User login
Stem cell transplant superior to DMTs for secondary progressive MS
new research suggests.
Results from a retrospective study show that more than 60% of patients with SPMS who received AHSCT were free from disability progression at 5 years. Also for these patients, improvement was more likely to be maintained for years after treatment.
The investigators noted that patients with secondary progressive disease often show little benefit from other DMTs, so interest in other treatments is high. While AHSCT is known to offer good results for patients with relapsing remitting MS, studies of its efficacy for SPMS have yielded conflicting results.
The new findings suggest it may be time to take another look at this therapy for patients with active, more severe disease, the researchers wrote.
“AHSCT may become a treatment option in secondary progressive MS patients with inflammatory activity who have failed available treatments,” said coinvestigator Matilde Inglese, MD, PhD, professor of neurology at the University of Genoa (Italy).
“Patients selection is very important to ensure the best treatment response and minimize safety issues, including transplant-related mortality,” Dr. Inglese added.
The findings were published online in Neurology.
Class III evidence
In the retrospective, propensity-matching study, researchers used two Italian registries to identify 79 patients who were treated off label with AHSCT and 1,975 patients who received another therapy.
Other DMTs included in the control-group analysis were beta-interferons, azathioprine, glatiramer acetate, mitoxantrone, fingolimod, natalizumab, methotrexate, teriflunomide, cyclophosphamide, dimethyl fumarate, or alemtuzumab.
Results showed that time to first disability progression was significantly longer for patients who had received transplants (hazard ratio, 0.5; P = .005); 61.7% of the AHSCT group were free of disability progression at 5 years versus 46.3% of the control group.
Among patients who received AHSCT, relapse rates were lower in comparison with those who received other DMTs (P < .001), and disability scores were lower over 10 years (P < .001).
The transplant group was also significantly more likely than the other-DMTs group to achieve sustained improvement in disability 3 years after treatment (34.7% vs. 4.6%; P < .001).
“This study provides Class III evidence that autologous hematopoietic stem cell transplants prolonged the time to confirmed disability progression compared to other disease-modifying therapies,” the investigators wrote.
Extends the treatment population
Commenting on the study, Jeff Cohen, MD, director of experimental therapeutics at the Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, said the research “extends the population for which hematopoietic stem cell transplant should be considered.”
Although previous studies did not show a benefit for patients with severe progressive MS, participants in the current study had secondary progressive MS and superimposed relapse activity, said Dr. Cohen, who was not involved with the research.
“We think that indicates a greater likelihood of benefit” from AHSCT, he noted. “The fact that someone has overt progression or somewhat more severe disability doesn’t preclude the use of stem cell transplant.”
Dr. Cohen pointed out, however, that the study is not without limitations. The exclusion of patients taking B-cell therapies from the SPMS control group raises the question of whether similar results would come from a comparison with AHSCT.
In addition, Dr. Cohen noted there are safety concerns about the therapy, which has yielded higher transplant-related mortality among patients with SPMS – although only one patient in the current study died following the transplant.
Still, the findings are promising, Dr. Cohen added.
“I think as more data accumulate that supports its benefit and reasonable safety in a variety of populations, we’ll see it used more,” he said.
The study was funded by the Italian Multiple Sclerosis Foundation. Dr. Inglese has received fees for consultation from Roche, Genzyme, Merck, Biogen, and Novartis. Dr. Cohen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Results from a retrospective study show that more than 60% of patients with SPMS who received AHSCT were free from disability progression at 5 years. Also for these patients, improvement was more likely to be maintained for years after treatment.
The investigators noted that patients with secondary progressive disease often show little benefit from other DMTs, so interest in other treatments is high. While AHSCT is known to offer good results for patients with relapsing remitting MS, studies of its efficacy for SPMS have yielded conflicting results.
The new findings suggest it may be time to take another look at this therapy for patients with active, more severe disease, the researchers wrote.
“AHSCT may become a treatment option in secondary progressive MS patients with inflammatory activity who have failed available treatments,” said coinvestigator Matilde Inglese, MD, PhD, professor of neurology at the University of Genoa (Italy).
“Patients selection is very important to ensure the best treatment response and minimize safety issues, including transplant-related mortality,” Dr. Inglese added.
The findings were published online in Neurology.
Class III evidence
In the retrospective, propensity-matching study, researchers used two Italian registries to identify 79 patients who were treated off label with AHSCT and 1,975 patients who received another therapy.
Other DMTs included in the control-group analysis were beta-interferons, azathioprine, glatiramer acetate, mitoxantrone, fingolimod, natalizumab, methotrexate, teriflunomide, cyclophosphamide, dimethyl fumarate, or alemtuzumab.
Results showed that time to first disability progression was significantly longer for patients who had received transplants (hazard ratio, 0.5; P = .005); 61.7% of the AHSCT group were free of disability progression at 5 years versus 46.3% of the control group.
Among patients who received AHSCT, relapse rates were lower in comparison with those who received other DMTs (P < .001), and disability scores were lower over 10 years (P < .001).
The transplant group was also significantly more likely than the other-DMTs group to achieve sustained improvement in disability 3 years after treatment (34.7% vs. 4.6%; P < .001).
“This study provides Class III evidence that autologous hematopoietic stem cell transplants prolonged the time to confirmed disability progression compared to other disease-modifying therapies,” the investigators wrote.
Extends the treatment population
Commenting on the study, Jeff Cohen, MD, director of experimental therapeutics at the Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, said the research “extends the population for which hematopoietic stem cell transplant should be considered.”
Although previous studies did not show a benefit for patients with severe progressive MS, participants in the current study had secondary progressive MS and superimposed relapse activity, said Dr. Cohen, who was not involved with the research.
“We think that indicates a greater likelihood of benefit” from AHSCT, he noted. “The fact that someone has overt progression or somewhat more severe disability doesn’t preclude the use of stem cell transplant.”
Dr. Cohen pointed out, however, that the study is not without limitations. The exclusion of patients taking B-cell therapies from the SPMS control group raises the question of whether similar results would come from a comparison with AHSCT.
In addition, Dr. Cohen noted there are safety concerns about the therapy, which has yielded higher transplant-related mortality among patients with SPMS – although only one patient in the current study died following the transplant.
Still, the findings are promising, Dr. Cohen added.
“I think as more data accumulate that supports its benefit and reasonable safety in a variety of populations, we’ll see it used more,” he said.
The study was funded by the Italian Multiple Sclerosis Foundation. Dr. Inglese has received fees for consultation from Roche, Genzyme, Merck, Biogen, and Novartis. Dr. Cohen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
Results from a retrospective study show that more than 60% of patients with SPMS who received AHSCT were free from disability progression at 5 years. Also for these patients, improvement was more likely to be maintained for years after treatment.
The investigators noted that patients with secondary progressive disease often show little benefit from other DMTs, so interest in other treatments is high. While AHSCT is known to offer good results for patients with relapsing remitting MS, studies of its efficacy for SPMS have yielded conflicting results.
The new findings suggest it may be time to take another look at this therapy for patients with active, more severe disease, the researchers wrote.
“AHSCT may become a treatment option in secondary progressive MS patients with inflammatory activity who have failed available treatments,” said coinvestigator Matilde Inglese, MD, PhD, professor of neurology at the University of Genoa (Italy).
“Patients selection is very important to ensure the best treatment response and minimize safety issues, including transplant-related mortality,” Dr. Inglese added.
The findings were published online in Neurology.
Class III evidence
In the retrospective, propensity-matching study, researchers used two Italian registries to identify 79 patients who were treated off label with AHSCT and 1,975 patients who received another therapy.
Other DMTs included in the control-group analysis were beta-interferons, azathioprine, glatiramer acetate, mitoxantrone, fingolimod, natalizumab, methotrexate, teriflunomide, cyclophosphamide, dimethyl fumarate, or alemtuzumab.
Results showed that time to first disability progression was significantly longer for patients who had received transplants (hazard ratio, 0.5; P = .005); 61.7% of the AHSCT group were free of disability progression at 5 years versus 46.3% of the control group.
Among patients who received AHSCT, relapse rates were lower in comparison with those who received other DMTs (P < .001), and disability scores were lower over 10 years (P < .001).
The transplant group was also significantly more likely than the other-DMTs group to achieve sustained improvement in disability 3 years after treatment (34.7% vs. 4.6%; P < .001).
“This study provides Class III evidence that autologous hematopoietic stem cell transplants prolonged the time to confirmed disability progression compared to other disease-modifying therapies,” the investigators wrote.
Extends the treatment population
Commenting on the study, Jeff Cohen, MD, director of experimental therapeutics at the Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, said the research “extends the population for which hematopoietic stem cell transplant should be considered.”
Although previous studies did not show a benefit for patients with severe progressive MS, participants in the current study had secondary progressive MS and superimposed relapse activity, said Dr. Cohen, who was not involved with the research.
“We think that indicates a greater likelihood of benefit” from AHSCT, he noted. “The fact that someone has overt progression or somewhat more severe disability doesn’t preclude the use of stem cell transplant.”
Dr. Cohen pointed out, however, that the study is not without limitations. The exclusion of patients taking B-cell therapies from the SPMS control group raises the question of whether similar results would come from a comparison with AHSCT.
In addition, Dr. Cohen noted there are safety concerns about the therapy, which has yielded higher transplant-related mortality among patients with SPMS – although only one patient in the current study died following the transplant.
Still, the findings are promising, Dr. Cohen added.
“I think as more data accumulate that supports its benefit and reasonable safety in a variety of populations, we’ll see it used more,” he said.
The study was funded by the Italian Multiple Sclerosis Foundation. Dr. Inglese has received fees for consultation from Roche, Genzyme, Merck, Biogen, and Novartis. Dr. Cohen reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM NEUROLOGY
STEMI times to treatment usually miss established goals
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).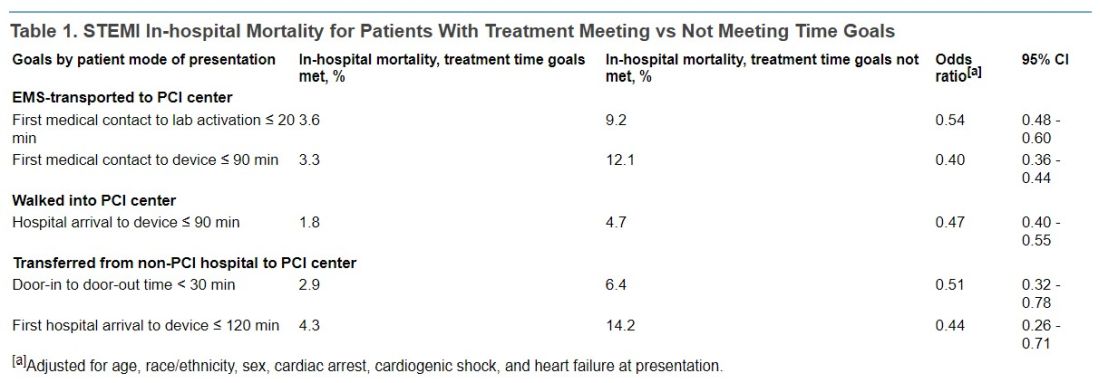
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
Therapy initiated within national treatment-time goals set a decade ago for patients with ST-segment elevation myocardial infarction (STEMI) remains associated with improved survival in recent years. But for many such patients, time from first symptoms to initiation of reperfusion therapy still fails to meet those goals, suggests a cross-sectional registry analysis.
For example, patients initially transported to centers with percutaneous coronary intervention (PCI) capability had a median treatment time of 148 minutes, in the analysis spanning the second quarter (Q2) of 2018 to the third quarter (Q3) of 2021. But the goal for centers called for treatment initiation within 90 minutes for at least 75% of such STEMI patients.
Moreover, overall STEMI treatment times and in-hospital mortality rose in tandem significantly from Q2 2018 through the first quarter (Q1) of 2021, which included the first year of the COVID-19 pandemic. Median time to treatment went from 86 minutes to 91 minutes during that period. Meanwhile, in-hospital mortality went from 5.6% to 8.7%, report the study authors led by James G. Jollis, MD, Duke University, Durham, N.C.
Their report, based on 114,871 STEMI patients at 601 US hospitals contributing to the Get With The Guidelines – Coronary Artery Disease registry, was published online in JAMA.
Of those patients, 25,085 had been transferred from non-PCI hospitals, 32,483 were walk-ins, and 57,303 arrived via emergency medical services (EMS). Their median times from symptom onset to PCI were 240, 195, and 148 minutes, respectively.
In-hospital mortality was significantly reduced in an adjusted analysis for patients treated within target times, compared with those whose treatment missed the time goals, regardless of whether they were transported by EMS, walked into a hospital with on-site PCI, or were transferred from a non-PCI center (Table 1).
Regardless of mode of patient presentation, treatment time goals were not met most of the time, the group reports. Patients who required interhospital transfer experienced the longest system delays; only 17% were treated within 120 minutes.
Among patients who received primary PCI, 20% had a registry-defined hospital-specified reason for delay, including cardiac arrest and/or need for intubation in 6.8%, “difficulty crossing the culprit lesion” in 3.8%, and “other reasons” in 5.8%, the group reports.
“In 2020, a new reason for delay was added to the registry, ‘need for additional personal protective equipment for suspected/confirmed infectious disease.’ This reason was most commonly used in the second quarter of 2020 (6%) and then declined over time to 1% in the final 2 quarters,” they write.
“Thus, active SARS-CoV-2 infection appeared to have a smaller direct role in longer treatment times or worse outcomes.” Rather, they continue, “the pandemic potentially had a significant indirect role as hospitals were overwhelmed with patients, EMS and hospitals were challenged in maintaining paramedic and nurse staffing and intensive care bed availability, and patients experienced delayed care due to barriers to access or perceived fear of becoming entangled in an overwhelmed medical system.”
Still an important quality metric
STEMI treatment times remain an important quality metric to which hospitals should continue to pay attention because shorter times improve patient care, Deepak Bhatt, MD, MPH, told this news organization.
“Having said that, as with all metrics, one needs to be thoughtful and realize that a difference of a couple of minutes is probably not a crucial thing,” said Dr. Bhatt, Brigham and Women’s Hospital and Harvard Medical School, Boston, who was not involved with the current study.
Interhospital transfers indeed involve longer delays, he observed, suggesting that regional integrated health systems should develop methods for optimizing STEMI care – even, for example, if they involve bypassing non-PCI centers or stopping patients briefly for stabilization followed by rapid transport to a PCI-capable facility.
“That, of course, requires cooperation among hospitals. Sometimes that requires hospitals putting aside economic considerations and just focusing on doing the right thing for that individual patient,” Dr. Bhatt said.
Transfer delays are common for patients presenting with STEMI at hospitals without PCI capability, he noted. “Having clear protocols in place that expedite that type of transfer, I think, could go a long way in reducing the time to treatment in patients that are presenting to the hospital without cath labs. That’s an important message that these data provide.”
The onset of COVID-19 led to widespread delays in STEMI time to treatment early in the pandemic. There were concerns about exposing cath lab personnel to SARS-CoV-2 and potential adverse consequences of sick personnel being unable to provide patient care in the subsequent weeks and months, Dr. Bhatt observed.
However, “All of that seems to have quieted down, and I don’t think COVID is impacting time to treatment right now.”
‘Suboptimal compliance’ with standards
The current findings of “suboptimal compliance with national targets underscore why reassessing quality metrics, in light of changing practice patterns and other secular trends, is critical,” write Andrew S. Oseran, MD, MBA, and Robert W. Yeh, MD, both of Harvard Medical School, in an accompanying editorial.
“While the importance of coordinated and expeditious care for this high-risk patient population is undeniable, the specific actions that hospitals can – or should – take to further improve overall STEMI outcomes are less clear,” they say.
“As physicians contemplate the optimal path forward in managing the care of STEMI patients, they must recognize the clinical and operational nuance that exists in caring for this diverse population and acknowledge the trade-offs associated with uniform quality metrics,” write the editorialists.
“Global reductions in time to treatment for STEMI patients has been one of health care’s great success stories. As we move forward, it may be time to consider whether efforts to achieve additional improvement in target treatment times will result in substantive benefits, or whether we have reached the point of diminishing returns.”
A version of this article first appeared on Medscape.com.
FROM JAMA
Innovations in Dermatology Fall Abstract Compendium
Fourth Study to Show Consistent Benefit of Highly Purified Eicosapentaenoic Acid on Cardiovascular Outcomes: Results From RESPECT-EPA
In this supplement to Cardiology News, John R. Nelson, MD, FACC, FNLA, FASNC, and Matthew J. Budoff, MD, discuss results from RESPECT-EPA and the existing evidence that purified eicosapentaenoic acid significantly reduces residual CV risk in patients with CVD.
In this supplement to Cardiology News, John R. Nelson, MD, FACC, FNLA, FASNC, and Matthew J. Budoff, MD, discuss results from RESPECT-EPA and the existing evidence that purified eicosapentaenoic acid significantly reduces residual CV risk in patients with CVD.
In this supplement to Cardiology News, John R. Nelson, MD, FACC, FNLA, FASNC, and Matthew J. Budoff, MD, discuss results from RESPECT-EPA and the existing evidence that purified eicosapentaenoic acid significantly reduces residual CV risk in patients with CVD.
“The Nail in the Coffin for Fibrates”: Futility of PROMINENT Trial Definitively Settles Debate on Avoiding Use of Fibrate Class of Medications for Cardiovascular Risk Reduction
In this supplement to Cardiology News, Payal Kohli, MD, FACC, and Nihar Desai, MD, MPH, discuss the PROMINENT trial and the debate on avoiding the use of fibrates for cardiovascular risk reduction.
In this supplement to Cardiology News, Payal Kohli, MD, FACC, and Nihar Desai, MD, MPH, discuss the PROMINENT trial and the debate on avoiding the use of fibrates for cardiovascular risk reduction.
In this supplement to Cardiology News, Payal Kohli, MD, FACC, and Nihar Desai, MD, MPH, discuss the PROMINENT trial and the debate on avoiding the use of fibrates for cardiovascular risk reduction.
Prodromal Parkinson’s disease tied to significant functional impairment
new research shows.
The new findings come from a large case-control study that analyzed Medicare claims data to evaluate functional limitations in prodromal Parkinson’s disease, leading the investigators to suggest prodromal Parkinson’s disease should be recognized as a distinct disease stage.
“It’s increasingly recognized as a stage of Parkinson’s and there is an argument here for that,” said lead investigator Cameron Miller-Patterson, MD, assistant professor of neurology at Virginia Commonwealth University, Richmond. “Because we’re finding that people with prodromal Parkinson’s disease may have functional limitations, identifying them sooner and getting them the appropriate symptomatic therapy could be helpful.”
The findings were published online in JAMA Neurology.
Improving quality of life
Individuals with prodromal Parkinson’s disease have symptoms of Parkinson’s disease, but not enough to meet diagnostic criteria. However, all patients with prodromal Parkinson’s disease eventually meet that threshold.
To evaluate whether functional limitations are present in individuals with Parkinson’s disease prior to diagnosis versus the general population, researchers analyzed Medicare-linked data on 6,674 individuals aged 65 years and older who participated in the National Health and Aging Trends Study, a longitudinal survey in the United States. Survey questions evaluated dexterity, eating, mobility, mood, pain, sleep, speech, strength, and vision.
Patients with incident Parkinson’s disease were defined as having two or more Medicare diagnoses. Controls were defined as those with Medicare eligibility at baseline and 2 or more years prior, with no diagnosis.
Compared with individuals who never had Parkinson’s disease, those who eventually received a diagnosis were less likely to report being able to walk 6 blocks (odds ratio, 0.34; 95% confidence interval, 0.15-0.82), stand independently from kneeling (OR, 0.30; 95% CI, 0.11-0.85) or lift a heavy object overhead (OR, 0.36; 95% CI, 0.15-0.87). They were also more likely to report imbalance (OR, 2.77; 95% CI, 1.24-6.20) 3 years prior to diagnosis.
“Generally, we don’t start treating people until we see them in the clinic and give them a diagnosis of Parkinson’s disease,” Dr. Miller-Patterson said. “If we identify them earlier, even before diagnosis, we may be able to improve their quality of life by treating them sooner.”
Serving patients better
Better recognition of prodromal Parkinson’s disease could also help identify participants for clinical trials of therapeutics that could slow disease progression, something that is beyond the ability of currently approved medications.
This, and growing support for distinguishing prodromal Parkinson’s disease as an official stage of Parkinson’s disease, makes findings such as these both timely and important, the authors of an accompanying commentary wrote .
“The recognition of a prodromal period has been viewed as potentially critical to the success of disease-modifying interventions, on the argument that it may be too late to enact meaningful clinical change once symptoms clinically manifest given the degree of neurodegeneration already present,” Ian O. Bledsoe, MD, Weill Institute for Neurosciences, University of California, San Francisco, and coauthors wrote.
One limitation, however, is that the study design didn’t allow researchers to determine if individuals with eventual Parkinson’s disease who reported parkinsonian symptoms had prodromal Parkinson’s disease or undiagnosed disease. The answer would clarify whether prodromal Parkinson’s disease is more common than previously thought or if Parkinson’s disease diagnosis is often delayed for years – or both.
“Despite the limitations of this study, its broader point and importance remain: People appear to have some markers of functional decline before they are diagnosed with Parkinson’s disease,” the editorialists wrote. “Additionally, motor dysfunction may arise at an earlier time point in the disease than we typically think. There is a potential opportunity to serve this population better.”
The study was funded by the National Institutes of Health. Dr. Miller-Patterson reported receiving other NIH grants during the course of the study. Dr. Bledsoe reported personal fees from Boston Scientific, Amneal Pharmaceuticals, IDEO, Accorda, Humancraft.com, and Putnam Associates, as well as grants from the National Institutes of Health, the Michael J. Fox Foundation, and Dystonia Medical.
A version of this article first appeared on Medscape.com.
new research shows.
The new findings come from a large case-control study that analyzed Medicare claims data to evaluate functional limitations in prodromal Parkinson’s disease, leading the investigators to suggest prodromal Parkinson’s disease should be recognized as a distinct disease stage.
“It’s increasingly recognized as a stage of Parkinson’s and there is an argument here for that,” said lead investigator Cameron Miller-Patterson, MD, assistant professor of neurology at Virginia Commonwealth University, Richmond. “Because we’re finding that people with prodromal Parkinson’s disease may have functional limitations, identifying them sooner and getting them the appropriate symptomatic therapy could be helpful.”
The findings were published online in JAMA Neurology.
Improving quality of life
Individuals with prodromal Parkinson’s disease have symptoms of Parkinson’s disease, but not enough to meet diagnostic criteria. However, all patients with prodromal Parkinson’s disease eventually meet that threshold.
To evaluate whether functional limitations are present in individuals with Parkinson’s disease prior to diagnosis versus the general population, researchers analyzed Medicare-linked data on 6,674 individuals aged 65 years and older who participated in the National Health and Aging Trends Study, a longitudinal survey in the United States. Survey questions evaluated dexterity, eating, mobility, mood, pain, sleep, speech, strength, and vision.
Patients with incident Parkinson’s disease were defined as having two or more Medicare diagnoses. Controls were defined as those with Medicare eligibility at baseline and 2 or more years prior, with no diagnosis.
Compared with individuals who never had Parkinson’s disease, those who eventually received a diagnosis were less likely to report being able to walk 6 blocks (odds ratio, 0.34; 95% confidence interval, 0.15-0.82), stand independently from kneeling (OR, 0.30; 95% CI, 0.11-0.85) or lift a heavy object overhead (OR, 0.36; 95% CI, 0.15-0.87). They were also more likely to report imbalance (OR, 2.77; 95% CI, 1.24-6.20) 3 years prior to diagnosis.
“Generally, we don’t start treating people until we see them in the clinic and give them a diagnosis of Parkinson’s disease,” Dr. Miller-Patterson said. “If we identify them earlier, even before diagnosis, we may be able to improve their quality of life by treating them sooner.”
Serving patients better
Better recognition of prodromal Parkinson’s disease could also help identify participants for clinical trials of therapeutics that could slow disease progression, something that is beyond the ability of currently approved medications.
This, and growing support for distinguishing prodromal Parkinson’s disease as an official stage of Parkinson’s disease, makes findings such as these both timely and important, the authors of an accompanying commentary wrote .
“The recognition of a prodromal period has been viewed as potentially critical to the success of disease-modifying interventions, on the argument that it may be too late to enact meaningful clinical change once symptoms clinically manifest given the degree of neurodegeneration already present,” Ian O. Bledsoe, MD, Weill Institute for Neurosciences, University of California, San Francisco, and coauthors wrote.
One limitation, however, is that the study design didn’t allow researchers to determine if individuals with eventual Parkinson’s disease who reported parkinsonian symptoms had prodromal Parkinson’s disease or undiagnosed disease. The answer would clarify whether prodromal Parkinson’s disease is more common than previously thought or if Parkinson’s disease diagnosis is often delayed for years – or both.
“Despite the limitations of this study, its broader point and importance remain: People appear to have some markers of functional decline before they are diagnosed with Parkinson’s disease,” the editorialists wrote. “Additionally, motor dysfunction may arise at an earlier time point in the disease than we typically think. There is a potential opportunity to serve this population better.”
The study was funded by the National Institutes of Health. Dr. Miller-Patterson reported receiving other NIH grants during the course of the study. Dr. Bledsoe reported personal fees from Boston Scientific, Amneal Pharmaceuticals, IDEO, Accorda, Humancraft.com, and Putnam Associates, as well as grants from the National Institutes of Health, the Michael J. Fox Foundation, and Dystonia Medical.
A version of this article first appeared on Medscape.com.
new research shows.
The new findings come from a large case-control study that analyzed Medicare claims data to evaluate functional limitations in prodromal Parkinson’s disease, leading the investigators to suggest prodromal Parkinson’s disease should be recognized as a distinct disease stage.
“It’s increasingly recognized as a stage of Parkinson’s and there is an argument here for that,” said lead investigator Cameron Miller-Patterson, MD, assistant professor of neurology at Virginia Commonwealth University, Richmond. “Because we’re finding that people with prodromal Parkinson’s disease may have functional limitations, identifying them sooner and getting them the appropriate symptomatic therapy could be helpful.”
The findings were published online in JAMA Neurology.
Improving quality of life
Individuals with prodromal Parkinson’s disease have symptoms of Parkinson’s disease, but not enough to meet diagnostic criteria. However, all patients with prodromal Parkinson’s disease eventually meet that threshold.
To evaluate whether functional limitations are present in individuals with Parkinson’s disease prior to diagnosis versus the general population, researchers analyzed Medicare-linked data on 6,674 individuals aged 65 years and older who participated in the National Health and Aging Trends Study, a longitudinal survey in the United States. Survey questions evaluated dexterity, eating, mobility, mood, pain, sleep, speech, strength, and vision.
Patients with incident Parkinson’s disease were defined as having two or more Medicare diagnoses. Controls were defined as those with Medicare eligibility at baseline and 2 or more years prior, with no diagnosis.
Compared with individuals who never had Parkinson’s disease, those who eventually received a diagnosis were less likely to report being able to walk 6 blocks (odds ratio, 0.34; 95% confidence interval, 0.15-0.82), stand independently from kneeling (OR, 0.30; 95% CI, 0.11-0.85) or lift a heavy object overhead (OR, 0.36; 95% CI, 0.15-0.87). They were also more likely to report imbalance (OR, 2.77; 95% CI, 1.24-6.20) 3 years prior to diagnosis.
“Generally, we don’t start treating people until we see them in the clinic and give them a diagnosis of Parkinson’s disease,” Dr. Miller-Patterson said. “If we identify them earlier, even before diagnosis, we may be able to improve their quality of life by treating them sooner.”
Serving patients better
Better recognition of prodromal Parkinson’s disease could also help identify participants for clinical trials of therapeutics that could slow disease progression, something that is beyond the ability of currently approved medications.
This, and growing support for distinguishing prodromal Parkinson’s disease as an official stage of Parkinson’s disease, makes findings such as these both timely and important, the authors of an accompanying commentary wrote .
“The recognition of a prodromal period has been viewed as potentially critical to the success of disease-modifying interventions, on the argument that it may be too late to enact meaningful clinical change once symptoms clinically manifest given the degree of neurodegeneration already present,” Ian O. Bledsoe, MD, Weill Institute for Neurosciences, University of California, San Francisco, and coauthors wrote.
One limitation, however, is that the study design didn’t allow researchers to determine if individuals with eventual Parkinson’s disease who reported parkinsonian symptoms had prodromal Parkinson’s disease or undiagnosed disease. The answer would clarify whether prodromal Parkinson’s disease is more common than previously thought or if Parkinson’s disease diagnosis is often delayed for years – or both.
“Despite the limitations of this study, its broader point and importance remain: People appear to have some markers of functional decline before they are diagnosed with Parkinson’s disease,” the editorialists wrote. “Additionally, motor dysfunction may arise at an earlier time point in the disease than we typically think. There is a potential opportunity to serve this population better.”
The study was funded by the National Institutes of Health. Dr. Miller-Patterson reported receiving other NIH grants during the course of the study. Dr. Bledsoe reported personal fees from Boston Scientific, Amneal Pharmaceuticals, IDEO, Accorda, Humancraft.com, and Putnam Associates, as well as grants from the National Institutes of Health, the Michael J. Fox Foundation, and Dystonia Medical.
A version of this article first appeared on Medscape.com.
FROM JAMA NEUROLOGY
Cervical cancer rise in White women: A ‘canary in the coal mine’
Cervical cancer appears to be rising more rapidly in White women than in Black women in the United States, according to two independent studies. Researchers puzzling over this counterintuitive finding say that, if true, the findings may be a “canary in the coal mine,” signaling problems with U.S. health care that go way beyond women’s health.
Cervical cancer incidence in the United States has plateaued since 2010 and now stands at 7.5 per 100,000 people. Well-known disparities exist: Compared with White women, Black women are more likely to have distant-stage disease at diagnosis and more commonly die of their cancer.
However, two unconnected studies published in the past 5 months suggest that White women are catching up fast.
Cervical cancer rates in White women aged 30-34 are rising 2.8% a year, but holding steady for Black women, according to a recent study led by Ashish A. Deshmukh, PhD, of the Medical University of South Carolina, Charleston. His team analyzed the 2001-2019 National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) dataset, which covers 98% of the U.S. population and 227,062 cervical cancer cases.
These findings were echoed by an analysis of the same database for 2001-2018 by the University of California, Los Angeles (UCLA), suggesting that distant-stage cervical cancer (defined as disease that has spread to the bladder and/or rectum at diagnosis) is climbing 1.69% a year in White people versus 0.67% in Black individuals.
The UCLA researchers, headed by Alex Francoeur, MD, a resident in the department of obstetrics and gynecology, found that disparities were most stark in adenocarcinoma, with an annual increase of 3.40% a year among White women and 1.71% in Black women.
Such findings have equity researchers scratching their heads. In cancer, it’s rare to see evidence that Black patients are doing better than their White counterparts.
One theoretical explanation is that the data are flawed, Ahmedin Jemal, DVM, PhD, senior vice president of surveillance and health equity science at the American Cancer Society, told this news organization. For example, the UCLA analysis may have been fogged by changes in staging definitions over time, Dr. Jemal said, although this would not explain the racial disparities per se.
Dr. Deshmukh stands by his data and said that, for him, the message is clear: “If rising incidence is not for localized-stage disease, but for advanced stages, that means it’s attributable to lack of screening,” he told this news organization.
However, this ‘simple’ explanation generates even more questions, Dr. Deshmukh said: “Screening is not a one-time procedure [but] a spectrum of timely cervical precancer treatment if [required]. We don’t know when exactly non-Hispanic White women are falling behind on that spectrum.”
The UCLA study supports Dr. Deshmukh’s conclusions. Using data from the Behavioral Risk Factor Surveillance System to calculate trends in “nonguideline screening,” the researchers found that White women were almost twice as likely to report that they were not following screening guidelines compared with Black women over the period of 2001-2016 (26.6% vs. 13.8%; P < .001).
“It’s not an artifact, it’s real,” said Timothy Rebbeck, PhD, the Vincent L. Gregory Jr. Professor of Cancer Prevention at Harvard TH Chan School of Public Health, Boston, who was not an author of either study and was approached for comment.
“The data are correct but there are so many things going on that might explain these patterns,” he told this news organization.
“This is a great example of complex changes in our social system, political system, health care system that are having really clear, measurable effects,” Dr. Rebbeck said. “Cervical cancer is almost a canary in the coal mine for some of this because it’s so preventable.”
(The saying “canary in a coal mine” is a warning of danger or trouble ahead. It comes from the time when coal miners would carry a caged canary down into the tunnels to warn them of noxious gases, which would kill the bird but give men time to escape.)
For example, Dr. Rebbeck said, recent turmoil in U.S. health care has left many people distrustful of the system. Although he acknowledged this was “high speculation,” he suggested that some women may have become less willing to participate in any mass health care intervention because of their political beliefs.
The UCLA study found that distant cervical cancer was rising fastest in middle-aged White women in the U.S. South, at a rate of 4.5% per year (P < .001).
Dr. Rebbeck also suggested that Medicaid expansion – the broadening of health insurance coverage in some states since the Affordable Care Act in 2014 – could be implicated. Of the 11 states that have not yet expanded Medicaid, eight are in the South.
“White populations who are in states that didn’t expand Medicaid are not getting a lot of the standard treatment and care that you would expect ...” Dr. Rebbeck said. “You could very well imagine that Medicaid nonexpanding states would have all kinds of patterns that would lead to more aggressive disease.”
In fact, there is already evidence that Medicaid expansion has affected racial disparities, disproportionately benefiting Black and Hispanic families, as for example, from an analysis of 65 studies by the Kaiser Family Foundation in 2020.
Commenting on these data, Dr. Rebbeck said, “Does that mean that the patterns of advanced cervical cancer had a smaller effect on Black women in this period because there was a greater shift in access to care? This is again a speculation, but it does fit with the ‘canary in a coal mine’ concept that advanced cervical cancer may be more rapidly influenced by health care access than other health conditions.”
The authors of the UCLA study suggested another explanation for their results: Differing enthusiasm for human papillomavirus (HPV) vaccination among White and Black families. The team also analyzed data on HPV vaccination, which offers protection against cervical cancer. The researchers found that vaccination rates were lowest, at 66.1%, among White teenagers aged 13-17 years, compared with Hispanics at 75.3%, Black teenagers at 74.6%, and Asians at 68.1%.
However, this theory was dismissed by both Dr. Jemal and Dr. Rebbeck due to timing. HPV vaccines have been around for approximately 15 years, so women who benefited (or didn’t benefit) from vaccination would be only in their late 20s today, they pointed out.
“Ninety-five percent of the cervical cancer cases we see now are in women who have not been vaccinated,” said Dr. Jemal, “So that’s out of the equation.”
Dr. Rebbeck agreed: “HPV may or may not be a thing here because it’s [got] such a latency.”
HPV vaccination may be out of the picture, but what about the epidemiology of HPV itself? Could the virus directly or indirectly be boosting advanced cervical cancer in White women?
Dr. Deshmukh thinks that it might be doing so.
He published an analysis of 2000-2018 SEER data showing that U.S. counties with the highest incidences of HPV-associated cancers also had the highest levels of smoking.
Other recent data suggest that middle-aged White women in the United States are more likely to reach for a smoke than are Black women.
Dr. Deshmukh acknowledges that the link is speculative but reasonable: “We don’t know exactly what the impact of smoking would be in terms of ... the ability to clear HPV infection. It may inhibit apoptosis, promoting tumor growth. There’s no causal association. It’s a cofactor risk.”
Dr. Rebbeck is also suspicious that smoking patterns might be a factor, pointing out that “smoking is certainly associated with both health behaviors and advanced cervical cancer.”
Both Dr. Rebbeck and Dr. Deshmukh concluded that, at this point, we can only speculate on what’s driving the puzzling acceleration of cervical cancer in White women in the United States.
However, whether it’s political aversion to screening, smoking-boosted HPV infection, Medicaid expansion or lack of it, or something else, they all agree that this canary in the coal mine clearly needs urgent medical attention.
Dr. Rebbeck and Dr. Jemal have declared no conflicts of interest. Dr. Deshmukh has declared consultant or advisory roles for Merck and Value Analytics Labs. None of the authors of the UCLA study have declared competing interests.
A version of this article first appeared on Medscape.com.
Cervical cancer appears to be rising more rapidly in White women than in Black women in the United States, according to two independent studies. Researchers puzzling over this counterintuitive finding say that, if true, the findings may be a “canary in the coal mine,” signaling problems with U.S. health care that go way beyond women’s health.
Cervical cancer incidence in the United States has plateaued since 2010 and now stands at 7.5 per 100,000 people. Well-known disparities exist: Compared with White women, Black women are more likely to have distant-stage disease at diagnosis and more commonly die of their cancer.
However, two unconnected studies published in the past 5 months suggest that White women are catching up fast.
Cervical cancer rates in White women aged 30-34 are rising 2.8% a year, but holding steady for Black women, according to a recent study led by Ashish A. Deshmukh, PhD, of the Medical University of South Carolina, Charleston. His team analyzed the 2001-2019 National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) dataset, which covers 98% of the U.S. population and 227,062 cervical cancer cases.
These findings were echoed by an analysis of the same database for 2001-2018 by the University of California, Los Angeles (UCLA), suggesting that distant-stage cervical cancer (defined as disease that has spread to the bladder and/or rectum at diagnosis) is climbing 1.69% a year in White people versus 0.67% in Black individuals.
The UCLA researchers, headed by Alex Francoeur, MD, a resident in the department of obstetrics and gynecology, found that disparities were most stark in adenocarcinoma, with an annual increase of 3.40% a year among White women and 1.71% in Black women.
Such findings have equity researchers scratching their heads. In cancer, it’s rare to see evidence that Black patients are doing better than their White counterparts.
One theoretical explanation is that the data are flawed, Ahmedin Jemal, DVM, PhD, senior vice president of surveillance and health equity science at the American Cancer Society, told this news organization. For example, the UCLA analysis may have been fogged by changes in staging definitions over time, Dr. Jemal said, although this would not explain the racial disparities per se.
Dr. Deshmukh stands by his data and said that, for him, the message is clear: “If rising incidence is not for localized-stage disease, but for advanced stages, that means it’s attributable to lack of screening,” he told this news organization.
However, this ‘simple’ explanation generates even more questions, Dr. Deshmukh said: “Screening is not a one-time procedure [but] a spectrum of timely cervical precancer treatment if [required]. We don’t know when exactly non-Hispanic White women are falling behind on that spectrum.”
The UCLA study supports Dr. Deshmukh’s conclusions. Using data from the Behavioral Risk Factor Surveillance System to calculate trends in “nonguideline screening,” the researchers found that White women were almost twice as likely to report that they were not following screening guidelines compared with Black women over the period of 2001-2016 (26.6% vs. 13.8%; P < .001).
“It’s not an artifact, it’s real,” said Timothy Rebbeck, PhD, the Vincent L. Gregory Jr. Professor of Cancer Prevention at Harvard TH Chan School of Public Health, Boston, who was not an author of either study and was approached for comment.
“The data are correct but there are so many things going on that might explain these patterns,” he told this news organization.
“This is a great example of complex changes in our social system, political system, health care system that are having really clear, measurable effects,” Dr. Rebbeck said. “Cervical cancer is almost a canary in the coal mine for some of this because it’s so preventable.”
(The saying “canary in a coal mine” is a warning of danger or trouble ahead. It comes from the time when coal miners would carry a caged canary down into the tunnels to warn them of noxious gases, which would kill the bird but give men time to escape.)
For example, Dr. Rebbeck said, recent turmoil in U.S. health care has left many people distrustful of the system. Although he acknowledged this was “high speculation,” he suggested that some women may have become less willing to participate in any mass health care intervention because of their political beliefs.
The UCLA study found that distant cervical cancer was rising fastest in middle-aged White women in the U.S. South, at a rate of 4.5% per year (P < .001).
Dr. Rebbeck also suggested that Medicaid expansion – the broadening of health insurance coverage in some states since the Affordable Care Act in 2014 – could be implicated. Of the 11 states that have not yet expanded Medicaid, eight are in the South.
“White populations who are in states that didn’t expand Medicaid are not getting a lot of the standard treatment and care that you would expect ...” Dr. Rebbeck said. “You could very well imagine that Medicaid nonexpanding states would have all kinds of patterns that would lead to more aggressive disease.”
In fact, there is already evidence that Medicaid expansion has affected racial disparities, disproportionately benefiting Black and Hispanic families, as for example, from an analysis of 65 studies by the Kaiser Family Foundation in 2020.
Commenting on these data, Dr. Rebbeck said, “Does that mean that the patterns of advanced cervical cancer had a smaller effect on Black women in this period because there was a greater shift in access to care? This is again a speculation, but it does fit with the ‘canary in a coal mine’ concept that advanced cervical cancer may be more rapidly influenced by health care access than other health conditions.”
The authors of the UCLA study suggested another explanation for their results: Differing enthusiasm for human papillomavirus (HPV) vaccination among White and Black families. The team also analyzed data on HPV vaccination, which offers protection against cervical cancer. The researchers found that vaccination rates were lowest, at 66.1%, among White teenagers aged 13-17 years, compared with Hispanics at 75.3%, Black teenagers at 74.6%, and Asians at 68.1%.
However, this theory was dismissed by both Dr. Jemal and Dr. Rebbeck due to timing. HPV vaccines have been around for approximately 15 years, so women who benefited (or didn’t benefit) from vaccination would be only in their late 20s today, they pointed out.
“Ninety-five percent of the cervical cancer cases we see now are in women who have not been vaccinated,” said Dr. Jemal, “So that’s out of the equation.”
Dr. Rebbeck agreed: “HPV may or may not be a thing here because it’s [got] such a latency.”
HPV vaccination may be out of the picture, but what about the epidemiology of HPV itself? Could the virus directly or indirectly be boosting advanced cervical cancer in White women?
Dr. Deshmukh thinks that it might be doing so.
He published an analysis of 2000-2018 SEER data showing that U.S. counties with the highest incidences of HPV-associated cancers also had the highest levels of smoking.
Other recent data suggest that middle-aged White women in the United States are more likely to reach for a smoke than are Black women.
Dr. Deshmukh acknowledges that the link is speculative but reasonable: “We don’t know exactly what the impact of smoking would be in terms of ... the ability to clear HPV infection. It may inhibit apoptosis, promoting tumor growth. There’s no causal association. It’s a cofactor risk.”
Dr. Rebbeck is also suspicious that smoking patterns might be a factor, pointing out that “smoking is certainly associated with both health behaviors and advanced cervical cancer.”
Both Dr. Rebbeck and Dr. Deshmukh concluded that, at this point, we can only speculate on what’s driving the puzzling acceleration of cervical cancer in White women in the United States.
However, whether it’s political aversion to screening, smoking-boosted HPV infection, Medicaid expansion or lack of it, or something else, they all agree that this canary in the coal mine clearly needs urgent medical attention.
Dr. Rebbeck and Dr. Jemal have declared no conflicts of interest. Dr. Deshmukh has declared consultant or advisory roles for Merck and Value Analytics Labs. None of the authors of the UCLA study have declared competing interests.
A version of this article first appeared on Medscape.com.
Cervical cancer appears to be rising more rapidly in White women than in Black women in the United States, according to two independent studies. Researchers puzzling over this counterintuitive finding say that, if true, the findings may be a “canary in the coal mine,” signaling problems with U.S. health care that go way beyond women’s health.
Cervical cancer incidence in the United States has plateaued since 2010 and now stands at 7.5 per 100,000 people. Well-known disparities exist: Compared with White women, Black women are more likely to have distant-stage disease at diagnosis and more commonly die of their cancer.
However, two unconnected studies published in the past 5 months suggest that White women are catching up fast.
Cervical cancer rates in White women aged 30-34 are rising 2.8% a year, but holding steady for Black women, according to a recent study led by Ashish A. Deshmukh, PhD, of the Medical University of South Carolina, Charleston. His team analyzed the 2001-2019 National Program of Cancer Registries (NPCR) and Surveillance, Epidemiology, and End Results (SEER) dataset, which covers 98% of the U.S. population and 227,062 cervical cancer cases.
These findings were echoed by an analysis of the same database for 2001-2018 by the University of California, Los Angeles (UCLA), suggesting that distant-stage cervical cancer (defined as disease that has spread to the bladder and/or rectum at diagnosis) is climbing 1.69% a year in White people versus 0.67% in Black individuals.
The UCLA researchers, headed by Alex Francoeur, MD, a resident in the department of obstetrics and gynecology, found that disparities were most stark in adenocarcinoma, with an annual increase of 3.40% a year among White women and 1.71% in Black women.
Such findings have equity researchers scratching their heads. In cancer, it’s rare to see evidence that Black patients are doing better than their White counterparts.
One theoretical explanation is that the data are flawed, Ahmedin Jemal, DVM, PhD, senior vice president of surveillance and health equity science at the American Cancer Society, told this news organization. For example, the UCLA analysis may have been fogged by changes in staging definitions over time, Dr. Jemal said, although this would not explain the racial disparities per se.
Dr. Deshmukh stands by his data and said that, for him, the message is clear: “If rising incidence is not for localized-stage disease, but for advanced stages, that means it’s attributable to lack of screening,” he told this news organization.
However, this ‘simple’ explanation generates even more questions, Dr. Deshmukh said: “Screening is not a one-time procedure [but] a spectrum of timely cervical precancer treatment if [required]. We don’t know when exactly non-Hispanic White women are falling behind on that spectrum.”
The UCLA study supports Dr. Deshmukh’s conclusions. Using data from the Behavioral Risk Factor Surveillance System to calculate trends in “nonguideline screening,” the researchers found that White women were almost twice as likely to report that they were not following screening guidelines compared with Black women over the period of 2001-2016 (26.6% vs. 13.8%; P < .001).
“It’s not an artifact, it’s real,” said Timothy Rebbeck, PhD, the Vincent L. Gregory Jr. Professor of Cancer Prevention at Harvard TH Chan School of Public Health, Boston, who was not an author of either study and was approached for comment.
“The data are correct but there are so many things going on that might explain these patterns,” he told this news organization.
“This is a great example of complex changes in our social system, political system, health care system that are having really clear, measurable effects,” Dr. Rebbeck said. “Cervical cancer is almost a canary in the coal mine for some of this because it’s so preventable.”
(The saying “canary in a coal mine” is a warning of danger or trouble ahead. It comes from the time when coal miners would carry a caged canary down into the tunnels to warn them of noxious gases, which would kill the bird but give men time to escape.)
For example, Dr. Rebbeck said, recent turmoil in U.S. health care has left many people distrustful of the system. Although he acknowledged this was “high speculation,” he suggested that some women may have become less willing to participate in any mass health care intervention because of their political beliefs.
The UCLA study found that distant cervical cancer was rising fastest in middle-aged White women in the U.S. South, at a rate of 4.5% per year (P < .001).
Dr. Rebbeck also suggested that Medicaid expansion – the broadening of health insurance coverage in some states since the Affordable Care Act in 2014 – could be implicated. Of the 11 states that have not yet expanded Medicaid, eight are in the South.
“White populations who are in states that didn’t expand Medicaid are not getting a lot of the standard treatment and care that you would expect ...” Dr. Rebbeck said. “You could very well imagine that Medicaid nonexpanding states would have all kinds of patterns that would lead to more aggressive disease.”
In fact, there is already evidence that Medicaid expansion has affected racial disparities, disproportionately benefiting Black and Hispanic families, as for example, from an analysis of 65 studies by the Kaiser Family Foundation in 2020.
Commenting on these data, Dr. Rebbeck said, “Does that mean that the patterns of advanced cervical cancer had a smaller effect on Black women in this period because there was a greater shift in access to care? This is again a speculation, but it does fit with the ‘canary in a coal mine’ concept that advanced cervical cancer may be more rapidly influenced by health care access than other health conditions.”
The authors of the UCLA study suggested another explanation for their results: Differing enthusiasm for human papillomavirus (HPV) vaccination among White and Black families. The team also analyzed data on HPV vaccination, which offers protection against cervical cancer. The researchers found that vaccination rates were lowest, at 66.1%, among White teenagers aged 13-17 years, compared with Hispanics at 75.3%, Black teenagers at 74.6%, and Asians at 68.1%.
However, this theory was dismissed by both Dr. Jemal and Dr. Rebbeck due to timing. HPV vaccines have been around for approximately 15 years, so women who benefited (or didn’t benefit) from vaccination would be only in their late 20s today, they pointed out.
“Ninety-five percent of the cervical cancer cases we see now are in women who have not been vaccinated,” said Dr. Jemal, “So that’s out of the equation.”
Dr. Rebbeck agreed: “HPV may or may not be a thing here because it’s [got] such a latency.”
HPV vaccination may be out of the picture, but what about the epidemiology of HPV itself? Could the virus directly or indirectly be boosting advanced cervical cancer in White women?
Dr. Deshmukh thinks that it might be doing so.
He published an analysis of 2000-2018 SEER data showing that U.S. counties with the highest incidences of HPV-associated cancers also had the highest levels of smoking.
Other recent data suggest that middle-aged White women in the United States are more likely to reach for a smoke than are Black women.
Dr. Deshmukh acknowledges that the link is speculative but reasonable: “We don’t know exactly what the impact of smoking would be in terms of ... the ability to clear HPV infection. It may inhibit apoptosis, promoting tumor growth. There’s no causal association. It’s a cofactor risk.”
Dr. Rebbeck is also suspicious that smoking patterns might be a factor, pointing out that “smoking is certainly associated with both health behaviors and advanced cervical cancer.”
Both Dr. Rebbeck and Dr. Deshmukh concluded that, at this point, we can only speculate on what’s driving the puzzling acceleration of cervical cancer in White women in the United States.
However, whether it’s political aversion to screening, smoking-boosted HPV infection, Medicaid expansion or lack of it, or something else, they all agree that this canary in the coal mine clearly needs urgent medical attention.
Dr. Rebbeck and Dr. Jemal have declared no conflicts of interest. Dr. Deshmukh has declared consultant or advisory roles for Merck and Value Analytics Labs. None of the authors of the UCLA study have declared competing interests.
A version of this article first appeared on Medscape.com.
Mediterranean diet linked with fewer pregnancy complications
Women in the United States who followed a Mediterranean-style diet – heavy on fresh foods, fish, and olive oil – around the time of conception had lower risk of developing a pregnancy complication, results of a large new study suggest.
The study included 7,798 women who had not given birth before. The group was geographically, racially, and ethnically diverse.
Researchers led by Nour Makarem, PhD, MS, with the department of epidemiology, Columbia University, New York, published their results in JAMA Network Open.
“Generally, higher intakes of vegetables, fruits, legumes, fish, and whole grains and lower intakes of red and processed meat were associated with lower risk of APOs [adverse pregnancy outcomes],” the authors wrote.
21% lower risk of complications
The investigators found that women in the study – who were part of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which enrolled 10,038 women between Oct. 1, 2010, and Sept. 30, 2013, and scored high on adherence to a Mediterranean diet – had a 21% lower risk of developing any adverse pregnancy outcome (APO) than those who had low adherence. And the better the adherence, the lower the risk of adverse outcomes, especially preeclampsia or eclampsia and gestational diabetes, the researchers wrote.
The research team also studied how following the diet correlated with gestational high blood pressure, preterm birth, delivery of a small-for-gestational-age infant, and stillbirth.
Women were scored on consumption of nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated to saturated fat ratio, red and processed meats, and alcohol.
No differences by race, ethnicity, or BMI
There were no differences in adverse pregnancy outcomes by race, ethnicity, or the woman’s body mass index before pregnancy, but associations were stronger in the women who were 35 years or older, according to the paper.
The authors pointed out that the women in the study had access to prenatal care at a large academic medical center during their first 3 months of pregnancy so the study may actually underestimate the importance of the diet in the pregnancy outcomes.
Christina Han, MD, division director of maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, said that the results make sense as the researchers looked at the time of conception, which is a time that reflects the way a person chooses to live their life.
“We know that your health state as you enter pregnancy can significantly affect your outcomes for that pregnancy,” she said. “We’ve known for decades now that a Mediterranean diet is good for just about everybody.”
Unequal access to foods on diet
Dr. Han said that, while it’s great the researchers were able to confirm the benefit of the Mediterranean diet, it highlights inequity as lower income people are not as likely to be able to afford fresh fruits and vegetables and fish.
“This is a call to arms for our food distribution system to even out the big divide in what patients have access to,” Dr. Han said.
She noted that most of the women in this study were married, non-Hispanic White, and had higher levels of education which may make it hard to generalize these results to the general population.
A limitation of the study is that the women were asked to report what they ate themselves, which can be less accurate than when researchers record what is eaten in a controlled setting.
The researchers suggested a next step: “Long-term intervention studies are needed to assess whether promoting a Mediterranean-style diet around the time of conception and throughout pregnancy can prevent APOs.”
Dr. Makarem reported receiving grants from the National Institutes of Health and the American Heart Association outside the submitted work. One coauthor reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor reported receiving personal fees for serving on the board of directors for iRhythm and from fees paid through Cedars-Sinai Medical Center from Abbott Diagnostics and Sanofi outside the submitted work, and one coauthor reported serving as a clinical end point committee member for GlaxoSmithKline outside the submitted work. No other disclosures were reported. Dr. Han reported no relevant financial relationships.
Women in the United States who followed a Mediterranean-style diet – heavy on fresh foods, fish, and olive oil – around the time of conception had lower risk of developing a pregnancy complication, results of a large new study suggest.
The study included 7,798 women who had not given birth before. The group was geographically, racially, and ethnically diverse.
Researchers led by Nour Makarem, PhD, MS, with the department of epidemiology, Columbia University, New York, published their results in JAMA Network Open.
“Generally, higher intakes of vegetables, fruits, legumes, fish, and whole grains and lower intakes of red and processed meat were associated with lower risk of APOs [adverse pregnancy outcomes],” the authors wrote.
21% lower risk of complications
The investigators found that women in the study – who were part of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which enrolled 10,038 women between Oct. 1, 2010, and Sept. 30, 2013, and scored high on adherence to a Mediterranean diet – had a 21% lower risk of developing any adverse pregnancy outcome (APO) than those who had low adherence. And the better the adherence, the lower the risk of adverse outcomes, especially preeclampsia or eclampsia and gestational diabetes, the researchers wrote.
The research team also studied how following the diet correlated with gestational high blood pressure, preterm birth, delivery of a small-for-gestational-age infant, and stillbirth.
Women were scored on consumption of nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated to saturated fat ratio, red and processed meats, and alcohol.
No differences by race, ethnicity, or BMI
There were no differences in adverse pregnancy outcomes by race, ethnicity, or the woman’s body mass index before pregnancy, but associations were stronger in the women who were 35 years or older, according to the paper.
The authors pointed out that the women in the study had access to prenatal care at a large academic medical center during their first 3 months of pregnancy so the study may actually underestimate the importance of the diet in the pregnancy outcomes.
Christina Han, MD, division director of maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, said that the results make sense as the researchers looked at the time of conception, which is a time that reflects the way a person chooses to live their life.
“We know that your health state as you enter pregnancy can significantly affect your outcomes for that pregnancy,” she said. “We’ve known for decades now that a Mediterranean diet is good for just about everybody.”
Unequal access to foods on diet
Dr. Han said that, while it’s great the researchers were able to confirm the benefit of the Mediterranean diet, it highlights inequity as lower income people are not as likely to be able to afford fresh fruits and vegetables and fish.
“This is a call to arms for our food distribution system to even out the big divide in what patients have access to,” Dr. Han said.
She noted that most of the women in this study were married, non-Hispanic White, and had higher levels of education which may make it hard to generalize these results to the general population.
A limitation of the study is that the women were asked to report what they ate themselves, which can be less accurate than when researchers record what is eaten in a controlled setting.
The researchers suggested a next step: “Long-term intervention studies are needed to assess whether promoting a Mediterranean-style diet around the time of conception and throughout pregnancy can prevent APOs.”
Dr. Makarem reported receiving grants from the National Institutes of Health and the American Heart Association outside the submitted work. One coauthor reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor reported receiving personal fees for serving on the board of directors for iRhythm and from fees paid through Cedars-Sinai Medical Center from Abbott Diagnostics and Sanofi outside the submitted work, and one coauthor reported serving as a clinical end point committee member for GlaxoSmithKline outside the submitted work. No other disclosures were reported. Dr. Han reported no relevant financial relationships.
Women in the United States who followed a Mediterranean-style diet – heavy on fresh foods, fish, and olive oil – around the time of conception had lower risk of developing a pregnancy complication, results of a large new study suggest.
The study included 7,798 women who had not given birth before. The group was geographically, racially, and ethnically diverse.
Researchers led by Nour Makarem, PhD, MS, with the department of epidemiology, Columbia University, New York, published their results in JAMA Network Open.
“Generally, higher intakes of vegetables, fruits, legumes, fish, and whole grains and lower intakes of red and processed meat were associated with lower risk of APOs [adverse pregnancy outcomes],” the authors wrote.
21% lower risk of complications
The investigators found that women in the study – who were part of the Nulliparous Pregnancy Outcomes Study: Monitoring Mothers-to-Be, which enrolled 10,038 women between Oct. 1, 2010, and Sept. 30, 2013, and scored high on adherence to a Mediterranean diet – had a 21% lower risk of developing any adverse pregnancy outcome (APO) than those who had low adherence. And the better the adherence, the lower the risk of adverse outcomes, especially preeclampsia or eclampsia and gestational diabetes, the researchers wrote.
The research team also studied how following the diet correlated with gestational high blood pressure, preterm birth, delivery of a small-for-gestational-age infant, and stillbirth.
Women were scored on consumption of nine components: vegetables (excluding potatoes), fruits, nuts, whole grains, legumes, fish, monounsaturated to saturated fat ratio, red and processed meats, and alcohol.
No differences by race, ethnicity, or BMI
There were no differences in adverse pregnancy outcomes by race, ethnicity, or the woman’s body mass index before pregnancy, but associations were stronger in the women who were 35 years or older, according to the paper.
The authors pointed out that the women in the study had access to prenatal care at a large academic medical center during their first 3 months of pregnancy so the study may actually underestimate the importance of the diet in the pregnancy outcomes.
Christina Han, MD, division director of maternal-fetal medicine at University of California, Los Angeles, who was not part of the study, said that the results make sense as the researchers looked at the time of conception, which is a time that reflects the way a person chooses to live their life.
“We know that your health state as you enter pregnancy can significantly affect your outcomes for that pregnancy,” she said. “We’ve known for decades now that a Mediterranean diet is good for just about everybody.”
Unequal access to foods on diet
Dr. Han said that, while it’s great the researchers were able to confirm the benefit of the Mediterranean diet, it highlights inequity as lower income people are not as likely to be able to afford fresh fruits and vegetables and fish.
“This is a call to arms for our food distribution system to even out the big divide in what patients have access to,” Dr. Han said.
She noted that most of the women in this study were married, non-Hispanic White, and had higher levels of education which may make it hard to generalize these results to the general population.
A limitation of the study is that the women were asked to report what they ate themselves, which can be less accurate than when researchers record what is eaten in a controlled setting.
The researchers suggested a next step: “Long-term intervention studies are needed to assess whether promoting a Mediterranean-style diet around the time of conception and throughout pregnancy can prevent APOs.”
Dr. Makarem reported receiving grants from the National Institutes of Health and the American Heart Association outside the submitted work. One coauthor reported receiving grants from the Eunice Kennedy Shriver National Institute of Child Health and Human Development during the study. One coauthor reported receiving personal fees for serving on the board of directors for iRhythm and from fees paid through Cedars-Sinai Medical Center from Abbott Diagnostics and Sanofi outside the submitted work, and one coauthor reported serving as a clinical end point committee member for GlaxoSmithKline outside the submitted work. No other disclosures were reported. Dr. Han reported no relevant financial relationships.
FROM JAMA NETWORK OPEN
SARS-CoV-2 seroprevalence grew rapidly in pandemic’s early waves
By August 2022, two-and-a-half years into the COVID-19 pandemic, most children as well as most adults under age 60 years had evidence of SARS-CoV-2 vaccination and/or infection, a Canadian seroprevalence study of almost 14,000 people over the first seven waves of the pandemic reports.
However, fewer than 50% people older than age 60, the age group most vulnerable to severe outcomes, showed evidence of immunity from infection or vaccination. The authors noted that older adults – who have the lowest infection rates but highest risk of severe outcomes – continue to warrant prioritized vaccination, writing online in the Canadian Medical Association Journal.
Previous evidence suggests that a combination of both infection and vaccination exposure may induce more robust and durable hybrid immunity than either exposure alone, according to lead author Danuta M. Skowronski, MD, MHSc, an epidemiologist at the British Columbia Centre for Disease Control in Vancouver.
“Our main objective was to chronicle the changing proportion of the population considered immunologically naive and therefore susceptible to SARS-CoV-2,” Dr. Skowronski said in an interview. “It’s relevant for risk assessment to know what proportion have acquired some priming for more efficient immune memory response to the virus because that reduces the likelihood of severe outcomes.” Standardized seroprevalence studies are essential to inform COVID-19 response, particularly in resource-limited regions.
The study
Conducted in British Columbia’s Greater Vancouver and Fraser Valley, the analysis found that in the first year of the pandemic, when extraordinary measures were in place to curtail transmission, virtually everyone remained immunologically naive. Thereafter, however, age-based vaccine rollouts dramatically changed the immuno-epidemiological landscape so that by September 2021, more than 80% of the study population had antibody evidence of immunological priming, while more than 85% remained uninfected.
By August 2022, after the Omicron-variant waves, overall vaccine- and infection-induced seroprevalence exceeded 95%, with 60% having been actually infected, including at least three-quarters of children. Fewer than 50% of older adults, however, showed immunological evidence of exposure.
The study results were based on anonymized residual sera from children and adults in an outpatient laboratory network. At least three immunoassays per serosurvey were used to detect antibodies to SARS-CoV-2 spike (from vaccine) and to nucleocapsid antibodies (from infection).
The researchers assessed any seroprevalence – vaccine-, infection-induced, or both – as defined by positivity on any two assays. Infection-induced seroprevalence was also defined by dual-assay positivity but required both antinucleocapsid and antispike detection. Their estimates of infection-induced seroprevalence indicated considerable under-ascertainment of infections by standard surveillance case reports.
Results
During the first year of the pandemic, fewer than 1% manifested seroprevalence during the first three snapshots and fewer than 5% by January 2021. With vaccine rollout, however, seroprevalence increased dramatically during the first half of 2021 to 56% by May/June 2021 and to 83% by September/October 2021.
In addition, infection-induced seroprevalence was low at less than 15% in September/October 2021 until the arrival of the Omicron waves, after which it rose to 42% by March 2022 and 61% by July/August 2022. Combined seroprevalence for vaccination or infection was more than 95% by the summer, with most children but less than half of adults older than 60 years showing evidence of having been infected.
“We found the highest infection rates among children, closely followed by young adults, which may reflect their greater interconnectedness, including between siblings and parents in the household, as well as with peers in schools and the community,” the authors wrote, adding that the low cumulative infection rates among older adults may reflect their higher vaccination rates and greater social isolation.
U.S. data show similar age-related infection rates, but data among children from other Canadian provinces are limited, the authors said.
Commenting on the survey but not involved in it, infectious diseases expert Marc Germain, MD, PhD, a vice president at Héma-Québec in Quebec City, believes the pattern observed in British Columbia is fairly representative of what happened across Canada and the United States, including the sweeping effect of the Omicron variant and the differential impact according to age.
“But regional differences might very well exist – for example, due to differential vaccine uptake – and are also probably related in part to the different testing platforms being used,” Dr. Germain told this news organization.
Caroline Quach-Tanh, MD, PhD, a pediatrician and epidemiologist/infectologist at the University of Montreal, pointed out that Quebec seroprevalence surveys using residual blood samples from children and adults visiting emergency departments for any reason showed higher rates of prior infection than did the BC surveys. “But Dr. Skowronski’s findings are likely applicable to settings where some nonpharmacological interventions were put in place, but without strict confinement – and thus are likely applicable to most settings in the U.S. and Canada.”
Dr. Quach-Tanh added that there is always a risk of bias with the use of residual blood samples, “but the fact that the study method was stable should have captured a similar population from time to time. It would be unlikely to result in a major overestimation in the proportion of individuals positive for SARS-CoV-2 antibodies.”
A recent global meta-analysis found that while global seroprevalence has risen considerably, albeit variably by region, more than a third of the world’s population is still seronegative to the SARS-CoV-2 virus.
Dr. Skowronski reported institutional grants from the Canadian Institutes of Health Research and the British Columbia Centre for Disease Control Foundation for Public Health for other SARSCoV-2 work. Coauthor Romina C. Reyes, MD, chairs the BC Diagnostic Accreditation Program committee. Coauthor Mel Krajden, MD, reported institutional grants from Roche, Hologic, and Siemens. Dr. Germain and Dr. Quach-Tanh disclosed no competing interests relevant to their comments.
By August 2022, two-and-a-half years into the COVID-19 pandemic, most children as well as most adults under age 60 years had evidence of SARS-CoV-2 vaccination and/or infection, a Canadian seroprevalence study of almost 14,000 people over the first seven waves of the pandemic reports.
However, fewer than 50% people older than age 60, the age group most vulnerable to severe outcomes, showed evidence of immunity from infection or vaccination. The authors noted that older adults – who have the lowest infection rates but highest risk of severe outcomes – continue to warrant prioritized vaccination, writing online in the Canadian Medical Association Journal.
Previous evidence suggests that a combination of both infection and vaccination exposure may induce more robust and durable hybrid immunity than either exposure alone, according to lead author Danuta M. Skowronski, MD, MHSc, an epidemiologist at the British Columbia Centre for Disease Control in Vancouver.
“Our main objective was to chronicle the changing proportion of the population considered immunologically naive and therefore susceptible to SARS-CoV-2,” Dr. Skowronski said in an interview. “It’s relevant for risk assessment to know what proportion have acquired some priming for more efficient immune memory response to the virus because that reduces the likelihood of severe outcomes.” Standardized seroprevalence studies are essential to inform COVID-19 response, particularly in resource-limited regions.
The study
Conducted in British Columbia’s Greater Vancouver and Fraser Valley, the analysis found that in the first year of the pandemic, when extraordinary measures were in place to curtail transmission, virtually everyone remained immunologically naive. Thereafter, however, age-based vaccine rollouts dramatically changed the immuno-epidemiological landscape so that by September 2021, more than 80% of the study population had antibody evidence of immunological priming, while more than 85% remained uninfected.
By August 2022, after the Omicron-variant waves, overall vaccine- and infection-induced seroprevalence exceeded 95%, with 60% having been actually infected, including at least three-quarters of children. Fewer than 50% of older adults, however, showed immunological evidence of exposure.
The study results were based on anonymized residual sera from children and adults in an outpatient laboratory network. At least three immunoassays per serosurvey were used to detect antibodies to SARS-CoV-2 spike (from vaccine) and to nucleocapsid antibodies (from infection).
The researchers assessed any seroprevalence – vaccine-, infection-induced, or both – as defined by positivity on any two assays. Infection-induced seroprevalence was also defined by dual-assay positivity but required both antinucleocapsid and antispike detection. Their estimates of infection-induced seroprevalence indicated considerable under-ascertainment of infections by standard surveillance case reports.
Results
During the first year of the pandemic, fewer than 1% manifested seroprevalence during the first three snapshots and fewer than 5% by January 2021. With vaccine rollout, however, seroprevalence increased dramatically during the first half of 2021 to 56% by May/June 2021 and to 83% by September/October 2021.
In addition, infection-induced seroprevalence was low at less than 15% in September/October 2021 until the arrival of the Omicron waves, after which it rose to 42% by March 2022 and 61% by July/August 2022. Combined seroprevalence for vaccination or infection was more than 95% by the summer, with most children but less than half of adults older than 60 years showing evidence of having been infected.
“We found the highest infection rates among children, closely followed by young adults, which may reflect their greater interconnectedness, including between siblings and parents in the household, as well as with peers in schools and the community,” the authors wrote, adding that the low cumulative infection rates among older adults may reflect their higher vaccination rates and greater social isolation.
U.S. data show similar age-related infection rates, but data among children from other Canadian provinces are limited, the authors said.
Commenting on the survey but not involved in it, infectious diseases expert Marc Germain, MD, PhD, a vice president at Héma-Québec in Quebec City, believes the pattern observed in British Columbia is fairly representative of what happened across Canada and the United States, including the sweeping effect of the Omicron variant and the differential impact according to age.
“But regional differences might very well exist – for example, due to differential vaccine uptake – and are also probably related in part to the different testing platforms being used,” Dr. Germain told this news organization.
Caroline Quach-Tanh, MD, PhD, a pediatrician and epidemiologist/infectologist at the University of Montreal, pointed out that Quebec seroprevalence surveys using residual blood samples from children and adults visiting emergency departments for any reason showed higher rates of prior infection than did the BC surveys. “But Dr. Skowronski’s findings are likely applicable to settings where some nonpharmacological interventions were put in place, but without strict confinement – and thus are likely applicable to most settings in the U.S. and Canada.”
Dr. Quach-Tanh added that there is always a risk of bias with the use of residual blood samples, “but the fact that the study method was stable should have captured a similar population from time to time. It would be unlikely to result in a major overestimation in the proportion of individuals positive for SARS-CoV-2 antibodies.”
A recent global meta-analysis found that while global seroprevalence has risen considerably, albeit variably by region, more than a third of the world’s population is still seronegative to the SARS-CoV-2 virus.
Dr. Skowronski reported institutional grants from the Canadian Institutes of Health Research and the British Columbia Centre for Disease Control Foundation for Public Health for other SARSCoV-2 work. Coauthor Romina C. Reyes, MD, chairs the BC Diagnostic Accreditation Program committee. Coauthor Mel Krajden, MD, reported institutional grants from Roche, Hologic, and Siemens. Dr. Germain and Dr. Quach-Tanh disclosed no competing interests relevant to their comments.
By August 2022, two-and-a-half years into the COVID-19 pandemic, most children as well as most adults under age 60 years had evidence of SARS-CoV-2 vaccination and/or infection, a Canadian seroprevalence study of almost 14,000 people over the first seven waves of the pandemic reports.
However, fewer than 50% people older than age 60, the age group most vulnerable to severe outcomes, showed evidence of immunity from infection or vaccination. The authors noted that older adults – who have the lowest infection rates but highest risk of severe outcomes – continue to warrant prioritized vaccination, writing online in the Canadian Medical Association Journal.
Previous evidence suggests that a combination of both infection and vaccination exposure may induce more robust and durable hybrid immunity than either exposure alone, according to lead author Danuta M. Skowronski, MD, MHSc, an epidemiologist at the British Columbia Centre for Disease Control in Vancouver.
“Our main objective was to chronicle the changing proportion of the population considered immunologically naive and therefore susceptible to SARS-CoV-2,” Dr. Skowronski said in an interview. “It’s relevant for risk assessment to know what proportion have acquired some priming for more efficient immune memory response to the virus because that reduces the likelihood of severe outcomes.” Standardized seroprevalence studies are essential to inform COVID-19 response, particularly in resource-limited regions.
The study
Conducted in British Columbia’s Greater Vancouver and Fraser Valley, the analysis found that in the first year of the pandemic, when extraordinary measures were in place to curtail transmission, virtually everyone remained immunologically naive. Thereafter, however, age-based vaccine rollouts dramatically changed the immuno-epidemiological landscape so that by September 2021, more than 80% of the study population had antibody evidence of immunological priming, while more than 85% remained uninfected.
By August 2022, after the Omicron-variant waves, overall vaccine- and infection-induced seroprevalence exceeded 95%, with 60% having been actually infected, including at least three-quarters of children. Fewer than 50% of older adults, however, showed immunological evidence of exposure.
The study results were based on anonymized residual sera from children and adults in an outpatient laboratory network. At least three immunoassays per serosurvey were used to detect antibodies to SARS-CoV-2 spike (from vaccine) and to nucleocapsid antibodies (from infection).
The researchers assessed any seroprevalence – vaccine-, infection-induced, or both – as defined by positivity on any two assays. Infection-induced seroprevalence was also defined by dual-assay positivity but required both antinucleocapsid and antispike detection. Their estimates of infection-induced seroprevalence indicated considerable under-ascertainment of infections by standard surveillance case reports.
Results
During the first year of the pandemic, fewer than 1% manifested seroprevalence during the first three snapshots and fewer than 5% by January 2021. With vaccine rollout, however, seroprevalence increased dramatically during the first half of 2021 to 56% by May/June 2021 and to 83% by September/October 2021.
In addition, infection-induced seroprevalence was low at less than 15% in September/October 2021 until the arrival of the Omicron waves, after which it rose to 42% by March 2022 and 61% by July/August 2022. Combined seroprevalence for vaccination or infection was more than 95% by the summer, with most children but less than half of adults older than 60 years showing evidence of having been infected.
“We found the highest infection rates among children, closely followed by young adults, which may reflect their greater interconnectedness, including between siblings and parents in the household, as well as with peers in schools and the community,” the authors wrote, adding that the low cumulative infection rates among older adults may reflect their higher vaccination rates and greater social isolation.
U.S. data show similar age-related infection rates, but data among children from other Canadian provinces are limited, the authors said.
Commenting on the survey but not involved in it, infectious diseases expert Marc Germain, MD, PhD, a vice president at Héma-Québec in Quebec City, believes the pattern observed in British Columbia is fairly representative of what happened across Canada and the United States, including the sweeping effect of the Omicron variant and the differential impact according to age.
“But regional differences might very well exist – for example, due to differential vaccine uptake – and are also probably related in part to the different testing platforms being used,” Dr. Germain told this news organization.
Caroline Quach-Tanh, MD, PhD, a pediatrician and epidemiologist/infectologist at the University of Montreal, pointed out that Quebec seroprevalence surveys using residual blood samples from children and adults visiting emergency departments for any reason showed higher rates of prior infection than did the BC surveys. “But Dr. Skowronski’s findings are likely applicable to settings where some nonpharmacological interventions were put in place, but without strict confinement – and thus are likely applicable to most settings in the U.S. and Canada.”
Dr. Quach-Tanh added that there is always a risk of bias with the use of residual blood samples, “but the fact that the study method was stable should have captured a similar population from time to time. It would be unlikely to result in a major overestimation in the proportion of individuals positive for SARS-CoV-2 antibodies.”
A recent global meta-analysis found that while global seroprevalence has risen considerably, albeit variably by region, more than a third of the world’s population is still seronegative to the SARS-CoV-2 virus.
Dr. Skowronski reported institutional grants from the Canadian Institutes of Health Research and the British Columbia Centre for Disease Control Foundation for Public Health for other SARSCoV-2 work. Coauthor Romina C. Reyes, MD, chairs the BC Diagnostic Accreditation Program committee. Coauthor Mel Krajden, MD, reported institutional grants from Roche, Hologic, and Siemens. Dr. Germain and Dr. Quach-Tanh disclosed no competing interests relevant to their comments.
FROM THE CANADIAN MEDICAL ASSOCIATION JOURNAL
Diabetes surge expected in young people
according to a new study published in Diabetes Care.
It is expected that as many as 526,000 people younger than 20 years in the United States will have diabetes by 2060, researchers from the Centers for Disease Control and Prevention report. Their projections found that the number of young people with diabetes will increase 12%, from 213,000 in 2017 to 239,000 in 2060.
The estimates include a 673% rise in the number of youth with type 2 diabetes and a 65% increase in cases of type 1 diabetes over the next 4 decades.
Most of the new cases are projected to occur among non-Hispanic Blacks, exacerbating the already significant racial disparities in type 2 diabetes in particular, the study found.
“This study’s startling projections of type 2 diabetes increases show why it is crucial to advance health equity and reduce the widespread disparities that already take a toll on people’s health,” Christopher Holliday, PhD, MPH, FACHE, director of CDC’s Division of Diabetes Translation, said in a press release about the new estimates.
Even if trends remain the same in coming decades, researchers said diagnoses of type 2 diabetes will rise almost 70% and that diagnoses of type 1 diabetes will increase by 3%.
The researchers attribute the increase in diabetes cases among youth to a variety of factors, including the growing prevalence of childhood obesity and the presence of diabetes in women of childbearing age, which is linked to obesity in their offspring.
Debra Houry, MD, MPH, acting principal director of the CDC, said the focus should be on prevention.
“This new research should serve as a wake-up call for all of us. It’s vital that we focus our efforts to ensure all Americans, especially our young people, are the healthiest they can be,” she said in a press release.
The findings come from the SEARCH for Diabetes in Youth study, funded by the CDC and the National Institutes of Health. Dr. Houry and Dr. Holliday report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study published in Diabetes Care.
It is expected that as many as 526,000 people younger than 20 years in the United States will have diabetes by 2060, researchers from the Centers for Disease Control and Prevention report. Their projections found that the number of young people with diabetes will increase 12%, from 213,000 in 2017 to 239,000 in 2060.
The estimates include a 673% rise in the number of youth with type 2 diabetes and a 65% increase in cases of type 1 diabetes over the next 4 decades.
Most of the new cases are projected to occur among non-Hispanic Blacks, exacerbating the already significant racial disparities in type 2 diabetes in particular, the study found.
“This study’s startling projections of type 2 diabetes increases show why it is crucial to advance health equity and reduce the widespread disparities that already take a toll on people’s health,” Christopher Holliday, PhD, MPH, FACHE, director of CDC’s Division of Diabetes Translation, said in a press release about the new estimates.
Even if trends remain the same in coming decades, researchers said diagnoses of type 2 diabetes will rise almost 70% and that diagnoses of type 1 diabetes will increase by 3%.
The researchers attribute the increase in diabetes cases among youth to a variety of factors, including the growing prevalence of childhood obesity and the presence of diabetes in women of childbearing age, which is linked to obesity in their offspring.
Debra Houry, MD, MPH, acting principal director of the CDC, said the focus should be on prevention.
“This new research should serve as a wake-up call for all of us. It’s vital that we focus our efforts to ensure all Americans, especially our young people, are the healthiest they can be,” she said in a press release.
The findings come from the SEARCH for Diabetes in Youth study, funded by the CDC and the National Institutes of Health. Dr. Houry and Dr. Holliday report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new study published in Diabetes Care.
It is expected that as many as 526,000 people younger than 20 years in the United States will have diabetes by 2060, researchers from the Centers for Disease Control and Prevention report. Their projections found that the number of young people with diabetes will increase 12%, from 213,000 in 2017 to 239,000 in 2060.
The estimates include a 673% rise in the number of youth with type 2 diabetes and a 65% increase in cases of type 1 diabetes over the next 4 decades.
Most of the new cases are projected to occur among non-Hispanic Blacks, exacerbating the already significant racial disparities in type 2 diabetes in particular, the study found.
“This study’s startling projections of type 2 diabetes increases show why it is crucial to advance health equity and reduce the widespread disparities that already take a toll on people’s health,” Christopher Holliday, PhD, MPH, FACHE, director of CDC’s Division of Diabetes Translation, said in a press release about the new estimates.
Even if trends remain the same in coming decades, researchers said diagnoses of type 2 diabetes will rise almost 70% and that diagnoses of type 1 diabetes will increase by 3%.
The researchers attribute the increase in diabetes cases among youth to a variety of factors, including the growing prevalence of childhood obesity and the presence of diabetes in women of childbearing age, which is linked to obesity in their offspring.
Debra Houry, MD, MPH, acting principal director of the CDC, said the focus should be on prevention.
“This new research should serve as a wake-up call for all of us. It’s vital that we focus our efforts to ensure all Americans, especially our young people, are the healthiest they can be,” she said in a press release.
The findings come from the SEARCH for Diabetes in Youth study, funded by the CDC and the National Institutes of Health. Dr. Houry and Dr. Holliday report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM DIABETES CARE