User login
In Case You Missed It: COVID
Many young kids with COVID may show no symptoms
BY WILL PASS
Just 14% of adults who tested positive for SARS-CoV-2 were asymptomatic, versus 37% of children aged 0-4 years, in the paper. This raises concern that parents, childcare providers, and preschools may be underestimating infection in seemingly healthy young kids who have been exposed to COVID, wrote lead author Ruth A. Karron, MD, and colleagues in JAMA Network Open.
Methods
The new research involved 690 individuals from 175 households in Maryland who were monitored closely between November 2020 and October 2021. Every week for 8 months, participants completed online symptom checks and underwent PCR testing using nasal swabs, with symptomatic individuals submitting additional swabs for analysis.
“What was different about our study [compared with previous studies] was the intensity of our collection, and the fact that we collected specimens from asymptomatic people,” said Dr. Karron, a pediatrician and professor in the department of international health, Johns Hopkins University, Baltimore, in an interview. “You shed more virus earlier in the infection than later, and the fact that we were sampling every single week meant that we could pick up those early infections.”
The study also stands out for its focus on young children, Dr. Karron said. Enrollment required all households to have at least one child aged 0-4 years, so 256 out of 690 participants (37.1%) were in this youngest age group. The remainder of the population consisted of 100 older children aged 5-17 years (14.5%) and 334 adults aged 18-74 years (48.4%).
Children 4 and under more than twice as likely to be asymptomatic
By the end of the study, 51 participants had tested positive for SARS-CoV-2, among whom 14 had no symptoms. A closer look showed that children 0-4 years of age who contracted COVID were more than twice as likely to be asymptomatic as infected adults (36.8% vs. 14.3%).
The relationship between symptoms and viral load also differed between adults and young children.
While adults with high viral loads – suggesting greater contagiousness – typically had more severe COVID symptoms, no correlation was found in young kids, meaning children with mild or no symptoms could still be highly contagious.
Dr. Karron said these findings should help parents and other stakeholders make better-informed decisions based on known risks. She recommended testing young, asymptomatic children for COVID if they have been exposed to infected individuals, then acting accordingly based on the results.
“If a family is infected with the virus, and the 2-year-old is asymptomatic, and people are thinking about a visit to elderly grandparents who may be frail, one shouldn’t assume that the 2-year-old is uninfected,” Dr. Karron said. “That child should be tested along with other family members.”
Testing should also be considered for young children exposed to COVID at childcare facilities, she added.
But not every expert consulted for this piece shared these opinions of Dr. Karron.
“I question whether that effort is worth it,” said Dean Blumberg, MD, professor and chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, Calif.
He noted that recent Food and Drug Administration guidance for COVID testing calls for three negative at-home antigen tests to confirm lack of infection.
“That would take 4 days to get those tests done,” he said. “So, it’s a lot of testing. It’s a lot of record keeping, it’s inconvenient, it’s uncomfortable to be tested, and I just question whether it’s worth that effort.”
Applicability of findings to today questioned
Dr. Blumberg also questioned whether the study, which was completed almost a year ago, reflects the current pandemic landscape.
“At the time this study was done, it was predominantly Delta [variant instead of Omicron],” Dr. Blumberg said. “The other issue [with the study] is that … most of the children didn’t have preexisting immunity, so you have to take that into account.”
Preexisting immunity – whether from exposure or vaccination – could lower viral loads, so asymptomatic children today really could be less contagious than they were when the study was done, according to Dr. Blumberg. Kids without symptoms are also less likely to spread the virus, because they aren’t coughing or sneezing, he added.
Sara R. Kim, MD, and Janet A. Englund, MD, of the Seattle Children’s Research Institute, University of Washington, said it’s challenging to know how applicable the findings are, although they sided more with the investigators than Dr. Blumberg.
“Given the higher rate of transmissibility and infectivity of the Omicron variant, it is difficult to make direct associations between findings reported during this study period and those present in the current era during which the Omicron variant is circulating,” they wrote in an accompanying editorial. “However, the higher rates of asymptomatic infection observed among children in this study are likely to be consistent with those observed for current and future viral variants.”
Although the experts offered different interpretations of the findings, they shared similar perspectives on vaccination.
“The most important thing that parents can do is get their kids vaccinated, be vaccinated themselves, and have everybody in the household vaccinated and up to date for all doses that are indicated,” Dr. Blumberg said.
Dr. Karron noted that vaccination will be increasingly important in the coming months.
“Summer is ending; school is starting,” she said. “We’re going to be in large groups indoors again very soon. To keep young children safe, I think it’s really important for them to get vaccinated.”
The study was funded by the CDC. The investigators disclosed no other relationships. Dr. Englund disclosed relationships with AstraZeneca, GlaxoSmithKline, Merck, and others. Dr. Kim and Dr. Blumberg disclosed no relevant conflicts of interest.
BY WILL PASS
Just 14% of adults who tested positive for SARS-CoV-2 were asymptomatic, versus 37% of children aged 0-4 years, in the paper. This raises concern that parents, childcare providers, and preschools may be underestimating infection in seemingly healthy young kids who have been exposed to COVID, wrote lead author Ruth A. Karron, MD, and colleagues in JAMA Network Open.
Methods
The new research involved 690 individuals from 175 households in Maryland who were monitored closely between November 2020 and October 2021. Every week for 8 months, participants completed online symptom checks and underwent PCR testing using nasal swabs, with symptomatic individuals submitting additional swabs for analysis.
“What was different about our study [compared with previous studies] was the intensity of our collection, and the fact that we collected specimens from asymptomatic people,” said Dr. Karron, a pediatrician and professor in the department of international health, Johns Hopkins University, Baltimore, in an interview. “You shed more virus earlier in the infection than later, and the fact that we were sampling every single week meant that we could pick up those early infections.”
The study also stands out for its focus on young children, Dr. Karron said. Enrollment required all households to have at least one child aged 0-4 years, so 256 out of 690 participants (37.1%) were in this youngest age group. The remainder of the population consisted of 100 older children aged 5-17 years (14.5%) and 334 adults aged 18-74 years (48.4%).
Children 4 and under more than twice as likely to be asymptomatic
By the end of the study, 51 participants had tested positive for SARS-CoV-2, among whom 14 had no symptoms. A closer look showed that children 0-4 years of age who contracted COVID were more than twice as likely to be asymptomatic as infected adults (36.8% vs. 14.3%).
The relationship between symptoms and viral load also differed between adults and young children.
While adults with high viral loads – suggesting greater contagiousness – typically had more severe COVID symptoms, no correlation was found in young kids, meaning children with mild or no symptoms could still be highly contagious.
Dr. Karron said these findings should help parents and other stakeholders make better-informed decisions based on known risks. She recommended testing young, asymptomatic children for COVID if they have been exposed to infected individuals, then acting accordingly based on the results.
“If a family is infected with the virus, and the 2-year-old is asymptomatic, and people are thinking about a visit to elderly grandparents who may be frail, one shouldn’t assume that the 2-year-old is uninfected,” Dr. Karron said. “That child should be tested along with other family members.”
Testing should also be considered for young children exposed to COVID at childcare facilities, she added.
But not every expert consulted for this piece shared these opinions of Dr. Karron.
“I question whether that effort is worth it,” said Dean Blumberg, MD, professor and chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, Calif.
He noted that recent Food and Drug Administration guidance for COVID testing calls for three negative at-home antigen tests to confirm lack of infection.
“That would take 4 days to get those tests done,” he said. “So, it’s a lot of testing. It’s a lot of record keeping, it’s inconvenient, it’s uncomfortable to be tested, and I just question whether it’s worth that effort.”
Applicability of findings to today questioned
Dr. Blumberg also questioned whether the study, which was completed almost a year ago, reflects the current pandemic landscape.
“At the time this study was done, it was predominantly Delta [variant instead of Omicron],” Dr. Blumberg said. “The other issue [with the study] is that … most of the children didn’t have preexisting immunity, so you have to take that into account.”
Preexisting immunity – whether from exposure or vaccination – could lower viral loads, so asymptomatic children today really could be less contagious than they were when the study was done, according to Dr. Blumberg. Kids without symptoms are also less likely to spread the virus, because they aren’t coughing or sneezing, he added.
Sara R. Kim, MD, and Janet A. Englund, MD, of the Seattle Children’s Research Institute, University of Washington, said it’s challenging to know how applicable the findings are, although they sided more with the investigators than Dr. Blumberg.
“Given the higher rate of transmissibility and infectivity of the Omicron variant, it is difficult to make direct associations between findings reported during this study period and those present in the current era during which the Omicron variant is circulating,” they wrote in an accompanying editorial. “However, the higher rates of asymptomatic infection observed among children in this study are likely to be consistent with those observed for current and future viral variants.”
Although the experts offered different interpretations of the findings, they shared similar perspectives on vaccination.
“The most important thing that parents can do is get their kids vaccinated, be vaccinated themselves, and have everybody in the household vaccinated and up to date for all doses that are indicated,” Dr. Blumberg said.
Dr. Karron noted that vaccination will be increasingly important in the coming months.
“Summer is ending; school is starting,” she said. “We’re going to be in large groups indoors again very soon. To keep young children safe, I think it’s really important for them to get vaccinated.”
The study was funded by the CDC. The investigators disclosed no other relationships. Dr. Englund disclosed relationships with AstraZeneca, GlaxoSmithKline, Merck, and others. Dr. Kim and Dr. Blumberg disclosed no relevant conflicts of interest.
BY WILL PASS
Just 14% of adults who tested positive for SARS-CoV-2 were asymptomatic, versus 37% of children aged 0-4 years, in the paper. This raises concern that parents, childcare providers, and preschools may be underestimating infection in seemingly healthy young kids who have been exposed to COVID, wrote lead author Ruth A. Karron, MD, and colleagues in JAMA Network Open.
Methods
The new research involved 690 individuals from 175 households in Maryland who were monitored closely between November 2020 and October 2021. Every week for 8 months, participants completed online symptom checks and underwent PCR testing using nasal swabs, with symptomatic individuals submitting additional swabs for analysis.
“What was different about our study [compared with previous studies] was the intensity of our collection, and the fact that we collected specimens from asymptomatic people,” said Dr. Karron, a pediatrician and professor in the department of international health, Johns Hopkins University, Baltimore, in an interview. “You shed more virus earlier in the infection than later, and the fact that we were sampling every single week meant that we could pick up those early infections.”
The study also stands out for its focus on young children, Dr. Karron said. Enrollment required all households to have at least one child aged 0-4 years, so 256 out of 690 participants (37.1%) were in this youngest age group. The remainder of the population consisted of 100 older children aged 5-17 years (14.5%) and 334 adults aged 18-74 years (48.4%).
Children 4 and under more than twice as likely to be asymptomatic
By the end of the study, 51 participants had tested positive for SARS-CoV-2, among whom 14 had no symptoms. A closer look showed that children 0-4 years of age who contracted COVID were more than twice as likely to be asymptomatic as infected adults (36.8% vs. 14.3%).
The relationship between symptoms and viral load also differed between adults and young children.
While adults with high viral loads – suggesting greater contagiousness – typically had more severe COVID symptoms, no correlation was found in young kids, meaning children with mild or no symptoms could still be highly contagious.
Dr. Karron said these findings should help parents and other stakeholders make better-informed decisions based on known risks. She recommended testing young, asymptomatic children for COVID if they have been exposed to infected individuals, then acting accordingly based on the results.
“If a family is infected with the virus, and the 2-year-old is asymptomatic, and people are thinking about a visit to elderly grandparents who may be frail, one shouldn’t assume that the 2-year-old is uninfected,” Dr. Karron said. “That child should be tested along with other family members.”
Testing should also be considered for young children exposed to COVID at childcare facilities, she added.
But not every expert consulted for this piece shared these opinions of Dr. Karron.
“I question whether that effort is worth it,” said Dean Blumberg, MD, professor and chief of the division of pediatric infectious diseases at UC Davis Health, Sacramento, Calif.
He noted that recent Food and Drug Administration guidance for COVID testing calls for three negative at-home antigen tests to confirm lack of infection.
“That would take 4 days to get those tests done,” he said. “So, it’s a lot of testing. It’s a lot of record keeping, it’s inconvenient, it’s uncomfortable to be tested, and I just question whether it’s worth that effort.”
Applicability of findings to today questioned
Dr. Blumberg also questioned whether the study, which was completed almost a year ago, reflects the current pandemic landscape.
“At the time this study was done, it was predominantly Delta [variant instead of Omicron],” Dr. Blumberg said. “The other issue [with the study] is that … most of the children didn’t have preexisting immunity, so you have to take that into account.”
Preexisting immunity – whether from exposure or vaccination – could lower viral loads, so asymptomatic children today really could be less contagious than they were when the study was done, according to Dr. Blumberg. Kids without symptoms are also less likely to spread the virus, because they aren’t coughing or sneezing, he added.
Sara R. Kim, MD, and Janet A. Englund, MD, of the Seattle Children’s Research Institute, University of Washington, said it’s challenging to know how applicable the findings are, although they sided more with the investigators than Dr. Blumberg.
“Given the higher rate of transmissibility and infectivity of the Omicron variant, it is difficult to make direct associations between findings reported during this study period and those present in the current era during which the Omicron variant is circulating,” they wrote in an accompanying editorial. “However, the higher rates of asymptomatic infection observed among children in this study are likely to be consistent with those observed for current and future viral variants.”
Although the experts offered different interpretations of the findings, they shared similar perspectives on vaccination.
“The most important thing that parents can do is get their kids vaccinated, be vaccinated themselves, and have everybody in the household vaccinated and up to date for all doses that are indicated,” Dr. Blumberg said.
Dr. Karron noted that vaccination will be increasingly important in the coming months.
“Summer is ending; school is starting,” she said. “We’re going to be in large groups indoors again very soon. To keep young children safe, I think it’s really important for them to get vaccinated.”
The study was funded by the CDC. The investigators disclosed no other relationships. Dr. Englund disclosed relationships with AstraZeneca, GlaxoSmithKline, Merck, and others. Dr. Kim and Dr. Blumberg disclosed no relevant conflicts of interest.
FROM JAMA NETWORK OPEN
FDA authorizes updated COVID boosters to target newest variants
The agency cited data to support the safety and efficacy of this next generation of mRNA vaccines targeted toward variants of concern.
The Pfizer EUA corresponds to the company’s combination booster shot that includes the original COVID-19 vaccine as well as a vaccine specifically designed to protect against the most recent Omicron variants, BA.4 and BA.5.
The Moderna combination vaccine will contain both the firm’s original COVID-19 vaccine and a vaccine to protect specifically against Omicron BA.4 and BA.5 subvariants.
As of Aug. 27, BA.4 and BA.4.6 account for about 11% of circulating variants and BA.5 accounts for almost all the remaining 89%, Centers for Disease Control and Prevention data show.
The next step will be review of the scientific data by the CDC’s Advisory Committee on Immunization Practices, which is set to meet Sept. 1 and 2. The final hurdle before distribution of the new vaccines will be sign-off on CDC recommendations for use by agency Director Rochelle Walensky, MD.
This is a developing story. A version of this article first appeared on WebMD.com.
The agency cited data to support the safety and efficacy of this next generation of mRNA vaccines targeted toward variants of concern.
The Pfizer EUA corresponds to the company’s combination booster shot that includes the original COVID-19 vaccine as well as a vaccine specifically designed to protect against the most recent Omicron variants, BA.4 and BA.5.
The Moderna combination vaccine will contain both the firm’s original COVID-19 vaccine and a vaccine to protect specifically against Omicron BA.4 and BA.5 subvariants.
As of Aug. 27, BA.4 and BA.4.6 account for about 11% of circulating variants and BA.5 accounts for almost all the remaining 89%, Centers for Disease Control and Prevention data show.
The next step will be review of the scientific data by the CDC’s Advisory Committee on Immunization Practices, which is set to meet Sept. 1 and 2. The final hurdle before distribution of the new vaccines will be sign-off on CDC recommendations for use by agency Director Rochelle Walensky, MD.
This is a developing story. A version of this article first appeared on WebMD.com.
The agency cited data to support the safety and efficacy of this next generation of mRNA vaccines targeted toward variants of concern.
The Pfizer EUA corresponds to the company’s combination booster shot that includes the original COVID-19 vaccine as well as a vaccine specifically designed to protect against the most recent Omicron variants, BA.4 and BA.5.
The Moderna combination vaccine will contain both the firm’s original COVID-19 vaccine and a vaccine to protect specifically against Omicron BA.4 and BA.5 subvariants.
As of Aug. 27, BA.4 and BA.4.6 account for about 11% of circulating variants and BA.5 accounts for almost all the remaining 89%, Centers for Disease Control and Prevention data show.
The next step will be review of the scientific data by the CDC’s Advisory Committee on Immunization Practices, which is set to meet Sept. 1 and 2. The final hurdle before distribution of the new vaccines will be sign-off on CDC recommendations for use by agency Director Rochelle Walensky, MD.
This is a developing story. A version of this article first appeared on WebMD.com.
Children and COVID: New cases increase; hospital admissions could follow
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.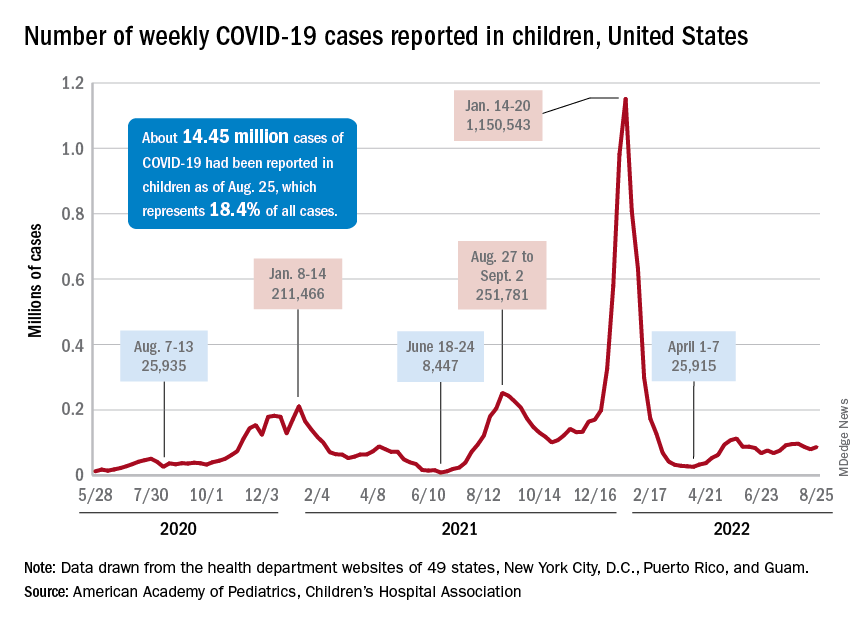
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.
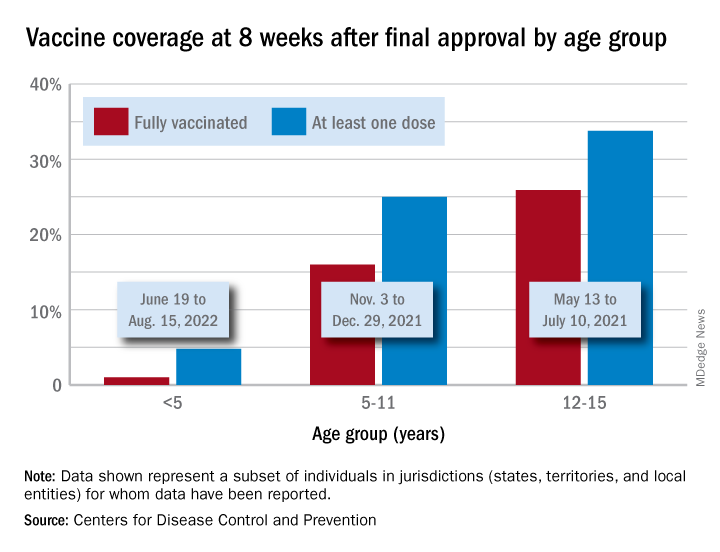
Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
New cases of COVID-19 in children were up again after 2 weeks of declines, and preliminary data suggest that hospitalizations may be on the rise as well.
, based on data collected by the American Academy of Pediatrics and the Children’s Hospital Association from state and territorial health departments.
A similar increase seems to be reflected by hospital-level data. The latest 7-day (Aug. 21-27) average is 305 new admissions with diagnosed COVID per day among children aged 0-17 years, compared with 290 per day for the week of Aug. 14-20, the Centers for Disease Control and Prevention reported, while also noting the potential for reporting delays in the most recent 7-day period.
Daily hospital admissions for COVID had been headed downward through the first half of August, falling from 0.46 per 100,000 population at the end of July to 0.40 on Aug. 19, the CDC said on its COVID Data Tracker. Since then, however, admissions have gone the other way, with the preliminary nature of the latest data suggesting that the numbers will be even higher as more hospitals report over the next few days.
Vaccine initiations continue to fall
Initiations among school-age children have fallen for 3 consecutive weeks since Aug. 3, when numbers receiving their first vaccinations reached late-summer highs for those aged 5-11 and 12-17 years. Children under age 5, included in the CDC data for the first time on Aug. 11 as separate groups – under 2 years and 2-4 years – have had vaccine initiations drop by 8.0% and 19.8% over the 2 following weeks, the CDC said.

Through their first 8 weeks of vaccine eligibility (June 19 to Aug. 15), 4.8% of children under 5 years of age had received a first vaccination and 1.0% were fully vaccinated. For the two other age groups (5-11 and 12-15) who became eligible after the very first emergency authorization back in 2020, the respective proportions were 25.0% and 16.0% (5-11) and 33.8% and 26.1% (12-15) through the first 8 weeks, according to CDC data.
Distorted time perception during the pandemic tied to stress, poor mental health
ranging from difficulty keeping track of the days of the week to feeling that the hours either crawled by or sped up, new research suggests.
Results showed the sense of present focus, blurring weekdays and weekends together, and uncertainly about the future were reported by over 65% of the 5,661 survey respondents. And more than half reported the experience of feeling “time speeding up or slowing down,” report the investigators, led by E. Alison Holman, PhD, professor at the University of California, Irvine.
Significant predictors of these time distortions included being exposed to daily pandemic-related media and having a mental health diagnosis prior to the pandemic; secondary stress such as school closures and lockdown; financial stress; lifetime stress; and lifetime trauma exposure.
“Continuity between past experiences, present life, and future hopes is critical to one’s well-being, and disruption of that synergy presents mental health challenges,” Dr. Holman said in a news release.
“We were able to measure this in a nationally representative sample of Americans as they were experiencing a protracted collective trauma, which has never been done before, and this study is the first to document the prevalence and early predictors of these time distortions,” added Dr. Holman.
The findings were published online in Psychological Trauma: Theory, Research, Practice, and Policy.
Unique opportunity
During the pandemic, many people’s time perspective (TP), defined as “our view of time as it spans from our past into the future,” shifted as they “focused on the immediate, present danger of the COVID-19 pandemic and future plans became uncertain,” the investigators wrote.
Studies of convenience samples “suggested that many people experienced time slowing down, stopping, and/or speeding up as they coped with the challenges of the pandemic” – a phenomenon known as temporal disintegration (TD) in psychiatric literature.
Dr. Holman said in an interview that she researched TD after the Sept.11, 2001 World Trade Center attacks.
“We found that people who experienced that early sense of TD, the sense of ‘time falling apart,’ were more prone to getting stuck in the past and staying focused on the past event,” which led to feeling “more distress over time,” she said.
Research examining the prevalence of and psychosocial factors predicting TD are “quite rare” and studies examining TD “during an unfolding, protracted collective trauma are even rarer,” the researchers note. The COVID pandemic “presented a unique opportunity to conduct such a study,” the researchers wrote.
For their study, the investigators surveyed participants in the NORC AmeriSpeak online panel, a “probability-based panel” of 35,000 U.S. households selected at random from across the country.
The study was conducted in two waves: the first survey was administered March–April 2020, the second in September–October 2020.
Speeding up, slowing down
At wave 2, participants completed a 7-item index of TD symptoms experienced over the previous 6 months. To adjust for psychological processes that may have predisposed individuals to experience TD during the pandemic, the researchers included a Wave 1 measure of future uncertainty as a covariate.
Prepandemic health data had been collected prior to the current study.
Wave 1 participants completed a checklist reporting personal, work, and community-wide exposure to the COVID outbreak, including contracting the virus, sheltering in place, and experiencing secondary stressors. The extent and type of pandemic-related media exposure were also assessed.
At wave 2, they reported the extent of exposure to the coronavirus, financial exposures, and secondary stressors. They also completed a non–COVID-related stress/trauma exposure checklist and were asked to indicate whether the trauma, disaster, or bereavement took place prior to or during the pandemic.
The final sample consisted of 5,661 adults (52% female) who completed the wave 2 survey. Participants were divided into four age groups: 18-34, 35-49, 50-64, and 65 and older.
The most common experiences (reported by more than 65% of respondents) included being focused on the present moment, feeling that weekdays and weekends were the same, and feeling uncertain about the future.
Over half of respondents (50.4%) reported feeling as though time was speeding up, and 55.2% reported feeling as though time was slowing down. Some also reported feeling uncertain about the time of day (46.4%) and forgetting events they had just experienced (35.2%).
When the researchers controlled for feeling uncertain about the future, they found that women reported more TD than men (b = 0.11; 95% confidence interval, 0.07-0.14; P < .001).
At wave 1, associations were found between TD and COVID-related media exposure, prepandemic mental health diagnoses, and prepandemic non–COVID-related stress and trauma. At wave 2, associations were found between TD and COVID-related secondary and financial stressors (P < .001 for all).
In contrast, COVID-related work exposure at wave 1, being 45-59 years old, and living in the Midwest region were negatively associated with TD.
“The sense of the flow of the past into the present, and the present into the future is important for our mental health,” Dr. Holman said. “We need to remember who we have been, how that shaped who we are today, and where we want to go with our lives.”
Staying in the present moment is “good, when you’re doing it mindfully. But you still need to feel you can shape and work toward the future and have some sense of control,” she added.
Dr. Homan also recommended time-perspective therapy, which helps patients with PTSD to “build continuity across time – to understand and learn from the past, live in the present, and move toward the future.”
Widespread distortion
In an interview, Ruth Ogden, PhD, a lecturer at Liverpool (England) John Moores University, said the findings “confirm those reported in Europe, South America, and the Middle East, that widespread distortion to time was common during the pandemic and that distortions to time were greatest amongst those most negatively affected by the pandemic.”
The results also support her own recent research in the United Kingdom “suggesting that distortions to time during the pandemic extend to our memory for the length of the pandemic, with most people believing that lockdowns lasted far longer than they actually did,” said Dr. Ogden, who was not involved with Dr. Holman and colleagues’ current study.
“This type of subjective lengthening of the pandemic may reinforce trauma by making the traumatic period seem longer, further damaging health and well-being,” she noted. “As the negative fallouts of the pandemic continue, it is important to establish the long-term effects of time distortions during the pandemic on mental health and well-being.”
The study was funded by U.S. National Science Foundation and the National Institute on Minority Health and Health Disparities. The investigators reported no relevant financial relationships. Dr. Ogden receives funding from the Wellcome Trust.
A version of this article first appeared on Medscape.com.
ranging from difficulty keeping track of the days of the week to feeling that the hours either crawled by or sped up, new research suggests.
Results showed the sense of present focus, blurring weekdays and weekends together, and uncertainly about the future were reported by over 65% of the 5,661 survey respondents. And more than half reported the experience of feeling “time speeding up or slowing down,” report the investigators, led by E. Alison Holman, PhD, professor at the University of California, Irvine.
Significant predictors of these time distortions included being exposed to daily pandemic-related media and having a mental health diagnosis prior to the pandemic; secondary stress such as school closures and lockdown; financial stress; lifetime stress; and lifetime trauma exposure.
“Continuity between past experiences, present life, and future hopes is critical to one’s well-being, and disruption of that synergy presents mental health challenges,” Dr. Holman said in a news release.
“We were able to measure this in a nationally representative sample of Americans as they were experiencing a protracted collective trauma, which has never been done before, and this study is the first to document the prevalence and early predictors of these time distortions,” added Dr. Holman.
The findings were published online in Psychological Trauma: Theory, Research, Practice, and Policy.
Unique opportunity
During the pandemic, many people’s time perspective (TP), defined as “our view of time as it spans from our past into the future,” shifted as they “focused on the immediate, present danger of the COVID-19 pandemic and future plans became uncertain,” the investigators wrote.
Studies of convenience samples “suggested that many people experienced time slowing down, stopping, and/or speeding up as they coped with the challenges of the pandemic” – a phenomenon known as temporal disintegration (TD) in psychiatric literature.
Dr. Holman said in an interview that she researched TD after the Sept.11, 2001 World Trade Center attacks.
“We found that people who experienced that early sense of TD, the sense of ‘time falling apart,’ were more prone to getting stuck in the past and staying focused on the past event,” which led to feeling “more distress over time,” she said.
Research examining the prevalence of and psychosocial factors predicting TD are “quite rare” and studies examining TD “during an unfolding, protracted collective trauma are even rarer,” the researchers note. The COVID pandemic “presented a unique opportunity to conduct such a study,” the researchers wrote.
For their study, the investigators surveyed participants in the NORC AmeriSpeak online panel, a “probability-based panel” of 35,000 U.S. households selected at random from across the country.
The study was conducted in two waves: the first survey was administered March–April 2020, the second in September–October 2020.
Speeding up, slowing down
At wave 2, participants completed a 7-item index of TD symptoms experienced over the previous 6 months. To adjust for psychological processes that may have predisposed individuals to experience TD during the pandemic, the researchers included a Wave 1 measure of future uncertainty as a covariate.
Prepandemic health data had been collected prior to the current study.
Wave 1 participants completed a checklist reporting personal, work, and community-wide exposure to the COVID outbreak, including contracting the virus, sheltering in place, and experiencing secondary stressors. The extent and type of pandemic-related media exposure were also assessed.
At wave 2, they reported the extent of exposure to the coronavirus, financial exposures, and secondary stressors. They also completed a non–COVID-related stress/trauma exposure checklist and were asked to indicate whether the trauma, disaster, or bereavement took place prior to or during the pandemic.
The final sample consisted of 5,661 adults (52% female) who completed the wave 2 survey. Participants were divided into four age groups: 18-34, 35-49, 50-64, and 65 and older.
The most common experiences (reported by more than 65% of respondents) included being focused on the present moment, feeling that weekdays and weekends were the same, and feeling uncertain about the future.
Over half of respondents (50.4%) reported feeling as though time was speeding up, and 55.2% reported feeling as though time was slowing down. Some also reported feeling uncertain about the time of day (46.4%) and forgetting events they had just experienced (35.2%).
When the researchers controlled for feeling uncertain about the future, they found that women reported more TD than men (b = 0.11; 95% confidence interval, 0.07-0.14; P < .001).
At wave 1, associations were found between TD and COVID-related media exposure, prepandemic mental health diagnoses, and prepandemic non–COVID-related stress and trauma. At wave 2, associations were found between TD and COVID-related secondary and financial stressors (P < .001 for all).
In contrast, COVID-related work exposure at wave 1, being 45-59 years old, and living in the Midwest region were negatively associated with TD.
“The sense of the flow of the past into the present, and the present into the future is important for our mental health,” Dr. Holman said. “We need to remember who we have been, how that shaped who we are today, and where we want to go with our lives.”
Staying in the present moment is “good, when you’re doing it mindfully. But you still need to feel you can shape and work toward the future and have some sense of control,” she added.
Dr. Homan also recommended time-perspective therapy, which helps patients with PTSD to “build continuity across time – to understand and learn from the past, live in the present, and move toward the future.”
Widespread distortion
In an interview, Ruth Ogden, PhD, a lecturer at Liverpool (England) John Moores University, said the findings “confirm those reported in Europe, South America, and the Middle East, that widespread distortion to time was common during the pandemic and that distortions to time were greatest amongst those most negatively affected by the pandemic.”
The results also support her own recent research in the United Kingdom “suggesting that distortions to time during the pandemic extend to our memory for the length of the pandemic, with most people believing that lockdowns lasted far longer than they actually did,” said Dr. Ogden, who was not involved with Dr. Holman and colleagues’ current study.
“This type of subjective lengthening of the pandemic may reinforce trauma by making the traumatic period seem longer, further damaging health and well-being,” she noted. “As the negative fallouts of the pandemic continue, it is important to establish the long-term effects of time distortions during the pandemic on mental health and well-being.”
The study was funded by U.S. National Science Foundation and the National Institute on Minority Health and Health Disparities. The investigators reported no relevant financial relationships. Dr. Ogden receives funding from the Wellcome Trust.
A version of this article first appeared on Medscape.com.
ranging from difficulty keeping track of the days of the week to feeling that the hours either crawled by or sped up, new research suggests.
Results showed the sense of present focus, blurring weekdays and weekends together, and uncertainly about the future were reported by over 65% of the 5,661 survey respondents. And more than half reported the experience of feeling “time speeding up or slowing down,” report the investigators, led by E. Alison Holman, PhD, professor at the University of California, Irvine.
Significant predictors of these time distortions included being exposed to daily pandemic-related media and having a mental health diagnosis prior to the pandemic; secondary stress such as school closures and lockdown; financial stress; lifetime stress; and lifetime trauma exposure.
“Continuity between past experiences, present life, and future hopes is critical to one’s well-being, and disruption of that synergy presents mental health challenges,” Dr. Holman said in a news release.
“We were able to measure this in a nationally representative sample of Americans as they were experiencing a protracted collective trauma, which has never been done before, and this study is the first to document the prevalence and early predictors of these time distortions,” added Dr. Holman.
The findings were published online in Psychological Trauma: Theory, Research, Practice, and Policy.
Unique opportunity
During the pandemic, many people’s time perspective (TP), defined as “our view of time as it spans from our past into the future,” shifted as they “focused on the immediate, present danger of the COVID-19 pandemic and future plans became uncertain,” the investigators wrote.
Studies of convenience samples “suggested that many people experienced time slowing down, stopping, and/or speeding up as they coped with the challenges of the pandemic” – a phenomenon known as temporal disintegration (TD) in psychiatric literature.
Dr. Holman said in an interview that she researched TD after the Sept.11, 2001 World Trade Center attacks.
“We found that people who experienced that early sense of TD, the sense of ‘time falling apart,’ were more prone to getting stuck in the past and staying focused on the past event,” which led to feeling “more distress over time,” she said.
Research examining the prevalence of and psychosocial factors predicting TD are “quite rare” and studies examining TD “during an unfolding, protracted collective trauma are even rarer,” the researchers note. The COVID pandemic “presented a unique opportunity to conduct such a study,” the researchers wrote.
For their study, the investigators surveyed participants in the NORC AmeriSpeak online panel, a “probability-based panel” of 35,000 U.S. households selected at random from across the country.
The study was conducted in two waves: the first survey was administered March–April 2020, the second in September–October 2020.
Speeding up, slowing down
At wave 2, participants completed a 7-item index of TD symptoms experienced over the previous 6 months. To adjust for psychological processes that may have predisposed individuals to experience TD during the pandemic, the researchers included a Wave 1 measure of future uncertainty as a covariate.
Prepandemic health data had been collected prior to the current study.
Wave 1 participants completed a checklist reporting personal, work, and community-wide exposure to the COVID outbreak, including contracting the virus, sheltering in place, and experiencing secondary stressors. The extent and type of pandemic-related media exposure were also assessed.
At wave 2, they reported the extent of exposure to the coronavirus, financial exposures, and secondary stressors. They also completed a non–COVID-related stress/trauma exposure checklist and were asked to indicate whether the trauma, disaster, or bereavement took place prior to or during the pandemic.
The final sample consisted of 5,661 adults (52% female) who completed the wave 2 survey. Participants were divided into four age groups: 18-34, 35-49, 50-64, and 65 and older.
The most common experiences (reported by more than 65% of respondents) included being focused on the present moment, feeling that weekdays and weekends were the same, and feeling uncertain about the future.
Over half of respondents (50.4%) reported feeling as though time was speeding up, and 55.2% reported feeling as though time was slowing down. Some also reported feeling uncertain about the time of day (46.4%) and forgetting events they had just experienced (35.2%).
When the researchers controlled for feeling uncertain about the future, they found that women reported more TD than men (b = 0.11; 95% confidence interval, 0.07-0.14; P < .001).
At wave 1, associations were found between TD and COVID-related media exposure, prepandemic mental health diagnoses, and prepandemic non–COVID-related stress and trauma. At wave 2, associations were found between TD and COVID-related secondary and financial stressors (P < .001 for all).
In contrast, COVID-related work exposure at wave 1, being 45-59 years old, and living in the Midwest region were negatively associated with TD.
“The sense of the flow of the past into the present, and the present into the future is important for our mental health,” Dr. Holman said. “We need to remember who we have been, how that shaped who we are today, and where we want to go with our lives.”
Staying in the present moment is “good, when you’re doing it mindfully. But you still need to feel you can shape and work toward the future and have some sense of control,” she added.
Dr. Homan also recommended time-perspective therapy, which helps patients with PTSD to “build continuity across time – to understand and learn from the past, live in the present, and move toward the future.”
Widespread distortion
In an interview, Ruth Ogden, PhD, a lecturer at Liverpool (England) John Moores University, said the findings “confirm those reported in Europe, South America, and the Middle East, that widespread distortion to time was common during the pandemic and that distortions to time were greatest amongst those most negatively affected by the pandemic.”
The results also support her own recent research in the United Kingdom “suggesting that distortions to time during the pandemic extend to our memory for the length of the pandemic, with most people believing that lockdowns lasted far longer than they actually did,” said Dr. Ogden, who was not involved with Dr. Holman and colleagues’ current study.
“This type of subjective lengthening of the pandemic may reinforce trauma by making the traumatic period seem longer, further damaging health and well-being,” she noted. “As the negative fallouts of the pandemic continue, it is important to establish the long-term effects of time distortions during the pandemic on mental health and well-being.”
The study was funded by U.S. National Science Foundation and the National Institute on Minority Health and Health Disparities. The investigators reported no relevant financial relationships. Dr. Ogden receives funding from the Wellcome Trust.
A version of this article first appeared on Medscape.com.
FROM PSYCHOLOGICAL TRAUMA: THEORY, RESEARCH, PRACTICE, AND POLICY
How do you live with COVID? One doctor’s personal experience
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Early in 2020, Anne Peters, MD, caught COVID-19. The author of Medscape’s “Peters on Diabetes” column was sick in March 2020 before state-mandated lockdowns, and well before there were any vaccines.
She remembers sitting in a small exam room with two patients who had flown to her Los Angeles office from New York. The elderly couple had hearing difficulties, so Dr. Peters sat close to them, putting on a continuous glucose monitor. “At that time, we didn’t think of COVID-19 as being in L.A.,” Dr. Peters recalled, “so I think we were not terribly consistent at mask-wearing due to the need to educate.”
“Several days later, I got COVID, but I didn’t know I had COVID per se. I felt crappy, had a terrible sore throat, lost my sense of taste and smell [which was not yet described as a COVID symptom], was completely exhausted, but had no fever or cough, which were the only criteria for getting COVID tested at the time. I didn’t know I had been exposed until 2 weeks later, when the patient’s assistant returned the sensor warning us to ‘be careful’ with it because the patient and his wife were recovering from COVID.”
That early battle with COVID-19 was just the beginning of what would become a 2-year struggle, including familial loss amid her own health problems and concerns about the under-resourced patients she cares for. Here, she shares her journey through the pandemic with this news organization.
Question: Thanks for talking to us. Let’s discuss your journey over these past 2.5 years.
Answer: Everybody has their own COVID story because we all went through this together. Some of us have worse COVID stories, and some of us have better ones, but all have been impacted.
I’m not a sick person. I’m a very healthy person but COVID made me so unwell for 2 years. The brain fog and fatigue were nothing compared to the autonomic neuropathy that affected my heart. It was really limiting for me. And I still don’t know the long-term implications, looking 20-30 years from now.
Q: When you initially had COVID, what were your symptoms? What was the impact?
A: I had all the symptoms of COVID, except for a cough and fever. I lost my sense of taste and smell. I had a horrible headache, a sore throat, and I was exhausted. I couldn’t get tested because I didn’t have the right symptoms.
Despite being sick, I never stopped working but just switched to telemedicine. I also took my regular monthly trip to our cabin in Montana. I unknowingly flew on a plane with COVID. I wore a well-fitted N95 mask, so I don’t think I gave anybody COVID. I didn’t give COVID to my partner, Eric, which is hard to believe as – at 77 – he’s older than me. He has diabetes, heart disease, and every other high-risk characteristic. If he’d gotten COVID back then, it would have been terrible, as there were no treatments, but luckily he didn’t get it.
Q: When were you officially diagnosed?
A: Two or 3 months after I thought I might have had COVID, I checked my antibodies, which tested strongly positive for a prior COVID infection. That was when I knew all the symptoms I’d had were due to the disease.
Q: Not only were you dealing with your own illness, but also that of those close to you. Can you talk about that?
A: In April 2020, my mother who was in her 90s and otherwise healthy except for dementia, got COVID. She could have gotten it from me. I visited often but wore a mask. She had all the horrible pulmonary symptoms. In her advance directive, she didn’t want to be hospitalized so I kept her in her home. She died from COVID in her own bed. It was fairly brutal, but at least I kept her where she felt comforted.
My 91-year-old dad was living in a different residential facility. Throughout COVID he had become very depressed because his social patterns had changed. Prior to COVID, they all ate together, but during the pandemic they were unable to. He missed his social connections, disliked being isolated in his room, hated everyone in masks.
He was a bit demented, but not so much that he couldn’t communicate with me or remember where his grandson was going to law school. I wasn’t allowed inside the facility, which was hard on him. I hadn’t told him his wife died because the hospice social workers advised me that I shouldn’t give him news that he couldn’t process readily until I could spend time with him. Unfortunately, that time never came. In December 2020, he got COVID. One of the people in that facility had gone to the hospital, came back, and tested negative, but actually had COVID and gave it to my dad. The guy who gave it to my dad didn’t die but my dad was terribly ill. He died 2 weeks short of getting his vaccine. He was coherent enough to have a conversation. I asked him: ‘Do you want to go to the hospital?’ And he said: ‘No, because it would be too scary,’ since he couldn’t be with me. I put him on hospice and held his hand as he died from pulmonary COVID, which was awful. I couldn’t give him enough morphine or valium to ease his breathing. But his last words to me were “I love you,” and at the very end he seemed peaceful, which was a blessing.
I got an autopsy, because he wanted one. Nothing else was wrong with him other than COVID. It destroyed his lungs. The rest of him was fine – no heart disease, cancer, or anything else. He died of COVID-19, the same as my mother.
That same week, my aunt, my only surviving older relative, who was in Des Moines, Iowa, died of COVID-19. All three family members died before the vaccine came out.
It was hard to lose my parents. I’m the only surviving child because my sister died in her 20s. It’s not been an easy pandemic. But what pandemic is easy? I just happened to have lost more people than most. Ironically, my grandfather was one of the legionnaires at the Bellevue-Stratford Hotel in Philadelphia in 1976 and died of Legionnaire’s disease before we knew what was causing the outbreak.
Q: Were you still struggling with COVID?
A: COVID impacted my whole body. I lost a lot of weight. I didn’t want to eat, and my gastrointestinal system was not happy. It took a while for my sense of taste and smell to come back. Nothing tasted good. I’m not a foodie; I don’t really care about food. We could get takeout or whatever, but none of it appealed to me. I’m not so sure it was a taste thing, I just didn’t feel like eating.
I didn’t realize I had “brain fog” per se, because I felt stressed and overwhelmed by the pandemic and my patients’ concerns. But one day, about 3 months after I had developed COVID, I woke up without the fog. Which made me aware that I hadn’t been feeling right up until that point.
The worst symptoms, however, were cardiac. I noticed also immediately that my heart rate went up very quickly with minimal exertion. My pulse has always been in the 55-60 bpm range, and suddenly just walking across a room made it go up to over 140 bpm. If I did any aerobic activity, it went up over 160 and would be associated with dyspnea and chest pain. I believed these were all post-COVID symptoms and felt validated when reports of others having similar issues were published in the literature.
Q: Did you continue seeing patients?
A: Yes, of course. Patients never needed their doctors more. In East L.A., where patients don’t have easy access to telemedicine, I kept going into clinic throughout the pandemic. In the more affluent Westside of Los Angeles, we switched to telemedicine, which was quite effective for most. However, because diabetes was associated with an increased risk of hospitalization and death from COVID, my patients were understandably afraid. I’ve never been busier, but (like all health care providers), I became more of a COVID provider than a diabetologist.
Q: Do you feel your battle with COVID impacted your work?
A: It didn’t affect me at work. If I was sitting still, I was fine. Sitting at home at a desk, I didn’t notice any symptoms. But as a habitual stair-user, I would be gasping for breath in the stairwell because I couldn’t go up the stairs to my office as I once could.
I think you empathize more with people who had COVID (when you’ve had it yourself). There was such a huge patient burden. And I think that’s been the thing that’s affected health care providers the most – no matter what specialty we’re in – that nobody has answers.
Q: What happened after you had your vaccine?
A: The vaccine itself was fine. I didn’t have any reaction to the first two doses. But the first booster made my cardiac issues worse.
By this point, my cardiac problems stopped me from exercising. I even went to the ER with chest pain once because I was having palpitations and chest pressure caused by simply taking my morning shower. Fortunately, I wasn’t having an MI, but I certainly wasn’t “normal.”
My measure of my fitness is the cross-country skiing trail I use in Montana. I know exactly how far I can ski. Usually I can do the loop in 35 minutes. After COVID, I lasted 10 minutes. I would be tachycardic, short of breath with chest pain radiating down my left arm. I would rest and try to keep going. But with each rest period, I only got worse. I would be laying in the snow and strangers would ask if I needed help.
Q: What helped you?
A: I’ve read a lot about long COVID and have tried to learn from the experts. Of course, I never went to a doctor directly, although I did ask colleagues for advice. What I learned was to never push myself. I forced myself to create an exercise schedule where I only exercised three times a week with rest days in between. When exercising, the second my heart rate went above 140 bpm, I stopped until I could get it back down. I would push against this new limit, even though my limit was low.
Additionally, I worked on my breathing patterns and did meditative breathing for 10 minutes twice daily using a commercially available app.
Although progress was slow, I did improve, and by June 2022, I seemed back to normal. I was not as fit as I was prior to COVID and needed to improve, but the tachycardic response to exercise and cardiac symptoms were gone. I felt like my normal self. Normal enough to go on a spot packing trip in the Sierras in August. (Horses carried us and a mule carried the gear over the 12,000-foot pass into the mountains, and then left my friend and me high in the Sierras for a week.) We were camped above 10,000 feet and every day hiked up to another high mountain lake where we fly-fished for trout that we ate for dinner. The hikes were a challenge, but not abnormally so. Not as they would have been while I had long COVID.
Q: What is the current atmosphere in your clinic?
A: COVID is much milder now in my vaccinated patients, but I feel most health care providers are exhausted. Many of my staff left when COVID hit because they didn’t want to keep working. It made practicing medicine exhausting. There’s been a shortage of nurses, a shortage of everything. We’ve been required to do a whole lot more than we ever did before. It’s much harder to be a doctor. This pandemic is the first time I’ve ever thought of quitting. Granted, I lost my whole family, or at least the older generation, but it’s just been almost overwhelming.
On the plus side, almost every one of my patients has been vaccinated, because early on, people would ask: “Do you trust this vaccine?” I would reply: “I saw my parents die from COVID when they weren’t vaccinated, so you’re getting vaccinated. This is real and the vaccines help.” It made me very good at convincing people to get vaccines because I knew what it was like to see someone dying from COVID up close.
Q: What advice do you have for those struggling with the COVID pandemic?
A: People need to decide what their own risk is for getting sick and how many times they want to get COVID. At this point, I want people to go out, but safely. In the beginning, when my patients said, “can I go visit my granddaughter?” I said, “no,” but that was before we had the vaccine. Now I feel it is safe to go out using common sense. I still have my patients wear masks on planes. I still have patients try to eat outside as much as possible. And I tell people to take the precautions that make sense, but I tell them to go out and do things because life is short.
I had a patient in his 70s who has many risk factors like heart disease and diabetes. His granddaughter’s Bat Mitzvah in Florida was coming up. He asked: “Can I go?” I told him “Yes,” but to be safe – to wear an N95 mask on the plane and at the event, and stay in his own hotel room, rather than with the whole family. I said, “You need to do this.” Earlier in the pandemic, I saw people who literally died from loneliness and isolation.
He and his wife flew there. He sent me a picture of himself with his granddaughter. When he returned, he showed me a handwritten note from her that said, “I love you so much. Everyone else canceled, which made me cry. You’re the only one who came. You have no idea how much this meant to me.”
He’s back in L.A., and he didn’t get COVID. He said, “It was the best thing I’ve done in years.” That’s what I need to help people with, navigating this world with COVID and assessing risks and benefits. As with all of medicine, my advice is individualized. My advice changes based on the major circulating variant and the rates of the virus in the population, as well as the risk factors of the individual.
Q: What are you doing now?
A: I’m trying to avoid getting COVID again, or another booster. I could get pre-exposure monoclonal antibodies but am waiting to do anything further until I see what happens over the fall and winter. I still wear a mask inside but now do a mix of in-person and telemedicine visits. I still try to go to outdoor restaurants, which is easy in California. But I’m flying to see my son in New York and plan to go to Europe this fall for a meeting. I also go to my cabin in Montana every month to get my “dose” of the wilderness. Overall, I travel for conferences and speaking engagements much less because I have learned the joy of staying home.
Thinking back on my life as a doctor, my career began as an intern at Stanford rotating through Ward 5B, the AIDS unit at San Francisco General Hospital, and will likely end with COVID. In spite of all our medical advances, my generation of physicians, much as many generations before us, has a front-row seat to the vulnerability of humans to infectious diseases and how far we still need to go to protect our patients from communicable illness.
A version of this article first appeared on Medscape.com.
Anne L. Peters, MD, is a professor of medicine at the University of Southern California, Los Angeles, and director of the USC clinical diabetes programs. She has published more than 200 articles, reviews, and abstracts; three books on diabetes; and has been an investigator for more than 40 research studies. She has spoken internationally at over 400 programs and serves on many committees of several professional organizations.
Autoimmune disease patients’ waxing, waning response to COVID vaccination studied in-depth
A new study in The Lancet Rheumatology examines the strength and duration of SARS-CoV-2 vaccine–induced immunoglobulin-G antibody responses over time for patients with a variety of autoimmune diseases, compared with healthy controls.
The presence of humoral antibodies to SARS-CoV-2 has been shown to correlate with protection against COVID infection. But for patients with immune-mediated inflammatory diseases (IMIDs), host response to COVID infection or to vaccination is affected by the immune dysfunction imposed by the IMID and by the use of immune-modulating drugs to treat it.
This new study finds a weaker – as shown previously – and less sustained immune response to SARS-CoV-2 vaccines in patients with a variety of IMIDs, including rheumatoid arthritis, spondyloarthritis, psoriasis, inflammatory bowel diseases, and other systemic autoimmune diseases such as lupus. It also points toward the possibility of adjusting treatment and vaccination schedules and strategies for these patients based on their antibody levels, among other factors, to preserve best protection against severe COVID.
“It is important to assess immune response in these patients to see if they still have protection against severe COVID infection,” said lead author David Simon, MD, senior clinical scientist in clinical immunology and rheumatology at University Hospital Erlangen (Germany). “We know that antibody response is an immune correlate. Therefore, it is important to see how large and durable the immune response is to the coronavirus vaccine in these IMID patients, and whether specific drugs or therapies have negative effects on their immune response.”
What was studied?
For this large prospective cohort study, researchers registered 5076 coronavirus-vaccinated individuals. They analyzed serum samples obtained between December 15, 2020, and December 1, 2021, from 2,535 patients diagnosed with IMIDs and participating in a prospective coronavirus study program at the Deutsches Zentrum Immuntherapie in Erlangen. The IMID patients had a mean age of 55.0 years, and 58.9% were women.
A healthy control group of 1,198 individuals without IMID who had a mean age of 40.7 years, including 53.8% men, was also recruited for the analysis. All approved coronavirus vaccines were included, following standard vaccination schedules. Antibody response was measured over time by an enzyme-linked immunosorbent assay from 8 weeks after first vaccination to week 40.
Among the findings, the healthy controls had higher postvaccine antibody levels than did those with IMIDs. But the majority of vaccinated patients with IMID were able to build up a humoral immune response to SARS-CoV-2. Patients who were taking B-cell inhibitors like rituximab (Rituxan, Genentech; and biosimilars) and T-cell inhibitors like abatacept (Orencia, Bristol Myers Squibb) for IMIDs had significantly poorer antibody response.
Greater age and the use of combination therapies for IMIDs, compared with monotherapy, further reduced immune response to the vaccine. In terms of vaccination modality, messenger RNA–based vaccines induced higher antibody levels than did vector-based vaccines. The researchers noted that patients with IMID who were given a third vaccine dose could actually catch up well with the antibody responses observed in healthy controls.
“We looked at whether different IMIDs had a different humoral response, and we also assessed if there are effects from different therapeutic strategies,” Dr. Simon explained. “It doesn’t matter so much what kind of IMID patients have; much more important is the specific drug treatment and its impact on their antibody response.” Some participants were advised to briefly stop taking some immunosuppressive treatments before or after vaccination.
One of Dr. Simon’s coauthors, statistician and rheumatologist Koray Tascilar, MD, added, “This research is important because we looked not only at who responded less, which has been previously established, but who are at greater risk of losing their immune response, and how quickly.”
Need to take care
“Most treatments we as rheumatologists give to our patients don’t affect their SARS-CoV-2 humoral response,” Dr. Simon said. “However, there are specific drugs that are associated with lower antibody response. With respect to those drugs, we have to be more careful.”
It is important to be able to tell patients which drugs are safe and won’t have a negative impact on their immune response to vaccinations, Dr. Tascilar said. “But it would be too strong to say we’re ready to choose therapies based on their potential impact on protection against COVID. Yes, there is a risk from catching COVID, but we need to balance that risk with the risk of not giving patients the medications that are necessary to treat their rheumatologic condition.”
These diseases are serious, sometimes life-threatening. “We might think of strategies for how to mitigate the risk of underprotection from COVID that is brought about by these treatments,” he said. For example, offering boosters sooner or more frequently, or prophylactically treating with monoclonal antibodies.
“This study, along other recent studies, has found that antibody levels in patients with immune-mediated diseases wane more rapidly than in healthy controls, and this is especially true of those on medications that interfere with the B and T cells and anticytokine therapies,” Rebecca Haberman, MD, assistant professor, division of rheumatology, New York University Langone Health, noted in an email to this news organization.
“While there is no known antibody level that specifically correlates with clinical protection, and each patient needs to be thought of individually, these findings support the use of supplemental booster dosing in patients with immune-mediated inflammatory diseases,” Dr. Haberman said, adding that her own research in this area has shown similar results.
“As a rheumatologist, I would be more likely to encourage my patients – especially those on immunomodulatory medications – to get boosted.”
Dr. Tascilar said his study does not directly answer the question of whether an earlier booster shot would be an effective strategy for patients with IMID. “In our department, we have an early boosting strategy, based on level of immune response.” But the decision of revaccination or not, and when, is based on a number of factors, not only on the level of antibodies. “It’s just part of the instruments we are using.”
The study was supported by the Deutsche Forschungsgemeinschaft. Dr. Simon and Dr. Tascilar declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study in The Lancet Rheumatology examines the strength and duration of SARS-CoV-2 vaccine–induced immunoglobulin-G antibody responses over time for patients with a variety of autoimmune diseases, compared with healthy controls.
The presence of humoral antibodies to SARS-CoV-2 has been shown to correlate with protection against COVID infection. But for patients with immune-mediated inflammatory diseases (IMIDs), host response to COVID infection or to vaccination is affected by the immune dysfunction imposed by the IMID and by the use of immune-modulating drugs to treat it.
This new study finds a weaker – as shown previously – and less sustained immune response to SARS-CoV-2 vaccines in patients with a variety of IMIDs, including rheumatoid arthritis, spondyloarthritis, psoriasis, inflammatory bowel diseases, and other systemic autoimmune diseases such as lupus. It also points toward the possibility of adjusting treatment and vaccination schedules and strategies for these patients based on their antibody levels, among other factors, to preserve best protection against severe COVID.
“It is important to assess immune response in these patients to see if they still have protection against severe COVID infection,” said lead author David Simon, MD, senior clinical scientist in clinical immunology and rheumatology at University Hospital Erlangen (Germany). “We know that antibody response is an immune correlate. Therefore, it is important to see how large and durable the immune response is to the coronavirus vaccine in these IMID patients, and whether specific drugs or therapies have negative effects on their immune response.”
What was studied?
For this large prospective cohort study, researchers registered 5076 coronavirus-vaccinated individuals. They analyzed serum samples obtained between December 15, 2020, and December 1, 2021, from 2,535 patients diagnosed with IMIDs and participating in a prospective coronavirus study program at the Deutsches Zentrum Immuntherapie in Erlangen. The IMID patients had a mean age of 55.0 years, and 58.9% were women.
A healthy control group of 1,198 individuals without IMID who had a mean age of 40.7 years, including 53.8% men, was also recruited for the analysis. All approved coronavirus vaccines were included, following standard vaccination schedules. Antibody response was measured over time by an enzyme-linked immunosorbent assay from 8 weeks after first vaccination to week 40.
Among the findings, the healthy controls had higher postvaccine antibody levels than did those with IMIDs. But the majority of vaccinated patients with IMID were able to build up a humoral immune response to SARS-CoV-2. Patients who were taking B-cell inhibitors like rituximab (Rituxan, Genentech; and biosimilars) and T-cell inhibitors like abatacept (Orencia, Bristol Myers Squibb) for IMIDs had significantly poorer antibody response.
Greater age and the use of combination therapies for IMIDs, compared with monotherapy, further reduced immune response to the vaccine. In terms of vaccination modality, messenger RNA–based vaccines induced higher antibody levels than did vector-based vaccines. The researchers noted that patients with IMID who were given a third vaccine dose could actually catch up well with the antibody responses observed in healthy controls.
“We looked at whether different IMIDs had a different humoral response, and we also assessed if there are effects from different therapeutic strategies,” Dr. Simon explained. “It doesn’t matter so much what kind of IMID patients have; much more important is the specific drug treatment and its impact on their antibody response.” Some participants were advised to briefly stop taking some immunosuppressive treatments before or after vaccination.
One of Dr. Simon’s coauthors, statistician and rheumatologist Koray Tascilar, MD, added, “This research is important because we looked not only at who responded less, which has been previously established, but who are at greater risk of losing their immune response, and how quickly.”
Need to take care
“Most treatments we as rheumatologists give to our patients don’t affect their SARS-CoV-2 humoral response,” Dr. Simon said. “However, there are specific drugs that are associated with lower antibody response. With respect to those drugs, we have to be more careful.”
It is important to be able to tell patients which drugs are safe and won’t have a negative impact on their immune response to vaccinations, Dr. Tascilar said. “But it would be too strong to say we’re ready to choose therapies based on their potential impact on protection against COVID. Yes, there is a risk from catching COVID, but we need to balance that risk with the risk of not giving patients the medications that are necessary to treat their rheumatologic condition.”
These diseases are serious, sometimes life-threatening. “We might think of strategies for how to mitigate the risk of underprotection from COVID that is brought about by these treatments,” he said. For example, offering boosters sooner or more frequently, or prophylactically treating with monoclonal antibodies.
“This study, along other recent studies, has found that antibody levels in patients with immune-mediated diseases wane more rapidly than in healthy controls, and this is especially true of those on medications that interfere with the B and T cells and anticytokine therapies,” Rebecca Haberman, MD, assistant professor, division of rheumatology, New York University Langone Health, noted in an email to this news organization.
“While there is no known antibody level that specifically correlates with clinical protection, and each patient needs to be thought of individually, these findings support the use of supplemental booster dosing in patients with immune-mediated inflammatory diseases,” Dr. Haberman said, adding that her own research in this area has shown similar results.
“As a rheumatologist, I would be more likely to encourage my patients – especially those on immunomodulatory medications – to get boosted.”
Dr. Tascilar said his study does not directly answer the question of whether an earlier booster shot would be an effective strategy for patients with IMID. “In our department, we have an early boosting strategy, based on level of immune response.” But the decision of revaccination or not, and when, is based on a number of factors, not only on the level of antibodies. “It’s just part of the instruments we are using.”
The study was supported by the Deutsche Forschungsgemeinschaft. Dr. Simon and Dr. Tascilar declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new study in The Lancet Rheumatology examines the strength and duration of SARS-CoV-2 vaccine–induced immunoglobulin-G antibody responses over time for patients with a variety of autoimmune diseases, compared with healthy controls.
The presence of humoral antibodies to SARS-CoV-2 has been shown to correlate with protection against COVID infection. But for patients with immune-mediated inflammatory diseases (IMIDs), host response to COVID infection or to vaccination is affected by the immune dysfunction imposed by the IMID and by the use of immune-modulating drugs to treat it.
This new study finds a weaker – as shown previously – and less sustained immune response to SARS-CoV-2 vaccines in patients with a variety of IMIDs, including rheumatoid arthritis, spondyloarthritis, psoriasis, inflammatory bowel diseases, and other systemic autoimmune diseases such as lupus. It also points toward the possibility of adjusting treatment and vaccination schedules and strategies for these patients based on their antibody levels, among other factors, to preserve best protection against severe COVID.
“It is important to assess immune response in these patients to see if they still have protection against severe COVID infection,” said lead author David Simon, MD, senior clinical scientist in clinical immunology and rheumatology at University Hospital Erlangen (Germany). “We know that antibody response is an immune correlate. Therefore, it is important to see how large and durable the immune response is to the coronavirus vaccine in these IMID patients, and whether specific drugs or therapies have negative effects on their immune response.”
What was studied?
For this large prospective cohort study, researchers registered 5076 coronavirus-vaccinated individuals. They analyzed serum samples obtained between December 15, 2020, and December 1, 2021, from 2,535 patients diagnosed with IMIDs and participating in a prospective coronavirus study program at the Deutsches Zentrum Immuntherapie in Erlangen. The IMID patients had a mean age of 55.0 years, and 58.9% were women.
A healthy control group of 1,198 individuals without IMID who had a mean age of 40.7 years, including 53.8% men, was also recruited for the analysis. All approved coronavirus vaccines were included, following standard vaccination schedules. Antibody response was measured over time by an enzyme-linked immunosorbent assay from 8 weeks after first vaccination to week 40.
Among the findings, the healthy controls had higher postvaccine antibody levels than did those with IMIDs. But the majority of vaccinated patients with IMID were able to build up a humoral immune response to SARS-CoV-2. Patients who were taking B-cell inhibitors like rituximab (Rituxan, Genentech; and biosimilars) and T-cell inhibitors like abatacept (Orencia, Bristol Myers Squibb) for IMIDs had significantly poorer antibody response.
Greater age and the use of combination therapies for IMIDs, compared with monotherapy, further reduced immune response to the vaccine. In terms of vaccination modality, messenger RNA–based vaccines induced higher antibody levels than did vector-based vaccines. The researchers noted that patients with IMID who were given a third vaccine dose could actually catch up well with the antibody responses observed in healthy controls.
“We looked at whether different IMIDs had a different humoral response, and we also assessed if there are effects from different therapeutic strategies,” Dr. Simon explained. “It doesn’t matter so much what kind of IMID patients have; much more important is the specific drug treatment and its impact on their antibody response.” Some participants were advised to briefly stop taking some immunosuppressive treatments before or after vaccination.
One of Dr. Simon’s coauthors, statistician and rheumatologist Koray Tascilar, MD, added, “This research is important because we looked not only at who responded less, which has been previously established, but who are at greater risk of losing their immune response, and how quickly.”
Need to take care
“Most treatments we as rheumatologists give to our patients don’t affect their SARS-CoV-2 humoral response,” Dr. Simon said. “However, there are specific drugs that are associated with lower antibody response. With respect to those drugs, we have to be more careful.”
It is important to be able to tell patients which drugs are safe and won’t have a negative impact on their immune response to vaccinations, Dr. Tascilar said. “But it would be too strong to say we’re ready to choose therapies based on their potential impact on protection against COVID. Yes, there is a risk from catching COVID, but we need to balance that risk with the risk of not giving patients the medications that are necessary to treat their rheumatologic condition.”
These diseases are serious, sometimes life-threatening. “We might think of strategies for how to mitigate the risk of underprotection from COVID that is brought about by these treatments,” he said. For example, offering boosters sooner or more frequently, or prophylactically treating with monoclonal antibodies.
“This study, along other recent studies, has found that antibody levels in patients with immune-mediated diseases wane more rapidly than in healthy controls, and this is especially true of those on medications that interfere with the B and T cells and anticytokine therapies,” Rebecca Haberman, MD, assistant professor, division of rheumatology, New York University Langone Health, noted in an email to this news organization.
“While there is no known antibody level that specifically correlates with clinical protection, and each patient needs to be thought of individually, these findings support the use of supplemental booster dosing in patients with immune-mediated inflammatory diseases,” Dr. Haberman said, adding that her own research in this area has shown similar results.
“As a rheumatologist, I would be more likely to encourage my patients – especially those on immunomodulatory medications – to get boosted.”
Dr. Tascilar said his study does not directly answer the question of whether an earlier booster shot would be an effective strategy for patients with IMID. “In our department, we have an early boosting strategy, based on level of immune response.” But the decision of revaccination or not, and when, is based on a number of factors, not only on the level of antibodies. “It’s just part of the instruments we are using.”
The study was supported by the Deutsche Forschungsgemeinschaft. Dr. Simon and Dr. Tascilar declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM THE LANCET RHEUMATOLOGY
Paxlovid reduces risk of COVID death by 79% in older adults
The antiviral drug Paxlovid appears to reduce the risk of dying from COVID-19 by 79% and decrease hospitalizations by 73% in at-risk patients who are ages 65 and older, according to a new study published in The New England Journal of Medicine.
The pill, which is a combination of the drugs nirmatrelvir and ritonavir, received FDA emergency use authorization in December 2021 to treat mild to moderate disease in ages 12 and older who face high risks for having severe COVID-19, hospitalization, and death.
“The results of the study show unequivocally that treatment with Paxlovid significantly reduces the risk of hospitalization and death from COVID-19,” Doron Netzer, MD, the senior study author and a researcher with Clalit Health Services, Tel Aviv, told The Jerusalem Post.
“We are the country’s leader in the provision of giving Paxlovid to relevant patients,” he said. “It was given to patients all over the country, with medical teams monitoring the patients who took the pills.”
, the news outlet reported. The research team analyzed information from Clalit’s electronic medical records. The health care organization covers about 52% of the Israeli population and almost two-thirds of older adults. More than 30,000 COVID-19 patients in Israel have been treated with the drug so far.
Dr. Netzer and colleagues looked at hospitalization and death data for at-risk COVID-19 patients ages 40 and older between Jan. 9 and March 31, when the original Omicron variant was the dominant strain in Israel. During that time, more than 1.1 million Clalit patients were infected with COVID-19, 109,000 patients were considered at-risk, and 3,900 patients received the drug.
The average age of the patients was 60, and 39% of the patients were 65 and older. Overall, 78% of the patients had previous COVID-19 immunity due to vaccination, prior infection, or both.
Among ages 65 and older, the rate of COVID-19 hospitalization was 14.7 cases per 100,000 person-days among treated patients, compared with 58.9 cases per 100,000 person-days among untreated patients. This represented a 73% lower chance of being hospitalized.
Among ages 40-64, the rate of hospitalization due to COVID-19 was 15.2 cases per 100,000 person-days among treated patients, compared with 15.8 cases per 100,000 person-days among untreated patients. The risk of hospitalization wasn’t significantly lower for this age group.
Among ages 65 and older, there were two deaths from COVID-19 in 2,484 treated patients, compared with 158 in the 40,337 untreated patients. This represented a 79% lower chance of dying from COVID-19.
Among ages 40-64, there was one death from COVID-19 in 1,418 treated patients, compared with 16 in the 65,015 untreated patients. The risk of death wasn’t significantly lower for this age group.
For both age groups, a lack of previous COVID-19 immunity and a previous hospitalization were most strongly linked to high rates of hospitalization during the Omicron wave.
The researchers noted that they didn’t break down the data on ages 40-64 who had cancer and other severe conditions that weaken the immune system. These patients may be more likely to benefit from Paxlovid, they said, though future studies will need to analyze the data.
The study didn’t receive any financial or in-kind support, the authors said.
A version of this article first appeared on WebMD.com.
The antiviral drug Paxlovid appears to reduce the risk of dying from COVID-19 by 79% and decrease hospitalizations by 73% in at-risk patients who are ages 65 and older, according to a new study published in The New England Journal of Medicine.
The pill, which is a combination of the drugs nirmatrelvir and ritonavir, received FDA emergency use authorization in December 2021 to treat mild to moderate disease in ages 12 and older who face high risks for having severe COVID-19, hospitalization, and death.
“The results of the study show unequivocally that treatment with Paxlovid significantly reduces the risk of hospitalization and death from COVID-19,” Doron Netzer, MD, the senior study author and a researcher with Clalit Health Services, Tel Aviv, told The Jerusalem Post.
“We are the country’s leader in the provision of giving Paxlovid to relevant patients,” he said. “It was given to patients all over the country, with medical teams monitoring the patients who took the pills.”
, the news outlet reported. The research team analyzed information from Clalit’s electronic medical records. The health care organization covers about 52% of the Israeli population and almost two-thirds of older adults. More than 30,000 COVID-19 patients in Israel have been treated with the drug so far.
Dr. Netzer and colleagues looked at hospitalization and death data for at-risk COVID-19 patients ages 40 and older between Jan. 9 and March 31, when the original Omicron variant was the dominant strain in Israel. During that time, more than 1.1 million Clalit patients were infected with COVID-19, 109,000 patients were considered at-risk, and 3,900 patients received the drug.
The average age of the patients was 60, and 39% of the patients were 65 and older. Overall, 78% of the patients had previous COVID-19 immunity due to vaccination, prior infection, or both.
Among ages 65 and older, the rate of COVID-19 hospitalization was 14.7 cases per 100,000 person-days among treated patients, compared with 58.9 cases per 100,000 person-days among untreated patients. This represented a 73% lower chance of being hospitalized.
Among ages 40-64, the rate of hospitalization due to COVID-19 was 15.2 cases per 100,000 person-days among treated patients, compared with 15.8 cases per 100,000 person-days among untreated patients. The risk of hospitalization wasn’t significantly lower for this age group.
Among ages 65 and older, there were two deaths from COVID-19 in 2,484 treated patients, compared with 158 in the 40,337 untreated patients. This represented a 79% lower chance of dying from COVID-19.
Among ages 40-64, there was one death from COVID-19 in 1,418 treated patients, compared with 16 in the 65,015 untreated patients. The risk of death wasn’t significantly lower for this age group.
For both age groups, a lack of previous COVID-19 immunity and a previous hospitalization were most strongly linked to high rates of hospitalization during the Omicron wave.
The researchers noted that they didn’t break down the data on ages 40-64 who had cancer and other severe conditions that weaken the immune system. These patients may be more likely to benefit from Paxlovid, they said, though future studies will need to analyze the data.
The study didn’t receive any financial or in-kind support, the authors said.
A version of this article first appeared on WebMD.com.
The antiviral drug Paxlovid appears to reduce the risk of dying from COVID-19 by 79% and decrease hospitalizations by 73% in at-risk patients who are ages 65 and older, according to a new study published in The New England Journal of Medicine.
The pill, which is a combination of the drugs nirmatrelvir and ritonavir, received FDA emergency use authorization in December 2021 to treat mild to moderate disease in ages 12 and older who face high risks for having severe COVID-19, hospitalization, and death.
“The results of the study show unequivocally that treatment with Paxlovid significantly reduces the risk of hospitalization and death from COVID-19,” Doron Netzer, MD, the senior study author and a researcher with Clalit Health Services, Tel Aviv, told The Jerusalem Post.
“We are the country’s leader in the provision of giving Paxlovid to relevant patients,” he said. “It was given to patients all over the country, with medical teams monitoring the patients who took the pills.”
, the news outlet reported. The research team analyzed information from Clalit’s electronic medical records. The health care organization covers about 52% of the Israeli population and almost two-thirds of older adults. More than 30,000 COVID-19 patients in Israel have been treated with the drug so far.
Dr. Netzer and colleagues looked at hospitalization and death data for at-risk COVID-19 patients ages 40 and older between Jan. 9 and March 31, when the original Omicron variant was the dominant strain in Israel. During that time, more than 1.1 million Clalit patients were infected with COVID-19, 109,000 patients were considered at-risk, and 3,900 patients received the drug.
The average age of the patients was 60, and 39% of the patients were 65 and older. Overall, 78% of the patients had previous COVID-19 immunity due to vaccination, prior infection, or both.
Among ages 65 and older, the rate of COVID-19 hospitalization was 14.7 cases per 100,000 person-days among treated patients, compared with 58.9 cases per 100,000 person-days among untreated patients. This represented a 73% lower chance of being hospitalized.
Among ages 40-64, the rate of hospitalization due to COVID-19 was 15.2 cases per 100,000 person-days among treated patients, compared with 15.8 cases per 100,000 person-days among untreated patients. The risk of hospitalization wasn’t significantly lower for this age group.
Among ages 65 and older, there were two deaths from COVID-19 in 2,484 treated patients, compared with 158 in the 40,337 untreated patients. This represented a 79% lower chance of dying from COVID-19.
Among ages 40-64, there was one death from COVID-19 in 1,418 treated patients, compared with 16 in the 65,015 untreated patients. The risk of death wasn’t significantly lower for this age group.
For both age groups, a lack of previous COVID-19 immunity and a previous hospitalization were most strongly linked to high rates of hospitalization during the Omicron wave.
The researchers noted that they didn’t break down the data on ages 40-64 who had cancer and other severe conditions that weaken the immune system. These patients may be more likely to benefit from Paxlovid, they said, though future studies will need to analyze the data.
The study didn’t receive any financial or in-kind support, the authors said.
A version of this article first appeared on WebMD.com.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
COVID-19 vaccine safe in patients with heart failure
Patients with heart failure (HF) who received two doses of COVID mRNA vaccines were not more likely to have worsening disease, venous thromboembolism, or myocarditis within 90 days than similar unvaccinated patients, in a case-control study in Denmark.
Moreover, in the 90 days after receiving the second shot, vaccinated patients were less likely to die of any cause, compared with unvaccinated patients during a similar 90-day period.
Caroline Sindet-Pedersen, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and colleagues presented these findings at the annual congress of the European Society of Cardiology.
Major risk is not receiving vaccine
These results “confirm that the major risk for patients with HF is not receiving vaccination for COVID-19,” Marco Metra, MD, who was not involved with this research, said in an interview.
Dr. Metra was coauthor of an ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic, published online ahead of print November 2021 in the European Heart Journal.
The guidance explains that patients with HF are at increased risk for hospitalization, need for mechanical ventilation, and death because of COVID-19, and that vaccination reduces the risk for serious illness from COVID-19, Dr. Sindet-Pedersen and colleagues explained in a press release from the ESC.
However, “concerns remain,” they added, “about the safety of the SARS-CoV-2 mRNA vaccines in heart failure patients, due to a perceived increased risk of cardiovascular side effects.”
The study findings suggest that “there should be no concern about cardiovascular side effects from mRNA vaccines in heart failure patients,” Dr. Sindet-Pedersen and colleagues summarized.
The results also “point to a beneficial effect of vaccination on mortality” and “indicate that patients with HF should be prioritized for COVID-19 vaccinations and boosters,” they added.
“There are ongoing concerns about the safety of COVID-19 vaccination in fragile patients and patients with heart failure,” said Dr. Metra, professor of cardiology and director of the Institute of Cardiology of the Civil Hospital and University of Brescia (Italy).
“These concerns are not based on evidence but just on reports of rare side effects (namely, myocarditis and pericarditis) in vaccinated people,” he added.
Dr. Metra also coauthored a position paper on COVID-19 vaccination in patients with HF from the Heart Failure Association of the ESC, which was published online October 2021 in the European Journal of Heart Failure.
“The current study,” he summarized, “shows a lower risk of mortality among patients vaccinated, compared with those not vaccinated.
“It has limitations,” he cautioned, “as it is not a prospective randomized study, but [rather] an observational one with comparison between vaccinated and not vaccinated patients with similar characteristics.
“However, it was done in a large population,” he noted, “and its results confirm that the major risk for patients with HF is not receiving vaccination for COVID-19.”
95% of patients with HF in Denmark double vaccinated
The group did not analyze the types of all-cause death in their study, Dr. Sindet-Pedersen clarified in an interview.
Other studies have shown that vaccines are associated with improved survival, she noted. For example, bacillus Calmette-Guérin vaccines and the measles vaccines have been linked with a decreased risk for nonspecific mortality in children, and influenza vaccines are associated with decreased all-cause mortality in patients with HF.
The rates of vaccination in this study were much higher than those for patients with HF in the United States.
In a study of 7,094 patients with HF seen at the Mount Sinai Health System between January 2021 and January 2022, 31% of patients were fully vaccinated with two doses and 14.8% had also received a booster, as per Centers for Disease Control and Prevention guidance. However, another 9.1% of patients were only partially vaccinated with one dose, and 45% remained unvaccinated by January 2022,
In the current study, “the uptake was very high,” Dr. Sindet-Pedersen noted, that is, “95% of the prevalent heart failure patients in 2021 received a vaccine.”
“It might be that the last 5% of the patients that did not receive a vaccine were too ill [terminal] to receive the vaccine,” she speculated, “or that was due to personal reasons.”
The researchers identified 50,893 patients with HF who were double vaccinated in 2021 and they matched them with 50,893 unvaccinated patients with HF in 2019 (prepandemic), with the same age, sex, HF duration, use of HF medications, ischemic heart disease, cancer, diabetes, atrial fibrillation, and admission with HF within 90 days.
Almost all patients in the vaccinated group received the Pfizer/BioNTech mRNA vaccine (92%) and the rest received the Moderna mRNA vaccine (8%), in 2021.
The patients had a mean age of 74, and 64% were men. They had HF for a median of 4.1 years.
During the 90-day follow-up, 1,311 patients in the unvaccinated cohort (2.56%) and 1,113 patients in the vaccinated cohort (2.23%) died; there was a significantly lower risk for all-cause death in the vaccinated cohort versus the unvaccinated cohort (–0.33 percentage points; 95% CI, –0.52 to –0.15 percentage points).
The risk for worsening heart failure was 1.1% in each group; myocarditis and venous thromboembolism were extremely rare, and risks for these conditions were not significantly different in the two groups.
The researchers and Dr. Metra declared they have no relevant financial disclosures. Dr. Metra is editor-in-chief of the European Journal of Heart Failure and senior consulting editor of the European Heart Journal.
A version of this article first appeared on Medscape.com.
Patients with heart failure (HF) who received two doses of COVID mRNA vaccines were not more likely to have worsening disease, venous thromboembolism, or myocarditis within 90 days than similar unvaccinated patients, in a case-control study in Denmark.
Moreover, in the 90 days after receiving the second shot, vaccinated patients were less likely to die of any cause, compared with unvaccinated patients during a similar 90-day period.
Caroline Sindet-Pedersen, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and colleagues presented these findings at the annual congress of the European Society of Cardiology.
Major risk is not receiving vaccine
These results “confirm that the major risk for patients with HF is not receiving vaccination for COVID-19,” Marco Metra, MD, who was not involved with this research, said in an interview.
Dr. Metra was coauthor of an ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic, published online ahead of print November 2021 in the European Heart Journal.
The guidance explains that patients with HF are at increased risk for hospitalization, need for mechanical ventilation, and death because of COVID-19, and that vaccination reduces the risk for serious illness from COVID-19, Dr. Sindet-Pedersen and colleagues explained in a press release from the ESC.
However, “concerns remain,” they added, “about the safety of the SARS-CoV-2 mRNA vaccines in heart failure patients, due to a perceived increased risk of cardiovascular side effects.”
The study findings suggest that “there should be no concern about cardiovascular side effects from mRNA vaccines in heart failure patients,” Dr. Sindet-Pedersen and colleagues summarized.
The results also “point to a beneficial effect of vaccination on mortality” and “indicate that patients with HF should be prioritized for COVID-19 vaccinations and boosters,” they added.
“There are ongoing concerns about the safety of COVID-19 vaccination in fragile patients and patients with heart failure,” said Dr. Metra, professor of cardiology and director of the Institute of Cardiology of the Civil Hospital and University of Brescia (Italy).
“These concerns are not based on evidence but just on reports of rare side effects (namely, myocarditis and pericarditis) in vaccinated people,” he added.
Dr. Metra also coauthored a position paper on COVID-19 vaccination in patients with HF from the Heart Failure Association of the ESC, which was published online October 2021 in the European Journal of Heart Failure.
“The current study,” he summarized, “shows a lower risk of mortality among patients vaccinated, compared with those not vaccinated.
“It has limitations,” he cautioned, “as it is not a prospective randomized study, but [rather] an observational one with comparison between vaccinated and not vaccinated patients with similar characteristics.
“However, it was done in a large population,” he noted, “and its results confirm that the major risk for patients with HF is not receiving vaccination for COVID-19.”
95% of patients with HF in Denmark double vaccinated
The group did not analyze the types of all-cause death in their study, Dr. Sindet-Pedersen clarified in an interview.
Other studies have shown that vaccines are associated with improved survival, she noted. For example, bacillus Calmette-Guérin vaccines and the measles vaccines have been linked with a decreased risk for nonspecific mortality in children, and influenza vaccines are associated with decreased all-cause mortality in patients with HF.
The rates of vaccination in this study were much higher than those for patients with HF in the United States.
In a study of 7,094 patients with HF seen at the Mount Sinai Health System between January 2021 and January 2022, 31% of patients were fully vaccinated with two doses and 14.8% had also received a booster, as per Centers for Disease Control and Prevention guidance. However, another 9.1% of patients were only partially vaccinated with one dose, and 45% remained unvaccinated by January 2022,
In the current study, “the uptake was very high,” Dr. Sindet-Pedersen noted, that is, “95% of the prevalent heart failure patients in 2021 received a vaccine.”
“It might be that the last 5% of the patients that did not receive a vaccine were too ill [terminal] to receive the vaccine,” she speculated, “or that was due to personal reasons.”
The researchers identified 50,893 patients with HF who were double vaccinated in 2021 and they matched them with 50,893 unvaccinated patients with HF in 2019 (prepandemic), with the same age, sex, HF duration, use of HF medications, ischemic heart disease, cancer, diabetes, atrial fibrillation, and admission with HF within 90 days.
Almost all patients in the vaccinated group received the Pfizer/BioNTech mRNA vaccine (92%) and the rest received the Moderna mRNA vaccine (8%), in 2021.
The patients had a mean age of 74, and 64% were men. They had HF for a median of 4.1 years.
During the 90-day follow-up, 1,311 patients in the unvaccinated cohort (2.56%) and 1,113 patients in the vaccinated cohort (2.23%) died; there was a significantly lower risk for all-cause death in the vaccinated cohort versus the unvaccinated cohort (–0.33 percentage points; 95% CI, –0.52 to –0.15 percentage points).
The risk for worsening heart failure was 1.1% in each group; myocarditis and venous thromboembolism were extremely rare, and risks for these conditions were not significantly different in the two groups.
The researchers and Dr. Metra declared they have no relevant financial disclosures. Dr. Metra is editor-in-chief of the European Journal of Heart Failure and senior consulting editor of the European Heart Journal.
A version of this article first appeared on Medscape.com.
Patients with heart failure (HF) who received two doses of COVID mRNA vaccines were not more likely to have worsening disease, venous thromboembolism, or myocarditis within 90 days than similar unvaccinated patients, in a case-control study in Denmark.
Moreover, in the 90 days after receiving the second shot, vaccinated patients were less likely to die of any cause, compared with unvaccinated patients during a similar 90-day period.
Caroline Sindet-Pedersen, PhD, Herlev and Gentofte Hospital, Hellerup, Denmark, and colleagues presented these findings at the annual congress of the European Society of Cardiology.
Major risk is not receiving vaccine
These results “confirm that the major risk for patients with HF is not receiving vaccination for COVID-19,” Marco Metra, MD, who was not involved with this research, said in an interview.
Dr. Metra was coauthor of an ESC guidance for the diagnosis and management of cardiovascular disease during the COVID-19 pandemic, published online ahead of print November 2021 in the European Heart Journal.
The guidance explains that patients with HF are at increased risk for hospitalization, need for mechanical ventilation, and death because of COVID-19, and that vaccination reduces the risk for serious illness from COVID-19, Dr. Sindet-Pedersen and colleagues explained in a press release from the ESC.
However, “concerns remain,” they added, “about the safety of the SARS-CoV-2 mRNA vaccines in heart failure patients, due to a perceived increased risk of cardiovascular side effects.”
The study findings suggest that “there should be no concern about cardiovascular side effects from mRNA vaccines in heart failure patients,” Dr. Sindet-Pedersen and colleagues summarized.
The results also “point to a beneficial effect of vaccination on mortality” and “indicate that patients with HF should be prioritized for COVID-19 vaccinations and boosters,” they added.
“There are ongoing concerns about the safety of COVID-19 vaccination in fragile patients and patients with heart failure,” said Dr. Metra, professor of cardiology and director of the Institute of Cardiology of the Civil Hospital and University of Brescia (Italy).
“These concerns are not based on evidence but just on reports of rare side effects (namely, myocarditis and pericarditis) in vaccinated people,” he added.
Dr. Metra also coauthored a position paper on COVID-19 vaccination in patients with HF from the Heart Failure Association of the ESC, which was published online October 2021 in the European Journal of Heart Failure.
“The current study,” he summarized, “shows a lower risk of mortality among patients vaccinated, compared with those not vaccinated.
“It has limitations,” he cautioned, “as it is not a prospective randomized study, but [rather] an observational one with comparison between vaccinated and not vaccinated patients with similar characteristics.
“However, it was done in a large population,” he noted, “and its results confirm that the major risk for patients with HF is not receiving vaccination for COVID-19.”
95% of patients with HF in Denmark double vaccinated
The group did not analyze the types of all-cause death in their study, Dr. Sindet-Pedersen clarified in an interview.
Other studies have shown that vaccines are associated with improved survival, she noted. For example, bacillus Calmette-Guérin vaccines and the measles vaccines have been linked with a decreased risk for nonspecific mortality in children, and influenza vaccines are associated with decreased all-cause mortality in patients with HF.
The rates of vaccination in this study were much higher than those for patients with HF in the United States.
In a study of 7,094 patients with HF seen at the Mount Sinai Health System between January 2021 and January 2022, 31% of patients were fully vaccinated with two doses and 14.8% had also received a booster, as per Centers for Disease Control and Prevention guidance. However, another 9.1% of patients were only partially vaccinated with one dose, and 45% remained unvaccinated by January 2022,
In the current study, “the uptake was very high,” Dr. Sindet-Pedersen noted, that is, “95% of the prevalent heart failure patients in 2021 received a vaccine.”
“It might be that the last 5% of the patients that did not receive a vaccine were too ill [terminal] to receive the vaccine,” she speculated, “or that was due to personal reasons.”
The researchers identified 50,893 patients with HF who were double vaccinated in 2021 and they matched them with 50,893 unvaccinated patients with HF in 2019 (prepandemic), with the same age, sex, HF duration, use of HF medications, ischemic heart disease, cancer, diabetes, atrial fibrillation, and admission with HF within 90 days.
Almost all patients in the vaccinated group received the Pfizer/BioNTech mRNA vaccine (92%) and the rest received the Moderna mRNA vaccine (8%), in 2021.
The patients had a mean age of 74, and 64% were men. They had HF for a median of 4.1 years.
During the 90-day follow-up, 1,311 patients in the unvaccinated cohort (2.56%) and 1,113 patients in the vaccinated cohort (2.23%) died; there was a significantly lower risk for all-cause death in the vaccinated cohort versus the unvaccinated cohort (–0.33 percentage points; 95% CI, –0.52 to –0.15 percentage points).
The risk for worsening heart failure was 1.1% in each group; myocarditis and venous thromboembolism were extremely rare, and risks for these conditions were not significantly different in the two groups.
The researchers and Dr. Metra declared they have no relevant financial disclosures. Dr. Metra is editor-in-chief of the European Journal of Heart Failure and senior consulting editor of the European Heart Journal.
A version of this article first appeared on Medscape.com.
FROM ESC CONGRESS 2022
Transverse Leukonychia and Beau Lines Following COVID-19 Vaccination
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).
Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.
She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.
Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).

Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.

She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.

Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
To the Editor:
Nail abnormalities associated with SARS-CoV-2 infection that have been reported in the medical literature include nail psoriasis,1 Beau lines,2 onychomadesis,3 heterogeneous red-white discoloration of the nail bed,4 transverse orange nail lesions,3 and the red half‐moon nail sign.3,5 It has been hypothesized that these nail findings may be an indication of microvascular injury to the distal subungual arcade of the digit or may be indicative of a procoagulant state.5,6 Currently, there is limited knowledge of the effect of COVID-19 vaccines on nail changes. We report a patient who presented with transverse leukonychia (Mees lines) and Beau lines shortly after each dose of the Pfizer-BioNTech COVID-19 messenger RNA vaccine was administered (with a total of 2 doses administered on presentation).
A 64-year-old woman with a history of rheumatoid arthritis presented with peeling of the fingernails and proximal white discoloration of several fingernails of 2 months’ duration. The patient first noticed whitening of the nails 3 weeks after she recevied the first dose of the COVID-19 vaccine. Five days after receiving the second, she presented to the dermatology clinic and exhibited transverse leukonychia in most fingernails (Figure 1).

Six weeks following the second dose of the COVID-19 vaccine, the patient returned to the dermatology clinic with Beau lines on the second and third fingernails on the right hand (Figure 2A). Subtle erythema of the proximal nail folds and distal fingers was observed in both hands. The patient also exhibited mild onychorrhexis of the left thumbnail and mottled red-brown discoloration of the third finger on the left hand (Figure 2B). Splinter hemorrhages and melanonychia of several fingernails also were observed. Our patient denied any known history of infection with SARS-CoV-2, which was confirmed by a negative COVID-19 polymerase chain reaction test result. She also denied fevers, chills, nausea, and vomiting, she and reported feeling generally well in the context of these postvaccination nail changes.

She reported no trauma or worsening of rheumatoid arthritis before or after COVID-19 vaccination. She was seronegative for rheumatoid arthritis and was being treated with hydroxychloroquine for the last year and methotrexate for the last 2 years. After each dose of the vaccine, methotrexate was withheld for 1 week and then resumed.
Subsequent follow-up examinations revealed the migration and resolution of transverse leukonychia and Beau lines. There also was interval improvement of the splinter hemorrhages. At 17 weeks following the second vaccine dose, all transverse leukonychia and Beau lines had resolved (Figure 3). The patient’s melanonychia remained unchanged.

Laboratory evaluations drawn 1 month following the first dose of the COVID-19 vaccine, including comprehensive metabolic panel; erythrocyte sedimentation rate; C-reactive protein; and vitamin B12, ferritin, and iron levels were within reference range. The complete blood cell count only showed a mildly decreased white blood cell count (3.55×103/µL [reference range, 4.16–9.95×103/µL]) and mildly elevated mean corpuscular volume (101.9 fL [reference range, 79.3–98.6 fL), both near the patient’s baseline values prior to vaccination.
Documented cutaneous manifestations of SARS‐CoV‐2 infection have included perniolike lesions (known as COVID toes) and vesicular, urticarial, petechial, livedoid, or retiform purpura eruptions. Less frequently, nail findings in patients infected with COVID-19 have been reported, including Beau lines,2 onychomadesis,3 transverse leukonychia,3,7 and the red half‐moon nail sign.3,5 Single or multiple nails may be affected. Although the pathogenesis of nail manifestations related to COVID-19 remains unclear, complement-mediated microvascular injury and thrombosis as well as the procoagulant state, which have been associated with COVID-19, may offer possible explanations.5,6 The presence of microvascular abnormalities was observed in a nail fold video capillaroscopy study of the nails of 82 patients with COVID-19, revealing pericapillary edema, capillary ectasia, sludge flow, meandering capillaries and microvascular derangement, and low capillary density.8
Our patient exhibited transverse leukonychia of the fingernails, which is thought to result from abnormal keratinization of the nail plate due to systemic disorders that induce a temporary dysfunction of nail growth.9 Fernandez-Nieto et al7 reported transverse leukonychia in a patient with COVID-19 that was hypothesized to be due to a transitory nail matrix injury.
Beau lines and onychomadesis, which represent nail matrix arrest, commonly are seen with systemic drug treatments such as chemotherapy and in infectious diseases that precipitate systemic illness, such as hand, foot, and mouth disease. Although histologic examination was not performed in our patient due to cosmetic concerns, we believe that inflammation induced by the vaccine response also can trigger nail abnormalities such as transverse leukonychia and Beau lines. Both SARS-CoV-2 infections and the COVID-19 messenger RNA vaccines can induce systemic inflammation largely due a TH1-dominant response, and they also can trigger other inflammatory conditions. Reports of lichen planus and psoriasis triggered by vaccination—the hepatitis B vaccine,10 influenza vaccine,11 and even COVID-19 vaccines1,12—have been reported. Beau lines have been observed to spontaneously resolve in a self-limiting manner in asymptomatic patients with COVID-19.
Interestingly, our patient only showed 2 nails with Beau lines. We hypothesize that the immune response triggered by vaccination was more subdued than that caused by SARS-CoV-2 infection. Additionally, our patient was already being treated with immunosuppressants, which may have been associated with a reduced immune response despite being withheld right before vaccination. One may debate whether the nail abnormalities observed in our patient constituted an isolated finding from COVID-19 vaccination or were caused by reactivation of rheumatoid arthritis. We favor the former, as the rheumatoid arthritis remained stable before and after COVID-19 vaccination. Laboratory evaluations and physical examination revealed no evidence of flares, and our patient was otherwise healthy. Although the splinter hemorrhages also improved, it is difficult to comment as to whether they were caused by the vaccine or had existed prior to vaccination. However, we believe the melanonychia observed in the nails was unrelated to the vaccine and was likely a chronic manifestation due to long-term hydroxychloroquine and/or methotrexate use.
Given accelerated global vaccination efforts to control the COVID-19 pandemic, more cases of adverse nail manifestations associated with COVID-19 vaccines are expected. Dermatologists should be aware of and use the reported nail findings to educate patients and reassure them that ungual abnormalities are potential adverse effects of COVID-19 vaccines, but they should not discourage vaccination because they usually are temporary and self-resolving.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
- Ricardo JW, Lipner SR. Case of de novo nail psoriasis triggered by the second dose of Pfizer-BioNTech BNT162b2 COVID-19 messenger RNA vaccine. JAAD Case Rep. 2021;17:18-20.
- Deng J, Ngo T, Zhu TH, et al. Telogen effluvium, Beau lines, and acral peeling associated with COVID-19 infection. JAAD Case Rep. 2021;13:138-140.
- Hadeler E, Morrison BW, Tosti A. A review of nail findings associated with COVID-19 infection. J Eur Acad Dermatol Venereol. 2021;35:E699-E709.
- Demir B, Yuksel EI, Cicek D, et al. Heterogeneous red-white discoloration of the nail bed and distal onycholysis in a patient with COVID-19. J Eur Acad Dermatol Venereol. 2021;35:E551-E553.
- Neri I, Guglielmo A, Virdi A, et al. The red half-moon nail sign: a novel manifestation of coronavirus infection. J Eur Acad Dermatol Venereol. 2020;34:E663-E665.
- Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res. 2020;220:1-13.
- Fernandez-Nieto D, Jimenez-Cauhe J, Ortega-Quijano D, et al. Transverse leukonychia (Mees’ lines) nail alterations in a COVID-19 patient. Dermatol Ther. 2020;33:E13863.
- Natalello G, De Luca G, Gigante L, et al. Nailfold capillaroscopy findings in patients with coronavirus disease 2019: broadening the spectrum of COVID-19 microvascular involvement [published online September 17, 2020]. Microvasc Res. doi:10.1016/j.mvr.2020.104071
- Piccolo V, Corneli P, Zalaudek I, et al. Mees’ lines because of chemotherapy for Hodgkin’s lymphoma. Int J Dermatol. 2020;59:E38.
- Miteva L. Bullous lichen planus with nail involvement induced by hepatitis B vaccine in a child. Int J Dermatol. 2005;44:142-144.
- Gunes AT, Fetil E, Akarsu S, et al. Possible triggering effect of influenza vaccination on psoriasis [published online August 25, 2015]. J Immunol Res. doi:10.1155/2015/258430
- Hiltun I, Sarriugarte J, Martínez-de-Espronceda I, et al. Lichen planus arising after COVID-19 vaccination. J Eur Acad Dermatol Venereol. 2021;35:e414-e415.
Practice Points
- Given accelerated global vaccination efforts to control the COVID-19 pandemic, cases of nail changes associated with COVID-19 vaccines are expected.
- Nail abnormalities are a potential general, temporary, and self-limiting adverse effect of COVID-19 vaccines that should not discourage patients from getting vaccinated.
Long COVID mimics other postviral conditions
When Jaime Seltzer first heard about a new virus that was spreading globally early in 2020, she was on full alert. As an advocate for the post-viral condition known as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), she worried about a new wave of people having long-term disabilities.
“The hair on my arms stood on end,” said Ms. Seltzer, director of scientific and medical outreach at the advocacy group MEAction and a consultant researcher at Stanford University.
Ms. Seltzer, who has had ME/CFS herself, said she wondered.
Sure enough, later in 2020, reports began emerging about people with extreme fatigue, postexertion crashes, brain fog, unrefreshing sleep, and dizziness when standing up months after a bout with the then-new viral illness. Those same symptoms had been designated as “core criteria” of ME/CFS by the National Academy of Medicine in a 2015 report.
Now, advocates like Ms. Seltzer are hoping the research and medical communities will give ME/CFS and other postviral illnesses the same attention they have increasingly focused on long COVID.
The emergence of long COVID was no surprise to researchers who study ME/CFS, because the same set of symptoms has arisen after many other viruses.
“This for all the world looks like ME/CFS. We think they are frighteningly similar, if not identical,” said David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital in Boston, who studies people with both diagnoses.
The actual numbers are hard to determine, since many people who meet ME/CFS criteria aren’t formally diagnosed. But a combined analysis of data from several studies published in March found that about one in three people had fatigue and about one in five reported having a hard time with thinking and memory 12 or more weeks after they had COVID-19.
According to some estimates, about half of people with long COVID will meet the criteria for ME/CFS, whether they’re given that specific diagnosis or not.
Other conditions that often exist with ME/CFS are also being seen in people with long COVID, including postural orthostatic tachycardia syndrome, which causes people to feel dizzy when they stand, along with other symptoms; other problems with the autonomic nervous system, which controls body systems such as heart rate, blood pressure, and digestion, known together as dysautonomia; and a condition related to allergies called mast cell activation disorder.
Post–acute infection syndromes have been linked to a long list of viruses, including Ebola, the 2003-2004 SARS virus, and Epstein-Barr – the virus most commonly associated with ME/CFS.
The problem in clinical medicine is that once an infection has cleared, the teaching has been that the person should no longer feel sick, said Nancy G. Klimas, MD, director of the Institute for Neuro-Immune Medicine at Nova Southeastern University in Miami. “I was taught that there has to be an antigen [such as a viral protein] in the system to drive the immune system to make it create sickness, and the immune system should shut off when it’s done,” she said.
Thus, if virus is gone and other routine lab tests come up negative, doctors often deem the person’s reported symptoms to be psychological, which can upset patients, Anthony Komaroff, MD, of Brigham and Women’s Hospital in Boston, wrote in July 2021.
Only recently have doctors started to appreciate the idea that the immune system may be overreacting long term, Dr. Klimas said.
Now, long COVID appears to be speeding up that recognition. Dr. Systrom said he has “absolutely” seen a change in attitude among fellow doctors who had been skeptical of ME/CFS as a “real” illness because there’s no test for it.
“I’m very keenly aware of a large group of health care professionals who really had not bought into the concept of ME/CFS as a real disease who have had an epiphany of sorts with long COVID and now, in a backwards way, have applied that same thinking to their very same patients with ME/CFS,” he said.
Science showing ‘frighteningly similar’ symptoms
Dr. Systrom has spent several years researching how ME/CFS patients cannot tolerate exercise and now is doing similar studies in people with long COVID. “Several months into the pandemic, we began receiving reports of patients who had survived COVID and maybe even had a relatively mild disease ... and as the summer of 2020 moved into the fall, it became apparent that there was a subset of patients who for all the world appeared to meet ME/CFS clinical criteria,” he said.
Using bicycle exercise tests on long COVID patients with catheters placed in their veins, Dr. Systrom and associates have shown a lack of exercise capacity that isn’t caused by heart or lung disease but instead is related to abnormal nerves and blood vessels, just as they’d shown previously in ME/CFS patient.
Avindra Nath, MD, senior investigator and clinical director of intramural research at the National Institute of Neurological Disorders and Stroke, Bethesda, Md., was doing a deep-dive scientific study on ME/CFS when the COVID-19 pandemic hit. Since then, he›s begun another study using the same protocol and sophisticated laboratory measurement to evaluate people with long COVID.
“As terrible as [long COVID] is, it’s kind of a blessing in disguise for ME/CFS because there’s just so much overlap between the two and they could very well be in many ways one in the same thing. The problem with studying ME/CFS is oftentimes you didn’t know what the trigger was. You see patients many years later, then try to backtrack and find out what happened,” said Dr. Nath, a neuroimmunologist.
With long COVID, on the other hand, “we know when they got infected and when their symptoms actually started, so it becomes much more uniform. ... It gives us an opportunity to maybe solve certain things in a much more well-defined population and try to find answers.”
Advocacy groups want to see more.
In February 2021, Solve M.E. launched the Long COVID Alliance, made up of several organizations, companies, and people with a goal to influence policy and speed up research into a range of postviral illnesses.
Solve M.E. has also pushed for inclusion of language regarding ME/CFS and related conditions into congressional bills addressing long COVID, including those that call for funding of research and clinical care.
“On the political front, we’ve really capitalized on a moment in time in which we have the spotlight,” said Emily Taylor, vice president of advocacy and engagement for Solve M.E.
“One of the hardest parts about ME/CFS is how to show that it’s real when it’s invisible. Most people agree that COVID is real and therefore if somebody gets ME/CFS after COVID, it’s real,” she said.
The advocacy groups are now pushing for non-COVID postinfection illnesses to be included in efforts aimed at helping people with long COVID, with mixed results. For example, the RECOVER Initiative, established in February 2021 with $1.5 billion in funding from Congress to the National Institutes of Health, is specifically for studying long COVID and does not fund research into other postinfection illnesses, although representatives from the ME/CFS community are advisers.
Language addressing ME/CFS and other postinfectious chronic illnesses has been included in several long COVID bills now pending in Congress, including the Care for Long COVID Act in the Senate and its companion COVID-19 Long Haulers Act in the House. “Our goal is to push for passage of a long COVID bill by the end of the year,” Ms. Taylor said.
A version of this article first appeared on WebMD.com.
When Jaime Seltzer first heard about a new virus that was spreading globally early in 2020, she was on full alert. As an advocate for the post-viral condition known as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), she worried about a new wave of people having long-term disabilities.
“The hair on my arms stood on end,” said Ms. Seltzer, director of scientific and medical outreach at the advocacy group MEAction and a consultant researcher at Stanford University.
Ms. Seltzer, who has had ME/CFS herself, said she wondered.
Sure enough, later in 2020, reports began emerging about people with extreme fatigue, postexertion crashes, brain fog, unrefreshing sleep, and dizziness when standing up months after a bout with the then-new viral illness. Those same symptoms had been designated as “core criteria” of ME/CFS by the National Academy of Medicine in a 2015 report.
Now, advocates like Ms. Seltzer are hoping the research and medical communities will give ME/CFS and other postviral illnesses the same attention they have increasingly focused on long COVID.
The emergence of long COVID was no surprise to researchers who study ME/CFS, because the same set of symptoms has arisen after many other viruses.
“This for all the world looks like ME/CFS. We think they are frighteningly similar, if not identical,” said David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital in Boston, who studies people with both diagnoses.
The actual numbers are hard to determine, since many people who meet ME/CFS criteria aren’t formally diagnosed. But a combined analysis of data from several studies published in March found that about one in three people had fatigue and about one in five reported having a hard time with thinking and memory 12 or more weeks after they had COVID-19.
According to some estimates, about half of people with long COVID will meet the criteria for ME/CFS, whether they’re given that specific diagnosis or not.
Other conditions that often exist with ME/CFS are also being seen in people with long COVID, including postural orthostatic tachycardia syndrome, which causes people to feel dizzy when they stand, along with other symptoms; other problems with the autonomic nervous system, which controls body systems such as heart rate, blood pressure, and digestion, known together as dysautonomia; and a condition related to allergies called mast cell activation disorder.
Post–acute infection syndromes have been linked to a long list of viruses, including Ebola, the 2003-2004 SARS virus, and Epstein-Barr – the virus most commonly associated with ME/CFS.
The problem in clinical medicine is that once an infection has cleared, the teaching has been that the person should no longer feel sick, said Nancy G. Klimas, MD, director of the Institute for Neuro-Immune Medicine at Nova Southeastern University in Miami. “I was taught that there has to be an antigen [such as a viral protein] in the system to drive the immune system to make it create sickness, and the immune system should shut off when it’s done,” she said.
Thus, if virus is gone and other routine lab tests come up negative, doctors often deem the person’s reported symptoms to be psychological, which can upset patients, Anthony Komaroff, MD, of Brigham and Women’s Hospital in Boston, wrote in July 2021.
Only recently have doctors started to appreciate the idea that the immune system may be overreacting long term, Dr. Klimas said.
Now, long COVID appears to be speeding up that recognition. Dr. Systrom said he has “absolutely” seen a change in attitude among fellow doctors who had been skeptical of ME/CFS as a “real” illness because there’s no test for it.
“I’m very keenly aware of a large group of health care professionals who really had not bought into the concept of ME/CFS as a real disease who have had an epiphany of sorts with long COVID and now, in a backwards way, have applied that same thinking to their very same patients with ME/CFS,” he said.
Science showing ‘frighteningly similar’ symptoms
Dr. Systrom has spent several years researching how ME/CFS patients cannot tolerate exercise and now is doing similar studies in people with long COVID. “Several months into the pandemic, we began receiving reports of patients who had survived COVID and maybe even had a relatively mild disease ... and as the summer of 2020 moved into the fall, it became apparent that there was a subset of patients who for all the world appeared to meet ME/CFS clinical criteria,” he said.
Using bicycle exercise tests on long COVID patients with catheters placed in their veins, Dr. Systrom and associates have shown a lack of exercise capacity that isn’t caused by heart or lung disease but instead is related to abnormal nerves and blood vessels, just as they’d shown previously in ME/CFS patient.
Avindra Nath, MD, senior investigator and clinical director of intramural research at the National Institute of Neurological Disorders and Stroke, Bethesda, Md., was doing a deep-dive scientific study on ME/CFS when the COVID-19 pandemic hit. Since then, he›s begun another study using the same protocol and sophisticated laboratory measurement to evaluate people with long COVID.
“As terrible as [long COVID] is, it’s kind of a blessing in disguise for ME/CFS because there’s just so much overlap between the two and they could very well be in many ways one in the same thing. The problem with studying ME/CFS is oftentimes you didn’t know what the trigger was. You see patients many years later, then try to backtrack and find out what happened,” said Dr. Nath, a neuroimmunologist.
With long COVID, on the other hand, “we know when they got infected and when their symptoms actually started, so it becomes much more uniform. ... It gives us an opportunity to maybe solve certain things in a much more well-defined population and try to find answers.”
Advocacy groups want to see more.
In February 2021, Solve M.E. launched the Long COVID Alliance, made up of several organizations, companies, and people with a goal to influence policy and speed up research into a range of postviral illnesses.
Solve M.E. has also pushed for inclusion of language regarding ME/CFS and related conditions into congressional bills addressing long COVID, including those that call for funding of research and clinical care.
“On the political front, we’ve really capitalized on a moment in time in which we have the spotlight,” said Emily Taylor, vice president of advocacy and engagement for Solve M.E.
“One of the hardest parts about ME/CFS is how to show that it’s real when it’s invisible. Most people agree that COVID is real and therefore if somebody gets ME/CFS after COVID, it’s real,” she said.
The advocacy groups are now pushing for non-COVID postinfection illnesses to be included in efforts aimed at helping people with long COVID, with mixed results. For example, the RECOVER Initiative, established in February 2021 with $1.5 billion in funding from Congress to the National Institutes of Health, is specifically for studying long COVID and does not fund research into other postinfection illnesses, although representatives from the ME/CFS community are advisers.
Language addressing ME/CFS and other postinfectious chronic illnesses has been included in several long COVID bills now pending in Congress, including the Care for Long COVID Act in the Senate and its companion COVID-19 Long Haulers Act in the House. “Our goal is to push for passage of a long COVID bill by the end of the year,” Ms. Taylor said.
A version of this article first appeared on WebMD.com.
When Jaime Seltzer first heard about a new virus that was spreading globally early in 2020, she was on full alert. As an advocate for the post-viral condition known as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), she worried about a new wave of people having long-term disabilities.
“The hair on my arms stood on end,” said Ms. Seltzer, director of scientific and medical outreach at the advocacy group MEAction and a consultant researcher at Stanford University.
Ms. Seltzer, who has had ME/CFS herself, said she wondered.
Sure enough, later in 2020, reports began emerging about people with extreme fatigue, postexertion crashes, brain fog, unrefreshing sleep, and dizziness when standing up months after a bout with the then-new viral illness. Those same symptoms had been designated as “core criteria” of ME/CFS by the National Academy of Medicine in a 2015 report.
Now, advocates like Ms. Seltzer are hoping the research and medical communities will give ME/CFS and other postviral illnesses the same attention they have increasingly focused on long COVID.
The emergence of long COVID was no surprise to researchers who study ME/CFS, because the same set of symptoms has arisen after many other viruses.
“This for all the world looks like ME/CFS. We think they are frighteningly similar, if not identical,” said David M. Systrom, MD, a pulmonary and critical care medicine specialist at Brigham and Women’s Hospital in Boston, who studies people with both diagnoses.
The actual numbers are hard to determine, since many people who meet ME/CFS criteria aren’t formally diagnosed. But a combined analysis of data from several studies published in March found that about one in three people had fatigue and about one in five reported having a hard time with thinking and memory 12 or more weeks after they had COVID-19.
According to some estimates, about half of people with long COVID will meet the criteria for ME/CFS, whether they’re given that specific diagnosis or not.
Other conditions that often exist with ME/CFS are also being seen in people with long COVID, including postural orthostatic tachycardia syndrome, which causes people to feel dizzy when they stand, along with other symptoms; other problems with the autonomic nervous system, which controls body systems such as heart rate, blood pressure, and digestion, known together as dysautonomia; and a condition related to allergies called mast cell activation disorder.
Post–acute infection syndromes have been linked to a long list of viruses, including Ebola, the 2003-2004 SARS virus, and Epstein-Barr – the virus most commonly associated with ME/CFS.
The problem in clinical medicine is that once an infection has cleared, the teaching has been that the person should no longer feel sick, said Nancy G. Klimas, MD, director of the Institute for Neuro-Immune Medicine at Nova Southeastern University in Miami. “I was taught that there has to be an antigen [such as a viral protein] in the system to drive the immune system to make it create sickness, and the immune system should shut off when it’s done,” she said.
Thus, if virus is gone and other routine lab tests come up negative, doctors often deem the person’s reported symptoms to be psychological, which can upset patients, Anthony Komaroff, MD, of Brigham and Women’s Hospital in Boston, wrote in July 2021.
Only recently have doctors started to appreciate the idea that the immune system may be overreacting long term, Dr. Klimas said.
Now, long COVID appears to be speeding up that recognition. Dr. Systrom said he has “absolutely” seen a change in attitude among fellow doctors who had been skeptical of ME/CFS as a “real” illness because there’s no test for it.
“I’m very keenly aware of a large group of health care professionals who really had not bought into the concept of ME/CFS as a real disease who have had an epiphany of sorts with long COVID and now, in a backwards way, have applied that same thinking to their very same patients with ME/CFS,” he said.
Science showing ‘frighteningly similar’ symptoms
Dr. Systrom has spent several years researching how ME/CFS patients cannot tolerate exercise and now is doing similar studies in people with long COVID. “Several months into the pandemic, we began receiving reports of patients who had survived COVID and maybe even had a relatively mild disease ... and as the summer of 2020 moved into the fall, it became apparent that there was a subset of patients who for all the world appeared to meet ME/CFS clinical criteria,” he said.
Using bicycle exercise tests on long COVID patients with catheters placed in their veins, Dr. Systrom and associates have shown a lack of exercise capacity that isn’t caused by heart or lung disease but instead is related to abnormal nerves and blood vessels, just as they’d shown previously in ME/CFS patient.
Avindra Nath, MD, senior investigator and clinical director of intramural research at the National Institute of Neurological Disorders and Stroke, Bethesda, Md., was doing a deep-dive scientific study on ME/CFS when the COVID-19 pandemic hit. Since then, he›s begun another study using the same protocol and sophisticated laboratory measurement to evaluate people with long COVID.
“As terrible as [long COVID] is, it’s kind of a blessing in disguise for ME/CFS because there’s just so much overlap between the two and they could very well be in many ways one in the same thing. The problem with studying ME/CFS is oftentimes you didn’t know what the trigger was. You see patients many years later, then try to backtrack and find out what happened,” said Dr. Nath, a neuroimmunologist.
With long COVID, on the other hand, “we know when they got infected and when their symptoms actually started, so it becomes much more uniform. ... It gives us an opportunity to maybe solve certain things in a much more well-defined population and try to find answers.”
Advocacy groups want to see more.
In February 2021, Solve M.E. launched the Long COVID Alliance, made up of several organizations, companies, and people with a goal to influence policy and speed up research into a range of postviral illnesses.
Solve M.E. has also pushed for inclusion of language regarding ME/CFS and related conditions into congressional bills addressing long COVID, including those that call for funding of research and clinical care.
“On the political front, we’ve really capitalized on a moment in time in which we have the spotlight,” said Emily Taylor, vice president of advocacy and engagement for Solve M.E.
“One of the hardest parts about ME/CFS is how to show that it’s real when it’s invisible. Most people agree that COVID is real and therefore if somebody gets ME/CFS after COVID, it’s real,” she said.
The advocacy groups are now pushing for non-COVID postinfection illnesses to be included in efforts aimed at helping people with long COVID, with mixed results. For example, the RECOVER Initiative, established in February 2021 with $1.5 billion in funding from Congress to the National Institutes of Health, is specifically for studying long COVID and does not fund research into other postinfection illnesses, although representatives from the ME/CFS community are advisers.
Language addressing ME/CFS and other postinfectious chronic illnesses has been included in several long COVID bills now pending in Congress, including the Care for Long COVID Act in the Senate and its companion COVID-19 Long Haulers Act in the House. “Our goal is to push for passage of a long COVID bill by the end of the year,” Ms. Taylor said.
A version of this article first appeared on WebMD.com.