User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Mood stabilizers, particularly lithium, potential lifesavers in bipolar disorder
Investigators led by Pao-Huan Chen, MD, of the department of psychiatry, Taipei Medical University Hospital, Taiwan, evaluated the association between the use of mood stabilizers and the risks for all-cause mortality, suicide, and natural mortality in more than 25,000 patients with BD and found that those with BD had higher mortality.
However, they also found that patients with BD had a significantly decreased adjusted 5-year risk of dying from any cause, suicide, and natural causes. Lithium was associated with the largest risk reduction compared with the other mood stabilizers.
“The present findings highlight the potential role of mood stabilizers, particularly lithium, in reducing mortality among patients with bipolar disorder,” the authors write.
“The findings of this study could inform future clinical and mechanistic research evaluating the multifaceted effects of mood stabilizers, particularly lithium, on the psychological and physiological statuses of patients with bipolar disorder,” they add.
The study was published online in Acta Psychiatrica Scandinavica.
Research gap
Patients with BD have an elevated risk for multiple comorbidities in addition to mood symptoms and neurocognitive dysfunction, with previous research suggesting a mortality rate due to suicide and natural causes that is at least twice as high as that of the general population, the authors write.
Lithium, in particular, has been associated with decreased risk for all-cause mortality and suicide in patients with BD, but findings regarding anticonvulsant mood stabilizers have been “inconsistent.”
To fill this research gap, the researchers evaluated 16 years of data from Taiwan’s National Health Insurance Research Database, which includes information about more than 23 million residents of Taiwan. The current study, which encompassed 25,787 patients with BD, looked at data from the 5-year period after index hospitalization.
The researchers hypothesized that mood stabilizers “would decrease the risk of mortality” among patients with BD and that “different mood stabilizers would exhibit different associations with mortality, owing to their varying effects on mood symptoms and physiological function.”
Covariates included sex, age, employment status, comorbidities, and concomitant drugs.
Of the patients with BD, 4,000 died within the 5-year period. Suicide and natural causes accounted for 19.0% and 73.7% of these deaths, respectively.
Cardioprotective effects?
The standardized mortality ratios (SMRs) – the ratios of observed mortality in the BD cohort to the number of expected deaths in the general population – were 5.26 for all causes (95% confidence interval, 5.10-5.43), 26.02 for suicide (95% CI, 24.20-27.93), and 4.68 for natural causes (95% CI, 4.51-4.85).
The cumulative mortality rate was higher among men vs. women, a difference that was even larger among patients who had died from any cause or natural causes (crude hazard ratios, .60 and .52, respectively; both Ps < .001).
The suicide risk peaked between ages 45 and 65 years, whereas the risks for all-cause and natural mortality increased with age and were highest in those older than 65 years.
Patients who had died from any cause or from natural causes had a higher risk for physical and psychiatric comorbidities, whereas those who had died by suicide had a higher risk for primarily psychiatric comorbidities.
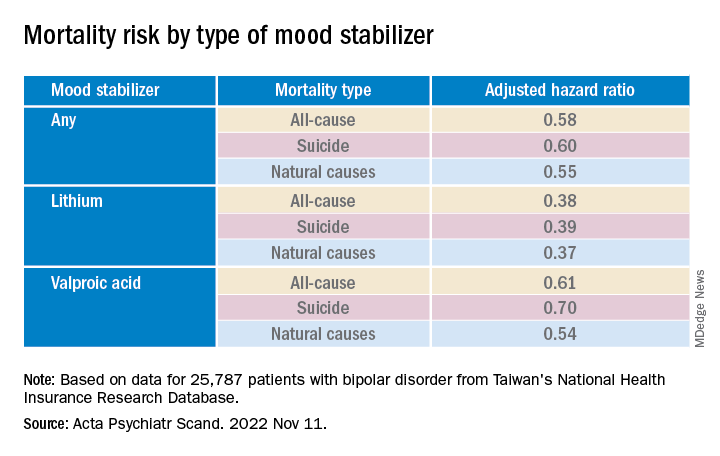
Mood stabilizers were associated with decreased risks for all-cause mortality and natural mortality, with lithium and valproic acid tied to the lowest risk for all three mortality types (all Ps < .001).
Lamotrigine and carbamazepine were “not significantly associated with any type of mortality,” the authors report.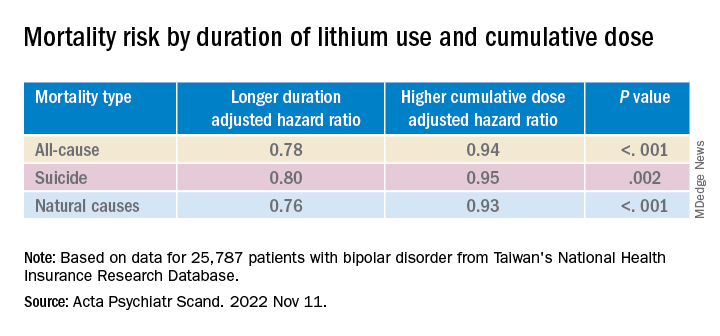
Longer duration of lithium use and a higher cumulative dose of lithium were both associated with lower risks for all three types of mortality (all Ps < .001).
Valproic acid was associated with dose-dependent decreases in all-cause and natural mortality risks.
The findings suggest that mood stabilizers “may improve not only psychosocial outcomes but also the physical health of patients with BD,” the investigators note.
The association between mood stabilizer use and reduced natural mortality risk “may be attributable to the potential benefits of psychiatric care” but may also “have resulted from the direct effects of mood stabilizers on physiological functions,” they add.
Some research suggests lithium treatment may reduce the risk for cardiovascular disease in patients with BD. Mechanistic studies have also pointed to potential cardioprotective effects from valproic acid.
The authors note several study limitations. Focusing on hospitalized patients “may have led to selection bias and overestimated mortality risk.” Moreover, the analyses were “based on the prescription, not the consumption, of mood stabilizers” and information regarding adherence was unavailable.
The absence of a protective mechanism of lamotrigine and carbamazepine may be attributable to “bias toward the relatively poor treatment responses” of these agents, neither of which is used as a first-line medication to treat BD in Taiwan. Patients taking these agents “may not receive medical care at a level equal to those taking lithium, who tend to receive closer surveillance, owing to the narrow therapeutic index.”
First-line treatment
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said that the data “add to a growing confluence of data from observational studies indicating that lithium especially is capable of reducing all-cause mortality, suicide mortality, and natural mortality.”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, agreed with the authors that lamotrigine is “not a very popular drug in Taiwan, therefore we may not have sufficient assay sensitivity to document the effect.”
But lamotrigine “does have recurrence prevention effects in BD, especially bipolar depression, and it would be expected that it would reduce suicide potentially especially in such a large sample.”
The study’s take-home message “is that the extant evidence now indicates that lithium should be a first-line treatment in persons who live with BD who are experiencing suicidal ideation and/or behavior and these data should inform algorithms of treatment selection and sequencing in clinical practice guidelines,” said Dr. McIntyre.
This research was supported by grants from the Ministry of Science and Technology in Taiwan and Taipei City Hospital. The authors declared no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China, and the Milken Institute; and speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe Pharma, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
Investigators led by Pao-Huan Chen, MD, of the department of psychiatry, Taipei Medical University Hospital, Taiwan, evaluated the association between the use of mood stabilizers and the risks for all-cause mortality, suicide, and natural mortality in more than 25,000 patients with BD and found that those with BD had higher mortality.
However, they also found that patients with BD had a significantly decreased adjusted 5-year risk of dying from any cause, suicide, and natural causes. Lithium was associated with the largest risk reduction compared with the other mood stabilizers.
“The present findings highlight the potential role of mood stabilizers, particularly lithium, in reducing mortality among patients with bipolar disorder,” the authors write.
“The findings of this study could inform future clinical and mechanistic research evaluating the multifaceted effects of mood stabilizers, particularly lithium, on the psychological and physiological statuses of patients with bipolar disorder,” they add.
The study was published online in Acta Psychiatrica Scandinavica.
Research gap
Patients with BD have an elevated risk for multiple comorbidities in addition to mood symptoms and neurocognitive dysfunction, with previous research suggesting a mortality rate due to suicide and natural causes that is at least twice as high as that of the general population, the authors write.

Lithium, in particular, has been associated with decreased risk for all-cause mortality and suicide in patients with BD, but findings regarding anticonvulsant mood stabilizers have been “inconsistent.”
To fill this research gap, the researchers evaluated 16 years of data from Taiwan’s National Health Insurance Research Database, which includes information about more than 23 million residents of Taiwan. The current study, which encompassed 25,787 patients with BD, looked at data from the 5-year period after index hospitalization.
The researchers hypothesized that mood stabilizers “would decrease the risk of mortality” among patients with BD and that “different mood stabilizers would exhibit different associations with mortality, owing to their varying effects on mood symptoms and physiological function.”
Covariates included sex, age, employment status, comorbidities, and concomitant drugs.
Of the patients with BD, 4,000 died within the 5-year period. Suicide and natural causes accounted for 19.0% and 73.7% of these deaths, respectively.
Cardioprotective effects?
The standardized mortality ratios (SMRs) – the ratios of observed mortality in the BD cohort to the number of expected deaths in the general population – were 5.26 for all causes (95% confidence interval, 5.10-5.43), 26.02 for suicide (95% CI, 24.20-27.93), and 4.68 for natural causes (95% CI, 4.51-4.85).
The cumulative mortality rate was higher among men vs. women, a difference that was even larger among patients who had died from any cause or natural causes (crude hazard ratios, .60 and .52, respectively; both Ps < .001).
The suicide risk peaked between ages 45 and 65 years, whereas the risks for all-cause and natural mortality increased with age and were highest in those older than 65 years.
Patients who had died from any cause or from natural causes had a higher risk for physical and psychiatric comorbidities, whereas those who had died by suicide had a higher risk for primarily psychiatric comorbidities.
Mood stabilizers were associated with decreased risks for all-cause mortality and natural mortality, with lithium and valproic acid tied to the lowest risk for all three mortality types (all Ps < .001).
Lamotrigine and carbamazepine were “not significantly associated with any type of mortality,” the authors report.
Longer duration of lithium use and a higher cumulative dose of lithium were both associated with lower risks for all three types of mortality (all Ps < .001).
Valproic acid was associated with dose-dependent decreases in all-cause and natural mortality risks.
The findings suggest that mood stabilizers “may improve not only psychosocial outcomes but also the physical health of patients with BD,” the investigators note.
The association between mood stabilizer use and reduced natural mortality risk “may be attributable to the potential benefits of psychiatric care” but may also “have resulted from the direct effects of mood stabilizers on physiological functions,” they add.
Some research suggests lithium treatment may reduce the risk for cardiovascular disease in patients with BD. Mechanistic studies have also pointed to potential cardioprotective effects from valproic acid.
The authors note several study limitations. Focusing on hospitalized patients “may have led to selection bias and overestimated mortality risk.” Moreover, the analyses were “based on the prescription, not the consumption, of mood stabilizers” and information regarding adherence was unavailable.
The absence of a protective mechanism of lamotrigine and carbamazepine may be attributable to “bias toward the relatively poor treatment responses” of these agents, neither of which is used as a first-line medication to treat BD in Taiwan. Patients taking these agents “may not receive medical care at a level equal to those taking lithium, who tend to receive closer surveillance, owing to the narrow therapeutic index.”
First-line treatment
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said that the data “add to a growing confluence of data from observational studies indicating that lithium especially is capable of reducing all-cause mortality, suicide mortality, and natural mortality.”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, agreed with the authors that lamotrigine is “not a very popular drug in Taiwan, therefore we may not have sufficient assay sensitivity to document the effect.”
But lamotrigine “does have recurrence prevention effects in BD, especially bipolar depression, and it would be expected that it would reduce suicide potentially especially in such a large sample.”
The study’s take-home message “is that the extant evidence now indicates that lithium should be a first-line treatment in persons who live with BD who are experiencing suicidal ideation and/or behavior and these data should inform algorithms of treatment selection and sequencing in clinical practice guidelines,” said Dr. McIntyre.
This research was supported by grants from the Ministry of Science and Technology in Taiwan and Taipei City Hospital. The authors declared no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China, and the Milken Institute; and speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe Pharma, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
Investigators led by Pao-Huan Chen, MD, of the department of psychiatry, Taipei Medical University Hospital, Taiwan, evaluated the association between the use of mood stabilizers and the risks for all-cause mortality, suicide, and natural mortality in more than 25,000 patients with BD and found that those with BD had higher mortality.
However, they also found that patients with BD had a significantly decreased adjusted 5-year risk of dying from any cause, suicide, and natural causes. Lithium was associated with the largest risk reduction compared with the other mood stabilizers.
“The present findings highlight the potential role of mood stabilizers, particularly lithium, in reducing mortality among patients with bipolar disorder,” the authors write.
“The findings of this study could inform future clinical and mechanistic research evaluating the multifaceted effects of mood stabilizers, particularly lithium, on the psychological and physiological statuses of patients with bipolar disorder,” they add.
The study was published online in Acta Psychiatrica Scandinavica.
Research gap
Patients with BD have an elevated risk for multiple comorbidities in addition to mood symptoms and neurocognitive dysfunction, with previous research suggesting a mortality rate due to suicide and natural causes that is at least twice as high as that of the general population, the authors write.

Lithium, in particular, has been associated with decreased risk for all-cause mortality and suicide in patients with BD, but findings regarding anticonvulsant mood stabilizers have been “inconsistent.”
To fill this research gap, the researchers evaluated 16 years of data from Taiwan’s National Health Insurance Research Database, which includes information about more than 23 million residents of Taiwan. The current study, which encompassed 25,787 patients with BD, looked at data from the 5-year period after index hospitalization.
The researchers hypothesized that mood stabilizers “would decrease the risk of mortality” among patients with BD and that “different mood stabilizers would exhibit different associations with mortality, owing to their varying effects on mood symptoms and physiological function.”
Covariates included sex, age, employment status, comorbidities, and concomitant drugs.
Of the patients with BD, 4,000 died within the 5-year period. Suicide and natural causes accounted for 19.0% and 73.7% of these deaths, respectively.
Cardioprotective effects?
The standardized mortality ratios (SMRs) – the ratios of observed mortality in the BD cohort to the number of expected deaths in the general population – were 5.26 for all causes (95% confidence interval, 5.10-5.43), 26.02 for suicide (95% CI, 24.20-27.93), and 4.68 for natural causes (95% CI, 4.51-4.85).
The cumulative mortality rate was higher among men vs. women, a difference that was even larger among patients who had died from any cause or natural causes (crude hazard ratios, .60 and .52, respectively; both Ps < .001).
The suicide risk peaked between ages 45 and 65 years, whereas the risks for all-cause and natural mortality increased with age and were highest in those older than 65 years.
Patients who had died from any cause or from natural causes had a higher risk for physical and psychiatric comorbidities, whereas those who had died by suicide had a higher risk for primarily psychiatric comorbidities.
Mood stabilizers were associated with decreased risks for all-cause mortality and natural mortality, with lithium and valproic acid tied to the lowest risk for all three mortality types (all Ps < .001).
Lamotrigine and carbamazepine were “not significantly associated with any type of mortality,” the authors report.
Longer duration of lithium use and a higher cumulative dose of lithium were both associated with lower risks for all three types of mortality (all Ps < .001).
Valproic acid was associated with dose-dependent decreases in all-cause and natural mortality risks.
The findings suggest that mood stabilizers “may improve not only psychosocial outcomes but also the physical health of patients with BD,” the investigators note.
The association between mood stabilizer use and reduced natural mortality risk “may be attributable to the potential benefits of psychiatric care” but may also “have resulted from the direct effects of mood stabilizers on physiological functions,” they add.
Some research suggests lithium treatment may reduce the risk for cardiovascular disease in patients with BD. Mechanistic studies have also pointed to potential cardioprotective effects from valproic acid.
The authors note several study limitations. Focusing on hospitalized patients “may have led to selection bias and overestimated mortality risk.” Moreover, the analyses were “based on the prescription, not the consumption, of mood stabilizers” and information regarding adherence was unavailable.
The absence of a protective mechanism of lamotrigine and carbamazepine may be attributable to “bias toward the relatively poor treatment responses” of these agents, neither of which is used as a first-line medication to treat BD in Taiwan. Patients taking these agents “may not receive medical care at a level equal to those taking lithium, who tend to receive closer surveillance, owing to the narrow therapeutic index.”
First-line treatment
Commenting on the study, Roger S. McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the mood disorders psychopharmacology unit, said that the data “add to a growing confluence of data from observational studies indicating that lithium especially is capable of reducing all-cause mortality, suicide mortality, and natural mortality.”
Dr. McIntyre, chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, who was not involved with the study, agreed with the authors that lamotrigine is “not a very popular drug in Taiwan, therefore we may not have sufficient assay sensitivity to document the effect.”
But lamotrigine “does have recurrence prevention effects in BD, especially bipolar depression, and it would be expected that it would reduce suicide potentially especially in such a large sample.”
The study’s take-home message “is that the extant evidence now indicates that lithium should be a first-line treatment in persons who live with BD who are experiencing suicidal ideation and/or behavior and these data should inform algorithms of treatment selection and sequencing in clinical practice guidelines,” said Dr. McIntyre.
This research was supported by grants from the Ministry of Science and Technology in Taiwan and Taipei City Hospital. The authors declared no relevant financial relationships. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China, and the Milken Institute; and speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Boehringer Ingelheim, Sage, Biogen, Mitsubishi Tanabe Pharma, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Viatris, AbbVie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
FROM ACTA PSYCHIATRICA SCANDINAVICA
Have you heard of VEXAS syndrome?
Its name is an acronym: Vacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic. The prevalence of this syndrome is unknown, but it is not so rare. As it is an X-linked disease, men are predominantly affected.
First identification
The NIH team screened the exomes and genomes of 2,560 individuals. Of this group, 1,477 had been referred because of undiagnosed recurrent fevers, systemic inflammation, or both, and 1,083 were affected by atypical, unclassified disorders. The researchers identified 25 men with a somatic mutation in the ubiquitin-like modifier activating enzyme 1 (UBA1) gene, which is involved in the protein ubiquitylation system. This posttranslational modification has a pleiotropic function that likely explains the clinical heterogeneity seen in VEXAS patients: regulation of protein turnover, especially those involved in the cell cycle, cell death, and signal transduction. Ubiquitylation is also involved in nonproteolytic functions, such as assembly of multiprotein complexes, intracellular signaling, inflammatory signaling, and DNA repair.
Clinical presentation
The clinicobiological presentation of VEXAS syndrome is very heterogeneous. Typically, patients present with a systemic inflammatory disease with unexplained episodes of fever, involvement of the lungs, skin, blood vessels, and joints. Molecular diagnosis is made by the sequencing of UBA1.
Most patients present with the characteristic clinical signs of other inflammatory diseases, such as polyarteritis nodosa and recurrent polychondritis. But VEXAS patients are at high risk of developing hematologic conditions. Indeed, the following were seen among the 25 participants in the NIH study: macrocytic anemia (96%), venous thromboembolism (44%), myelodysplastic syndrome (24%), and multiple myeloma or monoclonal gammopathy of undetermined significance (20%).
In VEXAS patients, levels of serum inflammatory markers are increased. These markers include tumor necrosis factor, interleukin-8, interleukin-6, interferon-inducible protein-10, interferon-gamma, C-reactive protein. In addition, there is aberrant activation of innate immune-signaling pathways.
In a large-scale analysis of a multicenter case series of 116 French patients, researchers found that VEXAS syndrome primarily affected men. The disease was progressive, and onset occurred after age 50 years. These patients can be divided into three phenotypically distinct clusters on the basis of integration of clinical and biological data. In the 58 cases in which myelodysplastic syndrome was present, the mortality rates were higher. The researchers also reported that the UBA1 p.Met41L mutation was associated with a better prognosis.
Treatment data
VEXAS syndrome resists the classical therapeutic arsenal. Patients require high-dose glucocorticoids, and prognosis appears to be poor. The available treatment data are retrospective. Of the 25 participants in the NIH study, 40% died within 5 years from disease-related causes or complications related to treatment. Among the promising therapeutic avenues is the use of inhibitors of the Janus kinase pathway.
This article was translated from Univadis France. A version of this article appeared on Medscape.com.
Its name is an acronym: Vacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic. The prevalence of this syndrome is unknown, but it is not so rare. As it is an X-linked disease, men are predominantly affected.
First identification
The NIH team screened the exomes and genomes of 2,560 individuals. Of this group, 1,477 had been referred because of undiagnosed recurrent fevers, systemic inflammation, or both, and 1,083 were affected by atypical, unclassified disorders. The researchers identified 25 men with a somatic mutation in the ubiquitin-like modifier activating enzyme 1 (UBA1) gene, which is involved in the protein ubiquitylation system. This posttranslational modification has a pleiotropic function that likely explains the clinical heterogeneity seen in VEXAS patients: regulation of protein turnover, especially those involved in the cell cycle, cell death, and signal transduction. Ubiquitylation is also involved in nonproteolytic functions, such as assembly of multiprotein complexes, intracellular signaling, inflammatory signaling, and DNA repair.
Clinical presentation
The clinicobiological presentation of VEXAS syndrome is very heterogeneous. Typically, patients present with a systemic inflammatory disease with unexplained episodes of fever, involvement of the lungs, skin, blood vessels, and joints. Molecular diagnosis is made by the sequencing of UBA1.
Most patients present with the characteristic clinical signs of other inflammatory diseases, such as polyarteritis nodosa and recurrent polychondritis. But VEXAS patients are at high risk of developing hematologic conditions. Indeed, the following were seen among the 25 participants in the NIH study: macrocytic anemia (96%), venous thromboembolism (44%), myelodysplastic syndrome (24%), and multiple myeloma or monoclonal gammopathy of undetermined significance (20%).
In VEXAS patients, levels of serum inflammatory markers are increased. These markers include tumor necrosis factor, interleukin-8, interleukin-6, interferon-inducible protein-10, interferon-gamma, C-reactive protein. In addition, there is aberrant activation of innate immune-signaling pathways.
In a large-scale analysis of a multicenter case series of 116 French patients, researchers found that VEXAS syndrome primarily affected men. The disease was progressive, and onset occurred after age 50 years. These patients can be divided into three phenotypically distinct clusters on the basis of integration of clinical and biological data. In the 58 cases in which myelodysplastic syndrome was present, the mortality rates were higher. The researchers also reported that the UBA1 p.Met41L mutation was associated with a better prognosis.
Treatment data
VEXAS syndrome resists the classical therapeutic arsenal. Patients require high-dose glucocorticoids, and prognosis appears to be poor. The available treatment data are retrospective. Of the 25 participants in the NIH study, 40% died within 5 years from disease-related causes or complications related to treatment. Among the promising therapeutic avenues is the use of inhibitors of the Janus kinase pathway.
This article was translated from Univadis France. A version of this article appeared on Medscape.com.
Its name is an acronym: Vacuoles, E1 enzyme, X-linked, Autoinflammatory, Somatic. The prevalence of this syndrome is unknown, but it is not so rare. As it is an X-linked disease, men are predominantly affected.
First identification
The NIH team screened the exomes and genomes of 2,560 individuals. Of this group, 1,477 had been referred because of undiagnosed recurrent fevers, systemic inflammation, or both, and 1,083 were affected by atypical, unclassified disorders. The researchers identified 25 men with a somatic mutation in the ubiquitin-like modifier activating enzyme 1 (UBA1) gene, which is involved in the protein ubiquitylation system. This posttranslational modification has a pleiotropic function that likely explains the clinical heterogeneity seen in VEXAS patients: regulation of protein turnover, especially those involved in the cell cycle, cell death, and signal transduction. Ubiquitylation is also involved in nonproteolytic functions, such as assembly of multiprotein complexes, intracellular signaling, inflammatory signaling, and DNA repair.
Clinical presentation
The clinicobiological presentation of VEXAS syndrome is very heterogeneous. Typically, patients present with a systemic inflammatory disease with unexplained episodes of fever, involvement of the lungs, skin, blood vessels, and joints. Molecular diagnosis is made by the sequencing of UBA1.
Most patients present with the characteristic clinical signs of other inflammatory diseases, such as polyarteritis nodosa and recurrent polychondritis. But VEXAS patients are at high risk of developing hematologic conditions. Indeed, the following were seen among the 25 participants in the NIH study: macrocytic anemia (96%), venous thromboembolism (44%), myelodysplastic syndrome (24%), and multiple myeloma or monoclonal gammopathy of undetermined significance (20%).
In VEXAS patients, levels of serum inflammatory markers are increased. These markers include tumor necrosis factor, interleukin-8, interleukin-6, interferon-inducible protein-10, interferon-gamma, C-reactive protein. In addition, there is aberrant activation of innate immune-signaling pathways.
In a large-scale analysis of a multicenter case series of 116 French patients, researchers found that VEXAS syndrome primarily affected men. The disease was progressive, and onset occurred after age 50 years. These patients can be divided into three phenotypically distinct clusters on the basis of integration of clinical and biological data. In the 58 cases in which myelodysplastic syndrome was present, the mortality rates were higher. The researchers also reported that the UBA1 p.Met41L mutation was associated with a better prognosis.
Treatment data
VEXAS syndrome resists the classical therapeutic arsenal. Patients require high-dose glucocorticoids, and prognosis appears to be poor. The available treatment data are retrospective. Of the 25 participants in the NIH study, 40% died within 5 years from disease-related causes or complications related to treatment. Among the promising therapeutic avenues is the use of inhibitors of the Janus kinase pathway.
This article was translated from Univadis France. A version of this article appeared on Medscape.com.
New AHA statement on complementary medicine in heart failure
There are some benefits and potentially serious risks associated with complementary and alternative medicines (CAM) patients with heart failure (HF) may use to manage symptoms, the American Heart Association noted in a new scientific statement on the topic.
For example, yoga and tai chi can be helpful for people with HF, and omega-3 polyunsaturated fatty acids may also have benefits. However, there are safety concerns with other commonly used over-the-counter CAM therapies, including vitamin D, blue cohosh, and Lily of the Valley, the writing group said.
It’s estimated that roughly one in three patients with HF use CAM. But often patients don’t report their CAM use to their clinicians and clinicians may not routinely ask about CAM use or have the resources to evaluate CAM therapies, writing group chair Sheryl L. Chow, PharmD, told this news organization.
“This represents a major public health problem given that consumers are frequently purchasing these potentially dangerous and minimally regulated products without the knowledge or advice from a health care professional,” said Dr. Chow, of Western University of Health Sciences, Pomona, Calif., and University of California, Irvine.
The 27-page statement was published online in Circulation.
CAM use common in HF
The statement defines CAM as medical practices, supplements, and approaches that do not conform to the standards of conventional, evidence-based practice guidelines. CAM products are available without prescriptions or medical guidance at pharmacies, health food stores, and online retailers.
“These agents are largely unregulated by the [Food and Drug Administration] and manufacturers do not need to demonstrate efficacy or safety. It is important that both health care professionals and consumers improve communication with respect to OTC therapies and are educated about potential efficacy and risk of harm so that shared and informed decision-making can occur,” Dr. Chow said.
The writing group reviewed research published before November 2021 on CAM among people with HF.
Omega-3 polyunsaturated fatty acids (PUFAs), such as fish oil, have the strongest evidence among CAM agents for clinical benefit in HF and may be used safely by patients in moderation and in consultation with their health care team, the writing group said.
Research has shown that omega-3 PUFAs are associated with a lower risk of developing HF as well as improvements in left ventricular systolic function in those with existing HF, they pointed out.
However, two clinical trials found a higher incidence of atrial fibrillation with high-dose omega-3 PUFA administration. “This risk appears to be dose-related and increased when exceeding 2 g/d of fish oil,” the writing group said.
Research suggests that yoga and tai chi, when added to standard HF treatment, may help improve exercise tolerance and quality of life and decrease blood pressure.
Inconclusive or potentially harmful CAM therapies
Other CAM therapies for HF have been shown as ineffective based on current data, have mixed findings, or appear to be harmful. The writers highlighted the following examples:
- Overall evidence regarding the value of vitamin D supplementation in patients with HF remains “inconclusive” and may be harmful when taken with HF medications such as digoxin, calcium channel blockers, and diuretics.
- Routine thiamine supplementation in patients with HF and without clinically significant thiamine deficiency may not be efficacious and should be avoided.
- Research on alcohol varies, with some data showing that drinking low-to-moderate amounts (one to two drinks per day) may help prevent HF, while habitual drinking or consuming higher amounts is known to contribute to HF.
- The literature is mixed on vitamin E. It may have some benefit in reducing the risk of HF with preserved ejection fraction but has also been associated with an increased risk of HF hospitalization.
- Coenzyme Q10 (Co-Q10), commonly taken as a dietary supplement, may help improve HF class, symptoms, and quality of life, but it also may interact with antihypertensive and anticoagulant medication. Co-Q10 remains of “uncertain” value in HF at this time. Large-scale randomized controlled trials are needed before any definitive conclusion can be reached.
- Hawthorn, a flowering shrub, has been shown in some studies to increase exercise tolerance and improve HF symptoms such as fatigue. Yet it also has the potential to worsen HF, and there is conflicting research about whether it interacts with digoxin.
- The herbal supplement blue cohosh, from the root of a flowering plant found in hardwood forests, could cause tachycardia, high blood pressure, chest pain, and increased blood glucose. It may also decrease the effect of medications taken to treat high blood pressure and type 2 diabetes, they noted.
- Lily of the Valley, the root, stems, and flower of which are used in supplements, has long been used in mild HF because it contains active chemicals similar to digoxin. But when taken with digoxin, it could lead to hypokalemia.
In an AHA news release, Dr. Chow said, “Overall, more quality research and well-powered randomized controlled trials are needed to better understand the risks and benefits” of CAM therapies for HF.
“This scientific statement provides critical information to health care professionals who treat people with heart failure and may be used as a resource for consumers about the potential benefit and harm associated with complementary and alternative medicine products,” Dr. Chow added.
The writing group encourages health care professionals to routinely ask their HF patients about their use of CAM therapies. They also say pharmacists should be included in the multidisciplinary health care team to provide consultations about the use of CAM therapies for HF patients.
The scientific statement does not include cannabis or traditional Chinese medicine, which have also been used in HF.
In 2020, the AHA published a separate scientific statement on the use of medical marijuana and recreational cannabis on cardiovascular health, as reported previously by this news organization.
The scientific statement on CAM for HF was prepared by the volunteer writing group on behalf of the AHA Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; the Council on Epidemiology and Prevention; and the Council on Cardiovascular and Stroke Nursing.
A version of this article first appeared on Medscape.com.
There are some benefits and potentially serious risks associated with complementary and alternative medicines (CAM) patients with heart failure (HF) may use to manage symptoms, the American Heart Association noted in a new scientific statement on the topic.
For example, yoga and tai chi can be helpful for people with HF, and omega-3 polyunsaturated fatty acids may also have benefits. However, there are safety concerns with other commonly used over-the-counter CAM therapies, including vitamin D, blue cohosh, and Lily of the Valley, the writing group said.
It’s estimated that roughly one in three patients with HF use CAM. But often patients don’t report their CAM use to their clinicians and clinicians may not routinely ask about CAM use or have the resources to evaluate CAM therapies, writing group chair Sheryl L. Chow, PharmD, told this news organization.
“This represents a major public health problem given that consumers are frequently purchasing these potentially dangerous and minimally regulated products without the knowledge or advice from a health care professional,” said Dr. Chow, of Western University of Health Sciences, Pomona, Calif., and University of California, Irvine.
The 27-page statement was published online in Circulation.
CAM use common in HF
The statement defines CAM as medical practices, supplements, and approaches that do not conform to the standards of conventional, evidence-based practice guidelines. CAM products are available without prescriptions or medical guidance at pharmacies, health food stores, and online retailers.
“These agents are largely unregulated by the [Food and Drug Administration] and manufacturers do not need to demonstrate efficacy or safety. It is important that both health care professionals and consumers improve communication with respect to OTC therapies and are educated about potential efficacy and risk of harm so that shared and informed decision-making can occur,” Dr. Chow said.
The writing group reviewed research published before November 2021 on CAM among people with HF.
Omega-3 polyunsaturated fatty acids (PUFAs), such as fish oil, have the strongest evidence among CAM agents for clinical benefit in HF and may be used safely by patients in moderation and in consultation with their health care team, the writing group said.
Research has shown that omega-3 PUFAs are associated with a lower risk of developing HF as well as improvements in left ventricular systolic function in those with existing HF, they pointed out.
However, two clinical trials found a higher incidence of atrial fibrillation with high-dose omega-3 PUFA administration. “This risk appears to be dose-related and increased when exceeding 2 g/d of fish oil,” the writing group said.
Research suggests that yoga and tai chi, when added to standard HF treatment, may help improve exercise tolerance and quality of life and decrease blood pressure.
Inconclusive or potentially harmful CAM therapies
Other CAM therapies for HF have been shown as ineffective based on current data, have mixed findings, or appear to be harmful. The writers highlighted the following examples:
- Overall evidence regarding the value of vitamin D supplementation in patients with HF remains “inconclusive” and may be harmful when taken with HF medications such as digoxin, calcium channel blockers, and diuretics.
- Routine thiamine supplementation in patients with HF and without clinically significant thiamine deficiency may not be efficacious and should be avoided.
- Research on alcohol varies, with some data showing that drinking low-to-moderate amounts (one to two drinks per day) may help prevent HF, while habitual drinking or consuming higher amounts is known to contribute to HF.
- The literature is mixed on vitamin E. It may have some benefit in reducing the risk of HF with preserved ejection fraction but has also been associated with an increased risk of HF hospitalization.
- Coenzyme Q10 (Co-Q10), commonly taken as a dietary supplement, may help improve HF class, symptoms, and quality of life, but it also may interact with antihypertensive and anticoagulant medication. Co-Q10 remains of “uncertain” value in HF at this time. Large-scale randomized controlled trials are needed before any definitive conclusion can be reached.
- Hawthorn, a flowering shrub, has been shown in some studies to increase exercise tolerance and improve HF symptoms such as fatigue. Yet it also has the potential to worsen HF, and there is conflicting research about whether it interacts with digoxin.
- The herbal supplement blue cohosh, from the root of a flowering plant found in hardwood forests, could cause tachycardia, high blood pressure, chest pain, and increased blood glucose. It may also decrease the effect of medications taken to treat high blood pressure and type 2 diabetes, they noted.
- Lily of the Valley, the root, stems, and flower of which are used in supplements, has long been used in mild HF because it contains active chemicals similar to digoxin. But when taken with digoxin, it could lead to hypokalemia.
In an AHA news release, Dr. Chow said, “Overall, more quality research and well-powered randomized controlled trials are needed to better understand the risks and benefits” of CAM therapies for HF.
“This scientific statement provides critical information to health care professionals who treat people with heart failure and may be used as a resource for consumers about the potential benefit and harm associated with complementary and alternative medicine products,” Dr. Chow added.
The writing group encourages health care professionals to routinely ask their HF patients about their use of CAM therapies. They also say pharmacists should be included in the multidisciplinary health care team to provide consultations about the use of CAM therapies for HF patients.
The scientific statement does not include cannabis or traditional Chinese medicine, which have also been used in HF.
In 2020, the AHA published a separate scientific statement on the use of medical marijuana and recreational cannabis on cardiovascular health, as reported previously by this news organization.
The scientific statement on CAM for HF was prepared by the volunteer writing group on behalf of the AHA Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; the Council on Epidemiology and Prevention; and the Council on Cardiovascular and Stroke Nursing.
A version of this article first appeared on Medscape.com.
There are some benefits and potentially serious risks associated with complementary and alternative medicines (CAM) patients with heart failure (HF) may use to manage symptoms, the American Heart Association noted in a new scientific statement on the topic.
For example, yoga and tai chi can be helpful for people with HF, and omega-3 polyunsaturated fatty acids may also have benefits. However, there are safety concerns with other commonly used over-the-counter CAM therapies, including vitamin D, blue cohosh, and Lily of the Valley, the writing group said.
It’s estimated that roughly one in three patients with HF use CAM. But often patients don’t report their CAM use to their clinicians and clinicians may not routinely ask about CAM use or have the resources to evaluate CAM therapies, writing group chair Sheryl L. Chow, PharmD, told this news organization.
“This represents a major public health problem given that consumers are frequently purchasing these potentially dangerous and minimally regulated products without the knowledge or advice from a health care professional,” said Dr. Chow, of Western University of Health Sciences, Pomona, Calif., and University of California, Irvine.
The 27-page statement was published online in Circulation.
CAM use common in HF
The statement defines CAM as medical practices, supplements, and approaches that do not conform to the standards of conventional, evidence-based practice guidelines. CAM products are available without prescriptions or medical guidance at pharmacies, health food stores, and online retailers.
“These agents are largely unregulated by the [Food and Drug Administration] and manufacturers do not need to demonstrate efficacy or safety. It is important that both health care professionals and consumers improve communication with respect to OTC therapies and are educated about potential efficacy and risk of harm so that shared and informed decision-making can occur,” Dr. Chow said.
The writing group reviewed research published before November 2021 on CAM among people with HF.
Omega-3 polyunsaturated fatty acids (PUFAs), such as fish oil, have the strongest evidence among CAM agents for clinical benefit in HF and may be used safely by patients in moderation and in consultation with their health care team, the writing group said.
Research has shown that omega-3 PUFAs are associated with a lower risk of developing HF as well as improvements in left ventricular systolic function in those with existing HF, they pointed out.
However, two clinical trials found a higher incidence of atrial fibrillation with high-dose omega-3 PUFA administration. “This risk appears to be dose-related and increased when exceeding 2 g/d of fish oil,” the writing group said.
Research suggests that yoga and tai chi, when added to standard HF treatment, may help improve exercise tolerance and quality of life and decrease blood pressure.
Inconclusive or potentially harmful CAM therapies
Other CAM therapies for HF have been shown as ineffective based on current data, have mixed findings, or appear to be harmful. The writers highlighted the following examples:
- Overall evidence regarding the value of vitamin D supplementation in patients with HF remains “inconclusive” and may be harmful when taken with HF medications such as digoxin, calcium channel blockers, and diuretics.
- Routine thiamine supplementation in patients with HF and without clinically significant thiamine deficiency may not be efficacious and should be avoided.
- Research on alcohol varies, with some data showing that drinking low-to-moderate amounts (one to two drinks per day) may help prevent HF, while habitual drinking or consuming higher amounts is known to contribute to HF.
- The literature is mixed on vitamin E. It may have some benefit in reducing the risk of HF with preserved ejection fraction but has also been associated with an increased risk of HF hospitalization.
- Coenzyme Q10 (Co-Q10), commonly taken as a dietary supplement, may help improve HF class, symptoms, and quality of life, but it also may interact with antihypertensive and anticoagulant medication. Co-Q10 remains of “uncertain” value in HF at this time. Large-scale randomized controlled trials are needed before any definitive conclusion can be reached.
- Hawthorn, a flowering shrub, has been shown in some studies to increase exercise tolerance and improve HF symptoms such as fatigue. Yet it also has the potential to worsen HF, and there is conflicting research about whether it interacts with digoxin.
- The herbal supplement blue cohosh, from the root of a flowering plant found in hardwood forests, could cause tachycardia, high blood pressure, chest pain, and increased blood glucose. It may also decrease the effect of medications taken to treat high blood pressure and type 2 diabetes, they noted.
- Lily of the Valley, the root, stems, and flower of which are used in supplements, has long been used in mild HF because it contains active chemicals similar to digoxin. But when taken with digoxin, it could lead to hypokalemia.
In an AHA news release, Dr. Chow said, “Overall, more quality research and well-powered randomized controlled trials are needed to better understand the risks and benefits” of CAM therapies for HF.
“This scientific statement provides critical information to health care professionals who treat people with heart failure and may be used as a resource for consumers about the potential benefit and harm associated with complementary and alternative medicine products,” Dr. Chow added.
The writing group encourages health care professionals to routinely ask their HF patients about their use of CAM therapies. They also say pharmacists should be included in the multidisciplinary health care team to provide consultations about the use of CAM therapies for HF patients.
The scientific statement does not include cannabis or traditional Chinese medicine, which have also been used in HF.
In 2020, the AHA published a separate scientific statement on the use of medical marijuana and recreational cannabis on cardiovascular health, as reported previously by this news organization.
The scientific statement on CAM for HF was prepared by the volunteer writing group on behalf of the AHA Clinical Pharmacology Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; the Council on Epidemiology and Prevention; and the Council on Cardiovascular and Stroke Nursing.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Statins tied to lower ICH risk regardless of bleed location
A new study has provided further reassurance on questions about the risk of intracerebral hemorrhage (ICH) with statins.
The Danish case-control study, which compared statin use in 2,164 case patients with ICH and in 86,255 matched control persons, found that current statin use was associated with a lower risk of having a first ICH and that the risk was further reduced with longer duration of statin use.
The study also showed that statin use was linked to a lower risk of ICH in the more superficial lobar areas of the brain and in the deeper, nonlobar locations. There was no difference in the magnitude of risk reduction between the two locations.
“Although this study is observational, I feel these data are strong, and the results are reassuring. It certainly does not suggest any increased risk of ICH with statins,” senior author David Gaist, PhD, Odense University Hospital, Denmark, said in an interview.
“On the contrary, it indicates a lower risk, which seems to be independent of the location of the bleed.”
The study was published online in Neurology.
The authors note that statins effectively reduce the occurrence of cardiovascular events and ischemic stroke in high-risk populations, but early randomized trials raised concerns of an increased risk of ICH among statin users who have a history of stroke.
Subsequent observational studies, including four meta-analyses, included patients with and those without prior stroke. The results were inconsistent, although most found no increase in bleeding. More recent studies have found a lower risk of ICH among statin users; the risk was inversely associated with the duration and intensity of statin treatment.
However, the researchers point out that few studies have assessed the association between statin use and the location of ICH. Hemorrhages that occur in the lobar region of the brain and those that occur in the nonlobar areas can have different pathophysiologies. Arteriolosclerosis, which is strongly associated with hypertension, is a common histologic finding in patients with ICH, regardless of hemorrhage location, while cerebral amyloid angiopathy (CAA) is associated with lobar but not nonlobar ICH.
The current study was conducted to look more closely at the relationship between statin use and hematoma location as a reflection of differences in the underlying pathophysiologies of lobar versus nonlobar ICH.
The researchers used Danish registries to identify all first-ever cases of spontaneous ICH that occurred between 2009 and 2018 in persons older than 55 years in the Southern Denmark region. Patients with traumatic ICH or ICH related to vascular malformations and tumors were excluded.
These cases were verified through medical records. ICH diagnoses were classified as having a lobar or nonlobar location, and patients were matched for age, sex, and calendar year to general population control persons. The nationwide prescription registry was also analyzed to ascertain use of statins and other medications.
The study included 989 patients with lobar ICH who were matched to 39,500 control persons and 1,175 patients with nonlobar ICH who were matched to 46,755 control persons.
Results showed that current statin use was associated with a 16%-17% relative reduction in ICH risk. There was no difference with respect to ICH location.
For lobar ICH, statin use showed an adjusted odds ratio of 0.83 (95% confidence interval, 0.70-0.98); for nonlobar ICH, the adjusted odds ratio was 0.84 (95% CI, 0.72-0.98).
Longer duration of statin use was associated with a greater reduction in risk of ICH; use for more than 5 years was associated with a relative reduction of ICH of 33%-38%, again with no difference with regard to ICH location.
For lobar ICH, statin use for more than 5 years showed an adjusted odds ratio of 0.67 (95% CI, 0.51-0.87); and for nonlobar ICH, the adjusted odds ratio was 0.62 (95% CI, 0.48-0.80).
“We suspected that statins may have more of an effect in reducing nonlobar ICH, as this type is considered to be more associated with arteriosclerosis, compared with lobar ICH,” Dr. Gaist explained. “But we didn’t find that. We found that taking statins was associated with a similar reduction in risk of both lobar and nonlobar ICH.”
Although amyloid angiopathy can contribute to lobar ICH, arteriosclerosis is still involved in the majority of cases, he noted. He cited a recent population-based U.K. study that showed that while histologically verified CAA was present in 58% of patients with a lobar ICH, most also had evidence of arteriosclerosis, with only 13% having isolated CAA pathology.
“If statins exert their effect on reducing ICH by reducing arteriosclerosis, which is likely, then this observation of arteriosclerosis pathology being prevalent in both lobar and nonlobar ICH locations would explain our results,” Dr. Gaist commented.
“Strengths of our study include the large numbers involved and the fact that the patients are unselected. We tried to find everyone who had had a first ICH in a well-defined region of Denmark, so issues of selection are less of a concern than in some other studies,” he noted.
He also pointed out that all the ICH diagnoses were verified from medical records and that in a substudy, brain scans were evaluated, with investigators masked to clinical data to evaluate the location and characteristics of the hematoma. In addition, data on statin use were collected prospectively from a nationwide prescription registry.
Interaction with antihypertensives, anticoagulants?
Other results from the study suggest a possible interaction between statin use and antihypertensive and anticoagulant drugs.
Data showed that the lower ICH risk was restricted to patients who received statins and antihypertensive drugs concurrently. Conversely, only patients who were not concurrently taking anticoagulants had a lower risk of ICH in association with statin use.
Dr. Gaist suggested that the lack of a reduction in ICH with statins among patients taking anticoagulants could be because the increased risk of ICH with anticoagulants was stronger than the reduced risk with statins.
Regarding the fact that the reduced risk of ICH with statins was only observed among individuals who were also taking antihypertensive medication, Dr. Gaist noted that because hypertension is such an important risk factor for ICH, “it may be that to get the true benefit of statins, patients have to have their hypertension controlled.”
However, an alternative explanation could that the finding is a result of “healthy adherer” bias, in which people who take antihypertensive medication and follow a healthy lifestyle as advised would be more likely to take statins.
“The observational nature of our study does not allow us to determine the extent to which associations are causal,” the authors say.
Dr. Gaist also noted that an important caveat in this study is that they focused on individuals who had had a first ICH.
“This data does not inform us about those who have already had an ICH and are taking statins. But we are planning to look at this in our next study,” he said.
The study was funded by the Novo Nordisk Foundation. Dr. Gaist has received speaker honorarium from Bristol-Myers Squibb and Pfizer unrelated to this work.
A version of this article first appeared on Medscape.com.
A new study has provided further reassurance on questions about the risk of intracerebral hemorrhage (ICH) with statins.
The Danish case-control study, which compared statin use in 2,164 case patients with ICH and in 86,255 matched control persons, found that current statin use was associated with a lower risk of having a first ICH and that the risk was further reduced with longer duration of statin use.
The study also showed that statin use was linked to a lower risk of ICH in the more superficial lobar areas of the brain and in the deeper, nonlobar locations. There was no difference in the magnitude of risk reduction between the two locations.
“Although this study is observational, I feel these data are strong, and the results are reassuring. It certainly does not suggest any increased risk of ICH with statins,” senior author David Gaist, PhD, Odense University Hospital, Denmark, said in an interview.
“On the contrary, it indicates a lower risk, which seems to be independent of the location of the bleed.”
The study was published online in Neurology.
The authors note that statins effectively reduce the occurrence of cardiovascular events and ischemic stroke in high-risk populations, but early randomized trials raised concerns of an increased risk of ICH among statin users who have a history of stroke.
Subsequent observational studies, including four meta-analyses, included patients with and those without prior stroke. The results were inconsistent, although most found no increase in bleeding. More recent studies have found a lower risk of ICH among statin users; the risk was inversely associated with the duration and intensity of statin treatment.
However, the researchers point out that few studies have assessed the association between statin use and the location of ICH. Hemorrhages that occur in the lobar region of the brain and those that occur in the nonlobar areas can have different pathophysiologies. Arteriolosclerosis, which is strongly associated with hypertension, is a common histologic finding in patients with ICH, regardless of hemorrhage location, while cerebral amyloid angiopathy (CAA) is associated with lobar but not nonlobar ICH.
The current study was conducted to look more closely at the relationship between statin use and hematoma location as a reflection of differences in the underlying pathophysiologies of lobar versus nonlobar ICH.
The researchers used Danish registries to identify all first-ever cases of spontaneous ICH that occurred between 2009 and 2018 in persons older than 55 years in the Southern Denmark region. Patients with traumatic ICH or ICH related to vascular malformations and tumors were excluded.
These cases were verified through medical records. ICH diagnoses were classified as having a lobar or nonlobar location, and patients were matched for age, sex, and calendar year to general population control persons. The nationwide prescription registry was also analyzed to ascertain use of statins and other medications.
The study included 989 patients with lobar ICH who were matched to 39,500 control persons and 1,175 patients with nonlobar ICH who were matched to 46,755 control persons.
Results showed that current statin use was associated with a 16%-17% relative reduction in ICH risk. There was no difference with respect to ICH location.
For lobar ICH, statin use showed an adjusted odds ratio of 0.83 (95% confidence interval, 0.70-0.98); for nonlobar ICH, the adjusted odds ratio was 0.84 (95% CI, 0.72-0.98).
Longer duration of statin use was associated with a greater reduction in risk of ICH; use for more than 5 years was associated with a relative reduction of ICH of 33%-38%, again with no difference with regard to ICH location.
For lobar ICH, statin use for more than 5 years showed an adjusted odds ratio of 0.67 (95% CI, 0.51-0.87); and for nonlobar ICH, the adjusted odds ratio was 0.62 (95% CI, 0.48-0.80).
“We suspected that statins may have more of an effect in reducing nonlobar ICH, as this type is considered to be more associated with arteriosclerosis, compared with lobar ICH,” Dr. Gaist explained. “But we didn’t find that. We found that taking statins was associated with a similar reduction in risk of both lobar and nonlobar ICH.”
Although amyloid angiopathy can contribute to lobar ICH, arteriosclerosis is still involved in the majority of cases, he noted. He cited a recent population-based U.K. study that showed that while histologically verified CAA was present in 58% of patients with a lobar ICH, most also had evidence of arteriosclerosis, with only 13% having isolated CAA pathology.
“If statins exert their effect on reducing ICH by reducing arteriosclerosis, which is likely, then this observation of arteriosclerosis pathology being prevalent in both lobar and nonlobar ICH locations would explain our results,” Dr. Gaist commented.
“Strengths of our study include the large numbers involved and the fact that the patients are unselected. We tried to find everyone who had had a first ICH in a well-defined region of Denmark, so issues of selection are less of a concern than in some other studies,” he noted.
He also pointed out that all the ICH diagnoses were verified from medical records and that in a substudy, brain scans were evaluated, with investigators masked to clinical data to evaluate the location and characteristics of the hematoma. In addition, data on statin use were collected prospectively from a nationwide prescription registry.
Interaction with antihypertensives, anticoagulants?
Other results from the study suggest a possible interaction between statin use and antihypertensive and anticoagulant drugs.
Data showed that the lower ICH risk was restricted to patients who received statins and antihypertensive drugs concurrently. Conversely, only patients who were not concurrently taking anticoagulants had a lower risk of ICH in association with statin use.
Dr. Gaist suggested that the lack of a reduction in ICH with statins among patients taking anticoagulants could be because the increased risk of ICH with anticoagulants was stronger than the reduced risk with statins.
Regarding the fact that the reduced risk of ICH with statins was only observed among individuals who were also taking antihypertensive medication, Dr. Gaist noted that because hypertension is such an important risk factor for ICH, “it may be that to get the true benefit of statins, patients have to have their hypertension controlled.”
However, an alternative explanation could that the finding is a result of “healthy adherer” bias, in which people who take antihypertensive medication and follow a healthy lifestyle as advised would be more likely to take statins.
“The observational nature of our study does not allow us to determine the extent to which associations are causal,” the authors say.
Dr. Gaist also noted that an important caveat in this study is that they focused on individuals who had had a first ICH.
“This data does not inform us about those who have already had an ICH and are taking statins. But we are planning to look at this in our next study,” he said.
The study was funded by the Novo Nordisk Foundation. Dr. Gaist has received speaker honorarium from Bristol-Myers Squibb and Pfizer unrelated to this work.
A version of this article first appeared on Medscape.com.
A new study has provided further reassurance on questions about the risk of intracerebral hemorrhage (ICH) with statins.
The Danish case-control study, which compared statin use in 2,164 case patients with ICH and in 86,255 matched control persons, found that current statin use was associated with a lower risk of having a first ICH and that the risk was further reduced with longer duration of statin use.
The study also showed that statin use was linked to a lower risk of ICH in the more superficial lobar areas of the brain and in the deeper, nonlobar locations. There was no difference in the magnitude of risk reduction between the two locations.
“Although this study is observational, I feel these data are strong, and the results are reassuring. It certainly does not suggest any increased risk of ICH with statins,” senior author David Gaist, PhD, Odense University Hospital, Denmark, said in an interview.
“On the contrary, it indicates a lower risk, which seems to be independent of the location of the bleed.”
The study was published online in Neurology.
The authors note that statins effectively reduce the occurrence of cardiovascular events and ischemic stroke in high-risk populations, but early randomized trials raised concerns of an increased risk of ICH among statin users who have a history of stroke.
Subsequent observational studies, including four meta-analyses, included patients with and those without prior stroke. The results were inconsistent, although most found no increase in bleeding. More recent studies have found a lower risk of ICH among statin users; the risk was inversely associated with the duration and intensity of statin treatment.
However, the researchers point out that few studies have assessed the association between statin use and the location of ICH. Hemorrhages that occur in the lobar region of the brain and those that occur in the nonlobar areas can have different pathophysiologies. Arteriolosclerosis, which is strongly associated with hypertension, is a common histologic finding in patients with ICH, regardless of hemorrhage location, while cerebral amyloid angiopathy (CAA) is associated with lobar but not nonlobar ICH.
The current study was conducted to look more closely at the relationship between statin use and hematoma location as a reflection of differences in the underlying pathophysiologies of lobar versus nonlobar ICH.
The researchers used Danish registries to identify all first-ever cases of spontaneous ICH that occurred between 2009 and 2018 in persons older than 55 years in the Southern Denmark region. Patients with traumatic ICH or ICH related to vascular malformations and tumors were excluded.
These cases were verified through medical records. ICH diagnoses were classified as having a lobar or nonlobar location, and patients were matched for age, sex, and calendar year to general population control persons. The nationwide prescription registry was also analyzed to ascertain use of statins and other medications.
The study included 989 patients with lobar ICH who were matched to 39,500 control persons and 1,175 patients with nonlobar ICH who were matched to 46,755 control persons.
Results showed that current statin use was associated with a 16%-17% relative reduction in ICH risk. There was no difference with respect to ICH location.
For lobar ICH, statin use showed an adjusted odds ratio of 0.83 (95% confidence interval, 0.70-0.98); for nonlobar ICH, the adjusted odds ratio was 0.84 (95% CI, 0.72-0.98).
Longer duration of statin use was associated with a greater reduction in risk of ICH; use for more than 5 years was associated with a relative reduction of ICH of 33%-38%, again with no difference with regard to ICH location.
For lobar ICH, statin use for more than 5 years showed an adjusted odds ratio of 0.67 (95% CI, 0.51-0.87); and for nonlobar ICH, the adjusted odds ratio was 0.62 (95% CI, 0.48-0.80).
“We suspected that statins may have more of an effect in reducing nonlobar ICH, as this type is considered to be more associated with arteriosclerosis, compared with lobar ICH,” Dr. Gaist explained. “But we didn’t find that. We found that taking statins was associated with a similar reduction in risk of both lobar and nonlobar ICH.”
Although amyloid angiopathy can contribute to lobar ICH, arteriosclerosis is still involved in the majority of cases, he noted. He cited a recent population-based U.K. study that showed that while histologically verified CAA was present in 58% of patients with a lobar ICH, most also had evidence of arteriosclerosis, with only 13% having isolated CAA pathology.
“If statins exert their effect on reducing ICH by reducing arteriosclerosis, which is likely, then this observation of arteriosclerosis pathology being prevalent in both lobar and nonlobar ICH locations would explain our results,” Dr. Gaist commented.
“Strengths of our study include the large numbers involved and the fact that the patients are unselected. We tried to find everyone who had had a first ICH in a well-defined region of Denmark, so issues of selection are less of a concern than in some other studies,” he noted.
He also pointed out that all the ICH diagnoses were verified from medical records and that in a substudy, brain scans were evaluated, with investigators masked to clinical data to evaluate the location and characteristics of the hematoma. In addition, data on statin use were collected prospectively from a nationwide prescription registry.
Interaction with antihypertensives, anticoagulants?
Other results from the study suggest a possible interaction between statin use and antihypertensive and anticoagulant drugs.
Data showed that the lower ICH risk was restricted to patients who received statins and antihypertensive drugs concurrently. Conversely, only patients who were not concurrently taking anticoagulants had a lower risk of ICH in association with statin use.
Dr. Gaist suggested that the lack of a reduction in ICH with statins among patients taking anticoagulants could be because the increased risk of ICH with anticoagulants was stronger than the reduced risk with statins.
Regarding the fact that the reduced risk of ICH with statins was only observed among individuals who were also taking antihypertensive medication, Dr. Gaist noted that because hypertension is such an important risk factor for ICH, “it may be that to get the true benefit of statins, patients have to have their hypertension controlled.”
However, an alternative explanation could that the finding is a result of “healthy adherer” bias, in which people who take antihypertensive medication and follow a healthy lifestyle as advised would be more likely to take statins.
“The observational nature of our study does not allow us to determine the extent to which associations are causal,” the authors say.
Dr. Gaist also noted that an important caveat in this study is that they focused on individuals who had had a first ICH.
“This data does not inform us about those who have already had an ICH and are taking statins. But we are planning to look at this in our next study,” he said.
The study was funded by the Novo Nordisk Foundation. Dr. Gaist has received speaker honorarium from Bristol-Myers Squibb and Pfizer unrelated to this work.
A version of this article first appeared on Medscape.com.
No, you can’t see a different doctor: We need zero tolerance of patient bias
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
It was 1970. I was in my second year of medical school. I can remember the hurt and embarrassment as if it were yesterday.
Coming from the Deep South, I was very familiar with racial bias, but I did not expect it at that level and in that environment. From that point on, I was anxious at each patient encounter, concerned that this might happen again. And it did several times during my residency and fellowship.
The Occupational Safety and Health Administration defines workplace violence as “any act or threat of physical violence, harassment, intimidation, or other threatening disruptive behavior that occurs at the work site. It ranges from threats and verbal abuse to physical assaults.”
There is considerable media focus on incidents of physical violence against health care workers, but when patients, their families, or visitors openly display bias and request a different doctor, nurse, or technician for nonmedical reasons, the impact is profound. This is extremely hurtful to a professional who has worked long and hard to acquire skills and expertise. And, while speech may not constitute violence in the strictest sense of the word, there is growing evidence that it can be physically harmful through its effect on the nervous system, even if no physical contact is involved.
Incidents of bias occur regularly and are clearly on the rise. In most cases the request for a different health care worker is granted to honor the rights of the patient. The healthcare worker is left alone and emotionally wounded; the healthcare institutions are complicit.
This bias is mostly racial but can also be based on religion, sexual orientation, age, disability, body size, accent, or gender.
An entire issue of the American Medical Association Journal of Ethics was devoted to this topic. From recognizing that there are limits to what clinicians should be expected to tolerate when patients’ preferences express unjust bias, the issue also explored where those limits should be placed, why, and who is obliged to enforce them.
The newly adopted Mass General Patient Code of Conduct is evidence that health care systems are beginning to recognize this problem and that such behavior will not be tolerated.
But having a zero-tolerance policy is not enough. We must have procedures in place to discourage and mitigate the impact of patient bias.
A clear definition of what constitutes a bias incident is essential. All team members must be made aware of the procedures for reporting such incidents and the chain of command for escalation. Reporting should be encouraged, and resources must be made available to impacted team members. Surveillance, monitoring, and review are also essential as is clarification on when patient preferences should be honored.
The Mayo Clinic 5 Step Plan is an excellent example of a protocol to deal with patient bias against health care workers and is based on a thoughtful analysis of what constitutes an unreasonable request for a different clinician. I’m pleased to report that my health care system (Inova Health) is developing a similar protocol.
The health care setting should be a bias-free zone for both patients and health care workers. I have been a strong advocate of patients’ rights and worked hard to guard against bias and eliminate disparities in care, but health care workers have rights as well.
We should expect to be treated with respect.
The views expressed by the author are those of the author alone and do not represent the views of the Inova Health System. Dr. Francis is a cardiologist at Inova Heart and Vascular Institute, McLean, Va. He disclosed no conflicts of interest.
A version of this article first appeared on Medscape.com.
States cracking down harder on docs who sexually abuse patients
It’s the latest example of states taking doctor sexual misconduct more seriously after longstanding criticism that medical boards have been too lenient.
The law, which takes effect in January 2023, requires the state’s medical board to permanently revoke these doctors’ licenses instead of allowing them to petition the board for reinstatement after 3 years.
“Physician licenses should not be reinstated after egregious sexual misconduct with patients. The doctor-patient relationship has to remain sacrosanct and trusted,” said Peter Yellowlees, MD, a professor of psychiatry at the University of California, Davis.
Although the vast majority of the nation’s estimated 1 million doctors don’t sexually abuse patients, the problem is a national one.
The Federation of State Medical Boards defines sexual misconduct as the exploitation of the physician-patient relationship in a sexual way. The exploitation may be verbal or physical and can occur in person or virtually.
The FSMB conducted a 2-year review of how medical boards handled cases of sexual misconduct, issuing a report in 2020 that contained 38 recommended actions.
Four states in addition to California have enacted laws that incorporate some FSMB recommendations. These include revoking doctors’ licenses after a single egregious act of sexual misconduct (including sexual assault), regardless of whether the physician was charged or convicted; increased reporting by hospitals and doctors of sexual misconduct; and training of physicians to recognize and report sexual misconduct.
The four state laws are:
- Georgia’s HB 458. It was signed into law in May 2021, and it authorizes the medical board to revoke or suspend a license if a physician is found guilty of sexually assaulting a patient in a criminal case. Doctors are required to report other doctors who have sexually abused patients and to take continuing medical education (CME) units on sexual misconduct.
- Florida’s SB 1934. This legislation was signed into law in June 2021, and it bars physicians charged with serious crimes such as sexual assault, sexual misconduct against patients, or possession of child pornography from seeing patients until those charges are resolved by the legal system.
- West Virginia’s SB 603. Signed into law in March 2022 it prohibits the medical board from issuing a license to a physician who engaged in sexual activity or misconduct with a patient whose license was revoked in another state or was involved in other violations.
- Tennessee HB 1045. It was signed into law in May 2021, and authorizes the medical board, upon learning of an indictment against a physician for a controlled substance violation or sexual offense, to immediately suspend the doctor’s ability to prescribe controlled substances until the doctor’s case is resolved.
A published study identified a total of 1,721 reports of physician sexual misconduct that were submitted to the National Practitioner Data Bank between 2000 and 2019. The annual incidence of sexual misconduct reports averaged 10.8 per 100,000 U.S. physician licensees, said the researchers.
In a groundbreaking 2016 investigation, the Atlanta Journal-Constitution reviewed thousands of documents and found more than 2,400 doctors whose sexual misconduct cases clearly involved patients since 1999.
Physician sexual misconduct is likely underreported
The actual incidence of physician-patient sexual misconduct is likely higher as a result of underreporting, according to the researchers.
Because a substantial power differential exists between patients and their physicians, the researchers noted, it follows that patient victims, like other sexual assault victims, may be unwilling or unable to report the incident in question.
Many violations involving physician sexual misconduct of patients never came to the attention of state regulators, according to the Journal-Constitution investigation. Reporting showed that hospitals, clinics, and fellow doctors fail to report sexual misconduct to regulators, despite laws in most states requiring them to do so.
Media investigations highlight medical board shortcomings
Public pressure on the California Medical Board increased after the Los Angeles Times investigated what happened to doctors who surrendered or had their licenses revoked after being reported for sexual abuse with patients. The Times revealed in 2021 that the board reinstated 10 of 17 doctors who petitioned for reinstatement.
They include Esmail Nadjmabadi, MD, of Bakersfield, Calif., who had sexually abused six female patients, including one in her mid-teens. The Times reported that, in 2009, he pleaded no contest to a criminal charge that he sexually exploited two or more women and surrendered his medical license the following year.
Five years later, Dr. Nadjmabadi petitioned the medical board to be reinstated and the board approved his request.
The California board has also reinstated several doctors who underwent sex offender rehabilitation. Board members rely heavily on a doctor’s evidence of rehabilitation, usually with the testimony of therapists hired by the doctor, and no input from the patients who were harmed, according to the Times’ investigation.
High-profile sexual misconduct or abuse cases involving Larry Nassar, MD, and Robert Anderson, MD, in Michigan; Richard Strauss, MD, in Ohio; and Ricardo Cruciani, MD, in New York, added to the mounting criticism that medical boards were too lenient in their handling of complaints of sexual misconduct.
Another state tackles sexual misconduct
Ohio’s medical board created an administrative rule stating that licensed physicians have a legal and ethical duty to report colleagues for sexual misconduct with patients and to complete a 1-hour CME training. Failure to report sexual misconduct complaints can lead to a doctor being permanently stripped of his license.
This happened to Robert S. Geiger, MD, in 2016 after not reporting his colleague James Bressi, MD, to the medical board after receiving complaints that Dr. Bressi was sexually abusing female patients at their pain clinic.
Dr. Bressi was convicted of sexual misconduct with a patient, stripped of his medical license, and sentenced to 59 days in prison.
“I think all of these reforms are a step in the right direction and will help to deter doctors from committing sexual misconduct to some extent,” said California activist Marian Hollingsworth, cofounder of the Patient Safety League.
But there’s room for improvement, she said, since “most states fall short in not requiring medical boards to notify law enforcement when they get a complaint of doctor sexual misconduct so the public can be aware of it.”
A version of this article first appeared on Medscape.com.
It’s the latest example of states taking doctor sexual misconduct more seriously after longstanding criticism that medical boards have been too lenient.
The law, which takes effect in January 2023, requires the state’s medical board to permanently revoke these doctors’ licenses instead of allowing them to petition the board for reinstatement after 3 years.
“Physician licenses should not be reinstated after egregious sexual misconduct with patients. The doctor-patient relationship has to remain sacrosanct and trusted,” said Peter Yellowlees, MD, a professor of psychiatry at the University of California, Davis.
Although the vast majority of the nation’s estimated 1 million doctors don’t sexually abuse patients, the problem is a national one.
The Federation of State Medical Boards defines sexual misconduct as the exploitation of the physician-patient relationship in a sexual way. The exploitation may be verbal or physical and can occur in person or virtually.
The FSMB conducted a 2-year review of how medical boards handled cases of sexual misconduct, issuing a report in 2020 that contained 38 recommended actions.
Four states in addition to California have enacted laws that incorporate some FSMB recommendations. These include revoking doctors’ licenses after a single egregious act of sexual misconduct (including sexual assault), regardless of whether the physician was charged or convicted; increased reporting by hospitals and doctors of sexual misconduct; and training of physicians to recognize and report sexual misconduct.
The four state laws are:
- Georgia’s HB 458. It was signed into law in May 2021, and it authorizes the medical board to revoke or suspend a license if a physician is found guilty of sexually assaulting a patient in a criminal case. Doctors are required to report other doctors who have sexually abused patients and to take continuing medical education (CME) units on sexual misconduct.
- Florida’s SB 1934. This legislation was signed into law in June 2021, and it bars physicians charged with serious crimes such as sexual assault, sexual misconduct against patients, or possession of child pornography from seeing patients until those charges are resolved by the legal system.
- West Virginia’s SB 603. Signed into law in March 2022 it prohibits the medical board from issuing a license to a physician who engaged in sexual activity or misconduct with a patient whose license was revoked in another state or was involved in other violations.
- Tennessee HB 1045. It was signed into law in May 2021, and authorizes the medical board, upon learning of an indictment against a physician for a controlled substance violation or sexual offense, to immediately suspend the doctor’s ability to prescribe controlled substances until the doctor’s case is resolved.
A published study identified a total of 1,721 reports of physician sexual misconduct that were submitted to the National Practitioner Data Bank between 2000 and 2019. The annual incidence of sexual misconduct reports averaged 10.8 per 100,000 U.S. physician licensees, said the researchers.
In a groundbreaking 2016 investigation, the Atlanta Journal-Constitution reviewed thousands of documents and found more than 2,400 doctors whose sexual misconduct cases clearly involved patients since 1999.
Physician sexual misconduct is likely underreported
The actual incidence of physician-patient sexual misconduct is likely higher as a result of underreporting, according to the researchers.
Because a substantial power differential exists between patients and their physicians, the researchers noted, it follows that patient victims, like other sexual assault victims, may be unwilling or unable to report the incident in question.
Many violations involving physician sexual misconduct of patients never came to the attention of state regulators, according to the Journal-Constitution investigation. Reporting showed that hospitals, clinics, and fellow doctors fail to report sexual misconduct to regulators, despite laws in most states requiring them to do so.
Media investigations highlight medical board shortcomings
Public pressure on the California Medical Board increased after the Los Angeles Times investigated what happened to doctors who surrendered or had their licenses revoked after being reported for sexual abuse with patients. The Times revealed in 2021 that the board reinstated 10 of 17 doctors who petitioned for reinstatement.
They include Esmail Nadjmabadi, MD, of Bakersfield, Calif., who had sexually abused six female patients, including one in her mid-teens. The Times reported that, in 2009, he pleaded no contest to a criminal charge that he sexually exploited two or more women and surrendered his medical license the following year.
Five years later, Dr. Nadjmabadi petitioned the medical board to be reinstated and the board approved his request.
The California board has also reinstated several doctors who underwent sex offender rehabilitation. Board members rely heavily on a doctor’s evidence of rehabilitation, usually with the testimony of therapists hired by the doctor, and no input from the patients who were harmed, according to the Times’ investigation.
High-profile sexual misconduct or abuse cases involving Larry Nassar, MD, and Robert Anderson, MD, in Michigan; Richard Strauss, MD, in Ohio; and Ricardo Cruciani, MD, in New York, added to the mounting criticism that medical boards were too lenient in their handling of complaints of sexual misconduct.
Another state tackles sexual misconduct
Ohio’s medical board created an administrative rule stating that licensed physicians have a legal and ethical duty to report colleagues for sexual misconduct with patients and to complete a 1-hour CME training. Failure to report sexual misconduct complaints can lead to a doctor being permanently stripped of his license.
This happened to Robert S. Geiger, MD, in 2016 after not reporting his colleague James Bressi, MD, to the medical board after receiving complaints that Dr. Bressi was sexually abusing female patients at their pain clinic.
Dr. Bressi was convicted of sexual misconduct with a patient, stripped of his medical license, and sentenced to 59 days in prison.
“I think all of these reforms are a step in the right direction and will help to deter doctors from committing sexual misconduct to some extent,” said California activist Marian Hollingsworth, cofounder of the Patient Safety League.
But there’s room for improvement, she said, since “most states fall short in not requiring medical boards to notify law enforcement when they get a complaint of doctor sexual misconduct so the public can be aware of it.”
A version of this article first appeared on Medscape.com.
It’s the latest example of states taking doctor sexual misconduct more seriously after longstanding criticism that medical boards have been too lenient.
The law, which takes effect in January 2023, requires the state’s medical board to permanently revoke these doctors’ licenses instead of allowing them to petition the board for reinstatement after 3 years.
“Physician licenses should not be reinstated after egregious sexual misconduct with patients. The doctor-patient relationship has to remain sacrosanct and trusted,” said Peter Yellowlees, MD, a professor of psychiatry at the University of California, Davis.
Although the vast majority of the nation’s estimated 1 million doctors don’t sexually abuse patients, the problem is a national one.
The Federation of State Medical Boards defines sexual misconduct as the exploitation of the physician-patient relationship in a sexual way. The exploitation may be verbal or physical and can occur in person or virtually.
The FSMB conducted a 2-year review of how medical boards handled cases of sexual misconduct, issuing a report in 2020 that contained 38 recommended actions.
Four states in addition to California have enacted laws that incorporate some FSMB recommendations. These include revoking doctors’ licenses after a single egregious act of sexual misconduct (including sexual assault), regardless of whether the physician was charged or convicted; increased reporting by hospitals and doctors of sexual misconduct; and training of physicians to recognize and report sexual misconduct.
The four state laws are:
- Georgia’s HB 458. It was signed into law in May 2021, and it authorizes the medical board to revoke or suspend a license if a physician is found guilty of sexually assaulting a patient in a criminal case. Doctors are required to report other doctors who have sexually abused patients and to take continuing medical education (CME) units on sexual misconduct.
- Florida’s SB 1934. This legislation was signed into law in June 2021, and it bars physicians charged with serious crimes such as sexual assault, sexual misconduct against patients, or possession of child pornography from seeing patients until those charges are resolved by the legal system.
- West Virginia’s SB 603. Signed into law in March 2022 it prohibits the medical board from issuing a license to a physician who engaged in sexual activity or misconduct with a patient whose license was revoked in another state or was involved in other violations.
- Tennessee HB 1045. It was signed into law in May 2021, and authorizes the medical board, upon learning of an indictment against a physician for a controlled substance violation or sexual offense, to immediately suspend the doctor’s ability to prescribe controlled substances until the doctor’s case is resolved.
A published study identified a total of 1,721 reports of physician sexual misconduct that were submitted to the National Practitioner Data Bank between 2000 and 2019. The annual incidence of sexual misconduct reports averaged 10.8 per 100,000 U.S. physician licensees, said the researchers.
In a groundbreaking 2016 investigation, the Atlanta Journal-Constitution reviewed thousands of documents and found more than 2,400 doctors whose sexual misconduct cases clearly involved patients since 1999.
Physician sexual misconduct is likely underreported
The actual incidence of physician-patient sexual misconduct is likely higher as a result of underreporting, according to the researchers.
Because a substantial power differential exists between patients and their physicians, the researchers noted, it follows that patient victims, like other sexual assault victims, may be unwilling or unable to report the incident in question.
Many violations involving physician sexual misconduct of patients never came to the attention of state regulators, according to the Journal-Constitution investigation. Reporting showed that hospitals, clinics, and fellow doctors fail to report sexual misconduct to regulators, despite laws in most states requiring them to do so.
Media investigations highlight medical board shortcomings
Public pressure on the California Medical Board increased after the Los Angeles Times investigated what happened to doctors who surrendered or had their licenses revoked after being reported for sexual abuse with patients. The Times revealed in 2021 that the board reinstated 10 of 17 doctors who petitioned for reinstatement.
They include Esmail Nadjmabadi, MD, of Bakersfield, Calif., who had sexually abused six female patients, including one in her mid-teens. The Times reported that, in 2009, he pleaded no contest to a criminal charge that he sexually exploited two or more women and surrendered his medical license the following year.
Five years later, Dr. Nadjmabadi petitioned the medical board to be reinstated and the board approved his request.
The California board has also reinstated several doctors who underwent sex offender rehabilitation. Board members rely heavily on a doctor’s evidence of rehabilitation, usually with the testimony of therapists hired by the doctor, and no input from the patients who were harmed, according to the Times’ investigation.
High-profile sexual misconduct or abuse cases involving Larry Nassar, MD, and Robert Anderson, MD, in Michigan; Richard Strauss, MD, in Ohio; and Ricardo Cruciani, MD, in New York, added to the mounting criticism that medical boards were too lenient in their handling of complaints of sexual misconduct.
Another state tackles sexual misconduct
Ohio’s medical board created an administrative rule stating that licensed physicians have a legal and ethical duty to report colleagues for sexual misconduct with patients and to complete a 1-hour CME training. Failure to report sexual misconduct complaints can lead to a doctor being permanently stripped of his license.
This happened to Robert S. Geiger, MD, in 2016 after not reporting his colleague James Bressi, MD, to the medical board after receiving complaints that Dr. Bressi was sexually abusing female patients at their pain clinic.
Dr. Bressi was convicted of sexual misconduct with a patient, stripped of his medical license, and sentenced to 59 days in prison.
“I think all of these reforms are a step in the right direction and will help to deter doctors from committing sexual misconduct to some extent,” said California activist Marian Hollingsworth, cofounder of the Patient Safety League.
But there’s room for improvement, she said, since “most states fall short in not requiring medical boards to notify law enforcement when they get a complaint of doctor sexual misconduct so the public can be aware of it.”
A version of this article first appeared on Medscape.com.
Guideline stresses new strategies for hypoglycemia management
The Endocrine Society has issued an updated clinical practice guideline on the prevention and management of hypoglycemia in patients with diabetes who are at high risk, addressing the wide variety of treatment advances, such as insulin pumps and continuous glucose monitoring (CGM) systems, that have appeared since the publication of the society’s last guideline on hypoglycemia, in 2009.
“CGM and insulin pumps have been much more commonly used in the last decade among people with diabetes, including children, and there are new forms of glucagon available,” said Anthony L. McCall, MD, PhD, chair of the panel that wrote the guideline.
“We had to update our guideline to match these developments in the diabetes field,” noted Dr. McCall, University of Virginia, Charlottesville, in a press statement.
The new guideline, developed by a multidisciplinary panel of clinical experts and published in the Journal of Clinical Endocrinology and Metabolism, addresses 10 key clinical questions regarding current issues relevant to hypoglycemia prevention and treatment in adult or pediatric patients with either type 1 or type 2 diabetes in the outpatient or inpatient setting.
Key guideline recommendations
The recommendations are based on factors including critical outcomes, implementation feasibility, and patient preferences.
Key guideline recommendations that are considered “strong,” based on evidence, include:
- The use of CGM rather than self-monitoring of blood glucose by fingerstick for patients with type 1 diabetes receiving multiple daily injections. The panel underscored that “comprehensive patient education on how to use and troubleshoot CGM devices and interpret these data is critically important for maximum benefit and successful outcomes.”
The use of a structured program for patient education versus unstructured advice for adult and pediatric outpatients with type 1 diabetes or type 2 diabetes receiving insulin therapy.
- Structured education on how to avoid repeated hypoglycemia is critical, and this education should be performed by experienced diabetes clinicians,” the panel asserts. “Moreover, insurance coverage for education should be available for all insulin-using patients.”
- The use of glucagon preparations that do not have to be reconstituted, as opposed to those that do (that is, available as a powder and diluent) in the treatment of outpatients with severe hypoglycemia.
Guideline recommendations that received conditional recommendations include:
- Use of real-time CGM and algorithm-driven insulin pumps in people with type 1 diabetes.
- Use of CGM for outpatients with type 2 diabetes at high risk for hypoglycemia.
- Use of long-acting and rapid-acting insulin analogs for patients at high risk for hypoglycemia.
Noting that there is “moderate-certainty” evidence for severe hypoglycemia reduction as an outcome in those using long-acting analog insulins versus human neutral protamine Hagedorn (NPH) insulin, the panel cautions that “most studies of long-acting analog insulins do not assess for significant adverse effects, including cardiovascular outcomes, and that many studies were designed to demonstrate noninferiority of analog insulin, compared with human NPH insulin.”
- Initiation of and continuation of CGM for select inpatient populations at high risk for hypoglycemia.
Hypoglycemia: One of top three preventable adverse drug reactions
The updated guidelines are especially important considering the common incidence of hypoglycemia, which the U.S. Department of Health and Human Services has determined to be one of the top 3 preventable adverse drug reactions, the panel says.
They note that between January 2007 and December 2011, emergency department visits for therapy-associated hypoglycemia among Medicare beneficiaries resulted in more than $600 million in spending.
Meanwhile, many people with type 1 or 2 diabetes may not experience or recognize the symptoms of hypoglycemia, which, in severe cases, can lead to unconsciousness or seizures, in addition to affecting quality of life, social life, work productivity, and ability to drive safely.
The key to accurate diagnosis of those patients is assessment of the three levels of hypoglycemia, described in a 2018 consensus statement:
- Level 1: Glucose less than 70 mg/dL (3.9 mmol/L) and greater than or equal to 54 mg/dL (3.0 mmol/L). This level of hypoglycemia should alert patients that they may need to ingest carbohydrate to prevent progressive hypoglycemia.
- Level 2: Glucose less than 54 mg/dL (3.0 mmol/L). This level of hypoglycemia is associated with increased risk for cognitive dysfunction and mortality.
- Level 3: A severe event characterized by altered mental and/or physical status requiring assistance. This level of hypoglycemia is life-threatening and requires emergent treatment, typically with glucagon.
Ultimately, “new technology and medications will help reduce hypoglycemia, and [clinicians] can better treat patients now with new, easier glucagons,” Dr. McCall told this news organization.
“People with diabetes, their caregivers, and diabetes specialists will all benefit from our guideline with a better understanding of best practices and interventions,” the panel notes.
Disparities still exist in access to insulin pumps
Separately, new research shows that while use of insulin pumps to manage type 1 diabetes has grown over 20 years, there has been no improvement in racial, ethnic, and socioeconomic disparities in their use in the United States. The findings are reported in Diabetes Technology & Therapeutics.
Using data from the SEARCH for Diabetes Youth Study across four time periods between 2001 and 2019, the researchers show that by the end of the period studied, insulin pump use was 67% among non-Hispanic White people, 41% among Hispanic people, 29% among Black people, and 46% among other racial and ethnic groups.
In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000-$74,999, 51% with $25,000-$49,999, and 41% with less than $25,000 used the pumps.
“Diabetes technology has numerous benefits for patients with type 1 diabetes, but the problem is that there is a huge divide in who actually has access to these technologies,” said study lead Estelle Everett, MD, assistant professor of medicine in the division of endocrinology, diabetes & metabolism at the University of California, Los Angeles.
A version of this article first appeared on Medscape.com.
The Endocrine Society has issued an updated clinical practice guideline on the prevention and management of hypoglycemia in patients with diabetes who are at high risk, addressing the wide variety of treatment advances, such as insulin pumps and continuous glucose monitoring (CGM) systems, that have appeared since the publication of the society’s last guideline on hypoglycemia, in 2009.
“CGM and insulin pumps have been much more commonly used in the last decade among people with diabetes, including children, and there are new forms of glucagon available,” said Anthony L. McCall, MD, PhD, chair of the panel that wrote the guideline.
“We had to update our guideline to match these developments in the diabetes field,” noted Dr. McCall, University of Virginia, Charlottesville, in a press statement.
The new guideline, developed by a multidisciplinary panel of clinical experts and published in the Journal of Clinical Endocrinology and Metabolism, addresses 10 key clinical questions regarding current issues relevant to hypoglycemia prevention and treatment in adult or pediatric patients with either type 1 or type 2 diabetes in the outpatient or inpatient setting.
Key guideline recommendations
The recommendations are based on factors including critical outcomes, implementation feasibility, and patient preferences.
Key guideline recommendations that are considered “strong,” based on evidence, include:
- The use of CGM rather than self-monitoring of blood glucose by fingerstick for patients with type 1 diabetes receiving multiple daily injections. The panel underscored that “comprehensive patient education on how to use and troubleshoot CGM devices and interpret these data is critically important for maximum benefit and successful outcomes.”
The use of a structured program for patient education versus unstructured advice for adult and pediatric outpatients with type 1 diabetes or type 2 diabetes receiving insulin therapy.
- Structured education on how to avoid repeated hypoglycemia is critical, and this education should be performed by experienced diabetes clinicians,” the panel asserts. “Moreover, insurance coverage for education should be available for all insulin-using patients.”
- The use of glucagon preparations that do not have to be reconstituted, as opposed to those that do (that is, available as a powder and diluent) in the treatment of outpatients with severe hypoglycemia.
Guideline recommendations that received conditional recommendations include:
- Use of real-time CGM and algorithm-driven insulin pumps in people with type 1 diabetes.
- Use of CGM for outpatients with type 2 diabetes at high risk for hypoglycemia.
- Use of long-acting and rapid-acting insulin analogs for patients at high risk for hypoglycemia.
Noting that there is “moderate-certainty” evidence for severe hypoglycemia reduction as an outcome in those using long-acting analog insulins versus human neutral protamine Hagedorn (NPH) insulin, the panel cautions that “most studies of long-acting analog insulins do not assess for significant adverse effects, including cardiovascular outcomes, and that many studies were designed to demonstrate noninferiority of analog insulin, compared with human NPH insulin.”
- Initiation of and continuation of CGM for select inpatient populations at high risk for hypoglycemia.
Hypoglycemia: One of top three preventable adverse drug reactions
The updated guidelines are especially important considering the common incidence of hypoglycemia, which the U.S. Department of Health and Human Services has determined to be one of the top 3 preventable adverse drug reactions, the panel says.
They note that between January 2007 and December 2011, emergency department visits for therapy-associated hypoglycemia among Medicare beneficiaries resulted in more than $600 million in spending.
Meanwhile, many people with type 1 or 2 diabetes may not experience or recognize the symptoms of hypoglycemia, which, in severe cases, can lead to unconsciousness or seizures, in addition to affecting quality of life, social life, work productivity, and ability to drive safely.
The key to accurate diagnosis of those patients is assessment of the three levels of hypoglycemia, described in a 2018 consensus statement:
- Level 1: Glucose less than 70 mg/dL (3.9 mmol/L) and greater than or equal to 54 mg/dL (3.0 mmol/L). This level of hypoglycemia should alert patients that they may need to ingest carbohydrate to prevent progressive hypoglycemia.
- Level 2: Glucose less than 54 mg/dL (3.0 mmol/L). This level of hypoglycemia is associated with increased risk for cognitive dysfunction and mortality.
- Level 3: A severe event characterized by altered mental and/or physical status requiring assistance. This level of hypoglycemia is life-threatening and requires emergent treatment, typically with glucagon.
Ultimately, “new technology and medications will help reduce hypoglycemia, and [clinicians] can better treat patients now with new, easier glucagons,” Dr. McCall told this news organization.
“People with diabetes, their caregivers, and diabetes specialists will all benefit from our guideline with a better understanding of best practices and interventions,” the panel notes.
Disparities still exist in access to insulin pumps
Separately, new research shows that while use of insulin pumps to manage type 1 diabetes has grown over 20 years, there has been no improvement in racial, ethnic, and socioeconomic disparities in their use in the United States. The findings are reported in Diabetes Technology & Therapeutics.
Using data from the SEARCH for Diabetes Youth Study across four time periods between 2001 and 2019, the researchers show that by the end of the period studied, insulin pump use was 67% among non-Hispanic White people, 41% among Hispanic people, 29% among Black people, and 46% among other racial and ethnic groups.
In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000-$74,999, 51% with $25,000-$49,999, and 41% with less than $25,000 used the pumps.
“Diabetes technology has numerous benefits for patients with type 1 diabetes, but the problem is that there is a huge divide in who actually has access to these technologies,” said study lead Estelle Everett, MD, assistant professor of medicine in the division of endocrinology, diabetes & metabolism at the University of California, Los Angeles.
A version of this article first appeared on Medscape.com.
The Endocrine Society has issued an updated clinical practice guideline on the prevention and management of hypoglycemia in patients with diabetes who are at high risk, addressing the wide variety of treatment advances, such as insulin pumps and continuous glucose monitoring (CGM) systems, that have appeared since the publication of the society’s last guideline on hypoglycemia, in 2009.
“CGM and insulin pumps have been much more commonly used in the last decade among people with diabetes, including children, and there are new forms of glucagon available,” said Anthony L. McCall, MD, PhD, chair of the panel that wrote the guideline.
“We had to update our guideline to match these developments in the diabetes field,” noted Dr. McCall, University of Virginia, Charlottesville, in a press statement.
The new guideline, developed by a multidisciplinary panel of clinical experts and published in the Journal of Clinical Endocrinology and Metabolism, addresses 10 key clinical questions regarding current issues relevant to hypoglycemia prevention and treatment in adult or pediatric patients with either type 1 or type 2 diabetes in the outpatient or inpatient setting.
Key guideline recommendations
The recommendations are based on factors including critical outcomes, implementation feasibility, and patient preferences.
Key guideline recommendations that are considered “strong,” based on evidence, include:
- The use of CGM rather than self-monitoring of blood glucose by fingerstick for patients with type 1 diabetes receiving multiple daily injections. The panel underscored that “comprehensive patient education on how to use and troubleshoot CGM devices and interpret these data is critically important for maximum benefit and successful outcomes.”
The use of a structured program for patient education versus unstructured advice for adult and pediatric outpatients with type 1 diabetes or type 2 diabetes receiving insulin therapy.
- Structured education on how to avoid repeated hypoglycemia is critical, and this education should be performed by experienced diabetes clinicians,” the panel asserts. “Moreover, insurance coverage for education should be available for all insulin-using patients.”
- The use of glucagon preparations that do not have to be reconstituted, as opposed to those that do (that is, available as a powder and diluent) in the treatment of outpatients with severe hypoglycemia.
Guideline recommendations that received conditional recommendations include:
- Use of real-time CGM and algorithm-driven insulin pumps in people with type 1 diabetes.
- Use of CGM for outpatients with type 2 diabetes at high risk for hypoglycemia.
- Use of long-acting and rapid-acting insulin analogs for patients at high risk for hypoglycemia.
Noting that there is “moderate-certainty” evidence for severe hypoglycemia reduction as an outcome in those using long-acting analog insulins versus human neutral protamine Hagedorn (NPH) insulin, the panel cautions that “most studies of long-acting analog insulins do not assess for significant adverse effects, including cardiovascular outcomes, and that many studies were designed to demonstrate noninferiority of analog insulin, compared with human NPH insulin.”
- Initiation of and continuation of CGM for select inpatient populations at high risk for hypoglycemia.
Hypoglycemia: One of top three preventable adverse drug reactions
The updated guidelines are especially important considering the common incidence of hypoglycemia, which the U.S. Department of Health and Human Services has determined to be one of the top 3 preventable adverse drug reactions, the panel says.
They note that between January 2007 and December 2011, emergency department visits for therapy-associated hypoglycemia among Medicare beneficiaries resulted in more than $600 million in spending.
Meanwhile, many people with type 1 or 2 diabetes may not experience or recognize the symptoms of hypoglycemia, which, in severe cases, can lead to unconsciousness or seizures, in addition to affecting quality of life, social life, work productivity, and ability to drive safely.
The key to accurate diagnosis of those patients is assessment of the three levels of hypoglycemia, described in a 2018 consensus statement:
- Level 1: Glucose less than 70 mg/dL (3.9 mmol/L) and greater than or equal to 54 mg/dL (3.0 mmol/L). This level of hypoglycemia should alert patients that they may need to ingest carbohydrate to prevent progressive hypoglycemia.
- Level 2: Glucose less than 54 mg/dL (3.0 mmol/L). This level of hypoglycemia is associated with increased risk for cognitive dysfunction and mortality.
- Level 3: A severe event characterized by altered mental and/or physical status requiring assistance. This level of hypoglycemia is life-threatening and requires emergent treatment, typically with glucagon.
Ultimately, “new technology and medications will help reduce hypoglycemia, and [clinicians] can better treat patients now with new, easier glucagons,” Dr. McCall told this news organization.
“People with diabetes, their caregivers, and diabetes specialists will all benefit from our guideline with a better understanding of best practices and interventions,” the panel notes.
Disparities still exist in access to insulin pumps
Separately, new research shows that while use of insulin pumps to manage type 1 diabetes has grown over 20 years, there has been no improvement in racial, ethnic, and socioeconomic disparities in their use in the United States. The findings are reported in Diabetes Technology & Therapeutics.
Using data from the SEARCH for Diabetes Youth Study across four time periods between 2001 and 2019, the researchers show that by the end of the period studied, insulin pump use was 67% among non-Hispanic White people, 41% among Hispanic people, 29% among Black people, and 46% among other racial and ethnic groups.
In addition, 70% of people with bachelor’s degrees or higher used the pumps, compared with 56% among those with some college, 40% among holders of high school degrees, and 18% among those with no high school education. By income level, 74% of those with household incomes of $75,000 or more, 66% with $50,000-$74,999, 51% with $25,000-$49,999, and 41% with less than $25,000 used the pumps.
“Diabetes technology has numerous benefits for patients with type 1 diabetes, but the problem is that there is a huge divide in who actually has access to these technologies,” said study lead Estelle Everett, MD, assistant professor of medicine in the division of endocrinology, diabetes & metabolism at the University of California, Los Angeles.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ENDOCRINOLOGY AND METABOLISM
Multidrug-resistant gram-negative infections treatable with newer antibiotics, but guidance is needed
Multidrug-resistant gram-negative infections (MDRGNIs) are an emerging and deadly threat worldwide. Some of these infections are now resistant to nearly all antibiotics, and very few treatment options exist. Some of the remaining antibiotics for these MDRGNIs can cause acute kidney injury and have other toxic effects and can worsen antibiotic resistance. When deciding which drugs to use, clinicians need to juggle the possible lethality of the infection with the dangers of its treatment.
Samuel Windham, MD, and Marin H. Kollef, MD, authors of a recent article in Current Opinion in Infectious Diseases, express this urgency. They offer recommendations based on current guidelines and recently published research for treating MDRGNIs with some of the newer antibiotics.
Dr. Kollef, professor of pulmonary and critical care medicine at Washington University in St. Louis, said in an email, “Our recommendations differ in that they offer an approach that is based on disease severity, local resistance prevalence in MDRGNIs, and patient risk factors for infection with MDRGNIs. For patients with severe infection and risk factors for infection with MDRGNIs, we suggest empiric coverage for MDRGNIs until susceptibility data are available or based on rapid molecular testing. Selection of antibiotic therapy would be based on which MDRGNIs predominate locally.”
In their article, the authors discuss how to best utilize the newer antibiotics of ceftazidime-avibactam (CZA), cefiderocol, ceftolozane-tazobactam (C/T), meropenem-vaborbactam (MVB), imipenem-relebactam (I-R), aztreonam-avibactam (ATM-AVI), eravacycline, and plazomicin.
The scope of the problem
Bacterial infections are deadly and are becoming less treatable. The Centers for Disease Control and Prevention reported in 2022 that the COVID-19 pandemic has reversed years of decreases in health care–associated infections. Much of the increase has been caused by multidrug-resistant organisms.
In November 2022, authors of an article published in The Lancet estimated worldwide deaths from 33 bacterial genera across 11 infectious syndromes. They found that these infections were the second leading cause of death worldwide in 2019 (ischemic heart disease was the first). Furthermore, they discovered that 54.9% of these deaths were attributable to just five pathogens – Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Three of those five bacterial species – E. coli, K. pneumoniae, and P. aeruginosa – are gram-negative and are highly prone to drug resistance.
The CDC classified each of those three pathogens as an “urgent threat” in its 2019 Antibiotic Resistance Threats in the United States report. Of particular concern are gram-negative infections that have become resistant to carbapenems, a heavy-hitting class of antibiotics.
Regarding organisms that cause MDRGNIs, known as serine-beta-lactamases (OXA, KPC, and CTX-M) and metallo-beta-lactamases (NDM, VIM, and IMP). Carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumanii also produce carbapenemases, rendering them invulnerable to carbapenem antibiotics.
Traditionally, a common alternative used for carbapenem-resistant infections has been colistin, an older and very toxic antibiotic. The authors cite recent research demonstrating that CZA yields significantly better outcomes with regard to patient mortality and acute kidney injury than colistin and that CZA plus aztreonam can even decrease mortality and length of hospital stay for patients who have bloodstream infections with metallo-beta-lactamase-producing Enterobacterales, which are some of the hardest infections to treat.
“CZA has been demonstrated to have excellent activity against MDR Pseudomonas aeruginosa and KPC Enterobacterales. It should be the preferred agent for use, compared with colistin, for the treatment of carbapenem-resistant gram-negative bacteria susceptible to CZA. Moreover, CZA combined with aztreonam has been shown to be an effective treatment for metallo-beta-lactamase MDRGNIs,” Dr. Kollef said.
Four key recommendations for treating MDRGNIs
The authors base their recommendations, in addition to the recent studies they cite concerning CZA, upon two major guidelines on the treatment of MDRGNIs: the European Society of Clinical Microbiology and Infectious Diseases’ Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli, and the Infectious Diseases Society of America’s (IDSA’s) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections (multiple documents, found here and here).
Dr. Windham and Dr. Kollef present a table showing the spectrum of activity of the newer antibiotics, as well as an algorithm for decision-making. They summarize their treatment recommendations, which are based upon the bacterial infection cultures or on historical risk (previous infection or colonization history). They encourage empiric treatment if there is an increased risk of death or the presence of shock. By pathogen, they recommend the following:
- For carbapenem-resistant Enterobacterales, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or meropenem-vaborbactam.
- For carbapenem-resistant Pseudomonas aeruginosa, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or ceftolozane-tazobactam.
- For carbapenem-resistant Acinetobacter baumanii, clinicians should treat patients with a cefiderocol backbone with or without the addition of plazomicin, eravacycline, or other older antibacterials.
- For metallo-beta-lactamase-producing organisms, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, aztreonam, imipenem-cilastatin-relabactam, aztreonam, or aztreonam-avibactam. The authors acknowledge that evidence is limited on treating these infections.
“In general, ceftazidime-avibactam works pretty well in patients with MDRGNIs, and there is no evidence that any of the other new agents is conclusively better in treatment responses. CZA and ceftolozane-tazobactam were the first of the new antibiotics active against highly MDRGN to get approved, and they have been most widely used,” Cornelius “Neil” J. Clancy, MD, chief of the Infectious Diseases Section at the VA Pittsburgh Health Care System, explained. Dr. Clancy was not involved in the Windham-Kollef review article.
“As such, it is not surprising that resistance has emerged and that it has been reported more commonly than for some other agents. The issue of resistance will be considered again as IDSA puts together their update,” Dr. Clancy said.
“The IDSA guidelines are regularly updated. The next updated iteration will be online in early 2023,” said Dr. Clancy, who is also affiliated with IDSA. “Clinical and resistance data that have appeared since the last update in 2022 will be considered as the guidance is put together.”
In general, Dr. Kollef also recommends using a facility’s antibiogram. “They are useful in determining which MDRGN’s predominate locally,” he said.
Dr. Kollef is a consultant for Pfizer, Merck, and Shionogi. Dr. Clancy has received research funding from Merck and from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Multidrug-resistant gram-negative infections (MDRGNIs) are an emerging and deadly threat worldwide. Some of these infections are now resistant to nearly all antibiotics, and very few treatment options exist. Some of the remaining antibiotics for these MDRGNIs can cause acute kidney injury and have other toxic effects and can worsen antibiotic resistance. When deciding which drugs to use, clinicians need to juggle the possible lethality of the infection with the dangers of its treatment.
Samuel Windham, MD, and Marin H. Kollef, MD, authors of a recent article in Current Opinion in Infectious Diseases, express this urgency. They offer recommendations based on current guidelines and recently published research for treating MDRGNIs with some of the newer antibiotics.
Dr. Kollef, professor of pulmonary and critical care medicine at Washington University in St. Louis, said in an email, “Our recommendations differ in that they offer an approach that is based on disease severity, local resistance prevalence in MDRGNIs, and patient risk factors for infection with MDRGNIs. For patients with severe infection and risk factors for infection with MDRGNIs, we suggest empiric coverage for MDRGNIs until susceptibility data are available or based on rapid molecular testing. Selection of antibiotic therapy would be based on which MDRGNIs predominate locally.”
In their article, the authors discuss how to best utilize the newer antibiotics of ceftazidime-avibactam (CZA), cefiderocol, ceftolozane-tazobactam (C/T), meropenem-vaborbactam (MVB), imipenem-relebactam (I-R), aztreonam-avibactam (ATM-AVI), eravacycline, and plazomicin.
The scope of the problem
Bacterial infections are deadly and are becoming less treatable. The Centers for Disease Control and Prevention reported in 2022 that the COVID-19 pandemic has reversed years of decreases in health care–associated infections. Much of the increase has been caused by multidrug-resistant organisms.
In November 2022, authors of an article published in The Lancet estimated worldwide deaths from 33 bacterial genera across 11 infectious syndromes. They found that these infections were the second leading cause of death worldwide in 2019 (ischemic heart disease was the first). Furthermore, they discovered that 54.9% of these deaths were attributable to just five pathogens – Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Three of those five bacterial species – E. coli, K. pneumoniae, and P. aeruginosa – are gram-negative and are highly prone to drug resistance.
The CDC classified each of those three pathogens as an “urgent threat” in its 2019 Antibiotic Resistance Threats in the United States report. Of particular concern are gram-negative infections that have become resistant to carbapenems, a heavy-hitting class of antibiotics.
Regarding organisms that cause MDRGNIs, known as serine-beta-lactamases (OXA, KPC, and CTX-M) and metallo-beta-lactamases (NDM, VIM, and IMP). Carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumanii also produce carbapenemases, rendering them invulnerable to carbapenem antibiotics.
Traditionally, a common alternative used for carbapenem-resistant infections has been colistin, an older and very toxic antibiotic. The authors cite recent research demonstrating that CZA yields significantly better outcomes with regard to patient mortality and acute kidney injury than colistin and that CZA plus aztreonam can even decrease mortality and length of hospital stay for patients who have bloodstream infections with metallo-beta-lactamase-producing Enterobacterales, which are some of the hardest infections to treat.
“CZA has been demonstrated to have excellent activity against MDR Pseudomonas aeruginosa and KPC Enterobacterales. It should be the preferred agent for use, compared with colistin, for the treatment of carbapenem-resistant gram-negative bacteria susceptible to CZA. Moreover, CZA combined with aztreonam has been shown to be an effective treatment for metallo-beta-lactamase MDRGNIs,” Dr. Kollef said.
Four key recommendations for treating MDRGNIs
The authors base their recommendations, in addition to the recent studies they cite concerning CZA, upon two major guidelines on the treatment of MDRGNIs: the European Society of Clinical Microbiology and Infectious Diseases’ Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli, and the Infectious Diseases Society of America’s (IDSA’s) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections (multiple documents, found here and here).
Dr. Windham and Dr. Kollef present a table showing the spectrum of activity of the newer antibiotics, as well as an algorithm for decision-making. They summarize their treatment recommendations, which are based upon the bacterial infection cultures or on historical risk (previous infection or colonization history). They encourage empiric treatment if there is an increased risk of death or the presence of shock. By pathogen, they recommend the following:
- For carbapenem-resistant Enterobacterales, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or meropenem-vaborbactam.
- For carbapenem-resistant Pseudomonas aeruginosa, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or ceftolozane-tazobactam.
- For carbapenem-resistant Acinetobacter baumanii, clinicians should treat patients with a cefiderocol backbone with or without the addition of plazomicin, eravacycline, or other older antibacterials.
- For metallo-beta-lactamase-producing organisms, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, aztreonam, imipenem-cilastatin-relabactam, aztreonam, or aztreonam-avibactam. The authors acknowledge that evidence is limited on treating these infections.
“In general, ceftazidime-avibactam works pretty well in patients with MDRGNIs, and there is no evidence that any of the other new agents is conclusively better in treatment responses. CZA and ceftolozane-tazobactam were the first of the new antibiotics active against highly MDRGN to get approved, and they have been most widely used,” Cornelius “Neil” J. Clancy, MD, chief of the Infectious Diseases Section at the VA Pittsburgh Health Care System, explained. Dr. Clancy was not involved in the Windham-Kollef review article.
“As such, it is not surprising that resistance has emerged and that it has been reported more commonly than for some other agents. The issue of resistance will be considered again as IDSA puts together their update,” Dr. Clancy said.
“The IDSA guidelines are regularly updated. The next updated iteration will be online in early 2023,” said Dr. Clancy, who is also affiliated with IDSA. “Clinical and resistance data that have appeared since the last update in 2022 will be considered as the guidance is put together.”
In general, Dr. Kollef also recommends using a facility’s antibiogram. “They are useful in determining which MDRGN’s predominate locally,” he said.
Dr. Kollef is a consultant for Pfizer, Merck, and Shionogi. Dr. Clancy has received research funding from Merck and from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
Multidrug-resistant gram-negative infections (MDRGNIs) are an emerging and deadly threat worldwide. Some of these infections are now resistant to nearly all antibiotics, and very few treatment options exist. Some of the remaining antibiotics for these MDRGNIs can cause acute kidney injury and have other toxic effects and can worsen antibiotic resistance. When deciding which drugs to use, clinicians need to juggle the possible lethality of the infection with the dangers of its treatment.
Samuel Windham, MD, and Marin H. Kollef, MD, authors of a recent article in Current Opinion in Infectious Diseases, express this urgency. They offer recommendations based on current guidelines and recently published research for treating MDRGNIs with some of the newer antibiotics.
Dr. Kollef, professor of pulmonary and critical care medicine at Washington University in St. Louis, said in an email, “Our recommendations differ in that they offer an approach that is based on disease severity, local resistance prevalence in MDRGNIs, and patient risk factors for infection with MDRGNIs. For patients with severe infection and risk factors for infection with MDRGNIs, we suggest empiric coverage for MDRGNIs until susceptibility data are available or based on rapid molecular testing. Selection of antibiotic therapy would be based on which MDRGNIs predominate locally.”
In their article, the authors discuss how to best utilize the newer antibiotics of ceftazidime-avibactam (CZA), cefiderocol, ceftolozane-tazobactam (C/T), meropenem-vaborbactam (MVB), imipenem-relebactam (I-R), aztreonam-avibactam (ATM-AVI), eravacycline, and plazomicin.
The scope of the problem
Bacterial infections are deadly and are becoming less treatable. The Centers for Disease Control and Prevention reported in 2022 that the COVID-19 pandemic has reversed years of decreases in health care–associated infections. Much of the increase has been caused by multidrug-resistant organisms.
In November 2022, authors of an article published in The Lancet estimated worldwide deaths from 33 bacterial genera across 11 infectious syndromes. They found that these infections were the second leading cause of death worldwide in 2019 (ischemic heart disease was the first). Furthermore, they discovered that 54.9% of these deaths were attributable to just five pathogens – Staphylococcus aureus, Escherichia coli, Streptococcus pneumoniae, Klebsiella pneumoniae, and Pseudomonas aeruginosa. Three of those five bacterial species – E. coli, K. pneumoniae, and P. aeruginosa – are gram-negative and are highly prone to drug resistance.
The CDC classified each of those three pathogens as an “urgent threat” in its 2019 Antibiotic Resistance Threats in the United States report. Of particular concern are gram-negative infections that have become resistant to carbapenems, a heavy-hitting class of antibiotics.
Regarding organisms that cause MDRGNIs, known as serine-beta-lactamases (OXA, KPC, and CTX-M) and metallo-beta-lactamases (NDM, VIM, and IMP). Carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant Acinetobacter baumanii also produce carbapenemases, rendering them invulnerable to carbapenem antibiotics.
Traditionally, a common alternative used for carbapenem-resistant infections has been colistin, an older and very toxic antibiotic. The authors cite recent research demonstrating that CZA yields significantly better outcomes with regard to patient mortality and acute kidney injury than colistin and that CZA plus aztreonam can even decrease mortality and length of hospital stay for patients who have bloodstream infections with metallo-beta-lactamase-producing Enterobacterales, which are some of the hardest infections to treat.
“CZA has been demonstrated to have excellent activity against MDR Pseudomonas aeruginosa and KPC Enterobacterales. It should be the preferred agent for use, compared with colistin, for the treatment of carbapenem-resistant gram-negative bacteria susceptible to CZA. Moreover, CZA combined with aztreonam has been shown to be an effective treatment for metallo-beta-lactamase MDRGNIs,” Dr. Kollef said.
Four key recommendations for treating MDRGNIs
The authors base their recommendations, in addition to the recent studies they cite concerning CZA, upon two major guidelines on the treatment of MDRGNIs: the European Society of Clinical Microbiology and Infectious Diseases’ Guidelines for the Treatment of Infections Caused by Multidrug-Resistant Gram-Negative Bacilli, and the Infectious Diseases Society of America’s (IDSA’s) Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections (multiple documents, found here and here).
Dr. Windham and Dr. Kollef present a table showing the spectrum of activity of the newer antibiotics, as well as an algorithm for decision-making. They summarize their treatment recommendations, which are based upon the bacterial infection cultures or on historical risk (previous infection or colonization history). They encourage empiric treatment if there is an increased risk of death or the presence of shock. By pathogen, they recommend the following:
- For carbapenem-resistant Enterobacterales, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or meropenem-vaborbactam.
- For carbapenem-resistant Pseudomonas aeruginosa, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, imipenem-cilastatin-relabactam, or ceftolozane-tazobactam.
- For carbapenem-resistant Acinetobacter baumanii, clinicians should treat patients with a cefiderocol backbone with or without the addition of plazomicin, eravacycline, or other older antibacterials.
- For metallo-beta-lactamase-producing organisms, clinicians should treat patients with cefiderocol, ceftazidime-avibactam, aztreonam, imipenem-cilastatin-relabactam, aztreonam, or aztreonam-avibactam. The authors acknowledge that evidence is limited on treating these infections.
“In general, ceftazidime-avibactam works pretty well in patients with MDRGNIs, and there is no evidence that any of the other new agents is conclusively better in treatment responses. CZA and ceftolozane-tazobactam were the first of the new antibiotics active against highly MDRGN to get approved, and they have been most widely used,” Cornelius “Neil” J. Clancy, MD, chief of the Infectious Diseases Section at the VA Pittsburgh Health Care System, explained. Dr. Clancy was not involved in the Windham-Kollef review article.
“As such, it is not surprising that resistance has emerged and that it has been reported more commonly than for some other agents. The issue of resistance will be considered again as IDSA puts together their update,” Dr. Clancy said.
“The IDSA guidelines are regularly updated. The next updated iteration will be online in early 2023,” said Dr. Clancy, who is also affiliated with IDSA. “Clinical and resistance data that have appeared since the last update in 2022 will be considered as the guidance is put together.”
In general, Dr. Kollef also recommends using a facility’s antibiogram. “They are useful in determining which MDRGN’s predominate locally,” he said.
Dr. Kollef is a consultant for Pfizer, Merck, and Shionogi. Dr. Clancy has received research funding from Merck and from the National Institutes of Health.
A version of this article first appeared on Medscape.com.
FROM CURRENT OPINION IN INFECTIOUS DISEASES
Poison centers fielding more calls about teen cannabis use
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Poison control centers in the United States now receive more calls about adolescents abusing cannabis than alcohol or any other substance, according to a new study.
Many helpline calls about cannabis involve edible products, the researchers noted.
Over-the-counter medications – especially dextromethorphan-containing cough and cold medications and oral antihistamines, such as Benadryl – are other commonly abused substances.
But cannabis recently started topping the list.
“Since 2018, the most reported misused/abused substance involved exposure to marijuana,” according to the study, which was published online in Clinical Toxicology.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health & Science University, Portland, and colleagues analyzed calls to United States poison control centers between 2000 and 2020. They focused on 338,000 calls about intentional substance abuse or misuse, including for the purpose of getting high, in individuals aged 6-18 years.
The calls were made to 55 certified helplines for health professionals, public health agencies, and members of the public seeking guidance about exposures to various substances.
Cannabis vs. alcohol
In 2000, alcohol was the substance involved in the largest number of cases (1,318, or 9.8% of all calls). Between 2000 and 2013, cases of alcohol abuse exceeded the number of cannabis cases each year.
But that changed in 2014, when cannabis overtook alcohol.
Over the 20-year study period, calls about exposure to cannabis increased 245%, from 510 in 2000 to 1,761 in 2020.
Edibles played a key role.
“Edible marijuana preparations accounted for the highest increase in call rates, compared with all other forms of marijuana,” the researchers reported.
Edible products are “often marketed in ways that are attractive to young people, and they are considered more discrete and convenient,” Dr. Hughes said. But they can have “unpredictable” effects.
“Compared to smoking cannabis, which typically results in an immediate high, intoxication from edible forms usually takes several hours, which may lead some individuals to consume greater amounts and experience unexpected and unpredictable highs,” she said.
For example, prior research has shown that edible cannabis consumption may lead to more acute psychiatric symptoms and cardiovascular events than does inhaled cannabis.
Trends in alcohol use may have held relatively steady, despite some minor declines in the poison center data, Dr. Hughes said.
“Anecdotally, there hasn’t been an obvious notable reduction in alcohol cases in the emergency department,” she said. “However, I wouldn’t expect a huge change given our data only found a slow mild decline in alcohol cases over the study period.”
The increase in cannabis-related calls coincides with more states legalizing or decriminalizing the drug for medical or recreational purposes. Currently, 21 states have approved recreational cannabis for adults who are at least 21 years old.
What are the risks?
Parents typically call a poison center about cannabis exposure after they see or suspect that their child has ingested loose cannabis leaves or edibles containing the substance, Dr. Hughes said.
“The poison center provides guidance to parents about whether or not their child can be watched at home or requires referral to a health care facility,” she said. “While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products.”
Intentional misuse or abuse tends to occur in older children and teens.
Nonprescription drugs have a high potential for abuse because they are legal and may be perceived as safe, Dr. Hughes said.
If a child has a history of misusing or abusing substances or if a parent is worried that their child is at high risk for this behavior, they should consider securing medicines in a lock box, she advised.
That applies to cannabis too.
“I would recommend that parents also consider locking up their cannabis products,” she said.
The National Poison Data System relies on voluntary reporting, and the data are not expected to represent the actual number of intentional misuse and abuse exposures, the researchers noted.
Poison control centers in the United States are available for consultation about patients with known or suspected cannabis ingestion or other suspected poisonings (1-800-222-1222).
The researchers had no disclosures.
A version of this article first appeared on Medscape.com.
Rise of the fungi: Pandemic tied to increasing fungal infections
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.
COVID-19 has lifted the lid on the risks of secondary pulmonary fungal infections in patients with severe respiratory viral illness – even previously immunocompetent individuals – and highlighted the importance of vigilant investigation to achieve early diagnoses, leading experts say.
Most fungi are not under surveillance in the United States, leaving experts without a national picture of the true burden of infection through the pandemic. However, a collection of published case series, cohort studies, and reviews from Europe, the United States, and throughout the world – mainly pre-Omicron – show that fungal disease has affected a significant portion of critically ill patients with COVID-19, with concerning excess mortality, these experts say.
COVID-associated pulmonary aspergillosis (CAPA) has been the predominant fungal coinfection in the United States and internationally. But COVID-associated mucormycosis (CAM) – the infection that surged in India in early 2021 – has also affected some patients in the United States, published data show. So have Pneumocystitis pneumonia, cryptococcosis, histoplasmosis, and Candida infections (which mainly affect the bloodstream and abdomen), say the experts who were interviewed.
“We had predicted [a rise in] aspergillosis, but we saw more than we thought we’d see. Most fungal infections became more common with COVID-19,” said George Thompson, MD, professor of clinical medicine at the University of California, Davis, and cochair of the University of Alabama–based Mycoses Study Group Education Committee, a group of experts in medical mycology. Pneumocystitis, for instance, “has historically been associated with AIDS or different types of leukemia or lymphoma, and is not an infection we’ve typically seen in our otherwise healthy ICU patients,” he noted. “But we did see more of it [with COVID-19].”
More recently, with fewer patients during the Omicron phase in intensive care units with acute respiratory failure, the profile of fungal disease secondary to COVID-19 has changed. Increasing proportions of patients have traditional risk factors for aspergillosis, such as hematologic malignancies and longer-term, pre-COVID use of systemic corticosteroids – a change that makes the contribution of the viral illness harder to distinguish.
Moving forward, the lessons of the COVID era – the fungal risks to patients with serious viral infections and the persistence needed to diagnose aspergillosis and other pulmonary fungal infections using bronchoscopy and imperfect noninvasive tests – should be taken to heart, experts say.
“Fungal diseases are not rare. They’re just not diagnosed because no one thinks to look for them,” said Dr. Thompson, a contributor to a recently released World Health Organization report naming a “fungal priority pathogens” list.
“We’re going to continue to see [secondary fungal infections] with other respiratory viruses,” he said. And overall, given environmental and other changes, “we’re going to see more and more fungal disease in the patients we take care of.”
CAPA not a surprise
CAPA is “not an unfamiliar story” in the world of fungal disease, given a history of influenza-associated pulmonary aspergillosis (IAPA), said Kieren A. Marr, MD, MBA, adjunct professor of medicine and past director of the transplant and oncology infectious diseases program at Johns Hopkins University, Baltimore, who has long researched invasive fungal disease.
European researchers, she said, have led the way in describing a high incidence of IAPA in patients admitted to ICUs with influenza. In a retrospective multicenter cohort study reported in 2018 by the Dutch-Belgian Mycosis Study group, for instance, almost 20% of 432 influenza patients admitted to the ICU, including patients who were otherwise healthy and not immunocompromised, had the diagnosis a median of 3 days after ICU admission. (Across other cohort studies, rates of IAPA have ranged from 7% to 30%.)
Mortality was significant: 51% of patients with influenza and invasive pulmonary aspergillosis died within 90 days, compared with 28% of patients with influenza and no invasive pulmonary aspergillosis.
Reports from Europe early in the pandemic indicated that CAPA was a similarly serious problem, prompting establishment at Johns Hopkins University of an aggressive screening program utilizing biomarker-based testing of blood and bronchoalveolar lavage (BAL) fluid. Of 396 mechanically ventilated COVID-19 patients admitted to Johns Hopkins University hospitals between March and August 2020, 39 met the institution’s criteria for CAPA, Dr. Marr and her colleagues reported this year in what might be the largest U.S. cohort study of CAPA published to date.
“We now know definitively that people with severe influenza and with severe COVID also have high risks for both invasive and airway disease caused by airborne fungi, most commonly aspergilliosis,” Dr. Marr said.
More recent unpublished analyses of patients from the start of the pandemic to June 2021 show persistent risk, said Nitipong Permpalung, MD, MPH, assistant professor in transplant and oncology infectious diseases at Johns Hopkins University and lead author of the cohort study. Among 832 patients with COVID-19 who were mechanically ventilated in Johns Hopkins University hospitals, 11.8% had CAPA, he said. (Also, 3.2% had invasive candidiasis, and 1.1% had other invasive fungal infections.)
Other sources said in interviews that these CAPA prevalence rates generally mirror reports from Europe, though some investigators in Europe have reported CAPA rates more toward 15%.
(The Mycoses Study Group recently collected data from its consortium of U.S. medical centers on the prevalence of CAPA, with funding support from the CDC, but at press time the data had not yet been released. Dr. Thompson said he suspected the prevalence will be lower than earlier papers have suggested, “but still will reflect a significant burden of disease.”)
Patients in the published Johns Hopkins University study who had CAPA were more likely than those with COVID-19 but no CAPA to have underlying pulmonary disease, liver disease, coagulopathy, solid tumors, multiple myeloma, and COVID-19–directed corticosteroids. And they had uniformly worse outcomes with regards to severity of illness and length of intubation.
How much of CAPA is driven by the SARS-CoV-2 virus itself and how much is a consequence of COVID-19 treatments is a topic of active discussion and research. Martin Hoenigl, MD, of the University of Graz, Austria, a leading researcher in medical mycology, said research shows corticosteroids and anti–IL-6 treatments, such as tocilizumab, used to treat COVID-19–driven acute respiratory failure clearly have contributed to CAPA. But he contends that “a number of other mechanisms” are involved as well.
“The immunologic mechanisms are definitely different in these patients with viral illness than in other ICU patients [who develop aspergilliosis]. It’s not just the corticosteroids. The more we learn, we see the virus plays a role as well, suppressing the interferon pathway,” for example, said Dr. Hoenigl, associate professor in the division of infectious diseases and the European Confederation of Medical Mycology (ECMM) Center of Excellence at the university. The earliest reports of CAPA came “when ICUs weren’t using dexamethasone or tocilizumab,” he noted.
In a paper published recently in Lancet Respiratory Medicine that Dr. Hoenigl and others point to, Belgian researchers reported a “three-level breach” in innate antifungal immunity in both IAPA and CAPA, affecting the integrity of the epithelial barrier, the capacity to phagocytose and kill Aspergillus spores, and the ability to destroy Aspergillus hyphae, which is mainly mediated by neutrophils.
The researchers ran a host of genetic and protein analyses on lung samples (most collected via BAL) of 169 patients with influenza or COVID-19, with and without aspergillosis. They found that patients with CAPA had significantly lower neutrophil cell fractions than patients with COVID-19 only, and patients with IAPA or CAPA had reduced type II IFN signaling and increased concentrations of fibrosis-associated growth factors in the lower respiratory tracts (Lancet Respir Med. 2022 Aug 24).
Tom Chiller, MD, MPH, chief of the Center for Disease Control and Prevention’s Mycotic Disease Branch, said he’s watching such research with interest. For now, he said, it’s important to also consider that “data on COVID show that almost all patients going into the ICUs with pneumonia and COVID are getting broad-spectrum antibiotics” in addition to corticosteroids.
By wiping out good bacteria, the antibiotics could be “creating a perfect niche for fungi to grow,” he said.
Diagnostic challenges
Aspergillus that has invaded the lung tissue in patients with COVID-19 appears to grow there for some time – around 8-10 days, much longer than in IAPA – before becoming angioinvasive, said Dr. Hoenigl. Such a pathophysiology “implicates that we should try to diagnose it while it’s in the lung tissue, using the BAL fluid, and not yet in the blood,” he said.
Some multicenter studies, including one from Europe on Aspergillus test profiles in critically ill COVID-19 patients, have shown mortality rates of close to 90% in patients with CAPA who have positive serum biomarkers, despite appropriate antifungal therapy. “If diagnosed while confined to the lung, however, mortality rates are more like 40%-50% with antifungal therapy,” Dr. Hoenigl said. (Cohort studies published thus far have fairly consistently reported mortality rates in patients with CAPA greater than 40%, he said.)
Bronchoscopy isn’t always pragmatic or possible, however, and is variably used. Some patients with severe COVID-19 may be too unstable for any invasive procedure, said Dr. Permpalung.
Dr. Permpalung looks for CAPA using serum (1-3) beta-D-glucan (BDG, a generic fungal test not specific to Aspergillus), serum galactomannan (GM, specific for Aspergillus), and respiratory cultures (sputum or endotracheal aspirate if intubated) as initial screening tests in the ICU. If there are concerns for CAPA – based on these tests and/or the clinical picture – “a thoughtful risk-benefit discussion is required to determine if patients would benefit from a bronchoscopy or if we should just start them on empiric antifungal therapy.”
Unfortunately, the sensitivity of serum GM is relatively low in CAPA – lower than with classic invasive aspergillosis in the nonviral setting, sources said. BDG, on the other hand, can be falsely positive in the setting of antimicrobials and within the ICU. And the utility of imaging for CAPA is limited. Both the clinical picture and radiological findings of CAPA have resembled those of severe COVID – with the caveat of cavitary lung lesions visible on imaging.
“Cavities or nodules are a highly suspicious finding that could indicate possible fungal infection,” said pulmonologist Amir A. Zeki, MD, MAS, professor of medicine at the University of California, Davis, and codirector of the UC Davis Asthma Network Clinic, who has cared for patients with CAPA.
Cavitation has been described in only a proportion of patients with CAPA, however. So in patients not doing well, “your suspicion has to be raised if you’re not seeing cavities,” he said.
Early in the pandemic, when patients worsened or failed to progress on mechanical ventilation, clinicians at the University of California, Davis, quickly learned not to pin blame too quickly on COVID-19 alone. This remains good advice today, Dr. Zeki said.
“If you have a patient who’s not doing well on a ventilator, not getting better [over weeks], has to be reintubated, has infiltrates or lung nodules that are evolving, or certainly, if they have a cavity, you have to suspect fungal infection,” said Dr. Zeki, who also practices at the Veterans Affairs Medical Center in San Diego. “Think about it for those patients who just aren’t moving forward and are continuing to struggle. Have a high index of suspicion, and consult with your infectious disease colleagues.”
Empiric treatment is warranted in some cases if a patient is doing poorly and suspicion for fungal infection is high based on clinical, radiographic, and/or laboratory evidence, he said.
The CDC’s Dr. Chiller said that screening and diagnostic algorithms currently vary from institution to institution, and that diagnostic challenges likely dissuade clinicians from thinking about fungi. “Clinicians often don’t want to deal with fungi – they’re difficult to diagnose, the treatments are limited and can be toxic. But fungi get pushed back until it’s too late,” he said.
“Fungal diagnostics is an area we all need a lot more help with,” and new diagnostics are in the pipeline, he said. In the meantime, he said, “there are tools out there, and we just need to use them more, and improve how they’re used.”
While reported CAPA thus far has typically occurred in the setting of ICU care and mechanical ventilation, it’s not always the case, Dr. Permpalung said. Lung and other solid organ transplant (SOT) recipients with COVID-19 are developing CAPA and other invasive secondary invasive fungal infections despite not being intubated, he said.
Of 276 SOT recipients with COVID-19 who required inpatient treatment at Johns Hopkins University hospitals from the beginning of the pandemic to March 2022, 23 patients developed invasive fungal infections (13 CAPA). Only a fraction – 38 of the 276 – had been intubated, he said.
Mucormycosis resistance
After CAPA, candidiasis and COVID-19-associated mucormycosis (CAM) – most frequently, rhino-orbital-cerebral disease or pulmonary disease – have been the leading reported fungal coinfections in COVID-19, said Dr. Hoenigl, who described the incidence, timeline, risk factors, and pathogenesis of these infections in a review published this year in Nature Microbiology. .
In India, where there has long been high exposure to Mucorales spores and a greater burden of invasive fungal disease, the rate of mucormycosis doubled in 2021, with rhino-orbital-cerebral disease reported almost exclusively, he said. Pulmonary disease has occurred almost exclusively in the ICU setting and has been present in about 50% of cases outside of India, including Europe and the United States.
A preprint meta-analysis of CAM cases posted by the Lancet in July 2022, in which investigators analyzed individual data of 556 reported cases of COVID-19–associated CAM, shows diabetes and history of corticosteroid use present in most patients, and an overall mortality rate of 44.4%, most of which stems from cases of pulmonary or disseminated disease. Thirteen of the 556 reported cases were from the United States.
An important take-away from the analysis, Dr. Hoenigl said, is that Aspergillus coinfection was seen in 7% of patients and was associated with higher mortality. “It’s important to consider that coinfections [of Aspergillus and Mucorales] can exist,” Dr. Hoenigl said, noting that like CAPA, pulmonary CAM is likely underdiagnosed and underreported.
As with CAPA, the clinical and radiological features of pulmonary CAM largely overlap with those associated with COVID-19, and bronchoscopy plays a central role in definitive diagnosis. In the United States, a Mucorales PCR test for blood and BAL fluid is commercially available and used at some centers, Dr. Hoenigl said.
“Mucormycosis is always difficult to treat ... a lot of the treatments don’t work particularly well,” said Dr. Thompson. “With aspergillosis, we have better treatment options.”
Dr. Thompson worries, however, about treatment resistance becoming widespread. Resistance to azole antifungal agents “is already pretty widespread in northern Europe, particularly in the Netherlands and part of the U.K.” because of injudicious use of antifungals in agriculture, he said. “We’ve started to see a few cases [of azole-resistant aspergillosis in the United States] and know it will be more widespread soon.”
Treatment resistance is a focus of the new WHO fungal priority pathogens list – the first such report from the organization. Of the 19 fungi on the list, 4 were ranked as critical: Cryptococcus neoformans, Candida auris, Aspergillus fumigatus, and Candida albicans. Like Dr. Thompson, Dr. Hoenigl contributed to the WHO report.
Dr. Hoenigl reported grant/research support from Astellas, Merck, F2G, Gilread, Pfizer, and Scynexis. Dr. Marr disclosed employment and equity in Pearl Diagnostics and Sfunga Therapeutics. Dr. Thompson, Dr. Permpalung, and Dr. Zeki reported that they have no relevant financial disclosures.