User login
The Role of Dietary Antioxidants in Melanoma and Nonmelanoma Skin Cancer
Nonmelanoma skin cancer (NMSC) is the most common cancer in the United States, and cutaneous melanoma is projected to be the fifth most common form of cancer in 2022, with increasing incidence and high potential for mortality.1-3 Estimates indicate that 35% to 45% of all cancers in White patients are cutaneous, with 4% to 5% occurring in Hispanic patients, 2% to 4% in Asian patients, and 1% to 2% in Black patients.4 Of the keratinocyte carcinomas, basal cell carcinoma (BCC) is the most prevalent, projected to affect approximately 33% to 39% of White males and 23% to 28% of White females in the United States during their lifetimes. Squamous cell carcinoma (SCC) is the second most common skin malignancy, with a lifetime risk of 9% to 14% for White males and 4% to 9% for White females in the United States.5 The incidence of melanoma continues to increase, with approximately 99,780 new cases expected in the United States in 2022.1
UV-induced DNA damage plays a key role in the pathogenesis and development of various skin malignancies.6 UV radiation from sunlight or tanning devices causes photocarcinogenesis due to molecular and cellular effects, including the generation of reactive oxygen species, DNA damage due to the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone, melanogenesis, apoptosis, and the increased expression of harmful genes and proteins.6 The summation of this damage can result in skin malignancies, including NMSC and melanoma.6,7 Dietary antioxidants theoretically help prevent oxidative reactions from occurring within the body, and it has been suggested that intake of dietary antioxidants may decrease DNA damage and prevent tumorigenesis secondary to UV radiation.8 Antioxidants exist naturally in the body but can be acquired exogenously. Investigators have studied dietary antioxidants in preventing skin cancer formation with promising results in the laboratory setting.8-11 Recently, more robust human studies have been initiated to further delineate this relationship. We present clinical evidence of several frequently utilized antioxidant vitamins and their effects on melanoma and NMSC.
Antioxidants
Vitamin A—Vitamin A is a fat-soluble vitamin found in animal sources, including fish, liver, and eggs. Carotenoids, such as beta carotene, are provitamin A plant derivatives found in fruits and vegetables that are converted into biologically active retinol and retinoic acid.12 Retinols play a key role in cellular growth and differentiation and are thought to be protective against skin cancer via the inactivation of free radicals and immunologic enhancement due to their antiproliferative, antioxidative, and antiapoptotic effects.13-16 Animal studies have demonstrated this protective effect and the ability of retinoids to suppress carcinogenesis; however, human studies reveal conflicting results.17,18
Greenberg et al19 investigated the use of beta carotene in preventing the formation of NMSC. Patients (N=1805) were randomized to receive 50 mg of beta carotene daily or placebo. Over a 5-year period, there was no significant reduction in the occurrence of NMSC (relative risk [RR], 1.05; 95% CI, 0.91-1.22).19 Frieling et al20 conducted a similar randomized, double-blind, placebo-controlled trial investigating beta carotene for primary prevention of NMSC in 22,071 healthy male physicians. The study group received 50 mg of beta carotene every other day for 12 years’ duration, and there was no significant effect on the incidence of first NMSC development (RR, 0.98; 95% CI, 0.92-1.05).20
A case-control study by Naldi et al21 found an inverse association between vitamin A intake and development of melanoma. Study participants were stratified into quartiles based on level of dietary intake and found an odds ratio (OR) of 0.71 for beta carotene (95% CI, 0.50-1.02), 0.57 for retinol (95% CI, 0.39-0.83), and 0.51 for total vitamin A (95% CI, 0.35-0.75) when comparing the upper quartile of vitamin A intake to the lower quartile. Upper-quartile cutoff values of vitamin A intake were 214 µg/d for beta carotene, 149 µg/d for retinol, and 359 µg/d for total vitamin A.21 More recently, a meta-analysis by Zhang et al22 pooled data from 8 case-control studies and 2 prospective studies. Intake of retinol but not total vitamin A or beta carotene was associated with a reduced risk for development of melanoma (retinol: OR, 0.80; 95% CI, 0.69-0.92; total vitamin A: OR, 0.86; 95% CI, 0.59-1.25; beta carotene: OR, 0.87; 95% CI, 0.62-1.20).22 Feskanich et al23 demonstrated similar findings with use of food-frequency questionnaires in White women, suggesting that retinol intake from food combined with supplements may be protective for women who were otherwise at a low risk for melanoma based on nondietary factors. These factors included painful or blistering sunburns during childhood, history of more than 6 sunburns, more than 3 moles on the left arm, having red or blonde hair, and having a parent or sibling with melanoma (P=.01). However, this relationship did not hold true when looking at women at an intermediate or high risk for melanoma (P=.16 and P=.46).23
When looking at high-risk patients, such as transplant patients, oral retinoids have been beneficial in preventing NMSC.24-27 Bavinck et al24 investigated 44 renal transplant patients with a history of more than 10 NMSCs treated with 30 mg of acitretin daily vs placebo. Patients receiving oral retinoid supplementation developed fewer NMSCs over a 6-month treatment period (P=.01).24 Similarly, George et al25 investigated acitretin in renal transplant patients and found a statistically significant decrease in number of SCCs in patients on supplementation (P=.002). Solomon-Cohen et al26 performed a retrospective case-crossover study in solid organ transplant recipients and found that those treated with 10 mg of acitretin daily for 2 years had a significant reduction in the number of new keratinocyte carcinomas (P=.002). Other investigators have demonstrated similar results, and in 2006, Otley et al27 proposed standardized dosing of acitretin for chemoprevention in high-risk patients, including patients developing 5 to 10 NMSCs per year, solid organ transplant recipients, and those with syndromes associated with the development of NMSC.28,29 Overall, in the general population, vitamin A and related compounds have not demonstrated a significant association with decreased development of NMSC; however, oral retinoids have proven useful for high-risk patients. Furthermore, several studies have suggested a negative association between vitamin A levels and the incidence of melanoma, specifically in the retinol formulation.
Vitamin B3—Nicotinamide (also known as niacinamide) is a water-soluble form of vitamin B3 and is obtained from animal-based and plant-based foods, such as meat, fish, and legumes.30 Nicotinamide plays a key role in cellular metabolism, cellular signaling, and DNA repair, including protection from UV damage within keratinocytes.31,32 Early mouse models demonstrated decreased formation of skin tumors in mice treated with topical or oral nicotinamide.32,33 A number of human studies have revealed similar results.34-36
Chen et al34 conducted the ONTRAC study, a phase 3, double-blind, randomized controlled trial (RCT) looking at 386 participants with a history of at least 2 NMSCs in the preceding 5 years. At 12 months, those treated with 500 mg of nicotinamide twice daily demonstrated a statistically significant decreased rate of SCC formation (P=.05). A decreased incidence of BCC development was noted; however, this trend did not reach statistical significance (P=.12). Precancerous skin lesions also were found to be decreased in the treatment group, with 20% lower incidence of actinic keratoses (AKs) after 9 months of treatment (P<.001).34 Drago et al35 specifically studied the incidence of AKs in 38 transplant recipients—8 liver and 30 kidney—and found that previously noted AKs had decreased in size for 18 of 19 patients taking 500 mg of nicotinamide daily when originally photographed AKs were remeasured at 6-month follow-up, with 7 of these 18 patients demonstrating complete clinical regression. Of those on nicotinamide supplementation, no new AKs developed compared to the control group, which demonstrated increased size of AKs or development of new AKs in 91% of patients, with 7 AKs progressing into SCC.35
Nicotinamide has been demonstrated to be useful in preventing skin cancer in high-risk populations, such as transplant patients or those with a high incidence of NMSC.34,36 Despite promising results within the laboratory setting, nicotinamide’s effects on melanoma in humans remains less clear.31,37 Studies suggest that nicotinamide enhances tumor-infiltrating lymphocytes and DNA repair mechanisms in melanocytes, which may translate into nicotinamide, providing chemoprevention for melanoma, but research in human patients is limited.31,37
Vitamin B9—Folate, the natural form of vitamin B9, is a water-soluble compound that is found in many foods, especially green leafy vegetables, and often is supplemented because of its health benefits.38,39 In the skin, folic acid plays a key role in cellular replication and proliferation.38 Controversy exists regarding folate’s effects on cellular growth and turnover with respect to cancer incidence.38,40 Donnenfeld et al41 conducted a prospective study assessing dietary folic acid intake and development of NMSC. A total of 5880 participants completed dietary records throughout the first 2 years of the study. After an average follow-up period of 12.6 years, there was an overall increased incidence of skin cancer in those with increased dietary folate (P=.03). Furthermore, when striating by skin cancer type, there was an increased incidence of NMSC overall as well as BCC when analyzing by type of NMSC (P=.03 for NMSC; P=.05 for BCC). However, when stratifying by gender, these findings only held true for women.41 Similar effects were observed by Fung et al,42 who prospectively studied the intake of various vitamins in relationship to the development of BCC in women. During 12 years of follow-up, a positive association was observed between folate intake and BCC development (OR, 1.2; 95% CI, 1.10-1.31).42 Fung et al43 also investigated the role of several vitamins in the development of SCC and found that folate showed a negative association, which did not reach statistical significance (RR, 0.79; 95% CI, 0.56-1.11). Furthermore, Vollset et al40 conducted a meta-analysis comparing folic acid to placebo in the incidence of various types of cancer. The study excluded NMSC but reported no significant association between the development of melanoma and folic acid supplementation.40 In summary, the effects of folate have diverse consequences, potentially promoting the formation of NMSC, but studies suggest that an individual’s gender and other genetic and environmental factors also may play a role.
Vitamin C—Vitamin C (also known as ascorbic acid) is a water-soluble vitamin with antioxidant immune-mediating effects. It is found in various fruits and vegetables and serves as a cofactor for enzymes within the body playing a key role in immune function and collagen formation.44,45 It has been postulated that ascorbic acid can provide protection from UV radiation damage via its intracellular activity but conversely can contribute to oxidative damage.44 Multiple in vitro laboratory studies and animal models have demonstrated photoprotective effects of ascorbic acid.46-48 Despite these findings, minimal photoprotective effects have been found in the human population.
Kune et al49 performed a case-control study of 88 males with previously diagnosed NMSC undergoing surgical removal and investigated patients’ prior dietary habits. Patients with NMSC had a statistically significantly lower level of vitamin C–containing food in their diet than those without NMSC (P=.004).49 In addition, Vural et al50 analyzed plasma samples and blood cells of patients with AK and BCC and found a significant decrease in ascorbic acid levels in both the AK (P<.001) and BCC (P<.001) groups compared with controls. However, studies have found that consumption of certain dietary compounds can rapidly increase plasma concentration levels, which may serve as a major confounding variable in this study. Plasma concentrations of ascorbic acid and beta carotene were found to be significantly increased following consumption of a high-antioxidant diet for as short a duration as 2 weeks (P<.05).51 More recently, Heinen et al52 performed a prospective study on 1001 adults. In patients without a history of skin cancer, they found that vitamin C from food sources plus dietary supplements was positively associated with the development of BCC (P=.03).52 Similarly, Fung et al42 performed a study in women and found a positive association between vitamin C intake and the development of BCC (OR, 1.13; 95% CI, 1.03-1.23).
The relationship between vitamin C intake—either in dietary or supplemental form—and melanoma remains controversial. Mice-based studies found that high concentrations of orally administered vitamin C induce cytotoxicity in melanoma cell lines, but at low concentrations they promote tumor growth of malignant melanoma.53 Feskanich et al23 examined the relationship between vitamin C intake and melanoma development via food frequency questionnaires in White women and found that vitamin C was associated with a higher risk for melanoma (P=.05), and furthermore, a positive dose response with frequency of orange juice intake was observed (P=.008). Overall, despite promising laboratory studies, there is a lack of RCTs investigating the use of vitamin C supplementation for prevention of NMSC and melanoma in humans, and the oral benefits of vitamin C for chemoprevention remain unclear.
Vitamin D—Vitamin D is a fat-soluble vitamin that is found in fish, liver, egg, and cheese, and is endogenously produced when UV radiation from sun exposure interacts with the skin, triggering the synthesis of vitamin D.54 Vitamin D is biologically inactive and must be converted to its active form 1,25-dihydroxyvitamin D after entering the body. Vitamin D modulates many genes involved in cellular proliferation and differentiation.54 Vitamin D receptors are expressed on keratinocytes and melanocytes.55 Animal studies have demonstrated a potentially protective effect of vitamin D in the development of NMSC.56 In a mouse model, Ellison et al56 found that mice without vitamin D receptors developed skin tumors more rapidly than those with vitamin D receptors.
Unfortunately, these findings have not been demonstrated in humans, and studies have even reported an increased risk for development of NMSC in patients with normal or increased vitamin D levels compared with those with low levels of vitamin D.57-60 Eide et al57 studied 3223 patients seeking advice for low bone density by recording their vitamin D levels at the time of presentation and monitoring development of NMSC. Vitamin D levels greater than 15 ng/mL were positively associated with the development of NMSC (OR, 1.7; 95% CI, 1.04-2.7). This association held true for both SCC and BCC, with a higher risk estimated for SCC (OR, 3.2; 95% CI, 0.4-24.0 for SCC; OR, 1.7; 95% CI, 0.5-5.8 for BCC).57 An increased vitamin D serum level also was found to be significantly associated with a higher risk for BCC and melanoma by van der Pols et al.58 This prospective study looked at the incidence of skin cancer over 11 years. Study participants with vitamin D levels over 75 nmol/L more frequently developed BCC (P=.01) and melanoma (P=.05). In contrast, SCC was less frequently observed in participants with these high levels of vitamin D (P=.07).58 Furthermore, Park et al60 looked at vitamin D and skin cancer risk for men and women in the United States and found no association with risk for SCC or melanoma but a positive association with BCC (P=.05 for total vitamin D; P<.01 for dietary vitamin D). Additional studies have been performed with inconsistent results, and multiple authors suggest the possible confounding relationship between vitamin D levels and UV radiation exposure.59-62 Furthermore, some studies have even demonstrated a negative association between vitamin D and NMSC. Tang et al63 performed a retrospective case-control study in elderly males, investigating serum levels of vitamin D and patients’ self-reported history of NMSC, which demonstrated that higher levels of vitamin D were associated with a decreased risk for NMSC. Overall, the relationship between vitamin D and skin cancer development remains unclear for both melanoma and NMSC.
Vitamin E—Vitamin E is a fat-soluble vitamin that is found in plant-based oils, nuts, seeds, fruits, and vegetables.64 It works as an antioxidant to protect against free radicals and heighten immune function, and it also serves as a pro-oxidant.65,66 Vitamin E naturally exists in 8 chemical forms, of which gamma-tocopherol is the most frequently obtained form in the diet, and alpha-tocopherol is the most abundant form found in the body.64,65
Early animal studies demonstrated the inhibition of UV-induced damage in mice receiving vitamin E supplementation.67,68 Human studies have not consistently shown these effects. Vural et al50 investigated plasma samples and blood cells of patients with AKs and BCCs and reported a significant decrease in alpha-tocopherol levels in both the AK (P<.05) and BCC (P<.001) groups compared with controls. However, studies also have demonstrated a positive association between vitamin E intake and the development of BCC, including one by Fung et al,42 which found a significant association in women (OR, 1.15; 95% CI, 1.06-1.26).
Vitamin E has been found to inhibit melanin synthesis in the laboratory, suggesting a potentially protective effect in melanoma.69,70 However, in the study performed by Feskanich et al23 examining vitamin intake and melanoma incidence via food-frequency questionnaires, vitamin E was not associated with a lower risk for melanoma. Despite promising laboratory studies, the data surrounding the use of a vitamin E supplement for prevention of melanoma and NMSC in humans remains unclear.
Selenium—Selenium is a trace mineral found in plants, meat, and fish. It plays a key role in reproduction, hormone metabolism, DNA synthesis, and protection from oxidative damage.71 In mice studies, lack of selenium-containing proteins resulted in skin abnormalities, including the development of a hyperplastic epidermis and aberrant hair follicle morphogenesis with alopecia after birth, and numerous experimental studies have demonstrated a negative association between selenium intake and cancer.72,73 However, human studies have yielded alternative results.
The Nutritional Prevention of Cancer Study Group analyzed 1312 dermatology patients with a history of NMSC.74 The study population was obtained from 7 dermatology clinics with randomization to control for confounding variables. Study participants received either 200 μg of selenium daily or placebo.74 Baseline characteristics of each study group were overall balanced. Selenium intake was found to have no effect on the development of BCC (hazard ratio [HR], 1.09; 95% CI, 0.94-1.26) but an increased risk for developing SCC (HR, 1.25; 95% CI, 1.03-1.51) and total NMSC (HR, 1.17; 95% CI, 1.02-1.34).74,75 Similarly, Reid et al76 performed an RCT comparing patients treated with 400 μg/d of selenium to those treated with 200 μg/d of selenium. When compared with placebo, those treated with 200 μg/d of selenium had a statistically significantly increased incidence of NMSC (P=.006); however, those treated with 400 μg/d of selenium had no significant change in total incidence of NMSC (P=.51).76 Furthermore, Vinceti et al77 performed a review of 83 studies from the literature investigating the effect of dietary selenium, and from the RCTs, there was no beneficial effect of selenium in reducing cancer risk in general; however, some studies demonstrated an increased incidence of other types of cancer, including melanoma. Of the RCTs included in the study investigating NMSC incidence specifically, it was found that the incidence was not affected by selenium administration (RR, 1.16; 95% CI, 0.30-4.42; 2 studies, 2027 participants).77 Despite data from several studies demonstrating an increased risk for NMSC, the effects of selenium on the risk for NMSC and melanoma remain unclear.
Combination Antioxidant Studies
In addition to investigating the use of single antioxidants in skin cancer prevention, studies utilizing the combination of various antioxidants or other dietary minerals have been conducted. Hercberg et al78 performed a randomized, double-blinded, placebo-controlled trial of 13,017 adults (7876 women and 5141 men) receiving a combination of 120 mg vitamin C, 30 mg vitamin E, 100 μg selenium, 6 mg beta carotene, and 20 mg zinc. Study participants were followed for an average of 7.5 years, and the development of skin cancers were recorded. Overall, the incidence rate of skin cancer did not differ between the 2 treatment groups; however, when segregated by gender, the study found that there was an increased risk for developing skin cancer in women taking the antioxidant supplement combination compared with placebo (P=.03). This difference was not observed in the 2 treatment groups of male patients (P=.11). When looking specifically at NMSC, there was no difference between treatment groups for male or female patients (P=.39 for males; P=.15 for females). In contrast, there was a higher incidence of melanoma identified in female patients taking the combination antioxidant supplement (P=.01), but this was not seen within the male study population (P=.51).78 In addition, Chang et al79 performed a meta-analysis of 10 previously published RCTs. Analysis revealed that treatment with a variety of supplements, including vitamins A, C, E, and beta carotene, were found to have no preventative effects on the incidence of skin cancer development (RR, 0.98; CI, 0.98-1.03). Notable limitations to this study included the variability in protocols of the studies included in this meta-analysis, the limited number of RCTs investigating vitamin supplementation and the risk for skin cancer development, and the influence of dietary intake on study outcomes.79
Other Dietary Agents
Furocoumarins—Furocoumarins are botanical substances found in various fruits and plants, including many citrus products. Furocoumarins are activated by UV light radiation and can lead to development of a phototoxic eruption. Several studies have suggested a pharmacogenetic effect of furocoumarins.80 Sun et al80 collected dietary data from 47,453 men and 75,291 women on furocoumarin intake and correlation with the development of NMSC. Overall, the study suggested that the intake of furocoumarins may lead to an increase in the development of BCC (HR, 1.16; 95% CI, 1.11-1.21; P=.002); however, there was no significant association identified between total intake of furocoumarins in the risk for SCC or melanoma.80 Furthermore, Sakaki et al81 conducted a survey study looking at the consumption of citrus products and the development of NMSC. The group found that there was an increased risk for NMSC in those consuming an increased amount of citrus products (P=.007).81
Conclusion
Dietary antioxidants have been investigated for their potential role in the prevention of tumorigenesis. Specific antioxidant vitamins, such as vitamin A derivatives and niacinamide, have demonstrated clinical utility in the prevention of NMSC in high-risk populations. Retinol also has been associated with a reduced incidence of melanoma. Numerous antioxidants have demonstrated promising data within the laboratory setting; however, inconsistent results have been appreciated in humans. Furthermore, several research studies suggest that folate, vitamin D, and furocoumarins may be associated with an increased risk for skin cancer development; however, these studies are inconclusive, and dietary studies are challenging to conduct. Overall, RCTs investigating the role of antioxidants for chemoprevention are limited. Moreover, the study of dietary antioxidants and vitamins may be affected by various confounding variables that can be difficult to account for because of patients’ potentially poor recall of dietary intake and the effect of dietary intake in supplemental studies. Given the increasing prevalence of skin cancer worldwide, further research into the clinical utility of antioxidants in skin cancer prevention is warranted.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
- Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768.
- Leiter U, Keim U, Garbe C. Epidemiology of skin cancer: update 2019. In: Reichrath J, ed. Sunlight, Vitamin D and Skin Cancer. Springer International Publishing; 2020:123-139.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
- Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2017;76(3S1):S100-S109.
- Pleasance ED, Cheetham RK, Stephens PJ, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191-196.
- Baek J, Lee MG. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016;21:164-169.
- Katta R, Brown DN. Diet and skin cancer: the potential role of dietary antioxidants in nonmelanoma skin cancer prevention. J Skin Cancer. 2015;2015:893149.
- Stoj V, Shahriari N, Shao K, et al. Nutrition and nonmelanoma skin cancers. Clin Dermatol. 2022;40:173-185.
- O’Connor EA, Evans CV, Ivlev I, et al. Vitamin and mineral supplements for the primary prevention of cardiovascular disease and cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;327:2334-2347.
- National Institutes of Health Office of Dietary Supplements. Vitamin A and carotenoids. fact sheet for health professionals. Updated June 15, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/
- Keller KL, Fenske NA. Uses of vitamins A, C, and E and related compounds in dermatology: a review. J Am Acad Dermatol. 1998;39:611-625.
- Wright TI, Spencer JM, Flowers FP. Chemoprevention of nonmelanoma skin cancer. J Am Acad Dermatol. 2006;54:933-946; quiz 947-950.
- Bushue N, Wan YJY. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62:1285-1298.
- Stahl W, Sies H. β-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr. 2012;96:1179S-1184S.
- Bukhari MH, Qureshi SS, Niazi S, et al. Chemotherapeutic/chemopreventive role of retinoids in chemically induced skin carcinogenesis in albino mice. Int J Dermatol. 2007;46:1160-1165.
- Lambert LA, Wamer WG, Wei RR, et al. The protective but nonsynergistic effect of dietary beta-carotene and vitamin E on skin tumorigenesis in Skh mice. Nutr Cancer. 1994;21:1-12.
- Greenberg ER, Baron JA, Stukel TA, et al. A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. The Skin Cancer Prevention Study Group. N Engl J Med. 1990;323:789-795.
- Frieling UM, Schaumberg DA, Kupper TS, et al. A randomized, 12-year primary-prevention trial of beta carotene supplementation for nonmelanoma skin cancer in the physician’s health study. Arch Dermatol. 2000;136:179-184.
- Naldi L, Gallus S, Tavani A, et al; Oncology Study Group of the Italian Group for Epidemiologic Research in Dermatology. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13:503-508.
- Zhang YP, Chu RX, Liu H. Vitamin A intake and risk of melanoma: a meta-analysis. PloS One. 2014;9:e102527.
- Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381-1387.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273.
- Solomon-Cohen E, Reiss-Huss S, Hodak E, et al. Low-dose acitretin for secondary prevention of keratinocyte carcinomas in solid-organ transplant recipients. Dermatology. 2022;238:161-166.
- Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568.
- Kadakia KC, Barton DL, Loprinzi CL, et al. Randomized controlled trial of acitretin versus placebo in patients at high-risk for basal cell or squamous cell carcinoma of the skin (North Central Cancer Treatment Group Study 969251). Cancer. 2012;118:2128-2137.
- McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660.
- National Institutes of Health Office of Dietary Supplements. Niacin: fact sheet for health professionals. Updated August 23, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/
- Malesu R, Martin AJ, Lyons JG, et al. Nicotinamide for skin cancer chemoprevention: effects of nicotinamide on melanoma in vitro and in vivo. Photochem Photobiol Sci. 2020;19:171-179.
- Gensler HL. Prevention of photoimmunosuppression and photocarcinogenesis by topical nicotinamide. Nutr Cancer. 1997;29:157-162.
- Gensler HL, Williams T, Huang AC, et al. Oral niacin prevents photocarcinogenesis and photoimmunosuppression in mice. Nutr Cancer. 1999;34:36-41.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Drago F, Ciccarese G, Cogorno L, et al. Prevention of non-melanoma skin cancers with nicotinamide in transplant recipients: a case-control study. Eur J Dermatol. 2017;27:382-385.
- Yélamos O, Halpern AC, Weinstock MA. Reply to “A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients.” Br J Dermatol. 2017;176:551-552.
- Scatozza F, Moschella F, D’Arcangelo D, et al. Nicotinamide inhibits melanoma in vitro and in vivo. J Exp Clin Cancer Res. 2020;39:211.
- National Institutes of Health Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 1, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/
- Butzbach K, Epe B. Photogenotoxicity of folic acid. Free Radic Biol Med. 2013;65:821-827.
- Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036.
- Donnenfeld M, Deschasaux M, Latino-Martel P, et al. Prospective association between dietary folate intake and skin cancer risk: results from the Supplémentation en Vitamines et Minéraux Antioxydants cohort. Am J Clin Nutr. 2015;102:471-478.
- Fung TT, Hunter DJ, Spiegelman D, et al. Vitamins and carotenoids intake and the risk of basal cell carcinoma of the skin in women (United States). Cancer Causes Control. 2002;13:221-230.
- Fung TT, Spiegelman D, Egan KM, et al. Vitamin and carotenoid intake and risk of squamous cell carcinoma of the skin. Int J Cancer. 2003;103:110-115.
- National Institutes of Health Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/
- Spoelstra-de Man AME, Elbers PWG, Oudemans-Van Straaten HM. Vitamin C: should we supplement? Curr Opin Crit Care. 2018;24:248-255.
- Moison RMW, Beijersbergen van Henegouwen GMJ. Topical antioxidant vitamins C and E prevent UVB-radiation-induced peroxidation of eicosapentaenoic acid in pig skin. Radiat Res. 2002;157:402-409.
- Lin JY, Selim MA, Shea CR, et al. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J Am Acad Dermatol. 2003;48:866-874.
- Pauling L, Willoughby R, Reynolds R, et al. Incidence of squamous cell carcinoma in hairless mice irradiated with ultraviolet light in relation to intake of ascorbic acid (vitamin C) and of D, L-alpha-tocopheryl acetate (vitamin E). Int J Vitam Nutr Res Suppl. 1982;23:53-82.
- Kune GA, Bannerman S, Field B, et al. Diet, alcohol, smoking, serum beta-carotene, and vitamin A in male nonmelanocytic skin cancer patients and controls. Nutr Cancer. 1992;18:237-244.
- Vural P, Canbaz M, Selçuki D. Plasma antioxidant defense in actinic keratosis and basal cell carcinoma. J Eur Acad Dermatol Venereol. 1999;13:96-101.
- Record IR, Dreosti IE, McInerney JK. Changes in plasma antioxidant status following consumption of diets high or low in fruit and vegetables or following dietary supplementation with an antioxidant mixture. Br J Nutr. 2001;85:459-464.
- Heinen MM, Hughes MC, Ibiebele TI, et al. Intake of antioxidant nutrients and the risk of skin cancer. Eur J Cancer. 2007;43:2707-2716.
- Yang G, Yan Y, Ma Y, et al. Vitamin C at high concentrations induces cytotoxicity in malignant melanoma but promotes tumor growth at low concentrations. Mol Carcinog. 2017;56:1965-1976.
- National Institutes of Health Office of Dietary Supplements. Vitamin D: fact sheet for health professionals. Updated August 12, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Reichrath J, Saternus R, Vogt T. Endocrine actions of vitamin D in skin: relevance for photocarcinogenesis of non-melanoma skin cancer, and beyond. Mol Cell Endocrinol. 2017;453:96-102.
- Ellison TI, Smith MK, Gilliam AC, et al. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008;128:2508-2517.
- Eide MJ, Johnson DA, Jacobsen GR, et al. Vitamin D and nonmelanoma skin cancer in a health maintenance organization cohort. Arch Dermatol. 2011;147:1379-1384.
- van der Pols JC, Russell A, Bauer U, et al. Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community. J Invest Dermatol. 2013;133:637-641.
- Caini S, Gnagnarella P, Stanganelli I, et al. Vitamin D and the risk of non-melanoma skin cancer: a systematic literature review and meta-analysis on behalf of the Italian Melanoma Intergroup. Cancers (Basel). 2021;13:4815.
- Park SM, Li T, Wu S, et al. Vitamin D intake and risk of skin cancer in US women and men. PLoS One. 2016;11:e0160308.
- Afzal S, Nordestgaard BG, Bojesen SE. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: a prospective cohort study. J Invest Dermatol. 2013;133:629-636.
- Asgari MM, Tang J, Warton ME, et al. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J Invest Dermatol. 2010;130:1438-1443.
- Tang JY, Parimi N, Wu A, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387-391.
- Keen MA, Hassan I. Vitamin E in dermatology. Indian Dermatol Online J. 2016;7:311-315.
- National Institutes of Health Office of Dietary Supplements. Vitamin E: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/
- Pearson P, Lewis SA, Britton J, et al. The pro-oxidant activity of high-dose vitamin E supplements in vivo. BioDrugs. 2006;20:271-273.
- Gerrish KE, Gensler HL. Prevention of photocarcinogenesis by dietary vitamin E. Nutr Cancer. 1993;19:125-133.
- McVean M, Liebler DC. Prevention of DNA photodamage by vitamin E compounds and sunscreens: roles of ultraviolet absorbance and cellular uptake. Mol Carcinog. 1999;24:169-176.
- Prasad KN, Cohrs RJ, Sharma OK. Decreased expressions of c-myc and H-ras oncogenes in vitamin E succinate induced morphologically differentiated murine B-16 melanoma cells in culture. Biochem Cell Biol. 1990;68:1250-1255.
- Funasaka Y, Komoto M, Ichihashi M. Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment Cell Res. 2000;13(suppl 8):170-174.
- National Institutes of Health Office of Dietary Supplements. Selenium: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/
- Sengupta A, Lichti UF, Carlson BA, et al. Selenoproteins are essential for proper keratinocyte function and skin development. PLoS One. 2010;5:e12249.
- Das RK, Hossain SKU, Bhattacharya S. Diphenylmethyl selenocyanate inhibits DMBA-croton oil induced two-stage mouse skin carcinogenesis by inducing apoptosis and inhibiting cutaneous cell proliferation. Cancer Lett. 2005;230:90-101.
- Clark LC, Combs GF Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957-1963.
- Duffield-Lillico AJ, Slate EH, Reid ME, et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477-1481.
- Reid ME, Duffield-Lillico AJ, Slate E, et al. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr Cancer. 2008;60:155-163.
- Vinceti M, Filippini T, Del Giovane C, et al. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018;1:CD005195.
- Hercberg S, Ezzedine K, Guinot C, et al. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr. 2007;137:2098-2105.
- Chang YJ, Myung SK, Chung ST, et al. Effects of vitamin treatment or supplements with purported antioxidant properties on skin cancer prevention: a meta-analysis of randomized controlled trials. Dermatology. 2011;223:36-44.
- Sun W, Rice MS, Park MK, et al. Intake of furocoumarins and risk of skin cancer in 2 prospective US cohort studies. J Nutr. 2020;150:1535-1544.
- Sakaki JR, Melough MM, Roberts MB, et al. Citrus consumption and the risk of non-melanoma skin cancer in the Women’s Health Initiative. Cancers (Basel). 2021;13:2173.
Nonmelanoma skin cancer (NMSC) is the most common cancer in the United States, and cutaneous melanoma is projected to be the fifth most common form of cancer in 2022, with increasing incidence and high potential for mortality.1-3 Estimates indicate that 35% to 45% of all cancers in White patients are cutaneous, with 4% to 5% occurring in Hispanic patients, 2% to 4% in Asian patients, and 1% to 2% in Black patients.4 Of the keratinocyte carcinomas, basal cell carcinoma (BCC) is the most prevalent, projected to affect approximately 33% to 39% of White males and 23% to 28% of White females in the United States during their lifetimes. Squamous cell carcinoma (SCC) is the second most common skin malignancy, with a lifetime risk of 9% to 14% for White males and 4% to 9% for White females in the United States.5 The incidence of melanoma continues to increase, with approximately 99,780 new cases expected in the United States in 2022.1
UV-induced DNA damage plays a key role in the pathogenesis and development of various skin malignancies.6 UV radiation from sunlight or tanning devices causes photocarcinogenesis due to molecular and cellular effects, including the generation of reactive oxygen species, DNA damage due to the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone, melanogenesis, apoptosis, and the increased expression of harmful genes and proteins.6 The summation of this damage can result in skin malignancies, including NMSC and melanoma.6,7 Dietary antioxidants theoretically help prevent oxidative reactions from occurring within the body, and it has been suggested that intake of dietary antioxidants may decrease DNA damage and prevent tumorigenesis secondary to UV radiation.8 Antioxidants exist naturally in the body but can be acquired exogenously. Investigators have studied dietary antioxidants in preventing skin cancer formation with promising results in the laboratory setting.8-11 Recently, more robust human studies have been initiated to further delineate this relationship. We present clinical evidence of several frequently utilized antioxidant vitamins and their effects on melanoma and NMSC.
Antioxidants
Vitamin A—Vitamin A is a fat-soluble vitamin found in animal sources, including fish, liver, and eggs. Carotenoids, such as beta carotene, are provitamin A plant derivatives found in fruits and vegetables that are converted into biologically active retinol and retinoic acid.12 Retinols play a key role in cellular growth and differentiation and are thought to be protective against skin cancer via the inactivation of free radicals and immunologic enhancement due to their antiproliferative, antioxidative, and antiapoptotic effects.13-16 Animal studies have demonstrated this protective effect and the ability of retinoids to suppress carcinogenesis; however, human studies reveal conflicting results.17,18
Greenberg et al19 investigated the use of beta carotene in preventing the formation of NMSC. Patients (N=1805) were randomized to receive 50 mg of beta carotene daily or placebo. Over a 5-year period, there was no significant reduction in the occurrence of NMSC (relative risk [RR], 1.05; 95% CI, 0.91-1.22).19 Frieling et al20 conducted a similar randomized, double-blind, placebo-controlled trial investigating beta carotene for primary prevention of NMSC in 22,071 healthy male physicians. The study group received 50 mg of beta carotene every other day for 12 years’ duration, and there was no significant effect on the incidence of first NMSC development (RR, 0.98; 95% CI, 0.92-1.05).20
A case-control study by Naldi et al21 found an inverse association between vitamin A intake and development of melanoma. Study participants were stratified into quartiles based on level of dietary intake and found an odds ratio (OR) of 0.71 for beta carotene (95% CI, 0.50-1.02), 0.57 for retinol (95% CI, 0.39-0.83), and 0.51 for total vitamin A (95% CI, 0.35-0.75) when comparing the upper quartile of vitamin A intake to the lower quartile. Upper-quartile cutoff values of vitamin A intake were 214 µg/d for beta carotene, 149 µg/d for retinol, and 359 µg/d for total vitamin A.21 More recently, a meta-analysis by Zhang et al22 pooled data from 8 case-control studies and 2 prospective studies. Intake of retinol but not total vitamin A or beta carotene was associated with a reduced risk for development of melanoma (retinol: OR, 0.80; 95% CI, 0.69-0.92; total vitamin A: OR, 0.86; 95% CI, 0.59-1.25; beta carotene: OR, 0.87; 95% CI, 0.62-1.20).22 Feskanich et al23 demonstrated similar findings with use of food-frequency questionnaires in White women, suggesting that retinol intake from food combined with supplements may be protective for women who were otherwise at a low risk for melanoma based on nondietary factors. These factors included painful or blistering sunburns during childhood, history of more than 6 sunburns, more than 3 moles on the left arm, having red or blonde hair, and having a parent or sibling with melanoma (P=.01). However, this relationship did not hold true when looking at women at an intermediate or high risk for melanoma (P=.16 and P=.46).23
When looking at high-risk patients, such as transplant patients, oral retinoids have been beneficial in preventing NMSC.24-27 Bavinck et al24 investigated 44 renal transplant patients with a history of more than 10 NMSCs treated with 30 mg of acitretin daily vs placebo. Patients receiving oral retinoid supplementation developed fewer NMSCs over a 6-month treatment period (P=.01).24 Similarly, George et al25 investigated acitretin in renal transplant patients and found a statistically significant decrease in number of SCCs in patients on supplementation (P=.002). Solomon-Cohen et al26 performed a retrospective case-crossover study in solid organ transplant recipients and found that those treated with 10 mg of acitretin daily for 2 years had a significant reduction in the number of new keratinocyte carcinomas (P=.002). Other investigators have demonstrated similar results, and in 2006, Otley et al27 proposed standardized dosing of acitretin for chemoprevention in high-risk patients, including patients developing 5 to 10 NMSCs per year, solid organ transplant recipients, and those with syndromes associated with the development of NMSC.28,29 Overall, in the general population, vitamin A and related compounds have not demonstrated a significant association with decreased development of NMSC; however, oral retinoids have proven useful for high-risk patients. Furthermore, several studies have suggested a negative association between vitamin A levels and the incidence of melanoma, specifically in the retinol formulation.
Vitamin B3—Nicotinamide (also known as niacinamide) is a water-soluble form of vitamin B3 and is obtained from animal-based and plant-based foods, such as meat, fish, and legumes.30 Nicotinamide plays a key role in cellular metabolism, cellular signaling, and DNA repair, including protection from UV damage within keratinocytes.31,32 Early mouse models demonstrated decreased formation of skin tumors in mice treated with topical or oral nicotinamide.32,33 A number of human studies have revealed similar results.34-36
Chen et al34 conducted the ONTRAC study, a phase 3, double-blind, randomized controlled trial (RCT) looking at 386 participants with a history of at least 2 NMSCs in the preceding 5 years. At 12 months, those treated with 500 mg of nicotinamide twice daily demonstrated a statistically significant decreased rate of SCC formation (P=.05). A decreased incidence of BCC development was noted; however, this trend did not reach statistical significance (P=.12). Precancerous skin lesions also were found to be decreased in the treatment group, with 20% lower incidence of actinic keratoses (AKs) after 9 months of treatment (P<.001).34 Drago et al35 specifically studied the incidence of AKs in 38 transplant recipients—8 liver and 30 kidney—and found that previously noted AKs had decreased in size for 18 of 19 patients taking 500 mg of nicotinamide daily when originally photographed AKs were remeasured at 6-month follow-up, with 7 of these 18 patients demonstrating complete clinical regression. Of those on nicotinamide supplementation, no new AKs developed compared to the control group, which demonstrated increased size of AKs or development of new AKs in 91% of patients, with 7 AKs progressing into SCC.35
Nicotinamide has been demonstrated to be useful in preventing skin cancer in high-risk populations, such as transplant patients or those with a high incidence of NMSC.34,36 Despite promising results within the laboratory setting, nicotinamide’s effects on melanoma in humans remains less clear.31,37 Studies suggest that nicotinamide enhances tumor-infiltrating lymphocytes and DNA repair mechanisms in melanocytes, which may translate into nicotinamide, providing chemoprevention for melanoma, but research in human patients is limited.31,37
Vitamin B9—Folate, the natural form of vitamin B9, is a water-soluble compound that is found in many foods, especially green leafy vegetables, and often is supplemented because of its health benefits.38,39 In the skin, folic acid plays a key role in cellular replication and proliferation.38 Controversy exists regarding folate’s effects on cellular growth and turnover with respect to cancer incidence.38,40 Donnenfeld et al41 conducted a prospective study assessing dietary folic acid intake and development of NMSC. A total of 5880 participants completed dietary records throughout the first 2 years of the study. After an average follow-up period of 12.6 years, there was an overall increased incidence of skin cancer in those with increased dietary folate (P=.03). Furthermore, when striating by skin cancer type, there was an increased incidence of NMSC overall as well as BCC when analyzing by type of NMSC (P=.03 for NMSC; P=.05 for BCC). However, when stratifying by gender, these findings only held true for women.41 Similar effects were observed by Fung et al,42 who prospectively studied the intake of various vitamins in relationship to the development of BCC in women. During 12 years of follow-up, a positive association was observed between folate intake and BCC development (OR, 1.2; 95% CI, 1.10-1.31).42 Fung et al43 also investigated the role of several vitamins in the development of SCC and found that folate showed a negative association, which did not reach statistical significance (RR, 0.79; 95% CI, 0.56-1.11). Furthermore, Vollset et al40 conducted a meta-analysis comparing folic acid to placebo in the incidence of various types of cancer. The study excluded NMSC but reported no significant association between the development of melanoma and folic acid supplementation.40 In summary, the effects of folate have diverse consequences, potentially promoting the formation of NMSC, but studies suggest that an individual’s gender and other genetic and environmental factors also may play a role.
Vitamin C—Vitamin C (also known as ascorbic acid) is a water-soluble vitamin with antioxidant immune-mediating effects. It is found in various fruits and vegetables and serves as a cofactor for enzymes within the body playing a key role in immune function and collagen formation.44,45 It has been postulated that ascorbic acid can provide protection from UV radiation damage via its intracellular activity but conversely can contribute to oxidative damage.44 Multiple in vitro laboratory studies and animal models have demonstrated photoprotective effects of ascorbic acid.46-48 Despite these findings, minimal photoprotective effects have been found in the human population.
Kune et al49 performed a case-control study of 88 males with previously diagnosed NMSC undergoing surgical removal and investigated patients’ prior dietary habits. Patients with NMSC had a statistically significantly lower level of vitamin C–containing food in their diet than those without NMSC (P=.004).49 In addition, Vural et al50 analyzed plasma samples and blood cells of patients with AK and BCC and found a significant decrease in ascorbic acid levels in both the AK (P<.001) and BCC (P<.001) groups compared with controls. However, studies have found that consumption of certain dietary compounds can rapidly increase plasma concentration levels, which may serve as a major confounding variable in this study. Plasma concentrations of ascorbic acid and beta carotene were found to be significantly increased following consumption of a high-antioxidant diet for as short a duration as 2 weeks (P<.05).51 More recently, Heinen et al52 performed a prospective study on 1001 adults. In patients without a history of skin cancer, they found that vitamin C from food sources plus dietary supplements was positively associated with the development of BCC (P=.03).52 Similarly, Fung et al42 performed a study in women and found a positive association between vitamin C intake and the development of BCC (OR, 1.13; 95% CI, 1.03-1.23).
The relationship between vitamin C intake—either in dietary or supplemental form—and melanoma remains controversial. Mice-based studies found that high concentrations of orally administered vitamin C induce cytotoxicity in melanoma cell lines, but at low concentrations they promote tumor growth of malignant melanoma.53 Feskanich et al23 examined the relationship between vitamin C intake and melanoma development via food frequency questionnaires in White women and found that vitamin C was associated with a higher risk for melanoma (P=.05), and furthermore, a positive dose response with frequency of orange juice intake was observed (P=.008). Overall, despite promising laboratory studies, there is a lack of RCTs investigating the use of vitamin C supplementation for prevention of NMSC and melanoma in humans, and the oral benefits of vitamin C for chemoprevention remain unclear.
Vitamin D—Vitamin D is a fat-soluble vitamin that is found in fish, liver, egg, and cheese, and is endogenously produced when UV radiation from sun exposure interacts with the skin, triggering the synthesis of vitamin D.54 Vitamin D is biologically inactive and must be converted to its active form 1,25-dihydroxyvitamin D after entering the body. Vitamin D modulates many genes involved in cellular proliferation and differentiation.54 Vitamin D receptors are expressed on keratinocytes and melanocytes.55 Animal studies have demonstrated a potentially protective effect of vitamin D in the development of NMSC.56 In a mouse model, Ellison et al56 found that mice without vitamin D receptors developed skin tumors more rapidly than those with vitamin D receptors.
Unfortunately, these findings have not been demonstrated in humans, and studies have even reported an increased risk for development of NMSC in patients with normal or increased vitamin D levels compared with those with low levels of vitamin D.57-60 Eide et al57 studied 3223 patients seeking advice for low bone density by recording their vitamin D levels at the time of presentation and monitoring development of NMSC. Vitamin D levels greater than 15 ng/mL were positively associated with the development of NMSC (OR, 1.7; 95% CI, 1.04-2.7). This association held true for both SCC and BCC, with a higher risk estimated for SCC (OR, 3.2; 95% CI, 0.4-24.0 for SCC; OR, 1.7; 95% CI, 0.5-5.8 for BCC).57 An increased vitamin D serum level also was found to be significantly associated with a higher risk for BCC and melanoma by van der Pols et al.58 This prospective study looked at the incidence of skin cancer over 11 years. Study participants with vitamin D levels over 75 nmol/L more frequently developed BCC (P=.01) and melanoma (P=.05). In contrast, SCC was less frequently observed in participants with these high levels of vitamin D (P=.07).58 Furthermore, Park et al60 looked at vitamin D and skin cancer risk for men and women in the United States and found no association with risk for SCC or melanoma but a positive association with BCC (P=.05 for total vitamin D; P<.01 for dietary vitamin D). Additional studies have been performed with inconsistent results, and multiple authors suggest the possible confounding relationship between vitamin D levels and UV radiation exposure.59-62 Furthermore, some studies have even demonstrated a negative association between vitamin D and NMSC. Tang et al63 performed a retrospective case-control study in elderly males, investigating serum levels of vitamin D and patients’ self-reported history of NMSC, which demonstrated that higher levels of vitamin D were associated with a decreased risk for NMSC. Overall, the relationship between vitamin D and skin cancer development remains unclear for both melanoma and NMSC.
Vitamin E—Vitamin E is a fat-soluble vitamin that is found in plant-based oils, nuts, seeds, fruits, and vegetables.64 It works as an antioxidant to protect against free radicals and heighten immune function, and it also serves as a pro-oxidant.65,66 Vitamin E naturally exists in 8 chemical forms, of which gamma-tocopherol is the most frequently obtained form in the diet, and alpha-tocopherol is the most abundant form found in the body.64,65
Early animal studies demonstrated the inhibition of UV-induced damage in mice receiving vitamin E supplementation.67,68 Human studies have not consistently shown these effects. Vural et al50 investigated plasma samples and blood cells of patients with AKs and BCCs and reported a significant decrease in alpha-tocopherol levels in both the AK (P<.05) and BCC (P<.001) groups compared with controls. However, studies also have demonstrated a positive association between vitamin E intake and the development of BCC, including one by Fung et al,42 which found a significant association in women (OR, 1.15; 95% CI, 1.06-1.26).
Vitamin E has been found to inhibit melanin synthesis in the laboratory, suggesting a potentially protective effect in melanoma.69,70 However, in the study performed by Feskanich et al23 examining vitamin intake and melanoma incidence via food-frequency questionnaires, vitamin E was not associated with a lower risk for melanoma. Despite promising laboratory studies, the data surrounding the use of a vitamin E supplement for prevention of melanoma and NMSC in humans remains unclear.
Selenium—Selenium is a trace mineral found in plants, meat, and fish. It plays a key role in reproduction, hormone metabolism, DNA synthesis, and protection from oxidative damage.71 In mice studies, lack of selenium-containing proteins resulted in skin abnormalities, including the development of a hyperplastic epidermis and aberrant hair follicle morphogenesis with alopecia after birth, and numerous experimental studies have demonstrated a negative association between selenium intake and cancer.72,73 However, human studies have yielded alternative results.
The Nutritional Prevention of Cancer Study Group analyzed 1312 dermatology patients with a history of NMSC.74 The study population was obtained from 7 dermatology clinics with randomization to control for confounding variables. Study participants received either 200 μg of selenium daily or placebo.74 Baseline characteristics of each study group were overall balanced. Selenium intake was found to have no effect on the development of BCC (hazard ratio [HR], 1.09; 95% CI, 0.94-1.26) but an increased risk for developing SCC (HR, 1.25; 95% CI, 1.03-1.51) and total NMSC (HR, 1.17; 95% CI, 1.02-1.34).74,75 Similarly, Reid et al76 performed an RCT comparing patients treated with 400 μg/d of selenium to those treated with 200 μg/d of selenium. When compared with placebo, those treated with 200 μg/d of selenium had a statistically significantly increased incidence of NMSC (P=.006); however, those treated with 400 μg/d of selenium had no significant change in total incidence of NMSC (P=.51).76 Furthermore, Vinceti et al77 performed a review of 83 studies from the literature investigating the effect of dietary selenium, and from the RCTs, there was no beneficial effect of selenium in reducing cancer risk in general; however, some studies demonstrated an increased incidence of other types of cancer, including melanoma. Of the RCTs included in the study investigating NMSC incidence specifically, it was found that the incidence was not affected by selenium administration (RR, 1.16; 95% CI, 0.30-4.42; 2 studies, 2027 participants).77 Despite data from several studies demonstrating an increased risk for NMSC, the effects of selenium on the risk for NMSC and melanoma remain unclear.
Combination Antioxidant Studies
In addition to investigating the use of single antioxidants in skin cancer prevention, studies utilizing the combination of various antioxidants or other dietary minerals have been conducted. Hercberg et al78 performed a randomized, double-blinded, placebo-controlled trial of 13,017 adults (7876 women and 5141 men) receiving a combination of 120 mg vitamin C, 30 mg vitamin E, 100 μg selenium, 6 mg beta carotene, and 20 mg zinc. Study participants were followed for an average of 7.5 years, and the development of skin cancers were recorded. Overall, the incidence rate of skin cancer did not differ between the 2 treatment groups; however, when segregated by gender, the study found that there was an increased risk for developing skin cancer in women taking the antioxidant supplement combination compared with placebo (P=.03). This difference was not observed in the 2 treatment groups of male patients (P=.11). When looking specifically at NMSC, there was no difference between treatment groups for male or female patients (P=.39 for males; P=.15 for females). In contrast, there was a higher incidence of melanoma identified in female patients taking the combination antioxidant supplement (P=.01), but this was not seen within the male study population (P=.51).78 In addition, Chang et al79 performed a meta-analysis of 10 previously published RCTs. Analysis revealed that treatment with a variety of supplements, including vitamins A, C, E, and beta carotene, were found to have no preventative effects on the incidence of skin cancer development (RR, 0.98; CI, 0.98-1.03). Notable limitations to this study included the variability in protocols of the studies included in this meta-analysis, the limited number of RCTs investigating vitamin supplementation and the risk for skin cancer development, and the influence of dietary intake on study outcomes.79
Other Dietary Agents
Furocoumarins—Furocoumarins are botanical substances found in various fruits and plants, including many citrus products. Furocoumarins are activated by UV light radiation and can lead to development of a phototoxic eruption. Several studies have suggested a pharmacogenetic effect of furocoumarins.80 Sun et al80 collected dietary data from 47,453 men and 75,291 women on furocoumarin intake and correlation with the development of NMSC. Overall, the study suggested that the intake of furocoumarins may lead to an increase in the development of BCC (HR, 1.16; 95% CI, 1.11-1.21; P=.002); however, there was no significant association identified between total intake of furocoumarins in the risk for SCC or melanoma.80 Furthermore, Sakaki et al81 conducted a survey study looking at the consumption of citrus products and the development of NMSC. The group found that there was an increased risk for NMSC in those consuming an increased amount of citrus products (P=.007).81
Conclusion
Dietary antioxidants have been investigated for their potential role in the prevention of tumorigenesis. Specific antioxidant vitamins, such as vitamin A derivatives and niacinamide, have demonstrated clinical utility in the prevention of NMSC in high-risk populations. Retinol also has been associated with a reduced incidence of melanoma. Numerous antioxidants have demonstrated promising data within the laboratory setting; however, inconsistent results have been appreciated in humans. Furthermore, several research studies suggest that folate, vitamin D, and furocoumarins may be associated with an increased risk for skin cancer development; however, these studies are inconclusive, and dietary studies are challenging to conduct. Overall, RCTs investigating the role of antioxidants for chemoprevention are limited. Moreover, the study of dietary antioxidants and vitamins may be affected by various confounding variables that can be difficult to account for because of patients’ potentially poor recall of dietary intake and the effect of dietary intake in supplemental studies. Given the increasing prevalence of skin cancer worldwide, further research into the clinical utility of antioxidants in skin cancer prevention is warranted.
Nonmelanoma skin cancer (NMSC) is the most common cancer in the United States, and cutaneous melanoma is projected to be the fifth most common form of cancer in 2022, with increasing incidence and high potential for mortality.1-3 Estimates indicate that 35% to 45% of all cancers in White patients are cutaneous, with 4% to 5% occurring in Hispanic patients, 2% to 4% in Asian patients, and 1% to 2% in Black patients.4 Of the keratinocyte carcinomas, basal cell carcinoma (BCC) is the most prevalent, projected to affect approximately 33% to 39% of White males and 23% to 28% of White females in the United States during their lifetimes. Squamous cell carcinoma (SCC) is the second most common skin malignancy, with a lifetime risk of 9% to 14% for White males and 4% to 9% for White females in the United States.5 The incidence of melanoma continues to increase, with approximately 99,780 new cases expected in the United States in 2022.1
UV-induced DNA damage plays a key role in the pathogenesis and development of various skin malignancies.6 UV radiation from sunlight or tanning devices causes photocarcinogenesis due to molecular and cellular effects, including the generation of reactive oxygen species, DNA damage due to the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone, melanogenesis, apoptosis, and the increased expression of harmful genes and proteins.6 The summation of this damage can result in skin malignancies, including NMSC and melanoma.6,7 Dietary antioxidants theoretically help prevent oxidative reactions from occurring within the body, and it has been suggested that intake of dietary antioxidants may decrease DNA damage and prevent tumorigenesis secondary to UV radiation.8 Antioxidants exist naturally in the body but can be acquired exogenously. Investigators have studied dietary antioxidants in preventing skin cancer formation with promising results in the laboratory setting.8-11 Recently, more robust human studies have been initiated to further delineate this relationship. We present clinical evidence of several frequently utilized antioxidant vitamins and their effects on melanoma and NMSC.
Antioxidants
Vitamin A—Vitamin A is a fat-soluble vitamin found in animal sources, including fish, liver, and eggs. Carotenoids, such as beta carotene, are provitamin A plant derivatives found in fruits and vegetables that are converted into biologically active retinol and retinoic acid.12 Retinols play a key role in cellular growth and differentiation and are thought to be protective against skin cancer via the inactivation of free radicals and immunologic enhancement due to their antiproliferative, antioxidative, and antiapoptotic effects.13-16 Animal studies have demonstrated this protective effect and the ability of retinoids to suppress carcinogenesis; however, human studies reveal conflicting results.17,18
Greenberg et al19 investigated the use of beta carotene in preventing the formation of NMSC. Patients (N=1805) were randomized to receive 50 mg of beta carotene daily or placebo. Over a 5-year period, there was no significant reduction in the occurrence of NMSC (relative risk [RR], 1.05; 95% CI, 0.91-1.22).19 Frieling et al20 conducted a similar randomized, double-blind, placebo-controlled trial investigating beta carotene for primary prevention of NMSC in 22,071 healthy male physicians. The study group received 50 mg of beta carotene every other day for 12 years’ duration, and there was no significant effect on the incidence of first NMSC development (RR, 0.98; 95% CI, 0.92-1.05).20
A case-control study by Naldi et al21 found an inverse association between vitamin A intake and development of melanoma. Study participants were stratified into quartiles based on level of dietary intake and found an odds ratio (OR) of 0.71 for beta carotene (95% CI, 0.50-1.02), 0.57 for retinol (95% CI, 0.39-0.83), and 0.51 for total vitamin A (95% CI, 0.35-0.75) when comparing the upper quartile of vitamin A intake to the lower quartile. Upper-quartile cutoff values of vitamin A intake were 214 µg/d for beta carotene, 149 µg/d for retinol, and 359 µg/d for total vitamin A.21 More recently, a meta-analysis by Zhang et al22 pooled data from 8 case-control studies and 2 prospective studies. Intake of retinol but not total vitamin A or beta carotene was associated with a reduced risk for development of melanoma (retinol: OR, 0.80; 95% CI, 0.69-0.92; total vitamin A: OR, 0.86; 95% CI, 0.59-1.25; beta carotene: OR, 0.87; 95% CI, 0.62-1.20).22 Feskanich et al23 demonstrated similar findings with use of food-frequency questionnaires in White women, suggesting that retinol intake from food combined with supplements may be protective for women who were otherwise at a low risk for melanoma based on nondietary factors. These factors included painful or blistering sunburns during childhood, history of more than 6 sunburns, more than 3 moles on the left arm, having red or blonde hair, and having a parent or sibling with melanoma (P=.01). However, this relationship did not hold true when looking at women at an intermediate or high risk for melanoma (P=.16 and P=.46).23
When looking at high-risk patients, such as transplant patients, oral retinoids have been beneficial in preventing NMSC.24-27 Bavinck et al24 investigated 44 renal transplant patients with a history of more than 10 NMSCs treated with 30 mg of acitretin daily vs placebo. Patients receiving oral retinoid supplementation developed fewer NMSCs over a 6-month treatment period (P=.01).24 Similarly, George et al25 investigated acitretin in renal transplant patients and found a statistically significant decrease in number of SCCs in patients on supplementation (P=.002). Solomon-Cohen et al26 performed a retrospective case-crossover study in solid organ transplant recipients and found that those treated with 10 mg of acitretin daily for 2 years had a significant reduction in the number of new keratinocyte carcinomas (P=.002). Other investigators have demonstrated similar results, and in 2006, Otley et al27 proposed standardized dosing of acitretin for chemoprevention in high-risk patients, including patients developing 5 to 10 NMSCs per year, solid organ transplant recipients, and those with syndromes associated with the development of NMSC.28,29 Overall, in the general population, vitamin A and related compounds have not demonstrated a significant association with decreased development of NMSC; however, oral retinoids have proven useful for high-risk patients. Furthermore, several studies have suggested a negative association between vitamin A levels and the incidence of melanoma, specifically in the retinol formulation.
Vitamin B3—Nicotinamide (also known as niacinamide) is a water-soluble form of vitamin B3 and is obtained from animal-based and plant-based foods, such as meat, fish, and legumes.30 Nicotinamide plays a key role in cellular metabolism, cellular signaling, and DNA repair, including protection from UV damage within keratinocytes.31,32 Early mouse models demonstrated decreased formation of skin tumors in mice treated with topical or oral nicotinamide.32,33 A number of human studies have revealed similar results.34-36
Chen et al34 conducted the ONTRAC study, a phase 3, double-blind, randomized controlled trial (RCT) looking at 386 participants with a history of at least 2 NMSCs in the preceding 5 years. At 12 months, those treated with 500 mg of nicotinamide twice daily demonstrated a statistically significant decreased rate of SCC formation (P=.05). A decreased incidence of BCC development was noted; however, this trend did not reach statistical significance (P=.12). Precancerous skin lesions also were found to be decreased in the treatment group, with 20% lower incidence of actinic keratoses (AKs) after 9 months of treatment (P<.001).34 Drago et al35 specifically studied the incidence of AKs in 38 transplant recipients—8 liver and 30 kidney—and found that previously noted AKs had decreased in size for 18 of 19 patients taking 500 mg of nicotinamide daily when originally photographed AKs were remeasured at 6-month follow-up, with 7 of these 18 patients demonstrating complete clinical regression. Of those on nicotinamide supplementation, no new AKs developed compared to the control group, which demonstrated increased size of AKs or development of new AKs in 91% of patients, with 7 AKs progressing into SCC.35
Nicotinamide has been demonstrated to be useful in preventing skin cancer in high-risk populations, such as transplant patients or those with a high incidence of NMSC.34,36 Despite promising results within the laboratory setting, nicotinamide’s effects on melanoma in humans remains less clear.31,37 Studies suggest that nicotinamide enhances tumor-infiltrating lymphocytes and DNA repair mechanisms in melanocytes, which may translate into nicotinamide, providing chemoprevention for melanoma, but research in human patients is limited.31,37
Vitamin B9—Folate, the natural form of vitamin B9, is a water-soluble compound that is found in many foods, especially green leafy vegetables, and often is supplemented because of its health benefits.38,39 In the skin, folic acid plays a key role in cellular replication and proliferation.38 Controversy exists regarding folate’s effects on cellular growth and turnover with respect to cancer incidence.38,40 Donnenfeld et al41 conducted a prospective study assessing dietary folic acid intake and development of NMSC. A total of 5880 participants completed dietary records throughout the first 2 years of the study. After an average follow-up period of 12.6 years, there was an overall increased incidence of skin cancer in those with increased dietary folate (P=.03). Furthermore, when striating by skin cancer type, there was an increased incidence of NMSC overall as well as BCC when analyzing by type of NMSC (P=.03 for NMSC; P=.05 for BCC). However, when stratifying by gender, these findings only held true for women.41 Similar effects were observed by Fung et al,42 who prospectively studied the intake of various vitamins in relationship to the development of BCC in women. During 12 years of follow-up, a positive association was observed between folate intake and BCC development (OR, 1.2; 95% CI, 1.10-1.31).42 Fung et al43 also investigated the role of several vitamins in the development of SCC and found that folate showed a negative association, which did not reach statistical significance (RR, 0.79; 95% CI, 0.56-1.11). Furthermore, Vollset et al40 conducted a meta-analysis comparing folic acid to placebo in the incidence of various types of cancer. The study excluded NMSC but reported no significant association between the development of melanoma and folic acid supplementation.40 In summary, the effects of folate have diverse consequences, potentially promoting the formation of NMSC, but studies suggest that an individual’s gender and other genetic and environmental factors also may play a role.
Vitamin C—Vitamin C (also known as ascorbic acid) is a water-soluble vitamin with antioxidant immune-mediating effects. It is found in various fruits and vegetables and serves as a cofactor for enzymes within the body playing a key role in immune function and collagen formation.44,45 It has been postulated that ascorbic acid can provide protection from UV radiation damage via its intracellular activity but conversely can contribute to oxidative damage.44 Multiple in vitro laboratory studies and animal models have demonstrated photoprotective effects of ascorbic acid.46-48 Despite these findings, minimal photoprotective effects have been found in the human population.
Kune et al49 performed a case-control study of 88 males with previously diagnosed NMSC undergoing surgical removal and investigated patients’ prior dietary habits. Patients with NMSC had a statistically significantly lower level of vitamin C–containing food in their diet than those without NMSC (P=.004).49 In addition, Vural et al50 analyzed plasma samples and blood cells of patients with AK and BCC and found a significant decrease in ascorbic acid levels in both the AK (P<.001) and BCC (P<.001) groups compared with controls. However, studies have found that consumption of certain dietary compounds can rapidly increase plasma concentration levels, which may serve as a major confounding variable in this study. Plasma concentrations of ascorbic acid and beta carotene were found to be significantly increased following consumption of a high-antioxidant diet for as short a duration as 2 weeks (P<.05).51 More recently, Heinen et al52 performed a prospective study on 1001 adults. In patients without a history of skin cancer, they found that vitamin C from food sources plus dietary supplements was positively associated with the development of BCC (P=.03).52 Similarly, Fung et al42 performed a study in women and found a positive association between vitamin C intake and the development of BCC (OR, 1.13; 95% CI, 1.03-1.23).
The relationship between vitamin C intake—either in dietary or supplemental form—and melanoma remains controversial. Mice-based studies found that high concentrations of orally administered vitamin C induce cytotoxicity in melanoma cell lines, but at low concentrations they promote tumor growth of malignant melanoma.53 Feskanich et al23 examined the relationship between vitamin C intake and melanoma development via food frequency questionnaires in White women and found that vitamin C was associated with a higher risk for melanoma (P=.05), and furthermore, a positive dose response with frequency of orange juice intake was observed (P=.008). Overall, despite promising laboratory studies, there is a lack of RCTs investigating the use of vitamin C supplementation for prevention of NMSC and melanoma in humans, and the oral benefits of vitamin C for chemoprevention remain unclear.
Vitamin D—Vitamin D is a fat-soluble vitamin that is found in fish, liver, egg, and cheese, and is endogenously produced when UV radiation from sun exposure interacts with the skin, triggering the synthesis of vitamin D.54 Vitamin D is biologically inactive and must be converted to its active form 1,25-dihydroxyvitamin D after entering the body. Vitamin D modulates many genes involved in cellular proliferation and differentiation.54 Vitamin D receptors are expressed on keratinocytes and melanocytes.55 Animal studies have demonstrated a potentially protective effect of vitamin D in the development of NMSC.56 In a mouse model, Ellison et al56 found that mice without vitamin D receptors developed skin tumors more rapidly than those with vitamin D receptors.
Unfortunately, these findings have not been demonstrated in humans, and studies have even reported an increased risk for development of NMSC in patients with normal or increased vitamin D levels compared with those with low levels of vitamin D.57-60 Eide et al57 studied 3223 patients seeking advice for low bone density by recording their vitamin D levels at the time of presentation and monitoring development of NMSC. Vitamin D levels greater than 15 ng/mL were positively associated with the development of NMSC (OR, 1.7; 95% CI, 1.04-2.7). This association held true for both SCC and BCC, with a higher risk estimated for SCC (OR, 3.2; 95% CI, 0.4-24.0 for SCC; OR, 1.7; 95% CI, 0.5-5.8 for BCC).57 An increased vitamin D serum level also was found to be significantly associated with a higher risk for BCC and melanoma by van der Pols et al.58 This prospective study looked at the incidence of skin cancer over 11 years. Study participants with vitamin D levels over 75 nmol/L more frequently developed BCC (P=.01) and melanoma (P=.05). In contrast, SCC was less frequently observed in participants with these high levels of vitamin D (P=.07).58 Furthermore, Park et al60 looked at vitamin D and skin cancer risk for men and women in the United States and found no association with risk for SCC or melanoma but a positive association with BCC (P=.05 for total vitamin D; P<.01 for dietary vitamin D). Additional studies have been performed with inconsistent results, and multiple authors suggest the possible confounding relationship between vitamin D levels and UV radiation exposure.59-62 Furthermore, some studies have even demonstrated a negative association between vitamin D and NMSC. Tang et al63 performed a retrospective case-control study in elderly males, investigating serum levels of vitamin D and patients’ self-reported history of NMSC, which demonstrated that higher levels of vitamin D were associated with a decreased risk for NMSC. Overall, the relationship between vitamin D and skin cancer development remains unclear for both melanoma and NMSC.
Vitamin E—Vitamin E is a fat-soluble vitamin that is found in plant-based oils, nuts, seeds, fruits, and vegetables.64 It works as an antioxidant to protect against free radicals and heighten immune function, and it also serves as a pro-oxidant.65,66 Vitamin E naturally exists in 8 chemical forms, of which gamma-tocopherol is the most frequently obtained form in the diet, and alpha-tocopherol is the most abundant form found in the body.64,65
Early animal studies demonstrated the inhibition of UV-induced damage in mice receiving vitamin E supplementation.67,68 Human studies have not consistently shown these effects. Vural et al50 investigated plasma samples and blood cells of patients with AKs and BCCs and reported a significant decrease in alpha-tocopherol levels in both the AK (P<.05) and BCC (P<.001) groups compared with controls. However, studies also have demonstrated a positive association between vitamin E intake and the development of BCC, including one by Fung et al,42 which found a significant association in women (OR, 1.15; 95% CI, 1.06-1.26).
Vitamin E has been found to inhibit melanin synthesis in the laboratory, suggesting a potentially protective effect in melanoma.69,70 However, in the study performed by Feskanich et al23 examining vitamin intake and melanoma incidence via food-frequency questionnaires, vitamin E was not associated with a lower risk for melanoma. Despite promising laboratory studies, the data surrounding the use of a vitamin E supplement for prevention of melanoma and NMSC in humans remains unclear.
Selenium—Selenium is a trace mineral found in plants, meat, and fish. It plays a key role in reproduction, hormone metabolism, DNA synthesis, and protection from oxidative damage.71 In mice studies, lack of selenium-containing proteins resulted in skin abnormalities, including the development of a hyperplastic epidermis and aberrant hair follicle morphogenesis with alopecia after birth, and numerous experimental studies have demonstrated a negative association between selenium intake and cancer.72,73 However, human studies have yielded alternative results.
The Nutritional Prevention of Cancer Study Group analyzed 1312 dermatology patients with a history of NMSC.74 The study population was obtained from 7 dermatology clinics with randomization to control for confounding variables. Study participants received either 200 μg of selenium daily or placebo.74 Baseline characteristics of each study group were overall balanced. Selenium intake was found to have no effect on the development of BCC (hazard ratio [HR], 1.09; 95% CI, 0.94-1.26) but an increased risk for developing SCC (HR, 1.25; 95% CI, 1.03-1.51) and total NMSC (HR, 1.17; 95% CI, 1.02-1.34).74,75 Similarly, Reid et al76 performed an RCT comparing patients treated with 400 μg/d of selenium to those treated with 200 μg/d of selenium. When compared with placebo, those treated with 200 μg/d of selenium had a statistically significantly increased incidence of NMSC (P=.006); however, those treated with 400 μg/d of selenium had no significant change in total incidence of NMSC (P=.51).76 Furthermore, Vinceti et al77 performed a review of 83 studies from the literature investigating the effect of dietary selenium, and from the RCTs, there was no beneficial effect of selenium in reducing cancer risk in general; however, some studies demonstrated an increased incidence of other types of cancer, including melanoma. Of the RCTs included in the study investigating NMSC incidence specifically, it was found that the incidence was not affected by selenium administration (RR, 1.16; 95% CI, 0.30-4.42; 2 studies, 2027 participants).77 Despite data from several studies demonstrating an increased risk for NMSC, the effects of selenium on the risk for NMSC and melanoma remain unclear.
Combination Antioxidant Studies
In addition to investigating the use of single antioxidants in skin cancer prevention, studies utilizing the combination of various antioxidants or other dietary minerals have been conducted. Hercberg et al78 performed a randomized, double-blinded, placebo-controlled trial of 13,017 adults (7876 women and 5141 men) receiving a combination of 120 mg vitamin C, 30 mg vitamin E, 100 μg selenium, 6 mg beta carotene, and 20 mg zinc. Study participants were followed for an average of 7.5 years, and the development of skin cancers were recorded. Overall, the incidence rate of skin cancer did not differ between the 2 treatment groups; however, when segregated by gender, the study found that there was an increased risk for developing skin cancer in women taking the antioxidant supplement combination compared with placebo (P=.03). This difference was not observed in the 2 treatment groups of male patients (P=.11). When looking specifically at NMSC, there was no difference between treatment groups for male or female patients (P=.39 for males; P=.15 for females). In contrast, there was a higher incidence of melanoma identified in female patients taking the combination antioxidant supplement (P=.01), but this was not seen within the male study population (P=.51).78 In addition, Chang et al79 performed a meta-analysis of 10 previously published RCTs. Analysis revealed that treatment with a variety of supplements, including vitamins A, C, E, and beta carotene, were found to have no preventative effects on the incidence of skin cancer development (RR, 0.98; CI, 0.98-1.03). Notable limitations to this study included the variability in protocols of the studies included in this meta-analysis, the limited number of RCTs investigating vitamin supplementation and the risk for skin cancer development, and the influence of dietary intake on study outcomes.79
Other Dietary Agents
Furocoumarins—Furocoumarins are botanical substances found in various fruits and plants, including many citrus products. Furocoumarins are activated by UV light radiation and can lead to development of a phototoxic eruption. Several studies have suggested a pharmacogenetic effect of furocoumarins.80 Sun et al80 collected dietary data from 47,453 men and 75,291 women on furocoumarin intake and correlation with the development of NMSC. Overall, the study suggested that the intake of furocoumarins may lead to an increase in the development of BCC (HR, 1.16; 95% CI, 1.11-1.21; P=.002); however, there was no significant association identified between total intake of furocoumarins in the risk for SCC or melanoma.80 Furthermore, Sakaki et al81 conducted a survey study looking at the consumption of citrus products and the development of NMSC. The group found that there was an increased risk for NMSC in those consuming an increased amount of citrus products (P=.007).81
Conclusion
Dietary antioxidants have been investigated for their potential role in the prevention of tumorigenesis. Specific antioxidant vitamins, such as vitamin A derivatives and niacinamide, have demonstrated clinical utility in the prevention of NMSC in high-risk populations. Retinol also has been associated with a reduced incidence of melanoma. Numerous antioxidants have demonstrated promising data within the laboratory setting; however, inconsistent results have been appreciated in humans. Furthermore, several research studies suggest that folate, vitamin D, and furocoumarins may be associated with an increased risk for skin cancer development; however, these studies are inconclusive, and dietary studies are challenging to conduct. Overall, RCTs investigating the role of antioxidants for chemoprevention are limited. Moreover, the study of dietary antioxidants and vitamins may be affected by various confounding variables that can be difficult to account for because of patients’ potentially poor recall of dietary intake and the effect of dietary intake in supplemental studies. Given the increasing prevalence of skin cancer worldwide, further research into the clinical utility of antioxidants in skin cancer prevention is warranted.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
- Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768.
- Leiter U, Keim U, Garbe C. Epidemiology of skin cancer: update 2019. In: Reichrath J, ed. Sunlight, Vitamin D and Skin Cancer. Springer International Publishing; 2020:123-139.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
- Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2017;76(3S1):S100-S109.
- Pleasance ED, Cheetham RK, Stephens PJ, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191-196.
- Baek J, Lee MG. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016;21:164-169.
- Katta R, Brown DN. Diet and skin cancer: the potential role of dietary antioxidants in nonmelanoma skin cancer prevention. J Skin Cancer. 2015;2015:893149.
- Stoj V, Shahriari N, Shao K, et al. Nutrition and nonmelanoma skin cancers. Clin Dermatol. 2022;40:173-185.
- O’Connor EA, Evans CV, Ivlev I, et al. Vitamin and mineral supplements for the primary prevention of cardiovascular disease and cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;327:2334-2347.
- National Institutes of Health Office of Dietary Supplements. Vitamin A and carotenoids. fact sheet for health professionals. Updated June 15, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/
- Keller KL, Fenske NA. Uses of vitamins A, C, and E and related compounds in dermatology: a review. J Am Acad Dermatol. 1998;39:611-625.
- Wright TI, Spencer JM, Flowers FP. Chemoprevention of nonmelanoma skin cancer. J Am Acad Dermatol. 2006;54:933-946; quiz 947-950.
- Bushue N, Wan YJY. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62:1285-1298.
- Stahl W, Sies H. β-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr. 2012;96:1179S-1184S.
- Bukhari MH, Qureshi SS, Niazi S, et al. Chemotherapeutic/chemopreventive role of retinoids in chemically induced skin carcinogenesis in albino mice. Int J Dermatol. 2007;46:1160-1165.
- Lambert LA, Wamer WG, Wei RR, et al. The protective but nonsynergistic effect of dietary beta-carotene and vitamin E on skin tumorigenesis in Skh mice. Nutr Cancer. 1994;21:1-12.
- Greenberg ER, Baron JA, Stukel TA, et al. A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. The Skin Cancer Prevention Study Group. N Engl J Med. 1990;323:789-795.
- Frieling UM, Schaumberg DA, Kupper TS, et al. A randomized, 12-year primary-prevention trial of beta carotene supplementation for nonmelanoma skin cancer in the physician’s health study. Arch Dermatol. 2000;136:179-184.
- Naldi L, Gallus S, Tavani A, et al; Oncology Study Group of the Italian Group for Epidemiologic Research in Dermatology. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13:503-508.
- Zhang YP, Chu RX, Liu H. Vitamin A intake and risk of melanoma: a meta-analysis. PloS One. 2014;9:e102527.
- Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381-1387.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273.
- Solomon-Cohen E, Reiss-Huss S, Hodak E, et al. Low-dose acitretin for secondary prevention of keratinocyte carcinomas in solid-organ transplant recipients. Dermatology. 2022;238:161-166.
- Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568.
- Kadakia KC, Barton DL, Loprinzi CL, et al. Randomized controlled trial of acitretin versus placebo in patients at high-risk for basal cell or squamous cell carcinoma of the skin (North Central Cancer Treatment Group Study 969251). Cancer. 2012;118:2128-2137.
- McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660.
- National Institutes of Health Office of Dietary Supplements. Niacin: fact sheet for health professionals. Updated August 23, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/
- Malesu R, Martin AJ, Lyons JG, et al. Nicotinamide for skin cancer chemoprevention: effects of nicotinamide on melanoma in vitro and in vivo. Photochem Photobiol Sci. 2020;19:171-179.
- Gensler HL. Prevention of photoimmunosuppression and photocarcinogenesis by topical nicotinamide. Nutr Cancer. 1997;29:157-162.
- Gensler HL, Williams T, Huang AC, et al. Oral niacin prevents photocarcinogenesis and photoimmunosuppression in mice. Nutr Cancer. 1999;34:36-41.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Drago F, Ciccarese G, Cogorno L, et al. Prevention of non-melanoma skin cancers with nicotinamide in transplant recipients: a case-control study. Eur J Dermatol. 2017;27:382-385.
- Yélamos O, Halpern AC, Weinstock MA. Reply to “A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients.” Br J Dermatol. 2017;176:551-552.
- Scatozza F, Moschella F, D’Arcangelo D, et al. Nicotinamide inhibits melanoma in vitro and in vivo. J Exp Clin Cancer Res. 2020;39:211.
- National Institutes of Health Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 1, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/
- Butzbach K, Epe B. Photogenotoxicity of folic acid. Free Radic Biol Med. 2013;65:821-827.
- Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036.
- Donnenfeld M, Deschasaux M, Latino-Martel P, et al. Prospective association between dietary folate intake and skin cancer risk: results from the Supplémentation en Vitamines et Minéraux Antioxydants cohort. Am J Clin Nutr. 2015;102:471-478.
- Fung TT, Hunter DJ, Spiegelman D, et al. Vitamins and carotenoids intake and the risk of basal cell carcinoma of the skin in women (United States). Cancer Causes Control. 2002;13:221-230.
- Fung TT, Spiegelman D, Egan KM, et al. Vitamin and carotenoid intake and risk of squamous cell carcinoma of the skin. Int J Cancer. 2003;103:110-115.
- National Institutes of Health Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/
- Spoelstra-de Man AME, Elbers PWG, Oudemans-Van Straaten HM. Vitamin C: should we supplement? Curr Opin Crit Care. 2018;24:248-255.
- Moison RMW, Beijersbergen van Henegouwen GMJ. Topical antioxidant vitamins C and E prevent UVB-radiation-induced peroxidation of eicosapentaenoic acid in pig skin. Radiat Res. 2002;157:402-409.
- Lin JY, Selim MA, Shea CR, et al. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J Am Acad Dermatol. 2003;48:866-874.
- Pauling L, Willoughby R, Reynolds R, et al. Incidence of squamous cell carcinoma in hairless mice irradiated with ultraviolet light in relation to intake of ascorbic acid (vitamin C) and of D, L-alpha-tocopheryl acetate (vitamin E). Int J Vitam Nutr Res Suppl. 1982;23:53-82.
- Kune GA, Bannerman S, Field B, et al. Diet, alcohol, smoking, serum beta-carotene, and vitamin A in male nonmelanocytic skin cancer patients and controls. Nutr Cancer. 1992;18:237-244.
- Vural P, Canbaz M, Selçuki D. Plasma antioxidant defense in actinic keratosis and basal cell carcinoma. J Eur Acad Dermatol Venereol. 1999;13:96-101.
- Record IR, Dreosti IE, McInerney JK. Changes in plasma antioxidant status following consumption of diets high or low in fruit and vegetables or following dietary supplementation with an antioxidant mixture. Br J Nutr. 2001;85:459-464.
- Heinen MM, Hughes MC, Ibiebele TI, et al. Intake of antioxidant nutrients and the risk of skin cancer. Eur J Cancer. 2007;43:2707-2716.
- Yang G, Yan Y, Ma Y, et al. Vitamin C at high concentrations induces cytotoxicity in malignant melanoma but promotes tumor growth at low concentrations. Mol Carcinog. 2017;56:1965-1976.
- National Institutes of Health Office of Dietary Supplements. Vitamin D: fact sheet for health professionals. Updated August 12, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Reichrath J, Saternus R, Vogt T. Endocrine actions of vitamin D in skin: relevance for photocarcinogenesis of non-melanoma skin cancer, and beyond. Mol Cell Endocrinol. 2017;453:96-102.
- Ellison TI, Smith MK, Gilliam AC, et al. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008;128:2508-2517.
- Eide MJ, Johnson DA, Jacobsen GR, et al. Vitamin D and nonmelanoma skin cancer in a health maintenance organization cohort. Arch Dermatol. 2011;147:1379-1384.
- van der Pols JC, Russell A, Bauer U, et al. Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community. J Invest Dermatol. 2013;133:637-641.
- Caini S, Gnagnarella P, Stanganelli I, et al. Vitamin D and the risk of non-melanoma skin cancer: a systematic literature review and meta-analysis on behalf of the Italian Melanoma Intergroup. Cancers (Basel). 2021;13:4815.
- Park SM, Li T, Wu S, et al. Vitamin D intake and risk of skin cancer in US women and men. PLoS One. 2016;11:e0160308.
- Afzal S, Nordestgaard BG, Bojesen SE. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: a prospective cohort study. J Invest Dermatol. 2013;133:629-636.
- Asgari MM, Tang J, Warton ME, et al. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J Invest Dermatol. 2010;130:1438-1443.
- Tang JY, Parimi N, Wu A, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387-391.
- Keen MA, Hassan I. Vitamin E in dermatology. Indian Dermatol Online J. 2016;7:311-315.
- National Institutes of Health Office of Dietary Supplements. Vitamin E: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/
- Pearson P, Lewis SA, Britton J, et al. The pro-oxidant activity of high-dose vitamin E supplements in vivo. BioDrugs. 2006;20:271-273.
- Gerrish KE, Gensler HL. Prevention of photocarcinogenesis by dietary vitamin E. Nutr Cancer. 1993;19:125-133.
- McVean M, Liebler DC. Prevention of DNA photodamage by vitamin E compounds and sunscreens: roles of ultraviolet absorbance and cellular uptake. Mol Carcinog. 1999;24:169-176.
- Prasad KN, Cohrs RJ, Sharma OK. Decreased expressions of c-myc and H-ras oncogenes in vitamin E succinate induced morphologically differentiated murine B-16 melanoma cells in culture. Biochem Cell Biol. 1990;68:1250-1255.
- Funasaka Y, Komoto M, Ichihashi M. Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment Cell Res. 2000;13(suppl 8):170-174.
- National Institutes of Health Office of Dietary Supplements. Selenium: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/
- Sengupta A, Lichti UF, Carlson BA, et al. Selenoproteins are essential for proper keratinocyte function and skin development. PLoS One. 2010;5:e12249.
- Das RK, Hossain SKU, Bhattacharya S. Diphenylmethyl selenocyanate inhibits DMBA-croton oil induced two-stage mouse skin carcinogenesis by inducing apoptosis and inhibiting cutaneous cell proliferation. Cancer Lett. 2005;230:90-101.
- Clark LC, Combs GF Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957-1963.
- Duffield-Lillico AJ, Slate EH, Reid ME, et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477-1481.
- Reid ME, Duffield-Lillico AJ, Slate E, et al. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr Cancer. 2008;60:155-163.
- Vinceti M, Filippini T, Del Giovane C, et al. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018;1:CD005195.
- Hercberg S, Ezzedine K, Guinot C, et al. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr. 2007;137:2098-2105.
- Chang YJ, Myung SK, Chung ST, et al. Effects of vitamin treatment or supplements with purported antioxidant properties on skin cancer prevention: a meta-analysis of randomized controlled trials. Dermatology. 2011;223:36-44.
- Sun W, Rice MS, Park MK, et al. Intake of furocoumarins and risk of skin cancer in 2 prospective US cohort studies. J Nutr. 2020;150:1535-1544.
- Sakaki JR, Melough MM, Roberts MB, et al. Citrus consumption and the risk of non-melanoma skin cancer in the Women’s Health Initiative. Cancers (Basel). 2021;13:2173.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
- Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768.
- Leiter U, Keim U, Garbe C. Epidemiology of skin cancer: update 2019. In: Reichrath J, ed. Sunlight, Vitamin D and Skin Cancer. Springer International Publishing; 2020:123-139.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
- Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2017;76(3S1):S100-S109.
- Pleasance ED, Cheetham RK, Stephens PJ, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191-196.
- Baek J, Lee MG. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016;21:164-169.
- Katta R, Brown DN. Diet and skin cancer: the potential role of dietary antioxidants in nonmelanoma skin cancer prevention. J Skin Cancer. 2015;2015:893149.
- Stoj V, Shahriari N, Shao K, et al. Nutrition and nonmelanoma skin cancers. Clin Dermatol. 2022;40:173-185.
- O’Connor EA, Evans CV, Ivlev I, et al. Vitamin and mineral supplements for the primary prevention of cardiovascular disease and cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;327:2334-2347.
- National Institutes of Health Office of Dietary Supplements. Vitamin A and carotenoids. fact sheet for health professionals. Updated June 15, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/
- Keller KL, Fenske NA. Uses of vitamins A, C, and E and related compounds in dermatology: a review. J Am Acad Dermatol. 1998;39:611-625.
- Wright TI, Spencer JM, Flowers FP. Chemoprevention of nonmelanoma skin cancer. J Am Acad Dermatol. 2006;54:933-946; quiz 947-950.
- Bushue N, Wan YJY. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62:1285-1298.
- Stahl W, Sies H. β-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr. 2012;96:1179S-1184S.
- Bukhari MH, Qureshi SS, Niazi S, et al. Chemotherapeutic/chemopreventive role of retinoids in chemically induced skin carcinogenesis in albino mice. Int J Dermatol. 2007;46:1160-1165.
- Lambert LA, Wamer WG, Wei RR, et al. The protective but nonsynergistic effect of dietary beta-carotene and vitamin E on skin tumorigenesis in Skh mice. Nutr Cancer. 1994;21:1-12.
- Greenberg ER, Baron JA, Stukel TA, et al. A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. The Skin Cancer Prevention Study Group. N Engl J Med. 1990;323:789-795.
- Frieling UM, Schaumberg DA, Kupper TS, et al. A randomized, 12-year primary-prevention trial of beta carotene supplementation for nonmelanoma skin cancer in the physician’s health study. Arch Dermatol. 2000;136:179-184.
- Naldi L, Gallus S, Tavani A, et al; Oncology Study Group of the Italian Group for Epidemiologic Research in Dermatology. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13:503-508.
- Zhang YP, Chu RX, Liu H. Vitamin A intake and risk of melanoma: a meta-analysis. PloS One. 2014;9:e102527.
- Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381-1387.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273.
- Solomon-Cohen E, Reiss-Huss S, Hodak E, et al. Low-dose acitretin for secondary prevention of keratinocyte carcinomas in solid-organ transplant recipients. Dermatology. 2022;238:161-166.
- Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568.
- Kadakia KC, Barton DL, Loprinzi CL, et al. Randomized controlled trial of acitretin versus placebo in patients at high-risk for basal cell or squamous cell carcinoma of the skin (North Central Cancer Treatment Group Study 969251). Cancer. 2012;118:2128-2137.
- McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660.
- National Institutes of Health Office of Dietary Supplements. Niacin: fact sheet for health professionals. Updated August 23, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/
- Malesu R, Martin AJ, Lyons JG, et al. Nicotinamide for skin cancer chemoprevention: effects of nicotinamide on melanoma in vitro and in vivo. Photochem Photobiol Sci. 2020;19:171-179.
- Gensler HL. Prevention of photoimmunosuppression and photocarcinogenesis by topical nicotinamide. Nutr Cancer. 1997;29:157-162.
- Gensler HL, Williams T, Huang AC, et al. Oral niacin prevents photocarcinogenesis and photoimmunosuppression in mice. Nutr Cancer. 1999;34:36-41.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Drago F, Ciccarese G, Cogorno L, et al. Prevention of non-melanoma skin cancers with nicotinamide in transplant recipients: a case-control study. Eur J Dermatol. 2017;27:382-385.
- Yélamos O, Halpern AC, Weinstock MA. Reply to “A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients.” Br J Dermatol. 2017;176:551-552.
- Scatozza F, Moschella F, D’Arcangelo D, et al. Nicotinamide inhibits melanoma in vitro and in vivo. J Exp Clin Cancer Res. 2020;39:211.
- National Institutes of Health Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 1, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/
- Butzbach K, Epe B. Photogenotoxicity of folic acid. Free Radic Biol Med. 2013;65:821-827.
- Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036.
- Donnenfeld M, Deschasaux M, Latino-Martel P, et al. Prospective association between dietary folate intake and skin cancer risk: results from the Supplémentation en Vitamines et Minéraux Antioxydants cohort. Am J Clin Nutr. 2015;102:471-478.
- Fung TT, Hunter DJ, Spiegelman D, et al. Vitamins and carotenoids intake and the risk of basal cell carcinoma of the skin in women (United States). Cancer Causes Control. 2002;13:221-230.
- Fung TT, Spiegelman D, Egan KM, et al. Vitamin and carotenoid intake and risk of squamous cell carcinoma of the skin. Int J Cancer. 2003;103:110-115.
- National Institutes of Health Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/
- Spoelstra-de Man AME, Elbers PWG, Oudemans-Van Straaten HM. Vitamin C: should we supplement? Curr Opin Crit Care. 2018;24:248-255.
- Moison RMW, Beijersbergen van Henegouwen GMJ. Topical antioxidant vitamins C and E prevent UVB-radiation-induced peroxidation of eicosapentaenoic acid in pig skin. Radiat Res. 2002;157:402-409.
- Lin JY, Selim MA, Shea CR, et al. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J Am Acad Dermatol. 2003;48:866-874.
- Pauling L, Willoughby R, Reynolds R, et al. Incidence of squamous cell carcinoma in hairless mice irradiated with ultraviolet light in relation to intake of ascorbic acid (vitamin C) and of D, L-alpha-tocopheryl acetate (vitamin E). Int J Vitam Nutr Res Suppl. 1982;23:53-82.
- Kune GA, Bannerman S, Field B, et al. Diet, alcohol, smoking, serum beta-carotene, and vitamin A in male nonmelanocytic skin cancer patients and controls. Nutr Cancer. 1992;18:237-244.
- Vural P, Canbaz M, Selçuki D. Plasma antioxidant defense in actinic keratosis and basal cell carcinoma. J Eur Acad Dermatol Venereol. 1999;13:96-101.
- Record IR, Dreosti IE, McInerney JK. Changes in plasma antioxidant status following consumption of diets high or low in fruit and vegetables or following dietary supplementation with an antioxidant mixture. Br J Nutr. 2001;85:459-464.
- Heinen MM, Hughes MC, Ibiebele TI, et al. Intake of antioxidant nutrients and the risk of skin cancer. Eur J Cancer. 2007;43:2707-2716.
- Yang G, Yan Y, Ma Y, et al. Vitamin C at high concentrations induces cytotoxicity in malignant melanoma but promotes tumor growth at low concentrations. Mol Carcinog. 2017;56:1965-1976.
- National Institutes of Health Office of Dietary Supplements. Vitamin D: fact sheet for health professionals. Updated August 12, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Reichrath J, Saternus R, Vogt T. Endocrine actions of vitamin D in skin: relevance for photocarcinogenesis of non-melanoma skin cancer, and beyond. Mol Cell Endocrinol. 2017;453:96-102.
- Ellison TI, Smith MK, Gilliam AC, et al. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008;128:2508-2517.
- Eide MJ, Johnson DA, Jacobsen GR, et al. Vitamin D and nonmelanoma skin cancer in a health maintenance organization cohort. Arch Dermatol. 2011;147:1379-1384.
- van der Pols JC, Russell A, Bauer U, et al. Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community. J Invest Dermatol. 2013;133:637-641.
- Caini S, Gnagnarella P, Stanganelli I, et al. Vitamin D and the risk of non-melanoma skin cancer: a systematic literature review and meta-analysis on behalf of the Italian Melanoma Intergroup. Cancers (Basel). 2021;13:4815.
- Park SM, Li T, Wu S, et al. Vitamin D intake and risk of skin cancer in US women and men. PLoS One. 2016;11:e0160308.
- Afzal S, Nordestgaard BG, Bojesen SE. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: a prospective cohort study. J Invest Dermatol. 2013;133:629-636.
- Asgari MM, Tang J, Warton ME, et al. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J Invest Dermatol. 2010;130:1438-1443.
- Tang JY, Parimi N, Wu A, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387-391.
- Keen MA, Hassan I. Vitamin E in dermatology. Indian Dermatol Online J. 2016;7:311-315.
- National Institutes of Health Office of Dietary Supplements. Vitamin E: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/
- Pearson P, Lewis SA, Britton J, et al. The pro-oxidant activity of high-dose vitamin E supplements in vivo. BioDrugs. 2006;20:271-273.
- Gerrish KE, Gensler HL. Prevention of photocarcinogenesis by dietary vitamin E. Nutr Cancer. 1993;19:125-133.
- McVean M, Liebler DC. Prevention of DNA photodamage by vitamin E compounds and sunscreens: roles of ultraviolet absorbance and cellular uptake. Mol Carcinog. 1999;24:169-176.
- Prasad KN, Cohrs RJ, Sharma OK. Decreased expressions of c-myc and H-ras oncogenes in vitamin E succinate induced morphologically differentiated murine B-16 melanoma cells in culture. Biochem Cell Biol. 1990;68:1250-1255.
- Funasaka Y, Komoto M, Ichihashi M. Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment Cell Res. 2000;13(suppl 8):170-174.
- National Institutes of Health Office of Dietary Supplements. Selenium: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/
- Sengupta A, Lichti UF, Carlson BA, et al. Selenoproteins are essential for proper keratinocyte function and skin development. PLoS One. 2010;5:e12249.
- Das RK, Hossain SKU, Bhattacharya S. Diphenylmethyl selenocyanate inhibits DMBA-croton oil induced two-stage mouse skin carcinogenesis by inducing apoptosis and inhibiting cutaneous cell proliferation. Cancer Lett. 2005;230:90-101.
- Clark LC, Combs GF Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957-1963.
- Duffield-Lillico AJ, Slate EH, Reid ME, et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477-1481.
- Reid ME, Duffield-Lillico AJ, Slate E, et al. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr Cancer. 2008;60:155-163.
- Vinceti M, Filippini T, Del Giovane C, et al. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018;1:CD005195.
- Hercberg S, Ezzedine K, Guinot C, et al. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr. 2007;137:2098-2105.
- Chang YJ, Myung SK, Chung ST, et al. Effects of vitamin treatment or supplements with purported antioxidant properties on skin cancer prevention: a meta-analysis of randomized controlled trials. Dermatology. 2011;223:36-44.
- Sun W, Rice MS, Park MK, et al. Intake of furocoumarins and risk of skin cancer in 2 prospective US cohort studies. J Nutr. 2020;150:1535-1544.
- Sakaki JR, Melough MM, Roberts MB, et al. Citrus consumption and the risk of non-melanoma skin cancer in the Women’s Health Initiative. Cancers (Basel). 2021;13:2173.
Practice Points
- Melanoma and nonmelanoma skin cancer (NMSC) are 2 of the most frequently diagnosed cancers in the United States. UV radiation plays a key role in the pathogenesis of both.
- Dietary antioxidants may mechanistically decrease DNA damage caused by UV radiation and could play a potential role in the prevention or development of melanoma and NMSC.
Epidermal Growth Factor Receptor Inhibitor–Induced Symmetrical Drug-Related Intertriginous and Flexural Exanthema: Should You Discontinue the Offending Agent?
Epidermal growth factor receptor (EGFR) inhibitors cause numerous cutaneous adverse events (AEs), including papulopustular eruptions, paronychia, acral fissures, xerosis, alopecia, and trichomegaly.1 Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an uncommon type IV hypersensitivity reaction reported most commonly in association with β-lactam antibiotics and other medications.2 Treatment of SDRIFE generally involves withdrawing the inciting medication; however, in SDRIFE secondary to oncologic therapies, medication withdrawal may not be feasible or desirable. We present 2 cases of SDRIFE secondary to EGFR inhibitors in which treatment was continued alongside supportive skin-directed therapies. We also review the literature.
Case Reports
Patient 1—A 65-year-old man with stage IV non–small cell lung cancer presented to the dermatology clinic with an eruption of 2 months’ duration that began in the periumbilical area and spread to the perianal area within 2 weeks of starting treatment with lazertinib and amivantamab. Physical examination was notable for Common Terminology Criteria for Adverse Events (CTCAE) Grade 2 periumbilical erythema and erosions as well as symmetric red-brown patches with linear erosions in the gluteal cleft (Figure 1) and Grade 2 facial papulopustular rash. Herpes simplex virus polymerase chain reaction and bacterial culture were negative. A skin biopsy from the left buttock revealed dermal edema and a perivascular lymphocytic infiltrate compatible with SDRIFE. Triamcinolone ointment 0.1% twice daily was initiated, then uptitrated to betamethasone ointment 0.05% twice daily with moderate improvement. The patient had a treatment interruption due to malignancy complications, at which time his skin improved, with recurrence of the eruption after treatment re-initiation. He resumed skin-directed treatment and was maintained on betamethasone ointment 0.05% and tacrolimus ointment 0.1% twice daily on alternating days. This treatment was continued for 4 months before the patient died from complications of the malignancy.
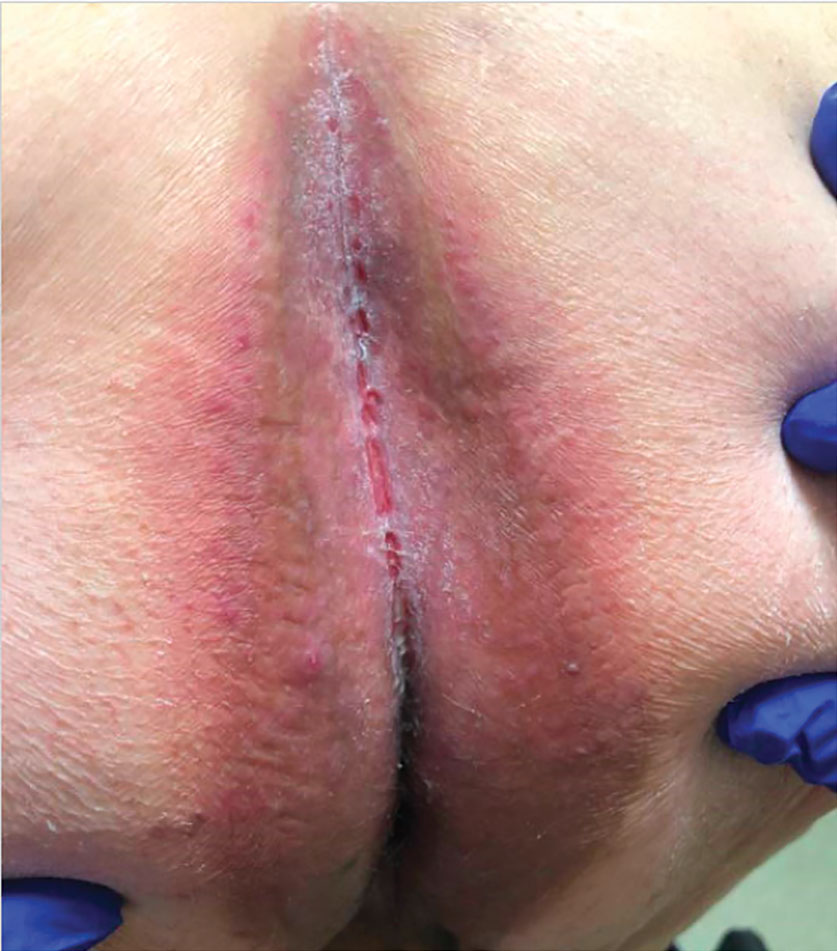
Patient 2—A 68-year-old woman with stage IV lung adenocarcinoma presented to the dermatology clinic with a rash of 3 weeks’ duration. Treatment with osimertinib was initiated 8 months prior to presentation, and there were no recent medication changes. Physical examination revealed CTCAE Grade 2 erythematous patches in the inguinal folds (Figure 2A), inframammary folds (Figure 2B), and on the nasal tip, as well as Grade 2 paronychia. The patient was managed with hydrocortisone cream 1% twice daily, and osimertinib was continued. At follow-up 4 weeks later, the erythema had faded to hyperpigmentation in affected areas with resolution of symptoms. No further treatment was required.
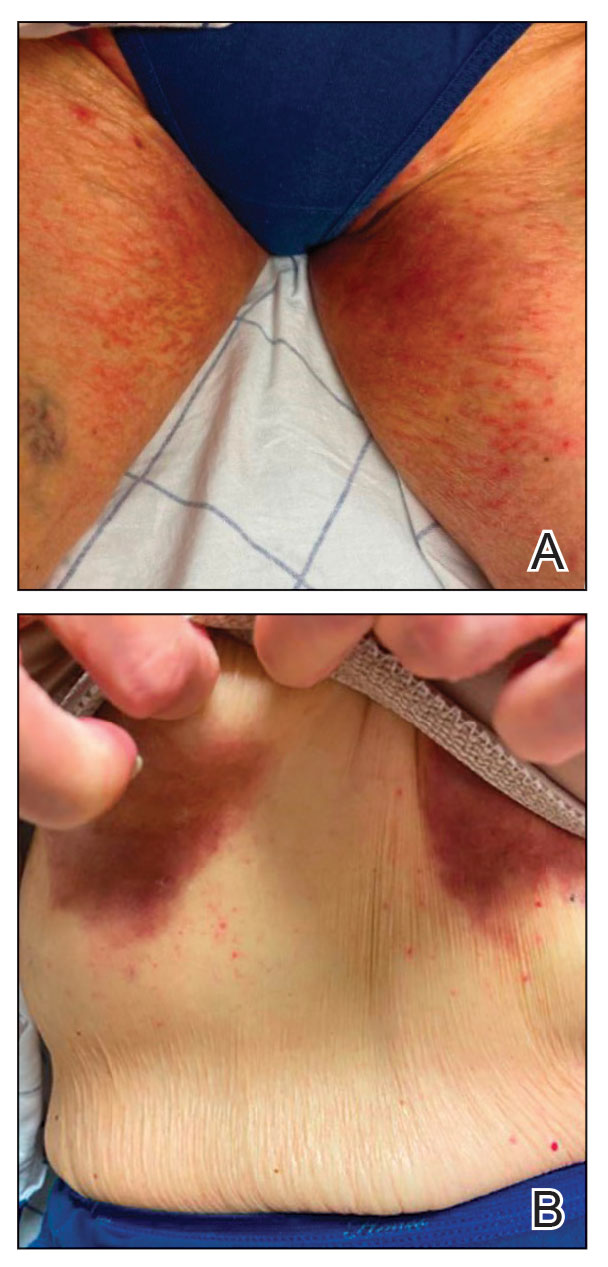
Comment
Supportive oncodermatologists and dermatology hospitalists should be aware of SDRIFE as an uncommon but increasingly recognized cutaneous AE of EGFR inhibitors. Other cases of SDRIFE secondary to EGFR inhibition are described in the Table.2-5 Although SDRIFE typically is treated by discontinuation of the offending agent, in all reported cases of EGFR inhibitor–associated SDRIFE the rash was CTCAE Grade 2, meaning that it did not interfere with instrumental activities of daily living. In 5 of 6 cases, EGFR therapy was continued while skin-directed therapies were used for symptom management.
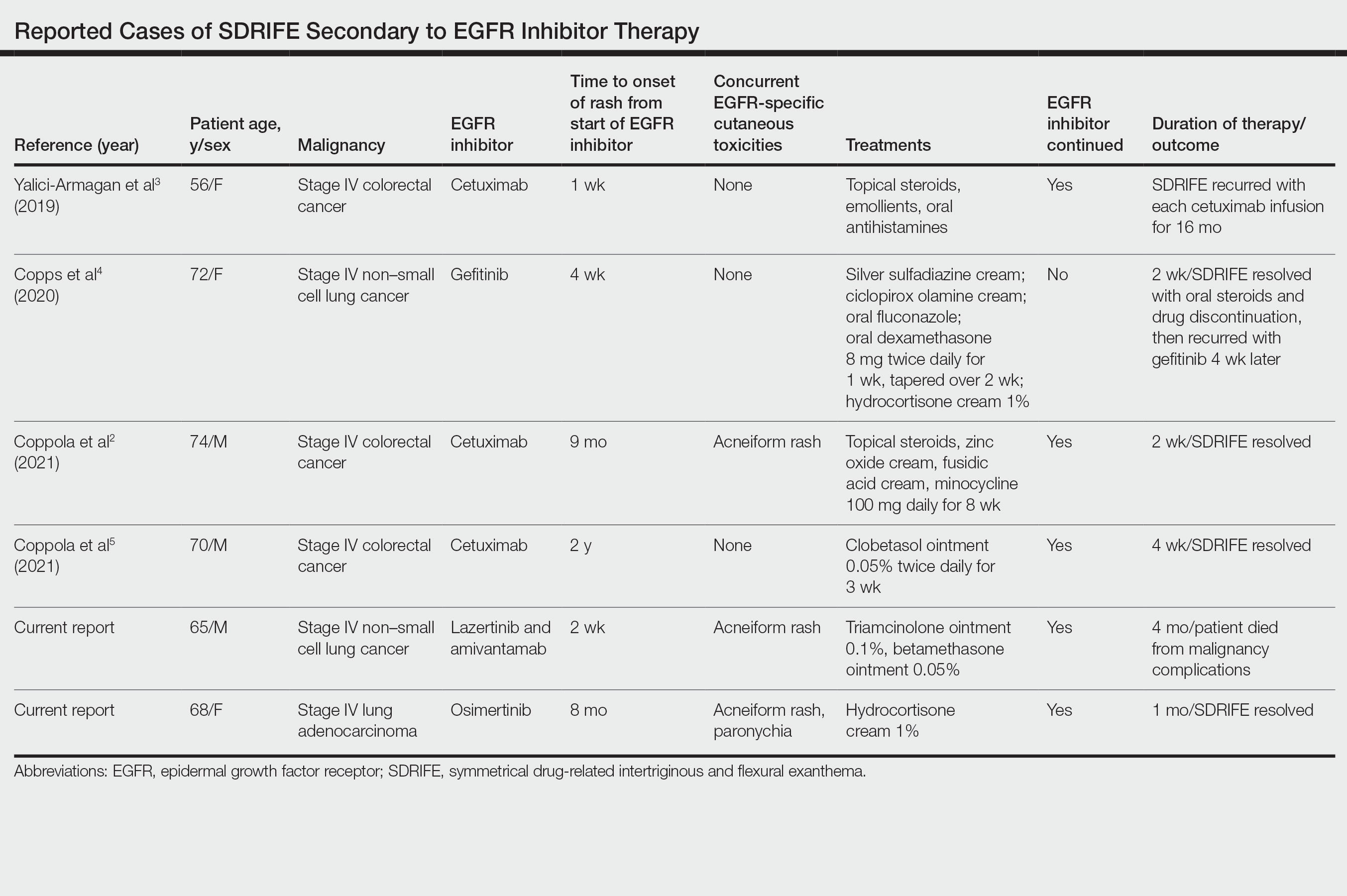
Presentation of SDRIFE—Symmetrical drug-related intertriginous and flexural exanthema is characterized by a symmetric, sharply demarcated erythema in the inguinal, gluteal, or perianal area with at least 1 other flexural localization involved in the absence of systemic signs. It is observed most frequently at initial exposure or re-exposure to a medication. Onset typically is within a few hours to a few days after exposure to a medication.6 Interestingly, in this case series, half of reported SDRIFE cases developed 8 months or more after EGFR inhibitor initiation.
Pathophysiology of SDRIFE—The mechanism of SDRIFE has not been clearly elucidated; it generally is accepted to be a delayed-type hypersensitivity drug reaction, though other proposed pathophysiologic mechanisms for the distribution of SDRIFE include recall phenomenon or predisposing anatomic factors such as temperature, humidity, and apocrine or eccrine gland density.6,7 Epidermal growth factor receptor plays a critical role in regulating differentiation and proliferation of epidermal keratinocytes, hair follicles, and the sweat gland apparatus. Additionally, it has been hypothesized that EGFR inhibitor use may affect the microflora of the skin and that EGFR inhibitors directly affect the immune system, as demonstrated in an experiment showing EGFR inhibitor–treated mice had enhanced skin inflammation and contact hypersensitivity responses.8 How these disparate mechanisms may interact to produce SDRIFE and the reason for the notably delayed presentation of SDRIFE in half of the cases we reviewed is not known. Other delayed cutaneous AEs of EGFR inhibitor therapy, such as paronychia, are thought to be secondary to development of skin fragility and decreased keratinocyte proliferation with secondary infection.1 It is conceivable that a combination of proliferative, immunologic, and microbiome-related factors may each be playing a role in EGFR inhibitor–related SDRIFE.
Dermatology Inpatient Considerations—As seen in our cases, dermatologists can play a valuable role in diagnosing, grading, and managing cutaneous AEs associated with the administration of oncologic therapies. The array of cutaneous AEs has grown as cancer treatment options have expanded from conventional antimetabolite agents to kinase inhibitors and immune checkpoint inhibitors. Dermatologists may play an important role in differentiating the etiology of a skin finding (eg, infectious vs inflammatory) and can identify serious or dose-limiting reactions, such as Stevens-Johnson syndrome or drug reaction with eosinophilia and systemic symptoms (DRESS). If cutaneous AEs appear to occur secondary to administration of a chemotherapeutic agent, use of the National Cancer Institute CTCAE should be employed. For certain AEs (eg, alopecia, acneiform rashes, bullous dermatitis), specific grading has been developed based on a combination of body surface area involved, psychosocial impact, symptoms, and other associated morbidity.9
In management of chemotherapy-associated cutaneous AEs, dermatologists are likely to be the members of the health care team most comfortable with prescribing high-potency anti-inflammatory topical medications. Dermatologic consultation for management of cutaneous AEs has been shown to both reduce the need for systemic immunosuppression and limit interruptions in oncologic treatment.10
Conclusion
Epidermal growth factor receptor inhibitors commonly are prescribed for colorectal cancer, non–small cell lung cancer, and squamous cell carcinoma of the head and neck. They are associated with a variety of cutaneous AEs, including acneiform eruptions, paronychia, and xerosis, which rarely necessitate stopping EGFR inhibitor therapy. Our cases support an approach to managing EGFR inhibitor–related SDRIFE that does not involve discontinuation of the offending agent. Further studies are needed on the best supportive topical and systemic regimens for EGFR inhibitor–associated SDRIFE.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317-326.
- Coppola R, Santo B, Silipigni S, et al. Symmetrical drug-related intertriginous and flexural exanthema and acneiform eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:331-332.
- Yalici-Armagan B, Ayanoglu BT, Demirdag HG. Targeted tumour therapy induced papulopustular rash and other dermatologic side effects: a retrospective study. Cutan Ocul Toxicol. 2019;38:261-266.
- Copps B, Lacroix JP, Sasseville D. Symmetrical drug-related intertriginous and flexural exanthema secondary to epidermal growth factor receptor inhibitor gefitinib. JAAD Case Rep. 2020;6:172-175.
- Coppola R, Santo B, Ramella S, et al. Novel skin toxicity of epidermal growth factor receptor inhibitors: a case of intertrigo-like eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:91-92.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events: (CTCAE), Version 5.0. US Department of Health and Human Services; 2017. Accessed December 16, 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- Chen ST, Molina GE, Lo JA, et al. Dermatology consultation reduces interruption of oncologic management among hospitalized patients with immune-related adverse events: a retrospective cohort study. J Am Acad Dermatol. 2020;82:994-996.
Epidermal growth factor receptor (EGFR) inhibitors cause numerous cutaneous adverse events (AEs), including papulopustular eruptions, paronychia, acral fissures, xerosis, alopecia, and trichomegaly.1 Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an uncommon type IV hypersensitivity reaction reported most commonly in association with β-lactam antibiotics and other medications.2 Treatment of SDRIFE generally involves withdrawing the inciting medication; however, in SDRIFE secondary to oncologic therapies, medication withdrawal may not be feasible or desirable. We present 2 cases of SDRIFE secondary to EGFR inhibitors in which treatment was continued alongside supportive skin-directed therapies. We also review the literature.
Case Reports
Patient 1—A 65-year-old man with stage IV non–small cell lung cancer presented to the dermatology clinic with an eruption of 2 months’ duration that began in the periumbilical area and spread to the perianal area within 2 weeks of starting treatment with lazertinib and amivantamab. Physical examination was notable for Common Terminology Criteria for Adverse Events (CTCAE) Grade 2 periumbilical erythema and erosions as well as symmetric red-brown patches with linear erosions in the gluteal cleft (Figure 1) and Grade 2 facial papulopustular rash. Herpes simplex virus polymerase chain reaction and bacterial culture were negative. A skin biopsy from the left buttock revealed dermal edema and a perivascular lymphocytic infiltrate compatible with SDRIFE. Triamcinolone ointment 0.1% twice daily was initiated, then uptitrated to betamethasone ointment 0.05% twice daily with moderate improvement. The patient had a treatment interruption due to malignancy complications, at which time his skin improved, with recurrence of the eruption after treatment re-initiation. He resumed skin-directed treatment and was maintained on betamethasone ointment 0.05% and tacrolimus ointment 0.1% twice daily on alternating days. This treatment was continued for 4 months before the patient died from complications of the malignancy.

Patient 2—A 68-year-old woman with stage IV lung adenocarcinoma presented to the dermatology clinic with a rash of 3 weeks’ duration. Treatment with osimertinib was initiated 8 months prior to presentation, and there were no recent medication changes. Physical examination revealed CTCAE Grade 2 erythematous patches in the inguinal folds (Figure 2A), inframammary folds (Figure 2B), and on the nasal tip, as well as Grade 2 paronychia. The patient was managed with hydrocortisone cream 1% twice daily, and osimertinib was continued. At follow-up 4 weeks later, the erythema had faded to hyperpigmentation in affected areas with resolution of symptoms. No further treatment was required.

Comment
Supportive oncodermatologists and dermatology hospitalists should be aware of SDRIFE as an uncommon but increasingly recognized cutaneous AE of EGFR inhibitors. Other cases of SDRIFE secondary to EGFR inhibition are described in the Table.2-5 Although SDRIFE typically is treated by discontinuation of the offending agent, in all reported cases of EGFR inhibitor–associated SDRIFE the rash was CTCAE Grade 2, meaning that it did not interfere with instrumental activities of daily living. In 5 of 6 cases, EGFR therapy was continued while skin-directed therapies were used for symptom management.

Presentation of SDRIFE—Symmetrical drug-related intertriginous and flexural exanthema is characterized by a symmetric, sharply demarcated erythema in the inguinal, gluteal, or perianal area with at least 1 other flexural localization involved in the absence of systemic signs. It is observed most frequently at initial exposure or re-exposure to a medication. Onset typically is within a few hours to a few days after exposure to a medication.6 Interestingly, in this case series, half of reported SDRIFE cases developed 8 months or more after EGFR inhibitor initiation.
Pathophysiology of SDRIFE—The mechanism of SDRIFE has not been clearly elucidated; it generally is accepted to be a delayed-type hypersensitivity drug reaction, though other proposed pathophysiologic mechanisms for the distribution of SDRIFE include recall phenomenon or predisposing anatomic factors such as temperature, humidity, and apocrine or eccrine gland density.6,7 Epidermal growth factor receptor plays a critical role in regulating differentiation and proliferation of epidermal keratinocytes, hair follicles, and the sweat gland apparatus. Additionally, it has been hypothesized that EGFR inhibitor use may affect the microflora of the skin and that EGFR inhibitors directly affect the immune system, as demonstrated in an experiment showing EGFR inhibitor–treated mice had enhanced skin inflammation and contact hypersensitivity responses.8 How these disparate mechanisms may interact to produce SDRIFE and the reason for the notably delayed presentation of SDRIFE in half of the cases we reviewed is not known. Other delayed cutaneous AEs of EGFR inhibitor therapy, such as paronychia, are thought to be secondary to development of skin fragility and decreased keratinocyte proliferation with secondary infection.1 It is conceivable that a combination of proliferative, immunologic, and microbiome-related factors may each be playing a role in EGFR inhibitor–related SDRIFE.
Dermatology Inpatient Considerations—As seen in our cases, dermatologists can play a valuable role in diagnosing, grading, and managing cutaneous AEs associated with the administration of oncologic therapies. The array of cutaneous AEs has grown as cancer treatment options have expanded from conventional antimetabolite agents to kinase inhibitors and immune checkpoint inhibitors. Dermatologists may play an important role in differentiating the etiology of a skin finding (eg, infectious vs inflammatory) and can identify serious or dose-limiting reactions, such as Stevens-Johnson syndrome or drug reaction with eosinophilia and systemic symptoms (DRESS). If cutaneous AEs appear to occur secondary to administration of a chemotherapeutic agent, use of the National Cancer Institute CTCAE should be employed. For certain AEs (eg, alopecia, acneiform rashes, bullous dermatitis), specific grading has been developed based on a combination of body surface area involved, psychosocial impact, symptoms, and other associated morbidity.9
In management of chemotherapy-associated cutaneous AEs, dermatologists are likely to be the members of the health care team most comfortable with prescribing high-potency anti-inflammatory topical medications. Dermatologic consultation for management of cutaneous AEs has been shown to both reduce the need for systemic immunosuppression and limit interruptions in oncologic treatment.10
Conclusion
Epidermal growth factor receptor inhibitors commonly are prescribed for colorectal cancer, non–small cell lung cancer, and squamous cell carcinoma of the head and neck. They are associated with a variety of cutaneous AEs, including acneiform eruptions, paronychia, and xerosis, which rarely necessitate stopping EGFR inhibitor therapy. Our cases support an approach to managing EGFR inhibitor–related SDRIFE that does not involve discontinuation of the offending agent. Further studies are needed on the best supportive topical and systemic regimens for EGFR inhibitor–associated SDRIFE.
Epidermal growth factor receptor (EGFR) inhibitors cause numerous cutaneous adverse events (AEs), including papulopustular eruptions, paronychia, acral fissures, xerosis, alopecia, and trichomegaly.1 Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an uncommon type IV hypersensitivity reaction reported most commonly in association with β-lactam antibiotics and other medications.2 Treatment of SDRIFE generally involves withdrawing the inciting medication; however, in SDRIFE secondary to oncologic therapies, medication withdrawal may not be feasible or desirable. We present 2 cases of SDRIFE secondary to EGFR inhibitors in which treatment was continued alongside supportive skin-directed therapies. We also review the literature.
Case Reports
Patient 1—A 65-year-old man with stage IV non–small cell lung cancer presented to the dermatology clinic with an eruption of 2 months’ duration that began in the periumbilical area and spread to the perianal area within 2 weeks of starting treatment with lazertinib and amivantamab. Physical examination was notable for Common Terminology Criteria for Adverse Events (CTCAE) Grade 2 periumbilical erythema and erosions as well as symmetric red-brown patches with linear erosions in the gluteal cleft (Figure 1) and Grade 2 facial papulopustular rash. Herpes simplex virus polymerase chain reaction and bacterial culture were negative. A skin biopsy from the left buttock revealed dermal edema and a perivascular lymphocytic infiltrate compatible with SDRIFE. Triamcinolone ointment 0.1% twice daily was initiated, then uptitrated to betamethasone ointment 0.05% twice daily with moderate improvement. The patient had a treatment interruption due to malignancy complications, at which time his skin improved, with recurrence of the eruption after treatment re-initiation. He resumed skin-directed treatment and was maintained on betamethasone ointment 0.05% and tacrolimus ointment 0.1% twice daily on alternating days. This treatment was continued for 4 months before the patient died from complications of the malignancy.

Patient 2—A 68-year-old woman with stage IV lung adenocarcinoma presented to the dermatology clinic with a rash of 3 weeks’ duration. Treatment with osimertinib was initiated 8 months prior to presentation, and there were no recent medication changes. Physical examination revealed CTCAE Grade 2 erythematous patches in the inguinal folds (Figure 2A), inframammary folds (Figure 2B), and on the nasal tip, as well as Grade 2 paronychia. The patient was managed with hydrocortisone cream 1% twice daily, and osimertinib was continued. At follow-up 4 weeks later, the erythema had faded to hyperpigmentation in affected areas with resolution of symptoms. No further treatment was required.

Comment
Supportive oncodermatologists and dermatology hospitalists should be aware of SDRIFE as an uncommon but increasingly recognized cutaneous AE of EGFR inhibitors. Other cases of SDRIFE secondary to EGFR inhibition are described in the Table.2-5 Although SDRIFE typically is treated by discontinuation of the offending agent, in all reported cases of EGFR inhibitor–associated SDRIFE the rash was CTCAE Grade 2, meaning that it did not interfere with instrumental activities of daily living. In 5 of 6 cases, EGFR therapy was continued while skin-directed therapies were used for symptom management.

Presentation of SDRIFE—Symmetrical drug-related intertriginous and flexural exanthema is characterized by a symmetric, sharply demarcated erythema in the inguinal, gluteal, or perianal area with at least 1 other flexural localization involved in the absence of systemic signs. It is observed most frequently at initial exposure or re-exposure to a medication. Onset typically is within a few hours to a few days after exposure to a medication.6 Interestingly, in this case series, half of reported SDRIFE cases developed 8 months or more after EGFR inhibitor initiation.
Pathophysiology of SDRIFE—The mechanism of SDRIFE has not been clearly elucidated; it generally is accepted to be a delayed-type hypersensitivity drug reaction, though other proposed pathophysiologic mechanisms for the distribution of SDRIFE include recall phenomenon or predisposing anatomic factors such as temperature, humidity, and apocrine or eccrine gland density.6,7 Epidermal growth factor receptor plays a critical role in regulating differentiation and proliferation of epidermal keratinocytes, hair follicles, and the sweat gland apparatus. Additionally, it has been hypothesized that EGFR inhibitor use may affect the microflora of the skin and that EGFR inhibitors directly affect the immune system, as demonstrated in an experiment showing EGFR inhibitor–treated mice had enhanced skin inflammation and contact hypersensitivity responses.8 How these disparate mechanisms may interact to produce SDRIFE and the reason for the notably delayed presentation of SDRIFE in half of the cases we reviewed is not known. Other delayed cutaneous AEs of EGFR inhibitor therapy, such as paronychia, are thought to be secondary to development of skin fragility and decreased keratinocyte proliferation with secondary infection.1 It is conceivable that a combination of proliferative, immunologic, and microbiome-related factors may each be playing a role in EGFR inhibitor–related SDRIFE.
Dermatology Inpatient Considerations—As seen in our cases, dermatologists can play a valuable role in diagnosing, grading, and managing cutaneous AEs associated with the administration of oncologic therapies. The array of cutaneous AEs has grown as cancer treatment options have expanded from conventional antimetabolite agents to kinase inhibitors and immune checkpoint inhibitors. Dermatologists may play an important role in differentiating the etiology of a skin finding (eg, infectious vs inflammatory) and can identify serious or dose-limiting reactions, such as Stevens-Johnson syndrome or drug reaction with eosinophilia and systemic symptoms (DRESS). If cutaneous AEs appear to occur secondary to administration of a chemotherapeutic agent, use of the National Cancer Institute CTCAE should be employed. For certain AEs (eg, alopecia, acneiform rashes, bullous dermatitis), specific grading has been developed based on a combination of body surface area involved, psychosocial impact, symptoms, and other associated morbidity.9
In management of chemotherapy-associated cutaneous AEs, dermatologists are likely to be the members of the health care team most comfortable with prescribing high-potency anti-inflammatory topical medications. Dermatologic consultation for management of cutaneous AEs has been shown to both reduce the need for systemic immunosuppression and limit interruptions in oncologic treatment.10
Conclusion
Epidermal growth factor receptor inhibitors commonly are prescribed for colorectal cancer, non–small cell lung cancer, and squamous cell carcinoma of the head and neck. They are associated with a variety of cutaneous AEs, including acneiform eruptions, paronychia, and xerosis, which rarely necessitate stopping EGFR inhibitor therapy. Our cases support an approach to managing EGFR inhibitor–related SDRIFE that does not involve discontinuation of the offending agent. Further studies are needed on the best supportive topical and systemic regimens for EGFR inhibitor–associated SDRIFE.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317-326.
- Coppola R, Santo B, Silipigni S, et al. Symmetrical drug-related intertriginous and flexural exanthema and acneiform eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:331-332.
- Yalici-Armagan B, Ayanoglu BT, Demirdag HG. Targeted tumour therapy induced papulopustular rash and other dermatologic side effects: a retrospective study. Cutan Ocul Toxicol. 2019;38:261-266.
- Copps B, Lacroix JP, Sasseville D. Symmetrical drug-related intertriginous and flexural exanthema secondary to epidermal growth factor receptor inhibitor gefitinib. JAAD Case Rep. 2020;6:172-175.
- Coppola R, Santo B, Ramella S, et al. Novel skin toxicity of epidermal growth factor receptor inhibitors: a case of intertrigo-like eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:91-92.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events: (CTCAE), Version 5.0. US Department of Health and Human Services; 2017. Accessed December 16, 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- Chen ST, Molina GE, Lo JA, et al. Dermatology consultation reduces interruption of oncologic management among hospitalized patients with immune-related adverse events: a retrospective cohort study. J Am Acad Dermatol. 2020;82:994-996.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317-326.
- Coppola R, Santo B, Silipigni S, et al. Symmetrical drug-related intertriginous and flexural exanthema and acneiform eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:331-332.
- Yalici-Armagan B, Ayanoglu BT, Demirdag HG. Targeted tumour therapy induced papulopustular rash and other dermatologic side effects: a retrospective study. Cutan Ocul Toxicol. 2019;38:261-266.
- Copps B, Lacroix JP, Sasseville D. Symmetrical drug-related intertriginous and flexural exanthema secondary to epidermal growth factor receptor inhibitor gefitinib. JAAD Case Rep. 2020;6:172-175.
- Coppola R, Santo B, Ramella S, et al. Novel skin toxicity of epidermal growth factor receptor inhibitors: a case of intertrigo-like eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:91-92.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events: (CTCAE), Version 5.0. US Department of Health and Human Services; 2017. Accessed December 16, 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- Chen ST, Molina GE, Lo JA, et al. Dermatology consultation reduces interruption of oncologic management among hospitalized patients with immune-related adverse events: a retrospective cohort study. J Am Acad Dermatol. 2020;82:994-996.
Practice Points
- Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an uncommon but increasingly recognized cutaneous adverse event (AE) of epidermal growth factor receptor (EGFR) inhibitors.
- Epidermal growth factor receptor inhibitor–associated SDRIFE may be approached similarly to other EGFR inhibitor–related cutaneous AEs in that it may not require discontinuation of the offending agent.
Topical treatment options for acne continue to expand
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
SAN DIEGO – , according to Lawrence F. Eichenfield, MD.
The product, known as IDP-126 and being developed by Ortho Dermatologics, is a fixed dose triple combination of clindamycin 1.2% plus benzoyl peroxide 3.1% and adapalene 0.15% being evaluated in patients nine years of age and older. According to a 2021 press release from the company, results from a second 12-week pivotal phase 3 trial showed a treatment success of 50.5% and 20.5% for IDP-126 and its vehicle, respectively, along with significant changes from baseline in inflammatory lesion count and non-inflammatory lesion count.
More recently, researchers led by Linda Stein Gold, MD, conducted a 12-week multicenter, randomized, double-blind study of IDP-126 in 741 children, adolescents, and adults with moderate to severe acne. They reported 52.5% of patients treated with IDP-126 gel achieved treatment success by week 12, with over 70% reduction in inflammatory and noninflammatory lesions.
“This will be interesting to follow as it moves along,” Dr. Eichenfield, chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, said at the annual Masters of Aesthetics Symposium in a presentation on the newest topical acne treatments.
“If approved, we probably will be able to decrease our need for systemic therapies in some individuals,” he said. “It’s something that may become important in practices that mix and match between medical and procedural or surgical approaches to acne.”
Dr. Eichenfield highlighted other products for the topical treatment of acne:
- Trifarotene cream 0.005% (Aklief). In 2019, Food and Drug Administration approval made trifarotene cream the first new retinoid indicated for acne in several decades. It is indicated for the topical treatment of acne vulgaris in patients age 9 and older and has been studied in acne of the face, chest, and back.
- Tazarotene lotion 0.045% (Arazlo). The 0.1% formulation of tazarotene is commonly used for acne, but it can cause skin irritation, dryness, and erythema. The new 0.045% formulation was developed in a three-dimensional mesh matrix, with ingredients from an oil-in-water emulsion. “Many of the new acne products come with a background of vehicle delivery systems that minimize the concentration gradient, so it decreases irritation,” said Dr. Eichenfield, one of the authors of a 2021 review article on the management of acne vulgaris in JAMA. “This has very good efficacy without the traditional irritation of other tazarotene products,” Dr. Eichenfield said.
- Minocycline 4% topical foam (Amzeeq). The 2019 U.S. approval marked the first and so far only topical minocycline prescription treatment for acne. “Its hydrophobic composition allows for stable and efficient delivery of inherently unstable pharmaceutical ingredients,” he said. “It’s generally well tolerated.”
- Clascoterone cream 1% (Winlevi). This first-in-class topical androgen receptor inhibitor is approved for the treatment of acne in patients 12 years and older. It competes with dihydrotestosterone and selectively targets androgen receptors in sebocytes and hair papilla cells. “It is safe for use in men, has been studied on the face and trunk, and has been shown to inhibit sebum production, reduce secretion of inflammatory cytokines, and inhibit inflammatory pathways,” Dr. Eichenfield said.
- Micro-encapsulated benzoyl peroxide 3% and tretinoin 0.1% cream (Twyneo). This is a once-daily fixed-dose combination of tretinoin and benzoyl peroxide indicated for the treatment of acne vulgaris in patients age 9 and older. According to a press release from Sol-Gel, the manufacturer, silica (silicon dioxide) core shell structures separate micro-encapsulate tretinoin crystals and benzoyl peroxide crystals, enabling inclusion of the two active ingredients in the cream.
Dr. Eichenfield disclosed that he has been an investigator and/or consultant for Almirall, Cassiopea, Dermata, Galderma, and Ortho Dermatologics.
AT MOAS 2022
Rituximab worsens COVID-19 outcomes in RA
Key clinical point: The use of rituximab in patients with rheumatoid arthritis (RA) was associated with worse COVID-19 outcomes compared with the use of conventional synthetic disease-modifying antirheumatic drugs (csDMARD).
Major finding: The risk for COVID-19-related hospitalization (adjusted odds ratio [aOR] 2.14; 95% CI 1.51-3.04), intensive care unit admission (aOR 5.22; 95% CI 1.77-15.41), and invasive ventilation (aOR 2.74; 95% CI 1.36-5.51) was significantly higher with baseline use of rituximab vs csDMARD.
Study details: The data come from a retrospective cohort study including 69,549 patients with RA, of which 22,956 patients were diagnosed with COVID-19 and 364 patients were exposed to rituximab prior to the first COVID-19 diagnosis.
Disclosures: This study did not report the source of funding. JA Singh reported receiving consulting fees, owning stock options, serving on speaker’s bureaus, and being a member of various committees.
Source: Singh N et al. Rituximab is associated with worse COVID-19 outcomes in patients with rheumatoid arthritis: A retrospective, nationally sampled cohort study from the U.S. National COVID Cohort Collaborative (N3C). Semin Arthritis Rheum. 2022;58:152149 (Dec 8). Doi: 10.1016/j.semarthrit.2022.152149
Key clinical point: The use of rituximab in patients with rheumatoid arthritis (RA) was associated with worse COVID-19 outcomes compared with the use of conventional synthetic disease-modifying antirheumatic drugs (csDMARD).
Major finding: The risk for COVID-19-related hospitalization (adjusted odds ratio [aOR] 2.14; 95% CI 1.51-3.04), intensive care unit admission (aOR 5.22; 95% CI 1.77-15.41), and invasive ventilation (aOR 2.74; 95% CI 1.36-5.51) was significantly higher with baseline use of rituximab vs csDMARD.
Study details: The data come from a retrospective cohort study including 69,549 patients with RA, of which 22,956 patients were diagnosed with COVID-19 and 364 patients were exposed to rituximab prior to the first COVID-19 diagnosis.
Disclosures: This study did not report the source of funding. JA Singh reported receiving consulting fees, owning stock options, serving on speaker’s bureaus, and being a member of various committees.
Source: Singh N et al. Rituximab is associated with worse COVID-19 outcomes in patients with rheumatoid arthritis: A retrospective, nationally sampled cohort study from the U.S. National COVID Cohort Collaborative (N3C). Semin Arthritis Rheum. 2022;58:152149 (Dec 8). Doi: 10.1016/j.semarthrit.2022.152149
Key clinical point: The use of rituximab in patients with rheumatoid arthritis (RA) was associated with worse COVID-19 outcomes compared with the use of conventional synthetic disease-modifying antirheumatic drugs (csDMARD).
Major finding: The risk for COVID-19-related hospitalization (adjusted odds ratio [aOR] 2.14; 95% CI 1.51-3.04), intensive care unit admission (aOR 5.22; 95% CI 1.77-15.41), and invasive ventilation (aOR 2.74; 95% CI 1.36-5.51) was significantly higher with baseline use of rituximab vs csDMARD.
Study details: The data come from a retrospective cohort study including 69,549 patients with RA, of which 22,956 patients were diagnosed with COVID-19 and 364 patients were exposed to rituximab prior to the first COVID-19 diagnosis.
Disclosures: This study did not report the source of funding. JA Singh reported receiving consulting fees, owning stock options, serving on speaker’s bureaus, and being a member of various committees.
Source: Singh N et al. Rituximab is associated with worse COVID-19 outcomes in patients with rheumatoid arthritis: A retrospective, nationally sampled cohort study from the U.S. National COVID Cohort Collaborative (N3C). Semin Arthritis Rheum. 2022;58:152149 (Dec 8). Doi: 10.1016/j.semarthrit.2022.152149
Losing muscle mass tied to physical dysfunction in early RA
Key clinical point: Myopenia was prevalent and associated with physical dysfunction in patients with early rheumatoid arthritis (RA).
Major finding: The prevalence of myopenia was higher in patients with early RA vs matched control individuals (41.3% vs 15.8%; P < .0167) but similar among patients with early and established RA. Myopenia was independently associated with physical dysfunction in patients with early RA (adjusted odds ratio 2.983; 95% CI 1.192-7.465).
Study details: This cross-sectional study included 1008 patients with RA (early RA n = 190; established RA n = 818) and 2017 control individuals who were age- and sex-matched for comparison.
Disclosures: This study was supported by the National Natural Science Foundation of China and other sources. Two authors declared being employees of Shanghai Healthcare Co. Ltd.
Source: Pan J et al. Muscle mass loss is associated with physical dysfunction in patients with early rheumatoid arthritis. Front Nutr. 2022;9:1007184 (Nov 23). Doi: 10.3389/fnut.2022.1007184
Key clinical point: Myopenia was prevalent and associated with physical dysfunction in patients with early rheumatoid arthritis (RA).
Major finding: The prevalence of myopenia was higher in patients with early RA vs matched control individuals (41.3% vs 15.8%; P < .0167) but similar among patients with early and established RA. Myopenia was independently associated with physical dysfunction in patients with early RA (adjusted odds ratio 2.983; 95% CI 1.192-7.465).
Study details: This cross-sectional study included 1008 patients with RA (early RA n = 190; established RA n = 818) and 2017 control individuals who were age- and sex-matched for comparison.
Disclosures: This study was supported by the National Natural Science Foundation of China and other sources. Two authors declared being employees of Shanghai Healthcare Co. Ltd.
Source: Pan J et al. Muscle mass loss is associated with physical dysfunction in patients with early rheumatoid arthritis. Front Nutr. 2022;9:1007184 (Nov 23). Doi: 10.3389/fnut.2022.1007184
Key clinical point: Myopenia was prevalent and associated with physical dysfunction in patients with early rheumatoid arthritis (RA).
Major finding: The prevalence of myopenia was higher in patients with early RA vs matched control individuals (41.3% vs 15.8%; P < .0167) but similar among patients with early and established RA. Myopenia was independently associated with physical dysfunction in patients with early RA (adjusted odds ratio 2.983; 95% CI 1.192-7.465).
Study details: This cross-sectional study included 1008 patients with RA (early RA n = 190; established RA n = 818) and 2017 control individuals who were age- and sex-matched for comparison.
Disclosures: This study was supported by the National Natural Science Foundation of China and other sources. Two authors declared being employees of Shanghai Healthcare Co. Ltd.
Source: Pan J et al. Muscle mass loss is associated with physical dysfunction in patients with early rheumatoid arthritis. Front Nutr. 2022;9:1007184 (Nov 23). Doi: 10.3389/fnut.2022.1007184
Inflammatory activity needs careful monitoring in late-onset rheumatoid arthritis
Key clinical point: Patients with late-onset rheumatoid arthritis (LORA; disease onset at ≥60 years) had poor control of inflammation after diagnosis compared with those with young-onset RA (YORA), with late onset being associated with a greater cumulative inflammatory burden over time.
Major finding: Moderate-high inflammatory activity (P = .018) was more frequent in LORA vs YORA. The median C-reactive protein (P = .039) and interleukin-6 (P = .045) levels at onset were significantly higher in patients with LORA vs YORA, and late-onset was significantly associated with a high cumulative inflammatory activity of RA (odds ratio 4.694; P = .008).
Study details: This was a nested cohort study of a prospective cohort including 110 patients with incident RA (LORA n = 22; YORA n = 88) and 110 age- and sex-matched control individuals.
Disclosures: This study was supported by grants from Fondos FEDER, University of Malaga, and other sources. The authors declared no conflicts of interest.
Source: Mena-Vázquez N et al. Inflammatory profile of incident cases of late-onset compared with young-onset rheumatoid arthritis: A nested cohort study. Front Med (Lausanne). 2022;9:1016159 (Nov 8). Doi: 10.3389/fmed.2022.1016159
Key clinical point: Patients with late-onset rheumatoid arthritis (LORA; disease onset at ≥60 years) had poor control of inflammation after diagnosis compared with those with young-onset RA (YORA), with late onset being associated with a greater cumulative inflammatory burden over time.
Major finding: Moderate-high inflammatory activity (P = .018) was more frequent in LORA vs YORA. The median C-reactive protein (P = .039) and interleukin-6 (P = .045) levels at onset were significantly higher in patients with LORA vs YORA, and late-onset was significantly associated with a high cumulative inflammatory activity of RA (odds ratio 4.694; P = .008).
Study details: This was a nested cohort study of a prospective cohort including 110 patients with incident RA (LORA n = 22; YORA n = 88) and 110 age- and sex-matched control individuals.
Disclosures: This study was supported by grants from Fondos FEDER, University of Malaga, and other sources. The authors declared no conflicts of interest.
Source: Mena-Vázquez N et al. Inflammatory profile of incident cases of late-onset compared with young-onset rheumatoid arthritis: A nested cohort study. Front Med (Lausanne). 2022;9:1016159 (Nov 8). Doi: 10.3389/fmed.2022.1016159
Key clinical point: Patients with late-onset rheumatoid arthritis (LORA; disease onset at ≥60 years) had poor control of inflammation after diagnosis compared with those with young-onset RA (YORA), with late onset being associated with a greater cumulative inflammatory burden over time.
Major finding: Moderate-high inflammatory activity (P = .018) was more frequent in LORA vs YORA. The median C-reactive protein (P = .039) and interleukin-6 (P = .045) levels at onset were significantly higher in patients with LORA vs YORA, and late-onset was significantly associated with a high cumulative inflammatory activity of RA (odds ratio 4.694; P = .008).
Study details: This was a nested cohort study of a prospective cohort including 110 patients with incident RA (LORA n = 22; YORA n = 88) and 110 age- and sex-matched control individuals.
Disclosures: This study was supported by grants from Fondos FEDER, University of Malaga, and other sources. The authors declared no conflicts of interest.
Source: Mena-Vázquez N et al. Inflammatory profile of incident cases of late-onset compared with young-onset rheumatoid arthritis: A nested cohort study. Front Med (Lausanne). 2022;9:1016159 (Nov 8). Doi: 10.3389/fmed.2022.1016159
Alopecia Areata in Skin of Color Patients: New Considerations Sparked by the Approval of Baricitinib
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
Anti-protein arginine deiminase antibodies associated with radiographic injury in RA
Key clinical point: Anti-protein arginine deiminase 3/4 (anti-PAD3/4) antibodies could help identify patients with rheumatoid arthritis (RA) having higher radiographic injury and bone erosion.
Major finding: Anti-PAD4 antibody levels were significantly associated with radiographic injury (P = .027). Patients with anti-PAD3/4 double positivity vs double negativity had a significantly higher radiographic injury (mean Simple Erosion Narrowing Score 48.7 vs 19.4; P = .04) and joint erosion (mean Joint Erosion Score 25.7 vs 9.3; P = .037).
Study details: This was a retrospective cross-sectional study including 71 patients with RA.
Disclosures: This study did not receive any specific funding. M Mahler and L Martinez-Prat declared being employees of Werfen, a company selling autoimmune diagnostic tests.
Source: Palterer B et al. Anti-protein arginine deiminase antibodies are distinctly associated with joint and lung involvement in rheumatoid arthritis. Rheumatology (Oxford). 2022 (Nov 28). Doi: 10.1093/rheumatology/keac667
Key clinical point: Anti-protein arginine deiminase 3/4 (anti-PAD3/4) antibodies could help identify patients with rheumatoid arthritis (RA) having higher radiographic injury and bone erosion.
Major finding: Anti-PAD4 antibody levels were significantly associated with radiographic injury (P = .027). Patients with anti-PAD3/4 double positivity vs double negativity had a significantly higher radiographic injury (mean Simple Erosion Narrowing Score 48.7 vs 19.4; P = .04) and joint erosion (mean Joint Erosion Score 25.7 vs 9.3; P = .037).
Study details: This was a retrospective cross-sectional study including 71 patients with RA.
Disclosures: This study did not receive any specific funding. M Mahler and L Martinez-Prat declared being employees of Werfen, a company selling autoimmune diagnostic tests.
Source: Palterer B et al. Anti-protein arginine deiminase antibodies are distinctly associated with joint and lung involvement in rheumatoid arthritis. Rheumatology (Oxford). 2022 (Nov 28). Doi: 10.1093/rheumatology/keac667
Key clinical point: Anti-protein arginine deiminase 3/4 (anti-PAD3/4) antibodies could help identify patients with rheumatoid arthritis (RA) having higher radiographic injury and bone erosion.
Major finding: Anti-PAD4 antibody levels were significantly associated with radiographic injury (P = .027). Patients with anti-PAD3/4 double positivity vs double negativity had a significantly higher radiographic injury (mean Simple Erosion Narrowing Score 48.7 vs 19.4; P = .04) and joint erosion (mean Joint Erosion Score 25.7 vs 9.3; P = .037).
Study details: This was a retrospective cross-sectional study including 71 patients with RA.
Disclosures: This study did not receive any specific funding. M Mahler and L Martinez-Prat declared being employees of Werfen, a company selling autoimmune diagnostic tests.
Source: Palterer B et al. Anti-protein arginine deiminase antibodies are distinctly associated with joint and lung involvement in rheumatoid arthritis. Rheumatology (Oxford). 2022 (Nov 28). Doi: 10.1093/rheumatology/keac667
Methotrexate protective against lung function decline and mortality in patients with RA
Key clinical point: The use of methotrexate was protective against lung function decline and mortality in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD).
Major finding: Use of methotrexate was protective against decline in lung function (adjusted odds ratio [aOR] 0.269; P = .014) and mortality (aOR 0.284; P = .029). However, a high erythrocyte sedimentation rate at baseline was a risk factor for decline in lung function (aOR 3.056; P = .021). Age ≥65 years (aOR 2.723; P = .024) and radiologic pattern of usual interstitial pneumonia (UIP) or probable UIP (aOR 3.948; P = .005) were risk factors for mortality.
Study details: The findings are from a retrospective cohort study including 170 patients with RA-ILD who underwent at least one spirometry test and chest computed tomography scan and were treated with methotrexate or oral glucocorticoids.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Kim K et al. Protective effect of methotrexate on lung function and mortality in rheumatoid arthritis–related interstitial lung disease: A retrospective cohort study. Ther Adv Respir Dis. 2022;16:17534666221135314 (Nov 8). Doi: 10.1177/17534666221135314
Key clinical point: The use of methotrexate was protective against lung function decline and mortality in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD).
Major finding: Use of methotrexate was protective against decline in lung function (adjusted odds ratio [aOR] 0.269; P = .014) and mortality (aOR 0.284; P = .029). However, a high erythrocyte sedimentation rate at baseline was a risk factor for decline in lung function (aOR 3.056; P = .021). Age ≥65 years (aOR 2.723; P = .024) and radiologic pattern of usual interstitial pneumonia (UIP) or probable UIP (aOR 3.948; P = .005) were risk factors for mortality.
Study details: The findings are from a retrospective cohort study including 170 patients with RA-ILD who underwent at least one spirometry test and chest computed tomography scan and were treated with methotrexate or oral glucocorticoids.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Kim K et al. Protective effect of methotrexate on lung function and mortality in rheumatoid arthritis–related interstitial lung disease: A retrospective cohort study. Ther Adv Respir Dis. 2022;16:17534666221135314 (Nov 8). Doi: 10.1177/17534666221135314
Key clinical point: The use of methotrexate was protective against lung function decline and mortality in patients with rheumatoid arthritis-associated interstitial lung disease (RA-ILD).
Major finding: Use of methotrexate was protective against decline in lung function (adjusted odds ratio [aOR] 0.269; P = .014) and mortality (aOR 0.284; P = .029). However, a high erythrocyte sedimentation rate at baseline was a risk factor for decline in lung function (aOR 3.056; P = .021). Age ≥65 years (aOR 2.723; P = .024) and radiologic pattern of usual interstitial pneumonia (UIP) or probable UIP (aOR 3.948; P = .005) were risk factors for mortality.
Study details: The findings are from a retrospective cohort study including 170 patients with RA-ILD who underwent at least one spirometry test and chest computed tomography scan and were treated with methotrexate or oral glucocorticoids.
Disclosures: This study did not receive any funding. The authors declared no conflicts of interest.
Source: Kim K et al. Protective effect of methotrexate on lung function and mortality in rheumatoid arthritis–related interstitial lung disease: A retrospective cohort study. Ther Adv Respir Dis. 2022;16:17534666221135314 (Nov 8). Doi: 10.1177/17534666221135314
Sarilumab improves disproportionate articular pain in RA
Key clinical point: In patients with active rheumatoid arthritis (RA), disproportionate articular pain (DP) was prevalent and sarilumab fared better than adalimumab or placebo in reducing DP and achieving clinical outcomes.
Major finding: At baseline, 23% of patients had DP. At week 24, a numerically lower proportion of patients experienced DP (15% vs 27%) and a higher proportion of patients achieved a Clinical Disease Activity Index of ≤10 (40% vs 17%) and American College of Rheumatology 50 response (42% vs 28%) with sarilumab vs adalimumab, with results being similar for sarilumab vs placebo.
Study details: This post hoc analysis of three phase 3 trials and their open label extension studies included 1531 patients with RA and intolerance or inadequate response to methotrexate/tumor necrosis factor inhibitors who received sarilumab, adalimumab, or placebo.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. Three authors declared being employees of or holding stock or stock options in Sanofi. Several authors reported ties with Sanofi, Regeneron Pharmaceuticals, and other sources.
Source: Choy E et al. Disproportionate articular pain is a frequent phenomenon in rheumatoid arthritis and responds to treatment with sarilumab. Rheumatology (Oxford). 2022 (Nov 22). Doi: 10.1093/rheumatology/keac659
Key clinical point: In patients with active rheumatoid arthritis (RA), disproportionate articular pain (DP) was prevalent and sarilumab fared better than adalimumab or placebo in reducing DP and achieving clinical outcomes.
Major finding: At baseline, 23% of patients had DP. At week 24, a numerically lower proportion of patients experienced DP (15% vs 27%) and a higher proportion of patients achieved a Clinical Disease Activity Index of ≤10 (40% vs 17%) and American College of Rheumatology 50 response (42% vs 28%) with sarilumab vs adalimumab, with results being similar for sarilumab vs placebo.
Study details: This post hoc analysis of three phase 3 trials and their open label extension studies included 1531 patients with RA and intolerance or inadequate response to methotrexate/tumor necrosis factor inhibitors who received sarilumab, adalimumab, or placebo.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. Three authors declared being employees of or holding stock or stock options in Sanofi. Several authors reported ties with Sanofi, Regeneron Pharmaceuticals, and other sources.
Source: Choy E et al. Disproportionate articular pain is a frequent phenomenon in rheumatoid arthritis and responds to treatment with sarilumab. Rheumatology (Oxford). 2022 (Nov 22). Doi: 10.1093/rheumatology/keac659
Key clinical point: In patients with active rheumatoid arthritis (RA), disproportionate articular pain (DP) was prevalent and sarilumab fared better than adalimumab or placebo in reducing DP and achieving clinical outcomes.
Major finding: At baseline, 23% of patients had DP. At week 24, a numerically lower proportion of patients experienced DP (15% vs 27%) and a higher proportion of patients achieved a Clinical Disease Activity Index of ≤10 (40% vs 17%) and American College of Rheumatology 50 response (42% vs 28%) with sarilumab vs adalimumab, with results being similar for sarilumab vs placebo.
Study details: This post hoc analysis of three phase 3 trials and their open label extension studies included 1531 patients with RA and intolerance or inadequate response to methotrexate/tumor necrosis factor inhibitors who received sarilumab, adalimumab, or placebo.
Disclosures: This study was funded by Sanofi and Regeneron Pharmaceuticals, Inc. Three authors declared being employees of or holding stock or stock options in Sanofi. Several authors reported ties with Sanofi, Regeneron Pharmaceuticals, and other sources.
Source: Choy E et al. Disproportionate articular pain is a frequent phenomenon in rheumatoid arthritis and responds to treatment with sarilumab. Rheumatology (Oxford). 2022 (Nov 22). Doi: 10.1093/rheumatology/keac659