User login
Formerly Skin & Allergy News
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')]
The leading independent newspaper covering dermatology news and commentary.
Common drug with lots of surprising side effects
A 55-year-old woman comes to clinic for follow-up. She reports her family is worried that she isn’t getting enough sleep and is more tired than usual. The patient reports she is sleeping 8 hours a night and wakes up feeling rested, but she has noticed she has been yawning much more frequently than she remembers in the past.
Past medical history: gastroesophageal reflux disease, hypertension, generalized anxiety disorder, hypothyroidism, and osteoporosis. Medications: amlodipine, lansoprazole, irbesartan, escitalopram, levothyroxine, and alendronate. Physical examination: blood pressure 110/70 mm Hg, pulse 60 bpm. Lower extremities: 1+ edema.
What is the likely cause of her increased yawning?
A. Amlodipine.
B. Alendronate.
C. Irbesartan.
D. Escitalopram.
E. Lansoprazole.
The correct answer here is escitalopram. Selective serotonin reuptake inhibitors in general are well tolerated. Given how commonly these drugs are used, however, there are a number of lesser-known side effects that you are likely to see.
In the above case, this patient has yawning caused by her SSRI. Roncero et al. described a case of yawning in a patient on escitalopram that resolved when the dose of escitalopram was reduced.1 Paroxetine has been reported to cause yawning at both low and high doses.2
In a review of drug-induced yawning, SSRIs as a class were most frequently involved, and sertraline and fluoxetine were implicated in addition to paroxetine.3 The serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine have also been associated with yawning.4,5
Hyperhydrosis has also been linked to SSRIs and SNRIs, and both yawning and hyperhidrosis may occur because of an underlying thermoregulatory dysfunction.6
SSRIs have been linked to increased bleeding risk, especially increased risk of upper gastrointestinal hemorrhage. Laporte and colleagues showed an association of SSRI use and risk of bleeding in a meta-analysis of 42 observational studies, with an odds ratio of 1.41 (95% confidence interval, 1.27-1.57; P less than .0001).7 The risk of upper gastrointestinal (UGI) bleeding is further increased if patients are also taking NSAIDs.
Anglin et al. looked at 15 case-control studies and 4 cohort studies and found an OR of 1.66 for UGI bleeding with SSRI use, and an OR of 4.25 for UGI bleeding if SSRI use was combined with NSAID use.8 The number needed to harm is 3,177 for NSAID use in populations at low risk for GI bleeding, but it is much lower (881) in higher-risk populations.8 Make sure to think about patients’ bleeding risks when starting SSRIs.
An issue that comes up frequently is: What is the risk of bleeding in patients on SSRIs who are also on anticoagulants? Dr. Quinn and colleagues looked at the bleeding risk of anticoagulated patients also taking SSRIs in the ROCKET AF trial.9 They found 737 patients who received SSRIs and matched them with other patients not on SSRIs in the trial. All patients in the trial were either receiving rivaroxaban or warfarin for stroke prophylaxis. They found no significant increase risk in bleeding in the patients on SSRIs and anticoagulants.
Take-home points:
- Yawning and hyperhidrosis are interesting side effects of SSRIs.
- Bleeding risk is increased in patients on SSRIs, especially when combined with NSAIDs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Neurologia. 2013 Nov-Dec;28(9):589-90.
2. Psychiatry Clin Neurosci. 2006 Apr;60(2):260.
3. Presse Med. 2014 Oct;43(10 Pt 1):1135-6.
4. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33(4):747.
5. Ann Pharmacother. 2011 Oct;45(10):1297-301.
6. Depress Anxiety. 2017 Dec;34(12):1134-46.
7. Pharmacol Res. 2017 Apr;118:19-32.
8. Am J Gastroenterol. 2014 Jun;109(6):811-9.
9. J Am Heart Assoc. 2018 Aug 7;7(15):e008755.
A 55-year-old woman comes to clinic for follow-up. She reports her family is worried that she isn’t getting enough sleep and is more tired than usual. The patient reports she is sleeping 8 hours a night and wakes up feeling rested, but she has noticed she has been yawning much more frequently than she remembers in the past.
Past medical history: gastroesophageal reflux disease, hypertension, generalized anxiety disorder, hypothyroidism, and osteoporosis. Medications: amlodipine, lansoprazole, irbesartan, escitalopram, levothyroxine, and alendronate. Physical examination: blood pressure 110/70 mm Hg, pulse 60 bpm. Lower extremities: 1+ edema.
What is the likely cause of her increased yawning?
A. Amlodipine.
B. Alendronate.
C. Irbesartan.
D. Escitalopram.
E. Lansoprazole.
The correct answer here is escitalopram. Selective serotonin reuptake inhibitors in general are well tolerated. Given how commonly these drugs are used, however, there are a number of lesser-known side effects that you are likely to see.
In the above case, this patient has yawning caused by her SSRI. Roncero et al. described a case of yawning in a patient on escitalopram that resolved when the dose of escitalopram was reduced.1 Paroxetine has been reported to cause yawning at both low and high doses.2
In a review of drug-induced yawning, SSRIs as a class were most frequently involved, and sertraline and fluoxetine were implicated in addition to paroxetine.3 The serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine have also been associated with yawning.4,5
Hyperhydrosis has also been linked to SSRIs and SNRIs, and both yawning and hyperhidrosis may occur because of an underlying thermoregulatory dysfunction.6
SSRIs have been linked to increased bleeding risk, especially increased risk of upper gastrointestinal hemorrhage. Laporte and colleagues showed an association of SSRI use and risk of bleeding in a meta-analysis of 42 observational studies, with an odds ratio of 1.41 (95% confidence interval, 1.27-1.57; P less than .0001).7 The risk of upper gastrointestinal (UGI) bleeding is further increased if patients are also taking NSAIDs.
Anglin et al. looked at 15 case-control studies and 4 cohort studies and found an OR of 1.66 for UGI bleeding with SSRI use, and an OR of 4.25 for UGI bleeding if SSRI use was combined with NSAID use.8 The number needed to harm is 3,177 for NSAID use in populations at low risk for GI bleeding, but it is much lower (881) in higher-risk populations.8 Make sure to think about patients’ bleeding risks when starting SSRIs.
An issue that comes up frequently is: What is the risk of bleeding in patients on SSRIs who are also on anticoagulants? Dr. Quinn and colleagues looked at the bleeding risk of anticoagulated patients also taking SSRIs in the ROCKET AF trial.9 They found 737 patients who received SSRIs and matched them with other patients not on SSRIs in the trial. All patients in the trial were either receiving rivaroxaban or warfarin for stroke prophylaxis. They found no significant increase risk in bleeding in the patients on SSRIs and anticoagulants.
Take-home points:
- Yawning and hyperhidrosis are interesting side effects of SSRIs.
- Bleeding risk is increased in patients on SSRIs, especially when combined with NSAIDs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Neurologia. 2013 Nov-Dec;28(9):589-90.
2. Psychiatry Clin Neurosci. 2006 Apr;60(2):260.
3. Presse Med. 2014 Oct;43(10 Pt 1):1135-6.
4. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33(4):747.
5. Ann Pharmacother. 2011 Oct;45(10):1297-301.
6. Depress Anxiety. 2017 Dec;34(12):1134-46.
7. Pharmacol Res. 2017 Apr;118:19-32.
8. Am J Gastroenterol. 2014 Jun;109(6):811-9.
9. J Am Heart Assoc. 2018 Aug 7;7(15):e008755.
A 55-year-old woman comes to clinic for follow-up. She reports her family is worried that she isn’t getting enough sleep and is more tired than usual. The patient reports she is sleeping 8 hours a night and wakes up feeling rested, but she has noticed she has been yawning much more frequently than she remembers in the past.
Past medical history: gastroesophageal reflux disease, hypertension, generalized anxiety disorder, hypothyroidism, and osteoporosis. Medications: amlodipine, lansoprazole, irbesartan, escitalopram, levothyroxine, and alendronate. Physical examination: blood pressure 110/70 mm Hg, pulse 60 bpm. Lower extremities: 1+ edema.
What is the likely cause of her increased yawning?
A. Amlodipine.
B. Alendronate.
C. Irbesartan.
D. Escitalopram.
E. Lansoprazole.
The correct answer here is escitalopram. Selective serotonin reuptake inhibitors in general are well tolerated. Given how commonly these drugs are used, however, there are a number of lesser-known side effects that you are likely to see.
In the above case, this patient has yawning caused by her SSRI. Roncero et al. described a case of yawning in a patient on escitalopram that resolved when the dose of escitalopram was reduced.1 Paroxetine has been reported to cause yawning at both low and high doses.2
In a review of drug-induced yawning, SSRIs as a class were most frequently involved, and sertraline and fluoxetine were implicated in addition to paroxetine.3 The serotonin-norepinephrine reuptake inhibitors duloxetine and venlafaxine have also been associated with yawning.4,5
Hyperhydrosis has also been linked to SSRIs and SNRIs, and both yawning and hyperhidrosis may occur because of an underlying thermoregulatory dysfunction.6
SSRIs have been linked to increased bleeding risk, especially increased risk of upper gastrointestinal hemorrhage. Laporte and colleagues showed an association of SSRI use and risk of bleeding in a meta-analysis of 42 observational studies, with an odds ratio of 1.41 (95% confidence interval, 1.27-1.57; P less than .0001).7 The risk of upper gastrointestinal (UGI) bleeding is further increased if patients are also taking NSAIDs.
Anglin et al. looked at 15 case-control studies and 4 cohort studies and found an OR of 1.66 for UGI bleeding with SSRI use, and an OR of 4.25 for UGI bleeding if SSRI use was combined with NSAID use.8 The number needed to harm is 3,177 for NSAID use in populations at low risk for GI bleeding, but it is much lower (881) in higher-risk populations.8 Make sure to think about patients’ bleeding risks when starting SSRIs.
An issue that comes up frequently is: What is the risk of bleeding in patients on SSRIs who are also on anticoagulants? Dr. Quinn and colleagues looked at the bleeding risk of anticoagulated patients also taking SSRIs in the ROCKET AF trial.9 They found 737 patients who received SSRIs and matched them with other patients not on SSRIs in the trial. All patients in the trial were either receiving rivaroxaban or warfarin for stroke prophylaxis. They found no significant increase risk in bleeding in the patients on SSRIs and anticoagulants.
Take-home points:
- Yawning and hyperhidrosis are interesting side effects of SSRIs.
- Bleeding risk is increased in patients on SSRIs, especially when combined with NSAIDs.
Dr. Paauw is professor of medicine in the division of general internal medicine at the University of Washington, Seattle, and he serves as third-year medical student clerkship director at the University of Washington. Contact Dr. Paauw at [email protected].
References
1. Neurologia. 2013 Nov-Dec;28(9):589-90.
2. Psychiatry Clin Neurosci. 2006 Apr;60(2):260.
3. Presse Med. 2014 Oct;43(10 Pt 1):1135-6.
4. Prog Neuropsychopharmacol Biol Psychiatry. 2009 Jun 15;33(4):747.
5. Ann Pharmacother. 2011 Oct;45(10):1297-301.
6. Depress Anxiety. 2017 Dec;34(12):1134-46.
7. Pharmacol Res. 2017 Apr;118:19-32.
8. Am J Gastroenterol. 2014 Jun;109(6):811-9.
9. J Am Heart Assoc. 2018 Aug 7;7(15):e008755.
ACP maps two potential paths to universal health care
The American College of Physicians is recommending either a single-payer system or a public option within a regulated private insurance system to help deliver universal and affordable access to health care for all Americans.
“We came to the conclusion that two directions or approaches could get us to where we need to be,” ACP President Robert McLean, MD, said in an interview. “We need ... a system that provides universal, affordable access to care.”
After examining the evidence, ACP discarded one option: a direct market-based approach.
“Direct market-based approaches won’t work,” Dr. McLean explained. “If you look at where direct marketplace approaches ... have been implemented, they just will not get you to a place where you are going to get universal coverage, portability, essential benefits, and preexisting condition protection and administrative simplification.”
Dr. McLean highlighted two paths that could achieve universal coverage and better access to health care: a single-payer–financed system, or a publicly financed coverage option within a system of regulated private insurance.
It’s the first time ACP has endorsed a single-payer approach. The college supported the public option that wasn’t included as part of the Affordable Care Act. But ACP’s latest publicly financed proposal offers a deeper level of detail on how to make that option work in the context of a private insurance system.
While the health reform conversation may be a political, ACP doesn’t want to make it a partisan one. ACP’s policy recommendations represent a carefully researched series of ideas backed by evidence-based research, Dr. McLean said.
“There is a lot of nuance behind” the two recommendations, he noted, and those nuances are explored in a series of articles and editorials published Jan. 21 in Annals of Internal Medicine.
Sizing up single payer
The ACP acknowledges that for its single-payer system, the transition could be “politically difficult and strain the federal budget,” according to Ryan A. Crowley, senior analyst at ACP, and colleagues in an article outlining the organization’s vision. “Taxes would probably replace premiums, and private insurance would have a reduced role or be eliminated altogether.”
However, the authors note that a single-payer system could be designed to address concerns from a generally skeptical public, such as providing bulk funding or setting minimum standards to guide state operations. It also could include private insurance to provide supplemental coverage.
Even so, “adopting a single-payer system would be highly disruptive and could lead to price controls that would perpetuate flaws in the current Medicare payment system, including the undervaluation of primary care,” Mr. Crowley and colleagues wrote. “If prices are set too low, it could lead to shortages and longer wait times for services. Without sufficient cost controls, however, the cost of a single-payer system could be too high to be feasible.”
Pondering the public option
Given a single-payer plan’s potential challenges, ACP also is endorsing a public option model, which provides the choice of a government-sponsored health insurance plan to compete with existing private insurance options.
“Depending on its structure and implementation, a public choice (or public option) model available to all could help to achieve universal coverage, better access, and improved outcomes without the disruption of a single-payer approach,” the ACP authors noted.
The public option has its own drawbacks, they acknowledge. Those include an inability to achieve better savings on prescription drugs, compared with a single-payer system. The public option approach also doesn’t do away with the current administrative burden, and access issues related to narrow provider networks would persist.
Dr. McLean noted that a more highly regulated insurance market would be needed to help make the public option model work.
“Insurance companies don’t have regulation in a lot of things that they do,” Dr. McLean said. “We see that as quite problematic. They are kind of running amok at this point.”
Expanding the role of primary care
In either reform scenario, primary care would play a much greater role.
“We need to promote primary care,” Dr. McLean said. That includes better incentives to draw physicians to it. “We have to pay them enough,” he added.
The health care models will need to move away from higher pay to specialties for high-cost, high-volume procedural reimbursement. And they’ll need to recognize the need for placing a higher value on the cognitive services provided at the primary care level.
Also in need of change: physicians’ administrative burdens. Reforms need to address the burden created by value-based care and the poor application and misapplication of quality measures.
Migration to a single-payer environment could would make reducing the administrative burden a lot easier, Dr. McLean said. But it also could be done with a public option approach.
That’s where regulators can play a big role in working with insurers to help address administrative burden – streamlining prior authorization of procedures, the types of forms used, and other policies, Dr. McLean explained.
“The number of insurers and their ability to have their own rules and regulations [make it] incredibly complex for patients as well as physicians trying to figure out how to deliver the care that they need,” he noted.
Dr. McLean hopes that the ACP’s papers will spark conversation, particularly among legislators and regulators.
“The bottom line is we cannot afford to not do something bold,” he cautioned. “It is just not working. Our patients deserve better, and we can do better.”
The American College of Physicians is recommending either a single-payer system or a public option within a regulated private insurance system to help deliver universal and affordable access to health care for all Americans.
“We came to the conclusion that two directions or approaches could get us to where we need to be,” ACP President Robert McLean, MD, said in an interview. “We need ... a system that provides universal, affordable access to care.”
After examining the evidence, ACP discarded one option: a direct market-based approach.
“Direct market-based approaches won’t work,” Dr. McLean explained. “If you look at where direct marketplace approaches ... have been implemented, they just will not get you to a place where you are going to get universal coverage, portability, essential benefits, and preexisting condition protection and administrative simplification.”
Dr. McLean highlighted two paths that could achieve universal coverage and better access to health care: a single-payer–financed system, or a publicly financed coverage option within a system of regulated private insurance.
It’s the first time ACP has endorsed a single-payer approach. The college supported the public option that wasn’t included as part of the Affordable Care Act. But ACP’s latest publicly financed proposal offers a deeper level of detail on how to make that option work in the context of a private insurance system.
While the health reform conversation may be a political, ACP doesn’t want to make it a partisan one. ACP’s policy recommendations represent a carefully researched series of ideas backed by evidence-based research, Dr. McLean said.
“There is a lot of nuance behind” the two recommendations, he noted, and those nuances are explored in a series of articles and editorials published Jan. 21 in Annals of Internal Medicine.
Sizing up single payer
The ACP acknowledges that for its single-payer system, the transition could be “politically difficult and strain the federal budget,” according to Ryan A. Crowley, senior analyst at ACP, and colleagues in an article outlining the organization’s vision. “Taxes would probably replace premiums, and private insurance would have a reduced role or be eliminated altogether.”
However, the authors note that a single-payer system could be designed to address concerns from a generally skeptical public, such as providing bulk funding or setting minimum standards to guide state operations. It also could include private insurance to provide supplemental coverage.
Even so, “adopting a single-payer system would be highly disruptive and could lead to price controls that would perpetuate flaws in the current Medicare payment system, including the undervaluation of primary care,” Mr. Crowley and colleagues wrote. “If prices are set too low, it could lead to shortages and longer wait times for services. Without sufficient cost controls, however, the cost of a single-payer system could be too high to be feasible.”
Pondering the public option
Given a single-payer plan’s potential challenges, ACP also is endorsing a public option model, which provides the choice of a government-sponsored health insurance plan to compete with existing private insurance options.
“Depending on its structure and implementation, a public choice (or public option) model available to all could help to achieve universal coverage, better access, and improved outcomes without the disruption of a single-payer approach,” the ACP authors noted.
The public option has its own drawbacks, they acknowledge. Those include an inability to achieve better savings on prescription drugs, compared with a single-payer system. The public option approach also doesn’t do away with the current administrative burden, and access issues related to narrow provider networks would persist.
Dr. McLean noted that a more highly regulated insurance market would be needed to help make the public option model work.
“Insurance companies don’t have regulation in a lot of things that they do,” Dr. McLean said. “We see that as quite problematic. They are kind of running amok at this point.”
Expanding the role of primary care
In either reform scenario, primary care would play a much greater role.
“We need to promote primary care,” Dr. McLean said. That includes better incentives to draw physicians to it. “We have to pay them enough,” he added.
The health care models will need to move away from higher pay to specialties for high-cost, high-volume procedural reimbursement. And they’ll need to recognize the need for placing a higher value on the cognitive services provided at the primary care level.
Also in need of change: physicians’ administrative burdens. Reforms need to address the burden created by value-based care and the poor application and misapplication of quality measures.
Migration to a single-payer environment could would make reducing the administrative burden a lot easier, Dr. McLean said. But it also could be done with a public option approach.
That’s where regulators can play a big role in working with insurers to help address administrative burden – streamlining prior authorization of procedures, the types of forms used, and other policies, Dr. McLean explained.
“The number of insurers and their ability to have their own rules and regulations [make it] incredibly complex for patients as well as physicians trying to figure out how to deliver the care that they need,” he noted.
Dr. McLean hopes that the ACP’s papers will spark conversation, particularly among legislators and regulators.
“The bottom line is we cannot afford to not do something bold,” he cautioned. “It is just not working. Our patients deserve better, and we can do better.”
The American College of Physicians is recommending either a single-payer system or a public option within a regulated private insurance system to help deliver universal and affordable access to health care for all Americans.
“We came to the conclusion that two directions or approaches could get us to where we need to be,” ACP President Robert McLean, MD, said in an interview. “We need ... a system that provides universal, affordable access to care.”
After examining the evidence, ACP discarded one option: a direct market-based approach.
“Direct market-based approaches won’t work,” Dr. McLean explained. “If you look at where direct marketplace approaches ... have been implemented, they just will not get you to a place where you are going to get universal coverage, portability, essential benefits, and preexisting condition protection and administrative simplification.”
Dr. McLean highlighted two paths that could achieve universal coverage and better access to health care: a single-payer–financed system, or a publicly financed coverage option within a system of regulated private insurance.
It’s the first time ACP has endorsed a single-payer approach. The college supported the public option that wasn’t included as part of the Affordable Care Act. But ACP’s latest publicly financed proposal offers a deeper level of detail on how to make that option work in the context of a private insurance system.
While the health reform conversation may be a political, ACP doesn’t want to make it a partisan one. ACP’s policy recommendations represent a carefully researched series of ideas backed by evidence-based research, Dr. McLean said.
“There is a lot of nuance behind” the two recommendations, he noted, and those nuances are explored in a series of articles and editorials published Jan. 21 in Annals of Internal Medicine.
Sizing up single payer
The ACP acknowledges that for its single-payer system, the transition could be “politically difficult and strain the federal budget,” according to Ryan A. Crowley, senior analyst at ACP, and colleagues in an article outlining the organization’s vision. “Taxes would probably replace premiums, and private insurance would have a reduced role or be eliminated altogether.”
However, the authors note that a single-payer system could be designed to address concerns from a generally skeptical public, such as providing bulk funding or setting minimum standards to guide state operations. It also could include private insurance to provide supplemental coverage.
Even so, “adopting a single-payer system would be highly disruptive and could lead to price controls that would perpetuate flaws in the current Medicare payment system, including the undervaluation of primary care,” Mr. Crowley and colleagues wrote. “If prices are set too low, it could lead to shortages and longer wait times for services. Without sufficient cost controls, however, the cost of a single-payer system could be too high to be feasible.”
Pondering the public option
Given a single-payer plan’s potential challenges, ACP also is endorsing a public option model, which provides the choice of a government-sponsored health insurance plan to compete with existing private insurance options.
“Depending on its structure and implementation, a public choice (or public option) model available to all could help to achieve universal coverage, better access, and improved outcomes without the disruption of a single-payer approach,” the ACP authors noted.
The public option has its own drawbacks, they acknowledge. Those include an inability to achieve better savings on prescription drugs, compared with a single-payer system. The public option approach also doesn’t do away with the current administrative burden, and access issues related to narrow provider networks would persist.
Dr. McLean noted that a more highly regulated insurance market would be needed to help make the public option model work.
“Insurance companies don’t have regulation in a lot of things that they do,” Dr. McLean said. “We see that as quite problematic. They are kind of running amok at this point.”
Expanding the role of primary care
In either reform scenario, primary care would play a much greater role.
“We need to promote primary care,” Dr. McLean said. That includes better incentives to draw physicians to it. “We have to pay them enough,” he added.
The health care models will need to move away from higher pay to specialties for high-cost, high-volume procedural reimbursement. And they’ll need to recognize the need for placing a higher value on the cognitive services provided at the primary care level.
Also in need of change: physicians’ administrative burdens. Reforms need to address the burden created by value-based care and the poor application and misapplication of quality measures.
Migration to a single-payer environment could would make reducing the administrative burden a lot easier, Dr. McLean said. But it also could be done with a public option approach.
That’s where regulators can play a big role in working with insurers to help address administrative burden – streamlining prior authorization of procedures, the types of forms used, and other policies, Dr. McLean explained.
“The number of insurers and their ability to have their own rules and regulations [make it] incredibly complex for patients as well as physicians trying to figure out how to deliver the care that they need,” he noted.
Dr. McLean hopes that the ACP’s papers will spark conversation, particularly among legislators and regulators.
“The bottom line is we cannot afford to not do something bold,” he cautioned. “It is just not working. Our patients deserve better, and we can do better.”
FROM ANNALS OF INTERNAL MEDICINE
CD1a and cosmetic-related contact dermatitis
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
As industries develop more chemical extraction techniques for synthetic or purified botanical ingredients to include in cosmetic and personal care products, the incidence of contact dermatitis is rising. Contact dermatitis (irritant or allergic) is the most common occupational skin disease, with current lifetime incidence exceeding 50%. For allergic contact dermatitis, type IV hypersensitivity (or delayed-type hypersensitivity) is thought to be the immunologic mediated pathway in which a T cell–mediated response occurs approximately 72 hours after exposure to the contact allergen. Diagnosis currently is predominately made clinically, after identifying the potential allergen or via patch testing. Treatment typically involves topical steroids or anti-inflammatories should a rash occur, and avoidance of the identified allergen.
In delayed-type hypersensitivity, most T-cell receptors recognize a peptide antigen bound to major histocompatibility complex (MHC) I or MHC II proteins, which stimulates a subsequent inflammatory immune response. However, in a recently published study, the authors wrote that “most known contact allergens are nonpeptidic small molecules, cations, or metals that are typically delivered to skin as drugs, oils, cosmetics, skin creams, or fragrances.” The chemical nature and structure of contact allergens “does not match the chemical structures of most antigens commonly recognized within the TCR-peptide-MHC axis,” they added. Thus, the mechanism by which nonpeptide molecules found in cosmetics cause a T cell–mediated hypersensitivity is poorly understood.
In that study, investigators from Brigham and Women’s Hospital, Boston; Columbia University, New York; and Monash University, Melbourne, looked at whether a protein found in immune cells – CD1a – could be involved in these allergic reactions. In a press release describing the results, cosenior author D. Branch Moody, MD, a principal investigator and physician in Brigham and Women’s division of rheumatology, inflammation, and immunity, noted that they “questioned the prevailing paradigm that T cell–mediated allergic reaction is only triggered when T cells respond to proteins or peptide antigens,” and found “a mechanism through which fragrance can initiate a T-cell response through a protein called CD1a.”
In their study, CD1a was identified as the and personal care products. Specifically, balsam of Peru (a tree oil commonly found in cosmetics and toothpaste), benzyl benzoate, benzyl cinnamate, and farnesol (often present in “fragrance”) after positive patch tests were found to elicit a CD1a-mediated immune response. Their findings suggest that, for these hydrophobic contact allergens, in forming CD1a-farnesol (or other) complexes, displacement of self-lipids normally bound to CD1a occurs, exposing T cell–stimulatory surface regions of CD1a that are normally hidden, thereby eliciting T cell–mediated hypersensitivity reactions.
The authors note that having a better understanding of how these ingredients elicit an immune response on a molecular level can help us potentially identify other molecules that can potentially block this response in humans, thereby treating or potentially mitigating allergic skin disease from these ingredients.
Dr. Wesley and Dr. Talakoub are cocontributors to this column. Dr. Wesley practices dermatology in Beverly Hills, Calif. Dr. Talakoub is in private practice in McLean, Va. This month’s column is by Dr. Wesley. Write to them at [email protected]. They had no relevant disclosures.
Resource
Nicolai S et al. Sci Immunol. 2020 Jan 3. doi: 10.1126/sciimmunol.aax5430.
Medscape survey points to generational differences in physician burnout
Burnout among physicians appears to have decreased slightly in the past few years, but remains a significant problem for the medical profession, according to the Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide.
A survey of more than 15,000 physicians revealed that 42% reported being burned out, down from 46% who responded to the survey 5 years ago. However, there are variations in the rates based on certain demographic factors such as specialty, age, and gender.
Urology sits at the top of the list as the specialty that is experiencing the highest rate of burnout, with 54% of urologists responding to the survey reporting burnout. Neurology and nephrology followed with rates of burnout at 50% and 49%, respectively. The next five specialties on the list all reported burnout rates of 46%: diabetes and endocrinology, family medicine, radiology, ob.gyn., and rheumatology. Pulmonology specialists reported a burnout rate of 41%. Gastroenterologists reported burnout rates of 37%.
The survey divided participants into three age categories – Millennial (ages 25-39 years), Generation X (ages 40-54 years), and Baby Boomer (ages 55-73 years). Both Millennials and Baby Boomers reported similar rates of burnout (38% and 39%, respectively) and those in Generation X reported a higher rate of burnout (48%).
This higher rate is not unexpected. The survey results cite Carol Bernstein, MD, of the Albert Einstein College of Medicine, New York, as noting that midcareer “is typically the time of highest burnout, which is where Gen Xers are in their career trajectory, suggesting a number of factors outside of work such as caring for children and elderly parents, planning for retirement, can play a role in contributing to burnout.”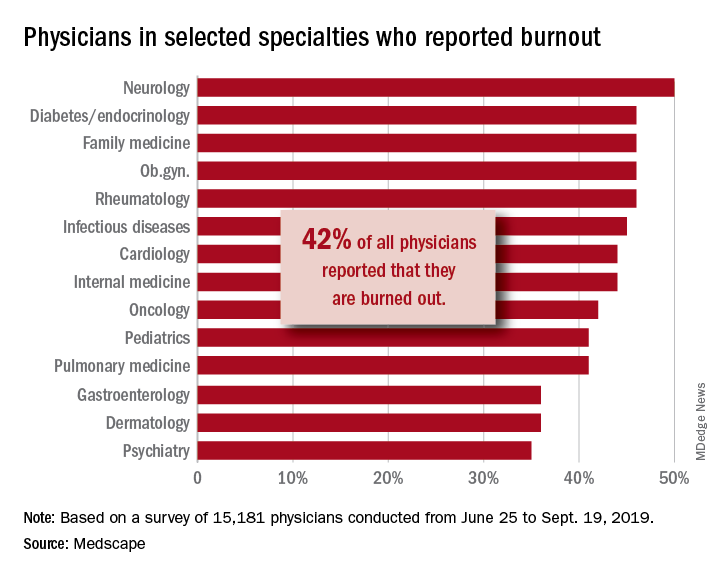
Women also reported a higher rate of burnout, although the rate has dropped from the survey conducted 5 years ago. The rate of burnout among women reported for the 2020 survey was 48%, down from 51% reported 5 years ago. By comparison, the rate of burnout for men was 37% in 2020, down from 43% in 2015.
In terms of what is causing burnout, the biggest contributor is the bureaucratic tasks (charting and paperwork, for example) that physicians must complete, which 55% of respondents to the survey said was the leading cause of burnout. Next was spending too many hours at work (33%); lack of respect from administrators, employers, colleagues, and staff (32%); and the increased computerization of the practice, including the use of electronic health records (30%).
When broken down by age category, the bureaucratic tasks was tops in all three groups (57% for Millennials, 56% for Generation X, and 54% for Baby Boomers), but what ranks next differs slightly by age group. For Millennials, the next two factors were too many hours at work (38%) and lack of respect (35%). Generation X respondents cited the same two factors, both at 33%. Baby Boomers cited computerization as their second-highest factor (41%) and spending too many hours at work as the third-highest factor (31%).
The generations had different approaches to coping with burnout. Millennials (56%) reported sleep as their top-ranked coping strategy, while Gen Xers and Baby Boomers ranked exercise and personal isolation as their top choice. For these two older groups, sleep was ranked last, after other activities such as talking with family and friends.
The survey also asked about depression, and respondents reported a similar rate across all age groups (15%, 18%, and 16%, respectively). Among those who said they were depressed, the three age groups had similar rates of suicidal thoughts (21%, 24%, and 22%).
Perhaps the most striking finding of the survey is the number of physicians who would take a pay cut to achieve a better work-life balance. Among Millennials, 52% would accept a pay cut, compared with 48% of Generation X and 49% of Baby Boomers. A surprising number (36%, 34%, and 31%, respectively, reported that they would accept a $10,000-$20,000 pay cut to have a 20% reduction in work hours. [email protected]
*This story was updated on 1/22/2020.
SOURCE: Kane L et al. Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide. Medscape. 2020 Jan 15.
Burnout among physicians appears to have decreased slightly in the past few years, but remains a significant problem for the medical profession, according to the Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide.
A survey of more than 15,000 physicians revealed that 42% reported being burned out, down from 46% who responded to the survey 5 years ago. However, there are variations in the rates based on certain demographic factors such as specialty, age, and gender.
Urology sits at the top of the list as the specialty that is experiencing the highest rate of burnout, with 54% of urologists responding to the survey reporting burnout. Neurology and nephrology followed with rates of burnout at 50% and 49%, respectively. The next five specialties on the list all reported burnout rates of 46%: diabetes and endocrinology, family medicine, radiology, ob.gyn., and rheumatology. Pulmonology specialists reported a burnout rate of 41%. Gastroenterologists reported burnout rates of 37%.
The survey divided participants into three age categories – Millennial (ages 25-39 years), Generation X (ages 40-54 years), and Baby Boomer (ages 55-73 years). Both Millennials and Baby Boomers reported similar rates of burnout (38% and 39%, respectively) and those in Generation X reported a higher rate of burnout (48%).
This higher rate is not unexpected. The survey results cite Carol Bernstein, MD, of the Albert Einstein College of Medicine, New York, as noting that midcareer “is typically the time of highest burnout, which is where Gen Xers are in their career trajectory, suggesting a number of factors outside of work such as caring for children and elderly parents, planning for retirement, can play a role in contributing to burnout.”
Women also reported a higher rate of burnout, although the rate has dropped from the survey conducted 5 years ago. The rate of burnout among women reported for the 2020 survey was 48%, down from 51% reported 5 years ago. By comparison, the rate of burnout for men was 37% in 2020, down from 43% in 2015.
In terms of what is causing burnout, the biggest contributor is the bureaucratic tasks (charting and paperwork, for example) that physicians must complete, which 55% of respondents to the survey said was the leading cause of burnout. Next was spending too many hours at work (33%); lack of respect from administrators, employers, colleagues, and staff (32%); and the increased computerization of the practice, including the use of electronic health records (30%).
When broken down by age category, the bureaucratic tasks was tops in all three groups (57% for Millennials, 56% for Generation X, and 54% for Baby Boomers), but what ranks next differs slightly by age group. For Millennials, the next two factors were too many hours at work (38%) and lack of respect (35%). Generation X respondents cited the same two factors, both at 33%. Baby Boomers cited computerization as their second-highest factor (41%) and spending too many hours at work as the third-highest factor (31%).
The generations had different approaches to coping with burnout. Millennials (56%) reported sleep as their top-ranked coping strategy, while Gen Xers and Baby Boomers ranked exercise and personal isolation as their top choice. For these two older groups, sleep was ranked last, after other activities such as talking with family and friends.
The survey also asked about depression, and respondents reported a similar rate across all age groups (15%, 18%, and 16%, respectively). Among those who said they were depressed, the three age groups had similar rates of suicidal thoughts (21%, 24%, and 22%).
Perhaps the most striking finding of the survey is the number of physicians who would take a pay cut to achieve a better work-life balance. Among Millennials, 52% would accept a pay cut, compared with 48% of Generation X and 49% of Baby Boomers. A surprising number (36%, 34%, and 31%, respectively, reported that they would accept a $10,000-$20,000 pay cut to have a 20% reduction in work hours. [email protected]
*This story was updated on 1/22/2020.
SOURCE: Kane L et al. Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide. Medscape. 2020 Jan 15.
Burnout among physicians appears to have decreased slightly in the past few years, but remains a significant problem for the medical profession, according to the Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide.
A survey of more than 15,000 physicians revealed that 42% reported being burned out, down from 46% who responded to the survey 5 years ago. However, there are variations in the rates based on certain demographic factors such as specialty, age, and gender.
Urology sits at the top of the list as the specialty that is experiencing the highest rate of burnout, with 54% of urologists responding to the survey reporting burnout. Neurology and nephrology followed with rates of burnout at 50% and 49%, respectively. The next five specialties on the list all reported burnout rates of 46%: diabetes and endocrinology, family medicine, radiology, ob.gyn., and rheumatology. Pulmonology specialists reported a burnout rate of 41%. Gastroenterologists reported burnout rates of 37%.
The survey divided participants into three age categories – Millennial (ages 25-39 years), Generation X (ages 40-54 years), and Baby Boomer (ages 55-73 years). Both Millennials and Baby Boomers reported similar rates of burnout (38% and 39%, respectively) and those in Generation X reported a higher rate of burnout (48%).
This higher rate is not unexpected. The survey results cite Carol Bernstein, MD, of the Albert Einstein College of Medicine, New York, as noting that midcareer “is typically the time of highest burnout, which is where Gen Xers are in their career trajectory, suggesting a number of factors outside of work such as caring for children and elderly parents, planning for retirement, can play a role in contributing to burnout.”
Women also reported a higher rate of burnout, although the rate has dropped from the survey conducted 5 years ago. The rate of burnout among women reported for the 2020 survey was 48%, down from 51% reported 5 years ago. By comparison, the rate of burnout for men was 37% in 2020, down from 43% in 2015.
In terms of what is causing burnout, the biggest contributor is the bureaucratic tasks (charting and paperwork, for example) that physicians must complete, which 55% of respondents to the survey said was the leading cause of burnout. Next was spending too many hours at work (33%); lack of respect from administrators, employers, colleagues, and staff (32%); and the increased computerization of the practice, including the use of electronic health records (30%).
When broken down by age category, the bureaucratic tasks was tops in all three groups (57% for Millennials, 56% for Generation X, and 54% for Baby Boomers), but what ranks next differs slightly by age group. For Millennials, the next two factors were too many hours at work (38%) and lack of respect (35%). Generation X respondents cited the same two factors, both at 33%. Baby Boomers cited computerization as their second-highest factor (41%) and spending too many hours at work as the third-highest factor (31%).
The generations had different approaches to coping with burnout. Millennials (56%) reported sleep as their top-ranked coping strategy, while Gen Xers and Baby Boomers ranked exercise and personal isolation as their top choice. For these two older groups, sleep was ranked last, after other activities such as talking with family and friends.
The survey also asked about depression, and respondents reported a similar rate across all age groups (15%, 18%, and 16%, respectively). Among those who said they were depressed, the three age groups had similar rates of suicidal thoughts (21%, 24%, and 22%).
Perhaps the most striking finding of the survey is the number of physicians who would take a pay cut to achieve a better work-life balance. Among Millennials, 52% would accept a pay cut, compared with 48% of Generation X and 49% of Baby Boomers. A surprising number (36%, 34%, and 31%, respectively, reported that they would accept a $10,000-$20,000 pay cut to have a 20% reduction in work hours. [email protected]
*This story was updated on 1/22/2020.
SOURCE: Kane L et al. Medscape National Physician Burnout & Suicide Report 2020: The Generational Divide. Medscape. 2020 Jan 15.
Alan Alda, Scripps Research join forces to improve science communication
LA JOLLA, CALIF. – The first time that legendary actor Alan Alda conducted an interview for “Scientific American Frontiers” on PBS, an award-winning series that ran for more than a decade, he remembers learning a lesson in humility.

“I wasn’t as smart as I thought I was,” he told a crowd of largely scientists and medical professionals who gathered in a small auditorium on the campus of Scripps Research on Jan. 16, 2020. “I didn’t realize the value of ignorance. I have a natural supply of it. I began to use it and say [to interviewees]: ‘I don’t understand what that means.’ Sometimes it would be basic physics and they’d look at me like I was a school child. I am a very curious person. What I discovered was, I was bringing out their humanity by my own curiosity, by the way I related to them, which I developed through studying improvisation as an actor, and relating as an actor to other actors.”
Mr. Alda, 83, appeared on the research campus to announce that Scripps Research is the new West Coast home of Alda Communication Training, which will work in tandem with the Alan Alda Center for Communicating Science at the State University of New York at Stony Brook, a nonprofit organization that Mr. Alda helped found in 2009.
Immersive training experience
“This will be a center where people can come to get training in effective communication,” said Mr. Alda, who is the winner of six Emmy Awards and six Golden Globe awards. “It’s an experiential kind of training. We don’t give tips. We don’t give lectures. We put you through exercises that are fun and actually make you laugh, but turn you into a better communicator, so you’re better able to connect to the people you’re talking to.”
To date, the Alan Alda Center for Communicating Science has trained more than 15,000 scientific leaders in the United States and other countries. The location at Scripps Research makes it more convenient for West Coast–based researchers and industry leaders to participate. “One of the things we wished, for years, we had was a place where we could train scientists and researchers and medical professionals all up and down the West Coast,” he said.
Recently, more than 30 of Scripps Research scientists participated in Mr. Alda’s training program, an immersive and engaging experience that helps participants learn to empathize with an audience and present their work in a way that connects with different stakeholders. The skills and strategies can help participants relate to prospective investors and philanthropists, government officials, members of the media, peers across scientific disciplines, and the general public.
Earlier in the day that he spoke on the Scripps campus, Mr. Alda encountered some of the Scripps researchers who had participated in that training. “One group of scientists came in and we shook hands,” he said. “They introduced themselves and said: ‘We’re working on infectious diseases.’ I said: ‘Oh my God; I just shook hands with you!’ No matter what I asked them, they had a clear way to express what they did. Then I realized they had studied with Alda Communications.”
Why communication matters
During the early stages of forming what became the Alan Alda Center for Communicating Science, one Nobel Prize winner at a major university dismissed the importance of improving the communication skills of young scientists. “He said to me: ‘We don’t have time for that; we have too much science to teach,’ ” said Mr. Alda, who played Army surgeon “Hawkeye” Pierce on the TV series “M*A*S*H”. “But communication is the essence of science. How can you do science unless you communicate with other scientists? There’s a stereotype that scientists are not as good at communicating as other people are. It’s true that they often speak a language that a lot of us don’t understand, but we all speak a language that is hard for other people to understand if we know something in great depth. We want to tell all the details; we want to speak in our special language because it makes us feel good.”
He underscored the importance of scientists being able to effectively communicate with the general public, “because the public needs to understand how important science is to their lives. It matters because at a place like [Scripps Research], understanding how nature works is put to work to keep our health secure.” Members of the public, he continued, “are busy living their lives; they’re busy working and bringing up their children. They haven’t spent 20, 30, 40 years devoted to a single aspect of nature the way scientists have. We can’t expect them to know as much as professional scientists, so we have to help them understand it. I hope we find ways to increase curiosity. I don’t know how to do that. I wish somebody would do a study on it, how you can take someone with a modicum of curiosity and help them enlarge it so it gives them the pleasure of discovering things about nature or understanding things about nature that other people don’t discover. Curiosity is the key to staying alive. That would bring us to a point of more people understanding science.”
Cultivating a sense of responsibility is another key to effective communication. “It’s the job of the person leading the discussion to make clear to the person listening,” Mr. Alda said. “You get the impression that ‘this person is my responsibility. I have to take care of them, so they understand what’s going on.’ ”
Parkinson’s disease diagnosis
During a question-and-answer session, Mr. Alda opened up about his Parkinson’s disease, which he said was diagnosed about 5 years ago. In 2018, he decided to speak publicly about his diagnosis for the first time.
“The reason was that I wanted to communicate to people who had recently been diagnosed not to believe or give into the stereotype that when you get a diagnosis, your life is over,” said Mr. Alda, who received the Public Welfare Medal from the National Academy of Sciences in 2016. “Under the burden of that belief, some people won’t tell their family or workplace colleagues. There are exercises you can do and medications you can take to prolong the time it takes before Parkinson’s gets much more serious. It’s not to diminish the fact that it can get really bad; but to think that your life is over as soon as you get a diagnosis is wrong.”
He added: “I’ve gone 5 years and I’m almost busier than I’ve ever been. I’m getting a lot accomplished and I look forward to I don’t know how many years. As long as I have them, I’m going to be grateful. It’s amazing how great it feels not to keep the diagnosis a secret.”
The first 2-day training session at Scripps Research will be held in June 2020. Additional sessions are scheduled to take place in October and December. Registration is available at aldacommunicationtraining.com/workshops.
LA JOLLA, CALIF. – The first time that legendary actor Alan Alda conducted an interview for “Scientific American Frontiers” on PBS, an award-winning series that ran for more than a decade, he remembers learning a lesson in humility.

“I wasn’t as smart as I thought I was,” he told a crowd of largely scientists and medical professionals who gathered in a small auditorium on the campus of Scripps Research on Jan. 16, 2020. “I didn’t realize the value of ignorance. I have a natural supply of it. I began to use it and say [to interviewees]: ‘I don’t understand what that means.’ Sometimes it would be basic physics and they’d look at me like I was a school child. I am a very curious person. What I discovered was, I was bringing out their humanity by my own curiosity, by the way I related to them, which I developed through studying improvisation as an actor, and relating as an actor to other actors.”
Mr. Alda, 83, appeared on the research campus to announce that Scripps Research is the new West Coast home of Alda Communication Training, which will work in tandem with the Alan Alda Center for Communicating Science at the State University of New York at Stony Brook, a nonprofit organization that Mr. Alda helped found in 2009.
Immersive training experience
“This will be a center where people can come to get training in effective communication,” said Mr. Alda, who is the winner of six Emmy Awards and six Golden Globe awards. “It’s an experiential kind of training. We don’t give tips. We don’t give lectures. We put you through exercises that are fun and actually make you laugh, but turn you into a better communicator, so you’re better able to connect to the people you’re talking to.”
To date, the Alan Alda Center for Communicating Science has trained more than 15,000 scientific leaders in the United States and other countries. The location at Scripps Research makes it more convenient for West Coast–based researchers and industry leaders to participate. “One of the things we wished, for years, we had was a place where we could train scientists and researchers and medical professionals all up and down the West Coast,” he said.
Recently, more than 30 of Scripps Research scientists participated in Mr. Alda’s training program, an immersive and engaging experience that helps participants learn to empathize with an audience and present their work in a way that connects with different stakeholders. The skills and strategies can help participants relate to prospective investors and philanthropists, government officials, members of the media, peers across scientific disciplines, and the general public.
Earlier in the day that he spoke on the Scripps campus, Mr. Alda encountered some of the Scripps researchers who had participated in that training. “One group of scientists came in and we shook hands,” he said. “They introduced themselves and said: ‘We’re working on infectious diseases.’ I said: ‘Oh my God; I just shook hands with you!’ No matter what I asked them, they had a clear way to express what they did. Then I realized they had studied with Alda Communications.”
Why communication matters
During the early stages of forming what became the Alan Alda Center for Communicating Science, one Nobel Prize winner at a major university dismissed the importance of improving the communication skills of young scientists. “He said to me: ‘We don’t have time for that; we have too much science to teach,’ ” said Mr. Alda, who played Army surgeon “Hawkeye” Pierce on the TV series “M*A*S*H”. “But communication is the essence of science. How can you do science unless you communicate with other scientists? There’s a stereotype that scientists are not as good at communicating as other people are. It’s true that they often speak a language that a lot of us don’t understand, but we all speak a language that is hard for other people to understand if we know something in great depth. We want to tell all the details; we want to speak in our special language because it makes us feel good.”
He underscored the importance of scientists being able to effectively communicate with the general public, “because the public needs to understand how important science is to their lives. It matters because at a place like [Scripps Research], understanding how nature works is put to work to keep our health secure.” Members of the public, he continued, “are busy living their lives; they’re busy working and bringing up their children. They haven’t spent 20, 30, 40 years devoted to a single aspect of nature the way scientists have. We can’t expect them to know as much as professional scientists, so we have to help them understand it. I hope we find ways to increase curiosity. I don’t know how to do that. I wish somebody would do a study on it, how you can take someone with a modicum of curiosity and help them enlarge it so it gives them the pleasure of discovering things about nature or understanding things about nature that other people don’t discover. Curiosity is the key to staying alive. That would bring us to a point of more people understanding science.”
Cultivating a sense of responsibility is another key to effective communication. “It’s the job of the person leading the discussion to make clear to the person listening,” Mr. Alda said. “You get the impression that ‘this person is my responsibility. I have to take care of them, so they understand what’s going on.’ ”
Parkinson’s disease diagnosis
During a question-and-answer session, Mr. Alda opened up about his Parkinson’s disease, which he said was diagnosed about 5 years ago. In 2018, he decided to speak publicly about his diagnosis for the first time.
“The reason was that I wanted to communicate to people who had recently been diagnosed not to believe or give into the stereotype that when you get a diagnosis, your life is over,” said Mr. Alda, who received the Public Welfare Medal from the National Academy of Sciences in 2016. “Under the burden of that belief, some people won’t tell their family or workplace colleagues. There are exercises you can do and medications you can take to prolong the time it takes before Parkinson’s gets much more serious. It’s not to diminish the fact that it can get really bad; but to think that your life is over as soon as you get a diagnosis is wrong.”
He added: “I’ve gone 5 years and I’m almost busier than I’ve ever been. I’m getting a lot accomplished and I look forward to I don’t know how many years. As long as I have them, I’m going to be grateful. It’s amazing how great it feels not to keep the diagnosis a secret.”
The first 2-day training session at Scripps Research will be held in June 2020. Additional sessions are scheduled to take place in October and December. Registration is available at aldacommunicationtraining.com/workshops.
LA JOLLA, CALIF. – The first time that legendary actor Alan Alda conducted an interview for “Scientific American Frontiers” on PBS, an award-winning series that ran for more than a decade, he remembers learning a lesson in humility.

“I wasn’t as smart as I thought I was,” he told a crowd of largely scientists and medical professionals who gathered in a small auditorium on the campus of Scripps Research on Jan. 16, 2020. “I didn’t realize the value of ignorance. I have a natural supply of it. I began to use it and say [to interviewees]: ‘I don’t understand what that means.’ Sometimes it would be basic physics and they’d look at me like I was a school child. I am a very curious person. What I discovered was, I was bringing out their humanity by my own curiosity, by the way I related to them, which I developed through studying improvisation as an actor, and relating as an actor to other actors.”
Mr. Alda, 83, appeared on the research campus to announce that Scripps Research is the new West Coast home of Alda Communication Training, which will work in tandem with the Alan Alda Center for Communicating Science at the State University of New York at Stony Brook, a nonprofit organization that Mr. Alda helped found in 2009.
Immersive training experience
“This will be a center where people can come to get training in effective communication,” said Mr. Alda, who is the winner of six Emmy Awards and six Golden Globe awards. “It’s an experiential kind of training. We don’t give tips. We don’t give lectures. We put you through exercises that are fun and actually make you laugh, but turn you into a better communicator, so you’re better able to connect to the people you’re talking to.”
To date, the Alan Alda Center for Communicating Science has trained more than 15,000 scientific leaders in the United States and other countries. The location at Scripps Research makes it more convenient for West Coast–based researchers and industry leaders to participate. “One of the things we wished, for years, we had was a place where we could train scientists and researchers and medical professionals all up and down the West Coast,” he said.
Recently, more than 30 of Scripps Research scientists participated in Mr. Alda’s training program, an immersive and engaging experience that helps participants learn to empathize with an audience and present their work in a way that connects with different stakeholders. The skills and strategies can help participants relate to prospective investors and philanthropists, government officials, members of the media, peers across scientific disciplines, and the general public.
Earlier in the day that he spoke on the Scripps campus, Mr. Alda encountered some of the Scripps researchers who had participated in that training. “One group of scientists came in and we shook hands,” he said. “They introduced themselves and said: ‘We’re working on infectious diseases.’ I said: ‘Oh my God; I just shook hands with you!’ No matter what I asked them, they had a clear way to express what they did. Then I realized they had studied with Alda Communications.”
Why communication matters
During the early stages of forming what became the Alan Alda Center for Communicating Science, one Nobel Prize winner at a major university dismissed the importance of improving the communication skills of young scientists. “He said to me: ‘We don’t have time for that; we have too much science to teach,’ ” said Mr. Alda, who played Army surgeon “Hawkeye” Pierce on the TV series “M*A*S*H”. “But communication is the essence of science. How can you do science unless you communicate with other scientists? There’s a stereotype that scientists are not as good at communicating as other people are. It’s true that they often speak a language that a lot of us don’t understand, but we all speak a language that is hard for other people to understand if we know something in great depth. We want to tell all the details; we want to speak in our special language because it makes us feel good.”
He underscored the importance of scientists being able to effectively communicate with the general public, “because the public needs to understand how important science is to their lives. It matters because at a place like [Scripps Research], understanding how nature works is put to work to keep our health secure.” Members of the public, he continued, “are busy living their lives; they’re busy working and bringing up their children. They haven’t spent 20, 30, 40 years devoted to a single aspect of nature the way scientists have. We can’t expect them to know as much as professional scientists, so we have to help them understand it. I hope we find ways to increase curiosity. I don’t know how to do that. I wish somebody would do a study on it, how you can take someone with a modicum of curiosity and help them enlarge it so it gives them the pleasure of discovering things about nature or understanding things about nature that other people don’t discover. Curiosity is the key to staying alive. That would bring us to a point of more people understanding science.”
Cultivating a sense of responsibility is another key to effective communication. “It’s the job of the person leading the discussion to make clear to the person listening,” Mr. Alda said. “You get the impression that ‘this person is my responsibility. I have to take care of them, so they understand what’s going on.’ ”
Parkinson’s disease diagnosis
During a question-and-answer session, Mr. Alda opened up about his Parkinson’s disease, which he said was diagnosed about 5 years ago. In 2018, he decided to speak publicly about his diagnosis for the first time.
“The reason was that I wanted to communicate to people who had recently been diagnosed not to believe or give into the stereotype that when you get a diagnosis, your life is over,” said Mr. Alda, who received the Public Welfare Medal from the National Academy of Sciences in 2016. “Under the burden of that belief, some people won’t tell their family or workplace colleagues. There are exercises you can do and medications you can take to prolong the time it takes before Parkinson’s gets much more serious. It’s not to diminish the fact that it can get really bad; but to think that your life is over as soon as you get a diagnosis is wrong.”
He added: “I’ve gone 5 years and I’m almost busier than I’ve ever been. I’m getting a lot accomplished and I look forward to I don’t know how many years. As long as I have them, I’m going to be grateful. It’s amazing how great it feels not to keep the diagnosis a secret.”
The first 2-day training session at Scripps Research will be held in June 2020. Additional sessions are scheduled to take place in October and December. Registration is available at aldacommunicationtraining.com/workshops.
European marketing of Picato suspended while skin cancer risk reviewed
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
As a precaution, the European Medicines Agency (EMA) has recommended that patients stop using ingenol mebutate (Picato) while the agency continues to review the safety of the topical treatment, which is indicated for the treatment of actinic keratosis in Europe and the United States.
No such action has been taken in the United States.
The EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) is reviewing data on skin cancer in patients treated with ingenol mebutate. In a trial comparing Picato and imiquimod, skin cancer was more common in the areas treated with Picato than in areas treated with imiquimod, the statement said.
“While uncertainties remain, the EMA said in a Jan. 17 news release. “The PRAC has therefore recommended suspending the medicine’s marketing authorization as a precaution and noted that alternative treatments are available.”
FDA is looking at the situation
LEO Pharma, the company that markets Picato, announced on Jan. 9 that it was initiating voluntary withdrawal of marketing authorization and possible voluntary withdrawal of Picato in the European Union (EU) and European Economic Area (EEA). The statement says, however, that “LEO Pharma has carefully reviewed the information received from PRAC, and the company disagrees with the ongoing assessment of PRAC.” There are “no additional safety data and it is LEO Pharma’s position that there is no evidence of a causal relationship or plausible mechanism hypothesis between the use of Picato and the development of skin malignancies.” An update added to the press release on Jan. 17 restates that the company disagrees with the assessment of PRAC.
“This matter does not affect Picato in the U.S., and there are no new developments in the [United States]. Picato continues to be available to patients in the U.S. We remain in dialogue with the U.S. Food and Drug Administration about Picato in the EU/EEA,” Rhonda Sciarra, associate director of global external communications for LEO Pharma, said in an email. “We remain committed to ensuring patient safety, rigorous pharmacovigilance monitoring, and transparency,” she added.
The FDA “is gathering data and information to investigate the safety concern related to Picato,” a spokesperson for the FDA told Dermatology News. “We are committed to sharing relevant findings when we have sufficient understanding of the situation and of what actions should be taken,” he added.
Examining the data
The EMA announcement described data about the risk of skin cancer in studies of Picato. A 3-year study in 484 patients found a higher incidence of skin malignancy with ingenol mebutate than with the comparator, imiquimod. In all, 3.3% of patients developed cancer in the ingenol mebutate group, compared with 0.4% in the comparator group.
In an 8-week vehicle-controlled trial in 1,262 patients, there were more skin tumors in patients who received ingenol mebutate than in those in the vehicle arm (1.0% vs. 0.1%).
In addition, according to the EMA statement, in four trials of a related ester that included 1,234 patients, a higher incidence of skin tumors occurred with the related drug, ingenol disoxate, than with a vehicle control (7.7% vs. 2.9%). PRAC considered these data because ingenol disoxate and ingenol mebutate are closely related, the EMA said.
“Health care professionals should stop prescribing Picato and consider different treatment options while authorities review the data,” according to the European agency. “Health care professionals should advise patients to be vigilant for any skin lesions developing and to seek medical advice promptly should any occur,” the statement adds.
Picato has been authorized in the EU since 2012, and the FDA approved Picato the same year. Patients have received about 2.8 million treatment courses in that time, according to the LEO Pharma press release.
Cognitive screening of older physicians: What’s fair?
Cognitive screening of 141 clinicians 70 years or older at Yale New Haven (Conn.) Hospital identified 18 with cognitive deficits likely to impair their ability to practice medicine. Six retired and 12 agreed to limit their practice to closely proctored environments, according to a report in JAMA.
It was part of a program to screen all practitioners 70 years or older who apply for reappointment to the medical staff, and every 2 years thereafter, due to “concerns about the potentially compromised ability of older clinicians,” said the authors, Yale rheumatologist and geriatrician Leo M. Cooney Jr., MD, and Thomas Balcezak, MD, Yale New Haven’s chief medical officer.
Yale is not alone. Intermountain Healthcare, Stanford Hospitals and Clinics, Scripps Health Care, Penn Medicine, and the University of California, San Diego, are among the institutions with similar programs.
The move is being driven by the aging of the medical community. About 15% of U.S. physicians are over 65 years old, a tripling from 23,000 in 1980 to 73,000 in 2012-2016, and the number is growing, according to an editorial by Jeffrey L. Saver, MD, professor of neurology and senior associate vice president of neurology at the University of California, Los Angeles.
Given the trend, “it is not surprising that the issue of screening aging physicians for cognitive deficits has gained attention over the last decade,” Katrina Armstrong, MD, chair of the department of medicine at Massachusetts General Hospital, Boston, and Eileen E. Reynolds, MD, associate professor of medicine at Beth Israel Deaconess Medical Center, Boston, noted in a second editorial.
“Cognitive decline often accompanies aging, and the prevalence of dementia increases rapidly after age 70 years,” they said.
The data on whether older clinicians pose a risk to patients is limited and somewhat mixed. An analysis of 736,537 Medicare hospitalizations found no association between physician age and 30-day patient mortality among physicians 60 years or older with more than 201 admissions per year, but higher mortality among older physicians with lower volumes.
A meta-analysis of 62 studies showed that “older physicians have less factual knowledge, are less likely to adhere to appropriate standards of care, and may also have poorer patient outcomes.”
The new Yale data, meanwhile, suggests that “approximately 13% [18 of 141] of physicians and other clinicians older than 70 years should not be practicing independently,” Dr. Armstrong and Dr. Reynolds said in their editorial.
There is support for screening efforts. “As a profession that deals with human life, medical practitioners must obviously have the cognitive capacity to safely practice medicine. I applaud the approach taken by Yale New Haven Hospital in that cognitive abilities themselves, and not simply funds of knowledge, are assessed,” said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic Arizona, Scottsdale, and a leader of the Alzheimer’s disease program there.
However, it’s not hard to imagine highly competent but older physicians taking umbrage at cognitive screening, and there’s been pushback. Stanford was considering a Yale-like approach but opted instead for peer review after opposition. Objections from the Utah Medical Association led Utah to enact a law banning age-based physician screening. In 2015, the American Medical Association issued a report calling for the development of guidelines and standards for assessing competency in aging physicians, but the AMA House of Delegates shelved it pending further study.
There are concerns about age discrimination, discounting the accumulated wisdom of long-practicing physicians, and misclassifying competent physicians, particularly those who provide quality care in rural and other underserved areas. Indeed, 8 of 14 clinicians who screened positive at Yale and underwent more extensive testing were allowed to recredential, “suggesting that the false-positive screening rate could be as high as 57%,” Dr. Armstrong and Dr. Reynolds noted.
The consensus seems to be that there probably is a need for some sort of screening, but it must be both sound and fair. Rather than a piecemeal institutional approach, perhaps there is “an important opportunity for other groups, including specialty boards and state licensing boards” to standardize the process, they said.
Among other things, assessments could focus less on test scores and more on the practice of medicine. For instance, fine motor skill/motor planning assessments for surgeons, and intermediate results could trigger a more extensive assessment of actual clinical performance, perhaps even direct observation, Dr. Saver said in his editorial.
As far as clinical performance goes, none of the 18 clinicians at Yale had previous performance problems. “Was this a failure of the system to report impaired physicians or were these physicians compensating sufficiently to avoid detection?” In either case, “cognitive testing should be a red flag that triggers other clinical assessments,” said Carl I. Cohen, MD, professor and director of the division of geriatric psychiatry at the State University of New York, Brooklyn.
The original plan at Yale was for neurologic and ophthalmologic examinations beginning at age 70, but ultimately it was decided to go with a battery of 16 tests to assess visual scanning and psychomotor efficiency, processing speed under pressure, concentration, and working memory, among other things. Testing takes about 50-90 minutes, and is graded by single neuropsychologist to ensure consistency. Results were compared with normative scores from both older and younger clinicians.
To prevent clinicians from preparing for it, Yale isn’t releasing its test battery.
Suboptimal performance triggered additional evaluations, including in-depth assessment of intellectual, memory, and executive function. Final reviews and recommendations were made by a committee that included a geriatrician, the clinician’s section or department chair, and current and past chief medical officers.
Among the 18 providers who demonstrated deficits impairing their ability to practice medicine, 5 were 70-74 years old; 4 were 75-79; and 9 were 80 years or older. Minor abnormalities were found in 34 other candidates (24.1%); they were allowed to recredential but were scheduled for rescreening at 1-year intervals, instead of every 2 years.
The mean age among the 141 screened clinicians was 74.3 years and ranged from 69 to 92 years; 86% were men. Applicants included 125 physicians (88.7%) as well as 5 advanced practice registered nurses; 4 dentists; 3 psychologists; 2 podiatrists; 1 physician associate; and 1 midwife.
The authors had no relevant disclosures.
SOURCE: Cooney L et al. JAMA. 2020 Jan 14;323(2):179-80.
Cognitive screening of 141 clinicians 70 years or older at Yale New Haven (Conn.) Hospital identified 18 with cognitive deficits likely to impair their ability to practice medicine. Six retired and 12 agreed to limit their practice to closely proctored environments, according to a report in JAMA.
It was part of a program to screen all practitioners 70 years or older who apply for reappointment to the medical staff, and every 2 years thereafter, due to “concerns about the potentially compromised ability of older clinicians,” said the authors, Yale rheumatologist and geriatrician Leo M. Cooney Jr., MD, and Thomas Balcezak, MD, Yale New Haven’s chief medical officer.
Yale is not alone. Intermountain Healthcare, Stanford Hospitals and Clinics, Scripps Health Care, Penn Medicine, and the University of California, San Diego, are among the institutions with similar programs.
The move is being driven by the aging of the medical community. About 15% of U.S. physicians are over 65 years old, a tripling from 23,000 in 1980 to 73,000 in 2012-2016, and the number is growing, according to an editorial by Jeffrey L. Saver, MD, professor of neurology and senior associate vice president of neurology at the University of California, Los Angeles.
Given the trend, “it is not surprising that the issue of screening aging physicians for cognitive deficits has gained attention over the last decade,” Katrina Armstrong, MD, chair of the department of medicine at Massachusetts General Hospital, Boston, and Eileen E. Reynolds, MD, associate professor of medicine at Beth Israel Deaconess Medical Center, Boston, noted in a second editorial.
“Cognitive decline often accompanies aging, and the prevalence of dementia increases rapidly after age 70 years,” they said.
The data on whether older clinicians pose a risk to patients is limited and somewhat mixed. An analysis of 736,537 Medicare hospitalizations found no association between physician age and 30-day patient mortality among physicians 60 years or older with more than 201 admissions per year, but higher mortality among older physicians with lower volumes.
A meta-analysis of 62 studies showed that “older physicians have less factual knowledge, are less likely to adhere to appropriate standards of care, and may also have poorer patient outcomes.”
The new Yale data, meanwhile, suggests that “approximately 13% [18 of 141] of physicians and other clinicians older than 70 years should not be practicing independently,” Dr. Armstrong and Dr. Reynolds said in their editorial.
There is support for screening efforts. “As a profession that deals with human life, medical practitioners must obviously have the cognitive capacity to safely practice medicine. I applaud the approach taken by Yale New Haven Hospital in that cognitive abilities themselves, and not simply funds of knowledge, are assessed,” said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic Arizona, Scottsdale, and a leader of the Alzheimer’s disease program there.
However, it’s not hard to imagine highly competent but older physicians taking umbrage at cognitive screening, and there’s been pushback. Stanford was considering a Yale-like approach but opted instead for peer review after opposition. Objections from the Utah Medical Association led Utah to enact a law banning age-based physician screening. In 2015, the American Medical Association issued a report calling for the development of guidelines and standards for assessing competency in aging physicians, but the AMA House of Delegates shelved it pending further study.
There are concerns about age discrimination, discounting the accumulated wisdom of long-practicing physicians, and misclassifying competent physicians, particularly those who provide quality care in rural and other underserved areas. Indeed, 8 of 14 clinicians who screened positive at Yale and underwent more extensive testing were allowed to recredential, “suggesting that the false-positive screening rate could be as high as 57%,” Dr. Armstrong and Dr. Reynolds noted.
The consensus seems to be that there probably is a need for some sort of screening, but it must be both sound and fair. Rather than a piecemeal institutional approach, perhaps there is “an important opportunity for other groups, including specialty boards and state licensing boards” to standardize the process, they said.
Among other things, assessments could focus less on test scores and more on the practice of medicine. For instance, fine motor skill/motor planning assessments for surgeons, and intermediate results could trigger a more extensive assessment of actual clinical performance, perhaps even direct observation, Dr. Saver said in his editorial.
As far as clinical performance goes, none of the 18 clinicians at Yale had previous performance problems. “Was this a failure of the system to report impaired physicians or were these physicians compensating sufficiently to avoid detection?” In either case, “cognitive testing should be a red flag that triggers other clinical assessments,” said Carl I. Cohen, MD, professor and director of the division of geriatric psychiatry at the State University of New York, Brooklyn.
The original plan at Yale was for neurologic and ophthalmologic examinations beginning at age 70, but ultimately it was decided to go with a battery of 16 tests to assess visual scanning and psychomotor efficiency, processing speed under pressure, concentration, and working memory, among other things. Testing takes about 50-90 minutes, and is graded by single neuropsychologist to ensure consistency. Results were compared with normative scores from both older and younger clinicians.
To prevent clinicians from preparing for it, Yale isn’t releasing its test battery.
Suboptimal performance triggered additional evaluations, including in-depth assessment of intellectual, memory, and executive function. Final reviews and recommendations were made by a committee that included a geriatrician, the clinician’s section or department chair, and current and past chief medical officers.
Among the 18 providers who demonstrated deficits impairing their ability to practice medicine, 5 were 70-74 years old; 4 were 75-79; and 9 were 80 years or older. Minor abnormalities were found in 34 other candidates (24.1%); they were allowed to recredential but were scheduled for rescreening at 1-year intervals, instead of every 2 years.
The mean age among the 141 screened clinicians was 74.3 years and ranged from 69 to 92 years; 86% were men. Applicants included 125 physicians (88.7%) as well as 5 advanced practice registered nurses; 4 dentists; 3 psychologists; 2 podiatrists; 1 physician associate; and 1 midwife.
The authors had no relevant disclosures.
SOURCE: Cooney L et al. JAMA. 2020 Jan 14;323(2):179-80.
Cognitive screening of 141 clinicians 70 years or older at Yale New Haven (Conn.) Hospital identified 18 with cognitive deficits likely to impair their ability to practice medicine. Six retired and 12 agreed to limit their practice to closely proctored environments, according to a report in JAMA.
It was part of a program to screen all practitioners 70 years or older who apply for reappointment to the medical staff, and every 2 years thereafter, due to “concerns about the potentially compromised ability of older clinicians,” said the authors, Yale rheumatologist and geriatrician Leo M. Cooney Jr., MD, and Thomas Balcezak, MD, Yale New Haven’s chief medical officer.
Yale is not alone. Intermountain Healthcare, Stanford Hospitals and Clinics, Scripps Health Care, Penn Medicine, and the University of California, San Diego, are among the institutions with similar programs.
The move is being driven by the aging of the medical community. About 15% of U.S. physicians are over 65 years old, a tripling from 23,000 in 1980 to 73,000 in 2012-2016, and the number is growing, according to an editorial by Jeffrey L. Saver, MD, professor of neurology and senior associate vice president of neurology at the University of California, Los Angeles.
Given the trend, “it is not surprising that the issue of screening aging physicians for cognitive deficits has gained attention over the last decade,” Katrina Armstrong, MD, chair of the department of medicine at Massachusetts General Hospital, Boston, and Eileen E. Reynolds, MD, associate professor of medicine at Beth Israel Deaconess Medical Center, Boston, noted in a second editorial.
“Cognitive decline often accompanies aging, and the prevalence of dementia increases rapidly after age 70 years,” they said.
The data on whether older clinicians pose a risk to patients is limited and somewhat mixed. An analysis of 736,537 Medicare hospitalizations found no association between physician age and 30-day patient mortality among physicians 60 years or older with more than 201 admissions per year, but higher mortality among older physicians with lower volumes.
A meta-analysis of 62 studies showed that “older physicians have less factual knowledge, are less likely to adhere to appropriate standards of care, and may also have poorer patient outcomes.”
The new Yale data, meanwhile, suggests that “approximately 13% [18 of 141] of physicians and other clinicians older than 70 years should not be practicing independently,” Dr. Armstrong and Dr. Reynolds said in their editorial.
There is support for screening efforts. “As a profession that deals with human life, medical practitioners must obviously have the cognitive capacity to safely practice medicine. I applaud the approach taken by Yale New Haven Hospital in that cognitive abilities themselves, and not simply funds of knowledge, are assessed,” said Richard J. Caselli, MD, professor of neurology at the Mayo Clinic Arizona, Scottsdale, and a leader of the Alzheimer’s disease program there.
However, it’s not hard to imagine highly competent but older physicians taking umbrage at cognitive screening, and there’s been pushback. Stanford was considering a Yale-like approach but opted instead for peer review after opposition. Objections from the Utah Medical Association led Utah to enact a law banning age-based physician screening. In 2015, the American Medical Association issued a report calling for the development of guidelines and standards for assessing competency in aging physicians, but the AMA House of Delegates shelved it pending further study.
There are concerns about age discrimination, discounting the accumulated wisdom of long-practicing physicians, and misclassifying competent physicians, particularly those who provide quality care in rural and other underserved areas. Indeed, 8 of 14 clinicians who screened positive at Yale and underwent more extensive testing were allowed to recredential, “suggesting that the false-positive screening rate could be as high as 57%,” Dr. Armstrong and Dr. Reynolds noted.
The consensus seems to be that there probably is a need for some sort of screening, but it must be both sound and fair. Rather than a piecemeal institutional approach, perhaps there is “an important opportunity for other groups, including specialty boards and state licensing boards” to standardize the process, they said.
Among other things, assessments could focus less on test scores and more on the practice of medicine. For instance, fine motor skill/motor planning assessments for surgeons, and intermediate results could trigger a more extensive assessment of actual clinical performance, perhaps even direct observation, Dr. Saver said in his editorial.
As far as clinical performance goes, none of the 18 clinicians at Yale had previous performance problems. “Was this a failure of the system to report impaired physicians or were these physicians compensating sufficiently to avoid detection?” In either case, “cognitive testing should be a red flag that triggers other clinical assessments,” said Carl I. Cohen, MD, professor and director of the division of geriatric psychiatry at the State University of New York, Brooklyn.
The original plan at Yale was for neurologic and ophthalmologic examinations beginning at age 70, but ultimately it was decided to go with a battery of 16 tests to assess visual scanning and psychomotor efficiency, processing speed under pressure, concentration, and working memory, among other things. Testing takes about 50-90 minutes, and is graded by single neuropsychologist to ensure consistency. Results were compared with normative scores from both older and younger clinicians.
To prevent clinicians from preparing for it, Yale isn’t releasing its test battery.
Suboptimal performance triggered additional evaluations, including in-depth assessment of intellectual, memory, and executive function. Final reviews and recommendations were made by a committee that included a geriatrician, the clinician’s section or department chair, and current and past chief medical officers.
Among the 18 providers who demonstrated deficits impairing their ability to practice medicine, 5 were 70-74 years old; 4 were 75-79; and 9 were 80 years or older. Minor abnormalities were found in 34 other candidates (24.1%); they were allowed to recredential but were scheduled for rescreening at 1-year intervals, instead of every 2 years.
The mean age among the 141 screened clinicians was 74.3 years and ranged from 69 to 92 years; 86% were men. Applicants included 125 physicians (88.7%) as well as 5 advanced practice registered nurses; 4 dentists; 3 psychologists; 2 podiatrists; 1 physician associate; and 1 midwife.
The authors had no relevant disclosures.
SOURCE: Cooney L et al. JAMA. 2020 Jan 14;323(2):179-80.
FROM JAMA
Pyrrolidone carboxylic acid may be a key cutaneous biomarker
Pyrrolidone carboxylic acid (PCA), the primary constituent of the natural moisturizing factor (NMF),1 including its derivatives – such as simple2 and novel3 esters as well as sugar complexes4 – is the subject of great interest and research regarding its capacity to moisturize the stratum corneum via topical application.
Creams and lotions containing the sodium salt of PCA are widely reported to aid in hydrating the skin and ameliorating dry flaky skin conditions.5,6 In addition, the zinc salt of L-pyrrolidone carboxylate is a longtime cosmetic ingredient due to antimicrobial and astringent qualities. This column briefly addresses the role of PCA in skin health.7
Dry skin
In a comprehensive literature review from 1981, Clar and Fourtanier reported conclusive evidence that PCA acts as a hydrating agent and that all the cosmetic formulations with a minimum of 2% PCA and PCA salt that they tested in their own 8-year study enhanced dry skin in short- and long-term conditions given suitable vehicles (no aqueous solutions).6
In a 2014 clinical study of 64 healthy white women with either normal or cosmetic dry skin, Feng et al. noted that tape stripped samples of stratum corneum revealed significantly lower ratios of free amino acids to protein and PCA to protein. This was associated with decreased hydration levels compared with normal skin. The investigators concluded that lower NMF levels across the depth of the stratum corneum and reduced cohesivity characterize cosmetic dry skin and that these clinical endpoints merit attention in evaluating the usefulness of treatments for dry skin.8
In 2016, Wei et al. reported on their assessment of the barrier function, hydration, and dryness of the lower leg skin of 25 female patients during the winter and then in the subsequent summer. They found that PCA levels were significantly greater during the summer, as were keratins. Hydration was also higher during the summer, while transepidermal water loss and visual dryness grades were substantially lower.9
Atopic dermatitis
A 2014 clinical study by Brandt et al. in patients with skin prone to developing atopic dermatitis (AD) revealed that a body wash composed of the filaggrin metabolites arginine and PCA was well tolerated and diminished pruritus. Patients reported liking the product and suggested that it improved their quality of life.10
Later that year, Jung et al. characterized the relationship of PCA levels, and other factors, with the clinical severity of AD. Specifically, in a study of 73 subjects (21 with mild AD, 21 with moderate to severe AD, 13 with X-linked ichthyosis as a negative control for filaggrin gene mutation, and 18 healthy controls), the investigators assessed transepidermal water loss, stratum corneum hydration, and skin surface pH. They found that PCA levels and caspase-14 were lower in inflammatory lesions compared with nonlesional skin in subjects with AD. These levels also were associated with clinical AD severity as measured by eczema area and severity index scores as well as skin barrier function.11
PCA as a biomarker
In 2009, Kezic et al. determined that the use of tape stripping to cull PCA in the stratum corneum was effective in revealing that PCA concentration in the outermost skin layer is a viable biomarker of filaggrin genotype.12
Raj et al. conducted an interesting study in 2016 in which they set out to describe the various markers for total NMF levels and link them to the activities of plasmin and corneocyte maturation in the photoexposed cheek and photoprotected postauricular regions of healthy white, black African, and albino African women in South Africa. PCA levels were highest among the albino African group, followed by black African and then white participants. The investigators also found that bleomycin hydrolase was linked to PCA synthesis, as suggested by higher bleomycin levels in albino African participants. In this group, corneocyte maturation was also observed to be impeded.13
The next year, the same team studied stratum corneum physiology and biochemistry of the cheeks in 48 white women with sensitive skin. The goal was to ascertain the connections between bleomycin hydrolase and calpain-1, PCA levels, corneocyte maturation, and transglutaminase and plasmin activities. Capsaicin sensitivity was observed in 52% of subjects, with PCA levels and bleomycin hydrolase activity found to be lower in the capsaicin-sensitive panel and correlated in subjects not sensitive to capsaicin. The researchers concluded that reduced levels of PCA, bleomycin hydrolase, and transglutaminase combined with a larger volume of immature corneocytes suggest comparatively poor stratum corneum maturation in individuals with sensitive skin.14
Other uses
In 2012, Takino et al. used cultured normal human dermal fibroblasts to show that zinc l-pyrrolidone carboxylate blocked UVA induction of activator protein-1, diminished matrix metalloproteinase-1 synthesis, and spurred type I collagen production. The researchers suggested that such results suggest the potential of zinc PCA for further investigation as an agent to combat photoaging.7
Conclusion
. Recent research suggests that it may serve as an important biomarker of filaggrin, NMF levels, and skin hydration. In addition, new data point to its usefulness as a gauge for ADs. More investigations are necessary to ascertain the feasibility of adjusting PCA levels through topical administration and what effects topically applied PCA may have on various skin parameters.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Björklund S et al. Soft Matter. 2014 Jul 7;10(25):4535-46.
2. Hall KJ, Hill JC. J Soc Cosmet Chem. 1986;37(6):397-407.
3. Tezuka T et al. Dermatology. 1994;188(1):21-4.
4. Kwoya Hakko Kogyo Co. Pyrrolidone carboxylic acid esters containing composition used to prevent loss of moisture from the skin. Patent JA 48 82 046 (1982).
5. Org Santerre. l-pyrrolidone carboxylic acid-sugar compounds as rehydrating ingredients in cosmetics. Patent Fr 2 277 823 (1977).
6. Clar EJ, Fourtanier A. Int J Cosmet Sci. 1981 Jun;3(3):101-13.
7. Takino Y et al. Int J Cosmet Sci. 2012 Feb;34(1):23-8.
8. Feng L et al. Int J Cosmet Sci. 2014 Jun;36(3):231-8.
9. Wei KS et al. J Cosmet Sci. 2016 May-Jun;67(3):185-203.
10. Brandt S et al. J Drugs Dermatol. 2014 Sep;13(9):1108-11.
11. Jung M et al. J Dermatol Sci. 2014 Dec;76(3):231-9.
12. Kezic S et al. Br J Dermatol. 2009 Nov;161(5):1098-104.
13. Raj N et al. Int J Cosmet Sci. 2016 Dec;38(6):567-75.
14. Raj N et al. Int J Cosmet Sci. 2017 Feb;39(1):2-10.
Pyrrolidone carboxylic acid (PCA), the primary constituent of the natural moisturizing factor (NMF),1 including its derivatives – such as simple2 and novel3 esters as well as sugar complexes4 – is the subject of great interest and research regarding its capacity to moisturize the stratum corneum via topical application.
Creams and lotions containing the sodium salt of PCA are widely reported to aid in hydrating the skin and ameliorating dry flaky skin conditions.5,6 In addition, the zinc salt of L-pyrrolidone carboxylate is a longtime cosmetic ingredient due to antimicrobial and astringent qualities. This column briefly addresses the role of PCA in skin health.7
Dry skin
In a comprehensive literature review from 1981, Clar and Fourtanier reported conclusive evidence that PCA acts as a hydrating agent and that all the cosmetic formulations with a minimum of 2% PCA and PCA salt that they tested in their own 8-year study enhanced dry skin in short- and long-term conditions given suitable vehicles (no aqueous solutions).6
In a 2014 clinical study of 64 healthy white women with either normal or cosmetic dry skin, Feng et al. noted that tape stripped samples of stratum corneum revealed significantly lower ratios of free amino acids to protein and PCA to protein. This was associated with decreased hydration levels compared with normal skin. The investigators concluded that lower NMF levels across the depth of the stratum corneum and reduced cohesivity characterize cosmetic dry skin and that these clinical endpoints merit attention in evaluating the usefulness of treatments for dry skin.8
In 2016, Wei et al. reported on their assessment of the barrier function, hydration, and dryness of the lower leg skin of 25 female patients during the winter and then in the subsequent summer. They found that PCA levels were significantly greater during the summer, as were keratins. Hydration was also higher during the summer, while transepidermal water loss and visual dryness grades were substantially lower.9
Atopic dermatitis
A 2014 clinical study by Brandt et al. in patients with skin prone to developing atopic dermatitis (AD) revealed that a body wash composed of the filaggrin metabolites arginine and PCA was well tolerated and diminished pruritus. Patients reported liking the product and suggested that it improved their quality of life.10
Later that year, Jung et al. characterized the relationship of PCA levels, and other factors, with the clinical severity of AD. Specifically, in a study of 73 subjects (21 with mild AD, 21 with moderate to severe AD, 13 with X-linked ichthyosis as a negative control for filaggrin gene mutation, and 18 healthy controls), the investigators assessed transepidermal water loss, stratum corneum hydration, and skin surface pH. They found that PCA levels and caspase-14 were lower in inflammatory lesions compared with nonlesional skin in subjects with AD. These levels also were associated with clinical AD severity as measured by eczema area and severity index scores as well as skin barrier function.11
PCA as a biomarker
In 2009, Kezic et al. determined that the use of tape stripping to cull PCA in the stratum corneum was effective in revealing that PCA concentration in the outermost skin layer is a viable biomarker of filaggrin genotype.12
Raj et al. conducted an interesting study in 2016 in which they set out to describe the various markers for total NMF levels and link them to the activities of plasmin and corneocyte maturation in the photoexposed cheek and photoprotected postauricular regions of healthy white, black African, and albino African women in South Africa. PCA levels were highest among the albino African group, followed by black African and then white participants. The investigators also found that bleomycin hydrolase was linked to PCA synthesis, as suggested by higher bleomycin levels in albino African participants. In this group, corneocyte maturation was also observed to be impeded.13
The next year, the same team studied stratum corneum physiology and biochemistry of the cheeks in 48 white women with sensitive skin. The goal was to ascertain the connections between bleomycin hydrolase and calpain-1, PCA levels, corneocyte maturation, and transglutaminase and plasmin activities. Capsaicin sensitivity was observed in 52% of subjects, with PCA levels and bleomycin hydrolase activity found to be lower in the capsaicin-sensitive panel and correlated in subjects not sensitive to capsaicin. The researchers concluded that reduced levels of PCA, bleomycin hydrolase, and transglutaminase combined with a larger volume of immature corneocytes suggest comparatively poor stratum corneum maturation in individuals with sensitive skin.14
Other uses
In 2012, Takino et al. used cultured normal human dermal fibroblasts to show that zinc l-pyrrolidone carboxylate blocked UVA induction of activator protein-1, diminished matrix metalloproteinase-1 synthesis, and spurred type I collagen production. The researchers suggested that such results suggest the potential of zinc PCA for further investigation as an agent to combat photoaging.7
Conclusion
. Recent research suggests that it may serve as an important biomarker of filaggrin, NMF levels, and skin hydration. In addition, new data point to its usefulness as a gauge for ADs. More investigations are necessary to ascertain the feasibility of adjusting PCA levels through topical administration and what effects topically applied PCA may have on various skin parameters.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Björklund S et al. Soft Matter. 2014 Jul 7;10(25):4535-46.
2. Hall KJ, Hill JC. J Soc Cosmet Chem. 1986;37(6):397-407.
3. Tezuka T et al. Dermatology. 1994;188(1):21-4.
4. Kwoya Hakko Kogyo Co. Pyrrolidone carboxylic acid esters containing composition used to prevent loss of moisture from the skin. Patent JA 48 82 046 (1982).
5. Org Santerre. l-pyrrolidone carboxylic acid-sugar compounds as rehydrating ingredients in cosmetics. Patent Fr 2 277 823 (1977).
6. Clar EJ, Fourtanier A. Int J Cosmet Sci. 1981 Jun;3(3):101-13.
7. Takino Y et al. Int J Cosmet Sci. 2012 Feb;34(1):23-8.
8. Feng L et al. Int J Cosmet Sci. 2014 Jun;36(3):231-8.
9. Wei KS et al. J Cosmet Sci. 2016 May-Jun;67(3):185-203.
10. Brandt S et al. J Drugs Dermatol. 2014 Sep;13(9):1108-11.
11. Jung M et al. J Dermatol Sci. 2014 Dec;76(3):231-9.
12. Kezic S et al. Br J Dermatol. 2009 Nov;161(5):1098-104.
13. Raj N et al. Int J Cosmet Sci. 2016 Dec;38(6):567-75.
14. Raj N et al. Int J Cosmet Sci. 2017 Feb;39(1):2-10.
Pyrrolidone carboxylic acid (PCA), the primary constituent of the natural moisturizing factor (NMF),1 including its derivatives – such as simple2 and novel3 esters as well as sugar complexes4 – is the subject of great interest and research regarding its capacity to moisturize the stratum corneum via topical application.
Creams and lotions containing the sodium salt of PCA are widely reported to aid in hydrating the skin and ameliorating dry flaky skin conditions.5,6 In addition, the zinc salt of L-pyrrolidone carboxylate is a longtime cosmetic ingredient due to antimicrobial and astringent qualities. This column briefly addresses the role of PCA in skin health.7
Dry skin
In a comprehensive literature review from 1981, Clar and Fourtanier reported conclusive evidence that PCA acts as a hydrating agent and that all the cosmetic formulations with a minimum of 2% PCA and PCA salt that they tested in their own 8-year study enhanced dry skin in short- and long-term conditions given suitable vehicles (no aqueous solutions).6
In a 2014 clinical study of 64 healthy white women with either normal or cosmetic dry skin, Feng et al. noted that tape stripped samples of stratum corneum revealed significantly lower ratios of free amino acids to protein and PCA to protein. This was associated with decreased hydration levels compared with normal skin. The investigators concluded that lower NMF levels across the depth of the stratum corneum and reduced cohesivity characterize cosmetic dry skin and that these clinical endpoints merit attention in evaluating the usefulness of treatments for dry skin.8
In 2016, Wei et al. reported on their assessment of the barrier function, hydration, and dryness of the lower leg skin of 25 female patients during the winter and then in the subsequent summer. They found that PCA levels were significantly greater during the summer, as were keratins. Hydration was also higher during the summer, while transepidermal water loss and visual dryness grades were substantially lower.9
Atopic dermatitis
A 2014 clinical study by Brandt et al. in patients with skin prone to developing atopic dermatitis (AD) revealed that a body wash composed of the filaggrin metabolites arginine and PCA was well tolerated and diminished pruritus. Patients reported liking the product and suggested that it improved their quality of life.10
Later that year, Jung et al. characterized the relationship of PCA levels, and other factors, with the clinical severity of AD. Specifically, in a study of 73 subjects (21 with mild AD, 21 with moderate to severe AD, 13 with X-linked ichthyosis as a negative control for filaggrin gene mutation, and 18 healthy controls), the investigators assessed transepidermal water loss, stratum corneum hydration, and skin surface pH. They found that PCA levels and caspase-14 were lower in inflammatory lesions compared with nonlesional skin in subjects with AD. These levels also were associated with clinical AD severity as measured by eczema area and severity index scores as well as skin barrier function.11
PCA as a biomarker
In 2009, Kezic et al. determined that the use of tape stripping to cull PCA in the stratum corneum was effective in revealing that PCA concentration in the outermost skin layer is a viable biomarker of filaggrin genotype.12
Raj et al. conducted an interesting study in 2016 in which they set out to describe the various markers for total NMF levels and link them to the activities of plasmin and corneocyte maturation in the photoexposed cheek and photoprotected postauricular regions of healthy white, black African, and albino African women in South Africa. PCA levels were highest among the albino African group, followed by black African and then white participants. The investigators also found that bleomycin hydrolase was linked to PCA synthesis, as suggested by higher bleomycin levels in albino African participants. In this group, corneocyte maturation was also observed to be impeded.13
The next year, the same team studied stratum corneum physiology and biochemistry of the cheeks in 48 white women with sensitive skin. The goal was to ascertain the connections between bleomycin hydrolase and calpain-1, PCA levels, corneocyte maturation, and transglutaminase and plasmin activities. Capsaicin sensitivity was observed in 52% of subjects, with PCA levels and bleomycin hydrolase activity found to be lower in the capsaicin-sensitive panel and correlated in subjects not sensitive to capsaicin. The researchers concluded that reduced levels of PCA, bleomycin hydrolase, and transglutaminase combined with a larger volume of immature corneocytes suggest comparatively poor stratum corneum maturation in individuals with sensitive skin.14
Other uses
In 2012, Takino et al. used cultured normal human dermal fibroblasts to show that zinc l-pyrrolidone carboxylate blocked UVA induction of activator protein-1, diminished matrix metalloproteinase-1 synthesis, and spurred type I collagen production. The researchers suggested that such results suggest the potential of zinc PCA for further investigation as an agent to combat photoaging.7
Conclusion
. Recent research suggests that it may serve as an important biomarker of filaggrin, NMF levels, and skin hydration. In addition, new data point to its usefulness as a gauge for ADs. More investigations are necessary to ascertain the feasibility of adjusting PCA levels through topical administration and what effects topically applied PCA may have on various skin parameters.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks, “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014), as well as a New York Times Best Sellers book for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC. Write to her at [email protected].
References
1. Björklund S et al. Soft Matter. 2014 Jul 7;10(25):4535-46.
2. Hall KJ, Hill JC. J Soc Cosmet Chem. 1986;37(6):397-407.
3. Tezuka T et al. Dermatology. 1994;188(1):21-4.
4. Kwoya Hakko Kogyo Co. Pyrrolidone carboxylic acid esters containing composition used to prevent loss of moisture from the skin. Patent JA 48 82 046 (1982).
5. Org Santerre. l-pyrrolidone carboxylic acid-sugar compounds as rehydrating ingredients in cosmetics. Patent Fr 2 277 823 (1977).
6. Clar EJ, Fourtanier A. Int J Cosmet Sci. 1981 Jun;3(3):101-13.
7. Takino Y et al. Int J Cosmet Sci. 2012 Feb;34(1):23-8.
8. Feng L et al. Int J Cosmet Sci. 2014 Jun;36(3):231-8.
9. Wei KS et al. J Cosmet Sci. 2016 May-Jun;67(3):185-203.
10. Brandt S et al. J Drugs Dermatol. 2014 Sep;13(9):1108-11.
11. Jung M et al. J Dermatol Sci. 2014 Dec;76(3):231-9.
12. Kezic S et al. Br J Dermatol. 2009 Nov;161(5):1098-104.
13. Raj N et al. Int J Cosmet Sci. 2016 Dec;38(6):567-75.
14. Raj N et al. Int J Cosmet Sci. 2017 Feb;39(1):2-10.
Seasonality
Did you notice that your practice slows down in February? In fact, if you plot your patient census over a few years, you may find that it dips every February. And you will discover other slow periods, perhaps in December, and busy months during other parts of the year.
Seasonality is yet another of those basic business concepts that most physicians have never heard of, because of the conspicuous lack of business training in medical schools. . Why are people more or less willing to spend money at certain times of the year? Analysts usually blame slow business during January or February on reluctance to buy products or services after the holiday season. They attribute summer peaks to everything from warm weather to an increased propensity to buy when students are out of school, and summer slumps to vacationing customers. It is not always easy – or necessary – to explain seasonality. The point is that such behavior patterns do exist.
It would seem that this behavior would be easy to change, by running some ads, or doing an e-mail blast; but unfortunately, altering a seasonal pattern is not an option for a small private practice. It can be done, but it is a deep pockets game requiring long, expensive campaigns that are only practical for large corporations.
For example, soup was traditionally consumed during the winter months since time immemorial. After years of pervasive advertising extolling its nutritional virtues (remember “Soup is Good Food”?), the soup industry succeeded in convincing the public to use their product year-round. Obviously, that kind of large-scale behavior modification is not practical for a local medical practice.
Does that mean there is nothing we can do about our practices’ seasonal variations? Not at all; but we must work within the realities of our patients’ seasonal behavior, rather than attempting to change that behavior outright.
First, you need to know what that behavior is, because it varies from practice to practice, even within the same state or city. Plotting your seasonality is easy; you can make a graph on Excel in a few minutes. Ask your office manager or accountant for month-by-month billing figures for the last 2 or 3 years. (Make sure it’s the amount billed, not collected, since the latter lags the former by several weeks at least.) Plot those figures on the vertical arm and time (in months) on the horizontal. Alternatively you can plot patient visits per month, if you wish; I do both.
Once you know your seasonality, review your options. Modify your own habits when necessary. If you typically take a vacation in August, for example, that’s not a great idea if August is one of your busiest months; consider vacationing during predictable slow periods instead.
Though I have said that you can’t change most seasonal behavior, it is possible to “retrain” some of your long-time, loyal patients to come in during your slower periods for at least some of their care. Use insurance company rules as a financial incentive, where possible. Many of my patients are on Medicare, so I send a notice to all of them in early November each year, urging them to come in during December (one of my light months) before their deductible has to be paid again.
If you advertise your services, do the bulk of it during your busiest months. That might seem counterintuitive; why not advertise during slow periods to fill those empty slots? But once again, you cannot change seasonal behavior with a low-budget, local advertising campaign; physicians who attempt it invariably get a poor response to their ads. So don’t try to move the mountain to Mohammed. Advertise during your busy periods, when seasonal patterns predict that potential patients are more willing to spend money and are more likely to respond to your message.
In short, then, try to “flatten” your seasonal dips by persuading as many existing patients as possible to return during slower seasons. You can then encourage new patients to make appointments when they are receptive to purchasing new services, your seasonal peaks. Once in your practice, some of them can then be shifted into your slower periods, especially for predictable, periodic care.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Did you notice that your practice slows down in February? In fact, if you plot your patient census over a few years, you may find that it dips every February. And you will discover other slow periods, perhaps in December, and busy months during other parts of the year.
Seasonality is yet another of those basic business concepts that most physicians have never heard of, because of the conspicuous lack of business training in medical schools. . Why are people more or less willing to spend money at certain times of the year? Analysts usually blame slow business during January or February on reluctance to buy products or services after the holiday season. They attribute summer peaks to everything from warm weather to an increased propensity to buy when students are out of school, and summer slumps to vacationing customers. It is not always easy – or necessary – to explain seasonality. The point is that such behavior patterns do exist.
It would seem that this behavior would be easy to change, by running some ads, or doing an e-mail blast; but unfortunately, altering a seasonal pattern is not an option for a small private practice. It can be done, but it is a deep pockets game requiring long, expensive campaigns that are only practical for large corporations.
For example, soup was traditionally consumed during the winter months since time immemorial. After years of pervasive advertising extolling its nutritional virtues (remember “Soup is Good Food”?), the soup industry succeeded in convincing the public to use their product year-round. Obviously, that kind of large-scale behavior modification is not practical for a local medical practice.
Does that mean there is nothing we can do about our practices’ seasonal variations? Not at all; but we must work within the realities of our patients’ seasonal behavior, rather than attempting to change that behavior outright.
First, you need to know what that behavior is, because it varies from practice to practice, even within the same state or city. Plotting your seasonality is easy; you can make a graph on Excel in a few minutes. Ask your office manager or accountant for month-by-month billing figures for the last 2 or 3 years. (Make sure it’s the amount billed, not collected, since the latter lags the former by several weeks at least.) Plot those figures on the vertical arm and time (in months) on the horizontal. Alternatively you can plot patient visits per month, if you wish; I do both.
Once you know your seasonality, review your options. Modify your own habits when necessary. If you typically take a vacation in August, for example, that’s not a great idea if August is one of your busiest months; consider vacationing during predictable slow periods instead.
Though I have said that you can’t change most seasonal behavior, it is possible to “retrain” some of your long-time, loyal patients to come in during your slower periods for at least some of their care. Use insurance company rules as a financial incentive, where possible. Many of my patients are on Medicare, so I send a notice to all of them in early November each year, urging them to come in during December (one of my light months) before their deductible has to be paid again.
If you advertise your services, do the bulk of it during your busiest months. That might seem counterintuitive; why not advertise during slow periods to fill those empty slots? But once again, you cannot change seasonal behavior with a low-budget, local advertising campaign; physicians who attempt it invariably get a poor response to their ads. So don’t try to move the mountain to Mohammed. Advertise during your busy periods, when seasonal patterns predict that potential patients are more willing to spend money and are more likely to respond to your message.
In short, then, try to “flatten” your seasonal dips by persuading as many existing patients as possible to return during slower seasons. You can then encourage new patients to make appointments when they are receptive to purchasing new services, your seasonal peaks. Once in your practice, some of them can then be shifted into your slower periods, especially for predictable, periodic care.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
Did you notice that your practice slows down in February? In fact, if you plot your patient census over a few years, you may find that it dips every February. And you will discover other slow periods, perhaps in December, and busy months during other parts of the year.
Seasonality is yet another of those basic business concepts that most physicians have never heard of, because of the conspicuous lack of business training in medical schools. . Why are people more or less willing to spend money at certain times of the year? Analysts usually blame slow business during January or February on reluctance to buy products or services after the holiday season. They attribute summer peaks to everything from warm weather to an increased propensity to buy when students are out of school, and summer slumps to vacationing customers. It is not always easy – or necessary – to explain seasonality. The point is that such behavior patterns do exist.
It would seem that this behavior would be easy to change, by running some ads, or doing an e-mail blast; but unfortunately, altering a seasonal pattern is not an option for a small private practice. It can be done, but it is a deep pockets game requiring long, expensive campaigns that are only practical for large corporations.
For example, soup was traditionally consumed during the winter months since time immemorial. After years of pervasive advertising extolling its nutritional virtues (remember “Soup is Good Food”?), the soup industry succeeded in convincing the public to use their product year-round. Obviously, that kind of large-scale behavior modification is not practical for a local medical practice.
Does that mean there is nothing we can do about our practices’ seasonal variations? Not at all; but we must work within the realities of our patients’ seasonal behavior, rather than attempting to change that behavior outright.
First, you need to know what that behavior is, because it varies from practice to practice, even within the same state or city. Plotting your seasonality is easy; you can make a graph on Excel in a few minutes. Ask your office manager or accountant for month-by-month billing figures for the last 2 or 3 years. (Make sure it’s the amount billed, not collected, since the latter lags the former by several weeks at least.) Plot those figures on the vertical arm and time (in months) on the horizontal. Alternatively you can plot patient visits per month, if you wish; I do both.
Once you know your seasonality, review your options. Modify your own habits when necessary. If you typically take a vacation in August, for example, that’s not a great idea if August is one of your busiest months; consider vacationing during predictable slow periods instead.
Though I have said that you can’t change most seasonal behavior, it is possible to “retrain” some of your long-time, loyal patients to come in during your slower periods for at least some of their care. Use insurance company rules as a financial incentive, where possible. Many of my patients are on Medicare, so I send a notice to all of them in early November each year, urging them to come in during December (one of my light months) before their deductible has to be paid again.
If you advertise your services, do the bulk of it during your busiest months. That might seem counterintuitive; why not advertise during slow periods to fill those empty slots? But once again, you cannot change seasonal behavior with a low-budget, local advertising campaign; physicians who attempt it invariably get a poor response to their ads. So don’t try to move the mountain to Mohammed. Advertise during your busy periods, when seasonal patterns predict that potential patients are more willing to spend money and are more likely to respond to your message.
In short, then, try to “flatten” your seasonal dips by persuading as many existing patients as possible to return during slower seasons. You can then encourage new patients to make appointments when they are receptive to purchasing new services, your seasonal peaks. Once in your practice, some of them can then be shifted into your slower periods, especially for predictable, periodic care.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J. He is the author of numerous articles and textbook chapters, and is a longtime monthly columnist for Dermatology News. Write to him at [email protected].
The power and promise of person-generated health data – part 1
The time shared during clinical encounters provides small peeks into patients’ lives that get documented as episodic snapshots in electronic health records. But there is little information about how patients are doing outside of the office. With increasing emphasis on filling out mandatory parts of the EHR, there is less time available for in-depth, in-office conversations and phone follow-ups.
At the same time, it has become clear that it is not just the medicines we prescribe that affect our patients’ lives. Their behaviors outside of the office – being physically active, eating well, getting a good night’s rest, and adhering to medications – also impact their health outcomes.
The explosion of technology and personal data in our increasingly connected world provides powerful new sources of health and behavior information that generate new understanding of patients’ lives in their everyday settings.
The ubiquity and remarkable technological progress of personal computing devices – including wearables, smartphones, and tablets – along with the multitude of sensor modalities embedded within these devices, has enabled us to establish a continuous connection with people who want to share information about their behavior and daily life.
Such rich, longitudinal information, known as person-generated health data (PGHD), can be searched for physiological and behavioral signatures that can be used in combination with traditional clinical information to predict, diagnose, and treat disease. It can also be used to understand the safety and effectiveness of medical interventions.
PGHD is defined as wellness and/or health-related data created, recorded, or gathered by individuals. It reflects events and interactions that occur during an person’s everyday life. Systematically gathering this information and organizing it to better understand patients’ approach to their health or their unique experience living with disease provides meaningful insights that complement the data traditionally collected as part of clinical trials or periodic office visits.
PGHD can produce a rich picture of a person’s health or symptom burden with disease. It allows the opportunity to measure the real human burden of a patient’s disease and how it changes over time, with an opportunity to detect changes in symptoms in real time.
PGHD can also enable participation in health research.
An example would be the work of Evidation Health in San Mateo, Calif. Evidation provides a platform to run research studies utilizing technology and systems to measure health in everyday life. Its app, Achievement, collects continuous behavior-related data from smartphones, wearables, connected devices, and apps. That provides opportunities for participants to join research studies that develop novel measures designed to quantify health outcomes in a way that more accurately reflects an individual’s day-to-day activities and experience. All data collected are at the direction of and with the permission of the individual.
“Achievers” are given points for taking health-related actions such as tracking steps or their sleep, which convert to cash that can be kept or donated to their favorite charities. Achievement’s 3.5 million diverse participants also receive offers to join research studies. This paradigm shift dramatically expands access to research to increase diversity, shortens the time to first data through rapid recruitment, and enhances retention rates by making it easier to engage. To date, more than 1 million users have chosen to participate in research studies. The technology is bringing new data and insights to health research; it supports important questions about quality of life, medical products’ real-world effectiveness, and the development of hyperpersonalized health care services.
This new type of data is transforming medical research by creating real-world studies of unprecedented size, such as the Apple Heart Study – a virtual study with more than 400,000 enrolled participants – which was designed to test the accuracy of Apple Watches in safely identifying atrial fibrillation. The FDA has cleared two features on the Apple Watch: the device’s ability to detect and notify the user of an irregular heart rhythm, and the ability to take a single-lead EKG feature that can provide a rhythm strip for a clinician to review.
The FDA clearance letters specify that the apps are “not intended to replace traditional methods of diagnosis or treatment.” They provide extra information, and that information might be helpful – but the apps won’t replace a doctor’s visit. It remains to be seen how these data will be used, but they have the potential to identify atrial fibrillation early, leading to treatment that may prevent devastating strokes.
Another example of home-generated health data is a tool that has obtained FDA clearance as a diagnostic device with insurance reimbursement: WatchPAT, a portable sleep apnea diagnostic device. WatchPAT is worn like a simple wristwatch, with no need for belts, wires, or nasal cannulas.
Over time, in-home tests like these that are of minimal inconvenience to the patient and reflect a real-world experience may eclipse traditional sleep studies that require patients to spend the night in a clinic while attached to wires and monitors.
Health data generated by connected populations will yield novel insights that may help us better predict, diagnose, and treat disease. These are examples of innovations that can extend clinicians’ abilities to remotely monitor or diagnose health conditions, and we can expect that more will continue to be integrated into the clinical and research settings in the near future.
In part 2 of this series, we will discuss novel digital measures and studies utilizing PGHD to impact population health.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, family medicine residency program, Abington (Pa.) Jefferson Health. Dr. Foschini is cofounder and chief data scientist at Evidation Health in San Mateo, Calif. Bray Patrick-Lake is a patient thought leader and director, strategic partnerships, at Evidation Health.
References
Determining real-world data’s fitness for use and the role of reliability, September 2019. Duke-Margolis Center for Health Policy.
N Engl J Med. 2019 Nov 14;381(20):1909-17.
The time shared during clinical encounters provides small peeks into patients’ lives that get documented as episodic snapshots in electronic health records. But there is little information about how patients are doing outside of the office. With increasing emphasis on filling out mandatory parts of the EHR, there is less time available for in-depth, in-office conversations and phone follow-ups.
At the same time, it has become clear that it is not just the medicines we prescribe that affect our patients’ lives. Their behaviors outside of the office – being physically active, eating well, getting a good night’s rest, and adhering to medications – also impact their health outcomes.
The explosion of technology and personal data in our increasingly connected world provides powerful new sources of health and behavior information that generate new understanding of patients’ lives in their everyday settings.
The ubiquity and remarkable technological progress of personal computing devices – including wearables, smartphones, and tablets – along with the multitude of sensor modalities embedded within these devices, has enabled us to establish a continuous connection with people who want to share information about their behavior and daily life.
Such rich, longitudinal information, known as person-generated health data (PGHD), can be searched for physiological and behavioral signatures that can be used in combination with traditional clinical information to predict, diagnose, and treat disease. It can also be used to understand the safety and effectiveness of medical interventions.
PGHD is defined as wellness and/or health-related data created, recorded, or gathered by individuals. It reflects events and interactions that occur during an person’s everyday life. Systematically gathering this information and organizing it to better understand patients’ approach to their health or their unique experience living with disease provides meaningful insights that complement the data traditionally collected as part of clinical trials or periodic office visits.
PGHD can produce a rich picture of a person’s health or symptom burden with disease. It allows the opportunity to measure the real human burden of a patient’s disease and how it changes over time, with an opportunity to detect changes in symptoms in real time.
PGHD can also enable participation in health research.
An example would be the work of Evidation Health in San Mateo, Calif. Evidation provides a platform to run research studies utilizing technology and systems to measure health in everyday life. Its app, Achievement, collects continuous behavior-related data from smartphones, wearables, connected devices, and apps. That provides opportunities for participants to join research studies that develop novel measures designed to quantify health outcomes in a way that more accurately reflects an individual’s day-to-day activities and experience. All data collected are at the direction of and with the permission of the individual.
“Achievers” are given points for taking health-related actions such as tracking steps or their sleep, which convert to cash that can be kept or donated to their favorite charities. Achievement’s 3.5 million diverse participants also receive offers to join research studies. This paradigm shift dramatically expands access to research to increase diversity, shortens the time to first data through rapid recruitment, and enhances retention rates by making it easier to engage. To date, more than 1 million users have chosen to participate in research studies. The technology is bringing new data and insights to health research; it supports important questions about quality of life, medical products’ real-world effectiveness, and the development of hyperpersonalized health care services.
This new type of data is transforming medical research by creating real-world studies of unprecedented size, such as the Apple Heart Study – a virtual study with more than 400,000 enrolled participants – which was designed to test the accuracy of Apple Watches in safely identifying atrial fibrillation. The FDA has cleared two features on the Apple Watch: the device’s ability to detect and notify the user of an irregular heart rhythm, and the ability to take a single-lead EKG feature that can provide a rhythm strip for a clinician to review.
The FDA clearance letters specify that the apps are “not intended to replace traditional methods of diagnosis or treatment.” They provide extra information, and that information might be helpful – but the apps won’t replace a doctor’s visit. It remains to be seen how these data will be used, but they have the potential to identify atrial fibrillation early, leading to treatment that may prevent devastating strokes.
Another example of home-generated health data is a tool that has obtained FDA clearance as a diagnostic device with insurance reimbursement: WatchPAT, a portable sleep apnea diagnostic device. WatchPAT is worn like a simple wristwatch, with no need for belts, wires, or nasal cannulas.
Over time, in-home tests like these that are of minimal inconvenience to the patient and reflect a real-world experience may eclipse traditional sleep studies that require patients to spend the night in a clinic while attached to wires and monitors.
Health data generated by connected populations will yield novel insights that may help us better predict, diagnose, and treat disease. These are examples of innovations that can extend clinicians’ abilities to remotely monitor or diagnose health conditions, and we can expect that more will continue to be integrated into the clinical and research settings in the near future.
In part 2 of this series, we will discuss novel digital measures and studies utilizing PGHD to impact population health.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, family medicine residency program, Abington (Pa.) Jefferson Health. Dr. Foschini is cofounder and chief data scientist at Evidation Health in San Mateo, Calif. Bray Patrick-Lake is a patient thought leader and director, strategic partnerships, at Evidation Health.
References
Determining real-world data’s fitness for use and the role of reliability, September 2019. Duke-Margolis Center for Health Policy.
N Engl J Med. 2019 Nov 14;381(20):1909-17.
The time shared during clinical encounters provides small peeks into patients’ lives that get documented as episodic snapshots in electronic health records. But there is little information about how patients are doing outside of the office. With increasing emphasis on filling out mandatory parts of the EHR, there is less time available for in-depth, in-office conversations and phone follow-ups.
At the same time, it has become clear that it is not just the medicines we prescribe that affect our patients’ lives. Their behaviors outside of the office – being physically active, eating well, getting a good night’s rest, and adhering to medications – also impact their health outcomes.
The explosion of technology and personal data in our increasingly connected world provides powerful new sources of health and behavior information that generate new understanding of patients’ lives in their everyday settings.
The ubiquity and remarkable technological progress of personal computing devices – including wearables, smartphones, and tablets – along with the multitude of sensor modalities embedded within these devices, has enabled us to establish a continuous connection with people who want to share information about their behavior and daily life.
Such rich, longitudinal information, known as person-generated health data (PGHD), can be searched for physiological and behavioral signatures that can be used in combination with traditional clinical information to predict, diagnose, and treat disease. It can also be used to understand the safety and effectiveness of medical interventions.
PGHD is defined as wellness and/or health-related data created, recorded, or gathered by individuals. It reflects events and interactions that occur during an person’s everyday life. Systematically gathering this information and organizing it to better understand patients’ approach to their health or their unique experience living with disease provides meaningful insights that complement the data traditionally collected as part of clinical trials or periodic office visits.
PGHD can produce a rich picture of a person’s health or symptom burden with disease. It allows the opportunity to measure the real human burden of a patient’s disease and how it changes over time, with an opportunity to detect changes in symptoms in real time.
PGHD can also enable participation in health research.
An example would be the work of Evidation Health in San Mateo, Calif. Evidation provides a platform to run research studies utilizing technology and systems to measure health in everyday life. Its app, Achievement, collects continuous behavior-related data from smartphones, wearables, connected devices, and apps. That provides opportunities for participants to join research studies that develop novel measures designed to quantify health outcomes in a way that more accurately reflects an individual’s day-to-day activities and experience. All data collected are at the direction of and with the permission of the individual.
“Achievers” are given points for taking health-related actions such as tracking steps or their sleep, which convert to cash that can be kept or donated to their favorite charities. Achievement’s 3.5 million diverse participants also receive offers to join research studies. This paradigm shift dramatically expands access to research to increase diversity, shortens the time to first data through rapid recruitment, and enhances retention rates by making it easier to engage. To date, more than 1 million users have chosen to participate in research studies. The technology is bringing new data and insights to health research; it supports important questions about quality of life, medical products’ real-world effectiveness, and the development of hyperpersonalized health care services.
This new type of data is transforming medical research by creating real-world studies of unprecedented size, such as the Apple Heart Study – a virtual study with more than 400,000 enrolled participants – which was designed to test the accuracy of Apple Watches in safely identifying atrial fibrillation. The FDA has cleared two features on the Apple Watch: the device’s ability to detect and notify the user of an irregular heart rhythm, and the ability to take a single-lead EKG feature that can provide a rhythm strip for a clinician to review.
The FDA clearance letters specify that the apps are “not intended to replace traditional methods of diagnosis or treatment.” They provide extra information, and that information might be helpful – but the apps won’t replace a doctor’s visit. It remains to be seen how these data will be used, but they have the potential to identify atrial fibrillation early, leading to treatment that may prevent devastating strokes.
Another example of home-generated health data is a tool that has obtained FDA clearance as a diagnostic device with insurance reimbursement: WatchPAT, a portable sleep apnea diagnostic device. WatchPAT is worn like a simple wristwatch, with no need for belts, wires, or nasal cannulas.
Over time, in-home tests like these that are of minimal inconvenience to the patient and reflect a real-world experience may eclipse traditional sleep studies that require patients to spend the night in a clinic while attached to wires and monitors.
Health data generated by connected populations will yield novel insights that may help us better predict, diagnose, and treat disease. These are examples of innovations that can extend clinicians’ abilities to remotely monitor or diagnose health conditions, and we can expect that more will continue to be integrated into the clinical and research settings in the near future.
In part 2 of this series, we will discuss novel digital measures and studies utilizing PGHD to impact population health.
Dr. Skolnik is professor of family and community medicine at Sidney Kimmel Medical College, Thomas Jefferson University, Philadelphia, and associate director, family medicine residency program, Abington (Pa.) Jefferson Health. Dr. Foschini is cofounder and chief data scientist at Evidation Health in San Mateo, Calif. Bray Patrick-Lake is a patient thought leader and director, strategic partnerships, at Evidation Health.
References
Determining real-world data’s fitness for use and the role of reliability, September 2019. Duke-Margolis Center for Health Policy.
N Engl J Med. 2019 Nov 14;381(20):1909-17.