User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Painless Vulvar Nodule
The Diagnosis: Proximal-Type Epithelioid Sarcoma
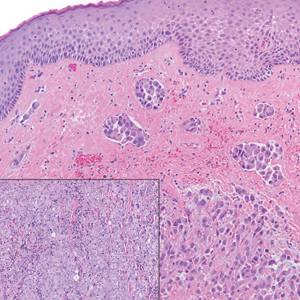
Proximal-type epithelioid sarcoma (PES) is a rare high-grade sarcoma of uncertain histogenesis that may present with a benign clinical appearance. Proximal-type epithelioid sarcoma peaks at 20 to 40 years of age and has a slight male predominance. This tumor exhibits aggressive behavior with both local recurrence and metastasis.1 The average overall survival is poor; however, tumor size less than 5 cm and complete excision with tumor-free margin status improves the prognosis.2 Proximal-type epithelioid sarcoma should not be confused with distal-type epithelioid sarcoma, which has a better prognosis and occurs in younger patients.1 Treatment of PES is wide excision, and chemotherapy trials with tazemetostat are ongoing.3
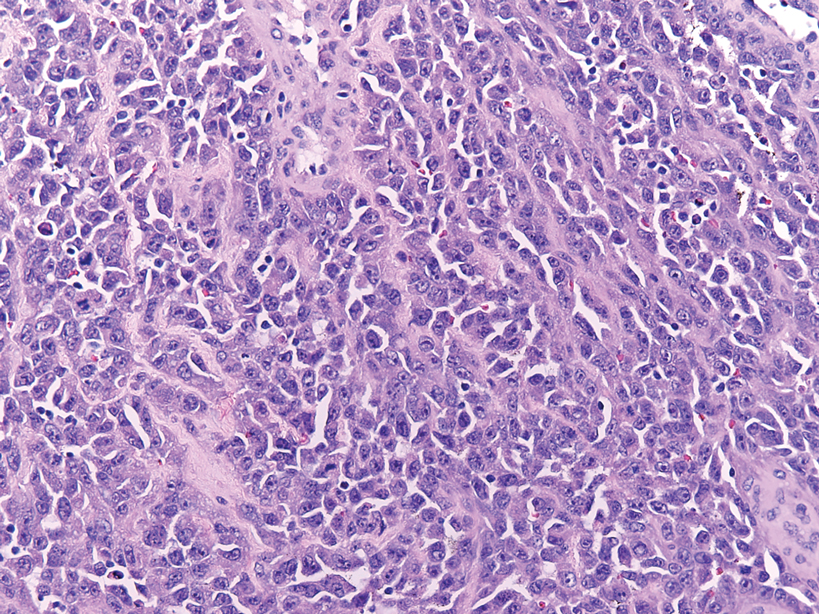
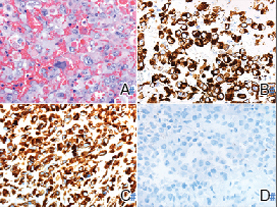
The deceptively banal clinical appearance of PES may delay appropriate diagnosis and treatment. Proximal-type epithelioid sarcoma often grows in sheets (quiz image [top] inset) and loose nests1 but also may take on a more corded appearance mimicking myoepithelial carcinoma. The cells themselves are plump dyscohesive epithelioid cells (quiz image [top]) with large nucleoli and eosinophilic cytoplasm or hyaline globules1 (quiz image [bottom]), but cells also may be focally spindled. Myxoid stroma, hemorrhage, and necrosis often are prominent features. Epithelioid sarcomas characteristically demonstrate positive immunostaining for both epithelial and mesenchymal markers (pan-cytokeratin and vimentin),1 with the majority having loss of expression of integrase interactor 1 (INI-1).2 Histology in this case was positive for cytokeratin monoclonal antibodies CAM5.2 and OSCAR, epithelial membrane antigen, and vimentin; it showed loss of INI-1 staining (quiz image [bottom]). Negative stains included S-100, p63, cytokeratins 7 and 20, CD34, CD31, ERG, glial fibrillary acidic protein, transducin-like enhancer of split 1, CD117, myogenin, synaptophysin, chromogranin, CD10, inhibin, CD99, and estrogen receptor.
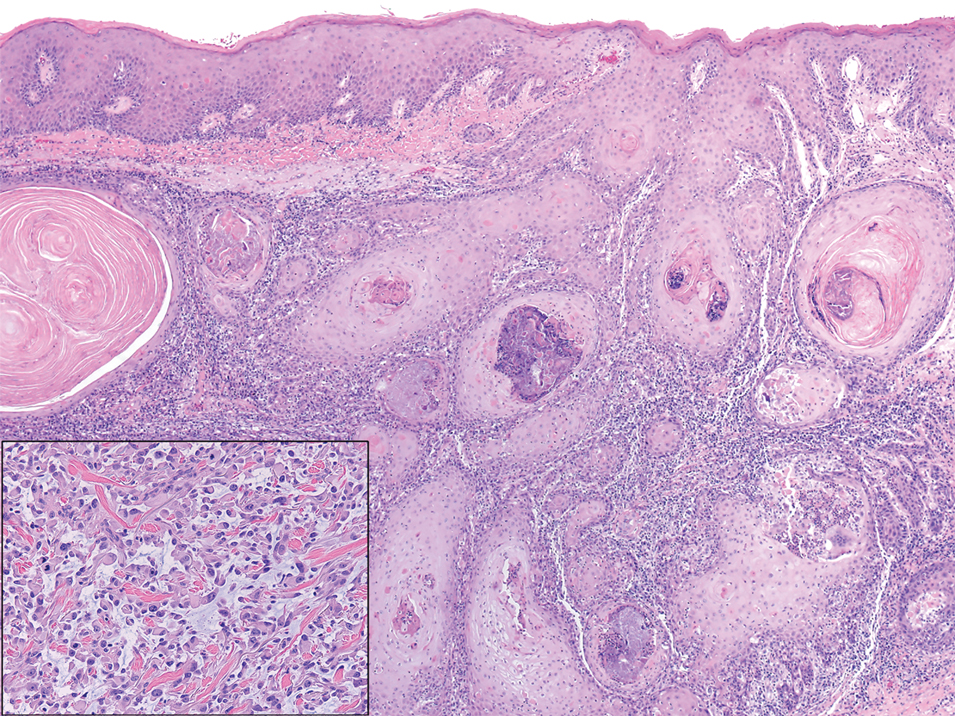
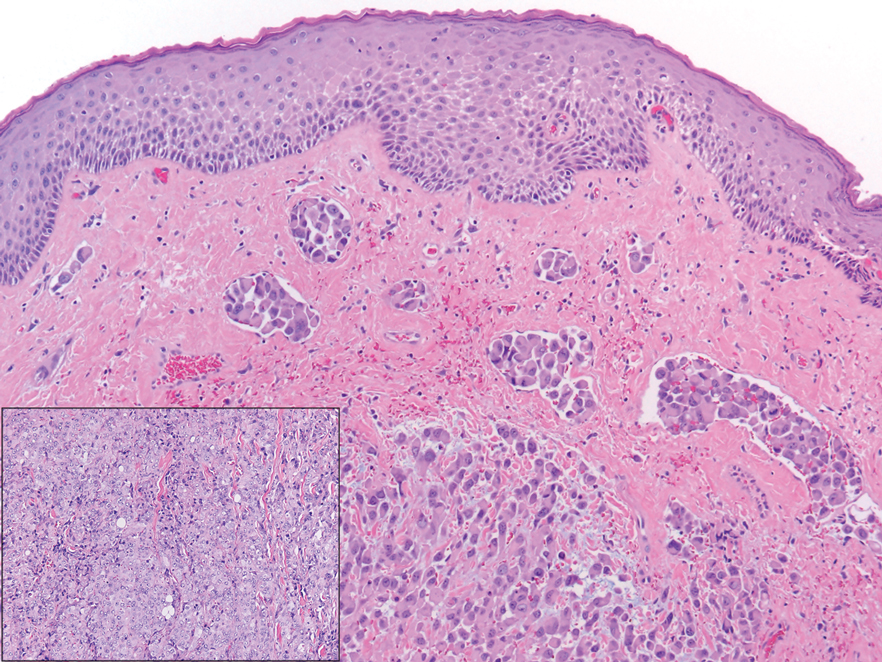
The differential diagnosis of PES includes poorly differentiated squamous cell carcinoma (Figure 1 [inset]), melanoma, myoepithelial carcinoma, and epithelioid angiosarcoma. Primary squamous cell carcinoma of the vulva presents as an endophytic or exophytic mass with raised borders. Vulvar cancer is uncommon among gynecologic malignancies, with squamous cell carcinoma being the most commonly encountered.4 Vulvar intraepithelial neoplasia (VIN) is increasing in incidence, while the occurrence of invasive squamous cell carcinoma remains stable.5 Human papillomavirus–related VIN (usual-type VIN) is less likely to progress to squamous cell carcinoma than differentiated VIN (d-VIN), a dysplasia that is unrelated to human papillomavirus that frequently harbors p53 mutations.4 The presence of histologic epidermal involvement can help distinguish squamous cell carcinoma from PES (Figure 1). As opposed to PES, metastatic squamous cell carcinoma is characterized by intercellular bridges and often at least focal keratinization (Figure 1). Squamous cell carcinoma demonstrates positivity with p63 and p40 immunohistochemical stains, while PES rarely stains for either.6
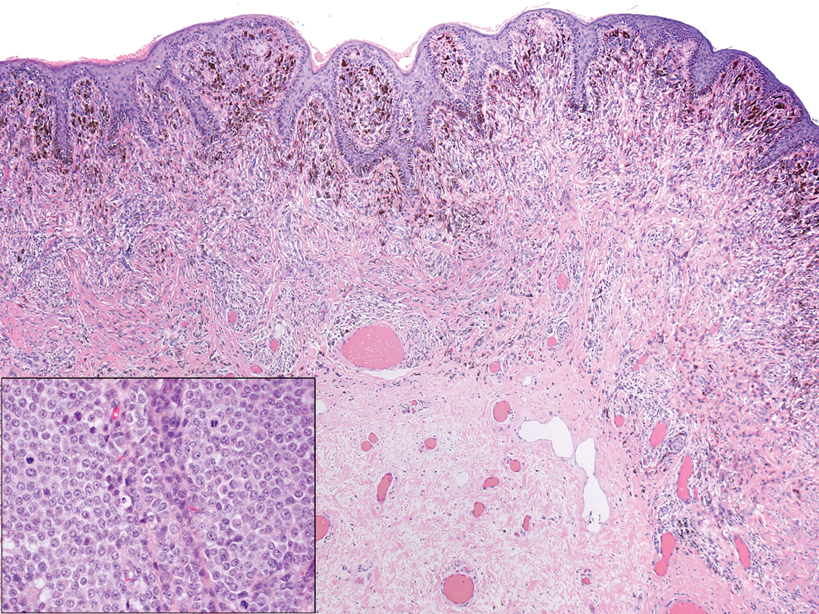
Melanoma is the second most common vulvar malignancy. Vulvar melanoma tends to occur in women of advanced age but has been reported in girls as young as 10 years old.7 There is some evidence that patients with lichen sclerosus may be at an increased risk for the development of vulvar melanoma.8 Compared to PES, primary vulvar melanoma usually demonstrates epidermal involvement as well as clinical findings of a pigmented lesion (Figure 2). A notable minority of vulvar melanomas are amelanotic.9 Melanoma may be distinguished from PES with a panel of melanocytic markers—human melanoma black 45, Melan-A, SRY-box transcription factor 10, S-100, and microphthalmia transcription factor—that rarely are expressed in the latter. Both PES and rhabdoid melanoma have eosinophilic and tinctorial cytoplasmic inclusions.10 Melanin pigment and more cohesive nests are helpful clues that may point to melanoma when present.
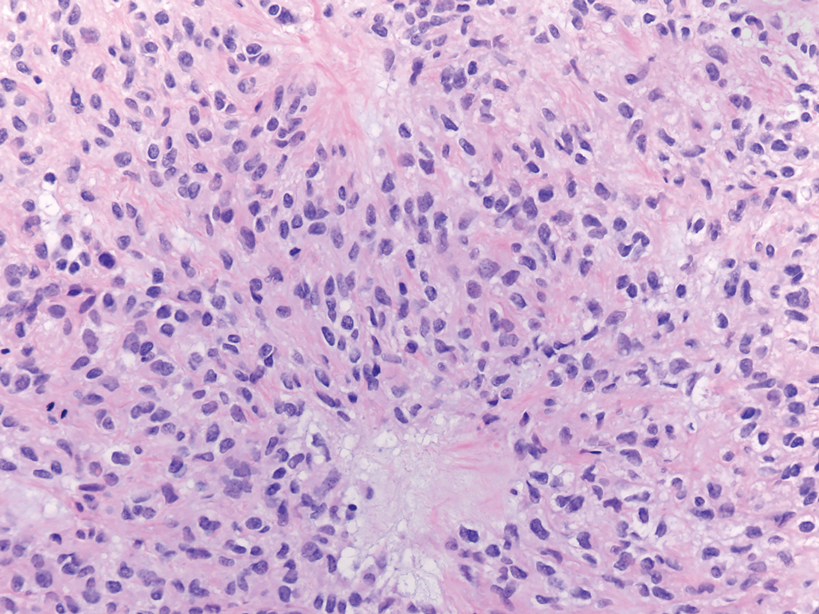
Myoepithelial carcinoma of the vulva is rare.11 Myoepithelial carcinoma of soft tissue is more aggressive than its benign counterpart, with up to a 50% metastasis rate.12 The presence of prominent corded or trabecular growth in a myxoid or hyaline background may point to the diagnosis (Figure 3). Similar to PES, myoepithelial carcinoma may lose expression of nuclear INI-1, while myoepithelial carcinoma is more likely to express S-100 and glial fibrillary acidic protein.13 Rearrangements of EWS RNA binding protein 1, EWSR1, have been found in half of myoepithelial neoplasms.12
Angiosarcomas represent 5% of cutaneous sarcomas and rarely have been reported in the vulva, primarily occurring in the setting of long-standing lymphedema and radiation.14 Angiosarcoma more often occurs on the head and neck, breasts, or extremities. Additional risk factors for the development of angiosarcoma include toxin exposure (eg, polyvinyl chloride, thorium dioxide, arsenic), anabolic steroids, and filariasis, as well as genetic disorders (eg, neurofibromatosis type 1, BRCA gene mutations, Maffucci syndrome).15 Epithelioid angiosarcoma is an infiltrative tumor composed of irregular anastomosing vascular channels with extravasated erythrocytes (Figure 4). Solid growth and necrosis may be present in more aggressive tumors. The cells themselves are pleomorphic endothelial cells with vesicular chromatin and prominent nucleoli. Epithelioid angiosarcoma may resemble carcinoma and have focal keratin expression. However, the characteristic eosinophilic cytoplasm seen in PES should not be identified in epithelioid angiosarcoma. Unlike PES, epithelioid angiosarcoma is positive for CD31 and has retained expression for INI-1. Both angiosarcoma and proximal-type epithelioid sarcoma may express vascular markers CD34 and FLI-116; thus an expanded panel of immunohistochemical studies may be of utility.
- Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130-146.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: a clinicopathological study of 20 cases. Mod Pathol. 2001;14:655-663.
- Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers. 2020:12:2112.
- Hoang LH, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291-302.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339-352.
- Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161-1168.
- Boer FL, Eikelder MLGT, Kapitejn EH, et al. Vulvar malignant melanoma: pathogenesis, clinical behavior and management: review of the literature. Cancer Treat Rev. 2019;73:91-103.
- Hieta N, Rintala SKM, Soderlund J, et al. Association of vulvar melanoma with lichen sclerosus. Acta Derm Venereol. 2019;99:339-340.
- Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;23:449-457.
- Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology. Elsevier Limited; 2021.
- Kyriazi MA, Carvounis EE, Kitsou M, et al. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: case report and review of literature. Int J Gynecol Pathol. 2010:29:501-504.
- Jo VY, Fletcher CD. Myoepithelial neoplasma of soft tissue: an updated review of the clinicopathological, immunophenotypic, and genetic features. Head Neck Pathol. 2015;9:32-38.
- Rekhi B, Sable M, Jambhekar NA. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461:687-697.
- Yost S, Bradish J, Grossheim L, et al. Epithelioid angiosarcoma of the vulva: a case report. Gynecol Oncol Rep. 2017;21:91-93.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Stockman DL, Hornick JL, Deavers MT, et al. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2014;27:496-501.
The Diagnosis: Proximal-Type Epithelioid Sarcoma
Proximal-type epithelioid sarcoma (PES) is a rare high-grade sarcoma of uncertain histogenesis that may present with a benign clinical appearance. Proximal-type epithelioid sarcoma peaks at 20 to 40 years of age and has a slight male predominance. This tumor exhibits aggressive behavior with both local recurrence and metastasis.1 The average overall survival is poor; however, tumor size less than 5 cm and complete excision with tumor-free margin status improves the prognosis.2 Proximal-type epithelioid sarcoma should not be confused with distal-type epithelioid sarcoma, which has a better prognosis and occurs in younger patients.1 Treatment of PES is wide excision, and chemotherapy trials with tazemetostat are ongoing.3
The deceptively banal clinical appearance of PES may delay appropriate diagnosis and treatment. Proximal-type epithelioid sarcoma often grows in sheets (quiz image [top] inset) and loose nests1 but also may take on a more corded appearance mimicking myoepithelial carcinoma. The cells themselves are plump dyscohesive epithelioid cells (quiz image [top]) with large nucleoli and eosinophilic cytoplasm or hyaline globules1 (quiz image [bottom]), but cells also may be focally spindled. Myxoid stroma, hemorrhage, and necrosis often are prominent features. Epithelioid sarcomas characteristically demonstrate positive immunostaining for both epithelial and mesenchymal markers (pan-cytokeratin and vimentin),1 with the majority having loss of expression of integrase interactor 1 (INI-1).2 Histology in this case was positive for cytokeratin monoclonal antibodies CAM5.2 and OSCAR, epithelial membrane antigen, and vimentin; it showed loss of INI-1 staining (quiz image [bottom]). Negative stains included S-100, p63, cytokeratins 7 and 20, CD34, CD31, ERG, glial fibrillary acidic protein, transducin-like enhancer of split 1, CD117, myogenin, synaptophysin, chromogranin, CD10, inhibin, CD99, and estrogen receptor.
The differential diagnosis of PES includes poorly differentiated squamous cell carcinoma (Figure 1 [inset]), melanoma, myoepithelial carcinoma, and epithelioid angiosarcoma. Primary squamous cell carcinoma of the vulva presents as an endophytic or exophytic mass with raised borders. Vulvar cancer is uncommon among gynecologic malignancies, with squamous cell carcinoma being the most commonly encountered.4 Vulvar intraepithelial neoplasia (VIN) is increasing in incidence, while the occurrence of invasive squamous cell carcinoma remains stable.5 Human papillomavirus–related VIN (usual-type VIN) is less likely to progress to squamous cell carcinoma than differentiated VIN (d-VIN), a dysplasia that is unrelated to human papillomavirus that frequently harbors p53 mutations.4 The presence of histologic epidermal involvement can help distinguish squamous cell carcinoma from PES (Figure 1). As opposed to PES, metastatic squamous cell carcinoma is characterized by intercellular bridges and often at least focal keratinization (Figure 1). Squamous cell carcinoma demonstrates positivity with p63 and p40 immunohistochemical stains, while PES rarely stains for either.6

Melanoma is the second most common vulvar malignancy. Vulvar melanoma tends to occur in women of advanced age but has been reported in girls as young as 10 years old.7 There is some evidence that patients with lichen sclerosus may be at an increased risk for the development of vulvar melanoma.8 Compared to PES, primary vulvar melanoma usually demonstrates epidermal involvement as well as clinical findings of a pigmented lesion (Figure 2). A notable minority of vulvar melanomas are amelanotic.9 Melanoma may be distinguished from PES with a panel of melanocytic markers—human melanoma black 45, Melan-A, SRY-box transcription factor 10, S-100, and microphthalmia transcription factor—that rarely are expressed in the latter. Both PES and rhabdoid melanoma have eosinophilic and tinctorial cytoplasmic inclusions.10 Melanin pigment and more cohesive nests are helpful clues that may point to melanoma when present.

Myoepithelial carcinoma of the vulva is rare.11 Myoepithelial carcinoma of soft tissue is more aggressive than its benign counterpart, with up to a 50% metastasis rate.12 The presence of prominent corded or trabecular growth in a myxoid or hyaline background may point to the diagnosis (Figure 3). Similar to PES, myoepithelial carcinoma may lose expression of nuclear INI-1, while myoepithelial carcinoma is more likely to express S-100 and glial fibrillary acidic protein.13 Rearrangements of EWS RNA binding protein 1, EWSR1, have been found in half of myoepithelial neoplasms.12

Angiosarcomas represent 5% of cutaneous sarcomas and rarely have been reported in the vulva, primarily occurring in the setting of long-standing lymphedema and radiation.14 Angiosarcoma more often occurs on the head and neck, breasts, or extremities. Additional risk factors for the development of angiosarcoma include toxin exposure (eg, polyvinyl chloride, thorium dioxide, arsenic), anabolic steroids, and filariasis, as well as genetic disorders (eg, neurofibromatosis type 1, BRCA gene mutations, Maffucci syndrome).15 Epithelioid angiosarcoma is an infiltrative tumor composed of irregular anastomosing vascular channels with extravasated erythrocytes (Figure 4). Solid growth and necrosis may be present in more aggressive tumors. The cells themselves are pleomorphic endothelial cells with vesicular chromatin and prominent nucleoli. Epithelioid angiosarcoma may resemble carcinoma and have focal keratin expression. However, the characteristic eosinophilic cytoplasm seen in PES should not be identified in epithelioid angiosarcoma. Unlike PES, epithelioid angiosarcoma is positive for CD31 and has retained expression for INI-1. Both angiosarcoma and proximal-type epithelioid sarcoma may express vascular markers CD34 and FLI-116; thus an expanded panel of immunohistochemical studies may be of utility.

The Diagnosis: Proximal-Type Epithelioid Sarcoma
Proximal-type epithelioid sarcoma (PES) is a rare high-grade sarcoma of uncertain histogenesis that may present with a benign clinical appearance. Proximal-type epithelioid sarcoma peaks at 20 to 40 years of age and has a slight male predominance. This tumor exhibits aggressive behavior with both local recurrence and metastasis.1 The average overall survival is poor; however, tumor size less than 5 cm and complete excision with tumor-free margin status improves the prognosis.2 Proximal-type epithelioid sarcoma should not be confused with distal-type epithelioid sarcoma, which has a better prognosis and occurs in younger patients.1 Treatment of PES is wide excision, and chemotherapy trials with tazemetostat are ongoing.3
The deceptively banal clinical appearance of PES may delay appropriate diagnosis and treatment. Proximal-type epithelioid sarcoma often grows in sheets (quiz image [top] inset) and loose nests1 but also may take on a more corded appearance mimicking myoepithelial carcinoma. The cells themselves are plump dyscohesive epithelioid cells (quiz image [top]) with large nucleoli and eosinophilic cytoplasm or hyaline globules1 (quiz image [bottom]), but cells also may be focally spindled. Myxoid stroma, hemorrhage, and necrosis often are prominent features. Epithelioid sarcomas characteristically demonstrate positive immunostaining for both epithelial and mesenchymal markers (pan-cytokeratin and vimentin),1 with the majority having loss of expression of integrase interactor 1 (INI-1).2 Histology in this case was positive for cytokeratin monoclonal antibodies CAM5.2 and OSCAR, epithelial membrane antigen, and vimentin; it showed loss of INI-1 staining (quiz image [bottom]). Negative stains included S-100, p63, cytokeratins 7 and 20, CD34, CD31, ERG, glial fibrillary acidic protein, transducin-like enhancer of split 1, CD117, myogenin, synaptophysin, chromogranin, CD10, inhibin, CD99, and estrogen receptor.
The differential diagnosis of PES includes poorly differentiated squamous cell carcinoma (Figure 1 [inset]), melanoma, myoepithelial carcinoma, and epithelioid angiosarcoma. Primary squamous cell carcinoma of the vulva presents as an endophytic or exophytic mass with raised borders. Vulvar cancer is uncommon among gynecologic malignancies, with squamous cell carcinoma being the most commonly encountered.4 Vulvar intraepithelial neoplasia (VIN) is increasing in incidence, while the occurrence of invasive squamous cell carcinoma remains stable.5 Human papillomavirus–related VIN (usual-type VIN) is less likely to progress to squamous cell carcinoma than differentiated VIN (d-VIN), a dysplasia that is unrelated to human papillomavirus that frequently harbors p53 mutations.4 The presence of histologic epidermal involvement can help distinguish squamous cell carcinoma from PES (Figure 1). As opposed to PES, metastatic squamous cell carcinoma is characterized by intercellular bridges and often at least focal keratinization (Figure 1). Squamous cell carcinoma demonstrates positivity with p63 and p40 immunohistochemical stains, while PES rarely stains for either.6

Melanoma is the second most common vulvar malignancy. Vulvar melanoma tends to occur in women of advanced age but has been reported in girls as young as 10 years old.7 There is some evidence that patients with lichen sclerosus may be at an increased risk for the development of vulvar melanoma.8 Compared to PES, primary vulvar melanoma usually demonstrates epidermal involvement as well as clinical findings of a pigmented lesion (Figure 2). A notable minority of vulvar melanomas are amelanotic.9 Melanoma may be distinguished from PES with a panel of melanocytic markers—human melanoma black 45, Melan-A, SRY-box transcription factor 10, S-100, and microphthalmia transcription factor—that rarely are expressed in the latter. Both PES and rhabdoid melanoma have eosinophilic and tinctorial cytoplasmic inclusions.10 Melanin pigment and more cohesive nests are helpful clues that may point to melanoma when present.

Myoepithelial carcinoma of the vulva is rare.11 Myoepithelial carcinoma of soft tissue is more aggressive than its benign counterpart, with up to a 50% metastasis rate.12 The presence of prominent corded or trabecular growth in a myxoid or hyaline background may point to the diagnosis (Figure 3). Similar to PES, myoepithelial carcinoma may lose expression of nuclear INI-1, while myoepithelial carcinoma is more likely to express S-100 and glial fibrillary acidic protein.13 Rearrangements of EWS RNA binding protein 1, EWSR1, have been found in half of myoepithelial neoplasms.12

Angiosarcomas represent 5% of cutaneous sarcomas and rarely have been reported in the vulva, primarily occurring in the setting of long-standing lymphedema and radiation.14 Angiosarcoma more often occurs on the head and neck, breasts, or extremities. Additional risk factors for the development of angiosarcoma include toxin exposure (eg, polyvinyl chloride, thorium dioxide, arsenic), anabolic steroids, and filariasis, as well as genetic disorders (eg, neurofibromatosis type 1, BRCA gene mutations, Maffucci syndrome).15 Epithelioid angiosarcoma is an infiltrative tumor composed of irregular anastomosing vascular channels with extravasated erythrocytes (Figure 4). Solid growth and necrosis may be present in more aggressive tumors. The cells themselves are pleomorphic endothelial cells with vesicular chromatin and prominent nucleoli. Epithelioid angiosarcoma may resemble carcinoma and have focal keratin expression. However, the characteristic eosinophilic cytoplasm seen in PES should not be identified in epithelioid angiosarcoma. Unlike PES, epithelioid angiosarcoma is positive for CD31 and has retained expression for INI-1. Both angiosarcoma and proximal-type epithelioid sarcoma may express vascular markers CD34 and FLI-116; thus an expanded panel of immunohistochemical studies may be of utility.

- Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130-146.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: a clinicopathological study of 20 cases. Mod Pathol. 2001;14:655-663.
- Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers. 2020:12:2112.
- Hoang LH, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291-302.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339-352.
- Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161-1168.
- Boer FL, Eikelder MLGT, Kapitejn EH, et al. Vulvar malignant melanoma: pathogenesis, clinical behavior and management: review of the literature. Cancer Treat Rev. 2019;73:91-103.
- Hieta N, Rintala SKM, Soderlund J, et al. Association of vulvar melanoma with lichen sclerosus. Acta Derm Venereol. 2019;99:339-340.
- Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;23:449-457.
- Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology. Elsevier Limited; 2021.
- Kyriazi MA, Carvounis EE, Kitsou M, et al. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: case report and review of literature. Int J Gynecol Pathol. 2010:29:501-504.
- Jo VY, Fletcher CD. Myoepithelial neoplasma of soft tissue: an updated review of the clinicopathological, immunophenotypic, and genetic features. Head Neck Pathol. 2015;9:32-38.
- Rekhi B, Sable M, Jambhekar NA. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461:687-697.
- Yost S, Bradish J, Grossheim L, et al. Epithelioid angiosarcoma of the vulva: a case report. Gynecol Oncol Rep. 2017;21:91-93.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Stockman DL, Hornick JL, Deavers MT, et al. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2014;27:496-501.
- Guillou L, Wadden C, Coindre JM, et al. “Proximal-type” epithelioid sarcoma, a distinctive aggressive neoplasm showing rhabdoid features. clinicopathologic, immunohistochemical, and ultrastructural study of a series. Am J Surg Pathol. 1997;21:130-146.
- Hasegawa T, Matsuno Y, Shimoda T, et al. Proximal-type epithelioid sarcoma: a clinicopathological study of 20 cases. Mod Pathol. 2001;14:655-663.
- Czarnecka AM, Sobczuk P, Kostrzanowski M, et al. Epithelioid sarcoma—from genetics to clinical practice. Cancers. 2020:12:2112.
- Hoang LH, Park KJ, Soslow RA, et al. Squamous precursor lesions of the vulva: current classification and diagnostic challenges. Pathology. 2016;48:291-302.
- Allbritton J. Vulvar neoplasms, benign and malignant. Obstet Gynecol Clin North Am. 2017;44:339-352.
- Laskin WB, Miettinen M. Epithelioid sarcoma: new insights based on an extended immunohistochemical analysis. Arch Pathol Lab Med. 2003;127:1161-1168.
- Boer FL, Eikelder MLGT, Kapitejn EH, et al. Vulvar malignant melanoma: pathogenesis, clinical behavior and management: review of the literature. Cancer Treat Rev. 2019;73:91-103.
- Hieta N, Rintala SKM, Soderlund J, et al. Association of vulvar melanoma with lichen sclerosus. Acta Derm Venereol. 2019;99:339-340.
- Edwards L. Pigmented vulvar lesions. Dermatol Ther. 2010;23:449-457.
- Patterson JW, Hosler GA, Prenshaw KL, eds. Weedon's Skin Pathology. Elsevier Limited; 2021.
- Kyriazi MA, Carvounis EE, Kitsou M, et al. Myoepithelial carcinoma of the vulva mimicking Bartholin gland abscess in a pregnant woman: case report and review of literature. Int J Gynecol Pathol. 2010:29:501-504.
- Jo VY, Fletcher CD. Myoepithelial neoplasma of soft tissue: an updated review of the clinicopathological, immunophenotypic, and genetic features. Head Neck Pathol. 2015;9:32-38.
- Rekhi B, Sable M, Jambhekar NA. Histopathological, immunohistochemical and molecular spectrum of myoepithelial tumours of soft tissues. Virchows Arch. 2012;461:687-697.
- Yost S, Bradish J, Grossheim L, et al. Epithelioid angiosarcoma of the vulva: a case report. Gynecol Oncol Rep. 2017;21:91-93.
- Young RJ, Brown NJ, Reed MW, et al. Angiosarcoma. Lancet Oncol. 2010;11:983-991.
- Stockman DL, Hornick JL, Deavers MT, et al. ERG and FLI1 protein expression in epithelioid sarcoma. Mod Pathol. 2014;27:496-501.
A 45-year-old woman with no notable medical history presented with a small nodule in the left pubic region lateral to the left labia majora. The lesion grew to 8 cm over the course of several months, and she underwent a simple excision for what clinically appeared to be a cyst.
Online Information About Hydroquinone: An Assessment of Accuracy and Readability
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1
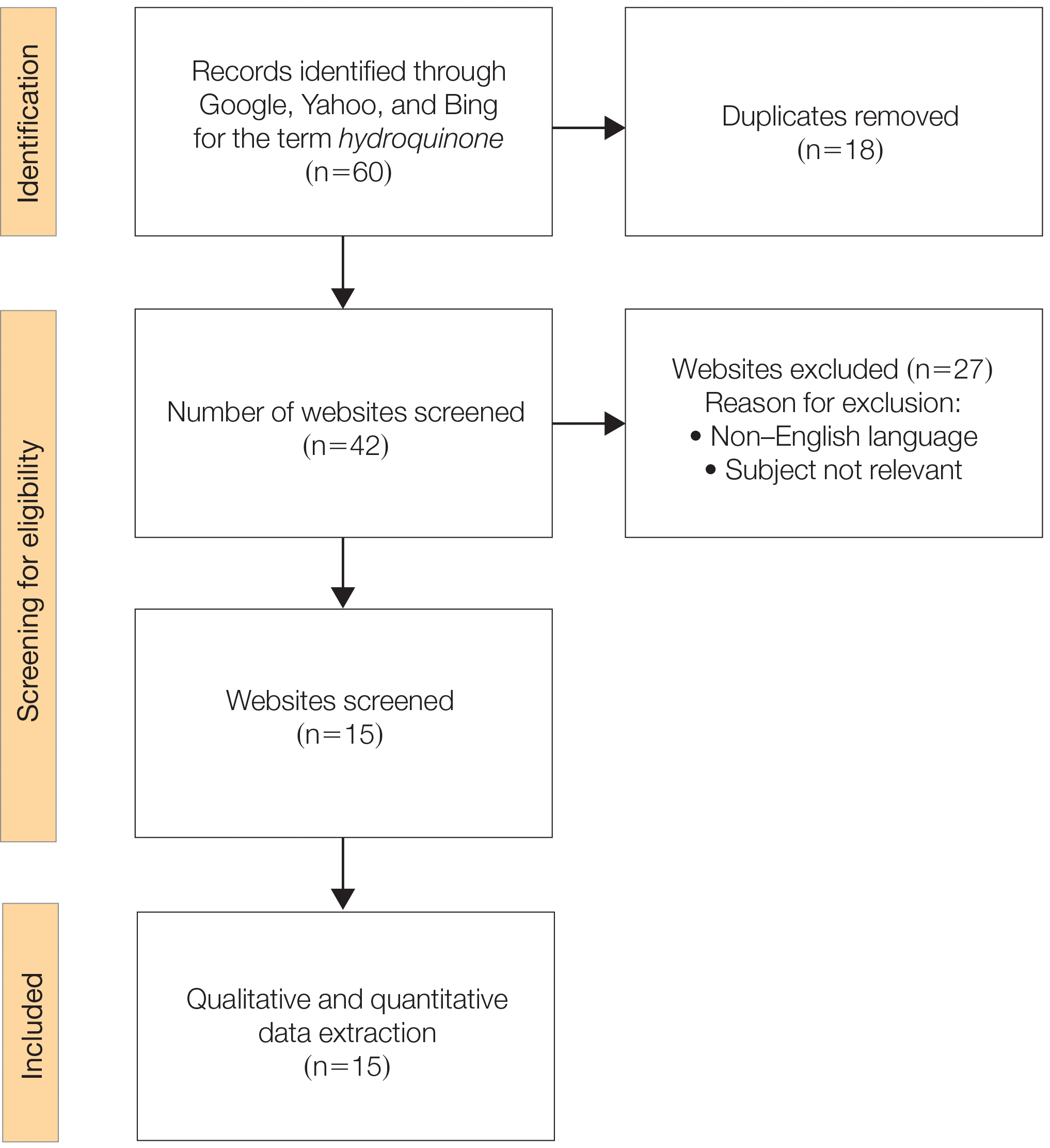
We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was
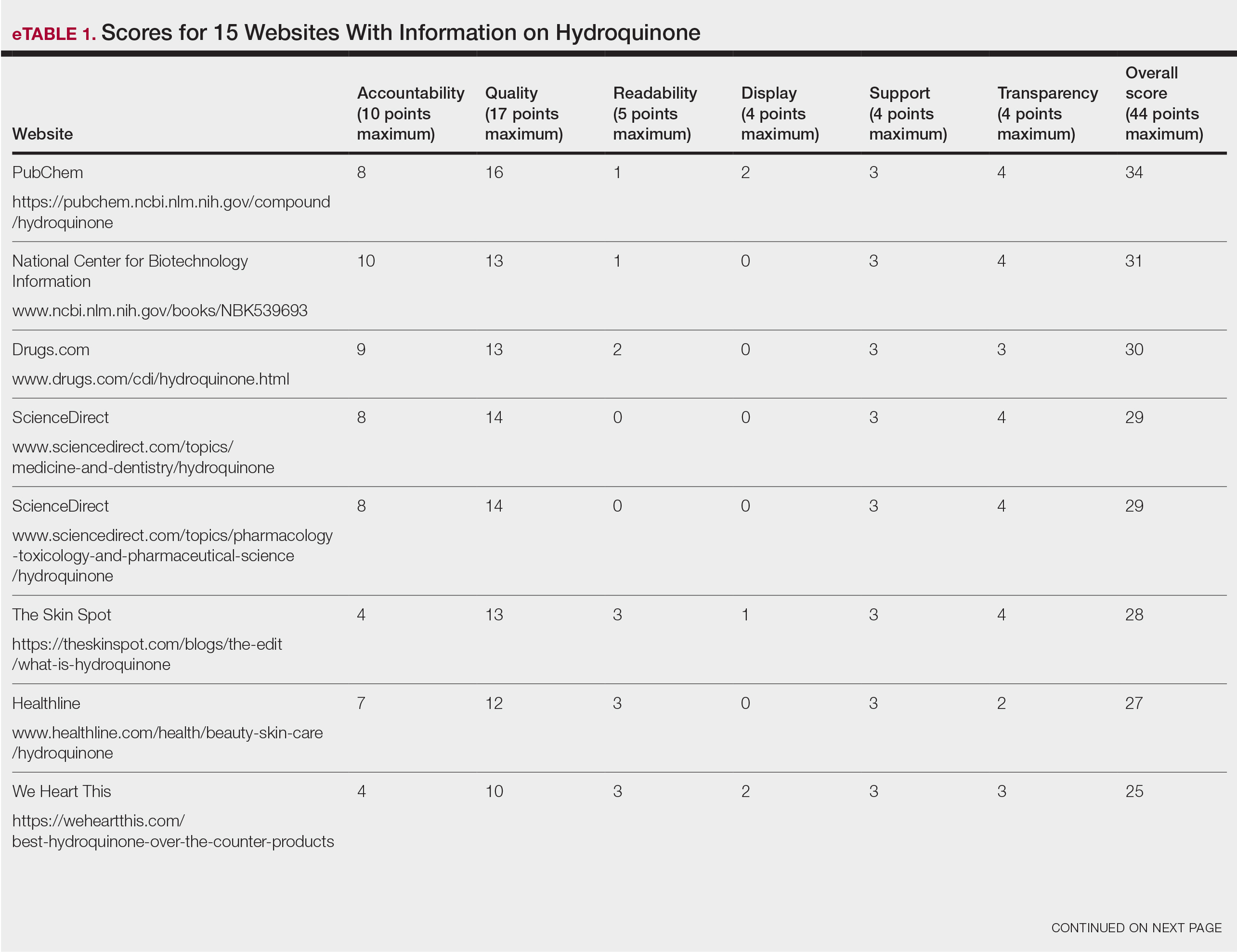
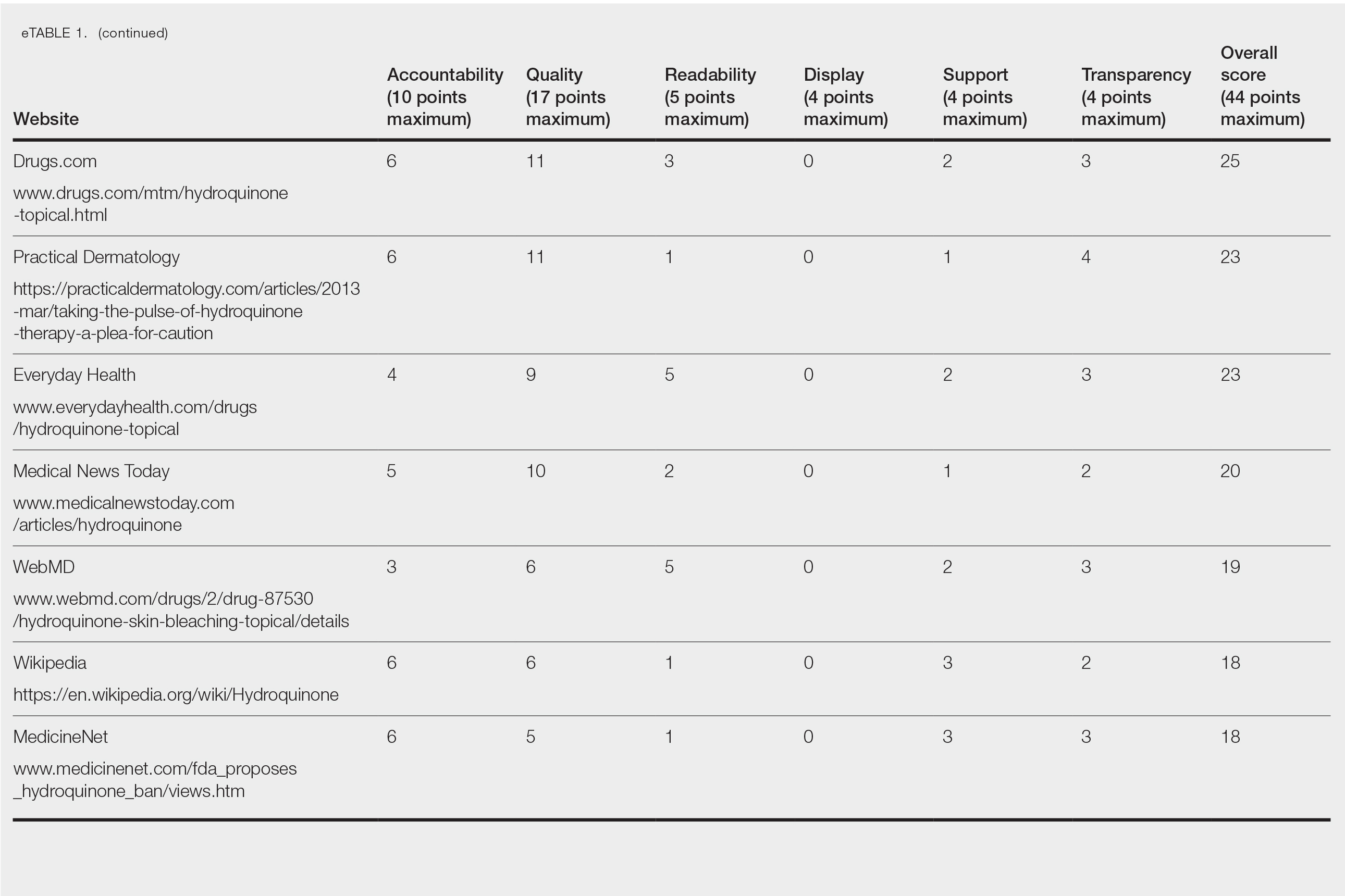
The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.
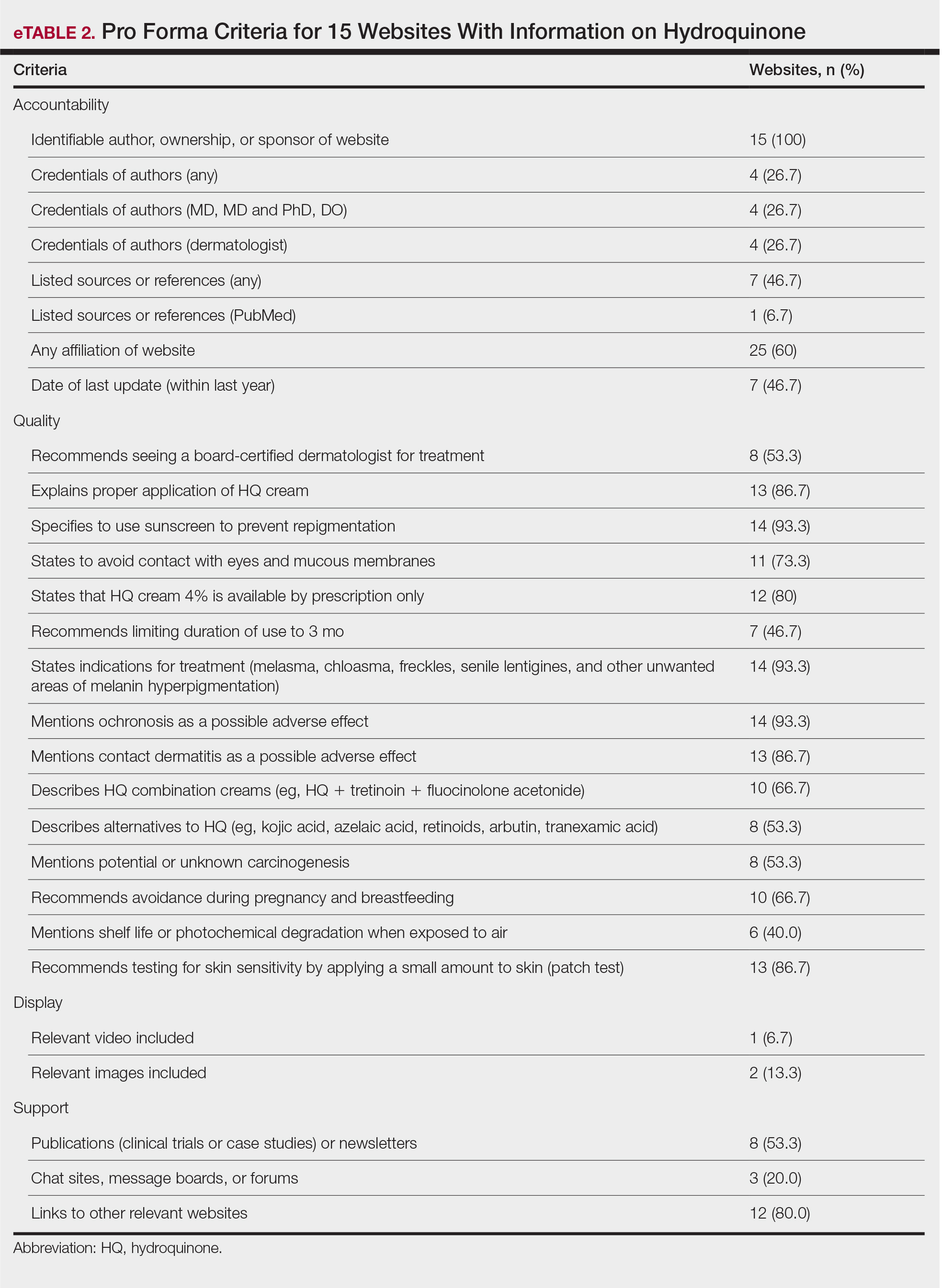
The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1

We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was


The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.

The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
To the Editor:
The internet is a popular resource for patients seeking information about dermatologic treatments. Hydroquinone (HQ) cream 4% is approved by the US Food and Drug Administration for skin hyperpigmentation.1 The agency enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform on September 25, 2020, to restrict distribution of OTC HQ.2 Exogenous ochronosis is listed as a potential adverse effect in the prescribing information for HQ.1

We sought to assess online resources on HQ for accuracy of information, including the recent OTC ban, as well as readability. The word hydroquinone was searched on 3 internet search engines—Google, Yahoo, and Bing—on December 12, 2020, each for the first 20 URLs (ie, websites)(total of 60 URLs). Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA)(Figure) reporting guidelines were used to assess a list of relevant websites to include in the final analysis. Website data were reviewed by both authors. Eighteen duplicates and 27 irrelevant and non–English-language URLs were excluded. The remaining 15 websites were analyzed. Based on a previously published and validated tool, a pro forma was designed to evaluate information on HQ for each website based on accountability, quality, readability, display, support, and transparency (Table).1,3

Scores for all 15 websites are listed in eTable 1. The mean overall (total) score was


The mean display score was 0.3 (of a possible 4; range, 0–2); 66.7% of websites (10/15) had advertisements or irrelevant material. Only 6.7% and 13.3% of websites included relevant videos or images, respectively, on applying HQ (eTable 2). We identified only 3 photographs—across all 15 websites—that depicted skin, all of which were Fitzpatrick skin types II or III. Therefore, none of the websites included a diversity of images to indicate broad ethnic relatability.

The average support score was 2.5 (of a possible 4; range, 1–3); 20% (3/15) of URLs included chat sites, message boards, or forums, and approximately half (8/15 [53.3%]) included references. Only 7 URLs (46.7%) had been updated in the last 12 months. Only 4 (26.7%) were written by a board-certified dermatologist (eTable 2). Most (60%) websites contained advertising, though none were sponsored by a pharmaceutical company that manufactures HQ.
Only 46.7% (7/15) of websites recommended limiting a course of HQ treatment to 3 months; only 40% (6/15) mentioned shelf life or photochemical degradation when exposed to air. Although 93.3% (14/15) of URLs mentioned ochronosis, a clinical description of the condition was provided in only 33.3% (5/15)—none with images.
Only 2 sites (13.3%; Everyday Health and WebMD) met the accepted 7th-grade reading level for online patient education material; those sites scored lower on quality (9 of 17 and 6 of 17, respectively) than sites with higher overall scores.
None of the 15 websites studied, therefore, demonstrated optimal features on combined measures of accountability, quality, readability, display, support, and transparency regarding HQ. Notably, the American Academy of Dermatology website (www.aad.org) was not among the 15 websites studied; the AAD website mentions HQ in a section on melasma, but only minimal detail is provided.
Limitations of this study include the small number of websites analyzed and possible selection bias because only 3 internet search engines were used to identify websites for study and analysis.
Previously, we analyzed content about HQ on the video-sharing and social media platform YouTube.4 The most viewed YouTube videos on HQ had poor-quality information (ie, only 20% mentioned ochronosis and only 28.6% recommended sunscreen [N=70]). However, average reading level of these videos was 7th grade.4,5 Therefore, YouTube HQ content, though comprehensible, generally is of poor quality.
By conducting a search for website content about HQ, we found that the most popular URLs had either accurate information with poor readability or lower-quality educational material that was more comprehensible. We conclude that there is a need to develop online patient education materials on HQ that are characterized by high-quality, up-to-date medical information; have been written by board-certified dermatologists; are comprehensible (ie, no more than approximately 1200 words and written at a 7th-grade reading level); and contain relevant clinical images and references. We encourage dermatologists to recognize the limitations of online patient education resources on HQ and educate patients on the proper use of the drug as well as its potential adverse effects
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
- US National Library of Medicine. Label: hydroquinone cream. DailyMed website. Updated November 24, 2020. Accessed May 19, 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=dc72c0b2-4505-4dcf-8a69-889cd9f41693
- US Congress. H.R.748 - CARES Act. 116th Congress (2019-2020). Updated March 27, 2020. Accessed May 19, 2022. https://www.congress.gov/bill/116th-congress/house-bill/748/text?fbclid=IwAR3ZxGP6AKUl6ce-dlWSU6D5MfCLD576nWNBV5YTE7R2a0IdLY4Usw4oOv4
- Kang R, Lipner S. Evaluation of onychomycosis information on the internet. J Drugs Dermatol. 2019;18:484-487.
- Ishack S, Lipner SR. Assessing the impact and educational value of YouTube as a source of information on hydroquinone: a content-quality and readability analysis. J Dermatolog Treat. 2020:1-3. doi:10.1080/09546634.2020.1782318
- Weiss BD. Health Literacy: A Manual for Clinicians. American Medical Association Foundation and American Medical Association; 2003. Accessed May 19, 2022. http://lib.ncfh.org/pdfs/6617.pdf
Practice Points
- Hydroquinone (HQ) 4% is US Food and Drug Administration (FDA) approved for skin hyperpigmentation including melasma.
- In September 2020, the FDA enforced the CARES (Coronavirus Aid, Relief, and Economic Security) Act and OTC (over-the-counter) Monograph Reform, announcing that HQ is not classified as Category II/not generally recognized as safe and effective, thus prohibiting the distribution of OTC HQ products.
- Exogenous ochronosis is a potential side effect associated with HQ.
- There is a need for dermatologists to develop online patient education materials on HQ that are characterized by high-quality and up-to-date medical information.
Cutaneous Body Image: How the Mental Health Benefits of Treating Dermatologic Disease Support Military Readiness in Service Members
According to the US Department of Defense, the term readiness refers to the ability to recruit, train, deploy, and sustain military forces that will be ready to “fight tonight” and succeed in combat. Readiness is a top priority for military medicine, which functions to diagnose, treat, and rehabilitate service members so that they can return to the fight. This central concept drives programs across the military—from operational training events to the establishment of medical and dental standards. Readiness is tracked and scrutinized constantly, and although it is a shared responsibility, efforts to increase and sustain readiness often fall on support staff and military medical providers.
In recent years, there has been a greater awareness of the negative effects of mental illness, low morale, and suicidality on military readiness. In 2013, suicide accounted for 28.1% of all deaths that occurred in the US Armed Forces.1 Put frankly, suicide was one of the leading causes of death among military members.
The most recent Marine Corps Order regarding the Marine Corps Suicide Prevention Program stated that “suicidal behaviors are a barrier to readiness that have lasting effects on Marines and Service Members attached to Marine Commands. . .Families, and the Marine Corps.” It goes on to say that “[e]ffective suicide prevention requires coordinated efforts within a prevention framework dedicated to promoting mental, physical, spiritual, and social fitness. . .[and] mitigating stressors that interfere with mission readiness.”2 This statement supports the notion that preventing suicide is not just about treating mental illness; it also involves maximizing physical, spiritual, and social fitness. Although it is well established that various mental health disorders are associated with an increased risk for suicide, it is worth noting that, in one study, only half of individuals who died by suicide had a mental health disorder diagnosed prior to their death.3 These statistics translate to the military. The 2015 Department of Defense Suicide Event Report noted that only 28% of service members who died by suicide and 22% of members with attempted suicide had been documented as having sought mental health care and disclosed their potential for self-harm prior to the event.1,4 In 2018, a study published by Ursano et al5 showed that 36.3% of US soldiers with a documented suicide attempt (N=9650) had no prior mental health diagnoses.
Expanding the scope to include mental health issues in general, only 29% of service members who reported experiencing a mental health problem actually sought mental health care in that same period. Overall, approximately 40% of service members with a reported perceived need for mental health care actually sought care over their entire course of service time,1 which raises concern for a large population of undiagnosed and undertreated mental illnesses across the military. In response to these statistics, Reger et al3 posited that it is “essential that suicide prevention efforts move outside the silo of mental health.” The authors went on to challenge health care providers across all specialties and civilians alike to take responsibility in understanding, recognizing, and mitigating risk factors for suicide in the general population.3 Although treating a service member’s acne or offering to stand duty for a service member who has been under a great deal of stress in their personal life may appear to be indirect ways of reducing suicide in the US military, they actually may be the most critical means of prevention in a culture that emphasizes resilience and self-reliance, where seeking help for mental health struggles could be perceived as weakness.1
In this review article, we discuss the concept of cutaneous body image (CBI) and its associated outcomes on health, satisfaction, and quality of life in military service members. We then examine the intersections between common dermatologic conditions, CBI, and mental health and explore the ability and role of the military dermatologist to serve as a positive influence on military readiness.
What is cutaneous body image?
Cutaneous body image is “the individual’s mental perception of his or her skin and its appendages (ie, hair, nails).”6 It is measured objectively using the Cutaneous Body Image Scale, a questionnaire that includes 7 items related to the overall satisfaction with the appearance of skin, color of skin, skin of the face, complexion of the face, hair, fingernails, and toenails. Each question is rated using a 10-point Likert scale (0=not at all; 10=very markedly).6
Some degree of CBI dissatisfaction is expected and has been shown in the general population at large; for example, more than 56% of women older than 30 years report some degree of dissatisfaction with their skin. Similarly, data from the American Society of Plastic Surgeons showed that while 10.9 million cosmetic procedures were performed in 2006, 9.1 million of them involved minimally invasive procedures such as botulinum toxin type A injections with the purpose of skin rejuvenation and improvement of facial appearance.7 However, lower than average CBI can contribute to considerable psychosocial morbidity. Dissatisfaction with CBI is associated with self-consciousness, feelings of inferiority, and social exclusion. These symptoms can be grouped into a construct called interpersonal sensitivity (IS). A 2013 study by Gupta and Gupta6 investigated the relationship between CBI, IS, and suicidal ideation among 312 consenting nonclinical participants in Canada. The study found that greater dissatisfaction with an individual’s CBI correlated to increased IS and increased rates of suicidal ideation and intentional self-injury.6
Cutaneous body image is particularly relevant to dermatologists, as many common dermatoses can cause cosmetically disfiguring skin conditions; for example, acne and rosacea have the propensity to cause notable disfigurement to the facial unit. Other common conditions such as atopic dermatitis or psoriasis can flare with stress and thereby throw patients into a vicious cycle of physical and psychosocial stress caused by social stigma, cosmetic disfigurement, and reduced CBI, in turn leading to worsening of the disease at hand. Dermatologists need to be aware that common dermatoses can impact a patient’s mental health via poor CBI.8 Similarly, dermatologists may be empowered by the awareness that treating common dermatoses, especially those associated with poor cosmesis, have 2-fold benefits—on the skin condition itself and on the patient’s mental health.
How are common dermatoses associated with mental health?
Acne—Acne is one of the most common skin diseases, so much so that in many cases acne has become an accepted and expected part of adolescence and young adulthood. Studies estimate that 85% of the US population aged 12 to 25 years have acne.9 For some adults, acne persists even longer, with 1% to 5% of adults reporting to have active lesions at 40 years of age.10 Acne is a multifactorial skin disease of the pilosebaceous unit that results in the development of inflammatory papules, pustules, and cysts. These lesions are most common on the face but can extend to other areas of the body, such as the chest and back.11 Although the active lesions can be painful and disfiguring, if left untreated, acne may lead to permanent disfigurement and scarring, which can have long-lasting psychosocial impacts.
Individuals with acne have an increased likelihood of self-consciousness, social isolation, depression, and suicidal ideation. This relationship has been well established for decades. In the 1990s, a small study reported that 7 of 16 (43.8%) cases of completed suicide in dermatology patients were in patients with acne.12 In a recent meta-analysis including 2,276,798 participants across 5 separate studies, researchers found that suicide was positively associated with acne, carrying an odds ratio of 1.50 (95% CI, 1.09-2.06).13
Rosacea—Rosacea is a common chronic inflammatory skin disease characterized by facial erythema, telangiectasia, phymatous changes, papules, pustules, and ocular irritation. The estimated worldwide prevalence is 5.5%.14 In addition to discomfort and irritation of the skin and eyes, rosacea often carries a higher risk of psychological and psychosocial distress due to its potentially disfiguring nature. Rosacea patients are at greater risk for having anxiety disorders and depression,15 and a 2018 study by Alinia et al16 showed that there is a direct relationship between rosacea severity and the actual level of depression.Although disease improvement certainly leads to improvements in quality of life and psychosocial status, Alinia et al16 noted that depression often is associated with poor treatment adherence due to poor motivation and hopelessness. It is critical that dermatologists are aware of these associations and maintain close follow-up with patients, even when the condition is not life-threatening, such as rosacea.
Hidradenitis Suppurativa—Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit that is characterized by the development of painful, malodorous, draining abscesses, fistulas, sinus tracts, and scars in sensitive areas such as the axillae, breasts, groin, and perineum.17 In severe cases, surgery may be required to excise affected areas. Compared to other cutaneous disease, HS is considered one of the most life-impacting disorders.18 The physical symptoms themselves often are debilitating, and patients often report considerable psychosocial and psychological impairment with decreased quality of life. Major depression frequently is noted, with 1 in 4 adults with HS also being depressed. In a large cross-sectional analysis of 38,140 adults and 1162 pediatric patients with HS, Wright et al17 reported the prevalence of depression among adults with HS as 30.0% compared to 16.9% in healthy controls. In children, the prevalence of depression was 11.7% compared to 4.1% in the general population.17 Similarly, 1 out of every 5 patients with HS experiences anxiety.18
In the military population, HS often can be duty limiting. The disease requires constant attention to wound care and frequent medical visits. For many service members operating in field training or combat environments, opportunities for and access to showers and basic hygiene is limited. Uniforms and additional necessary combat gear often are thick and occlusive. Taken as a whole, these factors may contribute to worsening of the disease and in severe cases are simply not conducive to the successful management of the condition. However, given the most commonly involved body areas and the nature of the disease, many service members with HS may feel embarrassed to disclose their condition. In uniform, the disease is not easily visible, and for unaware persons, the frequency of medical visits and limited duty status may seem unnecessary. This perception of a service member’s lack of productivity due to an unseen disease may further add to the psychosocial stress they experience.
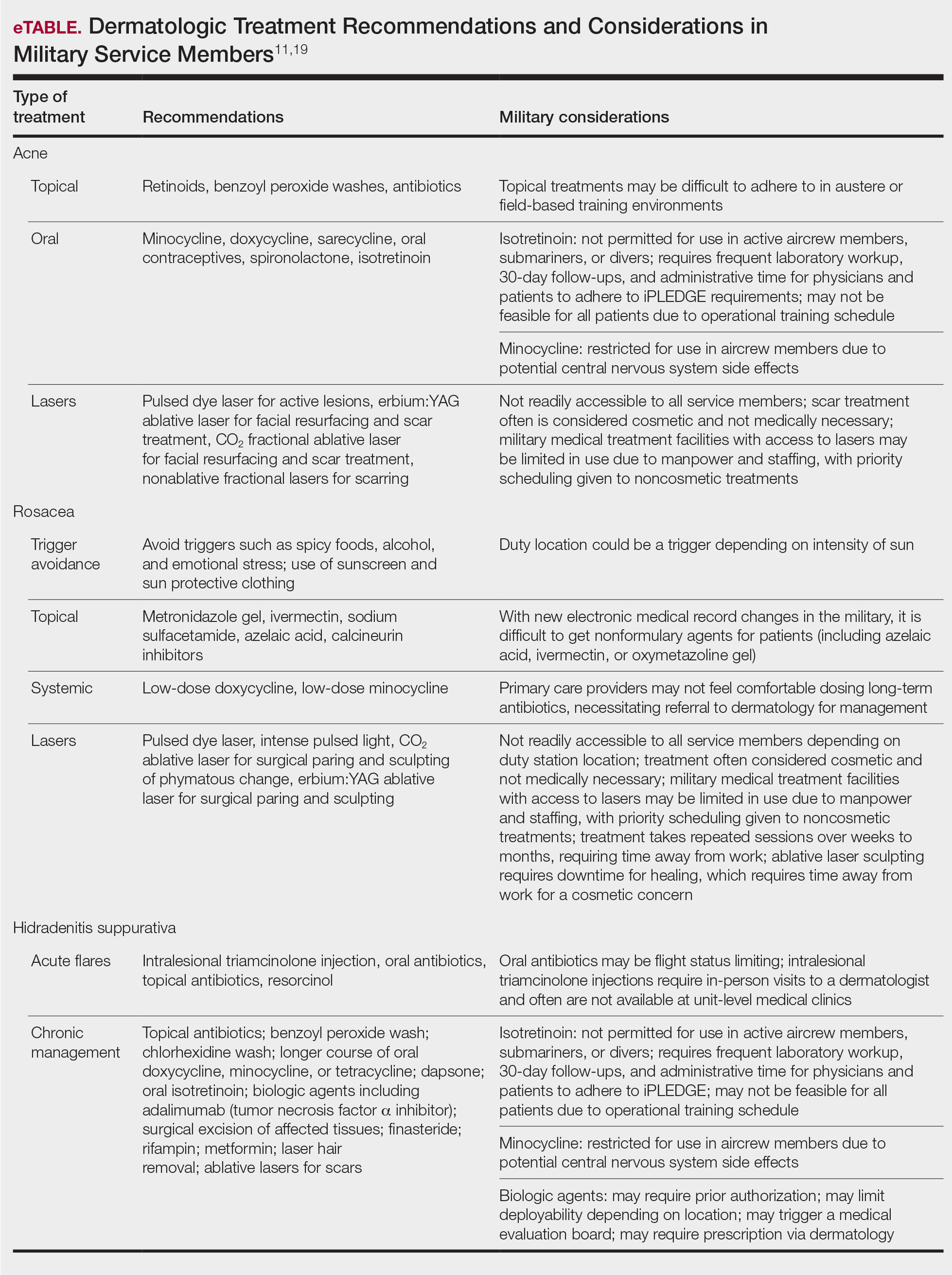
The treatments for acne, rosacea, and HS are outlined in the eTable.11,19 Also noted are specific considerations when managing an active-duty service member due to various operational duty restrictions and constraints.
Final Thoughts
Maintaining readiness in the military is essential to the ability to not only “fight tonight” but also to win tonight in whatever operational or combat mission a service member may be. Although many factors impact readiness, the rates of suicide within the armed forces cannot be ignored. Suicide not only eliminates the readiness of the deceased service member but has lasting ripple effects on the overall readiness of their unit and command at large. Most suicides in the military occur in personnel with no prior documented mental health diagnoses or treatment. Therefore, it is the responsibility of all service members to recognize and mitigate stressors and risk factors that may lead to mental health distress and suicidality. In the medical corps, this translates to a responsibility of all medical specialists to recognize and understand unique risk factors for suicidality and to do as much as they can to reduce these risks. For military dermatologists and for civilian physicians treating military service members, it is imperative to predict and understand the relationship between common dermatoses; reduced satisfaction with CBI; and increased risk for mental health illness, self-harm, and suicide. Military dermatologists, as well as other specialists, may be limited in the care they are able to provide due to manpower, staffing, demand, and institutional guidelines; however, to better serve those who serve in a holistic manner, consideration must be given to rethink what is “medically essential” and “cosmetic” and leverage the available skills, techniques, and equipment to increase the readiness of the force.

- Ghahramanlou-Holloway M, LaCroix JM, Koss K, et al. Outpatient mental health treatment utilization and military career impact in the United States Marine Corps. Int J Environ Res Public Health. 2018;15:828. doi:10.3390/ijerph15040828
- Ottignon DA. Marine Corps Suicide Prevention System (MCSPS). Marine Corps Order 1720.2A. 2021. Headquarters United States Marine Corps. Published August 2, 2021. Accessed May 25, 2022. https://www.marines.mil/Portals/1/Publications/MCO%201720.2A.pdf?ver=QPxZ_qMS-X-d037B65N9Tg%3d%3d
- Reger MA, Smolenski DJ, Carter SP. Suicide prevention in the US Army: a mission for more than mental health clinicians. JAMA Psychiatry. 2018;75:991-992. doi:10.1001/jamapsychiatry.2018.2042
- Pruitt LD, Smolenski DJ, Bush NE, et al. Department of Defense Suicide Event Report Calendar Year 2015 Annual Report. National Center for Telehealth & Technology (T2); 2016. Accessed May 20, 2022. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Psychological-Health-Center-of-Excellence/Department-of-Defense-Suicide-Event-Report
- Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry. 2018;75:1022-1032. doi:10.1001/jamapsychiatry.2018.2069
- Gupta MA, Gupta AK. Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J Psychosom Res. 2013;75:55-59. doi:10.1016/j.jpsychores.2013.01.015
- Gupta MA, Gupta AK. Evaluation of cutaneous body image dissatisfaction in the dermatology patient. Clin Dermatol. 2013;31:72-79. doi:10.1016/j.clindermatol.2011.11.010
- Hinkley SB, Holub SC, Menter A. The validity of cutaneous body image as a construct and as a mediator of the relationship between cutaneous disease and mental health. Dermatol Ther (Heidelb). 2020;10:203-211. doi:10.1007/s13555-020-00351-5
- Stamu-O’Brien C, Jafferany M, Carniciu S, et al. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20:1080-1083. doi:10.1111/jocd.13765
- Sood S, Jafferany M, Vinaya Kumar S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19:3177-3182. doi:10.1111/jocd.13753
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246-250.
- Xu S, Zhu Y, Hu H, et al. The analysis of acne increasing suicide risk. Medicine (Baltimore). 2021;100:E26035. doi:10.1097/MD.0000000000026035
- Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China [published online June 16, 2021]. Front Psychiatry. doi:10.3389/fpsyt.2021.659171
- Incel Uysal P, Akdogan N, Hayran Y, et al. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94:704-709. doi:10.1016/j.abd.2019.03.002
- Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102:36-38.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.06.843
- Misitzis A, Goldust M, Jafferany M, et al. Psychiatric comorbidities in patients with hidradenitis suppurativa. Dermatol Ther. 2020;33:E13541. doi:10.1111/dth.13541
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017.
According to the US Department of Defense, the term readiness refers to the ability to recruit, train, deploy, and sustain military forces that will be ready to “fight tonight” and succeed in combat. Readiness is a top priority for military medicine, which functions to diagnose, treat, and rehabilitate service members so that they can return to the fight. This central concept drives programs across the military—from operational training events to the establishment of medical and dental standards. Readiness is tracked and scrutinized constantly, and although it is a shared responsibility, efforts to increase and sustain readiness often fall on support staff and military medical providers.
In recent years, there has been a greater awareness of the negative effects of mental illness, low morale, and suicidality on military readiness. In 2013, suicide accounted for 28.1% of all deaths that occurred in the US Armed Forces.1 Put frankly, suicide was one of the leading causes of death among military members.
The most recent Marine Corps Order regarding the Marine Corps Suicide Prevention Program stated that “suicidal behaviors are a barrier to readiness that have lasting effects on Marines and Service Members attached to Marine Commands. . .Families, and the Marine Corps.” It goes on to say that “[e]ffective suicide prevention requires coordinated efforts within a prevention framework dedicated to promoting mental, physical, spiritual, and social fitness. . .[and] mitigating stressors that interfere with mission readiness.”2 This statement supports the notion that preventing suicide is not just about treating mental illness; it also involves maximizing physical, spiritual, and social fitness. Although it is well established that various mental health disorders are associated with an increased risk for suicide, it is worth noting that, in one study, only half of individuals who died by suicide had a mental health disorder diagnosed prior to their death.3 These statistics translate to the military. The 2015 Department of Defense Suicide Event Report noted that only 28% of service members who died by suicide and 22% of members with attempted suicide had been documented as having sought mental health care and disclosed their potential for self-harm prior to the event.1,4 In 2018, a study published by Ursano et al5 showed that 36.3% of US soldiers with a documented suicide attempt (N=9650) had no prior mental health diagnoses.
Expanding the scope to include mental health issues in general, only 29% of service members who reported experiencing a mental health problem actually sought mental health care in that same period. Overall, approximately 40% of service members with a reported perceived need for mental health care actually sought care over their entire course of service time,1 which raises concern for a large population of undiagnosed and undertreated mental illnesses across the military. In response to these statistics, Reger et al3 posited that it is “essential that suicide prevention efforts move outside the silo of mental health.” The authors went on to challenge health care providers across all specialties and civilians alike to take responsibility in understanding, recognizing, and mitigating risk factors for suicide in the general population.3 Although treating a service member’s acne or offering to stand duty for a service member who has been under a great deal of stress in their personal life may appear to be indirect ways of reducing suicide in the US military, they actually may be the most critical means of prevention in a culture that emphasizes resilience and self-reliance, where seeking help for mental health struggles could be perceived as weakness.1
In this review article, we discuss the concept of cutaneous body image (CBI) and its associated outcomes on health, satisfaction, and quality of life in military service members. We then examine the intersections between common dermatologic conditions, CBI, and mental health and explore the ability and role of the military dermatologist to serve as a positive influence on military readiness.
What is cutaneous body image?
Cutaneous body image is “the individual’s mental perception of his or her skin and its appendages (ie, hair, nails).”6 It is measured objectively using the Cutaneous Body Image Scale, a questionnaire that includes 7 items related to the overall satisfaction with the appearance of skin, color of skin, skin of the face, complexion of the face, hair, fingernails, and toenails. Each question is rated using a 10-point Likert scale (0=not at all; 10=very markedly).6
Some degree of CBI dissatisfaction is expected and has been shown in the general population at large; for example, more than 56% of women older than 30 years report some degree of dissatisfaction with their skin. Similarly, data from the American Society of Plastic Surgeons showed that while 10.9 million cosmetic procedures were performed in 2006, 9.1 million of them involved minimally invasive procedures such as botulinum toxin type A injections with the purpose of skin rejuvenation and improvement of facial appearance.7 However, lower than average CBI can contribute to considerable psychosocial morbidity. Dissatisfaction with CBI is associated with self-consciousness, feelings of inferiority, and social exclusion. These symptoms can be grouped into a construct called interpersonal sensitivity (IS). A 2013 study by Gupta and Gupta6 investigated the relationship between CBI, IS, and suicidal ideation among 312 consenting nonclinical participants in Canada. The study found that greater dissatisfaction with an individual’s CBI correlated to increased IS and increased rates of suicidal ideation and intentional self-injury.6
Cutaneous body image is particularly relevant to dermatologists, as many common dermatoses can cause cosmetically disfiguring skin conditions; for example, acne and rosacea have the propensity to cause notable disfigurement to the facial unit. Other common conditions such as atopic dermatitis or psoriasis can flare with stress and thereby throw patients into a vicious cycle of physical and psychosocial stress caused by social stigma, cosmetic disfigurement, and reduced CBI, in turn leading to worsening of the disease at hand. Dermatologists need to be aware that common dermatoses can impact a patient’s mental health via poor CBI.8 Similarly, dermatologists may be empowered by the awareness that treating common dermatoses, especially those associated with poor cosmesis, have 2-fold benefits—on the skin condition itself and on the patient’s mental health.
How are common dermatoses associated with mental health?
Acne—Acne is one of the most common skin diseases, so much so that in many cases acne has become an accepted and expected part of adolescence and young adulthood. Studies estimate that 85% of the US population aged 12 to 25 years have acne.9 For some adults, acne persists even longer, with 1% to 5% of adults reporting to have active lesions at 40 years of age.10 Acne is a multifactorial skin disease of the pilosebaceous unit that results in the development of inflammatory papules, pustules, and cysts. These lesions are most common on the face but can extend to other areas of the body, such as the chest and back.11 Although the active lesions can be painful and disfiguring, if left untreated, acne may lead to permanent disfigurement and scarring, which can have long-lasting psychosocial impacts.
Individuals with acne have an increased likelihood of self-consciousness, social isolation, depression, and suicidal ideation. This relationship has been well established for decades. In the 1990s, a small study reported that 7 of 16 (43.8%) cases of completed suicide in dermatology patients were in patients with acne.12 In a recent meta-analysis including 2,276,798 participants across 5 separate studies, researchers found that suicide was positively associated with acne, carrying an odds ratio of 1.50 (95% CI, 1.09-2.06).13
Rosacea—Rosacea is a common chronic inflammatory skin disease characterized by facial erythema, telangiectasia, phymatous changes, papules, pustules, and ocular irritation. The estimated worldwide prevalence is 5.5%.14 In addition to discomfort and irritation of the skin and eyes, rosacea often carries a higher risk of psychological and psychosocial distress due to its potentially disfiguring nature. Rosacea patients are at greater risk for having anxiety disorders and depression,15 and a 2018 study by Alinia et al16 showed that there is a direct relationship between rosacea severity and the actual level of depression.Although disease improvement certainly leads to improvements in quality of life and psychosocial status, Alinia et al16 noted that depression often is associated with poor treatment adherence due to poor motivation and hopelessness. It is critical that dermatologists are aware of these associations and maintain close follow-up with patients, even when the condition is not life-threatening, such as rosacea.
Hidradenitis Suppurativa—Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit that is characterized by the development of painful, malodorous, draining abscesses, fistulas, sinus tracts, and scars in sensitive areas such as the axillae, breasts, groin, and perineum.17 In severe cases, surgery may be required to excise affected areas. Compared to other cutaneous disease, HS is considered one of the most life-impacting disorders.18 The physical symptoms themselves often are debilitating, and patients often report considerable psychosocial and psychological impairment with decreased quality of life. Major depression frequently is noted, with 1 in 4 adults with HS also being depressed. In a large cross-sectional analysis of 38,140 adults and 1162 pediatric patients with HS, Wright et al17 reported the prevalence of depression among adults with HS as 30.0% compared to 16.9% in healthy controls. In children, the prevalence of depression was 11.7% compared to 4.1% in the general population.17 Similarly, 1 out of every 5 patients with HS experiences anxiety.18
In the military population, HS often can be duty limiting. The disease requires constant attention to wound care and frequent medical visits. For many service members operating in field training or combat environments, opportunities for and access to showers and basic hygiene is limited. Uniforms and additional necessary combat gear often are thick and occlusive. Taken as a whole, these factors may contribute to worsening of the disease and in severe cases are simply not conducive to the successful management of the condition. However, given the most commonly involved body areas and the nature of the disease, many service members with HS may feel embarrassed to disclose their condition. In uniform, the disease is not easily visible, and for unaware persons, the frequency of medical visits and limited duty status may seem unnecessary. This perception of a service member’s lack of productivity due to an unseen disease may further add to the psychosocial stress they experience.
The treatments for acne, rosacea, and HS are outlined in the eTable.11,19 Also noted are specific considerations when managing an active-duty service member due to various operational duty restrictions and constraints.

Final Thoughts
Maintaining readiness in the military is essential to the ability to not only “fight tonight” but also to win tonight in whatever operational or combat mission a service member may be. Although many factors impact readiness, the rates of suicide within the armed forces cannot be ignored. Suicide not only eliminates the readiness of the deceased service member but has lasting ripple effects on the overall readiness of their unit and command at large. Most suicides in the military occur in personnel with no prior documented mental health diagnoses or treatment. Therefore, it is the responsibility of all service members to recognize and mitigate stressors and risk factors that may lead to mental health distress and suicidality. In the medical corps, this translates to a responsibility of all medical specialists to recognize and understand unique risk factors for suicidality and to do as much as they can to reduce these risks. For military dermatologists and for civilian physicians treating military service members, it is imperative to predict and understand the relationship between common dermatoses; reduced satisfaction with CBI; and increased risk for mental health illness, self-harm, and suicide. Military dermatologists, as well as other specialists, may be limited in the care they are able to provide due to manpower, staffing, demand, and institutional guidelines; however, to better serve those who serve in a holistic manner, consideration must be given to rethink what is “medically essential” and “cosmetic” and leverage the available skills, techniques, and equipment to increase the readiness of the force.

According to the US Department of Defense, the term readiness refers to the ability to recruit, train, deploy, and sustain military forces that will be ready to “fight tonight” and succeed in combat. Readiness is a top priority for military medicine, which functions to diagnose, treat, and rehabilitate service members so that they can return to the fight. This central concept drives programs across the military—from operational training events to the establishment of medical and dental standards. Readiness is tracked and scrutinized constantly, and although it is a shared responsibility, efforts to increase and sustain readiness often fall on support staff and military medical providers.
In recent years, there has been a greater awareness of the negative effects of mental illness, low morale, and suicidality on military readiness. In 2013, suicide accounted for 28.1% of all deaths that occurred in the US Armed Forces.1 Put frankly, suicide was one of the leading causes of death among military members.
The most recent Marine Corps Order regarding the Marine Corps Suicide Prevention Program stated that “suicidal behaviors are a barrier to readiness that have lasting effects on Marines and Service Members attached to Marine Commands. . .Families, and the Marine Corps.” It goes on to say that “[e]ffective suicide prevention requires coordinated efforts within a prevention framework dedicated to promoting mental, physical, spiritual, and social fitness. . .[and] mitigating stressors that interfere with mission readiness.”2 This statement supports the notion that preventing suicide is not just about treating mental illness; it also involves maximizing physical, spiritual, and social fitness. Although it is well established that various mental health disorders are associated with an increased risk for suicide, it is worth noting that, in one study, only half of individuals who died by suicide had a mental health disorder diagnosed prior to their death.3 These statistics translate to the military. The 2015 Department of Defense Suicide Event Report noted that only 28% of service members who died by suicide and 22% of members with attempted suicide had been documented as having sought mental health care and disclosed their potential for self-harm prior to the event.1,4 In 2018, a study published by Ursano et al5 showed that 36.3% of US soldiers with a documented suicide attempt (N=9650) had no prior mental health diagnoses.
Expanding the scope to include mental health issues in general, only 29% of service members who reported experiencing a mental health problem actually sought mental health care in that same period. Overall, approximately 40% of service members with a reported perceived need for mental health care actually sought care over their entire course of service time,1 which raises concern for a large population of undiagnosed and undertreated mental illnesses across the military. In response to these statistics, Reger et al3 posited that it is “essential that suicide prevention efforts move outside the silo of mental health.” The authors went on to challenge health care providers across all specialties and civilians alike to take responsibility in understanding, recognizing, and mitigating risk factors for suicide in the general population.3 Although treating a service member’s acne or offering to stand duty for a service member who has been under a great deal of stress in their personal life may appear to be indirect ways of reducing suicide in the US military, they actually may be the most critical means of prevention in a culture that emphasizes resilience and self-reliance, where seeking help for mental health struggles could be perceived as weakness.1
In this review article, we discuss the concept of cutaneous body image (CBI) and its associated outcomes on health, satisfaction, and quality of life in military service members. We then examine the intersections between common dermatologic conditions, CBI, and mental health and explore the ability and role of the military dermatologist to serve as a positive influence on military readiness.
What is cutaneous body image?
Cutaneous body image is “the individual’s mental perception of his or her skin and its appendages (ie, hair, nails).”6 It is measured objectively using the Cutaneous Body Image Scale, a questionnaire that includes 7 items related to the overall satisfaction with the appearance of skin, color of skin, skin of the face, complexion of the face, hair, fingernails, and toenails. Each question is rated using a 10-point Likert scale (0=not at all; 10=very markedly).6
Some degree of CBI dissatisfaction is expected and has been shown in the general population at large; for example, more than 56% of women older than 30 years report some degree of dissatisfaction with their skin. Similarly, data from the American Society of Plastic Surgeons showed that while 10.9 million cosmetic procedures were performed in 2006, 9.1 million of them involved minimally invasive procedures such as botulinum toxin type A injections with the purpose of skin rejuvenation and improvement of facial appearance.7 However, lower than average CBI can contribute to considerable psychosocial morbidity. Dissatisfaction with CBI is associated with self-consciousness, feelings of inferiority, and social exclusion. These symptoms can be grouped into a construct called interpersonal sensitivity (IS). A 2013 study by Gupta and Gupta6 investigated the relationship between CBI, IS, and suicidal ideation among 312 consenting nonclinical participants in Canada. The study found that greater dissatisfaction with an individual’s CBI correlated to increased IS and increased rates of suicidal ideation and intentional self-injury.6
Cutaneous body image is particularly relevant to dermatologists, as many common dermatoses can cause cosmetically disfiguring skin conditions; for example, acne and rosacea have the propensity to cause notable disfigurement to the facial unit. Other common conditions such as atopic dermatitis or psoriasis can flare with stress and thereby throw patients into a vicious cycle of physical and psychosocial stress caused by social stigma, cosmetic disfigurement, and reduced CBI, in turn leading to worsening of the disease at hand. Dermatologists need to be aware that common dermatoses can impact a patient’s mental health via poor CBI.8 Similarly, dermatologists may be empowered by the awareness that treating common dermatoses, especially those associated with poor cosmesis, have 2-fold benefits—on the skin condition itself and on the patient’s mental health.
How are common dermatoses associated with mental health?
Acne—Acne is one of the most common skin diseases, so much so that in many cases acne has become an accepted and expected part of adolescence and young adulthood. Studies estimate that 85% of the US population aged 12 to 25 years have acne.9 For some adults, acne persists even longer, with 1% to 5% of adults reporting to have active lesions at 40 years of age.10 Acne is a multifactorial skin disease of the pilosebaceous unit that results in the development of inflammatory papules, pustules, and cysts. These lesions are most common on the face but can extend to other areas of the body, such as the chest and back.11 Although the active lesions can be painful and disfiguring, if left untreated, acne may lead to permanent disfigurement and scarring, which can have long-lasting psychosocial impacts.
Individuals with acne have an increased likelihood of self-consciousness, social isolation, depression, and suicidal ideation. This relationship has been well established for decades. In the 1990s, a small study reported that 7 of 16 (43.8%) cases of completed suicide in dermatology patients were in patients with acne.12 In a recent meta-analysis including 2,276,798 participants across 5 separate studies, researchers found that suicide was positively associated with acne, carrying an odds ratio of 1.50 (95% CI, 1.09-2.06).13
Rosacea—Rosacea is a common chronic inflammatory skin disease characterized by facial erythema, telangiectasia, phymatous changes, papules, pustules, and ocular irritation. The estimated worldwide prevalence is 5.5%.14 In addition to discomfort and irritation of the skin and eyes, rosacea often carries a higher risk of psychological and psychosocial distress due to its potentially disfiguring nature. Rosacea patients are at greater risk for having anxiety disorders and depression,15 and a 2018 study by Alinia et al16 showed that there is a direct relationship between rosacea severity and the actual level of depression.Although disease improvement certainly leads to improvements in quality of life and psychosocial status, Alinia et al16 noted that depression often is associated with poor treatment adherence due to poor motivation and hopelessness. It is critical that dermatologists are aware of these associations and maintain close follow-up with patients, even when the condition is not life-threatening, such as rosacea.
Hidradenitis Suppurativa—Hidradenitis suppurativa (HS) is a chronic inflammatory disease of the pilosebaceous unit that is characterized by the development of painful, malodorous, draining abscesses, fistulas, sinus tracts, and scars in sensitive areas such as the axillae, breasts, groin, and perineum.17 In severe cases, surgery may be required to excise affected areas. Compared to other cutaneous disease, HS is considered one of the most life-impacting disorders.18 The physical symptoms themselves often are debilitating, and patients often report considerable psychosocial and psychological impairment with decreased quality of life. Major depression frequently is noted, with 1 in 4 adults with HS also being depressed. In a large cross-sectional analysis of 38,140 adults and 1162 pediatric patients with HS, Wright et al17 reported the prevalence of depression among adults with HS as 30.0% compared to 16.9% in healthy controls. In children, the prevalence of depression was 11.7% compared to 4.1% in the general population.17 Similarly, 1 out of every 5 patients with HS experiences anxiety.18
In the military population, HS often can be duty limiting. The disease requires constant attention to wound care and frequent medical visits. For many service members operating in field training or combat environments, opportunities for and access to showers and basic hygiene is limited. Uniforms and additional necessary combat gear often are thick and occlusive. Taken as a whole, these factors may contribute to worsening of the disease and in severe cases are simply not conducive to the successful management of the condition. However, given the most commonly involved body areas and the nature of the disease, many service members with HS may feel embarrassed to disclose their condition. In uniform, the disease is not easily visible, and for unaware persons, the frequency of medical visits and limited duty status may seem unnecessary. This perception of a service member’s lack of productivity due to an unseen disease may further add to the psychosocial stress they experience.
The treatments for acne, rosacea, and HS are outlined in the eTable.11,19 Also noted are specific considerations when managing an active-duty service member due to various operational duty restrictions and constraints.

Final Thoughts
Maintaining readiness in the military is essential to the ability to not only “fight tonight” but also to win tonight in whatever operational or combat mission a service member may be. Although many factors impact readiness, the rates of suicide within the armed forces cannot be ignored. Suicide not only eliminates the readiness of the deceased service member but has lasting ripple effects on the overall readiness of their unit and command at large. Most suicides in the military occur in personnel with no prior documented mental health diagnoses or treatment. Therefore, it is the responsibility of all service members to recognize and mitigate stressors and risk factors that may lead to mental health distress and suicidality. In the medical corps, this translates to a responsibility of all medical specialists to recognize and understand unique risk factors for suicidality and to do as much as they can to reduce these risks. For military dermatologists and for civilian physicians treating military service members, it is imperative to predict and understand the relationship between common dermatoses; reduced satisfaction with CBI; and increased risk for mental health illness, self-harm, and suicide. Military dermatologists, as well as other specialists, may be limited in the care they are able to provide due to manpower, staffing, demand, and institutional guidelines; however, to better serve those who serve in a holistic manner, consideration must be given to rethink what is “medically essential” and “cosmetic” and leverage the available skills, techniques, and equipment to increase the readiness of the force.

- Ghahramanlou-Holloway M, LaCroix JM, Koss K, et al. Outpatient mental health treatment utilization and military career impact in the United States Marine Corps. Int J Environ Res Public Health. 2018;15:828. doi:10.3390/ijerph15040828
- Ottignon DA. Marine Corps Suicide Prevention System (MCSPS). Marine Corps Order 1720.2A. 2021. Headquarters United States Marine Corps. Published August 2, 2021. Accessed May 25, 2022. https://www.marines.mil/Portals/1/Publications/MCO%201720.2A.pdf?ver=QPxZ_qMS-X-d037B65N9Tg%3d%3d
- Reger MA, Smolenski DJ, Carter SP. Suicide prevention in the US Army: a mission for more than mental health clinicians. JAMA Psychiatry. 2018;75:991-992. doi:10.1001/jamapsychiatry.2018.2042
- Pruitt LD, Smolenski DJ, Bush NE, et al. Department of Defense Suicide Event Report Calendar Year 2015 Annual Report. National Center for Telehealth & Technology (T2); 2016. Accessed May 20, 2022. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Psychological-Health-Center-of-Excellence/Department-of-Defense-Suicide-Event-Report
- Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry. 2018;75:1022-1032. doi:10.1001/jamapsychiatry.2018.2069
- Gupta MA, Gupta AK. Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J Psychosom Res. 2013;75:55-59. doi:10.1016/j.jpsychores.2013.01.015
- Gupta MA, Gupta AK. Evaluation of cutaneous body image dissatisfaction in the dermatology patient. Clin Dermatol. 2013;31:72-79. doi:10.1016/j.clindermatol.2011.11.010
- Hinkley SB, Holub SC, Menter A. The validity of cutaneous body image as a construct and as a mediator of the relationship between cutaneous disease and mental health. Dermatol Ther (Heidelb). 2020;10:203-211. doi:10.1007/s13555-020-00351-5
- Stamu-O’Brien C, Jafferany M, Carniciu S, et al. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20:1080-1083. doi:10.1111/jocd.13765
- Sood S, Jafferany M, Vinaya Kumar S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19:3177-3182. doi:10.1111/jocd.13753
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246-250.
- Xu S, Zhu Y, Hu H, et al. The analysis of acne increasing suicide risk. Medicine (Baltimore). 2021;100:E26035. doi:10.1097/MD.0000000000026035
- Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China [published online June 16, 2021]. Front Psychiatry. doi:10.3389/fpsyt.2021.659171
- Incel Uysal P, Akdogan N, Hayran Y, et al. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94:704-709. doi:10.1016/j.abd.2019.03.002
- Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102:36-38.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.06.843
- Misitzis A, Goldust M, Jafferany M, et al. Psychiatric comorbidities in patients with hidradenitis suppurativa. Dermatol Ther. 2020;33:E13541. doi:10.1111/dth.13541
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017.
- Ghahramanlou-Holloway M, LaCroix JM, Koss K, et al. Outpatient mental health treatment utilization and military career impact in the United States Marine Corps. Int J Environ Res Public Health. 2018;15:828. doi:10.3390/ijerph15040828
- Ottignon DA. Marine Corps Suicide Prevention System (MCSPS). Marine Corps Order 1720.2A. 2021. Headquarters United States Marine Corps. Published August 2, 2021. Accessed May 25, 2022. https://www.marines.mil/Portals/1/Publications/MCO%201720.2A.pdf?ver=QPxZ_qMS-X-d037B65N9Tg%3d%3d
- Reger MA, Smolenski DJ, Carter SP. Suicide prevention in the US Army: a mission for more than mental health clinicians. JAMA Psychiatry. 2018;75:991-992. doi:10.1001/jamapsychiatry.2018.2042
- Pruitt LD, Smolenski DJ, Bush NE, et al. Department of Defense Suicide Event Report Calendar Year 2015 Annual Report. National Center for Telehealth & Technology (T2); 2016. Accessed May 20, 2022. https://health.mil/Military-Health-Topics/Centers-of-Excellence/Psychological-Health-Center-of-Excellence/Department-of-Defense-Suicide-Event-Report
- Ursano RJ, Kessler RC, Naifeh JA, et al. Risk factors associated with attempted suicide among US Army soldiers without a history of mental health diagnosis. JAMA Psychiatry. 2018;75:1022-1032. doi:10.1001/jamapsychiatry.2018.2069
- Gupta MA, Gupta AK. Cutaneous body image dissatisfaction and suicidal ideation: mediation by interpersonal sensitivity. J Psychosom Res. 2013;75:55-59. doi:10.1016/j.jpsychores.2013.01.015
- Gupta MA, Gupta AK. Evaluation of cutaneous body image dissatisfaction in the dermatology patient. Clin Dermatol. 2013;31:72-79. doi:10.1016/j.clindermatol.2011.11.010
- Hinkley SB, Holub SC, Menter A. The validity of cutaneous body image as a construct and as a mediator of the relationship between cutaneous disease and mental health. Dermatol Ther (Heidelb). 2020;10:203-211. doi:10.1007/s13555-020-00351-5
- Stamu-O’Brien C, Jafferany M, Carniciu S, et al. Psychodermatology of acne: psychological aspects and effects of acne vulgaris. J Cosmet Dermatol. 2021;20:1080-1083. doi:10.1111/jocd.13765
- Sood S, Jafferany M, Vinaya Kumar S. Depression, psychiatric comorbidities, and psychosocial implications associated with acne vulgaris. J Cosmet Dermatol. 2020;19:3177-3182. doi:10.1111/jocd.13753
- Brahe C, Peters K. Fighting acne for the fighting forces. Cutis. 2020;106:18-20, 22. doi:10.12788/cutis.0057
- Cotterill JA, Cunliffe WJ. Suicide in dermatological patients. Br J Dermatol. 1997;137:246-250.
- Xu S, Zhu Y, Hu H, et al. The analysis of acne increasing suicide risk. Medicine (Baltimore). 2021;100:E26035. doi:10.1097/MD.0000000000026035
- Chen M, Deng Z, Huang Y, et al. Prevalence and risk factors of anxiety and depression in rosacea patients: a cross-sectional study in China [published online June 16, 2021]. Front Psychiatry. doi:10.3389/fpsyt.2021.659171
- Incel Uysal P, Akdogan N, Hayran Y, et al. Rosacea associated with increased risk of generalized anxiety disorder: a case-control study of prevalence and risk of anxiety in patients with rosacea. An Bras Dermatol. 2019;94:704-709. doi:10.1016/j.abd.2019.03.002
- Alinia H, Cardwell LA, Tuchayi SM, et al. Screening for depression in rosacea patients. Cutis. 2018;102:36-38.
- Wright S, Strunk A, Garg A. Prevalence of depression among children, adolescents, and adults with hidradenitis suppurativa [published online June 16, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.06.843
- Misitzis A, Goldust M, Jafferany M, et al. Psychiatric comorbidities in patients with hidradenitis suppurativa. Dermatol Ther. 2020;33:E13541. doi:10.1111/dth.13541
- Bolognia J, Schaffer J, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2017.
Practice Points
- The term readiness refers to the ability to recruit, train, deploy, and sustain military forces that are ready to “fight tonight” and succeed in combat.
- Maintaining readiness requires a holistic approach, as it is directly affected by physical and mental health outcomes.
- Cutaneous body image (CBI) refers to an individual’s mental perception of the condition of their hair, nails, and skin. Positive CBI is related to increased quality of life, while negative CBI, which often is associated with dermatologic disease, is associated with poorer health outcomes and even self-injury.
- Treatment of dermatologic disease in the context of active-duty military members can positively influence CBI, which may in turn increase service members’ quality of life and overall military readiness.
Assessing Treatment Delays for Vitiligo Patients: A Retrospective Chart Review
Similar to other dermatologic conditions, barriers to early care in patients with vitiligo can exacerbate health disparities.1 Delayed treatment of vitiligo is known to hamper successful disease stabilization and repigmentation, as therapies tend to work more effectively in early stages of the disease.2
To investigate the factors associated with treatment delays for patients with vitiligo, we conducted a retrospective chart review of 102 consecutive patients with vitiligo attending an academic outpatient clinic in Austin, Texas, over 36 months.
Methods
Our sample included 102 consecutive patients with vitiligo who attended an academic outpatient clinic in Austin, Texas, from January 2017 to January 2020. Demographic information, clinical characteristics of vitiligo, and treatment data were self-reported via a standardized questionnaire given to all patients with vitiligo and gathered from medical chart review. Patient characteristics are outlined in the Table. The delay to treatment was the time (in months) from the date the patient first noticed the lesion to the start date of first treatment. This retrospective chart review was reviewed by the University of Texas at Austin institutional review board and was determined to be exempt.
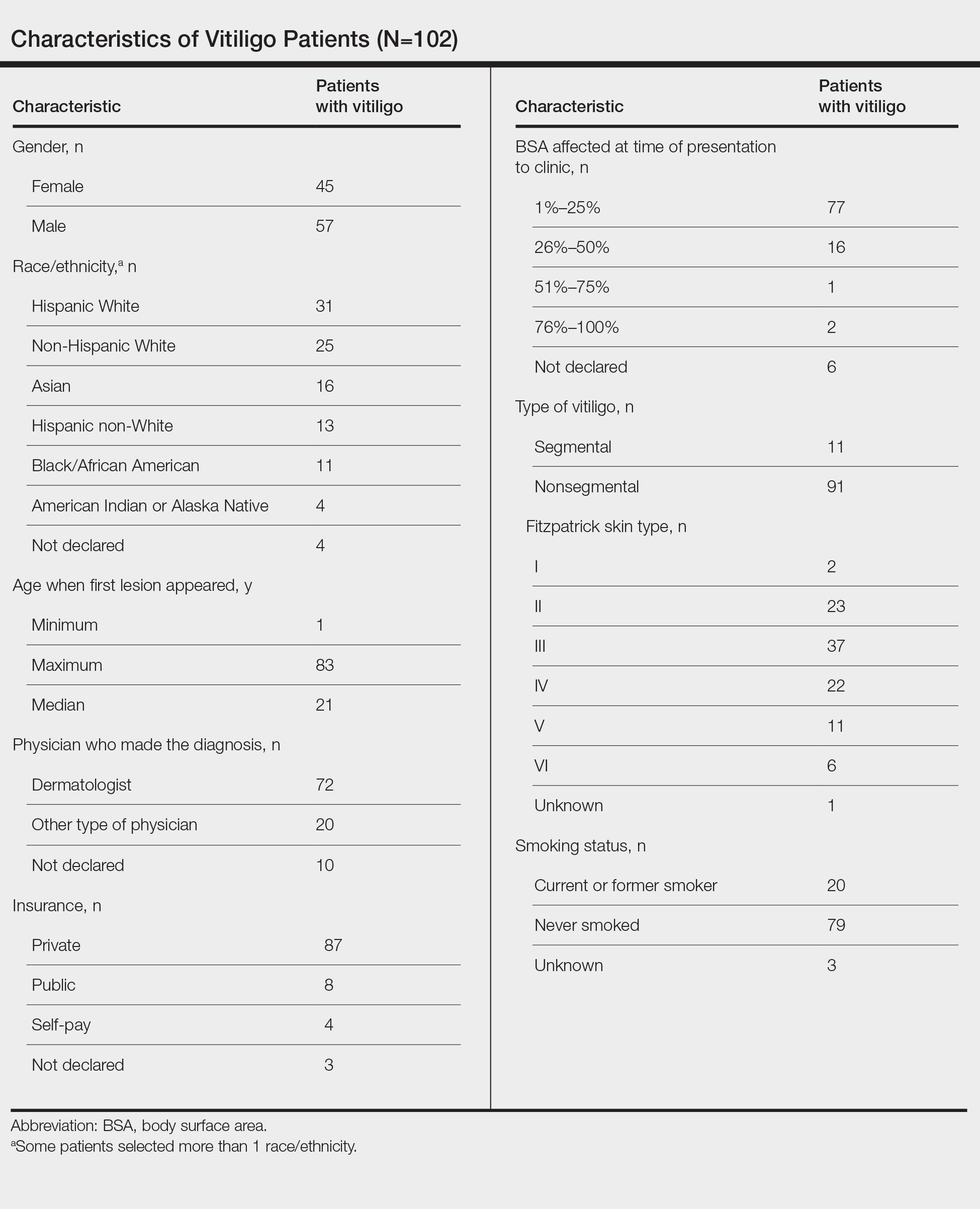
Statistical Analysis—The data were analyzed descriptively with a Wilcoxon rank sum test (type I error rate of .05).
Results
Of the 102 charts that were analyzed, 45 were females and 57 were males. More than half of the patients (54.9% [56/102]) were White. Sixteen were Asian, 13 were Hispanic non-White, 11 were Black/African American, and 4 were American Indian/Alaska Native. The median age of disease onset was 21 years, minimum age was 1 year, and maximum age was 83 years. The diagnosis of vitiligo was made by a dermatologist for 72 patients and by a physician of another specialty for 20 patients. Ten patients did not declare the specialty of the diagnosing physician.
Individuals older than 21 years when their disease started had a shorter delay to treatment than individuals who noticed their first lesion at an age younger than 21 years (median, 75 months vs 13 months; P<.01). Individuals diagnosed by a dermatologist had a shorter delay to treatment than individuals diagnosed by a physician of another specialty (median, 13 months vs 58 months; P<.05). White individuals had a shorter delay to treatment than individuals with skin of color (median, 13 months vs 31 months; P=.08), though this trend did not reach statistical significance. Individuals with 1% to 25% of body surface area (BSA) affected at time of presentation to clinic also had a shorter delay to treatment than those with a greater BSA affected (median, 13 months vs 74 months; P<.06), though this trend did not reach statistical significance. Type of vitiligo (P<.8), Fitzpatrick skin type (P<.6), and smoking status (P<.7) were not associated with differential delays.
Comment
Impact of Age on Vitiligo Treatment—Our data suggest that individuals who develop vitiligo at a younger age experience longer treatment delays compared to older individuals. Reasons for this are uncertain but could include access issues, medical decision-making agency, and younger patients not remembering being treated during their youth. Our data also could be influenced by some of the adult patients in our study first noticing their lesions many years ago when treatments for vitiligo were more limited. Nevertheless, detrimental effects on quality of life in children and adolescents with vitiligo suggest that motivating younger individuals with vitiligo to seek treatment or proactively making them aware of treatment opportunities may be beneficial.3
Diagnosis of Vitiligo by Nondermatologists—The increase in delay to treatment when a nondermatologist diagnoses vitiligo suggests that prompt initiation of treatment or referrals to dermatology by primary care providers may not routinely be occurring.4 Our data indicate the need to educate primary care providers on treatment opportunities for individuals with vitiligo and that early treatment generally is more effective.5
Impact of Race/Ethnicity on Vitiligo Treatment—Our data also show trends for longer treatment delays for individuals with skin of color. Although this did not reach statistical significance, we hope future studies will investigate this issue, especially because patients with skin of color experience more stigmatization and quality-of-life impacts by vitiligo than White patients.5
Impact of BSA on Vitiligo Treatment—Our data show that patients with a smaller BSA had a shorter delay to treatment than those with a greater BSA affected. This was a unique finding given it initially was hypothesized that patients with greater BSA would seek treatment earlier because of the associated increase in quality of life impact. This trend was not statistically significant, but further investigation would be helpful to analyze the reason behind treatment delays in patients with greater BSA affected.
Conclusion
The delay to treatment in our study population was correlated with the diagnosing physician’s specialty and patient age at disease onset, with trends also observed for race and BSA affected. These findings emphasize the need to investigate specific causes of barriers to early care to promote health equity among individuals with vitiligo.
- Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020;83:854-859.
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28:656-661.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55:608-614.
- Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol. 2011;165:1308-1313.
Similar to other dermatologic conditions, barriers to early care in patients with vitiligo can exacerbate health disparities.1 Delayed treatment of vitiligo is known to hamper successful disease stabilization and repigmentation, as therapies tend to work more effectively in early stages of the disease.2
To investigate the factors associated with treatment delays for patients with vitiligo, we conducted a retrospective chart review of 102 consecutive patients with vitiligo attending an academic outpatient clinic in Austin, Texas, over 36 months.
Methods
Our sample included 102 consecutive patients with vitiligo who attended an academic outpatient clinic in Austin, Texas, from January 2017 to January 2020. Demographic information, clinical characteristics of vitiligo, and treatment data were self-reported via a standardized questionnaire given to all patients with vitiligo and gathered from medical chart review. Patient characteristics are outlined in the Table. The delay to treatment was the time (in months) from the date the patient first noticed the lesion to the start date of first treatment. This retrospective chart review was reviewed by the University of Texas at Austin institutional review board and was determined to be exempt.

Statistical Analysis—The data were analyzed descriptively with a Wilcoxon rank sum test (type I error rate of .05).
Results
Of the 102 charts that were analyzed, 45 were females and 57 were males. More than half of the patients (54.9% [56/102]) were White. Sixteen were Asian, 13 were Hispanic non-White, 11 were Black/African American, and 4 were American Indian/Alaska Native. The median age of disease onset was 21 years, minimum age was 1 year, and maximum age was 83 years. The diagnosis of vitiligo was made by a dermatologist for 72 patients and by a physician of another specialty for 20 patients. Ten patients did not declare the specialty of the diagnosing physician.
Individuals older than 21 years when their disease started had a shorter delay to treatment than individuals who noticed their first lesion at an age younger than 21 years (median, 75 months vs 13 months; P<.01). Individuals diagnosed by a dermatologist had a shorter delay to treatment than individuals diagnosed by a physician of another specialty (median, 13 months vs 58 months; P<.05). White individuals had a shorter delay to treatment than individuals with skin of color (median, 13 months vs 31 months; P=.08), though this trend did not reach statistical significance. Individuals with 1% to 25% of body surface area (BSA) affected at time of presentation to clinic also had a shorter delay to treatment than those with a greater BSA affected (median, 13 months vs 74 months; P<.06), though this trend did not reach statistical significance. Type of vitiligo (P<.8), Fitzpatrick skin type (P<.6), and smoking status (P<.7) were not associated with differential delays.
Comment
Impact of Age on Vitiligo Treatment—Our data suggest that individuals who develop vitiligo at a younger age experience longer treatment delays compared to older individuals. Reasons for this are uncertain but could include access issues, medical decision-making agency, and younger patients not remembering being treated during their youth. Our data also could be influenced by some of the adult patients in our study first noticing their lesions many years ago when treatments for vitiligo were more limited. Nevertheless, detrimental effects on quality of life in children and adolescents with vitiligo suggest that motivating younger individuals with vitiligo to seek treatment or proactively making them aware of treatment opportunities may be beneficial.3
Diagnosis of Vitiligo by Nondermatologists—The increase in delay to treatment when a nondermatologist diagnoses vitiligo suggests that prompt initiation of treatment or referrals to dermatology by primary care providers may not routinely be occurring.4 Our data indicate the need to educate primary care providers on treatment opportunities for individuals with vitiligo and that early treatment generally is more effective.5
Impact of Race/Ethnicity on Vitiligo Treatment—Our data also show trends for longer treatment delays for individuals with skin of color. Although this did not reach statistical significance, we hope future studies will investigate this issue, especially because patients with skin of color experience more stigmatization and quality-of-life impacts by vitiligo than White patients.5
Impact of BSA on Vitiligo Treatment—Our data show that patients with a smaller BSA had a shorter delay to treatment than those with a greater BSA affected. This was a unique finding given it initially was hypothesized that patients with greater BSA would seek treatment earlier because of the associated increase in quality of life impact. This trend was not statistically significant, but further investigation would be helpful to analyze the reason behind treatment delays in patients with greater BSA affected.
Conclusion
The delay to treatment in our study population was correlated with the diagnosing physician’s specialty and patient age at disease onset, with trends also observed for race and BSA affected. These findings emphasize the need to investigate specific causes of barriers to early care to promote health equity among individuals with vitiligo.
Similar to other dermatologic conditions, barriers to early care in patients with vitiligo can exacerbate health disparities.1 Delayed treatment of vitiligo is known to hamper successful disease stabilization and repigmentation, as therapies tend to work more effectively in early stages of the disease.2
To investigate the factors associated with treatment delays for patients with vitiligo, we conducted a retrospective chart review of 102 consecutive patients with vitiligo attending an academic outpatient clinic in Austin, Texas, over 36 months.
Methods
Our sample included 102 consecutive patients with vitiligo who attended an academic outpatient clinic in Austin, Texas, from January 2017 to January 2020. Demographic information, clinical characteristics of vitiligo, and treatment data were self-reported via a standardized questionnaire given to all patients with vitiligo and gathered from medical chart review. Patient characteristics are outlined in the Table. The delay to treatment was the time (in months) from the date the patient first noticed the lesion to the start date of first treatment. This retrospective chart review was reviewed by the University of Texas at Austin institutional review board and was determined to be exempt.

Statistical Analysis—The data were analyzed descriptively with a Wilcoxon rank sum test (type I error rate of .05).
Results
Of the 102 charts that were analyzed, 45 were females and 57 were males. More than half of the patients (54.9% [56/102]) were White. Sixteen were Asian, 13 were Hispanic non-White, 11 were Black/African American, and 4 were American Indian/Alaska Native. The median age of disease onset was 21 years, minimum age was 1 year, and maximum age was 83 years. The diagnosis of vitiligo was made by a dermatologist for 72 patients and by a physician of another specialty for 20 patients. Ten patients did not declare the specialty of the diagnosing physician.
Individuals older than 21 years when their disease started had a shorter delay to treatment than individuals who noticed their first lesion at an age younger than 21 years (median, 75 months vs 13 months; P<.01). Individuals diagnosed by a dermatologist had a shorter delay to treatment than individuals diagnosed by a physician of another specialty (median, 13 months vs 58 months; P<.05). White individuals had a shorter delay to treatment than individuals with skin of color (median, 13 months vs 31 months; P=.08), though this trend did not reach statistical significance. Individuals with 1% to 25% of body surface area (BSA) affected at time of presentation to clinic also had a shorter delay to treatment than those with a greater BSA affected (median, 13 months vs 74 months; P<.06), though this trend did not reach statistical significance. Type of vitiligo (P<.8), Fitzpatrick skin type (P<.6), and smoking status (P<.7) were not associated with differential delays.
Comment
Impact of Age on Vitiligo Treatment—Our data suggest that individuals who develop vitiligo at a younger age experience longer treatment delays compared to older individuals. Reasons for this are uncertain but could include access issues, medical decision-making agency, and younger patients not remembering being treated during their youth. Our data also could be influenced by some of the adult patients in our study first noticing their lesions many years ago when treatments for vitiligo were more limited. Nevertheless, detrimental effects on quality of life in children and adolescents with vitiligo suggest that motivating younger individuals with vitiligo to seek treatment or proactively making them aware of treatment opportunities may be beneficial.3
Diagnosis of Vitiligo by Nondermatologists—The increase in delay to treatment when a nondermatologist diagnoses vitiligo suggests that prompt initiation of treatment or referrals to dermatology by primary care providers may not routinely be occurring.4 Our data indicate the need to educate primary care providers on treatment opportunities for individuals with vitiligo and that early treatment generally is more effective.5
Impact of Race/Ethnicity on Vitiligo Treatment—Our data also show trends for longer treatment delays for individuals with skin of color. Although this did not reach statistical significance, we hope future studies will investigate this issue, especially because patients with skin of color experience more stigmatization and quality-of-life impacts by vitiligo than White patients.5
Impact of BSA on Vitiligo Treatment—Our data show that patients with a smaller BSA had a shorter delay to treatment than those with a greater BSA affected. This was a unique finding given it initially was hypothesized that patients with greater BSA would seek treatment earlier because of the associated increase in quality of life impact. This trend was not statistically significant, but further investigation would be helpful to analyze the reason behind treatment delays in patients with greater BSA affected.
Conclusion
The delay to treatment in our study population was correlated with the diagnosing physician’s specialty and patient age at disease onset, with trends also observed for race and BSA affected. These findings emphasize the need to investigate specific causes of barriers to early care to promote health equity among individuals with vitiligo.
- Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020;83:854-859.
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28:656-661.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55:608-614.
- Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol. 2011;165:1308-1313.
- Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. J Am Acad Dermatol. 2020;83:854-859.
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28:656-661.
- Silverberg JI, Silverberg NB. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31:309-318.
- Amer AA, Gao XH. Quality of life in patients with vitiligo: an analysis of the dermatology life quality index outcome over the past two decades. Int J Dermatol. 2016;55:608-614.
- Weibel L, Laguda B, Atherton D, et al. Misdiagnosis and delay in referral of children with localized scleroderma. Br J Dermatol. 2011;165:1308-1313.
Practice Points
- The medical community should be aware of factors associated with delay to treatment in patients with vitiligo, such as the diagnosing physician’s specialty and patient age at disease onset.
- Race and percentage of body surface area affected at time of presentation also demonstrate trends regarding treatment delays in patients with vitiligo.
FDA OKs first systemic treatment for alopecia areata
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
.
The disorder with the hallmark signs of patchy baldness affects more than 300,000 people in the United States each year. In patients with the autoimmune disorder, the body attacks its own hair follicles and hair falls out, often in clumps. In February, the FDA granted priority review for baricitinib in adults with severe AA.
Baricitinib (Olumiant) is a Janus kinase (JAK) inhibitor, which blocks the activity of one or more enzymes, interfering with the pathway that leads to inflammation.
The FDA reports the most common side effects include upper respiratory tract infections, headache, acne, hyperlipidemia, increase of creatinine phosphokinase, urinary tract infection, elevated liver enzymes, inflammation of hair follicles, fatigue, lower respiratory tract infections, nausea, Candida infections, anemia, neutropenia, abdominal pain, herpes zoster (shingles), and weight gain. The labeling for baricitinib includes a boxed warning for serious infections, mortality, malignancy, major adverse cardiovascular events, and thrombosis.
Evidence from two trials led to announcement
The decision came after review of the results from two randomized, double-blind, placebo-controlled trials (BRAVE AA-1 and BRAVE AA-2) with patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool (SALT score) for more than 6 months.
Patients in these trials got either a placebo, 2 mg of baricitinib, or 4 mg of baricitinib every day. The primary endpoint for both trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In BRAVE AA-1, 22% of the 184 patients who received 2 mg of baricitinib and 35% of the 281 patients who received 4 mg of baricitinib achieved at least 80% scalp hair coverage, compared with 5% of the 189 patients in the placebo group.
In BRAVE AA-2, 17% of the 156 patients who received 2 mg of baricitinib and 32% of the 234 patients who received 4 mg achieved at least 80% scalp hair coverage, compared with 3% of the 156 patients in the placebo group.
The results were reported at the annual meeting of the American Academy of Dermatology meeting in March.
Baricitinib was originally approved in 2018 as a treatment for adult patients with moderately to severely active rheumatoid arthritis who have had an inadequate response to one or more tumor necrosis factor (TNF)–blockers. It is also approved for treating COVID-19 in certain hospitalized adults.
Two other companies, Pfizer and Concert Pharmaceuticals, have JAK inhibitors in late-stage development for AA. The drugs are already on the market for treating rheumatoid arthritis and other autoimmune diseases. FDA approval is important for insurance coverage of the drugs, which have a list price of nearly $2,500 a month, according to The New York Times.
Until now, the only treatments for moderate to severe AA approved by the FDA have been intralesional steroid injections, contact sensitization, and systemic immunosuppressants, but they have demonstrated limited efficacy, are inconvenient for patients to take, and have been unsuitable for use long term.
“Today’s approval will help fulfill a significant unmet need for patients with severe alopecia areata,” Kendall Marcus, MD, director of the Division of Dermatology and Dentistry in the FDA’s Center for Drug Evaluation and Research, said in the press release.
As Medscape reported last month, The European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) has recommended approval of baricitinib for adults with severe AA.
AA received widespread international attention earlier this year at the Academy Awards ceremony, when actor Will Smith walked from the audience up onto the stage and slapped comedian Chris Rock in the face after he directed a joke at Mr. Smith’s wife, Jada Pinkett Smith, about her shaved head. Mrs. Pinkett Smith has AA and has been public about her struggles with the disease.
A version of this article first appeared on Medscape.com.
Surprising link between herpes zoster and dementia
Herpes zoster does not appear to increase dementia risk – on the contrary, the viral infection may offer some protection, a large population-based study suggests.
“We were surprised by these results [and] the reasons for the decreased risk are unclear,” study author Sigrun Alba Johannesdottir Schmidt, MD, PhD, with Aarhus (Denmark) University Hospital, said in a news release.
The study was published online in Neurology.
Conflicting findings
Herpes zoster (HZ) is an acute, cutaneous viral infection caused by the reactivation of varicella-zoster virus (VZV). Previous population-based studies have reported both decreased and increased risks of dementia after having HZ.
It’s thought that HZ may contribute to the development of dementia through neuroinflammation, cerebral vasculopathy, or direct neural damage, but epidemiologic evidence is limited.
To investigate further, Dr. Schmidt and colleagues used Danish medical registries to identify 247,305 people who had visited a hospital for HZ or were prescribed antiviral medication for HZ over a 20-year period and matched them to 1,235,890 people who did not have HZ. For both cohorts, the median age was 64 years, and 61% were women.
Dementia was diagnosed in 9.7% of zoster patients and 10.3% of matched control persons during up to 21 years of follow-up.
Contrary to the researchers’ expectation, HZ was associated with a small (7%) decreased relative risk of all-cause dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.90-0.95).
There was no increased long-term risk of dementia in subgroup analyses, except possibly among those with HZ that involved the central nervous system (HR, 1.94; 95% CI, 0.78-4.80), which has been shown before.
However, the population attributable fraction of dementia caused by this rare complication is low (< 1%), suggesting that universal vaccination against VZV in the elderly has limited potential to reduce dementia risk, the investigators noted.
Nonetheless, Dr. Schmidt said shingles vaccination should be encouraged in older people because it can prevent complications from the disease.
The research team admitted that the slightly decreased long-term risk of dementia, including Alzheimer’s disease, was “unexpected.” The reasons for this decreased risk are unclear, they say, and could be explained by missed diagnoses of shingles in people with undiagnosed dementia.
They were not able to examine whether antiviral treatment modifies the association between HZ and dementia and said that this topic merits further research.
The study was supported by the Edel and Wilhelm Daubenmerkls Charitable Foundation. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Herpes zoster does not appear to increase dementia risk – on the contrary, the viral infection may offer some protection, a large population-based study suggests.
“We were surprised by these results [and] the reasons for the decreased risk are unclear,” study author Sigrun Alba Johannesdottir Schmidt, MD, PhD, with Aarhus (Denmark) University Hospital, said in a news release.
The study was published online in Neurology.
Conflicting findings
Herpes zoster (HZ) is an acute, cutaneous viral infection caused by the reactivation of varicella-zoster virus (VZV). Previous population-based studies have reported both decreased and increased risks of dementia after having HZ.
It’s thought that HZ may contribute to the development of dementia through neuroinflammation, cerebral vasculopathy, or direct neural damage, but epidemiologic evidence is limited.
To investigate further, Dr. Schmidt and colleagues used Danish medical registries to identify 247,305 people who had visited a hospital for HZ or were prescribed antiviral medication for HZ over a 20-year period and matched them to 1,235,890 people who did not have HZ. For both cohorts, the median age was 64 years, and 61% were women.
Dementia was diagnosed in 9.7% of zoster patients and 10.3% of matched control persons during up to 21 years of follow-up.
Contrary to the researchers’ expectation, HZ was associated with a small (7%) decreased relative risk of all-cause dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.90-0.95).
There was no increased long-term risk of dementia in subgroup analyses, except possibly among those with HZ that involved the central nervous system (HR, 1.94; 95% CI, 0.78-4.80), which has been shown before.
However, the population attributable fraction of dementia caused by this rare complication is low (< 1%), suggesting that universal vaccination against VZV in the elderly has limited potential to reduce dementia risk, the investigators noted.
Nonetheless, Dr. Schmidt said shingles vaccination should be encouraged in older people because it can prevent complications from the disease.
The research team admitted that the slightly decreased long-term risk of dementia, including Alzheimer’s disease, was “unexpected.” The reasons for this decreased risk are unclear, they say, and could be explained by missed diagnoses of shingles in people with undiagnosed dementia.
They were not able to examine whether antiviral treatment modifies the association between HZ and dementia and said that this topic merits further research.
The study was supported by the Edel and Wilhelm Daubenmerkls Charitable Foundation. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Herpes zoster does not appear to increase dementia risk – on the contrary, the viral infection may offer some protection, a large population-based study suggests.
“We were surprised by these results [and] the reasons for the decreased risk are unclear,” study author Sigrun Alba Johannesdottir Schmidt, MD, PhD, with Aarhus (Denmark) University Hospital, said in a news release.
The study was published online in Neurology.
Conflicting findings
Herpes zoster (HZ) is an acute, cutaneous viral infection caused by the reactivation of varicella-zoster virus (VZV). Previous population-based studies have reported both decreased and increased risks of dementia after having HZ.
It’s thought that HZ may contribute to the development of dementia through neuroinflammation, cerebral vasculopathy, or direct neural damage, but epidemiologic evidence is limited.
To investigate further, Dr. Schmidt and colleagues used Danish medical registries to identify 247,305 people who had visited a hospital for HZ or were prescribed antiviral medication for HZ over a 20-year period and matched them to 1,235,890 people who did not have HZ. For both cohorts, the median age was 64 years, and 61% were women.
Dementia was diagnosed in 9.7% of zoster patients and 10.3% of matched control persons during up to 21 years of follow-up.
Contrary to the researchers’ expectation, HZ was associated with a small (7%) decreased relative risk of all-cause dementia during follow-up (hazard ratio, 0.93; 95% confidence interval, 0.90-0.95).
There was no increased long-term risk of dementia in subgroup analyses, except possibly among those with HZ that involved the central nervous system (HR, 1.94; 95% CI, 0.78-4.80), which has been shown before.
However, the population attributable fraction of dementia caused by this rare complication is low (< 1%), suggesting that universal vaccination against VZV in the elderly has limited potential to reduce dementia risk, the investigators noted.
Nonetheless, Dr. Schmidt said shingles vaccination should be encouraged in older people because it can prevent complications from the disease.
The research team admitted that the slightly decreased long-term risk of dementia, including Alzheimer’s disease, was “unexpected.” The reasons for this decreased risk are unclear, they say, and could be explained by missed diagnoses of shingles in people with undiagnosed dementia.
They were not able to examine whether antiviral treatment modifies the association between HZ and dementia and said that this topic merits further research.
The study was supported by the Edel and Wilhelm Daubenmerkls Charitable Foundation. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Current monkeypox outbreak marked by unconventional spread, clinical features
When Esther Freeman, MD, PhD, thinks back on what she learned about monkeypox during her training as a dermatologist and an infectious disease epidemiologist, it was widely considered a viral disease with rare outbreaks limited primarily to Central and Western Africa.
“Monkeypox is something we have traditionally only seen very rarely in the U.S.,” Dr. Freeman, director of Global Health Dermatology at Massachusetts General Hospital and Harvard Medical School, Boston, said in an interview. “In the past, outbreaks in the U.S. have been related to international travel or import of exotic pets, which is very different than what we’re seeing as a global community now.”

Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. According to the Centers for Disease Control and Prevention, symptoms develop 5-21 days after infection and may include fever, chills, and swollen lymph nodes. Typically, within 1-3 days of the fever, a rash develops, followed by the formation of monkeypox lesions. These lesions progress from macules to papules, vesicles, pustules, and scabs, before falling off. The illness typically lasts 2-4 weeks.
What makes the 2022 monkeypox outbreak different from others is clear evidence of community transmission. According to worldwide data from the CDC, as of June 9, 2022, there were 1,356 confirmed cases in 31 countries, including 44 cases in the United States. This means that person-to-person spread of the monkeypox virus is occurring among individuals who have not traveled outside of their own country.
“This is likely an underestimate, especially when we think about the U.S., which only has 44 confirmed cases at this time,” Dr. Freeman said. “However, at present, monkeypox cases have to be confirmed by the CDC, so there are a lot more suspected cases that are likely to be confirmed in the coming days. As with any outbreak, it’s a rapidly changing situation.”
A different clinical presentation
The clinical presentation of monkeypox cases in the current outbreak also differs from that of previous outbreaks. In the past, monkeypox rashes often morphed from a macule to a pustule and commonly affected the face, hands, feet, and trunk, with some patients harboring as many as 200 lesions at once. That pattern still occurs, but increasingly, the presentation is characterized by a more localized spread, especially in the genital region, which Dr. Freeman described as “unusual and not an area we traditionally thought of in the past as a focus for monkeypox.”
Also, affected individuals in the current outbreak may develop fewer lesions, sometimes between 1 and 5 instead of up to 200. “This doesn’t apply to everybody, but it is a bit of a different picture than what we’ve seen in case descriptions and photographs in the past from places like Central Africa,” she said. “What’s being reported out of case clusters from the United Kingdom and Spain is a mix, where some people are having more generalized involvement while others have more localized involvement.” Visual examples of the monkey pox rash can be found in photos from the United Kingdom, the country with the highest number of confirmed cases, on the CDC’s website, and in a report from Spain.
Clusters of monkeypox cases have been reported worldwide in men who have sex with men, “but this is not limited to a particular subgroup of people,” emphasizes Dr. Freeman, who is also a member of the American Academy of Dermatology’s Ad Hoc Task Force to Develop Monkeypox Content, which created an online resource for clinicians. “There are several mechanisms of spread, but direct contact with lesions or infected fluids is one,” she notes.
Moreover, If you have a patient with a new genital lesion and you’re not sure what it is, testing for monkeypox in addition to classic sexually transmitted infections like HSV or syphilis would be reasonable during the current outbreak situation.”
The 2022 monkeypox outbreak may pale in comparison to the spread of COVID-19 in terms of case numbers and societal impact, but dermatologists may be the first point of contact for a person infected with the monkeypox virus. “It’s important for dermatologists to be able to recognize monkeypox, because by recognizing cases, we can stop the outbreak,” Dr. Freeman said. “In theory, an infected person could show up in your clinic, regardless of where you practice in the U.S. But at the same time, it’s important not to panic. This is not COVID-19 all over again; this is different. Yes, it is an outbreak, but we already have a vaccine that works against monkeypox, and while one of the possible modes of transmission for monkeypox is respiratory, it’s much harder to transmit that way than SARS-CoV-2 – it requires closer and longer contact.”
Confirmation of a monkeypox virus infection is based on results of a PCR test based on swabs of a lesion. The AAD task force recommends contacting the local hospital epidemiologist, infection control personnel, and/or state health department about suspected cases, “as different locations will have different regulations on where to send the [PCR] test. If appropriate, the state health department will contact the CDC.”
According to the CDC, current recommendations for personal protective equipment for possible and confirmed monkeypox cases include gown, gloves, a National Institute for Occupational Safety and Health-approved N-95 mask, and eye protection.
Topical antiviral an option
If the lesions in a patient with suspected monkeypox have turned into pustules while waiting for the PCR test results, one option is to prescribe 3%-5% topical cidofovir, according to Stephen K. Tyring, MD, PhD, of the departments of dermatology, microbiology & molecular genetics, and internal medicine at the University of Texas Health Science Center, Houston. “That’s the effective antiviral that is most available,” he said. Generic cidofovir is also now available.
Dr. Tyring recommends rapid referral of immunocompromised patients with suspected monkeypox to an infectious disease expert and/or consulting with the CDC. “The pediatric population also seems to be at somewhat more risk, as has been seen in sub-Saharan Africa,” said Dr. Tyring, who is one of the editors of the textbook Tropical Dermatology. “Also, by definition, pregnant women are at more risk because their immune systems aren’t up to par. You also want to make sure that if monkeypox is on a person’s skin that they don’t get it in their eyes, because they could lose their vision.” He added that sub-Saharan Africa has a monkeypox mortality of up to 10%, “which is something we don’t see in the U.S. or Europe. Those of us who grew up in the 20th century got routine smallpox vaccines, and we therefore probably have a degree of immunity to monkeypox. But for the past 40 years or so, unless you are in the military, you are not going to get a routine vaccine to prevent smallpox.”
Incubation period, appearance of lesions
Monkeypox has a long incubation period. According to Dr. Freeman, from the point of exposure to the development of symptomatic lesions is typically 7-14 days but can vary from 5-21 days. “It’s important for people to be aware that their exposure may have been in the more distant past, not just a few days ago” she said. “Identifying cases as quickly as possible gives us a window where we can vaccinate close contacts.”
Dr. Freeman and Dr. Tyring reported having no relevant financial disclosures.
CDC guidance on vaccination before and after exposure to monkeypox can be found here . A general Q&A for health care professionals from the CDC can be found here.
When Esther Freeman, MD, PhD, thinks back on what she learned about monkeypox during her training as a dermatologist and an infectious disease epidemiologist, it was widely considered a viral disease with rare outbreaks limited primarily to Central and Western Africa.
“Monkeypox is something we have traditionally only seen very rarely in the U.S.,” Dr. Freeman, director of Global Health Dermatology at Massachusetts General Hospital and Harvard Medical School, Boston, said in an interview. “In the past, outbreaks in the U.S. have been related to international travel or import of exotic pets, which is very different than what we’re seeing as a global community now.”

Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. According to the Centers for Disease Control and Prevention, symptoms develop 5-21 days after infection and may include fever, chills, and swollen lymph nodes. Typically, within 1-3 days of the fever, a rash develops, followed by the formation of monkeypox lesions. These lesions progress from macules to papules, vesicles, pustules, and scabs, before falling off. The illness typically lasts 2-4 weeks.
What makes the 2022 monkeypox outbreak different from others is clear evidence of community transmission. According to worldwide data from the CDC, as of June 9, 2022, there were 1,356 confirmed cases in 31 countries, including 44 cases in the United States. This means that person-to-person spread of the monkeypox virus is occurring among individuals who have not traveled outside of their own country.
“This is likely an underestimate, especially when we think about the U.S., which only has 44 confirmed cases at this time,” Dr. Freeman said. “However, at present, monkeypox cases have to be confirmed by the CDC, so there are a lot more suspected cases that are likely to be confirmed in the coming days. As with any outbreak, it’s a rapidly changing situation.”
A different clinical presentation
The clinical presentation of monkeypox cases in the current outbreak also differs from that of previous outbreaks. In the past, monkeypox rashes often morphed from a macule to a pustule and commonly affected the face, hands, feet, and trunk, with some patients harboring as many as 200 lesions at once. That pattern still occurs, but increasingly, the presentation is characterized by a more localized spread, especially in the genital region, which Dr. Freeman described as “unusual and not an area we traditionally thought of in the past as a focus for monkeypox.”
Also, affected individuals in the current outbreak may develop fewer lesions, sometimes between 1 and 5 instead of up to 200. “This doesn’t apply to everybody, but it is a bit of a different picture than what we’ve seen in case descriptions and photographs in the past from places like Central Africa,” she said. “What’s being reported out of case clusters from the United Kingdom and Spain is a mix, where some people are having more generalized involvement while others have more localized involvement.” Visual examples of the monkey pox rash can be found in photos from the United Kingdom, the country with the highest number of confirmed cases, on the CDC’s website, and in a report from Spain.
Clusters of monkeypox cases have been reported worldwide in men who have sex with men, “but this is not limited to a particular subgroup of people,” emphasizes Dr. Freeman, who is also a member of the American Academy of Dermatology’s Ad Hoc Task Force to Develop Monkeypox Content, which created an online resource for clinicians. “There are several mechanisms of spread, but direct contact with lesions or infected fluids is one,” she notes.
Moreover, If you have a patient with a new genital lesion and you’re not sure what it is, testing for monkeypox in addition to classic sexually transmitted infections like HSV or syphilis would be reasonable during the current outbreak situation.”
The 2022 monkeypox outbreak may pale in comparison to the spread of COVID-19 in terms of case numbers and societal impact, but dermatologists may be the first point of contact for a person infected with the monkeypox virus. “It’s important for dermatologists to be able to recognize monkeypox, because by recognizing cases, we can stop the outbreak,” Dr. Freeman said. “In theory, an infected person could show up in your clinic, regardless of where you practice in the U.S. But at the same time, it’s important not to panic. This is not COVID-19 all over again; this is different. Yes, it is an outbreak, but we already have a vaccine that works against monkeypox, and while one of the possible modes of transmission for monkeypox is respiratory, it’s much harder to transmit that way than SARS-CoV-2 – it requires closer and longer contact.”
Confirmation of a monkeypox virus infection is based on results of a PCR test based on swabs of a lesion. The AAD task force recommends contacting the local hospital epidemiologist, infection control personnel, and/or state health department about suspected cases, “as different locations will have different regulations on where to send the [PCR] test. If appropriate, the state health department will contact the CDC.”
According to the CDC, current recommendations for personal protective equipment for possible and confirmed monkeypox cases include gown, gloves, a National Institute for Occupational Safety and Health-approved N-95 mask, and eye protection.
Topical antiviral an option
If the lesions in a patient with suspected monkeypox have turned into pustules while waiting for the PCR test results, one option is to prescribe 3%-5% topical cidofovir, according to Stephen K. Tyring, MD, PhD, of the departments of dermatology, microbiology & molecular genetics, and internal medicine at the University of Texas Health Science Center, Houston. “That’s the effective antiviral that is most available,” he said. Generic cidofovir is also now available.
Dr. Tyring recommends rapid referral of immunocompromised patients with suspected monkeypox to an infectious disease expert and/or consulting with the CDC. “The pediatric population also seems to be at somewhat more risk, as has been seen in sub-Saharan Africa,” said Dr. Tyring, who is one of the editors of the textbook Tropical Dermatology. “Also, by definition, pregnant women are at more risk because their immune systems aren’t up to par. You also want to make sure that if monkeypox is on a person’s skin that they don’t get it in their eyes, because they could lose their vision.” He added that sub-Saharan Africa has a monkeypox mortality of up to 10%, “which is something we don’t see in the U.S. or Europe. Those of us who grew up in the 20th century got routine smallpox vaccines, and we therefore probably have a degree of immunity to monkeypox. But for the past 40 years or so, unless you are in the military, you are not going to get a routine vaccine to prevent smallpox.”
Incubation period, appearance of lesions
Monkeypox has a long incubation period. According to Dr. Freeman, from the point of exposure to the development of symptomatic lesions is typically 7-14 days but can vary from 5-21 days. “It’s important for people to be aware that their exposure may have been in the more distant past, not just a few days ago” she said. “Identifying cases as quickly as possible gives us a window where we can vaccinate close contacts.”
Dr. Freeman and Dr. Tyring reported having no relevant financial disclosures.
CDC guidance on vaccination before and after exposure to monkeypox can be found here . A general Q&A for health care professionals from the CDC can be found here.
When Esther Freeman, MD, PhD, thinks back on what she learned about monkeypox during her training as a dermatologist and an infectious disease epidemiologist, it was widely considered a viral disease with rare outbreaks limited primarily to Central and Western Africa.
“Monkeypox is something we have traditionally only seen very rarely in the U.S.,” Dr. Freeman, director of Global Health Dermatology at Massachusetts General Hospital and Harvard Medical School, Boston, said in an interview. “In the past, outbreaks in the U.S. have been related to international travel or import of exotic pets, which is very different than what we’re seeing as a global community now.”

Monkeypox virus belongs to the Orthopoxvirus genus in the family Poxviridae. According to the Centers for Disease Control and Prevention, symptoms develop 5-21 days after infection and may include fever, chills, and swollen lymph nodes. Typically, within 1-3 days of the fever, a rash develops, followed by the formation of monkeypox lesions. These lesions progress from macules to papules, vesicles, pustules, and scabs, before falling off. The illness typically lasts 2-4 weeks.
What makes the 2022 monkeypox outbreak different from others is clear evidence of community transmission. According to worldwide data from the CDC, as of June 9, 2022, there were 1,356 confirmed cases in 31 countries, including 44 cases in the United States. This means that person-to-person spread of the monkeypox virus is occurring among individuals who have not traveled outside of their own country.
“This is likely an underestimate, especially when we think about the U.S., which only has 44 confirmed cases at this time,” Dr. Freeman said. “However, at present, monkeypox cases have to be confirmed by the CDC, so there are a lot more suspected cases that are likely to be confirmed in the coming days. As with any outbreak, it’s a rapidly changing situation.”
A different clinical presentation
The clinical presentation of monkeypox cases in the current outbreak also differs from that of previous outbreaks. In the past, monkeypox rashes often morphed from a macule to a pustule and commonly affected the face, hands, feet, and trunk, with some patients harboring as many as 200 lesions at once. That pattern still occurs, but increasingly, the presentation is characterized by a more localized spread, especially in the genital region, which Dr. Freeman described as “unusual and not an area we traditionally thought of in the past as a focus for monkeypox.”
Also, affected individuals in the current outbreak may develop fewer lesions, sometimes between 1 and 5 instead of up to 200. “This doesn’t apply to everybody, but it is a bit of a different picture than what we’ve seen in case descriptions and photographs in the past from places like Central Africa,” she said. “What’s being reported out of case clusters from the United Kingdom and Spain is a mix, where some people are having more generalized involvement while others have more localized involvement.” Visual examples of the monkey pox rash can be found in photos from the United Kingdom, the country with the highest number of confirmed cases, on the CDC’s website, and in a report from Spain.
Clusters of monkeypox cases have been reported worldwide in men who have sex with men, “but this is not limited to a particular subgroup of people,” emphasizes Dr. Freeman, who is also a member of the American Academy of Dermatology’s Ad Hoc Task Force to Develop Monkeypox Content, which created an online resource for clinicians. “There are several mechanisms of spread, but direct contact with lesions or infected fluids is one,” she notes.
Moreover, If you have a patient with a new genital lesion and you’re not sure what it is, testing for monkeypox in addition to classic sexually transmitted infections like HSV or syphilis would be reasonable during the current outbreak situation.”
The 2022 monkeypox outbreak may pale in comparison to the spread of COVID-19 in terms of case numbers and societal impact, but dermatologists may be the first point of contact for a person infected with the monkeypox virus. “It’s important for dermatologists to be able to recognize monkeypox, because by recognizing cases, we can stop the outbreak,” Dr. Freeman said. “In theory, an infected person could show up in your clinic, regardless of where you practice in the U.S. But at the same time, it’s important not to panic. This is not COVID-19 all over again; this is different. Yes, it is an outbreak, but we already have a vaccine that works against monkeypox, and while one of the possible modes of transmission for monkeypox is respiratory, it’s much harder to transmit that way than SARS-CoV-2 – it requires closer and longer contact.”
Confirmation of a monkeypox virus infection is based on results of a PCR test based on swabs of a lesion. The AAD task force recommends contacting the local hospital epidemiologist, infection control personnel, and/or state health department about suspected cases, “as different locations will have different regulations on where to send the [PCR] test. If appropriate, the state health department will contact the CDC.”
According to the CDC, current recommendations for personal protective equipment for possible and confirmed monkeypox cases include gown, gloves, a National Institute for Occupational Safety and Health-approved N-95 mask, and eye protection.
Topical antiviral an option
If the lesions in a patient with suspected monkeypox have turned into pustules while waiting for the PCR test results, one option is to prescribe 3%-5% topical cidofovir, according to Stephen K. Tyring, MD, PhD, of the departments of dermatology, microbiology & molecular genetics, and internal medicine at the University of Texas Health Science Center, Houston. “That’s the effective antiviral that is most available,” he said. Generic cidofovir is also now available.
Dr. Tyring recommends rapid referral of immunocompromised patients with suspected monkeypox to an infectious disease expert and/or consulting with the CDC. “The pediatric population also seems to be at somewhat more risk, as has been seen in sub-Saharan Africa,” said Dr. Tyring, who is one of the editors of the textbook Tropical Dermatology. “Also, by definition, pregnant women are at more risk because their immune systems aren’t up to par. You also want to make sure that if monkeypox is on a person’s skin that they don’t get it in their eyes, because they could lose their vision.” He added that sub-Saharan Africa has a monkeypox mortality of up to 10%, “which is something we don’t see in the U.S. or Europe. Those of us who grew up in the 20th century got routine smallpox vaccines, and we therefore probably have a degree of immunity to monkeypox. But for the past 40 years or so, unless you are in the military, you are not going to get a routine vaccine to prevent smallpox.”
Incubation period, appearance of lesions
Monkeypox has a long incubation period. According to Dr. Freeman, from the point of exposure to the development of symptomatic lesions is typically 7-14 days but can vary from 5-21 days. “It’s important for people to be aware that their exposure may have been in the more distant past, not just a few days ago” she said. “Identifying cases as quickly as possible gives us a window where we can vaccinate close contacts.”
Dr. Freeman and Dr. Tyring reported having no relevant financial disclosures.
CDC guidance on vaccination before and after exposure to monkeypox can be found here . A general Q&A for health care professionals from the CDC can be found here.
‘My malpractice insurance doubled!’ Why, when fewer patients are suing?
Angela Intili, MD, an ob.gyn., was used to seeing her medical malpractice insurance premium rise slightly every couple of years. But she was shocked by the drastic rise she recently experienced.
In the last 2 years, Dr. Intili’s premiums shot from $60,000 to $130,000, she said.
“After 30 years of practice, this is the first time I’ve asked myself if I can even afford to continue practicing obstetrics and gynecology,” said Dr. Intili, 62, of Joliet, Ill. “It’s gotten very difficult to make ends meet as far as overhead because of the liability costs. I still love what I’m doing but I don’t know if I can afford to do it anymore.”
Even more frustrating for Dr. Intili was learning that claims in Illinois have sharply declined. From 2016 to 2020, tort filings in Illinois decreased by 43%, according to a state report.
“If claims are going down, I don’t understand why premium payments are going up,” she said.
Physicians across the country are experiencing a similar paradox. Claims are down, yet premiums are rising.
Medscape’s Malpractice Report 2021 found that 42% of primary care physicians were sued in 2020 through mid-2021, down from 52% in 2019. Fifty-six percent of specialists were sued in 2020 through mid-2021 compared with 62% in 2019, the report found. The pandemic was undoubtedly behind the decrease in suits, according to legal experts.
Yet, physicians paid higher premiums in 2021 and are on track for increases again in 2022, according to data and analysts.
According to Conning, direct premiums written for physicians increased 7.0% in 2021 (from $5.01 billion to $5.36 billion). Conning, an investment management firm that serves the insurance industry, analyzes annual financial reports filed by insurers to state insurance departments. The Medical Liability Monitor’s 2021 report found that premiums for internists, surgeons, and ob.gyns. in states without Patient Compensation Funds rose by an average of 2% in 2021.
The disparities raise questions about why physicians are paying higher premiums when having fewer claims is likely saving insurers’ money. Shouldn’t physicians’ rates reflect the reduction in claims?
Cases plummet during pandemic
During the pandemic, the volume of new medical malpractice claims dwindled to nearly nothing, said Michael Matray, editor of the Medical Liability Monitor, a national publication that analyzes medical liability insurance premiums.
“The court system closed for a while,” he said. “No elective procedures were being done in 2020 and the early parts of 2021. If you have no treatment, you have no malpractice, so of course, claims frequency tumbled down to a trickle.”
The number of large awards also decreased during the pandemic, noted Bill Burns, a director of insurance research at Conning.
“For claims that were already in the system, many of them could not be resolved because of the court closures, inability to take statements and depositions, etc.,” he said. “This resulted in a drop in verdicts.”
In 2021, there were 16 medical malpractice verdicts of $10 million or more in the United States, according to TransRe, an international reinsurance company that tracks large verdicts. In 2020, there were six verdicts of $10 million or more, TransRe research found. This is down from 52 verdicts of $10 million or more in 2019 and 46 verdicts of $10 million or more in 2018.
But although the pandemic lowered claims and decreased the number of payouts, one important aspect was untouched by the COVID era, said Richard E. Anderson, MD, chairman and CEO for The Doctors Company, a national medical liability insurer, and TDC Group.
“It’s a fair question: If claims are down, why are premiums continuing to go up?” Dr. Anderson said. “The answer is severity.”
High-dollar verdicts pave expensive path
The upward trend in severity has continued for about 6 years and has not slowed, Dr. Anderson said. Severity refers to high-dollar verdicts and settlements.
“We’re seeing record-high verdicts all over the country,” he said. “We used to have maps that showed the top 10 medical malpractice verdicts or awards, and they would be clustered where you’d expect them to be, New York, Florida, Illinois, and so forth. Now, if you look at those top 10 verdicts, they could be anywhere in the country.”
In Minnesota for instance, a jury awarded a record $111 million in damages to a college student in May after finding a hospital and an orthopedic surgeon negligent in treating his broken leg. In April, a Kansas City jury awarded a family $25 million after finding that an ob.gyn. and hospital failed to properly treat a mother in labor, causing brain damage to her infant.
Such record payouts factor into premium costs, said Ned Rand Jr., CEO for ProAssurance, a national medical liability insurer. Though only a minority of claims reach that level, when a high award occurs, it puts pressure on the ultimate cost to resolve claims, he said. The frequency of claims filed is also expected to soon rebound, he noted.
“As we price the product sitting here today, we have to factor both of those in,” Mr. Rand said. “That’s why we, as an industry, continue to see, by and large, rates going up. And we fell behind. Some of this severity, in particular, as an industry, we weren’t pricing fully for, so we’ve been playing catch-up.”
High-dollar awards – also called nuclear verdicts – set the arena for future settlements in similar cases, Dr. Anderson added.
“If it was an orthopedic case for instance, and there was a similar injury in another case, that’s the trial lawyers’ starting point for the award,” he said. “Now, they’re not going to get it, but it distorts the negotiations. As we have more and more nuclear verdicts, it becomes harder to settle claims for reasonable amounts.”
What does 2022 have in store?
Analysts say the backlog of malpractice claims in the court system could prove calamitous for premiums and the liability landscape.
Courts are slogging through the pileup caused by the pandemic, but it’s estimated that there is still about a one-third larger case backlog than normal, according to Mr. Matray.
Such delayed claims may end up costing more because of social inflation, said Mr. Burns.
“People look at the world differently than they did 2 years ago,” he said. “A jury may have awarded $5 million for a claim a few years ago. But then the pandemic hits, and we have the George Floyd incident, and we have people out of work and a shortage in baby formula. Yet, companies are still making a lot of money and many insurance companies are turning record profits. Today, that jury may look at a sympathetic malpractice victim and award $10 million for the same claim.”
Concerns also exist about a potential surge of new malpractice claims. Mr. Rand compares the possible wave to a large bubble.
“I liken it to a cartoon, when one character grabs the hose and a big bubble forms as the water builds up,” he said. “Then the character releases, and water comes flooding out. As an industry, we wait, wondering: Is there going to be this flood of claims as the court systems reopen and the statute of limitations approach around some of these claims? That’s an ongoing concern.”
As for impending premiums, physicians can expect rises in 2022 and again in 2023, according to Chris Wojciechowski, a partner at TigerRisk Partners, a reinsurance broker.
“In general, there is a lot of uncertainty around the state of the economy, the tort environment, litigation post COVID, and overall volatility across the capital markets,” he said. “Furthermore, thanks to social and financial inflation, the potential for very severe verdicts has increased dramatically, and as courthouses reopen, the trends are not looking favorable. While many of the physician carriers have strong balance sheets, they can’t lose money on an underwriting basis forever.”
For Dr. Intili, the Illinois ob.gyn., news of another impending increase in 2022 is distressing. She expects another 10%-20% rise in 2022, she said. If she were younger and earlier in her career, she might’ve considered moving, she said, but her family lives in Illinois and she cares for her older parents.
“I’m not ready to retire,” Dr. Intili said. “I’m looking into options, possibly becoming a hospitalist or doing locum tenens work. I’ve been a solo practitioner for 27 years and I love the autonomy. But these high premiums are making it almost impossible to continue.”
A version of this article first appeared on Medscape.com.
Angela Intili, MD, an ob.gyn., was used to seeing her medical malpractice insurance premium rise slightly every couple of years. But she was shocked by the drastic rise she recently experienced.
In the last 2 years, Dr. Intili’s premiums shot from $60,000 to $130,000, she said.
“After 30 years of practice, this is the first time I’ve asked myself if I can even afford to continue practicing obstetrics and gynecology,” said Dr. Intili, 62, of Joliet, Ill. “It’s gotten very difficult to make ends meet as far as overhead because of the liability costs. I still love what I’m doing but I don’t know if I can afford to do it anymore.”
Even more frustrating for Dr. Intili was learning that claims in Illinois have sharply declined. From 2016 to 2020, tort filings in Illinois decreased by 43%, according to a state report.
“If claims are going down, I don’t understand why premium payments are going up,” she said.
Physicians across the country are experiencing a similar paradox. Claims are down, yet premiums are rising.
Medscape’s Malpractice Report 2021 found that 42% of primary care physicians were sued in 2020 through mid-2021, down from 52% in 2019. Fifty-six percent of specialists were sued in 2020 through mid-2021 compared with 62% in 2019, the report found. The pandemic was undoubtedly behind the decrease in suits, according to legal experts.
Yet, physicians paid higher premiums in 2021 and are on track for increases again in 2022, according to data and analysts.
According to Conning, direct premiums written for physicians increased 7.0% in 2021 (from $5.01 billion to $5.36 billion). Conning, an investment management firm that serves the insurance industry, analyzes annual financial reports filed by insurers to state insurance departments. The Medical Liability Monitor’s 2021 report found that premiums for internists, surgeons, and ob.gyns. in states without Patient Compensation Funds rose by an average of 2% in 2021.
The disparities raise questions about why physicians are paying higher premiums when having fewer claims is likely saving insurers’ money. Shouldn’t physicians’ rates reflect the reduction in claims?
Cases plummet during pandemic
During the pandemic, the volume of new medical malpractice claims dwindled to nearly nothing, said Michael Matray, editor of the Medical Liability Monitor, a national publication that analyzes medical liability insurance premiums.
“The court system closed for a while,” he said. “No elective procedures were being done in 2020 and the early parts of 2021. If you have no treatment, you have no malpractice, so of course, claims frequency tumbled down to a trickle.”
The number of large awards also decreased during the pandemic, noted Bill Burns, a director of insurance research at Conning.
“For claims that were already in the system, many of them could not be resolved because of the court closures, inability to take statements and depositions, etc.,” he said. “This resulted in a drop in verdicts.”
In 2021, there were 16 medical malpractice verdicts of $10 million or more in the United States, according to TransRe, an international reinsurance company that tracks large verdicts. In 2020, there were six verdicts of $10 million or more, TransRe research found. This is down from 52 verdicts of $10 million or more in 2019 and 46 verdicts of $10 million or more in 2018.
But although the pandemic lowered claims and decreased the number of payouts, one important aspect was untouched by the COVID era, said Richard E. Anderson, MD, chairman and CEO for The Doctors Company, a national medical liability insurer, and TDC Group.
“It’s a fair question: If claims are down, why are premiums continuing to go up?” Dr. Anderson said. “The answer is severity.”
High-dollar verdicts pave expensive path
The upward trend in severity has continued for about 6 years and has not slowed, Dr. Anderson said. Severity refers to high-dollar verdicts and settlements.
“We’re seeing record-high verdicts all over the country,” he said. “We used to have maps that showed the top 10 medical malpractice verdicts or awards, and they would be clustered where you’d expect them to be, New York, Florida, Illinois, and so forth. Now, if you look at those top 10 verdicts, they could be anywhere in the country.”
In Minnesota for instance, a jury awarded a record $111 million in damages to a college student in May after finding a hospital and an orthopedic surgeon negligent in treating his broken leg. In April, a Kansas City jury awarded a family $25 million after finding that an ob.gyn. and hospital failed to properly treat a mother in labor, causing brain damage to her infant.
Such record payouts factor into premium costs, said Ned Rand Jr., CEO for ProAssurance, a national medical liability insurer. Though only a minority of claims reach that level, when a high award occurs, it puts pressure on the ultimate cost to resolve claims, he said. The frequency of claims filed is also expected to soon rebound, he noted.
“As we price the product sitting here today, we have to factor both of those in,” Mr. Rand said. “That’s why we, as an industry, continue to see, by and large, rates going up. And we fell behind. Some of this severity, in particular, as an industry, we weren’t pricing fully for, so we’ve been playing catch-up.”
High-dollar awards – also called nuclear verdicts – set the arena for future settlements in similar cases, Dr. Anderson added.
“If it was an orthopedic case for instance, and there was a similar injury in another case, that’s the trial lawyers’ starting point for the award,” he said. “Now, they’re not going to get it, but it distorts the negotiations. As we have more and more nuclear verdicts, it becomes harder to settle claims for reasonable amounts.”
What does 2022 have in store?
Analysts say the backlog of malpractice claims in the court system could prove calamitous for premiums and the liability landscape.
Courts are slogging through the pileup caused by the pandemic, but it’s estimated that there is still about a one-third larger case backlog than normal, according to Mr. Matray.
Such delayed claims may end up costing more because of social inflation, said Mr. Burns.
“People look at the world differently than they did 2 years ago,” he said. “A jury may have awarded $5 million for a claim a few years ago. But then the pandemic hits, and we have the George Floyd incident, and we have people out of work and a shortage in baby formula. Yet, companies are still making a lot of money and many insurance companies are turning record profits. Today, that jury may look at a sympathetic malpractice victim and award $10 million for the same claim.”
Concerns also exist about a potential surge of new malpractice claims. Mr. Rand compares the possible wave to a large bubble.
“I liken it to a cartoon, when one character grabs the hose and a big bubble forms as the water builds up,” he said. “Then the character releases, and water comes flooding out. As an industry, we wait, wondering: Is there going to be this flood of claims as the court systems reopen and the statute of limitations approach around some of these claims? That’s an ongoing concern.”
As for impending premiums, physicians can expect rises in 2022 and again in 2023, according to Chris Wojciechowski, a partner at TigerRisk Partners, a reinsurance broker.
“In general, there is a lot of uncertainty around the state of the economy, the tort environment, litigation post COVID, and overall volatility across the capital markets,” he said. “Furthermore, thanks to social and financial inflation, the potential for very severe verdicts has increased dramatically, and as courthouses reopen, the trends are not looking favorable. While many of the physician carriers have strong balance sheets, they can’t lose money on an underwriting basis forever.”
For Dr. Intili, the Illinois ob.gyn., news of another impending increase in 2022 is distressing. She expects another 10%-20% rise in 2022, she said. If she were younger and earlier in her career, she might’ve considered moving, she said, but her family lives in Illinois and she cares for her older parents.
“I’m not ready to retire,” Dr. Intili said. “I’m looking into options, possibly becoming a hospitalist or doing locum tenens work. I’ve been a solo practitioner for 27 years and I love the autonomy. But these high premiums are making it almost impossible to continue.”
A version of this article first appeared on Medscape.com.
Angela Intili, MD, an ob.gyn., was used to seeing her medical malpractice insurance premium rise slightly every couple of years. But she was shocked by the drastic rise she recently experienced.
In the last 2 years, Dr. Intili’s premiums shot from $60,000 to $130,000, she said.
“After 30 years of practice, this is the first time I’ve asked myself if I can even afford to continue practicing obstetrics and gynecology,” said Dr. Intili, 62, of Joliet, Ill. “It’s gotten very difficult to make ends meet as far as overhead because of the liability costs. I still love what I’m doing but I don’t know if I can afford to do it anymore.”
Even more frustrating for Dr. Intili was learning that claims in Illinois have sharply declined. From 2016 to 2020, tort filings in Illinois decreased by 43%, according to a state report.
“If claims are going down, I don’t understand why premium payments are going up,” she said.
Physicians across the country are experiencing a similar paradox. Claims are down, yet premiums are rising.
Medscape’s Malpractice Report 2021 found that 42% of primary care physicians were sued in 2020 through mid-2021, down from 52% in 2019. Fifty-six percent of specialists were sued in 2020 through mid-2021 compared with 62% in 2019, the report found. The pandemic was undoubtedly behind the decrease in suits, according to legal experts.
Yet, physicians paid higher premiums in 2021 and are on track for increases again in 2022, according to data and analysts.
According to Conning, direct premiums written for physicians increased 7.0% in 2021 (from $5.01 billion to $5.36 billion). Conning, an investment management firm that serves the insurance industry, analyzes annual financial reports filed by insurers to state insurance departments. The Medical Liability Monitor’s 2021 report found that premiums for internists, surgeons, and ob.gyns. in states without Patient Compensation Funds rose by an average of 2% in 2021.
The disparities raise questions about why physicians are paying higher premiums when having fewer claims is likely saving insurers’ money. Shouldn’t physicians’ rates reflect the reduction in claims?
Cases plummet during pandemic
During the pandemic, the volume of new medical malpractice claims dwindled to nearly nothing, said Michael Matray, editor of the Medical Liability Monitor, a national publication that analyzes medical liability insurance premiums.
“The court system closed for a while,” he said. “No elective procedures were being done in 2020 and the early parts of 2021. If you have no treatment, you have no malpractice, so of course, claims frequency tumbled down to a trickle.”
The number of large awards also decreased during the pandemic, noted Bill Burns, a director of insurance research at Conning.
“For claims that were already in the system, many of them could not be resolved because of the court closures, inability to take statements and depositions, etc.,” he said. “This resulted in a drop in verdicts.”
In 2021, there were 16 medical malpractice verdicts of $10 million or more in the United States, according to TransRe, an international reinsurance company that tracks large verdicts. In 2020, there were six verdicts of $10 million or more, TransRe research found. This is down from 52 verdicts of $10 million or more in 2019 and 46 verdicts of $10 million or more in 2018.
But although the pandemic lowered claims and decreased the number of payouts, one important aspect was untouched by the COVID era, said Richard E. Anderson, MD, chairman and CEO for The Doctors Company, a national medical liability insurer, and TDC Group.
“It’s a fair question: If claims are down, why are premiums continuing to go up?” Dr. Anderson said. “The answer is severity.”
High-dollar verdicts pave expensive path
The upward trend in severity has continued for about 6 years and has not slowed, Dr. Anderson said. Severity refers to high-dollar verdicts and settlements.
“We’re seeing record-high verdicts all over the country,” he said. “We used to have maps that showed the top 10 medical malpractice verdicts or awards, and they would be clustered where you’d expect them to be, New York, Florida, Illinois, and so forth. Now, if you look at those top 10 verdicts, they could be anywhere in the country.”
In Minnesota for instance, a jury awarded a record $111 million in damages to a college student in May after finding a hospital and an orthopedic surgeon negligent in treating his broken leg. In April, a Kansas City jury awarded a family $25 million after finding that an ob.gyn. and hospital failed to properly treat a mother in labor, causing brain damage to her infant.
Such record payouts factor into premium costs, said Ned Rand Jr., CEO for ProAssurance, a national medical liability insurer. Though only a minority of claims reach that level, when a high award occurs, it puts pressure on the ultimate cost to resolve claims, he said. The frequency of claims filed is also expected to soon rebound, he noted.
“As we price the product sitting here today, we have to factor both of those in,” Mr. Rand said. “That’s why we, as an industry, continue to see, by and large, rates going up. And we fell behind. Some of this severity, in particular, as an industry, we weren’t pricing fully for, so we’ve been playing catch-up.”
High-dollar awards – also called nuclear verdicts – set the arena for future settlements in similar cases, Dr. Anderson added.
“If it was an orthopedic case for instance, and there was a similar injury in another case, that’s the trial lawyers’ starting point for the award,” he said. “Now, they’re not going to get it, but it distorts the negotiations. As we have more and more nuclear verdicts, it becomes harder to settle claims for reasonable amounts.”
What does 2022 have in store?
Analysts say the backlog of malpractice claims in the court system could prove calamitous for premiums and the liability landscape.
Courts are slogging through the pileup caused by the pandemic, but it’s estimated that there is still about a one-third larger case backlog than normal, according to Mr. Matray.
Such delayed claims may end up costing more because of social inflation, said Mr. Burns.
“People look at the world differently than they did 2 years ago,” he said. “A jury may have awarded $5 million for a claim a few years ago. But then the pandemic hits, and we have the George Floyd incident, and we have people out of work and a shortage in baby formula. Yet, companies are still making a lot of money and many insurance companies are turning record profits. Today, that jury may look at a sympathetic malpractice victim and award $10 million for the same claim.”
Concerns also exist about a potential surge of new malpractice claims. Mr. Rand compares the possible wave to a large bubble.
“I liken it to a cartoon, when one character grabs the hose and a big bubble forms as the water builds up,” he said. “Then the character releases, and water comes flooding out. As an industry, we wait, wondering: Is there going to be this flood of claims as the court systems reopen and the statute of limitations approach around some of these claims? That’s an ongoing concern.”
As for impending premiums, physicians can expect rises in 2022 and again in 2023, according to Chris Wojciechowski, a partner at TigerRisk Partners, a reinsurance broker.
“In general, there is a lot of uncertainty around the state of the economy, the tort environment, litigation post COVID, and overall volatility across the capital markets,” he said. “Furthermore, thanks to social and financial inflation, the potential for very severe verdicts has increased dramatically, and as courthouses reopen, the trends are not looking favorable. While many of the physician carriers have strong balance sheets, they can’t lose money on an underwriting basis forever.”
For Dr. Intili, the Illinois ob.gyn., news of another impending increase in 2022 is distressing. She expects another 10%-20% rise in 2022, she said. If she were younger and earlier in her career, she might’ve considered moving, she said, but her family lives in Illinois and she cares for her older parents.
“I’m not ready to retire,” Dr. Intili said. “I’m looking into options, possibly becoming a hospitalist or doing locum tenens work. I’ve been a solo practitioner for 27 years and I love the autonomy. But these high premiums are making it almost impossible to continue.”
A version of this article first appeared on Medscape.com.
FDA cautions against using OTC products to remove skin spots, moles
Those moles, skin tags, and liver spots should stay on your skin until you see a doctor, according to a new alert from the U.S. Food and Drug Administration. The alert warns against the use of over-the-counter products for removing moles, seborrheic keratoses (wart-like growths that are often brown), or skin tags, emphasizing that none are approved by the FDA for at-home use.
Dermatologists and the FDA say these products may lead to scarring and disfigurement.
Risks include “skin injuries, infection requiring antibiotics, scarring, and delayed skin cancer diagnosis and treatment,” according to the alert, which adds that the agency has received reports of people “who developed permanent skin injuries and infections after using products marketed as mole or skin tag removers. “
These products come in the form of gels, liquids, sticks, or ointments and commonly contain ingredients like salicylic acid, which are cytotoxic, or cell-killing. These chemicals are what make the products potentially dangerous, as each contains unregulated, and likely very high, amounts of these corrosive agents. Even products marketed as natural or organic have these same issues, said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, who notes that bloodroot is another ingredient found in these products.
Dr. Friedman explained that using these products without the supervision of a health care provider can create a chemical burn in the skin, leading to scarring. He’s treated patients for open wounds and infected ulcers caused by these products. “Over my career, I’ve seen many cases of patients coming in with self-inflicted harm due to using these quote, unquote, safe and natural products to remove benign, or even worse, potentially malignant neoplasms,” he told this news organization.
Another concern is that these spots on the skin are often the only sign of a serious issue – cancer. Early signs of melanoma, a type of skin cancer, include large, misshapen, or rapidly changing moles. Dr. Friedman said that if a patient uses one of these products on what is actually a cancerous mole, they will likely only remove the surface, and in turn, destroy the only sign of cancer – effectively killing the canary in the coal mine.
There’s a good chance that the root of the mole has been left intact under the skin surface, and as a result, the cancer has the potential to spread unnoticed. “If people aren’t going to a dermatologist to be properly diagnosed and properly managed, they’re going to cause more harm by thinking that they’ve taken care of a problem,” he said.
If you are concerned about any type of spot on your skin, a visit to the dermatologist will prove much simpler and safer for treating it than doing so at home. In the office, Dr. Friedman said, providers can use a range of highly studied techniques to remove skin lesions with minimal pain and scarring. From freezing, burning, snipping, or a quick moment under a scalpel, you’ll be healed in no time.
Anyone who has experienced an adverse event with one of these products and health care professionals should report cases to the FDA’s MedWatch Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
Those moles, skin tags, and liver spots should stay on your skin until you see a doctor, according to a new alert from the U.S. Food and Drug Administration. The alert warns against the use of over-the-counter products for removing moles, seborrheic keratoses (wart-like growths that are often brown), or skin tags, emphasizing that none are approved by the FDA for at-home use.
Dermatologists and the FDA say these products may lead to scarring and disfigurement.
Risks include “skin injuries, infection requiring antibiotics, scarring, and delayed skin cancer diagnosis and treatment,” according to the alert, which adds that the agency has received reports of people “who developed permanent skin injuries and infections after using products marketed as mole or skin tag removers. “
These products come in the form of gels, liquids, sticks, or ointments and commonly contain ingredients like salicylic acid, which are cytotoxic, or cell-killing. These chemicals are what make the products potentially dangerous, as each contains unregulated, and likely very high, amounts of these corrosive agents. Even products marketed as natural or organic have these same issues, said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, who notes that bloodroot is another ingredient found in these products.
Dr. Friedman explained that using these products without the supervision of a health care provider can create a chemical burn in the skin, leading to scarring. He’s treated patients for open wounds and infected ulcers caused by these products. “Over my career, I’ve seen many cases of patients coming in with self-inflicted harm due to using these quote, unquote, safe and natural products to remove benign, or even worse, potentially malignant neoplasms,” he told this news organization.
Another concern is that these spots on the skin are often the only sign of a serious issue – cancer. Early signs of melanoma, a type of skin cancer, include large, misshapen, or rapidly changing moles. Dr. Friedman said that if a patient uses one of these products on what is actually a cancerous mole, they will likely only remove the surface, and in turn, destroy the only sign of cancer – effectively killing the canary in the coal mine.
There’s a good chance that the root of the mole has been left intact under the skin surface, and as a result, the cancer has the potential to spread unnoticed. “If people aren’t going to a dermatologist to be properly diagnosed and properly managed, they’re going to cause more harm by thinking that they’ve taken care of a problem,” he said.
If you are concerned about any type of spot on your skin, a visit to the dermatologist will prove much simpler and safer for treating it than doing so at home. In the office, Dr. Friedman said, providers can use a range of highly studied techniques to remove skin lesions with minimal pain and scarring. From freezing, burning, snipping, or a quick moment under a scalpel, you’ll be healed in no time.
Anyone who has experienced an adverse event with one of these products and health care professionals should report cases to the FDA’s MedWatch Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
Those moles, skin tags, and liver spots should stay on your skin until you see a doctor, according to a new alert from the U.S. Food and Drug Administration. The alert warns against the use of over-the-counter products for removing moles, seborrheic keratoses (wart-like growths that are often brown), or skin tags, emphasizing that none are approved by the FDA for at-home use.
Dermatologists and the FDA say these products may lead to scarring and disfigurement.
Risks include “skin injuries, infection requiring antibiotics, scarring, and delayed skin cancer diagnosis and treatment,” according to the alert, which adds that the agency has received reports of people “who developed permanent skin injuries and infections after using products marketed as mole or skin tag removers. “
These products come in the form of gels, liquids, sticks, or ointments and commonly contain ingredients like salicylic acid, which are cytotoxic, or cell-killing. These chemicals are what make the products potentially dangerous, as each contains unregulated, and likely very high, amounts of these corrosive agents. Even products marketed as natural or organic have these same issues, said Adam Friedman, MD, professor and chief of dermatology at George Washington University, Washington, who notes that bloodroot is another ingredient found in these products.
Dr. Friedman explained that using these products without the supervision of a health care provider can create a chemical burn in the skin, leading to scarring. He’s treated patients for open wounds and infected ulcers caused by these products. “Over my career, I’ve seen many cases of patients coming in with self-inflicted harm due to using these quote, unquote, safe and natural products to remove benign, or even worse, potentially malignant neoplasms,” he told this news organization.
Another concern is that these spots on the skin are often the only sign of a serious issue – cancer. Early signs of melanoma, a type of skin cancer, include large, misshapen, or rapidly changing moles. Dr. Friedman said that if a patient uses one of these products on what is actually a cancerous mole, they will likely only remove the surface, and in turn, destroy the only sign of cancer – effectively killing the canary in the coal mine.
There’s a good chance that the root of the mole has been left intact under the skin surface, and as a result, the cancer has the potential to spread unnoticed. “If people aren’t going to a dermatologist to be properly diagnosed and properly managed, they’re going to cause more harm by thinking that they’ve taken care of a problem,” he said.
If you are concerned about any type of spot on your skin, a visit to the dermatologist will prove much simpler and safer for treating it than doing so at home. In the office, Dr. Friedman said, providers can use a range of highly studied techniques to remove skin lesions with minimal pain and scarring. From freezing, burning, snipping, or a quick moment under a scalpel, you’ll be healed in no time.
Anyone who has experienced an adverse event with one of these products and health care professionals should report cases to the FDA’s MedWatch Adverse Event Reporting Program.
A version of this article first appeared on Medscape.com.
Atopic dermatitis: Options abound, and more are coming
, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
More and more treatment options are available and even more are in the pipeline, said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of dermatology at the University of California, San Diego and Rady Children’s Hospital. As he put it: “We got pills, injections, things to smear on the skin.”
Those options are welcome and needed, as AD affects up to 20% of children and up to 10% of adults. The course is variable, as is severity, and quality of life is impacted.
Besides new treatment options, there is a new understanding about comorbidities, environmental effects, and triggers, Dr. Eichenfield said. Among the potential comorbidities health care providers should be aware of are allergies, such as food allergies; asthma; rhinitis; mental health issues (depression, anxiety, ADHD, learning disabilities, or in adults, substance abuse); bone health; skin infections; immune disorders such as alopecia areata or urticaria; and cardiovascular issues that could affect adults.
Environmental effects can play a role in aggravating AD, as providers learned after visits for AD increased after Northern California wildfires and also in other areas with high air pollution, Dr. Eichenfield said. “I actually discuss this with my families,” when making them aware of factors that may affect AD, he noted.
Dr. Eichenfield provided an overview of available treatment options, and what treatments may be coming next. Among the highlights:
Topical ruxolitinib: A JAK1,2 inhibitor in a cream formulation, it is now approved for patients with mild to moderate AD aged 12 years and older in the United States. Of the two strengths studied, the higher strength, 1.5%, was approved, Dr. Eichenfield said. How well did it work? In two phase 3 studies in patients aged 12 and older, of those on 1.5%, 53% were clear or almost clear at 8 weeks, versus 11% in the control group given the vehicle; 52% had at least a 4-point reduction in itch from baseline, versus 15.4% on vehicle. Quality of life improved in up to 73.2% of those given the medication versus 19.7% of those on the vehicle. There was a marked and quick improvement in itch, as early as 12 hours, and safety measures also look good, he said.
Topical tapinarof: Approved in May 2022, for adults with plaque psoriasis, phase 3 trials began in September, 2021, for adults and children with AD, according to the manufacturer. Activation of the aryl hydrocarbon receptor mediates its anti-inflammatory properties.
Topical roflumilast: A potent PDE-4 inhibitor, phase 3 AD studies are underway. It appears to be well tolerated, Dr. Eichenfield said.
Dupilumab: An IL-4/13 blocker, this biologic produced an itch reduction of 50% and EASI of 80%, improved quality of life, and reduced anxiety and depression. The drug “led the revolution in systemic therapy for atopic dermatitis,” he said. First approved for treating AD in patients aged 18 years and up in 2017, approval for patients 12 years and up followed in March 2019, then for age 6 years and up May 2020.
At the meeting on June 3, Dr. Eichenfield said that approval in children 5 years and under was imminent, and on June 7, the FDA approved dupilumab for use in children aged 6 months to 5 years. In a phase 3, 16-week trial, 28% of children treated with dupilumab added on to low-potency topical corticosteroids met the endpoint of clear or nearly clear skin, compared with 4% of those on the corticosteroids alone (P < .0001).
Tralokinumab: There is no approved indication yet for adolescents, but the injected biologic, an interleukin-13 antagonist, is approved for adults with moderate to severe AD who are not well-controlled with topicals, or who cannot use topicals.
Oral JAK inhibitors: These include abrocitinib and upadacitinib, both approved by the FDA in January 2022 for treating moderate to severe AD, and baricitinib (the latter not in the United States). “For AD, you probably won’t see it in the U.S.,” Dr. Eichenfield said, referring to baricitinib. However, it might get approved for alopecia areata, he noted.
Upadacitinib is approved for adolescents 12 and older with AD. Abrocitinib is approved for adults 18 and older with AD.
Regarding safety and tolerance concerns with oral JAK inhibitors, Dr. Eichenfield cites headache, acne, nausea, and upper respiratory tract infections as relatively common, while herpes zoster, venous thromboembolism, and lab anomalies (neutropenia, elevated CPK) are uncommon.
As the options for AD treatments increase, and expectations by families and clinicians change, Dr. Eichenfield said he often focuses on “bucket duty” – whether a specific patient should be in the topical bucket or the systemic one. It’s a decision that will continue to be crucial, he said.
When presented with treatment options, patients – and parents – often worry about side effects, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas Medical Center, Little Rock, who also spoke at the meeting. She gently tells them: “The worst side effect you can have is probably not treating the disease itself.”
Medscape Live and this news organization are owned by the same parent company. Dr. Eichenfield is a consultant or investigator for numerous companies that manufacture treatments for AD, but based his discussion on evidence-based recommendations and public presentations or publications.
, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
More and more treatment options are available and even more are in the pipeline, said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of dermatology at the University of California, San Diego and Rady Children’s Hospital. As he put it: “We got pills, injections, things to smear on the skin.”
Those options are welcome and needed, as AD affects up to 20% of children and up to 10% of adults. The course is variable, as is severity, and quality of life is impacted.
Besides new treatment options, there is a new understanding about comorbidities, environmental effects, and triggers, Dr. Eichenfield said. Among the potential comorbidities health care providers should be aware of are allergies, such as food allergies; asthma; rhinitis; mental health issues (depression, anxiety, ADHD, learning disabilities, or in adults, substance abuse); bone health; skin infections; immune disorders such as alopecia areata or urticaria; and cardiovascular issues that could affect adults.
Environmental effects can play a role in aggravating AD, as providers learned after visits for AD increased after Northern California wildfires and also in other areas with high air pollution, Dr. Eichenfield said. “I actually discuss this with my families,” when making them aware of factors that may affect AD, he noted.
Dr. Eichenfield provided an overview of available treatment options, and what treatments may be coming next. Among the highlights:
Topical ruxolitinib: A JAK1,2 inhibitor in a cream formulation, it is now approved for patients with mild to moderate AD aged 12 years and older in the United States. Of the two strengths studied, the higher strength, 1.5%, was approved, Dr. Eichenfield said. How well did it work? In two phase 3 studies in patients aged 12 and older, of those on 1.5%, 53% were clear or almost clear at 8 weeks, versus 11% in the control group given the vehicle; 52% had at least a 4-point reduction in itch from baseline, versus 15.4% on vehicle. Quality of life improved in up to 73.2% of those given the medication versus 19.7% of those on the vehicle. There was a marked and quick improvement in itch, as early as 12 hours, and safety measures also look good, he said.
Topical tapinarof: Approved in May 2022, for adults with plaque psoriasis, phase 3 trials began in September, 2021, for adults and children with AD, according to the manufacturer. Activation of the aryl hydrocarbon receptor mediates its anti-inflammatory properties.
Topical roflumilast: A potent PDE-4 inhibitor, phase 3 AD studies are underway. It appears to be well tolerated, Dr. Eichenfield said.
Dupilumab: An IL-4/13 blocker, this biologic produced an itch reduction of 50% and EASI of 80%, improved quality of life, and reduced anxiety and depression. The drug “led the revolution in systemic therapy for atopic dermatitis,” he said. First approved for treating AD in patients aged 18 years and up in 2017, approval for patients 12 years and up followed in March 2019, then for age 6 years and up May 2020.
At the meeting on June 3, Dr. Eichenfield said that approval in children 5 years and under was imminent, and on June 7, the FDA approved dupilumab for use in children aged 6 months to 5 years. In a phase 3, 16-week trial, 28% of children treated with dupilumab added on to low-potency topical corticosteroids met the endpoint of clear or nearly clear skin, compared with 4% of those on the corticosteroids alone (P < .0001).
Tralokinumab: There is no approved indication yet for adolescents, but the injected biologic, an interleukin-13 antagonist, is approved for adults with moderate to severe AD who are not well-controlled with topicals, or who cannot use topicals.
Oral JAK inhibitors: These include abrocitinib and upadacitinib, both approved by the FDA in January 2022 for treating moderate to severe AD, and baricitinib (the latter not in the United States). “For AD, you probably won’t see it in the U.S.,” Dr. Eichenfield said, referring to baricitinib. However, it might get approved for alopecia areata, he noted.
Upadacitinib is approved for adolescents 12 and older with AD. Abrocitinib is approved for adults 18 and older with AD.
Regarding safety and tolerance concerns with oral JAK inhibitors, Dr. Eichenfield cites headache, acne, nausea, and upper respiratory tract infections as relatively common, while herpes zoster, venous thromboembolism, and lab anomalies (neutropenia, elevated CPK) are uncommon.
As the options for AD treatments increase, and expectations by families and clinicians change, Dr. Eichenfield said he often focuses on “bucket duty” – whether a specific patient should be in the topical bucket or the systemic one. It’s a decision that will continue to be crucial, he said.
When presented with treatment options, patients – and parents – often worry about side effects, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas Medical Center, Little Rock, who also spoke at the meeting. She gently tells them: “The worst side effect you can have is probably not treating the disease itself.”
Medscape Live and this news organization are owned by the same parent company. Dr. Eichenfield is a consultant or investigator for numerous companies that manufacture treatments for AD, but based his discussion on evidence-based recommendations and public presentations or publications.
, said at MedscapeLive’s Women’s & Pediatric Dermatology Seminar.
More and more treatment options are available and even more are in the pipeline, said Dr. Eichenfield, professor of dermatology and pediatrics and vice chair of dermatology at the University of California, San Diego and Rady Children’s Hospital. As he put it: “We got pills, injections, things to smear on the skin.”
Those options are welcome and needed, as AD affects up to 20% of children and up to 10% of adults. The course is variable, as is severity, and quality of life is impacted.
Besides new treatment options, there is a new understanding about comorbidities, environmental effects, and triggers, Dr. Eichenfield said. Among the potential comorbidities health care providers should be aware of are allergies, such as food allergies; asthma; rhinitis; mental health issues (depression, anxiety, ADHD, learning disabilities, or in adults, substance abuse); bone health; skin infections; immune disorders such as alopecia areata or urticaria; and cardiovascular issues that could affect adults.
Environmental effects can play a role in aggravating AD, as providers learned after visits for AD increased after Northern California wildfires and also in other areas with high air pollution, Dr. Eichenfield said. “I actually discuss this with my families,” when making them aware of factors that may affect AD, he noted.
Dr. Eichenfield provided an overview of available treatment options, and what treatments may be coming next. Among the highlights:
Topical ruxolitinib: A JAK1,2 inhibitor in a cream formulation, it is now approved for patients with mild to moderate AD aged 12 years and older in the United States. Of the two strengths studied, the higher strength, 1.5%, was approved, Dr. Eichenfield said. How well did it work? In two phase 3 studies in patients aged 12 and older, of those on 1.5%, 53% were clear or almost clear at 8 weeks, versus 11% in the control group given the vehicle; 52% had at least a 4-point reduction in itch from baseline, versus 15.4% on vehicle. Quality of life improved in up to 73.2% of those given the medication versus 19.7% of those on the vehicle. There was a marked and quick improvement in itch, as early as 12 hours, and safety measures also look good, he said.
Topical tapinarof: Approved in May 2022, for adults with plaque psoriasis, phase 3 trials began in September, 2021, for adults and children with AD, according to the manufacturer. Activation of the aryl hydrocarbon receptor mediates its anti-inflammatory properties.
Topical roflumilast: A potent PDE-4 inhibitor, phase 3 AD studies are underway. It appears to be well tolerated, Dr. Eichenfield said.
Dupilumab: An IL-4/13 blocker, this biologic produced an itch reduction of 50% and EASI of 80%, improved quality of life, and reduced anxiety and depression. The drug “led the revolution in systemic therapy for atopic dermatitis,” he said. First approved for treating AD in patients aged 18 years and up in 2017, approval for patients 12 years and up followed in March 2019, then for age 6 years and up May 2020.
At the meeting on June 3, Dr. Eichenfield said that approval in children 5 years and under was imminent, and on June 7, the FDA approved dupilumab for use in children aged 6 months to 5 years. In a phase 3, 16-week trial, 28% of children treated with dupilumab added on to low-potency topical corticosteroids met the endpoint of clear or nearly clear skin, compared with 4% of those on the corticosteroids alone (P < .0001).
Tralokinumab: There is no approved indication yet for adolescents, but the injected biologic, an interleukin-13 antagonist, is approved for adults with moderate to severe AD who are not well-controlled with topicals, or who cannot use topicals.
Oral JAK inhibitors: These include abrocitinib and upadacitinib, both approved by the FDA in January 2022 for treating moderate to severe AD, and baricitinib (the latter not in the United States). “For AD, you probably won’t see it in the U.S.,” Dr. Eichenfield said, referring to baricitinib. However, it might get approved for alopecia areata, he noted.
Upadacitinib is approved for adolescents 12 and older with AD. Abrocitinib is approved for adults 18 and older with AD.
Regarding safety and tolerance concerns with oral JAK inhibitors, Dr. Eichenfield cites headache, acne, nausea, and upper respiratory tract infections as relatively common, while herpes zoster, venous thromboembolism, and lab anomalies (neutropenia, elevated CPK) are uncommon.
As the options for AD treatments increase, and expectations by families and clinicians change, Dr. Eichenfield said he often focuses on “bucket duty” – whether a specific patient should be in the topical bucket or the systemic one. It’s a decision that will continue to be crucial, he said.
When presented with treatment options, patients – and parents – often worry about side effects, said Vivian Shi, MD, associate professor of dermatology at the University of Arkansas Medical Center, Little Rock, who also spoke at the meeting. She gently tells them: “The worst side effect you can have is probably not treating the disease itself.”
Medscape Live and this news organization are owned by the same parent company. Dr. Eichenfield is a consultant or investigator for numerous companies that manufacture treatments for AD, but based his discussion on evidence-based recommendations and public presentations or publications.
FROM MEDSCAPELIVE WOMEN’S & PEDIATRIC DERMATOLOGY SEMINAR