User login
In Case You Missed It: COVID
Despite limits, COVID vaccines protect CLL patients
These findings don’t reveal whether the T-cell boost actually provides extra protection against COVID-19. Still, the study suggests that patients with CLL should be vaccinated no matter which medications they’re taking, coauthor and hematologist/oncologist Clemens-Martin Wendtner, MD, of the Munich (Germany) Clinic, said in an interview.
“Do not defer or pause treatment,” said Dr. Wendtner, whose study was published in Blood Advances.
Patients with CLL appear to have among the weakest responses to the COVID-19 vaccine among people with various types of blood cancer. A meta-analysis published in 2022 found that seropositivity rates following vaccination were just 51% in patients with CLL, compared with 80%-90% in those with acute leukemia and 76%-80% of those with myeloma.
“Usually, the response rate to vaccination among the nonimmunocompromised would be 95%,” Dr. Wendtner said.
Research has also suggested that patients treated with B-cell pathway inhibitors and anti-CD20 antibodies are especially likely to have poorer responses to COVID-19 vaccines, no surprise considering that their job is to dampen the immune system. But there’s an unanswered question, according to Dr. Wendtner: Does “just measuring B-cell response tell us everything about the immune response?”
The new prospective, single-institution study aims to answer that question in patients who each received two types of vaccines. Researchers compared peripheral blood mononuclear cell transcriptional response with antibody and T-cell response rates in 15 patients with CLL/small lymphocytic lymphoma following vaccination with both the Pfizer-BioNTech and AstraZeneca vaccines.
The average antibody response was limited. “Overall, 7/15 of patients failed to mount a humoral response even after three-dose vaccination,” the researchers reported. All of the patients were “heavily pretreated” with CLL medications such as venetoclax, an anti-CD20 monoclonal antibody.
By contrast, the T-cell response was much stronger: 80% of patients (12/15) had a robust response, a number that grew to 90% (14/15) after a booster. This response is “almost ideal” considering that the response in a nonimmunocompromised person would be about 99%, Dr. Wendtner said.
The study also revealed that vaccine responses were weaker in patients who took a combination of a Bruton tyrosine kinase inhibitor and venetoclax within a year.
Four patients developed COVID-19 infections with the Omicron variant about 6 months after vaccination. All had mild symptoms. A lone patient had a history of COVID-19 infection prior to vaccination.
The researchers noted that the study had several limitations, including its small size, its reliance on a single institution, and the differences in treatments and vaccination protocols among the patient population.
Broadly speaking, the study showed that “a vaccine is not in vain” in patients with CLL, “although the doctor might not detect an antibody response,” Dr. Wendtner said. He added that mixing vaccine types should provide more protection. Start with a viral vector vaccine followed by an mRNA vaccine or vice versa, he suggested.
In an interview, infectious disease physician Joshua A. Hill, MD, from Fred Hutchinson Cancer Center, Seattle, who wasn’t involved with the study, said it makes “important and interesting observations to reinforce other studies with similar findings.”
Specifically, Dr. Hill said, “despite the absence of a robust antibody response some of these patients who are on active treatment, patients can still generate robust cellular immune responses in the form of T-cell immunity. Our understanding is that having T cell immunity will provide important additional protection for developing severe disease, although is less easily tested.”
As for the best vaccination strategies, Dr. Hill said “patients should get vaccinated as soon as they are eligible, according to standard guidelines. If patients have not yet started therapy, they should get their indicated vaccines before starting treatment whenever possible.”
The German study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the Bavarian State Ministry of Science and Art. Dr. Wendtner disclosed consultant fees from AstraZeneca and BioNTech, and another author disclosed consultant fees from AstraZeneca. The other authors reported no disclosures. Dr. Hill disclosed consultant fees from Moderna, Pfizer, and Gilead.
These findings don’t reveal whether the T-cell boost actually provides extra protection against COVID-19. Still, the study suggests that patients with CLL should be vaccinated no matter which medications they’re taking, coauthor and hematologist/oncologist Clemens-Martin Wendtner, MD, of the Munich (Germany) Clinic, said in an interview.
“Do not defer or pause treatment,” said Dr. Wendtner, whose study was published in Blood Advances.
Patients with CLL appear to have among the weakest responses to the COVID-19 vaccine among people with various types of blood cancer. A meta-analysis published in 2022 found that seropositivity rates following vaccination were just 51% in patients with CLL, compared with 80%-90% in those with acute leukemia and 76%-80% of those with myeloma.
“Usually, the response rate to vaccination among the nonimmunocompromised would be 95%,” Dr. Wendtner said.
Research has also suggested that patients treated with B-cell pathway inhibitors and anti-CD20 antibodies are especially likely to have poorer responses to COVID-19 vaccines, no surprise considering that their job is to dampen the immune system. But there’s an unanswered question, according to Dr. Wendtner: Does “just measuring B-cell response tell us everything about the immune response?”
The new prospective, single-institution study aims to answer that question in patients who each received two types of vaccines. Researchers compared peripheral blood mononuclear cell transcriptional response with antibody and T-cell response rates in 15 patients with CLL/small lymphocytic lymphoma following vaccination with both the Pfizer-BioNTech and AstraZeneca vaccines.
The average antibody response was limited. “Overall, 7/15 of patients failed to mount a humoral response even after three-dose vaccination,” the researchers reported. All of the patients were “heavily pretreated” with CLL medications such as venetoclax, an anti-CD20 monoclonal antibody.
By contrast, the T-cell response was much stronger: 80% of patients (12/15) had a robust response, a number that grew to 90% (14/15) after a booster. This response is “almost ideal” considering that the response in a nonimmunocompromised person would be about 99%, Dr. Wendtner said.
The study also revealed that vaccine responses were weaker in patients who took a combination of a Bruton tyrosine kinase inhibitor and venetoclax within a year.
Four patients developed COVID-19 infections with the Omicron variant about 6 months after vaccination. All had mild symptoms. A lone patient had a history of COVID-19 infection prior to vaccination.
The researchers noted that the study had several limitations, including its small size, its reliance on a single institution, and the differences in treatments and vaccination protocols among the patient population.
Broadly speaking, the study showed that “a vaccine is not in vain” in patients with CLL, “although the doctor might not detect an antibody response,” Dr. Wendtner said. He added that mixing vaccine types should provide more protection. Start with a viral vector vaccine followed by an mRNA vaccine or vice versa, he suggested.
In an interview, infectious disease physician Joshua A. Hill, MD, from Fred Hutchinson Cancer Center, Seattle, who wasn’t involved with the study, said it makes “important and interesting observations to reinforce other studies with similar findings.”
Specifically, Dr. Hill said, “despite the absence of a robust antibody response some of these patients who are on active treatment, patients can still generate robust cellular immune responses in the form of T-cell immunity. Our understanding is that having T cell immunity will provide important additional protection for developing severe disease, although is less easily tested.”
As for the best vaccination strategies, Dr. Hill said “patients should get vaccinated as soon as they are eligible, according to standard guidelines. If patients have not yet started therapy, they should get their indicated vaccines before starting treatment whenever possible.”
The German study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the Bavarian State Ministry of Science and Art. Dr. Wendtner disclosed consultant fees from AstraZeneca and BioNTech, and another author disclosed consultant fees from AstraZeneca. The other authors reported no disclosures. Dr. Hill disclosed consultant fees from Moderna, Pfizer, and Gilead.
These findings don’t reveal whether the T-cell boost actually provides extra protection against COVID-19. Still, the study suggests that patients with CLL should be vaccinated no matter which medications they’re taking, coauthor and hematologist/oncologist Clemens-Martin Wendtner, MD, of the Munich (Germany) Clinic, said in an interview.
“Do not defer or pause treatment,” said Dr. Wendtner, whose study was published in Blood Advances.
Patients with CLL appear to have among the weakest responses to the COVID-19 vaccine among people with various types of blood cancer. A meta-analysis published in 2022 found that seropositivity rates following vaccination were just 51% in patients with CLL, compared with 80%-90% in those with acute leukemia and 76%-80% of those with myeloma.
“Usually, the response rate to vaccination among the nonimmunocompromised would be 95%,” Dr. Wendtner said.
Research has also suggested that patients treated with B-cell pathway inhibitors and anti-CD20 antibodies are especially likely to have poorer responses to COVID-19 vaccines, no surprise considering that their job is to dampen the immune system. But there’s an unanswered question, according to Dr. Wendtner: Does “just measuring B-cell response tell us everything about the immune response?”
The new prospective, single-institution study aims to answer that question in patients who each received two types of vaccines. Researchers compared peripheral blood mononuclear cell transcriptional response with antibody and T-cell response rates in 15 patients with CLL/small lymphocytic lymphoma following vaccination with both the Pfizer-BioNTech and AstraZeneca vaccines.
The average antibody response was limited. “Overall, 7/15 of patients failed to mount a humoral response even after three-dose vaccination,” the researchers reported. All of the patients were “heavily pretreated” with CLL medications such as venetoclax, an anti-CD20 monoclonal antibody.
By contrast, the T-cell response was much stronger: 80% of patients (12/15) had a robust response, a number that grew to 90% (14/15) after a booster. This response is “almost ideal” considering that the response in a nonimmunocompromised person would be about 99%, Dr. Wendtner said.
The study also revealed that vaccine responses were weaker in patients who took a combination of a Bruton tyrosine kinase inhibitor and venetoclax within a year.
Four patients developed COVID-19 infections with the Omicron variant about 6 months after vaccination. All had mild symptoms. A lone patient had a history of COVID-19 infection prior to vaccination.
The researchers noted that the study had several limitations, including its small size, its reliance on a single institution, and the differences in treatments and vaccination protocols among the patient population.
Broadly speaking, the study showed that “a vaccine is not in vain” in patients with CLL, “although the doctor might not detect an antibody response,” Dr. Wendtner said. He added that mixing vaccine types should provide more protection. Start with a viral vector vaccine followed by an mRNA vaccine or vice versa, he suggested.
In an interview, infectious disease physician Joshua A. Hill, MD, from Fred Hutchinson Cancer Center, Seattle, who wasn’t involved with the study, said it makes “important and interesting observations to reinforce other studies with similar findings.”
Specifically, Dr. Hill said, “despite the absence of a robust antibody response some of these patients who are on active treatment, patients can still generate robust cellular immune responses in the form of T-cell immunity. Our understanding is that having T cell immunity will provide important additional protection for developing severe disease, although is less easily tested.”
As for the best vaccination strategies, Dr. Hill said “patients should get vaccinated as soon as they are eligible, according to standard guidelines. If patients have not yet started therapy, they should get their indicated vaccines before starting treatment whenever possible.”
The German study was funded by the National Institute of Diabetes and Digestive and Kidney Diseases and the Bavarian State Ministry of Science and Art. Dr. Wendtner disclosed consultant fees from AstraZeneca and BioNTech, and another author disclosed consultant fees from AstraZeneca. The other authors reported no disclosures. Dr. Hill disclosed consultant fees from Moderna, Pfizer, and Gilead.
FROM BLOOD ADVANCES
New developments and barriers to palliative care
As we enter into this new year, it is a good time to review the past few years of living through a pandemic and the impact this has had on the field of palliative care.
According to the World Health Organization, “Palliative care is an approach that improves the quality of life of patients and their families who are facing the problems associated with life-threatening illness, by the prevention and relief of suffering through early identification, assessment and treatment of pain and other problems whether physical, psychosocial and spiritual.”1 They identify a global need and recognize palliative care as a “human right to health and as a standard of care particularly for individuals living with a serious illness.1 However, the WHO goes further to recognize palliative care as an essential part of the response team during crises and health emergencies like a pandemic, noting that a response team without palliative care is “medically deficient and ethically indefensible.”2
The need for palliative care in the United States is projected to grow significantly in the next decades.3 However, there has been insufficient staffing to meet these needs, even prior to the pandemic.4 The demand for palliative care reached further unprecedented levels during the pandemic as palliative care teams played an integral role and were well situated to support not only patients and families with COVID-19,5 but to also support the well-being of health care teams caring for COVID-19 patients.6,7
A recent survey that was conducted by the Center to Advance Palliative Care among palliative care leadership captured the experiences of leading their teams through a pandemic. Below are the results of this survey, which highlighted important issues and developments to palliative care during the pandemic.6
Increasing need for palliative care
One of the main findings from the national survey of palliative care leaders corroborated that the demands for palliative care have increased significantly from 2020 through the pandemic.
As with many areas in the health care system, the pandemic has emphasized the strain and short staffing of the palliative care teams. In the survey, 61% of leaders reported that palliative care consults significantly increased from prepandemic levels. But only 26% of these leaders said they had the staffing support to meet these needs.
Value of palliative care
The value of palliative care along with understanding of the role of palliative care has been better recognized during the pandemic and has been evidenced by the increase in palliative care referrals from clinical providers, compared with prepandemic levels. In addition, data collected showed that earlier palliative care consultations reduced length of hospital stay, decreased ICU admissions, and improved patient, family, and provider satisfaction.
Well-being of the workforce
The pandemic has been a tremendously stressful time for the health care workforce that has undoubtedly led to burnout. A nationwide study of physicians,8 found that 61% of physicians experienced burnout. This is a significant increase from prepandemic levels with impacts on mental health (that is, anxiety, depression). This study did not include palliative care specialists, but the CAPC survey indicates a similar feeling of burnout. Because of this, some palliative care specialists have left the field altogether, or are leaving leadership positions because of burnout and exhaustion from the pandemic. This was featured as a concern among palliative care leaders, where 93% reported concern for the emotional well-being of the palliative care team.
Telehealth
A permanent operational change that has been well-utilized and implemented across multiple health care settings has been providing palliative care through telehealth. Prior to the pandemic, the baseline use of telehealth was less than 5% with the use now greater than 75% – a modality that is favored by both patients and clinicians. This has offered a broader scope of practice, reaching individuals who may have no other means, have limitations to accessing palliative care, or were in circumstances where patients required isolation during the pandemic. However, there are limitations to this platform, including in equity of access to devices and ease of use for those with limited exposure to technology.9
Barriers to implementation
Although the important role and value of palliative care has been well recognized, there have been barriers identified in a qualitative study of the integration of palliative care into COVID-19 action plans that are mentioned below.5
- Patients and families were identified as barriers to integration of palliative care if they were not open to palliative care referral, mainly because of misperceptions of palliative care as end-of-life care.
- Palliative care knowledge among providers was identified as another barrier to integration of palliative care. There are still misperceptions among providers that palliative care is end-of-life care and palliative care involvement is stigmatized as hastening death. In addition, some felt that COVID-19 was not a traditional “palliative diagnosis” thus were less likely to integrate palliative care into care plans.
- Lack of availability of a primary provider to conduct primary palliative care and lack of motivation “not to give up” were identified as other barriers. On the other hand, palliative care provider availability and accessibility to care teams affected the integration into COVID-19 care plans.
- COVID-19 itself was identified to be a barrier because of the uncertainty of illness trajectory and outcomes, which made it difficult for doctors to ascertain when to involve palliative care.
- Leadership and institution were important factors to consider in integration of palliative care into long-term care plans, which depended on leadership engagement and institutional culture.
Takeaways
The past few years have taught us a lot, but there is still much to learn. The COVID-19 pandemic has called attention to the challenges and barriers of health care delivery and has magnified the needs of the health care system including its infrastructure, preparedness, and staffing, including the field of palliative care. More work needs to be done, but leaders have taken steps to initiate national and international preparedness plans including the integration of palliative care, which has been identified as a vital role in any humanitarian crises.10,11
Dr. Kang is a geriatrician and palliative care provider at the University of Washington, Seattle, in the division of geriatrics and gerontology. She has no conflicts related to the content of this article.
References
1. Palliative care. World Health Organization. Aug 5, 2020. https://www.who.int/news-room/fact-sheets/detail/palliative-care
2. World Health Organization. Integrating palliative care and symptom relief into the response to humanitarian emergencies and crises: A WHO guide. Geneva: World Health Organization, 2018. https://apps.who.int/iris/handle/10665/274565.
3. Hughes MT, Smith TJ. The growth of palliative care in the United States. Annual Review Public Health. 2014;35:459-75.
4. Pastrana T et al. The impact of COVID-19 on palliative care workers across the world: A qualitative analysis of responses to open-ended questions. Palliative and Supportive Care. 2021:1-6.
5. Wentlandt K et al. Identifying barriers and facilitators to palliative care integration in the management of hospitalized patients with COVID-19: A qualitative study. Palliat Med. 2022;36(6):945-54.
6. Rogers M et al. Palliative care leadership during the pandemic: Results from a recent survey. Center to Advance Palliative Care. 2022 Sept 8. https://www.capc.org/blog/palliative-care-leadership-during-the-pandemic-results-from-a-recent-survey
7. Fogelman P. Reflections form a palliative care program leader two years into the pandemic. Center to Advance Palliative Care. 2023 Jan 15. https://www.capc.org/blog/reflections-from-a-palliative-care-program-leader-two-years-into-the-pandemic
8. 2021 survey of America’s physicians Covid-19 impact edition: A year later. The Physicians Foundation. 2021.
9. Caraceni A et al. Telemedicine for outpatient palliative care during Covid-19 pandemics: A longitudinal study. BMJ Supportive & Palliative Care. 2022;0:1-7.
10. Bausewein C et al. National strategy for palliative care of severely ill and dying people and their relatives in pandemics (PallPan) in Germany – study protocol of a mixed-methods project. BMC Palliative Care. 2022;21(10).
11. Powell RA et al. Palliative care in humanitarian crises: Always something to offer. The Lancet. 2017;389(10078):1498-9.
As we enter into this new year, it is a good time to review the past few years of living through a pandemic and the impact this has had on the field of palliative care.
According to the World Health Organization, “Palliative care is an approach that improves the quality of life of patients and their families who are facing the problems associated with life-threatening illness, by the prevention and relief of suffering through early identification, assessment and treatment of pain and other problems whether physical, psychosocial and spiritual.”1 They identify a global need and recognize palliative care as a “human right to health and as a standard of care particularly for individuals living with a serious illness.1 However, the WHO goes further to recognize palliative care as an essential part of the response team during crises and health emergencies like a pandemic, noting that a response team without palliative care is “medically deficient and ethically indefensible.”2
The need for palliative care in the United States is projected to grow significantly in the next decades.3 However, there has been insufficient staffing to meet these needs, even prior to the pandemic.4 The demand for palliative care reached further unprecedented levels during the pandemic as palliative care teams played an integral role and were well situated to support not only patients and families with COVID-19,5 but to also support the well-being of health care teams caring for COVID-19 patients.6,7
A recent survey that was conducted by the Center to Advance Palliative Care among palliative care leadership captured the experiences of leading their teams through a pandemic. Below are the results of this survey, which highlighted important issues and developments to palliative care during the pandemic.6
Increasing need for palliative care
One of the main findings from the national survey of palliative care leaders corroborated that the demands for palliative care have increased significantly from 2020 through the pandemic.
As with many areas in the health care system, the pandemic has emphasized the strain and short staffing of the palliative care teams. In the survey, 61% of leaders reported that palliative care consults significantly increased from prepandemic levels. But only 26% of these leaders said they had the staffing support to meet these needs.
Value of palliative care
The value of palliative care along with understanding of the role of palliative care has been better recognized during the pandemic and has been evidenced by the increase in palliative care referrals from clinical providers, compared with prepandemic levels. In addition, data collected showed that earlier palliative care consultations reduced length of hospital stay, decreased ICU admissions, and improved patient, family, and provider satisfaction.
Well-being of the workforce
The pandemic has been a tremendously stressful time for the health care workforce that has undoubtedly led to burnout. A nationwide study of physicians,8 found that 61% of physicians experienced burnout. This is a significant increase from prepandemic levels with impacts on mental health (that is, anxiety, depression). This study did not include palliative care specialists, but the CAPC survey indicates a similar feeling of burnout. Because of this, some palliative care specialists have left the field altogether, or are leaving leadership positions because of burnout and exhaustion from the pandemic. This was featured as a concern among palliative care leaders, where 93% reported concern for the emotional well-being of the palliative care team.
Telehealth
A permanent operational change that has been well-utilized and implemented across multiple health care settings has been providing palliative care through telehealth. Prior to the pandemic, the baseline use of telehealth was less than 5% with the use now greater than 75% – a modality that is favored by both patients and clinicians. This has offered a broader scope of practice, reaching individuals who may have no other means, have limitations to accessing palliative care, or were in circumstances where patients required isolation during the pandemic. However, there are limitations to this platform, including in equity of access to devices and ease of use for those with limited exposure to technology.9
Barriers to implementation
Although the important role and value of palliative care has been well recognized, there have been barriers identified in a qualitative study of the integration of palliative care into COVID-19 action plans that are mentioned below.5
- Patients and families were identified as barriers to integration of palliative care if they were not open to palliative care referral, mainly because of misperceptions of palliative care as end-of-life care.
- Palliative care knowledge among providers was identified as another barrier to integration of palliative care. There are still misperceptions among providers that palliative care is end-of-life care and palliative care involvement is stigmatized as hastening death. In addition, some felt that COVID-19 was not a traditional “palliative diagnosis” thus were less likely to integrate palliative care into care plans.
- Lack of availability of a primary provider to conduct primary palliative care and lack of motivation “not to give up” were identified as other barriers. On the other hand, palliative care provider availability and accessibility to care teams affected the integration into COVID-19 care plans.
- COVID-19 itself was identified to be a barrier because of the uncertainty of illness trajectory and outcomes, which made it difficult for doctors to ascertain when to involve palliative care.
- Leadership and institution were important factors to consider in integration of palliative care into long-term care plans, which depended on leadership engagement and institutional culture.
Takeaways
The past few years have taught us a lot, but there is still much to learn. The COVID-19 pandemic has called attention to the challenges and barriers of health care delivery and has magnified the needs of the health care system including its infrastructure, preparedness, and staffing, including the field of palliative care. More work needs to be done, but leaders have taken steps to initiate national and international preparedness plans including the integration of palliative care, which has been identified as a vital role in any humanitarian crises.10,11
Dr. Kang is a geriatrician and palliative care provider at the University of Washington, Seattle, in the division of geriatrics and gerontology. She has no conflicts related to the content of this article.
References
1. Palliative care. World Health Organization. Aug 5, 2020. https://www.who.int/news-room/fact-sheets/detail/palliative-care
2. World Health Organization. Integrating palliative care and symptom relief into the response to humanitarian emergencies and crises: A WHO guide. Geneva: World Health Organization, 2018. https://apps.who.int/iris/handle/10665/274565.
3. Hughes MT, Smith TJ. The growth of palliative care in the United States. Annual Review Public Health. 2014;35:459-75.
4. Pastrana T et al. The impact of COVID-19 on palliative care workers across the world: A qualitative analysis of responses to open-ended questions. Palliative and Supportive Care. 2021:1-6.
5. Wentlandt K et al. Identifying barriers and facilitators to palliative care integration in the management of hospitalized patients with COVID-19: A qualitative study. Palliat Med. 2022;36(6):945-54.
6. Rogers M et al. Palliative care leadership during the pandemic: Results from a recent survey. Center to Advance Palliative Care. 2022 Sept 8. https://www.capc.org/blog/palliative-care-leadership-during-the-pandemic-results-from-a-recent-survey
7. Fogelman P. Reflections form a palliative care program leader two years into the pandemic. Center to Advance Palliative Care. 2023 Jan 15. https://www.capc.org/blog/reflections-from-a-palliative-care-program-leader-two-years-into-the-pandemic
8. 2021 survey of America’s physicians Covid-19 impact edition: A year later. The Physicians Foundation. 2021.
9. Caraceni A et al. Telemedicine for outpatient palliative care during Covid-19 pandemics: A longitudinal study. BMJ Supportive & Palliative Care. 2022;0:1-7.
10. Bausewein C et al. National strategy for palliative care of severely ill and dying people and their relatives in pandemics (PallPan) in Germany – study protocol of a mixed-methods project. BMC Palliative Care. 2022;21(10).
11. Powell RA et al. Palliative care in humanitarian crises: Always something to offer. The Lancet. 2017;389(10078):1498-9.
As we enter into this new year, it is a good time to review the past few years of living through a pandemic and the impact this has had on the field of palliative care.
According to the World Health Organization, “Palliative care is an approach that improves the quality of life of patients and their families who are facing the problems associated with life-threatening illness, by the prevention and relief of suffering through early identification, assessment and treatment of pain and other problems whether physical, psychosocial and spiritual.”1 They identify a global need and recognize palliative care as a “human right to health and as a standard of care particularly for individuals living with a serious illness.1 However, the WHO goes further to recognize palliative care as an essential part of the response team during crises and health emergencies like a pandemic, noting that a response team without palliative care is “medically deficient and ethically indefensible.”2
The need for palliative care in the United States is projected to grow significantly in the next decades.3 However, there has been insufficient staffing to meet these needs, even prior to the pandemic.4 The demand for palliative care reached further unprecedented levels during the pandemic as palliative care teams played an integral role and were well situated to support not only patients and families with COVID-19,5 but to also support the well-being of health care teams caring for COVID-19 patients.6,7
A recent survey that was conducted by the Center to Advance Palliative Care among palliative care leadership captured the experiences of leading their teams through a pandemic. Below are the results of this survey, which highlighted important issues and developments to palliative care during the pandemic.6
Increasing need for palliative care
One of the main findings from the national survey of palliative care leaders corroborated that the demands for palliative care have increased significantly from 2020 through the pandemic.
As with many areas in the health care system, the pandemic has emphasized the strain and short staffing of the palliative care teams. In the survey, 61% of leaders reported that palliative care consults significantly increased from prepandemic levels. But only 26% of these leaders said they had the staffing support to meet these needs.
Value of palliative care
The value of palliative care along with understanding of the role of palliative care has been better recognized during the pandemic and has been evidenced by the increase in palliative care referrals from clinical providers, compared with prepandemic levels. In addition, data collected showed that earlier palliative care consultations reduced length of hospital stay, decreased ICU admissions, and improved patient, family, and provider satisfaction.
Well-being of the workforce
The pandemic has been a tremendously stressful time for the health care workforce that has undoubtedly led to burnout. A nationwide study of physicians,8 found that 61% of physicians experienced burnout. This is a significant increase from prepandemic levels with impacts on mental health (that is, anxiety, depression). This study did not include palliative care specialists, but the CAPC survey indicates a similar feeling of burnout. Because of this, some palliative care specialists have left the field altogether, or are leaving leadership positions because of burnout and exhaustion from the pandemic. This was featured as a concern among palliative care leaders, where 93% reported concern for the emotional well-being of the palliative care team.
Telehealth
A permanent operational change that has been well-utilized and implemented across multiple health care settings has been providing palliative care through telehealth. Prior to the pandemic, the baseline use of telehealth was less than 5% with the use now greater than 75% – a modality that is favored by both patients and clinicians. This has offered a broader scope of practice, reaching individuals who may have no other means, have limitations to accessing palliative care, or were in circumstances where patients required isolation during the pandemic. However, there are limitations to this platform, including in equity of access to devices and ease of use for those with limited exposure to technology.9
Barriers to implementation
Although the important role and value of palliative care has been well recognized, there have been barriers identified in a qualitative study of the integration of palliative care into COVID-19 action plans that are mentioned below.5
- Patients and families were identified as barriers to integration of palliative care if they were not open to palliative care referral, mainly because of misperceptions of palliative care as end-of-life care.
- Palliative care knowledge among providers was identified as another barrier to integration of palliative care. There are still misperceptions among providers that palliative care is end-of-life care and palliative care involvement is stigmatized as hastening death. In addition, some felt that COVID-19 was not a traditional “palliative diagnosis” thus were less likely to integrate palliative care into care plans.
- Lack of availability of a primary provider to conduct primary palliative care and lack of motivation “not to give up” were identified as other barriers. On the other hand, palliative care provider availability and accessibility to care teams affected the integration into COVID-19 care plans.
- COVID-19 itself was identified to be a barrier because of the uncertainty of illness trajectory and outcomes, which made it difficult for doctors to ascertain when to involve palliative care.
- Leadership and institution were important factors to consider in integration of palliative care into long-term care plans, which depended on leadership engagement and institutional culture.
Takeaways
The past few years have taught us a lot, but there is still much to learn. The COVID-19 pandemic has called attention to the challenges and barriers of health care delivery and has magnified the needs of the health care system including its infrastructure, preparedness, and staffing, including the field of palliative care. More work needs to be done, but leaders have taken steps to initiate national and international preparedness plans including the integration of palliative care, which has been identified as a vital role in any humanitarian crises.10,11
Dr. Kang is a geriatrician and palliative care provider at the University of Washington, Seattle, in the division of geriatrics and gerontology. She has no conflicts related to the content of this article.
References
1. Palliative care. World Health Organization. Aug 5, 2020. https://www.who.int/news-room/fact-sheets/detail/palliative-care
2. World Health Organization. Integrating palliative care and symptom relief into the response to humanitarian emergencies and crises: A WHO guide. Geneva: World Health Organization, 2018. https://apps.who.int/iris/handle/10665/274565.
3. Hughes MT, Smith TJ. The growth of palliative care in the United States. Annual Review Public Health. 2014;35:459-75.
4. Pastrana T et al. The impact of COVID-19 on palliative care workers across the world: A qualitative analysis of responses to open-ended questions. Palliative and Supportive Care. 2021:1-6.
5. Wentlandt K et al. Identifying barriers and facilitators to palliative care integration in the management of hospitalized patients with COVID-19: A qualitative study. Palliat Med. 2022;36(6):945-54.
6. Rogers M et al. Palliative care leadership during the pandemic: Results from a recent survey. Center to Advance Palliative Care. 2022 Sept 8. https://www.capc.org/blog/palliative-care-leadership-during-the-pandemic-results-from-a-recent-survey
7. Fogelman P. Reflections form a palliative care program leader two years into the pandemic. Center to Advance Palliative Care. 2023 Jan 15. https://www.capc.org/blog/reflections-from-a-palliative-care-program-leader-two-years-into-the-pandemic
8. 2021 survey of America’s physicians Covid-19 impact edition: A year later. The Physicians Foundation. 2021.
9. Caraceni A et al. Telemedicine for outpatient palliative care during Covid-19 pandemics: A longitudinal study. BMJ Supportive & Palliative Care. 2022;0:1-7.
10. Bausewein C et al. National strategy for palliative care of severely ill and dying people and their relatives in pandemics (PallPan) in Germany – study protocol of a mixed-methods project. BMC Palliative Care. 2022;21(10).
11. Powell RA et al. Palliative care in humanitarian crises: Always something to offer. The Lancet. 2017;389(10078):1498-9.
More data back Guillain-Barré risk with Janssen COVID shot
Over 14 months, GBS reporting rates within 21 and 42 days of administration of Janssen’s replication-incompetent adenoviral vector vaccine were approximately 9 to 12 times higher than after administration of the Pfizer-BioNTech (BNT162b2) or the Moderna (mRNA-1273) mRNA COVID vaccines.
Additionally, observed GBS cases after the Janssen shot were 2 to 3 times greater than expected, based on background rates within 21 and 42 days of vaccination.
Conversely, and confirming prior data, there was no increased risk for GBS with the Pfizer or Moderna vaccines and no significant difference between observed and expected numbers of GBS cases after either mRNA COVID-19 vaccine.
The findings were published online in JAMA Network Open.
More precise risk estimates
Winston Abara, MD, with the U.S. Centers for Disease Control and Prevention, and colleagues analyzed GBS reports submitted to the VAERS between December 2020 and January 2022.
Among 487.6 million COVID-19 vaccine doses administered, 3.7% were Janssen’s Ad26.COV2.S vaccine, 54.7% were Pfizer’s BNT162b2 vaccine, and 41.6% were Moderna’s mRNA-1273 vaccine.
There were 295 verified reports of GBS identified after COVID-19 vaccination. Of these, 209 occurred within 21 days of vaccination and 253 within 42 days.
Within 21 days of vaccination, GBS reporting rates per 1 million doses were 3.29 for the Janssen vaccine versus 0.29 and 0.35 for the Pfizer and Moderna vaccines, respectively. Within 42 days of vaccination, reporting rates per 1 million doses were 4.07, 0.34, and 0.44, respectively.
Also within 21 days of vaccination, GBS reporting rates were significantly higher with the Janssen vaccine than the Pfizer vaccine (reporting rate ratio, 11.40) and the Moderna vaccine (RRR, 9.26). Similar findings were observed within 42 days after vaccination.
The observed-to-expected ratios were 3.79 for 21-day and 2.34 for 42-day intervals after receipt of the Janssen vaccine, and less than 1 (not significant) after the Pfizer or Moderna vaccine within both post-vaccination periods.
“Unlike prior studies, our analysis included all U.S. reports of verified GBS cases that met the Brighton Collaboration GBS case definition criteria (Brighton Levels 1, 2, and 3) submitted over a 14-month surveillance period to the to the Vaccine Adverse Event Reporting System,” Dr. Abara said in an interview. “Because we used all U.S. reports, the sample of verified GBS cases in this analysis is larger than other studies. Therefore, it may provide a more precise estimate of the GBS risk within 21 and 42 days after mRNA and Ad26.COV2.S vaccination,” he said.
‘Remarkably low’ use
Nicola Klein, MD, PhD, Kaiser Permanente Vaccine Study Center, Oakland, Calif., noted that this is a “nice confirmatory analysis that supports and further expands what’s been observed before.”
Last year, as reported by this news organization, Dr. Klein and colleagues reported data from the Vaccine Safety Datalink confirming a small but statistically significant increased risk for GBS in the 3 weeks after receipt of the Janssen COVID-19 vaccine but not the Pfizer or Moderna vaccines.
Unlike VAERS, the Vaccine Safety Datalink is not a reporting system. It’s an active surveillance of medical records in the Kaiser Permanente system. The VAERS is a passive system, so it requires individuals to report GBS cases to the VAERS team, Dr. Klein explained.
So although the two studies are slightly different, overall, the VAERS data is “consistent with what we found,” she said.
Also weighing in, C. Buddy Creech, MD, MPH, director of the Vanderbilt Vaccine Research Program and professor of pediatrics at the Vanderbilt University School of Medicine, Nashville, Tenn., said it is “important to realize that GBS had been observed after adenovirus-vectored vaccines earlier in the pandemic, both for the AstraZeneca vaccine and the Janssen vaccine.”
The Advisory Committee on Immunization Practices (ACIP) preferentially recommends that people age 18 years and older receive an mRNA COVID-19 vaccine rather than the Janssen adenoviral vector vaccine when both types of COVID-19 vaccine are available.
“Thus, the use of the Janssen vaccine is remarkably low in the U.S. right now,” Dr. Creech said.
“Nevertheless, we have a firm commitment, both scientifically and ethically, to track potential side effects after vaccination and to make sure that the vaccines in use for COVID, and other important infectious diseases, are safe and effective,” he added.
The study had no commercial funding. Dr. Abara and Dr. Creech have reported no relevant financial relationships. Dr. Klein reported having received grants from Pfizer research support for a COVID vaccine clinical trial, as well as grants from Merck, GlaxoSmithKline, Sanofi Pasteur, and Protein Science (now Sanofi Pasteur).
A version of this article first appeared on Medscape.com.
Over 14 months, GBS reporting rates within 21 and 42 days of administration of Janssen’s replication-incompetent adenoviral vector vaccine were approximately 9 to 12 times higher than after administration of the Pfizer-BioNTech (BNT162b2) or the Moderna (mRNA-1273) mRNA COVID vaccines.
Additionally, observed GBS cases after the Janssen shot were 2 to 3 times greater than expected, based on background rates within 21 and 42 days of vaccination.
Conversely, and confirming prior data, there was no increased risk for GBS with the Pfizer or Moderna vaccines and no significant difference between observed and expected numbers of GBS cases after either mRNA COVID-19 vaccine.
The findings were published online in JAMA Network Open.
More precise risk estimates
Winston Abara, MD, with the U.S. Centers for Disease Control and Prevention, and colleagues analyzed GBS reports submitted to the VAERS between December 2020 and January 2022.
Among 487.6 million COVID-19 vaccine doses administered, 3.7% were Janssen’s Ad26.COV2.S vaccine, 54.7% were Pfizer’s BNT162b2 vaccine, and 41.6% were Moderna’s mRNA-1273 vaccine.
There were 295 verified reports of GBS identified after COVID-19 vaccination. Of these, 209 occurred within 21 days of vaccination and 253 within 42 days.
Within 21 days of vaccination, GBS reporting rates per 1 million doses were 3.29 for the Janssen vaccine versus 0.29 and 0.35 for the Pfizer and Moderna vaccines, respectively. Within 42 days of vaccination, reporting rates per 1 million doses were 4.07, 0.34, and 0.44, respectively.
Also within 21 days of vaccination, GBS reporting rates were significantly higher with the Janssen vaccine than the Pfizer vaccine (reporting rate ratio, 11.40) and the Moderna vaccine (RRR, 9.26). Similar findings were observed within 42 days after vaccination.
The observed-to-expected ratios were 3.79 for 21-day and 2.34 for 42-day intervals after receipt of the Janssen vaccine, and less than 1 (not significant) after the Pfizer or Moderna vaccine within both post-vaccination periods.
“Unlike prior studies, our analysis included all U.S. reports of verified GBS cases that met the Brighton Collaboration GBS case definition criteria (Brighton Levels 1, 2, and 3) submitted over a 14-month surveillance period to the to the Vaccine Adverse Event Reporting System,” Dr. Abara said in an interview. “Because we used all U.S. reports, the sample of verified GBS cases in this analysis is larger than other studies. Therefore, it may provide a more precise estimate of the GBS risk within 21 and 42 days after mRNA and Ad26.COV2.S vaccination,” he said.
‘Remarkably low’ use
Nicola Klein, MD, PhD, Kaiser Permanente Vaccine Study Center, Oakland, Calif., noted that this is a “nice confirmatory analysis that supports and further expands what’s been observed before.”
Last year, as reported by this news organization, Dr. Klein and colleagues reported data from the Vaccine Safety Datalink confirming a small but statistically significant increased risk for GBS in the 3 weeks after receipt of the Janssen COVID-19 vaccine but not the Pfizer or Moderna vaccines.
Unlike VAERS, the Vaccine Safety Datalink is not a reporting system. It’s an active surveillance of medical records in the Kaiser Permanente system. The VAERS is a passive system, so it requires individuals to report GBS cases to the VAERS team, Dr. Klein explained.
So although the two studies are slightly different, overall, the VAERS data is “consistent with what we found,” she said.
Also weighing in, C. Buddy Creech, MD, MPH, director of the Vanderbilt Vaccine Research Program and professor of pediatrics at the Vanderbilt University School of Medicine, Nashville, Tenn., said it is “important to realize that GBS had been observed after adenovirus-vectored vaccines earlier in the pandemic, both for the AstraZeneca vaccine and the Janssen vaccine.”
The Advisory Committee on Immunization Practices (ACIP) preferentially recommends that people age 18 years and older receive an mRNA COVID-19 vaccine rather than the Janssen adenoviral vector vaccine when both types of COVID-19 vaccine are available.
“Thus, the use of the Janssen vaccine is remarkably low in the U.S. right now,” Dr. Creech said.
“Nevertheless, we have a firm commitment, both scientifically and ethically, to track potential side effects after vaccination and to make sure that the vaccines in use for COVID, and other important infectious diseases, are safe and effective,” he added.
The study had no commercial funding. Dr. Abara and Dr. Creech have reported no relevant financial relationships. Dr. Klein reported having received grants from Pfizer research support for a COVID vaccine clinical trial, as well as grants from Merck, GlaxoSmithKline, Sanofi Pasteur, and Protein Science (now Sanofi Pasteur).
A version of this article first appeared on Medscape.com.
Over 14 months, GBS reporting rates within 21 and 42 days of administration of Janssen’s replication-incompetent adenoviral vector vaccine were approximately 9 to 12 times higher than after administration of the Pfizer-BioNTech (BNT162b2) or the Moderna (mRNA-1273) mRNA COVID vaccines.
Additionally, observed GBS cases after the Janssen shot were 2 to 3 times greater than expected, based on background rates within 21 and 42 days of vaccination.
Conversely, and confirming prior data, there was no increased risk for GBS with the Pfizer or Moderna vaccines and no significant difference between observed and expected numbers of GBS cases after either mRNA COVID-19 vaccine.
The findings were published online in JAMA Network Open.
More precise risk estimates
Winston Abara, MD, with the U.S. Centers for Disease Control and Prevention, and colleagues analyzed GBS reports submitted to the VAERS between December 2020 and January 2022.
Among 487.6 million COVID-19 vaccine doses administered, 3.7% were Janssen’s Ad26.COV2.S vaccine, 54.7% were Pfizer’s BNT162b2 vaccine, and 41.6% were Moderna’s mRNA-1273 vaccine.
There were 295 verified reports of GBS identified after COVID-19 vaccination. Of these, 209 occurred within 21 days of vaccination and 253 within 42 days.
Within 21 days of vaccination, GBS reporting rates per 1 million doses were 3.29 for the Janssen vaccine versus 0.29 and 0.35 for the Pfizer and Moderna vaccines, respectively. Within 42 days of vaccination, reporting rates per 1 million doses were 4.07, 0.34, and 0.44, respectively.
Also within 21 days of vaccination, GBS reporting rates were significantly higher with the Janssen vaccine than the Pfizer vaccine (reporting rate ratio, 11.40) and the Moderna vaccine (RRR, 9.26). Similar findings were observed within 42 days after vaccination.
The observed-to-expected ratios were 3.79 for 21-day and 2.34 for 42-day intervals after receipt of the Janssen vaccine, and less than 1 (not significant) after the Pfizer or Moderna vaccine within both post-vaccination periods.
“Unlike prior studies, our analysis included all U.S. reports of verified GBS cases that met the Brighton Collaboration GBS case definition criteria (Brighton Levels 1, 2, and 3) submitted over a 14-month surveillance period to the to the Vaccine Adverse Event Reporting System,” Dr. Abara said in an interview. “Because we used all U.S. reports, the sample of verified GBS cases in this analysis is larger than other studies. Therefore, it may provide a more precise estimate of the GBS risk within 21 and 42 days after mRNA and Ad26.COV2.S vaccination,” he said.
‘Remarkably low’ use
Nicola Klein, MD, PhD, Kaiser Permanente Vaccine Study Center, Oakland, Calif., noted that this is a “nice confirmatory analysis that supports and further expands what’s been observed before.”
Last year, as reported by this news organization, Dr. Klein and colleagues reported data from the Vaccine Safety Datalink confirming a small but statistically significant increased risk for GBS in the 3 weeks after receipt of the Janssen COVID-19 vaccine but not the Pfizer or Moderna vaccines.
Unlike VAERS, the Vaccine Safety Datalink is not a reporting system. It’s an active surveillance of medical records in the Kaiser Permanente system. The VAERS is a passive system, so it requires individuals to report GBS cases to the VAERS team, Dr. Klein explained.
So although the two studies are slightly different, overall, the VAERS data is “consistent with what we found,” she said.
Also weighing in, C. Buddy Creech, MD, MPH, director of the Vanderbilt Vaccine Research Program and professor of pediatrics at the Vanderbilt University School of Medicine, Nashville, Tenn., said it is “important to realize that GBS had been observed after adenovirus-vectored vaccines earlier in the pandemic, both for the AstraZeneca vaccine and the Janssen vaccine.”
The Advisory Committee on Immunization Practices (ACIP) preferentially recommends that people age 18 years and older receive an mRNA COVID-19 vaccine rather than the Janssen adenoviral vector vaccine when both types of COVID-19 vaccine are available.
“Thus, the use of the Janssen vaccine is remarkably low in the U.S. right now,” Dr. Creech said.
“Nevertheless, we have a firm commitment, both scientifically and ethically, to track potential side effects after vaccination and to make sure that the vaccines in use for COVID, and other important infectious diseases, are safe and effective,” he added.
The study had no commercial funding. Dr. Abara and Dr. Creech have reported no relevant financial relationships. Dr. Klein reported having received grants from Pfizer research support for a COVID vaccine clinical trial, as well as grants from Merck, GlaxoSmithKline, Sanofi Pasteur, and Protein Science (now Sanofi Pasteur).
A version of this article first appeared on Medscape.com.
Inflammation and immunity troubles top long-COVID suspect list
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
“I think that it’s a much more complex picture than just inflammation, or just autoimmunity, or just immune dysregulation. And it’s probably a combination of all three causing a cascade of effects that then manifests itself as brain fog, or shortness of breath, or chronic fatigue,” says Alexander Truong, MD, a pulmonologist and assistant professor at Emory University, Atlanta, who also runs a long-COVID clinic.
Long COVID, post–COVID-19 condition, and postacute sequelae of SARS-CoV-2 (PASC) are among the terms used by the National Institutes of Health to describe the long-term health issues faced by an estimated 10%-30% of people infected with COVID-19. Symptoms – as many as 200 – can range from inconvenient to crippling, damage multiple organ systems, come and go, and relapse. Long COVID increases the risk of worsening existing health problems and triggering new ones, including cardiovascular disease and type 2 diabetes.
So far, research suggests there is no single cause, condition, or disease that explains why some people have an extensive range of symptoms long after the early COVID-19 infection has cleared up. Many experts believe some combination of biological processes – including the virus hanging around in our bodies, inflammation, autoimmunity, tiny blood clots, immune system problems, and even the reactivation of dormant viruses such as the Epstein-Barr virus – could be the culprit, a theory also supported by a comprehensive and in-depth review of long-COVID studies published in the journal Nature Reviews Microbiology.
“It’s become clear over the last couple of years that there are different [symptoms] of long COVID … that cannot all be lumped together,” says Michael Peluso, MD, an assistant professor of medicine and an infectious diseases doctor at the University of California, San Francisco.
Inflammation and a virus that hangs around
Multiple studies have shown that the virus or pieces of it can remain in many parts of the body, including the kidneys, brain, heart, and gastrointestinal system, long after the early infection.
“One major question that I think is the area of most intense investigation now is whether there is viral persistence that is driving immune dysregulation and therefore symptoms,” says Dr. Peluso.
A small Harvard University study, for example, found evidence that reservoirs of the coronavirus could linger in patients up to a year after they’re first diagnosed.
An earlier German study found that patients with post-COVID-19 symptoms had higher levels of three cytokines – small proteins that tell the body’s immune system what to do and are involved in the growth and activity of immune system cells and blood cells. Researchers said the results supported the theory that there is persistent reprogramming of certain immune cells, and that the uncontrolled “self-fueled hyperinflammation” during the early COVID-19 infection can become continued immune cell disruption that drives long-COVID symptoms.
“Long COVID is more likely due to either an inflammatory response by the body or reservoirs of virus that the body is still trying to clear … and the symptoms we’re seeing are a side effect of that,” says Rainu Kaushal, MD, senior associate dean for clinical research at Weill Cornell Medicine in New York.
Australian researchers found that immune system recovery appeared different, compared with those who were infected with other common coronaviruses.
These findings also support concerns that some experts express over the long-term risks of COVID-19 infections in general, but especially repeat infections.
“Anything that kind of revs up inflammation in the body can boil that pot over and make the symptoms worse. That’s very easily an infection or some other insult to the body. So that’s the generalized hypothesis as to why insults to the body may worsen the symptoms,” says Dr. Truong.
An autoimmune condition?
But inflammation alone does not fully explain post–COVID-19 problems.
Dr. Truong and his team, for example, have been documenting inflammatory markers in patients at the post-COVID clinic he cofounded more than 2 years ago at Emory Executive Park in Atlanta. When the clinic was first launched, high-dose nonsteroidal anti-inflammatory drugs – including ibuprofen – and prednisone were prescribed to long-COVID patients.
“It didn’t make a difference at all for any of these folks,” he says, adding that there are signs that autoimmunity is at play. But he cautions that it is still too early to suggest treating long-COVID patients with medications used for other autoimmune conditions.
In autoimmune conditions such as rheumatoid arthritis, lupus, and type 1 diabetes, a person’s immune system can’t tell normal cells from foreign pathogens and attacks healthy cells. There is typically no single diagnostic test, and many share similar symptoms, making detection and diagnosis potentially difficult, according to Johns Hopkins Medicine.
A small study published in the journal Science Translational Medicine found that, among patients who failed to regain their sense of smell long after their initial infection, there was inflammation in the nose tissue where smell nerve cells are found, even though no detectable virus remained. Fewer olfactory sensory neurons were seen, as well – findings that researchers said resembled some kind of “autoimmune-like process.”
Meanwhile, scientists in Canada found signs of autoimmunity in blood samples taken from patients who still had fatigue and shortness of breath after their initial COVID-19 infection. Two specific proteins were present a year after infection in up to 30% of patients, many of whom still had shortness of breath and fatigue, the researchers reported in the Jan. 1 issue of the European Respiratory Journal. These patients had been healthy and had no autoimmune condition or other diseases before they were infected.
Immune system problems
A number of studies have suggested that a problematic immune response could also explain why symptoms persist for some people.
Researchers in France, for example, found that the immune response problems in those with severe COVID-19 infections caused exaggerated or uncontrolled formation of a type of bug-fighting defense mechanism called a neutrophil extracellular trap (NET), which in turn triggers harmful inflammation that can result in multiorgan damage. These traps are netlike structures made from fibers composed mostly of DNA strings that bind, or trap, pathogens.
Long COVID is not like an acute infectious disease, says Alexander Charney, MD, PhD, the lead principal investigator of the RECOVER adult cohort at Mount Sinai in New York, and an associate professor at Icahn School of Medicine at Mount Sinai. It is more similar to other complex chronic diseases that have taken decades to understand, such as heart disease, mental illness, and rheumatologic diseases, he says.
Biomarkers and blood clots
Scientists are homing in on biomarkers, or detectable and measurable traits – in this case, molecular indicators – that can make diagnosing long COVID easier and give better direction for treatment. These biomarkers are also key to helping sort out the complex biology of long COVID.
In one study, data from blood samples taken from hundreds of hospitalized COVID-19 patients suggests changes are happening at the molecular level during initial severe infections. These changes may be tied to the development of longer-term symptoms, according to the study by Dr. Charney and his team at Mount Sinai published in Nature Medicine
Blood clotting issues have also been detected in long COVID patients. At least one study found signs that long-COVID patients had higher levels of a type of auto-antibody linked to the abnormal formation of clots. Researchers suspect that tiny, persistent microclots – undetectable via regular pathology tests – may be cutting off oxygen flow to tissue by blocking capillaries – and could explain many of the post-COVID symptoms described by patients.
While enormous progress has been made toward understanding long COVID, the research is still considered early and faces many challenges, including varying criteria used to define the condition, the types and quality of data used, differences in how patients are defined and recruited, and the small size of many studies. Some research also appears to conflict with other studies. And while there are specialized tools for diagnosing some aspects of the condition, standard tests often don’t detect many of the signs seen in long-COVID patients. But given the urgency and global scale of the problem, experts say more funding and support should be prioritized.
“People are suffering now, and they want answers now. ... It’s not like with COVID, where the path towards a great and meaningful solution to this unbelievable problem was clear – we need a vaccine,” says Dr. Charney.
“It’s going to be a long haul to figure out what is going on.”
A version of this article originally appeared on WebMD.com.
COVID emergency orders ending: What’s next?
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
It’s the end of an era.
The orders spanned two presidencies. The Trump administration’s Health and Human Services Secretary Alex Azar issued a public health emergency in January 2020. Then-President Donald Trump declared the COVID-19 pandemic a national emergency 2 months later. Both emergency declarations – which remained in effect under President Joe Biden – are set to expire May 11.
Read on for an overview of how the end of the public health emergency will trigger multiple federal policy changes.
Changes that affect everyone
- There will be cost-sharing changes for COVID-19 vaccines, testing, and certain treatments. One hundred–percent coverage for COVID testing, including free at-home tests, will expire May 11.
- Telemedicine cannot be used to prescribe controlled substances after May 11, 2023.
- Enhanced federal funding will be phased down through Dec. 31, 2023. This extends the time states must receive federally matched funds for COVID-related services and products, through the Consolidated Appropriations Act of 2023. Otherwise, this would have expired June 30, 2023.
- Emergency use authorizations for COVID-19 treatments and vaccinations will not be affected and/or end on May 11.
Changes that affect people with private health insurance
- Many will likely see higher costs for COVID-19 tests, as free testing expires and cost-sharing begins in the coming months.
- COVID-19 vaccinations and boosters will continue to be covered until the federal government’s vaccination supply is depleted. If that happens, you will need an in-network provider.
- You will still have access to COVID-19 treatments – but that could change when the federal supply dwindles.
Changes that affect Medicare recipients
- Medicare telehealth flexibilities will be extended through Dec. 31, 2024, regardless of public health emergency status. This means people can access telehealth services from anywhere, not just rural areas; can use a smartphone for telehealth; and can access telehealth in their homes.
- Medicare cost-sharing for testing and treatments will expire May 11, except for oral antivirals.
Changes that affect Medicaid/CHIP recipients
- Medicaid and Children’s Health Insurance Program (CHIP) recipients will continue to receive approved vaccinations free of charge, but testing and treatment without cost-sharing will expire during the third quarter of 2024.
- The Medicaid continuous enrollment provision will be separated from the public health emergency, and continuous enrollment will end March 31, 2023.
Changes that affect uninsured people
- The uninsured will no longer have access to 100% coverage for these products and services (free COVID-19 treatments, vaccines, and testing).
Changes that affect health care providers
- There will be changes to how much providers get paid for diagnosing people with COVID-19, ending the enhanced Inpatient Prospective Payment System reimbursement rate, as of May 11, 2023.
- Health Insurance Portability and Accountability Act (HIPAA) potential penalty waivers will end. This allows providers to communicate with patients through telehealth on a smartphone, for example, without violating privacy laws and incurring penalties.
What the experts are saying
This news organization asked several health experts for their thoughts on ending the emergency health declarations for COVID, and what effects this could have. Many expressed concerns about the timing of the ending, saying that the move could limit access to COVID-related treatments. Others said the move was inevitable but raised concerns about federal guidance related to the decision.
Question: Do you agree with the timing of the end to the emergency order?
Answer: Robert Atmar, MD, professor of infectious diseases at Baylor College of Medicine in Houston: “A lead time to prepare and anticipate these consequences may ease the transition, compared to an abrupt declaration that ends the declaration.”
Answer: Georges C. Benjamin, MD, executive director of the American Public Health Association: “I think it’s time to do so. It has to be done in a great, thoughtful, and organized way because we’ve attached so many different things to this public health emergency. It’s going to take time for the system to adapt. [Centers for Disease Control and Prevention] data collection most likely will continue. People are used to reporting now. The CDC needs to give guidance to the states so that we’re clear about what we’re reporting, what we’re not. If we did that abruptly, it would just be a mess.”
Answer: Bruce Farber, MD, chief public health and epidemiology officer at Northwell Health in Manhasset, N.Y.: “I would have hoped to see it delayed.”
Answer: Steven Newmark, JD, chief legal officer and director of policy at the Global Healthy Living Foundation: “While we understand that an emergency cannot last forever, we hope that expanded services such as free vaccination, promotion of widespread vaccination, increased use of pharmacists to administer vaccines, telehealth availability and reimbursement, flexibility in work-from-home opportunities, and more continues. Access to equitable health care should never backtrack or be reduced.”
Q: What will the end of free COVID vaccinations and free testing mean?
A: Dr. Farber: “There will likely be a decrease in vaccinations and testing. The vaccination rates are very low to begin with, and this will likely lower it further.”
A: Dr. Atmar: “I think it will mean that fewer people will get tested and vaccinated,” which “could lead to increased transmission, although wastewater testing suggests that there is a lot of unrecognized infection already occurring.”
A: Dr. Benjamin: “That is a big concern. It means that for people, particularly for people who are uninsured and underinsured, we’ve got to make sure they have access to those. There’s a lot of discussion and debate about what the cost of those tests and vaccines will be, and it looks like the companies are going to impose very steep, increasing costs.”
Q: How will this affect higher-risk populations, like people with weakened immune systems?
A: Dr. Farber: “Without monoclonals [drugs to treat COVID] and free Paxlovid,” people with weakened immune systems “may be undertreated.”
A: Dr. Atmar: “The implications of ongoing widespread virus transmission are that immunocompromised individuals may be more likely to be exposed and infected and to suffer the consequences of such infection, including severe illness. However, to a certain degree, this may already be happening. We are still seeing about 500 deaths/day, primarily in persons at highest risk of severe disease.”
A: Dr. Benjamin: “People who have good insurance, can afford to get immunized, and have good relations with practitioners probably will continue to be covered. But lower-income individuals and people who really can’t afford to get tested or get immunized would likely become underimmunized and more infected.
“So even though the federal emergency declaration will go away, I’m hoping that the federal government will continue to encourage all of us to emphasize those populations at the highest risk – those with chronic disease and those who are immunocompromised.”
A: Mr. Newmark: “People who are immunocompromised by their chronic illness or the medicines they take to treat acute or chronic conditions remain at higher risk for COVID-19 and its serious complications. The administration needs to support continued development of effective treatments and updated vaccines to protect the individual and public health. We’re also concerned that increased health care services - such as vaccination or telehealth – may fall back to prepandemic levels while the burden of protection, such as masking, may fall to chronic disease patients alone, which adds to the burden of living with disease.”
Q: What effect will ending Medicaid expansion money have?
A: Dr. Benjamin: Anywhere from 16 to 20 million people are going to lose in coverage. I’m hoping that states will look at their experience over these last 2 years or so and come to the decision that there were improvements in healthier populations.
Q: Will this have any effect on how the public perceives the pandemic?
A: Dr. Farber: “It is likely to give the impression that COVID is gone, which clearly is not the case.”
A: Dr. Benjamin: “It’ll be another argument by some that the pandemic is over. People should think about this as kind of like a hurricane. A hurricane comes through and tragically tears up communities, and we have an emergency during that time. But then we have to go through a period of recovery. I’m hoping people will realize that even though the public health emergencies have gone away, that we still need to go through a period of transition ... and that means that they still need to protect themselves, get vaccinated, and wear a mask when appropriate.”
A: Dr. Atmar: “There needs to be messaging that while we are transitioning away from emergency management of COVID-19, it is still a significant public health concern.”
A version of this article originally appeared on WebMD.com.
Washington medical board charges doctor with spreading COVID misinformation
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Doctors and professional organizations are standing guard, hoping to protect patients from any harm that results from mistruths spread by colleagues.
Case in point: Several physicians and the American Board of Pathology filed complaints with Washington and Idaho medical boards alleging that Ryan Cole, MD, a board-certified pathologist who practices in Boise, Idaho, but who also holds a license in Washington, has spread antivaccine and pro-ivermectin statements on social media. Dr. Cole is one of the founders of America’s Frontline Doctors, a right-wing political organization. Dr. Cole did not respond to a request for comment.
Gary W. Procop, MD, CEO, American Board of Pathology, told this news organization that “as physicians and board-certified pathologists, we have a public trust, and we must be accountable to patients, society, and the profession. Misinformation can cause real harm to patients, which may include death. Misinformation diverts patients away from lifesaving vaccination and other preventive measures, promotes viral transmission, and recommends ineffective therapies that may be toxic instead of evidence-based medical care.”
Cavalcade of complaints
Several doctors also chimed in with formal complaints alleging that Cole is spreading unreliable information, according to a report from KTVB News. For example, a Boise doctor wrote in his complaint that Dr. Cole is “a major purveyor of misinformation” and called it “amazing” that the physician was continuing to publicly support debunked information about COVID-19 more than a year into the pandemic. The doctor also stated, “Cole is a health menace, abusing his status as a physician to mislead the public.”
As a result of such complaints, the Washington medical board has charged Cole with COVID-19–related violations. It is unclear whether or not the Idaho medical board will sanction the doctor. At least 12 medical boards have sanctioned doctors for similar violations since the start of the pandemic.
The statement of charges from the Washington medical board contends that since March 2021, Dr. Cole has made numerous misleading statements regarding the COVID-19 pandemic, vaccines, the use of ivermectin to treat COVID-19, and the effectiveness of masks.
In addition, the statement alleges that Dr. Cole treated several COVID-19 patients via telemedicine. During these sessions, he prescribed ivermectin, an antiparasite drug that has not been found to have any effectiveness in treating, curing, or preventing COVID-19. One of the patients died after receiving this treatment, according to the complaint.
Citing a study published in the New England Journal of Medicine, Dr. Procop pointed out that use of ivermectin, which is not approved by the U.S. Food and Drug Administration to treat COVID-19, is particularly troubling.
“There is a concern whenever an ineffective treatment is prescribed when more effective and scientifically proven therapies are available. Therapeutics have potential side effects, and toxicities have been associated with the use of ivermectin,” Dr. Procop said. “The benefits of therapy should always outweigh the risks of treatment.”
If the Washington medical board finds that Dr. Cole has engaged in unprofessional conduct, possible sanctions include revocation or suspension of his license. Washington state law also provides for a range of other possible sanctions, including restriction or limitation of his practice, requiring that he complete a specific program of remedial education or treatment, monitoring of his practice, censure or reprimand, probation, a fine of up to $5,000 for each violation, or refunding fees that his practice has billed to and collected from patients. Dr. Cole had until January 30 to respond to the medical board’s statement.
“The American Board of Pathology supports the actions of the Washington State Medical Board regarding their inquiries into any physician that holds license in their state who makes false and misleading medical claims, or provides medical care beyond their scope of practice, as indicated by their training,” Dr. Procop said.
Law in limbo
While medical boards are seeking to sanction professionals who spread falsehoods, the pause button has been hit on the California law that allows regulators to punish doctors for spreading false information about COVID-19 vaccinations and treatments.
The law went into effect Jan. 1 but was temporarily halted when U.S. District Judge William B. Shubb of the Eastern District of California granted a preliminary injunction against the law on Jan. 25, according to a report in the Sacramento Bee.
Mr. Shubb said the measure’s definition of “misinformation” was “unconstitutionally vague” under the due process clause of the 14th Amendment. He also criticized the law’s definition of “misinformation” as being “grammatically incoherent.”
A version of this article first appeared on Medscape.com.
Pandemic pregnancy-linked deaths up 35% from 2019
Pregnancy-associated deaths, including drug-related deaths and homicide, were up 35% in 2020, compared with prepandemic 2019, new research indicates.
The data also show a 7.1% decrease in pregnancy-related suicides in 2020 from 2019.
The study, led by Claire E. Margerison, PhD, with the department of epidemiology and biostatistics at Michigan State University, East Lansing, included 4,528 pregnancy-associated deaths. The rate of deaths per 100,000 live births from April to December 2020 was 66.9 (95% confidence interval, 63.9-70.1). The comparative rate from April to December 2019 was 49.6. Researchers looked at that time period because the pandemic started in March 2020.
The findings were published online in JAMA Open Network.
Drug-related deaths up 55.3%
During the study period, drug deaths increased 55.3% and deaths from homicide increased 41.2%. Deaths from obstetric and other causes (mainly vehicle crashes) increased 28.4% and 56.7%, respectively, according to Dr. Margerison's group.
“Although pregnancy-associated deaths increased over time, increases from 2019 to 2020 were substantially larger than increases from 2018 to 2019,” the authors wrote.
The findings align with deaths in the general population in that time frame, they added.
Another study – this one looking at all-cause and cause-specific mortality from 2019 to 2020 in recently pregnant women, also published in JAMA Network Open, found significant racial and ethnic disparities in rates and cause of death.
According to the study, “Compared with non-Hispanic White women, mortality rates were three- to fivefold higher among American Indian or Alaska Native women for every cause, including suicide. Likewise, these findings suggest that non-Hispanic Black women experienced significantly higher mortality rates across causes, with the highest rates for homicide.”
Dr. Margerison and colleagues did not try to answer what caused the increases but pointed to the fentanyl epidemic, the murder of George Floyd, and COVID-19–related economic strain as potential stressors. They also suggest fewer screenings during the pandemic may have played a role.
Prevention opportunities missed
“Although pregnancy is considered an opportunity for screening and prevention related to physical, mental, and behavioral health, our data suggest that such opportunities were missed for hundreds of pregnant people during the pandemic,” the authors wrote.
Researchers analyzed cross-sectional U.S. death certificates from Jan. 1, 2018, to Dec. 31, 2020, for female U.S. residents ages 15-44 years. They then obtained the count for live births for the same population and time frame from the Centers for Disease Control and Prevention WONDER database.
They were able to identify pregnancy-associated deaths as the 2003 Revised Death Certificate contains a standardized pregnancy checkbox that asks whether the person was pregnant at the time of death, within 42 days of death, or within 43 days to 1 year of death.
Researchers also included deaths with ICD-10 codes linked with death from obstetric causes.
Deaths from overdose, suicide, and homicide are making up large and growing proportions of all deaths during pregnancy and in the first year postpartum, the authors report.
Dr. Margerison and coauthors, in research published in 2022, reported that these causes account for more than one-fifth of all pregnancy-related deaths. They also reported that drug-related deaths and homicides in this population have increased over the past 10 years.
“Substantial racial and ethnic inequities in these deaths exist,” they wrote in that paper.
The authors concluded in the current research: “Our study findings suggest that there is a need for prevention and intervention efforts, including harm-reduction strategies, tailored to pregnant and postpartum women, particularly during times of population stress and decreased utilization of preventive care, such as a pandemic.”
Dr. Margerison and coauthors reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor received personal fees from the World Health Organization and Population Reference Bureau outside the submitted work. One coauthor reported receiving grant support from the National Institutes of Mental Health during the study.
*This story was updated on 2/1.
Pregnancy-associated deaths, including drug-related deaths and homicide, were up 35% in 2020, compared with prepandemic 2019, new research indicates.
The data also show a 7.1% decrease in pregnancy-related suicides in 2020 from 2019.
The study, led by Claire E. Margerison, PhD, with the department of epidemiology and biostatistics at Michigan State University, East Lansing, included 4,528 pregnancy-associated deaths. The rate of deaths per 100,000 live births from April to December 2020 was 66.9 (95% confidence interval, 63.9-70.1). The comparative rate from April to December 2019 was 49.6. Researchers looked at that time period because the pandemic started in March 2020.
The findings were published online in JAMA Open Network.
Drug-related deaths up 55.3%
During the study period, drug deaths increased 55.3% and deaths from homicide increased 41.2%. Deaths from obstetric and other causes (mainly vehicle crashes) increased 28.4% and 56.7%, respectively, according to Dr. Margerison's group.
“Although pregnancy-associated deaths increased over time, increases from 2019 to 2020 were substantially larger than increases from 2018 to 2019,” the authors wrote.
The findings align with deaths in the general population in that time frame, they added.
Another study – this one looking at all-cause and cause-specific mortality from 2019 to 2020 in recently pregnant women, also published in JAMA Network Open, found significant racial and ethnic disparities in rates and cause of death.
According to the study, “Compared with non-Hispanic White women, mortality rates were three- to fivefold higher among American Indian or Alaska Native women for every cause, including suicide. Likewise, these findings suggest that non-Hispanic Black women experienced significantly higher mortality rates across causes, with the highest rates for homicide.”
Dr. Margerison and colleagues did not try to answer what caused the increases but pointed to the fentanyl epidemic, the murder of George Floyd, and COVID-19–related economic strain as potential stressors. They also suggest fewer screenings during the pandemic may have played a role.
Prevention opportunities missed
“Although pregnancy is considered an opportunity for screening and prevention related to physical, mental, and behavioral health, our data suggest that such opportunities were missed for hundreds of pregnant people during the pandemic,” the authors wrote.
Researchers analyzed cross-sectional U.S. death certificates from Jan. 1, 2018, to Dec. 31, 2020, for female U.S. residents ages 15-44 years. They then obtained the count for live births for the same population and time frame from the Centers for Disease Control and Prevention WONDER database.
They were able to identify pregnancy-associated deaths as the 2003 Revised Death Certificate contains a standardized pregnancy checkbox that asks whether the person was pregnant at the time of death, within 42 days of death, or within 43 days to 1 year of death.
Researchers also included deaths with ICD-10 codes linked with death from obstetric causes.
Deaths from overdose, suicide, and homicide are making up large and growing proportions of all deaths during pregnancy and in the first year postpartum, the authors report.
Dr. Margerison and coauthors, in research published in 2022, reported that these causes account for more than one-fifth of all pregnancy-related deaths. They also reported that drug-related deaths and homicides in this population have increased over the past 10 years.
“Substantial racial and ethnic inequities in these deaths exist,” they wrote in that paper.
The authors concluded in the current research: “Our study findings suggest that there is a need for prevention and intervention efforts, including harm-reduction strategies, tailored to pregnant and postpartum women, particularly during times of population stress and decreased utilization of preventive care, such as a pandemic.”
Dr. Margerison and coauthors reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor received personal fees from the World Health Organization and Population Reference Bureau outside the submitted work. One coauthor reported receiving grant support from the National Institutes of Mental Health during the study.
*This story was updated on 2/1.
Pregnancy-associated deaths, including drug-related deaths and homicide, were up 35% in 2020, compared with prepandemic 2019, new research indicates.
The data also show a 7.1% decrease in pregnancy-related suicides in 2020 from 2019.
The study, led by Claire E. Margerison, PhD, with the department of epidemiology and biostatistics at Michigan State University, East Lansing, included 4,528 pregnancy-associated deaths. The rate of deaths per 100,000 live births from April to December 2020 was 66.9 (95% confidence interval, 63.9-70.1). The comparative rate from April to December 2019 was 49.6. Researchers looked at that time period because the pandemic started in March 2020.
The findings were published online in JAMA Open Network.
Drug-related deaths up 55.3%
During the study period, drug deaths increased 55.3% and deaths from homicide increased 41.2%. Deaths from obstetric and other causes (mainly vehicle crashes) increased 28.4% and 56.7%, respectively, according to Dr. Margerison's group.
“Although pregnancy-associated deaths increased over time, increases from 2019 to 2020 were substantially larger than increases from 2018 to 2019,” the authors wrote.
The findings align with deaths in the general population in that time frame, they added.
Another study – this one looking at all-cause and cause-specific mortality from 2019 to 2020 in recently pregnant women, also published in JAMA Network Open, found significant racial and ethnic disparities in rates and cause of death.
According to the study, “Compared with non-Hispanic White women, mortality rates were three- to fivefold higher among American Indian or Alaska Native women for every cause, including suicide. Likewise, these findings suggest that non-Hispanic Black women experienced significantly higher mortality rates across causes, with the highest rates for homicide.”
Dr. Margerison and colleagues did not try to answer what caused the increases but pointed to the fentanyl epidemic, the murder of George Floyd, and COVID-19–related economic strain as potential stressors. They also suggest fewer screenings during the pandemic may have played a role.
Prevention opportunities missed
“Although pregnancy is considered an opportunity for screening and prevention related to physical, mental, and behavioral health, our data suggest that such opportunities were missed for hundreds of pregnant people during the pandemic,” the authors wrote.
Researchers analyzed cross-sectional U.S. death certificates from Jan. 1, 2018, to Dec. 31, 2020, for female U.S. residents ages 15-44 years. They then obtained the count for live births for the same population and time frame from the Centers for Disease Control and Prevention WONDER database.
They were able to identify pregnancy-associated deaths as the 2003 Revised Death Certificate contains a standardized pregnancy checkbox that asks whether the person was pregnant at the time of death, within 42 days of death, or within 43 days to 1 year of death.
Researchers also included deaths with ICD-10 codes linked with death from obstetric causes.
Deaths from overdose, suicide, and homicide are making up large and growing proportions of all deaths during pregnancy and in the first year postpartum, the authors report.
Dr. Margerison and coauthors, in research published in 2022, reported that these causes account for more than one-fifth of all pregnancy-related deaths. They also reported that drug-related deaths and homicides in this population have increased over the past 10 years.
“Substantial racial and ethnic inequities in these deaths exist,” they wrote in that paper.
The authors concluded in the current research: “Our study findings suggest that there is a need for prevention and intervention efforts, including harm-reduction strategies, tailored to pregnant and postpartum women, particularly during times of population stress and decreased utilization of preventive care, such as a pandemic.”
Dr. Margerison and coauthors reported receiving grant support from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor received personal fees from the World Health Organization and Population Reference Bureau outside the submitted work. One coauthor reported receiving grant support from the National Institutes of Mental Health during the study.
*This story was updated on 2/1.
FROM JAMA NETWORK OPEN
Children and COVID: Weekly cases may have doubled in early January
Although new COVID-19 cases in children, as measured by the American Academy of Pediatrics and the Children’s Hospital Association, have remained fairly steady in recent months, data from the Centers for Diseases Control and Prevention suggest that weekly cases took a big jump in early January.
For the most recent week covered . New cases for the first 2 weeks of the year – 31,000 for the week of Dec. 30 to Jan. 5 and 26,000 during Jan. 6-12 – were consistent with the AAP/CHA assertion that “weekly reported child cases have plateaued at an average of about 32,000 cases ... over the past 4 months.”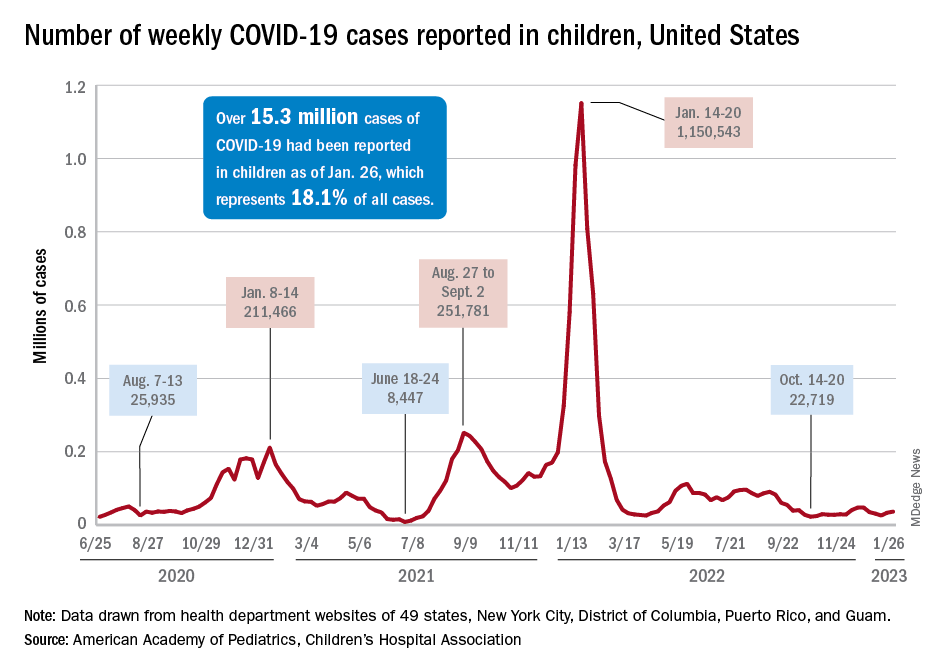
The CDC data, however, show that new cases doubled during the week of Jan. 1-7 to over 65,000, compared with the end of December, and stayed at that level for Jan. 8-14, and since CDC figures are subject to a 6-week reporting delay, the final numbers are likely to be even higher. The composition by age changed somewhat between the 2 weeks, though, as those aged 0-4 years went from almost half of all cases in the first week down to 40% in the second, while cases rose for children aged 5-11 and 12-15, based on data from the COVID-19 response team.
Emergency department visits for January do not show a corresponding increase. ED visits among children aged 0-11 years with COVID-19, measured as a percentage of all ED visits, declined over the course of the month, as did visits for 16- and 17-year-olds, while those aged 12-15 started the month at 1.4% and were at 1.4% on Jan. 27, with a slight dip down to 1.2% in between, the CDC said on its COVID Data Tracker. Daily hospitalizations for children aged 0-17 also declined through mid-January and did not reflect the jump in new cases.
Meanwhile, vaccinated children are still in the minority: 57% of those under age 18 have received no COVID vaccine yet, the AAP said in a separate report. Just 7.4% of children under age 2 years had received at least one dose as of Jan. 25, as had 10.1% of those aged 2-4 years, 39.6% of 5- to 11-year-olds and 71.8% of those 12-17 years old, according to the CDC, with corresponding figures for completion of the primary series at 3.5%, 5.3%, 32.5%, and 61.5%.
Although new COVID-19 cases in children, as measured by the American Academy of Pediatrics and the Children’s Hospital Association, have remained fairly steady in recent months, data from the Centers for Diseases Control and Prevention suggest that weekly cases took a big jump in early January.
For the most recent week covered . New cases for the first 2 weeks of the year – 31,000 for the week of Dec. 30 to Jan. 5 and 26,000 during Jan. 6-12 – were consistent with the AAP/CHA assertion that “weekly reported child cases have plateaued at an average of about 32,000 cases ... over the past 4 months.”
The CDC data, however, show that new cases doubled during the week of Jan. 1-7 to over 65,000, compared with the end of December, and stayed at that level for Jan. 8-14, and since CDC figures are subject to a 6-week reporting delay, the final numbers are likely to be even higher. The composition by age changed somewhat between the 2 weeks, though, as those aged 0-4 years went from almost half of all cases in the first week down to 40% in the second, while cases rose for children aged 5-11 and 12-15, based on data from the COVID-19 response team.
Emergency department visits for January do not show a corresponding increase. ED visits among children aged 0-11 years with COVID-19, measured as a percentage of all ED visits, declined over the course of the month, as did visits for 16- and 17-year-olds, while those aged 12-15 started the month at 1.4% and were at 1.4% on Jan. 27, with a slight dip down to 1.2% in between, the CDC said on its COVID Data Tracker. Daily hospitalizations for children aged 0-17 also declined through mid-January and did not reflect the jump in new cases.
Meanwhile, vaccinated children are still in the minority: 57% of those under age 18 have received no COVID vaccine yet, the AAP said in a separate report. Just 7.4% of children under age 2 years had received at least one dose as of Jan. 25, as had 10.1% of those aged 2-4 years, 39.6% of 5- to 11-year-olds and 71.8% of those 12-17 years old, according to the CDC, with corresponding figures for completion of the primary series at 3.5%, 5.3%, 32.5%, and 61.5%.
Although new COVID-19 cases in children, as measured by the American Academy of Pediatrics and the Children’s Hospital Association, have remained fairly steady in recent months, data from the Centers for Diseases Control and Prevention suggest that weekly cases took a big jump in early January.
For the most recent week covered . New cases for the first 2 weeks of the year – 31,000 for the week of Dec. 30 to Jan. 5 and 26,000 during Jan. 6-12 – were consistent with the AAP/CHA assertion that “weekly reported child cases have plateaued at an average of about 32,000 cases ... over the past 4 months.”
The CDC data, however, show that new cases doubled during the week of Jan. 1-7 to over 65,000, compared with the end of December, and stayed at that level for Jan. 8-14, and since CDC figures are subject to a 6-week reporting delay, the final numbers are likely to be even higher. The composition by age changed somewhat between the 2 weeks, though, as those aged 0-4 years went from almost half of all cases in the first week down to 40% in the second, while cases rose for children aged 5-11 and 12-15, based on data from the COVID-19 response team.
Emergency department visits for January do not show a corresponding increase. ED visits among children aged 0-11 years with COVID-19, measured as a percentage of all ED visits, declined over the course of the month, as did visits for 16- and 17-year-olds, while those aged 12-15 started the month at 1.4% and were at 1.4% on Jan. 27, with a slight dip down to 1.2% in between, the CDC said on its COVID Data Tracker. Daily hospitalizations for children aged 0-17 also declined through mid-January and did not reflect the jump in new cases.
Meanwhile, vaccinated children are still in the minority: 57% of those under age 18 have received no COVID vaccine yet, the AAP said in a separate report. Just 7.4% of children under age 2 years had received at least one dose as of Jan. 25, as had 10.1% of those aged 2-4 years, 39.6% of 5- to 11-year-olds and 71.8% of those 12-17 years old, according to the CDC, with corresponding figures for completion of the primary series at 3.5%, 5.3%, 32.5%, and 61.5%.
Managing respiratory symptoms in the ‘tripledemic’ era
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Is it COVID-19, flu, or even RSV? I recently described just such a patient, an obese woman with type 2 diabetes, presenting with fever, cough, myalgia, and fatigue. I asked readers whether they agreed with my management of this patient.
Thank you for your comments as we continue to react to high rates of URIs. Your comments highlight the importance of local resources and practice habits when managing patients with URI.
It was clear that readers value testing to distinguish between infections. However, access to testing is highly variable around the world and is likely to be routinely used only in high-income countries. The Kaiser Family Foundation performed a cost analysis of testing for SARS-CoV-2 in 2020 and found, not surprisingly, wide variability in the cost of testing. Medicare covers tests at rates of $36-$143 per test; a study of list prices for SARS-CoV-2 tests at 93 hospitals found a median cost of $148 per test. And this does not include collection or facility fees. About 20% of tests cost more than $300.
These costs are prohibitive for many health systems. However, more devices have been introduced since that analysis, and competition and evolving technology should drive down prices. Generally, multiplex polymerase chain reaction (PCR) testing for multiple pathogens is less expensive than ordering two or three separate molecular tests and is more convenient for patients and practices alike.
Other reader comments focused on the challenges of getting accurate data on viral epidemiology, and there is certainly a time lag between infection trends and public health reports. This is exacerbated by underreporting of symptoms and more testing at home using antigen tests.
But please do not give up on epidemiology! If a test such as PCR is 90% sensitive for identifying infection, the yield in terms of the number of individuals infected with a particular virus should be high, and that is true when infection is in broad circulation. If 20% of a population of 1,000 has an infection and the test sensitivity is 90%, the yield of testing is 180 true cases versus 20 false positives.
However, if just 2% of the population of 1,000 has the infection in this same scenario, then only 18 true cases are identified. The effect on public health is certainly less, and a lower prevalence rate means that confounding variables, such as how long an individual might shed viral particles and the method of sample collection, have an outsized effect on results. This reduces the validity of diagnostic tests.
Even trends on a national level can provide some insight regarding whom to test. Traditionally, our practice has been to not routinely test patients for influenza or RSV from late spring to early fall unless there was a compelling reason, such as recent travel to an area where these infections were more prevalent. The loss of temporality for these infections since 2020 has altered this approach and made us pay more attention to reports from public health organizations.
I also appreciate the discussion of how to treat Agnes’s symptoms as she waits to improve, and anyone who suffers with or treats a viral URI knows that there are few interventions effective for such symptoms as cough and congestion. A systematic review of 29 randomized controlled trials of over-the-counter medications for cough yielded mixed and largely negative results.
Antihistamines alone do not seem to work, and guaifenesin was successful in only one of three trials. Combinations of different drug classes appeared to be slightly more effective.
My personal favorite for the management of acute cough is something that kids generally love: honey. In a review of 14 studies, 9 of which were limited to pediatric patients, honey was associated with significant reductions in cough frequency, cough severity, and total symptom score. However, there was a moderate risk of bias in the included research, and evidence of honey’s benefit in placebo-controlled trials was limited. Honey used in this research came in a variety of forms, so the best dosage is uncertain.
Clearly, advancements are needed. Better symptom management in viral URI will almost certainly improve productivity across the population and will probably reduce the inappropriate use of antibiotics as well. I have said for years that the scientists who can solve the Gordian knot of pediatric mucus deserve three Nobel prizes. I look forward to that golden day.
Dr. Vega is a clinical professor of family medicine at the University of California, Irvine. He reported a conflict of interest with McNeil Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Biden to end COVID emergencies in May
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.
Doing so will have many effects, including the end of free vaccines and health services to fight the pandemic. The public health emergency has been renewed every 90 days since it was declared by the Trump administration in January 2020.
The declaration allowed major changes throughout the health care system to deal with the pandemic, including the free distribution of vaccines, testing, and treatments. In addition, telehealth services were expanded, and Medicaid and the Children’s Health Insurance Program were extended to millions more Americans.
Biden said the COVID-19 national emergency is set to expire March 1 while the declared public health emergency would currently expire on April 11. The president said both will be extended to end May 11.
There were nearly 300,000 newly reported COVID-19 cases in the United States for the week ending Jan. 25, according to CDC data, as well as more than 3,750 deaths.
A version of this article first appeared on WebMD.com.

