prior authorization approval, once granted, remains valid for as long as medically necessary to avoid disruptions in care.
- Conduct an annual review of utilization management policies.
- Ensure that coverage denials based on medical necessity be reviewed by health care professionals with relevant expertise before a denial can be issued.
Physician groups welcomed the changes. In a statement, the American Medical Association said that an initial reading of the rule suggested CMS had “taken important steps toward right-sizing the prior authorization process.”
The Medical Group Management Association praised CMS in a statement for having limited “dangerous disruptions and delays to necessary patient care” resulting from the cumbersome processes of prior approval. With the new rules, CMS will provide greater consistency across Advantage plans as well as traditional Medicare, said Anders Gilberg, MGMA’s senior vice president of government affairs, in a statement.
Peer consideration
The final rule did disappoint physician groups in one key way. CMS rebuffed requests to have CMS require Advantage plans to use reviewers of the same specialty as treating physicians in handling disputes about prior authorization. CMS said it expects plans to exercise judgment in finding reviewers with “sufficient expertise to make an informed and supportable decision.”
“In some instances, we expect that plans will use a physician or other health care professional of the same specialty or subspecialty as the treating physician,” CMS said. “In other instances, we expect that plans will utilize a reviewer with specialized training, certification, or clinical experience in the applicable field of medicine.”
Medicare Advantage marketing ‘sowing confusion’
With this final rule, CMS also sought to protect consumers from “potentially misleading marketing practices” used in promoting Medicare Advantage and Part D prescription drug plans.
The agency said it had received complaints about people who have received official-looking promotional materials for Medicare that directed them not to government sources of information but to Medicare Advantage and Part D plans or their agents and brokers.
Ads now must mention a specific plan name, and they cannot use the Medicare name, CMS logo, Medicare card, or other government information in a misleading way, CMS said.
“CMS can see no value or purpose in a non-governmental entity’s use of the Medicare logo or HHS logo except for the express purpose of sowing confusion and misrepresenting itself as the government,” the agency said.
A version of this article first appeared on Medscape.com.
A new federal rule seeks to reduce Medicare Advantage insurance plans’ prior authorization burdens on physicians while also ensuring that enrollees have the same access to necessary care that they would receive under traditional fee-for-service Medicare.
The prior authorization changes, announced this week, are part of the Centers for Medicare & Medicaid Services’ 2024 update of policy changes for Medicare Advantage and Part D pharmacy plans
Medicare Advantage plans’ business practices have raised significant concerns in recent years. More than 28 million Americans were enrolled in a Medicare Advantage plan in 2022, which is nearly half of all Medicare enrollees, according to the Kaiser Family Foundation.
Medicare pays a fixed amount per enrollee per year to these privately run managed care plans, in contrast to traditional fee-for-service Medicare. Medicare Advantage plans have been criticized for aggressive marketing, for overbilling the federal government for care, and for using prior authorization to inappropriately deny needed care to patients.
About 13% of prior authorization requests that are denied by Medicare Advantage plans actually met Medicare coverage rules and should have been approved, the Office of the Inspector General at the U.S. Department of Health & Human Services reported in 2022.
The newly finalized rule now requires Medicare Advantage plans to do the following.
- Ensure that a prior authorization approval, once granted, remains valid for as long as medically necessary to avoid disruptions in care.
- Conduct an annual review of utilization management policies.
- Ensure that coverage denials based on medical necessity be reviewed by health care professionals with relevant expertise before a denial can be issued.
Physician groups welcomed the changes. In a statement, the American Medical Association said that an initial reading of the rule suggested CMS had “taken important steps toward right-sizing the prior authorization process.”
The Medical Group Management Association praised CMS in a statement for having limited “dangerous disruptions and delays to necessary patient care” resulting from the cumbersome processes of prior approval. With the new rules, CMS will provide greater consistency across Advantage plans as well as traditional Medicare, said Anders Gilberg, MGMA’s senior vice president of government affairs, in a statement.
Peer consideration
The final rule did disappoint physician groups in one key way. CMS rebuffed requests to have CMS require Advantage plans to use reviewers of the same specialty as treating physicians in handling disputes about prior authorization. CMS said it expects plans to exercise judgment in finding reviewers with “sufficient expertise to make an informed and supportable decision.”
“In some instances, we expect that plans will use a physician or other health care professional of the same specialty or subspecialty as the treating physician,” CMS said. “In other instances, we expect that plans will utilize a reviewer with specialized training, certification, or clinical experience in the applicable field of medicine.”
Medicare Advantage marketing ‘sowing confusion’
With this final rule, CMS also sought to protect consumers from “potentially misleading marketing practices” used in promoting Medicare Advantage and Part D prescription drug plans.
The agency said it had received complaints about people who have received official-looking promotional materials for Medicare that directed them not to government sources of information but to Medicare Advantage and Part D plans or their agents and brokers.
Ads now must mention a specific plan name, and they cannot use the Medicare name, CMS logo, Medicare card, or other government information in a misleading way, CMS said.
“CMS can see no value or purpose in a non-governmental entity’s use of the Medicare logo or HHS logo except for the express purpose of sowing confusion and misrepresenting itself as the government,” the agency said.
A version of this article first appeared on Medscape.com.
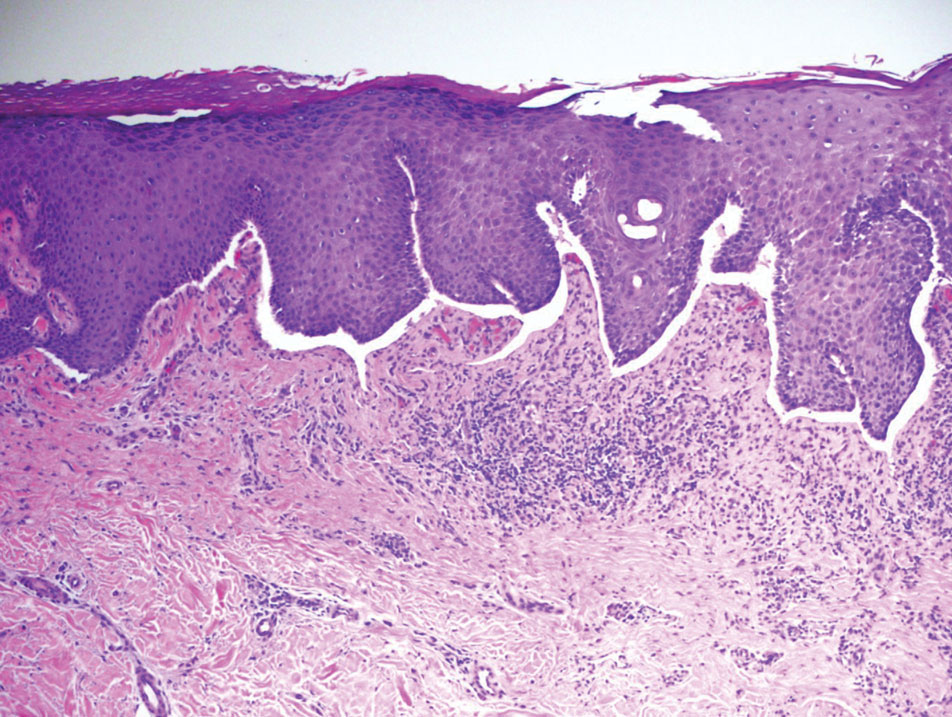
Study highlights potential skin cancer risk of UV nail polish dryers
Results of a study recently published in Nature Communications suggests that According to two experts, these findings raise concerns regarding the safety of frequent use of these nail dryers.
In the study, human and mouse cells were exposed to radiation from UV nail dryers. Exposing human and mice skin cells to UVA light for 20 minutes resulted in the death of 20%-30% of cells; three consecutive 20-minute sessions resulted in the death of 65%-70% of cells. Additionally, surviving cells suffered oxidative damage to their DNA and mitochondria, with mutational patterns similar to those seen in skin cancer, study investigator Maria Zhivagui, PhD, of the University of California, San Diego, and associates reported.
“This study showed that irradiation of human and mouse cell lines using UV nail polish dryers resulted in DNA damage and genome mutations,” Shari Lipner, MD, PhD, director of the nail division at New York–Presbyterian Hospital/Weill Cornell Medicine, New York, said in an interview. The study “ties together exposure to UV light from nail polish dryers and genetic mutations that are associated with skin cancers,” added Dr. Lipner, who was not involved with the study.
UV nail lamps are commonly used to dry and harden gel nail polish formulas. Often referred to as “mini tanning beds,” these devices emit UVA radiation, classified as a Group 1 Carcinogen by the International Agency for Research on Cancer.
“Both UVA and UVB are main drivers of both melanoma and keratinocyte carcinomas (basal cell carcinoma and squamous cell carcinoma),” said Anthony Rossi, MD, a dermatologic surgeon at Memorial Sloan Kettering Cancer Center, New York, who was also not a study investigator. UV irradiance “produces DNA mutations that are specific to forming types of skin cancer,” he said in an interview.
UVA wavelengths commonly used in nail dryers can penetrate all layers of the epidermis, the top layer of the skin, potentially affecting stem cells in the skin, according to the study.
Dr. Lipner noted that “there have been several case reports of patients with histories of gel manicures using UV nail polish dryers who later developed squamous cell carcinomas on the dorsal hands, fingers, and nails, and articles describing high UV emissions from nail polish dryers, but the direct connection between UV dryers and skin cancer development was tenuous.” The first of its kind, the new study investigated the impact of UV nail drying devices at a cellular level.
The results of this study, in combination with previous case reports suggesting the development of skin cancers following UVA dryer use, raise concern regarding the safety of these commonly used devices. The study, the authors wrote, “does not provide direct evidence for an increased cancer risk in human beings,” but their findings and “prior evidence strongly suggest that radiation emitted by UV nail polish dryers may cause cancers of the hand and that UV nail polish dryers, similar to tanning beds, may increase the risk of early onset skin cancer.”
Dr. Rossi said that, “while this study shows that the UV exposure does affect human cells and causes mutations, the study was not done in vivo in human beings, so further studies are needed to know at what dose and frequency gel manicures would be needed to cause detrimental effects.” However, for people who regularly receive gel manicures involving UV nail dryers, both Dr. Lipner and Dr. Rossi recommend applying a broad-spectrum sunscreen to protect the dorsal hands, fingertips, and skin surrounding the nails, or wearing UV-protective gloves.
The study was supported by an Alfred B. Sloan Research Fellowship to one of the authors and grants from the National Institutes of Health to two authors. One author reported being a compensated consultant and having an equity interest in io9. Dr. Lipner and Dr. Rossi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a study recently published in Nature Communications suggests that According to two experts, these findings raise concerns regarding the safety of frequent use of these nail dryers.
In the study, human and mouse cells were exposed to radiation from UV nail dryers. Exposing human and mice skin cells to UVA light for 20 minutes resulted in the death of 20%-30% of cells; three consecutive 20-minute sessions resulted in the death of 65%-70% of cells. Additionally, surviving cells suffered oxidative damage to their DNA and mitochondria, with mutational patterns similar to those seen in skin cancer, study investigator Maria Zhivagui, PhD, of the University of California, San Diego, and associates reported.
“This study showed that irradiation of human and mouse cell lines using UV nail polish dryers resulted in DNA damage and genome mutations,” Shari Lipner, MD, PhD, director of the nail division at New York–Presbyterian Hospital/Weill Cornell Medicine, New York, said in an interview. The study “ties together exposure to UV light from nail polish dryers and genetic mutations that are associated with skin cancers,” added Dr. Lipner, who was not involved with the study.
UV nail lamps are commonly used to dry and harden gel nail polish formulas. Often referred to as “mini tanning beds,” these devices emit UVA radiation, classified as a Group 1 Carcinogen by the International Agency for Research on Cancer.
“Both UVA and UVB are main drivers of both melanoma and keratinocyte carcinomas (basal cell carcinoma and squamous cell carcinoma),” said Anthony Rossi, MD, a dermatologic surgeon at Memorial Sloan Kettering Cancer Center, New York, who was also not a study investigator. UV irradiance “produces DNA mutations that are specific to forming types of skin cancer,” he said in an interview.
UVA wavelengths commonly used in nail dryers can penetrate all layers of the epidermis, the top layer of the skin, potentially affecting stem cells in the skin, according to the study.
Dr. Lipner noted that “there have been several case reports of patients with histories of gel manicures using UV nail polish dryers who later developed squamous cell carcinomas on the dorsal hands, fingers, and nails, and articles describing high UV emissions from nail polish dryers, but the direct connection between UV dryers and skin cancer development was tenuous.” The first of its kind, the new study investigated the impact of UV nail drying devices at a cellular level.
The results of this study, in combination with previous case reports suggesting the development of skin cancers following UVA dryer use, raise concern regarding the safety of these commonly used devices. The study, the authors wrote, “does not provide direct evidence for an increased cancer risk in human beings,” but their findings and “prior evidence strongly suggest that radiation emitted by UV nail polish dryers may cause cancers of the hand and that UV nail polish dryers, similar to tanning beds, may increase the risk of early onset skin cancer.”
Dr. Rossi said that, “while this study shows that the UV exposure does affect human cells and causes mutations, the study was not done in vivo in human beings, so further studies are needed to know at what dose and frequency gel manicures would be needed to cause detrimental effects.” However, for people who regularly receive gel manicures involving UV nail dryers, both Dr. Lipner and Dr. Rossi recommend applying a broad-spectrum sunscreen to protect the dorsal hands, fingertips, and skin surrounding the nails, or wearing UV-protective gloves.
The study was supported by an Alfred B. Sloan Research Fellowship to one of the authors and grants from the National Institutes of Health to two authors. One author reported being a compensated consultant and having an equity interest in io9. Dr. Lipner and Dr. Rossi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Results of a study recently published in Nature Communications suggests that According to two experts, these findings raise concerns regarding the safety of frequent use of these nail dryers.
In the study, human and mouse cells were exposed to radiation from UV nail dryers. Exposing human and mice skin cells to UVA light for 20 minutes resulted in the death of 20%-30% of cells; three consecutive 20-minute sessions resulted in the death of 65%-70% of cells. Additionally, surviving cells suffered oxidative damage to their DNA and mitochondria, with mutational patterns similar to those seen in skin cancer, study investigator Maria Zhivagui, PhD, of the University of California, San Diego, and associates reported.
“This study showed that irradiation of human and mouse cell lines using UV nail polish dryers resulted in DNA damage and genome mutations,” Shari Lipner, MD, PhD, director of the nail division at New York–Presbyterian Hospital/Weill Cornell Medicine, New York, said in an interview. The study “ties together exposure to UV light from nail polish dryers and genetic mutations that are associated with skin cancers,” added Dr. Lipner, who was not involved with the study.
UV nail lamps are commonly used to dry and harden gel nail polish formulas. Often referred to as “mini tanning beds,” these devices emit UVA radiation, classified as a Group 1 Carcinogen by the International Agency for Research on Cancer.
“Both UVA and UVB are main drivers of both melanoma and keratinocyte carcinomas (basal cell carcinoma and squamous cell carcinoma),” said Anthony Rossi, MD, a dermatologic surgeon at Memorial Sloan Kettering Cancer Center, New York, who was also not a study investigator. UV irradiance “produces DNA mutations that are specific to forming types of skin cancer,” he said in an interview.
UVA wavelengths commonly used in nail dryers can penetrate all layers of the epidermis, the top layer of the skin, potentially affecting stem cells in the skin, according to the study.
Dr. Lipner noted that “there have been several case reports of patients with histories of gel manicures using UV nail polish dryers who later developed squamous cell carcinomas on the dorsal hands, fingers, and nails, and articles describing high UV emissions from nail polish dryers, but the direct connection between UV dryers and skin cancer development was tenuous.” The first of its kind, the new study investigated the impact of UV nail drying devices at a cellular level.
The results of this study, in combination with previous case reports suggesting the development of skin cancers following UVA dryer use, raise concern regarding the safety of these commonly used devices. The study, the authors wrote, “does not provide direct evidence for an increased cancer risk in human beings,” but their findings and “prior evidence strongly suggest that radiation emitted by UV nail polish dryers may cause cancers of the hand and that UV nail polish dryers, similar to tanning beds, may increase the risk of early onset skin cancer.”
Dr. Rossi said that, “while this study shows that the UV exposure does affect human cells and causes mutations, the study was not done in vivo in human beings, so further studies are needed to know at what dose and frequency gel manicures would be needed to cause detrimental effects.” However, for people who regularly receive gel manicures involving UV nail dryers, both Dr. Lipner and Dr. Rossi recommend applying a broad-spectrum sunscreen to protect the dorsal hands, fingertips, and skin surrounding the nails, or wearing UV-protective gloves.
The study was supported by an Alfred B. Sloan Research Fellowship to one of the authors and grants from the National Institutes of Health to two authors. One author reported being a compensated consultant and having an equity interest in io9. Dr. Lipner and Dr. Rossi reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NATURE COMMUNICATIONS
What are the clinical implications of recent skin dysbiosis discoveries?
NEW ORLEANS – .
“There’s still a lot for us to learn,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said at the annual meeting of the American Academy of Dermatology. “Multiple factors contribute to the variability in the skin microbiota, including age, sex, environment, immune system, host genotype, lifestyle, and pathobiology. The question becomes, when do these factors or impacts on the microbiota become clinically significant?”
According to Dr. Friedman, there are 10 times more bacteria cells than human cells in the human body, “but it’s not a fight to the finish; it’s not us versus them,” he said. “Together, we are a super organism.” There are also more than 500 species of bacteria on human skin excluding viruses and fungi, and each person carries up to 5 pounds of bacteria, which is akin to finding a new organ in the body.
“What’s so unique is that we each have our own bacterial fingerprint,” he said. “Whoever is sitting next to you? Their microbiota makeup is different than yours.”
Beyond genetics and environment, activities that can contribute to alterations in skin flora or skin dysbiosis include topical application of steroids, antibiotics, retinoids, harsh soaps, chemical and physical exfoliants, and resurfacing techniques. “With anything we apply or do to the skin, we are literally changing the home of many microorganisms, for good or bad,” he said.
In the realm of atopic dermatitis (AD), Staphylococcus aureus has been implicated as an offender in the pathophysiology of the disease. “It’s not about one single species of Staphylococcus, though,” said Dr. Friedman, who also is director of translational research at George Washington University. “We’re finding out that, depending on the severity of disease, Staph. epidermis may be part of the problem as opposed to it just being about Staph. aureus. Furthermore, and more importantly, these changes in the microbiota, specifically a decrease in microbial diversity, has been shown to precede a disease flare, highlighting the central role of maintaining microbial diversity and by definition, supporting the living barrier in our management of AD.”
With this in mind, researchers in one study used high-throughput sequencing to evaluate the microbial communities associated with affected and unaffected skin of 49 patients with AD before and after emollient treatment. Following 84 days of emollient application, clinical symptoms of AD improved in 72% of the study population and Stenotrophomonas species were significantly more abundant among responders.
Prebiotics, probiotics
“Our treatments certainly can positively impact the microbiota, as we have seen even recently with some of our new targeted therapies, but we can also directly provide support,” he continued. Prebiotics, which he defined as supplements or foods that contain a nondigestible ingredient that selectively stimulates the growth and/or activity of indigenous bacteria, can be found in many over-the-counter moisturizers.
For example, colloidal oatmeal has been found to support the growth of S. epidermidis and enhance the production of lactic acid. “We really don’t know much about what these induced changes mean from a clinical perspective; that has yet to be elucidated,” Dr. Friedman said.
In light of the recent attention to the early application of moisturizers in infants at high risk of developing AD in an effort to prevent or limit AD, “maybe part of this has to do with applying something that’s nurturing an evolving microbiota,” Dr. Friedman noted. “It’s something to think about.”
Yet another area of study involves the use of probiotics, which Dr. Friedman defined as supplements or foods that contain viable microorganisms that alter the microflora of the host. In a first-of-its-kind trial, researchers evaluated the safety and efficacy of self-administered topical Roseomonas mucosa in 10 adults and 5 children with AD. No adverse events or treatment complications were observed, and the topical R. mucosa was associated with significant decreases in measures of disease severity, topical steroid requirement, and S. aureus burden
In a more recent randomized trial of 11 patients with AD, Richard L. Gallo, MD, PhD, chair of dermatology, University of California, San Diego, and colleagues found that application of a personalized topical cream formulated from coagulase-negative Staphylococcus with antimicrobial activity against S. aureus reduced colonization of S. aureus and improved disease severity.
And in another randomized, controlled trial, Italian researchers enrolled 80 adults with mild to severe AD to receive a placebo or a supplement that was a mixture of lactobacilli for 56 days. They found that adults in the treatment arm showed an improvement in skin smoothness, skin moisturization, self-perception, and a decrease in the SCORing Atopic Dermatitis (SCORAD) index as well as in levels of inflammatory markers associated with AD.
Dr. Friedman also discussed postbiotics, nonviable bacterial products or metabolic byproducts from probiotic microorganisms that have biologic activity in the host. In one trial, French researchers enrolled 75 people with AD who ranged in age from 6 to 70 years to receive a cream containing a 5% lysate of the nonpathogenic bacteria Vitreoscilla filiformis, or a vehicle cream for 30 days. They found that compared with the vehicle, V. filiformis lysate significantly decreased SCORAD levels and pruritus; active cream was shown to significantly decrease loss of sleep from day 0 to day 29.
Dr. Friedman characterized these novel approaches to AD as “an exciting area, one we need to pay attention to. But what I really want to know is, aside from these purposefully made and marketed products that have pre- and postprobiotics, is there a difference with some of the products we use already? My assumption is that there is, but we need to see that data.”
Dr. Friedman disclosed that he is a consultant and/or advisory board member for Medscape/SanovaWorks, Oakstone Institute, L’Oréal, La Roche Posay, Galderma, Aveeno, Ortho Dermatologic, Microcures, Pfizer, Novartis, Lilly, Hoth Therapeutics, Zylo Therapeutics, BMS, Vial, Janssen, Novocure, Dermavant, Regeneron/Sanofi, and Incyte. He has also received grants from Pfizer, the Dermatology Foundation, Lilly, Janssen, Incyte, and Galderma.
NEW ORLEANS – .
“There’s still a lot for us to learn,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said at the annual meeting of the American Academy of Dermatology. “Multiple factors contribute to the variability in the skin microbiota, including age, sex, environment, immune system, host genotype, lifestyle, and pathobiology. The question becomes, when do these factors or impacts on the microbiota become clinically significant?”
According to Dr. Friedman, there are 10 times more bacteria cells than human cells in the human body, “but it’s not a fight to the finish; it’s not us versus them,” he said. “Together, we are a super organism.” There are also more than 500 species of bacteria on human skin excluding viruses and fungi, and each person carries up to 5 pounds of bacteria, which is akin to finding a new organ in the body.
“What’s so unique is that we each have our own bacterial fingerprint,” he said. “Whoever is sitting next to you? Their microbiota makeup is different than yours.”
Beyond genetics and environment, activities that can contribute to alterations in skin flora or skin dysbiosis include topical application of steroids, antibiotics, retinoids, harsh soaps, chemical and physical exfoliants, and resurfacing techniques. “With anything we apply or do to the skin, we are literally changing the home of many microorganisms, for good or bad,” he said.
In the realm of atopic dermatitis (AD), Staphylococcus aureus has been implicated as an offender in the pathophysiology of the disease. “It’s not about one single species of Staphylococcus, though,” said Dr. Friedman, who also is director of translational research at George Washington University. “We’re finding out that, depending on the severity of disease, Staph. epidermis may be part of the problem as opposed to it just being about Staph. aureus. Furthermore, and more importantly, these changes in the microbiota, specifically a decrease in microbial diversity, has been shown to precede a disease flare, highlighting the central role of maintaining microbial diversity and by definition, supporting the living barrier in our management of AD.”
With this in mind, researchers in one study used high-throughput sequencing to evaluate the microbial communities associated with affected and unaffected skin of 49 patients with AD before and after emollient treatment. Following 84 days of emollient application, clinical symptoms of AD improved in 72% of the study population and Stenotrophomonas species were significantly more abundant among responders.
Prebiotics, probiotics
“Our treatments certainly can positively impact the microbiota, as we have seen even recently with some of our new targeted therapies, but we can also directly provide support,” he continued. Prebiotics, which he defined as supplements or foods that contain a nondigestible ingredient that selectively stimulates the growth and/or activity of indigenous bacteria, can be found in many over-the-counter moisturizers.
For example, colloidal oatmeal has been found to support the growth of S. epidermidis and enhance the production of lactic acid. “We really don’t know much about what these induced changes mean from a clinical perspective; that has yet to be elucidated,” Dr. Friedman said.
In light of the recent attention to the early application of moisturizers in infants at high risk of developing AD in an effort to prevent or limit AD, “maybe part of this has to do with applying something that’s nurturing an evolving microbiota,” Dr. Friedman noted. “It’s something to think about.”
Yet another area of study involves the use of probiotics, which Dr. Friedman defined as supplements or foods that contain viable microorganisms that alter the microflora of the host. In a first-of-its-kind trial, researchers evaluated the safety and efficacy of self-administered topical Roseomonas mucosa in 10 adults and 5 children with AD. No adverse events or treatment complications were observed, and the topical R. mucosa was associated with significant decreases in measures of disease severity, topical steroid requirement, and S. aureus burden
In a more recent randomized trial of 11 patients with AD, Richard L. Gallo, MD, PhD, chair of dermatology, University of California, San Diego, and colleagues found that application of a personalized topical cream formulated from coagulase-negative Staphylococcus with antimicrobial activity against S. aureus reduced colonization of S. aureus and improved disease severity.
And in another randomized, controlled trial, Italian researchers enrolled 80 adults with mild to severe AD to receive a placebo or a supplement that was a mixture of lactobacilli for 56 days. They found that adults in the treatment arm showed an improvement in skin smoothness, skin moisturization, self-perception, and a decrease in the SCORing Atopic Dermatitis (SCORAD) index as well as in levels of inflammatory markers associated with AD.
Dr. Friedman also discussed postbiotics, nonviable bacterial products or metabolic byproducts from probiotic microorganisms that have biologic activity in the host. In one trial, French researchers enrolled 75 people with AD who ranged in age from 6 to 70 years to receive a cream containing a 5% lysate of the nonpathogenic bacteria Vitreoscilla filiformis, or a vehicle cream for 30 days. They found that compared with the vehicle, V. filiformis lysate significantly decreased SCORAD levels and pruritus; active cream was shown to significantly decrease loss of sleep from day 0 to day 29.
Dr. Friedman characterized these novel approaches to AD as “an exciting area, one we need to pay attention to. But what I really want to know is, aside from these purposefully made and marketed products that have pre- and postprobiotics, is there a difference with some of the products we use already? My assumption is that there is, but we need to see that data.”
Dr. Friedman disclosed that he is a consultant and/or advisory board member for Medscape/SanovaWorks, Oakstone Institute, L’Oréal, La Roche Posay, Galderma, Aveeno, Ortho Dermatologic, Microcures, Pfizer, Novartis, Lilly, Hoth Therapeutics, Zylo Therapeutics, BMS, Vial, Janssen, Novocure, Dermavant, Regeneron/Sanofi, and Incyte. He has also received grants from Pfizer, the Dermatology Foundation, Lilly, Janssen, Incyte, and Galderma.
NEW ORLEANS – .
“There’s still a lot for us to learn,” Adam Friedman, MD, professor and chair of dermatology at George Washington University, Washington, said at the annual meeting of the American Academy of Dermatology. “Multiple factors contribute to the variability in the skin microbiota, including age, sex, environment, immune system, host genotype, lifestyle, and pathobiology. The question becomes, when do these factors or impacts on the microbiota become clinically significant?”
According to Dr. Friedman, there are 10 times more bacteria cells than human cells in the human body, “but it’s not a fight to the finish; it’s not us versus them,” he said. “Together, we are a super organism.” There are also more than 500 species of bacteria on human skin excluding viruses and fungi, and each person carries up to 5 pounds of bacteria, which is akin to finding a new organ in the body.
“What’s so unique is that we each have our own bacterial fingerprint,” he said. “Whoever is sitting next to you? Their microbiota makeup is different than yours.”
Beyond genetics and environment, activities that can contribute to alterations in skin flora or skin dysbiosis include topical application of steroids, antibiotics, retinoids, harsh soaps, chemical and physical exfoliants, and resurfacing techniques. “With anything we apply or do to the skin, we are literally changing the home of many microorganisms, for good or bad,” he said.
In the realm of atopic dermatitis (AD), Staphylococcus aureus has been implicated as an offender in the pathophysiology of the disease. “It’s not about one single species of Staphylococcus, though,” said Dr. Friedman, who also is director of translational research at George Washington University. “We’re finding out that, depending on the severity of disease, Staph. epidermis may be part of the problem as opposed to it just being about Staph. aureus. Furthermore, and more importantly, these changes in the microbiota, specifically a decrease in microbial diversity, has been shown to precede a disease flare, highlighting the central role of maintaining microbial diversity and by definition, supporting the living barrier in our management of AD.”
With this in mind, researchers in one study used high-throughput sequencing to evaluate the microbial communities associated with affected and unaffected skin of 49 patients with AD before and after emollient treatment. Following 84 days of emollient application, clinical symptoms of AD improved in 72% of the study population and Stenotrophomonas species were significantly more abundant among responders.
Prebiotics, probiotics
“Our treatments certainly can positively impact the microbiota, as we have seen even recently with some of our new targeted therapies, but we can also directly provide support,” he continued. Prebiotics, which he defined as supplements or foods that contain a nondigestible ingredient that selectively stimulates the growth and/or activity of indigenous bacteria, can be found in many over-the-counter moisturizers.
For example, colloidal oatmeal has been found to support the growth of S. epidermidis and enhance the production of lactic acid. “We really don’t know much about what these induced changes mean from a clinical perspective; that has yet to be elucidated,” Dr. Friedman said.
In light of the recent attention to the early application of moisturizers in infants at high risk of developing AD in an effort to prevent or limit AD, “maybe part of this has to do with applying something that’s nurturing an evolving microbiota,” Dr. Friedman noted. “It’s something to think about.”
Yet another area of study involves the use of probiotics, which Dr. Friedman defined as supplements or foods that contain viable microorganisms that alter the microflora of the host. In a first-of-its-kind trial, researchers evaluated the safety and efficacy of self-administered topical Roseomonas mucosa in 10 adults and 5 children with AD. No adverse events or treatment complications were observed, and the topical R. mucosa was associated with significant decreases in measures of disease severity, topical steroid requirement, and S. aureus burden
In a more recent randomized trial of 11 patients with AD, Richard L. Gallo, MD, PhD, chair of dermatology, University of California, San Diego, and colleagues found that application of a personalized topical cream formulated from coagulase-negative Staphylococcus with antimicrobial activity against S. aureus reduced colonization of S. aureus and improved disease severity.
And in another randomized, controlled trial, Italian researchers enrolled 80 adults with mild to severe AD to receive a placebo or a supplement that was a mixture of lactobacilli for 56 days. They found that adults in the treatment arm showed an improvement in skin smoothness, skin moisturization, self-perception, and a decrease in the SCORing Atopic Dermatitis (SCORAD) index as well as in levels of inflammatory markers associated with AD.
Dr. Friedman also discussed postbiotics, nonviable bacterial products or metabolic byproducts from probiotic microorganisms that have biologic activity in the host. In one trial, French researchers enrolled 75 people with AD who ranged in age from 6 to 70 years to receive a cream containing a 5% lysate of the nonpathogenic bacteria Vitreoscilla filiformis, or a vehicle cream for 30 days. They found that compared with the vehicle, V. filiformis lysate significantly decreased SCORAD levels and pruritus; active cream was shown to significantly decrease loss of sleep from day 0 to day 29.
Dr. Friedman characterized these novel approaches to AD as “an exciting area, one we need to pay attention to. But what I really want to know is, aside from these purposefully made and marketed products that have pre- and postprobiotics, is there a difference with some of the products we use already? My assumption is that there is, but we need to see that data.”
Dr. Friedman disclosed that he is a consultant and/or advisory board member for Medscape/SanovaWorks, Oakstone Institute, L’Oréal, La Roche Posay, Galderma, Aveeno, Ortho Dermatologic, Microcures, Pfizer, Novartis, Lilly, Hoth Therapeutics, Zylo Therapeutics, BMS, Vial, Janssen, Novocure, Dermavant, Regeneron/Sanofi, and Incyte. He has also received grants from Pfizer, the Dermatology Foundation, Lilly, Janssen, Incyte, and Galderma.
AT AAD 2023
Malpractice risks for docs who oversee NPs or PAs
Even in states that have abolished requirements that NPs be physician-supervised, physicians may still be liable by virtue of employing the NP, according to William P. Sullivan, DO, an attorney and emergency physician in Frankfort, Ill.
Indeed, the vast majority of lawsuits against NPs and PAs name the supervising physician. According to a study of claims against NPs from 2011 to 2016, 82% of the cases also named the supervising physician.
Employed or contracted physicians assigned to supervise NPs or PAs are also affected, Dr. Sullivan said. “The employed physicians’ contract with a hospital or staffing company may require them to assist in the selection, supervision, and/or training of NPs or PAs,” he said. He added that supervisory duties may also be assigned through hospital bylaws.
“The physician is usually not paid anything extra for this work and may not be given extra time to perform it,” Dr. Sullivan said. But still, he said, that physician could be named in a lawsuit and wind up bearing some responsibility for an NP’s or PA’s mistake.
In addition to facing medical malpractice suits, Dr. Sullivan said, doctors are often sanctioned by state licensure boards for improperly supervising NPs and PAs. Licensure boards often require extensive protocols for supervision of NPs and PAs.
Yet more states are removing supervision requirements
With the addition of Kansas and New York in 2022 and California in 2023, 27 states no longer require supervision for all or most NPs. Sixteen of those states, including New York and California, have instituted progressive practice authority that requires temporary supervision of new NPs but then removes supervision after a period of 6 months to 4 years, depending on the state, for the rest of their career.
“When it comes to NP independence, the horse is already out of the barn,” Dr. Sullivan said. “It’s unlikely that states will repeal laws granting NPs independence, and in fact, more states are likely to pass them.”
*PAs, in contrast, are well behind NPs in achieving independence, but the American Academy of Physician Associates (AAPA) is calling to eliminate a mandated relationship with a specific physician. So far, Utah, North Dakota and Wyoming have ended physician supervision of PAs, while California and Hawaii have eliminated mandated chart review. Other states are considering eliminating physician supervision of PAs, according to the AAPA.
In states that have abolished oversight requirements for NPs, “liability can then shift to the NP when the NP is fully independent,” Cathy Klein, an advanced practice registered nurse who helped found the NP profession 50 years ago, told this news organization. “More NPs are starting their own practices, and in many cases, patients actually prefer to see an NP.”
As more NPs became more autonomous, the average payment that NPs incurred in professional liability lawsuits rose by 10.5% from 2017 to 2022, to $332,187, according to the Nurses Service Organization (NSO), a nursing malpractice insurer.
The number of malpractice judgments against autonomous NPs alone has also been rising. From 2012 to 2017, autonomous NPs’ share of all NP cases rose from 7% to 16.4%, the NSO reported.
The good news for physicians is that states’ removal of restrictions on NPs has reduced physicians’ liability to some extent. A 2017 study found that enacting less restrictive scope-of-practice laws for NPs decreased the number of payments made by physicians in NP cases by as much as 31%.
However, the top location for NP payouts remains the physician’s office, not the autonomous NP’s practice, according to the latter NSO report. Plaintiffs sue NPs’ and PAs’ supervising physicians on the basis of legal concepts, such as vicarious liability and respondeat superior. Even if the physician-employer never saw the patient, he or she can be held liable.
Court cases in which supervising physician was found liable
There are plenty of judgments against supervising or collaborating physicians when the NP or PA made the error. Typically, the doctor was faulted for paying little attention to the NP or PA he or she was supposed to supervise.
Dr. Sullivan points to a 2016 case in which a New York jury held a physician 40% liable for a $7 million judgment in a malpractice case involving a PA’s care of a patient in the emergency department. The case is Shajan v. South Nassau Community Hospital in New York.
“The patient presented with nontraumatic leg pain to his lower leg, was diagnosed by the PA with a muscle strain, and discharged without a physician evaluation,” Dr. Sullivan said. The next day, the patient visited an orthopedist who immediately diagnosed compartment syndrome, an emergent condition in which pressure builds up in an affected extremity, damaging the muscles and nerves. “The patient developed irreversible nerve damage and chronic regional pain syndrome,” he said.
A malpractice lawsuit named the PA and the emergency physician he was supposed to be reporting to. Even though the physician had never seen the patient, he had signed off on the PA’s note from a patient’s ED visit. “Testimony during the trial focused on hospital protocols that the supervising physician was supposed to take,” Dr. Sullivan said.
When doctors share fault, they frequently failed to follow the collaborative agreement with the NP or PA. In Collip v. Ratts, a 2015 Indiana case in which the patient died from a drug interaction, the doctor’s certified public accountant stated that the doctor was required to review at least 5% of the NP’s charts every week to evaluate her prescriptive practices.
The doctor admitted that he never reviewed the NP’s charts on a weekly basis. He did conduct some cursory reviews of some of the NP’s notes, and in them he noted concerns for her prescribing practices and suggested she attend a narcotics-prescribing seminar, but he did not follow up to make sure she had done this.
Sometimes the NP or PA who made the mistake may actually be dropped from the lawsuit, leaving the supervising physician fully liable. In these cases, courts reason that a fully engaged supervisor could have prevented the error. In the 2006 case of Husak v. Siegal, the Florida Supreme Court dropped the NP from the case, ruling that the NP had provided the supervising doctor all the information he needed in order to tell her what to do for the patient.
The court noted the physician had failed to look at the chart, even though he was required to do so under his supervisory agreement with the NP. The doctor “could have made the correct diagnosis or referral had he been attentive,” the court said. Therefore, there was “no evidence of independent negligence” by the NP, even though she was the one who had made the incorrect diagnosis that harmed the patient.
When states require an autonomous NP to have a supervisory relationship with a doctor, the supervisor may be unavailable and may fail to designate a substitute. In Texas in January 2019, a 7-year-old girl died of pneumonia after being treated by an NP in an urgent care clinic. The NP had told the parents that the child could safely go home and only needed ibuprofen. The parents brought the girl back home, and she died 15 hours later. The Wattenbargers sued the NP, and the doctor’s supervision was a topic in the trial.
The supervising physician for the NP was out of the country at the time. He said that he had found a substitute, but the substitute doctor testified she had no idea she was designated to be the substitute, according to Niran Al-Agba, MD, a family physician in Silverdale, Wash., who has written on the Texas case. Dr. Al-Agba told this news organization the case appears to have been settled confidentially.
Different standards for expert witnesses
In many states, courts do not allow physicians to testify as expert witnesses in malpractice cases against NPs, arguing that nurses have a different set of standards than doctors have, Dr. Sullivan reported.
These states include Arkansas, Illinois, North Carolina, and New York, according to a report by SEAK Inc., an expert witness training program. The report said most other states allow physician experts in these cases, but they may still require that they have experience with the nursing standard of care.
Dr. Sullivan said some courts are whittling away at the ban on physician experts, and the ban may eventually disappear. He reported that in Oklahoma, which normally upholds the ban, a judge recently allowed a physician-expert to testify in a case involving the death of a 19-year-old woman, Alexus Ochoa, in an ED staffed by an NP. The judge reasoned that Ms. Ochoa’s parents assumed the ED was staffed by physicians and would adhere to medical standards.
Supervision pointers from a physician
Physicians who supervise NPs or PAs say it is important to keep track of their skills and help them sharpen their expertise. Their scope of practice and physicians’ supervisory responsibilities are included in the collaborative agreement.
Arthur Apolinario, MD, a family physician in Clinton, N.C., says his 10-physician practice, which employs six NPs and one PA, works under a collaborative agreement. “The agreement defines each person’s scope of practice. They can’t do certain procedures, such as surgery, and they need extra training before doing certain tasks alone, such as joint injection.
“You have to always figure that if there is a lawsuit against one of them, you as the supervising physician would be named,” said Dr. Apolinario, who is also president of the North Carolina Medical Society. “We try to avert mistakes by meeting regularly with our NPs and PAs and making sure they keep up to date.”
Collaborating with autonomous NPs
Even when NPs operate independently in states that have abolished supervision, physicians may still have some liability if they give NPs advice, Dr. Al-Agba said.
At her Washington state practice, Dr. Al-Agba shares an office with an autonomous NP. “We share overhead and a front desk, but we have separate patients,” Dr. Al-Agba said. “This arrangement works very well for both of us.”
The NP sometimes asks her for advice. When this occurs, Dr. Al-Agba said she always makes sure to see the patient first. “If you don’t actually see the patient, there could be a misunderstanding that could lead to an error,” she said.
Conclusion
Even though NPs now have autonomy in most states, supervising physicians may still be liable for NP malpractice by virtue of being their employers, and physicians in the remaining states are liable for NPs through state law and for PAs in virtually all the states. To determine the supervising physician’s fault, courts often study whether the physician has met the terms of the collaborative agreement.
Physicians can reduce collaborating NPs’ and PAs’ liability by properly training them, by verifying their scope of practice, by making themselves easily available for consultation, and by occasionally seeing their patients. If their NPs and PAs do commit malpractice, supervising physicians may be able to protect themselves from liability by adhering to all requirements of the collaborative agreement.
*Correction, 4/19/2023: An earlier version of this story misstated the name of the AAPA and the states that have ended physician supervision of PAs.
A version of this article first appeared on Medscape.com.
Even in states that have abolished requirements that NPs be physician-supervised, physicians may still be liable by virtue of employing the NP, according to William P. Sullivan, DO, an attorney and emergency physician in Frankfort, Ill.
Indeed, the vast majority of lawsuits against NPs and PAs name the supervising physician. According to a study of claims against NPs from 2011 to 2016, 82% of the cases also named the supervising physician.
Employed or contracted physicians assigned to supervise NPs or PAs are also affected, Dr. Sullivan said. “The employed physicians’ contract with a hospital or staffing company may require them to assist in the selection, supervision, and/or training of NPs or PAs,” he said. He added that supervisory duties may also be assigned through hospital bylaws.
“The physician is usually not paid anything extra for this work and may not be given extra time to perform it,” Dr. Sullivan said. But still, he said, that physician could be named in a lawsuit and wind up bearing some responsibility for an NP’s or PA’s mistake.
In addition to facing medical malpractice suits, Dr. Sullivan said, doctors are often sanctioned by state licensure boards for improperly supervising NPs and PAs. Licensure boards often require extensive protocols for supervision of NPs and PAs.
Yet more states are removing supervision requirements
With the addition of Kansas and New York in 2022 and California in 2023, 27 states no longer require supervision for all or most NPs. Sixteen of those states, including New York and California, have instituted progressive practice authority that requires temporary supervision of new NPs but then removes supervision after a period of 6 months to 4 years, depending on the state, for the rest of their career.
“When it comes to NP independence, the horse is already out of the barn,” Dr. Sullivan said. “It’s unlikely that states will repeal laws granting NPs independence, and in fact, more states are likely to pass them.”
*PAs, in contrast, are well behind NPs in achieving independence, but the American Academy of Physician Associates (AAPA) is calling to eliminate a mandated relationship with a specific physician. So far, Utah, North Dakota and Wyoming have ended physician supervision of PAs, while California and Hawaii have eliminated mandated chart review. Other states are considering eliminating physician supervision of PAs, according to the AAPA.
In states that have abolished oversight requirements for NPs, “liability can then shift to the NP when the NP is fully independent,” Cathy Klein, an advanced practice registered nurse who helped found the NP profession 50 years ago, told this news organization. “More NPs are starting their own practices, and in many cases, patients actually prefer to see an NP.”
As more NPs became more autonomous, the average payment that NPs incurred in professional liability lawsuits rose by 10.5% from 2017 to 2022, to $332,187, according to the Nurses Service Organization (NSO), a nursing malpractice insurer.
The number of malpractice judgments against autonomous NPs alone has also been rising. From 2012 to 2017, autonomous NPs’ share of all NP cases rose from 7% to 16.4%, the NSO reported.
The good news for physicians is that states’ removal of restrictions on NPs has reduced physicians’ liability to some extent. A 2017 study found that enacting less restrictive scope-of-practice laws for NPs decreased the number of payments made by physicians in NP cases by as much as 31%.
However, the top location for NP payouts remains the physician’s office, not the autonomous NP’s practice, according to the latter NSO report. Plaintiffs sue NPs’ and PAs’ supervising physicians on the basis of legal concepts, such as vicarious liability and respondeat superior. Even if the physician-employer never saw the patient, he or she can be held liable.
Court cases in which supervising physician was found liable
There are plenty of judgments against supervising or collaborating physicians when the NP or PA made the error. Typically, the doctor was faulted for paying little attention to the NP or PA he or she was supposed to supervise.
Dr. Sullivan points to a 2016 case in which a New York jury held a physician 40% liable for a $7 million judgment in a malpractice case involving a PA’s care of a patient in the emergency department. The case is Shajan v. South Nassau Community Hospital in New York.
“The patient presented with nontraumatic leg pain to his lower leg, was diagnosed by the PA with a muscle strain, and discharged without a physician evaluation,” Dr. Sullivan said. The next day, the patient visited an orthopedist who immediately diagnosed compartment syndrome, an emergent condition in which pressure builds up in an affected extremity, damaging the muscles and nerves. “The patient developed irreversible nerve damage and chronic regional pain syndrome,” he said.
A malpractice lawsuit named the PA and the emergency physician he was supposed to be reporting to. Even though the physician had never seen the patient, he had signed off on the PA’s note from a patient’s ED visit. “Testimony during the trial focused on hospital protocols that the supervising physician was supposed to take,” Dr. Sullivan said.
When doctors share fault, they frequently failed to follow the collaborative agreement with the NP or PA. In Collip v. Ratts, a 2015 Indiana case in which the patient died from a drug interaction, the doctor’s certified public accountant stated that the doctor was required to review at least 5% of the NP’s charts every week to evaluate her prescriptive practices.
The doctor admitted that he never reviewed the NP’s charts on a weekly basis. He did conduct some cursory reviews of some of the NP’s notes, and in them he noted concerns for her prescribing practices and suggested she attend a narcotics-prescribing seminar, but he did not follow up to make sure she had done this.
Sometimes the NP or PA who made the mistake may actually be dropped from the lawsuit, leaving the supervising physician fully liable. In these cases, courts reason that a fully engaged supervisor could have prevented the error. In the 2006 case of Husak v. Siegal, the Florida Supreme Court dropped the NP from the case, ruling that the NP had provided the supervising doctor all the information he needed in order to tell her what to do for the patient.
The court noted the physician had failed to look at the chart, even though he was required to do so under his supervisory agreement with the NP. The doctor “could have made the correct diagnosis or referral had he been attentive,” the court said. Therefore, there was “no evidence of independent negligence” by the NP, even though she was the one who had made the incorrect diagnosis that harmed the patient.
When states require an autonomous NP to have a supervisory relationship with a doctor, the supervisor may be unavailable and may fail to designate a substitute. In Texas in January 2019, a 7-year-old girl died of pneumonia after being treated by an NP in an urgent care clinic. The NP had told the parents that the child could safely go home and only needed ibuprofen. The parents brought the girl back home, and she died 15 hours later. The Wattenbargers sued the NP, and the doctor’s supervision was a topic in the trial.
The supervising physician for the NP was out of the country at the time. He said that he had found a substitute, but the substitute doctor testified she had no idea she was designated to be the substitute, according to Niran Al-Agba, MD, a family physician in Silverdale, Wash., who has written on the Texas case. Dr. Al-Agba told this news organization the case appears to have been settled confidentially.
Different standards for expert witnesses
In many states, courts do not allow physicians to testify as expert witnesses in malpractice cases against NPs, arguing that nurses have a different set of standards than doctors have, Dr. Sullivan reported.
These states include Arkansas, Illinois, North Carolina, and New York, according to a report by SEAK Inc., an expert witness training program. The report said most other states allow physician experts in these cases, but they may still require that they have experience with the nursing standard of care.
Dr. Sullivan said some courts are whittling away at the ban on physician experts, and the ban may eventually disappear. He reported that in Oklahoma, which normally upholds the ban, a judge recently allowed a physician-expert to testify in a case involving the death of a 19-year-old woman, Alexus Ochoa, in an ED staffed by an NP. The judge reasoned that Ms. Ochoa’s parents assumed the ED was staffed by physicians and would adhere to medical standards.
Supervision pointers from a physician
Physicians who supervise NPs or PAs say it is important to keep track of their skills and help them sharpen their expertise. Their scope of practice and physicians’ supervisory responsibilities are included in the collaborative agreement.
Arthur Apolinario, MD, a family physician in Clinton, N.C., says his 10-physician practice, which employs six NPs and one PA, works under a collaborative agreement. “The agreement defines each person’s scope of practice. They can’t do certain procedures, such as surgery, and they need extra training before doing certain tasks alone, such as joint injection.
“You have to always figure that if there is a lawsuit against one of them, you as the supervising physician would be named,” said Dr. Apolinario, who is also president of the North Carolina Medical Society. “We try to avert mistakes by meeting regularly with our NPs and PAs and making sure they keep up to date.”
Collaborating with autonomous NPs
Even when NPs operate independently in states that have abolished supervision, physicians may still have some liability if they give NPs advice, Dr. Al-Agba said.
At her Washington state practice, Dr. Al-Agba shares an office with an autonomous NP. “We share overhead and a front desk, but we have separate patients,” Dr. Al-Agba said. “This arrangement works very well for both of us.”
The NP sometimes asks her for advice. When this occurs, Dr. Al-Agba said she always makes sure to see the patient first. “If you don’t actually see the patient, there could be a misunderstanding that could lead to an error,” she said.
Conclusion
Even though NPs now have autonomy in most states, supervising physicians may still be liable for NP malpractice by virtue of being their employers, and physicians in the remaining states are liable for NPs through state law and for PAs in virtually all the states. To determine the supervising physician’s fault, courts often study whether the physician has met the terms of the collaborative agreement.
Physicians can reduce collaborating NPs’ and PAs’ liability by properly training them, by verifying their scope of practice, by making themselves easily available for consultation, and by occasionally seeing their patients. If their NPs and PAs do commit malpractice, supervising physicians may be able to protect themselves from liability by adhering to all requirements of the collaborative agreement.
*Correction, 4/19/2023: An earlier version of this story misstated the name of the AAPA and the states that have ended physician supervision of PAs.
A version of this article first appeared on Medscape.com.
Even in states that have abolished requirements that NPs be physician-supervised, physicians may still be liable by virtue of employing the NP, according to William P. Sullivan, DO, an attorney and emergency physician in Frankfort, Ill.
Indeed, the vast majority of lawsuits against NPs and PAs name the supervising physician. According to a study of claims against NPs from 2011 to 2016, 82% of the cases also named the supervising physician.
Employed or contracted physicians assigned to supervise NPs or PAs are also affected, Dr. Sullivan said. “The employed physicians’ contract with a hospital or staffing company may require them to assist in the selection, supervision, and/or training of NPs or PAs,” he said. He added that supervisory duties may also be assigned through hospital bylaws.
“The physician is usually not paid anything extra for this work and may not be given extra time to perform it,” Dr. Sullivan said. But still, he said, that physician could be named in a lawsuit and wind up bearing some responsibility for an NP’s or PA’s mistake.
In addition to facing medical malpractice suits, Dr. Sullivan said, doctors are often sanctioned by state licensure boards for improperly supervising NPs and PAs. Licensure boards often require extensive protocols for supervision of NPs and PAs.
Yet more states are removing supervision requirements
With the addition of Kansas and New York in 2022 and California in 2023, 27 states no longer require supervision for all or most NPs. Sixteen of those states, including New York and California, have instituted progressive practice authority that requires temporary supervision of new NPs but then removes supervision after a period of 6 months to 4 years, depending on the state, for the rest of their career.
“When it comes to NP independence, the horse is already out of the barn,” Dr. Sullivan said. “It’s unlikely that states will repeal laws granting NPs independence, and in fact, more states are likely to pass them.”
*PAs, in contrast, are well behind NPs in achieving independence, but the American Academy of Physician Associates (AAPA) is calling to eliminate a mandated relationship with a specific physician. So far, Utah, North Dakota and Wyoming have ended physician supervision of PAs, while California and Hawaii have eliminated mandated chart review. Other states are considering eliminating physician supervision of PAs, according to the AAPA.
In states that have abolished oversight requirements for NPs, “liability can then shift to the NP when the NP is fully independent,” Cathy Klein, an advanced practice registered nurse who helped found the NP profession 50 years ago, told this news organization. “More NPs are starting their own practices, and in many cases, patients actually prefer to see an NP.”
As more NPs became more autonomous, the average payment that NPs incurred in professional liability lawsuits rose by 10.5% from 2017 to 2022, to $332,187, according to the Nurses Service Organization (NSO), a nursing malpractice insurer.
The number of malpractice judgments against autonomous NPs alone has also been rising. From 2012 to 2017, autonomous NPs’ share of all NP cases rose from 7% to 16.4%, the NSO reported.
The good news for physicians is that states’ removal of restrictions on NPs has reduced physicians’ liability to some extent. A 2017 study found that enacting less restrictive scope-of-practice laws for NPs decreased the number of payments made by physicians in NP cases by as much as 31%.
However, the top location for NP payouts remains the physician’s office, not the autonomous NP’s practice, according to the latter NSO report. Plaintiffs sue NPs’ and PAs’ supervising physicians on the basis of legal concepts, such as vicarious liability and respondeat superior. Even if the physician-employer never saw the patient, he or she can be held liable.
Court cases in which supervising physician was found liable
There are plenty of judgments against supervising or collaborating physicians when the NP or PA made the error. Typically, the doctor was faulted for paying little attention to the NP or PA he or she was supposed to supervise.
Dr. Sullivan points to a 2016 case in which a New York jury held a physician 40% liable for a $7 million judgment in a malpractice case involving a PA’s care of a patient in the emergency department. The case is Shajan v. South Nassau Community Hospital in New York.
“The patient presented with nontraumatic leg pain to his lower leg, was diagnosed by the PA with a muscle strain, and discharged without a physician evaluation,” Dr. Sullivan said. The next day, the patient visited an orthopedist who immediately diagnosed compartment syndrome, an emergent condition in which pressure builds up in an affected extremity, damaging the muscles and nerves. “The patient developed irreversible nerve damage and chronic regional pain syndrome,” he said.
A malpractice lawsuit named the PA and the emergency physician he was supposed to be reporting to. Even though the physician had never seen the patient, he had signed off on the PA’s note from a patient’s ED visit. “Testimony during the trial focused on hospital protocols that the supervising physician was supposed to take,” Dr. Sullivan said.
When doctors share fault, they frequently failed to follow the collaborative agreement with the NP or PA. In Collip v. Ratts, a 2015 Indiana case in which the patient died from a drug interaction, the doctor’s certified public accountant stated that the doctor was required to review at least 5% of the NP’s charts every week to evaluate her prescriptive practices.
The doctor admitted that he never reviewed the NP’s charts on a weekly basis. He did conduct some cursory reviews of some of the NP’s notes, and in them he noted concerns for her prescribing practices and suggested she attend a narcotics-prescribing seminar, but he did not follow up to make sure she had done this.
Sometimes the NP or PA who made the mistake may actually be dropped from the lawsuit, leaving the supervising physician fully liable. In these cases, courts reason that a fully engaged supervisor could have prevented the error. In the 2006 case of Husak v. Siegal, the Florida Supreme Court dropped the NP from the case, ruling that the NP had provided the supervising doctor all the information he needed in order to tell her what to do for the patient.
The court noted the physician had failed to look at the chart, even though he was required to do so under his supervisory agreement with the NP. The doctor “could have made the correct diagnosis or referral had he been attentive,” the court said. Therefore, there was “no evidence of independent negligence” by the NP, even though she was the one who had made the incorrect diagnosis that harmed the patient.
When states require an autonomous NP to have a supervisory relationship with a doctor, the supervisor may be unavailable and may fail to designate a substitute. In Texas in January 2019, a 7-year-old girl died of pneumonia after being treated by an NP in an urgent care clinic. The NP had told the parents that the child could safely go home and only needed ibuprofen. The parents brought the girl back home, and she died 15 hours later. The Wattenbargers sued the NP, and the doctor’s supervision was a topic in the trial.
The supervising physician for the NP was out of the country at the time. He said that he had found a substitute, but the substitute doctor testified she had no idea she was designated to be the substitute, according to Niran Al-Agba, MD, a family physician in Silverdale, Wash., who has written on the Texas case. Dr. Al-Agba told this news organization the case appears to have been settled confidentially.
Different standards for expert witnesses
In many states, courts do not allow physicians to testify as expert witnesses in malpractice cases against NPs, arguing that nurses have a different set of standards than doctors have, Dr. Sullivan reported.
These states include Arkansas, Illinois, North Carolina, and New York, according to a report by SEAK Inc., an expert witness training program. The report said most other states allow physician experts in these cases, but they may still require that they have experience with the nursing standard of care.
Dr. Sullivan said some courts are whittling away at the ban on physician experts, and the ban may eventually disappear. He reported that in Oklahoma, which normally upholds the ban, a judge recently allowed a physician-expert to testify in a case involving the death of a 19-year-old woman, Alexus Ochoa, in an ED staffed by an NP. The judge reasoned that Ms. Ochoa’s parents assumed the ED was staffed by physicians and would adhere to medical standards.
Supervision pointers from a physician
Physicians who supervise NPs or PAs say it is important to keep track of their skills and help them sharpen their expertise. Their scope of practice and physicians’ supervisory responsibilities are included in the collaborative agreement.
Arthur Apolinario, MD, a family physician in Clinton, N.C., says his 10-physician practice, which employs six NPs and one PA, works under a collaborative agreement. “The agreement defines each person’s scope of practice. They can’t do certain procedures, such as surgery, and they need extra training before doing certain tasks alone, such as joint injection.
“You have to always figure that if there is a lawsuit against one of them, you as the supervising physician would be named,” said Dr. Apolinario, who is also president of the North Carolina Medical Society. “We try to avert mistakes by meeting regularly with our NPs and PAs and making sure they keep up to date.”
Collaborating with autonomous NPs
Even when NPs operate independently in states that have abolished supervision, physicians may still have some liability if they give NPs advice, Dr. Al-Agba said.
At her Washington state practice, Dr. Al-Agba shares an office with an autonomous NP. “We share overhead and a front desk, but we have separate patients,” Dr. Al-Agba said. “This arrangement works very well for both of us.”
The NP sometimes asks her for advice. When this occurs, Dr. Al-Agba said she always makes sure to see the patient first. “If you don’t actually see the patient, there could be a misunderstanding that could lead to an error,” she said.
Conclusion
Even though NPs now have autonomy in most states, supervising physicians may still be liable for NP malpractice by virtue of being their employers, and physicians in the remaining states are liable for NPs through state law and for PAs in virtually all the states. To determine the supervising physician’s fault, courts often study whether the physician has met the terms of the collaborative agreement.
Physicians can reduce collaborating NPs’ and PAs’ liability by properly training them, by verifying their scope of practice, by making themselves easily available for consultation, and by occasionally seeing their patients. If their NPs and PAs do commit malpractice, supervising physicians may be able to protect themselves from liability by adhering to all requirements of the collaborative agreement.
*Correction, 4/19/2023: An earlier version of this story misstated the name of the AAPA and the states that have ended physician supervision of PAs.
A version of this article first appeared on Medscape.com.
Lack of food for thought: Starve a bacterium, feed an infection
A whole new, tiny level of hangry
Ever been so hungry that everything just got on your nerves? Maybe you feel a little snappy right now? Like you’ll just lash out unless you get something to eat? Been there. And so have bacteria.
New research shows that some bacteria go into a full-on Hulk smash if they’re not getting the nutrients they need by releasing toxins into the body. Sounds like a bacterial temper tantrum.
Even though two cells may be genetically identical, they don’t always behave the same in a bacterial community. Some do their job and stay in line, but some evil twins rage out and make people sick by releasing toxins into the environment, Adam Rosenthal, PhD, of the University of North Carolina and his colleagues discovered.
To figure out why some cells were all business as usual while others were not, the investigators looked at Clostridium perfringens, a bacterium found in the intestines of humans and other vertebrates. When the C. perfringens cells were fed a little acetate to munch on, the hangry cells calmed down faster than a kid with a bag of fruit snacks, reducing toxin levels. Some cells even disappeared, falling in line with their model-citizen counterparts.
So what does this really mean? More research, duh. Now that we know nutrients play a role in toxicity, it may open the door to finding a way to fight against antibiotic resistance in humans and reduce antibiotic use in the food industry.
So think to yourself. Are you bothered for no reason? Getting a little testy with your friends and coworkers? Maybe you just haven’t eaten in a while. You’re literally not alone. Even a single-cell organism can behave based on its hunger levels.
Now go have a snack. Your bacteria are getting restless.
The very hangry iguana?
Imagine yourself on a warm, sunny tropical beach. You are enjoying a piece of cake as you take in the slow beat of the waves lapping against the shore. Life is as good as it could be.
Then you feel a presence nearby. Hostility. Hunger. A set of feral, covetous eyes in the nearby jungle. A reptilian beast stalks you, and its all-encompassing sweet tooth desires your cake.
Wait, hold on, what?
As an unfortunate 3-year-old on vacation in Costa Rica found out, there’s at least one iguana in the world out there with a taste for sugar (better than a taste for blood, we suppose).
While out on the beach, the lizard darted out of nowhere, bit the girl on the back of the hand, and stole her cake. Still not the worst party guest ever. The child was taken to a local clinic, where the wound was cleaned and a 5-day antibiotic treatment (lizards carry salmonella) was provided. Things seemed fine, and the girl returned home without incident.
But of course, that’s not the end of the story. Five months later, the girl’s parents noticed a red bump at the wound site. Over the next 3 months, the surrounding skin grew red and painful. A trip to the hospital in California revealed that she had a ganglion cyst and a discharge of pus. Turns out our cake-obsessed lizard friend did give the little girl a gift: the first known human case of Mycobacterium marinum infection following an iguana bite on record.
M. marinum, which causes a disease similar to tuberculosis, typically infects fish but can infect humans if skin wounds are exposed to contaminated water. It’s also resistant to most antibiotics, which is why the first round didn’t clear up the infection. A second round of more-potent antibiotics seems to be working well.
So, to sum up, this poor child got bitten by a lizard, had her cake stolen, and contracted a rare illness in exchange. For a 3-year-old, that’s gotta be in the top-10 worst days ever. Unless, of course, we’re actually living in the Marvel universe (sorry, multiverse at this point). Then we’re totally going to see the emergence of the new superhero Iguana Girl in 15 years or so. Keep your eyes open.
No allergies? Let them give up cake
Allergy season is already here – starting earlier every year, it seems – and many people are not happy about it. So unhappy, actually, that there’s a list of things they would be willing to give up for a year to get rid of their of allergies, according to a survey conducted by OnePoll on behalf of Flonase.
Nearly 40% of 2,000 respondents with allergies would go a year without eating cake or chocolate or playing video games in exchange for allergy-free status, the survey results show. Almost as many would forgo coffee (38%) or pizza (37%) for a year, while 36% would stay off social media and 31% would take a pay cut or give up their smartphones, the Independent reported.
More than half of the allergic Americans – 54%, to be exact – who were polled this past winter – Feb. 24 to March 1, to be exact – consider allergy symptoms to be the most frustrating part of the spring. Annoying things that were less frustrating to the group included mosquitoes (41%), filing tax returns (38%), and daylight savings time (37%).
The Trump arraignment circus, of course, occurred too late to make the list, as did the big “We’re going back to the office! No wait, we’re closing the office forever!” email extravaganza and emotional roller coaster. That second one, however, did not get nearly as much media coverage.
A whole new, tiny level of hangry
Ever been so hungry that everything just got on your nerves? Maybe you feel a little snappy right now? Like you’ll just lash out unless you get something to eat? Been there. And so have bacteria.
New research shows that some bacteria go into a full-on Hulk smash if they’re not getting the nutrients they need by releasing toxins into the body. Sounds like a bacterial temper tantrum.
Even though two cells may be genetically identical, they don’t always behave the same in a bacterial community. Some do their job and stay in line, but some evil twins rage out and make people sick by releasing toxins into the environment, Adam Rosenthal, PhD, of the University of North Carolina and his colleagues discovered.
To figure out why some cells were all business as usual while others were not, the investigators looked at Clostridium perfringens, a bacterium found in the intestines of humans and other vertebrates. When the C. perfringens cells were fed a little acetate to munch on, the hangry cells calmed down faster than a kid with a bag of fruit snacks, reducing toxin levels. Some cells even disappeared, falling in line with their model-citizen counterparts.
So what does this really mean? More research, duh. Now that we know nutrients play a role in toxicity, it may open the door to finding a way to fight against antibiotic resistance in humans and reduce antibiotic use in the food industry.
So think to yourself. Are you bothered for no reason? Getting a little testy with your friends and coworkers? Maybe you just haven’t eaten in a while. You’re literally not alone. Even a single-cell organism can behave based on its hunger levels.
Now go have a snack. Your bacteria are getting restless.
The very hangry iguana?
Imagine yourself on a warm, sunny tropical beach. You are enjoying a piece of cake as you take in the slow beat of the waves lapping against the shore. Life is as good as it could be.
Then you feel a presence nearby. Hostility. Hunger. A set of feral, covetous eyes in the nearby jungle. A reptilian beast stalks you, and its all-encompassing sweet tooth desires your cake.
Wait, hold on, what?
As an unfortunate 3-year-old on vacation in Costa Rica found out, there’s at least one iguana in the world out there with a taste for sugar (better than a taste for blood, we suppose).
While out on the beach, the lizard darted out of nowhere, bit the girl on the back of the hand, and stole her cake. Still not the worst party guest ever. The child was taken to a local clinic, where the wound was cleaned and a 5-day antibiotic treatment (lizards carry salmonella) was provided. Things seemed fine, and the girl returned home without incident.
But of course, that’s not the end of the story. Five months later, the girl’s parents noticed a red bump at the wound site. Over the next 3 months, the surrounding skin grew red and painful. A trip to the hospital in California revealed that she had a ganglion cyst and a discharge of pus. Turns out our cake-obsessed lizard friend did give the little girl a gift: the first known human case of Mycobacterium marinum infection following an iguana bite on record.
M. marinum, which causes a disease similar to tuberculosis, typically infects fish but can infect humans if skin wounds are exposed to contaminated water. It’s also resistant to most antibiotics, which is why the first round didn’t clear up the infection. A second round of more-potent antibiotics seems to be working well.
So, to sum up, this poor child got bitten by a lizard, had her cake stolen, and contracted a rare illness in exchange. For a 3-year-old, that’s gotta be in the top-10 worst days ever. Unless, of course, we’re actually living in the Marvel universe (sorry, multiverse at this point). Then we’re totally going to see the emergence of the new superhero Iguana Girl in 15 years or so. Keep your eyes open.
No allergies? Let them give up cake
Allergy season is already here – starting earlier every year, it seems – and many people are not happy about it. So unhappy, actually, that there’s a list of things they would be willing to give up for a year to get rid of their of allergies, according to a survey conducted by OnePoll on behalf of Flonase.
Nearly 40% of 2,000 respondents with allergies would go a year without eating cake or chocolate or playing video games in exchange for allergy-free status, the survey results show. Almost as many would forgo coffee (38%) or pizza (37%) for a year, while 36% would stay off social media and 31% would take a pay cut or give up their smartphones, the Independent reported.
More than half of the allergic Americans – 54%, to be exact – who were polled this past winter – Feb. 24 to March 1, to be exact – consider allergy symptoms to be the most frustrating part of the spring. Annoying things that were less frustrating to the group included mosquitoes (41%), filing tax returns (38%), and daylight savings time (37%).
The Trump arraignment circus, of course, occurred too late to make the list, as did the big “We’re going back to the office! No wait, we’re closing the office forever!” email extravaganza and emotional roller coaster. That second one, however, did not get nearly as much media coverage.
A whole new, tiny level of hangry
Ever been so hungry that everything just got on your nerves? Maybe you feel a little snappy right now? Like you’ll just lash out unless you get something to eat? Been there. And so have bacteria.
New research shows that some bacteria go into a full-on Hulk smash if they’re not getting the nutrients they need by releasing toxins into the body. Sounds like a bacterial temper tantrum.
Even though two cells may be genetically identical, they don’t always behave the same in a bacterial community. Some do their job and stay in line, but some evil twins rage out and make people sick by releasing toxins into the environment, Adam Rosenthal, PhD, of the University of North Carolina and his colleagues discovered.
To figure out why some cells were all business as usual while others were not, the investigators looked at Clostridium perfringens, a bacterium found in the intestines of humans and other vertebrates. When the C. perfringens cells were fed a little acetate to munch on, the hangry cells calmed down faster than a kid with a bag of fruit snacks, reducing toxin levels. Some cells even disappeared, falling in line with their model-citizen counterparts.
So what does this really mean? More research, duh. Now that we know nutrients play a role in toxicity, it may open the door to finding a way to fight against antibiotic resistance in humans and reduce antibiotic use in the food industry.
So think to yourself. Are you bothered for no reason? Getting a little testy with your friends and coworkers? Maybe you just haven’t eaten in a while. You’re literally not alone. Even a single-cell organism can behave based on its hunger levels.
Now go have a snack. Your bacteria are getting restless.
The very hangry iguana?
Imagine yourself on a warm, sunny tropical beach. You are enjoying a piece of cake as you take in the slow beat of the waves lapping against the shore. Life is as good as it could be.
Then you feel a presence nearby. Hostility. Hunger. A set of feral, covetous eyes in the nearby jungle. A reptilian beast stalks you, and its all-encompassing sweet tooth desires your cake.
Wait, hold on, what?
As an unfortunate 3-year-old on vacation in Costa Rica found out, there’s at least one iguana in the world out there with a taste for sugar (better than a taste for blood, we suppose).
While out on the beach, the lizard darted out of nowhere, bit the girl on the back of the hand, and stole her cake. Still not the worst party guest ever. The child was taken to a local clinic, where the wound was cleaned and a 5-day antibiotic treatment (lizards carry salmonella) was provided. Things seemed fine, and the girl returned home without incident.
But of course, that’s not the end of the story. Five months later, the girl’s parents noticed a red bump at the wound site. Over the next 3 months, the surrounding skin grew red and painful. A trip to the hospital in California revealed that she had a ganglion cyst and a discharge of pus. Turns out our cake-obsessed lizard friend did give the little girl a gift: the first known human case of Mycobacterium marinum infection following an iguana bite on record.
M. marinum, which causes a disease similar to tuberculosis, typically infects fish but can infect humans if skin wounds are exposed to contaminated water. It’s also resistant to most antibiotics, which is why the first round didn’t clear up the infection. A second round of more-potent antibiotics seems to be working well.
So, to sum up, this poor child got bitten by a lizard, had her cake stolen, and contracted a rare illness in exchange. For a 3-year-old, that’s gotta be in the top-10 worst days ever. Unless, of course, we’re actually living in the Marvel universe (sorry, multiverse at this point). Then we’re totally going to see the emergence of the new superhero Iguana Girl in 15 years or so. Keep your eyes open.
No allergies? Let them give up cake
Allergy season is already here – starting earlier every year, it seems – and many people are not happy about it. So unhappy, actually, that there’s a list of things they would be willing to give up for a year to get rid of their of allergies, according to a survey conducted by OnePoll on behalf of Flonase.
Nearly 40% of 2,000 respondents with allergies would go a year without eating cake or chocolate or playing video games in exchange for allergy-free status, the survey results show. Almost as many would forgo coffee (38%) or pizza (37%) for a year, while 36% would stay off social media and 31% would take a pay cut or give up their smartphones, the Independent reported.
More than half of the allergic Americans – 54%, to be exact – who were polled this past winter – Feb. 24 to March 1, to be exact – consider allergy symptoms to be the most frustrating part of the spring. Annoying things that were less frustrating to the group included mosquitoes (41%), filing tax returns (38%), and daylight savings time (37%).
The Trump arraignment circus, of course, occurred too late to make the list, as did the big “We’re going back to the office! No wait, we’re closing the office forever!” email extravaganza and emotional roller coaster. That second one, however, did not get nearly as much media coverage.
Practicing ethical medicine ‘is a requirement,’ not a luxury, expert says
NEW ORLEANS – , but results from a national survey of dermatology residency program directors suggest that ethics training is not a priority.
Of the 139 dermatology residency program or associate program directors surveyed in 2022, only 43% responded. Of these, 55% said that their program had no ethics curriculum. Among programs with an ethics curriculum, 75% were implemented in the past 10 years, and the most common settings for teaching ethics were formal didactics (32%) and ad hoc during clinical encounters (28%). Reported barriers to implementing and/or maintaining an ethics curriculum included a lack of time (30%), lack of faculty with expertise (24%), and lack of useful resources (20%).
“Clearly, medical ethics is needed more to be part of our dermatology residency curriculum,” one of the study authors, Jane M. Grant-Kels, MD, professor of dermatology, pathology, and pediatrics, and founding chair of dermatology at the University of Connecticut, Farmington, said during a plenary lecture at the annual meeting of the American Academy of Dermatology. “Why? Because even though we’re physicians, and some of us have big egos, we are just human beings. We have all the faults and frailties of other humans. What we do as doctors often has unintended consequences that impact patients and society at large.”
Dr. Grant-Kels, one of the editors of the textbook “Dermatoethics”, said that, while she does not believe that physicians are intentionally unethical, “we stumble into bad behavior because we fool ourselves. We think that we are ethical. We think our colleagues are ethical, and we don’t view them with a clear, transparent eye. This is referred to as ethical fading or bounded ethicality.”
Similar to religion and good behavior, one can’t really teach someone to be ethical, she continued. “But you can teach people to think about ethics and to recognize an ethical dilemma when they’re in one,” she said. “Most articles that are available [pertain to] whether ethics can be taught or not, but there are very few resources available on how to actually teach ethics.”
That, she added, has been her goal for the last 2 decades: “How do I teach ethics without sounding like I’m more ethical than anybody else, and how do I make it relevant and fun? It’s a difficult challenge.”
Pillars of medical ethics
Dr. Grant-Kels defined ethics as a way of determining how individuals ought to act based on concepts of right and wrong. An ethical dilemma is when an individual faces two competing possibilities: either both justifiable or both unjustifiable, and you have to make a decision. The four pillars of medical ethics, she noted, are beneficence (the notion that the patient’s best interests come first); nonmaleficence (do no intentional harm); autonomy (the patient’s right to refuse or choose a treatment); and justice (fairness in how health care is distributed).
“Medical ethics are the moral principles by which physicians should conduct themselves,” she said. “There is normative ethics, which involves decisions about which moral norms or ethical arguments should we accept and why; and applied ethics, or applications of these norms to specific problems or cases. No ethics is better than bad ethics, and we can see that even in today’s world. The lack of ethics, or poor ethics, or the wrong ethics has terrible consequences.”
Ethics instruction
Dr. Grant-Kels provided a “top 10 list” of tips for incorporating ethics instruction into dermatology residency programs and clinical practices:
- Make room for ethics in your curriculum. “It’s not science, and it needs to be discussed and developed with faculty and residents,” she said.
- Focus on real situations that residents will experience. Discuss what you should do, what you might have done, and why.
- Share stories and be truthful. Include other faculty members, “because you need different perspectives,” she said.
- Go beyond what is right and wrong, and the rationale. “You have to talk about the impact, because decisions you make have unintended consequences for individual patients and for patient care in general,” Dr. Grant-Kels said.
- Practice, practice, practice. Make time for discussions involving ethics, “because it takes a lot of education to be able to identify ethical issues and process them,” she said. “The truth is, we can rationalize almost anything and convince ourselves that we made the right choice. That’s why we need to continue to practice good ethics.”
- Challenge the residents. “Decisions are not always straightforward,” she said. “Pressures push us and we start to justify small decisions and then bigger decisions. This is a very gray zone. What’s ethical for one person may not be ethical to another.”
- Encourage residents and colleagues to ask the right questions and give them confidence to make the right decisions. “We have to work in an environment of ethics,” Dr. Grant-Kels said. “Many of us are role models, and we are not always behaving the way we should be. As role models, we need to be aware of that.”
- Expose residents to a variety of issues. Ethics vary depending on the situation, the people involved, and the information presented.
- Ethics cannot just come up in an ethics class. “We need to foster a culture of ethics,” she said. “If things go wrong and unethical behavior is noted, it needs to be brought to the floor and discussed.”
- Discuss the misguided pursuit of happiness and ethical decision-making. In the opinion of Dr. Grant-Kels, people can behave badly when they’re pursuing something like a career advancement, a new house, or an expensive object like a car or a boat. “They think that if they get that job or get that promotion or if they buy that big house or they buy that sports car, they’re going to be really happy,” she said.
“That’s called impact bias, which causes focalism, where you focus on that one thing, like ‘I’m going to make a lot of money’ or ‘I’m going to buy that big house on the mountain.’ The truth is, buying that car doesn’t make you happy. Buying that big house doesn’t make you happy. We need to combat focalism with professionalism, which means conducting oneself with responsibility, integrity, accountability, and excellence. Practicing ethical medicine is not a luxury; it’s a requirement. We should all try for aspirational ethics.”
Dr. Grant-Kels reported having no relevant financial disclosures.
NEW ORLEANS – , but results from a national survey of dermatology residency program directors suggest that ethics training is not a priority.
Of the 139 dermatology residency program or associate program directors surveyed in 2022, only 43% responded. Of these, 55% said that their program had no ethics curriculum. Among programs with an ethics curriculum, 75% were implemented in the past 10 years, and the most common settings for teaching ethics were formal didactics (32%) and ad hoc during clinical encounters (28%). Reported barriers to implementing and/or maintaining an ethics curriculum included a lack of time (30%), lack of faculty with expertise (24%), and lack of useful resources (20%).
“Clearly, medical ethics is needed more to be part of our dermatology residency curriculum,” one of the study authors, Jane M. Grant-Kels, MD, professor of dermatology, pathology, and pediatrics, and founding chair of dermatology at the University of Connecticut, Farmington, said during a plenary lecture at the annual meeting of the American Academy of Dermatology. “Why? Because even though we’re physicians, and some of us have big egos, we are just human beings. We have all the faults and frailties of other humans. What we do as doctors often has unintended consequences that impact patients and society at large.”
Dr. Grant-Kels, one of the editors of the textbook “Dermatoethics”, said that, while she does not believe that physicians are intentionally unethical, “we stumble into bad behavior because we fool ourselves. We think that we are ethical. We think our colleagues are ethical, and we don’t view them with a clear, transparent eye. This is referred to as ethical fading or bounded ethicality.”
Similar to religion and good behavior, one can’t really teach someone to be ethical, she continued. “But you can teach people to think about ethics and to recognize an ethical dilemma when they’re in one,” she said. “Most articles that are available [pertain to] whether ethics can be taught or not, but there are very few resources available on how to actually teach ethics.”
That, she added, has been her goal for the last 2 decades: “How do I teach ethics without sounding like I’m more ethical than anybody else, and how do I make it relevant and fun? It’s a difficult challenge.”
Pillars of medical ethics
Dr. Grant-Kels defined ethics as a way of determining how individuals ought to act based on concepts of right and wrong. An ethical dilemma is when an individual faces two competing possibilities: either both justifiable or both unjustifiable, and you have to make a decision. The four pillars of medical ethics, she noted, are beneficence (the notion that the patient’s best interests come first); nonmaleficence (do no intentional harm); autonomy (the patient’s right to refuse or choose a treatment); and justice (fairness in how health care is distributed).
“Medical ethics are the moral principles by which physicians should conduct themselves,” she said. “There is normative ethics, which involves decisions about which moral norms or ethical arguments should we accept and why; and applied ethics, or applications of these norms to specific problems or cases. No ethics is better than bad ethics, and we can see that even in today’s world. The lack of ethics, or poor ethics, or the wrong ethics has terrible consequences.”
Ethics instruction
Dr. Grant-Kels provided a “top 10 list” of tips for incorporating ethics instruction into dermatology residency programs and clinical practices:
- Make room for ethics in your curriculum. “It’s not science, and it needs to be discussed and developed with faculty and residents,” she said.
- Focus on real situations that residents will experience. Discuss what you should do, what you might have done, and why.
- Share stories and be truthful. Include other faculty members, “because you need different perspectives,” she said.
- Go beyond what is right and wrong, and the rationale. “You have to talk about the impact, because decisions you make have unintended consequences for individual patients and for patient care in general,” Dr. Grant-Kels said.
- Practice, practice, practice. Make time for discussions involving ethics, “because it takes a lot of education to be able to identify ethical issues and process them,” she said. “The truth is, we can rationalize almost anything and convince ourselves that we made the right choice. That’s why we need to continue to practice good ethics.”
- Challenge the residents. “Decisions are not always straightforward,” she said. “Pressures push us and we start to justify small decisions and then bigger decisions. This is a very gray zone. What’s ethical for one person may not be ethical to another.”
- Encourage residents and colleagues to ask the right questions and give them confidence to make the right decisions. “We have to work in an environment of ethics,” Dr. Grant-Kels said. “Many of us are role models, and we are not always behaving the way we should be. As role models, we need to be aware of that.”
- Expose residents to a variety of issues. Ethics vary depending on the situation, the people involved, and the information presented.
- Ethics cannot just come up in an ethics class. “We need to foster a culture of ethics,” she said. “If things go wrong and unethical behavior is noted, it needs to be brought to the floor and discussed.”
- Discuss the misguided pursuit of happiness and ethical decision-making. In the opinion of Dr. Grant-Kels, people can behave badly when they’re pursuing something like a career advancement, a new house, or an expensive object like a car or a boat. “They think that if they get that job or get that promotion or if they buy that big house or they buy that sports car, they’re going to be really happy,” she said.
“That’s called impact bias, which causes focalism, where you focus on that one thing, like ‘I’m going to make a lot of money’ or ‘I’m going to buy that big house on the mountain.’ The truth is, buying that car doesn’t make you happy. Buying that big house doesn’t make you happy. We need to combat focalism with professionalism, which means conducting oneself with responsibility, integrity, accountability, and excellence. Practicing ethical medicine is not a luxury; it’s a requirement. We should all try for aspirational ethics.”
Dr. Grant-Kels reported having no relevant financial disclosures.
NEW ORLEANS – , but results from a national survey of dermatology residency program directors suggest that ethics training is not a priority.
Of the 139 dermatology residency program or associate program directors surveyed in 2022, only 43% responded. Of these, 55% said that their program had no ethics curriculum. Among programs with an ethics curriculum, 75% were implemented in the past 10 years, and the most common settings for teaching ethics were formal didactics (32%) and ad hoc during clinical encounters (28%). Reported barriers to implementing and/or maintaining an ethics curriculum included a lack of time (30%), lack of faculty with expertise (24%), and lack of useful resources (20%).
“Clearly, medical ethics is needed more to be part of our dermatology residency curriculum,” one of the study authors, Jane M. Grant-Kels, MD, professor of dermatology, pathology, and pediatrics, and founding chair of dermatology at the University of Connecticut, Farmington, said during a plenary lecture at the annual meeting of the American Academy of Dermatology. “Why? Because even though we’re physicians, and some of us have big egos, we are just human beings. We have all the faults and frailties of other humans. What we do as doctors often has unintended consequences that impact patients and society at large.”
Dr. Grant-Kels, one of the editors of the textbook “Dermatoethics”, said that, while she does not believe that physicians are intentionally unethical, “we stumble into bad behavior because we fool ourselves. We think that we are ethical. We think our colleagues are ethical, and we don’t view them with a clear, transparent eye. This is referred to as ethical fading or bounded ethicality.”
Similar to religion and good behavior, one can’t really teach someone to be ethical, she continued. “But you can teach people to think about ethics and to recognize an ethical dilemma when they’re in one,” she said. “Most articles that are available [pertain to] whether ethics can be taught or not, but there are very few resources available on how to actually teach ethics.”
That, she added, has been her goal for the last 2 decades: “How do I teach ethics without sounding like I’m more ethical than anybody else, and how do I make it relevant and fun? It’s a difficult challenge.”
Pillars of medical ethics
Dr. Grant-Kels defined ethics as a way of determining how individuals ought to act based on concepts of right and wrong. An ethical dilemma is when an individual faces two competing possibilities: either both justifiable or both unjustifiable, and you have to make a decision. The four pillars of medical ethics, she noted, are beneficence (the notion that the patient’s best interests come first); nonmaleficence (do no intentional harm); autonomy (the patient’s right to refuse or choose a treatment); and justice (fairness in how health care is distributed).
“Medical ethics are the moral principles by which physicians should conduct themselves,” she said. “There is normative ethics, which involves decisions about which moral norms or ethical arguments should we accept and why; and applied ethics, or applications of these norms to specific problems or cases. No ethics is better than bad ethics, and we can see that even in today’s world. The lack of ethics, or poor ethics, or the wrong ethics has terrible consequences.”
Ethics instruction
Dr. Grant-Kels provided a “top 10 list” of tips for incorporating ethics instruction into dermatology residency programs and clinical practices:
- Make room for ethics in your curriculum. “It’s not science, and it needs to be discussed and developed with faculty and residents,” she said.
- Focus on real situations that residents will experience. Discuss what you should do, what you might have done, and why.
- Share stories and be truthful. Include other faculty members, “because you need different perspectives,” she said.
- Go beyond what is right and wrong, and the rationale. “You have to talk about the impact, because decisions you make have unintended consequences for individual patients and for patient care in general,” Dr. Grant-Kels said.
- Practice, practice, practice. Make time for discussions involving ethics, “because it takes a lot of education to be able to identify ethical issues and process them,” she said. “The truth is, we can rationalize almost anything and convince ourselves that we made the right choice. That’s why we need to continue to practice good ethics.”
- Challenge the residents. “Decisions are not always straightforward,” she said. “Pressures push us and we start to justify small decisions and then bigger decisions. This is a very gray zone. What’s ethical for one person may not be ethical to another.”
- Encourage residents and colleagues to ask the right questions and give them confidence to make the right decisions. “We have to work in an environment of ethics,” Dr. Grant-Kels said. “Many of us are role models, and we are not always behaving the way we should be. As role models, we need to be aware of that.”
- Expose residents to a variety of issues. Ethics vary depending on the situation, the people involved, and the information presented.
- Ethics cannot just come up in an ethics class. “We need to foster a culture of ethics,” she said. “If things go wrong and unethical behavior is noted, it needs to be brought to the floor and discussed.”
- Discuss the misguided pursuit of happiness and ethical decision-making. In the opinion of Dr. Grant-Kels, people can behave badly when they’re pursuing something like a career advancement, a new house, or an expensive object like a car or a boat. “They think that if they get that job or get that promotion or if they buy that big house or they buy that sports car, they’re going to be really happy,” she said.
“That’s called impact bias, which causes focalism, where you focus on that one thing, like ‘I’m going to make a lot of money’ or ‘I’m going to buy that big house on the mountain.’ The truth is, buying that car doesn’t make you happy. Buying that big house doesn’t make you happy. We need to combat focalism with professionalism, which means conducting oneself with responsibility, integrity, accountability, and excellence. Practicing ethical medicine is not a luxury; it’s a requirement. We should all try for aspirational ethics.”
Dr. Grant-Kels reported having no relevant financial disclosures.
AT AAD 2023
Melasma
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.
Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women aged 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
• Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
• Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
• Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
• First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
• Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or α-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
• Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or second-line topical therapy.
• Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
• Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
• Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and selfesteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out-of-pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
- Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
- Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies [published online January 30, 2017]. Dermatol Ther. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
- Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
- Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
- Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone /tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.

Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women aged 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
• Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
• Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
• Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
• First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
• Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or α-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
• Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or second-line topical therapy.
• Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
• Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
• Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and selfesteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out-of-pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
THE COMPARISON
A Melasma on the face of a Hispanic woman, with hyperpigmentation on the cheeks, bridge of the nose, and upper lip.
B Melasma on the face of a Malaysian woman, with hyperpigmentation on the upper cheeks and bridge of the nose.
C Melasma on the face of an African woman, with hyperpigmentation on the upper cheeks and lateral to the eyes.

Melasma (also known as chloasma) is a pigmentary disorder that causes chronic symmetric hyperpigmentation on the face. In patients with darker skin tones, centrofacial areas are affected.1 Increased deposition of melanin distributed in the dermis leads to dermal melanosis. Newer research suggests that mast cell and keratinocyte interactions, altered gene regulation, neovascularization, and disruptions in the basement membrane cause melasma.2 Patients present with epidermal or dermal melasma or a combination of both (mixed melasma).3 Wood lamp examination is helpful to distinguish between epidermal and dermal melasma. Dermal and mixed melasma can be difficult to treat and require multimodal treatments.
Epidemiology
Melasma commonly affects women aged 20 to 40 years,4 with a female to male ratio of 9:1.5 Potential triggers of melasma include hormones (eg, pregnancy, oral contraceptives, hormone replacement therapy) and exposure to UV light.2,5 Melasma occurs in patients of all racial and ethnic backgrounds; however, the prevalence is higher in patients with darker skin tones.2
Key clinical features in people with darker skin tones
Melasma commonly manifests as symmetrically distributed, reticulated (lacy), dark brown to grayish brown patches on the cheeks, nose, forehead, upper lip, and chin in patients with darker skin tones.5 The pigment can be tan brown in patients with lighter skin tones. Given that postinflammatory hyperpigmentation and other pigmentary disorders can cause a similar appearance, a biopsy sometimes is needed to confirm the diagnosis, but melasma is diagnosed via physical examination in most patients. Melasma can be misdiagnosed as postinflammatory hyperpigmentation, solar lentigines, exogenous ochronosis, and Hori nevus.5
Worth noting
Prevention
• Daily sunscreen use is critical to prevent worsening of melasma. Sunscreen may not appear cosmetically elegant on darker skin tones, which creates a barrier to its use.6 Protection from both sunlight and visible light is necessary. Visible light, including light from light bulbs and device-emitted blue light, can worsen melasma. Iron oxides in tinted sunscreen offer protection from visible light.
• Physicians can recommend sunscreens that are more transparent or tinted for a better cosmetic match.
• Severe flares of melasma can occur with sun exposure despite good control with medications and laser modalities.
Treatment
• First-line therapies include topical hydroquinone 2% to 4%, tretinoin, azelaic acid, kojic acid, or ascorbic acid (vitamin C). A popular topical compound is a steroid, tretinoin, and hydroquinone.1,5 Over-the-counter hydroquinone has been removed from the market due to safety concerns; however, it is still first line in the treatment of melasma. If hydroquinone is prescribed, treatment intervals of 6 to 8 weeks followed by a hydroquinone-free period is advised to reduce the risk for exogenous ochronosis (a paradoxical darkening of the skin).
• Chemical peels are second-line treatments that are effective for melasma. Improvement in epidermal melasma has been shown with chemical peels containing Jessner solution, salicylic acid, or α-hydroxy acid. Patients with dermal and mixed melasma have seen improvement with trichloroacetic acid 25% to 35% with or without Jessner solution.1
• Cysteamine is a topical treatment created from the degradation of coenzyme A. It disrupts the synthesis of melanin to create a more even skin tone. It may be recommended in combination with sunscreen as a first-line or second-line topical therapy.
• Oral tranexamic acid is a third-line treatment that is an analogue for lysine. It decreases prostaglandin production, which leads to a lower number of tyrosine precursors available for the creation of melanin. Tranexamic acid has been shown to lighten the appearance of melasma.7 The most common and dangerous adverse effect of tranexamic acid is blood clots and this treatment should be avoided in those on combination (estrogen and progestin) contraceptives or those with a personal or family history of clotting disorders.8
• Fourth-line treatments such as lasers (performed by dermatologists) can destroy the deposition of pigment while avoiding destruction of epidermal keratinocytes.1,9,10 They also are commonly employed in refractive melasma. The most common lasers are nonablative fractionated lasers and low-fluence Q-switched lasers. The Q-switched Nd:YAG and picosecond lasers are safe for treating melasma in darker skin tones. Ablative fractionated lasers such as CO2 lasers and erbium:YAG lasers also have been used in the treatment of melasma; however, there is still an extremely high risk for postinflammatory dyspigmentation 1 to 2 months after the procedure.10
• Although there is still a risk for rebound hyperpigmentation after laser treatment, use of topical hydroquinone pretreatment may help decrease postoperative hyperpigmentation.1,5 Patients who are treated with the incorrect laser or overtreated may develop postinflammatory hyperpigmentation, rebound hyperpigmentation, or hypopigmentation.
Health disparity highlight
Melasma, most common in patients with skin of color, is a common chronic pigmentation disorder that is cosmetically and psychologically burdensome,11 leading to decreased quality of life, emotional functioning, and selfesteem.12 Clinicians should counsel patients and work closely on long-term management. The treatment options for melasma are considered cosmetic and may be cost prohibitive for many to cover out-of-pocket. Topical treatments have been found to be the most cost-effective.13 Some compounding pharmacies and drug discount programs provide more affordable treatment pricing; however, some patients are still unable to afford these options.
- Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
- Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies [published online January 30, 2017]. Dermatol Ther. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
- Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
- Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
- Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone /tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
- Cunha PR, Kroumpouzos G. Melasma and vitiligo: novel and experimental therapies. J Clin Exp Derm Res. 2016;7:2. doi:10.4172/2155-9554.1000e106
- Rajanala S, Maymone MBC, Vashi NA. Melasma pathogenesis: a review of the latest research, pathological findings, and investigational therapies. Dermatol Online J. 2019;25:13030/qt47b7r28c.
- Grimes PE, Yamada N, Bhawan J. Light microscopic, immunohistochemical, and ultrastructural alterations in patients with melasma. Am J Dermatopathol. 2005;27:96-101.
- Achar A, Rathi SK. Melasma: a clinico-epidemiological study of 312 cases. Indian J Dermatol. 2011;56:380-382.
- Ogbechie-Godec OA, Elbuluk N. Melasma: an up-to-date comprehensive review. Dermatol Ther. 2017;7:305-318.
- Morquette AJ, Waples ER, Heath CR. The importance of cosmetically elegant sunscreen in skin of color populations. J Cosmet Dermatol. 2022;21:1337-1338.
- Taraz M, Nikham S, Ehsani AH. Tranexamic acid in treatment of melasma: a comprehensive review of clinical studies [published online January 30, 2017]. Dermatol Ther. doi:10.1111/dth.12465
- Bala HR, Lee S, Wong C, et al. Oral tranexamic acid for the treatment of melasma: a review. Dermatol Surg. 2018;44:814-825.
- Castanedo-Cazares JP, Hernandez-Blanco D, Carlos-Ortega B, et al. Near-visible light and UV photoprotection in the treatment of melasma: a double-blind randomized trial. Photodermatol Photoimmunol Photomed. 2014;30:35-42.
- Trivedi MK, Yang FC, Cho BK. A review of laser and light therapy in melasma. Int J Womens Dermatol. 2017;3:11-20.
- Dodmani PN, Deshmukh AR. Assessment of quality of life of melasma patients as per melasma quality of life scale (MELASQoL). Pigment Int. 2020;7:75-79.
- Balkrishnan R, McMichael A, Camacho FT, et al. Development and validation of a health‐related quality of life instrument for women with melasma. Br J Dermatol. 2003;149:572-577.
- Alikhan A, Daly M, Wu J, et al. Cost-effectiveness of a hydroquinone /tretinoin/fluocinolone acetonide cream combination in treating melasma in the United States. J Dermatolog Treat. 2010;21:276-281.
Recurrent Oral and Gluteal Cleft Erosions
The Diagnosis: Lichen Planus Pemphigoides
Lichen planus pemphigoides (LPP) is a rare acquired autoimmune blistering disorder with an estimated worldwide prevalence of approximately 1 in 1,000,000 individuals.1 It often manifests with overlapping features of both LP and bullous pemphigoid (BP). The condition usually presents in the fifth decade of life and has a slight female predominance.2 Although primarily idiopathic, it has been associated with certain medications and treatments, such as angiotensin-converting enzyme inhibitors, programmed cell death protein 1 inhibitors, programmed cell death ligand 1 inhibitors, labetalol, narrowband UVB, and psoralen plus UVA.3,4
Patients initially present with lesions of classic lichen planus (LP) with pink-purple, flat-topped, pruritic, polygonal papules and plaques.5 After weeks to months, tense vesicles and bullae usually develop on the sites of LP as well as on uninvolved skin. One study found a mean lag time of about 8.3 months for blistering to present after LP,5 but concurrent presentations of both have been reported.1 In addition, oral mucosal involvement has been seen in 36% of cases. The most commonly affected sites are the extremities; however, involvement can be widespread.2
The pathogenesis of LPP currently is unknown. It has been proposed that in LP, injury of basal keratinocytes exposes hidden basement membrane and hemidesmosome antigens including BP180, a 180 kDa transmembrane protein of the basement membrane zone (BMZ),6 which triggers an immune response where T cells recognize the extracellular portion of BP180 and antibodies are formed against the likely autoantigen.1 One study has suggested that the autoantigen in LPP is the MCW-4 epitope within the C-terminal end of the NC16A domain of BP180.7
Histopathology of LPP reveals characteristics of both LP as well as BP. Typical features of LP on hematoxylin and eosin (H&E) staining include lichenoid lymphocytic interface dermatitis, sawtooth rete ridges, wedge-shaped hypergranulosis, and colloid bodies, as demonstrated from the biopsy of our patient’s gluteal cleft lesion (quiz image 1), while the predominant feature of BP on H&E staining includes a subepidermal bulla with eosinophils.2 Typically, direct immunofluorescence (DIF) shows linear deposits of IgG and/or C3 along the BMZ. Indirect immunofluorescence (IIF) often reveals IgG against the roof of the BMZ in a human split-skin substrate.1 Antibodies against BP180 or uncommonly BP230 often are detected on enzyme-linked immunosorbent assay (ELISA). For our patient, IIF and ELISA tests were positive. Given the clinical presentation with recurrent oral and gluteal cleft erosions, histologic findings, and the results of our patient’s immunological testing, the diagnosis of LPP was made.
Topical steroids often are used to treat localized disease of LPP.8 Oral prednisone also may be given for widespread or unresponsive disease.9 Other treatments include azathioprine, mycophenolate mofetil, hydroxychloroquine, dapsone, tetracycline in combination with nicotinamide, acitretin, ustekinumab, baricitinib, and rituximab with intravenous immunoglobulin.3,8,10-12 Any potential medication culprits should be discontinued.9 Patients with oral involvement may require a soft diet to avoid further mucosal insult.10 Additionally, providers should consider dentistry, ophthalmology, and/or otolaryngology referrals depending on disease severity.
Bullous pemphigoid, the most common autoimmune blistering disease, has an estimated incidence of 10 to 43 per million individuals per year.2 Classically, it presents with tense bullae on the skin of the lower abdomen, thighs, groin, forearms, and axillae. Circulating antibodies against 2 BMZ proteins—BP180 and BP230—are important factors in BP pathogenesis.2 Diagnosis of BP is based on clinical features, histologic findings, and immunological studies including DIF, IIF, and ELISA. An eosinophil-rich subepidermal split typically is seen on H&E staining (Figure 1).
Direct immunofluorescence displays linear IgG and/ or C3 staining at the BMZ. Indirect immunofluorescence on a human salt-split skin substrate commonly shows linear BMZ deposition on the roof of the blister.2 Indirect immunofluorescence for IgG deposition on monkey esophagus substrate shows linear BMZ deposition. Antibodies against the NC16A domain of BP180 (NC16A-BP180) are dominant, but BP230 antibodies against BP230 also are detected with ELISA.2 Further studies have indicated that the NC16A epitopes of BP180 that are targeted in BP are MCW-0-3,2 different from the autoantigen MCW-4 that is targeted in LPP.7
Paraneoplastic pemphigus (PNP) is another diagnosis to consider. Patients with PNP initially present with oral findings—most commonly chronic, erosive, and painful mucositis—followed by cutaneous involvement, which varies from the development of bullae to the formation of plaques similar to those of LP.13 The latter, in combination with oral erosions, may appear clinically similar to LPP. The results of DIF in conjugation with IIF and ELISA may help to further differentiate these disorders. Direct immunofluorescence in PNP typically reveals positive intercellular and/or BMZ IgG and C3, while DIF in LPP reveals depositions along the BMZ alone. Indirect immunofluorescence performed on rat bladder epithelium is particularly useful, as binding of IgG to rat bladder epithelium is characteristic of PNP and not seen in other disorders.14 Lastly, patients with PNP may develop IgG antibodies to various antigens such as desmoplakin I, desmoplakin II, envoplakin, periplakin, BP230, desmoglein 1, and desmoglein 3, which would not be expected in LPP patients.15 Hematoxylin and eosin staining differs from LPP, primarily with the location of the blister being intraepidermal. Acantholysis with hemorrhagic bullae can be seen (Figure 2).
Classic LP is an inflammatory disorder that mainly affects adults, with an estimated incidence of less than 1%.16 The classic form presents with purple, flat-topped, pruritic, polygonal papules and plaques of varying size that often are characterized by Wickham striae. Lichen planus possesses a broad spectrum of subtypes involving different locations, though skin lesions usually are localized to the extremities. Despite an unknown etiology, activated T cells and T helper type 1 cytokines are considered key in keratinocyte injury. Compact orthokeratosis, wedge-shaped hypergranulosis, focal dyskeratosis, and colloid bodies typically are found on H&E staining, along with a dense bandlike lymphohistiocytic infiltrate at the dermoepidermal junction (DEJ)(Figure 3). Direct immunofluorescence typically shows a shaggy band of fibrinogen along the DEJ in addition to colloid bodies that stain with various autoantibodies including IgM, IgG, IgA, and C3.16
Bullous LP is a rare variant of LP that commonly develops on the oral mucosa and the legs, with blisters confined on pre-existing LP lesions.9 The pathogenesis is related to an epidermal inflammatory infiltrate that leads to basal layer destruction followed by dermal-epidermal separations that cause blistering.17 Bullous LP does not have positive DIF, IIF, or ELISA because the pathophysiology does not involve autoantibody production. Histopathology typically displays an extensive inflammatory infiltrate and degeneration of the basal keratinocytes, resulting in large dermal-epidermal separations called Max-Joseph spaces (Figure 4).17 Colloid bodies are prominent in bullous LP but rarely are seen in LPP; eosinophils also are much more prominent in LPP compared to bullous LP.18 Unlike in LPP, DIF usually is negative in bullous LP, though lichenoid lesions may exhibit globular deposition of IgM, IgG, and IgA in the colloid bodies of the lower epidermis and/or papillary dermis. Similar to LP, DIF of the biopsy specimen shows linear or shaggy deposits of fibrinogen at the DEJ.17
- Hübner F, Langan EA, Recke A. Lichen planus pemphigoides: from lichenoid inflammation to autoantibody-mediated blistering. Front Immunol. 2019;10:1389.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85:1-14.
- Hackländer K, Lehmann P, Hofmann SC. Successful treatment of lichen planus pemphigoides using acitretin as monotherapy. J Dtsch Dermatol Ges. 2014;12:818-819.
- Boyle M, Ashi S, Puiu T, et al. Lichen planus pemphigoides associated with PD-1 and PD-L1 inhibitors: a case series and review of the literature. Am J Dermatopathol. 2022;44:360-367.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018.
- Zillikens D, Caux F, Mascaru JM Jr, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Knisley RR, Petropolis AA, Mackey VT. Lichen planus pemphigoides treated with ustekinumab. Cutis. 2017;100:415-418.
- Liakopoulou A, Rallis E. Bullous lichen planus—a review. J Dermatol Case Rep. 2017;11:1-4.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149.
- Moussa A, Colla TG, Asfour L, et al. Effective treatment of refractory lichen planus pemphigoides with a Janus kinase-1/2 inhibitor. Clin Exp Dermatol. 2022;47:2040-2041.
- Brennan M, Baldissano M, King L, et al. Successful use of rituximab and intravenous gamma globulin to treat checkpoint inhibitor-induced severe lichen planus pemphigoides. Skinmed. 2020;18:246-249.
- Kim JH, Kim SC. Paraneoplastic pemphigus: paraneoplastic autoimmune disease of the skin and mucosa. Front Immunol. 2019;10:1259.
- Stevens SR, Griffiths CE, Anhalt GJ, et al. Paraneoplastic pemphigus presenting as a lichen planus pemphigoides-like eruption. Arch Dermatol. 1993;129:866-869.
- Ohzono A, Sogame R, Li X, et al. Clinical and immunological findings in 104 cases of paraneoplastic pemphigus. Br J Dermatol. 2015;173:1447-1452.
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Papara C, Danescu S, Sitaru C, et al. Challenges and pitfalls between lichen planus pemphigoides and bullous lichen planus. Australas J Dermatol. 2022;63:165-171.
- Tripathy DM, Vashisht D, Rathore G, et al. Bullous lichen planus vs lichen planus pemphigoides: a diagnostic dilemma. Indian Dermatol Online J. 2022;13:282-284.
The Diagnosis: Lichen Planus Pemphigoides
Lichen planus pemphigoides (LPP) is a rare acquired autoimmune blistering disorder with an estimated worldwide prevalence of approximately 1 in 1,000,000 individuals.1 It often manifests with overlapping features of both LP and bullous pemphigoid (BP). The condition usually presents in the fifth decade of life and has a slight female predominance.2 Although primarily idiopathic, it has been associated with certain medications and treatments, such as angiotensin-converting enzyme inhibitors, programmed cell death protein 1 inhibitors, programmed cell death ligand 1 inhibitors, labetalol, narrowband UVB, and psoralen plus UVA.3,4
Patients initially present with lesions of classic lichen planus (LP) with pink-purple, flat-topped, pruritic, polygonal papules and plaques.5 After weeks to months, tense vesicles and bullae usually develop on the sites of LP as well as on uninvolved skin. One study found a mean lag time of about 8.3 months for blistering to present after LP,5 but concurrent presentations of both have been reported.1 In addition, oral mucosal involvement has been seen in 36% of cases. The most commonly affected sites are the extremities; however, involvement can be widespread.2
The pathogenesis of LPP currently is unknown. It has been proposed that in LP, injury of basal keratinocytes exposes hidden basement membrane and hemidesmosome antigens including BP180, a 180 kDa transmembrane protein of the basement membrane zone (BMZ),6 which triggers an immune response where T cells recognize the extracellular portion of BP180 and antibodies are formed against the likely autoantigen.1 One study has suggested that the autoantigen in LPP is the MCW-4 epitope within the C-terminal end of the NC16A domain of BP180.7
Histopathology of LPP reveals characteristics of both LP as well as BP. Typical features of LP on hematoxylin and eosin (H&E) staining include lichenoid lymphocytic interface dermatitis, sawtooth rete ridges, wedge-shaped hypergranulosis, and colloid bodies, as demonstrated from the biopsy of our patient’s gluteal cleft lesion (quiz image 1), while the predominant feature of BP on H&E staining includes a subepidermal bulla with eosinophils.2 Typically, direct immunofluorescence (DIF) shows linear deposits of IgG and/or C3 along the BMZ. Indirect immunofluorescence (IIF) often reveals IgG against the roof of the BMZ in a human split-skin substrate.1 Antibodies against BP180 or uncommonly BP230 often are detected on enzyme-linked immunosorbent assay (ELISA). For our patient, IIF and ELISA tests were positive. Given the clinical presentation with recurrent oral and gluteal cleft erosions, histologic findings, and the results of our patient’s immunological testing, the diagnosis of LPP was made.
Topical steroids often are used to treat localized disease of LPP.8 Oral prednisone also may be given for widespread or unresponsive disease.9 Other treatments include azathioprine, mycophenolate mofetil, hydroxychloroquine, dapsone, tetracycline in combination with nicotinamide, acitretin, ustekinumab, baricitinib, and rituximab with intravenous immunoglobulin.3,8,10-12 Any potential medication culprits should be discontinued.9 Patients with oral involvement may require a soft diet to avoid further mucosal insult.10 Additionally, providers should consider dentistry, ophthalmology, and/or otolaryngology referrals depending on disease severity.
Bullous pemphigoid, the most common autoimmune blistering disease, has an estimated incidence of 10 to 43 per million individuals per year.2 Classically, it presents with tense bullae on the skin of the lower abdomen, thighs, groin, forearms, and axillae. Circulating antibodies against 2 BMZ proteins—BP180 and BP230—are important factors in BP pathogenesis.2 Diagnosis of BP is based on clinical features, histologic findings, and immunological studies including DIF, IIF, and ELISA. An eosinophil-rich subepidermal split typically is seen on H&E staining (Figure 1).

Direct immunofluorescence displays linear IgG and/ or C3 staining at the BMZ. Indirect immunofluorescence on a human salt-split skin substrate commonly shows linear BMZ deposition on the roof of the blister.2 Indirect immunofluorescence for IgG deposition on monkey esophagus substrate shows linear BMZ deposition. Antibodies against the NC16A domain of BP180 (NC16A-BP180) are dominant, but BP230 antibodies against BP230 also are detected with ELISA.2 Further studies have indicated that the NC16A epitopes of BP180 that are targeted in BP are MCW-0-3,2 different from the autoantigen MCW-4 that is targeted in LPP.7
Paraneoplastic pemphigus (PNP) is another diagnosis to consider. Patients with PNP initially present with oral findings—most commonly chronic, erosive, and painful mucositis—followed by cutaneous involvement, which varies from the development of bullae to the formation of plaques similar to those of LP.13 The latter, in combination with oral erosions, may appear clinically similar to LPP. The results of DIF in conjugation with IIF and ELISA may help to further differentiate these disorders. Direct immunofluorescence in PNP typically reveals positive intercellular and/or BMZ IgG and C3, while DIF in LPP reveals depositions along the BMZ alone. Indirect immunofluorescence performed on rat bladder epithelium is particularly useful, as binding of IgG to rat bladder epithelium is characteristic of PNP and not seen in other disorders.14 Lastly, patients with PNP may develop IgG antibodies to various antigens such as desmoplakin I, desmoplakin II, envoplakin, periplakin, BP230, desmoglein 1, and desmoglein 3, which would not be expected in LPP patients.15 Hematoxylin and eosin staining differs from LPP, primarily with the location of the blister being intraepidermal. Acantholysis with hemorrhagic bullae can be seen (Figure 2).

Classic LP is an inflammatory disorder that mainly affects adults, with an estimated incidence of less than 1%.16 The classic form presents with purple, flat-topped, pruritic, polygonal papules and plaques of varying size that often are characterized by Wickham striae. Lichen planus possesses a broad spectrum of subtypes involving different locations, though skin lesions usually are localized to the extremities. Despite an unknown etiology, activated T cells and T helper type 1 cytokines are considered key in keratinocyte injury. Compact orthokeratosis, wedge-shaped hypergranulosis, focal dyskeratosis, and colloid bodies typically are found on H&E staining, along with a dense bandlike lymphohistiocytic infiltrate at the dermoepidermal junction (DEJ)(Figure 3). Direct immunofluorescence typically shows a shaggy band of fibrinogen along the DEJ in addition to colloid bodies that stain with various autoantibodies including IgM, IgG, IgA, and C3.16

Bullous LP is a rare variant of LP that commonly develops on the oral mucosa and the legs, with blisters confined on pre-existing LP lesions.9 The pathogenesis is related to an epidermal inflammatory infiltrate that leads to basal layer destruction followed by dermal-epidermal separations that cause blistering.17 Bullous LP does not have positive DIF, IIF, or ELISA because the pathophysiology does not involve autoantibody production. Histopathology typically displays an extensive inflammatory infiltrate and degeneration of the basal keratinocytes, resulting in large dermal-epidermal separations called Max-Joseph spaces (Figure 4).17 Colloid bodies are prominent in bullous LP but rarely are seen in LPP; eosinophils also are much more prominent in LPP compared to bullous LP.18 Unlike in LPP, DIF usually is negative in bullous LP, though lichenoid lesions may exhibit globular deposition of IgM, IgG, and IgA in the colloid bodies of the lower epidermis and/or papillary dermis. Similar to LP, DIF of the biopsy specimen shows linear or shaggy deposits of fibrinogen at the DEJ.17

The Diagnosis: Lichen Planus Pemphigoides
Lichen planus pemphigoides (LPP) is a rare acquired autoimmune blistering disorder with an estimated worldwide prevalence of approximately 1 in 1,000,000 individuals.1 It often manifests with overlapping features of both LP and bullous pemphigoid (BP). The condition usually presents in the fifth decade of life and has a slight female predominance.2 Although primarily idiopathic, it has been associated with certain medications and treatments, such as angiotensin-converting enzyme inhibitors, programmed cell death protein 1 inhibitors, programmed cell death ligand 1 inhibitors, labetalol, narrowband UVB, and psoralen plus UVA.3,4
Patients initially present with lesions of classic lichen planus (LP) with pink-purple, flat-topped, pruritic, polygonal papules and plaques.5 After weeks to months, tense vesicles and bullae usually develop on the sites of LP as well as on uninvolved skin. One study found a mean lag time of about 8.3 months for blistering to present after LP,5 but concurrent presentations of both have been reported.1 In addition, oral mucosal involvement has been seen in 36% of cases. The most commonly affected sites are the extremities; however, involvement can be widespread.2
The pathogenesis of LPP currently is unknown. It has been proposed that in LP, injury of basal keratinocytes exposes hidden basement membrane and hemidesmosome antigens including BP180, a 180 kDa transmembrane protein of the basement membrane zone (BMZ),6 which triggers an immune response where T cells recognize the extracellular portion of BP180 and antibodies are formed against the likely autoantigen.1 One study has suggested that the autoantigen in LPP is the MCW-4 epitope within the C-terminal end of the NC16A domain of BP180.7
Histopathology of LPP reveals characteristics of both LP as well as BP. Typical features of LP on hematoxylin and eosin (H&E) staining include lichenoid lymphocytic interface dermatitis, sawtooth rete ridges, wedge-shaped hypergranulosis, and colloid bodies, as demonstrated from the biopsy of our patient’s gluteal cleft lesion (quiz image 1), while the predominant feature of BP on H&E staining includes a subepidermal bulla with eosinophils.2 Typically, direct immunofluorescence (DIF) shows linear deposits of IgG and/or C3 along the BMZ. Indirect immunofluorescence (IIF) often reveals IgG against the roof of the BMZ in a human split-skin substrate.1 Antibodies against BP180 or uncommonly BP230 often are detected on enzyme-linked immunosorbent assay (ELISA). For our patient, IIF and ELISA tests were positive. Given the clinical presentation with recurrent oral and gluteal cleft erosions, histologic findings, and the results of our patient’s immunological testing, the diagnosis of LPP was made.
Topical steroids often are used to treat localized disease of LPP.8 Oral prednisone also may be given for widespread or unresponsive disease.9 Other treatments include azathioprine, mycophenolate mofetil, hydroxychloroquine, dapsone, tetracycline in combination with nicotinamide, acitretin, ustekinumab, baricitinib, and rituximab with intravenous immunoglobulin.3,8,10-12 Any potential medication culprits should be discontinued.9 Patients with oral involvement may require a soft diet to avoid further mucosal insult.10 Additionally, providers should consider dentistry, ophthalmology, and/or otolaryngology referrals depending on disease severity.
Bullous pemphigoid, the most common autoimmune blistering disease, has an estimated incidence of 10 to 43 per million individuals per year.2 Classically, it presents with tense bullae on the skin of the lower abdomen, thighs, groin, forearms, and axillae. Circulating antibodies against 2 BMZ proteins—BP180 and BP230—are important factors in BP pathogenesis.2 Diagnosis of BP is based on clinical features, histologic findings, and immunological studies including DIF, IIF, and ELISA. An eosinophil-rich subepidermal split typically is seen on H&E staining (Figure 1).

Direct immunofluorescence displays linear IgG and/ or C3 staining at the BMZ. Indirect immunofluorescence on a human salt-split skin substrate commonly shows linear BMZ deposition on the roof of the blister.2 Indirect immunofluorescence for IgG deposition on monkey esophagus substrate shows linear BMZ deposition. Antibodies against the NC16A domain of BP180 (NC16A-BP180) are dominant, but BP230 antibodies against BP230 also are detected with ELISA.2 Further studies have indicated that the NC16A epitopes of BP180 that are targeted in BP are MCW-0-3,2 different from the autoantigen MCW-4 that is targeted in LPP.7
Paraneoplastic pemphigus (PNP) is another diagnosis to consider. Patients with PNP initially present with oral findings—most commonly chronic, erosive, and painful mucositis—followed by cutaneous involvement, which varies from the development of bullae to the formation of plaques similar to those of LP.13 The latter, in combination with oral erosions, may appear clinically similar to LPP. The results of DIF in conjugation with IIF and ELISA may help to further differentiate these disorders. Direct immunofluorescence in PNP typically reveals positive intercellular and/or BMZ IgG and C3, while DIF in LPP reveals depositions along the BMZ alone. Indirect immunofluorescence performed on rat bladder epithelium is particularly useful, as binding of IgG to rat bladder epithelium is characteristic of PNP and not seen in other disorders.14 Lastly, patients with PNP may develop IgG antibodies to various antigens such as desmoplakin I, desmoplakin II, envoplakin, periplakin, BP230, desmoglein 1, and desmoglein 3, which would not be expected in LPP patients.15 Hematoxylin and eosin staining differs from LPP, primarily with the location of the blister being intraepidermal. Acantholysis with hemorrhagic bullae can be seen (Figure 2).

Classic LP is an inflammatory disorder that mainly affects adults, with an estimated incidence of less than 1%.16 The classic form presents with purple, flat-topped, pruritic, polygonal papules and plaques of varying size that often are characterized by Wickham striae. Lichen planus possesses a broad spectrum of subtypes involving different locations, though skin lesions usually are localized to the extremities. Despite an unknown etiology, activated T cells and T helper type 1 cytokines are considered key in keratinocyte injury. Compact orthokeratosis, wedge-shaped hypergranulosis, focal dyskeratosis, and colloid bodies typically are found on H&E staining, along with a dense bandlike lymphohistiocytic infiltrate at the dermoepidermal junction (DEJ)(Figure 3). Direct immunofluorescence typically shows a shaggy band of fibrinogen along the DEJ in addition to colloid bodies that stain with various autoantibodies including IgM, IgG, IgA, and C3.16

Bullous LP is a rare variant of LP that commonly develops on the oral mucosa and the legs, with blisters confined on pre-existing LP lesions.9 The pathogenesis is related to an epidermal inflammatory infiltrate that leads to basal layer destruction followed by dermal-epidermal separations that cause blistering.17 Bullous LP does not have positive DIF, IIF, or ELISA because the pathophysiology does not involve autoantibody production. Histopathology typically displays an extensive inflammatory infiltrate and degeneration of the basal keratinocytes, resulting in large dermal-epidermal separations called Max-Joseph spaces (Figure 4).17 Colloid bodies are prominent in bullous LP but rarely are seen in LPP; eosinophils also are much more prominent in LPP compared to bullous LP.18 Unlike in LPP, DIF usually is negative in bullous LP, though lichenoid lesions may exhibit globular deposition of IgM, IgG, and IgA in the colloid bodies of the lower epidermis and/or papillary dermis. Similar to LP, DIF of the biopsy specimen shows linear or shaggy deposits of fibrinogen at the DEJ.17

- Hübner F, Langan EA, Recke A. Lichen planus pemphigoides: from lichenoid inflammation to autoantibody-mediated blistering. Front Immunol. 2019;10:1389.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85:1-14.
- Hackländer K, Lehmann P, Hofmann SC. Successful treatment of lichen planus pemphigoides using acitretin as monotherapy. J Dtsch Dermatol Ges. 2014;12:818-819.
- Boyle M, Ashi S, Puiu T, et al. Lichen planus pemphigoides associated with PD-1 and PD-L1 inhibitors: a case series and review of the literature. Am J Dermatopathol. 2022;44:360-367.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018.
- Zillikens D, Caux F, Mascaru JM Jr, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Knisley RR, Petropolis AA, Mackey VT. Lichen planus pemphigoides treated with ustekinumab. Cutis. 2017;100:415-418.
- Liakopoulou A, Rallis E. Bullous lichen planus—a review. J Dermatol Case Rep. 2017;11:1-4.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149.
- Moussa A, Colla TG, Asfour L, et al. Effective treatment of refractory lichen planus pemphigoides with a Janus kinase-1/2 inhibitor. Clin Exp Dermatol. 2022;47:2040-2041.
- Brennan M, Baldissano M, King L, et al. Successful use of rituximab and intravenous gamma globulin to treat checkpoint inhibitor-induced severe lichen planus pemphigoides. Skinmed. 2020;18:246-249.
- Kim JH, Kim SC. Paraneoplastic pemphigus: paraneoplastic autoimmune disease of the skin and mucosa. Front Immunol. 2019;10:1259.
- Stevens SR, Griffiths CE, Anhalt GJ, et al. Paraneoplastic pemphigus presenting as a lichen planus pemphigoides-like eruption. Arch Dermatol. 1993;129:866-869.
- Ohzono A, Sogame R, Li X, et al. Clinical and immunological findings in 104 cases of paraneoplastic pemphigus. Br J Dermatol. 2015;173:1447-1452.
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Papara C, Danescu S, Sitaru C, et al. Challenges and pitfalls between lichen planus pemphigoides and bullous lichen planus. Australas J Dermatol. 2022;63:165-171.
- Tripathy DM, Vashisht D, Rathore G, et al. Bullous lichen planus vs lichen planus pemphigoides: a diagnostic dilemma. Indian Dermatol Online J. 2022;13:282-284.
- Hübner F, Langan EA, Recke A. Lichen planus pemphigoides: from lichenoid inflammation to autoantibody-mediated blistering. Front Immunol. 2019;10:1389.
- Montagnon CM, Tolkachjov SN, Murrell DF, et al. Subepithelial autoimmune blistering dermatoses: clinical features and diagnosis. J Am Acad Dermatol. 2021;85:1-14.
- Hackländer K, Lehmann P, Hofmann SC. Successful treatment of lichen planus pemphigoides using acitretin as monotherapy. J Dtsch Dermatol Ges. 2014;12:818-819.
- Boyle M, Ashi S, Puiu T, et al. Lichen planus pemphigoides associated with PD-1 and PD-L1 inhibitors: a case series and review of the literature. Am J Dermatopathol. 2022;44:360-367.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018.
- Zillikens D, Caux F, Mascaru JM Jr, et al. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol. 1999;113:117-121.
- Knisley RR, Petropolis AA, Mackey VT. Lichen planus pemphigoides treated with ustekinumab. Cutis. 2017;100:415-418.
- Liakopoulou A, Rallis E. Bullous lichen planus—a review. J Dermatol Case Rep. 2017;11:1-4.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Womens Dermatol. 2015;1:140-149.
- Moussa A, Colla TG, Asfour L, et al. Effective treatment of refractory lichen planus pemphigoides with a Janus kinase-1/2 inhibitor. Clin Exp Dermatol. 2022;47:2040-2041.
- Brennan M, Baldissano M, King L, et al. Successful use of rituximab and intravenous gamma globulin to treat checkpoint inhibitor-induced severe lichen planus pemphigoides. Skinmed. 2020;18:246-249.
- Kim JH, Kim SC. Paraneoplastic pemphigus: paraneoplastic autoimmune disease of the skin and mucosa. Front Immunol. 2019;10:1259.
- Stevens SR, Griffiths CE, Anhalt GJ, et al. Paraneoplastic pemphigus presenting as a lichen planus pemphigoides-like eruption. Arch Dermatol. 1993;129:866-869.
- Ohzono A, Sogame R, Li X, et al. Clinical and immunological findings in 104 cases of paraneoplastic pemphigus. Br J Dermatol. 2015;173:1447-1452.
- Tziotzios C, Lee JYW, Brier T, et al. Lichen planus and lichenoid dermatoses: clinical overview and molecular basis. J Am Acad Dermatol. 2018;79:789-804.
- Papara C, Danescu S, Sitaru C, et al. Challenges and pitfalls between lichen planus pemphigoides and bullous lichen planus. Australas J Dermatol. 2022;63:165-171.
- Tripathy DM, Vashisht D, Rathore G, et al. Bullous lichen planus vs lichen planus pemphigoides: a diagnostic dilemma. Indian Dermatol Online J. 2022;13:282-284.
A 71-year-old woman with no relevant medical history presented with recurrent painful erosions on the gingivae and gluteal cleft of 1 year’s duration. She previously was diagnosed by her periodontist with erosive lichen planus and was prescribed topical and oral steroids with minimal improvement. She denied fever, chills, weakness, fatigue, vision changes, eye pain, and sore throat. Dermatologic examination revealed edematous and erythematous upper and lower gingivae with mild erosions, as well as thin, eroded, erythematous plaques within the gluteal cleft. Indirect immunofluorescence revealed IgG with epidermal localization in a human split-skin substrate, and an enzyme-linked immunosorbent assay revealed positive IgG to bullous pemphigoid (BP) 180 and negative IgG to BP230. A 4-mm punch biopsy of the gluteal cleft was performed.