User login
In Case You Missed It: COVID
COVID-19 vaccination in cancer patients: NCCN outlines priorities
Vaccination timing considerations vary based on factors such as cancer and treatment type, and reasons for delaying vaccination in the general public also apply to cancer patients (recent COVID-19 exposure, for example).
In general, however, patients with cancer should be assigned to Centers for Disease Control and Prevention priority group 1 b/c and immunized when vaccination is available to them, the guidelines state. Exceptions to this recommendation include:
- Patients undergoing hematopoietic stem cell transplant or receiving engineered cellular therapy such as chimeric antigen receptor T-cell therapy. Vaccination should be delayed for at least 3 months in these patients to maximize vaccine efficacy. Caregivers of these patients, however, should be immunized when possible.
- Patients with hematologic malignancies who are receiving intensive cytotoxic chemotherapy, such as cytarabine- or anthracycline-based regimens for acute myeloid leukemia. Vaccination in these patients should be delayed until absolute neutrophil count recovery.
- Patients undergoing major surgery. Vaccination should occur at least a few days before or after surgery.
- Patients who have experienced a severe or immediate adverse reaction to any of the ingredients in the mRNA COVID-19 vaccines.
Conversely, vaccination should occur when available in patients with hematologic malignancies and marrow failure who are expected to have limited or no recovery, patients with hematologic malignancies who are on long-term maintenance therapy, and patients with solid tumors who are receiving cytotoxic chemotherapy, targeted therapy, checkpoint inhibitors and other immunotherapy, or radiotherapy.
Caregivers, household contacts, and other close contacts who are 16 years of age and older should be vaccinated whenever they are eligible.
Unique concerns in patients with cancer
The NCCN recommendations were developed to address the unique issues and concerns with respect to patients with cancer, who have an increased risk of severe illness from SARS-CoV-2 infection. But the guidelines come with a caveat: “[t]here are limited safety and efficacy data in these patients,” the NCCN emphasized in a press statement.
“Right now, there is urgent need and limited data,” Steven Pergam, MD, co-leader of the NCCN COVID-19 Vaccination Committee, said in the statement.
“Our number one goal is helping to get the vaccine to as many people as we can,” Dr. Pergam said. “That means following existing national and regional directions for prioritizing people who are more likely to face death or severe illness from COVID-19.”
Dr. Pergam, associate professor at Fred Hutchinson Cancer Research Center in Seattle, further explained that “people receiving active cancer treatment are at greater risk for worse outcomes from COVID-19, particularly if they are older and have additional comorbidities, like immunosuppression.”
NCCN’s recommendations couldn’t have come at a better time for patients with cancer, according to Nora Disis, MD, a professor at the University of Washington in Seattle.
“The NCCN’s recommendations to prioritize COVID vaccinations for cancer patients on active treatment is an important step forward in protecting our patients from the infection,” Dr. Disis said in an interview.
“Cancer patients may be at higher risk for the complications seen with infection. In addition, cancer is a disease of older people, and a good number of our patients have the comorbidities that would predict a poorer outcome if they should become sick,” Dr. Disis added. “With the correct treatment, many patients with cancer will be long-term survivors. It is important that they be protected from infection with COVID to realize their best outcome.”
Additional vaccine considerations
The NCCN recommendations also address several other issues of importance for cancer patients, including:
- Deprioritizing other vaccines. COVID-19 vaccines should take precedence over other vaccines because data on dual vaccination are lacking. The NCCN recommends waiting 14 days after COVID-19 vaccination to deliver other vaccines.
- Vaccinating clinical trial participants. Trial leads should be consulted to prevent protocol violations or exclusions.
- Decision-making in the setting of limited vaccine availability. The NCCN noted that decisions on allocation must be made in accordance with state and local vaccine guidance but suggests prioritizing appropriate patients on active treatment, those planning to start treatment, and those who have just completed treatment. Additional risk factors for these patients, as well as other factors associated with risk for adverse COVID-19 outcomes, should also be considered. These include advanced age, comorbidities, and adverse social and demographic factors such as poverty and limited health care access.
- The need for ongoing prevention measures. Vaccines have been shown to decrease the incidence of COVID-19 and related complications, but it remains unclear whether vaccines prevent infection and subsequent transmission. This means everyone should continue following prevention recommendations, such as wearing masks and avoiding crowds.
The NCCN stressed that these recommendations are “intended to be a living document that is constantly evolving – it will be updated rapidly whenever new data comes out, as well as any potential new vaccines that may get approved in the future.” The NCCN also noted that the advisory committee will meet regularly to refine the recommendations as needed.
Dr. Pergam disclosed relationships with Chimerix Inc., Merck & Co., Global Life Technologies Inc., and Sanofi-Aventis. Dr. Disis disclosed grants from Pfizer, Bavarian Nordisk, Janssen, and Precigen. She is the founder of EpiThany and editor-in-chief of JAMA Oncology.
Vaccination timing considerations vary based on factors such as cancer and treatment type, and reasons for delaying vaccination in the general public also apply to cancer patients (recent COVID-19 exposure, for example).
In general, however, patients with cancer should be assigned to Centers for Disease Control and Prevention priority group 1 b/c and immunized when vaccination is available to them, the guidelines state. Exceptions to this recommendation include:
- Patients undergoing hematopoietic stem cell transplant or receiving engineered cellular therapy such as chimeric antigen receptor T-cell therapy. Vaccination should be delayed for at least 3 months in these patients to maximize vaccine efficacy. Caregivers of these patients, however, should be immunized when possible.
- Patients with hematologic malignancies who are receiving intensive cytotoxic chemotherapy, such as cytarabine- or anthracycline-based regimens for acute myeloid leukemia. Vaccination in these patients should be delayed until absolute neutrophil count recovery.
- Patients undergoing major surgery. Vaccination should occur at least a few days before or after surgery.
- Patients who have experienced a severe or immediate adverse reaction to any of the ingredients in the mRNA COVID-19 vaccines.
Conversely, vaccination should occur when available in patients with hematologic malignancies and marrow failure who are expected to have limited or no recovery, patients with hematologic malignancies who are on long-term maintenance therapy, and patients with solid tumors who are receiving cytotoxic chemotherapy, targeted therapy, checkpoint inhibitors and other immunotherapy, or radiotherapy.
Caregivers, household contacts, and other close contacts who are 16 years of age and older should be vaccinated whenever they are eligible.
Unique concerns in patients with cancer
The NCCN recommendations were developed to address the unique issues and concerns with respect to patients with cancer, who have an increased risk of severe illness from SARS-CoV-2 infection. But the guidelines come with a caveat: “[t]here are limited safety and efficacy data in these patients,” the NCCN emphasized in a press statement.
“Right now, there is urgent need and limited data,” Steven Pergam, MD, co-leader of the NCCN COVID-19 Vaccination Committee, said in the statement.
“Our number one goal is helping to get the vaccine to as many people as we can,” Dr. Pergam said. “That means following existing national and regional directions for prioritizing people who are more likely to face death or severe illness from COVID-19.”
Dr. Pergam, associate professor at Fred Hutchinson Cancer Research Center in Seattle, further explained that “people receiving active cancer treatment are at greater risk for worse outcomes from COVID-19, particularly if they are older and have additional comorbidities, like immunosuppression.”
NCCN’s recommendations couldn’t have come at a better time for patients with cancer, according to Nora Disis, MD, a professor at the University of Washington in Seattle.
“The NCCN’s recommendations to prioritize COVID vaccinations for cancer patients on active treatment is an important step forward in protecting our patients from the infection,” Dr. Disis said in an interview.
“Cancer patients may be at higher risk for the complications seen with infection. In addition, cancer is a disease of older people, and a good number of our patients have the comorbidities that would predict a poorer outcome if they should become sick,” Dr. Disis added. “With the correct treatment, many patients with cancer will be long-term survivors. It is important that they be protected from infection with COVID to realize their best outcome.”
Additional vaccine considerations
The NCCN recommendations also address several other issues of importance for cancer patients, including:
- Deprioritizing other vaccines. COVID-19 vaccines should take precedence over other vaccines because data on dual vaccination are lacking. The NCCN recommends waiting 14 days after COVID-19 vaccination to deliver other vaccines.
- Vaccinating clinical trial participants. Trial leads should be consulted to prevent protocol violations or exclusions.
- Decision-making in the setting of limited vaccine availability. The NCCN noted that decisions on allocation must be made in accordance with state and local vaccine guidance but suggests prioritizing appropriate patients on active treatment, those planning to start treatment, and those who have just completed treatment. Additional risk factors for these patients, as well as other factors associated with risk for adverse COVID-19 outcomes, should also be considered. These include advanced age, comorbidities, and adverse social and demographic factors such as poverty and limited health care access.
- The need for ongoing prevention measures. Vaccines have been shown to decrease the incidence of COVID-19 and related complications, but it remains unclear whether vaccines prevent infection and subsequent transmission. This means everyone should continue following prevention recommendations, such as wearing masks and avoiding crowds.
The NCCN stressed that these recommendations are “intended to be a living document that is constantly evolving – it will be updated rapidly whenever new data comes out, as well as any potential new vaccines that may get approved in the future.” The NCCN also noted that the advisory committee will meet regularly to refine the recommendations as needed.
Dr. Pergam disclosed relationships with Chimerix Inc., Merck & Co., Global Life Technologies Inc., and Sanofi-Aventis. Dr. Disis disclosed grants from Pfizer, Bavarian Nordisk, Janssen, and Precigen. She is the founder of EpiThany and editor-in-chief of JAMA Oncology.
Vaccination timing considerations vary based on factors such as cancer and treatment type, and reasons for delaying vaccination in the general public also apply to cancer patients (recent COVID-19 exposure, for example).
In general, however, patients with cancer should be assigned to Centers for Disease Control and Prevention priority group 1 b/c and immunized when vaccination is available to them, the guidelines state. Exceptions to this recommendation include:
- Patients undergoing hematopoietic stem cell transplant or receiving engineered cellular therapy such as chimeric antigen receptor T-cell therapy. Vaccination should be delayed for at least 3 months in these patients to maximize vaccine efficacy. Caregivers of these patients, however, should be immunized when possible.
- Patients with hematologic malignancies who are receiving intensive cytotoxic chemotherapy, such as cytarabine- or anthracycline-based regimens for acute myeloid leukemia. Vaccination in these patients should be delayed until absolute neutrophil count recovery.
- Patients undergoing major surgery. Vaccination should occur at least a few days before or after surgery.
- Patients who have experienced a severe or immediate adverse reaction to any of the ingredients in the mRNA COVID-19 vaccines.
Conversely, vaccination should occur when available in patients with hematologic malignancies and marrow failure who are expected to have limited or no recovery, patients with hematologic malignancies who are on long-term maintenance therapy, and patients with solid tumors who are receiving cytotoxic chemotherapy, targeted therapy, checkpoint inhibitors and other immunotherapy, or radiotherapy.
Caregivers, household contacts, and other close contacts who are 16 years of age and older should be vaccinated whenever they are eligible.
Unique concerns in patients with cancer
The NCCN recommendations were developed to address the unique issues and concerns with respect to patients with cancer, who have an increased risk of severe illness from SARS-CoV-2 infection. But the guidelines come with a caveat: “[t]here are limited safety and efficacy data in these patients,” the NCCN emphasized in a press statement.
“Right now, there is urgent need and limited data,” Steven Pergam, MD, co-leader of the NCCN COVID-19 Vaccination Committee, said in the statement.
“Our number one goal is helping to get the vaccine to as many people as we can,” Dr. Pergam said. “That means following existing national and regional directions for prioritizing people who are more likely to face death or severe illness from COVID-19.”
Dr. Pergam, associate professor at Fred Hutchinson Cancer Research Center in Seattle, further explained that “people receiving active cancer treatment are at greater risk for worse outcomes from COVID-19, particularly if they are older and have additional comorbidities, like immunosuppression.”
NCCN’s recommendations couldn’t have come at a better time for patients with cancer, according to Nora Disis, MD, a professor at the University of Washington in Seattle.
“The NCCN’s recommendations to prioritize COVID vaccinations for cancer patients on active treatment is an important step forward in protecting our patients from the infection,” Dr. Disis said in an interview.
“Cancer patients may be at higher risk for the complications seen with infection. In addition, cancer is a disease of older people, and a good number of our patients have the comorbidities that would predict a poorer outcome if they should become sick,” Dr. Disis added. “With the correct treatment, many patients with cancer will be long-term survivors. It is important that they be protected from infection with COVID to realize their best outcome.”
Additional vaccine considerations
The NCCN recommendations also address several other issues of importance for cancer patients, including:
- Deprioritizing other vaccines. COVID-19 vaccines should take precedence over other vaccines because data on dual vaccination are lacking. The NCCN recommends waiting 14 days after COVID-19 vaccination to deliver other vaccines.
- Vaccinating clinical trial participants. Trial leads should be consulted to prevent protocol violations or exclusions.
- Decision-making in the setting of limited vaccine availability. The NCCN noted that decisions on allocation must be made in accordance with state and local vaccine guidance but suggests prioritizing appropriate patients on active treatment, those planning to start treatment, and those who have just completed treatment. Additional risk factors for these patients, as well as other factors associated with risk for adverse COVID-19 outcomes, should also be considered. These include advanced age, comorbidities, and adverse social and demographic factors such as poverty and limited health care access.
- The need for ongoing prevention measures. Vaccines have been shown to decrease the incidence of COVID-19 and related complications, but it remains unclear whether vaccines prevent infection and subsequent transmission. This means everyone should continue following prevention recommendations, such as wearing masks and avoiding crowds.
The NCCN stressed that these recommendations are “intended to be a living document that is constantly evolving – it will be updated rapidly whenever new data comes out, as well as any potential new vaccines that may get approved in the future.” The NCCN also noted that the advisory committee will meet regularly to refine the recommendations as needed.
Dr. Pergam disclosed relationships with Chimerix Inc., Merck & Co., Global Life Technologies Inc., and Sanofi-Aventis. Dr. Disis disclosed grants from Pfizer, Bavarian Nordisk, Janssen, and Precigen. She is the founder of EpiThany and editor-in-chief of JAMA Oncology.
COVID-19 may alter gut microbiota
COVID-19 infection altered the gut microbiota of adult patients and caused depletion of several types of bacteria with known immunomodulatory properties, based on data from a cohort study of 100 patients with confirmed COVID-19 infections from two hospitals.
“As the GI tract is the largest immunological organ in the body and its resident microbiota are known to modulate host immune responses, we hypothesized that the gut microbiota is associated with host inflammatory immune responses in COVID19,” wrote Yun Kit Yeoh, PhD, of the Chinese University of Hong Kong, and colleagues.
In a study published in Gut, the researchers investigated patient microbiota by collecting blood, stool, and patient records between February and May 2020 from 100 confirmed SARS-CoV-2–infected patients in Hong Kong during hospitalization, as well as follow-up stool samples from 27 patients up to 30 days after they cleared the COVID-19 virus; these observations were compared with 78 non–COVID-19 controls.
Overall, 274 stool samples were sequenced. Samples collected from patients during hospitalization for COVID-19 were compared with non–COVID-19 controls. The presence of phylum Bacteroidetes was significantly higher in COVID-19 patients compared with controls (23.9% vs. 12.8%; P < .001), as were Actinobacteria (26.1% vs. 19.0%; P < .001).
After controlling for antibiotics, the investigators found that “differences between cohorts were primarily linked to enrichment of taxa such as Parabacteroides, Sutterella wadsworthensis, and Bacteroides caccae and depletion of Adlercreutzia equolifaciens, Dorea formicigenerans, and Clostridium leptum in COVID-19 relative to non-COVID-19” (P < .05). In addition, Faecalibacterium prausnitzii and Bifidobacterium bifidum were negatively correlated with COVID-19 severity after investigators controlled for patient age and antibiotic use (P < .05).
The researchers also examined bacteria in COVID-19 patients and controls in the context of cytokines and other inflammatory markers. “We hypothesized that these compositional changes play a role in exacerbating disease by contributing to dysregulation of the immune response,” they said.
In fact, species depleted in COVID-19 patients including included B. adolescentis, E. rectale, and F. prausnitzii were negatively correlated with inflammatory markers including CXCL10, IL-10, TNF-alpha, and CCL2.
In addition, 42 stool samples from 27 patients showed significantly distinct gut microbiota from controls up to 30 days (median, 6 days) after virus clearance, regardless of antibiotics use (P < .05), the researchers said.
Long-term data needed
The study findings were limited by several factors, including the potential confounding of microbial signatures associated with COVID-19 because of heterogeneous patient management in the clinical setting and the potential that gut microbiota reflects a patient’s health with no impact on disease severity, as well as lack of data on the role of antibiotics for severe and critical patients, the researchers noted. In addition, “gut microbiota composition is highly heterogeneous across human populations and changes in compositions reported here may not necessarily be reflected in patients with COVID-19 from other biogeographies,” they wrote.
The “longer follow-up of patients with COVID-19 (e.g., 3 months to 1 year after clearing the virus) is needed to address questions related to the duration of gut microbiota dysbiosis post recovery, link between microbiota dysbiosis and long-term persistent symptoms, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health problems,” they wrote.
However, the results suggest a likely role for gut microorganisms in host inflammatory responses to COVID-19 infection, and “underscore an urgent need to understand the specific roles of gut microorganisms in human immune function and systemic inflammation,” they concluded.
More than infectious
“A growing body of evidence suggests that severity of illness from COVID-19 is largely determined by the patient’s aberrant immune response to the virus,” Jatin Roper, MD, of Duke University, Durham, N.C., said in an interview. “Therefore, a critical question is: What patient factors determine this immune response? The gut microbiota closely interact with the host immune system and are altered in many immunological diseases,” he said. “Furthermore, the SARS-CoV-2 virus infects enterocytes in the intestine and causes symptomatic gastrointestinal disease in a subset of patients. Therefore, understanding a possible association between gut microbiota and COVID-19 may reveal microbial species involved in disease pathogenesis,” he emphasized.
In the current study, “I was surprised to find that COVID-19 infection is associated with depletion of immunomodulatory gut bacteria,” said Dr. Roper. “An open question is whether these changes are caused by the SARS-CoV-2 virus and then result in altered immune response. Alternatively, the changes in gut microbiota may be a result of the immune response or other changes associated with the disease,” he said.
“COVID-19 is an immunological disease, not just an infectious disease,” explained Dr. Roper. “The gut microbiota may play an important role in the pathogenesis of the disease. Thus, specific gut microbes could one day be analyzed to risk stratify patients, or even modified to treat the disease,” he noted.
Beyond COVID-19
“Given the impact of the gut microbiota on health and disease, as well as the impact of diseases on the microbiota, I am not at all surprised to find that there were significant changes in the microbiota of COVID-19 patients and that these changes are associated with inflammatory cytokines, chemokines, and blood markers of tissue damage,” said Anthony Sung, MD, also of Duke University.
According to Dr. Sung, researchers have already been investigating possible connections between gut microbiota and other conditions such as Alzheimer’s disease, and it’s been hypothesized that these connections are mediated by interactions between the gut microbiota and the immune system.
“While this is an important paper in our understanding of COVID-19, and highlights the microbiome as a potential therapeutic target, we need to conduct clinical trials of microbiota-based interventions before we can fully realize the clinical implications of these findings,” he said.
The study was supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, and donations from Hui Hoy & Chow Sin Lan Charity Fund Limited, Pine and Crane Company Limited, Mr. Hui Ming, and The D.H. Chen Foundation. The researchers had no financial conflicts to disclose. Dr. Roper and Dr. Sung had no financial conflicts to disclose.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
COVID-19 infection altered the gut microbiota of adult patients and caused depletion of several types of bacteria with known immunomodulatory properties, based on data from a cohort study of 100 patients with confirmed COVID-19 infections from two hospitals.
“As the GI tract is the largest immunological organ in the body and its resident microbiota are known to modulate host immune responses, we hypothesized that the gut microbiota is associated with host inflammatory immune responses in COVID19,” wrote Yun Kit Yeoh, PhD, of the Chinese University of Hong Kong, and colleagues.
In a study published in Gut, the researchers investigated patient microbiota by collecting blood, stool, and patient records between February and May 2020 from 100 confirmed SARS-CoV-2–infected patients in Hong Kong during hospitalization, as well as follow-up stool samples from 27 patients up to 30 days after they cleared the COVID-19 virus; these observations were compared with 78 non–COVID-19 controls.
Overall, 274 stool samples were sequenced. Samples collected from patients during hospitalization for COVID-19 were compared with non–COVID-19 controls. The presence of phylum Bacteroidetes was significantly higher in COVID-19 patients compared with controls (23.9% vs. 12.8%; P < .001), as were Actinobacteria (26.1% vs. 19.0%; P < .001).
After controlling for antibiotics, the investigators found that “differences between cohorts were primarily linked to enrichment of taxa such as Parabacteroides, Sutterella wadsworthensis, and Bacteroides caccae and depletion of Adlercreutzia equolifaciens, Dorea formicigenerans, and Clostridium leptum in COVID-19 relative to non-COVID-19” (P < .05). In addition, Faecalibacterium prausnitzii and Bifidobacterium bifidum were negatively correlated with COVID-19 severity after investigators controlled for patient age and antibiotic use (P < .05).
The researchers also examined bacteria in COVID-19 patients and controls in the context of cytokines and other inflammatory markers. “We hypothesized that these compositional changes play a role in exacerbating disease by contributing to dysregulation of the immune response,” they said.
In fact, species depleted in COVID-19 patients including included B. adolescentis, E. rectale, and F. prausnitzii were negatively correlated with inflammatory markers including CXCL10, IL-10, TNF-alpha, and CCL2.
In addition, 42 stool samples from 27 patients showed significantly distinct gut microbiota from controls up to 30 days (median, 6 days) after virus clearance, regardless of antibiotics use (P < .05), the researchers said.
Long-term data needed
The study findings were limited by several factors, including the potential confounding of microbial signatures associated with COVID-19 because of heterogeneous patient management in the clinical setting and the potential that gut microbiota reflects a patient’s health with no impact on disease severity, as well as lack of data on the role of antibiotics for severe and critical patients, the researchers noted. In addition, “gut microbiota composition is highly heterogeneous across human populations and changes in compositions reported here may not necessarily be reflected in patients with COVID-19 from other biogeographies,” they wrote.
The “longer follow-up of patients with COVID-19 (e.g., 3 months to 1 year after clearing the virus) is needed to address questions related to the duration of gut microbiota dysbiosis post recovery, link between microbiota dysbiosis and long-term persistent symptoms, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health problems,” they wrote.
However, the results suggest a likely role for gut microorganisms in host inflammatory responses to COVID-19 infection, and “underscore an urgent need to understand the specific roles of gut microorganisms in human immune function and systemic inflammation,” they concluded.
More than infectious
“A growing body of evidence suggests that severity of illness from COVID-19 is largely determined by the patient’s aberrant immune response to the virus,” Jatin Roper, MD, of Duke University, Durham, N.C., said in an interview. “Therefore, a critical question is: What patient factors determine this immune response? The gut microbiota closely interact with the host immune system and are altered in many immunological diseases,” he said. “Furthermore, the SARS-CoV-2 virus infects enterocytes in the intestine and causes symptomatic gastrointestinal disease in a subset of patients. Therefore, understanding a possible association between gut microbiota and COVID-19 may reveal microbial species involved in disease pathogenesis,” he emphasized.
In the current study, “I was surprised to find that COVID-19 infection is associated with depletion of immunomodulatory gut bacteria,” said Dr. Roper. “An open question is whether these changes are caused by the SARS-CoV-2 virus and then result in altered immune response. Alternatively, the changes in gut microbiota may be a result of the immune response or other changes associated with the disease,” he said.
“COVID-19 is an immunological disease, not just an infectious disease,” explained Dr. Roper. “The gut microbiota may play an important role in the pathogenesis of the disease. Thus, specific gut microbes could one day be analyzed to risk stratify patients, or even modified to treat the disease,” he noted.
Beyond COVID-19
“Given the impact of the gut microbiota on health and disease, as well as the impact of diseases on the microbiota, I am not at all surprised to find that there were significant changes in the microbiota of COVID-19 patients and that these changes are associated with inflammatory cytokines, chemokines, and blood markers of tissue damage,” said Anthony Sung, MD, also of Duke University.
According to Dr. Sung, researchers have already been investigating possible connections between gut microbiota and other conditions such as Alzheimer’s disease, and it’s been hypothesized that these connections are mediated by interactions between the gut microbiota and the immune system.
“While this is an important paper in our understanding of COVID-19, and highlights the microbiome as a potential therapeutic target, we need to conduct clinical trials of microbiota-based interventions before we can fully realize the clinical implications of these findings,” he said.
The study was supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, and donations from Hui Hoy & Chow Sin Lan Charity Fund Limited, Pine and Crane Company Limited, Mr. Hui Ming, and The D.H. Chen Foundation. The researchers had no financial conflicts to disclose. Dr. Roper and Dr. Sung had no financial conflicts to disclose.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
COVID-19 infection altered the gut microbiota of adult patients and caused depletion of several types of bacteria with known immunomodulatory properties, based on data from a cohort study of 100 patients with confirmed COVID-19 infections from two hospitals.
“As the GI tract is the largest immunological organ in the body and its resident microbiota are known to modulate host immune responses, we hypothesized that the gut microbiota is associated with host inflammatory immune responses in COVID19,” wrote Yun Kit Yeoh, PhD, of the Chinese University of Hong Kong, and colleagues.
In a study published in Gut, the researchers investigated patient microbiota by collecting blood, stool, and patient records between February and May 2020 from 100 confirmed SARS-CoV-2–infected patients in Hong Kong during hospitalization, as well as follow-up stool samples from 27 patients up to 30 days after they cleared the COVID-19 virus; these observations were compared with 78 non–COVID-19 controls.
Overall, 274 stool samples were sequenced. Samples collected from patients during hospitalization for COVID-19 were compared with non–COVID-19 controls. The presence of phylum Bacteroidetes was significantly higher in COVID-19 patients compared with controls (23.9% vs. 12.8%; P < .001), as were Actinobacteria (26.1% vs. 19.0%; P < .001).
After controlling for antibiotics, the investigators found that “differences between cohorts were primarily linked to enrichment of taxa such as Parabacteroides, Sutterella wadsworthensis, and Bacteroides caccae and depletion of Adlercreutzia equolifaciens, Dorea formicigenerans, and Clostridium leptum in COVID-19 relative to non-COVID-19” (P < .05). In addition, Faecalibacterium prausnitzii and Bifidobacterium bifidum were negatively correlated with COVID-19 severity after investigators controlled for patient age and antibiotic use (P < .05).
The researchers also examined bacteria in COVID-19 patients and controls in the context of cytokines and other inflammatory markers. “We hypothesized that these compositional changes play a role in exacerbating disease by contributing to dysregulation of the immune response,” they said.
In fact, species depleted in COVID-19 patients including included B. adolescentis, E. rectale, and F. prausnitzii were negatively correlated with inflammatory markers including CXCL10, IL-10, TNF-alpha, and CCL2.
In addition, 42 stool samples from 27 patients showed significantly distinct gut microbiota from controls up to 30 days (median, 6 days) after virus clearance, regardless of antibiotics use (P < .05), the researchers said.
Long-term data needed
The study findings were limited by several factors, including the potential confounding of microbial signatures associated with COVID-19 because of heterogeneous patient management in the clinical setting and the potential that gut microbiota reflects a patient’s health with no impact on disease severity, as well as lack of data on the role of antibiotics for severe and critical patients, the researchers noted. In addition, “gut microbiota composition is highly heterogeneous across human populations and changes in compositions reported here may not necessarily be reflected in patients with COVID-19 from other biogeographies,” they wrote.
The “longer follow-up of patients with COVID-19 (e.g., 3 months to 1 year after clearing the virus) is needed to address questions related to the duration of gut microbiota dysbiosis post recovery, link between microbiota dysbiosis and long-term persistent symptoms, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health problems,” they wrote.
However, the results suggest a likely role for gut microorganisms in host inflammatory responses to COVID-19 infection, and “underscore an urgent need to understand the specific roles of gut microorganisms in human immune function and systemic inflammation,” they concluded.
More than infectious
“A growing body of evidence suggests that severity of illness from COVID-19 is largely determined by the patient’s aberrant immune response to the virus,” Jatin Roper, MD, of Duke University, Durham, N.C., said in an interview. “Therefore, a critical question is: What patient factors determine this immune response? The gut microbiota closely interact with the host immune system and are altered in many immunological diseases,” he said. “Furthermore, the SARS-CoV-2 virus infects enterocytes in the intestine and causes symptomatic gastrointestinal disease in a subset of patients. Therefore, understanding a possible association between gut microbiota and COVID-19 may reveal microbial species involved in disease pathogenesis,” he emphasized.
In the current study, “I was surprised to find that COVID-19 infection is associated with depletion of immunomodulatory gut bacteria,” said Dr. Roper. “An open question is whether these changes are caused by the SARS-CoV-2 virus and then result in altered immune response. Alternatively, the changes in gut microbiota may be a result of the immune response or other changes associated with the disease,” he said.
“COVID-19 is an immunological disease, not just an infectious disease,” explained Dr. Roper. “The gut microbiota may play an important role in the pathogenesis of the disease. Thus, specific gut microbes could one day be analyzed to risk stratify patients, or even modified to treat the disease,” he noted.
Beyond COVID-19
“Given the impact of the gut microbiota on health and disease, as well as the impact of diseases on the microbiota, I am not at all surprised to find that there were significant changes in the microbiota of COVID-19 patients and that these changes are associated with inflammatory cytokines, chemokines, and blood markers of tissue damage,” said Anthony Sung, MD, also of Duke University.
According to Dr. Sung, researchers have already been investigating possible connections between gut microbiota and other conditions such as Alzheimer’s disease, and it’s been hypothesized that these connections are mediated by interactions between the gut microbiota and the immune system.
“While this is an important paper in our understanding of COVID-19, and highlights the microbiome as a potential therapeutic target, we need to conduct clinical trials of microbiota-based interventions before we can fully realize the clinical implications of these findings,” he said.
The study was supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, and donations from Hui Hoy & Chow Sin Lan Charity Fund Limited, Pine and Crane Company Limited, Mr. Hui Ming, and The D.H. Chen Foundation. The researchers had no financial conflicts to disclose. Dr. Roper and Dr. Sung had no financial conflicts to disclose.
For the latest clinical guidance, education, research and physician resources about coronavirus, visit the AGA COVID-19 Resource Center at www.gastro.org/COVID.
FROM GUT
Tough pain relief choices in the COVID-19 pandemic
More people with fever and body aches are turning to NSAIDs to ease symptoms, but the drugs have come under new scrutiny as investigators work to determine whether they are a safe way to relieve the pain of COVID-19 vaccination or symptoms of the disease.
Early on in the pandemic, French health officials warned that NSAIDs, such as ibuprofen, could worsen coronavirus disease, and they recommended switching to acetaminophen instead.
The National Health Service in the United Kingdom followed with a similar recommendation for acetaminophen.
But the European Medicines Agency took a different approach, reporting “no scientific evidence” that NSAIDs could worsen COVID-19. The U.S. Food and Drug Administration also opted not to take a stance.
The debate prompted discussion on social media, with various reactions from around the world. It also inspired Craig Wilen, MD, PhD, from Yale University, New Haven, Conn., and associates to examine the effect of NSAIDs on COVID-19 infection and immune response. Their findings were published online Jan.20 in the Journal of Virology.
“It really bothered me that non–evidence-based decisions were driving the conversation,” Dr. Wilen said. “Millions of people are taking NSAIDs every day and clinical decisions about their care shouldn’t be made on a hypothesis.”
One theory is that NSAIDs alter susceptibility to infection by modifying ACE2. The drugs might also change the cell entry receptor for SARS-CoV-2, alter virus replication, or even modify the immune response.
British researchers, also questioning the safety of NSAIDs in patients with COVID-19, delved into National Health Service records to study two large groups of patients, some of whom were taking the pain relievers.
“We were watching the controversy and the lack of evidence and wanted to contribute,” lead investigator Angel Wong, PhD, from the London School of Hygiene and Tropical Medicine, said in an interview.
And with nearly 11 million NSAID prescriptions dispensed in primary care in England alone in the past 12 months, the inconsistency was concerning.
The team compared COVID-19–related deaths in two groups: one group of more than 700,000 people taking NSAIDs, including patients with rheumatoid arthritis and osteoarthritis; and another of almost 3.5 million people not on the medication.
NSAIDs work by inhibiting cyclooxygenase-1 and COX-2 enzymes in the body, which are crucial for the generation of prostaglandins. These lipid molecules play a role in inflammation and are blocked by NSAIDs.
The investigators found no evidence of a harmful effect of NSAIDs on COVID-19-related deaths; their results were published online Jan. 21 in the Annals of the Rheumatic Diseases.
The results, they pointed out, are in line with a Danish study that also showed no evidence of a higher risk for severe COVID-19 outcomes with NSAID use.
“It’s reassuring,” Dr. Wong said, “that patients can safely continue treatment.”
More new evidence
Dr. Wilen’s team found that SARS-CoV-2 infection stimulated COX-2 expression in human and mice cells. However, suppression of COX-2 by two commonly used NSAIDs, ibuprofen and meloxicam, had no effect on ACE2 expression, viral entry, or viral replication.
In their mouse model of SARS-CoV-2 infection, the investigators saw that NSAIDs impaired the production of proinflammatory cytokines and neutralizing antibodies. The findings suggest that NSAIDs influence COVID-19 outcomes by dampening the inflammatory response and production of protective antibodies, rather than modifying susceptibility to infection or viral replication.
Understanding the effect of NSAIDs on cytokine production is critical, Dr. Wilen pointed out, because they might be protective early in COVID-19 but pathologic at later stages.
Timing is crucial in the case of other immunomodulatory drugs. For example, dexamethasone lowers mortality in COVID-19 patients on respiratory support but is potentially harmful for those with milder disease.
There still is a lot to learn, Dr. Wilen acknowledged. “We may be seeing something similar going on with NSAIDs, where the timing of treatment is important.”
A version of this article first appeared on Medscape.com.
More people with fever and body aches are turning to NSAIDs to ease symptoms, but the drugs have come under new scrutiny as investigators work to determine whether they are a safe way to relieve the pain of COVID-19 vaccination or symptoms of the disease.
Early on in the pandemic, French health officials warned that NSAIDs, such as ibuprofen, could worsen coronavirus disease, and they recommended switching to acetaminophen instead.
The National Health Service in the United Kingdom followed with a similar recommendation for acetaminophen.
But the European Medicines Agency took a different approach, reporting “no scientific evidence” that NSAIDs could worsen COVID-19. The U.S. Food and Drug Administration also opted not to take a stance.
The debate prompted discussion on social media, with various reactions from around the world. It also inspired Craig Wilen, MD, PhD, from Yale University, New Haven, Conn., and associates to examine the effect of NSAIDs on COVID-19 infection and immune response. Their findings were published online Jan.20 in the Journal of Virology.
“It really bothered me that non–evidence-based decisions were driving the conversation,” Dr. Wilen said. “Millions of people are taking NSAIDs every day and clinical decisions about their care shouldn’t be made on a hypothesis.”
One theory is that NSAIDs alter susceptibility to infection by modifying ACE2. The drugs might also change the cell entry receptor for SARS-CoV-2, alter virus replication, or even modify the immune response.
British researchers, also questioning the safety of NSAIDs in patients with COVID-19, delved into National Health Service records to study two large groups of patients, some of whom were taking the pain relievers.
“We were watching the controversy and the lack of evidence and wanted to contribute,” lead investigator Angel Wong, PhD, from the London School of Hygiene and Tropical Medicine, said in an interview.
And with nearly 11 million NSAID prescriptions dispensed in primary care in England alone in the past 12 months, the inconsistency was concerning.
The team compared COVID-19–related deaths in two groups: one group of more than 700,000 people taking NSAIDs, including patients with rheumatoid arthritis and osteoarthritis; and another of almost 3.5 million people not on the medication.
NSAIDs work by inhibiting cyclooxygenase-1 and COX-2 enzymes in the body, which are crucial for the generation of prostaglandins. These lipid molecules play a role in inflammation and are blocked by NSAIDs.
The investigators found no evidence of a harmful effect of NSAIDs on COVID-19-related deaths; their results were published online Jan. 21 in the Annals of the Rheumatic Diseases.
The results, they pointed out, are in line with a Danish study that also showed no evidence of a higher risk for severe COVID-19 outcomes with NSAID use.
“It’s reassuring,” Dr. Wong said, “that patients can safely continue treatment.”
More new evidence
Dr. Wilen’s team found that SARS-CoV-2 infection stimulated COX-2 expression in human and mice cells. However, suppression of COX-2 by two commonly used NSAIDs, ibuprofen and meloxicam, had no effect on ACE2 expression, viral entry, or viral replication.
In their mouse model of SARS-CoV-2 infection, the investigators saw that NSAIDs impaired the production of proinflammatory cytokines and neutralizing antibodies. The findings suggest that NSAIDs influence COVID-19 outcomes by dampening the inflammatory response and production of protective antibodies, rather than modifying susceptibility to infection or viral replication.
Understanding the effect of NSAIDs on cytokine production is critical, Dr. Wilen pointed out, because they might be protective early in COVID-19 but pathologic at later stages.
Timing is crucial in the case of other immunomodulatory drugs. For example, dexamethasone lowers mortality in COVID-19 patients on respiratory support but is potentially harmful for those with milder disease.
There still is a lot to learn, Dr. Wilen acknowledged. “We may be seeing something similar going on with NSAIDs, where the timing of treatment is important.”
A version of this article first appeared on Medscape.com.
More people with fever and body aches are turning to NSAIDs to ease symptoms, but the drugs have come under new scrutiny as investigators work to determine whether they are a safe way to relieve the pain of COVID-19 vaccination or symptoms of the disease.
Early on in the pandemic, French health officials warned that NSAIDs, such as ibuprofen, could worsen coronavirus disease, and they recommended switching to acetaminophen instead.
The National Health Service in the United Kingdom followed with a similar recommendation for acetaminophen.
But the European Medicines Agency took a different approach, reporting “no scientific evidence” that NSAIDs could worsen COVID-19. The U.S. Food and Drug Administration also opted not to take a stance.
The debate prompted discussion on social media, with various reactions from around the world. It also inspired Craig Wilen, MD, PhD, from Yale University, New Haven, Conn., and associates to examine the effect of NSAIDs on COVID-19 infection and immune response. Their findings were published online Jan.20 in the Journal of Virology.
“It really bothered me that non–evidence-based decisions were driving the conversation,” Dr. Wilen said. “Millions of people are taking NSAIDs every day and clinical decisions about their care shouldn’t be made on a hypothesis.”
One theory is that NSAIDs alter susceptibility to infection by modifying ACE2. The drugs might also change the cell entry receptor for SARS-CoV-2, alter virus replication, or even modify the immune response.
British researchers, also questioning the safety of NSAIDs in patients with COVID-19, delved into National Health Service records to study two large groups of patients, some of whom were taking the pain relievers.
“We were watching the controversy and the lack of evidence and wanted to contribute,” lead investigator Angel Wong, PhD, from the London School of Hygiene and Tropical Medicine, said in an interview.
And with nearly 11 million NSAID prescriptions dispensed in primary care in England alone in the past 12 months, the inconsistency was concerning.
The team compared COVID-19–related deaths in two groups: one group of more than 700,000 people taking NSAIDs, including patients with rheumatoid arthritis and osteoarthritis; and another of almost 3.5 million people not on the medication.
NSAIDs work by inhibiting cyclooxygenase-1 and COX-2 enzymes in the body, which are crucial for the generation of prostaglandins. These lipid molecules play a role in inflammation and are blocked by NSAIDs.
The investigators found no evidence of a harmful effect of NSAIDs on COVID-19-related deaths; their results were published online Jan. 21 in the Annals of the Rheumatic Diseases.
The results, they pointed out, are in line with a Danish study that also showed no evidence of a higher risk for severe COVID-19 outcomes with NSAID use.
“It’s reassuring,” Dr. Wong said, “that patients can safely continue treatment.”
More new evidence
Dr. Wilen’s team found that SARS-CoV-2 infection stimulated COX-2 expression in human and mice cells. However, suppression of COX-2 by two commonly used NSAIDs, ibuprofen and meloxicam, had no effect on ACE2 expression, viral entry, or viral replication.
In their mouse model of SARS-CoV-2 infection, the investigators saw that NSAIDs impaired the production of proinflammatory cytokines and neutralizing antibodies. The findings suggest that NSAIDs influence COVID-19 outcomes by dampening the inflammatory response and production of protective antibodies, rather than modifying susceptibility to infection or viral replication.
Understanding the effect of NSAIDs on cytokine production is critical, Dr. Wilen pointed out, because they might be protective early in COVID-19 but pathologic at later stages.
Timing is crucial in the case of other immunomodulatory drugs. For example, dexamethasone lowers mortality in COVID-19 patients on respiratory support but is potentially harmful for those with milder disease.
There still is a lot to learn, Dr. Wilen acknowledged. “We may be seeing something similar going on with NSAIDs, where the timing of treatment is important.”
A version of this article first appeared on Medscape.com.
Maternal COVID antibodies cross placenta, detected in newborns
Antibodies against SARS-CoV-2 cross the placenta during pregnancy and are detectable in most newborns born to mothers who had COVID-19 during pregnancy, according to findings from a study presented Jan. 28 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“I think the most striking finding is that we noticed a high degree of neutralizing response to natural infection even among asymptomatic infection, but of course a higher degree was seen in those with symptomatic infection,” Naima Joseph, MD, MPH, of Emory University, Atlanta, said in an interview.
“Our data demonstrate maternal capacity to mount an appropriate and robust immune response,” and maternal protective immunity lasted at least 28 days after infection, Dr. Joseph said. “Also, we noted higher neonatal cord blood titers in moms with higher titers, which suggests a relationship, but we need to better understand how transplacental transfer occurs as well as establish neonatal correlates of protection in order to see if and how maternal immunity may also benefit neonates.”
The researchers analyzed the amount of IgG and IgM antibodies in maternal and cord blood samples prospectively collected at delivery from women who tested positive for COVID-19 at any time while pregnant. They used enzyme-linked immunosorbent assay to assess for antibodies for the receptor binding domain of the SARS-CoV-2 spike protein.
The 32 pairs of mothers and infants in the study were predominantly non-Hispanic Black (72%) and Hispanic (25%), and 84% used Medicaid as their payer. Most of the mothers (72%) had at least one comorbidity, most commonly obesity, hypertension, and asthma or pulmonary disease. Just over half the women (53%) were symptomatic while they were infected, and 88% were ill with COVID-19 during the third trimester. The average time from infection to delivery was 28 days.
All the mothers had IgG antibodies, 94% had IgM antibodies, and 94% had neutralizing antibodies against SARS-CoV-2. Among the cord blood samples, 91% had IgG antibodies, 9% had IgM antibodies, and 25% had neutralizing antibodies.
“It’s reassuring that, so far, the physiological response is exactly what we expected it to be,” Judette Louis, MD, MPH, an associate professor of ob.gyn. and the ob.gyn. department chair at the University of South Florida, Tampa, said in an interview. “It’s what we would expect, but it’s always helpful to have more data to support that. Otherwise, you’re extrapolating from what you know from other conditions,” said Dr. Louis, who moderated the oral abstracts session.
Symptomatic infection was associated with significantly higher IgG titers than asymptomatic infection (P = .03), but no correlation was seen for IgM or neutralizing antibodies. In addition, although mothers who delivered more than 28 days after their infection had higher IgG titers (P = .05), no differences existed in IgM or neutralizing response.
Infants’ cord blood titers were significantly lower than their corresponding maternal samples, independently of symptoms or latency from infection to delivery (P < .001), Dr. Joseph reported.
“Transplacental efficiency in other pathogens has been shown to be correlated with neonatal immunity when the ratio of cord to maternal blood is greater than 1,” Dr. Joseph said in her presentation. Their data showed “suboptimal efficiency” at a ratio of 0.81.
The study’s small sample size and lack of a control group were weaknesses, but a major strength was having a population at disproportionately higher risk for infection and severe morbidity than the general population.
Implications for maternal COVID-19 vaccination
Although the data are not yet available, Dr. Joseph said they have expanded their protocol to include vaccinated pregnant women.
“The key to developing an effective vaccine [for pregnant people] is in really characterizing adaptive immunity in pregnancy,” Dr. Joseph told SMFM attendees. “I think that these findings inform further vaccine development in demonstrating that maternal immunity is robust.”
The World Health Organization recently recommended withholding COVID-19 vaccines from pregnant people, but the SMFM and American College of Obstetricians and Gynecologists subsequently issued a joint statement reaffirming that the COVID-19 vaccines authorized by the FDA “should not be withheld from pregnant individuals who choose to receive the vaccine.”
“One of the questions people ask is whether in pregnancy you’re going to mount a good response to the vaccine the way you would outside of pregnancy,” Dr. Louis said. “If we can demonstrate that you do, that may provide the information that some mothers need to make their decisions.” Data such as those from Dr. Joseph’s study can also inform recommendations on timing of maternal vaccination.
“For instance, Dr. Joseph demonstrated that, 28 days out from the infection, you had more antibodies, so there may be a scenario where we say this vaccine may be more beneficial in the middle of the pregnancy for the purpose of forming those antibodies,” Dr. Louis said.
Consensus emerging from maternal antibodies data
The findings from Dr. Joseph’s study mirror those reported in a study published online Jan. 29 in JAMA Pediatrics. That study, led by Dustin D. Flannery, DO, MSCE, of Children’s Hospital of Philadelphia, also examined maternal and neonatal levels of IgG and IgM antibodies against the receptor binding domain of the SARS-CoV-2 spike protein. They also found a positive correlation between cord blood and maternal IgG concentrations (P < .001), but notably, the ratio of cord to maternal blood titers was greater than 1, unlike in Dr. Joseph’s study.
For their study, Dr. Flannery and colleagues obtained maternal and cord blood sera at the time of delivery from 1471 pairs of mothers and infants, independently of COVID status during pregnancy. The average maternal age was 32 years, and just over a quarter of the population (26%) were Black, non-Hispanic women. About half (51%) were White, 12% were Hispanic, and 7% were Asian.
About 6% of the women had either IgG or IgM antibodies at delivery, and 87% of infants born to those mothers had measurable IgG in their cord blood. No infants had IgM antibodies. As with the study presented at SMFM, the mothers’ infections included asymptomatic, mild, moderate, and severe cases, and the degree of severity of cases had no apparent effect on infant antibody concentrations. Most of the women who tested positive for COVID-19 (60%) were asymptomatic.
Among the 11 mothers who had antibodies but whose infants’ cord blood did not, 5 had only IgM antibodies, and 6 had significantly lower IgG concentrations than those seen in the other mothers.
In a commentary about the JAMA Pediatrics study, Flor Munoz, MD, of the Baylor College of Medicine, Houston, suggested that the findings are grounds for optimism about a maternal vaccination strategy to protect infants from COVID-19.
“However, the timing of maternal vaccination to protect the infant, as opposed to the mother alone, would necessitate an adequate interval from vaccination to delivery (of at least 4 weeks), while vaccination early in gestation and even late in the third trimester could still be protective for the mother,” Dr. Munoz wrote.
Given the interval between two-dose vaccination regimens and the fact that transplacental transfer begins at about the 17th week of gestation, “maternal vaccination starting in the early second trimester of gestation might be optimal to achieve the highest levels of antibodies in the newborn,” Dr. Munoz wrote. But questions remain, such as how effective the neonatal antibodies would be in protecting against COVID-19 and how long they last after birth.
No external funding was used in Dr. Joseph’s study. Dr. Joseph and Dr. Louis have disclosed no relevant financial relationships. The JAMA Pediatrics study was funded by the Children’s Hospital of Philadelphia. One coauthor received consultancy fees from Sanofi Pasteur, Lumen, Novavax, and Merck unrelated to the study. Dr. Munoz served on the data and safety monitoring boards of Moderna, Pfizer, Virometix, and Meissa Vaccines and has received grants from Novavax Research and Gilead Research.
A version of this article first appeared on Medscape.com.
Antibodies against SARS-CoV-2 cross the placenta during pregnancy and are detectable in most newborns born to mothers who had COVID-19 during pregnancy, according to findings from a study presented Jan. 28 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“I think the most striking finding is that we noticed a high degree of neutralizing response to natural infection even among asymptomatic infection, but of course a higher degree was seen in those with symptomatic infection,” Naima Joseph, MD, MPH, of Emory University, Atlanta, said in an interview.
“Our data demonstrate maternal capacity to mount an appropriate and robust immune response,” and maternal protective immunity lasted at least 28 days after infection, Dr. Joseph said. “Also, we noted higher neonatal cord blood titers in moms with higher titers, which suggests a relationship, but we need to better understand how transplacental transfer occurs as well as establish neonatal correlates of protection in order to see if and how maternal immunity may also benefit neonates.”
The researchers analyzed the amount of IgG and IgM antibodies in maternal and cord blood samples prospectively collected at delivery from women who tested positive for COVID-19 at any time while pregnant. They used enzyme-linked immunosorbent assay to assess for antibodies for the receptor binding domain of the SARS-CoV-2 spike protein.
The 32 pairs of mothers and infants in the study were predominantly non-Hispanic Black (72%) and Hispanic (25%), and 84% used Medicaid as their payer. Most of the mothers (72%) had at least one comorbidity, most commonly obesity, hypertension, and asthma or pulmonary disease. Just over half the women (53%) were symptomatic while they were infected, and 88% were ill with COVID-19 during the third trimester. The average time from infection to delivery was 28 days.
All the mothers had IgG antibodies, 94% had IgM antibodies, and 94% had neutralizing antibodies against SARS-CoV-2. Among the cord blood samples, 91% had IgG antibodies, 9% had IgM antibodies, and 25% had neutralizing antibodies.
“It’s reassuring that, so far, the physiological response is exactly what we expected it to be,” Judette Louis, MD, MPH, an associate professor of ob.gyn. and the ob.gyn. department chair at the University of South Florida, Tampa, said in an interview. “It’s what we would expect, but it’s always helpful to have more data to support that. Otherwise, you’re extrapolating from what you know from other conditions,” said Dr. Louis, who moderated the oral abstracts session.
Symptomatic infection was associated with significantly higher IgG titers than asymptomatic infection (P = .03), but no correlation was seen for IgM or neutralizing antibodies. In addition, although mothers who delivered more than 28 days after their infection had higher IgG titers (P = .05), no differences existed in IgM or neutralizing response.
Infants’ cord blood titers were significantly lower than their corresponding maternal samples, independently of symptoms or latency from infection to delivery (P < .001), Dr. Joseph reported.
“Transplacental efficiency in other pathogens has been shown to be correlated with neonatal immunity when the ratio of cord to maternal blood is greater than 1,” Dr. Joseph said in her presentation. Their data showed “suboptimal efficiency” at a ratio of 0.81.
The study’s small sample size and lack of a control group were weaknesses, but a major strength was having a population at disproportionately higher risk for infection and severe morbidity than the general population.
Implications for maternal COVID-19 vaccination
Although the data are not yet available, Dr. Joseph said they have expanded their protocol to include vaccinated pregnant women.
“The key to developing an effective vaccine [for pregnant people] is in really characterizing adaptive immunity in pregnancy,” Dr. Joseph told SMFM attendees. “I think that these findings inform further vaccine development in demonstrating that maternal immunity is robust.”
The World Health Organization recently recommended withholding COVID-19 vaccines from pregnant people, but the SMFM and American College of Obstetricians and Gynecologists subsequently issued a joint statement reaffirming that the COVID-19 vaccines authorized by the FDA “should not be withheld from pregnant individuals who choose to receive the vaccine.”
“One of the questions people ask is whether in pregnancy you’re going to mount a good response to the vaccine the way you would outside of pregnancy,” Dr. Louis said. “If we can demonstrate that you do, that may provide the information that some mothers need to make their decisions.” Data such as those from Dr. Joseph’s study can also inform recommendations on timing of maternal vaccination.
“For instance, Dr. Joseph demonstrated that, 28 days out from the infection, you had more antibodies, so there may be a scenario where we say this vaccine may be more beneficial in the middle of the pregnancy for the purpose of forming those antibodies,” Dr. Louis said.
Consensus emerging from maternal antibodies data
The findings from Dr. Joseph’s study mirror those reported in a study published online Jan. 29 in JAMA Pediatrics. That study, led by Dustin D. Flannery, DO, MSCE, of Children’s Hospital of Philadelphia, also examined maternal and neonatal levels of IgG and IgM antibodies against the receptor binding domain of the SARS-CoV-2 spike protein. They also found a positive correlation between cord blood and maternal IgG concentrations (P < .001), but notably, the ratio of cord to maternal blood titers was greater than 1, unlike in Dr. Joseph’s study.
For their study, Dr. Flannery and colleagues obtained maternal and cord blood sera at the time of delivery from 1471 pairs of mothers and infants, independently of COVID status during pregnancy. The average maternal age was 32 years, and just over a quarter of the population (26%) were Black, non-Hispanic women. About half (51%) were White, 12% were Hispanic, and 7% were Asian.
About 6% of the women had either IgG or IgM antibodies at delivery, and 87% of infants born to those mothers had measurable IgG in their cord blood. No infants had IgM antibodies. As with the study presented at SMFM, the mothers’ infections included asymptomatic, mild, moderate, and severe cases, and the degree of severity of cases had no apparent effect on infant antibody concentrations. Most of the women who tested positive for COVID-19 (60%) were asymptomatic.
Among the 11 mothers who had antibodies but whose infants’ cord blood did not, 5 had only IgM antibodies, and 6 had significantly lower IgG concentrations than those seen in the other mothers.
In a commentary about the JAMA Pediatrics study, Flor Munoz, MD, of the Baylor College of Medicine, Houston, suggested that the findings are grounds for optimism about a maternal vaccination strategy to protect infants from COVID-19.
“However, the timing of maternal vaccination to protect the infant, as opposed to the mother alone, would necessitate an adequate interval from vaccination to delivery (of at least 4 weeks), while vaccination early in gestation and even late in the third trimester could still be protective for the mother,” Dr. Munoz wrote.
Given the interval between two-dose vaccination regimens and the fact that transplacental transfer begins at about the 17th week of gestation, “maternal vaccination starting in the early second trimester of gestation might be optimal to achieve the highest levels of antibodies in the newborn,” Dr. Munoz wrote. But questions remain, such as how effective the neonatal antibodies would be in protecting against COVID-19 and how long they last after birth.
No external funding was used in Dr. Joseph’s study. Dr. Joseph and Dr. Louis have disclosed no relevant financial relationships. The JAMA Pediatrics study was funded by the Children’s Hospital of Philadelphia. One coauthor received consultancy fees from Sanofi Pasteur, Lumen, Novavax, and Merck unrelated to the study. Dr. Munoz served on the data and safety monitoring boards of Moderna, Pfizer, Virometix, and Meissa Vaccines and has received grants from Novavax Research and Gilead Research.
A version of this article first appeared on Medscape.com.
Antibodies against SARS-CoV-2 cross the placenta during pregnancy and are detectable in most newborns born to mothers who had COVID-19 during pregnancy, according to findings from a study presented Jan. 28 at the meeting sponsored by the Society for Maternal-Fetal Medicine.
“I think the most striking finding is that we noticed a high degree of neutralizing response to natural infection even among asymptomatic infection, but of course a higher degree was seen in those with symptomatic infection,” Naima Joseph, MD, MPH, of Emory University, Atlanta, said in an interview.
“Our data demonstrate maternal capacity to mount an appropriate and robust immune response,” and maternal protective immunity lasted at least 28 days after infection, Dr. Joseph said. “Also, we noted higher neonatal cord blood titers in moms with higher titers, which suggests a relationship, but we need to better understand how transplacental transfer occurs as well as establish neonatal correlates of protection in order to see if and how maternal immunity may also benefit neonates.”
The researchers analyzed the amount of IgG and IgM antibodies in maternal and cord blood samples prospectively collected at delivery from women who tested positive for COVID-19 at any time while pregnant. They used enzyme-linked immunosorbent assay to assess for antibodies for the receptor binding domain of the SARS-CoV-2 spike protein.
The 32 pairs of mothers and infants in the study were predominantly non-Hispanic Black (72%) and Hispanic (25%), and 84% used Medicaid as their payer. Most of the mothers (72%) had at least one comorbidity, most commonly obesity, hypertension, and asthma or pulmonary disease. Just over half the women (53%) were symptomatic while they were infected, and 88% were ill with COVID-19 during the third trimester. The average time from infection to delivery was 28 days.
All the mothers had IgG antibodies, 94% had IgM antibodies, and 94% had neutralizing antibodies against SARS-CoV-2. Among the cord blood samples, 91% had IgG antibodies, 9% had IgM antibodies, and 25% had neutralizing antibodies.
“It’s reassuring that, so far, the physiological response is exactly what we expected it to be,” Judette Louis, MD, MPH, an associate professor of ob.gyn. and the ob.gyn. department chair at the University of South Florida, Tampa, said in an interview. “It’s what we would expect, but it’s always helpful to have more data to support that. Otherwise, you’re extrapolating from what you know from other conditions,” said Dr. Louis, who moderated the oral abstracts session.
Symptomatic infection was associated with significantly higher IgG titers than asymptomatic infection (P = .03), but no correlation was seen for IgM or neutralizing antibodies. In addition, although mothers who delivered more than 28 days after their infection had higher IgG titers (P = .05), no differences existed in IgM or neutralizing response.
Infants’ cord blood titers were significantly lower than their corresponding maternal samples, independently of symptoms or latency from infection to delivery (P < .001), Dr. Joseph reported.
“Transplacental efficiency in other pathogens has been shown to be correlated with neonatal immunity when the ratio of cord to maternal blood is greater than 1,” Dr. Joseph said in her presentation. Their data showed “suboptimal efficiency” at a ratio of 0.81.
The study’s small sample size and lack of a control group were weaknesses, but a major strength was having a population at disproportionately higher risk for infection and severe morbidity than the general population.
Implications for maternal COVID-19 vaccination
Although the data are not yet available, Dr. Joseph said they have expanded their protocol to include vaccinated pregnant women.
“The key to developing an effective vaccine [for pregnant people] is in really characterizing adaptive immunity in pregnancy,” Dr. Joseph told SMFM attendees. “I think that these findings inform further vaccine development in demonstrating that maternal immunity is robust.”
The World Health Organization recently recommended withholding COVID-19 vaccines from pregnant people, but the SMFM and American College of Obstetricians and Gynecologists subsequently issued a joint statement reaffirming that the COVID-19 vaccines authorized by the FDA “should not be withheld from pregnant individuals who choose to receive the vaccine.”
“One of the questions people ask is whether in pregnancy you’re going to mount a good response to the vaccine the way you would outside of pregnancy,” Dr. Louis said. “If we can demonstrate that you do, that may provide the information that some mothers need to make their decisions.” Data such as those from Dr. Joseph’s study can also inform recommendations on timing of maternal vaccination.
“For instance, Dr. Joseph demonstrated that, 28 days out from the infection, you had more antibodies, so there may be a scenario where we say this vaccine may be more beneficial in the middle of the pregnancy for the purpose of forming those antibodies,” Dr. Louis said.
Consensus emerging from maternal antibodies data
The findings from Dr. Joseph’s study mirror those reported in a study published online Jan. 29 in JAMA Pediatrics. That study, led by Dustin D. Flannery, DO, MSCE, of Children’s Hospital of Philadelphia, also examined maternal and neonatal levels of IgG and IgM antibodies against the receptor binding domain of the SARS-CoV-2 spike protein. They also found a positive correlation between cord blood and maternal IgG concentrations (P < .001), but notably, the ratio of cord to maternal blood titers was greater than 1, unlike in Dr. Joseph’s study.
For their study, Dr. Flannery and colleagues obtained maternal and cord blood sera at the time of delivery from 1471 pairs of mothers and infants, independently of COVID status during pregnancy. The average maternal age was 32 years, and just over a quarter of the population (26%) were Black, non-Hispanic women. About half (51%) were White, 12% were Hispanic, and 7% were Asian.
About 6% of the women had either IgG or IgM antibodies at delivery, and 87% of infants born to those mothers had measurable IgG in their cord blood. No infants had IgM antibodies. As with the study presented at SMFM, the mothers’ infections included asymptomatic, mild, moderate, and severe cases, and the degree of severity of cases had no apparent effect on infant antibody concentrations. Most of the women who tested positive for COVID-19 (60%) were asymptomatic.
Among the 11 mothers who had antibodies but whose infants’ cord blood did not, 5 had only IgM antibodies, and 6 had significantly lower IgG concentrations than those seen in the other mothers.
In a commentary about the JAMA Pediatrics study, Flor Munoz, MD, of the Baylor College of Medicine, Houston, suggested that the findings are grounds for optimism about a maternal vaccination strategy to protect infants from COVID-19.
“However, the timing of maternal vaccination to protect the infant, as opposed to the mother alone, would necessitate an adequate interval from vaccination to delivery (of at least 4 weeks), while vaccination early in gestation and even late in the third trimester could still be protective for the mother,” Dr. Munoz wrote.
Given the interval between two-dose vaccination regimens and the fact that transplacental transfer begins at about the 17th week of gestation, “maternal vaccination starting in the early second trimester of gestation might be optimal to achieve the highest levels of antibodies in the newborn,” Dr. Munoz wrote. But questions remain, such as how effective the neonatal antibodies would be in protecting against COVID-19 and how long they last after birth.
No external funding was used in Dr. Joseph’s study. Dr. Joseph and Dr. Louis have disclosed no relevant financial relationships. The JAMA Pediatrics study was funded by the Children’s Hospital of Philadelphia. One coauthor received consultancy fees from Sanofi Pasteur, Lumen, Novavax, and Merck unrelated to the study. Dr. Munoz served on the data and safety monitoring boards of Moderna, Pfizer, Virometix, and Meissa Vaccines and has received grants from Novavax Research and Gilead Research.
A version of this article first appeared on Medscape.com.
Dr. Fauci sees ‘wake-up call’ in emergence of new virus variants
New data on COVID-19 vaccines should serve as a “wake-up call” about the need to stop the spread of the SARS-CoV-2 virus among people and thus deprive it of opportunities to evolve its defenses, the top federal expert on infectious diseases said.
“The virus will continue to mutate and will mutate for its own selective advantage,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, at a Friday news conference organized by the White House.
The continued transmission of SARS-CoV-2 “gives the virus the chance to adapt to the forces, in this case the immune response, that’s trying to get rid of it,” Dr. Fauci said. “That’s where you get mutations.”
Federal health officials are working to boost the U.S. supply of COVID-19 vaccines, even as signals emerge about the extent that the virus is already evolving.
Data released this week about the Janssen/Johnson & Johnson (J&J) and Novavax COVID-19 vaccines in late-stage development provides further evidence that they may not protect as well against emerging variants, Dr. Fauci said.
“Mutations that lead to different lineage do have clinical consequences,” he said, while also emphasizing that the emerging vaccines appear to confer broad protection. Dr. Fauci earlier in the day addressed the “messaging challenge” for clinicians and researchers in discussing the results of the J&J vaccine trial, which appear to fall short of those reported for the two vaccines already approved and in use in the United States. He noted the benefits of possibly soon having more authorized vaccines to combat COVID-19. But continued community spread of the infection will foster conditions that can undermine the vaccines’ effectiveness.
“Even though the long-range effect in the sense of severe disease is still handled reasonably well by the vaccines, this is a wake-up call to all of us,” Dr. Fauci said.
Pharmaceutical scientists and executives and government health officials will need to work together to continue to develop vaccines that can outwit the emerging variants, he said.
On Jan. 29, J&J reported that its highly anticipated single-dose vaccine had shown its worst results in South Africa where many cases of COVID-19 were caused by infection with a SARS-CoV-2 variant from the B.1.351 lineage. The overall efficacy was 66% globally, 72% in the United States, and 57% in South Africa against moderate to severe SARS-CoV-2, J&J said.
Novavax on Jan. 28 reported an efficacy rate for its COVID-19 vaccine of 49.4% from a clinical trial conducted in South Africa, compared with an 89.3% rate from a U.K. study. There already have been attempts to estimate how well the Pfizer/BioNTech and Moderna vaccines can handle new variants of the virus. They both have been granted emergency-use authorization by the U.S. Food and Drug Administration.
‘Genomic surveillance’
The Centers for Disease Control and Prevention on Thursday reported the first U.S.-documented cases of the B.1.351 variant of SARS-CoV-2 in South Carolina. On Jan. 26, the first confirmed U.S. case of a highly transmissible Brazilian coronavirus variant was detected in Minnesota, state health officials said.
The CDC’s stepped-up “genomic surveillance” will help keep clinicians and researchers aware of how SARS-CoV-2 is changing, Dr. Fauci said.
Speaking at the same White House news conference, CDC director Rochelle Walensky, MD, MPH, said the two South Carolina cases of the B.1.351 variant were reported in different parts of the state and not believed to be epidemiologically linked. The people involved “did not have any travel history,” she added.
The SARS-CoV-2 mutations were expected to emerge at some point, as with any virus, but their appearance underscores the need for people to remain vigilant about precautions that can stop its spread, Dr. Walensky said.
She and Dr. Fauci both stressed the need for continued use of masks and social distancing and urged people to get COVID-19 vaccines as they become available. Continued community spread of the virus allows this global health threat to keep replicating, and thus increases its chances to thwart medical interventions, Dr. Fauci said.
“The virus has a playing field, as it were, to mutate,” Dr. Fauci said. “If you stop that and stop the replication, the viruses cannot mutate if they don’t replicate.”
A version of this article first appeared on Medscape.com.
New data on COVID-19 vaccines should serve as a “wake-up call” about the need to stop the spread of the SARS-CoV-2 virus among people and thus deprive it of opportunities to evolve its defenses, the top federal expert on infectious diseases said.
“The virus will continue to mutate and will mutate for its own selective advantage,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, at a Friday news conference organized by the White House.
The continued transmission of SARS-CoV-2 “gives the virus the chance to adapt to the forces, in this case the immune response, that’s trying to get rid of it,” Dr. Fauci said. “That’s where you get mutations.”
Federal health officials are working to boost the U.S. supply of COVID-19 vaccines, even as signals emerge about the extent that the virus is already evolving.
Data released this week about the Janssen/Johnson & Johnson (J&J) and Novavax COVID-19 vaccines in late-stage development provides further evidence that they may not protect as well against emerging variants, Dr. Fauci said.
“Mutations that lead to different lineage do have clinical consequences,” he said, while also emphasizing that the emerging vaccines appear to confer broad protection. Dr. Fauci earlier in the day addressed the “messaging challenge” for clinicians and researchers in discussing the results of the J&J vaccine trial, which appear to fall short of those reported for the two vaccines already approved and in use in the United States. He noted the benefits of possibly soon having more authorized vaccines to combat COVID-19. But continued community spread of the infection will foster conditions that can undermine the vaccines’ effectiveness.
“Even though the long-range effect in the sense of severe disease is still handled reasonably well by the vaccines, this is a wake-up call to all of us,” Dr. Fauci said.
Pharmaceutical scientists and executives and government health officials will need to work together to continue to develop vaccines that can outwit the emerging variants, he said.
On Jan. 29, J&J reported that its highly anticipated single-dose vaccine had shown its worst results in South Africa where many cases of COVID-19 were caused by infection with a SARS-CoV-2 variant from the B.1.351 lineage. The overall efficacy was 66% globally, 72% in the United States, and 57% in South Africa against moderate to severe SARS-CoV-2, J&J said.
Novavax on Jan. 28 reported an efficacy rate for its COVID-19 vaccine of 49.4% from a clinical trial conducted in South Africa, compared with an 89.3% rate from a U.K. study. There already have been attempts to estimate how well the Pfizer/BioNTech and Moderna vaccines can handle new variants of the virus. They both have been granted emergency-use authorization by the U.S. Food and Drug Administration.
‘Genomic surveillance’
The Centers for Disease Control and Prevention on Thursday reported the first U.S.-documented cases of the B.1.351 variant of SARS-CoV-2 in South Carolina. On Jan. 26, the first confirmed U.S. case of a highly transmissible Brazilian coronavirus variant was detected in Minnesota, state health officials said.
The CDC’s stepped-up “genomic surveillance” will help keep clinicians and researchers aware of how SARS-CoV-2 is changing, Dr. Fauci said.
Speaking at the same White House news conference, CDC director Rochelle Walensky, MD, MPH, said the two South Carolina cases of the B.1.351 variant were reported in different parts of the state and not believed to be epidemiologically linked. The people involved “did not have any travel history,” she added.
The SARS-CoV-2 mutations were expected to emerge at some point, as with any virus, but their appearance underscores the need for people to remain vigilant about precautions that can stop its spread, Dr. Walensky said.
She and Dr. Fauci both stressed the need for continued use of masks and social distancing and urged people to get COVID-19 vaccines as they become available. Continued community spread of the virus allows this global health threat to keep replicating, and thus increases its chances to thwart medical interventions, Dr. Fauci said.
“The virus has a playing field, as it were, to mutate,” Dr. Fauci said. “If you stop that and stop the replication, the viruses cannot mutate if they don’t replicate.”
A version of this article first appeared on Medscape.com.
New data on COVID-19 vaccines should serve as a “wake-up call” about the need to stop the spread of the SARS-CoV-2 virus among people and thus deprive it of opportunities to evolve its defenses, the top federal expert on infectious diseases said.
“The virus will continue to mutate and will mutate for its own selective advantage,” said Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases, at a Friday news conference organized by the White House.
The continued transmission of SARS-CoV-2 “gives the virus the chance to adapt to the forces, in this case the immune response, that’s trying to get rid of it,” Dr. Fauci said. “That’s where you get mutations.”
Federal health officials are working to boost the U.S. supply of COVID-19 vaccines, even as signals emerge about the extent that the virus is already evolving.
Data released this week about the Janssen/Johnson & Johnson (J&J) and Novavax COVID-19 vaccines in late-stage development provides further evidence that they may not protect as well against emerging variants, Dr. Fauci said.
“Mutations that lead to different lineage do have clinical consequences,” he said, while also emphasizing that the emerging vaccines appear to confer broad protection. Dr. Fauci earlier in the day addressed the “messaging challenge” for clinicians and researchers in discussing the results of the J&J vaccine trial, which appear to fall short of those reported for the two vaccines already approved and in use in the United States. He noted the benefits of possibly soon having more authorized vaccines to combat COVID-19. But continued community spread of the infection will foster conditions that can undermine the vaccines’ effectiveness.
“Even though the long-range effect in the sense of severe disease is still handled reasonably well by the vaccines, this is a wake-up call to all of us,” Dr. Fauci said.
Pharmaceutical scientists and executives and government health officials will need to work together to continue to develop vaccines that can outwit the emerging variants, he said.
On Jan. 29, J&J reported that its highly anticipated single-dose vaccine had shown its worst results in South Africa where many cases of COVID-19 were caused by infection with a SARS-CoV-2 variant from the B.1.351 lineage. The overall efficacy was 66% globally, 72% in the United States, and 57% in South Africa against moderate to severe SARS-CoV-2, J&J said.
Novavax on Jan. 28 reported an efficacy rate for its COVID-19 vaccine of 49.4% from a clinical trial conducted in South Africa, compared with an 89.3% rate from a U.K. study. There already have been attempts to estimate how well the Pfizer/BioNTech and Moderna vaccines can handle new variants of the virus. They both have been granted emergency-use authorization by the U.S. Food and Drug Administration.
‘Genomic surveillance’
The Centers for Disease Control and Prevention on Thursday reported the first U.S.-documented cases of the B.1.351 variant of SARS-CoV-2 in South Carolina. On Jan. 26, the first confirmed U.S. case of a highly transmissible Brazilian coronavirus variant was detected in Minnesota, state health officials said.
The CDC’s stepped-up “genomic surveillance” will help keep clinicians and researchers aware of how SARS-CoV-2 is changing, Dr. Fauci said.
Speaking at the same White House news conference, CDC director Rochelle Walensky, MD, MPH, said the two South Carolina cases of the B.1.351 variant were reported in different parts of the state and not believed to be epidemiologically linked. The people involved “did not have any travel history,” she added.
The SARS-CoV-2 mutations were expected to emerge at some point, as with any virus, but their appearance underscores the need for people to remain vigilant about precautions that can stop its spread, Dr. Walensky said.
She and Dr. Fauci both stressed the need for continued use of masks and social distancing and urged people to get COVID-19 vaccines as they become available. Continued community spread of the virus allows this global health threat to keep replicating, and thus increases its chances to thwart medical interventions, Dr. Fauci said.
“The virus has a playing field, as it were, to mutate,” Dr. Fauci said. “If you stop that and stop the replication, the viruses cannot mutate if they don’t replicate.”
A version of this article first appeared on Medscape.com.
The COVID-19 virus may prompt the body to attack itself
An international team of researchers studying COVID-19 has made a startling and pivotal discovery: The virus appears to cause the body to make weapons to attack its own tissues.
The finding could unlock a number of COVID-19’s clinical mysteries. They include the puzzling collection of symptoms that can come with the infection; the persistence of symptoms in some people for months after they clear the virus, a phenomenon dubbed long COVID-19; and why some children and adults have a serious inflammatory syndrome, called multisystem inflammatory syndrome in children (MIS-C) or MIS in adults (MIS-A), after their infections.
“It suggests that the virus might be directly causing autoimmunity, which would be fascinating,” says lead study author Paul Utz, MD, who studies immunology and autoimmunity at Stanford (Calif.) University.
The study also deepens the question of whether other respiratory viruses might also break the body’s tolerance to itself, setting people up for autoimmune diseases like multiple sclerosis, rheumatoid arthritis, and lupus later in life.
Dr. Utz said he and his team are next going to study flu patients to see if that virus might also cause this phenomenon.
“My prediction is that it isn’t going to be specific just to SARS-CoV-2. I’m willing to bet that we will find this with other respiratory viruses,” he said.
The study comes on the heels of a handful of smaller, detailed investigations that have come to similar conclusions.
The study included data from more than 300 patients from four hospitals: two in California, one in Pennsylvania, and another in Germany.
Researchers used blood tests to study their immune responses as their infections progressed. Researchers looked for autoantibodies – weapons of the immune system that go rogue and launch an attack against the body’s own tissues. They compared these autoantibodies with those found in people who were not infected with the virus that causes COVID.
As previous studies have found, autoantibodies were more common after COVID – 50% of people hospitalized for their infections had autoantibodies, compared with less than 15% of those who were healthy and uninfected.
Some people with autoantibodies had little change in them as their infections progressed. That suggests the autoantibodies were there to begin with, possibly allowing the infection to burn out of control in the body.
“Their body is set up to get bad COVID, and it’s probably caused by the autoantibodies,” Dr. Utz said.
But in others, about 20% of people who had them, the autoantibodies became more common as the infection progressed, suggesting they were directly related to the viral infection, instead of being a preexisting condition.
Some of these were antibodies that attack key components of the immune system’s weapons against the virus, like interferon. Interferons are proteins that help infected cells call for reinforcements and can also interfere with a virus’s ability to copy itself. Taking them out is a powerful evasive tactic, and previous studies have shown that people who are born with genes that cause them to have lower interferon function, or who make autoantibodies against these proteins, appear to be at higher risk for life-threatening COVID infections.
“It seems to give the virus a powerful advantage,” said study author, John Wherry, PhD, who directs the Institute for Immunology at the University of Pennsylvania, Philadelphia. “Now your immune system, instead of having a tiny little hill to climb, is staring at Mount Everest. That really is devious.”
In addition to those that sabotage the immune system, some people in the study had autoantibodies against muscles and connective tissues that are seen in some rare disorders.
Dr. Utz said they started the study after seeing COVID patients with strange collections of symptoms that looked more like autoimmune diseases than viral infections – skin rashes, joint pain, fatigue, aching muscles, brain swelling, dry eyes, blood that clots easily, and inflamed blood vessels.
“One thing that’s very important to note is that we don’t know if these patients are going to go on to develop autoimmune disease,” Dr. Utz said. “I think we’ll be able to answer that question in the next 6-12 months as we follow the long haulers and study their samples.”
Dr. Utz said it will be important to study autoantibodies in long haulers to see if they can identify exactly which ones seem to be at work in the condition. If you can catch them early, it might be possible to treat those at risk for enduring symptoms with drugs that suppress the immune system.
What this means, he said, is that COVID will be with us for a long, long time.
“We have to realize that there’s going to be long-term damage from this virus for the survivors. Not just the long haulers, but all the people who have lung damage and heart damage and everything else. We’re going to be studying this virus and it’s badness for decades,” Dr. Utz said.
A version of this article first appeared on WebMD.com.
An international team of researchers studying COVID-19 has made a startling and pivotal discovery: The virus appears to cause the body to make weapons to attack its own tissues.
The finding could unlock a number of COVID-19’s clinical mysteries. They include the puzzling collection of symptoms that can come with the infection; the persistence of symptoms in some people for months after they clear the virus, a phenomenon dubbed long COVID-19; and why some children and adults have a serious inflammatory syndrome, called multisystem inflammatory syndrome in children (MIS-C) or MIS in adults (MIS-A), after their infections.
“It suggests that the virus might be directly causing autoimmunity, which would be fascinating,” says lead study author Paul Utz, MD, who studies immunology and autoimmunity at Stanford (Calif.) University.
The study also deepens the question of whether other respiratory viruses might also break the body’s tolerance to itself, setting people up for autoimmune diseases like multiple sclerosis, rheumatoid arthritis, and lupus later in life.
Dr. Utz said he and his team are next going to study flu patients to see if that virus might also cause this phenomenon.
“My prediction is that it isn’t going to be specific just to SARS-CoV-2. I’m willing to bet that we will find this with other respiratory viruses,” he said.
The study comes on the heels of a handful of smaller, detailed investigations that have come to similar conclusions.
The study included data from more than 300 patients from four hospitals: two in California, one in Pennsylvania, and another in Germany.
Researchers used blood tests to study their immune responses as their infections progressed. Researchers looked for autoantibodies – weapons of the immune system that go rogue and launch an attack against the body’s own tissues. They compared these autoantibodies with those found in people who were not infected with the virus that causes COVID.
As previous studies have found, autoantibodies were more common after COVID – 50% of people hospitalized for their infections had autoantibodies, compared with less than 15% of those who were healthy and uninfected.
Some people with autoantibodies had little change in them as their infections progressed. That suggests the autoantibodies were there to begin with, possibly allowing the infection to burn out of control in the body.
“Their body is set up to get bad COVID, and it’s probably caused by the autoantibodies,” Dr. Utz said.
But in others, about 20% of people who had them, the autoantibodies became more common as the infection progressed, suggesting they were directly related to the viral infection, instead of being a preexisting condition.
Some of these were antibodies that attack key components of the immune system’s weapons against the virus, like interferon. Interferons are proteins that help infected cells call for reinforcements and can also interfere with a virus’s ability to copy itself. Taking them out is a powerful evasive tactic, and previous studies have shown that people who are born with genes that cause them to have lower interferon function, or who make autoantibodies against these proteins, appear to be at higher risk for life-threatening COVID infections.
“It seems to give the virus a powerful advantage,” said study author, John Wherry, PhD, who directs the Institute for Immunology at the University of Pennsylvania, Philadelphia. “Now your immune system, instead of having a tiny little hill to climb, is staring at Mount Everest. That really is devious.”
In addition to those that sabotage the immune system, some people in the study had autoantibodies against muscles and connective tissues that are seen in some rare disorders.
Dr. Utz said they started the study after seeing COVID patients with strange collections of symptoms that looked more like autoimmune diseases than viral infections – skin rashes, joint pain, fatigue, aching muscles, brain swelling, dry eyes, blood that clots easily, and inflamed blood vessels.
“One thing that’s very important to note is that we don’t know if these patients are going to go on to develop autoimmune disease,” Dr. Utz said. “I think we’ll be able to answer that question in the next 6-12 months as we follow the long haulers and study their samples.”
Dr. Utz said it will be important to study autoantibodies in long haulers to see if they can identify exactly which ones seem to be at work in the condition. If you can catch them early, it might be possible to treat those at risk for enduring symptoms with drugs that suppress the immune system.
What this means, he said, is that COVID will be with us for a long, long time.
“We have to realize that there’s going to be long-term damage from this virus for the survivors. Not just the long haulers, but all the people who have lung damage and heart damage and everything else. We’re going to be studying this virus and it’s badness for decades,” Dr. Utz said.
A version of this article first appeared on WebMD.com.
An international team of researchers studying COVID-19 has made a startling and pivotal discovery: The virus appears to cause the body to make weapons to attack its own tissues.
The finding could unlock a number of COVID-19’s clinical mysteries. They include the puzzling collection of symptoms that can come with the infection; the persistence of symptoms in some people for months after they clear the virus, a phenomenon dubbed long COVID-19; and why some children and adults have a serious inflammatory syndrome, called multisystem inflammatory syndrome in children (MIS-C) or MIS in adults (MIS-A), after their infections.
“It suggests that the virus might be directly causing autoimmunity, which would be fascinating,” says lead study author Paul Utz, MD, who studies immunology and autoimmunity at Stanford (Calif.) University.
The study also deepens the question of whether other respiratory viruses might also break the body’s tolerance to itself, setting people up for autoimmune diseases like multiple sclerosis, rheumatoid arthritis, and lupus later in life.
Dr. Utz said he and his team are next going to study flu patients to see if that virus might also cause this phenomenon.
“My prediction is that it isn’t going to be specific just to SARS-CoV-2. I’m willing to bet that we will find this with other respiratory viruses,” he said.
The study comes on the heels of a handful of smaller, detailed investigations that have come to similar conclusions.
The study included data from more than 300 patients from four hospitals: two in California, one in Pennsylvania, and another in Germany.
Researchers used blood tests to study their immune responses as their infections progressed. Researchers looked for autoantibodies – weapons of the immune system that go rogue and launch an attack against the body’s own tissues. They compared these autoantibodies with those found in people who were not infected with the virus that causes COVID.
As previous studies have found, autoantibodies were more common after COVID – 50% of people hospitalized for their infections had autoantibodies, compared with less than 15% of those who were healthy and uninfected.
Some people with autoantibodies had little change in them as their infections progressed. That suggests the autoantibodies were there to begin with, possibly allowing the infection to burn out of control in the body.
“Their body is set up to get bad COVID, and it’s probably caused by the autoantibodies,” Dr. Utz said.
But in others, about 20% of people who had them, the autoantibodies became more common as the infection progressed, suggesting they were directly related to the viral infection, instead of being a preexisting condition.
Some of these were antibodies that attack key components of the immune system’s weapons against the virus, like interferon. Interferons are proteins that help infected cells call for reinforcements and can also interfere with a virus’s ability to copy itself. Taking them out is a powerful evasive tactic, and previous studies have shown that people who are born with genes that cause them to have lower interferon function, or who make autoantibodies against these proteins, appear to be at higher risk for life-threatening COVID infections.
“It seems to give the virus a powerful advantage,” said study author, John Wherry, PhD, who directs the Institute for Immunology at the University of Pennsylvania, Philadelphia. “Now your immune system, instead of having a tiny little hill to climb, is staring at Mount Everest. That really is devious.”
In addition to those that sabotage the immune system, some people in the study had autoantibodies against muscles and connective tissues that are seen in some rare disorders.
Dr. Utz said they started the study after seeing COVID patients with strange collections of symptoms that looked more like autoimmune diseases than viral infections – skin rashes, joint pain, fatigue, aching muscles, brain swelling, dry eyes, blood that clots easily, and inflamed blood vessels.
“One thing that’s very important to note is that we don’t know if these patients are going to go on to develop autoimmune disease,” Dr. Utz said. “I think we’ll be able to answer that question in the next 6-12 months as we follow the long haulers and study their samples.”
Dr. Utz said it will be important to study autoantibodies in long haulers to see if they can identify exactly which ones seem to be at work in the condition. If you can catch them early, it might be possible to treat those at risk for enduring symptoms with drugs that suppress the immune system.
What this means, he said, is that COVID will be with us for a long, long time.
“We have to realize that there’s going to be long-term damage from this virus for the survivors. Not just the long haulers, but all the people who have lung damage and heart damage and everything else. We’re going to be studying this virus and it’s badness for decades,” Dr. Utz said.
A version of this article first appeared on WebMD.com.
Long-acting injectable antipsychotics during COVID-19
Long-acting injectable antipsychotics (LAIs) are an essential tool in the treatment of patients with psychotic disorders, allowing for periods of stable drug plasma concentration and confirmed adherence.1 The current coronavirus disease 2019 (COVID-19) pandemic presents unique challenges for administering LAIs and requires a thoughtful and prospective approach in order to ensure continuity of psychiatric care while minimizing the risk of infection with COVID-19. Ideally, patients should be seen in person as infrequently as clinically prudent during this public health emergency; however, LAI administration necessitates direct physical contact between patient and clinician.
Patients with serious mental illness (SMI), who comprise the majority of individuals who receive LAIs, are at heightened risk for cardiovascular and pulmonary comorbidities. These factors are the primary reason the life expectancy of a patient with SMI is nearly 30 years shorter than that of the general population.2-5 The risk of health care workers becoming infected or inadvertently spreading COVID-19 is heightened when working with patients in group living environments (ie, a shelter or group home), who have both increased exposure and increased risk of further transmission.6 Additional patient populations, including older adults, immunocompromised individuals, and those with preexisting conditions, are at heightened risk for serious complications if they were to contract COVID-19.7,8
Thus, the questions of whether LAIs should be administered, and how to do so safely (both during the ongoing, acute phase of the pandemic as well as during the subsequent recovery period until the pandemic abates) need to be carefully considered. In this article, we provide concrete advice for clinicians and clinics on these topics, with the goal of maintaining patients’ psychiatric stability while protecting patients, health care workers, and the broader society from COVID-19 infection. Table 1 summarizes the questions regarding LAIs that clinicians need to address during this crisis. While we focus on outpatient care, inpatient teams should keep these considerations in mind if they are starting and discharging a patient on an LAI. More than ever, close collaboration and communication between inpatient and outpatient teams is critical.
Should an LAI be continued?
An important first step to approaching this challenge is to create a spreadsheet for all patients receiving LAIs. Focusing on a population-based approach is helpful to be systematic and ensure that no patients fall through the cracks during this public health emergency.9 Once all patients have been identified, the treatment team should review each patient to determine if continuing to administer the antipsychotic as an LAI formulation is essential, taking into account the patient’s current psychiatric status, historical medication adherence, potential severity and dangerousness of decompensation if nonadherent, and structures to support stability. For example, can a patient move in with family who can monitor medication adherence during the pandemic? Is it possible for the group home to assume medication administration? Additional consideration should be given to the living environment and health-vulnerability of the patient and the individuals living with them.
If the risk calculation does not point strongly towards a need for continuing the LAI, it may be prudent to temporarily transition the patient to the corresponding oral antipsychotic preparation. Table 2 lists all LAIs available in the United States and their approximate equivalent oral dosing. It is important to note that such transitions are not without clinical risk, to emphasize to the patient that the transition is intended as a temporary measure, and to discuss a proposed timeline for re-initiating the LAI. Also, emphasize to the patient and family that this transition does not diminish the previous reasoning for needing an LAI, but is a temporary measure taken in light of weighing the risks and benefits during a pandemic.
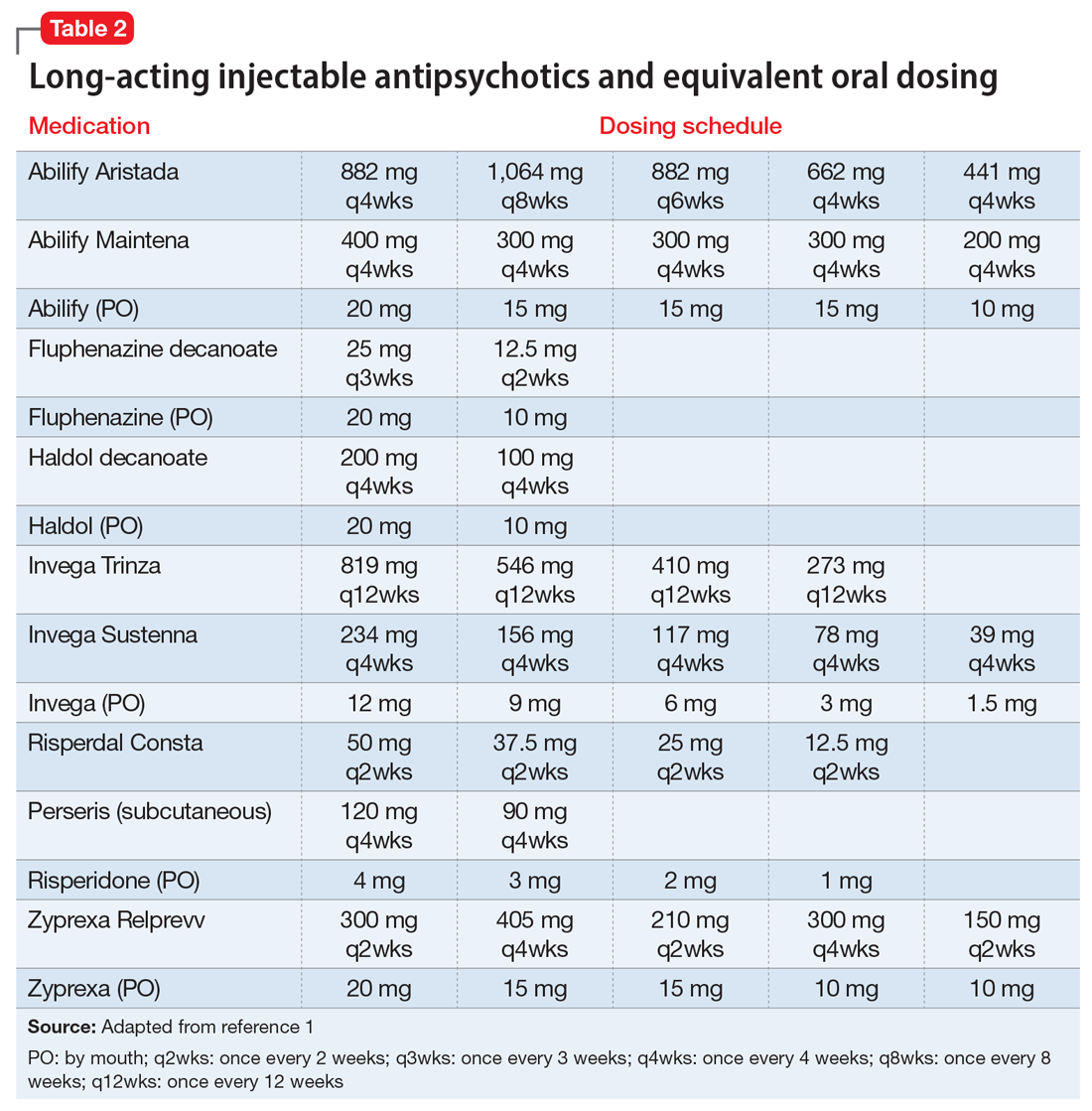
Which LAI should be administered?
If continuing the LAI is determined to be clinically necessary, consider switching the patient to a longer-acting preparation to maximize intervals between administrations and minimize the potential for infection. From a public health perspective, the longest clinically prudent interval between injections may be the most important consideration, provided the patient can receive a dose necessary to retain stability, and the LAI should be chosen accordingly. Deltoid injections may be able to be administered with reduced contact, or on a “drive-up” basis.10 Consider transitioning a patient who is receiving olanzapine pamoate to an alternate LAI or oral formulation, because the 3-hour observation period that is required after olanzapine pamoate administration is particularly problematic. While it may not be ideal to make medication changes during a pandemic, it is worth carefully weighing the patient’s stability and historical experience with other LAIs to determine if a safer/longer-spaced option is worth trying.11
We recommend only switching among similar antipsychotics (ie, risperidone to paliperidone), or between different preparations of the same drug (ie, Abilify Maintena to Aristada), if possible, as these are the lowest risk transitions with regards to relapse. Table 3 provides examples.
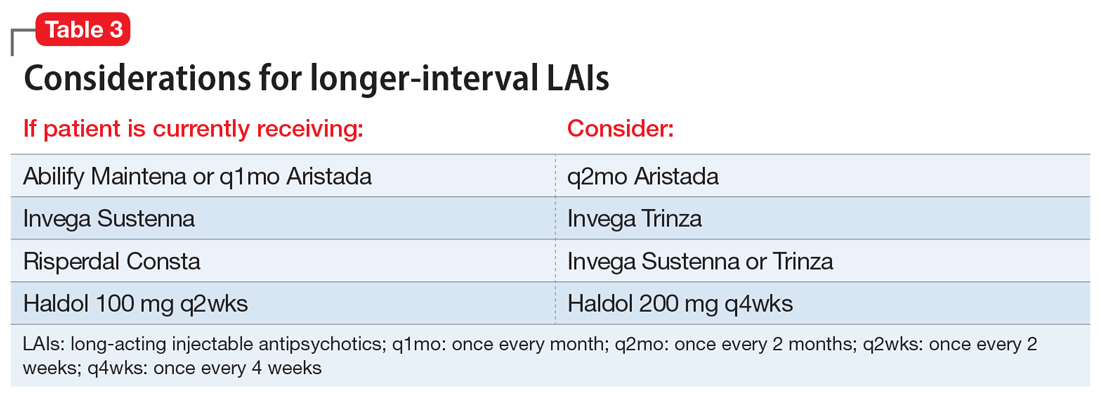
Continue to: When should the LAI be administered?
When should the LAI be administered?
The pharmacokinetics of LAIs allow for some flexibility in terms of when an LAI needs to be administered. The package inserts of all second-generation LAIs include missed-dose guidelines. These guidelines provide information on how long one can wait before the next injection is due, and what additional measures must be taken when beyond that date. Delaying an injection may be prudent, and the missed dose guidelines will indicate when one must consider supplementing with oral medications. For patients who are in quarantine, it may be better to delay an injection until the patient ends their quarantine than to deliver the dose during quarantine. Administering an injection earlier also is usually safe; off-cycle visits may help minimize patient contact (ie, if the patient happens to be coming into the vicinity of the clinic, or requires phlebotomy for therapeutic drug monitoring), and assist in planning for possible resurgences. When appropriate, and after considering the risk of worsening adverse effects, administering a higher dose than the usual maintenance dose would provide a buffer if the next injection was to be delayed. Therapeutic drug monitoring can help to optimize dosing and avoid low plasma drug levels, which may be not be sufficient, particularly during this time of stress.12 To provide optimal protection against relapse, consider administering a dose that puts patients at the higher range of plasma drug levels.
Where can the LAI be administered, and who can give it?
For patients who usually travel to a clinic, consider arranging for a more local injection (ie, at the patient’s primary care clinic in their hometown, or at a local mental health center), and explore if the patient may be able to receive their injection in their home through a visiting nurse association (VNA). In many states (approximately 30 currently), clinicians at pharmacies are also able to administer patient injections. Clinics would do well to at least plan for alternate staffing models in the event of staff illness. A pool of individuals should be available to give injections; consider training additional staff members (including MDs who may have never previously administered an LAI but could be quickly instructed to do so) to administer LAIs. Theoretically, during a public health emergency, family members, particularly those who have a background in health care, could be trained to give an injection and provided education on LAI storage and post-injection monitoring. This approach would not be consistent with FDA labeling, however, and should only be considered as a last resort.
What safety measures can be put in place?
Face-to-face time for injection administration should be kept as brief as possible. Before the encounter, obtain the patient’s clinical information, ideally through telehealth or from an acceptable distance. Medication should be drawn ahead of time, and not in an enclosed space with the patient present. Strongly consider abandoning the traditional enclosed room for the injection, and instead use larger spaces, doorways, or outside, if feasible. As previously noted, some clinics and clinicians have used a drive-up approach for LAI administration, particularly for deltoid injections.10 Individuals who administer the injections should wear personal protective equipment, and the clinic should obtain an adequate supply of this equipment well in advance.
Lessons learned at our clinic
In our community mental health center clinic, planning around these questions has allowed us to provide safe and continuous psychiatric care with LAIs during this public health emergency while reducing the risk of infection. We have worked to transfer LAI administration to VNAs and transition patients to longer-lasting formulations or oral medications where appropriate, which has resulted in an approximately 50% decrease in in-person visits. Reducing the number of in-person visits does not need to result in less frequent clinical follow-up. Telepsychiatry visits can make up for lost in-person visits and have generally been well accepted.
As we are preparing for the next phase, routine medical health monitoring (eg, metabolic monitoring, monitoring for tardive dyskinesia) that has not been at the forefront of concerns should be carefully reintroduced. Challenges encountered have included difficulty in having VNA accept patients for short-term LAI visits, changes to where on the body the injection is delivered, and patients with SMI and their families being reluctant to depart from previous routines and administration schedules.
Continue to: There is great value...
There is great value in the collective lessons learned during this public health emergency (eg, the need for a flexible, population health-based approach; acceptability of combination telehealth and in-person visits) that can lead to more person-centered and accessible care for patients with SMI.
Acknowledgments
The authors thank North Suffolk Mental Health Association, the Freedom Trail Clinic, and their patients.
Bottom Line
When caring for a patient with a psychotic illness during the coronavirus disease 2019 (COVID-19) pandemic, evaluate whether it is necessary to continue a longacting injectable antipsychotic (LAI). If yes, reconsider which LAI should be administered, when and where it should be given, and by whom. Implement safety measures to minimize the risk of COVID-19 exposure and transmission.
Related Resources
- American Association of Community Psychiatrists. Clinical Tip Series. Long acting antipsychotic medications. https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view?usp=sharing
- SMI Adviser. What are clinical considerations for giving LAIs during the COVID-19 public health emergency? https://smiadviser.org/knowledge_post/what-are-clinical-considerations-for-giving-lais-during-the-covid-19-public-health-emergency
- CDC. Infection control basics. https://www.cdc.gov/infectioncontrol/basics/index.html
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole for extended- release injectable suspension • Abilify Maintena
Aripiprazole lauroxil • Aristada
Haloperidol • Haldol
Haloperidol injection • Haldol decanoate
Olanzapine • Zyprexa
Olanzapine for extended-release injectable suspension • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate extended-release injectable suspension • Invega Sustenna
Paliperidone palmitate extended-release injectable suspension • Invega Trinza
Risperidone • Risperdal
Risperidone for extended- release injectable suspension • Perseris
Risperidone injection • Risperdal Consta
1. Freudenreich O. Long-acting injectable antipsychotics. In: Freudenreich O. Psychotic disorders: a practical guide. Springer; 2020:249-261.
2. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
3. Reilly S, Olier I, Planner C, et al. Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open. 2015;5(12):e009010.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
5. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66(2):183-194.
6. Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323(21):2191-2192.
7. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346.
8. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985
9. Etches V, Frank J, Di Ruggiero E, et al. Measuring population health: a review of indicators. Annu Rev Public Health. 2006;27:29-55.
10. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
11. Sajatovic M, Ross R, Legacy SN, et al. Initiating/maintaining long-acting injectable antipsychotics in schizophrenia/schizoaffective or bipolar disorder - expert consensus survey part 2. Neuropsychiatr Dis Treat. 2018;14:1475-1492.
12. Schoretsanitis G, Kane JM, Correll CU, et al; American Society of Clinical Psychopharmacology, Pharmakopsychiatrie TTDMTFOTAFNU. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81(3):19cs13169. doi: 10.4088/JCP.19cs13169
Long-acting injectable antipsychotics (LAIs) are an essential tool in the treatment of patients with psychotic disorders, allowing for periods of stable drug plasma concentration and confirmed adherence.1 The current coronavirus disease 2019 (COVID-19) pandemic presents unique challenges for administering LAIs and requires a thoughtful and prospective approach in order to ensure continuity of psychiatric care while minimizing the risk of infection with COVID-19. Ideally, patients should be seen in person as infrequently as clinically prudent during this public health emergency; however, LAI administration necessitates direct physical contact between patient and clinician.
Patients with serious mental illness (SMI), who comprise the majority of individuals who receive LAIs, are at heightened risk for cardiovascular and pulmonary comorbidities. These factors are the primary reason the life expectancy of a patient with SMI is nearly 30 years shorter than that of the general population.2-5 The risk of health care workers becoming infected or inadvertently spreading COVID-19 is heightened when working with patients in group living environments (ie, a shelter or group home), who have both increased exposure and increased risk of further transmission.6 Additional patient populations, including older adults, immunocompromised individuals, and those with preexisting conditions, are at heightened risk for serious complications if they were to contract COVID-19.7,8
Thus, the questions of whether LAIs should be administered, and how to do so safely (both during the ongoing, acute phase of the pandemic as well as during the subsequent recovery period until the pandemic abates) need to be carefully considered. In this article, we provide concrete advice for clinicians and clinics on these topics, with the goal of maintaining patients’ psychiatric stability while protecting patients, health care workers, and the broader society from COVID-19 infection. Table 1 summarizes the questions regarding LAIs that clinicians need to address during this crisis. While we focus on outpatient care, inpatient teams should keep these considerations in mind if they are starting and discharging a patient on an LAI. More than ever, close collaboration and communication between inpatient and outpatient teams is critical.
Should an LAI be continued?
An important first step to approaching this challenge is to create a spreadsheet for all patients receiving LAIs. Focusing on a population-based approach is helpful to be systematic and ensure that no patients fall through the cracks during this public health emergency.9 Once all patients have been identified, the treatment team should review each patient to determine if continuing to administer the antipsychotic as an LAI formulation is essential, taking into account the patient’s current psychiatric status, historical medication adherence, potential severity and dangerousness of decompensation if nonadherent, and structures to support stability. For example, can a patient move in with family who can monitor medication adherence during the pandemic? Is it possible for the group home to assume medication administration? Additional consideration should be given to the living environment and health-vulnerability of the patient and the individuals living with them.
If the risk calculation does not point strongly towards a need for continuing the LAI, it may be prudent to temporarily transition the patient to the corresponding oral antipsychotic preparation. Table 2 lists all LAIs available in the United States and their approximate equivalent oral dosing. It is important to note that such transitions are not without clinical risk, to emphasize to the patient that the transition is intended as a temporary measure, and to discuss a proposed timeline for re-initiating the LAI. Also, emphasize to the patient and family that this transition does not diminish the previous reasoning for needing an LAI, but is a temporary measure taken in light of weighing the risks and benefits during a pandemic.

Which LAI should be administered?
If continuing the LAI is determined to be clinically necessary, consider switching the patient to a longer-acting preparation to maximize intervals between administrations and minimize the potential for infection. From a public health perspective, the longest clinically prudent interval between injections may be the most important consideration, provided the patient can receive a dose necessary to retain stability, and the LAI should be chosen accordingly. Deltoid injections may be able to be administered with reduced contact, or on a “drive-up” basis.10 Consider transitioning a patient who is receiving olanzapine pamoate to an alternate LAI or oral formulation, because the 3-hour observation period that is required after olanzapine pamoate administration is particularly problematic. While it may not be ideal to make medication changes during a pandemic, it is worth carefully weighing the patient’s stability and historical experience with other LAIs to determine if a safer/longer-spaced option is worth trying.11
We recommend only switching among similar antipsychotics (ie, risperidone to paliperidone), or between different preparations of the same drug (ie, Abilify Maintena to Aristada), if possible, as these are the lowest risk transitions with regards to relapse. Table 3 provides examples.

Continue to: When should the LAI be administered?
When should the LAI be administered?
The pharmacokinetics of LAIs allow for some flexibility in terms of when an LAI needs to be administered. The package inserts of all second-generation LAIs include missed-dose guidelines. These guidelines provide information on how long one can wait before the next injection is due, and what additional measures must be taken when beyond that date. Delaying an injection may be prudent, and the missed dose guidelines will indicate when one must consider supplementing with oral medications. For patients who are in quarantine, it may be better to delay an injection until the patient ends their quarantine than to deliver the dose during quarantine. Administering an injection earlier also is usually safe; off-cycle visits may help minimize patient contact (ie, if the patient happens to be coming into the vicinity of the clinic, or requires phlebotomy for therapeutic drug monitoring), and assist in planning for possible resurgences. When appropriate, and after considering the risk of worsening adverse effects, administering a higher dose than the usual maintenance dose would provide a buffer if the next injection was to be delayed. Therapeutic drug monitoring can help to optimize dosing and avoid low plasma drug levels, which may be not be sufficient, particularly during this time of stress.12 To provide optimal protection against relapse, consider administering a dose that puts patients at the higher range of plasma drug levels.
Where can the LAI be administered, and who can give it?
For patients who usually travel to a clinic, consider arranging for a more local injection (ie, at the patient’s primary care clinic in their hometown, or at a local mental health center), and explore if the patient may be able to receive their injection in their home through a visiting nurse association (VNA). In many states (approximately 30 currently), clinicians at pharmacies are also able to administer patient injections. Clinics would do well to at least plan for alternate staffing models in the event of staff illness. A pool of individuals should be available to give injections; consider training additional staff members (including MDs who may have never previously administered an LAI but could be quickly instructed to do so) to administer LAIs. Theoretically, during a public health emergency, family members, particularly those who have a background in health care, could be trained to give an injection and provided education on LAI storage and post-injection monitoring. This approach would not be consistent with FDA labeling, however, and should only be considered as a last resort.
What safety measures can be put in place?
Face-to-face time for injection administration should be kept as brief as possible. Before the encounter, obtain the patient’s clinical information, ideally through telehealth or from an acceptable distance. Medication should be drawn ahead of time, and not in an enclosed space with the patient present. Strongly consider abandoning the traditional enclosed room for the injection, and instead use larger spaces, doorways, or outside, if feasible. As previously noted, some clinics and clinicians have used a drive-up approach for LAI administration, particularly for deltoid injections.10 Individuals who administer the injections should wear personal protective equipment, and the clinic should obtain an adequate supply of this equipment well in advance.
Lessons learned at our clinic
In our community mental health center clinic, planning around these questions has allowed us to provide safe and continuous psychiatric care with LAIs during this public health emergency while reducing the risk of infection. We have worked to transfer LAI administration to VNAs and transition patients to longer-lasting formulations or oral medications where appropriate, which has resulted in an approximately 50% decrease in in-person visits. Reducing the number of in-person visits does not need to result in less frequent clinical follow-up. Telepsychiatry visits can make up for lost in-person visits and have generally been well accepted.
As we are preparing for the next phase, routine medical health monitoring (eg, metabolic monitoring, monitoring for tardive dyskinesia) that has not been at the forefront of concerns should be carefully reintroduced. Challenges encountered have included difficulty in having VNA accept patients for short-term LAI visits, changes to where on the body the injection is delivered, and patients with SMI and their families being reluctant to depart from previous routines and administration schedules.
Continue to: There is great value...
There is great value in the collective lessons learned during this public health emergency (eg, the need for a flexible, population health-based approach; acceptability of combination telehealth and in-person visits) that can lead to more person-centered and accessible care for patients with SMI.
Acknowledgments
The authors thank North Suffolk Mental Health Association, the Freedom Trail Clinic, and their patients.
Bottom Line
When caring for a patient with a psychotic illness during the coronavirus disease 2019 (COVID-19) pandemic, evaluate whether it is necessary to continue a longacting injectable antipsychotic (LAI). If yes, reconsider which LAI should be administered, when and where it should be given, and by whom. Implement safety measures to minimize the risk of COVID-19 exposure and transmission.
Related Resources
- American Association of Community Psychiatrists. Clinical Tip Series. Long acting antipsychotic medications. https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view?usp=sharing
- SMI Adviser. What are clinical considerations for giving LAIs during the COVID-19 public health emergency? https://smiadviser.org/knowledge_post/what-are-clinical-considerations-for-giving-lais-during-the-covid-19-public-health-emergency
- CDC. Infection control basics. https://www.cdc.gov/infectioncontrol/basics/index.html
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole for extended- release injectable suspension • Abilify Maintena
Aripiprazole lauroxil • Aristada
Haloperidol • Haldol
Haloperidol injection • Haldol decanoate
Olanzapine • Zyprexa
Olanzapine for extended-release injectable suspension • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate extended-release injectable suspension • Invega Sustenna
Paliperidone palmitate extended-release injectable suspension • Invega Trinza
Risperidone • Risperdal
Risperidone for extended- release injectable suspension • Perseris
Risperidone injection • Risperdal Consta
Long-acting injectable antipsychotics (LAIs) are an essential tool in the treatment of patients with psychotic disorders, allowing for periods of stable drug plasma concentration and confirmed adherence.1 The current coronavirus disease 2019 (COVID-19) pandemic presents unique challenges for administering LAIs and requires a thoughtful and prospective approach in order to ensure continuity of psychiatric care while minimizing the risk of infection with COVID-19. Ideally, patients should be seen in person as infrequently as clinically prudent during this public health emergency; however, LAI administration necessitates direct physical contact between patient and clinician.
Patients with serious mental illness (SMI), who comprise the majority of individuals who receive LAIs, are at heightened risk for cardiovascular and pulmonary comorbidities. These factors are the primary reason the life expectancy of a patient with SMI is nearly 30 years shorter than that of the general population.2-5 The risk of health care workers becoming infected or inadvertently spreading COVID-19 is heightened when working with patients in group living environments (ie, a shelter or group home), who have both increased exposure and increased risk of further transmission.6 Additional patient populations, including older adults, immunocompromised individuals, and those with preexisting conditions, are at heightened risk for serious complications if they were to contract COVID-19.7,8
Thus, the questions of whether LAIs should be administered, and how to do so safely (both during the ongoing, acute phase of the pandemic as well as during the subsequent recovery period until the pandemic abates) need to be carefully considered. In this article, we provide concrete advice for clinicians and clinics on these topics, with the goal of maintaining patients’ psychiatric stability while protecting patients, health care workers, and the broader society from COVID-19 infection. Table 1 summarizes the questions regarding LAIs that clinicians need to address during this crisis. While we focus on outpatient care, inpatient teams should keep these considerations in mind if they are starting and discharging a patient on an LAI. More than ever, close collaboration and communication between inpatient and outpatient teams is critical.
Should an LAI be continued?
An important first step to approaching this challenge is to create a spreadsheet for all patients receiving LAIs. Focusing on a population-based approach is helpful to be systematic and ensure that no patients fall through the cracks during this public health emergency.9 Once all patients have been identified, the treatment team should review each patient to determine if continuing to administer the antipsychotic as an LAI formulation is essential, taking into account the patient’s current psychiatric status, historical medication adherence, potential severity and dangerousness of decompensation if nonadherent, and structures to support stability. For example, can a patient move in with family who can monitor medication adherence during the pandemic? Is it possible for the group home to assume medication administration? Additional consideration should be given to the living environment and health-vulnerability of the patient and the individuals living with them.
If the risk calculation does not point strongly towards a need for continuing the LAI, it may be prudent to temporarily transition the patient to the corresponding oral antipsychotic preparation. Table 2 lists all LAIs available in the United States and their approximate equivalent oral dosing. It is important to note that such transitions are not without clinical risk, to emphasize to the patient that the transition is intended as a temporary measure, and to discuss a proposed timeline for re-initiating the LAI. Also, emphasize to the patient and family that this transition does not diminish the previous reasoning for needing an LAI, but is a temporary measure taken in light of weighing the risks and benefits during a pandemic.

Which LAI should be administered?
If continuing the LAI is determined to be clinically necessary, consider switching the patient to a longer-acting preparation to maximize intervals between administrations and minimize the potential for infection. From a public health perspective, the longest clinically prudent interval between injections may be the most important consideration, provided the patient can receive a dose necessary to retain stability, and the LAI should be chosen accordingly. Deltoid injections may be able to be administered with reduced contact, or on a “drive-up” basis.10 Consider transitioning a patient who is receiving olanzapine pamoate to an alternate LAI or oral formulation, because the 3-hour observation period that is required after olanzapine pamoate administration is particularly problematic. While it may not be ideal to make medication changes during a pandemic, it is worth carefully weighing the patient’s stability and historical experience with other LAIs to determine if a safer/longer-spaced option is worth trying.11
We recommend only switching among similar antipsychotics (ie, risperidone to paliperidone), or between different preparations of the same drug (ie, Abilify Maintena to Aristada), if possible, as these are the lowest risk transitions with regards to relapse. Table 3 provides examples.

Continue to: When should the LAI be administered?
When should the LAI be administered?
The pharmacokinetics of LAIs allow for some flexibility in terms of when an LAI needs to be administered. The package inserts of all second-generation LAIs include missed-dose guidelines. These guidelines provide information on how long one can wait before the next injection is due, and what additional measures must be taken when beyond that date. Delaying an injection may be prudent, and the missed dose guidelines will indicate when one must consider supplementing with oral medications. For patients who are in quarantine, it may be better to delay an injection until the patient ends their quarantine than to deliver the dose during quarantine. Administering an injection earlier also is usually safe; off-cycle visits may help minimize patient contact (ie, if the patient happens to be coming into the vicinity of the clinic, or requires phlebotomy for therapeutic drug monitoring), and assist in planning for possible resurgences. When appropriate, and after considering the risk of worsening adverse effects, administering a higher dose than the usual maintenance dose would provide a buffer if the next injection was to be delayed. Therapeutic drug monitoring can help to optimize dosing and avoid low plasma drug levels, which may be not be sufficient, particularly during this time of stress.12 To provide optimal protection against relapse, consider administering a dose that puts patients at the higher range of plasma drug levels.
Where can the LAI be administered, and who can give it?
For patients who usually travel to a clinic, consider arranging for a more local injection (ie, at the patient’s primary care clinic in their hometown, or at a local mental health center), and explore if the patient may be able to receive their injection in their home through a visiting nurse association (VNA). In many states (approximately 30 currently), clinicians at pharmacies are also able to administer patient injections. Clinics would do well to at least plan for alternate staffing models in the event of staff illness. A pool of individuals should be available to give injections; consider training additional staff members (including MDs who may have never previously administered an LAI but could be quickly instructed to do so) to administer LAIs. Theoretically, during a public health emergency, family members, particularly those who have a background in health care, could be trained to give an injection and provided education on LAI storage and post-injection monitoring. This approach would not be consistent with FDA labeling, however, and should only be considered as a last resort.
What safety measures can be put in place?
Face-to-face time for injection administration should be kept as brief as possible. Before the encounter, obtain the patient’s clinical information, ideally through telehealth or from an acceptable distance. Medication should be drawn ahead of time, and not in an enclosed space with the patient present. Strongly consider abandoning the traditional enclosed room for the injection, and instead use larger spaces, doorways, or outside, if feasible. As previously noted, some clinics and clinicians have used a drive-up approach for LAI administration, particularly for deltoid injections.10 Individuals who administer the injections should wear personal protective equipment, and the clinic should obtain an adequate supply of this equipment well in advance.
Lessons learned at our clinic
In our community mental health center clinic, planning around these questions has allowed us to provide safe and continuous psychiatric care with LAIs during this public health emergency while reducing the risk of infection. We have worked to transfer LAI administration to VNAs and transition patients to longer-lasting formulations or oral medications where appropriate, which has resulted in an approximately 50% decrease in in-person visits. Reducing the number of in-person visits does not need to result in less frequent clinical follow-up. Telepsychiatry visits can make up for lost in-person visits and have generally been well accepted.
As we are preparing for the next phase, routine medical health monitoring (eg, metabolic monitoring, monitoring for tardive dyskinesia) that has not been at the forefront of concerns should be carefully reintroduced. Challenges encountered have included difficulty in having VNA accept patients for short-term LAI visits, changes to where on the body the injection is delivered, and patients with SMI and their families being reluctant to depart from previous routines and administration schedules.
Continue to: There is great value...
There is great value in the collective lessons learned during this public health emergency (eg, the need for a flexible, population health-based approach; acceptability of combination telehealth and in-person visits) that can lead to more person-centered and accessible care for patients with SMI.
Acknowledgments
The authors thank North Suffolk Mental Health Association, the Freedom Trail Clinic, and their patients.
Bottom Line
When caring for a patient with a psychotic illness during the coronavirus disease 2019 (COVID-19) pandemic, evaluate whether it is necessary to continue a longacting injectable antipsychotic (LAI). If yes, reconsider which LAI should be administered, when and where it should be given, and by whom. Implement safety measures to minimize the risk of COVID-19 exposure and transmission.
Related Resources
- American Association of Community Psychiatrists. Clinical Tip Series. Long acting antipsychotic medications. https://drive.google.com/file/d/1unigjmjFJkqZMbaZ_ftdj8oqog49awZs/view?usp=sharing
- SMI Adviser. What are clinical considerations for giving LAIs during the COVID-19 public health emergency? https://smiadviser.org/knowledge_post/what-are-clinical-considerations-for-giving-lais-during-the-covid-19-public-health-emergency
- CDC. Infection control basics. https://www.cdc.gov/infectioncontrol/basics/index.html
Drug Brand Names
Aripiprazole • Abilify
Aripiprazole for extended- release injectable suspension • Abilify Maintena
Aripiprazole lauroxil • Aristada
Haloperidol • Haldol
Haloperidol injection • Haldol decanoate
Olanzapine • Zyprexa
Olanzapine for extended-release injectable suspension • Zyprexa Relprevv
Paliperidone • Invega
Paliperidone palmitate extended-release injectable suspension • Invega Sustenna
Paliperidone palmitate extended-release injectable suspension • Invega Trinza
Risperidone • Risperdal
Risperidone for extended- release injectable suspension • Perseris
Risperidone injection • Risperdal Consta
1. Freudenreich O. Long-acting injectable antipsychotics. In: Freudenreich O. Psychotic disorders: a practical guide. Springer; 2020:249-261.
2. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
3. Reilly S, Olier I, Planner C, et al. Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open. 2015;5(12):e009010.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
5. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66(2):183-194.
6. Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323(21):2191-2192.
7. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346.
8. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985
9. Etches V, Frank J, Di Ruggiero E, et al. Measuring population health: a review of indicators. Annu Rev Public Health. 2006;27:29-55.
10. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
11. Sajatovic M, Ross R, Legacy SN, et al. Initiating/maintaining long-acting injectable antipsychotics in schizophrenia/schizoaffective or bipolar disorder - expert consensus survey part 2. Neuropsychiatr Dis Treat. 2018;14:1475-1492.
12. Schoretsanitis G, Kane JM, Correll CU, et al; American Society of Clinical Psychopharmacology, Pharmakopsychiatrie TTDMTFOTAFNU. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81(3):19cs13169. doi: 10.4088/JCP.19cs13169
1. Freudenreich O. Long-acting injectable antipsychotics. In: Freudenreich O. Psychotic disorders: a practical guide. Springer; 2020:249-261.
2. Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172-1181.
3. Reilly S, Olier I, Planner C, et al. Inequalities in physical comorbidity: a longitudinal comparative cohort study of people with severe mental illness in the UK. BMJ Open. 2015;5(12):e009010.
4. Brown S, Inskip H, Barraclough B. Causes of the excess mortality of schizophrenia. Br J Psychiatry. 2000;177:212-217.
5. Goff DC, Cather C, Evins AE, et al. Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry. 2005;66(2):183-194.
6. Baggett TP, Keyes H, Sporn N, et al. Prevalence of SARS-CoV-2 infection in residents of a large homeless shelter in Boston. JAMA. 2020;323(21):2191-2192.
7. CDC COVID-19 Response Team. Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346.
8. Docherty AB, Harrison EM, Green CA, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985
9. Etches V, Frank J, Di Ruggiero E, et al. Measuring population health: a review of indicators. Annu Rev Public Health. 2006;27:29-55.
10. Chepke C. Drive-up pharmacotherapy during the COVID-19 pandemic. Current Psychiatry. 2020;19(5):29-30.
11. Sajatovic M, Ross R, Legacy SN, et al. Initiating/maintaining long-acting injectable antipsychotics in schizophrenia/schizoaffective or bipolar disorder - expert consensus survey part 2. Neuropsychiatr Dis Treat. 2018;14:1475-1492.
12. Schoretsanitis G, Kane JM, Correll CU, et al; American Society of Clinical Psychopharmacology, Pharmakopsychiatrie TTDMTFOTAFNU. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81(3):19cs13169. doi: 10.4088/JCP.19cs13169
COVID-19 and the risk of homicide-suicide among older adults
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
On March 25, 2020, in Cambridge, United Kingdom, a 71-year-old man stabbed his 71-year-old wife before suffocating himself to death. The couple was reportedly anxious about the coronavirus disease 2019 (COVID-19) pandemic lockdown measures and were on the verge of running out of food and medicine.1
One week later, in Chicago, Illinois, a 54-year-old man shot and killed his female partner, age 54, before killing himself. The couple was tested for COVID-19 2 days earlier and the man believed they had contracted the virus; however, the test results for both of them had come back negative.2
Intimate partner homicide-suicide is the most dramatic domestic abuse outcome.3 Homicide-suicide is defined as “homicide committed by a person who subsequently commits suicide within one week of the homicide. In most cases the subsequent suicide occurs within a 24-hour period.”4 Approximately one-quarter of all homicide-suicides are committed by persons age ≥55 years.5,6 We believe that during the COVID-19 pandemic, the risk of homicide-suicide among older adults may be increased due to several factors, including:
- physical distancing and quarantine measures. Protocols established to slow the spread of the virus may be associated with increased rates of depression and anxiety7 and an increased risk of suicide among older adults8
- increased intimate partner violence9
- increased firearm ownership rates in the United States.10
In this article, we review studies that identified risk factors for homicide-suicide among older adults, discuss the impact the COVID-19 pandemic has had on these risks, and describe steps clinicians can take to intervene.
A review of the literature
To better characterize the perpetrators of older adult homicide-suicide, we conducted a literature search of relevant terms. We identified 9 original research publications that examined homicide-suicide in older adults.
Bourget et al11 (2010) reviewed coroners’ charts of individuals killed by an older (age ≥65) spouse or family member from 1992 through 2007 in Quebec, Canada. They identified 19 cases of homicide-suicide, 17 (90%) of which were perpetrated by men. Perpetrators and victims were married (63%), in common-law relationships (16%), or separated/divorced (16%). A history of domestic violence was documented in 4 (21%) cases. The authors found that 13 of 15 perpetrators (87%) had “major depression” and 2 perpetrators had a psychotic disorder. Substance use at the time of the event was confirmed in 6 (32%) cases. Firearms and strangulation were the top methods used to carry out the homicide-suicide.11
Cheung et al12 (2016) conducted a review of coroners’ records of homicide-suicide cases among individuals age ≥65 in New Zealand from 2007 through 2012. In all 4 cases, the perpetrators were men, and their victims were predominantly female, live-in family members. Two cases involved men with a history of domestic violence who were undergoing significant changes in their home and social lives. Both men had a history suggestive of depression and used a firearm to carry out the homicide-suicide.12
Continue to: Cohen et al
Cohen et al13 (1998) conducted a review of coroners’ records from 1988 through 1994 in 2 regions in Florida. They found 48 intimate partner homicide-suicide cases among “old couples” (age ≥55). All were perpetrated by men. The authors identified sociocultural differences in risk factors between the 2 regions. In west-central Florida, perpetrators and victims were predominantly white and in a spousal relationship. Domestic violence was documented in <4% of cases. Approximately 55% of the couples were reported to be ill, and a substantial proportion were documented to be declining in health. One-quarter of the perpetrators and one-third of the victims had “pain and suffering.” More than one-third of perpetrators were reported to have “depression,” 15% were reported to have talked about suicide, and 4% had a history of a suicide attempt. Only 11% of perpetrators were described as abusing substances.
The authors noted several differences in cases in southeastern Florida. Approximately two-thirds of the couples were Hispanic, and 14% had a history of domestic violence. A minority of the couples were in a live-in relationship. Less than 15% of the perpetrators and victims were described as having a decline in health. Additionally, only 19% of perpetrators were reported to have “depression,” and none of the perpetrators had a documented history of attempted suicide or substance abuse. No information was provided regarding the methods used to carry out the homicide-suicide in the southeastern region.13 Financial stress was not a factor in either region.
Malphurs et al14 (2001) used the same database described in the Cohen et al13 study to compare 27 perpetrators of homicide-suicide to 36 age-matched suicide decedents in west central Florida. They found that homicide-suicide perpetrators were significantly less likely to have health problems and were 3 times more likely to be caregivers to their spouses. Approximately 52% of perpetrators had at least 1 documented psychiatric symptom (“depression” and/or substance abuse or other), but only 5% were seeking mental health services at the time of death.14
De Koning and Piette15 (2014) conducted a retrospective medicolegal chart review from 1935 to 2010 to identify homicide-suicide cases in West and East Flanders, Belgium. They found 19 cases of intimate partner homicide-suicide committed by offenders age ≥55 years. Ninety-five percent of the perpetrators were men who killed their female partners. In one-quarter of the cases, either the perpetrator or the victim had a health issue; 21% of the perpetrators were documented as having depression and 27% had alcohol intoxication at the time of death. A motive was documented in 14 out of 19 cases; “mercy killing” was determined as the motive in 6 (43%) cases and “amorous jealousy” in 5 cases (36%).15 Starting in the 1970s, firearms were the most prevalent method used to kill a partner.
Logan et al16 (2019) used data from the National Violent Death Reporting System between 2003 and 2015 to identify characteristics that differentiated male suicide decedents from male perpetrators of intimate partner homicide-suicide. They found that men age 50 to 64 years were 3 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide, and that men age ≥65 years were approximately 5 times more likely than men age 18 to 34 years to commit intimate partner homicide-suicide. The authors found that approximately 22% of all perpetrators had a documented history of physical domestic violence, and close to 17% had a prior interaction with the criminal justice system. Furthermore, one-third of perpetrators had relationship difficulties and were in the process of a breakup. Health issues were prevalent in 34% of the victims and 26% of the perpetrators. Perpetrator-caregiver burden was reported as a contributing factor for homicide-suicide in 16% of cases. In 27% of cases, multiple health-related contributing factors were mentioned.16
Continue to: Malphurs and Cohen
Malphurs and Cohen5 (2002) reviewed American newspapers from 1997 through 1999 and identified 673 homicide-suicide events, of which 152 (27%) were committed by individuals age ≥55 years. The victims and perpetrators (95% of which were men) were intimate partners in three-quarters of cases. In nearly one-third of cases, caregiving was a contributing factor for the homicide-suicide. A history of or a pending divorce was reported in nearly 14% of cases. Substance use history was rarely recorded. Firearms were used in 88% of the homicide-suicide cases.5
Malphurs and Cohen17 (2005) reviewed coroner records between 1998 and 1999 in Florida and compared 20 cases of intimate partner homicide-suicide involving perpetrators age ≥55 years with matched suicide decedents. They found that 60% of homicide-suicide perpetrators had documented health issues. The authors reported that a “recent change in health status” was more prevalent among perpetrators compared with decedents. Perpetrators were also more likely to be caregivers to their spouses. The authors found that 65% of perpetrators were reported to have a “depressed mood” and 15% of perpetrators had reportedly threatened suicide prior to the incident. However, none of the perpetrators tested positive for antidepressants as documented on post-mortem toxicology reports. Firearms were used in 100% of homicide-suicide cases.17
Salari3 (2007) reviewed multiple American media sources and published police reports between 1999 and 2005 to retrieve data about intimate partner homicide-suicide events in the United States. There were 225 events identified where the perpetrator and/or the victim were age ≥60 years. Ninety-six percent of the perpetrators were men and most homicide-suicide events were committed at the home. A history of domestic violence was reported in 14% of homicide-suicide cases. Thirteen percent of couples were separated or divorced. The perpetrator and/or victim had health issues in 124 (55%) events. Dementia was reported in 7.5% of cases, but overwhelmingly among the victims. Substance abuse was rarely mentioned as a contributing factor. In three-quarters of cases where a motive was described, the perpetrator was “suicidal”; however, a “suicide pact” was mentioned in only 4% of cases. Firearms were used in 87% of cases.3
Focus on common risk factors
The scarcity and heterogeneity of research regarding older adult homicide-suicide were major limitations to our review. Because most of the studies we identified had a small sample size and limited information regarding the mental health of victims and perpetrators, it would be an overreach to claim to have identified a typical profile of an older perpetrator of homicide-suicide. However, the literature has repeatedly identified several common characteristics of such perpetrators. They are significantly more likely to be men who use firearms to murder their intimate partners and then die by suicide in their home (Table3,5,11-17). Health issues afflicting 1 or both individuals in the couple appear to be a contributing factor, particularly when the perpetrator is in a caregiving role. Relational discord, with or without a history of domestic violence, increases the risk of homicide-suicide. Finally, older perpetrators are highly likely to be depressed and have suicidal ideations.
How COVID-19 affects these risks
Although it is too early to determine if there is a causal relationship between the COVID-19 pandemic and an increase in homicide-suicide, the pandemic is likely to promote risk factors for these events, especially among older adults. Confinement measures put into place during the pandemic context have already been shown to increase rates of domestic violence18 and depression and anxiety among older individuals.7 Furthermore,
Continue to: Late-life psychiatric disorders
Late-life psychiatric disorders
Early recognition and effective treatment of late-life psychiatric disorders is crucial. Unfortunately, depression in geriatric patients is often underdiagnosed and undertreated.20 Older adults have more frequent contact with their primary care physicians, and rarely consult mental health professionals.21,22 Several models of integrated depression care within primary care settings have shown the positive impact of this collaborative approach in treating late-life depression and preventing suicide in older individuals.23 Additionally, because alcohol abuse is also a risk factor for domestic violence and breaking the law in this population,24,25 older adults should be screened for alcohol use disorders, and referred to treatment when necessary.
Take steps to keep patients safe
In the context of the COVID-19 pandemic, there are several steps clinicians need to keep in mind when interacting with older patients:
- Screen for depressive symptoms, suicidality, and alcohol and substance use disorders. Individuals who have tested positive for COVID-19 or who have been in contact with a carrier are a particularly vulnerable population.
- Screen for domestic violence and access to weapons at home.4 Any older adult who has a psychiatric disorder and/or suicide ideation should receive immediate intervention through a social worker that includes providing gun-risk education to other family members or contacting law-enforcement officials.26
- Refer patients with a suspected psychiatric disorder to specialized mental health clinicians. Telemental health services can provide rapid access to subspecialists, allowing patients to be treated from their homes.27 These services need to be promoted among older adults during this critical period and reimbursed by public and private insurance systems to ensure accessibility and affordability.28
- Create psychiatric inpatient units specifically designed for suicidal and/or homicidal patients with COVID-19.
Additionally, informing the public about these major health issues is crucial. The media can raise awareness about domestic violence and depression among older adults; however, this should be done responsibly and with accuracy to prevent the spread of misinformation, confusion, fear, and panic.29
Bottom Line
The mental health burden of the coronavirus disease 2019 pandemic has significantly impacted individuals who are older and most vulnerable. Reducing the incidence of homicide-suicide among older adults requires timely screening and interventions by primary care providers, mental health specialists, social workers, media, and governmental agencies.
Related Resources
- Saeed SA, Hebishi K. The psychiatric consequences of COVID-19: 8 studies. Current Psychiatry. 2020;19(11):22-24,28-30,32-35.
- Schwab-Reese LM, Murfree L, Coppola EC, et al. Homicidesuicide across the lifespan: a mixed methods examination of factors contributing to older adult perpetration. Aging Ment Health. 2020;20:1-9.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
1. Christodoulou H. LOCKDOWN ‘MURDER-SUICIDE’ OAP, 71, ‘stabbed wife to death then killed himself as he worried about coping with coronavirus lockdown.’ The Sun. Updated April 4, 2020. Accessed December 22, 2020. https://www.thesun.co.uk/news/11327095/coronavirus-lockdown-murder-suicide-cambridge/
2. Farberov S. Illinois man, 54, shoots dead his wife then kills himself in murder-suicide because he feared they had coronavirus - but tests later show the couple were NOT ill. Updated April 6, 2020. Accessed December 22, 2020. https://www.dailymail.co.uk/news/article-8191933/Man-kills-wife-feared-coronavirus.html
3. Salari S. Patterns of intimate partner homicide suicide in later life: strategies for prevention. Clin Interv Aging. 2007;2(3):441-452.
4. Kotzé C, Roos JL. Homicide–suicide: practical implications for risk reduction and support services at primary care level. South African Family Practice. 2019;61(4):165-169.
5. Malphurs JE, Cohen D. A newspaper surveillance study of homicide-suicide in the United States. Am J Forensic Med Pathol. 2002;23(2):142-148.
6. Eliason S. Murder-suicide: a review of the recent literature. J Am Acad Psychiatry Law. 2009;37(3):371-376.
7. Armitage R, Nellums LB. COVID-19 and the consequences of isolating the elderly. Lancet Public Health. 2020;5(5):e256. doi: 10.1016/S2468-2667(20)30061-X
8. Gunnell D, Appleby L, Arensman E, et al. Suicide risk and prevention during the COVID-19 pandemic. Lancet Psychiatry 2020;7(6):468-471.
9. Gosangi B, Park H, Thomas R, et al. Exacerbation of physical intimate partner violence during COVID-19 pandemic. Radiology. 2021;298(1):E38-E45.
10. Mannix R, Lee LK, Fleegler EW. Coronavirus disease 2019 (COVID-19) and firearms in the United States: will an epidemic of suicide follow? Ann Intern Med. 2020;173(3):228-229.
11. Bourget D, Gagne P, Whitehurst L. Domestic homicide and homicide-suicide: the older offender. J Am Acad Psychiatry Law. 2010;38(3):305-311.
12. Cheung G, Hatters Friedman S, Sundram F. Late-life homicide-suicide: a national case series in New Zealand. Psychogeriatrics. 2016;16(1):76-81.
13. Cohen D, Llorente M, Eisdorfer C. Homicide-suicide in older persons. Am J Psychiatry. 1998;155(3):390-396.
14. Malphurs JE, Eisdorfer C, Cohen D. A comparison of antecedents of homicide-suicide and suicide in older married men. Am J Geriatr Psychiatry. 2001;9(1):49-57.
15. De Koning E, Piette MHA. A retrospective study of murder–suicide at the Forensic Institute of Ghent University, Belgium: 1935–2010. Med Sci Law. 2014;54(2):88-98.
16. Logan JE, Ertl A, Bossarte R. Correlates of intimate partner homicide among male suicide decedents with known intimate partner problems. Suicide Life Threat Behav. 2019;49(6):1693-1706.
17. Malphurs JE, Cohen D. A statewide case-control study of spousal homicide-suicide in older persons. Am J Geriatr Psychiatry. 2005;13(3):211-217.
18. Sanford A. ‘Horrifying surge in domestic violence’ against women amid coronavirus-lockdowns, UN chief warns. Euronews. Published June 4, 2020. Accessed December 22, 2020. https://www.euronews.com/2020/04/06/horrifying-surge-in-domestic-violence-against-women-amid-coronavirus-lockdowns-un-chief-w
19. Appel JM. Intimate partner homicide in elderly populations. In: Friedman SH, ed. Family murder: pathologies of love and hate. American Psychiatric Association Publishing; 2019:131-142.
20. Hall CA, Reynolds-III CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147-152.
21. Unützer J. Diagnosis and treatment of older adults with depression in primary care. Biological Psychiatry. 2002;52(3):285-292.
22. Byers AL, Arean PA, Yaffe K. Low use of mental health services among older Americans with mood and anxiety disorders. Psychiatr Serv. 2012;63(1):66-72.
23. Bruce ML, Sirey JA. Integrated care for depression in older primary care patients. Can J Psychiatry. 2018;63(7):439-446.
24. Rao R, Roche A. Substance misuse in older people. BMJ. 2017;358:j3885. doi: 10.1136/bmj.j3885
25. Ghossoub E, Khoury R. Prevalence and correlates of criminal behavior among the non-institutionalized elderly: results from the National Survey on Drug Use and Health. J Geriatr Psychiatry Neurol. 2018;31(4):211-222.
26. Slater MAG. Older adults at risk for suicide. In: Berkman B. Handbook of social work in health and aging. Oxford University Press; 2006:149-161.
27. Hollander JE, Carr BG. Virtually perfect? Telemedicine for Covid-19. N Engl J Med. 2020;382(18):1679-1681.
28. Centers for Medicare & Medicaid Services. President Trump expands telehealth benefits for Medicare beneficiaries during COVID-19 outbreak. Published March 17, 2020. Accessed December 23, 2020. https://www.cms.gov/newsroom/press-releases/president-trump-expands-telehealth-benefits-medicare-beneficiaries-during-covid-19-outbreak
29. Mian A, Khan S. Coronavirus: the spread of misinformation. BMC Med. 2020;18(1):89.
Plagues that will haunt us long after the COVID-19 pandemic is gone
As we struggle to gradually emerge from the horrid coronavirus disease 2019 (COVID-19) pandemic that has disrupted our lives and killed hundreds of thousands of people in the United States, we harbor the hope that life will return to “normal.” But while it will certainly be a great relief to put this deadly virus behind us, many other epidemics will continue to plague our society and taint our culture.
Scientific ingenuity has led to the development of several vaccines in record time (aka “warp speed”) that will help defeat the deadly scourge of COVID-19. The pandemic is likely to peter out 2 years after its onset. We will all be grateful for such a rapid resolution of the worst health crisis the world has faced in a century, which will enable medical, economic, and social recovery. But as we eventually resume our lives and rejoice in resuming the pursuit of happiness, we will quickly realize that all is not well in our society just because the viral pandemic is gone.
Perhaps the ordeal of the COVID-19 pandemic, and the agony that was universally shared, will open our collective eyes to a jarring reality: many other epidemics will continue to permeate society and cause endless grief and suffering to many of our fellow humans. And thanks to our training as psychiatric physicians, we have developed extra “receptors” to the darker side of the human condition. As we help many of our psychiatric patients rendered sicker under the unbearable stress of the pandemic, we must not overlook the plight of so many others who do not show up in our clinics for health care, yet suffer enormously but imperceptibly. And no vaccine can come to the rescue of those who continue to live in quiet desperation.
Long-standing epidemics
It is truly unfortunate that many of the epidemics I am referring to have persisted for so long that they have become “fixtures” of contemporary societies. They have become “endemic epidemics” with no urgency to squelch them, as with the COVID-19 pandemic. The benign neglect that perpetuates these serious epidemics has had a malignant effect of “grudging resignation” that nothing can be done to reverse them. Unlike the viral epidemic that engulfed everyone around the world and triggered a massive and unified push to defeat the virus, these long-standing epidemics continue to afflict subgroups who are left to fend for themselves. These individuals deserve our empathy and warrant our determination to lift them from their miserable existence.
Consider some of the widespread epidemics that preceded the pandemic and will, in all likelihood, persist after the pandemic’s burden is lifted:
- millions of people living in poverty and hunger
- widespread racism
- smoldering social injustice
- appalling human trafficking, especially targeting children and women
- child abuse and neglect that leads to psychosis, depression, and suicide in adulthood
- gun violence, which kills many innocent people
- domestic violence that inflicts both physical and mental harm on families
- suicide, both attempts and completions, which continues to increase annually
- the festering stigma of mental illness that adds insult to injury for psychiatric patients
- alcohol and drug addictions, which destroy lives and corrode the fabric of society
- lack of access to mental health care for millions of people who need it
- lack of parity for psychiatric disorders, which is so unjust for our patients
- venomous political hatred and hyperpartisanship, which permeates our culture and can lead to violence, as we recently witnessed
- physician burnout, due to many causes, even before the stresses of COVID-19
- the ongoing agony of wars and terrorism, including dangerous cyberattacks
- the deleterious effect of social media on everyone, especially children.
Most of these epidemics claim thousands of lives each year, and yet no concerted public health effort is being mounted to counteract them, as we are seeing with the COVID-19 pandemic. Much is being written about each of them, but there has been little tangible action, so they persist. They have become a perpetual underbelly of our society that is essentially ignored or simply given the usual lip service.
It will take a herculean effort by policymakers, the judicial system, the medical establishment, and faith organizations to put an end to these life-threatening epidemics. It may appear too daunting to mount a war on so many fronts, but that should not deter us all from launching a strategic plan to create meaningful tactics and solutions. And just as was done with the COVID-19 pandemic, both mitigation measures as well as effective interventions must be employed in this campaign against the epidemic “hydra.”
Continue to: It is tragic...
It is tragic that so many fellow humans are allowed to suffer or die while the rest of us watch, or worse, turn a blind eye and never get involved. A civilized society must never neglect so many of its suffering citizens. As psychiatrists, we are aware of those human travesties around us, but we are often so overwhelmed with our work and personal responsibilities that few of us are passionately advocating or setting aside some time for those victimized by one or more of these endemic pandemics. And unless we all decide to be actively, meaningfully involved, many lives will continue to be lost every day, but without the daily “casualty count” displayed on television screens, as is the case with COVID-19 causalities.
Regrettably, maybe that old saw is true: out of sight, out of mind.
As we struggle to gradually emerge from the horrid coronavirus disease 2019 (COVID-19) pandemic that has disrupted our lives and killed hundreds of thousands of people in the United States, we harbor the hope that life will return to “normal.” But while it will certainly be a great relief to put this deadly virus behind us, many other epidemics will continue to plague our society and taint our culture.
Scientific ingenuity has led to the development of several vaccines in record time (aka “warp speed”) that will help defeat the deadly scourge of COVID-19. The pandemic is likely to peter out 2 years after its onset. We will all be grateful for such a rapid resolution of the worst health crisis the world has faced in a century, which will enable medical, economic, and social recovery. But as we eventually resume our lives and rejoice in resuming the pursuit of happiness, we will quickly realize that all is not well in our society just because the viral pandemic is gone.
Perhaps the ordeal of the COVID-19 pandemic, and the agony that was universally shared, will open our collective eyes to a jarring reality: many other epidemics will continue to permeate society and cause endless grief and suffering to many of our fellow humans. And thanks to our training as psychiatric physicians, we have developed extra “receptors” to the darker side of the human condition. As we help many of our psychiatric patients rendered sicker under the unbearable stress of the pandemic, we must not overlook the plight of so many others who do not show up in our clinics for health care, yet suffer enormously but imperceptibly. And no vaccine can come to the rescue of those who continue to live in quiet desperation.
Long-standing epidemics
It is truly unfortunate that many of the epidemics I am referring to have persisted for so long that they have become “fixtures” of contemporary societies. They have become “endemic epidemics” with no urgency to squelch them, as with the COVID-19 pandemic. The benign neglect that perpetuates these serious epidemics has had a malignant effect of “grudging resignation” that nothing can be done to reverse them. Unlike the viral epidemic that engulfed everyone around the world and triggered a massive and unified push to defeat the virus, these long-standing epidemics continue to afflict subgroups who are left to fend for themselves. These individuals deserve our empathy and warrant our determination to lift them from their miserable existence.
Consider some of the widespread epidemics that preceded the pandemic and will, in all likelihood, persist after the pandemic’s burden is lifted:
- millions of people living in poverty and hunger
- widespread racism
- smoldering social injustice
- appalling human trafficking, especially targeting children and women
- child abuse and neglect that leads to psychosis, depression, and suicide in adulthood
- gun violence, which kills many innocent people
- domestic violence that inflicts both physical and mental harm on families
- suicide, both attempts and completions, which continues to increase annually
- the festering stigma of mental illness that adds insult to injury for psychiatric patients
- alcohol and drug addictions, which destroy lives and corrode the fabric of society
- lack of access to mental health care for millions of people who need it
- lack of parity for psychiatric disorders, which is so unjust for our patients
- venomous political hatred and hyperpartisanship, which permeates our culture and can lead to violence, as we recently witnessed
- physician burnout, due to many causes, even before the stresses of COVID-19
- the ongoing agony of wars and terrorism, including dangerous cyberattacks
- the deleterious effect of social media on everyone, especially children.
Most of these epidemics claim thousands of lives each year, and yet no concerted public health effort is being mounted to counteract them, as we are seeing with the COVID-19 pandemic. Much is being written about each of them, but there has been little tangible action, so they persist. They have become a perpetual underbelly of our society that is essentially ignored or simply given the usual lip service.
It will take a herculean effort by policymakers, the judicial system, the medical establishment, and faith organizations to put an end to these life-threatening epidemics. It may appear too daunting to mount a war on so many fronts, but that should not deter us all from launching a strategic plan to create meaningful tactics and solutions. And just as was done with the COVID-19 pandemic, both mitigation measures as well as effective interventions must be employed in this campaign against the epidemic “hydra.”
Continue to: It is tragic...
It is tragic that so many fellow humans are allowed to suffer or die while the rest of us watch, or worse, turn a blind eye and never get involved. A civilized society must never neglect so many of its suffering citizens. As psychiatrists, we are aware of those human travesties around us, but we are often so overwhelmed with our work and personal responsibilities that few of us are passionately advocating or setting aside some time for those victimized by one or more of these endemic pandemics. And unless we all decide to be actively, meaningfully involved, many lives will continue to be lost every day, but without the daily “casualty count” displayed on television screens, as is the case with COVID-19 causalities.
Regrettably, maybe that old saw is true: out of sight, out of mind.
As we struggle to gradually emerge from the horrid coronavirus disease 2019 (COVID-19) pandemic that has disrupted our lives and killed hundreds of thousands of people in the United States, we harbor the hope that life will return to “normal.” But while it will certainly be a great relief to put this deadly virus behind us, many other epidemics will continue to plague our society and taint our culture.
Scientific ingenuity has led to the development of several vaccines in record time (aka “warp speed”) that will help defeat the deadly scourge of COVID-19. The pandemic is likely to peter out 2 years after its onset. We will all be grateful for such a rapid resolution of the worst health crisis the world has faced in a century, which will enable medical, economic, and social recovery. But as we eventually resume our lives and rejoice in resuming the pursuit of happiness, we will quickly realize that all is not well in our society just because the viral pandemic is gone.
Perhaps the ordeal of the COVID-19 pandemic, and the agony that was universally shared, will open our collective eyes to a jarring reality: many other epidemics will continue to permeate society and cause endless grief and suffering to many of our fellow humans. And thanks to our training as psychiatric physicians, we have developed extra “receptors” to the darker side of the human condition. As we help many of our psychiatric patients rendered sicker under the unbearable stress of the pandemic, we must not overlook the plight of so many others who do not show up in our clinics for health care, yet suffer enormously but imperceptibly. And no vaccine can come to the rescue of those who continue to live in quiet desperation.
Long-standing epidemics
It is truly unfortunate that many of the epidemics I am referring to have persisted for so long that they have become “fixtures” of contemporary societies. They have become “endemic epidemics” with no urgency to squelch them, as with the COVID-19 pandemic. The benign neglect that perpetuates these serious epidemics has had a malignant effect of “grudging resignation” that nothing can be done to reverse them. Unlike the viral epidemic that engulfed everyone around the world and triggered a massive and unified push to defeat the virus, these long-standing epidemics continue to afflict subgroups who are left to fend for themselves. These individuals deserve our empathy and warrant our determination to lift them from their miserable existence.
Consider some of the widespread epidemics that preceded the pandemic and will, in all likelihood, persist after the pandemic’s burden is lifted:
- millions of people living in poverty and hunger
- widespread racism
- smoldering social injustice
- appalling human trafficking, especially targeting children and women
- child abuse and neglect that leads to psychosis, depression, and suicide in adulthood
- gun violence, which kills many innocent people
- domestic violence that inflicts both physical and mental harm on families
- suicide, both attempts and completions, which continues to increase annually
- the festering stigma of mental illness that adds insult to injury for psychiatric patients
- alcohol and drug addictions, which destroy lives and corrode the fabric of society
- lack of access to mental health care for millions of people who need it
- lack of parity for psychiatric disorders, which is so unjust for our patients
- venomous political hatred and hyperpartisanship, which permeates our culture and can lead to violence, as we recently witnessed
- physician burnout, due to many causes, even before the stresses of COVID-19
- the ongoing agony of wars and terrorism, including dangerous cyberattacks
- the deleterious effect of social media on everyone, especially children.
Most of these epidemics claim thousands of lives each year, and yet no concerted public health effort is being mounted to counteract them, as we are seeing with the COVID-19 pandemic. Much is being written about each of them, but there has been little tangible action, so they persist. They have become a perpetual underbelly of our society that is essentially ignored or simply given the usual lip service.
It will take a herculean effort by policymakers, the judicial system, the medical establishment, and faith organizations to put an end to these life-threatening epidemics. It may appear too daunting to mount a war on so many fronts, but that should not deter us all from launching a strategic plan to create meaningful tactics and solutions. And just as was done with the COVID-19 pandemic, both mitigation measures as well as effective interventions must be employed in this campaign against the epidemic “hydra.”
Continue to: It is tragic...
It is tragic that so many fellow humans are allowed to suffer or die while the rest of us watch, or worse, turn a blind eye and never get involved. A civilized society must never neglect so many of its suffering citizens. As psychiatrists, we are aware of those human travesties around us, but we are often so overwhelmed with our work and personal responsibilities that few of us are passionately advocating or setting aside some time for those victimized by one or more of these endemic pandemics. And unless we all decide to be actively, meaningfully involved, many lives will continue to be lost every day, but without the daily “casualty count” displayed on television screens, as is the case with COVID-19 causalities.
Regrettably, maybe that old saw is true: out of sight, out of mind.
COVID-19 may alter gut microbiota
COVID-19 infection altered the gut microbiota of adult patients and caused depletion of several types of bacteria with known immunomodulatory properties, based on data from a cohort study of 100 patients with confirmed COVID-19 infections from two hospitals.
“As the GI tract is the largest immunological organ in the body and its resident microbiota are known to modulate host immune responses, we hypothesized that the gut microbiota is associated with host inflammatory immune responses in COVID19,” wrote Yun Kit Yeoh, PhD, of the Chinese University of Hong Kong, and colleagues.
In a study published in Gut, the researchers investigated patient microbiota by collecting blood, stool, and patient records between February and May 2020 from 100 confirmed SARS-CoV-2–infected patients in Hong Kong during hospitalization, as well as follow-up stool samples from 27 patients up to 30 days after they cleared the COVID-19 virus; these observations were compared with 78 non–COVID-19 controls.
Overall, 274 stool samples were sequenced. Samples collected from patients during hospitalization for COVID-19 were compared with non–COVID-19 controls. The presence of phylum Bacteroidetes was significantly higher in COVID-19 patients compared with controls (23.9% vs. 12.8%; P < .001), as were Actinobacteria (26.1% vs. 19.0%; P < .001).
After controlling for antibiotics, the investigators found that “differences between cohorts were primarily linked to enrichment of taxa such as Parabacteroides, Sutterella wadsworthensis, and Bacteroides caccae and depletion of Adlercreutzia equolifaciens, Dorea formicigenerans, and Clostridium leptum in COVID-19 relative to non-COVID-19” (P < .05). In addition, Faecalibacterium prausnitzii and Bifidobacterium bifidum were negatively correlated with COVID-19 severity after investigators controlled for patient age and antibiotic use (P < .05).
The researchers also examined bacteria in COVID-19 patients and controls in the context of cytokines and other inflammatory markers. “We hypothesized that these compositional changes play a role in exacerbating disease by contributing to dysregulation of the immune response,” they said.
In fact, species depleted in COVID-19 patients including included B. adolescentis, E. rectale, and F. prausnitzii were negatively correlated with inflammatory markers including CXCL10, IL-10, TNF-alpha, and CCL2.
In addition, 42 stool samples from 27 patients showed significantly distinct gut microbiota from controls up to 30 days (median, 6 days) after virus clearance, regardless of antibiotics use (P < .05), the researchers said.
Long-term data needed
The study findings were limited by several factors, including the potential confounding of microbial signatures associated with COVID-19 because of heterogeneous patient management in the clinical setting and the potential that gut microbiota reflects a patient’s health with no impact on disease severity, as well as lack of data on the role of antibiotics for severe and critical patients, the researchers noted. In addition, “gut microbiota composition is highly heterogeneous across human populations and changes in compositions reported here may not necessarily be reflected in patients with COVID-19 from other biogeographies,” they wrote.
The “longer follow-up of patients with COVID-19 (e.g., 3 months to 1 year after clearing the virus) is needed to address questions related to the duration of gut microbiota dysbiosis post recovery, link between microbiota dysbiosis and long-term persistent symptoms, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health problems,” they wrote.
However, the results suggest a likely role for gut microorganisms in host inflammatory responses to COVID-19 infection, and “underscore an urgent need to understand the specific roles of gut microorganisms in human immune function and systemic inflammation,” they concluded.
More than infectious
“A growing body of evidence suggests that severity of illness from COVID-19 is largely determined by the patient’s aberrant immune response to the virus,” Jatin Roper, MD, of Duke University, Durham, N.C., said in an interview. “Therefore, a critical question is: What patient factors determine this immune response? The gut microbiota closely interact with the host immune system and are altered in many immunological diseases,” he said. “Furthermore, the SARS-CoV-2 virus infects enterocytes in the intestine and causes symptomatic gastrointestinal disease in a subset of patients. Therefore, understanding a possible association between gut microbiota and COVID-19 may reveal microbial species involved in disease pathogenesis,” he emphasized.
In the current study, “I was surprised to find that COVID-19 infection is associated with depletion of immunomodulatory gut bacteria,” said Dr. Roper. “An open question is whether these changes are caused by the SARS-CoV-2 virus and then result in altered immune response. Alternatively, the changes in gut microbiota may be a result of the immune response or other changes associated with the disease,” he said.
“COVID-19 is an immunological disease, not just an infectious disease,” explained Dr. Roper. “The gut microbiota may play an important role in the pathogenesis of the disease. Thus, specific gut microbes could one day be analyzed to risk stratify patients, or even modified to treat the disease,” he noted.
Beyond COVID-19
“Given the impact of the gut microbiota on health and disease, as well as the impact of diseases on the microbiota, I am not at all surprised to find that there were significant changes in the microbiota of COVID-19 patients and that these changes are associated with inflammatory cytokines, chemokines, and blood markers of tissue damage,” said Anthony Sung, MD, also of Duke University.
According to Dr. Sung, researchers have already been investigating possible connections between gut microbiota and other conditions such as Alzheimer’s disease, and it’s been hypothesized that these connections are mediated by interactions between the gut microbiota and the immune system.
“While this is an important paper in our understanding of COVID-19, and highlights the microbiome as a potential therapeutic target, we need to conduct clinical trials of microbiota-based interventions before we can fully realize the clinical implications of these findings,” he said.
The study was supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, and donations from Hui Hoy & Chow Sin Lan Charity Fund Limited, Pine and Crane Company Limited, Mr. Hui Ming, and The D.H. Chen Foundation. The researchers had no financial conflicts to disclose. Dr. Roper and Dr. Sung had no financial conflicts to disclose.
COVID-19 infection altered the gut microbiota of adult patients and caused depletion of several types of bacteria with known immunomodulatory properties, based on data from a cohort study of 100 patients with confirmed COVID-19 infections from two hospitals.
“As the GI tract is the largest immunological organ in the body and its resident microbiota are known to modulate host immune responses, we hypothesized that the gut microbiota is associated with host inflammatory immune responses in COVID19,” wrote Yun Kit Yeoh, PhD, of the Chinese University of Hong Kong, and colleagues.
In a study published in Gut, the researchers investigated patient microbiota by collecting blood, stool, and patient records between February and May 2020 from 100 confirmed SARS-CoV-2–infected patients in Hong Kong during hospitalization, as well as follow-up stool samples from 27 patients up to 30 days after they cleared the COVID-19 virus; these observations were compared with 78 non–COVID-19 controls.
Overall, 274 stool samples were sequenced. Samples collected from patients during hospitalization for COVID-19 were compared with non–COVID-19 controls. The presence of phylum Bacteroidetes was significantly higher in COVID-19 patients compared with controls (23.9% vs. 12.8%; P < .001), as were Actinobacteria (26.1% vs. 19.0%; P < .001).
After controlling for antibiotics, the investigators found that “differences between cohorts were primarily linked to enrichment of taxa such as Parabacteroides, Sutterella wadsworthensis, and Bacteroides caccae and depletion of Adlercreutzia equolifaciens, Dorea formicigenerans, and Clostridium leptum in COVID-19 relative to non-COVID-19” (P < .05). In addition, Faecalibacterium prausnitzii and Bifidobacterium bifidum were negatively correlated with COVID-19 severity after investigators controlled for patient age and antibiotic use (P < .05).
The researchers also examined bacteria in COVID-19 patients and controls in the context of cytokines and other inflammatory markers. “We hypothesized that these compositional changes play a role in exacerbating disease by contributing to dysregulation of the immune response,” they said.
In fact, species depleted in COVID-19 patients including included B. adolescentis, E. rectale, and F. prausnitzii were negatively correlated with inflammatory markers including CXCL10, IL-10, TNF-alpha, and CCL2.
In addition, 42 stool samples from 27 patients showed significantly distinct gut microbiota from controls up to 30 days (median, 6 days) after virus clearance, regardless of antibiotics use (P < .05), the researchers said.
Long-term data needed
The study findings were limited by several factors, including the potential confounding of microbial signatures associated with COVID-19 because of heterogeneous patient management in the clinical setting and the potential that gut microbiota reflects a patient’s health with no impact on disease severity, as well as lack of data on the role of antibiotics for severe and critical patients, the researchers noted. In addition, “gut microbiota composition is highly heterogeneous across human populations and changes in compositions reported here may not necessarily be reflected in patients with COVID-19 from other biogeographies,” they wrote.
The “longer follow-up of patients with COVID-19 (e.g., 3 months to 1 year after clearing the virus) is needed to address questions related to the duration of gut microbiota dysbiosis post recovery, link between microbiota dysbiosis and long-term persistent symptoms, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health problems,” they wrote.
However, the results suggest a likely role for gut microorganisms in host inflammatory responses to COVID-19 infection, and “underscore an urgent need to understand the specific roles of gut microorganisms in human immune function and systemic inflammation,” they concluded.
More than infectious
“A growing body of evidence suggests that severity of illness from COVID-19 is largely determined by the patient’s aberrant immune response to the virus,” Jatin Roper, MD, of Duke University, Durham, N.C., said in an interview. “Therefore, a critical question is: What patient factors determine this immune response? The gut microbiota closely interact with the host immune system and are altered in many immunological diseases,” he said. “Furthermore, the SARS-CoV-2 virus infects enterocytes in the intestine and causes symptomatic gastrointestinal disease in a subset of patients. Therefore, understanding a possible association between gut microbiota and COVID-19 may reveal microbial species involved in disease pathogenesis,” he emphasized.
In the current study, “I was surprised to find that COVID-19 infection is associated with depletion of immunomodulatory gut bacteria,” said Dr. Roper. “An open question is whether these changes are caused by the SARS-CoV-2 virus and then result in altered immune response. Alternatively, the changes in gut microbiota may be a result of the immune response or other changes associated with the disease,” he said.
“COVID-19 is an immunological disease, not just an infectious disease,” explained Dr. Roper. “The gut microbiota may play an important role in the pathogenesis of the disease. Thus, specific gut microbes could one day be analyzed to risk stratify patients, or even modified to treat the disease,” he noted.
Beyond COVID-19
“Given the impact of the gut microbiota on health and disease, as well as the impact of diseases on the microbiota, I am not at all surprised to find that there were significant changes in the microbiota of COVID-19 patients and that these changes are associated with inflammatory cytokines, chemokines, and blood markers of tissue damage,” said Anthony Sung, MD, also of Duke University.
According to Dr. Sung, researchers have already been investigating possible connections between gut microbiota and other conditions such as Alzheimer’s disease, and it’s been hypothesized that these connections are mediated by interactions between the gut microbiota and the immune system.
“While this is an important paper in our understanding of COVID-19, and highlights the microbiome as a potential therapeutic target, we need to conduct clinical trials of microbiota-based interventions before we can fully realize the clinical implications of these findings,” he said.
The study was supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, and donations from Hui Hoy & Chow Sin Lan Charity Fund Limited, Pine and Crane Company Limited, Mr. Hui Ming, and The D.H. Chen Foundation. The researchers had no financial conflicts to disclose. Dr. Roper and Dr. Sung had no financial conflicts to disclose.
COVID-19 infection altered the gut microbiota of adult patients and caused depletion of several types of bacteria with known immunomodulatory properties, based on data from a cohort study of 100 patients with confirmed COVID-19 infections from two hospitals.
“As the GI tract is the largest immunological organ in the body and its resident microbiota are known to modulate host immune responses, we hypothesized that the gut microbiota is associated with host inflammatory immune responses in COVID19,” wrote Yun Kit Yeoh, PhD, of the Chinese University of Hong Kong, and colleagues.
In a study published in Gut, the researchers investigated patient microbiota by collecting blood, stool, and patient records between February and May 2020 from 100 confirmed SARS-CoV-2–infected patients in Hong Kong during hospitalization, as well as follow-up stool samples from 27 patients up to 30 days after they cleared the COVID-19 virus; these observations were compared with 78 non–COVID-19 controls.
Overall, 274 stool samples were sequenced. Samples collected from patients during hospitalization for COVID-19 were compared with non–COVID-19 controls. The presence of phylum Bacteroidetes was significantly higher in COVID-19 patients compared with controls (23.9% vs. 12.8%; P < .001), as were Actinobacteria (26.1% vs. 19.0%; P < .001).
After controlling for antibiotics, the investigators found that “differences between cohorts were primarily linked to enrichment of taxa such as Parabacteroides, Sutterella wadsworthensis, and Bacteroides caccae and depletion of Adlercreutzia equolifaciens, Dorea formicigenerans, and Clostridium leptum in COVID-19 relative to non-COVID-19” (P < .05). In addition, Faecalibacterium prausnitzii and Bifidobacterium bifidum were negatively correlated with COVID-19 severity after investigators controlled for patient age and antibiotic use (P < .05).
The researchers also examined bacteria in COVID-19 patients and controls in the context of cytokines and other inflammatory markers. “We hypothesized that these compositional changes play a role in exacerbating disease by contributing to dysregulation of the immune response,” they said.
In fact, species depleted in COVID-19 patients including included B. adolescentis, E. rectale, and F. prausnitzii were negatively correlated with inflammatory markers including CXCL10, IL-10, TNF-alpha, and CCL2.
In addition, 42 stool samples from 27 patients showed significantly distinct gut microbiota from controls up to 30 days (median, 6 days) after virus clearance, regardless of antibiotics use (P < .05), the researchers said.
Long-term data needed
The study findings were limited by several factors, including the potential confounding of microbial signatures associated with COVID-19 because of heterogeneous patient management in the clinical setting and the potential that gut microbiota reflects a patient’s health with no impact on disease severity, as well as lack of data on the role of antibiotics for severe and critical patients, the researchers noted. In addition, “gut microbiota composition is highly heterogeneous across human populations and changes in compositions reported here may not necessarily be reflected in patients with COVID-19 from other biogeographies,” they wrote.
The “longer follow-up of patients with COVID-19 (e.g., 3 months to 1 year after clearing the virus) is needed to address questions related to the duration of gut microbiota dysbiosis post recovery, link between microbiota dysbiosis and long-term persistent symptoms, and whether the dysbiosis or enrichment/depletion of specific gut microorganisms predisposes recovered individuals to future health problems,” they wrote.
However, the results suggest a likely role for gut microorganisms in host inflammatory responses to COVID-19 infection, and “underscore an urgent need to understand the specific roles of gut microorganisms in human immune function and systemic inflammation,” they concluded.
More than infectious
“A growing body of evidence suggests that severity of illness from COVID-19 is largely determined by the patient’s aberrant immune response to the virus,” Jatin Roper, MD, of Duke University, Durham, N.C., said in an interview. “Therefore, a critical question is: What patient factors determine this immune response? The gut microbiota closely interact with the host immune system and are altered in many immunological diseases,” he said. “Furthermore, the SARS-CoV-2 virus infects enterocytes in the intestine and causes symptomatic gastrointestinal disease in a subset of patients. Therefore, understanding a possible association between gut microbiota and COVID-19 may reveal microbial species involved in disease pathogenesis,” he emphasized.
In the current study, “I was surprised to find that COVID-19 infection is associated with depletion of immunomodulatory gut bacteria,” said Dr. Roper. “An open question is whether these changes are caused by the SARS-CoV-2 virus and then result in altered immune response. Alternatively, the changes in gut microbiota may be a result of the immune response or other changes associated with the disease,” he said.
“COVID-19 is an immunological disease, not just an infectious disease,” explained Dr. Roper. “The gut microbiota may play an important role in the pathogenesis of the disease. Thus, specific gut microbes could one day be analyzed to risk stratify patients, or even modified to treat the disease,” he noted.
Beyond COVID-19
“Given the impact of the gut microbiota on health and disease, as well as the impact of diseases on the microbiota, I am not at all surprised to find that there were significant changes in the microbiota of COVID-19 patients and that these changes are associated with inflammatory cytokines, chemokines, and blood markers of tissue damage,” said Anthony Sung, MD, also of Duke University.
According to Dr. Sung, researchers have already been investigating possible connections between gut microbiota and other conditions such as Alzheimer’s disease, and it’s been hypothesized that these connections are mediated by interactions between the gut microbiota and the immune system.
“While this is an important paper in our understanding of COVID-19, and highlights the microbiome as a potential therapeutic target, we need to conduct clinical trials of microbiota-based interventions before we can fully realize the clinical implications of these findings,” he said.
The study was supported by the Health and Medical Research Fund, the Food and Health Bureau, The Government of the Hong Kong Special Administrative Region, and donations from Hui Hoy & Chow Sin Lan Charity Fund Limited, Pine and Crane Company Limited, Mr. Hui Ming, and The D.H. Chen Foundation. The researchers had no financial conflicts to disclose. Dr. Roper and Dr. Sung had no financial conflicts to disclose.
FROM GUT