User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


When you see something ...
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Over the last several decades science has fallen off this country’s radar screen. Yes, STEM (science, technology, engineering, and mathematics) has recently had a brief moment in the spotlight as a buzzword de jour. But the critical importance of careful and systematic investigation into the world around us using observation and trial and error is a tough sell to a large segment of our population.
The COVID-19 pandemic is providing an excellent opportunity for science and medicine to showcase their star qualities. Of course some people in leadership positions persist in disregarding the value of scientific investigation. But I get the feeling that the fear generated by the pandemic is creating some converts among many previous science skeptics. This gathering enthusiasm among the general population is a predictably slow process because that’s the way science works. It often doesn’t provide quick answers. And it is difficult for the nonscientist to see the beauty in the reality that the things we thought were true 2 months ago are likely to be proven wrong today as more observations accumulate.
A recent New York Times article examines the career of one such unscrupulous physician/scientist whose recent exploits threaten to undo much of the positive image the pandemic has cast on science (“The Doctor Behind the Disputed Covid Data,” by Ellen Gabler and Roni Caryn Rabin, The New York Times, July 27, 2020). The subject of the article is the physician who was responsible for providing some of the large data sets on which several papers were published about the apparent ineffectiveness and danger of using hydroxychloroquine in COVID-19 patients. The authenticity of the data sets recently has been seriously questioned, and the articles have been retracted by the journals in which they had appeared.
Based on numerous interviews with coworkers, the Times reporters present a strong case that this individual’s long history of unreliability make his association with allegedly fraudulent data set not surprising but maybe even predictable. At one point in his training, there appears to have been serious questions about advancing the physician to the next level. Despite these concerns, he was allowed to continue and complete his specialty training. It is of note that in his last year of clinical practice, the physician became the subject of three serious malpractice claims that question his competence.
I suspect that some of you have crossed paths with physicians whose competence and/or moral character you found concerning. Were they peers? Were you the individual’s supervisor or was he or she your mentor? How did you respond? Did anyone respond at all?
There has been a lot written and said in recent months about how and when to respond to respond to sexual harassment in the workplace. But I don’t recall reading any articles that discuss how one should respond to incompetence. Of course competency can be a relative term, but in most cases significant incompetence is hard to miss because it tends to be repeated.
It is easy for the airports and subway systems to post signs that say “If you see something say something.” It’s a different story for hospitals and medical schools that may have systems in place for reporting and following up on poor practice. But my sense is that there are too many cases that slip through the cracks.
This is another example of a problem for which I don’t have a solution. However, if this column prompts just one of you who sees something to say something then I have had a good day.
Dr. Wilkoff practiced primary care pediatrics in Brunswick, Maine, for nearly 40 years. He has authored several books on behavioral pediatrics, including “How to Say No to Your Toddler.” Email him at [email protected].
Septicemia first among hospital inpatient costs
according to a recent analysis from the Agency for Healthcare Research and Quality.
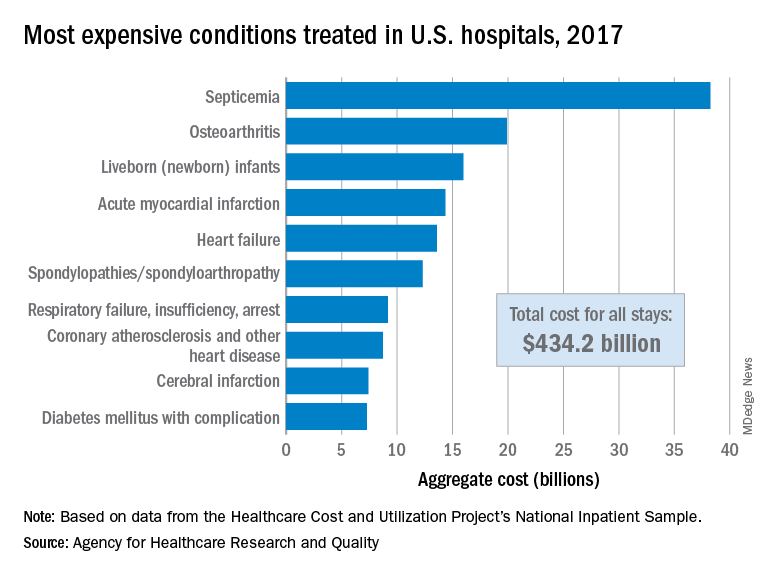
The single most expensive inpatient condition that year, representing about 8.8% of all hospital costs, was septicemia at $38.2 billion, nearly double the $19.9 billion spent on the next most expensive condition, osteoarthritis, Lan Liang, PhD, of the AHRQ, and associates said in a statistical brief.
These figures “represent the hospital’s costs to produce the services – not the amount paid for services by payers – and they do not include separately billed physician fees associated with the hospitalization,” they noted.
Third in overall cost for 2017 but first in total number of stays were live-born infants, with 3.7 million admissions costing just under $16 billion. Hospital costs for acute myocardial infarction ($14.3 billion) made it the fourth most expensive condition, with heart failure fifth at $13.6 billion, based on data from the Healthcare Cost and Utilization Project’s National Inpatient Sample.
The 20 most expensive conditions, which also included coronary atherosclerosis, pneumonia, renal failure, and lower-limb fracture, accounted for close to 47% of all hospital costs and over 43% of all stays in 2017. The total amount spent by hospitals that year, $1.1 trillion, constituted nearly a third of all health care expenditures and was 4.7% higher than in 2016, Dr. Liang and associates reported.
“Although this growth represented deceleration, compared with the 5.8% increase between 2014 and 2015, the consistent year-to-year rise in hospital-related expenses remains a central concern among policymakers,” they wrote.
according to a recent analysis from the Agency for Healthcare Research and Quality.

The single most expensive inpatient condition that year, representing about 8.8% of all hospital costs, was septicemia at $38.2 billion, nearly double the $19.9 billion spent on the next most expensive condition, osteoarthritis, Lan Liang, PhD, of the AHRQ, and associates said in a statistical brief.
These figures “represent the hospital’s costs to produce the services – not the amount paid for services by payers – and they do not include separately billed physician fees associated with the hospitalization,” they noted.
Third in overall cost for 2017 but first in total number of stays were live-born infants, with 3.7 million admissions costing just under $16 billion. Hospital costs for acute myocardial infarction ($14.3 billion) made it the fourth most expensive condition, with heart failure fifth at $13.6 billion, based on data from the Healthcare Cost and Utilization Project’s National Inpatient Sample.
The 20 most expensive conditions, which also included coronary atherosclerosis, pneumonia, renal failure, and lower-limb fracture, accounted for close to 47% of all hospital costs and over 43% of all stays in 2017. The total amount spent by hospitals that year, $1.1 trillion, constituted nearly a third of all health care expenditures and was 4.7% higher than in 2016, Dr. Liang and associates reported.
“Although this growth represented deceleration, compared with the 5.8% increase between 2014 and 2015, the consistent year-to-year rise in hospital-related expenses remains a central concern among policymakers,” they wrote.
according to a recent analysis from the Agency for Healthcare Research and Quality.

The single most expensive inpatient condition that year, representing about 8.8% of all hospital costs, was septicemia at $38.2 billion, nearly double the $19.9 billion spent on the next most expensive condition, osteoarthritis, Lan Liang, PhD, of the AHRQ, and associates said in a statistical brief.
These figures “represent the hospital’s costs to produce the services – not the amount paid for services by payers – and they do not include separately billed physician fees associated with the hospitalization,” they noted.
Third in overall cost for 2017 but first in total number of stays were live-born infants, with 3.7 million admissions costing just under $16 billion. Hospital costs for acute myocardial infarction ($14.3 billion) made it the fourth most expensive condition, with heart failure fifth at $13.6 billion, based on data from the Healthcare Cost and Utilization Project’s National Inpatient Sample.
The 20 most expensive conditions, which also included coronary atherosclerosis, pneumonia, renal failure, and lower-limb fracture, accounted for close to 47% of all hospital costs and over 43% of all stays in 2017. The total amount spent by hospitals that year, $1.1 trillion, constituted nearly a third of all health care expenditures and was 4.7% higher than in 2016, Dr. Liang and associates reported.
“Although this growth represented deceleration, compared with the 5.8% increase between 2014 and 2015, the consistent year-to-year rise in hospital-related expenses remains a central concern among policymakers,” they wrote.
Order errors not reduced with limiting number of open records
Background: An estimated 600,000 patients in U.S. hospitals had an order placed in their record that was meant for another patient in 2016. The Office of the National Coordinator for Health Information Technology and the Joint Commission recommend that EHRs limit the number of open records to one at a time based on expert opinion only. There is wide variation in the number of open records allowed among EHRs across the United States currently.
Study design: Randomized clinical trial.
Setting: Large health system in New York.
Synopsis: There were 3,356 clinicians (inpatient, outpatient, ED) randomized in a 1:1 ratio into either a restricted group (one open record at a time) or an unrestricted group (up to four open records at a time). In this study, 12,140,298 orders, in 4,486,631 order sessions, were analyzed with the Wrong-Patient Retract-and-Reorder (RAR) measure to identify wrong-patient orders. The proportion of wrong-patient order sessions were 90.7 vs. 88.0 per 100,000 order sessions for the restricted versus unrestricted groups (odds ratio, 1.03; 95% confidence interval, 0.90-1.20). There were no statistically significant differences in wrong-patient order sessions between the restricted and unrestricted groups in any clinical setting examined (inpatient, outpatient, ED).
Despite the ability to have up to four open records at one time in the unrestricted group, 66% of the order sessions were completed with only one record open in that group. This limited the power of the study to detect a difference in risk of order errors between the restricted and unrestricted groups.
Bottom line: Limiting clinicians to only one open record did not reduce the proportion of wrong-patient orders, compared with allowing up to four open records concurrently.
Citation: Adelman JS et al. Effect of restriction of the number of concurrently open records in an electronic health record on wrong-patient order errors: A randomized clinical trial. JAMA. 2019;32(18):1780-7.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
Background: An estimated 600,000 patients in U.S. hospitals had an order placed in their record that was meant for another patient in 2016. The Office of the National Coordinator for Health Information Technology and the Joint Commission recommend that EHRs limit the number of open records to one at a time based on expert opinion only. There is wide variation in the number of open records allowed among EHRs across the United States currently.
Study design: Randomized clinical trial.
Setting: Large health system in New York.
Synopsis: There were 3,356 clinicians (inpatient, outpatient, ED) randomized in a 1:1 ratio into either a restricted group (one open record at a time) or an unrestricted group (up to four open records at a time). In this study, 12,140,298 orders, in 4,486,631 order sessions, were analyzed with the Wrong-Patient Retract-and-Reorder (RAR) measure to identify wrong-patient orders. The proportion of wrong-patient order sessions were 90.7 vs. 88.0 per 100,000 order sessions for the restricted versus unrestricted groups (odds ratio, 1.03; 95% confidence interval, 0.90-1.20). There were no statistically significant differences in wrong-patient order sessions between the restricted and unrestricted groups in any clinical setting examined (inpatient, outpatient, ED).
Despite the ability to have up to four open records at one time in the unrestricted group, 66% of the order sessions were completed with only one record open in that group. This limited the power of the study to detect a difference in risk of order errors between the restricted and unrestricted groups.
Bottom line: Limiting clinicians to only one open record did not reduce the proportion of wrong-patient orders, compared with allowing up to four open records concurrently.
Citation: Adelman JS et al. Effect of restriction of the number of concurrently open records in an electronic health record on wrong-patient order errors: A randomized clinical trial. JAMA. 2019;32(18):1780-7.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
Background: An estimated 600,000 patients in U.S. hospitals had an order placed in their record that was meant for another patient in 2016. The Office of the National Coordinator for Health Information Technology and the Joint Commission recommend that EHRs limit the number of open records to one at a time based on expert opinion only. There is wide variation in the number of open records allowed among EHRs across the United States currently.
Study design: Randomized clinical trial.
Setting: Large health system in New York.
Synopsis: There were 3,356 clinicians (inpatient, outpatient, ED) randomized in a 1:1 ratio into either a restricted group (one open record at a time) or an unrestricted group (up to four open records at a time). In this study, 12,140,298 orders, in 4,486,631 order sessions, were analyzed with the Wrong-Patient Retract-and-Reorder (RAR) measure to identify wrong-patient orders. The proportion of wrong-patient order sessions were 90.7 vs. 88.0 per 100,000 order sessions for the restricted versus unrestricted groups (odds ratio, 1.03; 95% confidence interval, 0.90-1.20). There were no statistically significant differences in wrong-patient order sessions between the restricted and unrestricted groups in any clinical setting examined (inpatient, outpatient, ED).
Despite the ability to have up to four open records at one time in the unrestricted group, 66% of the order sessions were completed with only one record open in that group. This limited the power of the study to detect a difference in risk of order errors between the restricted and unrestricted groups.
Bottom line: Limiting clinicians to only one open record did not reduce the proportion of wrong-patient orders, compared with allowing up to four open records concurrently.
Citation: Adelman JS et al. Effect of restriction of the number of concurrently open records in an electronic health record on wrong-patient order errors: A randomized clinical trial. JAMA. 2019;32(18):1780-7.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
All NSAIDs raise post-MI risk but some are safer than others: Next chapter
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Patients on antithrombotics after an acute MI will face a greater risk for bleeding and secondary cardiovascular (CV) events if they start taking any nonaspirin NSAID, confirms a large observational study.
Like other research before it, the new study suggests those risks will be much lower for some nonaspirin NSAIDs than others. But it may also challenge at least some conventional thinking about the safety of these drugs, and is based solely on a large cohort in South Korea, a group for which such NSAID data has been in short supply.
“It was intriguing that our study presented better safety profiles with celecoxib and meloxicam versus other subtypes of NSAIDs,” noted the report, published online July 27 in the Journal of the American College of Cardiology.
Most of the NSAIDs included in the analysis, “including naproxen, conferred a significantly higher risk for cardiovascular and bleeding events, compared with celecoxib and meloxicam,” wrote the authors, led by Dong Oh Kang, MD, Korea University Guro Hospital, Seoul, South Korea.
A main contribution of the study “is the thorough and comprehensive evaluation of the Korean population by use of the nationwide prescription claims database that reflects real-world clinical practice,” senior author Cheol Ung Choi, MD, PhD, of the same institution, said in an interview.
“Because we included the largest number of patients of any comparable clinical studies on NSAID treatment after MI thus far, our study may allow the generalizability of the adverse events of NSAIDs to all patients by constituting global evidence encompassing different population groups,” Dr. Choi said.
The analysis has limitations along with its strengths, the authors acknowledged, including its observational design and potential for confounding not addressed in statistical adjustments.
Observers of the study concurred, but some cited evidence pointing to such confounding that is serious enough to question the entire study’s validity.
Among the cohort of more than 100,000 patients followed for an average of about 2.3 years after their MI, the adjusted risk of thromboembolic CV events went up almost 7 times for those who took any NSAID for at least 4 consecutive weeks, compared with those who didn’t take NSAIDs, based on prescription records.
Their adjusted risk of bleeding events – which included gastrointestinal, intracranial, respiratory, or urinary tract bleeding or posthemorrhagic anemia, the group writes – was increased 300%.
There was wide variance in the adjusted hazard ratios for outcomes by type of NSAID. The risk of CV events climbed from a low of about 3 with meloxicam and almost 5 for celecoxib to more than 10 and 12 for naproxen and dexibuprofen, respectively.
The hazard ratios for bleeding ranged from about 3 for both meloxicam and celecoxib to more than 6 for naproxen.
Of note, celecoxib and meloxicam both preferentially target the cyclooxygenase type 2 (COX-2) pathway, and naproxen among NSAIDs once had a reputation for relative cardiac safety, although subsequent studies have challenged that notion.
“On the basis of the contemporary guidelines, NSAID treatment should be limited as much as possible after MI; however, our data suggest that celecoxib and meloxicam could be considered possible alternative choices in patients with MI when NSAID prescription is unavoidable,” the group wrote.
They acknowledged some limitations of the analysis, including an observational design and the possibility of unidentified confounders; that mortality outcomes were not available from the National Health Insurance Service database used in the study; and that the 2009-2013 span for the data didn’t allow consideration of more contemporary antiplatelet agents and direct oral anticoagulants.
Also, NSAID use was based on prescriptions without regard to over-the-counter usage. Although use of over-the-counter NSAIDs is common in Korea, “most MI patients in Korea are prescribed most medications, including NSAIDs, in the hospital. So I think that usage of over-the-counter NSAIDs did not change the results,” Dr. Choi said.
“This study breaks new ground by demonstrating cardiovascular safety of meloxicam (and not only of celecoxib), probably because of its higher COX-2 selectivity,” wrote the authors of an accompanying editorial, Juan J. Badimon, PhD, and Carlos G. Santos-Gallego, MD, both of the Icahn School of Medicine at Mount Sinai, New York.
Notably, “this paper rejects the cardiovascular safety of naproxen, which had been suggested classically and in the previous Danish data, but that was not evident in this study.” The finding is consistent with the PRECISION trial, in which both bleeding and CV risk were increased with naproxen versus other NSAIDs, observed Dr. Badimon and Dr. Santos-Gallego.
They agreed with the authors in recommending that, “although NSAID treatment should be avoided in patients with MI, if the use of NSAIDs is inevitable due to comorbidities, the prescription of celecoxib and meloxicam could be considered as alternative options.”
But, “as no study is perfect, this article also presents some limitations,” the editorial agreed, citing some of the same issues noted by Dr. Kang and associates, along with potential confounding by indication and the lack of “clinical information to adjust (e.g., angiographic features, left ventricular function).”
“There’s undoubtedly residual confounding,” James M. Brophy, MD, PhD, a pharmacoepidemiologist at McGill University, Montreal, said in an interview.
The 400%-900% relative risks for CV events “are just too far in left field, compared to everything else we know,” he said. “There has never been a class of drugs that have shown this sort of magnitude of effect for adverse events.”
Even in PRECISION with its more than 24,000 high-coronary-risk patients randomized and followed for 5 years, Dr. Brophy observed, relative risks for the different NSAIDs varied by an order of magnitude of only 1-2.
“You should be interpreting things in the context of what is already known,” Dr. Brophy said. “The only conclusion I would draw is the paper is fatally flawed.”
The registry included 108,232 primarily male patients followed from their first diagnosed MI for CV and bleeding events. About 1.9% were prescribed at least one NSAID for 4 or more consecutive weeks during the follow-up period averaging 2.3 years, the group reported.
The most frequently prescribed NSAID was diclofenac, at about 72% of prescribed NSAIDs in the analysis for CV events and about 69% in the bleeding-event analysis.
Adding any NSAID to post-MI antithrombotic therapy led to an adjusted HR of 6.96 (P < .001) for CV events and 4.08 (P < .001) for bleeding events, compared with no NSAID treatment.
The 88% of the cohort who were on dual-antiplatelet therapy with aspirin and clopidogrel showed very nearly the same risk increases for both endpoints.
Further studies are needed to confirm the results “and ensure their generalizability to other populations,” Dr. Choi said. They should be validated especially using the claims data bases of countries near Korea, “such as Japan and Taiwan, to examine the reproducibility of the results in similar ethnic populations.”
That the study focused on a cohort in Korea is a strength, contended the authors as well as Dr. Badimon and Dr. Santos-Gallego, given “that most data about NSAIDs were extracted from Western populations, but the risk of thrombosis/bleeding post-MI varies according to ethnicity,” according to the editorial
Dr. Brophy agreed, but doubted that ethnic differences are responsible for variation in relative risks between the current results and other studies. “There are pharmacogenomic differences between different ethnicities as to how they activate these drugs. But I suspect that sort of difference is really minor. Maybe it leads to a 2% or a 5% difference in risks.”
Dr. Kang and associates, Dr. Badimon, Dr. Santos-Gallego, and Dr. Brophy disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Value of palliative care shines clearly in a crisis
Hospitalists have played a key role
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: [email protected].
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
Hospitalists have played a key role
Hospitalists have played a key role
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: [email protected].
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
For some palliative care professionals, the COVID-19 pandemic, particularly in viral hot spots like New York City, represents a “moment” that could lead to greater awareness of what this service offers to seriously ill patients in a crisis.
They say it has provided an opportunity to show what palliative care teams can contribute to the difficult circumstances of patients with severe symptoms, isolated and alone in quarantined hospitals, with poor survival rates, perhaps sedated for extended stays on scarce ventilators – and for their family members, who are able to visit them only virtually via telephone or tablet.
But it has also highlighted gaps – including insufficient staffing for some palliative care teams. Hospitalists and other clinicians in the hospital need to learn the basics of primary palliative care, such as how to communicate bad news, initiate goals of care conversations, and address common symptoms of serious illness, such as pain. That way, they could shoulder more of the demand for this kind of care when palliative care specialists are in short supply.
Hospitalists, some of whom also have pursued a specialization in palliative care, have played key roles in clarifying and redefining the new role for palliative care, whom it is meant for, and who should provide it. Central to this new role is the greater use of telemedicine – for talking to hospitalized patients without increasing viral exposure, for linking up with family members who can’t visit their loved ones in the hospital, and for helping frontline hospital staff who need a palliative care consultation – or just a chance to debrief on what they are seeing.
A pandemic wake-up call
Elizabeth Gundersen, MD, FHM, FAAHPM, director of the hospice and palliative medicine fellowship program at the Charles E. Schmidt College of Medicine at Florida Atlantic University (FAU) in Boca Raton, practiced hospital medicine for 10 years before pursuing a fellowship in hospice and palliative medicine and working as an academic palliative medicine physician. She calls the pandemic a wake-up call for gaps in care and all the things that weren’t working well in the health care system.
“Now we are seeing more clearly what’s lacking – or broken – and what we will carry forward from this experience into the post-COVID world,” she said. Some hospitalists do palliative care very well, and others don’t feel as comfortable in having these difficult conversations with patients. But in the uncertain course of the virus they get thrust into it.
Although FAU’s associated hospitals were not as inundated with COVID-19 patients in the early weeks of the pandemic as were other regions, the volume of other patients plummeted, Dr. Gundersen said, adding that “there’s still been incredible intensity and worry about the virus. For me, the basic role of palliative care hasn’t changed, and the phrase I have always used when introducing myself – ‘we’re an extra layer of support for the patient and family’ – still holds true,” she said.
“I try to make it clear to people that palliative care is not synonymous with end-of-life care. We don’t want people to think that a palliative care referral implies imminent death. The goal is not to get more people to have a do not attempt resuscitation (DNAR) order, but to determine the patient and family’s treatment goals and whether a DNAR order fits those goals.”
The tough conversations
Dr. Gundersen is cochair of SHM’s Palliative Care Special Interest Group, along with Rab Razzak, MD, clinical director of palliative medicine at University Hospitals Cleveland Medical Center, one of the hospitals affiliated with Case Western University in Cleveland. (Connect with them on Twitter: @Top_Gundersen and @rabrazzak.)
Dr. Razzak also transitioned from hospital medicine to palliative medicine 10 years ago. “As a hospitalist, I enjoyed the tough conversations and bringing the human element into my health care interactions,” he explained. “To me, palliative care is a philosophy of care that puts the person we call the patient at the center of the interaction, while we try to figure out how to best care for them as a person.”
When the pandemic hit, University Hospitals made 20 ICU beds available for COVID-19 patients, Dr. Razzak said. This unit has since been full but not overflowing, while overall hospital census went down. The palliative care team at the hospital includes four inpatient doctors, nurse practitioners, and a chaplain, as well as an outpatient team primarily focused on oncology.
“In some settings, palliative care has been at the forefront of difficult conversations, when things aren’t going well for the patient and there’s much uncertainty,” Dr. Razzak said. The interface between hospital medicine and palliative care can be complementary, he added. “We talk about primary palliative care, which we want every discipline to be able to do – lead meaningful conversations, help manage symptoms.”
The take-home message for hospitalists, he said, is to get training in how to have these discussions, using such resources as VitalTalk (https://www.vitaltalk.org/), a nonprofit organization that disseminates education in communication skills for difficult conversations, and the Center to Advance Palliative Care (www.capc.org) at Icahn School of Medicine at Mount Sinai in New York City. “Once you’ve mastered the conversation, it will get easier. But ask for help when you need it, and learn how to know when you need it.”
Dr. Gundersen added that hospital medicine groups and palliative care teams could reach out to each other and talk about what they did in the crisis and how they can work together in the future. She recommends frequent ongoing support and collaboration that could range from formal conferences or training sessions to informal team interactions, perhaps with sandwiches in the doctor’s lounge – provided that there’s room for social distancing. She has recently started giving talks in the community and grand rounds presentations in hospitals about palliative care.
Other approaches and applications
In New York City, the initial epicenter for the pandemic in the United States, the adult palliative care service of Columbia University Medical Center (CUMC) experienced a sevenfold increase in consultation requests at the apex of the crisis, said its director, Craig Blinderman, MD. That demand was impossible to meet with existing staff. So Dr. Blinderman and colleagues established a virtual consultation model, recruiting and deploying volunteer out-of-state palliative care specialists to staff it.
An eight-bed palliative care unit was opened at CUMC for COVID-19 patients whose surrogates had opted not to initiate or continue intubation or life-sustaining treatments. This helped to relieve some of the pressures on the ICUs while making it possible for in-person visits to the hospice unit by families – in full PPE. Palliative care staff were embedded in various units in the hospital.
A palliative care response team composed of a hospice and palliative medicine fellow and four psychiatry residents or fellows, based in the emergency department and with supervision from the palliative care team, provided time-critical goals of care conversations with families using telemedicine – and a forum for listening to their suffering. Dr. Blinderman and colleagues also have found time to write up their experience for medical journals.1,2
There’s no reason to think that hospitalists, with a little basic training, couldn’t be having these same goals of care conversations, Dr. Blinderman said. “But the fact that hospitalists, at the pandemic’s peak, along with ICU doctors, were seeing an unprecedented magnitude of dying on a daily basis generated a lot of moral distress for them.”
Palliative care professionals, because they engage with these issues in a different way, may be somewhat better equipped to deal with the sheer emotional demands when so many are dying, as at the peak of the surge in New York. “We don’t see dying as a failure on our part but an opportunity to relieve suffering,” Dr. Blinderman said. And the palliative care field also emphasizes the importance of self-care for its practitioners.
“How do we meet the incredible palliative care needs in the epicenter of a pandemic? That question also applies to other kinds of crises we could imagine, for example, climate-related disasters,” Dr. Blinderman said. “What lessons have we learned about the value of palliative care and how to start incorporating it more integrally into the delivery of hospital care? Here we showed that we could work collaboratively with our colleagues at other major medical centers, bringing together their expertise to help us when we didn’t have the bandwidth to meet the demand,” he said.
Scripts can help
“Also, it won’t make sense to just go back to normal (after the crisis fades),” Dr. Blinderman said. “We need to take a close look at how our society is functioning in the wake of the pandemic and the ways the health care system has failed us. We have learned that we’re all interconnected and we need to work together to serve our communities – locally and nationally – applying basic distributive justice.”
Could there be, for example, a national infrastructure for mobilizing and deploying palliative care resources to areas of greatest need, similar to what was done in New York?
At Northwestern Medicine in Chicago, a number of palliative care clinicians at the system’s hospitals worked together to develop scripts designed to help other clinicians start goals of care conversations with patients and families, for use in the hospital as well as in outpatient primary care and other settings, with results integrated into the system’s electronic health record.
Front-line clinicians may not have the time to ask for formal consults from palliative care because of high volume and rapidly changing patient status, explained Eytan Szmuilowicz, MD, director of the section of palliative medicine at Northwestern Memorial Hospital. Or they may not have access to specialty-level palliative care in their settings.
The scripts are aimed at primary care, emergency physicians, and hospitalists needing to consider critical care placement or attempted resuscitation and to ICU clinicians helping families make decisions about life-sustaining treatments. They also can help facilitate advance care planning discussions. An example is “CALMER,” a six-step mnemonic guide to promote goals of care discussions with hospitalized patients. For more information on these scripts, contact Dr. Szmuilowicz: [email protected].
Eerily quiet
The COVID-19 crisis has been quite a whirlwind for hospital medicine, said Jeanie Youngwerth, MD, a hospitalist and program director of the palliative care service at the University of Colorado in Denver, which was a significant viral hotspot early on.
“When it first started, things seemed to change almost overnight – starting on Friday, March 13. People had to take action right away to develop work flows and the technology to allow us to see as many patients as possible,” she said. By the time Monday came, it was a whole new ballgame.
Dr. Youngwerth and two colleagues worked quickly to develop inpatient telemedicine capacity where none existed. “We knew we would not be going into patients’ rooms, but most of our team showed up in the hospital to work with the primary care teams. Our job was to see what we could do that actually made a difference,” she said.
“The hospital became a very strange place. You’d walk down the hallway and it was eerily quiet. Everybody you came across was being so nice to each other.” Televisits became a powerful way to bring the human connection back to medical care.
“What we learned from families was that they were thirsting to have some kind of connection with their loved one, and to be able to talk about their loved one and who they were as a person,” she said. “We’d contact the family through video visits and then, when the family meeting ended, the nurse would bring an iPad into the patient’s room so the family could see their loved one on a ventilator. They would immediately start communicating with their loved one, praying aloud, singing, playing music. It would make a huge difference for the family – and for the staff.”
References
1. Nakagawa S et al. Pandemic palliative care consultations spanning state and institutional borders. J Am Geriatr Soc. 2020 May 22. doi: 10.1111/jgs.16643.
2. Lee J Abrukin L, Flores S. Early intervention of palliative care in the emergency department during the COVID-19 pandemic. JAMA Intern Med. 2020 Jun 5. doi: 10.1001/jamainternmed.2020.2713.
ACC panel defines, advises on heart failure with ‘recovered’ EF
Because heart failure patients with recovered ejection fraction are a complex and diverse group, little consensus has emerged on how to define, diagnose, and manage this growing population.
To provide some clarity for identifying and treating these patients, a Journal of the American College of Cardiology scientific expert panel has issued a consensus document. Published Aug. 3 in the Journal of the American College of Cardiology, it provides a working definition of heart failure with recovered ejection fraction (HFrecEF) and recommends approaches for treatment and follow-up.
Defining a new class of HF
“Part of the impetus of this was to bring attention to what we think is a new class of heart failure, and it requires different treatment modalities and different ways of thinking about it,” expert panel member Douglas L. Mann, MD, cardiologist-in-chief at Barnes Jewish Hospital in St. Louis, said in an interview. “It’s a newly discovered HF biology about which we know very little and it’s very confusing to just go on the ejection fraction alone.”
The panel, led by Jane E. Wilcox, MD, of Northwestern University, Chicago, recommends three components for a working definition of HFrecEF: 1) documentation of a decreased left ventricle ejection fraction (LVEF) of less than 40% at baseline; 2) a 10% or greater absolute improvement in LVEF; and 3) a second measurement of LVEF >40%.
“We try to give it a nomenclature that clearly indicates what it is,” Dr. Mann said. “There has been a lot of confusing terminology.” Among the terms the panel calls out in the lexicon of modestly recovered EF, in addition to HFrecEF, are HF improved EF, HF with preserved EF (HFpEF), borderline HFpEF and HF with mid-range EF (HFmrEF).
The panel also recommends that guideline-based medical and device therapies for HFrecEF should continue indefinitely until there’s a better understanding of the biology and clinical epidemiology of HFrecEF, and that these patients should have close clinical follow-up because of the high risk of HF relapse.
Determining EF’s ‘trajectory’
The findings presented in the statement should help cardiologists distinguish HFrecEF from HFpEF, Dr. Mann noted. “Because EF is moving, we also have to emphasize the importance of following the trajectory of EF,” he said. “It’s not enough to know where the EF is; you have to know where it came from – if it had been from a higher number or a lower number – because that will help inform you about the patient’s disease.”
In that regard, the panel states that the level of change in LVEF – the “trajectory” – will provide clues to the nature and extent of myocardial injury, degree and duration of LV remodeling, and the type of therapy that’s indicated. Clinicians should consider HFmrEF, a description the European Society of Cardiology has endorsed, as an entity different from HFrecEF without data on LVEF trajectory.
The statement delves into the biology of HFrecEF, defining reverse LV remodeling as the restoration of some normalization to cardiac myocyte size and LV chamber geometry that results in a leftward shift toward normalization of end-diastolic pressure volume. The panel also noted that cardiac remodeling in reverse LV remodeling and recovery of LV function is bidirectional and involves multiple molecular and cellular changes that contribute to changes in the heart’s size, shape and function, and explains the role gene expression has in HF-related LV changes.
The statement explores the recovery of LV function, noting that spontaneous recovery often occurs when the cause of the myocardial dysfunction resolves. Common causes are chronic tachycardia and thyroid disease.
That panel noted that “super responders” to cardiac resynchronization therapy can provide insight into HFrecEF. Favorable responders include women, patients with nonischemic HF, very wide ECG ventricular depolarization wavelength with left-bundle branch block morphology, and dyssynchrony on ECG.
The panel states that, “regardless of the definition of HFrecEF,” the evidence suggests that younger patients, women, and those with nonischemic disease, shorter disease duration, and relatively few comorbidities are more likely to recover LVEF – and their outcomes are typically better than those of patients with HF reduced EF (HFrEF) and HFpEF.
Clinicians should bear in mind, however, that patients on guideline-directed medical therapy (GDMT) who achieve complete normalization of LV structure and function are prone to recurrent LV dysfunction and HF. The panel explored the role of potential treatment for three different etiologies of HF. Little is known about Takotsubo cardiomyopathy, considered a transient form of LV dysfunction, in terms of how many of these patients will develop HFrEF or if they’ll benefit from GDMT. Alcohol-induced cardiomyopathy patients should continue on medical therapy even if they have HFrecEF, as should patients with fulminant and nonfulminant myocarditis.
Managing HFrecEF
Management should include assessment of jugular vein distention and signs of volume overload – “particularly concerning in HFrecEF” – the panel noted. ECG is cost effective, and signs of left-bundle branch block are predictors of low success with GDMT alone. The panel also recommended a family history going back three generations and consideration of genetic testing to determine the risk for sudden cardiac death. Two-dimensional ECG can help predict GDMT response and cardiovascular magnetic resonance imaging can provide information about myocardial substrate at the time of diagnosis of HFrEF.
The panel suggested four areas for future research: 1) improved phenotyping of HFrEF; 2) use of inception cohorts to better understand the natural history of HFrecEF; 3) clinical trials to better define those clinical care components most effective at maintaining remission; and 4) basic studies to better define the biology of HFrecEF. “The goal,” wrote Dr. Wilcox and colleagues, “is to develop new therapeutic targets that will enable patients with HFrecEF to experience a durable remission from HF.”
Dr. Wilcox reported receiving funding from the National Institutes of Health and the American Heart Association, and financial relationships with Abbott, Medtronic, and Cytokinetics. Dr. Mann has received funding from NIH and reports financial relationships with MyoKardia and Novartis. Coauthors reported funding from NIH and AHA and financial relationships with Novartis, Amgen, AstraZeneca, Thoratec Corporation (Abbott), Sanofi, Pfizer, MyoKardia and American Regent.
SOURCE: Wilcox JE et al. J Am Coll Cardiol. 2020;76:719-34.
Because heart failure patients with recovered ejection fraction are a complex and diverse group, little consensus has emerged on how to define, diagnose, and manage this growing population.
To provide some clarity for identifying and treating these patients, a Journal of the American College of Cardiology scientific expert panel has issued a consensus document. Published Aug. 3 in the Journal of the American College of Cardiology, it provides a working definition of heart failure with recovered ejection fraction (HFrecEF) and recommends approaches for treatment and follow-up.
Defining a new class of HF
“Part of the impetus of this was to bring attention to what we think is a new class of heart failure, and it requires different treatment modalities and different ways of thinking about it,” expert panel member Douglas L. Mann, MD, cardiologist-in-chief at Barnes Jewish Hospital in St. Louis, said in an interview. “It’s a newly discovered HF biology about which we know very little and it’s very confusing to just go on the ejection fraction alone.”
The panel, led by Jane E. Wilcox, MD, of Northwestern University, Chicago, recommends three components for a working definition of HFrecEF: 1) documentation of a decreased left ventricle ejection fraction (LVEF) of less than 40% at baseline; 2) a 10% or greater absolute improvement in LVEF; and 3) a second measurement of LVEF >40%.
“We try to give it a nomenclature that clearly indicates what it is,” Dr. Mann said. “There has been a lot of confusing terminology.” Among the terms the panel calls out in the lexicon of modestly recovered EF, in addition to HFrecEF, are HF improved EF, HF with preserved EF (HFpEF), borderline HFpEF and HF with mid-range EF (HFmrEF).
The panel also recommends that guideline-based medical and device therapies for HFrecEF should continue indefinitely until there’s a better understanding of the biology and clinical epidemiology of HFrecEF, and that these patients should have close clinical follow-up because of the high risk of HF relapse.
Determining EF’s ‘trajectory’
The findings presented in the statement should help cardiologists distinguish HFrecEF from HFpEF, Dr. Mann noted. “Because EF is moving, we also have to emphasize the importance of following the trajectory of EF,” he said. “It’s not enough to know where the EF is; you have to know where it came from – if it had been from a higher number or a lower number – because that will help inform you about the patient’s disease.”
In that regard, the panel states that the level of change in LVEF – the “trajectory” – will provide clues to the nature and extent of myocardial injury, degree and duration of LV remodeling, and the type of therapy that’s indicated. Clinicians should consider HFmrEF, a description the European Society of Cardiology has endorsed, as an entity different from HFrecEF without data on LVEF trajectory.
The statement delves into the biology of HFrecEF, defining reverse LV remodeling as the restoration of some normalization to cardiac myocyte size and LV chamber geometry that results in a leftward shift toward normalization of end-diastolic pressure volume. The panel also noted that cardiac remodeling in reverse LV remodeling and recovery of LV function is bidirectional and involves multiple molecular and cellular changes that contribute to changes in the heart’s size, shape and function, and explains the role gene expression has in HF-related LV changes.
The statement explores the recovery of LV function, noting that spontaneous recovery often occurs when the cause of the myocardial dysfunction resolves. Common causes are chronic tachycardia and thyroid disease.
That panel noted that “super responders” to cardiac resynchronization therapy can provide insight into HFrecEF. Favorable responders include women, patients with nonischemic HF, very wide ECG ventricular depolarization wavelength with left-bundle branch block morphology, and dyssynchrony on ECG.
The panel states that, “regardless of the definition of HFrecEF,” the evidence suggests that younger patients, women, and those with nonischemic disease, shorter disease duration, and relatively few comorbidities are more likely to recover LVEF – and their outcomes are typically better than those of patients with HF reduced EF (HFrEF) and HFpEF.
Clinicians should bear in mind, however, that patients on guideline-directed medical therapy (GDMT) who achieve complete normalization of LV structure and function are prone to recurrent LV dysfunction and HF. The panel explored the role of potential treatment for three different etiologies of HF. Little is known about Takotsubo cardiomyopathy, considered a transient form of LV dysfunction, in terms of how many of these patients will develop HFrEF or if they’ll benefit from GDMT. Alcohol-induced cardiomyopathy patients should continue on medical therapy even if they have HFrecEF, as should patients with fulminant and nonfulminant myocarditis.
Managing HFrecEF
Management should include assessment of jugular vein distention and signs of volume overload – “particularly concerning in HFrecEF” – the panel noted. ECG is cost effective, and signs of left-bundle branch block are predictors of low success with GDMT alone. The panel also recommended a family history going back three generations and consideration of genetic testing to determine the risk for sudden cardiac death. Two-dimensional ECG can help predict GDMT response and cardiovascular magnetic resonance imaging can provide information about myocardial substrate at the time of diagnosis of HFrEF.
The panel suggested four areas for future research: 1) improved phenotyping of HFrEF; 2) use of inception cohorts to better understand the natural history of HFrecEF; 3) clinical trials to better define those clinical care components most effective at maintaining remission; and 4) basic studies to better define the biology of HFrecEF. “The goal,” wrote Dr. Wilcox and colleagues, “is to develop new therapeutic targets that will enable patients with HFrecEF to experience a durable remission from HF.”
Dr. Wilcox reported receiving funding from the National Institutes of Health and the American Heart Association, and financial relationships with Abbott, Medtronic, and Cytokinetics. Dr. Mann has received funding from NIH and reports financial relationships with MyoKardia and Novartis. Coauthors reported funding from NIH and AHA and financial relationships with Novartis, Amgen, AstraZeneca, Thoratec Corporation (Abbott), Sanofi, Pfizer, MyoKardia and American Regent.
SOURCE: Wilcox JE et al. J Am Coll Cardiol. 2020;76:719-34.
Because heart failure patients with recovered ejection fraction are a complex and diverse group, little consensus has emerged on how to define, diagnose, and manage this growing population.
To provide some clarity for identifying and treating these patients, a Journal of the American College of Cardiology scientific expert panel has issued a consensus document. Published Aug. 3 in the Journal of the American College of Cardiology, it provides a working definition of heart failure with recovered ejection fraction (HFrecEF) and recommends approaches for treatment and follow-up.
Defining a new class of HF
“Part of the impetus of this was to bring attention to what we think is a new class of heart failure, and it requires different treatment modalities and different ways of thinking about it,” expert panel member Douglas L. Mann, MD, cardiologist-in-chief at Barnes Jewish Hospital in St. Louis, said in an interview. “It’s a newly discovered HF biology about which we know very little and it’s very confusing to just go on the ejection fraction alone.”
The panel, led by Jane E. Wilcox, MD, of Northwestern University, Chicago, recommends three components for a working definition of HFrecEF: 1) documentation of a decreased left ventricle ejection fraction (LVEF) of less than 40% at baseline; 2) a 10% or greater absolute improvement in LVEF; and 3) a second measurement of LVEF >40%.
“We try to give it a nomenclature that clearly indicates what it is,” Dr. Mann said. “There has been a lot of confusing terminology.” Among the terms the panel calls out in the lexicon of modestly recovered EF, in addition to HFrecEF, are HF improved EF, HF with preserved EF (HFpEF), borderline HFpEF and HF with mid-range EF (HFmrEF).
The panel also recommends that guideline-based medical and device therapies for HFrecEF should continue indefinitely until there’s a better understanding of the biology and clinical epidemiology of HFrecEF, and that these patients should have close clinical follow-up because of the high risk of HF relapse.
Determining EF’s ‘trajectory’
The findings presented in the statement should help cardiologists distinguish HFrecEF from HFpEF, Dr. Mann noted. “Because EF is moving, we also have to emphasize the importance of following the trajectory of EF,” he said. “It’s not enough to know where the EF is; you have to know where it came from – if it had been from a higher number or a lower number – because that will help inform you about the patient’s disease.”
In that regard, the panel states that the level of change in LVEF – the “trajectory” – will provide clues to the nature and extent of myocardial injury, degree and duration of LV remodeling, and the type of therapy that’s indicated. Clinicians should consider HFmrEF, a description the European Society of Cardiology has endorsed, as an entity different from HFrecEF without data on LVEF trajectory.
The statement delves into the biology of HFrecEF, defining reverse LV remodeling as the restoration of some normalization to cardiac myocyte size and LV chamber geometry that results in a leftward shift toward normalization of end-diastolic pressure volume. The panel also noted that cardiac remodeling in reverse LV remodeling and recovery of LV function is bidirectional and involves multiple molecular and cellular changes that contribute to changes in the heart’s size, shape and function, and explains the role gene expression has in HF-related LV changes.
The statement explores the recovery of LV function, noting that spontaneous recovery often occurs when the cause of the myocardial dysfunction resolves. Common causes are chronic tachycardia and thyroid disease.
That panel noted that “super responders” to cardiac resynchronization therapy can provide insight into HFrecEF. Favorable responders include women, patients with nonischemic HF, very wide ECG ventricular depolarization wavelength with left-bundle branch block morphology, and dyssynchrony on ECG.
The panel states that, “regardless of the definition of HFrecEF,” the evidence suggests that younger patients, women, and those with nonischemic disease, shorter disease duration, and relatively few comorbidities are more likely to recover LVEF – and their outcomes are typically better than those of patients with HF reduced EF (HFrEF) and HFpEF.
Clinicians should bear in mind, however, that patients on guideline-directed medical therapy (GDMT) who achieve complete normalization of LV structure and function are prone to recurrent LV dysfunction and HF. The panel explored the role of potential treatment for three different etiologies of HF. Little is known about Takotsubo cardiomyopathy, considered a transient form of LV dysfunction, in terms of how many of these patients will develop HFrEF or if they’ll benefit from GDMT. Alcohol-induced cardiomyopathy patients should continue on medical therapy even if they have HFrecEF, as should patients with fulminant and nonfulminant myocarditis.
Managing HFrecEF
Management should include assessment of jugular vein distention and signs of volume overload – “particularly concerning in HFrecEF” – the panel noted. ECG is cost effective, and signs of left-bundle branch block are predictors of low success with GDMT alone. The panel also recommended a family history going back three generations and consideration of genetic testing to determine the risk for sudden cardiac death. Two-dimensional ECG can help predict GDMT response and cardiovascular magnetic resonance imaging can provide information about myocardial substrate at the time of diagnosis of HFrEF.
The panel suggested four areas for future research: 1) improved phenotyping of HFrEF; 2) use of inception cohorts to better understand the natural history of HFrecEF; 3) clinical trials to better define those clinical care components most effective at maintaining remission; and 4) basic studies to better define the biology of HFrecEF. “The goal,” wrote Dr. Wilcox and colleagues, “is to develop new therapeutic targets that will enable patients with HFrecEF to experience a durable remission from HF.”
Dr. Wilcox reported receiving funding from the National Institutes of Health and the American Heart Association, and financial relationships with Abbott, Medtronic, and Cytokinetics. Dr. Mann has received funding from NIH and reports financial relationships with MyoKardia and Novartis. Coauthors reported funding from NIH and AHA and financial relationships with Novartis, Amgen, AstraZeneca, Thoratec Corporation (Abbott), Sanofi, Pfizer, MyoKardia and American Regent.
SOURCE: Wilcox JE et al. J Am Coll Cardiol. 2020;76:719-34.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF CARDIOLOGY
Hospital vs. outpatient management comparable for elderly syncope patients
Background: In the United States, there are over 1 million visits to EDs for syncope with a greater than 50% hospitalization rate for older adult patients. There remains uncertainty around which patients without an identified cause for the syncope could be discharged from the ED and managed as an outpatient.
Study design: Propensity score analysis.
Setting: EDs from 11 nonprofit academic hospitals.
Synopsis: Prospective data for 2,492 patients aged 60 years and older who did not have an identified cause in the ED for their presenting complaint of syncope were included in the propensity score analysis resulting in a sample size of 1,064 with 532 patients in each of the discharged and hospitalized groups. There was no significant difference in risk of 30-day post-ED serious adverse events between the hospitalized patients (4.89%; 95% confidence interval, 3.06%-6.72%) and discharged patients (2.82%; 95% CI, 1.41%-4.23%; risk difference 2.07%; 95% CI, –0.24% to 4.38%). There was also no statistically significant difference in 30-day mortality post–ED visit.
These results show no clinical benefit in hospitalization for older adults with unexplained syncope after ED evaluation suggesting that it would be reasonable to proceed with outpatient management and evaluation of these patients.
Bottom line: Consider discharging older patients home from the ED who do not have high risk factors and no identified cause of their syncope.
Citation: Probst MA et al. Clinical benefit of hospitalization for older adults with unexplained syncope: A propensity-matched analysis. Ann Emerg Med. 2019 Aug;74(2):260-9.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
Background: In the United States, there are over 1 million visits to EDs for syncope with a greater than 50% hospitalization rate for older adult patients. There remains uncertainty around which patients without an identified cause for the syncope could be discharged from the ED and managed as an outpatient.
Study design: Propensity score analysis.
Setting: EDs from 11 nonprofit academic hospitals.
Synopsis: Prospective data for 2,492 patients aged 60 years and older who did not have an identified cause in the ED for their presenting complaint of syncope were included in the propensity score analysis resulting in a sample size of 1,064 with 532 patients in each of the discharged and hospitalized groups. There was no significant difference in risk of 30-day post-ED serious adverse events between the hospitalized patients (4.89%; 95% confidence interval, 3.06%-6.72%) and discharged patients (2.82%; 95% CI, 1.41%-4.23%; risk difference 2.07%; 95% CI, –0.24% to 4.38%). There was also no statistically significant difference in 30-day mortality post–ED visit.
These results show no clinical benefit in hospitalization for older adults with unexplained syncope after ED evaluation suggesting that it would be reasonable to proceed with outpatient management and evaluation of these patients.
Bottom line: Consider discharging older patients home from the ED who do not have high risk factors and no identified cause of their syncope.
Citation: Probst MA et al. Clinical benefit of hospitalization for older adults with unexplained syncope: A propensity-matched analysis. Ann Emerg Med. 2019 Aug;74(2):260-9.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
Background: In the United States, there are over 1 million visits to EDs for syncope with a greater than 50% hospitalization rate for older adult patients. There remains uncertainty around which patients without an identified cause for the syncope could be discharged from the ED and managed as an outpatient.
Study design: Propensity score analysis.
Setting: EDs from 11 nonprofit academic hospitals.
Synopsis: Prospective data for 2,492 patients aged 60 years and older who did not have an identified cause in the ED for their presenting complaint of syncope were included in the propensity score analysis resulting in a sample size of 1,064 with 532 patients in each of the discharged and hospitalized groups. There was no significant difference in risk of 30-day post-ED serious adverse events between the hospitalized patients (4.89%; 95% confidence interval, 3.06%-6.72%) and discharged patients (2.82%; 95% CI, 1.41%-4.23%; risk difference 2.07%; 95% CI, –0.24% to 4.38%). There was also no statistically significant difference in 30-day mortality post–ED visit.
These results show no clinical benefit in hospitalization for older adults with unexplained syncope after ED evaluation suggesting that it would be reasonable to proceed with outpatient management and evaluation of these patients.
Bottom line: Consider discharging older patients home from the ED who do not have high risk factors and no identified cause of their syncope.
Citation: Probst MA et al. Clinical benefit of hospitalization for older adults with unexplained syncope: A propensity-matched analysis. Ann Emerg Med. 2019 Aug;74(2):260-9.
Dr. Field is a hospitalist at Ochsner Health System, New Orleans.
Reflections from PHM’s chief fellow
The education of a new generation of subspecialists
Editor’s note: The Hospitalist is excited to debut a quarterly Pediatric Hospital Medicine Fellows column with this article by pediatric hospitalist Dr. Adam Cohen.
In June 2019, I was offered the new role of chief fellow of pediatric hospital medicine at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. After messaging colleagues and friends at PHM fellowships across the country, I discovered that I wasn’t only Baylor’s first chief fellow of PHM, but I was the only chief fellow of PHM in the nation.
At first, this seemed to be a daunting prospect that left me wondering what my experiences would be like. However, as any good academician knows, the only way to properly answer a question with such existential considerations is a literature review.
While the role of chief fellow exists in other pediatric subspecialty fellowships, the literature on this role is not yet developed. I focused my literature review on using the chief resident role as a surrogate. The chief resident position is filled with opportunities to work administratively and educationally and even has the potential to drive interinstitutional educational change.1 However, many chief residents feel their administrative roles outweigh their educational ones.2,3 This worried me, as the administrative side of program leadership was something that I had little experience in. Would I be weighed down with answering emails and fielding grievances from other fellows? While I did occasionally have that responsibility, my experiences as a chief fellow meant being intimately involved in one program’s response and growth during a national change to PHM as a field, while also coaching those from other programs on how to respond to these many changes.
The dawn of this new era of PHM saw the first board-certified hospitalists crowned and the first fellowships accredited by the Accreditation Council for Graduate Medical Education within the past academic year. I experienced this in a unique position as a chief fellow – an insider as part of the administration and an outsider as a prospective specialist. Prior to the recent accreditation and certification, PHM fellowship graduates were becoming successful academic physicians. A 2014 study of over 80% of all graduated PHM fellows showed nearly all had academic positions in which they taught students and residents. Many of these graduates also participated in research, with two-thirds being the first author on at least one peer-reviewed article.4
However, we also know that, prior to accreditation, fellowship training was varied, with clinical time ranging from 20% to 65%, in addition to wide variability in billing practices, scholarly practices, and the ability to pursue advanced nonclinical training, such as coursework or master’s degrees in quality improvement or education.5 With PHM fellowships becoming accredited and hospitalists becoming board certified, this is going to change, hopefully for the better.
National accrediting bodies like the ACGME create standards for programs to follow, but as a field we have to make sure we know what those standards mean for our future fellows and our educators. At my own program, these standards meant a significant reduction in clinical time, which was the main way fellows obtained content mastery in PHM. There were also concerns from practicing hospitalists about what it would mean if they did not or could not “grandfather in” to board certification. Would they be pushed out of their jobs or forced into less desirable ones? Would they be able to continue teaching and working with fellows?
As I reflect on experiencing this tumultuous time of change for our specialty, my main takeaway is that board certification of PHM faculty and accreditation of fellowships is an important step to creating the next generation of productive academic hospitalists. The greatest benefit for PHM fellows is that ACGME accreditation mandates that they be treated as learners, and not just junior attendings who are paid less. Many programs rely on fellow billing to fund fellowships, which can create a culture where the focus falls away from exploring a wide variety of educational opportunities and toward an exclusive or near-exclusive service-learning model.
This old model can come at the expense of opportunities such as conferences or secondary degrees. Under ACGME accreditation, fellowships will also be required to provide a regimented system of mentorship and support, more than just nonclinical time, to allow fellows to follow their interests and passions, whether that be in clinical hospital medicine, education, quality, advocacy or more. When these fellows graduate and become board certified, they will truly have recognition as specialists in the field, and be able to advance the field in any setting they choose to practice.
Like any change, this shift in our field also comes with our fair share of risks. Fellowship programs have to be careful about what they take away from an accreditation process that can be incredibly time-consuming and difficult. Leadership at these programs need to look critically at the changes they are required to make, and ensure they are integrated intelligently in a way that benefits the fellows.
At Baylor, while a decrease in clinical time was required, our leadership saw it as an opportunity to implement active learning and assessment techniques to improve clinical mastery with less clinical time. While many programs may need to make significant changes to align with ACGME standards, a key lesson in education is that these changes also need to reflect the goal of the program, to create expert academicians, clinicians, and leaders in PHM.
One of the largest challenges brought about by these changes is how we take into account pediatric hospitalists with clinical expertise who either are not academically oriented or are not eligible for board certification. Excluding them from participating in fellowship training or as productive members of our groups can create a hidden curriculum that board certification and academic practice are the only way forward in our field. We also risk excluding those with the ability to fill the largest need in our specialty, those who practice clinically in the community.6
We must ensure that our desire to have productive academic faculty does not result in the loss of those with clinical expertise, both for the care of our patients and the education of our learners. Whether that solution lies with alternative certification procedures or through thoughtful hiring and educational policies is yet to be seen.
Overall, as PHM’s chief fellow this past academic year, I found that we have a lot to be excited for as our field continues to grow. With this growth, we need be careful about how we move forward with the standardization of our training, education, and faculty practices to align with our core values of excellent care for children and advancement of our field to meet their needs and the needs of our medical system. I am grateful to the many PHM leaders and providers who have thoughtfully stimulated so much growth in the field and paved the way for current and future generations of fellows to benefit from that growth.
Dr. Cohen is an assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine and Texas Children’s Hospital. He graduated from PHM fellowship in June 2020 at Baylor, dedicating himself to developing expertise in medical education. He would like to thank Dr. Michelle Lopez for her assistance in revising this article.
References
1. Myers RE et al. Pediatric chief resident exchange program: A novel method to share educational ideas across training programs. Acad Pediatr. 2019. doi: S1876-2859(19)30386-9.
2. Norris T et al. Do program directors and their chief residents view the role of chief resident similarly? Family Medicine. 1996;28(5):343-5.
3. Dabrow SM et al. Two perspectives on the educational and administrative roles of the pediatric chief resident. J Grad Med Educ. 2011;3(1):17-20.
4. Oshimura JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7.
5. Shah NH et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-8.
6. Leyenaar JK et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-9.
The education of a new generation of subspecialists
The education of a new generation of subspecialists
Editor’s note: The Hospitalist is excited to debut a quarterly Pediatric Hospital Medicine Fellows column with this article by pediatric hospitalist Dr. Adam Cohen.
In June 2019, I was offered the new role of chief fellow of pediatric hospital medicine at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. After messaging colleagues and friends at PHM fellowships across the country, I discovered that I wasn’t only Baylor’s first chief fellow of PHM, but I was the only chief fellow of PHM in the nation.
At first, this seemed to be a daunting prospect that left me wondering what my experiences would be like. However, as any good academician knows, the only way to properly answer a question with such existential considerations is a literature review.
While the role of chief fellow exists in other pediatric subspecialty fellowships, the literature on this role is not yet developed. I focused my literature review on using the chief resident role as a surrogate. The chief resident position is filled with opportunities to work administratively and educationally and even has the potential to drive interinstitutional educational change.1 However, many chief residents feel their administrative roles outweigh their educational ones.2,3 This worried me, as the administrative side of program leadership was something that I had little experience in. Would I be weighed down with answering emails and fielding grievances from other fellows? While I did occasionally have that responsibility, my experiences as a chief fellow meant being intimately involved in one program’s response and growth during a national change to PHM as a field, while also coaching those from other programs on how to respond to these many changes.
The dawn of this new era of PHM saw the first board-certified hospitalists crowned and the first fellowships accredited by the Accreditation Council for Graduate Medical Education within the past academic year. I experienced this in a unique position as a chief fellow – an insider as part of the administration and an outsider as a prospective specialist. Prior to the recent accreditation and certification, PHM fellowship graduates were becoming successful academic physicians. A 2014 study of over 80% of all graduated PHM fellows showed nearly all had academic positions in which they taught students and residents. Many of these graduates also participated in research, with two-thirds being the first author on at least one peer-reviewed article.4
However, we also know that, prior to accreditation, fellowship training was varied, with clinical time ranging from 20% to 65%, in addition to wide variability in billing practices, scholarly practices, and the ability to pursue advanced nonclinical training, such as coursework or master’s degrees in quality improvement or education.5 With PHM fellowships becoming accredited and hospitalists becoming board certified, this is going to change, hopefully for the better.
National accrediting bodies like the ACGME create standards for programs to follow, but as a field we have to make sure we know what those standards mean for our future fellows and our educators. At my own program, these standards meant a significant reduction in clinical time, which was the main way fellows obtained content mastery in PHM. There were also concerns from practicing hospitalists about what it would mean if they did not or could not “grandfather in” to board certification. Would they be pushed out of their jobs or forced into less desirable ones? Would they be able to continue teaching and working with fellows?
As I reflect on experiencing this tumultuous time of change for our specialty, my main takeaway is that board certification of PHM faculty and accreditation of fellowships is an important step to creating the next generation of productive academic hospitalists. The greatest benefit for PHM fellows is that ACGME accreditation mandates that they be treated as learners, and not just junior attendings who are paid less. Many programs rely on fellow billing to fund fellowships, which can create a culture where the focus falls away from exploring a wide variety of educational opportunities and toward an exclusive or near-exclusive service-learning model.
This old model can come at the expense of opportunities such as conferences or secondary degrees. Under ACGME accreditation, fellowships will also be required to provide a regimented system of mentorship and support, more than just nonclinical time, to allow fellows to follow their interests and passions, whether that be in clinical hospital medicine, education, quality, advocacy or more. When these fellows graduate and become board certified, they will truly have recognition as specialists in the field, and be able to advance the field in any setting they choose to practice.
Like any change, this shift in our field also comes with our fair share of risks. Fellowship programs have to be careful about what they take away from an accreditation process that can be incredibly time-consuming and difficult. Leadership at these programs need to look critically at the changes they are required to make, and ensure they are integrated intelligently in a way that benefits the fellows.
At Baylor, while a decrease in clinical time was required, our leadership saw it as an opportunity to implement active learning and assessment techniques to improve clinical mastery with less clinical time. While many programs may need to make significant changes to align with ACGME standards, a key lesson in education is that these changes also need to reflect the goal of the program, to create expert academicians, clinicians, and leaders in PHM.
One of the largest challenges brought about by these changes is how we take into account pediatric hospitalists with clinical expertise who either are not academically oriented or are not eligible for board certification. Excluding them from participating in fellowship training or as productive members of our groups can create a hidden curriculum that board certification and academic practice are the only way forward in our field. We also risk excluding those with the ability to fill the largest need in our specialty, those who practice clinically in the community.6
We must ensure that our desire to have productive academic faculty does not result in the loss of those with clinical expertise, both for the care of our patients and the education of our learners. Whether that solution lies with alternative certification procedures or through thoughtful hiring and educational policies is yet to be seen.
Overall, as PHM’s chief fellow this past academic year, I found that we have a lot to be excited for as our field continues to grow. With this growth, we need be careful about how we move forward with the standardization of our training, education, and faculty practices to align with our core values of excellent care for children and advancement of our field to meet their needs and the needs of our medical system. I am grateful to the many PHM leaders and providers who have thoughtfully stimulated so much growth in the field and paved the way for current and future generations of fellows to benefit from that growth.
Dr. Cohen is an assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine and Texas Children’s Hospital. He graduated from PHM fellowship in June 2020 at Baylor, dedicating himself to developing expertise in medical education. He would like to thank Dr. Michelle Lopez for her assistance in revising this article.
References
1. Myers RE et al. Pediatric chief resident exchange program: A novel method to share educational ideas across training programs. Acad Pediatr. 2019. doi: S1876-2859(19)30386-9.
2. Norris T et al. Do program directors and their chief residents view the role of chief resident similarly? Family Medicine. 1996;28(5):343-5.
3. Dabrow SM et al. Two perspectives on the educational and administrative roles of the pediatric chief resident. J Grad Med Educ. 2011;3(1):17-20.
4. Oshimura JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7.
5. Shah NH et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-8.
6. Leyenaar JK et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-9.
Editor’s note: The Hospitalist is excited to debut a quarterly Pediatric Hospital Medicine Fellows column with this article by pediatric hospitalist Dr. Adam Cohen.
In June 2019, I was offered the new role of chief fellow of pediatric hospital medicine at Baylor College of Medicine and Texas Children’s Hospital, both in Houston. After messaging colleagues and friends at PHM fellowships across the country, I discovered that I wasn’t only Baylor’s first chief fellow of PHM, but I was the only chief fellow of PHM in the nation.
At first, this seemed to be a daunting prospect that left me wondering what my experiences would be like. However, as any good academician knows, the only way to properly answer a question with such existential considerations is a literature review.
While the role of chief fellow exists in other pediatric subspecialty fellowships, the literature on this role is not yet developed. I focused my literature review on using the chief resident role as a surrogate. The chief resident position is filled with opportunities to work administratively and educationally and even has the potential to drive interinstitutional educational change.1 However, many chief residents feel their administrative roles outweigh their educational ones.2,3 This worried me, as the administrative side of program leadership was something that I had little experience in. Would I be weighed down with answering emails and fielding grievances from other fellows? While I did occasionally have that responsibility, my experiences as a chief fellow meant being intimately involved in one program’s response and growth during a national change to PHM as a field, while also coaching those from other programs on how to respond to these many changes.
The dawn of this new era of PHM saw the first board-certified hospitalists crowned and the first fellowships accredited by the Accreditation Council for Graduate Medical Education within the past academic year. I experienced this in a unique position as a chief fellow – an insider as part of the administration and an outsider as a prospective specialist. Prior to the recent accreditation and certification, PHM fellowship graduates were becoming successful academic physicians. A 2014 study of over 80% of all graduated PHM fellows showed nearly all had academic positions in which they taught students and residents. Many of these graduates also participated in research, with two-thirds being the first author on at least one peer-reviewed article.4
However, we also know that, prior to accreditation, fellowship training was varied, with clinical time ranging from 20% to 65%, in addition to wide variability in billing practices, scholarly practices, and the ability to pursue advanced nonclinical training, such as coursework or master’s degrees in quality improvement or education.5 With PHM fellowships becoming accredited and hospitalists becoming board certified, this is going to change, hopefully for the better.
National accrediting bodies like the ACGME create standards for programs to follow, but as a field we have to make sure we know what those standards mean for our future fellows and our educators. At my own program, these standards meant a significant reduction in clinical time, which was the main way fellows obtained content mastery in PHM. There were also concerns from practicing hospitalists about what it would mean if they did not or could not “grandfather in” to board certification. Would they be pushed out of their jobs or forced into less desirable ones? Would they be able to continue teaching and working with fellows?
As I reflect on experiencing this tumultuous time of change for our specialty, my main takeaway is that board certification of PHM faculty and accreditation of fellowships is an important step to creating the next generation of productive academic hospitalists. The greatest benefit for PHM fellows is that ACGME accreditation mandates that they be treated as learners, and not just junior attendings who are paid less. Many programs rely on fellow billing to fund fellowships, which can create a culture where the focus falls away from exploring a wide variety of educational opportunities and toward an exclusive or near-exclusive service-learning model.
This old model can come at the expense of opportunities such as conferences or secondary degrees. Under ACGME accreditation, fellowships will also be required to provide a regimented system of mentorship and support, more than just nonclinical time, to allow fellows to follow their interests and passions, whether that be in clinical hospital medicine, education, quality, advocacy or more. When these fellows graduate and become board certified, they will truly have recognition as specialists in the field, and be able to advance the field in any setting they choose to practice.
Like any change, this shift in our field also comes with our fair share of risks. Fellowship programs have to be careful about what they take away from an accreditation process that can be incredibly time-consuming and difficult. Leadership at these programs need to look critically at the changes they are required to make, and ensure they are integrated intelligently in a way that benefits the fellows.
At Baylor, while a decrease in clinical time was required, our leadership saw it as an opportunity to implement active learning and assessment techniques to improve clinical mastery with less clinical time. While many programs may need to make significant changes to align with ACGME standards, a key lesson in education is that these changes also need to reflect the goal of the program, to create expert academicians, clinicians, and leaders in PHM.
One of the largest challenges brought about by these changes is how we take into account pediatric hospitalists with clinical expertise who either are not academically oriented or are not eligible for board certification. Excluding them from participating in fellowship training or as productive members of our groups can create a hidden curriculum that board certification and academic practice are the only way forward in our field. We also risk excluding those with the ability to fill the largest need in our specialty, those who practice clinically in the community.6
We must ensure that our desire to have productive academic faculty does not result in the loss of those with clinical expertise, both for the care of our patients and the education of our learners. Whether that solution lies with alternative certification procedures or through thoughtful hiring and educational policies is yet to be seen.
Overall, as PHM’s chief fellow this past academic year, I found that we have a lot to be excited for as our field continues to grow. With this growth, we need be careful about how we move forward with the standardization of our training, education, and faculty practices to align with our core values of excellent care for children and advancement of our field to meet their needs and the needs of our medical system. I am grateful to the many PHM leaders and providers who have thoughtfully stimulated so much growth in the field and paved the way for current and future generations of fellows to benefit from that growth.
Dr. Cohen is an assistant professor of pediatrics in the section of hospital medicine at Baylor College of Medicine and Texas Children’s Hospital. He graduated from PHM fellowship in June 2020 at Baylor, dedicating himself to developing expertise in medical education. He would like to thank Dr. Michelle Lopez for her assistance in revising this article.
References
1. Myers RE et al. Pediatric chief resident exchange program: A novel method to share educational ideas across training programs. Acad Pediatr. 2019. doi: S1876-2859(19)30386-9.
2. Norris T et al. Do program directors and their chief residents view the role of chief resident similarly? Family Medicine. 1996;28(5):343-5.
3. Dabrow SM et al. Two perspectives on the educational and administrative roles of the pediatric chief resident. J Grad Med Educ. 2011;3(1):17-20.
4. Oshimura JM et al. Current roles and perceived needs of pediatric hospital medicine fellowship graduates. Hosp Pediatr. 2016;6(10):633-7.
5. Shah NH et al. The current state of pediatric hospital medicine fellowships: A survey of program directors. J Hosp Med. 2016;11(5):324-8.
6. Leyenaar JK et al. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743-9.
Global study to track COVID-19’s impact on the brain
At its annual meeting, the Alzheimer’s Association announced the launch of a global study to examine the impact of COVID-19 on the brain, as well as policy recommendations to better address the COVID-19 crisis in long-term care facilities. The study will be led by researchers at the Alzheimer’s Association and the University of Texas Health, San Antonio, with participation from more than 30 countries and technical guidance from the World Health Organization.
The target sample size is 20,000-40,000 total participants.
Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, announced the study’s launch during a COVID-19–focused panel discussion at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
“To build a strong foundation for this research, we will align with existing studies, such as the Framingham Heart Study, and clinicians from around the world on how the data are going to be collected, obtained, and shared. We are going to have cross-study collaborations to understand the impact of the virus on the brain directly,” said Dr. Carrillo. “We will have some very good data to present next year at AAIC.”
‘Frightening’ headlines
As previously reported, mounting evidence suggests that SARS-CoV-2 invades the central nervous system, causing a wide range of neurologic and neuropsychiatric complications, including stroke, psychosis, altered mental state, and dementia-like syndrome. It’s likely that “dementia does not increase the risk for COVID-19, just like dementia does not increase risk for the flu. But increased age, being in a long-term care setting, and common health conditions that often accompany dementia may increase the risk,” Dr. Carrillo said.
Panel member Beth Kallmyer, MSW, vice president of care and support at the Alzheimer’s Association, spoke about the ongoing challenges long-term care facilities are facing during the pandemic. “You’ve all seen the headlines, and they’re frightening, frankly,” she said. An estimated 59,000 residents and employees of long-term care have died as a result of COVID-19, which is 42% of all U.S. deaths.
The long-term care community is being impacted at “significantly greater rates than the rest of society and yet we don’t have things in place to protect them. We also know that individuals living with dementia make up a large percentage of those that are living in long-term care,” Ms. Kallmyer said.
She noted that infection control is always a challenge in long-term care settings, but infection control during a pandemic “takes it to a whole other level.” Quarantining is hard for anyone, “but when you layer dementia on top of that we have a real challenge.” One long-term care provider told Ms. Kallmyer that “we might be saving them from COVID, but we’re losing them to social isolation and cognitive decline.”
New recommendations
Ms. Kallmyer outlined new policy recommendations from the Alzheimer’s Association to address the COVID-19 crisis in long-term and community-based care settings. They include:
- Testing every resident, employee, and visitor each time they leave and come back, so residents would not need to be confined to their own rooms
- Having a single portal that is easy and efficient for reporting cases
- Developing “surge activation” protocols to respond to hot spots, including the possibility of “strike teams” that go in and help during an outbreak
- Making sure all long-term care providers have full access to all needed personal protective equipment (PPE)
“Five months in and long-term care providers still don’t have adequate PPE. This is unacceptable,” said Ms. Kallmyer. “We have to be able to provide them with PPE.”
Panel member Gregory A. Jicha, MD, PhD, Sanders-Brown Center on Aging, University of Kentucky, Lexington, spoke about the critical need to continue Alzheimer’s disease research during the pandemic, noting that the number of promising targets for Alzheimer’s disease and related dementias has “never been higher or more comprehensive.”
Measures to ensure safety of researchers and participants include screening for symptoms (50% effective), social distancing (93% effective), minimizing exposure time (50% effective), limiting staff to 50% (50% effective), cloth/paper masks (80% effective), and testing (99.25% effective), Dr. Jicha noted.
With no safety measures in place, the risk of getting COVID-19 from a research visit is 1 in 20; when all these safety measures are combined, the risk is 1 in over 1.5 million, so “we can essentially eradicate or minimize the risks for COVID to less that of a lightning strike,” he said.
Dr. Carrillo, Ms. Kallmyer, and Dr. Jicha disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
At its annual meeting, the Alzheimer’s Association announced the launch of a global study to examine the impact of COVID-19 on the brain, as well as policy recommendations to better address the COVID-19 crisis in long-term care facilities. The study will be led by researchers at the Alzheimer’s Association and the University of Texas Health, San Antonio, with participation from more than 30 countries and technical guidance from the World Health Organization.
The target sample size is 20,000-40,000 total participants.
Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, announced the study’s launch during a COVID-19–focused panel discussion at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
“To build a strong foundation for this research, we will align with existing studies, such as the Framingham Heart Study, and clinicians from around the world on how the data are going to be collected, obtained, and shared. We are going to have cross-study collaborations to understand the impact of the virus on the brain directly,” said Dr. Carrillo. “We will have some very good data to present next year at AAIC.”
‘Frightening’ headlines
As previously reported, mounting evidence suggests that SARS-CoV-2 invades the central nervous system, causing a wide range of neurologic and neuropsychiatric complications, including stroke, psychosis, altered mental state, and dementia-like syndrome. It’s likely that “dementia does not increase the risk for COVID-19, just like dementia does not increase risk for the flu. But increased age, being in a long-term care setting, and common health conditions that often accompany dementia may increase the risk,” Dr. Carrillo said.
Panel member Beth Kallmyer, MSW, vice president of care and support at the Alzheimer’s Association, spoke about the ongoing challenges long-term care facilities are facing during the pandemic. “You’ve all seen the headlines, and they’re frightening, frankly,” she said. An estimated 59,000 residents and employees of long-term care have died as a result of COVID-19, which is 42% of all U.S. deaths.
The long-term care community is being impacted at “significantly greater rates than the rest of society and yet we don’t have things in place to protect them. We also know that individuals living with dementia make up a large percentage of those that are living in long-term care,” Ms. Kallmyer said.
She noted that infection control is always a challenge in long-term care settings, but infection control during a pandemic “takes it to a whole other level.” Quarantining is hard for anyone, “but when you layer dementia on top of that we have a real challenge.” One long-term care provider told Ms. Kallmyer that “we might be saving them from COVID, but we’re losing them to social isolation and cognitive decline.”
New recommendations
Ms. Kallmyer outlined new policy recommendations from the Alzheimer’s Association to address the COVID-19 crisis in long-term and community-based care settings. They include:
- Testing every resident, employee, and visitor each time they leave and come back, so residents would not need to be confined to their own rooms
- Having a single portal that is easy and efficient for reporting cases
- Developing “surge activation” protocols to respond to hot spots, including the possibility of “strike teams” that go in and help during an outbreak
- Making sure all long-term care providers have full access to all needed personal protective equipment (PPE)
“Five months in and long-term care providers still don’t have adequate PPE. This is unacceptable,” said Ms. Kallmyer. “We have to be able to provide them with PPE.”
Panel member Gregory A. Jicha, MD, PhD, Sanders-Brown Center on Aging, University of Kentucky, Lexington, spoke about the critical need to continue Alzheimer’s disease research during the pandemic, noting that the number of promising targets for Alzheimer’s disease and related dementias has “never been higher or more comprehensive.”
Measures to ensure safety of researchers and participants include screening for symptoms (50% effective), social distancing (93% effective), minimizing exposure time (50% effective), limiting staff to 50% (50% effective), cloth/paper masks (80% effective), and testing (99.25% effective), Dr. Jicha noted.
With no safety measures in place, the risk of getting COVID-19 from a research visit is 1 in 20; when all these safety measures are combined, the risk is 1 in over 1.5 million, so “we can essentially eradicate or minimize the risks for COVID to less that of a lightning strike,” he said.
Dr. Carrillo, Ms. Kallmyer, and Dr. Jicha disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
At its annual meeting, the Alzheimer’s Association announced the launch of a global study to examine the impact of COVID-19 on the brain, as well as policy recommendations to better address the COVID-19 crisis in long-term care facilities. The study will be led by researchers at the Alzheimer’s Association and the University of Texas Health, San Antonio, with participation from more than 30 countries and technical guidance from the World Health Organization.
The target sample size is 20,000-40,000 total participants.
Maria C. Carrillo, PhD, chief science officer for the Alzheimer’s Association, announced the study’s launch during a COVID-19–focused panel discussion at the virtual annual meeting of the Alzheimer’s Association International Conference 2020.
“To build a strong foundation for this research, we will align with existing studies, such as the Framingham Heart Study, and clinicians from around the world on how the data are going to be collected, obtained, and shared. We are going to have cross-study collaborations to understand the impact of the virus on the brain directly,” said Dr. Carrillo. “We will have some very good data to present next year at AAIC.”
‘Frightening’ headlines
As previously reported, mounting evidence suggests that SARS-CoV-2 invades the central nervous system, causing a wide range of neurologic and neuropsychiatric complications, including stroke, psychosis, altered mental state, and dementia-like syndrome. It’s likely that “dementia does not increase the risk for COVID-19, just like dementia does not increase risk for the flu. But increased age, being in a long-term care setting, and common health conditions that often accompany dementia may increase the risk,” Dr. Carrillo said.
Panel member Beth Kallmyer, MSW, vice president of care and support at the Alzheimer’s Association, spoke about the ongoing challenges long-term care facilities are facing during the pandemic. “You’ve all seen the headlines, and they’re frightening, frankly,” she said. An estimated 59,000 residents and employees of long-term care have died as a result of COVID-19, which is 42% of all U.S. deaths.
The long-term care community is being impacted at “significantly greater rates than the rest of society and yet we don’t have things in place to protect them. We also know that individuals living with dementia make up a large percentage of those that are living in long-term care,” Ms. Kallmyer said.
She noted that infection control is always a challenge in long-term care settings, but infection control during a pandemic “takes it to a whole other level.” Quarantining is hard for anyone, “but when you layer dementia on top of that we have a real challenge.” One long-term care provider told Ms. Kallmyer that “we might be saving them from COVID, but we’re losing them to social isolation and cognitive decline.”
New recommendations
Ms. Kallmyer outlined new policy recommendations from the Alzheimer’s Association to address the COVID-19 crisis in long-term and community-based care settings. They include:
- Testing every resident, employee, and visitor each time they leave and come back, so residents would not need to be confined to their own rooms
- Having a single portal that is easy and efficient for reporting cases
- Developing “surge activation” protocols to respond to hot spots, including the possibility of “strike teams” that go in and help during an outbreak
- Making sure all long-term care providers have full access to all needed personal protective equipment (PPE)
“Five months in and long-term care providers still don’t have adequate PPE. This is unacceptable,” said Ms. Kallmyer. “We have to be able to provide them with PPE.”
Panel member Gregory A. Jicha, MD, PhD, Sanders-Brown Center on Aging, University of Kentucky, Lexington, spoke about the critical need to continue Alzheimer’s disease research during the pandemic, noting that the number of promising targets for Alzheimer’s disease and related dementias has “never been higher or more comprehensive.”
Measures to ensure safety of researchers and participants include screening for symptoms (50% effective), social distancing (93% effective), minimizing exposure time (50% effective), limiting staff to 50% (50% effective), cloth/paper masks (80% effective), and testing (99.25% effective), Dr. Jicha noted.
With no safety measures in place, the risk of getting COVID-19 from a research visit is 1 in 20; when all these safety measures are combined, the risk is 1 in over 1.5 million, so “we can essentially eradicate or minimize the risks for COVID to less that of a lightning strike,” he said.
Dr. Carrillo, Ms. Kallmyer, and Dr. Jicha disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM AAIC 2020
COVID-19 taking financial toll on people in U.S. with diabetes
The COVID-19 pandemic is taking a particularly severe financial toll on people with diabetes, new research from the United States suggests.
Results from a national online survey of 5,000 people with diabetes conducted between June 26 and July 1, 2020, were posted July 29 on the American Diabetes Association website.
The survey, conducted by the diabetes research company dQ&A in association with the ADA, revealed that Americans with diabetes are experiencing extreme financial pressures, leading to medication and supply rationing.
A high proportion of respondents had either lost income or are working in jobs that place them at risk for catching the novel coronavirus.
“These new numbers show the urgency needed to adopt measures to protect and assist the millions of people with diabetes who are suffering through this pandemic,” Tracey D. Brown, CEO of the ADA, said in a statement.
She called for states to extend health care coverage to people who have lost their jobs, for the eradication of insulin copays during the pandemic, and for increased COVID-19 testing capacity in high-risk communities.
“If these actions aren’t taken immediately, we will continue to see devastating impacts and outcomes for millions of vulnerable Americans,” Ms. Brown stressed.
COVID-19 has worsened financial pressures for people with diabetes
In the survey, 24% of respondents reported having used savings, loans, or stimulus check money to pay for diabetes care in the past 3 months. Among those who have lost income, half are using savings or stimulus money.
A quarter of respondents said they have been self-rationing supplies to cut costs.
Extrapolating to the entire U.S. population with diabetes, dQ&A estimated that roughly 650,000 are skipping insulin doses or taking less than prescribed, and 3 million are skipping blood glucose tests.
In June, the unemployment rate for people with diabetes was 18%, higher than the national rate of 12%.
Also higher is the proportion of those working prior to the pandemic who have since lost income: 33%, compared with 29% for the general population.
Among those who are self-employed, 7 in 10 of those with diabetes have lost some or all of their income.
Many with diabetes who are employed are vulnerable to exposure
Of those who remain employed, half said they can’t work from home.
Of those, 60% work in essential industries, with 22% in health care. A large majority, 90%, reported lack of social distancing at work and nearly a third work in places that don’t require masks.
“People with diabetes are helping to provide the services we all depend on during this pandemic, even as it puts their own well-being at risk,” the report said.
It concluded that “these numbers represent a conservative estimate of the pandemic’s impact. They are generated from an ongoing online study of the diabetes population amongst people who have opted in to participate.”
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic is taking a particularly severe financial toll on people with diabetes, new research from the United States suggests.
Results from a national online survey of 5,000 people with diabetes conducted between June 26 and July 1, 2020, were posted July 29 on the American Diabetes Association website.
The survey, conducted by the diabetes research company dQ&A in association with the ADA, revealed that Americans with diabetes are experiencing extreme financial pressures, leading to medication and supply rationing.
A high proportion of respondents had either lost income or are working in jobs that place them at risk for catching the novel coronavirus.
“These new numbers show the urgency needed to adopt measures to protect and assist the millions of people with diabetes who are suffering through this pandemic,” Tracey D. Brown, CEO of the ADA, said in a statement.
She called for states to extend health care coverage to people who have lost their jobs, for the eradication of insulin copays during the pandemic, and for increased COVID-19 testing capacity in high-risk communities.
“If these actions aren’t taken immediately, we will continue to see devastating impacts and outcomes for millions of vulnerable Americans,” Ms. Brown stressed.
COVID-19 has worsened financial pressures for people with diabetes
In the survey, 24% of respondents reported having used savings, loans, or stimulus check money to pay for diabetes care in the past 3 months. Among those who have lost income, half are using savings or stimulus money.
A quarter of respondents said they have been self-rationing supplies to cut costs.
Extrapolating to the entire U.S. population with diabetes, dQ&A estimated that roughly 650,000 are skipping insulin doses or taking less than prescribed, and 3 million are skipping blood glucose tests.
In June, the unemployment rate for people with diabetes was 18%, higher than the national rate of 12%.
Also higher is the proportion of those working prior to the pandemic who have since lost income: 33%, compared with 29% for the general population.
Among those who are self-employed, 7 in 10 of those with diabetes have lost some or all of their income.
Many with diabetes who are employed are vulnerable to exposure
Of those who remain employed, half said they can’t work from home.
Of those, 60% work in essential industries, with 22% in health care. A large majority, 90%, reported lack of social distancing at work and nearly a third work in places that don’t require masks.
“People with diabetes are helping to provide the services we all depend on during this pandemic, even as it puts their own well-being at risk,” the report said.
It concluded that “these numbers represent a conservative estimate of the pandemic’s impact. They are generated from an ongoing online study of the diabetes population amongst people who have opted in to participate.”
A version of this article originally appeared on Medscape.com.
The COVID-19 pandemic is taking a particularly severe financial toll on people with diabetes, new research from the United States suggests.
Results from a national online survey of 5,000 people with diabetes conducted between June 26 and July 1, 2020, were posted July 29 on the American Diabetes Association website.
The survey, conducted by the diabetes research company dQ&A in association with the ADA, revealed that Americans with diabetes are experiencing extreme financial pressures, leading to medication and supply rationing.
A high proportion of respondents had either lost income or are working in jobs that place them at risk for catching the novel coronavirus.
“These new numbers show the urgency needed to adopt measures to protect and assist the millions of people with diabetes who are suffering through this pandemic,” Tracey D. Brown, CEO of the ADA, said in a statement.
She called for states to extend health care coverage to people who have lost their jobs, for the eradication of insulin copays during the pandemic, and for increased COVID-19 testing capacity in high-risk communities.
“If these actions aren’t taken immediately, we will continue to see devastating impacts and outcomes for millions of vulnerable Americans,” Ms. Brown stressed.
COVID-19 has worsened financial pressures for people with diabetes
In the survey, 24% of respondents reported having used savings, loans, or stimulus check money to pay for diabetes care in the past 3 months. Among those who have lost income, half are using savings or stimulus money.
A quarter of respondents said they have been self-rationing supplies to cut costs.
Extrapolating to the entire U.S. population with diabetes, dQ&A estimated that roughly 650,000 are skipping insulin doses or taking less than prescribed, and 3 million are skipping blood glucose tests.
In June, the unemployment rate for people with diabetes was 18%, higher than the national rate of 12%.
Also higher is the proportion of those working prior to the pandemic who have since lost income: 33%, compared with 29% for the general population.
Among those who are self-employed, 7 in 10 of those with diabetes have lost some or all of their income.
Many with diabetes who are employed are vulnerable to exposure
Of those who remain employed, half said they can’t work from home.
Of those, 60% work in essential industries, with 22% in health care. A large majority, 90%, reported lack of social distancing at work and nearly a third work in places that don’t require masks.
“People with diabetes are helping to provide the services we all depend on during this pandemic, even as it puts their own well-being at risk,” the report said.
It concluded that “these numbers represent a conservative estimate of the pandemic’s impact. They are generated from an ongoing online study of the diabetes population amongst people who have opted in to participate.”
A version of this article originally appeared on Medscape.com.
















