User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
TNF inhibitors may dampen COVID-19 severity
Patients on a tumor necrosis factor inhibitor for their rheumatic disease when they became infected with COVID-19 were markedly less likely to subsequently require hospitalization, according to intriguing early evidence from the COVID-19 Global Rheumatology Alliance Registry.
On the other hand, those registry patients who were on 10 mg of prednisone or more daily when they got infected were more than twice as likely to be hospitalized than were those who were not on corticosteroids, even after controlling for the severity of their rheumatic disease and other potential confounders, Jinoos Yazdany, MD, reported at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
“We saw a signal with moderate to high-dose steroids. I think it’s something we’re going to have to keep an eye out on as more data come in,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
The global registry launched on March 24, 2020, and was quickly embraced by rheumatologists from around the world. By May 12, the registry included more than 1,300 patients with a range of rheumatic diseases, all with confirmed COVID-19 infection as a requisite for enrollment; the cases were submitted by more than 300 rheumatologists in 40 countries. The registry is supported by the ACR and European League Against Rheumatism.
Dr. Yazdany, a member of the registry steering committee, described the project’s two main goals: To learn the outcomes of COVID-19–infected patients with various rheumatic diseases and to make inferences regarding the impact of the immunosuppressive and antimalarial medications widely prescribed by rheumatologists.
She presented soon-to-be-published data on the characteristics and disposition of the first 600 patients, 46% of whom were hospitalized and 9% died. A caveat regarding the registry, she noted, is that these are observational data and thus potentially subject to unrecognized confounders. Also, the registry population is skewed toward the sicker end of the COVID-19 disease spectrum because while all participants have confirmed infection, testing for the infection has been notoriously uneven. Many people are infected asymptomatically and thus may not undergo testing even where readily available.
Early key findings from registry
The risk factors for more severe infection resulting in hospitalization in patients with rheumatic diseases are by and large the same drivers described in the general population: older age and comorbid conditions including diabetes, hypertension, cardiovascular disease, obesity, chronic kidney disease, and lung disease. Notably, however, patients on the equivalent of 10 mg/day of prednisone or more were at a 105% increased risk for hospitalization, compared with those not on corticosteroids after adjustment for age, comorbid conditions, and rheumatic disease severity.
Patients on a background tumor necrosis factor (TNF) inhibitor had an adjusted 60% reduction in risk of hospitalization. This apparent protective effect against more severe COVID-19 disease is mechanistically plausible: In animal studies, being on a TNF inhibitor has been associated with less severe infection following exposure to influenza virus, Dr. Yazdany observed.
COVID-infected patients on any biologic disease-modifying antirheumatic drug had a 54% decreased risk of hospitalization. However, in this early analysis, the study was sufficiently powered only to specifically assess the impact of TNF inhibitors, since those agents were by far the most commonly used biologics. As the registry grows, it will be possible to analyze the impact of other antirheumatic medications.
Being on hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization.
The only rheumatic disease diagnosis with an odds of hospitalization significantly different from that of RA patients was systemic lupus erythematosus (SLE). Lupus patients were at 80% increased risk of hospitalization. Although this was a statistically significant difference, Dr. Yazdany cautioned against making too much of it because of the strong potential for unmeasured confounding. In particular, lupus patients as a group are known to rate on the lower end of measures of social determinants of health, a status that is an established major risk factor for COVID-19 disease.
“A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” she said.
Being on background NSAIDs at the time of SARS-CoV-2 infection was not associated with increased risk of hospitalization; in fact, NSAID users were 36% less likely to be hospitalized for their COVID-19 disease, although this difference didn’t reach statistical significance.
Dr. Yazdany urged her fellow rheumatologists to enter their cases on the registry website: rheum-covid.org. There they can also join the registry mailing list and receive weekly updates.
Other recent insights on COVID-19 in rheumatology
An as-yet unpublished U.K. observational study involving electronic health record data on 17 million people included 885,000 individuals with RA, SLE, or psoriasis. After extensive statistical controlling for the known risk factors for severe COVID-19 infection, including a measure of socioeconomic deprivation, the group with one of these autoimmune diseases had an adjusted, statistically significant 23% increased risk of hospital death because of COVID-19 infection.
“This is the largest study of its kind to date. There’s potential for unmeasured confounding and selection bias here due to who gets tested. We’ll have to see where this study lands, but I think it does suggest there’s a slightly higher mortality risk in COVID-infected patients with rheumatic disease,” according to Dr. Yazdany.
On the other hand, there have been at least eight recently published patient surveys and case series of patients with rheumatic diseases in areas of the world hardest hit by the pandemic, and they paint a consistent picture.
“What we’ve learned from these studies was the infection rate was generally in the ballpark of people in the region. It doesn’t seem like there’s a dramatically higher infection rate in people with rheumatic disease in these surveys. The hospitalized rheumatology patients had many of the familiar comorbidities. This is the first glance at how likely people are to become infected and how they fared, and I think overall the data have been quite reassuring,” she said.
Dr. Yazdany reported serving as a consultant to AstraZeneca and Eli Lilly and receiving research funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention.
Patients on a tumor necrosis factor inhibitor for their rheumatic disease when they became infected with COVID-19 were markedly less likely to subsequently require hospitalization, according to intriguing early evidence from the COVID-19 Global Rheumatology Alliance Registry.
On the other hand, those registry patients who were on 10 mg of prednisone or more daily when they got infected were more than twice as likely to be hospitalized than were those who were not on corticosteroids, even after controlling for the severity of their rheumatic disease and other potential confounders, Jinoos Yazdany, MD, reported at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
“We saw a signal with moderate to high-dose steroids. I think it’s something we’re going to have to keep an eye out on as more data come in,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
The global registry launched on March 24, 2020, and was quickly embraced by rheumatologists from around the world. By May 12, the registry included more than 1,300 patients with a range of rheumatic diseases, all with confirmed COVID-19 infection as a requisite for enrollment; the cases were submitted by more than 300 rheumatologists in 40 countries. The registry is supported by the ACR and European League Against Rheumatism.
Dr. Yazdany, a member of the registry steering committee, described the project’s two main goals: To learn the outcomes of COVID-19–infected patients with various rheumatic diseases and to make inferences regarding the impact of the immunosuppressive and antimalarial medications widely prescribed by rheumatologists.
She presented soon-to-be-published data on the characteristics and disposition of the first 600 patients, 46% of whom were hospitalized and 9% died. A caveat regarding the registry, she noted, is that these are observational data and thus potentially subject to unrecognized confounders. Also, the registry population is skewed toward the sicker end of the COVID-19 disease spectrum because while all participants have confirmed infection, testing for the infection has been notoriously uneven. Many people are infected asymptomatically and thus may not undergo testing even where readily available.
Early key findings from registry
The risk factors for more severe infection resulting in hospitalization in patients with rheumatic diseases are by and large the same drivers described in the general population: older age and comorbid conditions including diabetes, hypertension, cardiovascular disease, obesity, chronic kidney disease, and lung disease. Notably, however, patients on the equivalent of 10 mg/day of prednisone or more were at a 105% increased risk for hospitalization, compared with those not on corticosteroids after adjustment for age, comorbid conditions, and rheumatic disease severity.
Patients on a background tumor necrosis factor (TNF) inhibitor had an adjusted 60% reduction in risk of hospitalization. This apparent protective effect against more severe COVID-19 disease is mechanistically plausible: In animal studies, being on a TNF inhibitor has been associated with less severe infection following exposure to influenza virus, Dr. Yazdany observed.
COVID-infected patients on any biologic disease-modifying antirheumatic drug had a 54% decreased risk of hospitalization. However, in this early analysis, the study was sufficiently powered only to specifically assess the impact of TNF inhibitors, since those agents were by far the most commonly used biologics. As the registry grows, it will be possible to analyze the impact of other antirheumatic medications.
Being on hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization.
The only rheumatic disease diagnosis with an odds of hospitalization significantly different from that of RA patients was systemic lupus erythematosus (SLE). Lupus patients were at 80% increased risk of hospitalization. Although this was a statistically significant difference, Dr. Yazdany cautioned against making too much of it because of the strong potential for unmeasured confounding. In particular, lupus patients as a group are known to rate on the lower end of measures of social determinants of health, a status that is an established major risk factor for COVID-19 disease.
“A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” she said.
Being on background NSAIDs at the time of SARS-CoV-2 infection was not associated with increased risk of hospitalization; in fact, NSAID users were 36% less likely to be hospitalized for their COVID-19 disease, although this difference didn’t reach statistical significance.
Dr. Yazdany urged her fellow rheumatologists to enter their cases on the registry website: rheum-covid.org. There they can also join the registry mailing list and receive weekly updates.
Other recent insights on COVID-19 in rheumatology
An as-yet unpublished U.K. observational study involving electronic health record data on 17 million people included 885,000 individuals with RA, SLE, or psoriasis. After extensive statistical controlling for the known risk factors for severe COVID-19 infection, including a measure of socioeconomic deprivation, the group with one of these autoimmune diseases had an adjusted, statistically significant 23% increased risk of hospital death because of COVID-19 infection.
“This is the largest study of its kind to date. There’s potential for unmeasured confounding and selection bias here due to who gets tested. We’ll have to see where this study lands, but I think it does suggest there’s a slightly higher mortality risk in COVID-infected patients with rheumatic disease,” according to Dr. Yazdany.
On the other hand, there have been at least eight recently published patient surveys and case series of patients with rheumatic diseases in areas of the world hardest hit by the pandemic, and they paint a consistent picture.
“What we’ve learned from these studies was the infection rate was generally in the ballpark of people in the region. It doesn’t seem like there’s a dramatically higher infection rate in people with rheumatic disease in these surveys. The hospitalized rheumatology patients had many of the familiar comorbidities. This is the first glance at how likely people are to become infected and how they fared, and I think overall the data have been quite reassuring,” she said.
Dr. Yazdany reported serving as a consultant to AstraZeneca and Eli Lilly and receiving research funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention.
Patients on a tumor necrosis factor inhibitor for their rheumatic disease when they became infected with COVID-19 were markedly less likely to subsequently require hospitalization, according to intriguing early evidence from the COVID-19 Global Rheumatology Alliance Registry.
On the other hand, those registry patients who were on 10 mg of prednisone or more daily when they got infected were more than twice as likely to be hospitalized than were those who were not on corticosteroids, even after controlling for the severity of their rheumatic disease and other potential confounders, Jinoos Yazdany, MD, reported at the virtual edition of the American College of Rheumatology’s 2020 State-of-the-Art Clinical Symposium.
“We saw a signal with moderate to high-dose steroids. I think it’s something we’re going to have to keep an eye out on as more data come in,” said Dr. Yazdany, professor of medicine at the University of California, San Francisco, and chief of rheumatology at San Francisco General Hospital.
The global registry launched on March 24, 2020, and was quickly embraced by rheumatologists from around the world. By May 12, the registry included more than 1,300 patients with a range of rheumatic diseases, all with confirmed COVID-19 infection as a requisite for enrollment; the cases were submitted by more than 300 rheumatologists in 40 countries. The registry is supported by the ACR and European League Against Rheumatism.
Dr. Yazdany, a member of the registry steering committee, described the project’s two main goals: To learn the outcomes of COVID-19–infected patients with various rheumatic diseases and to make inferences regarding the impact of the immunosuppressive and antimalarial medications widely prescribed by rheumatologists.
She presented soon-to-be-published data on the characteristics and disposition of the first 600 patients, 46% of whom were hospitalized and 9% died. A caveat regarding the registry, she noted, is that these are observational data and thus potentially subject to unrecognized confounders. Also, the registry population is skewed toward the sicker end of the COVID-19 disease spectrum because while all participants have confirmed infection, testing for the infection has been notoriously uneven. Many people are infected asymptomatically and thus may not undergo testing even where readily available.
Early key findings from registry
The risk factors for more severe infection resulting in hospitalization in patients with rheumatic diseases are by and large the same drivers described in the general population: older age and comorbid conditions including diabetes, hypertension, cardiovascular disease, obesity, chronic kidney disease, and lung disease. Notably, however, patients on the equivalent of 10 mg/day of prednisone or more were at a 105% increased risk for hospitalization, compared with those not on corticosteroids after adjustment for age, comorbid conditions, and rheumatic disease severity.
Patients on a background tumor necrosis factor (TNF) inhibitor had an adjusted 60% reduction in risk of hospitalization. This apparent protective effect against more severe COVID-19 disease is mechanistically plausible: In animal studies, being on a TNF inhibitor has been associated with less severe infection following exposure to influenza virus, Dr. Yazdany observed.
COVID-infected patients on any biologic disease-modifying antirheumatic drug had a 54% decreased risk of hospitalization. However, in this early analysis, the study was sufficiently powered only to specifically assess the impact of TNF inhibitors, since those agents were by far the most commonly used biologics. As the registry grows, it will be possible to analyze the impact of other antirheumatic medications.
Being on hydroxychloroquine or other antimalarials at the time of COVID-19 infection had no impact on hospitalization.
The only rheumatic disease diagnosis with an odds of hospitalization significantly different from that of RA patients was systemic lupus erythematosus (SLE). Lupus patients were at 80% increased risk of hospitalization. Although this was a statistically significant difference, Dr. Yazdany cautioned against making too much of it because of the strong potential for unmeasured confounding. In particular, lupus patients as a group are known to rate on the lower end of measures of social determinants of health, a status that is an established major risk factor for COVID-19 disease.
“A strength of the global registry has been that it provides timely data that’s been very helpful for rheumatologists to rapidly dispel misinformation that has been spread about hydroxychloroquine, especially statements about lupus patients not getting COVID-19. We know from these data that’s not true,” she said.
Being on background NSAIDs at the time of SARS-CoV-2 infection was not associated with increased risk of hospitalization; in fact, NSAID users were 36% less likely to be hospitalized for their COVID-19 disease, although this difference didn’t reach statistical significance.
Dr. Yazdany urged her fellow rheumatologists to enter their cases on the registry website: rheum-covid.org. There they can also join the registry mailing list and receive weekly updates.
Other recent insights on COVID-19 in rheumatology
An as-yet unpublished U.K. observational study involving electronic health record data on 17 million people included 885,000 individuals with RA, SLE, or psoriasis. After extensive statistical controlling for the known risk factors for severe COVID-19 infection, including a measure of socioeconomic deprivation, the group with one of these autoimmune diseases had an adjusted, statistically significant 23% increased risk of hospital death because of COVID-19 infection.
“This is the largest study of its kind to date. There’s potential for unmeasured confounding and selection bias here due to who gets tested. We’ll have to see where this study lands, but I think it does suggest there’s a slightly higher mortality risk in COVID-infected patients with rheumatic disease,” according to Dr. Yazdany.
On the other hand, there have been at least eight recently published patient surveys and case series of patients with rheumatic diseases in areas of the world hardest hit by the pandemic, and they paint a consistent picture.
“What we’ve learned from these studies was the infection rate was generally in the ballpark of people in the region. It doesn’t seem like there’s a dramatically higher infection rate in people with rheumatic disease in these surveys. The hospitalized rheumatology patients had many of the familiar comorbidities. This is the first glance at how likely people are to become infected and how they fared, and I think overall the data have been quite reassuring,” she said.
Dr. Yazdany reported serving as a consultant to AstraZeneca and Eli Lilly and receiving research funding from the National Institutes of Health, the Agency for Healthcare Research and Quality, and the Centers for Disease Control and Prevention.
REPORTING FROM SOTA 2020
Antibody testing suggests COVID-19 cases are being missed
The number of COVID-19 infections in the community may be “substantially greater” than totals confirmed by authorities, based on SARS-CoV-2 antibody testing among a random sample of adults in Los Angeles County, Calif.
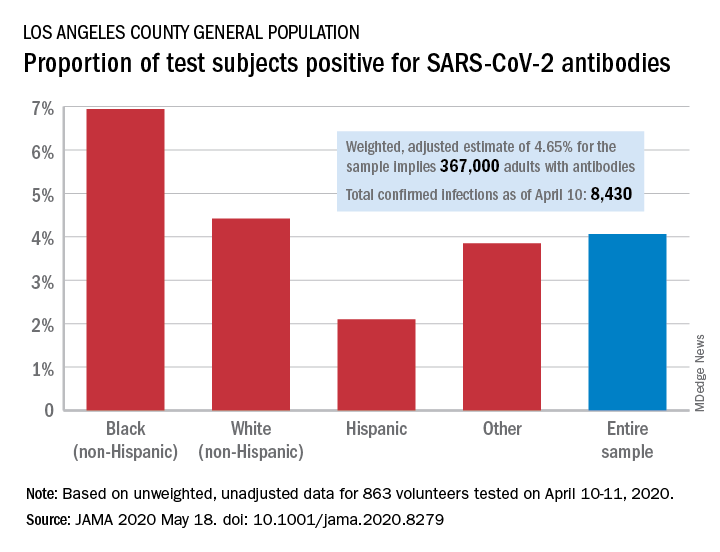
Testing of 863 people on April 10-11 revealed that 35 (4.06%) were positive for SARS-CoV-2–specific antibodies (IgM or IgG), and after adjustment for test sensitivity and specificity, the weighted prevalence for the entire sample was 4.65%, Neeraj Sood, PhD, of the University of Southern California, Los Angeles, and associates wrote in JAMA.
The estimate of 4.65% “implies that approximately 367,000 adults [in Los Angeles County] had SARS-CoV-2 antibodies, which is substantially greater than the 8,430 cumulative number of confirmed infections in the county on April 10,” they wrote.
It also suggests that fatality rates based on the larger number of infections may be lower than rates based on confirmed cases. “In addition, contact tracing methods to limit the spread of infection will face considerable challenges,” Dr. Sood and associates said.
Test positivity varied by race/ethnicity, sex, and income. The proportion of non-Hispanic blacks with a positive result was 6.94%, compared with 4.42% for non-Hispanic whites, 2.10% for Hispanics, and 3.85% for others. Men were much more likely than women to be positive for SARS-CoV-2: 5.18% vs. 3.31%, the investigators said.
Household income favored the middle ground. Those individuals making less than $50,000 a year had a positivity rate of 5.14% and those with an income of $100,000 or more had a rate of 4.90%, but only 1.58% of those making $50,000-$99,999 tested positive, they reported.
The authors reported numerous sources of nonprofit organization support.
SOURCE: Sood N et al. JAMA 2020 May 18. doi: 10.1001/jama.2020.8279.
The number of COVID-19 infections in the community may be “substantially greater” than totals confirmed by authorities, based on SARS-CoV-2 antibody testing among a random sample of adults in Los Angeles County, Calif.

Testing of 863 people on April 10-11 revealed that 35 (4.06%) were positive for SARS-CoV-2–specific antibodies (IgM or IgG), and after adjustment for test sensitivity and specificity, the weighted prevalence for the entire sample was 4.65%, Neeraj Sood, PhD, of the University of Southern California, Los Angeles, and associates wrote in JAMA.
The estimate of 4.65% “implies that approximately 367,000 adults [in Los Angeles County] had SARS-CoV-2 antibodies, which is substantially greater than the 8,430 cumulative number of confirmed infections in the county on April 10,” they wrote.
It also suggests that fatality rates based on the larger number of infections may be lower than rates based on confirmed cases. “In addition, contact tracing methods to limit the spread of infection will face considerable challenges,” Dr. Sood and associates said.
Test positivity varied by race/ethnicity, sex, and income. The proportion of non-Hispanic blacks with a positive result was 6.94%, compared with 4.42% for non-Hispanic whites, 2.10% for Hispanics, and 3.85% for others. Men were much more likely than women to be positive for SARS-CoV-2: 5.18% vs. 3.31%, the investigators said.
Household income favored the middle ground. Those individuals making less than $50,000 a year had a positivity rate of 5.14% and those with an income of $100,000 or more had a rate of 4.90%, but only 1.58% of those making $50,000-$99,999 tested positive, they reported.
The authors reported numerous sources of nonprofit organization support.
SOURCE: Sood N et al. JAMA 2020 May 18. doi: 10.1001/jama.2020.8279.
The number of COVID-19 infections in the community may be “substantially greater” than totals confirmed by authorities, based on SARS-CoV-2 antibody testing among a random sample of adults in Los Angeles County, Calif.

Testing of 863 people on April 10-11 revealed that 35 (4.06%) were positive for SARS-CoV-2–specific antibodies (IgM or IgG), and after adjustment for test sensitivity and specificity, the weighted prevalence for the entire sample was 4.65%, Neeraj Sood, PhD, of the University of Southern California, Los Angeles, and associates wrote in JAMA.
The estimate of 4.65% “implies that approximately 367,000 adults [in Los Angeles County] had SARS-CoV-2 antibodies, which is substantially greater than the 8,430 cumulative number of confirmed infections in the county on April 10,” they wrote.
It also suggests that fatality rates based on the larger number of infections may be lower than rates based on confirmed cases. “In addition, contact tracing methods to limit the spread of infection will face considerable challenges,” Dr. Sood and associates said.
Test positivity varied by race/ethnicity, sex, and income. The proportion of non-Hispanic blacks with a positive result was 6.94%, compared with 4.42% for non-Hispanic whites, 2.10% for Hispanics, and 3.85% for others. Men were much more likely than women to be positive for SARS-CoV-2: 5.18% vs. 3.31%, the investigators said.
Household income favored the middle ground. Those individuals making less than $50,000 a year had a positivity rate of 5.14% and those with an income of $100,000 or more had a rate of 4.90%, but only 1.58% of those making $50,000-$99,999 tested positive, they reported.
The authors reported numerous sources of nonprofit organization support.
SOURCE: Sood N et al. JAMA 2020 May 18. doi: 10.1001/jama.2020.8279.
FROM JAMA
COVID-19 in kids: Severe illness most common in infants, teens
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
Children and young adults in all age groups can develop severe illness after SARS-CoV-2 infection, but the oldest and youngest appear most likely to be hospitalized and possibly critically ill, based on data from a retrospective cohort study of 177 pediatric patients seen at a single center.
“Although children and young adults clearly are susceptible to SARS-CoV-2 infection, attention has focused primarily on their potential role in influencing spread and community transmission rather than the potential severity of infection in children and young adults themselves,” wrote Roberta L. DeBiasi, MD, chief of the division of pediatric infectious diseases at Children’s National Hospital, Washington, and colleagues.
In a study published in the Journal of Pediatrics, the researchers reviewed data from 44 hospitalized and 133 non-hospitalized children and young adults infected with SARS-CoV-2. Of the 44 hospitalized patients, 35 were noncritically ill and 9 were critically ill. The study population ranged from 0.1-34 years of age, with a median of 10 years, which was similar between hospitalized and nonhospitalized patients. However, the median age of critically ill patients was significantly higher, compared with noncritically ill patients (17 years vs. 4 years). All age groups were represented in all cohorts. “However, we noted a bimodal distribution of patients less than 1 year of age and patients greater than 15 years of age representing the largest proportion of patients within the SARS-CoV-2–infected hospitalized and critically ill cohorts,” the researchers noted. Children less than 1 year and adolescents/young adults over 15 years each represented 32% of the 44 hospitalized patients.
Overall, 39% of the 177 patients had underlying medical conditions, the most frequent of which was asthma (20%), which was not significantly more common between hospitalized/nonhospitalized patients or critically ill/noncritically ill patients. Patients also presented with neurologic conditions (6%), diabetes (3%), obesity (2%), cardiac conditions (3%), hematologic conditions (3%) and oncologic conditions (1%). Underlying conditions occurred more commonly in the hospitalized cohort (63%) than in the nonhospitalized cohort (32%).
Neurologic disorders, cardiac conditions, hematologic conditions, and oncologic conditions were significantly more common in hospitalized patients, but not significantly more common among those critically ill versus noncritically ill.
About 76% of the patients presented with respiratory symptoms including rhinorrhea, congestion, sore throat, cough, or shortness of breath – with or without fever; 66% had fevers; and 48% had both respiratory symptoms and fever. Shortness of breath was significantly more common among hospitalized patients versus nonhospitalized patients (26% vs. 12%), but less severe respiratory symptoms were significantly more common among nonhospitalized patients, the researchers noted.
Other symptoms – such as diarrhea, vomiting, chest pain, and loss of sense or smell occurred in a small percentage of patients – but were not more likely to occur in any of the cohorts.
Among the critically ill patients, eight of nine needed some level of respiratory support, and four were on ventilators.
“One patient had features consistent with the recently emerged Kawasaki disease–like presentation with hyperinflammatory state, hypotension, and profound myocardial depression,” Dr. DiBiasi and associates noted.
The researchers found coinfection with routine coronavirus, respiratory syncytial virus, or rhinovirus/enterovirus in 4 of 63 (6%) patients, but the clinical impact of these coinfections are unclear.
The study findings were limited by several factors including the retrospective design and the ongoing transmission of COVID-19 in the Washington area, the researchers noted. “One potential bias of this study is our regional role in providing critical care for young adults age 21-35 years with COVID-19.” In addition, “we plan to address the role of race and ethnicity after validation of current administrative data and have elected to defer this analysis until completed.”
“Our findings highlight the potential for severe disease in this age group and inform other regions to anticipate and prepare their COVID-19 response to include a significant burden of hospitalized and critically ill children and young adults. As SARS-CoV-2 spreads within the United States, regional differences may be apparent based on virus and host factors that are yet to be identified,” Dr. DeBiasi and colleagues concluded.
Robin Steinhorn, MD, serves as an associate editor for the Journal of Pediatrics. The other researchers declared no conflicts of interest.
SOURCE: DeBiasi RL et al. J Pediatr. 2020 May 6. doi: 10.1016/j.jpeds.2020.05.007.
This article was updated 5/19/20.
FROM THE JOURNAL OF PEDIATRICS
Dermatologic changes with COVID-19: What we know and don’t know
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
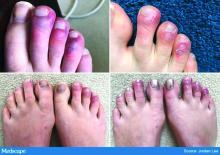
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
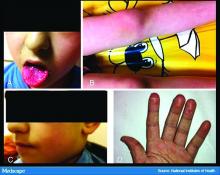
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
The dermatologic manifestations associated with SARS-CoV-2 are many and varied, with new information virtually daily. Graeme Lipper, MD, a member of the Medscape Dermatology advisory board, discussed what we know and what is still to be learned with Lindy Fox, MD, a professor of dermatology at University of California, San Francisco (UCSF) and a member of the American Academy of Dermatology’s COVID-19 Registry task force.
Graeme M. Lipper, MD
Earlier this spring, before there was any real talk about skin manifestations of COVID, my partner called me in to see an unusual case. His patient was a healthy 20-year-old who had just come back from college and had tender, purple discoloration and swelling on his toes. I shrugged and said “looks like chilblains,” but there was something weird about the case. It seemed more severe, with areas of blistering and erosions, and the discomfort was unusual for run-of-the-mill pernio. This young man had experienced a cough and shortness of breath a few weeks earlier but those symptoms had resolved when we saw him.
That evening, I was on a derm social media site and saw a series of pictures from Italy that blew me away. All of these pictures looked just like this kid’s toes. That’s the first I heard of “COVID toes,” but now they seem to be everywhere. How would you describe this presentation, and how does it differ from typical chilblains?
Lindy P. Fox, MD
I am so proud of dermatologists around the world who have really jumped into action to examine the pathophysiology and immunology behind these findings.
Your experience matches mine. Like you, I first heard about these pernio- or chilblains-like lesions when Europe was experiencing its surge in cases. And while it does indeed look like chilblains, I think the reality is that it is more severe and symptomatic than we would expect. I think your observation is exactly right. There are certainly clinicians who do not believe that this is an association with COVID-19 because the testing is often negative. But to my mind, there are just too many cases at the wrong time of year, all happening concomitantly, and simultaneous with a new virus for me to accept that they are not somehow related.
Dr. Lipper: Some have referred to this as “quarantine toes,” the result of more people at home and walking around barefoot. That doesn’t seem to make a whole lot of sense because it’s happening in both warm and cold climates.
Others have speculated that there is another, unrelated circulating virus causing these pernio cases, but that seems far-fetched.
But the idea of a reporting bias – more patients paying attention to these lesions because they’ve read something in the mass media or seen a report on television and are concerned, and thus present with mild lesions they might otherwise have ignored – may be contributing somewhat. But even that cannot be the sole reason behind the increase.
Dr. Fox: Agree.
Evaluation of the patient with chilblains – then and now
Dr. Lipper: In the past, how did you perform a workup for someone with chilblains?
Dr. Fox: Pre-COVID – and I think we all have divided our world into pre- and post-COVID – the most common thing that I’d be looking for would be a clotting disorder or an autoimmune disease, typically lupus. So I take a good history, review of systems, and look at the skin for signs of lupus or other autoimmune connective tissue diseases. My lab workup is probably limited to an antinuclear antibody (ANA). If the findings are severe and recurrent, I might check for hypercoagulability with an antiphospholipid antibody panel. But that was usually it unless there was something in the history or physical exam that would lead me to look for something less common – for example, cryoglobulins or an underlying hematologic disease that would lead to a predominance of lesions in acral sites.
My approach was the same. In New England, where I practice, I also always look at environmental factors. We would sometimes see chilblains in someone from a warmer climate who came home to the Northeast to ski.
Dr. Lipper: Now, in the post-COVID world, how do you assess these patients? What has changed?
Dr. Fox: That’s a great question. To be frank, our focus now is on not missing a secondary consequence of COVID infection that we might not have picked up before. I’m the first to admit that the workup that we have been doing at UCSF is extremely comprehensive. We may be ordering tests that don’t need to be done. But until we know better what might and might not be affected by COVID, we don’t actually have a sense of whether they’re worth looking for or not.
Right now, my workup includes nasal swab polymerase chain reaction (PCR) for COVID, as well as IgG and IgM serology if available. We have IgG easily available to us. IgM needs approval; at UCSF, it is primarily done in neonates as of now. I also do a workup for autoimmunity and cold-associated disease, which includes an ANA, rheumatoid factor, cryoglobulin, and cold agglutinins.
Because of reported concerns about hypercoagulability in COVID patients, particularly in those who are doing poorly in the hospital, we look for elevations in d-dimers and fibrinogen. We check antiphospholipid antibodies, anticardiolipin antibodies, erythrocyte sedimentation rate, and C-reactive protein. That is probably too much of a workup for the healthy young person, but as of yet, we are just unable to say that those things are universally normal.
There has also been concern that complement may be involved in patients who do poorly and tend to clot a lot. So we are also checking C3, C4, and CH50.
To date, in my patients who have had this workup, I have found one with a positive ANA that was significant (1:320) who also had low complements.
There have been a couple of patients at my institution, not my own patients, who are otherwise fine but have some slight elevation in d-dimers.
Dr. Lipper: Is COVID toes more than one condition?
Some of the initial reports of finger/toe cyanosis out of China were very alarming, with many patients developing skin necrosis or even gangrene. These were critically ill adults with pneumonia and blood markers of disseminated intravascular coagulation, and five out of seven died. In contrast, the cases of pseudo-pernio reported in Europe, and now the United States, seem to be much milder, usually occurring late in the illness or in asymptomatic young people. Do you think these are two different conditions?
Dr. Fox: I believe you have hit the nail on the head. I think it is really important that we don’t confuse those two things. In the inpatient setting, we are clearly seeing patients with a prothrombotic state with associated retiform purpura. For nondermatologists, that usually means star-like, stellate-like, or even lacy purpuric changes with potential for necrosis of the skin. In hospitalized patients, the fingers and toes are usually affected but, interestingly, also the buttocks. When these lesions are biopsied, as has been done by our colleague at Weill Cornell Medicine, New York, Joanna Harp, MD, we tend to find thrombosis.
A study of endothelial cell function in patients with COVID-19, published in the Lancet tried to determine whether viral particles could be found in endothelial cells. And the investigators did indeed find these particles. So it appears that the virus is endothelially active, and this might provide some insight into the thromboses seen in hospitalized patients. These patients can develop purple necrotic toes that may progress to gangrene. But that is completely different from what we’re seeing when we say pernio-like or chilblains-like lesions.
The chilblains-like lesions come in several forms. They may be purple, red bumps, often involving the tops of the toes and sometimes the bottom of the feet. Some have been described as target-like or erythema multiforme–like. In others, there may not be individual discrete lesions but rather a redness or bluish, purplish discoloration accompanied by edema of the entire toe or several toes.
Biopsies that I am aware of have identified features consistent with an inflammatory process, all of which can be seen in a typical biopsy of pernio. You can sometimes see lymphocytes surrounding a vessel (called lymphocytic vasculitis) that may damage a vessel and cause a small clot, but the primary process is an inflammatory rather than thrombotic one. You may get a clot in a little tiny vessel secondary to inflammation, and that may lead to some blisters or little areas of necrosis. But you’re not going to see digital necrosis and gangrene. I think that’s an important distinction.
The patients who get the pernio-like lesions are typically children or young adults and are otherwise healthy. Half of them didn’t even have COVID symptoms. If they did have COVID symptoms they were typically mild. So we think the pernio-like lesions are most often occurring in the late stage of the disease and now represent a secondary inflammatory response.
Managing COVID toes
Dr. Lipper: One question I’ve been struggling with is, what do we tell these otherwise healthy patients with purple toes, especially those with no other symptoms? Many of them are testing SARS-CoV-2 negative, both with viral swabs and serologies. Some have suggestive histories like known COVID exposure, recent cough, or travel to high-risk areas. Do we tell them they’re at risk of transmitting the virus? Should they self-quarantine, and for how long? Is there any consensus emerging?
Dr. Fox: This is a good opportunity to plug the American Academy of Dermatology’s COVID-19 Registry, which is run by Esther Freeman, MD, at Massachusetts General Hospital. She has done a phenomenal job in helping us figure out the answers to these exact questions.
I’d encourage any clinicians who have a suspected COVID patient with a skin finding, whether or not infection is confirmed with testing, to enter information about that patient into the registry. That is the only way we will figure out evidence-based answers to a lot of the questions that we’re talking about today.
Based on working with the registry, we know that, rarely, patients who develop pernio-like changes will do so before they get COVID symptoms or at the same time as more typical symptoms. Some patients with these findings are PCR positive, and it is therefore theoretically possible that you could be shedding virus while you’re having the pernio toes. However, more commonly – and this is the experience of most of my colleagues and what we’re seeing at UCSF – pernio is a later finding and most patients are no longer shedding the virus. It appears that pseudo-pernio is an immune reaction and most people are not actively infectious at that point.
The only way to know for sure is to send patients for both PCR testing and antibody testing. If the PCR is negative, the most likely interpretation is that the person is no longer shedding virus, though there can be some false negatives. Therefore, these patients do not need to isolate outside of what I call their COVID pod – family or roommates who have probably been with them the whole time. Any transmission likely would have already occurred.
I tell people who call me concerned about their toes that I do think they should be given a workup for COVID. However, I reassure them that it is usually a good prognostic sign.
What is puzzling is that even in patients with pseudo-chilblains who have a clinical history consistent with COVID or exposure to a COVID-positive family member, antibody testing is often – in fact, most often – negative. There are many hypotheses as to why this is. Maybe the tests just aren’t good. Maybe people with mild disease don’t generate enough antibodies to be detected, Maybe we’re testing at the wrong time. Those are all things that we’re trying to figure out.
But currently, I tell patients that they do not need to strictly isolate. They should still practice social distancing, wear a mask, practice good hand hygiene, and do all of the careful things that we should all be doing. However, they can live within their home environment and be reassured that most likely they are in the convalescent stage.
Dr. Lipper: I find the antibody issue both fascinating and confusing.
In my practice, we’ve noticed a range of symptoms associated with pseudo-pernio. Some people barely realize it’s there and only called because they saw a headline in the news. Others complain of severe burning, throbbing, or itching that keeps them up at night and can sometimes last for weeks. Are there any treatments that seem to help?
Dr. Fox: We can start by saying, as you note, that a lot of patients don’t need interventions. They want reassurance that their toes aren’t going to fall off, that nothing terrible is going to happen to them, and often that’s enough. So far, many patients have contacted us just because they heard about the link between what they were seeing on their feet and COVID. They were likely toward the end of any other symptoms they may have had. But moving forward, I think we’re going to be seeing patients at the more active stage as the public is more aware of this finding.
Most of the time we can manage with clobetasol ointment and low-dose aspirin. I wouldn’t give aspirin to a young child with a high fever, but otherwise I think aspirin is not harmful. A paper published in Mayo Clinic Proceedings in 2014, before COVID, by Jonathan Cappel, MD, and David Wetter, MD, provides a nice therapeutic algorithm. Assuming that the findings we are seeing now are inflammatory, then I think that algorithm should apply. Nifedipine 20-60 mg/day is an option. Hydroxychloroquine, a maximum of 5 mg/kg per day, is an option. I have used hydroxychloroquine most commonly, pre-COVID, in patients who have symptomatic pernio.
I also use pentoxifylline 400 mg three times a day, which has a slight anti-inflammatory effect, when I think a blood vessel is incidentally involved or the patient has a predisposition to clotting. Nicotinamide 500 mg three times a day can be used, though I have not used it.
Some topical options are nitroglycerin, tacrolimus, and minoxidil.
However, during this post-COVID period, I have not come across many with pseudo-pernio who needed anything more than a topical steroid and some aspirin. But I do know of other physicians who have been taking care of patients with much more symptomatic disease.
Dr. Lipper: That is a comprehensive list. You’ve mentioned some options that I’ve wondered about, especially pentoxifylline, which I have found to be very helpful for livedoid vasculopathy. I should note that these are all off-label uses.
Let’s talk about some other suspected skin manifestations of COVID. A prospective nationwide study in Spain of 375 patients reported on a number of different skin manifestations of COVID.
You’re part of a team doing critically important work with the American Academy of Dermatology COVID-19 Dermatology Registry. I know it’s early going, but what are some of the other common skin presentations you’re finding?
Dr. Fox: I’m glad you brought up that paper out of Spain. I think it is really good and does highlight the difference in acute versus convalescent cutaneous manifestations and prognosis. It confirms what we’re seeing. Retiform purpura is an early finding associated with ill patients in the hospital. Pseudo pernio-like lesions tend to be later-stage and in younger, healthier patients.
Interestingly, the vesicular eruption that those investigators describe – monomorphic vesicles on the trunk and extremity – can occur in the more acute phase. That’s fascinating to me because widespread vesicular eruptions are not a thing that we commonly see. If it is not an autoimmune blistering disease, and not a drug-induced blistering process, then you’re really left with viral. Rickettsialpox can do that, as can primary varicella, disseminated herpes, disseminated zoster, and now COVID. So that’s intriguing.
I got called to see a patient yesterday who had symptoms of COVID about a month ago. She was not PCR tested at the time but she is now negative. She has a widespread eruption of tiny vesicles on an erythematous base. An IgG for COVID is positive. How do we decide whether her skin lesions have active virus in them?
The many dermatologic manifestations of COVID-19
Dr. Lipper: In the series in Spain, almost 1 out of 10 patients were found to have a widespread vesicular rash. And just under half had maculopapular exanthems. The information arising from the AAD registry will be of great interest and build on this paper.
In England, the National Health Service and the Paediatric Intensive Care Society recently put out a warning about an alarming number of children with COVID-19 who developed symptoms mimicking Kawasaki disease (high fever, abdominal pain, rash, swollen lymph nodes, mucositis, and conjunctivitis). These kids have systemic inflammation and vasculitis and are critically ill. That was followed by an alert from the New York City Health Department about cases there, which as of May 6 numbered 64. Another 25 children with similar findings have been identified in France.
This is such a scary development, especially because children were supposed to be relatively “safe” from this virus. Any thoughts on who is at risk or why?
Dr. Fox: It’s very alarming. It appears that these cases look just like Kawasaki disease.
It was once hypothesized that Coronaviridae was the cause of Kawasaki disease. Then that got debunked. But these cases now raise the question of whether Kawasaki disease may be virally mediated. Is it an immune reaction to an infectious trigger? Is it actually Coronaviridae that triggers it?
As with these pernio cases, I think we’re going to learn about the pathophysiology of these diseases that we currently look at as secondary responses or immune reactions to unknown triggers. We’re going to learn a lot about them and about the immune system because of how this virus is acting on the immune system.
Dr. Lipper: As is the case with patients with pernio-like lesions, some of these children with Kawasaki-like disease are PCR negative for SARS-CoV-2. It will be interesting to see what happens with antibody testing in this population.
Dr. Fox: Agree. While some of the manufacturers of serology tests have claimed that they have very high sensitivity and specificity, that has not been my experience.
Dr. Lipper: I’ve had a number of patients with a clinical picture that strongly suggests COVID whose serology tests have been negative.
Dr. Fox: As have I. While this could be the result of faulty tests, my biggest worry is that it means that people with mild disease do not mount an antibody response. And if people who have disease can’t make antibodies, then there’s no herd immunity. If there’s no herd immunity, we’re stuck in lockdown until there’s a vaccine.
Dr. Lipper: That is a scary but real possibility. We need evidence – evidence like that provided by the AAD registry.
Dr. Fox: Agree. I look forward to sharing those results with you when we have them.
Dr. Lipper is a clinical assistant professor at the University of Vermont, Burlington, and a partner at Advanced DermCare in Danbury, Conn.
Dr. Fox is a professor in the department of dermatology at the University of California, San Francisco. She is a hospital-based dermatologist who specializes in the care of patients with complex skin conditions. She is immediate past president of the Medical Dermatology Society and current president of the Society of Dermatology Hospitalists.
This article was first published on Medscape.com.
Energy-based therapy plus oxymetazoline proves safe for rosacea
according to results from a phase 4 study.
“The current study was designed to evaluate the safety and tolerability of oxymetazoline when used as an adjunctive treatment with energy‐based therapy for patients with moderate to severe facial erythema associated with rosacea,” wrote Emil A. Tanghetti, MD, of the Center for Dermatology and Laser Surgery in Sacramento, and coauthors. The findings were published in Lasers in Surgery and Medicine.
The open-label, interventional study included 46 patients with rosacea, with moderate to severe facial erythema. Study participants received treatment with one of four energy-based devices: pulsed‐dye laser Vbeam Perfecta (PDL-Vbeam), pulsed‐dye laser Cynergy (PDL-Cynergy), intense pulsed-light therapy (IPL), or potassium titanyl phosphate laser (KTP laser), in combination with adjunctive oxymetazoline hydrochloride cream (1%).
On days 3-27 and 31-56, oxymetazoline, an alpha1A adrenoceptor agonist was applied once daily, while energy-based therapy was provided on day 1 and day 29.
The primary safety endpoints were the incidence of treatment‐emergent adverse events (TEAEs) and serious adverse events; the exploratory efficacy endpoint was the change in clinician erythema assessment (CEA) score from start of therapy measured over a 6-hour period post treatment.
Among 43 evaluable patients (who completed the study), CEA score was improved in 39 (90.7%) patients 6 hours post treatment on day 56 and in 30 (68.2%) patients pretreatment.
On day 31, of the 43 evaluable patients, “one‐grade or greater improvement was observed” in 26 (60.5%) patients before application of oxymetazoline, and in 38 (88.4%) of patients 6 hours post treatment, they wrote.
Overall, patient satisfaction increased over the course of the study, with 28 (65.1%) of patients reporting they were satisfied or very satisfied with the treatment on day 56.
Among 46 patients who received at least one treatment, 5 (10.9%) patients had one or more TEAEs (KTP laser, n = 1; PDL-Vbeam, n = 4), and 4 patients had one or more treatment‐related TEAEs (PDL-Vbeam, n = 4); All TEAEs were considered mild or moderate. “Three (6.5%) patients experienced TEAEs related to oxymetazoline; all led to study discontinuation,” the researchers reported.
The researchers acknowledged that a key limitation of the study was the use of multiple energy-based devices, delivered by different providers, which could have caused inconsistency in the results.
“Prospective clinical studies assessing the long‐term safety and efficacy of combined treatment with oxymetazoline and energy‐based therapies are needed,” they concluded.
The manuscript was funded by oxymetazoline manufacturer Aclaris Therapeutics. Several authors disclosed being an investigator, consultant, and/or laser manufacturers. One author was an employee of Aclaris at the time of the study.
SOURCE: Tanghetti EA et al. Lasers Surg Med. 2020 May 6. doi: 10.1002/lsm.23253.
according to results from a phase 4 study.
“The current study was designed to evaluate the safety and tolerability of oxymetazoline when used as an adjunctive treatment with energy‐based therapy for patients with moderate to severe facial erythema associated with rosacea,” wrote Emil A. Tanghetti, MD, of the Center for Dermatology and Laser Surgery in Sacramento, and coauthors. The findings were published in Lasers in Surgery and Medicine.
The open-label, interventional study included 46 patients with rosacea, with moderate to severe facial erythema. Study participants received treatment with one of four energy-based devices: pulsed‐dye laser Vbeam Perfecta (PDL-Vbeam), pulsed‐dye laser Cynergy (PDL-Cynergy), intense pulsed-light therapy (IPL), or potassium titanyl phosphate laser (KTP laser), in combination with adjunctive oxymetazoline hydrochloride cream (1%).
On days 3-27 and 31-56, oxymetazoline, an alpha1A adrenoceptor agonist was applied once daily, while energy-based therapy was provided on day 1 and day 29.
The primary safety endpoints were the incidence of treatment‐emergent adverse events (TEAEs) and serious adverse events; the exploratory efficacy endpoint was the change in clinician erythema assessment (CEA) score from start of therapy measured over a 6-hour period post treatment.
Among 43 evaluable patients (who completed the study), CEA score was improved in 39 (90.7%) patients 6 hours post treatment on day 56 and in 30 (68.2%) patients pretreatment.
On day 31, of the 43 evaluable patients, “one‐grade or greater improvement was observed” in 26 (60.5%) patients before application of oxymetazoline, and in 38 (88.4%) of patients 6 hours post treatment, they wrote.
Overall, patient satisfaction increased over the course of the study, with 28 (65.1%) of patients reporting they were satisfied or very satisfied with the treatment on day 56.
Among 46 patients who received at least one treatment, 5 (10.9%) patients had one or more TEAEs (KTP laser, n = 1; PDL-Vbeam, n = 4), and 4 patients had one or more treatment‐related TEAEs (PDL-Vbeam, n = 4); All TEAEs were considered mild or moderate. “Three (6.5%) patients experienced TEAEs related to oxymetazoline; all led to study discontinuation,” the researchers reported.
The researchers acknowledged that a key limitation of the study was the use of multiple energy-based devices, delivered by different providers, which could have caused inconsistency in the results.
“Prospective clinical studies assessing the long‐term safety and efficacy of combined treatment with oxymetazoline and energy‐based therapies are needed,” they concluded.
The manuscript was funded by oxymetazoline manufacturer Aclaris Therapeutics. Several authors disclosed being an investigator, consultant, and/or laser manufacturers. One author was an employee of Aclaris at the time of the study.
SOURCE: Tanghetti EA et al. Lasers Surg Med. 2020 May 6. doi: 10.1002/lsm.23253.
according to results from a phase 4 study.
“The current study was designed to evaluate the safety and tolerability of oxymetazoline when used as an adjunctive treatment with energy‐based therapy for patients with moderate to severe facial erythema associated with rosacea,” wrote Emil A. Tanghetti, MD, of the Center for Dermatology and Laser Surgery in Sacramento, and coauthors. The findings were published in Lasers in Surgery and Medicine.
The open-label, interventional study included 46 patients with rosacea, with moderate to severe facial erythema. Study participants received treatment with one of four energy-based devices: pulsed‐dye laser Vbeam Perfecta (PDL-Vbeam), pulsed‐dye laser Cynergy (PDL-Cynergy), intense pulsed-light therapy (IPL), or potassium titanyl phosphate laser (KTP laser), in combination with adjunctive oxymetazoline hydrochloride cream (1%).
On days 3-27 and 31-56, oxymetazoline, an alpha1A adrenoceptor agonist was applied once daily, while energy-based therapy was provided on day 1 and day 29.
The primary safety endpoints were the incidence of treatment‐emergent adverse events (TEAEs) and serious adverse events; the exploratory efficacy endpoint was the change in clinician erythema assessment (CEA) score from start of therapy measured over a 6-hour period post treatment.
Among 43 evaluable patients (who completed the study), CEA score was improved in 39 (90.7%) patients 6 hours post treatment on day 56 and in 30 (68.2%) patients pretreatment.
On day 31, of the 43 evaluable patients, “one‐grade or greater improvement was observed” in 26 (60.5%) patients before application of oxymetazoline, and in 38 (88.4%) of patients 6 hours post treatment, they wrote.
Overall, patient satisfaction increased over the course of the study, with 28 (65.1%) of patients reporting they were satisfied or very satisfied with the treatment on day 56.
Among 46 patients who received at least one treatment, 5 (10.9%) patients had one or more TEAEs (KTP laser, n = 1; PDL-Vbeam, n = 4), and 4 patients had one or more treatment‐related TEAEs (PDL-Vbeam, n = 4); All TEAEs were considered mild or moderate. “Three (6.5%) patients experienced TEAEs related to oxymetazoline; all led to study discontinuation,” the researchers reported.
The researchers acknowledged that a key limitation of the study was the use of multiple energy-based devices, delivered by different providers, which could have caused inconsistency in the results.
“Prospective clinical studies assessing the long‐term safety and efficacy of combined treatment with oxymetazoline and energy‐based therapies are needed,” they concluded.
The manuscript was funded by oxymetazoline manufacturer Aclaris Therapeutics. Several authors disclosed being an investigator, consultant, and/or laser manufacturers. One author was an employee of Aclaris at the time of the study.
SOURCE: Tanghetti EA et al. Lasers Surg Med. 2020 May 6. doi: 10.1002/lsm.23253.
FROM LASERS IN SURGERY AND MEDICINE
Planning for a psychiatric COVID-19–positive unit
Identifying key decision points is critical
Reports have emerged about the unique vulnerability of psychiatric hospitals to the ravages of COVID-19.
In a South Korea psychiatric hospital, 101 of 103 patients contracted SARS-CoV-2 during an outbreak; 7 eventually died.1,2 This report, among a few others, have led to the development of psychiatric COVID-19–positive units (PCU). However, it remains highly unclear how many are currently open, where they are located, or what their operations are like.
We knew that we could not allow a medically asymptomatic “covertly” COVID-19–positive patient to be introduced to the social community of our inpatient units because of the risks of transmission to other patients and staff.
In coordination with our health system infection prevention experts, we have therefore required a confirmed negative COVID-19 polymerase chain reaction nasal swab performed no more than 48 hours prior to the time/date of acute psychiatric inpatient admission. Furthermore, as part of the broad health system response and surge planning, we were asked by our respective incident command centers to begin planning for a Psychiatric COVID-19–positive Unit (PCU) that might allow us to safely care for a cohort of patients needing such hospitalization.
It is worth emphasizing that the typical patient who is a candidate for a PCU is so acutely psychiatrically ill that they cannot be managed in a less restrictive environment than an inpatient psychiatric unit and, at the same time, is likely to not be medically ill enough to warrant admission to an internal medicine service in a general acute care hospital.
We have identified eight principles and critical decision points that can help inpatient units plan for the safe care of COVID-19–positive patients on a PCU.
1. Triage: Patients admitted to a PCU should be medically stable, particularly with regard to COVID-19 and respiratory symptomatology. PCUs should establish clear criteria for admission and discharge (or medical transfer). Examples of potential exclusionary criteria to a PCU include:
- Respiratory distress, shortness of breath, hypoxia, requirement for supplemental oxygen, or requirement for respiratory therapy breathing treatments.
- Fever, or signs of sepsis, or systemic inflammatory response syndrome.
- Medical frailty, significant medical comorbidities, delirium, or altered mental status;
- Requirements for continuous vital sign monitoring or of a monitoring frequency beyond the capacity of the PCU.
Discharge criteria may also include a symptom-based strategy because emerging evidence suggests that patients may be less infectious by day 10-14 of the disease course,3 and viral lab testing is very sensitive and will be positive for periods of time after individuals are no longer infectious. The symptom-based strategy allows for patients to not require retesting prior to discharge. However, some receiving facilities (for example residential or skilled nursing facilities) may necessitate testing, in which case a testing-based strategy can be used. The Centers for Disease Control and Prevention provides guidelines for both types of strategies.4
2. Infection control and personal protective equipment: PCUs require modifications or departures from the typical inpatient free-ranging environment in which common areas are provided for patients to engage in a community of care, including group therapy (such as occupational, recreational, Alcoholics Anonymous, and social work groups).
- Isolation: PCUs must consider whether they will require patients to isolate to their rooms or to allow modified or limited access to “public” or “community” areas. While there do not appear to be standard recommendations from the CDC or other public health entities regarding negative pressure or any specific room ventilation requirements, it is prudent to work with local infectious disease experts on protocols. Important considerations include spatial planning for infection control areas to don and doff appropriate personal protective equipment (PPE) and appropriate workspace to prevent contamination of non–COVID-19 work areas. Approaches can include establishing clearly identified and visually demarcated infection control “zones” (often referred to as “hot, warm, and cold zones”) that correspond to specific PPE requirements for staff. In addition, individuals should eat in their own rooms or designated areas because use of common areas for meals can potentially lead to aerosolized spread of the virus.
- Cohorting: Generally, PCUs should consider admitting only COVID-19–positive patients to a PCU to avoid exposure to other patients. Hospitals and health systems should determine protocols and locations for testing and managing “patients under investigation” for COVID-19, which should precede admission to the PCU.
- PPE: It is important to clearly establish and communicate PPE requirements and procedures for direct physical contact versus no physical contact (for example, visual safety checks). Identify clear supply chains for PPE and hand sanitizer.
3. Medical management and consultation: PCUs should establish clear pathways for accessing consultation from medical consultants. It may be ideal, in addition to standard daily psychiatric physician rounding, to have daily internal medicine rounding and/or medical nursing staff working on the unit. Given the potential of COVID-19–positive patients to rapidly devolve from asymptomatic to acutely ill, it is necessary to establish protocols for the provision of urgent medical care 24/7 and streamlined processes for transfer to a medical unit.
Clear protocols should be established to address any potential signs of decompensation in the respiratory status of a PCU unit, including administration of oxygen and restrictions (or appropriate precautions) related to aerosolizing treatment such as nebulizers or positive airway pressure.
4. Code blue protocol: Any emergent medical issues, including acute respiratory decompensation, should trigger a Code Blue response that has been specifically designed for COVID-19–positive patients, including considerations for proper PPE during resuscitation efforts.
5. Psychiatric staffing and workflows: When possible, it may be preferable to engage volunteer medical and nursing staff for the PCU, as opposed to mandating participation. Take into consideration support needs, including education and training about safe PPE practices, processes for testing health care workers, return-to-work guidance, and potential alternate housing.
- Telehealth: Clinicians (such as physicians, social workers, occupational therapists) should leverage and maximize the use of telemedicine to minimize direct or prolonged exposure to infectious disease risks.
- Nursing: It is important to establish appropriate ratios of nursing and support staff for a COVID-19–positive psychiatry unit given the unique work flows related to isolation precautions and to ensure patient and staff safety. These ratios may take into account patient-specific needs, including the need for additional staff to perform constant observation for high-risk patients, management of agitated patients, and sufficient staff to allow for relief and break-time from PPE. Admission and routine care processes should be adapted in order to limit equipment entering the room, such as computer workstations on wheels.
- Medication administration procedures: Develop work flows related to PPE and infection control when retrieving and administering medications.
- Workspace: Designate appropriate workspace for PCU clinicians to access computers and documents and to minimize use of non–COVID-19 unit work areas.
6. Restraints and management of agitated patients: PCUs should develop plans for addressing agitated patients, including contingency plans for whether seclusion or restraints should be administered in the patient’s individual room or in a dedicated restraint room in the PCU. Staff training should include protocols specifically designed for managing agitated patients in the PCU.
7. Discharge processes: If patients remain medically well and clear their COVID-19 PCR tests, it is conceivable that they might be transferred to a non–COVID-19 psychiatric unit if sufficient isolation time has passed and the infectious disease consultants deem it appropriate. It is also possible that patients would be discharged from a PCU to home or other residential setting. Such patients should be assessed for ability to comply with continued self-quarantine if necessary. Discharge planning must take into consideration follow-up plans for COVID-19 illness and primary care appointments, as well as needed psychiatric follow-up.
8. Patients’ rights: The apparently highly infectious and transmissible nature of SARS-CoV-2 creates novel tensions between a wide range of individual rights and the rights of others. In addition to manifesting in our general society, there are potentially unique tensions in acute inpatient psychiatric settings. Certain patients’ rights may require modification in a PCU (for example, access to outdoor space, personal belongings, visitors, and possibly civil commitment judicial hearings). These discussions may require input from hospital compliance officers, ethics committees, risk managers, and the local department of mental health and also may be partly solved by using video communication platforms.
A few other “pearls” may be of value: Psychiatric hospitals that are colocated with a general acute care hospital or ED might be better situated to develop protocols to safely care for COVID-19–positive psychiatric patients, by virtue of the close proximity of full-spectrum acute general hospital services. Direct engagement by a command center and hospital or health system senior leadership also seems crucial as a means for assuring authorization to proceed with planning what may be a frightening or controversial (but necessary) adaptation of inpatient psychiatric unit(s) to the exigencies of the COVID-19 pandemic.
The resources of a robust community hospital or academic health system (including infection prevention leaders who engage in continuous liaison with local, county, state, and federal public health expertise) are crucial to the “learning health system” model, which requires flexibility, rapid adaptation to new knowledge, and accessibility to infectious disease and other consultation for special situations. Frequent and open communication with all professional stakeholders (through town halls, Q&A sessions, group discussions, and so on) is important in the planning process to socialize the principles and concepts that are critical for providing care in a PCU, reducing anxiety, and bolstering collegiality and staff morale.
References
1. Kim MJ. “ ‘It was a medical disaster’: The psychiatric ward that saw 100 patients with new coronavirus.” Independent. 2020 Mar 1.
2. Korean Society of Infectious Diseases et al. J Korean Med Sci. 2020 Mar 16;35(10):e112.
3. Centers for Disease Control and Prevention. Symptom-based strategy to discontinue isolation for persons with COVID-19. Decision Memo. 2020 May 3.
4. He X et al. Nature Medicine. 2020. 26:672-5.
Dr. Cheung is associate medical director and chief quality officer at the Stewart and Lynda Resnick Neuropsychiatric Hospital at the University of California, Los Angeles. He has no conflicts of interest. Dr. Strouse is medical director, UCLA Stewart and Lynda Resnick Neuropsychiatric Hospital and Maddie Katz Professor at the UCLA department of psychiatry/Semel Institute. He has no conflicts of interest. Dr. Li is associate medical director of quality improvement at Yale-New Haven Psychiatric Hospital in Connecticut. She also serves as medical director of clinical operations at the Yale-New Haven Health System. Dr. Li is a 2019-2020 Health and Aging Policy Fellow and receives funding support from the program.
Identifying key decision points is critical
Identifying key decision points is critical
Reports have emerged about the unique vulnerability of psychiatric hospitals to the ravages of COVID-19.
In a South Korea psychiatric hospital, 101 of 103 patients contracted SARS-CoV-2 during an outbreak; 7 eventually died.1,2 This report, among a few others, have led to the development of psychiatric COVID-19–positive units (PCU). However, it remains highly unclear how many are currently open, where they are located, or what their operations are like.
We knew that we could not allow a medically asymptomatic “covertly” COVID-19–positive patient to be introduced to the social community of our inpatient units because of the risks of transmission to other patients and staff.
In coordination with our health system infection prevention experts, we have therefore required a confirmed negative COVID-19 polymerase chain reaction nasal swab performed no more than 48 hours prior to the time/date of acute psychiatric inpatient admission. Furthermore, as part of the broad health system response and surge planning, we were asked by our respective incident command centers to begin planning for a Psychiatric COVID-19–positive Unit (PCU) that might allow us to safely care for a cohort of patients needing such hospitalization.
It is worth emphasizing that the typical patient who is a candidate for a PCU is so acutely psychiatrically ill that they cannot be managed in a less restrictive environment than an inpatient psychiatric unit and, at the same time, is likely to not be medically ill enough to warrant admission to an internal medicine service in a general acute care hospital.
We have identified eight principles and critical decision points that can help inpatient units plan for the safe care of COVID-19–positive patients on a PCU.
1. Triage: Patients admitted to a PCU should be medically stable, particularly with regard to COVID-19 and respiratory symptomatology. PCUs should establish clear criteria for admission and discharge (or medical transfer). Examples of potential exclusionary criteria to a PCU include:
- Respiratory distress, shortness of breath, hypoxia, requirement for supplemental oxygen, or requirement for respiratory therapy breathing treatments.
- Fever, or signs of sepsis, or systemic inflammatory response syndrome.
- Medical frailty, significant medical comorbidities, delirium, or altered mental status;
- Requirements for continuous vital sign monitoring or of a monitoring frequency beyond the capacity of the PCU.
Discharge criteria may also include a symptom-based strategy because emerging evidence suggests that patients may be less infectious by day 10-14 of the disease course,3 and viral lab testing is very sensitive and will be positive for periods of time after individuals are no longer infectious. The symptom-based strategy allows for patients to not require retesting prior to discharge. However, some receiving facilities (for example residential or skilled nursing facilities) may necessitate testing, in which case a testing-based strategy can be used. The Centers for Disease Control and Prevention provides guidelines for both types of strategies.4
2. Infection control and personal protective equipment: PCUs require modifications or departures from the typical inpatient free-ranging environment in which common areas are provided for patients to engage in a community of care, including group therapy (such as occupational, recreational, Alcoholics Anonymous, and social work groups).
- Isolation: PCUs must consider whether they will require patients to isolate to their rooms or to allow modified or limited access to “public” or “community” areas. While there do not appear to be standard recommendations from the CDC or other public health entities regarding negative pressure or any specific room ventilation requirements, it is prudent to work with local infectious disease experts on protocols. Important considerations include spatial planning for infection control areas to don and doff appropriate personal protective equipment (PPE) and appropriate workspace to prevent contamination of non–COVID-19 work areas. Approaches can include establishing clearly identified and visually demarcated infection control “zones” (often referred to as “hot, warm, and cold zones”) that correspond to specific PPE requirements for staff. In addition, individuals should eat in their own rooms or designated areas because use of common areas for meals can potentially lead to aerosolized spread of the virus.
- Cohorting: Generally, PCUs should consider admitting only COVID-19–positive patients to a PCU to avoid exposure to other patients. Hospitals and health systems should determine protocols and locations for testing and managing “patients under investigation” for COVID-19, which should precede admission to the PCU.
- PPE: It is important to clearly establish and communicate PPE requirements and procedures for direct physical contact versus no physical contact (for example, visual safety checks). Identify clear supply chains for PPE and hand sanitizer.
3. Medical management and consultation: PCUs should establish clear pathways for accessing consultation from medical consultants. It may be ideal, in addition to standard daily psychiatric physician rounding, to have daily internal medicine rounding and/or medical nursing staff working on the unit. Given the potential of COVID-19–positive patients to rapidly devolve from asymptomatic to acutely ill, it is necessary to establish protocols for the provision of urgent medical care 24/7 and streamlined processes for transfer to a medical unit.
Clear protocols should be established to address any potential signs of decompensation in the respiratory status of a PCU unit, including administration of oxygen and restrictions (or appropriate precautions) related to aerosolizing treatment such as nebulizers or positive airway pressure.
4. Code blue protocol: Any emergent medical issues, including acute respiratory decompensation, should trigger a Code Blue response that has been specifically designed for COVID-19–positive patients, including considerations for proper PPE during resuscitation efforts.
5. Psychiatric staffing and workflows: When possible, it may be preferable to engage volunteer medical and nursing staff for the PCU, as opposed to mandating participation. Take into consideration support needs, including education and training about safe PPE practices, processes for testing health care workers, return-to-work guidance, and potential alternate housing.
- Telehealth: Clinicians (such as physicians, social workers, occupational therapists) should leverage and maximize the use of telemedicine to minimize direct or prolonged exposure to infectious disease risks.
- Nursing: It is important to establish appropriate ratios of nursing and support staff for a COVID-19–positive psychiatry unit given the unique work flows related to isolation precautions and to ensure patient and staff safety. These ratios may take into account patient-specific needs, including the need for additional staff to perform constant observation for high-risk patients, management of agitated patients, and sufficient staff to allow for relief and break-time from PPE. Admission and routine care processes should be adapted in order to limit equipment entering the room, such as computer workstations on wheels.
- Medication administration procedures: Develop work flows related to PPE and infection control when retrieving and administering medications.
- Workspace: Designate appropriate workspace for PCU clinicians to access computers and documents and to minimize use of non–COVID-19 unit work areas.
6. Restraints and management of agitated patients: PCUs should develop plans for addressing agitated patients, including contingency plans for whether seclusion or restraints should be administered in the patient’s individual room or in a dedicated restraint room in the PCU. Staff training should include protocols specifically designed for managing agitated patients in the PCU.
7. Discharge processes: If patients remain medically well and clear their COVID-19 PCR tests, it is conceivable that they might be transferred to a non–COVID-19 psychiatric unit if sufficient isolation time has passed and the infectious disease consultants deem it appropriate. It is also possible that patients would be discharged from a PCU to home or other residential setting. Such patients should be assessed for ability to comply with continued self-quarantine if necessary. Discharge planning must take into consideration follow-up plans for COVID-19 illness and primary care appointments, as well as needed psychiatric follow-up.
8. Patients’ rights: The apparently highly infectious and transmissible nature of SARS-CoV-2 creates novel tensions between a wide range of individual rights and the rights of others. In addition to manifesting in our general society, there are potentially unique tensions in acute inpatient psychiatric settings. Certain patients’ rights may require modification in a PCU (for example, access to outdoor space, personal belongings, visitors, and possibly civil commitment judicial hearings). These discussions may require input from hospital compliance officers, ethics committees, risk managers, and the local department of mental health and also may be partly solved by using video communication platforms.
A few other “pearls” may be of value: Psychiatric hospitals that are colocated with a general acute care hospital or ED might be better situated to develop protocols to safely care for COVID-19–positive psychiatric patients, by virtue of the close proximity of full-spectrum acute general hospital services. Direct engagement by a command center and hospital or health system senior leadership also seems crucial as a means for assuring authorization to proceed with planning what may be a frightening or controversial (but necessary) adaptation of inpatient psychiatric unit(s) to the exigencies of the COVID-19 pandemic.
The resources of a robust community hospital or academic health system (including infection prevention leaders who engage in continuous liaison with local, county, state, and federal public health expertise) are crucial to the “learning health system” model, which requires flexibility, rapid adaptation to new knowledge, and accessibility to infectious disease and other consultation for special situations. Frequent and open communication with all professional stakeholders (through town halls, Q&A sessions, group discussions, and so on) is important in the planning process to socialize the principles and concepts that are critical for providing care in a PCU, reducing anxiety, and bolstering collegiality and staff morale.
References
1. Kim MJ. “ ‘It was a medical disaster’: The psychiatric ward that saw 100 patients with new coronavirus.” Independent. 2020 Mar 1.
2. Korean Society of Infectious Diseases et al. J Korean Med Sci. 2020 Mar 16;35(10):e112.
3. Centers for Disease Control and Prevention. Symptom-based strategy to discontinue isolation for persons with COVID-19. Decision Memo. 2020 May 3.
4. He X et al. Nature Medicine. 2020. 26:672-5.
Dr. Cheung is associate medical director and chief quality officer at the Stewart and Lynda Resnick Neuropsychiatric Hospital at the University of California, Los Angeles. He has no conflicts of interest. Dr. Strouse is medical director, UCLA Stewart and Lynda Resnick Neuropsychiatric Hospital and Maddie Katz Professor at the UCLA department of psychiatry/Semel Institute. He has no conflicts of interest. Dr. Li is associate medical director of quality improvement at Yale-New Haven Psychiatric Hospital in Connecticut. She also serves as medical director of clinical operations at the Yale-New Haven Health System. Dr. Li is a 2019-2020 Health and Aging Policy Fellow and receives funding support from the program.
Reports have emerged about the unique vulnerability of psychiatric hospitals to the ravages of COVID-19.
In a South Korea psychiatric hospital, 101 of 103 patients contracted SARS-CoV-2 during an outbreak; 7 eventually died.1,2 This report, among a few others, have led to the development of psychiatric COVID-19–positive units (PCU). However, it remains highly unclear how many are currently open, where they are located, or what their operations are like.
We knew that we could not allow a medically asymptomatic “covertly” COVID-19–positive patient to be introduced to the social community of our inpatient units because of the risks of transmission to other patients and staff.
In coordination with our health system infection prevention experts, we have therefore required a confirmed negative COVID-19 polymerase chain reaction nasal swab performed no more than 48 hours prior to the time/date of acute psychiatric inpatient admission. Furthermore, as part of the broad health system response and surge planning, we were asked by our respective incident command centers to begin planning for a Psychiatric COVID-19–positive Unit (PCU) that might allow us to safely care for a cohort of patients needing such hospitalization.
It is worth emphasizing that the typical patient who is a candidate for a PCU is so acutely psychiatrically ill that they cannot be managed in a less restrictive environment than an inpatient psychiatric unit and, at the same time, is likely to not be medically ill enough to warrant admission to an internal medicine service in a general acute care hospital.
We have identified eight principles and critical decision points that can help inpatient units plan for the safe care of COVID-19–positive patients on a PCU.
1. Triage: Patients admitted to a PCU should be medically stable, particularly with regard to COVID-19 and respiratory symptomatology. PCUs should establish clear criteria for admission and discharge (or medical transfer). Examples of potential exclusionary criteria to a PCU include:
- Respiratory distress, shortness of breath, hypoxia, requirement for supplemental oxygen, or requirement for respiratory therapy breathing treatments.
- Fever, or signs of sepsis, or systemic inflammatory response syndrome.
- Medical frailty, significant medical comorbidities, delirium, or altered mental status;
- Requirements for continuous vital sign monitoring or of a monitoring frequency beyond the capacity of the PCU.
Discharge criteria may also include a symptom-based strategy because emerging evidence suggests that patients may be less infectious by day 10-14 of the disease course,3 and viral lab testing is very sensitive and will be positive for periods of time after individuals are no longer infectious. The symptom-based strategy allows for patients to not require retesting prior to discharge. However, some receiving facilities (for example residential or skilled nursing facilities) may necessitate testing, in which case a testing-based strategy can be used. The Centers for Disease Control and Prevention provides guidelines for both types of strategies.4
2. Infection control and personal protective equipment: PCUs require modifications or departures from the typical inpatient free-ranging environment in which common areas are provided for patients to engage in a community of care, including group therapy (such as occupational, recreational, Alcoholics Anonymous, and social work groups).
- Isolation: PCUs must consider whether they will require patients to isolate to their rooms or to allow modified or limited access to “public” or “community” areas. While there do not appear to be standard recommendations from the CDC or other public health entities regarding negative pressure or any specific room ventilation requirements, it is prudent to work with local infectious disease experts on protocols. Important considerations include spatial planning for infection control areas to don and doff appropriate personal protective equipment (PPE) and appropriate workspace to prevent contamination of non–COVID-19 work areas. Approaches can include establishing clearly identified and visually demarcated infection control “zones” (often referred to as “hot, warm, and cold zones”) that correspond to specific PPE requirements for staff. In addition, individuals should eat in their own rooms or designated areas because use of common areas for meals can potentially lead to aerosolized spread of the virus.
- Cohorting: Generally, PCUs should consider admitting only COVID-19–positive patients to a PCU to avoid exposure to other patients. Hospitals and health systems should determine protocols and locations for testing and managing “patients under investigation” for COVID-19, which should precede admission to the PCU.
- PPE: It is important to clearly establish and communicate PPE requirements and procedures for direct physical contact versus no physical contact (for example, visual safety checks). Identify clear supply chains for PPE and hand sanitizer.
3. Medical management and consultation: PCUs should establish clear pathways for accessing consultation from medical consultants. It may be ideal, in addition to standard daily psychiatric physician rounding, to have daily internal medicine rounding and/or medical nursing staff working on the unit. Given the potential of COVID-19–positive patients to rapidly devolve from asymptomatic to acutely ill, it is necessary to establish protocols for the provision of urgent medical care 24/7 and streamlined processes for transfer to a medical unit.
Clear protocols should be established to address any potential signs of decompensation in the respiratory status of a PCU unit, including administration of oxygen and restrictions (or appropriate precautions) related to aerosolizing treatment such as nebulizers or positive airway pressure.
4. Code blue protocol: Any emergent medical issues, including acute respiratory decompensation, should trigger a Code Blue response that has been specifically designed for COVID-19–positive patients, including considerations for proper PPE during resuscitation efforts.
5. Psychiatric staffing and workflows: When possible, it may be preferable to engage volunteer medical and nursing staff for the PCU, as opposed to mandating participation. Take into consideration support needs, including education and training about safe PPE practices, processes for testing health care workers, return-to-work guidance, and potential alternate housing.
- Telehealth: Clinicians (such as physicians, social workers, occupational therapists) should leverage and maximize the use of telemedicine to minimize direct or prolonged exposure to infectious disease risks.
- Nursing: It is important to establish appropriate ratios of nursing and support staff for a COVID-19–positive psychiatry unit given the unique work flows related to isolation precautions and to ensure patient and staff safety. These ratios may take into account patient-specific needs, including the need for additional staff to perform constant observation for high-risk patients, management of agitated patients, and sufficient staff to allow for relief and break-time from PPE. Admission and routine care processes should be adapted in order to limit equipment entering the room, such as computer workstations on wheels.
- Medication administration procedures: Develop work flows related to PPE and infection control when retrieving and administering medications.
- Workspace: Designate appropriate workspace for PCU clinicians to access computers and documents and to minimize use of non–COVID-19 unit work areas.
6. Restraints and management of agitated patients: PCUs should develop plans for addressing agitated patients, including contingency plans for whether seclusion or restraints should be administered in the patient’s individual room or in a dedicated restraint room in the PCU. Staff training should include protocols specifically designed for managing agitated patients in the PCU.
7. Discharge processes: If patients remain medically well and clear their COVID-19 PCR tests, it is conceivable that they might be transferred to a non–COVID-19 psychiatric unit if sufficient isolation time has passed and the infectious disease consultants deem it appropriate. It is also possible that patients would be discharged from a PCU to home or other residential setting. Such patients should be assessed for ability to comply with continued self-quarantine if necessary. Discharge planning must take into consideration follow-up plans for COVID-19 illness and primary care appointments, as well as needed psychiatric follow-up.
8. Patients’ rights: The apparently highly infectious and transmissible nature of SARS-CoV-2 creates novel tensions between a wide range of individual rights and the rights of others. In addition to manifesting in our general society, there are potentially unique tensions in acute inpatient psychiatric settings. Certain patients’ rights may require modification in a PCU (for example, access to outdoor space, personal belongings, visitors, and possibly civil commitment judicial hearings). These discussions may require input from hospital compliance officers, ethics committees, risk managers, and the local department of mental health and also may be partly solved by using video communication platforms.
A few other “pearls” may be of value: Psychiatric hospitals that are colocated with a general acute care hospital or ED might be better situated to develop protocols to safely care for COVID-19–positive psychiatric patients, by virtue of the close proximity of full-spectrum acute general hospital services. Direct engagement by a command center and hospital or health system senior leadership also seems crucial as a means for assuring authorization to proceed with planning what may be a frightening or controversial (but necessary) adaptation of inpatient psychiatric unit(s) to the exigencies of the COVID-19 pandemic.
The resources of a robust community hospital or academic health system (including infection prevention leaders who engage in continuous liaison with local, county, state, and federal public health expertise) are crucial to the “learning health system” model, which requires flexibility, rapid adaptation to new knowledge, and accessibility to infectious disease and other consultation for special situations. Frequent and open communication with all professional stakeholders (through town halls, Q&A sessions, group discussions, and so on) is important in the planning process to socialize the principles and concepts that are critical for providing care in a PCU, reducing anxiety, and bolstering collegiality and staff morale.
References
1. Kim MJ. “ ‘It was a medical disaster’: The psychiatric ward that saw 100 patients with new coronavirus.” Independent. 2020 Mar 1.
2. Korean Society of Infectious Diseases et al. J Korean Med Sci. 2020 Mar 16;35(10):e112.
3. Centers for Disease Control and Prevention. Symptom-based strategy to discontinue isolation for persons with COVID-19. Decision Memo. 2020 May 3.
4. He X et al. Nature Medicine. 2020. 26:672-5.
Dr. Cheung is associate medical director and chief quality officer at the Stewart and Lynda Resnick Neuropsychiatric Hospital at the University of California, Los Angeles. He has no conflicts of interest. Dr. Strouse is medical director, UCLA Stewart and Lynda Resnick Neuropsychiatric Hospital and Maddie Katz Professor at the UCLA department of psychiatry/Semel Institute. He has no conflicts of interest. Dr. Li is associate medical director of quality improvement at Yale-New Haven Psychiatric Hospital in Connecticut. She also serves as medical director of clinical operations at the Yale-New Haven Health System. Dr. Li is a 2019-2020 Health and Aging Policy Fellow and receives funding support from the program.
FDA approves pomalidomide for Kaposi sarcoma
The Food and Drug Administration has granted accelerated approval to pomalidomide (Pomalyst, Bristol-Myers Squibb) for the treatment of AIDS-related Kaposi sarcoma that is resistant to highly active antiretroviral therapy (HAART) or that occurs in HIV-negative patients.
Pomalidomide is the only oral agent and first new treatment option for Kaposi sarcoma in more than 20 years, according to the company.
The drug, a thalidomide analogue, is already marketed for the treatment of multiple myeloma.
Pomalidomide has “shown positive results in Kaposi sarcoma patients, regardless of their HIV status,” said Robert Yarchoan, MD, chief of the HIV and AIDS Malignancy Branch, National Cancer Institute, in a press statement.
The conditional approval is based on the 71% overall response rate observed in a phase 1/2 open-label, single-arm clinical trial that involved 28 patients, 18 of whom were HIV positive and 10 of whom were HIV negative.
Most of the responses were partial (57%; 16/28); 14% (4/28) were complete. Median duration of response was 12.1 months. Additionally, for half of the patients who showed a response, that response was maintained for more than 12 months.
Patients received 5 mg of pomalidomide once daily for 21 of 28-day cycles until disease progression or unacceptable toxicity occurred.
Permanent discontinuation because of an adverse reaction occurred in 11% (3/28) of patients.
Adverse reactions (≥20%) included maculopapular rash (71%), constipation (71%), fatigue (68%), nausea (36%), diarrhea (32%), cough (29%), dyspnea (29%), peripheral edema (29%), upper respiratory tract infection (29%), muscle spasms (25%), hypothyroidism (21%), dry skin (21%), and chills (21%).
Grade 3 or 4 adverse reactions included maculopapular rash (3.6%), diarrhea (3.6%), and peripheral edema (3.6%).
Grade 3 or 4 laboratory abnormalities (≥5%) that worsened from baseline included decreased absolute neutrophil count (50%), decreased phosphate level (25%), elevated glucose level (7%), and elevated creatine kinase level (7%).
As a thalidomide analogue, pomalidomide includes a boxed warning in the prescribing information; thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. Deep vein thrombosis, pulmonary embolism, myocardial infarction, and stroke can occur in patients treated with pomalidomide; thromboprophylaxis is recommended.
Pomalidomide is available only through a restricted distribution program, Pomalyst REMS.
This article first appeared on Medscape.com.
The Food and Drug Administration has granted accelerated approval to pomalidomide (Pomalyst, Bristol-Myers Squibb) for the treatment of AIDS-related Kaposi sarcoma that is resistant to highly active antiretroviral therapy (HAART) or that occurs in HIV-negative patients.
Pomalidomide is the only oral agent and first new treatment option for Kaposi sarcoma in more than 20 years, according to the company.
The drug, a thalidomide analogue, is already marketed for the treatment of multiple myeloma.
Pomalidomide has “shown positive results in Kaposi sarcoma patients, regardless of their HIV status,” said Robert Yarchoan, MD, chief of the HIV and AIDS Malignancy Branch, National Cancer Institute, in a press statement.
The conditional approval is based on the 71% overall response rate observed in a phase 1/2 open-label, single-arm clinical trial that involved 28 patients, 18 of whom were HIV positive and 10 of whom were HIV negative.
Most of the responses were partial (57%; 16/28); 14% (4/28) were complete. Median duration of response was 12.1 months. Additionally, for half of the patients who showed a response, that response was maintained for more than 12 months.
Patients received 5 mg of pomalidomide once daily for 21 of 28-day cycles until disease progression or unacceptable toxicity occurred.
Permanent discontinuation because of an adverse reaction occurred in 11% (3/28) of patients.
Adverse reactions (≥20%) included maculopapular rash (71%), constipation (71%), fatigue (68%), nausea (36%), diarrhea (32%), cough (29%), dyspnea (29%), peripheral edema (29%), upper respiratory tract infection (29%), muscle spasms (25%), hypothyroidism (21%), dry skin (21%), and chills (21%).
Grade 3 or 4 adverse reactions included maculopapular rash (3.6%), diarrhea (3.6%), and peripheral edema (3.6%).
Grade 3 or 4 laboratory abnormalities (≥5%) that worsened from baseline included decreased absolute neutrophil count (50%), decreased phosphate level (25%), elevated glucose level (7%), and elevated creatine kinase level (7%).
As a thalidomide analogue, pomalidomide includes a boxed warning in the prescribing information; thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. Deep vein thrombosis, pulmonary embolism, myocardial infarction, and stroke can occur in patients treated with pomalidomide; thromboprophylaxis is recommended.
Pomalidomide is available only through a restricted distribution program, Pomalyst REMS.
This article first appeared on Medscape.com.
The Food and Drug Administration has granted accelerated approval to pomalidomide (Pomalyst, Bristol-Myers Squibb) for the treatment of AIDS-related Kaposi sarcoma that is resistant to highly active antiretroviral therapy (HAART) or that occurs in HIV-negative patients.
Pomalidomide is the only oral agent and first new treatment option for Kaposi sarcoma in more than 20 years, according to the company.
The drug, a thalidomide analogue, is already marketed for the treatment of multiple myeloma.
Pomalidomide has “shown positive results in Kaposi sarcoma patients, regardless of their HIV status,” said Robert Yarchoan, MD, chief of the HIV and AIDS Malignancy Branch, National Cancer Institute, in a press statement.
The conditional approval is based on the 71% overall response rate observed in a phase 1/2 open-label, single-arm clinical trial that involved 28 patients, 18 of whom were HIV positive and 10 of whom were HIV negative.
Most of the responses were partial (57%; 16/28); 14% (4/28) were complete. Median duration of response was 12.1 months. Additionally, for half of the patients who showed a response, that response was maintained for more than 12 months.
Patients received 5 mg of pomalidomide once daily for 21 of 28-day cycles until disease progression or unacceptable toxicity occurred.
Permanent discontinuation because of an adverse reaction occurred in 11% (3/28) of patients.
Adverse reactions (≥20%) included maculopapular rash (71%), constipation (71%), fatigue (68%), nausea (36%), diarrhea (32%), cough (29%), dyspnea (29%), peripheral edema (29%), upper respiratory tract infection (29%), muscle spasms (25%), hypothyroidism (21%), dry skin (21%), and chills (21%).
Grade 3 or 4 adverse reactions included maculopapular rash (3.6%), diarrhea (3.6%), and peripheral edema (3.6%).
Grade 3 or 4 laboratory abnormalities (≥5%) that worsened from baseline included decreased absolute neutrophil count (50%), decreased phosphate level (25%), elevated glucose level (7%), and elevated creatine kinase level (7%).
As a thalidomide analogue, pomalidomide includes a boxed warning in the prescribing information; thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. Deep vein thrombosis, pulmonary embolism, myocardial infarction, and stroke can occur in patients treated with pomalidomide; thromboprophylaxis is recommended.
Pomalidomide is available only through a restricted distribution program, Pomalyst REMS.
This article first appeared on Medscape.com.
Glucose control linked to COVID-19 outcomes in largest-yet study
The strong link between glucose control and COVID-19 outcomes has been reaffirmed in the largest study thus far of hospitalized patients with preexisting type 2 diabetes.
The retrospective, multicenter study, from 7,337 hospitalized patients with COVID-19, was published online in Cell Metabolism by Lihua Zhu, Renmin Hospital of Wuhan University, China, and colleagues.
The study finds that, while the presence of type 2 diabetes per se is a risk factor for worse COVID-19 outcomes, better glycemic control among those with preexisting type 2 diabetes appears to be associated with significant reductions in adverse outcomes and death.
“We were surprised to see such favorable outcomes in the well-controlled blood glucose group among patients with COVID-19 and preexisting type 2 diabetes,” senior author Hongliang Li, also of Renmin Hospital, said in a statement.
“Considering that people with diabetes had much higher risk for death and various complications, and there are no specific drugs for COVID-19, our findings indicate that controlling blood glucose well may act as an effective auxiliary approach to improve the prognosis of patients with COVID-19 and preexisting diabetes,” Dr. Li added.
Asked to comment on the findings, David Klonoff, MD, medical director of the Diabetes Research Institute at Mills–Peninsula Medical Center, San Mateo, Calif., cautioned that the way in which the “well-controlled” diabetes group was distinguished from the “poorly controlled” one in this study used a “nonstandard method for distinguishing these groups based on variability.”
So “there was a great deal of overlap between the two groups,” he observed.
Diabetes itself was associated with worse COVID-19 outcomes
Of the 7,337 participants with confirmed COVID-19 in the Chinese study, 13% (952) had preexisting type 2 diabetes while the other 6,385 did not have diabetes.
Median ages were 62 years for those with and 53 years for those without diabetes. As has been reported several times since the pandemic began, the presence of diabetes was associated with a worse COVID-19 prognosis.
Those with preexisting diabetes received significantly more antibiotics, antifungals, systemic corticosteroids, immunoglobulin, antihypertensive drugs, and vasoactive drugs than did those without diabetes. They were also more likely to receive oxygen inhalation (76.9% vs. 61.2%), noninvasive ventilation (10.2% vs. 3.9%), and invasive ventilation (3.6% vs. 0.7%).
Over 28 days starting with the day of admission, the type 2 diabetes group was significantly more likely to die compared with those without diabetes (7.8% vs. 2.7%; P < .001), with a crude hazard ratio of 2.90 (P < .001). After adjustments for age, gender, and COVID-19 severity, the diabetes group was still significantly more likely to die, with a hazard ratio of 1.49 (P = .005).
Those with diabetes were also significantly more likely to develop acute respiratory distress syndrome (adjusted hazard ratio, 1.44), acute kidney injury (3.01), and septic shock (1.95).
“The results were unequivocal to implicate diabetes mellitus in higher risk of death and other detrimental outcomes of COVID-19,” the authors wrote, although they caution “there were notable differences in the covariate distributions between the two groups.”
With T2D, tighter glycemic control predicted better outcome
Among the 952 with COVID-19 and type 2 diabetes, 282 individuals had “well-controlled” blood glucose, ranging from 3.9 to 10.0 mmol/L (~70 - 180 mg/dL) with median 6.4 mmol/L (115 mg/dL) and hemoglobin A1c of 7.3%.
The other 528 were “poorly controlled,” defined as the lowest fasting glucose level 3.9 mmol/L or above and the highest 2-hour postprandial glucose exceeding 10.0 mmol/L, with median 10.9 mmol/L (196 mg/dL) and HbA1c of 8.1%.
Just as with the diabetes vs. no diabetes comparison, those in the “well-controlled” blood glucose group had lower use of antivirals, antibiotics, antifungals, systemic corticosteroids, immunoglobulin, and vasoactive drugs.
They also were less likely to require oxygen inhalation (70.2% vs. 83.5%), non-invasive ventilation (4.6% vs. 11.9%), invasive ventilation (0% vs. 4.2%), and extracorporeal membrane oxygenation (0% vs. 0.8%).
In-hospital death was significantly lower in the “well-controlled” group (1.1% vs. 11.0%; crude hazard ratio, 0.09; P < .001). After adjustments for the previous factors plus site effect, the difference remained significant (0.13; P < .001). Adjusted hazard ratio for acute respiratory distress syndrome was 0.41 (P < .001) and for acute heart injury it was 0.21 (P = .003).
Stress hyperglycemia in COVID-19 associated with greater mortality
Klonoff was senior author on a previous study from the United States that showed that both diabetes and uncontrolled hyperglycemia among people without prior diabetes – the latter “presumably due to stress,” he said – were strong predictors of mortality among hospitalized patients with COVID-19.
The new Chinese research only looks at individuals with previously diagnosed type 2 diabetes, Klonoff pointed out in an interview.
“The article by Zhu et al. did not look at outcomes of hospitalized COVID-19 patients with uncontrolled hyperglycemia. Per [the U.S. study], in COVID-19 stress hyperglycemia, compared to diabetes, was associated with greater mortality.”
In addition, although international guidance now advises optimizing blood glucose levels in all patients with hyperglycemia and COVID-19, it’s actually not yet totally clear which in-target range improves COVID-19 prognosis the best, Dr. Klonoff said.
He is now working on a study aimed at answering that question.
The researchers have disclosed no relevant financial relationships. Dr. Klonoff is a consultant to Abbott, Ascensia, Dexcom, EOFlow, Fractyl, Lifecare, Novo, Roche, and ThirdWayv.
A version of this article originally appeared on Medscape.com.
The strong link between glucose control and COVID-19 outcomes has been reaffirmed in the largest study thus far of hospitalized patients with preexisting type 2 diabetes.
The retrospective, multicenter study, from 7,337 hospitalized patients with COVID-19, was published online in Cell Metabolism by Lihua Zhu, Renmin Hospital of Wuhan University, China, and colleagues.
The study finds that, while the presence of type 2 diabetes per se is a risk factor for worse COVID-19 outcomes, better glycemic control among those with preexisting type 2 diabetes appears to be associated with significant reductions in adverse outcomes and death.
“We were surprised to see such favorable outcomes in the well-controlled blood glucose group among patients with COVID-19 and preexisting type 2 diabetes,” senior author Hongliang Li, also of Renmin Hospital, said in a statement.
“Considering that people with diabetes had much higher risk for death and various complications, and there are no specific drugs for COVID-19, our findings indicate that controlling blood glucose well may act as an effective auxiliary approach to improve the prognosis of patients with COVID-19 and preexisting diabetes,” Dr. Li added.
Asked to comment on the findings, David Klonoff, MD, medical director of the Diabetes Research Institute at Mills–Peninsula Medical Center, San Mateo, Calif., cautioned that the way in which the “well-controlled” diabetes group was distinguished from the “poorly controlled” one in this study used a “nonstandard method for distinguishing these groups based on variability.”
So “there was a great deal of overlap between the two groups,” he observed.
Diabetes itself was associated with worse COVID-19 outcomes
Of the 7,337 participants with confirmed COVID-19 in the Chinese study, 13% (952) had preexisting type 2 diabetes while the other 6,385 did not have diabetes.
Median ages were 62 years for those with and 53 years for those without diabetes. As has been reported several times since the pandemic began, the presence of diabetes was associated with a worse COVID-19 prognosis.
Those with preexisting diabetes received significantly more antibiotics, antifungals, systemic corticosteroids, immunoglobulin, antihypertensive drugs, and vasoactive drugs than did those without diabetes. They were also more likely to receive oxygen inhalation (76.9% vs. 61.2%), noninvasive ventilation (10.2% vs. 3.9%), and invasive ventilation (3.6% vs. 0.7%).
Over 28 days starting with the day of admission, the type 2 diabetes group was significantly more likely to die compared with those without diabetes (7.8% vs. 2.7%; P < .001), with a crude hazard ratio of 2.90 (P < .001). After adjustments for age, gender, and COVID-19 severity, the diabetes group was still significantly more likely to die, with a hazard ratio of 1.49 (P = .005).
Those with diabetes were also significantly more likely to develop acute respiratory distress syndrome (adjusted hazard ratio, 1.44), acute kidney injury (3.01), and septic shock (1.95).
“The results were unequivocal to implicate diabetes mellitus in higher risk of death and other detrimental outcomes of COVID-19,” the authors wrote, although they caution “there were notable differences in the covariate distributions between the two groups.”
With T2D, tighter glycemic control predicted better outcome
Among the 952 with COVID-19 and type 2 diabetes, 282 individuals had “well-controlled” blood glucose, ranging from 3.9 to 10.0 mmol/L (~70 - 180 mg/dL) with median 6.4 mmol/L (115 mg/dL) and hemoglobin A1c of 7.3%.
The other 528 were “poorly controlled,” defined as the lowest fasting glucose level 3.9 mmol/L or above and the highest 2-hour postprandial glucose exceeding 10.0 mmol/L, with median 10.9 mmol/L (196 mg/dL) and HbA1c of 8.1%.
Just as with the diabetes vs. no diabetes comparison, those in the “well-controlled” blood glucose group had lower use of antivirals, antibiotics, antifungals, systemic corticosteroids, immunoglobulin, and vasoactive drugs.
They also were less likely to require oxygen inhalation (70.2% vs. 83.5%), non-invasive ventilation (4.6% vs. 11.9%), invasive ventilation (0% vs. 4.2%), and extracorporeal membrane oxygenation (0% vs. 0.8%).
In-hospital death was significantly lower in the “well-controlled” group (1.1% vs. 11.0%; crude hazard ratio, 0.09; P < .001). After adjustments for the previous factors plus site effect, the difference remained significant (0.13; P < .001). Adjusted hazard ratio for acute respiratory distress syndrome was 0.41 (P < .001) and for acute heart injury it was 0.21 (P = .003).
Stress hyperglycemia in COVID-19 associated with greater mortality
Klonoff was senior author on a previous study from the United States that showed that both diabetes and uncontrolled hyperglycemia among people without prior diabetes – the latter “presumably due to stress,” he said – were strong predictors of mortality among hospitalized patients with COVID-19.
The new Chinese research only looks at individuals with previously diagnosed type 2 diabetes, Klonoff pointed out in an interview.
“The article by Zhu et al. did not look at outcomes of hospitalized COVID-19 patients with uncontrolled hyperglycemia. Per [the U.S. study], in COVID-19 stress hyperglycemia, compared to diabetes, was associated with greater mortality.”
In addition, although international guidance now advises optimizing blood glucose levels in all patients with hyperglycemia and COVID-19, it’s actually not yet totally clear which in-target range improves COVID-19 prognosis the best, Dr. Klonoff said.
He is now working on a study aimed at answering that question.
The researchers have disclosed no relevant financial relationships. Dr. Klonoff is a consultant to Abbott, Ascensia, Dexcom, EOFlow, Fractyl, Lifecare, Novo, Roche, and ThirdWayv.
A version of this article originally appeared on Medscape.com.
The strong link between glucose control and COVID-19 outcomes has been reaffirmed in the largest study thus far of hospitalized patients with preexisting type 2 diabetes.
The retrospective, multicenter study, from 7,337 hospitalized patients with COVID-19, was published online in Cell Metabolism by Lihua Zhu, Renmin Hospital of Wuhan University, China, and colleagues.
The study finds that, while the presence of type 2 diabetes per se is a risk factor for worse COVID-19 outcomes, better glycemic control among those with preexisting type 2 diabetes appears to be associated with significant reductions in adverse outcomes and death.
“We were surprised to see such favorable outcomes in the well-controlled blood glucose group among patients with COVID-19 and preexisting type 2 diabetes,” senior author Hongliang Li, also of Renmin Hospital, said in a statement.
“Considering that people with diabetes had much higher risk for death and various complications, and there are no specific drugs for COVID-19, our findings indicate that controlling blood glucose well may act as an effective auxiliary approach to improve the prognosis of patients with COVID-19 and preexisting diabetes,” Dr. Li added.
Asked to comment on the findings, David Klonoff, MD, medical director of the Diabetes Research Institute at Mills–Peninsula Medical Center, San Mateo, Calif., cautioned that the way in which the “well-controlled” diabetes group was distinguished from the “poorly controlled” one in this study used a “nonstandard method for distinguishing these groups based on variability.”
So “there was a great deal of overlap between the two groups,” he observed.
Diabetes itself was associated with worse COVID-19 outcomes
Of the 7,337 participants with confirmed COVID-19 in the Chinese study, 13% (952) had preexisting type 2 diabetes while the other 6,385 did not have diabetes.
Median ages were 62 years for those with and 53 years for those without diabetes. As has been reported several times since the pandemic began, the presence of diabetes was associated with a worse COVID-19 prognosis.
Those with preexisting diabetes received significantly more antibiotics, antifungals, systemic corticosteroids, immunoglobulin, antihypertensive drugs, and vasoactive drugs than did those without diabetes. They were also more likely to receive oxygen inhalation (76.9% vs. 61.2%), noninvasive ventilation (10.2% vs. 3.9%), and invasive ventilation (3.6% vs. 0.7%).
Over 28 days starting with the day of admission, the type 2 diabetes group was significantly more likely to die compared with those without diabetes (7.8% vs. 2.7%; P < .001), with a crude hazard ratio of 2.90 (P < .001). After adjustments for age, gender, and COVID-19 severity, the diabetes group was still significantly more likely to die, with a hazard ratio of 1.49 (P = .005).
Those with diabetes were also significantly more likely to develop acute respiratory distress syndrome (adjusted hazard ratio, 1.44), acute kidney injury (3.01), and septic shock (1.95).
“The results were unequivocal to implicate diabetes mellitus in higher risk of death and other detrimental outcomes of COVID-19,” the authors wrote, although they caution “there were notable differences in the covariate distributions between the two groups.”
With T2D, tighter glycemic control predicted better outcome
Among the 952 with COVID-19 and type 2 diabetes, 282 individuals had “well-controlled” blood glucose, ranging from 3.9 to 10.0 mmol/L (~70 - 180 mg/dL) with median 6.4 mmol/L (115 mg/dL) and hemoglobin A1c of 7.3%.
The other 528 were “poorly controlled,” defined as the lowest fasting glucose level 3.9 mmol/L or above and the highest 2-hour postprandial glucose exceeding 10.0 mmol/L, with median 10.9 mmol/L (196 mg/dL) and HbA1c of 8.1%.
Just as with the diabetes vs. no diabetes comparison, those in the “well-controlled” blood glucose group had lower use of antivirals, antibiotics, antifungals, systemic corticosteroids, immunoglobulin, and vasoactive drugs.
They also were less likely to require oxygen inhalation (70.2% vs. 83.5%), non-invasive ventilation (4.6% vs. 11.9%), invasive ventilation (0% vs. 4.2%), and extracorporeal membrane oxygenation (0% vs. 0.8%).
In-hospital death was significantly lower in the “well-controlled” group (1.1% vs. 11.0%; crude hazard ratio, 0.09; P < .001). After adjustments for the previous factors plus site effect, the difference remained significant (0.13; P < .001). Adjusted hazard ratio for acute respiratory distress syndrome was 0.41 (P < .001) and for acute heart injury it was 0.21 (P = .003).
Stress hyperglycemia in COVID-19 associated with greater mortality
Klonoff was senior author on a previous study from the United States that showed that both diabetes and uncontrolled hyperglycemia among people without prior diabetes – the latter “presumably due to stress,” he said – were strong predictors of mortality among hospitalized patients with COVID-19.
The new Chinese research only looks at individuals with previously diagnosed type 2 diabetes, Klonoff pointed out in an interview.
“The article by Zhu et al. did not look at outcomes of hospitalized COVID-19 patients with uncontrolled hyperglycemia. Per [the U.S. study], in COVID-19 stress hyperglycemia, compared to diabetes, was associated with greater mortality.”
In addition, although international guidance now advises optimizing blood glucose levels in all patients with hyperglycemia and COVID-19, it’s actually not yet totally clear which in-target range improves COVID-19 prognosis the best, Dr. Klonoff said.
He is now working on a study aimed at answering that question.
The researchers have disclosed no relevant financial relationships. Dr. Klonoff is a consultant to Abbott, Ascensia, Dexcom, EOFlow, Fractyl, Lifecare, Novo, Roche, and ThirdWayv.
A version of this article originally appeared on Medscape.com.
Laser surgery precautions as clinics begin to reopen amid COVID-19
Protective measures recommended for cosmetic procedures have recently been published by Dover et al. in Facial Plastic Surgery & Aesthetic Medicine. The manuscript, titled “A path to resume aesthetic care Project AesCert Guidance Supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” provides thorough, detailed recommendations on all aspects of protection and preparedness for aesthetic clinical practices.
in this uncharted territory. During the last pandemic, the 1918 Spanish flu, caused by an H1N1 virus, laser procedures didn’t exist. Discussion among dermatologists and laser surgeons, including the aforementioned publication, have led to the following initial office recommendations (subject to change).
Office preparation and safety including:
- Prescreening patients for symptoms.
- Social distancing in the office, including waiting room areas (or eliminating waiting areas and bringing patients into exam rooms upon arrival).
- Decreasing patient load and increasing length of appointment times.
- Having no additional visitors during patient appointments, unless necessary (minor, caregiver).
- Patients wearing masks to appointments and hand washing/sanitizing upon arrival/departure.
- Providers wearing appropriate personal protective equipment (PPE) during visits.
- Instituting office disinfectant checklists.
For nonablative laser surgery specifically, especially for therapy of the face and neck, recommendations include the following:
- Lasers and office areas are thoroughly sanitized between each procedure.
- Providers wear appropriate PPE, including N95 masks if possible, wraparound safety glasses, gloves, as well as strong consideration of face shields).
- The duration and number of procedures should be limited, as should intraprocedure conversations and close face-to-face proximity with patient’s airways.
- Lasers with increased plume, including laser tattoo removal and laser hair removal, are the procedures with the most concern with regards to viral particle or infection transmission.
PPE is recommended (including masks – N95 if available – gloves, and face shield), as well as evacuator suction systems of the two-stage filtration type, and/or negative room pressure if available. For air-filtration evacuator suction systems, the device vacuum must be held within 2 inches of the treatment area for the best efficacy. Some have suggested performing laser tattoo removal through a hydrogel patch to help eliminate plume, which may also increase the cost of the procedure and may depend on the availability of the patches themselves. Nothing has been published on the use of the hydrogel patch in laser hair removal. Shaving or trimming of hairs prior to the procedure is critical.
While pulse dye and intense pulsed light (IPL) lasers have generally been deemed safer to use during the COVID-19 pandemic – with appropriate protective gear and general office precautions – I would recommend being mindful of potential plume created when using these lasers in hair-bearing areas. IPL is generally avoided in these regions, unless specific filters are used for hair removal treatment. But if use an IPL in a hair-bearing region, shaving or trimming of the hairs with the above precautions should be done first to reduce plume. As with all face-to-face procedures, the above PPE, contact, and intraprocedure conversation precautions should be taken.
Nonablative fractional resurfacing lasers are areas in which more questions lie. Some providers are comfortable performing nonablative fractional lasers with protective gear and air filtration systems, while others are recommending delaying these procedures until more information is available. The question essentially involves whether infection risk is higher with these procedures because of plume and if depth of penetration of the laser can release viral particles.
In addition to the other precautions above, with the high transmissibility of COVID-19, I would recommend considering precleansing the treatment area with soap and water or a sterile prep that won’t irritate the skin, which has activity against coronaviruses. A study by Kampf et al. demonstrated that coronaviruses can persist on surfaces such as metal, glass, or plastic for up to 9 days (human skin surface unknown) but can be effectively inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents that may be more tolerable on the skin surface, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. Washing the face with soap and water may be the most tolerated and easiest cleansing method. Face-to-face respiratory transmission should be mitigated by the aforementioned methods.
Ablative laser surgery
Most laser surgeons agree that ablative laser surgery procedures should likely be delayed until the virus has waned more, because of the increased invasiveness of and recovery of wound healing from the procedure. There is increased evidence of SARS-CoV-2 infecting endothelial cells, raising concern about transmission via blood. A study of the cardiovascular manifestations seen in COVID-19 infection, published in The Lancet, showed the virus directly targets the endothelial cells that line blood vessels. Ablative laser surgery (fractional and fully ablative) is associated with blood or serous fluid on the skin surface immediately after the procedure and for up to 5-7 days post procedure, particularly with Er:Yag than with the CO2 laser. Antibacterial and antiviral prophylaxis often is used with these procedures. While the aforementioned protocols for other nonablative lasers may help with ablative laser treatment, there is currently no known effective and available antiviral prophylactic medication against SARS-CoV-2, if needed.
PPE
Personal protective equipment shortages are still a concern. Many hospitals are sterilizing and reusing traditionally disposable N95 masks in the inpatient setting, which is unprecedented. Resterilization will likely be necessary in outpatient medical offices as well, if the supply of masks does not increase. The supply chain will be a factor in considering PPE use in outpatient offices affecting the availability of PPE for emergency medicine, inpatient hospital, and ICU providers in direct contact with known COVID-19 patients.
With asymptomatic spread and the lack of adequate testing for COVID-19, as practices reopen, all practitioners will be on the front lines and should treat their practice and protect their patients, staff and themselves as such.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
References:
Dover JS et al. Facial Plast Surg Aesthet Med. 2020 May 5. doi: 10.1089/fpsam.2020.0239.
Kampf G et al. J Hosp Infect. 2020 Mar;104(3):246-51.
Varga Z et al. Lancet. 2020 May 2;395(10234):1417-8.
Protective measures recommended for cosmetic procedures have recently been published by Dover et al. in Facial Plastic Surgery & Aesthetic Medicine. The manuscript, titled “A path to resume aesthetic care Project AesCert Guidance Supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” provides thorough, detailed recommendations on all aspects of protection and preparedness for aesthetic clinical practices.
in this uncharted territory. During the last pandemic, the 1918 Spanish flu, caused by an H1N1 virus, laser procedures didn’t exist. Discussion among dermatologists and laser surgeons, including the aforementioned publication, have led to the following initial office recommendations (subject to change).
Office preparation and safety including:
- Prescreening patients for symptoms.
- Social distancing in the office, including waiting room areas (or eliminating waiting areas and bringing patients into exam rooms upon arrival).
- Decreasing patient load and increasing length of appointment times.
- Having no additional visitors during patient appointments, unless necessary (minor, caregiver).
- Patients wearing masks to appointments and hand washing/sanitizing upon arrival/departure.
- Providers wearing appropriate personal protective equipment (PPE) during visits.
- Instituting office disinfectant checklists.
For nonablative laser surgery specifically, especially for therapy of the face and neck, recommendations include the following:
- Lasers and office areas are thoroughly sanitized between each procedure.
- Providers wear appropriate PPE, including N95 masks if possible, wraparound safety glasses, gloves, as well as strong consideration of face shields).
- The duration and number of procedures should be limited, as should intraprocedure conversations and close face-to-face proximity with patient’s airways.
- Lasers with increased plume, including laser tattoo removal and laser hair removal, are the procedures with the most concern with regards to viral particle or infection transmission.
PPE is recommended (including masks – N95 if available – gloves, and face shield), as well as evacuator suction systems of the two-stage filtration type, and/or negative room pressure if available. For air-filtration evacuator suction systems, the device vacuum must be held within 2 inches of the treatment area for the best efficacy. Some have suggested performing laser tattoo removal through a hydrogel patch to help eliminate plume, which may also increase the cost of the procedure and may depend on the availability of the patches themselves. Nothing has been published on the use of the hydrogel patch in laser hair removal. Shaving or trimming of hairs prior to the procedure is critical.
While pulse dye and intense pulsed light (IPL) lasers have generally been deemed safer to use during the COVID-19 pandemic – with appropriate protective gear and general office precautions – I would recommend being mindful of potential plume created when using these lasers in hair-bearing areas. IPL is generally avoided in these regions, unless specific filters are used for hair removal treatment. But if use an IPL in a hair-bearing region, shaving or trimming of the hairs with the above precautions should be done first to reduce plume. As with all face-to-face procedures, the above PPE, contact, and intraprocedure conversation precautions should be taken.
Nonablative fractional resurfacing lasers are areas in which more questions lie. Some providers are comfortable performing nonablative fractional lasers with protective gear and air filtration systems, while others are recommending delaying these procedures until more information is available. The question essentially involves whether infection risk is higher with these procedures because of plume and if depth of penetration of the laser can release viral particles.
In addition to the other precautions above, with the high transmissibility of COVID-19, I would recommend considering precleansing the treatment area with soap and water or a sterile prep that won’t irritate the skin, which has activity against coronaviruses. A study by Kampf et al. demonstrated that coronaviruses can persist on surfaces such as metal, glass, or plastic for up to 9 days (human skin surface unknown) but can be effectively inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents that may be more tolerable on the skin surface, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. Washing the face with soap and water may be the most tolerated and easiest cleansing method. Face-to-face respiratory transmission should be mitigated by the aforementioned methods.
Ablative laser surgery
Most laser surgeons agree that ablative laser surgery procedures should likely be delayed until the virus has waned more, because of the increased invasiveness of and recovery of wound healing from the procedure. There is increased evidence of SARS-CoV-2 infecting endothelial cells, raising concern about transmission via blood. A study of the cardiovascular manifestations seen in COVID-19 infection, published in The Lancet, showed the virus directly targets the endothelial cells that line blood vessels. Ablative laser surgery (fractional and fully ablative) is associated with blood or serous fluid on the skin surface immediately after the procedure and for up to 5-7 days post procedure, particularly with Er:Yag than with the CO2 laser. Antibacterial and antiviral prophylaxis often is used with these procedures. While the aforementioned protocols for other nonablative lasers may help with ablative laser treatment, there is currently no known effective and available antiviral prophylactic medication against SARS-CoV-2, if needed.
PPE
Personal protective equipment shortages are still a concern. Many hospitals are sterilizing and reusing traditionally disposable N95 masks in the inpatient setting, which is unprecedented. Resterilization will likely be necessary in outpatient medical offices as well, if the supply of masks does not increase. The supply chain will be a factor in considering PPE use in outpatient offices affecting the availability of PPE for emergency medicine, inpatient hospital, and ICU providers in direct contact with known COVID-19 patients.
With asymptomatic spread and the lack of adequate testing for COVID-19, as practices reopen, all practitioners will be on the front lines and should treat their practice and protect their patients, staff and themselves as such.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
References:
Dover JS et al. Facial Plast Surg Aesthet Med. 2020 May 5. doi: 10.1089/fpsam.2020.0239.
Kampf G et al. J Hosp Infect. 2020 Mar;104(3):246-51.
Varga Z et al. Lancet. 2020 May 2;395(10234):1417-8.
Protective measures recommended for cosmetic procedures have recently been published by Dover et al. in Facial Plastic Surgery & Aesthetic Medicine. The manuscript, titled “A path to resume aesthetic care Project AesCert Guidance Supplement – practical considerations for aesthetic medicine professionals supporting clinic preparedness in response to the SARS-CoV-2 outbreak,” provides thorough, detailed recommendations on all aspects of protection and preparedness for aesthetic clinical practices.
in this uncharted territory. During the last pandemic, the 1918 Spanish flu, caused by an H1N1 virus, laser procedures didn’t exist. Discussion among dermatologists and laser surgeons, including the aforementioned publication, have led to the following initial office recommendations (subject to change).
Office preparation and safety including:
- Prescreening patients for symptoms.
- Social distancing in the office, including waiting room areas (or eliminating waiting areas and bringing patients into exam rooms upon arrival).
- Decreasing patient load and increasing length of appointment times.
- Having no additional visitors during patient appointments, unless necessary (minor, caregiver).
- Patients wearing masks to appointments and hand washing/sanitizing upon arrival/departure.
- Providers wearing appropriate personal protective equipment (PPE) during visits.
- Instituting office disinfectant checklists.
For nonablative laser surgery specifically, especially for therapy of the face and neck, recommendations include the following:
- Lasers and office areas are thoroughly sanitized between each procedure.
- Providers wear appropriate PPE, including N95 masks if possible, wraparound safety glasses, gloves, as well as strong consideration of face shields).
- The duration and number of procedures should be limited, as should intraprocedure conversations and close face-to-face proximity with patient’s airways.
- Lasers with increased plume, including laser tattoo removal and laser hair removal, are the procedures with the most concern with regards to viral particle or infection transmission.
PPE is recommended (including masks – N95 if available – gloves, and face shield), as well as evacuator suction systems of the two-stage filtration type, and/or negative room pressure if available. For air-filtration evacuator suction systems, the device vacuum must be held within 2 inches of the treatment area for the best efficacy. Some have suggested performing laser tattoo removal through a hydrogel patch to help eliminate plume, which may also increase the cost of the procedure and may depend on the availability of the patches themselves. Nothing has been published on the use of the hydrogel patch in laser hair removal. Shaving or trimming of hairs prior to the procedure is critical.
While pulse dye and intense pulsed light (IPL) lasers have generally been deemed safer to use during the COVID-19 pandemic – with appropriate protective gear and general office precautions – I would recommend being mindful of potential plume created when using these lasers in hair-bearing areas. IPL is generally avoided in these regions, unless specific filters are used for hair removal treatment. But if use an IPL in a hair-bearing region, shaving or trimming of the hairs with the above precautions should be done first to reduce plume. As with all face-to-face procedures, the above PPE, contact, and intraprocedure conversation precautions should be taken.
Nonablative fractional resurfacing lasers are areas in which more questions lie. Some providers are comfortable performing nonablative fractional lasers with protective gear and air filtration systems, while others are recommending delaying these procedures until more information is available. The question essentially involves whether infection risk is higher with these procedures because of plume and if depth of penetration of the laser can release viral particles.
In addition to the other precautions above, with the high transmissibility of COVID-19, I would recommend considering precleansing the treatment area with soap and water or a sterile prep that won’t irritate the skin, which has activity against coronaviruses. A study by Kampf et al. demonstrated that coronaviruses can persist on surfaces such as metal, glass, or plastic for up to 9 days (human skin surface unknown) but can be effectively inactivated by surface disinfection procedures with 62%-71% ethanol, 0.5% hydrogen peroxide, or 0.1% sodium hypochlorite within 1 minute. Other biocidal agents that may be more tolerable on the skin surface, such as 0.05%-0.2% benzalkonium chloride or 0.02% chlorhexidine digluconate were less effective. Washing the face with soap and water may be the most tolerated and easiest cleansing method. Face-to-face respiratory transmission should be mitigated by the aforementioned methods.
Ablative laser surgery
Most laser surgeons agree that ablative laser surgery procedures should likely be delayed until the virus has waned more, because of the increased invasiveness of and recovery of wound healing from the procedure. There is increased evidence of SARS-CoV-2 infecting endothelial cells, raising concern about transmission via blood. A study of the cardiovascular manifestations seen in COVID-19 infection, published in The Lancet, showed the virus directly targets the endothelial cells that line blood vessels. Ablative laser surgery (fractional and fully ablative) is associated with blood or serous fluid on the skin surface immediately after the procedure and for up to 5-7 days post procedure, particularly with Er:Yag than with the CO2 laser. Antibacterial and antiviral prophylaxis often is used with these procedures. While the aforementioned protocols for other nonablative lasers may help with ablative laser treatment, there is currently no known effective and available antiviral prophylactic medication against SARS-CoV-2, if needed.
PPE
Personal protective equipment shortages are still a concern. Many hospitals are sterilizing and reusing traditionally disposable N95 masks in the inpatient setting, which is unprecedented. Resterilization will likely be necessary in outpatient medical offices as well, if the supply of masks does not increase. The supply chain will be a factor in considering PPE use in outpatient offices affecting the availability of PPE for emergency medicine, inpatient hospital, and ICU providers in direct contact with known COVID-19 patients.
With asymptomatic spread and the lack of adequate testing for COVID-19, as practices reopen, all practitioners will be on the front lines and should treat their practice and protect their patients, staff and themselves as such.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They have no relevant disclosures.
References:
Dover JS et al. Facial Plast Surg Aesthet Med. 2020 May 5. doi: 10.1089/fpsam.2020.0239.
Kampf G et al. J Hosp Infect. 2020 Mar;104(3):246-51.
Varga Z et al. Lancet. 2020 May 2;395(10234):1417-8.
Lessons learned during the COVID-19 pandemic
Each day, we’re inundated with news about the COVID-19 pandemic and how it continues to strain our health care system and resources. With more than 1.15 million positive cases in the United States and over 67,000 deaths as of this writing, it has been a scary yet humbling experience for everyone. There is no doubt this pandemic will be a defining moment in health care for several reasons. From supply chain disruptions and personal protective equipment (PPE) and ventilator shortages to exhausted caregivers – both physically and mentally – this event has pushed the envelope on finding answers from federal and state authorities. Hospital administrations are working harder than ever to rise to the challenge and do what is best for their frontline staff and, more importantly, the patients and the communities they serve.
The provider experience during COVID-19
Hospitalists are in a unique situation as frontline providers. Managing daily throughput of patients has always been a key role for the specialty. They also play an integral role in their own care teams alongside nurses, trainees, case managers, pharmacists, and others in cohorted COVID-19 units. Now more than ever, such a geographic placement of patients is quickly emerging as a must-have staffing model to reduce risk of cross-contamination and preserving critical PPE supplies. This heightened awareness, coupled with anxiety, sometimes leads to added stress and burnout risk for hospitalists.
Communication is critical in creating situational awareness and reducing anxiety within the teams. This is exactly where hospitalists can lead:
- Active presence in hospital incident command centers and infection control boards
- Close coordination with emergency medicine colleagues and bed placement navigators
- Developing protocols for appropriate testing
- Frequent daily huddles to discuss current state- and hospital-level testing guidelines
- Close involvement in the hospital operations committee
- Advocating for or securing more testing or supplies, especially PPE
- Effective communication about changes in PPE requirements and conservation strategies as per the Centers for Disease Control and Prevention, State Department of Health, and the hospital infection control board
- Crisis-driven changes, including development and review of triage and treatment protocols and elective procedure cancellations
- Census numbers and capacity/staffing adjustments within the team to meet temporary dips and surges in on-service patient volumes
- Frontline caregiver mental and physical health assessment
Daily huddles at key times (e.g., at shift start and end times) can help to identify these barriers. If operational issues arise, there should be a clear channel to escalate them to senior leadership.
Hospitalists could also use several strategies proven to improve staff morale and resilience. For instance, take this time to connect with friends and family virtually, unplug when off from work, explore one’s spiritual self through meditation and prayers, spend time with nature, exercise daily, seek humor, and develop or work on one’s hobby.
The patient experience during COVID-19
Some intriguing data is also being released about patient experience during the pandemic. A Press Ganey analysis of 350,000 comments between January and March 2020 shows that patients are looking for more information about their condition, primarily COVID-19 test delays and result notification time. There is also hypervigilance in patients’ minds about hand hygiene and overall cleanliness of the hospital. Patients also seek clarification and transparent explanation of their caregiver’s bedside mannerisms – for example, why did they gown up before entering – and their daily care plans.
Patients have been appreciative of providers and recognize the personal risk frontline staff put themselves through. Communication transparency seems to mitigate concerns about delays of care especially caused by operational challenges as a result of the pandemic.
In surveys specifically related to experiences including COVID-19, patients were more likely to rate more areas of service lower than in surveys that did not mention COVID-19. The patients also seemed to put more value on the quality of instructions and information they received and on perception of providers’ respect and listening abilities. These insights could prove invaluable in improving care delivery by hospitalists.
Isolation of patients has been shown in multiple studies to have negative outcomes. These patients are up to twice as likely to have an adverse event, and seven times more likely to have treatment-related avoidable adversity, poorer perceived patient experience, and overall perception of being cared for “less.” Add to this a higher level of depression and mental strain, and these patients quickly become “unsatisfied.”
At the ED level, the willingness to let family be present for care was the key area of concern listed – a metric that has changed rapidly since the early days of the pandemic.
The bottom line is these are trying times for everyone – both for providers and patients. Both look up to health system and group leadership for reassurance. Patients and families recognize the risks frontline providers are assuming. However, transparent communication across all levels is the key. Silos are disappearing and team based care is taking center stage.
Beyond the current public health crisis, these efforts will go a long way to create unshakable trust between health systems, providers, patients, and their loved ones.
Dr. Singh is currently the chief of inpatient operations at Adena Health System in Chillicothe, Ohio, where he also has key roles in medical informatics and health IT. He is also the president-elect of the Central Ohio Chapter of SHM.
Each day, we’re inundated with news about the COVID-19 pandemic and how it continues to strain our health care system and resources. With more than 1.15 million positive cases in the United States and over 67,000 deaths as of this writing, it has been a scary yet humbling experience for everyone. There is no doubt this pandemic will be a defining moment in health care for several reasons. From supply chain disruptions and personal protective equipment (PPE) and ventilator shortages to exhausted caregivers – both physically and mentally – this event has pushed the envelope on finding answers from federal and state authorities. Hospital administrations are working harder than ever to rise to the challenge and do what is best for their frontline staff and, more importantly, the patients and the communities they serve.
The provider experience during COVID-19
Hospitalists are in a unique situation as frontline providers. Managing daily throughput of patients has always been a key role for the specialty. They also play an integral role in their own care teams alongside nurses, trainees, case managers, pharmacists, and others in cohorted COVID-19 units. Now more than ever, such a geographic placement of patients is quickly emerging as a must-have staffing model to reduce risk of cross-contamination and preserving critical PPE supplies. This heightened awareness, coupled with anxiety, sometimes leads to added stress and burnout risk for hospitalists.
Communication is critical in creating situational awareness and reducing anxiety within the teams. This is exactly where hospitalists can lead:
- Active presence in hospital incident command centers and infection control boards
- Close coordination with emergency medicine colleagues and bed placement navigators
- Developing protocols for appropriate testing
- Frequent daily huddles to discuss current state- and hospital-level testing guidelines
- Close involvement in the hospital operations committee
- Advocating for or securing more testing or supplies, especially PPE
- Effective communication about changes in PPE requirements and conservation strategies as per the Centers for Disease Control and Prevention, State Department of Health, and the hospital infection control board
- Crisis-driven changes, including development and review of triage and treatment protocols and elective procedure cancellations
- Census numbers and capacity/staffing adjustments within the team to meet temporary dips and surges in on-service patient volumes
- Frontline caregiver mental and physical health assessment
Daily huddles at key times (e.g., at shift start and end times) can help to identify these barriers. If operational issues arise, there should be a clear channel to escalate them to senior leadership.
Hospitalists could also use several strategies proven to improve staff morale and resilience. For instance, take this time to connect with friends and family virtually, unplug when off from work, explore one’s spiritual self through meditation and prayers, spend time with nature, exercise daily, seek humor, and develop or work on one’s hobby.
The patient experience during COVID-19
Some intriguing data is also being released about patient experience during the pandemic. A Press Ganey analysis of 350,000 comments between January and March 2020 shows that patients are looking for more information about their condition, primarily COVID-19 test delays and result notification time. There is also hypervigilance in patients’ minds about hand hygiene and overall cleanliness of the hospital. Patients also seek clarification and transparent explanation of their caregiver’s bedside mannerisms – for example, why did they gown up before entering – and their daily care plans.
Patients have been appreciative of providers and recognize the personal risk frontline staff put themselves through. Communication transparency seems to mitigate concerns about delays of care especially caused by operational challenges as a result of the pandemic.
In surveys specifically related to experiences including COVID-19, patients were more likely to rate more areas of service lower than in surveys that did not mention COVID-19. The patients also seemed to put more value on the quality of instructions and information they received and on perception of providers’ respect and listening abilities. These insights could prove invaluable in improving care delivery by hospitalists.
Isolation of patients has been shown in multiple studies to have negative outcomes. These patients are up to twice as likely to have an adverse event, and seven times more likely to have treatment-related avoidable adversity, poorer perceived patient experience, and overall perception of being cared for “less.” Add to this a higher level of depression and mental strain, and these patients quickly become “unsatisfied.”
At the ED level, the willingness to let family be present for care was the key area of concern listed – a metric that has changed rapidly since the early days of the pandemic.
The bottom line is these are trying times for everyone – both for providers and patients. Both look up to health system and group leadership for reassurance. Patients and families recognize the risks frontline providers are assuming. However, transparent communication across all levels is the key. Silos are disappearing and team based care is taking center stage.
Beyond the current public health crisis, these efforts will go a long way to create unshakable trust between health systems, providers, patients, and their loved ones.
Dr. Singh is currently the chief of inpatient operations at Adena Health System in Chillicothe, Ohio, where he also has key roles in medical informatics and health IT. He is also the president-elect of the Central Ohio Chapter of SHM.
Each day, we’re inundated with news about the COVID-19 pandemic and how it continues to strain our health care system and resources. With more than 1.15 million positive cases in the United States and over 67,000 deaths as of this writing, it has been a scary yet humbling experience for everyone. There is no doubt this pandemic will be a defining moment in health care for several reasons. From supply chain disruptions and personal protective equipment (PPE) and ventilator shortages to exhausted caregivers – both physically and mentally – this event has pushed the envelope on finding answers from federal and state authorities. Hospital administrations are working harder than ever to rise to the challenge and do what is best for their frontline staff and, more importantly, the patients and the communities they serve.
The provider experience during COVID-19
Hospitalists are in a unique situation as frontline providers. Managing daily throughput of patients has always been a key role for the specialty. They also play an integral role in their own care teams alongside nurses, trainees, case managers, pharmacists, and others in cohorted COVID-19 units. Now more than ever, such a geographic placement of patients is quickly emerging as a must-have staffing model to reduce risk of cross-contamination and preserving critical PPE supplies. This heightened awareness, coupled with anxiety, sometimes leads to added stress and burnout risk for hospitalists.
Communication is critical in creating situational awareness and reducing anxiety within the teams. This is exactly where hospitalists can lead:
- Active presence in hospital incident command centers and infection control boards
- Close coordination with emergency medicine colleagues and bed placement navigators
- Developing protocols for appropriate testing
- Frequent daily huddles to discuss current state- and hospital-level testing guidelines
- Close involvement in the hospital operations committee
- Advocating for or securing more testing or supplies, especially PPE
- Effective communication about changes in PPE requirements and conservation strategies as per the Centers for Disease Control and Prevention, State Department of Health, and the hospital infection control board
- Crisis-driven changes, including development and review of triage and treatment protocols and elective procedure cancellations
- Census numbers and capacity/staffing adjustments within the team to meet temporary dips and surges in on-service patient volumes
- Frontline caregiver mental and physical health assessment
Daily huddles at key times (e.g., at shift start and end times) can help to identify these barriers. If operational issues arise, there should be a clear channel to escalate them to senior leadership.
Hospitalists could also use several strategies proven to improve staff morale and resilience. For instance, take this time to connect with friends and family virtually, unplug when off from work, explore one’s spiritual self through meditation and prayers, spend time with nature, exercise daily, seek humor, and develop or work on one’s hobby.
The patient experience during COVID-19
Some intriguing data is also being released about patient experience during the pandemic. A Press Ganey analysis of 350,000 comments between January and March 2020 shows that patients are looking for more information about their condition, primarily COVID-19 test delays and result notification time. There is also hypervigilance in patients’ minds about hand hygiene and overall cleanliness of the hospital. Patients also seek clarification and transparent explanation of their caregiver’s bedside mannerisms – for example, why did they gown up before entering – and their daily care plans.
Patients have been appreciative of providers and recognize the personal risk frontline staff put themselves through. Communication transparency seems to mitigate concerns about delays of care especially caused by operational challenges as a result of the pandemic.
In surveys specifically related to experiences including COVID-19, patients were more likely to rate more areas of service lower than in surveys that did not mention COVID-19. The patients also seemed to put more value on the quality of instructions and information they received and on perception of providers’ respect and listening abilities. These insights could prove invaluable in improving care delivery by hospitalists.
Isolation of patients has been shown in multiple studies to have negative outcomes. These patients are up to twice as likely to have an adverse event, and seven times more likely to have treatment-related avoidable adversity, poorer perceived patient experience, and overall perception of being cared for “less.” Add to this a higher level of depression and mental strain, and these patients quickly become “unsatisfied.”
At the ED level, the willingness to let family be present for care was the key area of concern listed – a metric that has changed rapidly since the early days of the pandemic.
The bottom line is these are trying times for everyone – both for providers and patients. Both look up to health system and group leadership for reassurance. Patients and families recognize the risks frontline providers are assuming. However, transparent communication across all levels is the key. Silos are disappearing and team based care is taking center stage.
Beyond the current public health crisis, these efforts will go a long way to create unshakable trust between health systems, providers, patients, and their loved ones.
Dr. Singh is currently the chief of inpatient operations at Adena Health System in Chillicothe, Ohio, where he also has key roles in medical informatics and health IT. He is also the president-elect of the Central Ohio Chapter of SHM.