User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Ready to make a difference, dermatologist takes the helm as AMA president
Jack S. Resneck Jr., MD, is not usually the loudest voice in the room, but when he speaks, his words carry a heft and an appeal that is straightforward and undeniable.
That was on full display as.
He did not mince words when it came to describing the current landscape. “I doubt you imagined a divided country such as this, where physicians and public health officials often face antiscience aggression and threats of violence simply for doing our jobs,” he said. “You probably didn’t plan on insurers questioning every prescription and every procedure you asked for. Or government criminalizing routine and vital health care, enshrining discrimination against our LGBTQ patients or attacking a woman’s right to control health care decisions that should only be between her and her doctor,” said Dr. Resneck.
But, he added, all was not lost. “While it would be easy to get overwhelmed by despair as I begin this new role, I’ve never been prouder of my physician colleagues,” he said.
Dr. Resneck is the first dermatologist to lead the 175-year-old organization since 1925. Colleagues in the field speak of pride in having one of their own at the top, and they are even more complimentary about his depth of health policy knowledge, his communications skills, and his ability to find common ground.
“He loves looking at both sides,” said Marta J. Van Beek, MD, clinical professor of dermatology at the University of Iowa, Iowa City. “That’s how he builds consensus,” said Dr. Van Beek, who has known Dr. Resneck for more than 20 years, since they were chief dermatology residents – she at Iowa and he at UCSF.
Dr. Van Beek and Dr. Resneck have a deep interest in health policy and have long worked side by side on committees at both the American Academy of Dermatology (AAD) and the AMA. She looks back to the lead-up period before the 2010 passage of the Affordable Care Act as one of Dr. Resneck’s shining moments. “Those were contentious times in medicine,” Dr. Van Beek told this news organization. Dr. Resneck, as chair of the AAD’s Council on Government Affairs and Health Policy from 2008 to 2012, rallied the board to agree on a set of health care reform principles, she noted.
Dr. Resneck is “really very unifying,” agreed Bruce A. Brod, MD, clinical professor of dermatology at the University of Pennsylvania, Philadelphia. Dr. Brod has worked with Dr. Resneck for 2 decades on various committees at the AAD. They’ve also known each other through the AMA House of Delegates.
Now Dr. Brod is following in Dr. Resneck’s footsteps as chair of the AAD’s Council on Government Affairs and Health Policy. “Big shoes to fill there,” said Dr. Brod. He said he’s been inspired by Dr. Resneck’s always-positive approach, punctuated by his ever-constant belief that “there’s a lot more common ground than meets the eye here.”
“I really think he’s the perfect leader at this time,” he said.
Outgoing AMA President Gerald E. Harmon, MD, said that Dr. Resneck’s long experience as a teacher and a mentor, and what he describes as a “good, active listening talent,” have been integral to his success as a leader. He expects those qualities to make Dr. Resneck an effective advocate for all of medicine. “He identifies the problem, he identifies the gap, and then he establishes a workable, executable plan to close that gap,” Dr. Harmon told this news organization, adding that he’s seen this at work in AMA board meetings.
“He was a good teacher for me,” said Dr. Harmon, who had known Dr. Resneck through the House of Delegates and various AMA councils for at least a decade before they both joined the AMA board. “He can be such a mentor to all age groups, including senior physicians like myself,” said Dr. Harmon.
Dr. Resneck is excited, but also measured. “This has been a tumultuous couple of years in the country with the pandemic and with the fractured politics,” he said in an interview. Thinking about taking on the AMA presidency, he said, “I’ve had some moments of trepidation. I wanted to be sure that I was going to be able to make a difference.”
Long interest in health policy
Growing up in Shreveport, La., as the son of a dermatologist, Dr. Resneck said, “at first, I swore I was going to do something other than medicine.” It was not out of rebellion. He got along fine with his father. He wanted to pursue his own journey.
Dr. Resneck began his long love affair with health policy at Brown University, graduating magna cum laude with honors in public policy. A 1991 U.S. Department of Health & Human Services internship between his junior and senior years helped inform his honors thesis on health care financing policy.
Ultimately, Dr. Resneck did not stray far from his father’s career path. While still at Brown, he decided to go to medical school, entering UCSF in the fall of 1993. “At the end of the day, there was this undeniable influence that he loved his job,” Dr. Resneck said in the interview. His father was energized by the work and helping patients. “If burnout was in his vocabulary, I never heard it,” said Dr. Resneck.
Initially, he did a 1-year internal medicine residency at UCSF, but then switched to dermatology and became chief resident in 2000.
He was quickly pegged as a leader and an inspirational speaker. The AAD gave him its Young Physician Leadership Development Award in 2001, and the AMA gave Dr. Resneck its Excellence in Medicine National Award for Young Physician Leadership in 2004. He began giving talks at national meetings in 2002 and has been busy ever since, addressing the AMA, the AAD, state medical and dermatology societies, subspecialty groups such as the American College of Mohs Surgery and the Association of Professors of Dermatology, and other organizations such as the American Telemedicine Association.
He’s a sought-after speaker in part because of his ability to simply communicate health policy, said Dr. Brod. “He can connect the real-world issues really well to the policy needs and communicate it very well, and come in at just the right level,” he said.
Dr. Resneck has been immersed in policy and practice issues since the start of his career, serving on AAD and AMA committees addressing quality measures, data collection, access to care, workforce issues, and telemedicine. He started writing about dermatology workforce challenges in 2001 and has revisited that topic with regularity.
He has published often on the difficulties of patient access, looking at wait times for appointments, among other issues. In 2018, he expressed concern in a commentary in JAMA Dermatology that private equity purchases of dermatology practices might lead to an improper focus on profits over patients and that it could reduce the diversity of practice models.
At heart, Dr. Resneck is an institutionalist, someone who believes that the “collaborative, collective voice can make change,” said Dr. Brod. Dr. Resneck said as much in his inaugural speech. “I believe those who show up can use levers of power to confront our system’s flaws,” he said. “This is the nerdy policy part of my life, which my friends will force me to admit is most of my life,” said Dr. Resneck.
Prior authorization, telemedicine, equity
Beneath the reserved exterior lies an intense yearning to act on his passions, both at work and at play.
Dr. Resneck notes almost in passing that he likes to ski when he’s not practicing medicine or serving on a committee. Dr. Van Beek – whose family has vacationed with Dr. Resneck, his wife, Ellen Hufbauer, MD, a family medicine physician in Concord, Calif., and their two teenaged children Zachary and Amelia – said he’s “a very good skier.”
She noted that he and his children share the same enthusiasm for researching every aspect of wherever they travel, including the best places to eat. “He embraces work and life with an incredible amount of intellectual curiosity,” Dr. Van Beek said.
That curiosity – and the passion to make a difference – has driven his deep dives into what he sees as the corrosive practice of prior authorization and the promise of telemedicine, which he has explained and supported in testimony on Capitol Hill.
Dr. Brod said that Dr. Resneck was among those who helped convince the federal government to expand coverage for telemedicine during the COVID-19 pandemic. “He brought together his patient experience in dermatology and his policy experience,” and was able to deftly explain how it could increase access during the shutdown, said Dr. Brod.
Prior authorization gets him fired up. “We’ve reached a point where there’s almost not anything I will write a prescription for that doesn’t oftentimes require all of these hoops that we have to jump through,” he told this news organization. Prescriptions for generic topical cortisones that have been around for 50 years “now all of the sudden require a week of arguing,” said Dr. Resneck. In the meantime, patients aren’t getting treatment, he said.
Dr. Resneck’s passion for health equity is borne in part out of the racism witnessed by him and his family, including an uncle who started an antisegregationist newspaper and was kicked out of medical school for his views in the 1950s. Dr. Resneck said he had a rudimentary understanding of racism as a youngster. But he told the AMA delegates, “I knew enough at age 16 to write an op-ed in our city’s newspaper about the need to remove Confederate monuments from our courthouse lawn.” Added Dr. Resneck, “You can imagine how that went over in 1987.”
The pandemic shined a bright light on inequities and heightened awareness of “the institutionalized systems that have perpetuated racism and gender discrimination in medicine for as far back as we want to look,” Dr. Resneck said during his inaugural speech. Pointedly, he told his colleagues that “the AMA has not always been on the right side of history,” adding, “Each of us must do our part to eliminate health inequities by engaging in antiracist and antisexist work.”
That kind of talk is signature Resneck, said Dr. Brod. “He’s not afraid to exhibit very brave leadership,” especially “when there are issues that jeopardize the needs of patient access or patient safety,” he said.
“He’ll be a tremendous AMA president,” said Dr. Van Beek. “He’s present, he’s devoted, and he’s typically the expert in the room.”
Dr. Harmon said that Dr. Resneck is “cut from the same cloth that I am. He believes that if you get an opportunity to make things better, you take it.”
His advice to Dr. Resneck: “Rarely turn an opportunity down.” Dr. Harmon has little doubt that Dr. Resneck is up to the job but said, “I’m going to encourage him to keep that energy and enthusiasm up.”
Dr. Resneck told physician colleagues not to worry: “I will keep relentlessly showing up.”
A version of this article first appeared on Medscape.com.
Jack S. Resneck Jr., MD, is not usually the loudest voice in the room, but when he speaks, his words carry a heft and an appeal that is straightforward and undeniable.
That was on full display as.
He did not mince words when it came to describing the current landscape. “I doubt you imagined a divided country such as this, where physicians and public health officials often face antiscience aggression and threats of violence simply for doing our jobs,” he said. “You probably didn’t plan on insurers questioning every prescription and every procedure you asked for. Or government criminalizing routine and vital health care, enshrining discrimination against our LGBTQ patients or attacking a woman’s right to control health care decisions that should only be between her and her doctor,” said Dr. Resneck.
But, he added, all was not lost. “While it would be easy to get overwhelmed by despair as I begin this new role, I’ve never been prouder of my physician colleagues,” he said.
Dr. Resneck is the first dermatologist to lead the 175-year-old organization since 1925. Colleagues in the field speak of pride in having one of their own at the top, and they are even more complimentary about his depth of health policy knowledge, his communications skills, and his ability to find common ground.
“He loves looking at both sides,” said Marta J. Van Beek, MD, clinical professor of dermatology at the University of Iowa, Iowa City. “That’s how he builds consensus,” said Dr. Van Beek, who has known Dr. Resneck for more than 20 years, since they were chief dermatology residents – she at Iowa and he at UCSF.
Dr. Van Beek and Dr. Resneck have a deep interest in health policy and have long worked side by side on committees at both the American Academy of Dermatology (AAD) and the AMA. She looks back to the lead-up period before the 2010 passage of the Affordable Care Act as one of Dr. Resneck’s shining moments. “Those were contentious times in medicine,” Dr. Van Beek told this news organization. Dr. Resneck, as chair of the AAD’s Council on Government Affairs and Health Policy from 2008 to 2012, rallied the board to agree on a set of health care reform principles, she noted.
Dr. Resneck is “really very unifying,” agreed Bruce A. Brod, MD, clinical professor of dermatology at the University of Pennsylvania, Philadelphia. Dr. Brod has worked with Dr. Resneck for 2 decades on various committees at the AAD. They’ve also known each other through the AMA House of Delegates.
Now Dr. Brod is following in Dr. Resneck’s footsteps as chair of the AAD’s Council on Government Affairs and Health Policy. “Big shoes to fill there,” said Dr. Brod. He said he’s been inspired by Dr. Resneck’s always-positive approach, punctuated by his ever-constant belief that “there’s a lot more common ground than meets the eye here.”
“I really think he’s the perfect leader at this time,” he said.
Outgoing AMA President Gerald E. Harmon, MD, said that Dr. Resneck’s long experience as a teacher and a mentor, and what he describes as a “good, active listening talent,” have been integral to his success as a leader. He expects those qualities to make Dr. Resneck an effective advocate for all of medicine. “He identifies the problem, he identifies the gap, and then he establishes a workable, executable plan to close that gap,” Dr. Harmon told this news organization, adding that he’s seen this at work in AMA board meetings.
“He was a good teacher for me,” said Dr. Harmon, who had known Dr. Resneck through the House of Delegates and various AMA councils for at least a decade before they both joined the AMA board. “He can be such a mentor to all age groups, including senior physicians like myself,” said Dr. Harmon.
Dr. Resneck is excited, but also measured. “This has been a tumultuous couple of years in the country with the pandemic and with the fractured politics,” he said in an interview. Thinking about taking on the AMA presidency, he said, “I’ve had some moments of trepidation. I wanted to be sure that I was going to be able to make a difference.”
Long interest in health policy
Growing up in Shreveport, La., as the son of a dermatologist, Dr. Resneck said, “at first, I swore I was going to do something other than medicine.” It was not out of rebellion. He got along fine with his father. He wanted to pursue his own journey.
Dr. Resneck began his long love affair with health policy at Brown University, graduating magna cum laude with honors in public policy. A 1991 U.S. Department of Health & Human Services internship between his junior and senior years helped inform his honors thesis on health care financing policy.
Ultimately, Dr. Resneck did not stray far from his father’s career path. While still at Brown, he decided to go to medical school, entering UCSF in the fall of 1993. “At the end of the day, there was this undeniable influence that he loved his job,” Dr. Resneck said in the interview. His father was energized by the work and helping patients. “If burnout was in his vocabulary, I never heard it,” said Dr. Resneck.
Initially, he did a 1-year internal medicine residency at UCSF, but then switched to dermatology and became chief resident in 2000.
He was quickly pegged as a leader and an inspirational speaker. The AAD gave him its Young Physician Leadership Development Award in 2001, and the AMA gave Dr. Resneck its Excellence in Medicine National Award for Young Physician Leadership in 2004. He began giving talks at national meetings in 2002 and has been busy ever since, addressing the AMA, the AAD, state medical and dermatology societies, subspecialty groups such as the American College of Mohs Surgery and the Association of Professors of Dermatology, and other organizations such as the American Telemedicine Association.
He’s a sought-after speaker in part because of his ability to simply communicate health policy, said Dr. Brod. “He can connect the real-world issues really well to the policy needs and communicate it very well, and come in at just the right level,” he said.
Dr. Resneck has been immersed in policy and practice issues since the start of his career, serving on AAD and AMA committees addressing quality measures, data collection, access to care, workforce issues, and telemedicine. He started writing about dermatology workforce challenges in 2001 and has revisited that topic with regularity.
He has published often on the difficulties of patient access, looking at wait times for appointments, among other issues. In 2018, he expressed concern in a commentary in JAMA Dermatology that private equity purchases of dermatology practices might lead to an improper focus on profits over patients and that it could reduce the diversity of practice models.
At heart, Dr. Resneck is an institutionalist, someone who believes that the “collaborative, collective voice can make change,” said Dr. Brod. Dr. Resneck said as much in his inaugural speech. “I believe those who show up can use levers of power to confront our system’s flaws,” he said. “This is the nerdy policy part of my life, which my friends will force me to admit is most of my life,” said Dr. Resneck.
Prior authorization, telemedicine, equity
Beneath the reserved exterior lies an intense yearning to act on his passions, both at work and at play.
Dr. Resneck notes almost in passing that he likes to ski when he’s not practicing medicine or serving on a committee. Dr. Van Beek – whose family has vacationed with Dr. Resneck, his wife, Ellen Hufbauer, MD, a family medicine physician in Concord, Calif., and their two teenaged children Zachary and Amelia – said he’s “a very good skier.”
She noted that he and his children share the same enthusiasm for researching every aspect of wherever they travel, including the best places to eat. “He embraces work and life with an incredible amount of intellectual curiosity,” Dr. Van Beek said.
That curiosity – and the passion to make a difference – has driven his deep dives into what he sees as the corrosive practice of prior authorization and the promise of telemedicine, which he has explained and supported in testimony on Capitol Hill.
Dr. Brod said that Dr. Resneck was among those who helped convince the federal government to expand coverage for telemedicine during the COVID-19 pandemic. “He brought together his patient experience in dermatology and his policy experience,” and was able to deftly explain how it could increase access during the shutdown, said Dr. Brod.
Prior authorization gets him fired up. “We’ve reached a point where there’s almost not anything I will write a prescription for that doesn’t oftentimes require all of these hoops that we have to jump through,” he told this news organization. Prescriptions for generic topical cortisones that have been around for 50 years “now all of the sudden require a week of arguing,” said Dr. Resneck. In the meantime, patients aren’t getting treatment, he said.
Dr. Resneck’s passion for health equity is borne in part out of the racism witnessed by him and his family, including an uncle who started an antisegregationist newspaper and was kicked out of medical school for his views in the 1950s. Dr. Resneck said he had a rudimentary understanding of racism as a youngster. But he told the AMA delegates, “I knew enough at age 16 to write an op-ed in our city’s newspaper about the need to remove Confederate monuments from our courthouse lawn.” Added Dr. Resneck, “You can imagine how that went over in 1987.”
The pandemic shined a bright light on inequities and heightened awareness of “the institutionalized systems that have perpetuated racism and gender discrimination in medicine for as far back as we want to look,” Dr. Resneck said during his inaugural speech. Pointedly, he told his colleagues that “the AMA has not always been on the right side of history,” adding, “Each of us must do our part to eliminate health inequities by engaging in antiracist and antisexist work.”
That kind of talk is signature Resneck, said Dr. Brod. “He’s not afraid to exhibit very brave leadership,” especially “when there are issues that jeopardize the needs of patient access or patient safety,” he said.
“He’ll be a tremendous AMA president,” said Dr. Van Beek. “He’s present, he’s devoted, and he’s typically the expert in the room.”
Dr. Harmon said that Dr. Resneck is “cut from the same cloth that I am. He believes that if you get an opportunity to make things better, you take it.”
His advice to Dr. Resneck: “Rarely turn an opportunity down.” Dr. Harmon has little doubt that Dr. Resneck is up to the job but said, “I’m going to encourage him to keep that energy and enthusiasm up.”
Dr. Resneck told physician colleagues not to worry: “I will keep relentlessly showing up.”
A version of this article first appeared on Medscape.com.
Jack S. Resneck Jr., MD, is not usually the loudest voice in the room, but when he speaks, his words carry a heft and an appeal that is straightforward and undeniable.
That was on full display as.
He did not mince words when it came to describing the current landscape. “I doubt you imagined a divided country such as this, where physicians and public health officials often face antiscience aggression and threats of violence simply for doing our jobs,” he said. “You probably didn’t plan on insurers questioning every prescription and every procedure you asked for. Or government criminalizing routine and vital health care, enshrining discrimination against our LGBTQ patients or attacking a woman’s right to control health care decisions that should only be between her and her doctor,” said Dr. Resneck.
But, he added, all was not lost. “While it would be easy to get overwhelmed by despair as I begin this new role, I’ve never been prouder of my physician colleagues,” he said.
Dr. Resneck is the first dermatologist to lead the 175-year-old organization since 1925. Colleagues in the field speak of pride in having one of their own at the top, and they are even more complimentary about his depth of health policy knowledge, his communications skills, and his ability to find common ground.
“He loves looking at both sides,” said Marta J. Van Beek, MD, clinical professor of dermatology at the University of Iowa, Iowa City. “That’s how he builds consensus,” said Dr. Van Beek, who has known Dr. Resneck for more than 20 years, since they were chief dermatology residents – she at Iowa and he at UCSF.
Dr. Van Beek and Dr. Resneck have a deep interest in health policy and have long worked side by side on committees at both the American Academy of Dermatology (AAD) and the AMA. She looks back to the lead-up period before the 2010 passage of the Affordable Care Act as one of Dr. Resneck’s shining moments. “Those were contentious times in medicine,” Dr. Van Beek told this news organization. Dr. Resneck, as chair of the AAD’s Council on Government Affairs and Health Policy from 2008 to 2012, rallied the board to agree on a set of health care reform principles, she noted.
Dr. Resneck is “really very unifying,” agreed Bruce A. Brod, MD, clinical professor of dermatology at the University of Pennsylvania, Philadelphia. Dr. Brod has worked with Dr. Resneck for 2 decades on various committees at the AAD. They’ve also known each other through the AMA House of Delegates.
Now Dr. Brod is following in Dr. Resneck’s footsteps as chair of the AAD’s Council on Government Affairs and Health Policy. “Big shoes to fill there,” said Dr. Brod. He said he’s been inspired by Dr. Resneck’s always-positive approach, punctuated by his ever-constant belief that “there’s a lot more common ground than meets the eye here.”
“I really think he’s the perfect leader at this time,” he said.
Outgoing AMA President Gerald E. Harmon, MD, said that Dr. Resneck’s long experience as a teacher and a mentor, and what he describes as a “good, active listening talent,” have been integral to his success as a leader. He expects those qualities to make Dr. Resneck an effective advocate for all of medicine. “He identifies the problem, he identifies the gap, and then he establishes a workable, executable plan to close that gap,” Dr. Harmon told this news organization, adding that he’s seen this at work in AMA board meetings.
“He was a good teacher for me,” said Dr. Harmon, who had known Dr. Resneck through the House of Delegates and various AMA councils for at least a decade before they both joined the AMA board. “He can be such a mentor to all age groups, including senior physicians like myself,” said Dr. Harmon.
Dr. Resneck is excited, but also measured. “This has been a tumultuous couple of years in the country with the pandemic and with the fractured politics,” he said in an interview. Thinking about taking on the AMA presidency, he said, “I’ve had some moments of trepidation. I wanted to be sure that I was going to be able to make a difference.”
Long interest in health policy
Growing up in Shreveport, La., as the son of a dermatologist, Dr. Resneck said, “at first, I swore I was going to do something other than medicine.” It was not out of rebellion. He got along fine with his father. He wanted to pursue his own journey.
Dr. Resneck began his long love affair with health policy at Brown University, graduating magna cum laude with honors in public policy. A 1991 U.S. Department of Health & Human Services internship between his junior and senior years helped inform his honors thesis on health care financing policy.
Ultimately, Dr. Resneck did not stray far from his father’s career path. While still at Brown, he decided to go to medical school, entering UCSF in the fall of 1993. “At the end of the day, there was this undeniable influence that he loved his job,” Dr. Resneck said in the interview. His father was energized by the work and helping patients. “If burnout was in his vocabulary, I never heard it,” said Dr. Resneck.
Initially, he did a 1-year internal medicine residency at UCSF, but then switched to dermatology and became chief resident in 2000.
He was quickly pegged as a leader and an inspirational speaker. The AAD gave him its Young Physician Leadership Development Award in 2001, and the AMA gave Dr. Resneck its Excellence in Medicine National Award for Young Physician Leadership in 2004. He began giving talks at national meetings in 2002 and has been busy ever since, addressing the AMA, the AAD, state medical and dermatology societies, subspecialty groups such as the American College of Mohs Surgery and the Association of Professors of Dermatology, and other organizations such as the American Telemedicine Association.
He’s a sought-after speaker in part because of his ability to simply communicate health policy, said Dr. Brod. “He can connect the real-world issues really well to the policy needs and communicate it very well, and come in at just the right level,” he said.
Dr. Resneck has been immersed in policy and practice issues since the start of his career, serving on AAD and AMA committees addressing quality measures, data collection, access to care, workforce issues, and telemedicine. He started writing about dermatology workforce challenges in 2001 and has revisited that topic with regularity.
He has published often on the difficulties of patient access, looking at wait times for appointments, among other issues. In 2018, he expressed concern in a commentary in JAMA Dermatology that private equity purchases of dermatology practices might lead to an improper focus on profits over patients and that it could reduce the diversity of practice models.
At heart, Dr. Resneck is an institutionalist, someone who believes that the “collaborative, collective voice can make change,” said Dr. Brod. Dr. Resneck said as much in his inaugural speech. “I believe those who show up can use levers of power to confront our system’s flaws,” he said. “This is the nerdy policy part of my life, which my friends will force me to admit is most of my life,” said Dr. Resneck.
Prior authorization, telemedicine, equity
Beneath the reserved exterior lies an intense yearning to act on his passions, both at work and at play.
Dr. Resneck notes almost in passing that he likes to ski when he’s not practicing medicine or serving on a committee. Dr. Van Beek – whose family has vacationed with Dr. Resneck, his wife, Ellen Hufbauer, MD, a family medicine physician in Concord, Calif., and their two teenaged children Zachary and Amelia – said he’s “a very good skier.”
She noted that he and his children share the same enthusiasm for researching every aspect of wherever they travel, including the best places to eat. “He embraces work and life with an incredible amount of intellectual curiosity,” Dr. Van Beek said.
That curiosity – and the passion to make a difference – has driven his deep dives into what he sees as the corrosive practice of prior authorization and the promise of telemedicine, which he has explained and supported in testimony on Capitol Hill.
Dr. Brod said that Dr. Resneck was among those who helped convince the federal government to expand coverage for telemedicine during the COVID-19 pandemic. “He brought together his patient experience in dermatology and his policy experience,” and was able to deftly explain how it could increase access during the shutdown, said Dr. Brod.
Prior authorization gets him fired up. “We’ve reached a point where there’s almost not anything I will write a prescription for that doesn’t oftentimes require all of these hoops that we have to jump through,” he told this news organization. Prescriptions for generic topical cortisones that have been around for 50 years “now all of the sudden require a week of arguing,” said Dr. Resneck. In the meantime, patients aren’t getting treatment, he said.
Dr. Resneck’s passion for health equity is borne in part out of the racism witnessed by him and his family, including an uncle who started an antisegregationist newspaper and was kicked out of medical school for his views in the 1950s. Dr. Resneck said he had a rudimentary understanding of racism as a youngster. But he told the AMA delegates, “I knew enough at age 16 to write an op-ed in our city’s newspaper about the need to remove Confederate monuments from our courthouse lawn.” Added Dr. Resneck, “You can imagine how that went over in 1987.”
The pandemic shined a bright light on inequities and heightened awareness of “the institutionalized systems that have perpetuated racism and gender discrimination in medicine for as far back as we want to look,” Dr. Resneck said during his inaugural speech. Pointedly, he told his colleagues that “the AMA has not always been on the right side of history,” adding, “Each of us must do our part to eliminate health inequities by engaging in antiracist and antisexist work.”
That kind of talk is signature Resneck, said Dr. Brod. “He’s not afraid to exhibit very brave leadership,” especially “when there are issues that jeopardize the needs of patient access or patient safety,” he said.
“He’ll be a tremendous AMA president,” said Dr. Van Beek. “He’s present, he’s devoted, and he’s typically the expert in the room.”
Dr. Harmon said that Dr. Resneck is “cut from the same cloth that I am. He believes that if you get an opportunity to make things better, you take it.”
His advice to Dr. Resneck: “Rarely turn an opportunity down.” Dr. Harmon has little doubt that Dr. Resneck is up to the job but said, “I’m going to encourage him to keep that energy and enthusiasm up.”
Dr. Resneck told physician colleagues not to worry: “I will keep relentlessly showing up.”
A version of this article first appeared on Medscape.com.
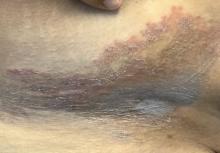
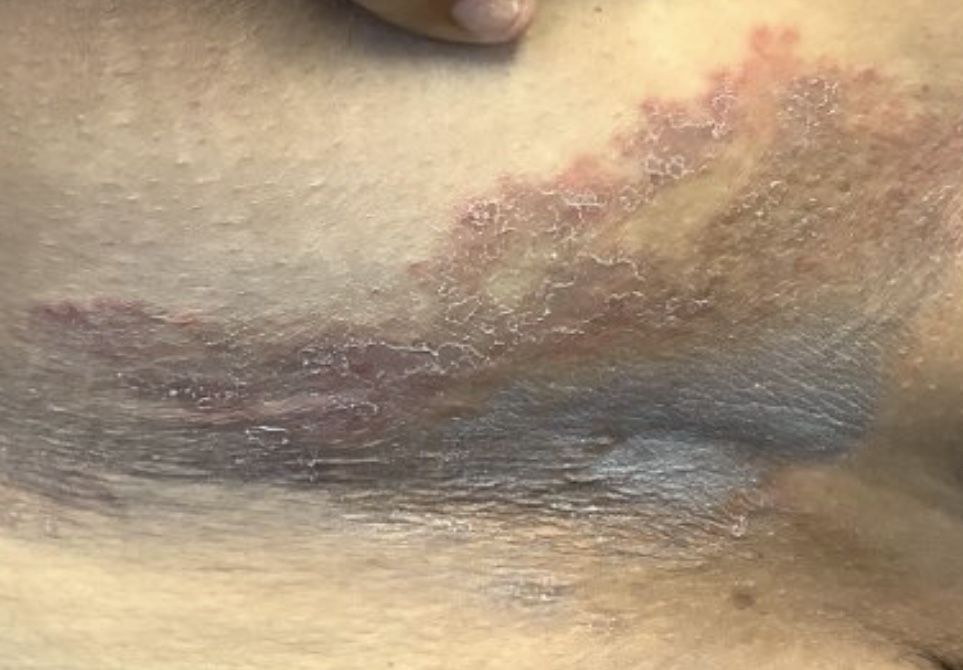
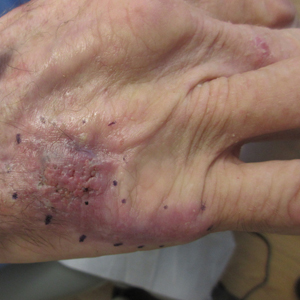
A Hispanic male presented with a 3-month history of a spreading, itchy rash
, more often on exposed skin. In the United States, Trichophyton rubrum, T. mentagrophytes, and Microsporum canis are the most common causal organisms. People can become infected from contact with other people, animals, or soil. Variants of tinea corporis include tinea imbricata (caused by T. concentricum), bullous tinea corporis, tinea gladiatorum (seen in wrestlers), tinea incognito (atypical tinea resulting from topical steroid use), and Majocchi’s granuloma. Widespread tinea may be secondary to underlying immunodeficiency such as HIV/AIDS or treatment with topical or oral steroids.
The typical presentation of tinea corporis is scaly erythematous or hypopigmented annular patches with a raised border and central clearing. In tinea imbricata, which is more commonly seen in southeast Asia, India, and Central America, concentric circles and serpiginous plaques are present. Majocchi’s granuloma has a deeper involvement of fungus in the hair follicles, presenting with papules and pustules at the periphery of the patches. Lesions of tinea incognito may lack a scaly border and can be more widespread.
Diagnosis can be confirmed with a skin scraping and potassium hydroxide (KOH) staining, which will reveal septate and branching hyphae. Biopsy is often helpful, especially in tinea incognito. Classically, a “sandwich sign” is seen: hyphae between orthokeratosis and compact hyperkeratosis or parakeratosis. In this patient, a biopsy from the left hip revealed dermatophytosis, with PAS positive for organisms.
Localized lesions respond to topical antifungal creams such as azoles or topical terbinafine. More extensive tinea will often require a systemic antifungal with griseofulvin, terbinafine, itraconazole, or fluconazole. This patient responded to topical ketoconazole cream and oral terbinafine. A workup for underlying immunodeficiency was negative.
Dr. Bilu Martin provided this case and photo.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
, more often on exposed skin. In the United States, Trichophyton rubrum, T. mentagrophytes, and Microsporum canis are the most common causal organisms. People can become infected from contact with other people, animals, or soil. Variants of tinea corporis include tinea imbricata (caused by T. concentricum), bullous tinea corporis, tinea gladiatorum (seen in wrestlers), tinea incognito (atypical tinea resulting from topical steroid use), and Majocchi’s granuloma. Widespread tinea may be secondary to underlying immunodeficiency such as HIV/AIDS or treatment with topical or oral steroids.
The typical presentation of tinea corporis is scaly erythematous or hypopigmented annular patches with a raised border and central clearing. In tinea imbricata, which is more commonly seen in southeast Asia, India, and Central America, concentric circles and serpiginous plaques are present. Majocchi’s granuloma has a deeper involvement of fungus in the hair follicles, presenting with papules and pustules at the periphery of the patches. Lesions of tinea incognito may lack a scaly border and can be more widespread.
Diagnosis can be confirmed with a skin scraping and potassium hydroxide (KOH) staining, which will reveal septate and branching hyphae. Biopsy is often helpful, especially in tinea incognito. Classically, a “sandwich sign” is seen: hyphae between orthokeratosis and compact hyperkeratosis or parakeratosis. In this patient, a biopsy from the left hip revealed dermatophytosis, with PAS positive for organisms.
Localized lesions respond to topical antifungal creams such as azoles or topical terbinafine. More extensive tinea will often require a systemic antifungal with griseofulvin, terbinafine, itraconazole, or fluconazole. This patient responded to topical ketoconazole cream and oral terbinafine. A workup for underlying immunodeficiency was negative.
Dr. Bilu Martin provided this case and photo.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
, more often on exposed skin. In the United States, Trichophyton rubrum, T. mentagrophytes, and Microsporum canis are the most common causal organisms. People can become infected from contact with other people, animals, or soil. Variants of tinea corporis include tinea imbricata (caused by T. concentricum), bullous tinea corporis, tinea gladiatorum (seen in wrestlers), tinea incognito (atypical tinea resulting from topical steroid use), and Majocchi’s granuloma. Widespread tinea may be secondary to underlying immunodeficiency such as HIV/AIDS or treatment with topical or oral steroids.
The typical presentation of tinea corporis is scaly erythematous or hypopigmented annular patches with a raised border and central clearing. In tinea imbricata, which is more commonly seen in southeast Asia, India, and Central America, concentric circles and serpiginous plaques are present. Majocchi’s granuloma has a deeper involvement of fungus in the hair follicles, presenting with papules and pustules at the periphery of the patches. Lesions of tinea incognito may lack a scaly border and can be more widespread.
Diagnosis can be confirmed with a skin scraping and potassium hydroxide (KOH) staining, which will reveal septate and branching hyphae. Biopsy is often helpful, especially in tinea incognito. Classically, a “sandwich sign” is seen: hyphae between orthokeratosis and compact hyperkeratosis or parakeratosis. In this patient, a biopsy from the left hip revealed dermatophytosis, with PAS positive for organisms.
Localized lesions respond to topical antifungal creams such as azoles or topical terbinafine. More extensive tinea will often require a systemic antifungal with griseofulvin, terbinafine, itraconazole, or fluconazole. This patient responded to topical ketoconazole cream and oral terbinafine. A workup for underlying immunodeficiency was negative.
Dr. Bilu Martin provided this case and photo.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at MDedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
Microbiome’s new happy place: The beer gut
Your gut microbiome will thank you later
A healthy gut seems like the new catch-all to better overall health these days. Nutrition and diet culture has us drinking kombucha and ginger tea and coffee, but what if we told you that going to happy hour might also help?
In a recent double-blind study published in the Journal of Agricultural and Food Chemistry, 19 men were divided into two groups and asked to drink 11 ounces of alcoholic lager (5.2% by volume) or nonalcoholic lager with dinner for 4 weeks.
Beer? Yes. Beer.
We humans have trillions of microorganisms running rampant through our digestive tracts. When they’re happy, we have a lower chance of developing heart disease and diabetes. You know what else has millions of happy microorganisms from fermentation? Beer. It also has polyphenols that can help the body’s tissues fight cancers, as well as heart disease and inflammation. So beer is looking a little more healthy now, isn’t it?
In the study, the researchers found that both the alcoholic- and nonalcoholic-lager groups had a boost in bacterial diversity in the gut and higher fecal alkaline phosphatase levels, which showed improved intestinal health. They acknowledged, however, that the nonalcoholic route would be safer and healthier for overall health.
So add a lager to the list of gut-healthy foods that you should be consuming. It may give the phrase “beer gut” a whole new meaning.
We’ve lost our minds, but at least we know how fast they’re going
The phrase “quantum consciousness” sounds like something out of a particularly cheesy episode of Star Trek: “Oh no, Captain, the quantum consciousness has invaded our computer, and the only way to drive it out is to reverse the polarity of a focused tachyon beam.”
When it comes to understanding such basic existential issues as the origin of consciousness, however, quantum mechanics wasn’t off the table. The theory of the quantum origin of consciousness dates back to the 1990s (thanks in part to noted physician Roger Penrose), and goes something like this: There are microtubules within neurons in the brain that are small enough and isolated enough from the warm, wet, and chaotic brain environment where quantum effects can briefly come into play. We’re talking miniscule fractions of a second here, but still, long enough for quantum calculations to take place in the form of system wavefunction collapse, courtesy of gravity.
To plunge even deeper into the rabbit hole of quantum mechanics, the reason Schrödinger’s cat doesn’t occur in real life is wavefunction collapse; the more massive a quantum system is, the more likely it is to collapse into one state or another (alive or dead, in the cat’s case). The quantum origin of consciousness, or Orch OR theory, holds that human consciousness arises from electrical oscillations within the neuronal microtubules caused by the computations stemming from the collapse of small quantum systems.
That is an awful lot of overly simplified explanation, especially considering the study that just came out essentially disproved it. Oops. The research, published in Physics of Life Reviews, is pretty simple. The researchers went to a lab deep underground to avoid interference from cosmic rays, and sat around for months, observing a chunk of germanium for signs of spontaneous radiation, attributable to the same sort of wavefunction collapse that is supposedly occurring in our brains. They found nothing out of the ordinary, pretty definitively disproving most of Orch OR theory.
The researchers were unwilling to completely dismiss the idea (this is quantum mechanics, after all, uncertainty kind of goes with the territory), but it does seem like we’ll have to search elsewhere for sources of human consciousness. Personally, we’re big fans of the cymbal-playing monkey.
Missing links: A real fish story
Dear LOTME:
Ear’s a question that’s been keeping me up at night. Is the human middle ear the result of top-secret government experiments involving alien technology, Abraham Lincoln, and the Illuminati?
Restless in Roswell
Dear Restless:
The paleoanthropologic community has been sorting through this mystery for decades, and fossils discovered in China over the past 20 years finally provide a much less conspiratorially satisfying answer.
For some time now, experts in the field have believed that the bones of the human middle ear evolved from the spiracular gill of a fish. The spiracle is a small hole behind each eye that opens to the mouth in some fishes and was used to breathe air in the earliest, most primitive species. But how did we get from spiracle to ear?
The missing links come in the form of the cranial anatomy of Shuyu, a 438-million-year-old, fingernail-sized skull of a jawless fish, and the 419-million-year-old fossil of a completely preserved fish with gill filaments in the first branchial chamber.
“These fossils provided the first anatomical and fossil evidence for a vertebrate spiracle originating from fish gills,” senior author Gai Zhikun, PhD, of the Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, said in a written statement.
In many ways, it seems, we are fish: “Many important structures of human beings can be traced back to our fish ancestors, such as our teeth, jaws, middle ears, etc,” added Zhu Min, PhD, also of the institute.
So, Restless, the next time you hear the soothing sounds of an angry mob storming the Capitol or you chew on a slab, slice, or chunk of mutant, laboratory-produced chicken in your favorite fast-food restaurant, be sure to thank Shuyu.
Can you lend me an ear?
If you thought locusts were only a nuisance, think again. They have their uses. If you take a locust’s ear and put it inside a robot, the robot will be able to hear and receive signals. Who knew?
Researchers from Tel Aviv University in Israel showed the robot’s hearing abilities by giving clap signals that told the robot what to do: One clap means go forward, two claps mean move back. What do you think the robot would do if it heard the clap break from Cha Cha Slide?
“Our task was to replace the robot’s electronic microphone with a dead insect’s ear, use the ear’s ability to detect the electrical signals from the environment, in this case vibrations in the air, and, using a special chip, convert the insect input to that of the robot,” Ben M. Maoz, PhD, said in a statement from the university.
And how does a dead locust ear work in a robot? Well, Dr. Maoz explained: “My laboratory has developed a special device – Ear-on-a-Chip – that allows the ear to be kept alive throughout the experiment by supplying oxygen and food to the organ while allowing the electrical signals to be taken out of the locust’s ear and amplified and transmitted to the robot.”
The research won’t stop at hearing, he said, as the other four senses also will be taken into consideration. This could help us sense dangers in the future, such as earthquakes or diseases. We said it before and we’ll say it again: We’re rooting for you, science!
Your gut microbiome will thank you later
A healthy gut seems like the new catch-all to better overall health these days. Nutrition and diet culture has us drinking kombucha and ginger tea and coffee, but what if we told you that going to happy hour might also help?
In a recent double-blind study published in the Journal of Agricultural and Food Chemistry, 19 men were divided into two groups and asked to drink 11 ounces of alcoholic lager (5.2% by volume) or nonalcoholic lager with dinner for 4 weeks.
Beer? Yes. Beer.
We humans have trillions of microorganisms running rampant through our digestive tracts. When they’re happy, we have a lower chance of developing heart disease and diabetes. You know what else has millions of happy microorganisms from fermentation? Beer. It also has polyphenols that can help the body’s tissues fight cancers, as well as heart disease and inflammation. So beer is looking a little more healthy now, isn’t it?
In the study, the researchers found that both the alcoholic- and nonalcoholic-lager groups had a boost in bacterial diversity in the gut and higher fecal alkaline phosphatase levels, which showed improved intestinal health. They acknowledged, however, that the nonalcoholic route would be safer and healthier for overall health.
So add a lager to the list of gut-healthy foods that you should be consuming. It may give the phrase “beer gut” a whole new meaning.
We’ve lost our minds, but at least we know how fast they’re going
The phrase “quantum consciousness” sounds like something out of a particularly cheesy episode of Star Trek: “Oh no, Captain, the quantum consciousness has invaded our computer, and the only way to drive it out is to reverse the polarity of a focused tachyon beam.”
When it comes to understanding such basic existential issues as the origin of consciousness, however, quantum mechanics wasn’t off the table. The theory of the quantum origin of consciousness dates back to the 1990s (thanks in part to noted physician Roger Penrose), and goes something like this: There are microtubules within neurons in the brain that are small enough and isolated enough from the warm, wet, and chaotic brain environment where quantum effects can briefly come into play. We’re talking miniscule fractions of a second here, but still, long enough for quantum calculations to take place in the form of system wavefunction collapse, courtesy of gravity.
To plunge even deeper into the rabbit hole of quantum mechanics, the reason Schrödinger’s cat doesn’t occur in real life is wavefunction collapse; the more massive a quantum system is, the more likely it is to collapse into one state or another (alive or dead, in the cat’s case). The quantum origin of consciousness, or Orch OR theory, holds that human consciousness arises from electrical oscillations within the neuronal microtubules caused by the computations stemming from the collapse of small quantum systems.
That is an awful lot of overly simplified explanation, especially considering the study that just came out essentially disproved it. Oops. The research, published in Physics of Life Reviews, is pretty simple. The researchers went to a lab deep underground to avoid interference from cosmic rays, and sat around for months, observing a chunk of germanium for signs of spontaneous radiation, attributable to the same sort of wavefunction collapse that is supposedly occurring in our brains. They found nothing out of the ordinary, pretty definitively disproving most of Orch OR theory.
The researchers were unwilling to completely dismiss the idea (this is quantum mechanics, after all, uncertainty kind of goes with the territory), but it does seem like we’ll have to search elsewhere for sources of human consciousness. Personally, we’re big fans of the cymbal-playing monkey.
Missing links: A real fish story
Dear LOTME:
Ear’s a question that’s been keeping me up at night. Is the human middle ear the result of top-secret government experiments involving alien technology, Abraham Lincoln, and the Illuminati?
Restless in Roswell
Dear Restless:
The paleoanthropologic community has been sorting through this mystery for decades, and fossils discovered in China over the past 20 years finally provide a much less conspiratorially satisfying answer.
For some time now, experts in the field have believed that the bones of the human middle ear evolved from the spiracular gill of a fish. The spiracle is a small hole behind each eye that opens to the mouth in some fishes and was used to breathe air in the earliest, most primitive species. But how did we get from spiracle to ear?
The missing links come in the form of the cranial anatomy of Shuyu, a 438-million-year-old, fingernail-sized skull of a jawless fish, and the 419-million-year-old fossil of a completely preserved fish with gill filaments in the first branchial chamber.
“These fossils provided the first anatomical and fossil evidence for a vertebrate spiracle originating from fish gills,” senior author Gai Zhikun, PhD, of the Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, said in a written statement.
In many ways, it seems, we are fish: “Many important structures of human beings can be traced back to our fish ancestors, such as our teeth, jaws, middle ears, etc,” added Zhu Min, PhD, also of the institute.
So, Restless, the next time you hear the soothing sounds of an angry mob storming the Capitol or you chew on a slab, slice, or chunk of mutant, laboratory-produced chicken in your favorite fast-food restaurant, be sure to thank Shuyu.
Can you lend me an ear?
If you thought locusts were only a nuisance, think again. They have their uses. If you take a locust’s ear and put it inside a robot, the robot will be able to hear and receive signals. Who knew?
Researchers from Tel Aviv University in Israel showed the robot’s hearing abilities by giving clap signals that told the robot what to do: One clap means go forward, two claps mean move back. What do you think the robot would do if it heard the clap break from Cha Cha Slide?
“Our task was to replace the robot’s electronic microphone with a dead insect’s ear, use the ear’s ability to detect the electrical signals from the environment, in this case vibrations in the air, and, using a special chip, convert the insect input to that of the robot,” Ben M. Maoz, PhD, said in a statement from the university.
And how does a dead locust ear work in a robot? Well, Dr. Maoz explained: “My laboratory has developed a special device – Ear-on-a-Chip – that allows the ear to be kept alive throughout the experiment by supplying oxygen and food to the organ while allowing the electrical signals to be taken out of the locust’s ear and amplified and transmitted to the robot.”
The research won’t stop at hearing, he said, as the other four senses also will be taken into consideration. This could help us sense dangers in the future, such as earthquakes or diseases. We said it before and we’ll say it again: We’re rooting for you, science!
Your gut microbiome will thank you later
A healthy gut seems like the new catch-all to better overall health these days. Nutrition and diet culture has us drinking kombucha and ginger tea and coffee, but what if we told you that going to happy hour might also help?
In a recent double-blind study published in the Journal of Agricultural and Food Chemistry, 19 men were divided into two groups and asked to drink 11 ounces of alcoholic lager (5.2% by volume) or nonalcoholic lager with dinner for 4 weeks.
Beer? Yes. Beer.
We humans have trillions of microorganisms running rampant through our digestive tracts. When they’re happy, we have a lower chance of developing heart disease and diabetes. You know what else has millions of happy microorganisms from fermentation? Beer. It also has polyphenols that can help the body’s tissues fight cancers, as well as heart disease and inflammation. So beer is looking a little more healthy now, isn’t it?
In the study, the researchers found that both the alcoholic- and nonalcoholic-lager groups had a boost in bacterial diversity in the gut and higher fecal alkaline phosphatase levels, which showed improved intestinal health. They acknowledged, however, that the nonalcoholic route would be safer and healthier for overall health.
So add a lager to the list of gut-healthy foods that you should be consuming. It may give the phrase “beer gut” a whole new meaning.
We’ve lost our minds, but at least we know how fast they’re going
The phrase “quantum consciousness” sounds like something out of a particularly cheesy episode of Star Trek: “Oh no, Captain, the quantum consciousness has invaded our computer, and the only way to drive it out is to reverse the polarity of a focused tachyon beam.”
When it comes to understanding such basic existential issues as the origin of consciousness, however, quantum mechanics wasn’t off the table. The theory of the quantum origin of consciousness dates back to the 1990s (thanks in part to noted physician Roger Penrose), and goes something like this: There are microtubules within neurons in the brain that are small enough and isolated enough from the warm, wet, and chaotic brain environment where quantum effects can briefly come into play. We’re talking miniscule fractions of a second here, but still, long enough for quantum calculations to take place in the form of system wavefunction collapse, courtesy of gravity.
To plunge even deeper into the rabbit hole of quantum mechanics, the reason Schrödinger’s cat doesn’t occur in real life is wavefunction collapse; the more massive a quantum system is, the more likely it is to collapse into one state or another (alive or dead, in the cat’s case). The quantum origin of consciousness, or Orch OR theory, holds that human consciousness arises from electrical oscillations within the neuronal microtubules caused by the computations stemming from the collapse of small quantum systems.
That is an awful lot of overly simplified explanation, especially considering the study that just came out essentially disproved it. Oops. The research, published in Physics of Life Reviews, is pretty simple. The researchers went to a lab deep underground to avoid interference from cosmic rays, and sat around for months, observing a chunk of germanium for signs of spontaneous radiation, attributable to the same sort of wavefunction collapse that is supposedly occurring in our brains. They found nothing out of the ordinary, pretty definitively disproving most of Orch OR theory.
The researchers were unwilling to completely dismiss the idea (this is quantum mechanics, after all, uncertainty kind of goes with the territory), but it does seem like we’ll have to search elsewhere for sources of human consciousness. Personally, we’re big fans of the cymbal-playing monkey.
Missing links: A real fish story
Dear LOTME:
Ear’s a question that’s been keeping me up at night. Is the human middle ear the result of top-secret government experiments involving alien technology, Abraham Lincoln, and the Illuminati?
Restless in Roswell
Dear Restless:
The paleoanthropologic community has been sorting through this mystery for decades, and fossils discovered in China over the past 20 years finally provide a much less conspiratorially satisfying answer.
For some time now, experts in the field have believed that the bones of the human middle ear evolved from the spiracular gill of a fish. The spiracle is a small hole behind each eye that opens to the mouth in some fishes and was used to breathe air in the earliest, most primitive species. But how did we get from spiracle to ear?
The missing links come in the form of the cranial anatomy of Shuyu, a 438-million-year-old, fingernail-sized skull of a jawless fish, and the 419-million-year-old fossil of a completely preserved fish with gill filaments in the first branchial chamber.
“These fossils provided the first anatomical and fossil evidence for a vertebrate spiracle originating from fish gills,” senior author Gai Zhikun, PhD, of the Institute of Vertebrate Paleontology and Paleoanthropology, Beijing, said in a written statement.
In many ways, it seems, we are fish: “Many important structures of human beings can be traced back to our fish ancestors, such as our teeth, jaws, middle ears, etc,” added Zhu Min, PhD, also of the institute.
So, Restless, the next time you hear the soothing sounds of an angry mob storming the Capitol or you chew on a slab, slice, or chunk of mutant, laboratory-produced chicken in your favorite fast-food restaurant, be sure to thank Shuyu.
Can you lend me an ear?
If you thought locusts were only a nuisance, think again. They have their uses. If you take a locust’s ear and put it inside a robot, the robot will be able to hear and receive signals. Who knew?
Researchers from Tel Aviv University in Israel showed the robot’s hearing abilities by giving clap signals that told the robot what to do: One clap means go forward, two claps mean move back. What do you think the robot would do if it heard the clap break from Cha Cha Slide?
“Our task was to replace the robot’s electronic microphone with a dead insect’s ear, use the ear’s ability to detect the electrical signals from the environment, in this case vibrations in the air, and, using a special chip, convert the insect input to that of the robot,” Ben M. Maoz, PhD, said in a statement from the university.
And how does a dead locust ear work in a robot? Well, Dr. Maoz explained: “My laboratory has developed a special device – Ear-on-a-Chip – that allows the ear to be kept alive throughout the experiment by supplying oxygen and food to the organ while allowing the electrical signals to be taken out of the locust’s ear and amplified and transmitted to the robot.”
The research won’t stop at hearing, he said, as the other four senses also will be taken into consideration. This could help us sense dangers in the future, such as earthquakes or diseases. We said it before and we’ll say it again: We’re rooting for you, science!
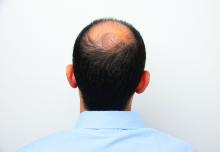
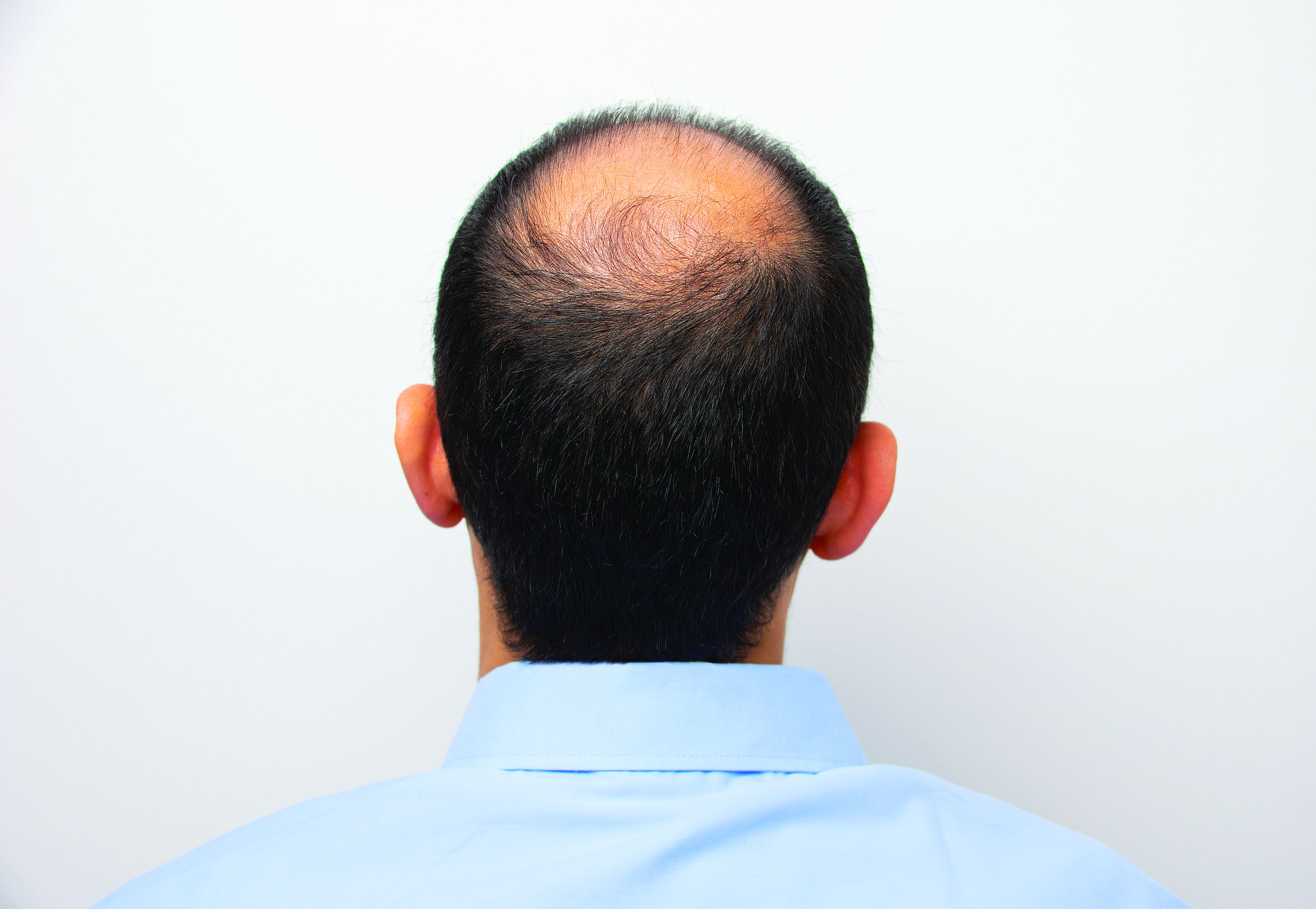
Hair disorder treatments are evolving
“No matter who the patient is, whether a child, adolescent, or adult, the key to figuring out hair disease is getting a good history,” Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said at the Medscape Live Women’s and Pediatric Dermatology Seminar.
. She also urged physicians and other health care providers to use the electronic medical record and to be thorough in documenting information – noting nutrition, hair care habits, supplement use, and other details.
Lab tests should be selected based on that history, she said. For instance, low iron stores can be associated with hair shedding; and thyroid function studies might be needed.
Other highlights of her presentation included comments on different types of alopecia, and some new treatment approaches:
Androgenetic alopecia. In a meta-analysis and systematic review published in 2017, all treatments tested (2% and 5% minoxidil in men, 1 mg finasteride in men, 2% minoxidil in women, and low-level laser light therapy in men) were superior to placebo. Several photobiomodulation (PBM) devices (also known as low-level laser light) for home use have been cleared for androgenetic alopecia by the Food and Drug Administration; a clinician’s guide, published in 2018, provides information on these devices.
Hair and hormones. Combination therapy for female-pattern hair loss – low-dose minoxidil and spironolactone – is important to know about, she said, adding there are data from an observational pilot study supporting this treatment. Women should not become pregnant while on this treatment, Dr. Hordinsky cautioned.
PRP (platelet rich plasma). This treatment for hair loss can be costly, she cautioned, as it’s viewed as a cosmetic technique, “but it actually can work rather well.”
Hair regrowth measures. Traditionally, measures center on global assessment, the patient’s self-assessment, investigator assessment, and an independent photo review. Enter the dermatoscope. “We can now get pictures as a baseline. Patients can see, and also see the health of their scalp,” and if treatments make it look better or worse, she noted.
Alopecia areata (AA). Patients and families need to be made aware that this is an autoimmune disease that can recur, and if it does recur, the extent of hair loss is not predictable. According to Dr. Hordinsky, the most widely used tool to halt disease activity has been treatment with a corticosteroid (topical, intralesional, oral, or even intravenous corticosteroids).
Clinical trials and publications from 2018 to 2020 have triggered interest in off-label use and further studies of JAK inhibitors for treating AA, which include baricitinib, ruxolitinib, and tofacitinib. At the American Academy of Dermatology meeting in March 2022, results of the ALLEGRO phase 2b/3 trial found that the JAK inhibitor ritlecitinib (50 mg or 20 mg daily, with or without a 200-mg loading dose), was efficacious in adults and adolescents with AA, compared with placebo, with no safety concerns noted. “This looks to be very, very promising,” she said, “and also very safe.” Two phase 3 trials of baricitinib also presented at the same meeting found it was superior to placebo for hair regrowth in adults with severe AA at 36 weeks. (On June 13, shortly after Dr. Hordinsky spoke at the meeting, the FDA approved baricitinib for treating AA in adults, making this the first systemic treatment to be approved for AA).
Research on topical JAK inhibitors for AA has been disappointing, Dr. Hordinsky said.
Alopecia areata and atopic dermatitis. For patients with both AA and AD, dupilumab may provide relief, she said. She referred to a recently published phase 2a trial in patients with AA (including some with both AA and AD), which found that Severity of Alopecia Tool (SALT) scores improved after 48 weeks of treatment, with higher response rates among those with baseline IgE levels of 200 IU/mL or higher. “If your patient has both, and their immunoglobulin-E level is greater than 200, then they may be a good candidate for dupilumab and both diseases may respond,” she said.
Scalp symptoms. It can be challenging when patients complain of itch, pain, or burning on the scalp, but have no obvious skin disease, Dr. Hordinsky said. Her tips: Some of these patients may be experiencing scalp symptoms secondary to a neuropathy; others may have mast cell degranulation, but for others, the basis of the symptoms may be unclear. Special nerve studies may be needed. For relief, a trial of antihistamines or topical or oral gabapentin may be needed, she said.
Frontal fibrosing alopecia (FFA). This condition, first described in postmenopausal women, is now reported in men and in younger women. While sunscreen has been suspected, there are no good data that have proven that link, she said. Cosmetics are also considered a possible culprit. For treatment, “the first thing we try to do is treat the inflammation,” Dr. Hordinsky said. Treatment options include topical high-potency corticosteroids, intralesional steroids, and topical nonsteroid anti-inflammatory creams (tier 1); hydroxychloroquine, low-dose antibiotics, and acitretin (tier 2); and cyclosporin and mycophenolate mofetil (tier 3).
In an observational study of mostly women with FFA, she noted, treatment with dutasteride was more effective than commonly used systemic treatments.
“Don’t forget to address the psychosocial needs of the hair loss patient,” Dr. Hordinsky advised. “Hair loss patients are very distressed, and you have to learn how to be fast and nimble and address those needs.” Working with a behavioral health specialist or therapist can help, she said.
She also recommended directing patients to appropriate organizations such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, as well as conferences, such as the upcoming NAAF conference in Washington. “These organizations do give good information that should complement what you are doing.”
Medscape Live and this news organization are owned by the same parent company. Dr. Hordinsky reported no disclosures.
“No matter who the patient is, whether a child, adolescent, or adult, the key to figuring out hair disease is getting a good history,” Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said at the Medscape Live Women’s and Pediatric Dermatology Seminar.
. She also urged physicians and other health care providers to use the electronic medical record and to be thorough in documenting information – noting nutrition, hair care habits, supplement use, and other details.
Lab tests should be selected based on that history, she said. For instance, low iron stores can be associated with hair shedding; and thyroid function studies might be needed.
Other highlights of her presentation included comments on different types of alopecia, and some new treatment approaches:
Androgenetic alopecia. In a meta-analysis and systematic review published in 2017, all treatments tested (2% and 5% minoxidil in men, 1 mg finasteride in men, 2% minoxidil in women, and low-level laser light therapy in men) were superior to placebo. Several photobiomodulation (PBM) devices (also known as low-level laser light) for home use have been cleared for androgenetic alopecia by the Food and Drug Administration; a clinician’s guide, published in 2018, provides information on these devices.
Hair and hormones. Combination therapy for female-pattern hair loss – low-dose minoxidil and spironolactone – is important to know about, she said, adding there are data from an observational pilot study supporting this treatment. Women should not become pregnant while on this treatment, Dr. Hordinsky cautioned.
PRP (platelet rich plasma). This treatment for hair loss can be costly, she cautioned, as it’s viewed as a cosmetic technique, “but it actually can work rather well.”
Hair regrowth measures. Traditionally, measures center on global assessment, the patient’s self-assessment, investigator assessment, and an independent photo review. Enter the dermatoscope. “We can now get pictures as a baseline. Patients can see, and also see the health of their scalp,” and if treatments make it look better or worse, she noted.
Alopecia areata (AA). Patients and families need to be made aware that this is an autoimmune disease that can recur, and if it does recur, the extent of hair loss is not predictable. According to Dr. Hordinsky, the most widely used tool to halt disease activity has been treatment with a corticosteroid (topical, intralesional, oral, or even intravenous corticosteroids).
Clinical trials and publications from 2018 to 2020 have triggered interest in off-label use and further studies of JAK inhibitors for treating AA, which include baricitinib, ruxolitinib, and tofacitinib. At the American Academy of Dermatology meeting in March 2022, results of the ALLEGRO phase 2b/3 trial found that the JAK inhibitor ritlecitinib (50 mg or 20 mg daily, with or without a 200-mg loading dose), was efficacious in adults and adolescents with AA, compared with placebo, with no safety concerns noted. “This looks to be very, very promising,” she said, “and also very safe.” Two phase 3 trials of baricitinib also presented at the same meeting found it was superior to placebo for hair regrowth in adults with severe AA at 36 weeks. (On June 13, shortly after Dr. Hordinsky spoke at the meeting, the FDA approved baricitinib for treating AA in adults, making this the first systemic treatment to be approved for AA).
Research on topical JAK inhibitors for AA has been disappointing, Dr. Hordinsky said.
Alopecia areata and atopic dermatitis. For patients with both AA and AD, dupilumab may provide relief, she said. She referred to a recently published phase 2a trial in patients with AA (including some with both AA and AD), which found that Severity of Alopecia Tool (SALT) scores improved after 48 weeks of treatment, with higher response rates among those with baseline IgE levels of 200 IU/mL or higher. “If your patient has both, and their immunoglobulin-E level is greater than 200, then they may be a good candidate for dupilumab and both diseases may respond,” she said.
Scalp symptoms. It can be challenging when patients complain of itch, pain, or burning on the scalp, but have no obvious skin disease, Dr. Hordinsky said. Her tips: Some of these patients may be experiencing scalp symptoms secondary to a neuropathy; others may have mast cell degranulation, but for others, the basis of the symptoms may be unclear. Special nerve studies may be needed. For relief, a trial of antihistamines or topical or oral gabapentin may be needed, she said.
Frontal fibrosing alopecia (FFA). This condition, first described in postmenopausal women, is now reported in men and in younger women. While sunscreen has been suspected, there are no good data that have proven that link, she said. Cosmetics are also considered a possible culprit. For treatment, “the first thing we try to do is treat the inflammation,” Dr. Hordinsky said. Treatment options include topical high-potency corticosteroids, intralesional steroids, and topical nonsteroid anti-inflammatory creams (tier 1); hydroxychloroquine, low-dose antibiotics, and acitretin (tier 2); and cyclosporin and mycophenolate mofetil (tier 3).
In an observational study of mostly women with FFA, she noted, treatment with dutasteride was more effective than commonly used systemic treatments.
“Don’t forget to address the psychosocial needs of the hair loss patient,” Dr. Hordinsky advised. “Hair loss patients are very distressed, and you have to learn how to be fast and nimble and address those needs.” Working with a behavioral health specialist or therapist can help, she said.
She also recommended directing patients to appropriate organizations such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, as well as conferences, such as the upcoming NAAF conference in Washington. “These organizations do give good information that should complement what you are doing.”
Medscape Live and this news organization are owned by the same parent company. Dr. Hordinsky reported no disclosures.
“No matter who the patient is, whether a child, adolescent, or adult, the key to figuring out hair disease is getting a good history,” Maria Hordinsky, MD, professor and chair of the department of dermatology at the University of Minnesota, Minneapolis, said at the Medscape Live Women’s and Pediatric Dermatology Seminar.
. She also urged physicians and other health care providers to use the electronic medical record and to be thorough in documenting information – noting nutrition, hair care habits, supplement use, and other details.
Lab tests should be selected based on that history, she said. For instance, low iron stores can be associated with hair shedding; and thyroid function studies might be needed.
Other highlights of her presentation included comments on different types of alopecia, and some new treatment approaches:
Androgenetic alopecia. In a meta-analysis and systematic review published in 2017, all treatments tested (2% and 5% minoxidil in men, 1 mg finasteride in men, 2% minoxidil in women, and low-level laser light therapy in men) were superior to placebo. Several photobiomodulation (PBM) devices (also known as low-level laser light) for home use have been cleared for androgenetic alopecia by the Food and Drug Administration; a clinician’s guide, published in 2018, provides information on these devices.
Hair and hormones. Combination therapy for female-pattern hair loss – low-dose minoxidil and spironolactone – is important to know about, she said, adding there are data from an observational pilot study supporting this treatment. Women should not become pregnant while on this treatment, Dr. Hordinsky cautioned.
PRP (platelet rich plasma). This treatment for hair loss can be costly, she cautioned, as it’s viewed as a cosmetic technique, “but it actually can work rather well.”
Hair regrowth measures. Traditionally, measures center on global assessment, the patient’s self-assessment, investigator assessment, and an independent photo review. Enter the dermatoscope. “We can now get pictures as a baseline. Patients can see, and also see the health of their scalp,” and if treatments make it look better or worse, she noted.
Alopecia areata (AA). Patients and families need to be made aware that this is an autoimmune disease that can recur, and if it does recur, the extent of hair loss is not predictable. According to Dr. Hordinsky, the most widely used tool to halt disease activity has been treatment with a corticosteroid (topical, intralesional, oral, or even intravenous corticosteroids).
Clinical trials and publications from 2018 to 2020 have triggered interest in off-label use and further studies of JAK inhibitors for treating AA, which include baricitinib, ruxolitinib, and tofacitinib. At the American Academy of Dermatology meeting in March 2022, results of the ALLEGRO phase 2b/3 trial found that the JAK inhibitor ritlecitinib (50 mg or 20 mg daily, with or without a 200-mg loading dose), was efficacious in adults and adolescents with AA, compared with placebo, with no safety concerns noted. “This looks to be very, very promising,” she said, “and also very safe.” Two phase 3 trials of baricitinib also presented at the same meeting found it was superior to placebo for hair regrowth in adults with severe AA at 36 weeks. (On June 13, shortly after Dr. Hordinsky spoke at the meeting, the FDA approved baricitinib for treating AA in adults, making this the first systemic treatment to be approved for AA).
Research on topical JAK inhibitors for AA has been disappointing, Dr. Hordinsky said.
Alopecia areata and atopic dermatitis. For patients with both AA and AD, dupilumab may provide relief, she said. She referred to a recently published phase 2a trial in patients with AA (including some with both AA and AD), which found that Severity of Alopecia Tool (SALT) scores improved after 48 weeks of treatment, with higher response rates among those with baseline IgE levels of 200 IU/mL or higher. “If your patient has both, and their immunoglobulin-E level is greater than 200, then they may be a good candidate for dupilumab and both diseases may respond,” she said.
Scalp symptoms. It can be challenging when patients complain of itch, pain, or burning on the scalp, but have no obvious skin disease, Dr. Hordinsky said. Her tips: Some of these patients may be experiencing scalp symptoms secondary to a neuropathy; others may have mast cell degranulation, but for others, the basis of the symptoms may be unclear. Special nerve studies may be needed. For relief, a trial of antihistamines or topical or oral gabapentin may be needed, she said.
Frontal fibrosing alopecia (FFA). This condition, first described in postmenopausal women, is now reported in men and in younger women. While sunscreen has been suspected, there are no good data that have proven that link, she said. Cosmetics are also considered a possible culprit. For treatment, “the first thing we try to do is treat the inflammation,” Dr. Hordinsky said. Treatment options include topical high-potency corticosteroids, intralesional steroids, and topical nonsteroid anti-inflammatory creams (tier 1); hydroxychloroquine, low-dose antibiotics, and acitretin (tier 2); and cyclosporin and mycophenolate mofetil (tier 3).
In an observational study of mostly women with FFA, she noted, treatment with dutasteride was more effective than commonly used systemic treatments.
“Don’t forget to address the psychosocial needs of the hair loss patient,” Dr. Hordinsky advised. “Hair loss patients are very distressed, and you have to learn how to be fast and nimble and address those needs.” Working with a behavioral health specialist or therapist can help, she said.
She also recommended directing patients to appropriate organizations such as the National Alopecia Areata Foundation and the Scarring Alopecia Foundation, as well as conferences, such as the upcoming NAAF conference in Washington. “These organizations do give good information that should complement what you are doing.”
Medscape Live and this news organization are owned by the same parent company. Dr. Hordinsky reported no disclosures.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR
Bimekizumab calms psoriatic arthritis in phase 3 ‘BE’ trials
COPENHAGEN – For patients with active psoriatic arthritis for whom tumor necrosis factor (TNF) inhibitors failed to produce an adequate response, use of the dual interleukin-17 (IL-17) inhibitor bimekizumab (Bimzelx) was associated with significant improvement in joint, skin, and health-related quality-of-life parameters, compared with placebo, reported investigators in the phase 3, double-blind, randomized BE COMPLETE trial.
The primary endpoint, which was the percentage of patients who had 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, was achieved in 43.4% of patients assigned to receive bimekizumab 160 mg every 4 weeks, compared with 6.8% among patients who received placebo, reported Joseph F. Merola, MD, a dermatologist and rheumatologist at Brigham and Women’s Hospital in Boston.
“The high-level and exciting take-home [message is] that BE COMPLETE did meet all primary and all ranked secondary endpoints at week 16,” he said at the annual European Congress of Rheumatology.
Also at the congress, Iain McInnes, MD, PhD, of the Institute of Infection, Immunity, and Inflammation at the University of Glasgow, Scotland, presented data from a second phase 3, double-blind, randomized trial called BE OPTIMAL that showed similar benefits for patients with psoriatic arthritis who had not previously received biologic disease-modifying antirheumatic drugs.
“This is a new mode of action, inhibiting two cytokines simultaneously,” he said in a late-breaking oral abstract session.
As previously reported by this news organization, use of bimekizumab led to rapid reductions in signs and symptoms of radiographic axial spondyloarthritis in the phase 3 trial called BE MOBILE 2.
Bimekizumab is a monoclonal immunoglobulin G1 antibody that selectively inhibits IL-17A and IL-17F. It is approved in the European Union for treating adults with moderate to severe plaque psoriasis.
BE COMPLETE efficacy
Inclusion criteria comprised adult-onset psoriatic arthritis meeting Classification Criteria for Psoriatic Arthritis (CASPAR) for at least 6 months; tender and swollen joint counts of at least 3/68; one or more active psoriatic lesions; and/or a documented history of psoriasis characterized by intolerance to one or two TNF inhibitors or failure of TNF inhibitors. Patients were randomly assigned in a 2:1 ratio to receive either bimekizumab 160 mg every 4 weeks (n = 267) or placebo (n = 133) for 16 weeks.
Some participants are being followed in the extension BE VITAL study, which will evaluate response to treatment and long-term safety. Patients who do enroll in the extension study will be followed for safety for a period of 20 weeks after the last dose.
As noted before, the trial met its primary endpoint of a significant improvement over placebo in ACR50 (hazard ratio, 11.1; P < .001).
In addition, the trial met all ranked secondary endpoints, including the Health Assessment Questionnaire–Disability Index change from baseline, 90% improvement in the Psoriasis Area and Severity Index (PASI90), Short-Form 36-Item Health Survey, and minimal disease activity (P < .001 for all comparisons).
Improvement with bimekizumab was rapid; curves began to separate from placebo by week 4, Dr. Merola said.
BE OPTIMAL efficacy
In this study, which had the same eligibility criteria as BE COMPLETE, patients were randomly assigned in a 2:3:1 ratio to receive 16 weeks of treatment with either placebo, bimekizumab 160 mg every 4 weeks, or adalimumab 40 mg every 2 weeks as a reference treatment.
This trial also met its primary and ranked secondary endpoints, which were similar to those of BE COMPLETE but also included measures of pooled resolution of enthesitis and dactylitis and change from baseline in van der Heijde modified total Sharp score (P < .001 for all comparisons).
In all, 43.9% of patients who received bimekizumab and 45.7% who received adalimumab achieved ACR50 at week 16, compared with 10% of patients who received placebo. The difference between the placebo and bimekizumab groups was significant (P < .001).
Safety
More patients who received the two active agents in this trial had treatment-emergent adverse events (TEAEs) in comparison with those in the placebo arm, but the incidence of serious TEAEs was less than 2% in each arm.
The most frequent events were nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and hypertension.
Patients tolerated bimekizumab well, and there were no unexpected safety signals, Dr. McInnes said.
Clues to efficacy
In the question-and-answer session following Dr. McInnes’ presentation, Ronald Van Vollenhoven, MD, of the University of Amsterdam, said, “I have a question that is sort of generic in studies of psoriatic arthritis, so it does not only apply to this study, but the skin responses seem to be excellent – PASI90 sounds wonderful – but given that this is the case, is it reasonable to claim that the study is double-blinded in respect to the joints?”
Dr. McInnes replied that while he has considered this conundrum for many years in trials of drugs for psoriatic arthritis, “it doesn’t seem to be a major determinant of the outcome.”
The studies were supported by UCB Pharma. Dr. Merola and Dr. McInnes have consulted for UCB and other pharmaceutical companies that market drugs for psoriatic arthritis and psoriasis. Dr. Van Vollenhoven has received research support, has consulted for, and has spoken on behalf of UCB and other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
COPENHAGEN – For patients with active psoriatic arthritis for whom tumor necrosis factor (TNF) inhibitors failed to produce an adequate response, use of the dual interleukin-17 (IL-17) inhibitor bimekizumab (Bimzelx) was associated with significant improvement in joint, skin, and health-related quality-of-life parameters, compared with placebo, reported investigators in the phase 3, double-blind, randomized BE COMPLETE trial.
The primary endpoint, which was the percentage of patients who had 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, was achieved in 43.4% of patients assigned to receive bimekizumab 160 mg every 4 weeks, compared with 6.8% among patients who received placebo, reported Joseph F. Merola, MD, a dermatologist and rheumatologist at Brigham and Women’s Hospital in Boston.
“The high-level and exciting take-home [message is] that BE COMPLETE did meet all primary and all ranked secondary endpoints at week 16,” he said at the annual European Congress of Rheumatology.
Also at the congress, Iain McInnes, MD, PhD, of the Institute of Infection, Immunity, and Inflammation at the University of Glasgow, Scotland, presented data from a second phase 3, double-blind, randomized trial called BE OPTIMAL that showed similar benefits for patients with psoriatic arthritis who had not previously received biologic disease-modifying antirheumatic drugs.
“This is a new mode of action, inhibiting two cytokines simultaneously,” he said in a late-breaking oral abstract session.
As previously reported by this news organization, use of bimekizumab led to rapid reductions in signs and symptoms of radiographic axial spondyloarthritis in the phase 3 trial called BE MOBILE 2.
Bimekizumab is a monoclonal immunoglobulin G1 antibody that selectively inhibits IL-17A and IL-17F. It is approved in the European Union for treating adults with moderate to severe plaque psoriasis.
BE COMPLETE efficacy
Inclusion criteria comprised adult-onset psoriatic arthritis meeting Classification Criteria for Psoriatic Arthritis (CASPAR) for at least 6 months; tender and swollen joint counts of at least 3/68; one or more active psoriatic lesions; and/or a documented history of psoriasis characterized by intolerance to one or two TNF inhibitors or failure of TNF inhibitors. Patients were randomly assigned in a 2:1 ratio to receive either bimekizumab 160 mg every 4 weeks (n = 267) or placebo (n = 133) for 16 weeks.
Some participants are being followed in the extension BE VITAL study, which will evaluate response to treatment and long-term safety. Patients who do enroll in the extension study will be followed for safety for a period of 20 weeks after the last dose.
As noted before, the trial met its primary endpoint of a significant improvement over placebo in ACR50 (hazard ratio, 11.1; P < .001).
In addition, the trial met all ranked secondary endpoints, including the Health Assessment Questionnaire–Disability Index change from baseline, 90% improvement in the Psoriasis Area and Severity Index (PASI90), Short-Form 36-Item Health Survey, and minimal disease activity (P < .001 for all comparisons).
Improvement with bimekizumab was rapid; curves began to separate from placebo by week 4, Dr. Merola said.
BE OPTIMAL efficacy
In this study, which had the same eligibility criteria as BE COMPLETE, patients were randomly assigned in a 2:3:1 ratio to receive 16 weeks of treatment with either placebo, bimekizumab 160 mg every 4 weeks, or adalimumab 40 mg every 2 weeks as a reference treatment.
This trial also met its primary and ranked secondary endpoints, which were similar to those of BE COMPLETE but also included measures of pooled resolution of enthesitis and dactylitis and change from baseline in van der Heijde modified total Sharp score (P < .001 for all comparisons).
In all, 43.9% of patients who received bimekizumab and 45.7% who received adalimumab achieved ACR50 at week 16, compared with 10% of patients who received placebo. The difference between the placebo and bimekizumab groups was significant (P < .001).
Safety
More patients who received the two active agents in this trial had treatment-emergent adverse events (TEAEs) in comparison with those in the placebo arm, but the incidence of serious TEAEs was less than 2% in each arm.
The most frequent events were nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and hypertension.
Patients tolerated bimekizumab well, and there were no unexpected safety signals, Dr. McInnes said.
Clues to efficacy
In the question-and-answer session following Dr. McInnes’ presentation, Ronald Van Vollenhoven, MD, of the University of Amsterdam, said, “I have a question that is sort of generic in studies of psoriatic arthritis, so it does not only apply to this study, but the skin responses seem to be excellent – PASI90 sounds wonderful – but given that this is the case, is it reasonable to claim that the study is double-blinded in respect to the joints?”
Dr. McInnes replied that while he has considered this conundrum for many years in trials of drugs for psoriatic arthritis, “it doesn’t seem to be a major determinant of the outcome.”
The studies were supported by UCB Pharma. Dr. Merola and Dr. McInnes have consulted for UCB and other pharmaceutical companies that market drugs for psoriatic arthritis and psoriasis. Dr. Van Vollenhoven has received research support, has consulted for, and has spoken on behalf of UCB and other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
COPENHAGEN – For patients with active psoriatic arthritis for whom tumor necrosis factor (TNF) inhibitors failed to produce an adequate response, use of the dual interleukin-17 (IL-17) inhibitor bimekizumab (Bimzelx) was associated with significant improvement in joint, skin, and health-related quality-of-life parameters, compared with placebo, reported investigators in the phase 3, double-blind, randomized BE COMPLETE trial.
The primary endpoint, which was the percentage of patients who had 50% improvement in American College of Rheumatology response criteria (ACR50) at 16 weeks, was achieved in 43.4% of patients assigned to receive bimekizumab 160 mg every 4 weeks, compared with 6.8% among patients who received placebo, reported Joseph F. Merola, MD, a dermatologist and rheumatologist at Brigham and Women’s Hospital in Boston.
“The high-level and exciting take-home [message is] that BE COMPLETE did meet all primary and all ranked secondary endpoints at week 16,” he said at the annual European Congress of Rheumatology.
Also at the congress, Iain McInnes, MD, PhD, of the Institute of Infection, Immunity, and Inflammation at the University of Glasgow, Scotland, presented data from a second phase 3, double-blind, randomized trial called BE OPTIMAL that showed similar benefits for patients with psoriatic arthritis who had not previously received biologic disease-modifying antirheumatic drugs.
“This is a new mode of action, inhibiting two cytokines simultaneously,” he said in a late-breaking oral abstract session.
As previously reported by this news organization, use of bimekizumab led to rapid reductions in signs and symptoms of radiographic axial spondyloarthritis in the phase 3 trial called BE MOBILE 2.
Bimekizumab is a monoclonal immunoglobulin G1 antibody that selectively inhibits IL-17A and IL-17F. It is approved in the European Union for treating adults with moderate to severe plaque psoriasis.
BE COMPLETE efficacy
Inclusion criteria comprised adult-onset psoriatic arthritis meeting Classification Criteria for Psoriatic Arthritis (CASPAR) for at least 6 months; tender and swollen joint counts of at least 3/68; one or more active psoriatic lesions; and/or a documented history of psoriasis characterized by intolerance to one or two TNF inhibitors or failure of TNF inhibitors. Patients were randomly assigned in a 2:1 ratio to receive either bimekizumab 160 mg every 4 weeks (n = 267) or placebo (n = 133) for 16 weeks.
Some participants are being followed in the extension BE VITAL study, which will evaluate response to treatment and long-term safety. Patients who do enroll in the extension study will be followed for safety for a period of 20 weeks after the last dose.
As noted before, the trial met its primary endpoint of a significant improvement over placebo in ACR50 (hazard ratio, 11.1; P < .001).
In addition, the trial met all ranked secondary endpoints, including the Health Assessment Questionnaire–Disability Index change from baseline, 90% improvement in the Psoriasis Area and Severity Index (PASI90), Short-Form 36-Item Health Survey, and minimal disease activity (P < .001 for all comparisons).
Improvement with bimekizumab was rapid; curves began to separate from placebo by week 4, Dr. Merola said.
BE OPTIMAL efficacy
In this study, which had the same eligibility criteria as BE COMPLETE, patients were randomly assigned in a 2:3:1 ratio to receive 16 weeks of treatment with either placebo, bimekizumab 160 mg every 4 weeks, or adalimumab 40 mg every 2 weeks as a reference treatment.
This trial also met its primary and ranked secondary endpoints, which were similar to those of BE COMPLETE but also included measures of pooled resolution of enthesitis and dactylitis and change from baseline in van der Heijde modified total Sharp score (P < .001 for all comparisons).
In all, 43.9% of patients who received bimekizumab and 45.7% who received adalimumab achieved ACR50 at week 16, compared with 10% of patients who received placebo. The difference between the placebo and bimekizumab groups was significant (P < .001).
Safety
More patients who received the two active agents in this trial had treatment-emergent adverse events (TEAEs) in comparison with those in the placebo arm, but the incidence of serious TEAEs was less than 2% in each arm.
The most frequent events were nasopharyngitis, upper respiratory tract infection, headache, diarrhea, and hypertension.
Patients tolerated bimekizumab well, and there were no unexpected safety signals, Dr. McInnes said.
Clues to efficacy
In the question-and-answer session following Dr. McInnes’ presentation, Ronald Van Vollenhoven, MD, of the University of Amsterdam, said, “I have a question that is sort of generic in studies of psoriatic arthritis, so it does not only apply to this study, but the skin responses seem to be excellent – PASI90 sounds wonderful – but given that this is the case, is it reasonable to claim that the study is double-blinded in respect to the joints?”
Dr. McInnes replied that while he has considered this conundrum for many years in trials of drugs for psoriatic arthritis, “it doesn’t seem to be a major determinant of the outcome.”
The studies were supported by UCB Pharma. Dr. Merola and Dr. McInnes have consulted for UCB and other pharmaceutical companies that market drugs for psoriatic arthritis and psoriasis. Dr. Van Vollenhoven has received research support, has consulted for, and has spoken on behalf of UCB and other pharmaceutical companies.
A version of this article first appeared on Medscape.com.
AT THE EULAR 2022 CONGRESS
Nonhealing Violaceous Plaque of the Hand Following a Splinter Injury
The Diagnosis: Chromoblastomycosis
This case highlights the importance of routine skin biopsy and tissue culture when clinical suspicion for mycotic infection is high. Despite nonspecific biopsy results (Figure), a diagnosis of chromoblastomycosis (CBM) was reached based on tissue culture. Surgical excision was not possible in our patient due to the size and location of the lesion. The patient was referred to infectious disease, with the plan to start long-term itraconazole for at least 6 to 12 months.
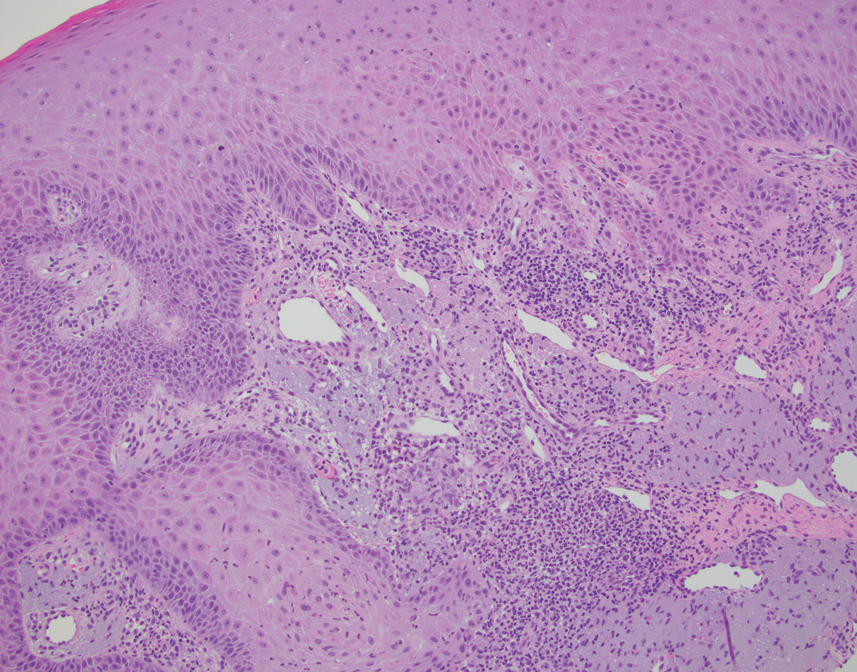
Cases of CBM were first documented in 1914 and distinguished by the appearance of spherical, brown, muriform cells on skin biopsy—features that now serve as the hallmark of CBM diagnoses.1,2 The implantation mycosis commonly is caused by agents such as Fonsecaea pedrosoi and Fonsecaea monophora of the bantiana-clade, as classified according to molecular phylogeny2; these agents have been isolated from soil, plants, and wood sources in tropical and subtropical regions and are strongly associated with agricultural activities.3
Chromoblastomycosis lesions tend to be asymptomatic with a variable amount of time between inoculation and lesion presentation, delaying medical care by months to years.3 The fungus causes a granulomatous reaction after skin damage, with noticeable pseudoepitheliomatous hyperplasia of the epidermis and granulomas formed by epithelioid and Langerhans cells in the dermis.4 Typically, CBM initially presents as an erythematous macular skin lesion, which then progresses to become more pink, papular, and sometimes pruritic.2 Muriform (sclerotic) bodies, which reflect fungal components, extrude transepidermally and appear as black dots on the lesion’s surface.4 Chromoblastomycosis is limited to the subcutaneous tissue and has been classified into 5 types of lesions: nodular, tumoral, verrucous, scarring, and plaque.2 Diagnosis is established using fungal tests such as potassium hydroxide direct microscopy, which exposes muriform bodies often in combination with dematiaceous hyphae, while fungal culture of F pedrosoi in Sabouraud agar produces velvety dark colonies.3 Although an immune response to CBM infection remains unclear, it has been demonstrated that the response differs based on the severity of the infection. The severe form of CBM produces high levels of IL-10, low levels of IFN-γ, and inefficient T-cell proliferation, while milder forms of CBM display low levels of IL-10, high levels of IFN-γ, and efficient T-cell proliferation.5 Complications of CBM include chronic lymphedema, ankylosis, and secondary bacterial infections, which largely are observed in advanced cases; malignant transformation to squamous cell carcinoma, though rare, also has been observed.6
Several therapeutic methods have been implemented in the treatment of CBM, but lesions often remain refractory, especially in advanced cases.6 Approaches to treatment can be divided into antifungal and physical methods. Commonly employed antifungal agents include itraconazole and terbinafine, which must be taken daily for a period ranging from 6 months to 1 year or longer; flucytosine with or without amphotericin also has been employed.4 Among the physical methods, surgical excision is not suggested due to possible dissemination of disease; other options include cryotherapy, thermotherapy, and laser vaporization.6 The prognosis has improved since the use of extended-spectrum triazoles, but high rates of refractory disease remain unchanged.2
The differential diagnosis includes other infections. Nocardiosis is a bacterial infection in which cutaneous disease can result in actinomycetoma, which presents with grains that are small, round, and stain blue on hematoxylin and eosin with eosinophilic rays at the periphery.7 Although the clinical features and pseudoepitheliomatous hyperplasia seen in CBM can mimic squamous cell carcinoma, the latter would show variable degrees of differentiation, keratinization, nuclear atypia, and architectural atypia with a negative tissue culture.8 Eumycetoma is a fungal infection that typically is not caused by F pedrosoi but rather most commonly Madurella mycetomatis.9 Leishmaniasis is a parasitic infection in which a biopsy of cutaneous lesions often displays parasite-filled histiocytes.10
- Rudolph M. Über die brasilianische “figueira” (vorläufige mitteilung). Arch Schiffs Trop Hyg. 1914;18:498-499.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- Kurien G, Sugumar K, Chandran V. Chromoblastomycosis. StatPearls. StatPearls Publishing; 2021. Accessed June 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470253/
- Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708-713. doi:10.1016/j.micinf.2005.01.006
- Krzys´ciak PM, Pindycka-Piaszczyn´ska M, Piaszczyn´ski M. Chromoblastomycosis. Postepy Dermatol Alergol. 2014;31:310-321. doi:10.5114/pdia.2014.40949
- Siddig EE, van de Sande WWJ, Fahal AH. Actinomycetoma laboratory-based diagnosis: a mini-review. Trans R Soc Trop Med Hyg. 2021;115:355-363.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll .2017.06.003
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- Saliba M, Shalhoub A, Taraif S, et al. Cutaneous leishmaniasis: an evolving disease with ancient roots. Int J Dermatol. 2019;58:834-843. doi:10.1111/ijd.14451
The Diagnosis: Chromoblastomycosis
This case highlights the importance of routine skin biopsy and tissue culture when clinical suspicion for mycotic infection is high. Despite nonspecific biopsy results (Figure), a diagnosis of chromoblastomycosis (CBM) was reached based on tissue culture. Surgical excision was not possible in our patient due to the size and location of the lesion. The patient was referred to infectious disease, with the plan to start long-term itraconazole for at least 6 to 12 months.

Cases of CBM were first documented in 1914 and distinguished by the appearance of spherical, brown, muriform cells on skin biopsy—features that now serve as the hallmark of CBM diagnoses.1,2 The implantation mycosis commonly is caused by agents such as Fonsecaea pedrosoi and Fonsecaea monophora of the bantiana-clade, as classified according to molecular phylogeny2; these agents have been isolated from soil, plants, and wood sources in tropical and subtropical regions and are strongly associated with agricultural activities.3
Chromoblastomycosis lesions tend to be asymptomatic with a variable amount of time between inoculation and lesion presentation, delaying medical care by months to years.3 The fungus causes a granulomatous reaction after skin damage, with noticeable pseudoepitheliomatous hyperplasia of the epidermis and granulomas formed by epithelioid and Langerhans cells in the dermis.4 Typically, CBM initially presents as an erythematous macular skin lesion, which then progresses to become more pink, papular, and sometimes pruritic.2 Muriform (sclerotic) bodies, which reflect fungal components, extrude transepidermally and appear as black dots on the lesion’s surface.4 Chromoblastomycosis is limited to the subcutaneous tissue and has been classified into 5 types of lesions: nodular, tumoral, verrucous, scarring, and plaque.2 Diagnosis is established using fungal tests such as potassium hydroxide direct microscopy, which exposes muriform bodies often in combination with dematiaceous hyphae, while fungal culture of F pedrosoi in Sabouraud agar produces velvety dark colonies.3 Although an immune response to CBM infection remains unclear, it has been demonstrated that the response differs based on the severity of the infection. The severe form of CBM produces high levels of IL-10, low levels of IFN-γ, and inefficient T-cell proliferation, while milder forms of CBM display low levels of IL-10, high levels of IFN-γ, and efficient T-cell proliferation.5 Complications of CBM include chronic lymphedema, ankylosis, and secondary bacterial infections, which largely are observed in advanced cases; malignant transformation to squamous cell carcinoma, though rare, also has been observed.6
Several therapeutic methods have been implemented in the treatment of CBM, but lesions often remain refractory, especially in advanced cases.6 Approaches to treatment can be divided into antifungal and physical methods. Commonly employed antifungal agents include itraconazole and terbinafine, which must be taken daily for a period ranging from 6 months to 1 year or longer; flucytosine with or without amphotericin also has been employed.4 Among the physical methods, surgical excision is not suggested due to possible dissemination of disease; other options include cryotherapy, thermotherapy, and laser vaporization.6 The prognosis has improved since the use of extended-spectrum triazoles, but high rates of refractory disease remain unchanged.2
The differential diagnosis includes other infections. Nocardiosis is a bacterial infection in which cutaneous disease can result in actinomycetoma, which presents with grains that are small, round, and stain blue on hematoxylin and eosin with eosinophilic rays at the periphery.7 Although the clinical features and pseudoepitheliomatous hyperplasia seen in CBM can mimic squamous cell carcinoma, the latter would show variable degrees of differentiation, keratinization, nuclear atypia, and architectural atypia with a negative tissue culture.8 Eumycetoma is a fungal infection that typically is not caused by F pedrosoi but rather most commonly Madurella mycetomatis.9 Leishmaniasis is a parasitic infection in which a biopsy of cutaneous lesions often displays parasite-filled histiocytes.10
The Diagnosis: Chromoblastomycosis
This case highlights the importance of routine skin biopsy and tissue culture when clinical suspicion for mycotic infection is high. Despite nonspecific biopsy results (Figure), a diagnosis of chromoblastomycosis (CBM) was reached based on tissue culture. Surgical excision was not possible in our patient due to the size and location of the lesion. The patient was referred to infectious disease, with the plan to start long-term itraconazole for at least 6 to 12 months.

Cases of CBM were first documented in 1914 and distinguished by the appearance of spherical, brown, muriform cells on skin biopsy—features that now serve as the hallmark of CBM diagnoses.1,2 The implantation mycosis commonly is caused by agents such as Fonsecaea pedrosoi and Fonsecaea monophora of the bantiana-clade, as classified according to molecular phylogeny2; these agents have been isolated from soil, plants, and wood sources in tropical and subtropical regions and are strongly associated with agricultural activities.3
Chromoblastomycosis lesions tend to be asymptomatic with a variable amount of time between inoculation and lesion presentation, delaying medical care by months to years.3 The fungus causes a granulomatous reaction after skin damage, with noticeable pseudoepitheliomatous hyperplasia of the epidermis and granulomas formed by epithelioid and Langerhans cells in the dermis.4 Typically, CBM initially presents as an erythematous macular skin lesion, which then progresses to become more pink, papular, and sometimes pruritic.2 Muriform (sclerotic) bodies, which reflect fungal components, extrude transepidermally and appear as black dots on the lesion’s surface.4 Chromoblastomycosis is limited to the subcutaneous tissue and has been classified into 5 types of lesions: nodular, tumoral, verrucous, scarring, and plaque.2 Diagnosis is established using fungal tests such as potassium hydroxide direct microscopy, which exposes muriform bodies often in combination with dematiaceous hyphae, while fungal culture of F pedrosoi in Sabouraud agar produces velvety dark colonies.3 Although an immune response to CBM infection remains unclear, it has been demonstrated that the response differs based on the severity of the infection. The severe form of CBM produces high levels of IL-10, low levels of IFN-γ, and inefficient T-cell proliferation, while milder forms of CBM display low levels of IL-10, high levels of IFN-γ, and efficient T-cell proliferation.5 Complications of CBM include chronic lymphedema, ankylosis, and secondary bacterial infections, which largely are observed in advanced cases; malignant transformation to squamous cell carcinoma, though rare, also has been observed.6
Several therapeutic methods have been implemented in the treatment of CBM, but lesions often remain refractory, especially in advanced cases.6 Approaches to treatment can be divided into antifungal and physical methods. Commonly employed antifungal agents include itraconazole and terbinafine, which must be taken daily for a period ranging from 6 months to 1 year or longer; flucytosine with or without amphotericin also has been employed.4 Among the physical methods, surgical excision is not suggested due to possible dissemination of disease; other options include cryotherapy, thermotherapy, and laser vaporization.6 The prognosis has improved since the use of extended-spectrum triazoles, but high rates of refractory disease remain unchanged.2
The differential diagnosis includes other infections. Nocardiosis is a bacterial infection in which cutaneous disease can result in actinomycetoma, which presents with grains that are small, round, and stain blue on hematoxylin and eosin with eosinophilic rays at the periphery.7 Although the clinical features and pseudoepitheliomatous hyperplasia seen in CBM can mimic squamous cell carcinoma, the latter would show variable degrees of differentiation, keratinization, nuclear atypia, and architectural atypia with a negative tissue culture.8 Eumycetoma is a fungal infection that typically is not caused by F pedrosoi but rather most commonly Madurella mycetomatis.9 Leishmaniasis is a parasitic infection in which a biopsy of cutaneous lesions often displays parasite-filled histiocytes.10
- Rudolph M. Über die brasilianische “figueira” (vorläufige mitteilung). Arch Schiffs Trop Hyg. 1914;18:498-499.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- Kurien G, Sugumar K, Chandran V. Chromoblastomycosis. StatPearls. StatPearls Publishing; 2021. Accessed June 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470253/
- Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708-713. doi:10.1016/j.micinf.2005.01.006
- Krzys´ciak PM, Pindycka-Piaszczyn´ska M, Piaszczyn´ski M. Chromoblastomycosis. Postepy Dermatol Alergol. 2014;31:310-321. doi:10.5114/pdia.2014.40949
- Siddig EE, van de Sande WWJ, Fahal AH. Actinomycetoma laboratory-based diagnosis: a mini-review. Trans R Soc Trop Med Hyg. 2021;115:355-363.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll .2017.06.003
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- Saliba M, Shalhoub A, Taraif S, et al. Cutaneous leishmaniasis: an evolving disease with ancient roots. Int J Dermatol. 2019;58:834-843. doi:10.1111/ijd.14451
- Rudolph M. Über die brasilianische “figueira” (vorläufige mitteilung). Arch Schiffs Trop Hyg. 1914;18:498-499.
- Queiroz-Telles F, de Hoog S, Santos DW, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Brito AC, Bittencourt MJS. Chromoblastomycosis: an etiological, epidemiological, clinical, diagnostic, and treatment update. An Bras Dermatol. 2018;93:495-506. doi:10.1590/abd1806-4841.20187321
- Kurien G, Sugumar K, Chandran V. Chromoblastomycosis. StatPearls. StatPearls Publishing; 2021. Accessed June 4, 2022. https://www.ncbi.nlm.nih.gov/books/NBK470253/
- Mazo Fávero Gimenes V, Da Glória de Souza M, Ferreira KS, et al. Cytokines and lymphocyte proliferation in patients with different clinical forms of chromoblastomycosis. Microbes Infect. 2005;7:708-713. doi:10.1016/j.micinf.2005.01.006
- Krzys´ciak PM, Pindycka-Piaszczyn´ska M, Piaszczyn´ski M. Chromoblastomycosis. Postepy Dermatol Alergol. 2014;31:310-321. doi:10.5114/pdia.2014.40949
- Siddig EE, van de Sande WWJ, Fahal AH. Actinomycetoma laboratory-based diagnosis: a mini-review. Trans R Soc Trop Med Hyg. 2021;115:355-363.
- Parekh V, Seykora JT. Cutaneous squamous cell carcinoma. Clin Lab Med. 2017;37:503-525. doi:10.1016/j.cll .2017.06.003
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- Saliba M, Shalhoub A, Taraif S, et al. Cutaneous leishmaniasis: an evolving disease with ancient roots. Int J Dermatol. 2019;58:834-843. doi:10.1111/ijd.14451
A 70-year-old immunocompetent man presented to the dermatology department with a progressive asymptomatic hand wound of 2 years’ duration following a splinter injury in Belize. Prior treatment included oral antibiotics without improvement. Physical examination revealed a 5.1×3.0 cm, pink to violaceous, nonpurulent plaque with a cobblestonelike appearance on the dorsal aspect of the right hand. Both the initial and a repeat skin biopsy revealed nonspecific changes, including hyperkeratosis, hypergranulosis, acute and chronic inflammation, and vascular ectasia. Grocott-Gomori methenamine-silver staining was negative for fungal organisms. One month after the repeat biopsy, a tissue culture returned positive for the rare Fonsecaea pedrosoi.
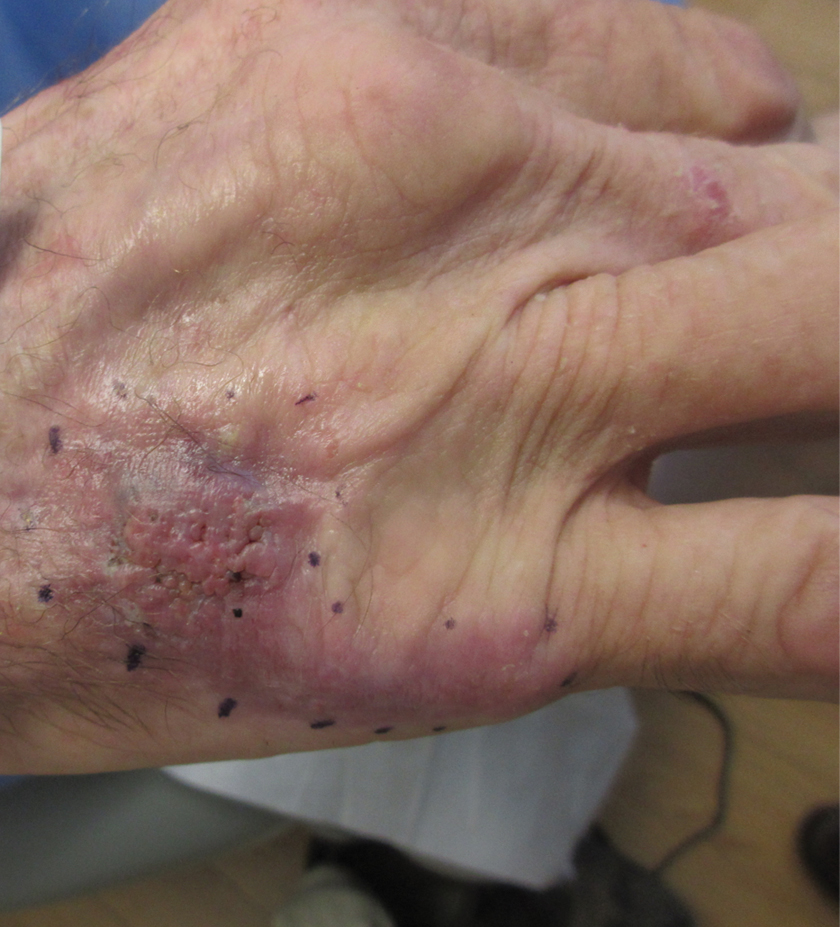
Private Payer Engagement
Payer Advocacy in Dermatology
Frustrations with payers is a common source of annoyance among dermatologists. Payment rules can seem arbitrary, ever-changing, and not uniform among the various payers. Keeping track of payer requirements can be nearly impossible.
To assist members in handling these concerns, the American Academy of Dermatology Association (AADA) created the Patient Access and Payer Relations (PAPR) committee, which seeks to promote patient access to dermatologic care by addressing issues that may arise with private payers. The committee utilizes a multipronged approach to develop strategies to educate payers on the value of dermatology, addressing systematic payment issues as they arise over time, and building relationships with insurers and employers to promote coverage and payment policies allowing for the highest quality of dermatologic care. The committee is comprised of practicing dermatologists who meet regularly to help guide and implement the AADA’s payer advocacy initiatives.
Identifying payer contacts and forging working relationships is a cornerstone of payer advocacy. In addition to patient access to quality dermatologic services, fair reimbursement is always a primary concern.
Hot Topics in Payer Advocacy
How to Use Modifier −25 Appropriately—The AADA has been advocating for appropriate coverage and reimbursement for services billed by dermatologists; recent examples include assuring appropriate payment for services reported with modifier −25, which is used when a procedure such as a biopsy is performed on the same day as a separate and unrelated evaluation and management (E/M) service, such as psoriasis management. Some payers claim the concurrent nature of the services results in an overlap of office expenses such that these claims should be paid at a lesser amount; however, when procedure codes are frequently billed in association with an office visit, that overlap has already been accounted for as part of the code valuation process, negating the need for additional reduction.
The AADA PAPR committee has created numerous resources for our members to ensure they are using modifier −25 appropriately, particularly now that the US Department of Health and Human Services Office of the Inspector General (OIG) has announced a work plan to audit dermatologists claims reporting modifier −25.1 The AADA immediately formed a work group, including PAPR committee members, to develop and employ a strategy to educate key decision-makers on the correct use of modifier −25 and highlight appropriate resources to guide members. An introductory call was held with the OIG audit team to discuss the appropriate use of modifier −25 in dermatology as the OIG prepares to develop the parameters of its audit sometime in the future (AADA, unpublished data, 2021).
Working With Dermatology Societies on Payer Issues—The American Academy of Dermatology Association PAPR committee works collaboratively with members of the American Academy of Dermatology, state and local dermatology societies, and private payers to alleviate administrative burdens for dermatologists, maintain appropriate reimbursement for furnished services, and ensure patients can access covered quality care. Collaboration with state dermatology societies is essential to address payer issues that impact their members and provide guidance on effective engagement with their state payers. Recent examples include working with dermatology societies in Massachusetts, Rhode Island, and Florida on strategies to advocate against modifier −25 payment reductions by insurance carriers (AADA, unpublished data, 2021). Additionally, the AADA PAPR committee has been able to provide guidance and technical support as needed to state dermatology societies, such as to the Rhode Island Dermatology Society and the Pennsylvania Academy of Dermatology and Dermatologic Surgery to address payer quality metrics and access to laboratory services, respectively (AADA, unpublished data, 2021).
Patient Access to Affordable Treatments—American Academy of Dermatology Association payer advocacy is anchored to published position statements and clinical guidelines. To strengthen AADA advocacy on payer-mandated drug substitutions for nonmedical reasons and to preserve patient access to medications, the PAPR committee collaborated with the American Academy of Dermatology’s Drug Pricing and Transparency Task Force to update the AADA Position Statement on Patient Access to Affordable Treatments2 to address this issue. Essentially, patients who are stable on a medication should be allowed to keep using the same medication without payers changing their coverage for nonmedical reasons or by offering financial incentives to switch.
Relationships With Major Insurance Carriers—Integral to the PAPR committee’s private payer advocacy success are our proactive relationships with major insurance carriers. In 2021, the PAPR committee established quarterly dermatology-specific meetings with the major national carriers. In nurturing these relationships, the PAPR committee has been able to expand on opportunities to provide payer policy reviews as well as identify dermatologists as subject matter experts available to payers to assist with physician panels or policy reviews. These regular contacts also have proved beneficial in addressing issues raised by members; a few such examples include when one major payer reversed its denials on dermatologists’ claims for Current Procedural Terminology code 88304 (surgical pathology, gross and microscopic tissue exam) after it was brought to their attention by the AADA (AADA, unpublished data, 2021). This payer worked with its external vendor to correct the denials. When the AADA learned that another major payer was improperly denying payment for claims for 1 stage of Mohs micrographic surgery reported using Current Procedural Terminology code 17311, we worked with contacts at this payer to resolve the issue. They were receptive to our concerns and readily researched the issue. Leadership of the PAPR committee continued working with the AADA coding team and this payer to develop training guidance to prevent future denials, and the payer has reviewed prior denials and reprocessed claims for payment (AADA, unpublished data, 2021).
E/M Coding Issues
Another issue under consideration by several national insurers is E/M-level reassignment. Payers are reviewing claims from providers who are identified as coding at a higher E/M level as compared to their specialty peers. Some insurance carriers are using proprietary algorithms that attempt to link specific diagnoses to certain levels of E/M, triggering claim edits within their claim processing systems (AADA, unpublished data, 2021). The carrier will then either deny the claim or adjust reimbursement to a lower-level E/M service. In discussions with a national carrier on its E/M Leveling Program, the AADA has offered to work with them on appropriate E/M documentation and reporting (AADA, unpublished data, 2021). The AADA also has extensive member resources for guidance on E/M reporting as well as preparing for audits and appealing payer downcoding developed by the coding staff in conjunction with the Coding and Reimbursement Committee.
Recent Efforts From the AADA
Within the AADA, the PAPR committee works closely with the coding, practice management, and regulatory teams to address payer issues and develop resources for members. Recent examples include resources for dermatology practices on the No Surprises Act and what practices need to do to comply (AADA, unpublished data, 2021). The PAPR committee also works collaboratively with other AADA committees and task forces on payer issues as needed; for example, the PAPR committee has been working with the Dermatopathology Rapid Response committee to address member concerns regarding access to the pathology laboratory of their choice. Many payers are seeking to consolidate and save money by requiring the use of preferred laboratories, which impacts patient access to physician office laboratories and physician-recommended reference laboratories. The AADA, along with other medical specialties, has advocated for payers to not create a restrictive network of pathology laboratories within their provider networks and to support dermatologists’ laboratories of choice (AADA, unpublished data, 2021).
Within the payer space, the role of employers in impacting payment and coverage policies continues to rise. In 2021, the AADA leadership approved the employer outreach strategy to engage employers. The overall objectives are to advocate to employers on the value of dermatologic care and access to care provided by board-certified dermatologists. This is a long-term project that is just getting underway (AADA, unpublished data, 2021).
Payer Resource Center for AADA Members
To ensure that AADA members have the resources they need to advocate with payers as well as to keep the PAPR committee aware of emerging payer issues, the AADA created a new private payer resource center for members (https://www.aad.org/member/advocacy/priorities/payer-advocacy), which assists AADA members with common dermatologic concerns with insurers as well as contracting issues. The website also includes an email address for members to report payer issues ([email protected]). This information helps the PAPR committee identify and prioritize issues of concern.
Final Thoughts
Given the control that private insurance companies exert over the health care that dermatology patients can access, the AADA in general and the PAPR committee specifically play a valuable role in advocating access to care for dermatology patients.
- US Department of Health and Human Services Office of the Inspector General. Dermatologist claims for evaluation and management services on the same day as minor surgical procedures. Accessed May 16, 2022. https://www.oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000577.asp
- American Academy of Dermatology Association. Position Statement on Patient Access to Affordable Treatments. Updated November 4, 2017. Accessed May 24, 2022. https://server.aad.org/forms/policies/uploads/ps/ps%20-%20patient%20access%20to%20affordable%20treatments.pdf?)
Payer Advocacy in Dermatology
Frustrations with payers is a common source of annoyance among dermatologists. Payment rules can seem arbitrary, ever-changing, and not uniform among the various payers. Keeping track of payer requirements can be nearly impossible.
To assist members in handling these concerns, the American Academy of Dermatology Association (AADA) created the Patient Access and Payer Relations (PAPR) committee, which seeks to promote patient access to dermatologic care by addressing issues that may arise with private payers. The committee utilizes a multipronged approach to develop strategies to educate payers on the value of dermatology, addressing systematic payment issues as they arise over time, and building relationships with insurers and employers to promote coverage and payment policies allowing for the highest quality of dermatologic care. The committee is comprised of practicing dermatologists who meet regularly to help guide and implement the AADA’s payer advocacy initiatives.
Identifying payer contacts and forging working relationships is a cornerstone of payer advocacy. In addition to patient access to quality dermatologic services, fair reimbursement is always a primary concern.
Hot Topics in Payer Advocacy
How to Use Modifier −25 Appropriately—The AADA has been advocating for appropriate coverage and reimbursement for services billed by dermatologists; recent examples include assuring appropriate payment for services reported with modifier −25, which is used when a procedure such as a biopsy is performed on the same day as a separate and unrelated evaluation and management (E/M) service, such as psoriasis management. Some payers claim the concurrent nature of the services results in an overlap of office expenses such that these claims should be paid at a lesser amount; however, when procedure codes are frequently billed in association with an office visit, that overlap has already been accounted for as part of the code valuation process, negating the need for additional reduction.
The AADA PAPR committee has created numerous resources for our members to ensure they are using modifier −25 appropriately, particularly now that the US Department of Health and Human Services Office of the Inspector General (OIG) has announced a work plan to audit dermatologists claims reporting modifier −25.1 The AADA immediately formed a work group, including PAPR committee members, to develop and employ a strategy to educate key decision-makers on the correct use of modifier −25 and highlight appropriate resources to guide members. An introductory call was held with the OIG audit team to discuss the appropriate use of modifier −25 in dermatology as the OIG prepares to develop the parameters of its audit sometime in the future (AADA, unpublished data, 2021).
Working With Dermatology Societies on Payer Issues—The American Academy of Dermatology Association PAPR committee works collaboratively with members of the American Academy of Dermatology, state and local dermatology societies, and private payers to alleviate administrative burdens for dermatologists, maintain appropriate reimbursement for furnished services, and ensure patients can access covered quality care. Collaboration with state dermatology societies is essential to address payer issues that impact their members and provide guidance on effective engagement with their state payers. Recent examples include working with dermatology societies in Massachusetts, Rhode Island, and Florida on strategies to advocate against modifier −25 payment reductions by insurance carriers (AADA, unpublished data, 2021). Additionally, the AADA PAPR committee has been able to provide guidance and technical support as needed to state dermatology societies, such as to the Rhode Island Dermatology Society and the Pennsylvania Academy of Dermatology and Dermatologic Surgery to address payer quality metrics and access to laboratory services, respectively (AADA, unpublished data, 2021).
Patient Access to Affordable Treatments—American Academy of Dermatology Association payer advocacy is anchored to published position statements and clinical guidelines. To strengthen AADA advocacy on payer-mandated drug substitutions for nonmedical reasons and to preserve patient access to medications, the PAPR committee collaborated with the American Academy of Dermatology’s Drug Pricing and Transparency Task Force to update the AADA Position Statement on Patient Access to Affordable Treatments2 to address this issue. Essentially, patients who are stable on a medication should be allowed to keep using the same medication without payers changing their coverage for nonmedical reasons or by offering financial incentives to switch.
Relationships With Major Insurance Carriers—Integral to the PAPR committee’s private payer advocacy success are our proactive relationships with major insurance carriers. In 2021, the PAPR committee established quarterly dermatology-specific meetings with the major national carriers. In nurturing these relationships, the PAPR committee has been able to expand on opportunities to provide payer policy reviews as well as identify dermatologists as subject matter experts available to payers to assist with physician panels or policy reviews. These regular contacts also have proved beneficial in addressing issues raised by members; a few such examples include when one major payer reversed its denials on dermatologists’ claims for Current Procedural Terminology code 88304 (surgical pathology, gross and microscopic tissue exam) after it was brought to their attention by the AADA (AADA, unpublished data, 2021). This payer worked with its external vendor to correct the denials. When the AADA learned that another major payer was improperly denying payment for claims for 1 stage of Mohs micrographic surgery reported using Current Procedural Terminology code 17311, we worked with contacts at this payer to resolve the issue. They were receptive to our concerns and readily researched the issue. Leadership of the PAPR committee continued working with the AADA coding team and this payer to develop training guidance to prevent future denials, and the payer has reviewed prior denials and reprocessed claims for payment (AADA, unpublished data, 2021).
E/M Coding Issues
Another issue under consideration by several national insurers is E/M-level reassignment. Payers are reviewing claims from providers who are identified as coding at a higher E/M level as compared to their specialty peers. Some insurance carriers are using proprietary algorithms that attempt to link specific diagnoses to certain levels of E/M, triggering claim edits within their claim processing systems (AADA, unpublished data, 2021). The carrier will then either deny the claim or adjust reimbursement to a lower-level E/M service. In discussions with a national carrier on its E/M Leveling Program, the AADA has offered to work with them on appropriate E/M documentation and reporting (AADA, unpublished data, 2021). The AADA also has extensive member resources for guidance on E/M reporting as well as preparing for audits and appealing payer downcoding developed by the coding staff in conjunction with the Coding and Reimbursement Committee.
Recent Efforts From the AADA
Within the AADA, the PAPR committee works closely with the coding, practice management, and regulatory teams to address payer issues and develop resources for members. Recent examples include resources for dermatology practices on the No Surprises Act and what practices need to do to comply (AADA, unpublished data, 2021). The PAPR committee also works collaboratively with other AADA committees and task forces on payer issues as needed; for example, the PAPR committee has been working with the Dermatopathology Rapid Response committee to address member concerns regarding access to the pathology laboratory of their choice. Many payers are seeking to consolidate and save money by requiring the use of preferred laboratories, which impacts patient access to physician office laboratories and physician-recommended reference laboratories. The AADA, along with other medical specialties, has advocated for payers to not create a restrictive network of pathology laboratories within their provider networks and to support dermatologists’ laboratories of choice (AADA, unpublished data, 2021).
Within the payer space, the role of employers in impacting payment and coverage policies continues to rise. In 2021, the AADA leadership approved the employer outreach strategy to engage employers. The overall objectives are to advocate to employers on the value of dermatologic care and access to care provided by board-certified dermatologists. This is a long-term project that is just getting underway (AADA, unpublished data, 2021).
Payer Resource Center for AADA Members
To ensure that AADA members have the resources they need to advocate with payers as well as to keep the PAPR committee aware of emerging payer issues, the AADA created a new private payer resource center for members (https://www.aad.org/member/advocacy/priorities/payer-advocacy), which assists AADA members with common dermatologic concerns with insurers as well as contracting issues. The website also includes an email address for members to report payer issues ([email protected]). This information helps the PAPR committee identify and prioritize issues of concern.
Final Thoughts
Given the control that private insurance companies exert over the health care that dermatology patients can access, the AADA in general and the PAPR committee specifically play a valuable role in advocating access to care for dermatology patients.
Payer Advocacy in Dermatology
Frustrations with payers is a common source of annoyance among dermatologists. Payment rules can seem arbitrary, ever-changing, and not uniform among the various payers. Keeping track of payer requirements can be nearly impossible.
To assist members in handling these concerns, the American Academy of Dermatology Association (AADA) created the Patient Access and Payer Relations (PAPR) committee, which seeks to promote patient access to dermatologic care by addressing issues that may arise with private payers. The committee utilizes a multipronged approach to develop strategies to educate payers on the value of dermatology, addressing systematic payment issues as they arise over time, and building relationships with insurers and employers to promote coverage and payment policies allowing for the highest quality of dermatologic care. The committee is comprised of practicing dermatologists who meet regularly to help guide and implement the AADA’s payer advocacy initiatives.
Identifying payer contacts and forging working relationships is a cornerstone of payer advocacy. In addition to patient access to quality dermatologic services, fair reimbursement is always a primary concern.
Hot Topics in Payer Advocacy
How to Use Modifier −25 Appropriately—The AADA has been advocating for appropriate coverage and reimbursement for services billed by dermatologists; recent examples include assuring appropriate payment for services reported with modifier −25, which is used when a procedure such as a biopsy is performed on the same day as a separate and unrelated evaluation and management (E/M) service, such as psoriasis management. Some payers claim the concurrent nature of the services results in an overlap of office expenses such that these claims should be paid at a lesser amount; however, when procedure codes are frequently billed in association with an office visit, that overlap has already been accounted for as part of the code valuation process, negating the need for additional reduction.
The AADA PAPR committee has created numerous resources for our members to ensure they are using modifier −25 appropriately, particularly now that the US Department of Health and Human Services Office of the Inspector General (OIG) has announced a work plan to audit dermatologists claims reporting modifier −25.1 The AADA immediately formed a work group, including PAPR committee members, to develop and employ a strategy to educate key decision-makers on the correct use of modifier −25 and highlight appropriate resources to guide members. An introductory call was held with the OIG audit team to discuss the appropriate use of modifier −25 in dermatology as the OIG prepares to develop the parameters of its audit sometime in the future (AADA, unpublished data, 2021).
Working With Dermatology Societies on Payer Issues—The American Academy of Dermatology Association PAPR committee works collaboratively with members of the American Academy of Dermatology, state and local dermatology societies, and private payers to alleviate administrative burdens for dermatologists, maintain appropriate reimbursement for furnished services, and ensure patients can access covered quality care. Collaboration with state dermatology societies is essential to address payer issues that impact their members and provide guidance on effective engagement with their state payers. Recent examples include working with dermatology societies in Massachusetts, Rhode Island, and Florida on strategies to advocate against modifier −25 payment reductions by insurance carriers (AADA, unpublished data, 2021). Additionally, the AADA PAPR committee has been able to provide guidance and technical support as needed to state dermatology societies, such as to the Rhode Island Dermatology Society and the Pennsylvania Academy of Dermatology and Dermatologic Surgery to address payer quality metrics and access to laboratory services, respectively (AADA, unpublished data, 2021).
Patient Access to Affordable Treatments—American Academy of Dermatology Association payer advocacy is anchored to published position statements and clinical guidelines. To strengthen AADA advocacy on payer-mandated drug substitutions for nonmedical reasons and to preserve patient access to medications, the PAPR committee collaborated with the American Academy of Dermatology’s Drug Pricing and Transparency Task Force to update the AADA Position Statement on Patient Access to Affordable Treatments2 to address this issue. Essentially, patients who are stable on a medication should be allowed to keep using the same medication without payers changing their coverage for nonmedical reasons or by offering financial incentives to switch.
Relationships With Major Insurance Carriers—Integral to the PAPR committee’s private payer advocacy success are our proactive relationships with major insurance carriers. In 2021, the PAPR committee established quarterly dermatology-specific meetings with the major national carriers. In nurturing these relationships, the PAPR committee has been able to expand on opportunities to provide payer policy reviews as well as identify dermatologists as subject matter experts available to payers to assist with physician panels or policy reviews. These regular contacts also have proved beneficial in addressing issues raised by members; a few such examples include when one major payer reversed its denials on dermatologists’ claims for Current Procedural Terminology code 88304 (surgical pathology, gross and microscopic tissue exam) after it was brought to their attention by the AADA (AADA, unpublished data, 2021). This payer worked with its external vendor to correct the denials. When the AADA learned that another major payer was improperly denying payment for claims for 1 stage of Mohs micrographic surgery reported using Current Procedural Terminology code 17311, we worked with contacts at this payer to resolve the issue. They were receptive to our concerns and readily researched the issue. Leadership of the PAPR committee continued working with the AADA coding team and this payer to develop training guidance to prevent future denials, and the payer has reviewed prior denials and reprocessed claims for payment (AADA, unpublished data, 2021).
E/M Coding Issues
Another issue under consideration by several national insurers is E/M-level reassignment. Payers are reviewing claims from providers who are identified as coding at a higher E/M level as compared to their specialty peers. Some insurance carriers are using proprietary algorithms that attempt to link specific diagnoses to certain levels of E/M, triggering claim edits within their claim processing systems (AADA, unpublished data, 2021). The carrier will then either deny the claim or adjust reimbursement to a lower-level E/M service. In discussions with a national carrier on its E/M Leveling Program, the AADA has offered to work with them on appropriate E/M documentation and reporting (AADA, unpublished data, 2021). The AADA also has extensive member resources for guidance on E/M reporting as well as preparing for audits and appealing payer downcoding developed by the coding staff in conjunction with the Coding and Reimbursement Committee.
Recent Efforts From the AADA
Within the AADA, the PAPR committee works closely with the coding, practice management, and regulatory teams to address payer issues and develop resources for members. Recent examples include resources for dermatology practices on the No Surprises Act and what practices need to do to comply (AADA, unpublished data, 2021). The PAPR committee also works collaboratively with other AADA committees and task forces on payer issues as needed; for example, the PAPR committee has been working with the Dermatopathology Rapid Response committee to address member concerns regarding access to the pathology laboratory of their choice. Many payers are seeking to consolidate and save money by requiring the use of preferred laboratories, which impacts patient access to physician office laboratories and physician-recommended reference laboratories. The AADA, along with other medical specialties, has advocated for payers to not create a restrictive network of pathology laboratories within their provider networks and to support dermatologists’ laboratories of choice (AADA, unpublished data, 2021).
Within the payer space, the role of employers in impacting payment and coverage policies continues to rise. In 2021, the AADA leadership approved the employer outreach strategy to engage employers. The overall objectives are to advocate to employers on the value of dermatologic care and access to care provided by board-certified dermatologists. This is a long-term project that is just getting underway (AADA, unpublished data, 2021).
Payer Resource Center for AADA Members
To ensure that AADA members have the resources they need to advocate with payers as well as to keep the PAPR committee aware of emerging payer issues, the AADA created a new private payer resource center for members (https://www.aad.org/member/advocacy/priorities/payer-advocacy), which assists AADA members with common dermatologic concerns with insurers as well as contracting issues. The website also includes an email address for members to report payer issues ([email protected]). This information helps the PAPR committee identify and prioritize issues of concern.
Final Thoughts
Given the control that private insurance companies exert over the health care that dermatology patients can access, the AADA in general and the PAPR committee specifically play a valuable role in advocating access to care for dermatology patients.
- US Department of Health and Human Services Office of the Inspector General. Dermatologist claims for evaluation and management services on the same day as minor surgical procedures. Accessed May 16, 2022. https://www.oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000577.asp
- American Academy of Dermatology Association. Position Statement on Patient Access to Affordable Treatments. Updated November 4, 2017. Accessed May 24, 2022. https://server.aad.org/forms/policies/uploads/ps/ps%20-%20patient%20access%20to%20affordable%20treatments.pdf?)
- US Department of Health and Human Services Office of the Inspector General. Dermatologist claims for evaluation and management services on the same day as minor surgical procedures. Accessed May 16, 2022. https://www.oig.hhs.gov/reports-and-publications/workplan/summary/wp-summary-0000577.asp
- American Academy of Dermatology Association. Position Statement on Patient Access to Affordable Treatments. Updated November 4, 2017. Accessed May 24, 2022. https://server.aad.org/forms/policies/uploads/ps/ps%20-%20patient%20access%20to%20affordable%20treatments.pdf?)
Practice Points
- The American Academy of Dermatology Association routinely interacts with private medical payers on behalf of dermatologists and to insure access to dermatologic care for patients.
- Members of the American Academy of Dermatology are encouraged to work with the association when issues with payers arise.
Autoimmune disease linked to better late-stage breast cancer survival
CHICAGO – Comorbid autoimmune disease is associated with a greater chance of survival among women with stage IV breast cancer, according to a retrospective study presented at the annual meeting of the American Society of Clinical Oncology.
“It’s counterintuitive that, if you have two diseases instead of one, that you live longer, so then we had to scratch our heads a little bit and think about why these people are living longer,” said lead author Demitrios Dedousis, MD, University Hospitals, Case Medical Center, Cleveland.
Dr. Dedousis and colleagues conducted a retrospective analysis of patients from Surveillance, Epidemiology, and End Results–Medicare databases between 2007 and 2014 with breast cancer. The study included data from 137,324 patients diagnosed between 2007 and 2012, before the widespread use of immunotherapy. 27% of patients had an autoimmune disease, most commonly rheumatoid arthritis (23%), psoriasis (2.4%), and systemic lupus erythematosus (1.1%).
When all patients were included in the analysis, those with autoimmune disorders had slightly longer survival times, but these weren’t clinically significant. A subanalysis found a greater difference in survival.
The association appears more pronounced in metastatic cancer. Patients with stage 4 breast cancer and autoimmune disease had a longer mean overall survival (36 months vs. 30 months; hazard ratio, 1.46; P < .0001. Cancer-specific survival: HR, 1.39; P < .0001). Patients with autoimmune disease and stage 1-3 breast cancer had lower overall survival (P < .0001, P < 0.0001, and P = 0.026 respectively), compared with patients without autoimmune disease.
“What we thought was happening is that the lack of increased survival in stages 1 through 3 was hiding the increase in survival among the stage IV patients when looking at the overall cohort,” Dr. Dedousis said.
The retrospective nature of the study makes it impossible to draw any firm conclusions about causation. It could be that patients who have already been diagnosed with an autoimmune disease are more vigilant about going to health checkups. “There are other possible explanations, but the one that’s most interesting to us is that their immune system is involved in fighting the cancer. Our study certainly didn’t prove that, but it’s suggesting that’s a possibility,” Dr. Dedousis said.
He and his coauthors anticipate conducting similar studies in other cancers to see if there are similar relationships. Some preliminary work has already suggested something similar in lung cancer. “I think demonstrating this in a few kinds of cancer goes part of the way towards showing that this is a real biological phenomenon,” he said.
Another research avenue is to examine the immune systems and pathology specimens in patients with both an autoimmune disease and cancer to see if there is a greater immune response within the tumor. If so, that could suggest new immunotherapy strategies.
Another possibility is to look at the specific immune pathways within “protective” autoimmune conditions. “For the sake of argument, if we find a particular autoimmune condition is improving survival across multiple kinds of cancers, we could look at those pathways that are specifically involved in that autoimmune condition. It might help us identify a target for drug development,” Dr. Dedousis said.
Asked why a potential benefit might be more apparent in late-stage disease, he suggested that, in early-stage breast cancer, surgery and other treatments may be so effective that the immune system’s role only rarely makes a difference. It could play a larger role in late-stage disease when there are less effective therapies. It could also be that the immune system doesn’t recognize the cancer until it has spread beyond the regional lymph nodes on its way to metastasizing.
According to the National Cancer Institute, 10%-30% of people with cancer also have an autoimmune disease.
Dr. Dedousis has no relevant financial disclosures.
CHICAGO – Comorbid autoimmune disease is associated with a greater chance of survival among women with stage IV breast cancer, according to a retrospective study presented at the annual meeting of the American Society of Clinical Oncology.
“It’s counterintuitive that, if you have two diseases instead of one, that you live longer, so then we had to scratch our heads a little bit and think about why these people are living longer,” said lead author Demitrios Dedousis, MD, University Hospitals, Case Medical Center, Cleveland.
Dr. Dedousis and colleagues conducted a retrospective analysis of patients from Surveillance, Epidemiology, and End Results–Medicare databases between 2007 and 2014 with breast cancer. The study included data from 137,324 patients diagnosed between 2007 and 2012, before the widespread use of immunotherapy. 27% of patients had an autoimmune disease, most commonly rheumatoid arthritis (23%), psoriasis (2.4%), and systemic lupus erythematosus (1.1%).
When all patients were included in the analysis, those with autoimmune disorders had slightly longer survival times, but these weren’t clinically significant. A subanalysis found a greater difference in survival.
The association appears more pronounced in metastatic cancer. Patients with stage 4 breast cancer and autoimmune disease had a longer mean overall survival (36 months vs. 30 months; hazard ratio, 1.46; P < .0001. Cancer-specific survival: HR, 1.39; P < .0001). Patients with autoimmune disease and stage 1-3 breast cancer had lower overall survival (P < .0001, P < 0.0001, and P = 0.026 respectively), compared with patients without autoimmune disease.
“What we thought was happening is that the lack of increased survival in stages 1 through 3 was hiding the increase in survival among the stage IV patients when looking at the overall cohort,” Dr. Dedousis said.
The retrospective nature of the study makes it impossible to draw any firm conclusions about causation. It could be that patients who have already been diagnosed with an autoimmune disease are more vigilant about going to health checkups. “There are other possible explanations, but the one that’s most interesting to us is that their immune system is involved in fighting the cancer. Our study certainly didn’t prove that, but it’s suggesting that’s a possibility,” Dr. Dedousis said.
He and his coauthors anticipate conducting similar studies in other cancers to see if there are similar relationships. Some preliminary work has already suggested something similar in lung cancer. “I think demonstrating this in a few kinds of cancer goes part of the way towards showing that this is a real biological phenomenon,” he said.
Another research avenue is to examine the immune systems and pathology specimens in patients with both an autoimmune disease and cancer to see if there is a greater immune response within the tumor. If so, that could suggest new immunotherapy strategies.
Another possibility is to look at the specific immune pathways within “protective” autoimmune conditions. “For the sake of argument, if we find a particular autoimmune condition is improving survival across multiple kinds of cancers, we could look at those pathways that are specifically involved in that autoimmune condition. It might help us identify a target for drug development,” Dr. Dedousis said.
Asked why a potential benefit might be more apparent in late-stage disease, he suggested that, in early-stage breast cancer, surgery and other treatments may be so effective that the immune system’s role only rarely makes a difference. It could play a larger role in late-stage disease when there are less effective therapies. It could also be that the immune system doesn’t recognize the cancer until it has spread beyond the regional lymph nodes on its way to metastasizing.
According to the National Cancer Institute, 10%-30% of people with cancer also have an autoimmune disease.
Dr. Dedousis has no relevant financial disclosures.
CHICAGO – Comorbid autoimmune disease is associated with a greater chance of survival among women with stage IV breast cancer, according to a retrospective study presented at the annual meeting of the American Society of Clinical Oncology.
“It’s counterintuitive that, if you have two diseases instead of one, that you live longer, so then we had to scratch our heads a little bit and think about why these people are living longer,” said lead author Demitrios Dedousis, MD, University Hospitals, Case Medical Center, Cleveland.
Dr. Dedousis and colleagues conducted a retrospective analysis of patients from Surveillance, Epidemiology, and End Results–Medicare databases between 2007 and 2014 with breast cancer. The study included data from 137,324 patients diagnosed between 2007 and 2012, before the widespread use of immunotherapy. 27% of patients had an autoimmune disease, most commonly rheumatoid arthritis (23%), psoriasis (2.4%), and systemic lupus erythematosus (1.1%).
When all patients were included in the analysis, those with autoimmune disorders had slightly longer survival times, but these weren’t clinically significant. A subanalysis found a greater difference in survival.
The association appears more pronounced in metastatic cancer. Patients with stage 4 breast cancer and autoimmune disease had a longer mean overall survival (36 months vs. 30 months; hazard ratio, 1.46; P < .0001. Cancer-specific survival: HR, 1.39; P < .0001). Patients with autoimmune disease and stage 1-3 breast cancer had lower overall survival (P < .0001, P < 0.0001, and P = 0.026 respectively), compared with patients without autoimmune disease.
“What we thought was happening is that the lack of increased survival in stages 1 through 3 was hiding the increase in survival among the stage IV patients when looking at the overall cohort,” Dr. Dedousis said.
The retrospective nature of the study makes it impossible to draw any firm conclusions about causation. It could be that patients who have already been diagnosed with an autoimmune disease are more vigilant about going to health checkups. “There are other possible explanations, but the one that’s most interesting to us is that their immune system is involved in fighting the cancer. Our study certainly didn’t prove that, but it’s suggesting that’s a possibility,” Dr. Dedousis said.
He and his coauthors anticipate conducting similar studies in other cancers to see if there are similar relationships. Some preliminary work has already suggested something similar in lung cancer. “I think demonstrating this in a few kinds of cancer goes part of the way towards showing that this is a real biological phenomenon,” he said.
Another research avenue is to examine the immune systems and pathology specimens in patients with both an autoimmune disease and cancer to see if there is a greater immune response within the tumor. If so, that could suggest new immunotherapy strategies.
Another possibility is to look at the specific immune pathways within “protective” autoimmune conditions. “For the sake of argument, if we find a particular autoimmune condition is improving survival across multiple kinds of cancers, we could look at those pathways that are specifically involved in that autoimmune condition. It might help us identify a target for drug development,” Dr. Dedousis said.
Asked why a potential benefit might be more apparent in late-stage disease, he suggested that, in early-stage breast cancer, surgery and other treatments may be so effective that the immune system’s role only rarely makes a difference. It could play a larger role in late-stage disease when there are less effective therapies. It could also be that the immune system doesn’t recognize the cancer until it has spread beyond the regional lymph nodes on its way to metastasizing.
According to the National Cancer Institute, 10%-30% of people with cancer also have an autoimmune disease.
Dr. Dedousis has no relevant financial disclosures.
AT ASCO 2022
A fish tale? More on that seafood, melanoma study
A recent study linking the consumption of fish to melanoma had sushi eaters looking up the number of the nearest dermatologist. But experts said the findings had several important limitations and that no one should change their diet based on the results.
“It wouldn’t impact my fish consumption at all,” said Sancy Leachman, MD, PhD, who directs the melanoma program at Oregon Health & Science University’s Knight Cancer Institute in Portland. “Fish are part of a healthy diet,” particularly if it replaces less healthy proteins such as beef.
Even the authors of the study advised caution when interpreting the findings.
“I wouldn’t encourage anyone to change their fish consumption habits just because of this paper,” said study leader Eunyoung Cho, ScD, an epidemiologist at Brown University, Providence, R.I. “Fish is cardioprotective and is related to reduced risk of developing certain cancers too.”
Solid findings or fishing expedition?
The study quickly generated headlines and was the most viewed article of the journal Cancer Causes & Control within a day of its publication.
Dr. Cho, who is on the editorial board of the journal, analyzed the results of a study funded by the National Institutes of Health and the Association for the Advancement of Retired Persons that began in the 1990s and explored possible links between fish consumption and developing different forms of cancer.
Previous research from this project had shown eating fish was associated with an increased risk of melanoma – but no other type of cancer.
Dr. Cho speculated that the risk is due to contaminants, such as mercury and arsenic, in fish. But she was curious to see if she could find a connection between the amount of fish consumed and the risk of developing skin cancer.
At the beginning of the study people reported how much fish they had consumed over the previous year, which varied widely by person. Then researchers tracked participants for 15 years, recording who developed cancer and who did not.
Dietary recall can be difficult; people often say they can’t remember what they ate yesterday, much less 1 year ago. Still, dietitian Clare Collins, PhD, of the University of Newcastle (Australia), noted that previous research showed that the dietary recall tool for this study is reliable.
NIH researchers never checked to see how their diet changed over time, as this was a study meant to observe changes in health status starting from a baseline point. The researchers assumed that the level of fish intake recorded at the beginning of the study remained steady.
Of 491,000 people tracked in the study, 5,000 developed malignant melanoma and another 3,300 developed melanoma cells on the skin surface. People who ate the most fish – an average of almost 43 g, or about 1.5 ounces, per day – were 23% more likely to develop melanoma than the lightest fish eaters, who averaged 3 g per day.
That risk is modest, Dr. Leachman said, especially for people with red hair who, she said, are 400% more likely to get melanoma than others. “A 23% increase, in the whole scheme of things, is not nearly as important as not getting sunburned if you’re a redhead,” Dr. Leachman said.
And what about the sun? Sun exposure is the principal risk factor for developing melanoma, and the researchers didn’t account for that factor. People who developed melanoma during the study may simply have spent more time in tanning beds or on the beach – or perhaps fishing and then eating their catch.
Dr. Cho and colleagues tried to consider the effects of solar radiation by looking at average sun exposure times in the places where the participants in the study were living when the research began. Using that framework, variation in sun exposure made no difference to melanoma risk, but Dr. Leachman said the technique isn’t foolproof.
“They assumed that they were going to get a certain amount of ultraviolet light just based on where they lived. We don’t know how long they lived there or if they really had ultraviolet exposure or not,” Dr. Leachman said. Someone in presumably less sunny Pennsylvania could get more sun than someone in sun-drenched Arizona depending on their lifestyle and habits.
The kind of study Dr. Cho published cannot account for individual behaviors related to sun exposure, a limitation her team readily acknowledges. Nor does it include information about moles or hair color – important considerations for skin cancers. It may be that redheads with moles who barely ate any fish developed melanoma at higher rates than anyone else, but the data don’t allow for that kind of analysis.
Dr. Cho’s group found that canned tuna and any type of fish that wasn’t fried were associated with a higher risk of developing melanoma, as people reported eating more of those products. However, people who said they ate greater quantities of fried fish had a lower risk of melanoma, a counterintuitive finding that she said warrants further research.
Given that the study showed only a modest chance of developing melanoma regardless of fish intake, and left so many questions unanswered, what was the point?
Other journals declined to publish this paper, Dr. Cho acknowledged, but she defended the article as a step toward better understanding the health impact of environmental contaminants in fish.
Dr. Leachman agreed. “These kinds of studies are very important to do. They have large data sets, where you can start to see trends that may be important,” she said. “They can help you identify things that might be related. These experiments are hypothesis generating.”
“I already published an article showing that total mercury level is related to skin cancer, and we know very well that in the U.S. fish consumption is the major source of mercury contamination,” Dr. Cho said. “So, I naturally thought that fish consumption may be associated with increased risk of skin cancer too.” Dr. Cho said she believed the findings confirm that hypothesis.
Dr. Cho said the next step would be to measure blood levels of different contaminants such as mercury and arsenic in people with melanoma, to determine which toxin is the biggest driver of melanoma. She said she plans to seek funding for that research.
Meanwhile, pass the salmon – but go light on the salt.
Dr. Cho and Dr. Leachman reported no relevant financial relationships. Dr. Cho is on the editorial board of Cancer Causes & Control.
A version of this article first appeared on Medscape.com.
A recent study linking the consumption of fish to melanoma had sushi eaters looking up the number of the nearest dermatologist. But experts said the findings had several important limitations and that no one should change their diet based on the results.
“It wouldn’t impact my fish consumption at all,” said Sancy Leachman, MD, PhD, who directs the melanoma program at Oregon Health & Science University’s Knight Cancer Institute in Portland. “Fish are part of a healthy diet,” particularly if it replaces less healthy proteins such as beef.
Even the authors of the study advised caution when interpreting the findings.
“I wouldn’t encourage anyone to change their fish consumption habits just because of this paper,” said study leader Eunyoung Cho, ScD, an epidemiologist at Brown University, Providence, R.I. “Fish is cardioprotective and is related to reduced risk of developing certain cancers too.”
Solid findings or fishing expedition?
The study quickly generated headlines and was the most viewed article of the journal Cancer Causes & Control within a day of its publication.
Dr. Cho, who is on the editorial board of the journal, analyzed the results of a study funded by the National Institutes of Health and the Association for the Advancement of Retired Persons that began in the 1990s and explored possible links between fish consumption and developing different forms of cancer.
Previous research from this project had shown eating fish was associated with an increased risk of melanoma – but no other type of cancer.
Dr. Cho speculated that the risk is due to contaminants, such as mercury and arsenic, in fish. But she was curious to see if she could find a connection between the amount of fish consumed and the risk of developing skin cancer.
At the beginning of the study people reported how much fish they had consumed over the previous year, which varied widely by person. Then researchers tracked participants for 15 years, recording who developed cancer and who did not.
Dietary recall can be difficult; people often say they can’t remember what they ate yesterday, much less 1 year ago. Still, dietitian Clare Collins, PhD, of the University of Newcastle (Australia), noted that previous research showed that the dietary recall tool for this study is reliable.
NIH researchers never checked to see how their diet changed over time, as this was a study meant to observe changes in health status starting from a baseline point. The researchers assumed that the level of fish intake recorded at the beginning of the study remained steady.
Of 491,000 people tracked in the study, 5,000 developed malignant melanoma and another 3,300 developed melanoma cells on the skin surface. People who ate the most fish – an average of almost 43 g, or about 1.5 ounces, per day – were 23% more likely to develop melanoma than the lightest fish eaters, who averaged 3 g per day.
That risk is modest, Dr. Leachman said, especially for people with red hair who, she said, are 400% more likely to get melanoma than others. “A 23% increase, in the whole scheme of things, is not nearly as important as not getting sunburned if you’re a redhead,” Dr. Leachman said.
And what about the sun? Sun exposure is the principal risk factor for developing melanoma, and the researchers didn’t account for that factor. People who developed melanoma during the study may simply have spent more time in tanning beds or on the beach – or perhaps fishing and then eating their catch.
Dr. Cho and colleagues tried to consider the effects of solar radiation by looking at average sun exposure times in the places where the participants in the study were living when the research began. Using that framework, variation in sun exposure made no difference to melanoma risk, but Dr. Leachman said the technique isn’t foolproof.
“They assumed that they were going to get a certain amount of ultraviolet light just based on where they lived. We don’t know how long they lived there or if they really had ultraviolet exposure or not,” Dr. Leachman said. Someone in presumably less sunny Pennsylvania could get more sun than someone in sun-drenched Arizona depending on their lifestyle and habits.
The kind of study Dr. Cho published cannot account for individual behaviors related to sun exposure, a limitation her team readily acknowledges. Nor does it include information about moles or hair color – important considerations for skin cancers. It may be that redheads with moles who barely ate any fish developed melanoma at higher rates than anyone else, but the data don’t allow for that kind of analysis.
Dr. Cho’s group found that canned tuna and any type of fish that wasn’t fried were associated with a higher risk of developing melanoma, as people reported eating more of those products. However, people who said they ate greater quantities of fried fish had a lower risk of melanoma, a counterintuitive finding that she said warrants further research.
Given that the study showed only a modest chance of developing melanoma regardless of fish intake, and left so many questions unanswered, what was the point?
Other journals declined to publish this paper, Dr. Cho acknowledged, but she defended the article as a step toward better understanding the health impact of environmental contaminants in fish.
Dr. Leachman agreed. “These kinds of studies are very important to do. They have large data sets, where you can start to see trends that may be important,” she said. “They can help you identify things that might be related. These experiments are hypothesis generating.”
“I already published an article showing that total mercury level is related to skin cancer, and we know very well that in the U.S. fish consumption is the major source of mercury contamination,” Dr. Cho said. “So, I naturally thought that fish consumption may be associated with increased risk of skin cancer too.” Dr. Cho said she believed the findings confirm that hypothesis.
Dr. Cho said the next step would be to measure blood levels of different contaminants such as mercury and arsenic in people with melanoma, to determine which toxin is the biggest driver of melanoma. She said she plans to seek funding for that research.
Meanwhile, pass the salmon – but go light on the salt.
Dr. Cho and Dr. Leachman reported no relevant financial relationships. Dr. Cho is on the editorial board of Cancer Causes & Control.
A version of this article first appeared on Medscape.com.
A recent study linking the consumption of fish to melanoma had sushi eaters looking up the number of the nearest dermatologist. But experts said the findings had several important limitations and that no one should change their diet based on the results.
“It wouldn’t impact my fish consumption at all,” said Sancy Leachman, MD, PhD, who directs the melanoma program at Oregon Health & Science University’s Knight Cancer Institute in Portland. “Fish are part of a healthy diet,” particularly if it replaces less healthy proteins such as beef.
Even the authors of the study advised caution when interpreting the findings.
“I wouldn’t encourage anyone to change their fish consumption habits just because of this paper,” said study leader Eunyoung Cho, ScD, an epidemiologist at Brown University, Providence, R.I. “Fish is cardioprotective and is related to reduced risk of developing certain cancers too.”
Solid findings or fishing expedition?
The study quickly generated headlines and was the most viewed article of the journal Cancer Causes & Control within a day of its publication.
Dr. Cho, who is on the editorial board of the journal, analyzed the results of a study funded by the National Institutes of Health and the Association for the Advancement of Retired Persons that began in the 1990s and explored possible links between fish consumption and developing different forms of cancer.
Previous research from this project had shown eating fish was associated with an increased risk of melanoma – but no other type of cancer.
Dr. Cho speculated that the risk is due to contaminants, such as mercury and arsenic, in fish. But she was curious to see if she could find a connection between the amount of fish consumed and the risk of developing skin cancer.
At the beginning of the study people reported how much fish they had consumed over the previous year, which varied widely by person. Then researchers tracked participants for 15 years, recording who developed cancer and who did not.
Dietary recall can be difficult; people often say they can’t remember what they ate yesterday, much less 1 year ago. Still, dietitian Clare Collins, PhD, of the University of Newcastle (Australia), noted that previous research showed that the dietary recall tool for this study is reliable.
NIH researchers never checked to see how their diet changed over time, as this was a study meant to observe changes in health status starting from a baseline point. The researchers assumed that the level of fish intake recorded at the beginning of the study remained steady.
Of 491,000 people tracked in the study, 5,000 developed malignant melanoma and another 3,300 developed melanoma cells on the skin surface. People who ate the most fish – an average of almost 43 g, or about 1.5 ounces, per day – were 23% more likely to develop melanoma than the lightest fish eaters, who averaged 3 g per day.
That risk is modest, Dr. Leachman said, especially for people with red hair who, she said, are 400% more likely to get melanoma than others. “A 23% increase, in the whole scheme of things, is not nearly as important as not getting sunburned if you’re a redhead,” Dr. Leachman said.
And what about the sun? Sun exposure is the principal risk factor for developing melanoma, and the researchers didn’t account for that factor. People who developed melanoma during the study may simply have spent more time in tanning beds or on the beach – or perhaps fishing and then eating their catch.
Dr. Cho and colleagues tried to consider the effects of solar radiation by looking at average sun exposure times in the places where the participants in the study were living when the research began. Using that framework, variation in sun exposure made no difference to melanoma risk, but Dr. Leachman said the technique isn’t foolproof.
“They assumed that they were going to get a certain amount of ultraviolet light just based on where they lived. We don’t know how long they lived there or if they really had ultraviolet exposure or not,” Dr. Leachman said. Someone in presumably less sunny Pennsylvania could get more sun than someone in sun-drenched Arizona depending on their lifestyle and habits.
The kind of study Dr. Cho published cannot account for individual behaviors related to sun exposure, a limitation her team readily acknowledges. Nor does it include information about moles or hair color – important considerations for skin cancers. It may be that redheads with moles who barely ate any fish developed melanoma at higher rates than anyone else, but the data don’t allow for that kind of analysis.
Dr. Cho’s group found that canned tuna and any type of fish that wasn’t fried were associated with a higher risk of developing melanoma, as people reported eating more of those products. However, people who said they ate greater quantities of fried fish had a lower risk of melanoma, a counterintuitive finding that she said warrants further research.
Given that the study showed only a modest chance of developing melanoma regardless of fish intake, and left so many questions unanswered, what was the point?
Other journals declined to publish this paper, Dr. Cho acknowledged, but she defended the article as a step toward better understanding the health impact of environmental contaminants in fish.
Dr. Leachman agreed. “These kinds of studies are very important to do. They have large data sets, where you can start to see trends that may be important,” she said. “They can help you identify things that might be related. These experiments are hypothesis generating.”
“I already published an article showing that total mercury level is related to skin cancer, and we know very well that in the U.S. fish consumption is the major source of mercury contamination,” Dr. Cho said. “So, I naturally thought that fish consumption may be associated with increased risk of skin cancer too.” Dr. Cho said she believed the findings confirm that hypothesis.
Dr. Cho said the next step would be to measure blood levels of different contaminants such as mercury and arsenic in people with melanoma, to determine which toxin is the biggest driver of melanoma. She said she plans to seek funding for that research.
Meanwhile, pass the salmon – but go light on the salt.
Dr. Cho and Dr. Leachman reported no relevant financial relationships. Dr. Cho is on the editorial board of Cancer Causes & Control.
A version of this article first appeared on Medscape.com.
Basal Cell Carcinoma
THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
Basal cell carcinoma is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals.5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (Figure, A) as in non-Hispanic White individuals.7 Any skin cancer can present with ulcerations, so while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ ethnic variability. In my practice in San Antonio (R.P.U.), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (Figure, A). There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes. 1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and SCC found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non- Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (Figure, B) should prompt consideration of skin cancer regardless of skin color.
- Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. doi:10.1007/s40257-021-00662-z
- Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/S0190-9622(81)70067-7
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/S0190-9622(96)90007-9
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study [published online July 9, 2021]. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005

THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
Basal cell carcinoma is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals.5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (Figure, A) as in non-Hispanic White individuals.7 Any skin cancer can present with ulcerations, so while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ ethnic variability. In my practice in San Antonio (R.P.U.), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (Figure, A). There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes. 1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and SCC found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non- Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (Figure, B) should prompt consideration of skin cancer regardless of skin color.

THE COMPARISON
A Nodular basal cell carcinoma (BCC) with a pearly rolled border, central pigmentation, and telangiectasia on the forehead of an 80-year-old Hispanic woman (light skin tone).
B Nodular BCC on the cheek of a 64-year-old Black man. The dark nonhealing ulcer had a subtle, pearly, rolled border and no visible telangiectasia.
Basal cell carcinoma is most prevalent in individuals with lighter skin tones and rarely affects those with darker skin tones. Unfortunately, the lower incidence and lack of surveillance frequently result in a delayed diagnosis and increased morbidity for the skin of color population.1
Epidemiology
Basal cell carcinoma is the most common skin cancer in White, Asian, and Hispanic individuals and the second most common in Black individuals. Squamous cell carcinoma is the most common skin cancer in Black individuals.2
Although BCCs are rare in individuals with darker skin tones, they most often develop in sun-exposed areas of the head and neck region.1 In one study in an academic urban medical center, BCCs were more likely to occur in lightly pigmented vs darkly pigmented Black individuals.3
Key clinical features in people with darker skin tones
The classic BCC manifestation of a pearly papule with rolled borders and telangiectasia may not be seen in the skin of color population, especially among those with darker skin tones.4 In patient A, a Hispanic woman, these features are present along with hyperpigmentation. More than 50% of BCCs are pigmented in patients with skin of color vs only 5% in White individuals.5-7 The incidence of a pigmented BCC is twice as frequent in Hispanic individuals (Figure, A) as in non-Hispanic White individuals.7 Any skin cancer can present with ulcerations, so while this is not specific to BCC, it is a reason to consider biopsy.
Worth noting
Pigmented BCC can mimic melanoma clinically and even when viewed with a dermatoscope, but such a suspicious lesion should prompt the clinician to perform a biopsy regardless of the type of suspected cancer. With experience and training, however, physicians can use dermoscopy to help make this distinction.
Note that skin of color is found in a heterogeneous population with a spectrum of skin tones and genetic/ ethnic variability. In my practice in San Antonio (R.P.U.), BCC is uncommon in Black patients and relatively common in Hispanic patients with lighter skin tones (Figure, A). There is speculation that a lower incidence of BCC in the skin of color population leads to a low index of suspicion, which contributes to delayed diagnoses with poorer outcomes. 1 There are no firm data to support this because the rare occurrence of BCC in darker skin tones makes this a challenge to study.
Health disparity highlight
In general, barriers to health care include poverty, lack of education, lack of health insurance, and systemic racism. One study on keratinocyte skin cancers including BCC and SCC found that these cancers were more costly to treat and required more health care resources, such as ambulatory visits and medication costs, in non-Hispanic Black and Hispanic White patients compared to non- Hispanic White patients.8
Final thoughts
Efforts are needed to achieve health equity through education of patients and health care providers about the appearance of BCC in skin of color with the goal of earlier diagnosis. Any nonhealing ulcer on the skin (Figure, B) should prompt consideration of skin cancer regardless of skin color.
- Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. doi:10.1007/s40257-021-00662-z
- Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/S0190-9622(81)70067-7
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/S0190-9622(96)90007-9
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study [published online July 9, 2021]. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005
- Ahluwalia J, Hadjicharalambous E, Mehregan D. Basal cell carcinoma in skin of color. J Drugs Dermatol. 2012;11:484-486.
- Zakhem GA, Pulavarty AN, Lester JC, et al. Skin cancer in people of color: a systematic review. Am J Clin Dermatol. 2022;23:137-151. doi:10.1007/s40257-021-00662-z
- Halder RM, Bang KM. Skin cancer in blacks in the United States. Dermatol Clin. 1988;6:397-405.
- Hogue L, Harvey VM. Basal cell carcinoma, squamous cell carcinoma, and cutaneous melanoma in skin of color patients. Dermatol Clin. 2019;37:519-526. doi:10.1016/j.det.2019.05.009
- Agbai ON, Buster K, Sanchez M, et al. Skin cancer and photoprotection in people of color: a review and recommendations for physicians and the public. J Am Acad Dermatol. 2014;70:748-762. doi:10.1016/j.jaad.2013.11.038
- Matsuoka LY, Schauer PK, Sordillo PP. Basal cell carcinoma in black patients. J Am Acad Dermatol. 1981;4:670-672. doi:10.1016/S0190-9622(81)70067-7
- Bigler C, Feldman J, Hall E, et al. Pigmented basal cell carcinoma in Hispanics. J Am Acad Dermatol. 1996;34:751-752. doi:10.1016/S0190-9622(96)90007-9
- Sierro TJ, Blumenthal LY, Hekmatjah J, et al. Differences in health care resource utilization and costs for keratinocyte carcinoma among racioethnic groups: a population-based study [published online July 9, 2021]. J Am Acad Dermatol. 2022;86:373-378. doi:10.1016/j.jaad.2021.07.005