User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Remdesivir may keep unvaccinated out of the hospital: Study
The antiviral remdesivir, an intravenous drug given mostly to seriously ill COVID-19 patients in hospitals, could keep unvaccinated people who become infected out of the hospital if given on an outpatient basis, a new study says.
Researchers studied 562 unvaccinated people from September 2020 to April 2021, according to the study published in the New England Journal of Medicine. The study determined the risk of hospitalization or death was 87% lower in study participants who were given remdesivir than participants who received a placebo.
All participants were at high risk of developing severe COVID-19 because of their age – they were over 60 – or because they had an underlying medical condition such as diabetes or obesity.
An important caveat: The findings are based on data collected before the Delta variant surged in the summer of 2021 or the Omicron variant surged late in the year, the Washington Post reported.
The new study says the drug could be helpful in keeping vaccinated as well as unvaccinated people out of the hospital – an important factor as the Omicron surge threatens to overwhelm health systems around the world.
Remdesivir could be a boon for COVID-19 patients in parts of the world that don’t have vaccines or for patients with immunocompromised systems.
“These data provide evidence that a 3-day course of remdesivir could play a critical role in helping COVID-19 patients stay out of the hospital,” Robert L. Gottlieb, MD, PhD, the therapeutic lead for COVID-19 research at Baylor Scott & White Health in Dallas, said in a news release from Gilead Pharmaceuticals. “While our hospitals are ready to assist patients in need, prevention and early intervention are preferable to reduce the risk of disease progression and allow patients not requiring oxygen to recover from home when appropriate.”
Remdesivir was the first antiviral for COVID-19 authorized by the Food and Drug Administration. It was given to then-President Donald Trump when he was hospitalized with COVID-19 in October 2020.
Gilead released the study findings in September.
A version of this article first appeared on WebMD.com.
The antiviral remdesivir, an intravenous drug given mostly to seriously ill COVID-19 patients in hospitals, could keep unvaccinated people who become infected out of the hospital if given on an outpatient basis, a new study says.
Researchers studied 562 unvaccinated people from September 2020 to April 2021, according to the study published in the New England Journal of Medicine. The study determined the risk of hospitalization or death was 87% lower in study participants who were given remdesivir than participants who received a placebo.
All participants were at high risk of developing severe COVID-19 because of their age – they were over 60 – or because they had an underlying medical condition such as diabetes or obesity.
An important caveat: The findings are based on data collected before the Delta variant surged in the summer of 2021 or the Omicron variant surged late in the year, the Washington Post reported.
The new study says the drug could be helpful in keeping vaccinated as well as unvaccinated people out of the hospital – an important factor as the Omicron surge threatens to overwhelm health systems around the world.
Remdesivir could be a boon for COVID-19 patients in parts of the world that don’t have vaccines or for patients with immunocompromised systems.
“These data provide evidence that a 3-day course of remdesivir could play a critical role in helping COVID-19 patients stay out of the hospital,” Robert L. Gottlieb, MD, PhD, the therapeutic lead for COVID-19 research at Baylor Scott & White Health in Dallas, said in a news release from Gilead Pharmaceuticals. “While our hospitals are ready to assist patients in need, prevention and early intervention are preferable to reduce the risk of disease progression and allow patients not requiring oxygen to recover from home when appropriate.”
Remdesivir was the first antiviral for COVID-19 authorized by the Food and Drug Administration. It was given to then-President Donald Trump when he was hospitalized with COVID-19 in October 2020.
Gilead released the study findings in September.
A version of this article first appeared on WebMD.com.
The antiviral remdesivir, an intravenous drug given mostly to seriously ill COVID-19 patients in hospitals, could keep unvaccinated people who become infected out of the hospital if given on an outpatient basis, a new study says.
Researchers studied 562 unvaccinated people from September 2020 to April 2021, according to the study published in the New England Journal of Medicine. The study determined the risk of hospitalization or death was 87% lower in study participants who were given remdesivir than participants who received a placebo.
All participants were at high risk of developing severe COVID-19 because of their age – they were over 60 – or because they had an underlying medical condition such as diabetes or obesity.
An important caveat: The findings are based on data collected before the Delta variant surged in the summer of 2021 or the Omicron variant surged late in the year, the Washington Post reported.
The new study says the drug could be helpful in keeping vaccinated as well as unvaccinated people out of the hospital – an important factor as the Omicron surge threatens to overwhelm health systems around the world.
Remdesivir could be a boon for COVID-19 patients in parts of the world that don’t have vaccines or for patients with immunocompromised systems.
“These data provide evidence that a 3-day course of remdesivir could play a critical role in helping COVID-19 patients stay out of the hospital,” Robert L. Gottlieb, MD, PhD, the therapeutic lead for COVID-19 research at Baylor Scott & White Health in Dallas, said in a news release from Gilead Pharmaceuticals. “While our hospitals are ready to assist patients in need, prevention and early intervention are preferable to reduce the risk of disease progression and allow patients not requiring oxygen to recover from home when appropriate.”
Remdesivir was the first antiviral for COVID-19 authorized by the Food and Drug Administration. It was given to then-President Donald Trump when he was hospitalized with COVID-19 in October 2020.
Gilead released the study findings in September.
A version of this article first appeared on WebMD.com.
New studies suggest Omicron infections are less severe than Delta ones
People who get COVID-19 infections caused by the Omicron variant are less likely to need hospital care, compared with those infected by the Delta variant, according to two large new studies from the U.K. and South Africa.
The findings, which were released ahead of peer review, add to previous glimmers of evidence suggesting that Omicron – while extremely contagious -– may result in less severe symptoms than its predecessors.
“This is helping us quantify how much less severe Omicron is than Delta, and it appears to be between 40 to 75% reduced risk of hospitalizations, adjusted for many factors, which is very good,” said Eric Topol, MD, the editor-in-chief of Medscape and a cardiologist at Scripps Research Translational Institute in La Jolla, CA.
The first analysis, which was done by the World Health Organization Collaborating Centre for Infectious Disease Modelling and Imperial College London, found that overall, people infected by Omicron had about a 20% reduced risk of needing any hospital care for their infections and a 40% lower risk of an overnight hospital stay, compared to those infected with Delta.
Meanwhile, people who were re-infected – meaning they caught Omicron after recovering from a previous COVID-19 infection – had a 50%-60% lower risk of needing hospital care, likely reflecting the benefits of having some prior immunity against the same family of viruses.
The study included everyone with polymerase chain reaction-confirmed COVID-19 in the U.K. during the first 2 weeks of December – roughly 56,000 Omicron cases and 269,000 Delta infections.
The second study, from researchers at the National Institute for Communicable Diseases in South Africa, included more than 29,000 COVID-19 cases that had lab results highly suggestive of Omicron infections. Compared to people infected with the Delta variant, those with presumed Omicron infections were about 70% less likely to have severe disease.
While the news is hopeful for individuals, on a population level, health care systems may still be stressed, the study authors warned.
“Given the high transmissibility of the Omicron virus, there remains the potential for health services to face increasing demand if Omicron cases continue to grow at the rate that has been seen in recent weeks,” said study author Neil Ferguson, PhD, who studies how infectious diseases spread at Imperial College London.
The study authors say their findings are specific to the U.K. and South Africa, where substantial portions of the population have some immune protection from past infection. In other words, they may not apply to countries where fewer people have been vaccinated or recovered from a bout with COVID-19.
A version of this article first appeared on WebMD.com.
People who get COVID-19 infections caused by the Omicron variant are less likely to need hospital care, compared with those infected by the Delta variant, according to two large new studies from the U.K. and South Africa.
The findings, which were released ahead of peer review, add to previous glimmers of evidence suggesting that Omicron – while extremely contagious -– may result in less severe symptoms than its predecessors.
“This is helping us quantify how much less severe Omicron is than Delta, and it appears to be between 40 to 75% reduced risk of hospitalizations, adjusted for many factors, which is very good,” said Eric Topol, MD, the editor-in-chief of Medscape and a cardiologist at Scripps Research Translational Institute in La Jolla, CA.
The first analysis, which was done by the World Health Organization Collaborating Centre for Infectious Disease Modelling and Imperial College London, found that overall, people infected by Omicron had about a 20% reduced risk of needing any hospital care for their infections and a 40% lower risk of an overnight hospital stay, compared to those infected with Delta.
Meanwhile, people who were re-infected – meaning they caught Omicron after recovering from a previous COVID-19 infection – had a 50%-60% lower risk of needing hospital care, likely reflecting the benefits of having some prior immunity against the same family of viruses.
The study included everyone with polymerase chain reaction-confirmed COVID-19 in the U.K. during the first 2 weeks of December – roughly 56,000 Omicron cases and 269,000 Delta infections.
The second study, from researchers at the National Institute for Communicable Diseases in South Africa, included more than 29,000 COVID-19 cases that had lab results highly suggestive of Omicron infections. Compared to people infected with the Delta variant, those with presumed Omicron infections were about 70% less likely to have severe disease.
While the news is hopeful for individuals, on a population level, health care systems may still be stressed, the study authors warned.
“Given the high transmissibility of the Omicron virus, there remains the potential for health services to face increasing demand if Omicron cases continue to grow at the rate that has been seen in recent weeks,” said study author Neil Ferguson, PhD, who studies how infectious diseases spread at Imperial College London.
The study authors say their findings are specific to the U.K. and South Africa, where substantial portions of the population have some immune protection from past infection. In other words, they may not apply to countries where fewer people have been vaccinated or recovered from a bout with COVID-19.
A version of this article first appeared on WebMD.com.
People who get COVID-19 infections caused by the Omicron variant are less likely to need hospital care, compared with those infected by the Delta variant, according to two large new studies from the U.K. and South Africa.
The findings, which were released ahead of peer review, add to previous glimmers of evidence suggesting that Omicron – while extremely contagious -– may result in less severe symptoms than its predecessors.
“This is helping us quantify how much less severe Omicron is than Delta, and it appears to be between 40 to 75% reduced risk of hospitalizations, adjusted for many factors, which is very good,” said Eric Topol, MD, the editor-in-chief of Medscape and a cardiologist at Scripps Research Translational Institute in La Jolla, CA.
The first analysis, which was done by the World Health Organization Collaborating Centre for Infectious Disease Modelling and Imperial College London, found that overall, people infected by Omicron had about a 20% reduced risk of needing any hospital care for their infections and a 40% lower risk of an overnight hospital stay, compared to those infected with Delta.
Meanwhile, people who were re-infected – meaning they caught Omicron after recovering from a previous COVID-19 infection – had a 50%-60% lower risk of needing hospital care, likely reflecting the benefits of having some prior immunity against the same family of viruses.
The study included everyone with polymerase chain reaction-confirmed COVID-19 in the U.K. during the first 2 weeks of December – roughly 56,000 Omicron cases and 269,000 Delta infections.
The second study, from researchers at the National Institute for Communicable Diseases in South Africa, included more than 29,000 COVID-19 cases that had lab results highly suggestive of Omicron infections. Compared to people infected with the Delta variant, those with presumed Omicron infections were about 70% less likely to have severe disease.
While the news is hopeful for individuals, on a population level, health care systems may still be stressed, the study authors warned.
“Given the high transmissibility of the Omicron virus, there remains the potential for health services to face increasing demand if Omicron cases continue to grow at the rate that has been seen in recent weeks,” said study author Neil Ferguson, PhD, who studies how infectious diseases spread at Imperial College London.
The study authors say their findings are specific to the U.K. and South Africa, where substantial portions of the population have some immune protection from past infection. In other words, they may not apply to countries where fewer people have been vaccinated or recovered from a bout with COVID-19.
A version of this article first appeared on WebMD.com.
Last call? Moderate alcohol’s health benefits look increasingly doubtful
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
Epilepsy in older adults: Misdiagnosis and case complexity are common
, a neurologist told an audience at the annual meeting of the American Epilepsy Society. She urged colleagues to focus on possible interactions with other neurological conditions, consider various complicating factors, and embrace a team strategy.
“There are lots of nuances,” said Rebecca O’Dwyer, MD, an adult epilepsy specialist with Rush Epilepsy Center in Chicago. “It takes a lot of time and requires a multidisciplinary approach. Taking care of older individuals with epilepsy truly is a team sport.”
According to a 2014 report highlighted by Dr. O’Dwyer, “nearly 25% of new-onset seizures occur after age 65. The incidence of epilepsy in this age group is almost twice the rate in children, and in people over age 80, it is triple the rate in children.”
Research suggests it can take up to 2 years to correctly diagnose epilepsy in older people, Dr. O’Dwyer said, and nearly two-thirds of cases may be misdiagnosed. “Some of it is just limited awareness. There’s this perception in the public that epilepsy is something that occurs in younger adults or young children, and that when you come to a certain age, you cannot have epilepsy. Also, there are differences in the clinical manifestations of their seizures, and many comorbid possibilities could also present in similar fashion to epilepsy. Some of our usual tools that we use to come to the diagnosis such as EEG are also known to be less sensitive in this age group.”
According to the 2014 report, research finds that the elderly are much more likely than young adults to have postictal sleepiness or unresponsiveness and seizures manifesting as brief moments of subtle confusion. They’re much less likely to have epileptic aura and generalized tonic seizures.
“An epileptic seizure in an older adult tends to be less dramatic with fewer motor manifestations, and they often tend to be monophasic. They may be so subtle that they’re missed by family members and other medical providers,” Dr. O’Dwyer said. “I had a patient whose seizure consisted of her tapping her left shoulder. She had been doing this for at least 6 months, and she came to my clinic after her daughter realized that she was a little confused afterward. She’d already seen a behavioral neurologist and been given the diagnosis of dementia. We were fortunate enough to catch one of these episodes while we were doing an EEG, and we diagnosed her with focal epilepsy. With one antiseizure medication, we stopped the seizures, and her memory came back.”
Make sure to take detailed histories and keep an eye out for descriptions of behaviors that are episodic but perhaps not typical of seizures, she said.
Epilepsy can be misdiagnosed as a variety of conditions, she said, such as syncope, Alzheimer’s disease, stroke, Parkinson’s disease, and atrial fibrillation. “When you do diagnose somebody older with new-onset epilepsy, you should work them up for a stroke. Because we know that within the first 4 weeks after their first seizure the likelihood that they could have a stroke is three times higher.”
It’s also possible that neurological conditions can be followed by new-onset epilepsy, she said, making dementia even worse. Low-dose antiepileptic drugs can be helpful in these patients.
But seniors are especially vulnerable to side effects of antiepileptic drugs such as sedation, dizziness, and cardiac-conduction abnormalities. “You must adhere to the mantra of going low and going slow because they are exquisitely susceptible,” Dr. O’Dwyer said.
She recommends lamotrigine, which is well tolerated with helpful mood-stabilizing effects, and levetiracetam, which attenuates cognitive decline in dementia but may cause side effects such as irritable mood. Zonisamide is showing promise in patients with parkinsonian syndromes, she said, and it may be helpful to maximize drugs that patients are already taking such as gabapentin or pregabalin.
Finally, Dr. O’Dwyer urged colleagues to work in teams that include caregivers, primary care doctors, social workers, and pharmacists. “Sometimes in all this,” she said, “my job is the easiest.”
Dr. O’Dwyer discloses research support from the Shapiro Foundation.
, a neurologist told an audience at the annual meeting of the American Epilepsy Society. She urged colleagues to focus on possible interactions with other neurological conditions, consider various complicating factors, and embrace a team strategy.
“There are lots of nuances,” said Rebecca O’Dwyer, MD, an adult epilepsy specialist with Rush Epilepsy Center in Chicago. “It takes a lot of time and requires a multidisciplinary approach. Taking care of older individuals with epilepsy truly is a team sport.”
According to a 2014 report highlighted by Dr. O’Dwyer, “nearly 25% of new-onset seizures occur after age 65. The incidence of epilepsy in this age group is almost twice the rate in children, and in people over age 80, it is triple the rate in children.”
Research suggests it can take up to 2 years to correctly diagnose epilepsy in older people, Dr. O’Dwyer said, and nearly two-thirds of cases may be misdiagnosed. “Some of it is just limited awareness. There’s this perception in the public that epilepsy is something that occurs in younger adults or young children, and that when you come to a certain age, you cannot have epilepsy. Also, there are differences in the clinical manifestations of their seizures, and many comorbid possibilities could also present in similar fashion to epilepsy. Some of our usual tools that we use to come to the diagnosis such as EEG are also known to be less sensitive in this age group.”
According to the 2014 report, research finds that the elderly are much more likely than young adults to have postictal sleepiness or unresponsiveness and seizures manifesting as brief moments of subtle confusion. They’re much less likely to have epileptic aura and generalized tonic seizures.
“An epileptic seizure in an older adult tends to be less dramatic with fewer motor manifestations, and they often tend to be monophasic. They may be so subtle that they’re missed by family members and other medical providers,” Dr. O’Dwyer said. “I had a patient whose seizure consisted of her tapping her left shoulder. She had been doing this for at least 6 months, and she came to my clinic after her daughter realized that she was a little confused afterward. She’d already seen a behavioral neurologist and been given the diagnosis of dementia. We were fortunate enough to catch one of these episodes while we were doing an EEG, and we diagnosed her with focal epilepsy. With one antiseizure medication, we stopped the seizures, and her memory came back.”
Make sure to take detailed histories and keep an eye out for descriptions of behaviors that are episodic but perhaps not typical of seizures, she said.
Epilepsy can be misdiagnosed as a variety of conditions, she said, such as syncope, Alzheimer’s disease, stroke, Parkinson’s disease, and atrial fibrillation. “When you do diagnose somebody older with new-onset epilepsy, you should work them up for a stroke. Because we know that within the first 4 weeks after their first seizure the likelihood that they could have a stroke is three times higher.”
It’s also possible that neurological conditions can be followed by new-onset epilepsy, she said, making dementia even worse. Low-dose antiepileptic drugs can be helpful in these patients.
But seniors are especially vulnerable to side effects of antiepileptic drugs such as sedation, dizziness, and cardiac-conduction abnormalities. “You must adhere to the mantra of going low and going slow because they are exquisitely susceptible,” Dr. O’Dwyer said.
She recommends lamotrigine, which is well tolerated with helpful mood-stabilizing effects, and levetiracetam, which attenuates cognitive decline in dementia but may cause side effects such as irritable mood. Zonisamide is showing promise in patients with parkinsonian syndromes, she said, and it may be helpful to maximize drugs that patients are already taking such as gabapentin or pregabalin.
Finally, Dr. O’Dwyer urged colleagues to work in teams that include caregivers, primary care doctors, social workers, and pharmacists. “Sometimes in all this,” she said, “my job is the easiest.”
Dr. O’Dwyer discloses research support from the Shapiro Foundation.
, a neurologist told an audience at the annual meeting of the American Epilepsy Society. She urged colleagues to focus on possible interactions with other neurological conditions, consider various complicating factors, and embrace a team strategy.
“There are lots of nuances,” said Rebecca O’Dwyer, MD, an adult epilepsy specialist with Rush Epilepsy Center in Chicago. “It takes a lot of time and requires a multidisciplinary approach. Taking care of older individuals with epilepsy truly is a team sport.”
According to a 2014 report highlighted by Dr. O’Dwyer, “nearly 25% of new-onset seizures occur after age 65. The incidence of epilepsy in this age group is almost twice the rate in children, and in people over age 80, it is triple the rate in children.”
Research suggests it can take up to 2 years to correctly diagnose epilepsy in older people, Dr. O’Dwyer said, and nearly two-thirds of cases may be misdiagnosed. “Some of it is just limited awareness. There’s this perception in the public that epilepsy is something that occurs in younger adults or young children, and that when you come to a certain age, you cannot have epilepsy. Also, there are differences in the clinical manifestations of their seizures, and many comorbid possibilities could also present in similar fashion to epilepsy. Some of our usual tools that we use to come to the diagnosis such as EEG are also known to be less sensitive in this age group.”
According to the 2014 report, research finds that the elderly are much more likely than young adults to have postictal sleepiness or unresponsiveness and seizures manifesting as brief moments of subtle confusion. They’re much less likely to have epileptic aura and generalized tonic seizures.
“An epileptic seizure in an older adult tends to be less dramatic with fewer motor manifestations, and they often tend to be monophasic. They may be so subtle that they’re missed by family members and other medical providers,” Dr. O’Dwyer said. “I had a patient whose seizure consisted of her tapping her left shoulder. She had been doing this for at least 6 months, and she came to my clinic after her daughter realized that she was a little confused afterward. She’d already seen a behavioral neurologist and been given the diagnosis of dementia. We were fortunate enough to catch one of these episodes while we were doing an EEG, and we diagnosed her with focal epilepsy. With one antiseizure medication, we stopped the seizures, and her memory came back.”
Make sure to take detailed histories and keep an eye out for descriptions of behaviors that are episodic but perhaps not typical of seizures, she said.
Epilepsy can be misdiagnosed as a variety of conditions, she said, such as syncope, Alzheimer’s disease, stroke, Parkinson’s disease, and atrial fibrillation. “When you do diagnose somebody older with new-onset epilepsy, you should work them up for a stroke. Because we know that within the first 4 weeks after their first seizure the likelihood that they could have a stroke is three times higher.”
It’s also possible that neurological conditions can be followed by new-onset epilepsy, she said, making dementia even worse. Low-dose antiepileptic drugs can be helpful in these patients.
But seniors are especially vulnerable to side effects of antiepileptic drugs such as sedation, dizziness, and cardiac-conduction abnormalities. “You must adhere to the mantra of going low and going slow because they are exquisitely susceptible,” Dr. O’Dwyer said.
She recommends lamotrigine, which is well tolerated with helpful mood-stabilizing effects, and levetiracetam, which attenuates cognitive decline in dementia but may cause side effects such as irritable mood. Zonisamide is showing promise in patients with parkinsonian syndromes, she said, and it may be helpful to maximize drugs that patients are already taking such as gabapentin or pregabalin.
Finally, Dr. O’Dwyer urged colleagues to work in teams that include caregivers, primary care doctors, social workers, and pharmacists. “Sometimes in all this,” she said, “my job is the easiest.”
Dr. O’Dwyer discloses research support from the Shapiro Foundation.
FROM AES 2021
FDA authorizes Pfizer antiviral pill for COVID-19
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
Newborn Screening for Spinal Muscular Atrophy in the United States
Click here to read the supplement
Newborn screening for spinal muscular atrophy (SMA) is essential to achieve optimal outcomes for patients with SMA by facilitating early identification and treatment before the onset of irreversible motor neuron loss.
Click here to read the supplement
Newborn screening for spinal muscular atrophy (SMA) is essential to achieve optimal outcomes for patients with SMA by facilitating early identification and treatment before the onset of irreversible motor neuron loss.
Click here to read the supplement
Newborn screening for spinal muscular atrophy (SMA) is essential to achieve optimal outcomes for patients with SMA by facilitating early identification and treatment before the onset of irreversible motor neuron loss.
Sleep disturbances more profound in older adults with atopic dermatitis
especially trouble staying asleep.
Those are key findings from a cross-sectional study that Jaya Manjunath, BS, and Jonathan I. Silverberg, MD, PhD, MPH, presented during a poster session at the Revolutionizing Atopic Dermatitis symposium.
“Atopic dermatitis is a chronic, pruritic skin disease associated with sleep disturbance and fatigue affecting adults of all ages,” they wrote. “When caring for geriatric patients, several factors such as sleep disturbance, polypharmacy, cognition, social support, and mobility should be considered. However, little is known about the characteristics of atopic dermatitis in the geriatric population.”
Ms. Manjunath, a student at George Washington University, Washington, and Dr. Silverberg, director of clinical research in the department of dermatology at GWU, recruited patients with AD aged 18 years and older diagnosed by Hanifin-Rajka criteria who were evaluated at an academic medical center between 2014 and 2019. They underwent full body skin exams and completed electronic questionnaires. AD severity was assessed with the Eczema Area and Severity Index (EASI), Scoring Atopic Dermatitis (SCORAD) total and itch subscores, Investigator’s Global Assessment (IGA), patient-reported Global Assessment of AD severity, and the Patient-Oriented Eczema Measure (POEM).
The researchers also assessed the frequency of sleep disturbances, including difficulty falling asleep and staying asleep, and used multivariable logistic regression models to evaluate associations of age (65 and older vs. 18-64 years) with AD severity, sleep disturbance or fatigue, controlling for total POEM score, sex, and race.
Using adjusted odds ratios, Ms. Manjunath and Dr. Silverberg found that being 65 or older was not associated with AD severity on the EASI (adjusted odds ratio, 1.47); total SCORAD (aOR, 1.10), and itch subscore (aOR, 1.00); IGA (aOR, 1.87); patient-reported Global Assessment of AD severity (aOR, 0.80), or the patient-oriented eczema measure (aOR, 0.55), associations that were not statistically significant.
However, the researchers found that older adult age was associated with an increased number of nights of sleep disturbance from AD in the past week (aOR, 2.14; P = .0142), as well as increased fatigue in the past 7 days (aOR, 1.81; P = .0313), trouble sleeping in the past 7 days (aOR, 1.98; P = .0118), and trouble staying asleep in the past 7 days (aOR, 2.26; P = .0030), but not with difficulty falling asleep in the last 7 days (aOR, 1.16; P = .5996).
“Future studies are needed to determine why geriatric AD patients have increased sleep disturbance and optimal interventions to address their sleep disturbance,” the researchers concluded.
The study was supported by the Agency for Healthcare Research and Quality, the Dermatology Foundation, and by an unrestricted grant from Galderma. Ms. Manjunath disclosed no relevant financial relationships. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
A version of this article first appeared on Medscape.com.
especially trouble staying asleep.
Those are key findings from a cross-sectional study that Jaya Manjunath, BS, and Jonathan I. Silverberg, MD, PhD, MPH, presented during a poster session at the Revolutionizing Atopic Dermatitis symposium.
“Atopic dermatitis is a chronic, pruritic skin disease associated with sleep disturbance and fatigue affecting adults of all ages,” they wrote. “When caring for geriatric patients, several factors such as sleep disturbance, polypharmacy, cognition, social support, and mobility should be considered. However, little is known about the characteristics of atopic dermatitis in the geriatric population.”
Ms. Manjunath, a student at George Washington University, Washington, and Dr. Silverberg, director of clinical research in the department of dermatology at GWU, recruited patients with AD aged 18 years and older diagnosed by Hanifin-Rajka criteria who were evaluated at an academic medical center between 2014 and 2019. They underwent full body skin exams and completed electronic questionnaires. AD severity was assessed with the Eczema Area and Severity Index (EASI), Scoring Atopic Dermatitis (SCORAD) total and itch subscores, Investigator’s Global Assessment (IGA), patient-reported Global Assessment of AD severity, and the Patient-Oriented Eczema Measure (POEM).
The researchers also assessed the frequency of sleep disturbances, including difficulty falling asleep and staying asleep, and used multivariable logistic regression models to evaluate associations of age (65 and older vs. 18-64 years) with AD severity, sleep disturbance or fatigue, controlling for total POEM score, sex, and race.
Using adjusted odds ratios, Ms. Manjunath and Dr. Silverberg found that being 65 or older was not associated with AD severity on the EASI (adjusted odds ratio, 1.47); total SCORAD (aOR, 1.10), and itch subscore (aOR, 1.00); IGA (aOR, 1.87); patient-reported Global Assessment of AD severity (aOR, 0.80), or the patient-oriented eczema measure (aOR, 0.55), associations that were not statistically significant.
However, the researchers found that older adult age was associated with an increased number of nights of sleep disturbance from AD in the past week (aOR, 2.14; P = .0142), as well as increased fatigue in the past 7 days (aOR, 1.81; P = .0313), trouble sleeping in the past 7 days (aOR, 1.98; P = .0118), and trouble staying asleep in the past 7 days (aOR, 2.26; P = .0030), but not with difficulty falling asleep in the last 7 days (aOR, 1.16; P = .5996).
“Future studies are needed to determine why geriatric AD patients have increased sleep disturbance and optimal interventions to address their sleep disturbance,” the researchers concluded.
The study was supported by the Agency for Healthcare Research and Quality, the Dermatology Foundation, and by an unrestricted grant from Galderma. Ms. Manjunath disclosed no relevant financial relationships. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
A version of this article first appeared on Medscape.com.
especially trouble staying asleep.
Those are key findings from a cross-sectional study that Jaya Manjunath, BS, and Jonathan I. Silverberg, MD, PhD, MPH, presented during a poster session at the Revolutionizing Atopic Dermatitis symposium.
“Atopic dermatitis is a chronic, pruritic skin disease associated with sleep disturbance and fatigue affecting adults of all ages,” they wrote. “When caring for geriatric patients, several factors such as sleep disturbance, polypharmacy, cognition, social support, and mobility should be considered. However, little is known about the characteristics of atopic dermatitis in the geriatric population.”
Ms. Manjunath, a student at George Washington University, Washington, and Dr. Silverberg, director of clinical research in the department of dermatology at GWU, recruited patients with AD aged 18 years and older diagnosed by Hanifin-Rajka criteria who were evaluated at an academic medical center between 2014 and 2019. They underwent full body skin exams and completed electronic questionnaires. AD severity was assessed with the Eczema Area and Severity Index (EASI), Scoring Atopic Dermatitis (SCORAD) total and itch subscores, Investigator’s Global Assessment (IGA), patient-reported Global Assessment of AD severity, and the Patient-Oriented Eczema Measure (POEM).
The researchers also assessed the frequency of sleep disturbances, including difficulty falling asleep and staying asleep, and used multivariable logistic regression models to evaluate associations of age (65 and older vs. 18-64 years) with AD severity, sleep disturbance or fatigue, controlling for total POEM score, sex, and race.
Using adjusted odds ratios, Ms. Manjunath and Dr. Silverberg found that being 65 or older was not associated with AD severity on the EASI (adjusted odds ratio, 1.47); total SCORAD (aOR, 1.10), and itch subscore (aOR, 1.00); IGA (aOR, 1.87); patient-reported Global Assessment of AD severity (aOR, 0.80), or the patient-oriented eczema measure (aOR, 0.55), associations that were not statistically significant.
However, the researchers found that older adult age was associated with an increased number of nights of sleep disturbance from AD in the past week (aOR, 2.14; P = .0142), as well as increased fatigue in the past 7 days (aOR, 1.81; P = .0313), trouble sleeping in the past 7 days (aOR, 1.98; P = .0118), and trouble staying asleep in the past 7 days (aOR, 2.26; P = .0030), but not with difficulty falling asleep in the last 7 days (aOR, 1.16; P = .5996).
“Future studies are needed to determine why geriatric AD patients have increased sleep disturbance and optimal interventions to address their sleep disturbance,” the researchers concluded.
The study was supported by the Agency for Healthcare Research and Quality, the Dermatology Foundation, and by an unrestricted grant from Galderma. Ms. Manjunath disclosed no relevant financial relationships. Dr. Silverberg reported that he is a consultant to and/or an advisory board member for several pharmaceutical companies. He is also a speaker for Regeneron and Sanofi and has received a grant from Galderma.
A version of this article first appeared on Medscape.com.
FROM REVOLUTIONIZING AD 2021
Even light physical activity linked to lower dementia risk
Older adults who participate in even light physical activity (LPA) may have a lower risk of developing dementia, new research suggests.
In a retrospective analysis of more than 62,000 individuals aged 65 or older without preexisting dementia, 6% developed dementia.
Compared with inactive individuals, “insufficiently active,” “active,” and “highly active” individuals all had a 10%, 20%, and 28% lower risk for dementia, respectively. And this association was consistent regardless of age, sex, other comorbidities, or after the researchers censored for stroke.
Even the lowest amount of LPA was associated with reduced dementia risk, investigators noted.
“In older adults, an increased physical activity level, including a low amount of LPA, was associated with a reduced risk of dementia,” Minjae Yoon, MD, division of cardiology, Severance Cardiovascular Hospital, Yonsei University, Seoul, South Korea, and colleagues wrote.
“Promotion of LPA might reduce the risk of dementia in older adults,” they added.
The findings were published online in JAMA Network Open.
Reverse causation?
Physical activity has been shown previously to be associated with reduced dementia risk. Current World Health Organization guidelines recommend that adults with normal cognition should engage in PA to reduce their risk for cognitive decline.
However, some studies have not yielded this result, “suggesting that previous findings showing a lower risk of dementia in physically active people could be attributed to reverse causation,” the investigators noted. Additionally, previous research regarding exercise intensity has been “inconsistent” concerning the role of LPA in reducing dementia risk.
Many older adults with frailty and comorbidity cannot perform intense or even moderate PA, therefore “these adults would have to gain the benefits of physical activity from LPA,” the researchers noted.
To clarify the potential association between PA and new-onset dementia, they focused specifically on the “dose-response association” between PA and dementia – especially LPA.
Between 2009 and 2012, the investigators enrolled 62,286 older individuals (60.4% women; mean age, 73.2 years) with available health checkup data from the National Health Insurance Service–Senior Database of Korea. All had no history of dementia.
Leisure-time PA was assessed with self-report questionnaires that used a 7-day recall method and included three questions regarding usual frequency (in days per week):
- Vigorous PA (VPA) for at least 20 minutes
- Moderate-intensity PA (MPA) for at least 30 minutes
- LPA for at least 30 minutes
VPA was defined as “intense exercise that caused severe shortness of breath, MPA was defined as activity causing mild shortness of breath, and LPA was defined as “walking at a slow or leisurely pace.”
PA-related energy expenditure was also calculated in metabolic equivalent (MET) minutes per week by “summing the product of frequency, intensity, and duration,” the investigators noted.
Participants were stratified on the basis of their weekly total PA levels into the following groups:
- Inactive (no LPA beyond basic movements)
- Insufficiently active (less than the recommended target range of 1-499 MET-min/wk)
- Active (meeting the recommended target range of 500-999 MET-min/wk)
- Highly active (exceeding the recommended target range of at least 1,000 MET-min/wk)
Of all participants, 35% were categorized as inactive, 25% were insufficiently active, 24.4% were active, and 15.2% were highly active.
Controversy remains
During the total median follow-up of 42 months, 6% of participants had all-cause dementia. After the researchers excluded the first 2 years, incidence of dementia was 21.6 per 1000 person-years during follow-up.
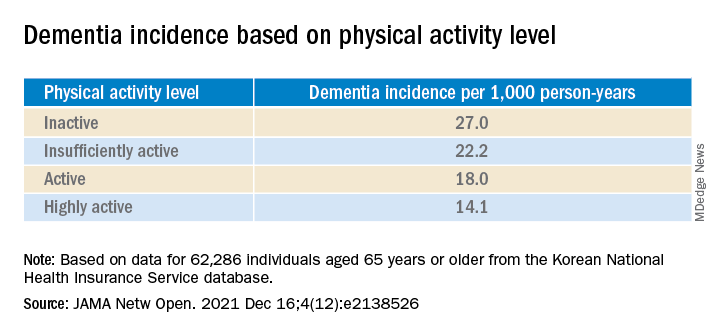
“The cumulative incidence of dementia was associated with a progressively decreasing trend with increasing physical activity” (P = .001 for trend), the investigators reported.
When using a competing-risk multivariable regression model, they found that higher levels of PA were associated with lower risk for dementia, compared with the inactive group.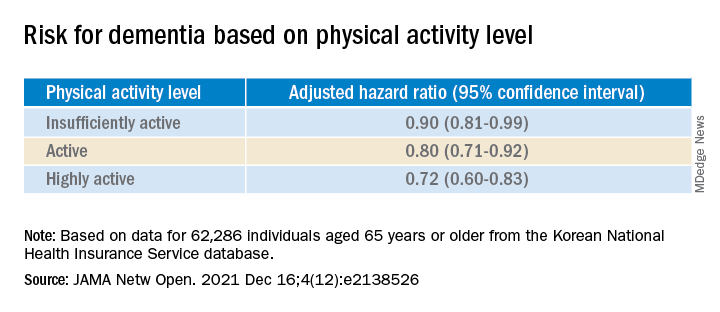
Similar findings were obtained after censoring for stroke, and were consistent for all follow-up periods. In subgroup analysis, the association between PA level and dementia risk remained consistent, regardless of age, sex, and comorbidities.
Even a low amount of LPA (1-299 MET-min/wk) was linked to reduced risk for dementia versus total sedentary behavior (adjusted HR, 0.86; 95% CI, 0.74-0.99).
The investigators noted that some “controversy” remains regarding the possibility of reverse causation and, because their study was observational in nature, “it cannot be used to establish causal relationship.”
Nevertheless, the study had important strengths, including the large number of older adults with available data, the assessment of dose-response association between PA and dementia, and the sensitivity analyses they performed, the researchers added.
Piece of important evidence
Commenting on the findings, Takashi Tarumi, PhD, senior research investigator, National Institute of Advanced Industrial Science and Technology, Ibaraki, Japan, said previous studies have suggested “an inverse association between physical activity and dementia risk, such that older adults performing a higher dose of exercise may have a greater benefit for reducing the dementia risk.”
Dr. Tarumi, an associate editor at the Journal of Alzheimer’s Disease, added the current study “significantly extends our knowledge by showing that dementia risk can also be reduced by light physical activities when they are performed for longer hours.”
This provides “another piece of important evidence” to support clinicians recommending regular physical activity for the prevention of dementia in later life, said Dr. Tarumi, who was not involved with the research.
Also commenting, Martin Underwood, MD, Warwick Medical School, Coventry, England, described the association between reduced physical inactivity and dementia as well established – and noted the current study “appears to confirm earlier observational data showing this relationship.”
The current results have “still not been able to fully exclude the possibility of reverse causation,” said Dr. Underwood, who was also not associated with the study.
However, the finding that more physically active individuals are less likely to develop dementia “only becomes of real interest if we can show that increased physical activity prevents the onset, or slows the progression, of dementia,” he noted.
“To my knowledge this has not yet been established” in randomized clinical trials, Dr. Underwood added.
The study was supported by grants from the Patient-Centered Clinical Research Coordinating Center, funded by the Ministry of Health & Welfare, Republic of Korea; and by a research grant from Yonsei University. One coauthor reported serving as a speaker for Bayer, Bristol-Myers Squibb/Pfizer, Medtronic, and Daiichi-Sankyo, and receiving research funds from Medtronic and Abbott. No other author disclosures were reported. Dr. Tarumi and Dr. Underwood have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Older adults who participate in even light physical activity (LPA) may have a lower risk of developing dementia, new research suggests.
In a retrospective analysis of more than 62,000 individuals aged 65 or older without preexisting dementia, 6% developed dementia.
Compared with inactive individuals, “insufficiently active,” “active,” and “highly active” individuals all had a 10%, 20%, and 28% lower risk for dementia, respectively. And this association was consistent regardless of age, sex, other comorbidities, or after the researchers censored for stroke.
Even the lowest amount of LPA was associated with reduced dementia risk, investigators noted.
“In older adults, an increased physical activity level, including a low amount of LPA, was associated with a reduced risk of dementia,” Minjae Yoon, MD, division of cardiology, Severance Cardiovascular Hospital, Yonsei University, Seoul, South Korea, and colleagues wrote.
“Promotion of LPA might reduce the risk of dementia in older adults,” they added.
The findings were published online in JAMA Network Open.
Reverse causation?
Physical activity has been shown previously to be associated with reduced dementia risk. Current World Health Organization guidelines recommend that adults with normal cognition should engage in PA to reduce their risk for cognitive decline.
However, some studies have not yielded this result, “suggesting that previous findings showing a lower risk of dementia in physically active people could be attributed to reverse causation,” the investigators noted. Additionally, previous research regarding exercise intensity has been “inconsistent” concerning the role of LPA in reducing dementia risk.
Many older adults with frailty and comorbidity cannot perform intense or even moderate PA, therefore “these adults would have to gain the benefits of physical activity from LPA,” the researchers noted.
To clarify the potential association between PA and new-onset dementia, they focused specifically on the “dose-response association” between PA and dementia – especially LPA.
Between 2009 and 2012, the investigators enrolled 62,286 older individuals (60.4% women; mean age, 73.2 years) with available health checkup data from the National Health Insurance Service–Senior Database of Korea. All had no history of dementia.
Leisure-time PA was assessed with self-report questionnaires that used a 7-day recall method and included three questions regarding usual frequency (in days per week):
- Vigorous PA (VPA) for at least 20 minutes
- Moderate-intensity PA (MPA) for at least 30 minutes
- LPA for at least 30 minutes
VPA was defined as “intense exercise that caused severe shortness of breath, MPA was defined as activity causing mild shortness of breath, and LPA was defined as “walking at a slow or leisurely pace.”
PA-related energy expenditure was also calculated in metabolic equivalent (MET) minutes per week by “summing the product of frequency, intensity, and duration,” the investigators noted.
Participants were stratified on the basis of their weekly total PA levels into the following groups:
- Inactive (no LPA beyond basic movements)
- Insufficiently active (less than the recommended target range of 1-499 MET-min/wk)
- Active (meeting the recommended target range of 500-999 MET-min/wk)
- Highly active (exceeding the recommended target range of at least 1,000 MET-min/wk)
Of all participants, 35% were categorized as inactive, 25% were insufficiently active, 24.4% were active, and 15.2% were highly active.
Controversy remains
During the total median follow-up of 42 months, 6% of participants had all-cause dementia. After the researchers excluded the first 2 years, incidence of dementia was 21.6 per 1000 person-years during follow-up.

“The cumulative incidence of dementia was associated with a progressively decreasing trend with increasing physical activity” (P = .001 for trend), the investigators reported.
When using a competing-risk multivariable regression model, they found that higher levels of PA were associated with lower risk for dementia, compared with the inactive group.
Similar findings were obtained after censoring for stroke, and were consistent for all follow-up periods. In subgroup analysis, the association between PA level and dementia risk remained consistent, regardless of age, sex, and comorbidities.
Even a low amount of LPA (1-299 MET-min/wk) was linked to reduced risk for dementia versus total sedentary behavior (adjusted HR, 0.86; 95% CI, 0.74-0.99).
The investigators noted that some “controversy” remains regarding the possibility of reverse causation and, because their study was observational in nature, “it cannot be used to establish causal relationship.”
Nevertheless, the study had important strengths, including the large number of older adults with available data, the assessment of dose-response association between PA and dementia, and the sensitivity analyses they performed, the researchers added.
Piece of important evidence
Commenting on the findings, Takashi Tarumi, PhD, senior research investigator, National Institute of Advanced Industrial Science and Technology, Ibaraki, Japan, said previous studies have suggested “an inverse association between physical activity and dementia risk, such that older adults performing a higher dose of exercise may have a greater benefit for reducing the dementia risk.”
Dr. Tarumi, an associate editor at the Journal of Alzheimer’s Disease, added the current study “significantly extends our knowledge by showing that dementia risk can also be reduced by light physical activities when they are performed for longer hours.”
This provides “another piece of important evidence” to support clinicians recommending regular physical activity for the prevention of dementia in later life, said Dr. Tarumi, who was not involved with the research.
Also commenting, Martin Underwood, MD, Warwick Medical School, Coventry, England, described the association between reduced physical inactivity and dementia as well established – and noted the current study “appears to confirm earlier observational data showing this relationship.”
The current results have “still not been able to fully exclude the possibility of reverse causation,” said Dr. Underwood, who was also not associated with the study.
However, the finding that more physically active individuals are less likely to develop dementia “only becomes of real interest if we can show that increased physical activity prevents the onset, or slows the progression, of dementia,” he noted.
“To my knowledge this has not yet been established” in randomized clinical trials, Dr. Underwood added.
The study was supported by grants from the Patient-Centered Clinical Research Coordinating Center, funded by the Ministry of Health & Welfare, Republic of Korea; and by a research grant from Yonsei University. One coauthor reported serving as a speaker for Bayer, Bristol-Myers Squibb/Pfizer, Medtronic, and Daiichi-Sankyo, and receiving research funds from Medtronic and Abbott. No other author disclosures were reported. Dr. Tarumi and Dr. Underwood have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Older adults who participate in even light physical activity (LPA) may have a lower risk of developing dementia, new research suggests.
In a retrospective analysis of more than 62,000 individuals aged 65 or older without preexisting dementia, 6% developed dementia.
Compared with inactive individuals, “insufficiently active,” “active,” and “highly active” individuals all had a 10%, 20%, and 28% lower risk for dementia, respectively. And this association was consistent regardless of age, sex, other comorbidities, or after the researchers censored for stroke.
Even the lowest amount of LPA was associated with reduced dementia risk, investigators noted.
“In older adults, an increased physical activity level, including a low amount of LPA, was associated with a reduced risk of dementia,” Minjae Yoon, MD, division of cardiology, Severance Cardiovascular Hospital, Yonsei University, Seoul, South Korea, and colleagues wrote.
“Promotion of LPA might reduce the risk of dementia in older adults,” they added.
The findings were published online in JAMA Network Open.
Reverse causation?
Physical activity has been shown previously to be associated with reduced dementia risk. Current World Health Organization guidelines recommend that adults with normal cognition should engage in PA to reduce their risk for cognitive decline.
However, some studies have not yielded this result, “suggesting that previous findings showing a lower risk of dementia in physically active people could be attributed to reverse causation,” the investigators noted. Additionally, previous research regarding exercise intensity has been “inconsistent” concerning the role of LPA in reducing dementia risk.
Many older adults with frailty and comorbidity cannot perform intense or even moderate PA, therefore “these adults would have to gain the benefits of physical activity from LPA,” the researchers noted.
To clarify the potential association between PA and new-onset dementia, they focused specifically on the “dose-response association” between PA and dementia – especially LPA.
Between 2009 and 2012, the investigators enrolled 62,286 older individuals (60.4% women; mean age, 73.2 years) with available health checkup data from the National Health Insurance Service–Senior Database of Korea. All had no history of dementia.
Leisure-time PA was assessed with self-report questionnaires that used a 7-day recall method and included three questions regarding usual frequency (in days per week):
- Vigorous PA (VPA) for at least 20 minutes
- Moderate-intensity PA (MPA) for at least 30 minutes
- LPA for at least 30 minutes
VPA was defined as “intense exercise that caused severe shortness of breath, MPA was defined as activity causing mild shortness of breath, and LPA was defined as “walking at a slow or leisurely pace.”
PA-related energy expenditure was also calculated in metabolic equivalent (MET) minutes per week by “summing the product of frequency, intensity, and duration,” the investigators noted.
Participants were stratified on the basis of their weekly total PA levels into the following groups:
- Inactive (no LPA beyond basic movements)
- Insufficiently active (less than the recommended target range of 1-499 MET-min/wk)
- Active (meeting the recommended target range of 500-999 MET-min/wk)
- Highly active (exceeding the recommended target range of at least 1,000 MET-min/wk)
Of all participants, 35% were categorized as inactive, 25% were insufficiently active, 24.4% were active, and 15.2% were highly active.
Controversy remains
During the total median follow-up of 42 months, 6% of participants had all-cause dementia. After the researchers excluded the first 2 years, incidence of dementia was 21.6 per 1000 person-years during follow-up.

“The cumulative incidence of dementia was associated with a progressively decreasing trend with increasing physical activity” (P = .001 for trend), the investigators reported.
When using a competing-risk multivariable regression model, they found that higher levels of PA were associated with lower risk for dementia, compared with the inactive group.
Similar findings were obtained after censoring for stroke, and were consistent for all follow-up periods. In subgroup analysis, the association between PA level and dementia risk remained consistent, regardless of age, sex, and comorbidities.
Even a low amount of LPA (1-299 MET-min/wk) was linked to reduced risk for dementia versus total sedentary behavior (adjusted HR, 0.86; 95% CI, 0.74-0.99).
The investigators noted that some “controversy” remains regarding the possibility of reverse causation and, because their study was observational in nature, “it cannot be used to establish causal relationship.”
Nevertheless, the study had important strengths, including the large number of older adults with available data, the assessment of dose-response association between PA and dementia, and the sensitivity analyses they performed, the researchers added.
Piece of important evidence
Commenting on the findings, Takashi Tarumi, PhD, senior research investigator, National Institute of Advanced Industrial Science and Technology, Ibaraki, Japan, said previous studies have suggested “an inverse association between physical activity and dementia risk, such that older adults performing a higher dose of exercise may have a greater benefit for reducing the dementia risk.”
Dr. Tarumi, an associate editor at the Journal of Alzheimer’s Disease, added the current study “significantly extends our knowledge by showing that dementia risk can also be reduced by light physical activities when they are performed for longer hours.”
This provides “another piece of important evidence” to support clinicians recommending regular physical activity for the prevention of dementia in later life, said Dr. Tarumi, who was not involved with the research.
Also commenting, Martin Underwood, MD, Warwick Medical School, Coventry, England, described the association between reduced physical inactivity and dementia as well established – and noted the current study “appears to confirm earlier observational data showing this relationship.”
The current results have “still not been able to fully exclude the possibility of reverse causation,” said Dr. Underwood, who was also not associated with the study.
However, the finding that more physically active individuals are less likely to develop dementia “only becomes of real interest if we can show that increased physical activity prevents the onset, or slows the progression, of dementia,” he noted.
“To my knowledge this has not yet been established” in randomized clinical trials, Dr. Underwood added.
The study was supported by grants from the Patient-Centered Clinical Research Coordinating Center, funded by the Ministry of Health & Welfare, Republic of Korea; and by a research grant from Yonsei University. One coauthor reported serving as a speaker for Bayer, Bristol-Myers Squibb/Pfizer, Medtronic, and Daiichi-Sankyo, and receiving research funds from Medtronic and Abbott. No other author disclosures were reported. Dr. Tarumi and Dr. Underwood have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A common problem improved but not solved
Phoenix has only a few months each year to use my hot tub, so winter is when I catch up on a lot of my reading. Recently I was reading the November Lancet, which had some interesting statistics about migraine.
- It’s the second leading cause (behind back pain) of years lived with disability.
- There are 10 million people with migraines in the United Kingdom (population roughly 70 million).
- In the last 5 years, migraine use of emergency rooms has increased 14%.
- According to the U.K. National Health Service, over 16,000 ER visits for migraine could be avoided.
These are compelling statistics, and probably (taking into account population differences) similar to numbers here in the United States or Canada.
Like all neurologists, I see my share of migraine.
Like many neurologists, I also get migraines. Not many, maybe 2-3 per month, effectively treated with a triptan. So I have a decent understanding that they aren’t pleasant.
Fortunately, migraine advances have been impressive, with seven new CGRP drugs in the last 3 years, bringing successful treatment closer for many.
But the problem is far from solved, a point that was driven home yesterday.
I awoke early yesterday morning with a migraine, and took an Imitrex. But instead of feeling better in an hour, it kept worsening until I was literally disabled by it. I took some Excedrin Migraine. The last time I had a migraine this bad was in 1998, during my fellowship, and my attending had to drive me home (thanks, Joe).
It was showing no signs of letting up. I thought about going to emergency department. After all, aren’t we trained for that when we hear “worst headache of my life?” but figured it was more likely just a migraine, and didn’t want to bog down my ED colleagues in the midst of another COVID-19 wave.
I took another Imitrex. I found a sample of Ubrelvy that I’d brought home out of curiosity, and took that, too. I think I have an old, nearly empty, bottle of Norco, somewhere, from a 2014 dental surgery, but was too photophobic to go looking for it (if I still have it at all).
I lay down in bed under the ceiling fan, and somehow fell asleep.
When I woke about 90 minutes later it was gone, like a switch had been flipped. Maybe it was all, or just one of, the meds I’d taken. I’ll never know. I could now resume my regularly scheduled program.
The migraine had cost me 7 hours. Like most small business owners, I’m trying to get all the year-end paperwork wrapped up, in addition to reviewing cases, writing up reports, and spending time with my family. So none of that happened that Saturday morning. If I’d had to see patients that morning there’s no way I could have done it.
Fortunately, as I said, that’s only the second time that’s happened to me, and it’s been 25 years since the last one.
But I’m lucky. There are those who have them far more frequently, limiting their ability to work, raise families, spend time with friends. … Have a life.
Migraine is far from a deadly disease. In neurology we treat far worse conditions. But in sheer numbers migraine affects far more people, and (indirectly) an even larger group of coworkers, parents, friends, and children who have to cover unpredictably when the other person is out with one.
For all of them,
Phoenix has only a few months each year to use my hot tub, so winter is when I catch up on a lot of my reading. Recently I was reading the November Lancet, which had some interesting statistics about migraine.
- It’s the second leading cause (behind back pain) of years lived with disability.
- There are 10 million people with migraines in the United Kingdom (population roughly 70 million).
- In the last 5 years, migraine use of emergency rooms has increased 14%.
- According to the U.K. National Health Service, over 16,000 ER visits for migraine could be avoided.
These are compelling statistics, and probably (taking into account population differences) similar to numbers here in the United States or Canada.
Like all neurologists, I see my share of migraine.
Like many neurologists, I also get migraines. Not many, maybe 2-3 per month, effectively treated with a triptan. So I have a decent understanding that they aren’t pleasant.
Fortunately, migraine advances have been impressive, with seven new CGRP drugs in the last 3 years, bringing successful treatment closer for many.
But the problem is far from solved, a point that was driven home yesterday.
I awoke early yesterday morning with a migraine, and took an Imitrex. But instead of feeling better in an hour, it kept worsening until I was literally disabled by it. I took some Excedrin Migraine. The last time I had a migraine this bad was in 1998, during my fellowship, and my attending had to drive me home (thanks, Joe).
It was showing no signs of letting up. I thought about going to emergency department. After all, aren’t we trained for that when we hear “worst headache of my life?” but figured it was more likely just a migraine, and didn’t want to bog down my ED colleagues in the midst of another COVID-19 wave.
I took another Imitrex. I found a sample of Ubrelvy that I’d brought home out of curiosity, and took that, too. I think I have an old, nearly empty, bottle of Norco, somewhere, from a 2014 dental surgery, but was too photophobic to go looking for it (if I still have it at all).
I lay down in bed under the ceiling fan, and somehow fell asleep.
When I woke about 90 minutes later it was gone, like a switch had been flipped. Maybe it was all, or just one of, the meds I’d taken. I’ll never know. I could now resume my regularly scheduled program.
The migraine had cost me 7 hours. Like most small business owners, I’m trying to get all the year-end paperwork wrapped up, in addition to reviewing cases, writing up reports, and spending time with my family. So none of that happened that Saturday morning. If I’d had to see patients that morning there’s no way I could have done it.
Fortunately, as I said, that’s only the second time that’s happened to me, and it’s been 25 years since the last one.
But I’m lucky. There are those who have them far more frequently, limiting their ability to work, raise families, spend time with friends. … Have a life.
Migraine is far from a deadly disease. In neurology we treat far worse conditions. But in sheer numbers migraine affects far more people, and (indirectly) an even larger group of coworkers, parents, friends, and children who have to cover unpredictably when the other person is out with one.
For all of them,
Phoenix has only a few months each year to use my hot tub, so winter is when I catch up on a lot of my reading. Recently I was reading the November Lancet, which had some interesting statistics about migraine.
- It’s the second leading cause (behind back pain) of years lived with disability.
- There are 10 million people with migraines in the United Kingdom (population roughly 70 million).
- In the last 5 years, migraine use of emergency rooms has increased 14%.
- According to the U.K. National Health Service, over 16,000 ER visits for migraine could be avoided.
These are compelling statistics, and probably (taking into account population differences) similar to numbers here in the United States or Canada.
Like all neurologists, I see my share of migraine.
Like many neurologists, I also get migraines. Not many, maybe 2-3 per month, effectively treated with a triptan. So I have a decent understanding that they aren’t pleasant.
Fortunately, migraine advances have been impressive, with seven new CGRP drugs in the last 3 years, bringing successful treatment closer for many.
But the problem is far from solved, a point that was driven home yesterday.
I awoke early yesterday morning with a migraine, and took an Imitrex. But instead of feeling better in an hour, it kept worsening until I was literally disabled by it. I took some Excedrin Migraine. The last time I had a migraine this bad was in 1998, during my fellowship, and my attending had to drive me home (thanks, Joe).
It was showing no signs of letting up. I thought about going to emergency department. After all, aren’t we trained for that when we hear “worst headache of my life?” but figured it was more likely just a migraine, and didn’t want to bog down my ED colleagues in the midst of another COVID-19 wave.
I took another Imitrex. I found a sample of Ubrelvy that I’d brought home out of curiosity, and took that, too. I think I have an old, nearly empty, bottle of Norco, somewhere, from a 2014 dental surgery, but was too photophobic to go looking for it (if I still have it at all).
I lay down in bed under the ceiling fan, and somehow fell asleep.
When I woke about 90 minutes later it was gone, like a switch had been flipped. Maybe it was all, or just one of, the meds I’d taken. I’ll never know. I could now resume my regularly scheduled program.
The migraine had cost me 7 hours. Like most small business owners, I’m trying to get all the year-end paperwork wrapped up, in addition to reviewing cases, writing up reports, and spending time with my family. So none of that happened that Saturday morning. If I’d had to see patients that morning there’s no way I could have done it.
Fortunately, as I said, that’s only the second time that’s happened to me, and it’s been 25 years since the last one.
But I’m lucky. There are those who have them far more frequently, limiting their ability to work, raise families, spend time with friends. … Have a life.
Migraine is far from a deadly disease. In neurology we treat far worse conditions. But in sheer numbers migraine affects far more people, and (indirectly) an even larger group of coworkers, parents, friends, and children who have to cover unpredictably when the other person is out with one.
For all of them,
Ophthalmologist who developed medical botox dies at 89
his family confirmed to National Public Radio.
Four decades ago, Alan Brown Scott, MD, a native of Berkeley, Calif., turned the drug, once a deadly poison, into a revolutionary treatment for obscure eye diseases. It later became a well-known blockbuster treatment for reducing the appearance of wrinkles and treating hyperhidrosis (excessive sweating). Other approved medical uses include treatment of overactive bladder and urinary incontinence.
According to the American Society of Plastic Surgeons, its popularity for cosmetic use was boosted further during the pandemic and it was the No. 1 minimally invasive cosmetic procedure performed in 2020. Among the 13.3 million procedures, 4.4 million involved Botox.
According to Bloomberg Businessweek, Ed Schantz, who was working in the military’s biological weapons program, was the one to first send the toxin to Dr. Scott, who wanted to explore its properties for medical use.
The same Bloomberg article also noted that the original botulinum toxin itself “is so powerful that a tiny amount can suffocate a person by paralyzing the muscles used for breathing.”
Dr. Scott was looking for a way to help his patients avoid extensive surgeries.
“Specifically, he was aiming to treat people with strabismus, or cross-eyes, and blepharospasm, which is an uncontrollable closure of eyes. Today, it’s also used as a treatment to help with migraines, hair loss, and drooling,” NPR reported.
The New York Times once described Botox as “medicine’s answer to duct tape.”
Dr. Scott was the executive director of the Smith-Kettlewell Eye Research Institute in San Francisco when he did his pioneering research with botulinum toxin in the 1970s and 1980s, according to a 2002 article in SFGate.
In 1991, Dr. Scott sold the drug to Allergan, when it was called Oculinum. The next year, the name was officially changed to Botox.
In 2002, Dr. Scott told SFGate, when asked about the more popular use for the drug, “I think that’s a charming, slightly frivolous use,” adding, “but it’s not along the lines of what I was into, applications for serious disorders.”
According to Scientific American in 2016, Dr. Scott, then age 83, kept working on the noncosmetic benefits of botulism-toxin injections for eye-related disorders at the Strabismus Research Foundation,
He told Scientific American he was proud that his efforts “are directly helpful to people.”
“There are interesting and difficult problems still to be solved, and I’m a practicing physician and I see them every day,” he said.
Dr. Scott’s daughter, Ann Scott, told NPR: “He definitely loved his work and he was also a really great father.” She said her dad involved his children in his research and work.
She added, “He was a really calm, more of a quiet reserved person,” and said he was committed to teaching his students, many of them international students.
“That was what he really loved,” she said.
Dr. Scott, who died Dec. 16, was in intensive care for the last 10 days from an unspecified illness, his daughter told NPR.
A version of this article first appeared on Medscape.com.
his family confirmed to National Public Radio.
Four decades ago, Alan Brown Scott, MD, a native of Berkeley, Calif., turned the drug, once a deadly poison, into a revolutionary treatment for obscure eye diseases. It later became a well-known blockbuster treatment for reducing the appearance of wrinkles and treating hyperhidrosis (excessive sweating). Other approved medical uses include treatment of overactive bladder and urinary incontinence.
According to the American Society of Plastic Surgeons, its popularity for cosmetic use was boosted further during the pandemic and it was the No. 1 minimally invasive cosmetic procedure performed in 2020. Among the 13.3 million procedures, 4.4 million involved Botox.
According to Bloomberg Businessweek, Ed Schantz, who was working in the military’s biological weapons program, was the one to first send the toxin to Dr. Scott, who wanted to explore its properties for medical use.
The same Bloomberg article also noted that the original botulinum toxin itself “is so powerful that a tiny amount can suffocate a person by paralyzing the muscles used for breathing.”
Dr. Scott was looking for a way to help his patients avoid extensive surgeries.
“Specifically, he was aiming to treat people with strabismus, or cross-eyes, and blepharospasm, which is an uncontrollable closure of eyes. Today, it’s also used as a treatment to help with migraines, hair loss, and drooling,” NPR reported.
The New York Times once described Botox as “medicine’s answer to duct tape.”
Dr. Scott was the executive director of the Smith-Kettlewell Eye Research Institute in San Francisco when he did his pioneering research with botulinum toxin in the 1970s and 1980s, according to a 2002 article in SFGate.
In 1991, Dr. Scott sold the drug to Allergan, when it was called Oculinum. The next year, the name was officially changed to Botox.
In 2002, Dr. Scott told SFGate, when asked about the more popular use for the drug, “I think that’s a charming, slightly frivolous use,” adding, “but it’s not along the lines of what I was into, applications for serious disorders.”
According to Scientific American in 2016, Dr. Scott, then age 83, kept working on the noncosmetic benefits of botulism-toxin injections for eye-related disorders at the Strabismus Research Foundation,
He told Scientific American he was proud that his efforts “are directly helpful to people.”
“There are interesting and difficult problems still to be solved, and I’m a practicing physician and I see them every day,” he said.
Dr. Scott’s daughter, Ann Scott, told NPR: “He definitely loved his work and he was also a really great father.” She said her dad involved his children in his research and work.
She added, “He was a really calm, more of a quiet reserved person,” and said he was committed to teaching his students, many of them international students.
“That was what he really loved,” she said.
Dr. Scott, who died Dec. 16, was in intensive care for the last 10 days from an unspecified illness, his daughter told NPR.
A version of this article first appeared on Medscape.com.
his family confirmed to National Public Radio.
Four decades ago, Alan Brown Scott, MD, a native of Berkeley, Calif., turned the drug, once a deadly poison, into a revolutionary treatment for obscure eye diseases. It later became a well-known blockbuster treatment for reducing the appearance of wrinkles and treating hyperhidrosis (excessive sweating). Other approved medical uses include treatment of overactive bladder and urinary incontinence.
According to the American Society of Plastic Surgeons, its popularity for cosmetic use was boosted further during the pandemic and it was the No. 1 minimally invasive cosmetic procedure performed in 2020. Among the 13.3 million procedures, 4.4 million involved Botox.
According to Bloomberg Businessweek, Ed Schantz, who was working in the military’s biological weapons program, was the one to first send the toxin to Dr. Scott, who wanted to explore its properties for medical use.
The same Bloomberg article also noted that the original botulinum toxin itself “is so powerful that a tiny amount can suffocate a person by paralyzing the muscles used for breathing.”
Dr. Scott was looking for a way to help his patients avoid extensive surgeries.
“Specifically, he was aiming to treat people with strabismus, or cross-eyes, and blepharospasm, which is an uncontrollable closure of eyes. Today, it’s also used as a treatment to help with migraines, hair loss, and drooling,” NPR reported.
The New York Times once described Botox as “medicine’s answer to duct tape.”
Dr. Scott was the executive director of the Smith-Kettlewell Eye Research Institute in San Francisco when he did his pioneering research with botulinum toxin in the 1970s and 1980s, according to a 2002 article in SFGate.
In 1991, Dr. Scott sold the drug to Allergan, when it was called Oculinum. The next year, the name was officially changed to Botox.
In 2002, Dr. Scott told SFGate, when asked about the more popular use for the drug, “I think that’s a charming, slightly frivolous use,” adding, “but it’s not along the lines of what I was into, applications for serious disorders.”
According to Scientific American in 2016, Dr. Scott, then age 83, kept working on the noncosmetic benefits of botulism-toxin injections for eye-related disorders at the Strabismus Research Foundation,
He told Scientific American he was proud that his efforts “are directly helpful to people.”
“There are interesting and difficult problems still to be solved, and I’m a practicing physician and I see them every day,” he said.
Dr. Scott’s daughter, Ann Scott, told NPR: “He definitely loved his work and he was also a really great father.” She said her dad involved his children in his research and work.
She added, “He was a really calm, more of a quiet reserved person,” and said he was committed to teaching his students, many of them international students.
“That was what he really loved,” she said.
Dr. Scott, who died Dec. 16, was in intensive care for the last 10 days from an unspecified illness, his daughter told NPR.
A version of this article first appeared on Medscape.com.





