User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Botanical Briefs: Primula obconica Dermatitis
Etiology
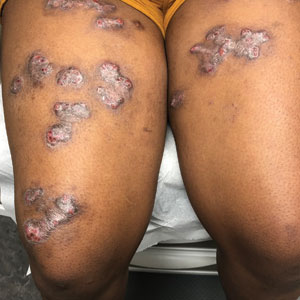
Calcareous soils of central and southwest China are home to Primula obconica1 (also known as German primrose and Libre Magenta).2 Primula obconica was introduced to Europe in the 1880s, where it became a popular ornamental and decorative household plant (Figure).3 It also is a frequent resident of greenhouses.

Primula obconica is a member of the family Primulaceae, which comprises semi-evergreen perennials. The genus name Primula is derived from Latin meaning “first”; obconica refers to the conelike shape of the plant’s vivid, cerise-red flowers.
Allergens From P obconica
The allergens primin (2-methoxy-6-pentyl-1,4-benzoquinone) and miconidin (2-methoxy-6-pentyl-1, 4-dihydroxybenzene) have been isolated from P obconica stems, leaves, and flowers. Allergies to P obconica are much more commonly detected in Europe than in the United States because the plant is part of standard allergen screening in dermatology clinics in Europe.4 In a British patch test study of 234 patients with hand dermatitis, 34 displayed immediate or delayed sensitization to P obconica allergens.5 However, in another study, researchers who surveyed the incidence of P obconica allergic contact dermatitis (CD) in the United Kingdom found a notable decline in the number of primin-positive patch tests from 1995 to 2000, which likely was attributable to a decrease in the number of plant retailers who stocked P obconica and the availability of primin-free varieties from 50% of suppliers.3 Furthermore, a study in the United States of 567 consecutive patch tests that included primin as part of standard screening found only 1 positive reaction, suggesting that routine patch testing for P obconica in the United States would have a low yield unless the patient has a relevant history.4
Cutaneous Presentation
Clinical features of P obconica–induced dermatitis include fingertip dermatitis, as well as facial, hand, and forearm dermatitis.6 Patients typically present with lichenification and fissuring of the fingertips; fingertip vesicular dermatitis; or linear erythematous streaks, vesicles, and bullae on the forearms, hands, and face. Vesicles and bullae can be hemorrhagic in patients with pompholyxlike lesions.7
Some patients have been reported to present with facial angioedema; the clinical diagnosis of CD can be challenging when facial edema is more prominent than eczema.6 Furthermore, in a reported case of P obconica CD, the patient’s vesicular hand dermatitis became pustular and spread to the face.8
Allergy Testing
Patch testing is performed with synthetic primin to detect allergens of P obconica in patients who are sensitive to them, which can be useful because Primula dermatitis can have variable presentations and cases can be missed if patch testing is not performed.9 Diagnostic mimics—herpes simplex, pompholyx, seborrheic dermatitis, and scabies—should be considered before patch testing.7
Prevention and Treatment
Preventive Measures—Ideally, once CD occurs in response to P obconica, handling of and other exposure to the plant should be halted; thus, prevention becomes the mainstay of treatment. Alternatively, when exposure is a necessary occupational hazard, nitrile gloves should be worn; allergenicity can be decreased by overwatering or introducing more primin-free varieties.3,10
Cultivating the plant outdoors during the winter in milder climates can potentially decrease sensitivity because allergen production is lowest during cold months and highest during summer.11 Because P obconica is commonly grown indoors, allergenicity can persist year-round.
Pharmacotherapy—Drawing on experience treating CD caused by other plants, acute and chronic P obconica CD are primarily treated with a topical steroid or, if the face or genitals are affected, with a steroid-sparing agent, such as tacrolimus.12 A cool compress of water, saline, or Burow solution (aluminum acetate in water) can help decrease acute inflammation, especially in the setting of vesiculation.13
Mild CD also can be treated with a barrier cream and lipid-rich moisturizer. Their effectiveness likely is due to increased hydration and aiding impaired skin-barrier repair.14
Some success in treating chronic CD also has been reported with psoralen plus UVA and UVB light therapy, which function as local immunosuppressants, thus decreasing inflammation.15
Final Thoughts
Contact dermatitis caused by P obconica is common in Europe but less common in the United States and therefore often is underrecognized. Avoiding contact with the plant should be strongly recommended to allergic persons. Primula obconica allergic CD can be treated with a topical steroid.
- Nan P, Shi S, Peng S, et al. Genetic diversity in Primula obconica (Primulaceae) from Central and South‐west China as revealed by ISSR markers. Ann Bot. 2003;91:329-333. doi:10.1093/AOB/MCG018
- Primula obconica “Libre Magenta” (Ob). The Royal Horticultural Society. Accessed February 14, 2023. https://www.rhs.org.uk/plants/131697/i-primula-obconica-i-libre-magenta-(ob)/details
- Connolly M, McCune J, Dauncey E, et al. Primula obconica—is contact allergy on the decline? Contact Dermatitis. 2004;51:167-171. doi:10.1111/J.0105-1873.2004.00427.X
- Mowad C. Routine testing for Primula obconica: is it useful in the United States? Am J Contact Dermat. 1998;9:231-233.
- Agrup C, Fregert S, Rorsman H. Sensitization by routine patch testing with ether extract of Primula obconica. Br J Dermatol. 1969;81:897-898. doi:10.1111/J.1365-2133.1969.TB15970.X
- Lleonart Bellfill R, Casas Ramisa R, Nevot Falcó S. Primula dermatitis. Allergol Immunopathol (Madr). 1999;27:29-31.
- Thomson KF, Charles-Holmes R, Beck MH. Primula dermatitis mimicking herpes simplex. Contact Dermatitis. 1997;37:185-186. doi:10.1111/J.1600-0536.1997.TB00200.X
- Tabar AI, Quirce S, García BE, et al. Primula dermatitis: versatility in its clinical presentation and the advantages of patch tests with synthetic primin. Contact Dermatitis. 1994;30:47-48. doi:10.1111/J.1600-0536.1994.tb00734.X
- Apted JH. Primula obconica sensitivity and testing with primin. Australas J Dermatol. 1988;29:161-162. doi:10.1111/J.1440-0960.1988.TB00390.X
- Aplin CG, Lovell CR. Contact dermatitis due to hardy Primula species and their cultivars. Contact Dermatitis. 2001;44:23-29. doi:10.1034/J.1600-0536.2001.440105.X
- Christensen LP, Larsen E. Direct emission of the allergen primin from intact Primula obconica plants. Contact Dermatitis. 2000;42:149-153. doi:10.1034/J.1600-0536.2000.042003149.X
- Esser PR, Mueller S, Martin SF. Plant allergen-induced contact dermatitis. Planta Med. 2019;85:528-534. doi:10.1055/A-0873-1494
- Levin CY, Maibach HI. Do cool water or physiologic saline compresses enhance resolution of experimentally-induced irritant contact dermatitis? Contact Dermatitis. 2001;45:146-150. doi:10.1034/J.1600-0536.2001.045003146.X
- M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71:79-82.
- Levin CY, Maibach HI. Irritant contact dermatitis: is there an immunologic component? Int Immunopharmacol. 2002;2:183-189. doi:10.1016/S1567-5769(01)00171-0
Etiology
Calcareous soils of central and southwest China are home to Primula obconica1 (also known as German primrose and Libre Magenta).2 Primula obconica was introduced to Europe in the 1880s, where it became a popular ornamental and decorative household plant (Figure).3 It also is a frequent resident of greenhouses.

Primula obconica is a member of the family Primulaceae, which comprises semi-evergreen perennials. The genus name Primula is derived from Latin meaning “first”; obconica refers to the conelike shape of the plant’s vivid, cerise-red flowers.
Allergens From P obconica
The allergens primin (2-methoxy-6-pentyl-1,4-benzoquinone) and miconidin (2-methoxy-6-pentyl-1, 4-dihydroxybenzene) have been isolated from P obconica stems, leaves, and flowers. Allergies to P obconica are much more commonly detected in Europe than in the United States because the plant is part of standard allergen screening in dermatology clinics in Europe.4 In a British patch test study of 234 patients with hand dermatitis, 34 displayed immediate or delayed sensitization to P obconica allergens.5 However, in another study, researchers who surveyed the incidence of P obconica allergic contact dermatitis (CD) in the United Kingdom found a notable decline in the number of primin-positive patch tests from 1995 to 2000, which likely was attributable to a decrease in the number of plant retailers who stocked P obconica and the availability of primin-free varieties from 50% of suppliers.3 Furthermore, a study in the United States of 567 consecutive patch tests that included primin as part of standard screening found only 1 positive reaction, suggesting that routine patch testing for P obconica in the United States would have a low yield unless the patient has a relevant history.4
Cutaneous Presentation
Clinical features of P obconica–induced dermatitis include fingertip dermatitis, as well as facial, hand, and forearm dermatitis.6 Patients typically present with lichenification and fissuring of the fingertips; fingertip vesicular dermatitis; or linear erythematous streaks, vesicles, and bullae on the forearms, hands, and face. Vesicles and bullae can be hemorrhagic in patients with pompholyxlike lesions.7
Some patients have been reported to present with facial angioedema; the clinical diagnosis of CD can be challenging when facial edema is more prominent than eczema.6 Furthermore, in a reported case of P obconica CD, the patient’s vesicular hand dermatitis became pustular and spread to the face.8
Allergy Testing
Patch testing is performed with synthetic primin to detect allergens of P obconica in patients who are sensitive to them, which can be useful because Primula dermatitis can have variable presentations and cases can be missed if patch testing is not performed.9 Diagnostic mimics—herpes simplex, pompholyx, seborrheic dermatitis, and scabies—should be considered before patch testing.7
Prevention and Treatment
Preventive Measures—Ideally, once CD occurs in response to P obconica, handling of and other exposure to the plant should be halted; thus, prevention becomes the mainstay of treatment. Alternatively, when exposure is a necessary occupational hazard, nitrile gloves should be worn; allergenicity can be decreased by overwatering or introducing more primin-free varieties.3,10
Cultivating the plant outdoors during the winter in milder climates can potentially decrease sensitivity because allergen production is lowest during cold months and highest during summer.11 Because P obconica is commonly grown indoors, allergenicity can persist year-round.
Pharmacotherapy—Drawing on experience treating CD caused by other plants, acute and chronic P obconica CD are primarily treated with a topical steroid or, if the face or genitals are affected, with a steroid-sparing agent, such as tacrolimus.12 A cool compress of water, saline, or Burow solution (aluminum acetate in water) can help decrease acute inflammation, especially in the setting of vesiculation.13
Mild CD also can be treated with a barrier cream and lipid-rich moisturizer. Their effectiveness likely is due to increased hydration and aiding impaired skin-barrier repair.14
Some success in treating chronic CD also has been reported with psoralen plus UVA and UVB light therapy, which function as local immunosuppressants, thus decreasing inflammation.15
Final Thoughts
Contact dermatitis caused by P obconica is common in Europe but less common in the United States and therefore often is underrecognized. Avoiding contact with the plant should be strongly recommended to allergic persons. Primula obconica allergic CD can be treated with a topical steroid.
Etiology
Calcareous soils of central and southwest China are home to Primula obconica1 (also known as German primrose and Libre Magenta).2 Primula obconica was introduced to Europe in the 1880s, where it became a popular ornamental and decorative household plant (Figure).3 It also is a frequent resident of greenhouses.

Primula obconica is a member of the family Primulaceae, which comprises semi-evergreen perennials. The genus name Primula is derived from Latin meaning “first”; obconica refers to the conelike shape of the plant’s vivid, cerise-red flowers.
Allergens From P obconica
The allergens primin (2-methoxy-6-pentyl-1,4-benzoquinone) and miconidin (2-methoxy-6-pentyl-1, 4-dihydroxybenzene) have been isolated from P obconica stems, leaves, and flowers. Allergies to P obconica are much more commonly detected in Europe than in the United States because the plant is part of standard allergen screening in dermatology clinics in Europe.4 In a British patch test study of 234 patients with hand dermatitis, 34 displayed immediate or delayed sensitization to P obconica allergens.5 However, in another study, researchers who surveyed the incidence of P obconica allergic contact dermatitis (CD) in the United Kingdom found a notable decline in the number of primin-positive patch tests from 1995 to 2000, which likely was attributable to a decrease in the number of plant retailers who stocked P obconica and the availability of primin-free varieties from 50% of suppliers.3 Furthermore, a study in the United States of 567 consecutive patch tests that included primin as part of standard screening found only 1 positive reaction, suggesting that routine patch testing for P obconica in the United States would have a low yield unless the patient has a relevant history.4
Cutaneous Presentation
Clinical features of P obconica–induced dermatitis include fingertip dermatitis, as well as facial, hand, and forearm dermatitis.6 Patients typically present with lichenification and fissuring of the fingertips; fingertip vesicular dermatitis; or linear erythematous streaks, vesicles, and bullae on the forearms, hands, and face. Vesicles and bullae can be hemorrhagic in patients with pompholyxlike lesions.7
Some patients have been reported to present with facial angioedema; the clinical diagnosis of CD can be challenging when facial edema is more prominent than eczema.6 Furthermore, in a reported case of P obconica CD, the patient’s vesicular hand dermatitis became pustular and spread to the face.8
Allergy Testing
Patch testing is performed with synthetic primin to detect allergens of P obconica in patients who are sensitive to them, which can be useful because Primula dermatitis can have variable presentations and cases can be missed if patch testing is not performed.9 Diagnostic mimics—herpes simplex, pompholyx, seborrheic dermatitis, and scabies—should be considered before patch testing.7
Prevention and Treatment
Preventive Measures—Ideally, once CD occurs in response to P obconica, handling of and other exposure to the plant should be halted; thus, prevention becomes the mainstay of treatment. Alternatively, when exposure is a necessary occupational hazard, nitrile gloves should be worn; allergenicity can be decreased by overwatering or introducing more primin-free varieties.3,10
Cultivating the plant outdoors during the winter in milder climates can potentially decrease sensitivity because allergen production is lowest during cold months and highest during summer.11 Because P obconica is commonly grown indoors, allergenicity can persist year-round.
Pharmacotherapy—Drawing on experience treating CD caused by other plants, acute and chronic P obconica CD are primarily treated with a topical steroid or, if the face or genitals are affected, with a steroid-sparing agent, such as tacrolimus.12 A cool compress of water, saline, or Burow solution (aluminum acetate in water) can help decrease acute inflammation, especially in the setting of vesiculation.13
Mild CD also can be treated with a barrier cream and lipid-rich moisturizer. Their effectiveness likely is due to increased hydration and aiding impaired skin-barrier repair.14
Some success in treating chronic CD also has been reported with psoralen plus UVA and UVB light therapy, which function as local immunosuppressants, thus decreasing inflammation.15
Final Thoughts
Contact dermatitis caused by P obconica is common in Europe but less common in the United States and therefore often is underrecognized. Avoiding contact with the plant should be strongly recommended to allergic persons. Primula obconica allergic CD can be treated with a topical steroid.
- Nan P, Shi S, Peng S, et al. Genetic diversity in Primula obconica (Primulaceae) from Central and South‐west China as revealed by ISSR markers. Ann Bot. 2003;91:329-333. doi:10.1093/AOB/MCG018
- Primula obconica “Libre Magenta” (Ob). The Royal Horticultural Society. Accessed February 14, 2023. https://www.rhs.org.uk/plants/131697/i-primula-obconica-i-libre-magenta-(ob)/details
- Connolly M, McCune J, Dauncey E, et al. Primula obconica—is contact allergy on the decline? Contact Dermatitis. 2004;51:167-171. doi:10.1111/J.0105-1873.2004.00427.X
- Mowad C. Routine testing for Primula obconica: is it useful in the United States? Am J Contact Dermat. 1998;9:231-233.
- Agrup C, Fregert S, Rorsman H. Sensitization by routine patch testing with ether extract of Primula obconica. Br J Dermatol. 1969;81:897-898. doi:10.1111/J.1365-2133.1969.TB15970.X
- Lleonart Bellfill R, Casas Ramisa R, Nevot Falcó S. Primula dermatitis. Allergol Immunopathol (Madr). 1999;27:29-31.
- Thomson KF, Charles-Holmes R, Beck MH. Primula dermatitis mimicking herpes simplex. Contact Dermatitis. 1997;37:185-186. doi:10.1111/J.1600-0536.1997.TB00200.X
- Tabar AI, Quirce S, García BE, et al. Primula dermatitis: versatility in its clinical presentation and the advantages of patch tests with synthetic primin. Contact Dermatitis. 1994;30:47-48. doi:10.1111/J.1600-0536.1994.tb00734.X
- Apted JH. Primula obconica sensitivity and testing with primin. Australas J Dermatol. 1988;29:161-162. doi:10.1111/J.1440-0960.1988.TB00390.X
- Aplin CG, Lovell CR. Contact dermatitis due to hardy Primula species and their cultivars. Contact Dermatitis. 2001;44:23-29. doi:10.1034/J.1600-0536.2001.440105.X
- Christensen LP, Larsen E. Direct emission of the allergen primin from intact Primula obconica plants. Contact Dermatitis. 2000;42:149-153. doi:10.1034/J.1600-0536.2000.042003149.X
- Esser PR, Mueller S, Martin SF. Plant allergen-induced contact dermatitis. Planta Med. 2019;85:528-534. doi:10.1055/A-0873-1494
- Levin CY, Maibach HI. Do cool water or physiologic saline compresses enhance resolution of experimentally-induced irritant contact dermatitis? Contact Dermatitis. 2001;45:146-150. doi:10.1034/J.1600-0536.2001.045003146.X
- M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71:79-82.
- Levin CY, Maibach HI. Irritant contact dermatitis: is there an immunologic component? Int Immunopharmacol. 2002;2:183-189. doi:10.1016/S1567-5769(01)00171-0
- Nan P, Shi S, Peng S, et al. Genetic diversity in Primula obconica (Primulaceae) from Central and South‐west China as revealed by ISSR markers. Ann Bot. 2003;91:329-333. doi:10.1093/AOB/MCG018
- Primula obconica “Libre Magenta” (Ob). The Royal Horticultural Society. Accessed February 14, 2023. https://www.rhs.org.uk/plants/131697/i-primula-obconica-i-libre-magenta-(ob)/details
- Connolly M, McCune J, Dauncey E, et al. Primula obconica—is contact allergy on the decline? Contact Dermatitis. 2004;51:167-171. doi:10.1111/J.0105-1873.2004.00427.X
- Mowad C. Routine testing for Primula obconica: is it useful in the United States? Am J Contact Dermat. 1998;9:231-233.
- Agrup C, Fregert S, Rorsman H. Sensitization by routine patch testing with ether extract of Primula obconica. Br J Dermatol. 1969;81:897-898. doi:10.1111/J.1365-2133.1969.TB15970.X
- Lleonart Bellfill R, Casas Ramisa R, Nevot Falcó S. Primula dermatitis. Allergol Immunopathol (Madr). 1999;27:29-31.
- Thomson KF, Charles-Holmes R, Beck MH. Primula dermatitis mimicking herpes simplex. Contact Dermatitis. 1997;37:185-186. doi:10.1111/J.1600-0536.1997.TB00200.X
- Tabar AI, Quirce S, García BE, et al. Primula dermatitis: versatility in its clinical presentation and the advantages of patch tests with synthetic primin. Contact Dermatitis. 1994;30:47-48. doi:10.1111/J.1600-0536.1994.tb00734.X
- Apted JH. Primula obconica sensitivity and testing with primin. Australas J Dermatol. 1988;29:161-162. doi:10.1111/J.1440-0960.1988.TB00390.X
- Aplin CG, Lovell CR. Contact dermatitis due to hardy Primula species and their cultivars. Contact Dermatitis. 2001;44:23-29. doi:10.1034/J.1600-0536.2001.440105.X
- Christensen LP, Larsen E. Direct emission of the allergen primin from intact Primula obconica plants. Contact Dermatitis. 2000;42:149-153. doi:10.1034/J.1600-0536.2000.042003149.X
- Esser PR, Mueller S, Martin SF. Plant allergen-induced contact dermatitis. Planta Med. 2019;85:528-534. doi:10.1055/A-0873-1494
- Levin CY, Maibach HI. Do cool water or physiologic saline compresses enhance resolution of experimentally-induced irritant contact dermatitis? Contact Dermatitis. 2001;45:146-150. doi:10.1034/J.1600-0536.2001.045003146.X
- M, Lindberg M. The influence of a single application of different moisturizers on the skin capacitance. Acta Derm Venereol. 1991;71:79-82.
- Levin CY, Maibach HI. Irritant contact dermatitis: is there an immunologic component? Int Immunopharmacol. 2002;2:183-189. doi:10.1016/S1567-5769(01)00171-0
Practice Points
- Primula obconica is a household plant that can cause contact dermatitis (CD). Spent blossoms must be pinched off to keep the plant blooming, resulting in fingertip dermatitis.
- In the United States, P obconica is not a component of routine patch testing; therefore, it might be missed as the cause of an allergic reaction.
- Primin and miconidin are the principal allergens known to be responsible for causing P obconica dermatitis.
- Treatment of this condition is similar to the usual treatment of plant-induced CD: avoiding exposure to the plant and applying a topical steroid.
How to Advise Medical Students Interested in Dermatology: A Survey of Academic Dermatology Mentors
Dermatology remains one of the most competitive specialties in medicine. In 2022, there were 851 applicants (613 doctor of medicine seniors, 85 doctor of osteopathic medicine seniors) for 492 postgraduate year (PGY) 2 positions.1 During the 2022 application season, the average matched dermatology candidate had 7.2 research experiences; 20.9 abstracts, presentations, or publications; 11 volunteer experiences; and a US Medical Licensing Examination (USMLE) Step 2 Clinical Knowledge score of 257.1 With hopes of matching into such a competitive field, students often seek advice from academic dermatology mentors. Such advice may substantially differ based on each mentor and may or may not be evidence based.
We sought to analyze the range of advice given to medical students applying to dermatology residency programs via a survey to members of the Association of Professors of Dermatology (APD) with the intent to help applicants and mentors understand how letters of intent, letters of recommendation (LORs), and Electronic Residency Application Service (ERAS) supplemental applications are used by dermatology programs nationwide.
Methods
The study was reviewed by The Ohio State University institutional review board and was deemed exempt. A branching-logic survey with common questions from medical students while applying to dermatology residency programs (Table) was sent to all members of APD through the email listserve. Study data were collected and managed using REDCap electronic data capture tools hosted at The Ohio State University (Columbus, Ohio) to ensure data security.

The survey was distributed from August 28, 2022, to September 12, 2022. A total of 101 surveys were returned from 646 listserve members (15.6%). Given the branching-logic questions, differing numbers of responses were collected for each question. Descriptive statistics were utilized to analyze and report the results.
Results
Residency Program Number—Members of the APD were asked if they recommend students apply to a certain number of programs, and if so, how many programs. Of members who responded, 62.2% (61/98) either always (22.4% [22/98]) or sometimes (40.2% [39/97]) suggested students apply to a certain number of programs. When mentors made a recommendation, 54.1% (33/61) recommended applying to 59 or fewer programs, with only 9.8% (6/61) recommending students apply to 80 or more programs.
Gap Year—We queried mentors about their recommendations for a research gap year and asked which applicants should pursue this extra year. Our survey found that 74.5% of mentors (73/98) almost always (4.1% [4/98]) or sometimes (70.4% [69/98]) recommended a research gap year, most commonly for those applicants with a strong research interest (71.8% [51/71]). Other reasons mentors recommended a dedicated research year during medical school included low USMLE Step scores (50.7% [36/71]), low grades (45.1% [32/71]), little research (46.5% [33/71]), and no home program (43.7% [31/71]).
Internship Choices—Our survey results indicated that nearly two-thirds (63.3% [62/98]) of mentors did not give applicants a recommendation on type of internship (PGY-1). If a recommendation was given, academic dermatologists more commonly recommended an internal medicine preliminary year (29.6% [29/98]) over a transitional year (7.1% [7/98]).
Communication of Interest Via a Letter of Intent—We asked mentors if they recommended applicants send a letter of intent and conversely if receiving a letter of intent impacted their rank list. Nearly half (48.5% [47/97]) of mentors indicated they did not recommend sending a letter of intent, with only 15.5% (15/97) of mentors regularly recommending this practice. Additionally, 75.8% of mentors indicated that a letter of intent never (42.1% [40/95]) or rarely (33.7% [32/95]) impacted their rank list.
Rotation Choices—We queried mentors if they recommended students complete away rotations, and if so, how many rotations did they recommend. We found that 85.9% (85/99) of mentors recommended students complete an away rotation; 63.1% (53/84) of them recommended performing 2 away rotations, and 14.3% (12/84) of respondents recommended students complete 3 away rotations. More than a quarter of mentors (27.1% [23/85]) indicated their home medical schools limited the number of away rotations a medical student could complete in any 1 specialty, and 42.4% (36/85) of respondents were unsure if such a limitation existed.
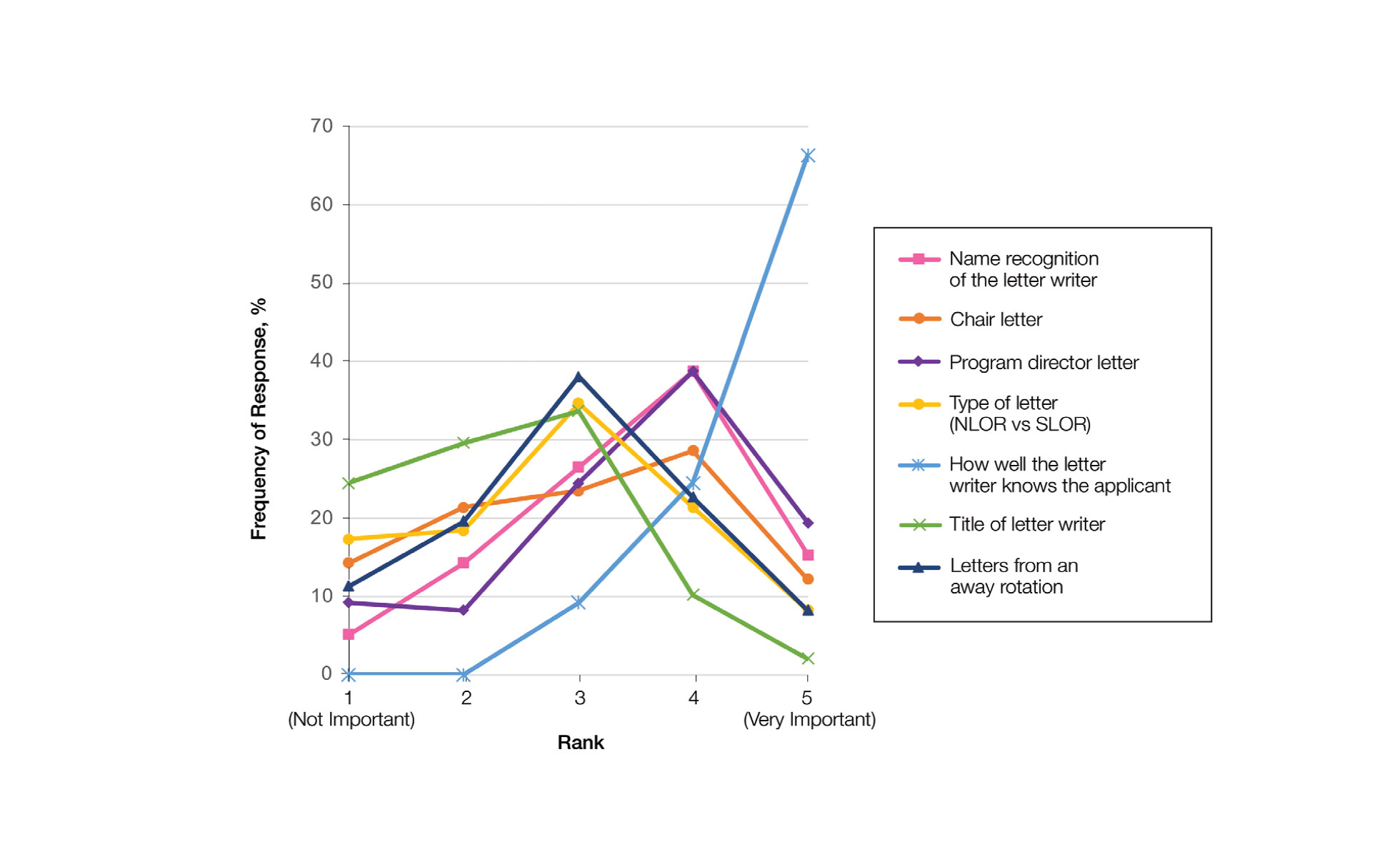
Letters of Recommendation—Our survey asked respondents to rank various factors on a 5-point scale (1=not important; 5=very important) when deciding who should write the students’ LORs. Mentors indicated that the most important factor for letter-writer selection was how well the letter writer knows the applicant, with 90.8% (89/98) of mentors rating the importance of this quality as a 4 or 5 (Figure). More than half of respondents rated the name recognition of the letter writer and program director letter as a 4 or 5 in importance (54.1% [53/98] and 58.2% [57/98], respectively). Type of letter (standardized vs nonstandardized), title of letter writer, letters from an away rotation, and chair letter scored lower, with fewer than half of mentors rating these as a 4 or 5 in importance.
Supplemental Application—When asked about the 2022 application cycle, respondents of our survey reported that the supplemental application was overall more important in deciding which applicants to interview vs which to rank highly. Prior experiences were important (ranked 4 or 5) for 58.8% (57/97) of respondents in choosing applicants to interview, and 49.4% (48/97) of respondents thought prior experiences were important for ranking. Similarly, 34.0% (33/97) of mentors indicated geographic preference was important (ranked 4 or 5) for interview compared with only 23.8% (23/97) for ranking. Finally, 57.7% (56/97) of our survey respondents denoted that program signals were important or very important in choosing which applicants to interview, while 32.0% (31/97) indicated that program signals were important in ranking applicants.
Comment
Residency Programs: Which Ones, and How Many?—The number of applications for dermatology residency programs has increased 33.9% from 2010 to 2019.2 The American Association of Medical Colleges Apply Smart data from 2013 to 2017 indicate that dermatology applicants arrive at a point of diminishing return between 37 and 62 applications, with variation within that range based on USMLE Step 1 score,3 and our data support this with nearly two-thirds of dermatology advisors recommending students apply within this range. Despite this data, dermatology residency applicants applied to more programs over the last decade (64.8 vs 77.0),2 likely to maximize their chance of matching.
Research Gap Years During Medical School—Prior research has shown that nearly half of faculty indicated that a research year during medical school can distinguish similar applicants, and close to 25% of applicants completed a research gap year.4,5 However, available data indicate that taking a research gap year has no effect on match rate or number of interview invites but does correlate with match rates at the highest ranked dermatology residency programs.6-8
Our data indicate that the most commonly recommended reason for a research gap year was an applicants’ strong interest in research. However, nearly half of dermatology mentors recommended research years during medical school for reasons other than an interest in research. As research gap years increase in popularity, future research is needed to confirm the consequence of this additional year and which applicants, if any, will benefit from such a year.
Preferences for Intern Year—Prior research suggests that dermatology residency program directors favor PGY-1 preliminary medicine internships because of the rigor of training.9,10 Our data continue to show a preference for internal medicine preliminary years over transitional years. However, given nearly two-thirds of dermatology mentors do not give applicants any recommendations on PGY-1 year, this preference may be fading.
Letters of Intent Not Recommended—Research in 2022 found that 78.8% of dermatology applicants sent a letter of intent communicating a plan to rank that program number 1, with nearly 13% sending such a letter to more than 1 program.11 With nearly half of mentors in our survey actively discouraging this process and more than 75% of mentors not utilizing this letter, the APD issued a brief statement on the 2022-2023 application cycle stating, “Post-interview communication of preference—including ‘letters of intent’ and thank you letters—should not be sent to programs. These types of communication are typically not used by residency programs in decision-making and lead to downstream pressures on applicants.”12
Away Rotations—Prior to the COVID-19 pandemic, data demonstrated that nearly one-third of dermatology applicants (29%) matched at their home institution, and nearly one-fifth (18%) matched where they completed an away rotation.13 In-person away rotations were eliminated in 2020 and restricted to 1 away rotation in 2021. Restrictions regarding away rotations were removed in 2022. Our data indicate that dermatology mentors strongly supported an away rotation, with more than half of them recommending at least 2 away rotations.
Further research is needed to determine the effect numerous away rotations have on minimizing students’ exposure to other specialties outside their chosen field. Additionally, further studies are needed to determine the impact away rotations have on economically disadvantaged students, students without home programs, and students with families. In an effort to standardize the number of away rotations, the APD issued a statement for the 2023-2024 application cycle indicating that dermatology applicants should limit away rotations to 2 in-person electives. Students without a home dermatology program could consider completing up to 3 electives.14
Who Should Write LORs?—Research in 2014 demonstrated that LORs were very important in determining applicants to interview, with a strong preference for LORs from academic dermatologists and colleagues.15 Our data strongly indicated applicants should predominantly ask for letters from writers who know them well. The majority of mentors did not give value to the rank of the letter writer (eg, assistant professor, associate professor, professor), type of letter, chair letters, or letters from an away rotation. These data may help alleviate stress many students feel as they search for letter writers.
How is the Supplemental Application Used?—In 2022, the ERAS supplemental application was introduced, which allowed applicants to detail 5 meaningful experiences, describe impactful life challenges, and indicate preferences for geographic region. Dermatology residency applicants also were able to choose 3 residency programs to signal interest in that program. Our data found that the supplemental application was utilized predominantly to select applicants to interview, which is in line with the Association of American Medical Colleges’ and APD guidelines indicating that this tool is solely meant to assist with application review.16 Further research and data will hopefully inform approaches to best utilize the ERAS supplemental application data.
Limitations—Our data were limited by response rate and sample size, as only academic dermatologists belonging to the APD were queried. Additionally, we did not track personal information of the mentors, so more than 1 mentor may have responded from a single institution, making it possible that our data may not be broadly applicable to all institutions.
Conclusion
Although there is no algorithmic method of advising medical students who are interested in dermatology, our survey data help to describe the range of advice currently given to students, which can improve and guide future recommendations. Additionally, some of our data demonstrate a discrepancy between mentor advice and current medical student practice for the number of applications and use of a letter of intent. We hope our data will assist academic dermatology mentors in the provision of advice to mentees as well as inform organizations seeking to create standards and official recommendations regarding aspects of the application process.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. May 2022. Accessed February 21, 2023. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Secrest AM, Coman GC, Swink JM, et al. Limiting residency applications to dermatology benefits nearly everyone. J Clin Aesthet Dermatol. 2021;14:30-32.
- Apply smart for residency. Association of American Medical Colleges website. Accessed February 21, 2023. https://students-residents.aamc.org/apply-smart-residency
- Shamloul N, Grandhi R, Hossler E. Perceived importance of dermatology research fellowships. Presented at: Dermatology Teachers Exchange Group; October 3, 2020.
- Runge M, Jairath NK, Renati S, et al. Pursuit of a research year or dual degree by dermatology residency applicants: a cross-sectional study. Cutis. 2022;109:E12-E13.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230.
- Ramachandran V, Nguyen HY, Dao H Jr. Does it match? analyzing self-reported online dermatology match data to charting outcomes in the Match. Dermatol Online J. 2020;26:13030/qt4604h1w4.
- Hopkins C, Jalali O, Guffey D, et al. A survey of dermatology residents and program directors assessing the transition to dermatology residency. Proc (Bayl Univ Med Center). 2021;34:59-62.
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Brumfiel CM, Jefferson IS, Rinderknecht FA, et al. Current perspectives of and potential reforms to the dermatology residency application process: a nationwide survey of program directors and applicants. Clin Dermatol. 2022;40:595-601.
- Association of Professors of Dermatology. Residency Program Directors Section. Updated Information Regarding the 2022-2023 Application Cycle. Updated October 18, 2022. Accessed February 24, 2023. https://www.dermatologyprofessors.org/files/APD%20statement%20on%202022-2023%20application%20cycle_updated%20Oct.pdf
- Narang J, Morgan F, Eversman A, et al. Trends in geographic and home program preferences in the dermatology residency match: a retrospective cohort analysis. J Am Acad Dermatol. 2022;86:645-647.
- Association of Professors of Dermatology Residency Program Directors Section. Recommendations Regarding Away Electives. Updated December 14, 2022. Accessed February 24, 2022. https://www.dermatologyprofessors.org/files/APD%20recommendations%20on%20away%20rotations%202023-2024.pdf
- Kaffenberger BH, Kaffenberger JA, Zirwas MJ. Academic dermatologists’ views on the value of residency letters of recommendation. J Am Acad Dermatol. 2014;71:395-396.
- Supplemental ERAS Application: Guide for Residency Program. Association of American Medical Colleges; June 2022.
Dermatology remains one of the most competitive specialties in medicine. In 2022, there were 851 applicants (613 doctor of medicine seniors, 85 doctor of osteopathic medicine seniors) for 492 postgraduate year (PGY) 2 positions.1 During the 2022 application season, the average matched dermatology candidate had 7.2 research experiences; 20.9 abstracts, presentations, or publications; 11 volunteer experiences; and a US Medical Licensing Examination (USMLE) Step 2 Clinical Knowledge score of 257.1 With hopes of matching into such a competitive field, students often seek advice from academic dermatology mentors. Such advice may substantially differ based on each mentor and may or may not be evidence based.
We sought to analyze the range of advice given to medical students applying to dermatology residency programs via a survey to members of the Association of Professors of Dermatology (APD) with the intent to help applicants and mentors understand how letters of intent, letters of recommendation (LORs), and Electronic Residency Application Service (ERAS) supplemental applications are used by dermatology programs nationwide.
Methods
The study was reviewed by The Ohio State University institutional review board and was deemed exempt. A branching-logic survey with common questions from medical students while applying to dermatology residency programs (Table) was sent to all members of APD through the email listserve. Study data were collected and managed using REDCap electronic data capture tools hosted at The Ohio State University (Columbus, Ohio) to ensure data security.

The survey was distributed from August 28, 2022, to September 12, 2022. A total of 101 surveys were returned from 646 listserve members (15.6%). Given the branching-logic questions, differing numbers of responses were collected for each question. Descriptive statistics were utilized to analyze and report the results.
Results
Residency Program Number—Members of the APD were asked if they recommend students apply to a certain number of programs, and if so, how many programs. Of members who responded, 62.2% (61/98) either always (22.4% [22/98]) or sometimes (40.2% [39/97]) suggested students apply to a certain number of programs. When mentors made a recommendation, 54.1% (33/61) recommended applying to 59 or fewer programs, with only 9.8% (6/61) recommending students apply to 80 or more programs.
Gap Year—We queried mentors about their recommendations for a research gap year and asked which applicants should pursue this extra year. Our survey found that 74.5% of mentors (73/98) almost always (4.1% [4/98]) or sometimes (70.4% [69/98]) recommended a research gap year, most commonly for those applicants with a strong research interest (71.8% [51/71]). Other reasons mentors recommended a dedicated research year during medical school included low USMLE Step scores (50.7% [36/71]), low grades (45.1% [32/71]), little research (46.5% [33/71]), and no home program (43.7% [31/71]).
Internship Choices—Our survey results indicated that nearly two-thirds (63.3% [62/98]) of mentors did not give applicants a recommendation on type of internship (PGY-1). If a recommendation was given, academic dermatologists more commonly recommended an internal medicine preliminary year (29.6% [29/98]) over a transitional year (7.1% [7/98]).
Communication of Interest Via a Letter of Intent—We asked mentors if they recommended applicants send a letter of intent and conversely if receiving a letter of intent impacted their rank list. Nearly half (48.5% [47/97]) of mentors indicated they did not recommend sending a letter of intent, with only 15.5% (15/97) of mentors regularly recommending this practice. Additionally, 75.8% of mentors indicated that a letter of intent never (42.1% [40/95]) or rarely (33.7% [32/95]) impacted their rank list.
Rotation Choices—We queried mentors if they recommended students complete away rotations, and if so, how many rotations did they recommend. We found that 85.9% (85/99) of mentors recommended students complete an away rotation; 63.1% (53/84) of them recommended performing 2 away rotations, and 14.3% (12/84) of respondents recommended students complete 3 away rotations. More than a quarter of mentors (27.1% [23/85]) indicated their home medical schools limited the number of away rotations a medical student could complete in any 1 specialty, and 42.4% (36/85) of respondents were unsure if such a limitation existed.
Letters of Recommendation—Our survey asked respondents to rank various factors on a 5-point scale (1=not important; 5=very important) when deciding who should write the students’ LORs. Mentors indicated that the most important factor for letter-writer selection was how well the letter writer knows the applicant, with 90.8% (89/98) of mentors rating the importance of this quality as a 4 or 5 (Figure). More than half of respondents rated the name recognition of the letter writer and program director letter as a 4 or 5 in importance (54.1% [53/98] and 58.2% [57/98], respectively). Type of letter (standardized vs nonstandardized), title of letter writer, letters from an away rotation, and chair letter scored lower, with fewer than half of mentors rating these as a 4 or 5 in importance.

Supplemental Application—When asked about the 2022 application cycle, respondents of our survey reported that the supplemental application was overall more important in deciding which applicants to interview vs which to rank highly. Prior experiences were important (ranked 4 or 5) for 58.8% (57/97) of respondents in choosing applicants to interview, and 49.4% (48/97) of respondents thought prior experiences were important for ranking. Similarly, 34.0% (33/97) of mentors indicated geographic preference was important (ranked 4 or 5) for interview compared with only 23.8% (23/97) for ranking. Finally, 57.7% (56/97) of our survey respondents denoted that program signals were important or very important in choosing which applicants to interview, while 32.0% (31/97) indicated that program signals were important in ranking applicants.
Comment
Residency Programs: Which Ones, and How Many?—The number of applications for dermatology residency programs has increased 33.9% from 2010 to 2019.2 The American Association of Medical Colleges Apply Smart data from 2013 to 2017 indicate that dermatology applicants arrive at a point of diminishing return between 37 and 62 applications, with variation within that range based on USMLE Step 1 score,3 and our data support this with nearly two-thirds of dermatology advisors recommending students apply within this range. Despite this data, dermatology residency applicants applied to more programs over the last decade (64.8 vs 77.0),2 likely to maximize their chance of matching.
Research Gap Years During Medical School—Prior research has shown that nearly half of faculty indicated that a research year during medical school can distinguish similar applicants, and close to 25% of applicants completed a research gap year.4,5 However, available data indicate that taking a research gap year has no effect on match rate or number of interview invites but does correlate with match rates at the highest ranked dermatology residency programs.6-8
Our data indicate that the most commonly recommended reason for a research gap year was an applicants’ strong interest in research. However, nearly half of dermatology mentors recommended research years during medical school for reasons other than an interest in research. As research gap years increase in popularity, future research is needed to confirm the consequence of this additional year and which applicants, if any, will benefit from such a year.
Preferences for Intern Year—Prior research suggests that dermatology residency program directors favor PGY-1 preliminary medicine internships because of the rigor of training.9,10 Our data continue to show a preference for internal medicine preliminary years over transitional years. However, given nearly two-thirds of dermatology mentors do not give applicants any recommendations on PGY-1 year, this preference may be fading.
Letters of Intent Not Recommended—Research in 2022 found that 78.8% of dermatology applicants sent a letter of intent communicating a plan to rank that program number 1, with nearly 13% sending such a letter to more than 1 program.11 With nearly half of mentors in our survey actively discouraging this process and more than 75% of mentors not utilizing this letter, the APD issued a brief statement on the 2022-2023 application cycle stating, “Post-interview communication of preference—including ‘letters of intent’ and thank you letters—should not be sent to programs. These types of communication are typically not used by residency programs in decision-making and lead to downstream pressures on applicants.”12
Away Rotations—Prior to the COVID-19 pandemic, data demonstrated that nearly one-third of dermatology applicants (29%) matched at their home institution, and nearly one-fifth (18%) matched where they completed an away rotation.13 In-person away rotations were eliminated in 2020 and restricted to 1 away rotation in 2021. Restrictions regarding away rotations were removed in 2022. Our data indicate that dermatology mentors strongly supported an away rotation, with more than half of them recommending at least 2 away rotations.
Further research is needed to determine the effect numerous away rotations have on minimizing students’ exposure to other specialties outside their chosen field. Additionally, further studies are needed to determine the impact away rotations have on economically disadvantaged students, students without home programs, and students with families. In an effort to standardize the number of away rotations, the APD issued a statement for the 2023-2024 application cycle indicating that dermatology applicants should limit away rotations to 2 in-person electives. Students without a home dermatology program could consider completing up to 3 electives.14
Who Should Write LORs?—Research in 2014 demonstrated that LORs were very important in determining applicants to interview, with a strong preference for LORs from academic dermatologists and colleagues.15 Our data strongly indicated applicants should predominantly ask for letters from writers who know them well. The majority of mentors did not give value to the rank of the letter writer (eg, assistant professor, associate professor, professor), type of letter, chair letters, or letters from an away rotation. These data may help alleviate stress many students feel as they search for letter writers.
How is the Supplemental Application Used?—In 2022, the ERAS supplemental application was introduced, which allowed applicants to detail 5 meaningful experiences, describe impactful life challenges, and indicate preferences for geographic region. Dermatology residency applicants also were able to choose 3 residency programs to signal interest in that program. Our data found that the supplemental application was utilized predominantly to select applicants to interview, which is in line with the Association of American Medical Colleges’ and APD guidelines indicating that this tool is solely meant to assist with application review.16 Further research and data will hopefully inform approaches to best utilize the ERAS supplemental application data.
Limitations—Our data were limited by response rate and sample size, as only academic dermatologists belonging to the APD were queried. Additionally, we did not track personal information of the mentors, so more than 1 mentor may have responded from a single institution, making it possible that our data may not be broadly applicable to all institutions.
Conclusion
Although there is no algorithmic method of advising medical students who are interested in dermatology, our survey data help to describe the range of advice currently given to students, which can improve and guide future recommendations. Additionally, some of our data demonstrate a discrepancy between mentor advice and current medical student practice for the number of applications and use of a letter of intent. We hope our data will assist academic dermatology mentors in the provision of advice to mentees as well as inform organizations seeking to create standards and official recommendations regarding aspects of the application process.
Dermatology remains one of the most competitive specialties in medicine. In 2022, there were 851 applicants (613 doctor of medicine seniors, 85 doctor of osteopathic medicine seniors) for 492 postgraduate year (PGY) 2 positions.1 During the 2022 application season, the average matched dermatology candidate had 7.2 research experiences; 20.9 abstracts, presentations, or publications; 11 volunteer experiences; and a US Medical Licensing Examination (USMLE) Step 2 Clinical Knowledge score of 257.1 With hopes of matching into such a competitive field, students often seek advice from academic dermatology mentors. Such advice may substantially differ based on each mentor and may or may not be evidence based.
We sought to analyze the range of advice given to medical students applying to dermatology residency programs via a survey to members of the Association of Professors of Dermatology (APD) with the intent to help applicants and mentors understand how letters of intent, letters of recommendation (LORs), and Electronic Residency Application Service (ERAS) supplemental applications are used by dermatology programs nationwide.
Methods
The study was reviewed by The Ohio State University institutional review board and was deemed exempt. A branching-logic survey with common questions from medical students while applying to dermatology residency programs (Table) was sent to all members of APD through the email listserve. Study data were collected and managed using REDCap electronic data capture tools hosted at The Ohio State University (Columbus, Ohio) to ensure data security.

The survey was distributed from August 28, 2022, to September 12, 2022. A total of 101 surveys were returned from 646 listserve members (15.6%). Given the branching-logic questions, differing numbers of responses were collected for each question. Descriptive statistics were utilized to analyze and report the results.
Results
Residency Program Number—Members of the APD were asked if they recommend students apply to a certain number of programs, and if so, how many programs. Of members who responded, 62.2% (61/98) either always (22.4% [22/98]) or sometimes (40.2% [39/97]) suggested students apply to a certain number of programs. When mentors made a recommendation, 54.1% (33/61) recommended applying to 59 or fewer programs, with only 9.8% (6/61) recommending students apply to 80 or more programs.
Gap Year—We queried mentors about their recommendations for a research gap year and asked which applicants should pursue this extra year. Our survey found that 74.5% of mentors (73/98) almost always (4.1% [4/98]) or sometimes (70.4% [69/98]) recommended a research gap year, most commonly for those applicants with a strong research interest (71.8% [51/71]). Other reasons mentors recommended a dedicated research year during medical school included low USMLE Step scores (50.7% [36/71]), low grades (45.1% [32/71]), little research (46.5% [33/71]), and no home program (43.7% [31/71]).
Internship Choices—Our survey results indicated that nearly two-thirds (63.3% [62/98]) of mentors did not give applicants a recommendation on type of internship (PGY-1). If a recommendation was given, academic dermatologists more commonly recommended an internal medicine preliminary year (29.6% [29/98]) over a transitional year (7.1% [7/98]).
Communication of Interest Via a Letter of Intent—We asked mentors if they recommended applicants send a letter of intent and conversely if receiving a letter of intent impacted their rank list. Nearly half (48.5% [47/97]) of mentors indicated they did not recommend sending a letter of intent, with only 15.5% (15/97) of mentors regularly recommending this practice. Additionally, 75.8% of mentors indicated that a letter of intent never (42.1% [40/95]) or rarely (33.7% [32/95]) impacted their rank list.
Rotation Choices—We queried mentors if they recommended students complete away rotations, and if so, how many rotations did they recommend. We found that 85.9% (85/99) of mentors recommended students complete an away rotation; 63.1% (53/84) of them recommended performing 2 away rotations, and 14.3% (12/84) of respondents recommended students complete 3 away rotations. More than a quarter of mentors (27.1% [23/85]) indicated their home medical schools limited the number of away rotations a medical student could complete in any 1 specialty, and 42.4% (36/85) of respondents were unsure if such a limitation existed.
Letters of Recommendation—Our survey asked respondents to rank various factors on a 5-point scale (1=not important; 5=very important) when deciding who should write the students’ LORs. Mentors indicated that the most important factor for letter-writer selection was how well the letter writer knows the applicant, with 90.8% (89/98) of mentors rating the importance of this quality as a 4 or 5 (Figure). More than half of respondents rated the name recognition of the letter writer and program director letter as a 4 or 5 in importance (54.1% [53/98] and 58.2% [57/98], respectively). Type of letter (standardized vs nonstandardized), title of letter writer, letters from an away rotation, and chair letter scored lower, with fewer than half of mentors rating these as a 4 or 5 in importance.

Supplemental Application—When asked about the 2022 application cycle, respondents of our survey reported that the supplemental application was overall more important in deciding which applicants to interview vs which to rank highly. Prior experiences were important (ranked 4 or 5) for 58.8% (57/97) of respondents in choosing applicants to interview, and 49.4% (48/97) of respondents thought prior experiences were important for ranking. Similarly, 34.0% (33/97) of mentors indicated geographic preference was important (ranked 4 or 5) for interview compared with only 23.8% (23/97) for ranking. Finally, 57.7% (56/97) of our survey respondents denoted that program signals were important or very important in choosing which applicants to interview, while 32.0% (31/97) indicated that program signals were important in ranking applicants.
Comment
Residency Programs: Which Ones, and How Many?—The number of applications for dermatology residency programs has increased 33.9% from 2010 to 2019.2 The American Association of Medical Colleges Apply Smart data from 2013 to 2017 indicate that dermatology applicants arrive at a point of diminishing return between 37 and 62 applications, with variation within that range based on USMLE Step 1 score,3 and our data support this with nearly two-thirds of dermatology advisors recommending students apply within this range. Despite this data, dermatology residency applicants applied to more programs over the last decade (64.8 vs 77.0),2 likely to maximize their chance of matching.
Research Gap Years During Medical School—Prior research has shown that nearly half of faculty indicated that a research year during medical school can distinguish similar applicants, and close to 25% of applicants completed a research gap year.4,5 However, available data indicate that taking a research gap year has no effect on match rate or number of interview invites but does correlate with match rates at the highest ranked dermatology residency programs.6-8
Our data indicate that the most commonly recommended reason for a research gap year was an applicants’ strong interest in research. However, nearly half of dermatology mentors recommended research years during medical school for reasons other than an interest in research. As research gap years increase in popularity, future research is needed to confirm the consequence of this additional year and which applicants, if any, will benefit from such a year.
Preferences for Intern Year—Prior research suggests that dermatology residency program directors favor PGY-1 preliminary medicine internships because of the rigor of training.9,10 Our data continue to show a preference for internal medicine preliminary years over transitional years. However, given nearly two-thirds of dermatology mentors do not give applicants any recommendations on PGY-1 year, this preference may be fading.
Letters of Intent Not Recommended—Research in 2022 found that 78.8% of dermatology applicants sent a letter of intent communicating a plan to rank that program number 1, with nearly 13% sending such a letter to more than 1 program.11 With nearly half of mentors in our survey actively discouraging this process and more than 75% of mentors not utilizing this letter, the APD issued a brief statement on the 2022-2023 application cycle stating, “Post-interview communication of preference—including ‘letters of intent’ and thank you letters—should not be sent to programs. These types of communication are typically not used by residency programs in decision-making and lead to downstream pressures on applicants.”12
Away Rotations—Prior to the COVID-19 pandemic, data demonstrated that nearly one-third of dermatology applicants (29%) matched at their home institution, and nearly one-fifth (18%) matched where they completed an away rotation.13 In-person away rotations were eliminated in 2020 and restricted to 1 away rotation in 2021. Restrictions regarding away rotations were removed in 2022. Our data indicate that dermatology mentors strongly supported an away rotation, with more than half of them recommending at least 2 away rotations.
Further research is needed to determine the effect numerous away rotations have on minimizing students’ exposure to other specialties outside their chosen field. Additionally, further studies are needed to determine the impact away rotations have on economically disadvantaged students, students without home programs, and students with families. In an effort to standardize the number of away rotations, the APD issued a statement for the 2023-2024 application cycle indicating that dermatology applicants should limit away rotations to 2 in-person electives. Students without a home dermatology program could consider completing up to 3 electives.14
Who Should Write LORs?—Research in 2014 demonstrated that LORs were very important in determining applicants to interview, with a strong preference for LORs from academic dermatologists and colleagues.15 Our data strongly indicated applicants should predominantly ask for letters from writers who know them well. The majority of mentors did not give value to the rank of the letter writer (eg, assistant professor, associate professor, professor), type of letter, chair letters, or letters from an away rotation. These data may help alleviate stress many students feel as they search for letter writers.
How is the Supplemental Application Used?—In 2022, the ERAS supplemental application was introduced, which allowed applicants to detail 5 meaningful experiences, describe impactful life challenges, and indicate preferences for geographic region. Dermatology residency applicants also were able to choose 3 residency programs to signal interest in that program. Our data found that the supplemental application was utilized predominantly to select applicants to interview, which is in line with the Association of American Medical Colleges’ and APD guidelines indicating that this tool is solely meant to assist with application review.16 Further research and data will hopefully inform approaches to best utilize the ERAS supplemental application data.
Limitations—Our data were limited by response rate and sample size, as only academic dermatologists belonging to the APD were queried. Additionally, we did not track personal information of the mentors, so more than 1 mentor may have responded from a single institution, making it possible that our data may not be broadly applicable to all institutions.
Conclusion
Although there is no algorithmic method of advising medical students who are interested in dermatology, our survey data help to describe the range of advice currently given to students, which can improve and guide future recommendations. Additionally, some of our data demonstrate a discrepancy between mentor advice and current medical student practice for the number of applications and use of a letter of intent. We hope our data will assist academic dermatology mentors in the provision of advice to mentees as well as inform organizations seeking to create standards and official recommendations regarding aspects of the application process.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. May 2022. Accessed February 21, 2023. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Secrest AM, Coman GC, Swink JM, et al. Limiting residency applications to dermatology benefits nearly everyone. J Clin Aesthet Dermatol. 2021;14:30-32.
- Apply smart for residency. Association of American Medical Colleges website. Accessed February 21, 2023. https://students-residents.aamc.org/apply-smart-residency
- Shamloul N, Grandhi R, Hossler E. Perceived importance of dermatology research fellowships. Presented at: Dermatology Teachers Exchange Group; October 3, 2020.
- Runge M, Jairath NK, Renati S, et al. Pursuit of a research year or dual degree by dermatology residency applicants: a cross-sectional study. Cutis. 2022;109:E12-E13.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230.
- Ramachandran V, Nguyen HY, Dao H Jr. Does it match? analyzing self-reported online dermatology match data to charting outcomes in the Match. Dermatol Online J. 2020;26:13030/qt4604h1w4.
- Hopkins C, Jalali O, Guffey D, et al. A survey of dermatology residents and program directors assessing the transition to dermatology residency. Proc (Bayl Univ Med Center). 2021;34:59-62.
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Brumfiel CM, Jefferson IS, Rinderknecht FA, et al. Current perspectives of and potential reforms to the dermatology residency application process: a nationwide survey of program directors and applicants. Clin Dermatol. 2022;40:595-601.
- Association of Professors of Dermatology. Residency Program Directors Section. Updated Information Regarding the 2022-2023 Application Cycle. Updated October 18, 2022. Accessed February 24, 2023. https://www.dermatologyprofessors.org/files/APD%20statement%20on%202022-2023%20application%20cycle_updated%20Oct.pdf
- Narang J, Morgan F, Eversman A, et al. Trends in geographic and home program preferences in the dermatology residency match: a retrospective cohort analysis. J Am Acad Dermatol. 2022;86:645-647.
- Association of Professors of Dermatology Residency Program Directors Section. Recommendations Regarding Away Electives. Updated December 14, 2022. Accessed February 24, 2022. https://www.dermatologyprofessors.org/files/APD%20recommendations%20on%20away%20rotations%202023-2024.pdf
- Kaffenberger BH, Kaffenberger JA, Zirwas MJ. Academic dermatologists’ views on the value of residency letters of recommendation. J Am Acad Dermatol. 2014;71:395-396.
- Supplemental ERAS Application: Guide for Residency Program. Association of American Medical Colleges; June 2022.
- National Resident Matching Program. Results and Data: 2022 Main Residency Match. May 2022. Accessed February 21, 2023. https://www.nrmp.org/wp-content/uploads/2022/05/2022-Main-Match-Results-and-Data_Final.pdf
- Secrest AM, Coman GC, Swink JM, et al. Limiting residency applications to dermatology benefits nearly everyone. J Clin Aesthet Dermatol. 2021;14:30-32.
- Apply smart for residency. Association of American Medical Colleges website. Accessed February 21, 2023. https://students-residents.aamc.org/apply-smart-residency
- Shamloul N, Grandhi R, Hossler E. Perceived importance of dermatology research fellowships. Presented at: Dermatology Teachers Exchange Group; October 3, 2020.
- Runge M, Jairath NK, Renati S, et al. Pursuit of a research year or dual degree by dermatology residency applicants: a cross-sectional study. Cutis. 2022;109:E12-E13.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role of race and ethnicity in the dermatology applicant match process. J Natl Med Assoc. 2022;113:666-670.
- Costello CM, Harvey JA, Besch-Stokes JG, et al. The role research gap years play in a successful dermatology match. Int J Dermatol. 2022;61:226-230.
- Ramachandran V, Nguyen HY, Dao H Jr. Does it match? analyzing self-reported online dermatology match data to charting outcomes in the Match. Dermatol Online J. 2020;26:13030/qt4604h1w4.
- Hopkins C, Jalali O, Guffey D, et al. A survey of dermatology residents and program directors assessing the transition to dermatology residency. Proc (Bayl Univ Med Center). 2021;34:59-62.
- Stratman EJ, Ness RM. Factors associated with successful matching to dermatology residency programs by reapplicants and other applicants who previously graduated from medical school. Arch Dermatol. 2011;147:196-202.
- Brumfiel CM, Jefferson IS, Rinderknecht FA, et al. Current perspectives of and potential reforms to the dermatology residency application process: a nationwide survey of program directors and applicants. Clin Dermatol. 2022;40:595-601.
- Association of Professors of Dermatology. Residency Program Directors Section. Updated Information Regarding the 2022-2023 Application Cycle. Updated October 18, 2022. Accessed February 24, 2023. https://www.dermatologyprofessors.org/files/APD%20statement%20on%202022-2023%20application%20cycle_updated%20Oct.pdf
- Narang J, Morgan F, Eversman A, et al. Trends in geographic and home program preferences in the dermatology residency match: a retrospective cohort analysis. J Am Acad Dermatol. 2022;86:645-647.
- Association of Professors of Dermatology Residency Program Directors Section. Recommendations Regarding Away Electives. Updated December 14, 2022. Accessed February 24, 2022. https://www.dermatologyprofessors.org/files/APD%20recommendations%20on%20away%20rotations%202023-2024.pdf
- Kaffenberger BH, Kaffenberger JA, Zirwas MJ. Academic dermatologists’ views on the value of residency letters of recommendation. J Am Acad Dermatol. 2014;71:395-396.
- Supplemental ERAS Application: Guide for Residency Program. Association of American Medical Colleges; June 2022.
Practice Points
- Dermatology mentors recommend students apply to 60 or fewer programs, with only a small percentage of faculty routinely recommending students apply to more than 80 programs.
- Dermatology mentors strongly recommend that students should not send a letter of intent to programs, as it rarely is used in the ranking process.
- Dermatology mentors encourage students to ask for letters of recommendation from writers who know them the best, irrespective of the letter writer’s rank or title. The type of letter (standardized vs nonstandardized), chair letter, or letters from an away rotation do not hold as much importance.
Inequity, Bias, Racism, and Physician Burnout: Staying Connected to Purpose and Identity as an Antidote
“Where are you really from?”
When I tell patients I am from Casper, Wyoming—wh ere I have lived the majority of my life—it’smet with disbelief. The subtext: YOU can’t be from THERE.
I didn’t used to think much of comments like this, but as I have continued to hear them, I find myself feeling tired—tired of explaining myself, tired of being treated differently than my colleagues, and tired of justifying myself. My experiences as a woman of color sadly are not uncommon in medicine.
Sara Martinez-Garcia, BA
Racial bias and racism are steeped in the culture of medicine—from the medical school admissions process1,2 to the medical training itself.3 More than half of medical students who identify as underrepresented in medicine (UIM) experience microaggressions.4 Experiencing racism and sexism in the learning environment can lead to burnout, and microaggressions promote feelings of self-doubt and isolation. Medical students who experience microaggressions are more likely to report feelings of burnout and impaired learning.4 These experiences can leave one feeling as if “You do not belong” and “You are unworthy of being in this position.”
Addressing physician burnout already is complex, and addressing burnout caused by inequity, bias, and racism is even more so. In an ideal world, we would eliminate inequity, bias, and racism in medicine through institutional and individual actions. There has been movement to do so. For example, the Accreditation Council for Graduate Medical Education (ACGME), which oversees standards for US resident and fellow training, launched ACGME Equity Matters (https://www.acgme.org/what-we-do/diversity-equity-and-inclusion/ACGME-Equity-Matters/), an initiative aimed to improve diversity, equity, and antiracism practices within graduate medical eduation. However, we know that education alone isn’t enough to fix this monumental problem. Traditional diversity training as we have known it has never been demonstrated to contribute to lasting changes in behavior; it takes much more extensive and complex interventions to meaningfully reduce bias.5 In the meantime, we need action. As a medical community, we need to be better about not turning the other way when we see these things happening in our classrooms and in our hospitals. As individuals, we must self-reflect on the role that we each play in contributing to or combatting injustices and seek out bystander training to empower us to speak out against acts of bias such as sexism or racism. Whether it is supporting a fellow colleague or speaking out against an inappropriate interaction, we can all do our part. A very brief list of actions and resources to support our UIM students and colleagues are listed in the Table; those interested in more in-depth resources are encouraged to explore the Association of American Medical Colleges Diversity and Inclusion Toolkit (https://www.aamc.org/professional-development/affinity-groups/cfas/diversity-inclusion-toolkit/resources).

We can’t change the culture of medicine quickly or even in our lifetime. In the meantime, those who are UIM will continue to experience these events that erode our well-being. They will continue to need support. Discussing mental health has long been stigmatized, and physicians are no exception. Many physicians are hesitant to discuss mental health issues out of fear of judgement and perceived or even real repercussions on their careers.10 However, times are changing and evolving with the current generation of medical students. It’s no secret that medicine is stressful. Most medical schools provide free counseling services, which lowers the barrier for discussions of mental health from the beginning. Making talk about mental health just as normal as talking about other aspects of health takes away the fear that “something is wrong with me” if someone seeks out counseling and mental health services. Faculty should actively check in and maintain open lines of communication, which can be invaluable for UIM students and their training experience. Creating an environment where trainees can be real and honest about the struggles they face in and out of the classroom can make everyone feel like they are not alone.
Addressing burnout in medicine is going to require an all-hands-on-deck approach. At an institutional level, there is a lot of room for improvement—improving systems for physicians so they are able to operate at their highest level (eg, addressing the burdens of prior authorizations and the electronic medical record), setting reasonable expectations around productivity, and creating work structures that respect work-life balance.11 But what can we do for ourselves? We believe that one of the most important ways to protect ourselves from burnout is to remember why. As a medical student, there is enormous pressure—pressure to learn an enormous volume of information, pass examinations, get involved in extracurricular activities, make connections, and seek research opportunities, while also cooking healthy food, grocery shopping, maintaining relationships with loved ones, and generally taking care of oneself. At times it can feel as if our lives outside of medical school are not important enough or valuable enough to make time for, but the pieces of our identity outside of medicine are what shape us into who we are today and are the roots of our purpose in medicine. Sometimes you can feel the most motivated, valued, and supported when you make time to have dinner with friends, call a family member, or simply spend time alone in the outdoors. Who you are and how you got to this point in your life are your identity. Reminding yourself of that can help when experiencing microaggressions or when that voice tries to tell you that you are not worthy. As you progress further in your career, maintaining that relationship with who you are outside of medicine can be your armor against burnout.
- Capers Q IV, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369.
- Lucey CR, Saguil A. The consequences of structural racism on MCAT scores and medical school admissions: the past is prologue. Acad Med. 2020;95:351-356.
- Nguemeni Tiako MJ, South EC, Ray V. Medical schools as racialized organizations: a primer. Ann Intern Med. 2021;174:1143-1144.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2021;113:310-314.
- Dobbin F, Kalev A. Why doesn’t diversity training work? the challenge for industry and academia. Anthropology Now. 2018;10:48-55.
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Int J Womens Dermatol. 2020;6:57-60.
- Hackworth JM, Kotagal M, Bignall ONR, et al. Microaggressions: privileged observers’ duty to act and what they can do [published online December 1, 2021]. Pediatrics. doi:10.1542/peds.2021-052758.
- Wheeler DJ, Zapata J, Davis D, et al. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2019;41:1112-1117.
- Scott K. Just Work: How to Root Out Bias, Prejudice, and Bullying to Build a Kick-Ass Culture of Inclusivity. St. Martin’s Press; 2021.
- Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289:3161-3166.
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
“Where are you really from?”
When I tell patients I am from Casper, Wyoming—wh ere I have lived the majority of my life—it’smet with disbelief. The subtext: YOU can’t be from THERE.
I didn’t used to think much of comments like this, but as I have continued to hear them, I find myself feeling tired—tired of explaining myself, tired of being treated differently than my colleagues, and tired of justifying myself. My experiences as a woman of color sadly are not uncommon in medicine.
Sara Martinez-Garcia, BA
Racial bias and racism are steeped in the culture of medicine—from the medical school admissions process1,2 to the medical training itself.3 More than half of medical students who identify as underrepresented in medicine (UIM) experience microaggressions.4 Experiencing racism and sexism in the learning environment can lead to burnout, and microaggressions promote feelings of self-doubt and isolation. Medical students who experience microaggressions are more likely to report feelings of burnout and impaired learning.4 These experiences can leave one feeling as if “You do not belong” and “You are unworthy of being in this position.”
Addressing physician burnout already is complex, and addressing burnout caused by inequity, bias, and racism is even more so. In an ideal world, we would eliminate inequity, bias, and racism in medicine through institutional and individual actions. There has been movement to do so. For example, the Accreditation Council for Graduate Medical Education (ACGME), which oversees standards for US resident and fellow training, launched ACGME Equity Matters (https://www.acgme.org/what-we-do/diversity-equity-and-inclusion/ACGME-Equity-Matters/), an initiative aimed to improve diversity, equity, and antiracism practices within graduate medical eduation. However, we know that education alone isn’t enough to fix this monumental problem. Traditional diversity training as we have known it has never been demonstrated to contribute to lasting changes in behavior; it takes much more extensive and complex interventions to meaningfully reduce bias.5 In the meantime, we need action. As a medical community, we need to be better about not turning the other way when we see these things happening in our classrooms and in our hospitals. As individuals, we must self-reflect on the role that we each play in contributing to or combatting injustices and seek out bystander training to empower us to speak out against acts of bias such as sexism or racism. Whether it is supporting a fellow colleague or speaking out against an inappropriate interaction, we can all do our part. A very brief list of actions and resources to support our UIM students and colleagues are listed in the Table; those interested in more in-depth resources are encouraged to explore the Association of American Medical Colleges Diversity and Inclusion Toolkit (https://www.aamc.org/professional-development/affinity-groups/cfas/diversity-inclusion-toolkit/resources).

We can’t change the culture of medicine quickly or even in our lifetime. In the meantime, those who are UIM will continue to experience these events that erode our well-being. They will continue to need support. Discussing mental health has long been stigmatized, and physicians are no exception. Many physicians are hesitant to discuss mental health issues out of fear of judgement and perceived or even real repercussions on their careers.10 However, times are changing and evolving with the current generation of medical students. It’s no secret that medicine is stressful. Most medical schools provide free counseling services, which lowers the barrier for discussions of mental health from the beginning. Making talk about mental health just as normal as talking about other aspects of health takes away the fear that “something is wrong with me” if someone seeks out counseling and mental health services. Faculty should actively check in and maintain open lines of communication, which can be invaluable for UIM students and their training experience. Creating an environment where trainees can be real and honest about the struggles they face in and out of the classroom can make everyone feel like they are not alone.
Addressing burnout in medicine is going to require an all-hands-on-deck approach. At an institutional level, there is a lot of room for improvement—improving systems for physicians so they are able to operate at their highest level (eg, addressing the burdens of prior authorizations and the electronic medical record), setting reasonable expectations around productivity, and creating work structures that respect work-life balance.11 But what can we do for ourselves? We believe that one of the most important ways to protect ourselves from burnout is to remember why. As a medical student, there is enormous pressure—pressure to learn an enormous volume of information, pass examinations, get involved in extracurricular activities, make connections, and seek research opportunities, while also cooking healthy food, grocery shopping, maintaining relationships with loved ones, and generally taking care of oneself. At times it can feel as if our lives outside of medical school are not important enough or valuable enough to make time for, but the pieces of our identity outside of medicine are what shape us into who we are today and are the roots of our purpose in medicine. Sometimes you can feel the most motivated, valued, and supported when you make time to have dinner with friends, call a family member, or simply spend time alone in the outdoors. Who you are and how you got to this point in your life are your identity. Reminding yourself of that can help when experiencing microaggressions or when that voice tries to tell you that you are not worthy. As you progress further in your career, maintaining that relationship with who you are outside of medicine can be your armor against burnout.
“Where are you really from?”
When I tell patients I am from Casper, Wyoming—wh ere I have lived the majority of my life—it’smet with disbelief. The subtext: YOU can’t be from THERE.
I didn’t used to think much of comments like this, but as I have continued to hear them, I find myself feeling tired—tired of explaining myself, tired of being treated differently than my colleagues, and tired of justifying myself. My experiences as a woman of color sadly are not uncommon in medicine.
Sara Martinez-Garcia, BA
Racial bias and racism are steeped in the culture of medicine—from the medical school admissions process1,2 to the medical training itself.3 More than half of medical students who identify as underrepresented in medicine (UIM) experience microaggressions.4 Experiencing racism and sexism in the learning environment can lead to burnout, and microaggressions promote feelings of self-doubt and isolation. Medical students who experience microaggressions are more likely to report feelings of burnout and impaired learning.4 These experiences can leave one feeling as if “You do not belong” and “You are unworthy of being in this position.”
Addressing physician burnout already is complex, and addressing burnout caused by inequity, bias, and racism is even more so. In an ideal world, we would eliminate inequity, bias, and racism in medicine through institutional and individual actions. There has been movement to do so. For example, the Accreditation Council for Graduate Medical Education (ACGME), which oversees standards for US resident and fellow training, launched ACGME Equity Matters (https://www.acgme.org/what-we-do/diversity-equity-and-inclusion/ACGME-Equity-Matters/), an initiative aimed to improve diversity, equity, and antiracism practices within graduate medical eduation. However, we know that education alone isn’t enough to fix this monumental problem. Traditional diversity training as we have known it has never been demonstrated to contribute to lasting changes in behavior; it takes much more extensive and complex interventions to meaningfully reduce bias.5 In the meantime, we need action. As a medical community, we need to be better about not turning the other way when we see these things happening in our classrooms and in our hospitals. As individuals, we must self-reflect on the role that we each play in contributing to or combatting injustices and seek out bystander training to empower us to speak out against acts of bias such as sexism or racism. Whether it is supporting a fellow colleague or speaking out against an inappropriate interaction, we can all do our part. A very brief list of actions and resources to support our UIM students and colleagues are listed in the Table; those interested in more in-depth resources are encouraged to explore the Association of American Medical Colleges Diversity and Inclusion Toolkit (https://www.aamc.org/professional-development/affinity-groups/cfas/diversity-inclusion-toolkit/resources).

We can’t change the culture of medicine quickly or even in our lifetime. In the meantime, those who are UIM will continue to experience these events that erode our well-being. They will continue to need support. Discussing mental health has long been stigmatized, and physicians are no exception. Many physicians are hesitant to discuss mental health issues out of fear of judgement and perceived or even real repercussions on their careers.10 However, times are changing and evolving with the current generation of medical students. It’s no secret that medicine is stressful. Most medical schools provide free counseling services, which lowers the barrier for discussions of mental health from the beginning. Making talk about mental health just as normal as talking about other aspects of health takes away the fear that “something is wrong with me” if someone seeks out counseling and mental health services. Faculty should actively check in and maintain open lines of communication, which can be invaluable for UIM students and their training experience. Creating an environment where trainees can be real and honest about the struggles they face in and out of the classroom can make everyone feel like they are not alone.
Addressing burnout in medicine is going to require an all-hands-on-deck approach. At an institutional level, there is a lot of room for improvement—improving systems for physicians so they are able to operate at their highest level (eg, addressing the burdens of prior authorizations and the electronic medical record), setting reasonable expectations around productivity, and creating work structures that respect work-life balance.11 But what can we do for ourselves? We believe that one of the most important ways to protect ourselves from burnout is to remember why. As a medical student, there is enormous pressure—pressure to learn an enormous volume of information, pass examinations, get involved in extracurricular activities, make connections, and seek research opportunities, while also cooking healthy food, grocery shopping, maintaining relationships with loved ones, and generally taking care of oneself. At times it can feel as if our lives outside of medical school are not important enough or valuable enough to make time for, but the pieces of our identity outside of medicine are what shape us into who we are today and are the roots of our purpose in medicine. Sometimes you can feel the most motivated, valued, and supported when you make time to have dinner with friends, call a family member, or simply spend time alone in the outdoors. Who you are and how you got to this point in your life are your identity. Reminding yourself of that can help when experiencing microaggressions or when that voice tries to tell you that you are not worthy. As you progress further in your career, maintaining that relationship with who you are outside of medicine can be your armor against burnout.
- Capers Q IV, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369.
- Lucey CR, Saguil A. The consequences of structural racism on MCAT scores and medical school admissions: the past is prologue. Acad Med. 2020;95:351-356.
- Nguemeni Tiako MJ, South EC, Ray V. Medical schools as racialized organizations: a primer. Ann Intern Med. 2021;174:1143-1144.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2021;113:310-314.
- Dobbin F, Kalev A. Why doesn’t diversity training work? the challenge for industry and academia. Anthropology Now. 2018;10:48-55.
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Int J Womens Dermatol. 2020;6:57-60.
- Hackworth JM, Kotagal M, Bignall ONR, et al. Microaggressions: privileged observers’ duty to act and what they can do [published online December 1, 2021]. Pediatrics. doi:10.1542/peds.2021-052758.
- Wheeler DJ, Zapata J, Davis D, et al. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2019;41:1112-1117.
- Scott K. Just Work: How to Root Out Bias, Prejudice, and Bullying to Build a Kick-Ass Culture of Inclusivity. St. Martin’s Press; 2021.
- Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289:3161-3166.
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
- Capers Q IV, Clinchot D, McDougle L, et al. Implicit racial bias in medical school admissions. Acad Med. 2017;92:365-369.
- Lucey CR, Saguil A. The consequences of structural racism on MCAT scores and medical school admissions: the past is prologue. Acad Med. 2020;95:351-356.
- Nguemeni Tiako MJ, South EC, Ray V. Medical schools as racialized organizations: a primer. Ann Intern Med. 2021;174:1143-1144.
- Chisholm LP, Jackson KR, Davidson HA, et al. Evaluation of racial microaggressions experienced during medical school training and the effect on medical student education and burnout: a validation study. J Natl Med Assoc. 2021;113:310-314.
- Dobbin F, Kalev A. Why doesn’t diversity training work? the challenge for industry and academia. Anthropology Now. 2018;10:48-55.
- Okoye GA. Supporting underrepresented minority women in academic dermatology. Int J Womens Dermatol. 2020;6:57-60.
- Hackworth JM, Kotagal M, Bignall ONR, et al. Microaggressions: privileged observers’ duty to act and what they can do [published online December 1, 2021]. Pediatrics. doi:10.1542/peds.2021-052758.
- Wheeler DJ, Zapata J, Davis D, et al. Twelve tips for responding to microaggressions and overt discrimination: when the patient offends the learner. Med Teach. 2019;41:1112-1117.
- Scott K. Just Work: How to Root Out Bias, Prejudice, and Bullying to Build a Kick-Ass Culture of Inclusivity. St. Martin’s Press; 2021.
- Center C, Davis M, Detre T, et al. Confronting depression and suicide in physicians: a consensus statement. JAMA. 2003;289:3161-3166.
- West CP, Dyrbye LN, Shanafelt TD. Physician burnout: contributors, consequences and solutions. J Intern Med. 2018;283:516-529.
Bridging the Digital Divide in Teledermatology Usage: A Retrospective Review of Patient Visits
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).
Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.
Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.
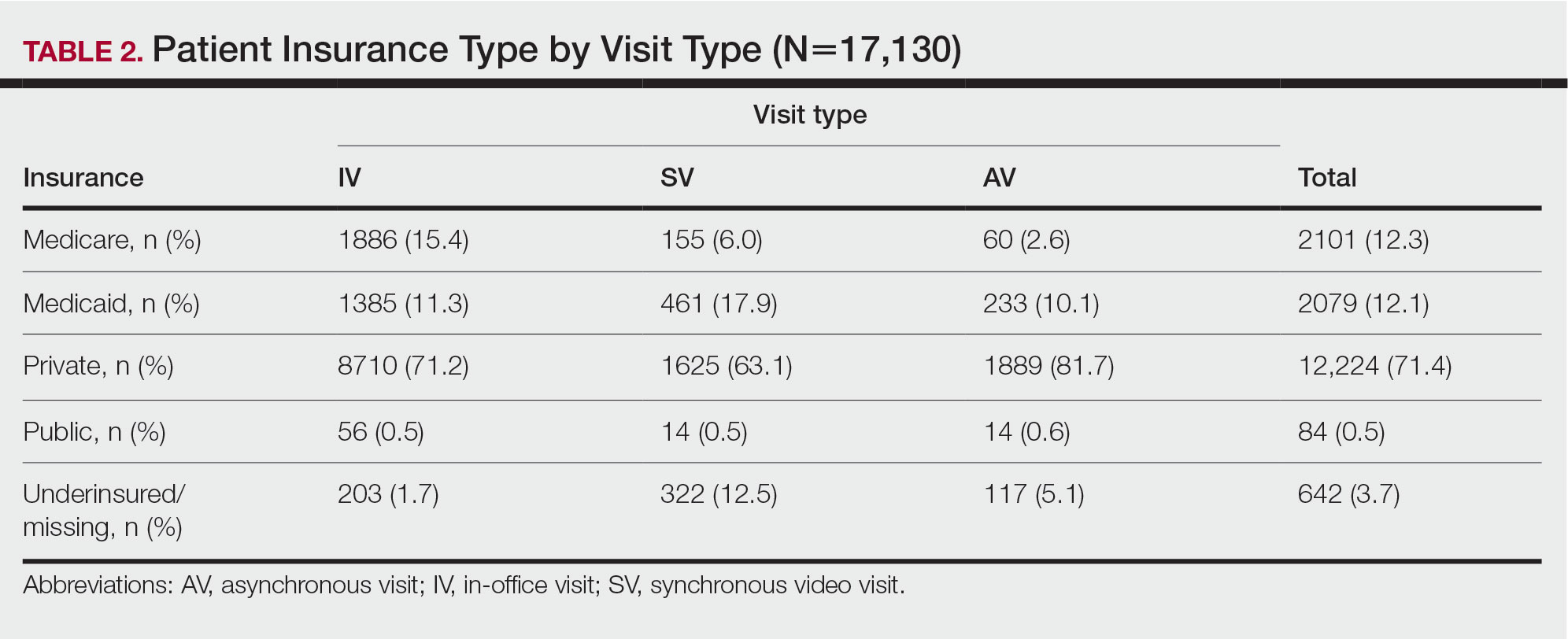
Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).

Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.

Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.

Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
Teledermatology is an effective patient care model for the delivery of high-quality dermatologic care.1 Teledermatology can occur using synchronous, asynchronous, and hybrid models of care. In asynchronous visits (AVs), patients or health professionals submit photographs and information for dermatologists to review and provide treatment recommendations. With synchronous visits (SVs), patients have a visit with a dermatology health professional in real time via live video conferencing software. Hybrid models incorporate asynchronous strategies for patient intake forms and skin photograph submissions as well as synchronous methods for live video consultation in a single visit.1 However, remarkable inequities in internet access limit telemedicine usage among medically marginalized patient populations, including racialized, elderly, and low socioeconomic status groups.2
Synchronous visits, a relatively newer teledermatology format, allow for communication with dermatology professionals from the convenience of a patient’s selected location. The live interaction of SVs allows dermatology professionals to answer questions, provide treatment recommendations, and build therapeutic relationships with patients. Concerns for dermatologist reimbursement, malpractice/liability, and technological challenges stalled large-scale uptake of teledermatology platforms.3 The COVID-19 pandemic led to a drastic increase in teledermatology usage of approximately 587.2%, largely due to public safety measures and Medicaid reimbursement parity between SV and in-office visits (IVs).3,4
With the implementation of SVs as a patient care model, we investigated the demographics of patients who utilized SVs, AVs, or IVs, and we propose strategies to promote equity in dermatologic care access.
Methods
This study was approved by the University of Pittsburgh institutional review board (STUDY20110043). We performed a retrospective electronic medical record review of deidentified data from the University of Pittsburgh Medical Center, a tertiary care center in Allegheny County, Pennsylvania, with an established asynchronous teledermatology program. Hybrid SVs were integrated into the University of Pittsburgh Medical Center patient care visit options in March 2020. Patients were instructed to upload photographs of their skin conditions prior to SV appointments. The study included visits occurring between July and December 2020. Visit types included SVs, AVs, and IVs.
We analyzed the initial dermatology visits of 17,130 patients aged 17.5 years and older. Recorded data included diagnosis, age, sex, race, ethnicity, and insurance type for each visit type. Patients without a reported race (990 patients) or ethnicity (1712 patients) were excluded from analysis of race/ethnicity data. Patient zip codes were compared with the zip codes of Allegheny County municipalities as reported by the Allegheny County Elections Division.
Statistical Analysis—Descriptive statistics were calculated; frequency with percentage was used to report categorical variables, and the mean (SD) was used for normally distributed continuous variables. Univariate analysis was performed using the χ2 test for categorical variables. One-way analysis of variance was used to compare age among visit types. Statistical significance was defined as P<.05. IBM SPSS Statistics for Windows, Version 24 (IBM Corp) was used for all statistical analyses.
Results
In our study population, 81.2% (13,916) of patients were residents of Allegheny County, where 51.6% of residents are female and 81.4% are older than 18 years according to data from 2020.5 The racial and ethnic demographics of Allegheny County were 13.4% African American/Black, 0.2% American Indian/Alaska Native, 4.2% Asian, 2.3% Hispanic/Latino, and 79.6% White. The percentage of residents who identified as Native Hawaiian/Pacific Islander was reported to be greater than 0% but less than 0.5%.5
In our analysis, IVs were the most utilized visit type, accounting for 71.5% (12,240) of visits, followed by 15.0% (2577) for SVs and 13.5% (2313) for AVs. The mean age (SD) of IV patients was 51.0 (18.8) years compared with 39.9 (16.9) years for SV patients and 37.5 (14.3) years for AV patients (eTable). The majority of patients for all visits were female: 62.1% (7599) for IVs, 71.4% (1652) for AVs, and 72.8% (1877) for SVs. The largest racial or ethnic group for all visit types included White patients (83.8% [13,524] of all patients), followed by Black (12.4% [2007]), Hispanic/Latino (1.4% [209]), Asian (3.4% [555]), American Indian/Alaska Native (0.2% [35]), and Native Hawaiian/Other Pacific Islander patients (0.1% [19]).

Asian patients, who comprised 4.2% of Allegheny County residents,5 accounted for 2.7% (334) of IVs, 4.9% (113) of AVs, and 4.2% (108) of SVs. Black patients, who were reported as 13.4% of the Allegheny County population,5 were more likely to utilize SVs (19% [490])compared with AVs (7.5% [174]) and IVs (11% [1343]). Hispanic/Latino patients had a disproportionally lower utilization of dermatologic care in all settings, comprising 1.4% (209) of all patients in our study compared with 2.3% of Allegheny County residents.5 White patients, who comprised 79.6% of Allegheny County residents, accounted for 81.1% (9928) of IVs, 67.4% (1737) of SVs, and 80.4% (1859) of AVs. There was no significant difference in the percentage of American Indian/Alaska Native and Native Hawaiian/Other Pacific Islander patients among visit types.
The 3 most common diagnoses for IVs were skin cancer screening, seborrheic keratosis, and melanocytic nevus (Table 1). Skin cancer screening was the most common diagnosis, accounting for 12.2% (8530) of 69,812 IVs. The 3 most common diagnoses for SVs were acne vulgaris, dermatitis, and psoriasis. The 3 most common diagnoses for AVs were acne vulgaris, dermatitis, and perioral dermatitis.

Private insurance was the most common insurance type among all patients (71.4% [12,224])(Table 2). A higher percentage of patients with Medicaid insurance (17.9% [461]) utilized SVs compared with AVs (10.1% [233]) and IVs (11.3% 1385]). Similarly, a higher percentage of patients with no insurance or no insurance listed were seen via SVs (12.5% [322]) compared with AVs (5.1% [117]) and IVs (1.7% [203]). Patients with Medicare insurance used IVs (15.4% [1886]) more than SVs (6.0% [155]) or AVs (2.6% [60]). There was no significant difference among visit type usage for patients with public insurance.

Comment
Teledermatology Benefits—In this retrospective review of medical records of patients who obtained dermatologic care after the implementation of SVs at our institution, we found a proportionally higher use of SVs among Black patients, patients with Medicaid, and patients who are underinsured. Benefits of teledermatology include decreases in patient transportation and associated costs, time away from work or home, and need for childcare.6 The SV format provides the additional advantage of direct live interaction and the development of a patient-physician or patient–physician assistant relationship. Although the prerequisite technology, internet, and broadband connectivity preclude use of teledermatology for many vulnerable patients,2 its convenience ultimately may reduce inequities in access.
Disparities in Dermatologic Care—Hispanic ethnicity and male sex are among described patient demographics associated with decreased rates of outpatient dermatologic care.7 We reported disparities in dermatologic care utilization across all visit types among Hispanic patients and males. Patients identifying as Hispanic/Latino composed only 1.4% (n=209) of our study population compared with 2.3% of Allegheny County residents.5 During our study period, most patients seen were female, accounting for 62.1% to 72.8% of visits, compared with 51.6% of Allegheny County residents.5 These disparities in dermatologic care use may have implications for increased skin-associated morbidity and provide impetus for dermatologists to increase engagement with these patient groups.
Characteristics of Patients Using Teledermatology—Patients using SVs and AVs were significantly younger (mean age [SD], 39.9 [16.9] years and 37.5 [14.3] years, respectively) compared with those using IVs (51.0 [18.8] years). This finding reflects known digital knowledge barriers among older patients.8,9 The synchronous communication format of SVs simulates the traditional visit style of IVs, which may be preferable for some patients. Continued patient education and advocacy for broadband access may increase teledermatology use among older patients and patients with limited technology resources.8
Teledermatology visits were used most frequently for acne and dermatitis, while IVs were used for skin cancer screenings and examination of concerning lesions. This usage pattern is consistent with a previously described consensus among dermatologists on the conditions most amenable to teledermatology evaluation.3
Medicaid reimbursement parity for SVs is in effect nationally until the end of the COVID-19 public health emergency declaration in the United States.10 As of February 2023, the public health emergency declaration has been renewed 12 times since January 2020, with the most recent renewal on January 11, 2023.11 As of January 2023, 21 states have enacted legislation providing permanent reimbursement parity for SV services. Six additional states have some payment parity in place, each with its own qualifying criteria, and 23 states have no payment parity.12 Only 25 Medicaid programs currently provide reimbursement for AV services.13
Study Limitations—Our study was limited by lack of data on patients who are multiracial and those who identify as nonbinary and transgender. Because of the low numbers of Hispanic patients associated with each race category and a high number of patients who did not report an ethnicity or race, race and ethnicity data were analyzed separately. For SVs, patients were instructed to upload photographs prior to their visit; however, the percentage of patients who uploaded photographs was not analyzed.
Conclusion
Expansion of teledermatology services, including SVs and AVs, patient outreach and education, advocacy for broadband access, and Medicaid payment parity, may improve dermatologic care access for medically marginalized groups. Teledermatology has the potential to serve as an effective health care option for patients who are racially minoritized, older, and underinsured. To further assess the effectiveness of teledermatology, we plan to analyze the number of SVs and AVs that were referred to IVs. Future studies also will investigate the impact of implementing patient education and patient-reported outcomes of teledermatology visits.
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
- Lee JJ, English JC. Teledermatology: a review and update. Am J Clin Dermatol. 2018;19:253-260.
- Bakhtiar M, Elbuluk N, Lipoff JB. The digital divide: how COVID-19’s telemedicine expansion could exacerbate disparities. J Am Acad Dermatol. 2020;83:E345-E346.
- Kennedy J, Arey S, Hopkins Z, et al. dermatologist perceptions of teledermatology implementation and future use after COVID-19: demographics, barriers, and insights. JAMA Dermatol. 2021;157:595-597.
- Centers for Disease Control and Prevention. Using telehealth to expand access to essential health services during the COVID-19 pandemic. Updated June 10, 2020. Accessed February 10, 2023. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html
- United States Census Bureau. QuickFacts: Allegheny County, Pennsylvania. Accessed August 12, 2021. https://www.census.gov/quickfacts/alleghenycountypennsylvania
- Moore HW. Teledermatology—access to specialized care via a different model. Dermatology Advisor. November 12, 2019. Accessed February 10, 2023. https://www.dermatologyadvisor.com/home/topics/practice-management/teledermatology-access-to-specialized-care-via-a-different-model/
- Tripathi R, Knusel KD, Ezaldein HH, et al. Association of demographic and socioeconomic characteristics with differences in use of outpatient dermatology services in the United States. JAMA Dermatol. 2018;154:1286-1291.
- Nouri S, Khoong EC, Lyles CR, et al. Addressing equity in telemedicine for chronic disease management during the COVID-19 pandemic [published online May 4, 2020]. NEJM Catal Innov Care Deliv. doi:10.1056/CAT.20.0123
- Swenson K, Ghertner R. People in low-income households have less access to internet services—2019 update. Office of the Assistant Secretary for Planning and Evaluation; US Department of Health and Human Services. March 2021. Accessed February 10, 2023. https://aspe.hhs.gov/sites/default/files/private/pdf/263601/internet-access-among-low-income-2019.pdf
- Centers for Medicare and Medicaid Services. COVID-19 frequently asked questions (FAQs) on Medicare fee-for-service (FFS) billing. Updated August 16, 2022. Accessed February 10, 2023. https://www.cms.gov/files/document/03092020-covid-19-faqs-508.pdf
- US Department of Health and Human Services. Renewal of determination that a public health emergency exists. Updated February 9, 2023. Accessed February 20, 2023. https://aspr.hhs.gov/legal/PHE/Pages/COVID19-9Feb2023.aspx?
- Augenstein J, Smith JM. Executive summary: tracking telehealth changes state-by-state in response to COVID-19. Updated January 27, 2023. Accessed February 10, 2023. https://www.manatt.com/insights/newsletters/covid-19-update/executive-summary-tracking-telehealth-changes-stat
- Center for Connected Health Policy. Policy trend maps: store and forward Medicaid reimbursement. Accessed June 23, 2022. https://www.cchpca.org/policy-trends/
Practice Points
- There is increased use of synchronous video visits (SVs) among Black patients, patients with Medicaid, and patients who are underinsured.
- Synchronous video visits may increase dermatologic care utilization for medically marginalized groups.
- Efforts are needed to increase engagement with dermatologic care for Hispanic and male patients.
A “Solution” for Patients Unable to Swallow a Pill: Crushed Terbinafine Mixed With Syrup
Practice Gap
Terbinafine can be used safely and effectively in adult and pediatric patients to treat superficial fungal infections, including onychomycosis.1 These superficial fungal infections have become increasingly prevalent in children and often require oral therapy2; however, children are frequently unable to swallow a pill.
Until 2016, terbinafine was available as oral granules that could be sprinkled on food, but this formulation has been discontinued.3 In addition, terbinafine tablets have a bitter taste. Therefore, the inability to swallow a pill—typical of young children and other patients with pill dysphagia—is a barrier to prescribing terbinafine.
The Technique
For patients who cannot swallow a pill, a terbinafine tablet can be crushed and mixed with food or a syrup without loss of efficacy. Terbinafine in tablet form has been shown to have relatively unchanged properties after being crushed and mixed in solution, even several weeks after preparation.4 Crushing and mixing a terbinafine tablet with food or a syrup therefore is an effective option for patients who cannot swallow a pill but can safely swallow food.
The food or syrup used for this purpose should have a pH of at least 5 because greater acidity reduces absorption of terbinafine. Therefore, avoid mixing it with fruit juices, applesauce, or soda. Given the bitter taste of the terbinafine tablet, mixing it with a sweet food or syrup improves taste and compliance, which makes pudding a particularly good food option for this purpose.
However, because younger patients might not finish an entire serving of pudding or other food into which the tablet has been crushed and mixed, inconsistent dosing might result. Therefore, we recommend mixing the crushed terbinafine tablet with 1 oz (30 mL) of chocolate syrup or corn syrup (Figure). This solution is sweet, easy to prepare and consume, widely available, and affordable (as low as $0.28/oz for corn syrup and as low as $0.10/oz for chocolate syrup, as priced on Amazon).

The tablet can be crushed using a pill crusher ($5–$10 at pharmacies or on Amazon) or by placing it on a piece of paper and crushing it with the back of a metal spoon. For children, the recommended dosing of terbinafine with a 250-mg tablet is based on weight: one-quarter of a tablet for a child weighing 10 to 20 kg; one-half of a tablet for a child weighing 20 to 40 kg; and a full tablet for a child weighing more than 40 kg.5 Because terbinafine tablets are not scored, a combined pill splitter–crusher can be used (also available at pharmacies or on Amazon; the price of this device is within the same price range as a pill crusher).
Practical Implication
Use of this method for crushing and mixing the terbinafine tablet allows patients who are unable to swallow a pill to safely and effectively use oral terbinafine.
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85:1042-1044. doi:10.1016/j.jaad.2021.01.073
- Lamisil (terbinafine hydrochloride) oral granules. Prescribing information. Novartis Pharmaceutical Corporation; 2013. Accessed February 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022071s009lbl.pdf
- Abdel-Rahman SM, Nahata MC. Stability of terbinafine hydrochloride in an extemporaneously prepared oral suspension at 25 and 4 degrees C. Am J Health Syst Pharm. 1999;56:243-245. doi:10.1093/ajhp/56.3.243
- Gupta AK, Adamiak A, Cooper EA. The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003;17:627-640. doi: 10.1046/j.1468-3083.2003.00691.x
Practice Gap
Terbinafine can be used safely and effectively in adult and pediatric patients to treat superficial fungal infections, including onychomycosis.1 These superficial fungal infections have become increasingly prevalent in children and often require oral therapy2; however, children are frequently unable to swallow a pill.
Until 2016, terbinafine was available as oral granules that could be sprinkled on food, but this formulation has been discontinued.3 In addition, terbinafine tablets have a bitter taste. Therefore, the inability to swallow a pill—typical of young children and other patients with pill dysphagia—is a barrier to prescribing terbinafine.
The Technique
For patients who cannot swallow a pill, a terbinafine tablet can be crushed and mixed with food or a syrup without loss of efficacy. Terbinafine in tablet form has been shown to have relatively unchanged properties after being crushed and mixed in solution, even several weeks after preparation.4 Crushing and mixing a terbinafine tablet with food or a syrup therefore is an effective option for patients who cannot swallow a pill but can safely swallow food.
The food or syrup used for this purpose should have a pH of at least 5 because greater acidity reduces absorption of terbinafine. Therefore, avoid mixing it with fruit juices, applesauce, or soda. Given the bitter taste of the terbinafine tablet, mixing it with a sweet food or syrup improves taste and compliance, which makes pudding a particularly good food option for this purpose.
However, because younger patients might not finish an entire serving of pudding or other food into which the tablet has been crushed and mixed, inconsistent dosing might result. Therefore, we recommend mixing the crushed terbinafine tablet with 1 oz (30 mL) of chocolate syrup or corn syrup (Figure). This solution is sweet, easy to prepare and consume, widely available, and affordable (as low as $0.28/oz for corn syrup and as low as $0.10/oz for chocolate syrup, as priced on Amazon).

The tablet can be crushed using a pill crusher ($5–$10 at pharmacies or on Amazon) or by placing it on a piece of paper and crushing it with the back of a metal spoon. For children, the recommended dosing of terbinafine with a 250-mg tablet is based on weight: one-quarter of a tablet for a child weighing 10 to 20 kg; one-half of a tablet for a child weighing 20 to 40 kg; and a full tablet for a child weighing more than 40 kg.5 Because terbinafine tablets are not scored, a combined pill splitter–crusher can be used (also available at pharmacies or on Amazon; the price of this device is within the same price range as a pill crusher).
Practical Implication
Use of this method for crushing and mixing the terbinafine tablet allows patients who are unable to swallow a pill to safely and effectively use oral terbinafine.
Practice Gap
Terbinafine can be used safely and effectively in adult and pediatric patients to treat superficial fungal infections, including onychomycosis.1 These superficial fungal infections have become increasingly prevalent in children and often require oral therapy2; however, children are frequently unable to swallow a pill.
Until 2016, terbinafine was available as oral granules that could be sprinkled on food, but this formulation has been discontinued.3 In addition, terbinafine tablets have a bitter taste. Therefore, the inability to swallow a pill—typical of young children and other patients with pill dysphagia—is a barrier to prescribing terbinafine.
The Technique
For patients who cannot swallow a pill, a terbinafine tablet can be crushed and mixed with food or a syrup without loss of efficacy. Terbinafine in tablet form has been shown to have relatively unchanged properties after being crushed and mixed in solution, even several weeks after preparation.4 Crushing and mixing a terbinafine tablet with food or a syrup therefore is an effective option for patients who cannot swallow a pill but can safely swallow food.
The food or syrup used for this purpose should have a pH of at least 5 because greater acidity reduces absorption of terbinafine. Therefore, avoid mixing it with fruit juices, applesauce, or soda. Given the bitter taste of the terbinafine tablet, mixing it with a sweet food or syrup improves taste and compliance, which makes pudding a particularly good food option for this purpose.
However, because younger patients might not finish an entire serving of pudding or other food into which the tablet has been crushed and mixed, inconsistent dosing might result. Therefore, we recommend mixing the crushed terbinafine tablet with 1 oz (30 mL) of chocolate syrup or corn syrup (Figure). This solution is sweet, easy to prepare and consume, widely available, and affordable (as low as $0.28/oz for corn syrup and as low as $0.10/oz for chocolate syrup, as priced on Amazon).

The tablet can be crushed using a pill crusher ($5–$10 at pharmacies or on Amazon) or by placing it on a piece of paper and crushing it with the back of a metal spoon. For children, the recommended dosing of terbinafine with a 250-mg tablet is based on weight: one-quarter of a tablet for a child weighing 10 to 20 kg; one-half of a tablet for a child weighing 20 to 40 kg; and a full tablet for a child weighing more than 40 kg.5 Because terbinafine tablets are not scored, a combined pill splitter–crusher can be used (also available at pharmacies or on Amazon; the price of this device is within the same price range as a pill crusher).
Practical Implication
Use of this method for crushing and mixing the terbinafine tablet allows patients who are unable to swallow a pill to safely and effectively use oral terbinafine.
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85:1042-1044. doi:10.1016/j.jaad.2021.01.073
- Lamisil (terbinafine hydrochloride) oral granules. Prescribing information. Novartis Pharmaceutical Corporation; 2013. Accessed February 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022071s009lbl.pdf
- Abdel-Rahman SM, Nahata MC. Stability of terbinafine hydrochloride in an extemporaneously prepared oral suspension at 25 and 4 degrees C. Am J Health Syst Pharm. 1999;56:243-245. doi:10.1093/ajhp/56.3.243
- Gupta AK, Adamiak A, Cooper EA. The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003;17:627-640. doi: 10.1046/j.1468-3083.2003.00691.x
- Solís-Arias MP, García-Romero MT. Onychomycosis in children. a review. Int J Dermatol. 2017;56:123-130. doi:10.1111/ijd.13392
- Wang Y, Lipner SR. Retrospective analysis of abnormal laboratory test results in pediatric patients prescribed terbinafine for superficial fungal infections. J Am Acad Dermatol. 2021;85:1042-1044. doi:10.1016/j.jaad.2021.01.073
- Lamisil (terbinafine hydrochloride) oral granules. Prescribing information. Novartis Pharmaceutical Corporation; 2013. Accessed February 6, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022071s009lbl.pdf
- Abdel-Rahman SM, Nahata MC. Stability of terbinafine hydrochloride in an extemporaneously prepared oral suspension at 25 and 4 degrees C. Am J Health Syst Pharm. 1999;56:243-245. doi:10.1093/ajhp/56.3.243
- Gupta AK, Adamiak A, Cooper EA. The efficacy and safety of terbinafine in children. J Eur Acad Dermatol Venereol. 2003;17:627-640. doi: 10.1046/j.1468-3083.2003.00691.x
Spreading Painful Lesions on the Legs
The Diagnosis: Cutaneous Leishmaniasis
A punch biopsy of the skin showed pseudoepitheliomatous hyperplasia of the epidermis with dermal granulomatous and suppurative inflammation; tissue cultures remained sterile. Polymerase chain reaction testing of the skin revealed the presence of Leishmania guyanensis complex. Leishmaniasis is a widespread parasitic disease transmitted via sandflies that often is seen in children and young adults.1 Although leishmaniasis is endemic to several countries within Southeast Asia, East Africa, and Latin America, an increase in international travel has brought the disease to nonendemic regions. Therefore, it is crucial to obtain a detailed history of travel and exposure to sandflies in patients who have recently returned from endemic regions.
Leishmaniasis may present in 3 forms: cutaneous, mucocutaneous, or visceral. Cutaneous clinical findings vary depending on disease stage, causative species, and host immune activation. Presentation following a sandfly bite typically includes a papule that progresses to an erythematous nodule. Cutaneous leishmaniasis commonly occurs in areas of the body that are easily accessible to sandflies, such as the face, neck, and limbs. Mucocutaneous leishmaniasis presents with nasal or oral involvement several years after the onset of cutaneous leishmaniasis; however, it can coexist with cutaneous involvement. Without treatment, mucocutaneous leishmaniasis may lead to perforation of the nasal septum, destruction of the mouth, and life-threatening airway obstruction.1 Determining the specific species is important due to the variation in treatment options and prognosis. Because Leishmania organisms are fastidious, obtaining a positive culture often is challenging. Polymerase chain reaction can be utilized for identification, with detection rates of 97%.1 Systemic treatment is indicated for patients with multiple or large lesions; lesions on the hands, feet, face, or joints; or immunocompromised patients. Antimonial drugs are the first-line treatment for most forms of leishmaniasis, though increasing resistance has led to a decrease in efficacy.1 Our patient ultimately was treated with 4 weeks of miltefosine 50 mg 3 times daily. She obtained full resolution of the lesions with no further treatment indicated.
Pemphigus vegetans may present with various clinical manifestations that often can lead to a delay in diagnosis. The Hallopeau subtype typically presents as pustular lesions, while the Neumann subtype may present as large vesiculobullous erosive lesions that rupture and form verrucous, crusted, vegetative plaques. The groin, inguinal folds, axillae, thighs, and flexural areas commonly are affected, but reports of nasal, vaginal, and conjunctival involvement also exist.2
Granuloma inguinale is a sexually transmitted ulcerative disease that is caused by infection with Klebsiella granulomatis. It typically is found in tropical and subtropical climates, including Australia, Brazil, India, and South Africa. The initial presentation includes a single papule or multiple papules or nodules in the genital area that progress to a painless ulcer. It can be diagnosed via biopsies or tissue smears, which will demonstrate the presence of inclusion bodies known as Donovan bodies.3
Cutaneous tuberculosis (TB) can have variable clinical presentations and may be acquired exogenously or endogenously. Cutaneous TB can be divided into 2 categories: exogenous TB caused by inoculation and endogenous TB due to direct spread or autoinoculation. Exogenous TB subtypes include tuberculous chancre and TB verrucosa cutis, while endogenous TB includes scrofuloderma, orificial TB, and lupus vulgaris.4 Patches and plaques are found in patients with lupus vulgaris and TB verrucosa cutis. Scrofuloderma, tuberculous chancre, and orificial TB can present as ulcerative or erosive lesions. Cutaneous TB infection can be diagnosed through a smear, culture, or polymerase chain reaction.4
Deep cutaneous fungal infections most commonly present in immunocompromised individuals, particularly those who are severely neutropenic and are receiving broad-spectrum systemic antimicrobial agents. Deep cutaneous fungal infections initially present as a papule and evolve into a pustule followed by a necrotic ulcer. The lesions typically are accompanied by a fever and/or vital sign abnormalities.5
- Pace D. Leishmaniasis [published online September 17, 2014]. J Infect. 2014;69(suppl 1):S10-S18. doi:10.1016/j.jinf.2014.07.016
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022.
- Ornelas J, Kiuru M, Konia T, et al. Granuloma inguinale in a 51-year-old man. Dermatol Online J. 2016;22:13030/qt52k0c4hj.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37:192-199.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
The Diagnosis: Cutaneous Leishmaniasis
A punch biopsy of the skin showed pseudoepitheliomatous hyperplasia of the epidermis with dermal granulomatous and suppurative inflammation; tissue cultures remained sterile. Polymerase chain reaction testing of the skin revealed the presence of Leishmania guyanensis complex. Leishmaniasis is a widespread parasitic disease transmitted via sandflies that often is seen in children and young adults.1 Although leishmaniasis is endemic to several countries within Southeast Asia, East Africa, and Latin America, an increase in international travel has brought the disease to nonendemic regions. Therefore, it is crucial to obtain a detailed history of travel and exposure to sandflies in patients who have recently returned from endemic regions.
Leishmaniasis may present in 3 forms: cutaneous, mucocutaneous, or visceral. Cutaneous clinical findings vary depending on disease stage, causative species, and host immune activation. Presentation following a sandfly bite typically includes a papule that progresses to an erythematous nodule. Cutaneous leishmaniasis commonly occurs in areas of the body that are easily accessible to sandflies, such as the face, neck, and limbs. Mucocutaneous leishmaniasis presents with nasal or oral involvement several years after the onset of cutaneous leishmaniasis; however, it can coexist with cutaneous involvement. Without treatment, mucocutaneous leishmaniasis may lead to perforation of the nasal septum, destruction of the mouth, and life-threatening airway obstruction.1 Determining the specific species is important due to the variation in treatment options and prognosis. Because Leishmania organisms are fastidious, obtaining a positive culture often is challenging. Polymerase chain reaction can be utilized for identification, with detection rates of 97%.1 Systemic treatment is indicated for patients with multiple or large lesions; lesions on the hands, feet, face, or joints; or immunocompromised patients. Antimonial drugs are the first-line treatment for most forms of leishmaniasis, though increasing resistance has led to a decrease in efficacy.1 Our patient ultimately was treated with 4 weeks of miltefosine 50 mg 3 times daily. She obtained full resolution of the lesions with no further treatment indicated.
Pemphigus vegetans may present with various clinical manifestations that often can lead to a delay in diagnosis. The Hallopeau subtype typically presents as pustular lesions, while the Neumann subtype may present as large vesiculobullous erosive lesions that rupture and form verrucous, crusted, vegetative plaques. The groin, inguinal folds, axillae, thighs, and flexural areas commonly are affected, but reports of nasal, vaginal, and conjunctival involvement also exist.2
Granuloma inguinale is a sexually transmitted ulcerative disease that is caused by infection with Klebsiella granulomatis. It typically is found in tropical and subtropical climates, including Australia, Brazil, India, and South Africa. The initial presentation includes a single papule or multiple papules or nodules in the genital area that progress to a painless ulcer. It can be diagnosed via biopsies or tissue smears, which will demonstrate the presence of inclusion bodies known as Donovan bodies.3
Cutaneous tuberculosis (TB) can have variable clinical presentations and may be acquired exogenously or endogenously. Cutaneous TB can be divided into 2 categories: exogenous TB caused by inoculation and endogenous TB due to direct spread or autoinoculation. Exogenous TB subtypes include tuberculous chancre and TB verrucosa cutis, while endogenous TB includes scrofuloderma, orificial TB, and lupus vulgaris.4 Patches and plaques are found in patients with lupus vulgaris and TB verrucosa cutis. Scrofuloderma, tuberculous chancre, and orificial TB can present as ulcerative or erosive lesions. Cutaneous TB infection can be diagnosed through a smear, culture, or polymerase chain reaction.4
Deep cutaneous fungal infections most commonly present in immunocompromised individuals, particularly those who are severely neutropenic and are receiving broad-spectrum systemic antimicrobial agents. Deep cutaneous fungal infections initially present as a papule and evolve into a pustule followed by a necrotic ulcer. The lesions typically are accompanied by a fever and/or vital sign abnormalities.5
The Diagnosis: Cutaneous Leishmaniasis
A punch biopsy of the skin showed pseudoepitheliomatous hyperplasia of the epidermis with dermal granulomatous and suppurative inflammation; tissue cultures remained sterile. Polymerase chain reaction testing of the skin revealed the presence of Leishmania guyanensis complex. Leishmaniasis is a widespread parasitic disease transmitted via sandflies that often is seen in children and young adults.1 Although leishmaniasis is endemic to several countries within Southeast Asia, East Africa, and Latin America, an increase in international travel has brought the disease to nonendemic regions. Therefore, it is crucial to obtain a detailed history of travel and exposure to sandflies in patients who have recently returned from endemic regions.
Leishmaniasis may present in 3 forms: cutaneous, mucocutaneous, or visceral. Cutaneous clinical findings vary depending on disease stage, causative species, and host immune activation. Presentation following a sandfly bite typically includes a papule that progresses to an erythematous nodule. Cutaneous leishmaniasis commonly occurs in areas of the body that are easily accessible to sandflies, such as the face, neck, and limbs. Mucocutaneous leishmaniasis presents with nasal or oral involvement several years after the onset of cutaneous leishmaniasis; however, it can coexist with cutaneous involvement. Without treatment, mucocutaneous leishmaniasis may lead to perforation of the nasal septum, destruction of the mouth, and life-threatening airway obstruction.1 Determining the specific species is important due to the variation in treatment options and prognosis. Because Leishmania organisms are fastidious, obtaining a positive culture often is challenging. Polymerase chain reaction can be utilized for identification, with detection rates of 97%.1 Systemic treatment is indicated for patients with multiple or large lesions; lesions on the hands, feet, face, or joints; or immunocompromised patients. Antimonial drugs are the first-line treatment for most forms of leishmaniasis, though increasing resistance has led to a decrease in efficacy.1 Our patient ultimately was treated with 4 weeks of miltefosine 50 mg 3 times daily. She obtained full resolution of the lesions with no further treatment indicated.
Pemphigus vegetans may present with various clinical manifestations that often can lead to a delay in diagnosis. The Hallopeau subtype typically presents as pustular lesions, while the Neumann subtype may present as large vesiculobullous erosive lesions that rupture and form verrucous, crusted, vegetative plaques. The groin, inguinal folds, axillae, thighs, and flexural areas commonly are affected, but reports of nasal, vaginal, and conjunctival involvement also exist.2
Granuloma inguinale is a sexually transmitted ulcerative disease that is caused by infection with Klebsiella granulomatis. It typically is found in tropical and subtropical climates, including Australia, Brazil, India, and South Africa. The initial presentation includes a single papule or multiple papules or nodules in the genital area that progress to a painless ulcer. It can be diagnosed via biopsies or tissue smears, which will demonstrate the presence of inclusion bodies known as Donovan bodies.3
Cutaneous tuberculosis (TB) can have variable clinical presentations and may be acquired exogenously or endogenously. Cutaneous TB can be divided into 2 categories: exogenous TB caused by inoculation and endogenous TB due to direct spread or autoinoculation. Exogenous TB subtypes include tuberculous chancre and TB verrucosa cutis, while endogenous TB includes scrofuloderma, orificial TB, and lupus vulgaris.4 Patches and plaques are found in patients with lupus vulgaris and TB verrucosa cutis. Scrofuloderma, tuberculous chancre, and orificial TB can present as ulcerative or erosive lesions. Cutaneous TB infection can be diagnosed through a smear, culture, or polymerase chain reaction.4
Deep cutaneous fungal infections most commonly present in immunocompromised individuals, particularly those who are severely neutropenic and are receiving broad-spectrum systemic antimicrobial agents. Deep cutaneous fungal infections initially present as a papule and evolve into a pustule followed by a necrotic ulcer. The lesions typically are accompanied by a fever and/or vital sign abnormalities.5
- Pace D. Leishmaniasis [published online September 17, 2014]. J Infect. 2014;69(suppl 1):S10-S18. doi:10.1016/j.jinf.2014.07.016
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022.
- Ornelas J, Kiuru M, Konia T, et al. Granuloma inguinale in a 51-year-old man. Dermatol Online J. 2016;22:13030/qt52k0c4hj.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37:192-199.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
- Pace D. Leishmaniasis [published online September 17, 2014]. J Infect. 2014;69(suppl 1):S10-S18. doi:10.1016/j.jinf.2014.07.016
- Messersmith L, Krauland K. Pemphigus vegetans. StatPearls. StatPearls Publishing; 2022.
- Ornelas J, Kiuru M, Konia T, et al. Granuloma inguinale in a 51-year-old man. Dermatol Online J. 2016;22:13030/qt52k0c4hj.
- Chen Q, Chen W, Hao F. Cutaneous tuberculosis: a great imitator. Clin Dermatol. 2019;37:192-199.
- Marcoux D, Jafarian F, Joncas V, et al. Deep cutaneous fungal infections in immunocompromised children. J Am Acad Dermatol. 2009;61:857-864.
A 14-year-old adolescent girl presented with spreading painful lesions on the legs and left forearm of 2 years’ duration. Her travel history included several countries in South and Central America, traversing the Colombian jungle on foot. Near the end of the jungle trip, she noted a skin lesion on the left forearm around the site of an insect bite. Within 1 month, the lesions spread to the legs. She was treated with topical corticosteroids without improvement. Physical examination revealed verrucous, reddish-brown plaques on the legs and left forearm. Intranasal examination revealed a red rounded lesion inside the left nostril.
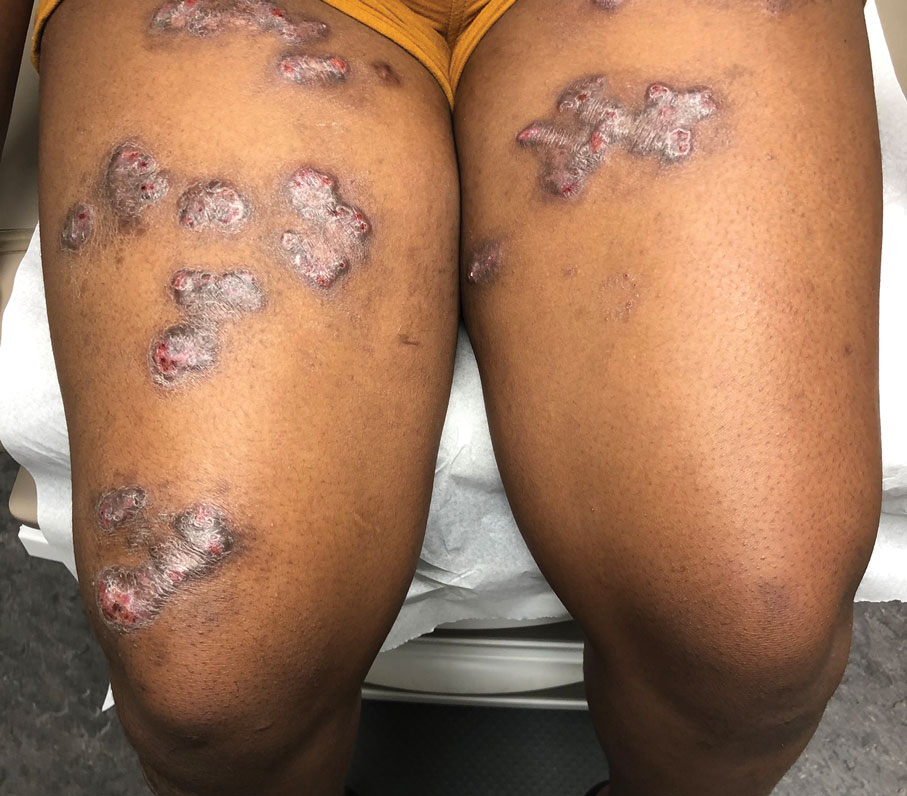
How to help pediatricians apply peanut allergy guidelines
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
Despite the profound shift in guidelines for preventing peanut allergies in infants after the landmark LEAP study, national surveys in 2021 showed that 70% of parents and caregivers said that they hadn’t heard the new recommendations, and fewer than one-third of pediatricians were following them.
Now, in a 5-year National Institutes of Health–funded study called iREACH, researchers are testing whether a two-part intervention, which includes training videos and a clinical decision support tool, helps pediatricians follow the guidelines and ultimately reduces peanut allergy.
Early results from iREACH, presented at the American Academy of Allergy, Asthma, and Immunology 2023 annual meeting in San Antonio, showed mixed results with a sharp rise in clinician knowledge of the guidelines but only a modest increase in their real-world implementation with high-risk infants.
Raising a food-allergic child while working as a pediatrician herself, Ruchi Gupta, MD, MPH, director of the Center for Food Allergy and Asthma Research at Northwestern University, Chicago, understands the importance and challenge of translating published findings into practice.
During a typical 4- to 6-month well-child visit, pediatricians must check the baby’s growth, perform a physical exam, discuss milestones, field questions about sleep and poop and colic and – if they’re up on the latest guidelines – explain why it’s important to feed peanuts early and often.
“Pediatricians get stuff from every single specialty, and guidelines are always changing,” she told this news organization.
The current feeding guidelines, published in 2017 after the landmark LEAP study, switched from “ ‘don’t introduce peanuts until age 3’ to ‘introduce peanuts now,’ ” said Dr. Gupta.
But the recommendations aren’t entirely straightforward. They require pediatricians to make an assessment when the baby is around 4 months old. If the child is high-risk (has severe eczema or an egg allergy), they need a peanut-specific immunoglobulin E (IgE) test. If the test is negative, the pediatrician should encourage peanut introduction. If positive, they should refer the child to an allergist.
“It’s a little complicated,” Dr. Gupta said.
To boost understanding and adherence, Dr. Gupta’s team created the intervention tested in the iREACH study. It includes a set of training videos, a clinical decision support tool that embeds into the electronic health record (EHR) with pop-ups reminding the physician to discuss early introduction, menus for ordering peanut IgE tests or referring to an allergist if needed, and a caregiver handout that explains how to add peanuts to the baby’s diet. (These resources can be found here.)
The study enrolled 290 pediatric clinicians at 30 local practices, examining 18,460 babies from diverse backgrounds, about one-quarter of whom were from families on public insurance. About half of the clinicians received the intervention, whereas the other half served as the control arm.
The training videos seemed effective. Clinicians’ knowledge of the guidelines rose from 72.6% at baseline to 94.5% after the intervention, and their ability to identify severe eczema went up from 63.4% to 97.6%. This translated to 70.4% success with applying the guidelines when presented various clinical scenarios, up from 29% at baseline. These results are in press at JAMA Network Open.
The next set of analyses, preliminary and unpublished, monitored real-world adherence using natural language processing to pull EHR data from 4- and 6-month well-check visits. It was “AI [artificial intelligence] for notes,” Dr. Gupta said.
For low-risk infants, the training and EHR-embedded support tool greatly improved clinician adherence. Eighty percent of clinicians in the intervention arm followed the guidelines, compared with 26% in the control group.
In high-risk infants, the impact was much weaker. Even after the video-based training, only 17% of pediatric clinicians followed the guidelines – that is, ordered a peanut IgE test or referred to an allergist – compared with 8% in the control group.
Why such a low uptake?
Pediatricians are time-pressed. “How do you add [early introduction] to the other 10 or 15 things you want to talk to a parent about at the 4-month visit?” said Jonathan Necheles, MD, MPH, a pediatrician at Children’s Healthcare Associates in Chicago.
It can also be hard to tell if a baby’s eczema is “severe” or “mild to moderate.” The EHR-integrated support tool included a scorecard for judging eczema severity across a range of skin tones. The condition can be hard to recognize in patients of color. “You don’t get the redness in the same way,” said Dr. Necheles, who worked with Dr. Gupta to develop the iREACH intervention.
Curiously, even though the AI analysis found that less than one-fifth of pediatricians put the guidelines into action for high-risk infants, 69% of them recommended peanut introduction.
One interpretation is that busy pediatricians may be “doing the minimum” – introducing the concept of early introduction and telling parents to try it “but not giving any additional sort of guidance as far as who’s high risk, who’s low risk, who should see the allergist, who should get screened,” said Edwin Kim, MD, allergist-immunologist and director of the Food Allergy Initiative at the University of North Carolina at Chapel Hill.
The ultimate impact of iREACH has yet to be seen. “The end goal is, if pediatricians recommend, will parents follow, and will we reduce peanut allergy?” Dr. Gupta said.
Dr. Gupta consults or serves as an advisor for Genentech, Novartis, Aimmune, Allergenis, and Food Allergy Research & Education; receives research funding from Novartis, Genentech, FARE, Melchiorre Family Foundation, and Sunshine Charitable Foundation; and reports ownership interest from Yobee Care. Dr. Necheles reports no financial disclosures. Dr. Kim reports consultancy with Allergy Therapeutics, Belhaven Biopharma, Duke Clinical Research Institute, Genentech, Nutricia, and Revolo; advisory board membership with ALK, Kenota Health, and Ukko; and grant support from the National Institute of Allergy and Infectious Diseases, Immune Tolerance Network, and Food Allergy Research and Education.
A version of this article first appeared on Medscape.com.
FROM AAAAI 2023
Skin reactions from melanoma targeted and immune therapies range from pruritus to SJS
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
SAN DIEGO – A
“These skin reactions can cause pain, itching, and emotional and social distress that may severely impact activities of daily living,” Aleksandr Itkin, MD, a dermatologist at Scripps MD Anderson Cancer Center, San Diego, said at the annual Cutaneous Malignancy Update. An estimated 30%-50% of patients on immune checkpoint inhibitors (ICIs) experience cutaneous adverse events, he said, which leads to dose reduction or discontinuation of ICIs in 20% of cases.
Clinicians first observed these side effects in 2011, with the Food and Drug Administration approval of ipilimumab, a human cytotoxic T-lymphocyte antigen 4 (CTLA-4)–blocking antibody, for metastatic melanoma, followed by the programmed death receptor-1 (PD-1) inhibitors nivolumab and pembrolizumab, which were approved in 2014 for the same indication.
Since then, more ICIs showing similar adverse cutaneous reactions have been approved by the FDA. These include avelumab, atezolizumab in combination with cobimetinib and vemurafenib, and a combination of relatlimab, an anti-LAG-3 antibody, with nivolumab.
Among the targeted therapies, the BRAF inhibitors vemurafenib and dabrafenib alone or in combination with MAPK pathway inhibitors cobimetinib and trametinib, which are a first-line therapy for V600 BRAF mutated metastatic melanoma, are associated with their own set of cutaneous reactions. The oncolytic modified herpes simplex virus T-VEC (talimogene laherparepvec), approved by the FDA in 2015 for the treatment of unresectable stage IIIB-IV metastatic melanoma, also results in cutaneous reactions that have been found useful in assessing the therapeutic outcome of this agent.
According to a 2020 CME article on the dermatologic adverse events that occur after treatment initiation with ICIs, the time of onset of psoriasiform rash is within the first 3 weeks, maculopapular rash and pruritus in the first 4-6 weeks, lichenoid eruption in the first 7-12 weeks, and bullous pemphigoid in weeks 13-15. The most severe reactions – SJS, toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) – usually occur after 2-3 months of treatment.
A subsequent retrospective cohort study of patients in the United States treated with ICIs for a variety of systemic malignancies and matched controls found that the ICI-treated group had a significantly higher incidence of pruritus, mucositis, erythroderma, maculopapular eruption, vitiligo, lichen planus, bullous pemphigoid, Grover disease, rash, other nonspecific eruptions, and drug eruption or other nonspecific drug reaction. Patients with melanoma and renal cell carcinoma and those receiving combination therapy were at a higher risk of cutaneous immune-related adverse events.
Another study, a prospective trial of 617 patients with various advanced cancers (including melanoma), found that both severe and mild skin toxicities were significantly associated with improved progression-free and overall survival.
According to Dr. Itkin, erythema multiforme, SJS, and TEN have been reported with anti-PD1, anti-CTLA4, and BRAF inhibitors. In TEN induced by vemurafenib, an in vitro analysis showed cross-activation of lymphocytes with dabrafenib and with sulfamethoxazole. “This means you that may want to avoid sulfonamides in patients with serious hypersensitivity to vemurafenib, and vice versa,” he said at the meeting hosted by Scripps MD Anderson Cancer Center.
Acneiform eruptions
In addition, the use of MAPK inhibitors can trigger acneiform eruptions. In one study, 77% of patients on trametinib developed acneiform eruption, but only 10% of those on trametinib in combination with dabrafenib developed acneiform eruption. “Inhibition of the MAPK pathway leads to decreased proliferative markers, further leading to decreased keratinocyte replication, increased inflammatory cytokine, apoptosis, thinning and abnormal epidermal differentiation, follicular rupture, and papule/pustule formation,” he said. For these cases, “treatment options are similar to what we use for regular acne except for here, use of systemic steroids is sometimes needed, especially in more severe cases. The reaction may be so severe as to lead to dose reduction or discontinuation of antineoplastic treatment.”
Effects on nail, hair
Paronychia and onycholysis are additional potential adverse events of MEK inhibitors and BRAF inhibitors alone or in combination, Dr. Itkin continued. Onycholysis is associated with dabrafenib alone or in combination with trametinib, while vemurafenib has been shown to induce acute paronychia and brittle nails. He said that secondary infections in these cases can be treated with the options familiar to dermatologists in their daily practice: oral doxycycline, azole antifungals, vinegar soaks, topical superpotent corticosteroids under occlusion, nail avulsion, and phenol nail matrix ablation.
Dr. Itkin noted that while PD-1 and PD-L1 inhibitors can cause hair repigmentation, CTLA-4 and PD-1 inhibitors are more likely to cause vitiligo. Appearance of vitiligo is regarded as a good prognostic factor in the treatment of melanoma with various checkpoint inhibitors alone or in combination with each other or with radiation therapy. “About 5% of melanoma patients treated with ipilimumab will develop vitiligo,” he said.
ICI-induced vitiligo differs from conventional vitiligo in that there is no family or personal history of autoimmunity; it presents as a flecked pattern of lesion on photo-exposed skin, and it lacks the Koebner phenomenon. In addition, induction of squamous neoplasms can occur with BRAF inhibitors, especially in patients with a high frequency of RAS mutations.
He said that coadministration of MEK inhibitors such as trametinib and cobimetinib may prevent induction of keratinocytic neoplasms.
Dr. Itkin reported having no relevant financial disclosures.
AT MELANOMA 2023
NP-PA turf fights: Where the relationship can improve
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
– The U.S. Bureau of Labor Statistics forecasts a 40% increase in the NP workforce by 2031, coupled with a 28% rise in PAs.
In recent reports on the quality of the relationships involving these health care professions, survey respondents mostly gave positive accounts of collaboration, using words such as like “comradery,” “teamwork,” “congenial,” and “cohesion.” But all was not perfect. Where and how could these important health care provider relationships improve?
PAs: “Competition and collaboration’ with RNs
In a Medscape survey of more than 770 PAs about their working relationships with other health care professionals; 83% of them supported the idea of PAs and NPs practicing more independently from physicians, but sometimes it’s not easy to stay in their individual lanes.
One PA respondent complained that NPs get “more opportunities and preference,” another pointed to PA-NP “turf issues,” and a third griped about NPs’ “strong unions,” which have stoked more fighting about practice abilities and available settings.
Robert Blumm, MA, PA-C, a retired surgical and emergency medicine PA who regards himself as an advocate for both PAs and NPs, describes their interaction as a “mixture of competition and collaboration.”
On one hand, the two groups typically “cooperate and do an excellent job, incurring patient errors similar to or less than physician colleagues or senior residents.” On the other hand, Mr. Blumm conceded, there is some jealousy among PAs over NPs’ advantage in staffing and hiring decisions, “since they don’t need [direct physician] supervision ... and there are limits on how many PAs can be supervised by one physician.”
Most PA-NP interactions are collaborative, although many people emphasize the relatively few conflicts, said Jennifer Orozco, DMSc, PA-C, president and chair of the American Academy of PAs.
“We see that a lot in this country,” she said. “People try to drive a wedge, but it’s often a misnomer that there’s a lot of arguing and infighting.”
NPs: Different backgrounds, same goal
The Medscape survey also included information from 750 NPs on working relationships; 93% of them favored nurses and PAs working more independently from doctors.
April Kapu, DNP, ARPN, has worked closely with PAs for more than 20 years. “In my experience ... they complement one another as health team members, although the education and training are somewhat different,” said Ms. Kapu, , president of the American Association of Nurse Practitioners.
Some respondents noted the different educational trajectories for NPs and PAs. “Doctors and PAs are taught using the same model, but NPs are taught under the nursing model,” wrote a family medicine PA.
In emergency departments where Mr. Blumm has worked, ICU NPs have an edge over PAs in terms of preparation, organization, and the tabulation of formulas. On the other hand, some of Mr. Blumm’s fellow PAs were also emergency medicine technicians or respiratory therapists, who had “2 years of classroom training, on par with that of medical students.”
Must these differences in training and education foment conflict between NPs and PAs? “We all bring something different to the table,” said Ms. Kapu, who also is associate dean for clinical and community partnerships at Vanderbilt University, Nashville, Tenn. “It is important to respect each person’s entry point, education, and training.”
Differing personalities and environments
Numerous PA respondents said that individual personalities and work environments are more likely to trigger issues with NPs than are differences in training.
“It depends on the team and situation and who the people are, not the letters behind their names,” an emergency medicine PA wrote. A surgical PA noted that “group dynamics and work culture differ from place to place,” while a third PA agreed that “it’s personality dependent, not title dependent.”
No single formula will resolve areas of NP-PA conflict, Ms. Orozco said. “What works in Chicago might not work in rural Colorado or Texas or California, but we do have to come together. The overall focus should be on greater flexibility for PAs and NPs. Patients will fare better.”
Joint research, publishing could help
About a decade ago, Mr. Blumm joined with another PA and an NP to form the American College of Clinicians, the first joint PA-NP national professional organization. Although it disbanded after 6 years, owing to low membership, he hopes a similar collaboration will take off in the future.
“I also recommend that PAs and NPs publish articles together, with research as an excellent place to start,” he added. “PAs and NPs should stand together and be a source of healing for all our patients. Regardless of our titles, our responsibility is to bring healing together.”
A version of this article first appeared on Medscape.com.
Docs struggle to keep up with the flood of new medical knowledge. Here’s advice
making it much tougher for physicians to identify innovative findings and newer guidelines for helping patients. Yet not keeping up with the latest information can put doctors at risk.
“Most doctors are feeling lost about keeping up to date,” said John P.A. Ioannidis, MD, professor of medicine at Stanford (Calif.) University School of Medicine. “The vast majority of new studies are either wrong or not useful, but physicians cannot sort out which are those studies.”
The sheer number of new studies may even force some doctors to retreat from areas where they have not kept up, said Stephen A. Martin, MD, professor of family medicine and community health at the University of Massachusetts, Worcester. “When doctors don’t feel they can stay current, they may refer more cases to specialists or narrow their focus,” he said.
Some specialties have a greater challenge than others
Dr. Martin said the deluge of studies heavily impacts generalists because they have a wider field of information to keep up with. However, certain specialties like oncology are particularly flooded with new findings.
Specialties with the greatest number of published studies are reportedly oncology, cardiology, and neurology. A 2021 study found that the number of articles with the word “stroke” in them increased five times from 2000 to 2020. And investigative treatments targeting cancer nearly quadrupled just between 2010 and 2020.
What’s more, physicians spend a great deal of time sifting through studies that are ultimately useless. In a survey of internists by Univadis, which is part of WebMD/Medscape, 82% said that fewer than half of the studies they read actually had an impact on how they practice medicine.
“You often have to dig into an article and learn more about a finding before you now whether it’s useful,” Dr. Martin said. “And in the end, relatively few new findings are truly novel ones that are useful for patient care.”
So what can a physician do? First, find out what you don’t know
Looking for new findings needs to be carried out systematically, according to William B. Cutrer, MD, MEd, a pediatric intensivist who is associate dean for undergraduate medical education at Vanderbilt University School of Medicine, Nashville, Tenn.
“Before you start, you have to know what you don’t know, and that’s often not so easy,” he said. “You may get a spark about what you don’t know in an encounter with a patient or colleague or through patient outcomes data,” he said.
Dr. Martin, on the other hand, advocates a broad approach that involves finding out at least a little about everything in one’s field. “If you have a good base, you’re not starting from zero when you encounter a new clinical situation,” he said.
“The idea is that you don’t need to memorize most things, but you do need to know how to access them,” Dr. Martin said. “I memorize the things I do all the time, such as dosing or indicated testing, but I look up things that I don’t see that often and ones that have some complexity.”
Updating the old ways
For generations, doctors have stayed current by going to meetings, conversing with colleagues, and reading journals, but many physicians have updated these methods through various resources on the internet.
For example, meetings went virtual during the pandemic, and now that face-to-face meetings are back, many of them retain a virtual option, said Kevin Campbell, MD, a cardiologist at Health First Medical Group, Melbourne, Fla. “I typically go to one or two conferences a year, but I also learn a lot digitally,” he said.
As to journal reading, “assessing an article is an essential skill,” Dr. Cutrer said. “It’s important to quickly decide whether a journal article is worth reading or not. One answer to this problem is to consult summaries of important articles. But summaries are sometimes unhelpful, and it is hard to know which articles are significant. Therefore, doctors have been reaching out to others who can research the articles for them.”
For many years, some physicians have pooled their resources in journal clubs. “You get a chance to cross-cultivate your skills with others,” Dr. Ioannidis said. “But you need someone who is well informed and dedicated to run the journal club, using evidence-based principles.”
Dr. Cutrer said physicians like to cast their net wide because they are understandably wary of changing their practice based on one study. “Unless there is one large study that is really well designed, doctors will need two or more findings to be convinced,” he said. This requires having the ability to match studies across many journals.
Using research summaries
In the past two decades, physicians have gained access to countless summaries of journal articles prepared by armies of clinical experts working for review services such as the New England Journal of Medicine’s “Journal Watch,” Annals of Internal Medicine’s “In the Clinic,” and BMJ’s “State of the Arts.”
In addition to summarizing findings from a wide variety of journals in plain language, reviewers may compare them to similar studies and assess the validity of the finding by assigning a level of evidence.
Some commercial ventures provide similar services. Betsy Jones, executive vice president of clinical decisions at EBSCO, said the DynaMed service is now available through an app on the physician’s smartphone or through the electronic health record.
Physicians like this approach. Many specialists have noted that reading full-length articles was not an efficient use of their time, while even more said that reviews are efficient.
Exchanging information online
Physicians are increasingly keeping current by using the internet, especially on social media, Dr. Cutrer said. “Young doctors in particular are more likely to keep up digitally,” he said.
Internet-based information has become so widespread that disparities in health care from region to region have somewhat abated, according to Stuart J. Fischer, MD, an orthopedic surgeon at Summit Orthopaedics and Sports Medicine, New Jersey. “One positive outcome of this plethora of information today is that geographic disparities in clinical practice are not as great as they used to be,” he said.
Rather than chatting up colleagues in the hallway, many physicians have come to rely on internet-based discussion boards.
Blogs, podcasts, and Twitter
Blogs and podcasts, often focused on a specialty, can be a great way for physicians to keep up, said UMass Chan professor Dr. Martin. “Podcasts in particular have enhanced the ability to stay current,” he said. “You want to find someone you trust.”
Internal medicine podcasts include Annals on Call, where doctors discuss articles in the Annals of Internal Medicine, and the Curbsiders, where two internists interview a guest expert.
Orthopedic surgeons can visit podcasts like Nailed it, Orthobullets, the Ortho Show, and Inside Orthopedics. Neurologists can consult Brainwaves, Neurology Podcast, Practical Neurology Podcast, and Clinical Neurology with KD. And pediatricians can drop in on Talking Pediatrics, The Cribsiders, and PedsCases.
Meanwhile, Twitter has become a particularly effective way to broadcast new findings, speeding up the transition from the bench to the bedside, said Dr. Campbell, the Florida cardiologist.
“I visit cardio-specific resources on Twitter,” he said. “They can be real-time video chats or posted messages. They spur discussion like a journal club. Colleagues present cases and drop in and out of the discussion.”
Others are not as enthusiastic. Although Stanford’s Dr. Ioannidis is in the heart of the Silicon Valley, he is leery of some of the new digital methods. “I don’t use Twitter,” he says. “You just add more people to the process, which could only make things more confusing. I want to be able to think a lot about it.”
Cutting-edge knowledge at the point of care
Consulting the literature often takes place at the point of care, when a particular patient requires treatment. This can be done by using clinical decision support (CDS) and by using clinical practice guidelines (CPGs), which are typically developed by panels of doctors at specialty societies.
“It used to be that the doctor was expected to know everything,” said Ms. Jones at DynaMed. “Today there is no way to keep up with it all. Doctors often need a quick memory jog.”
Ms. Jones said the CDS result always requires the doctor’s interpretation. “It is up to the doctor to decide whether a new finding is the best choice for his or her patient,” she said.
Dr. Martin recommends going easy on point-of-care resources. “They can be used for showing a patient a differential diagnosis list or checking the cost of a procedure, but they are harder to use for novel developments that require time and context to evaluate their impact,” he said.
CPGs, meanwhile, have a high profile in the research world. In a 2018 study, Dr. Ioannidis found that 8 of the 15 most-cited articles were CPGs, disease definitions, or disease statistics.
Dr. Fischer said CPGs are typically based on thorough reviews of the literature, but they do involve experts’ interpretation of the science. “It can be difficult to obtain specific answers to some medical questions, especially for problems with complex treatments or variations,” he said.
As a result, Dr. Fischer said doctors have to use their judgment in applying CPGs to a specific patient. “For example, the orthopedic surgeon would normally recommend a total hip replacement for patients with a bad hip, but it might not be appropriate for an overweight patient.”
Stay skeptical
There are many novel ways for physicians to keep current, including summaries of articles, discussion boards, blogs, podcasts, Twitter, clinical decision support, and clinical practice guidelines.
Even with all these new services, though, doctors need to retain a healthy amount of skepticism about new research findings, Dr. Ioannidis said. “Ask yourself questions such as: Does it deal with a real problem? Am I getting the real information? Is it relevant to real patients? Is it offering good value for money?”
A version of this article first appeared on Medscape.com.
making it much tougher for physicians to identify innovative findings and newer guidelines for helping patients. Yet not keeping up with the latest information can put doctors at risk.
“Most doctors are feeling lost about keeping up to date,” said John P.A. Ioannidis, MD, professor of medicine at Stanford (Calif.) University School of Medicine. “The vast majority of new studies are either wrong or not useful, but physicians cannot sort out which are those studies.”
The sheer number of new studies may even force some doctors to retreat from areas where they have not kept up, said Stephen A. Martin, MD, professor of family medicine and community health at the University of Massachusetts, Worcester. “When doctors don’t feel they can stay current, they may refer more cases to specialists or narrow their focus,” he said.
Some specialties have a greater challenge than others
Dr. Martin said the deluge of studies heavily impacts generalists because they have a wider field of information to keep up with. However, certain specialties like oncology are particularly flooded with new findings.
Specialties with the greatest number of published studies are reportedly oncology, cardiology, and neurology. A 2021 study found that the number of articles with the word “stroke” in them increased five times from 2000 to 2020. And investigative treatments targeting cancer nearly quadrupled just between 2010 and 2020.
What’s more, physicians spend a great deal of time sifting through studies that are ultimately useless. In a survey of internists by Univadis, which is part of WebMD/Medscape, 82% said that fewer than half of the studies they read actually had an impact on how they practice medicine.
“You often have to dig into an article and learn more about a finding before you now whether it’s useful,” Dr. Martin said. “And in the end, relatively few new findings are truly novel ones that are useful for patient care.”
So what can a physician do? First, find out what you don’t know
Looking for new findings needs to be carried out systematically, according to William B. Cutrer, MD, MEd, a pediatric intensivist who is associate dean for undergraduate medical education at Vanderbilt University School of Medicine, Nashville, Tenn.
“Before you start, you have to know what you don’t know, and that’s often not so easy,” he said. “You may get a spark about what you don’t know in an encounter with a patient or colleague or through patient outcomes data,” he said.
Dr. Martin, on the other hand, advocates a broad approach that involves finding out at least a little about everything in one’s field. “If you have a good base, you’re not starting from zero when you encounter a new clinical situation,” he said.
“The idea is that you don’t need to memorize most things, but you do need to know how to access them,” Dr. Martin said. “I memorize the things I do all the time, such as dosing or indicated testing, but I look up things that I don’t see that often and ones that have some complexity.”
Updating the old ways
For generations, doctors have stayed current by going to meetings, conversing with colleagues, and reading journals, but many physicians have updated these methods through various resources on the internet.
For example, meetings went virtual during the pandemic, and now that face-to-face meetings are back, many of them retain a virtual option, said Kevin Campbell, MD, a cardiologist at Health First Medical Group, Melbourne, Fla. “I typically go to one or two conferences a year, but I also learn a lot digitally,” he said.
As to journal reading, “assessing an article is an essential skill,” Dr. Cutrer said. “It’s important to quickly decide whether a journal article is worth reading or not. One answer to this problem is to consult summaries of important articles. But summaries are sometimes unhelpful, and it is hard to know which articles are significant. Therefore, doctors have been reaching out to others who can research the articles for them.”
For many years, some physicians have pooled their resources in journal clubs. “You get a chance to cross-cultivate your skills with others,” Dr. Ioannidis said. “But you need someone who is well informed and dedicated to run the journal club, using evidence-based principles.”
Dr. Cutrer said physicians like to cast their net wide because they are understandably wary of changing their practice based on one study. “Unless there is one large study that is really well designed, doctors will need two or more findings to be convinced,” he said. This requires having the ability to match studies across many journals.
Using research summaries
In the past two decades, physicians have gained access to countless summaries of journal articles prepared by armies of clinical experts working for review services such as the New England Journal of Medicine’s “Journal Watch,” Annals of Internal Medicine’s “In the Clinic,” and BMJ’s “State of the Arts.”
In addition to summarizing findings from a wide variety of journals in plain language, reviewers may compare them to similar studies and assess the validity of the finding by assigning a level of evidence.
Some commercial ventures provide similar services. Betsy Jones, executive vice president of clinical decisions at EBSCO, said the DynaMed service is now available through an app on the physician’s smartphone or through the electronic health record.
Physicians like this approach. Many specialists have noted that reading full-length articles was not an efficient use of their time, while even more said that reviews are efficient.
Exchanging information online
Physicians are increasingly keeping current by using the internet, especially on social media, Dr. Cutrer said. “Young doctors in particular are more likely to keep up digitally,” he said.
Internet-based information has become so widespread that disparities in health care from region to region have somewhat abated, according to Stuart J. Fischer, MD, an orthopedic surgeon at Summit Orthopaedics and Sports Medicine, New Jersey. “One positive outcome of this plethora of information today is that geographic disparities in clinical practice are not as great as they used to be,” he said.
Rather than chatting up colleagues in the hallway, many physicians have come to rely on internet-based discussion boards.
Blogs, podcasts, and Twitter
Blogs and podcasts, often focused on a specialty, can be a great way for physicians to keep up, said UMass Chan professor Dr. Martin. “Podcasts in particular have enhanced the ability to stay current,” he said. “You want to find someone you trust.”
Internal medicine podcasts include Annals on Call, where doctors discuss articles in the Annals of Internal Medicine, and the Curbsiders, where two internists interview a guest expert.
Orthopedic surgeons can visit podcasts like Nailed it, Orthobullets, the Ortho Show, and Inside Orthopedics. Neurologists can consult Brainwaves, Neurology Podcast, Practical Neurology Podcast, and Clinical Neurology with KD. And pediatricians can drop in on Talking Pediatrics, The Cribsiders, and PedsCases.
Meanwhile, Twitter has become a particularly effective way to broadcast new findings, speeding up the transition from the bench to the bedside, said Dr. Campbell, the Florida cardiologist.
“I visit cardio-specific resources on Twitter,” he said. “They can be real-time video chats or posted messages. They spur discussion like a journal club. Colleagues present cases and drop in and out of the discussion.”
Others are not as enthusiastic. Although Stanford’s Dr. Ioannidis is in the heart of the Silicon Valley, he is leery of some of the new digital methods. “I don’t use Twitter,” he says. “You just add more people to the process, which could only make things more confusing. I want to be able to think a lot about it.”
Cutting-edge knowledge at the point of care
Consulting the literature often takes place at the point of care, when a particular patient requires treatment. This can be done by using clinical decision support (CDS) and by using clinical practice guidelines (CPGs), which are typically developed by panels of doctors at specialty societies.
“It used to be that the doctor was expected to know everything,” said Ms. Jones at DynaMed. “Today there is no way to keep up with it all. Doctors often need a quick memory jog.”
Ms. Jones said the CDS result always requires the doctor’s interpretation. “It is up to the doctor to decide whether a new finding is the best choice for his or her patient,” she said.
Dr. Martin recommends going easy on point-of-care resources. “They can be used for showing a patient a differential diagnosis list or checking the cost of a procedure, but they are harder to use for novel developments that require time and context to evaluate their impact,” he said.
CPGs, meanwhile, have a high profile in the research world. In a 2018 study, Dr. Ioannidis found that 8 of the 15 most-cited articles were CPGs, disease definitions, or disease statistics.
Dr. Fischer said CPGs are typically based on thorough reviews of the literature, but they do involve experts’ interpretation of the science. “It can be difficult to obtain specific answers to some medical questions, especially for problems with complex treatments or variations,” he said.
As a result, Dr. Fischer said doctors have to use their judgment in applying CPGs to a specific patient. “For example, the orthopedic surgeon would normally recommend a total hip replacement for patients with a bad hip, but it might not be appropriate for an overweight patient.”
Stay skeptical
There are many novel ways for physicians to keep current, including summaries of articles, discussion boards, blogs, podcasts, Twitter, clinical decision support, and clinical practice guidelines.
Even with all these new services, though, doctors need to retain a healthy amount of skepticism about new research findings, Dr. Ioannidis said. “Ask yourself questions such as: Does it deal with a real problem? Am I getting the real information? Is it relevant to real patients? Is it offering good value for money?”
A version of this article first appeared on Medscape.com.
making it much tougher for physicians to identify innovative findings and newer guidelines for helping patients. Yet not keeping up with the latest information can put doctors at risk.
“Most doctors are feeling lost about keeping up to date,” said John P.A. Ioannidis, MD, professor of medicine at Stanford (Calif.) University School of Medicine. “The vast majority of new studies are either wrong or not useful, but physicians cannot sort out which are those studies.”
The sheer number of new studies may even force some doctors to retreat from areas where they have not kept up, said Stephen A. Martin, MD, professor of family medicine and community health at the University of Massachusetts, Worcester. “When doctors don’t feel they can stay current, they may refer more cases to specialists or narrow their focus,” he said.
Some specialties have a greater challenge than others
Dr. Martin said the deluge of studies heavily impacts generalists because they have a wider field of information to keep up with. However, certain specialties like oncology are particularly flooded with new findings.
Specialties with the greatest number of published studies are reportedly oncology, cardiology, and neurology. A 2021 study found that the number of articles with the word “stroke” in them increased five times from 2000 to 2020. And investigative treatments targeting cancer nearly quadrupled just between 2010 and 2020.
What’s more, physicians spend a great deal of time sifting through studies that are ultimately useless. In a survey of internists by Univadis, which is part of WebMD/Medscape, 82% said that fewer than half of the studies they read actually had an impact on how they practice medicine.
“You often have to dig into an article and learn more about a finding before you now whether it’s useful,” Dr. Martin said. “And in the end, relatively few new findings are truly novel ones that are useful for patient care.”
So what can a physician do? First, find out what you don’t know
Looking for new findings needs to be carried out systematically, according to William B. Cutrer, MD, MEd, a pediatric intensivist who is associate dean for undergraduate medical education at Vanderbilt University School of Medicine, Nashville, Tenn.
“Before you start, you have to know what you don’t know, and that’s often not so easy,” he said. “You may get a spark about what you don’t know in an encounter with a patient or colleague or through patient outcomes data,” he said.
Dr. Martin, on the other hand, advocates a broad approach that involves finding out at least a little about everything in one’s field. “If you have a good base, you’re not starting from zero when you encounter a new clinical situation,” he said.
“The idea is that you don’t need to memorize most things, but you do need to know how to access them,” Dr. Martin said. “I memorize the things I do all the time, such as dosing or indicated testing, but I look up things that I don’t see that often and ones that have some complexity.”
Updating the old ways
For generations, doctors have stayed current by going to meetings, conversing with colleagues, and reading journals, but many physicians have updated these methods through various resources on the internet.
For example, meetings went virtual during the pandemic, and now that face-to-face meetings are back, many of them retain a virtual option, said Kevin Campbell, MD, a cardiologist at Health First Medical Group, Melbourne, Fla. “I typically go to one or two conferences a year, but I also learn a lot digitally,” he said.
As to journal reading, “assessing an article is an essential skill,” Dr. Cutrer said. “It’s important to quickly decide whether a journal article is worth reading or not. One answer to this problem is to consult summaries of important articles. But summaries are sometimes unhelpful, and it is hard to know which articles are significant. Therefore, doctors have been reaching out to others who can research the articles for them.”
For many years, some physicians have pooled their resources in journal clubs. “You get a chance to cross-cultivate your skills with others,” Dr. Ioannidis said. “But you need someone who is well informed and dedicated to run the journal club, using evidence-based principles.”
Dr. Cutrer said physicians like to cast their net wide because they are understandably wary of changing their practice based on one study. “Unless there is one large study that is really well designed, doctors will need two or more findings to be convinced,” he said. This requires having the ability to match studies across many journals.
Using research summaries
In the past two decades, physicians have gained access to countless summaries of journal articles prepared by armies of clinical experts working for review services such as the New England Journal of Medicine’s “Journal Watch,” Annals of Internal Medicine’s “In the Clinic,” and BMJ’s “State of the Arts.”
In addition to summarizing findings from a wide variety of journals in plain language, reviewers may compare them to similar studies and assess the validity of the finding by assigning a level of evidence.
Some commercial ventures provide similar services. Betsy Jones, executive vice president of clinical decisions at EBSCO, said the DynaMed service is now available through an app on the physician’s smartphone or through the electronic health record.
Physicians like this approach. Many specialists have noted that reading full-length articles was not an efficient use of their time, while even more said that reviews are efficient.
Exchanging information online
Physicians are increasingly keeping current by using the internet, especially on social media, Dr. Cutrer said. “Young doctors in particular are more likely to keep up digitally,” he said.
Internet-based information has become so widespread that disparities in health care from region to region have somewhat abated, according to Stuart J. Fischer, MD, an orthopedic surgeon at Summit Orthopaedics and Sports Medicine, New Jersey. “One positive outcome of this plethora of information today is that geographic disparities in clinical practice are not as great as they used to be,” he said.
Rather than chatting up colleagues in the hallway, many physicians have come to rely on internet-based discussion boards.
Blogs, podcasts, and Twitter
Blogs and podcasts, often focused on a specialty, can be a great way for physicians to keep up, said UMass Chan professor Dr. Martin. “Podcasts in particular have enhanced the ability to stay current,” he said. “You want to find someone you trust.”
Internal medicine podcasts include Annals on Call, where doctors discuss articles in the Annals of Internal Medicine, and the Curbsiders, where two internists interview a guest expert.
Orthopedic surgeons can visit podcasts like Nailed it, Orthobullets, the Ortho Show, and Inside Orthopedics. Neurologists can consult Brainwaves, Neurology Podcast, Practical Neurology Podcast, and Clinical Neurology with KD. And pediatricians can drop in on Talking Pediatrics, The Cribsiders, and PedsCases.
Meanwhile, Twitter has become a particularly effective way to broadcast new findings, speeding up the transition from the bench to the bedside, said Dr. Campbell, the Florida cardiologist.
“I visit cardio-specific resources on Twitter,” he said. “They can be real-time video chats or posted messages. They spur discussion like a journal club. Colleagues present cases and drop in and out of the discussion.”
Others are not as enthusiastic. Although Stanford’s Dr. Ioannidis is in the heart of the Silicon Valley, he is leery of some of the new digital methods. “I don’t use Twitter,” he says. “You just add more people to the process, which could only make things more confusing. I want to be able to think a lot about it.”
Cutting-edge knowledge at the point of care
Consulting the literature often takes place at the point of care, when a particular patient requires treatment. This can be done by using clinical decision support (CDS) and by using clinical practice guidelines (CPGs), which are typically developed by panels of doctors at specialty societies.
“It used to be that the doctor was expected to know everything,” said Ms. Jones at DynaMed. “Today there is no way to keep up with it all. Doctors often need a quick memory jog.”
Ms. Jones said the CDS result always requires the doctor’s interpretation. “It is up to the doctor to decide whether a new finding is the best choice for his or her patient,” she said.
Dr. Martin recommends going easy on point-of-care resources. “They can be used for showing a patient a differential diagnosis list or checking the cost of a procedure, but they are harder to use for novel developments that require time and context to evaluate their impact,” he said.
CPGs, meanwhile, have a high profile in the research world. In a 2018 study, Dr. Ioannidis found that 8 of the 15 most-cited articles were CPGs, disease definitions, or disease statistics.
Dr. Fischer said CPGs are typically based on thorough reviews of the literature, but they do involve experts’ interpretation of the science. “It can be difficult to obtain specific answers to some medical questions, especially for problems with complex treatments or variations,” he said.
As a result, Dr. Fischer said doctors have to use their judgment in applying CPGs to a specific patient. “For example, the orthopedic surgeon would normally recommend a total hip replacement for patients with a bad hip, but it might not be appropriate for an overweight patient.”
Stay skeptical
There are many novel ways for physicians to keep current, including summaries of articles, discussion boards, blogs, podcasts, Twitter, clinical decision support, and clinical practice guidelines.
Even with all these new services, though, doctors need to retain a healthy amount of skepticism about new research findings, Dr. Ioannidis said. “Ask yourself questions such as: Does it deal with a real problem? Am I getting the real information? Is it relevant to real patients? Is it offering good value for money?”
A version of this article first appeared on Medscape.com.