User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Study estimates carbon footprint reduction of virtual isotretinoin visits
: A reduction of 5,137 kg of greenhouse gas emissions in carbon dioxide equivalents.
In what they say is “one of the first studies to evaluate the environmental impact of any aspect of dermatology,” the authors of the retrospective cross-sectional study identified patients who had virtual visits for isotretinoin management between March 25 and May 29, 2020, – the period during which all such visits were conducted virtually in keeping with hospital recommendations to minimize the spread of COVID-19.
The investigators, from the department of dermatology and the department of civil and environmental engineering at West Virginia University, Morgantown, then counted the number of virtual visits that occurred during this period and through Dec. 1, 2020, (175 virtual visits), calculated the distance patients would have traveled round-trip had these visits been in-person, and converted miles saved into the environmental impact using U.S. Environmental Protection Agency and Federal Highway Administration data and relevant EPA standards.
Most patients had elected to continue virtual visits after May 29, the point at which patients were given the option to return to the WVUH clinic. (Patients who initiated treatment during the 2-month identification period were not included.)
The investigators determined that virtual management of isotretinoin saved a median of 37.8 miles per visit during the study period of March 25 to Dec. 1, and estimated that the virtual visits reduced total travel by 14,450 miles. For the analysis, patients were assumed to use light-duty vehicles.
In addition to calculating the reduction in emissions during the study period (5,137 kg of CO2equivalents) they used patient census data from 2019 to 2020 and data from the study period to project the mileage – and the associated emissions – that would be saved annually if all in-person visits for isotretinoin management occurred virtually.
Their calculation for a projected emissions reduction with 1 year of all-virtual isotretinoin management was 49,400 kg of greenhouse gas emissions in CO2equivalents. This is the emission load released when 24,690 kg of coal are burned or 6.3 million smartphones are charged, the researchers wrote.
“Considering that more than 1,000,000 prescriptions of isotretinoin are authorized annually in the United States, the environmental impact could be magnified if virtual delivery of isotretinoin care is adopted on a national scale,” they commented.“Given the serious consequences of global climate change, analysis of the environmental impact of all fields of medicine, including dermatology, is warranted,” they added.
The reduced greenhouse gas emissions are “definitely [being taken] into consideration for future decisions about virtual visits” in the department of dermatology, said Zachary Zinn, MD, residency director and associate professor in the department of dermatology at West Virginia University, Morgantown, who is the senior author of the study. “The main benefit of virtual care in my opinion,” he said in an interview, “is the potential to reduce our carbon footprint.”
Justin Lee, MD, an intern at WVU and the study’s first author, said that the research team was motivated to think about how they “could reduce the negative environmental impact of practicing dermatology” after they read a paper about the environmental impact of endoscopy, written by a gastroenterologist.
In the study, no pregnancies occurred and monthly tests were performed, but “formal assessment of pregnancy risk with virtual isotretinoin management would be warranted,” Dr. Lee and coauthors wrote, noting too that, while no differences were seen with respect to isotretinoin side effects, these were not formally analyzed.
Dr. Zinn said that he and colleagues at WVUH are currently conducting clinical trials to assess the quality and efficacy of virtual care for patients with acne, atopic dermatitis, and psoriasis. Delivering care virtually “will be easier to do if there are data supporting [its] quality and efficacy,” he said. Rosacea is another condition that may be amendable to virtual care, he noted.
Meanwhile, he said, isotretinoin management is “well suited” for virtual visits. When initiating isotretinoin treatment, Dr. Zinn now “proactively inquires” if patients would like to pursue their follow-up visits virtually. “I’ll note that it will save the time and decrease the burden of travel, including the financial cost as well as the environmental cost of travel,” he said, estimating that about half of their management visits are currently virtual.
Asked about the study, Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said the reduced carbon footprint calculated by the researchers and its downstream health benefits “should be taken into consideration by [dermatology] departments, insurers and policymakers” when making decisions about teledermatology.
While environmental impact is “not something I think most institutions are considering for virtual versus in-person care, they should be. And some are,” said Dr. Rosenbach, a founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues.
Limitations of the study include the generalizability of the results. The impact of virtual isotretinoin management “may be less in predominantly urban areas” than it is in predominately rural West Virginia, the study authors note. And in the case of West Virginia, travel to a local laboratory and pharmacy offsets some of the environmental benefits for the virtual care, they noted. Such travel wasn’t accounted for in the study, but it was found to be a fraction of travel to the WVU hospital clinic. (Patients traveled a median of 5.8 miles to a lab 2.4 times from March 25 to Dec. 1, 2020.)
Dr. Lee will start his dermatology residency at WVU next year. The study was funded by a grant from the U.S. National Science Foundation. The authors have no relevant conflicts of interest, according to Dr. Lee.
: A reduction of 5,137 kg of greenhouse gas emissions in carbon dioxide equivalents.
In what they say is “one of the first studies to evaluate the environmental impact of any aspect of dermatology,” the authors of the retrospective cross-sectional study identified patients who had virtual visits for isotretinoin management between March 25 and May 29, 2020, – the period during which all such visits were conducted virtually in keeping with hospital recommendations to minimize the spread of COVID-19.
The investigators, from the department of dermatology and the department of civil and environmental engineering at West Virginia University, Morgantown, then counted the number of virtual visits that occurred during this period and through Dec. 1, 2020, (175 virtual visits), calculated the distance patients would have traveled round-trip had these visits been in-person, and converted miles saved into the environmental impact using U.S. Environmental Protection Agency and Federal Highway Administration data and relevant EPA standards.
Most patients had elected to continue virtual visits after May 29, the point at which patients were given the option to return to the WVUH clinic. (Patients who initiated treatment during the 2-month identification period were not included.)
The investigators determined that virtual management of isotretinoin saved a median of 37.8 miles per visit during the study period of March 25 to Dec. 1, and estimated that the virtual visits reduced total travel by 14,450 miles. For the analysis, patients were assumed to use light-duty vehicles.
In addition to calculating the reduction in emissions during the study period (5,137 kg of CO2equivalents) they used patient census data from 2019 to 2020 and data from the study period to project the mileage – and the associated emissions – that would be saved annually if all in-person visits for isotretinoin management occurred virtually.
Their calculation for a projected emissions reduction with 1 year of all-virtual isotretinoin management was 49,400 kg of greenhouse gas emissions in CO2equivalents. This is the emission load released when 24,690 kg of coal are burned or 6.3 million smartphones are charged, the researchers wrote.
“Considering that more than 1,000,000 prescriptions of isotretinoin are authorized annually in the United States, the environmental impact could be magnified if virtual delivery of isotretinoin care is adopted on a national scale,” they commented.“Given the serious consequences of global climate change, analysis of the environmental impact of all fields of medicine, including dermatology, is warranted,” they added.
The reduced greenhouse gas emissions are “definitely [being taken] into consideration for future decisions about virtual visits” in the department of dermatology, said Zachary Zinn, MD, residency director and associate professor in the department of dermatology at West Virginia University, Morgantown, who is the senior author of the study. “The main benefit of virtual care in my opinion,” he said in an interview, “is the potential to reduce our carbon footprint.”
Justin Lee, MD, an intern at WVU and the study’s first author, said that the research team was motivated to think about how they “could reduce the negative environmental impact of practicing dermatology” after they read a paper about the environmental impact of endoscopy, written by a gastroenterologist.
In the study, no pregnancies occurred and monthly tests were performed, but “formal assessment of pregnancy risk with virtual isotretinoin management would be warranted,” Dr. Lee and coauthors wrote, noting too that, while no differences were seen with respect to isotretinoin side effects, these were not formally analyzed.
Dr. Zinn said that he and colleagues at WVUH are currently conducting clinical trials to assess the quality and efficacy of virtual care for patients with acne, atopic dermatitis, and psoriasis. Delivering care virtually “will be easier to do if there are data supporting [its] quality and efficacy,” he said. Rosacea is another condition that may be amendable to virtual care, he noted.
Meanwhile, he said, isotretinoin management is “well suited” for virtual visits. When initiating isotretinoin treatment, Dr. Zinn now “proactively inquires” if patients would like to pursue their follow-up visits virtually. “I’ll note that it will save the time and decrease the burden of travel, including the financial cost as well as the environmental cost of travel,” he said, estimating that about half of their management visits are currently virtual.
Asked about the study, Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said the reduced carbon footprint calculated by the researchers and its downstream health benefits “should be taken into consideration by [dermatology] departments, insurers and policymakers” when making decisions about teledermatology.
While environmental impact is “not something I think most institutions are considering for virtual versus in-person care, they should be. And some are,” said Dr. Rosenbach, a founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues.
Limitations of the study include the generalizability of the results. The impact of virtual isotretinoin management “may be less in predominantly urban areas” than it is in predominately rural West Virginia, the study authors note. And in the case of West Virginia, travel to a local laboratory and pharmacy offsets some of the environmental benefits for the virtual care, they noted. Such travel wasn’t accounted for in the study, but it was found to be a fraction of travel to the WVU hospital clinic. (Patients traveled a median of 5.8 miles to a lab 2.4 times from March 25 to Dec. 1, 2020.)
Dr. Lee will start his dermatology residency at WVU next year. The study was funded by a grant from the U.S. National Science Foundation. The authors have no relevant conflicts of interest, according to Dr. Lee.
: A reduction of 5,137 kg of greenhouse gas emissions in carbon dioxide equivalents.
In what they say is “one of the first studies to evaluate the environmental impact of any aspect of dermatology,” the authors of the retrospective cross-sectional study identified patients who had virtual visits for isotretinoin management between March 25 and May 29, 2020, – the period during which all such visits were conducted virtually in keeping with hospital recommendations to minimize the spread of COVID-19.
The investigators, from the department of dermatology and the department of civil and environmental engineering at West Virginia University, Morgantown, then counted the number of virtual visits that occurred during this period and through Dec. 1, 2020, (175 virtual visits), calculated the distance patients would have traveled round-trip had these visits been in-person, and converted miles saved into the environmental impact using U.S. Environmental Protection Agency and Federal Highway Administration data and relevant EPA standards.
Most patients had elected to continue virtual visits after May 29, the point at which patients were given the option to return to the WVUH clinic. (Patients who initiated treatment during the 2-month identification period were not included.)
The investigators determined that virtual management of isotretinoin saved a median of 37.8 miles per visit during the study period of March 25 to Dec. 1, and estimated that the virtual visits reduced total travel by 14,450 miles. For the analysis, patients were assumed to use light-duty vehicles.
In addition to calculating the reduction in emissions during the study period (5,137 kg of CO2equivalents) they used patient census data from 2019 to 2020 and data from the study period to project the mileage – and the associated emissions – that would be saved annually if all in-person visits for isotretinoin management occurred virtually.
Their calculation for a projected emissions reduction with 1 year of all-virtual isotretinoin management was 49,400 kg of greenhouse gas emissions in CO2equivalents. This is the emission load released when 24,690 kg of coal are burned or 6.3 million smartphones are charged, the researchers wrote.
“Considering that more than 1,000,000 prescriptions of isotretinoin are authorized annually in the United States, the environmental impact could be magnified if virtual delivery of isotretinoin care is adopted on a national scale,” they commented.“Given the serious consequences of global climate change, analysis of the environmental impact of all fields of medicine, including dermatology, is warranted,” they added.
The reduced greenhouse gas emissions are “definitely [being taken] into consideration for future decisions about virtual visits” in the department of dermatology, said Zachary Zinn, MD, residency director and associate professor in the department of dermatology at West Virginia University, Morgantown, who is the senior author of the study. “The main benefit of virtual care in my opinion,” he said in an interview, “is the potential to reduce our carbon footprint.”
Justin Lee, MD, an intern at WVU and the study’s first author, said that the research team was motivated to think about how they “could reduce the negative environmental impact of practicing dermatology” after they read a paper about the environmental impact of endoscopy, written by a gastroenterologist.
In the study, no pregnancies occurred and monthly tests were performed, but “formal assessment of pregnancy risk with virtual isotretinoin management would be warranted,” Dr. Lee and coauthors wrote, noting too that, while no differences were seen with respect to isotretinoin side effects, these were not formally analyzed.
Dr. Zinn said that he and colleagues at WVUH are currently conducting clinical trials to assess the quality and efficacy of virtual care for patients with acne, atopic dermatitis, and psoriasis. Delivering care virtually “will be easier to do if there are data supporting [its] quality and efficacy,” he said. Rosacea is another condition that may be amendable to virtual care, he noted.
Meanwhile, he said, isotretinoin management is “well suited” for virtual visits. When initiating isotretinoin treatment, Dr. Zinn now “proactively inquires” if patients would like to pursue their follow-up visits virtually. “I’ll note that it will save the time and decrease the burden of travel, including the financial cost as well as the environmental cost of travel,” he said, estimating that about half of their management visits are currently virtual.
Asked about the study, Misha Rosenbach, MD, associate professor of dermatology at the University of Pennsylvania, Philadelphia, said the reduced carbon footprint calculated by the researchers and its downstream health benefits “should be taken into consideration by [dermatology] departments, insurers and policymakers” when making decisions about teledermatology.
While environmental impact is “not something I think most institutions are considering for virtual versus in-person care, they should be. And some are,” said Dr. Rosenbach, a founder and cochair of the American Academy of Dermatology Expert Resource Group for Climate Change and Environmental Issues.
Limitations of the study include the generalizability of the results. The impact of virtual isotretinoin management “may be less in predominantly urban areas” than it is in predominately rural West Virginia, the study authors note. And in the case of West Virginia, travel to a local laboratory and pharmacy offsets some of the environmental benefits for the virtual care, they noted. Such travel wasn’t accounted for in the study, but it was found to be a fraction of travel to the WVU hospital clinic. (Patients traveled a median of 5.8 miles to a lab 2.4 times from March 25 to Dec. 1, 2020.)
Dr. Lee will start his dermatology residency at WVU next year. The study was funded by a grant from the U.S. National Science Foundation. The authors have no relevant conflicts of interest, according to Dr. Lee.
FROM PEDIATRIC DERMATOLOGY
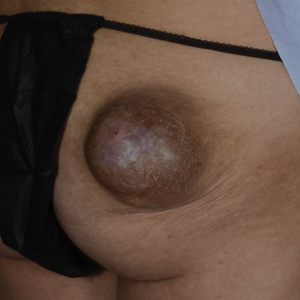
Formaldehyde-Induced Contact Dermatitis From an N95 Respirator Mask
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19

Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19

Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
The COVID-19 pandemic has overwhelmed health care facilities and health care providers (HCPs) due to the limited resources available to treat a rapidly expanding patient population. Health care providers have been required to work long hours and put themselves at increased risk of infection by coming into frequent contact with infected patients. In addition to the risk of becoming infected with severe acute respiratory syndrome coronavirus 2, HCPs might be required to wear personal protective equipment (PPE) for the entirety of the workday, which can cause a variety of adverse effects.
During the COVID-19 pandemic, there has been an increase in reported cases of facial acne, pressure injury, urticaria, allergic contact dermatitis (ACD), irritant contact dermatitis (ICD), and exacerbation of underlying cutaneous conditions among health care workers.1-4 This increase in dermatologic disorders among HCPs has been associated with the increased utilization of and duration of exposure to PPE—particularly N95 respirator masks and surgical masks.5-7 Most studies of these reactions have attributed them to local pressure, friction, hyperhydration, elevated pH, and occlusion caused by prolonged wearing of the masks, resulting ultimately in acne and other rashes8-10; however, a few studies have suggested that formaldehyde is a potential culprit underlying the increase in skin reactions to face masks.11-14
Formaldehyde is a known skin irritant and has been found to cause ACD and ICD from exposure to textiles and cosmetics treated with this chemical.15-18 Both N95 and surgical masks previously have been found to contain sufficient levels of formaldehyde or formaldehyde-releasing resins (FRRs) to induce ACD or ICD in susceptible people.12-14 In this article, we focus on the role of formaldehyde in N95 masks as a potential cause of ACD and ICD in HCPs who have been wearing PPE during the COVID-19 pandemic.
Formaldehyde: Benefits With Significant Problems
Formaldehyde is nearly ubiquitous in the textile industry because it confers advantageous properties, including resistance to flames, water, and wrinkling.15 Despite these advantages, it has long been established that consumers can become sensitized to formaldehyde and FRRs in textiles after chronic exposure.15-18
A study of Australian HCPs found that 5.2% of those tested had ACD in response to formaldehyde, which was attributed to their PPE.11 In a case report of ACD caused by FRRs, Donovan and Skotnicki-Grant12 suggested that individuals who are sensitive to formaldehyde are vulnerable to reactions that are exacerbated by friction, warmth, moisture, and tight-fitting materials—all of which can occur when wearing an N95 mask. In that report, a formaldehyde-sensitive patient had a strong positive reaction on patch testing to melamine formaldehyde and to a piece of her N95 mask while taking prednisone 8 mg/d, suggesting that some sensitized patients have a strong reaction to their mask even when they are immunosuppressed.12
This finding, along with the known formaldehyde content of some N95 masks, suggests that these masks might be a cause of contact dermatitis in some HCPs. Somewhat complicating the situation is that false-negative patch testing can occur in and might contribute to the underdiagnosis of formaldehyde-induced N95 mask facial dermatitis.12,13 Some HCPs have reported mild respiratory symptoms and eye irritation associated with the use of an N95 mask—symptoms that are consistent with formaldehyde exposure. In some cases, those symptoms have caused discomfort sufficient to prompt HCPs to take leave from work.13,14
Development of contact dermatitis in response to an N95 mask is not novel; this problem also was observed during the severe acute respiratory syndrome pandemic of the early 2000s.9,17 Some HCPs noticed onset of skin reactions after they were required to wear an N95 mask in the workplace, which some studies attributed to material in the mask increasing the likelihood of developing an adverse reaction.2,6,8 The components of N95 masks and the materials from which they are manufactured are listed in the Table.19

Other studies have shown that formaldehyde-sensitive individuals had positive patch test reactions to the fabric of N95 and surgical masks, which was found to contain free formaldehyde or FRRs.12-14 However, there are limited reports in the literature confirming the presence of formaldehyde in N95 masks, suggesting the need for (1) more patch testing of N95 mask fabric and (2) correlative high-performance liquid chromatography analysis of the masks to confirm that formaldehyde-sensitive individuals are at risk of formaldehyde-related dermatosis in response to an N95 mask. The absence of any regulatory requirements to list the chemical components of N95 masks makes it impossible for mask users to avoid exposure to potential irritants or carcinogens.
Face Masks, Adverse Reactions, and Formaldehyde
Allergic contact dermatitis and ICD typically are rare responses to wearing facial masks, but the recent COVID-19 pandemic has forced HCPs to wear masks for longer than 6 hours at a time and to reuse a single mask, which has been shown to increase the likelihood of adverse reactions.1,4,6 Additionally, humid environments, tight-fitting materials, and skin abrasions—all of which can be induced by wearing an N95 mask—have been found to increase the likelihood of formaldehyde-related contact dermatitis by increasing the release of free formaldehyde or by enhancing its penetration into the skin.6,20,21
Formaldehyde is an ubiquitous chemical agent that is part of indoor and outdoor working and residential environments. Health care professionals have many opportunities to be exposed to formaldehyde, which is a well-known mucous membrane irritant and a primary skin-sensitizing agent associated with both contact dermatitis (type IV hypersensitivity reaction), and an immediate anaphylactic reaction (type I hypersensitivity reaction).22-25 Exposure to formaldehyde by inhalation has been identified as a potential cause of asthma.26,27 More studies on the prevalence of formaldehyde-induced hypersensitivity reactions would be beneficial to HCPs for early diagnosis of hypersensitivity, adequate prophylaxis, and occupational risk assessment.
N95 mask dermatitis also heightens the potential for breaches of PPE protocols. The discomfort that HCPs experience in response to adverse skin reactions to masks can cause an increased rate of inappropriate mask-wearing, face-touching during mask adjustment, and removal of the mask in the health care setting.28 These acts of face-touching and PPE adjustment have been shown to increase microbial transmission and to reduce the efficacy of PPE in blocking pathogens.29,30
Considering the mounting evidence that widespread use of masks effectively prevents viral transmission, it is crucial that all HCPs wear appropriate PPE when treating patients during the COVID-19 pandemic.31,32 The recent surge in ACD and ICD among HCPs in response to wearing N95 masks creates a need to determine the underlying cause of these dermatoses and find methods of mitigating sensitization of HCPs to the offending agents. The current epidemiology of COVID-19 in the United States suggests that PPE will be necessary for much longer than originally anticipated and will continue to be worn for long hours by HCPs.
Formaldehyde-Free Alternatives?
Some researchers have proposed that using materials that are free of allergens like formaldehyde might be a long-term solution to the development of contact dermatitis.15,33 Formaldehyde is used in the finishing process of N95 masks for wrinkle and crease resistance and to prevent mildew. It is possible that formaldehyde could be completely removed from the manufacturing process, although no studies on the effects of such alternatives on mask efficacy have been performed.
Formaldehyde-free alternatives that would confer similar properties on textiles have been explored; the most promising alternative to formaldehyde in cross-linking cellulose fibers is polycarboxylic acid in combination with sodium hypophosphite, which can help avoid the adverse health outcomes and environmental impact of formaldehyde.34-36 Studies of such alternatives in the manufacturing of N95 masks would be needed to establish the efficacy and durability of formaldehyde-free PPE.
Final Thoughts
Additional studies are needed to confirm the presence of formaldehyde in N95 masks and to confirm that the mask material yields a positive patch test in sensitized individuals. The paucity of available studies that quantify formaldehyde or FRR content of N95 and surgical masks makes it difficult to establish an association between the chemical content of masks and the prevalence of mask dermatitis among HCPs; however, available reports of skin reactions, including contact dermatitis, from PPE suggest that formaldehyde sensitivity might be at least part of the problem. As such, we propose that manufacturers of N95 and surgical masks be required to reveal the chemical components of their products so that consumers can make educated purchasing decisions.
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
- Lan J, Song Z, Miao X, et al. Skin damage among health care workers managing coronavirus disease-2019. letter. J Am Acad Dermatol. 2020;82:1215-1216. doi:10.1016/j.jaad.2020.03.014
- Yan Y, Chen H, Chen L, et al. Consensus of Chinese experts on protection of skin and mucous membrane barrier for health-care workers fighting against coronavirus disease 2019. Dermatol Ther. 2020;33:e13310. doi:10.1111/dth.13310
- Elston DM. Occupational skin disease among health care workers during the coronavirus (COVID-19) epidemic. J Am Acad Dermatol. 2020;82:1085-1086. doi:10.1016/j.jaad.2020.03.012
- Balato A, Ayala F, Bruze M, et al. European Task Force on Contact Dermatitis statement on coronavirus disease-19 (COVID-19) outbreak and the risk of adverse cutaneous reactions. J Eur Acad Dermatol Venereol. 2020;34:E353-E354. doi:10.1111/jdv.16557
- Hu K, Fan J, Li X, et al. The adverse skin reactions of health care workers using personal protective equipment for COVID-19. Medicine (Baltimore). 2020;99:e20603. doi:10.1097/MD.0000000000020603
- Singh M, Pawar M, Bothra A, et al. Personal protective equipment induced facial dermatoses in healthcare workers managing coronavirus disease 2019. J Eur Acad Dermatol Venereol. 2020;34:E378-E380. doi:10.1111/jdv.16628
- Zhou P, Huang Z, Xiao Y, et al. Protecting Chinese healthcare workers while combating the 2019 novel coronavirus. Infect Control Hosp Epidemiol. 2020;41:745-746. doi:10.1017/ice.2020.60
- Hua W, Zuo Y, Wan R, et al. Short-term skin reactions following use of N95 respirators and medical masks. Contact Dermatitis. 2020;83:115-121. doi:10.1111/cod.13601
- Foo CCI, Goon ATJ, Leow Y-H, et al. Adverse skin reactions to personal protective equipment against severe acute respiratory syndrome—a descriptive study in Singapore. Contact Dermatitis. 2006;55:291-294. doi:10.1111/j.1600-0536.2006.00953.x
- Zuo Y, Hua W, Luo Y, et al. Skin reactions of N95 masks and medial masks among health-care personnel: a self‐report questionnaire survey in China. Contact Dermatitis. 2020;83:145-147. doi:10.1111/cod.13555
- Higgins CL, Palmer AM, Cahill JL, et al. Occupational skin disease among Australian healthcare workers: a retrospective analysis from an occupational dermatology clinic, 1993-2014. Contact Dermatitis. 2016;75:213-222. doi:10.1111/cod.12616
- Donovan J, Skotnicki-Grant S. Allergic contact dermatitis from formaldehyde textile resins in surgical uniforms and nonwoven textile masks. Dermatitis. 2007;18:40-44. doi:10.2310/6620.2007.05003
- Donovan J, Kudla I, Holness LD, et al. Skin reactions following use of N95 facial masks. meeting abstract. Dermatitis. 2007;18:104.
- Aerts O, Dendooven E, Foubert K, et al. Surgical mask dermatitis caused by formaldehyde (releasers) during the COVID-19 pandemic. Contact Dermatitis. 2020;83:172-1173. doi:10.1111/cod.13626
- Fowler JF. Formaldehyde as a textile allergen. Curr Probl Dermatol. 2003;31:156-165. doi:10.1159/000072245
- Schorr WF, Keran E, Plotka E. Formaldehyde allergy: the quantitative analysis of American clothing for free formaldehyde and its relevance in clinical practice. Arch Dermatol. 1974;110:73-76.
- Slodownik D, Williams J, Tate B, et al. Textile allergy—the Melbourne experience. Contact Dermatitis. 2011;65:38-42. doi:10.1111/j.1600-0536.2010.01861.x
- O’Quinn SE, Kennedy CB. Contact dermatitis due to formaldehyde in clothing textiles. JAMA. 1965;194:593-596.
- Technical specification sheet—3M™ Particulate Respirator 8210, N95. Published 2018. 3M website. Accessed July 12, 2021. https://multimedia.3m.com/mws/media/1425070O/3m-particulate-respirator-8210-n95-technical-specifications.pdf
- Bhoyrul B, Lecamwasam K, Wilkinson M, et al. A review of non‐glove personal protective equipment‐related occupational dermatoses reported to EPIDERM between 1993 and 2013. Contact Dermatitis. 2019;80:217-221. doi: 10.1111/cod.13177
- Lyapina M, Kissselova-Yaneva A, Krasteva A, et al. Allergic contact dermatitis from formaldehyde exposure. Journal of IMAB - Annual Proceeding (Scientific Papers). 2012;18:255-262. doi:10.5272/jimab.2012184.255
- Foussereau J, Cavelier C, Selig D. Occupational eczema from para-tertiary-butylphenol formaldehyde resins: a review of the sensitizing resins. Contact Dermatitis. 1976;2:254-258. doi:10.1111/j.1600-0536.1976.tb03043.x
- Frølich KW, Andersen LM, Knutsen A, et al. Phenoxyethanol as a nontoxic substitute for formaldehyde in long-term preservation of human anatomical specimens for dissection and demonstration purposes. Anat Rec. 1984;208:271-278. doi:10.1002/ar.1092080214
- Bolt HM. Experimental toxicology of formaldehyde. J Cancer Res Clin Oncol. 1987;113:305-309. doi:10.1007/BF00397713
- Arts JHE, Rennen MAJ, de Heer C. Inhaled formaldehyde: evaluation of sensory irritation in relation to carcinogenicity. Regul Toxicol Pharmacol. 2006;44:144-160. doi:10.1016/j.yrtph.2005.11.006
- Kim CW, Song JS, Ahn YS, et al. Occupational asthma due to formaldehyde. Yonsei Med J. 2001;42:440-445. doi:10.3349/ymj.2001.42.4.440
- Nordman H, Keskinen H, Tuppurainen M. Formaldehyde asthma—rare or overlooked? J Allergy Clin Immunol. 1985;75(1 pt 1):91-99. doi:10.1016/0091-6749(85)90018-1
- Kantor J. Behavioral considerations and impact on personal protective equipment use: early lessons from the coronavirus (COVID-19) pandemic. J Am Acad Dermatol. 2020;82:1087-1088. doi:10.1016/j.jaad.2020.03.013
- Kwok YLA, Gralton J, McLaws M-L. Face touching: a frequent habit that has implications for hand hygiene. Am J Infect Control. 2015;43:112-114. doi:10.1016/j.ajic.2014.10.015
- Nicas M, Best D. A study quantifying the hand-to-face contact rate and its potential application to predicting respiratory tract infection. J Occup Environ Hyg. 2008;5:347-352. doi:10.1080/15459620802003896
- MacIntyre CR, Chughtai AA. A rapid systematic review of the efficacy of face masks and respirators against coronaviruses and other respiratory transmissible viruses for the community, healthcare workers and sick patients. Int J Nurs Stud. 2020;108:103629. doi:10.1016/j.ijnurstu.2020.103629
- Garcia Godoy LR, Jones AE, Anderson TN, et al. Facial protection for healthcare workers during pandemics: a scoping review. BMJ Glob Health. 2020;5:e002553. doi:10.1136/bmjgh-2020-002553
- Svedman C, Engfeldt M, Malinauskiene L. Textile contact dermatitis: how fabrics can induce ermatitis. Curr Treat Options Allergy. 2019;6:103-111. doi:10.1007/s40521-019-0197-5
- Yang CQ, Wang X, Kang I-S. Ester crosslinking of cotton fabric by polymeric carboxylic acids and citric acid. Textile Res J. 1997;67:334-342. https://doi.org/10.1177/004051759706700505
- Welch CM. Formaldehyde-free durable-press finishes. Rev Prog Coloration Related Top. 1992;22:32-41. https://doi.org/10.1111/j.1478-4408.1992.tb00087.x
- Peng H, Yang CQ, Wang S. Nonformaldehyde durable press finishing of cotton fabrics using the combination of maleic acid and sodium hypophosphite. Carbohydrate Polymers. 2012;87:491-499. doi:10.1016/j.carbpol.2011.08.013
Practice Points
- Prolonged wearing of N95 respirator masks has been associated with causing or complicating a number of facial inflammatory dermatoses.
- Consider the possibility of contact dermatitis secondary to formaldehyde exposure in individuals wearing N95 masks for prolonged periods.
- Information on the chemical components of N95 masks would be useful for clinicians tasked with evaluating patients with facial inflammatory dermatoses.
Phototherapy: Safe and Effective for Challenging Skin Conditions in Older Adults
Identifying safe, effective, and affordable evidence-based dermatologic treatments for older adults can be challenging because of age-related changes in the skin, comorbidities, polypharmacy, mobility issues, and cognitive changes. Phototherapy has been shown to be an effective nonpharmacologic treatment option for multiple challenging dermatologic conditions1-8; however, few studies have specifically examined its effectiveness in older adults. The challenge for older patients with psoriasis and dermatitis is that the conditions can be difficult to control and often require multiple treatment modalities.9,10 Patients with psoriasis also have a higher risk for diabetes, dyslipidemia, and cardiovascular disease compared to other older patients,11,12 which poses treatment challenges and makes nonpharmacologic treatments even more appealing.
Recent studies show that phototherapy can help decrease the use of dermatologic medications. Foerster and colleagues2 found that adults with psoriasis who were treated with phototherapy significantly decreased their use of topical steroids (24.5% fewer patients required steroid creams and 31.1% fewer patients required psoriasis-specific topicals)(P<.01) while their use of non–psoriasis-specific medications did not change. Click and colleagues13 identified a decrease in medication costs, health care utilization, and risk for immunosuppression in patients treated with phototherapy when compared to those treated with biologics and apremilast. Methotrexate is a common dermatologic medication that is highly associated with increased risks in elderly patients because of impaired immune system function and the presence of comorbidities (eg, kidney disease, obesity, diabetes, fatty liver),14 which increase in prevalence with age. Combining phototherapy with methotrexate can substantially decrease the amount of methotrexate needed to achieve disease control,15 thereby decreasing the methotrexate-associated risks. Findings from these studies suggest that a safe, effective, cost-effective, and well-tolerated nonpharmacologic alternative, such as phototherapy, is highly desirable and should be optimized. Unfortunately, most studies that report the effectiveness of phototherapy are in younger populations.
This retrospective study aimed to (1) identify the most common dermatologic conditions treated with phototherapy in older adults, (2) examine the effectiveness and safety of phototherapy in older adults
Methods
Design, Setting, Sample, and Statistical Analysis
The institutional review boards of Kaiser Permanente Washington Health Research Institute, Seattle, and the University of Washington, Seattle, approved this study. It was conducted in a large US multispecialty health care system (Group Health, Seattle, Washington [now Kaiser Permanente Washington]) serving approximately 600,000 patients, using billing records to identify all patients treated with phototherapy between January 1, 2015, and December 31, 2015, all who received narrowband UVB (NB-UVB) phototherapy. All adults 65 years and older who received phototherapy treatment during the 12-month study period were included. Patients were included regardless of comorbidities and other dermatologic treatments to maintain as much uniformity as possible between the present study and 2 prior studies examining phototherapy in older adult populations in the United Kingdom16 and Turkey.17 Demographic and clinical factors were presented using frequencies (percentages) or means and medians as appropriate. Comparisons of dermatologic conditions and clearance levels used a Fisher exact test. The number of phototherapy treatments to clearance and total number of treatments were compared between groups of patients using independent sample t tests.
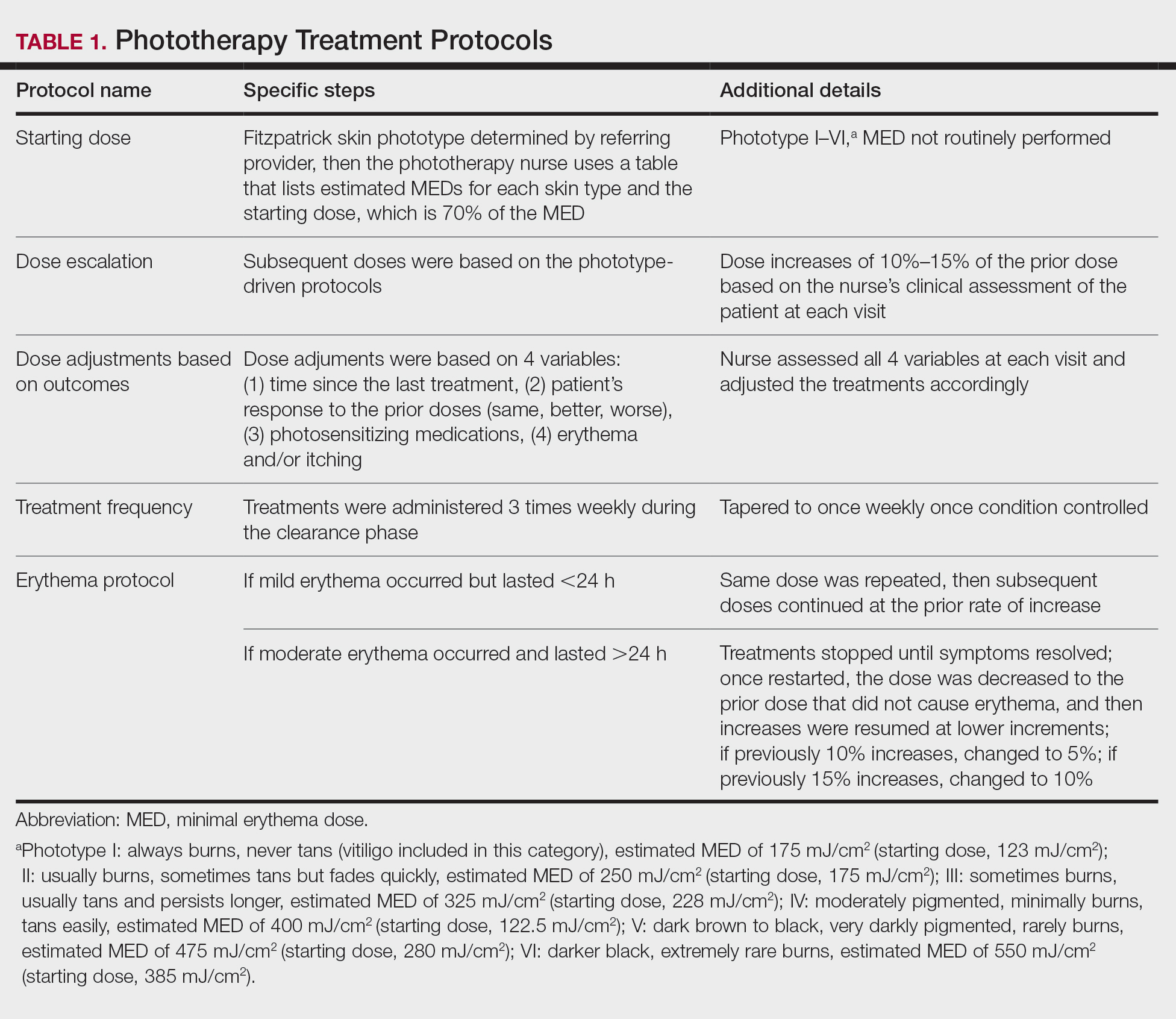
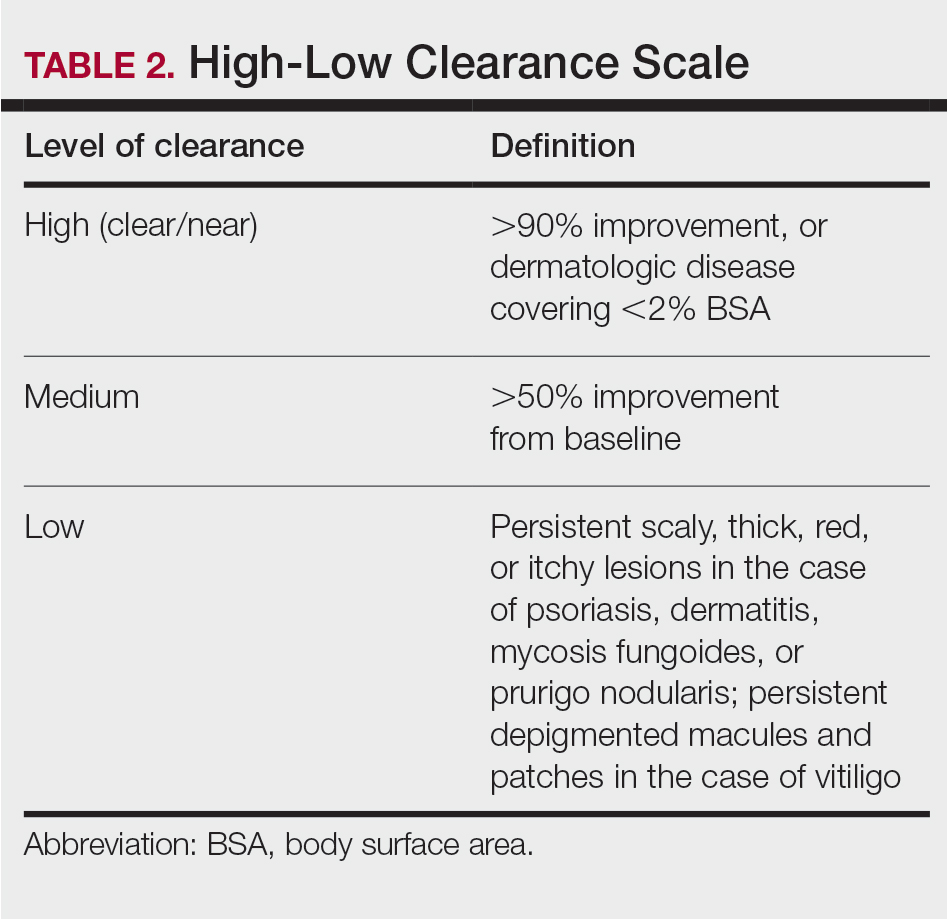
Phototherapy Protocol
All patients received treatments administered by specially trained phototherapy nurses using a Daavlin UV Series (The Daavlin Company) or an Ultralite unit (Ultralite Enterprises, Inc), both with 48 lamps. All phototherapy nurses had been previously trained to provide treatments based on standardized protocols (Table 1) and to determine the patient’s level of disease clearance using a high to low clearance scale (Table 2). Daavlin’s treatment protocols were built into the software that accompanied the units and were developed based on the American Academy of Dermatology guidelines. The starting dose for an individual patient was determined based on the estimated
Results
Patients
Billing records identified 229 total patients who received phototherapy in 2015, of whom 52 (22.7%) were at least 65 years old. The median age was 70 years (range, 65–91 years). Twenty-nine (56%) were men and 35 (67%) had previously received phototherapy treatments.
Dermatologic Conditions Treated With Phototherapy
Our primary aim was to identify the most common dermatologic conditions treated with phototherapy in older adults. Psoriasis and dermatitis were the most common conditions treated in the sample (50% [26/52] and 21% [11/52], respectively), with mycosis fungoides being the third most common (10% [5/52]) and vitiligo tied with prurigo nodularis as fourth most common (6% [3/52])(Figure 1).

Effectiveness and Safety of Phototherapy
Our secondary aim was to examine the effectiveness and safety of phototherapy in older adults. Phototherapy was effective in this population, with 50 of 52 patients (96%) achieving a high or medium level of clearance. The degree of clearance for each of the dermatologic conditions is shown in Figure 2. Psoriasis and dermatitis achieved high clearance rates in 81% (21/26) and 82% (9/11) of patients, respectively. Overall, conditions did not have significant differences in clearances rates (Fisher exact test, P=.10). On average, it took patients 33 treatments to achieve medium or high rates of clearance. Psoriasis cleared more quickly, with an average of 30.4 treatments vs 36.1 treatments for other conditions, but the difference was not significant (t test, P=.26). Patients received an average of 98 total phototherapy treatments; the median number of treatments was 81 due to many being on maintenance therapy over several months. There was no relationship between a history of treatment with phototherapy and the total number of treatments needed to achieve clearance (t test, P=.40), but interestingly, those who had a history of phototherapy took approximately 5 more treatments to achieve clearance. The present study found that a slightly larger number of men were being treated for psoriasis (15 men vs 11 women), but there was no significant difference in response rate based on gender.
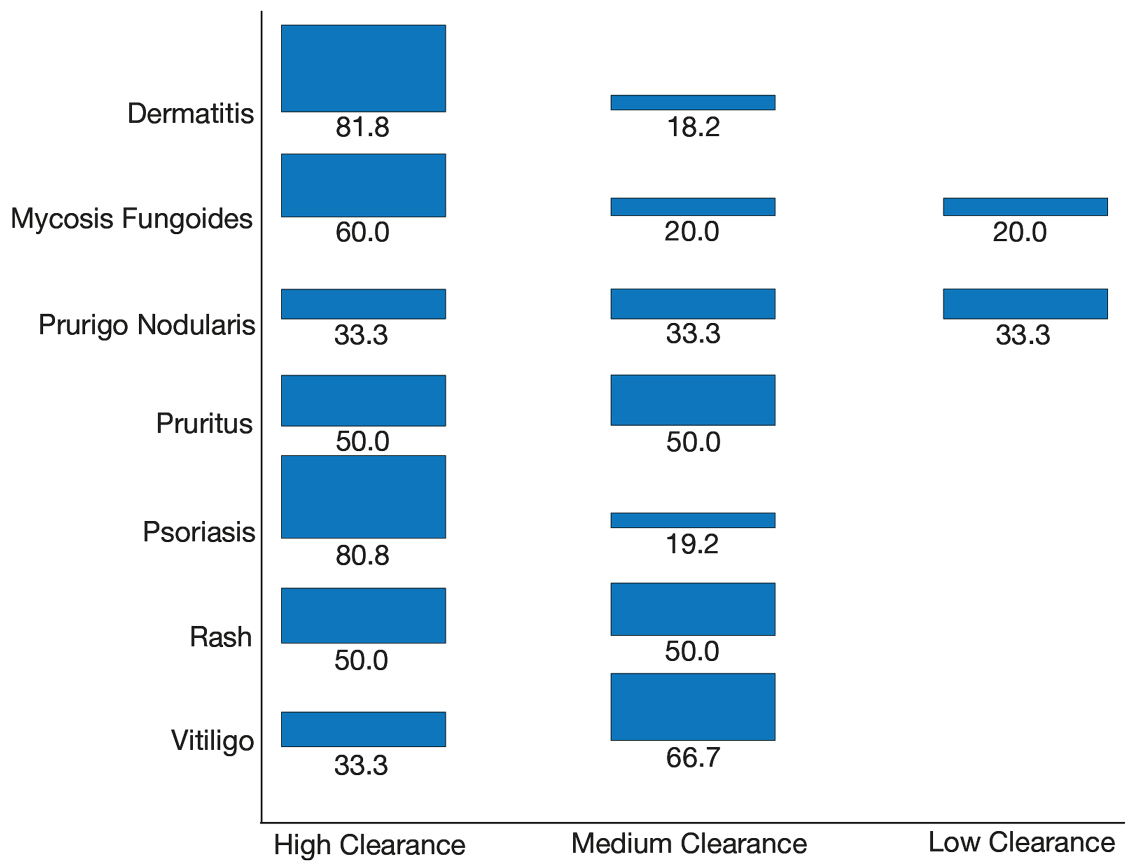
Side effects from phototherapy were minimal; 24 patients (46%) experienced grade 1 (mild) erythema at some point during their treatment course. Thirteen (25%) patients experienced grade 2 erythema, but this was a rare event for most patients. Only 1 (2%) patient experienced grade 3 erythema 1 time. Three patients experienced increased itching (6%). Thirteen (25%) patients had no side effects. None developed severe erythema or blisters, and none discontinued phototherapy because of side effects. Over the course of the study year, we found a high degree of acceptance of phototherapy treatments by older patients: 22 (42%) completed therapy after achieving clearance, 10 (19%) were continuing ongoing treatments (maintenance), and 15 (29%) stopped because of life circumstances (eg, other health issues, moving out of the area). Only 4 (8%) stopped because of a lack of effectiveness, and 1 (2%) patient because the treatments were burdensome.
Comparison of Outcomes
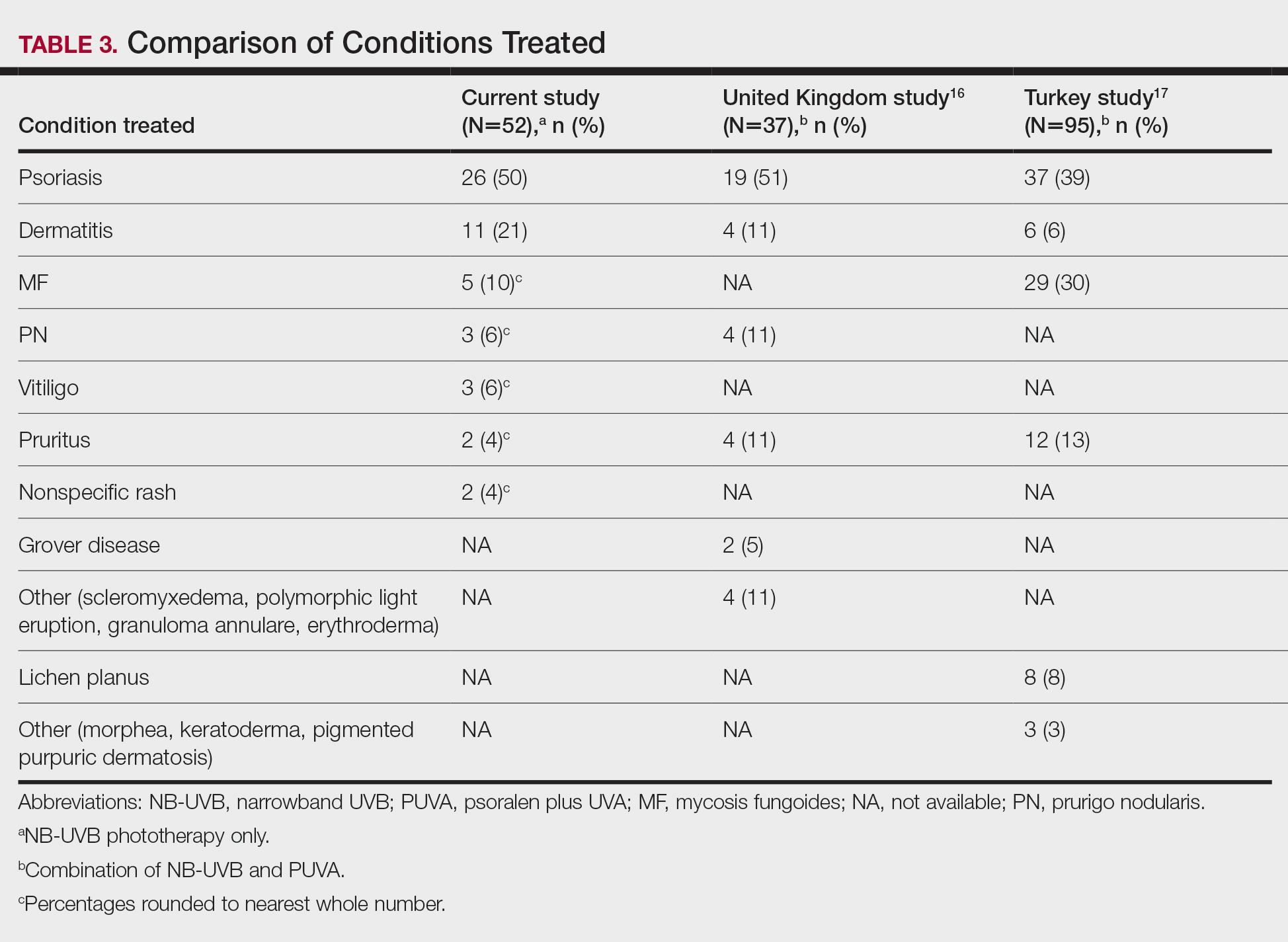
Our third aim was to compare the outcomes with similar studies in the United Kingdom16 and Turkey.17 This study confirmed that phototherapy is being used in older adults (22.7% of this study’s total patients) and is an effective treatment for older patients experiencing a range of challenging inflammatory and proliferative skin diseases similar to studies in the general population. Prior phototherapy studies in elderly patients also found psoriasis to be the most common skin condition treated, with 1 study finding that 51% (19/37) of older phototherapy patients had psoriasis,16 while another reported 58% (37/95) of older phototherapy patients had psoriasis.17 These numbers are similar to those in our study, which showed 50% (26/52) of elderly phototherapy patients had psoriasis. Psoriasis is the main indication for treatment with NB-UVB phototherapy in the general population,19 and because the risk for psoriasis increases with age,20 it is not surprising that all 3 studies found psoriasis to be the most common indication in elderly phototherapy patients. Table 3 provides further details on conditions treated in all 3 studies.
Comment
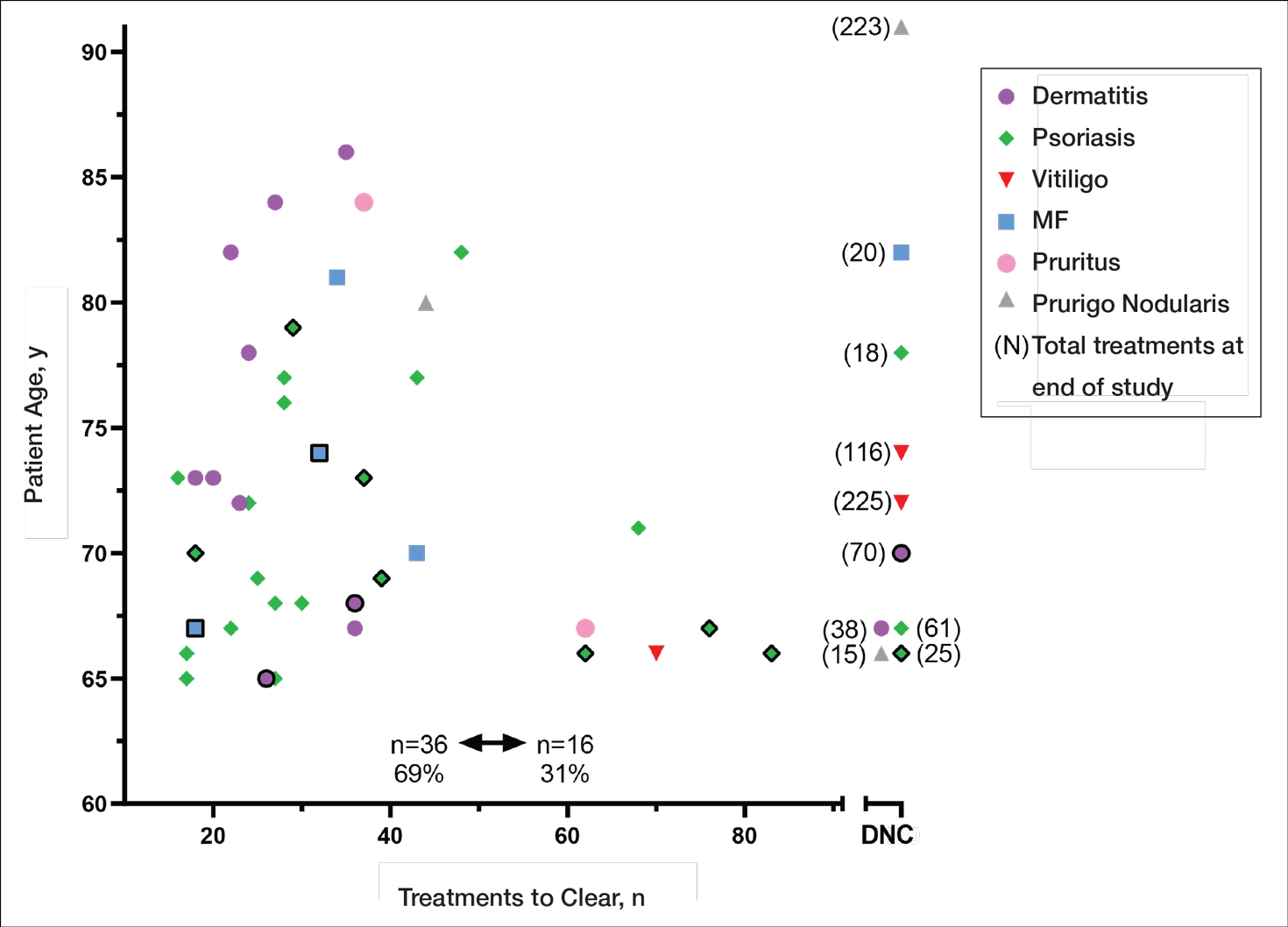
Our study found that 94% of patients with psoriasis achieved clearance with an average of 30.4 treatments, which is comparable to the reported 91% response rate with an average of 30 treatments in the United Kingdom.16 The other similar study in Turkey17 reported 73.7% of psoriasis patients achieved a 75% or more improvement from baseline with an average of 42 treatments, which may reflect underlying differences in regional skin type. Of note, the scatter chart (Figure 3) shows that several patients in the present study’s analysis are listed as not clear, but many of those patients had low treatment numbers below the mean time to clearance. Thus, the present study’s response rate may have been underestimated.
In the general population, studies show that psoriasis treated with standardized phototherapy protocols typically clears with an average of 20.6 treatments.21 The levels of clearance were similar in our study’s older population, but more treatments were required to achieve those results, with an average of 10 more treatments needed (an additional 3.3 weeks). Similar results were found in this sample for dermatitis and mycosis fungoides, indicating comparable clearance rates and levels but a need for more treatments to achieve similar results compared to the general population.
Additionally, in the current study more patients experienced grade 1 (mild) erythema (46%) and grade 2 erythema (25%) at some point in their treatment compared with the United Kingdom16 (1.89%) and Turkey17 (35%) studies, though these side effects did not impact the clearance rate. Interestingly, the current study’s scatter chart (Figure 3) illustrates that this side effect did not seem to increase with aging in this population. If anything, the erythema response was more prevalent in the median or younger patients in the sample. Erythema may have been due to the frequent use of photosensitizing medications in older adults in the United States, some of which typically get discontinued in patients 75 years and older (eg, statins). Other potential causes might include the use of phototype vs minimal erythema dose–driven protocols, the standard utilization of protocols originally designed for psoriasis vs other condition-specific protocols, missed treatments leading to increased sensitivity, or possibly shielding mishaps (eg, not wearing a prescribed face shield). Given the number of potential causes and the possibility of overlapping factors, careful analysis is important. With NB-UVB phototherapy, near-erythemogenic doses are optimal to achieve effective treatments, but this delicate balance may be more problematic for older adults. Future studies are needed to fully determine the factors at play for this population. In the interim, it is important for phototherapy-trained nurses to consider this risk carefully in the older population. They must follow the prescribed protocols that guide them to query patients about their responses to the prior treatment (eg, erythema, tenderness, itching), photosensitizing medications, missed treatments, and placement of shielding, and then adjust the treatment dosing accordingly.
Limitations
This study had several limitations. Although clinical outcomes were recorded prospectively, the analysis was retrospective, unblinded, and not placebo controlled. It was conducted in a single organization (Group Health [now Kaiser Permanente Washington]) but did analyze data from 4 medical centers in different cities with diverse demographics and a variety of nursing staff providing the treatments. Although the vitiligo treatment protocol likely slowed the response rate for those patients with vitiligo, the numbers were small (ie, only 3 of 52 patients), so the researchers chose to include them in the current study. The sample population was relatively small, but when these data are evaluated alongside the studies in the United Kingdom16 and Turkey,17 they show a consistent picture illustrating the effectiveness and safety of phototherapy in the older population. Further epidemiologic studies could be helpful to further describe the usefulness of this modality compared with other treatments for a variety of dermatoses in this age group. Supplementary analysis specifically examining the relationship between the number and type of photosensitizing medications, frequency of erythema, and time to clearance also could be useful.
Conclusion
Older adults with a variety of dermatoses respond well to phototherapy and should have the opportunity to use it, particularly considering the potential for increased complications and costs from other treatment modalities, such as commonly used immunosuppressive pharmaceuticals. However, the current study and the comparison studies indicate that it is important to carefully consider the slower clearance rates and the potential risk for increased erythema in this population and adjust patient education and treatment dosing accordingly.
Unfortunately, many dermatology centers do not offer phototherapy because of infrastructure limitations such as space and specially trained nursing staff. Increasing accessibility of phototherapy for older adults through home treatments may be an alternative, given its effectiveness in the general population.22,23 In addition, home phototherapy may be worth pursuing for the older population considering the challenges they may face with transportation to the clinic setting and their increased risk for serious illness if exposed to infections such as COVID-19. The COVID-19 pandemic has brought to light the need for reliable, safe, and effective treatments that can be utilized in the safety of patients’ homes and should therefore be considered as an option for older adults. Issues such as mobility and cognitive decline could pose some complicating factors, but with the help of a well-trained family member or caregiver, home phototherapy could be a viable option that improves accessibility for older patients. Future research opportunities include further examination of the slower but ultimately equivalent response to phototherapy in the older population, the influence of photosensitizing medications on phototherapy effects, and the impact of phototherapy on utilization of immunosuppressive pharmaceuticals in older adults.
- British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327-330.
Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE. 2017;12:e0181813. doi:10.1371/journal.pone.0181813 - Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2015;29:19-23.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593. - Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Martin JA, Laube S, Edwards C, et al. Rate of acute adverse events for narrow-band UVB and psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:68-72.
- Mokos ZB, Jovic A, Ceovic R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Exp Opin Biol Ther. 2018;18:897-903.
- Napolitano M, Balato N, Ayala F, et al. Psoriasis in elderly and non-elderly population: clinical and molecular features. G Ital Dermatol Venereol. 2016;151:587-595.
- Grozdev IS, Van Voorhees AS, Gottlieb AB, et al. Psoriasis in the elderly: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2011;65:537-545.
- Click J, Alabaster A, Postlethwaite D, et al. Effect of availability of at-home phototherapy on the use of systemic medications for psoriasis.
Photodermatol Photoimmunol Photomed. 2017;33:345-346. - Piaserico S, Conti A, Lo Console F, et al.
Efficacy and safety of systemic treatments for psoriasis in elderly. Acta Derm Venereol. 2014;94:293-297. - Soliman A, Nofal E, Nofal A, et al. Combination therapy of methotrexate plus NB-UVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat. 2015;26:528-534.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:66-80.
- Ibbotson SH. A perspective on the use of NB-UVB phototherapy vs. PUVA photochemotherapy. Front Med (Lausanne). 2018;5:184.
- Bell LM, Sedlack R, Beard CM, et al. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127:1184-1187.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413.
- Cameron H, Yule S, Dawe RS, et al. Review of an established UK home phototherapy service 1998-2011: improving access to a cost-effective treatment for chronic skin disease. Public Health. 2014;128:317-324.
- Matthews SW, Simmer M, Williams L, et al. Transition of patients with psoriasis from office-based phototherapy to nurse-supported home phototherapy: a pilot study. JDNA. 2018;10:29-41.
Identifying safe, effective, and affordable evidence-based dermatologic treatments for older adults can be challenging because of age-related changes in the skin, comorbidities, polypharmacy, mobility issues, and cognitive changes. Phototherapy has been shown to be an effective nonpharmacologic treatment option for multiple challenging dermatologic conditions1-8; however, few studies have specifically examined its effectiveness in older adults. The challenge for older patients with psoriasis and dermatitis is that the conditions can be difficult to control and often require multiple treatment modalities.9,10 Patients with psoriasis also have a higher risk for diabetes, dyslipidemia, and cardiovascular disease compared to other older patients,11,12 which poses treatment challenges and makes nonpharmacologic treatments even more appealing.
Recent studies show that phototherapy can help decrease the use of dermatologic medications. Foerster and colleagues2 found that adults with psoriasis who were treated with phototherapy significantly decreased their use of topical steroids (24.5% fewer patients required steroid creams and 31.1% fewer patients required psoriasis-specific topicals)(P<.01) while their use of non–psoriasis-specific medications did not change. Click and colleagues13 identified a decrease in medication costs, health care utilization, and risk for immunosuppression in patients treated with phototherapy when compared to those treated with biologics and apremilast. Methotrexate is a common dermatologic medication that is highly associated with increased risks in elderly patients because of impaired immune system function and the presence of comorbidities (eg, kidney disease, obesity, diabetes, fatty liver),14 which increase in prevalence with age. Combining phototherapy with methotrexate can substantially decrease the amount of methotrexate needed to achieve disease control,15 thereby decreasing the methotrexate-associated risks. Findings from these studies suggest that a safe, effective, cost-effective, and well-tolerated nonpharmacologic alternative, such as phototherapy, is highly desirable and should be optimized. Unfortunately, most studies that report the effectiveness of phototherapy are in younger populations.
This retrospective study aimed to (1) identify the most common dermatologic conditions treated with phototherapy in older adults, (2) examine the effectiveness and safety of phototherapy in older adults
Methods
Design, Setting, Sample, and Statistical Analysis
The institutional review boards of Kaiser Permanente Washington Health Research Institute, Seattle, and the University of Washington, Seattle, approved this study. It was conducted in a large US multispecialty health care system (Group Health, Seattle, Washington [now Kaiser Permanente Washington]) serving approximately 600,000 patients, using billing records to identify all patients treated with phototherapy between January 1, 2015, and December 31, 2015, all who received narrowband UVB (NB-UVB) phototherapy. All adults 65 years and older who received phototherapy treatment during the 12-month study period were included. Patients were included regardless of comorbidities and other dermatologic treatments to maintain as much uniformity as possible between the present study and 2 prior studies examining phototherapy in older adult populations in the United Kingdom16 and Turkey.17 Demographic and clinical factors were presented using frequencies (percentages) or means and medians as appropriate. Comparisons of dermatologic conditions and clearance levels used a Fisher exact test. The number of phototherapy treatments to clearance and total number of treatments were compared between groups of patients using independent sample t tests.
Phototherapy Protocol
All patients received treatments administered by specially trained phototherapy nurses using a Daavlin UV Series (The Daavlin Company) or an Ultralite unit (Ultralite Enterprises, Inc), both with 48 lamps. All phototherapy nurses had been previously trained to provide treatments based on standardized protocols (Table 1) and to determine the patient’s level of disease clearance using a high to low clearance scale (Table 2). Daavlin’s treatment protocols were built into the software that accompanied the units and were developed based on the American Academy of Dermatology guidelines. The starting dose for an individual patient was determined based on the estimated


Results
Patients
Billing records identified 229 total patients who received phototherapy in 2015, of whom 52 (22.7%) were at least 65 years old. The median age was 70 years (range, 65–91 years). Twenty-nine (56%) were men and 35 (67%) had previously received phototherapy treatments.
Dermatologic Conditions Treated With Phototherapy
Our primary aim was to identify the most common dermatologic conditions treated with phototherapy in older adults. Psoriasis and dermatitis were the most common conditions treated in the sample (50% [26/52] and 21% [11/52], respectively), with mycosis fungoides being the third most common (10% [5/52]) and vitiligo tied with prurigo nodularis as fourth most common (6% [3/52])(Figure 1).

Effectiveness and Safety of Phototherapy
Our secondary aim was to examine the effectiveness and safety of phototherapy in older adults. Phototherapy was effective in this population, with 50 of 52 patients (96%) achieving a high or medium level of clearance. The degree of clearance for each of the dermatologic conditions is shown in Figure 2. Psoriasis and dermatitis achieved high clearance rates in 81% (21/26) and 82% (9/11) of patients, respectively. Overall, conditions did not have significant differences in clearances rates (Fisher exact test, P=.10). On average, it took patients 33 treatments to achieve medium or high rates of clearance. Psoriasis cleared more quickly, with an average of 30.4 treatments vs 36.1 treatments for other conditions, but the difference was not significant (t test, P=.26). Patients received an average of 98 total phototherapy treatments; the median number of treatments was 81 due to many being on maintenance therapy over several months. There was no relationship between a history of treatment with phototherapy and the total number of treatments needed to achieve clearance (t test, P=.40), but interestingly, those who had a history of phototherapy took approximately 5 more treatments to achieve clearance. The present study found that a slightly larger number of men were being treated for psoriasis (15 men vs 11 women), but there was no significant difference in response rate based on gender.

Side effects from phototherapy were minimal; 24 patients (46%) experienced grade 1 (mild) erythema at some point during their treatment course. Thirteen (25%) patients experienced grade 2 erythema, but this was a rare event for most patients. Only 1 (2%) patient experienced grade 3 erythema 1 time. Three patients experienced increased itching (6%). Thirteen (25%) patients had no side effects. None developed severe erythema or blisters, and none discontinued phototherapy because of side effects. Over the course of the study year, we found a high degree of acceptance of phototherapy treatments by older patients: 22 (42%) completed therapy after achieving clearance, 10 (19%) were continuing ongoing treatments (maintenance), and 15 (29%) stopped because of life circumstances (eg, other health issues, moving out of the area). Only 4 (8%) stopped because of a lack of effectiveness, and 1 (2%) patient because the treatments were burdensome.
Comparison of Outcomes
Our third aim was to compare the outcomes with similar studies in the United Kingdom16 and Turkey.17 This study confirmed that phototherapy is being used in older adults (22.7% of this study’s total patients) and is an effective treatment for older patients experiencing a range of challenging inflammatory and proliferative skin diseases similar to studies in the general population. Prior phototherapy studies in elderly patients also found psoriasis to be the most common skin condition treated, with 1 study finding that 51% (19/37) of older phototherapy patients had psoriasis,16 while another reported 58% (37/95) of older phototherapy patients had psoriasis.17 These numbers are similar to those in our study, which showed 50% (26/52) of elderly phototherapy patients had psoriasis. Psoriasis is the main indication for treatment with NB-UVB phototherapy in the general population,19 and because the risk for psoriasis increases with age,20 it is not surprising that all 3 studies found psoriasis to be the most common indication in elderly phototherapy patients. Table 3 provides further details on conditions treated in all 3 studies.

Comment
Our study found that 94% of patients with psoriasis achieved clearance with an average of 30.4 treatments, which is comparable to the reported 91% response rate with an average of 30 treatments in the United Kingdom.16 The other similar study in Turkey17 reported 73.7% of psoriasis patients achieved a 75% or more improvement from baseline with an average of 42 treatments, which may reflect underlying differences in regional skin type. Of note, the scatter chart (Figure 3) shows that several patients in the present study’s analysis are listed as not clear, but many of those patients had low treatment numbers below the mean time to clearance. Thus, the present study’s response rate may have been underestimated.

In the general population, studies show that psoriasis treated with standardized phototherapy protocols typically clears with an average of 20.6 treatments.21 The levels of clearance were similar in our study’s older population, but more treatments were required to achieve those results, with an average of 10 more treatments needed (an additional 3.3 weeks). Similar results were found in this sample for dermatitis and mycosis fungoides, indicating comparable clearance rates and levels but a need for more treatments to achieve similar results compared to the general population.
Additionally, in the current study more patients experienced grade 1 (mild) erythema (46%) and grade 2 erythema (25%) at some point in their treatment compared with the United Kingdom16 (1.89%) and Turkey17 (35%) studies, though these side effects did not impact the clearance rate. Interestingly, the current study’s scatter chart (Figure 3) illustrates that this side effect did not seem to increase with aging in this population. If anything, the erythema response was more prevalent in the median or younger patients in the sample. Erythema may have been due to the frequent use of photosensitizing medications in older adults in the United States, some of which typically get discontinued in patients 75 years and older (eg, statins). Other potential causes might include the use of phototype vs minimal erythema dose–driven protocols, the standard utilization of protocols originally designed for psoriasis vs other condition-specific protocols, missed treatments leading to increased sensitivity, or possibly shielding mishaps (eg, not wearing a prescribed face shield). Given the number of potential causes and the possibility of overlapping factors, careful analysis is important. With NB-UVB phototherapy, near-erythemogenic doses are optimal to achieve effective treatments, but this delicate balance may be more problematic for older adults. Future studies are needed to fully determine the factors at play for this population. In the interim, it is important for phototherapy-trained nurses to consider this risk carefully in the older population. They must follow the prescribed protocols that guide them to query patients about their responses to the prior treatment (eg, erythema, tenderness, itching), photosensitizing medications, missed treatments, and placement of shielding, and then adjust the treatment dosing accordingly.
Limitations
This study had several limitations. Although clinical outcomes were recorded prospectively, the analysis was retrospective, unblinded, and not placebo controlled. It was conducted in a single organization (Group Health [now Kaiser Permanente Washington]) but did analyze data from 4 medical centers in different cities with diverse demographics and a variety of nursing staff providing the treatments. Although the vitiligo treatment protocol likely slowed the response rate for those patients with vitiligo, the numbers were small (ie, only 3 of 52 patients), so the researchers chose to include them in the current study. The sample population was relatively small, but when these data are evaluated alongside the studies in the United Kingdom16 and Turkey,17 they show a consistent picture illustrating the effectiveness and safety of phototherapy in the older population. Further epidemiologic studies could be helpful to further describe the usefulness of this modality compared with other treatments for a variety of dermatoses in this age group. Supplementary analysis specifically examining the relationship between the number and type of photosensitizing medications, frequency of erythema, and time to clearance also could be useful.
Conclusion
Older adults with a variety of dermatoses respond well to phototherapy and should have the opportunity to use it, particularly considering the potential for increased complications and costs from other treatment modalities, such as commonly used immunosuppressive pharmaceuticals. However, the current study and the comparison studies indicate that it is important to carefully consider the slower clearance rates and the potential risk for increased erythema in this population and adjust patient education and treatment dosing accordingly.
Unfortunately, many dermatology centers do not offer phototherapy because of infrastructure limitations such as space and specially trained nursing staff. Increasing accessibility of phototherapy for older adults through home treatments may be an alternative, given its effectiveness in the general population.22,23 In addition, home phototherapy may be worth pursuing for the older population considering the challenges they may face with transportation to the clinic setting and their increased risk for serious illness if exposed to infections such as COVID-19. The COVID-19 pandemic has brought to light the need for reliable, safe, and effective treatments that can be utilized in the safety of patients’ homes and should therefore be considered as an option for older adults. Issues such as mobility and cognitive decline could pose some complicating factors, but with the help of a well-trained family member or caregiver, home phototherapy could be a viable option that improves accessibility for older patients. Future research opportunities include further examination of the slower but ultimately equivalent response to phototherapy in the older population, the influence of photosensitizing medications on phototherapy effects, and the impact of phototherapy on utilization of immunosuppressive pharmaceuticals in older adults.
Identifying safe, effective, and affordable evidence-based dermatologic treatments for older adults can be challenging because of age-related changes in the skin, comorbidities, polypharmacy, mobility issues, and cognitive changes. Phototherapy has been shown to be an effective nonpharmacologic treatment option for multiple challenging dermatologic conditions1-8; however, few studies have specifically examined its effectiveness in older adults. The challenge for older patients with psoriasis and dermatitis is that the conditions can be difficult to control and often require multiple treatment modalities.9,10 Patients with psoriasis also have a higher risk for diabetes, dyslipidemia, and cardiovascular disease compared to other older patients,11,12 which poses treatment challenges and makes nonpharmacologic treatments even more appealing.
Recent studies show that phototherapy can help decrease the use of dermatologic medications. Foerster and colleagues2 found that adults with psoriasis who were treated with phototherapy significantly decreased their use of topical steroids (24.5% fewer patients required steroid creams and 31.1% fewer patients required psoriasis-specific topicals)(P<.01) while their use of non–psoriasis-specific medications did not change. Click and colleagues13 identified a decrease in medication costs, health care utilization, and risk for immunosuppression in patients treated with phototherapy when compared to those treated with biologics and apremilast. Methotrexate is a common dermatologic medication that is highly associated with increased risks in elderly patients because of impaired immune system function and the presence of comorbidities (eg, kidney disease, obesity, diabetes, fatty liver),14 which increase in prevalence with age. Combining phototherapy with methotrexate can substantially decrease the amount of methotrexate needed to achieve disease control,15 thereby decreasing the methotrexate-associated risks. Findings from these studies suggest that a safe, effective, cost-effective, and well-tolerated nonpharmacologic alternative, such as phototherapy, is highly desirable and should be optimized. Unfortunately, most studies that report the effectiveness of phototherapy are in younger populations.
This retrospective study aimed to (1) identify the most common dermatologic conditions treated with phototherapy in older adults, (2) examine the effectiveness and safety of phototherapy in older adults
Methods
Design, Setting, Sample, and Statistical Analysis
The institutional review boards of Kaiser Permanente Washington Health Research Institute, Seattle, and the University of Washington, Seattle, approved this study. It was conducted in a large US multispecialty health care system (Group Health, Seattle, Washington [now Kaiser Permanente Washington]) serving approximately 600,000 patients, using billing records to identify all patients treated with phototherapy between January 1, 2015, and December 31, 2015, all who received narrowband UVB (NB-UVB) phototherapy. All adults 65 years and older who received phototherapy treatment during the 12-month study period were included. Patients were included regardless of comorbidities and other dermatologic treatments to maintain as much uniformity as possible between the present study and 2 prior studies examining phototherapy in older adult populations in the United Kingdom16 and Turkey.17 Demographic and clinical factors were presented using frequencies (percentages) or means and medians as appropriate. Comparisons of dermatologic conditions and clearance levels used a Fisher exact test. The number of phototherapy treatments to clearance and total number of treatments were compared between groups of patients using independent sample t tests.
Phototherapy Protocol
All patients received treatments administered by specially trained phototherapy nurses using a Daavlin UV Series (The Daavlin Company) or an Ultralite unit (Ultralite Enterprises, Inc), both with 48 lamps. All phototherapy nurses had been previously trained to provide treatments based on standardized protocols (Table 1) and to determine the patient’s level of disease clearance using a high to low clearance scale (Table 2). Daavlin’s treatment protocols were built into the software that accompanied the units and were developed based on the American Academy of Dermatology guidelines. The starting dose for an individual patient was determined based on the estimated


Results
Patients
Billing records identified 229 total patients who received phototherapy in 2015, of whom 52 (22.7%) were at least 65 years old. The median age was 70 years (range, 65–91 years). Twenty-nine (56%) were men and 35 (67%) had previously received phototherapy treatments.
Dermatologic Conditions Treated With Phototherapy
Our primary aim was to identify the most common dermatologic conditions treated with phototherapy in older adults. Psoriasis and dermatitis were the most common conditions treated in the sample (50% [26/52] and 21% [11/52], respectively), with mycosis fungoides being the third most common (10% [5/52]) and vitiligo tied with prurigo nodularis as fourth most common (6% [3/52])(Figure 1).

Effectiveness and Safety of Phototherapy
Our secondary aim was to examine the effectiveness and safety of phototherapy in older adults. Phototherapy was effective in this population, with 50 of 52 patients (96%) achieving a high or medium level of clearance. The degree of clearance for each of the dermatologic conditions is shown in Figure 2. Psoriasis and dermatitis achieved high clearance rates in 81% (21/26) and 82% (9/11) of patients, respectively. Overall, conditions did not have significant differences in clearances rates (Fisher exact test, P=.10). On average, it took patients 33 treatments to achieve medium or high rates of clearance. Psoriasis cleared more quickly, with an average of 30.4 treatments vs 36.1 treatments for other conditions, but the difference was not significant (t test, P=.26). Patients received an average of 98 total phototherapy treatments; the median number of treatments was 81 due to many being on maintenance therapy over several months. There was no relationship between a history of treatment with phototherapy and the total number of treatments needed to achieve clearance (t test, P=.40), but interestingly, those who had a history of phototherapy took approximately 5 more treatments to achieve clearance. The present study found that a slightly larger number of men were being treated for psoriasis (15 men vs 11 women), but there was no significant difference in response rate based on gender.

Side effects from phototherapy were minimal; 24 patients (46%) experienced grade 1 (mild) erythema at some point during their treatment course. Thirteen (25%) patients experienced grade 2 erythema, but this was a rare event for most patients. Only 1 (2%) patient experienced grade 3 erythema 1 time. Three patients experienced increased itching (6%). Thirteen (25%) patients had no side effects. None developed severe erythema or blisters, and none discontinued phototherapy because of side effects. Over the course of the study year, we found a high degree of acceptance of phototherapy treatments by older patients: 22 (42%) completed therapy after achieving clearance, 10 (19%) were continuing ongoing treatments (maintenance), and 15 (29%) stopped because of life circumstances (eg, other health issues, moving out of the area). Only 4 (8%) stopped because of a lack of effectiveness, and 1 (2%) patient because the treatments were burdensome.
Comparison of Outcomes
Our third aim was to compare the outcomes with similar studies in the United Kingdom16 and Turkey.17 This study confirmed that phototherapy is being used in older adults (22.7% of this study’s total patients) and is an effective treatment for older patients experiencing a range of challenging inflammatory and proliferative skin diseases similar to studies in the general population. Prior phototherapy studies in elderly patients also found psoriasis to be the most common skin condition treated, with 1 study finding that 51% (19/37) of older phototherapy patients had psoriasis,16 while another reported 58% (37/95) of older phototherapy patients had psoriasis.17 These numbers are similar to those in our study, which showed 50% (26/52) of elderly phototherapy patients had psoriasis. Psoriasis is the main indication for treatment with NB-UVB phototherapy in the general population,19 and because the risk for psoriasis increases with age,20 it is not surprising that all 3 studies found psoriasis to be the most common indication in elderly phototherapy patients. Table 3 provides further details on conditions treated in all 3 studies.

Comment
Our study found that 94% of patients with psoriasis achieved clearance with an average of 30.4 treatments, which is comparable to the reported 91% response rate with an average of 30 treatments in the United Kingdom.16 The other similar study in Turkey17 reported 73.7% of psoriasis patients achieved a 75% or more improvement from baseline with an average of 42 treatments, which may reflect underlying differences in regional skin type. Of note, the scatter chart (Figure 3) shows that several patients in the present study’s analysis are listed as not clear, but many of those patients had low treatment numbers below the mean time to clearance. Thus, the present study’s response rate may have been underestimated.

In the general population, studies show that psoriasis treated with standardized phototherapy protocols typically clears with an average of 20.6 treatments.21 The levels of clearance were similar in our study’s older population, but more treatments were required to achieve those results, with an average of 10 more treatments needed (an additional 3.3 weeks). Similar results were found in this sample for dermatitis and mycosis fungoides, indicating comparable clearance rates and levels but a need for more treatments to achieve similar results compared to the general population.
Additionally, in the current study more patients experienced grade 1 (mild) erythema (46%) and grade 2 erythema (25%) at some point in their treatment compared with the United Kingdom16 (1.89%) and Turkey17 (35%) studies, though these side effects did not impact the clearance rate. Interestingly, the current study’s scatter chart (Figure 3) illustrates that this side effect did not seem to increase with aging in this population. If anything, the erythema response was more prevalent in the median or younger patients in the sample. Erythema may have been due to the frequent use of photosensitizing medications in older adults in the United States, some of which typically get discontinued in patients 75 years and older (eg, statins). Other potential causes might include the use of phototype vs minimal erythema dose–driven protocols, the standard utilization of protocols originally designed for psoriasis vs other condition-specific protocols, missed treatments leading to increased sensitivity, or possibly shielding mishaps (eg, not wearing a prescribed face shield). Given the number of potential causes and the possibility of overlapping factors, careful analysis is important. With NB-UVB phototherapy, near-erythemogenic doses are optimal to achieve effective treatments, but this delicate balance may be more problematic for older adults. Future studies are needed to fully determine the factors at play for this population. In the interim, it is important for phototherapy-trained nurses to consider this risk carefully in the older population. They must follow the prescribed protocols that guide them to query patients about their responses to the prior treatment (eg, erythema, tenderness, itching), photosensitizing medications, missed treatments, and placement of shielding, and then adjust the treatment dosing accordingly.
Limitations
This study had several limitations. Although clinical outcomes were recorded prospectively, the analysis was retrospective, unblinded, and not placebo controlled. It was conducted in a single organization (Group Health [now Kaiser Permanente Washington]) but did analyze data from 4 medical centers in different cities with diverse demographics and a variety of nursing staff providing the treatments. Although the vitiligo treatment protocol likely slowed the response rate for those patients with vitiligo, the numbers were small (ie, only 3 of 52 patients), so the researchers chose to include them in the current study. The sample population was relatively small, but when these data are evaluated alongside the studies in the United Kingdom16 and Turkey,17 they show a consistent picture illustrating the effectiveness and safety of phototherapy in the older population. Further epidemiologic studies could be helpful to further describe the usefulness of this modality compared with other treatments for a variety of dermatoses in this age group. Supplementary analysis specifically examining the relationship between the number and type of photosensitizing medications, frequency of erythema, and time to clearance also could be useful.
Conclusion
Older adults with a variety of dermatoses respond well to phototherapy and should have the opportunity to use it, particularly considering the potential for increased complications and costs from other treatment modalities, such as commonly used immunosuppressive pharmaceuticals. However, the current study and the comparison studies indicate that it is important to carefully consider the slower clearance rates and the potential risk for increased erythema in this population and adjust patient education and treatment dosing accordingly.
Unfortunately, many dermatology centers do not offer phototherapy because of infrastructure limitations such as space and specially trained nursing staff. Increasing accessibility of phototherapy for older adults through home treatments may be an alternative, given its effectiveness in the general population.22,23 In addition, home phototherapy may be worth pursuing for the older population considering the challenges they may face with transportation to the clinic setting and their increased risk for serious illness if exposed to infections such as COVID-19. The COVID-19 pandemic has brought to light the need for reliable, safe, and effective treatments that can be utilized in the safety of patients’ homes and should therefore be considered as an option for older adults. Issues such as mobility and cognitive decline could pose some complicating factors, but with the help of a well-trained family member or caregiver, home phototherapy could be a viable option that improves accessibility for older patients. Future research opportunities include further examination of the slower but ultimately equivalent response to phototherapy in the older population, the influence of photosensitizing medications on phototherapy effects, and the impact of phototherapy on utilization of immunosuppressive pharmaceuticals in older adults.
- British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327-330.
Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE. 2017;12:e0181813. doi:10.1371/journal.pone.0181813 - Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2015;29:19-23.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593. - Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Martin JA, Laube S, Edwards C, et al. Rate of acute adverse events for narrow-band UVB and psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:68-72.
- Mokos ZB, Jovic A, Ceovic R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Exp Opin Biol Ther. 2018;18:897-903.
- Napolitano M, Balato N, Ayala F, et al. Psoriasis in elderly and non-elderly population: clinical and molecular features. G Ital Dermatol Venereol. 2016;151:587-595.
- Grozdev IS, Van Voorhees AS, Gottlieb AB, et al. Psoriasis in the elderly: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2011;65:537-545.
- Click J, Alabaster A, Postlethwaite D, et al. Effect of availability of at-home phototherapy on the use of systemic medications for psoriasis.
Photodermatol Photoimmunol Photomed. 2017;33:345-346. - Piaserico S, Conti A, Lo Console F, et al.
Efficacy and safety of systemic treatments for psoriasis in elderly. Acta Derm Venereol. 2014;94:293-297. - Soliman A, Nofal E, Nofal A, et al. Combination therapy of methotrexate plus NB-UVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat. 2015;26:528-534.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:66-80.
- Ibbotson SH. A perspective on the use of NB-UVB phototherapy vs. PUVA photochemotherapy. Front Med (Lausanne). 2018;5:184.
- Bell LM, Sedlack R, Beard CM, et al. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127:1184-1187.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413.
- Cameron H, Yule S, Dawe RS, et al. Review of an established UK home phototherapy service 1998-2011: improving access to a cost-effective treatment for chronic skin disease. Public Health. 2014;128:317-324.
- Matthews SW, Simmer M, Williams L, et al. Transition of patients with psoriasis from office-based phototherapy to nurse-supported home phototherapy: a pilot study. JDNA. 2018;10:29-41.
- British Photodermatology Group. An appraisal of narrowband (TL-01) UVB phototherapy. British Photodermatology Group Workshop Report (April 1996). Br J Dermatol. 1997;137:327-330.
Foerster J, Boswell K, West J, et al. Narrowband UVB treatment is highly effective and causes a strong reduction in the use of steroid and other creams in psoriasis patients in clinical practice. PLoS ONE. 2017;12:e0181813. doi:10.1371/journal.pone.0181813 - Fernández-Guarino M, Aboin-Gonzalez S, Barchino L, et al. Treatment of moderate and severe adult chronic atopic dermatitis with narrow-band UVB and the combination of narrow-band UVB/UVA phototherapy. Dermatol Ther. 2015;29:19-23.
- Ryu HH, Choe YS, Jo S, et al. Remission period in psoriasis after multiple cycles of narrowband ultraviolet B phototherapy. J Dermatol. 2014;41:622-627.
Tintle S, Shemer A, Suárez-Fariñas M, et al. Reversal of atopic dermatitis with narrow-band UVB phototherapy and biomarkers for therapeutic response. J Allergy Clin Immunol. 2011;128:583-593. - Gambichler T, Breuckmann F, Boms S, et al. Narrowband UVB phototherapy in skin conditions beyond psoriasis. J Am Acad Dermatol. 2005;52:660-670.
- Schneider LA, Hinrichs R, Scharffetter-Kochanek K. Phototherapy and photochemotherapy. Clin Dermatol. 2008;26:464-476.
- Martin JA, Laube S, Edwards C, et al. Rate of acute adverse events for narrow-band UVB and psoralen-UVA phototherapy. Photodermatol Photoimmunol Photomed. 2007;23:68-72.
- Mokos ZB, Jovic A, Ceovic R, et al. Therapeutic challenges in the mature patient. Clin Dermatol. 2018;36:128-139.
- Di Lernia V, Goldust M. An overview of the efficacy and safety of systemic treatments for psoriasis in the elderly. Exp Opin Biol Ther. 2018;18:897-903.
- Napolitano M, Balato N, Ayala F, et al. Psoriasis in elderly and non-elderly population: clinical and molecular features. G Ital Dermatol Venereol. 2016;151:587-595.
- Grozdev IS, Van Voorhees AS, Gottlieb AB, et al. Psoriasis in the elderly: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2011;65:537-545.
- Click J, Alabaster A, Postlethwaite D, et al. Effect of availability of at-home phototherapy on the use of systemic medications for psoriasis.
Photodermatol Photoimmunol Photomed. 2017;33:345-346. - Piaserico S, Conti A, Lo Console F, et al.
Efficacy and safety of systemic treatments for psoriasis in elderly. Acta Derm Venereol. 2014;94:293-297. - Soliman A, Nofal E, Nofal A, et al. Combination therapy of methotrexate plus NB-UVB phototherapy is more effective than methotrexate monotherapy in the treatment of chronic plaque psoriasis. J Dermatol Treat. 2015;26:528-534.
- Powell JB, Gach JE. Phototherapy in the elderly. Clin Exp Dermatol. 2015;40:605-610.
- Bulur I, Erdogan HK, Aksu AE, et al. The efficacy and safety of phototherapy in geriatric patients: a retrospective study. An Bras Dermatol. 2018;93:33-38.
- Madigan LM, Al-Jamal M, Hamzavi I. Exploring the gaps in the evidence-based application of narrowband UVB for the treatment of vitiligo. Photodermatol Photoimmunol Photomed. 2016;32:66-80.
- Ibbotson SH. A perspective on the use of NB-UVB phototherapy vs. PUVA photochemotherapy. Front Med (Lausanne). 2018;5:184.
- Bell LM, Sedlack R, Beard CM, et al. Incidence of psoriasis in Rochester, Minn, 1980-1983. Arch Dermatol. 1991;127:1184-1187.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413.
- Cameron H, Yule S, Dawe RS, et al. Review of an established UK home phototherapy service 1998-2011: improving access to a cost-effective treatment for chronic skin disease. Public Health. 2014;128:317-324.
- Matthews SW, Simmer M, Williams L, et al. Transition of patients with psoriasis from office-based phototherapy to nurse-supported home phototherapy: a pilot study. JDNA. 2018;10:29-41.
Practice Points
- With appropriate nursing care, phototherapy can be safe and effective for a variety of conditions in elderly patients.
- Compared to younger patients, elderly patients may need more sessions to achieve comparable clearance rates.
- The increased prevalence of photosensitizing medications in the elderly population will require careful adjustments in dosing.
Are you at legal risk for speaking at conferences?
When Jerry Gardner, MD, and a junior colleague received the acceptance notification for their abstract to be presented at Digestive Diseases Week® (DDW) 2021, a clause in the mandatory participation agreement gave Dr. Gardner pause. It required his colleague, as the submitting author, to completely accept any and all legal responsibility for any claims that might arise out of their presentation.
The clause was a red flag to Dr. Gardner, president of Science for Organizations, a Mill Valley, Calif.–based consulting firm. The gastroenterologist and former head of the digestive diseases branch at the National Institute of Diabetes and Digestive and Kidney Diseases – who has made hundreds of presentations and had participated in DDW for 40 years – had never encountered such a broad indemnity clause.
This news organization investigated just how risky it is to make a presentation at a conference – more than a dozen professional societies were contacted. Although DDW declined to discuss its agreement, Houston health care attorney Rachel V. Rose said that Dr. Gardner was smart to be cautious. “I would not sign that agreement. I have never seen anything that broad and all encompassing,” she said.
The DDW requirement “means that participants must put themselves at great potential financial risk in order to present their work,” Dr. Gardner said. He added that he and his colleague would not have submitted an abstract had they known about the indemnification clause up front.
Dr. Gardner advised his colleague not to sign the DDW agreement. She did not, and both missed the meeting.
Speakers ‘have to be careful’
Dr. Gardner may be an exception. How many doctors are willing to forgo a presentation because of a concern about something in an agreement?
John Mandrola, MD, said he operates under the assumption that if he does not sign the agreement, then he won’t be able to give his presentation. He admits that he generally just signs them and is careful with his presentations. “I’ve never really paid much attention to them,” said Dr. Mandrola, a cardiac electrophysiologist in Louisville, Ky., and chief cardiology correspondent for Medscape.
Not everyone takes that approach. “I do think that people read them, but they also take them with a grain of salt,” said E. Magnus Ohman, MBBS, professor of medicine at Duke University, Durham, N.C. He said he’s pragmatic and regards the agreements as a necessary evil in a litigious nation. Speakers “have to be careful, obviously,” Dr. Ohman said in an interview.
Some argue that the requirements are not only fair but also understandable. David Johnson, MD, a former president of the American College of Gastroenterology, said he has never had questions about agreements for meetings he has been involved with. “To me, this is not anything other than standard operating procedure,” he said.
Presenters participate by invitation, noted Dr. Johnson, a professor of medicine and chief of gastroenterology at the Eastern Virginia Medical School, Norfolk, who is a contributor to this news organization. “If they stand up and do something egregious, I would concur that the society should not be liable,” he said.
Big asks, big secrecy
Even for those who generally agree with Dr. Johnson’s position, it may be hard to completely understand what’s at stake without an attorney.
Although many declined to discuss their policies, a handful of professional societies provided their agreements for review. In general, the agreements appear to offer broad protection and rights to the organizers and large liability exposure for the participants. Participants are charged with a wide range of responsibilities, such as ensuring against copyright violations and intellectual property infringement, and that they also agree to unlimited use of their presentations and their name and likeness.
The American Academy of Neurology, which held its meeting virtually in 2021, required participants to indemnify the organization against all “losses, expenses, damages, or liabilities,” including “reasonable attorneys’ fees.” Federal employees, however, could opt out of indemnification.
The American Society of Clinical Oncology said that it does not usually require indemnification from its meeting participants. However, a spokesperson noted that ASCO did require participants at its 2021 virtual meeting to abide by the terms of use for content posted to the ASCO website. Those terms specify that users agree to indemnify ASCO from damages related to posts.
The American Psychiatric Association said it does not require any indemnification but did not make its agreement available. The American Academy of Pediatrics also said it did not require indemnification but would not share its agreement.
An American Diabetes Association spokesperson said that “every association is different in what they ask or require from speakers,” but would not share its requirements.
The American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American College of Physicians, and the Endocrine Society all declined to participate.
The organizations that withheld agreements “probably don’t want anybody picking apart their documents,” said Kyle Claussen, CEO of the Resolve Physician Agency, which reviews employment contracts and other contracts for physicians. “The more fair a document, the more likely they would be willing to disclose that, because they have nothing to hide,” he said.
‘It’s all on you’
Requiring indemnification for any and all aspects of a presentation appears to be increasingly common, said the attorneys interviewed for this article. As organizations repackage meeting presentations for sale, they put the content further out into the world and for a longer period, which increases liability exposure.
“If I’m the attorney for DDW, I certainly think I’d want to have this in place,” said Mr. Claussen.
“It’s good business sense for them because it reduces their risk,” said Courtney H. A. Thompson, an attorney with Fredrikson & Byron in Minneapolis, who advises regional and national corporations and ad agencies on advertising, marketing, and trademark law. She also works with clients who speak at meetings and who thus encounter meeting agreements.
Ms. Thompson said indemnity clauses have become fairly common over the past decade, especially as more companies and organizations have sought to protect trademarks, copyrights, and intellectual property and to minimize litigation costs.
A conference organizer “doesn’t want a third party to come after them for intellectual property, privacy, or publicity right infringement based on the participation of the customer or, in this case, the speaker,” said Ms. Thompson.
The agreements also reflect America’s litigation-prone culture.
Dean Fanelli, a patent attorney in the Washington, D.C., office of Cooley LLP, said the agreements he’s been asked to sign as a speaker increasingly seem “overly lawyerly.”
Two decades ago, a speaker might have been asked to sign a paragraph or a one-page form. Now “they often look more like formalized legal agreements,” Mr. Fanelli told this news organization.
The DDW agreement, for instance, ran four pages and contained 21 detailed clauses.
The increasingly complicated agreements “are a little over the top,” said Mr. Fanelli. But as an attorney who works with clients in the pharmaceutical industry, he said he understands that meeting organizers want to protect their rights.
DDW’s main indemnification clause requires the participant to indemnify DDW and its agents, directors, and employees “against any and all claims, demands, causes of action, losses, damages, liabilities, costs, and expenses,” including attorneys’ fees “arising out of a claim, action or proceeding” based on a breach or “alleged breach” by the participant.
“You’re releasing this information to them and then you’re also giving them blanket indemnity back, saying if there’s any type of intellectual property violation on your end – if you’ve included any type of work that’s protected, if this causes any problems – it’s all on you,” said Mr. Claussen.
Other potential pitfalls
Aside from indemnification, participation agreements can contain other potentially worrisome clauses, including onerous terms for cancellation and reuse of content without remuneration.
DDW requires royalty-free licensing of a speaker’s content; the organization can reproduce it in perpetuity without royalties. Many organizations have such a clause in their agreements, including the AAN and the American College of Cardiology.
ASCO’s general authorization form for meeting participants requires that they assign to ASCO rights to their content “perpetually, irrevocably, worldwide and royalty free.” Participants can contact the organization if they seek to opt out, but it’s not clear whether ASCO grants such requests.
Participants in the upcoming American Heart Association annual meeting can deny permission to record their presentation. But if they allow recording and do not agree to assign all rights and copyright ownership to the AHA, the work will be excluded from publication in the meeting program, e-posters, and the meeting supplement in Circulation.
Mr. Claussen said granting royalty-free rights presents a conundrum. Having content reproduced in various formats “might be better for your personal brand,” but it’s not likely to result in any direct compensation and could increase liability exposure, he said.
How presenters must prepare
Mr. Claussen and Ms. Rose said speakers should be vigilant about their own rights and responsibilities, including ensuring that they do not violate copyrights or infringe on intellectual property rights.
“I would recommend that folks be meticulous about what is in their slide deck and materials,” said Ms. Thompson. He said that presenters should be sure they have the right to share material. Technologies crawl the internet seeking out infringement, which often leads to cease and desist letters from attorneys, she said.
It’s better to head off such a letter, Ms. Thompson said. “You need to defend it whether or not it’s a viable claim,” and that can be costly, she said.
Both Ms. Thompson and Mr. Fanelli also warn about disclosing anything that might be considered a trade secret. Many agreements prohibit presenters from engaging in commercial promotion, but if a talk includes information about a drug or device, the manufacturer will want to review the presentation before it’s made public, said Mr. Fanelli.
Many organizations prohibit attendees from photographing, recording, or tweeting at meetings and often require speakers to warn the audience about doing so. DDW goes further by holding presenters liable if someone violates the rule.
“That’s a huge problem,” said Dr. Mandrola. He noted that although it might be easy to police journalists attending a meeting, “it seems hard to enforce that rule amongst just regular attendees.”
Accept or negotiate?
Individuals who submit work to an organization might feel they must sign an agreement as is, especially if they are looking to advance their career or expand knowledge by presenting work at a meeting. But some attorneys said it might be possible to negotiate with meeting organizers.
“My personal opinion is that it never hurts to ask,” said Ms. Thompson. If she were speaking at a legal conference, she would mark up a contract and “see what happens.” The more times pushback is accepted – say, if it works with three out of five speaking engagements – the more it reduces overall liability exposure.
Mr. Fanelli, however, said that although he always reads over an agreement, he typically signs without negotiating. “I don’t usually worry about it because I’m just trying to talk at a particular seminar,” he said.
Prospective presenters “have to weigh that balance – do you want to talk at a seminar, or are you concerned about the legal issues?” said Mr. Fanelli.
If in doubt, talk with a lawyer.
“If you ever have a question on whether or not you should consult an attorney, the answer is always yes,” said Mr. Claussen. It would be “an ounce of prevention,” especially if it’s just a short agreement, he said.
Dr. Ohman, however, said that he believed “it would be fairly costly” and potentially unwieldy. “You can’t litigate everything in life,” he added.
As for Dr. Gardner, he said he would not be as likely to attend DDW in the future if he has to agree to cover any and all liability. “I can’t conceive of ever agreeing to personally indemnify DDW in order to make a presentation at the annual meeting,” he said.
A version of this article first appeared on Medscape.com.
When Jerry Gardner, MD, and a junior colleague received the acceptance notification for their abstract to be presented at Digestive Diseases Week® (DDW) 2021, a clause in the mandatory participation agreement gave Dr. Gardner pause. It required his colleague, as the submitting author, to completely accept any and all legal responsibility for any claims that might arise out of their presentation.
The clause was a red flag to Dr. Gardner, president of Science for Organizations, a Mill Valley, Calif.–based consulting firm. The gastroenterologist and former head of the digestive diseases branch at the National Institute of Diabetes and Digestive and Kidney Diseases – who has made hundreds of presentations and had participated in DDW for 40 years – had never encountered such a broad indemnity clause.
This news organization investigated just how risky it is to make a presentation at a conference – more than a dozen professional societies were contacted. Although DDW declined to discuss its agreement, Houston health care attorney Rachel V. Rose said that Dr. Gardner was smart to be cautious. “I would not sign that agreement. I have never seen anything that broad and all encompassing,” she said.
The DDW requirement “means that participants must put themselves at great potential financial risk in order to present their work,” Dr. Gardner said. He added that he and his colleague would not have submitted an abstract had they known about the indemnification clause up front.
Dr. Gardner advised his colleague not to sign the DDW agreement. She did not, and both missed the meeting.
Speakers ‘have to be careful’
Dr. Gardner may be an exception. How many doctors are willing to forgo a presentation because of a concern about something in an agreement?
John Mandrola, MD, said he operates under the assumption that if he does not sign the agreement, then he won’t be able to give his presentation. He admits that he generally just signs them and is careful with his presentations. “I’ve never really paid much attention to them,” said Dr. Mandrola, a cardiac electrophysiologist in Louisville, Ky., and chief cardiology correspondent for Medscape.
Not everyone takes that approach. “I do think that people read them, but they also take them with a grain of salt,” said E. Magnus Ohman, MBBS, professor of medicine at Duke University, Durham, N.C. He said he’s pragmatic and regards the agreements as a necessary evil in a litigious nation. Speakers “have to be careful, obviously,” Dr. Ohman said in an interview.
Some argue that the requirements are not only fair but also understandable. David Johnson, MD, a former president of the American College of Gastroenterology, said he has never had questions about agreements for meetings he has been involved with. “To me, this is not anything other than standard operating procedure,” he said.
Presenters participate by invitation, noted Dr. Johnson, a professor of medicine and chief of gastroenterology at the Eastern Virginia Medical School, Norfolk, who is a contributor to this news organization. “If they stand up and do something egregious, I would concur that the society should not be liable,” he said.
Big asks, big secrecy
Even for those who generally agree with Dr. Johnson’s position, it may be hard to completely understand what’s at stake without an attorney.
Although many declined to discuss their policies, a handful of professional societies provided their agreements for review. In general, the agreements appear to offer broad protection and rights to the organizers and large liability exposure for the participants. Participants are charged with a wide range of responsibilities, such as ensuring against copyright violations and intellectual property infringement, and that they also agree to unlimited use of their presentations and their name and likeness.
The American Academy of Neurology, which held its meeting virtually in 2021, required participants to indemnify the organization against all “losses, expenses, damages, or liabilities,” including “reasonable attorneys’ fees.” Federal employees, however, could opt out of indemnification.
The American Society of Clinical Oncology said that it does not usually require indemnification from its meeting participants. However, a spokesperson noted that ASCO did require participants at its 2021 virtual meeting to abide by the terms of use for content posted to the ASCO website. Those terms specify that users agree to indemnify ASCO from damages related to posts.
The American Psychiatric Association said it does not require any indemnification but did not make its agreement available. The American Academy of Pediatrics also said it did not require indemnification but would not share its agreement.
An American Diabetes Association spokesperson said that “every association is different in what they ask or require from speakers,” but would not share its requirements.
The American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American College of Physicians, and the Endocrine Society all declined to participate.
The organizations that withheld agreements “probably don’t want anybody picking apart their documents,” said Kyle Claussen, CEO of the Resolve Physician Agency, which reviews employment contracts and other contracts for physicians. “The more fair a document, the more likely they would be willing to disclose that, because they have nothing to hide,” he said.
‘It’s all on you’
Requiring indemnification for any and all aspects of a presentation appears to be increasingly common, said the attorneys interviewed for this article. As organizations repackage meeting presentations for sale, they put the content further out into the world and for a longer period, which increases liability exposure.
“If I’m the attorney for DDW, I certainly think I’d want to have this in place,” said Mr. Claussen.
“It’s good business sense for them because it reduces their risk,” said Courtney H. A. Thompson, an attorney with Fredrikson & Byron in Minneapolis, who advises regional and national corporations and ad agencies on advertising, marketing, and trademark law. She also works with clients who speak at meetings and who thus encounter meeting agreements.
Ms. Thompson said indemnity clauses have become fairly common over the past decade, especially as more companies and organizations have sought to protect trademarks, copyrights, and intellectual property and to minimize litigation costs.
A conference organizer “doesn’t want a third party to come after them for intellectual property, privacy, or publicity right infringement based on the participation of the customer or, in this case, the speaker,” said Ms. Thompson.
The agreements also reflect America’s litigation-prone culture.
Dean Fanelli, a patent attorney in the Washington, D.C., office of Cooley LLP, said the agreements he’s been asked to sign as a speaker increasingly seem “overly lawyerly.”
Two decades ago, a speaker might have been asked to sign a paragraph or a one-page form. Now “they often look more like formalized legal agreements,” Mr. Fanelli told this news organization.
The DDW agreement, for instance, ran four pages and contained 21 detailed clauses.
The increasingly complicated agreements “are a little over the top,” said Mr. Fanelli. But as an attorney who works with clients in the pharmaceutical industry, he said he understands that meeting organizers want to protect their rights.
DDW’s main indemnification clause requires the participant to indemnify DDW and its agents, directors, and employees “against any and all claims, demands, causes of action, losses, damages, liabilities, costs, and expenses,” including attorneys’ fees “arising out of a claim, action or proceeding” based on a breach or “alleged breach” by the participant.
“You’re releasing this information to them and then you’re also giving them blanket indemnity back, saying if there’s any type of intellectual property violation on your end – if you’ve included any type of work that’s protected, if this causes any problems – it’s all on you,” said Mr. Claussen.
Other potential pitfalls
Aside from indemnification, participation agreements can contain other potentially worrisome clauses, including onerous terms for cancellation and reuse of content without remuneration.
DDW requires royalty-free licensing of a speaker’s content; the organization can reproduce it in perpetuity without royalties. Many organizations have such a clause in their agreements, including the AAN and the American College of Cardiology.
ASCO’s general authorization form for meeting participants requires that they assign to ASCO rights to their content “perpetually, irrevocably, worldwide and royalty free.” Participants can contact the organization if they seek to opt out, but it’s not clear whether ASCO grants such requests.
Participants in the upcoming American Heart Association annual meeting can deny permission to record their presentation. But if they allow recording and do not agree to assign all rights and copyright ownership to the AHA, the work will be excluded from publication in the meeting program, e-posters, and the meeting supplement in Circulation.
Mr. Claussen said granting royalty-free rights presents a conundrum. Having content reproduced in various formats “might be better for your personal brand,” but it’s not likely to result in any direct compensation and could increase liability exposure, he said.
How presenters must prepare
Mr. Claussen and Ms. Rose said speakers should be vigilant about their own rights and responsibilities, including ensuring that they do not violate copyrights or infringe on intellectual property rights.
“I would recommend that folks be meticulous about what is in their slide deck and materials,” said Ms. Thompson. He said that presenters should be sure they have the right to share material. Technologies crawl the internet seeking out infringement, which often leads to cease and desist letters from attorneys, she said.
It’s better to head off such a letter, Ms. Thompson said. “You need to defend it whether or not it’s a viable claim,” and that can be costly, she said.
Both Ms. Thompson and Mr. Fanelli also warn about disclosing anything that might be considered a trade secret. Many agreements prohibit presenters from engaging in commercial promotion, but if a talk includes information about a drug or device, the manufacturer will want to review the presentation before it’s made public, said Mr. Fanelli.
Many organizations prohibit attendees from photographing, recording, or tweeting at meetings and often require speakers to warn the audience about doing so. DDW goes further by holding presenters liable if someone violates the rule.
“That’s a huge problem,” said Dr. Mandrola. He noted that although it might be easy to police journalists attending a meeting, “it seems hard to enforce that rule amongst just regular attendees.”
Accept or negotiate?
Individuals who submit work to an organization might feel they must sign an agreement as is, especially if they are looking to advance their career or expand knowledge by presenting work at a meeting. But some attorneys said it might be possible to negotiate with meeting organizers.
“My personal opinion is that it never hurts to ask,” said Ms. Thompson. If she were speaking at a legal conference, she would mark up a contract and “see what happens.” The more times pushback is accepted – say, if it works with three out of five speaking engagements – the more it reduces overall liability exposure.
Mr. Fanelli, however, said that although he always reads over an agreement, he typically signs without negotiating. “I don’t usually worry about it because I’m just trying to talk at a particular seminar,” he said.
Prospective presenters “have to weigh that balance – do you want to talk at a seminar, or are you concerned about the legal issues?” said Mr. Fanelli.
If in doubt, talk with a lawyer.
“If you ever have a question on whether or not you should consult an attorney, the answer is always yes,” said Mr. Claussen. It would be “an ounce of prevention,” especially if it’s just a short agreement, he said.
Dr. Ohman, however, said that he believed “it would be fairly costly” and potentially unwieldy. “You can’t litigate everything in life,” he added.
As for Dr. Gardner, he said he would not be as likely to attend DDW in the future if he has to agree to cover any and all liability. “I can’t conceive of ever agreeing to personally indemnify DDW in order to make a presentation at the annual meeting,” he said.
A version of this article first appeared on Medscape.com.
When Jerry Gardner, MD, and a junior colleague received the acceptance notification for their abstract to be presented at Digestive Diseases Week® (DDW) 2021, a clause in the mandatory participation agreement gave Dr. Gardner pause. It required his colleague, as the submitting author, to completely accept any and all legal responsibility for any claims that might arise out of their presentation.
The clause was a red flag to Dr. Gardner, president of Science for Organizations, a Mill Valley, Calif.–based consulting firm. The gastroenterologist and former head of the digestive diseases branch at the National Institute of Diabetes and Digestive and Kidney Diseases – who has made hundreds of presentations and had participated in DDW for 40 years – had never encountered such a broad indemnity clause.
This news organization investigated just how risky it is to make a presentation at a conference – more than a dozen professional societies were contacted. Although DDW declined to discuss its agreement, Houston health care attorney Rachel V. Rose said that Dr. Gardner was smart to be cautious. “I would not sign that agreement. I have never seen anything that broad and all encompassing,” she said.
The DDW requirement “means that participants must put themselves at great potential financial risk in order to present their work,” Dr. Gardner said. He added that he and his colleague would not have submitted an abstract had they known about the indemnification clause up front.
Dr. Gardner advised his colleague not to sign the DDW agreement. She did not, and both missed the meeting.
Speakers ‘have to be careful’
Dr. Gardner may be an exception. How many doctors are willing to forgo a presentation because of a concern about something in an agreement?
John Mandrola, MD, said he operates under the assumption that if he does not sign the agreement, then he won’t be able to give his presentation. He admits that he generally just signs them and is careful with his presentations. “I’ve never really paid much attention to them,” said Dr. Mandrola, a cardiac electrophysiologist in Louisville, Ky., and chief cardiology correspondent for Medscape.
Not everyone takes that approach. “I do think that people read them, but they also take them with a grain of salt,” said E. Magnus Ohman, MBBS, professor of medicine at Duke University, Durham, N.C. He said he’s pragmatic and regards the agreements as a necessary evil in a litigious nation. Speakers “have to be careful, obviously,” Dr. Ohman said in an interview.
Some argue that the requirements are not only fair but also understandable. David Johnson, MD, a former president of the American College of Gastroenterology, said he has never had questions about agreements for meetings he has been involved with. “To me, this is not anything other than standard operating procedure,” he said.
Presenters participate by invitation, noted Dr. Johnson, a professor of medicine and chief of gastroenterology at the Eastern Virginia Medical School, Norfolk, who is a contributor to this news organization. “If they stand up and do something egregious, I would concur that the society should not be liable,” he said.
Big asks, big secrecy
Even for those who generally agree with Dr. Johnson’s position, it may be hard to completely understand what’s at stake without an attorney.
Although many declined to discuss their policies, a handful of professional societies provided their agreements for review. In general, the agreements appear to offer broad protection and rights to the organizers and large liability exposure for the participants. Participants are charged with a wide range of responsibilities, such as ensuring against copyright violations and intellectual property infringement, and that they also agree to unlimited use of their presentations and their name and likeness.
The American Academy of Neurology, which held its meeting virtually in 2021, required participants to indemnify the organization against all “losses, expenses, damages, or liabilities,” including “reasonable attorneys’ fees.” Federal employees, however, could opt out of indemnification.
The American Society of Clinical Oncology said that it does not usually require indemnification from its meeting participants. However, a spokesperson noted that ASCO did require participants at its 2021 virtual meeting to abide by the terms of use for content posted to the ASCO website. Those terms specify that users agree to indemnify ASCO from damages related to posts.
The American Psychiatric Association said it does not require any indemnification but did not make its agreement available. The American Academy of Pediatrics also said it did not require indemnification but would not share its agreement.
An American Diabetes Association spokesperson said that “every association is different in what they ask or require from speakers,” but would not share its requirements.
The American Academy of Family Physicians, the American College of Obstetricians and Gynecologists, the American College of Physicians, and the Endocrine Society all declined to participate.
The organizations that withheld agreements “probably don’t want anybody picking apart their documents,” said Kyle Claussen, CEO of the Resolve Physician Agency, which reviews employment contracts and other contracts for physicians. “The more fair a document, the more likely they would be willing to disclose that, because they have nothing to hide,” he said.
‘It’s all on you’
Requiring indemnification for any and all aspects of a presentation appears to be increasingly common, said the attorneys interviewed for this article. As organizations repackage meeting presentations for sale, they put the content further out into the world and for a longer period, which increases liability exposure.
“If I’m the attorney for DDW, I certainly think I’d want to have this in place,” said Mr. Claussen.
“It’s good business sense for them because it reduces their risk,” said Courtney H. A. Thompson, an attorney with Fredrikson & Byron in Minneapolis, who advises regional and national corporations and ad agencies on advertising, marketing, and trademark law. She also works with clients who speak at meetings and who thus encounter meeting agreements.
Ms. Thompson said indemnity clauses have become fairly common over the past decade, especially as more companies and organizations have sought to protect trademarks, copyrights, and intellectual property and to minimize litigation costs.
A conference organizer “doesn’t want a third party to come after them for intellectual property, privacy, or publicity right infringement based on the participation of the customer or, in this case, the speaker,” said Ms. Thompson.
The agreements also reflect America’s litigation-prone culture.
Dean Fanelli, a patent attorney in the Washington, D.C., office of Cooley LLP, said the agreements he’s been asked to sign as a speaker increasingly seem “overly lawyerly.”
Two decades ago, a speaker might have been asked to sign a paragraph or a one-page form. Now “they often look more like formalized legal agreements,” Mr. Fanelli told this news organization.
The DDW agreement, for instance, ran four pages and contained 21 detailed clauses.
The increasingly complicated agreements “are a little over the top,” said Mr. Fanelli. But as an attorney who works with clients in the pharmaceutical industry, he said he understands that meeting organizers want to protect their rights.
DDW’s main indemnification clause requires the participant to indemnify DDW and its agents, directors, and employees “against any and all claims, demands, causes of action, losses, damages, liabilities, costs, and expenses,” including attorneys’ fees “arising out of a claim, action or proceeding” based on a breach or “alleged breach” by the participant.
“You’re releasing this information to them and then you’re also giving them blanket indemnity back, saying if there’s any type of intellectual property violation on your end – if you’ve included any type of work that’s protected, if this causes any problems – it’s all on you,” said Mr. Claussen.
Other potential pitfalls
Aside from indemnification, participation agreements can contain other potentially worrisome clauses, including onerous terms for cancellation and reuse of content without remuneration.
DDW requires royalty-free licensing of a speaker’s content; the organization can reproduce it in perpetuity without royalties. Many organizations have such a clause in their agreements, including the AAN and the American College of Cardiology.
ASCO’s general authorization form for meeting participants requires that they assign to ASCO rights to their content “perpetually, irrevocably, worldwide and royalty free.” Participants can contact the organization if they seek to opt out, but it’s not clear whether ASCO grants such requests.
Participants in the upcoming American Heart Association annual meeting can deny permission to record their presentation. But if they allow recording and do not agree to assign all rights and copyright ownership to the AHA, the work will be excluded from publication in the meeting program, e-posters, and the meeting supplement in Circulation.
Mr. Claussen said granting royalty-free rights presents a conundrum. Having content reproduced in various formats “might be better for your personal brand,” but it’s not likely to result in any direct compensation and could increase liability exposure, he said.
How presenters must prepare
Mr. Claussen and Ms. Rose said speakers should be vigilant about their own rights and responsibilities, including ensuring that they do not violate copyrights or infringe on intellectual property rights.
“I would recommend that folks be meticulous about what is in their slide deck and materials,” said Ms. Thompson. He said that presenters should be sure they have the right to share material. Technologies crawl the internet seeking out infringement, which often leads to cease and desist letters from attorneys, she said.
It’s better to head off such a letter, Ms. Thompson said. “You need to defend it whether or not it’s a viable claim,” and that can be costly, she said.
Both Ms. Thompson and Mr. Fanelli also warn about disclosing anything that might be considered a trade secret. Many agreements prohibit presenters from engaging in commercial promotion, but if a talk includes information about a drug or device, the manufacturer will want to review the presentation before it’s made public, said Mr. Fanelli.
Many organizations prohibit attendees from photographing, recording, or tweeting at meetings and often require speakers to warn the audience about doing so. DDW goes further by holding presenters liable if someone violates the rule.
“That’s a huge problem,” said Dr. Mandrola. He noted that although it might be easy to police journalists attending a meeting, “it seems hard to enforce that rule amongst just regular attendees.”
Accept or negotiate?
Individuals who submit work to an organization might feel they must sign an agreement as is, especially if they are looking to advance their career or expand knowledge by presenting work at a meeting. But some attorneys said it might be possible to negotiate with meeting organizers.
“My personal opinion is that it never hurts to ask,” said Ms. Thompson. If she were speaking at a legal conference, she would mark up a contract and “see what happens.” The more times pushback is accepted – say, if it works with three out of five speaking engagements – the more it reduces overall liability exposure.
Mr. Fanelli, however, said that although he always reads over an agreement, he typically signs without negotiating. “I don’t usually worry about it because I’m just trying to talk at a particular seminar,” he said.
Prospective presenters “have to weigh that balance – do you want to talk at a seminar, or are you concerned about the legal issues?” said Mr. Fanelli.
If in doubt, talk with a lawyer.
“If you ever have a question on whether or not you should consult an attorney, the answer is always yes,” said Mr. Claussen. It would be “an ounce of prevention,” especially if it’s just a short agreement, he said.
Dr. Ohman, however, said that he believed “it would be fairly costly” and potentially unwieldy. “You can’t litigate everything in life,” he added.
As for Dr. Gardner, he said he would not be as likely to attend DDW in the future if he has to agree to cover any and all liability. “I can’t conceive of ever agreeing to personally indemnify DDW in order to make a presentation at the annual meeting,” he said.
A version of this article first appeared on Medscape.com.
Common outcome measures for AD lack adequate reporting of race, skin tone
, according to results from a systematic review.
“AD is associated with considerable heterogeneity across different races and skin tones,” presenting study author Trisha Kaundinya said at the Revolutionizing Atopic Dermatitis symposium. “Compared with lighter skin tones, darker skin tones more commonly have diffuse xerosis, Dennis-Morgan lines, hyperlinearity of the palms, periorbital dark circles, lichenification, and prurigo nodularis. This heterogeneity can be challenging to assess in clinical trials and in practice.”
The Harmonizing Outcome Measures for Eczema (HOME) group has selected several scales by international consensus. For clinical trials, the group recommends the Patient-Oriented Eczema Measure (POEM), Eczema Area and Severity Index (EASI), and Dermatology Life Quality Index (DLQI). In clinical practice, the HOME group recommends the POEM, Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD), and the Numeric Rating Scale (NRS)-itch measures. “The psychometric validity and reliability of these outcome measures have undergone robust investigation before, but the validity and reliability of these outcome measures remains uncertain across different races, ethnicities, and skin tones,” Ms. Kaundinya said.
Jonathan Silverberg, MD, PhD, associate professor of dermatology at George Washington University, Washington, in collaboration with Andrew F. Alexis, MD, MPH, vice-chair for diversity and inclusion for the department of dermatology at Weill-Cornell Medicine, New York, and Jacob P. Thyssen, MD, PhD, at the University of Copenhagen, Denmark, sought to examine reporting of race, ethnicity, and skin tone, and to compare results across these groups from studies of psychometric properties for outcome measures in AD. Under the mentorship of Dr. Silverberg, Ms. Kaundinya, a medical student at Northwestern University, Chicago, and her research associates conducted a systematic review that searched PubMed and Embase and identified 165 relevant published studies of 41,146 individuals.
Of the individuals participating in these 165 studies, 73% had an unspecified racial background, 18% were White, 4% were Asian, 2% were Black, 2% were Hispanic, 1% were multiracial/other, and the remainder were American Indian/Alaskan Native. Only 55 of the studies (33%) reported the distribution of race or ethnicity, 5 (3%) reported the distribution of skin tone, and 16 (10%) reported psychometric differences in patients with different races, ethnicities, or skin tones. In addition, only 5 of 113 (4%) studies that did not report race, ethnicity, or skin tone–based differences acknowledged absence of stratification as a limitation.
Of note, significant differential item functioning was found between race subgroups for one or more items of the PO-SCORAD, the Patient-Reported Outcomes Measurement Information System (PROMIS) Itch Questionnaire (PIQ) Short Forms, POEM, DLQI, Hospital Anxiety and Depression Scale (HADS), Itchy Quality of Life (ItchyQOL) scale, 5-dimensions (5D) itch scale, Short Form (SF)-12, and NRS-itch. “Correlations of the POEM with the Investigator’s Global Assessment (IGA) differed the most between skin of color and lighter skin,” Ms. Kaundinya said.
“The POEM did seem to correlate similarly with the DLQI and the EASI in both white and nonwhite participants, which may indicate why this trifecta of instruments is recommended by the HOME group. One study found that substituting the erythema component of the EASI scale with greyness for darker skin, in which erythema is more challenging to assess, did not significantly improve the reliability of EASI. This indicates that further research is needed to investigate how EASI can be modified to perform better in darker skin tones.”
She pointed out that some studies of clinician-reported outcome measures were underpowered to detect meaningful differences between patient subgroups. “There were also insufficient data to perform meta-regression of differences between patient subgroups,” she said. “Overall, future studies are needed to determine whether outcome measures recommended by the HOME and other tools perform equally well across diverse patient populations. This systematic review indicates significant reporting and knowledge gaps for psychometric properties of outcome measures by race, ethnicity, or skin tone in AD.”
Ms. Kaundinya reported having no relevant financial disclosures. Dr. Silverberg, the study’s senior author, is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
, according to results from a systematic review.
“AD is associated with considerable heterogeneity across different races and skin tones,” presenting study author Trisha Kaundinya said at the Revolutionizing Atopic Dermatitis symposium. “Compared with lighter skin tones, darker skin tones more commonly have diffuse xerosis, Dennis-Morgan lines, hyperlinearity of the palms, periorbital dark circles, lichenification, and prurigo nodularis. This heterogeneity can be challenging to assess in clinical trials and in practice.”
The Harmonizing Outcome Measures for Eczema (HOME) group has selected several scales by international consensus. For clinical trials, the group recommends the Patient-Oriented Eczema Measure (POEM), Eczema Area and Severity Index (EASI), and Dermatology Life Quality Index (DLQI). In clinical practice, the HOME group recommends the POEM, Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD), and the Numeric Rating Scale (NRS)-itch measures. “The psychometric validity and reliability of these outcome measures have undergone robust investigation before, but the validity and reliability of these outcome measures remains uncertain across different races, ethnicities, and skin tones,” Ms. Kaundinya said.
Jonathan Silverberg, MD, PhD, associate professor of dermatology at George Washington University, Washington, in collaboration with Andrew F. Alexis, MD, MPH, vice-chair for diversity and inclusion for the department of dermatology at Weill-Cornell Medicine, New York, and Jacob P. Thyssen, MD, PhD, at the University of Copenhagen, Denmark, sought to examine reporting of race, ethnicity, and skin tone, and to compare results across these groups from studies of psychometric properties for outcome measures in AD. Under the mentorship of Dr. Silverberg, Ms. Kaundinya, a medical student at Northwestern University, Chicago, and her research associates conducted a systematic review that searched PubMed and Embase and identified 165 relevant published studies of 41,146 individuals.
Of the individuals participating in these 165 studies, 73% had an unspecified racial background, 18% were White, 4% were Asian, 2% were Black, 2% were Hispanic, 1% were multiracial/other, and the remainder were American Indian/Alaskan Native. Only 55 of the studies (33%) reported the distribution of race or ethnicity, 5 (3%) reported the distribution of skin tone, and 16 (10%) reported psychometric differences in patients with different races, ethnicities, or skin tones. In addition, only 5 of 113 (4%) studies that did not report race, ethnicity, or skin tone–based differences acknowledged absence of stratification as a limitation.
Of note, significant differential item functioning was found between race subgroups for one or more items of the PO-SCORAD, the Patient-Reported Outcomes Measurement Information System (PROMIS) Itch Questionnaire (PIQ) Short Forms, POEM, DLQI, Hospital Anxiety and Depression Scale (HADS), Itchy Quality of Life (ItchyQOL) scale, 5-dimensions (5D) itch scale, Short Form (SF)-12, and NRS-itch. “Correlations of the POEM with the Investigator’s Global Assessment (IGA) differed the most between skin of color and lighter skin,” Ms. Kaundinya said.
“The POEM did seem to correlate similarly with the DLQI and the EASI in both white and nonwhite participants, which may indicate why this trifecta of instruments is recommended by the HOME group. One study found that substituting the erythema component of the EASI scale with greyness for darker skin, in which erythema is more challenging to assess, did not significantly improve the reliability of EASI. This indicates that further research is needed to investigate how EASI can be modified to perform better in darker skin tones.”
She pointed out that some studies of clinician-reported outcome measures were underpowered to detect meaningful differences between patient subgroups. “There were also insufficient data to perform meta-regression of differences between patient subgroups,” she said. “Overall, future studies are needed to determine whether outcome measures recommended by the HOME and other tools perform equally well across diverse patient populations. This systematic review indicates significant reporting and knowledge gaps for psychometric properties of outcome measures by race, ethnicity, or skin tone in AD.”
Ms. Kaundinya reported having no relevant financial disclosures. Dr. Silverberg, the study’s senior author, is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
, according to results from a systematic review.
“AD is associated with considerable heterogeneity across different races and skin tones,” presenting study author Trisha Kaundinya said at the Revolutionizing Atopic Dermatitis symposium. “Compared with lighter skin tones, darker skin tones more commonly have diffuse xerosis, Dennis-Morgan lines, hyperlinearity of the palms, periorbital dark circles, lichenification, and prurigo nodularis. This heterogeneity can be challenging to assess in clinical trials and in practice.”
The Harmonizing Outcome Measures for Eczema (HOME) group has selected several scales by international consensus. For clinical trials, the group recommends the Patient-Oriented Eczema Measure (POEM), Eczema Area and Severity Index (EASI), and Dermatology Life Quality Index (DLQI). In clinical practice, the HOME group recommends the POEM, Patient-Oriented Scoring Atopic Dermatitis (PO-SCORAD), and the Numeric Rating Scale (NRS)-itch measures. “The psychometric validity and reliability of these outcome measures have undergone robust investigation before, but the validity and reliability of these outcome measures remains uncertain across different races, ethnicities, and skin tones,” Ms. Kaundinya said.
Jonathan Silverberg, MD, PhD, associate professor of dermatology at George Washington University, Washington, in collaboration with Andrew F. Alexis, MD, MPH, vice-chair for diversity and inclusion for the department of dermatology at Weill-Cornell Medicine, New York, and Jacob P. Thyssen, MD, PhD, at the University of Copenhagen, Denmark, sought to examine reporting of race, ethnicity, and skin tone, and to compare results across these groups from studies of psychometric properties for outcome measures in AD. Under the mentorship of Dr. Silverberg, Ms. Kaundinya, a medical student at Northwestern University, Chicago, and her research associates conducted a systematic review that searched PubMed and Embase and identified 165 relevant published studies of 41,146 individuals.
Of the individuals participating in these 165 studies, 73% had an unspecified racial background, 18% were White, 4% were Asian, 2% were Black, 2% were Hispanic, 1% were multiracial/other, and the remainder were American Indian/Alaskan Native. Only 55 of the studies (33%) reported the distribution of race or ethnicity, 5 (3%) reported the distribution of skin tone, and 16 (10%) reported psychometric differences in patients with different races, ethnicities, or skin tones. In addition, only 5 of 113 (4%) studies that did not report race, ethnicity, or skin tone–based differences acknowledged absence of stratification as a limitation.
Of note, significant differential item functioning was found between race subgroups for one or more items of the PO-SCORAD, the Patient-Reported Outcomes Measurement Information System (PROMIS) Itch Questionnaire (PIQ) Short Forms, POEM, DLQI, Hospital Anxiety and Depression Scale (HADS), Itchy Quality of Life (ItchyQOL) scale, 5-dimensions (5D) itch scale, Short Form (SF)-12, and NRS-itch. “Correlations of the POEM with the Investigator’s Global Assessment (IGA) differed the most between skin of color and lighter skin,” Ms. Kaundinya said.
“The POEM did seem to correlate similarly with the DLQI and the EASI in both white and nonwhite participants, which may indicate why this trifecta of instruments is recommended by the HOME group. One study found that substituting the erythema component of the EASI scale with greyness for darker skin, in which erythema is more challenging to assess, did not significantly improve the reliability of EASI. This indicates that further research is needed to investigate how EASI can be modified to perform better in darker skin tones.”
She pointed out that some studies of clinician-reported outcome measures were underpowered to detect meaningful differences between patient subgroups. “There were also insufficient data to perform meta-regression of differences between patient subgroups,” she said. “Overall, future studies are needed to determine whether outcome measures recommended by the HOME and other tools perform equally well across diverse patient populations. This systematic review indicates significant reporting and knowledge gaps for psychometric properties of outcome measures by race, ethnicity, or skin tone in AD.”
Ms. Kaundinya reported having no relevant financial disclosures. Dr. Silverberg, the study’s senior author, is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
FROM REVOLUTIONIZING AD 2021
COVID-19 vaccination does not increase risk of flare in patients with lupus
COVID-19 vaccinations appear to be well tolerated in patients with systemic lupus erythematosus (SLE) and come with a low risk of flare, according to the results of a global, web-based survey.
“Disseminating these reassuring data might prove crucial to increasing vaccine coverage in patients with SLE,” wrote lead author Renaud Felten, MD, of Strasbourg (France) University Hospital. Their results were published as a comment in Lancet Rheumatology.
To assess vaccine tolerability among lupus patients, the cross-sectional Tolerance and Consequences of Vaccination Against COVID-19 in Lupus Patients (VACOLUP) study analyzed a 43-question survey of 696 participants with a self-reported, medically confirmed diagnosis of SLE from 30 countries between March 22, 2021, and May 17, 2021. The cohort was 96% women, and their median age was 42 (interquartile range, 34-51). Nearly 36% of respondents were from Italy, 27% were from Chile, 13% were from France, and just under 9% were Americans. All participants received at least one dose of COVID-19 vaccine, and 49% received a second dose. The most common vaccines were Pfizer-BioNTech (57%), Sinovac (22%), AstraZeneca (10%), and Moderna (8%).
Only 21 participants (3%) reported a medically confirmed SLE flare after a median of 3 days (IQR, 0-29) post COVID vaccination, with most experiencing musculoskeletal symptoms (90%) and fatigue (86%). Of the 21 cases, 15 reported a subsequent change in SLE treatment and 4 were admitted to the hospital. A previous flare that occurred within a year before vaccination was associated with an increased risk of flare post vaccination (relative risk, 5.52; 95% confidence interval, 2.17-14.03; P < .0001).
Side effects – including swelling, soreness, fever, chills, fatigue, joint and muscle pain, nausea, and headache – were reported in 45% of participants (n = 316) after their first dose and in 53% of the 343 participants who received a second dose. There was no notable difference in the likelihood of side effects across gender and age or in patients who received mRNA vaccines, compared with vaccines with other modes of action. Patients who reported side effects after the first dose were more likely to also report them after the second, compared with those who reported none (109 [81%] of 135 vs. 72 [35%] of 205; RR, 2.30; 95% CI, 1.88-2.82; P < .0001).
In the majority of cases (2,232 of 2,683), the side effects were of minor or moderate intensity and did not affect the participants’ ability to perform daily tasks. The study found no significant association between side effects and a SLE flare and SLE medications or previous SLE disease manifestations.
When asked to comment on the study, Amit Saxena, MD, of the Lupus Center at New York University Langone Health, said: “What we are seeing is pretty mild to moderate in terms of follow-up side effects or lupus-related activity. Several studies have shown this amongst our autoimmune rheumatology cohort, as well as what I’ve seen clinically in my own patients. More than anything else, numbers are the most important, and this is a large study.”
He acknowledged the benefits of going directly to patients to gauge their responses and reactions, giving them the opportunity to share concerns that physicians may not think about.
“As rheumatologists, we tend to focus on certain things that might not necessarily be what the patients themselves focus on,” he said. “I think the fact that this questionnaire dealt with a lot of what people complain about – fatigue, sore arm, things that we know are part of getting the vaccine – they aren’t necessarily things we capture with tools that screen for lupus flares, for example.”
More than anything, Dr. Saxena commended the study’s timeliness. “Patients are constantly asking us about the vaccine, and there’s so much misinformation,” he said. “People say, ‘Because I have lupus, I was told not to get vaccinated.’ I don’t know where they get that information from; we are telling everyone to get it, especially our lupus patients.”
The authors recognized their study’s main limitation as the self-reported and subjective nature of the survey, which they attempted to mitigate by asking for medically confirmed flares only. They noted, however, that the short median time between vaccination and flare onset could be caused by patients confusing expected side effects for something more serious, meaning the 3% figure “could be an overestimation of the actual flare rate.”
“Vaccination is recommended for patients with rheumatic and musculoskeletal diseases according to the American College of Rheumatology,” they added, “irrespective of disease activity and severity.”
Several authors reported potential conflicts of interest, including receiving consultancy fees and grants from Pfizer, GlaxoSmithKline, AbbVie, and Janssen, all unrelated to the study.
COVID-19 vaccinations appear to be well tolerated in patients with systemic lupus erythematosus (SLE) and come with a low risk of flare, according to the results of a global, web-based survey.
“Disseminating these reassuring data might prove crucial to increasing vaccine coverage in patients with SLE,” wrote lead author Renaud Felten, MD, of Strasbourg (France) University Hospital. Their results were published as a comment in Lancet Rheumatology.
To assess vaccine tolerability among lupus patients, the cross-sectional Tolerance and Consequences of Vaccination Against COVID-19 in Lupus Patients (VACOLUP) study analyzed a 43-question survey of 696 participants with a self-reported, medically confirmed diagnosis of SLE from 30 countries between March 22, 2021, and May 17, 2021. The cohort was 96% women, and their median age was 42 (interquartile range, 34-51). Nearly 36% of respondents were from Italy, 27% were from Chile, 13% were from France, and just under 9% were Americans. All participants received at least one dose of COVID-19 vaccine, and 49% received a second dose. The most common vaccines were Pfizer-BioNTech (57%), Sinovac (22%), AstraZeneca (10%), and Moderna (8%).
Only 21 participants (3%) reported a medically confirmed SLE flare after a median of 3 days (IQR, 0-29) post COVID vaccination, with most experiencing musculoskeletal symptoms (90%) and fatigue (86%). Of the 21 cases, 15 reported a subsequent change in SLE treatment and 4 were admitted to the hospital. A previous flare that occurred within a year before vaccination was associated with an increased risk of flare post vaccination (relative risk, 5.52; 95% confidence interval, 2.17-14.03; P < .0001).
Side effects – including swelling, soreness, fever, chills, fatigue, joint and muscle pain, nausea, and headache – were reported in 45% of participants (n = 316) after their first dose and in 53% of the 343 participants who received a second dose. There was no notable difference in the likelihood of side effects across gender and age or in patients who received mRNA vaccines, compared with vaccines with other modes of action. Patients who reported side effects after the first dose were more likely to also report them after the second, compared with those who reported none (109 [81%] of 135 vs. 72 [35%] of 205; RR, 2.30; 95% CI, 1.88-2.82; P < .0001).
In the majority of cases (2,232 of 2,683), the side effects were of minor or moderate intensity and did not affect the participants’ ability to perform daily tasks. The study found no significant association between side effects and a SLE flare and SLE medications or previous SLE disease manifestations.
When asked to comment on the study, Amit Saxena, MD, of the Lupus Center at New York University Langone Health, said: “What we are seeing is pretty mild to moderate in terms of follow-up side effects or lupus-related activity. Several studies have shown this amongst our autoimmune rheumatology cohort, as well as what I’ve seen clinically in my own patients. More than anything else, numbers are the most important, and this is a large study.”
He acknowledged the benefits of going directly to patients to gauge their responses and reactions, giving them the opportunity to share concerns that physicians may not think about.
“As rheumatologists, we tend to focus on certain things that might not necessarily be what the patients themselves focus on,” he said. “I think the fact that this questionnaire dealt with a lot of what people complain about – fatigue, sore arm, things that we know are part of getting the vaccine – they aren’t necessarily things we capture with tools that screen for lupus flares, for example.”
More than anything, Dr. Saxena commended the study’s timeliness. “Patients are constantly asking us about the vaccine, and there’s so much misinformation,” he said. “People say, ‘Because I have lupus, I was told not to get vaccinated.’ I don’t know where they get that information from; we are telling everyone to get it, especially our lupus patients.”
The authors recognized their study’s main limitation as the self-reported and subjective nature of the survey, which they attempted to mitigate by asking for medically confirmed flares only. They noted, however, that the short median time between vaccination and flare onset could be caused by patients confusing expected side effects for something more serious, meaning the 3% figure “could be an overestimation of the actual flare rate.”
“Vaccination is recommended for patients with rheumatic and musculoskeletal diseases according to the American College of Rheumatology,” they added, “irrespective of disease activity and severity.”
Several authors reported potential conflicts of interest, including receiving consultancy fees and grants from Pfizer, GlaxoSmithKline, AbbVie, and Janssen, all unrelated to the study.
COVID-19 vaccinations appear to be well tolerated in patients with systemic lupus erythematosus (SLE) and come with a low risk of flare, according to the results of a global, web-based survey.
“Disseminating these reassuring data might prove crucial to increasing vaccine coverage in patients with SLE,” wrote lead author Renaud Felten, MD, of Strasbourg (France) University Hospital. Their results were published as a comment in Lancet Rheumatology.
To assess vaccine tolerability among lupus patients, the cross-sectional Tolerance and Consequences of Vaccination Against COVID-19 in Lupus Patients (VACOLUP) study analyzed a 43-question survey of 696 participants with a self-reported, medically confirmed diagnosis of SLE from 30 countries between March 22, 2021, and May 17, 2021. The cohort was 96% women, and their median age was 42 (interquartile range, 34-51). Nearly 36% of respondents were from Italy, 27% were from Chile, 13% were from France, and just under 9% were Americans. All participants received at least one dose of COVID-19 vaccine, and 49% received a second dose. The most common vaccines were Pfizer-BioNTech (57%), Sinovac (22%), AstraZeneca (10%), and Moderna (8%).
Only 21 participants (3%) reported a medically confirmed SLE flare after a median of 3 days (IQR, 0-29) post COVID vaccination, with most experiencing musculoskeletal symptoms (90%) and fatigue (86%). Of the 21 cases, 15 reported a subsequent change in SLE treatment and 4 were admitted to the hospital. A previous flare that occurred within a year before vaccination was associated with an increased risk of flare post vaccination (relative risk, 5.52; 95% confidence interval, 2.17-14.03; P < .0001).
Side effects – including swelling, soreness, fever, chills, fatigue, joint and muscle pain, nausea, and headache – were reported in 45% of participants (n = 316) after their first dose and in 53% of the 343 participants who received a second dose. There was no notable difference in the likelihood of side effects across gender and age or in patients who received mRNA vaccines, compared with vaccines with other modes of action. Patients who reported side effects after the first dose were more likely to also report them after the second, compared with those who reported none (109 [81%] of 135 vs. 72 [35%] of 205; RR, 2.30; 95% CI, 1.88-2.82; P < .0001).
In the majority of cases (2,232 of 2,683), the side effects were of minor or moderate intensity and did not affect the participants’ ability to perform daily tasks. The study found no significant association between side effects and a SLE flare and SLE medications or previous SLE disease manifestations.
When asked to comment on the study, Amit Saxena, MD, of the Lupus Center at New York University Langone Health, said: “What we are seeing is pretty mild to moderate in terms of follow-up side effects or lupus-related activity. Several studies have shown this amongst our autoimmune rheumatology cohort, as well as what I’ve seen clinically in my own patients. More than anything else, numbers are the most important, and this is a large study.”
He acknowledged the benefits of going directly to patients to gauge their responses and reactions, giving them the opportunity to share concerns that physicians may not think about.
“As rheumatologists, we tend to focus on certain things that might not necessarily be what the patients themselves focus on,” he said. “I think the fact that this questionnaire dealt with a lot of what people complain about – fatigue, sore arm, things that we know are part of getting the vaccine – they aren’t necessarily things we capture with tools that screen for lupus flares, for example.”
More than anything, Dr. Saxena commended the study’s timeliness. “Patients are constantly asking us about the vaccine, and there’s so much misinformation,” he said. “People say, ‘Because I have lupus, I was told not to get vaccinated.’ I don’t know where they get that information from; we are telling everyone to get it, especially our lupus patients.”
The authors recognized their study’s main limitation as the self-reported and subjective nature of the survey, which they attempted to mitigate by asking for medically confirmed flares only. They noted, however, that the short median time between vaccination and flare onset could be caused by patients confusing expected side effects for something more serious, meaning the 3% figure “could be an overestimation of the actual flare rate.”
“Vaccination is recommended for patients with rheumatic and musculoskeletal diseases according to the American College of Rheumatology,” they added, “irrespective of disease activity and severity.”
Several authors reported potential conflicts of interest, including receiving consultancy fees and grants from Pfizer, GlaxoSmithKline, AbbVie, and Janssen, all unrelated to the study.
FROM THE LANCET RHEUMATOLOGY
Genetic testing for neurofibromatosis 1: An imperfect science
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
According to Peter Kannu, MB, ChB, DCH, PhD, a definitive diagnosis of NF1 can be made in most children using National Institutes of Health criteria published in 1988, which include the presence of two of the following:
- Six or more café au lait macules over 5 mm in diameter in prepubertal individuals and over 15 mm in greatest diameter in postpubertal individuals
- Two or more neurofibromas of any type or one plexiform neurofibroma
- Freckling in the axillary or inguinal regions
- Two or more Lisch nodules
- Optic glioma
- A distinctive osseous lesion such as sphenoid dysplasia or thinning of long bone cortex, with or without pseudarthrosis
- Having a first-degree relative with NF1
For example, in the case of an 8-year-old child who presents with multiple café au lait macules, axillary and inguinal freckling, Lisch nodules, and an optic glioma, “the diagnosis is secure and genetic testing is not going to change clinical management or surveillance,” Dr. Kannu, a clinical geneticist at the University of Alberta, Edmonton, said during the annual meeting of the Society for Pediatric Dermatology. “The only reason for genetic testing in this situation is so that we know the mutation in order to inform reproductive risk counseling in the future.”
However, while a diagnosis of NF1 may be suspected in a 6- to 12-month-old presenting with only café au lait macules, “the diagnosis is not secure because the clinical criteria cannot be met. In this situation, a genetic test can speed up the diagnosis,” he added. “Or, if the test is negative, it can decrease your suspicion for NF1 and you wouldn’t refer the child on to an NF1 screening clinic for intensive surveillance.”
Dr. Kannu based his remarks largely on his 5 years working at the multidisciplinary Genodermatoses Clinic at the Hospital for Sick Children, Toronto. Founded in 2015, the clinic is a “one-stop shop” designed to reduce the wait time for diagnosis and management and the number of hospital visits. The team – composed of a dermatologist, medical geneticist, genetic counselor, residents, and fellows – meets to review the charts of each patient before the appointment, and decides on a preliminary management plan. All children are then seen by one of the trainees in the clinic who devises a differential diagnosis that is presented to staff physicians, at which point genetic testing is decided on. A genetics counselor handles follow-up for those who do have genetic testing.
In 2018, Dr. Kannu and colleagues conducted an informal review of 300 patients who had been seen in the clinic. The mean age at referral was about 6 years, 51% were female, and the top three referral sources were pediatricians (51%), dermatologists (18%), and family physicians (18%). Of the 300 children, 84 (28%) were confirmed to have a diagnosis of NF1. Two patients were diagnosed with NF2 and 5% of the total cohort was diagnosed with mosaic NF1 (MNF1), “which is higher than what you would expect based on the incidence of MNF1 in the literature,” he said.
He separates genetic tests for NF1 into one of two categories: Conventional testing, which is offered by most labs in North America; and comprehensive testing, which is offered by the medical genomics lab at the University of Alabama at Birmingham. Conventional testing focuses on the exons, “the protein coding regions of the gene where most of the mutations lie,” he said. “The test also sequences about 20 base pairs or so of the intron exon boundary and may pick up some intronic mutations. But this test will not detect anything that’s hidden deep in the intronic region.”
Comprehensive testing, meanwhile, checks for mutations in both introns and exons.
Dr. Kannu and colleagues published a case of a paraspinal ganglioneuroma in the proband of a large family with mild cutaneous manifestations of NF1, carrying a deep NF1 intronic mutation. “The clinicians were suspicious that this was NF1, rightly so. The diagnosis was only confirmed after we sent samples to the University of Alabama lab where the deep intronic mutation was found,” he said.
The other situation where conventional genetic testing may be negative is in the case of MNF1, where there “are mutations in some cells but not all cells,” Dr. Kannu explained. “It may only be present in the melanocytes of the skin but not present in the lymphocytes in the blood. Mosaicism is characterized by the regional distribution of pigmentary or other NF1 associated findings. Mosaicism may be detected in the blood if it’s more than 20%. Anything less than that is not detected with conventional genetic testing using DNA from blood and requires extracting DNA from a punch biopsy sample of a café au lait macule.”
The differential diagnosis of café au lait macules includes several conditions associated mutations in the RAS pathway. “Neurofibromin is a key signal of molecules which regulates the activation of RAS,” Dr. Kannu said. “A close binding partner of NF1 is SPRED 1. We know that mutations in this gene cause Legius syndrome, a condition which presents with multiple café au lait macules.”
Two key receptors in the RAS pathway include EGFR and KITL, he continued. Mutations in the EGFR receptor cause a rare condition known as neonatal skin and bowel disease, while mutations in the KITL receptor cause familial progressive hyperpigmentation with or without hypopigmentation. “Looking into the pathway and focusing downstream of RAS, we have genes such as RAF and CBL, which are mutated in Noonan syndrome,” he said. “Further along in the pathway you have mutations in PTEN, which cause Cowden syndrome, and mutations in TSC1 and TSC2, which cause tuberous sclerosis. Mutations in any of these genes can also present with café au lait macules.”
During a question-and-answer session Dr. Kannu was asked to comment about revised diagnostic criteria for NF1 based on an international consensus recommendation, such as changes in the eye that require a formal opthalmologic examination, which were recently published.
“We are understanding more about the phenotype,” he said. “If you fulfill diagnostic criteria for NF1, the main reasons for doing genetic testing are, one, if the family wants to know that information, and two, it informs our reproductive risk counseling. Genotype-phenotype correlations do exist in NF1 but they’re not very robust, so that information is not clinically useful.”
Dr. Kannu disclosed that he has been an advisory board member for Ipsen, Novartis, and Alexion. He has also been a primary investigator for QED and Clementia.
FROM SPD 2021
Mayo, Cleveland Clinics top latest U.S. News & World Report hospital rankings
This year’s expanded report debuts new ratings for seven “important procedures and conditions to help patients, in consultation with their doctors, narrow down their choice of hospital based on the specific type of care they need,” Ben Harder, managing editor and chief of health analysis, said in a news release.
With new ratings for myocardial infarction, stroke, hip fracture, and back surgery (spinal fusion), the report now ranks 17 procedures and conditions.
Also new to the 2021 report, which marks the 32nd edition, is a look at racial disparities in health care and the inclusion of health equity measures alongside the hospital rankings.
The new measures examine whether the patients each hospital has treated reflect the racial and ethnic diversity of the surrounding community, among other aspects of health equity.
“At roughly four out of five hospitals, we found that the community’s minority residents were underrepresented among patients receiving services such as joint replacement, cancer surgery and common heart procedures,” Mr. Harder said.
“Against this backdrop, however, we found important exceptions – hospitals that provide care to a disproportionate share of their community’s minority residents. These metrics are just a beginning; we aim to expand on our measurement of health equity in the future,” Mr. Harder added.
Mayo and Cleveland Clinic remain tops
Following the Mayo Clinic, the Cleveland Clinic once again takes the No. 2 spot in the magazine’s latest annual honor roll of best hospitals, which highlights hospitals that deliver exceptional treatment across multiple areas of care.
UCLA Medical Center, Los Angeles, holds the No. 3 spot in 2021. In 2020, UCLA Medical Center and New York–Presbyterian Hospital–Columbia and Cornell, New York, sat in a tie at No. 4.
In 2021, Johns Hopkins Hospital, Baltimore, which held the No. 3 spot in 2020, drops to No. 4, while Massachusetts General Hospital in Boston takes the No. 5 spot, up from No. 6 in 2020.
Rounding out the top 10 (in order) are Cedars-Sinai Medical Center, Los Angeles; New York–Presbyterian Hospital–Columbia and Cornell, New York; NYU Langone Hospitals, New York; UCSF Medical Center, San Francisco; and Northwestern Memorial Hospital, Chicago.
2021-2022 Best Hospitals honor roll
1. Mayo Clinic, Rochester, Minn.
2. Cleveland Clinic, Cleveland
3. UCLA Medical Center, Los Angeles
4. Johns Hopkins Hospital, Baltimore
5. Massachusetts General Hospital, Boston
6. Cedars-Sinai Medical Center, San Francisco
7. New York–Presbyterian Hospital–Columbia and Cornell, New York
8. NYU Langone Hospitals, New York
9. UCSF Medical Center, San Francisco
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor.
12. Stanford Health Care–Stanford Hospital, Palo Alto, Calif.
13. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
14. Brigham and Women’s Hospital, Boston
15. Mayo Clinic–Phoenix, Phoenix
16. Houston Methodist Hospital, Houston
17. (tie) Barnes-Jewish Hospital, St. Louis
17. (tie) Mount Sinai Hospital, New York Rush University Medical Center, Chicago
19. Rush University Medical Center, Chicago
20. Vanderbilt University Medical Center, Nashville, Tenn.
For the 2021-2022 rankings and ratings, the magazine compared more than 4,750 hospitals nationwide in 15 specialties and 17 procedures and conditions.
At least 2,039 hospitals received a high performance rating in at least one of the services rated; 11 hospitals received high performance in all 17. A total of 175 hospitals were nationally ranked in at least one specialty
For specialty rankings, the University of Texas MD Anderson Cancer Center continues to hold the No. 1 spot in cancer care, the Hospital for Special Surgery continues to be No. 1 in orthopedics, and the Cleveland Clinic continues to be No. 1 in cardiology and heart surgery.
Top five for cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York
3. Mayo Clinic, Rochester, Minn.
4. Dana-Farber/Brigham & Women’s Cancer Center, Boston
5. Cleveland Clinic, Cleveland
Top five for cardiology and heart surgery
1. Cleveland Clinic, Cleveland
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, New York
5. NYU Langone Hospitals, New York
Top five for orthopedics
1. Hospital for Special Surgery, New York
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York
5. UCLA Medical Center, Los Angeles
The magazine noted that data for the 2021-2022 Best Hospitals rankings and ratings were not affected by the COVID-19 pandemic, which began after the end of the data collection period.
The methodologies used in determining the rankings are based largely on objective measures, such as risk-adjusted survival, discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report is available online.
A version of this article first appeared on Medscape.com.
This year’s expanded report debuts new ratings for seven “important procedures and conditions to help patients, in consultation with their doctors, narrow down their choice of hospital based on the specific type of care they need,” Ben Harder, managing editor and chief of health analysis, said in a news release.
With new ratings for myocardial infarction, stroke, hip fracture, and back surgery (spinal fusion), the report now ranks 17 procedures and conditions.
Also new to the 2021 report, which marks the 32nd edition, is a look at racial disparities in health care and the inclusion of health equity measures alongside the hospital rankings.
The new measures examine whether the patients each hospital has treated reflect the racial and ethnic diversity of the surrounding community, among other aspects of health equity.
“At roughly four out of five hospitals, we found that the community’s minority residents were underrepresented among patients receiving services such as joint replacement, cancer surgery and common heart procedures,” Mr. Harder said.
“Against this backdrop, however, we found important exceptions – hospitals that provide care to a disproportionate share of their community’s minority residents. These metrics are just a beginning; we aim to expand on our measurement of health equity in the future,” Mr. Harder added.
Mayo and Cleveland Clinic remain tops
Following the Mayo Clinic, the Cleveland Clinic once again takes the No. 2 spot in the magazine’s latest annual honor roll of best hospitals, which highlights hospitals that deliver exceptional treatment across multiple areas of care.
UCLA Medical Center, Los Angeles, holds the No. 3 spot in 2021. In 2020, UCLA Medical Center and New York–Presbyterian Hospital–Columbia and Cornell, New York, sat in a tie at No. 4.
In 2021, Johns Hopkins Hospital, Baltimore, which held the No. 3 spot in 2020, drops to No. 4, while Massachusetts General Hospital in Boston takes the No. 5 spot, up from No. 6 in 2020.
Rounding out the top 10 (in order) are Cedars-Sinai Medical Center, Los Angeles; New York–Presbyterian Hospital–Columbia and Cornell, New York; NYU Langone Hospitals, New York; UCSF Medical Center, San Francisco; and Northwestern Memorial Hospital, Chicago.
2021-2022 Best Hospitals honor roll
1. Mayo Clinic, Rochester, Minn.
2. Cleveland Clinic, Cleveland
3. UCLA Medical Center, Los Angeles
4. Johns Hopkins Hospital, Baltimore
5. Massachusetts General Hospital, Boston
6. Cedars-Sinai Medical Center, San Francisco
7. New York–Presbyterian Hospital–Columbia and Cornell, New York
8. NYU Langone Hospitals, New York
9. UCSF Medical Center, San Francisco
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor.
12. Stanford Health Care–Stanford Hospital, Palo Alto, Calif.
13. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
14. Brigham and Women’s Hospital, Boston
15. Mayo Clinic–Phoenix, Phoenix
16. Houston Methodist Hospital, Houston
17. (tie) Barnes-Jewish Hospital, St. Louis
17. (tie) Mount Sinai Hospital, New York Rush University Medical Center, Chicago
19. Rush University Medical Center, Chicago
20. Vanderbilt University Medical Center, Nashville, Tenn.
For the 2021-2022 rankings and ratings, the magazine compared more than 4,750 hospitals nationwide in 15 specialties and 17 procedures and conditions.
At least 2,039 hospitals received a high performance rating in at least one of the services rated; 11 hospitals received high performance in all 17. A total of 175 hospitals were nationally ranked in at least one specialty
For specialty rankings, the University of Texas MD Anderson Cancer Center continues to hold the No. 1 spot in cancer care, the Hospital for Special Surgery continues to be No. 1 in orthopedics, and the Cleveland Clinic continues to be No. 1 in cardiology and heart surgery.
Top five for cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York
3. Mayo Clinic, Rochester, Minn.
4. Dana-Farber/Brigham & Women’s Cancer Center, Boston
5. Cleveland Clinic, Cleveland
Top five for cardiology and heart surgery
1. Cleveland Clinic, Cleveland
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, New York
5. NYU Langone Hospitals, New York
Top five for orthopedics
1. Hospital for Special Surgery, New York
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York
5. UCLA Medical Center, Los Angeles
The magazine noted that data for the 2021-2022 Best Hospitals rankings and ratings were not affected by the COVID-19 pandemic, which began after the end of the data collection period.
The methodologies used in determining the rankings are based largely on objective measures, such as risk-adjusted survival, discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report is available online.
A version of this article first appeared on Medscape.com.
This year’s expanded report debuts new ratings for seven “important procedures and conditions to help patients, in consultation with their doctors, narrow down their choice of hospital based on the specific type of care they need,” Ben Harder, managing editor and chief of health analysis, said in a news release.
With new ratings for myocardial infarction, stroke, hip fracture, and back surgery (spinal fusion), the report now ranks 17 procedures and conditions.
Also new to the 2021 report, which marks the 32nd edition, is a look at racial disparities in health care and the inclusion of health equity measures alongside the hospital rankings.
The new measures examine whether the patients each hospital has treated reflect the racial and ethnic diversity of the surrounding community, among other aspects of health equity.
“At roughly four out of five hospitals, we found that the community’s minority residents were underrepresented among patients receiving services such as joint replacement, cancer surgery and common heart procedures,” Mr. Harder said.
“Against this backdrop, however, we found important exceptions – hospitals that provide care to a disproportionate share of their community’s minority residents. These metrics are just a beginning; we aim to expand on our measurement of health equity in the future,” Mr. Harder added.
Mayo and Cleveland Clinic remain tops
Following the Mayo Clinic, the Cleveland Clinic once again takes the No. 2 spot in the magazine’s latest annual honor roll of best hospitals, which highlights hospitals that deliver exceptional treatment across multiple areas of care.
UCLA Medical Center, Los Angeles, holds the No. 3 spot in 2021. In 2020, UCLA Medical Center and New York–Presbyterian Hospital–Columbia and Cornell, New York, sat in a tie at No. 4.
In 2021, Johns Hopkins Hospital, Baltimore, which held the No. 3 spot in 2020, drops to No. 4, while Massachusetts General Hospital in Boston takes the No. 5 spot, up from No. 6 in 2020.
Rounding out the top 10 (in order) are Cedars-Sinai Medical Center, Los Angeles; New York–Presbyterian Hospital–Columbia and Cornell, New York; NYU Langone Hospitals, New York; UCSF Medical Center, San Francisco; and Northwestern Memorial Hospital, Chicago.
2021-2022 Best Hospitals honor roll
1. Mayo Clinic, Rochester, Minn.
2. Cleveland Clinic, Cleveland
3. UCLA Medical Center, Los Angeles
4. Johns Hopkins Hospital, Baltimore
5. Massachusetts General Hospital, Boston
6. Cedars-Sinai Medical Center, San Francisco
7. New York–Presbyterian Hospital–Columbia and Cornell, New York
8. NYU Langone Hospitals, New York
9. UCSF Medical Center, San Francisco
10. Northwestern Memorial Hospital, Chicago
11. University of Michigan Hospitals–Michigan Medicine, Ann Arbor.
12. Stanford Health Care–Stanford Hospital, Palo Alto, Calif.
13. Hospitals of the University of Pennsylvania–Penn Presbyterian, Philadelphia
14. Brigham and Women’s Hospital, Boston
15. Mayo Clinic–Phoenix, Phoenix
16. Houston Methodist Hospital, Houston
17. (tie) Barnes-Jewish Hospital, St. Louis
17. (tie) Mount Sinai Hospital, New York Rush University Medical Center, Chicago
19. Rush University Medical Center, Chicago
20. Vanderbilt University Medical Center, Nashville, Tenn.
For the 2021-2022 rankings and ratings, the magazine compared more than 4,750 hospitals nationwide in 15 specialties and 17 procedures and conditions.
At least 2,039 hospitals received a high performance rating in at least one of the services rated; 11 hospitals received high performance in all 17. A total of 175 hospitals were nationally ranked in at least one specialty
For specialty rankings, the University of Texas MD Anderson Cancer Center continues to hold the No. 1 spot in cancer care, the Hospital for Special Surgery continues to be No. 1 in orthopedics, and the Cleveland Clinic continues to be No. 1 in cardiology and heart surgery.
Top five for cancer
1. University of Texas MD Anderson Cancer Center, Houston
2. Memorial Sloan Kettering Cancer Center, New York
3. Mayo Clinic, Rochester, Minn.
4. Dana-Farber/Brigham & Women’s Cancer Center, Boston
5. Cleveland Clinic, Cleveland
Top five for cardiology and heart surgery
1. Cleveland Clinic, Cleveland
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. New York–Presbyterian Hospital–Columbia and Cornell, New York
5. NYU Langone Hospitals, New York
Top five for orthopedics
1. Hospital for Special Surgery, New York
2. Mayo Clinic, Rochester, Minn.
3. Cedars-Sinai Medical Center, Los Angeles
4. NYU Langone Orthopedic Hospital, New York
5. UCLA Medical Center, Los Angeles
The magazine noted that data for the 2021-2022 Best Hospitals rankings and ratings were not affected by the COVID-19 pandemic, which began after the end of the data collection period.
The methodologies used in determining the rankings are based largely on objective measures, such as risk-adjusted survival, discharge-to-home rates, volume, and quality of nursing, among other care-related indicators.
The full report is available online.
A version of this article first appeared on Medscape.com.
AMA, 55 other groups urge health care vax mandate
As COVID-19 cases, hospitalizations, and deaths mount again across the country, the American Medical Association (AMA), the American Nursing Association, and 54 other
This injunction, issued July 26, covers everyone in healthcare, Emanuel Ezekiel, MD, PhD, chair of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and the organizer of the joint statement, said in an interview.
That includes not only hospitals, but also physician offices, ambulatory surgery centers, home care agencies, skilled nursing facilities, pharmacies, laboratories, and imaging centers, he said.
The exhortation to get vaccinated also extends to federal and state healthcare facilities, including those of the military health system — TRICARE and the Department of Veterans Affairs — which instituted a mandate the same day.
The American Hospital Association (AHA) and other hospital groups recently said they supported hospitals and health systems that required their personnel to get vaccinated. Several dozen healthcare organizations have already done so, including some of the nation’s largest health systems.
A substantial fraction of U.S. healthcare workers have not yet gotten vaccinated, although how many are unvaccinated is unclear. An analysis by WebMD and Medscape Medical News estimated that 25% of hospital workers who had contact with patients were unvaccinated at the end of May.
More than 38% of nursing workers were not fully vaccinated by July 11, according to an analysis of Centers for Medicare & Medicaid Services data by LeadingAge, which was cited by the Washington Post. And more than 40% of nursing home employees have not been fully vaccinated, according to the Centers for Disease Control and Prevention.
The joint statement did not give any indication of how many employees of physician practices have failed to get COVID shots. However, a recent AMA survey shows that 96% of physicians have been fully vaccinated.
Ethical commitment
The main reason for vaccine mandates, according to the healthcare associations’ statement, is “the ethical commitment to put patients as well as residents of long-term care facilities first and take all steps necessary to ensure their health and well-being.”
In addition, the statement noted, vaccination can protect healthcare workers and their families from getting COVID-19.
The statement also pointed out that many healthcare and long-term care organizations already require vaccinations for influenza, hepatitis B, and pertussis.
Workers who have certain medical conditions should be exempt from the vaccination mandates, the statement added.
While recognizing the “historical mistrust of health care institutions” among some healthcare workers, the statement said, “We must continue to address workers’ concerns, engage with marginalized populations, and work with trusted messengers to improve vaccine acceptance.”
There has been some skepticism about the legality of requiring healthcare workers to get vaccinated as a condition of employment, partly because the U.S. Food and Drug Administration has not yet fully authorized any of the COVID-19 vaccines.
But in June, a federal judge turned down a legal challenge to Houston Methodist’s vaccination mandate.
“It is critical that all people in the health care workforce get vaccinated against COVID-19 for the safety of our patients and our colleagues. With more than 300 million doses administered in the United States and nearly 4 billion doses administered worldwide, we know the vaccines are safe and highly effective at preventing severe illness and death from COVID-19.
“Increased vaccinations among health care personnel will not only reduce the spread of COVID-19 but also reduce the harmful toll this virus is taking within the health care workforce and those we are striving to serve,” Susan Bailey, MD, immediate past president of the AMA, said in a news release.
A version of this article first appeared on Medscape.com.
As COVID-19 cases, hospitalizations, and deaths mount again across the country, the American Medical Association (AMA), the American Nursing Association, and 54 other
This injunction, issued July 26, covers everyone in healthcare, Emanuel Ezekiel, MD, PhD, chair of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and the organizer of the joint statement, said in an interview.
That includes not only hospitals, but also physician offices, ambulatory surgery centers, home care agencies, skilled nursing facilities, pharmacies, laboratories, and imaging centers, he said.
The exhortation to get vaccinated also extends to federal and state healthcare facilities, including those of the military health system — TRICARE and the Department of Veterans Affairs — which instituted a mandate the same day.
The American Hospital Association (AHA) and other hospital groups recently said they supported hospitals and health systems that required their personnel to get vaccinated. Several dozen healthcare organizations have already done so, including some of the nation’s largest health systems.
A substantial fraction of U.S. healthcare workers have not yet gotten vaccinated, although how many are unvaccinated is unclear. An analysis by WebMD and Medscape Medical News estimated that 25% of hospital workers who had contact with patients were unvaccinated at the end of May.
More than 38% of nursing workers were not fully vaccinated by July 11, according to an analysis of Centers for Medicare & Medicaid Services data by LeadingAge, which was cited by the Washington Post. And more than 40% of nursing home employees have not been fully vaccinated, according to the Centers for Disease Control and Prevention.
The joint statement did not give any indication of how many employees of physician practices have failed to get COVID shots. However, a recent AMA survey shows that 96% of physicians have been fully vaccinated.
Ethical commitment
The main reason for vaccine mandates, according to the healthcare associations’ statement, is “the ethical commitment to put patients as well as residents of long-term care facilities first and take all steps necessary to ensure their health and well-being.”
In addition, the statement noted, vaccination can protect healthcare workers and their families from getting COVID-19.
The statement also pointed out that many healthcare and long-term care organizations already require vaccinations for influenza, hepatitis B, and pertussis.
Workers who have certain medical conditions should be exempt from the vaccination mandates, the statement added.
While recognizing the “historical mistrust of health care institutions” among some healthcare workers, the statement said, “We must continue to address workers’ concerns, engage with marginalized populations, and work with trusted messengers to improve vaccine acceptance.”
There has been some skepticism about the legality of requiring healthcare workers to get vaccinated as a condition of employment, partly because the U.S. Food and Drug Administration has not yet fully authorized any of the COVID-19 vaccines.
But in June, a federal judge turned down a legal challenge to Houston Methodist’s vaccination mandate.
“It is critical that all people in the health care workforce get vaccinated against COVID-19 for the safety of our patients and our colleagues. With more than 300 million doses administered in the United States and nearly 4 billion doses administered worldwide, we know the vaccines are safe and highly effective at preventing severe illness and death from COVID-19.
“Increased vaccinations among health care personnel will not only reduce the spread of COVID-19 but also reduce the harmful toll this virus is taking within the health care workforce and those we are striving to serve,” Susan Bailey, MD, immediate past president of the AMA, said in a news release.
A version of this article first appeared on Medscape.com.
As COVID-19 cases, hospitalizations, and deaths mount again across the country, the American Medical Association (AMA), the American Nursing Association, and 54 other
This injunction, issued July 26, covers everyone in healthcare, Emanuel Ezekiel, MD, PhD, chair of the department of medical ethics and health policy at the University of Pennsylvania, Philadelphia, and the organizer of the joint statement, said in an interview.
That includes not only hospitals, but also physician offices, ambulatory surgery centers, home care agencies, skilled nursing facilities, pharmacies, laboratories, and imaging centers, he said.
The exhortation to get vaccinated also extends to federal and state healthcare facilities, including those of the military health system — TRICARE and the Department of Veterans Affairs — which instituted a mandate the same day.
The American Hospital Association (AHA) and other hospital groups recently said they supported hospitals and health systems that required their personnel to get vaccinated. Several dozen healthcare organizations have already done so, including some of the nation’s largest health systems.
A substantial fraction of U.S. healthcare workers have not yet gotten vaccinated, although how many are unvaccinated is unclear. An analysis by WebMD and Medscape Medical News estimated that 25% of hospital workers who had contact with patients were unvaccinated at the end of May.
More than 38% of nursing workers were not fully vaccinated by July 11, according to an analysis of Centers for Medicare & Medicaid Services data by LeadingAge, which was cited by the Washington Post. And more than 40% of nursing home employees have not been fully vaccinated, according to the Centers for Disease Control and Prevention.
The joint statement did not give any indication of how many employees of physician practices have failed to get COVID shots. However, a recent AMA survey shows that 96% of physicians have been fully vaccinated.
Ethical commitment
The main reason for vaccine mandates, according to the healthcare associations’ statement, is “the ethical commitment to put patients as well as residents of long-term care facilities first and take all steps necessary to ensure their health and well-being.”
In addition, the statement noted, vaccination can protect healthcare workers and their families from getting COVID-19.
The statement also pointed out that many healthcare and long-term care organizations already require vaccinations for influenza, hepatitis B, and pertussis.
Workers who have certain medical conditions should be exempt from the vaccination mandates, the statement added.
While recognizing the “historical mistrust of health care institutions” among some healthcare workers, the statement said, “We must continue to address workers’ concerns, engage with marginalized populations, and work with trusted messengers to improve vaccine acceptance.”
There has been some skepticism about the legality of requiring healthcare workers to get vaccinated as a condition of employment, partly because the U.S. Food and Drug Administration has not yet fully authorized any of the COVID-19 vaccines.
But in June, a federal judge turned down a legal challenge to Houston Methodist’s vaccination mandate.
“It is critical that all people in the health care workforce get vaccinated against COVID-19 for the safety of our patients and our colleagues. With more than 300 million doses administered in the United States and nearly 4 billion doses administered worldwide, we know the vaccines are safe and highly effective at preventing severe illness and death from COVID-19.
“Increased vaccinations among health care personnel will not only reduce the spread of COVID-19 but also reduce the harmful toll this virus is taking within the health care workforce and those we are striving to serve,” Susan Bailey, MD, immediate past president of the AMA, said in a news release.
A version of this article first appeared on Medscape.com.
Exophytic Tumor on the Buttock
The Diagnosis: Hidradenocarcinoma
An excisional biopsy revealed a neoplasm in the dermis with focal invasion into the adjacent soft tissue (Figure 1). The tumor consisted of sheets of cells with cytoplasmic vacuoles and ductal differentiation (Figure 2), as well as cells with mild atypia, mild pleomorphism, rare mitotic figures, and abundant pale cytoplasm. Immunohistochemical staining was positive for cytokeratin (CK) 5, CK7, CK20, CK AE1/AE3, and p63 (Figure 3). The culmination of features including the large tumor size, immunohistochemical staining pattern, and mild pleomorphism with focal invasion into the soft tissue supported the diagnosis of hidradenocarcinoma.

Hidradenocarcinoma is an exceedingly rare malignant tumor of eccrine and/or apocrine origin.1 It accounts for less than 0.001% of all tumors and 1 of 13,000 skin biopsies.2 It usually arises in the head and neck region and most commonly affects older adults aged 50 to 70 years.3 The size of hidradenocarcinomas can vary; however, they typically are large, often growing to be greater than 5 cm in diameter.2 It tends to be an aggressive tumor that generally spreads to regional lymph nodes and distant viscera.4 Although it most commonly arises de novo, it may occasionally derive from a benign hidradenoma.1 The diagnosis of hidradenocarcinoma is made based on the tumor’s morphologic and pathologic characteristics. Histologically, it is characterized by an infiltrative and invasive proliferation of lobules made of large clear cells with atypical mitotic figures and nuclear pleomorphism as well as immunohistochemical features displaying various positive markers, such as carcinoembryonic antigen, epithelial membrane antigen, S-100 protein, and CKs AE1/AE3 and 5/6.2 Invasion of the adjacent soft tissue can be present and helps to confirm the diagnosis.
The differential diagnosis for hidradenocarcinoma primarily is the benign hidradenoma, which is similar both clinically and histologically with a few important differences. Hidradenocarcinomas often are larger and ulcerated. Histologically, they usually are more pleomorphic with the presence of mitotic figures in clear cells and tend to invade locally into the surrounding soft tissue. Other similar lesions such as spiradenoma, Merkel cell carcinoma, lymphangioma, cutaneous Crohn disease, tumors metastatic to the skin, and metastatic clear cell carcinomas originating from other organs also are included in the differential diagnosis.2
Spiradenomas are dermal tumors originating from the sweat glands. They typically present as bluish, painful, solitary nodules on the ventral surfaces of the upper body, though multiple nodules also are reported.5 Spiradenomas manifest as a central constellation of pale large cells surrounded by small, dark, basaloid cells containing hyperchromatic nuclei. The microscopic appearance of the blue basaloid cells contrasts with the clear cells seen in hidradenoma.5
Merkel cell carcinoma is a cutaneous neuroendocrine tumor affecting elderly or immunosuppressed individuals. It arises in sun-exposed areas and often is associated with Merkel cell polyomavirus infection. The histologic features display small and round cells that stain positive for CK8, CK18, CK19, and CK20 but stain negative for CK7, a marker that often is positive in hidradenocarcinoma.6
Lymphangioma, particularly cavernous lymphangioma, may resemble the gross appearance of hidradenoma/ hidradenocarcinoma. It usually presents as irregular clear blue papules and nodules in the skin and subcutaneous tissue.7 The key histopathologic finding in this tumor is the endothelium-lined channels that stain positive for D2-40, a lymphatic endothelium marker.7,8
Cutaneous Crohn disease is classified as noncaseating granulomatous skin lesions that are noncontinuous with the gastrointestinal tract.9 Clinical presentations in addition to skin edema include erythematous plaques, ulcerations, and erosions. Histopathology reveals sterile noncaseating granulomas made of Langerhans giant cells, epithelioid histocytes, and plasma cells.9
Metastatic clear cell carcinomas, such as renal cell carcinoma, can be differentiated by a history of primary carcinoma, demonstration of histologic vascular stroma, and other features related to metastatic clear cell carcinoma.2
There are no well-established therapeutic guidelines for hidradenocarcinoma. Wide local excision with margins greater than 2 cm is the preferred initial treatment and often is performed in conjunction with sentinel lymph node biopsy. External beam radiotherapy and adjunctive chemotherapy have been used for tumors that could not be surgically cleared. However, the efficacy of these treatments has not been well established.2 Targeted therapies recently have emerged as an alternative treatment choice for hidradenocarcinoma due to the utilization of immunohistochemical and genomic testing. The discovery of specific gene mutations or the expression of hormonal receptors in this tumor have paved the way for targeting HER2-expressing hidradenocarcinomas with trastuzumab and those expressing estrogen receptor with the estrogen receptor inhibitor tamoxifen.1 Epidermal growth factor receptor inhibitors and PI3K/Akt/mTOR (phosphatidylinositol-3-kinase/AKT/mammalian target of rapamycin) pathway inhibitors also have been used to target various signal transduction pathways.2
Wide excision with 2.5-cm margins was performed on our patient, and a positron emission tomography– computed tomography scan revealed no metastatic disease. She declined sentinel lymph node biopsy and additional treatment. Due to the risk for recurrence, she was monitored closely with skin examinations and positron emission tomography–computed tomography every 3 months for the first year and every 6 months thereafter. Thus far, she has had no evidence of local or regional recurrence.
- Miller DH, Peterson JL, Buskirk SJ, et al. Management of metastatic apocrine hidradenocarcinoma with chemotherapy and radiation. Rare Tumors. 2015;7:6082.
- Soni A, Bansal N, Kaushal V, et al. Current management approach to hidradenocarcinoma: a comprehensive review of the literature. Ecancermedicalscience. 2015;9:517.
- Jinnah AH, Emory CL, Mai NH, et al. Hidradenocarcinoma presenting as soft tissue mass: case report with cytomorphologic description, histologic correlation, and differential diagnosis. Diagn Cytopathol. 2016;44:438-441.
- Khan BM, Mansha MA, Ali N, et al. Hidradenocarcinoma: five years of local and systemic control of a rare sweat gland neoplasm with nodal metastasis. Cureus. 2018;10:E2884.
- Miceli A, Ferrer-Bruker SJ. Spiradenoma. StatPearls. StatPearls Publishing; 2019.
- Banks PD, Sandhu S, Gyorki DE, et al. Recent insights and advances in the management of Merkel cell carcinoma. J Oncol Pract. 2016; 12:637-646.
- Flanagan BP, Helwig EB. Cutaneous lymphangioma. Arch Dermatol. 1977;113:24-30.
- Kalof AN, Cooper K. D2-40 immunohistochemistry—so far! Adv Anat Pathol. 2009;16:62-64.
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574.
The Diagnosis: Hidradenocarcinoma
An excisional biopsy revealed a neoplasm in the dermis with focal invasion into the adjacent soft tissue (Figure 1). The tumor consisted of sheets of cells with cytoplasmic vacuoles and ductal differentiation (Figure 2), as well as cells with mild atypia, mild pleomorphism, rare mitotic figures, and abundant pale cytoplasm. Immunohistochemical staining was positive for cytokeratin (CK) 5, CK7, CK20, CK AE1/AE3, and p63 (Figure 3). The culmination of features including the large tumor size, immunohistochemical staining pattern, and mild pleomorphism with focal invasion into the soft tissue supported the diagnosis of hidradenocarcinoma.



Hidradenocarcinoma is an exceedingly rare malignant tumor of eccrine and/or apocrine origin.1 It accounts for less than 0.001% of all tumors and 1 of 13,000 skin biopsies.2 It usually arises in the head and neck region and most commonly affects older adults aged 50 to 70 years.3 The size of hidradenocarcinomas can vary; however, they typically are large, often growing to be greater than 5 cm in diameter.2 It tends to be an aggressive tumor that generally spreads to regional lymph nodes and distant viscera.4 Although it most commonly arises de novo, it may occasionally derive from a benign hidradenoma.1 The diagnosis of hidradenocarcinoma is made based on the tumor’s morphologic and pathologic characteristics. Histologically, it is characterized by an infiltrative and invasive proliferation of lobules made of large clear cells with atypical mitotic figures and nuclear pleomorphism as well as immunohistochemical features displaying various positive markers, such as carcinoembryonic antigen, epithelial membrane antigen, S-100 protein, and CKs AE1/AE3 and 5/6.2 Invasion of the adjacent soft tissue can be present and helps to confirm the diagnosis.
The differential diagnosis for hidradenocarcinoma primarily is the benign hidradenoma, which is similar both clinically and histologically with a few important differences. Hidradenocarcinomas often are larger and ulcerated. Histologically, they usually are more pleomorphic with the presence of mitotic figures in clear cells and tend to invade locally into the surrounding soft tissue. Other similar lesions such as spiradenoma, Merkel cell carcinoma, lymphangioma, cutaneous Crohn disease, tumors metastatic to the skin, and metastatic clear cell carcinomas originating from other organs also are included in the differential diagnosis.2
Spiradenomas are dermal tumors originating from the sweat glands. They typically present as bluish, painful, solitary nodules on the ventral surfaces of the upper body, though multiple nodules also are reported.5 Spiradenomas manifest as a central constellation of pale large cells surrounded by small, dark, basaloid cells containing hyperchromatic nuclei. The microscopic appearance of the blue basaloid cells contrasts with the clear cells seen in hidradenoma.5
Merkel cell carcinoma is a cutaneous neuroendocrine tumor affecting elderly or immunosuppressed individuals. It arises in sun-exposed areas and often is associated with Merkel cell polyomavirus infection. The histologic features display small and round cells that stain positive for CK8, CK18, CK19, and CK20 but stain negative for CK7, a marker that often is positive in hidradenocarcinoma.6
Lymphangioma, particularly cavernous lymphangioma, may resemble the gross appearance of hidradenoma/ hidradenocarcinoma. It usually presents as irregular clear blue papules and nodules in the skin and subcutaneous tissue.7 The key histopathologic finding in this tumor is the endothelium-lined channels that stain positive for D2-40, a lymphatic endothelium marker.7,8
Cutaneous Crohn disease is classified as noncaseating granulomatous skin lesions that are noncontinuous with the gastrointestinal tract.9 Clinical presentations in addition to skin edema include erythematous plaques, ulcerations, and erosions. Histopathology reveals sterile noncaseating granulomas made of Langerhans giant cells, epithelioid histocytes, and plasma cells.9
Metastatic clear cell carcinomas, such as renal cell carcinoma, can be differentiated by a history of primary carcinoma, demonstration of histologic vascular stroma, and other features related to metastatic clear cell carcinoma.2
There are no well-established therapeutic guidelines for hidradenocarcinoma. Wide local excision with margins greater than 2 cm is the preferred initial treatment and often is performed in conjunction with sentinel lymph node biopsy. External beam radiotherapy and adjunctive chemotherapy have been used for tumors that could not be surgically cleared. However, the efficacy of these treatments has not been well established.2 Targeted therapies recently have emerged as an alternative treatment choice for hidradenocarcinoma due to the utilization of immunohistochemical and genomic testing. The discovery of specific gene mutations or the expression of hormonal receptors in this tumor have paved the way for targeting HER2-expressing hidradenocarcinomas with trastuzumab and those expressing estrogen receptor with the estrogen receptor inhibitor tamoxifen.1 Epidermal growth factor receptor inhibitors and PI3K/Akt/mTOR (phosphatidylinositol-3-kinase/AKT/mammalian target of rapamycin) pathway inhibitors also have been used to target various signal transduction pathways.2
Wide excision with 2.5-cm margins was performed on our patient, and a positron emission tomography– computed tomography scan revealed no metastatic disease. She declined sentinel lymph node biopsy and additional treatment. Due to the risk for recurrence, she was monitored closely with skin examinations and positron emission tomography–computed tomography every 3 months for the first year and every 6 months thereafter. Thus far, she has had no evidence of local or regional recurrence.
The Diagnosis: Hidradenocarcinoma
An excisional biopsy revealed a neoplasm in the dermis with focal invasion into the adjacent soft tissue (Figure 1). The tumor consisted of sheets of cells with cytoplasmic vacuoles and ductal differentiation (Figure 2), as well as cells with mild atypia, mild pleomorphism, rare mitotic figures, and abundant pale cytoplasm. Immunohistochemical staining was positive for cytokeratin (CK) 5, CK7, CK20, CK AE1/AE3, and p63 (Figure 3). The culmination of features including the large tumor size, immunohistochemical staining pattern, and mild pleomorphism with focal invasion into the soft tissue supported the diagnosis of hidradenocarcinoma.



Hidradenocarcinoma is an exceedingly rare malignant tumor of eccrine and/or apocrine origin.1 It accounts for less than 0.001% of all tumors and 1 of 13,000 skin biopsies.2 It usually arises in the head and neck region and most commonly affects older adults aged 50 to 70 years.3 The size of hidradenocarcinomas can vary; however, they typically are large, often growing to be greater than 5 cm in diameter.2 It tends to be an aggressive tumor that generally spreads to regional lymph nodes and distant viscera.4 Although it most commonly arises de novo, it may occasionally derive from a benign hidradenoma.1 The diagnosis of hidradenocarcinoma is made based on the tumor’s morphologic and pathologic characteristics. Histologically, it is characterized by an infiltrative and invasive proliferation of lobules made of large clear cells with atypical mitotic figures and nuclear pleomorphism as well as immunohistochemical features displaying various positive markers, such as carcinoembryonic antigen, epithelial membrane antigen, S-100 protein, and CKs AE1/AE3 and 5/6.2 Invasion of the adjacent soft tissue can be present and helps to confirm the diagnosis.
The differential diagnosis for hidradenocarcinoma primarily is the benign hidradenoma, which is similar both clinically and histologically with a few important differences. Hidradenocarcinomas often are larger and ulcerated. Histologically, they usually are more pleomorphic with the presence of mitotic figures in clear cells and tend to invade locally into the surrounding soft tissue. Other similar lesions such as spiradenoma, Merkel cell carcinoma, lymphangioma, cutaneous Crohn disease, tumors metastatic to the skin, and metastatic clear cell carcinomas originating from other organs also are included in the differential diagnosis.2
Spiradenomas are dermal tumors originating from the sweat glands. They typically present as bluish, painful, solitary nodules on the ventral surfaces of the upper body, though multiple nodules also are reported.5 Spiradenomas manifest as a central constellation of pale large cells surrounded by small, dark, basaloid cells containing hyperchromatic nuclei. The microscopic appearance of the blue basaloid cells contrasts with the clear cells seen in hidradenoma.5
Merkel cell carcinoma is a cutaneous neuroendocrine tumor affecting elderly or immunosuppressed individuals. It arises in sun-exposed areas and often is associated with Merkel cell polyomavirus infection. The histologic features display small and round cells that stain positive for CK8, CK18, CK19, and CK20 but stain negative for CK7, a marker that often is positive in hidradenocarcinoma.6
Lymphangioma, particularly cavernous lymphangioma, may resemble the gross appearance of hidradenoma/ hidradenocarcinoma. It usually presents as irregular clear blue papules and nodules in the skin and subcutaneous tissue.7 The key histopathologic finding in this tumor is the endothelium-lined channels that stain positive for D2-40, a lymphatic endothelium marker.7,8
Cutaneous Crohn disease is classified as noncaseating granulomatous skin lesions that are noncontinuous with the gastrointestinal tract.9 Clinical presentations in addition to skin edema include erythematous plaques, ulcerations, and erosions. Histopathology reveals sterile noncaseating granulomas made of Langerhans giant cells, epithelioid histocytes, and plasma cells.9
Metastatic clear cell carcinomas, such as renal cell carcinoma, can be differentiated by a history of primary carcinoma, demonstration of histologic vascular stroma, and other features related to metastatic clear cell carcinoma.2
There are no well-established therapeutic guidelines for hidradenocarcinoma. Wide local excision with margins greater than 2 cm is the preferred initial treatment and often is performed in conjunction with sentinel lymph node biopsy. External beam radiotherapy and adjunctive chemotherapy have been used for tumors that could not be surgically cleared. However, the efficacy of these treatments has not been well established.2 Targeted therapies recently have emerged as an alternative treatment choice for hidradenocarcinoma due to the utilization of immunohistochemical and genomic testing. The discovery of specific gene mutations or the expression of hormonal receptors in this tumor have paved the way for targeting HER2-expressing hidradenocarcinomas with trastuzumab and those expressing estrogen receptor with the estrogen receptor inhibitor tamoxifen.1 Epidermal growth factor receptor inhibitors and PI3K/Akt/mTOR (phosphatidylinositol-3-kinase/AKT/mammalian target of rapamycin) pathway inhibitors also have been used to target various signal transduction pathways.2
Wide excision with 2.5-cm margins was performed on our patient, and a positron emission tomography– computed tomography scan revealed no metastatic disease. She declined sentinel lymph node biopsy and additional treatment. Due to the risk for recurrence, she was monitored closely with skin examinations and positron emission tomography–computed tomography every 3 months for the first year and every 6 months thereafter. Thus far, she has had no evidence of local or regional recurrence.
- Miller DH, Peterson JL, Buskirk SJ, et al. Management of metastatic apocrine hidradenocarcinoma with chemotherapy and radiation. Rare Tumors. 2015;7:6082.
- Soni A, Bansal N, Kaushal V, et al. Current management approach to hidradenocarcinoma: a comprehensive review of the literature. Ecancermedicalscience. 2015;9:517.
- Jinnah AH, Emory CL, Mai NH, et al. Hidradenocarcinoma presenting as soft tissue mass: case report with cytomorphologic description, histologic correlation, and differential diagnosis. Diagn Cytopathol. 2016;44:438-441.
- Khan BM, Mansha MA, Ali N, et al. Hidradenocarcinoma: five years of local and systemic control of a rare sweat gland neoplasm with nodal metastasis. Cureus. 2018;10:E2884.
- Miceli A, Ferrer-Bruker SJ. Spiradenoma. StatPearls. StatPearls Publishing; 2019.
- Banks PD, Sandhu S, Gyorki DE, et al. Recent insights and advances in the management of Merkel cell carcinoma. J Oncol Pract. 2016; 12:637-646.
- Flanagan BP, Helwig EB. Cutaneous lymphangioma. Arch Dermatol. 1977;113:24-30.
- Kalof AN, Cooper K. D2-40 immunohistochemistry—so far! Adv Anat Pathol. 2009;16:62-64.
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574.
- Miller DH, Peterson JL, Buskirk SJ, et al. Management of metastatic apocrine hidradenocarcinoma with chemotherapy and radiation. Rare Tumors. 2015;7:6082.
- Soni A, Bansal N, Kaushal V, et al. Current management approach to hidradenocarcinoma: a comprehensive review of the literature. Ecancermedicalscience. 2015;9:517.
- Jinnah AH, Emory CL, Mai NH, et al. Hidradenocarcinoma presenting as soft tissue mass: case report with cytomorphologic description, histologic correlation, and differential diagnosis. Diagn Cytopathol. 2016;44:438-441.
- Khan BM, Mansha MA, Ali N, et al. Hidradenocarcinoma: five years of local and systemic control of a rare sweat gland neoplasm with nodal metastasis. Cureus. 2018;10:E2884.
- Miceli A, Ferrer-Bruker SJ. Spiradenoma. StatPearls. StatPearls Publishing; 2019.
- Banks PD, Sandhu S, Gyorki DE, et al. Recent insights and advances in the management of Merkel cell carcinoma. J Oncol Pract. 2016; 12:637-646.
- Flanagan BP, Helwig EB. Cutaneous lymphangioma. Arch Dermatol. 1977;113:24-30.
- Kalof AN, Cooper K. D2-40 immunohistochemistry—so far! Adv Anat Pathol. 2009;16:62-64.
- Schneider SL, Foster K, Patel D, et al. Cutaneous manifestations of metastatic Crohn’s disease. Pediatr Dermatol. 2018;35:566-574.
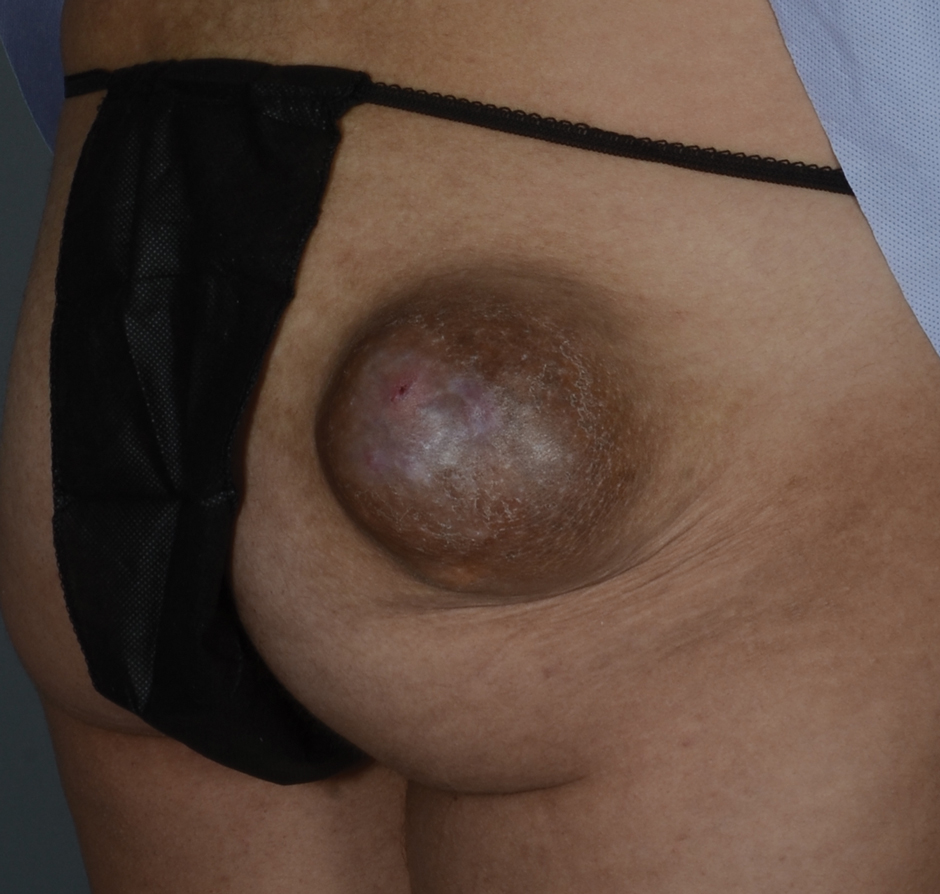
A 20-year-old woman with no notable medical history presented to the dermatology clinic with an enlarging mass on the right buttock that had been growing over the course of several years. The mass progressed from a small, mildly tender nodule to a 10×10-cm, hyperpigmented, exophytic tumor. There were no other abnormal findings on physical examination, and the patient denied any systemic symptoms.