User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Hairdressers have ‘excess risk’ of contact allergies
.
“Research has shown that up to 70% of hairdressers suffer from work-related skin damage, mostly hand dermatitis, at some point during their career,” write Wolfgang Uter of Friedrich-Alexander University Erlangen-Nürnberg and coauthors. In general, they write, occupational skin diseases such as hand dermatitis represent up to 35% of reported occupational diseases. The study was published online in Contact Dermatitis.
Wet work and skin contact with detergents and hairdressing chemicals are top risk factors for developing occupational skin disease in this population, according to the researchers.
To further understand the burden of occupational contact allergy in hairdressers, the investigators gathered evidence published since 2000 on contact allergies to hair cosmetic chemicals. They searched the literature for nine substances selected beforehand by experts and stakeholders. The researchers also examined the prevalence of sensitization between hairdressers and other individuals given skin patch tests.
Substance by substance
Common potentially sensitizing cosmetic ingredients reported across studies included p-phenylenediamine (PPD), persulfates (mostly ammonium persulfate [APS]), glyceryl thioglycolate (GMTG), and ammonium thioglycolate (ATG).
In a pooled analysis, the overall prevalence of contact allergy to PPD was 4.3% in consecutively patch-tested patients, but in hairdressers specifically, the overall prevalence of contact allergy to this ingredient was 28.6%, reviewers reported.
The pooled prevalence of contact allergy to APS was 5.5% in consumers and 17.2% in hairdressers. In other review studies, contact allergy risks to APS, GMTG, and ATG were also elevated in hairdressers compared with all controls.
The calculated relative risk (RR) of contact allergy to PPD was approximately 5.4 higher for hairdressers, while the RR for ATG sensitization was 3.4 in hairdressers compared with consumers.
Commenting on these findings, James A. Yiannias, MD, professor of dermatology at the Mayo Medical School, Phoenix, told this news organization in an email that many providers and patients are concerned only about hair dye molecules such as PPD and aminophenol, as well as permanent, wave, and straightening chemicals such as GMTG.
“Although these are common allergens in hairdressers, allergens such as fragrances and some preservatives found in daily hair care products such as shampoos, conditioners, and hair sprays are also common causes of contact dermatitis,” said Dr. Yiannias, who wasn’t involved in the research.
Consequences of exposure
Dr. Yiannias explained that progressive worsening of the dermatitis can occur with ongoing allergen exposure and, if not properly mitigated, can lead to bigger issues. “Initial nuisances of mild irritation and hyperkeratosis can evolve to a state of fissuring with the risk of bleeding and significant pain,” he said.
But once severe and untreated dermatitis occurs, Dr. Yiannias said that hairdressers “may need to change careers” or at least face short- or long-term unemployment.
The researchers suggest reducing exposure to the allergen is key for prevention of symptoms, adding that adequate guidance on the safe use of new products is needed. Also, the researchers suggested that vocational schools should more rigorously implement education for hairdressers that addresses how to protect the skin appropriately at work.
“Hairdressers are taught during their training to be cautious about allergen exposure by avoiding touching high-risk ingredients such as hair dyes,” Dr. Yiannias added. “However, in practice, this is very difficult since the wearing of gloves can impair the tactile sensations that hairdressers often feel is essential in performing their job.”
The study received no industry funding. Dr. Yiannias reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
.
“Research has shown that up to 70% of hairdressers suffer from work-related skin damage, mostly hand dermatitis, at some point during their career,” write Wolfgang Uter of Friedrich-Alexander University Erlangen-Nürnberg and coauthors. In general, they write, occupational skin diseases such as hand dermatitis represent up to 35% of reported occupational diseases. The study was published online in Contact Dermatitis.
Wet work and skin contact with detergents and hairdressing chemicals are top risk factors for developing occupational skin disease in this population, according to the researchers.
To further understand the burden of occupational contact allergy in hairdressers, the investigators gathered evidence published since 2000 on contact allergies to hair cosmetic chemicals. They searched the literature for nine substances selected beforehand by experts and stakeholders. The researchers also examined the prevalence of sensitization between hairdressers and other individuals given skin patch tests.
Substance by substance
Common potentially sensitizing cosmetic ingredients reported across studies included p-phenylenediamine (PPD), persulfates (mostly ammonium persulfate [APS]), glyceryl thioglycolate (GMTG), and ammonium thioglycolate (ATG).
In a pooled analysis, the overall prevalence of contact allergy to PPD was 4.3% in consecutively patch-tested patients, but in hairdressers specifically, the overall prevalence of contact allergy to this ingredient was 28.6%, reviewers reported.
The pooled prevalence of contact allergy to APS was 5.5% in consumers and 17.2% in hairdressers. In other review studies, contact allergy risks to APS, GMTG, and ATG were also elevated in hairdressers compared with all controls.
The calculated relative risk (RR) of contact allergy to PPD was approximately 5.4 higher for hairdressers, while the RR for ATG sensitization was 3.4 in hairdressers compared with consumers.
Commenting on these findings, James A. Yiannias, MD, professor of dermatology at the Mayo Medical School, Phoenix, told this news organization in an email that many providers and patients are concerned only about hair dye molecules such as PPD and aminophenol, as well as permanent, wave, and straightening chemicals such as GMTG.
“Although these are common allergens in hairdressers, allergens such as fragrances and some preservatives found in daily hair care products such as shampoos, conditioners, and hair sprays are also common causes of contact dermatitis,” said Dr. Yiannias, who wasn’t involved in the research.
Consequences of exposure
Dr. Yiannias explained that progressive worsening of the dermatitis can occur with ongoing allergen exposure and, if not properly mitigated, can lead to bigger issues. “Initial nuisances of mild irritation and hyperkeratosis can evolve to a state of fissuring with the risk of bleeding and significant pain,” he said.
But once severe and untreated dermatitis occurs, Dr. Yiannias said that hairdressers “may need to change careers” or at least face short- or long-term unemployment.
The researchers suggest reducing exposure to the allergen is key for prevention of symptoms, adding that adequate guidance on the safe use of new products is needed. Also, the researchers suggested that vocational schools should more rigorously implement education for hairdressers that addresses how to protect the skin appropriately at work.
“Hairdressers are taught during their training to be cautious about allergen exposure by avoiding touching high-risk ingredients such as hair dyes,” Dr. Yiannias added. “However, in practice, this is very difficult since the wearing of gloves can impair the tactile sensations that hairdressers often feel is essential in performing their job.”
The study received no industry funding. Dr. Yiannias reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
.
“Research has shown that up to 70% of hairdressers suffer from work-related skin damage, mostly hand dermatitis, at some point during their career,” write Wolfgang Uter of Friedrich-Alexander University Erlangen-Nürnberg and coauthors. In general, they write, occupational skin diseases such as hand dermatitis represent up to 35% of reported occupational diseases. The study was published online in Contact Dermatitis.
Wet work and skin contact with detergents and hairdressing chemicals are top risk factors for developing occupational skin disease in this population, according to the researchers.
To further understand the burden of occupational contact allergy in hairdressers, the investigators gathered evidence published since 2000 on contact allergies to hair cosmetic chemicals. They searched the literature for nine substances selected beforehand by experts and stakeholders. The researchers also examined the prevalence of sensitization between hairdressers and other individuals given skin patch tests.
Substance by substance
Common potentially sensitizing cosmetic ingredients reported across studies included p-phenylenediamine (PPD), persulfates (mostly ammonium persulfate [APS]), glyceryl thioglycolate (GMTG), and ammonium thioglycolate (ATG).
In a pooled analysis, the overall prevalence of contact allergy to PPD was 4.3% in consecutively patch-tested patients, but in hairdressers specifically, the overall prevalence of contact allergy to this ingredient was 28.6%, reviewers reported.
The pooled prevalence of contact allergy to APS was 5.5% in consumers and 17.2% in hairdressers. In other review studies, contact allergy risks to APS, GMTG, and ATG were also elevated in hairdressers compared with all controls.
The calculated relative risk (RR) of contact allergy to PPD was approximately 5.4 higher for hairdressers, while the RR for ATG sensitization was 3.4 in hairdressers compared with consumers.
Commenting on these findings, James A. Yiannias, MD, professor of dermatology at the Mayo Medical School, Phoenix, told this news organization in an email that many providers and patients are concerned only about hair dye molecules such as PPD and aminophenol, as well as permanent, wave, and straightening chemicals such as GMTG.
“Although these are common allergens in hairdressers, allergens such as fragrances and some preservatives found in daily hair care products such as shampoos, conditioners, and hair sprays are also common causes of contact dermatitis,” said Dr. Yiannias, who wasn’t involved in the research.
Consequences of exposure
Dr. Yiannias explained that progressive worsening of the dermatitis can occur with ongoing allergen exposure and, if not properly mitigated, can lead to bigger issues. “Initial nuisances of mild irritation and hyperkeratosis can evolve to a state of fissuring with the risk of bleeding and significant pain,” he said.
But once severe and untreated dermatitis occurs, Dr. Yiannias said that hairdressers “may need to change careers” or at least face short- or long-term unemployment.
The researchers suggest reducing exposure to the allergen is key for prevention of symptoms, adding that adequate guidance on the safe use of new products is needed. Also, the researchers suggested that vocational schools should more rigorously implement education for hairdressers that addresses how to protect the skin appropriately at work.
“Hairdressers are taught during their training to be cautious about allergen exposure by avoiding touching high-risk ingredients such as hair dyes,” Dr. Yiannias added. “However, in practice, this is very difficult since the wearing of gloves can impair the tactile sensations that hairdressers often feel is essential in performing their job.”
The study received no industry funding. Dr. Yiannias reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cutaneous and Subcutaneous Perineuriomas in 2 Pediatric Patients
Perineuriomas are benign, slow-growing tumors derived from perineurial cells,1 which form the structurally supportive perineurium that surrounds individual nerve fascicles.2,3 Perineuriomas are classified into 2 main forms: intraneural or extraneural.4 Intraneural perineuriomas are found within the border of the peripheral nerve,5 while extraneural perineuriomas usually are found in soft tissue and skin. Extraneural perineuriomas can be further classified into variants based on their histologic appearance, including reticular, sclerosing, and plexiform subtypes. Extraneural perineuriomas usually present on the extremities or trunk of young to middle-aged adults as a well-circumscribed, painless, subcutaneous masses.1 These tumors are especially unusual in children.4 We present 2 extraneural perineurioma cases in children, and we review the pertinent diagnostic features of perineurioma as well as the presentation in the pediatric population.
Case Reports
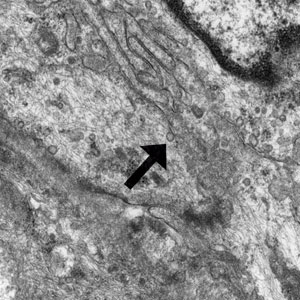
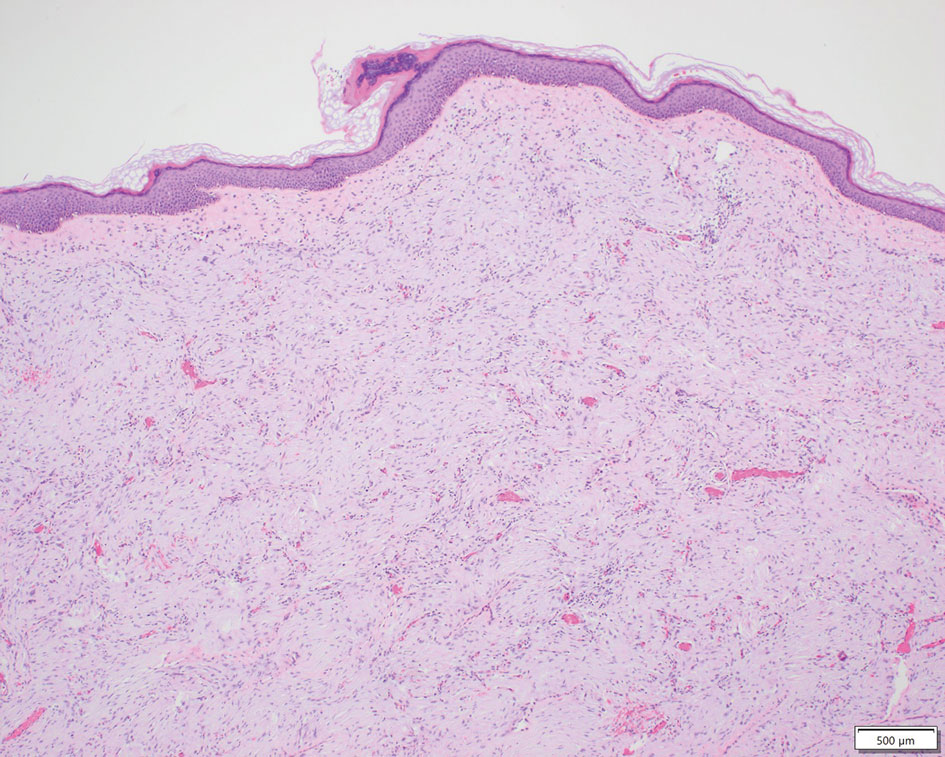
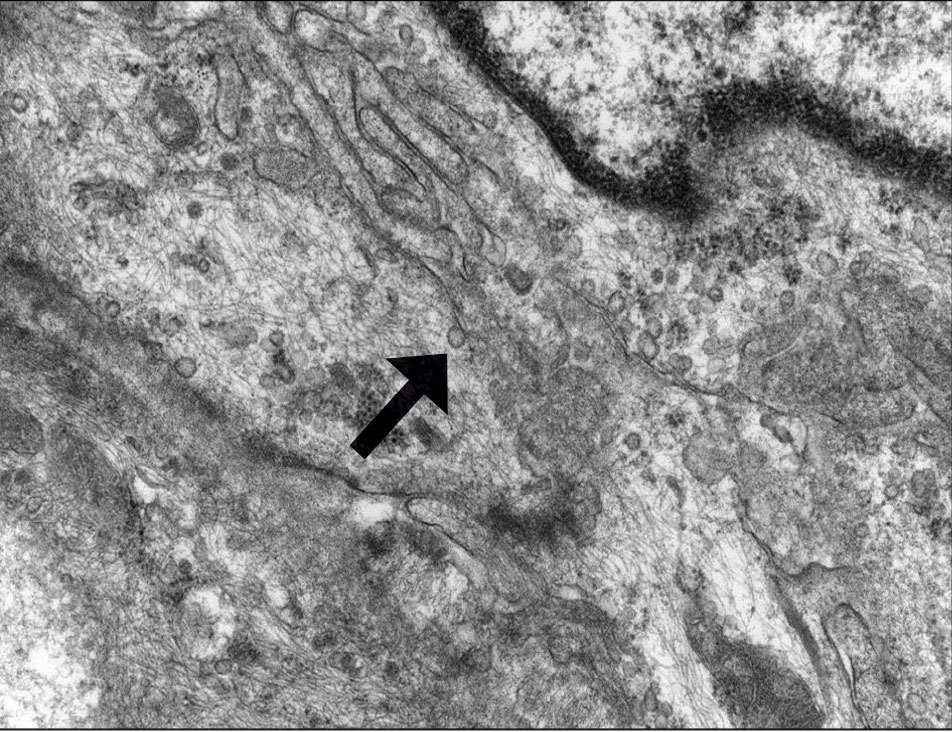
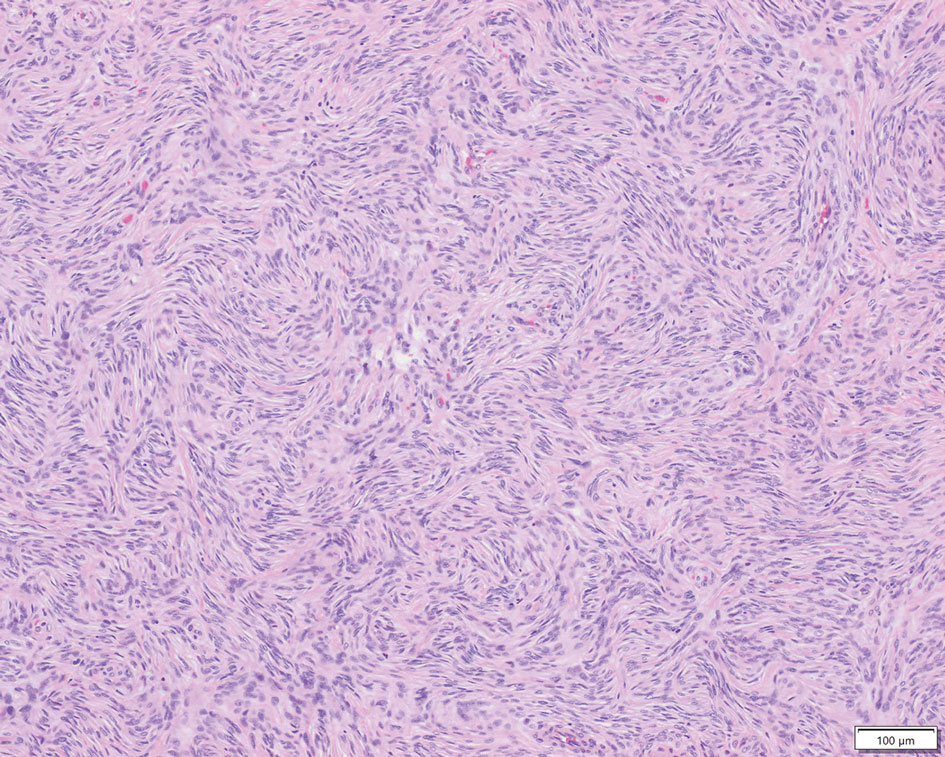
Patient 1—A 10-year-old boy with a history of cerebral palsy and related comorbidities presented to the clinic for evaluation of a lesion on the thigh with no associated pain, irritation, erythema, or drainage. Physical examination revealed a soft, pedunculated, mobile nodule on the right medial thigh. An elliptical excision was performed. Gross examination demonstrated a 2.0×2.0×1.8-cm polypoid nodule. Histologic examination showed a dermal-based proliferation of bland spindle cells (Figure 1). The cytomorphology was characterized by elongated tapering nuclei and many areas with delicate bipolar cytoplasmic processes. The constituent cells were arranged in a whorled pattern in a variably myxoid to collagenous stroma. The tumor cells were multifocally positive for CD34; focally positive for smooth muscle actin (SMA); and negative for S-100, epithelial membrane antigen (EMA), GLUT1, claudin-1, STAT6, and desmin. Rb protein was intact. The CD34 immunostain highlighted the cytoplasmic processes. Electron microscopy was performed because the immunohistochemical results were nonspecific despite the favorable histologic features for perineurioma and showed pinocytic vesicles with delicate cytoplasmic processes, characteristic of perineurioma (Figure 2). Follow-up visits were related to the management of multiple comorbidities; no known recurrence of the lesion was documented.
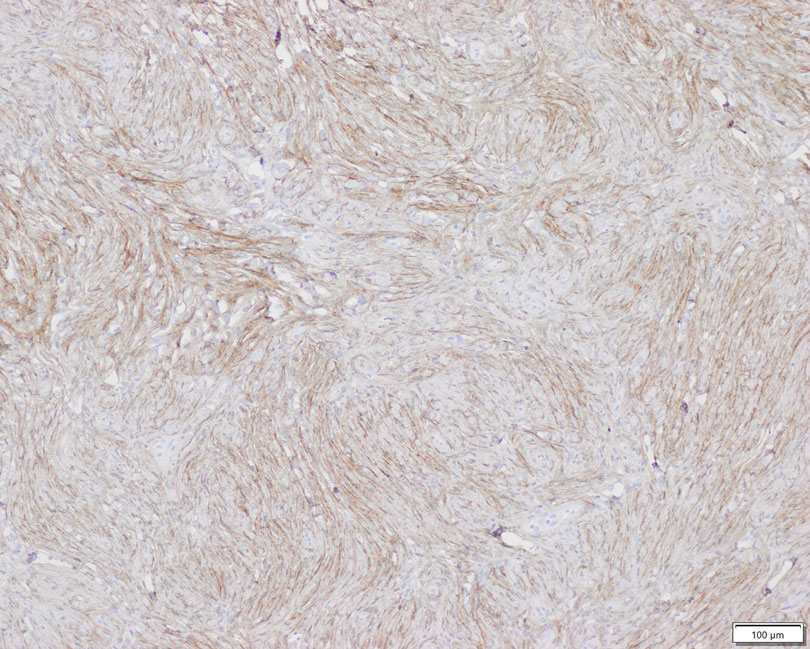
Patient 2—A 15-year-old adolescent boy with no notable medical history presented to the pediatric clinic for a bump on the right upper arm of 4 to 5 months’ duration. He did not recall an injury to the area and denied change in size, redness, bruising, or pain of the lesion. Ultrasonography demonstrated a 2.6×2.3×1.3-cm hypoechoic and slightly heterogeneous, well-circumscribed, subcutaneous mass with internal vascularity. The patient was then referred to a pediatric surgeon. The clinical differential included a lipoma, lymphadenopathy, or sebaceous cyst. An excision was performed. Gross inspection demonstrated a 7-g, 2.8×2.6×1.8-cm, homogeneous, tan-pink, rubbery nodule with minimal surrounding soft tissue. Histologic examination showed a bland proliferation of spindle cells with storiform and whorled patterns (Figure 3). No notable nuclear atypia or necrosis was identified. The tumor cells were focally positive for EMA (Figure 4), claudin-1, and CD34 and negative for S-100, SOX10, GLUT1, desmin, STAT6, pankeratin AE1/AE3, and SMA. The diagnosis of perineurioma was rendered. No recurrence of the lesion was appreciated clinically on a 6-month follow-up examination.
Comment
Characteristics of Perineuriomas—On gross evaluation, perineuriomas are firm, gray-white, and well circumscribed but not encapsulated. Histologically, perineuriomas can have a storiform, whorled, or lamellar pattern of spindle cells. Perivascular whorls can be a histologic clue. The spindle cells are bland appearing and typically are elongated and slender but can appear slightly ovoid and plump. The background stroma can be myxoid, collagenous, or mixed. There usually is no atypia, and mitotic figures are rare.2,3,6,7 Intraneural perineuriomas vary architecturally in that they display a unique onion bulb–like appearance in which whorls of cytoplasmic material of variable sizes surround central axons.3
Diagnosis—The diagnosis of perineuriomas usually requires characteristic immunohistochemical and sometimes ultrastructural features. Perineuriomas are positive for EMA and GLUT1 and variable for CD34.6 Approximately 20% to 91% will be positive for claudin-1, a tight junction protein associated with perineuriomas.8 Of note, EMA and GLUT1 usually are positive in both neoplastic and nonneoplastic perineurial cells.9,10 Occasionally, these tumors can be focally positive for SMA and negative for S-100 and glial fibrillary acidic protein. The bipolar, thin, delicate, cytoplasmic processes with long-tapering nuclei may be easier to appreciate on electron microscopy than on conventional light microscopy. In addition, the cells contain pinocytotic vesicles and a discontinuous external lamina, which may be helpful for diagnosis.10
Genetics—Genetic alterations in perineurioma continue to be elucidated. Although many soft tissue perineuriomas possess deletion of chromosome 22q material, this is not a consistent finding and is not pathognomonic. Notably, the NF2 tumor suppressor gene is found on chromosome 22.11 For the sclerosing variant of perineurioma, rearrangements or deletions of chromosome 10q have been described. A study of 14 soft tissue/extraneural perineuriomas using whole-exome sequencing and single nucleotide polymorphism array showed 6 cases of recurrent chromosome 22q deletions containing the NF2 locus and 4 cases with a previously unreported finding of chromosome 17q deletions containing the NF1 locus that were mutually exclusive events in all but 1 case.12 Although perineuriomas can harbor NF1 or NF2 mutations, perineuriomas are not considered to be associated with neurofibromatosis type 1 or 2 (NF1 or NF2, respectively). Patients with NF1 or NF2 and perineurioma are exceedingly rare. One pediatric patient with both soft tissue perineurioma and NF1 has been reported in the literature.13
Differential Diagnosis—Perineuriomas should be distinguished from other benign neural neoplasms of the skin and soft tissue. Commonly considered in the differential diagnosis is schwannoma and neurofibroma. Schwannomas are encapsulated epineurial nerve sheath tumors comprised of a neoplastic proliferation of Schwann cells. Schwannomas morphologically differ from perineuriomas because of the presence of the hypercellular Antoni A with Verocay bodies and the hypocellular myxoid Antoni B patterns of spindle cells with elongated wavy nuclei and tapered ends. Other features include hyalinized vessels, hemosiderin deposition, cystic degeneration, and/or degenerative atypia.3,14 Importantly, the constituent cells of schwannomas are positive for S-100 and SOX10 and negative for EMA.3 Neurofibromas consist of fascicles and whorls of Schwann cells in a background myxoid stroma with scattered mast cells, lymphocytes, fibroblasts, and perineurial cells. Similar to schwannomas, neurofibromas also are positive for S-100 and negative for EMA.3,14 Neurofibromas can have either a somatic or germline mutation of the biallelic NF1 gene on chromosome 17q11.2 with subsequent loss of protein neurofibromin activity.15 Less common but still a consideration are the hybrid peripheral nerve sheath tumors that may present with a biphasic or intermingled morphology. Combinations include neurofibroma-schwannoma, schwannoma-perineurioma, and neurofibroma-perineurioma. The hybrid schwannoma-perineurioma has a mixture of thin and plump spindle cells with tapered nuclei as well as patchy S-100 positivity corresponding to schwannian areas. Similarly, S-100 will highlight the wavy Schwann cells in neurofibroma-perineurioma as well as CD34-highlighting fibroblasts.7,15 In both aforementioned hybrid tumors, EMA will be positive in the perineurial areas. Another potential diagnostic consideration that can occur in both pediatric and adult populations is dermatofibrosarcoma protuberans (DFSP), which is comprised of a dermal proliferation of monomorphic fusiform spindle cells. Although both perineuriomas and DFSP can have a storiform architecture, DFSP is more asymmetric and infiltrative. Dermatofibrosarcoma protuberans is recognized in areas of individual adipocyte trapping, referred to as honeycombing. Dermatofibrosarcoma protuberans typically does not express EMA, though the sclerosing variant of DFSP has been reported to sometimes demonstrate focal EMA reactivity.11,14,16 For morphologically challenging cases, cytogenetic studies will show t(17;22) translocation fusing the COL1A1 and PDGFRB genes.16 Finally, for subcutaneous or deep-seated tumors, one also may consider other mesenchymal neoplasms, including solitary fibrous tumor, low-grade fibromyxoid sarcoma, or low-grade malignant peripheral nerve sheath tumor (MPNST).11
Management—Perineuriomas are considered benign. The presence of mitotic figures, pleomorphism, and degenerative nuclear atypia akin to ancient change, as seen in ancient schwannoma, does not affect their benign clinical behavior. Treatment of a perineurioma typically is surgical excision with conservative margins and minimal chance of recurrence.1,11 So-called malignant perineuriomas are better classified as MPNSTs with perineural differentiation or perineurial MPNST. They also are positive for EMA and may be distinguished from perineurioma by the presence of major atypia and an infiltrative growth pattern.17,18
Considerations in the Pediatric Population—Few pediatric soft tissue perineuriomas have been reported. A clinicopathologic analysis by Hornick and Fletcher1 of patients with soft tissue perineurioma showed that only 6 of 81 patients were younger than 20 years. The youngest reported case of perineurioma occurred as an extraneural perineurioma on the scalp in an infant.19 Only 1 soft tissue perineural MPNST has been reported in the pediatric population, arising on the face of an 11-year-old boy. In a case series of 11 pediatric perineuriomas, including extraneural and intraneural, there was no evidence of recurrence or metastasis at follow-up.4
Conclusion
Perineuriomas are rare benign peripheral nerve sheath tumors with unique histologic and immunohistochemical features. Soft tissue perineuriomas in the pediatric population are an important diagnostic consideration, especially for the pediatrician or dermatologist when encountering a well-circumscribed nodular soft tissue lesion of the extremity or when encountering a neural-appearing tumor in the subcutaneous tissue.
Acknowledgment—We would like to thank Christopher Fletcher, MD (Boston, Massachusetts), for his expertise in outside consultation for patient 1.
- Hornick J, Fletcher C. Soft tissue perineurioma. Am J Surg Pathol. 2005;29:845-858.
- Tsang WY, Chan JK, Chow LT, et al. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756-763.
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Balarezo FS, Muller RC, Weiss RG, et al. Soft tissue perineuriomas in children: report of three cases and review of the literature. Pediatr Dev Pathol. 2003;6:137-141. Published correction appears in Pediatr Dev Pathol. 2003;6:following 364.
- Macarenco R, Ellinger F, Oliveira A. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625-636.
- Agaimy A, Buslei R, Coras R, et al. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60-70.
- Greenson JK, Hornick JL, Longacre TA, et al. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer; 2015.
- Folpe A, Billings S, McKenney J, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620-1626.
- Hirose T, Tani T, Shimada T, et al. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293-298.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Perineurioma. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013:176-178.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Saunders; 2013.
- Carter JM, Wu Y, Blessing MM, et al. Recurrent genomic alterations in soft tissue perineuriomas. Am J Surg Pathol. 2018;42:1708-1714.
- Al-Adnani M. Soft tissue perineurioma in a child with neurofibromatosis type 1: a case report and review of the literature. Pediatr Dev Pathol. 2017;20:444-448.
- Reddy VB, David O, Spitz DJ, et al. Gattuso’s Differential Diagnosis in Surgical Pathology. Elsevier Saunders; 2022.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Abdaljaleel MY, North JP. Sclerosing dermatofibrosarcoma protuberans shows significant overlap with sclerotic fibroma in both routine and immunohistochemical analysis: a potential diagnostic pitfall. Am J Dermatopathol. 2017;39:83-88.
- Rosenberg AS, Langee CL, Stevens GL, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma.” J Cutan Pathol. 2002;29:362-367.
- Mitchell A, Scheithauer BW, Doyon J, et al. Malignant perineurioma (malignant peripheral nerve sheath tumor with perineural differentiation). Clin Neuropathol. 2012;31:424-429.
- Duhan A, Rana P, Beniwal K, et al. Perineurioma of scalp in an infant: a case report with short review of literature. Asian J Neurosurg. 2016;11:81-83.
Perineuriomas are benign, slow-growing tumors derived from perineurial cells,1 which form the structurally supportive perineurium that surrounds individual nerve fascicles.2,3 Perineuriomas are classified into 2 main forms: intraneural or extraneural.4 Intraneural perineuriomas are found within the border of the peripheral nerve,5 while extraneural perineuriomas usually are found in soft tissue and skin. Extraneural perineuriomas can be further classified into variants based on their histologic appearance, including reticular, sclerosing, and plexiform subtypes. Extraneural perineuriomas usually present on the extremities or trunk of young to middle-aged adults as a well-circumscribed, painless, subcutaneous masses.1 These tumors are especially unusual in children.4 We present 2 extraneural perineurioma cases in children, and we review the pertinent diagnostic features of perineurioma as well as the presentation in the pediatric population.

Case Reports
Patient 1—A 10-year-old boy with a history of cerebral palsy and related comorbidities presented to the clinic for evaluation of a lesion on the thigh with no associated pain, irritation, erythema, or drainage. Physical examination revealed a soft, pedunculated, mobile nodule on the right medial thigh. An elliptical excision was performed. Gross examination demonstrated a 2.0×2.0×1.8-cm polypoid nodule. Histologic examination showed a dermal-based proliferation of bland spindle cells (Figure 1). The cytomorphology was characterized by elongated tapering nuclei and many areas with delicate bipolar cytoplasmic processes. The constituent cells were arranged in a whorled pattern in a variably myxoid to collagenous stroma. The tumor cells were multifocally positive for CD34; focally positive for smooth muscle actin (SMA); and negative for S-100, epithelial membrane antigen (EMA), GLUT1, claudin-1, STAT6, and desmin. Rb protein was intact. The CD34 immunostain highlighted the cytoplasmic processes. Electron microscopy was performed because the immunohistochemical results were nonspecific despite the favorable histologic features for perineurioma and showed pinocytic vesicles with delicate cytoplasmic processes, characteristic of perineurioma (Figure 2). Follow-up visits were related to the management of multiple comorbidities; no known recurrence of the lesion was documented.

Patient 2—A 15-year-old adolescent boy with no notable medical history presented to the pediatric clinic for a bump on the right upper arm of 4 to 5 months’ duration. He did not recall an injury to the area and denied change in size, redness, bruising, or pain of the lesion. Ultrasonography demonstrated a 2.6×2.3×1.3-cm hypoechoic and slightly heterogeneous, well-circumscribed, subcutaneous mass with internal vascularity. The patient was then referred to a pediatric surgeon. The clinical differential included a lipoma, lymphadenopathy, or sebaceous cyst. An excision was performed. Gross inspection demonstrated a 7-g, 2.8×2.6×1.8-cm, homogeneous, tan-pink, rubbery nodule with minimal surrounding soft tissue. Histologic examination showed a bland proliferation of spindle cells with storiform and whorled patterns (Figure 3). No notable nuclear atypia or necrosis was identified. The tumor cells were focally positive for EMA (Figure 4), claudin-1, and CD34 and negative for S-100, SOX10, GLUT1, desmin, STAT6, pankeratin AE1/AE3, and SMA. The diagnosis of perineurioma was rendered. No recurrence of the lesion was appreciated clinically on a 6-month follow-up examination.

Comment
Characteristics of Perineuriomas—On gross evaluation, perineuriomas are firm, gray-white, and well circumscribed but not encapsulated. Histologically, perineuriomas can have a storiform, whorled, or lamellar pattern of spindle cells. Perivascular whorls can be a histologic clue. The spindle cells are bland appearing and typically are elongated and slender but can appear slightly ovoid and plump. The background stroma can be myxoid, collagenous, or mixed. There usually is no atypia, and mitotic figures are rare.2,3,6,7 Intraneural perineuriomas vary architecturally in that they display a unique onion bulb–like appearance in which whorls of cytoplasmic material of variable sizes surround central axons.3

Diagnosis—The diagnosis of perineuriomas usually requires characteristic immunohistochemical and sometimes ultrastructural features. Perineuriomas are positive for EMA and GLUT1 and variable for CD34.6 Approximately 20% to 91% will be positive for claudin-1, a tight junction protein associated with perineuriomas.8 Of note, EMA and GLUT1 usually are positive in both neoplastic and nonneoplastic perineurial cells.9,10 Occasionally, these tumors can be focally positive for SMA and negative for S-100 and glial fibrillary acidic protein. The bipolar, thin, delicate, cytoplasmic processes with long-tapering nuclei may be easier to appreciate on electron microscopy than on conventional light microscopy. In addition, the cells contain pinocytotic vesicles and a discontinuous external lamina, which may be helpful for diagnosis.10
Genetics—Genetic alterations in perineurioma continue to be elucidated. Although many soft tissue perineuriomas possess deletion of chromosome 22q material, this is not a consistent finding and is not pathognomonic. Notably, the NF2 tumor suppressor gene is found on chromosome 22.11 For the sclerosing variant of perineurioma, rearrangements or deletions of chromosome 10q have been described. A study of 14 soft tissue/extraneural perineuriomas using whole-exome sequencing and single nucleotide polymorphism array showed 6 cases of recurrent chromosome 22q deletions containing the NF2 locus and 4 cases with a previously unreported finding of chromosome 17q deletions containing the NF1 locus that were mutually exclusive events in all but 1 case.12 Although perineuriomas can harbor NF1 or NF2 mutations, perineuriomas are not considered to be associated with neurofibromatosis type 1 or 2 (NF1 or NF2, respectively). Patients with NF1 or NF2 and perineurioma are exceedingly rare. One pediatric patient with both soft tissue perineurioma and NF1 has been reported in the literature.13
Differential Diagnosis—Perineuriomas should be distinguished from other benign neural neoplasms of the skin and soft tissue. Commonly considered in the differential diagnosis is schwannoma and neurofibroma. Schwannomas are encapsulated epineurial nerve sheath tumors comprised of a neoplastic proliferation of Schwann cells. Schwannomas morphologically differ from perineuriomas because of the presence of the hypercellular Antoni A with Verocay bodies and the hypocellular myxoid Antoni B patterns of spindle cells with elongated wavy nuclei and tapered ends. Other features include hyalinized vessels, hemosiderin deposition, cystic degeneration, and/or degenerative atypia.3,14 Importantly, the constituent cells of schwannomas are positive for S-100 and SOX10 and negative for EMA.3 Neurofibromas consist of fascicles and whorls of Schwann cells in a background myxoid stroma with scattered mast cells, lymphocytes, fibroblasts, and perineurial cells. Similar to schwannomas, neurofibromas also are positive for S-100 and negative for EMA.3,14 Neurofibromas can have either a somatic or germline mutation of the biallelic NF1 gene on chromosome 17q11.2 with subsequent loss of protein neurofibromin activity.15 Less common but still a consideration are the hybrid peripheral nerve sheath tumors that may present with a biphasic or intermingled morphology. Combinations include neurofibroma-schwannoma, schwannoma-perineurioma, and neurofibroma-perineurioma. The hybrid schwannoma-perineurioma has a mixture of thin and plump spindle cells with tapered nuclei as well as patchy S-100 positivity corresponding to schwannian areas. Similarly, S-100 will highlight the wavy Schwann cells in neurofibroma-perineurioma as well as CD34-highlighting fibroblasts.7,15 In both aforementioned hybrid tumors, EMA will be positive in the perineurial areas. Another potential diagnostic consideration that can occur in both pediatric and adult populations is dermatofibrosarcoma protuberans (DFSP), which is comprised of a dermal proliferation of monomorphic fusiform spindle cells. Although both perineuriomas and DFSP can have a storiform architecture, DFSP is more asymmetric and infiltrative. Dermatofibrosarcoma protuberans is recognized in areas of individual adipocyte trapping, referred to as honeycombing. Dermatofibrosarcoma protuberans typically does not express EMA, though the sclerosing variant of DFSP has been reported to sometimes demonstrate focal EMA reactivity.11,14,16 For morphologically challenging cases, cytogenetic studies will show t(17;22) translocation fusing the COL1A1 and PDGFRB genes.16 Finally, for subcutaneous or deep-seated tumors, one also may consider other mesenchymal neoplasms, including solitary fibrous tumor, low-grade fibromyxoid sarcoma, or low-grade malignant peripheral nerve sheath tumor (MPNST).11
Management—Perineuriomas are considered benign. The presence of mitotic figures, pleomorphism, and degenerative nuclear atypia akin to ancient change, as seen in ancient schwannoma, does not affect their benign clinical behavior. Treatment of a perineurioma typically is surgical excision with conservative margins and minimal chance of recurrence.1,11 So-called malignant perineuriomas are better classified as MPNSTs with perineural differentiation or perineurial MPNST. They also are positive for EMA and may be distinguished from perineurioma by the presence of major atypia and an infiltrative growth pattern.17,18
Considerations in the Pediatric Population—Few pediatric soft tissue perineuriomas have been reported. A clinicopathologic analysis by Hornick and Fletcher1 of patients with soft tissue perineurioma showed that only 6 of 81 patients were younger than 20 years. The youngest reported case of perineurioma occurred as an extraneural perineurioma on the scalp in an infant.19 Only 1 soft tissue perineural MPNST has been reported in the pediatric population, arising on the face of an 11-year-old boy. In a case series of 11 pediatric perineuriomas, including extraneural and intraneural, there was no evidence of recurrence or metastasis at follow-up.4
Conclusion
Perineuriomas are rare benign peripheral nerve sheath tumors with unique histologic and immunohistochemical features. Soft tissue perineuriomas in the pediatric population are an important diagnostic consideration, especially for the pediatrician or dermatologist when encountering a well-circumscribed nodular soft tissue lesion of the extremity or when encountering a neural-appearing tumor in the subcutaneous tissue.
Acknowledgment—We would like to thank Christopher Fletcher, MD (Boston, Massachusetts), for his expertise in outside consultation for patient 1.
Perineuriomas are benign, slow-growing tumors derived from perineurial cells,1 which form the structurally supportive perineurium that surrounds individual nerve fascicles.2,3 Perineuriomas are classified into 2 main forms: intraneural or extraneural.4 Intraneural perineuriomas are found within the border of the peripheral nerve,5 while extraneural perineuriomas usually are found in soft tissue and skin. Extraneural perineuriomas can be further classified into variants based on their histologic appearance, including reticular, sclerosing, and plexiform subtypes. Extraneural perineuriomas usually present on the extremities or trunk of young to middle-aged adults as a well-circumscribed, painless, subcutaneous masses.1 These tumors are especially unusual in children.4 We present 2 extraneural perineurioma cases in children, and we review the pertinent diagnostic features of perineurioma as well as the presentation in the pediatric population.

Case Reports
Patient 1—A 10-year-old boy with a history of cerebral palsy and related comorbidities presented to the clinic for evaluation of a lesion on the thigh with no associated pain, irritation, erythema, or drainage. Physical examination revealed a soft, pedunculated, mobile nodule on the right medial thigh. An elliptical excision was performed. Gross examination demonstrated a 2.0×2.0×1.8-cm polypoid nodule. Histologic examination showed a dermal-based proliferation of bland spindle cells (Figure 1). The cytomorphology was characterized by elongated tapering nuclei and many areas with delicate bipolar cytoplasmic processes. The constituent cells were arranged in a whorled pattern in a variably myxoid to collagenous stroma. The tumor cells were multifocally positive for CD34; focally positive for smooth muscle actin (SMA); and negative for S-100, epithelial membrane antigen (EMA), GLUT1, claudin-1, STAT6, and desmin. Rb protein was intact. The CD34 immunostain highlighted the cytoplasmic processes. Electron microscopy was performed because the immunohistochemical results were nonspecific despite the favorable histologic features for perineurioma and showed pinocytic vesicles with delicate cytoplasmic processes, characteristic of perineurioma (Figure 2). Follow-up visits were related to the management of multiple comorbidities; no known recurrence of the lesion was documented.

Patient 2—A 15-year-old adolescent boy with no notable medical history presented to the pediatric clinic for a bump on the right upper arm of 4 to 5 months’ duration. He did not recall an injury to the area and denied change in size, redness, bruising, or pain of the lesion. Ultrasonography demonstrated a 2.6×2.3×1.3-cm hypoechoic and slightly heterogeneous, well-circumscribed, subcutaneous mass with internal vascularity. The patient was then referred to a pediatric surgeon. The clinical differential included a lipoma, lymphadenopathy, or sebaceous cyst. An excision was performed. Gross inspection demonstrated a 7-g, 2.8×2.6×1.8-cm, homogeneous, tan-pink, rubbery nodule with minimal surrounding soft tissue. Histologic examination showed a bland proliferation of spindle cells with storiform and whorled patterns (Figure 3). No notable nuclear atypia or necrosis was identified. The tumor cells were focally positive for EMA (Figure 4), claudin-1, and CD34 and negative for S-100, SOX10, GLUT1, desmin, STAT6, pankeratin AE1/AE3, and SMA. The diagnosis of perineurioma was rendered. No recurrence of the lesion was appreciated clinically on a 6-month follow-up examination.

Comment
Characteristics of Perineuriomas—On gross evaluation, perineuriomas are firm, gray-white, and well circumscribed but not encapsulated. Histologically, perineuriomas can have a storiform, whorled, or lamellar pattern of spindle cells. Perivascular whorls can be a histologic clue. The spindle cells are bland appearing and typically are elongated and slender but can appear slightly ovoid and plump. The background stroma can be myxoid, collagenous, or mixed. There usually is no atypia, and mitotic figures are rare.2,3,6,7 Intraneural perineuriomas vary architecturally in that they display a unique onion bulb–like appearance in which whorls of cytoplasmic material of variable sizes surround central axons.3

Diagnosis—The diagnosis of perineuriomas usually requires characteristic immunohistochemical and sometimes ultrastructural features. Perineuriomas are positive for EMA and GLUT1 and variable for CD34.6 Approximately 20% to 91% will be positive for claudin-1, a tight junction protein associated with perineuriomas.8 Of note, EMA and GLUT1 usually are positive in both neoplastic and nonneoplastic perineurial cells.9,10 Occasionally, these tumors can be focally positive for SMA and negative for S-100 and glial fibrillary acidic protein. The bipolar, thin, delicate, cytoplasmic processes with long-tapering nuclei may be easier to appreciate on electron microscopy than on conventional light microscopy. In addition, the cells contain pinocytotic vesicles and a discontinuous external lamina, which may be helpful for diagnosis.10
Genetics—Genetic alterations in perineurioma continue to be elucidated. Although many soft tissue perineuriomas possess deletion of chromosome 22q material, this is not a consistent finding and is not pathognomonic. Notably, the NF2 tumor suppressor gene is found on chromosome 22.11 For the sclerosing variant of perineurioma, rearrangements or deletions of chromosome 10q have been described. A study of 14 soft tissue/extraneural perineuriomas using whole-exome sequencing and single nucleotide polymorphism array showed 6 cases of recurrent chromosome 22q deletions containing the NF2 locus and 4 cases with a previously unreported finding of chromosome 17q deletions containing the NF1 locus that were mutually exclusive events in all but 1 case.12 Although perineuriomas can harbor NF1 or NF2 mutations, perineuriomas are not considered to be associated with neurofibromatosis type 1 or 2 (NF1 or NF2, respectively). Patients with NF1 or NF2 and perineurioma are exceedingly rare. One pediatric patient with both soft tissue perineurioma and NF1 has been reported in the literature.13
Differential Diagnosis—Perineuriomas should be distinguished from other benign neural neoplasms of the skin and soft tissue. Commonly considered in the differential diagnosis is schwannoma and neurofibroma. Schwannomas are encapsulated epineurial nerve sheath tumors comprised of a neoplastic proliferation of Schwann cells. Schwannomas morphologically differ from perineuriomas because of the presence of the hypercellular Antoni A with Verocay bodies and the hypocellular myxoid Antoni B patterns of spindle cells with elongated wavy nuclei and tapered ends. Other features include hyalinized vessels, hemosiderin deposition, cystic degeneration, and/or degenerative atypia.3,14 Importantly, the constituent cells of schwannomas are positive for S-100 and SOX10 and negative for EMA.3 Neurofibromas consist of fascicles and whorls of Schwann cells in a background myxoid stroma with scattered mast cells, lymphocytes, fibroblasts, and perineurial cells. Similar to schwannomas, neurofibromas also are positive for S-100 and negative for EMA.3,14 Neurofibromas can have either a somatic or germline mutation of the biallelic NF1 gene on chromosome 17q11.2 with subsequent loss of protein neurofibromin activity.15 Less common but still a consideration are the hybrid peripheral nerve sheath tumors that may present with a biphasic or intermingled morphology. Combinations include neurofibroma-schwannoma, schwannoma-perineurioma, and neurofibroma-perineurioma. The hybrid schwannoma-perineurioma has a mixture of thin and plump spindle cells with tapered nuclei as well as patchy S-100 positivity corresponding to schwannian areas. Similarly, S-100 will highlight the wavy Schwann cells in neurofibroma-perineurioma as well as CD34-highlighting fibroblasts.7,15 In both aforementioned hybrid tumors, EMA will be positive in the perineurial areas. Another potential diagnostic consideration that can occur in both pediatric and adult populations is dermatofibrosarcoma protuberans (DFSP), which is comprised of a dermal proliferation of monomorphic fusiform spindle cells. Although both perineuriomas and DFSP can have a storiform architecture, DFSP is more asymmetric and infiltrative. Dermatofibrosarcoma protuberans is recognized in areas of individual adipocyte trapping, referred to as honeycombing. Dermatofibrosarcoma protuberans typically does not express EMA, though the sclerosing variant of DFSP has been reported to sometimes demonstrate focal EMA reactivity.11,14,16 For morphologically challenging cases, cytogenetic studies will show t(17;22) translocation fusing the COL1A1 and PDGFRB genes.16 Finally, for subcutaneous or deep-seated tumors, one also may consider other mesenchymal neoplasms, including solitary fibrous tumor, low-grade fibromyxoid sarcoma, or low-grade malignant peripheral nerve sheath tumor (MPNST).11
Management—Perineuriomas are considered benign. The presence of mitotic figures, pleomorphism, and degenerative nuclear atypia akin to ancient change, as seen in ancient schwannoma, does not affect their benign clinical behavior. Treatment of a perineurioma typically is surgical excision with conservative margins and minimal chance of recurrence.1,11 So-called malignant perineuriomas are better classified as MPNSTs with perineural differentiation or perineurial MPNST. They also are positive for EMA and may be distinguished from perineurioma by the presence of major atypia and an infiltrative growth pattern.17,18
Considerations in the Pediatric Population—Few pediatric soft tissue perineuriomas have been reported. A clinicopathologic analysis by Hornick and Fletcher1 of patients with soft tissue perineurioma showed that only 6 of 81 patients were younger than 20 years. The youngest reported case of perineurioma occurred as an extraneural perineurioma on the scalp in an infant.19 Only 1 soft tissue perineural MPNST has been reported in the pediatric population, arising on the face of an 11-year-old boy. In a case series of 11 pediatric perineuriomas, including extraneural and intraneural, there was no evidence of recurrence or metastasis at follow-up.4
Conclusion
Perineuriomas are rare benign peripheral nerve sheath tumors with unique histologic and immunohistochemical features. Soft tissue perineuriomas in the pediatric population are an important diagnostic consideration, especially for the pediatrician or dermatologist when encountering a well-circumscribed nodular soft tissue lesion of the extremity or when encountering a neural-appearing tumor in the subcutaneous tissue.
Acknowledgment—We would like to thank Christopher Fletcher, MD (Boston, Massachusetts), for his expertise in outside consultation for patient 1.
- Hornick J, Fletcher C. Soft tissue perineurioma. Am J Surg Pathol. 2005;29:845-858.
- Tsang WY, Chan JK, Chow LT, et al. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756-763.
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Balarezo FS, Muller RC, Weiss RG, et al. Soft tissue perineuriomas in children: report of three cases and review of the literature. Pediatr Dev Pathol. 2003;6:137-141. Published correction appears in Pediatr Dev Pathol. 2003;6:following 364.
- Macarenco R, Ellinger F, Oliveira A. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625-636.
- Agaimy A, Buslei R, Coras R, et al. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60-70.
- Greenson JK, Hornick JL, Longacre TA, et al. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer; 2015.
- Folpe A, Billings S, McKenney J, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620-1626.
- Hirose T, Tani T, Shimada T, et al. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293-298.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Perineurioma. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013:176-178.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Saunders; 2013.
- Carter JM, Wu Y, Blessing MM, et al. Recurrent genomic alterations in soft tissue perineuriomas. Am J Surg Pathol. 2018;42:1708-1714.
- Al-Adnani M. Soft tissue perineurioma in a child with neurofibromatosis type 1: a case report and review of the literature. Pediatr Dev Pathol. 2017;20:444-448.
- Reddy VB, David O, Spitz DJ, et al. Gattuso’s Differential Diagnosis in Surgical Pathology. Elsevier Saunders; 2022.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Abdaljaleel MY, North JP. Sclerosing dermatofibrosarcoma protuberans shows significant overlap with sclerotic fibroma in both routine and immunohistochemical analysis: a potential diagnostic pitfall. Am J Dermatopathol. 2017;39:83-88.
- Rosenberg AS, Langee CL, Stevens GL, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma.” J Cutan Pathol. 2002;29:362-367.
- Mitchell A, Scheithauer BW, Doyon J, et al. Malignant perineurioma (malignant peripheral nerve sheath tumor with perineural differentiation). Clin Neuropathol. 2012;31:424-429.
- Duhan A, Rana P, Beniwal K, et al. Perineurioma of scalp in an infant: a case report with short review of literature. Asian J Neurosurg. 2016;11:81-83.
- Hornick J, Fletcher C. Soft tissue perineurioma. Am J Surg Pathol. 2005;29:845-858.
- Tsang WY, Chan JK, Chow LT, et al. Perineurioma: an uncommon soft tissue neoplasm distinct from localized hypertrophic neuropathy and neurofibroma. Am J Surg Pathol. 1992;16:756-763.
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Balarezo FS, Muller RC, Weiss RG, et al. Soft tissue perineuriomas in children: report of three cases and review of the literature. Pediatr Dev Pathol. 2003;6:137-141. Published correction appears in Pediatr Dev Pathol. 2003;6:following 364.
- Macarenco R, Ellinger F, Oliveira A. Perineurioma: a distinctive and underrecognized peripheral nerve sheath neoplasm. Arch Pathol Lab Med. 2007;131:625-636.
- Agaimy A, Buslei R, Coras R, et al. Comparative study of soft tissue perineurioma and meningioma using a five-marker immunohistochemical panel. Histopathology. 2014;65:60-70.
- Greenson JK, Hornick JL, Longacre TA, et al. Sternberg’s Diagnostic Surgical Pathology. Wolters Kluwer; 2015.
- Folpe A, Billings S, McKenney J, et al. Expression of claudin-1, a recently described tight junction-associated protein, distinguishes soft tissue perineurioma from potential mimics. Am J Surg Pathol. 2002;26:1620-1626.
- Hirose T, Tani T, Shimada T, et al. Immunohistochemical demonstration of EMA/Glut1-positive perineurial cells and CD34-positive fibroblastic cells in peripheral nerve sheath tumors. Mod Pathol. 2003;16:293-298.
- Fletcher CDM, Bridge JA, Hogendoorn PCW, et al. Perineurioma. WHO Classification of Tumours of Soft Tissue and Bone. IARC Press; 2013:176-178.
- Hornick JL. Practical Soft Tissue Pathology: A Diagnostic Approach. Elsevier Saunders; 2013.
- Carter JM, Wu Y, Blessing MM, et al. Recurrent genomic alterations in soft tissue perineuriomas. Am J Surg Pathol. 2018;42:1708-1714.
- Al-Adnani M. Soft tissue perineurioma in a child with neurofibromatosis type 1: a case report and review of the literature. Pediatr Dev Pathol. 2017;20:444-448.
- Reddy VB, David O, Spitz DJ, et al. Gattuso’s Differential Diagnosis in Surgical Pathology. Elsevier Saunders; 2022.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Abdaljaleel MY, North JP. Sclerosing dermatofibrosarcoma protuberans shows significant overlap with sclerotic fibroma in both routine and immunohistochemical analysis: a potential diagnostic pitfall. Am J Dermatopathol. 2017;39:83-88.
- Rosenberg AS, Langee CL, Stevens GL, et al. Malignant peripheral nerve sheath tumor with perineurial differentiation: “malignant perineurioma.” J Cutan Pathol. 2002;29:362-367.
- Mitchell A, Scheithauer BW, Doyon J, et al. Malignant perineurioma (malignant peripheral nerve sheath tumor with perineural differentiation). Clin Neuropathol. 2012;31:424-429.
- Duhan A, Rana P, Beniwal K, et al. Perineurioma of scalp in an infant: a case report with short review of literature. Asian J Neurosurg. 2016;11:81-83.
Practice Points
- Perineuriomas are rare benign peripheral nerve sheath tumors that most commonly occur in young to middle-aged adults but rarely can present in children.
- Immunohistochemically, perineuriomas show positive staining with epithelial membrane antigen, GLUT1, claudin-1, and frequently with CD34; they are negative for S-100 and glial fibrillary acidic protein.
- Perineuriomas should be considered in the differential diagnosis in children who present with a well-circumscribed nodular lesion in the subcutaneous tissue.
Past, Present, and Future of Pediatric Atopic Dermatitis Management
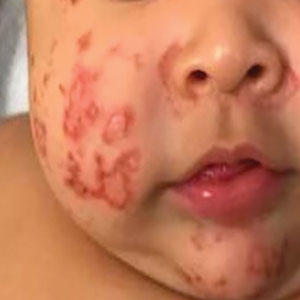
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
Atopic dermatitis (AD), or eczema, is a common inflammatory skin disease notorious for its chronic, relapsing, and often frustrating disease course. Although as many as 25% of children in the United States are affected by this condition and its impact on the quality of life of affected patients and families is profound,1-3 therapeutic advances in the pediatric population have been fairly limited until recently.
Over the last 10 years, there has been robust investigation into pediatric AD therapeutics, with many topical and systemic medications either recently approved or under clinical investigation. These developments are changing the landscape of the management of pediatric AD and raise a set of fascinating questions about how early and aggressive intervention might change the course of this disease. We discuss current limitations in the field that may be addressed with additional research.
New Topical Medications
In the last several years, there has been a rapid increase in efforts to develop new topical agents to manage AD. Until the beginning of the 21st century, the dermatologist’s arsenal was limited to topical corticosteroids (TCs). In the early 2000s, attention shifted to topical calcineurin inhibitors as nonsteroidal alternatives when the US Food and Drug Administration (FDA) approved topical tacrolimus and pimecrolimus for AD. In 2016, crisaborole (a phosphodiesterase-4 [PDE4] inhibitor) was approved by the FDA for use in mild to moderate AD in patients 2 years and older, marking a new age of development for topical AD therapies. In 2021, the FDA approved ruxolitinib (a topical Janus kinase [JAK] 1/2 inhibitor) for use in mild to moderate AD in patients 12 years and older.
Roflumilast (ARQ-151) and difamilast (OPA-15406)(members of the PDE4 inhibitor class) are undergoing investigation for pediatric AD. A phase 3 clinical trial for roflumilast for AD is underway (ClinicalTrial.gov Identifier: NCT04845620); it is already approved for psoriasis in patients 12 years and older. A phase 3 trial of difamilast (NCT03911401) was recently completed, with results supporting the drug’s safety and efficacy in AD management.4 Efforts to synthesize new better-targeted PDE4 inhibitors are ongoing.5
Tapinarof (a novel aryl hydrocarbon receptor-modulating agent) is approved for psoriasis in adults, and a phase 3 trial for management of pediatric AD is underway (NCT05032859) after phase 2 trials revealed promising results.6
Lastly, the microbiome is a target for AD topical therapies. A recently completed phase 1 trial of bacteriotherapy with Staphylococcus hominis A9 transplant lotion showed promising results (NCT03151148).7 Although this bacteriotherapy technique is early in development and has been studied only in adult patients, results are exciting because they represent a gateway to a largely unexplored realm of potential future therapies.
Standard of Care—How will these new topical therapies impact our standard of care for pediatric AD patients? Topical corticosteroids are still a pillar of topical AD therapy, but the potential for nonsteroidal topical agents as alternatives and used in combination therapeutic regimens has expanded exponentially. It is uncertain how we might individualize regimens tailored to patient-specific factors because the standard approach has been to test drugs as monotherapy, with vehicle comparisons or with reference medications in Europe.
Newer topical nonsteroidal agents may offer several opportunities. First, they may help avoid local and systemic adverse effects that often limit the use of current standard therapy.8 This capability may prove essential in bridging TC treatments and serving as long-term maintenance therapies to decrease the frequency of eczema flares. Second, they can alleviate the need for different medication strengths for different body regions, thereby allowing for simplification of regimens and potentially increased adherence and decreased disease burden—a boon to affected patients and caregivers.
Although the efficacy and long-term safety profile of these new drugs require further study, it does not seem unreasonable to look forward to achieving levels of optimization and individualization with topical regimens for AD in the near future that makes flares in patients with mild to moderate AD a phenomenon of the past.
Advances in Systemic Therapy
Systemic therapeutics in pediatric AD also recently entered an exciting era of development. Traditional systemic agents, including cyclosporine, methotrexate, azathioprine, and mycophenolate mofetil, have existed for decades but have not been widely utilized for moderate to severe AD in the United States, especially in the pediatric population, likely because these drugs lacked FDA approval and they can cause a range of adverse effects, including notable immunosuppression.9
Introduction and approval of dupilumab in 2017 by the FDA was revolutionary in this field. As a monoclonal antibody targeted against IL-4 and IL-13, dupilumab has consistently demonstrated strong long-term efficacy for pediatric AD and has an acceptable safety profile in children and adolescents.10-14 Expansion of the label to include children as young as 6 months with moderate to severe AD seems an important milestone in pediatric AD care.
Since the approval of dupilumab for adolescents and children aged 6 to 12 years, global experience has supported expanded use of systemic agents for patients who have an inadequate response to TCs and previously approved nonsteroidal topical agents. How expansive the use of systemics will be in younger children depends on how their long-term use impacts the disease course, whether therapy is disease modifying, and whether early use can curb the development of comorbidities.
Investigations into targeted systemic therapeutics for eczematous dermatitis are not limited to dupilumab. In a study of adolescents as young as 12 years, tralokinumab (an IL-13 pathway inhibitor) demonstrated an Eczema Area Severity Index-75 of 27.8% to 28.6% and a mean decrease in the SCORing Atopic Dermatitis index of 27.5 to 29.1, with minimal adverse effects.15 Lebrikizumab, another biologic IL-13 inhibitor with strong published safety and efficacy data in adults, has completed short- and longer-term studies in adolescents (NCT04178967 and NCT04146363).16 The drug received FDA Fast Track designation for moderate to severe AD in patients 12 years and older after showing positive data.17
This push to targeted therapy stretches beyond monoclonal antibodies. In the last few years, oral JAK inhibitors have emerged as a new class of systemic therapy for eczematous dermatitis. Upadacitinib, a JAK1 selective inhibitor, was approved by the FDA in 2022 for patients 12 years and older with AD and has data that supports its efficacy in adolescents and adults.18 Other JAK inhibitors including the selective JAK1 inhibitor abrocitinib and the combined JAK1/2 inhibitor baricitinib are being studied for pediatric AD (NCT04564755, NCT03422822, and NCT03952559), with most evidence to date supporting their safety and efficacy, at least over the short-term.19
The study of these and other advanced systemic therapies for eczematous dermatitis is transforming the toolbox for pediatric AD care. Although long-term data are lacking for some of these medications, it is possible that newer agents may decrease reliance on older immunosuppressants, such as systemic corticosteroids, cyclosporine, and methotrexate. Unanswered questions include: How and which systemic medications may alter the course of the disease? What is the disease modification for AD? What is the impact on comorbidities over time?
What’s Missing?
The field of pediatric AD has experienced exciting new developments with the emergence of targeted therapeutics, but those new agents require more long-term study, though we already have longer-term data on crisaborole and dupilumab.10-14,20 Studies of the long-term use of these new treatments on comorbidities of pediatric AD—mental health outcomes, cardiovascular disease, effects on the family, and other allergic conditions—are needed.21 Furthermore, clinical guidelines that address indications, timing of use, tapering, and discontinuation of new treatments depend on long-term experience and data collection.
Therefore, it is prudent that investigators, companies, payers, patients, and families support phase 4, long-term extension, and registry studies, which will expand our knowledge of AD medications and their impact on the disease over time.
Final Thoughts
Medications to treat AD are reaching a new level of advancement—from topical agents that target novel pathways to revolutionary biologics and systemic medications. Although there are knowledge gaps on these new therapeutics, the standard of care is already rapidly changing as the expectations of clinicians, patients, and families advance with each addition to the provider’s toolbox.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: part 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Kiebert G, Sorensen SV, Revicki D, et al. Atopic dermatitis is associated with a decrement in health-related quality of life. Int J Dermatol. 2002;41:151-158. doi:10.1046/j.1365-4362.2002.01436.x
- Al Shobaili HA. The impact of childhood atopic dermatitis on the patients’ family. Pediatr Dermatol. 2010;27:618-623. doi:10.1111/j.1525-1470.2010.01215.x
- Saeki H, Baba N, Ito K, et al. Difamilast, a selective phosphodiesterase 4 inhibitor, ointment in paediatric patients with atopic dermatitis: a phase III randomized double-blind, vehicle-controlled trial [published online November 1, 2021]. Br J Dermatol. 2022;186:40-49. doi:10.1111/bjd.20655
- Chu Z, Xu Q, Zhu Q, et al. Design, synthesis and biological evaluation of novel benzoxaborole derivatives as potent PDE4 inhibitors for topical treatment of atopic dermatitis. Eur J Med Chem. 2021;213:113171. doi:10.1016/j.ejmech.2021.113171
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: part 2. management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132. doi:10.1016/j.jaad.2014.03.023
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: part 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Gooderham MJ, Hong HC-H, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Blauvelt A, Guttman-Yassky E, Paller AS, et al. Long-term efficacy and safety of dupilumab in adolescents with moderate-to-severe atopicdermatitis: results through week 52 from a phase III open-label extension trial (LIBERTY AD PED-OLE). Am J Clin Dermatol. 2022;23:365-383. doi:10.1007/s40257-022-00683-2
- Cork MJ, D, Eichenfield LF, et al. Dupilumab provides favourable long-term safety and efficacy in children aged ≥ 6 to < 12 years with uncontrolled severe atopic dermatitis: results from an open-label phase IIa study and subsequent phase III open-label extension study. Br J Dermatol. 2021;184:857-870. doi:10.1111/bjd.19460
- Simpson EL, Paller AS, Siegfried EC, et al. Dupilumab demonstrates rapid and consistent improvement in extent and signs of atopic dermatitis across all anatomical regions in pediatric patients 6 years of age and older. Dermatol Ther (Heidelb). 2021;11:1643-1656. doi:10.1007/s13555-021-00568-y
- Paller A, Blauvelt A, Soong W, et al. Efficacy and safety of tralokinumab in adolescents with moderate-to-severe atopic dermatitis: results of the phase 3 ECZTRA 6 trial. SKIN. 2022;6:S29. doi:10.25251/skin.6.supp.s29
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Lebrikizumab dosed every four weeks maintained durable skin clearance in Lilly’s phase 3 monotherapy atopic dermatitis trials [news release]. Eli Lilly and Company; September 8, 2022. Accessed October 19, 2022. https://investor.lilly.com/news-releases/news-release-details/lebrikizumab-dosed-every-four-weeks-maintained-durable-skin
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168. doi:10.1016/S0140-6736(21)00588-2
- Chovatiya R, Paller AS. JAK inhibitors in the treatment of atopic dermatitis. J Allergy Clin Immunol. 2021;148:927-940. doi:10.1016/j.jaci.2021.08.009
- Geng B, Hebert AA, Takiya L, et al. Efficacy and safety trends with continuous, long-term crisaborole use in patients aged ≥ 2 years with mild-to-moderate atopic dermatitis. Dermatol Ther (Heidelb). 2021;11:1667-1678. doi:10.1007/s13555-021-00584-y
- Appiah MM, Haft MA, Kleinman E, et al. Atopic dermatitis: review of comorbidities and therapeutics. Ann Allergy Asthma Immunol. 2022;129:142-149. doi:10.1016/j.anai.2022.05.015
PRACTICE POINTS
- Pediatric atopic dermatitis (AD) therapeutics have rapidly evolved over the last decade and dermatologists should be aware of new tools in their treatment arsenal.
- New topical nonsteroidal agents serve as useful alternatives to topical corticosteroids through mitigating adverse effects from current standard therapy and potentially simplifying topical regimens.
- Monoclonal antibodies and Janus kinase inhibitors are part of an important set of new systemic therapeutics for pediatric AD.
- Long-term data on these new therapeutics is required to better understand their impact on pediatric AD comorbidities and impact on the longitudinal disease course.
Acquired Acrodermatitis Enteropathica in an Infant
Acrodermatitis enteropathica (AE) is a rare disorder of zinc metabolism that typically presents in infancy.1 Although it is clinically characterized by acral and periorificial dermatitis, alopecia, and diarrhea, only 20% of cases present with this triad.2 Zinc deficiency in AE can either be acquired or inborn (congenital). Acquired forms can occur from dietary inadequacy or malabsorption, whereas genetic causes are related to an autosomal-recessive disorder affecting zinc transporters.1 We report a case of a 3-month-old female infant with acquired AE who was successfully treated with zinc supplementation over the course of 3 weeks.
Case Report
A 3-month-old female infant presented to the emergency department with a rash of 2 weeks’ duration. She was born full term with no birth complications. The patient’s mother reported that the rash started on the cheeks, then enlarged and spread to the neck, back, and perineum. The patient also had been having diarrhea during this time. She previously had received mupirocin and cephalexin with no response to treatment. Maternal history was negative for lupus, and the mother’s diet consisted of a variety of foods but not many vegetables. The patient was exclusively breastfed, and there was no pertinent history of similar rashes occurring in other family members.
Physical examination revealed the patient had annular and polycyclic, hyperkeratotic, crusted papules and plaques on the cheeks, neck, back, and axillae, as well as the perineum/groin and perianal regions (Figure 1). The differential diagnosis at the time included neonatal lupus, zinc deficiency, and syphilis. Relevant laboratory testing and a shave biopsy of the left axilla were obtained.
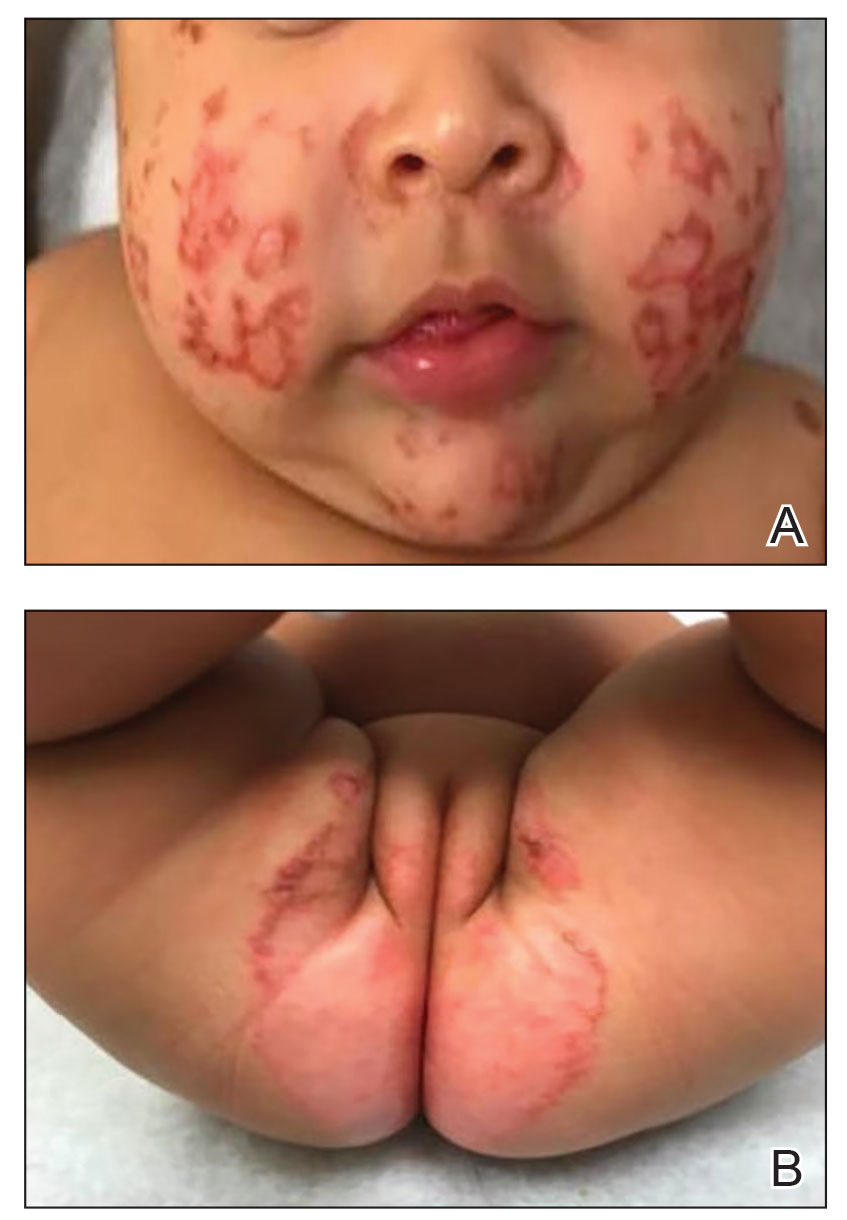
Pertinent laboratory findings included a low zinc level (23 μg/dL [reference range, 26–141 μg/dL]), low alkaline phosphatase level (74 U/L [reference range, 94–486 U/L]), and thrombocytosis (826×109/L [reference range, 150–400×109/L). Results for antinuclear antibody and anti–Sjögren syndrome–related antigen A and B antibody testing were negative. A rapid plasma reagin test was nonreactive. Histologic examination revealed psoriasiform hyperplasia with overlying confluent parakeratosis, focal spongiosis, multiple dyskeratotic keratinocytes, and mitotic figures (Figure 2). Ballooning was evident in focal cells in the subcorneal region in addition to an accompanying lymphocytic infiltrate and occasional neutrophils.
The patient was given a 10-mg/mL suspension of elemental zinc and was advised to take 1 mL (10 mg) by mouth twice daily with food. This dosage equated to 3 mg/kg/d. On follow-up 3 weeks later, the skin began to clear (Figure 3). Follow-up laboratory testing showed an increase in zinc (114 μg/dL) and alkaline phosphatase levels (313 U/L). The patient was able to discontinue the zinc supplementation, and follow-up during the next year revealed no recurrence.
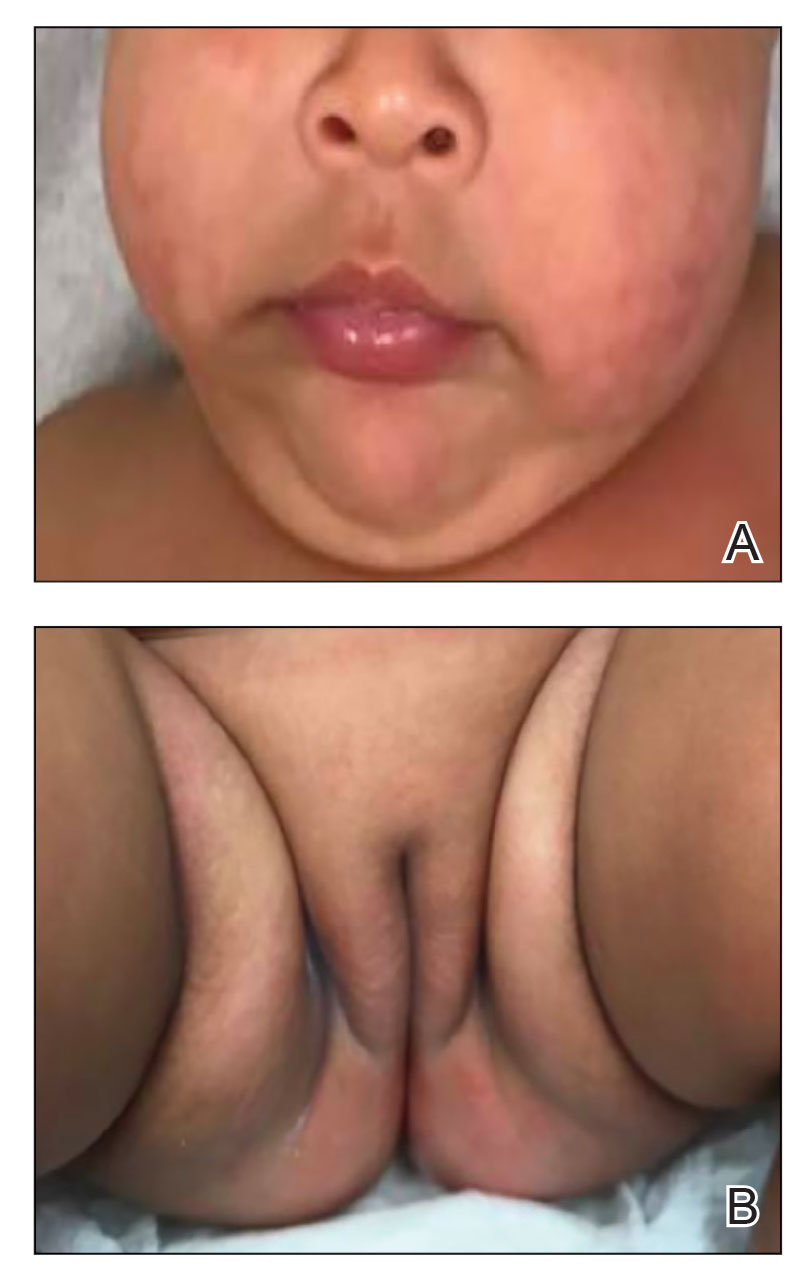
Comment
Etiology of AE—Acrodermatitis enteropathica was first identified in 1942 as an acral rash associated with diarrhea3; in 1973, Barnes and Moynahan4 discovered zinc deficiency as a causal agent for these findings. The causes of AE are further subclassified as either an acquired or inborn etiology. Congenital causes commonly are seen in infants within the first few months of life, whereas acquired forms are seen at any age. Acquired forms in infants can occur from failure of the mother to secrete zinc in breast milk, low maternal serum zinc levels, or other reasons causing low nutritional intake. A single mutation in the SLC30A2 gene has been found to markedly reduce zinc concentrations in breast milk, thus causing zinc deficiency in breastfed infants.5 Other acquired forms can be caused by malabsorption, sometimes after surgery such as intestinal bypass or from intravenous nutrition without sufficient zinc.1 The congenital form of AE is an autosomal-recessive disorder occurring from mutations in the SLC39A4 gene located on band 8q24.3. Affected individuals have a decreased ability to absorb zinc in the small intestine because of defects in zinc transporters ZIP and ZnT.6 Based on our patient’s laboratory findings and history, it is believed that the zinc deficiency was acquired, as the condition normalized with repletion and has not required any supplementation in the year of follow-up. In addition, the absence of a pertinent family history supported an acquired diagnosis, which has various etiologies, whereas the congenital form primarily is a genetic disease.
Management—Treatment of AE includes supplementation with oral elemental zinc; however, there are scant evidence-based recommendations on the exact dose of zinc to be given. Generally, the recommended amount is 3 mg/kg/d.8 For individuals with the congenital form of AE, lifelong zinc supplementation is additionally recommended.9 It is important to recognize this presentation because the patient can develop worsening irritability, severe diarrhea, nail dystrophy, hair loss, immune dysfunction, and numerous ophthalmic disorders if left untreated. Acute zinc toxicity due to excess administration is rare, with symptoms of nausea and vomiting occurring with dosages of 50 to 100 mg/d. Additionally, dosages of up to 70 mg twice weekly have been provided without any toxic effect.10 In our case, 3 mg/kg/d of oral zinc supplementation proved to be effective in resolving the patient’s symptoms of acquired zinc deficiency.
Differential Diagnosis—It is important to note that deficiencies of other nutrients may present as an AE-like eruption called acrodermatitis dysmetabolica (AD). Both diseases may present with the triad of dermatitis, alopecia, and diarrhea; however, AD is associated with inborn errors of metabolism. There have been cases that describe AD in patients with a zinc deficiency in conjunction with a deficiency of branched-chain amino acids.11,12 It is important to consider AD in the differential diagnosis of an AE eruption, especially in the context of a metabolic disorder, as it may affect the treatment plan. One case described the dermatitis of AD as not responding to zinc supplementation alone, while another described improvement after increasing an isoleucine supplementation dose.11,12
Other considerations in the differential diagnoses include AE-like conditions such as biotinidase deficiency, multiple carboxylase deficiency, and essential fatty acid deficiency. An AE-like condition may present with the triad of dermatitis, alopecia, and diarrhea. However, unlike in true AE, zinc and alkaline phosphatase levels tend to be normal in these conditions. Other features seen in AE-like conditions depend on the underlying cause but often include failure to thrive, neurologic defects, ophthalmic abnormalities, and metabolic abnormalities.13
- Acrodermatitis enteropathica. National Organization for Rare Disorders. Accessed October 16, 2022. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica/
- Perafán-Riveros C, França LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Danbolt N. Acrodermatitis enteropathica. Br J Dermatol. 1979;100:37-40.
- Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329.
- Lee S, Zhou Y, Gill DL, et al. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci Rep. 2018;8:3542.
- Kaur S, Sangwan A, Sahu P, et al. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol. 2016;17:35-37.
- Dela Rosa KM, James WD. Acrodermatitis enteropathica workup. Medscape. Updated June 4, 2021. Accessed October 16, 2022. https://emedicine.medscape.com/article/1102575-workup#showall
- Ngan V, Gangakhedkar A, Oakley A. Acrodermatitis enteropathica. DermNet. Accessed October 16, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica/
- Ranugha P, Sethi P, Veeranna S. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018;24:13030/qt1w9002sr.
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26:356-365.
- Samady JA, Schwartz RA, Shih LY, et al. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. 2000;27:604-608.
- Flores K, Chikowski R, Morrell DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. 2016;41:651-654.
- Jones L, Oakley A. Acrodermatitis enteropathica-like conditions. DermNet. Accessed August 30, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica-like-conditions
Acrodermatitis enteropathica (AE) is a rare disorder of zinc metabolism that typically presents in infancy.1 Although it is clinically characterized by acral and periorificial dermatitis, alopecia, and diarrhea, only 20% of cases present with this triad.2 Zinc deficiency in AE can either be acquired or inborn (congenital). Acquired forms can occur from dietary inadequacy or malabsorption, whereas genetic causes are related to an autosomal-recessive disorder affecting zinc transporters.1 We report a case of a 3-month-old female infant with acquired AE who was successfully treated with zinc supplementation over the course of 3 weeks.
Case Report
A 3-month-old female infant presented to the emergency department with a rash of 2 weeks’ duration. She was born full term with no birth complications. The patient’s mother reported that the rash started on the cheeks, then enlarged and spread to the neck, back, and perineum. The patient also had been having diarrhea during this time. She previously had received mupirocin and cephalexin with no response to treatment. Maternal history was negative for lupus, and the mother’s diet consisted of a variety of foods but not many vegetables. The patient was exclusively breastfed, and there was no pertinent history of similar rashes occurring in other family members.
Physical examination revealed the patient had annular and polycyclic, hyperkeratotic, crusted papules and plaques on the cheeks, neck, back, and axillae, as well as the perineum/groin and perianal regions (Figure 1). The differential diagnosis at the time included neonatal lupus, zinc deficiency, and syphilis. Relevant laboratory testing and a shave biopsy of the left axilla were obtained.

Pertinent laboratory findings included a low zinc level (23 μg/dL [reference range, 26–141 μg/dL]), low alkaline phosphatase level (74 U/L [reference range, 94–486 U/L]), and thrombocytosis (826×109/L [reference range, 150–400×109/L). Results for antinuclear antibody and anti–Sjögren syndrome–related antigen A and B antibody testing were negative. A rapid plasma reagin test was nonreactive. Histologic examination revealed psoriasiform hyperplasia with overlying confluent parakeratosis, focal spongiosis, multiple dyskeratotic keratinocytes, and mitotic figures (Figure 2). Ballooning was evident in focal cells in the subcorneal region in addition to an accompanying lymphocytic infiltrate and occasional neutrophils.

The patient was given a 10-mg/mL suspension of elemental zinc and was advised to take 1 mL (10 mg) by mouth twice daily with food. This dosage equated to 3 mg/kg/d. On follow-up 3 weeks later, the skin began to clear (Figure 3). Follow-up laboratory testing showed an increase in zinc (114 μg/dL) and alkaline phosphatase levels (313 U/L). The patient was able to discontinue the zinc supplementation, and follow-up during the next year revealed no recurrence.

Comment
Etiology of AE—Acrodermatitis enteropathica was first identified in 1942 as an acral rash associated with diarrhea3; in 1973, Barnes and Moynahan4 discovered zinc deficiency as a causal agent for these findings. The causes of AE are further subclassified as either an acquired or inborn etiology. Congenital causes commonly are seen in infants within the first few months of life, whereas acquired forms are seen at any age. Acquired forms in infants can occur from failure of the mother to secrete zinc in breast milk, low maternal serum zinc levels, or other reasons causing low nutritional intake. A single mutation in the SLC30A2 gene has been found to markedly reduce zinc concentrations in breast milk, thus causing zinc deficiency in breastfed infants.5 Other acquired forms can be caused by malabsorption, sometimes after surgery such as intestinal bypass or from intravenous nutrition without sufficient zinc.1 The congenital form of AE is an autosomal-recessive disorder occurring from mutations in the SLC39A4 gene located on band 8q24.3. Affected individuals have a decreased ability to absorb zinc in the small intestine because of defects in zinc transporters ZIP and ZnT.6 Based on our patient’s laboratory findings and history, it is believed that the zinc deficiency was acquired, as the condition normalized with repletion and has not required any supplementation in the year of follow-up. In addition, the absence of a pertinent family history supported an acquired diagnosis, which has various etiologies, whereas the congenital form primarily is a genetic disease.
Management—Treatment of AE includes supplementation with oral elemental zinc; however, there are scant evidence-based recommendations on the exact dose of zinc to be given. Generally, the recommended amount is 3 mg/kg/d.8 For individuals with the congenital form of AE, lifelong zinc supplementation is additionally recommended.9 It is important to recognize this presentation because the patient can develop worsening irritability, severe diarrhea, nail dystrophy, hair loss, immune dysfunction, and numerous ophthalmic disorders if left untreated. Acute zinc toxicity due to excess administration is rare, with symptoms of nausea and vomiting occurring with dosages of 50 to 100 mg/d. Additionally, dosages of up to 70 mg twice weekly have been provided without any toxic effect.10 In our case, 3 mg/kg/d of oral zinc supplementation proved to be effective in resolving the patient’s symptoms of acquired zinc deficiency.
Differential Diagnosis—It is important to note that deficiencies of other nutrients may present as an AE-like eruption called acrodermatitis dysmetabolica (AD). Both diseases may present with the triad of dermatitis, alopecia, and diarrhea; however, AD is associated with inborn errors of metabolism. There have been cases that describe AD in patients with a zinc deficiency in conjunction with a deficiency of branched-chain amino acids.11,12 It is important to consider AD in the differential diagnosis of an AE eruption, especially in the context of a metabolic disorder, as it may affect the treatment plan. One case described the dermatitis of AD as not responding to zinc supplementation alone, while another described improvement after increasing an isoleucine supplementation dose.11,12
Other considerations in the differential diagnoses include AE-like conditions such as biotinidase deficiency, multiple carboxylase deficiency, and essential fatty acid deficiency. An AE-like condition may present with the triad of dermatitis, alopecia, and diarrhea. However, unlike in true AE, zinc and alkaline phosphatase levels tend to be normal in these conditions. Other features seen in AE-like conditions depend on the underlying cause but often include failure to thrive, neurologic defects, ophthalmic abnormalities, and metabolic abnormalities.13
Acrodermatitis enteropathica (AE) is a rare disorder of zinc metabolism that typically presents in infancy.1 Although it is clinically characterized by acral and periorificial dermatitis, alopecia, and diarrhea, only 20% of cases present with this triad.2 Zinc deficiency in AE can either be acquired or inborn (congenital). Acquired forms can occur from dietary inadequacy or malabsorption, whereas genetic causes are related to an autosomal-recessive disorder affecting zinc transporters.1 We report a case of a 3-month-old female infant with acquired AE who was successfully treated with zinc supplementation over the course of 3 weeks.
Case Report
A 3-month-old female infant presented to the emergency department with a rash of 2 weeks’ duration. She was born full term with no birth complications. The patient’s mother reported that the rash started on the cheeks, then enlarged and spread to the neck, back, and perineum. The patient also had been having diarrhea during this time. She previously had received mupirocin and cephalexin with no response to treatment. Maternal history was negative for lupus, and the mother’s diet consisted of a variety of foods but not many vegetables. The patient was exclusively breastfed, and there was no pertinent history of similar rashes occurring in other family members.
Physical examination revealed the patient had annular and polycyclic, hyperkeratotic, crusted papules and plaques on the cheeks, neck, back, and axillae, as well as the perineum/groin and perianal regions (Figure 1). The differential diagnosis at the time included neonatal lupus, zinc deficiency, and syphilis. Relevant laboratory testing and a shave biopsy of the left axilla were obtained.

Pertinent laboratory findings included a low zinc level (23 μg/dL [reference range, 26–141 μg/dL]), low alkaline phosphatase level (74 U/L [reference range, 94–486 U/L]), and thrombocytosis (826×109/L [reference range, 150–400×109/L). Results for antinuclear antibody and anti–Sjögren syndrome–related antigen A and B antibody testing were negative. A rapid plasma reagin test was nonreactive. Histologic examination revealed psoriasiform hyperplasia with overlying confluent parakeratosis, focal spongiosis, multiple dyskeratotic keratinocytes, and mitotic figures (Figure 2). Ballooning was evident in focal cells in the subcorneal region in addition to an accompanying lymphocytic infiltrate and occasional neutrophils.

The patient was given a 10-mg/mL suspension of elemental zinc and was advised to take 1 mL (10 mg) by mouth twice daily with food. This dosage equated to 3 mg/kg/d. On follow-up 3 weeks later, the skin began to clear (Figure 3). Follow-up laboratory testing showed an increase in zinc (114 μg/dL) and alkaline phosphatase levels (313 U/L). The patient was able to discontinue the zinc supplementation, and follow-up during the next year revealed no recurrence.

Comment
Etiology of AE—Acrodermatitis enteropathica was first identified in 1942 as an acral rash associated with diarrhea3; in 1973, Barnes and Moynahan4 discovered zinc deficiency as a causal agent for these findings. The causes of AE are further subclassified as either an acquired or inborn etiology. Congenital causes commonly are seen in infants within the first few months of life, whereas acquired forms are seen at any age. Acquired forms in infants can occur from failure of the mother to secrete zinc in breast milk, low maternal serum zinc levels, or other reasons causing low nutritional intake. A single mutation in the SLC30A2 gene has been found to markedly reduce zinc concentrations in breast milk, thus causing zinc deficiency in breastfed infants.5 Other acquired forms can be caused by malabsorption, sometimes after surgery such as intestinal bypass or from intravenous nutrition without sufficient zinc.1 The congenital form of AE is an autosomal-recessive disorder occurring from mutations in the SLC39A4 gene located on band 8q24.3. Affected individuals have a decreased ability to absorb zinc in the small intestine because of defects in zinc transporters ZIP and ZnT.6 Based on our patient’s laboratory findings and history, it is believed that the zinc deficiency was acquired, as the condition normalized with repletion and has not required any supplementation in the year of follow-up. In addition, the absence of a pertinent family history supported an acquired diagnosis, which has various etiologies, whereas the congenital form primarily is a genetic disease.
Management—Treatment of AE includes supplementation with oral elemental zinc; however, there are scant evidence-based recommendations on the exact dose of zinc to be given. Generally, the recommended amount is 3 mg/kg/d.8 For individuals with the congenital form of AE, lifelong zinc supplementation is additionally recommended.9 It is important to recognize this presentation because the patient can develop worsening irritability, severe diarrhea, nail dystrophy, hair loss, immune dysfunction, and numerous ophthalmic disorders if left untreated. Acute zinc toxicity due to excess administration is rare, with symptoms of nausea and vomiting occurring with dosages of 50 to 100 mg/d. Additionally, dosages of up to 70 mg twice weekly have been provided without any toxic effect.10 In our case, 3 mg/kg/d of oral zinc supplementation proved to be effective in resolving the patient’s symptoms of acquired zinc deficiency.
Differential Diagnosis—It is important to note that deficiencies of other nutrients may present as an AE-like eruption called acrodermatitis dysmetabolica (AD). Both diseases may present with the triad of dermatitis, alopecia, and diarrhea; however, AD is associated with inborn errors of metabolism. There have been cases that describe AD in patients with a zinc deficiency in conjunction with a deficiency of branched-chain amino acids.11,12 It is important to consider AD in the differential diagnosis of an AE eruption, especially in the context of a metabolic disorder, as it may affect the treatment plan. One case described the dermatitis of AD as not responding to zinc supplementation alone, while another described improvement after increasing an isoleucine supplementation dose.11,12
Other considerations in the differential diagnoses include AE-like conditions such as biotinidase deficiency, multiple carboxylase deficiency, and essential fatty acid deficiency. An AE-like condition may present with the triad of dermatitis, alopecia, and diarrhea. However, unlike in true AE, zinc and alkaline phosphatase levels tend to be normal in these conditions. Other features seen in AE-like conditions depend on the underlying cause but often include failure to thrive, neurologic defects, ophthalmic abnormalities, and metabolic abnormalities.13
- Acrodermatitis enteropathica. National Organization for Rare Disorders. Accessed October 16, 2022. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica/
- Perafán-Riveros C, França LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Danbolt N. Acrodermatitis enteropathica. Br J Dermatol. 1979;100:37-40.
- Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329.
- Lee S, Zhou Y, Gill DL, et al. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci Rep. 2018;8:3542.
- Kaur S, Sangwan A, Sahu P, et al. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol. 2016;17:35-37.
- Dela Rosa KM, James WD. Acrodermatitis enteropathica workup. Medscape. Updated June 4, 2021. Accessed October 16, 2022. https://emedicine.medscape.com/article/1102575-workup#showall
- Ngan V, Gangakhedkar A, Oakley A. Acrodermatitis enteropathica. DermNet. Accessed October 16, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica/
- Ranugha P, Sethi P, Veeranna S. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018;24:13030/qt1w9002sr.
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26:356-365.
- Samady JA, Schwartz RA, Shih LY, et al. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. 2000;27:604-608.
- Flores K, Chikowski R, Morrell DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. 2016;41:651-654.
- Jones L, Oakley A. Acrodermatitis enteropathica-like conditions. DermNet. Accessed August 30, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica-like-conditions
- Acrodermatitis enteropathica. National Organization for Rare Disorders. Accessed October 16, 2022. https://rarediseases.org/rare-diseases/acrodermatitis-enteropathica/
- Perafán-Riveros C, França LFS, Alves ACF, et al. Acrodermatitis enteropathica: case report and review of the literature. Pediatr Dermatol. 2002;19:426-431.
- Danbolt N. Acrodermatitis enteropathica. Br J Dermatol. 1979;100:37-40.
- Barnes PM, Moynahan EJ. Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med. 1973;66:327-329.
- Lee S, Zhou Y, Gill DL, et al. A genetic variant in SLC30A2 causes breast dysfunction during lactation by inducing ER stress, oxidative stress and epithelial barrier defects. Sci Rep. 2018;8:3542.
- Kaur S, Sangwan A, Sahu P, et al. Clinical variants of acrodermatitis enteropathica and its co-relation with genetics. Indian J Paediatr Dermatol. 2016;17:35-37.
- Dela Rosa KM, James WD. Acrodermatitis enteropathica workup. Medscape. Updated June 4, 2021. Accessed October 16, 2022. https://emedicine.medscape.com/article/1102575-workup#showall
- Ngan V, Gangakhedkar A, Oakley A. Acrodermatitis enteropathica. DermNet. Accessed October 16, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica/
- Ranugha P, Sethi P, Veeranna S. Acrodermatitis enteropathica: the need for sustained high dose zinc supplementation. Dermatol Online J. 2018;24:13030/qt1w9002sr.
- Larson CP, Roy SK, Khan AI, et al. Zinc treatment to under-five children: applications to improve child survival and reduce burden of disease. J Health Popul Nutr. 2008;26:356-365.
- Samady JA, Schwartz RA, Shih LY, et al. Acrodermatitis enteropathica-like eruption in an infant with nonketotic hyperglycinemia. J Dermatol. 2000;27:604-608.
- Flores K, Chikowski R, Morrell DS. Acrodermatitis dysmetabolica in an infant with maple syrup urine disease. Clin Exp Dermatol. 2016;41:651-654.
- Jones L, Oakley A. Acrodermatitis enteropathica-like conditions. DermNet. Accessed August 30, 2022. https://dermnetnz.org/topics/acrodermatitis-enteropathica-like-conditions
Practice Points
- Although clinically characterized by the triad of acral and periorificial dermatitis, alopecia, and diarrhea, most cases of acrodermatitis enteropathica (AE) present with only partial features of this syndrome.
- Low levels of zinc-dependent enzymes such as alkaline phosphatase may support the diagnosis of AE.
Photoallergic Contact Dermatitis: No Fun in the Sun
Photoallergic contact dermatitis (PACD), a subtype of allergic contact dermatitis that occurs because of the specific combination of exposure to an exogenous chemical applied topically to the skin and UV radiation, may be more common than was once thought.1 Although the incidence in the general population is unknown, current research points to approximately 20% to 40% of patients with suspected photosensitivity having a PACD diagnosis.2 Recently, the North American Contact Dermatitis Group (NACDG) reported that 21% of 373 patients undergoing photopatch testing (PPT) were diagnosed with PACD2; however, PPT is not routinely performed, which may contribute to underdiagnosis.
Mechanism of Disease
Similar to allergic contact dermatitis, PACD is a delayed type IV hypersensitivity reaction; however, it only occurs when an exogenous chemical is applied topically to the skin with concomitant exposure to UV radiation, usually in the UVA range (315–400 nm).3,4 When exposed to UV radiation, it is thought that the exogenous chemical combines with a protein in the skin and transforms into a photoantigen. In the sensitization phase, the photoantigen is taken up by antigen-presenting cells in the epidermis and transported to local lymph nodes where antigen-specific T cells are generated.5 In the elicitation phase, the inflammatory reaction of PACD occurs upon subsequent exposure to the same chemical plus UV radiation.4 Development of PACD does not necessarily depend on the dose of the chemical or the amount of UV radiation.6 Why certain individuals may be more susceptible is unknown, though major histocompatibility complex haplotypes could be influential.7,8
Clinical Manifestations
Photoallergic contact dermatitis primarily presents in sun-exposed areas of the skin (eg, face, neck, V area of the chest, dorsal upper extremities) with sparing of naturally photoprotected sites, such as the upper eyelids and nasolabial and retroauricular folds. Other than its characteristic photodistribution, PACD often is clinically indistinguishable from routine allergic contact dermatitis. It manifests as a pruritic, poorly demarcated, eczematous or sometimes vesiculobullous eruption that develops in a delayed fashion—24 to 72 hours after sun exposure. The dermatitis may extend to other parts of the body either through spread of the chemical agent by the hands or clothing or due to the systemic nature of the immune response. The severity of the presentation can vary depending on multiple factors, such as concentration and absorption of the agent, length of exposure, intensity and duration of UV radiation exposure, and individual susceptibility.4 Chronic PACD may become lichenified. Generally, rashes resolve after discontinuation of the causative agent; however, long-term exposure may lead to development of chronic actinic dermatitis, with persistent photodistributed eczema regardless of contact with the initial inciting agent.9
Differential Diagnosis
The differential diagnosis for patients presenting with photodistributed dermatitis is broad; therefore, taking a thorough history is important. Considerations include age of onset, timing and persistence of reactions, use of topical and systemic medications (both prescription and over-the-counter [OTC]), personal care products, occupation, and hobbies, as well as a thorough review of systems.
It is important to distinguish PACD from phototoxic contact dermatitis (PTCD)(also known as photoirritant contact dermatitis)(Table). Asking about the onset and timing of the eruption may be critical for distinction, as PTCD can occur within minutes to hours of the first exposure to a chemical and UV radiation, while there is a sensitization delay in PACD.6 Phytophotodermatitis is a well-known type of PTCD caused by exposure to furocoumarin-containing plants, most commonly limes.10 Other causes of PTCD include tar products and certain medications.11 Importantly, PPT to a known phototoxic chemical should never be performed because it will cause a strong reaction in anyone tested, regardless of exposure history.
Other diagnoses to consider include photoaggravated dermatoses (eg, atopic dermatitis, lupus erythematosus, dermatomyositis) and idiopathic photodermatoses (eg, chronic actinic dermatitis, actinic prurigo, polymorphous light eruption). Although atopic dermatitis usually improves with UV light exposure, photoaggravated atopic dermatitis is suggested in eczema patients who flare with sun exposure, in a seasonal pattern, or after phototherapy; this condition is challenging to differentiate from PACD if PPT is not performed.12 The diagnosis of idiopathic photodermatoses is nuanced; however, asking about the timeline of the reaction including onset, duration, and persistence, as well as characterization of unique clinical features, can help in differentiation.13 In certain scenarios, a biopsy may be helpful. A thorough review of systems will help to assess for autoimmune connective tissue disorders, and relevant serologies should be checked as indicated.
Diagnosis
Histologically, PACD presents similarly to allergic contact dermatitis with spongiotic dermatitis; therefore, biopsy cannot be relied upon to make the diagnosis.6 Photopatch testing is required for definitive diagnosis. It is reasonable to perform PPT in any patient with chronic dermatitis primarily affecting sun-exposed areas without a clear alternative diagnosis.14,15 Of note, at present there are no North American consensus guidelines for PPT, but typically duplicate sets of photoallergens are applied to both sides of the patient’s back and one side is exposed to UVA radiation. The reactions are compared after 48 to 96 hours.15 A positive reaction only at the irradiated site is consistent with photoallergy, while a reaction of equal strength at both the irradiated and nonirradiated sites indicates regular contact allergy. The case of a reaction occurring at both sites with a stronger response at the irradiated site is known as photoaggravated contact allergy, which can be thought of as allergic contact dermatitis that worsens but does not solely occur with exposure to sunlight.
Although PPT is necessary for the accurate diagnosis of PACD, it is infrequently used. Two surveys of 112 and 117 American Contact Dermatitis Society members, respectively, have revealed that only around half performed PPT, most of them testing fewer than 20 times per year.16,17 Additionally, there was variability in the test methodology and allergens employed. Nevertheless, most respondents tested sunscreens, nonsteroidal anti-inflammatory drugs (NSAIDs), fragrances, and their patients’ own products.16,17 The most common reasons for not performing PPT were lack of equipment, insufficient skills, rare clinical suspicion, and cost. Dermatologists at academic centers performed more PPT than those in other practice settings, including multispecialty group practices and private offices.16 These findings highlight multiple factors that may contribute to reduced patient access to PPT and thus potential underdiagnosis of PACD.
Common Photoallergens
The most common photoallergens change over time in response to market trends; for example, fragrance was once a top photoallergen in the United States in the 1970s and 1980s but declined in prominence after musk ambrette—the primary allergen associated with PACD at the time—was removed as an ingredient in fragrances.18
In the largest and most recent PPT series from North America (1999-2009),2 sunscreens comprised 7 of the top 10 most common photoallergens, which is consistent with other studies showing sunscreens to be the most common North American photoallergens.19-22 The frequency of PACD due to sunscreens likely relates to their increasing use worldwide as awareness of photocarcinogenesis and photoaging grows, as well as the common use of UV filters in nonsunscreen personal care products, ranging from lip balms to perfumes and bodywashes. Chemical (organic) UV filters—in particular oxybenzone (benzophenone-3) and avobenzone (butyl methoxydibenzoylmethane)—are the most common sunscreen photoallergens.2,23 Para-aminobenzoic acid was once a common photoallergen, but it is no longer used in US sunscreens due to safety concerns.19,20 The physical (inorganic) UV filters zinc oxide and titanium dioxide are not known photosensitizers.
Methylisothiazolinone (MI) is a highly allergenic preservative commonly used in a wide array of personal care products, including sunscreens.24 In the most recent NACDG patch test data, MI was the second most common contact allergen.25 Allergic contact dermatitis caused by MI in sunscreen can mimic PACD.26 In addition, MI can cause photoaggravated contact dermatitis, with some affected patients experiencing ongoing photosensitivity even after avoiding this allergen.26-30 The European Union and Canada have introduced restrictions on the use of MI in personal care products, but no such regulatory measures have been taken in the United States to date.25,31,32
After sunscreens, another common cause of PACD are topical NSAIDs, which are frequently used for musculoskeletal pain relief. These are of particular concern in Europe, where a variety of formulations are widely available OTC.33 Ketoprofen and etofenamate are responsible for the largest number of PACD reactions in Europe.2,34,35 Meanwhile, the only OTC topical NSAID available in the United States is diclofenac gel, which was approved in 2020. Cases of PACD due to use of diclofenac gel have been reported in the literature, but testing in larger populations is needed.36-39
Notably, ketoprofen may co- or cross-react with certain UV filters—oxybenzone and octocrylene—and the lipid-lowering agent fenofibrate due to chemical similarities.40-43 Despite the relatively high number of photoallergic reactions to ketoprofen in the NACDG photopatch series, only 25% (5/20) were considered clinically relevant (ie, the allergen could not be verified as present in the known skin contactants of the patient, and the patient was not exposed to circumstances in which contact with materials known to contain the allergen would likely occur), which suggests that they likely represented cross-reactions in patients sensitized to sunscreens.2
Other agents that may cause PACD include antimicrobials, plants and plant derivatives, and pesticides.2,4,18 The antimicrobial fentichlor is a common cause of positive PPT reactions, but it rarely is clinically relevant.44
Treatment
The primary management of PACD centers on identification of the causative photoallergen to avoid future exposure. Patients should be educated on the various names by which the causative allergen can be identified on product labels and should be given a list of safe products that are free from relevant allergens and cross-reacting chemicals.45 Additionally, sun protection education should be provided. Exposure to UVA radiation can occur through windows, making the use of broad-spectrum sunscreens and protective clothing crucial. In cases of sunscreen-induced PACD, the responsible chemical UV filter(s) should be avoided, or alternatively, patients may use physical sunscreens containing only zinc oxide and/or titanium dioxide as active ingredients, as these are not known to cause PACD.4
When avoidance alone is insufficient, topical corticosteroids are the usual first-line treatment for localized PACD. When steroid-sparing treatments are preferred, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used. If PACD is more widespread and severe, systemic therapy using steroids or steroid-sparing agents may be necessary to provide symptomatic relief.4
Final Interpretation
Photoallergic contact dermatitis is not uncommon, particularly among photosensitive patients. Most cases are due to sunscreens or topical NSAIDs. Consideration of PPT should be given in any patient with a chronic photodistributed dermatitis to evaluate for the possibility of PACD.
- Darvay A, White IR, Rycroft RJ, et al. Photoallergic contact dermatitis is uncommon. Br J Dermatol. 2001;145:597-601.
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291.
- Kerr A, Ferguson J. Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2010;26:56-65.
- As¸kın Ö, Cesur SK, Engin B, et al. Photoallergic contact dermatitis. Curr Derm Rep. 2019;8:157-163.
- Wilm A, Berneburg M. Photoallergy. J Dtsch Dermatol Ges. 2015;13:7-13.
- DeLeo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Imai S, Atarashi K, Ikesue K, et al. Establishment of murine model of allergic photocontact dermatitis to ketoprofen and characterization of pathogenic T cells. J Dermatol Sci. 2006;41:127-136.
- Tokura Y, Yagi H, Satoh T, et al. Inhibitory effect of melanin pigment on sensitization and elicitation of murine contact photosensitivity: mechanism of low responsiveness in C57BL/10 background mice. J Invest Dermatol. 1993;101:673-678.
- Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007;6:431-443.
- Janusz SC, Schwartz RA. Botanical briefs: phytophotodermatitis is an occupational and recreational dermatosis in the limelight. Cutis. 2021;107:187-189.
- Atwal SK, Chen A, Adler BL. Phototoxic contact dermatitis from over-the-counter 8-methoxypsoralen. Cutis. 2022;109:E2-E3.
- Rutter KJ, Farrar MD, Marjanovic EJ, et al. Clinicophotobiological characterization of photoaggravated atopic dermatitis [published online July 27, 2022]. JAMA Dermatol. doi:10.1001/jamadermatol.2022.2823
- Lecha M. Idiopathic photodermatoses: clinical, diagnostic and therapeutic aspects. J Eur Acad Dermatol Venereol. 2001;15:499-505.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers; 2016.
- Bruynzeel DP, Ferguson J, Andersen K, et al. Photopatch testing: a consensus methodology for Europe. J Eur Acad Dermatol Venereol. 2004;18:679-682.
- Kim T, Taylor JS, Maibach HI, et al. Photopatch testing among members of the American Contact Dermatitis Society. Dermatitis. 2020;31:59-67.
- Asemota E, Crawford G, Kovarik C, et al. A survey examining photopatch test and phototest methodologies of contact dermatologists in the United States: platform for developing a consensus. Dermatitis. 2017;28:265-269.
- Scalf LA, Davis MD, Rohlinger AL, et al. Photopatch testing of 182 patients: a 6-year experience at the Mayo Clinic. Dermatitis. 2009;20:44-52.
- Greenspoon J, Ahluwalia R, Juma N, et al. Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis. 2013;24:29-32.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens. review of a 15-year experience and of the literature. Contact Dermatitis. 1997;37:221-232.
- Collaris EJ, Frank J. Photoallergic contact dermatitis caused by ultraviolet filters in different sunscreens. Int J Dermatol. 2008;47(suppl 1):35-37.
- Heurung AR, Raju SI, Warshaw EM. Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis. 2014;25:289-326.
- Reeder M, Atwater AR. Methylisothiazolinone and isothiazolinone allergy. Cutis. 2019;104:94-96.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123.
- Kullberg SA, Voller LM, Warshaw EM. Methylisothiazolinone in “dermatology-recommended” sunscreens: an important mimicker of photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2021;37:366-370.
- Herman A, Aerts O, de Montjoye L, et al. Isothiazolinone derivatives and allergic contact dermatitis: a review and update. J Eur Acad Dermatol Venereol. 2019;33:267-276.
- Adler BL, Houle MC, Pratt M. Photoaggravated contact dermatitis to methylisothiazolinone and associated photosensitivity: a case series [published online January 25, 2022]. Dermatitis. doi:10.1097/DER.0000000000000833
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Pirmez R, Fernandes AL, Melo MG. Photoaggravated contact dermatitis to Kathon CG (methylchloroisothiazolinone/methylisothiazolinone): a novel pattern of involvement in a growing epidemic?. Br J Dermatol. 2015;173:1343-1344.
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures.J Eur Acad Dermatol Venereol. 2020;34:333-339.
- Government of Canada. Changes to the cosmetic ingredient hotlist. December 3, 2019. Updated August 26, 2022. Accessed October 20, 2022. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/changes.html
- Barkin RL. Topical nonsteroidal anti-inflammatory drugs: the importance of drug, delivery, and therapeutic outcome. Am J Ther. 2015;22:388-407.
- European Multicentre Photopatch Test Study (EMCPPTS) Taskforce. A European multicentre photopatch test study. Br J Dermatol. 2012;166:1002-1009.
- Ophaswongse S, Maibach H. Topical nonsteroidal antiinflammatory drugs: allergic and photoallergic contact dermatitis and phototoxicity. Contact Dermatitis. 1993;29:57-64.
- Kowalzick L, Ziegler H. Photoallergic contact dermatitis from topical diclofenac in Solaraze gel. Contact Dermatitis. 2006;54:348-349.
- Montoro J, Rodríguez M, Díaz M, et al. Photoallergic contact dermatitis due to diclofenac. Contact Dermatitis. 2003;48:115.
- Fernández-Jorge B, Goday-Buján JJ, Murga M, et al. Photoallergic contact dermatitis due to diclofenac with cross-reaction to aceclofenac: two case reports. Contact Dermatitis. 2009;61:236-237.
- Akat PB. Severe photosensitivity reaction induced by topical diclofenac. Indian J Pharmacol. 2013;45:408-409.
- Leroy D, Dompmartin A, Szczurko C, et al. Photodermatitis from ketoprofen with cross-reactivity to fenofibrate and benzophenones. Photodermatol Photoimmunol Photomed. 1997;13:93-97.
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166.
- Matsushita T, Kamide R. Five cases of photocontact dermatitisdue to topical ketoprofen: photopatch testing and cross-reaction study. Photodermatol Photoimmunol Photomed. 2001;17:26-31.
- de Groot AC, Roberts DW. Contact and photocontact allergy to octocrylene: a review. Contact Dermatitis. 2014;70:193-204.
- Wolverton JE, Soter NA, Cohen DE. Fentichlor photocontact dermatitis: a persistent enigma. Dermatitis. 2013;24:77-81.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
Photoallergic contact dermatitis (PACD), a subtype of allergic contact dermatitis that occurs because of the specific combination of exposure to an exogenous chemical applied topically to the skin and UV radiation, may be more common than was once thought.1 Although the incidence in the general population is unknown, current research points to approximately 20% to 40% of patients with suspected photosensitivity having a PACD diagnosis.2 Recently, the North American Contact Dermatitis Group (NACDG) reported that 21% of 373 patients undergoing photopatch testing (PPT) were diagnosed with PACD2; however, PPT is not routinely performed, which may contribute to underdiagnosis.
Mechanism of Disease
Similar to allergic contact dermatitis, PACD is a delayed type IV hypersensitivity reaction; however, it only occurs when an exogenous chemical is applied topically to the skin with concomitant exposure to UV radiation, usually in the UVA range (315–400 nm).3,4 When exposed to UV radiation, it is thought that the exogenous chemical combines with a protein in the skin and transforms into a photoantigen. In the sensitization phase, the photoantigen is taken up by antigen-presenting cells in the epidermis and transported to local lymph nodes where antigen-specific T cells are generated.5 In the elicitation phase, the inflammatory reaction of PACD occurs upon subsequent exposure to the same chemical plus UV radiation.4 Development of PACD does not necessarily depend on the dose of the chemical or the amount of UV radiation.6 Why certain individuals may be more susceptible is unknown, though major histocompatibility complex haplotypes could be influential.7,8
Clinical Manifestations
Photoallergic contact dermatitis primarily presents in sun-exposed areas of the skin (eg, face, neck, V area of the chest, dorsal upper extremities) with sparing of naturally photoprotected sites, such as the upper eyelids and nasolabial and retroauricular folds. Other than its characteristic photodistribution, PACD often is clinically indistinguishable from routine allergic contact dermatitis. It manifests as a pruritic, poorly demarcated, eczematous or sometimes vesiculobullous eruption that develops in a delayed fashion—24 to 72 hours after sun exposure. The dermatitis may extend to other parts of the body either through spread of the chemical agent by the hands or clothing or due to the systemic nature of the immune response. The severity of the presentation can vary depending on multiple factors, such as concentration and absorption of the agent, length of exposure, intensity and duration of UV radiation exposure, and individual susceptibility.4 Chronic PACD may become lichenified. Generally, rashes resolve after discontinuation of the causative agent; however, long-term exposure may lead to development of chronic actinic dermatitis, with persistent photodistributed eczema regardless of contact with the initial inciting agent.9
Differential Diagnosis
The differential diagnosis for patients presenting with photodistributed dermatitis is broad; therefore, taking a thorough history is important. Considerations include age of onset, timing and persistence of reactions, use of topical and systemic medications (both prescription and over-the-counter [OTC]), personal care products, occupation, and hobbies, as well as a thorough review of systems.
It is important to distinguish PACD from phototoxic contact dermatitis (PTCD)(also known as photoirritant contact dermatitis)(Table). Asking about the onset and timing of the eruption may be critical for distinction, as PTCD can occur within minutes to hours of the first exposure to a chemical and UV radiation, while there is a sensitization delay in PACD.6 Phytophotodermatitis is a well-known type of PTCD caused by exposure to furocoumarin-containing plants, most commonly limes.10 Other causes of PTCD include tar products and certain medications.11 Importantly, PPT to a known phototoxic chemical should never be performed because it will cause a strong reaction in anyone tested, regardless of exposure history.

Other diagnoses to consider include photoaggravated dermatoses (eg, atopic dermatitis, lupus erythematosus, dermatomyositis) and idiopathic photodermatoses (eg, chronic actinic dermatitis, actinic prurigo, polymorphous light eruption). Although atopic dermatitis usually improves with UV light exposure, photoaggravated atopic dermatitis is suggested in eczema patients who flare with sun exposure, in a seasonal pattern, or after phototherapy; this condition is challenging to differentiate from PACD if PPT is not performed.12 The diagnosis of idiopathic photodermatoses is nuanced; however, asking about the timeline of the reaction including onset, duration, and persistence, as well as characterization of unique clinical features, can help in differentiation.13 In certain scenarios, a biopsy may be helpful. A thorough review of systems will help to assess for autoimmune connective tissue disorders, and relevant serologies should be checked as indicated.
Diagnosis
Histologically, PACD presents similarly to allergic contact dermatitis with spongiotic dermatitis; therefore, biopsy cannot be relied upon to make the diagnosis.6 Photopatch testing is required for definitive diagnosis. It is reasonable to perform PPT in any patient with chronic dermatitis primarily affecting sun-exposed areas without a clear alternative diagnosis.14,15 Of note, at present there are no North American consensus guidelines for PPT, but typically duplicate sets of photoallergens are applied to both sides of the patient’s back and one side is exposed to UVA radiation. The reactions are compared after 48 to 96 hours.15 A positive reaction only at the irradiated site is consistent with photoallergy, while a reaction of equal strength at both the irradiated and nonirradiated sites indicates regular contact allergy. The case of a reaction occurring at both sites with a stronger response at the irradiated site is known as photoaggravated contact allergy, which can be thought of as allergic contact dermatitis that worsens but does not solely occur with exposure to sunlight.
Although PPT is necessary for the accurate diagnosis of PACD, it is infrequently used. Two surveys of 112 and 117 American Contact Dermatitis Society members, respectively, have revealed that only around half performed PPT, most of them testing fewer than 20 times per year.16,17 Additionally, there was variability in the test methodology and allergens employed. Nevertheless, most respondents tested sunscreens, nonsteroidal anti-inflammatory drugs (NSAIDs), fragrances, and their patients’ own products.16,17 The most common reasons for not performing PPT were lack of equipment, insufficient skills, rare clinical suspicion, and cost. Dermatologists at academic centers performed more PPT than those in other practice settings, including multispecialty group practices and private offices.16 These findings highlight multiple factors that may contribute to reduced patient access to PPT and thus potential underdiagnosis of PACD.
Common Photoallergens
The most common photoallergens change over time in response to market trends; for example, fragrance was once a top photoallergen in the United States in the 1970s and 1980s but declined in prominence after musk ambrette—the primary allergen associated with PACD at the time—was removed as an ingredient in fragrances.18
In the largest and most recent PPT series from North America (1999-2009),2 sunscreens comprised 7 of the top 10 most common photoallergens, which is consistent with other studies showing sunscreens to be the most common North American photoallergens.19-22 The frequency of PACD due to sunscreens likely relates to their increasing use worldwide as awareness of photocarcinogenesis and photoaging grows, as well as the common use of UV filters in nonsunscreen personal care products, ranging from lip balms to perfumes and bodywashes. Chemical (organic) UV filters—in particular oxybenzone (benzophenone-3) and avobenzone (butyl methoxydibenzoylmethane)—are the most common sunscreen photoallergens.2,23 Para-aminobenzoic acid was once a common photoallergen, but it is no longer used in US sunscreens due to safety concerns.19,20 The physical (inorganic) UV filters zinc oxide and titanium dioxide are not known photosensitizers.
Methylisothiazolinone (MI) is a highly allergenic preservative commonly used in a wide array of personal care products, including sunscreens.24 In the most recent NACDG patch test data, MI was the second most common contact allergen.25 Allergic contact dermatitis caused by MI in sunscreen can mimic PACD.26 In addition, MI can cause photoaggravated contact dermatitis, with some affected patients experiencing ongoing photosensitivity even after avoiding this allergen.26-30 The European Union and Canada have introduced restrictions on the use of MI in personal care products, but no such regulatory measures have been taken in the United States to date.25,31,32
After sunscreens, another common cause of PACD are topical NSAIDs, which are frequently used for musculoskeletal pain relief. These are of particular concern in Europe, where a variety of formulations are widely available OTC.33 Ketoprofen and etofenamate are responsible for the largest number of PACD reactions in Europe.2,34,35 Meanwhile, the only OTC topical NSAID available in the United States is diclofenac gel, which was approved in 2020. Cases of PACD due to use of diclofenac gel have been reported in the literature, but testing in larger populations is needed.36-39
Notably, ketoprofen may co- or cross-react with certain UV filters—oxybenzone and octocrylene—and the lipid-lowering agent fenofibrate due to chemical similarities.40-43 Despite the relatively high number of photoallergic reactions to ketoprofen in the NACDG photopatch series, only 25% (5/20) were considered clinically relevant (ie, the allergen could not be verified as present in the known skin contactants of the patient, and the patient was not exposed to circumstances in which contact with materials known to contain the allergen would likely occur), which suggests that they likely represented cross-reactions in patients sensitized to sunscreens.2
Other agents that may cause PACD include antimicrobials, plants and plant derivatives, and pesticides.2,4,18 The antimicrobial fentichlor is a common cause of positive PPT reactions, but it rarely is clinically relevant.44
Treatment
The primary management of PACD centers on identification of the causative photoallergen to avoid future exposure. Patients should be educated on the various names by which the causative allergen can be identified on product labels and should be given a list of safe products that are free from relevant allergens and cross-reacting chemicals.45 Additionally, sun protection education should be provided. Exposure to UVA radiation can occur through windows, making the use of broad-spectrum sunscreens and protective clothing crucial. In cases of sunscreen-induced PACD, the responsible chemical UV filter(s) should be avoided, or alternatively, patients may use physical sunscreens containing only zinc oxide and/or titanium dioxide as active ingredients, as these are not known to cause PACD.4
When avoidance alone is insufficient, topical corticosteroids are the usual first-line treatment for localized PACD. When steroid-sparing treatments are preferred, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used. If PACD is more widespread and severe, systemic therapy using steroids or steroid-sparing agents may be necessary to provide symptomatic relief.4
Final Interpretation
Photoallergic contact dermatitis is not uncommon, particularly among photosensitive patients. Most cases are due to sunscreens or topical NSAIDs. Consideration of PPT should be given in any patient with a chronic photodistributed dermatitis to evaluate for the possibility of PACD.
Photoallergic contact dermatitis (PACD), a subtype of allergic contact dermatitis that occurs because of the specific combination of exposure to an exogenous chemical applied topically to the skin and UV radiation, may be more common than was once thought.1 Although the incidence in the general population is unknown, current research points to approximately 20% to 40% of patients with suspected photosensitivity having a PACD diagnosis.2 Recently, the North American Contact Dermatitis Group (NACDG) reported that 21% of 373 patients undergoing photopatch testing (PPT) were diagnosed with PACD2; however, PPT is not routinely performed, which may contribute to underdiagnosis.
Mechanism of Disease
Similar to allergic contact dermatitis, PACD is a delayed type IV hypersensitivity reaction; however, it only occurs when an exogenous chemical is applied topically to the skin with concomitant exposure to UV radiation, usually in the UVA range (315–400 nm).3,4 When exposed to UV radiation, it is thought that the exogenous chemical combines with a protein in the skin and transforms into a photoantigen. In the sensitization phase, the photoantigen is taken up by antigen-presenting cells in the epidermis and transported to local lymph nodes where antigen-specific T cells are generated.5 In the elicitation phase, the inflammatory reaction of PACD occurs upon subsequent exposure to the same chemical plus UV radiation.4 Development of PACD does not necessarily depend on the dose of the chemical or the amount of UV radiation.6 Why certain individuals may be more susceptible is unknown, though major histocompatibility complex haplotypes could be influential.7,8
Clinical Manifestations
Photoallergic contact dermatitis primarily presents in sun-exposed areas of the skin (eg, face, neck, V area of the chest, dorsal upper extremities) with sparing of naturally photoprotected sites, such as the upper eyelids and nasolabial and retroauricular folds. Other than its characteristic photodistribution, PACD often is clinically indistinguishable from routine allergic contact dermatitis. It manifests as a pruritic, poorly demarcated, eczematous or sometimes vesiculobullous eruption that develops in a delayed fashion—24 to 72 hours after sun exposure. The dermatitis may extend to other parts of the body either through spread of the chemical agent by the hands or clothing or due to the systemic nature of the immune response. The severity of the presentation can vary depending on multiple factors, such as concentration and absorption of the agent, length of exposure, intensity and duration of UV radiation exposure, and individual susceptibility.4 Chronic PACD may become lichenified. Generally, rashes resolve after discontinuation of the causative agent; however, long-term exposure may lead to development of chronic actinic dermatitis, with persistent photodistributed eczema regardless of contact with the initial inciting agent.9
Differential Diagnosis
The differential diagnosis for patients presenting with photodistributed dermatitis is broad; therefore, taking a thorough history is important. Considerations include age of onset, timing and persistence of reactions, use of topical and systemic medications (both prescription and over-the-counter [OTC]), personal care products, occupation, and hobbies, as well as a thorough review of systems.
It is important to distinguish PACD from phototoxic contact dermatitis (PTCD)(also known as photoirritant contact dermatitis)(Table). Asking about the onset and timing of the eruption may be critical for distinction, as PTCD can occur within minutes to hours of the first exposure to a chemical and UV radiation, while there is a sensitization delay in PACD.6 Phytophotodermatitis is a well-known type of PTCD caused by exposure to furocoumarin-containing plants, most commonly limes.10 Other causes of PTCD include tar products and certain medications.11 Importantly, PPT to a known phototoxic chemical should never be performed because it will cause a strong reaction in anyone tested, regardless of exposure history.

Other diagnoses to consider include photoaggravated dermatoses (eg, atopic dermatitis, lupus erythematosus, dermatomyositis) and idiopathic photodermatoses (eg, chronic actinic dermatitis, actinic prurigo, polymorphous light eruption). Although atopic dermatitis usually improves with UV light exposure, photoaggravated atopic dermatitis is suggested in eczema patients who flare with sun exposure, in a seasonal pattern, or after phototherapy; this condition is challenging to differentiate from PACD if PPT is not performed.12 The diagnosis of idiopathic photodermatoses is nuanced; however, asking about the timeline of the reaction including onset, duration, and persistence, as well as characterization of unique clinical features, can help in differentiation.13 In certain scenarios, a biopsy may be helpful. A thorough review of systems will help to assess for autoimmune connective tissue disorders, and relevant serologies should be checked as indicated.
Diagnosis
Histologically, PACD presents similarly to allergic contact dermatitis with spongiotic dermatitis; therefore, biopsy cannot be relied upon to make the diagnosis.6 Photopatch testing is required for definitive diagnosis. It is reasonable to perform PPT in any patient with chronic dermatitis primarily affecting sun-exposed areas without a clear alternative diagnosis.14,15 Of note, at present there are no North American consensus guidelines for PPT, but typically duplicate sets of photoallergens are applied to both sides of the patient’s back and one side is exposed to UVA radiation. The reactions are compared after 48 to 96 hours.15 A positive reaction only at the irradiated site is consistent with photoallergy, while a reaction of equal strength at both the irradiated and nonirradiated sites indicates regular contact allergy. The case of a reaction occurring at both sites with a stronger response at the irradiated site is known as photoaggravated contact allergy, which can be thought of as allergic contact dermatitis that worsens but does not solely occur with exposure to sunlight.
Although PPT is necessary for the accurate diagnosis of PACD, it is infrequently used. Two surveys of 112 and 117 American Contact Dermatitis Society members, respectively, have revealed that only around half performed PPT, most of them testing fewer than 20 times per year.16,17 Additionally, there was variability in the test methodology and allergens employed. Nevertheless, most respondents tested sunscreens, nonsteroidal anti-inflammatory drugs (NSAIDs), fragrances, and their patients’ own products.16,17 The most common reasons for not performing PPT were lack of equipment, insufficient skills, rare clinical suspicion, and cost. Dermatologists at academic centers performed more PPT than those in other practice settings, including multispecialty group practices and private offices.16 These findings highlight multiple factors that may contribute to reduced patient access to PPT and thus potential underdiagnosis of PACD.
Common Photoallergens
The most common photoallergens change over time in response to market trends; for example, fragrance was once a top photoallergen in the United States in the 1970s and 1980s but declined in prominence after musk ambrette—the primary allergen associated with PACD at the time—was removed as an ingredient in fragrances.18
In the largest and most recent PPT series from North America (1999-2009),2 sunscreens comprised 7 of the top 10 most common photoallergens, which is consistent with other studies showing sunscreens to be the most common North American photoallergens.19-22 The frequency of PACD due to sunscreens likely relates to their increasing use worldwide as awareness of photocarcinogenesis and photoaging grows, as well as the common use of UV filters in nonsunscreen personal care products, ranging from lip balms to perfumes and bodywashes. Chemical (organic) UV filters—in particular oxybenzone (benzophenone-3) and avobenzone (butyl methoxydibenzoylmethane)—are the most common sunscreen photoallergens.2,23 Para-aminobenzoic acid was once a common photoallergen, but it is no longer used in US sunscreens due to safety concerns.19,20 The physical (inorganic) UV filters zinc oxide and titanium dioxide are not known photosensitizers.
Methylisothiazolinone (MI) is a highly allergenic preservative commonly used in a wide array of personal care products, including sunscreens.24 In the most recent NACDG patch test data, MI was the second most common contact allergen.25 Allergic contact dermatitis caused by MI in sunscreen can mimic PACD.26 In addition, MI can cause photoaggravated contact dermatitis, with some affected patients experiencing ongoing photosensitivity even after avoiding this allergen.26-30 The European Union and Canada have introduced restrictions on the use of MI in personal care products, but no such regulatory measures have been taken in the United States to date.25,31,32
After sunscreens, another common cause of PACD are topical NSAIDs, which are frequently used for musculoskeletal pain relief. These are of particular concern in Europe, where a variety of formulations are widely available OTC.33 Ketoprofen and etofenamate are responsible for the largest number of PACD reactions in Europe.2,34,35 Meanwhile, the only OTC topical NSAID available in the United States is diclofenac gel, which was approved in 2020. Cases of PACD due to use of diclofenac gel have been reported in the literature, but testing in larger populations is needed.36-39
Notably, ketoprofen may co- or cross-react with certain UV filters—oxybenzone and octocrylene—and the lipid-lowering agent fenofibrate due to chemical similarities.40-43 Despite the relatively high number of photoallergic reactions to ketoprofen in the NACDG photopatch series, only 25% (5/20) were considered clinically relevant (ie, the allergen could not be verified as present in the known skin contactants of the patient, and the patient was not exposed to circumstances in which contact with materials known to contain the allergen would likely occur), which suggests that they likely represented cross-reactions in patients sensitized to sunscreens.2
Other agents that may cause PACD include antimicrobials, plants and plant derivatives, and pesticides.2,4,18 The antimicrobial fentichlor is a common cause of positive PPT reactions, but it rarely is clinically relevant.44
Treatment
The primary management of PACD centers on identification of the causative photoallergen to avoid future exposure. Patients should be educated on the various names by which the causative allergen can be identified on product labels and should be given a list of safe products that are free from relevant allergens and cross-reacting chemicals.45 Additionally, sun protection education should be provided. Exposure to UVA radiation can occur through windows, making the use of broad-spectrum sunscreens and protective clothing crucial. In cases of sunscreen-induced PACD, the responsible chemical UV filter(s) should be avoided, or alternatively, patients may use physical sunscreens containing only zinc oxide and/or titanium dioxide as active ingredients, as these are not known to cause PACD.4
When avoidance alone is insufficient, topical corticosteroids are the usual first-line treatment for localized PACD. When steroid-sparing treatments are preferred, topical calcineurin inhibitors such as tacrolimus and pimecrolimus may be used. If PACD is more widespread and severe, systemic therapy using steroids or steroid-sparing agents may be necessary to provide symptomatic relief.4
Final Interpretation
Photoallergic contact dermatitis is not uncommon, particularly among photosensitive patients. Most cases are due to sunscreens or topical NSAIDs. Consideration of PPT should be given in any patient with a chronic photodistributed dermatitis to evaluate for the possibility of PACD.
- Darvay A, White IR, Rycroft RJ, et al. Photoallergic contact dermatitis is uncommon. Br J Dermatol. 2001;145:597-601.
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291.
- Kerr A, Ferguson J. Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2010;26:56-65.
- As¸kın Ö, Cesur SK, Engin B, et al. Photoallergic contact dermatitis. Curr Derm Rep. 2019;8:157-163.
- Wilm A, Berneburg M. Photoallergy. J Dtsch Dermatol Ges. 2015;13:7-13.
- DeLeo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Imai S, Atarashi K, Ikesue K, et al. Establishment of murine model of allergic photocontact dermatitis to ketoprofen and characterization of pathogenic T cells. J Dermatol Sci. 2006;41:127-136.
- Tokura Y, Yagi H, Satoh T, et al. Inhibitory effect of melanin pigment on sensitization and elicitation of murine contact photosensitivity: mechanism of low responsiveness in C57BL/10 background mice. J Invest Dermatol. 1993;101:673-678.
- Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007;6:431-443.
- Janusz SC, Schwartz RA. Botanical briefs: phytophotodermatitis is an occupational and recreational dermatosis in the limelight. Cutis. 2021;107:187-189.
- Atwal SK, Chen A, Adler BL. Phototoxic contact dermatitis from over-the-counter 8-methoxypsoralen. Cutis. 2022;109:E2-E3.
- Rutter KJ, Farrar MD, Marjanovic EJ, et al. Clinicophotobiological characterization of photoaggravated atopic dermatitis [published online July 27, 2022]. JAMA Dermatol. doi:10.1001/jamadermatol.2022.2823
- Lecha M. Idiopathic photodermatoses: clinical, diagnostic and therapeutic aspects. J Eur Acad Dermatol Venereol. 2001;15:499-505.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers; 2016.
- Bruynzeel DP, Ferguson J, Andersen K, et al. Photopatch testing: a consensus methodology for Europe. J Eur Acad Dermatol Venereol. 2004;18:679-682.
- Kim T, Taylor JS, Maibach HI, et al. Photopatch testing among members of the American Contact Dermatitis Society. Dermatitis. 2020;31:59-67.
- Asemota E, Crawford G, Kovarik C, et al. A survey examining photopatch test and phototest methodologies of contact dermatologists in the United States: platform for developing a consensus. Dermatitis. 2017;28:265-269.
- Scalf LA, Davis MD, Rohlinger AL, et al. Photopatch testing of 182 patients: a 6-year experience at the Mayo Clinic. Dermatitis. 2009;20:44-52.
- Greenspoon J, Ahluwalia R, Juma N, et al. Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis. 2013;24:29-32.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens. review of a 15-year experience and of the literature. Contact Dermatitis. 1997;37:221-232.
- Collaris EJ, Frank J. Photoallergic contact dermatitis caused by ultraviolet filters in different sunscreens. Int J Dermatol. 2008;47(suppl 1):35-37.
- Heurung AR, Raju SI, Warshaw EM. Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis. 2014;25:289-326.
- Reeder M, Atwater AR. Methylisothiazolinone and isothiazolinone allergy. Cutis. 2019;104:94-96.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123.
- Kullberg SA, Voller LM, Warshaw EM. Methylisothiazolinone in “dermatology-recommended” sunscreens: an important mimicker of photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2021;37:366-370.
- Herman A, Aerts O, de Montjoye L, et al. Isothiazolinone derivatives and allergic contact dermatitis: a review and update. J Eur Acad Dermatol Venereol. 2019;33:267-276.
- Adler BL, Houle MC, Pratt M. Photoaggravated contact dermatitis to methylisothiazolinone and associated photosensitivity: a case series [published online January 25, 2022]. Dermatitis. doi:10.1097/DER.0000000000000833
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Pirmez R, Fernandes AL, Melo MG. Photoaggravated contact dermatitis to Kathon CG (methylchloroisothiazolinone/methylisothiazolinone): a novel pattern of involvement in a growing epidemic?. Br J Dermatol. 2015;173:1343-1344.
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures.J Eur Acad Dermatol Venereol. 2020;34:333-339.
- Government of Canada. Changes to the cosmetic ingredient hotlist. December 3, 2019. Updated August 26, 2022. Accessed October 20, 2022. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/changes.html
- Barkin RL. Topical nonsteroidal anti-inflammatory drugs: the importance of drug, delivery, and therapeutic outcome. Am J Ther. 2015;22:388-407.
- European Multicentre Photopatch Test Study (EMCPPTS) Taskforce. A European multicentre photopatch test study. Br J Dermatol. 2012;166:1002-1009.
- Ophaswongse S, Maibach H. Topical nonsteroidal antiinflammatory drugs: allergic and photoallergic contact dermatitis and phototoxicity. Contact Dermatitis. 1993;29:57-64.
- Kowalzick L, Ziegler H. Photoallergic contact dermatitis from topical diclofenac in Solaraze gel. Contact Dermatitis. 2006;54:348-349.
- Montoro J, Rodríguez M, Díaz M, et al. Photoallergic contact dermatitis due to diclofenac. Contact Dermatitis. 2003;48:115.
- Fernández-Jorge B, Goday-Buján JJ, Murga M, et al. Photoallergic contact dermatitis due to diclofenac with cross-reaction to aceclofenac: two case reports. Contact Dermatitis. 2009;61:236-237.
- Akat PB. Severe photosensitivity reaction induced by topical diclofenac. Indian J Pharmacol. 2013;45:408-409.
- Leroy D, Dompmartin A, Szczurko C, et al. Photodermatitis from ketoprofen with cross-reactivity to fenofibrate and benzophenones. Photodermatol Photoimmunol Photomed. 1997;13:93-97.
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166.
- Matsushita T, Kamide R. Five cases of photocontact dermatitisdue to topical ketoprofen: photopatch testing and cross-reaction study. Photodermatol Photoimmunol Photomed. 2001;17:26-31.
- de Groot AC, Roberts DW. Contact and photocontact allergy to octocrylene: a review. Contact Dermatitis. 2014;70:193-204.
- Wolverton JE, Soter NA, Cohen DE. Fentichlor photocontact dermatitis: a persistent enigma. Dermatitis. 2013;24:77-81.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
- Darvay A, White IR, Rycroft RJ, et al. Photoallergic contact dermatitis is uncommon. Br J Dermatol. 2001;145:597-601.
- DeLeo VA, Adler BL, Warshaw EM, et al. Photopatch test results of the North American contact dermatitis group, 1999-2009. Photodermatol Photoimmunol Photomed. 2022;38:288-291.
- Kerr A, Ferguson J. Photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2010;26:56-65.
- As¸kın Ö, Cesur SK, Engin B, et al. Photoallergic contact dermatitis. Curr Derm Rep. 2019;8:157-163.
- Wilm A, Berneburg M. Photoallergy. J Dtsch Dermatol Ges. 2015;13:7-13.
- DeLeo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- Imai S, Atarashi K, Ikesue K, et al. Establishment of murine model of allergic photocontact dermatitis to ketoprofen and characterization of pathogenic T cells. J Dermatol Sci. 2006;41:127-136.
- Tokura Y, Yagi H, Satoh T, et al. Inhibitory effect of melanin pigment on sensitization and elicitation of murine contact photosensitivity: mechanism of low responsiveness in C57BL/10 background mice. J Invest Dermatol. 1993;101:673-678.
- Stein KR, Scheinfeld NS. Drug-induced photoallergic and phototoxic reactions. Expert Opin Drug Saf. 2007;6:431-443.
- Janusz SC, Schwartz RA. Botanical briefs: phytophotodermatitis is an occupational and recreational dermatosis in the limelight. Cutis. 2021;107:187-189.
- Atwal SK, Chen A, Adler BL. Phototoxic contact dermatitis from over-the-counter 8-methoxypsoralen. Cutis. 2022;109:E2-E3.
- Rutter KJ, Farrar MD, Marjanovic EJ, et al. Clinicophotobiological characterization of photoaggravated atopic dermatitis [published online July 27, 2022]. JAMA Dermatol. doi:10.1001/jamadermatol.2022.2823
- Lecha M. Idiopathic photodermatoses: clinical, diagnostic and therapeutic aspects. J Eur Acad Dermatol Venereol. 2001;15:499-505.
- Marks JG Jr, Anderson BE, DeLeo VA. Contact & Occupational Dermatology. 4th ed. Jaypee Brothers; 2016.
- Bruynzeel DP, Ferguson J, Andersen K, et al. Photopatch testing: a consensus methodology for Europe. J Eur Acad Dermatol Venereol. 2004;18:679-682.
- Kim T, Taylor JS, Maibach HI, et al. Photopatch testing among members of the American Contact Dermatitis Society. Dermatitis. 2020;31:59-67.
- Asemota E, Crawford G, Kovarik C, et al. A survey examining photopatch test and phototest methodologies of contact dermatologists in the United States: platform for developing a consensus. Dermatitis. 2017;28:265-269.
- Scalf LA, Davis MD, Rohlinger AL, et al. Photopatch testing of 182 patients: a 6-year experience at the Mayo Clinic. Dermatitis. 2009;20:44-52.
- Greenspoon J, Ahluwalia R, Juma N, et al. Allergic and photoallergic contact dermatitis: a 10-year experience. Dermatitis. 2013;24:29-32.
- Victor FC, Cohen DE, Soter NA. A 20-year analysis of previous and emerging allergens that elicit photoallergic contact dermatitis. J Am Acad Dermatol. 2010;62:605-610.
- Schauder S, Ippen H. Contact and photocontact sensitivity to sunscreens. review of a 15-year experience and of the literature. Contact Dermatitis. 1997;37:221-232.
- Collaris EJ, Frank J. Photoallergic contact dermatitis caused by ultraviolet filters in different sunscreens. Int J Dermatol. 2008;47(suppl 1):35-37.
- Heurung AR, Raju SI, Warshaw EM. Adverse reactions to sunscreen agents: epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis. 2014;25:289-326.
- Reeder M, Atwater AR. Methylisothiazolinone and isothiazolinone allergy. Cutis. 2019;104:94-96.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group Patch Test Results: 2017-2018. Dermatitis. 2021;32:111-123.
- Kullberg SA, Voller LM, Warshaw EM. Methylisothiazolinone in “dermatology-recommended” sunscreens: an important mimicker of photoallergic contact dermatitis. Photodermatol Photoimmunol Photomed. 2021;37:366-370.
- Herman A, Aerts O, de Montjoye L, et al. Isothiazolinone derivatives and allergic contact dermatitis: a review and update. J Eur Acad Dermatol Venereol. 2019;33:267-276.
- Adler BL, Houle MC, Pratt M. Photoaggravated contact dermatitis to methylisothiazolinone and associated photosensitivity: a case series [published online January 25, 2022]. Dermatitis. doi:10.1097/DER.0000000000000833
- Aerts O, Goossens A, Marguery MC, et al. Photoaggravated allergic contact dermatitis and transient photosensitivity caused by methylisothiazolinone. Contact Dermatitis. 2018;78:241-245.
- Pirmez R, Fernandes AL, Melo MG. Photoaggravated contact dermatitis to Kathon CG (methylchloroisothiazolinone/methylisothiazolinone): a novel pattern of involvement in a growing epidemic?. Br J Dermatol. 2015;173:1343-1344.
- Uter W, Aalto-Korte K, Agner T, et al. The epidemic of methylisothiazolinone contact allergy in Europe: follow-up on changing exposures.J Eur Acad Dermatol Venereol. 2020;34:333-339.
- Government of Canada. Changes to the cosmetic ingredient hotlist. December 3, 2019. Updated August 26, 2022. Accessed October 20, 2022. https://www.canada.ca/en/health-canada/services/consumer-product-safety/cosmetics/cosmetic-ingredient-hotlist-prohibited-restricted-ingredients/changes.html
- Barkin RL. Topical nonsteroidal anti-inflammatory drugs: the importance of drug, delivery, and therapeutic outcome. Am J Ther. 2015;22:388-407.
- European Multicentre Photopatch Test Study (EMCPPTS) Taskforce. A European multicentre photopatch test study. Br J Dermatol. 2012;166:1002-1009.
- Ophaswongse S, Maibach H. Topical nonsteroidal antiinflammatory drugs: allergic and photoallergic contact dermatitis and phototoxicity. Contact Dermatitis. 1993;29:57-64.
- Kowalzick L, Ziegler H. Photoallergic contact dermatitis from topical diclofenac in Solaraze gel. Contact Dermatitis. 2006;54:348-349.
- Montoro J, Rodríguez M, Díaz M, et al. Photoallergic contact dermatitis due to diclofenac. Contact Dermatitis. 2003;48:115.
- Fernández-Jorge B, Goday-Buján JJ, Murga M, et al. Photoallergic contact dermatitis due to diclofenac with cross-reaction to aceclofenac: two case reports. Contact Dermatitis. 2009;61:236-237.
- Akat PB. Severe photosensitivity reaction induced by topical diclofenac. Indian J Pharmacol. 2013;45:408-409.
- Leroy D, Dompmartin A, Szczurko C, et al. Photodermatitis from ketoprofen with cross-reactivity to fenofibrate and benzophenones. Photodermatol Photoimmunol Photomed. 1997;13:93-97.
- Devleeschouwer V, Roelandts R, Garmyn M, et al. Allergic and photoallergic contact dermatitis from ketoprofen: results of (photo) patch testing and follow-up of 42 patients. Contact Dermatitis. 2008;58:159-166.
- Matsushita T, Kamide R. Five cases of photocontact dermatitisdue to topical ketoprofen: photopatch testing and cross-reaction study. Photodermatol Photoimmunol Photomed. 2001;17:26-31.
- de Groot AC, Roberts DW. Contact and photocontact allergy to octocrylene: a review. Contact Dermatitis. 2014;70:193-204.
- Wolverton JE, Soter NA, Cohen DE. Fentichlor photocontact dermatitis: a persistent enigma. Dermatitis. 2013;24:77-81.
- Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient management and education. J Am Acad Dermatol. 2016;74:1043-1054.
Practice Points
- Photoallergic contact dermatitis (PACD) presents clinically and histologically similar to allergic contact dermatitis but is concentrated in sun-exposed body sites.
- Sunscreens currently are the most common photoallergens in North America, whereas topical nonsteroidal anti-inflammatory drugs are more common culprits in Europe.
- Photopatch testing is required to diagnose PACD; however, it is infrequently performed, and there currently are no North American consensus guidelines.
Update on Tinea Capitis Diagnosis and Treatment
Tinea capitis (TC) most often is caused by Trichophyton tonsurans and Microsporum canis. The peak incidence is between 3 and 7 years of age. Noninflammatory TC typically presents as fine scaling with single or multiple scaly patches of circular alopecia (grey patches); diffuse or patchy, fine, white, adherent scaling of the scalp resembling generalized dandruff with subtle hair loss; or single or multiple patches of well-demarcated areas of alopecia with fine scale studded with broken-off hairs at the scalp surface, resulting in a black dot appearance. Inflammatory variants of TC include kerion and favus.1 Herein, updates on diagnosis, treatment, and monitoring of TC are provided, as well as a discussion of changes in the fungal microbiome associated with TC. Lastly, insights to some queries that practitioners may encounter when treating children with TC are provided.
Genetic Susceptibility
Molecular techniques have identified a number of macrophage regulator, leukocyte activation and migration, and cutaneous permeability genes associated with susceptibility to TC. These findings indicate that genetically determined deficiency in adaptive immune responses may affect the predisposition to dermatophyte infections.2
Clinical Varieties of Infection
Dermatophytes causing ringworm are capable of invading the hair shafts and can simultaneously invade smooth or glabrous skin (eg, T tonsurans, Trichophyton schoenleinii, Trichophyton violaceum). Some causative dermatophytes can even penetrate the nails (eg, Trichophyton soudanense). The clinical presentation is dependent on 3 main patterns of hair invasion3:
• Ectothrix: A mid-follicular pattern of invasion with hyphae growing down to the hair bulb that commonly is caused by Microsporum species. It clinically presents with scaling and inflammation with hair shafts breaking 2 to 3 mm above the scalp level.
• Endothrix: This pattern is nonfluorescent on Wood lamp examination, and hairs often break at the scalp level (black dot type). Trichophyton tonsurans, T soudanense, Trichophyton rubrum, and T violaceum are common causes.
• Favus: In this pattern, T schoenleinii is a common cause, and hairs grow to considerable lengths above the scalp with less damage than the other patterns. The hair shafts present with characteristic air spaces, and hyphae form clusters at the level of the epidermis.
Diagnosis
Optimal treatment of TC relies on proper identification of the causative agent. Fungal culture remains the gold standard of mycologic diagnosis regardless of its delayed results, which may take up to 4 weeks for proper identification of the fungal colonies and require ample expertise to interpret the morphologic features of the grown colonies.4
Other tests such as the potassium hydroxide preparation are nonspecific and do not identify the dermatophyte species. Although this method has been reported to have 5% to 15% false-negative results in routine practice depending on the skill of the observer and the quality of sampling, microscopic examination is essential, as it may allow the clinician to start treatment sooner pending culture results. The use of a Wood lamp is not suitable for definitive species identification, as this technique primarily is useful for observing fluorescence in ectothrix infection caused by Microsporum species, with the exception of T schoenleinii; otherwise, Trichophyton species, which cause endothrix infections, do not fluoresce.5Polymerase chain reaction is a sensitive technique that can help identify both the genus and species of common dermatophytes. Common target sequences include the ribosomal internal transcribed spacer and translation elongation factor 1α. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry also has become popular for dermatophyte identification.6Trichoscopic diagnosis of TC, which is simple and noninvasive, is becoming increasingly popular. Features such as short, broken, black dot, comma, corkscrew, and/or zigzag hairs, as well as perifollicular scaling, are helpful for diagnosing TC (Figure). Moreover, trichoscopy can be useful for differentiating other common causes of hair loss, such as trichotillomania and alopecia areata. It had been reported that the trichoscopic features of TC can be seen as early as 2 weeks after starting treatment and therefore this can be a reliable period in which to follow-up with the patient to evaluate progress. The disappearance of black dots and comma hairs can be appreciated from 2 weeks onwards by trichoscopic evaluation.4
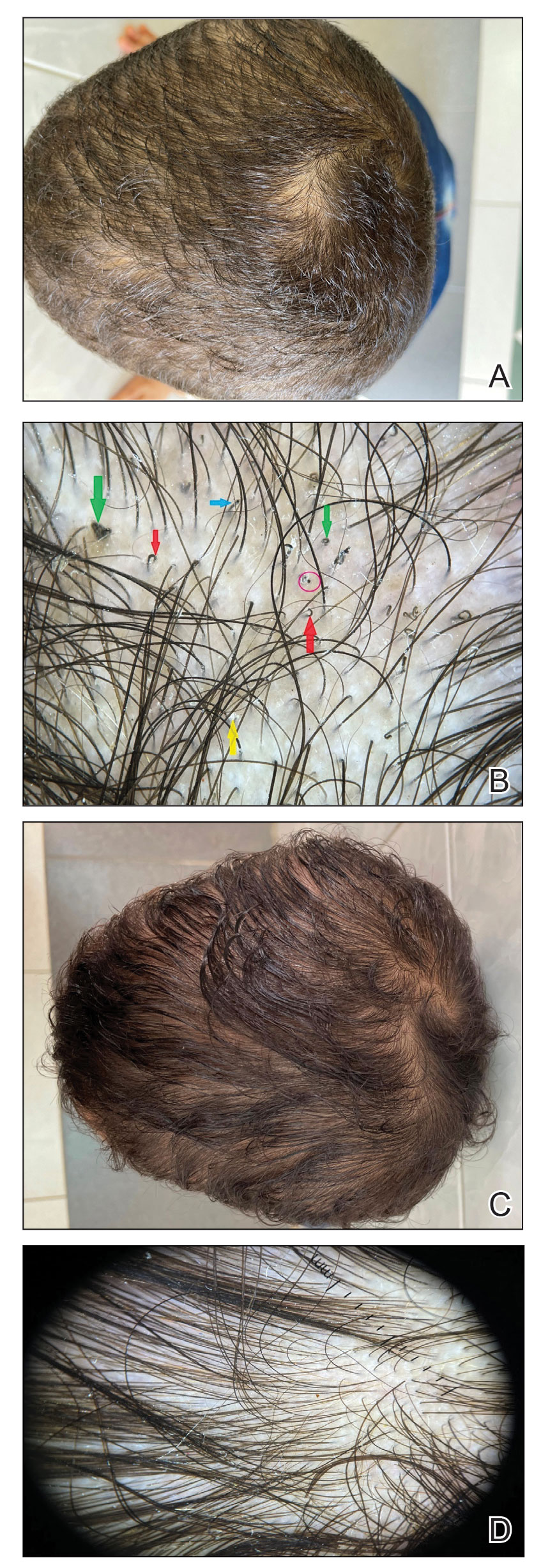
Treatment
The common recommendation for first-line treatment of TC is the use of systemic antifungals with the use of a topical agent as an adjuvant to prevent the spread of fungal spores. For almost 6 decades, griseofulvin had been the gold-standard fungistatic used for treating TC in patients older than 2 years until the 2007 US Food and Drug Administration (FDA) approval of terbinafine fungicidal oral granules for treatment of TC in patients older than 4 years.7
Meta-analyses have demonstrated comparable efficacy for a 4-week course of terbinafine compared to 6 weeks of griseofulvin for TC based on the infectious organism. Terbinafine demonstrated superiority in treating T tonsurans and a similar efficacy in treating T violaceum, while griseofulvin was superior in treating M canis and other Microsporum species.8,9
The off-label use of fluconazole and itraconazole to treat TC is gaining popularity, with limited trials showing increased evidence of their effectiveness. There is not much clinical evidence to support the use of other oral antifungals, including the newer azoles such as voriconazole or posaconazole.9
Newer limited evidence has shown the off-label use of photodynamic therapy to be a promising alternative to systemic antifungal therapy in treating TC, pending validation by larger sample trials.10In my practice, I have found that severe cases of TC demonstrating inflammation or possible widespread id reactions are better treated with oral steroids. Ketoconazole shampoo or selenium sulfide used 2 to 3 times weekly to prevent spread in the early phases of therapy is a good adjunct to systemic treatment. Cases with kerions should be assessed for the possibility of a coexisting bacterial infection under the crusts, and if confirmed, antibiotics should be started.9The commonly used systemic antifungals generally are safe with a low side-effect profile, but there is a risk for hepatotoxicity. The FDA recommends that baseline alanine transaminase and aspartate transaminase levels should be obtained prior to beginning a terbinafine-based treatment regimen.11 The American Academy of Pediatrics has specifically stated that laboratory testing of serum hepatic enzymes is not a requirement if a griseofulvin-based regimen does not exceed 8 weeks; however, transaminase levels (alanine transaminase and aspartate transaminase) should be considered in patients using terbinafine at baseline or if treatment is prolonged beyond 4 to 6 weeks.12 In agreement with the FDA guidelines, the Canadian Pediatric Society has suggested that liver enzymes should be periodically monitored in patients being treated with terbinafine beyond 4 to 6 weeks.13
Changes in the Fungal Microbiome
Research has shown that changes in the fungal microbiome were associated with an altered bacterial community in patients with TC. During fungal infection, the relative abundances of Cutibacterium and Corynebacterium increased, and the relative abundance of Streptococcus decreased. In addition, some uncommon bacterial genera such as Herbaspirillum and Methylorubrum were detected on the scalp in TC.14
Carrier State
Carrier state is determined for those siblings and contacts of cases with a clinically normal scalp that are positive on culture. Those individuals could represent a potential reservoir responsible for contamination (or recontamination) of the patient as well as treatment failure. Opinions remain divided as to whether to use oral antifungal therapy in these carriers or maintain therapy on antifungal shampoos containing ketoconazole or povidone-iodine. Due to the paucity of available data, my experience has shown that it is sufficient to use antifungal shampoos for such carriers. In zoophilic infections, it is important to identify and treat the animal source.6-9
Final Thoughts
Successful treatment of TC requires accurate identification of the pathogen, which commonly is achieved via fungal culture. Despite its practical value, the conventional identification of dermatophytes based on morphologic features can be highly challenging due to the low positive rate and delayed results. Trichoscopy is a quick, handy, and noninvasive tool that can better indicate the diagnosis and also is helpful for follow-up on treatment progress. Due to better understanding of the immunology and genetic susceptibility associated with TC spread, the current treatment pipeline holds more insight into better control of this condition. Increased surveillance, prompt diagnosis, and early onset of systemic treatment are the key to proper prevention of spread of TC.
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68.
- Abdel-Rahman SM, Preuett BL. Genetic predictors of susceptibility to cutaneous fungal infections: a pilot genome wide association study to refine a candidate gene search. J Dermatol Sci. 2012;67:147-152.
- Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182:87-93.
- Wahbah HR, Atallah RB, Eldahshan RM, et al. A prospective clinical and trichoscopic study of tinea capitis in children during treatment [published online May 23, 2022]. Dermatol Ther. 2022;35:E15582. doi:10.1111/dth.15582
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Molecular epidemiology, genetic diversity, and antifungal susceptibility of major pathogenic dermatophytes isolated from human dermatophytosis. Front Microbiol. 2021;12:643509.
- Lamisil. Package insert. Novartis; 2011. Accessed October 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64:663-670.
- Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: an update. Pediatr Dermatol. 2022;39:167-172.
- Aspiroz C, Melcon B, Cerro PA, et al. Tinea capitis caused by Microsporum canis treated with methyl-aminolevulinate daylight photodynamic therapy and ketoconazole shampooing. Photodermatol Photoimmunol Photomed. 2021;37:567-568.
- Aleohin N, Bar J, Bar-Ilan E, et al. Laboratory monitoring during antifungal treatment of paediatric tinea capitis. Mycoses. 2021;64:157-161.
- Kimberlin DW, Brady MT, Jackson MA, et al, eds. Tinea capitis. In: Red Book 2018-2021: Report of the Committee of Infectious Diseases. American Academy of Pediatrics; 2018:798-801.
- Bortolussi R, Martin S, Audcent T, et al. Antifungal agents for common outpatient paediatric infections. Canadian Paediatric Society website. Published June 20, 2019. Accessed October 4, 2022. https://www.cps.ca/en/documents/position/antifungal-agents-common-infections
- Tao R, Zhu P, Zhou Y, et al. Altered skin fungal and bacterial community compositions in tinea capitis. Mycoses. 2022;65:834-840.
Tinea capitis (TC) most often is caused by Trichophyton tonsurans and Microsporum canis. The peak incidence is between 3 and 7 years of age. Noninflammatory TC typically presents as fine scaling with single or multiple scaly patches of circular alopecia (grey patches); diffuse or patchy, fine, white, adherent scaling of the scalp resembling generalized dandruff with subtle hair loss; or single or multiple patches of well-demarcated areas of alopecia with fine scale studded with broken-off hairs at the scalp surface, resulting in a black dot appearance. Inflammatory variants of TC include kerion and favus.1 Herein, updates on diagnosis, treatment, and monitoring of TC are provided, as well as a discussion of changes in the fungal microbiome associated with TC. Lastly, insights to some queries that practitioners may encounter when treating children with TC are provided.
Genetic Susceptibility
Molecular techniques have identified a number of macrophage regulator, leukocyte activation and migration, and cutaneous permeability genes associated with susceptibility to TC. These findings indicate that genetically determined deficiency in adaptive immune responses may affect the predisposition to dermatophyte infections.2
Clinical Varieties of Infection
Dermatophytes causing ringworm are capable of invading the hair shafts and can simultaneously invade smooth or glabrous skin (eg, T tonsurans, Trichophyton schoenleinii, Trichophyton violaceum). Some causative dermatophytes can even penetrate the nails (eg, Trichophyton soudanense). The clinical presentation is dependent on 3 main patterns of hair invasion3:
• Ectothrix: A mid-follicular pattern of invasion with hyphae growing down to the hair bulb that commonly is caused by Microsporum species. It clinically presents with scaling and inflammation with hair shafts breaking 2 to 3 mm above the scalp level.
• Endothrix: This pattern is nonfluorescent on Wood lamp examination, and hairs often break at the scalp level (black dot type). Trichophyton tonsurans, T soudanense, Trichophyton rubrum, and T violaceum are common causes.
• Favus: In this pattern, T schoenleinii is a common cause, and hairs grow to considerable lengths above the scalp with less damage than the other patterns. The hair shafts present with characteristic air spaces, and hyphae form clusters at the level of the epidermis.
Diagnosis
Optimal treatment of TC relies on proper identification of the causative agent. Fungal culture remains the gold standard of mycologic diagnosis regardless of its delayed results, which may take up to 4 weeks for proper identification of the fungal colonies and require ample expertise to interpret the morphologic features of the grown colonies.4
Other tests such as the potassium hydroxide preparation are nonspecific and do not identify the dermatophyte species. Although this method has been reported to have 5% to 15% false-negative results in routine practice depending on the skill of the observer and the quality of sampling, microscopic examination is essential, as it may allow the clinician to start treatment sooner pending culture results. The use of a Wood lamp is not suitable for definitive species identification, as this technique primarily is useful for observing fluorescence in ectothrix infection caused by Microsporum species, with the exception of T schoenleinii; otherwise, Trichophyton species, which cause endothrix infections, do not fluoresce.5Polymerase chain reaction is a sensitive technique that can help identify both the genus and species of common dermatophytes. Common target sequences include the ribosomal internal transcribed spacer and translation elongation factor 1α. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry also has become popular for dermatophyte identification.6Trichoscopic diagnosis of TC, which is simple and noninvasive, is becoming increasingly popular. Features such as short, broken, black dot, comma, corkscrew, and/or zigzag hairs, as well as perifollicular scaling, are helpful for diagnosing TC (Figure). Moreover, trichoscopy can be useful for differentiating other common causes of hair loss, such as trichotillomania and alopecia areata. It had been reported that the trichoscopic features of TC can be seen as early as 2 weeks after starting treatment and therefore this can be a reliable period in which to follow-up with the patient to evaluate progress. The disappearance of black dots and comma hairs can be appreciated from 2 weeks onwards by trichoscopic evaluation.4

Treatment
The common recommendation for first-line treatment of TC is the use of systemic antifungals with the use of a topical agent as an adjuvant to prevent the spread of fungal spores. For almost 6 decades, griseofulvin had been the gold-standard fungistatic used for treating TC in patients older than 2 years until the 2007 US Food and Drug Administration (FDA) approval of terbinafine fungicidal oral granules for treatment of TC in patients older than 4 years.7
Meta-analyses have demonstrated comparable efficacy for a 4-week course of terbinafine compared to 6 weeks of griseofulvin for TC based on the infectious organism. Terbinafine demonstrated superiority in treating T tonsurans and a similar efficacy in treating T violaceum, while griseofulvin was superior in treating M canis and other Microsporum species.8,9
The off-label use of fluconazole and itraconazole to treat TC is gaining popularity, with limited trials showing increased evidence of their effectiveness. There is not much clinical evidence to support the use of other oral antifungals, including the newer azoles such as voriconazole or posaconazole.9
Newer limited evidence has shown the off-label use of photodynamic therapy to be a promising alternative to systemic antifungal therapy in treating TC, pending validation by larger sample trials.10In my practice, I have found that severe cases of TC demonstrating inflammation or possible widespread id reactions are better treated with oral steroids. Ketoconazole shampoo or selenium sulfide used 2 to 3 times weekly to prevent spread in the early phases of therapy is a good adjunct to systemic treatment. Cases with kerions should be assessed for the possibility of a coexisting bacterial infection under the crusts, and if confirmed, antibiotics should be started.9The commonly used systemic antifungals generally are safe with a low side-effect profile, but there is a risk for hepatotoxicity. The FDA recommends that baseline alanine transaminase and aspartate transaminase levels should be obtained prior to beginning a terbinafine-based treatment regimen.11 The American Academy of Pediatrics has specifically stated that laboratory testing of serum hepatic enzymes is not a requirement if a griseofulvin-based regimen does not exceed 8 weeks; however, transaminase levels (alanine transaminase and aspartate transaminase) should be considered in patients using terbinafine at baseline or if treatment is prolonged beyond 4 to 6 weeks.12 In agreement with the FDA guidelines, the Canadian Pediatric Society has suggested that liver enzymes should be periodically monitored in patients being treated with terbinafine beyond 4 to 6 weeks.13
Changes in the Fungal Microbiome
Research has shown that changes in the fungal microbiome were associated with an altered bacterial community in patients with TC. During fungal infection, the relative abundances of Cutibacterium and Corynebacterium increased, and the relative abundance of Streptococcus decreased. In addition, some uncommon bacterial genera such as Herbaspirillum and Methylorubrum were detected on the scalp in TC.14
Carrier State
Carrier state is determined for those siblings and contacts of cases with a clinically normal scalp that are positive on culture. Those individuals could represent a potential reservoir responsible for contamination (or recontamination) of the patient as well as treatment failure. Opinions remain divided as to whether to use oral antifungal therapy in these carriers or maintain therapy on antifungal shampoos containing ketoconazole or povidone-iodine. Due to the paucity of available data, my experience has shown that it is sufficient to use antifungal shampoos for such carriers. In zoophilic infections, it is important to identify and treat the animal source.6-9
Final Thoughts
Successful treatment of TC requires accurate identification of the pathogen, which commonly is achieved via fungal culture. Despite its practical value, the conventional identification of dermatophytes based on morphologic features can be highly challenging due to the low positive rate and delayed results. Trichoscopy is a quick, handy, and noninvasive tool that can better indicate the diagnosis and also is helpful for follow-up on treatment progress. Due to better understanding of the immunology and genetic susceptibility associated with TC spread, the current treatment pipeline holds more insight into better control of this condition. Increased surveillance, prompt diagnosis, and early onset of systemic treatment are the key to proper prevention of spread of TC.
Tinea capitis (TC) most often is caused by Trichophyton tonsurans and Microsporum canis. The peak incidence is between 3 and 7 years of age. Noninflammatory TC typically presents as fine scaling with single or multiple scaly patches of circular alopecia (grey patches); diffuse or patchy, fine, white, adherent scaling of the scalp resembling generalized dandruff with subtle hair loss; or single or multiple patches of well-demarcated areas of alopecia with fine scale studded with broken-off hairs at the scalp surface, resulting in a black dot appearance. Inflammatory variants of TC include kerion and favus.1 Herein, updates on diagnosis, treatment, and monitoring of TC are provided, as well as a discussion of changes in the fungal microbiome associated with TC. Lastly, insights to some queries that practitioners may encounter when treating children with TC are provided.
Genetic Susceptibility
Molecular techniques have identified a number of macrophage regulator, leukocyte activation and migration, and cutaneous permeability genes associated with susceptibility to TC. These findings indicate that genetically determined deficiency in adaptive immune responses may affect the predisposition to dermatophyte infections.2
Clinical Varieties of Infection
Dermatophytes causing ringworm are capable of invading the hair shafts and can simultaneously invade smooth or glabrous skin (eg, T tonsurans, Trichophyton schoenleinii, Trichophyton violaceum). Some causative dermatophytes can even penetrate the nails (eg, Trichophyton soudanense). The clinical presentation is dependent on 3 main patterns of hair invasion3:
• Ectothrix: A mid-follicular pattern of invasion with hyphae growing down to the hair bulb that commonly is caused by Microsporum species. It clinically presents with scaling and inflammation with hair shafts breaking 2 to 3 mm above the scalp level.
• Endothrix: This pattern is nonfluorescent on Wood lamp examination, and hairs often break at the scalp level (black dot type). Trichophyton tonsurans, T soudanense, Trichophyton rubrum, and T violaceum are common causes.
• Favus: In this pattern, T schoenleinii is a common cause, and hairs grow to considerable lengths above the scalp with less damage than the other patterns. The hair shafts present with characteristic air spaces, and hyphae form clusters at the level of the epidermis.
Diagnosis
Optimal treatment of TC relies on proper identification of the causative agent. Fungal culture remains the gold standard of mycologic diagnosis regardless of its delayed results, which may take up to 4 weeks for proper identification of the fungal colonies and require ample expertise to interpret the morphologic features of the grown colonies.4
Other tests such as the potassium hydroxide preparation are nonspecific and do not identify the dermatophyte species. Although this method has been reported to have 5% to 15% false-negative results in routine practice depending on the skill of the observer and the quality of sampling, microscopic examination is essential, as it may allow the clinician to start treatment sooner pending culture results. The use of a Wood lamp is not suitable for definitive species identification, as this technique primarily is useful for observing fluorescence in ectothrix infection caused by Microsporum species, with the exception of T schoenleinii; otherwise, Trichophyton species, which cause endothrix infections, do not fluoresce.5Polymerase chain reaction is a sensitive technique that can help identify both the genus and species of common dermatophytes. Common target sequences include the ribosomal internal transcribed spacer and translation elongation factor 1α. The use of matrix-assisted laser desorption/ionization time-of-flight mass spectrometry also has become popular for dermatophyte identification.6Trichoscopic diagnosis of TC, which is simple and noninvasive, is becoming increasingly popular. Features such as short, broken, black dot, comma, corkscrew, and/or zigzag hairs, as well as perifollicular scaling, are helpful for diagnosing TC (Figure). Moreover, trichoscopy can be useful for differentiating other common causes of hair loss, such as trichotillomania and alopecia areata. It had been reported that the trichoscopic features of TC can be seen as early as 2 weeks after starting treatment and therefore this can be a reliable period in which to follow-up with the patient to evaluate progress. The disappearance of black dots and comma hairs can be appreciated from 2 weeks onwards by trichoscopic evaluation.4

Treatment
The common recommendation for first-line treatment of TC is the use of systemic antifungals with the use of a topical agent as an adjuvant to prevent the spread of fungal spores. For almost 6 decades, griseofulvin had been the gold-standard fungistatic used for treating TC in patients older than 2 years until the 2007 US Food and Drug Administration (FDA) approval of terbinafine fungicidal oral granules for treatment of TC in patients older than 4 years.7
Meta-analyses have demonstrated comparable efficacy for a 4-week course of terbinafine compared to 6 weeks of griseofulvin for TC based on the infectious organism. Terbinafine demonstrated superiority in treating T tonsurans and a similar efficacy in treating T violaceum, while griseofulvin was superior in treating M canis and other Microsporum species.8,9
The off-label use of fluconazole and itraconazole to treat TC is gaining popularity, with limited trials showing increased evidence of their effectiveness. There is not much clinical evidence to support the use of other oral antifungals, including the newer azoles such as voriconazole or posaconazole.9
Newer limited evidence has shown the off-label use of photodynamic therapy to be a promising alternative to systemic antifungal therapy in treating TC, pending validation by larger sample trials.10In my practice, I have found that severe cases of TC demonstrating inflammation or possible widespread id reactions are better treated with oral steroids. Ketoconazole shampoo or selenium sulfide used 2 to 3 times weekly to prevent spread in the early phases of therapy is a good adjunct to systemic treatment. Cases with kerions should be assessed for the possibility of a coexisting bacterial infection under the crusts, and if confirmed, antibiotics should be started.9The commonly used systemic antifungals generally are safe with a low side-effect profile, but there is a risk for hepatotoxicity. The FDA recommends that baseline alanine transaminase and aspartate transaminase levels should be obtained prior to beginning a terbinafine-based treatment regimen.11 The American Academy of Pediatrics has specifically stated that laboratory testing of serum hepatic enzymes is not a requirement if a griseofulvin-based regimen does not exceed 8 weeks; however, transaminase levels (alanine transaminase and aspartate transaminase) should be considered in patients using terbinafine at baseline or if treatment is prolonged beyond 4 to 6 weeks.12 In agreement with the FDA guidelines, the Canadian Pediatric Society has suggested that liver enzymes should be periodically monitored in patients being treated with terbinafine beyond 4 to 6 weeks.13
Changes in the Fungal Microbiome
Research has shown that changes in the fungal microbiome were associated with an altered bacterial community in patients with TC. During fungal infection, the relative abundances of Cutibacterium and Corynebacterium increased, and the relative abundance of Streptococcus decreased. In addition, some uncommon bacterial genera such as Herbaspirillum and Methylorubrum were detected on the scalp in TC.14
Carrier State
Carrier state is determined for those siblings and contacts of cases with a clinically normal scalp that are positive on culture. Those individuals could represent a potential reservoir responsible for contamination (or recontamination) of the patient as well as treatment failure. Opinions remain divided as to whether to use oral antifungal therapy in these carriers or maintain therapy on antifungal shampoos containing ketoconazole or povidone-iodine. Due to the paucity of available data, my experience has shown that it is sufficient to use antifungal shampoos for such carriers. In zoophilic infections, it is important to identify and treat the animal source.6-9
Final Thoughts
Successful treatment of TC requires accurate identification of the pathogen, which commonly is achieved via fungal culture. Despite its practical value, the conventional identification of dermatophytes based on morphologic features can be highly challenging due to the low positive rate and delayed results. Trichoscopy is a quick, handy, and noninvasive tool that can better indicate the diagnosis and also is helpful for follow-up on treatment progress. Due to better understanding of the immunology and genetic susceptibility associated with TC spread, the current treatment pipeline holds more insight into better control of this condition. Increased surveillance, prompt diagnosis, and early onset of systemic treatment are the key to proper prevention of spread of TC.
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68.
- Abdel-Rahman SM, Preuett BL. Genetic predictors of susceptibility to cutaneous fungal infections: a pilot genome wide association study to refine a candidate gene search. J Dermatol Sci. 2012;67:147-152.
- Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182:87-93.
- Wahbah HR, Atallah RB, Eldahshan RM, et al. A prospective clinical and trichoscopic study of tinea capitis in children during treatment [published online May 23, 2022]. Dermatol Ther. 2022;35:E15582. doi:10.1111/dth.15582
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Molecular epidemiology, genetic diversity, and antifungal susceptibility of major pathogenic dermatophytes isolated from human dermatophytosis. Front Microbiol. 2021;12:643509.
- Lamisil. Package insert. Novartis; 2011. Accessed October 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64:663-670.
- Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: an update. Pediatr Dermatol. 2022;39:167-172.
- Aspiroz C, Melcon B, Cerro PA, et al. Tinea capitis caused by Microsporum canis treated with methyl-aminolevulinate daylight photodynamic therapy and ketoconazole shampooing. Photodermatol Photoimmunol Photomed. 2021;37:567-568.
- Aleohin N, Bar J, Bar-Ilan E, et al. Laboratory monitoring during antifungal treatment of paediatric tinea capitis. Mycoses. 2021;64:157-161.
- Kimberlin DW, Brady MT, Jackson MA, et al, eds. Tinea capitis. In: Red Book 2018-2021: Report of the Committee of Infectious Diseases. American Academy of Pediatrics; 2018:798-801.
- Bortolussi R, Martin S, Audcent T, et al. Antifungal agents for common outpatient paediatric infections. Canadian Paediatric Society website. Published June 20, 2019. Accessed October 4, 2022. https://www.cps.ca/en/documents/position/antifungal-agents-common-infections
- Tao R, Zhu P, Zhou Y, et al. Altered skin fungal and bacterial community compositions in tinea capitis. Mycoses. 2022;65:834-840.
- Leung AKC, Hon KL, Leong KF, et al. Tinea capitis: an updated review. Recent Pat Inflamm Allergy Drug Discov. 2020;14:58-68.
- Abdel-Rahman SM, Preuett BL. Genetic predictors of susceptibility to cutaneous fungal infections: a pilot genome wide association study to refine a candidate gene search. J Dermatol Sci. 2012;67:147-152.
- Hay RJ. Tinea capitis: current status. Mycopathologia. 2017;182:87-93.
- Wahbah HR, Atallah RB, Eldahshan RM, et al. A prospective clinical and trichoscopic study of tinea capitis in children during treatment [published online May 23, 2022]. Dermatol Ther. 2022;35:E15582. doi:10.1111/dth.15582
- Salehi Z, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Molecular epidemiology, genetic diversity, and antifungal susceptibility of major pathogenic dermatophytes isolated from human dermatophytosis. Front Microbiol. 2021;12:643509.
- Lamisil. Package insert. Novartis; 2011. Accessed October 17, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020539s021lbl.pdf
- Gupta AK, Drummond-Main C. Meta-analysis of randomized, controlled trials comparing particular doses of griseofulvin and terbinafine for the treatment of tinea capitis. Pediatr Dermatol. 2013;30:1-6.
- Tey HL, Tan AS, Chan YC. Meta-analysis of randomized, controlled trials comparing griseofulvin and terbinafine in the treatment of tinea capitis. J Am Acad Dermatol. 2011;64:663-670.
- Gupta AK, Friedlander SF, Simkovich AJ. Tinea capitis: an update. Pediatr Dermatol. 2022;39:167-172.
- Aspiroz C, Melcon B, Cerro PA, et al. Tinea capitis caused by Microsporum canis treated with methyl-aminolevulinate daylight photodynamic therapy and ketoconazole shampooing. Photodermatol Photoimmunol Photomed. 2021;37:567-568.
- Aleohin N, Bar J, Bar-Ilan E, et al. Laboratory monitoring during antifungal treatment of paediatric tinea capitis. Mycoses. 2021;64:157-161.
- Kimberlin DW, Brady MT, Jackson MA, et al, eds. Tinea capitis. In: Red Book 2018-2021: Report of the Committee of Infectious Diseases. American Academy of Pediatrics; 2018:798-801.
- Bortolussi R, Martin S, Audcent T, et al. Antifungal agents for common outpatient paediatric infections. Canadian Paediatric Society website. Published June 20, 2019. Accessed October 4, 2022. https://www.cps.ca/en/documents/position/antifungal-agents-common-infections
- Tao R, Zhu P, Zhou Y, et al. Altered skin fungal and bacterial community compositions in tinea capitis. Mycoses. 2022;65:834-840.
A 95-year-old White male with hypertension presented with itchy patches and bullae on the trunk and extremities
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
and is associated with various predisposing factors, including HLA genes, comorbidities, aging, and trigger factors such as drugs, trauma, radiation, chemotherapy, and infections. The autoimmune reaction is mediated by a dysregulation of T cells in which IgG and IgE autoantibodies form against hemidesmosomal proteins (BP180 and BP230). These autoantibodies induce neutrophil activation, recruitment, and degradation in the basement membrane of the skin.
Typically, patients present with intense pruritus followed by an urticarial or eczematous eruption. Tense blisters and bullae occur commonly on the trunk and extremities. Drug-associated bullous pemphigoid (DABP) is a common manifestation of the disease with histologic and immunologic features similar to those of the idiopathic version. Eruptions can be triggered by systemic or topical medications, and incidence of these reactions may be related to a genetic predisposition for the disease.
Some research suggests that drug-induced changes to the antigenic properties of the epidermal basement membrane result in an augmented immune response, while others point to structural modification in these zones that stimulate the immune system. Thiol- and phenol-based drugs have been largely implicated in the development of DABP because they are capable of structural modification and disruption of the dermo-epidermal junction in the basement membrane.
DABP often presents with patients taking multiple medications. Some of the most common medications are gliptins, PD-1 inhibitors, diuretics, antibiotics, anti-inflammatory drugs, and ACE-inhibitors, and other cardiovascular drugs. DABP may present with mucosal eruptions unlike its idiopathic counterpart that is mostly contained to the skin.
On this patient, two punch biopsies were taken. Histopathology revealed an eosinophil-rich subepidermal blister with a smooth epidermal undersurface consistent with bullous pemphigoid. Direct immunofluorescence was positive with a deposition of IgG and C3 at the epidermal side of salt split basement membrane zone.
Treatment for BP includes high potency topical and systemic steroids. Tetracyclines and niacinamide have been reported to improve the condition. Treatment is tailored to allow for cutaneous healing and control pruritus, but the physician must be mindful of the patient’s comorbidities and capacity for self-care. Prognosis is often better for DABP as withdrawal of the medication greatly accelerates clearance of the lesions. Worse prognosis is related to increased number of comorbidities and older age. Our patient’s BP is controlled currently with topical steroids and oral doxycycline.
This case and photo were submitted by Lucas Shapiro, BS, Nova Southeastern University College of Osteopathic Medicine, Tampa, and Dr. Bilu Martin.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Miyamoto D et al. An Bras Dermatol. 2019 Mar-Apr;94(2):133-46.
2. Moro et al. Biomolecules. 2020 Oct 10;10(10):1432.
3. Verheyden M et al. Acta Derm Venereol. 2020 Aug 17;100(15):adv00224.
The truth of alcohol consequences
Bad drinking consequence No. 87: Joining the LOTME team
Alcohol and college students go together like peanut butter and jelly. Or peanut butter and chocolate. Or peanut butter and toothpaste. Peanut butter goes with a lot of things.
Naturally, when you combine alcohol and college students, bad decisions are sure to follow. But have you ever wondered just how many bad decisions alcohol causes? A team of researchers from Penn State University, the undisputed champion of poor drinking decisions (trust us, we know), sure has. They’ve even conducted a 4-year study of 1,700 students as they carved a drunken swath through the many fine local drinking establishments, such as East Halls or that one frat house that hosts medieval battle–style ping pong tournaments.
The students were surveyed twice a year throughout the study, and the researchers compiled a list of all the various consequences their subjects experienced. Ultimately, college students will experience an average of 102 consequences from drinking during their 4-year college careers, which is an impressive number. Try thinking up a hundred consequences for anything.
Some consequences are less common than others – we imagine “missing the Renaissance Faire because you felt drunker the morning after than while you were drinking” is pretty low on the list – but more than 96% of students reported that they’d experienced a hangover and that drinking had caused them to say or do embarrassing things. Also, more than 70% said they needed additional alcohol to feel any effect, a potential sign of alcohol use disorder.
Once they had their list, the researchers focused on 12 of the more common and severe consequences, such as blacking out, hangovers, and missing work/class, and asked the study participants how their parents would react to their drinking and those specific consequences. Students who believed their parents would disapprove of alcohol-related consequences actually experienced fewer consequences overall.
College students, it seems, really do care what their parents think, even if they don’t express it, the researchers said. That gives space for parents to offer advice about the consequences of hard drinking, making decisions while drunk, or bringing godawful Fireball whiskey to parties. Seriously, don’t do that. Stuff’s bad, and you should feel bad for bringing it. Your parents raised you better than that.
COVID ‘expert’ discusses data sharing
We interrupt our regularly scheduled programming to bring you this special news event. Elon Musk, the world’s second-most annoying human, is holding a press conference to discuss, of all things, COVID-19.
Reporter: Hey, Mr. Musketeer, what qualifies you to talk about a global pandemic?
EM: As the official king of the Twitterverse, I’m pretty much an expert on any topic.
Reporter: Okay then, Mr. Muskmelon, what can you tell us about the new study in Agricultural Economics, which looked at consumers’ knowledge of local COVID infection rates and their willingness to eat at restaurants?
EM: Well, I know that one of the investigators, Rigoberto Lopez, PhD, of the University of Connecticut, said “no news is bad news.” Restaurants located in cities where local regulations required COVID tracking recovered faster than those in areas that did not, according to data from 87 restaurants in 10 Chinese cities that were gathered between Dec. 1, 2019, and March 27, 2020. Having access to local infection rate data made customers more comfortable going out to eat, the investigators explained.
Second reporter: Interesting, Mr. Muskox, but how about this headline from CNN: “Workers flee China’s biggest iPhone factory over Covid outbreak”? Do you agree with analysts, who said that “the chaos at Zhengzhou could jeopardize Apple and Foxconn’s output in the coming weeks,” as CNN put it?
EM: I did see that a manager at Foxconn, which owns the factory and is known to its friends as Hon Hai Precision Industry, told a Chinese media outlet that “workers are panicking over the spread of the virus at the factory and lack of access to official information.” As we’ve already discussed, no news is bad news.
That’s all the time I have to chat with you today. I’m off to fire some more Twitter employees.
In case you hadn’t already guessed, Vlad Putin is officially more annoying than Elon Musk. We now return to this week’s typical LOTME shenanigans, already in progress.
The deadliest month
With climate change making the world hotter, leading to more heat stroke and organ failure, you would think the summer months would be the most deadly. In reality, though, it’s quite the opposite.
There are multiple factors that make January the most deadly month out of the year, as LiveScience discovered in a recent analysis.
Let’s go through them, shall we?
Respiratory viruses: Robert Glatter, MD, of Lenox Hill Hospital in New York, told LiveScence that winter is the time for illnesses like the flu, bacterial pneumonia, and RSV. Millions of people worldwide die from the flu, according to the CDC. And the World Health Organization reported lower respiratory infections as the fourth-leading cause of death worldwide before COVID came along.
Heart disease: Heart conditions are actually more fatal in the winter months, according to a study published in Circulation. The cold puts more stress on the heart to keep the body warm, which can be a challenge for people who already have preexisting heart conditions.
Space heaters: Dr. Glatter also told Live Science that the use of space heaters could be a factor in the cold winter months since they can lead to carbon monoxide poisoning and even fires. Silent killers.
Holiday season: A time for joy and merriment, certainly, but Christmas et al. have their downsides. By January we’re coming off a 3-month food and alcohol binge, which leads to cardiac stress. There’s also the psychological stress that comes with the season. Sometimes the most wonderful time of the year just isn’t.
So even though summer is hot, fall has hurricanes, and spring tends to have the highest suicide rate, winter still ends up being the deadliest season.
Bad drinking consequence No. 87: Joining the LOTME team
Alcohol and college students go together like peanut butter and jelly. Or peanut butter and chocolate. Or peanut butter and toothpaste. Peanut butter goes with a lot of things.
Naturally, when you combine alcohol and college students, bad decisions are sure to follow. But have you ever wondered just how many bad decisions alcohol causes? A team of researchers from Penn State University, the undisputed champion of poor drinking decisions (trust us, we know), sure has. They’ve even conducted a 4-year study of 1,700 students as they carved a drunken swath through the many fine local drinking establishments, such as East Halls or that one frat house that hosts medieval battle–style ping pong tournaments.
The students were surveyed twice a year throughout the study, and the researchers compiled a list of all the various consequences their subjects experienced. Ultimately, college students will experience an average of 102 consequences from drinking during their 4-year college careers, which is an impressive number. Try thinking up a hundred consequences for anything.
Some consequences are less common than others – we imagine “missing the Renaissance Faire because you felt drunker the morning after than while you were drinking” is pretty low on the list – but more than 96% of students reported that they’d experienced a hangover and that drinking had caused them to say or do embarrassing things. Also, more than 70% said they needed additional alcohol to feel any effect, a potential sign of alcohol use disorder.
Once they had their list, the researchers focused on 12 of the more common and severe consequences, such as blacking out, hangovers, and missing work/class, and asked the study participants how their parents would react to their drinking and those specific consequences. Students who believed their parents would disapprove of alcohol-related consequences actually experienced fewer consequences overall.
College students, it seems, really do care what their parents think, even if they don’t express it, the researchers said. That gives space for parents to offer advice about the consequences of hard drinking, making decisions while drunk, or bringing godawful Fireball whiskey to parties. Seriously, don’t do that. Stuff’s bad, and you should feel bad for bringing it. Your parents raised you better than that.
COVID ‘expert’ discusses data sharing
We interrupt our regularly scheduled programming to bring you this special news event. Elon Musk, the world’s second-most annoying human, is holding a press conference to discuss, of all things, COVID-19.
Reporter: Hey, Mr. Musketeer, what qualifies you to talk about a global pandemic?
EM: As the official king of the Twitterverse, I’m pretty much an expert on any topic.
Reporter: Okay then, Mr. Muskmelon, what can you tell us about the new study in Agricultural Economics, which looked at consumers’ knowledge of local COVID infection rates and their willingness to eat at restaurants?
EM: Well, I know that one of the investigators, Rigoberto Lopez, PhD, of the University of Connecticut, said “no news is bad news.” Restaurants located in cities where local regulations required COVID tracking recovered faster than those in areas that did not, according to data from 87 restaurants in 10 Chinese cities that were gathered between Dec. 1, 2019, and March 27, 2020. Having access to local infection rate data made customers more comfortable going out to eat, the investigators explained.
Second reporter: Interesting, Mr. Muskox, but how about this headline from CNN: “Workers flee China’s biggest iPhone factory over Covid outbreak”? Do you agree with analysts, who said that “the chaos at Zhengzhou could jeopardize Apple and Foxconn’s output in the coming weeks,” as CNN put it?
EM: I did see that a manager at Foxconn, which owns the factory and is known to its friends as Hon Hai Precision Industry, told a Chinese media outlet that “workers are panicking over the spread of the virus at the factory and lack of access to official information.” As we’ve already discussed, no news is bad news.
That’s all the time I have to chat with you today. I’m off to fire some more Twitter employees.
In case you hadn’t already guessed, Vlad Putin is officially more annoying than Elon Musk. We now return to this week’s typical LOTME shenanigans, already in progress.
The deadliest month
With climate change making the world hotter, leading to more heat stroke and organ failure, you would think the summer months would be the most deadly. In reality, though, it’s quite the opposite.
There are multiple factors that make January the most deadly month out of the year, as LiveScience discovered in a recent analysis.
Let’s go through them, shall we?
Respiratory viruses: Robert Glatter, MD, of Lenox Hill Hospital in New York, told LiveScence that winter is the time for illnesses like the flu, bacterial pneumonia, and RSV. Millions of people worldwide die from the flu, according to the CDC. And the World Health Organization reported lower respiratory infections as the fourth-leading cause of death worldwide before COVID came along.
Heart disease: Heart conditions are actually more fatal in the winter months, according to a study published in Circulation. The cold puts more stress on the heart to keep the body warm, which can be a challenge for people who already have preexisting heart conditions.
Space heaters: Dr. Glatter also told Live Science that the use of space heaters could be a factor in the cold winter months since they can lead to carbon monoxide poisoning and even fires. Silent killers.
Holiday season: A time for joy and merriment, certainly, but Christmas et al. have their downsides. By January we’re coming off a 3-month food and alcohol binge, which leads to cardiac stress. There’s also the psychological stress that comes with the season. Sometimes the most wonderful time of the year just isn’t.
So even though summer is hot, fall has hurricanes, and spring tends to have the highest suicide rate, winter still ends up being the deadliest season.
Bad drinking consequence No. 87: Joining the LOTME team
Alcohol and college students go together like peanut butter and jelly. Or peanut butter and chocolate. Or peanut butter and toothpaste. Peanut butter goes with a lot of things.
Naturally, when you combine alcohol and college students, bad decisions are sure to follow. But have you ever wondered just how many bad decisions alcohol causes? A team of researchers from Penn State University, the undisputed champion of poor drinking decisions (trust us, we know), sure has. They’ve even conducted a 4-year study of 1,700 students as they carved a drunken swath through the many fine local drinking establishments, such as East Halls or that one frat house that hosts medieval battle–style ping pong tournaments.
The students were surveyed twice a year throughout the study, and the researchers compiled a list of all the various consequences their subjects experienced. Ultimately, college students will experience an average of 102 consequences from drinking during their 4-year college careers, which is an impressive number. Try thinking up a hundred consequences for anything.
Some consequences are less common than others – we imagine “missing the Renaissance Faire because you felt drunker the morning after than while you were drinking” is pretty low on the list – but more than 96% of students reported that they’d experienced a hangover and that drinking had caused them to say or do embarrassing things. Also, more than 70% said they needed additional alcohol to feel any effect, a potential sign of alcohol use disorder.
Once they had their list, the researchers focused on 12 of the more common and severe consequences, such as blacking out, hangovers, and missing work/class, and asked the study participants how their parents would react to their drinking and those specific consequences. Students who believed their parents would disapprove of alcohol-related consequences actually experienced fewer consequences overall.
College students, it seems, really do care what their parents think, even if they don’t express it, the researchers said. That gives space for parents to offer advice about the consequences of hard drinking, making decisions while drunk, or bringing godawful Fireball whiskey to parties. Seriously, don’t do that. Stuff’s bad, and you should feel bad for bringing it. Your parents raised you better than that.
COVID ‘expert’ discusses data sharing
We interrupt our regularly scheduled programming to bring you this special news event. Elon Musk, the world’s second-most annoying human, is holding a press conference to discuss, of all things, COVID-19.
Reporter: Hey, Mr. Musketeer, what qualifies you to talk about a global pandemic?
EM: As the official king of the Twitterverse, I’m pretty much an expert on any topic.
Reporter: Okay then, Mr. Muskmelon, what can you tell us about the new study in Agricultural Economics, which looked at consumers’ knowledge of local COVID infection rates and their willingness to eat at restaurants?
EM: Well, I know that one of the investigators, Rigoberto Lopez, PhD, of the University of Connecticut, said “no news is bad news.” Restaurants located in cities where local regulations required COVID tracking recovered faster than those in areas that did not, according to data from 87 restaurants in 10 Chinese cities that were gathered between Dec. 1, 2019, and March 27, 2020. Having access to local infection rate data made customers more comfortable going out to eat, the investigators explained.
Second reporter: Interesting, Mr. Muskox, but how about this headline from CNN: “Workers flee China’s biggest iPhone factory over Covid outbreak”? Do you agree with analysts, who said that “the chaos at Zhengzhou could jeopardize Apple and Foxconn’s output in the coming weeks,” as CNN put it?
EM: I did see that a manager at Foxconn, which owns the factory and is known to its friends as Hon Hai Precision Industry, told a Chinese media outlet that “workers are panicking over the spread of the virus at the factory and lack of access to official information.” As we’ve already discussed, no news is bad news.
That’s all the time I have to chat with you today. I’m off to fire some more Twitter employees.
In case you hadn’t already guessed, Vlad Putin is officially more annoying than Elon Musk. We now return to this week’s typical LOTME shenanigans, already in progress.
The deadliest month
With climate change making the world hotter, leading to more heat stroke and organ failure, you would think the summer months would be the most deadly. In reality, though, it’s quite the opposite.
There are multiple factors that make January the most deadly month out of the year, as LiveScience discovered in a recent analysis.
Let’s go through them, shall we?
Respiratory viruses: Robert Glatter, MD, of Lenox Hill Hospital in New York, told LiveScence that winter is the time for illnesses like the flu, bacterial pneumonia, and RSV. Millions of people worldwide die from the flu, according to the CDC. And the World Health Organization reported lower respiratory infections as the fourth-leading cause of death worldwide before COVID came along.
Heart disease: Heart conditions are actually more fatal in the winter months, according to a study published in Circulation. The cold puts more stress on the heart to keep the body warm, which can be a challenge for people who already have preexisting heart conditions.
Space heaters: Dr. Glatter also told Live Science that the use of space heaters could be a factor in the cold winter months since they can lead to carbon monoxide poisoning and even fires. Silent killers.
Holiday season: A time for joy and merriment, certainly, but Christmas et al. have their downsides. By January we’re coming off a 3-month food and alcohol binge, which leads to cardiac stress. There’s also the psychological stress that comes with the season. Sometimes the most wonderful time of the year just isn’t.
So even though summer is hot, fall has hurricanes, and spring tends to have the highest suicide rate, winter still ends up being the deadliest season.
Hyaluronidase for Skin Necrosis Induced by Amiodarone
To the Editor:
Amiodarone is an oral or intravenous (IV) drug commonly used to treat supraventricular and ventricular arrhythmia as well as atrial fibrillation.1 Adverse drug reactions associated with the use of amiodarone include pulmonary, gastrointestinal, thyroid, ocular, neurologic, and cutaneous reactions.1 Long-term use of amiodarone—typically more than 4 months—can lead to slate-gray skin discoloration and photosensitivity, both of which can be reversed with drug withdrawal.2,3 Phlebitis also has been described in less than 3% of patients who receive peripheral IV administration of amiodarone.4
Amiodarone-induced skin necrosis due to extravasation is a rare complication of this antiarrhythmic medication, with only 3 reported cases in the literature according to a PubMed search of articles indexed for MEDLINE using the search terms amiodarone and skin and (necrosis or ischemia or extravasation or reaction).5–7 Although hyaluronidase is a known therapy for extravasation of fluids, including parenteral nutrition and chemotherapy, its use for the treatment of extravasation from amiodarone is not well documented.6 We report a case of skin necrosis of the left dorsal forearm and the left dorsal and ventral hand following infusion of amiodarone through a peripheral IV line, which was treated with injections of hyaluronidase.
A 77-year-old man was admitted to the emergency department for sepsis secondary to cholangitis in the setting of an obstructive gallbladder stone. His medical history was notable for multivessel coronary artery disease and atrial flutter treated with ablation. One day after admission, endoscopic retrograde cholangiopancreatography was attempted and aborted due to atrial fibrillation with rapid ventricular response. A second endoscopic retrograde cholangiopancreatography attempt was made 4 days later, during which the patient underwent cardiac arrest. During this event, amiodarone was administered in a 200-mL solution (1.8 mg/mL) in 5% dextrose through a peripheral IV line in the left forearm. The patient was stabilized and transferred to the intensive care unit.
Twenty-four hours after amiodarone administration, erythema was noted on the left dorsal forearm. Within hours, the digits of the hand became a dark, dusky color, which spread to involve the forearm. Surgical debridement was not deemed necessary; the left arm was elevated, and warm compresses were applied regularly. Within the next week, the skin of the left hand and dorsal forearm had progressively worsened and took on a well-demarcated, dusky blue hue surrounded by an erythematous border involving the proximal forearm and upper arm (Figure 1A). The skin was fragile and had overlying bullae (Figure 1B).
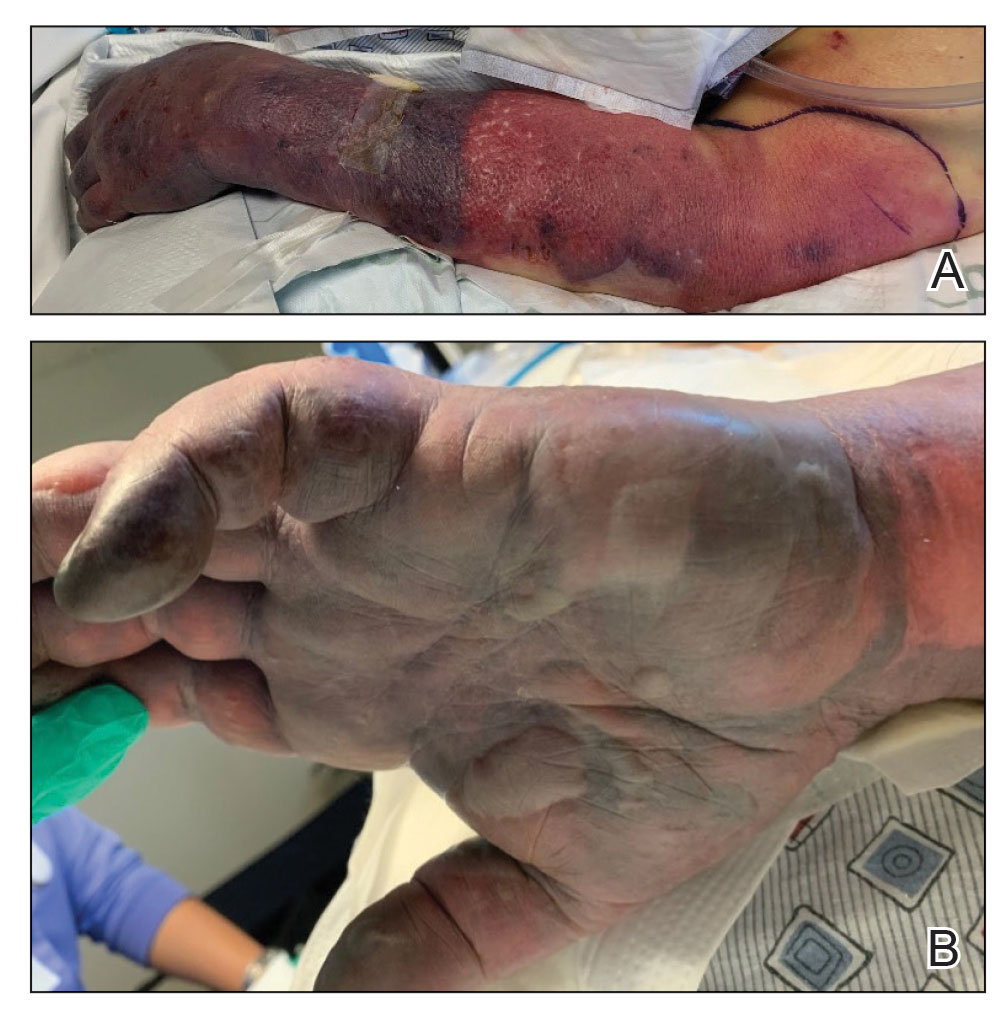
Hyaluronidase (1000 U) was injected into the surrounding areas of erythema, which resolved from the left proximal forearm to the elbow within 2 days after injection (Figure 2). The dusky violaceous patches were persistent, and the necrotic bullae were unchanged. Hyaluronidase (1000 U) was injected into necrotic skin of the left dorsal forearm and dorsal and ventral hand. No improvement was noted on subsequent evaluations of this area. While still an inpatient, he received wound care and twice-daily Doppler ultrasounds in the areas of necrosis. The patient lost sensation in the left hand with increased soft tissue necrosis and developed an eschar on the left dorsal forearm. Due to the progressive loss of function and necrosis, a partial forearm amputation was performed that healed well, and the patient experienced improvement in range of motion of the left upper extremity.
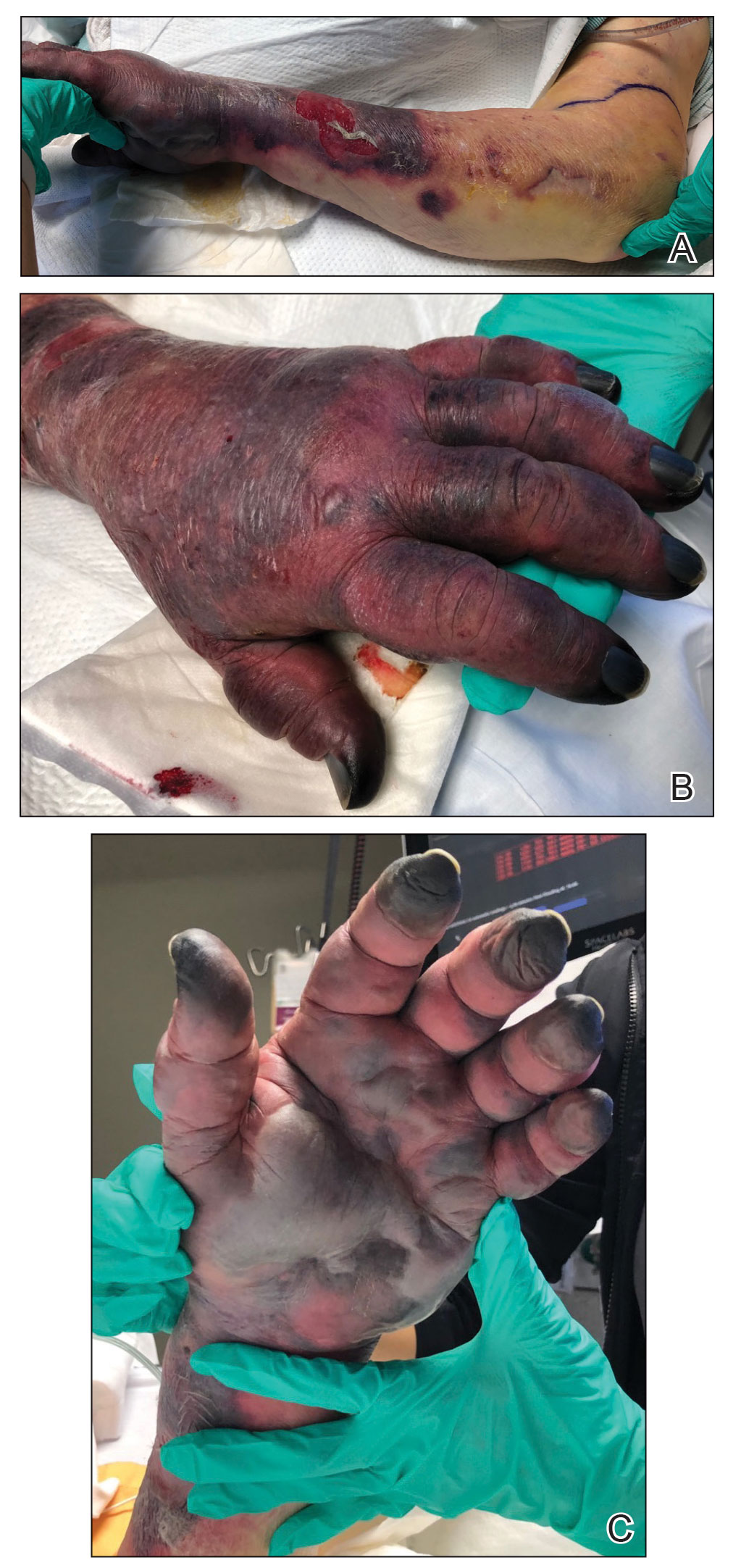
Well-known adverse reactions of amiodarone treatment include pulmonary fibrosis, hepatic dysfunction, hypothyroidism and hyperthyroidism, peripheral neuropathy, and corneal deposits.1 Cutaneous adverse reactions include photosensitivity (phototoxic and photoallergic reactions), hyperpigmentation, pseudoporphyria, and linear IgA bullous dermatosis. Less commonly, it also can cause urticaria, pruritus, erythema nodosum, purpura, and toxic epidermal necrolysis.3 Amiodarone-induced skin necrosis is rare, first described by Russell and Saltissi5 in 2006 in a 60-year-old man who developed dark discoloration and edema of the forearm 24 hours after initiation of an amiodarone peripheral IV. The patient was treated with hot or cold packs and steroid cream per the pharmaceutical company’s recommendations; however, patient outcomes were not discussed.5 A 77-year-old man who received subcutaneous amiodarone due to misplaced vascular access developed edema and bullae of the forearm followed by tissue necrosis, resulting in notably reduced mobility.6 Fox et al7 described a 60-year-old man who developed atrial fibrillation after emergent spinal fusion and laminectomy. He received intradermal hyaluronidase administration within 24 hours of developing severe pain from extravasation induced by amiodarone with no adverse outcomes and full recovery.7
There are numerous properties of amiodarone that may have resulted in the skin necrosis seen in these cases. The acidic pH (3.5–4.5) of amiodarone can contribute to coagulative necrosis, cellular desiccation, eschar formation, and edema.8 It also can contain additives such as polysorbate and benzyl alcohol, which may contribute to the drug’s vesicant properties.9
Current recommendations for IV administration of amiodarone include delivery through a central vein with high concentrations (>2 mg/mL) because peripheral infusion is slower and may cause phlebitis.4 In-line filters also may be a potential method of preventing phlebitis with peripheral IV administration of amiodarone.10 Extravasation of amiodarone can be treated nonpharmacologically with limb elevation and warm compresses, as these methods may promote vasodilation and enhance drug removal.5-7 However, when extravasation leads to progressive erythema and skin necrosis or is refractory to these therapies, intradermal injection of hyaluronidase should be considered. Hyaluronidase mediates the degradation of hyaluronic acid in the extracellular matrix, allowing for increased permeability of injected fluids into tissues and diluting the concentration of toxins at the site of exposure.9,11 It has been used to treat extravasation of fluids such as parenteral nutrition, electrolyte infusion, antibiotics, aminophylline, mannitol, and chemotherapy.11 Although hyaluronidase has been recognized as therapeutic for extravasation, there is no established consistent dosing or proper technique. In the setting of infiltration of chemotherapy, doses of hyaluronidase ranging from 150 to 1500 U/mL can be subcutaneously or intradermally injected into the site within 1 hour of extravasation. Side effects of using hyaluronidase are rare, including local pruritus, allergic reactions, urticaria, and angioedema.12
The patient described by Fox et al7 who fully recovered from amiodarone extravasation after hyaluronidase injections likely benefited from quick intervention, as he received amiodarone within 24 hours of the care team identifying initial erythema. Although our patient did have improvement of the areas of erythema on the forearm, evidence of skin and subcutaneous tissue necrosis on the left hand and proximal forearm was already apparent and not reversible, most likely caused by late intervention of intradermal hyaluronidase almost a week after the extravasation event. It is important to identify amiodarone as the source of extravasation and administer intradermal hyaluronidase in a timely fashion for extravasation refractory to conventional measurements to prevent progression to severe tissue damage.
Our case draws attention to the risk for skin necrosis with peripheral IV administration of amiodarone. Interventions include limb elevation, warm compresses, and consideration of intradermal hyaluronidase within 24 hours of extravasation, as this may reduce the severity of subsequent tissue damage with minimal side effects.
- Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468-475. doi:10.1016/j.amjmed.2015.08.039
- Harris L, McKenna WJ, Rowland E, et al. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45-51. doi:10.1161/01.cir.67.1.45
- Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369-2372. doi:10.12659/MSM.890881
- Kowey Peter R, Marinchak Roger A, Rials Seth J, et al. Intravenous amiodarone. J Am Coll Cardiol. 1997;29:1190-1198. doi:10.1016/S0735-1097(97)00069-7
- Russell SJ, Saltissi S. Amiodarone induced skin necrosis. Heart. 2006;92:1395. doi:10.1136/hrt.2005.086157
- Grove EL. Skin necrosis and consequences of accidental subcutaneous administration of amiodarone. Ugeskr Laeger. 2015;177:V66928.
- Fox AN, Villanueva R, Miller JL. Management of amiodarone extravasation with intradermal hyaluronidase. Am J Health Syst Pharm. 2017;74:1545-1548. doi:10.2146/ajhp160737
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632. doi:https://doi.org/10.1002/phar.1396
- Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870-886. doi:10.1177/1060028014527820
- Slim AM, Roth JE, Duffy B, et al. The incidence of phlebitis with intravenous amiodarone at guideline dose recommendations. Mil Med. 2007;172:1279-1283.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921-1943. doi:10.1016/j.lfs.2007.02.037
- Jung H. Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300. doi:10.5999/aps.2020.00752
To the Editor:
Amiodarone is an oral or intravenous (IV) drug commonly used to treat supraventricular and ventricular arrhythmia as well as atrial fibrillation.1 Adverse drug reactions associated with the use of amiodarone include pulmonary, gastrointestinal, thyroid, ocular, neurologic, and cutaneous reactions.1 Long-term use of amiodarone—typically more than 4 months—can lead to slate-gray skin discoloration and photosensitivity, both of which can be reversed with drug withdrawal.2,3 Phlebitis also has been described in less than 3% of patients who receive peripheral IV administration of amiodarone.4
Amiodarone-induced skin necrosis due to extravasation is a rare complication of this antiarrhythmic medication, with only 3 reported cases in the literature according to a PubMed search of articles indexed for MEDLINE using the search terms amiodarone and skin and (necrosis or ischemia or extravasation or reaction).5–7 Although hyaluronidase is a known therapy for extravasation of fluids, including parenteral nutrition and chemotherapy, its use for the treatment of extravasation from amiodarone is not well documented.6 We report a case of skin necrosis of the left dorsal forearm and the left dorsal and ventral hand following infusion of amiodarone through a peripheral IV line, which was treated with injections of hyaluronidase.
A 77-year-old man was admitted to the emergency department for sepsis secondary to cholangitis in the setting of an obstructive gallbladder stone. His medical history was notable for multivessel coronary artery disease and atrial flutter treated with ablation. One day after admission, endoscopic retrograde cholangiopancreatography was attempted and aborted due to atrial fibrillation with rapid ventricular response. A second endoscopic retrograde cholangiopancreatography attempt was made 4 days later, during which the patient underwent cardiac arrest. During this event, amiodarone was administered in a 200-mL solution (1.8 mg/mL) in 5% dextrose through a peripheral IV line in the left forearm. The patient was stabilized and transferred to the intensive care unit.
Twenty-four hours after amiodarone administration, erythema was noted on the left dorsal forearm. Within hours, the digits of the hand became a dark, dusky color, which spread to involve the forearm. Surgical debridement was not deemed necessary; the left arm was elevated, and warm compresses were applied regularly. Within the next week, the skin of the left hand and dorsal forearm had progressively worsened and took on a well-demarcated, dusky blue hue surrounded by an erythematous border involving the proximal forearm and upper arm (Figure 1A). The skin was fragile and had overlying bullae (Figure 1B).

Hyaluronidase (1000 U) was injected into the surrounding areas of erythema, which resolved from the left proximal forearm to the elbow within 2 days after injection (Figure 2). The dusky violaceous patches were persistent, and the necrotic bullae were unchanged. Hyaluronidase (1000 U) was injected into necrotic skin of the left dorsal forearm and dorsal and ventral hand. No improvement was noted on subsequent evaluations of this area. While still an inpatient, he received wound care and twice-daily Doppler ultrasounds in the areas of necrosis. The patient lost sensation in the left hand with increased soft tissue necrosis and developed an eschar on the left dorsal forearm. Due to the progressive loss of function and necrosis, a partial forearm amputation was performed that healed well, and the patient experienced improvement in range of motion of the left upper extremity.

Well-known adverse reactions of amiodarone treatment include pulmonary fibrosis, hepatic dysfunction, hypothyroidism and hyperthyroidism, peripheral neuropathy, and corneal deposits.1 Cutaneous adverse reactions include photosensitivity (phototoxic and photoallergic reactions), hyperpigmentation, pseudoporphyria, and linear IgA bullous dermatosis. Less commonly, it also can cause urticaria, pruritus, erythema nodosum, purpura, and toxic epidermal necrolysis.3 Amiodarone-induced skin necrosis is rare, first described by Russell and Saltissi5 in 2006 in a 60-year-old man who developed dark discoloration and edema of the forearm 24 hours after initiation of an amiodarone peripheral IV. The patient was treated with hot or cold packs and steroid cream per the pharmaceutical company’s recommendations; however, patient outcomes were not discussed.5 A 77-year-old man who received subcutaneous amiodarone due to misplaced vascular access developed edema and bullae of the forearm followed by tissue necrosis, resulting in notably reduced mobility.6 Fox et al7 described a 60-year-old man who developed atrial fibrillation after emergent spinal fusion and laminectomy. He received intradermal hyaluronidase administration within 24 hours of developing severe pain from extravasation induced by amiodarone with no adverse outcomes and full recovery.7
There are numerous properties of amiodarone that may have resulted in the skin necrosis seen in these cases. The acidic pH (3.5–4.5) of amiodarone can contribute to coagulative necrosis, cellular desiccation, eschar formation, and edema.8 It also can contain additives such as polysorbate and benzyl alcohol, which may contribute to the drug’s vesicant properties.9
Current recommendations for IV administration of amiodarone include delivery through a central vein with high concentrations (>2 mg/mL) because peripheral infusion is slower and may cause phlebitis.4 In-line filters also may be a potential method of preventing phlebitis with peripheral IV administration of amiodarone.10 Extravasation of amiodarone can be treated nonpharmacologically with limb elevation and warm compresses, as these methods may promote vasodilation and enhance drug removal.5-7 However, when extravasation leads to progressive erythema and skin necrosis or is refractory to these therapies, intradermal injection of hyaluronidase should be considered. Hyaluronidase mediates the degradation of hyaluronic acid in the extracellular matrix, allowing for increased permeability of injected fluids into tissues and diluting the concentration of toxins at the site of exposure.9,11 It has been used to treat extravasation of fluids such as parenteral nutrition, electrolyte infusion, antibiotics, aminophylline, mannitol, and chemotherapy.11 Although hyaluronidase has been recognized as therapeutic for extravasation, there is no established consistent dosing or proper technique. In the setting of infiltration of chemotherapy, doses of hyaluronidase ranging from 150 to 1500 U/mL can be subcutaneously or intradermally injected into the site within 1 hour of extravasation. Side effects of using hyaluronidase are rare, including local pruritus, allergic reactions, urticaria, and angioedema.12
The patient described by Fox et al7 who fully recovered from amiodarone extravasation after hyaluronidase injections likely benefited from quick intervention, as he received amiodarone within 24 hours of the care team identifying initial erythema. Although our patient did have improvement of the areas of erythema on the forearm, evidence of skin and subcutaneous tissue necrosis on the left hand and proximal forearm was already apparent and not reversible, most likely caused by late intervention of intradermal hyaluronidase almost a week after the extravasation event. It is important to identify amiodarone as the source of extravasation and administer intradermal hyaluronidase in a timely fashion for extravasation refractory to conventional measurements to prevent progression to severe tissue damage.
Our case draws attention to the risk for skin necrosis with peripheral IV administration of amiodarone. Interventions include limb elevation, warm compresses, and consideration of intradermal hyaluronidase within 24 hours of extravasation, as this may reduce the severity of subsequent tissue damage with minimal side effects.
To the Editor:
Amiodarone is an oral or intravenous (IV) drug commonly used to treat supraventricular and ventricular arrhythmia as well as atrial fibrillation.1 Adverse drug reactions associated with the use of amiodarone include pulmonary, gastrointestinal, thyroid, ocular, neurologic, and cutaneous reactions.1 Long-term use of amiodarone—typically more than 4 months—can lead to slate-gray skin discoloration and photosensitivity, both of which can be reversed with drug withdrawal.2,3 Phlebitis also has been described in less than 3% of patients who receive peripheral IV administration of amiodarone.4
Amiodarone-induced skin necrosis due to extravasation is a rare complication of this antiarrhythmic medication, with only 3 reported cases in the literature according to a PubMed search of articles indexed for MEDLINE using the search terms amiodarone and skin and (necrosis or ischemia or extravasation or reaction).5–7 Although hyaluronidase is a known therapy for extravasation of fluids, including parenteral nutrition and chemotherapy, its use for the treatment of extravasation from amiodarone is not well documented.6 We report a case of skin necrosis of the left dorsal forearm and the left dorsal and ventral hand following infusion of amiodarone through a peripheral IV line, which was treated with injections of hyaluronidase.
A 77-year-old man was admitted to the emergency department for sepsis secondary to cholangitis in the setting of an obstructive gallbladder stone. His medical history was notable for multivessel coronary artery disease and atrial flutter treated with ablation. One day after admission, endoscopic retrograde cholangiopancreatography was attempted and aborted due to atrial fibrillation with rapid ventricular response. A second endoscopic retrograde cholangiopancreatography attempt was made 4 days later, during which the patient underwent cardiac arrest. During this event, amiodarone was administered in a 200-mL solution (1.8 mg/mL) in 5% dextrose through a peripheral IV line in the left forearm. The patient was stabilized and transferred to the intensive care unit.
Twenty-four hours after amiodarone administration, erythema was noted on the left dorsal forearm. Within hours, the digits of the hand became a dark, dusky color, which spread to involve the forearm. Surgical debridement was not deemed necessary; the left arm was elevated, and warm compresses were applied regularly. Within the next week, the skin of the left hand and dorsal forearm had progressively worsened and took on a well-demarcated, dusky blue hue surrounded by an erythematous border involving the proximal forearm and upper arm (Figure 1A). The skin was fragile and had overlying bullae (Figure 1B).

Hyaluronidase (1000 U) was injected into the surrounding areas of erythema, which resolved from the left proximal forearm to the elbow within 2 days after injection (Figure 2). The dusky violaceous patches were persistent, and the necrotic bullae were unchanged. Hyaluronidase (1000 U) was injected into necrotic skin of the left dorsal forearm and dorsal and ventral hand. No improvement was noted on subsequent evaluations of this area. While still an inpatient, he received wound care and twice-daily Doppler ultrasounds in the areas of necrosis. The patient lost sensation in the left hand with increased soft tissue necrosis and developed an eschar on the left dorsal forearm. Due to the progressive loss of function and necrosis, a partial forearm amputation was performed that healed well, and the patient experienced improvement in range of motion of the left upper extremity.

Well-known adverse reactions of amiodarone treatment include pulmonary fibrosis, hepatic dysfunction, hypothyroidism and hyperthyroidism, peripheral neuropathy, and corneal deposits.1 Cutaneous adverse reactions include photosensitivity (phototoxic and photoallergic reactions), hyperpigmentation, pseudoporphyria, and linear IgA bullous dermatosis. Less commonly, it also can cause urticaria, pruritus, erythema nodosum, purpura, and toxic epidermal necrolysis.3 Amiodarone-induced skin necrosis is rare, first described by Russell and Saltissi5 in 2006 in a 60-year-old man who developed dark discoloration and edema of the forearm 24 hours after initiation of an amiodarone peripheral IV. The patient was treated with hot or cold packs and steroid cream per the pharmaceutical company’s recommendations; however, patient outcomes were not discussed.5 A 77-year-old man who received subcutaneous amiodarone due to misplaced vascular access developed edema and bullae of the forearm followed by tissue necrosis, resulting in notably reduced mobility.6 Fox et al7 described a 60-year-old man who developed atrial fibrillation after emergent spinal fusion and laminectomy. He received intradermal hyaluronidase administration within 24 hours of developing severe pain from extravasation induced by amiodarone with no adverse outcomes and full recovery.7
There are numerous properties of amiodarone that may have resulted in the skin necrosis seen in these cases. The acidic pH (3.5–4.5) of amiodarone can contribute to coagulative necrosis, cellular desiccation, eschar formation, and edema.8 It also can contain additives such as polysorbate and benzyl alcohol, which may contribute to the drug’s vesicant properties.9
Current recommendations for IV administration of amiodarone include delivery through a central vein with high concentrations (>2 mg/mL) because peripheral infusion is slower and may cause phlebitis.4 In-line filters also may be a potential method of preventing phlebitis with peripheral IV administration of amiodarone.10 Extravasation of amiodarone can be treated nonpharmacologically with limb elevation and warm compresses, as these methods may promote vasodilation and enhance drug removal.5-7 However, when extravasation leads to progressive erythema and skin necrosis or is refractory to these therapies, intradermal injection of hyaluronidase should be considered. Hyaluronidase mediates the degradation of hyaluronic acid in the extracellular matrix, allowing for increased permeability of injected fluids into tissues and diluting the concentration of toxins at the site of exposure.9,11 It has been used to treat extravasation of fluids such as parenteral nutrition, electrolyte infusion, antibiotics, aminophylline, mannitol, and chemotherapy.11 Although hyaluronidase has been recognized as therapeutic for extravasation, there is no established consistent dosing or proper technique. In the setting of infiltration of chemotherapy, doses of hyaluronidase ranging from 150 to 1500 U/mL can be subcutaneously or intradermally injected into the site within 1 hour of extravasation. Side effects of using hyaluronidase are rare, including local pruritus, allergic reactions, urticaria, and angioedema.12
The patient described by Fox et al7 who fully recovered from amiodarone extravasation after hyaluronidase injections likely benefited from quick intervention, as he received amiodarone within 24 hours of the care team identifying initial erythema. Although our patient did have improvement of the areas of erythema on the forearm, evidence of skin and subcutaneous tissue necrosis on the left hand and proximal forearm was already apparent and not reversible, most likely caused by late intervention of intradermal hyaluronidase almost a week after the extravasation event. It is important to identify amiodarone as the source of extravasation and administer intradermal hyaluronidase in a timely fashion for extravasation refractory to conventional measurements to prevent progression to severe tissue damage.
Our case draws attention to the risk for skin necrosis with peripheral IV administration of amiodarone. Interventions include limb elevation, warm compresses, and consideration of intradermal hyaluronidase within 24 hours of extravasation, as this may reduce the severity of subsequent tissue damage with minimal side effects.
- Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468-475. doi:10.1016/j.amjmed.2015.08.039
- Harris L, McKenna WJ, Rowland E, et al. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45-51. doi:10.1161/01.cir.67.1.45
- Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369-2372. doi:10.12659/MSM.890881
- Kowey Peter R, Marinchak Roger A, Rials Seth J, et al. Intravenous amiodarone. J Am Coll Cardiol. 1997;29:1190-1198. doi:10.1016/S0735-1097(97)00069-7
- Russell SJ, Saltissi S. Amiodarone induced skin necrosis. Heart. 2006;92:1395. doi:10.1136/hrt.2005.086157
- Grove EL. Skin necrosis and consequences of accidental subcutaneous administration of amiodarone. Ugeskr Laeger. 2015;177:V66928.
- Fox AN, Villanueva R, Miller JL. Management of amiodarone extravasation with intradermal hyaluronidase. Am J Health Syst Pharm. 2017;74:1545-1548. doi:10.2146/ajhp160737
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632. doi:https://doi.org/10.1002/phar.1396
- Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870-886. doi:10.1177/1060028014527820
- Slim AM, Roth JE, Duffy B, et al. The incidence of phlebitis with intravenous amiodarone at guideline dose recommendations. Mil Med. 2007;172:1279-1283.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921-1943. doi:10.1016/j.lfs.2007.02.037
- Jung H. Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300. doi:10.5999/aps.2020.00752
- Epstein AE, Olshansky B, Naccarelli GV, et al. Practical management guide for clinicians who treat patients with amiodarone. Am J Med. 2016;129:468-475. doi:10.1016/j.amjmed.2015.08.039
- Harris L, McKenna WJ, Rowland E, et al. Side effects of long-term amiodarone therapy. Circulation. 1983;67:45-51. doi:10.1161/01.cir.67.1.45
- Jaworski K, Walecka I, Rudnicka L, et al. Cutaneous adverse reactions of amiodarone. Med Sci Monit. 2014;20:2369-2372. doi:10.12659/MSM.890881
- Kowey Peter R, Marinchak Roger A, Rials Seth J, et al. Intravenous amiodarone. J Am Coll Cardiol. 1997;29:1190-1198. doi:10.1016/S0735-1097(97)00069-7
- Russell SJ, Saltissi S. Amiodarone induced skin necrosis. Heart. 2006;92:1395. doi:10.1136/hrt.2005.086157
- Grove EL. Skin necrosis and consequences of accidental subcutaneous administration of amiodarone. Ugeskr Laeger. 2015;177:V66928.
- Fox AN, Villanueva R, Miller JL. Management of amiodarone extravasation with intradermal hyaluronidase. Am J Health Syst Pharm. 2017;74:1545-1548. doi:10.2146/ajhp160737
- Reynolds PM, MacLaren R, Mueller SW, et al. Management of extravasation injuries: a focused evaluation of noncytotoxic medications. Pharmacotherapy. 2014;34:617-632. doi:https://doi.org/10.1002/phar.1396
- Le A, Patel S. Extravasation of noncytotoxic drugs: a review of the literature. Ann Pharmacother. 2014;48:870-886. doi:10.1177/1060028014527820
- Slim AM, Roth JE, Duffy B, et al. The incidence of phlebitis with intravenous amiodarone at guideline dose recommendations. Mil Med. 2007;172:1279-1283.
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921-1943. doi:10.1016/j.lfs.2007.02.037
- Jung H. Hyaluronidase: an overview of its properties, applications, and side effects. Arch Plast Surg. 2020;47:297-300. doi:10.5999/aps.2020.00752
Practice Points
- Intravenous amiodarone administered peripherally can induce skin extravasation, leading to necrosis.
- Dermatologists should be aware that early intervention with intradermal hyaluronidase may reduce the severity of tissue damage caused by amiodarone-induced skin necrosis.
Genital Lentiginosis: A Benign Pigmentary Abnormality Often Raising Concern for Melanoma
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.
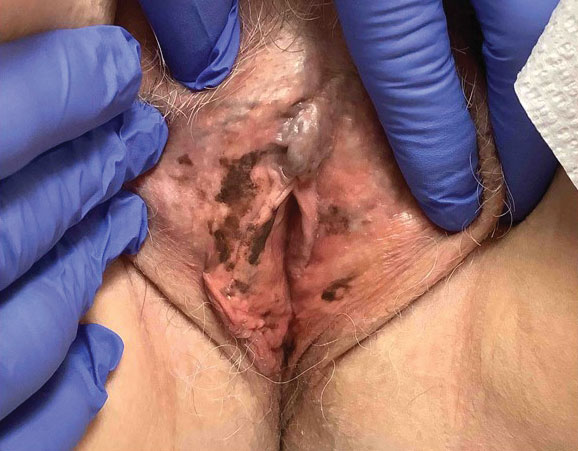
Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.
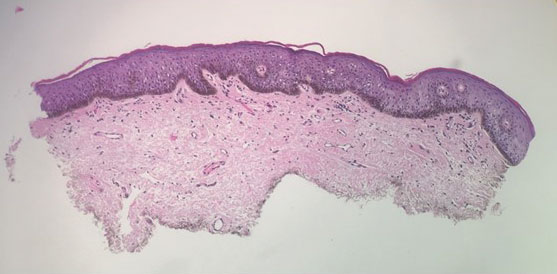
A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.
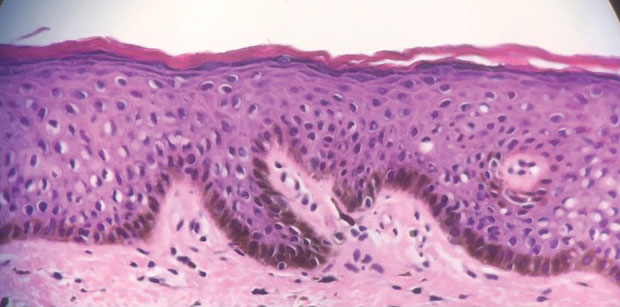
Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.

Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.

A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.

Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
To the Editor:
Genital lentiginosis (also known as mucosal melanotic macules, vulvar melanosis, penile melanosis, and penile lentigines) occurs in men and women.1 Lesions present in adult life as multifocal, asymmetrical, pigmented patches that can have a mottled appearance or exhibit skip areas. The irregular appearance of the pigmented areas often raises concern for melanoma. Biopsy reveals increased pigmentation along the basal layer of the epidermis; the irregular distribution of single melanocytes and pagetoid spread typical of melanoma in situ is not identified.

Genital lentiginosis usually occurs as an isolated finding; however, the condition can be a manifestation of Laugier-Hunziker syndrome, Carney complex, and Bannayan-Riley-Ruvalcaba syndrome.1-3 When it occurs as an isolated finding, the patient can be reassured and treatment is unnecessary. Because genital lentiginosis may mimic the appearance of melanoma, it is important for physicians to differentiate the two and make a correct diagnosis. We present a case of genital lentiginosis that mimicked vulvar melanoma.

A 64-year-old woman was referred by her gynecologist to dermatology to rule out vulvar melanoma. The patient had a history of hypothyroidism and hypercholesterolemia but was otherwise in good health. Genital examination revealed asymptomatic pigmented macules and patches of unknown duration (Figure 1). Specimens were taken from 3 areas by punch biopsy to clarify the diagnosis. All 3 specimens showed identical features including basilar pigmentation, occasional melanophages in the papillary dermis, and no evidence of nests or pagetoid spread of atypical melanocytes (Figures 2 and 3). Histologic findings were diagnostic for genital lentiginosis. The patient was reassured, and no treatment was provided. At 6-month follow-up there was no change in clinical appearance.

Genital lentiginosis is characterized by brown lesions that can have a mottled appearance and often are associated with skip areas.1 Lesions can be strikingly irregular and darkly pigmented.
Although the lesions of genital lentiginosis most often are isolated findings, they can be a clue to several uncommon syndromes such as autosomal-dominant Bannayan-Riley-Ruvalcaba syndrome, which is associated with genital lentiginosis, intestinal polyposis, and macrocephaly.3 Vascular malformations, lipomatosis, verrucal keratoses, and acrochordons can occur. Bannayan-Riley-Ruvalcaba syndrome and Cowden syndrome may share genetic linkage; mutations in the tumor suppressor PTEN (phosphatase and tensin homolog deleted on chromosome ten) has been implicated in both syndromes.4 Underlying Carney complex should be excluded when genital lentiginosis is encountered.
Genital lentiginosis is idiopathic in most instances, but reports of lesions occurring after annular lichen planus suggest a possible mechanism.5 The disappearance of lentigines after imatinib therapy suggests a role for c-kit, a receptor tyrosine kinase that is involved in intracellular signaling, in some cases.6 At times, lesions can simulate trichrome vitiligo or have a reticulate pattern.7
Men and women present at different points in the course of disease. Men often present with penile lesions 14 years after onset, on average; they notice a gradual increase in the size of lesions. Because women can have greater difficulty self-examining the genital region, they tend to present much later in the course but often within a few months after initial inspection.1,8
Genital lentiginosis can mimic melanoma with nonhomogeneous pigmentation, asymmetry, and unilateral distribution, which makes dermoscopic assessment of colors helpful in narrowing the differential diagnosis. Melanoma is associated with combinations of gray, red, blue, and white, which are not found in genital lentiginosis.9
Biopsy of a genital lentigo is diagnostic, distinguishing the lesion from melanoma—failing to reveal the atypical melanocytes and pagetoid spread characteristic of melanoma in situ. Histologic findings can cause diagnostic difficulties when concurrent lichen sclerosus is associated with genital lentigines or nevi.10
Lentigines on sun-damaged skin or in the setting of xeroderma pigmentosum have been associated with melanoma,11-13 but genital lentigines are not considered a form of precancerous melanosis. In women, early diagnosis is important when there is concern for melanoma because the prognosis for vulvar melanoma is improved in thin lesions.14
Other entities in the differential include secondary syphilis, which commonly presents as macules and scaly papules and can be found on mucosal surfaces such as the oral cavity,15 as well as Kaposi sarcoma, which is characterized by purplish, brown, or black macules, plaques, and nodules, more commonly in immunosuppressed patients.16
To avoid unwarranted concern and unnecessary surgery, dermatologists should be aware of genital lentigines and their characteristic presentation in adults.
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
- Hwang L, Wilson H, Orengo I. Off-center fold: irregular, pigmented genital macules. Arch Dermatol. 2000;136:1559-1564. doi:10.1001/archderm.136.12.1559-b
- Rhodes AR, Silverman RA, Harrist TJ, et al. Mucocutaneous lentigines, cardiomucocutaneous myxomas, and multiple blue nevi: the “LAMB” syndrome. J Am Acad Dermatol. 1984;10:72-82. doi:10.1016/s0190-9622(84)80047-x
- Erkek E, Hizel S, Sanl C, et al. Clinical and histopathological findings in Bannayan-Riley-Ruvalcaba syndrome. J Am Acad Dermatol. 2005;53:639-643. doi:10.1016/j.jaad.2005.06.022
- Blum RR, Rahimizadeh A, Kardon N, et al. Genital lentigines in a 6-year-old boy with a family history of Cowden’s disease: clinical and genetic evidence of the linkage between Bannayan-Riley-Ruvalcaba syndrome and Cowden’s disease. J Cutan Med Surg. 2001;5:228-230. doi:10.1177/120347540100500307
- Isbary G, Dyall-Smith D, Coras-Stepanek B, et al. Penile lentigo (genital mucosal macule) following annular lichen planus: a possible association? Australas J Dermatol. 2014;55:159-161. doi:10.1111/ajd.12169
- Campbell T, Felsten L, Moore J. Disappearance of lentigines in a patient receiving imatinib treatment for familial gastrointestinal stromal tumor syndrome. Arch Dermatol. 2009;145:1313-1316. doi:10.1001/archdermatol.2009.263
- Romero- A, R, , et al. Reticulate genital pigmentation associated with localized vitiligo. Arch Dermatol. 2010; 146:574-575. doi:10.1001/archdermatol.2010.69
- Barnhill RL, Albert LS, Shama SK, et al. Genital lentiginosis: a clinical and histopathologic study. J Am Acad Dermatol. 1990;22:453-460. doi:10.1016/0190-9622(90)70064-o
- De Giorgi V, Gori A, Salvati L, et al. Clinical and dermoscopic features of vulvar melanosis over the last 20 years. JAMA Dermatol. 2020;156:1185–1191. doi:10.1001/jamadermatol.2020.2528
- El Shabrawi-Caelen L, Soyer HP, Schaeppi H, et al. Genital lentigines and melanocytic nevi with superimposed lichen sclerosus: a diagnostic challenge. J Am Acad Dermatol. 2004;50:690-694. doi:10.1016/j.jaad.2003.09.034
- Shatkin M, Helm MF, Muhlbauer A, et al. Solar lentigo evolving into fatal metastatic melanoma in a patient who initially refused surgery. N A J Med Sci. 2020;1:28-31. doi:10.7156/najms.2020.1301028
- Stern JB, Peck GL, Haupt HM, et al. Malignant melanoma in xeroderma pigmentosum: search for a precursor lesion. J Am Acad Dermatol. 1993;28:591-594. doi:10.1016/0190-9622(93)70079-9
- Byrom L, Barksdale S, Weedon D, et al. Unstable solar lentigo: a defined separate entity. Australas J Dermatol. 2016;57:229-234. doi:10.1111/ajd.12447
- Panizzon RG. Vulvar melanoma. Semin Dermatol. 1996;15:67-70. doi:10.1016/s1085-5629(96)80021-6
- Chapel TA. The signs and symptoms of secondary syphilis. Sex Transm Dis. 1980;7:161-164. doi:10.1097/00007435-198010000-00002
- Schwartz RA. Kaposi’s sarcoma: an update. J Surg Oncol. 2004;87:146-151. doi:10.1002/jso.20090
Practice Points
- The irregular appearance of genital lentiginosis—multifocal, asymmetric, irregular, and darkly pigmented patches—often raises concern for melanoma but is benign.
- Certain genetic conditions can present with genital lentiginosis.
- Dermoscopic assessment of the lesion color is highly helpful in narrowing the differential diagnosis; seeing no gray, red, blue, or white makes melanoma less likely.
- Be aware of genital lentigines and their characteristic presentation in adulthood to avoid unwarranted concern and unneeded surgery.