User login
Bringing you the latest news, research and reviews, exclusive interviews, podcasts, quizzes, and more.
div[contains(@class, 'read-next-article')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
nav[contains(@class, 'nav-ce-stack nav-ce-stack__large-screen')]
header[@id='header']
div[contains(@class, 'header__large-screen')]
div[contains(@class, 'read-next-article')]
div[contains(@class, 'main-prefix')]
div[contains(@class, 'nav-primary')]
nav[contains(@class, 'nav-primary')]
section[contains(@class, 'footer-nav-section-wrapper')]
footer[@id='footer']
section[contains(@class, 'nav-hidden')]
div[contains(@class, 'ce-card-content')]
nav[contains(@class, 'nav-ce-stack')]
div[contains(@class, 'view-medstat-quiz-listing-panes')]
div[contains(@class, 'pane-article-sidebar-latest-news')]
Excessive drooling is a sign of greater dysfunction in patients with Parkinson’s disease
, new research shows. “Sialorrhea is not just a cosmetic problem,” study investigator Francesca Morgante, MD, associate professor of neurology, St. George’s University, London, told this news organization.
“We need to understand the relationship between sialorrhea and these speech and swallowing disturbances and whether treatment for sialorrhea improves that,” Dr. Morgante added.
The findings were presented at the 2021 Congress of the European Academy of Neurology.
Underrecognized symptom
Sialorrhea is an underrecognized nonmotor symptom that can affect up to 70% of patients with Parkinson’s disease, said co-investigator Ioana Cociasu, PhD, postdoctoral research fellow, Neurosciences Research Center, St. George’s University. The impact on quality of life increases with disease severity, she said.
The current study included 101 consecutive patients attending an advanced Parkinson’s disease disorders clinic. Researchers collected demographic data that included information on gender, age, age at Parkinson’s disease onset, and disease duration. They also gathered data on motor symptoms by assessing total levodopa equivalent daily dose (LEDD) and LEDD dopamine agonists. They also assessed results on the Unified Parkinson’s Disease Rating Scale (UPDRS) part III and the Hoehn and Yahr scale for on- and off-medication states.
Nonmotor functioning was assessed using the Non-Motor Symptoms Scale (NMSS) and Scales for Outcomes in Parkinson’s disease–autonomic dysfunction (SCOPA-AUT) questionnaire. Among patients with Parkinson’s disease, autonomic dysfunction can precede motor impairment and can involve orthostatic and postprandial hypotension, among other symptoms, the investigators noted.
Health status and quality of life were assessed using the Parkinson’s disease questionnaire–39 items (PDQ-39). The Radboud Oral Motor Inventory for PD (ROMP) was used to measure orofacial symptoms. ROMP is a self-administered questionnaire that evaluates speech, swallowing disturbances, and drooling of saliva. The Montreal Cognitive Assessment test was also used.
Investigators compared participants with sialorrhea to those without sialorrhea, described as droolers and nondroolers. Droolers were defined as those scoring higher than 1 on the UPDRS-II item 6. This signified slight but definite presence of saliva in the mouth and/or the possibility of nighttime drooling.
Greater impairment
Among the participants, 65 (64.4%) were classified as droolers, and 36 (35.6%) as nondroolers.
Patients with both Parkinson’s disease and sialorrhea were significantly more impaired in terms of motor functioning than those without sialorrhea. In these patients, the UPDRS-III was more severe in both the off- (P = .03) and on-states (P = .002), and they had less improvement with the levodopa challenge test (P = .007).
Droolers were also more severely affected by nonmotor problems. They had more severe speech dysfunction (P < .0001) and swallowing dysfunction (P < .05), and they had higher scores on the NMSS (P = .0008) and SCOPA-AUT (P = .003) and poorer quality-of-life scores on the PDQ-39 (P = .049).
To evaluate respiratory tract infections, the researchers used electronic health records. About 15.4% of the study population had had a documented respiratory infection since they were diagnosed with Parkinson’s disease.
Upper and lower respiratory tract infections were more frequent among droolers than nondroolers (P = .05).
“Infections might arise from swallowing disturbances leading to aspiration and drooling,” Dr. Morgante noted.
The drooling did not appear to affect cognition or sleep in these patients.
Treatment options?
Following the study presentation, session co-chair Philippe G. Damier, MD, PhD, professor of neurology, University Hospital, Nantes, France, asked about the best treatment for sialorrhea for these patients.
In general, those with milder disease might try chewing gum to improve swallowing; patients with more severe cases may benefit from botulinum toxin injections, said Dr. Cociasu. The treatment choice, she added, “very much depends on the severity of the sialorrhea.”
Botulinum toxin therapy involves injections into the salivary gland to reduce saliva production. It is typically administered about every 4 months.
The second session co-chair, Elena Moro, MD, PhD, director of the Movement Disorders Unit at Grenoble Alpes University, France, pointed out that chewing gum may be a swallowing hazard for patients with PD and severe dementia.
Asked by Dr. Moro whether patients with higher scores on balance and posture were more likely to have sialorrhea, Dr. Cociasu said she and her colleagues are currently looking into this.
Dr. Morgante said that the current study did not examine the effect of treatment on speech disorders associated with sialorrhea. “We are running another study now to understand the effect of treatment of sialorrhea on these features,” she said.
Dr. Morgante and Dr. Cociasu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. “Sialorrhea is not just a cosmetic problem,” study investigator Francesca Morgante, MD, associate professor of neurology, St. George’s University, London, told this news organization.
“We need to understand the relationship between sialorrhea and these speech and swallowing disturbances and whether treatment for sialorrhea improves that,” Dr. Morgante added.
The findings were presented at the 2021 Congress of the European Academy of Neurology.
Underrecognized symptom
Sialorrhea is an underrecognized nonmotor symptom that can affect up to 70% of patients with Parkinson’s disease, said co-investigator Ioana Cociasu, PhD, postdoctoral research fellow, Neurosciences Research Center, St. George’s University. The impact on quality of life increases with disease severity, she said.
The current study included 101 consecutive patients attending an advanced Parkinson’s disease disorders clinic. Researchers collected demographic data that included information on gender, age, age at Parkinson’s disease onset, and disease duration. They also gathered data on motor symptoms by assessing total levodopa equivalent daily dose (LEDD) and LEDD dopamine agonists. They also assessed results on the Unified Parkinson’s Disease Rating Scale (UPDRS) part III and the Hoehn and Yahr scale for on- and off-medication states.
Nonmotor functioning was assessed using the Non-Motor Symptoms Scale (NMSS) and Scales for Outcomes in Parkinson’s disease–autonomic dysfunction (SCOPA-AUT) questionnaire. Among patients with Parkinson’s disease, autonomic dysfunction can precede motor impairment and can involve orthostatic and postprandial hypotension, among other symptoms, the investigators noted.
Health status and quality of life were assessed using the Parkinson’s disease questionnaire–39 items (PDQ-39). The Radboud Oral Motor Inventory for PD (ROMP) was used to measure orofacial symptoms. ROMP is a self-administered questionnaire that evaluates speech, swallowing disturbances, and drooling of saliva. The Montreal Cognitive Assessment test was also used.
Investigators compared participants with sialorrhea to those without sialorrhea, described as droolers and nondroolers. Droolers were defined as those scoring higher than 1 on the UPDRS-II item 6. This signified slight but definite presence of saliva in the mouth and/or the possibility of nighttime drooling.
Greater impairment
Among the participants, 65 (64.4%) were classified as droolers, and 36 (35.6%) as nondroolers.
Patients with both Parkinson’s disease and sialorrhea were significantly more impaired in terms of motor functioning than those without sialorrhea. In these patients, the UPDRS-III was more severe in both the off- (P = .03) and on-states (P = .002), and they had less improvement with the levodopa challenge test (P = .007).
Droolers were also more severely affected by nonmotor problems. They had more severe speech dysfunction (P < .0001) and swallowing dysfunction (P < .05), and they had higher scores on the NMSS (P = .0008) and SCOPA-AUT (P = .003) and poorer quality-of-life scores on the PDQ-39 (P = .049).
To evaluate respiratory tract infections, the researchers used electronic health records. About 15.4% of the study population had had a documented respiratory infection since they were diagnosed with Parkinson’s disease.
Upper and lower respiratory tract infections were more frequent among droolers than nondroolers (P = .05).
“Infections might arise from swallowing disturbances leading to aspiration and drooling,” Dr. Morgante noted.
The drooling did not appear to affect cognition or sleep in these patients.
Treatment options?
Following the study presentation, session co-chair Philippe G. Damier, MD, PhD, professor of neurology, University Hospital, Nantes, France, asked about the best treatment for sialorrhea for these patients.
In general, those with milder disease might try chewing gum to improve swallowing; patients with more severe cases may benefit from botulinum toxin injections, said Dr. Cociasu. The treatment choice, she added, “very much depends on the severity of the sialorrhea.”
Botulinum toxin therapy involves injections into the salivary gland to reduce saliva production. It is typically administered about every 4 months.
The second session co-chair, Elena Moro, MD, PhD, director of the Movement Disorders Unit at Grenoble Alpes University, France, pointed out that chewing gum may be a swallowing hazard for patients with PD and severe dementia.
Asked by Dr. Moro whether patients with higher scores on balance and posture were more likely to have sialorrhea, Dr. Cociasu said she and her colleagues are currently looking into this.
Dr. Morgante said that the current study did not examine the effect of treatment on speech disorders associated with sialorrhea. “We are running another study now to understand the effect of treatment of sialorrhea on these features,” she said.
Dr. Morgante and Dr. Cociasu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
, new research shows. “Sialorrhea is not just a cosmetic problem,” study investigator Francesca Morgante, MD, associate professor of neurology, St. George’s University, London, told this news organization.
“We need to understand the relationship between sialorrhea and these speech and swallowing disturbances and whether treatment for sialorrhea improves that,” Dr. Morgante added.
The findings were presented at the 2021 Congress of the European Academy of Neurology.
Underrecognized symptom
Sialorrhea is an underrecognized nonmotor symptom that can affect up to 70% of patients with Parkinson’s disease, said co-investigator Ioana Cociasu, PhD, postdoctoral research fellow, Neurosciences Research Center, St. George’s University. The impact on quality of life increases with disease severity, she said.
The current study included 101 consecutive patients attending an advanced Parkinson’s disease disorders clinic. Researchers collected demographic data that included information on gender, age, age at Parkinson’s disease onset, and disease duration. They also gathered data on motor symptoms by assessing total levodopa equivalent daily dose (LEDD) and LEDD dopamine agonists. They also assessed results on the Unified Parkinson’s Disease Rating Scale (UPDRS) part III and the Hoehn and Yahr scale for on- and off-medication states.
Nonmotor functioning was assessed using the Non-Motor Symptoms Scale (NMSS) and Scales for Outcomes in Parkinson’s disease–autonomic dysfunction (SCOPA-AUT) questionnaire. Among patients with Parkinson’s disease, autonomic dysfunction can precede motor impairment and can involve orthostatic and postprandial hypotension, among other symptoms, the investigators noted.
Health status and quality of life were assessed using the Parkinson’s disease questionnaire–39 items (PDQ-39). The Radboud Oral Motor Inventory for PD (ROMP) was used to measure orofacial symptoms. ROMP is a self-administered questionnaire that evaluates speech, swallowing disturbances, and drooling of saliva. The Montreal Cognitive Assessment test was also used.
Investigators compared participants with sialorrhea to those without sialorrhea, described as droolers and nondroolers. Droolers were defined as those scoring higher than 1 on the UPDRS-II item 6. This signified slight but definite presence of saliva in the mouth and/or the possibility of nighttime drooling.
Greater impairment
Among the participants, 65 (64.4%) were classified as droolers, and 36 (35.6%) as nondroolers.
Patients with both Parkinson’s disease and sialorrhea were significantly more impaired in terms of motor functioning than those without sialorrhea. In these patients, the UPDRS-III was more severe in both the off- (P = .03) and on-states (P = .002), and they had less improvement with the levodopa challenge test (P = .007).
Droolers were also more severely affected by nonmotor problems. They had more severe speech dysfunction (P < .0001) and swallowing dysfunction (P < .05), and they had higher scores on the NMSS (P = .0008) and SCOPA-AUT (P = .003) and poorer quality-of-life scores on the PDQ-39 (P = .049).
To evaluate respiratory tract infections, the researchers used electronic health records. About 15.4% of the study population had had a documented respiratory infection since they were diagnosed with Parkinson’s disease.
Upper and lower respiratory tract infections were more frequent among droolers than nondroolers (P = .05).
“Infections might arise from swallowing disturbances leading to aspiration and drooling,” Dr. Morgante noted.
The drooling did not appear to affect cognition or sleep in these patients.
Treatment options?
Following the study presentation, session co-chair Philippe G. Damier, MD, PhD, professor of neurology, University Hospital, Nantes, France, asked about the best treatment for sialorrhea for these patients.
In general, those with milder disease might try chewing gum to improve swallowing; patients with more severe cases may benefit from botulinum toxin injections, said Dr. Cociasu. The treatment choice, she added, “very much depends on the severity of the sialorrhea.”
Botulinum toxin therapy involves injections into the salivary gland to reduce saliva production. It is typically administered about every 4 months.
The second session co-chair, Elena Moro, MD, PhD, director of the Movement Disorders Unit at Grenoble Alpes University, France, pointed out that chewing gum may be a swallowing hazard for patients with PD and severe dementia.
Asked by Dr. Moro whether patients with higher scores on balance and posture were more likely to have sialorrhea, Dr. Cociasu said she and her colleagues are currently looking into this.
Dr. Morgante said that the current study did not examine the effect of treatment on speech disorders associated with sialorrhea. “We are running another study now to understand the effect of treatment of sialorrhea on these features,” she said.
Dr. Morgante and Dr. Cociasu have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From EAN 2021
AI-based software demonstrates accuracy in diagnosis of autism
A software program based on artificial intelligence (AI) is effective for distinguishing young children with autism spectrum disorder (ASD) from those with other conditions, according to results of a pivotal trial presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
The AI-based software, which will be submitted to regulatory approval as a device, employs an algorithm that assembles inputs from a caregiver questionnaire, a video, and a clinician questionnaire, according to Sharief Taraman, MD, a pediatric neurologist at CHOC, a pediatric health care system in Orange County, Calif.
Although the device could be employed in a variety of settings, it is envisioned for use by primary care physicians. This will circumvent the need for specialist evaluation except in challenging cases. Currently, nearly all children with ASD are diagnosed in specialty care, according to data cited by Dr. Taraman.
“The lack of diagnostic tools for ASD in primary care settings contributes to an average delay of 3 years between first parental concern and diagnosis and to long wait lists for specialty evaluation,” he reported at the virtual meeting, presented by MedscapeLive.
When used with clinical judgment and criteria from the American Psychiatric Association’s 5th edition of the Diagnostic and Statistical Manual (DSM-5), the data from the trial suggest the diagnostic tool in the hands of primary care physicians “could efficiently and accurately assess ASD in children 18 to 72 months old,” said Dr. Taraman, also an associate clinical professor of pediatrics at the University of California, Irvine.*
The AI-assisted software was evaluated in 425 children at 14 sites in 6 states. The study population was reflective of U.S. demographics. Although only 36% of the children were female, this is consistent with ASD prevalence. Only 60% of the subjects were White. Nearly 30% were Black or Latinx and other populations, such as those of Asian heritage, were represented.
Children between the ages of 18 and 72 months were eligible if both a caregiver and a health care professional were concerned that the child had ASD. About the same time that a caregiver completed a 20-item questionnaire and the primary care physician completed a 15-item questionnaire on a mobile device, the caregiver uploaded two videos of 1-2 minutes in length.
This information, along with a 33-item questionnaire completed by an analyst of the submitted videos, was then processed by the software algorithm. It provided a patient status of positive or negative for ASD, or it concluded that the status was indeterminate.
“To reduce the risk of false classifications, the indeterminate status was included as a safety feature,” Dr. Taraman explained. However, Dr. Taraman considers an indeterminate designation potentially actionable. Rather than a negative result, this status suggests a complex neurodevelopmental disorder and indicates the need for further evaluation.
The reference standard diagnosis, completed in all participants in this study, was a specialist evaluation completed independently by two experts. The presence or absence of ASD was confirmed if the experts agreed. If they did not, a third specialist made the final determination.
In comparison to the specialist determinations, all were correctly classified except for one child, in which the software was determined to have made a false-negative diagnosis. A diagnosis of ASD was reached in 29% of the study participants.
For those with a determinate designation, the sensitivity was 98.4% and the specificity was 78.9%. This translated into positive predictive and negative predictive values of 80.8% and 98.3%, respectively.
Of those identified as indeterminate by the AI-assisted algorithm, 91% were ultimately considered by specialist evaluation to have complex issues. In this group, ASD was part of the complex clinical picture in 20%. The others had non-ASD neurodevelopmental conditions, according to Dr. Taraman.
When the accuracy was evaluated across ages, ethnicity, and factors such as parent education or family income, the tool performed consistently, Dr. Taraman reported. This is important, he said, because the presence or absence of ASD is misdiagnosed in many underserved populations.
The focus on developing a methodology specific for use in primary care was based on evidence that the delay in the diagnosis of ASD is attributable to long wait times for specialty evaluations.
“There will never be enough specialists. There is a need for a way to streamline the diagnosis of ASD,” Dr. Taraman maintained. This is helpful not only to parents concerned about their children, he said, but also there are data to suggest that early intervention improves outcomes.
A specialist in ASD, Paul Carbone, MD, medical director of the child development program at the University of Utah, Salt Lake City, agreed. He said early diagnosis and intervention should be a goal.
“Reducing the age of ASD diagnosis is a priority because early entry into autism-specific interventions is a strong predictor of optimal developmental outcomes for children,” Dr. Carbone said.
Although he is not familiar with this experimental AI-assisted diagnostic program, he has published on the feasibility of ASD diagnosis at the primary care level. In his study, Dr. Carbone examined the Modified Checklist for Autism in Toddlers (M-CHAT) as one of several methodologies that might be considered.
Diagnosis of ASD “can be achieved through systematic processes within primary care that facilitate universal development surveillance and autism screening followed by prompt and timely diagnostic evaluations of at-risk children,” Dr. Carbone said.
MedscapeLive and this news organization are owned by the same parent company. Dr. Taraman reported a financial relationship with Cognoa, the company that is developing the ASD software for clinical use. Dr. Carbone reported that he has no conflicts of interest.
*Updated, 7/7/21
A software program based on artificial intelligence (AI) is effective for distinguishing young children with autism spectrum disorder (ASD) from those with other conditions, according to results of a pivotal trial presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
The AI-based software, which will be submitted to regulatory approval as a device, employs an algorithm that assembles inputs from a caregiver questionnaire, a video, and a clinician questionnaire, according to Sharief Taraman, MD, a pediatric neurologist at CHOC, a pediatric health care system in Orange County, Calif.
Although the device could be employed in a variety of settings, it is envisioned for use by primary care physicians. This will circumvent the need for specialist evaluation except in challenging cases. Currently, nearly all children with ASD are diagnosed in specialty care, according to data cited by Dr. Taraman.
“The lack of diagnostic tools for ASD in primary care settings contributes to an average delay of 3 years between first parental concern and diagnosis and to long wait lists for specialty evaluation,” he reported at the virtual meeting, presented by MedscapeLive.
When used with clinical judgment and criteria from the American Psychiatric Association’s 5th edition of the Diagnostic and Statistical Manual (DSM-5), the data from the trial suggest the diagnostic tool in the hands of primary care physicians “could efficiently and accurately assess ASD in children 18 to 72 months old,” said Dr. Taraman, also an associate clinical professor of pediatrics at the University of California, Irvine.*
The AI-assisted software was evaluated in 425 children at 14 sites in 6 states. The study population was reflective of U.S. demographics. Although only 36% of the children were female, this is consistent with ASD prevalence. Only 60% of the subjects were White. Nearly 30% were Black or Latinx and other populations, such as those of Asian heritage, were represented.
Children between the ages of 18 and 72 months were eligible if both a caregiver and a health care professional were concerned that the child had ASD. About the same time that a caregiver completed a 20-item questionnaire and the primary care physician completed a 15-item questionnaire on a mobile device, the caregiver uploaded two videos of 1-2 minutes in length.
This information, along with a 33-item questionnaire completed by an analyst of the submitted videos, was then processed by the software algorithm. It provided a patient status of positive or negative for ASD, or it concluded that the status was indeterminate.
“To reduce the risk of false classifications, the indeterminate status was included as a safety feature,” Dr. Taraman explained. However, Dr. Taraman considers an indeterminate designation potentially actionable. Rather than a negative result, this status suggests a complex neurodevelopmental disorder and indicates the need for further evaluation.
The reference standard diagnosis, completed in all participants in this study, was a specialist evaluation completed independently by two experts. The presence or absence of ASD was confirmed if the experts agreed. If they did not, a third specialist made the final determination.
In comparison to the specialist determinations, all were correctly classified except for one child, in which the software was determined to have made a false-negative diagnosis. A diagnosis of ASD was reached in 29% of the study participants.
For those with a determinate designation, the sensitivity was 98.4% and the specificity was 78.9%. This translated into positive predictive and negative predictive values of 80.8% and 98.3%, respectively.
Of those identified as indeterminate by the AI-assisted algorithm, 91% were ultimately considered by specialist evaluation to have complex issues. In this group, ASD was part of the complex clinical picture in 20%. The others had non-ASD neurodevelopmental conditions, according to Dr. Taraman.
When the accuracy was evaluated across ages, ethnicity, and factors such as parent education or family income, the tool performed consistently, Dr. Taraman reported. This is important, he said, because the presence or absence of ASD is misdiagnosed in many underserved populations.
The focus on developing a methodology specific for use in primary care was based on evidence that the delay in the diagnosis of ASD is attributable to long wait times for specialty evaluations.
“There will never be enough specialists. There is a need for a way to streamline the diagnosis of ASD,” Dr. Taraman maintained. This is helpful not only to parents concerned about their children, he said, but also there are data to suggest that early intervention improves outcomes.
A specialist in ASD, Paul Carbone, MD, medical director of the child development program at the University of Utah, Salt Lake City, agreed. He said early diagnosis and intervention should be a goal.
“Reducing the age of ASD diagnosis is a priority because early entry into autism-specific interventions is a strong predictor of optimal developmental outcomes for children,” Dr. Carbone said.
Although he is not familiar with this experimental AI-assisted diagnostic program, he has published on the feasibility of ASD diagnosis at the primary care level. In his study, Dr. Carbone examined the Modified Checklist for Autism in Toddlers (M-CHAT) as one of several methodologies that might be considered.
Diagnosis of ASD “can be achieved through systematic processes within primary care that facilitate universal development surveillance and autism screening followed by prompt and timely diagnostic evaluations of at-risk children,” Dr. Carbone said.
MedscapeLive and this news organization are owned by the same parent company. Dr. Taraman reported a financial relationship with Cognoa, the company that is developing the ASD software for clinical use. Dr. Carbone reported that he has no conflicts of interest.
*Updated, 7/7/21
A software program based on artificial intelligence (AI) is effective for distinguishing young children with autism spectrum disorder (ASD) from those with other conditions, according to results of a pivotal trial presented by Current Psychiatry and the American Academy of Clinical Psychiatrists.
The AI-based software, which will be submitted to regulatory approval as a device, employs an algorithm that assembles inputs from a caregiver questionnaire, a video, and a clinician questionnaire, according to Sharief Taraman, MD, a pediatric neurologist at CHOC, a pediatric health care system in Orange County, Calif.
Although the device could be employed in a variety of settings, it is envisioned for use by primary care physicians. This will circumvent the need for specialist evaluation except in challenging cases. Currently, nearly all children with ASD are diagnosed in specialty care, according to data cited by Dr. Taraman.
“The lack of diagnostic tools for ASD in primary care settings contributes to an average delay of 3 years between first parental concern and diagnosis and to long wait lists for specialty evaluation,” he reported at the virtual meeting, presented by MedscapeLive.
When used with clinical judgment and criteria from the American Psychiatric Association’s 5th edition of the Diagnostic and Statistical Manual (DSM-5), the data from the trial suggest the diagnostic tool in the hands of primary care physicians “could efficiently and accurately assess ASD in children 18 to 72 months old,” said Dr. Taraman, also an associate clinical professor of pediatrics at the University of California, Irvine.*
The AI-assisted software was evaluated in 425 children at 14 sites in 6 states. The study population was reflective of U.S. demographics. Although only 36% of the children were female, this is consistent with ASD prevalence. Only 60% of the subjects were White. Nearly 30% were Black or Latinx and other populations, such as those of Asian heritage, were represented.
Children between the ages of 18 and 72 months were eligible if both a caregiver and a health care professional were concerned that the child had ASD. About the same time that a caregiver completed a 20-item questionnaire and the primary care physician completed a 15-item questionnaire on a mobile device, the caregiver uploaded two videos of 1-2 minutes in length.
This information, along with a 33-item questionnaire completed by an analyst of the submitted videos, was then processed by the software algorithm. It provided a patient status of positive or negative for ASD, or it concluded that the status was indeterminate.
“To reduce the risk of false classifications, the indeterminate status was included as a safety feature,” Dr. Taraman explained. However, Dr. Taraman considers an indeterminate designation potentially actionable. Rather than a negative result, this status suggests a complex neurodevelopmental disorder and indicates the need for further evaluation.
The reference standard diagnosis, completed in all participants in this study, was a specialist evaluation completed independently by two experts. The presence or absence of ASD was confirmed if the experts agreed. If they did not, a third specialist made the final determination.
In comparison to the specialist determinations, all were correctly classified except for one child, in which the software was determined to have made a false-negative diagnosis. A diagnosis of ASD was reached in 29% of the study participants.
For those with a determinate designation, the sensitivity was 98.4% and the specificity was 78.9%. This translated into positive predictive and negative predictive values of 80.8% and 98.3%, respectively.
Of those identified as indeterminate by the AI-assisted algorithm, 91% were ultimately considered by specialist evaluation to have complex issues. In this group, ASD was part of the complex clinical picture in 20%. The others had non-ASD neurodevelopmental conditions, according to Dr. Taraman.
When the accuracy was evaluated across ages, ethnicity, and factors such as parent education or family income, the tool performed consistently, Dr. Taraman reported. This is important, he said, because the presence or absence of ASD is misdiagnosed in many underserved populations.
The focus on developing a methodology specific for use in primary care was based on evidence that the delay in the diagnosis of ASD is attributable to long wait times for specialty evaluations.
“There will never be enough specialists. There is a need for a way to streamline the diagnosis of ASD,” Dr. Taraman maintained. This is helpful not only to parents concerned about their children, he said, but also there are data to suggest that early intervention improves outcomes.
A specialist in ASD, Paul Carbone, MD, medical director of the child development program at the University of Utah, Salt Lake City, agreed. He said early diagnosis and intervention should be a goal.
“Reducing the age of ASD diagnosis is a priority because early entry into autism-specific interventions is a strong predictor of optimal developmental outcomes for children,” Dr. Carbone said.
Although he is not familiar with this experimental AI-assisted diagnostic program, he has published on the feasibility of ASD diagnosis at the primary care level. In his study, Dr. Carbone examined the Modified Checklist for Autism in Toddlers (M-CHAT) as one of several methodologies that might be considered.
Diagnosis of ASD “can be achieved through systematic processes within primary care that facilitate universal development surveillance and autism screening followed by prompt and timely diagnostic evaluations of at-risk children,” Dr. Carbone said.
MedscapeLive and this news organization are owned by the same parent company. Dr. Taraman reported a financial relationship with Cognoa, the company that is developing the ASD software for clinical use. Dr. Carbone reported that he has no conflicts of interest.
*Updated, 7/7/21
FROM CP/AACP PSYCHIATRY UPDATE
Few clinical guidelines exist for treating post-COVID symptoms
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
As doctors struggled through several surges of COVID-19 infections, most of what we learned was acquired through real-life experience. While many treatment options were promoted, most flat-out failed to be real therapeutics at all. Now that we have a safe and effective vaccine, we can prevent many infections from this virus. However, we are still left to manage the many post-COVID symptoms our patients continue to suffer with.
Symptoms following infection can last for months and range widely from “brain fog,” fatigue, dyspnea, chest pain, generalized weakness, depression, and a host of others. Patients may experience one or all of these symptoms, and there is currently no good way to predict who will go on to become a COVID “long hauler”.
Following the example of being educated by COVID as it happened, the same is true for managing post-COVID symptoms. The medical community still has a poor understanding of why some people develop it and there are few evidence-based studies to support any treatment modalities.
which they define as “new, recurring, or ongoing symptoms more than 4 weeks after infection, sometimes after initial symptom recovery.” It is important to note that these symptoms can occur in any degree of sickness during the acute infection, including in those who were asymptomatic. Even the actual name of this post-COVID syndrome is still being developed, with several other names being used for it as well.
While the guidelines are quite extensive, the actual clinical recommendations are still vague. For example, it is advised to let the patient know that post-COVID symptoms are still not well understood. While it is important to be transparent with patients, this does little to reassure them. Patients look to doctors, especially their primary care physicians, to guide them on the best treatment paths. Yet, we currently have none for post-COVID syndrome.
It is also advised to treat the patients’ symptoms and help improve functioning. For many diseases, doctors like to get to the root cause of the problem. Treating a symptom often masks an underlying condition. It may make the patient feel better and improve what they are capable of doing, which is important, but it also fails to unmask the real problem. It is also important to note that symptoms can be out of proportion to clinical findings and should not be dismissed: we just don’t have the answers yet.
One helpful recommendation is having a patient keep a diary of their symptoms. This will help both the patient and doctor learn what may be triggering factors. If it is, for example, exertion that induces breathlessness, perhaps the patient can gradually increase their level of activity to minimize symptoms. Additionally, a “comprehensive rehabilitation program” is also advised and this can greatly assist addressing all the issues a patient is experiencing, physically and medically.
It is also advised that management of underlying medical conditions be optimized. While this is very important, it is not something specific to post-COVID syndrome: All patients should have their underlying medical conditions well controlled. It might be that the patient is paying more attention to their overall health, which is a good thing. However, this does not necessarily reduce the current symptoms a patient is experiencing.
The CDC makes a good attempt to offer guidance in the frustrating management of post-COVID syndrome. However, their clinical guidelines fail to offer specific management tools specific to treating post-COVID patients. The recommendations offered are more helpful to health in general. The fact that more specific recommendations are lacking is simply caused by the lack of knowledge of this condition at present. As more research is conducted and more knowledge obtained, new guidelines should become more detailed.
Dr. Girgis practices family medicine in South River, N.J., and is a clinical assistant professor of family medicine at Robert Wood Johnson Medical School, New Brunswick, N.J. You can contact her at [email protected].
Novel NSAID–triptan drug effectively relieves migraine pain
, new research suggests. Results from the phase 3 INTERCEPT trial show that the treatment, known as AXS-07 (Axsome Therapeutics), also provided greater relief from the patients’ most bothersome symptom (MBS) compared with placebo.
In addition, about 74% of patients who received AXS-07 experienced no progression of pain from 2 to 24 hours after dosing and were less than half as likely to use rescue medication through 24 hours than those who received placebo.
Similar to a previous formulation combining naproxen sodium and sumatriptan, AXS-07 combines a nonsteroidal anti-inflammatory drug with a triptan. The combination is synergistic, investigators note, because one drug addresses pain mechanisms that the other does not.
“Rizatriptan’s primary mechanism is peripheral, and NSAIDs have both peripheral and central benefit,” said study investigator Stewart J. Tepper, MD, professor of neurology, Geisel School of Medicine at Dartmouth, Hanover, N.H. “That is why the whole is greater than the sum of the parts,” Dr. Tepper added.
The findings were presented at the American Headache Society’s 2021 annual meeting.
Acute treatments needed
For many patients, current migraine treatments are inadequate. In addition, suboptimal acute treatment can increase risk for progression from episodic migraine to chronic migraine. It also increases the risk for medication-overuse headache.
The search for optimal acute treatments is therefore “really important for patients,” Dr. Tepper noted.
Because it contains rizatriptan, AXS-07 is believed to inhibit the release of calcitonin gene-related peptide, reverse the vasodilation that it causes, and decrease the transmission of pain signals. Meloxicam, on the other hand, is thought to reduce neuroinflammation and reverse central sensitization, which maintains chronic pain.
In the phase 3, double-blind INTERCEPT trial, the investigators examined AXS-07 for early treatment of migraine. Eligible patients were aged 18 to 65 years, had been diagnosed with migraine in accordance with ICHD-3 criteria, and averaged two to eight migraines per month.
The researchers randomly assigned a single dose of AXS-07 (n = 152) or placebo (n = 150). Participants were asked to administer treatment to themselves at the earliest sign of migraine pain.
The trial’s two primary endpoints were pain freedom and freedom from the MBS 2 hours after dosing. Secondary endpoints included sustained pain freedom and freedom from pain progression, functional disability, and use of rescue medication.
Demographic characteristics of the study population reflected those of the general population of people with migraine, according to the researchers. More than 85% of participants were women, and the study group’s mean age was 41 years. There were no demographic differences between the two treatment groups.
Reduced pain progression
Results showed that 2 hours after treatment, rate of pain freedom was 32.6% in the AXS-07 group and 16.3% in the placebo group (P = .002). At the same time point, rate of freedom from MBS was 43.9% and 26.7%, respectively (P = .003).
Approximately 64% of patients who received AXS-07 were pain free at 12 hours, and 69% were pain free at 24 hours. In contrast, 42% of the placebo group were pain free at 12 hours, and 47% were pain free at 24 hours (P < .001 for both comparisons).
The benefits AXS-07 provided were sustained; 22.7% of the active-treatment group achieved sustained pain freedom from 2 to 24 hours after treatment, compared with 12.6% of the placebo group (P = .03). Results were similar for sustained pain freedom from 2 to 48 hours after treatment (20.5% vs. 9.6%; P = .013).
In addition, 73.5% of patients who received AXS-07 had freedom from pain progression from 2 to 24 hours after treatment, versus 47.4% of those who received placebo (P < .001). The rate of rescue medication use through 24 hours was 15.3% and 42.2%, respectively (P < .001).
AXS-07 was also linked to significant reductions in functional disability. About 74% of patients who received it reported no disability at 24 hours, compared with 47% of patients who received placebo (P < .001). Scores on the Patient Global Impression of Change scale were very much improved or much improved 2 hours after dosing for 52.4% of the AXS-07 group, versus 27.7% of the placebo group (P < .001).
The overall rate of treatment-emergent adverse events (AEs) was 17.9% in the active group and 7.7% among the control group. The rate of somnolence was 4.3%, versus 2.1%; the rate of dizziness was 2.9%, versus 1.4%; and the rate of paresthesia was 2.1%, versus 0%. There were no serious AEs.
“Unexpectedly, and it’s hard to interpret this, but the nausea associated with the use of AXS-07 is less than with either of the active components or the placebo,” said Dr. Tepper. “It’s not dramatically different for dizziness.”
Improved adherence?
Meloxicam is generally used not as an acute medication but for prevention, Dr. Tepper noted. The drug is often administered to reduce inflammation in conditions such as chronic arthritis.
AXS-07 incorporates an altered pharmacokinetic delivery system to provide a quicker onset of effect for meloxicam.
“Most headache specialists would say that of all the oral triptans, rizatriptan is the fastest,” said Dr. Tepper.
The idea for the new agent was to hasten the onset of meloxicam’s effect so that both active components would work rapidly. “We know that there is a synergy between NSAIDs and triptans, in terms of complete headache response,” Dr. Tepper said.
Data indicate that when neurologists recommend that patients take an NSAID and triptan together at the beginning of an attack, patients rarely comply. “It’s a big adherence issue,” said Dr. Tepper. “They’re more likely to get a complete response if they take them together, especially if the tablet is designed to deliver the two products together in an optimal way.”
Uncertain therapeutic advantage
Commenting on the findings, Robert Shapiro, MD, PhD, professor of neurologic science at the University of Vermont, Burlington, noted that because of favorable data from past studies for the combination of 85 mg of sumatriptan with 500 mg of naproxen sodium, the coadministration of a triptan with an NSAID has been a standard of care for the past decade.
“It’s therefore unsurprising that a combination of rizatriptan 10 mg plus meloxicam 20 mg in a proprietary MoSEIC formulation might also prove to be more effective than either individual medication taken alone for acute migraine attacks,” said Dr. Shapiro, who was not involved with the research.
It is not possible to compare the efficacy and tolerability of AXS-07 with those of sumatriptan–naproxen sodium without head-to-head trials. However, the available data suggest that the latter formulation is superior, he added.
In 2008, researchers conducted two parallel-group, placebo-controlled trials of sumatriptan–naproxen sodium taken early in a migraine attack. These trials had protocols comparable to that of the current INTERCEPT trial for AXS-07, said Dr. Shapiro.
For the key primary endpoint of 2-hour pain freedom, the two sumatriptan–naproxen sodium trials found therapeutic gains of 35% and 36%, respectively, versus 16.3% for the AXS-07 trial. The placebo response rates (17% and 15% for sumatriptan–naproxen sodium, vs. 16.3% for the AXS-07 trial) were comparable.
Similarly, for the endpoint of 2- to 24-hour pain freedom, the sumatriptan–naproxen sodium trials found therapeutic gains of 33% and 26%, respectively, versus 15.1% for the AXS-07 trial. Again, response rates for placebo were comparable (12% and 14% for sumatriptan–naproxen sodium, vs. 12.6% for AXS-07).
The placebo-adjusted differences for reporting any treatment-emergent AE, otherwise known as “therapeutic penalty,” was 10.2% for AXS-07 in the INTERCEPT trial, versus 7% and 5%, respectively for participants in the two sumatriptan–naproxen sodium trials.
“In light of these data, it’s not immediately apparent what advantage AXS-07 might offer over sumatriptan–naproxen sodium,” said Dr. Shapiro.
“Furthermore, sumatriptan–naproxen sodium is currently available in generic form,” he added.
The study was funded by Axsome Therapeutics. Dr. Tepper is a consultant to Axsome Therapeutics. Dr. Shapiro has previously performed research consulting for Lilly and Lundbeck.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from the phase 3 INTERCEPT trial show that the treatment, known as AXS-07 (Axsome Therapeutics), also provided greater relief from the patients’ most bothersome symptom (MBS) compared with placebo.
In addition, about 74% of patients who received AXS-07 experienced no progression of pain from 2 to 24 hours after dosing and were less than half as likely to use rescue medication through 24 hours than those who received placebo.
Similar to a previous formulation combining naproxen sodium and sumatriptan, AXS-07 combines a nonsteroidal anti-inflammatory drug with a triptan. The combination is synergistic, investigators note, because one drug addresses pain mechanisms that the other does not.
“Rizatriptan’s primary mechanism is peripheral, and NSAIDs have both peripheral and central benefit,” said study investigator Stewart J. Tepper, MD, professor of neurology, Geisel School of Medicine at Dartmouth, Hanover, N.H. “That is why the whole is greater than the sum of the parts,” Dr. Tepper added.
The findings were presented at the American Headache Society’s 2021 annual meeting.
Acute treatments needed
For many patients, current migraine treatments are inadequate. In addition, suboptimal acute treatment can increase risk for progression from episodic migraine to chronic migraine. It also increases the risk for medication-overuse headache.
The search for optimal acute treatments is therefore “really important for patients,” Dr. Tepper noted.
Because it contains rizatriptan, AXS-07 is believed to inhibit the release of calcitonin gene-related peptide, reverse the vasodilation that it causes, and decrease the transmission of pain signals. Meloxicam, on the other hand, is thought to reduce neuroinflammation and reverse central sensitization, which maintains chronic pain.
In the phase 3, double-blind INTERCEPT trial, the investigators examined AXS-07 for early treatment of migraine. Eligible patients were aged 18 to 65 years, had been diagnosed with migraine in accordance with ICHD-3 criteria, and averaged two to eight migraines per month.
The researchers randomly assigned a single dose of AXS-07 (n = 152) or placebo (n = 150). Participants were asked to administer treatment to themselves at the earliest sign of migraine pain.
The trial’s two primary endpoints were pain freedom and freedom from the MBS 2 hours after dosing. Secondary endpoints included sustained pain freedom and freedom from pain progression, functional disability, and use of rescue medication.
Demographic characteristics of the study population reflected those of the general population of people with migraine, according to the researchers. More than 85% of participants were women, and the study group’s mean age was 41 years. There were no demographic differences between the two treatment groups.
Reduced pain progression
Results showed that 2 hours after treatment, rate of pain freedom was 32.6% in the AXS-07 group and 16.3% in the placebo group (P = .002). At the same time point, rate of freedom from MBS was 43.9% and 26.7%, respectively (P = .003).
Approximately 64% of patients who received AXS-07 were pain free at 12 hours, and 69% were pain free at 24 hours. In contrast, 42% of the placebo group were pain free at 12 hours, and 47% were pain free at 24 hours (P < .001 for both comparisons).
The benefits AXS-07 provided were sustained; 22.7% of the active-treatment group achieved sustained pain freedom from 2 to 24 hours after treatment, compared with 12.6% of the placebo group (P = .03). Results were similar for sustained pain freedom from 2 to 48 hours after treatment (20.5% vs. 9.6%; P = .013).
In addition, 73.5% of patients who received AXS-07 had freedom from pain progression from 2 to 24 hours after treatment, versus 47.4% of those who received placebo (P < .001). The rate of rescue medication use through 24 hours was 15.3% and 42.2%, respectively (P < .001).
AXS-07 was also linked to significant reductions in functional disability. About 74% of patients who received it reported no disability at 24 hours, compared with 47% of patients who received placebo (P < .001). Scores on the Patient Global Impression of Change scale were very much improved or much improved 2 hours after dosing for 52.4% of the AXS-07 group, versus 27.7% of the placebo group (P < .001).
The overall rate of treatment-emergent adverse events (AEs) was 17.9% in the active group and 7.7% among the control group. The rate of somnolence was 4.3%, versus 2.1%; the rate of dizziness was 2.9%, versus 1.4%; and the rate of paresthesia was 2.1%, versus 0%. There were no serious AEs.
“Unexpectedly, and it’s hard to interpret this, but the nausea associated with the use of AXS-07 is less than with either of the active components or the placebo,” said Dr. Tepper. “It’s not dramatically different for dizziness.”
Improved adherence?
Meloxicam is generally used not as an acute medication but for prevention, Dr. Tepper noted. The drug is often administered to reduce inflammation in conditions such as chronic arthritis.
AXS-07 incorporates an altered pharmacokinetic delivery system to provide a quicker onset of effect for meloxicam.
“Most headache specialists would say that of all the oral triptans, rizatriptan is the fastest,” said Dr. Tepper.
The idea for the new agent was to hasten the onset of meloxicam’s effect so that both active components would work rapidly. “We know that there is a synergy between NSAIDs and triptans, in terms of complete headache response,” Dr. Tepper said.
Data indicate that when neurologists recommend that patients take an NSAID and triptan together at the beginning of an attack, patients rarely comply. “It’s a big adherence issue,” said Dr. Tepper. “They’re more likely to get a complete response if they take them together, especially if the tablet is designed to deliver the two products together in an optimal way.”
Uncertain therapeutic advantage
Commenting on the findings, Robert Shapiro, MD, PhD, professor of neurologic science at the University of Vermont, Burlington, noted that because of favorable data from past studies for the combination of 85 mg of sumatriptan with 500 mg of naproxen sodium, the coadministration of a triptan with an NSAID has been a standard of care for the past decade.
“It’s therefore unsurprising that a combination of rizatriptan 10 mg plus meloxicam 20 mg in a proprietary MoSEIC formulation might also prove to be more effective than either individual medication taken alone for acute migraine attacks,” said Dr. Shapiro, who was not involved with the research.
It is not possible to compare the efficacy and tolerability of AXS-07 with those of sumatriptan–naproxen sodium without head-to-head trials. However, the available data suggest that the latter formulation is superior, he added.
In 2008, researchers conducted two parallel-group, placebo-controlled trials of sumatriptan–naproxen sodium taken early in a migraine attack. These trials had protocols comparable to that of the current INTERCEPT trial for AXS-07, said Dr. Shapiro.
For the key primary endpoint of 2-hour pain freedom, the two sumatriptan–naproxen sodium trials found therapeutic gains of 35% and 36%, respectively, versus 16.3% for the AXS-07 trial. The placebo response rates (17% and 15% for sumatriptan–naproxen sodium, vs. 16.3% for the AXS-07 trial) were comparable.
Similarly, for the endpoint of 2- to 24-hour pain freedom, the sumatriptan–naproxen sodium trials found therapeutic gains of 33% and 26%, respectively, versus 15.1% for the AXS-07 trial. Again, response rates for placebo were comparable (12% and 14% for sumatriptan–naproxen sodium, vs. 12.6% for AXS-07).
The placebo-adjusted differences for reporting any treatment-emergent AE, otherwise known as “therapeutic penalty,” was 10.2% for AXS-07 in the INTERCEPT trial, versus 7% and 5%, respectively for participants in the two sumatriptan–naproxen sodium trials.
“In light of these data, it’s not immediately apparent what advantage AXS-07 might offer over sumatriptan–naproxen sodium,” said Dr. Shapiro.
“Furthermore, sumatriptan–naproxen sodium is currently available in generic form,” he added.
The study was funded by Axsome Therapeutics. Dr. Tepper is a consultant to Axsome Therapeutics. Dr. Shapiro has previously performed research consulting for Lilly and Lundbeck.
A version of this article first appeared on Medscape.com.
, new research suggests. Results from the phase 3 INTERCEPT trial show that the treatment, known as AXS-07 (Axsome Therapeutics), also provided greater relief from the patients’ most bothersome symptom (MBS) compared with placebo.
In addition, about 74% of patients who received AXS-07 experienced no progression of pain from 2 to 24 hours after dosing and were less than half as likely to use rescue medication through 24 hours than those who received placebo.
Similar to a previous formulation combining naproxen sodium and sumatriptan, AXS-07 combines a nonsteroidal anti-inflammatory drug with a triptan. The combination is synergistic, investigators note, because one drug addresses pain mechanisms that the other does not.
“Rizatriptan’s primary mechanism is peripheral, and NSAIDs have both peripheral and central benefit,” said study investigator Stewart J. Tepper, MD, professor of neurology, Geisel School of Medicine at Dartmouth, Hanover, N.H. “That is why the whole is greater than the sum of the parts,” Dr. Tepper added.
The findings were presented at the American Headache Society’s 2021 annual meeting.
Acute treatments needed
For many patients, current migraine treatments are inadequate. In addition, suboptimal acute treatment can increase risk for progression from episodic migraine to chronic migraine. It also increases the risk for medication-overuse headache.
The search for optimal acute treatments is therefore “really important for patients,” Dr. Tepper noted.
Because it contains rizatriptan, AXS-07 is believed to inhibit the release of calcitonin gene-related peptide, reverse the vasodilation that it causes, and decrease the transmission of pain signals. Meloxicam, on the other hand, is thought to reduce neuroinflammation and reverse central sensitization, which maintains chronic pain.
In the phase 3, double-blind INTERCEPT trial, the investigators examined AXS-07 for early treatment of migraine. Eligible patients were aged 18 to 65 years, had been diagnosed with migraine in accordance with ICHD-3 criteria, and averaged two to eight migraines per month.
The researchers randomly assigned a single dose of AXS-07 (n = 152) or placebo (n = 150). Participants were asked to administer treatment to themselves at the earliest sign of migraine pain.
The trial’s two primary endpoints were pain freedom and freedom from the MBS 2 hours after dosing. Secondary endpoints included sustained pain freedom and freedom from pain progression, functional disability, and use of rescue medication.
Demographic characteristics of the study population reflected those of the general population of people with migraine, according to the researchers. More than 85% of participants were women, and the study group’s mean age was 41 years. There were no demographic differences between the two treatment groups.
Reduced pain progression
Results showed that 2 hours after treatment, rate of pain freedom was 32.6% in the AXS-07 group and 16.3% in the placebo group (P = .002). At the same time point, rate of freedom from MBS was 43.9% and 26.7%, respectively (P = .003).
Approximately 64% of patients who received AXS-07 were pain free at 12 hours, and 69% were pain free at 24 hours. In contrast, 42% of the placebo group were pain free at 12 hours, and 47% were pain free at 24 hours (P < .001 for both comparisons).
The benefits AXS-07 provided were sustained; 22.7% of the active-treatment group achieved sustained pain freedom from 2 to 24 hours after treatment, compared with 12.6% of the placebo group (P = .03). Results were similar for sustained pain freedom from 2 to 48 hours after treatment (20.5% vs. 9.6%; P = .013).
In addition, 73.5% of patients who received AXS-07 had freedom from pain progression from 2 to 24 hours after treatment, versus 47.4% of those who received placebo (P < .001). The rate of rescue medication use through 24 hours was 15.3% and 42.2%, respectively (P < .001).
AXS-07 was also linked to significant reductions in functional disability. About 74% of patients who received it reported no disability at 24 hours, compared with 47% of patients who received placebo (P < .001). Scores on the Patient Global Impression of Change scale were very much improved or much improved 2 hours after dosing for 52.4% of the AXS-07 group, versus 27.7% of the placebo group (P < .001).
The overall rate of treatment-emergent adverse events (AEs) was 17.9% in the active group and 7.7% among the control group. The rate of somnolence was 4.3%, versus 2.1%; the rate of dizziness was 2.9%, versus 1.4%; and the rate of paresthesia was 2.1%, versus 0%. There were no serious AEs.
“Unexpectedly, and it’s hard to interpret this, but the nausea associated with the use of AXS-07 is less than with either of the active components or the placebo,” said Dr. Tepper. “It’s not dramatically different for dizziness.”
Improved adherence?
Meloxicam is generally used not as an acute medication but for prevention, Dr. Tepper noted. The drug is often administered to reduce inflammation in conditions such as chronic arthritis.
AXS-07 incorporates an altered pharmacokinetic delivery system to provide a quicker onset of effect for meloxicam.
“Most headache specialists would say that of all the oral triptans, rizatriptan is the fastest,” said Dr. Tepper.
The idea for the new agent was to hasten the onset of meloxicam’s effect so that both active components would work rapidly. “We know that there is a synergy between NSAIDs and triptans, in terms of complete headache response,” Dr. Tepper said.
Data indicate that when neurologists recommend that patients take an NSAID and triptan together at the beginning of an attack, patients rarely comply. “It’s a big adherence issue,” said Dr. Tepper. “They’re more likely to get a complete response if they take them together, especially if the tablet is designed to deliver the two products together in an optimal way.”
Uncertain therapeutic advantage
Commenting on the findings, Robert Shapiro, MD, PhD, professor of neurologic science at the University of Vermont, Burlington, noted that because of favorable data from past studies for the combination of 85 mg of sumatriptan with 500 mg of naproxen sodium, the coadministration of a triptan with an NSAID has been a standard of care for the past decade.
“It’s therefore unsurprising that a combination of rizatriptan 10 mg plus meloxicam 20 mg in a proprietary MoSEIC formulation might also prove to be more effective than either individual medication taken alone for acute migraine attacks,” said Dr. Shapiro, who was not involved with the research.
It is not possible to compare the efficacy and tolerability of AXS-07 with those of sumatriptan–naproxen sodium without head-to-head trials. However, the available data suggest that the latter formulation is superior, he added.
In 2008, researchers conducted two parallel-group, placebo-controlled trials of sumatriptan–naproxen sodium taken early in a migraine attack. These trials had protocols comparable to that of the current INTERCEPT trial for AXS-07, said Dr. Shapiro.
For the key primary endpoint of 2-hour pain freedom, the two sumatriptan–naproxen sodium trials found therapeutic gains of 35% and 36%, respectively, versus 16.3% for the AXS-07 trial. The placebo response rates (17% and 15% for sumatriptan–naproxen sodium, vs. 16.3% for the AXS-07 trial) were comparable.
Similarly, for the endpoint of 2- to 24-hour pain freedom, the sumatriptan–naproxen sodium trials found therapeutic gains of 33% and 26%, respectively, versus 15.1% for the AXS-07 trial. Again, response rates for placebo were comparable (12% and 14% for sumatriptan–naproxen sodium, vs. 12.6% for AXS-07).
The placebo-adjusted differences for reporting any treatment-emergent AE, otherwise known as “therapeutic penalty,” was 10.2% for AXS-07 in the INTERCEPT trial, versus 7% and 5%, respectively for participants in the two sumatriptan–naproxen sodium trials.
“In light of these data, it’s not immediately apparent what advantage AXS-07 might offer over sumatriptan–naproxen sodium,” said Dr. Shapiro.
“Furthermore, sumatriptan–naproxen sodium is currently available in generic form,” he added.
The study was funded by Axsome Therapeutics. Dr. Tepper is a consultant to Axsome Therapeutics. Dr. Shapiro has previously performed research consulting for Lilly and Lundbeck.
A version of this article first appeared on Medscape.com.
From AHS 2021
FDA to add myocarditis warning to mRNA COVID-19 vaccines
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
The Food and Drug Administration is adding a warning to mRNA COVID-19 vaccines’ fact sheets as medical experts continue to investigate cases of heart inflammation, which are rare but are more likely to occur in young men and teen boys.
Doran Fink, MD, PhD, deputy director of the FDA’s division of vaccines and related products applications, told a Centers for Disease Control and Prevention expert panel on June 23 that the FDA is finalizing language on a warning statement for health care providers, vaccine recipients, and parents or caregivers of teens.
The incidents are more likely to follow the second dose of the Pfizer or Moderna vaccine, with chest pain and other symptoms occurring within several days to a week, the warning will note.
“Based on limited follow-up, most cases appear to have been associated with resolution of symptoms, but limited information is available about potential long-term sequelae,” Dr. Fink said, describing the statement to the Advisory Committee on Immunization Practices, independent experts who advise the CDC.
“Symptoms suggestive of myocarditis or pericarditis should result in vaccine recipients seeking medical attention,” he said.
Benefits outweigh risks
Although no formal vote occurred after the meeting, the ACIP members delivered a strong endorsement for continuing to vaccinate 12- to 29-year-olds with the Pfizer and Moderna vaccines despite the warning.
“To me it’s clear, based on current information, that the benefits of vaccine clearly outweigh the risks,” said ACIP member Veronica McNally, president and CEO of the Franny Strong Foundation in Bloomfield, Mich., a sentiment echoed by other members.
As ACIP was meeting, leaders of the nation’s major physician, nurse, and public health associations issued a statement supporting continued vaccination: “The facts are clear: this is an extremely rare side effect, and only an exceedingly small number of people will experience it after vaccination.
“Importantly, for the young people who do, most cases are mild, and individuals recover often on their own or with minimal treatment. In addition, we know that myocarditis and pericarditis are much more common if you get COVID-19, and the risks to the heart from COVID-19 infection can be more severe.”
ACIP heard the evidence behind that claim. According to the Vaccine Safety Datalink, which contains data from more than 12 million medical records, myocarditis or pericarditis occurs in 12- to 39-year-olds at a rate of 8 per 1 million after the second Pfizer dose and 19.8 per 1 million after the second Moderna dose.
The CDC continues to investigate the link between the mRNA vaccines and heart inflammation, including any differences between the vaccines.
Most of the symptoms resolved quickly, said Tom Shimabukuro, deputy director of CDC’s Immunization Safety Office. Of 323 cases analyzed by the CDC, 309 were hospitalized, 295 were discharged, and 218, or 79%, had recovered from symptoms.
“Most postvaccine myocarditis has been responding to minimal treatment,” pediatric cardiologist Matthew Oster, MD, MPH, from Children’s Healthcare of Atlanta, told the panel.
COVID ‘risks are higher’
Overall, the CDC has reported 2,767 COVID-19 deaths among people aged 12-29 years, and there have been 4,018 reported cases of the COVID-linked inflammatory disorder MIS-C since the beginning of the pandemic.
That amounts to 1 MIS-C case in every 3,200 COVID infections – 36% of them among teens aged 12-20 years and 62% among children who are Hispanic or Black and non-Hispanic, according to a CDC presentation.
The CDC estimated that every 1 million second-dose COVID vaccines administered to 12- to 17-year-old boys could prevent 5,700 cases of COVID-19, 215 hospitalizations, 71 ICU admissions, and 2 deaths. There could also be 56-69 myocarditis cases.
The emergence of new variants in the United States and the skewed pattern of vaccination around the country also may increase the risk to unvaccinated young people, noted Grace Lee, MD, MPH, chair of the ACIP’s COVID-19 Vaccine Safety Technical Subgroup and a pediatric infectious disease physician at Stanford (Calif.) Children’s Health.
“If you’re in an area with low vaccination, the risks are higher,” she said. “The benefits [of the vaccine] are going to be far, far greater than any risk.”
Individuals, parents, and their clinicians should consider the full scope of risk when making decisions about vaccination, she said.
As the pandemic evolves, medical experts have to balance the known risks and benefits while they gather more information, said William Schaffner, MD, an infectious disease physician at Vanderbilt University, Nashville, Tenn., and medical director of the National Foundation for Infectious Diseases.
“The story is not over,” Dr. Schaffner said in an interview. “Clearly, we are still working in the face of a pandemic, so there’s urgency to continue vaccinating. But they would like to know more about the long-term consequences of the myocarditis.”
Booster possibilities
Meanwhile, ACIP began conversations on the parameters for a possible vaccine booster. For now, there are simply questions: Would a third vaccine help the immunocompromised gain protection? Should people get a different type of vaccine – mRNA versus adenovirus vector – for their booster? Most important, how long do antibodies last?
“Prior to going around giving everyone boosters, we really need to improve the overall vaccination coverage,” said Helen Keipp Talbot, MD, associate professor of medicine at Vanderbilt University. “That will protect everyone.”
A version of this article first appeared on Medscape.com.
Gray hair goes away and squids go to space
Goodbye stress, goodbye gray hair
Last year was a doozy, so it wouldn’t be too surprising if we all had a few new gray strands in our hair. But what if we told you that you don’t need to start dying them or plucking them out? What if they could magically go back to the way they were? Well, it may be possible, sans magic and sans stress.
Investigators recently discovered that the age-old belief that stress will permanently turn your hair gray may not be true after all. There’s a strong possibility that it could turn back to its original color once the stressful agent is eliminated.
“Understanding the mechanisms that allow ‘old’ gray hairs to return to their ‘young’ pigmented states could yield new clues about the malleability of human aging in general and how it is influenced by stress,” said senior author Martin Picard, PhD, of Columbia University, New York.
For the study, 14 volunteers were asked to keep a stress diary and review their levels of stress throughout the week. The researchers used a new method of viewing and capturing the images of tiny parts of the hairs to see how much graying took place in each part of the strand. And what they found – some strands naturally turning back to the original color – had never been documented before.
How did it happen? Our good friend the mitochondria. We haven’t really heard that word since eighth-grade biology, but it’s actually the key link between stress hormones and hair pigmentation. Think of them as little radars picking up all different kinds of signals in your body, like mental/emotional stress. They get a big enough alert and they’re going to react, thus gray hair.
So that’s all it takes? Cut the stress and a full head of gray can go back to brown? Not exactly. The researchers said there may be a “threshold because of biological age and other factors.” They believe middle age is near that threshold and it could easily be pushed over due to stress and could potentially go back. But if you’ve been rocking the salt and pepper or silver fox for a number of years and are looking for change, you might want to just eliminate the stress and pick up a bottle of dye.
One small step for squid
Space does a number on the human body. Forget the obvious like going for a walk outside without a spacesuit, or even the well-known risks like the degradation of bone in microgravity; there are numerous smaller but still important changes to the body during spaceflight, like the disruption of the symbiotic relationship between gut bacteria and the human body. This causes the immune system to lose the ability to recognize threats, and illnesses spread more easily.
Naturally, if astronauts are going to undertake years-long journeys to Mars and beyond, a thorough understanding of this disturbance is necessary, and that’s why NASA has sent a bunch of squid to the International Space Station.
When it comes to animal studies, squid aren’t the usual culprits, but there’s a reason NASA chose calamari over the alternatives: The Hawaiian bobtail squid has a symbiotic relationship with bacteria that regulate their bioluminescence in much the same way that we have a symbiotic relationship with our gut bacteria, but the squid is a much simpler animal. If the bioluminescence-regulating bacteria are disturbed during their time in space, it will be much easier to figure out what’s going wrong.
The experiment is ongoing, but we should salute the brave squid who have taken a giant leap for squidkind. Though if NASA didn’t send them up in a giant bubble, we’re going to be very disappointed.
Less plastic, more vanilla
Have you been racked by guilt over the number of plastic water bottles you use? What about the amount of ice cream you eat? Well, this one’s for you.
Plastic isn’t the first thing you think about when you open up a pint of vanilla ice cream and catch the sweet, spicy vanilla scent, or when you smell those fresh vanilla scones coming out of the oven at the coffee shop, but a new study shows that the flavor of vanilla can come from water bottles.
Here’s the deal. A compound called vanillin is responsible for the scent of vanilla, and it can come naturally from the bean or it can be made synthetically. Believe it or not, 85% of vanillin is made synthetically from fossil fuels!
We’ve definitely grown accustomed to our favorite vanilla scents, foods, and cosmetics. In 2018, the global demand for vanillin was about 40,800 tons and is expected to grow to 65,000 tons by 2025, which far exceeds the supply of natural vanilla.
So what can we do? Well, we can use genetically engineered bacteria to turn plastic water bottles into vanillin, according to a study published in the journal Green Chemistry.
The plastic can be broken down into terephthalic acid, which is very similar, chemically speaking, to vanillin. Similar enough that a bit of bioengineering produced Escherichia coli that could convert the acid into the tasty treat, according to researchers at the University of Edinburgh.
A perfect solution? Decreasing plastic waste while producing a valued food product? The thought of consuming plastic isn’t appetizing, so just eat your ice cream and try to forget about it.
No withdrawals from this bank
Into each life, some milestones must fall: High school graduation, birth of a child, first house, 50th wedding anniversary, COVID-19. One LOTME staffer got really excited – way too excited, actually – when his Nissan Sentra reached 300,000 miles.
Well, there are milestones, and then there are milestones. “1,000 Reasons for Hope” is a report celebrating the first 1,000 brains donated to the VA-BU-CLF Brain Bank. For those of you keeping score at home, that would be the Department of Veterans Affairs, Boston University, and the Concussion Legacy Foundation.
The Brain Bank, created in 2008 to study concussions and chronic traumatic encephalopathy, is the brainchild – yes, we went there – of Chris Nowinski, PhD, a former professional wrestler, and Ann McKee, MD, an expert on neurogenerative disease. “Our discoveries have already inspired changes to sports that will prevent many future cases of CTE in the next generation of athletes,” Dr. Nowinski, the CEO of CLF, said in a written statement.
Data from the first thousand brains show that 706 men, including 305 former NFL players, had football as their primary exposure to head impacts. Women were underrepresented, making up only 2.8% of brain donations, so recruiting females is a priority. Anyone interested in pledging can go to PledgeMyBrain.org or call 617-992-0615 for the 24-hour emergency donation pager.
LOTME wanted to help, so we called the Brain Bank to find out about donating. They asked a few questions and we told them what we do for a living. “Oh, you’re with LOTME? Yeah, we’ve … um, seen that before. It’s, um … funny. Can we put you on hold?” We’re starting to get a little sick of the on-hold music by now.
Goodbye stress, goodbye gray hair
Last year was a doozy, so it wouldn’t be too surprising if we all had a few new gray strands in our hair. But what if we told you that you don’t need to start dying them or plucking them out? What if they could magically go back to the way they were? Well, it may be possible, sans magic and sans stress.
Investigators recently discovered that the age-old belief that stress will permanently turn your hair gray may not be true after all. There’s a strong possibility that it could turn back to its original color once the stressful agent is eliminated.
“Understanding the mechanisms that allow ‘old’ gray hairs to return to their ‘young’ pigmented states could yield new clues about the malleability of human aging in general and how it is influenced by stress,” said senior author Martin Picard, PhD, of Columbia University, New York.
For the study, 14 volunteers were asked to keep a stress diary and review their levels of stress throughout the week. The researchers used a new method of viewing and capturing the images of tiny parts of the hairs to see how much graying took place in each part of the strand. And what they found – some strands naturally turning back to the original color – had never been documented before.
How did it happen? Our good friend the mitochondria. We haven’t really heard that word since eighth-grade biology, but it’s actually the key link between stress hormones and hair pigmentation. Think of them as little radars picking up all different kinds of signals in your body, like mental/emotional stress. They get a big enough alert and they’re going to react, thus gray hair.
So that’s all it takes? Cut the stress and a full head of gray can go back to brown? Not exactly. The researchers said there may be a “threshold because of biological age and other factors.” They believe middle age is near that threshold and it could easily be pushed over due to stress and could potentially go back. But if you’ve been rocking the salt and pepper or silver fox for a number of years and are looking for change, you might want to just eliminate the stress and pick up a bottle of dye.
One small step for squid
Space does a number on the human body. Forget the obvious like going for a walk outside without a spacesuit, or even the well-known risks like the degradation of bone in microgravity; there are numerous smaller but still important changes to the body during spaceflight, like the disruption of the symbiotic relationship between gut bacteria and the human body. This causes the immune system to lose the ability to recognize threats, and illnesses spread more easily.
Naturally, if astronauts are going to undertake years-long journeys to Mars and beyond, a thorough understanding of this disturbance is necessary, and that’s why NASA has sent a bunch of squid to the International Space Station.
When it comes to animal studies, squid aren’t the usual culprits, but there’s a reason NASA chose calamari over the alternatives: The Hawaiian bobtail squid has a symbiotic relationship with bacteria that regulate their bioluminescence in much the same way that we have a symbiotic relationship with our gut bacteria, but the squid is a much simpler animal. If the bioluminescence-regulating bacteria are disturbed during their time in space, it will be much easier to figure out what’s going wrong.
The experiment is ongoing, but we should salute the brave squid who have taken a giant leap for squidkind. Though if NASA didn’t send them up in a giant bubble, we’re going to be very disappointed.
Less plastic, more vanilla
Have you been racked by guilt over the number of plastic water bottles you use? What about the amount of ice cream you eat? Well, this one’s for you.
Plastic isn’t the first thing you think about when you open up a pint of vanilla ice cream and catch the sweet, spicy vanilla scent, or when you smell those fresh vanilla scones coming out of the oven at the coffee shop, but a new study shows that the flavor of vanilla can come from water bottles.
Here’s the deal. A compound called vanillin is responsible for the scent of vanilla, and it can come naturally from the bean or it can be made synthetically. Believe it or not, 85% of vanillin is made synthetically from fossil fuels!
We’ve definitely grown accustomed to our favorite vanilla scents, foods, and cosmetics. In 2018, the global demand for vanillin was about 40,800 tons and is expected to grow to 65,000 tons by 2025, which far exceeds the supply of natural vanilla.
So what can we do? Well, we can use genetically engineered bacteria to turn plastic water bottles into vanillin, according to a study published in the journal Green Chemistry.
The plastic can be broken down into terephthalic acid, which is very similar, chemically speaking, to vanillin. Similar enough that a bit of bioengineering produced Escherichia coli that could convert the acid into the tasty treat, according to researchers at the University of Edinburgh.
A perfect solution? Decreasing plastic waste while producing a valued food product? The thought of consuming plastic isn’t appetizing, so just eat your ice cream and try to forget about it.
No withdrawals from this bank
Into each life, some milestones must fall: High school graduation, birth of a child, first house, 50th wedding anniversary, COVID-19. One LOTME staffer got really excited – way too excited, actually – when his Nissan Sentra reached 300,000 miles.
Well, there are milestones, and then there are milestones. “1,000 Reasons for Hope” is a report celebrating the first 1,000 brains donated to the VA-BU-CLF Brain Bank. For those of you keeping score at home, that would be the Department of Veterans Affairs, Boston University, and the Concussion Legacy Foundation.
The Brain Bank, created in 2008 to study concussions and chronic traumatic encephalopathy, is the brainchild – yes, we went there – of Chris Nowinski, PhD, a former professional wrestler, and Ann McKee, MD, an expert on neurogenerative disease. “Our discoveries have already inspired changes to sports that will prevent many future cases of CTE in the next generation of athletes,” Dr. Nowinski, the CEO of CLF, said in a written statement.
Data from the first thousand brains show that 706 men, including 305 former NFL players, had football as their primary exposure to head impacts. Women were underrepresented, making up only 2.8% of brain donations, so recruiting females is a priority. Anyone interested in pledging can go to PledgeMyBrain.org or call 617-992-0615 for the 24-hour emergency donation pager.
LOTME wanted to help, so we called the Brain Bank to find out about donating. They asked a few questions and we told them what we do for a living. “Oh, you’re with LOTME? Yeah, we’ve … um, seen that before. It’s, um … funny. Can we put you on hold?” We’re starting to get a little sick of the on-hold music by now.
Goodbye stress, goodbye gray hair
Last year was a doozy, so it wouldn’t be too surprising if we all had a few new gray strands in our hair. But what if we told you that you don’t need to start dying them or plucking them out? What if they could magically go back to the way they were? Well, it may be possible, sans magic and sans stress.
Investigators recently discovered that the age-old belief that stress will permanently turn your hair gray may not be true after all. There’s a strong possibility that it could turn back to its original color once the stressful agent is eliminated.
“Understanding the mechanisms that allow ‘old’ gray hairs to return to their ‘young’ pigmented states could yield new clues about the malleability of human aging in general and how it is influenced by stress,” said senior author Martin Picard, PhD, of Columbia University, New York.
For the study, 14 volunteers were asked to keep a stress diary and review their levels of stress throughout the week. The researchers used a new method of viewing and capturing the images of tiny parts of the hairs to see how much graying took place in each part of the strand. And what they found – some strands naturally turning back to the original color – had never been documented before.
How did it happen? Our good friend the mitochondria. We haven’t really heard that word since eighth-grade biology, but it’s actually the key link between stress hormones and hair pigmentation. Think of them as little radars picking up all different kinds of signals in your body, like mental/emotional stress. They get a big enough alert and they’re going to react, thus gray hair.
So that’s all it takes? Cut the stress and a full head of gray can go back to brown? Not exactly. The researchers said there may be a “threshold because of biological age and other factors.” They believe middle age is near that threshold and it could easily be pushed over due to stress and could potentially go back. But if you’ve been rocking the salt and pepper or silver fox for a number of years and are looking for change, you might want to just eliminate the stress and pick up a bottle of dye.
One small step for squid
Space does a number on the human body. Forget the obvious like going for a walk outside without a spacesuit, or even the well-known risks like the degradation of bone in microgravity; there are numerous smaller but still important changes to the body during spaceflight, like the disruption of the symbiotic relationship between gut bacteria and the human body. This causes the immune system to lose the ability to recognize threats, and illnesses spread more easily.
Naturally, if astronauts are going to undertake years-long journeys to Mars and beyond, a thorough understanding of this disturbance is necessary, and that’s why NASA has sent a bunch of squid to the International Space Station.
When it comes to animal studies, squid aren’t the usual culprits, but there’s a reason NASA chose calamari over the alternatives: The Hawaiian bobtail squid has a symbiotic relationship with bacteria that regulate their bioluminescence in much the same way that we have a symbiotic relationship with our gut bacteria, but the squid is a much simpler animal. If the bioluminescence-regulating bacteria are disturbed during their time in space, it will be much easier to figure out what’s going wrong.
The experiment is ongoing, but we should salute the brave squid who have taken a giant leap for squidkind. Though if NASA didn’t send them up in a giant bubble, we’re going to be very disappointed.
Less plastic, more vanilla
Have you been racked by guilt over the number of plastic water bottles you use? What about the amount of ice cream you eat? Well, this one’s for you.
Plastic isn’t the first thing you think about when you open up a pint of vanilla ice cream and catch the sweet, spicy vanilla scent, or when you smell those fresh vanilla scones coming out of the oven at the coffee shop, but a new study shows that the flavor of vanilla can come from water bottles.
Here’s the deal. A compound called vanillin is responsible for the scent of vanilla, and it can come naturally from the bean or it can be made synthetically. Believe it or not, 85% of vanillin is made synthetically from fossil fuels!
We’ve definitely grown accustomed to our favorite vanilla scents, foods, and cosmetics. In 2018, the global demand for vanillin was about 40,800 tons and is expected to grow to 65,000 tons by 2025, which far exceeds the supply of natural vanilla.
So what can we do? Well, we can use genetically engineered bacteria to turn plastic water bottles into vanillin, according to a study published in the journal Green Chemistry.
The plastic can be broken down into terephthalic acid, which is very similar, chemically speaking, to vanillin. Similar enough that a bit of bioengineering produced Escherichia coli that could convert the acid into the tasty treat, according to researchers at the University of Edinburgh.
A perfect solution? Decreasing plastic waste while producing a valued food product? The thought of consuming plastic isn’t appetizing, so just eat your ice cream and try to forget about it.
No withdrawals from this bank
Into each life, some milestones must fall: High school graduation, birth of a child, first house, 50th wedding anniversary, COVID-19. One LOTME staffer got really excited – way too excited, actually – when his Nissan Sentra reached 300,000 miles.
Well, there are milestones, and then there are milestones. “1,000 Reasons for Hope” is a report celebrating the first 1,000 brains donated to the VA-BU-CLF Brain Bank. For those of you keeping score at home, that would be the Department of Veterans Affairs, Boston University, and the Concussion Legacy Foundation.
The Brain Bank, created in 2008 to study concussions and chronic traumatic encephalopathy, is the brainchild – yes, we went there – of Chris Nowinski, PhD, a former professional wrestler, and Ann McKee, MD, an expert on neurogenerative disease. “Our discoveries have already inspired changes to sports that will prevent many future cases of CTE in the next generation of athletes,” Dr. Nowinski, the CEO of CLF, said in a written statement.
Data from the first thousand brains show that 706 men, including 305 former NFL players, had football as their primary exposure to head impacts. Women were underrepresented, making up only 2.8% of brain donations, so recruiting females is a priority. Anyone interested in pledging can go to PledgeMyBrain.org or call 617-992-0615 for the 24-hour emergency donation pager.
LOTME wanted to help, so we called the Brain Bank to find out about donating. They asked a few questions and we told them what we do for a living. “Oh, you’re with LOTME? Yeah, we’ve … um, seen that before. It’s, um … funny. Can we put you on hold?” We’re starting to get a little sick of the on-hold music by now.
Women with migraine are ‘high-risk’ patients during pregnancy
new research suggests. Although pregnancy is generally considered a “safe period” for women with migraine, “we actually found they have more diabetes, more hypertension, more blood clots, more complications during their delivery, and more postpartum complications,” said study investigator Nirit Lev, MD, PhD, head, department of neurology, Meir Medical Center, Kfar Saba Sackler Faculty of Medicine, Tel Aviv University.
The results highlight the need for clinicians “to take people with migraines seriously” and reinforce the idea that migraine is not “just a headache,” said Dr. Lev.
Pregnant women with migraine should be considered high risk and have specialized neurologic follow-up during pregnancy and the postpartum period, she added.
The findings were presented at the 2021 Congress of the European Academy of Neurology.
Prevalent, disabling
Migraine is one of the most prevalent and disabling neurologic disorders. Such disorders are major causes of death and disability.
In childhood, there’s no difference between the sexes in terms of migraine prevalence, but after puberty, migraine is about three times more common in women than men. Fluctuating levels of estrogen and progesterone likely explain these differences, said Dr. Lev.
The prevalence of migraine among females peaks during their reproductive years. Most female migraine patients report an improvement in headache symptoms during pregnancy, with some experiencing a “complete remission.” However, a minority report worsening of migraine when expecting a child, said Dr. Lev.
Some patients have their first aura during pregnancy. The most common migraine aura is visual, a problem with the visual field that can affect motor and sensory functioning, said Dr. Lev.
Managing migraine during pregnancy is “very complicated,” said Dr. Lev. She said the first-line treatment is paracetamol (acetaminophen) and stressed that taking opioids should be avoided.
Retrospective database study
For the study, the researchers retrospectively reviewed pregnancy and delivery records from a database of Clalit Medical Services, which has more than 4.5 million members and is the largest such database in Israel. They collected demographic data and information on mode of delivery, medical and obstetric complications, hospitalizations, emergency department visits, use of medications, laboratory reports, and medical consultations.
The study included 145,102 women who gave birth from 2014 to 2020.
Of these, 10,646 had migraine without aura, and 1,576 had migraine with aura. The migraine diagnoses, which were based on International Headache Society criteria and diagnostic codes, were made prior to pregnancy.
Dr. Lev noted that the number of patients with migraine is likely an underestimation because migraine is “not always diagnosed.”
Results showed that the risk for obstetric complications was higher among pregnant women with migraine, especially those with aura, in comparison with women without migraine. About 6.9% of patients with migraine without aura were admitted to high-risk hospital departments, compared with 6% of pregnant control patients who did not have migraine (P < .0001). For patients with migraine with aura, the risk for admissions was even higher (8.7%; P < .0001 vs. control patients and P < .03 vs. patients with migraine without aura) and was “very highly statistically significant,” said Dr. Lev.
Pregnant women with migraine were at significantly increased risk for gestational diabetes, hyperlipidemia, and being diagnosed with a psychiatric disorder (all P < .0001). These women were also more likely to experience preeclampsia and blood clots (P < .0001).
Unexpected finding
The finding that the risk for diabetes was higher was “unexpected,” inasmuch as older women with migraine are typically at increased risk for metabolic syndrome and higher body mass index, said Dr. Lev.
Migraine patients had significantly more consultations with family physicians, gynecologists, and neurologists (P < .0001). In addition, they were more likely to utilize emergency services; take more medications, mostly analgesics; and undergo more laboratory studies and brain imaging.
Those with aura had significantly more specialist consultations and took more medications compared with migraine patients without aura.
There was a statistically significant increase in the use of epidural anesthesia for migraine patients (40.5% of women without migraine; 45.7% of those with migraine accompanied by aura; and 47.5% of migraine patients without aura).
This was an “interesting” finding, said Dr. Lev. “We didn’t know what to expect; people with migraine are used to pain, so the question was, will they tolerate pain better or be more afraid of pain?”
Women with migraine also experienced more assisted deliveries with increased use of vacuums and forceps.
During the 3-month postpartum period, women with migraine sought more medical consultations and used more medications compared with control patients. They also underwent more lab examinations and more brain imaging during this period.
Dr. Lev noted that some of these evaluations may have been postponed because of the pregnancy.
Women with migraine also had a greater risk for postpartum depression, which Dr. Lev found “concerning.” She noted that depression is often underreported but is treatable. Women with migraine should be monitored for depression post partum, she said.
It’s unclear which factors contribute to the increased risk for pregnancy complications in women with migraine. Dr. Lev said she doesn’t believe it’s drug related.
“Although they’re taking more medications than people who don’t have migraine, we still are giving very low doses and only safe medicines, so I don’t think these increased risks are side effects,” she said.
She noted that women with migraine have more cardiovascular complications, including stroke and myocardial infarction, although these generally affect older patients.
Dr. Lev also noted that pain, especially chronic pain, can cause depression. “We know that people with migraine have more depression and anxiety, so maybe that also affects them during their pregnancy and after,” she said.
She suggested that pregnant women with migraine be considered high risk and be managed via specialized clinics.
Room for improvement
Commenting on the research, Lauren Doyle Strauss, DO, associate professor of neurology, Wake Forest University, Winston-Salem, N.C., who has written about the management of migraine during pregnancy, said studies such as this help raise awareness about pregnancy risks in migraine patients. Dr. Strauss did not attend the live presentation but is aware of the findings.
The increased use of epidurals during delivery among migraine patients in the study makes some sense, said Dr. Strauss. “It kind of shows a comfort level with medicines.”
She expressed concern that such research may be “skewed” because it includes patients with more severe migraine. If less severe cases were included in this research, “maybe there would still be higher risks, but not as high as what we have been finding in some of our studies,” she said.
Dr. Strauss said she feels the medical community should do a better job of identifying and diagnosing migraine. She said she would like to see migraine screening become a routine part of obstetric/gynecologic care. Doctors should counsel migraine patients who wish to become pregnant about potential risks, said Dr. Strauss. “We need to be up front in telling them when to seek care and when to report symptoms and not to wait for it to become super severe,” she said.
She also believes doctors should be “proactive” in helping patients develop a treatment plan before becoming pregnant, because the limited pain control options available for pregnant patients can take time to have an effect.
Also commenting on the study findings, Nina Riggins, MD, PhD, clinical associate professor of neurology at the University of California, San Francisco, said the study raises “important questions” and has “important aims.”
She believes the study reinforces the importance of collaboration between experts in primary care, obstetrics/gynecology, and neurology. However, she was surprised at some of the investigators’ assertions that there are no differences in migraine among prepubertal children and that the course of migraine for men is stable throughout their life span.
“There is literature that supports the view that the prevalence in boys is higher in prepuberty, and studies do show that migraine prevalence decreases in older adults – men and women,” she said.
There is still not enough evidence to determine that antiemetics and triptans are safe during pregnancy or that pregnant women with migraine should be taking acetylsalicylic acid, said Dr. Riggins.
The investigators, Dr. Strauss, and Dr. Riggins have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests. Although pregnancy is generally considered a “safe period” for women with migraine, “we actually found they have more diabetes, more hypertension, more blood clots, more complications during their delivery, and more postpartum complications,” said study investigator Nirit Lev, MD, PhD, head, department of neurology, Meir Medical Center, Kfar Saba Sackler Faculty of Medicine, Tel Aviv University.
The results highlight the need for clinicians “to take people with migraines seriously” and reinforce the idea that migraine is not “just a headache,” said Dr. Lev.
Pregnant women with migraine should be considered high risk and have specialized neurologic follow-up during pregnancy and the postpartum period, she added.
The findings were presented at the 2021 Congress of the European Academy of Neurology.
Prevalent, disabling
Migraine is one of the most prevalent and disabling neurologic disorders. Such disorders are major causes of death and disability.
In childhood, there’s no difference between the sexes in terms of migraine prevalence, but after puberty, migraine is about three times more common in women than men. Fluctuating levels of estrogen and progesterone likely explain these differences, said Dr. Lev.
The prevalence of migraine among females peaks during their reproductive years. Most female migraine patients report an improvement in headache symptoms during pregnancy, with some experiencing a “complete remission.” However, a minority report worsening of migraine when expecting a child, said Dr. Lev.
Some patients have their first aura during pregnancy. The most common migraine aura is visual, a problem with the visual field that can affect motor and sensory functioning, said Dr. Lev.
Managing migraine during pregnancy is “very complicated,” said Dr. Lev. She said the first-line treatment is paracetamol (acetaminophen) and stressed that taking opioids should be avoided.
Retrospective database study
For the study, the researchers retrospectively reviewed pregnancy and delivery records from a database of Clalit Medical Services, which has more than 4.5 million members and is the largest such database in Israel. They collected demographic data and information on mode of delivery, medical and obstetric complications, hospitalizations, emergency department visits, use of medications, laboratory reports, and medical consultations.
The study included 145,102 women who gave birth from 2014 to 2020.
Of these, 10,646 had migraine without aura, and 1,576 had migraine with aura. The migraine diagnoses, which were based on International Headache Society criteria and diagnostic codes, were made prior to pregnancy.
Dr. Lev noted that the number of patients with migraine is likely an underestimation because migraine is “not always diagnosed.”
Results showed that the risk for obstetric complications was higher among pregnant women with migraine, especially those with aura, in comparison with women without migraine. About 6.9% of patients with migraine without aura were admitted to high-risk hospital departments, compared with 6% of pregnant control patients who did not have migraine (P < .0001). For patients with migraine with aura, the risk for admissions was even higher (8.7%; P < .0001 vs. control patients and P < .03 vs. patients with migraine without aura) and was “very highly statistically significant,” said Dr. Lev.
Pregnant women with migraine were at significantly increased risk for gestational diabetes, hyperlipidemia, and being diagnosed with a psychiatric disorder (all P < .0001). These women were also more likely to experience preeclampsia and blood clots (P < .0001).
Unexpected finding
The finding that the risk for diabetes was higher was “unexpected,” inasmuch as older women with migraine are typically at increased risk for metabolic syndrome and higher body mass index, said Dr. Lev.
Migraine patients had significantly more consultations with family physicians, gynecologists, and neurologists (P < .0001). In addition, they were more likely to utilize emergency services; take more medications, mostly analgesics; and undergo more laboratory studies and brain imaging.
Those with aura had significantly more specialist consultations and took more medications compared with migraine patients without aura.
There was a statistically significant increase in the use of epidural anesthesia for migraine patients (40.5% of women without migraine; 45.7% of those with migraine accompanied by aura; and 47.5% of migraine patients without aura).
This was an “interesting” finding, said Dr. Lev. “We didn’t know what to expect; people with migraine are used to pain, so the question was, will they tolerate pain better or be more afraid of pain?”
Women with migraine also experienced more assisted deliveries with increased use of vacuums and forceps.
During the 3-month postpartum period, women with migraine sought more medical consultations and used more medications compared with control patients. They also underwent more lab examinations and more brain imaging during this period.
Dr. Lev noted that some of these evaluations may have been postponed because of the pregnancy.
Women with migraine also had a greater risk for postpartum depression, which Dr. Lev found “concerning.” She noted that depression is often underreported but is treatable. Women with migraine should be monitored for depression post partum, she said.
It’s unclear which factors contribute to the increased risk for pregnancy complications in women with migraine. Dr. Lev said she doesn’t believe it’s drug related.
“Although they’re taking more medications than people who don’t have migraine, we still are giving very low doses and only safe medicines, so I don’t think these increased risks are side effects,” she said.
She noted that women with migraine have more cardiovascular complications, including stroke and myocardial infarction, although these generally affect older patients.
Dr. Lev also noted that pain, especially chronic pain, can cause depression. “We know that people with migraine have more depression and anxiety, so maybe that also affects them during their pregnancy and after,” she said.
She suggested that pregnant women with migraine be considered high risk and be managed via specialized clinics.
Room for improvement
Commenting on the research, Lauren Doyle Strauss, DO, associate professor of neurology, Wake Forest University, Winston-Salem, N.C., who has written about the management of migraine during pregnancy, said studies such as this help raise awareness about pregnancy risks in migraine patients. Dr. Strauss did not attend the live presentation but is aware of the findings.
The increased use of epidurals during delivery among migraine patients in the study makes some sense, said Dr. Strauss. “It kind of shows a comfort level with medicines.”
She expressed concern that such research may be “skewed” because it includes patients with more severe migraine. If less severe cases were included in this research, “maybe there would still be higher risks, but not as high as what we have been finding in some of our studies,” she said.
Dr. Strauss said she feels the medical community should do a better job of identifying and diagnosing migraine. She said she would like to see migraine screening become a routine part of obstetric/gynecologic care. Doctors should counsel migraine patients who wish to become pregnant about potential risks, said Dr. Strauss. “We need to be up front in telling them when to seek care and when to report symptoms and not to wait for it to become super severe,” she said.
She also believes doctors should be “proactive” in helping patients develop a treatment plan before becoming pregnant, because the limited pain control options available for pregnant patients can take time to have an effect.
Also commenting on the study findings, Nina Riggins, MD, PhD, clinical associate professor of neurology at the University of California, San Francisco, said the study raises “important questions” and has “important aims.”
She believes the study reinforces the importance of collaboration between experts in primary care, obstetrics/gynecology, and neurology. However, she was surprised at some of the investigators’ assertions that there are no differences in migraine among prepubertal children and that the course of migraine for men is stable throughout their life span.
“There is literature that supports the view that the prevalence in boys is higher in prepuberty, and studies do show that migraine prevalence decreases in older adults – men and women,” she said.
There is still not enough evidence to determine that antiemetics and triptans are safe during pregnancy or that pregnant women with migraine should be taking acetylsalicylic acid, said Dr. Riggins.
The investigators, Dr. Strauss, and Dr. Riggins have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests. Although pregnancy is generally considered a “safe period” for women with migraine, “we actually found they have more diabetes, more hypertension, more blood clots, more complications during their delivery, and more postpartum complications,” said study investigator Nirit Lev, MD, PhD, head, department of neurology, Meir Medical Center, Kfar Saba Sackler Faculty of Medicine, Tel Aviv University.
The results highlight the need for clinicians “to take people with migraines seriously” and reinforce the idea that migraine is not “just a headache,” said Dr. Lev.
Pregnant women with migraine should be considered high risk and have specialized neurologic follow-up during pregnancy and the postpartum period, she added.
The findings were presented at the 2021 Congress of the European Academy of Neurology.
Prevalent, disabling
Migraine is one of the most prevalent and disabling neurologic disorders. Such disorders are major causes of death and disability.
In childhood, there’s no difference between the sexes in terms of migraine prevalence, but after puberty, migraine is about three times more common in women than men. Fluctuating levels of estrogen and progesterone likely explain these differences, said Dr. Lev.
The prevalence of migraine among females peaks during their reproductive years. Most female migraine patients report an improvement in headache symptoms during pregnancy, with some experiencing a “complete remission.” However, a minority report worsening of migraine when expecting a child, said Dr. Lev.
Some patients have their first aura during pregnancy. The most common migraine aura is visual, a problem with the visual field that can affect motor and sensory functioning, said Dr. Lev.
Managing migraine during pregnancy is “very complicated,” said Dr. Lev. She said the first-line treatment is paracetamol (acetaminophen) and stressed that taking opioids should be avoided.
Retrospective database study
For the study, the researchers retrospectively reviewed pregnancy and delivery records from a database of Clalit Medical Services, which has more than 4.5 million members and is the largest such database in Israel. They collected demographic data and information on mode of delivery, medical and obstetric complications, hospitalizations, emergency department visits, use of medications, laboratory reports, and medical consultations.
The study included 145,102 women who gave birth from 2014 to 2020.
Of these, 10,646 had migraine without aura, and 1,576 had migraine with aura. The migraine diagnoses, which were based on International Headache Society criteria and diagnostic codes, were made prior to pregnancy.
Dr. Lev noted that the number of patients with migraine is likely an underestimation because migraine is “not always diagnosed.”
Results showed that the risk for obstetric complications was higher among pregnant women with migraine, especially those with aura, in comparison with women without migraine. About 6.9% of patients with migraine without aura were admitted to high-risk hospital departments, compared with 6% of pregnant control patients who did not have migraine (P < .0001). For patients with migraine with aura, the risk for admissions was even higher (8.7%; P < .0001 vs. control patients and P < .03 vs. patients with migraine without aura) and was “very highly statistically significant,” said Dr. Lev.
Pregnant women with migraine were at significantly increased risk for gestational diabetes, hyperlipidemia, and being diagnosed with a psychiatric disorder (all P < .0001). These women were also more likely to experience preeclampsia and blood clots (P < .0001).
Unexpected finding
The finding that the risk for diabetes was higher was “unexpected,” inasmuch as older women with migraine are typically at increased risk for metabolic syndrome and higher body mass index, said Dr. Lev.
Migraine patients had significantly more consultations with family physicians, gynecologists, and neurologists (P < .0001). In addition, they were more likely to utilize emergency services; take more medications, mostly analgesics; and undergo more laboratory studies and brain imaging.
Those with aura had significantly more specialist consultations and took more medications compared with migraine patients without aura.
There was a statistically significant increase in the use of epidural anesthesia for migraine patients (40.5% of women without migraine; 45.7% of those with migraine accompanied by aura; and 47.5% of migraine patients without aura).
This was an “interesting” finding, said Dr. Lev. “We didn’t know what to expect; people with migraine are used to pain, so the question was, will they tolerate pain better or be more afraid of pain?”
Women with migraine also experienced more assisted deliveries with increased use of vacuums and forceps.
During the 3-month postpartum period, women with migraine sought more medical consultations and used more medications compared with control patients. They also underwent more lab examinations and more brain imaging during this period.
Dr. Lev noted that some of these evaluations may have been postponed because of the pregnancy.
Women with migraine also had a greater risk for postpartum depression, which Dr. Lev found “concerning.” She noted that depression is often underreported but is treatable. Women with migraine should be monitored for depression post partum, she said.
It’s unclear which factors contribute to the increased risk for pregnancy complications in women with migraine. Dr. Lev said she doesn’t believe it’s drug related.
“Although they’re taking more medications than people who don’t have migraine, we still are giving very low doses and only safe medicines, so I don’t think these increased risks are side effects,” she said.
She noted that women with migraine have more cardiovascular complications, including stroke and myocardial infarction, although these generally affect older patients.
Dr. Lev also noted that pain, especially chronic pain, can cause depression. “We know that people with migraine have more depression and anxiety, so maybe that also affects them during their pregnancy and after,” she said.
She suggested that pregnant women with migraine be considered high risk and be managed via specialized clinics.
Room for improvement
Commenting on the research, Lauren Doyle Strauss, DO, associate professor of neurology, Wake Forest University, Winston-Salem, N.C., who has written about the management of migraine during pregnancy, said studies such as this help raise awareness about pregnancy risks in migraine patients. Dr. Strauss did not attend the live presentation but is aware of the findings.
The increased use of epidurals during delivery among migraine patients in the study makes some sense, said Dr. Strauss. “It kind of shows a comfort level with medicines.”
She expressed concern that such research may be “skewed” because it includes patients with more severe migraine. If less severe cases were included in this research, “maybe there would still be higher risks, but not as high as what we have been finding in some of our studies,” she said.
Dr. Strauss said she feels the medical community should do a better job of identifying and diagnosing migraine. She said she would like to see migraine screening become a routine part of obstetric/gynecologic care. Doctors should counsel migraine patients who wish to become pregnant about potential risks, said Dr. Strauss. “We need to be up front in telling them when to seek care and when to report symptoms and not to wait for it to become super severe,” she said.
She also believes doctors should be “proactive” in helping patients develop a treatment plan before becoming pregnant, because the limited pain control options available for pregnant patients can take time to have an effect.
Also commenting on the study findings, Nina Riggins, MD, PhD, clinical associate professor of neurology at the University of California, San Francisco, said the study raises “important questions” and has “important aims.”
She believes the study reinforces the importance of collaboration between experts in primary care, obstetrics/gynecology, and neurology. However, she was surprised at some of the investigators’ assertions that there are no differences in migraine among prepubertal children and that the course of migraine for men is stable throughout their life span.
“There is literature that supports the view that the prevalence in boys is higher in prepuberty, and studies do show that migraine prevalence decreases in older adults – men and women,” she said.
There is still not enough evidence to determine that antiemetics and triptans are safe during pregnancy or that pregnant women with migraine should be taking acetylsalicylic acid, said Dr. Riggins.
The investigators, Dr. Strauss, and Dr. Riggins have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
From EAN 2021
No overall statin effect seen on dementia, cognition in ASPREE analysis
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”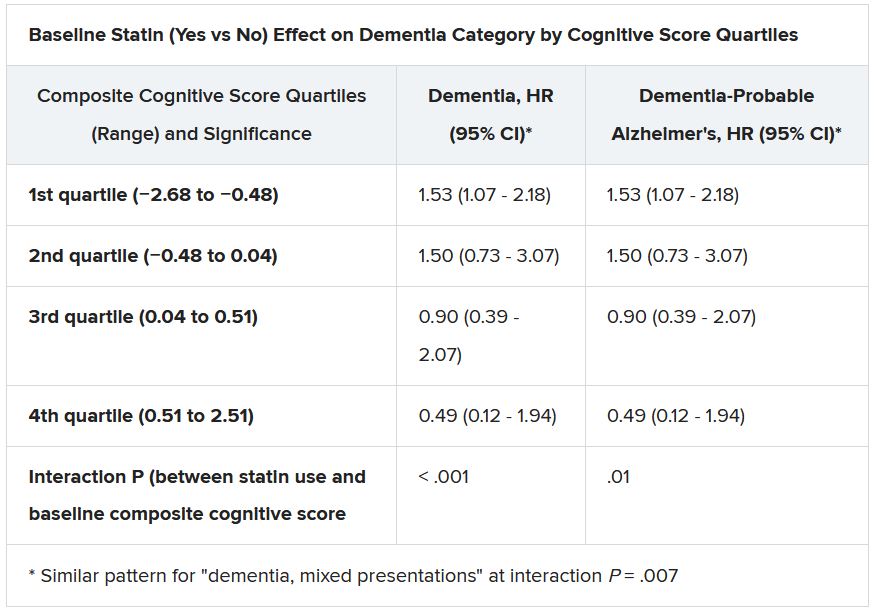
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Statin therapy likely didn’t lead to dementia or even mild cognitive impairment (MCI) in older patients taking the drugs for cardiovascular (CV) primary prevention in a post hoc analysis of a trial that required normal cognitive ability for entry.
Nor did statins, whether lipophilic or hydrophilic, appear to influence changes in cognition or affect separate domains of mental performance, such as memory, language ability, or executive function, over the trial’s follow-up, which averaged almost 5 years.
Although such findings aren’t novel – they are consistent with observations from a number of earlier studies – the new analysis included a possible signal for a statin association with new-onset dementia in a subgroup of more than 18,000 patients. Researchers attribute the retrospective finding, from a trial not designed to explore the issue, to confounding or chance.
Still, the adjusted risk for dementia seemed to go up by a third among statin users who at baseline placed in the lowest quartile for cognitive function, based on a composite test score, in the ASPREE trial, a test of primary-prevention low-dose aspirin in patients 65 or older. The better the baseline cognitive score by quartile, the lower the risk for dementia ( interaction P < .001).
The bottom-quartile association of statins with dementia was driven by new diagnoses of Alzheimer’s disease, as opposed to the study’s other “mixed presentation” dementia subtype, wrote the authors of analysis, published June 21, 2021, in the Journal of the American College of Cardiology), led by Zhen Zhou, PhD, Menzies Institute for Medical Research, University of Tasmania, Hobart, Australia.
“I wouldn’t overinterpret that,” said senior author Mark R. Nelson, MBBS, PhD, of the same institution. Indeed, it should be “reassuring” for physicians prescribing statins to older patients that there was no overall statin effect on cognition or new-onset dementia, he said in an interview.
“This is a post hoc analysis within a dataset, although a very-high-quality dataset, it must be said.” The patients were prospectively followed for a range of cognition domains, and the results were adjudicated, Dr. Nelson observed. Although the question of statins and dementia risk is thought to be largely settled, the analysis “was just too tempting not to do.”
On the basis of the current analysis and the bulk of preceding evidence, “lipid lowering in the short term does not appear to result in improvement or deterioration of cognition irrespective of baseline LDL cholesterol levels and medication used,” Christie M. Ballantyne, MD, and Vijay Nambi, MD, PhD, both from Baylor College of Medicine, Houston, wrote in an accompanying editorial.
The current study “provides additional information that the lipo- or hydrophilicity of the statin does not affect changes in cognition. However, the potential increased risk for Alzheimer’s disease, especially among patients with baseline cognitive impairment, requires further investigation.”
The current analysis is reassuring that the likelihood of such statin effects on cognition “is vanishingly small,” Neil J. Stone MD, Northwestern University, Chicago, said in an interview. In fact, its primary finding of no such association “best summarizes what we know in 2021 about statin therapy” after exploration of the issue in a number of prospective trials and systematic reviews, said Dr. Stone, who was not a coauthor on the report.
The observed interaction between statin use and baseline neurocognitive ability “is hypothesis raising at best. It should be explored in randomized, controlled trials that can look at this question in an unbiased manner,” he agreed.
If patients believe or suspect that a statin is causing symptoms that suggest cognitive dysfunction, “what they really need to do is to stop it for 3 weeks and check out other causes. And in rechallenging, the guidelines say, if they think that it’s causing a memory problem that occurs anecdotally, then they can be given another statin, usually, which doesn’t cause it.”
ASPREE compared daily low-dose aspirin with placebo in a community-based older population numbering about 19,000 in Australia and the United States. Patients were initially without known CV disease, dementia, or physical disabilities. It did not randomize patients by statin therapy.
Of note, entry to the trial required a score of at least 78 on the Modified Mini-Mental State Examination (3MS), corresponding to normal cognition.
Aspirin showed no significant benefit for disability-free survival, an endpoint that included death and dementia, or CV events over a median of 4.7 years. It was associated with slightly more cases of major hemorrhage, as previously reported.
A subsequent ASPREE analysis suggested that the aspirin had no effect on risks of mild cognitive impairment, cognitive decline, or dementia.
Of the 18,846 patients in the current post hoc analysis, the average age of the patients was 74 years, and 56.4% were women; 31.3% were taking statins at baseline. The incidence of dementia per 1,000 person-years for those taking statins in comparison with those not taking statins was 6.91 and 6.48, respectively. Any cognitive changes were tracked by the 3MS and three other validated tests in different domains of cognition, with results contributing to the composite score.
The corresponding incidence of dementia considered probable Alzheimer’s disease was 2.97 and 2.65 for those receiving versus not receiving statins, respectively. The incidence of dementia with mixed presentation was 3.94 and 3.84, respectively.
There were no significant differences in risk for dementia overall or for either dementia subtype in multivariate analyses. Adjustments included demographics, CV lifestyle risk factors, family medical history, including dementia, ASPREE randomization group, and individual scores on the four tests of cognition.
Results for development of MCI mirrored those for dementia, as did results stratified for baseline lipids and for use of lipophilic statins, such as atorvastatin or simvastatin versus hydrophilic statins, including pravastatin and rosuvastatin.
Significant interactions were observed between composite cognitive scores and statin therapy at baseline; as scores increased, indicating better cognitive performance, the risks for dementia and its subtypes went down. Statins were associated with incident dementia at the lowest cognitive performance quartile.
That association is probably a function of the cohort’s advanced age, Dr. Nelson said. “If you get into old age, and you’ve got high cognitive scores, you’ve probably got protective factors. That’s how I would interpret that.”
Dr. Ballantyne and Dr. Nambi also emphasized the difficulties of controlling for potential biases even with extensive covariate adjustments. The statin dosages at which patients were treated were not part of the analysis, “and achieved LDL [cholesterol levels over the study period were not known,” they wrote.
“Furthermore, patients who were treated with statins were more likely to have diabetes, hypertension, chronic kidney disease, and obesity, all of which are known to increase risk for cognitive decline, and, as might have been predicted, statin users therefore had significantly lower scores for global cognition and episodic memory.”
Dr. Nelson pointed to an ongoing prospective atorvastatin trial that includes dementia in its primary endpoint and should be “the definitive study.” STAREE (Statin Therapy for Reducing Events in the Elderly) is running throughout Australia with a projected enrollment of 18,000 and primary completion by the end of 2022. “We’ve already enrolled 8,000 patients.”
Less far along is the PREVENTABLE (Pragmatic Evaluation of Events and Benefits of Lipid-Lowering in Older Adults) trial, based in the United States and also randomizing to atorvastatin or placebo, that will have an estimated 20,000 older patients and completion in 5 years. The primary endpoint is new dementia or persistent disability.
Both trials “are powered to enable firm conclusions concerning any statin effects,” said Dr. Ballantyne and Dr. Nambi. “In the meantime, practicing clinicians can have confidence and share with their patients that short-term lipid-lowering therapy in older patients, including with statins, is unlikely to have a major impact on cognition.”
ASPREE was supported by grants from the U.S. National Institute on Aging and the National Cancer Institute and the National Health and Medical Research Council of Australia, by Monash University, and by the Victorian Cancer Agency. Dr. Nelson reported receiving honoraria from Sanofi and Amgen; support from Bayer for ASPREE; and grant support for STAREE. Disclosures for the other authors are in the report. Dr. Ballantyne disclosed grant and research support from Abbott Diagnostic, Akcea, Amgen, Esperion, Ionis, Novartis, Regeneron, and Roche Diagnostics; and consulting for Abbott Diagnostics, Althera, Amarin, Amgen, Arrowhead, AstraZeneca, Corvidia, Denka Seiken, Esperion, Genentech, Gilead, Matinas BioPharma, New Amsterdam, Novartis, Novo Nordisk, Pfizer, Regeneron, Roche Diagnostics, and Sanofi-Synthelabo. Dr. Nambi is a coinvestigator on a provisional patent along with Baylor College of Medicine and Roche on the use of biomarkers to predict heart failure, and a site principal investigator for studies sponsored by Amgen and Merck. Dr. Stone had no disclosures.
A version of this article first appeared on Medscape.com.
Tardive dyskinesia: The role of targeted therapy
Tardive dyskinesia is a movement disorder characterized by involuntary and repetitive movements of the tongue, lips, face, trunk, and extremities. It is caused by the use of dopamine receptor-blocking drugs, most often antipsychotics, but has also been associated with treatments for some gastrointestinal disorders.
Dr. Peter LeWitt, Sastry Foundation Endowed Chair in Neurology at Wayne State University School of Medicine in Detroit, MI, walks through some of the known causes of tardive dyskinesia as well as steps physicians can take in making a differential diagnosis.
Next, Dr. LeWitt dives into the role of targeted therapy in the management of tardive dyskinesia. VMAT2 (vesicular monoamine transporter type 2) inhibitors are emerging as a treatment class of choice that help suppress tardive dyskinesia symptoms by depleting presynaptic dopamine, while other patients may benefit more from benzodiazepines, botulinum toxin, or deep brain stimulation.
--
Dr. Peter LeWitt, Sastry Foundation Endowed Chair in Neurology at Wayne State University School of Medicine in Detroit, MI.
Peter A. LeWitt reports advisory roles for: Abide, Acorda Therapeutics, Adamas, Biogen, Cavion, Denali, Intec Pharma, Jazz Pharmaceuticals, Lundbeck, Neurocrine, Mitsubishi NeuroDerm Ltd, Prexton, Revance, Sage, and US WorldMeds.
Lecture fees from: Acorda, American Academy of Neurology, Kyowa Hakko Kirin, and US WorldMeds.
Research grant support from: Abide, Acorda, Amneal, Lundbeck, Michael J. Fox Foundation for Parkinson's Research, Mitsubishi NeuroDerm Ltd, Parkinson Study Group; Pharma 2B, Revance, Hoffmann-La Roche; Sunovion, Sun Pharma, and US WorldMeds.
Tardive dyskinesia is a movement disorder characterized by involuntary and repetitive movements of the tongue, lips, face, trunk, and extremities. It is caused by the use of dopamine receptor-blocking drugs, most often antipsychotics, but has also been associated with treatments for some gastrointestinal disorders.
Dr. Peter LeWitt, Sastry Foundation Endowed Chair in Neurology at Wayne State University School of Medicine in Detroit, MI, walks through some of the known causes of tardive dyskinesia as well as steps physicians can take in making a differential diagnosis.
Next, Dr. LeWitt dives into the role of targeted therapy in the management of tardive dyskinesia. VMAT2 (vesicular monoamine transporter type 2) inhibitors are emerging as a treatment class of choice that help suppress tardive dyskinesia symptoms by depleting presynaptic dopamine, while other patients may benefit more from benzodiazepines, botulinum toxin, or deep brain stimulation.
--
Dr. Peter LeWitt, Sastry Foundation Endowed Chair in Neurology at Wayne State University School of Medicine in Detroit, MI.
Peter A. LeWitt reports advisory roles for: Abide, Acorda Therapeutics, Adamas, Biogen, Cavion, Denali, Intec Pharma, Jazz Pharmaceuticals, Lundbeck, Neurocrine, Mitsubishi NeuroDerm Ltd, Prexton, Revance, Sage, and US WorldMeds.
Lecture fees from: Acorda, American Academy of Neurology, Kyowa Hakko Kirin, and US WorldMeds.
Research grant support from: Abide, Acorda, Amneal, Lundbeck, Michael J. Fox Foundation for Parkinson's Research, Mitsubishi NeuroDerm Ltd, Parkinson Study Group; Pharma 2B, Revance, Hoffmann-La Roche; Sunovion, Sun Pharma, and US WorldMeds.
Tardive dyskinesia is a movement disorder characterized by involuntary and repetitive movements of the tongue, lips, face, trunk, and extremities. It is caused by the use of dopamine receptor-blocking drugs, most often antipsychotics, but has also been associated with treatments for some gastrointestinal disorders.
Dr. Peter LeWitt, Sastry Foundation Endowed Chair in Neurology at Wayne State University School of Medicine in Detroit, MI, walks through some of the known causes of tardive dyskinesia as well as steps physicians can take in making a differential diagnosis.
Next, Dr. LeWitt dives into the role of targeted therapy in the management of tardive dyskinesia. VMAT2 (vesicular monoamine transporter type 2) inhibitors are emerging as a treatment class of choice that help suppress tardive dyskinesia symptoms by depleting presynaptic dopamine, while other patients may benefit more from benzodiazepines, botulinum toxin, or deep brain stimulation.
--
Dr. Peter LeWitt, Sastry Foundation Endowed Chair in Neurology at Wayne State University School of Medicine in Detroit, MI.
Peter A. LeWitt reports advisory roles for: Abide, Acorda Therapeutics, Adamas, Biogen, Cavion, Denali, Intec Pharma, Jazz Pharmaceuticals, Lundbeck, Neurocrine, Mitsubishi NeuroDerm Ltd, Prexton, Revance, Sage, and US WorldMeds.
Lecture fees from: Acorda, American Academy of Neurology, Kyowa Hakko Kirin, and US WorldMeds.
Research grant support from: Abide, Acorda, Amneal, Lundbeck, Michael J. Fox Foundation for Parkinson's Research, Mitsubishi NeuroDerm Ltd, Parkinson Study Group; Pharma 2B, Revance, Hoffmann-La Roche; Sunovion, Sun Pharma, and US WorldMeds.

Depression remains common among dystonia patients
About one-third of individuals with adult-onset idiopathic dystonia experience major depression or dysthymia, data from a meta-analysis of 54 studies show.
Adult-onset idiopathic dystonia (AOID) is the third-most common movement disorder after essential tremor and Parkinson’s disease, and data show that depression and anxiety are the largest contributors to reduced quality of life in these patients, wrote Alex Medina Escobar, MD, of the University of Calgary (Alta.), and colleagues. However, “the pathogenic mechanisms of depression and anxiety in AOID remain unclear” and might involve a combination of biologic factors, as well as social stigma.
In the meta-analysis, published in Neuroscience and Biobehavioral Reviews, the researchers examined the point prevalence of supraclinical threshold depressive symptoms/depressive disorders in AOID using 54 studies. The resulting study population included 12,635 patients: 6,977 with cervical dystonia, 732 with cranial dystonia, 4,504 with mixed forms, 303 with laryngeal dystonia, and 119 with upper-limb dystonia. The studies were published between 1988 and 2020, and included patients from 21 countries in 52 single-center studies and 2 multicenter studies.
Overall, the pooled prevalence of either supraclinical threshold depressive symptoms or depressive disorders was 31.5% for cervical dystonia, 29.2 % for cranial dystonia, and 33.6 % for clinical samples with mixed forms of AOID.
Among patients with cervical dystonia, major depressive disorder was more prevalent than dysthymia, but among patients with cranial dystonia, dysthymia was more prevalent. Among patients with mixed forms, the prevalence of major depressive disorder was higher than dysthymia. Heterogeneity varied among the studies but was higher in studies that used rating scales.
Treatment of patients with AOID does not take into account the impact of depression on quality of life, Dr. Escobar and colleagues reported.
“ Such model appears to be inefficient to guarantee resources to address these comorbidities within secondary or tertiary care, or through shared care pathways engaging both primary and hospital-based care.” They also said the use of antidepressants and cognitive-behavioral therapy as a way to target negative body concept or social stigma among these patients are “underexplored and underutilized.”
The study findings were limited by several factors, including the inclusion only of studies published in English. In addition, most of the studies were conducted at movement disorders clinics, which may have yielded a patient population with more severe AOID. Further limitations included the inability to perform subgroup analysis based on demographic and clinical factors, and the insufficient number of studies for meta-analysis of laryngeal and hand dystonia, Dr. Escobar and colleagues added.
However, the results represent the first pooled estimate of depression prevalence in AOID and confirm a high prevalence across different clinical forms, the researchers said. The heterogeneity across studies highlights the need for standardized screening for depression and improved diagnosis of mood disorders in AOID.
“The meta-analytic estimates provided here will be highly useful for the planning of future mechanistic and interventional studies, as well as for the redefinition of current models of care,” they concluded.
The study received no outside funding. Dr. Escobar and colleagues had no disclosures.
About one-third of individuals with adult-onset idiopathic dystonia experience major depression or dysthymia, data from a meta-analysis of 54 studies show.
Adult-onset idiopathic dystonia (AOID) is the third-most common movement disorder after essential tremor and Parkinson’s disease, and data show that depression and anxiety are the largest contributors to reduced quality of life in these patients, wrote Alex Medina Escobar, MD, of the University of Calgary (Alta.), and colleagues. However, “the pathogenic mechanisms of depression and anxiety in AOID remain unclear” and might involve a combination of biologic factors, as well as social stigma.
In the meta-analysis, published in Neuroscience and Biobehavioral Reviews, the researchers examined the point prevalence of supraclinical threshold depressive symptoms/depressive disorders in AOID using 54 studies. The resulting study population included 12,635 patients: 6,977 with cervical dystonia, 732 with cranial dystonia, 4,504 with mixed forms, 303 with laryngeal dystonia, and 119 with upper-limb dystonia. The studies were published between 1988 and 2020, and included patients from 21 countries in 52 single-center studies and 2 multicenter studies.
Overall, the pooled prevalence of either supraclinical threshold depressive symptoms or depressive disorders was 31.5% for cervical dystonia, 29.2 % for cranial dystonia, and 33.6 % for clinical samples with mixed forms of AOID.
Among patients with cervical dystonia, major depressive disorder was more prevalent than dysthymia, but among patients with cranial dystonia, dysthymia was more prevalent. Among patients with mixed forms, the prevalence of major depressive disorder was higher than dysthymia. Heterogeneity varied among the studies but was higher in studies that used rating scales.
Treatment of patients with AOID does not take into account the impact of depression on quality of life, Dr. Escobar and colleagues reported.
“ Such model appears to be inefficient to guarantee resources to address these comorbidities within secondary or tertiary care, or through shared care pathways engaging both primary and hospital-based care.” They also said the use of antidepressants and cognitive-behavioral therapy as a way to target negative body concept or social stigma among these patients are “underexplored and underutilized.”
The study findings were limited by several factors, including the inclusion only of studies published in English. In addition, most of the studies were conducted at movement disorders clinics, which may have yielded a patient population with more severe AOID. Further limitations included the inability to perform subgroup analysis based on demographic and clinical factors, and the insufficient number of studies for meta-analysis of laryngeal and hand dystonia, Dr. Escobar and colleagues added.
However, the results represent the first pooled estimate of depression prevalence in AOID and confirm a high prevalence across different clinical forms, the researchers said. The heterogeneity across studies highlights the need for standardized screening for depression and improved diagnosis of mood disorders in AOID.
“The meta-analytic estimates provided here will be highly useful for the planning of future mechanistic and interventional studies, as well as for the redefinition of current models of care,” they concluded.
The study received no outside funding. Dr. Escobar and colleagues had no disclosures.
About one-third of individuals with adult-onset idiopathic dystonia experience major depression or dysthymia, data from a meta-analysis of 54 studies show.
Adult-onset idiopathic dystonia (AOID) is the third-most common movement disorder after essential tremor and Parkinson’s disease, and data show that depression and anxiety are the largest contributors to reduced quality of life in these patients, wrote Alex Medina Escobar, MD, of the University of Calgary (Alta.), and colleagues. However, “the pathogenic mechanisms of depression and anxiety in AOID remain unclear” and might involve a combination of biologic factors, as well as social stigma.
In the meta-analysis, published in Neuroscience and Biobehavioral Reviews, the researchers examined the point prevalence of supraclinical threshold depressive symptoms/depressive disorders in AOID using 54 studies. The resulting study population included 12,635 patients: 6,977 with cervical dystonia, 732 with cranial dystonia, 4,504 with mixed forms, 303 with laryngeal dystonia, and 119 with upper-limb dystonia. The studies were published between 1988 and 2020, and included patients from 21 countries in 52 single-center studies and 2 multicenter studies.
Overall, the pooled prevalence of either supraclinical threshold depressive symptoms or depressive disorders was 31.5% for cervical dystonia, 29.2 % for cranial dystonia, and 33.6 % for clinical samples with mixed forms of AOID.
Among patients with cervical dystonia, major depressive disorder was more prevalent than dysthymia, but among patients with cranial dystonia, dysthymia was more prevalent. Among patients with mixed forms, the prevalence of major depressive disorder was higher than dysthymia. Heterogeneity varied among the studies but was higher in studies that used rating scales.
Treatment of patients with AOID does not take into account the impact of depression on quality of life, Dr. Escobar and colleagues reported.
“ Such model appears to be inefficient to guarantee resources to address these comorbidities within secondary or tertiary care, or through shared care pathways engaging both primary and hospital-based care.” They also said the use of antidepressants and cognitive-behavioral therapy as a way to target negative body concept or social stigma among these patients are “underexplored and underutilized.”
The study findings were limited by several factors, including the inclusion only of studies published in English. In addition, most of the studies were conducted at movement disorders clinics, which may have yielded a patient population with more severe AOID. Further limitations included the inability to perform subgroup analysis based on demographic and clinical factors, and the insufficient number of studies for meta-analysis of laryngeal and hand dystonia, Dr. Escobar and colleagues added.
However, the results represent the first pooled estimate of depression prevalence in AOID and confirm a high prevalence across different clinical forms, the researchers said. The heterogeneity across studies highlights the need for standardized screening for depression and improved diagnosis of mood disorders in AOID.
“The meta-analytic estimates provided here will be highly useful for the planning of future mechanistic and interventional studies, as well as for the redefinition of current models of care,” they concluded.
The study received no outside funding. Dr. Escobar and colleagues had no disclosures.
FROM NEUROSCIENCE AND BIOBEHAVIORAL REVIEWS