User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Atopic Dermatitis Topical Therapies: Study of YouTube Videos as a Source of Patient Information
To the Editor:
Atopic dermatitis (eczema) affects approximately 20% of children worldwide.1 In atopic dermatitis management, patient education is crucial for optimal outcomes.2 The COVID-19 pandemic has impacted patient-physician interactions. To ensure safety of patients and physicians, visits may have been canceled, postponed, or conducted virtually, leaving less time for discussion and questions.3 As a consequence, patients may seek information about atopic dermatitis from alternative sources, including YouTube videos. We performed a cross-sectional study to analyze YouTube videos about topical treatments for atopic dermatitis.
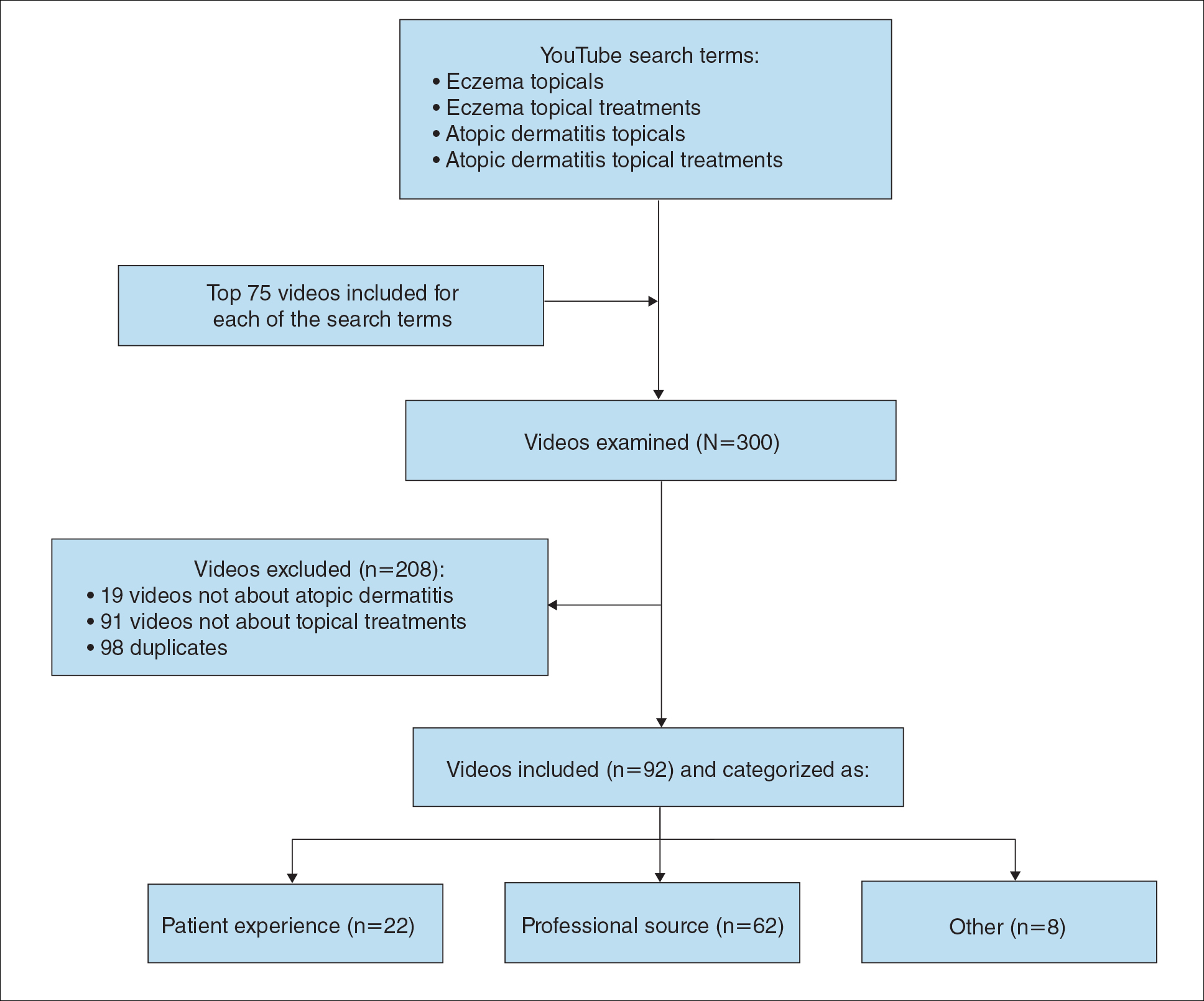
During the week of July 16, 2020, we performed 4 private browser YouTube searches with default filters using the following terms: eczema topicals, eczema topical treatments, atopic dermatitis topicals, and atopic dermatitis topical treatments. For video selection, we defined topical treatments as topical corticosteroids, topical calcineurin inhibitors, crisaborole, emollients, wet wraps, and any prospective treatment topically administered. For each of the 4 searches, 2 researchers (A.M. and A.T.) independently examined the top 75 videos, yielding a total of 300 videos. Of them, 98 videos were duplicates, 19 videos were not about atopic dermatitis, and 91 videos were not about topical treatments, leaving a total of 92 videos for analysis (Figure 1).
For the 92 included videos, the length; upload year; number of views, likes, dislikes, and comments; interaction ratio (IR)(the sum of likes, dislikes, and comments divided by the number of views); and video content were determined. The videos were placed into mutually exclusive categories as follows: (1) patient experience, defined as a video about patient perspective; (2) professional source, defined as a video featuring a physician, physician extender, pharmacist, or scientist, or produced by a formal organization; or (3) other. The DISCERN Instrument was used for grading the reliability and quality of the 92 included videos. This instrument consists of 16 questions with the responses rated on a scale of 1 to 5.4 For analysis of DISCERN scores, patient experience and other videos were grouped together as nonprofessional source videos. A 2-sample t-test was used to compare DISCERN scores between professional source and nonprofessional source videos.
Most videos were uploaded in 2017 (n=19), 2018 (n=23), and 2019 (n=25), but 20 were uploaded in 2012-2016 and 5 were uploaded in 2020. The 92 videos had a mean length of 8 minutes and 35 seconds (range, 30 seconds to 62 minutes and 23 seconds).
Patient experience videos accounted for 23.9% (n=22) of videos. These videos discussed topical steroid withdrawal (TSW)(n=16), instructions for making emollients (n=2), and treatment successes (n=4). Professional source videos represented 67.4% (n=62) of videos. Of them, 40.3% (n=25) were physician oriented, defined as having extensive medical terminology or qualifying for continuing medical education credit. Three (4.8%) of the professional source videos were sponsored by a drug company. Other constituted the remaining 8.7% (n=8) of videos. Patient experience videos had more views (median views [interquartile range], 6865 [10,307]) and higher engagement (median IR [interquartile range], 0.038 [0.022]) than professional source videos (views: median views [interquartile range], 1052.5 [10,610.5]; engagement: median IR [interquartile range], 0.006 [0.008]).
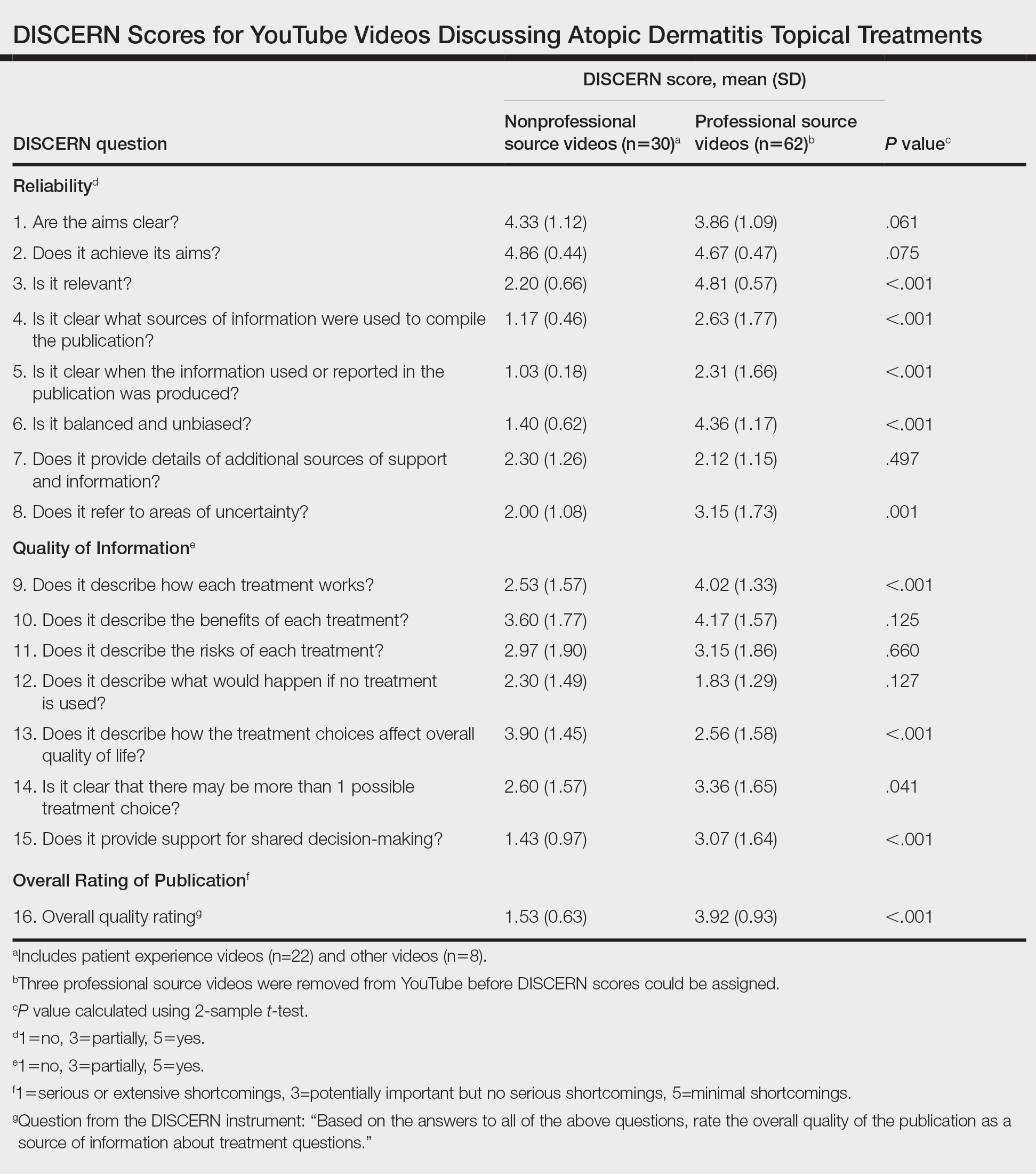
Although less popular, professional source videos had a significantly higher DISCERN overall quality rating score (question 16) compared to those categorized as nonprofessional source (3.92 vs 1.53; P<.001). In contrast, nonprofessional source videos scored significantly higher on the quality-of-life question (question 13) compared to professional source videos (3.90 vs 2.56; P<.001)(eTable). (Three professional source videos were removed from YouTube before DISCERN scores could be assigned.)
Notably, 20.7% (n=19) of the 92 videos discussed TSW, and most of them were patient experiences (n=16). Other categories included topical steroids excluding TSW (n=11), steroid phobia (n=2), topical calcineurin inhibitors (n=2), crisaborole (n=6), news broadcast (n=7), wet wraps (n=5), product advertisement (n=7), and research (n=11)(Figure 2). Interestingly, there were no videos focusing on the calcineurin inhibitor black box warning.
Similar to prior studies, our results indicate preference for patient-generated videos over videos produced by or including a professional source.5 Additionally, only 3 of 19 videos about TSW were from a professional source, increasing the potential for patient misconceptions about topical corticosteroids. Future studies should examine the educational impact of patient-generated videos as well as features that make the patient experience videos more desirable for viewing.
- Mueller SM, Hongler VNS, Jungo P, et al. Fiction, falsehoods, and few facts: cross-sectional study on the content-related quality of atopic eczema-related videos on YouTube. J Med Internet Res. 2020;22:e15599. doi:10.2196/15599
- Torres T, Ferreira EO, Gonçalo M, et al. Update on atopic dermatitis. Acta Med Port. 2019;32:606-613. doi:10.20344/amp.11963
- Vogler SA, Lightner AL. Rethinking how we care for our patients in a time of social distancing during the COVID-19 pandemic. Br J Surg. 2020;107:937-939. doi:10.1002/bjs.11636
- The DISCERN Instrument. discern online. Accessed January 22, 2021. http://www.discern.org.uk/discern_instrument.php
- Pithadia DJ, Reynolds KA, Lee EB, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube? [published online April 16, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1755418
To the Editor:
Atopic dermatitis (eczema) affects approximately 20% of children worldwide.1 In atopic dermatitis management, patient education is crucial for optimal outcomes.2 The COVID-19 pandemic has impacted patient-physician interactions. To ensure safety of patients and physicians, visits may have been canceled, postponed, or conducted virtually, leaving less time for discussion and questions.3 As a consequence, patients may seek information about atopic dermatitis from alternative sources, including YouTube videos. We performed a cross-sectional study to analyze YouTube videos about topical treatments for atopic dermatitis.
During the week of July 16, 2020, we performed 4 private browser YouTube searches with default filters using the following terms: eczema topicals, eczema topical treatments, atopic dermatitis topicals, and atopic dermatitis topical treatments. For video selection, we defined topical treatments as topical corticosteroids, topical calcineurin inhibitors, crisaborole, emollients, wet wraps, and any prospective treatment topically administered. For each of the 4 searches, 2 researchers (A.M. and A.T.) independently examined the top 75 videos, yielding a total of 300 videos. Of them, 98 videos were duplicates, 19 videos were not about atopic dermatitis, and 91 videos were not about topical treatments, leaving a total of 92 videos for analysis (Figure 1).
For the 92 included videos, the length; upload year; number of views, likes, dislikes, and comments; interaction ratio (IR)(the sum of likes, dislikes, and comments divided by the number of views); and video content were determined. The videos were placed into mutually exclusive categories as follows: (1) patient experience, defined as a video about patient perspective; (2) professional source, defined as a video featuring a physician, physician extender, pharmacist, or scientist, or produced by a formal organization; or (3) other. The DISCERN Instrument was used for grading the reliability and quality of the 92 included videos. This instrument consists of 16 questions with the responses rated on a scale of 1 to 5.4 For analysis of DISCERN scores, patient experience and other videos were grouped together as nonprofessional source videos. A 2-sample t-test was used to compare DISCERN scores between professional source and nonprofessional source videos.
Most videos were uploaded in 2017 (n=19), 2018 (n=23), and 2019 (n=25), but 20 were uploaded in 2012-2016 and 5 were uploaded in 2020. The 92 videos had a mean length of 8 minutes and 35 seconds (range, 30 seconds to 62 minutes and 23 seconds).
Patient experience videos accounted for 23.9% (n=22) of videos. These videos discussed topical steroid withdrawal (TSW)(n=16), instructions for making emollients (n=2), and treatment successes (n=4). Professional source videos represented 67.4% (n=62) of videos. Of them, 40.3% (n=25) were physician oriented, defined as having extensive medical terminology or qualifying for continuing medical education credit. Three (4.8%) of the professional source videos were sponsored by a drug company. Other constituted the remaining 8.7% (n=8) of videos. Patient experience videos had more views (median views [interquartile range], 6865 [10,307]) and higher engagement (median IR [interquartile range], 0.038 [0.022]) than professional source videos (views: median views [interquartile range], 1052.5 [10,610.5]; engagement: median IR [interquartile range], 0.006 [0.008]).
Although less popular, professional source videos had a significantly higher DISCERN overall quality rating score (question 16) compared to those categorized as nonprofessional source (3.92 vs 1.53; P<.001). In contrast, nonprofessional source videos scored significantly higher on the quality-of-life question (question 13) compared to professional source videos (3.90 vs 2.56; P<.001)(eTable). (Three professional source videos were removed from YouTube before DISCERN scores could be assigned.)
Notably, 20.7% (n=19) of the 92 videos discussed TSW, and most of them were patient experiences (n=16). Other categories included topical steroids excluding TSW (n=11), steroid phobia (n=2), topical calcineurin inhibitors (n=2), crisaborole (n=6), news broadcast (n=7), wet wraps (n=5), product advertisement (n=7), and research (n=11)(Figure 2). Interestingly, there were no videos focusing on the calcineurin inhibitor black box warning.
Similar to prior studies, our results indicate preference for patient-generated videos over videos produced by or including a professional source.5 Additionally, only 3 of 19 videos about TSW were from a professional source, increasing the potential for patient misconceptions about topical corticosteroids. Future studies should examine the educational impact of patient-generated videos as well as features that make the patient experience videos more desirable for viewing.
To the Editor:
Atopic dermatitis (eczema) affects approximately 20% of children worldwide.1 In atopic dermatitis management, patient education is crucial for optimal outcomes.2 The COVID-19 pandemic has impacted patient-physician interactions. To ensure safety of patients and physicians, visits may have been canceled, postponed, or conducted virtually, leaving less time for discussion and questions.3 As a consequence, patients may seek information about atopic dermatitis from alternative sources, including YouTube videos. We performed a cross-sectional study to analyze YouTube videos about topical treatments for atopic dermatitis.
During the week of July 16, 2020, we performed 4 private browser YouTube searches with default filters using the following terms: eczema topicals, eczema topical treatments, atopic dermatitis topicals, and atopic dermatitis topical treatments. For video selection, we defined topical treatments as topical corticosteroids, topical calcineurin inhibitors, crisaborole, emollients, wet wraps, and any prospective treatment topically administered. For each of the 4 searches, 2 researchers (A.M. and A.T.) independently examined the top 75 videos, yielding a total of 300 videos. Of them, 98 videos were duplicates, 19 videos were not about atopic dermatitis, and 91 videos were not about topical treatments, leaving a total of 92 videos for analysis (Figure 1).
For the 92 included videos, the length; upload year; number of views, likes, dislikes, and comments; interaction ratio (IR)(the sum of likes, dislikes, and comments divided by the number of views); and video content were determined. The videos were placed into mutually exclusive categories as follows: (1) patient experience, defined as a video about patient perspective; (2) professional source, defined as a video featuring a physician, physician extender, pharmacist, or scientist, or produced by a formal organization; or (3) other. The DISCERN Instrument was used for grading the reliability and quality of the 92 included videos. This instrument consists of 16 questions with the responses rated on a scale of 1 to 5.4 For analysis of DISCERN scores, patient experience and other videos were grouped together as nonprofessional source videos. A 2-sample t-test was used to compare DISCERN scores between professional source and nonprofessional source videos.
Most videos were uploaded in 2017 (n=19), 2018 (n=23), and 2019 (n=25), but 20 were uploaded in 2012-2016 and 5 were uploaded in 2020. The 92 videos had a mean length of 8 minutes and 35 seconds (range, 30 seconds to 62 minutes and 23 seconds).
Patient experience videos accounted for 23.9% (n=22) of videos. These videos discussed topical steroid withdrawal (TSW)(n=16), instructions for making emollients (n=2), and treatment successes (n=4). Professional source videos represented 67.4% (n=62) of videos. Of them, 40.3% (n=25) were physician oriented, defined as having extensive medical terminology or qualifying for continuing medical education credit. Three (4.8%) of the professional source videos were sponsored by a drug company. Other constituted the remaining 8.7% (n=8) of videos. Patient experience videos had more views (median views [interquartile range], 6865 [10,307]) and higher engagement (median IR [interquartile range], 0.038 [0.022]) than professional source videos (views: median views [interquartile range], 1052.5 [10,610.5]; engagement: median IR [interquartile range], 0.006 [0.008]).
Although less popular, professional source videos had a significantly higher DISCERN overall quality rating score (question 16) compared to those categorized as nonprofessional source (3.92 vs 1.53; P<.001). In contrast, nonprofessional source videos scored significantly higher on the quality-of-life question (question 13) compared to professional source videos (3.90 vs 2.56; P<.001)(eTable). (Three professional source videos were removed from YouTube before DISCERN scores could be assigned.)
Notably, 20.7% (n=19) of the 92 videos discussed TSW, and most of them were patient experiences (n=16). Other categories included topical steroids excluding TSW (n=11), steroid phobia (n=2), topical calcineurin inhibitors (n=2), crisaborole (n=6), news broadcast (n=7), wet wraps (n=5), product advertisement (n=7), and research (n=11)(Figure 2). Interestingly, there were no videos focusing on the calcineurin inhibitor black box warning.
Similar to prior studies, our results indicate preference for patient-generated videos over videos produced by or including a professional source.5 Additionally, only 3 of 19 videos about TSW were from a professional source, increasing the potential for patient misconceptions about topical corticosteroids. Future studies should examine the educational impact of patient-generated videos as well as features that make the patient experience videos more desirable for viewing.
- Mueller SM, Hongler VNS, Jungo P, et al. Fiction, falsehoods, and few facts: cross-sectional study on the content-related quality of atopic eczema-related videos on YouTube. J Med Internet Res. 2020;22:e15599. doi:10.2196/15599
- Torres T, Ferreira EO, Gonçalo M, et al. Update on atopic dermatitis. Acta Med Port. 2019;32:606-613. doi:10.20344/amp.11963
- Vogler SA, Lightner AL. Rethinking how we care for our patients in a time of social distancing during the COVID-19 pandemic. Br J Surg. 2020;107:937-939. doi:10.1002/bjs.11636
- The DISCERN Instrument. discern online. Accessed January 22, 2021. http://www.discern.org.uk/discern_instrument.php
- Pithadia DJ, Reynolds KA, Lee EB, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube? [published online April 16, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1755418
- Mueller SM, Hongler VNS, Jungo P, et al. Fiction, falsehoods, and few facts: cross-sectional study on the content-related quality of atopic eczema-related videos on YouTube. J Med Internet Res. 2020;22:e15599. doi:10.2196/15599
- Torres T, Ferreira EO, Gonçalo M, et al. Update on atopic dermatitis. Acta Med Port. 2019;32:606-613. doi:10.20344/amp.11963
- Vogler SA, Lightner AL. Rethinking how we care for our patients in a time of social distancing during the COVID-19 pandemic. Br J Surg. 2020;107:937-939. doi:10.1002/bjs.11636
- The DISCERN Instrument. discern online. Accessed January 22, 2021. http://www.discern.org.uk/discern_instrument.php
- Pithadia DJ, Reynolds KA, Lee EB, et al. Dupilumab for atopic dermatitis: what are patients learning on YouTube? [published online April 16, 2020]. J Dermatolog Treat. doi:10.1080/09546634.2020.1755418
Practice Points
- YouTube is a readily accessible resource for educating patients about topical treatments for atopic dermatitis.
- Although professional source videos comprised a larger percentage of the videos included within our study, patient experience videos had a higher number of views and engagement.
- Twenty-one percent (19/92) of the videos examined in our study discussed topical steroid withdrawal, and the majority of them were patient experience videos.
Plant Dermatitis: More Than Just Poison Ivy
Plants can contribute to a variety of dermatoses. The Toxicodendron genus, which includes poison ivy, poison oak, and poison sumac, is a well-known and common cause of allergic contact dermatitis (ACD), but many other plants can cause direct or airborne contact dermatitis, especially in gardeners, florists, and farmers. This article provides an overview of different plant-related dermatoses and culprit plants as well as how these dermatoses should be diagnosed and treated.
Epidemiology
Plant dermatoses affect more than 50 million individuals each year.1,2 In the United States, the Toxicodendron genus causes ACD in more than 70% of exposed individuals, leading to medical visits.3 An urgent care visit for a plant-related dermatitis is estimated to cost $168, while an emergency department visit can cost 3 times as much.4 Although less common, Compositae plants are another important culprit of plant dermatitis, particularly in gardeners, florists, and farmers. Data from the 2017-2018 North American Contact Dermatitis Group screening series (N=4947) showed sesquiterpene lactones and Compositae to be positive in 0.5% of patch-tested patients.5
Plant Dermatitis Classifications
Plant dermatitis can be classified into 5 main categories: ACD, mechanical irritant contact dermatitis, chemical irritant contact dermatitis, light-mediated dermatitis, and pseudophytodermatitis.6
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The common molecular allergens in plants include phenols, α-methylene-γ-butyrolactones, quinones, terpenes, disulfides, isothiocyanates, and polyacetylenic derivatives.6
Plant contact dermatitis due to mechanical and chemical irritants is precipitated by multiple mechanisms, including disruption of the epidermal barrier and subsequent cytokine release from keratinocytes.7 Nonimmunologic contact urticaria from plants is thought to be a type of irritant reaction precipitated by mechanical or chemical trauma.8
Light-mediated dermatitis includes phytophotodermatitis and photoallergic contact dermatitis. Phytophotodermatitis is a phototoxic reaction triggered by exposure to both plant-derived furanocoumarin and UVA light.9 By contrast, photoallergic contact dermatitis is a delayed hypersensitivity reaction from prior sensitization to a light-activated antigen.10
Pseudophytodermatitis, as its name implies, is not truly mediated by an allergen or irritant intrinsic to the plant but rather by dyes, waxes, insecticides, or arthropods that inhabit the plant or are secondarily applied.6
Common Plant Allergens
Anacardiaceae Family
Most of the allergenic plants within the Anacardiaceae family belong to the Toxicodendron genus, which encompasses poison ivy (Toxicodendron radicans), poison oak (Toxicodendron pubescens,Toxicodendron quercifolium, Toxicodendron diversiloum), and poison sumac (Toxicodendron vernix). Poison ivy is the celebrity of the Anacardiaceae family and contributes to most cases of plant-related ACD. It is found in every state in the continental United States. Poison oak is another common culprit found in the western and southeastern United States.11 Plants within the Anacardiaceae family contain an oleoresin called urushiol, which is the primary sensitizing substance. Although poison ivy and poison oak grow well in full sun to partial shade, poison sumac typically is found in damp swampy areas east of the Rocky Mountains. Most cases of ACD related to Anacardiaceae species are due to direct contact with urushiol from a Toxicodendron plant, but burning of brush containing Toxicodendron can cause airborne exposure when urushiol oil is carried by smoke particles.12 Sensitization to Toxicodendron can cause ACD to other Anacardiaceae species such as the Japanese lacquer tree (Toxicodendron vernicifluum), mango tree (Mangifera indica), cashew tree (Anacardium occidentale), and Indian marking nut tree (Semecarpus anacardium).6 Cross-reactions to components of the ginkgo tree (Ginkgo biloba) also are possible.
Toxicodendron plants can be more easily identified and avoided with knowledge of their characteristic leaf patterns. The most dependable way to identify poison ivy and poison oak species is to look for plants with 3 leaves, giving rise to the common saying, “Leaves of three, leave them be.” Poison sumac plants have groups of 7 to 13 leaves arranged as pairs along a central rib. Another helpful finding is a black deposit that Toxicodendron species leave behind following trauma to the leaves. Urushiol oxidizes when exposed to air and turns into a black deposit that can be seen on damaged leaves themselves or can be demonstrated in a black spot test to verify if a plant is a Toxicodendron species. The test is performed by gathering (carefully, without direct contact) a few leaves in a paper towel and crushing them to release sap. Within minutes, the sap will turn black if the plant is indeed a Toxicodendron species.13Pruritic, edematous, erythematous papules, plaques, and eventual vesicles in a linear distribution are suspicious for Toxicodendron exposure. Although your pet will not develop Toxicodendron ACD, oleoresin-contaminated pets can transfer the oils to their owners after coming into contact with these plants. Toxicodendron dermatitis also can be acquired from oleoresin-contaminated fomites such as clothing and shoes worn in the garden or when hiking. Toxicodendron dermatitis can appear at different sites on the body at different times depending on the amount of oleoresin exposure as well as epidermal thickness. For example, the oleoresin can be transferred from the hands to body areas with a thinner stratum corneum (eg, genitalia) and cause subsequent dermatitis.1
Compositae Family
The Compositae family (also known as Asteraceae) is a large plant family with more than 20,000 species, including numerous weeds, wildflowers, and vegetables. The flowers, leaves, stems, and pollens of the Compositae family are coated by cyclic esters called sesquiterpene lactones. Mitchell and Dupuis14 showed that sesquiterpene lactones are the allergens responsible for ACD to various Compositae plants, including ragweed (Ambrosia), sneezeweed (Helenium), and chrysanthemums (Chrysanthemum). Common Compositae vegetables such as lettuce (Lactuca sativa) have been reported to cause ACD in chefs, grocery store produce handlers, gardeners, and even owners of lettuce-eating pet guinea pigs and turtles.15 Similarly, artichokes (Cynara scolymus) can cause ACD in gardeners.16 Exposure to Compositae species also has been implicated in photoallergic reactions, and studies have demonstrated that some patients with chronic actinic dermatitis also have positive patch test reactions to Compositae species and/or sesquiterpene lactones.17,18
In addition to direct contact with Compositae plants, airborne exposure to sesquiterpene lactones can cause ACD.14 The pattern of airborne contact dermatitis typically involves exposed areas such as the eyelids, central face, and/or neck. The beak sign also can be a clue to airborne contact dermatitis, which involves dermatitis of the face that spares the nasal tip and/or nasal ridge. It is thought that the beak sign may result from increased sebaceous gland concentration on the nose, which prevents penetration of allergens and irritants.19 Unlike photoallergic contact dermatitis, which also can involve the face, airborne ACD frequently involves photoprotected areas such as the submandibular chin and the upper lip. Davies and Kersey20 reported the case of a groundsman who was cutting grass with dandelions (Taraxacum officinale) and was found to have associated airborne ACD of the face, neck, and forearms due to Compositae allergy. In a different setting, the aromas of chamomile (Matricaria chamomilla) have been reported to cause airborne ACD in a tea drinker.21 Paulsen22 found that ingestion of chamomile tea can induce systemic ACD in sensitized individuals.
Alstroemeriaceae, Liliaceae, and Primulaceae
Florists are exposed to many plant species and have a high prevalence of ACD. Thiboutot et al23 found that 15 of 57 (26%) floral workers experienced hand dermatitis that cleared with time away from work. The Peruvian lily (Alstroemeria, Alstroemeriaceae family), which contains tuliposide A, was found to be the leading cause of sensitization.23 Tulips (Tulipa, Liliaceae family), as the flower name suggests, also contain tuliposide A, which along with mechanical irritation from the course tecta fibers on the bulbs lead to a dermatitis known as tulip fingers.24,25 Poison primrose (Primula obconica, Primulaceae family), cultivated for its highly colorful flowers, contains the contact allergen primin.6 A common clinical presentation of ACD for any of these culprit flowers is localized dermatitis of the thumb and index finger in a florist or gardener.
Plants That Cause Irritant Reactions
Cactuses
Although the long spines of the Cactaceae family of cactuses is a warning for passersby, it is the small and nearly invisible barbed hairs (glochids) that inflict a more dramatic cutaneous reaction. The prickly pear cactus (Opuntia species) is a good example of such a plant, as its glochids cause mechanical irritation but also can become embedded in the skin and result in subcutaneous granulomas known as sabra dermatitis.26
Stinging Nettle
The dermatologic term urticaria owes its namesake to the stinging nettle plant, which comes from the family Urticaceae. The stinging nettle has small hairs on its leaves, referred to as stinging trichomes, which have needlelike tips that pierce the skin and inject a mix of histamine, formic acid, and acetylcholine, causing a pruritic dermatitis that may last up to 12 hours.27 The plant is found worldwide and is a common weed in North America.
Phytophotodermatitis
Lemons and limes (Rutaceae family) are common culprits of phytophotodermatitis, often causing what is known as a margarita burn after outdoor consumption or preparation of this tasty citrus beverage.28 An accidental spray of lime juice on the skin while adding it to a beer, guacamole, salsa, or any other food or beverage also can cause phytophotodermatitis.29-31 Although the juice of lemons and limes contains psoralens, the rind can contain a 6- to 186-fold increased concentration.32 Psoralen is the photoactive agent in Rutaceae plants that intercalates in double-stranded DNA and promotes intrastrand cross-links when exposed to UVA light, which ultimately leads to dermatitis.9 Phytophotodermatitis commonly causes erythema, edema, and painful bullae on sun-exposed areas and classically heals with hyperpigmentation.
Pseudophytodermatitis can occur in grain farmers and harvesters who handle wheat and/or barley and incidentally come in contact with insects and chemicals on the plant material. Pseudophytodermatitis from mites in the wheat and/or barley plant can occur at harvest time when contact with the plant material is high. Insects such as the North American itch mite (Pediculoides ventricosus) can cause petechiae, wheals, and pustules. In addition, insecticides such as malathion and arsenical sprays that are applied to plant leaves can cause pseudophytodermatitis, which may be initially diagnosed as dermatitis to the plant itself.6
Patch Testing to Plants
When a patient presents with recurrent or persistent dermatitis and a plant contact allergen is suspected, patch testing is indicated. Most comprehensive patch test series contain various plant allergens, such as sesquiterpene lactones, Compositae mix, and limonene hydroperoxides, and patch testing to a specialized plant series may be necessary. Poison ivy/oak/sumac allergens typically are not included in patch test series because of the high prevalence of allergic reactions to these chemicals and the likelihood of sensitization when patch testing with urushiol. Compositae contact sensitization can be difficult to diagnose because neither sesquiterpene lactone mix 0.1% nor parthenolide 0.1% are sensitive enough to pick up all Compositae allergies.33,34 Paulsen and Andersen34 proposed that if Compositae sensitization is suspected, testing should include sesquiterpene lactone, parthenolide, and Compositae mix II 2.5%, as well as other potential Compositae allergens based on the patient’s history.34
Because plants can have geographic variability and contain potentially unknown allergens,35 testing to plant components may increase the diagnostic yield of patch testing. Dividing the plant into component parts (ie, stem, bulb, leaf, flower) is helpful, as different components have different allergen concentrations. It is important to consult expert resources before proceeding with plant component patch testing because irritant reactions are frequent and may confound the testing.36
Prevention and Treatment
For all plant dermatoses, the mainstay of prevention is to avoid contact with the offending plant material. Gloves can be an important protective tool for plant dermatitis prevention; the correct material depends on the plant species being handled. Rubber gloves should not be worn to protect against Toxicodendron plants since the catechols in urushiol are soluble in rubber; vinyl gloves should be worn instead.6 Marks37 found that tuliposide A, the allergen in the Peruvian lily (Alstroemeria), penetrates both vinyl and latex gloves; it does not penetrate nitrile gloves. If exposed, the risk of dermatitis can be decreased if the allergen is washed away with soap and water as soon as possible. Some allergens such as Toxicodendron are absorbed quickly and need to be washed off within 10 minutes of exposure.6 Importantly, exposed gardening gloves may continue to perpetuate ACD if the allergen is not also washed off the gloves themselves.
For light-mediated dermatoses, sun avoidance or use of an effective sunscreen can reduce symptoms in an individual who has already been exposed.10 UVA light activates psoralen-mediated dermatitis but not until 30 to 120 minutes after absorption into the skin.38
Barrier creams are thought to be protective against plant ACD through a variety of mechanisms. The cream itself is meant to reduce skin contact to an allergen or irritant. Additionally, barrier creams contain active ingredients such as silicone, hydrocarbons, and aluminum chlorohydrate, which are thought to trap or transform offending agents before contacting the skin. When contact with a Toxicodendron species is anticipated, Marks et al39 found that dermatitis was absent or significantly reduced when 144 patients were pretreated with quaternium-18 bentonite lotion 5% (P<.0001).
Although allergen avoidance and use of gloves and barrier creams are the mainstays of preventing plant dermatoses, treatment often is required to control postexposure symptoms. For all plant dermatoses, topical corticosteroids can be used to reduce inflammation and pruritus. In some cases, systemic steroids may be necessary. To prevent rebound of dermatitis, patients often require a 3-week or longer course of oral steroids to quell the reaction, particularly if the dermatitis is vigorous or an id reaction is present.40 Antihistamines and cold compresses also can provide symptomatic relief.
Final Interpretation
Plants can cause a variety of dermatoses. Although Toxicodendron plants are the most frequent cause of ACD, it is important to keep in mind that florists, gardeners, and farmers are exposed to a large variety of allergens, irritants, and phototoxic agents that cause dermatoses as well. Confirmation of plant-induced ACD involves patch testing against suspected species. Prevention involves use of appropriate barriers and avoidance of implicated plants. Treatment includes topical steroids, antihistamines, and prednisone.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128.
- Pariser D, Ceilley R, Lefkovits A, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Wolff K, Johnson R. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. McGraw Hill Education; 2009.
- Zomorodi N, Butt M, Maczuga S, et al. Cost and diagnostic characteristics of Toxicodendron dermatitis in the USA: a retrospective cross-sectional analysis. Br J Dermatol. 2020;183:772-773.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123.
- Fowler JF, Zirwas MJ. Fisher’s Contact Dermatitis. 7th ed. Contact Dermatitis Institute; 2019.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Wakelin SH. Contact urticaria. Clin Exp Dermatol. 2001;26:132-136.
- Ellis CR, Elston DM. Psoralen-induced phytophotodermatitis. Dermatitis. 2021;32:140-143.
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- National Institute for Occupational Safety and Health. Poisonous plants. Centers for Disease Control and Prevention website. Updated June 1, 2018. Accessed August 10, 2021. https://www.cdc.gov/niosh/topics/plants/geographic.html
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mitchell J, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150.
- Paulsen E, Andersen KE. Lettuce contact allergy. Contact Dermatitis. 2016;74:67-75.
- Samaran Q, Clark E, Dereure O, et al. Airborne allergic contact dermatitis caused by artichoke. Contact Dermatitis. 2020;82:395-397.
- Du H, Ross JS, Norris PG, et al. Contact and photocontact sensitization in chronic actinic dermatitis: sesquiterpene lactone mix is an important allergen. Br J Dermatol. 1995;132:543-547.
- Wrangsjo K, Marie Ros A, Walhberg JE. Contact allergy to Compositae plants in patients with summer-exacerbated dermatitis. Contact Dermatitis. 1990;22:148-154.
- Staser K, Ezra N, Sheehan MP, et al. The beak sign: a clinical clue to airborne contact dermatitis. Dermatitis. 2014;25:97-98.
- Davies M, Kersey J. Contact allergy to yarrow and dandelion. Contact Dermatitis. 1986;14:256-257.
- Anzai A, Vázquez Herrera NE, Tosti A. Airborne allergic contact dermatitis caused by chamomile tea. Contact Dermatitis. 2015;72:254-255.
- Paulsen E. Systemic allergic dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2017;76:1-10.
- Thiboutot DM, Hamory BH, Marks JG. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58.
- Hjorth N, Wilkinson DS. Contact dermatitis IV. tulip fingers, hyacinth itch and lily rash. Br J Dermatol. 1968;80:696-698.
- Guin JD, Franks H. Fingertip dermatitis in a retail florist. Cutis. 2001;67:328-330.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Cummings AJ, Olsen M. Mechanism of action of stinging nettles. Wilderness Environ Med. 2011;22:136-139.
- Maniam G, Light KML, Wilson J. Margarita burn: recognition and treatment of phytophotodermatitis. J Am Board Fam Med. 2021;34:398-401.
- Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
- Kung AC, Stephens MB, Darling T. Phytophotodermatitis: bulla formation and hyperpigmentation during spring break. Mil Med. 2009;174:657-661.
- Smith LG. Phytophotodermatitis. Images Emerg Med. 2017;1:146-147.
- Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
- Green C, Ferguson J. Sesquiterpene lactone mix is not an adequate screen for Compositae allergy. Contact Dermatitis. 1994;31:151-153.
- Paulsen E, Andersen KE. Screening for Compositae contact sensitization with sesquiterpene lactones and Compositae mix 2.5% pet. Contact Dermatitis. 2019;81:368-373.
- Paulsen E, Andersen KE. Patch testing with constituents of Compositae mixes. Contact Dermatitis. 2012;66:241-246.
- Frosch PJ, Geier J, Uter W, et al. Patch testing with the patients’ own products. Contact Dermatitis. 2011:929-941.
- Marks JG. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Moreau JF, English JC, Gehris RP. Phytophotodermatitis. J Pediatr Adolesc Gynecol. 2014;27:93-94.
- Marks JG, Fowler JF, Sherertz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216.
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (rhus)? J Fam Pract. 2006;55:166-167.
Plants can contribute to a variety of dermatoses. The Toxicodendron genus, which includes poison ivy, poison oak, and poison sumac, is a well-known and common cause of allergic contact dermatitis (ACD), but many other plants can cause direct or airborne contact dermatitis, especially in gardeners, florists, and farmers. This article provides an overview of different plant-related dermatoses and culprit plants as well as how these dermatoses should be diagnosed and treated.
Epidemiology
Plant dermatoses affect more than 50 million individuals each year.1,2 In the United States, the Toxicodendron genus causes ACD in more than 70% of exposed individuals, leading to medical visits.3 An urgent care visit for a plant-related dermatitis is estimated to cost $168, while an emergency department visit can cost 3 times as much.4 Although less common, Compositae plants are another important culprit of plant dermatitis, particularly in gardeners, florists, and farmers. Data from the 2017-2018 North American Contact Dermatitis Group screening series (N=4947) showed sesquiterpene lactones and Compositae to be positive in 0.5% of patch-tested patients.5
Plant Dermatitis Classifications
Plant dermatitis can be classified into 5 main categories: ACD, mechanical irritant contact dermatitis, chemical irritant contact dermatitis, light-mediated dermatitis, and pseudophytodermatitis.6
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The common molecular allergens in plants include phenols, α-methylene-γ-butyrolactones, quinones, terpenes, disulfides, isothiocyanates, and polyacetylenic derivatives.6
Plant contact dermatitis due to mechanical and chemical irritants is precipitated by multiple mechanisms, including disruption of the epidermal barrier and subsequent cytokine release from keratinocytes.7 Nonimmunologic contact urticaria from plants is thought to be a type of irritant reaction precipitated by mechanical or chemical trauma.8
Light-mediated dermatitis includes phytophotodermatitis and photoallergic contact dermatitis. Phytophotodermatitis is a phototoxic reaction triggered by exposure to both plant-derived furanocoumarin and UVA light.9 By contrast, photoallergic contact dermatitis is a delayed hypersensitivity reaction from prior sensitization to a light-activated antigen.10
Pseudophytodermatitis, as its name implies, is not truly mediated by an allergen or irritant intrinsic to the plant but rather by dyes, waxes, insecticides, or arthropods that inhabit the plant or are secondarily applied.6
Common Plant Allergens
Anacardiaceae Family
Most of the allergenic plants within the Anacardiaceae family belong to the Toxicodendron genus, which encompasses poison ivy (Toxicodendron radicans), poison oak (Toxicodendron pubescens,Toxicodendron quercifolium, Toxicodendron diversiloum), and poison sumac (Toxicodendron vernix). Poison ivy is the celebrity of the Anacardiaceae family and contributes to most cases of plant-related ACD. It is found in every state in the continental United States. Poison oak is another common culprit found in the western and southeastern United States.11 Plants within the Anacardiaceae family contain an oleoresin called urushiol, which is the primary sensitizing substance. Although poison ivy and poison oak grow well in full sun to partial shade, poison sumac typically is found in damp swampy areas east of the Rocky Mountains. Most cases of ACD related to Anacardiaceae species are due to direct contact with urushiol from a Toxicodendron plant, but burning of brush containing Toxicodendron can cause airborne exposure when urushiol oil is carried by smoke particles.12 Sensitization to Toxicodendron can cause ACD to other Anacardiaceae species such as the Japanese lacquer tree (Toxicodendron vernicifluum), mango tree (Mangifera indica), cashew tree (Anacardium occidentale), and Indian marking nut tree (Semecarpus anacardium).6 Cross-reactions to components of the ginkgo tree (Ginkgo biloba) also are possible.
Toxicodendron plants can be more easily identified and avoided with knowledge of their characteristic leaf patterns. The most dependable way to identify poison ivy and poison oak species is to look for plants with 3 leaves, giving rise to the common saying, “Leaves of three, leave them be.” Poison sumac plants have groups of 7 to 13 leaves arranged as pairs along a central rib. Another helpful finding is a black deposit that Toxicodendron species leave behind following trauma to the leaves. Urushiol oxidizes when exposed to air and turns into a black deposit that can be seen on damaged leaves themselves or can be demonstrated in a black spot test to verify if a plant is a Toxicodendron species. The test is performed by gathering (carefully, without direct contact) a few leaves in a paper towel and crushing them to release sap. Within minutes, the sap will turn black if the plant is indeed a Toxicodendron species.13Pruritic, edematous, erythematous papules, plaques, and eventual vesicles in a linear distribution are suspicious for Toxicodendron exposure. Although your pet will not develop Toxicodendron ACD, oleoresin-contaminated pets can transfer the oils to their owners after coming into contact with these plants. Toxicodendron dermatitis also can be acquired from oleoresin-contaminated fomites such as clothing and shoes worn in the garden or when hiking. Toxicodendron dermatitis can appear at different sites on the body at different times depending on the amount of oleoresin exposure as well as epidermal thickness. For example, the oleoresin can be transferred from the hands to body areas with a thinner stratum corneum (eg, genitalia) and cause subsequent dermatitis.1
Compositae Family
The Compositae family (also known as Asteraceae) is a large plant family with more than 20,000 species, including numerous weeds, wildflowers, and vegetables. The flowers, leaves, stems, and pollens of the Compositae family are coated by cyclic esters called sesquiterpene lactones. Mitchell and Dupuis14 showed that sesquiterpene lactones are the allergens responsible for ACD to various Compositae plants, including ragweed (Ambrosia), sneezeweed (Helenium), and chrysanthemums (Chrysanthemum). Common Compositae vegetables such as lettuce (Lactuca sativa) have been reported to cause ACD in chefs, grocery store produce handlers, gardeners, and even owners of lettuce-eating pet guinea pigs and turtles.15 Similarly, artichokes (Cynara scolymus) can cause ACD in gardeners.16 Exposure to Compositae species also has been implicated in photoallergic reactions, and studies have demonstrated that some patients with chronic actinic dermatitis also have positive patch test reactions to Compositae species and/or sesquiterpene lactones.17,18
In addition to direct contact with Compositae plants, airborne exposure to sesquiterpene lactones can cause ACD.14 The pattern of airborne contact dermatitis typically involves exposed areas such as the eyelids, central face, and/or neck. The beak sign also can be a clue to airborne contact dermatitis, which involves dermatitis of the face that spares the nasal tip and/or nasal ridge. It is thought that the beak sign may result from increased sebaceous gland concentration on the nose, which prevents penetration of allergens and irritants.19 Unlike photoallergic contact dermatitis, which also can involve the face, airborne ACD frequently involves photoprotected areas such as the submandibular chin and the upper lip. Davies and Kersey20 reported the case of a groundsman who was cutting grass with dandelions (Taraxacum officinale) and was found to have associated airborne ACD of the face, neck, and forearms due to Compositae allergy. In a different setting, the aromas of chamomile (Matricaria chamomilla) have been reported to cause airborne ACD in a tea drinker.21 Paulsen22 found that ingestion of chamomile tea can induce systemic ACD in sensitized individuals.
Alstroemeriaceae, Liliaceae, and Primulaceae
Florists are exposed to many plant species and have a high prevalence of ACD. Thiboutot et al23 found that 15 of 57 (26%) floral workers experienced hand dermatitis that cleared with time away from work. The Peruvian lily (Alstroemeria, Alstroemeriaceae family), which contains tuliposide A, was found to be the leading cause of sensitization.23 Tulips (Tulipa, Liliaceae family), as the flower name suggests, also contain tuliposide A, which along with mechanical irritation from the course tecta fibers on the bulbs lead to a dermatitis known as tulip fingers.24,25 Poison primrose (Primula obconica, Primulaceae family), cultivated for its highly colorful flowers, contains the contact allergen primin.6 A common clinical presentation of ACD for any of these culprit flowers is localized dermatitis of the thumb and index finger in a florist or gardener.
Plants That Cause Irritant Reactions
Cactuses
Although the long spines of the Cactaceae family of cactuses is a warning for passersby, it is the small and nearly invisible barbed hairs (glochids) that inflict a more dramatic cutaneous reaction. The prickly pear cactus (Opuntia species) is a good example of such a plant, as its glochids cause mechanical irritation but also can become embedded in the skin and result in subcutaneous granulomas known as sabra dermatitis.26
Stinging Nettle
The dermatologic term urticaria owes its namesake to the stinging nettle plant, which comes from the family Urticaceae. The stinging nettle has small hairs on its leaves, referred to as stinging trichomes, which have needlelike tips that pierce the skin and inject a mix of histamine, formic acid, and acetylcholine, causing a pruritic dermatitis that may last up to 12 hours.27 The plant is found worldwide and is a common weed in North America.
Phytophotodermatitis
Lemons and limes (Rutaceae family) are common culprits of phytophotodermatitis, often causing what is known as a margarita burn after outdoor consumption or preparation of this tasty citrus beverage.28 An accidental spray of lime juice on the skin while adding it to a beer, guacamole, salsa, or any other food or beverage also can cause phytophotodermatitis.29-31 Although the juice of lemons and limes contains psoralens, the rind can contain a 6- to 186-fold increased concentration.32 Psoralen is the photoactive agent in Rutaceae plants that intercalates in double-stranded DNA and promotes intrastrand cross-links when exposed to UVA light, which ultimately leads to dermatitis.9 Phytophotodermatitis commonly causes erythema, edema, and painful bullae on sun-exposed areas and classically heals with hyperpigmentation.
Pseudophytodermatitis can occur in grain farmers and harvesters who handle wheat and/or barley and incidentally come in contact with insects and chemicals on the plant material. Pseudophytodermatitis from mites in the wheat and/or barley plant can occur at harvest time when contact with the plant material is high. Insects such as the North American itch mite (Pediculoides ventricosus) can cause petechiae, wheals, and pustules. In addition, insecticides such as malathion and arsenical sprays that are applied to plant leaves can cause pseudophytodermatitis, which may be initially diagnosed as dermatitis to the plant itself.6
Patch Testing to Plants
When a patient presents with recurrent or persistent dermatitis and a plant contact allergen is suspected, patch testing is indicated. Most comprehensive patch test series contain various plant allergens, such as sesquiterpene lactones, Compositae mix, and limonene hydroperoxides, and patch testing to a specialized plant series may be necessary. Poison ivy/oak/sumac allergens typically are not included in patch test series because of the high prevalence of allergic reactions to these chemicals and the likelihood of sensitization when patch testing with urushiol. Compositae contact sensitization can be difficult to diagnose because neither sesquiterpene lactone mix 0.1% nor parthenolide 0.1% are sensitive enough to pick up all Compositae allergies.33,34 Paulsen and Andersen34 proposed that if Compositae sensitization is suspected, testing should include sesquiterpene lactone, parthenolide, and Compositae mix II 2.5%, as well as other potential Compositae allergens based on the patient’s history.34
Because plants can have geographic variability and contain potentially unknown allergens,35 testing to plant components may increase the diagnostic yield of patch testing. Dividing the plant into component parts (ie, stem, bulb, leaf, flower) is helpful, as different components have different allergen concentrations. It is important to consult expert resources before proceeding with plant component patch testing because irritant reactions are frequent and may confound the testing.36
Prevention and Treatment
For all plant dermatoses, the mainstay of prevention is to avoid contact with the offending plant material. Gloves can be an important protective tool for plant dermatitis prevention; the correct material depends on the plant species being handled. Rubber gloves should not be worn to protect against Toxicodendron plants since the catechols in urushiol are soluble in rubber; vinyl gloves should be worn instead.6 Marks37 found that tuliposide A, the allergen in the Peruvian lily (Alstroemeria), penetrates both vinyl and latex gloves; it does not penetrate nitrile gloves. If exposed, the risk of dermatitis can be decreased if the allergen is washed away with soap and water as soon as possible. Some allergens such as Toxicodendron are absorbed quickly and need to be washed off within 10 minutes of exposure.6 Importantly, exposed gardening gloves may continue to perpetuate ACD if the allergen is not also washed off the gloves themselves.
For light-mediated dermatoses, sun avoidance or use of an effective sunscreen can reduce symptoms in an individual who has already been exposed.10 UVA light activates psoralen-mediated dermatitis but not until 30 to 120 minutes after absorption into the skin.38
Barrier creams are thought to be protective against plant ACD through a variety of mechanisms. The cream itself is meant to reduce skin contact to an allergen or irritant. Additionally, barrier creams contain active ingredients such as silicone, hydrocarbons, and aluminum chlorohydrate, which are thought to trap or transform offending agents before contacting the skin. When contact with a Toxicodendron species is anticipated, Marks et al39 found that dermatitis was absent or significantly reduced when 144 patients were pretreated with quaternium-18 bentonite lotion 5% (P<.0001).
Although allergen avoidance and use of gloves and barrier creams are the mainstays of preventing plant dermatoses, treatment often is required to control postexposure symptoms. For all plant dermatoses, topical corticosteroids can be used to reduce inflammation and pruritus. In some cases, systemic steroids may be necessary. To prevent rebound of dermatitis, patients often require a 3-week or longer course of oral steroids to quell the reaction, particularly if the dermatitis is vigorous or an id reaction is present.40 Antihistamines and cold compresses also can provide symptomatic relief.
Final Interpretation
Plants can cause a variety of dermatoses. Although Toxicodendron plants are the most frequent cause of ACD, it is important to keep in mind that florists, gardeners, and farmers are exposed to a large variety of allergens, irritants, and phototoxic agents that cause dermatoses as well. Confirmation of plant-induced ACD involves patch testing against suspected species. Prevention involves use of appropriate barriers and avoidance of implicated plants. Treatment includes topical steroids, antihistamines, and prednisone.
Plants can contribute to a variety of dermatoses. The Toxicodendron genus, which includes poison ivy, poison oak, and poison sumac, is a well-known and common cause of allergic contact dermatitis (ACD), but many other plants can cause direct or airborne contact dermatitis, especially in gardeners, florists, and farmers. This article provides an overview of different plant-related dermatoses and culprit plants as well as how these dermatoses should be diagnosed and treated.
Epidemiology
Plant dermatoses affect more than 50 million individuals each year.1,2 In the United States, the Toxicodendron genus causes ACD in more than 70% of exposed individuals, leading to medical visits.3 An urgent care visit for a plant-related dermatitis is estimated to cost $168, while an emergency department visit can cost 3 times as much.4 Although less common, Compositae plants are another important culprit of plant dermatitis, particularly in gardeners, florists, and farmers. Data from the 2017-2018 North American Contact Dermatitis Group screening series (N=4947) showed sesquiterpene lactones and Compositae to be positive in 0.5% of patch-tested patients.5
Plant Dermatitis Classifications
Plant dermatitis can be classified into 5 main categories: ACD, mechanical irritant contact dermatitis, chemical irritant contact dermatitis, light-mediated dermatitis, and pseudophytodermatitis.6
Allergic contact dermatitis is an immune-mediated type IV delayed hypersensitivity reaction. The common molecular allergens in plants include phenols, α-methylene-γ-butyrolactones, quinones, terpenes, disulfides, isothiocyanates, and polyacetylenic derivatives.6
Plant contact dermatitis due to mechanical and chemical irritants is precipitated by multiple mechanisms, including disruption of the epidermal barrier and subsequent cytokine release from keratinocytes.7 Nonimmunologic contact urticaria from plants is thought to be a type of irritant reaction precipitated by mechanical or chemical trauma.8
Light-mediated dermatitis includes phytophotodermatitis and photoallergic contact dermatitis. Phytophotodermatitis is a phototoxic reaction triggered by exposure to both plant-derived furanocoumarin and UVA light.9 By contrast, photoallergic contact dermatitis is a delayed hypersensitivity reaction from prior sensitization to a light-activated antigen.10
Pseudophytodermatitis, as its name implies, is not truly mediated by an allergen or irritant intrinsic to the plant but rather by dyes, waxes, insecticides, or arthropods that inhabit the plant or are secondarily applied.6
Common Plant Allergens
Anacardiaceae Family
Most of the allergenic plants within the Anacardiaceae family belong to the Toxicodendron genus, which encompasses poison ivy (Toxicodendron radicans), poison oak (Toxicodendron pubescens,Toxicodendron quercifolium, Toxicodendron diversiloum), and poison sumac (Toxicodendron vernix). Poison ivy is the celebrity of the Anacardiaceae family and contributes to most cases of plant-related ACD. It is found in every state in the continental United States. Poison oak is another common culprit found in the western and southeastern United States.11 Plants within the Anacardiaceae family contain an oleoresin called urushiol, which is the primary sensitizing substance. Although poison ivy and poison oak grow well in full sun to partial shade, poison sumac typically is found in damp swampy areas east of the Rocky Mountains. Most cases of ACD related to Anacardiaceae species are due to direct contact with urushiol from a Toxicodendron plant, but burning of brush containing Toxicodendron can cause airborne exposure when urushiol oil is carried by smoke particles.12 Sensitization to Toxicodendron can cause ACD to other Anacardiaceae species such as the Japanese lacquer tree (Toxicodendron vernicifluum), mango tree (Mangifera indica), cashew tree (Anacardium occidentale), and Indian marking nut tree (Semecarpus anacardium).6 Cross-reactions to components of the ginkgo tree (Ginkgo biloba) also are possible.
Toxicodendron plants can be more easily identified and avoided with knowledge of their characteristic leaf patterns. The most dependable way to identify poison ivy and poison oak species is to look for plants with 3 leaves, giving rise to the common saying, “Leaves of three, leave them be.” Poison sumac plants have groups of 7 to 13 leaves arranged as pairs along a central rib. Another helpful finding is a black deposit that Toxicodendron species leave behind following trauma to the leaves. Urushiol oxidizes when exposed to air and turns into a black deposit that can be seen on damaged leaves themselves or can be demonstrated in a black spot test to verify if a plant is a Toxicodendron species. The test is performed by gathering (carefully, without direct contact) a few leaves in a paper towel and crushing them to release sap. Within minutes, the sap will turn black if the plant is indeed a Toxicodendron species.13Pruritic, edematous, erythematous papules, plaques, and eventual vesicles in a linear distribution are suspicious for Toxicodendron exposure. Although your pet will not develop Toxicodendron ACD, oleoresin-contaminated pets can transfer the oils to their owners after coming into contact with these plants. Toxicodendron dermatitis also can be acquired from oleoresin-contaminated fomites such as clothing and shoes worn in the garden or when hiking. Toxicodendron dermatitis can appear at different sites on the body at different times depending on the amount of oleoresin exposure as well as epidermal thickness. For example, the oleoresin can be transferred from the hands to body areas with a thinner stratum corneum (eg, genitalia) and cause subsequent dermatitis.1
Compositae Family
The Compositae family (also known as Asteraceae) is a large plant family with more than 20,000 species, including numerous weeds, wildflowers, and vegetables. The flowers, leaves, stems, and pollens of the Compositae family are coated by cyclic esters called sesquiterpene lactones. Mitchell and Dupuis14 showed that sesquiterpene lactones are the allergens responsible for ACD to various Compositae plants, including ragweed (Ambrosia), sneezeweed (Helenium), and chrysanthemums (Chrysanthemum). Common Compositae vegetables such as lettuce (Lactuca sativa) have been reported to cause ACD in chefs, grocery store produce handlers, gardeners, and even owners of lettuce-eating pet guinea pigs and turtles.15 Similarly, artichokes (Cynara scolymus) can cause ACD in gardeners.16 Exposure to Compositae species also has been implicated in photoallergic reactions, and studies have demonstrated that some patients with chronic actinic dermatitis also have positive patch test reactions to Compositae species and/or sesquiterpene lactones.17,18
In addition to direct contact with Compositae plants, airborne exposure to sesquiterpene lactones can cause ACD.14 The pattern of airborne contact dermatitis typically involves exposed areas such as the eyelids, central face, and/or neck. The beak sign also can be a clue to airborne contact dermatitis, which involves dermatitis of the face that spares the nasal tip and/or nasal ridge. It is thought that the beak sign may result from increased sebaceous gland concentration on the nose, which prevents penetration of allergens and irritants.19 Unlike photoallergic contact dermatitis, which also can involve the face, airborne ACD frequently involves photoprotected areas such as the submandibular chin and the upper lip. Davies and Kersey20 reported the case of a groundsman who was cutting grass with dandelions (Taraxacum officinale) and was found to have associated airborne ACD of the face, neck, and forearms due to Compositae allergy. In a different setting, the aromas of chamomile (Matricaria chamomilla) have been reported to cause airborne ACD in a tea drinker.21 Paulsen22 found that ingestion of chamomile tea can induce systemic ACD in sensitized individuals.
Alstroemeriaceae, Liliaceae, and Primulaceae
Florists are exposed to many plant species and have a high prevalence of ACD. Thiboutot et al23 found that 15 of 57 (26%) floral workers experienced hand dermatitis that cleared with time away from work. The Peruvian lily (Alstroemeria, Alstroemeriaceae family), which contains tuliposide A, was found to be the leading cause of sensitization.23 Tulips (Tulipa, Liliaceae family), as the flower name suggests, also contain tuliposide A, which along with mechanical irritation from the course tecta fibers on the bulbs lead to a dermatitis known as tulip fingers.24,25 Poison primrose (Primula obconica, Primulaceae family), cultivated for its highly colorful flowers, contains the contact allergen primin.6 A common clinical presentation of ACD for any of these culprit flowers is localized dermatitis of the thumb and index finger in a florist or gardener.
Plants That Cause Irritant Reactions
Cactuses
Although the long spines of the Cactaceae family of cactuses is a warning for passersby, it is the small and nearly invisible barbed hairs (glochids) that inflict a more dramatic cutaneous reaction. The prickly pear cactus (Opuntia species) is a good example of such a plant, as its glochids cause mechanical irritation but also can become embedded in the skin and result in subcutaneous granulomas known as sabra dermatitis.26
Stinging Nettle
The dermatologic term urticaria owes its namesake to the stinging nettle plant, which comes from the family Urticaceae. The stinging nettle has small hairs on its leaves, referred to as stinging trichomes, which have needlelike tips that pierce the skin and inject a mix of histamine, formic acid, and acetylcholine, causing a pruritic dermatitis that may last up to 12 hours.27 The plant is found worldwide and is a common weed in North America.
Phytophotodermatitis
Lemons and limes (Rutaceae family) are common culprits of phytophotodermatitis, often causing what is known as a margarita burn after outdoor consumption or preparation of this tasty citrus beverage.28 An accidental spray of lime juice on the skin while adding it to a beer, guacamole, salsa, or any other food or beverage also can cause phytophotodermatitis.29-31 Although the juice of lemons and limes contains psoralens, the rind can contain a 6- to 186-fold increased concentration.32 Psoralen is the photoactive agent in Rutaceae plants that intercalates in double-stranded DNA and promotes intrastrand cross-links when exposed to UVA light, which ultimately leads to dermatitis.9 Phytophotodermatitis commonly causes erythema, edema, and painful bullae on sun-exposed areas and classically heals with hyperpigmentation.
Pseudophytodermatitis can occur in grain farmers and harvesters who handle wheat and/or barley and incidentally come in contact with insects and chemicals on the plant material. Pseudophytodermatitis from mites in the wheat and/or barley plant can occur at harvest time when contact with the plant material is high. Insects such as the North American itch mite (Pediculoides ventricosus) can cause petechiae, wheals, and pustules. In addition, insecticides such as malathion and arsenical sprays that are applied to plant leaves can cause pseudophytodermatitis, which may be initially diagnosed as dermatitis to the plant itself.6
Patch Testing to Plants
When a patient presents with recurrent or persistent dermatitis and a plant contact allergen is suspected, patch testing is indicated. Most comprehensive patch test series contain various plant allergens, such as sesquiterpene lactones, Compositae mix, and limonene hydroperoxides, and patch testing to a specialized plant series may be necessary. Poison ivy/oak/sumac allergens typically are not included in patch test series because of the high prevalence of allergic reactions to these chemicals and the likelihood of sensitization when patch testing with urushiol. Compositae contact sensitization can be difficult to diagnose because neither sesquiterpene lactone mix 0.1% nor parthenolide 0.1% are sensitive enough to pick up all Compositae allergies.33,34 Paulsen and Andersen34 proposed that if Compositae sensitization is suspected, testing should include sesquiterpene lactone, parthenolide, and Compositae mix II 2.5%, as well as other potential Compositae allergens based on the patient’s history.34
Because plants can have geographic variability and contain potentially unknown allergens,35 testing to plant components may increase the diagnostic yield of patch testing. Dividing the plant into component parts (ie, stem, bulb, leaf, flower) is helpful, as different components have different allergen concentrations. It is important to consult expert resources before proceeding with plant component patch testing because irritant reactions are frequent and may confound the testing.36
Prevention and Treatment
For all plant dermatoses, the mainstay of prevention is to avoid contact with the offending plant material. Gloves can be an important protective tool for plant dermatitis prevention; the correct material depends on the plant species being handled. Rubber gloves should not be worn to protect against Toxicodendron plants since the catechols in urushiol are soluble in rubber; vinyl gloves should be worn instead.6 Marks37 found that tuliposide A, the allergen in the Peruvian lily (Alstroemeria), penetrates both vinyl and latex gloves; it does not penetrate nitrile gloves. If exposed, the risk of dermatitis can be decreased if the allergen is washed away with soap and water as soon as possible. Some allergens such as Toxicodendron are absorbed quickly and need to be washed off within 10 minutes of exposure.6 Importantly, exposed gardening gloves may continue to perpetuate ACD if the allergen is not also washed off the gloves themselves.
For light-mediated dermatoses, sun avoidance or use of an effective sunscreen can reduce symptoms in an individual who has already been exposed.10 UVA light activates psoralen-mediated dermatitis but not until 30 to 120 minutes after absorption into the skin.38
Barrier creams are thought to be protective against plant ACD through a variety of mechanisms. The cream itself is meant to reduce skin contact to an allergen or irritant. Additionally, barrier creams contain active ingredients such as silicone, hydrocarbons, and aluminum chlorohydrate, which are thought to trap or transform offending agents before contacting the skin. When contact with a Toxicodendron species is anticipated, Marks et al39 found that dermatitis was absent or significantly reduced when 144 patients were pretreated with quaternium-18 bentonite lotion 5% (P<.0001).
Although allergen avoidance and use of gloves and barrier creams are the mainstays of preventing plant dermatoses, treatment often is required to control postexposure symptoms. For all plant dermatoses, topical corticosteroids can be used to reduce inflammation and pruritus. In some cases, systemic steroids may be necessary. To prevent rebound of dermatitis, patients often require a 3-week or longer course of oral steroids to quell the reaction, particularly if the dermatitis is vigorous or an id reaction is present.40 Antihistamines and cold compresses also can provide symptomatic relief.
Final Interpretation
Plants can cause a variety of dermatoses. Although Toxicodendron plants are the most frequent cause of ACD, it is important to keep in mind that florists, gardeners, and farmers are exposed to a large variety of allergens, irritants, and phototoxic agents that cause dermatoses as well. Confirmation of plant-induced ACD involves patch testing against suspected species. Prevention involves use of appropriate barriers and avoidance of implicated plants. Treatment includes topical steroids, antihistamines, and prednisone.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128.
- Pariser D, Ceilley R, Lefkovits A, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Wolff K, Johnson R. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. McGraw Hill Education; 2009.
- Zomorodi N, Butt M, Maczuga S, et al. Cost and diagnostic characteristics of Toxicodendron dermatitis in the USA: a retrospective cross-sectional analysis. Br J Dermatol. 2020;183:772-773.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123.
- Fowler JF, Zirwas MJ. Fisher’s Contact Dermatitis. 7th ed. Contact Dermatitis Institute; 2019.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Wakelin SH. Contact urticaria. Clin Exp Dermatol. 2001;26:132-136.
- Ellis CR, Elston DM. Psoralen-induced phytophotodermatitis. Dermatitis. 2021;32:140-143.
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- National Institute for Occupational Safety and Health. Poisonous plants. Centers for Disease Control and Prevention website. Updated June 1, 2018. Accessed August 10, 2021. https://www.cdc.gov/niosh/topics/plants/geographic.html
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mitchell J, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150.
- Paulsen E, Andersen KE. Lettuce contact allergy. Contact Dermatitis. 2016;74:67-75.
- Samaran Q, Clark E, Dereure O, et al. Airborne allergic contact dermatitis caused by artichoke. Contact Dermatitis. 2020;82:395-397.
- Du H, Ross JS, Norris PG, et al. Contact and photocontact sensitization in chronic actinic dermatitis: sesquiterpene lactone mix is an important allergen. Br J Dermatol. 1995;132:543-547.
- Wrangsjo K, Marie Ros A, Walhberg JE. Contact allergy to Compositae plants in patients with summer-exacerbated dermatitis. Contact Dermatitis. 1990;22:148-154.
- Staser K, Ezra N, Sheehan MP, et al. The beak sign: a clinical clue to airborne contact dermatitis. Dermatitis. 2014;25:97-98.
- Davies M, Kersey J. Contact allergy to yarrow and dandelion. Contact Dermatitis. 1986;14:256-257.
- Anzai A, Vázquez Herrera NE, Tosti A. Airborne allergic contact dermatitis caused by chamomile tea. Contact Dermatitis. 2015;72:254-255.
- Paulsen E. Systemic allergic dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2017;76:1-10.
- Thiboutot DM, Hamory BH, Marks JG. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58.
- Hjorth N, Wilkinson DS. Contact dermatitis IV. tulip fingers, hyacinth itch and lily rash. Br J Dermatol. 1968;80:696-698.
- Guin JD, Franks H. Fingertip dermatitis in a retail florist. Cutis. 2001;67:328-330.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Cummings AJ, Olsen M. Mechanism of action of stinging nettles. Wilderness Environ Med. 2011;22:136-139.
- Maniam G, Light KML, Wilson J. Margarita burn: recognition and treatment of phytophotodermatitis. J Am Board Fam Med. 2021;34:398-401.
- Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
- Kung AC, Stephens MB, Darling T. Phytophotodermatitis: bulla formation and hyperpigmentation during spring break. Mil Med. 2009;174:657-661.
- Smith LG. Phytophotodermatitis. Images Emerg Med. 2017;1:146-147.
- Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
- Green C, Ferguson J. Sesquiterpene lactone mix is not an adequate screen for Compositae allergy. Contact Dermatitis. 1994;31:151-153.
- Paulsen E, Andersen KE. Screening for Compositae contact sensitization with sesquiterpene lactones and Compositae mix 2.5% pet. Contact Dermatitis. 2019;81:368-373.
- Paulsen E, Andersen KE. Patch testing with constituents of Compositae mixes. Contact Dermatitis. 2012;66:241-246.
- Frosch PJ, Geier J, Uter W, et al. Patch testing with the patients’ own products. Contact Dermatitis. 2011:929-941.
- Marks JG. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Moreau JF, English JC, Gehris RP. Phytophotodermatitis. J Pediatr Adolesc Gynecol. 2014;27:93-94.
- Marks JG, Fowler JF, Sherertz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216.
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (rhus)? J Fam Pract. 2006;55:166-167.
- Gladman AC. Toxicodendron dermatitis: poison ivy, oak, and sumac. Wilderness Environ Med. 2006;17:120-128.
- Pariser D, Ceilley R, Lefkovits A, et al. Poison ivy, oak and sumac. Derm Insights. 2003;4:26-28.
- Wolff K, Johnson R. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 6th ed. McGraw Hill Education; 2009.
- Zomorodi N, Butt M, Maczuga S, et al. Cost and diagnostic characteristics of Toxicodendron dermatitis in the USA: a retrospective cross-sectional analysis. Br J Dermatol. 2020;183:772-773.
- DeKoven JG, Silverberg JI, Warshaw EM, et al. North American Contact Dermatitis Group patch test results: 2017-2018. Dermatitis. 2021;32:111-123.
- Fowler JF, Zirwas MJ. Fisher’s Contact Dermatitis. 7th ed. Contact Dermatitis Institute; 2019.
- Smith HR, Basketter DA, McFadden JP. Irritant dermatitis, irritancy and its role in allergic contact dermatitis. Clin Exp Dermatol. 2002;27:138-146.
- Wakelin SH. Contact urticaria. Clin Exp Dermatol. 2001;26:132-136.
- Ellis CR, Elston DM. Psoralen-induced phytophotodermatitis. Dermatitis. 2021;32:140-143.
- Deleo VA. Photocontact dermatitis. Dermatol Ther. 2004;17:279-288.
- National Institute for Occupational Safety and Health. Poisonous plants. Centers for Disease Control and Prevention website. Updated June 1, 2018. Accessed August 10, 2021. https://www.cdc.gov/niosh/topics/plants/geographic.html
- Schloemer JA, Zirwas MJ, Burkhart CG. Airborne contact dermatitis: common causes in the USA. Int J Dermatol. 2015;54:271-274.
- Guin JD. The black spot test for recognizing poison ivy and related species. J Am Acad Dermatol. 1980;2:332-333.
- Mitchell J, Dupuis G. Allergic contact dermatitis from sesquiterpenoids of the Compositae family of plants. Br J Dermatol. 1971;84:139-150.
- Paulsen E, Andersen KE. Lettuce contact allergy. Contact Dermatitis. 2016;74:67-75.
- Samaran Q, Clark E, Dereure O, et al. Airborne allergic contact dermatitis caused by artichoke. Contact Dermatitis. 2020;82:395-397.
- Du H, Ross JS, Norris PG, et al. Contact and photocontact sensitization in chronic actinic dermatitis: sesquiterpene lactone mix is an important allergen. Br J Dermatol. 1995;132:543-547.
- Wrangsjo K, Marie Ros A, Walhberg JE. Contact allergy to Compositae plants in patients with summer-exacerbated dermatitis. Contact Dermatitis. 1990;22:148-154.
- Staser K, Ezra N, Sheehan MP, et al. The beak sign: a clinical clue to airborne contact dermatitis. Dermatitis. 2014;25:97-98.
- Davies M, Kersey J. Contact allergy to yarrow and dandelion. Contact Dermatitis. 1986;14:256-257.
- Anzai A, Vázquez Herrera NE, Tosti A. Airborne allergic contact dermatitis caused by chamomile tea. Contact Dermatitis. 2015;72:254-255.
- Paulsen E. Systemic allergic dermatitis caused by sesquiterpene lactones. Contact Dermatitis. 2017;76:1-10.
- Thiboutot DM, Hamory BH, Marks JG. Dermatoses among floral shop workers. J Am Acad Dermatol. 1990;22:54-58.
- Hjorth N, Wilkinson DS. Contact dermatitis IV. tulip fingers, hyacinth itch and lily rash. Br J Dermatol. 1968;80:696-698.
- Guin JD, Franks H. Fingertip dermatitis in a retail florist. Cutis. 2001;67:328-330.
- Magro C, Lipner S. Sabra dermatitis: combined features of delayed hypersensitivity and foreign body reaction to implanted glochidia. Dermatol Online J. 2020;26:13030/qt2157f9g0.
- Cummings AJ, Olsen M. Mechanism of action of stinging nettles. Wilderness Environ Med. 2011;22:136-139.
- Maniam G, Light KML, Wilson J. Margarita burn: recognition and treatment of phytophotodermatitis. J Am Board Fam Med. 2021;34:398-401.
- Flugman SL. Mexican beer dermatitis: a unique variant of lime phytophotodermatitis attributable to contemporary beer-drinking practices. Arch Dermatol. 2010;146:1194-1195.
- Kung AC, Stephens MB, Darling T. Phytophotodermatitis: bulla formation and hyperpigmentation during spring break. Mil Med. 2009;174:657-661.
- Smith LG. Phytophotodermatitis. Images Emerg Med. 2017;1:146-147.
- Wagner AM, Wu JJ, Hansen RC, et al. Bullous phytophotodermatitis associated with high natural concentrations of furanocoumarins in limes. Am J Contact Dermat. 2002;13:10-14.
- Green C, Ferguson J. Sesquiterpene lactone mix is not an adequate screen for Compositae allergy. Contact Dermatitis. 1994;31:151-153.
- Paulsen E, Andersen KE. Screening for Compositae contact sensitization with sesquiterpene lactones and Compositae mix 2.5% pet. Contact Dermatitis. 2019;81:368-373.
- Paulsen E, Andersen KE. Patch testing with constituents of Compositae mixes. Contact Dermatitis. 2012;66:241-246.
- Frosch PJ, Geier J, Uter W, et al. Patch testing with the patients’ own products. Contact Dermatitis. 2011:929-941.
- Marks JG. Allergic contact dermatitis to Alstroemeria. Arch Dermatol. 1988;124:914-916.
- Moreau JF, English JC, Gehris RP. Phytophotodermatitis. J Pediatr Adolesc Gynecol. 2014;27:93-94.
- Marks JG, Fowler JF, Sherertz EF, et al. Prevention of poison ivy and poison oak allergic contact dermatitis by quaternium-18 bentonite. J Am Acad Dermatol. 1995;33:212-216.
- Craig K, Meadows SE. What is the best duration of steroid therapy for contact dermatitis (rhus)? J Fam Pract. 2006;55:166-167.
Practice Points
- Gardeners, florists, farmers, and outdoor enthusiasts are at risk for various plant dermatoses, which can be classified into 5 main categories: allergic contact dermatitis (ACD), mechanical irritant contact dermatitis, chemical irritant contact dermatitis, light-mediated dermatitis, and pseudophytodermatitis.
- Poison ivy, from the Toxicodendron genus, is the leading cause of plant ACD; however, a myriad of other plants also can cause dermatoses.
- Patch testing can be used to identify the source of immune-mediated type IV delayed hypersensitivity reactions to various plant species in individuals with recurrent or persistent dermatitis.
- Treatment options for all plant dermatoses can include topical steroids, antihistamines, and oral prednisone. Prevention involves avoidance or use of an effective barrier.
Large study affirms what we already know: Masks work to prevent COVID-19
It also shows that surgical masks are more effective than cloth face coverings.
The study, which was published ahead of peer review, demonstrates the power of careful investigation and offers a host of lessons about mask wearing that will be important worldwide. One key finding of the study, for example, is that wearing a mask doesn’t lead people to abandon social distancing, something public health officials had feared might happen if masks gave people a false sense of security.
“What we really were able to achieve is to demonstrate that masks are effective against COVID-19, even under a rigorous and systematic evaluation that was done in the throes of the pandemic,” said Ashley Styczynski, MD, who was an infectious disease fellow at Stanford (Calif.) University when she collaborated on the study with other colleagues at Stanford, Yale, and Innovations for Poverty Action, a large research and policy nonprofit organization that currently works in 22 countries.
“And so, I think people who have been holding out on wearing masks because [they] felt like there wasn’t enough evidence for it, we’re hoping this will really help bridge that gap for them,” she said.
It included more than 600 unions – or local governmental districts in Bangladesh – and roughly 340,000 people.
Half of the districts were given cloth or surgical face masks along with continual reminders to wear them properly; the other half were tracked with no intervention. Blood tests of people who developed symptoms during the study verified their infections.
Compared to villages that didn’t mask, those in which masks of any type were worn had about 9% fewer symptomatic cases of COVID-19. The finding was statistically significant and was unlikely to have occurred by chance alone.
“Somebody could read this study and say, ‘OK, you reduced COVID-19 by 9%. Big deal.’ And what I would respond to that would be that, if anything, we think that that is a substantial underestimate,” Dr. Styczynski said.
One reason they think they underestimated the effectiveness of masks is that they tested only people who were having symptoms, so people who had only very mild or asymptomatic infections were missed.
Another reason is that, among people who had symptoms, only one-third agreed to undergo a blood test. The effect may have been bigger had participation been universal.
Local transmission may have played a role, too. Rates of COVID-19 in Bangladesh were relatively low during the study. Most infections were caused by the B.1.1.7, or Alpha, variant.
Since then, Delta has taken over. Delta is thought to be more transmissible, and some studies have suggested that people infected with Delta shed more viral particles. Masks may be more effective when more virus is circulating.
The investigators also found important differences by age and by the type of mask. Villages in which surgical masks were worn had 11% fewer COVID-19 cases than villages in which masks were not worn. In villages in which cloth masks were worn, on the other hand, infections were reduced by only 5%.
The cloth masks were substantial. Each had three layers – two layers of fabric with an outer layer of polypropylene. On testing, the filtration efficiency of the cloth masks was only about 37%, compared with 95% for the three-layer surgical masks, which were also made of polypropylene.
Masks were most effective for older individuals. People aged 50-60 years who wore surgical masks were 23% less likely to test positive for COVID, compared with their peers who didn’t wear masks. For people older than 60, the reduction in risk was greater – 35%.
Rigorous research
The study took place over a period of 8 weeks in each district. The interventions were rolled out in waves, with the first starting in November 2020 and the last in January 2021.
Investigators gave each household free cloth or surgical face masks and showed families a video about proper mask wearing with promotional messages from the prime minister, a head imam, and a national cricket star. They also handed out free masks.
Previous studies have shown that people aren’t always truthful about wearing masks in public. In Kenya, for example, 88% of people answering a phone survey said that they wore masks regularly, but researchers determined that only 10% of them actually did so.
Investigators in the Bangladesh study didn’t just ask people if they’d worn masks, they stationed themselves in public markets, mosques, tea stalls, and on roads that were the main entrances to the villages and took notes.
They also tested various ways to educate people and to remind them to wear masks. They found that four factors were effective at promoting the wearing of masks, and they gave them an acronym – NORM.
- N for no-cost masks.
- O for offering information through the video and local leaders.
- R for regular reminders to people by investigators who stand in public markets and offer masks or encourage anyone who wasn’t wearing one or wasn’t wearing it correctly.
- M for modeling, in which local leaders, such as imams, wear masks and remind their followers to wear them.
These four measures tripled the wearing of masks in the intervention communities, from a baseline level of 13% to 42%. People continued to wear their masks properly for about 2 weeks after the study ended, indicating that they’d gotten used to wearing them.
Dr. Styczynski said that nothing else – not text message reminders, or signs posted in public places, or local incentives – moved the needle on mask wearing.
Saved lives and money
The study found that the strategy was cost effective, too. Giving masks to a large population and getting people to use them costs about $10,000 per life saved from COVID, on par with the cost of deploying mosquito nets to save people from malaria, Dr. Styczynski said.
“I think that what we’ve been able to show is that this is a really important tool to be used globally, especially as countries have delays in getting access to vaccines and rolling them out,” she said.
Dr. Styczynski said masks will continue to be important even in countries such as the United States, where vaccines aren’t stopping transmission 100% and there are still large portions of the population who are unvaccinated, such as children.
“If we want to reduce COVID-19 here, it’s really important that we consider the ongoing utility of masks, in addition to vaccines, and not really thinking of them as one or the other,” she said.
The study was funded by a grant from GiveWell.org. The funder had no role in the study design, interpretation, or the decision to publish.
A version of this article first appeared on Medscape.com.
It also shows that surgical masks are more effective than cloth face coverings.
The study, which was published ahead of peer review, demonstrates the power of careful investigation and offers a host of lessons about mask wearing that will be important worldwide. One key finding of the study, for example, is that wearing a mask doesn’t lead people to abandon social distancing, something public health officials had feared might happen if masks gave people a false sense of security.
“What we really were able to achieve is to demonstrate that masks are effective against COVID-19, even under a rigorous and systematic evaluation that was done in the throes of the pandemic,” said Ashley Styczynski, MD, who was an infectious disease fellow at Stanford (Calif.) University when she collaborated on the study with other colleagues at Stanford, Yale, and Innovations for Poverty Action, a large research and policy nonprofit organization that currently works in 22 countries.
“And so, I think people who have been holding out on wearing masks because [they] felt like there wasn’t enough evidence for it, we’re hoping this will really help bridge that gap for them,” she said.
It included more than 600 unions – or local governmental districts in Bangladesh – and roughly 340,000 people.
Half of the districts were given cloth or surgical face masks along with continual reminders to wear them properly; the other half were tracked with no intervention. Blood tests of people who developed symptoms during the study verified their infections.
Compared to villages that didn’t mask, those in which masks of any type were worn had about 9% fewer symptomatic cases of COVID-19. The finding was statistically significant and was unlikely to have occurred by chance alone.
“Somebody could read this study and say, ‘OK, you reduced COVID-19 by 9%. Big deal.’ And what I would respond to that would be that, if anything, we think that that is a substantial underestimate,” Dr. Styczynski said.
One reason they think they underestimated the effectiveness of masks is that they tested only people who were having symptoms, so people who had only very mild or asymptomatic infections were missed.
Another reason is that, among people who had symptoms, only one-third agreed to undergo a blood test. The effect may have been bigger had participation been universal.
Local transmission may have played a role, too. Rates of COVID-19 in Bangladesh were relatively low during the study. Most infections were caused by the B.1.1.7, or Alpha, variant.
Since then, Delta has taken over. Delta is thought to be more transmissible, and some studies have suggested that people infected with Delta shed more viral particles. Masks may be more effective when more virus is circulating.
The investigators also found important differences by age and by the type of mask. Villages in which surgical masks were worn had 11% fewer COVID-19 cases than villages in which masks were not worn. In villages in which cloth masks were worn, on the other hand, infections were reduced by only 5%.
The cloth masks were substantial. Each had three layers – two layers of fabric with an outer layer of polypropylene. On testing, the filtration efficiency of the cloth masks was only about 37%, compared with 95% for the three-layer surgical masks, which were also made of polypropylene.
Masks were most effective for older individuals. People aged 50-60 years who wore surgical masks were 23% less likely to test positive for COVID, compared with their peers who didn’t wear masks. For people older than 60, the reduction in risk was greater – 35%.
Rigorous research
The study took place over a period of 8 weeks in each district. The interventions were rolled out in waves, with the first starting in November 2020 and the last in January 2021.
Investigators gave each household free cloth or surgical face masks and showed families a video about proper mask wearing with promotional messages from the prime minister, a head imam, and a national cricket star. They also handed out free masks.
Previous studies have shown that people aren’t always truthful about wearing masks in public. In Kenya, for example, 88% of people answering a phone survey said that they wore masks regularly, but researchers determined that only 10% of them actually did so.
Investigators in the Bangladesh study didn’t just ask people if they’d worn masks, they stationed themselves in public markets, mosques, tea stalls, and on roads that were the main entrances to the villages and took notes.
They also tested various ways to educate people and to remind them to wear masks. They found that four factors were effective at promoting the wearing of masks, and they gave them an acronym – NORM.
- N for no-cost masks.
- O for offering information through the video and local leaders.
- R for regular reminders to people by investigators who stand in public markets and offer masks or encourage anyone who wasn’t wearing one or wasn’t wearing it correctly.
- M for modeling, in which local leaders, such as imams, wear masks and remind their followers to wear them.
These four measures tripled the wearing of masks in the intervention communities, from a baseline level of 13% to 42%. People continued to wear their masks properly for about 2 weeks after the study ended, indicating that they’d gotten used to wearing them.
Dr. Styczynski said that nothing else – not text message reminders, or signs posted in public places, or local incentives – moved the needle on mask wearing.
Saved lives and money
The study found that the strategy was cost effective, too. Giving masks to a large population and getting people to use them costs about $10,000 per life saved from COVID, on par with the cost of deploying mosquito nets to save people from malaria, Dr. Styczynski said.
“I think that what we’ve been able to show is that this is a really important tool to be used globally, especially as countries have delays in getting access to vaccines and rolling them out,” she said.
Dr. Styczynski said masks will continue to be important even in countries such as the United States, where vaccines aren’t stopping transmission 100% and there are still large portions of the population who are unvaccinated, such as children.
“If we want to reduce COVID-19 here, it’s really important that we consider the ongoing utility of masks, in addition to vaccines, and not really thinking of them as one or the other,” she said.
The study was funded by a grant from GiveWell.org. The funder had no role in the study design, interpretation, or the decision to publish.
A version of this article first appeared on Medscape.com.
It also shows that surgical masks are more effective than cloth face coverings.
The study, which was published ahead of peer review, demonstrates the power of careful investigation and offers a host of lessons about mask wearing that will be important worldwide. One key finding of the study, for example, is that wearing a mask doesn’t lead people to abandon social distancing, something public health officials had feared might happen if masks gave people a false sense of security.
“What we really were able to achieve is to demonstrate that masks are effective against COVID-19, even under a rigorous and systematic evaluation that was done in the throes of the pandemic,” said Ashley Styczynski, MD, who was an infectious disease fellow at Stanford (Calif.) University when she collaborated on the study with other colleagues at Stanford, Yale, and Innovations for Poverty Action, a large research and policy nonprofit organization that currently works in 22 countries.
“And so, I think people who have been holding out on wearing masks because [they] felt like there wasn’t enough evidence for it, we’re hoping this will really help bridge that gap for them,” she said.
It included more than 600 unions – or local governmental districts in Bangladesh – and roughly 340,000 people.
Half of the districts were given cloth or surgical face masks along with continual reminders to wear them properly; the other half were tracked with no intervention. Blood tests of people who developed symptoms during the study verified their infections.
Compared to villages that didn’t mask, those in which masks of any type were worn had about 9% fewer symptomatic cases of COVID-19. The finding was statistically significant and was unlikely to have occurred by chance alone.
“Somebody could read this study and say, ‘OK, you reduced COVID-19 by 9%. Big deal.’ And what I would respond to that would be that, if anything, we think that that is a substantial underestimate,” Dr. Styczynski said.
One reason they think they underestimated the effectiveness of masks is that they tested only people who were having symptoms, so people who had only very mild or asymptomatic infections were missed.
Another reason is that, among people who had symptoms, only one-third agreed to undergo a blood test. The effect may have been bigger had participation been universal.
Local transmission may have played a role, too. Rates of COVID-19 in Bangladesh were relatively low during the study. Most infections were caused by the B.1.1.7, or Alpha, variant.
Since then, Delta has taken over. Delta is thought to be more transmissible, and some studies have suggested that people infected with Delta shed more viral particles. Masks may be more effective when more virus is circulating.
The investigators also found important differences by age and by the type of mask. Villages in which surgical masks were worn had 11% fewer COVID-19 cases than villages in which masks were not worn. In villages in which cloth masks were worn, on the other hand, infections were reduced by only 5%.
The cloth masks were substantial. Each had three layers – two layers of fabric with an outer layer of polypropylene. On testing, the filtration efficiency of the cloth masks was only about 37%, compared with 95% for the three-layer surgical masks, which were also made of polypropylene.
Masks were most effective for older individuals. People aged 50-60 years who wore surgical masks were 23% less likely to test positive for COVID, compared with their peers who didn’t wear masks. For people older than 60, the reduction in risk was greater – 35%.
Rigorous research
The study took place over a period of 8 weeks in each district. The interventions were rolled out in waves, with the first starting in November 2020 and the last in January 2021.
Investigators gave each household free cloth or surgical face masks and showed families a video about proper mask wearing with promotional messages from the prime minister, a head imam, and a national cricket star. They also handed out free masks.
Previous studies have shown that people aren’t always truthful about wearing masks in public. In Kenya, for example, 88% of people answering a phone survey said that they wore masks regularly, but researchers determined that only 10% of them actually did so.
Investigators in the Bangladesh study didn’t just ask people if they’d worn masks, they stationed themselves in public markets, mosques, tea stalls, and on roads that were the main entrances to the villages and took notes.
They also tested various ways to educate people and to remind them to wear masks. They found that four factors were effective at promoting the wearing of masks, and they gave them an acronym – NORM.
- N for no-cost masks.
- O for offering information through the video and local leaders.
- R for regular reminders to people by investigators who stand in public markets and offer masks or encourage anyone who wasn’t wearing one or wasn’t wearing it correctly.
- M for modeling, in which local leaders, such as imams, wear masks and remind their followers to wear them.
These four measures tripled the wearing of masks in the intervention communities, from a baseline level of 13% to 42%. People continued to wear their masks properly for about 2 weeks after the study ended, indicating that they’d gotten used to wearing them.
Dr. Styczynski said that nothing else – not text message reminders, or signs posted in public places, or local incentives – moved the needle on mask wearing.
Saved lives and money
The study found that the strategy was cost effective, too. Giving masks to a large population and getting people to use them costs about $10,000 per life saved from COVID, on par with the cost of deploying mosquito nets to save people from malaria, Dr. Styczynski said.
“I think that what we’ve been able to show is that this is a really important tool to be used globally, especially as countries have delays in getting access to vaccines and rolling them out,” she said.
Dr. Styczynski said masks will continue to be important even in countries such as the United States, where vaccines aren’t stopping transmission 100% and there are still large portions of the population who are unvaccinated, such as children.
“If we want to reduce COVID-19 here, it’s really important that we consider the ongoing utility of masks, in addition to vaccines, and not really thinking of them as one or the other,” she said.
The study was funded by a grant from GiveWell.org. The funder had no role in the study design, interpretation, or the decision to publish.
A version of this article first appeared on Medscape.com.
Atopic Dermatitis: Evolution and Revolution in Therapy
Atopic dermatitis (AD) is an incredibly common chronic skin disease, affecting up to 25% of children and 7% of adults in the United States.1,2 Despite the prevalence of this disease and its impact on patient quality of life, research and scholarly work in AD has been limited until recent years. A PubMed search of articles indexed for MEDLINE using the term atopic dermatitis showed that there were fewer than 500 articles published in 2000 and 965 in 2010; with our more recent acceleration in research, there were 2168 articles published in 2020 and more than 1300 published in just the first half of 2021 (through June). This new research includes insights into the pathogenesis of AD and study of the disease impact and comorbidities as well as an extensive amount of drug development and clinical trial work for new topical and systemic therapies.
New Agents to Treat AD
The 2016 approval of crisaborole,3 a phosphodiesterase 4 inhibitor, followed by the approval of dupilumab, an IL-4 and IL-13 pathway inhibitor and the first biologic agent approved for AD,4 ushered in a new age of therapy. We currently are awaiting the incorporation of a new set of topical nonsteroidal agents, oral Janus kinase (JAK) inhibitors, and new biologic agents for AD, several of which have completed phase 3 trials and extended safety evaluations. How these new drugs will impact our standard treatment across the spectrum of care for AD is not yet known.
The emergence of new systemic therapies is timely, as the most used systemic medications previously were oral corticosteroids, despite their use being advised against in standard practice guidelines. Other agents such as methotrexate, cyclosporine, azathioprine, and mycophenolate are discussed in the literature and AD treatment guidelines as being potentially useful, though absence of US Food and Drug Administration (FDA) approval and the need for frequent laboratory monitoring, as well as drug-specific side effects and an increased risk of infection, limit their use in the United States, especially in pediatric and adolescent populations.5
The approval of dupilumab as a systemic therapy—initially for adults and subsequently for teenagers (12–17 years of age) and then children (6–11 years of age)—has markedly influenced the standard of care for moderate to severe AD. This agent has been shown to have a considerable impact on disease severity and quality of life, with a good safety profile and the added benefit of not requiring continuous (or any) laboratory monitoring.6-8 Ongoing studies of dupilumab in children (ClinicalTrials.gov identifiers NCT02612454, NCT03346434), including those younger than 1 year,9 raise the question of how commonly this medication might be incorporated into care across the entire age spectrum of patients with AD. What standards will there be for assessment of severity, disease impact, and persistence to warrant use in younger ages? Will early treatment with novel systemic agents change the overall course of the disease and minimize the development of comorbidities? The answers to these questions remain to be seen.
JAK Inhibitors for AD
Additional novel therapeutics currently are undergoing studies for treatment of AD, most notably the oral JAK inhibitors upadacitinib,10 baricitinib,11 and abrocitinib.12 Each of these agents has completed phase 3 trials for AD. Two of these agents—upadacitinib and baricitinib—have prior FDA approval for use in other disease states. Of note, baricitinib is already approved for treatment of moderate to severe AD in adults in more than 40 countries13; however, the use of these agents in other diseases brings about concerns of malignancy, severe infection, and thrombosis. In the clinical trials for AD, many of these events have not been seen, but the number of patients treated is limited, and longer-term safety assessment is important.10,11
How will the oral JAK inhibitors be incorporated into care compared to biologic agents such as dupilumab? Tolerance and more serious potential adverse events are concerns, with nausea, headaches, and acneform eruptions being associated with some of the medications, in addition to potential issues with herpes simplex and zoster infections. However, oral JAK inhibitors have the benefit of not requiring injections, something that many patients may prefer, and data show that these drugs generally are associated with a rapid reduction in pruritus and, depending on the drug, very quick and profound effects on objective signs of AD.10-12 Two head-to-head studies have been completed comparing dupilumab to oral JAK inhibitors in adults: the JADE COMPARE trial examining dupilumab vs abrocitinib12 and the Heads UP trial comparing dupilumab vs upadacitinib.14 Compared to dupilumab, higher-dose abrocitinib showed more rapid responses, superiority in itch response, and similarity or superiority in other outcomes depending on the time point of the evaluation. Adverse event profiles differed; for example, abrocitinib was associated with more nausea, acneform eruptions, and herpes zoster, while dupilumab had higher rates of conjunctivitis.12 Upadacitinib, which was only studied at higher dosing (30 mg daily), showed superiority to dupilumab in itch response and in improvement in AD severity in multiple outcome measures; however, there were increases in serious infections, eczema herpeticum, herpes zoster, and laboratory-related adverse events.14 Dupilumab has the advantage of studies of extended use along with real-world experience, generally with excellent safety and tolerance other than injection-site reactions and conjunctivitis.8 Biologics targeting IL-13—tralokinumab and lebrikizumab—also are to be added to our armamentarium.15,16 The addition of these agents and JAK inhibitors as new systemic treatment options points to the quickly evolving future of AD treatment for patients with extensive disease.
New topical therapies in development provide even more treatment options. New nonsteroidal topicals include topical JAK inhibitors such as ruxolitinib17; tapinarof,18 an aryl hydrocarbon receptor modulator; and phosphodiesterase 4 inhibitors. These agents may be useful either as monotherapy, as studied, potentially without the regional limitations associated with stronger topical corticosteroids, but also should be useful in clinical practice as part of therapeutic regimens with other topical steroid and nonsteroidal agents.
The Microbiome and AD
In addition, research looking at topical microbes as specific interventions that may mediate the microbiome and inflammation of AD are intriguing. A recent phase 1 trial from the University of California San Diego19 indicated that topical bacteriotherapy directed at decreasing Staphylococcus aureus may provide an impact in AD. Observations by Kong et al20 showed that gram-negative microbiome differences are seen in AD patients compared to unaffected individuals, which has fueled studies showing that Roseomonas mucosa, a gram-negative skin commensal, when applied as a topical live biotherapeutic agent has improved disease severity in children and adults with AD.21 Although further studies are underway, these initial data suggest a role for microbiome-modifying therapies as AD treatment.
Chronic Hand Eczema
Chronic hand eczema (CHE), which has considerable overlap with AD in many patients, especially children and adolescents,22-24 is another area of interesting research. This high-prevalence condition is associated with allergic and irritant contact dermatitis24-26—conditions that are both considered alternative diagnoses for and exacerbators of AD27—and is a disease process currently being targeted for new therapies. Delgocitinib (NCT04872101, NCT04871711), the novel JAK inhibitor ARQ-252 (NCT04378569), among other topical agents, as well as systemic therapeutics such as gusacitinib (NCT03728504), are in active trials for CHE. Given CHE’s impact on quality of life28 and its overlap with AD, investigation into this disorder can help drive future AD research as well as lead to better knowledge and treatment of CHE.
Final Thoughts
Despite the promising results of these myriad new therapies in AD, there are many factors that influence how and when we use these drugs, including their approval status, FDA labeling, and the ability of patients to access and afford treatment. Additionally, continued study is needed to evaluate the long-term safety and extended efficacy of newer drugs, such as the oral JAK inhibitors. Despite these hurdles, the current landscape of research and development is rapidly evolving. Compared to the many years when only one main group of therapies was a reasonable option for patients, the future of AD treatment looks bright.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- FDA approves Eucrisa for eczema. News release. US Food and Drug Administration; December 14, 2016. Accessed August 16, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-eucrisa-eczema
- Gooderham MJ, Hong HC, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293. doi:10.1016/j.jaad.2020.06.054
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82:377-388. doi:10.1016/j.jaad.2019.07.074
- Paller AS, Siegfried EC, Simpson EL, et al. A phase 2, open-label study of single-dose dupilumab in children aged 6 months to <6 years with severe uncontrolled atopic dermatitis: pharmacokinetics, safety and efficacy. J Eur Acad Dermatol Venereol. 2021;35:464-475. doi: 10.1111/jdv.16928
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181. doi:10.1016/S0140-6736(21)00589-4
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70. doi:10.1016/j.jaad.2021.02.028
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112. doi:10.1056/NEJMoa2019380
- Lilly and Incyte provide update on supplemental New Drug Application for baricitinib for the treatment of moderate to severe atopic dermatitis. News release. Eli Lilly and Company; July 16, 2021. Accessed August 16, 2021. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-provide-update-supplemental new-drug
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial [published online August 4, 2021]. JAMA Dermatol. doi:10.1001/jamadermatol.2021.3023
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Silverberg JI, Toth D, Bieber T, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre,placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450-463. doi:10.1111/bjd.19573
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies [published online May 4, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.085
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji, T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial [published online February 22, 2021]. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850-859. doi:10.1101/gr.131029.111
- Myles IA, Castillo CR, Barbian KD, et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci Transl Med. 2020;12:eaaz8631. doi:10.1126/scitranslmed.aaz8631
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, et al. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis. Br J Dermatol. 2001;144:523-532. doi:10.1046/j.1365-2133.2001.04078.x
- Grönhagen C, Lidén C, Wahlgren CF, et al. Hand eczema and atopic dermatitis in adolescents: a prospective cohort study from the BAMSE project. Br J Dermatol. 2015;173:1175-1182. doi:10.1111/bjd.14019
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, et al. Contact allergy and allergic contact dermatitis in adolescents: prevalence measures and associations. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS). Acta Derm Venereol. 2002;82:352-358. doi:10.1080/000155502320624087
- Isaksson M, Olhardt S, Rådehed J, et al. Children with atopic dermatitis should always be patch-tested if they have hand or foot dermatitis. Acta Derm Venereol. 2015;95:583-586. doi:10.2340/00015555-1995
- Silverberg JI, Warshaw EM, Maibach HI, et al. Hand eczema in children referred for patch testing: North American Contact Dermatitis Group Data, 2000-2016. Br J Dermatol. 2021;185:185-194. doi:10.1111/bjd.19818
- Agner T, Elsner P. Hand eczema: epidemiology, prognosis and prevention. J Eur Acad Dermatol Venereol. 2020;34(suppl 1):4-12. doi:10.1111/jdv.16061
- Cazzaniga S, Ballmer-Weber BK, Gräni N, et al. Medical, psychological and socio-economic implications of chronic hand eczema: a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:628-637. doi:10.1111/jdv.13479
Atopic dermatitis (AD) is an incredibly common chronic skin disease, affecting up to 25% of children and 7% of adults in the United States.1,2 Despite the prevalence of this disease and its impact on patient quality of life, research and scholarly work in AD has been limited until recent years. A PubMed search of articles indexed for MEDLINE using the term atopic dermatitis showed that there were fewer than 500 articles published in 2000 and 965 in 2010; with our more recent acceleration in research, there were 2168 articles published in 2020 and more than 1300 published in just the first half of 2021 (through June). This new research includes insights into the pathogenesis of AD and study of the disease impact and comorbidities as well as an extensive amount of drug development and clinical trial work for new topical and systemic therapies.
New Agents to Treat AD
The 2016 approval of crisaborole,3 a phosphodiesterase 4 inhibitor, followed by the approval of dupilumab, an IL-4 and IL-13 pathway inhibitor and the first biologic agent approved for AD,4 ushered in a new age of therapy. We currently are awaiting the incorporation of a new set of topical nonsteroidal agents, oral Janus kinase (JAK) inhibitors, and new biologic agents for AD, several of which have completed phase 3 trials and extended safety evaluations. How these new drugs will impact our standard treatment across the spectrum of care for AD is not yet known.
The emergence of new systemic therapies is timely, as the most used systemic medications previously were oral corticosteroids, despite their use being advised against in standard practice guidelines. Other agents such as methotrexate, cyclosporine, azathioprine, and mycophenolate are discussed in the literature and AD treatment guidelines as being potentially useful, though absence of US Food and Drug Administration (FDA) approval and the need for frequent laboratory monitoring, as well as drug-specific side effects and an increased risk of infection, limit their use in the United States, especially in pediatric and adolescent populations.5
The approval of dupilumab as a systemic therapy—initially for adults and subsequently for teenagers (12–17 years of age) and then children (6–11 years of age)—has markedly influenced the standard of care for moderate to severe AD. This agent has been shown to have a considerable impact on disease severity and quality of life, with a good safety profile and the added benefit of not requiring continuous (or any) laboratory monitoring.6-8 Ongoing studies of dupilumab in children (ClinicalTrials.gov identifiers NCT02612454, NCT03346434), including those younger than 1 year,9 raise the question of how commonly this medication might be incorporated into care across the entire age spectrum of patients with AD. What standards will there be for assessment of severity, disease impact, and persistence to warrant use in younger ages? Will early treatment with novel systemic agents change the overall course of the disease and minimize the development of comorbidities? The answers to these questions remain to be seen.
JAK Inhibitors for AD
Additional novel therapeutics currently are undergoing studies for treatment of AD, most notably the oral JAK inhibitors upadacitinib,10 baricitinib,11 and abrocitinib.12 Each of these agents has completed phase 3 trials for AD. Two of these agents—upadacitinib and baricitinib—have prior FDA approval for use in other disease states. Of note, baricitinib is already approved for treatment of moderate to severe AD in adults in more than 40 countries13; however, the use of these agents in other diseases brings about concerns of malignancy, severe infection, and thrombosis. In the clinical trials for AD, many of these events have not been seen, but the number of patients treated is limited, and longer-term safety assessment is important.10,11
How will the oral JAK inhibitors be incorporated into care compared to biologic agents such as dupilumab? Tolerance and more serious potential adverse events are concerns, with nausea, headaches, and acneform eruptions being associated with some of the medications, in addition to potential issues with herpes simplex and zoster infections. However, oral JAK inhibitors have the benefit of not requiring injections, something that many patients may prefer, and data show that these drugs generally are associated with a rapid reduction in pruritus and, depending on the drug, very quick and profound effects on objective signs of AD.10-12 Two head-to-head studies have been completed comparing dupilumab to oral JAK inhibitors in adults: the JADE COMPARE trial examining dupilumab vs abrocitinib12 and the Heads UP trial comparing dupilumab vs upadacitinib.14 Compared to dupilumab, higher-dose abrocitinib showed more rapid responses, superiority in itch response, and similarity or superiority in other outcomes depending on the time point of the evaluation. Adverse event profiles differed; for example, abrocitinib was associated with more nausea, acneform eruptions, and herpes zoster, while dupilumab had higher rates of conjunctivitis.12 Upadacitinib, which was only studied at higher dosing (30 mg daily), showed superiority to dupilumab in itch response and in improvement in AD severity in multiple outcome measures; however, there were increases in serious infections, eczema herpeticum, herpes zoster, and laboratory-related adverse events.14 Dupilumab has the advantage of studies of extended use along with real-world experience, generally with excellent safety and tolerance other than injection-site reactions and conjunctivitis.8 Biologics targeting IL-13—tralokinumab and lebrikizumab—also are to be added to our armamentarium.15,16 The addition of these agents and JAK inhibitors as new systemic treatment options points to the quickly evolving future of AD treatment for patients with extensive disease.
New topical therapies in development provide even more treatment options. New nonsteroidal topicals include topical JAK inhibitors such as ruxolitinib17; tapinarof,18 an aryl hydrocarbon receptor modulator; and phosphodiesterase 4 inhibitors. These agents may be useful either as monotherapy, as studied, potentially without the regional limitations associated with stronger topical corticosteroids, but also should be useful in clinical practice as part of therapeutic regimens with other topical steroid and nonsteroidal agents.
The Microbiome and AD
In addition, research looking at topical microbes as specific interventions that may mediate the microbiome and inflammation of AD are intriguing. A recent phase 1 trial from the University of California San Diego19 indicated that topical bacteriotherapy directed at decreasing Staphylococcus aureus may provide an impact in AD. Observations by Kong et al20 showed that gram-negative microbiome differences are seen in AD patients compared to unaffected individuals, which has fueled studies showing that Roseomonas mucosa, a gram-negative skin commensal, when applied as a topical live biotherapeutic agent has improved disease severity in children and adults with AD.21 Although further studies are underway, these initial data suggest a role for microbiome-modifying therapies as AD treatment.
Chronic Hand Eczema
Chronic hand eczema (CHE), which has considerable overlap with AD in many patients, especially children and adolescents,22-24 is another area of interesting research. This high-prevalence condition is associated with allergic and irritant contact dermatitis24-26—conditions that are both considered alternative diagnoses for and exacerbators of AD27—and is a disease process currently being targeted for new therapies. Delgocitinib (NCT04872101, NCT04871711), the novel JAK inhibitor ARQ-252 (NCT04378569), among other topical agents, as well as systemic therapeutics such as gusacitinib (NCT03728504), are in active trials for CHE. Given CHE’s impact on quality of life28 and its overlap with AD, investigation into this disorder can help drive future AD research as well as lead to better knowledge and treatment of CHE.
Final Thoughts
Despite the promising results of these myriad new therapies in AD, there are many factors that influence how and when we use these drugs, including their approval status, FDA labeling, and the ability of patients to access and afford treatment. Additionally, continued study is needed to evaluate the long-term safety and extended efficacy of newer drugs, such as the oral JAK inhibitors. Despite these hurdles, the current landscape of research and development is rapidly evolving. Compared to the many years when only one main group of therapies was a reasonable option for patients, the future of AD treatment looks bright.
Atopic dermatitis (AD) is an incredibly common chronic skin disease, affecting up to 25% of children and 7% of adults in the United States.1,2 Despite the prevalence of this disease and its impact on patient quality of life, research and scholarly work in AD has been limited until recent years. A PubMed search of articles indexed for MEDLINE using the term atopic dermatitis showed that there were fewer than 500 articles published in 2000 and 965 in 2010; with our more recent acceleration in research, there were 2168 articles published in 2020 and more than 1300 published in just the first half of 2021 (through June). This new research includes insights into the pathogenesis of AD and study of the disease impact and comorbidities as well as an extensive amount of drug development and clinical trial work for new topical and systemic therapies.
New Agents to Treat AD
The 2016 approval of crisaborole,3 a phosphodiesterase 4 inhibitor, followed by the approval of dupilumab, an IL-4 and IL-13 pathway inhibitor and the first biologic agent approved for AD,4 ushered in a new age of therapy. We currently are awaiting the incorporation of a new set of topical nonsteroidal agents, oral Janus kinase (JAK) inhibitors, and new biologic agents for AD, several of which have completed phase 3 trials and extended safety evaluations. How these new drugs will impact our standard treatment across the spectrum of care for AD is not yet known.
The emergence of new systemic therapies is timely, as the most used systemic medications previously were oral corticosteroids, despite their use being advised against in standard practice guidelines. Other agents such as methotrexate, cyclosporine, azathioprine, and mycophenolate are discussed in the literature and AD treatment guidelines as being potentially useful, though absence of US Food and Drug Administration (FDA) approval and the need for frequent laboratory monitoring, as well as drug-specific side effects and an increased risk of infection, limit their use in the United States, especially in pediatric and adolescent populations.5
The approval of dupilumab as a systemic therapy—initially for adults and subsequently for teenagers (12–17 years of age) and then children (6–11 years of age)—has markedly influenced the standard of care for moderate to severe AD. This agent has been shown to have a considerable impact on disease severity and quality of life, with a good safety profile and the added benefit of not requiring continuous (or any) laboratory monitoring.6-8 Ongoing studies of dupilumab in children (ClinicalTrials.gov identifiers NCT02612454, NCT03346434), including those younger than 1 year,9 raise the question of how commonly this medication might be incorporated into care across the entire age spectrum of patients with AD. What standards will there be for assessment of severity, disease impact, and persistence to warrant use in younger ages? Will early treatment with novel systemic agents change the overall course of the disease and minimize the development of comorbidities? The answers to these questions remain to be seen.
JAK Inhibitors for AD
Additional novel therapeutics currently are undergoing studies for treatment of AD, most notably the oral JAK inhibitors upadacitinib,10 baricitinib,11 and abrocitinib.12 Each of these agents has completed phase 3 trials for AD. Two of these agents—upadacitinib and baricitinib—have prior FDA approval for use in other disease states. Of note, baricitinib is already approved for treatment of moderate to severe AD in adults in more than 40 countries13; however, the use of these agents in other diseases brings about concerns of malignancy, severe infection, and thrombosis. In the clinical trials for AD, many of these events have not been seen, but the number of patients treated is limited, and longer-term safety assessment is important.10,11
How will the oral JAK inhibitors be incorporated into care compared to biologic agents such as dupilumab? Tolerance and more serious potential adverse events are concerns, with nausea, headaches, and acneform eruptions being associated with some of the medications, in addition to potential issues with herpes simplex and zoster infections. However, oral JAK inhibitors have the benefit of not requiring injections, something that many patients may prefer, and data show that these drugs generally are associated with a rapid reduction in pruritus and, depending on the drug, very quick and profound effects on objective signs of AD.10-12 Two head-to-head studies have been completed comparing dupilumab to oral JAK inhibitors in adults: the JADE COMPARE trial examining dupilumab vs abrocitinib12 and the Heads UP trial comparing dupilumab vs upadacitinib.14 Compared to dupilumab, higher-dose abrocitinib showed more rapid responses, superiority in itch response, and similarity or superiority in other outcomes depending on the time point of the evaluation. Adverse event profiles differed; for example, abrocitinib was associated with more nausea, acneform eruptions, and herpes zoster, while dupilumab had higher rates of conjunctivitis.12 Upadacitinib, which was only studied at higher dosing (30 mg daily), showed superiority to dupilumab in itch response and in improvement in AD severity in multiple outcome measures; however, there were increases in serious infections, eczema herpeticum, herpes zoster, and laboratory-related adverse events.14 Dupilumab has the advantage of studies of extended use along with real-world experience, generally with excellent safety and tolerance other than injection-site reactions and conjunctivitis.8 Biologics targeting IL-13—tralokinumab and lebrikizumab—also are to be added to our armamentarium.15,16 The addition of these agents and JAK inhibitors as new systemic treatment options points to the quickly evolving future of AD treatment for patients with extensive disease.
New topical therapies in development provide even more treatment options. New nonsteroidal topicals include topical JAK inhibitors such as ruxolitinib17; tapinarof,18 an aryl hydrocarbon receptor modulator; and phosphodiesterase 4 inhibitors. These agents may be useful either as monotherapy, as studied, potentially without the regional limitations associated with stronger topical corticosteroids, but also should be useful in clinical practice as part of therapeutic regimens with other topical steroid and nonsteroidal agents.
The Microbiome and AD
In addition, research looking at topical microbes as specific interventions that may mediate the microbiome and inflammation of AD are intriguing. A recent phase 1 trial from the University of California San Diego19 indicated that topical bacteriotherapy directed at decreasing Staphylococcus aureus may provide an impact in AD. Observations by Kong et al20 showed that gram-negative microbiome differences are seen in AD patients compared to unaffected individuals, which has fueled studies showing that Roseomonas mucosa, a gram-negative skin commensal, when applied as a topical live biotherapeutic agent has improved disease severity in children and adults with AD.21 Although further studies are underway, these initial data suggest a role for microbiome-modifying therapies as AD treatment.
Chronic Hand Eczema
Chronic hand eczema (CHE), which has considerable overlap with AD in many patients, especially children and adolescents,22-24 is another area of interesting research. This high-prevalence condition is associated with allergic and irritant contact dermatitis24-26—conditions that are both considered alternative diagnoses for and exacerbators of AD27—and is a disease process currently being targeted for new therapies. Delgocitinib (NCT04872101, NCT04871711), the novel JAK inhibitor ARQ-252 (NCT04378569), among other topical agents, as well as systemic therapeutics such as gusacitinib (NCT03728504), are in active trials for CHE. Given CHE’s impact on quality of life28 and its overlap with AD, investigation into this disorder can help drive future AD research as well as lead to better knowledge and treatment of CHE.
Final Thoughts
Despite the promising results of these myriad new therapies in AD, there are many factors that influence how and when we use these drugs, including their approval status, FDA labeling, and the ability of patients to access and afford treatment. Additionally, continued study is needed to evaluate the long-term safety and extended efficacy of newer drugs, such as the oral JAK inhibitors. Despite these hurdles, the current landscape of research and development is rapidly evolving. Compared to the many years when only one main group of therapies was a reasonable option for patients, the future of AD treatment looks bright.
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- FDA approves Eucrisa for eczema. News release. US Food and Drug Administration; December 14, 2016. Accessed August 16, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-eucrisa-eczema
- Gooderham MJ, Hong HC, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293. doi:10.1016/j.jaad.2020.06.054
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82:377-388. doi:10.1016/j.jaad.2019.07.074
- Paller AS, Siegfried EC, Simpson EL, et al. A phase 2, open-label study of single-dose dupilumab in children aged 6 months to <6 years with severe uncontrolled atopic dermatitis: pharmacokinetics, safety and efficacy. J Eur Acad Dermatol Venereol. 2021;35:464-475. doi: 10.1111/jdv.16928
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181. doi:10.1016/S0140-6736(21)00589-4
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70. doi:10.1016/j.jaad.2021.02.028
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112. doi:10.1056/NEJMoa2019380
- Lilly and Incyte provide update on supplemental New Drug Application for baricitinib for the treatment of moderate to severe atopic dermatitis. News release. Eli Lilly and Company; July 16, 2021. Accessed August 16, 2021. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-provide-update-supplemental new-drug
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial [published online August 4, 2021]. JAMA Dermatol. doi:10.1001/jamadermatol.2021.3023
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Silverberg JI, Toth D, Bieber T, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre,placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450-463. doi:10.1111/bjd.19573
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies [published online May 4, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.085
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji, T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial [published online February 22, 2021]. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850-859. doi:10.1101/gr.131029.111
- Myles IA, Castillo CR, Barbian KD, et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci Transl Med. 2020;12:eaaz8631. doi:10.1126/scitranslmed.aaz8631
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, et al. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis. Br J Dermatol. 2001;144:523-532. doi:10.1046/j.1365-2133.2001.04078.x
- Grönhagen C, Lidén C, Wahlgren CF, et al. Hand eczema and atopic dermatitis in adolescents: a prospective cohort study from the BAMSE project. Br J Dermatol. 2015;173:1175-1182. doi:10.1111/bjd.14019
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, et al. Contact allergy and allergic contact dermatitis in adolescents: prevalence measures and associations. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS). Acta Derm Venereol. 2002;82:352-358. doi:10.1080/000155502320624087
- Isaksson M, Olhardt S, Rådehed J, et al. Children with atopic dermatitis should always be patch-tested if they have hand or foot dermatitis. Acta Derm Venereol. 2015;95:583-586. doi:10.2340/00015555-1995
- Silverberg JI, Warshaw EM, Maibach HI, et al. Hand eczema in children referred for patch testing: North American Contact Dermatitis Group Data, 2000-2016. Br J Dermatol. 2021;185:185-194. doi:10.1111/bjd.19818
- Agner T, Elsner P. Hand eczema: epidemiology, prognosis and prevention. J Eur Acad Dermatol Venereol. 2020;34(suppl 1):4-12. doi:10.1111/jdv.16061
- Cazzaniga S, Ballmer-Weber BK, Gräni N, et al. Medical, psychological and socio-economic implications of chronic hand eczema: a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:628-637. doi:10.1111/jdv.13479
- Eichenfield LF, Tom WL, Chamlin SL, et al. Guidelines of care for the management of atopic dermatitis: section 1. diagnosis and assessment of atopic dermatitis. J Am Acad Dermatol. 2014;70:338-351. doi:10.1016/j.jaad.2013.10.010
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590. doi:10.1016/j.jid.2018.08.028
- FDA approves Eucrisa for eczema. News release. US Food and Drug Administration; December 14, 2016. Accessed August 16, 2021. https://www.fda.gov/news-events/press-announcements/fda-approves-eucrisa-eczema
- Gooderham MJ, Hong HC, Eshtiaghi P, et al. Dupilumab: a review of its use in the treatment of atopic dermatitis. J Am Acad Dermatol. 2018;78(3 suppl 1):S28-S36. doi:10.1016/j.jaad.2017.12.022
- Sidbury R, Davis DM, Cohen DE, et al. Guidelines of care for the management of atopic dermatitis: section 3. management and treatment with phototherapy and systemic agents. J Am Acad Dermatol. 2014;71:327-349. doi:10.1016/j.jaad.2014.03.030
- Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled phase 3 trial. J Am Acad Dermatol. 2020;83:1282-1293. doi:10.1016/j.jaad.2020.06.054
- Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156:44-56. doi:10.1001/jamadermatol.2019.3336
- Deleuran M, Thaçi D, Beck LA, et al. Dupilumab shows long-term safety and efficacy in patients with moderate to severe atopic dermatitis enrolled in a phase 3 open-label extension study. J Am Acad Dermatol. 2020;82:377-388. doi:10.1016/j.jaad.2019.07.074
- Paller AS, Siegfried EC, Simpson EL, et al. A phase 2, open-label study of single-dose dupilumab in children aged 6 months to <6 years with severe uncontrolled atopic dermatitis: pharmacokinetics, safety and efficacy. J Eur Acad Dermatol Venereol. 2021;35:464-475. doi: 10.1111/jdv.16928
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181. doi:10.1016/S0140-6736(21)00589-4
- Simpson EL, Forman S, Silverberg JI, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis: results from a randomized monotherapy phase 3 trial in the United States and Canada (BREEZE-AD5). J Am Acad Dermatol. 2021;85:62-70. doi:10.1016/j.jaad.2021.02.028
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112. doi:10.1056/NEJMoa2019380
- Lilly and Incyte provide update on supplemental New Drug Application for baricitinib for the treatment of moderate to severe atopic dermatitis. News release. Eli Lilly and Company; July 16, 2021. Accessed August 16, 2021. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-provide-update-supplemental new-drug
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial [published online August 4, 2021]. JAMA Dermatol. doi:10.1001/jamadermatol.2021.3023
- Guttman-Yassky E, Blauvelt A, Eichenfield LF, et al. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411-420. doi:10.1001/jamadermatol.2020.0079
- Silverberg JI, Toth D, Bieber T, et al. Tralokinumab plus topical corticosteroids for the treatment of moderate-to-severe atopic dermatitis: results from the double-blind, randomized, multicentre,placebo-controlled phase III ECZTRA 3 trial. Br J Dermatol. 2021;184:450-463. doi:10.1111/bjd.19573
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies [published online May 4, 2021]. J Am Acad Dermatol. doi:10.1016/j.jaad.2021.04.085
- Paller AS, Stein Gold L, Soung J, et al. Efficacy and patient-reported outcomes from a phase 2b, randomized clinical trial of tapinarof cream for the treatment of adolescents and adults with atopic dermatitis. J Am Acad Dermatol. 2021;84:632-638. doi:10.1016/j.jaad.2020.05.135
- Nakatsuji, T, Hata TR, Tong Y, et al. Development of a human skin commensal microbe for bacteriotherapy of atopic dermatitis and use in a phase 1 randomized clinical trial [published online February 22, 2021]. Nat Med. 2021;27:700-709. doi:10.1038/s41591-021-01256-2
- Kong HH, Oh J, Deming C, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22:850-859. doi:10.1101/gr.131029.111
- Myles IA, Castillo CR, Barbian KD, et al. Therapeutic responses to Roseomonas mucosa in atopic dermatitis may involve lipid-mediated TNF-related epithelial repair. Sci Transl Med. 2020;12:eaaz8631. doi:10.1126/scitranslmed.aaz8631
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, et al. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis. Br J Dermatol. 2001;144:523-532. doi:10.1046/j.1365-2133.2001.04078.x
- Grönhagen C, Lidén C, Wahlgren CF, et al. Hand eczema and atopic dermatitis in adolescents: a prospective cohort study from the BAMSE project. Br J Dermatol. 2015;173:1175-1182. doi:10.1111/bjd.14019
- Mortz CG, Lauritsen JM, Bindslev-Jensen C, et al. Contact allergy and allergic contact dermatitis in adolescents: prevalence measures and associations. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis (TOACS). Acta Derm Venereol. 2002;82:352-358. doi:10.1080/000155502320624087
- Isaksson M, Olhardt S, Rådehed J, et al. Children with atopic dermatitis should always be patch-tested if they have hand or foot dermatitis. Acta Derm Venereol. 2015;95:583-586. doi:10.2340/00015555-1995
- Silverberg JI, Warshaw EM, Maibach HI, et al. Hand eczema in children referred for patch testing: North American Contact Dermatitis Group Data, 2000-2016. Br J Dermatol. 2021;185:185-194. doi:10.1111/bjd.19818
- Agner T, Elsner P. Hand eczema: epidemiology, prognosis and prevention. J Eur Acad Dermatol Venereol. 2020;34(suppl 1):4-12. doi:10.1111/jdv.16061
- Cazzaniga S, Ballmer-Weber BK, Gräni N, et al. Medical, psychological and socio-economic implications of chronic hand eczema: a cross-sectional study. J Eur Acad Dermatol Venereol. 2016;30:628-637. doi:10.1111/jdv.13479
Lesions on the Thigh After an Organ Transplant
The Diagnosis: Microcystic Lymphatic Malformation
The shave biopsy demonstrated numerous thin-walled vascular spaces filled with lymphatic fluid within the dermis (Figure), consistent with a diagnosis of microcystic lymphatic malformation (LM). Lymphatic malformations represent a class of benign vascular lesions consisting of anomalous or dilated lymphatic vessels, which can be broadly categorized as macrocystic (formerly cavernous lymphangioma or cystic hygroma), microcystic (formerly lymphangioma circumscriptum), or mixed.1 Patients often will present with pruritus, crusting, secondary infection, edema, or oozing.2 The superficial blebs of microcystic LMs resemble frog spawn and range in color from clear to pink, brawny, or deep maroon.3 Although the lymphatic vessels involved in microcystic LMs appear disconnected from the major lymphatic circulation,3 systemic fluid overload could plausibly promote lesional swelling and tenderness; we attributed our patient's worsening symptoms to the cumulative 7.8 L of intravenous fluid he received intraoperatively during his cardiac transplant. The excess fluid allowed communication between lymphatic cisterns and thin-walled vesicles on the skin surface through dilated channels. Overall, LMs represent roughly 26% of pediatric benign vascular tumors and approximately 4% of all vascular tumors.4
Although microcystic LMs may appear especially vascular or verrucous, the differential diagnosis for our patient's LM included condyloma acuminatum,5,6 condyloma lata,7 epidermal nevus, and lymphangiosarcoma. Epidermal nevi are congenital lesions, varying in appearance from velvety to verrucous patches and plaques that often evolve during puberty and become thicker, more verrucous, and hyperpigmented. Keratinocytic epidermal nevus syndromes and other entities such as nevus sebaceous have been associated with somatic mutations affecting proteins in the fibroblast growth factor receptor signaling pathway (eg, FGFR3, HRAS).8 Although the clinical appearance alone may be similar, lymphangiosarcoma can be distinguished from LM via biopsy.
There are several methods to diagnose LM. Duplex sonography is possibly the best noninvasive method to identify the flow between venous valves. Magnetic resonance imaging can detect larger occurrences of LM, and lymphangiography can be utilized to confirm a normal or abnormal lymphatic network.4 Treatment options are broad, including surgical excision, laser ablation, and topical sirolimus. Hypertonic saline sclerotherapy can be injected into the afflicted lymphatic channels to decrease inflammation, erythema, and hyperpigmentation without further treatment or major side effects.4
However, the benefits of sclerotherapy alone in the treatment of LM often come gradually, and radiofrequency ablation may need to be utilized to achieve more immediate results.2 Overall, outcomes are highly variable, but favorable outcomes often can be difficult to obtain due to a high recurrence rate.2,8 Our patient's symptoms improved during his postoperative recovery, and he declined further intervention.
- Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23:178-185. doi:10.1053/j.sempedsurg.2014.07.002
- Niti K, Manish P. Microcystic lymphatic malformation (lymphangioma circumscriptum) treated using a minimally invasive technique of radiofrequency ablation and sclerotherapy. Dermatol Surg. 2010;36:1711-1717. doi:10.1111/j.1524-4725.2010.01723.x
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111 /j.1365-4632.2009.04226.x
- Bikowski JB, Dumont AM. Lymphangioma circumscriptum: treatment with hypertonic saline sclerotherapy. J Am Acad Dermatol. 2005;53:442-444. doi:10.1016/j.jaad.2005.04.086
- Costa-Silva M, Fernandes I, Rodrigues AG, et al. Anogenital warts in pediatric population. An Bras Dermatol. 2017;92:675-681. doi:10.1590 /abd1806-4841.201756411
- Darmstadt GL. Perianal lymphangioma circumscriptum mistaken for genital warts. Pediatrics 1996;98;461.
- Bruins FG, van Deudekom FJA, de Vries HJC. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259. doi:10.1136 /bmj.h1259
- Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi:10.1111 /pde.13273
The Diagnosis: Microcystic Lymphatic Malformation
The shave biopsy demonstrated numerous thin-walled vascular spaces filled with lymphatic fluid within the dermis (Figure), consistent with a diagnosis of microcystic lymphatic malformation (LM). Lymphatic malformations represent a class of benign vascular lesions consisting of anomalous or dilated lymphatic vessels, which can be broadly categorized as macrocystic (formerly cavernous lymphangioma or cystic hygroma), microcystic (formerly lymphangioma circumscriptum), or mixed.1 Patients often will present with pruritus, crusting, secondary infection, edema, or oozing.2 The superficial blebs of microcystic LMs resemble frog spawn and range in color from clear to pink, brawny, or deep maroon.3 Although the lymphatic vessels involved in microcystic LMs appear disconnected from the major lymphatic circulation,3 systemic fluid overload could plausibly promote lesional swelling and tenderness; we attributed our patient's worsening symptoms to the cumulative 7.8 L of intravenous fluid he received intraoperatively during his cardiac transplant. The excess fluid allowed communication between lymphatic cisterns and thin-walled vesicles on the skin surface through dilated channels. Overall, LMs represent roughly 26% of pediatric benign vascular tumors and approximately 4% of all vascular tumors.4
Although microcystic LMs may appear especially vascular or verrucous, the differential diagnosis for our patient's LM included condyloma acuminatum,5,6 condyloma lata,7 epidermal nevus, and lymphangiosarcoma. Epidermal nevi are congenital lesions, varying in appearance from velvety to verrucous patches and plaques that often evolve during puberty and become thicker, more verrucous, and hyperpigmented. Keratinocytic epidermal nevus syndromes and other entities such as nevus sebaceous have been associated with somatic mutations affecting proteins in the fibroblast growth factor receptor signaling pathway (eg, FGFR3, HRAS).8 Although the clinical appearance alone may be similar, lymphangiosarcoma can be distinguished from LM via biopsy.
There are several methods to diagnose LM. Duplex sonography is possibly the best noninvasive method to identify the flow between venous valves. Magnetic resonance imaging can detect larger occurrences of LM, and lymphangiography can be utilized to confirm a normal or abnormal lymphatic network.4 Treatment options are broad, including surgical excision, laser ablation, and topical sirolimus. Hypertonic saline sclerotherapy can be injected into the afflicted lymphatic channels to decrease inflammation, erythema, and hyperpigmentation without further treatment or major side effects.4
However, the benefits of sclerotherapy alone in the treatment of LM often come gradually, and radiofrequency ablation may need to be utilized to achieve more immediate results.2 Overall, outcomes are highly variable, but favorable outcomes often can be difficult to obtain due to a high recurrence rate.2,8 Our patient's symptoms improved during his postoperative recovery, and he declined further intervention.
The Diagnosis: Microcystic Lymphatic Malformation
The shave biopsy demonstrated numerous thin-walled vascular spaces filled with lymphatic fluid within the dermis (Figure), consistent with a diagnosis of microcystic lymphatic malformation (LM). Lymphatic malformations represent a class of benign vascular lesions consisting of anomalous or dilated lymphatic vessels, which can be broadly categorized as macrocystic (formerly cavernous lymphangioma or cystic hygroma), microcystic (formerly lymphangioma circumscriptum), or mixed.1 Patients often will present with pruritus, crusting, secondary infection, edema, or oozing.2 The superficial blebs of microcystic LMs resemble frog spawn and range in color from clear to pink, brawny, or deep maroon.3 Although the lymphatic vessels involved in microcystic LMs appear disconnected from the major lymphatic circulation,3 systemic fluid overload could plausibly promote lesional swelling and tenderness; we attributed our patient's worsening symptoms to the cumulative 7.8 L of intravenous fluid he received intraoperatively during his cardiac transplant. The excess fluid allowed communication between lymphatic cisterns and thin-walled vesicles on the skin surface through dilated channels. Overall, LMs represent roughly 26% of pediatric benign vascular tumors and approximately 4% of all vascular tumors.4
Although microcystic LMs may appear especially vascular or verrucous, the differential diagnosis for our patient's LM included condyloma acuminatum,5,6 condyloma lata,7 epidermal nevus, and lymphangiosarcoma. Epidermal nevi are congenital lesions, varying in appearance from velvety to verrucous patches and plaques that often evolve during puberty and become thicker, more verrucous, and hyperpigmented. Keratinocytic epidermal nevus syndromes and other entities such as nevus sebaceous have been associated with somatic mutations affecting proteins in the fibroblast growth factor receptor signaling pathway (eg, FGFR3, HRAS).8 Although the clinical appearance alone may be similar, lymphangiosarcoma can be distinguished from LM via biopsy.
There are several methods to diagnose LM. Duplex sonography is possibly the best noninvasive method to identify the flow between venous valves. Magnetic resonance imaging can detect larger occurrences of LM, and lymphangiography can be utilized to confirm a normal or abnormal lymphatic network.4 Treatment options are broad, including surgical excision, laser ablation, and topical sirolimus. Hypertonic saline sclerotherapy can be injected into the afflicted lymphatic channels to decrease inflammation, erythema, and hyperpigmentation without further treatment or major side effects.4
However, the benefits of sclerotherapy alone in the treatment of LM often come gradually, and radiofrequency ablation may need to be utilized to achieve more immediate results.2 Overall, outcomes are highly variable, but favorable outcomes often can be difficult to obtain due to a high recurrence rate.2,8 Our patient's symptoms improved during his postoperative recovery, and he declined further intervention.
- Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23:178-185. doi:10.1053/j.sempedsurg.2014.07.002
- Niti K, Manish P. Microcystic lymphatic malformation (lymphangioma circumscriptum) treated using a minimally invasive technique of radiofrequency ablation and sclerotherapy. Dermatol Surg. 2010;36:1711-1717. doi:10.1111/j.1524-4725.2010.01723.x
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111 /j.1365-4632.2009.04226.x
- Bikowski JB, Dumont AM. Lymphangioma circumscriptum: treatment with hypertonic saline sclerotherapy. J Am Acad Dermatol. 2005;53:442-444. doi:10.1016/j.jaad.2005.04.086
- Costa-Silva M, Fernandes I, Rodrigues AG, et al. Anogenital warts in pediatric population. An Bras Dermatol. 2017;92:675-681. doi:10.1590 /abd1806-4841.201756411
- Darmstadt GL. Perianal lymphangioma circumscriptum mistaken for genital warts. Pediatrics 1996;98;461.
- Bruins FG, van Deudekom FJA, de Vries HJC. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259. doi:10.1136 /bmj.h1259
- Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi:10.1111 /pde.13273
- Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23:178-185. doi:10.1053/j.sempedsurg.2014.07.002
- Niti K, Manish P. Microcystic lymphatic malformation (lymphangioma circumscriptum) treated using a minimally invasive technique of radiofrequency ablation and sclerotherapy. Dermatol Surg. 2010;36:1711-1717. doi:10.1111/j.1524-4725.2010.01723.x
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111 /j.1365-4632.2009.04226.x
- Bikowski JB, Dumont AM. Lymphangioma circumscriptum: treatment with hypertonic saline sclerotherapy. J Am Acad Dermatol. 2005;53:442-444. doi:10.1016/j.jaad.2005.04.086
- Costa-Silva M, Fernandes I, Rodrigues AG, et al. Anogenital warts in pediatric population. An Bras Dermatol. 2017;92:675-681. doi:10.1590 /abd1806-4841.201756411
- Darmstadt GL. Perianal lymphangioma circumscriptum mistaken for genital warts. Pediatrics 1996;98;461.
- Bruins FG, van Deudekom FJA, de Vries HJC. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259. doi:10.1136 /bmj.h1259
- Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi:10.1111 /pde.13273
A 17-year-old adolescent boy presented with increasingly painful genital warts on the right thigh, groin, and scrotum that had been present since birth. The patient had a medical history of cardiac transplantation in the months prior to presentation and was on immunosuppressive therapy. The lesions had become more swollen and bothersome in the weeks following the transplantation and now prevented him from ambulating due to discomfort. He denied any history of sexual contact or oral lesions. Physical examination revealed numerous translucent and hemorrhagic vesicles clustered and linearly distributed on the right medial thigh. A shave biopsy of a vesicle was performed.
I did peer review: I saw turf wars, ego, and unfairness
After making an insulting comment to a surgery scheduler, a surgeon become the subject of a peer review investigation.
The surgeon had been called in on a Saturday morning for surgery, but when he arrived at the hospital, staff informed him that the operating room had been incorrectly booked and asked him to come back that afternoon. When the surgeon returned, the room still wasn’t ready, recounted David Beran, DO, a peer reviewer and medical director for the emergency department at University Medical Center New Orleans, in Louisiana. After more waiting and staff uncertainty about which operating room was going to open, the surgeon became frustrated and said to the scheduler: “Any idiot could figure this out!”
During his peer review, the surgeon acknowledged that he shouldn’t have made the rude remark to the scheduler, Dr. Beran said. His exasperation stemmed from an ongoing problem – operating rooms at the hospital were being inefficiently managed.
“The surgeon acknowledged that even though there was a systems issue at the root, that’s not justification to speak to people unprofessionally,” Dr. Beran said. “So, there was education for the surgeon, but the surgeon was also able to explain the frustration that led to that point.”
System problems are commonly encountered by peer reviewers, said Dr. Beran.
“There’s a huge gap between administration and clinical professionals when it comes to peer review,” he said. “So many times, bad situations, whether they’re clinical or behavioral, often boil down to systems issues or some inadequacy, whether it’s an EMR [electronic medical record] problem, an inefficacy, or how complicated a process is for an end user. But having a peer review situation that then leads to a system-level change that prevents that problem from happening again is really unlikely. There’s a huge disconnect between those two.”
Peer review is generally a process that goes on behind closed doors. Although structures may differ, peer review is generally described as the process by which physicians assess the quality of their peers’ work to ensure that standards of care are being met. The process is often used to evaluate issues regarding clinical care as well as behavioral complaints against physicians.
Doctors who undergo peer review frequently share their experiences, but reviewers themselves rarely speak out. For this story,
“Peer review processes are in place to build stronger institutions and stronger practices, and they’re supposed to be helpful,” Dr. Beran said. “But because of how opaque they are, it immediately puts physicians on the defensive, and it doesn’t always succeed in what it’s trying to do. I think that’s one of the biggest challenges.”
Biased reviewers taint evaluations
A peer reviewer on and off throughout her career, Indiana family physician Lana Patch, MD, said she always strived to be fair when evaluating fellow physicians. But not every reviewer she encountered operated the same way, she said. Some were biased.
In one case, Dr. Patch peer reviewed a general surgeon who had performed a hysterectomy on a 16-year-old girl. The surgeon believed the teenager likely had an acute appendicitis, but it turned out she had a uterine pathology, Dr. Patch said. The surgeon saved the girl’s life, but the case came under review because of the patient’s age and the fact that her uterus was removed. A local obstetrician-gynecologist weighed in on the case.
“The local ob.gyn. saw it as a turf battle,” recalled Dr. Patch, who is now retired after 30 years of practice in eastern Indiana. “The doctor had nothing but bad to say about the surgeon. He was a competitor.”
Because it was a small hospital, the committee sometimes had trouble finding a specialist who was qualified to give an opinion and who wasn’t in competition with the physician in question, said Dr. Patch. Eventually they found an outside pediatric gynecologist who reviewed the case and concluded that the surgeon had followed the standard of care.
Personal agendas in can come from different directions, said Robert Marder, MD, the author of several books on peer review. Dr. Marder is a consultant who assists with peer review redesign. He has worked with hundreds of medical staff leaders and is a former vice president at the Greeley Company, a consulting firm in Danvers, Mass., that performs peer review redesign. Dr. Marder is president of Robert J. Marder Consulting.
“It goes both ways,” Dr. Marder said. “I’ve seen where somebody with a personal view decides to bring things to the peer review committee specifically because they want the peer review committee to have an adverse view of this person and get them off the medical staff. And I’ve seen hospitals that are uncomfortable with a certain person for whatever reason and want the peer review committee to address it, as opposed to addressing it from a human resource standpoint.”
Dr. Patch recalled a case in which reviewers and hospital leaders were at odds over the credentialing of a physician. Fifteen years earlier, while driving in California, the psychiatrist had been pulled over and was found with an ounce of marijuana, she said.
“We wanted to privilege him,” Dr. Patch said. “As staff physicians, we felt that was 15 years ago, people change over time. Doctors are human beings, too. He seemed to have good credentials and good training. The hospital said, ‘Oh no, we can’t have somebody like this.’ “
The psychiatrist was placed on probation and had to undergo a review every 90 days for about 3 years. Eventually, he was privileged, Dr. Patch said.
Bias among reviewers, including unintentional bias, is also a challenge, Dr. Marder noted. Some initial reviewers score a physician too harshly, he said, whereas others underscore.
“Underscoring is more insidious and more difficult to deal with,” Dr. Marder said. “Underscoring is where the reviewer is too nice. They tend to dismiss things from their colleagues rather than recognize them as an opportunity to help them improve. With underscoring, a lot of committees, if the initial reviewer says the care was appropriate, they don’t even look at the case. They just take that one person’s word for it.”
Reviewers: Looks can be deceiving
When first examining the documented details of a case, it can be easy for peer reviewers to make a quick judgment about what happened, Dr. Beran said.
“You get these complaints, and you read through it, and you think, ‘Oh man, this person really messed up,’ “ he said. “Then you hear the doctor’s side of it, and you realize, ‘No, there’s a much bigger picture at play.’ You realize both sides have valid perspectives on it.”
In one case, for example, Dr. Beran recalled a complaint against a physician who made a snarky remark to a nurse. The doctor had asked the nurse for a piece of equipment, and the nurse said she was busy preparing the room for a patient. The doctor made a comment along the lines of, “Well, would you like me to do that for you and also intubate the patient while you do some charting?!”
At first glance, it appeared that the physician lashed out inappropriately at the nurse. But when reviewers heard from the doctor, they learned that the nurses knew that a trauma patient was coming by ambulance and that he would likely require a ventilator, Dr. Beran said. As the minutes ticked by, however, the nurses were seen in the break room chatting. Nothing had been prepared in the room, including any airway supply.
“The patient had a prolonged course and a very difficult intubation and could have very easily wound up with a much worse outcome for something the nurses had been warned about prior to the patient’s arrival,” he said. “I can see anybody getting upset in that situation if I warned them 5 or 10 minutes beforehand, ‘Get this stuff ready,’ and then nothing was done.”
There was no direct penalty for the physician.
Just as some complaints can be misleading, the clinical record in some peer review cases can also lead reviewers astray.
Physicians frequently include too much irrelevant information in the record, which can cloud a peer review, said Hans Duvefelt, MD, a family physician at Pines Health Services, in Van Buren, Maine. Dr. Duvefelt is a former medical director at Bucksport Regional Health Center, in Ellsworth, Maine. Both facilities are federally qualified health centers where continuous, random peer reviews are required.
In one case, Dr. Duvefelt was peer reviewing a physician’s office note regarding an elderly patient with a low-grade fever. The final diagnosis was urinary tract infection. Dr. Duvefelt said he had trouble following the doctor’s line of thinking because of a plethora of unnecessary data in the 10-page document. The office note included past medical history, prior lab and imaging test results, and an extensive narrative section that included a mixture of active medical problems and ongoing relationships with specialists, he said.
After reading through the printout three times, Dr. Duvefelt said he finally found mention of increased urinary dribbling and details about an enlarged prostate. He also spotted a same-day urinalysis among nearly a dozen other previous lab tests that had no connection to body temperature. Dr. Duvefelt gave the physician a passing grade but also left a scathing note about all the irrelevant information.
“It’s very common,” Dr. Duvefelt said. “It’s a disaster. Other doctors can’t follow your thinking. A reviewer has a hard time determining whether the doctor acted reasonably.”
Slackers make bad reviewers
Although dedicated reviewers work hard to get to the bottom of cases, it’s not uncommon for some committee members to hardly work at all, according to experts.
Dr. Marder said he’s seen many instances in which reviewers were assigned a review but did not complete it for months. Most committees have set time frames in which reviewers must complete their review.
“That delays that review, and by that time, the review is older and it’s harder to remember things,” he said. “It’s not fair to the physician. If there was a problem the physician could fix and you don’t tell him for 3 or 4 months what it is, he may do the same thing again. The case might come before the committee again and it looks like he’s repeated something, but you never gave him the opportunity to improve.”
Other reviewers fail to attend meetings regularly. Peer review committee members are generally volunteers, and meetings are usually held in the early mornings or late evenings.
“There are reasons for not attending occasionally, but some people put on a committee just don’t take it seriously,” Dr. Marder said. “They don’t fulfill their responsibilities as well as they should. If you accept the job, do the job.”
For physicians considering becoming a peer reviewer, Dr. Beran offers these tips: Be transparent, help physicians understand next steps, and make yourself as available as allowed to answer questions.
Know your committee’s policies and procedures, and follow them, added Dr. Marder. It’s also a good idea to work with your hospital’s quality staff, he said.
Reviewers should keep in mind that they may not always be the one assessing someone else, Dr. Beran said.
“Realize very easily you could be on the other side of that table for things that are outside your control,” he said. “How would you want to be treated?”
A version of this article first appeared on Medscape.com.
After making an insulting comment to a surgery scheduler, a surgeon become the subject of a peer review investigation.
The surgeon had been called in on a Saturday morning for surgery, but when he arrived at the hospital, staff informed him that the operating room had been incorrectly booked and asked him to come back that afternoon. When the surgeon returned, the room still wasn’t ready, recounted David Beran, DO, a peer reviewer and medical director for the emergency department at University Medical Center New Orleans, in Louisiana. After more waiting and staff uncertainty about which operating room was going to open, the surgeon became frustrated and said to the scheduler: “Any idiot could figure this out!”
During his peer review, the surgeon acknowledged that he shouldn’t have made the rude remark to the scheduler, Dr. Beran said. His exasperation stemmed from an ongoing problem – operating rooms at the hospital were being inefficiently managed.
“The surgeon acknowledged that even though there was a systems issue at the root, that’s not justification to speak to people unprofessionally,” Dr. Beran said. “So, there was education for the surgeon, but the surgeon was also able to explain the frustration that led to that point.”
System problems are commonly encountered by peer reviewers, said Dr. Beran.
“There’s a huge gap between administration and clinical professionals when it comes to peer review,” he said. “So many times, bad situations, whether they’re clinical or behavioral, often boil down to systems issues or some inadequacy, whether it’s an EMR [electronic medical record] problem, an inefficacy, or how complicated a process is for an end user. But having a peer review situation that then leads to a system-level change that prevents that problem from happening again is really unlikely. There’s a huge disconnect between those two.”
Peer review is generally a process that goes on behind closed doors. Although structures may differ, peer review is generally described as the process by which physicians assess the quality of their peers’ work to ensure that standards of care are being met. The process is often used to evaluate issues regarding clinical care as well as behavioral complaints against physicians.
Doctors who undergo peer review frequently share their experiences, but reviewers themselves rarely speak out. For this story,
“Peer review processes are in place to build stronger institutions and stronger practices, and they’re supposed to be helpful,” Dr. Beran said. “But because of how opaque they are, it immediately puts physicians on the defensive, and it doesn’t always succeed in what it’s trying to do. I think that’s one of the biggest challenges.”
Biased reviewers taint evaluations
A peer reviewer on and off throughout her career, Indiana family physician Lana Patch, MD, said she always strived to be fair when evaluating fellow physicians. But not every reviewer she encountered operated the same way, she said. Some were biased.
In one case, Dr. Patch peer reviewed a general surgeon who had performed a hysterectomy on a 16-year-old girl. The surgeon believed the teenager likely had an acute appendicitis, but it turned out she had a uterine pathology, Dr. Patch said. The surgeon saved the girl’s life, but the case came under review because of the patient’s age and the fact that her uterus was removed. A local obstetrician-gynecologist weighed in on the case.
“The local ob.gyn. saw it as a turf battle,” recalled Dr. Patch, who is now retired after 30 years of practice in eastern Indiana. “The doctor had nothing but bad to say about the surgeon. He was a competitor.”
Because it was a small hospital, the committee sometimes had trouble finding a specialist who was qualified to give an opinion and who wasn’t in competition with the physician in question, said Dr. Patch. Eventually they found an outside pediatric gynecologist who reviewed the case and concluded that the surgeon had followed the standard of care.
Personal agendas in can come from different directions, said Robert Marder, MD, the author of several books on peer review. Dr. Marder is a consultant who assists with peer review redesign. He has worked with hundreds of medical staff leaders and is a former vice president at the Greeley Company, a consulting firm in Danvers, Mass., that performs peer review redesign. Dr. Marder is president of Robert J. Marder Consulting.
“It goes both ways,” Dr. Marder said. “I’ve seen where somebody with a personal view decides to bring things to the peer review committee specifically because they want the peer review committee to have an adverse view of this person and get them off the medical staff. And I’ve seen hospitals that are uncomfortable with a certain person for whatever reason and want the peer review committee to address it, as opposed to addressing it from a human resource standpoint.”
Dr. Patch recalled a case in which reviewers and hospital leaders were at odds over the credentialing of a physician. Fifteen years earlier, while driving in California, the psychiatrist had been pulled over and was found with an ounce of marijuana, she said.
“We wanted to privilege him,” Dr. Patch said. “As staff physicians, we felt that was 15 years ago, people change over time. Doctors are human beings, too. He seemed to have good credentials and good training. The hospital said, ‘Oh no, we can’t have somebody like this.’ “
The psychiatrist was placed on probation and had to undergo a review every 90 days for about 3 years. Eventually, he was privileged, Dr. Patch said.
Bias among reviewers, including unintentional bias, is also a challenge, Dr. Marder noted. Some initial reviewers score a physician too harshly, he said, whereas others underscore.
“Underscoring is more insidious and more difficult to deal with,” Dr. Marder said. “Underscoring is where the reviewer is too nice. They tend to dismiss things from their colleagues rather than recognize them as an opportunity to help them improve. With underscoring, a lot of committees, if the initial reviewer says the care was appropriate, they don’t even look at the case. They just take that one person’s word for it.”
Reviewers: Looks can be deceiving
When first examining the documented details of a case, it can be easy for peer reviewers to make a quick judgment about what happened, Dr. Beran said.
“You get these complaints, and you read through it, and you think, ‘Oh man, this person really messed up,’ “ he said. “Then you hear the doctor’s side of it, and you realize, ‘No, there’s a much bigger picture at play.’ You realize both sides have valid perspectives on it.”
In one case, for example, Dr. Beran recalled a complaint against a physician who made a snarky remark to a nurse. The doctor had asked the nurse for a piece of equipment, and the nurse said she was busy preparing the room for a patient. The doctor made a comment along the lines of, “Well, would you like me to do that for you and also intubate the patient while you do some charting?!”
At first glance, it appeared that the physician lashed out inappropriately at the nurse. But when reviewers heard from the doctor, they learned that the nurses knew that a trauma patient was coming by ambulance and that he would likely require a ventilator, Dr. Beran said. As the minutes ticked by, however, the nurses were seen in the break room chatting. Nothing had been prepared in the room, including any airway supply.
“The patient had a prolonged course and a very difficult intubation and could have very easily wound up with a much worse outcome for something the nurses had been warned about prior to the patient’s arrival,” he said. “I can see anybody getting upset in that situation if I warned them 5 or 10 minutes beforehand, ‘Get this stuff ready,’ and then nothing was done.”
There was no direct penalty for the physician.
Just as some complaints can be misleading, the clinical record in some peer review cases can also lead reviewers astray.
Physicians frequently include too much irrelevant information in the record, which can cloud a peer review, said Hans Duvefelt, MD, a family physician at Pines Health Services, in Van Buren, Maine. Dr. Duvefelt is a former medical director at Bucksport Regional Health Center, in Ellsworth, Maine. Both facilities are federally qualified health centers where continuous, random peer reviews are required.
In one case, Dr. Duvefelt was peer reviewing a physician’s office note regarding an elderly patient with a low-grade fever. The final diagnosis was urinary tract infection. Dr. Duvefelt said he had trouble following the doctor’s line of thinking because of a plethora of unnecessary data in the 10-page document. The office note included past medical history, prior lab and imaging test results, and an extensive narrative section that included a mixture of active medical problems and ongoing relationships with specialists, he said.
After reading through the printout three times, Dr. Duvefelt said he finally found mention of increased urinary dribbling and details about an enlarged prostate. He also spotted a same-day urinalysis among nearly a dozen other previous lab tests that had no connection to body temperature. Dr. Duvefelt gave the physician a passing grade but also left a scathing note about all the irrelevant information.
“It’s very common,” Dr. Duvefelt said. “It’s a disaster. Other doctors can’t follow your thinking. A reviewer has a hard time determining whether the doctor acted reasonably.”
Slackers make bad reviewers
Although dedicated reviewers work hard to get to the bottom of cases, it’s not uncommon for some committee members to hardly work at all, according to experts.
Dr. Marder said he’s seen many instances in which reviewers were assigned a review but did not complete it for months. Most committees have set time frames in which reviewers must complete their review.
“That delays that review, and by that time, the review is older and it’s harder to remember things,” he said. “It’s not fair to the physician. If there was a problem the physician could fix and you don’t tell him for 3 or 4 months what it is, he may do the same thing again. The case might come before the committee again and it looks like he’s repeated something, but you never gave him the opportunity to improve.”
Other reviewers fail to attend meetings regularly. Peer review committee members are generally volunteers, and meetings are usually held in the early mornings or late evenings.
“There are reasons for not attending occasionally, but some people put on a committee just don’t take it seriously,” Dr. Marder said. “They don’t fulfill their responsibilities as well as they should. If you accept the job, do the job.”
For physicians considering becoming a peer reviewer, Dr. Beran offers these tips: Be transparent, help physicians understand next steps, and make yourself as available as allowed to answer questions.
Know your committee’s policies and procedures, and follow them, added Dr. Marder. It’s also a good idea to work with your hospital’s quality staff, he said.
Reviewers should keep in mind that they may not always be the one assessing someone else, Dr. Beran said.
“Realize very easily you could be on the other side of that table for things that are outside your control,” he said. “How would you want to be treated?”
A version of this article first appeared on Medscape.com.
After making an insulting comment to a surgery scheduler, a surgeon become the subject of a peer review investigation.
The surgeon had been called in on a Saturday morning for surgery, but when he arrived at the hospital, staff informed him that the operating room had been incorrectly booked and asked him to come back that afternoon. When the surgeon returned, the room still wasn’t ready, recounted David Beran, DO, a peer reviewer and medical director for the emergency department at University Medical Center New Orleans, in Louisiana. After more waiting and staff uncertainty about which operating room was going to open, the surgeon became frustrated and said to the scheduler: “Any idiot could figure this out!”
During his peer review, the surgeon acknowledged that he shouldn’t have made the rude remark to the scheduler, Dr. Beran said. His exasperation stemmed from an ongoing problem – operating rooms at the hospital were being inefficiently managed.
“The surgeon acknowledged that even though there was a systems issue at the root, that’s not justification to speak to people unprofessionally,” Dr. Beran said. “So, there was education for the surgeon, but the surgeon was also able to explain the frustration that led to that point.”
System problems are commonly encountered by peer reviewers, said Dr. Beran.
“There’s a huge gap between administration and clinical professionals when it comes to peer review,” he said. “So many times, bad situations, whether they’re clinical or behavioral, often boil down to systems issues or some inadequacy, whether it’s an EMR [electronic medical record] problem, an inefficacy, or how complicated a process is for an end user. But having a peer review situation that then leads to a system-level change that prevents that problem from happening again is really unlikely. There’s a huge disconnect between those two.”
Peer review is generally a process that goes on behind closed doors. Although structures may differ, peer review is generally described as the process by which physicians assess the quality of their peers’ work to ensure that standards of care are being met. The process is often used to evaluate issues regarding clinical care as well as behavioral complaints against physicians.
Doctors who undergo peer review frequently share their experiences, but reviewers themselves rarely speak out. For this story,
“Peer review processes are in place to build stronger institutions and stronger practices, and they’re supposed to be helpful,” Dr. Beran said. “But because of how opaque they are, it immediately puts physicians on the defensive, and it doesn’t always succeed in what it’s trying to do. I think that’s one of the biggest challenges.”
Biased reviewers taint evaluations
A peer reviewer on and off throughout her career, Indiana family physician Lana Patch, MD, said she always strived to be fair when evaluating fellow physicians. But not every reviewer she encountered operated the same way, she said. Some were biased.
In one case, Dr. Patch peer reviewed a general surgeon who had performed a hysterectomy on a 16-year-old girl. The surgeon believed the teenager likely had an acute appendicitis, but it turned out she had a uterine pathology, Dr. Patch said. The surgeon saved the girl’s life, but the case came under review because of the patient’s age and the fact that her uterus was removed. A local obstetrician-gynecologist weighed in on the case.
“The local ob.gyn. saw it as a turf battle,” recalled Dr. Patch, who is now retired after 30 years of practice in eastern Indiana. “The doctor had nothing but bad to say about the surgeon. He was a competitor.”
Because it was a small hospital, the committee sometimes had trouble finding a specialist who was qualified to give an opinion and who wasn’t in competition with the physician in question, said Dr. Patch. Eventually they found an outside pediatric gynecologist who reviewed the case and concluded that the surgeon had followed the standard of care.
Personal agendas in can come from different directions, said Robert Marder, MD, the author of several books on peer review. Dr. Marder is a consultant who assists with peer review redesign. He has worked with hundreds of medical staff leaders and is a former vice president at the Greeley Company, a consulting firm in Danvers, Mass., that performs peer review redesign. Dr. Marder is president of Robert J. Marder Consulting.
“It goes both ways,” Dr. Marder said. “I’ve seen where somebody with a personal view decides to bring things to the peer review committee specifically because they want the peer review committee to have an adverse view of this person and get them off the medical staff. And I’ve seen hospitals that are uncomfortable with a certain person for whatever reason and want the peer review committee to address it, as opposed to addressing it from a human resource standpoint.”
Dr. Patch recalled a case in which reviewers and hospital leaders were at odds over the credentialing of a physician. Fifteen years earlier, while driving in California, the psychiatrist had been pulled over and was found with an ounce of marijuana, she said.
“We wanted to privilege him,” Dr. Patch said. “As staff physicians, we felt that was 15 years ago, people change over time. Doctors are human beings, too. He seemed to have good credentials and good training. The hospital said, ‘Oh no, we can’t have somebody like this.’ “
The psychiatrist was placed on probation and had to undergo a review every 90 days for about 3 years. Eventually, he was privileged, Dr. Patch said.
Bias among reviewers, including unintentional bias, is also a challenge, Dr. Marder noted. Some initial reviewers score a physician too harshly, he said, whereas others underscore.
“Underscoring is more insidious and more difficult to deal with,” Dr. Marder said. “Underscoring is where the reviewer is too nice. They tend to dismiss things from their colleagues rather than recognize them as an opportunity to help them improve. With underscoring, a lot of committees, if the initial reviewer says the care was appropriate, they don’t even look at the case. They just take that one person’s word for it.”
Reviewers: Looks can be deceiving
When first examining the documented details of a case, it can be easy for peer reviewers to make a quick judgment about what happened, Dr. Beran said.
“You get these complaints, and you read through it, and you think, ‘Oh man, this person really messed up,’ “ he said. “Then you hear the doctor’s side of it, and you realize, ‘No, there’s a much bigger picture at play.’ You realize both sides have valid perspectives on it.”
In one case, for example, Dr. Beran recalled a complaint against a physician who made a snarky remark to a nurse. The doctor had asked the nurse for a piece of equipment, and the nurse said she was busy preparing the room for a patient. The doctor made a comment along the lines of, “Well, would you like me to do that for you and also intubate the patient while you do some charting?!”
At first glance, it appeared that the physician lashed out inappropriately at the nurse. But when reviewers heard from the doctor, they learned that the nurses knew that a trauma patient was coming by ambulance and that he would likely require a ventilator, Dr. Beran said. As the minutes ticked by, however, the nurses were seen in the break room chatting. Nothing had been prepared in the room, including any airway supply.
“The patient had a prolonged course and a very difficult intubation and could have very easily wound up with a much worse outcome for something the nurses had been warned about prior to the patient’s arrival,” he said. “I can see anybody getting upset in that situation if I warned them 5 or 10 minutes beforehand, ‘Get this stuff ready,’ and then nothing was done.”
There was no direct penalty for the physician.
Just as some complaints can be misleading, the clinical record in some peer review cases can also lead reviewers astray.
Physicians frequently include too much irrelevant information in the record, which can cloud a peer review, said Hans Duvefelt, MD, a family physician at Pines Health Services, in Van Buren, Maine. Dr. Duvefelt is a former medical director at Bucksport Regional Health Center, in Ellsworth, Maine. Both facilities are federally qualified health centers where continuous, random peer reviews are required.
In one case, Dr. Duvefelt was peer reviewing a physician’s office note regarding an elderly patient with a low-grade fever. The final diagnosis was urinary tract infection. Dr. Duvefelt said he had trouble following the doctor’s line of thinking because of a plethora of unnecessary data in the 10-page document. The office note included past medical history, prior lab and imaging test results, and an extensive narrative section that included a mixture of active medical problems and ongoing relationships with specialists, he said.
After reading through the printout three times, Dr. Duvefelt said he finally found mention of increased urinary dribbling and details about an enlarged prostate. He also spotted a same-day urinalysis among nearly a dozen other previous lab tests that had no connection to body temperature. Dr. Duvefelt gave the physician a passing grade but also left a scathing note about all the irrelevant information.
“It’s very common,” Dr. Duvefelt said. “It’s a disaster. Other doctors can’t follow your thinking. A reviewer has a hard time determining whether the doctor acted reasonably.”
Slackers make bad reviewers
Although dedicated reviewers work hard to get to the bottom of cases, it’s not uncommon for some committee members to hardly work at all, according to experts.
Dr. Marder said he’s seen many instances in which reviewers were assigned a review but did not complete it for months. Most committees have set time frames in which reviewers must complete their review.
“That delays that review, and by that time, the review is older and it’s harder to remember things,” he said. “It’s not fair to the physician. If there was a problem the physician could fix and you don’t tell him for 3 or 4 months what it is, he may do the same thing again. The case might come before the committee again and it looks like he’s repeated something, but you never gave him the opportunity to improve.”
Other reviewers fail to attend meetings regularly. Peer review committee members are generally volunteers, and meetings are usually held in the early mornings or late evenings.
“There are reasons for not attending occasionally, but some people put on a committee just don’t take it seriously,” Dr. Marder said. “They don’t fulfill their responsibilities as well as they should. If you accept the job, do the job.”
For physicians considering becoming a peer reviewer, Dr. Beran offers these tips: Be transparent, help physicians understand next steps, and make yourself as available as allowed to answer questions.
Know your committee’s policies and procedures, and follow them, added Dr. Marder. It’s also a good idea to work with your hospital’s quality staff, he said.
Reviewers should keep in mind that they may not always be the one assessing someone else, Dr. Beran said.
“Realize very easily you could be on the other side of that table for things that are outside your control,” he said. “How would you want to be treated?”
A version of this article first appeared on Medscape.com.
Six shifts driving the future of medicine, strategist says
Contact lenses that detect glucose in tears. Capsules embedded in clothes that can be used to counteract the risk of sensitive skin conditions.
These are just .
At the annual meeting of the Society for Pediatric Dermatology, Zayna Khayat, PhD, said that the future of medicine is driven by six shifts pulling society from a past oriented around the health care system – the buildings, clinicians, and payers – to a patient-oriented perspective. “That doesn’t just happen on its own,” said Dr. Khayat, a future strategist at Toronto-based SE Health. “There are big forces that are pulling us to the future whether we want it to or not. One is that patients have woken up. They have grown to have power in many other complex decisions in their life, and they’re expecting no less from our health care system.”
During her presentation, she discussed the six shifts:
1. The timing of service placement. The traditional model of medicine is “an intermittent and interventional science that waits for the symptoms and goes in and either fixes or manages them,” she said. “So, it’s not really health care; it’s sick care. That’s been fine in the industrial era when we needed to get medicine to stop catastrophic events. Not only is it shifting to be proactive and preventative but it’s shifting to a new science of medicine called predictive medicine.”
As for proactive and preventative care, she continued, each patient’s choice of behaviors related to diet, exercise, and stress “mingles with DNA to produce health, yet we spend about 90% of our resources on sick care. Now, health systems are moving their resources to things like education, housing, transportation, food security, equity, and racial divides. ... This is trickling down to how we train health care professionals. We know that patients live very little of their time in formal care settings, so all of their health is created – or destroyed – well outside of the clinical setting. We train our health professionals mostly in a clinical setting. Health systems are now starting to reimagine how training happens so we can train people to understand the fully loaded context of their patients’ lives.”
2. A shift in precision. For all its advances and science breakthroughs, medicine “is still quite crude,” said Dr. Khayat, who is also an adjunct professor in the Rotman School of Management at the University of Toronto. “It’s very analog, based on a one-size-fits-all approach. In the business world, we call this a segment of one: the idea that in some clinical trial, a result was produced that was based on the average of everybody, and therefore we just give everybody what worked for the average. ... We don’t need to have that trade-off anymore, because it won’t be a trade-off of higher cost to tailor down to an N of 1. It will be highly personalized, intelligent medicine, very precise.”
3. A shift from institution-centered to person-centered care. “The artifacts that health care was built on are very analog and are going to get decentralized out of buildings, dephysicalized, disintermediated,” she predicted. “We’ll have a seamless digital physical experience, expanded channels through which patients can access their services. Pick a channel that makes sense for the patient and don’t let care follow the place but rather let care follow the person.”
4. A shift in care duration, from episodic and intermittent care to more continuous care. “With very little input you should know what’s going on at any point in time instead of time-sharing access to diagnostics and to clinicians,” Dr. Khayat said. Wrist-worn devices that gather personal omics “are now really democratized, with every aspect of a diagnostic clinic available within or connected to a smartphone. This allows for data to be gathered and shared with clinicians, including tools under the skin that can get some of the biochemical data in real time instead of poking and prodding and waiting for a diagnostic lab.” These devices, she said, will become easier to use, cheaper, and will work faster, and provide much better data “at almost zero cost.”
Technologies being developed include tattoos that can read biomarkers, innovations in clothing that can detect biochemical reactions in the skin, underwear that can read vital signs, and contact lenses that can measure glucose levels. “The skin will become a major noninvasive way to obtain information,” she said.
5. A shift in power from providers to patients. “It’s estimated that about 80% of health care decisions could be self-managed by people in their communities,” Dr. Khayat said.
6. A shift from volume-based to value-based care. “Because we’ve been obsessed with the system, we’ve paid for stuff like visits, pills, MRI scans, et cetera,” she said. “We don’t need to do that anymore. Health systems don’t want to keep paying for stuff if they don’t see the results. Because of all the other shifts, we can pay for results. Some call this value-based care. I call it fee-for-health.”
She noted that the future of medicine is underpinned by innovations in AI/predictalytics, voice recognition, virtual reality, blockchain, IoT sensors, 3D printing, omics, robotics, autonomous transport, neurotechnology, nanobiology, and cellular therapy. “They’re moving at a very fast pace because they don’t need the kind of cost, capital, and expertise that the previous tools did,” she said. “This is the promise that technology can bring.”
Dr. Khayat disclosed that she has been a workshop participant for Roche Canada.
Contact lenses that detect glucose in tears. Capsules embedded in clothes that can be used to counteract the risk of sensitive skin conditions.
These are just .
At the annual meeting of the Society for Pediatric Dermatology, Zayna Khayat, PhD, said that the future of medicine is driven by six shifts pulling society from a past oriented around the health care system – the buildings, clinicians, and payers – to a patient-oriented perspective. “That doesn’t just happen on its own,” said Dr. Khayat, a future strategist at Toronto-based SE Health. “There are big forces that are pulling us to the future whether we want it to or not. One is that patients have woken up. They have grown to have power in many other complex decisions in their life, and they’re expecting no less from our health care system.”
During her presentation, she discussed the six shifts:
1. The timing of service placement. The traditional model of medicine is “an intermittent and interventional science that waits for the symptoms and goes in and either fixes or manages them,” she said. “So, it’s not really health care; it’s sick care. That’s been fine in the industrial era when we needed to get medicine to stop catastrophic events. Not only is it shifting to be proactive and preventative but it’s shifting to a new science of medicine called predictive medicine.”
As for proactive and preventative care, she continued, each patient’s choice of behaviors related to diet, exercise, and stress “mingles with DNA to produce health, yet we spend about 90% of our resources on sick care. Now, health systems are moving their resources to things like education, housing, transportation, food security, equity, and racial divides. ... This is trickling down to how we train health care professionals. We know that patients live very little of their time in formal care settings, so all of their health is created – or destroyed – well outside of the clinical setting. We train our health professionals mostly in a clinical setting. Health systems are now starting to reimagine how training happens so we can train people to understand the fully loaded context of their patients’ lives.”
2. A shift in precision. For all its advances and science breakthroughs, medicine “is still quite crude,” said Dr. Khayat, who is also an adjunct professor in the Rotman School of Management at the University of Toronto. “It’s very analog, based on a one-size-fits-all approach. In the business world, we call this a segment of one: the idea that in some clinical trial, a result was produced that was based on the average of everybody, and therefore we just give everybody what worked for the average. ... We don’t need to have that trade-off anymore, because it won’t be a trade-off of higher cost to tailor down to an N of 1. It will be highly personalized, intelligent medicine, very precise.”
3. A shift from institution-centered to person-centered care. “The artifacts that health care was built on are very analog and are going to get decentralized out of buildings, dephysicalized, disintermediated,” she predicted. “We’ll have a seamless digital physical experience, expanded channels through which patients can access their services. Pick a channel that makes sense for the patient and don’t let care follow the place but rather let care follow the person.”
4. A shift in care duration, from episodic and intermittent care to more continuous care. “With very little input you should know what’s going on at any point in time instead of time-sharing access to diagnostics and to clinicians,” Dr. Khayat said. Wrist-worn devices that gather personal omics “are now really democratized, with every aspect of a diagnostic clinic available within or connected to a smartphone. This allows for data to be gathered and shared with clinicians, including tools under the skin that can get some of the biochemical data in real time instead of poking and prodding and waiting for a diagnostic lab.” These devices, she said, will become easier to use, cheaper, and will work faster, and provide much better data “at almost zero cost.”
Technologies being developed include tattoos that can read biomarkers, innovations in clothing that can detect biochemical reactions in the skin, underwear that can read vital signs, and contact lenses that can measure glucose levels. “The skin will become a major noninvasive way to obtain information,” she said.
5. A shift in power from providers to patients. “It’s estimated that about 80% of health care decisions could be self-managed by people in their communities,” Dr. Khayat said.
6. A shift from volume-based to value-based care. “Because we’ve been obsessed with the system, we’ve paid for stuff like visits, pills, MRI scans, et cetera,” she said. “We don’t need to do that anymore. Health systems don’t want to keep paying for stuff if they don’t see the results. Because of all the other shifts, we can pay for results. Some call this value-based care. I call it fee-for-health.”
She noted that the future of medicine is underpinned by innovations in AI/predictalytics, voice recognition, virtual reality, blockchain, IoT sensors, 3D printing, omics, robotics, autonomous transport, neurotechnology, nanobiology, and cellular therapy. “They’re moving at a very fast pace because they don’t need the kind of cost, capital, and expertise that the previous tools did,” she said. “This is the promise that technology can bring.”
Dr. Khayat disclosed that she has been a workshop participant for Roche Canada.
Contact lenses that detect glucose in tears. Capsules embedded in clothes that can be used to counteract the risk of sensitive skin conditions.
These are just .
At the annual meeting of the Society for Pediatric Dermatology, Zayna Khayat, PhD, said that the future of medicine is driven by six shifts pulling society from a past oriented around the health care system – the buildings, clinicians, and payers – to a patient-oriented perspective. “That doesn’t just happen on its own,” said Dr. Khayat, a future strategist at Toronto-based SE Health. “There are big forces that are pulling us to the future whether we want it to or not. One is that patients have woken up. They have grown to have power in many other complex decisions in their life, and they’re expecting no less from our health care system.”
During her presentation, she discussed the six shifts:
1. The timing of service placement. The traditional model of medicine is “an intermittent and interventional science that waits for the symptoms and goes in and either fixes or manages them,” she said. “So, it’s not really health care; it’s sick care. That’s been fine in the industrial era when we needed to get medicine to stop catastrophic events. Not only is it shifting to be proactive and preventative but it’s shifting to a new science of medicine called predictive medicine.”
As for proactive and preventative care, she continued, each patient’s choice of behaviors related to diet, exercise, and stress “mingles with DNA to produce health, yet we spend about 90% of our resources on sick care. Now, health systems are moving their resources to things like education, housing, transportation, food security, equity, and racial divides. ... This is trickling down to how we train health care professionals. We know that patients live very little of their time in formal care settings, so all of their health is created – or destroyed – well outside of the clinical setting. We train our health professionals mostly in a clinical setting. Health systems are now starting to reimagine how training happens so we can train people to understand the fully loaded context of their patients’ lives.”
2. A shift in precision. For all its advances and science breakthroughs, medicine “is still quite crude,” said Dr. Khayat, who is also an adjunct professor in the Rotman School of Management at the University of Toronto. “It’s very analog, based on a one-size-fits-all approach. In the business world, we call this a segment of one: the idea that in some clinical trial, a result was produced that was based on the average of everybody, and therefore we just give everybody what worked for the average. ... We don’t need to have that trade-off anymore, because it won’t be a trade-off of higher cost to tailor down to an N of 1. It will be highly personalized, intelligent medicine, very precise.”
3. A shift from institution-centered to person-centered care. “The artifacts that health care was built on are very analog and are going to get decentralized out of buildings, dephysicalized, disintermediated,” she predicted. “We’ll have a seamless digital physical experience, expanded channels through which patients can access their services. Pick a channel that makes sense for the patient and don’t let care follow the place but rather let care follow the person.”
4. A shift in care duration, from episodic and intermittent care to more continuous care. “With very little input you should know what’s going on at any point in time instead of time-sharing access to diagnostics and to clinicians,” Dr. Khayat said. Wrist-worn devices that gather personal omics “are now really democratized, with every aspect of a diagnostic clinic available within or connected to a smartphone. This allows for data to be gathered and shared with clinicians, including tools under the skin that can get some of the biochemical data in real time instead of poking and prodding and waiting for a diagnostic lab.” These devices, she said, will become easier to use, cheaper, and will work faster, and provide much better data “at almost zero cost.”
Technologies being developed include tattoos that can read biomarkers, innovations in clothing that can detect biochemical reactions in the skin, underwear that can read vital signs, and contact lenses that can measure glucose levels. “The skin will become a major noninvasive way to obtain information,” she said.
5. A shift in power from providers to patients. “It’s estimated that about 80% of health care decisions could be self-managed by people in their communities,” Dr. Khayat said.
6. A shift from volume-based to value-based care. “Because we’ve been obsessed with the system, we’ve paid for stuff like visits, pills, MRI scans, et cetera,” she said. “We don’t need to do that anymore. Health systems don’t want to keep paying for stuff if they don’t see the results. Because of all the other shifts, we can pay for results. Some call this value-based care. I call it fee-for-health.”
She noted that the future of medicine is underpinned by innovations in AI/predictalytics, voice recognition, virtual reality, blockchain, IoT sensors, 3D printing, omics, robotics, autonomous transport, neurotechnology, nanobiology, and cellular therapy. “They’re moving at a very fast pace because they don’t need the kind of cost, capital, and expertise that the previous tools did,” she said. “This is the promise that technology can bring.”
Dr. Khayat disclosed that she has been a workshop participant for Roche Canada.
FROM SPD 2021
Targeted therapies for vascular anomalies continue to be refined
“The medicines we had were believed to be antiangiogenic and they were used not only for tumors but for all sorts of malformations,” Dr. Adams, a pediatric hematologist-oncologist at Children’s Hospital of Philadelphia, recalled during the annual meeting of the Society for Pediatric Dermatology. “I didn’t understand how so many different phenotypes could respond to the same medicine. Not all of them did, but some did have some response.”
She also grew frustrated by the lack of clinical trials and collaborative research groups involving patients with vascular anomalies. “I called this the chicken soup of medical management,” she said. “As we got more involved in vascular anomalies, the power of one patient or that power of a few patients led us in a direction for improved medical management. Or knowledge was gained by one patient who failed all noted medical management and led us into a direction repurposing a drug that actually wound up working.”
Propranolol, for example, became a key medicine for the treatment of vascular anomalies when it was found to improve hemangiomas in children who were given the drug for other reasons. “From this observation a key prospective study was performed and this beta-blocker became FDA approved for the treatment of complicated hemangiomas,” said Dr. Adams, who directs the hospital’s Comprehensive Vascular Anomalies Program. “That was how a bedside observation let to bench intervention, and how presently we are investigating bench interventions related to the mechanism of propranolol therapy.”
Then there is the story of the mammalian target of rapamycin (mTOR) inhibitor sirolimus. In her previous role as medical director of the Hemangioma and Vascular Malformation Center at Cincinnati Children’s Hospital, Dr. Adams and colleagues cared for an infant who presented with a Kaposiform hemangioendothelioma (KHE). “At that time, she was given our standard of practice for the treatment, but our standard of practice was not good enough,” she said.
While other options were being discussed for this patient, “we had been doing some collaborative work with pathology and nephrology on the PIKC3A pathway, because we knew that germline mutations of TEK were involved in this pathway, and we knew that 50% of patients with PTEN mutations had vascular anomalies. So, we hypothesized that this pathway was involved in vascular anomalies.”
They also had earlier success using mTOR inhibition for tuberous sclerosis patients with angiomyolipomas and patients with neurofibromatosis. “We needed a medicine that could be given orally because we did not think this patient was going to do well, so we started her on sirolimus,” Dr. Adams said. “She had a great response. This was followed by a phase 2 study, which proved efficacy and led to discovery of biomarkers.” This is where the angiopoietin-2 story started, she said, noting that this biomarker is now used “to differentiate KLA [Kaposiform lymphangiomatosis] from KHE and KLAs and KHE from other disorders.”
This bedside work helped researchers to better understand the mechanism of action in other disorders, such as observing somatic mutations in PIK3CA in patients with CLOVES syndrome. “This meant that we could now correlate the phenotype to the genotype, and it opened up targeted therapy with developmental therapeutics that were already in use for oncology,” Dr. Adams said. “We know we had mTOR inhibition with sirolimus and everolimus. We now have an AKT inhibitor, a PIK3CA inhibitor, and we now have another side of the pathway which deals with RASopathies, and some other medicines that we can use.”
Miransertib, a potent PAN-AKT inhibitor initially used for breast cancer, is currently being evaluated in open-label, phase 1 and 2 trials in patients with PIK3CA-related overgrowth spectrum (PROS) and Proteus syndrome. The dose used in a pilot study is about one-sixth of the dose used for oncology patients, Dr. Adams said.
She and her colleagues used miransertib to treat a 3-year-old with CLOVES syndrome who had lipomatous infiltration of the abdomen and retroperitoneum with failure to thrive. “He was not eating and was G-tube dependent,” she recalled. “After a month of therapy, he started eating and had improvement in his quality of life,” although despite this improvement volumetric MRI remained unchanged.
Advances in bench to bedside approaches are also under way. Hakon Hakonarson, MD, PhD, the founding director of the Center for Applied Genomics at CHOP, has discovered several genes with in vitro testing and zebra fish modeling, which has been followed by testing medicines on patients.
One such patient, according to Dr. Adams, had a severe central conducting lymphatic anomaly, with a pericardial effusion and significant dysfunction of the central conducting system. The patient was found to have an ARAF mutation, which induces ERK activation. “ERK is downstream of MEK, so the question was whether a MEK inhibitor, trametinib, could be used to treat this patient,” she said. “Trametinib was first used in tissue culture, then used in a zebra fish model and it showed some positive results. Then it was taken to the patient, who had improvement of pulmonary function, remodeling of the lymphatic system, and decrease in the size of his legs.”
Other antiangiogenic agents being used for the treatment of vascular anomalies include bevacizumab, which is being used in hereditary hemorrhagic telangiectasia, and thalidomide for HHT and arteriovenous malformations. For more information, Dr. Adams recommended a comprehensive review of vascular anomalies, related genes, and treatments that was published in Circulation Research.
The goal of future drug therapies is to support normal growth, “so we don’t need a maximum tolerated dose,” Dr. Adams said. “We need to be very careful of short-term and long-term side effects.”
Going forward, she said that she would like to see more natural history studies of vascular anomalies, improved outcome measures for clinical trials, adaptive study design, preclinical testing, animal model studies, universal availability of genomic testing, improvement of NIH funding, research collaboration nationally and internationally, and industry support.
Dr. Adams disclosed that she is a consultant to Venthera and Novartis.
“The medicines we had were believed to be antiangiogenic and they were used not only for tumors but for all sorts of malformations,” Dr. Adams, a pediatric hematologist-oncologist at Children’s Hospital of Philadelphia, recalled during the annual meeting of the Society for Pediatric Dermatology. “I didn’t understand how so many different phenotypes could respond to the same medicine. Not all of them did, but some did have some response.”
She also grew frustrated by the lack of clinical trials and collaborative research groups involving patients with vascular anomalies. “I called this the chicken soup of medical management,” she said. “As we got more involved in vascular anomalies, the power of one patient or that power of a few patients led us in a direction for improved medical management. Or knowledge was gained by one patient who failed all noted medical management and led us into a direction repurposing a drug that actually wound up working.”
Propranolol, for example, became a key medicine for the treatment of vascular anomalies when it was found to improve hemangiomas in children who were given the drug for other reasons. “From this observation a key prospective study was performed and this beta-blocker became FDA approved for the treatment of complicated hemangiomas,” said Dr. Adams, who directs the hospital’s Comprehensive Vascular Anomalies Program. “That was how a bedside observation let to bench intervention, and how presently we are investigating bench interventions related to the mechanism of propranolol therapy.”
Then there is the story of the mammalian target of rapamycin (mTOR) inhibitor sirolimus. In her previous role as medical director of the Hemangioma and Vascular Malformation Center at Cincinnati Children’s Hospital, Dr. Adams and colleagues cared for an infant who presented with a Kaposiform hemangioendothelioma (KHE). “At that time, she was given our standard of practice for the treatment, but our standard of practice was not good enough,” she said.
While other options were being discussed for this patient, “we had been doing some collaborative work with pathology and nephrology on the PIKC3A pathway, because we knew that germline mutations of TEK were involved in this pathway, and we knew that 50% of patients with PTEN mutations had vascular anomalies. So, we hypothesized that this pathway was involved in vascular anomalies.”
They also had earlier success using mTOR inhibition for tuberous sclerosis patients with angiomyolipomas and patients with neurofibromatosis. “We needed a medicine that could be given orally because we did not think this patient was going to do well, so we started her on sirolimus,” Dr. Adams said. “She had a great response. This was followed by a phase 2 study, which proved efficacy and led to discovery of biomarkers.” This is where the angiopoietin-2 story started, she said, noting that this biomarker is now used “to differentiate KLA [Kaposiform lymphangiomatosis] from KHE and KLAs and KHE from other disorders.”
This bedside work helped researchers to better understand the mechanism of action in other disorders, such as observing somatic mutations in PIK3CA in patients with CLOVES syndrome. “This meant that we could now correlate the phenotype to the genotype, and it opened up targeted therapy with developmental therapeutics that were already in use for oncology,” Dr. Adams said. “We know we had mTOR inhibition with sirolimus and everolimus. We now have an AKT inhibitor, a PIK3CA inhibitor, and we now have another side of the pathway which deals with RASopathies, and some other medicines that we can use.”
Miransertib, a potent PAN-AKT inhibitor initially used for breast cancer, is currently being evaluated in open-label, phase 1 and 2 trials in patients with PIK3CA-related overgrowth spectrum (PROS) and Proteus syndrome. The dose used in a pilot study is about one-sixth of the dose used for oncology patients, Dr. Adams said.
She and her colleagues used miransertib to treat a 3-year-old with CLOVES syndrome who had lipomatous infiltration of the abdomen and retroperitoneum with failure to thrive. “He was not eating and was G-tube dependent,” she recalled. “After a month of therapy, he started eating and had improvement in his quality of life,” although despite this improvement volumetric MRI remained unchanged.
Advances in bench to bedside approaches are also under way. Hakon Hakonarson, MD, PhD, the founding director of the Center for Applied Genomics at CHOP, has discovered several genes with in vitro testing and zebra fish modeling, which has been followed by testing medicines on patients.
One such patient, according to Dr. Adams, had a severe central conducting lymphatic anomaly, with a pericardial effusion and significant dysfunction of the central conducting system. The patient was found to have an ARAF mutation, which induces ERK activation. “ERK is downstream of MEK, so the question was whether a MEK inhibitor, trametinib, could be used to treat this patient,” she said. “Trametinib was first used in tissue culture, then used in a zebra fish model and it showed some positive results. Then it was taken to the patient, who had improvement of pulmonary function, remodeling of the lymphatic system, and decrease in the size of his legs.”
Other antiangiogenic agents being used for the treatment of vascular anomalies include bevacizumab, which is being used in hereditary hemorrhagic telangiectasia, and thalidomide for HHT and arteriovenous malformations. For more information, Dr. Adams recommended a comprehensive review of vascular anomalies, related genes, and treatments that was published in Circulation Research.
The goal of future drug therapies is to support normal growth, “so we don’t need a maximum tolerated dose,” Dr. Adams said. “We need to be very careful of short-term and long-term side effects.”
Going forward, she said that she would like to see more natural history studies of vascular anomalies, improved outcome measures for clinical trials, adaptive study design, preclinical testing, animal model studies, universal availability of genomic testing, improvement of NIH funding, research collaboration nationally and internationally, and industry support.
Dr. Adams disclosed that she is a consultant to Venthera and Novartis.
“The medicines we had were believed to be antiangiogenic and they were used not only for tumors but for all sorts of malformations,” Dr. Adams, a pediatric hematologist-oncologist at Children’s Hospital of Philadelphia, recalled during the annual meeting of the Society for Pediatric Dermatology. “I didn’t understand how so many different phenotypes could respond to the same medicine. Not all of them did, but some did have some response.”
She also grew frustrated by the lack of clinical trials and collaborative research groups involving patients with vascular anomalies. “I called this the chicken soup of medical management,” she said. “As we got more involved in vascular anomalies, the power of one patient or that power of a few patients led us in a direction for improved medical management. Or knowledge was gained by one patient who failed all noted medical management and led us into a direction repurposing a drug that actually wound up working.”
Propranolol, for example, became a key medicine for the treatment of vascular anomalies when it was found to improve hemangiomas in children who were given the drug for other reasons. “From this observation a key prospective study was performed and this beta-blocker became FDA approved for the treatment of complicated hemangiomas,” said Dr. Adams, who directs the hospital’s Comprehensive Vascular Anomalies Program. “That was how a bedside observation let to bench intervention, and how presently we are investigating bench interventions related to the mechanism of propranolol therapy.”
Then there is the story of the mammalian target of rapamycin (mTOR) inhibitor sirolimus. In her previous role as medical director of the Hemangioma and Vascular Malformation Center at Cincinnati Children’s Hospital, Dr. Adams and colleagues cared for an infant who presented with a Kaposiform hemangioendothelioma (KHE). “At that time, she was given our standard of practice for the treatment, but our standard of practice was not good enough,” she said.
While other options were being discussed for this patient, “we had been doing some collaborative work with pathology and nephrology on the PIKC3A pathway, because we knew that germline mutations of TEK were involved in this pathway, and we knew that 50% of patients with PTEN mutations had vascular anomalies. So, we hypothesized that this pathway was involved in vascular anomalies.”
They also had earlier success using mTOR inhibition for tuberous sclerosis patients with angiomyolipomas and patients with neurofibromatosis. “We needed a medicine that could be given orally because we did not think this patient was going to do well, so we started her on sirolimus,” Dr. Adams said. “She had a great response. This was followed by a phase 2 study, which proved efficacy and led to discovery of biomarkers.” This is where the angiopoietin-2 story started, she said, noting that this biomarker is now used “to differentiate KLA [Kaposiform lymphangiomatosis] from KHE and KLAs and KHE from other disorders.”
This bedside work helped researchers to better understand the mechanism of action in other disorders, such as observing somatic mutations in PIK3CA in patients with CLOVES syndrome. “This meant that we could now correlate the phenotype to the genotype, and it opened up targeted therapy with developmental therapeutics that were already in use for oncology,” Dr. Adams said. “We know we had mTOR inhibition with sirolimus and everolimus. We now have an AKT inhibitor, a PIK3CA inhibitor, and we now have another side of the pathway which deals with RASopathies, and some other medicines that we can use.”
Miransertib, a potent PAN-AKT inhibitor initially used for breast cancer, is currently being evaluated in open-label, phase 1 and 2 trials in patients with PIK3CA-related overgrowth spectrum (PROS) and Proteus syndrome. The dose used in a pilot study is about one-sixth of the dose used for oncology patients, Dr. Adams said.
She and her colleagues used miransertib to treat a 3-year-old with CLOVES syndrome who had lipomatous infiltration of the abdomen and retroperitoneum with failure to thrive. “He was not eating and was G-tube dependent,” she recalled. “After a month of therapy, he started eating and had improvement in his quality of life,” although despite this improvement volumetric MRI remained unchanged.
Advances in bench to bedside approaches are also under way. Hakon Hakonarson, MD, PhD, the founding director of the Center for Applied Genomics at CHOP, has discovered several genes with in vitro testing and zebra fish modeling, which has been followed by testing medicines on patients.
One such patient, according to Dr. Adams, had a severe central conducting lymphatic anomaly, with a pericardial effusion and significant dysfunction of the central conducting system. The patient was found to have an ARAF mutation, which induces ERK activation. “ERK is downstream of MEK, so the question was whether a MEK inhibitor, trametinib, could be used to treat this patient,” she said. “Trametinib was first used in tissue culture, then used in a zebra fish model and it showed some positive results. Then it was taken to the patient, who had improvement of pulmonary function, remodeling of the lymphatic system, and decrease in the size of his legs.”
Other antiangiogenic agents being used for the treatment of vascular anomalies include bevacizumab, which is being used in hereditary hemorrhagic telangiectasia, and thalidomide for HHT and arteriovenous malformations. For more information, Dr. Adams recommended a comprehensive review of vascular anomalies, related genes, and treatments that was published in Circulation Research.
The goal of future drug therapies is to support normal growth, “so we don’t need a maximum tolerated dose,” Dr. Adams said. “We need to be very careful of short-term and long-term side effects.”
Going forward, she said that she would like to see more natural history studies of vascular anomalies, improved outcome measures for clinical trials, adaptive study design, preclinical testing, animal model studies, universal availability of genomic testing, improvement of NIH funding, research collaboration nationally and internationally, and industry support.
Dr. Adams disclosed that she is a consultant to Venthera and Novartis.
FROM SPD 2021
Changing minds: What moves the needle for the unvaccinated?
Not so long ago, Heather Simpson of Dallas was known as the anti-vaccine mom who dressed as “the measles” for Halloween. She painted red spots on her face and posted her photo on Facebook, joking: “Was trying to think of the least scary thing I could be for Halloween … so I became the measles.” It went viral with the anti-vaccine crowd.
But between that Halloween and today, a series of “aha” moments transformed Ms. Simpson’s attitudes toward vaccines.
In January 2021, one of those moments involved her daughter, now 4, who was scratched by a feral cat, raising concerns about tetanus. Her daughter had been bitten by a dog when she was just 1, and Ms. Simpson turned down advice then to get a tetanus shot. “I was convinced the tetanus shot would kill her faster than the tetanus.”
After the cat incident, the anxiety was so exhausting, she listened to the nurse practitioner at the clinic, whom she trusted. The nurse gently reassured Ms. Simpson that the shot was less risky than the possibility of tetanus – but did not bombard her with statistics – and that won over Ms. Simpson and triggered an overall rethinking of her vaccine stance.
Fast-forward to February, and that “aha” turned into action when Ms. Simpson launched a “Back to the Vax” effort with a fellow former vaccine opponent. Through their website, Facebook page, and podcasts, they now encourage people to get the COVID-19 vaccine, as well as other immunizations.
Challenge: Reaching the rest
With just over 52% of those eligible in the United States fully vaccinated as of Sept. 1,
Recent data and a poll show some movement in the right direction, as immunizations are increasing and hesitancy is declining among certain groups. According to federal officials, about 14 million people in the United States got their first dose in August, an increase of 4 million, compared to the numbers who got it in July.
And a new poll from the Axios-IPSOS Coronavirus Index found only one in five Americans, or 20%, say they are not likely to get the vaccine, while “hard opposition,” those not at all likely, has dropped to 14% of those adults.
But there is still a lot of work to do. So, how do medical professionals or concerned citizens reach those who haven’t gotten vaccinated yet, whatever their reason?
Many experts in communication and persuasion that this news organization talked to agree that throwing statistics at people hesitant to get the COVID-19 vaccine is generally useless and often backfires.
So what does work, according to these experts?
- Emphasizing the trends of more people getting vaccinated.
- Focusing on everyone’s freedom of choice.
- Listening to concerns without judgment.
- Offering credible information.
- Correcting myths when necessary.
- Helping them fit vaccination into their “world view.”
Stories over statistics
Talking about the trends of vaccinations can definitely change minds about getting vaccinated, said Robert Cialdini, PhD, regents professor emeritus of psychology and marketing at Arizona State University, Tempe, and author of the recently updated book, “Influence: The Psychology of Persuasion,” which has sold over 5 million copies since it was first published in 1984.
Face-to-face with a hesitant patient, a doctor can say: “More and more people are being vaccinated every day,” Dr. Cialdini says. “The reason you say more and more is [that] it conveys a trend. When people see a trend, they project it into the future that it is going to get even larger.”
A focus on choice can also help people change their minds and accept the vaccine, he says. “A lot of conspiracy theorists claim they don’t want to do it because they are being pushed or forced by the government, and they are resisting that.”
If that’s the case, presenting people with new information, such as the increased infectiousness of the Delta variant, and suggesting that a decision be made based on the new information, can work, Dr. Cialdini says, but be sure to end with: “It’s completely up to you.”
“This removes all their sense of being pushed. It says, ‘Here is all the evidence.’ ” At this point, a doctor’s personal recommendation with a patient who trusts him or her may sway them, Dr. Cialdini said. “I think you have to personalize the communication in both directions. That is, to say, ‘For someone in your situation, I would personally recommend that you get the vaccine.’ ” A health care professional’s authority and expertise can carry the day, he says, although “not always.”
This approach worked, Dr. Cialdini says, with a friend of the family hesitant about the COVID-19 vaccine. “I told him: ‘We have gotten it. You trust us, right?’ ” He waited for the person to say yes.
Then: “For someone in your position, my personal recommendation is to get vaccinated. There is new information about the vaccine, and more and more people are getting vaccinated. And of course, it is completely up to you.”
The person decided to get the vaccine.
‘Live in that space’
“People develop negative attitudes [about vaccines] by accessing alternative sources of information, anecdotes, and personal stories,” said Matthew Seeger, PhD, dean of the College of Fine, Performing, and Communication Arts and codirector of the Center for Emerging Infectious Diseases at Wayne State University in Detroit.
“If we are going to change their opinion, we need to live in that space.” That means listening first, he says. Ask: “Where did you get that information? How credible do you think the sources are? What do you mean about the vaccine changing DNA?”
Then, you might respond, he said, by addressing that specific information, such as, “We have no cases of DNA being changed.”
Dr. Seeger recalls that his mother would simply talk louder when she couldn’t understand someone who wasn’t a native English speaker. “That’s what we are trying to do with the vaccine-hesitant,” he says. “In some cases, we are yelling at them.” Instead, he says, probe their sources of information.
For some who are vaccine-hesitant, Dr. Seeger said, it is not just about the vaccine. The attitude about vaccines is tied in, often, with a distrust of government and feelings about personal freedom. “That’s one reason it’s so hard to change the attitude.” For some, getting the vaccine in a family against the vaccine might also disrupt their social structure or even get them ostracized.
For these people, a health care provider might give opportunities to get the vaccine without affecting either what they see as their political stance or upsetting family harmony. “There are places you can go, make an appointment, get a vaccine, and nobody knows,” Dr. Seeger said.
One Missouri doctor told CNN that some people calling for a vaccine appointment do request privacy, such as going through a drive-thru or having the shot as they sit in their cars. She said the hospital tries to accommodate them, reasoning that every additional vaccine shot is a win.
Dr. Seeger agrees. “Of course there are still public records,” he says, “but you can still claim you are a vaccine denier. It’s very difficult to persuade people to give up their whole world. Vaccine denial is part of that world. At this point, we need to do whatever we can to get people vaccinated.”
From peer to peer
A theme that runs through many of these persuasion techniques is peer pressure.
One example, while a bit more profane and confrontational than some groups, is COVIDAteMyFace, a subgroup, or “subreddit,” of the popular online site Reddit, which hosts numerous forums inviting users to share news and comments on a variety of topics. The subreddit has over 20,000 members. Its purpose, says the sub’s creator, “was to document the folks who denied COVID, then got bitten in the ass by it.” Reports are of actual cases.
“It’s interesting and powerful that Reddit users are taking this on,” Dr. Seeger said. And this kind of peer pressure, or peer-to-peer information, can be persuasive, he says. “We often seek consensual validation from peers about risk messages and risk behaviors.”
For instance, hurricane evacuation notices are more effective, he said, when people learn their neighbors are leaving.
Peer information – “the number of others who are doing or believing or responding to something – definitely persuades people,” agreed Dr. Cialdini. “When a lot of others are responding in a particular way – for example, getting vaccinated – people follow for three reasons: The action seems more appropriate or correct, it appears more feasible to perform, and it avoids social disapproval from those others.”
Let them talk, give them time
Gladys Jimenez is a contact tracer and “vaccine ambassador” for Tracing Health, a partnership between the Oregon Public Health Institute and the Public Health Institute that has nearly 300 bilingual contract tracers who serve the ethnic communities they’re from. During a typical week, she talks to 50 people or more, and promoting the vaccine is top of mind.
The conversations, Ms. Jimenez said, are like a dance. She presents information, then steps back and lets them talk. “I want to hear the person talk, where they are coming from, where they are at.” Depending on what they say, she gives them more information or corrects their misinformation. “They often will say, ‘Oh, I didn’t know that.’ ”
It’s rarely one conversation that convinces hesitant people, she said. “I’m planting this seed in their brain. ... people want someone to listen to them ... they want to vent.”
Once you let them do that, Ms. Jimenez said, “I can tell the person is in a different state of mind.” She also knows that people “will make the decision in their own time.”
With time, people can change their minds, as a Southern California woman who resisted at first (and asked to remain anonymous) can attest. “When the vaccine first came out, I remember thinking [that] it was a quick fix to a very big problem,” she said. The lack of full FDA approval, which has since been granted, was also an issue. She doesn’t oppose vaccines, she said, but was leery just of the COVID-19 vaccine.
When her longtime partner got his vaccine, he urged her to go right away for hers. She stalled. He got his second dose and grew impatient with her hesitancy. It began to wear on the relationship. Finally, the woman talked to two health care professionals she knew socially. They both follow the science, and “they both could explain vaccination to me in a way that resonated. The information was coming from sources I already trusted.”
Those conversations are what convinced her to get vaccinated this summer.
Simpson’s transformation
Ms. Simpson of Back to the Vax got her first COVID-19 immunization April 16. She had an allergic reaction, including severe itchiness and a bad headache, and needed emergency care, she said. Even so, she scheduled her second shot appointment.
Like many who turned against vaccines as adults, Ms. Simpson had all her childhood vaccines, but she developed a distrust after watching a lengthy documentary series that warned of vaccine dangers as an adult.
Looking back at that documentary, she thought about how it seems to blame everything – childhood cancer, ADHD, autism, allergies – on vaccinations. That suddenly seemed like sketchy science to her.
So did the claim from a family friend who said she knew someone who got the flu shot and began walking backward. She researched on her own, and with time, she decided to be pro-vaccines.
These days, she continues to find that stories, not statistics, are changing the minds of many who decide to get vaccinated. If the nurse practitioner urging the tetanus shot for her daughter had told her that the tetanus shot is linked with problems in one of a specific number of people who get it, no matter how large that second number was, Ms. Simpson said she would have thought: “What if she is that one?”
So she relies on stories that point out how universally vulnerable people are to COVID-19 first, facts next.
“Facts help once you are already moved,” Ms. Simpson said.
A version of this article first appeared on WebMD.com.
Not so long ago, Heather Simpson of Dallas was known as the anti-vaccine mom who dressed as “the measles” for Halloween. She painted red spots on her face and posted her photo on Facebook, joking: “Was trying to think of the least scary thing I could be for Halloween … so I became the measles.” It went viral with the anti-vaccine crowd.
But between that Halloween and today, a series of “aha” moments transformed Ms. Simpson’s attitudes toward vaccines.
In January 2021, one of those moments involved her daughter, now 4, who was scratched by a feral cat, raising concerns about tetanus. Her daughter had been bitten by a dog when she was just 1, and Ms. Simpson turned down advice then to get a tetanus shot. “I was convinced the tetanus shot would kill her faster than the tetanus.”
After the cat incident, the anxiety was so exhausting, she listened to the nurse practitioner at the clinic, whom she trusted. The nurse gently reassured Ms. Simpson that the shot was less risky than the possibility of tetanus – but did not bombard her with statistics – and that won over Ms. Simpson and triggered an overall rethinking of her vaccine stance.
Fast-forward to February, and that “aha” turned into action when Ms. Simpson launched a “Back to the Vax” effort with a fellow former vaccine opponent. Through their website, Facebook page, and podcasts, they now encourage people to get the COVID-19 vaccine, as well as other immunizations.
Challenge: Reaching the rest
With just over 52% of those eligible in the United States fully vaccinated as of Sept. 1,
Recent data and a poll show some movement in the right direction, as immunizations are increasing and hesitancy is declining among certain groups. According to federal officials, about 14 million people in the United States got their first dose in August, an increase of 4 million, compared to the numbers who got it in July.
And a new poll from the Axios-IPSOS Coronavirus Index found only one in five Americans, or 20%, say they are not likely to get the vaccine, while “hard opposition,” those not at all likely, has dropped to 14% of those adults.
But there is still a lot of work to do. So, how do medical professionals or concerned citizens reach those who haven’t gotten vaccinated yet, whatever their reason?
Many experts in communication and persuasion that this news organization talked to agree that throwing statistics at people hesitant to get the COVID-19 vaccine is generally useless and often backfires.
So what does work, according to these experts?
- Emphasizing the trends of more people getting vaccinated.
- Focusing on everyone’s freedom of choice.
- Listening to concerns without judgment.
- Offering credible information.
- Correcting myths when necessary.
- Helping them fit vaccination into their “world view.”
Stories over statistics
Talking about the trends of vaccinations can definitely change minds about getting vaccinated, said Robert Cialdini, PhD, regents professor emeritus of psychology and marketing at Arizona State University, Tempe, and author of the recently updated book, “Influence: The Psychology of Persuasion,” which has sold over 5 million copies since it was first published in 1984.
Face-to-face with a hesitant patient, a doctor can say: “More and more people are being vaccinated every day,” Dr. Cialdini says. “The reason you say more and more is [that] it conveys a trend. When people see a trend, they project it into the future that it is going to get even larger.”
A focus on choice can also help people change their minds and accept the vaccine, he says. “A lot of conspiracy theorists claim they don’t want to do it because they are being pushed or forced by the government, and they are resisting that.”
If that’s the case, presenting people with new information, such as the increased infectiousness of the Delta variant, and suggesting that a decision be made based on the new information, can work, Dr. Cialdini says, but be sure to end with: “It’s completely up to you.”
“This removes all their sense of being pushed. It says, ‘Here is all the evidence.’ ” At this point, a doctor’s personal recommendation with a patient who trusts him or her may sway them, Dr. Cialdini said. “I think you have to personalize the communication in both directions. That is, to say, ‘For someone in your situation, I would personally recommend that you get the vaccine.’ ” A health care professional’s authority and expertise can carry the day, he says, although “not always.”
This approach worked, Dr. Cialdini says, with a friend of the family hesitant about the COVID-19 vaccine. “I told him: ‘We have gotten it. You trust us, right?’ ” He waited for the person to say yes.
Then: “For someone in your position, my personal recommendation is to get vaccinated. There is new information about the vaccine, and more and more people are getting vaccinated. And of course, it is completely up to you.”
The person decided to get the vaccine.
‘Live in that space’
“People develop negative attitudes [about vaccines] by accessing alternative sources of information, anecdotes, and personal stories,” said Matthew Seeger, PhD, dean of the College of Fine, Performing, and Communication Arts and codirector of the Center for Emerging Infectious Diseases at Wayne State University in Detroit.
“If we are going to change their opinion, we need to live in that space.” That means listening first, he says. Ask: “Where did you get that information? How credible do you think the sources are? What do you mean about the vaccine changing DNA?”
Then, you might respond, he said, by addressing that specific information, such as, “We have no cases of DNA being changed.”
Dr. Seeger recalls that his mother would simply talk louder when she couldn’t understand someone who wasn’t a native English speaker. “That’s what we are trying to do with the vaccine-hesitant,” he says. “In some cases, we are yelling at them.” Instead, he says, probe their sources of information.
For some who are vaccine-hesitant, Dr. Seeger said, it is not just about the vaccine. The attitude about vaccines is tied in, often, with a distrust of government and feelings about personal freedom. “That’s one reason it’s so hard to change the attitude.” For some, getting the vaccine in a family against the vaccine might also disrupt their social structure or even get them ostracized.
For these people, a health care provider might give opportunities to get the vaccine without affecting either what they see as their political stance or upsetting family harmony. “There are places you can go, make an appointment, get a vaccine, and nobody knows,” Dr. Seeger said.
One Missouri doctor told CNN that some people calling for a vaccine appointment do request privacy, such as going through a drive-thru or having the shot as they sit in their cars. She said the hospital tries to accommodate them, reasoning that every additional vaccine shot is a win.
Dr. Seeger agrees. “Of course there are still public records,” he says, “but you can still claim you are a vaccine denier. It’s very difficult to persuade people to give up their whole world. Vaccine denial is part of that world. At this point, we need to do whatever we can to get people vaccinated.”
From peer to peer
A theme that runs through many of these persuasion techniques is peer pressure.
One example, while a bit more profane and confrontational than some groups, is COVIDAteMyFace, a subgroup, or “subreddit,” of the popular online site Reddit, which hosts numerous forums inviting users to share news and comments on a variety of topics. The subreddit has over 20,000 members. Its purpose, says the sub’s creator, “was to document the folks who denied COVID, then got bitten in the ass by it.” Reports are of actual cases.
“It’s interesting and powerful that Reddit users are taking this on,” Dr. Seeger said. And this kind of peer pressure, or peer-to-peer information, can be persuasive, he says. “We often seek consensual validation from peers about risk messages and risk behaviors.”
For instance, hurricane evacuation notices are more effective, he said, when people learn their neighbors are leaving.
Peer information – “the number of others who are doing or believing or responding to something – definitely persuades people,” agreed Dr. Cialdini. “When a lot of others are responding in a particular way – for example, getting vaccinated – people follow for three reasons: The action seems more appropriate or correct, it appears more feasible to perform, and it avoids social disapproval from those others.”
Let them talk, give them time
Gladys Jimenez is a contact tracer and “vaccine ambassador” for Tracing Health, a partnership between the Oregon Public Health Institute and the Public Health Institute that has nearly 300 bilingual contract tracers who serve the ethnic communities they’re from. During a typical week, she talks to 50 people or more, and promoting the vaccine is top of mind.
The conversations, Ms. Jimenez said, are like a dance. She presents information, then steps back and lets them talk. “I want to hear the person talk, where they are coming from, where they are at.” Depending on what they say, she gives them more information or corrects their misinformation. “They often will say, ‘Oh, I didn’t know that.’ ”
It’s rarely one conversation that convinces hesitant people, she said. “I’m planting this seed in their brain. ... people want someone to listen to them ... they want to vent.”
Once you let them do that, Ms. Jimenez said, “I can tell the person is in a different state of mind.” She also knows that people “will make the decision in their own time.”
With time, people can change their minds, as a Southern California woman who resisted at first (and asked to remain anonymous) can attest. “When the vaccine first came out, I remember thinking [that] it was a quick fix to a very big problem,” she said. The lack of full FDA approval, which has since been granted, was also an issue. She doesn’t oppose vaccines, she said, but was leery just of the COVID-19 vaccine.
When her longtime partner got his vaccine, he urged her to go right away for hers. She stalled. He got his second dose and grew impatient with her hesitancy. It began to wear on the relationship. Finally, the woman talked to two health care professionals she knew socially. They both follow the science, and “they both could explain vaccination to me in a way that resonated. The information was coming from sources I already trusted.”
Those conversations are what convinced her to get vaccinated this summer.
Simpson’s transformation
Ms. Simpson of Back to the Vax got her first COVID-19 immunization April 16. She had an allergic reaction, including severe itchiness and a bad headache, and needed emergency care, she said. Even so, she scheduled her second shot appointment.
Like many who turned against vaccines as adults, Ms. Simpson had all her childhood vaccines, but she developed a distrust after watching a lengthy documentary series that warned of vaccine dangers as an adult.
Looking back at that documentary, she thought about how it seems to blame everything – childhood cancer, ADHD, autism, allergies – on vaccinations. That suddenly seemed like sketchy science to her.
So did the claim from a family friend who said she knew someone who got the flu shot and began walking backward. She researched on her own, and with time, she decided to be pro-vaccines.
These days, she continues to find that stories, not statistics, are changing the minds of many who decide to get vaccinated. If the nurse practitioner urging the tetanus shot for her daughter had told her that the tetanus shot is linked with problems in one of a specific number of people who get it, no matter how large that second number was, Ms. Simpson said she would have thought: “What if she is that one?”
So she relies on stories that point out how universally vulnerable people are to COVID-19 first, facts next.
“Facts help once you are already moved,” Ms. Simpson said.
A version of this article first appeared on WebMD.com.
Not so long ago, Heather Simpson of Dallas was known as the anti-vaccine mom who dressed as “the measles” for Halloween. She painted red spots on her face and posted her photo on Facebook, joking: “Was trying to think of the least scary thing I could be for Halloween … so I became the measles.” It went viral with the anti-vaccine crowd.
But between that Halloween and today, a series of “aha” moments transformed Ms. Simpson’s attitudes toward vaccines.
In January 2021, one of those moments involved her daughter, now 4, who was scratched by a feral cat, raising concerns about tetanus. Her daughter had been bitten by a dog when she was just 1, and Ms. Simpson turned down advice then to get a tetanus shot. “I was convinced the tetanus shot would kill her faster than the tetanus.”
After the cat incident, the anxiety was so exhausting, she listened to the nurse practitioner at the clinic, whom she trusted. The nurse gently reassured Ms. Simpson that the shot was less risky than the possibility of tetanus – but did not bombard her with statistics – and that won over Ms. Simpson and triggered an overall rethinking of her vaccine stance.
Fast-forward to February, and that “aha” turned into action when Ms. Simpson launched a “Back to the Vax” effort with a fellow former vaccine opponent. Through their website, Facebook page, and podcasts, they now encourage people to get the COVID-19 vaccine, as well as other immunizations.
Challenge: Reaching the rest
With just over 52% of those eligible in the United States fully vaccinated as of Sept. 1,
Recent data and a poll show some movement in the right direction, as immunizations are increasing and hesitancy is declining among certain groups. According to federal officials, about 14 million people in the United States got their first dose in August, an increase of 4 million, compared to the numbers who got it in July.
And a new poll from the Axios-IPSOS Coronavirus Index found only one in five Americans, or 20%, say they are not likely to get the vaccine, while “hard opposition,” those not at all likely, has dropped to 14% of those adults.
But there is still a lot of work to do. So, how do medical professionals or concerned citizens reach those who haven’t gotten vaccinated yet, whatever their reason?
Many experts in communication and persuasion that this news organization talked to agree that throwing statistics at people hesitant to get the COVID-19 vaccine is generally useless and often backfires.
So what does work, according to these experts?
- Emphasizing the trends of more people getting vaccinated.
- Focusing on everyone’s freedom of choice.
- Listening to concerns without judgment.
- Offering credible information.
- Correcting myths when necessary.
- Helping them fit vaccination into their “world view.”
Stories over statistics
Talking about the trends of vaccinations can definitely change minds about getting vaccinated, said Robert Cialdini, PhD, regents professor emeritus of psychology and marketing at Arizona State University, Tempe, and author of the recently updated book, “Influence: The Psychology of Persuasion,” which has sold over 5 million copies since it was first published in 1984.
Face-to-face with a hesitant patient, a doctor can say: “More and more people are being vaccinated every day,” Dr. Cialdini says. “The reason you say more and more is [that] it conveys a trend. When people see a trend, they project it into the future that it is going to get even larger.”
A focus on choice can also help people change their minds and accept the vaccine, he says. “A lot of conspiracy theorists claim they don’t want to do it because they are being pushed or forced by the government, and they are resisting that.”
If that’s the case, presenting people with new information, such as the increased infectiousness of the Delta variant, and suggesting that a decision be made based on the new information, can work, Dr. Cialdini says, but be sure to end with: “It’s completely up to you.”
“This removes all their sense of being pushed. It says, ‘Here is all the evidence.’ ” At this point, a doctor’s personal recommendation with a patient who trusts him or her may sway them, Dr. Cialdini said. “I think you have to personalize the communication in both directions. That is, to say, ‘For someone in your situation, I would personally recommend that you get the vaccine.’ ” A health care professional’s authority and expertise can carry the day, he says, although “not always.”
This approach worked, Dr. Cialdini says, with a friend of the family hesitant about the COVID-19 vaccine. “I told him: ‘We have gotten it. You trust us, right?’ ” He waited for the person to say yes.
Then: “For someone in your position, my personal recommendation is to get vaccinated. There is new information about the vaccine, and more and more people are getting vaccinated. And of course, it is completely up to you.”
The person decided to get the vaccine.
‘Live in that space’
“People develop negative attitudes [about vaccines] by accessing alternative sources of information, anecdotes, and personal stories,” said Matthew Seeger, PhD, dean of the College of Fine, Performing, and Communication Arts and codirector of the Center for Emerging Infectious Diseases at Wayne State University in Detroit.
“If we are going to change their opinion, we need to live in that space.” That means listening first, he says. Ask: “Where did you get that information? How credible do you think the sources are? What do you mean about the vaccine changing DNA?”
Then, you might respond, he said, by addressing that specific information, such as, “We have no cases of DNA being changed.”
Dr. Seeger recalls that his mother would simply talk louder when she couldn’t understand someone who wasn’t a native English speaker. “That’s what we are trying to do with the vaccine-hesitant,” he says. “In some cases, we are yelling at them.” Instead, he says, probe their sources of information.
For some who are vaccine-hesitant, Dr. Seeger said, it is not just about the vaccine. The attitude about vaccines is tied in, often, with a distrust of government and feelings about personal freedom. “That’s one reason it’s so hard to change the attitude.” For some, getting the vaccine in a family against the vaccine might also disrupt their social structure or even get them ostracized.
For these people, a health care provider might give opportunities to get the vaccine without affecting either what they see as their political stance or upsetting family harmony. “There are places you can go, make an appointment, get a vaccine, and nobody knows,” Dr. Seeger said.
One Missouri doctor told CNN that some people calling for a vaccine appointment do request privacy, such as going through a drive-thru or having the shot as they sit in their cars. She said the hospital tries to accommodate them, reasoning that every additional vaccine shot is a win.
Dr. Seeger agrees. “Of course there are still public records,” he says, “but you can still claim you are a vaccine denier. It’s very difficult to persuade people to give up their whole world. Vaccine denial is part of that world. At this point, we need to do whatever we can to get people vaccinated.”
From peer to peer
A theme that runs through many of these persuasion techniques is peer pressure.
One example, while a bit more profane and confrontational than some groups, is COVIDAteMyFace, a subgroup, or “subreddit,” of the popular online site Reddit, which hosts numerous forums inviting users to share news and comments on a variety of topics. The subreddit has over 20,000 members. Its purpose, says the sub’s creator, “was to document the folks who denied COVID, then got bitten in the ass by it.” Reports are of actual cases.
“It’s interesting and powerful that Reddit users are taking this on,” Dr. Seeger said. And this kind of peer pressure, or peer-to-peer information, can be persuasive, he says. “We often seek consensual validation from peers about risk messages and risk behaviors.”
For instance, hurricane evacuation notices are more effective, he said, when people learn their neighbors are leaving.
Peer information – “the number of others who are doing or believing or responding to something – definitely persuades people,” agreed Dr. Cialdini. “When a lot of others are responding in a particular way – for example, getting vaccinated – people follow for three reasons: The action seems more appropriate or correct, it appears more feasible to perform, and it avoids social disapproval from those others.”
Let them talk, give them time
Gladys Jimenez is a contact tracer and “vaccine ambassador” for Tracing Health, a partnership between the Oregon Public Health Institute and the Public Health Institute that has nearly 300 bilingual contract tracers who serve the ethnic communities they’re from. During a typical week, she talks to 50 people or more, and promoting the vaccine is top of mind.
The conversations, Ms. Jimenez said, are like a dance. She presents information, then steps back and lets them talk. “I want to hear the person talk, where they are coming from, where they are at.” Depending on what they say, she gives them more information or corrects their misinformation. “They often will say, ‘Oh, I didn’t know that.’ ”
It’s rarely one conversation that convinces hesitant people, she said. “I’m planting this seed in their brain. ... people want someone to listen to them ... they want to vent.”
Once you let them do that, Ms. Jimenez said, “I can tell the person is in a different state of mind.” She also knows that people “will make the decision in their own time.”
With time, people can change their minds, as a Southern California woman who resisted at first (and asked to remain anonymous) can attest. “When the vaccine first came out, I remember thinking [that] it was a quick fix to a very big problem,” she said. The lack of full FDA approval, which has since been granted, was also an issue. She doesn’t oppose vaccines, she said, but was leery just of the COVID-19 vaccine.
When her longtime partner got his vaccine, he urged her to go right away for hers. She stalled. He got his second dose and grew impatient with her hesitancy. It began to wear on the relationship. Finally, the woman talked to two health care professionals she knew socially. They both follow the science, and “they both could explain vaccination to me in a way that resonated. The information was coming from sources I already trusted.”
Those conversations are what convinced her to get vaccinated this summer.
Simpson’s transformation
Ms. Simpson of Back to the Vax got her first COVID-19 immunization April 16. She had an allergic reaction, including severe itchiness and a bad headache, and needed emergency care, she said. Even so, she scheduled her second shot appointment.
Like many who turned against vaccines as adults, Ms. Simpson had all her childhood vaccines, but she developed a distrust after watching a lengthy documentary series that warned of vaccine dangers as an adult.
Looking back at that documentary, she thought about how it seems to blame everything – childhood cancer, ADHD, autism, allergies – on vaccinations. That suddenly seemed like sketchy science to her.
So did the claim from a family friend who said she knew someone who got the flu shot and began walking backward. She researched on her own, and with time, she decided to be pro-vaccines.
These days, she continues to find that stories, not statistics, are changing the minds of many who decide to get vaccinated. If the nurse practitioner urging the tetanus shot for her daughter had told her that the tetanus shot is linked with problems in one of a specific number of people who get it, no matter how large that second number was, Ms. Simpson said she would have thought: “What if she is that one?”
So she relies on stories that point out how universally vulnerable people are to COVID-19 first, facts next.
“Facts help once you are already moved,” Ms. Simpson said.
A version of this article first appeared on WebMD.com.
Politics or protection? What’s behind the push for boosters?
That plan, which was first announced on Aug. 18, has raised eyebrows because it comes in advance of regulatory reviews by the Food and Drug Administration and recommendations from the Centers for Disease Control and Prevention. Those reviews are needed to determine whether third doses of these vaccines are effective or even safe. The move could have important legal ramifications for doctors and patients, too.
On Aug. 31, two high-level officials in the FDA’s Office of Vaccines Research and Review abruptly resigned amid reports that they were angry that the Biden administration was making decisions that should be left up to that agency.
So far, data show that the vaccines are highly effective at preventing the most severe consequences of COVID-19 – hospitalization and death – even regarding the Delta variant. The World Health Organization has urged wealthy nations such as the United States not to offer boosters so that the limited supply of vaccines can be directed to countries with fewer resources.
White House supports boosters
In a recent press briefing, Jeff Zients, the White House COVID-19 response coordinator, defended the move.
“You know, the booster decision, which you referenced ... was made by and announced by the nation’s leading public health officials, including Dr. Walensky; Dr. Fauci; Surgeon General Vivek Murthy; Dr. Janet Woodcock; the FDA acting commissioner, Dr. Francis Collins; Dr. Kessler; and others,” Mr. Zients said.
“And as our medical experts laid out, having reviewed all of the available data, it is in their clinical judgment that it is time to prepare Americans for a booster shot.”
He said a target date of Sept. 20 was announced so as to give states and practitioners time to prepare. He also said the move to give boosters was meant to help the United States stay ahead of a rapidly changing virus. Mr. Zients added that whether boosters will be administered starting on Sept. 20 depends on the FDA’s and CDC’s giving the go-ahead.
“Booster doses are going to be handled the same way all vaccines are handled,” said Kristen Nordlund, a CDC spokesperson. “Companies will have to provide data to FDA. FDA will have to make a decision and authorize the use of those, and ACIP [the Advisory Committee on Immunization Practices] will have to look at the evidence as well and make recommendations on top of FDA’s regulatory action,” she said.
Ms. Nordlund agreed that the planned Sept. 20 start date for boosters was something to which they aspired and was not necessarily set.
Historically, the FDA has needed at least 4 months to review a change to a vaccine’s approval, even on an accelerated schedule. Reviewers use that time to assess data regarding individual patients in a study, to review raw data, and essentially to check a drug company’s math and conclusions. The Biden administration’s timeline would shorten that review period from months to just a few weeks.
‘FDA in a very difficult position’
After the FDA approves, the ACIP of the CDC must meet to review the evidence and make recommendations on the use of the boosters in the United States.
Pfizer says it completed its submission for a supplemental biologics license application to the FDA on Aug. 27. To meet a Sept. 20 timeline, the entire process would have to be completed within 3 weeks.
“I don’t think that was handled, you know, ideally,” said Peter Lurie, MD, president of the Center for Science in the Public Interest and former associate commissioner of public health strategy and analysis at the FDA.
“It puts FDA in a very difficult position,” Dr. Lurie said. “It’s almost as if the decision has been made and they’re just checking a box, and that is, you know, contrary to the what FDA – at least the internal people at FDA – have been trying to do for ages.”
He said the agency took great pains with the emergency use authorizations and the full approvals of the vaccines to work as rapidly but thoroughly as possible. They did not skip steps.
“I think all of that reflected very well on the agency,” Dr. Lurie said. “And I think it worked out well in terms of trust in the vaccines.”
Although additional doses of vaccine are expected to be safe, little is known about side effects or adverse events after a third dose.
“It’s critical to wait for additional data and regulatory allowance for booster doses,” Sara Oliver, MD, a member of the CDC’s epidemic intelligence service, said in an Aug. 30 presentation to the ACIP, which is charged with making recommendations for use of all vaccines in the United States.
Boosters already being given
But after the White House announced that boosters were on the way, many people are not waiting.
Many health care practitioners and pharmacies have already been giving people third doses of vaccines, even if they are not among the immunocompromised – the group for which the shots are currently approved.
“You can walk into a pharmacy and ask for a third dose. Depending on which pharmacy you go to, you may get it,” said Helen Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., and a member of the ACIP.
She says she has a friend who recently went for a checkup and was offered a third dose. His physician is already giving extra doses to everyone who is older than 65.
Dr. Talbot said that in fairness, pharmacies in the United States are throwing away doses of vaccine because they are expiring before they get used.
“Many of us may or may not be ready to give a third dose but would rather give someone a third dose than throw a vaccine away,” she said.
Consequences of a third shot
But giving or getting a third dose before approval by the FDA may have legal consequences.
In the ACIP meeting on Aug. 30, Demetre Daskalakis, MD, who leads vaccine equity efforts at the CDC, cautioned that physicians who give extra doses of the vaccine before the FDA and CDC have signed off may be in violation of practitioner agreements with the federal government and might not be covered by the federal PREP Act. The PREP Act provides immunity from lawsuits for people who administer COVID-19 vaccines and compensates patients in the event of injury. Patients who get a vaccine and suffer a rare but serious side effect may lose the ability to claim compensation offered by the act.
“Many of us gasped when he said that,” Dr. Talbot said, “because that’s a big deal.”
The ACIP signaled that it is considering recommending boosters for a much narrower slice of the American population than the Biden administration has suggested.
They said that so far, the data point only to the need for boosters for seniors, who are the patients most likely to experience breakthrough infections that require hospitalization, and health care workers, who are needed now more than ever and cannot work if they’re sick.
In a White House news briefing Aug. 31, CDC Director Rochelle Walensky, MD, was asked about the ACIP’s conclusions and whether she believed there were enough data to recommend booster shots for most Americans 8 months after their last dose.
“The ACIP did not review international data that actually has led us to be even more concerned about increased risk of vaccine effectiveness waning against hospitalization, severe disease, and death. They will be reviewing that as well,” she said.
A version of this article first appeared on Medscape.com.
That plan, which was first announced on Aug. 18, has raised eyebrows because it comes in advance of regulatory reviews by the Food and Drug Administration and recommendations from the Centers for Disease Control and Prevention. Those reviews are needed to determine whether third doses of these vaccines are effective or even safe. The move could have important legal ramifications for doctors and patients, too.
On Aug. 31, two high-level officials in the FDA’s Office of Vaccines Research and Review abruptly resigned amid reports that they were angry that the Biden administration was making decisions that should be left up to that agency.
So far, data show that the vaccines are highly effective at preventing the most severe consequences of COVID-19 – hospitalization and death – even regarding the Delta variant. The World Health Organization has urged wealthy nations such as the United States not to offer boosters so that the limited supply of vaccines can be directed to countries with fewer resources.
White House supports boosters
In a recent press briefing, Jeff Zients, the White House COVID-19 response coordinator, defended the move.
“You know, the booster decision, which you referenced ... was made by and announced by the nation’s leading public health officials, including Dr. Walensky; Dr. Fauci; Surgeon General Vivek Murthy; Dr. Janet Woodcock; the FDA acting commissioner, Dr. Francis Collins; Dr. Kessler; and others,” Mr. Zients said.
“And as our medical experts laid out, having reviewed all of the available data, it is in their clinical judgment that it is time to prepare Americans for a booster shot.”
He said a target date of Sept. 20 was announced so as to give states and practitioners time to prepare. He also said the move to give boosters was meant to help the United States stay ahead of a rapidly changing virus. Mr. Zients added that whether boosters will be administered starting on Sept. 20 depends on the FDA’s and CDC’s giving the go-ahead.
“Booster doses are going to be handled the same way all vaccines are handled,” said Kristen Nordlund, a CDC spokesperson. “Companies will have to provide data to FDA. FDA will have to make a decision and authorize the use of those, and ACIP [the Advisory Committee on Immunization Practices] will have to look at the evidence as well and make recommendations on top of FDA’s regulatory action,” she said.
Ms. Nordlund agreed that the planned Sept. 20 start date for boosters was something to which they aspired and was not necessarily set.
Historically, the FDA has needed at least 4 months to review a change to a vaccine’s approval, even on an accelerated schedule. Reviewers use that time to assess data regarding individual patients in a study, to review raw data, and essentially to check a drug company’s math and conclusions. The Biden administration’s timeline would shorten that review period from months to just a few weeks.
‘FDA in a very difficult position’
After the FDA approves, the ACIP of the CDC must meet to review the evidence and make recommendations on the use of the boosters in the United States.
Pfizer says it completed its submission for a supplemental biologics license application to the FDA on Aug. 27. To meet a Sept. 20 timeline, the entire process would have to be completed within 3 weeks.
“I don’t think that was handled, you know, ideally,” said Peter Lurie, MD, president of the Center for Science in the Public Interest and former associate commissioner of public health strategy and analysis at the FDA.
“It puts FDA in a very difficult position,” Dr. Lurie said. “It’s almost as if the decision has been made and they’re just checking a box, and that is, you know, contrary to the what FDA – at least the internal people at FDA – have been trying to do for ages.”
He said the agency took great pains with the emergency use authorizations and the full approvals of the vaccines to work as rapidly but thoroughly as possible. They did not skip steps.
“I think all of that reflected very well on the agency,” Dr. Lurie said. “And I think it worked out well in terms of trust in the vaccines.”
Although additional doses of vaccine are expected to be safe, little is known about side effects or adverse events after a third dose.
“It’s critical to wait for additional data and regulatory allowance for booster doses,” Sara Oliver, MD, a member of the CDC’s epidemic intelligence service, said in an Aug. 30 presentation to the ACIP, which is charged with making recommendations for use of all vaccines in the United States.
Boosters already being given
But after the White House announced that boosters were on the way, many people are not waiting.
Many health care practitioners and pharmacies have already been giving people third doses of vaccines, even if they are not among the immunocompromised – the group for which the shots are currently approved.
“You can walk into a pharmacy and ask for a third dose. Depending on which pharmacy you go to, you may get it,” said Helen Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., and a member of the ACIP.
She says she has a friend who recently went for a checkup and was offered a third dose. His physician is already giving extra doses to everyone who is older than 65.
Dr. Talbot said that in fairness, pharmacies in the United States are throwing away doses of vaccine because they are expiring before they get used.
“Many of us may or may not be ready to give a third dose but would rather give someone a third dose than throw a vaccine away,” she said.
Consequences of a third shot
But giving or getting a third dose before approval by the FDA may have legal consequences.
In the ACIP meeting on Aug. 30, Demetre Daskalakis, MD, who leads vaccine equity efforts at the CDC, cautioned that physicians who give extra doses of the vaccine before the FDA and CDC have signed off may be in violation of practitioner agreements with the federal government and might not be covered by the federal PREP Act. The PREP Act provides immunity from lawsuits for people who administer COVID-19 vaccines and compensates patients in the event of injury. Patients who get a vaccine and suffer a rare but serious side effect may lose the ability to claim compensation offered by the act.
“Many of us gasped when he said that,” Dr. Talbot said, “because that’s a big deal.”
The ACIP signaled that it is considering recommending boosters for a much narrower slice of the American population than the Biden administration has suggested.
They said that so far, the data point only to the need for boosters for seniors, who are the patients most likely to experience breakthrough infections that require hospitalization, and health care workers, who are needed now more than ever and cannot work if they’re sick.
In a White House news briefing Aug. 31, CDC Director Rochelle Walensky, MD, was asked about the ACIP’s conclusions and whether she believed there were enough data to recommend booster shots for most Americans 8 months after their last dose.
“The ACIP did not review international data that actually has led us to be even more concerned about increased risk of vaccine effectiveness waning against hospitalization, severe disease, and death. They will be reviewing that as well,” she said.
A version of this article first appeared on Medscape.com.
That plan, which was first announced on Aug. 18, has raised eyebrows because it comes in advance of regulatory reviews by the Food and Drug Administration and recommendations from the Centers for Disease Control and Prevention. Those reviews are needed to determine whether third doses of these vaccines are effective or even safe. The move could have important legal ramifications for doctors and patients, too.
On Aug. 31, two high-level officials in the FDA’s Office of Vaccines Research and Review abruptly resigned amid reports that they were angry that the Biden administration was making decisions that should be left up to that agency.
So far, data show that the vaccines are highly effective at preventing the most severe consequences of COVID-19 – hospitalization and death – even regarding the Delta variant. The World Health Organization has urged wealthy nations such as the United States not to offer boosters so that the limited supply of vaccines can be directed to countries with fewer resources.
White House supports boosters
In a recent press briefing, Jeff Zients, the White House COVID-19 response coordinator, defended the move.
“You know, the booster decision, which you referenced ... was made by and announced by the nation’s leading public health officials, including Dr. Walensky; Dr. Fauci; Surgeon General Vivek Murthy; Dr. Janet Woodcock; the FDA acting commissioner, Dr. Francis Collins; Dr. Kessler; and others,” Mr. Zients said.
“And as our medical experts laid out, having reviewed all of the available data, it is in their clinical judgment that it is time to prepare Americans for a booster shot.”
He said a target date of Sept. 20 was announced so as to give states and practitioners time to prepare. He also said the move to give boosters was meant to help the United States stay ahead of a rapidly changing virus. Mr. Zients added that whether boosters will be administered starting on Sept. 20 depends on the FDA’s and CDC’s giving the go-ahead.
“Booster doses are going to be handled the same way all vaccines are handled,” said Kristen Nordlund, a CDC spokesperson. “Companies will have to provide data to FDA. FDA will have to make a decision and authorize the use of those, and ACIP [the Advisory Committee on Immunization Practices] will have to look at the evidence as well and make recommendations on top of FDA’s regulatory action,” she said.
Ms. Nordlund agreed that the planned Sept. 20 start date for boosters was something to which they aspired and was not necessarily set.
Historically, the FDA has needed at least 4 months to review a change to a vaccine’s approval, even on an accelerated schedule. Reviewers use that time to assess data regarding individual patients in a study, to review raw data, and essentially to check a drug company’s math and conclusions. The Biden administration’s timeline would shorten that review period from months to just a few weeks.
‘FDA in a very difficult position’
After the FDA approves, the ACIP of the CDC must meet to review the evidence and make recommendations on the use of the boosters in the United States.
Pfizer says it completed its submission for a supplemental biologics license application to the FDA on Aug. 27. To meet a Sept. 20 timeline, the entire process would have to be completed within 3 weeks.
“I don’t think that was handled, you know, ideally,” said Peter Lurie, MD, president of the Center for Science in the Public Interest and former associate commissioner of public health strategy and analysis at the FDA.
“It puts FDA in a very difficult position,” Dr. Lurie said. “It’s almost as if the decision has been made and they’re just checking a box, and that is, you know, contrary to the what FDA – at least the internal people at FDA – have been trying to do for ages.”
He said the agency took great pains with the emergency use authorizations and the full approvals of the vaccines to work as rapidly but thoroughly as possible. They did not skip steps.
“I think all of that reflected very well on the agency,” Dr. Lurie said. “And I think it worked out well in terms of trust in the vaccines.”
Although additional doses of vaccine are expected to be safe, little is known about side effects or adverse events after a third dose.
“It’s critical to wait for additional data and regulatory allowance for booster doses,” Sara Oliver, MD, a member of the CDC’s epidemic intelligence service, said in an Aug. 30 presentation to the ACIP, which is charged with making recommendations for use of all vaccines in the United States.
Boosters already being given
But after the White House announced that boosters were on the way, many people are not waiting.
Many health care practitioners and pharmacies have already been giving people third doses of vaccines, even if they are not among the immunocompromised – the group for which the shots are currently approved.
“You can walk into a pharmacy and ask for a third dose. Depending on which pharmacy you go to, you may get it,” said Helen Talbot, MD, associate professor of medicine at Vanderbilt University, Nashville, Tenn., and a member of the ACIP.
She says she has a friend who recently went for a checkup and was offered a third dose. His physician is already giving extra doses to everyone who is older than 65.
Dr. Talbot said that in fairness, pharmacies in the United States are throwing away doses of vaccine because they are expiring before they get used.
“Many of us may or may not be ready to give a third dose but would rather give someone a third dose than throw a vaccine away,” she said.
Consequences of a third shot
But giving or getting a third dose before approval by the FDA may have legal consequences.
In the ACIP meeting on Aug. 30, Demetre Daskalakis, MD, who leads vaccine equity efforts at the CDC, cautioned that physicians who give extra doses of the vaccine before the FDA and CDC have signed off may be in violation of practitioner agreements with the federal government and might not be covered by the federal PREP Act. The PREP Act provides immunity from lawsuits for people who administer COVID-19 vaccines and compensates patients in the event of injury. Patients who get a vaccine and suffer a rare but serious side effect may lose the ability to claim compensation offered by the act.
“Many of us gasped when he said that,” Dr. Talbot said, “because that’s a big deal.”
The ACIP signaled that it is considering recommending boosters for a much narrower slice of the American population than the Biden administration has suggested.
They said that so far, the data point only to the need for boosters for seniors, who are the patients most likely to experience breakthrough infections that require hospitalization, and health care workers, who are needed now more than ever and cannot work if they’re sick.
In a White House news briefing Aug. 31, CDC Director Rochelle Walensky, MD, was asked about the ACIP’s conclusions and whether she believed there were enough data to recommend booster shots for most Americans 8 months after their last dose.
“The ACIP did not review international data that actually has led us to be even more concerned about increased risk of vaccine effectiveness waning against hospitalization, severe disease, and death. They will be reviewing that as well,” she said.
A version of this article first appeared on Medscape.com.