User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Psoriasis patients with mental illness report lower satisfaction with physicians
according to a retrospective analysis of survey data.
The findings highlight the importance of clinicians being supportive and adaptable in their communication style when interacting with psoriasis patients with mental illness.
“This study aims to evaluate whether an association exists between a patient’s psychological state and the perception of patient-clinician encounters,” wrote Charlotte Read, MBBS, of Imperial College London, and April W. Armstrong, MD, MPH, of the University of Southern California, Los Angeles, in JAMA Dermatology.
The researchers retrospectively analyzed longitudinal data from over 8.8 million U.S. adults (unweighted, 652) with psoriasis who participated in the Medical Expenditure Panel Survey from 2004 to 2017. The nationally representative database includes various clinical information, such as data on patient demographics, health care use, and mental health comorbidities.
The primary outcome, patient satisfaction with their physician, was assessed using a patient-physician communication composite score. Mental health comorbidities were evaluated using standard questionnaires.
The mean age of study patients was 52.1 years (range, 0.7 years), and most were female (54%). In all, 73% of participants had no or mild psychological distress symptoms, and 27% had moderate or severe symptoms.
After analysis, the researchers found that patients with moderate psychological distress symptoms were 2.8 times more likely to report lower satisfaction with their physician than were those with no or mild symptoms (adjusted odds ratio, 2.8; P = .001). They also reported that patients with severe symptoms were more likely to report lower satisfaction (aOR, 2.3; P = .03).
“Patients with moderate or severe depression symptoms were less satisfied with their clinicians, compared with those with no or mild depression symptoms,” they further explained.
Based on the results, the coinvestigators emphasized the importance of bettering the patient experience for those with mental illness given the potential association with improved health outcomes.
“Because depressed patients can be more sensitive to negative communication, the clinician needs to be more conscious about using a positive and supportive communication style,” they recommended.
The authors acknowledged the inadequacy of evaluating clinician performance using patient satisfaction alone. As a result, the findings may not be generalizable to all clinical settings.
The study was funded by the National Psoriasis Foundation. Dr. Armstrong reported financial affiliations with several pharmaceutical companies.
SOURCE: Read C, Armstrong AW. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.1054.
according to a retrospective analysis of survey data.
The findings highlight the importance of clinicians being supportive and adaptable in their communication style when interacting with psoriasis patients with mental illness.
“This study aims to evaluate whether an association exists between a patient’s psychological state and the perception of patient-clinician encounters,” wrote Charlotte Read, MBBS, of Imperial College London, and April W. Armstrong, MD, MPH, of the University of Southern California, Los Angeles, in JAMA Dermatology.
The researchers retrospectively analyzed longitudinal data from over 8.8 million U.S. adults (unweighted, 652) with psoriasis who participated in the Medical Expenditure Panel Survey from 2004 to 2017. The nationally representative database includes various clinical information, such as data on patient demographics, health care use, and mental health comorbidities.
The primary outcome, patient satisfaction with their physician, was assessed using a patient-physician communication composite score. Mental health comorbidities were evaluated using standard questionnaires.
The mean age of study patients was 52.1 years (range, 0.7 years), and most were female (54%). In all, 73% of participants had no or mild psychological distress symptoms, and 27% had moderate or severe symptoms.
After analysis, the researchers found that patients with moderate psychological distress symptoms were 2.8 times more likely to report lower satisfaction with their physician than were those with no or mild symptoms (adjusted odds ratio, 2.8; P = .001). They also reported that patients with severe symptoms were more likely to report lower satisfaction (aOR, 2.3; P = .03).
“Patients with moderate or severe depression symptoms were less satisfied with their clinicians, compared with those with no or mild depression symptoms,” they further explained.
Based on the results, the coinvestigators emphasized the importance of bettering the patient experience for those with mental illness given the potential association with improved health outcomes.
“Because depressed patients can be more sensitive to negative communication, the clinician needs to be more conscious about using a positive and supportive communication style,” they recommended.
The authors acknowledged the inadequacy of evaluating clinician performance using patient satisfaction alone. As a result, the findings may not be generalizable to all clinical settings.
The study was funded by the National Psoriasis Foundation. Dr. Armstrong reported financial affiliations with several pharmaceutical companies.
SOURCE: Read C, Armstrong AW. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.1054.
according to a retrospective analysis of survey data.
The findings highlight the importance of clinicians being supportive and adaptable in their communication style when interacting with psoriasis patients with mental illness.
“This study aims to evaluate whether an association exists between a patient’s psychological state and the perception of patient-clinician encounters,” wrote Charlotte Read, MBBS, of Imperial College London, and April W. Armstrong, MD, MPH, of the University of Southern California, Los Angeles, in JAMA Dermatology.
The researchers retrospectively analyzed longitudinal data from over 8.8 million U.S. adults (unweighted, 652) with psoriasis who participated in the Medical Expenditure Panel Survey from 2004 to 2017. The nationally representative database includes various clinical information, such as data on patient demographics, health care use, and mental health comorbidities.
The primary outcome, patient satisfaction with their physician, was assessed using a patient-physician communication composite score. Mental health comorbidities were evaluated using standard questionnaires.
The mean age of study patients was 52.1 years (range, 0.7 years), and most were female (54%). In all, 73% of participants had no or mild psychological distress symptoms, and 27% had moderate or severe symptoms.
After analysis, the researchers found that patients with moderate psychological distress symptoms were 2.8 times more likely to report lower satisfaction with their physician than were those with no or mild symptoms (adjusted odds ratio, 2.8; P = .001). They also reported that patients with severe symptoms were more likely to report lower satisfaction (aOR, 2.3; P = .03).
“Patients with moderate or severe depression symptoms were less satisfied with their clinicians, compared with those with no or mild depression symptoms,” they further explained.
Based on the results, the coinvestigators emphasized the importance of bettering the patient experience for those with mental illness given the potential association with improved health outcomes.
“Because depressed patients can be more sensitive to negative communication, the clinician needs to be more conscious about using a positive and supportive communication style,” they recommended.
The authors acknowledged the inadequacy of evaluating clinician performance using patient satisfaction alone. As a result, the findings may not be generalizable to all clinical settings.
The study was funded by the National Psoriasis Foundation. Dr. Armstrong reported financial affiliations with several pharmaceutical companies.
SOURCE: Read C, Armstrong AW. JAMA Dermatol. 2020 May 6. doi: 10.1001/jamadermatol.2020.1054.
FROM JAMA DERMATOLOGY
Sericin, a versatile silk protein, has multiple potential roles in dermatology
Inexpensively obtained as a silk industry by-product, sericin is a glycoprotein found to confer various biologic effects.1 The globular protein sericin has also long been known to exhibit antityrosinase and immunomodulatory activities.2,3 This column focuses on the wide range of emerging and potential applications of sericin in cutaneous treatments.
Protection against solar radiation and photoaging
Studies in mice to evaluate the potential antioxidant and skin-protective effects of sericin by Zhaorigetu et al. in 2003 revealed that, by diminishing oxidative stress, cyclooxygenase-2 protein, and cell proliferation, sericin exerted a photoprotective effect against acute harm and tumor promotion elicited by UVB.4
Using mouse skin models, Dash et al. showed in 2008 that the silk protein sericin derived from the tropical tasar silkworm is a robust antioxidant and photoprotective agent, displaying a capacity to block UVB-induced apoptosis in irradiated (30 mJ/cm2 UVB) human keratinocytes and, as compared with the mulberry silkworm, yielding protection against oxidative stress.5,6
In 2015, Berardesca et al. conducted a randomized, double-blind, vehicle-controlled, split-face study over 8 weeks in 40 women (ages 40-70 years) to assess the antiaging effects of topically applied combination therapy including gold silk sericin, niacinamide, and signaline. The investigators observed significant improvements in stratum corneum hydration, barrier function, skin elasticity, and roughness as compared with skin treated with the control formulation. They concluded that this combination formulation featuring gold silk sericin warrants attention in the arsenal for ameliorating signs of aging female facial skin.7
A year earlier, Aramwit and Bang introduced a bacterial nanocellulose gel shown to effectively release silk sericin for facial treatment. Formulated at a pH of 4.5, the bioactive mask exhibited an ultrafine and pure fiber network structure. The authors noted that the gel was less adhesive than the commercially available paper mask, while the silk sericin product displayed greater moisture absorption capacity. In vitro cytotoxicity assessments also revealed that the product is safe for facial treatments.8
Cosmeceutical antioxidant for hyperpigmentation
In 2019, Kumar et al. demonstrated the inhibitory effect of topically applied silk sericin derived from Antheraea assamensis against UV-induced melanogenesis in mouse melanoma. They suggested that the formulation shows promise as a cosmeceutical antioxidant agent designed to address hyperpigmentation.3
The previous year, Aramwit et al. demonstrated using an in vitro model that urea-extracted sericin displays a capacity to inhibit melanogenesis by hindering tyrosinase activity, attenuating inflammation and allergic reactions, and reducing the expression of microphthalmia-associated transcription factor, a marker of melanogenesis regulation, in melanocytes and keratinocytes.2
Potential use as an adjunct psoriasis treatment
A combination of naringin (from Citrus maxima) and sericin (from Bombyx mori) was evaluated in 2019 by Deenonpoe et al. for the treatment of psoriasis. They isolated human peripheral blood mononuclear cells from 10 healthy subjects and 10 patients with psoriasis. The combination formulation was much more effective than either compound alone in significantly reducing mRNA expression and the synthesis of proinflammatory cytokines in samples from psoriasis patients. The investigators concluded that the down-regulation of proinflammatory cytokines imparted by the naringin/sericin product points toward its possible clinical use as a complementary treatment for psoriasis and other inflammation-mediated conditions.9
Uremic pruritus and burn wounds
A randomized, double-blind, placebo-controlled 6-week study in 2012 conducted by Aramwit et al. assessed the use of sericin cream versus a cream base placebo in the treatment of uremic pruritus in 50 hemodialysis patients, 47 of whom completed the study. Significant differences in the creams were identified, with hydration vastly improved in patients using the sericin cream. Significant reductions in pruritus and dyspigmentation were also observed in the treatment group, with an overall quality of life improvement noted in relation to pain score.10
The ensuing year, Aramwit et al. showed that silk sericin promoted wound healing in vitro and, when added to silver sulfadiazine cream and evaluated in a randomized, double-blind, standard-controlled study, demonstrated clinical efficacy in healing burn wounds.11
Wound healing
An expanding body of research suggests the role of sericin in wound healing. In 2007, Aramwit et al. found that sericin, which boasts notable hydrophilic qualities, was effective as a wound-healing agent in rats. The tested sericin cream successfully reduced wound size and wound healing time was substantially shorter than in animals treated with control formula. Treatment for 15 days yielded complete healing, no ulceration, and higher collagen levels, as determined by histologic examination, in comparison with control.12 Other studies using sericin hydrogel as well as a sericin-based nanofibrous matrix with chitosan have demonstrated success in wound healing in mice.13,14
Human studies
In 2018, Napavichayanun et al. reported on the clinical efficacy and safety of bacterial cellulose wound dressings including silk sericin and PHMB as compared with Bactigras (an antiseptic dressing) as a control in split-thickness skin graft donor-site wound treatment. In this single-blinded, randomized, controlled study of 21 patients, pain scores were significantly lower and wound quality higher in the skin treated with the sericin product. The test formulation was protected against infection without inducing adverse effects.15
Previously, a silk sericin–releasing wound dressing introduced in 2014 was found to significantly diminish pain and promote more rapid healing in patients with split-thickness skin graft donor sites as compared with treatment with the Bactigras wound dressing.16
Sericin in tissue repair and as a drug delivery carrier
Sericin is associated with antioxidant and moisturizing properties as well as a mitogenic influence on mammalian cells, with a particular impact on keratinocytes and fibroblasts that render it useful in biomaterials designed for skin tissue repair.17
Wang et al. have cross-linked dialdehyde carboxymethyl cellulose with silk sericin derived from the B. mori cocoon to develop a film with impressive blood compatibility and cytocompatibility that shows potential for use as a wound dressing, artificial skin, and in tissue engineering.18
Similarly, Liang et al. have been successful in preparing a medical tissue glue incorporating a gelatin, sericin, and carboxymethyl chitosan blend solution, cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. The tissue glue has been found to offer notable biocompatibility and structural traits at low cost.19
Sericin protein also evinces potential as a biocompatible, bioviable carrier for drug delivery. Suktham et al. showed that resveratrol-loaded sericin nanoparticles robustly hindered growth of colorectal adenocarcinoma cells while cytotoxic to skin fibroblasts, suggesting the viability or potential of sericin nanoparticles as bionanocarriers in a drug delivery system.20 In addition, Tao et al. found silk sericin to be effective when blended with poly(vinyl alcohol) in a hydrogel with antibacterial properties as a drug delivery carrier with potential for use as wound dressing.21
Conclusion
Much more research is necessary, though, to explore how the antioxidant and moisturizing activities of the protein may be harnessed to confer skin-protective effects, especially against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
2. Aramwit P et al. Biol Res. 2018 Nov 29;51(1):54.
3. Kumar JP, Mandal BB. Photochem Photobiol Sci. 2019 Oct 9:18(10):2497-508.
4. Zhaorigetu S et al. J Photochem Photobiol B. 2003 Oct 15;71(1-3):11-7.
5. Dash R et al. Mol Cell Biochem. 2008 Apr;311(1-2):111-9.
6. Dash R et al. BMB Rep. 2008 Mar 31;41(3):236-41.
7. Berardesca E et al. Int J Cosmet Sci. 2015 Dec;37(6):606-12.
8. Aramwit P, Bang N. BMC Biotechnol. 2014 Dec 9;14:104.
9. Deenonpoe R et al. BMC Complement Altern Med. 2019 Jul 10;19(1):168.
10. Aramwit P et al. BMC Nephrol. 2012 Sep 24;13:119.
11. Aramwit P et al. Arch Dermatol Res. 2013 Sep;305(7):585-94.
12. Aramwit P, Sangcakul A. Biosci Biotechnol Biochem. 2007 Oct;71(10):2473-7.
13. Qi C et al. Biomater Sci. 2018 Nov 1;6(11):2859-70.
14. Sapru S et al. Acta Biomater. 2018 Sep 15;78:137-50.
15. Napavichayanun S et al. Arch Dermatol Res. 2018 Dec;310(10):795-805.
16. Siritientong T et al. Pharm Res. 2014 Jan;31(1):104-16.
17. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
18. Wang P et al. Carbohydr Polym. 2019 May 15;212:403-11.
19. Liang M et al. J Appl Biomater Funct Mater. 2018 Apr;16(2):97-106.
20. Suktham K et al. Int J Pharm. 2018 Feb 15;537(1-2):48-56.
21. Tao G et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:341-51.
Inexpensively obtained as a silk industry by-product, sericin is a glycoprotein found to confer various biologic effects.1 The globular protein sericin has also long been known to exhibit antityrosinase and immunomodulatory activities.2,3 This column focuses on the wide range of emerging and potential applications of sericin in cutaneous treatments.
Protection against solar radiation and photoaging
Studies in mice to evaluate the potential antioxidant and skin-protective effects of sericin by Zhaorigetu et al. in 2003 revealed that, by diminishing oxidative stress, cyclooxygenase-2 protein, and cell proliferation, sericin exerted a photoprotective effect against acute harm and tumor promotion elicited by UVB.4
Using mouse skin models, Dash et al. showed in 2008 that the silk protein sericin derived from the tropical tasar silkworm is a robust antioxidant and photoprotective agent, displaying a capacity to block UVB-induced apoptosis in irradiated (30 mJ/cm2 UVB) human keratinocytes and, as compared with the mulberry silkworm, yielding protection against oxidative stress.5,6
In 2015, Berardesca et al. conducted a randomized, double-blind, vehicle-controlled, split-face study over 8 weeks in 40 women (ages 40-70 years) to assess the antiaging effects of topically applied combination therapy including gold silk sericin, niacinamide, and signaline. The investigators observed significant improvements in stratum corneum hydration, barrier function, skin elasticity, and roughness as compared with skin treated with the control formulation. They concluded that this combination formulation featuring gold silk sericin warrants attention in the arsenal for ameliorating signs of aging female facial skin.7
A year earlier, Aramwit and Bang introduced a bacterial nanocellulose gel shown to effectively release silk sericin for facial treatment. Formulated at a pH of 4.5, the bioactive mask exhibited an ultrafine and pure fiber network structure. The authors noted that the gel was less adhesive than the commercially available paper mask, while the silk sericin product displayed greater moisture absorption capacity. In vitro cytotoxicity assessments also revealed that the product is safe for facial treatments.8
Cosmeceutical antioxidant for hyperpigmentation
In 2019, Kumar et al. demonstrated the inhibitory effect of topically applied silk sericin derived from Antheraea assamensis against UV-induced melanogenesis in mouse melanoma. They suggested that the formulation shows promise as a cosmeceutical antioxidant agent designed to address hyperpigmentation.3
The previous year, Aramwit et al. demonstrated using an in vitro model that urea-extracted sericin displays a capacity to inhibit melanogenesis by hindering tyrosinase activity, attenuating inflammation and allergic reactions, and reducing the expression of microphthalmia-associated transcription factor, a marker of melanogenesis regulation, in melanocytes and keratinocytes.2
Potential use as an adjunct psoriasis treatment
A combination of naringin (from Citrus maxima) and sericin (from Bombyx mori) was evaluated in 2019 by Deenonpoe et al. for the treatment of psoriasis. They isolated human peripheral blood mononuclear cells from 10 healthy subjects and 10 patients with psoriasis. The combination formulation was much more effective than either compound alone in significantly reducing mRNA expression and the synthesis of proinflammatory cytokines in samples from psoriasis patients. The investigators concluded that the down-regulation of proinflammatory cytokines imparted by the naringin/sericin product points toward its possible clinical use as a complementary treatment for psoriasis and other inflammation-mediated conditions.9
Uremic pruritus and burn wounds
A randomized, double-blind, placebo-controlled 6-week study in 2012 conducted by Aramwit et al. assessed the use of sericin cream versus a cream base placebo in the treatment of uremic pruritus in 50 hemodialysis patients, 47 of whom completed the study. Significant differences in the creams were identified, with hydration vastly improved in patients using the sericin cream. Significant reductions in pruritus and dyspigmentation were also observed in the treatment group, with an overall quality of life improvement noted in relation to pain score.10
The ensuing year, Aramwit et al. showed that silk sericin promoted wound healing in vitro and, when added to silver sulfadiazine cream and evaluated in a randomized, double-blind, standard-controlled study, demonstrated clinical efficacy in healing burn wounds.11
Wound healing
An expanding body of research suggests the role of sericin in wound healing. In 2007, Aramwit et al. found that sericin, which boasts notable hydrophilic qualities, was effective as a wound-healing agent in rats. The tested sericin cream successfully reduced wound size and wound healing time was substantially shorter than in animals treated with control formula. Treatment for 15 days yielded complete healing, no ulceration, and higher collagen levels, as determined by histologic examination, in comparison with control.12 Other studies using sericin hydrogel as well as a sericin-based nanofibrous matrix with chitosan have demonstrated success in wound healing in mice.13,14
Human studies
In 2018, Napavichayanun et al. reported on the clinical efficacy and safety of bacterial cellulose wound dressings including silk sericin and PHMB as compared with Bactigras (an antiseptic dressing) as a control in split-thickness skin graft donor-site wound treatment. In this single-blinded, randomized, controlled study of 21 patients, pain scores were significantly lower and wound quality higher in the skin treated with the sericin product. The test formulation was protected against infection without inducing adverse effects.15
Previously, a silk sericin–releasing wound dressing introduced in 2014 was found to significantly diminish pain and promote more rapid healing in patients with split-thickness skin graft donor sites as compared with treatment with the Bactigras wound dressing.16
Sericin in tissue repair and as a drug delivery carrier
Sericin is associated with antioxidant and moisturizing properties as well as a mitogenic influence on mammalian cells, with a particular impact on keratinocytes and fibroblasts that render it useful in biomaterials designed for skin tissue repair.17
Wang et al. have cross-linked dialdehyde carboxymethyl cellulose with silk sericin derived from the B. mori cocoon to develop a film with impressive blood compatibility and cytocompatibility that shows potential for use as a wound dressing, artificial skin, and in tissue engineering.18
Similarly, Liang et al. have been successful in preparing a medical tissue glue incorporating a gelatin, sericin, and carboxymethyl chitosan blend solution, cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. The tissue glue has been found to offer notable biocompatibility and structural traits at low cost.19
Sericin protein also evinces potential as a biocompatible, bioviable carrier for drug delivery. Suktham et al. showed that resveratrol-loaded sericin nanoparticles robustly hindered growth of colorectal adenocarcinoma cells while cytotoxic to skin fibroblasts, suggesting the viability or potential of sericin nanoparticles as bionanocarriers in a drug delivery system.20 In addition, Tao et al. found silk sericin to be effective when blended with poly(vinyl alcohol) in a hydrogel with antibacterial properties as a drug delivery carrier with potential for use as wound dressing.21
Conclusion
Much more research is necessary, though, to explore how the antioxidant and moisturizing activities of the protein may be harnessed to confer skin-protective effects, especially against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
2. Aramwit P et al. Biol Res. 2018 Nov 29;51(1):54.
3. Kumar JP, Mandal BB. Photochem Photobiol Sci. 2019 Oct 9:18(10):2497-508.
4. Zhaorigetu S et al. J Photochem Photobiol B. 2003 Oct 15;71(1-3):11-7.
5. Dash R et al. Mol Cell Biochem. 2008 Apr;311(1-2):111-9.
6. Dash R et al. BMB Rep. 2008 Mar 31;41(3):236-41.
7. Berardesca E et al. Int J Cosmet Sci. 2015 Dec;37(6):606-12.
8. Aramwit P, Bang N. BMC Biotechnol. 2014 Dec 9;14:104.
9. Deenonpoe R et al. BMC Complement Altern Med. 2019 Jul 10;19(1):168.
10. Aramwit P et al. BMC Nephrol. 2012 Sep 24;13:119.
11. Aramwit P et al. Arch Dermatol Res. 2013 Sep;305(7):585-94.
12. Aramwit P, Sangcakul A. Biosci Biotechnol Biochem. 2007 Oct;71(10):2473-7.
13. Qi C et al. Biomater Sci. 2018 Nov 1;6(11):2859-70.
14. Sapru S et al. Acta Biomater. 2018 Sep 15;78:137-50.
15. Napavichayanun S et al. Arch Dermatol Res. 2018 Dec;310(10):795-805.
16. Siritientong T et al. Pharm Res. 2014 Jan;31(1):104-16.
17. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
18. Wang P et al. Carbohydr Polym. 2019 May 15;212:403-11.
19. Liang M et al. J Appl Biomater Funct Mater. 2018 Apr;16(2):97-106.
20. Suktham K et al. Int J Pharm. 2018 Feb 15;537(1-2):48-56.
21. Tao G et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:341-51.
Inexpensively obtained as a silk industry by-product, sericin is a glycoprotein found to confer various biologic effects.1 The globular protein sericin has also long been known to exhibit antityrosinase and immunomodulatory activities.2,3 This column focuses on the wide range of emerging and potential applications of sericin in cutaneous treatments.
Protection against solar radiation and photoaging
Studies in mice to evaluate the potential antioxidant and skin-protective effects of sericin by Zhaorigetu et al. in 2003 revealed that, by diminishing oxidative stress, cyclooxygenase-2 protein, and cell proliferation, sericin exerted a photoprotective effect against acute harm and tumor promotion elicited by UVB.4
Using mouse skin models, Dash et al. showed in 2008 that the silk protein sericin derived from the tropical tasar silkworm is a robust antioxidant and photoprotective agent, displaying a capacity to block UVB-induced apoptosis in irradiated (30 mJ/cm2 UVB) human keratinocytes and, as compared with the mulberry silkworm, yielding protection against oxidative stress.5,6
In 2015, Berardesca et al. conducted a randomized, double-blind, vehicle-controlled, split-face study over 8 weeks in 40 women (ages 40-70 years) to assess the antiaging effects of topically applied combination therapy including gold silk sericin, niacinamide, and signaline. The investigators observed significant improvements in stratum corneum hydration, barrier function, skin elasticity, and roughness as compared with skin treated with the control formulation. They concluded that this combination formulation featuring gold silk sericin warrants attention in the arsenal for ameliorating signs of aging female facial skin.7
A year earlier, Aramwit and Bang introduced a bacterial nanocellulose gel shown to effectively release silk sericin for facial treatment. Formulated at a pH of 4.5, the bioactive mask exhibited an ultrafine and pure fiber network structure. The authors noted that the gel was less adhesive than the commercially available paper mask, while the silk sericin product displayed greater moisture absorption capacity. In vitro cytotoxicity assessments also revealed that the product is safe for facial treatments.8
Cosmeceutical antioxidant for hyperpigmentation
In 2019, Kumar et al. demonstrated the inhibitory effect of topically applied silk sericin derived from Antheraea assamensis against UV-induced melanogenesis in mouse melanoma. They suggested that the formulation shows promise as a cosmeceutical antioxidant agent designed to address hyperpigmentation.3
The previous year, Aramwit et al. demonstrated using an in vitro model that urea-extracted sericin displays a capacity to inhibit melanogenesis by hindering tyrosinase activity, attenuating inflammation and allergic reactions, and reducing the expression of microphthalmia-associated transcription factor, a marker of melanogenesis regulation, in melanocytes and keratinocytes.2
Potential use as an adjunct psoriasis treatment
A combination of naringin (from Citrus maxima) and sericin (from Bombyx mori) was evaluated in 2019 by Deenonpoe et al. for the treatment of psoriasis. They isolated human peripheral blood mononuclear cells from 10 healthy subjects and 10 patients with psoriasis. The combination formulation was much more effective than either compound alone in significantly reducing mRNA expression and the synthesis of proinflammatory cytokines in samples from psoriasis patients. The investigators concluded that the down-regulation of proinflammatory cytokines imparted by the naringin/sericin product points toward its possible clinical use as a complementary treatment for psoriasis and other inflammation-mediated conditions.9
Uremic pruritus and burn wounds
A randomized, double-blind, placebo-controlled 6-week study in 2012 conducted by Aramwit et al. assessed the use of sericin cream versus a cream base placebo in the treatment of uremic pruritus in 50 hemodialysis patients, 47 of whom completed the study. Significant differences in the creams were identified, with hydration vastly improved in patients using the sericin cream. Significant reductions in pruritus and dyspigmentation were also observed in the treatment group, with an overall quality of life improvement noted in relation to pain score.10
The ensuing year, Aramwit et al. showed that silk sericin promoted wound healing in vitro and, when added to silver sulfadiazine cream and evaluated in a randomized, double-blind, standard-controlled study, demonstrated clinical efficacy in healing burn wounds.11
Wound healing
An expanding body of research suggests the role of sericin in wound healing. In 2007, Aramwit et al. found that sericin, which boasts notable hydrophilic qualities, was effective as a wound-healing agent in rats. The tested sericin cream successfully reduced wound size and wound healing time was substantially shorter than in animals treated with control formula. Treatment for 15 days yielded complete healing, no ulceration, and higher collagen levels, as determined by histologic examination, in comparison with control.12 Other studies using sericin hydrogel as well as a sericin-based nanofibrous matrix with chitosan have demonstrated success in wound healing in mice.13,14
Human studies
In 2018, Napavichayanun et al. reported on the clinical efficacy and safety of bacterial cellulose wound dressings including silk sericin and PHMB as compared with Bactigras (an antiseptic dressing) as a control in split-thickness skin graft donor-site wound treatment. In this single-blinded, randomized, controlled study of 21 patients, pain scores were significantly lower and wound quality higher in the skin treated with the sericin product. The test formulation was protected against infection without inducing adverse effects.15
Previously, a silk sericin–releasing wound dressing introduced in 2014 was found to significantly diminish pain and promote more rapid healing in patients with split-thickness skin graft donor sites as compared with treatment with the Bactigras wound dressing.16
Sericin in tissue repair and as a drug delivery carrier
Sericin is associated with antioxidant and moisturizing properties as well as a mitogenic influence on mammalian cells, with a particular impact on keratinocytes and fibroblasts that render it useful in biomaterials designed for skin tissue repair.17
Wang et al. have cross-linked dialdehyde carboxymethyl cellulose with silk sericin derived from the B. mori cocoon to develop a film with impressive blood compatibility and cytocompatibility that shows potential for use as a wound dressing, artificial skin, and in tissue engineering.18
Similarly, Liang et al. have been successful in preparing a medical tissue glue incorporating a gelatin, sericin, and carboxymethyl chitosan blend solution, cross-linked with 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide. The tissue glue has been found to offer notable biocompatibility and structural traits at low cost.19
Sericin protein also evinces potential as a biocompatible, bioviable carrier for drug delivery. Suktham et al. showed that resveratrol-loaded sericin nanoparticles robustly hindered growth of colorectal adenocarcinoma cells while cytotoxic to skin fibroblasts, suggesting the viability or potential of sericin nanoparticles as bionanocarriers in a drug delivery system.20 In addition, Tao et al. found silk sericin to be effective when blended with poly(vinyl alcohol) in a hydrogel with antibacterial properties as a drug delivery carrier with potential for use as wound dressing.21
Conclusion
Much more research is necessary, though, to explore how the antioxidant and moisturizing activities of the protein may be harnessed to confer skin-protective effects, especially against UV damage.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002), and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), and a New York Times Best Sellers book for consumers,“The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems. Write to her at [email protected]
References
1. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
2. Aramwit P et al. Biol Res. 2018 Nov 29;51(1):54.
3. Kumar JP, Mandal BB. Photochem Photobiol Sci. 2019 Oct 9:18(10):2497-508.
4. Zhaorigetu S et al. J Photochem Photobiol B. 2003 Oct 15;71(1-3):11-7.
5. Dash R et al. Mol Cell Biochem. 2008 Apr;311(1-2):111-9.
6. Dash R et al. BMB Rep. 2008 Mar 31;41(3):236-41.
7. Berardesca E et al. Int J Cosmet Sci. 2015 Dec;37(6):606-12.
8. Aramwit P, Bang N. BMC Biotechnol. 2014 Dec 9;14:104.
9. Deenonpoe R et al. BMC Complement Altern Med. 2019 Jul 10;19(1):168.
10. Aramwit P et al. BMC Nephrol. 2012 Sep 24;13:119.
11. Aramwit P et al. Arch Dermatol Res. 2013 Sep;305(7):585-94.
12. Aramwit P, Sangcakul A. Biosci Biotechnol Biochem. 2007 Oct;71(10):2473-7.
13. Qi C et al. Biomater Sci. 2018 Nov 1;6(11):2859-70.
14. Sapru S et al. Acta Biomater. 2018 Sep 15;78:137-50.
15. Napavichayanun S et al. Arch Dermatol Res. 2018 Dec;310(10):795-805.
16. Siritientong T et al. Pharm Res. 2014 Jan;31(1):104-16.
17. Lamboni L et al. Biotechnol Adv. 2015 Dec;33(8):1855-67.
18. Wang P et al. Carbohydr Polym. 2019 May 15;212:403-11.
19. Liang M et al. J Appl Biomater Funct Mater. 2018 Apr;16(2):97-106.
20. Suktham K et al. Int J Pharm. 2018 Feb 15;537(1-2):48-56.
21. Tao G et al. Mater Sci Eng C Mater Biol Appl. 2019 Aug;101:341-51.
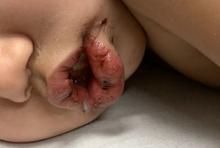
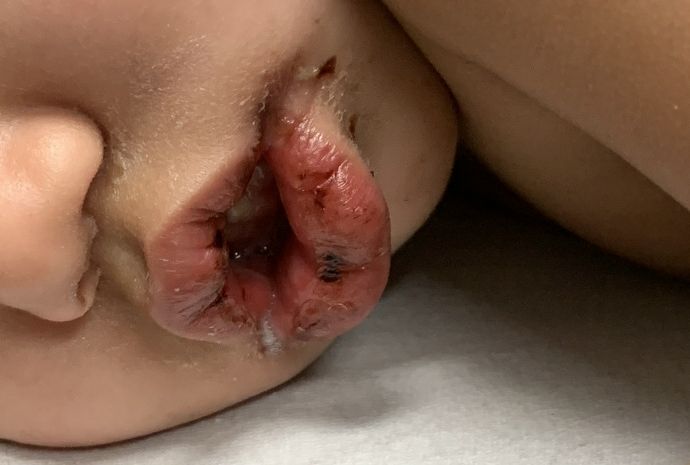
What's your diagnosis?
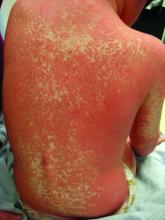
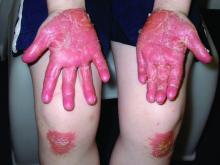
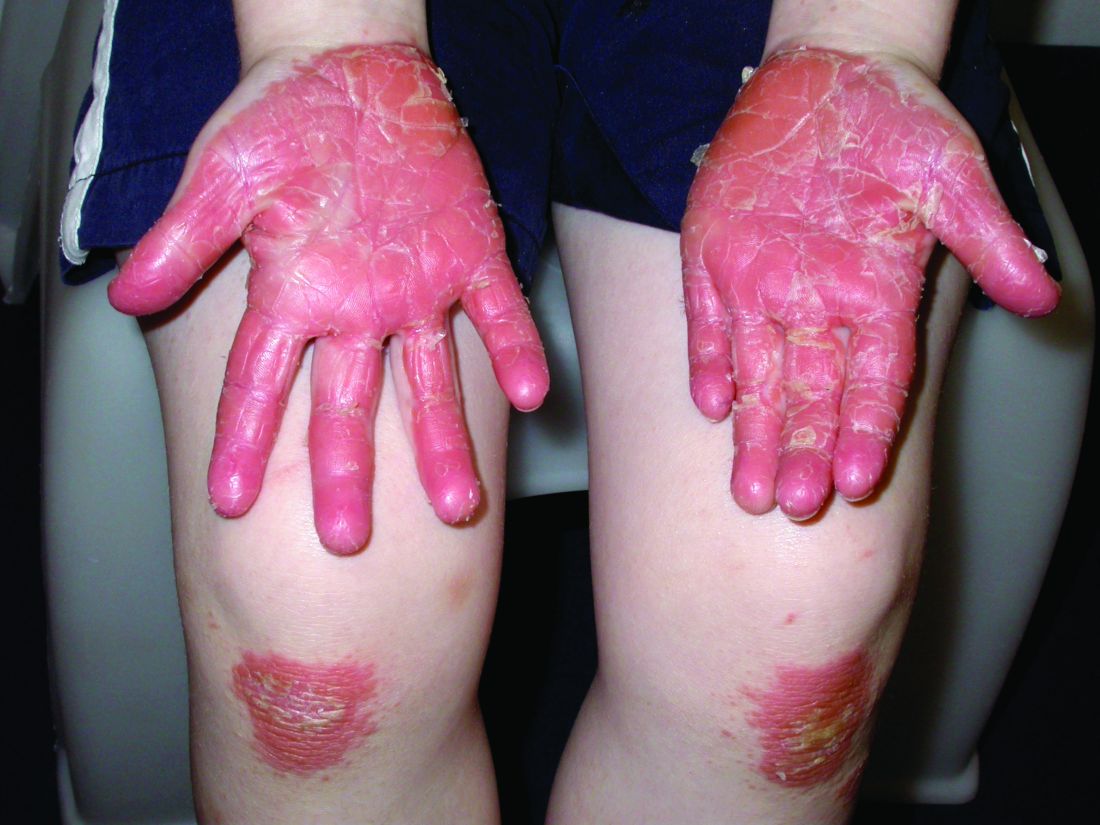
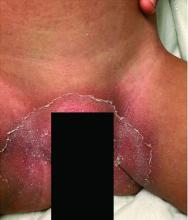
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at [email protected].
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at [email protected].
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
Pityriasis rubra pilaris (PRP) is the name given to a heterogeneous group of rare inflammatory papulosquamous dermatoses. There are six sub-types that can present with various skin findings, however, the cardinal features across sub-types include well-defined, red-orange hued plaques with varying scale, palmoplantar keratoderma, and follicular keratosis. In the more generalized subtypes, there is a characteristic feature of intervening areas of unaffected skin often referred to as “islands of sparing.” The plaques may cover the entire body or just parts of the body such as the elbows and knees, palms and soles. Lesions are generally asymptomatic; occasionally patients complain of mild pruritus.
The etiology and pathophysiology of this group of disorders is not well understood. However, there are several hypotheses including dysfunction in vitamin A metabolism, autoimmune dysregulation, as well as environmental and immunologic triggers such as infection and ultraviolet exposure. Although most cases are sporadic, genetics do seem to play a role in the development of some cases. Caspase recruitment domain-containing protein 14 (CARD14) mutations are seen in familial PRP, and occasionally in patients with sporadic PRP, with gain of function mutations. Interestingly, CARD14 mutations are also associated with psoriasis in some individuals.1 The type-VI PRP variant has been associated with HIV, although this is incredibly rare in pediatrics.2
PRP shows significant clinical diversity, with six subtypes defined by age of onset, distribution, and appearance of lesions, and presence of HIV. This includes type I (classical adult onset), type II (atypical adult onset), type III (classical juvenile onset), type IV (circumscribed juvenile onset), type V (atypical juvenile onset), and type VI (HIV-associated). As mentioned earlier, shared features that appear across subtypes in variable degrees include red-orange papules and plaques, hyperkeratotic follicular papules, and palmoplantar hyperkeratosis.
Of the six subtypes, type III, IV, and V occur in the pediatric population. Type III, classic juvenile PRP, typically occurs within the first 2 years of life or in adolescence. Only 10% of cases fall into this category. It shares similar features to type I PRP including red-orange plaques; islands of sparing, perifollicular hyperkeratotic papules; waxy palmoplantar keratoderma; and the distribution of affected skin is more diffuse overall. While some children clear within a few years, more recent studies stress a more prolonged course similar to the type IV variant.2
Type-IV PRP, also known as circumscribed juvenile PRP, is a focal variant, usually seen in prepubertal children and making up 25% of total cases. Clinically, these patients tend to have sharply demarcated grouped erythematous, follicular papules on the elbows, knees and over bony prominences.2
Type-V PRP is an atypical generalized juvenile variant which affects 5% of patients. It is a non-remitting hereditary condition with classic characteristics similar to type III with additional scleroderma-like changes involving the palms and soles.2
Diagnosis of PRP is based on clinical recognition and biopsy can be important to secure a diagnosis.
PRP, in many cases is self-limited and asymptomatic, and therefore does not necessarily require treatment. In other patients treatment can be challenging, and referral to a pediatric dermatology specialist is reasonable. Most practitioners recommend combination therapy with topical agents (emollients, topical corticosteroids, tazarotene, topical calcineurin inhibitors, and keratolytic agents such as urea, salicylic acid, or alpha-hydroxy acids) for symptomatic management and systemic therapies (methotrexate, isotretinoin) aimed at reducing inflammation. There is some data that CARD14-associated PRP can respond well to targeted biologic therapies.1
The subtypes of PRP can present in a myriad of ways and often the disease is misdiagnosed. Depending on the particular subtype and findings present, the differential can vary considerably. Commonly, physicians need to consider: psoriasis, seborrheic dermatitis, atopic dermatitis, ichthyoses, and other conditions which can cause erythroderma.3 The characteristic red-orange color and variable associated edema helps to distinguish keratoderma of PRP from psoriasis, atopic dermatitis, ichthyosis, and hereditary palmoplantar keratoderma. Scalp involvement of PRP should be differentiated from the waxy scale of seborrheic dermatitis and the well demarcated silvery scale of psoriasis. History alone may assist in distinguishing PRP from other major causes of generalized erythroderma, although biopsy is warranted in these cases.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Tracy is a research fellow in pediatric dermatology at Rady Children’s Hospital-San Diego and the University of California, San Diego. They have no relevant financial disclosures. Email them at [email protected].
References
1. J Am Acad Dermatol. 2018 Sep;79(3):487-94.
2. “Pityriasis Rubra Pilaris” (Treasure Island, Fla.: StatPearls Publishing, July 20, 2019). 3. JAMA Dermatol. 2016 Jun 1;152(6):670-5.
A 10-year-old, otherwise healthy female with no prior significant medical history is brought into clinic for evaluation of orange-red scaly papules and plaques that first started on the face, neck, and fingers and began spreading to the trunk, arms, and knees. The mother of the patient also had noticed thickening of the skin on her palms and soles. The rash has been present for 2 months. Patient does not appear to be itchy, and otherwise is in normal state without pain, fever, drainage from sites, or known exposures. She was initially treated with topical triamcinolone with minimal improvement.
On physical exam, she is noted to have reddish-orange hyperkeratotic scaling papules coalescing into large plaques with follicular prominence diffusely on the face, neck, trunk, and upper extremities with smaller islands of skin that are normal-appearing. There is diffuse fine scale throughout the scalp and thickening of the skin on the palms and soles with a yellowish waxy appearance.
Summary of the IDSA guidelines on the diagnosis of COVID-19
These guidelines were developed using a rigorous evidence-based approach, the GRADE framework, which involved identifying the important questions that need to be addressed ahead of time and, later, integrating the best available evidence into the recommendations.
The Food and Drug Administration’s Emergency Use Authorization is useful for understanding any recommendations related to COVID-19 testing. Under usual FDA approval, a manufacturer has to submit data on the performance of a test in human subjects. Under the Emergency Use Authorization for development and approval of SARS-CoV-2 testing, approval is based on “acceptable analytical accuracy,” meaning that a test is assessed using manufactured reagents. The approved test is not tested in real-world clinical situations prior to FDA approval, and the test’s sensitivity and specificity are not well described.
IDSA formulated 15 recommendations, of which the most relevant to primary care clinicians are described and discussed below. The complete set of recommendations can be viewed on the IDSA website:
Recommendation 1
The IDSA panel recommends a SARS-CoV-2 nucleic acid amplification test in symptomatic individuals in the community suspected of having COVID-19, even when the clinical suspicion is low (strong recommendation, very low certainty of evidence). The panel placed a high value on accurate assessment of COVID-19 with the intent of minimizing overdiagnosis of COVID-19 using clinical diagnosis alone. Without testing, the rate of overdiagnosis ranges from 62% to 98%.
If patients are misdiagnosed as having COVID-19, they may spend unnecessary time in quarantine and then may stop taking appropriate safety precautions to protect themselves from infection.
Recommendation 2
The IDSA panel suggests collecting nasopharyngeal, or mid-turbinate or nasal swabs, rather than oropharyngeal swabs or saliva alone for SARS-CoV-2 RNA testing in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, very low certainty of evidence).
The rationale for this recommendation is that comparative data showed a much lower sensitivity for oral sampling, compared with nasopharyngeal, mid-turbinate, or nasal sampling.
The average sensitivity of oral swabs is 56%, compared with nasopharyngeal at 97%, mid-turbinate at 100%, and nasal sampling at 95%. Given these test characteristics, there are far less false-negative tests with nasopharyngeal, mid-turbinate, and nasal swabs. Fewer false negatives means fewer instances of incorrectly telling COVID-19–positive patients that they do not have the illness. An exciting new area of testing that is being evaluated is saliva, which appears to have a sensitivity of 85%.
Recommendation 3
The IDSA panel suggests that nasal and mid-turbinate swab specimens may be collected for SARS-CoV-2 RNA testing by either patients or health care providers in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, low certainty of evidence).
This recommendation is particularly exciting because patient self-collection provides the potential for health care personnel to avoid exposure to infection, as can occur when health care personnel are swabbing a patient; this is ow testing has been done at most testing centers.
While the data are limited, it appears that patient self-collection of nasal or mid-turbinate swabs results in similar detection rates as occurs with health care personnel–collected nasopharyngeal swabs.
Recommendation 6
The IDSA panel suggests repeating viral RNA testing when the initial test is negative (versus performing a single test) in symptomatic individuals with an intermediate or high clinical suspicion of COVID-19 (conditional recommendation, low certainty of evidence).
Since none of the tests are perfect and any can have false negatives, the panel places a high value on detecting infection when present. If there is a low clinical likelihood of disease, the panel recommends not retesting. When the clinical likelihood of COVID-19 is moderate to high, in the event that the initial test is negative, the panel recommends retesting for COVID-19 1-2 days after the initial test.
Recommendation 8
The IDSA panel suggests SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to COVID-19 (conditional recommendation, very low certainty of evidence).
For this recommendation, a known contact is defined as someone who has had direct contact with a confirmed case.
A suspected exposure occurs when someone is working or living in a congregate setting such as long-term care, a correctional facility, or a cruise ship in which there is an outbreak. The time frame during which to do post-exposure testing is five to seven days after the exposure.
Recommendation 10
The IDSA panel recommends direct SARS-CoV-2 RNA testing in asymptomatic individuals with no known contact with COVID-19 who are being hospitalized in areas with a high prevalence of COVID-19 in the community (conditional recommendation, very low certainty of evidence).
The idea is to do rapid testing to identify individuals entering the hospital either for other illnesses or for procedures, in order to be able to institute appropriate precautions and decrease the likelihood of nosocomial transmission and/or transmission to health care personnel. It is worth noting that the recommendations do not address testing in areas with a low or intermediate prevalence of COVID-19. In the absence of an official guideline-based-recommendation, the decision about testing needs to made by the local hospital system.
Recommendations 11, 12, and 13
The IDSA panel recommends SARS-CoV-2 RNA testing in immunocompromised asymptomatic individuals who are being admitted to the hospital and in asymptomatic individuals prior to receiving immunosuppressive therapy regardless of exposure to COVID-19. It is also recommended to test asymptomatic individuals planning to undergo major surgery.
The rationale for this recommendation is that patients who are to receive chemotherapy, other immunosuppressive procedures, or surgery are at high risk if they have COVID-19 and may be better off delaying the procedure.
Some additional issues were addressed, though not in the form of additional recommendations. It was clarified that some individuals remain nucleic acid positive after their symptoms resolve, and sometimes even after seroconversion. It is not clear if those individuals remain infectious to others. The recommendations did not address serologic testing for public health surveillance.
Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
SOURCE: Hanson KE et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19.
These guidelines were developed using a rigorous evidence-based approach, the GRADE framework, which involved identifying the important questions that need to be addressed ahead of time and, later, integrating the best available evidence into the recommendations.
The Food and Drug Administration’s Emergency Use Authorization is useful for understanding any recommendations related to COVID-19 testing. Under usual FDA approval, a manufacturer has to submit data on the performance of a test in human subjects. Under the Emergency Use Authorization for development and approval of SARS-CoV-2 testing, approval is based on “acceptable analytical accuracy,” meaning that a test is assessed using manufactured reagents. The approved test is not tested in real-world clinical situations prior to FDA approval, and the test’s sensitivity and specificity are not well described.
IDSA formulated 15 recommendations, of which the most relevant to primary care clinicians are described and discussed below. The complete set of recommendations can be viewed on the IDSA website:
Recommendation 1
The IDSA panel recommends a SARS-CoV-2 nucleic acid amplification test in symptomatic individuals in the community suspected of having COVID-19, even when the clinical suspicion is low (strong recommendation, very low certainty of evidence). The panel placed a high value on accurate assessment of COVID-19 with the intent of minimizing overdiagnosis of COVID-19 using clinical diagnosis alone. Without testing, the rate of overdiagnosis ranges from 62% to 98%.
If patients are misdiagnosed as having COVID-19, they may spend unnecessary time in quarantine and then may stop taking appropriate safety precautions to protect themselves from infection.
Recommendation 2
The IDSA panel suggests collecting nasopharyngeal, or mid-turbinate or nasal swabs, rather than oropharyngeal swabs or saliva alone for SARS-CoV-2 RNA testing in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, very low certainty of evidence).
The rationale for this recommendation is that comparative data showed a much lower sensitivity for oral sampling, compared with nasopharyngeal, mid-turbinate, or nasal sampling.
The average sensitivity of oral swabs is 56%, compared with nasopharyngeal at 97%, mid-turbinate at 100%, and nasal sampling at 95%. Given these test characteristics, there are far less false-negative tests with nasopharyngeal, mid-turbinate, and nasal swabs. Fewer false negatives means fewer instances of incorrectly telling COVID-19–positive patients that they do not have the illness. An exciting new area of testing that is being evaluated is saliva, which appears to have a sensitivity of 85%.
Recommendation 3
The IDSA panel suggests that nasal and mid-turbinate swab specimens may be collected for SARS-CoV-2 RNA testing by either patients or health care providers in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, low certainty of evidence).
This recommendation is particularly exciting because patient self-collection provides the potential for health care personnel to avoid exposure to infection, as can occur when health care personnel are swabbing a patient; this is ow testing has been done at most testing centers.
While the data are limited, it appears that patient self-collection of nasal or mid-turbinate swabs results in similar detection rates as occurs with health care personnel–collected nasopharyngeal swabs.
Recommendation 6
The IDSA panel suggests repeating viral RNA testing when the initial test is negative (versus performing a single test) in symptomatic individuals with an intermediate or high clinical suspicion of COVID-19 (conditional recommendation, low certainty of evidence).
Since none of the tests are perfect and any can have false negatives, the panel places a high value on detecting infection when present. If there is a low clinical likelihood of disease, the panel recommends not retesting. When the clinical likelihood of COVID-19 is moderate to high, in the event that the initial test is negative, the panel recommends retesting for COVID-19 1-2 days after the initial test.
Recommendation 8
The IDSA panel suggests SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to COVID-19 (conditional recommendation, very low certainty of evidence).
For this recommendation, a known contact is defined as someone who has had direct contact with a confirmed case.
A suspected exposure occurs when someone is working or living in a congregate setting such as long-term care, a correctional facility, or a cruise ship in which there is an outbreak. The time frame during which to do post-exposure testing is five to seven days after the exposure.
Recommendation 10
The IDSA panel recommends direct SARS-CoV-2 RNA testing in asymptomatic individuals with no known contact with COVID-19 who are being hospitalized in areas with a high prevalence of COVID-19 in the community (conditional recommendation, very low certainty of evidence).
The idea is to do rapid testing to identify individuals entering the hospital either for other illnesses or for procedures, in order to be able to institute appropriate precautions and decrease the likelihood of nosocomial transmission and/or transmission to health care personnel. It is worth noting that the recommendations do not address testing in areas with a low or intermediate prevalence of COVID-19. In the absence of an official guideline-based-recommendation, the decision about testing needs to made by the local hospital system.
Recommendations 11, 12, and 13
The IDSA panel recommends SARS-CoV-2 RNA testing in immunocompromised asymptomatic individuals who are being admitted to the hospital and in asymptomatic individuals prior to receiving immunosuppressive therapy regardless of exposure to COVID-19. It is also recommended to test asymptomatic individuals planning to undergo major surgery.
The rationale for this recommendation is that patients who are to receive chemotherapy, other immunosuppressive procedures, or surgery are at high risk if they have COVID-19 and may be better off delaying the procedure.
Some additional issues were addressed, though not in the form of additional recommendations. It was clarified that some individuals remain nucleic acid positive after their symptoms resolve, and sometimes even after seroconversion. It is not clear if those individuals remain infectious to others. The recommendations did not address serologic testing for public health surveillance.
Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
SOURCE: Hanson KE et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19.
These guidelines were developed using a rigorous evidence-based approach, the GRADE framework, which involved identifying the important questions that need to be addressed ahead of time and, later, integrating the best available evidence into the recommendations.
The Food and Drug Administration’s Emergency Use Authorization is useful for understanding any recommendations related to COVID-19 testing. Under usual FDA approval, a manufacturer has to submit data on the performance of a test in human subjects. Under the Emergency Use Authorization for development and approval of SARS-CoV-2 testing, approval is based on “acceptable analytical accuracy,” meaning that a test is assessed using manufactured reagents. The approved test is not tested in real-world clinical situations prior to FDA approval, and the test’s sensitivity and specificity are not well described.
IDSA formulated 15 recommendations, of which the most relevant to primary care clinicians are described and discussed below. The complete set of recommendations can be viewed on the IDSA website:
Recommendation 1
The IDSA panel recommends a SARS-CoV-2 nucleic acid amplification test in symptomatic individuals in the community suspected of having COVID-19, even when the clinical suspicion is low (strong recommendation, very low certainty of evidence). The panel placed a high value on accurate assessment of COVID-19 with the intent of minimizing overdiagnosis of COVID-19 using clinical diagnosis alone. Without testing, the rate of overdiagnosis ranges from 62% to 98%.
If patients are misdiagnosed as having COVID-19, they may spend unnecessary time in quarantine and then may stop taking appropriate safety precautions to protect themselves from infection.
Recommendation 2
The IDSA panel suggests collecting nasopharyngeal, or mid-turbinate or nasal swabs, rather than oropharyngeal swabs or saliva alone for SARS-CoV-2 RNA testing in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, very low certainty of evidence).
The rationale for this recommendation is that comparative data showed a much lower sensitivity for oral sampling, compared with nasopharyngeal, mid-turbinate, or nasal sampling.
The average sensitivity of oral swabs is 56%, compared with nasopharyngeal at 97%, mid-turbinate at 100%, and nasal sampling at 95%. Given these test characteristics, there are far less false-negative tests with nasopharyngeal, mid-turbinate, and nasal swabs. Fewer false negatives means fewer instances of incorrectly telling COVID-19–positive patients that they do not have the illness. An exciting new area of testing that is being evaluated is saliva, which appears to have a sensitivity of 85%.
Recommendation 3
The IDSA panel suggests that nasal and mid-turbinate swab specimens may be collected for SARS-CoV-2 RNA testing by either patients or health care providers in symptomatic individuals with upper respiratory tract infection or influenza-like illness suspected of having COVID-19 (conditional recommendation, low certainty of evidence).
This recommendation is particularly exciting because patient self-collection provides the potential for health care personnel to avoid exposure to infection, as can occur when health care personnel are swabbing a patient; this is ow testing has been done at most testing centers.
While the data are limited, it appears that patient self-collection of nasal or mid-turbinate swabs results in similar detection rates as occurs with health care personnel–collected nasopharyngeal swabs.
Recommendation 6
The IDSA panel suggests repeating viral RNA testing when the initial test is negative (versus performing a single test) in symptomatic individuals with an intermediate or high clinical suspicion of COVID-19 (conditional recommendation, low certainty of evidence).
Since none of the tests are perfect and any can have false negatives, the panel places a high value on detecting infection when present. If there is a low clinical likelihood of disease, the panel recommends not retesting. When the clinical likelihood of COVID-19 is moderate to high, in the event that the initial test is negative, the panel recommends retesting for COVID-19 1-2 days after the initial test.
Recommendation 8
The IDSA panel suggests SARS-CoV-2 RNA testing in asymptomatic individuals who are either known or suspected to have been exposed to COVID-19 (conditional recommendation, very low certainty of evidence).
For this recommendation, a known contact is defined as someone who has had direct contact with a confirmed case.
A suspected exposure occurs when someone is working or living in a congregate setting such as long-term care, a correctional facility, or a cruise ship in which there is an outbreak. The time frame during which to do post-exposure testing is five to seven days after the exposure.
Recommendation 10
The IDSA panel recommends direct SARS-CoV-2 RNA testing in asymptomatic individuals with no known contact with COVID-19 who are being hospitalized in areas with a high prevalence of COVID-19 in the community (conditional recommendation, very low certainty of evidence).
The idea is to do rapid testing to identify individuals entering the hospital either for other illnesses or for procedures, in order to be able to institute appropriate precautions and decrease the likelihood of nosocomial transmission and/or transmission to health care personnel. It is worth noting that the recommendations do not address testing in areas with a low or intermediate prevalence of COVID-19. In the absence of an official guideline-based-recommendation, the decision about testing needs to made by the local hospital system.
Recommendations 11, 12, and 13
The IDSA panel recommends SARS-CoV-2 RNA testing in immunocompromised asymptomatic individuals who are being admitted to the hospital and in asymptomatic individuals prior to receiving immunosuppressive therapy regardless of exposure to COVID-19. It is also recommended to test asymptomatic individuals planning to undergo major surgery.
The rationale for this recommendation is that patients who are to receive chemotherapy, other immunosuppressive procedures, or surgery are at high risk if they have COVID-19 and may be better off delaying the procedure.
Some additional issues were addressed, though not in the form of additional recommendations. It was clarified that some individuals remain nucleic acid positive after their symptoms resolve, and sometimes even after seroconversion. It is not clear if those individuals remain infectious to others. The recommendations did not address serologic testing for public health surveillance.
Dr. Skolnik is professor of family and community medicine at the Sidney Kimmel Medical College of Thomas Jefferson University, Philadelphia, and associate director of the family medicine residency program at Abington (Pa.) Jefferson Health.
SOURCE: Hanson KE et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID-19.
ER docs ask, “Where are our patients?”
according to an expert panel on unanticipated consequences of pandemic care hosted by the presidents of the Society of Critical Care Medicine and the American College of Emergency Physicians.*
“At the peak of exposure to COVID-19 illness or infection, ED volumes in my system, which are really not much different from others across the country, were cut in half, if not more. And those changes happened across virtually every form of ED presentation, from the highest acuity to the lowest. We’re now beyond our highest level of exposure to COVID-19 clinically symptomatic patients in western Pennsylvania, but that recovery in volume hasn’t occurred yet, although there are some embers,” explained Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh.
He and other panelists also addressed some of the other unanticipated developments in the COVID-19 pandemic, including a recently recognized childhood manifestation called for now COVID-associated pediatric multisystem inflammatory syndrome, an anticipated massive second wave of non-COVID patients expected to present late to EDs and primary care clinics after having avoided needed medical care out of fear of infection, and the pandemic’s negative impact upon medical education.
Who’s not showing up in the ED
Dr. Yealy said that across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more.
“You might say, if I was designing from the insurance side, this is exactly what I’d hope for. I’ve heard that some people on the insurance-only side of the business really are experiencing a pretty good deal right now: They’re collecting premiums and not having to pay out on the ED or hospital side,” he said.
Tweaking the public health message on seeking medical care
“One of the unanticipated casualties of the pandemic are the patients who don’t have it. It will take a whole lot of work and coordinated effort to re-engage with those patients,” predicted SCCM President Lewis J. Kaplan, MD, professor of surgery at the University of Pennsylvania, Philadelphia.
Evie G. Marcolini, MD, described what she believes is necessary now: “We need to have a big focus on getting the word out to the public that acute MI, stroke, and other acute injuries are still a time-sensitive problem and they warrant at least a call to their physician or consideration of coming in to the ED.
“I think when we started out, we were telling people, ‘Don’t come in.’ Now we’re trying to dial it back a little bit and say, ‘Listen, there are things you really do need to come in for. And we will keep you safe,’” said Dr. Marcolini, an emergency medicine and neurocritical care specialist at Dartmouth-Hitchcock Medical Center, Hanover, N.H.
“It is safe,” Dr. Yealy agreed. “The safest place in the world to be right now is the ED. Everybody’s cordoned off. There’s way more PPE [personal protective equipment]. There’s a level of precision now that should have existed but never did in our previous influenza seasons. So we have something very unique to offer, and we can put people’s minds at rest.”
He spoke of a coming “tsunami of untreated illness.”
“My concern is there is a significant subset of people who are not only eschewing ED care but staying away from their primary care provider. My fear is that we’re not as well aware of this,” he said. “Together with our primary care partners, we have to figure out ways to reach the people who are ignoring illnesses and injuries that they’re making long-term decisions about without realizing it. We have to find a way to reach those people and say it’s okay to reach for care.”
SCCM Immediate Past President Heatherlee Bailey, MD, also sees a problematic looming wave.
“I’m quite concerned about the coming second wave of non-COVID patients who’ve sat home with their worsening renal failure that’s gone from 2 to 5 because they’ve been taking a lot of NSAIDs, or the individual who’s had several TIAs that self-resolved, and we’ve missed an opportunity to prevent some significant disease. At some point they’re going to come back, and we need to figure out how to get these individuals hooked up with care, either through the ED or with their primary care provider, to prevent these potential bad outcomes,” said Dr. Bailey of the Durham (N.C.) Veterans Affairs Medical Center.
Interim guidance for pediatricians on an alarming new syndrome
Edward E. Conway Jr., MD, recalled that early in the U.S. pandemic, pediatricians felt a sense of relief that children appeared to be spared from severe COVID-19 disease. But, in just the past few weeks, a new syndrome has emerged. New York City has recorded more than 100 cases of what’s provisionally being called COVID-associated pediatric multisystem inflammatory syndrome. Dr. Conway and others are working with the Centers for Disease Control and Prevention to develop a case definition for the syndrome, first reported by pediatricians in Italy and the United Kingdom.
“We’re trying to get the word out to general pediatricians as to the common signs and symptoms that should prompt parents to bring their children in for medical care,” according to Dr. Conway, chief of pediatric critical care medicine and vice-chair of pediatrics at Jacobi Medical Center in New York.
Ninety percent of affected children have abdominal symptoms early on, including abdominal pain, diarrhea, emesis, or enteritis upon imaging. A nondescript rash, headache, conjunctivitis, and irritability are common, cough much less so – under 25%.
“The thought is that if any one of these is associated with a fever lasting more than 4 days, we suggest these children be brought in and seen by a pediatrician. We don’t have a formal guideline – we’re working on that – but basically the current recommendation is to screen them initially with a CBC with differential, a chem 10, and liver function tests, but also to look for inflammatory markers that we see in our COVID patients. We’ve been quite surprised: These patients have C-reactive proteins of about 240 mg/L on average, ferritin is quite high at around 1,200 ng/mL, and d-dimers of 2,300 ng/mL. We’ve also found very high brain natriuretic peptides and troponins in these patients,” according to Dr. Conway.
Analogies have been made between this COVID-19 pediatric syndrome and Kawasaki disease. Dr. Conway is unconvinced.
“This is quite different from Kawasaki in that these children are usually thrombocytopenic and usually present with DIC [disseminated intravascular coagulation], and the d-dimers are extraordinarily high, compared to what we’re used to seeing in pediatric patients,” he said.
Symptomatic children with laboratory red flags should be hospitalized. Most of the affected New York City children have recovered after 5 or 6 days in the pediatric ICU with empiric treatment using intravenous immunoglobulin (IVIG), corticosteroids, and/or interleukin-6 inhibitors. However, five recent deaths are now under study.
Dr. Yealy commented that this new pediatric syndrome is “really interesting,” but to date, it affects only a very small percentage of children, and children overall have been much less affected by the pandemic than are adults.
“The populations being disproportionately impacted are the elderly, the elderly, the elderly, and then other vulnerable populations, particularly congregants and the poor,” he said. “At my site, three-quarters of the patients coming in are either patients at assisted-living facilities or work at one of those congregant facilities.”
The pandemic’s impact on medical education
In many hospitals, grand rounds are being done virtually via videoconferencing, often with attendant challenges in asking and answering questions. Hospital patient volumes are diminished. Medical students aren’t coming in to do clinical rotations. Medical students and residents can’t travel to interview for future residencies or jobs.
“It’s affecting education across all of the components of medicine. It’s hard to say how long this pandemic is going to last. We’re all trying to be innovative in using online tools, but I believe it’s going to have a long-lasting effect on our education system,” Dr. Marcolini predicted.
Remote interface while working from home has become frustrating, especially during peak Internet use hours.
“It’s staggering how slow my home system has become in comparison to what’s wired at work. Now many times when you try to get into your work system from home, you time out while you’re waiting for the next piece of information to come across,” Dr. Kaplan commented.
All panel participants reported having no financial conflicts of interest.
*Correction, 5/15/20: An earlier version of this article misstated the name of the American College of Emergency Physicians.)
according to an expert panel on unanticipated consequences of pandemic care hosted by the presidents of the Society of Critical Care Medicine and the American College of Emergency Physicians.*
“At the peak of exposure to COVID-19 illness or infection, ED volumes in my system, which are really not much different from others across the country, were cut in half, if not more. And those changes happened across virtually every form of ED presentation, from the highest acuity to the lowest. We’re now beyond our highest level of exposure to COVID-19 clinically symptomatic patients in western Pennsylvania, but that recovery in volume hasn’t occurred yet, although there are some embers,” explained Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh.
He and other panelists also addressed some of the other unanticipated developments in the COVID-19 pandemic, including a recently recognized childhood manifestation called for now COVID-associated pediatric multisystem inflammatory syndrome, an anticipated massive second wave of non-COVID patients expected to present late to EDs and primary care clinics after having avoided needed medical care out of fear of infection, and the pandemic’s negative impact upon medical education.
Who’s not showing up in the ED
Dr. Yealy said that across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more.
“You might say, if I was designing from the insurance side, this is exactly what I’d hope for. I’ve heard that some people on the insurance-only side of the business really are experiencing a pretty good deal right now: They’re collecting premiums and not having to pay out on the ED or hospital side,” he said.
Tweaking the public health message on seeking medical care
“One of the unanticipated casualties of the pandemic are the patients who don’t have it. It will take a whole lot of work and coordinated effort to re-engage with those patients,” predicted SCCM President Lewis J. Kaplan, MD, professor of surgery at the University of Pennsylvania, Philadelphia.
Evie G. Marcolini, MD, described what she believes is necessary now: “We need to have a big focus on getting the word out to the public that acute MI, stroke, and other acute injuries are still a time-sensitive problem and they warrant at least a call to their physician or consideration of coming in to the ED.
“I think when we started out, we were telling people, ‘Don’t come in.’ Now we’re trying to dial it back a little bit and say, ‘Listen, there are things you really do need to come in for. And we will keep you safe,’” said Dr. Marcolini, an emergency medicine and neurocritical care specialist at Dartmouth-Hitchcock Medical Center, Hanover, N.H.
“It is safe,” Dr. Yealy agreed. “The safest place in the world to be right now is the ED. Everybody’s cordoned off. There’s way more PPE [personal protective equipment]. There’s a level of precision now that should have existed but never did in our previous influenza seasons. So we have something very unique to offer, and we can put people’s minds at rest.”
He spoke of a coming “tsunami of untreated illness.”
“My concern is there is a significant subset of people who are not only eschewing ED care but staying away from their primary care provider. My fear is that we’re not as well aware of this,” he said. “Together with our primary care partners, we have to figure out ways to reach the people who are ignoring illnesses and injuries that they’re making long-term decisions about without realizing it. We have to find a way to reach those people and say it’s okay to reach for care.”
SCCM Immediate Past President Heatherlee Bailey, MD, also sees a problematic looming wave.
“I’m quite concerned about the coming second wave of non-COVID patients who’ve sat home with their worsening renal failure that’s gone from 2 to 5 because they’ve been taking a lot of NSAIDs, or the individual who’s had several TIAs that self-resolved, and we’ve missed an opportunity to prevent some significant disease. At some point they’re going to come back, and we need to figure out how to get these individuals hooked up with care, either through the ED or with their primary care provider, to prevent these potential bad outcomes,” said Dr. Bailey of the Durham (N.C.) Veterans Affairs Medical Center.
Interim guidance for pediatricians on an alarming new syndrome
Edward E. Conway Jr., MD, recalled that early in the U.S. pandemic, pediatricians felt a sense of relief that children appeared to be spared from severe COVID-19 disease. But, in just the past few weeks, a new syndrome has emerged. New York City has recorded more than 100 cases of what’s provisionally being called COVID-associated pediatric multisystem inflammatory syndrome. Dr. Conway and others are working with the Centers for Disease Control and Prevention to develop a case definition for the syndrome, first reported by pediatricians in Italy and the United Kingdom.
“We’re trying to get the word out to general pediatricians as to the common signs and symptoms that should prompt parents to bring their children in for medical care,” according to Dr. Conway, chief of pediatric critical care medicine and vice-chair of pediatrics at Jacobi Medical Center in New York.
Ninety percent of affected children have abdominal symptoms early on, including abdominal pain, diarrhea, emesis, or enteritis upon imaging. A nondescript rash, headache, conjunctivitis, and irritability are common, cough much less so – under 25%.
“The thought is that if any one of these is associated with a fever lasting more than 4 days, we suggest these children be brought in and seen by a pediatrician. We don’t have a formal guideline – we’re working on that – but basically the current recommendation is to screen them initially with a CBC with differential, a chem 10, and liver function tests, but also to look for inflammatory markers that we see in our COVID patients. We’ve been quite surprised: These patients have C-reactive proteins of about 240 mg/L on average, ferritin is quite high at around 1,200 ng/mL, and d-dimers of 2,300 ng/mL. We’ve also found very high brain natriuretic peptides and troponins in these patients,” according to Dr. Conway.
Analogies have been made between this COVID-19 pediatric syndrome and Kawasaki disease. Dr. Conway is unconvinced.
“This is quite different from Kawasaki in that these children are usually thrombocytopenic and usually present with DIC [disseminated intravascular coagulation], and the d-dimers are extraordinarily high, compared to what we’re used to seeing in pediatric patients,” he said.
Symptomatic children with laboratory red flags should be hospitalized. Most of the affected New York City children have recovered after 5 or 6 days in the pediatric ICU with empiric treatment using intravenous immunoglobulin (IVIG), corticosteroids, and/or interleukin-6 inhibitors. However, five recent deaths are now under study.
Dr. Yealy commented that this new pediatric syndrome is “really interesting,” but to date, it affects only a very small percentage of children, and children overall have been much less affected by the pandemic than are adults.
“The populations being disproportionately impacted are the elderly, the elderly, the elderly, and then other vulnerable populations, particularly congregants and the poor,” he said. “At my site, three-quarters of the patients coming in are either patients at assisted-living facilities or work at one of those congregant facilities.”
The pandemic’s impact on medical education
In many hospitals, grand rounds are being done virtually via videoconferencing, often with attendant challenges in asking and answering questions. Hospital patient volumes are diminished. Medical students aren’t coming in to do clinical rotations. Medical students and residents can’t travel to interview for future residencies or jobs.
“It’s affecting education across all of the components of medicine. It’s hard to say how long this pandemic is going to last. We’re all trying to be innovative in using online tools, but I believe it’s going to have a long-lasting effect on our education system,” Dr. Marcolini predicted.
Remote interface while working from home has become frustrating, especially during peak Internet use hours.
“It’s staggering how slow my home system has become in comparison to what’s wired at work. Now many times when you try to get into your work system from home, you time out while you’re waiting for the next piece of information to come across,” Dr. Kaplan commented.
All panel participants reported having no financial conflicts of interest.
*Correction, 5/15/20: An earlier version of this article misstated the name of the American College of Emergency Physicians.)
according to an expert panel on unanticipated consequences of pandemic care hosted by the presidents of the Society of Critical Care Medicine and the American College of Emergency Physicians.*
“At the peak of exposure to COVID-19 illness or infection, ED volumes in my system, which are really not much different from others across the country, were cut in half, if not more. And those changes happened across virtually every form of ED presentation, from the highest acuity to the lowest. We’re now beyond our highest level of exposure to COVID-19 clinically symptomatic patients in western Pennsylvania, but that recovery in volume hasn’t occurred yet, although there are some embers,” explained Donald M. Yealy, MD, professor and chair of the department of emergency medicine at the University of Pittsburgh.
He and other panelists also addressed some of the other unanticipated developments in the COVID-19 pandemic, including a recently recognized childhood manifestation called for now COVID-associated pediatric multisystem inflammatory syndrome, an anticipated massive second wave of non-COVID patients expected to present late to EDs and primary care clinics after having avoided needed medical care out of fear of infection, and the pandemic’s negative impact upon medical education.
Who’s not showing up in the ED
Dr. Yealy said that across the country, the number of patients arriving in EDs with acute ST-elevation MI, stroke, trauma, and other highest-acuity presentations is down substantially. But the volume of patients with more routine, bread-and-butter conditions typically seen in EDs is down even more.
“You might say, if I was designing from the insurance side, this is exactly what I’d hope for. I’ve heard that some people on the insurance-only side of the business really are experiencing a pretty good deal right now: They’re collecting premiums and not having to pay out on the ED or hospital side,” he said.
Tweaking the public health message on seeking medical care
“One of the unanticipated casualties of the pandemic are the patients who don’t have it. It will take a whole lot of work and coordinated effort to re-engage with those patients,” predicted SCCM President Lewis J. Kaplan, MD, professor of surgery at the University of Pennsylvania, Philadelphia.
Evie G. Marcolini, MD, described what she believes is necessary now: “We need to have a big focus on getting the word out to the public that acute MI, stroke, and other acute injuries are still a time-sensitive problem and they warrant at least a call to their physician or consideration of coming in to the ED.
“I think when we started out, we were telling people, ‘Don’t come in.’ Now we’re trying to dial it back a little bit and say, ‘Listen, there are things you really do need to come in for. And we will keep you safe,’” said Dr. Marcolini, an emergency medicine and neurocritical care specialist at Dartmouth-Hitchcock Medical Center, Hanover, N.H.
“It is safe,” Dr. Yealy agreed. “The safest place in the world to be right now is the ED. Everybody’s cordoned off. There’s way more PPE [personal protective equipment]. There’s a level of precision now that should have existed but never did in our previous influenza seasons. So we have something very unique to offer, and we can put people’s minds at rest.”
He spoke of a coming “tsunami of untreated illness.”
“My concern is there is a significant subset of people who are not only eschewing ED care but staying away from their primary care provider. My fear is that we’re not as well aware of this,” he said. “Together with our primary care partners, we have to figure out ways to reach the people who are ignoring illnesses and injuries that they’re making long-term decisions about without realizing it. We have to find a way to reach those people and say it’s okay to reach for care.”
SCCM Immediate Past President Heatherlee Bailey, MD, also sees a problematic looming wave.
“I’m quite concerned about the coming second wave of non-COVID patients who’ve sat home with their worsening renal failure that’s gone from 2 to 5 because they’ve been taking a lot of NSAIDs, or the individual who’s had several TIAs that self-resolved, and we’ve missed an opportunity to prevent some significant disease. At some point they’re going to come back, and we need to figure out how to get these individuals hooked up with care, either through the ED or with their primary care provider, to prevent these potential bad outcomes,” said Dr. Bailey of the Durham (N.C.) Veterans Affairs Medical Center.
Interim guidance for pediatricians on an alarming new syndrome
Edward E. Conway Jr., MD, recalled that early in the U.S. pandemic, pediatricians felt a sense of relief that children appeared to be spared from severe COVID-19 disease. But, in just the past few weeks, a new syndrome has emerged. New York City has recorded more than 100 cases of what’s provisionally being called COVID-associated pediatric multisystem inflammatory syndrome. Dr. Conway and others are working with the Centers for Disease Control and Prevention to develop a case definition for the syndrome, first reported by pediatricians in Italy and the United Kingdom.
“We’re trying to get the word out to general pediatricians as to the common signs and symptoms that should prompt parents to bring their children in for medical care,” according to Dr. Conway, chief of pediatric critical care medicine and vice-chair of pediatrics at Jacobi Medical Center in New York.
Ninety percent of affected children have abdominal symptoms early on, including abdominal pain, diarrhea, emesis, or enteritis upon imaging. A nondescript rash, headache, conjunctivitis, and irritability are common, cough much less so – under 25%.
“The thought is that if any one of these is associated with a fever lasting more than 4 days, we suggest these children be brought in and seen by a pediatrician. We don’t have a formal guideline – we’re working on that – but basically the current recommendation is to screen them initially with a CBC with differential, a chem 10, and liver function tests, but also to look for inflammatory markers that we see in our COVID patients. We’ve been quite surprised: These patients have C-reactive proteins of about 240 mg/L on average, ferritin is quite high at around 1,200 ng/mL, and d-dimers of 2,300 ng/mL. We’ve also found very high brain natriuretic peptides and troponins in these patients,” according to Dr. Conway.
Analogies have been made between this COVID-19 pediatric syndrome and Kawasaki disease. Dr. Conway is unconvinced.
“This is quite different from Kawasaki in that these children are usually thrombocytopenic and usually present with DIC [disseminated intravascular coagulation], and the d-dimers are extraordinarily high, compared to what we’re used to seeing in pediatric patients,” he said.
Symptomatic children with laboratory red flags should be hospitalized. Most of the affected New York City children have recovered after 5 or 6 days in the pediatric ICU with empiric treatment using intravenous immunoglobulin (IVIG), corticosteroids, and/or interleukin-6 inhibitors. However, five recent deaths are now under study.
Dr. Yealy commented that this new pediatric syndrome is “really interesting,” but to date, it affects only a very small percentage of children, and children overall have been much less affected by the pandemic than are adults.
“The populations being disproportionately impacted are the elderly, the elderly, the elderly, and then other vulnerable populations, particularly congregants and the poor,” he said. “At my site, three-quarters of the patients coming in are either patients at assisted-living facilities or work at one of those congregant facilities.”
The pandemic’s impact on medical education
In many hospitals, grand rounds are being done virtually via videoconferencing, often with attendant challenges in asking and answering questions. Hospital patient volumes are diminished. Medical students aren’t coming in to do clinical rotations. Medical students and residents can’t travel to interview for future residencies or jobs.
“It’s affecting education across all of the components of medicine. It’s hard to say how long this pandemic is going to last. We’re all trying to be innovative in using online tools, but I believe it’s going to have a long-lasting effect on our education system,” Dr. Marcolini predicted.
Remote interface while working from home has become frustrating, especially during peak Internet use hours.
“It’s staggering how slow my home system has become in comparison to what’s wired at work. Now many times when you try to get into your work system from home, you time out while you’re waiting for the next piece of information to come across,” Dr. Kaplan commented.
All panel participants reported having no financial conflicts of interest.
*Correction, 5/15/20: An earlier version of this article misstated the name of the American College of Emergency Physicians.)
Even with mild COVID-19, athletes need cardiac testing before returning to play
Potential risks of cardiac injury posed by coronavirus disease 2019 (COVID-19) infection warrant a cautious return-to-play for highly active people and competitive athletes who test positive, according to leading sports cardiologists.
To prevent cardiac injury, athletes should rest for at least 2 weeks after symptoms resolve, then undergo cardiac testing before returning high-level competitive sports, reported lead author Dermot Phelan, MD, PhD, of Atrium Health in Charlotte, N.C., and colleagues.
These recommendations, which were published in JAMA Cardiology, are part of a clinical algorithm that sorts athletes based on coronavirus test status and symptom severity. The algorithm offers a clear timeline for resumption of activity, with management decisions for symptomatic individuals based on additional diagnostics, such as high-sensitivity troponin testing and electrocardiogram.
Despite a scarcity of relevant clinical data, Dr. Phelan said that he and his colleagues wanted to offer their best recommendations to the athletic community, who had been reaching out for help.
“We were getting calls and messages from amateur and professional sporting organizations from around the country asking for guidance about what to do,” Dr. Phelan said. “So a number of us from the American College of Cardiology Sports and Exercise Council decided that we really should provide some guidance even in the absence of good, strong data, for what we feel is a reasonable approach.”
The recommendations were based on what is known of other viral infections, as well as risks posed by COVID-19 that may be worsened by athletic activity.
“We know that, when people have an active infection, vigorous exercise can lower immunity, and that can make the infection worse,” Dr. Phelan said. “That really applies very strongly in people who have had myocarditis. If you exercise when you have myocarditis, it actually increases viral replication and results in increased necrosis of the heart muscle. We really want to avoid exercising during that active infection phase.”
Myocarditis is one of the top causes of sudden cardiac death among young athletes, Dr. Phelan said, “so that’s a major concern for us.”
According to Dr. Phelan, existing data suggest a wide range of incidence of 7%-33% for cardiac injury among patients hospitalized for COVID-19. Even the low end of this range, at 7%, is significantly higher than the incidence rate of 1% found in patients with non–COVID-19 acute viral infections.
“This particular virus appears to cause more cardiac insults than other viruses,” Dr. Phelan said.
The incidence of cardiac injury among nonhospitalized patients remains unknown, leaving a wide knowledge gap that shaped the conservative nature of the present recommendations.
With more information, however, the guidance may “change dramatically,” Dr. Phelan said.
“If the data come back and show that no nonhospitalized patients got cardiac injury, then we would be much more comfortable allowing return to play without the need for cardiac testing,” he said.
Conversely, if cardiac injury is more common than anticipated, then more extensive testing may be needed, he added.
As the algorithm stands, high-sensitivity troponin testing and/or cardiac studies are recommended for all symptomatic athletes; if troponin levels are greater than the 99th percentile or a cardiac study is abnormal, then clinicians should follow return-to-play guidelines for myocarditis. For athletes with normal tests, slow resumption of activity is recommended, including close monitoring for clinical deterioration.
As Dr. Phelan discussed these recommendations in a broader context, he emphasized the need for caution, both preventively, and for cardiologists working with recovering athletes.
“For the early stage of this reentry into normal life while this is still an active pandemic, we need to be cautious,” Dr. Phelan said. “We need to follow the regular CDC guidelines, in terms of social distancing and handwashing, but we also need to consider that those people who have suffered from COVID-19 may have had cardiac injury. We don’t know that yet. But we need to be cautious with these individuals and test them before they return to high-level competitive sports.”
One author disclosed a relationship with the Atlanta Falcons.
SOURCE: Phelan D et al. JAMA Cardiology. 2020 Apr 13. doi: 10.1001/jamacardio.2020.2136.
Potential risks of cardiac injury posed by coronavirus disease 2019 (COVID-19) infection warrant a cautious return-to-play for highly active people and competitive athletes who test positive, according to leading sports cardiologists.
To prevent cardiac injury, athletes should rest for at least 2 weeks after symptoms resolve, then undergo cardiac testing before returning high-level competitive sports, reported lead author Dermot Phelan, MD, PhD, of Atrium Health in Charlotte, N.C., and colleagues.
These recommendations, which were published in JAMA Cardiology, are part of a clinical algorithm that sorts athletes based on coronavirus test status and symptom severity. The algorithm offers a clear timeline for resumption of activity, with management decisions for symptomatic individuals based on additional diagnostics, such as high-sensitivity troponin testing and electrocardiogram.
Despite a scarcity of relevant clinical data, Dr. Phelan said that he and his colleagues wanted to offer their best recommendations to the athletic community, who had been reaching out for help.
“We were getting calls and messages from amateur and professional sporting organizations from around the country asking for guidance about what to do,” Dr. Phelan said. “So a number of us from the American College of Cardiology Sports and Exercise Council decided that we really should provide some guidance even in the absence of good, strong data, for what we feel is a reasonable approach.”
The recommendations were based on what is known of other viral infections, as well as risks posed by COVID-19 that may be worsened by athletic activity.
“We know that, when people have an active infection, vigorous exercise can lower immunity, and that can make the infection worse,” Dr. Phelan said. “That really applies very strongly in people who have had myocarditis. If you exercise when you have myocarditis, it actually increases viral replication and results in increased necrosis of the heart muscle. We really want to avoid exercising during that active infection phase.”
Myocarditis is one of the top causes of sudden cardiac death among young athletes, Dr. Phelan said, “so that’s a major concern for us.”
According to Dr. Phelan, existing data suggest a wide range of incidence of 7%-33% for cardiac injury among patients hospitalized for COVID-19. Even the low end of this range, at 7%, is significantly higher than the incidence rate of 1% found in patients with non–COVID-19 acute viral infections.
“This particular virus appears to cause more cardiac insults than other viruses,” Dr. Phelan said.
The incidence of cardiac injury among nonhospitalized patients remains unknown, leaving a wide knowledge gap that shaped the conservative nature of the present recommendations.
With more information, however, the guidance may “change dramatically,” Dr. Phelan said.
“If the data come back and show that no nonhospitalized patients got cardiac injury, then we would be much more comfortable allowing return to play without the need for cardiac testing,” he said.
Conversely, if cardiac injury is more common than anticipated, then more extensive testing may be needed, he added.
As the algorithm stands, high-sensitivity troponin testing and/or cardiac studies are recommended for all symptomatic athletes; if troponin levels are greater than the 99th percentile or a cardiac study is abnormal, then clinicians should follow return-to-play guidelines for myocarditis. For athletes with normal tests, slow resumption of activity is recommended, including close monitoring for clinical deterioration.
As Dr. Phelan discussed these recommendations in a broader context, he emphasized the need for caution, both preventively, and for cardiologists working with recovering athletes.
“For the early stage of this reentry into normal life while this is still an active pandemic, we need to be cautious,” Dr. Phelan said. “We need to follow the regular CDC guidelines, in terms of social distancing and handwashing, but we also need to consider that those people who have suffered from COVID-19 may have had cardiac injury. We don’t know that yet. But we need to be cautious with these individuals and test them before they return to high-level competitive sports.”
One author disclosed a relationship with the Atlanta Falcons.
SOURCE: Phelan D et al. JAMA Cardiology. 2020 Apr 13. doi: 10.1001/jamacardio.2020.2136.
Potential risks of cardiac injury posed by coronavirus disease 2019 (COVID-19) infection warrant a cautious return-to-play for highly active people and competitive athletes who test positive, according to leading sports cardiologists.
To prevent cardiac injury, athletes should rest for at least 2 weeks after symptoms resolve, then undergo cardiac testing before returning high-level competitive sports, reported lead author Dermot Phelan, MD, PhD, of Atrium Health in Charlotte, N.C., and colleagues.
These recommendations, which were published in JAMA Cardiology, are part of a clinical algorithm that sorts athletes based on coronavirus test status and symptom severity. The algorithm offers a clear timeline for resumption of activity, with management decisions for symptomatic individuals based on additional diagnostics, such as high-sensitivity troponin testing and electrocardiogram.
Despite a scarcity of relevant clinical data, Dr. Phelan said that he and his colleagues wanted to offer their best recommendations to the athletic community, who had been reaching out for help.
“We were getting calls and messages from amateur and professional sporting organizations from around the country asking for guidance about what to do,” Dr. Phelan said. “So a number of us from the American College of Cardiology Sports and Exercise Council decided that we really should provide some guidance even in the absence of good, strong data, for what we feel is a reasonable approach.”
The recommendations were based on what is known of other viral infections, as well as risks posed by COVID-19 that may be worsened by athletic activity.
“We know that, when people have an active infection, vigorous exercise can lower immunity, and that can make the infection worse,” Dr. Phelan said. “That really applies very strongly in people who have had myocarditis. If you exercise when you have myocarditis, it actually increases viral replication and results in increased necrosis of the heart muscle. We really want to avoid exercising during that active infection phase.”
Myocarditis is one of the top causes of sudden cardiac death among young athletes, Dr. Phelan said, “so that’s a major concern for us.”
According to Dr. Phelan, existing data suggest a wide range of incidence of 7%-33% for cardiac injury among patients hospitalized for COVID-19. Even the low end of this range, at 7%, is significantly higher than the incidence rate of 1% found in patients with non–COVID-19 acute viral infections.
“This particular virus appears to cause more cardiac insults than other viruses,” Dr. Phelan said.
The incidence of cardiac injury among nonhospitalized patients remains unknown, leaving a wide knowledge gap that shaped the conservative nature of the present recommendations.
With more information, however, the guidance may “change dramatically,” Dr. Phelan said.
“If the data come back and show that no nonhospitalized patients got cardiac injury, then we would be much more comfortable allowing return to play without the need for cardiac testing,” he said.
Conversely, if cardiac injury is more common than anticipated, then more extensive testing may be needed, he added.
As the algorithm stands, high-sensitivity troponin testing and/or cardiac studies are recommended for all symptomatic athletes; if troponin levels are greater than the 99th percentile or a cardiac study is abnormal, then clinicians should follow return-to-play guidelines for myocarditis. For athletes with normal tests, slow resumption of activity is recommended, including close monitoring for clinical deterioration.
As Dr. Phelan discussed these recommendations in a broader context, he emphasized the need for caution, both preventively, and for cardiologists working with recovering athletes.
“For the early stage of this reentry into normal life while this is still an active pandemic, we need to be cautious,” Dr. Phelan said. “We need to follow the regular CDC guidelines, in terms of social distancing and handwashing, but we also need to consider that those people who have suffered from COVID-19 may have had cardiac injury. We don’t know that yet. But we need to be cautious with these individuals and test them before they return to high-level competitive sports.”
One author disclosed a relationship with the Atlanta Falcons.
SOURCE: Phelan D et al. JAMA Cardiology. 2020 Apr 13. doi: 10.1001/jamacardio.2020.2136.
FROM JAMA CARDIOLOGY
Comparing COVID-19, flu death tolls ‘extremely dangerous’
The number of COVID-19 deaths cannot be directly compared to the number of seasonal influenza deaths because they are calculated differently, researchers say in a report released today.
Whereas COVID-19 death rates are determined from actual counts of people who have died, seasonal influenza death rates are estimated by the Centers for Disease Control and Prevention (CDC) using population modeling algorithms, explains Jeremy Samuel Faust, MD, with Harvard Medical School and Brigham and Women’s Hospital, Division of Health Policy and Public Health in Boston, Massachusetts.
The CDC estimates that between 24,000 and 62,000 people died from influenza during the 2019-2020 season (through April 4). At the time of the analysis (as of April 28), COVID-19 deaths had reached 65,000 in the United States.
But making that comparison “is extremely dangerous,” Faust told Medscape Medical News.
“COVID-19 is far more dangerous and is wreaking far more havoc than seasonal influenza ever has,” he said.
Faust coauthored the perspective article, published online in JAMA Internal Medicine, with Carlos del Rio, MD, Division of Infectious Diseases at Emory University School of Medicine in Atlanta, Georgia.
The message and methodology of Faust’s and del Rio’s article are on target, according to Jonathan L. Temte, MD, PhD, who has been working in influenza surveillance for almost 25 years.
Current flu data draw on limited information from primary care practices and hospitals, said Dr. Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison. The estimates help bridge the gaps, he said, but the system is inherently vulnerable to error.
“Comparing them – as so many people in this country have done – to try to diminish the impact of SARS-CoV2 is not fair,” he said.
Estimated versus actual influenza deaths
The authors illustrate the difference in the way rates of death from influenza are calculated: “Between 2013-2014 and 2018-2019, the reported yearly estimated influenza deaths ranged from 23,000 to 61,000. Over that same time period, however, the number of counted influenza deaths was between 3,448 and 15,620 yearly.”
“It’s apparent [the CDC has] been overestimating,” Faust said. “If you publish a number on the higher end of the estimate, people might take your public health messages more seriously, such as, it’s important to get your yearly flu shot.”
He added that until influenza death rates started to be compared with COVID-19 rates, “there was never really a downside” to reporting estimates.
Dr. Temte said he doesn’t regard overestimating flu deaths as intentional but rather the result of a longstanding “bias against the elderly in this country” that the estimates are meant to account for.
For example, he says, reporting influenza deaths is mandatory when such deaths involve persons younger than 18 years but not when they involve adults.
Also, traditionally, influenza has been seen “as a cause of death in people with multiple comorbidities that was just part and parcel of wintertime,” Dr. Temte said.
“The likelihood of being tested for influenza goes down greatly when you’re older,” he said. “This is slowly changing.”
The CDC acknowledges on its website that it “does not know the exact number of people who have been sick and affected by influenza because influenza is not a reportable disease in most areas of the US.”
It adds that the burden is estimated through the US Influenza Surveillance System, which covers approximately 8.5% of the US population.
Comparing recorded deaths
It’s more accurate and meaningful to compare actual numbers of deaths for the diseases, Dr. Faust and Dr. del Rio say in their article.
When the authors made that comparison, they drew a stark contrast.
There were 15,455 recorded COVID-19 deaths in the week that ended April 21. The week before, the number of recorded deaths was 14,478, they found. (Those were the two most recent weeks before they submitted their article for publication.)
In comparison, counted deaths ranged from 351 to 1,626 during the peak week of the seven influenza seasons between 2013-2014 and 2019-2020. The average counted deaths for the peak week of the seven seasons was 752.4 (95% confidence interval, 558.8-946.1).
“These statistics on counted deaths suggest that the number of COVID-19 deaths for the week ending April 21 was 9.5-fold to 44.1-fold greater than the peak week of counted influenza deaths during the past seven influenza seasons in the US, with a 20.5-fold mean increase (95% CI, 16.3-27.7),” the authors write.
However, Natasha Chida, MD, MSPH, an infectious disease physician and assistant professor at the Johns Hopkins University School of Medicine in Baltimore, Maryland, said in an interview that the actual number of deaths doesn’t tell the complete flu story either. That count would miss people who later died from secondary complications associated with influenza, she said.
“There’s just no way to reliably count influenza deaths,” she said. “I think if we required it as a reported illness, that would be the ideal situation, but there’s so much flu every year that that probably would not be practical.”
She said she agrees that rates of influenza deaths and rates of COVID-19 deaths cannot be fairly compared.
What the authors don’t touch on, she said, is that flu season lasts 4 to 6 months a year, and just 3 months into the coronavirus pandemic, US deaths due to COVID-19 are already higher than those for seasonal influenza.
“Even if we look at it in the way that people who think we can compare flu and coronavirus do, it’s still not going to work out in their favor from a numbers standpoint,” she said.
The article clarifies the differences for “people who don’t live in the flu world,” she said.
“It is not accurate to compare the two for the reasons the authors described and also because they are very different diseases,” she added.
Real-life validation
Dr. Faust said in an interview that real-life experiences add external validity to their analysis.
Differences in the way deaths are calculated does not reflect frontline clinical conditions during the COVID-19 crisis, with hospitals stretched past their limits, ventilator shortages, and bodies stacking up in some overwhelmed facilities, the authors say.
Dr. Temte said the external validation of the numbers also rings true in light of his own experience.
He said that, in the past 2 months, he has known two people who have had family members who died of COVID-19.
Conversely, “I would have to search long and hard to come up with people I have known or have been one degree of separation from” who have died from influenza, Dr. Temte said.
The authors, Dr. Temte, and Dr. Chida report no relevant financial relationships.
This article first appeared on Medscape.com.
The number of COVID-19 deaths cannot be directly compared to the number of seasonal influenza deaths because they are calculated differently, researchers say in a report released today.
Whereas COVID-19 death rates are determined from actual counts of people who have died, seasonal influenza death rates are estimated by the Centers for Disease Control and Prevention (CDC) using population modeling algorithms, explains Jeremy Samuel Faust, MD, with Harvard Medical School and Brigham and Women’s Hospital, Division of Health Policy and Public Health in Boston, Massachusetts.
The CDC estimates that between 24,000 and 62,000 people died from influenza during the 2019-2020 season (through April 4). At the time of the analysis (as of April 28), COVID-19 deaths had reached 65,000 in the United States.
But making that comparison “is extremely dangerous,” Faust told Medscape Medical News.
“COVID-19 is far more dangerous and is wreaking far more havoc than seasonal influenza ever has,” he said.
Faust coauthored the perspective article, published online in JAMA Internal Medicine, with Carlos del Rio, MD, Division of Infectious Diseases at Emory University School of Medicine in Atlanta, Georgia.
The message and methodology of Faust’s and del Rio’s article are on target, according to Jonathan L. Temte, MD, PhD, who has been working in influenza surveillance for almost 25 years.
Current flu data draw on limited information from primary care practices and hospitals, said Dr. Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison. The estimates help bridge the gaps, he said, but the system is inherently vulnerable to error.
“Comparing them – as so many people in this country have done – to try to diminish the impact of SARS-CoV2 is not fair,” he said.
Estimated versus actual influenza deaths
The authors illustrate the difference in the way rates of death from influenza are calculated: “Between 2013-2014 and 2018-2019, the reported yearly estimated influenza deaths ranged from 23,000 to 61,000. Over that same time period, however, the number of counted influenza deaths was between 3,448 and 15,620 yearly.”
“It’s apparent [the CDC has] been overestimating,” Faust said. “If you publish a number on the higher end of the estimate, people might take your public health messages more seriously, such as, it’s important to get your yearly flu shot.”
He added that until influenza death rates started to be compared with COVID-19 rates, “there was never really a downside” to reporting estimates.
Dr. Temte said he doesn’t regard overestimating flu deaths as intentional but rather the result of a longstanding “bias against the elderly in this country” that the estimates are meant to account for.
For example, he says, reporting influenza deaths is mandatory when such deaths involve persons younger than 18 years but not when they involve adults.
Also, traditionally, influenza has been seen “as a cause of death in people with multiple comorbidities that was just part and parcel of wintertime,” Dr. Temte said.
“The likelihood of being tested for influenza goes down greatly when you’re older,” he said. “This is slowly changing.”
The CDC acknowledges on its website that it “does not know the exact number of people who have been sick and affected by influenza because influenza is not a reportable disease in most areas of the US.”
It adds that the burden is estimated through the US Influenza Surveillance System, which covers approximately 8.5% of the US population.
Comparing recorded deaths
It’s more accurate and meaningful to compare actual numbers of deaths for the diseases, Dr. Faust and Dr. del Rio say in their article.
When the authors made that comparison, they drew a stark contrast.
There were 15,455 recorded COVID-19 deaths in the week that ended April 21. The week before, the number of recorded deaths was 14,478, they found. (Those were the two most recent weeks before they submitted their article for publication.)
In comparison, counted deaths ranged from 351 to 1,626 during the peak week of the seven influenza seasons between 2013-2014 and 2019-2020. The average counted deaths for the peak week of the seven seasons was 752.4 (95% confidence interval, 558.8-946.1).
“These statistics on counted deaths suggest that the number of COVID-19 deaths for the week ending April 21 was 9.5-fold to 44.1-fold greater than the peak week of counted influenza deaths during the past seven influenza seasons in the US, with a 20.5-fold mean increase (95% CI, 16.3-27.7),” the authors write.
However, Natasha Chida, MD, MSPH, an infectious disease physician and assistant professor at the Johns Hopkins University School of Medicine in Baltimore, Maryland, said in an interview that the actual number of deaths doesn’t tell the complete flu story either. That count would miss people who later died from secondary complications associated with influenza, she said.
“There’s just no way to reliably count influenza deaths,” she said. “I think if we required it as a reported illness, that would be the ideal situation, but there’s so much flu every year that that probably would not be practical.”
She said she agrees that rates of influenza deaths and rates of COVID-19 deaths cannot be fairly compared.
What the authors don’t touch on, she said, is that flu season lasts 4 to 6 months a year, and just 3 months into the coronavirus pandemic, US deaths due to COVID-19 are already higher than those for seasonal influenza.
“Even if we look at it in the way that people who think we can compare flu and coronavirus do, it’s still not going to work out in their favor from a numbers standpoint,” she said.
The article clarifies the differences for “people who don’t live in the flu world,” she said.
“It is not accurate to compare the two for the reasons the authors described and also because they are very different diseases,” she added.
Real-life validation
Dr. Faust said in an interview that real-life experiences add external validity to their analysis.
Differences in the way deaths are calculated does not reflect frontline clinical conditions during the COVID-19 crisis, with hospitals stretched past their limits, ventilator shortages, and bodies stacking up in some overwhelmed facilities, the authors say.
Dr. Temte said the external validation of the numbers also rings true in light of his own experience.
He said that, in the past 2 months, he has known two people who have had family members who died of COVID-19.
Conversely, “I would have to search long and hard to come up with people I have known or have been one degree of separation from” who have died from influenza, Dr. Temte said.
The authors, Dr. Temte, and Dr. Chida report no relevant financial relationships.
This article first appeared on Medscape.com.
The number of COVID-19 deaths cannot be directly compared to the number of seasonal influenza deaths because they are calculated differently, researchers say in a report released today.
Whereas COVID-19 death rates are determined from actual counts of people who have died, seasonal influenza death rates are estimated by the Centers for Disease Control and Prevention (CDC) using population modeling algorithms, explains Jeremy Samuel Faust, MD, with Harvard Medical School and Brigham and Women’s Hospital, Division of Health Policy and Public Health in Boston, Massachusetts.
The CDC estimates that between 24,000 and 62,000 people died from influenza during the 2019-2020 season (through April 4). At the time of the analysis (as of April 28), COVID-19 deaths had reached 65,000 in the United States.
But making that comparison “is extremely dangerous,” Faust told Medscape Medical News.
“COVID-19 is far more dangerous and is wreaking far more havoc than seasonal influenza ever has,” he said.
Faust coauthored the perspective article, published online in JAMA Internal Medicine, with Carlos del Rio, MD, Division of Infectious Diseases at Emory University School of Medicine in Atlanta, Georgia.
The message and methodology of Faust’s and del Rio’s article are on target, according to Jonathan L. Temte, MD, PhD, who has been working in influenza surveillance for almost 25 years.
Current flu data draw on limited information from primary care practices and hospitals, said Dr. Temte, associate dean for public health and community engagement at the University of Wisconsin School of Medicine and Public Health in Madison. The estimates help bridge the gaps, he said, but the system is inherently vulnerable to error.
“Comparing them – as so many people in this country have done – to try to diminish the impact of SARS-CoV2 is not fair,” he said.
Estimated versus actual influenza deaths
The authors illustrate the difference in the way rates of death from influenza are calculated: “Between 2013-2014 and 2018-2019, the reported yearly estimated influenza deaths ranged from 23,000 to 61,000. Over that same time period, however, the number of counted influenza deaths was between 3,448 and 15,620 yearly.”
“It’s apparent [the CDC has] been overestimating,” Faust said. “If you publish a number on the higher end of the estimate, people might take your public health messages more seriously, such as, it’s important to get your yearly flu shot.”
He added that until influenza death rates started to be compared with COVID-19 rates, “there was never really a downside” to reporting estimates.
Dr. Temte said he doesn’t regard overestimating flu deaths as intentional but rather the result of a longstanding “bias against the elderly in this country” that the estimates are meant to account for.
For example, he says, reporting influenza deaths is mandatory when such deaths involve persons younger than 18 years but not when they involve adults.
Also, traditionally, influenza has been seen “as a cause of death in people with multiple comorbidities that was just part and parcel of wintertime,” Dr. Temte said.
“The likelihood of being tested for influenza goes down greatly when you’re older,” he said. “This is slowly changing.”
The CDC acknowledges on its website that it “does not know the exact number of people who have been sick and affected by influenza because influenza is not a reportable disease in most areas of the US.”
It adds that the burden is estimated through the US Influenza Surveillance System, which covers approximately 8.5% of the US population.
Comparing recorded deaths
It’s more accurate and meaningful to compare actual numbers of deaths for the diseases, Dr. Faust and Dr. del Rio say in their article.
When the authors made that comparison, they drew a stark contrast.
There were 15,455 recorded COVID-19 deaths in the week that ended April 21. The week before, the number of recorded deaths was 14,478, they found. (Those were the two most recent weeks before they submitted their article for publication.)
In comparison, counted deaths ranged from 351 to 1,626 during the peak week of the seven influenza seasons between 2013-2014 and 2019-2020. The average counted deaths for the peak week of the seven seasons was 752.4 (95% confidence interval, 558.8-946.1).
“These statistics on counted deaths suggest that the number of COVID-19 deaths for the week ending April 21 was 9.5-fold to 44.1-fold greater than the peak week of counted influenza deaths during the past seven influenza seasons in the US, with a 20.5-fold mean increase (95% CI, 16.3-27.7),” the authors write.
However, Natasha Chida, MD, MSPH, an infectious disease physician and assistant professor at the Johns Hopkins University School of Medicine in Baltimore, Maryland, said in an interview that the actual number of deaths doesn’t tell the complete flu story either. That count would miss people who later died from secondary complications associated with influenza, she said.
“There’s just no way to reliably count influenza deaths,” she said. “I think if we required it as a reported illness, that would be the ideal situation, but there’s so much flu every year that that probably would not be practical.”
She said she agrees that rates of influenza deaths and rates of COVID-19 deaths cannot be fairly compared.
What the authors don’t touch on, she said, is that flu season lasts 4 to 6 months a year, and just 3 months into the coronavirus pandemic, US deaths due to COVID-19 are already higher than those for seasonal influenza.
“Even if we look at it in the way that people who think we can compare flu and coronavirus do, it’s still not going to work out in their favor from a numbers standpoint,” she said.
The article clarifies the differences for “people who don’t live in the flu world,” she said.
“It is not accurate to compare the two for the reasons the authors described and also because they are very different diseases,” she added.
Real-life validation
Dr. Faust said in an interview that real-life experiences add external validity to their analysis.
Differences in the way deaths are calculated does not reflect frontline clinical conditions during the COVID-19 crisis, with hospitals stretched past their limits, ventilator shortages, and bodies stacking up in some overwhelmed facilities, the authors say.
Dr. Temte said the external validation of the numbers also rings true in light of his own experience.
He said that, in the past 2 months, he has known two people who have had family members who died of COVID-19.
Conversely, “I would have to search long and hard to come up with people I have known or have been one degree of separation from” who have died from influenza, Dr. Temte said.
The authors, Dr. Temte, and Dr. Chida report no relevant financial relationships.
This article first appeared on Medscape.com.
Masks, fear, and loss of connection in the era of COVID-19
Over the din of the negative pressure machine, I shouted goodbye to my patient and zipped my way out of one of the little plastic enclosures in our ED and carefully shed my gloves, gown, and face shield, leaving on my precious mask. I discarded the rest with disgust and a bit of fear. I thought, “This is a whole new world, and I hate it.”
I feel as if I am constantly battling the fear of dying from COVID-19 but am doing the best I can, given the circumstances at hand. I have the proper equipment and use it well. My work still brings meaning: I serve those in need without hesitation. The problem is that deep feeling of connection with patients, which is such an important part of this work, feels like fraying threads moving further apart because of the havoc this virus has wrought. A few weeks ago, the intricate fabric of what it is to be human connected me to patients through the basics: touch, facial expressions, a physical proximity, and openhearted, honest dialogue. Much of that’s gone, and while I can carry on, I will surely burn out if I can’t figure out how to get at least some of that connection back.
Overwhelmed by the amount of information I need to process daily, I had not been thinking about the interpersonal side of the pandemic for the first weeks. I felt it leaving the ED that morning and later that day, and I felt it again with Ms. Z, who was not even suspected of having COVID. She is a 62-year-old I interviewed with the help of a translator phone. At the end of our encounter, she said “But doctor, will you make my tumor go away?” From across the room, I said, “I will try.” I saw her eyes dampen as I made a hasty exit, following protocol to limit time in the room of all patients.
Typically, leaving a patient’s room, I would feel a fullness associated with a sense of meaning. How did I feel after that? In that moment, mostly ashamed at my lack of compassion during my time with Ms. Z. Then, with further reflection, tense from all things COVID-19! Having an amped-up sympathetic nervous system is understandable, but it’s not where we want to be for our compassion to flow.
We connect best when our parasympathetic nervous system is predominant. So much of the stimuli we need to activate that part of the nervous system is gone. There is a virtuous cycle, much of it unconscious, where something positive leads to more positivity, which is crucial to meaningful patient encounters. We read each other’s facial expressions, hear the tone of voice, and as we pick up subtle cues from our patient, our nervous system is further engaged and our hearts opened.
The specter of COVID-19 has us battling a negative spiral of stress and fear. For the most part, I try to keep that from consuming me, but it clearly saps my energy during encounters. In the same way we need to marshal our resources to battle both the stress and the disease itself, we need to actively engage pro-social elements of providing care to maintain our compassion. Clearly, I needed a more concerted effort to kick start this virtuous cycle of compassion.
My next patient was Ms. J., a 55-year-old with advanced chronic obstructive pulmonary disease (COPD) who came in the night before with shortness of breath. Her slight frame shook from coughing as I entered the room. I did not think she had COVID-19, but we were ruling it out.
We reviewed how she felt since admission, and I performed a hasty exam and stepped back across the room. She coughed again and said, “I feel so weak, and the world feels so crazy; tell it to me straight.” Then looking in my eyes, “I am going to make it, doc?”
I took my cue from her; I walked back to the bedside, placed a gloved hand on her shoulder and with the other, I took her hand. I bent forward just a little. Making eye contact and attempting a comforting tone of voice, I said, “Everyone is a little scared, including me. We need each other more than ever these days. We will do our best for you. That means thoughtful medical care and a whole lot of love! And, truly, I don’t think you are dying; this is just one of your COPD flares.”
“God bless you!” she said, squeezing my hand as a tear rolled down her cheek.
“Bless you, too. We all need blessing with this madness going on,” I replied. Despite the mask, I am sure she saw the smile in my eyes. “Thanks for being the beautiful person you are and opening up to me. That’s the way we will make it through this. I will see you tomorrow.” Backing away, hands together in prayer, I gave a little bow and left the room.
With Ms. J.’s help, I began to figure it out. To tackle the stress of COVID, we need to be very direct – almost to the point of exaggeration – to make sure our words and actions convey what we need to express. William James, the father of psychology, believed that if you force a smile, your emotions would follow. The neural pathways could work backward in that way. He said, “If you want a quality, act as if you have it.” The modern translation would be, “Fake it ’til you make it.’ ” You may be feeling stressed, but with a deep breath and a moment’s reflection on the suffering of that patient you are about to see, you can turn the tide on anxiety and give those under your care what they need.
These are unprecedented times; anxiety abounds. While we can aspire to positivity, there are times when we simply can’t muster showing it. Alternatively, as I experienced with Ms. J., honesty and vulnerability can open the door to meaningful connection. This can be quite powerful when we, as physicians, open up to our patients.
People are yearning for deep connection, and we should attempt to deliver it with:
- Touch (as we can) to convey connection.
- Body language that adds emphasis to our message and our emotions that may go above and beyond what we are used to.
- Tone of voice that enhances our words.
- Talk that emphasizes the big stuff, such as love, fear, connection and community
With gloves, masks, distance, and fear between and us and our patients, we need to actively engage our pro-social tools to turn the negative spiral of fear into the virtuous cycle of positive emotions that promotes healing of our patients and emotional engagement for those providing their care.
Dr. Hass was trained in family medicine at University of California, San Francisco, after receiving his medical degree from the McGill University faculty of medicine, Montreal. He works as a hospitalist with Sutter Health in Oakland, Calif. He is an adviser on health and health care for the Greater Good Science Center at UC Berkeley and clinical faculty at UCSF School of Medicine. This article appeared initially at The Hospital Leader, the official blog of SHM.
Over the din of the negative pressure machine, I shouted goodbye to my patient and zipped my way out of one of the little plastic enclosures in our ED and carefully shed my gloves, gown, and face shield, leaving on my precious mask. I discarded the rest with disgust and a bit of fear. I thought, “This is a whole new world, and I hate it.”
I feel as if I am constantly battling the fear of dying from COVID-19 but am doing the best I can, given the circumstances at hand. I have the proper equipment and use it well. My work still brings meaning: I serve those in need without hesitation. The problem is that deep feeling of connection with patients, which is such an important part of this work, feels like fraying threads moving further apart because of the havoc this virus has wrought. A few weeks ago, the intricate fabric of what it is to be human connected me to patients through the basics: touch, facial expressions, a physical proximity, and openhearted, honest dialogue. Much of that’s gone, and while I can carry on, I will surely burn out if I can’t figure out how to get at least some of that connection back.
Overwhelmed by the amount of information I need to process daily, I had not been thinking about the interpersonal side of the pandemic for the first weeks. I felt it leaving the ED that morning and later that day, and I felt it again with Ms. Z, who was not even suspected of having COVID. She is a 62-year-old I interviewed with the help of a translator phone. At the end of our encounter, she said “But doctor, will you make my tumor go away?” From across the room, I said, “I will try.” I saw her eyes dampen as I made a hasty exit, following protocol to limit time in the room of all patients.
Typically, leaving a patient’s room, I would feel a fullness associated with a sense of meaning. How did I feel after that? In that moment, mostly ashamed at my lack of compassion during my time with Ms. Z. Then, with further reflection, tense from all things COVID-19! Having an amped-up sympathetic nervous system is understandable, but it’s not where we want to be for our compassion to flow.
We connect best when our parasympathetic nervous system is predominant. So much of the stimuli we need to activate that part of the nervous system is gone. There is a virtuous cycle, much of it unconscious, where something positive leads to more positivity, which is crucial to meaningful patient encounters. We read each other’s facial expressions, hear the tone of voice, and as we pick up subtle cues from our patient, our nervous system is further engaged and our hearts opened.
The specter of COVID-19 has us battling a negative spiral of stress and fear. For the most part, I try to keep that from consuming me, but it clearly saps my energy during encounters. In the same way we need to marshal our resources to battle both the stress and the disease itself, we need to actively engage pro-social elements of providing care to maintain our compassion. Clearly, I needed a more concerted effort to kick start this virtuous cycle of compassion.
My next patient was Ms. J., a 55-year-old with advanced chronic obstructive pulmonary disease (COPD) who came in the night before with shortness of breath. Her slight frame shook from coughing as I entered the room. I did not think she had COVID-19, but we were ruling it out.
We reviewed how she felt since admission, and I performed a hasty exam and stepped back across the room. She coughed again and said, “I feel so weak, and the world feels so crazy; tell it to me straight.” Then looking in my eyes, “I am going to make it, doc?”
I took my cue from her; I walked back to the bedside, placed a gloved hand on her shoulder and with the other, I took her hand. I bent forward just a little. Making eye contact and attempting a comforting tone of voice, I said, “Everyone is a little scared, including me. We need each other more than ever these days. We will do our best for you. That means thoughtful medical care and a whole lot of love! And, truly, I don’t think you are dying; this is just one of your COPD flares.”
“God bless you!” she said, squeezing my hand as a tear rolled down her cheek.
“Bless you, too. We all need blessing with this madness going on,” I replied. Despite the mask, I am sure she saw the smile in my eyes. “Thanks for being the beautiful person you are and opening up to me. That’s the way we will make it through this. I will see you tomorrow.” Backing away, hands together in prayer, I gave a little bow and left the room.
With Ms. J.’s help, I began to figure it out. To tackle the stress of COVID, we need to be very direct – almost to the point of exaggeration – to make sure our words and actions convey what we need to express. William James, the father of psychology, believed that if you force a smile, your emotions would follow. The neural pathways could work backward in that way. He said, “If you want a quality, act as if you have it.” The modern translation would be, “Fake it ’til you make it.’ ” You may be feeling stressed, but with a deep breath and a moment’s reflection on the suffering of that patient you are about to see, you can turn the tide on anxiety and give those under your care what they need.
These are unprecedented times; anxiety abounds. While we can aspire to positivity, there are times when we simply can’t muster showing it. Alternatively, as I experienced with Ms. J., honesty and vulnerability can open the door to meaningful connection. This can be quite powerful when we, as physicians, open up to our patients.
People are yearning for deep connection, and we should attempt to deliver it with:
- Touch (as we can) to convey connection.
- Body language that adds emphasis to our message and our emotions that may go above and beyond what we are used to.
- Tone of voice that enhances our words.
- Talk that emphasizes the big stuff, such as love, fear, connection and community
With gloves, masks, distance, and fear between and us and our patients, we need to actively engage our pro-social tools to turn the negative spiral of fear into the virtuous cycle of positive emotions that promotes healing of our patients and emotional engagement for those providing their care.
Dr. Hass was trained in family medicine at University of California, San Francisco, after receiving his medical degree from the McGill University faculty of medicine, Montreal. He works as a hospitalist with Sutter Health in Oakland, Calif. He is an adviser on health and health care for the Greater Good Science Center at UC Berkeley and clinical faculty at UCSF School of Medicine. This article appeared initially at The Hospital Leader, the official blog of SHM.
Over the din of the negative pressure machine, I shouted goodbye to my patient and zipped my way out of one of the little plastic enclosures in our ED and carefully shed my gloves, gown, and face shield, leaving on my precious mask. I discarded the rest with disgust and a bit of fear. I thought, “This is a whole new world, and I hate it.”
I feel as if I am constantly battling the fear of dying from COVID-19 but am doing the best I can, given the circumstances at hand. I have the proper equipment and use it well. My work still brings meaning: I serve those in need without hesitation. The problem is that deep feeling of connection with patients, which is such an important part of this work, feels like fraying threads moving further apart because of the havoc this virus has wrought. A few weeks ago, the intricate fabric of what it is to be human connected me to patients through the basics: touch, facial expressions, a physical proximity, and openhearted, honest dialogue. Much of that’s gone, and while I can carry on, I will surely burn out if I can’t figure out how to get at least some of that connection back.
Overwhelmed by the amount of information I need to process daily, I had not been thinking about the interpersonal side of the pandemic for the first weeks. I felt it leaving the ED that morning and later that day, and I felt it again with Ms. Z, who was not even suspected of having COVID. She is a 62-year-old I interviewed with the help of a translator phone. At the end of our encounter, she said “But doctor, will you make my tumor go away?” From across the room, I said, “I will try.” I saw her eyes dampen as I made a hasty exit, following protocol to limit time in the room of all patients.
Typically, leaving a patient’s room, I would feel a fullness associated with a sense of meaning. How did I feel after that? In that moment, mostly ashamed at my lack of compassion during my time with Ms. Z. Then, with further reflection, tense from all things COVID-19! Having an amped-up sympathetic nervous system is understandable, but it’s not where we want to be for our compassion to flow.
We connect best when our parasympathetic nervous system is predominant. So much of the stimuli we need to activate that part of the nervous system is gone. There is a virtuous cycle, much of it unconscious, where something positive leads to more positivity, which is crucial to meaningful patient encounters. We read each other’s facial expressions, hear the tone of voice, and as we pick up subtle cues from our patient, our nervous system is further engaged and our hearts opened.
The specter of COVID-19 has us battling a negative spiral of stress and fear. For the most part, I try to keep that from consuming me, but it clearly saps my energy during encounters. In the same way we need to marshal our resources to battle both the stress and the disease itself, we need to actively engage pro-social elements of providing care to maintain our compassion. Clearly, I needed a more concerted effort to kick start this virtuous cycle of compassion.
My next patient was Ms. J., a 55-year-old with advanced chronic obstructive pulmonary disease (COPD) who came in the night before with shortness of breath. Her slight frame shook from coughing as I entered the room. I did not think she had COVID-19, but we were ruling it out.
We reviewed how she felt since admission, and I performed a hasty exam and stepped back across the room. She coughed again and said, “I feel so weak, and the world feels so crazy; tell it to me straight.” Then looking in my eyes, “I am going to make it, doc?”
I took my cue from her; I walked back to the bedside, placed a gloved hand on her shoulder and with the other, I took her hand. I bent forward just a little. Making eye contact and attempting a comforting tone of voice, I said, “Everyone is a little scared, including me. We need each other more than ever these days. We will do our best for you. That means thoughtful medical care and a whole lot of love! And, truly, I don’t think you are dying; this is just one of your COPD flares.”
“God bless you!” she said, squeezing my hand as a tear rolled down her cheek.
“Bless you, too. We all need blessing with this madness going on,” I replied. Despite the mask, I am sure she saw the smile in my eyes. “Thanks for being the beautiful person you are and opening up to me. That’s the way we will make it through this. I will see you tomorrow.” Backing away, hands together in prayer, I gave a little bow and left the room.
With Ms. J.’s help, I began to figure it out. To tackle the stress of COVID, we need to be very direct – almost to the point of exaggeration – to make sure our words and actions convey what we need to express. William James, the father of psychology, believed that if you force a smile, your emotions would follow. The neural pathways could work backward in that way. He said, “If you want a quality, act as if you have it.” The modern translation would be, “Fake it ’til you make it.’ ” You may be feeling stressed, but with a deep breath and a moment’s reflection on the suffering of that patient you are about to see, you can turn the tide on anxiety and give those under your care what they need.
These are unprecedented times; anxiety abounds. While we can aspire to positivity, there are times when we simply can’t muster showing it. Alternatively, as I experienced with Ms. J., honesty and vulnerability can open the door to meaningful connection. This can be quite powerful when we, as physicians, open up to our patients.
People are yearning for deep connection, and we should attempt to deliver it with:
- Touch (as we can) to convey connection.
- Body language that adds emphasis to our message and our emotions that may go above and beyond what we are used to.
- Tone of voice that enhances our words.
- Talk that emphasizes the big stuff, such as love, fear, connection and community
With gloves, masks, distance, and fear between and us and our patients, we need to actively engage our pro-social tools to turn the negative spiral of fear into the virtuous cycle of positive emotions that promotes healing of our patients and emotional engagement for those providing their care.
Dr. Hass was trained in family medicine at University of California, San Francisco, after receiving his medical degree from the McGill University faculty of medicine, Montreal. He works as a hospitalist with Sutter Health in Oakland, Calif. He is an adviser on health and health care for the Greater Good Science Center at UC Berkeley and clinical faculty at UCSF School of Medicine. This article appeared initially at The Hospital Leader, the official blog of SHM.
A toddler with a fever and desquamating perineal rash
Kawasaki disease
Given (KD). An echocardiogram revealed diffuse dilation of the left anterior descending artery without evidence of an aneurysm. The patient was promptly started on 2 g/kg IVIG and high-dose aspirin. She was later transitioned to low-dose aspirin. Long-term follow-up thus far has revealed no cardiac sequelae.
KD, or mucocutaneous lymph node syndrome, is a multisystem vasculitis with predilection for the coronary arteries that most commonly affects children between 6 months and 5 years of age.1 While the etiology remains unclear, the pathogenesis is thought to be the result of an immune response to an infection in the setting of genetic susceptibility.1 Approximately 90% of patients have mucocutaneous manifestations, highlighting the important role dermatologists play in the diagnosis and early intervention to prevent cardiovascular morbidity.
The diagnostic criteria include fever for at least 5 days accompanied by at least four of the following:
- Bilateral bulbar conjunctival injection without exudate that is classically limbal sparing.
- Oral mucosal changes with cracked fissured lips, “strawberry tongue,” or erythema of the lips and mucosa.
- Changes in the extremities: erythema, swelling, or periungual peeling.
- Polymorphous exanthem.
- Cervical lymphadenopathy, often unilateral (greater than 1.5 cm).
Although nonspecific for diagnosis, laboratory abnormalities are common, including anemia, thrombocytosis, leukocytosis, elevated inflammatory markers, elevated alanine aminotransferase (ALT), hypoalbuminemia, and sterile pyuria on urine analysis.1
Notably, a classic finding of KD is perineal dermatitis with desquamation occurring in the acute phase of disease in 80%-90% of patients.2-5 In a retrospective review, up to 67% of patients with KD developed a perineal rash in the first week, most often beginning in the diaper area.2 The perineal rash classically desquamates early during the acute phase of the disease.1
While most individuals with KD follow a benign disease course, it is the most common cause of acquired heart disease in the United States.1 Treatment is aimed at decreasing the risk of developing coronary abnormalities through the prompt administration of IVIG and high-dose aspirin initiated early in the acute phase.6 A second dose of IVIG may be given to patients who remain febrile within 24-48 hours after treatment.6 Infliximab has been used safely and effectively in patients with refractory KD.7 Long-term cardiac follow-up of KD patients is recommended.
Recently, there has been an emerging association between COVID-19 and pediatric multi-system inflammatory syndrome, which shares features with KD. Patients with pediatric multi-system inflammatory syndrome who meet clinical criteria for KD should be promptly treated with IVIG and aspirin to avoid long-term cardiac sequelae.
This case and the photos were submitted by Dr. Elizabeth H. Cusick and Dr. Molly E. Plovanich, both with the department of dermatology at the University of Rochester (N.Y.). Dr. Donna Bilu Martin edited the case.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bayers S et al. (2013). J Am Acad Dermatol. 2013 Oct;69(4):501.e1-11.
2. Friter BS and Lucky AW. Arch Dermatol. 1988 Dec;124(12):1805-10.
3. Urbach AH et al. Am J Dis Child. 1988 Nov;142(11):1174-6.
4. Fink CW. Pediatr Infect Dis. 1983 Mar-Apr; 2(2):140-1.
5. Aballi A J and Bisken LC. Pediatr Infect Dis. 1984 Mar-Apr;3(2):187.
6. McCrindle BW et al. Circulation. 2017 Apr 25;135(17):e927-e99.
7.Sauvaget E et al. J Pediatr. 2012 May; 160(5),875-6.
Kawasaki disease
Given (KD). An echocardiogram revealed diffuse dilation of the left anterior descending artery without evidence of an aneurysm. The patient was promptly started on 2 g/kg IVIG and high-dose aspirin. She was later transitioned to low-dose aspirin. Long-term follow-up thus far has revealed no cardiac sequelae.
KD, or mucocutaneous lymph node syndrome, is a multisystem vasculitis with predilection for the coronary arteries that most commonly affects children between 6 months and 5 years of age.1 While the etiology remains unclear, the pathogenesis is thought to be the result of an immune response to an infection in the setting of genetic susceptibility.1 Approximately 90% of patients have mucocutaneous manifestations, highlighting the important role dermatologists play in the diagnosis and early intervention to prevent cardiovascular morbidity.
The diagnostic criteria include fever for at least 5 days accompanied by at least four of the following:
- Bilateral bulbar conjunctival injection without exudate that is classically limbal sparing.
- Oral mucosal changes with cracked fissured lips, “strawberry tongue,” or erythema of the lips and mucosa.
- Changes in the extremities: erythema, swelling, or periungual peeling.
- Polymorphous exanthem.
- Cervical lymphadenopathy, often unilateral (greater than 1.5 cm).
Although nonspecific for diagnosis, laboratory abnormalities are common, including anemia, thrombocytosis, leukocytosis, elevated inflammatory markers, elevated alanine aminotransferase (ALT), hypoalbuminemia, and sterile pyuria on urine analysis.1
Notably, a classic finding of KD is perineal dermatitis with desquamation occurring in the acute phase of disease in 80%-90% of patients.2-5 In a retrospective review, up to 67% of patients with KD developed a perineal rash in the first week, most often beginning in the diaper area.2 The perineal rash classically desquamates early during the acute phase of the disease.1
While most individuals with KD follow a benign disease course, it is the most common cause of acquired heart disease in the United States.1 Treatment is aimed at decreasing the risk of developing coronary abnormalities through the prompt administration of IVIG and high-dose aspirin initiated early in the acute phase.6 A second dose of IVIG may be given to patients who remain febrile within 24-48 hours after treatment.6 Infliximab has been used safely and effectively in patients with refractory KD.7 Long-term cardiac follow-up of KD patients is recommended.
Recently, there has been an emerging association between COVID-19 and pediatric multi-system inflammatory syndrome, which shares features with KD. Patients with pediatric multi-system inflammatory syndrome who meet clinical criteria for KD should be promptly treated with IVIG and aspirin to avoid long-term cardiac sequelae.
This case and the photos were submitted by Dr. Elizabeth H. Cusick and Dr. Molly E. Plovanich, both with the department of dermatology at the University of Rochester (N.Y.). Dr. Donna Bilu Martin edited the case.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bayers S et al. (2013). J Am Acad Dermatol. 2013 Oct;69(4):501.e1-11.
2. Friter BS and Lucky AW. Arch Dermatol. 1988 Dec;124(12):1805-10.
3. Urbach AH et al. Am J Dis Child. 1988 Nov;142(11):1174-6.
4. Fink CW. Pediatr Infect Dis. 1983 Mar-Apr; 2(2):140-1.
5. Aballi A J and Bisken LC. Pediatr Infect Dis. 1984 Mar-Apr;3(2):187.
6. McCrindle BW et al. Circulation. 2017 Apr 25;135(17):e927-e99.
7.Sauvaget E et al. J Pediatr. 2012 May; 160(5),875-6.
Kawasaki disease
Given (KD). An echocardiogram revealed diffuse dilation of the left anterior descending artery without evidence of an aneurysm. The patient was promptly started on 2 g/kg IVIG and high-dose aspirin. She was later transitioned to low-dose aspirin. Long-term follow-up thus far has revealed no cardiac sequelae.
KD, or mucocutaneous lymph node syndrome, is a multisystem vasculitis with predilection for the coronary arteries that most commonly affects children between 6 months and 5 years of age.1 While the etiology remains unclear, the pathogenesis is thought to be the result of an immune response to an infection in the setting of genetic susceptibility.1 Approximately 90% of patients have mucocutaneous manifestations, highlighting the important role dermatologists play in the diagnosis and early intervention to prevent cardiovascular morbidity.
The diagnostic criteria include fever for at least 5 days accompanied by at least four of the following:
- Bilateral bulbar conjunctival injection without exudate that is classically limbal sparing.
- Oral mucosal changes with cracked fissured lips, “strawberry tongue,” or erythema of the lips and mucosa.
- Changes in the extremities: erythema, swelling, or periungual peeling.
- Polymorphous exanthem.
- Cervical lymphadenopathy, often unilateral (greater than 1.5 cm).
Although nonspecific for diagnosis, laboratory abnormalities are common, including anemia, thrombocytosis, leukocytosis, elevated inflammatory markers, elevated alanine aminotransferase (ALT), hypoalbuminemia, and sterile pyuria on urine analysis.1
Notably, a classic finding of KD is perineal dermatitis with desquamation occurring in the acute phase of disease in 80%-90% of patients.2-5 In a retrospective review, up to 67% of patients with KD developed a perineal rash in the first week, most often beginning in the diaper area.2 The perineal rash classically desquamates early during the acute phase of the disease.1
While most individuals with KD follow a benign disease course, it is the most common cause of acquired heart disease in the United States.1 Treatment is aimed at decreasing the risk of developing coronary abnormalities through the prompt administration of IVIG and high-dose aspirin initiated early in the acute phase.6 A second dose of IVIG may be given to patients who remain febrile within 24-48 hours after treatment.6 Infliximab has been used safely and effectively in patients with refractory KD.7 Long-term cardiac follow-up of KD patients is recommended.
Recently, there has been an emerging association between COVID-19 and pediatric multi-system inflammatory syndrome, which shares features with KD. Patients with pediatric multi-system inflammatory syndrome who meet clinical criteria for KD should be promptly treated with IVIG and aspirin to avoid long-term cardiac sequelae.
This case and the photos were submitted by Dr. Elizabeth H. Cusick and Dr. Molly E. Plovanich, both with the department of dermatology at the University of Rochester (N.Y.). Dr. Donna Bilu Martin edited the case.
Dr. Bilu Martin is a board-certified dermatologist in private practice at Premier Dermatology, MD, in Aventura, Fla. More diagnostic cases are available at mdedge.com/dermatology. To submit a case for possible publication, send an email to [email protected].
References
1. Bayers S et al. (2013). J Am Acad Dermatol. 2013 Oct;69(4):501.e1-11.
2. Friter BS and Lucky AW. Arch Dermatol. 1988 Dec;124(12):1805-10.
3. Urbach AH et al. Am J Dis Child. 1988 Nov;142(11):1174-6.
4. Fink CW. Pediatr Infect Dis. 1983 Mar-Apr; 2(2):140-1.
5. Aballi A J and Bisken LC. Pediatr Infect Dis. 1984 Mar-Apr;3(2):187.
6. McCrindle BW et al. Circulation. 2017 Apr 25;135(17):e927-e99.
7.Sauvaget E et al. J Pediatr. 2012 May; 160(5),875-6.
An otherwise healthy 18-month-old female presented to the emergency department with 5 days of fever, erythema, fissuring of the lips, conjunctival injection, and a desquamating perineal rash. In addition, she had nasal congestion and cough for which she was started on amoxicillin 2 days prior to presentation given concern for pneumonia.
On exam, she was also noted to have several palpable cervical lymph nodes and edematous hands with overlying erythema. Laboratory evaluation was notable for respiratory syncytial virus positivity by polymerase chain reaction assay, leukocytosis, and elevated inflammatory markers (erythrocyte sedimentation rate and C-reactive protein).
Doctors advise asthmatics to continue therapy during pandemic
“In fact, there’s no data to support this at this time. Maintaining adequate asthma control is the current CDC recommendation,” said pediatric pulmonologist John Carl, MD, of Cleveland Clinic Children’s Hospital. Patients, he said, should be advised to “follow your asthma action plan as outlined by your primary care or specialty clinician and communicate about evolving symptoms, such as fever rather than just congestion, wheezing, and coughing, etc.”
Dr. Carl spoke in a May 7 webinar about asthma and COVID-19 with Lakiea Wright, M.D., a physician specializing in internal medicine and allergy and immunology at Brigham and Women’s Hospital in Boston and medical director of clinical affairs for Thermo Fisher Scientific’s ImmunoDiagnostics division. The webinar, sponsored by Thermo Fisher Scientific, included discussion of COVID-19 risks, disease management, and distinguishing between the virus and asthma.
In a follow-up interview, Dr. Wright said she’s hearing from patients and parents who are concerned about whether people with asthma face a higher risk of COVID-19 infection. There’s no evidence that they do, she said, but “the CDC states that individuals with moderate to severe asthma may be higher risk for moderate to severe disease from COVID-19 if they were to become infected.”
Indeed, she said, “it is well established that viruses can trigger asthma.” But, as she also noted, early research about the risk in patients with asthma is conflicting.
“Some studies suggest asthma may be a risk factor for hospitalization while other data suggests asthma is not a common risk factor for those hospitalized,” Dr. Wright said.
She highlighted a recent study that suggests people with allergic asthma have “a reduced ACE2 gene expression in airway cells and thus decreased susceptibility to infection” by the novel coronavirus (J Allergy Clin Immunol. 2020 Apr 22. doi: 10.1016/j.jaci.2020.04.009).
Dr. Wright cautioned, however, that “this is a hypothesis and will need to be studied more.”
For now, she said, patients “should follow their asthma action plan and take their inhalers, including inhaled corticosteroids, as prescribed by their health care providers.”
Most patients are reasonable and do comply when their physicians explain why they should take a medication,” she noted.
Dr. Carl agreed, and added that a short course of oral corticosteroids are also recommended to manage minor exacerbations and “prevent patients from having to arrive as inpatients in more acute settings and risk health system–related exposures to the current pandemic.”
He cautioned, however, that metered-dose inhalers are preferable to nebulizers, and side vent ports should be avoided since they can aerosolize infectious agents and put health care providers and family members at risk.
Unfortunately, he said, there’s been a shortage of short-acting beta agonist albuterol inhalers. This has been linked to hospitals trying to avoid the use of nebulizers.
Dr. Wright advised colleagues to focus on unique symptoms first, then address overlapping symptoms and other symptoms to differentiate between COVID-19 and asthma/allergy.
She noted that environmental allergy symptoms alone do not cause fever, a hallmark of COVID-19. Shortness of breath can be a distinguishing symptom for the virus, because this is not a common symptom of environmental allergies unless the patient has asthma, Dr. Wright said.
Cough can be an overlapping symptom because in environmental allergies, postnasal drip from allergic rhinitis can trigger cough, she explained. Nasal congestion and/or runny nose can develop with viral illnesses in general, but these are symptoms not included in the CDC’s list of the most common COVID-19 symptoms. Severe fatigue and body aches aren’t symptoms consistent with environmental allergies, Dr. Wright said.
Both Dr. Carl and Dr. Wright emphasized the importance of continuing routine asthma therapy during the pandemic.
“When discussing the importance of taking their inhaled steroids with patients, I also remind patients that asthma management is comprehensive,” Dr. Wright said. “I want them to take their medications, but I also want them avoid or minimize exposure to triggers. Allergic and nonallergic triggers such as environmental tobacco smoke can exacerbate asthma.”
In addition, she said, “it’s important to take a detailed medical history to identify triggers. And it’s important to conduct allergy testing to common environmental allergens to help identify allergic triggers and tailor environmental allergen control strategies based on the results. All of these strategies help patients keep their asthma well-controlled.”
Dr. Carl and Dr. Wright report having no relevant disclosures.
“In fact, there’s no data to support this at this time. Maintaining adequate asthma control is the current CDC recommendation,” said pediatric pulmonologist John Carl, MD, of Cleveland Clinic Children’s Hospital. Patients, he said, should be advised to “follow your asthma action plan as outlined by your primary care or specialty clinician and communicate about evolving symptoms, such as fever rather than just congestion, wheezing, and coughing, etc.”
Dr. Carl spoke in a May 7 webinar about asthma and COVID-19 with Lakiea Wright, M.D., a physician specializing in internal medicine and allergy and immunology at Brigham and Women’s Hospital in Boston and medical director of clinical affairs for Thermo Fisher Scientific’s ImmunoDiagnostics division. The webinar, sponsored by Thermo Fisher Scientific, included discussion of COVID-19 risks, disease management, and distinguishing between the virus and asthma.
In a follow-up interview, Dr. Wright said she’s hearing from patients and parents who are concerned about whether people with asthma face a higher risk of COVID-19 infection. There’s no evidence that they do, she said, but “the CDC states that individuals with moderate to severe asthma may be higher risk for moderate to severe disease from COVID-19 if they were to become infected.”
Indeed, she said, “it is well established that viruses can trigger asthma.” But, as she also noted, early research about the risk in patients with asthma is conflicting.
“Some studies suggest asthma may be a risk factor for hospitalization while other data suggests asthma is not a common risk factor for those hospitalized,” Dr. Wright said.
She highlighted a recent study that suggests people with allergic asthma have “a reduced ACE2 gene expression in airway cells and thus decreased susceptibility to infection” by the novel coronavirus (J Allergy Clin Immunol. 2020 Apr 22. doi: 10.1016/j.jaci.2020.04.009).
Dr. Wright cautioned, however, that “this is a hypothesis and will need to be studied more.”
For now, she said, patients “should follow their asthma action plan and take their inhalers, including inhaled corticosteroids, as prescribed by their health care providers.”
Most patients are reasonable and do comply when their physicians explain why they should take a medication,” she noted.
Dr. Carl agreed, and added that a short course of oral corticosteroids are also recommended to manage minor exacerbations and “prevent patients from having to arrive as inpatients in more acute settings and risk health system–related exposures to the current pandemic.”
He cautioned, however, that metered-dose inhalers are preferable to nebulizers, and side vent ports should be avoided since they can aerosolize infectious agents and put health care providers and family members at risk.
Unfortunately, he said, there’s been a shortage of short-acting beta agonist albuterol inhalers. This has been linked to hospitals trying to avoid the use of nebulizers.
Dr. Wright advised colleagues to focus on unique symptoms first, then address overlapping symptoms and other symptoms to differentiate between COVID-19 and asthma/allergy.
She noted that environmental allergy symptoms alone do not cause fever, a hallmark of COVID-19. Shortness of breath can be a distinguishing symptom for the virus, because this is not a common symptom of environmental allergies unless the patient has asthma, Dr. Wright said.
Cough can be an overlapping symptom because in environmental allergies, postnasal drip from allergic rhinitis can trigger cough, she explained. Nasal congestion and/or runny nose can develop with viral illnesses in general, but these are symptoms not included in the CDC’s list of the most common COVID-19 symptoms. Severe fatigue and body aches aren’t symptoms consistent with environmental allergies, Dr. Wright said.
Both Dr. Carl and Dr. Wright emphasized the importance of continuing routine asthma therapy during the pandemic.
“When discussing the importance of taking their inhaled steroids with patients, I also remind patients that asthma management is comprehensive,” Dr. Wright said. “I want them to take their medications, but I also want them avoid or minimize exposure to triggers. Allergic and nonallergic triggers such as environmental tobacco smoke can exacerbate asthma.”
In addition, she said, “it’s important to take a detailed medical history to identify triggers. And it’s important to conduct allergy testing to common environmental allergens to help identify allergic triggers and tailor environmental allergen control strategies based on the results. All of these strategies help patients keep their asthma well-controlled.”
Dr. Carl and Dr. Wright report having no relevant disclosures.
“In fact, there’s no data to support this at this time. Maintaining adequate asthma control is the current CDC recommendation,” said pediatric pulmonologist John Carl, MD, of Cleveland Clinic Children’s Hospital. Patients, he said, should be advised to “follow your asthma action plan as outlined by your primary care or specialty clinician and communicate about evolving symptoms, such as fever rather than just congestion, wheezing, and coughing, etc.”
Dr. Carl spoke in a May 7 webinar about asthma and COVID-19 with Lakiea Wright, M.D., a physician specializing in internal medicine and allergy and immunology at Brigham and Women’s Hospital in Boston and medical director of clinical affairs for Thermo Fisher Scientific’s ImmunoDiagnostics division. The webinar, sponsored by Thermo Fisher Scientific, included discussion of COVID-19 risks, disease management, and distinguishing between the virus and asthma.
In a follow-up interview, Dr. Wright said she’s hearing from patients and parents who are concerned about whether people with asthma face a higher risk of COVID-19 infection. There’s no evidence that they do, she said, but “the CDC states that individuals with moderate to severe asthma may be higher risk for moderate to severe disease from COVID-19 if they were to become infected.”
Indeed, she said, “it is well established that viruses can trigger asthma.” But, as she also noted, early research about the risk in patients with asthma is conflicting.
“Some studies suggest asthma may be a risk factor for hospitalization while other data suggests asthma is not a common risk factor for those hospitalized,” Dr. Wright said.
She highlighted a recent study that suggests people with allergic asthma have “a reduced ACE2 gene expression in airway cells and thus decreased susceptibility to infection” by the novel coronavirus (J Allergy Clin Immunol. 2020 Apr 22. doi: 10.1016/j.jaci.2020.04.009).
Dr. Wright cautioned, however, that “this is a hypothesis and will need to be studied more.”
For now, she said, patients “should follow their asthma action plan and take their inhalers, including inhaled corticosteroids, as prescribed by their health care providers.”
Most patients are reasonable and do comply when their physicians explain why they should take a medication,” she noted.
Dr. Carl agreed, and added that a short course of oral corticosteroids are also recommended to manage minor exacerbations and “prevent patients from having to arrive as inpatients in more acute settings and risk health system–related exposures to the current pandemic.”
He cautioned, however, that metered-dose inhalers are preferable to nebulizers, and side vent ports should be avoided since they can aerosolize infectious agents and put health care providers and family members at risk.
Unfortunately, he said, there’s been a shortage of short-acting beta agonist albuterol inhalers. This has been linked to hospitals trying to avoid the use of nebulizers.
Dr. Wright advised colleagues to focus on unique symptoms first, then address overlapping symptoms and other symptoms to differentiate between COVID-19 and asthma/allergy.
She noted that environmental allergy symptoms alone do not cause fever, a hallmark of COVID-19. Shortness of breath can be a distinguishing symptom for the virus, because this is not a common symptom of environmental allergies unless the patient has asthma, Dr. Wright said.
Cough can be an overlapping symptom because in environmental allergies, postnasal drip from allergic rhinitis can trigger cough, she explained. Nasal congestion and/or runny nose can develop with viral illnesses in general, but these are symptoms not included in the CDC’s list of the most common COVID-19 symptoms. Severe fatigue and body aches aren’t symptoms consistent with environmental allergies, Dr. Wright said.
Both Dr. Carl and Dr. Wright emphasized the importance of continuing routine asthma therapy during the pandemic.
“When discussing the importance of taking their inhaled steroids with patients, I also remind patients that asthma management is comprehensive,” Dr. Wright said. “I want them to take their medications, but I also want them avoid or minimize exposure to triggers. Allergic and nonallergic triggers such as environmental tobacco smoke can exacerbate asthma.”
In addition, she said, “it’s important to take a detailed medical history to identify triggers. And it’s important to conduct allergy testing to common environmental allergens to help identify allergic triggers and tailor environmental allergen control strategies based on the results. All of these strategies help patients keep their asthma well-controlled.”
Dr. Carl and Dr. Wright report having no relevant disclosures.