User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
div[contains(@class, 'main-prefix')]
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
Flu hospitalizations drop amid signs of an early peak
It’s beginning to look less like an epidemic as seasonal flu activity “appears to be declining in some areas,” according to the Centers for Disease Control and Prevention.
Declines in a few states and territories were enough to lower national activity, as measured by outpatient visits for influenza-like illness, for the second consecutive week. This reduced the weekly number of hospital admissions for the first time in the 2022-2023 season, according to the CDC influenza division’s weekly FluView report.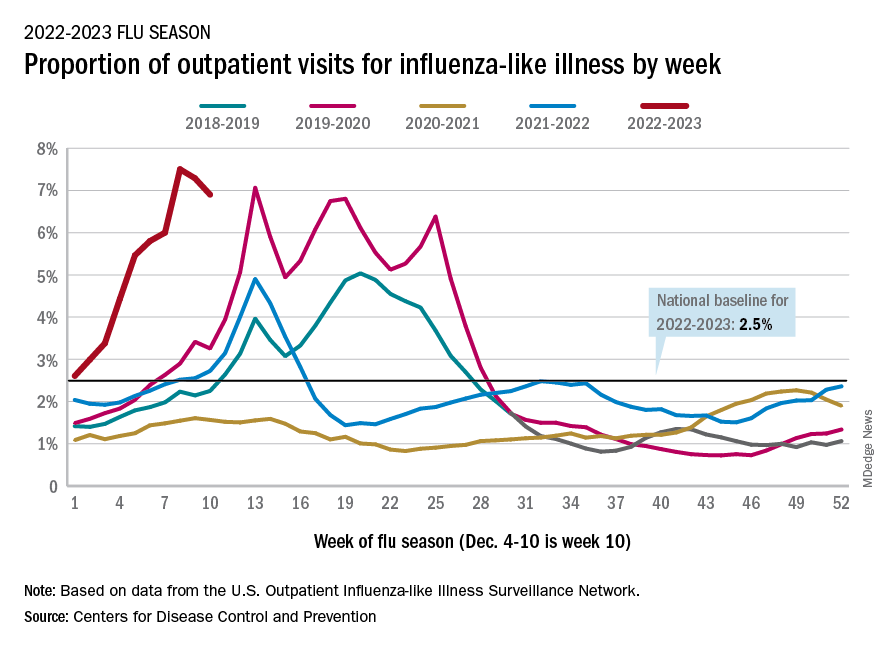
Flu-related hospital admissions slipped to about 23,500 during the week of Dec. 4-10, after topping 26,000 the week before, based on data reported by 5,000 hospitals from all states and territories.
which was still higher than any other December rate from all previous seasons going back to 2009-10, CDC data shows.
Visits for flu-like illness represented 6.9% of all outpatient visits reported to the CDC during the week of Dec. 4-10. The rate reached 7.5% during the last full week of November before dropping to 7.3%, the CDC said.
There were 28 states or territories with “very high” activity for the latest reporting week, compared with 32 the previous week. Eight states – Colorado, Idaho, Kentucky, Nebraska, New Mexico, Oklahoma, Tennessee, and Washington – and New York City were at the very highest level on the CDC’s 1-13 scale of activity, compared with 14 areas the week before, the agency reported.
So far for the 2022-2023 season, the CDC estimated there have been at least 15 million cases of the flu, 150,000 hospitalizations, and 9,300 deaths. Among those deaths have been 30 reported in children, compared with 44 for the entire 2021-22 season and just 1 for 2020-21.
A version of this article first appeared on WebMD.com.
It’s beginning to look less like an epidemic as seasonal flu activity “appears to be declining in some areas,” according to the Centers for Disease Control and Prevention.
Declines in a few states and territories were enough to lower national activity, as measured by outpatient visits for influenza-like illness, for the second consecutive week. This reduced the weekly number of hospital admissions for the first time in the 2022-2023 season, according to the CDC influenza division’s weekly FluView report.
Flu-related hospital admissions slipped to about 23,500 during the week of Dec. 4-10, after topping 26,000 the week before, based on data reported by 5,000 hospitals from all states and territories.
which was still higher than any other December rate from all previous seasons going back to 2009-10, CDC data shows.
Visits for flu-like illness represented 6.9% of all outpatient visits reported to the CDC during the week of Dec. 4-10. The rate reached 7.5% during the last full week of November before dropping to 7.3%, the CDC said.
There were 28 states or territories with “very high” activity for the latest reporting week, compared with 32 the previous week. Eight states – Colorado, Idaho, Kentucky, Nebraska, New Mexico, Oklahoma, Tennessee, and Washington – and New York City were at the very highest level on the CDC’s 1-13 scale of activity, compared with 14 areas the week before, the agency reported.
So far for the 2022-2023 season, the CDC estimated there have been at least 15 million cases of the flu, 150,000 hospitalizations, and 9,300 deaths. Among those deaths have been 30 reported in children, compared with 44 for the entire 2021-22 season and just 1 for 2020-21.
A version of this article first appeared on WebMD.com.
It’s beginning to look less like an epidemic as seasonal flu activity “appears to be declining in some areas,” according to the Centers for Disease Control and Prevention.
Declines in a few states and territories were enough to lower national activity, as measured by outpatient visits for influenza-like illness, for the second consecutive week. This reduced the weekly number of hospital admissions for the first time in the 2022-2023 season, according to the CDC influenza division’s weekly FluView report.
Flu-related hospital admissions slipped to about 23,500 during the week of Dec. 4-10, after topping 26,000 the week before, based on data reported by 5,000 hospitals from all states and territories.
which was still higher than any other December rate from all previous seasons going back to 2009-10, CDC data shows.
Visits for flu-like illness represented 6.9% of all outpatient visits reported to the CDC during the week of Dec. 4-10. The rate reached 7.5% during the last full week of November before dropping to 7.3%, the CDC said.
There were 28 states or territories with “very high” activity for the latest reporting week, compared with 32 the previous week. Eight states – Colorado, Idaho, Kentucky, Nebraska, New Mexico, Oklahoma, Tennessee, and Washington – and New York City were at the very highest level on the CDC’s 1-13 scale of activity, compared with 14 areas the week before, the agency reported.
So far for the 2022-2023 season, the CDC estimated there have been at least 15 million cases of the flu, 150,000 hospitalizations, and 9,300 deaths. Among those deaths have been 30 reported in children, compared with 44 for the entire 2021-22 season and just 1 for 2020-21.
A version of this article first appeared on WebMD.com.
Docs treating other doctors: What can go wrong?
It’s not unusual for physicians to see other doctors as patients – often they’re colleagues or even friends.
“When doctors don’t get the proper care, that’s when things go south. Any time physicians lower their standard of care, there is a risk of missing something that could affect their differential diagnosis, ultimate working diagnosis, and treatment plan,” said Michael Myers, MD, professor of clinical psychiatry at State University of New York, Brooklyn, who saw only medical students, physicians, and their family members in his private practice for over 3 decades.
Of the more than 200 physicians who responded to a recent Medscape poll, more than half said they treated physician-patients differently from other patients.
They granted their peers special privileges: They spent more time with them than other patients, gave out their personal contact information, and granted them professional courtesy by waiving or discounting their fees.
Published studies have reported that special treatment of physician-patients, such as giving personal contact information or avoiding uncomfortable testing, can create challenges for the treating physicians who may feel pressure to deviate from the standard of care.
The American Medical Association has recognized the challenges that physicians have when they treat other physicians they know personally or professionally, including a potential loss of objectivity, privacy, or confidentiality.
The AMA recommends that physicians treat physician-patients the same way they would other patients. The guidance states that the treating physician should exercise objective professional judgment and make unbiased treatment recommendations; be sensitive to the potential psychological discomfort of the physician-patient, and respect the physical and informational privacy of physician-patients.
Dr. Myers recalled that one doctor-patient said his primary care physician was his business partner in the practice. They ordered tests for each other and occasionally examined each other, but the patient never felt comfortable asking his partner for a full physical, said Dr. Myers, the author of “Becoming a Doctors’ Doctor: A Memoir.”
“I recommended that he choose a primary care doctor whom he didn’t know so that he could truly be a patient and the doctor could truly be a treating doctor,” said Dr. Myers.
Physician-patients may also be concerned about running into their physicians and being judged, or that they will break confidentiality and tell their spouse or another colleague, said Dr. Myers.
“When your doctor is a complete and total stranger, and especially if you live in a sizable community and your paths never cross, you don’t have that added worry,” he said.
Do docs expect special treatment as patients?
Some doctors expect special treatment from other doctors when they’re patients – 14% of physician poll respondents said that was their experience.
Dr. Myers recommends setting boundaries with doctor-patients early on in the relationship. “Some doctors expected me to go over my regular appointment time and when they realized that I started and stopped on time, they got upset. Once, one doctor insisted to my answering service that he had to talk to me although I was at home. When he started talking, I interrupted him and asked if the matter was urgent. He said no, so I offered to fit him in before his next appointment if he felt it couldn’t wait,” said Dr. Myers.
Some doctors also give physician-patients “professional courtesy” when it comes to payment. One in four poll respondents said they waived or discounted their professional fees for a doctor-patient. As most doctors have health insurance, doctors may waive copayments or other out-of-pocket fees, according to the American Academy of Pediatrics.
However, waiving or discounting health insurance fees, especially for government funded insurance, may be illegal under federal anti-fraud and abuse laws and payer contracts as well as state laws, the AAP says. It’s best to check with an attorney.
Treating other physicians can be rewarding
“Physicians can be the most rewarding patients because they are allies and partners in the effort to overcome whatever is ailing them,” said one doctor who responded to the Medscape poll.
Over two-thirds of respondents said that doctor-patients participated much more in their care than did other patients – typically, they discussed their care in more depth than did other patients.
Most doctors also felt that it was easier to communicate with their physician-patients than other patients because they understood medicine and were knowledgeable about their conditions.
Being judged by your peers can be stressful
How physicians feel about treating physician-patients is complicated. Nearly half of respondents said that it was more stressful than treating other patients.
One respondent said, “If we are honest, treating other physicians as patients is more stressful because we know that our skills are being assessed by someone who is at our level. There is no training for treating physicians, as there is for the Pope’s confessor. And we can be challenging in more ways than one!”
About one-third of poll respondents said they were afraid of disappointing their physician-patients.
“I’m not surprised,” said Dr. Myers, when told of that poll response. “This is why some doctors are reluctant to treat other physicians; they may wonder whether they’re up to speed. I have always thrived on having a high bar set for me – it spurs me on to really stay current with the literature and be humble,” he said.
A version of this article first appeared on Medscape.com.
It’s not unusual for physicians to see other doctors as patients – often they’re colleagues or even friends.
“When doctors don’t get the proper care, that’s when things go south. Any time physicians lower their standard of care, there is a risk of missing something that could affect their differential diagnosis, ultimate working diagnosis, and treatment plan,” said Michael Myers, MD, professor of clinical psychiatry at State University of New York, Brooklyn, who saw only medical students, physicians, and their family members in his private practice for over 3 decades.
Of the more than 200 physicians who responded to a recent Medscape poll, more than half said they treated physician-patients differently from other patients.
They granted their peers special privileges: They spent more time with them than other patients, gave out their personal contact information, and granted them professional courtesy by waiving or discounting their fees.
Published studies have reported that special treatment of physician-patients, such as giving personal contact information or avoiding uncomfortable testing, can create challenges for the treating physicians who may feel pressure to deviate from the standard of care.
The American Medical Association has recognized the challenges that physicians have when they treat other physicians they know personally or professionally, including a potential loss of objectivity, privacy, or confidentiality.
The AMA recommends that physicians treat physician-patients the same way they would other patients. The guidance states that the treating physician should exercise objective professional judgment and make unbiased treatment recommendations; be sensitive to the potential psychological discomfort of the physician-patient, and respect the physical and informational privacy of physician-patients.
Dr. Myers recalled that one doctor-patient said his primary care physician was his business partner in the practice. They ordered tests for each other and occasionally examined each other, but the patient never felt comfortable asking his partner for a full physical, said Dr. Myers, the author of “Becoming a Doctors’ Doctor: A Memoir.”
“I recommended that he choose a primary care doctor whom he didn’t know so that he could truly be a patient and the doctor could truly be a treating doctor,” said Dr. Myers.
Physician-patients may also be concerned about running into their physicians and being judged, or that they will break confidentiality and tell their spouse or another colleague, said Dr. Myers.
“When your doctor is a complete and total stranger, and especially if you live in a sizable community and your paths never cross, you don’t have that added worry,” he said.
Do docs expect special treatment as patients?
Some doctors expect special treatment from other doctors when they’re patients – 14% of physician poll respondents said that was their experience.
Dr. Myers recommends setting boundaries with doctor-patients early on in the relationship. “Some doctors expected me to go over my regular appointment time and when they realized that I started and stopped on time, they got upset. Once, one doctor insisted to my answering service that he had to talk to me although I was at home. When he started talking, I interrupted him and asked if the matter was urgent. He said no, so I offered to fit him in before his next appointment if he felt it couldn’t wait,” said Dr. Myers.
Some doctors also give physician-patients “professional courtesy” when it comes to payment. One in four poll respondents said they waived or discounted their professional fees for a doctor-patient. As most doctors have health insurance, doctors may waive copayments or other out-of-pocket fees, according to the American Academy of Pediatrics.
However, waiving or discounting health insurance fees, especially for government funded insurance, may be illegal under federal anti-fraud and abuse laws and payer contracts as well as state laws, the AAP says. It’s best to check with an attorney.
Treating other physicians can be rewarding
“Physicians can be the most rewarding patients because they are allies and partners in the effort to overcome whatever is ailing them,” said one doctor who responded to the Medscape poll.
Over two-thirds of respondents said that doctor-patients participated much more in their care than did other patients – typically, they discussed their care in more depth than did other patients.
Most doctors also felt that it was easier to communicate with their physician-patients than other patients because they understood medicine and were knowledgeable about their conditions.
Being judged by your peers can be stressful
How physicians feel about treating physician-patients is complicated. Nearly half of respondents said that it was more stressful than treating other patients.
One respondent said, “If we are honest, treating other physicians as patients is more stressful because we know that our skills are being assessed by someone who is at our level. There is no training for treating physicians, as there is for the Pope’s confessor. And we can be challenging in more ways than one!”
About one-third of poll respondents said they were afraid of disappointing their physician-patients.
“I’m not surprised,” said Dr. Myers, when told of that poll response. “This is why some doctors are reluctant to treat other physicians; they may wonder whether they’re up to speed. I have always thrived on having a high bar set for me – it spurs me on to really stay current with the literature and be humble,” he said.
A version of this article first appeared on Medscape.com.
It’s not unusual for physicians to see other doctors as patients – often they’re colleagues or even friends.
“When doctors don’t get the proper care, that’s when things go south. Any time physicians lower their standard of care, there is a risk of missing something that could affect their differential diagnosis, ultimate working diagnosis, and treatment plan,” said Michael Myers, MD, professor of clinical psychiatry at State University of New York, Brooklyn, who saw only medical students, physicians, and their family members in his private practice for over 3 decades.
Of the more than 200 physicians who responded to a recent Medscape poll, more than half said they treated physician-patients differently from other patients.
They granted their peers special privileges: They spent more time with them than other patients, gave out their personal contact information, and granted them professional courtesy by waiving or discounting their fees.
Published studies have reported that special treatment of physician-patients, such as giving personal contact information or avoiding uncomfortable testing, can create challenges for the treating physicians who may feel pressure to deviate from the standard of care.
The American Medical Association has recognized the challenges that physicians have when they treat other physicians they know personally or professionally, including a potential loss of objectivity, privacy, or confidentiality.
The AMA recommends that physicians treat physician-patients the same way they would other patients. The guidance states that the treating physician should exercise objective professional judgment and make unbiased treatment recommendations; be sensitive to the potential psychological discomfort of the physician-patient, and respect the physical and informational privacy of physician-patients.
Dr. Myers recalled that one doctor-patient said his primary care physician was his business partner in the practice. They ordered tests for each other and occasionally examined each other, but the patient never felt comfortable asking his partner for a full physical, said Dr. Myers, the author of “Becoming a Doctors’ Doctor: A Memoir.”
“I recommended that he choose a primary care doctor whom he didn’t know so that he could truly be a patient and the doctor could truly be a treating doctor,” said Dr. Myers.
Physician-patients may also be concerned about running into their physicians and being judged, or that they will break confidentiality and tell their spouse or another colleague, said Dr. Myers.
“When your doctor is a complete and total stranger, and especially if you live in a sizable community and your paths never cross, you don’t have that added worry,” he said.
Do docs expect special treatment as patients?
Some doctors expect special treatment from other doctors when they’re patients – 14% of physician poll respondents said that was their experience.
Dr. Myers recommends setting boundaries with doctor-patients early on in the relationship. “Some doctors expected me to go over my regular appointment time and when they realized that I started and stopped on time, they got upset. Once, one doctor insisted to my answering service that he had to talk to me although I was at home. When he started talking, I interrupted him and asked if the matter was urgent. He said no, so I offered to fit him in before his next appointment if he felt it couldn’t wait,” said Dr. Myers.
Some doctors also give physician-patients “professional courtesy” when it comes to payment. One in four poll respondents said they waived or discounted their professional fees for a doctor-patient. As most doctors have health insurance, doctors may waive copayments or other out-of-pocket fees, according to the American Academy of Pediatrics.
However, waiving or discounting health insurance fees, especially for government funded insurance, may be illegal under federal anti-fraud and abuse laws and payer contracts as well as state laws, the AAP says. It’s best to check with an attorney.
Treating other physicians can be rewarding
“Physicians can be the most rewarding patients because they are allies and partners in the effort to overcome whatever is ailing them,” said one doctor who responded to the Medscape poll.
Over two-thirds of respondents said that doctor-patients participated much more in their care than did other patients – typically, they discussed their care in more depth than did other patients.
Most doctors also felt that it was easier to communicate with their physician-patients than other patients because they understood medicine and were knowledgeable about their conditions.
Being judged by your peers can be stressful
How physicians feel about treating physician-patients is complicated. Nearly half of respondents said that it was more stressful than treating other patients.
One respondent said, “If we are honest, treating other physicians as patients is more stressful because we know that our skills are being assessed by someone who is at our level. There is no training for treating physicians, as there is for the Pope’s confessor. And we can be challenging in more ways than one!”
About one-third of poll respondents said they were afraid of disappointing their physician-patients.
“I’m not surprised,” said Dr. Myers, when told of that poll response. “This is why some doctors are reluctant to treat other physicians; they may wonder whether they’re up to speed. I have always thrived on having a high bar set for me – it spurs me on to really stay current with the literature and be humble,” he said.
A version of this article first appeared on Medscape.com.
New test that detects 14 cancers focuses on sugars, not DNA
The leader in this field is the Galleri test (from GRAIL) which is already in clinical use in some health care networks across the United States. That test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA (cfDNA) in a blood sample.
The new test, under development by Swedish biotechnology company Elypta AB, has a different premise. It can detect 14 cancer types based on the analysis of glycosaminoglycans, which are a diverse group of polysaccharides that are altered by the presence of tumors. Using plasma and urine samples, the method had a 41.6%-62.3% sensitivity for detecting stage I cancer at 95% specificity.
In comparison, say the authors, other assays have reported 39%-73% sensitivity to stage I cancers, but these estimates are usually limited to 12 cancer types that are considered “high-signal,” and the assays perform poorly in cancers that emit little cfDNA, such as genitourinary and brain malignancies.
“The main advantage of glycosaminoglycans appears to be that they change in the blood and urine at the earliest stages of cancer,” said study author Francesco Gatto, PhD, founder and chief scientific officer at Elypta. “Consequently, this method showed an impressive detection rate in stage I compared to other emerging methods.”
The study was published online in Proceedings of the National Academy of Sciences.
Combine tests?
Dr. Gatto commented that he “could envision that one day we may be able to combine these methods.”
“The same blood specimen could be used to test both glycosaminoglycans and genomic biomarkers,” said Dr. Gatto. “This strategy could hopefully detect even more cancers than with either method alone, and the resulting performance may well be sufficient as a one-stop-shop screening program.”
So how does the new test from Elypta compare with the Galleri test?
“Galleri and similar methods mostly focused on information coming from molecules of DNA naturally floating in the blood,” explained Dr. Gatto. “It makes sense to conduct research there because cancers typically start with events in the DNA.”
He noted that the current study explored a new layer of information, molecules called glycosaminoglycans, that participate in the metabolism of cancer.
“This method detected many cancers that the previous methods missed, and a substantial proportion of these were at stage I,” said Dr. Gatto. “Cancer is a complex disease, so the most layers of information we can probe noninvasively, say with a blood test, the more likely we can catch more cancers at its earliest stage.”
Other platforms typically rely on sequencing and detecting cancer-derived fractions of cfDNA, but these methods have challenges that can interfere with their usage. For example, some cancer types do not shed sufficient cfDNA and it cannot be accurately measured.
“An advantage on focusing on glycosaminoglycans is that the method does not require next-generation sequencing or similarly complex assays because glycosaminoglycans are informative with less than 10 simultaneous measurements as opposed to Galleri that looks at over 1 million DNA methylation sites,” he said.
“This makes the assay behind the test much cheaper and robust – we estimated a 5-10 times lower cost difference,” Dr. Gatto said.
Prospective and comparative data needed
In a comment, Eric Klein, MD, emeritus chair of the Glickman Urological and Kidney Institute at the Cleveland Clinic explained that the “only accurate way to know how a test will perform in an intended-use population is to actually test it in that population. It’s not possible to extrapolate results directly from a case-control study.”
Cancers shed many different biologic markers into body fluids, but which of these signals will be best to serve as the basis of an MCED (multi-cancer detection test) that has clinical utility in a screening population has yet to be determined, he noted. “And it’s possible that no single test will be optimum for every clinical situation.”
“The results of this study appear promising, but it is not possible to claim superiority of one test over another based on individual case-control studies because of uncontrolled differences in the selected populations,” Dr. Klein continued. “The only scientifically accurate way to do this is to perform different tests on the same patient samples in a head-to-head comparison.”
There is only one study that he is aware of that has done this recently, in which multiple different assays looking at various signals in cell-free DNA were directly compared on the same samples (Cancer cell. 2022;40:1537-49.e12). “A targeted methylation assay that is the basis for Galleri was best for the lowest limit of detection and for predicting cancer site of origin,” said Dr. Klein.
Another expert agreed that a direct head-to-head study is needed to compare assays. “Based on this data, you cannot say that this method is better than the other one because that requires a comparative study,” said Fred Hirsch, MD, PhD, executive director of the Center for Thoracic Oncology, Tisch Cancer Institute at Mount Sinai, New York.
Metabolomics is interesting, and the data are encouraging, he continued. “But this is a multicancer early detection test and metabolism changes may vary from cancer type to cancer type. I’m not sure that the metabolism of lung cancer is the same as that of a gynecologic cancer.”
Dr. Hirsch also pointed out that there could also be confounding factors. “They have excluded inflammatory disease, but there can be other variables such as smoking,” he said. “Overall it gives some interesting perspectives but I would like to see more prospective validation and studies in specific disease groups, and eventually comparative studies with other methodologies.”
Study details
The authors evaluated if plasma and urine free GAGomes (free glycosaminoglycan profiles) deviated from baseline physiological levels in 14 cancer types and could serve as metabolic cancer biomarkers. They also then validated using free GAGomes for MCED in an external population with 2,064 samples obtained from 1,260 patients with cancer and healthy individuals.
In an in vivo cancer progression model, they observed widespread cancer-specific changes in biofluidic free GAGomes and then developed three machine-learning models based on urine (nurine = 220 cancer vs. 360 healthy) and plasma (nplasma = 517 cancer vs. 425 healthy) free GAGomes that were able to detect any cancer with an area under the receiver operating characteristic curve of 0.83-0.93 (with up to 62% sensitivity to stage I disease at 95% specificity).
To assess if altered GAGome features associated with cancer suggested more aggressive tumor biology, they correlated each score with overall survival. The median follow-up time was 17 months in the plasma cohort (n = 370 across 13 cancer types), 15 months in the urine cohort (n = 162 across 4 cancer types), and 15 months in the combined cohort (n = 152 across 4 cancer types).
They found that all three scores independently predicted overall survival in a multivariable analysis (hazard ratio, 1.29; P = .0009 for plasma; HR, 1.79; P = .0009 for urine; HR, 1.91; P = .0004 for combined) after adjusting for cancer type, age, sex, and stage IV or high-grade disease.
These findings showed an association of free GAGome alterations with aggressive cancer phenotypes and suggested that scores below the 95% specificity cutoff might have a better prognosis, the authors comment.
In addition, other analyses showed that free GAGomes predicted the putative cancer location with 89% accuracy. And finally, to confirm whether the free GAGome MCED scores could be used for screening, a validation analysis was conducted using a typical “screening population,” which requires at least 99% specificity. The combined free GAGomes were able to predict a poor prognosis of any cancer type within 18 months and with 43% sensitivity (21% in stage I; n = 121 and 49 cases).
Dr. Gatto believes that these results, as well as those from other studies looking at glycosaminoglycans as cancer biomarkers, will lead to the next steps of development. “But I speculate that this test could be most useful to assess in a cheap, practical, and noninvasive manner if a person at increased risk of cancer should be selected for cancer screening as part of established or emerging screening programs.”
The study was sponsored by Elypta. Dr. Gatto is listed as an inventor in patent applications related to the biomarkers described in this study and later assigned to Elypta, and is a shareholder and employed at Elypta. Dr. Hirsch reports no relevant financial relationships. Dr. Klein is a consultant for GRAIL and an investigator for CCGA and Pathfinder.
A version of this article first appeared on Medscape.com.
The leader in this field is the Galleri test (from GRAIL) which is already in clinical use in some health care networks across the United States. That test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA (cfDNA) in a blood sample.
The new test, under development by Swedish biotechnology company Elypta AB, has a different premise. It can detect 14 cancer types based on the analysis of glycosaminoglycans, which are a diverse group of polysaccharides that are altered by the presence of tumors. Using plasma and urine samples, the method had a 41.6%-62.3% sensitivity for detecting stage I cancer at 95% specificity.
In comparison, say the authors, other assays have reported 39%-73% sensitivity to stage I cancers, but these estimates are usually limited to 12 cancer types that are considered “high-signal,” and the assays perform poorly in cancers that emit little cfDNA, such as genitourinary and brain malignancies.
“The main advantage of glycosaminoglycans appears to be that they change in the blood and urine at the earliest stages of cancer,” said study author Francesco Gatto, PhD, founder and chief scientific officer at Elypta. “Consequently, this method showed an impressive detection rate in stage I compared to other emerging methods.”
The study was published online in Proceedings of the National Academy of Sciences.
Combine tests?
Dr. Gatto commented that he “could envision that one day we may be able to combine these methods.”
“The same blood specimen could be used to test both glycosaminoglycans and genomic biomarkers,” said Dr. Gatto. “This strategy could hopefully detect even more cancers than with either method alone, and the resulting performance may well be sufficient as a one-stop-shop screening program.”
So how does the new test from Elypta compare with the Galleri test?
“Galleri and similar methods mostly focused on information coming from molecules of DNA naturally floating in the blood,” explained Dr. Gatto. “It makes sense to conduct research there because cancers typically start with events in the DNA.”
He noted that the current study explored a new layer of information, molecules called glycosaminoglycans, that participate in the metabolism of cancer.
“This method detected many cancers that the previous methods missed, and a substantial proportion of these were at stage I,” said Dr. Gatto. “Cancer is a complex disease, so the most layers of information we can probe noninvasively, say with a blood test, the more likely we can catch more cancers at its earliest stage.”
Other platforms typically rely on sequencing and detecting cancer-derived fractions of cfDNA, but these methods have challenges that can interfere with their usage. For example, some cancer types do not shed sufficient cfDNA and it cannot be accurately measured.
“An advantage on focusing on glycosaminoglycans is that the method does not require next-generation sequencing or similarly complex assays because glycosaminoglycans are informative with less than 10 simultaneous measurements as opposed to Galleri that looks at over 1 million DNA methylation sites,” he said.
“This makes the assay behind the test much cheaper and robust – we estimated a 5-10 times lower cost difference,” Dr. Gatto said.
Prospective and comparative data needed
In a comment, Eric Klein, MD, emeritus chair of the Glickman Urological and Kidney Institute at the Cleveland Clinic explained that the “only accurate way to know how a test will perform in an intended-use population is to actually test it in that population. It’s not possible to extrapolate results directly from a case-control study.”
Cancers shed many different biologic markers into body fluids, but which of these signals will be best to serve as the basis of an MCED (multi-cancer detection test) that has clinical utility in a screening population has yet to be determined, he noted. “And it’s possible that no single test will be optimum for every clinical situation.”
“The results of this study appear promising, but it is not possible to claim superiority of one test over another based on individual case-control studies because of uncontrolled differences in the selected populations,” Dr. Klein continued. “The only scientifically accurate way to do this is to perform different tests on the same patient samples in a head-to-head comparison.”
There is only one study that he is aware of that has done this recently, in which multiple different assays looking at various signals in cell-free DNA were directly compared on the same samples (Cancer cell. 2022;40:1537-49.e12). “A targeted methylation assay that is the basis for Galleri was best for the lowest limit of detection and for predicting cancer site of origin,” said Dr. Klein.
Another expert agreed that a direct head-to-head study is needed to compare assays. “Based on this data, you cannot say that this method is better than the other one because that requires a comparative study,” said Fred Hirsch, MD, PhD, executive director of the Center for Thoracic Oncology, Tisch Cancer Institute at Mount Sinai, New York.
Metabolomics is interesting, and the data are encouraging, he continued. “But this is a multicancer early detection test and metabolism changes may vary from cancer type to cancer type. I’m not sure that the metabolism of lung cancer is the same as that of a gynecologic cancer.”
Dr. Hirsch also pointed out that there could also be confounding factors. “They have excluded inflammatory disease, but there can be other variables such as smoking,” he said. “Overall it gives some interesting perspectives but I would like to see more prospective validation and studies in specific disease groups, and eventually comparative studies with other methodologies.”
Study details
The authors evaluated if plasma and urine free GAGomes (free glycosaminoglycan profiles) deviated from baseline physiological levels in 14 cancer types and could serve as metabolic cancer biomarkers. They also then validated using free GAGomes for MCED in an external population with 2,064 samples obtained from 1,260 patients with cancer and healthy individuals.
In an in vivo cancer progression model, they observed widespread cancer-specific changes in biofluidic free GAGomes and then developed three machine-learning models based on urine (nurine = 220 cancer vs. 360 healthy) and plasma (nplasma = 517 cancer vs. 425 healthy) free GAGomes that were able to detect any cancer with an area under the receiver operating characteristic curve of 0.83-0.93 (with up to 62% sensitivity to stage I disease at 95% specificity).
To assess if altered GAGome features associated with cancer suggested more aggressive tumor biology, they correlated each score with overall survival. The median follow-up time was 17 months in the plasma cohort (n = 370 across 13 cancer types), 15 months in the urine cohort (n = 162 across 4 cancer types), and 15 months in the combined cohort (n = 152 across 4 cancer types).
They found that all three scores independently predicted overall survival in a multivariable analysis (hazard ratio, 1.29; P = .0009 for plasma; HR, 1.79; P = .0009 for urine; HR, 1.91; P = .0004 for combined) after adjusting for cancer type, age, sex, and stage IV or high-grade disease.
These findings showed an association of free GAGome alterations with aggressive cancer phenotypes and suggested that scores below the 95% specificity cutoff might have a better prognosis, the authors comment.
In addition, other analyses showed that free GAGomes predicted the putative cancer location with 89% accuracy. And finally, to confirm whether the free GAGome MCED scores could be used for screening, a validation analysis was conducted using a typical “screening population,” which requires at least 99% specificity. The combined free GAGomes were able to predict a poor prognosis of any cancer type within 18 months and with 43% sensitivity (21% in stage I; n = 121 and 49 cases).
Dr. Gatto believes that these results, as well as those from other studies looking at glycosaminoglycans as cancer biomarkers, will lead to the next steps of development. “But I speculate that this test could be most useful to assess in a cheap, practical, and noninvasive manner if a person at increased risk of cancer should be selected for cancer screening as part of established or emerging screening programs.”
The study was sponsored by Elypta. Dr. Gatto is listed as an inventor in patent applications related to the biomarkers described in this study and later assigned to Elypta, and is a shareholder and employed at Elypta. Dr. Hirsch reports no relevant financial relationships. Dr. Klein is a consultant for GRAIL and an investigator for CCGA and Pathfinder.
A version of this article first appeared on Medscape.com.
The leader in this field is the Galleri test (from GRAIL) which is already in clinical use in some health care networks across the United States. That test uses next-generation sequencing to analyze the arrangement of methyl groups on circulating tumor (or cell-free) DNA (cfDNA) in a blood sample.
The new test, under development by Swedish biotechnology company Elypta AB, has a different premise. It can detect 14 cancer types based on the analysis of glycosaminoglycans, which are a diverse group of polysaccharides that are altered by the presence of tumors. Using plasma and urine samples, the method had a 41.6%-62.3% sensitivity for detecting stage I cancer at 95% specificity.
In comparison, say the authors, other assays have reported 39%-73% sensitivity to stage I cancers, but these estimates are usually limited to 12 cancer types that are considered “high-signal,” and the assays perform poorly in cancers that emit little cfDNA, such as genitourinary and brain malignancies.
“The main advantage of glycosaminoglycans appears to be that they change in the blood and urine at the earliest stages of cancer,” said study author Francesco Gatto, PhD, founder and chief scientific officer at Elypta. “Consequently, this method showed an impressive detection rate in stage I compared to other emerging methods.”
The study was published online in Proceedings of the National Academy of Sciences.
Combine tests?
Dr. Gatto commented that he “could envision that one day we may be able to combine these methods.”
“The same blood specimen could be used to test both glycosaminoglycans and genomic biomarkers,” said Dr. Gatto. “This strategy could hopefully detect even more cancers than with either method alone, and the resulting performance may well be sufficient as a one-stop-shop screening program.”
So how does the new test from Elypta compare with the Galleri test?
“Galleri and similar methods mostly focused on information coming from molecules of DNA naturally floating in the blood,” explained Dr. Gatto. “It makes sense to conduct research there because cancers typically start with events in the DNA.”
He noted that the current study explored a new layer of information, molecules called glycosaminoglycans, that participate in the metabolism of cancer.
“This method detected many cancers that the previous methods missed, and a substantial proportion of these were at stage I,” said Dr. Gatto. “Cancer is a complex disease, so the most layers of information we can probe noninvasively, say with a blood test, the more likely we can catch more cancers at its earliest stage.”
Other platforms typically rely on sequencing and detecting cancer-derived fractions of cfDNA, but these methods have challenges that can interfere with their usage. For example, some cancer types do not shed sufficient cfDNA and it cannot be accurately measured.
“An advantage on focusing on glycosaminoglycans is that the method does not require next-generation sequencing or similarly complex assays because glycosaminoglycans are informative with less than 10 simultaneous measurements as opposed to Galleri that looks at over 1 million DNA methylation sites,” he said.
“This makes the assay behind the test much cheaper and robust – we estimated a 5-10 times lower cost difference,” Dr. Gatto said.
Prospective and comparative data needed
In a comment, Eric Klein, MD, emeritus chair of the Glickman Urological and Kidney Institute at the Cleveland Clinic explained that the “only accurate way to know how a test will perform in an intended-use population is to actually test it in that population. It’s not possible to extrapolate results directly from a case-control study.”
Cancers shed many different biologic markers into body fluids, but which of these signals will be best to serve as the basis of an MCED (multi-cancer detection test) that has clinical utility in a screening population has yet to be determined, he noted. “And it’s possible that no single test will be optimum for every clinical situation.”
“The results of this study appear promising, but it is not possible to claim superiority of one test over another based on individual case-control studies because of uncontrolled differences in the selected populations,” Dr. Klein continued. “The only scientifically accurate way to do this is to perform different tests on the same patient samples in a head-to-head comparison.”
There is only one study that he is aware of that has done this recently, in which multiple different assays looking at various signals in cell-free DNA were directly compared on the same samples (Cancer cell. 2022;40:1537-49.e12). “A targeted methylation assay that is the basis for Galleri was best for the lowest limit of detection and for predicting cancer site of origin,” said Dr. Klein.
Another expert agreed that a direct head-to-head study is needed to compare assays. “Based on this data, you cannot say that this method is better than the other one because that requires a comparative study,” said Fred Hirsch, MD, PhD, executive director of the Center for Thoracic Oncology, Tisch Cancer Institute at Mount Sinai, New York.
Metabolomics is interesting, and the data are encouraging, he continued. “But this is a multicancer early detection test and metabolism changes may vary from cancer type to cancer type. I’m not sure that the metabolism of lung cancer is the same as that of a gynecologic cancer.”
Dr. Hirsch also pointed out that there could also be confounding factors. “They have excluded inflammatory disease, but there can be other variables such as smoking,” he said. “Overall it gives some interesting perspectives but I would like to see more prospective validation and studies in specific disease groups, and eventually comparative studies with other methodologies.”
Study details
The authors evaluated if plasma and urine free GAGomes (free glycosaminoglycan profiles) deviated from baseline physiological levels in 14 cancer types and could serve as metabolic cancer biomarkers. They also then validated using free GAGomes for MCED in an external population with 2,064 samples obtained from 1,260 patients with cancer and healthy individuals.
In an in vivo cancer progression model, they observed widespread cancer-specific changes in biofluidic free GAGomes and then developed three machine-learning models based on urine (nurine = 220 cancer vs. 360 healthy) and plasma (nplasma = 517 cancer vs. 425 healthy) free GAGomes that were able to detect any cancer with an area under the receiver operating characteristic curve of 0.83-0.93 (with up to 62% sensitivity to stage I disease at 95% specificity).
To assess if altered GAGome features associated with cancer suggested more aggressive tumor biology, they correlated each score with overall survival. The median follow-up time was 17 months in the plasma cohort (n = 370 across 13 cancer types), 15 months in the urine cohort (n = 162 across 4 cancer types), and 15 months in the combined cohort (n = 152 across 4 cancer types).
They found that all three scores independently predicted overall survival in a multivariable analysis (hazard ratio, 1.29; P = .0009 for plasma; HR, 1.79; P = .0009 for urine; HR, 1.91; P = .0004 for combined) after adjusting for cancer type, age, sex, and stage IV or high-grade disease.
These findings showed an association of free GAGome alterations with aggressive cancer phenotypes and suggested that scores below the 95% specificity cutoff might have a better prognosis, the authors comment.
In addition, other analyses showed that free GAGomes predicted the putative cancer location with 89% accuracy. And finally, to confirm whether the free GAGome MCED scores could be used for screening, a validation analysis was conducted using a typical “screening population,” which requires at least 99% specificity. The combined free GAGomes were able to predict a poor prognosis of any cancer type within 18 months and with 43% sensitivity (21% in stage I; n = 121 and 49 cases).
Dr. Gatto believes that these results, as well as those from other studies looking at glycosaminoglycans as cancer biomarkers, will lead to the next steps of development. “But I speculate that this test could be most useful to assess in a cheap, practical, and noninvasive manner if a person at increased risk of cancer should be selected for cancer screening as part of established or emerging screening programs.”
The study was sponsored by Elypta. Dr. Gatto is listed as an inventor in patent applications related to the biomarkers described in this study and later assigned to Elypta, and is a shareholder and employed at Elypta. Dr. Hirsch reports no relevant financial relationships. Dr. Klein is a consultant for GRAIL and an investigator for CCGA and Pathfinder.
A version of this article first appeared on Medscape.com.
FROM PROCEEDINGS OF THE NATIONAL ACADEMY OF SCIENCES
Have you heard the one about the cow in the doctor’s office?
Maybe the cow was late for its appointment
It’s been a long day running the front desk at your doctor’s office. People calling in prescriptions, a million appointments, you’ve been running yourself ragged keeping things together. Finally, it’s almost closing time. The last patient of the day has just checked out and you turn back to the waiting room, expecting to see it blessedly empty.
Instead, a 650-pound cow is staring at you.
“I’m sorry, sir or madam, we’re about to close.”
Moo.
“I understand it’s important, but seriously, the doctor’s about to …”
Moo.
“Fine, I’ll see what we can do for you. What’s your insurance?”
Moo Cross Moo Shield.
“Sorry, we don’t take that. You’ll have to go someplace else.”
This is probably not how things went down recently at Orange (Va.) Family Physicians, when they had a cow break into the office. Cows don’t have health insurance.
The intrepid bovine was being transferred to a new home when it jumped off the trailer and wandered an eighth of a mile to Orange Family Physicians, where the cow wranglers found it hanging around outside. Unfortunately, this was a smart cow, and it bolted as it saw the wranglers, crashing through the glass doors into the doctor’s office. Though neither man had ever wrangled a cow from inside a building, they ultimately secured a rope around the cow’s neck and escorted it back outside, tying it to a nearby pole to keep it from further adventures.
One of the wranglers summed up the situation quite nicely on his Facebook page: “You ain’t no cowboy if you don’t rope a calf out of a [doctor’s] office.”
We can see that decision in your eyes
The cliché that eyes are the windows to the soul doesn’t tell the whole story about how telling eyes really are. It’s all about how they move. In a recent study, researchers determined that a type of eye movement known as a saccade reveals your choice before you even decide.
Saccades involve the eyes jumping from one fixation point to another, senior author Alaa Ahmed of the University of Colorado, Boulder, explained in a statement from the university. Saccade vigor was the key in how aligned the type of decisions were made by the 22 study participants.
In the study, subjects walked on a treadmill at varied inclines for a period of time. Then they sat in front of a monitor and a high-speed camera that tracked their eye movements as the monitor presented them with a series of exercise options. The participants had only 4 seconds to choose between them.
After they made their choices, participants went back on the treadmill to perform the exercises they had chosen. The researchers found that participants’ eyes jumped between the options slowly then faster to the option they eventually picked. The more impulsive decision-makers also tended to move their eyes even more rapidly before slowing down after a decision was made, making it pretty conclusive that the eyes were revealing their choices.
The way your eyes shift gives you away without saying a thing. Might be wise, then, to wear sunglasses to your next poker tournament.
Let them eat soap
Okay, we admit it: LOTME spends a lot of time in the bathroom. Today, though, we’re interested in the sinks. Specifically, the P-traps under the sinks. You know, the curvy bit that keeps sewer gas from wafting back into the room?
Well, researchers from the University of Reading (England) recently found some fungi while examining a bunch of sinks on the university’s Whiteknights campus. “It isn’t a big surprise to find fungi in a warm, wet environment. But sinks and P-traps have thus far been overlooked as potential reservoirs of these microorganisms,” they said in a written statement.
Samples collected from 289 P-traps contained “a very similar community of yeasts and molds, showing that sinks in use in public environments share a role as reservoirs of fungal organisms,” they noted.
The fungi living in the traps survived conditions with high temperatures, low pH, and little in the way of nutrients. So what were they eating? Some varieties, they said, “use detergents, found in soap, as a source of carbon-rich food.” We’ll repeat that last part: They used the soap as food.
WARNING: Rant Ahead.
There are a lot of cleaning products for sale that say they will make your home safe by killing 99.9% of germs and bacteria. Not fungi, exactly, but we’re still talking microorganisms. Molds, bacteria, and viruses are all stuff that can infect humans and make them sick.
So you kill 99.9% of them. Great, but that leaves 0.1% that you just made angry. And what do they do next? They learn to eat soap. Then University of Reading investigators find out that all the extra hand washing going on during the COVID-19 pandemic was “clogging up sinks with nasty disease-causing bacteria.”
These are microorganisms we’re talking about people. They’ve been at this for a billion years! Rats can’t beat them, cockroaches won’t stop them – Earth’s ultimate survivors are powerless against the invisible horde.
We’re doomed.
Maybe the cow was late for its appointment
It’s been a long day running the front desk at your doctor’s office. People calling in prescriptions, a million appointments, you’ve been running yourself ragged keeping things together. Finally, it’s almost closing time. The last patient of the day has just checked out and you turn back to the waiting room, expecting to see it blessedly empty.
Instead, a 650-pound cow is staring at you.
“I’m sorry, sir or madam, we’re about to close.”
Moo.
“I understand it’s important, but seriously, the doctor’s about to …”
Moo.
“Fine, I’ll see what we can do for you. What’s your insurance?”
Moo Cross Moo Shield.
“Sorry, we don’t take that. You’ll have to go someplace else.”
This is probably not how things went down recently at Orange (Va.) Family Physicians, when they had a cow break into the office. Cows don’t have health insurance.
The intrepid bovine was being transferred to a new home when it jumped off the trailer and wandered an eighth of a mile to Orange Family Physicians, where the cow wranglers found it hanging around outside. Unfortunately, this was a smart cow, and it bolted as it saw the wranglers, crashing through the glass doors into the doctor’s office. Though neither man had ever wrangled a cow from inside a building, they ultimately secured a rope around the cow’s neck and escorted it back outside, tying it to a nearby pole to keep it from further adventures.
One of the wranglers summed up the situation quite nicely on his Facebook page: “You ain’t no cowboy if you don’t rope a calf out of a [doctor’s] office.”
We can see that decision in your eyes
The cliché that eyes are the windows to the soul doesn’t tell the whole story about how telling eyes really are. It’s all about how they move. In a recent study, researchers determined that a type of eye movement known as a saccade reveals your choice before you even decide.
Saccades involve the eyes jumping from one fixation point to another, senior author Alaa Ahmed of the University of Colorado, Boulder, explained in a statement from the university. Saccade vigor was the key in how aligned the type of decisions were made by the 22 study participants.
In the study, subjects walked on a treadmill at varied inclines for a period of time. Then they sat in front of a monitor and a high-speed camera that tracked their eye movements as the monitor presented them with a series of exercise options. The participants had only 4 seconds to choose between them.
After they made their choices, participants went back on the treadmill to perform the exercises they had chosen. The researchers found that participants’ eyes jumped between the options slowly then faster to the option they eventually picked. The more impulsive decision-makers also tended to move their eyes even more rapidly before slowing down after a decision was made, making it pretty conclusive that the eyes were revealing their choices.
The way your eyes shift gives you away without saying a thing. Might be wise, then, to wear sunglasses to your next poker tournament.
Let them eat soap
Okay, we admit it: LOTME spends a lot of time in the bathroom. Today, though, we’re interested in the sinks. Specifically, the P-traps under the sinks. You know, the curvy bit that keeps sewer gas from wafting back into the room?
Well, researchers from the University of Reading (England) recently found some fungi while examining a bunch of sinks on the university’s Whiteknights campus. “It isn’t a big surprise to find fungi in a warm, wet environment. But sinks and P-traps have thus far been overlooked as potential reservoirs of these microorganisms,” they said in a written statement.
Samples collected from 289 P-traps contained “a very similar community of yeasts and molds, showing that sinks in use in public environments share a role as reservoirs of fungal organisms,” they noted.
The fungi living in the traps survived conditions with high temperatures, low pH, and little in the way of nutrients. So what were they eating? Some varieties, they said, “use detergents, found in soap, as a source of carbon-rich food.” We’ll repeat that last part: They used the soap as food.
WARNING: Rant Ahead.
There are a lot of cleaning products for sale that say they will make your home safe by killing 99.9% of germs and bacteria. Not fungi, exactly, but we’re still talking microorganisms. Molds, bacteria, and viruses are all stuff that can infect humans and make them sick.
So you kill 99.9% of them. Great, but that leaves 0.1% that you just made angry. And what do they do next? They learn to eat soap. Then University of Reading investigators find out that all the extra hand washing going on during the COVID-19 pandemic was “clogging up sinks with nasty disease-causing bacteria.”
These are microorganisms we’re talking about people. They’ve been at this for a billion years! Rats can’t beat them, cockroaches won’t stop them – Earth’s ultimate survivors are powerless against the invisible horde.
We’re doomed.
Maybe the cow was late for its appointment
It’s been a long day running the front desk at your doctor’s office. People calling in prescriptions, a million appointments, you’ve been running yourself ragged keeping things together. Finally, it’s almost closing time. The last patient of the day has just checked out and you turn back to the waiting room, expecting to see it blessedly empty.
Instead, a 650-pound cow is staring at you.
“I’m sorry, sir or madam, we’re about to close.”
Moo.
“I understand it’s important, but seriously, the doctor’s about to …”
Moo.
“Fine, I’ll see what we can do for you. What’s your insurance?”
Moo Cross Moo Shield.
“Sorry, we don’t take that. You’ll have to go someplace else.”
This is probably not how things went down recently at Orange (Va.) Family Physicians, when they had a cow break into the office. Cows don’t have health insurance.
The intrepid bovine was being transferred to a new home when it jumped off the trailer and wandered an eighth of a mile to Orange Family Physicians, where the cow wranglers found it hanging around outside. Unfortunately, this was a smart cow, and it bolted as it saw the wranglers, crashing through the glass doors into the doctor’s office. Though neither man had ever wrangled a cow from inside a building, they ultimately secured a rope around the cow’s neck and escorted it back outside, tying it to a nearby pole to keep it from further adventures.
One of the wranglers summed up the situation quite nicely on his Facebook page: “You ain’t no cowboy if you don’t rope a calf out of a [doctor’s] office.”
We can see that decision in your eyes
The cliché that eyes are the windows to the soul doesn’t tell the whole story about how telling eyes really are. It’s all about how they move. In a recent study, researchers determined that a type of eye movement known as a saccade reveals your choice before you even decide.
Saccades involve the eyes jumping from one fixation point to another, senior author Alaa Ahmed of the University of Colorado, Boulder, explained in a statement from the university. Saccade vigor was the key in how aligned the type of decisions were made by the 22 study participants.
In the study, subjects walked on a treadmill at varied inclines for a period of time. Then they sat in front of a monitor and a high-speed camera that tracked their eye movements as the monitor presented them with a series of exercise options. The participants had only 4 seconds to choose between them.
After they made their choices, participants went back on the treadmill to perform the exercises they had chosen. The researchers found that participants’ eyes jumped between the options slowly then faster to the option they eventually picked. The more impulsive decision-makers also tended to move their eyes even more rapidly before slowing down after a decision was made, making it pretty conclusive that the eyes were revealing their choices.
The way your eyes shift gives you away without saying a thing. Might be wise, then, to wear sunglasses to your next poker tournament.
Let them eat soap
Okay, we admit it: LOTME spends a lot of time in the bathroom. Today, though, we’re interested in the sinks. Specifically, the P-traps under the sinks. You know, the curvy bit that keeps sewer gas from wafting back into the room?
Well, researchers from the University of Reading (England) recently found some fungi while examining a bunch of sinks on the university’s Whiteknights campus. “It isn’t a big surprise to find fungi in a warm, wet environment. But sinks and P-traps have thus far been overlooked as potential reservoirs of these microorganisms,” they said in a written statement.
Samples collected from 289 P-traps contained “a very similar community of yeasts and molds, showing that sinks in use in public environments share a role as reservoirs of fungal organisms,” they noted.
The fungi living in the traps survived conditions with high temperatures, low pH, and little in the way of nutrients. So what were they eating? Some varieties, they said, “use detergents, found in soap, as a source of carbon-rich food.” We’ll repeat that last part: They used the soap as food.
WARNING: Rant Ahead.
There are a lot of cleaning products for sale that say they will make your home safe by killing 99.9% of germs and bacteria. Not fungi, exactly, but we’re still talking microorganisms. Molds, bacteria, and viruses are all stuff that can infect humans and make them sick.
So you kill 99.9% of them. Great, but that leaves 0.1% that you just made angry. And what do they do next? They learn to eat soap. Then University of Reading investigators find out that all the extra hand washing going on during the COVID-19 pandemic was “clogging up sinks with nasty disease-causing bacteria.”
These are microorganisms we’re talking about people. They’ve been at this for a billion years! Rats can’t beat them, cockroaches won’t stop them – Earth’s ultimate survivors are powerless against the invisible horde.
We’re doomed.
Should you quit employment to open a practice? These docs share how they did it
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
“Everyone said private practice is dying,” said Omar Maniya, MD, an emergency physician who left his hospital job for family practice in New Jersey. “But I think it could be one of the best models we have to advance our health care system and prevent burnout – and bring joy back to the practice of medicine.”
But employment doesn’t necessarily mean happiness. In the Medscape “Employed Physicians: Loving the Focus, Hating the Bureaucracy” ” report, more than 1,350 U.S. physicians employed by a health care organization, hospital, large group practice, or other medical group were surveyedabout their work. As the subtitle suggests, many are torn.
In the survey, employed doctors cited three main downsides to the lifestyle: They have less autonomy, more corporate rules than they’d like, and lower earning potential. Nearly one-third say they’re unhappy about their work-life balance, too, which raises the risk for burnout.
Some physicians find that employment has more cons than pros and turn to private practice instead.
A system skewed toward employment
In the mid-1990s, when James Milford, MD, completed his residency, going straight into private practice was the norm. The family physician bucked that trend by joining a large regional medical center in Wisconsin. He spent the next 20+ years working to establish a network of medical clinics.
“It was very satisfying,” Dr. Milford said. “When I started, I had a lot of input, a lot of control.”
Since then, the pendulum has been swinging toward employment. Brieanna Seefeldt, DO, a family physician outside Denver, completed her residency in 2012.
“I told the recruiter I wanted my own practice,” Dr. Seefeldt said, “They said if you’re not independently wealthy, there’s no way.”
Sonal G. Patel, MD, a pediatric neurologist in Bethesda, finished her residency the same year as Dr. Seefeldt. Dr. Patel never even considered private practice.
“I always thought I would have a certain amount of clinic time where I have my regular patients,” she said, “but I’d also be doing hospital rounds and reading EEG studies at the hospital.”
For Dr. Maniya, who completed his residency in 2021, the choice was simple. Growing up, he watched his immigrant parents, both doctors in private practice, struggle to keep up.
“I opted for a big, sophisticated health system,” he said. “I thought we’d be pushing the envelope of what was possible in medicine.”
Becoming disillusioned with employment
All four of these physicians are now in private practice and are much happier.
Within a few years of starting her job, Dr. Seefeldt was one of the top producers in her area but felt tremendous pressure to see more and more patients. The last straw came after an unpaid maternity leave.
“They told me I owed them for my maternity leave, for lack of productivity,” she said. “I was in practice for only 4 years, but already feeling the effects of burnout.”
Dr. Patel only lasted 2 years before realizing employment didn’t suit her.
“There was an excessive amount of hospital calls,” she said. “And there were bureaucratic issues that made it very difficult to practice the way I thought my practice would be.”
It took just 18 months for Dr. Maniya’s light-bulb moment. He was working at a hospital when COVID-19 hit.
“At my big health care system, it took 9 months to come up with a way to get COVID swabs for free,” he said. “At the same time, I was helping out the family business, a private practice. It took me two calls and 48 hours to get free swabs for not just the practice, not just our patients, but the entire city of Hamilton, New Jersey.”
Milford lasted the longest as an employee – nearly 25 years. The end came after a healthcare company with hospitals in 30 states bought out the medical center.
“My control gradually eroded,” he said. “It got to the point where I had no input regarding things like employees or processes we wanted to improve.”
Making the leap to private practice
Private practice can take different forms.
Dr. Seefeldt opted for direct primary care, a model in which her patients pay a set monthly fee for care whenever needed. Her practice doesn’t take any insurance besides Medicaid.
“Direct primary care is about working directly with the patient and cost-conscious, transparent care,” she said. “And I don’t have to deal with insurance.”
For Dr. Patel, working with an accountable care organization made the transition easier. She owns her practice solo but works with a company called Privia for administrative needs. Privia sent a consultant to set up her office in the company’s electronic medical record. Things were up and running within the first week.
Dr. Maniya joined his mother’s practice, easing his way in over 18 months.
And then there’s what Milford did, building a private practice from the ground up.
“We did a lot of Googling, a lot of meeting with accountants, meeting with small business development from the state of Wisconsin,” he said. “We asked people that were in business, ‘What are the things businesses fail on? Not medical practices, but businesses.’” All that research helped him launch successfully.
Making the dollars and cents add up
Moving from employment into private practice takes time, effort, and of course, money. How much of each varies depending on where you live, your specialty, whether you choose to rent or buy office space, staffing needs, and other factors.
Dr. Seefeldt, Dr. Patel, Dr. Milford, and Dr. Maniya illustrate the range.
- Dr. Seefeldt got a home equity loan of $50,000 to cover startup costs – and paid it back within 6 months.
- Purchasing EEG equipment added to Dr. Patel’s budget; she spent $130,000 of her own money to launch her practice in a temporary office and took out a $150,000 loan to finance the buildout of her final space. It took her 3 years to pay it back.
- When Dr. Milford left employment, he borrowed the buildout and startup costs for his practice from his father, a retired surgeon, to the tune of $500,000.
- Dr. Maniya assumed the largest risk. When he took over the family practice, he borrowed $1.5 million to modernize and build a new office. The practice has now quintupled in size. “It’s going great,” he said. “One of our questions is, should we pay back the loan at a faster pace rather than make the minimum payments?”
Several years in, Dr. Patel reports she’s easily making three to four times as much as she would have at a hospital. However, Dr. Maniya’s guaranteed compensation is 10% less than his old job.
“But as a partner in a private practice, if it succeeds, it could be 100%-150% more in a good year,” he said. On the flip side, if the practice runs into financial trouble, so does he. “Does the risk keep me up at night, give me heartburn? You betcha.”
Dr. Milford and Dr. Seefeldt have both chosen to take less compensation than they could, opting to reinvest in and nurture their practices.
“I love it,” said Dr. Milford. “I joke that I have half as much in my pocketbook, twice as much in my heart. But it’s not really half as much, 5 years in. If I weren’t growing the business, I’d be making more than before.”
Private practice is not without challenges
Being the big cheese does have drawbacks. In the current climate, staffing is a persistent issue for doctors in private practice – both maintaining a full staff and managing their employees.
And without the backing of a large corporation, doctors are sometimes called on to do less than pleasant tasks.
“If the toilet gets clogged and the plumber can’t come for a few hours, the patients still need a bathroom,” Dr. Maniya said. “I’ll go in with my $400 shoes and snake the toilet.”
Dr. Milford pointed out that when the buck stops with you, small mistakes can have enormous ramifications. “But with the bad comes the great potential for good. You have the ability to positively affect patients and healthcare, and to make a difference for people. It creates great personal satisfaction.”
Is running your own practice all it’s cracked up to be?
If it’s not yet apparent, all four doctors highly recommend moving from employment to private practice when possible. The autonomy and the improved work-life balance have helped them find the satisfaction they’d been missing while making burnout less likely.
“When you don’t have to spend 30% of your day apologizing to patients for how bad the health care system is, it reignites your passion for why you went into medicine in the first place,” said Dr. Maniya. In his practice, he’s made a conscious decision to pursue a mix of demographics. “Thirty percent of our patients are Medicaid. The vast majority are middle to low income.”
For physicians who are also parents, the ability to set their own schedules is life-changing.
“My son got an award ... and the teacher invited me to the assembly. In a corporate-based world, I’d struggle to be able to go,” said Dr. Seefeldt. As her own boss, she didn’t have to forgo this special event. Instead, she coordinated directly with her scheduled patient to make time for it.
In Medscape’s report, 61% of employed physicians indicated that they don’t have a say on key management decisions. However, doctors who launch private practices embrace the chance to set their own standards.
“We make sure from the minute someone calls they know they’re in good hands, we’re responsive, we address concerns right away. That’s the difference with private practice – the one-on-one connection is huge,” said Dr. Patel.
“This is exactly what I always wanted. It brings me joy knowing we’ve made a difference in these children’s lives, in their parents’ lives,” she concluded.
A version of this article first appeared on Medscape.com.
U.S. sees most flu hospitalizations in a decade
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.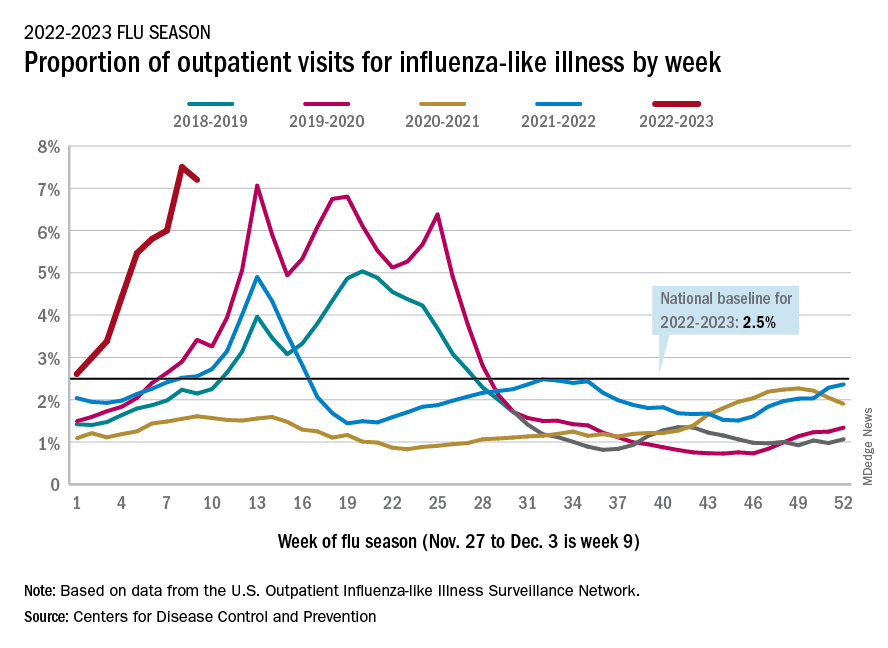
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
But the number of deaths and outpatient visits for flu or flu-like illnesses was down slightly from the week before, the CDC said in its weekly FluView report.
There were almost 26,000 new hospital admissions involving laboratory-confirmed influenza over those 7 days, up by over 31% from the previous week, based on data from 5,000 hospitals in the HHS Protect system, which tracks and shares COVID-19 data.
The cumulative hospitalization rate for the 2022-2023 season is 26.0 per 100,000 people, the highest seen at this time of year since 2010-2011, the CDC said, based on data from its Influenza Hospitalization Surveillance Network, which includes hospitals in select counties in 13 states.
At this point in the 2019-2020 season, just before the COVID-19 pandemic began, the cumulative rate was 3.1 per 100,000 people, the CDC’s data show.
On the positive side, the proportion of outpatient visits for influenza-like illness dropped slightly to 7.2%, from 7.5% the week before. But these cases from the CDC’s Outpatient Influenza-like Illness Surveillance Network are not laboratory confirmed, so the data could include people with the flu, COVID-19, or respiratory syncytial virus.
The number of confirmed flu deaths for the week of Nov. 27 to Dec. 3 also fell slightly from the last full week of November, 246 vs. 255, but the number of pediatric deaths rose from 2 to 7, and total deaths in children are already up to 21 for 2022-2023. That’s compared to 44 that were reported during all of the 2021-2022 season, the CDC said.
“So far this season, there have been at least 13 million illnesses, 120,000 hospitalizations, and 7,300 deaths from flu,” the agency estimated.
A version of this article first appeared on Medscape.com.
As COVID treatments dwindle, are new ones waiting in the wings?
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
It was the last monoclonal antibody treatment standing. But less than 10 months after the U.S. Food and Drug Administration gave bebtelovimab its emergency use authorization (EUA) to fight COVID-19, it earlier this month de-authorized it, just as it had for other monoclonal antibody treatments, and for the same reason:
Bebtelovimab couldn’t neutralize the Omicron subvariants BQ.1 and BQ.1.1, the cause of nearly 60% of COVID cases nationally as of November 30.
Next on the chopping block, some predict, will be Evusheld, the combination of tixagevimab and cilgavimab given as a preventive monoclonal antibody to people who are immunocompromised and at high risk of contracting COVID and to those who can’t take the vaccine. In October, the FDA warned that Evusheld was not neutralizing circulating COVID variants.
As the options for treating and preventing COVID decline, will companies rally quickly to develop new ones, or cut their losses in developing treatments that may work for only a few months, given the speed of viral mutations?
But although monoclonal antibody treatments are off the table, at least for now, antiviral drugs – including Paxlovid – are still very much available, and some say underused.
Others suggest it’s time to resurrect interest in convalescent plasma, a treatment used early in the pandemic before drugs or vaccines were here and still authorized for use in those who are immunosuppressed or receiving immunosuppressive treatment.
And on the prevention front, staying up to date with booster vaccines, masking, and taking other precautions should be stressed more, others say, regardless of the number of treatment options, and especially now, as cases rise and people gather for the winter holidays.
‘A major setback’
The bebtelovimab de-authorization was “a major setback,” but an understandable one, said Arturo Casadevall, MD, PhD, professor and chair of molecular microbiology and immunology at the Johns Hopkins Bloomberg School of Public Health in Baltimore. “Monoclonal antibodies are great drugs. We are in an unfortunate situation in that they are vulnerable to changes in the virus” and can’t offer long-lasting protection.
Supplies of bebtelovimab will be retained, according to the FDA, in case variants susceptible to it return.
“What happened to bebtelovimab is no surprise,” agreed Amesh Adalja, MD, senior scholar at Johns Hopkins Center for Health Security. “This is what is going to happen when you are targeting a virus that mutates a lot.”
Monoclonal antibodies work by binding to the spike protein on the virus surface to prevent it from entering cells.
However, Dr. Adalja doesn’t view the disappearance of monoclonal antibody treatments as a major setback. Monoclonal antibodies were not the primary way COVID was treated, he said.
While he does believe it’s important that more monoclonal antibody treatments be developed, “I think it’s important to remember we still have Paxlovid while everyone is lamenting the loss of bebtelovimab.’’
Antivirals: What’s here, what’s coming
Compared with monoclonal antibodies, “Paxlovid remains a much easier drug to give,” Dr. Adalja told this news organization, because it is taken orally, not intravenously.
And it’s effective. In a recent study, researchers found that adults diagnosed with COVID given Paxlovid within 5 days of diagnosis had a 51% lower hospitalization rate within the next 30 days than those not given it. Another study shows it could also reduce a person’s risk of developing long COVID by 26%.
Paxlovid is underused, Dr. Adalja said, partly because the rebound potential got more press than the effectiveness. When a celebrity got rebound from Paxlovid, he said, that would make the news, overshadowing the research on its effectiveness.
Besides Paxlovid, the antivirals remdesivir (Veklury), given intravenously for 3 days, and molnupiravir (Lagevrio), taken orally, are also still available. Antivirals work by targeting specific parts of the virus to prevent it from multiplying.
In the lab, remdesivir, molnupiravir, and another antiviral, nirmatrelvir, all appear to be effective against both BQ.1.1 (a BA.5 subvariant) and XBB (a BA.2 subvariant), both rapidly rising in the United States, according to a report last week in the New England Journal of Medicine.
The researchers also tested several monoclonal antibodies and found they did not neutralize either of the subvariants BQ.1.1 and XBB.
A new oral antiviral, Xocova (ensitrelvir fumaric acid), from Japanese manufacturer Shionogi, received emergency approval in Japan on November 22. It’s taken once a day for 5 days. The goal is to expand access to it globally, according to the company.
Pardes Biosciences launched a phase 2 trial in September for its oral antiviral drug (PBI-0451), under study as a treatment and preventive for COVID. It expects data by the first quarter of 2023.
Pfizer, which makes Paxlovid, has partnered with Clear Creek Bio to develop another oral antiviral COVID drug.
Other approaches
A receptor protein known as ACE2 (angiotensin-converting enzyme 2) is the main “doorway” that SARS-CoV-2 uses to enter and infect cells.
Dana-Farber Cancer Institute scientists are developing a “decoy” drug that works by mimicking the ACE2 receptor on the surface of cells; when the virus tries to bind to it, the spike protein is destroyed. Human trials have not yet started.
Other researchers are investigating whether an already-approved drug used to treat a liver disease, Actigall (UDCA/ursodeoxycholic acid), could protect against COVID infection by reducing ACE2.
So far, the researchers have found in early research that people taking UDCA for liver conditions were less likely than those not taking the drug to have severe COVID. They also found that UDCA reduced SARS-CoV-2 infection in human lungs maintained outside the body.
Monoclonal antibody treatments?
After the FDA decision to withdraw the bebtelovimab EUA, which Eli Lilly said it agreed with, the company issued a statement, promising it wasn’t giving up on monoclonal antibody treatments.
“Lilly will continue to search and evaluate monoclonal antibodies to identify potential candidates for clinical development against new variants,” it read in part.
AstraZeneca, which makes Evusheld, is also continuing to work on monoclonal antibody development. According to a spokesperson, “We are also developing a new long-acting antibody combination – AZD5156 – which has been shown in the lab to neutralize emerging new variants and all known variants to date. We are working to accelerate the development of AZD5156 to make it available at the end of 2023.”
The AstraZeneca spokesperson said he could share no more information about what the combination would include.
A convalescent plasma comeback?
Although Paxlovid can help, there are many contraindications to it, such as drug-drug interactions, Dr. Casadevall told this news organization. And now that the monoclonal antibody treatments have been paused, convalescent plasma “is the only antibody-based therapy that is reliably available. Convalescent plasma includes thousands of different antibodies.”
With his colleagues, Dr. Casadevall evaluated plasma samples from 740 patients. Some had received booster vaccines and been infected with Omicron, others had received boosters and not been infected, and still others had not been vaccinated and became infected.
In a report (not yet peer-reviewed), they found the plasma from those who had been infected or boosted within the past 6 months neutralized the new Omicron variants BQ.1.1, XBB.1, and BF.7.
A push for boosters, masks
To get through the coming months, taking precautions like masking and distancing and staying up to date on booster vaccinations, especially for older adults, can make a difference, other experts say.
In a Twitter thread in early December, Peter Hotez, MD, PhD, professor of pediatrics and molecular virology and microbiology at Baylor College of Medicine, Houston, urged people to take COVID seriously as holiday parties and gatherings occur.
“The single most impactful thing you can do is get your bivalent booster,” he tweeted, as well as give your kids the booster, citing preliminary research that the bivalent mRNA booster broadens immunity against the Omicron subvariants.
For seniors, he said, ‘‘if you get breakthrough COVID, [it’s] really important to get Paxlovid.” Masks will help not only for COVID but also influenza, respiratory syncytial virus (RSV), and other conditions.
Mitigation measures have largely been abandoned, according to Eric Topol, MD, director of the Scripps Research Translational Institute, La Jolla, Calif., and editor-in-chief of Medscape. In an op-ed in the Los Angeles Times, and on his Twitter feed, he reminds people about masking and urges people to get the bivalent booster.
According to the Centers for Disease Control and Prevention, as of Dec. 8, only 13.5% of people aged 5 and older have gotten an updated booster, despite research that shows an increase in antibodies to BQ.1.1. Recent research has found that the bivalent booster increases antibodies to BQ.1.1 by up to 10-fold, Dr. Topol said.
Dr. Adalja is on advisory boards for Shionogi, GSK, and Pardes. Dr. Casadevall reports no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Cardiologist sues hospital, claims he was fired in retaliation
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
alleging that he was fired and maligned after raising concerns about poorly performed surgeries and poor ethical practices at the hospital.
Dr. Zelman, from Barnstable, Mass., has been affiliated with Cape Cod Hospital in Hyannis, Mass., for more than 30 years. He helped found the hospital’s Heart and Vascular Institute and has served as its medical director since 2018.
In his lawsuit filed Dec. 6, Dr. Zelman alleges that the defendants, under Mr. Lauf’s leadership, “placed profit above all else, including by prioritizing revenue generation over patient safety and public health.”
Dr. Zelman says the defendants supported him “to the extent his actions were profitable.”
Yet, when he raised patient safety concerns that harmed that bottom line, Dr. Zelman says the defendants retaliated against him, including by threatening his career and reputation and unlawfully terminating his employment with the hospital.
The complaint notes Dr. Zelman is bringing this action “to recover damages for violations of the Massachusetts Healthcare Provider Whistleblower Statute ... as well as for breach of contract and common law claims.”
Dr. Zelman’s complaint alleges the defendants refused to adequately address the “dangerous care and violations of the professional standards of practice” that he reported, “resulting in harmful and tragic consequences.”
It also alleges Mr. Lauf restricted the use of a cerebral protection device used in patients undergoing transcatheter aortic-valve replacement (TAVR) deemed to be at high risk for periprocedural stroke to only those patients whose insurance reimbursed at higher rates.
Dr. Zelman says he objected to this prohibition “in accordance with his contractual and ethical obligations to ensure treatment of patients without regard to their ability to pay.”
Dr. Zelman’s lawsuit further alleges that Mr. Lauf launched a “trumped-up” and “baseless, biased, and retaliatory sham” investigation against him.
In a statement sent to the Boston Globe, Cape Cod Hospital denied Dr. Zelman’s claims that the cardiologist was retaliated against for raising patient safety issues, or that the hospital didn’t take action to improve cardiac care at the facility.
Voiced concerns
In a statement sent to this news organization, Dr. Zelman, now in private practice, said, “Over the past 25 years, I have been instrumental in bringing advanced cardiac care to Cape Cod. My commitment has always been to delivering the same quality outcomes and safety as the academic centers in Boston.
“Unfortunately, over the past 5 years, there has been inadequate oversight by the hospital administration and problems have occurred that in my opinion have led to serious patient consequences,” Dr. Zelman stated.
He said he has “voiced concerns over several years and they have been ignored.”
He added that Cape Cod Hospital offered him a million-dollar contract as long as he agreed to immediately issue a written statement endorsing the quality and safety of the cardiac surgical program that no longer exists.
“No amount of money was going to buy my silence,” Dr. Zelman told this news organization.
In his lawsuit, Dr. Zelman is seeking an undisclosed amount in damages, including back and front pay, lost benefits, physical and emotional distress, and attorneys’ fees.
This news organization reached out to Cape Cod Hospital for comment but has not yet received a response.
A version of this article first appeared on Medscape.com.
‘Striking’ rate of mental health comorbidities in epilepsy
NASHVILLE, TENN. – , new research reveals.
“We hope these results inspire epileptologists and neurologists to both recognize and screen for suicide ideation and behaviors in their adolescent patients,” said study investigator Hadley Greenwood, a third-year medical student at New York University.
The new data should also encourage providers “to become more comfortable” providing support to patients, “be that by increasing their familiarity with prescribing different antidepressants or by being well versed in how to connect patients to resources within their community,” said Mr. Greenwood.
The findings were presented here at the annual meeting of the American Epilepsy Society.
Little research
Previous studies have reported on the prevalence of suicidality as well as depression and anxiety among adults with epilepsy. “We wanted to look at adolescents because there’s much less in the literature out there about psychiatric comorbidity, and specifically suicidality, in this population,” said Mr. Greenwood.
Researchers used data from the Human Epilepsy Project, a study that collected data from 34 sites in the United States, Canada, Europe, and Australia from 2012 to 2017.
From a cohort of more than 400 participants, researchers identified 67 patients aged 11-17 years who were enrolled within 4 months of starting treatment for focal epilepsy.
Participants completed the Columbia–Suicide Severity Rating Scale (C-SSRS) at enrollment and at follow-ups over 36 months. The C-SSRS measures suicidal ideation and severity, said Mr. Greenwood.
“It’s scaled from passive suicide ideation, such as thoughts of ‘I wish I were dead’ without active intent, all the way up to active suicidal ideation with a plan and intent.”
Researchers were able to distinguish individuals with passive suicide ideation from those with more serious intentions, said Mr. Greenwood. They used medical records to evaluate the prevalence of suicidal ideation and behavior.
The investigators found that more than one in five (20.9%) teens endorsed any lifetime suicide ideation. This, said Mr. Greenwood, is “roughly equivalent” to the prevalence reported earlier in the adult cohort of the Human Epilepsy Project (21.6%).
‘Striking’ rate
The fact that one in five adolescents had any lifetime suicide ideation is “definitely a striking number,” said Mr. Greenwood.
Researchers found that 15% of patients experienced active suicide ideation, 7.5% exhibited preparatory or suicidal behaviors, and 3% had made a prior suicide attempt.
All of these percentages increased at 3 years: Thirty-one percent for suicide ideation; 25% for active suicide behavior, 15% for preparatory or suicide behaviors, and 5% for prior suicide attempt.
The fact that nearly one in three adolescents endorsed suicide ideation at 3 years is another “striking” finding, said Mr. Greenwood.
Of the 53 adolescents who had never had suicide ideation at the time of enrollment, 7 endorsed new-onset suicide ideation in the follow-up period. Five of 14 who had had suicide ideation at some point prior to enrollment continued to endorse it.
“The value of the study is identifying the prevalence and identifying the significant number of adolescents with epilepsy who are endorsing either suicide ideation or suicidal behaviors,” said Mr. Greenwood.
The researchers found that among younger teens (aged 11–14 years) rates of suicide ideation were higher than among their older counterparts (aged 15–17 years).
The study does not shed light on the biological connection between epilepsy and suicidality, but Mr. Greenwood noted that prior research has suggested a bidirectional relationship.
“Depression and other psychiatric comorbidities might exist prior to epileptic activity and actually predispose to epileptic activity.”
Mr. Greenwood noted that suicide ideation has “spiked” recently across the general population, and so it’s difficult to compare the prevalence in her study with “today’s prevalence.”
However, other research generally shows that the suicide ideation rate in the general adolescent population is much lower than in teens with epilepsy.
Unique aspects of the current study are that it reports suicide ideation and behaviors at around the time of an epilepsy diagnosis and documents how suicidality progresses or resolves over time, said Mr. Greenwood.
Underdiagnosed, undertreated
Commenting on the research, Elizabeth Donner, MD, director of the comprehensive epilepsy program, Hospital for Sick Children, and associate professor, department of pediatrics, University of Toronto, said a “key point” from the study is that the suicidality rate among teens with epilepsy exceeds that of children not living with epilepsy.
“We are significantly underdiagnosing and undertreating the mental health comorbidities in epilepsy,” said Dr. Donner. “Epilepsy is a brain disease and so are mental health disorders, so it shouldn’t come as any surprise that they coexist in individuals with epilepsy.”
The new results contribute to what is already known about the significant mortality rates among persons with epilepsy, said Dr. Donner. She referred to a 2018 study that showed that people with epilepsy were 3.5 times more likely to die by suicide.
Other research has shown that people with epilepsy are 10 times more likely to die by drowning, mostly in the bathtub, said Dr. Donner.
“You would think that we’re educating these people about risks related to their epilepsy, but either the messages don’t get through, or they don’t know how to keep themselves safe,” she said.
“This needs to be seen in a bigger picture, and the bigger picture is we need to recognize comorbid mental health issues; we need to address them once recognized; and then we need to counsel and support people to live safely with their epilepsy.
The study received funding from the Epilepsy Study Consortium, Finding a Cure for Epilepsy and Seizures (FACES) and other related foundations, UCB, Pfizer, Eisai, Lundbeck, and Sunovion. Mr. Greenwood and Dr. Donner report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – , new research reveals.
“We hope these results inspire epileptologists and neurologists to both recognize and screen for suicide ideation and behaviors in their adolescent patients,” said study investigator Hadley Greenwood, a third-year medical student at New York University.
The new data should also encourage providers “to become more comfortable” providing support to patients, “be that by increasing their familiarity with prescribing different antidepressants or by being well versed in how to connect patients to resources within their community,” said Mr. Greenwood.
The findings were presented here at the annual meeting of the American Epilepsy Society.
Little research
Previous studies have reported on the prevalence of suicidality as well as depression and anxiety among adults with epilepsy. “We wanted to look at adolescents because there’s much less in the literature out there about psychiatric comorbidity, and specifically suicidality, in this population,” said Mr. Greenwood.
Researchers used data from the Human Epilepsy Project, a study that collected data from 34 sites in the United States, Canada, Europe, and Australia from 2012 to 2017.
From a cohort of more than 400 participants, researchers identified 67 patients aged 11-17 years who were enrolled within 4 months of starting treatment for focal epilepsy.
Participants completed the Columbia–Suicide Severity Rating Scale (C-SSRS) at enrollment and at follow-ups over 36 months. The C-SSRS measures suicidal ideation and severity, said Mr. Greenwood.
“It’s scaled from passive suicide ideation, such as thoughts of ‘I wish I were dead’ without active intent, all the way up to active suicidal ideation with a plan and intent.”
Researchers were able to distinguish individuals with passive suicide ideation from those with more serious intentions, said Mr. Greenwood. They used medical records to evaluate the prevalence of suicidal ideation and behavior.
The investigators found that more than one in five (20.9%) teens endorsed any lifetime suicide ideation. This, said Mr. Greenwood, is “roughly equivalent” to the prevalence reported earlier in the adult cohort of the Human Epilepsy Project (21.6%).
‘Striking’ rate
The fact that one in five adolescents had any lifetime suicide ideation is “definitely a striking number,” said Mr. Greenwood.
Researchers found that 15% of patients experienced active suicide ideation, 7.5% exhibited preparatory or suicidal behaviors, and 3% had made a prior suicide attempt.
All of these percentages increased at 3 years: Thirty-one percent for suicide ideation; 25% for active suicide behavior, 15% for preparatory or suicide behaviors, and 5% for prior suicide attempt.
The fact that nearly one in three adolescents endorsed suicide ideation at 3 years is another “striking” finding, said Mr. Greenwood.
Of the 53 adolescents who had never had suicide ideation at the time of enrollment, 7 endorsed new-onset suicide ideation in the follow-up period. Five of 14 who had had suicide ideation at some point prior to enrollment continued to endorse it.
“The value of the study is identifying the prevalence and identifying the significant number of adolescents with epilepsy who are endorsing either suicide ideation or suicidal behaviors,” said Mr. Greenwood.
The researchers found that among younger teens (aged 11–14 years) rates of suicide ideation were higher than among their older counterparts (aged 15–17 years).
The study does not shed light on the biological connection between epilepsy and suicidality, but Mr. Greenwood noted that prior research has suggested a bidirectional relationship.
“Depression and other psychiatric comorbidities might exist prior to epileptic activity and actually predispose to epileptic activity.”
Mr. Greenwood noted that suicide ideation has “spiked” recently across the general population, and so it’s difficult to compare the prevalence in her study with “today’s prevalence.”
However, other research generally shows that the suicide ideation rate in the general adolescent population is much lower than in teens with epilepsy.
Unique aspects of the current study are that it reports suicide ideation and behaviors at around the time of an epilepsy diagnosis and documents how suicidality progresses or resolves over time, said Mr. Greenwood.
Underdiagnosed, undertreated
Commenting on the research, Elizabeth Donner, MD, director of the comprehensive epilepsy program, Hospital for Sick Children, and associate professor, department of pediatrics, University of Toronto, said a “key point” from the study is that the suicidality rate among teens with epilepsy exceeds that of children not living with epilepsy.
“We are significantly underdiagnosing and undertreating the mental health comorbidities in epilepsy,” said Dr. Donner. “Epilepsy is a brain disease and so are mental health disorders, so it shouldn’t come as any surprise that they coexist in individuals with epilepsy.”
The new results contribute to what is already known about the significant mortality rates among persons with epilepsy, said Dr. Donner. She referred to a 2018 study that showed that people with epilepsy were 3.5 times more likely to die by suicide.
Other research has shown that people with epilepsy are 10 times more likely to die by drowning, mostly in the bathtub, said Dr. Donner.
“You would think that we’re educating these people about risks related to their epilepsy, but either the messages don’t get through, or they don’t know how to keep themselves safe,” she said.
“This needs to be seen in a bigger picture, and the bigger picture is we need to recognize comorbid mental health issues; we need to address them once recognized; and then we need to counsel and support people to live safely with their epilepsy.
The study received funding from the Epilepsy Study Consortium, Finding a Cure for Epilepsy and Seizures (FACES) and other related foundations, UCB, Pfizer, Eisai, Lundbeck, and Sunovion. Mr. Greenwood and Dr. Donner report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NASHVILLE, TENN. – , new research reveals.
“We hope these results inspire epileptologists and neurologists to both recognize and screen for suicide ideation and behaviors in their adolescent patients,” said study investigator Hadley Greenwood, a third-year medical student at New York University.
The new data should also encourage providers “to become more comfortable” providing support to patients, “be that by increasing their familiarity with prescribing different antidepressants or by being well versed in how to connect patients to resources within their community,” said Mr. Greenwood.
The findings were presented here at the annual meeting of the American Epilepsy Society.
Little research
Previous studies have reported on the prevalence of suicidality as well as depression and anxiety among adults with epilepsy. “We wanted to look at adolescents because there’s much less in the literature out there about psychiatric comorbidity, and specifically suicidality, in this population,” said Mr. Greenwood.
Researchers used data from the Human Epilepsy Project, a study that collected data from 34 sites in the United States, Canada, Europe, and Australia from 2012 to 2017.
From a cohort of more than 400 participants, researchers identified 67 patients aged 11-17 years who were enrolled within 4 months of starting treatment for focal epilepsy.
Participants completed the Columbia–Suicide Severity Rating Scale (C-SSRS) at enrollment and at follow-ups over 36 months. The C-SSRS measures suicidal ideation and severity, said Mr. Greenwood.
“It’s scaled from passive suicide ideation, such as thoughts of ‘I wish I were dead’ without active intent, all the way up to active suicidal ideation with a plan and intent.”
Researchers were able to distinguish individuals with passive suicide ideation from those with more serious intentions, said Mr. Greenwood. They used medical records to evaluate the prevalence of suicidal ideation and behavior.
The investigators found that more than one in five (20.9%) teens endorsed any lifetime suicide ideation. This, said Mr. Greenwood, is “roughly equivalent” to the prevalence reported earlier in the adult cohort of the Human Epilepsy Project (21.6%).
‘Striking’ rate
The fact that one in five adolescents had any lifetime suicide ideation is “definitely a striking number,” said Mr. Greenwood.
Researchers found that 15% of patients experienced active suicide ideation, 7.5% exhibited preparatory or suicidal behaviors, and 3% had made a prior suicide attempt.
All of these percentages increased at 3 years: Thirty-one percent for suicide ideation; 25% for active suicide behavior, 15% for preparatory or suicide behaviors, and 5% for prior suicide attempt.
The fact that nearly one in three adolescents endorsed suicide ideation at 3 years is another “striking” finding, said Mr. Greenwood.
Of the 53 adolescents who had never had suicide ideation at the time of enrollment, 7 endorsed new-onset suicide ideation in the follow-up period. Five of 14 who had had suicide ideation at some point prior to enrollment continued to endorse it.
“The value of the study is identifying the prevalence and identifying the significant number of adolescents with epilepsy who are endorsing either suicide ideation or suicidal behaviors,” said Mr. Greenwood.
The researchers found that among younger teens (aged 11–14 years) rates of suicide ideation were higher than among their older counterparts (aged 15–17 years).
The study does not shed light on the biological connection between epilepsy and suicidality, but Mr. Greenwood noted that prior research has suggested a bidirectional relationship.
“Depression and other psychiatric comorbidities might exist prior to epileptic activity and actually predispose to epileptic activity.”
Mr. Greenwood noted that suicide ideation has “spiked” recently across the general population, and so it’s difficult to compare the prevalence in her study with “today’s prevalence.”
However, other research generally shows that the suicide ideation rate in the general adolescent population is much lower than in teens with epilepsy.
Unique aspects of the current study are that it reports suicide ideation and behaviors at around the time of an epilepsy diagnosis and documents how suicidality progresses or resolves over time, said Mr. Greenwood.
Underdiagnosed, undertreated
Commenting on the research, Elizabeth Donner, MD, director of the comprehensive epilepsy program, Hospital for Sick Children, and associate professor, department of pediatrics, University of Toronto, said a “key point” from the study is that the suicidality rate among teens with epilepsy exceeds that of children not living with epilepsy.
“We are significantly underdiagnosing and undertreating the mental health comorbidities in epilepsy,” said Dr. Donner. “Epilepsy is a brain disease and so are mental health disorders, so it shouldn’t come as any surprise that they coexist in individuals with epilepsy.”
The new results contribute to what is already known about the significant mortality rates among persons with epilepsy, said Dr. Donner. She referred to a 2018 study that showed that people with epilepsy were 3.5 times more likely to die by suicide.
Other research has shown that people with epilepsy are 10 times more likely to die by drowning, mostly in the bathtub, said Dr. Donner.
“You would think that we’re educating these people about risks related to their epilepsy, but either the messages don’t get through, or they don’t know how to keep themselves safe,” she said.
“This needs to be seen in a bigger picture, and the bigger picture is we need to recognize comorbid mental health issues; we need to address them once recognized; and then we need to counsel and support people to live safely with their epilepsy.
The study received funding from the Epilepsy Study Consortium, Finding a Cure for Epilepsy and Seizures (FACES) and other related foundations, UCB, Pfizer, Eisai, Lundbeck, and Sunovion. Mr. Greenwood and Dr. Donner report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
AT AES 2022
Why doctors are losing trust in patients; what should be done?
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University.
I want to talk about a paper that my colleagues in my division just published in Health Affairs.
As they pointed out, there’s a large amount of literature about what makes patients trust their doctor. There are many studies that show that, although patients sometimes have become more critical of the medical profession, in general they still try to trust their individual physician. Nurses remain in fairly high esteem among those who are getting hospital care.
What isn’t studied, as this paper properly points out, is, what can the doctor and the nurse do to trust the patient? How can that be assessed? Isn’t that just as important as saying that patients have to trust their doctors to do and comply with what they’re told?
What if doctors are afraid of violence? What if doctors are fearful that they can’t trust patients to listen, pay attention, or do what they’re being told? What if they think that patients are coming in with all kinds of disinformation, false information, or things they pick up on the Internet, so that even though you try your best to get across accurate and complete information about what to do about infectious diseases, taking care of a kid with strep throat, or whatever it might be, you’re thinking, Can I trust this patient to do what it is that I want them to do?
One particular problem that’s causing distrust is that more and more patients are showing stress and dependence on drugs and alcohol. That doesn’t make them less trustworthy per se, but it means they can’t regulate their own behavior as well.
That obviously has to be something that the physician or the nurse is thinking about. Is this person going to be able to contain anger? Is this person going to be able to handle bad news? Is this person going to deal with me when I tell them that some of the things they believe to be true about what’s good for their health care are false?
I think we have to really start to push administrators and people in positions of power to teach doctors and nurses how to defuse situations and how to make people more comfortable when they come in and the doctor suspects that they might be under the influence, impaired, or angry because of things they’ve seen on social media, whatever those might be – including concerns about racism, bigotry, and bias, which some patients are bringing into the clinic and the hospital setting.
We need more training. We’ve got to address this as a serious issue. What can we do to defuse situations where the doctor or the nurse rightly thinks that they can’t control or they can’t trust what the patient is thinking or how the patient might behave?
It’s also the case that I think we need more backup and quick access to security so that people feel safe and comfortable in providing care. We have to make sure that if you need someone to restrain a patient or to get somebody out of a situation, that they can get there quickly and respond rapidly, and that they know what to do to deescalate a situation.
It’s sad to say, but security in today’s health care world has to be something that we really test and check – not because we’re worried, as many places are, about a shooter entering the premises, which is its own bit of concern – but I’m just talking about when the doctor or the nurse says that this patient might be acting up, could get violent, or is someone I can’t trust.
My coauthors are basically saying that it’s not a one-way street. Yes, we have to figure out ways to make sure that our patients can trust what we say. Trust is absolutely the lubricant that makes health care flow. If patients don’t trust their doctors, they’re not going to do what they say. They’re not going to get their prescriptions filled. They’re not going to be compliant. They’re not going to try to lose weight or control their diabetes.
It also goes the other way. The doctor or the nurse has to trust the patient. They have to believe that they’re safe. They have to believe that the patient is capable of controlling themselves. They have to believe that the patient is capable of listening and hearing what they’re saying, and that they’re competent to follow up on instructions, including to come back if that’s what’s required.
Everybody has to feel secure in the environment in which they’re working. Security, sadly, has to be a priority if we’re going to have a health care workforce that really feels safe and comfortable dealing with a patient population that is increasingly aggressive and perhaps not as trustworthy.
That’s not news I like to read when my colleagues write it up, but it’s important and we have to take it seriously.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University.
I want to talk about a paper that my colleagues in my division just published in Health Affairs.
As they pointed out, there’s a large amount of literature about what makes patients trust their doctor. There are many studies that show that, although patients sometimes have become more critical of the medical profession, in general they still try to trust their individual physician. Nurses remain in fairly high esteem among those who are getting hospital care.
What isn’t studied, as this paper properly points out, is, what can the doctor and the nurse do to trust the patient? How can that be assessed? Isn’t that just as important as saying that patients have to trust their doctors to do and comply with what they’re told?
What if doctors are afraid of violence? What if doctors are fearful that they can’t trust patients to listen, pay attention, or do what they’re being told? What if they think that patients are coming in with all kinds of disinformation, false information, or things they pick up on the Internet, so that even though you try your best to get across accurate and complete information about what to do about infectious diseases, taking care of a kid with strep throat, or whatever it might be, you’re thinking, Can I trust this patient to do what it is that I want them to do?
One particular problem that’s causing distrust is that more and more patients are showing stress and dependence on drugs and alcohol. That doesn’t make them less trustworthy per se, but it means they can’t regulate their own behavior as well.
That obviously has to be something that the physician or the nurse is thinking about. Is this person going to be able to contain anger? Is this person going to be able to handle bad news? Is this person going to deal with me when I tell them that some of the things they believe to be true about what’s good for their health care are false?
I think we have to really start to push administrators and people in positions of power to teach doctors and nurses how to defuse situations and how to make people more comfortable when they come in and the doctor suspects that they might be under the influence, impaired, or angry because of things they’ve seen on social media, whatever those might be – including concerns about racism, bigotry, and bias, which some patients are bringing into the clinic and the hospital setting.
We need more training. We’ve got to address this as a serious issue. What can we do to defuse situations where the doctor or the nurse rightly thinks that they can’t control or they can’t trust what the patient is thinking or how the patient might behave?
It’s also the case that I think we need more backup and quick access to security so that people feel safe and comfortable in providing care. We have to make sure that if you need someone to restrain a patient or to get somebody out of a situation, that they can get there quickly and respond rapidly, and that they know what to do to deescalate a situation.
It’s sad to say, but security in today’s health care world has to be something that we really test and check – not because we’re worried, as many places are, about a shooter entering the premises, which is its own bit of concern – but I’m just talking about when the doctor or the nurse says that this patient might be acting up, could get violent, or is someone I can’t trust.
My coauthors are basically saying that it’s not a one-way street. Yes, we have to figure out ways to make sure that our patients can trust what we say. Trust is absolutely the lubricant that makes health care flow. If patients don’t trust their doctors, they’re not going to do what they say. They’re not going to get their prescriptions filled. They’re not going to be compliant. They’re not going to try to lose weight or control their diabetes.
It also goes the other way. The doctor or the nurse has to trust the patient. They have to believe that they’re safe. They have to believe that the patient is capable of controlling themselves. They have to believe that the patient is capable of listening and hearing what they’re saying, and that they’re competent to follow up on instructions, including to come back if that’s what’s required.
Everybody has to feel secure in the environment in which they’re working. Security, sadly, has to be a priority if we’re going to have a health care workforce that really feels safe and comfortable dealing with a patient population that is increasingly aggressive and perhaps not as trustworthy.
That’s not news I like to read when my colleagues write it up, but it’s important and we have to take it seriously.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Art Caplan. I’m at the division of medical ethics at New York University.
I want to talk about a paper that my colleagues in my division just published in Health Affairs.
As they pointed out, there’s a large amount of literature about what makes patients trust their doctor. There are many studies that show that, although patients sometimes have become more critical of the medical profession, in general they still try to trust their individual physician. Nurses remain in fairly high esteem among those who are getting hospital care.
What isn’t studied, as this paper properly points out, is, what can the doctor and the nurse do to trust the patient? How can that be assessed? Isn’t that just as important as saying that patients have to trust their doctors to do and comply with what they’re told?
What if doctors are afraid of violence? What if doctors are fearful that they can’t trust patients to listen, pay attention, or do what they’re being told? What if they think that patients are coming in with all kinds of disinformation, false information, or things they pick up on the Internet, so that even though you try your best to get across accurate and complete information about what to do about infectious diseases, taking care of a kid with strep throat, or whatever it might be, you’re thinking, Can I trust this patient to do what it is that I want them to do?
One particular problem that’s causing distrust is that more and more patients are showing stress and dependence on drugs and alcohol. That doesn’t make them less trustworthy per se, but it means they can’t regulate their own behavior as well.
That obviously has to be something that the physician or the nurse is thinking about. Is this person going to be able to contain anger? Is this person going to be able to handle bad news? Is this person going to deal with me when I tell them that some of the things they believe to be true about what’s good for their health care are false?
I think we have to really start to push administrators and people in positions of power to teach doctors and nurses how to defuse situations and how to make people more comfortable when they come in and the doctor suspects that they might be under the influence, impaired, or angry because of things they’ve seen on social media, whatever those might be – including concerns about racism, bigotry, and bias, which some patients are bringing into the clinic and the hospital setting.
We need more training. We’ve got to address this as a serious issue. What can we do to defuse situations where the doctor or the nurse rightly thinks that they can’t control or they can’t trust what the patient is thinking or how the patient might behave?
It’s also the case that I think we need more backup and quick access to security so that people feel safe and comfortable in providing care. We have to make sure that if you need someone to restrain a patient or to get somebody out of a situation, that they can get there quickly and respond rapidly, and that they know what to do to deescalate a situation.
It’s sad to say, but security in today’s health care world has to be something that we really test and check – not because we’re worried, as many places are, about a shooter entering the premises, which is its own bit of concern – but I’m just talking about when the doctor or the nurse says that this patient might be acting up, could get violent, or is someone I can’t trust.
My coauthors are basically saying that it’s not a one-way street. Yes, we have to figure out ways to make sure that our patients can trust what we say. Trust is absolutely the lubricant that makes health care flow. If patients don’t trust their doctors, they’re not going to do what they say. They’re not going to get their prescriptions filled. They’re not going to be compliant. They’re not going to try to lose weight or control their diabetes.
It also goes the other way. The doctor or the nurse has to trust the patient. They have to believe that they’re safe. They have to believe that the patient is capable of controlling themselves. They have to believe that the patient is capable of listening and hearing what they’re saying, and that they’re competent to follow up on instructions, including to come back if that’s what’s required.
Everybody has to feel secure in the environment in which they’re working. Security, sadly, has to be a priority if we’re going to have a health care workforce that really feels safe and comfortable dealing with a patient population that is increasingly aggressive and perhaps not as trustworthy.
That’s not news I like to read when my colleagues write it up, but it’s important and we have to take it seriously.
Dr. Caplan disclosed that he has served as a director, officer, partner, employee, adviser, consultant, or trustee for Johnson & Johnson’s Panel for Compassionate Drug Use (unpaid position), and is a contributing author and adviser for Medscape. A version of this article first appeared on Medscape.com.



