User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Dupilumab hits the mark for severe AD in younger children
The monoclonal antibody , according to new clinical trial results.
In a cohort of children with severe AD, 33% achieved clear or nearly clear skin after 16 weeks of treatment with every 4-week dosing of the injectable medication, while 30% also achieved that mark when receiving a weight-based dose every 2 weeks. Both groups had results that were significantly better than those receiving placebo, with 11% of these children had clear or nearly clear skin by 16 weeks of dupilumab (Dupixent) therapy (P less than .0001 for both therapy arms versus placebo).
“Dupilumab with a topical corticosteroid showed clinically meaningful and statistically significant improvement in the atopic dermatitis signs and symptoms in children aged 6 to less than 12 years of age with severe atopic dermatitis,” said Amy Paller, MD, the Walter J. Hamlin professor and chair of the department of dermatology at Northwestern University, Chicago, presenting the results at the Revolutionizing Atopic Dermatitis virtual symposium. Portions of the conference, which has been rescheduled to December 2020, in Chicago, were presented virtually because of the COVID-19 pandemic.
The phase 3 trial of subcutaneously injected dupilumab for atopic dermatitis, dubbed LIBERTY AD PEDS, included children aged 6-11 years with severe AD. The study’s primary endpoint was the proportion of patients achieving a score of 0 or 1 (clear or almost clear skin) on the Investigator’s Global Assessment (IGA) scale by study week 16.
For the purposes of reporting results to the European Medicines Agency, the investigators added a coprimary endpoint of patients reaching 75% clearing on the Eczema Area and Severity Index (EASI-75) by week 16.
The randomized, double-blind, placebo-controlled trial enrolled 367 children with IGA scores of 4, denoting severe AD. The EASI score had to be at least 21 and patients had to endorse peak pruritus of at least 4 on a 0-10 numeric rating scale; body surface involvement had to be at least 15%. Patients went through a washout period of any systemic therapies before beginning the trial, which randomized patients 1:1:1 to receive placebo, dupilumab 300 mg every 4 weeks, or dupilumab every 2 weeks with weight-dependent dosing. All participants were also permitted topical corticosteroids.
Patients were an average of aged 8 years, about half were female, and about two-thirds were white. Most participants had developed AD within their first year of life. Patients were about evenly divided between weighing over and under 30 kg, which was the cutoff for 100 mcg versus 200 mcg dupilumab for the every-2-week dosing group.
Over 90% of patients had other atopic comorbidities, and the mean EASI score was about 38 with average weekly peak pruritus averaging 7.8 on the numeric rating scale.
“When we’re talking about how severe this population is, it’s interesting to note that about 30 to 35% were all that had been previously treated with either systemic steroids or some systemic nonsteroidal immunosuppressants,” Dr. Paller pointed out. “I think that reflects the fact that so many of these very severely affected children are not put on a systemic therapy, but are still staying on topical therapies to try to control their disease.”
Looking at the proportion of patients reaching EASI-75, both dosing strategies for dupilumab out-performed placebo, with 70% of the every 4-week group and 67% of the every 2-week group reaching EASI-75 at 16 weeks, compared with 27% of those on placebo (P less than .0001 for both active arms). “These differences were seen very early on; by 2 weeks already, we can see that we’re starting to see a difference in both of these arms,” noted Dr. Paller, adding that the difference was statistically significant by 4 weeks into the study.
The overall group of dupilumab participants saw their EASI scores drop by about 80%, while those taking placebo saw a 49% drop in EASI scores.
For the group of participants weighing less than 30 kg, the every 4-week strategy resulted in better clearing as measured by both IGA and EASI-75. This effect wasn’t seen for heavier patients. Trough dupilumab concentrations at 16 weeks were higher for lighter patients with every 4-week dosing and for heavier patients with the biweekly strategy, noted Dr. Paller.
In terms of itch, 60% to 68% of participants receiving dupilumab had a drop of at least 3 points in peak pruritus on the numeric rating scale, compared with 21% of those receiving placebo (P less than .001), while about half of the dupilumab groups and 12% of the placebo group saw pruritus improvements of 4 points or more (P less than .001). Pruritus improved early in the active arms of the study, becoming statistically significant at the 2 to 4 week range.
Treatment-emergent adverse events were numerically higher in patients in the placebo group, including infections and adjudicated skin infections. Conjunctivitis occurred more frequently in the dupilumab group, as did injection-site reactions.
“Overall, dupilumab was well tolerated, and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Paller said.
Dupilumab has been approved by the Food and Drug Administration to treat moderate to severe AD in those aged 12 years and older whose disease can’t be adequately controlled with topical prescription medications, or when those treatments are not advisable.
The fully human monoclonal antibody blocks a shared receptor component for interleukin-4 and interleukin-13, which contribute to inflammation in AD, as well as asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis.
Dr. Paller reported receiving support from multiple pharmaceutical companies including Sanofi and Regeneron Pharmaceuticals, which sponsored the study.
The monoclonal antibody , according to new clinical trial results.
In a cohort of children with severe AD, 33% achieved clear or nearly clear skin after 16 weeks of treatment with every 4-week dosing of the injectable medication, while 30% also achieved that mark when receiving a weight-based dose every 2 weeks. Both groups had results that were significantly better than those receiving placebo, with 11% of these children had clear or nearly clear skin by 16 weeks of dupilumab (Dupixent) therapy (P less than .0001 for both therapy arms versus placebo).
“Dupilumab with a topical corticosteroid showed clinically meaningful and statistically significant improvement in the atopic dermatitis signs and symptoms in children aged 6 to less than 12 years of age with severe atopic dermatitis,” said Amy Paller, MD, the Walter J. Hamlin professor and chair of the department of dermatology at Northwestern University, Chicago, presenting the results at the Revolutionizing Atopic Dermatitis virtual symposium. Portions of the conference, which has been rescheduled to December 2020, in Chicago, were presented virtually because of the COVID-19 pandemic.
The phase 3 trial of subcutaneously injected dupilumab for atopic dermatitis, dubbed LIBERTY AD PEDS, included children aged 6-11 years with severe AD. The study’s primary endpoint was the proportion of patients achieving a score of 0 or 1 (clear or almost clear skin) on the Investigator’s Global Assessment (IGA) scale by study week 16.
For the purposes of reporting results to the European Medicines Agency, the investigators added a coprimary endpoint of patients reaching 75% clearing on the Eczema Area and Severity Index (EASI-75) by week 16.
The randomized, double-blind, placebo-controlled trial enrolled 367 children with IGA scores of 4, denoting severe AD. The EASI score had to be at least 21 and patients had to endorse peak pruritus of at least 4 on a 0-10 numeric rating scale; body surface involvement had to be at least 15%. Patients went through a washout period of any systemic therapies before beginning the trial, which randomized patients 1:1:1 to receive placebo, dupilumab 300 mg every 4 weeks, or dupilumab every 2 weeks with weight-dependent dosing. All participants were also permitted topical corticosteroids.
Patients were an average of aged 8 years, about half were female, and about two-thirds were white. Most participants had developed AD within their first year of life. Patients were about evenly divided between weighing over and under 30 kg, which was the cutoff for 100 mcg versus 200 mcg dupilumab for the every-2-week dosing group.
Over 90% of patients had other atopic comorbidities, and the mean EASI score was about 38 with average weekly peak pruritus averaging 7.8 on the numeric rating scale.
“When we’re talking about how severe this population is, it’s interesting to note that about 30 to 35% were all that had been previously treated with either systemic steroids or some systemic nonsteroidal immunosuppressants,” Dr. Paller pointed out. “I think that reflects the fact that so many of these very severely affected children are not put on a systemic therapy, but are still staying on topical therapies to try to control their disease.”
Looking at the proportion of patients reaching EASI-75, both dosing strategies for dupilumab out-performed placebo, with 70% of the every 4-week group and 67% of the every 2-week group reaching EASI-75 at 16 weeks, compared with 27% of those on placebo (P less than .0001 for both active arms). “These differences were seen very early on; by 2 weeks already, we can see that we’re starting to see a difference in both of these arms,” noted Dr. Paller, adding that the difference was statistically significant by 4 weeks into the study.
The overall group of dupilumab participants saw their EASI scores drop by about 80%, while those taking placebo saw a 49% drop in EASI scores.
For the group of participants weighing less than 30 kg, the every 4-week strategy resulted in better clearing as measured by both IGA and EASI-75. This effect wasn’t seen for heavier patients. Trough dupilumab concentrations at 16 weeks were higher for lighter patients with every 4-week dosing and for heavier patients with the biweekly strategy, noted Dr. Paller.
In terms of itch, 60% to 68% of participants receiving dupilumab had a drop of at least 3 points in peak pruritus on the numeric rating scale, compared with 21% of those receiving placebo (P less than .001), while about half of the dupilumab groups and 12% of the placebo group saw pruritus improvements of 4 points or more (P less than .001). Pruritus improved early in the active arms of the study, becoming statistically significant at the 2 to 4 week range.
Treatment-emergent adverse events were numerically higher in patients in the placebo group, including infections and adjudicated skin infections. Conjunctivitis occurred more frequently in the dupilumab group, as did injection-site reactions.
“Overall, dupilumab was well tolerated, and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Paller said.
Dupilumab has been approved by the Food and Drug Administration to treat moderate to severe AD in those aged 12 years and older whose disease can’t be adequately controlled with topical prescription medications, or when those treatments are not advisable.
The fully human monoclonal antibody blocks a shared receptor component for interleukin-4 and interleukin-13, which contribute to inflammation in AD, as well as asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis.
Dr. Paller reported receiving support from multiple pharmaceutical companies including Sanofi and Regeneron Pharmaceuticals, which sponsored the study.
The monoclonal antibody , according to new clinical trial results.
In a cohort of children with severe AD, 33% achieved clear or nearly clear skin after 16 weeks of treatment with every 4-week dosing of the injectable medication, while 30% also achieved that mark when receiving a weight-based dose every 2 weeks. Both groups had results that were significantly better than those receiving placebo, with 11% of these children had clear or nearly clear skin by 16 weeks of dupilumab (Dupixent) therapy (P less than .0001 for both therapy arms versus placebo).
“Dupilumab with a topical corticosteroid showed clinically meaningful and statistically significant improvement in the atopic dermatitis signs and symptoms in children aged 6 to less than 12 years of age with severe atopic dermatitis,” said Amy Paller, MD, the Walter J. Hamlin professor and chair of the department of dermatology at Northwestern University, Chicago, presenting the results at the Revolutionizing Atopic Dermatitis virtual symposium. Portions of the conference, which has been rescheduled to December 2020, in Chicago, were presented virtually because of the COVID-19 pandemic.
The phase 3 trial of subcutaneously injected dupilumab for atopic dermatitis, dubbed LIBERTY AD PEDS, included children aged 6-11 years with severe AD. The study’s primary endpoint was the proportion of patients achieving a score of 0 or 1 (clear or almost clear skin) on the Investigator’s Global Assessment (IGA) scale by study week 16.
For the purposes of reporting results to the European Medicines Agency, the investigators added a coprimary endpoint of patients reaching 75% clearing on the Eczema Area and Severity Index (EASI-75) by week 16.
The randomized, double-blind, placebo-controlled trial enrolled 367 children with IGA scores of 4, denoting severe AD. The EASI score had to be at least 21 and patients had to endorse peak pruritus of at least 4 on a 0-10 numeric rating scale; body surface involvement had to be at least 15%. Patients went through a washout period of any systemic therapies before beginning the trial, which randomized patients 1:1:1 to receive placebo, dupilumab 300 mg every 4 weeks, or dupilumab every 2 weeks with weight-dependent dosing. All participants were also permitted topical corticosteroids.
Patients were an average of aged 8 years, about half were female, and about two-thirds were white. Most participants had developed AD within their first year of life. Patients were about evenly divided between weighing over and under 30 kg, which was the cutoff for 100 mcg versus 200 mcg dupilumab for the every-2-week dosing group.
Over 90% of patients had other atopic comorbidities, and the mean EASI score was about 38 with average weekly peak pruritus averaging 7.8 on the numeric rating scale.
“When we’re talking about how severe this population is, it’s interesting to note that about 30 to 35% were all that had been previously treated with either systemic steroids or some systemic nonsteroidal immunosuppressants,” Dr. Paller pointed out. “I think that reflects the fact that so many of these very severely affected children are not put on a systemic therapy, but are still staying on topical therapies to try to control their disease.”
Looking at the proportion of patients reaching EASI-75, both dosing strategies for dupilumab out-performed placebo, with 70% of the every 4-week group and 67% of the every 2-week group reaching EASI-75 at 16 weeks, compared with 27% of those on placebo (P less than .0001 for both active arms). “These differences were seen very early on; by 2 weeks already, we can see that we’re starting to see a difference in both of these arms,” noted Dr. Paller, adding that the difference was statistically significant by 4 weeks into the study.
The overall group of dupilumab participants saw their EASI scores drop by about 80%, while those taking placebo saw a 49% drop in EASI scores.
For the group of participants weighing less than 30 kg, the every 4-week strategy resulted in better clearing as measured by both IGA and EASI-75. This effect wasn’t seen for heavier patients. Trough dupilumab concentrations at 16 weeks were higher for lighter patients with every 4-week dosing and for heavier patients with the biweekly strategy, noted Dr. Paller.
In terms of itch, 60% to 68% of participants receiving dupilumab had a drop of at least 3 points in peak pruritus on the numeric rating scale, compared with 21% of those receiving placebo (P less than .001), while about half of the dupilumab groups and 12% of the placebo group saw pruritus improvements of 4 points or more (P less than .001). Pruritus improved early in the active arms of the study, becoming statistically significant at the 2 to 4 week range.
Treatment-emergent adverse events were numerically higher in patients in the placebo group, including infections and adjudicated skin infections. Conjunctivitis occurred more frequently in the dupilumab group, as did injection-site reactions.
“Overall, dupilumab was well tolerated, and data were consistent with the known dupilumab safety profile observed in adults and adolescents,” Dr. Paller said.
Dupilumab has been approved by the Food and Drug Administration to treat moderate to severe AD in those aged 12 years and older whose disease can’t be adequately controlled with topical prescription medications, or when those treatments are not advisable.
The fully human monoclonal antibody blocks a shared receptor component for interleukin-4 and interleukin-13, which contribute to inflammation in AD, as well as asthma, chronic rhinosinusitis with nasal polyps, and eosinophilic esophagitis.
Dr. Paller reported receiving support from multiple pharmaceutical companies including Sanofi and Regeneron Pharmaceuticals, which sponsored the study.
FROM REVOLUTIONIZING AD 2020
D.C.-area blacks face increased risk of mortality from SJS/TEN
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
(TEN), compared with nonblack patients, results from a single-center study showed.

Adam Swigost, MD, presented data on behalf of the study’s principal investigator, Helena B. Pasieka, MD, and associates at MedStar Health Georgetown University in Washington in a video presentation during a virtual meeting held by the George Washington University department of dermatology. The virtual meeting included presentations slated for the annual meeting of the American Academy of Dermatology, which was canceled because of the COVID-19 pandemic.
According to the 2009-2012 Nationwide Inpatient Survey, there were 12,195 cases of SJS, 2,373 cases of SJS/TEN overlap, and 2,675 cases of TEN. In 2016, researchers led by Derek Y. Hsu, MD, of Northwestern University, Chicago, found that SJS/TEN was associated with nonwhite race, particularly Asians (odds ratio, 3.27) and blacks (OR, 2.01) (J Invest Dermatol. 2016;136[7]:1387-97).
“This led Dr. Pasieka and our team to ask the question: Are there differences in SJS/TEN outcomes in self-reported blacks in the U.S.?” said Dr. Swigost, a resident in the department of dermatology at MedStar Health Georgetown University.
To find out, he and his colleagues retrospectively analyzed records from 74 patients with SJS/TEN who were treated at Washington Hospital Center in Washington, D.C., from 2009 to 2019. They drew data from clinical diagnoses with histopathologic evaluation, when available, and performed a multivariate analysis adjusted for age, HIV status, black race, and offending drug category.
Of the 75 patients, 43 were female, 45 were black, 16 were white, 6 were Asian, 5 were Indian, 1 was Native American, and 1 was South Asian. Multivariate analysis revealed that black race was the only significant variable associated with an elevated risk of mortality from SJS/TEN (OR, 4.81; P = .04).
Of the 45 black patients in the study, 33 were HIV negative and 12 were HIV positive. “While this variable was not statistically significant, it did seem to have an elevated risk for mortality in HIV-positive patients [4 of 12; 33%], compared with 8 of 33 HIV-negative patients [25%],” Dr. Swigost said.
Next, the researchers investigated the culprit medications in the black patients. As a reference, they compared their data with a 2015 study that set out to document the clinical profile, etiologies, and outcomes of SJS and TEN in hospitals in four sub-Saharan African countries (Int J Dermatol. 2013 May;52[5]:575-9). In the 2015 study, sulfonamides were the most-used drugs (38%) followed by the antiretroviral drug nevirapine (20%) and tuberculosis drugs (6%). In the study by Dr. Swigost and colleagues, the most frequently implicated drugs were sulfonamides (24%), followed by other antibiotics (24%), and anticonvulsants (17%).
“Our patients at MedStar Washington Hospital Center are going to have different comorbidities and medical problems that dictate different medications being used in different proportions,” Dr. Swigost explained.
Delayed detection is one possible reason for the increased mortality observed in black patients. “Dermatology education on a national level is biased most commonly toward white skin,” he said. “Often, diseases can be missed in skin of color. It’s possible that the diagnoses are being delayed and so treatment is being delayed.”
Socioeconomics and access to health care could also play a role in the poor outcome we observed. “Those are variables we want to further analyze in this data,” Dr. Swigost said. “Other things to consider are genetic variations between African and American black patient populations, because in the U.S. our black population is likely more heterogeneous than African patient populations are. It’s possible that there are HLA [human leukocyte antigen] differences that are contributing. Lastly, further characterization and stratification of SJS/TEN risk factors are required.”
Dr. Swigost and Dr. Pasieka reported having no disclosures.
Comorbidities the rule in New York’s COVID-19 deaths
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.
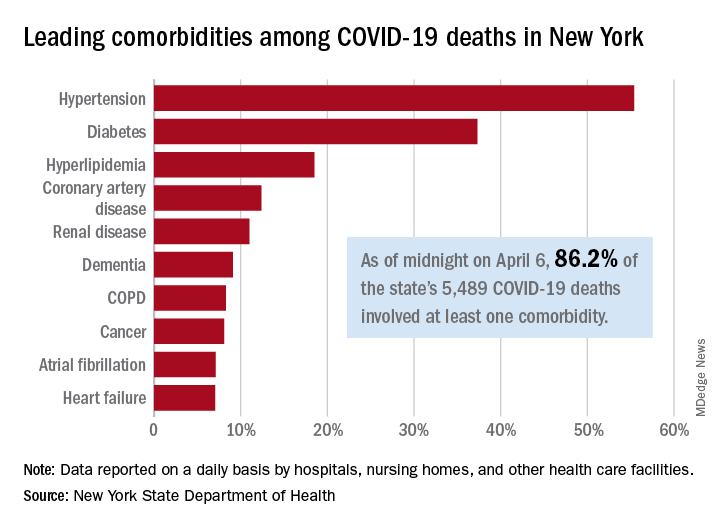
As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.

As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
In New York state, just over 86% of reported COVID-19 deaths involved at least one comorbidity, according to the state’s department of health.

As of midnight on April 6, there had been 5,489 fatalities caused by COVID-19 in the state, of which 86.2% (4,732) had at least one underlying condition, the New York State Department of Health reported April 7 on its COVID-19 tracker.
The leading comorbidity, seen in 55.4% of all deaths, was hypertension. In comparison, a recent estimate from the U.S. Department of Health & Human Services put the prevalence of high blood pressure at about 45% in the overall adult population.
In New York, the rest of the 10 most common comorbidities in COVID-19 fatalities were diabetes (37.3%), hyperlipidemia (18.5%), coronary artery disease (12.4%), renal disease (11.0%), dementia (9.1%), chronic obstructive pulmonary disease (8.3%), cancer (8.1%), atrial fibrillation (7.1%), and heart failure (7.1%), the NYSDOH said.
Other data on the tracker site show that 63% of all deaths involved a patient who was aged 70 years or older and that 61% of COVID-19 patients who have died in New York were male and 38.8% were female (sex unknown for 0.2%). Among all individuals who have tested positive, 54.8% were male and 44.6% were female (sex unknown for 0.6%).
As of the end of day on April 6, a total of 340,058 persons had been tested in the state and 40.8% (138,863) were positive for the SARS-CoV-2 virus. By county, the highest positive rates are in New York City: Queens at 57.4%, Brooklyn at 52.4%, and the Bronx at 52.3%, according to the NYSDOH.
SARS-CoV-2 escapes cotton, surgical masks of infected
June 9, 2020 — Editor’s note: The study on which this news story is based has been retracted by the journal. The retraction notice can be found here.
according to Seongman Bae, MD, of the University of Ulsan College of Medicine in Seoul, South Korea, and associates.
The report was published in Annals of Internal Medicine.
Because the COVID-19 pandemic has caused a shortage of N95 and surgical masks, cotton masks have gained interest as a substitute, as surgical masks have been shown to effectively filter influenza virus, the researchers wrote. However, the size of and concentrations of SARS-CoV-2 in aerosols generated during coughing are unknown.
To compare the effectiveness of cotton and surgical masks, a group of patients infected with SARS-CoV-2 coughed into petri dishes while wearing no mask, a surgical mask, and a cotton mask. The mask surfaces were swabbed afterward to assess viral positivity on the mask itself.
The median nasopharyngeal and saliva viral load was 5.66 log copies/mL and 4.00 log copies/mL, respectively. The median viral loads after coughing was 2.56 log copies/mL without a mask, 2.42 log copies/mL with a surgical mask, and 1.85 log copies/mL with a cotton mask. All outer surfaces of the mask were positive for SARS-CoV-2, while most inner surfaces were negative.
The investigators acknowledged that the test did not include N95 masks and does not reflect the actual infection transmission, and that they didn’t know whether cotton or surgical masks shorten the travel distance of droplets while coughing.
“Further study is needed to recommend whether face masks decrease transmission of virus from asymptomatic individuals or those with suspected COVID-19 who are not coughing,” they added.
The study was funded by a grant from the government-wide R&D Fund Project for Infectious Disease Research. The investigators reported that they had no conflicts of interest.
SOURCE: Bae S et al. Ann Intern Med. 2020 Apr 6. doi: 10.7326/M20-1342.
Correction, 4/9/20: The headline of an earlier version of this article misstated a finding of this study. Whether cotton and surgical masks can block transmission was not investigated.
June 9, 2020 — Editor’s note: The study on which this news story is based has been retracted by the journal. The retraction notice can be found here.
according to Seongman Bae, MD, of the University of Ulsan College of Medicine in Seoul, South Korea, and associates.
The report was published in Annals of Internal Medicine.
Because the COVID-19 pandemic has caused a shortage of N95 and surgical masks, cotton masks have gained interest as a substitute, as surgical masks have been shown to effectively filter influenza virus, the researchers wrote. However, the size of and concentrations of SARS-CoV-2 in aerosols generated during coughing are unknown.
To compare the effectiveness of cotton and surgical masks, a group of patients infected with SARS-CoV-2 coughed into petri dishes while wearing no mask, a surgical mask, and a cotton mask. The mask surfaces were swabbed afterward to assess viral positivity on the mask itself.
The median nasopharyngeal and saliva viral load was 5.66 log copies/mL and 4.00 log copies/mL, respectively. The median viral loads after coughing was 2.56 log copies/mL without a mask, 2.42 log copies/mL with a surgical mask, and 1.85 log copies/mL with a cotton mask. All outer surfaces of the mask were positive for SARS-CoV-2, while most inner surfaces were negative.
The investigators acknowledged that the test did not include N95 masks and does not reflect the actual infection transmission, and that they didn’t know whether cotton or surgical masks shorten the travel distance of droplets while coughing.
“Further study is needed to recommend whether face masks decrease transmission of virus from asymptomatic individuals or those with suspected COVID-19 who are not coughing,” they added.
The study was funded by a grant from the government-wide R&D Fund Project for Infectious Disease Research. The investigators reported that they had no conflicts of interest.
SOURCE: Bae S et al. Ann Intern Med. 2020 Apr 6. doi: 10.7326/M20-1342.
Correction, 4/9/20: The headline of an earlier version of this article misstated a finding of this study. Whether cotton and surgical masks can block transmission was not investigated.
June 9, 2020 — Editor’s note: The study on which this news story is based has been retracted by the journal. The retraction notice can be found here.
according to Seongman Bae, MD, of the University of Ulsan College of Medicine in Seoul, South Korea, and associates.
The report was published in Annals of Internal Medicine.
Because the COVID-19 pandemic has caused a shortage of N95 and surgical masks, cotton masks have gained interest as a substitute, as surgical masks have been shown to effectively filter influenza virus, the researchers wrote. However, the size of and concentrations of SARS-CoV-2 in aerosols generated during coughing are unknown.
To compare the effectiveness of cotton and surgical masks, a group of patients infected with SARS-CoV-2 coughed into petri dishes while wearing no mask, a surgical mask, and a cotton mask. The mask surfaces were swabbed afterward to assess viral positivity on the mask itself.
The median nasopharyngeal and saliva viral load was 5.66 log copies/mL and 4.00 log copies/mL, respectively. The median viral loads after coughing was 2.56 log copies/mL without a mask, 2.42 log copies/mL with a surgical mask, and 1.85 log copies/mL with a cotton mask. All outer surfaces of the mask were positive for SARS-CoV-2, while most inner surfaces were negative.
The investigators acknowledged that the test did not include N95 masks and does not reflect the actual infection transmission, and that they didn’t know whether cotton or surgical masks shorten the travel distance of droplets while coughing.
“Further study is needed to recommend whether face masks decrease transmission of virus from asymptomatic individuals or those with suspected COVID-19 who are not coughing,” they added.
The study was funded by a grant from the government-wide R&D Fund Project for Infectious Disease Research. The investigators reported that they had no conflicts of interest.
SOURCE: Bae S et al. Ann Intern Med. 2020 Apr 6. doi: 10.7326/M20-1342.
Correction, 4/9/20: The headline of an earlier version of this article misstated a finding of this study. Whether cotton and surgical masks can block transmission was not investigated.
FROM ANNALS OF INTERNAL MEDICINE
Belimumab may improve skin in scleroderma
MAUI, HAWAII – Belimumab shows promise as a novel biologic treatment for skin involvement in early diffuse cutaneous systemic sclerosis, Janet E. Pope, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
She highlighted a single-center, double-blind, placebo-controlled, New York pilot study including 20 patients with early diffuse cutaneous systemic sclerosis and moderate skin involvement. Participants had recently started on background mycophenolate mofetil (MMF) at 1,000 mg twice daily and were then randomized to add-on belimumab (Benlysta) at the dosing approved for systemic lupus erythematosus or to infusions of normal saline.
At 52 weeks, the modified Rodnan skin thickness score (mRSS) decreased by a median of 10 points from a baseline of 27 in the belimumab group, compared with just a 3-point reduction in controls on MMF plus placebo.
This small study raises several key points. It definitely warrants confirmation in a large phase 3 trial, according to Dr. Pope, professor of medicine at the University of Western Ontario and head of the division of rheumatology at St. Joseph’s Health Care, both in London.
For one thing, the pilot study makes a good case for multidrug therapy in scleroderma. “In rheumatoid arthritis, if in general one drug is not as good as two, why would we ever think, in our most difficult-to-treat disease, one drug would be okay?” the rheumatologist observed.
The belimumab study also highlights the role of abnormalities in B-cell function in the pathogenesis of skin involvement in early diffuse cutaneous systemic sclerosis. Belimumab is a fully human monoclonal antibody which binds to soluble B-lymphocyte stimulator and inhibits autoantibody production.
Belimumab’s mechanism of benefit was as expected: The improvement in skin scores in the belimumab group was accompanied by decreased expression of profibrotic genes and B-cell signaling, changes that didn’t occur in the controls on MMF alone.
The belimumab study makes another important point: MMF, despite its growing popularity for treatment of skin manifestations of scleroderma, is actually a wimpy drug for that purpose, achieving a mere 3-point reduction in mRSS.
“To be quite honest, mycophenolate mofetil is not all that great on skin,” Dr. Pope said.
Nonetheless, when she and her coworkers recently polled 170 scleroderma experts as to their favored treatments directed at various target organs impaired by the disease, as she had previously done in 2012, a clear trend was evident. “There’s a shift in that mycophenolate mofetil is moving to first-line treatment across the board for skin,” Dr. Pope observed.
Indeed, in the more recent survey, 71% of the experts agreed upon a scleroderma skin involvement treatment algorithm in which the first-line treatment for severe skin disease as defined by an mRSS of 32 was MMF, with methotrexate as second line, intravenous cyclophosphamide third, and autologous stem cell transplantation as fourth line for the small number of patients who qualify for it.
For moderate skin involvement, with an mRSS of 24, methotrexate was endorsed as first line, although by the narrowest of margins, over MMF, with intravenous cyclophosphamide as third line. For mild disease, with an mRSS of 10, methotrexate again narrowly beat out MMF by expert consensus as the preferred first-line therapy.
When asked about concomitant use of corticosteroids for treatment of skin involvement, 35% of experts said they never prescribe them for that indication, 33% do so occasionally, 19% sometimes, and 13% routinely. There was an even split on dosing among those who prescribe steroids: 49% suggested using prednisone at less than 7.5 mg/day, and 51% recommended 7.5-20 mg/day.
The purpose in polling the experts, who were drawn from the Scleroderma Clinical Trials Consortium and the Canadian Scleroderma Research Group, was to provide treatment guidance to general rheumatologists and dermatologists who may not see many patients with scleroderma. In contrast, the great majority of the polled experts see more than 50 scleroderma patients per year. And they had a high level of total agreement for treatment algorithms addressing not only skin disease, but also pulmonary arterial hypertension, interstitial lung disease, Raynaud’s phenomenon, renal crisis, digital ulcers, inflammatory arthritis, cardiac involvement, and gastrointestinal disease, Dr. Pope noted.
She attributed the experts’ rising enthusiasm for MMF for scleroderma skin involvement to the results of the Scleroderma Lung Study II, the first randomized, controlled trial to compare MMF and cyclophosphamide for the treatment of symptomatic scleroderma interstitial lung disease. Two years of MMF improved forced vital capacity as much as 1 year of oral cyclophosphamide. At 2 years of follow-up, the mRSS dropped modestly from baseline by an average of 6.1 points in the cyclophosphamide group and 2.9 points with MMF, a nonsignificant difference. But the incidence of serious adverse events was roughly three times higher and deaths were twice as frequent in the cyclophosphamide group.
“I think mycophenolate mofetil is surging for treatment of skin because of the lung protection and it was safer, but it’s hard for me to know if the deaths were more common in the cyclophosphamide group because of the cyclophosphamide or because of no treatment in year 2,” Dr. Pope commented.
She reported receiving research grants from Bristol-Myers Squibb, Merck, Roche, Seattle Genetics, and UCB, and serving as a consultant to more than a dozen pharmaceutical companies.
MAUI, HAWAII – Belimumab shows promise as a novel biologic treatment for skin involvement in early diffuse cutaneous systemic sclerosis, Janet E. Pope, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
She highlighted a single-center, double-blind, placebo-controlled, New York pilot study including 20 patients with early diffuse cutaneous systemic sclerosis and moderate skin involvement. Participants had recently started on background mycophenolate mofetil (MMF) at 1,000 mg twice daily and were then randomized to add-on belimumab (Benlysta) at the dosing approved for systemic lupus erythematosus or to infusions of normal saline.
At 52 weeks, the modified Rodnan skin thickness score (mRSS) decreased by a median of 10 points from a baseline of 27 in the belimumab group, compared with just a 3-point reduction in controls on MMF plus placebo.
This small study raises several key points. It definitely warrants confirmation in a large phase 3 trial, according to Dr. Pope, professor of medicine at the University of Western Ontario and head of the division of rheumatology at St. Joseph’s Health Care, both in London.
For one thing, the pilot study makes a good case for multidrug therapy in scleroderma. “In rheumatoid arthritis, if in general one drug is not as good as two, why would we ever think, in our most difficult-to-treat disease, one drug would be okay?” the rheumatologist observed.
The belimumab study also highlights the role of abnormalities in B-cell function in the pathogenesis of skin involvement in early diffuse cutaneous systemic sclerosis. Belimumab is a fully human monoclonal antibody which binds to soluble B-lymphocyte stimulator and inhibits autoantibody production.
Belimumab’s mechanism of benefit was as expected: The improvement in skin scores in the belimumab group was accompanied by decreased expression of profibrotic genes and B-cell signaling, changes that didn’t occur in the controls on MMF alone.
The belimumab study makes another important point: MMF, despite its growing popularity for treatment of skin manifestations of scleroderma, is actually a wimpy drug for that purpose, achieving a mere 3-point reduction in mRSS.
“To be quite honest, mycophenolate mofetil is not all that great on skin,” Dr. Pope said.
Nonetheless, when she and her coworkers recently polled 170 scleroderma experts as to their favored treatments directed at various target organs impaired by the disease, as she had previously done in 2012, a clear trend was evident. “There’s a shift in that mycophenolate mofetil is moving to first-line treatment across the board for skin,” Dr. Pope observed.
Indeed, in the more recent survey, 71% of the experts agreed upon a scleroderma skin involvement treatment algorithm in which the first-line treatment for severe skin disease as defined by an mRSS of 32 was MMF, with methotrexate as second line, intravenous cyclophosphamide third, and autologous stem cell transplantation as fourth line for the small number of patients who qualify for it.
For moderate skin involvement, with an mRSS of 24, methotrexate was endorsed as first line, although by the narrowest of margins, over MMF, with intravenous cyclophosphamide as third line. For mild disease, with an mRSS of 10, methotrexate again narrowly beat out MMF by expert consensus as the preferred first-line therapy.
When asked about concomitant use of corticosteroids for treatment of skin involvement, 35% of experts said they never prescribe them for that indication, 33% do so occasionally, 19% sometimes, and 13% routinely. There was an even split on dosing among those who prescribe steroids: 49% suggested using prednisone at less than 7.5 mg/day, and 51% recommended 7.5-20 mg/day.
The purpose in polling the experts, who were drawn from the Scleroderma Clinical Trials Consortium and the Canadian Scleroderma Research Group, was to provide treatment guidance to general rheumatologists and dermatologists who may not see many patients with scleroderma. In contrast, the great majority of the polled experts see more than 50 scleroderma patients per year. And they had a high level of total agreement for treatment algorithms addressing not only skin disease, but also pulmonary arterial hypertension, interstitial lung disease, Raynaud’s phenomenon, renal crisis, digital ulcers, inflammatory arthritis, cardiac involvement, and gastrointestinal disease, Dr. Pope noted.
She attributed the experts’ rising enthusiasm for MMF for scleroderma skin involvement to the results of the Scleroderma Lung Study II, the first randomized, controlled trial to compare MMF and cyclophosphamide for the treatment of symptomatic scleroderma interstitial lung disease. Two years of MMF improved forced vital capacity as much as 1 year of oral cyclophosphamide. At 2 years of follow-up, the mRSS dropped modestly from baseline by an average of 6.1 points in the cyclophosphamide group and 2.9 points with MMF, a nonsignificant difference. But the incidence of serious adverse events was roughly three times higher and deaths were twice as frequent in the cyclophosphamide group.
“I think mycophenolate mofetil is surging for treatment of skin because of the lung protection and it was safer, but it’s hard for me to know if the deaths were more common in the cyclophosphamide group because of the cyclophosphamide or because of no treatment in year 2,” Dr. Pope commented.
She reported receiving research grants from Bristol-Myers Squibb, Merck, Roche, Seattle Genetics, and UCB, and serving as a consultant to more than a dozen pharmaceutical companies.
MAUI, HAWAII – Belimumab shows promise as a novel biologic treatment for skin involvement in early diffuse cutaneous systemic sclerosis, Janet E. Pope, MD, said at the 2020 Rheumatology Winter Clinical Symposium.
She highlighted a single-center, double-blind, placebo-controlled, New York pilot study including 20 patients with early diffuse cutaneous systemic sclerosis and moderate skin involvement. Participants had recently started on background mycophenolate mofetil (MMF) at 1,000 mg twice daily and were then randomized to add-on belimumab (Benlysta) at the dosing approved for systemic lupus erythematosus or to infusions of normal saline.
At 52 weeks, the modified Rodnan skin thickness score (mRSS) decreased by a median of 10 points from a baseline of 27 in the belimumab group, compared with just a 3-point reduction in controls on MMF plus placebo.
This small study raises several key points. It definitely warrants confirmation in a large phase 3 trial, according to Dr. Pope, professor of medicine at the University of Western Ontario and head of the division of rheumatology at St. Joseph’s Health Care, both in London.
For one thing, the pilot study makes a good case for multidrug therapy in scleroderma. “In rheumatoid arthritis, if in general one drug is not as good as two, why would we ever think, in our most difficult-to-treat disease, one drug would be okay?” the rheumatologist observed.
The belimumab study also highlights the role of abnormalities in B-cell function in the pathogenesis of skin involvement in early diffuse cutaneous systemic sclerosis. Belimumab is a fully human monoclonal antibody which binds to soluble B-lymphocyte stimulator and inhibits autoantibody production.
Belimumab’s mechanism of benefit was as expected: The improvement in skin scores in the belimumab group was accompanied by decreased expression of profibrotic genes and B-cell signaling, changes that didn’t occur in the controls on MMF alone.
The belimumab study makes another important point: MMF, despite its growing popularity for treatment of skin manifestations of scleroderma, is actually a wimpy drug for that purpose, achieving a mere 3-point reduction in mRSS.
“To be quite honest, mycophenolate mofetil is not all that great on skin,” Dr. Pope said.
Nonetheless, when she and her coworkers recently polled 170 scleroderma experts as to their favored treatments directed at various target organs impaired by the disease, as she had previously done in 2012, a clear trend was evident. “There’s a shift in that mycophenolate mofetil is moving to first-line treatment across the board for skin,” Dr. Pope observed.
Indeed, in the more recent survey, 71% of the experts agreed upon a scleroderma skin involvement treatment algorithm in which the first-line treatment for severe skin disease as defined by an mRSS of 32 was MMF, with methotrexate as second line, intravenous cyclophosphamide third, and autologous stem cell transplantation as fourth line for the small number of patients who qualify for it.
For moderate skin involvement, with an mRSS of 24, methotrexate was endorsed as first line, although by the narrowest of margins, over MMF, with intravenous cyclophosphamide as third line. For mild disease, with an mRSS of 10, methotrexate again narrowly beat out MMF by expert consensus as the preferred first-line therapy.
When asked about concomitant use of corticosteroids for treatment of skin involvement, 35% of experts said they never prescribe them for that indication, 33% do so occasionally, 19% sometimes, and 13% routinely. There was an even split on dosing among those who prescribe steroids: 49% suggested using prednisone at less than 7.5 mg/day, and 51% recommended 7.5-20 mg/day.
The purpose in polling the experts, who were drawn from the Scleroderma Clinical Trials Consortium and the Canadian Scleroderma Research Group, was to provide treatment guidance to general rheumatologists and dermatologists who may not see many patients with scleroderma. In contrast, the great majority of the polled experts see more than 50 scleroderma patients per year. And they had a high level of total agreement for treatment algorithms addressing not only skin disease, but also pulmonary arterial hypertension, interstitial lung disease, Raynaud’s phenomenon, renal crisis, digital ulcers, inflammatory arthritis, cardiac involvement, and gastrointestinal disease, Dr. Pope noted.
She attributed the experts’ rising enthusiasm for MMF for scleroderma skin involvement to the results of the Scleroderma Lung Study II, the first randomized, controlled trial to compare MMF and cyclophosphamide for the treatment of symptomatic scleroderma interstitial lung disease. Two years of MMF improved forced vital capacity as much as 1 year of oral cyclophosphamide. At 2 years of follow-up, the mRSS dropped modestly from baseline by an average of 6.1 points in the cyclophosphamide group and 2.9 points with MMF, a nonsignificant difference. But the incidence of serious adverse events was roughly three times higher and deaths were twice as frequent in the cyclophosphamide group.
“I think mycophenolate mofetil is surging for treatment of skin because of the lung protection and it was safer, but it’s hard for me to know if the deaths were more common in the cyclophosphamide group because of the cyclophosphamide or because of no treatment in year 2,” Dr. Pope commented.
She reported receiving research grants from Bristol-Myers Squibb, Merck, Roche, Seattle Genetics, and UCB, and serving as a consultant to more than a dozen pharmaceutical companies.
REPORTING FROM RWCS 2020
Treatment for RA, SpA may not affect COVID-19 severity
Patients being treated for RA or spondyloarthritis who develop symptoms of COVID-19 do not appear to be at higher risk of respiratory or life-threatening complications, results from a new study in Italy suggest.
Such patients, the study authors wrote, do not need to be taken off their immunosuppressive medications if they develop COVID-19 symptoms.
In a letter published in Annals of the Rheumatic Diseases, Sara Monti, MD, and colleagues in the rheumatology department of the Fondazione IRCCS Policlinico in San Matteo, Italy, described results from an observational cohort of 320 patients (68% women; mean age, 55 years) with RA or spondyloarthritis from a single outpatient clinic. The vast majority of subjects (92%) were taking biologic disease-modifying antirheumatic drugs (bDMARD), including tumor necrosis factor inhibitors, while the rest were taking targeted synthetic DMARDs (tsDMARD).
Four patients in the cohort developed laboratory-confirmed COVID-19; another four developed symptoms highly suggestive of the disease but did not receive confirmatory testing, and five had contact with a confirmed COVID-19 case but did not develop symptoms of COVID-19.
Among the eight confirmed and suspected COVID-19 patients, only one was hospitalized. All temporarily withdrew bDMARD or tsDMARD treatment at symptom onset.
“To date, there have been no significant relapses of the rheumatic disease,” Dr. Monti and colleagues reported. “None of the patients with a confirmed diagnosis of COVID-19 or with a highly suggestive clinical picture developed severe respiratory complications or died. Only one patient, aged 65, required admission to hospital and low-flow oxygen supplementation for a few days.”
The findings “do not allow any conclusions on the incidence rate of SARS-CoV-2 infection in patients with rheumatic diseases, nor on the overall outcome of immunocompromised patients affected by COVID-19,” the investigators cautioned, adding that such patients should receive careful attention and follow-up. “However, our preliminary experience shows that patients with chronic arthritis treated with bDMARDs or tsDMARDs do not seem to be at increased risk of respiratory or life-threatening complications from SARS-CoV-2, compared with the general population.”
Dr. Monti and colleagues noted that, during previous outbreaks of other coronaviruses, no increased mortality was reported for people taking immunosuppressive drugs for a range of conditions, including autoimmune diseases.
“These data can support rheumatologists [in] avoiding the unjustifiable preventive withdrawal of DMARDs, which could lead to an increased risk of relapses and morbidity from the chronic rheumatological condition,” the researchers concluded.
Dr. Monti and colleagues reported no outside funding or financial conflicts of interest.
SOURCE: Monti S et al. Ann Rheum Dis. 2020 April 2. doi: 10.1136/annrheumdis-2020-217424.
Patients being treated for RA or spondyloarthritis who develop symptoms of COVID-19 do not appear to be at higher risk of respiratory or life-threatening complications, results from a new study in Italy suggest.
Such patients, the study authors wrote, do not need to be taken off their immunosuppressive medications if they develop COVID-19 symptoms.
In a letter published in Annals of the Rheumatic Diseases, Sara Monti, MD, and colleagues in the rheumatology department of the Fondazione IRCCS Policlinico in San Matteo, Italy, described results from an observational cohort of 320 patients (68% women; mean age, 55 years) with RA or spondyloarthritis from a single outpatient clinic. The vast majority of subjects (92%) were taking biologic disease-modifying antirheumatic drugs (bDMARD), including tumor necrosis factor inhibitors, while the rest were taking targeted synthetic DMARDs (tsDMARD).
Four patients in the cohort developed laboratory-confirmed COVID-19; another four developed symptoms highly suggestive of the disease but did not receive confirmatory testing, and five had contact with a confirmed COVID-19 case but did not develop symptoms of COVID-19.
Among the eight confirmed and suspected COVID-19 patients, only one was hospitalized. All temporarily withdrew bDMARD or tsDMARD treatment at symptom onset.
“To date, there have been no significant relapses of the rheumatic disease,” Dr. Monti and colleagues reported. “None of the patients with a confirmed diagnosis of COVID-19 or with a highly suggestive clinical picture developed severe respiratory complications or died. Only one patient, aged 65, required admission to hospital and low-flow oxygen supplementation for a few days.”
The findings “do not allow any conclusions on the incidence rate of SARS-CoV-2 infection in patients with rheumatic diseases, nor on the overall outcome of immunocompromised patients affected by COVID-19,” the investigators cautioned, adding that such patients should receive careful attention and follow-up. “However, our preliminary experience shows that patients with chronic arthritis treated with bDMARDs or tsDMARDs do not seem to be at increased risk of respiratory or life-threatening complications from SARS-CoV-2, compared with the general population.”
Dr. Monti and colleagues noted that, during previous outbreaks of other coronaviruses, no increased mortality was reported for people taking immunosuppressive drugs for a range of conditions, including autoimmune diseases.
“These data can support rheumatologists [in] avoiding the unjustifiable preventive withdrawal of DMARDs, which could lead to an increased risk of relapses and morbidity from the chronic rheumatological condition,” the researchers concluded.
Dr. Monti and colleagues reported no outside funding or financial conflicts of interest.
SOURCE: Monti S et al. Ann Rheum Dis. 2020 April 2. doi: 10.1136/annrheumdis-2020-217424.
Patients being treated for RA or spondyloarthritis who develop symptoms of COVID-19 do not appear to be at higher risk of respiratory or life-threatening complications, results from a new study in Italy suggest.
Such patients, the study authors wrote, do not need to be taken off their immunosuppressive medications if they develop COVID-19 symptoms.
In a letter published in Annals of the Rheumatic Diseases, Sara Monti, MD, and colleagues in the rheumatology department of the Fondazione IRCCS Policlinico in San Matteo, Italy, described results from an observational cohort of 320 patients (68% women; mean age, 55 years) with RA or spondyloarthritis from a single outpatient clinic. The vast majority of subjects (92%) were taking biologic disease-modifying antirheumatic drugs (bDMARD), including tumor necrosis factor inhibitors, while the rest were taking targeted synthetic DMARDs (tsDMARD).
Four patients in the cohort developed laboratory-confirmed COVID-19; another four developed symptoms highly suggestive of the disease but did not receive confirmatory testing, and five had contact with a confirmed COVID-19 case but did not develop symptoms of COVID-19.
Among the eight confirmed and suspected COVID-19 patients, only one was hospitalized. All temporarily withdrew bDMARD or tsDMARD treatment at symptom onset.
“To date, there have been no significant relapses of the rheumatic disease,” Dr. Monti and colleagues reported. “None of the patients with a confirmed diagnosis of COVID-19 or with a highly suggestive clinical picture developed severe respiratory complications or died. Only one patient, aged 65, required admission to hospital and low-flow oxygen supplementation for a few days.”
The findings “do not allow any conclusions on the incidence rate of SARS-CoV-2 infection in patients with rheumatic diseases, nor on the overall outcome of immunocompromised patients affected by COVID-19,” the investigators cautioned, adding that such patients should receive careful attention and follow-up. “However, our preliminary experience shows that patients with chronic arthritis treated with bDMARDs or tsDMARDs do not seem to be at increased risk of respiratory or life-threatening complications from SARS-CoV-2, compared with the general population.”
Dr. Monti and colleagues noted that, during previous outbreaks of other coronaviruses, no increased mortality was reported for people taking immunosuppressive drugs for a range of conditions, including autoimmune diseases.
“These data can support rheumatologists [in] avoiding the unjustifiable preventive withdrawal of DMARDs, which could lead to an increased risk of relapses and morbidity from the chronic rheumatological condition,” the researchers concluded.
Dr. Monti and colleagues reported no outside funding or financial conflicts of interest.
SOURCE: Monti S et al. Ann Rheum Dis. 2020 April 2. doi: 10.1136/annrheumdis-2020-217424.
FROM ANNALS OF THE RHEUMATIC DISEASES
‘The kids will be all right,’ won’t they?
Pediatric patients and COVID-19
The coronavirus disease 2019 (COVID-19) pandemic affects us in many ways. Pediatric patients, interestingly, are largely unaffected clinically by this disease. Less than 1% of documented infections occur in children under 10 years old, according to a review of over 72,000 cases from China.1 In that review, most children were asymptomatic or had mild illness, only three required intensive care, and only one death had been reported as of March 10, 2020. This is in stark contrast to the shocking morbidity and mortality statistics we are becoming all too familiar with on the adult side.
From a social standpoint, however, our pediatric patients’ lives have been turned upside down. Their schedules and routines upended, their education and friendships interrupted, and many are likely experiencing real anxiety and fear.2 For countless children, school is a major source of social, emotional, and nutritional support that has been cut off. Some will lose parents, grandparents, or other loved ones to this disease. Parents will lose jobs and will be unable to afford necessities. Pediatric patients will experience delays of procedures or treatments because of the pandemic. Some have projected that rates of child abuse will increase as has been reported during natural disasters.3
Pediatricians around the country are coming together to tackle these issues in creative ways, including the rapid expansion of virtual/telehealth programs. The school systems are developing strategies to deliver online content, and even food, to their students’ homes. Hopefully these tactics will mitigate some of the potential effects on the mental and physical well-being of these patients.
How about my kids? Will they be all right? I am lucky that my husband and I will have jobs throughout this ordeal. Unfortunately, given my role as a hospitalist and my husband’s as a pulmonary/critical care physician, these same jobs that will keep our kids nourished and supported pose the greatest threat to them. As health care workers, we are worried about protecting our families, which may include vulnerable members. The Spanish health ministry announced that medical professionals account for approximately one in eight documented COVID-19 infections in Spain.4 With inadequate supplies of personal protective equipment (PPE) in our own nation, we are concerned that our statistics could be similar.
There are multiple strategies to protect ourselves and our families during this difficult time. First, appropriate PPE is essential and integrity with the process must be maintained always. Hospital leaders can protect us by tirelessly working to acquire PPE. In Grand Rapids, Mich., our health system has partnered with multiple local manufacturing companies, including Steelcase, who are producing PPE for our workforce.5 Leaders can diligently update their system’s PPE recommendations to be in line with the latest CDC recommendations and disseminate the information regularly. Hospitalists should frequently check with their Infection Prevention department to make sure they understand if there have been any changes to the recommendations. Innovative solutions for sterilization of PPE, stethoscopes, badges and other equipment, such as with the use of UV boxes or hydrogen peroxide vapor,6 should be explored to minimize contamination. Hospitalists should bring a set of clothes and shoes to change into upon arrival to work and to change out of prior to leaving the hospital.
We must also keep our heads strong. Currently the anxiety amongst physicians is palpable but there is solidarity. Hospital leaders must ensure that hospitalists have easy access to free mental health resources, such as virtual counseling. Wellness teams must rise to the occasion with innovative tactics to support us. For example, Spectrum Health’s wellness team is sponsoring a blog where physicians can discuss COVID-19–related challenges openly. Hospitalist leaders should ensure that there is a structure for debriefing after critical incidents, which are sure to increase in frequency. Email lists and discussion boards sponsored by professional society also provide a collaborative venue for some of these discussions. We must take advantage of these resources and communicate with each other.
For me, in the end it comes back to the kids. My kids and most pediatric patients are not likely to be hospitalized from COVID-19, but they are also not immune to the toll that fighting this pandemic will take on our families. We took an oath to protect our patients, but what do we owe to our own children? At a minimum we can optimize how we protect ourselves every day, both physically and mentally. As we come together as a strong community to fight this pandemic, in addition to saving lives, we are working to ensure that, in the end, the kids will be all right.
Dr. Hadley is chief of pediatric hospital medicine at Spectrum Health/Helen DeVos Children’s Hospital in Grand Rapids, Mich., and clinical assistant professor at Michigan State University, East Lansing.
References
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
2. Hagan JF Jr; American Academy of Pediatrics Committee on Psychosocial Aspects of Child and Family Health; Task Force on Terrorism. Psychosocial implications of disaster or terrorism on children: A guide for the pediatrician. Pediatrics. 2005;116(3):787-795.
3. Gearhart S et al. The impact of natural disasters on domestic violence: An analysis of reports of simple assault in Florida (1997-2007). Violence Gend. 2018 Jun. doi: 10.1089/vio.2017.0077.
4. Minder R, Peltier E. Virus knocks thousands of health workers out of action in Europe. The New York Times. March 24, 2020.
5. McVicar B. West Michigan businesses hustle to produce medical supplies amid coronavirus pandemic. MLive. March 25, 2020.
6. Kenney PA et al. Hydrogen Peroxide Vapor sterilization of N95 respirators for reuse. medRxiv preprint. 2020 Mar. doi: 10.1101/2020.03.24.20041087.
Pediatric patients and COVID-19
Pediatric patients and COVID-19
The coronavirus disease 2019 (COVID-19) pandemic affects us in many ways. Pediatric patients, interestingly, are largely unaffected clinically by this disease. Less than 1% of documented infections occur in children under 10 years old, according to a review of over 72,000 cases from China.1 In that review, most children were asymptomatic or had mild illness, only three required intensive care, and only one death had been reported as of March 10, 2020. This is in stark contrast to the shocking morbidity and mortality statistics we are becoming all too familiar with on the adult side.
From a social standpoint, however, our pediatric patients’ lives have been turned upside down. Their schedules and routines upended, their education and friendships interrupted, and many are likely experiencing real anxiety and fear.2 For countless children, school is a major source of social, emotional, and nutritional support that has been cut off. Some will lose parents, grandparents, or other loved ones to this disease. Parents will lose jobs and will be unable to afford necessities. Pediatric patients will experience delays of procedures or treatments because of the pandemic. Some have projected that rates of child abuse will increase as has been reported during natural disasters.3
Pediatricians around the country are coming together to tackle these issues in creative ways, including the rapid expansion of virtual/telehealth programs. The school systems are developing strategies to deliver online content, and even food, to their students’ homes. Hopefully these tactics will mitigate some of the potential effects on the mental and physical well-being of these patients.
How about my kids? Will they be all right? I am lucky that my husband and I will have jobs throughout this ordeal. Unfortunately, given my role as a hospitalist and my husband’s as a pulmonary/critical care physician, these same jobs that will keep our kids nourished and supported pose the greatest threat to them. As health care workers, we are worried about protecting our families, which may include vulnerable members. The Spanish health ministry announced that medical professionals account for approximately one in eight documented COVID-19 infections in Spain.4 With inadequate supplies of personal protective equipment (PPE) in our own nation, we are concerned that our statistics could be similar.
There are multiple strategies to protect ourselves and our families during this difficult time. First, appropriate PPE is essential and integrity with the process must be maintained always. Hospital leaders can protect us by tirelessly working to acquire PPE. In Grand Rapids, Mich., our health system has partnered with multiple local manufacturing companies, including Steelcase, who are producing PPE for our workforce.5 Leaders can diligently update their system’s PPE recommendations to be in line with the latest CDC recommendations and disseminate the information regularly. Hospitalists should frequently check with their Infection Prevention department to make sure they understand if there have been any changes to the recommendations. Innovative solutions for sterilization of PPE, stethoscopes, badges and other equipment, such as with the use of UV boxes or hydrogen peroxide vapor,6 should be explored to minimize contamination. Hospitalists should bring a set of clothes and shoes to change into upon arrival to work and to change out of prior to leaving the hospital.
We must also keep our heads strong. Currently the anxiety amongst physicians is palpable but there is solidarity. Hospital leaders must ensure that hospitalists have easy access to free mental health resources, such as virtual counseling. Wellness teams must rise to the occasion with innovative tactics to support us. For example, Spectrum Health’s wellness team is sponsoring a blog where physicians can discuss COVID-19–related challenges openly. Hospitalist leaders should ensure that there is a structure for debriefing after critical incidents, which are sure to increase in frequency. Email lists and discussion boards sponsored by professional society also provide a collaborative venue for some of these discussions. We must take advantage of these resources and communicate with each other.
For me, in the end it comes back to the kids. My kids and most pediatric patients are not likely to be hospitalized from COVID-19, but they are also not immune to the toll that fighting this pandemic will take on our families. We took an oath to protect our patients, but what do we owe to our own children? At a minimum we can optimize how we protect ourselves every day, both physically and mentally. As we come together as a strong community to fight this pandemic, in addition to saving lives, we are working to ensure that, in the end, the kids will be all right.
Dr. Hadley is chief of pediatric hospital medicine at Spectrum Health/Helen DeVos Children’s Hospital in Grand Rapids, Mich., and clinical assistant professor at Michigan State University, East Lansing.
References
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
2. Hagan JF Jr; American Academy of Pediatrics Committee on Psychosocial Aspects of Child and Family Health; Task Force on Terrorism. Psychosocial implications of disaster or terrorism on children: A guide for the pediatrician. Pediatrics. 2005;116(3):787-795.
3. Gearhart S et al. The impact of natural disasters on domestic violence: An analysis of reports of simple assault in Florida (1997-2007). Violence Gend. 2018 Jun. doi: 10.1089/vio.2017.0077.
4. Minder R, Peltier E. Virus knocks thousands of health workers out of action in Europe. The New York Times. March 24, 2020.
5. McVicar B. West Michigan businesses hustle to produce medical supplies amid coronavirus pandemic. MLive. March 25, 2020.
6. Kenney PA et al. Hydrogen Peroxide Vapor sterilization of N95 respirators for reuse. medRxiv preprint. 2020 Mar. doi: 10.1101/2020.03.24.20041087.
The coronavirus disease 2019 (COVID-19) pandemic affects us in many ways. Pediatric patients, interestingly, are largely unaffected clinically by this disease. Less than 1% of documented infections occur in children under 10 years old, according to a review of over 72,000 cases from China.1 In that review, most children were asymptomatic or had mild illness, only three required intensive care, and only one death had been reported as of March 10, 2020. This is in stark contrast to the shocking morbidity and mortality statistics we are becoming all too familiar with on the adult side.
From a social standpoint, however, our pediatric patients’ lives have been turned upside down. Their schedules and routines upended, their education and friendships interrupted, and many are likely experiencing real anxiety and fear.2 For countless children, school is a major source of social, emotional, and nutritional support that has been cut off. Some will lose parents, grandparents, or other loved ones to this disease. Parents will lose jobs and will be unable to afford necessities. Pediatric patients will experience delays of procedures or treatments because of the pandemic. Some have projected that rates of child abuse will increase as has been reported during natural disasters.3
Pediatricians around the country are coming together to tackle these issues in creative ways, including the rapid expansion of virtual/telehealth programs. The school systems are developing strategies to deliver online content, and even food, to their students’ homes. Hopefully these tactics will mitigate some of the potential effects on the mental and physical well-being of these patients.
How about my kids? Will they be all right? I am lucky that my husband and I will have jobs throughout this ordeal. Unfortunately, given my role as a hospitalist and my husband’s as a pulmonary/critical care physician, these same jobs that will keep our kids nourished and supported pose the greatest threat to them. As health care workers, we are worried about protecting our families, which may include vulnerable members. The Spanish health ministry announced that medical professionals account for approximately one in eight documented COVID-19 infections in Spain.4 With inadequate supplies of personal protective equipment (PPE) in our own nation, we are concerned that our statistics could be similar.
There are multiple strategies to protect ourselves and our families during this difficult time. First, appropriate PPE is essential and integrity with the process must be maintained always. Hospital leaders can protect us by tirelessly working to acquire PPE. In Grand Rapids, Mich., our health system has partnered with multiple local manufacturing companies, including Steelcase, who are producing PPE for our workforce.5 Leaders can diligently update their system’s PPE recommendations to be in line with the latest CDC recommendations and disseminate the information regularly. Hospitalists should frequently check with their Infection Prevention department to make sure they understand if there have been any changes to the recommendations. Innovative solutions for sterilization of PPE, stethoscopes, badges and other equipment, such as with the use of UV boxes or hydrogen peroxide vapor,6 should be explored to minimize contamination. Hospitalists should bring a set of clothes and shoes to change into upon arrival to work and to change out of prior to leaving the hospital.
We must also keep our heads strong. Currently the anxiety amongst physicians is palpable but there is solidarity. Hospital leaders must ensure that hospitalists have easy access to free mental health resources, such as virtual counseling. Wellness teams must rise to the occasion with innovative tactics to support us. For example, Spectrum Health’s wellness team is sponsoring a blog where physicians can discuss COVID-19–related challenges openly. Hospitalist leaders should ensure that there is a structure for debriefing after critical incidents, which are sure to increase in frequency. Email lists and discussion boards sponsored by professional society also provide a collaborative venue for some of these discussions. We must take advantage of these resources and communicate with each other.
For me, in the end it comes back to the kids. My kids and most pediatric patients are not likely to be hospitalized from COVID-19, but they are also not immune to the toll that fighting this pandemic will take on our families. We took an oath to protect our patients, but what do we owe to our own children? At a minimum we can optimize how we protect ourselves every day, both physically and mentally. As we come together as a strong community to fight this pandemic, in addition to saving lives, we are working to ensure that, in the end, the kids will be all right.
Dr. Hadley is chief of pediatric hospital medicine at Spectrum Health/Helen DeVos Children’s Hospital in Grand Rapids, Mich., and clinical assistant professor at Michigan State University, East Lansing.
References
1. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020 Feb 24. doi: 10.1001/jama.2020.2648.
2. Hagan JF Jr; American Academy of Pediatrics Committee on Psychosocial Aspects of Child and Family Health; Task Force on Terrorism. Psychosocial implications of disaster or terrorism on children: A guide for the pediatrician. Pediatrics. 2005;116(3):787-795.
3. Gearhart S et al. The impact of natural disasters on domestic violence: An analysis of reports of simple assault in Florida (1997-2007). Violence Gend. 2018 Jun. doi: 10.1089/vio.2017.0077.
4. Minder R, Peltier E. Virus knocks thousands of health workers out of action in Europe. The New York Times. March 24, 2020.
5. McVicar B. West Michigan businesses hustle to produce medical supplies amid coronavirus pandemic. MLive. March 25, 2020.
6. Kenney PA et al. Hydrogen Peroxide Vapor sterilization of N95 respirators for reuse. medRxiv preprint. 2020 Mar. doi: 10.1101/2020.03.24.20041087.
COVID-19 linked to multiple cardiovascular presentations
It’s becoming clear that COVID-19 infection can involve the cardiovascular system in many different ways, and this has “evolving” potential implications for treatment, say a team of cardiologists on the frontlines of the COVID-19 battle in New York City.
In an article published online April 3 in Circulation, Justin Fried, MD, Division of Cardiology, Columbia University, New York City, and colleagues present four case studies of COVID-19 patients with various cardiovascular presentations.
Case 1 is a 64-year-old woman whose predominant symptoms on admission were cardiac in nature, including chest pain and ST elevation, but without fever, cough, or other symptoms suggestive of COVID-19.
“In patients presenting with what appears to be a typical cardiac syndrome, COVID-19 infection should be in the differential during the current pandemic, even in the absence of fever or cough,” the clinicians advise.
Case 2 is a 38-year-old man with cardiogenic shock and acute respiratory distress with profound hypoxia who was rescued with veno-arterial-venous extracorporeal membrane oxygenation (VV ECMO).
The initial presentation of this patient was more characteristic of severe COVID-19 disease, and cardiac involvement only became apparent after the initiation of ECMO, Fried and colleagues report.
Based on this case, they advise a “low threshold” to assess for cardiogenic shock in patients with acute systolic heart failure related to COVID-19. If inotropic support fails in these patients, intra-aortic balloon pump should be considered first for mechanical circulatory support because it requires the least maintenance from medical support staff.
In addition, in their experience, when a patient on VV ECMO develops superimposed cardiogenic shock, adding an arterial conduit at a relatively low blood flow rate may provide the necessary circulatory support without inducing left ventricular distension, they note.
“Our experience confirms that rescue of patients even with profound cardiogenic or mixed shock may be possible with temporary hemodynamic support at centers with availability of such devices,” Fried and colleagues report.
Case 3 is a 64-year-old woman with underlying cardiac disease who developed profound decompensation with COVID-19 infection.
This case demonstrates that the infection can cause decompensation of underlying heart failure and may lead to mixed shock, the clinicians say.
“Invasive hemodynamic monitoring, if feasible, may be helpful to manage the cardiac component of shock in such cases. Medications that prolong the QT interval are being considered for COVID-19 patients and may require closer monitoring in patients with underlying structural heart disease,” they note.
Case 4 is a 51-year-old man who underwent a heart transplant in 2007 and a kidney transplant in 2010. He had COVID-19 symptoms akin to those seen in nonimmunosuppressed patients with COVID-19.
The COVID-19 pandemic presents a “unique challenge” for solid organ transplant recipients, with only “limited” data on how to adjust immunosuppression during COVID-19 infection, Fried and colleagues say.
The pandemic also creates a challenge for the management of heart failure patients on the heart transplant wait list; the risks of delaying a transplant need to be balanced against the risks of donor infection and uncertainty regarding the impact of post-transplant immunosuppression protocols, they note.
As reported by Medscape Medical News, the American Heart Association has developed a COVID-19 patient registry to collect data on cardiovascular conditions and outcomes related to COVID-19 infection.
To participate in the registry, contact [email protected].
This article first appeared on Medscape.com.
It’s becoming clear that COVID-19 infection can involve the cardiovascular system in many different ways, and this has “evolving” potential implications for treatment, say a team of cardiologists on the frontlines of the COVID-19 battle in New York City.
In an article published online April 3 in Circulation, Justin Fried, MD, Division of Cardiology, Columbia University, New York City, and colleagues present four case studies of COVID-19 patients with various cardiovascular presentations.
Case 1 is a 64-year-old woman whose predominant symptoms on admission were cardiac in nature, including chest pain and ST elevation, but without fever, cough, or other symptoms suggestive of COVID-19.
“In patients presenting with what appears to be a typical cardiac syndrome, COVID-19 infection should be in the differential during the current pandemic, even in the absence of fever or cough,” the clinicians advise.
Case 2 is a 38-year-old man with cardiogenic shock and acute respiratory distress with profound hypoxia who was rescued with veno-arterial-venous extracorporeal membrane oxygenation (VV ECMO).
The initial presentation of this patient was more characteristic of severe COVID-19 disease, and cardiac involvement only became apparent after the initiation of ECMO, Fried and colleagues report.
Based on this case, they advise a “low threshold” to assess for cardiogenic shock in patients with acute systolic heart failure related to COVID-19. If inotropic support fails in these patients, intra-aortic balloon pump should be considered first for mechanical circulatory support because it requires the least maintenance from medical support staff.
In addition, in their experience, when a patient on VV ECMO develops superimposed cardiogenic shock, adding an arterial conduit at a relatively low blood flow rate may provide the necessary circulatory support without inducing left ventricular distension, they note.
“Our experience confirms that rescue of patients even with profound cardiogenic or mixed shock may be possible with temporary hemodynamic support at centers with availability of such devices,” Fried and colleagues report.
Case 3 is a 64-year-old woman with underlying cardiac disease who developed profound decompensation with COVID-19 infection.
This case demonstrates that the infection can cause decompensation of underlying heart failure and may lead to mixed shock, the clinicians say.
“Invasive hemodynamic monitoring, if feasible, may be helpful to manage the cardiac component of shock in such cases. Medications that prolong the QT interval are being considered for COVID-19 patients and may require closer monitoring in patients with underlying structural heart disease,” they note.
Case 4 is a 51-year-old man who underwent a heart transplant in 2007 and a kidney transplant in 2010. He had COVID-19 symptoms akin to those seen in nonimmunosuppressed patients with COVID-19.
The COVID-19 pandemic presents a “unique challenge” for solid organ transplant recipients, with only “limited” data on how to adjust immunosuppression during COVID-19 infection, Fried and colleagues say.
The pandemic also creates a challenge for the management of heart failure patients on the heart transplant wait list; the risks of delaying a transplant need to be balanced against the risks of donor infection and uncertainty regarding the impact of post-transplant immunosuppression protocols, they note.
As reported by Medscape Medical News, the American Heart Association has developed a COVID-19 patient registry to collect data on cardiovascular conditions and outcomes related to COVID-19 infection.
To participate in the registry, contact [email protected].
This article first appeared on Medscape.com.
It’s becoming clear that COVID-19 infection can involve the cardiovascular system in many different ways, and this has “evolving” potential implications for treatment, say a team of cardiologists on the frontlines of the COVID-19 battle in New York City.
In an article published online April 3 in Circulation, Justin Fried, MD, Division of Cardiology, Columbia University, New York City, and colleagues present four case studies of COVID-19 patients with various cardiovascular presentations.
Case 1 is a 64-year-old woman whose predominant symptoms on admission were cardiac in nature, including chest pain and ST elevation, but without fever, cough, or other symptoms suggestive of COVID-19.
“In patients presenting with what appears to be a typical cardiac syndrome, COVID-19 infection should be in the differential during the current pandemic, even in the absence of fever or cough,” the clinicians advise.
Case 2 is a 38-year-old man with cardiogenic shock and acute respiratory distress with profound hypoxia who was rescued with veno-arterial-venous extracorporeal membrane oxygenation (VV ECMO).
The initial presentation of this patient was more characteristic of severe COVID-19 disease, and cardiac involvement only became apparent after the initiation of ECMO, Fried and colleagues report.
Based on this case, they advise a “low threshold” to assess for cardiogenic shock in patients with acute systolic heart failure related to COVID-19. If inotropic support fails in these patients, intra-aortic balloon pump should be considered first for mechanical circulatory support because it requires the least maintenance from medical support staff.
In addition, in their experience, when a patient on VV ECMO develops superimposed cardiogenic shock, adding an arterial conduit at a relatively low blood flow rate may provide the necessary circulatory support without inducing left ventricular distension, they note.
“Our experience confirms that rescue of patients even with profound cardiogenic or mixed shock may be possible with temporary hemodynamic support at centers with availability of such devices,” Fried and colleagues report.
Case 3 is a 64-year-old woman with underlying cardiac disease who developed profound decompensation with COVID-19 infection.
This case demonstrates that the infection can cause decompensation of underlying heart failure and may lead to mixed shock, the clinicians say.
“Invasive hemodynamic monitoring, if feasible, may be helpful to manage the cardiac component of shock in such cases. Medications that prolong the QT interval are being considered for COVID-19 patients and may require closer monitoring in patients with underlying structural heart disease,” they note.
Case 4 is a 51-year-old man who underwent a heart transplant in 2007 and a kidney transplant in 2010. He had COVID-19 symptoms akin to those seen in nonimmunosuppressed patients with COVID-19.
The COVID-19 pandemic presents a “unique challenge” for solid organ transplant recipients, with only “limited” data on how to adjust immunosuppression during COVID-19 infection, Fried and colleagues say.
The pandemic also creates a challenge for the management of heart failure patients on the heart transplant wait list; the risks of delaying a transplant need to be balanced against the risks of donor infection and uncertainty regarding the impact of post-transplant immunosuppression protocols, they note.
As reported by Medscape Medical News, the American Heart Association has developed a COVID-19 patient registry to collect data on cardiovascular conditions and outcomes related to COVID-19 infection.
To participate in the registry, contact [email protected].
This article first appeared on Medscape.com.
Tips for self-care during the COVID-19 crisis
I think it’s fair to say, none of us have seen anything like this before. Yet here we are, and we must lead. We are many weeks into the COVID-19 crisis. We moved our offices home and tried not to miss a beat. Our patients need us more than ever – and in different ways.
Lest we become like the shoemaker’s daughter who has no shoes, let’s make sure we take care of ourselves. The shock waves from this pandemic are going to be massive and long lasting. I am already witnessing massive psychological growth on the part of my patients, and I hope, myself and my family. We must be strong as individuals and as a group of professionals.
Now more than ever, we need to set boundaries. So many are suffering. We must take stock of our own lives. Many of us are extremely fortunate. We have homes, families, and plenty of food. We are doctors performing essential services, and we can do so without risking our lives.
The priority is to make sure you are safe, and keeping your family and loved ones safe. As physicians, we have learned to distance ourselves from illness, but the coronavirus has affected us in disproportionate numbers.
To be physically and mentally strong, we must get enough sleep. This is exhausting for some and energizing for others. It is definitely a marathon not a sprint, so pace yourself. Eat well. This is no time for empty calories, and that goes for alcohol as well.
Create new routines. Exercise at the same time each day or perhaps twice a day. Try to be productive during certain hours, and relax at other times. Eat at similar times each day. We must strive to quickly create a “new normal” as we spend our days at home.
Find safe alternatives to your usual workout routine. Use YouTube and Instagram to help you find ways to stay fit in your own home. Ask friends for tips and consider sharing workout time with them via Zoom or FaceTime. New options are coming on line daily.
Make sure you are getting enough information to stay safe, and follow the advice of experts. Then turn off the news. I offer the same advice for financial worries. Try not to stress too much about finances right now. Most of us are feeling the pain of lost income and lost savings. Many of us have spouses or partners who suddenly found themselves out of work. Most likely, we will have ample ability to recover financially as we move forward and find ourselves with more work than ever.
Meditate. This may be advice you have been telling your patients for years but never found the time to try yourself. You can begin very simply with an app called Headspace or Calm. Google “5-minute meditation” on YouTube or find a meditation of any length you desire. If not now, when?
Reach out to one another. We can all use a caring word, or some humor or advice about how to move our practices online.
You may find your concentration is decreased, so be realistic in your expectations of yourself. I am finding shorter sessions more often are providing more comfort to some patients. Other patients are digging deeper than ever emotionally, and the work is becoming more rewarding.
Make sure you take a break to engage in positive activities. Read a book. Listen to soft music. Dim the lights. Watch the sunset, or be in nature if you can do so safely. Watch a TedTalk. Brush up on a foreign language. Take a deep breath. Journal. Puzzles, games, cooking, magazines, and humor all provide much needed respite from the stress. If you are lucky enough to be with family, try to take advantage of this unique time.
Try to avoid or minimize conflict with others. We need one another now more than ever. If you lose your cool, forgive yourself and make amends.
Even in these most challenging times, we must focus on what we are grateful for. Express gratitude to those around you as it will lift their mood as well. I know I am extremely grateful to be able to continue meaningful work when so many are unable to do so.
The next waves of this virus will be hitting our specialty directly so be strong and be prepared. It is an honor to serve, and we must rise to the occasion.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018), and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
I think it’s fair to say, none of us have seen anything like this before. Yet here we are, and we must lead. We are many weeks into the COVID-19 crisis. We moved our offices home and tried not to miss a beat. Our patients need us more than ever – and in different ways.
Lest we become like the shoemaker’s daughter who has no shoes, let’s make sure we take care of ourselves. The shock waves from this pandemic are going to be massive and long lasting. I am already witnessing massive psychological growth on the part of my patients, and I hope, myself and my family. We must be strong as individuals and as a group of professionals.
Now more than ever, we need to set boundaries. So many are suffering. We must take stock of our own lives. Many of us are extremely fortunate. We have homes, families, and plenty of food. We are doctors performing essential services, and we can do so without risking our lives.
The priority is to make sure you are safe, and keeping your family and loved ones safe. As physicians, we have learned to distance ourselves from illness, but the coronavirus has affected us in disproportionate numbers.
To be physically and mentally strong, we must get enough sleep. This is exhausting for some and energizing for others. It is definitely a marathon not a sprint, so pace yourself. Eat well. This is no time for empty calories, and that goes for alcohol as well.
Create new routines. Exercise at the same time each day or perhaps twice a day. Try to be productive during certain hours, and relax at other times. Eat at similar times each day. We must strive to quickly create a “new normal” as we spend our days at home.
Find safe alternatives to your usual workout routine. Use YouTube and Instagram to help you find ways to stay fit in your own home. Ask friends for tips and consider sharing workout time with them via Zoom or FaceTime. New options are coming on line daily.
Make sure you are getting enough information to stay safe, and follow the advice of experts. Then turn off the news. I offer the same advice for financial worries. Try not to stress too much about finances right now. Most of us are feeling the pain of lost income and lost savings. Many of us have spouses or partners who suddenly found themselves out of work. Most likely, we will have ample ability to recover financially as we move forward and find ourselves with more work than ever.
Meditate. This may be advice you have been telling your patients for years but never found the time to try yourself. You can begin very simply with an app called Headspace or Calm. Google “5-minute meditation” on YouTube or find a meditation of any length you desire. If not now, when?
Reach out to one another. We can all use a caring word, or some humor or advice about how to move our practices online.
You may find your concentration is decreased, so be realistic in your expectations of yourself. I am finding shorter sessions more often are providing more comfort to some patients. Other patients are digging deeper than ever emotionally, and the work is becoming more rewarding.
Make sure you take a break to engage in positive activities. Read a book. Listen to soft music. Dim the lights. Watch the sunset, or be in nature if you can do so safely. Watch a TedTalk. Brush up on a foreign language. Take a deep breath. Journal. Puzzles, games, cooking, magazines, and humor all provide much needed respite from the stress. If you are lucky enough to be with family, try to take advantage of this unique time.
Try to avoid or minimize conflict with others. We need one another now more than ever. If you lose your cool, forgive yourself and make amends.
Even in these most challenging times, we must focus on what we are grateful for. Express gratitude to those around you as it will lift their mood as well. I know I am extremely grateful to be able to continue meaningful work when so many are unable to do so.
The next waves of this virus will be hitting our specialty directly so be strong and be prepared. It is an honor to serve, and we must rise to the occasion.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018), and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
I think it’s fair to say, none of us have seen anything like this before. Yet here we are, and we must lead. We are many weeks into the COVID-19 crisis. We moved our offices home and tried not to miss a beat. Our patients need us more than ever – and in different ways.
Lest we become like the shoemaker’s daughter who has no shoes, let’s make sure we take care of ourselves. The shock waves from this pandemic are going to be massive and long lasting. I am already witnessing massive psychological growth on the part of my patients, and I hope, myself and my family. We must be strong as individuals and as a group of professionals.
Now more than ever, we need to set boundaries. So many are suffering. We must take stock of our own lives. Many of us are extremely fortunate. We have homes, families, and plenty of food. We are doctors performing essential services, and we can do so without risking our lives.
The priority is to make sure you are safe, and keeping your family and loved ones safe. As physicians, we have learned to distance ourselves from illness, but the coronavirus has affected us in disproportionate numbers.
To be physically and mentally strong, we must get enough sleep. This is exhausting for some and energizing for others. It is definitely a marathon not a sprint, so pace yourself. Eat well. This is no time for empty calories, and that goes for alcohol as well.
Create new routines. Exercise at the same time each day or perhaps twice a day. Try to be productive during certain hours, and relax at other times. Eat at similar times each day. We must strive to quickly create a “new normal” as we spend our days at home.
Find safe alternatives to your usual workout routine. Use YouTube and Instagram to help you find ways to stay fit in your own home. Ask friends for tips and consider sharing workout time with them via Zoom or FaceTime. New options are coming on line daily.
Make sure you are getting enough information to stay safe, and follow the advice of experts. Then turn off the news. I offer the same advice for financial worries. Try not to stress too much about finances right now. Most of us are feeling the pain of lost income and lost savings. Many of us have spouses or partners who suddenly found themselves out of work. Most likely, we will have ample ability to recover financially as we move forward and find ourselves with more work than ever.
Meditate. This may be advice you have been telling your patients for years but never found the time to try yourself. You can begin very simply with an app called Headspace or Calm. Google “5-minute meditation” on YouTube or find a meditation of any length you desire. If not now, when?
Reach out to one another. We can all use a caring word, or some humor or advice about how to move our practices online.
You may find your concentration is decreased, so be realistic in your expectations of yourself. I am finding shorter sessions more often are providing more comfort to some patients. Other patients are digging deeper than ever emotionally, and the work is becoming more rewarding.
Make sure you take a break to engage in positive activities. Read a book. Listen to soft music. Dim the lights. Watch the sunset, or be in nature if you can do so safely. Watch a TedTalk. Brush up on a foreign language. Take a deep breath. Journal. Puzzles, games, cooking, magazines, and humor all provide much needed respite from the stress. If you are lucky enough to be with family, try to take advantage of this unique time.
Try to avoid or minimize conflict with others. We need one another now more than ever. If you lose your cool, forgive yourself and make amends.
Even in these most challenging times, we must focus on what we are grateful for. Express gratitude to those around you as it will lift their mood as well. I know I am extremely grateful to be able to continue meaningful work when so many are unable to do so.
The next waves of this virus will be hitting our specialty directly so be strong and be prepared. It is an honor to serve, and we must rise to the occasion.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach, Fla. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018), and is the founder of the Bekindr Global Initiative, a movement aimed at cultivating kindness in the world. Dr. Ritvo also is the cofounder of the Bold Beauty Project, a nonprofit group that pairs women with disabilities with photographers who create art exhibitions to raise awareness.
AMA president calls for greater reliance on science in COVID-19 fight
The president of the American Medical Association is calling on politicians and the media to rely on science and evidence to help the public through the COVID-19 pandemic.
“We live in a time when misinformation, falsehoods, and outright lies spread like viruses online, through social media and even, at times, in the media at large,” Patrice A. Harris, MD, said during an April 7 address. “We have witnessed a concerning shift over the last several decades where policy decisions seem to be driven by ideology and politics instead of facts and evidence. The result is a growing mistrust in American institutions, in science, and in the counsel of leading experts whose lives are dedicated to the pursuit of evidence and reason.”
To that end, she called on everyone – from politicians to the general public – to trust the scientific evidence.
Dr. Harris noted that the scientific data on COVID-19 have already yielded important lessons about who is more likely to be affected and how easily the virus can spread. The data also point to the effectiveness of stay-at-home and shelter-in-place orders. “This is our best chance to slow the spread of the virus,” she said, adding that the enhanced emphasis on hand washing and other hygiene practices “may seem ‘simplistic,’ but they are, in fact, based in science and evidence.”
And, as the pandemic continues, Dr. Harris said that now is the time to rely on science. She said the AMA “calls on all elected officials to affirm science, evidence, and fact in their words and actions,” and she urged that the government’s scientific institutions be led by experts who are “protected from political influence.”
It is incumbent upon everyone to actively work to contain and stop the spread of misinformation related to COVID-19, she said. “We must ensure the war is against the virus and not against science,” Dr. Harris said.
The president of the American Medical Association is calling on politicians and the media to rely on science and evidence to help the public through the COVID-19 pandemic.
“We live in a time when misinformation, falsehoods, and outright lies spread like viruses online, through social media and even, at times, in the media at large,” Patrice A. Harris, MD, said during an April 7 address. “We have witnessed a concerning shift over the last several decades where policy decisions seem to be driven by ideology and politics instead of facts and evidence. The result is a growing mistrust in American institutions, in science, and in the counsel of leading experts whose lives are dedicated to the pursuit of evidence and reason.”
To that end, she called on everyone – from politicians to the general public – to trust the scientific evidence.
Dr. Harris noted that the scientific data on COVID-19 have already yielded important lessons about who is more likely to be affected and how easily the virus can spread. The data also point to the effectiveness of stay-at-home and shelter-in-place orders. “This is our best chance to slow the spread of the virus,” she said, adding that the enhanced emphasis on hand washing and other hygiene practices “may seem ‘simplistic,’ but they are, in fact, based in science and evidence.”
And, as the pandemic continues, Dr. Harris said that now is the time to rely on science. She said the AMA “calls on all elected officials to affirm science, evidence, and fact in their words and actions,” and she urged that the government’s scientific institutions be led by experts who are “protected from political influence.”
It is incumbent upon everyone to actively work to contain and stop the spread of misinformation related to COVID-19, she said. “We must ensure the war is against the virus and not against science,” Dr. Harris said.
The president of the American Medical Association is calling on politicians and the media to rely on science and evidence to help the public through the COVID-19 pandemic.
“We live in a time when misinformation, falsehoods, and outright lies spread like viruses online, through social media and even, at times, in the media at large,” Patrice A. Harris, MD, said during an April 7 address. “We have witnessed a concerning shift over the last several decades where policy decisions seem to be driven by ideology and politics instead of facts and evidence. The result is a growing mistrust in American institutions, in science, and in the counsel of leading experts whose lives are dedicated to the pursuit of evidence and reason.”
To that end, she called on everyone – from politicians to the general public – to trust the scientific evidence.
Dr. Harris noted that the scientific data on COVID-19 have already yielded important lessons about who is more likely to be affected and how easily the virus can spread. The data also point to the effectiveness of stay-at-home and shelter-in-place orders. “This is our best chance to slow the spread of the virus,” she said, adding that the enhanced emphasis on hand washing and other hygiene practices “may seem ‘simplistic,’ but they are, in fact, based in science and evidence.”
And, as the pandemic continues, Dr. Harris said that now is the time to rely on science. She said the AMA “calls on all elected officials to affirm science, evidence, and fact in their words and actions,” and she urged that the government’s scientific institutions be led by experts who are “protected from political influence.”
It is incumbent upon everyone to actively work to contain and stop the spread of misinformation related to COVID-19, she said. “We must ensure the war is against the virus and not against science,” Dr. Harris said.







