User login
Official news magazine of the Society of Hospital Medicine
Copyright by Society of Hospital Medicine or related companies. All rights reserved. ISSN 1553-085X
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
div[contains(@class, 'pane-pub-article-hospitalist')]


Residents, fellows will get minimum 6 weeks leave for caregiving
the American Board of Medical Specialties has announced.
The “ABMS Policy on Parental, Caregiver and Family Leave” announced July 13 was developed after a report from the Accreditation Council for Graduate Medical Education’s Council of Review Committee Residents in June 2019.
Richard E. Hawkins, MD, ABMS President and CEO, said in a statement that “the growing shifts in viewpoints regarding work-life balance and parental roles had a great influence in the creation of this policy, which fosters an environment that supports our trainees’ ability to care not only for patients, but also for themselves and their families.”
Specifically, the time can be taken for birth and care of a newborn, adopting a child, or becoming a foster parent; care of a child, spouse, or parent with a serious health condition; or the trainee’s own serious health condition. The policy applies to member boards with training programs of at least 2 years.
Boards must communicate when a leave will require an official extension to avoid disruptions to a physician’s career trajectory, a delay in starting a fellowship, or moving into a salaried position.
Work/life balance was by far the biggest challenge reported in the Medscape Residents Lifestyle & Happiness Report 2019.
Several member boards had already implemented policies that offered more flexibility without unduly delaying board certification; now ABMS is extending that to all boards.
ABMS says member boards may limit the maximum time away in a single year or level of training and directed member boards to “make reasonable testing accommodations” – for example, by allowing candidates to take an exam provided the candidate completes all training requirements by a certain date.
Kristy Rialon, MD, an author of the ACGME report and assistant professor of surgery at Baylor College of Medicine and the Texas Children’s Hospital, both in Houston, noted the significance of the change in a news release.
“By virtue of their ages, residents and fellows – male and female – often find themselves having and raising children, as well as serving as family members’ caregivers,” Dr. Rialon said. “By adopting more realistic and compassionate approaches, the ABMS member boards will significantly improve the quality of life for residents and fellows. This also will support our female physicians, helping to narrow the gender gap in their career advancement by allowing for greater leave flexibility.”
A Medscape survey published July 15 said work-life balance was the No. 1 concern of female physicians, far outpacing pay.
A version of this article originally appeared on Medscape.com.
the American Board of Medical Specialties has announced.
The “ABMS Policy on Parental, Caregiver and Family Leave” announced July 13 was developed after a report from the Accreditation Council for Graduate Medical Education’s Council of Review Committee Residents in June 2019.
Richard E. Hawkins, MD, ABMS President and CEO, said in a statement that “the growing shifts in viewpoints regarding work-life balance and parental roles had a great influence in the creation of this policy, which fosters an environment that supports our trainees’ ability to care not only for patients, but also for themselves and their families.”
Specifically, the time can be taken for birth and care of a newborn, adopting a child, or becoming a foster parent; care of a child, spouse, or parent with a serious health condition; or the trainee’s own serious health condition. The policy applies to member boards with training programs of at least 2 years.
Boards must communicate when a leave will require an official extension to avoid disruptions to a physician’s career trajectory, a delay in starting a fellowship, or moving into a salaried position.
Work/life balance was by far the biggest challenge reported in the Medscape Residents Lifestyle & Happiness Report 2019.
Several member boards had already implemented policies that offered more flexibility without unduly delaying board certification; now ABMS is extending that to all boards.
ABMS says member boards may limit the maximum time away in a single year or level of training and directed member boards to “make reasonable testing accommodations” – for example, by allowing candidates to take an exam provided the candidate completes all training requirements by a certain date.
Kristy Rialon, MD, an author of the ACGME report and assistant professor of surgery at Baylor College of Medicine and the Texas Children’s Hospital, both in Houston, noted the significance of the change in a news release.
“By virtue of their ages, residents and fellows – male and female – often find themselves having and raising children, as well as serving as family members’ caregivers,” Dr. Rialon said. “By adopting more realistic and compassionate approaches, the ABMS member boards will significantly improve the quality of life for residents and fellows. This also will support our female physicians, helping to narrow the gender gap in their career advancement by allowing for greater leave flexibility.”
A Medscape survey published July 15 said work-life balance was the No. 1 concern of female physicians, far outpacing pay.
A version of this article originally appeared on Medscape.com.
the American Board of Medical Specialties has announced.
The “ABMS Policy on Parental, Caregiver and Family Leave” announced July 13 was developed after a report from the Accreditation Council for Graduate Medical Education’s Council of Review Committee Residents in June 2019.
Richard E. Hawkins, MD, ABMS President and CEO, said in a statement that “the growing shifts in viewpoints regarding work-life balance and parental roles had a great influence in the creation of this policy, which fosters an environment that supports our trainees’ ability to care not only for patients, but also for themselves and their families.”
Specifically, the time can be taken for birth and care of a newborn, adopting a child, or becoming a foster parent; care of a child, spouse, or parent with a serious health condition; or the trainee’s own serious health condition. The policy applies to member boards with training programs of at least 2 years.
Boards must communicate when a leave will require an official extension to avoid disruptions to a physician’s career trajectory, a delay in starting a fellowship, or moving into a salaried position.
Work/life balance was by far the biggest challenge reported in the Medscape Residents Lifestyle & Happiness Report 2019.
Several member boards had already implemented policies that offered more flexibility without unduly delaying board certification; now ABMS is extending that to all boards.
ABMS says member boards may limit the maximum time away in a single year or level of training and directed member boards to “make reasonable testing accommodations” – for example, by allowing candidates to take an exam provided the candidate completes all training requirements by a certain date.
Kristy Rialon, MD, an author of the ACGME report and assistant professor of surgery at Baylor College of Medicine and the Texas Children’s Hospital, both in Houston, noted the significance of the change in a news release.
“By virtue of their ages, residents and fellows – male and female – often find themselves having and raising children, as well as serving as family members’ caregivers,” Dr. Rialon said. “By adopting more realistic and compassionate approaches, the ABMS member boards will significantly improve the quality of life for residents and fellows. This also will support our female physicians, helping to narrow the gender gap in their career advancement by allowing for greater leave flexibility.”
A Medscape survey published July 15 said work-life balance was the No. 1 concern of female physicians, far outpacing pay.
A version of this article originally appeared on Medscape.com.
Guidance addresses elders with diabetes during COVID-19
Two experts in geriatric diabetes are offering some contemporary practical recommendations for diabetes management in older adults during the COVID-19 pandemic.
The viewpoint, entitled, “Caring for Older Adults With Diabetes During the COVID-19 Pandemic,” was published online in JAMA Internal Medicine by Medha N. Munshi, MD, director of the geriatrics program at the Joslin Diabetes Center, Boston, and Sarah L. Sy, MD, a geriatrician in the same program.
Adults aged 70 years and older with comorbidities such as diabetes are among those at highest risk for adverse outcomes and mortality due to COVID-19.
At the same time, those who don’t have the illness face major challenges in avoiding it, including disruptions in normal activities and barriers to receiving health care.
Although telemedicine has become much more widely adopted in diabetes management since the pandemic began, older adults may not be as tech savvy, may not have computer or Internet access, and/or may have cognitive dysfunction that precludes its use.
“These unprecedented times pose a great challenge to this heterogeneous population with varying levels of complexity, frailty, and multimorbidity,” Munshi and Sy point out, noting that “clinicians can lessen the load by guiding, reassuring, and supporting them through this pandemic time.”
Because the pandemic could last for several months longer, the authors offer the following advice for clinicians who care for older adults with diabetes.
- Accessibility to health care: When possible, use telemedicine, diabetes care apps, or platforms to obtain data from glucose meters, continuous glucose monitors, and/or pumps. When use of technology isn’t possible, schedule telephone appointments and have the patient or caregiver read the glucose values.
- Multicomplexity and geriatric syndromes: Identify high-risk patients, such as those with or recurrent , and prioritize patient goals. If appropriate, simplify the diabetes treatment plan and reinforce with repeated education and instructions. Glucose goals may need to be liberalized. Advise patients to stay hydrated to minimize the risk of dehydration and falls. Take steps to avoid hypoglycemia, reduce polypharmacy, and consolidate medication doses.
- Burden of diabetes self-care: Bloodwork for can be delayed by a few months. Patients with can decrease the frequency of blood glucose checks if their glucose levels are generally within acceptable range. Encourage patients to eat healthily with regular meals rather than optimizing the diet for glucose levels, and adjust medications for any changes in diet. Advise safe options for physical activity such as walking inside the home or walking in place for 10 minutes, three times per day, and incorporating strength training, such as with resistance bands. Online exercise programs are another option.
- Psychological stress: Check in with patients and encourage them to stay as connected as possible using technology (phone, video chat, text message), letters, or cards with family, friends, and/or religious communities. Screen for , using either the Geriatric Depression Scale or Patient Health Questionnaire-2, and refer to mental health colleagues if appropriate. Speak or email with caregivers to assess the patient’s mental health state and offer local support resources, if needed.
- Medication and equipment issues: Refill 90-day prescriptions and equipment, and request mail or home (contactless) delivery. Patients should also have backups in case of equipment failures, such as syringes and long-acting insulin in case of pump failure, and test strips/meter for continuous glucose monitor problems.
Munshi and Sy conclude: “Many of the recommendations presented in this article are practical and will continue to be relevant after COVID-19. When this is all over, patients will remember how we made them feel, and how we kept them safe and healthy at home.”
Munshi is a consultant for Sanofi and Lilly. Sy has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Two experts in geriatric diabetes are offering some contemporary practical recommendations for diabetes management in older adults during the COVID-19 pandemic.
The viewpoint, entitled, “Caring for Older Adults With Diabetes During the COVID-19 Pandemic,” was published online in JAMA Internal Medicine by Medha N. Munshi, MD, director of the geriatrics program at the Joslin Diabetes Center, Boston, and Sarah L. Sy, MD, a geriatrician in the same program.
Adults aged 70 years and older with comorbidities such as diabetes are among those at highest risk for adverse outcomes and mortality due to COVID-19.
At the same time, those who don’t have the illness face major challenges in avoiding it, including disruptions in normal activities and barriers to receiving health care.
Although telemedicine has become much more widely adopted in diabetes management since the pandemic began, older adults may not be as tech savvy, may not have computer or Internet access, and/or may have cognitive dysfunction that precludes its use.
“These unprecedented times pose a great challenge to this heterogeneous population with varying levels of complexity, frailty, and multimorbidity,” Munshi and Sy point out, noting that “clinicians can lessen the load by guiding, reassuring, and supporting them through this pandemic time.”
Because the pandemic could last for several months longer, the authors offer the following advice for clinicians who care for older adults with diabetes.
- Accessibility to health care: When possible, use telemedicine, diabetes care apps, or platforms to obtain data from glucose meters, continuous glucose monitors, and/or pumps. When use of technology isn’t possible, schedule telephone appointments and have the patient or caregiver read the glucose values.
- Multicomplexity and geriatric syndromes: Identify high-risk patients, such as those with or recurrent , and prioritize patient goals. If appropriate, simplify the diabetes treatment plan and reinforce with repeated education and instructions. Glucose goals may need to be liberalized. Advise patients to stay hydrated to minimize the risk of dehydration and falls. Take steps to avoid hypoglycemia, reduce polypharmacy, and consolidate medication doses.
- Burden of diabetes self-care: Bloodwork for can be delayed by a few months. Patients with can decrease the frequency of blood glucose checks if their glucose levels are generally within acceptable range. Encourage patients to eat healthily with regular meals rather than optimizing the diet for glucose levels, and adjust medications for any changes in diet. Advise safe options for physical activity such as walking inside the home or walking in place for 10 minutes, three times per day, and incorporating strength training, such as with resistance bands. Online exercise programs are another option.
- Psychological stress: Check in with patients and encourage them to stay as connected as possible using technology (phone, video chat, text message), letters, or cards with family, friends, and/or religious communities. Screen for , using either the Geriatric Depression Scale or Patient Health Questionnaire-2, and refer to mental health colleagues if appropriate. Speak or email with caregivers to assess the patient’s mental health state and offer local support resources, if needed.
- Medication and equipment issues: Refill 90-day prescriptions and equipment, and request mail or home (contactless) delivery. Patients should also have backups in case of equipment failures, such as syringes and long-acting insulin in case of pump failure, and test strips/meter for continuous glucose monitor problems.
Munshi and Sy conclude: “Many of the recommendations presented in this article are practical and will continue to be relevant after COVID-19. When this is all over, patients will remember how we made them feel, and how we kept them safe and healthy at home.”
Munshi is a consultant for Sanofi and Lilly. Sy has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Two experts in geriatric diabetes are offering some contemporary practical recommendations for diabetes management in older adults during the COVID-19 pandemic.
The viewpoint, entitled, “Caring for Older Adults With Diabetes During the COVID-19 Pandemic,” was published online in JAMA Internal Medicine by Medha N. Munshi, MD, director of the geriatrics program at the Joslin Diabetes Center, Boston, and Sarah L. Sy, MD, a geriatrician in the same program.
Adults aged 70 years and older with comorbidities such as diabetes are among those at highest risk for adverse outcomes and mortality due to COVID-19.
At the same time, those who don’t have the illness face major challenges in avoiding it, including disruptions in normal activities and barriers to receiving health care.
Although telemedicine has become much more widely adopted in diabetes management since the pandemic began, older adults may not be as tech savvy, may not have computer or Internet access, and/or may have cognitive dysfunction that precludes its use.
“These unprecedented times pose a great challenge to this heterogeneous population with varying levels of complexity, frailty, and multimorbidity,” Munshi and Sy point out, noting that “clinicians can lessen the load by guiding, reassuring, and supporting them through this pandemic time.”
Because the pandemic could last for several months longer, the authors offer the following advice for clinicians who care for older adults with diabetes.
- Accessibility to health care: When possible, use telemedicine, diabetes care apps, or platforms to obtain data from glucose meters, continuous glucose monitors, and/or pumps. When use of technology isn’t possible, schedule telephone appointments and have the patient or caregiver read the glucose values.
- Multicomplexity and geriatric syndromes: Identify high-risk patients, such as those with or recurrent , and prioritize patient goals. If appropriate, simplify the diabetes treatment plan and reinforce with repeated education and instructions. Glucose goals may need to be liberalized. Advise patients to stay hydrated to minimize the risk of dehydration and falls. Take steps to avoid hypoglycemia, reduce polypharmacy, and consolidate medication doses.
- Burden of diabetes self-care: Bloodwork for can be delayed by a few months. Patients with can decrease the frequency of blood glucose checks if their glucose levels are generally within acceptable range. Encourage patients to eat healthily with regular meals rather than optimizing the diet for glucose levels, and adjust medications for any changes in diet. Advise safe options for physical activity such as walking inside the home or walking in place for 10 minutes, three times per day, and incorporating strength training, such as with resistance bands. Online exercise programs are another option.
- Psychological stress: Check in with patients and encourage them to stay as connected as possible using technology (phone, video chat, text message), letters, or cards with family, friends, and/or religious communities. Screen for , using either the Geriatric Depression Scale or Patient Health Questionnaire-2, and refer to mental health colleagues if appropriate. Speak or email with caregivers to assess the patient’s mental health state and offer local support resources, if needed.
- Medication and equipment issues: Refill 90-day prescriptions and equipment, and request mail or home (contactless) delivery. Patients should also have backups in case of equipment failures, such as syringes and long-acting insulin in case of pump failure, and test strips/meter for continuous glucose monitor problems.
Munshi and Sy conclude: “Many of the recommendations presented in this article are practical and will continue to be relevant after COVID-19. When this is all over, patients will remember how we made them feel, and how we kept them safe and healthy at home.”
Munshi is a consultant for Sanofi and Lilly. Sy has reported no relevant financial relationships.
This article first appeared on Medscape.com.
Creating a student-staffed family call line to alleviate clinical burden
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
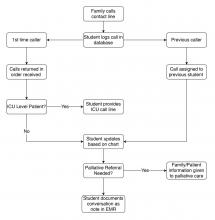
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
The coronavirus pandemic has fundamentally altered American health care. At our academic medical center in Brooklyn, a large safety net institution, clinical year medical students are normally integral members of the team consistent with the model of “value-added medical education.”1 With the suspension of clinical rotations on March 13, 2020, a key part of the workforce was suddenly withdrawn while demand skyrocketed.
In response, students self-organized into numerous remote support projects, including the project described below.
Under infection control regulations, a “no-visitor” policy was instituted. Concurrently, the dramatic increase in patient volume left clinicians unable to regularly update patients’ families. To address this gap, a family contact line was created.
A dedicated phone number was distributed to key hospital personnel to share with families seeking information. The work flow for returning calls is shown in the figure. After verifying patient information and the caller’s relation, students provide updates based on chart review. Calls are prefaced with the disclaimer that students are not part of the treatment team and can only give information that is accessible via the electronic medical record.
Students created a phone script in conjunction with faculty, as well as a referral system for those seeking specific information from other departments. This script undergoes daily revision after the student huddle to address new issues. Flow of information is bidirectional: students relay patient updates as well as quarantine precautions and obtain past medical history. This proved essential during the surge of patients, unknown to the hospital and frequently altered, arriving by ambulance. Students document these conversations in the EMR, including family concerns and whether immediate provider follow-up is needed.
Two key limitations were quickly addressed: First, patients requiring ICU-level care have fluctuating courses, and an update based solely on chart review is insufficient. In response, students worked with intensivist teams to create a dedicated call line staffed by providers.
Second, conversations regarding goals of care and end of life concerns were beyond students’ scope. Together with palliative care teams, students developed criteria for flagging families for follow-up by a consulting palliative care attending.
Through working the call line, students received a crash course in empathetically communicating over the phone. Particularly during the worst of the surge, families were afraid and often frustrated at the lack of communication up to that point. Navigating these emotions, learning how to update family members while removed from the teams, and educating callers on quarantine precautions and other concerns was a valuable learning experience.
As students, we have been exposed to many of the realities of communicating as a physician. Relaying updates and prognosis to family while also providing emotional support is not something we are taught in medical school, but is something we will be expected to handle our first night on the wards as an intern. This experience has prepared us well for that and has illuminated missing parts of the medical school curriculum we are working on emphasizing moving forward.
Over the first 2 weeks, students put in 848 volunteer-hours, making 1,438 calls which reached 1,114 different families. We hope our experience proves instructive for other academic medical centers facing similar concerns in coming months. This model allows medical students to be directly involved in patient care during this crisis and shifts these time-intensive conversations away from overwhelmed primary medical teams.
Reference
1. Gonzalo JD et al. Value-added clinical systems learning roles for 355 medical students that transform education and health: A guide for building partnerships between 356 medical schools and health systems. Acad Med. 2017;92(5):602-7.
Ms. Jaiman is an MD candidate at State University of New York, Brooklyn and a PhD candidate at the National Center of Biological Sciences in Bangalore, India. Mr. Hessburg is an MD/PhD candidate at State University of New York, Brooklyn. Dr. Egelko is a recent graduate of State University of New York, Brooklyn.
The public’s trust in science
Having been a bench research scientist 30 years ago, I am flabbergasted at what is and is not currently possible. In a few weeks, scientists sequenced a novel coronavirus and used the genetic sequence to select candidate molecules for a vaccine. But we still can’t reliably say how much protection a cloth mask provides. Worse yet, even if/when we could reliably quantify contagion, it isn’t clear that the public will believe us anyhow.
The good news is that the public worldwide did believe scientists about the threat of a pandemic and the need to flatten the curve. Saving lives has not been about the strength of an antibiotic or the skill in managing a ventilator, but the credibility of medical scientists. The degree of acceptance was variable and subject to a variety of delays caused by regional politicians, but The bad news is that the public’s trust in that scientific advice has waned, the willingness to accept onerous restrictions has fatigued, and the cooperation for maintaining these social changes is evaporating.
I will leave pontificating about the spread of COVID-19 to other experts in other forums. My focus is on the public’s trust in the professionalism of physicians, nurses, medical scientists, and the health care industry as a whole. That trust has been our most valuable tool in fighting the pandemic so far. There have been situations in which weaknesses in modern science have let society down during the pandemic of the century. In my February 2020 column, at the beginning of the outbreak, a month before it was declared a pandemic, when its magnitude was still unclear, I emphasized the importance of having a trusted scientific spokesperson providing timely, accurate information to the public. That, obviously, did not happen in the United States and the degree of the ensuing disaster is still to be revealed.
Scientists have made some wrong decisions about this novel threat. The advice on masks is an illustrative example. For many years, infection control nurses have insisted that medical students wear a mask to protect themselves, even if they were observing rounds from just inside the doorway of a room of a baby with bronchiolitis. The landfills are full of briefly worn surgical masks. Now the story goes: Surgical masks don’t protect staff; they protect others. Changes like that contribute to a credibility gap.
For 3 months, there was conflicting advice about the appropriateness of masks. In early March 2020, some health care workers were disciplined for wearing personal masks. Now, most scientists recommend the public use masks to reduce contagion. Significant subgroups in the U.S. population have refused, mostly to signal their contrarian politics. In June there was an anecdote of a success story from the Show Me state of Missouri, where a mask is credited for preventing an outbreak from a sick hair stylist.
It is hard to find something more reliable than an anecdote. On June 1, a meta-analysis funded by the World Health Organization was published online by Lancet. It supports the idea that masks are beneficial. It is mostly forest plots, so you can try to interpret it yourself. There were 172 observational studies in the systematic review, and the meta-analysis contains 44 relevant comparative studies and 0 randomized controlled trials. Most of those forest plots have an I2 of 75% or worse, which to me indicates that they are not much more reliable than a good anecdote. My primary conclusion was that modern academic science, in an era with a shortage of toilet paper, should convert to printing on soft tissue paper.
It is important to note that the guesstimated overall benefit of cloth masks was a relative risk of 0.30. That benefit is easily nullified if the false security of a mask causes people to congregate together in groups three times larger or for three times more minutes. N95 masks were more effective.
A different article was published in PNAS on June 11. Its senior author was awarded the Nobel Prize in Chemistry in 1995. That article touted the benefits of masks. The article is facing heavy criticism for flaws in methodology and flaws in the peer review process. A long list of signatories have joined a letter asking for the article’s retraction.
This article, when combined with the two instances of prominent articles being retracted in the prior month by the New England Journal of Medicine and The Lancet, is accumulating evidence the peer review system is not working as intended.
There are many heroes in this pandemic, from the frontline health care workers in hotspots to the grocery workers and cleaning staff. There is hope, indeed some faith, that medical scientists in the foreseeable future will provide treatments and a vaccine for this viral plague. This month, the credibility of scientists again plays a major role as communities respond to outbreaks related to reopening the economy. Let’s celebrate the victories, resolve to fix the impure system, and restore a high level of public trust in science. Lives depend on it.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He has no relevant financial disclosures. Email him at [email protected].
Having been a bench research scientist 30 years ago, I am flabbergasted at what is and is not currently possible. In a few weeks, scientists sequenced a novel coronavirus and used the genetic sequence to select candidate molecules for a vaccine. But we still can’t reliably say how much protection a cloth mask provides. Worse yet, even if/when we could reliably quantify contagion, it isn’t clear that the public will believe us anyhow.
The good news is that the public worldwide did believe scientists about the threat of a pandemic and the need to flatten the curve. Saving lives has not been about the strength of an antibiotic or the skill in managing a ventilator, but the credibility of medical scientists. The degree of acceptance was variable and subject to a variety of delays caused by regional politicians, but The bad news is that the public’s trust in that scientific advice has waned, the willingness to accept onerous restrictions has fatigued, and the cooperation for maintaining these social changes is evaporating.
I will leave pontificating about the spread of COVID-19 to other experts in other forums. My focus is on the public’s trust in the professionalism of physicians, nurses, medical scientists, and the health care industry as a whole. That trust has been our most valuable tool in fighting the pandemic so far. There have been situations in which weaknesses in modern science have let society down during the pandemic of the century. In my February 2020 column, at the beginning of the outbreak, a month before it was declared a pandemic, when its magnitude was still unclear, I emphasized the importance of having a trusted scientific spokesperson providing timely, accurate information to the public. That, obviously, did not happen in the United States and the degree of the ensuing disaster is still to be revealed.
Scientists have made some wrong decisions about this novel threat. The advice on masks is an illustrative example. For many years, infection control nurses have insisted that medical students wear a mask to protect themselves, even if they were observing rounds from just inside the doorway of a room of a baby with bronchiolitis. The landfills are full of briefly worn surgical masks. Now the story goes: Surgical masks don’t protect staff; they protect others. Changes like that contribute to a credibility gap.
For 3 months, there was conflicting advice about the appropriateness of masks. In early March 2020, some health care workers were disciplined for wearing personal masks. Now, most scientists recommend the public use masks to reduce contagion. Significant subgroups in the U.S. population have refused, mostly to signal their contrarian politics. In June there was an anecdote of a success story from the Show Me state of Missouri, where a mask is credited for preventing an outbreak from a sick hair stylist.
It is hard to find something more reliable than an anecdote. On June 1, a meta-analysis funded by the World Health Organization was published online by Lancet. It supports the idea that masks are beneficial. It is mostly forest plots, so you can try to interpret it yourself. There were 172 observational studies in the systematic review, and the meta-analysis contains 44 relevant comparative studies and 0 randomized controlled trials. Most of those forest plots have an I2 of 75% or worse, which to me indicates that they are not much more reliable than a good anecdote. My primary conclusion was that modern academic science, in an era with a shortage of toilet paper, should convert to printing on soft tissue paper.
It is important to note that the guesstimated overall benefit of cloth masks was a relative risk of 0.30. That benefit is easily nullified if the false security of a mask causes people to congregate together in groups three times larger or for three times more minutes. N95 masks were more effective.
A different article was published in PNAS on June 11. Its senior author was awarded the Nobel Prize in Chemistry in 1995. That article touted the benefits of masks. The article is facing heavy criticism for flaws in methodology and flaws in the peer review process. A long list of signatories have joined a letter asking for the article’s retraction.
This article, when combined with the two instances of prominent articles being retracted in the prior month by the New England Journal of Medicine and The Lancet, is accumulating evidence the peer review system is not working as intended.
There are many heroes in this pandemic, from the frontline health care workers in hotspots to the grocery workers and cleaning staff. There is hope, indeed some faith, that medical scientists in the foreseeable future will provide treatments and a vaccine for this viral plague. This month, the credibility of scientists again plays a major role as communities respond to outbreaks related to reopening the economy. Let’s celebrate the victories, resolve to fix the impure system, and restore a high level of public trust in science. Lives depend on it.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He has no relevant financial disclosures. Email him at [email protected].
Having been a bench research scientist 30 years ago, I am flabbergasted at what is and is not currently possible. In a few weeks, scientists sequenced a novel coronavirus and used the genetic sequence to select candidate molecules for a vaccine. But we still can’t reliably say how much protection a cloth mask provides. Worse yet, even if/when we could reliably quantify contagion, it isn’t clear that the public will believe us anyhow.
The good news is that the public worldwide did believe scientists about the threat of a pandemic and the need to flatten the curve. Saving lives has not been about the strength of an antibiotic or the skill in managing a ventilator, but the credibility of medical scientists. The degree of acceptance was variable and subject to a variety of delays caused by regional politicians, but The bad news is that the public’s trust in that scientific advice has waned, the willingness to accept onerous restrictions has fatigued, and the cooperation for maintaining these social changes is evaporating.
I will leave pontificating about the spread of COVID-19 to other experts in other forums. My focus is on the public’s trust in the professionalism of physicians, nurses, medical scientists, and the health care industry as a whole. That trust has been our most valuable tool in fighting the pandemic so far. There have been situations in which weaknesses in modern science have let society down during the pandemic of the century. In my February 2020 column, at the beginning of the outbreak, a month before it was declared a pandemic, when its magnitude was still unclear, I emphasized the importance of having a trusted scientific spokesperson providing timely, accurate information to the public. That, obviously, did not happen in the United States and the degree of the ensuing disaster is still to be revealed.
Scientists have made some wrong decisions about this novel threat. The advice on masks is an illustrative example. For many years, infection control nurses have insisted that medical students wear a mask to protect themselves, even if they were observing rounds from just inside the doorway of a room of a baby with bronchiolitis. The landfills are full of briefly worn surgical masks. Now the story goes: Surgical masks don’t protect staff; they protect others. Changes like that contribute to a credibility gap.
For 3 months, there was conflicting advice about the appropriateness of masks. In early March 2020, some health care workers were disciplined for wearing personal masks. Now, most scientists recommend the public use masks to reduce contagion. Significant subgroups in the U.S. population have refused, mostly to signal their contrarian politics. In June there was an anecdote of a success story from the Show Me state of Missouri, where a mask is credited for preventing an outbreak from a sick hair stylist.
It is hard to find something more reliable than an anecdote. On June 1, a meta-analysis funded by the World Health Organization was published online by Lancet. It supports the idea that masks are beneficial. It is mostly forest plots, so you can try to interpret it yourself. There were 172 observational studies in the systematic review, and the meta-analysis contains 44 relevant comparative studies and 0 randomized controlled trials. Most of those forest plots have an I2 of 75% or worse, which to me indicates that they are not much more reliable than a good anecdote. My primary conclusion was that modern academic science, in an era with a shortage of toilet paper, should convert to printing on soft tissue paper.
It is important to note that the guesstimated overall benefit of cloth masks was a relative risk of 0.30. That benefit is easily nullified if the false security of a mask causes people to congregate together in groups three times larger or for three times more minutes. N95 masks were more effective.
A different article was published in PNAS on June 11. Its senior author was awarded the Nobel Prize in Chemistry in 1995. That article touted the benefits of masks. The article is facing heavy criticism for flaws in methodology and flaws in the peer review process. A long list of signatories have joined a letter asking for the article’s retraction.
This article, when combined with the two instances of prominent articles being retracted in the prior month by the New England Journal of Medicine and The Lancet, is accumulating evidence the peer review system is not working as intended.
There are many heroes in this pandemic, from the frontline health care workers in hotspots to the grocery workers and cleaning staff. There is hope, indeed some faith, that medical scientists in the foreseeable future will provide treatments and a vaccine for this viral plague. This month, the credibility of scientists again plays a major role as communities respond to outbreaks related to reopening the economy. Let’s celebrate the victories, resolve to fix the impure system, and restore a high level of public trust in science. Lives depend on it.
Dr. Powell is a pediatric hospitalist and clinical ethics consultant living in St. Louis. He has no relevant financial disclosures. Email him at [email protected].
COVID-19 symptoms can linger for months
Clinicians and researchers have focused on the acute phase of COVID-19 infection, but it’s increasingly clear that some recovered patients discharged from acute care need continued monitoring for long-lasting effects, a study has found.
In a research letter published online July 9 in JAMA, Angelo Carfi, MD, and colleagues from the Gemelli Against COVID-19 Post–Acute Care Study Group in Rome, report that
Postdischarge assessments of patients who met criteria for SARS-CoV-2 negativity, including a reverse transcriptase–polymerase chain reaction test, were conducted from April 21 to May 29. Among the results:
- Only 12.6% of the 143 patients were completely free of any COVID-19 symptom
- About 32% of patients had one or two symptoms and 55% had three or more
- None had fever or other signs and symptoms of acute illness
- About 53% of patients still had fatigue, 43.4% had dyspnea, 27.3% had joint pain, and had 21.7% chest pain
- About 44% reported worsened quality of life on the EuroQol visual analog scale.
The sample cohort, assessed in a COVID-19 patient service recently established at the Fondazione Policlinico Universitario Agostino Gemelli had a mean age of 56.5 years and 37% were women. The mean length of hospital stay was 13.5 days. During their hospitalization, 72.7% of patients showed evidence of interstitial pneumonia. Noninvasive ventilation was given to 14.7% of patients and 4.9% received invasive ventilation.
The reality of lingering symptoms has led Dr. Carfi’s clinic to schedule a final “wrap-up visit” for patients after full assessment. “On that occasion the doctor prescribes anything necessary to correct the anomalies found during the full evaluation,” Dr. Carfi, a geriatrician at the Gemelli clinic, said in an interview. “These usually include vitamin supplementation and, in selected cases, a new drug prescription such as a blood thinner if necessary.”
Patients can also enroll in a training program in which breathing status is monitored.
In North America, doctors are also addressing the reality that the road to recovery can be a long and upward one, with persistent symptoms worse than those seen with acute influenza infection. “We see patients who were first diagnosed in March or April and still have symptoms in July,” said Zijian Chen, MD, an endocrinologist and medical director of Mount Sinai Health System’s Center for Post-COVID Care in New York.
“Persistent symptoms are much worse for COVID patients than flu patients. Even flu patients who spent time in the intensive care unit recover fully, and we can optimize their breathing before discharge,” Dr. Chen said in an interview.
As in the Italian study, Dr. Chen sees patients with COVID-19 who have ongoing shortness of breath, some requiring supplemental oxygen, or with persistent chest pain on exertion, blood clotting problems, poor concentration, gastrointestinal distress, and reduced muscle strength and impaired grasping power. He doesn’t rule out permanent lung damage in some. “Even asymptomatic individuals already show lung scarring on imaging,” he said.
The Mount Sinai program provides specialized interdisciplinary management that may include CT scans, endoscopy, and drugs such as respiratory medications or anticoagulants. It also offers training to combat the fatigue and deconditioning caused by the infection, symptoms that are not medically treatable but impact quality of life.
“These patients do get better, but I expect they may still have symptoms requiring monitoring after a year,” Dr. Chen said.
The study received no specific funding. Dr. Carfi and colleagues have disclosed no relevant financial relationships. Dr. Chen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Clinicians and researchers have focused on the acute phase of COVID-19 infection, but it’s increasingly clear that some recovered patients discharged from acute care need continued monitoring for long-lasting effects, a study has found.
In a research letter published online July 9 in JAMA, Angelo Carfi, MD, and colleagues from the Gemelli Against COVID-19 Post–Acute Care Study Group in Rome, report that
Postdischarge assessments of patients who met criteria for SARS-CoV-2 negativity, including a reverse transcriptase–polymerase chain reaction test, were conducted from April 21 to May 29. Among the results:
- Only 12.6% of the 143 patients were completely free of any COVID-19 symptom
- About 32% of patients had one or two symptoms and 55% had three or more
- None had fever or other signs and symptoms of acute illness
- About 53% of patients still had fatigue, 43.4% had dyspnea, 27.3% had joint pain, and had 21.7% chest pain
- About 44% reported worsened quality of life on the EuroQol visual analog scale.
The sample cohort, assessed in a COVID-19 patient service recently established at the Fondazione Policlinico Universitario Agostino Gemelli had a mean age of 56.5 years and 37% were women. The mean length of hospital stay was 13.5 days. During their hospitalization, 72.7% of patients showed evidence of interstitial pneumonia. Noninvasive ventilation was given to 14.7% of patients and 4.9% received invasive ventilation.
The reality of lingering symptoms has led Dr. Carfi’s clinic to schedule a final “wrap-up visit” for patients after full assessment. “On that occasion the doctor prescribes anything necessary to correct the anomalies found during the full evaluation,” Dr. Carfi, a geriatrician at the Gemelli clinic, said in an interview. “These usually include vitamin supplementation and, in selected cases, a new drug prescription such as a blood thinner if necessary.”
Patients can also enroll in a training program in which breathing status is monitored.
In North America, doctors are also addressing the reality that the road to recovery can be a long and upward one, with persistent symptoms worse than those seen with acute influenza infection. “We see patients who were first diagnosed in March or April and still have symptoms in July,” said Zijian Chen, MD, an endocrinologist and medical director of Mount Sinai Health System’s Center for Post-COVID Care in New York.
“Persistent symptoms are much worse for COVID patients than flu patients. Even flu patients who spent time in the intensive care unit recover fully, and we can optimize their breathing before discharge,” Dr. Chen said in an interview.
As in the Italian study, Dr. Chen sees patients with COVID-19 who have ongoing shortness of breath, some requiring supplemental oxygen, or with persistent chest pain on exertion, blood clotting problems, poor concentration, gastrointestinal distress, and reduced muscle strength and impaired grasping power. He doesn’t rule out permanent lung damage in some. “Even asymptomatic individuals already show lung scarring on imaging,” he said.
The Mount Sinai program provides specialized interdisciplinary management that may include CT scans, endoscopy, and drugs such as respiratory medications or anticoagulants. It also offers training to combat the fatigue and deconditioning caused by the infection, symptoms that are not medically treatable but impact quality of life.
“These patients do get better, but I expect they may still have symptoms requiring monitoring after a year,” Dr. Chen said.
The study received no specific funding. Dr. Carfi and colleagues have disclosed no relevant financial relationships. Dr. Chen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Clinicians and researchers have focused on the acute phase of COVID-19 infection, but it’s increasingly clear that some recovered patients discharged from acute care need continued monitoring for long-lasting effects, a study has found.
In a research letter published online July 9 in JAMA, Angelo Carfi, MD, and colleagues from the Gemelli Against COVID-19 Post–Acute Care Study Group in Rome, report that
Postdischarge assessments of patients who met criteria for SARS-CoV-2 negativity, including a reverse transcriptase–polymerase chain reaction test, were conducted from April 21 to May 29. Among the results:
- Only 12.6% of the 143 patients were completely free of any COVID-19 symptom
- About 32% of patients had one or two symptoms and 55% had three or more
- None had fever or other signs and symptoms of acute illness
- About 53% of patients still had fatigue, 43.4% had dyspnea, 27.3% had joint pain, and had 21.7% chest pain
- About 44% reported worsened quality of life on the EuroQol visual analog scale.
The sample cohort, assessed in a COVID-19 patient service recently established at the Fondazione Policlinico Universitario Agostino Gemelli had a mean age of 56.5 years and 37% were women. The mean length of hospital stay was 13.5 days. During their hospitalization, 72.7% of patients showed evidence of interstitial pneumonia. Noninvasive ventilation was given to 14.7% of patients and 4.9% received invasive ventilation.
The reality of lingering symptoms has led Dr. Carfi’s clinic to schedule a final “wrap-up visit” for patients after full assessment. “On that occasion the doctor prescribes anything necessary to correct the anomalies found during the full evaluation,” Dr. Carfi, a geriatrician at the Gemelli clinic, said in an interview. “These usually include vitamin supplementation and, in selected cases, a new drug prescription such as a blood thinner if necessary.”
Patients can also enroll in a training program in which breathing status is monitored.
In North America, doctors are also addressing the reality that the road to recovery can be a long and upward one, with persistent symptoms worse than those seen with acute influenza infection. “We see patients who were first diagnosed in March or April and still have symptoms in July,” said Zijian Chen, MD, an endocrinologist and medical director of Mount Sinai Health System’s Center for Post-COVID Care in New York.
“Persistent symptoms are much worse for COVID patients than flu patients. Even flu patients who spent time in the intensive care unit recover fully, and we can optimize their breathing before discharge,” Dr. Chen said in an interview.
As in the Italian study, Dr. Chen sees patients with COVID-19 who have ongoing shortness of breath, some requiring supplemental oxygen, or with persistent chest pain on exertion, blood clotting problems, poor concentration, gastrointestinal distress, and reduced muscle strength and impaired grasping power. He doesn’t rule out permanent lung damage in some. “Even asymptomatic individuals already show lung scarring on imaging,” he said.
The Mount Sinai program provides specialized interdisciplinary management that may include CT scans, endoscopy, and drugs such as respiratory medications or anticoagulants. It also offers training to combat the fatigue and deconditioning caused by the infection, symptoms that are not medically treatable but impact quality of life.
“These patients do get better, but I expect they may still have symptoms requiring monitoring after a year,” Dr. Chen said.
The study received no specific funding. Dr. Carfi and colleagues have disclosed no relevant financial relationships. Dr. Chen has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Intubation boxes may do more harm than good in COVID-19 risk
Clear aerosol boxes designed to keep COVID-19 patients’ airborne droplets from infecting health care workers during intubation may actually increase providers’ exposure to the virus, a small study suggests.
Joanna P. Simpson, MbChB, an intensivist in the department of anaesthesia and perioperative medicine at Eastern Health in Melbourne, and colleagues tested five models of barriers used for protection while intubating simulated “patients” with COVID-19 and compared the interventions with a control of having no protection. They published their findings online in Anaesthesia.
Coauthor Peter Chan, MBBS, also an intensivist at Eastern Health, said in an interview that the virus essentially concentrates inside the box and because the box has holes on the sides to allow providers’ arms in, the gaps “act as nozzles, so when a patient coughs, it creates a sudden wave of air that pushes all these particles out the path of least resistance” and into the face of the intubator.
Their institution stopped using any such aerosol-containment devices during intubation until safety can be proven.
Many forms for boxes
The boxes take different forms and are made by various designers and manufacturers around the world, including in the United States, but they generally cover the head and upper body of patients and allow providers to reach through holes to intubate.
The U.S. Food and Drug Administration on May 1 issued an emergency use authorization (EUA) for “protective barrier enclosures ... to prevent [health care provider] exposure to pathogenic biological airborne particulates by providing an extra layer of barrier protection in addition to personal protective equipment [PPE].”
Others refer to them as “intubation boxes.” A search of GoFundMe campaigns showed hundreds of campaigns for intubation boxes.
Dr. Simpson and colleagues used an in-situ simulation model to evaluate laryngoscopist exposure to airborne particles sized 0.3-5.0 mcm using five aerosol containment devices (aerosol box, sealed box with suction, sealed box without suction, vertical drapes, and horizontal drapes) compared with no aerosol containment device.
Nebulized saline was used in an aerosol-generating model for 300 seconds, at which point the devices were removed to gauge particle spread for another 60 seconds.
Compared with no device use, the sealed intubation box with suction resulted in a decreased exposure for particle sizes of 0.3, 0.5, 1.0, and 2.5 mcm – but not 5.0 mcm – over all time periods (P = .003 for all time periods, which ranged from 30 to 360 seconds).
Conversely, the aerosol box, compared with no device use, showed an increase in 1.0, 2.5, and 5.0 mcm airborne-particle exposure at 300 seconds (P = .002, 0.008, and .002, respectively). Compared with no device use, neither horizontal nor vertical drapes showed any difference in any particle size exposure at any time.
The researchers used seven volunteers who took turns acting as the patient or the intubator. As each of the seven volunteers did all six trials (the five interventions plus no intervention), the study generated 42 sets of results.
More evidence passive boxes are ineffective
Plastic surgeon Dave Turer, MD, MS, who is also an electrical and biomedical engineer, and some emergency physician colleagues had doubts about these boxes early on and wrote about the need for thorough testing.
He told this news organization, “I find it kind of infuriating that if you search for ‘intubation box’ there are all these companies making claims that are totally unsubstantiated.”
A desperate need to stop the virus is leading to unacceptable practices, he said.
His team at the University of Pittsburgh Medical Center in Pennsylvania tested commercially available boxes using white vapor to simulate patients› exhaled breath and found the vapor billowed into the surrounding environment.
He said Simpson and colleagues had similar findings: The boxes didn’t contain the patients’ breaths and may even increase the stream heading toward intubators.
Dr. Turer said his team has designed a different kind of box, without armholes for the intubators, and with active airflow and filtering and have submitted their design and research to the FDA for an EUA.
The FDA’s current EUA is for boxes “that are no different from a face shield or a splash shield,” Dr. Turer said, adding that “they specifically state that they are not designed or intended to contain aerosol.”
He said while this study is a good start, his team’s findings will help demonstrate why the common passive boxes should not be used.
One of the most prevalent designs, he pointed out, was one by Taiwanese anesthesiologist Hsien Yung Lai that was widely circulated in March.
David W. Kaczka, MD, PhD, associate professor of anesthesia, biomedical engineering, and radiology at University of Iowa in Iowa City, is one of the researchers who modified that design and made prototypes. He said in an interview he thinks the study conclusion by Simpson et al is “not as dismal as the authors are making it out to be.”
He pointed to the relative success of the sealed box with suction. His team’s adapted model added a suction port to generate a negative pressure field around the patient.
The biggest critique he had of the study, Dr. Kaczka said, was a lack of a true control group.
“They tested all their conditions with nebulized saline,” he pointed out. “I think a more appropriately designed study would have also looked at a group where no saline was being nebulized and see what the particle counts were afterwards. It’s not clear how the device would distinguish between a particle coming from a saline nebulizer vs. coming from a simulated patient vs. coming from the laryngoscopist.”
He also noted that what comes out of a patient is not going to be saline and will have different density and viscosity.
That said, the study by Dr. Simpson and colleagues highlights the need to take a hard look at these boxes with more research, he said, adding, “I think there’s some hope there.”
He noted that a letter to the editor by Boston researchers, published online April 3 in the New England Journal of Medicine, describes how they used fluorescent dye forced from a balloon to simulate a patient’s cough to see whether an aerosol box protected intubators.
That letter concludes, “We suggest that our ad hoc barrier enclosure provided a modicum of additional protection and could be considered to be an adjunct to standard PPE.”
The Anaesthesia findings come as a second global wave becomes more likely as does awareness of the potential of airborne droplets to spread the virus.
Scientists from 32 countries warned the World Health Organization that the spread of COVID-19 through airborne droplets may have been severely underestimated.
On Wednesday, the World Health Organization formally acknowledged evidence regarding potential spread of the virus through these droplets and on Thursday issued an updated brief.
Intellectual property surrounding the device invented by Dr. Turer’s team is owned by UPMC. Dr. Chan and Dr. Kaczka have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clear aerosol boxes designed to keep COVID-19 patients’ airborne droplets from infecting health care workers during intubation may actually increase providers’ exposure to the virus, a small study suggests.
Joanna P. Simpson, MbChB, an intensivist in the department of anaesthesia and perioperative medicine at Eastern Health in Melbourne, and colleagues tested five models of barriers used for protection while intubating simulated “patients” with COVID-19 and compared the interventions with a control of having no protection. They published their findings online in Anaesthesia.
Coauthor Peter Chan, MBBS, also an intensivist at Eastern Health, said in an interview that the virus essentially concentrates inside the box and because the box has holes on the sides to allow providers’ arms in, the gaps “act as nozzles, so when a patient coughs, it creates a sudden wave of air that pushes all these particles out the path of least resistance” and into the face of the intubator.
Their institution stopped using any such aerosol-containment devices during intubation until safety can be proven.
Many forms for boxes
The boxes take different forms and are made by various designers and manufacturers around the world, including in the United States, but they generally cover the head and upper body of patients and allow providers to reach through holes to intubate.
The U.S. Food and Drug Administration on May 1 issued an emergency use authorization (EUA) for “protective barrier enclosures ... to prevent [health care provider] exposure to pathogenic biological airborne particulates by providing an extra layer of barrier protection in addition to personal protective equipment [PPE].”
Others refer to them as “intubation boxes.” A search of GoFundMe campaigns showed hundreds of campaigns for intubation boxes.
Dr. Simpson and colleagues used an in-situ simulation model to evaluate laryngoscopist exposure to airborne particles sized 0.3-5.0 mcm using five aerosol containment devices (aerosol box, sealed box with suction, sealed box without suction, vertical drapes, and horizontal drapes) compared with no aerosol containment device.
Nebulized saline was used in an aerosol-generating model for 300 seconds, at which point the devices were removed to gauge particle spread for another 60 seconds.
Compared with no device use, the sealed intubation box with suction resulted in a decreased exposure for particle sizes of 0.3, 0.5, 1.0, and 2.5 mcm – but not 5.0 mcm – over all time periods (P = .003 for all time periods, which ranged from 30 to 360 seconds).
Conversely, the aerosol box, compared with no device use, showed an increase in 1.0, 2.5, and 5.0 mcm airborne-particle exposure at 300 seconds (P = .002, 0.008, and .002, respectively). Compared with no device use, neither horizontal nor vertical drapes showed any difference in any particle size exposure at any time.
The researchers used seven volunteers who took turns acting as the patient or the intubator. As each of the seven volunteers did all six trials (the five interventions plus no intervention), the study generated 42 sets of results.
More evidence passive boxes are ineffective
Plastic surgeon Dave Turer, MD, MS, who is also an electrical and biomedical engineer, and some emergency physician colleagues had doubts about these boxes early on and wrote about the need for thorough testing.
He told this news organization, “I find it kind of infuriating that if you search for ‘intubation box’ there are all these companies making claims that are totally unsubstantiated.”
A desperate need to stop the virus is leading to unacceptable practices, he said.
His team at the University of Pittsburgh Medical Center in Pennsylvania tested commercially available boxes using white vapor to simulate patients› exhaled breath and found the vapor billowed into the surrounding environment.
He said Simpson and colleagues had similar findings: The boxes didn’t contain the patients’ breaths and may even increase the stream heading toward intubators.
Dr. Turer said his team has designed a different kind of box, without armholes for the intubators, and with active airflow and filtering and have submitted their design and research to the FDA for an EUA.
The FDA’s current EUA is for boxes “that are no different from a face shield or a splash shield,” Dr. Turer said, adding that “they specifically state that they are not designed or intended to contain aerosol.”
He said while this study is a good start, his team’s findings will help demonstrate why the common passive boxes should not be used.
One of the most prevalent designs, he pointed out, was one by Taiwanese anesthesiologist Hsien Yung Lai that was widely circulated in March.
David W. Kaczka, MD, PhD, associate professor of anesthesia, biomedical engineering, and radiology at University of Iowa in Iowa City, is one of the researchers who modified that design and made prototypes. He said in an interview he thinks the study conclusion by Simpson et al is “not as dismal as the authors are making it out to be.”
He pointed to the relative success of the sealed box with suction. His team’s adapted model added a suction port to generate a negative pressure field around the patient.
The biggest critique he had of the study, Dr. Kaczka said, was a lack of a true control group.
“They tested all their conditions with nebulized saline,” he pointed out. “I think a more appropriately designed study would have also looked at a group where no saline was being nebulized and see what the particle counts were afterwards. It’s not clear how the device would distinguish between a particle coming from a saline nebulizer vs. coming from a simulated patient vs. coming from the laryngoscopist.”
He also noted that what comes out of a patient is not going to be saline and will have different density and viscosity.
That said, the study by Dr. Simpson and colleagues highlights the need to take a hard look at these boxes with more research, he said, adding, “I think there’s some hope there.”
He noted that a letter to the editor by Boston researchers, published online April 3 in the New England Journal of Medicine, describes how they used fluorescent dye forced from a balloon to simulate a patient’s cough to see whether an aerosol box protected intubators.
That letter concludes, “We suggest that our ad hoc barrier enclosure provided a modicum of additional protection and could be considered to be an adjunct to standard PPE.”
The Anaesthesia findings come as a second global wave becomes more likely as does awareness of the potential of airborne droplets to spread the virus.
Scientists from 32 countries warned the World Health Organization that the spread of COVID-19 through airborne droplets may have been severely underestimated.
On Wednesday, the World Health Organization formally acknowledged evidence regarding potential spread of the virus through these droplets and on Thursday issued an updated brief.
Intellectual property surrounding the device invented by Dr. Turer’s team is owned by UPMC. Dr. Chan and Dr. Kaczka have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Clear aerosol boxes designed to keep COVID-19 patients’ airborne droplets from infecting health care workers during intubation may actually increase providers’ exposure to the virus, a small study suggests.
Joanna P. Simpson, MbChB, an intensivist in the department of anaesthesia and perioperative medicine at Eastern Health in Melbourne, and colleagues tested five models of barriers used for protection while intubating simulated “patients” with COVID-19 and compared the interventions with a control of having no protection. They published their findings online in Anaesthesia.
Coauthor Peter Chan, MBBS, also an intensivist at Eastern Health, said in an interview that the virus essentially concentrates inside the box and because the box has holes on the sides to allow providers’ arms in, the gaps “act as nozzles, so when a patient coughs, it creates a sudden wave of air that pushes all these particles out the path of least resistance” and into the face of the intubator.
Their institution stopped using any such aerosol-containment devices during intubation until safety can be proven.
Many forms for boxes
The boxes take different forms and are made by various designers and manufacturers around the world, including in the United States, but they generally cover the head and upper body of patients and allow providers to reach through holes to intubate.
The U.S. Food and Drug Administration on May 1 issued an emergency use authorization (EUA) for “protective barrier enclosures ... to prevent [health care provider] exposure to pathogenic biological airborne particulates by providing an extra layer of barrier protection in addition to personal protective equipment [PPE].”
Others refer to them as “intubation boxes.” A search of GoFundMe campaigns showed hundreds of campaigns for intubation boxes.
Dr. Simpson and colleagues used an in-situ simulation model to evaluate laryngoscopist exposure to airborne particles sized 0.3-5.0 mcm using five aerosol containment devices (aerosol box, sealed box with suction, sealed box without suction, vertical drapes, and horizontal drapes) compared with no aerosol containment device.
Nebulized saline was used in an aerosol-generating model for 300 seconds, at which point the devices were removed to gauge particle spread for another 60 seconds.
Compared with no device use, the sealed intubation box with suction resulted in a decreased exposure for particle sizes of 0.3, 0.5, 1.0, and 2.5 mcm – but not 5.0 mcm – over all time periods (P = .003 for all time periods, which ranged from 30 to 360 seconds).
Conversely, the aerosol box, compared with no device use, showed an increase in 1.0, 2.5, and 5.0 mcm airborne-particle exposure at 300 seconds (P = .002, 0.008, and .002, respectively). Compared with no device use, neither horizontal nor vertical drapes showed any difference in any particle size exposure at any time.
The researchers used seven volunteers who took turns acting as the patient or the intubator. As each of the seven volunteers did all six trials (the five interventions plus no intervention), the study generated 42 sets of results.
More evidence passive boxes are ineffective
Plastic surgeon Dave Turer, MD, MS, who is also an electrical and biomedical engineer, and some emergency physician colleagues had doubts about these boxes early on and wrote about the need for thorough testing.
He told this news organization, “I find it kind of infuriating that if you search for ‘intubation box’ there are all these companies making claims that are totally unsubstantiated.”
A desperate need to stop the virus is leading to unacceptable practices, he said.
His team at the University of Pittsburgh Medical Center in Pennsylvania tested commercially available boxes using white vapor to simulate patients› exhaled breath and found the vapor billowed into the surrounding environment.
He said Simpson and colleagues had similar findings: The boxes didn’t contain the patients’ breaths and may even increase the stream heading toward intubators.
Dr. Turer said his team has designed a different kind of box, without armholes for the intubators, and with active airflow and filtering and have submitted their design and research to the FDA for an EUA.
The FDA’s current EUA is for boxes “that are no different from a face shield or a splash shield,” Dr. Turer said, adding that “they specifically state that they are not designed or intended to contain aerosol.”
He said while this study is a good start, his team’s findings will help demonstrate why the common passive boxes should not be used.
One of the most prevalent designs, he pointed out, was one by Taiwanese anesthesiologist Hsien Yung Lai that was widely circulated in March.
David W. Kaczka, MD, PhD, associate professor of anesthesia, biomedical engineering, and radiology at University of Iowa in Iowa City, is one of the researchers who modified that design and made prototypes. He said in an interview he thinks the study conclusion by Simpson et al is “not as dismal as the authors are making it out to be.”
He pointed to the relative success of the sealed box with suction. His team’s adapted model added a suction port to generate a negative pressure field around the patient.
The biggest critique he had of the study, Dr. Kaczka said, was a lack of a true control group.
“They tested all their conditions with nebulized saline,” he pointed out. “I think a more appropriately designed study would have also looked at a group where no saline was being nebulized and see what the particle counts were afterwards. It’s not clear how the device would distinguish between a particle coming from a saline nebulizer vs. coming from a simulated patient vs. coming from the laryngoscopist.”
He also noted that what comes out of a patient is not going to be saline and will have different density and viscosity.
That said, the study by Dr. Simpson and colleagues highlights the need to take a hard look at these boxes with more research, he said, adding, “I think there’s some hope there.”
He noted that a letter to the editor by Boston researchers, published online April 3 in the New England Journal of Medicine, describes how they used fluorescent dye forced from a balloon to simulate a patient’s cough to see whether an aerosol box protected intubators.
That letter concludes, “We suggest that our ad hoc barrier enclosure provided a modicum of additional protection and could be considered to be an adjunct to standard PPE.”
The Anaesthesia findings come as a second global wave becomes more likely as does awareness of the potential of airborne droplets to spread the virus.
Scientists from 32 countries warned the World Health Organization that the spread of COVID-19 through airborne droplets may have been severely underestimated.
On Wednesday, the World Health Organization formally acknowledged evidence regarding potential spread of the virus through these droplets and on Thursday issued an updated brief.
Intellectual property surrounding the device invented by Dr. Turer’s team is owned by UPMC. Dr. Chan and Dr. Kaczka have disclosed no relevant financial relationships.
This article first appeared on Medscape.com.
Wave, surge, or tsunami
Different COVID-19 models and predicting inpatient bed capacity
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
Different COVID-19 models and predicting inpatient bed capacity
Different COVID-19 models and predicting inpatient bed capacity
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
The COVID-19 pandemic is one of the defining moments in history for this generation’s health care leaders. In 2019, most of us wrongly assumed that this virus would be similar to the past viral epidemics and pandemics such as 2002 severe acute respiratory syndrome–CoV in Asia, 2009 H1N1 influenza in the United States, 2012 Middle East respiratory syndrome–CoV in Saudi Arabia, and 2014-2016 Ebola in West Africa. Moreover, we understood that the 50% fatality rate of Ebola, a single-stranded RNA virus, was deadly on the continent of Africa, but its transmission was through direct contact with blood or other bodily fluids. Hence, the infectivity of Ebola to the general public was lower than SARS-CoV-2, which is spread by respiratory droplets and contact routes in addition to being the virus that causes COVID-19.1 Many of us did not expect that SARS-CoV-2, a single-stranded RNA virus consisting of 32 kilobytes, would reach the shores of the United States from the Hubei province of China, the northern Lombardy region of Italy, or other initial hotspots. We could not imagine its effects would be so devastating from an economic and medical perspective. Until it did.
The first reported case of SARS-CoV-2 was on Jan. 20, 2020 in Snohomish County, Wash., and the first known death from COVID-19 occurred on Feb. 6, 2020 in Santa Clara County, Calif.2,3 Since then, the United States has lost over 135,000 people from COVID-19 with death(s) reported in every state and the highest number of overall deaths of any country in the world.4 At the beginning of 2020, at our institution, Wake Forest Baptist Health System in Winston-Salem, N.C., we began preparing for the wave, surge, or tsunami of inpatients that was coming. Plans were afoot to increase our staff, even perhaps by hiring out-of-state physicians and nurses if needed, and every possible bed was considered within the system. It was not an if, but rather a when, as to the arrival of COVID-19.
Epidemiologists and biostatisticians developed predictive COVID-19 models so that health care leaders could plan accordingly, especially those patients that required critical care or inpatient medical care. These predictive models have been used across the globe and can be categorized into three groups: Susceptible-Exposed-Infectious-Recovered, Agent-Based, and Curve Fitting Extrapolation.5 Our original predictions were based on the Institute for Health Metrics and Evaluation model from Washington state (Curve Fitting Extrapolation). It creates projections from COVID-19 mortality data and assumes a 3% infection rate. Other health systems in our region used the COVID-19 Hospital Impact Model for Epidemics–University of Pennsylvania model. It pins its suppositions on hospitalized COVID-19 patients, regional infection rates, and hospital market shares. Lastly, the agent-based mode, such as the Global Epidemic and Mobility Project, takes simulated populations and forecasts the spread of SARS-CoV-2 anchoring on the interplay of individuals and groups. The assumptions are created secondary to the interactions of people, time, health care interventions, and public health policies.
Based on these predictive simulations, health systems have spent countless hours of planning and have utilized resources for the anticipated needs related to beds, ventilators, supplies, and staffing. Frontline staff were retrained how to don and doff personal protective equipment. Our teams were ready if we saw a wave of 250, a surge of 500, or a tsunami of 750 COVID-19 inpatients. We were prepared to run into the fire fully knowing the personal risks and consequences.
But, as yet, the tsunami in North Carolina has never come. On April 21, 2020, the COVID-19 mortality data in North Carolina peaked at 34 deaths, with the total number of deaths standing at 1,510 as of July 13, 2020.6 A surge did not hit our institutional shores at Wake Forest Baptist Health. As we looked through the proverbial back window and hear about the tsunami in Houston, Texas, we are very thankful that the tsunami turned out to be a small wave so far in North Carolina. We are grateful that there were fewer deaths than expected. The dust is settling now and the question, spoken or unspoken, is: “How could we be so wrong with our predictions?”
Models have strengths and weaknesses and none are perfect.7 There is an old aphorism in statistics that is often attributed to George Box that says: “All models are wrong but some are useful.”8 Predictions and projections are good, but not perfect. Our measurements and tests should not only be accurate, but also be as precise as possible.9 Moreover, the assumptions we make should be on solid ground. Since the beginning of the pandemic, there may have been undercounts and delays in reporting. The assumptions of the effects of social distancing may have been inaccurate. Just as important, the lack of early testing in our pandemic and the relatively limited testing currently available provide challenges not only in attributing past deaths to COVID-19, but also with planning and public health measures. To be fair, the tsunami that turned out to be a small wave in North Carolina may be caused by the strong leadership from politicians, public health officials, and health system leaders for their stay-at-home decree and vigorous public health measures in our state.
Some of the health systems in the United States have created “reemergence plans” to care for those patients who have stayed at home for the past several months. Elective surgeries and procedures have begun in different regions of the United States and will likely continue reopening into the late summer. Nevertheless, challenges and opportunities continue to abound during these difficult times of COVID-19. The tsunamis or surges will continue to occur in the United States and the premature reopening of some of the public places and businesses have not helped our collective efforts. In addition, the personal costs have been and will be immeasurable. Many of us have lost loved ones, been laid off, or face mental health crises because of the social isolation and false news.
COVID-19 is here to stay and will be with us for the foreseeable future. Health care providers have been literally risking their lives to serve the public and we will continue to do so. Hitting the target of needed inpatient beds and critical care beds is critically important and is tough without accurate data. We simply have inadequate and unreliable data of COVID-19 incidence and prevalence rates in the communities that we serve. More available testing would allow frontline health care providers and health care leaders to match hospital demand to supply, at individual hospitals and within the health care system. Moreover, contact tracing capabilities would give us the opportunity to isolate individuals and extinguish population-based hotspots.
We may have seen the first wave, but other waves of COVID-19 in North Carolina are sure to come. Since the partial reopening of North Carolina on May 8, 2020, coupled with pockets of nonadherence to social distancing and mask wearing, we expect a second wave sooner rather than later. Interestingly, daily new lab-confirmed COVID-19 cases in North Carolina have been on the rise, with the highest one-day total occurring on June 12, 2020 with 1,768 cases reported.6 As a result, North Carolina Gov. Roy Cooper and Secretary of the North Carolina Department of Health and Human Services, Dr. Mandy Cohen, placed a temporary pause on the Phase 2 reopening plan and mandated masks in public on June 24, 2020. It is unclear whether these intermittent daily spikes in lab-confirmed COVID-19 cases are a foreshadowing of our next wave, surge, or tsunami, or just an anomaly. Only time will tell, but as Jim Kim, MD, PhD, has stated so well, there is still time for social distancing, contact tracing, testing, isolation, and treatment.10 There is still time for us, for our loved ones, for our hospital systems, and for our public health system.
Dr. Huang is the executive medical director and service line director of general medicine and hospital medicine within the Wake Forest Baptist Health System and associate professor of internal medicine at Wake Forest School of Medicine. Dr. Lippert is assistant professor of internal medicine at Wake Forest School of Medicine. Mr. Payne is the associate vice president of Wake Forest Baptist Health. He is responsible for engineering, facilities planning & design as well as environmental health and safety departments. Dr. Pariyadath is comedical director of the Patient Flow Operations Center which facilitates patient placement throughout the Wake Forest Baptist Health system. He is also the associate medical director for the adult emergency department. Dr. Sunkara is assistant professor of internal medicine at Wake Forest School of Medicine. He is the medical director for hospital medicine units and the newly established PUI unit.
Acknowledgments
The authors would like to thank Julie Freischlag, MD; Kevin High, MD, MS; Gary Rosenthal, MD; Wayne Meredith, MD;Russ Howerton, MD; Mike Waid, Andrea Fernandez, MD; Brian Hiestand, MD; the Wake Forest Baptist Health System COVID-19 task force, the Operations Center, and the countless frontline staff at all five hospitals within the Wake Forest Baptist Health System.
References
1. World Health Organization. Modes of transmission of virus causing COVID-19: Implications for IPC precaution recommendations. 2020 June 30. https://www.who.int/news-room/commentaries/detail/modes-of-transmission-of-virus-causing-covid-19-implications-for-ipc-precaution-recommendations.
2. Holshue et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382: 929-36.
3. Fuller T, Baker M. Coronavirus death in California came weeks before first known U.S. death. New York Times. 2020 Apr 22. https://www.nytimes.com/2020/04/22/us/coronavirus-first-united-states-death.html.
4. Johns Hopkins Coronavirus Resource Center. https://coronavirus.jhu.edu/us-map. Accessed 2020 May 28.
5. Michaud J et al. COVID-19 models: Can they tell us what we want to know? 2020 April 16. https://www.kff.org/coronavirus-policy-watch/covid-19-models.
6. Centers for Disease Control and Prevention. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed 2020 June 30.
7. Jewell N et al. Caution warranted: Using the Institute for Health Metrics and Evaluation Model for predicting the course of the COVID-19 pandemic. Ann Intern Med. 2020;173:1-3.
8. Box G. Science and statistics. J Am Stat Assoc. 1972;71:791-9.
9. Shapiro DE. The interpretation of diagnostic tests. Stat Methods Med Res. 1999;8:113-34.
10. Kim J. It is not too late to go on the offense against the coronavirus. The New Yorker. 2020 Apr 20. https://www.newyorker.com/science/medical-dispatch/its-not-too-late-to-go-on-offense-against-the-coronavirus.
Hep C sofosbuvir/daclatasvir combo promising for COVID-19
research from an open-label Iranian study shows.
And the good news is that the treatment combination “already has a well-established safety profile in the treatment of hepatitis C,” said investigator Andrew Hill, PhD, from the University of Liverpool, United Kingdom.
But although the results look promising, they are preliminary, he cautioned. The combination could follow the path of ritonavir plus lopinavir (Kaletra, AbbVie Pharmaceuticals) or hydroxychloroquine (Plaquenil, Sanofi Pharmaceuticals), which showed promise early but did not perform as hoped in large randomized controlled trials.
“We need to remember that conducting research amidst a pandemic with overwhelmed hospitals is a clear challenge, and we cannot be sure of success,” he added.
Three Trials, 176 Patients
Data collected during a four-site trial of the combination treatment in Tehran during an early spike in cases in Iran were presented at the Virtual COVID-19 Conference 2020 by Hannah Wentzel, a masters student in public health at Imperial College London and a member of Hill’s team.
All 66 study participants were diagnosed with moderate to severe COVID-19 and were treated with standard care, which consisted of hydroxychloroquine 200 mg twice daily with or without the combination of lopinavir plus ritonavir 250 mg twice daily.
The 33 patients randomized to the treatment group also received the combination of sofosbuvir plus daclatasvir 460 mg once daily. These patients were slightly younger and more likely to be men than were those in the standard-care group, but the differences were not significant.
All participants were treated for 14 days, and then the researchers assessed fever, respiration rate, and blood oxygen saturation.
More patients in the treatment group than in the standard-care group had recovered at 14 days (88% vs 67%), but the difference was not significant.
However, median time to clinical recovery, which took into account death as a competing risk, was significantly faster in the treatment group than in the standard-care group (6 vs 11 days; P = .041).
The researchers then pooled their Tehran data with those from two other trials of the sofosbuvir plus daclatasvir combination conducted in Iran: one in the city of Sari with 48 patients and one in the city of Abadan with 62 patients.
A meta-analysis showed that clinical recovery in 14 days was 14% better in the treatment group than in the control group in the Sari study, 32% better in the Tehran study, and 82% better in the Abadan study. However, in a sensitivity analysis, because “the trial in Abadan was not properly randomized,” only the improvements in the Sari and Tehran studies were significant, Wentzel reported.
The meta-analysis also showed that patients in the treatment groups were 70% more likely than those in the standard-care groups to survive.
However, the treatment regimens in the standard-care groups of the three studies were all different, reflecting evolving national treatment guidelines in Iran at the time. And SARS-CoV-2 viral loads were not measured in any of the trials, so the effects of the different drugs on the virus itself could not be assessed.
Still, overall, “sofosbuvir and daclatasvir is associated with faster discharge from hospital and improved survival,” Wentzel said.
These findings are hopeful, “provocative, and encouraging,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and he echoed Hill’s call to “get these kinds of studies into randomized controlled trials.”
But he cautioned that more data are needed before the sofosbuvir and daclatasvir combination can be added to the National Institutes of Health COVID-19 Treatment Guidelines, which clinicians who might be under-resourced and overwhelmed with spikes in COVID-19 cases rely on.
Results from three double-blind randomized controlled trials – one each in Iran, Egypt, and South Africa – with an estimated cumulative enrollment of about 2,000 patients, are expected in October, Hill reported.
“Having gone through feeling so desperate to help people and try new things, it’s really important to do these trials,” said Kristen Marks, MD, from Weill Cornell Medicine in New York City.
“You get tempted to just kind of throw anything at people. And I think we really have to have science to guide us,” she told Medscape Medical News.
This article first appeared on Medscape.com.
research from an open-label Iranian study shows.
And the good news is that the treatment combination “already has a well-established safety profile in the treatment of hepatitis C,” said investigator Andrew Hill, PhD, from the University of Liverpool, United Kingdom.
But although the results look promising, they are preliminary, he cautioned. The combination could follow the path of ritonavir plus lopinavir (Kaletra, AbbVie Pharmaceuticals) or hydroxychloroquine (Plaquenil, Sanofi Pharmaceuticals), which showed promise early but did not perform as hoped in large randomized controlled trials.
“We need to remember that conducting research amidst a pandemic with overwhelmed hospitals is a clear challenge, and we cannot be sure of success,” he added.
Three Trials, 176 Patients
Data collected during a four-site trial of the combination treatment in Tehran during an early spike in cases in Iran were presented at the Virtual COVID-19 Conference 2020 by Hannah Wentzel, a masters student in public health at Imperial College London and a member of Hill’s team.
All 66 study participants were diagnosed with moderate to severe COVID-19 and were treated with standard care, which consisted of hydroxychloroquine 200 mg twice daily with or without the combination of lopinavir plus ritonavir 250 mg twice daily.
The 33 patients randomized to the treatment group also received the combination of sofosbuvir plus daclatasvir 460 mg once daily. These patients were slightly younger and more likely to be men than were those in the standard-care group, but the differences were not significant.
All participants were treated for 14 days, and then the researchers assessed fever, respiration rate, and blood oxygen saturation.
More patients in the treatment group than in the standard-care group had recovered at 14 days (88% vs 67%), but the difference was not significant.
However, median time to clinical recovery, which took into account death as a competing risk, was significantly faster in the treatment group than in the standard-care group (6 vs 11 days; P = .041).
The researchers then pooled their Tehran data with those from two other trials of the sofosbuvir plus daclatasvir combination conducted in Iran: one in the city of Sari with 48 patients and one in the city of Abadan with 62 patients.
A meta-analysis showed that clinical recovery in 14 days was 14% better in the treatment group than in the control group in the Sari study, 32% better in the Tehran study, and 82% better in the Abadan study. However, in a sensitivity analysis, because “the trial in Abadan was not properly randomized,” only the improvements in the Sari and Tehran studies were significant, Wentzel reported.
The meta-analysis also showed that patients in the treatment groups were 70% more likely than those in the standard-care groups to survive.
However, the treatment regimens in the standard-care groups of the three studies were all different, reflecting evolving national treatment guidelines in Iran at the time. And SARS-CoV-2 viral loads were not measured in any of the trials, so the effects of the different drugs on the virus itself could not be assessed.
Still, overall, “sofosbuvir and daclatasvir is associated with faster discharge from hospital and improved survival,” Wentzel said.
These findings are hopeful, “provocative, and encouraging,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and he echoed Hill’s call to “get these kinds of studies into randomized controlled trials.”
But he cautioned that more data are needed before the sofosbuvir and daclatasvir combination can be added to the National Institutes of Health COVID-19 Treatment Guidelines, which clinicians who might be under-resourced and overwhelmed with spikes in COVID-19 cases rely on.
Results from three double-blind randomized controlled trials – one each in Iran, Egypt, and South Africa – with an estimated cumulative enrollment of about 2,000 patients, are expected in October, Hill reported.
“Having gone through feeling so desperate to help people and try new things, it’s really important to do these trials,” said Kristen Marks, MD, from Weill Cornell Medicine in New York City.
“You get tempted to just kind of throw anything at people. And I think we really have to have science to guide us,” she told Medscape Medical News.
This article first appeared on Medscape.com.
research from an open-label Iranian study shows.
And the good news is that the treatment combination “already has a well-established safety profile in the treatment of hepatitis C,” said investigator Andrew Hill, PhD, from the University of Liverpool, United Kingdom.
But although the results look promising, they are preliminary, he cautioned. The combination could follow the path of ritonavir plus lopinavir (Kaletra, AbbVie Pharmaceuticals) or hydroxychloroquine (Plaquenil, Sanofi Pharmaceuticals), which showed promise early but did not perform as hoped in large randomized controlled trials.
“We need to remember that conducting research amidst a pandemic with overwhelmed hospitals is a clear challenge, and we cannot be sure of success,” he added.
Three Trials, 176 Patients
Data collected during a four-site trial of the combination treatment in Tehran during an early spike in cases in Iran were presented at the Virtual COVID-19 Conference 2020 by Hannah Wentzel, a masters student in public health at Imperial College London and a member of Hill’s team.
All 66 study participants were diagnosed with moderate to severe COVID-19 and were treated with standard care, which consisted of hydroxychloroquine 200 mg twice daily with or without the combination of lopinavir plus ritonavir 250 mg twice daily.
The 33 patients randomized to the treatment group also received the combination of sofosbuvir plus daclatasvir 460 mg once daily. These patients were slightly younger and more likely to be men than were those in the standard-care group, but the differences were not significant.
All participants were treated for 14 days, and then the researchers assessed fever, respiration rate, and blood oxygen saturation.
More patients in the treatment group than in the standard-care group had recovered at 14 days (88% vs 67%), but the difference was not significant.
However, median time to clinical recovery, which took into account death as a competing risk, was significantly faster in the treatment group than in the standard-care group (6 vs 11 days; P = .041).
The researchers then pooled their Tehran data with those from two other trials of the sofosbuvir plus daclatasvir combination conducted in Iran: one in the city of Sari with 48 patients and one in the city of Abadan with 62 patients.
A meta-analysis showed that clinical recovery in 14 days was 14% better in the treatment group than in the control group in the Sari study, 32% better in the Tehran study, and 82% better in the Abadan study. However, in a sensitivity analysis, because “the trial in Abadan was not properly randomized,” only the improvements in the Sari and Tehran studies were significant, Wentzel reported.
The meta-analysis also showed that patients in the treatment groups were 70% more likely than those in the standard-care groups to survive.
However, the treatment regimens in the standard-care groups of the three studies were all different, reflecting evolving national treatment guidelines in Iran at the time. And SARS-CoV-2 viral loads were not measured in any of the trials, so the effects of the different drugs on the virus itself could not be assessed.
Still, overall, “sofosbuvir and daclatasvir is associated with faster discharge from hospital and improved survival,” Wentzel said.
These findings are hopeful, “provocative, and encouraging,” said Anthony Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, and he echoed Hill’s call to “get these kinds of studies into randomized controlled trials.”
But he cautioned that more data are needed before the sofosbuvir and daclatasvir combination can be added to the National Institutes of Health COVID-19 Treatment Guidelines, which clinicians who might be under-resourced and overwhelmed with spikes in COVID-19 cases rely on.
Results from three double-blind randomized controlled trials – one each in Iran, Egypt, and South Africa – with an estimated cumulative enrollment of about 2,000 patients, are expected in October, Hill reported.
“Having gone through feeling so desperate to help people and try new things, it’s really important to do these trials,” said Kristen Marks, MD, from Weill Cornell Medicine in New York City.
“You get tempted to just kind of throw anything at people. And I think we really have to have science to guide us,” she told Medscape Medical News.
This article first appeared on Medscape.com.
Calculations of an academic hospitalist
The term “academic hospitalist” has come to mean more than a mere affiliation to an academic medical center (AMC). Academic hospitalists perform various clinical roles like staffing house staff teams, covering nonteaching services, critical care services, procedure teams, night services, medical consultation, and comanagement services.
Over the last decade, academic hospitalists have successfully managed many nonclinical roles in areas like research, medical unit leadership, faculty development, faculty affairs, quality, safety, informatics, utilization review, clinical documentation, throughput, group management, hospital administration, and educational leadership. The role of an academic hospital is as clear as a chocolate martini these days. Here we present some recent trends in academic hospital medicine.
Compensation
From SHM’s State of Hospital Medicine report (SoHM)2014 to 2018 data, the median compensation for U.S. academic hospitalists has risen by an average of 5.15% every year, although increases vary by rank.1 From 2016 to 2018, clinical instructors saw the most significant growth, 11.23% per year, suggesting a need to remain competitive for junior hospitalists. Compensation also varies by geographic area, with the Southern region reporting the highest compensation. Over the last decade, academic hospitalists received, on average, a 28%-35% lower salary, compared with community hospitalists.
Patient population and census
Lower patient encounters and compensation of the academic hospitalists poses the chicken or the egg dilemma. In the 2018 SoHM report, academic hospitalists had an average of 17% fewer encounters. Of note, AMC patients tend to have higher complexity, as measured by the Case Mix Index (CMI – the average diagnosis-related group weight of a hospital).2 A higher CMI is a surrogate marker for the diagnostic diversity, clinical complexity, and resource needs of the patient population in the hospital.
Productivity and financial metrics
The financial bottom line is a critical aspect, and as a report in the Journal of Hospital Medicine described, all health care executives look at business metrics while making decisions.3 Below are some significant academic and community comparisons from SoHM 2018.
- Collections, encounters, and wRVUs (work relative value units) were highly correlated. All of them were lower for academic hospitalists, corroborating the fact that they see a smaller number of patients. Clinical full-time equivalents (cFTE) is a vernacular of how much of the faculty time is devoted to clinical activities. The academic data from SoHM achieves the same target, as it is standardized to 100% billable clinical activity, so the fact that many academic hospitalists do not work a full-time clinical schedule is not a factor in their lower production.
- Charges had a smaller gap likely because of sicker patients in AMCs. The higher acuity difference can also explain 12% higher wRVU/encounter for academic hospitalists.
- The wRVU/encounter ratio can indicate a few patterns: high acuity of patients in AMCs, higher levels of evaluation and management documentation, or both. As the encounters and charges have the same percentage differences, we would place our bets on the former.
- Compensation per encounter and compensation per wRVU showed that academic hospitalists do get a slight advantage.
CMI and wRVUs
Although the SoHM does not capture information on patient acuity or CMI, we speculate that the relationship between CMI and wRVUs may be more or less linear at lower levels of acuity. However, once level III E/M billing is achieved (assuming there is no critical care provided), wRVUs/encounter plateau, even as acuity continues to increase. This plateau effect may be seen more often in high-acuity AMC settings than in community hospitals.
So, in our opinion, compensation models based solely on wRVU production would not do justice for hospitalists in AMC settings since these models would fail to capture the extra work involved with very-high-acuity patients. SoHM 2018 shows the financial support per wRVU for AMC is $45.81, and for the community is $41.28, an 11% difference. We think the higher financial support per wRVU for academic practices may be related to the lost wRVU potential of caring for very-high-acuity patients.
Conclusion
In an academic setting, hospitalists are reforming the field of hospital medicine and defining the ways we could deliver care. They are the pillars of collaboration, education, research, innovation, quality, and safety. It would be increasingly crucial for academic hospitalist leaders to use comparative metrics from SoHM to advocate for their group. The bottom line can be explained by the title of the qualitative study in JHM referenced above: “Collaboration, not calculation.”3
Dr. Chadha is division chief for the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, scheduling, practice analysis, and operation of the group. He is a first-time member of the practice analysis committee. Ms. Dede is division administrator for the division of hospital medicine at the University of Kentucky Healthcare. She prepares and manages budgets, liaisons with the downstream revenue teams, and contributes to the building of academic compensation models. She is serving in the practice administrators committee for the second year and is currently vice chair of the Executive Council for the Practice Administrators special interest group.
References
1. State of Hospital Medicine Report. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
2. Deloitte Center for Health Solutions. Academic Medical Centers: Joining forces with community providers for broad benefits and positive outcomes. 2015. https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/academic-medical-centers-consolidation.html
3. White AA et al. Collaboration, not calculation: A qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(10):662‐7.
The term “academic hospitalist” has come to mean more than a mere affiliation to an academic medical center (AMC). Academic hospitalists perform various clinical roles like staffing house staff teams, covering nonteaching services, critical care services, procedure teams, night services, medical consultation, and comanagement services.
Over the last decade, academic hospitalists have successfully managed many nonclinical roles in areas like research, medical unit leadership, faculty development, faculty affairs, quality, safety, informatics, utilization review, clinical documentation, throughput, group management, hospital administration, and educational leadership. The role of an academic hospital is as clear as a chocolate martini these days. Here we present some recent trends in academic hospital medicine.
Compensation
From SHM’s State of Hospital Medicine report (SoHM)2014 to 2018 data, the median compensation for U.S. academic hospitalists has risen by an average of 5.15% every year, although increases vary by rank.1 From 2016 to 2018, clinical instructors saw the most significant growth, 11.23% per year, suggesting a need to remain competitive for junior hospitalists. Compensation also varies by geographic area, with the Southern region reporting the highest compensation. Over the last decade, academic hospitalists received, on average, a 28%-35% lower salary, compared with community hospitalists.
Patient population and census
Lower patient encounters and compensation of the academic hospitalists poses the chicken or the egg dilemma. In the 2018 SoHM report, academic hospitalists had an average of 17% fewer encounters. Of note, AMC patients tend to have higher complexity, as measured by the Case Mix Index (CMI – the average diagnosis-related group weight of a hospital).2 A higher CMI is a surrogate marker for the diagnostic diversity, clinical complexity, and resource needs of the patient population in the hospital.
Productivity and financial metrics
The financial bottom line is a critical aspect, and as a report in the Journal of Hospital Medicine described, all health care executives look at business metrics while making decisions.3 Below are some significant academic and community comparisons from SoHM 2018.
- Collections, encounters, and wRVUs (work relative value units) were highly correlated. All of them were lower for academic hospitalists, corroborating the fact that they see a smaller number of patients. Clinical full-time equivalents (cFTE) is a vernacular of how much of the faculty time is devoted to clinical activities. The academic data from SoHM achieves the same target, as it is standardized to 100% billable clinical activity, so the fact that many academic hospitalists do not work a full-time clinical schedule is not a factor in their lower production.
- Charges had a smaller gap likely because of sicker patients in AMCs. The higher acuity difference can also explain 12% higher wRVU/encounter for academic hospitalists.
- The wRVU/encounter ratio can indicate a few patterns: high acuity of patients in AMCs, higher levels of evaluation and management documentation, or both. As the encounters and charges have the same percentage differences, we would place our bets on the former.
- Compensation per encounter and compensation per wRVU showed that academic hospitalists do get a slight advantage.
CMI and wRVUs
Although the SoHM does not capture information on patient acuity or CMI, we speculate that the relationship between CMI and wRVUs may be more or less linear at lower levels of acuity. However, once level III E/M billing is achieved (assuming there is no critical care provided), wRVUs/encounter plateau, even as acuity continues to increase. This plateau effect may be seen more often in high-acuity AMC settings than in community hospitals.
So, in our opinion, compensation models based solely on wRVU production would not do justice for hospitalists in AMC settings since these models would fail to capture the extra work involved with very-high-acuity patients. SoHM 2018 shows the financial support per wRVU for AMC is $45.81, and for the community is $41.28, an 11% difference. We think the higher financial support per wRVU for academic practices may be related to the lost wRVU potential of caring for very-high-acuity patients.
Conclusion
In an academic setting, hospitalists are reforming the field of hospital medicine and defining the ways we could deliver care. They are the pillars of collaboration, education, research, innovation, quality, and safety. It would be increasingly crucial for academic hospitalist leaders to use comparative metrics from SoHM to advocate for their group. The bottom line can be explained by the title of the qualitative study in JHM referenced above: “Collaboration, not calculation.”3
Dr. Chadha is division chief for the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, scheduling, practice analysis, and operation of the group. He is a first-time member of the practice analysis committee. Ms. Dede is division administrator for the division of hospital medicine at the University of Kentucky Healthcare. She prepares and manages budgets, liaisons with the downstream revenue teams, and contributes to the building of academic compensation models. She is serving in the practice administrators committee for the second year and is currently vice chair of the Executive Council for the Practice Administrators special interest group.
References
1. State of Hospital Medicine Report. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
2. Deloitte Center for Health Solutions. Academic Medical Centers: Joining forces with community providers for broad benefits and positive outcomes. 2015. https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/academic-medical-centers-consolidation.html
3. White AA et al. Collaboration, not calculation: A qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(10):662‐7.
The term “academic hospitalist” has come to mean more than a mere affiliation to an academic medical center (AMC). Academic hospitalists perform various clinical roles like staffing house staff teams, covering nonteaching services, critical care services, procedure teams, night services, medical consultation, and comanagement services.
Over the last decade, academic hospitalists have successfully managed many nonclinical roles in areas like research, medical unit leadership, faculty development, faculty affairs, quality, safety, informatics, utilization review, clinical documentation, throughput, group management, hospital administration, and educational leadership. The role of an academic hospital is as clear as a chocolate martini these days. Here we present some recent trends in academic hospital medicine.
Compensation
From SHM’s State of Hospital Medicine report (SoHM)2014 to 2018 data, the median compensation for U.S. academic hospitalists has risen by an average of 5.15% every year, although increases vary by rank.1 From 2016 to 2018, clinical instructors saw the most significant growth, 11.23% per year, suggesting a need to remain competitive for junior hospitalists. Compensation also varies by geographic area, with the Southern region reporting the highest compensation. Over the last decade, academic hospitalists received, on average, a 28%-35% lower salary, compared with community hospitalists.
Patient population and census
Lower patient encounters and compensation of the academic hospitalists poses the chicken or the egg dilemma. In the 2018 SoHM report, academic hospitalists had an average of 17% fewer encounters. Of note, AMC patients tend to have higher complexity, as measured by the Case Mix Index (CMI – the average diagnosis-related group weight of a hospital).2 A higher CMI is a surrogate marker for the diagnostic diversity, clinical complexity, and resource needs of the patient population in the hospital.
Productivity and financial metrics
The financial bottom line is a critical aspect, and as a report in the Journal of Hospital Medicine described, all health care executives look at business metrics while making decisions.3 Below are some significant academic and community comparisons from SoHM 2018.
- Collections, encounters, and wRVUs (work relative value units) were highly correlated. All of them were lower for academic hospitalists, corroborating the fact that they see a smaller number of patients. Clinical full-time equivalents (cFTE) is a vernacular of how much of the faculty time is devoted to clinical activities. The academic data from SoHM achieves the same target, as it is standardized to 100% billable clinical activity, so the fact that many academic hospitalists do not work a full-time clinical schedule is not a factor in their lower production.
- Charges had a smaller gap likely because of sicker patients in AMCs. The higher acuity difference can also explain 12% higher wRVU/encounter for academic hospitalists.
- The wRVU/encounter ratio can indicate a few patterns: high acuity of patients in AMCs, higher levels of evaluation and management documentation, or both. As the encounters and charges have the same percentage differences, we would place our bets on the former.
- Compensation per encounter and compensation per wRVU showed that academic hospitalists do get a slight advantage.
CMI and wRVUs
Although the SoHM does not capture information on patient acuity or CMI, we speculate that the relationship between CMI and wRVUs may be more or less linear at lower levels of acuity. However, once level III E/M billing is achieved (assuming there is no critical care provided), wRVUs/encounter plateau, even as acuity continues to increase. This plateau effect may be seen more often in high-acuity AMC settings than in community hospitals.
So, in our opinion, compensation models based solely on wRVU production would not do justice for hospitalists in AMC settings since these models would fail to capture the extra work involved with very-high-acuity patients. SoHM 2018 shows the financial support per wRVU for AMC is $45.81, and for the community is $41.28, an 11% difference. We think the higher financial support per wRVU for academic practices may be related to the lost wRVU potential of caring for very-high-acuity patients.
Conclusion
In an academic setting, hospitalists are reforming the field of hospital medicine and defining the ways we could deliver care. They are the pillars of collaboration, education, research, innovation, quality, and safety. It would be increasingly crucial for academic hospitalist leaders to use comparative metrics from SoHM to advocate for their group. The bottom line can be explained by the title of the qualitative study in JHM referenced above: “Collaboration, not calculation.”3
Dr. Chadha is division chief for the division of hospital medicine at the University of Kentucky Healthcare, Lexington. He actively leads efforts of recruiting, scheduling, practice analysis, and operation of the group. He is a first-time member of the practice analysis committee. Ms. Dede is division administrator for the division of hospital medicine at the University of Kentucky Healthcare. She prepares and manages budgets, liaisons with the downstream revenue teams, and contributes to the building of academic compensation models. She is serving in the practice administrators committee for the second year and is currently vice chair of the Executive Council for the Practice Administrators special interest group.
References
1. State of Hospital Medicine Report. https://www.hospitalmedicine.org/practice-management/shms-state-of-hospital-medicine/
2. Deloitte Center for Health Solutions. Academic Medical Centers: Joining forces with community providers for broad benefits and positive outcomes. 2015. https://www2.deloitte.com/us/en/pages/life-sciences-and-health-care/articles/academic-medical-centers-consolidation.html
3. White AA et al. Collaboration, not calculation: A qualitative study of how hospital executives value hospital medicine groups. J Hosp Med. 2019;14(10):662‐7.
Medical societies advise on vitamin D in midst of COVID-19
Six medical societies from across the globe are emphasizing the importance of individuals obtaining the daily recommended dose of vitamin D, especially given the impact of the COVID-19 pandemic on outdoor time.
The statement, “Joint Guidance on Vitamin D in the Era of COVID-19,” is supported by the American Society for Bone and Mineral Research, the Endocrine Society, and the American Association of Clinical Endocrinologists, among others.
They felt the need to clarify the recommendations for clinicians. Central to the guidance is the recommendation to directly expose the skin to sunlight for 15-30 minutes per day, while taking care to avoid sunburn.
The statement noted that “vitamin D is very safe when taken at reasonable dosages and is important for musculoskeletal health. Levels are likely to decline as individuals reduce outside activity (sun exposure) during the pandemic.”
It added that “most older and younger adults can safely take 400-1000 IU daily to keep vitamin D levels within the optimal range as recommended by [the US] Institute of Medicine guidelines.”
The statement also noted that the scientific evidence clearly supports the benefits that vitamin D (in combination with calcium intake) plays in building a strong skeleton and preventing bone loss.
Other societies supporting the statement are the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
What role for vitamin D in COVID-19?
Over recent months, the role of vitamin D in relation to prevention of COVID-19 has been the subject of intense debate. Now, these societies have joined forces and endorsed evidence-based guidance to clarify the issue around obtaining the daily recommended dosage of vitamin D.
During the pandemic, orders to stay at home meant individuals were likely to spend less time outdoors and have less opportunity to draw their vitamin D directly from sunlight, which is its main source, other than a limited number of foods or as a dietary supplement, the societies explained.
However, they acknowledged that the role of vitamin D in COVID-19 remains unclear.
“The current data do not provide any evidence that vitamin D supplementation will help prevent or treat COVID-19 infection; however, our guidance does not preclude further study of the potential effects of vitamin D on COVID-19,” the joint statement said.
Research to date suggests that vitamin D may play a role in enhancing the immune response, and given prior work demonstrating a role for the activated form of vitamin D – 1,25(OH)2D – in immune responses, “further research into vitamin D supplementation in COVID-19 disease is warranted,” it added. “Trials to date have been observational and there have been no randomized, controlled trials from which firm conclusions about causal relationships can be drawn. Observational studies suggest associations between low vitamin D concentrations and higher rates of COVID-19 infection.”
Medscape Medical News previously reported on the existing observational data regarding vitamin D in COVID-19. A recent rapid evidence review by the National Institute for Health and Care Excellence failed to find any evidence that vitamin D supplementation reduces the risk or severity of COVID-19.
A version of this article originally appeared on Medscape.com.
Six medical societies from across the globe are emphasizing the importance of individuals obtaining the daily recommended dose of vitamin D, especially given the impact of the COVID-19 pandemic on outdoor time.
The statement, “Joint Guidance on Vitamin D in the Era of COVID-19,” is supported by the American Society for Bone and Mineral Research, the Endocrine Society, and the American Association of Clinical Endocrinologists, among others.
They felt the need to clarify the recommendations for clinicians. Central to the guidance is the recommendation to directly expose the skin to sunlight for 15-30 minutes per day, while taking care to avoid sunburn.
The statement noted that “vitamin D is very safe when taken at reasonable dosages and is important for musculoskeletal health. Levels are likely to decline as individuals reduce outside activity (sun exposure) during the pandemic.”
It added that “most older and younger adults can safely take 400-1000 IU daily to keep vitamin D levels within the optimal range as recommended by [the US] Institute of Medicine guidelines.”
The statement also noted that the scientific evidence clearly supports the benefits that vitamin D (in combination with calcium intake) plays in building a strong skeleton and preventing bone loss.
Other societies supporting the statement are the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
What role for vitamin D in COVID-19?
Over recent months, the role of vitamin D in relation to prevention of COVID-19 has been the subject of intense debate. Now, these societies have joined forces and endorsed evidence-based guidance to clarify the issue around obtaining the daily recommended dosage of vitamin D.
During the pandemic, orders to stay at home meant individuals were likely to spend less time outdoors and have less opportunity to draw their vitamin D directly from sunlight, which is its main source, other than a limited number of foods or as a dietary supplement, the societies explained.
However, they acknowledged that the role of vitamin D in COVID-19 remains unclear.
“The current data do not provide any evidence that vitamin D supplementation will help prevent or treat COVID-19 infection; however, our guidance does not preclude further study of the potential effects of vitamin D on COVID-19,” the joint statement said.
Research to date suggests that vitamin D may play a role in enhancing the immune response, and given prior work demonstrating a role for the activated form of vitamin D – 1,25(OH)2D – in immune responses, “further research into vitamin D supplementation in COVID-19 disease is warranted,” it added. “Trials to date have been observational and there have been no randomized, controlled trials from which firm conclusions about causal relationships can be drawn. Observational studies suggest associations between low vitamin D concentrations and higher rates of COVID-19 infection.”
Medscape Medical News previously reported on the existing observational data regarding vitamin D in COVID-19. A recent rapid evidence review by the National Institute for Health and Care Excellence failed to find any evidence that vitamin D supplementation reduces the risk or severity of COVID-19.
A version of this article originally appeared on Medscape.com.
Six medical societies from across the globe are emphasizing the importance of individuals obtaining the daily recommended dose of vitamin D, especially given the impact of the COVID-19 pandemic on outdoor time.
The statement, “Joint Guidance on Vitamin D in the Era of COVID-19,” is supported by the American Society for Bone and Mineral Research, the Endocrine Society, and the American Association of Clinical Endocrinologists, among others.
They felt the need to clarify the recommendations for clinicians. Central to the guidance is the recommendation to directly expose the skin to sunlight for 15-30 minutes per day, while taking care to avoid sunburn.
The statement noted that “vitamin D is very safe when taken at reasonable dosages and is important for musculoskeletal health. Levels are likely to decline as individuals reduce outside activity (sun exposure) during the pandemic.”
It added that “most older and younger adults can safely take 400-1000 IU daily to keep vitamin D levels within the optimal range as recommended by [the US] Institute of Medicine guidelines.”
The statement also noted that the scientific evidence clearly supports the benefits that vitamin D (in combination with calcium intake) plays in building a strong skeleton and preventing bone loss.
Other societies supporting the statement are the European Calcified Tissue Society, the National Osteoporosis Foundation, and the International Osteoporosis Foundation.
What role for vitamin D in COVID-19?
Over recent months, the role of vitamin D in relation to prevention of COVID-19 has been the subject of intense debate. Now, these societies have joined forces and endorsed evidence-based guidance to clarify the issue around obtaining the daily recommended dosage of vitamin D.
During the pandemic, orders to stay at home meant individuals were likely to spend less time outdoors and have less opportunity to draw their vitamin D directly from sunlight, which is its main source, other than a limited number of foods or as a dietary supplement, the societies explained.
However, they acknowledged that the role of vitamin D in COVID-19 remains unclear.
“The current data do not provide any evidence that vitamin D supplementation will help prevent or treat COVID-19 infection; however, our guidance does not preclude further study of the potential effects of vitamin D on COVID-19,” the joint statement said.
Research to date suggests that vitamin D may play a role in enhancing the immune response, and given prior work demonstrating a role for the activated form of vitamin D – 1,25(OH)2D – in immune responses, “further research into vitamin D supplementation in COVID-19 disease is warranted,” it added. “Trials to date have been observational and there have been no randomized, controlled trials from which firm conclusions about causal relationships can be drawn. Observational studies suggest associations between low vitamin D concentrations and higher rates of COVID-19 infection.”
Medscape Medical News previously reported on the existing observational data regarding vitamin D in COVID-19. A recent rapid evidence review by the National Institute for Health and Care Excellence failed to find any evidence that vitamin D supplementation reduces the risk or severity of COVID-19.
A version of this article originally appeared on Medscape.com.