User login
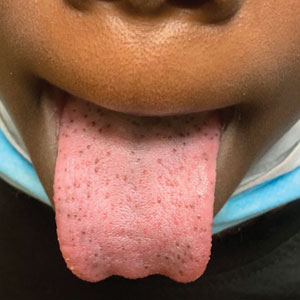
Hyperpigmented Papules on the Tongue of a Child
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
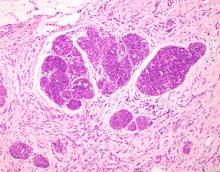
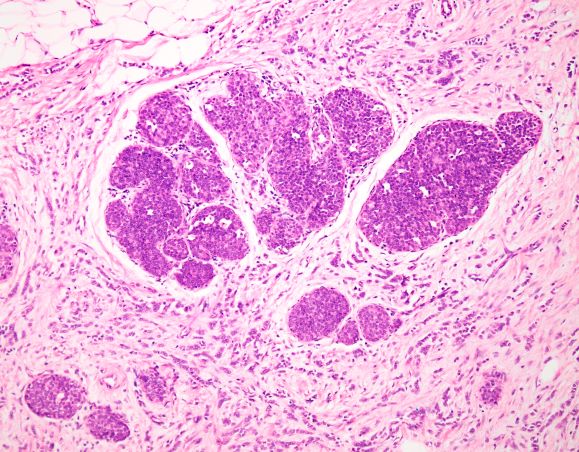
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
A 9-year-old Black boy presented to the dermatology clinic for evaluation of dark spots on the tongue. The family first noted these spots 5 months prior and reported that they remained stable during that time. The patient’s medical history was notable for autism spectrum disorder and multiple food allergies. His family history was negative for similar oral pigmentation or other pigmentary anomalies. A review of systems was positive only for selective eating and rare nosebleeds. Physical examination revealed numerous dark brown, pinpoint papules across the dorsal aspect of the tongue. No hyperpigmentation of the buccal mucosae, lips, palms, or soles was identified. Several light brown streaks were present on the fingernails and toenails, consistent with longitudinal melanonychia. A prior complete blood cell count was within reference range.
Time for a rest
“More than Jews have kept Shabbat, Shabbat has kept the Jews.” – Ahad Ha’am
You should all be well rested by now. After all, we’ve just come through the festive shutdown of the holiday season where all of your pumpkin/peppermint/marshmallow flavored coffees were sipped while walking around in your jimjams at 10 a.m. It was the time of year for you to take time off to get a proper rest and be energized to get back to work. Yet, I’m not feeling it from you.
So let’s talk about burnout – just kidding, that would only make it worse. “Burned-out’’ is a hackneyed and defective phrase to describe what many of us are feeling. We are not “destroyed, gutted by fire or by overheating.” No, we are, as one of our docs put it to me: “Just tired.” Ah, a much better Old English word! “Tired” captures it. It means to feel “in need of rest.” We are not ruined, we are just depleted. We don’t need discarding. We need some rest.
I asked some docs when they thought this feeling of exhaustion first began. We agreed that the pandemic, doubledemic, tripledemic, backlog have taken a toll. But The consumerization of medicine? All factors, but not the beginning. No, the beginning was before paper charts. Well, actually it was before paper. We have to go back to the 5th or 6th century BCE. That is when scholars believe the book of Genesis originated from the Yahwist source. In it, it is written that the 7th day be set aside as a day of rest from labor. It is not written that burnout would ensue if sabbath wasn’t observed; however, if you failed to keep it, then you might have been killed. They took rest seriously back then.
This innovation of setting aside a day each week to rest, reflect, and worship was such a good idea that it was codified as one of the 10 commandments. It spread widely. Early Christians kept the Jewish tradition of observing Shabbat from Friday sundown to Saturday until the ever practical Romans decided that Sunday would be a better day. Sunday was already the day to worship the sun god. The newly-converted Christian Emperor Constantine issued an edict on March 7th, 321 CE that all “city people and craftsmen shall rest from labor upon the venerable day of the sun.” And so Sunday it was.
Protestant Seventh-day denomination churches later shifted sabbath back to Saturday believing that Sunday must have been the Pope’s idea. The best deal seems to have been around 1273 when the Ethiopian Orthodox leader Ewostatewos decreed that both Saturday AND Sunday would be days of rest. (But when would one go to Costco?!) In Islam, there is Jumu’ah on Friday. Buddhists have Uposatha, a day of rest and observance every 7 or 8 days. Bah’ai keep Friday as a day of rest and worship. So vital are days of respite to the health of our communities that the state has made working on certain days a violation of the law, “blue laws” they are called. We’ve had blue laws on the books since the time of the Jamestown Colony in 1619 where the first Virginia Assembly required taking Sunday off for worship. Most of these laws have been repealed, although a few states, such as Rhode Island, still have blue laws prohibiting retail and grocery stores from opening on Thanksgiving or Christmas. So there – enjoy your two days off this year!
Ironically, this column, like most of mine, comes to you after my having written it on a Saturday and Sunday. I also just logged on to my EMR and checked results, renewed a few prescriptions, and answered a couple messages. If I didn’t, my Monday’s work would be crushingly heavy.
Maybe I need to be more efficient and finish my work during the week. Or maybe I need to realize that work has not let up since about 600 BCE and taking one day off each week to rest is an obligation to myself, my family and my community.
I wonder if I can choose Mondays.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“More than Jews have kept Shabbat, Shabbat has kept the Jews.” – Ahad Ha’am
You should all be well rested by now. After all, we’ve just come through the festive shutdown of the holiday season where all of your pumpkin/peppermint/marshmallow flavored coffees were sipped while walking around in your jimjams at 10 a.m. It was the time of year for you to take time off to get a proper rest and be energized to get back to work. Yet, I’m not feeling it from you.
So let’s talk about burnout – just kidding, that would only make it worse. “Burned-out’’ is a hackneyed and defective phrase to describe what many of us are feeling. We are not “destroyed, gutted by fire or by overheating.” No, we are, as one of our docs put it to me: “Just tired.” Ah, a much better Old English word! “Tired” captures it. It means to feel “in need of rest.” We are not ruined, we are just depleted. We don’t need discarding. We need some rest.
I asked some docs when they thought this feeling of exhaustion first began. We agreed that the pandemic, doubledemic, tripledemic, backlog have taken a toll. But The consumerization of medicine? All factors, but not the beginning. No, the beginning was before paper charts. Well, actually it was before paper. We have to go back to the 5th or 6th century BCE. That is when scholars believe the book of Genesis originated from the Yahwist source. In it, it is written that the 7th day be set aside as a day of rest from labor. It is not written that burnout would ensue if sabbath wasn’t observed; however, if you failed to keep it, then you might have been killed. They took rest seriously back then.
This innovation of setting aside a day each week to rest, reflect, and worship was such a good idea that it was codified as one of the 10 commandments. It spread widely. Early Christians kept the Jewish tradition of observing Shabbat from Friday sundown to Saturday until the ever practical Romans decided that Sunday would be a better day. Sunday was already the day to worship the sun god. The newly-converted Christian Emperor Constantine issued an edict on March 7th, 321 CE that all “city people and craftsmen shall rest from labor upon the venerable day of the sun.” And so Sunday it was.
Protestant Seventh-day denomination churches later shifted sabbath back to Saturday believing that Sunday must have been the Pope’s idea. The best deal seems to have been around 1273 when the Ethiopian Orthodox leader Ewostatewos decreed that both Saturday AND Sunday would be days of rest. (But when would one go to Costco?!) In Islam, there is Jumu’ah on Friday. Buddhists have Uposatha, a day of rest and observance every 7 or 8 days. Bah’ai keep Friday as a day of rest and worship. So vital are days of respite to the health of our communities that the state has made working on certain days a violation of the law, “blue laws” they are called. We’ve had blue laws on the books since the time of the Jamestown Colony in 1619 where the first Virginia Assembly required taking Sunday off for worship. Most of these laws have been repealed, although a few states, such as Rhode Island, still have blue laws prohibiting retail and grocery stores from opening on Thanksgiving or Christmas. So there – enjoy your two days off this year!
Ironically, this column, like most of mine, comes to you after my having written it on a Saturday and Sunday. I also just logged on to my EMR and checked results, renewed a few prescriptions, and answered a couple messages. If I didn’t, my Monday’s work would be crushingly heavy.
Maybe I need to be more efficient and finish my work during the week. Or maybe I need to realize that work has not let up since about 600 BCE and taking one day off each week to rest is an obligation to myself, my family and my community.
I wonder if I can choose Mondays.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
“More than Jews have kept Shabbat, Shabbat has kept the Jews.” – Ahad Ha’am
You should all be well rested by now. After all, we’ve just come through the festive shutdown of the holiday season where all of your pumpkin/peppermint/marshmallow flavored coffees were sipped while walking around in your jimjams at 10 a.m. It was the time of year for you to take time off to get a proper rest and be energized to get back to work. Yet, I’m not feeling it from you.
So let’s talk about burnout – just kidding, that would only make it worse. “Burned-out’’ is a hackneyed and defective phrase to describe what many of us are feeling. We are not “destroyed, gutted by fire or by overheating.” No, we are, as one of our docs put it to me: “Just tired.” Ah, a much better Old English word! “Tired” captures it. It means to feel “in need of rest.” We are not ruined, we are just depleted. We don’t need discarding. We need some rest.
I asked some docs when they thought this feeling of exhaustion first began. We agreed that the pandemic, doubledemic, tripledemic, backlog have taken a toll. But The consumerization of medicine? All factors, but not the beginning. No, the beginning was before paper charts. Well, actually it was before paper. We have to go back to the 5th or 6th century BCE. That is when scholars believe the book of Genesis originated from the Yahwist source. In it, it is written that the 7th day be set aside as a day of rest from labor. It is not written that burnout would ensue if sabbath wasn’t observed; however, if you failed to keep it, then you might have been killed. They took rest seriously back then.
This innovation of setting aside a day each week to rest, reflect, and worship was such a good idea that it was codified as one of the 10 commandments. It spread widely. Early Christians kept the Jewish tradition of observing Shabbat from Friday sundown to Saturday until the ever practical Romans decided that Sunday would be a better day. Sunday was already the day to worship the sun god. The newly-converted Christian Emperor Constantine issued an edict on March 7th, 321 CE that all “city people and craftsmen shall rest from labor upon the venerable day of the sun.” And so Sunday it was.
Protestant Seventh-day denomination churches later shifted sabbath back to Saturday believing that Sunday must have been the Pope’s idea. The best deal seems to have been around 1273 when the Ethiopian Orthodox leader Ewostatewos decreed that both Saturday AND Sunday would be days of rest. (But when would one go to Costco?!) In Islam, there is Jumu’ah on Friday. Buddhists have Uposatha, a day of rest and observance every 7 or 8 days. Bah’ai keep Friday as a day of rest and worship. So vital are days of respite to the health of our communities that the state has made working on certain days a violation of the law, “blue laws” they are called. We’ve had blue laws on the books since the time of the Jamestown Colony in 1619 where the first Virginia Assembly required taking Sunday off for worship. Most of these laws have been repealed, although a few states, such as Rhode Island, still have blue laws prohibiting retail and grocery stores from opening on Thanksgiving or Christmas. So there – enjoy your two days off this year!
Ironically, this column, like most of mine, comes to you after my having written it on a Saturday and Sunday. I also just logged on to my EMR and checked results, renewed a few prescriptions, and answered a couple messages. If I didn’t, my Monday’s work would be crushingly heavy.
Maybe I need to be more efficient and finish my work during the week. Or maybe I need to realize that work has not let up since about 600 BCE and taking one day off each week to rest is an obligation to myself, my family and my community.
I wonder if I can choose Mondays.
Dr. Benabio is director of Healthcare Transformation and chief of dermatology at Kaiser Permanente San Diego. The opinions expressed in this column are his own and do not represent those of Kaiser Permanente. Dr. Benabio is @Dermdoc on Twitter. Write to him at [email protected].
Commentary: Interstitial Lung Disease, Onset Time, and RA, January 2023
Though rheumatoid arthritis (RA)–associated interstitial lung disease (RA-ILD) is a feared complication that can significantly affect morbidity and mortality, the role of methotrexate in treatment and its possible contribution to ILD is yet unknown. Kim and colleagues performed a retrospective analysis of a series of 170 patients with RA-ILD to try to identify risk factors and protective factors for mortality and decline of lung function. Previously known risk factors included older age, smoking, and seropositivity for cyclic citrullinated peptide (CCP). In this series, patients who had exposure to methotrexate after a diagnosis of RA-ILD were found to have less progression of decline in lung function and decreased mortality compared with those who did not, which is a finding that warrants further examination. On the other hand, there was a suggestion that sulfasalazine use is associated with increased mortality, though this finding was not borne out in multivariate analysis.
A different group of authors also examined the association with conventional disease-modifying antirheumatic drugs (DMARD) with ILD progression in a prospective analysis of 143 patients in the multicenter Korean RA-ILD cohort. Patients were classified regarding exposure to methotrexate, leflunomide, or tacrolimus as well as biologic DMARD and glucocorticoid exposure, with a primary outcome of ILD progression based on pulmonary function tests or mortality. The study did not detect any difference in time to ILD progression with methotrexate exposure, though it is not clear that the study would be able to detect a protective effect as was possible in the prior study. However, patients who were exposed to leflunomide had a shorter time to ILD progression than did those who were not, though this did not persist in multivariate analysis, and tacrolimus exposure had a statistically insignificant impact on ILD progression. Because the study is small, other associations which could affect use of leflunomide in these patients were not examined, though prior studies have suggested an association with leflunomide in ILD progression in patients with existing RA-ILD.
Li and colleagues addressed the characteristics and prognosis of late-onset RA (LORA) in people 60 years or older compared with younger-onset RA (YORA) in a prospective cohort study using a Canadian RA registry. Patients in the registry were enrolled early in the course of their illness and clinical characteristics as well as time to Disease Activity Score (DAS28) remission were analyzed. Of note, YORA and LORA patients had similar times to remission but were on less aggressive medication regimens, such as conventional DMARD without biologic DMARD or Janus kinase (JAK) inhibitors. In this registry, a smaller percentage of LORA patients compared with YORA patients were seropositive, which, given the enrollment of patients early in their disease course, may affect the use of biologic DMARD and JAK inhibitors.
Finally, the issue of noninflammatory pain contributing to disease activity and quality of life in RA has received increased scrutiny recently. Choy and colleagues studied disproportionate articular pain (DP) and its response to sarilumab, adalimumab, or placebo in a post hoc analysis of data from prior randomized clinical trials. DP was defined as a tender joint count that exceeded swollen joint count by seven and was present in about 20% of patients in the three randomized clinical trials examined. In these studies, DP was reduced in patients treated with sarilumab compared with placebo or adalimumab. Although this finding is exciting in raising the possibility of an immunologic explanation for DP via interleukin 6 (IL-6), the results should be considered carefully in the context of this post hoc analysis, especially before considering sarilumab or other IL-6 inhibitors as viable treatment options for DP in RA.
Though rheumatoid arthritis (RA)–associated interstitial lung disease (RA-ILD) is a feared complication that can significantly affect morbidity and mortality, the role of methotrexate in treatment and its possible contribution to ILD is yet unknown. Kim and colleagues performed a retrospective analysis of a series of 170 patients with RA-ILD to try to identify risk factors and protective factors for mortality and decline of lung function. Previously known risk factors included older age, smoking, and seropositivity for cyclic citrullinated peptide (CCP). In this series, patients who had exposure to methotrexate after a diagnosis of RA-ILD were found to have less progression of decline in lung function and decreased mortality compared with those who did not, which is a finding that warrants further examination. On the other hand, there was a suggestion that sulfasalazine use is associated with increased mortality, though this finding was not borne out in multivariate analysis.
A different group of authors also examined the association with conventional disease-modifying antirheumatic drugs (DMARD) with ILD progression in a prospective analysis of 143 patients in the multicenter Korean RA-ILD cohort. Patients were classified regarding exposure to methotrexate, leflunomide, or tacrolimus as well as biologic DMARD and glucocorticoid exposure, with a primary outcome of ILD progression based on pulmonary function tests or mortality. The study did not detect any difference in time to ILD progression with methotrexate exposure, though it is not clear that the study would be able to detect a protective effect as was possible in the prior study. However, patients who were exposed to leflunomide had a shorter time to ILD progression than did those who were not, though this did not persist in multivariate analysis, and tacrolimus exposure had a statistically insignificant impact on ILD progression. Because the study is small, other associations which could affect use of leflunomide in these patients were not examined, though prior studies have suggested an association with leflunomide in ILD progression in patients with existing RA-ILD.
Li and colleagues addressed the characteristics and prognosis of late-onset RA (LORA) in people 60 years or older compared with younger-onset RA (YORA) in a prospective cohort study using a Canadian RA registry. Patients in the registry were enrolled early in the course of their illness and clinical characteristics as well as time to Disease Activity Score (DAS28) remission were analyzed. Of note, YORA and LORA patients had similar times to remission but were on less aggressive medication regimens, such as conventional DMARD without biologic DMARD or Janus kinase (JAK) inhibitors. In this registry, a smaller percentage of LORA patients compared with YORA patients were seropositive, which, given the enrollment of patients early in their disease course, may affect the use of biologic DMARD and JAK inhibitors.
Finally, the issue of noninflammatory pain contributing to disease activity and quality of life in RA has received increased scrutiny recently. Choy and colleagues studied disproportionate articular pain (DP) and its response to sarilumab, adalimumab, or placebo in a post hoc analysis of data from prior randomized clinical trials. DP was defined as a tender joint count that exceeded swollen joint count by seven and was present in about 20% of patients in the three randomized clinical trials examined. In these studies, DP was reduced in patients treated with sarilumab compared with placebo or adalimumab. Although this finding is exciting in raising the possibility of an immunologic explanation for DP via interleukin 6 (IL-6), the results should be considered carefully in the context of this post hoc analysis, especially before considering sarilumab or other IL-6 inhibitors as viable treatment options for DP in RA.
Though rheumatoid arthritis (RA)–associated interstitial lung disease (RA-ILD) is a feared complication that can significantly affect morbidity and mortality, the role of methotrexate in treatment and its possible contribution to ILD is yet unknown. Kim and colleagues performed a retrospective analysis of a series of 170 patients with RA-ILD to try to identify risk factors and protective factors for mortality and decline of lung function. Previously known risk factors included older age, smoking, and seropositivity for cyclic citrullinated peptide (CCP). In this series, patients who had exposure to methotrexate after a diagnosis of RA-ILD were found to have less progression of decline in lung function and decreased mortality compared with those who did not, which is a finding that warrants further examination. On the other hand, there was a suggestion that sulfasalazine use is associated with increased mortality, though this finding was not borne out in multivariate analysis.
A different group of authors also examined the association with conventional disease-modifying antirheumatic drugs (DMARD) with ILD progression in a prospective analysis of 143 patients in the multicenter Korean RA-ILD cohort. Patients were classified regarding exposure to methotrexate, leflunomide, or tacrolimus as well as biologic DMARD and glucocorticoid exposure, with a primary outcome of ILD progression based on pulmonary function tests or mortality. The study did not detect any difference in time to ILD progression with methotrexate exposure, though it is not clear that the study would be able to detect a protective effect as was possible in the prior study. However, patients who were exposed to leflunomide had a shorter time to ILD progression than did those who were not, though this did not persist in multivariate analysis, and tacrolimus exposure had a statistically insignificant impact on ILD progression. Because the study is small, other associations which could affect use of leflunomide in these patients were not examined, though prior studies have suggested an association with leflunomide in ILD progression in patients with existing RA-ILD.
Li and colleagues addressed the characteristics and prognosis of late-onset RA (LORA) in people 60 years or older compared with younger-onset RA (YORA) in a prospective cohort study using a Canadian RA registry. Patients in the registry were enrolled early in the course of their illness and clinical characteristics as well as time to Disease Activity Score (DAS28) remission were analyzed. Of note, YORA and LORA patients had similar times to remission but were on less aggressive medication regimens, such as conventional DMARD without biologic DMARD or Janus kinase (JAK) inhibitors. In this registry, a smaller percentage of LORA patients compared with YORA patients were seropositive, which, given the enrollment of patients early in their disease course, may affect the use of biologic DMARD and JAK inhibitors.
Finally, the issue of noninflammatory pain contributing to disease activity and quality of life in RA has received increased scrutiny recently. Choy and colleagues studied disproportionate articular pain (DP) and its response to sarilumab, adalimumab, or placebo in a post hoc analysis of data from prior randomized clinical trials. DP was defined as a tender joint count that exceeded swollen joint count by seven and was present in about 20% of patients in the three randomized clinical trials examined. In these studies, DP was reduced in patients treated with sarilumab compared with placebo or adalimumab. Although this finding is exciting in raising the possibility of an immunologic explanation for DP via interleukin 6 (IL-6), the results should be considered carefully in the context of this post hoc analysis, especially before considering sarilumab or other IL-6 inhibitors as viable treatment options for DP in RA.
ObGyns united in a divided post-Dobbs America
While many anticipated the fall of Roe v Wade after the leaked Supreme Court of the United States (SCOTUS) decision in the Dobbs v Jackson case, few may have fully comprehended the myriad of ways this ruling would create a national health care crisis overnight. Since the ruling, abortion has been banned, or a 6-week gestational age limit has been implemented, in a total of 13 states, all within the South
The 2022 American College of Obstetricians and Gynecologists (ACOG) Annual Clinical and Scientific Meeting, held shortly after the leaked SCOTUS opinion, was unlike most others. ACOG staff appropriately recognized the vastly different ways this ruling would affect patients and providers alike, simply based on the states in which they reside. ACOG staff organized the large group of attendees according to self-identified status (ie, whether they worked in states with protected, restricted, or threatened access to abortion care). Since this is such a vast topic, attendees also were asked to identify an area upon which to focus, such as the provision of health care, advocacy, or education. As a clinician practicing in Massachusetts, Dr. Bradley found herself meeting with an ACOG leader from California as they brainstormed how to best help our own communities. In conversing with attendees from other parts of the country, it became apparent the challenges others would be facing elsewhere were far more substantive than those we would be facing in “blue states.” After the Dobbs ruling, those predictions became harsh realities.
As we begin to see and hear reports of the devastating consequences of this ruling in “red states,” those of us in protected states have been struggling to try and ascertain how to help. Many of us have worked with our own legislatures to further enshrine protections for our patients and clinicians. New York and Massachusetts exemplify these efforts.6,7 These legislative efforts have included liability protections for patients and their clinicians who care for those who travel from restricted to protected states. Others involve codifying the principles of Roe and clarifying existing law to improve access. An online fundraiser organized by physicians to assist Dr. Bernard with her legal costs as she faces politically motivated investigation by Indiana state authorities has raised more than $260,000.8 Many expressed the potential legal and medical peril for examiners and examinees if the American Board of Obstetrics and Gynecology held in-person oral examinations in Texas as previously scheduled.9 An online petition to change the format to virtual had 728 signatories, and the format was changed back to virtual.10
The implications on medical schools, residencies, and fellowships cannot be overstated. The Dobbs ruling almost immediately affected nearly half of the training programs, which is particularly problematic given the Accreditation Council for Graduate Medical Education requirement that all ObGyn residents have access to abortion training.11 Other programs already are starting to try to meet this vast training need. The University of California San Francisco started offering training to physicians from Texas who were affected by the strict restrictions that predated Dobbs in the SB8 legislation, which turned ordinary citizens into vigilantes.12
ACOG has created an online resource (https://www.acog.org/advocacy/abortion-is-essential) with a number of different sections regarding clinical care, education and training, advocacy at the state level, and how to use effective language when talking about abortion in our communities. Planned Parenthood also suggests a myriad of ways those directly and indirectly affected could get involved:
- Donate to the National Network of Abortion Funds. This fund (https://secure.actblue.com/donate/fundabortionnow) facilitates care for those without the financial means to obtain it, supporting travel, lodging, and child care.
- Share #AbortionAccess posts on social media. These stories are a powerful reminder of the incredibly harmful impact this legislation can have on our patients.
- Donate to the If When How’s Legal Repro Defense Fund (https:/www.ifwhenhow.org/), which helps cover legal costs for those facing state persecution related to reproductive health care.
- Volunteer to help protect abortion health care at the state level.
- Engage with members of Congress in their home districts. (https://www.congress.gov/members/find-your-member)
- Contact the Planned Parenthood Local Engagement Team to facilitate your group, business, or organization’s involvement.
- Partner. Facilitate your organization and other companies to partner with Planned Parenthood and sign up for Bans off our Bodies (https://docs.google.com/forms/d/e/1FAIpQLSdrmxwMcwNXJ8I NE8S2gYjDDXuT76ws_Fr7CLm3 qbtR8dcZHw/viewform).
- Record your perspective about abortion (https://www.together.plannedparenthood.org/articles/6-share-abortion-story), whether it’s having had one, supported someone who had one, or advocated for others to have access to the procedure.13
ACOG also outlines several ways those of us in protected states could help shape the landscape in other communities in addition to advocating for state medical society resolutions, writing op-eds and letters to the editor, and utilizing ACOG’s social media graphics.14 In recognition of the often sensitive, polarizing nature of these discussions, ACOG is offering a workshop entitled “Building Evidence-Based Skills for Effective Conversations about Abortion.”15
Abortion traditionally was a policy issue other medical organizations shied away from developing official policy on and speaking out in support of, but recognizing the devastating scope of the public health crisis, 75 medical professional organizations recently released a strongly worded joint statement noting, “As leading medical and health care organizations dedicated to patient care and public health, we condemn this and all interference in the patient–clinician relationship.”16 Clinicians could work to expand this list to include all aspects of organized medicine. Initiatives to get out the vote may be helpful in vulnerable states, as well.
Clinicians in protected states are not necessarily directly affected in our daily interactions with patients, but we stand in solidarity with those who are. We must remain united as a profession as different state legislatures seek to divide us. We must support those who are struggling every day. Our colleagues and fellow citizens deserve nothing less. ●
- Tracking the states where abortion is now banned. New York Times. November 23, 2022. https://www.nytimes.com/interactive/2022/us/abortion-laws-roe-v-wade.html. Accessed November 28, 2022.
- Stanton A. ‘She’s 10’: child rape victims abortion denial spreads outrage on Twitter. Newsweek. July 2, 2022. https://www.newsweek.com/shes-10-child-rape-victims-abortion-denial-sparks-outrage-twitter-1721248. Accessed November 6, 2022.
- Judge-Golden C, Flink-Bochacki R. The burden of abortion restrictions and conservative diagnostic guidelines on patient-centered care for early pregnancy loss. Obstet Gynecol 2021;138:467071.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1. doi:10.1016/j.ajog.2022.06.060.
- Winter J. The Dobbs decision has unleashed legal chaos for doctors and patients. The New Yorker. July 2, 2022. https://www.newyorker.com/news/news-desk/the-dobbs-decision-has-unleashed-legal-chaos-for-doctors-and-patients. Accessed November 6, 2022.
- Lynch B, Mallow M, Bodde K, et al. Addressing a crisis in abortion access: a case study in advocacy. Obstet Gynecol. 2022;140:110-114.
- Evans M, Bradley T, Ireland L, et al. How the fall of Roe could change abortion care in Mass. Cognoscenti. July 26, 2022. https://www.wbur.org/cognoscenti/2022/07/26/dobbs-roe-abortion-massachusetts-megan-l-evans-erin-t-bradley-luu-ireland-chloe-zera. Accessed November 6, 2022.
- Spocchia G. Over $200k raised for doctor who performed abortion on 10-year-old rape victim. Independent. July 18, 2022. https://www.independent.co.uk/news/world/americas/fundriaser-ohio-abortion-doctor-rape-b2125621.html. Accessed November 6, 2022.
- ABOG petition: convert to online examination to protect OBGYN providers. Change.org website. https://www.change.org/p/abog-petition?original_footer_petition_id=33459909&algorithm=promoted&source_location=petition_footer&grid_position=8&pt=AVBldGl0aW9uAHgWBQIAAAAAYs65vIyhbUxhZGM0MWVhZg%3D%3D. Accessed November 6, 2022.
- D’Ambrosio A. Ob/Gyn board certification exam stays virtual in light of Dobbs. MedPageToday. July 15, 2022. https://www.medpagetoday.com/special-reports/features/99758. Accessed November 6, 2022.
- Weiner S. How the repeal of Roe v. Wade will affect training in abortion and reproductive health. AAMC News. June 24, 2022. https://www.aamc.org/news-insights/how-repeal-roe-v-wade-will-affect-training-abortion-and-reproductive-health. Accessed November 6, 2022.
- Anderson N. The fall of Roe scrambles abortion training for university hospitals. The Washington Post. June 30, 2022. https://www.washingtonpost.com/education/2022/06/30/abortion-training-upheaval-dobbs/. Accessed November 6, 2022.
- Bans off our bodies. Planned Parenthood website. https://www.plannedparenthoodaction.org/rightfully-ours/bans-off-our-bodies. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. Shape the public discourse. ACOG website. https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. Building evidence-based skills for effective conversations about abortion. ACOG website. https://www.acog.org/programs/impact/activities-initiatives/building-evidence-based-skills-for-effective-conversations-about-abortion. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. More than 75 health care organizations release joint statement in opposition to legislative interference. ACOG website. Published July 7, 2022. https://www.acog.org/news/news-releases/2022/07/more-than-75-health-care-organizations-release-joint-statement-in-opposition-to-legislative-interference. Accessed November 6, 2022.
While many anticipated the fall of Roe v Wade after the leaked Supreme Court of the United States (SCOTUS) decision in the Dobbs v Jackson case, few may have fully comprehended the myriad of ways this ruling would create a national health care crisis overnight. Since the ruling, abortion has been banned, or a 6-week gestational age limit has been implemented, in a total of 13 states, all within the South
The 2022 American College of Obstetricians and Gynecologists (ACOG) Annual Clinical and Scientific Meeting, held shortly after the leaked SCOTUS opinion, was unlike most others. ACOG staff appropriately recognized the vastly different ways this ruling would affect patients and providers alike, simply based on the states in which they reside. ACOG staff organized the large group of attendees according to self-identified status (ie, whether they worked in states with protected, restricted, or threatened access to abortion care). Since this is such a vast topic, attendees also were asked to identify an area upon which to focus, such as the provision of health care, advocacy, or education. As a clinician practicing in Massachusetts, Dr. Bradley found herself meeting with an ACOG leader from California as they brainstormed how to best help our own communities. In conversing with attendees from other parts of the country, it became apparent the challenges others would be facing elsewhere were far more substantive than those we would be facing in “blue states.” After the Dobbs ruling, those predictions became harsh realities.
As we begin to see and hear reports of the devastating consequences of this ruling in “red states,” those of us in protected states have been struggling to try and ascertain how to help. Many of us have worked with our own legislatures to further enshrine protections for our patients and clinicians. New York and Massachusetts exemplify these efforts.6,7 These legislative efforts have included liability protections for patients and their clinicians who care for those who travel from restricted to protected states. Others involve codifying the principles of Roe and clarifying existing law to improve access. An online fundraiser organized by physicians to assist Dr. Bernard with her legal costs as she faces politically motivated investigation by Indiana state authorities has raised more than $260,000.8 Many expressed the potential legal and medical peril for examiners and examinees if the American Board of Obstetrics and Gynecology held in-person oral examinations in Texas as previously scheduled.9 An online petition to change the format to virtual had 728 signatories, and the format was changed back to virtual.10
The implications on medical schools, residencies, and fellowships cannot be overstated. The Dobbs ruling almost immediately affected nearly half of the training programs, which is particularly problematic given the Accreditation Council for Graduate Medical Education requirement that all ObGyn residents have access to abortion training.11 Other programs already are starting to try to meet this vast training need. The University of California San Francisco started offering training to physicians from Texas who were affected by the strict restrictions that predated Dobbs in the SB8 legislation, which turned ordinary citizens into vigilantes.12
ACOG has created an online resource (https://www.acog.org/advocacy/abortion-is-essential) with a number of different sections regarding clinical care, education and training, advocacy at the state level, and how to use effective language when talking about abortion in our communities. Planned Parenthood also suggests a myriad of ways those directly and indirectly affected could get involved:
- Donate to the National Network of Abortion Funds. This fund (https://secure.actblue.com/donate/fundabortionnow) facilitates care for those without the financial means to obtain it, supporting travel, lodging, and child care.
- Share #AbortionAccess posts on social media. These stories are a powerful reminder of the incredibly harmful impact this legislation can have on our patients.
- Donate to the If When How’s Legal Repro Defense Fund (https:/www.ifwhenhow.org/), which helps cover legal costs for those facing state persecution related to reproductive health care.
- Volunteer to help protect abortion health care at the state level.
- Engage with members of Congress in their home districts. (https://www.congress.gov/members/find-your-member)
- Contact the Planned Parenthood Local Engagement Team to facilitate your group, business, or organization’s involvement.
- Partner. Facilitate your organization and other companies to partner with Planned Parenthood and sign up for Bans off our Bodies (https://docs.google.com/forms/d/e/1FAIpQLSdrmxwMcwNXJ8I NE8S2gYjDDXuT76ws_Fr7CLm3 qbtR8dcZHw/viewform).
- Record your perspective about abortion (https://www.together.plannedparenthood.org/articles/6-share-abortion-story), whether it’s having had one, supported someone who had one, or advocated for others to have access to the procedure.13
ACOG also outlines several ways those of us in protected states could help shape the landscape in other communities in addition to advocating for state medical society resolutions, writing op-eds and letters to the editor, and utilizing ACOG’s social media graphics.14 In recognition of the often sensitive, polarizing nature of these discussions, ACOG is offering a workshop entitled “Building Evidence-Based Skills for Effective Conversations about Abortion.”15
Abortion traditionally was a policy issue other medical organizations shied away from developing official policy on and speaking out in support of, but recognizing the devastating scope of the public health crisis, 75 medical professional organizations recently released a strongly worded joint statement noting, “As leading medical and health care organizations dedicated to patient care and public health, we condemn this and all interference in the patient–clinician relationship.”16 Clinicians could work to expand this list to include all aspects of organized medicine. Initiatives to get out the vote may be helpful in vulnerable states, as well.
Clinicians in protected states are not necessarily directly affected in our daily interactions with patients, but we stand in solidarity with those who are. We must remain united as a profession as different state legislatures seek to divide us. We must support those who are struggling every day. Our colleagues and fellow citizens deserve nothing less. ●
While many anticipated the fall of Roe v Wade after the leaked Supreme Court of the United States (SCOTUS) decision in the Dobbs v Jackson case, few may have fully comprehended the myriad of ways this ruling would create a national health care crisis overnight. Since the ruling, abortion has been banned, or a 6-week gestational age limit has been implemented, in a total of 13 states, all within the South
The 2022 American College of Obstetricians and Gynecologists (ACOG) Annual Clinical and Scientific Meeting, held shortly after the leaked SCOTUS opinion, was unlike most others. ACOG staff appropriately recognized the vastly different ways this ruling would affect patients and providers alike, simply based on the states in which they reside. ACOG staff organized the large group of attendees according to self-identified status (ie, whether they worked in states with protected, restricted, or threatened access to abortion care). Since this is such a vast topic, attendees also were asked to identify an area upon which to focus, such as the provision of health care, advocacy, or education. As a clinician practicing in Massachusetts, Dr. Bradley found herself meeting with an ACOG leader from California as they brainstormed how to best help our own communities. In conversing with attendees from other parts of the country, it became apparent the challenges others would be facing elsewhere were far more substantive than those we would be facing in “blue states.” After the Dobbs ruling, those predictions became harsh realities.
As we begin to see and hear reports of the devastating consequences of this ruling in “red states,” those of us in protected states have been struggling to try and ascertain how to help. Many of us have worked with our own legislatures to further enshrine protections for our patients and clinicians. New York and Massachusetts exemplify these efforts.6,7 These legislative efforts have included liability protections for patients and their clinicians who care for those who travel from restricted to protected states. Others involve codifying the principles of Roe and clarifying existing law to improve access. An online fundraiser organized by physicians to assist Dr. Bernard with her legal costs as she faces politically motivated investigation by Indiana state authorities has raised more than $260,000.8 Many expressed the potential legal and medical peril for examiners and examinees if the American Board of Obstetrics and Gynecology held in-person oral examinations in Texas as previously scheduled.9 An online petition to change the format to virtual had 728 signatories, and the format was changed back to virtual.10
The implications on medical schools, residencies, and fellowships cannot be overstated. The Dobbs ruling almost immediately affected nearly half of the training programs, which is particularly problematic given the Accreditation Council for Graduate Medical Education requirement that all ObGyn residents have access to abortion training.11 Other programs already are starting to try to meet this vast training need. The University of California San Francisco started offering training to physicians from Texas who were affected by the strict restrictions that predated Dobbs in the SB8 legislation, which turned ordinary citizens into vigilantes.12
ACOG has created an online resource (https://www.acog.org/advocacy/abortion-is-essential) with a number of different sections regarding clinical care, education and training, advocacy at the state level, and how to use effective language when talking about abortion in our communities. Planned Parenthood also suggests a myriad of ways those directly and indirectly affected could get involved:
- Donate to the National Network of Abortion Funds. This fund (https://secure.actblue.com/donate/fundabortionnow) facilitates care for those without the financial means to obtain it, supporting travel, lodging, and child care.
- Share #AbortionAccess posts on social media. These stories are a powerful reminder of the incredibly harmful impact this legislation can have on our patients.
- Donate to the If When How’s Legal Repro Defense Fund (https:/www.ifwhenhow.org/), which helps cover legal costs for those facing state persecution related to reproductive health care.
- Volunteer to help protect abortion health care at the state level.
- Engage with members of Congress in their home districts. (https://www.congress.gov/members/find-your-member)
- Contact the Planned Parenthood Local Engagement Team to facilitate your group, business, or organization’s involvement.
- Partner. Facilitate your organization and other companies to partner with Planned Parenthood and sign up for Bans off our Bodies (https://docs.google.com/forms/d/e/1FAIpQLSdrmxwMcwNXJ8I NE8S2gYjDDXuT76ws_Fr7CLm3 qbtR8dcZHw/viewform).
- Record your perspective about abortion (https://www.together.plannedparenthood.org/articles/6-share-abortion-story), whether it’s having had one, supported someone who had one, or advocated for others to have access to the procedure.13
ACOG also outlines several ways those of us in protected states could help shape the landscape in other communities in addition to advocating for state medical society resolutions, writing op-eds and letters to the editor, and utilizing ACOG’s social media graphics.14 In recognition of the often sensitive, polarizing nature of these discussions, ACOG is offering a workshop entitled “Building Evidence-Based Skills for Effective Conversations about Abortion.”15
Abortion traditionally was a policy issue other medical organizations shied away from developing official policy on and speaking out in support of, but recognizing the devastating scope of the public health crisis, 75 medical professional organizations recently released a strongly worded joint statement noting, “As leading medical and health care organizations dedicated to patient care and public health, we condemn this and all interference in the patient–clinician relationship.”16 Clinicians could work to expand this list to include all aspects of organized medicine. Initiatives to get out the vote may be helpful in vulnerable states, as well.
Clinicians in protected states are not necessarily directly affected in our daily interactions with patients, but we stand in solidarity with those who are. We must remain united as a profession as different state legislatures seek to divide us. We must support those who are struggling every day. Our colleagues and fellow citizens deserve nothing less. ●
- Tracking the states where abortion is now banned. New York Times. November 23, 2022. https://www.nytimes.com/interactive/2022/us/abortion-laws-roe-v-wade.html. Accessed November 28, 2022.
- Stanton A. ‘She’s 10’: child rape victims abortion denial spreads outrage on Twitter. Newsweek. July 2, 2022. https://www.newsweek.com/shes-10-child-rape-victims-abortion-denial-sparks-outrage-twitter-1721248. Accessed November 6, 2022.
- Judge-Golden C, Flink-Bochacki R. The burden of abortion restrictions and conservative diagnostic guidelines on patient-centered care for early pregnancy loss. Obstet Gynecol 2021;138:467071.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1. doi:10.1016/j.ajog.2022.06.060.
- Winter J. The Dobbs decision has unleashed legal chaos for doctors and patients. The New Yorker. July 2, 2022. https://www.newyorker.com/news/news-desk/the-dobbs-decision-has-unleashed-legal-chaos-for-doctors-and-patients. Accessed November 6, 2022.
- Lynch B, Mallow M, Bodde K, et al. Addressing a crisis in abortion access: a case study in advocacy. Obstet Gynecol. 2022;140:110-114.
- Evans M, Bradley T, Ireland L, et al. How the fall of Roe could change abortion care in Mass. Cognoscenti. July 26, 2022. https://www.wbur.org/cognoscenti/2022/07/26/dobbs-roe-abortion-massachusetts-megan-l-evans-erin-t-bradley-luu-ireland-chloe-zera. Accessed November 6, 2022.
- Spocchia G. Over $200k raised for doctor who performed abortion on 10-year-old rape victim. Independent. July 18, 2022. https://www.independent.co.uk/news/world/americas/fundriaser-ohio-abortion-doctor-rape-b2125621.html. Accessed November 6, 2022.
- ABOG petition: convert to online examination to protect OBGYN providers. Change.org website. https://www.change.org/p/abog-petition?original_footer_petition_id=33459909&algorithm=promoted&source_location=petition_footer&grid_position=8&pt=AVBldGl0aW9uAHgWBQIAAAAAYs65vIyhbUxhZGM0MWVhZg%3D%3D. Accessed November 6, 2022.
- D’Ambrosio A. Ob/Gyn board certification exam stays virtual in light of Dobbs. MedPageToday. July 15, 2022. https://www.medpagetoday.com/special-reports/features/99758. Accessed November 6, 2022.
- Weiner S. How the repeal of Roe v. Wade will affect training in abortion and reproductive health. AAMC News. June 24, 2022. https://www.aamc.org/news-insights/how-repeal-roe-v-wade-will-affect-training-abortion-and-reproductive-health. Accessed November 6, 2022.
- Anderson N. The fall of Roe scrambles abortion training for university hospitals. The Washington Post. June 30, 2022. https://www.washingtonpost.com/education/2022/06/30/abortion-training-upheaval-dobbs/. Accessed November 6, 2022.
- Bans off our bodies. Planned Parenthood website. https://www.plannedparenthoodaction.org/rightfully-ours/bans-off-our-bodies. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. Shape the public discourse. ACOG website. https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. Building evidence-based skills for effective conversations about abortion. ACOG website. https://www.acog.org/programs/impact/activities-initiatives/building-evidence-based-skills-for-effective-conversations-about-abortion. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. More than 75 health care organizations release joint statement in opposition to legislative interference. ACOG website. Published July 7, 2022. https://www.acog.org/news/news-releases/2022/07/more-than-75-health-care-organizations-release-joint-statement-in-opposition-to-legislative-interference. Accessed November 6, 2022.
- Tracking the states where abortion is now banned. New York Times. November 23, 2022. https://www.nytimes.com/interactive/2022/us/abortion-laws-roe-v-wade.html. Accessed November 28, 2022.
- Stanton A. ‘She’s 10’: child rape victims abortion denial spreads outrage on Twitter. Newsweek. July 2, 2022. https://www.newsweek.com/shes-10-child-rape-victims-abortion-denial-sparks-outrage-twitter-1721248. Accessed November 6, 2022.
- Judge-Golden C, Flink-Bochacki R. The burden of abortion restrictions and conservative diagnostic guidelines on patient-centered care for early pregnancy loss. Obstet Gynecol 2021;138:467071.
- Nambiar A, Patel S, Santiago-Munoz P, et al. Maternal morbidity and fetal outcomes among pregnant women at 22 weeks’ gestation or less with complications in 2 Texas hospitals after legislation on abortion. Am J Obstet Gynecol. 2022;227:648-650.e1. doi:10.1016/j.ajog.2022.06.060.
- Winter J. The Dobbs decision has unleashed legal chaos for doctors and patients. The New Yorker. July 2, 2022. https://www.newyorker.com/news/news-desk/the-dobbs-decision-has-unleashed-legal-chaos-for-doctors-and-patients. Accessed November 6, 2022.
- Lynch B, Mallow M, Bodde K, et al. Addressing a crisis in abortion access: a case study in advocacy. Obstet Gynecol. 2022;140:110-114.
- Evans M, Bradley T, Ireland L, et al. How the fall of Roe could change abortion care in Mass. Cognoscenti. July 26, 2022. https://www.wbur.org/cognoscenti/2022/07/26/dobbs-roe-abortion-massachusetts-megan-l-evans-erin-t-bradley-luu-ireland-chloe-zera. Accessed November 6, 2022.
- Spocchia G. Over $200k raised for doctor who performed abortion on 10-year-old rape victim. Independent. July 18, 2022. https://www.independent.co.uk/news/world/americas/fundriaser-ohio-abortion-doctor-rape-b2125621.html. Accessed November 6, 2022.
- ABOG petition: convert to online examination to protect OBGYN providers. Change.org website. https://www.change.org/p/abog-petition?original_footer_petition_id=33459909&algorithm=promoted&source_location=petition_footer&grid_position=8&pt=AVBldGl0aW9uAHgWBQIAAAAAYs65vIyhbUxhZGM0MWVhZg%3D%3D. Accessed November 6, 2022.
- D’Ambrosio A. Ob/Gyn board certification exam stays virtual in light of Dobbs. MedPageToday. July 15, 2022. https://www.medpagetoday.com/special-reports/features/99758. Accessed November 6, 2022.
- Weiner S. How the repeal of Roe v. Wade will affect training in abortion and reproductive health. AAMC News. June 24, 2022. https://www.aamc.org/news-insights/how-repeal-roe-v-wade-will-affect-training-abortion-and-reproductive-health. Accessed November 6, 2022.
- Anderson N. The fall of Roe scrambles abortion training for university hospitals. The Washington Post. June 30, 2022. https://www.washingtonpost.com/education/2022/06/30/abortion-training-upheaval-dobbs/. Accessed November 6, 2022.
- Bans off our bodies. Planned Parenthood website. https://www.plannedparenthoodaction.org/rightfully-ours/bans-off-our-bodies. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. Shape the public discourse. ACOG website. https://www.acog.org/advocacy/abortion-is-essential/connect-in-your-community. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. Building evidence-based skills for effective conversations about abortion. ACOG website. https://www.acog.org/programs/impact/activities-initiatives/building-evidence-based-skills-for-effective-conversations-about-abortion. Accessed November 6, 2022.
- American College of Obstetricians and Gynecologists. More than 75 health care organizations release joint statement in opposition to legislative interference. ACOG website. Published July 7, 2022. https://www.acog.org/news/news-releases/2022/07/more-than-75-health-care-organizations-release-joint-statement-in-opposition-to-legislative-interference. Accessed November 6, 2022.
Home births in the United States, 2019—2021


Weight loss management ... a new frontier?
Dear colleagues,
Treating obesity easily falls under our purview as gastroenterologists. But like the mouse who would bell the cat, our direct involvement has been limited. However, over the past decade, advances in endobariatrics and medical management have given us many options. But how do we choose from this growing armamentarium of minimally invasive procedures and weight loss medicines? What combination is best? And what about the standard “diet and exercise”?
In this issue of perspectives, Carolyn Newberry, MD, director of GI nutrition at Innovation Center for Health and Nutrition in Gastroenterology, Weill Cornell Medicine, New York, will emphasize the benefits of medical and lifestyle management. Pichamol Jirapinyo, MD, MPH, ABOM, director of bariatric endoscopy fellowship at Brigham and Women’s Hospital/Harvard Medical School, Boston, responds with robust data for endoscopic therapies. We hope that their expert perspectives will help guide you in your own approach to obesity management – certainly no one size fits all. I welcome your thoughts on this growing field in gastroenterology – share with us on Twitter @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Exciting time for endoscopic bariatric and metabolic therapies (EBMTs)
BY PICHAMOL JIRAPINYO, MD, MPH, ABOM
2022 was an exciting year for our field of endoscopic bariatric and metabolic therapy (EBMT). Not only did it mark the 10th year anniversary since the very first-in-human endoscopic sleeve gastroplasty (ESG) performed by Christopher Thompson and Robert Hawes in India, but also the MERIT trial (a randomized-controlled trial on ESG) was published.1 This decade of work led to the OverStitch Endoscopic Suturing System (Apollo Endosurgery, Austin, Tex.) being granted de novo authorization from the Food and Drug Administration for the treatment of obesity and weight regain following bariatric surgery.
Currently, at our institution, we offer four primary EBMTs for patients who are seeking endoscopic weight loss therapy and have not yet undergone prior bariatric surgery. These include the Orbera intragastric balloon (IGB) (Apollo Endosurgery), ESG (Apollo Endosurgery), primary obesity surgery endoluminal (POSE: USGI Medical, San Clemente, Calif.), and a gastric plication procedure using Endomina (Endo Tools Therapeutics, Gosselies, Belgium). While the former two have FDA approval, the latter two devices have FDA clearance for tissue approximation. The indication for primary EBMTs includes having a body mass index of at least 30 kg/m2.
From our experience, patients who present to our bariatric endoscopy clinic consist of three groups. First are those who have tried several anti-obesity medications (AOMs), but are unable to tolerate the side effects or their BMI remains greater than 30 kg/m2. Second are those who have heard about EBMTs and are interested in the procedures. Usually, these patients are either too light to qualify for bariatric surgery (BMI 30-35 kg/m2 or 35-40 kg/m2 without an obesity-related comorbidity) or are not interested in bariatric surgery for a variety of reasons, including its perceived invasiveness. The last group are those whose BMI falls within the “super obese” category, defined as a BMI ≥ 50 kg/m2, who are deemed too high risk to undergo medically necessary procedures, such as an orthopedic, colorectal, or transplant surgery.
During the initial consultation, I always discuss pros and cons of all treatment modalities for obesity with the patients, ranging from lifestyle modification to AOMs, EBMTs, and bariatric surgeries. While the data on AOMs are promising, especially with the most recent FDA-approved semaglutide (Wegovy: Novo Nordisk, Bagsvaerd, Denmark) yielding 14.9% total weight loss (TWL) at 1 year, in reality, the starting doses of this medication have been out of stock for over a year.2 Other AOMs, on the other hand, are associated with 6%-8% TWL and are frequently associated with intolerance due to side effects. In comparison, meta-analyses demonstrate that an IGB is associated with 11.3% TWL and ESG with 16.5% TWL at 1 year. Our recent publication describing a new technique for POSE, also known as a distal POSE procedure with a double-helix technique, demonstrates a 20.3% TWL at 1 year.3 The rate of serious adverse events for EBMTs is low with 0.1% for IGB and 1%-2% for ESG/POSE.
The question regarding a comparison between AOMs and EBMTs comes up quite frequently in clinical practice. In reality, I often encourage my patients to consider combination therapy where I prescribe an AOM at 3-6 months following EBMTs to augment the amount of weight loss. However, since this is a debate, I will highlight a few advantages of EBMTs. First, the amount of weight loss following EBMTs, especially with ESG/POSE (which is currently the most commonly-requested procedure in our practice), tends to be higher than that of most AOMs. Second, while we are eagerly awaiting the long-term safety and efficacy data for semaglutide, ESG has been shown to be durable with the patients maintaining 15.9% TWL at 5 years.4 Third, an EBMT is a one-time procedure. In contrast, AOMs rely on patients’ compliance with taking the medication(s) reliably and indefinitely. A study based on HMO pharmacy data of over a million patients who were prescribed AOMs showed that fewer than 2% completed 12 months of weight loss medication therapy.5 The long-term use of AOMs also has cost implications. Specifically, a month supply of semaglutide costs about $1,400, which translates to $16,800 in 1 year and $84,000 in 5 years, which clearly outweighs the cost of ESG/POSE that has been demonstrated to be durable up to at least 5 years. IGBs have limitations similar to those of AOMs upon removal. Nevertheless, with the average cost of an IGB being $8,000, placing one every year would still be less costly, although this would likely be unnecessary considering the weight loss trend after IGB.
There are a few hurdles that need to be overcome before EBMTs are widely adopted. Reimbursement remains a major issue at most centers in the United States. Currently, most EBMTs are offered as a self-pay procedure, making the majority of patients who are otherwise eligible and interested not able to afford the procedure. With the recently published MERIT trial, long-term data on ESG as well as several upcoming society guidelines on EBMTs, we are hopeful that insurance coverage for EBMTs is nearing. Another important aspect is training. While IGB placement and removal are simple procedures, performing a high-quality ESG/POSE requires rigorous training to ensure safety and optimal outcomes. Several professional societies are working hard to develop curriculums on EBMTs with a focus on hands-on training to ensure endoscopists are properly trained prior to starting their bariatric endoscopy program. At our institution, we have a dedicated training program focusing on bariatric endoscopy (i.e. separate from the traditional advanced endoscopy fellowship), where fellows learn advanced bariatric suturing and plication as well as multidisciplinary care for this patient population. I am hopeful that this kind of training will become more prevalent in the near future.
With mounting evidence supporting the benefits of EBMTs, bariatric endoscopy has revolutionized the care of patients suffering from obesity and its related comorbidities. Moving forward, the field will continue to evolve, and EBMT procedures will only become simpler, safer, and more effective. It is an exciting time for gastroenterologists to get involved.
Dr. Jirapinyo is the director of bariatric endoscopy fellowship at Brigham and Women’s Hospital/Harvard Medical School, Boston. She is board certified in internal medicine, gastroenterology, and obesity medicine and completed her bariatric endoscopy and advanced endoscopy fellowships at Brigham and Women’s Hospital. She serves as a consultant for Apollo Endosurgery, Spatz Medical, and ERBE, and she receives research support from USGI Medical, GI Dynamics, and Fractyl.
References
1. Abu Dayyeh BK et al. Lancet. 2022;400(10350):441-51.
2. Wilding JPH et al. N Engl J Med. 2021;384:989-1002.
3. Jirapinyo P and Thompson CC. Gastrointest Endosc. 2022;96(3):479-86.
4. Sharaiha RZ et al. Clin Gastroenterol Hepatol. 2021;19(5):1051-57.
5. Hemo B et al. Diabetes Res Clin Pract. 2011;94(2):269-75.
A new frontier for weight management: Assess your options carefully
BY CAROLYN NEWBERRY, MD
Considering the continued rise in obesity rates in this country coupled with an increase in associated digestive disease burden from conditions such as nonalcoholic fatty liver disease (NAFLD), gastroesophageal reflux disease (GERD), and select gastrointestinal malignancies, I believe it is now more important than ever for gastroenterologists to familiarize themselves with weight management principles and incorporation into clinical practice. A growing arsenal of tools is available for addressing excess weight, including medications and novel endobariatric techniques. Although the latter is an important consideration in patients with obesity, lifestyle counseling with or without weight loss medications sets the stage for sustainable weight loss success and may eliminate the need for procedural intervention. As such, current guidelines set forth by multiple societies, including the American Gastroenterological Association (AGA), emphasize the importance of lifestyle counseling targeting caloric restriction and increased physical activity along with medical augmentation via pharmacological agents in eligible patients.1,2 These guidelines underline the importance of medical weight management prior to consideration of procedural options, including both endobariatrics and more classic bariatric surgeries. This ensures patients understand approaches to weight loss via noninvasive means, reduces risk of weight regain by building foundational habits, and enhances overall success of procedures long term if they are pursued. In addition, newer pharmacological agents are now approaching total body weight loss percentages of currently available endobariatric techniques while still showing high tolerance rates and long-term efficacy, indicating some patients who previously would require procedures to meet weight loss goals may no longer need them.3 Alternatively, these medications may augment efforts prior to procedures, enhancing overall total body weight loss achieved. If patients are not introduced to such options initially and as a part of comprehensive care management planning, they may not achieve the same degree of weight loss success and metabolic optimization.
As a gastroenterologist co-leading a multidisciplinary weight management and lifestyle clinic, I have witnessed firsthand the enhanced outcomes in patients who pursue endobariatric procedures after establishing care with a clinical team and attempting (and succeeding) in weight loss via changes in diet, physical activity, and medication use. Patients should be encouraged to gain understanding of one’s own “personal relationship” with food and/or address medical and social barriers to weight loss maintenance prior to procedural intervention, which requires some lead time and ideally professional expertise from multiple team members, including a dietitian. Weight regain after anti-obesity surgery is common, with significant gain occurring in up to half of patients. Several factors have been associated with weight regain, including lack of consistent follow-up, excess calorie and simple carbohydrate intake, and inconsistent physical activity.4 As such, most insurance companies mandate a trial of at least 6 months of lifestyle and/or medical weight management prior to considering procedural reimbursement. Although robust longitudinal data for endobariatric outcomes is not yet available, it is reasonable to believe similar concepts may be in play. In fact, since endobariatric procedures are less invasive but also therefore more temporal (as in the case of endoscopic balloon placement, which is only approved for 6 months of continuous use), behavioral modification and medical management to reduce risk of significant weight regain is even more imperative. Even in the case of more durable procedures, such as endoscopic gastroplasty, lack of compliance with recommended dietary protocols can reduce efficacy by loosening and even ripping sutures prior to establishment of bridging fibrotic mucosal changes, which enhance longevity of the procedure and support continued gastric restriction and reduction in motility. Some patients who undergo endoscopic gastroplasty end up seeking out revision and repeat procedure later due to lack of results, which may be avoided with alternative dietary and lifestyle decisions in the postprocedural state.
The landscape of non-procedural weight management tools has changed in the last 1-2 years with the approval of newer injectable medications that disrupt insulin and hormonal pathways and produce sustainable weight loss similar to reported outcomes achieved with endobariatric procedures. These medications are becoming increasingly accessible and of interest to patients, with continued destigmatization of the use of weight loss drugs in practice, which had previous negative connotations and concerns regarding safety. New guidelines put forth by the AGA recommend adding pharmacological agents to lifestyle interventions over continuing lifestyle interventions alone if adequate weight loss has not been achieved with the latter.3 This further exemplifies the importance of a multifaceted approach to optimize medical weight management as first-line therapy for obesity and associated comorbidities.
In summary, although endobariatric procedures are an important tool for gastroenterologists to incorporate into their weight management plans, they must be implemented with care and only after lifestyle and medical interventions have failed to produce desired results. Shared decision making among providers and patients enhances weight loss efforts and augments sustainability of outcomes. Considering the rapidly evolving landscape of obesity medicine, gastroenterologists need to continue to stay up to date on best practices to improve patient care, reduce associated morbidity, and enhance outcomes of novel endobariatric procedures.
Dr. Newberry is with the Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), division of gastroenterology, Weill Cornell Medical Center, New York. She disclosed receiving speaker honorariums from Baxter International and InBody USA.
References
1. Acosta et al. Clin Gastroenterol Hepatol. 2017 May;15(5):631-49.
2. Jensen et al. Circulation. 2014;129:S102-38.
3. Grunvald et al. Gastroenterology. 2022;163(5):1198-225.
4. Athansiadis et al. Surg Endosc. 2021 Aug;35(8):4069-84.
Dear colleagues,
Treating obesity easily falls under our purview as gastroenterologists. But like the mouse who would bell the cat, our direct involvement has been limited. However, over the past decade, advances in endobariatrics and medical management have given us many options. But how do we choose from this growing armamentarium of minimally invasive procedures and weight loss medicines? What combination is best? And what about the standard “diet and exercise”?
In this issue of perspectives, Carolyn Newberry, MD, director of GI nutrition at Innovation Center for Health and Nutrition in Gastroenterology, Weill Cornell Medicine, New York, will emphasize the benefits of medical and lifestyle management. Pichamol Jirapinyo, MD, MPH, ABOM, director of bariatric endoscopy fellowship at Brigham and Women’s Hospital/Harvard Medical School, Boston, responds with robust data for endoscopic therapies. We hope that their expert perspectives will help guide you in your own approach to obesity management – certainly no one size fits all. I welcome your thoughts on this growing field in gastroenterology – share with us on Twitter @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Exciting time for endoscopic bariatric and metabolic therapies (EBMTs)
BY PICHAMOL JIRAPINYO, MD, MPH, ABOM
2022 was an exciting year for our field of endoscopic bariatric and metabolic therapy (EBMT). Not only did it mark the 10th year anniversary since the very first-in-human endoscopic sleeve gastroplasty (ESG) performed by Christopher Thompson and Robert Hawes in India, but also the MERIT trial (a randomized-controlled trial on ESG) was published.1 This decade of work led to the OverStitch Endoscopic Suturing System (Apollo Endosurgery, Austin, Tex.) being granted de novo authorization from the Food and Drug Administration for the treatment of obesity and weight regain following bariatric surgery.
Currently, at our institution, we offer four primary EBMTs for patients who are seeking endoscopic weight loss therapy and have not yet undergone prior bariatric surgery. These include the Orbera intragastric balloon (IGB) (Apollo Endosurgery), ESG (Apollo Endosurgery), primary obesity surgery endoluminal (POSE: USGI Medical, San Clemente, Calif.), and a gastric plication procedure using Endomina (Endo Tools Therapeutics, Gosselies, Belgium). While the former two have FDA approval, the latter two devices have FDA clearance for tissue approximation. The indication for primary EBMTs includes having a body mass index of at least 30 kg/m2.
From our experience, patients who present to our bariatric endoscopy clinic consist of three groups. First are those who have tried several anti-obesity medications (AOMs), but are unable to tolerate the side effects or their BMI remains greater than 30 kg/m2. Second are those who have heard about EBMTs and are interested in the procedures. Usually, these patients are either too light to qualify for bariatric surgery (BMI 30-35 kg/m2 or 35-40 kg/m2 without an obesity-related comorbidity) or are not interested in bariatric surgery for a variety of reasons, including its perceived invasiveness. The last group are those whose BMI falls within the “super obese” category, defined as a BMI ≥ 50 kg/m2, who are deemed too high risk to undergo medically necessary procedures, such as an orthopedic, colorectal, or transplant surgery.
During the initial consultation, I always discuss pros and cons of all treatment modalities for obesity with the patients, ranging from lifestyle modification to AOMs, EBMTs, and bariatric surgeries. While the data on AOMs are promising, especially with the most recent FDA-approved semaglutide (Wegovy: Novo Nordisk, Bagsvaerd, Denmark) yielding 14.9% total weight loss (TWL) at 1 year, in reality, the starting doses of this medication have been out of stock for over a year.2 Other AOMs, on the other hand, are associated with 6%-8% TWL and are frequently associated with intolerance due to side effects. In comparison, meta-analyses demonstrate that an IGB is associated with 11.3% TWL and ESG with 16.5% TWL at 1 year. Our recent publication describing a new technique for POSE, also known as a distal POSE procedure with a double-helix technique, demonstrates a 20.3% TWL at 1 year.3 The rate of serious adverse events for EBMTs is low with 0.1% for IGB and 1%-2% for ESG/POSE.
The question regarding a comparison between AOMs and EBMTs comes up quite frequently in clinical practice. In reality, I often encourage my patients to consider combination therapy where I prescribe an AOM at 3-6 months following EBMTs to augment the amount of weight loss. However, since this is a debate, I will highlight a few advantages of EBMTs. First, the amount of weight loss following EBMTs, especially with ESG/POSE (which is currently the most commonly-requested procedure in our practice), tends to be higher than that of most AOMs. Second, while we are eagerly awaiting the long-term safety and efficacy data for semaglutide, ESG has been shown to be durable with the patients maintaining 15.9% TWL at 5 years.4 Third, an EBMT is a one-time procedure. In contrast, AOMs rely on patients’ compliance with taking the medication(s) reliably and indefinitely. A study based on HMO pharmacy data of over a million patients who were prescribed AOMs showed that fewer than 2% completed 12 months of weight loss medication therapy.5 The long-term use of AOMs also has cost implications. Specifically, a month supply of semaglutide costs about $1,400, which translates to $16,800 in 1 year and $84,000 in 5 years, which clearly outweighs the cost of ESG/POSE that has been demonstrated to be durable up to at least 5 years. IGBs have limitations similar to those of AOMs upon removal. Nevertheless, with the average cost of an IGB being $8,000, placing one every year would still be less costly, although this would likely be unnecessary considering the weight loss trend after IGB.
There are a few hurdles that need to be overcome before EBMTs are widely adopted. Reimbursement remains a major issue at most centers in the United States. Currently, most EBMTs are offered as a self-pay procedure, making the majority of patients who are otherwise eligible and interested not able to afford the procedure. With the recently published MERIT trial, long-term data on ESG as well as several upcoming society guidelines on EBMTs, we are hopeful that insurance coverage for EBMTs is nearing. Another important aspect is training. While IGB placement and removal are simple procedures, performing a high-quality ESG/POSE requires rigorous training to ensure safety and optimal outcomes. Several professional societies are working hard to develop curriculums on EBMTs with a focus on hands-on training to ensure endoscopists are properly trained prior to starting their bariatric endoscopy program. At our institution, we have a dedicated training program focusing on bariatric endoscopy (i.e. separate from the traditional advanced endoscopy fellowship), where fellows learn advanced bariatric suturing and plication as well as multidisciplinary care for this patient population. I am hopeful that this kind of training will become more prevalent in the near future.
With mounting evidence supporting the benefits of EBMTs, bariatric endoscopy has revolutionized the care of patients suffering from obesity and its related comorbidities. Moving forward, the field will continue to evolve, and EBMT procedures will only become simpler, safer, and more effective. It is an exciting time for gastroenterologists to get involved.
Dr. Jirapinyo is the director of bariatric endoscopy fellowship at Brigham and Women’s Hospital/Harvard Medical School, Boston. She is board certified in internal medicine, gastroenterology, and obesity medicine and completed her bariatric endoscopy and advanced endoscopy fellowships at Brigham and Women’s Hospital. She serves as a consultant for Apollo Endosurgery, Spatz Medical, and ERBE, and she receives research support from USGI Medical, GI Dynamics, and Fractyl.
References
1. Abu Dayyeh BK et al. Lancet. 2022;400(10350):441-51.
2. Wilding JPH et al. N Engl J Med. 2021;384:989-1002.
3. Jirapinyo P and Thompson CC. Gastrointest Endosc. 2022;96(3):479-86.
4. Sharaiha RZ et al. Clin Gastroenterol Hepatol. 2021;19(5):1051-57.
5. Hemo B et al. Diabetes Res Clin Pract. 2011;94(2):269-75.
A new frontier for weight management: Assess your options carefully
BY CAROLYN NEWBERRY, MD
Considering the continued rise in obesity rates in this country coupled with an increase in associated digestive disease burden from conditions such as nonalcoholic fatty liver disease (NAFLD), gastroesophageal reflux disease (GERD), and select gastrointestinal malignancies, I believe it is now more important than ever for gastroenterologists to familiarize themselves with weight management principles and incorporation into clinical practice. A growing arsenal of tools is available for addressing excess weight, including medications and novel endobariatric techniques. Although the latter is an important consideration in patients with obesity, lifestyle counseling with or without weight loss medications sets the stage for sustainable weight loss success and may eliminate the need for procedural intervention. As such, current guidelines set forth by multiple societies, including the American Gastroenterological Association (AGA), emphasize the importance of lifestyle counseling targeting caloric restriction and increased physical activity along with medical augmentation via pharmacological agents in eligible patients.1,2 These guidelines underline the importance of medical weight management prior to consideration of procedural options, including both endobariatrics and more classic bariatric surgeries. This ensures patients understand approaches to weight loss via noninvasive means, reduces risk of weight regain by building foundational habits, and enhances overall success of procedures long term if they are pursued. In addition, newer pharmacological agents are now approaching total body weight loss percentages of currently available endobariatric techniques while still showing high tolerance rates and long-term efficacy, indicating some patients who previously would require procedures to meet weight loss goals may no longer need them.3 Alternatively, these medications may augment efforts prior to procedures, enhancing overall total body weight loss achieved. If patients are not introduced to such options initially and as a part of comprehensive care management planning, they may not achieve the same degree of weight loss success and metabolic optimization.
As a gastroenterologist co-leading a multidisciplinary weight management and lifestyle clinic, I have witnessed firsthand the enhanced outcomes in patients who pursue endobariatric procedures after establishing care with a clinical team and attempting (and succeeding) in weight loss via changes in diet, physical activity, and medication use. Patients should be encouraged to gain understanding of one’s own “personal relationship” with food and/or address medical and social barriers to weight loss maintenance prior to procedural intervention, which requires some lead time and ideally professional expertise from multiple team members, including a dietitian. Weight regain after anti-obesity surgery is common, with significant gain occurring in up to half of patients. Several factors have been associated with weight regain, including lack of consistent follow-up, excess calorie and simple carbohydrate intake, and inconsistent physical activity.4 As such, most insurance companies mandate a trial of at least 6 months of lifestyle and/or medical weight management prior to considering procedural reimbursement. Although robust longitudinal data for endobariatric outcomes is not yet available, it is reasonable to believe similar concepts may be in play. In fact, since endobariatric procedures are less invasive but also therefore more temporal (as in the case of endoscopic balloon placement, which is only approved for 6 months of continuous use), behavioral modification and medical management to reduce risk of significant weight regain is even more imperative. Even in the case of more durable procedures, such as endoscopic gastroplasty, lack of compliance with recommended dietary protocols can reduce efficacy by loosening and even ripping sutures prior to establishment of bridging fibrotic mucosal changes, which enhance longevity of the procedure and support continued gastric restriction and reduction in motility. Some patients who undergo endoscopic gastroplasty end up seeking out revision and repeat procedure later due to lack of results, which may be avoided with alternative dietary and lifestyle decisions in the postprocedural state.
The landscape of non-procedural weight management tools has changed in the last 1-2 years with the approval of newer injectable medications that disrupt insulin and hormonal pathways and produce sustainable weight loss similar to reported outcomes achieved with endobariatric procedures. These medications are becoming increasingly accessible and of interest to patients, with continued destigmatization of the use of weight loss drugs in practice, which had previous negative connotations and concerns regarding safety. New guidelines put forth by the AGA recommend adding pharmacological agents to lifestyle interventions over continuing lifestyle interventions alone if adequate weight loss has not been achieved with the latter.3 This further exemplifies the importance of a multifaceted approach to optimize medical weight management as first-line therapy for obesity and associated comorbidities.
In summary, although endobariatric procedures are an important tool for gastroenterologists to incorporate into their weight management plans, they must be implemented with care and only after lifestyle and medical interventions have failed to produce desired results. Shared decision making among providers and patients enhances weight loss efforts and augments sustainability of outcomes. Considering the rapidly evolving landscape of obesity medicine, gastroenterologists need to continue to stay up to date on best practices to improve patient care, reduce associated morbidity, and enhance outcomes of novel endobariatric procedures.
Dr. Newberry is with the Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), division of gastroenterology, Weill Cornell Medical Center, New York. She disclosed receiving speaker honorariums from Baxter International and InBody USA.
References
1. Acosta et al. Clin Gastroenterol Hepatol. 2017 May;15(5):631-49.
2. Jensen et al. Circulation. 2014;129:S102-38.
3. Grunvald et al. Gastroenterology. 2022;163(5):1198-225.
4. Athansiadis et al. Surg Endosc. 2021 Aug;35(8):4069-84.
Dear colleagues,
Treating obesity easily falls under our purview as gastroenterologists. But like the mouse who would bell the cat, our direct involvement has been limited. However, over the past decade, advances in endobariatrics and medical management have given us many options. But how do we choose from this growing armamentarium of minimally invasive procedures and weight loss medicines? What combination is best? And what about the standard “diet and exercise”?
In this issue of perspectives, Carolyn Newberry, MD, director of GI nutrition at Innovation Center for Health and Nutrition in Gastroenterology, Weill Cornell Medicine, New York, will emphasize the benefits of medical and lifestyle management. Pichamol Jirapinyo, MD, MPH, ABOM, director of bariatric endoscopy fellowship at Brigham and Women’s Hospital/Harvard Medical School, Boston, responds with robust data for endoscopic therapies. We hope that their expert perspectives will help guide you in your own approach to obesity management – certainly no one size fits all. I welcome your thoughts on this growing field in gastroenterology – share with us on Twitter @AGA_GIHN.
Gyanprakash A. Ketwaroo, MD, MSc, is associate professor of medicine, Yale University, New Haven, Conn., and chief of endoscopy at West Haven (Conn.) VA Medical Center. He is an associate editor for GI & Hepatology News.
Exciting time for endoscopic bariatric and metabolic therapies (EBMTs)
BY PICHAMOL JIRAPINYO, MD, MPH, ABOM
2022 was an exciting year for our field of endoscopic bariatric and metabolic therapy (EBMT). Not only did it mark the 10th year anniversary since the very first-in-human endoscopic sleeve gastroplasty (ESG) performed by Christopher Thompson and Robert Hawes in India, but also the MERIT trial (a randomized-controlled trial on ESG) was published.1 This decade of work led to the OverStitch Endoscopic Suturing System (Apollo Endosurgery, Austin, Tex.) being granted de novo authorization from the Food and Drug Administration for the treatment of obesity and weight regain following bariatric surgery.
Currently, at our institution, we offer four primary EBMTs for patients who are seeking endoscopic weight loss therapy and have not yet undergone prior bariatric surgery. These include the Orbera intragastric balloon (IGB) (Apollo Endosurgery), ESG (Apollo Endosurgery), primary obesity surgery endoluminal (POSE: USGI Medical, San Clemente, Calif.), and a gastric plication procedure using Endomina (Endo Tools Therapeutics, Gosselies, Belgium). While the former two have FDA approval, the latter two devices have FDA clearance for tissue approximation. The indication for primary EBMTs includes having a body mass index of at least 30 kg/m2.
From our experience, patients who present to our bariatric endoscopy clinic consist of three groups. First are those who have tried several anti-obesity medications (AOMs), but are unable to tolerate the side effects or their BMI remains greater than 30 kg/m2. Second are those who have heard about EBMTs and are interested in the procedures. Usually, these patients are either too light to qualify for bariatric surgery (BMI 30-35 kg/m2 or 35-40 kg/m2 without an obesity-related comorbidity) or are not interested in bariatric surgery for a variety of reasons, including its perceived invasiveness. The last group are those whose BMI falls within the “super obese” category, defined as a BMI ≥ 50 kg/m2, who are deemed too high risk to undergo medically necessary procedures, such as an orthopedic, colorectal, or transplant surgery.
During the initial consultation, I always discuss pros and cons of all treatment modalities for obesity with the patients, ranging from lifestyle modification to AOMs, EBMTs, and bariatric surgeries. While the data on AOMs are promising, especially with the most recent FDA-approved semaglutide (Wegovy: Novo Nordisk, Bagsvaerd, Denmark) yielding 14.9% total weight loss (TWL) at 1 year, in reality, the starting doses of this medication have been out of stock for over a year.2 Other AOMs, on the other hand, are associated with 6%-8% TWL and are frequently associated with intolerance due to side effects. In comparison, meta-analyses demonstrate that an IGB is associated with 11.3% TWL and ESG with 16.5% TWL at 1 year. Our recent publication describing a new technique for POSE, also known as a distal POSE procedure with a double-helix technique, demonstrates a 20.3% TWL at 1 year.3 The rate of serious adverse events for EBMTs is low with 0.1% for IGB and 1%-2% for ESG/POSE.
The question regarding a comparison between AOMs and EBMTs comes up quite frequently in clinical practice. In reality, I often encourage my patients to consider combination therapy where I prescribe an AOM at 3-6 months following EBMTs to augment the amount of weight loss. However, since this is a debate, I will highlight a few advantages of EBMTs. First, the amount of weight loss following EBMTs, especially with ESG/POSE (which is currently the most commonly-requested procedure in our practice), tends to be higher than that of most AOMs. Second, while we are eagerly awaiting the long-term safety and efficacy data for semaglutide, ESG has been shown to be durable with the patients maintaining 15.9% TWL at 5 years.4 Third, an EBMT is a one-time procedure. In contrast, AOMs rely on patients’ compliance with taking the medication(s) reliably and indefinitely. A study based on HMO pharmacy data of over a million patients who were prescribed AOMs showed that fewer than 2% completed 12 months of weight loss medication therapy.5 The long-term use of AOMs also has cost implications. Specifically, a month supply of semaglutide costs about $1,400, which translates to $16,800 in 1 year and $84,000 in 5 years, which clearly outweighs the cost of ESG/POSE that has been demonstrated to be durable up to at least 5 years. IGBs have limitations similar to those of AOMs upon removal. Nevertheless, with the average cost of an IGB being $8,000, placing one every year would still be less costly, although this would likely be unnecessary considering the weight loss trend after IGB.
There are a few hurdles that need to be overcome before EBMTs are widely adopted. Reimbursement remains a major issue at most centers in the United States. Currently, most EBMTs are offered as a self-pay procedure, making the majority of patients who are otherwise eligible and interested not able to afford the procedure. With the recently published MERIT trial, long-term data on ESG as well as several upcoming society guidelines on EBMTs, we are hopeful that insurance coverage for EBMTs is nearing. Another important aspect is training. While IGB placement and removal are simple procedures, performing a high-quality ESG/POSE requires rigorous training to ensure safety and optimal outcomes. Several professional societies are working hard to develop curriculums on EBMTs with a focus on hands-on training to ensure endoscopists are properly trained prior to starting their bariatric endoscopy program. At our institution, we have a dedicated training program focusing on bariatric endoscopy (i.e. separate from the traditional advanced endoscopy fellowship), where fellows learn advanced bariatric suturing and plication as well as multidisciplinary care for this patient population. I am hopeful that this kind of training will become more prevalent in the near future.
With mounting evidence supporting the benefits of EBMTs, bariatric endoscopy has revolutionized the care of patients suffering from obesity and its related comorbidities. Moving forward, the field will continue to evolve, and EBMT procedures will only become simpler, safer, and more effective. It is an exciting time for gastroenterologists to get involved.
Dr. Jirapinyo is the director of bariatric endoscopy fellowship at Brigham and Women’s Hospital/Harvard Medical School, Boston. She is board certified in internal medicine, gastroenterology, and obesity medicine and completed her bariatric endoscopy and advanced endoscopy fellowships at Brigham and Women’s Hospital. She serves as a consultant for Apollo Endosurgery, Spatz Medical, and ERBE, and she receives research support from USGI Medical, GI Dynamics, and Fractyl.
References
1. Abu Dayyeh BK et al. Lancet. 2022;400(10350):441-51.
2. Wilding JPH et al. N Engl J Med. 2021;384:989-1002.
3. Jirapinyo P and Thompson CC. Gastrointest Endosc. 2022;96(3):479-86.
4. Sharaiha RZ et al. Clin Gastroenterol Hepatol. 2021;19(5):1051-57.
5. Hemo B et al. Diabetes Res Clin Pract. 2011;94(2):269-75.
A new frontier for weight management: Assess your options carefully
BY CAROLYN NEWBERRY, MD
Considering the continued rise in obesity rates in this country coupled with an increase in associated digestive disease burden from conditions such as nonalcoholic fatty liver disease (NAFLD), gastroesophageal reflux disease (GERD), and select gastrointestinal malignancies, I believe it is now more important than ever for gastroenterologists to familiarize themselves with weight management principles and incorporation into clinical practice. A growing arsenal of tools is available for addressing excess weight, including medications and novel endobariatric techniques. Although the latter is an important consideration in patients with obesity, lifestyle counseling with or without weight loss medications sets the stage for sustainable weight loss success and may eliminate the need for procedural intervention. As such, current guidelines set forth by multiple societies, including the American Gastroenterological Association (AGA), emphasize the importance of lifestyle counseling targeting caloric restriction and increased physical activity along with medical augmentation via pharmacological agents in eligible patients.1,2 These guidelines underline the importance of medical weight management prior to consideration of procedural options, including both endobariatrics and more classic bariatric surgeries. This ensures patients understand approaches to weight loss via noninvasive means, reduces risk of weight regain by building foundational habits, and enhances overall success of procedures long term if they are pursued. In addition, newer pharmacological agents are now approaching total body weight loss percentages of currently available endobariatric techniques while still showing high tolerance rates and long-term efficacy, indicating some patients who previously would require procedures to meet weight loss goals may no longer need them.3 Alternatively, these medications may augment efforts prior to procedures, enhancing overall total body weight loss achieved. If patients are not introduced to such options initially and as a part of comprehensive care management planning, they may not achieve the same degree of weight loss success and metabolic optimization.
As a gastroenterologist co-leading a multidisciplinary weight management and lifestyle clinic, I have witnessed firsthand the enhanced outcomes in patients who pursue endobariatric procedures after establishing care with a clinical team and attempting (and succeeding) in weight loss via changes in diet, physical activity, and medication use. Patients should be encouraged to gain understanding of one’s own “personal relationship” with food and/or address medical and social barriers to weight loss maintenance prior to procedural intervention, which requires some lead time and ideally professional expertise from multiple team members, including a dietitian. Weight regain after anti-obesity surgery is common, with significant gain occurring in up to half of patients. Several factors have been associated with weight regain, including lack of consistent follow-up, excess calorie and simple carbohydrate intake, and inconsistent physical activity.4 As such, most insurance companies mandate a trial of at least 6 months of lifestyle and/or medical weight management prior to considering procedural reimbursement. Although robust longitudinal data for endobariatric outcomes is not yet available, it is reasonable to believe similar concepts may be in play. In fact, since endobariatric procedures are less invasive but also therefore more temporal (as in the case of endoscopic balloon placement, which is only approved for 6 months of continuous use), behavioral modification and medical management to reduce risk of significant weight regain is even more imperative. Even in the case of more durable procedures, such as endoscopic gastroplasty, lack of compliance with recommended dietary protocols can reduce efficacy by loosening and even ripping sutures prior to establishment of bridging fibrotic mucosal changes, which enhance longevity of the procedure and support continued gastric restriction and reduction in motility. Some patients who undergo endoscopic gastroplasty end up seeking out revision and repeat procedure later due to lack of results, which may be avoided with alternative dietary and lifestyle decisions in the postprocedural state.
The landscape of non-procedural weight management tools has changed in the last 1-2 years with the approval of newer injectable medications that disrupt insulin and hormonal pathways and produce sustainable weight loss similar to reported outcomes achieved with endobariatric procedures. These medications are becoming increasingly accessible and of interest to patients, with continued destigmatization of the use of weight loss drugs in practice, which had previous negative connotations and concerns regarding safety. New guidelines put forth by the AGA recommend adding pharmacological agents to lifestyle interventions over continuing lifestyle interventions alone if adequate weight loss has not been achieved with the latter.3 This further exemplifies the importance of a multifaceted approach to optimize medical weight management as first-line therapy for obesity and associated comorbidities.
In summary, although endobariatric procedures are an important tool for gastroenterologists to incorporate into their weight management plans, they must be implemented with care and only after lifestyle and medical interventions have failed to produce desired results. Shared decision making among providers and patients enhances weight loss efforts and augments sustainability of outcomes. Considering the rapidly evolving landscape of obesity medicine, gastroenterologists need to continue to stay up to date on best practices to improve patient care, reduce associated morbidity, and enhance outcomes of novel endobariatric procedures.
Dr. Newberry is with the Innovative Center for Health and Nutrition in Gastroenterology (ICHANGE), division of gastroenterology, Weill Cornell Medical Center, New York. She disclosed receiving speaker honorariums from Baxter International and InBody USA.
References
1. Acosta et al. Clin Gastroenterol Hepatol. 2017 May;15(5):631-49.
2. Jensen et al. Circulation. 2014;129:S102-38.
3. Grunvald et al. Gastroenterology. 2022;163(5):1198-225.
4. Athansiadis et al. Surg Endosc. 2021 Aug;35(8):4069-84.
From the editor: Building community - Introducing Member Spotlight
Happy New Year, everyone! In early December, I attended the 2022 AGA Women’s Leadership Collaboration Conference to discuss strategies to promote gender equity in our profession. It was an inspiring weekend and reminded me how many talented individuals we have in the field of gastroenterology, all with fascinating personal and professional stories and much to contribute. I think I speak for all attendees in saying that it was a privilege to have the opportunity to interact with this amazing group of women leaders, reflect on our shared experiences and visions for the future of GI, and expand our networks.
This month we are excited to launch a new recurring feature in the newspaper and online – the Member Spotlight column. AGA has more than16,000 members from varied backgrounds. Yet the reality is that each of our individual networks is much smaller, and we would all benefit from learning more about one other and building a greater sense of community. To that end, starting with this issue, we will feature a different AGA member each month in our Member Spotlight column. The goal of this new feature is to recognize AGA members’ accomplishments across all career stages and practice settings, to highlight the diversity of our membership, and to help AGA members feel more connected by learning more about each other. Our inaugural Member Spotlight column highlights Patricia Jones, MD, associate professor at the University of Miami and an accomplished hepatologist. We thank Dr. Jones for sharing her story with us.
This will be a recurring monthly feature, so please consider nominating your colleagues (including trainees, practicing GIs in academics and community practice, those with non-traditional careers or unique pursuits outside of medicine, and others) to be featured in future Member Spotlight columns! It’s a great way for the nominee’s accomplishments to be recognized and to build a sense of community among the broader AGA membership. To submit a nomination, please send the nominee’s name, email, and a brief description of why you are nominating them to: [email protected]. We look forward to reviewing your submissions and hope you will use these Member Spotlights as an opportunity to strike up a conversation with someone new and expand your networks.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Happy New Year, everyone! In early December, I attended the 2022 AGA Women’s Leadership Collaboration Conference to discuss strategies to promote gender equity in our profession. It was an inspiring weekend and reminded me how many talented individuals we have in the field of gastroenterology, all with fascinating personal and professional stories and much to contribute. I think I speak for all attendees in saying that it was a privilege to have the opportunity to interact with this amazing group of women leaders, reflect on our shared experiences and visions for the future of GI, and expand our networks.
This month we are excited to launch a new recurring feature in the newspaper and online – the Member Spotlight column. AGA has more than16,000 members from varied backgrounds. Yet the reality is that each of our individual networks is much smaller, and we would all benefit from learning more about one other and building a greater sense of community. To that end, starting with this issue, we will feature a different AGA member each month in our Member Spotlight column. The goal of this new feature is to recognize AGA members’ accomplishments across all career stages and practice settings, to highlight the diversity of our membership, and to help AGA members feel more connected by learning more about each other. Our inaugural Member Spotlight column highlights Patricia Jones, MD, associate professor at the University of Miami and an accomplished hepatologist. We thank Dr. Jones for sharing her story with us.
This will be a recurring monthly feature, so please consider nominating your colleagues (including trainees, practicing GIs in academics and community practice, those with non-traditional careers or unique pursuits outside of medicine, and others) to be featured in future Member Spotlight columns! It’s a great way for the nominee’s accomplishments to be recognized and to build a sense of community among the broader AGA membership. To submit a nomination, please send the nominee’s name, email, and a brief description of why you are nominating them to: [email protected]. We look forward to reviewing your submissions and hope you will use these Member Spotlights as an opportunity to strike up a conversation with someone new and expand your networks.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Happy New Year, everyone! In early December, I attended the 2022 AGA Women’s Leadership Collaboration Conference to discuss strategies to promote gender equity in our profession. It was an inspiring weekend and reminded me how many talented individuals we have in the field of gastroenterology, all with fascinating personal and professional stories and much to contribute. I think I speak for all attendees in saying that it was a privilege to have the opportunity to interact with this amazing group of women leaders, reflect on our shared experiences and visions for the future of GI, and expand our networks.
This month we are excited to launch a new recurring feature in the newspaper and online – the Member Spotlight column. AGA has more than16,000 members from varied backgrounds. Yet the reality is that each of our individual networks is much smaller, and we would all benefit from learning more about one other and building a greater sense of community. To that end, starting with this issue, we will feature a different AGA member each month in our Member Spotlight column. The goal of this new feature is to recognize AGA members’ accomplishments across all career stages and practice settings, to highlight the diversity of our membership, and to help AGA members feel more connected by learning more about each other. Our inaugural Member Spotlight column highlights Patricia Jones, MD, associate professor at the University of Miami and an accomplished hepatologist. We thank Dr. Jones for sharing her story with us.
This will be a recurring monthly feature, so please consider nominating your colleagues (including trainees, practicing GIs in academics and community practice, those with non-traditional careers or unique pursuits outside of medicine, and others) to be featured in future Member Spotlight columns! It’s a great way for the nominee’s accomplishments to be recognized and to build a sense of community among the broader AGA membership. To submit a nomination, please send the nominee’s name, email, and a brief description of why you are nominating them to: [email protected]. We look forward to reviewing your submissions and hope you will use these Member Spotlights as an opportunity to strike up a conversation with someone new and expand your networks.
Megan A. Adams, MD, JD, MSc
Editor-in-Chief
Miami hepatologist leverages Golden Rule to balance work, family, address health equities
Hepatologist Patricia Denise Jones, MD, recollects the balancing act of going through medical training while caring for her four children.
“I had them at every stage: my first one as a medical student; twins when I was a resident, and my last one at the end of fellowship. It was challenging, trying to put their needs first while trying to be a great doctor, learning how to do research,” said Dr. Jones, an associate professor at the University of Miami Health system.
She has no regrets. “I think I’m a better doctor and colleague because I have children. Showing my kids how important it is to help and serve others is one of the best legacies I can leave them.”

If there’s anything she’d like to fix, it’s the healthcare delivery system for patients disproportionately affected by liver disease.
Dr. Jones was selected as 1 of 10 scholars in the inaugural cohort of the National Institutes of Health–funded program Fostering Opportunities Resulting in Workforce and Research Diversity (FORWARD) where she participated in a 2-year program of leadership development, mentorship, and research skills development.
In an interview,
Q: Describe your current practice. What gives you the most joy in your day-to-day practice?
Dr. Jones: Being able to make a difference in the lives of patients. A lot of the patients that I take care of have difficulty navigating the health system. That’s the population I feel most inclined to serve. It’s always rewarding to help someone make a connection that they couldn’t make on their own or help them understand something that wasn’t clear. Knowing that you’re helping someone to live a healthier life is deeply gratifying.
Q: Tell me about your patient population.
Dr. Jones: My focus is patients with liver cancer, hepatocellular carcinoma specifically, and cirrhosis patients. They tend to be sick relative to most Americans. I also take care of people who have other forms of liver disease like fatty liver and viral hepatitis. I live in Miami, so most of the patients that I take care of are going to be Hispanic. A good percentage are immigrants with limited health literacy.
Q: What is your biggest practice-related challenge? What are you doing to address it?
Dr. Jones: Lack of insurance and underinsurance. One patient of mine with Medicare and Humana has a carve out: She can see me and some of my colleagues but not the oncologist or a radiation oncologist. For her to be seen in our center, she would have to get a referral from a doctor in a different county. This makes no sense. It’s a hard problem to solve. To me, that’s the most challenging thing – not being able to help when something is beyond my control, beyond what I understand, and translating it into action.
Q: What general principles guide you in your professional and personal life?
Dr. Jones: I try to think of the Golden Rule in every encounter with a person, either in clinic or in real life, as if they were my mother or sister. If I’m frustrated or having a bad day, what would I want that person’s experience to be with their doctor? I also try to assume the best possible intent with people.

Q: What teacher, mentor, or other influences had the greatest impact on you?
Dr. Jones: My father. He started out as a salesman, worked in legislation, and then retired early to focus on and build up our community, making sure that we were better off than we were before. In terms of my professional life, Robert Sandler, MD is one of my greatest mentors. He is at the University of North Carolina and was the division chief of gastroenterology. He saw potential in me and supports me to this day. If you need something, he’s there. If you need him to comment on your draft, he’s very reliable and gives you great, critical feedback.
Q: In 10 years, what do you hope you are doing or what do you hope you have accomplished?
Dr. Jones: In 10 years, I hope that my efforts will have revolutionized our approach to delivering care to vulnerable populations. Much of the work that has been done thus far in the field of disparities and liver disease has focused on describing the inequities. However, I have just started working in health equity. This will require partnering with patients and caregivers to get a better understanding of their needs and collaborating with legislators to increase funding directed towards building the infrastructure necessary to deliver health care to those who have been forgotten.
Lightning round questions
Favorite movie, show, or book
Forrest Gump, Blackish, anything by Toni Morrison
Favorite music genre
Hip Hop
Favorite food
Seafood
Favorite travel destination
Tanzania
Your ideal type of pet
Dog
Optimist or pessimist?
Optimist!
Dr. Jones is on Twitter @DrLiverPatty.
Hepatologist Patricia Denise Jones, MD, recollects the balancing act of going through medical training while caring for her four children.
“I had them at every stage: my first one as a medical student; twins when I was a resident, and my last one at the end of fellowship. It was challenging, trying to put their needs first while trying to be a great doctor, learning how to do research,” said Dr. Jones, an associate professor at the University of Miami Health system.
She has no regrets. “I think I’m a better doctor and colleague because I have children. Showing my kids how important it is to help and serve others is one of the best legacies I can leave them.”

If there’s anything she’d like to fix, it’s the healthcare delivery system for patients disproportionately affected by liver disease.
Dr. Jones was selected as 1 of 10 scholars in the inaugural cohort of the National Institutes of Health–funded program Fostering Opportunities Resulting in Workforce and Research Diversity (FORWARD) where she participated in a 2-year program of leadership development, mentorship, and research skills development.
In an interview,
Q: Describe your current practice. What gives you the most joy in your day-to-day practice?
Dr. Jones: Being able to make a difference in the lives of patients. A lot of the patients that I take care of have difficulty navigating the health system. That’s the population I feel most inclined to serve. It’s always rewarding to help someone make a connection that they couldn’t make on their own or help them understand something that wasn’t clear. Knowing that you’re helping someone to live a healthier life is deeply gratifying.
Q: Tell me about your patient population.
Dr. Jones: My focus is patients with liver cancer, hepatocellular carcinoma specifically, and cirrhosis patients. They tend to be sick relative to most Americans. I also take care of people who have other forms of liver disease like fatty liver and viral hepatitis. I live in Miami, so most of the patients that I take care of are going to be Hispanic. A good percentage are immigrants with limited health literacy.
Q: What is your biggest practice-related challenge? What are you doing to address it?
Dr. Jones: Lack of insurance and underinsurance. One patient of mine with Medicare and Humana has a carve out: She can see me and some of my colleagues but not the oncologist or a radiation oncologist. For her to be seen in our center, she would have to get a referral from a doctor in a different county. This makes no sense. It’s a hard problem to solve. To me, that’s the most challenging thing – not being able to help when something is beyond my control, beyond what I understand, and translating it into action.
Q: What general principles guide you in your professional and personal life?
Dr. Jones: I try to think of the Golden Rule in every encounter with a person, either in clinic or in real life, as if they were my mother or sister. If I’m frustrated or having a bad day, what would I want that person’s experience to be with their doctor? I also try to assume the best possible intent with people.

Q: What teacher, mentor, or other influences had the greatest impact on you?
Dr. Jones: My father. He started out as a salesman, worked in legislation, and then retired early to focus on and build up our community, making sure that we were better off than we were before. In terms of my professional life, Robert Sandler, MD is one of my greatest mentors. He is at the University of North Carolina and was the division chief of gastroenterology. He saw potential in me and supports me to this day. If you need something, he’s there. If you need him to comment on your draft, he’s very reliable and gives you great, critical feedback.
Q: In 10 years, what do you hope you are doing or what do you hope you have accomplished?
Dr. Jones: In 10 years, I hope that my efforts will have revolutionized our approach to delivering care to vulnerable populations. Much of the work that has been done thus far in the field of disparities and liver disease has focused on describing the inequities. However, I have just started working in health equity. This will require partnering with patients and caregivers to get a better understanding of their needs and collaborating with legislators to increase funding directed towards building the infrastructure necessary to deliver health care to those who have been forgotten.
Lightning round questions
Favorite movie, show, or book
Forrest Gump, Blackish, anything by Toni Morrison
Favorite music genre
Hip Hop
Favorite food
Seafood
Favorite travel destination
Tanzania
Your ideal type of pet
Dog
Optimist or pessimist?
Optimist!
Dr. Jones is on Twitter @DrLiverPatty.
Hepatologist Patricia Denise Jones, MD, recollects the balancing act of going through medical training while caring for her four children.
“I had them at every stage: my first one as a medical student; twins when I was a resident, and my last one at the end of fellowship. It was challenging, trying to put their needs first while trying to be a great doctor, learning how to do research,” said Dr. Jones, an associate professor at the University of Miami Health system.
She has no regrets. “I think I’m a better doctor and colleague because I have children. Showing my kids how important it is to help and serve others is one of the best legacies I can leave them.”

If there’s anything she’d like to fix, it’s the healthcare delivery system for patients disproportionately affected by liver disease.
Dr. Jones was selected as 1 of 10 scholars in the inaugural cohort of the National Institutes of Health–funded program Fostering Opportunities Resulting in Workforce and Research Diversity (FORWARD) where she participated in a 2-year program of leadership development, mentorship, and research skills development.
In an interview,
Q: Describe your current practice. What gives you the most joy in your day-to-day practice?
Dr. Jones: Being able to make a difference in the lives of patients. A lot of the patients that I take care of have difficulty navigating the health system. That’s the population I feel most inclined to serve. It’s always rewarding to help someone make a connection that they couldn’t make on their own or help them understand something that wasn’t clear. Knowing that you’re helping someone to live a healthier life is deeply gratifying.
Q: Tell me about your patient population.
Dr. Jones: My focus is patients with liver cancer, hepatocellular carcinoma specifically, and cirrhosis patients. They tend to be sick relative to most Americans. I also take care of people who have other forms of liver disease like fatty liver and viral hepatitis. I live in Miami, so most of the patients that I take care of are going to be Hispanic. A good percentage are immigrants with limited health literacy.
Q: What is your biggest practice-related challenge? What are you doing to address it?
Dr. Jones: Lack of insurance and underinsurance. One patient of mine with Medicare and Humana has a carve out: She can see me and some of my colleagues but not the oncologist or a radiation oncologist. For her to be seen in our center, she would have to get a referral from a doctor in a different county. This makes no sense. It’s a hard problem to solve. To me, that’s the most challenging thing – not being able to help when something is beyond my control, beyond what I understand, and translating it into action.
Q: What general principles guide you in your professional and personal life?
Dr. Jones: I try to think of the Golden Rule in every encounter with a person, either in clinic or in real life, as if they were my mother or sister. If I’m frustrated or having a bad day, what would I want that person’s experience to be with their doctor? I also try to assume the best possible intent with people.

Q: What teacher, mentor, or other influences had the greatest impact on you?
Dr. Jones: My father. He started out as a salesman, worked in legislation, and then retired early to focus on and build up our community, making sure that we were better off than we were before. In terms of my professional life, Robert Sandler, MD is one of my greatest mentors. He is at the University of North Carolina and was the division chief of gastroenterology. He saw potential in me and supports me to this day. If you need something, he’s there. If you need him to comment on your draft, he’s very reliable and gives you great, critical feedback.
Q: In 10 years, what do you hope you are doing or what do you hope you have accomplished?
Dr. Jones: In 10 years, I hope that my efforts will have revolutionized our approach to delivering care to vulnerable populations. Much of the work that has been done thus far in the field of disparities and liver disease has focused on describing the inequities. However, I have just started working in health equity. This will require partnering with patients and caregivers to get a better understanding of their needs and collaborating with legislators to increase funding directed towards building the infrastructure necessary to deliver health care to those who have been forgotten.
Lightning round questions
Favorite movie, show, or book
Forrest Gump, Blackish, anything by Toni Morrison
Favorite music genre
Hip Hop
Favorite food
Seafood
Favorite travel destination
Tanzania
Your ideal type of pet
Dog
Optimist or pessimist?
Optimist!
Dr. Jones is on Twitter @DrLiverPatty.
Unintentional weight loss
This patient's clinical presentation is consistent with a diagnosis of metastatic invasive lobular carcinoma, with nodal involvement.
Breast cancer is one of the most frequently diagnosed cancers worldwide. In Western countries, 1 in 8 women will be diagnosed with breast cancer at some point in their lives. Various histologic subtypes with specific clinical characteristics exist. Invasive lobular carcinoma (ILC) is the second most common subtype, accounting for an estimated 10%-15% of breast cancers. Over the past two decades, a significant increase has been observed in the incidence of ILC, particularly among postmenopausal women. Improved diagnostic techniques and the use of hormone replacement therapy may account for this increased incidence. White women have the highest incidence of ILC; however, compared with White women and women of other races, Black women experience the worst 5-year overall survival from ILC.
ILC arises in the mammary ducts (lobules) of the breast. Women with ILC are typically slightly older than women with invasive breast cancer of no special type at diagnosis (mean age 63.4 vs 59.5 years, respectively). Risk factors for ILC may include early menarche, use of progesterone-based hormone replacement therapy, late age at first live birth, and alcohol consumption.
In most cases, ILC does not form a discrete palpable mass until it has reached an advanced stage, making it more difficult to detect through physical examination or imaging. Patients often present with a large tumor and with nodal involvement. A slight thickening of the nipple, an exudative scab on the skin, or other changes in the skin, such as flushing or swelling, may be seen in patients presenting with advanced disease. Additionally, ILC tumors are often bilateral and multifocal.
ILC is predominantly a histopathologic diagnosis based on standard hematoxylin and eosin staining. Histologically, ILC is characterized by a proliferation of small cells that lack cohesion. These cells are often dispersed individually through a fibrous connective tissue; alternatively, they may be organized in single-file linear cords invading the stroma. A concentric pattern around normal ducts is often seen in the infiltrating cords. There is usually little host reaction of the background architecture. Round or notched ovoid nuclei are seen in the neoplastic cells, along with a thin rim of cytoplasm. Occasionally, an intracytoplasmic lumen is present and may harbor a central mucoid inclusion. Very few or no mitoses are seen.
Several variants of ILC exist, all of which lack cell-to-cell cohesion. These include:
• Solid type
• Pleomorphic lobular carcinoma
• Tubulo-lobular variant
• Alveolar variant
• Mixed type
Complete loss of E-cadherin expression occurs in most ILCs, which can help to differentiate it from invasive ductal cancers or ductal carcinomas in situ. Diffuse cortical thickening without hilar mass effect is often seen in nodal metastases associated with ILC.
Most classic ILCs are estrogen receptor– and progesterone receptor–positive. Conversely, HER2 overexpression and amplification rarely occurs in ILC.
Late relapses more than 10 years after remission may occur. In addition to frequent bone and liver metastasis, ILC is associated with metastatic spread to unusual sites, including the peritoneum, gastrointestinal tract, urinary tract, leptomeninges, skin, orbit, and ovaries.
Mastectomy is often indicated in ILC. In the neoadjuvant setting, ILC is associated with low pathologic complete response rates. Endocrine therapy in the neoadjuvant setting is an emerging approach for some patients with ILC. According to 2022 National Comprehensive Cancer Network guidelines, adjuvant chemotherapy followed by endocrine therapy or endocrine therapy alone should be considered for pre- and postmenopausal patients with ILC.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's clinical presentation is consistent with a diagnosis of metastatic invasive lobular carcinoma, with nodal involvement.
Breast cancer is one of the most frequently diagnosed cancers worldwide. In Western countries, 1 in 8 women will be diagnosed with breast cancer at some point in their lives. Various histologic subtypes with specific clinical characteristics exist. Invasive lobular carcinoma (ILC) is the second most common subtype, accounting for an estimated 10%-15% of breast cancers. Over the past two decades, a significant increase has been observed in the incidence of ILC, particularly among postmenopausal women. Improved diagnostic techniques and the use of hormone replacement therapy may account for this increased incidence. White women have the highest incidence of ILC; however, compared with White women and women of other races, Black women experience the worst 5-year overall survival from ILC.
ILC arises in the mammary ducts (lobules) of the breast. Women with ILC are typically slightly older than women with invasive breast cancer of no special type at diagnosis (mean age 63.4 vs 59.5 years, respectively). Risk factors for ILC may include early menarche, use of progesterone-based hormone replacement therapy, late age at first live birth, and alcohol consumption.
In most cases, ILC does not form a discrete palpable mass until it has reached an advanced stage, making it more difficult to detect through physical examination or imaging. Patients often present with a large tumor and with nodal involvement. A slight thickening of the nipple, an exudative scab on the skin, or other changes in the skin, such as flushing or swelling, may be seen in patients presenting with advanced disease. Additionally, ILC tumors are often bilateral and multifocal.
ILC is predominantly a histopathologic diagnosis based on standard hematoxylin and eosin staining. Histologically, ILC is characterized by a proliferation of small cells that lack cohesion. These cells are often dispersed individually through a fibrous connective tissue; alternatively, they may be organized in single-file linear cords invading the stroma. A concentric pattern around normal ducts is often seen in the infiltrating cords. There is usually little host reaction of the background architecture. Round or notched ovoid nuclei are seen in the neoplastic cells, along with a thin rim of cytoplasm. Occasionally, an intracytoplasmic lumen is present and may harbor a central mucoid inclusion. Very few or no mitoses are seen.
Several variants of ILC exist, all of which lack cell-to-cell cohesion. These include:
• Solid type
• Pleomorphic lobular carcinoma
• Tubulo-lobular variant
• Alveolar variant
• Mixed type
Complete loss of E-cadherin expression occurs in most ILCs, which can help to differentiate it from invasive ductal cancers or ductal carcinomas in situ. Diffuse cortical thickening without hilar mass effect is often seen in nodal metastases associated with ILC.
Most classic ILCs are estrogen receptor– and progesterone receptor–positive. Conversely, HER2 overexpression and amplification rarely occurs in ILC.
Late relapses more than 10 years after remission may occur. In addition to frequent bone and liver metastasis, ILC is associated with metastatic spread to unusual sites, including the peritoneum, gastrointestinal tract, urinary tract, leptomeninges, skin, orbit, and ovaries.
Mastectomy is often indicated in ILC. In the neoadjuvant setting, ILC is associated with low pathologic complete response rates. Endocrine therapy in the neoadjuvant setting is an emerging approach for some patients with ILC. According to 2022 National Comprehensive Cancer Network guidelines, adjuvant chemotherapy followed by endocrine therapy or endocrine therapy alone should be considered for pre- and postmenopausal patients with ILC.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
This patient's clinical presentation is consistent with a diagnosis of metastatic invasive lobular carcinoma, with nodal involvement.
Breast cancer is one of the most frequently diagnosed cancers worldwide. In Western countries, 1 in 8 women will be diagnosed with breast cancer at some point in their lives. Various histologic subtypes with specific clinical characteristics exist. Invasive lobular carcinoma (ILC) is the second most common subtype, accounting for an estimated 10%-15% of breast cancers. Over the past two decades, a significant increase has been observed in the incidence of ILC, particularly among postmenopausal women. Improved diagnostic techniques and the use of hormone replacement therapy may account for this increased incidence. White women have the highest incidence of ILC; however, compared with White women and women of other races, Black women experience the worst 5-year overall survival from ILC.
ILC arises in the mammary ducts (lobules) of the breast. Women with ILC are typically slightly older than women with invasive breast cancer of no special type at diagnosis (mean age 63.4 vs 59.5 years, respectively). Risk factors for ILC may include early menarche, use of progesterone-based hormone replacement therapy, late age at first live birth, and alcohol consumption.
In most cases, ILC does not form a discrete palpable mass until it has reached an advanced stage, making it more difficult to detect through physical examination or imaging. Patients often present with a large tumor and with nodal involvement. A slight thickening of the nipple, an exudative scab on the skin, or other changes in the skin, such as flushing or swelling, may be seen in patients presenting with advanced disease. Additionally, ILC tumors are often bilateral and multifocal.
ILC is predominantly a histopathologic diagnosis based on standard hematoxylin and eosin staining. Histologically, ILC is characterized by a proliferation of small cells that lack cohesion. These cells are often dispersed individually through a fibrous connective tissue; alternatively, they may be organized in single-file linear cords invading the stroma. A concentric pattern around normal ducts is often seen in the infiltrating cords. There is usually little host reaction of the background architecture. Round or notched ovoid nuclei are seen in the neoplastic cells, along with a thin rim of cytoplasm. Occasionally, an intracytoplasmic lumen is present and may harbor a central mucoid inclusion. Very few or no mitoses are seen.
Several variants of ILC exist, all of which lack cell-to-cell cohesion. These include:
• Solid type
• Pleomorphic lobular carcinoma
• Tubulo-lobular variant
• Alveolar variant
• Mixed type
Complete loss of E-cadherin expression occurs in most ILCs, which can help to differentiate it from invasive ductal cancers or ductal carcinomas in situ. Diffuse cortical thickening without hilar mass effect is often seen in nodal metastases associated with ILC.
Most classic ILCs are estrogen receptor– and progesterone receptor–positive. Conversely, HER2 overexpression and amplification rarely occurs in ILC.
Late relapses more than 10 years after remission may occur. In addition to frequent bone and liver metastasis, ILC is associated with metastatic spread to unusual sites, including the peritoneum, gastrointestinal tract, urinary tract, leptomeninges, skin, orbit, and ovaries.
Mastectomy is often indicated in ILC. In the neoadjuvant setting, ILC is associated with low pathologic complete response rates. Endocrine therapy in the neoadjuvant setting is an emerging approach for some patients with ILC. According to 2022 National Comprehensive Cancer Network guidelines, adjuvant chemotherapy followed by endocrine therapy or endocrine therapy alone should be considered for pre- and postmenopausal patients with ILC.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 47-year-old woman presented for her annual gynecologic examination. Her current height and weight were 5 ft 4 in and 133 lb. This reflected a 9-lb weight loss since the previous visit. At completion of the height and weight intake by a nurse, the patient reported being surprised by this unintentional weight loss. Her previous medical history was unremarkable except for an advanced maternal age pregnancy 5 years earlier and dental implant surgery approximately 1 month earlier. The patient believed that her weight loss was related to her diminished appetite and transient difficulty chewing following her dental surgery. Laboratory findings were all within normal ranges except for a hemoglobin level of 9.4 g/dL. Physical examination revealed a palpable mass in the right upper outer quadrant of the right breast with slight thickening of the nipple and a right axillary mass. The patient's last bilateral screening mammogram 3 months earlier did not reveal any suspicious masses or lesions.
An ultrasound-guided biopsy of the right breast and axillary lymph node was performed. Histopathologic findings included small tumor cells without cohesion arranged in single files, loss of the long arm of chromosome 16, and a complete loss of E-cadherin expression on immunohistochemistry. Additionally, the tumor was estrogen receptor–positive/progesterone receptor–positive and human epidermal growth factor receptor 2–negative (ER+/PR+/HER2-).
Botanical Briefs: Daffodils (Narcissus Species)
Contact dermatitis is a common problem in the floral bulb industry and is considered an occupational disease. Daffodils (Narcissus species)(Figure) are thought to be the most common cause of irritant contact dermatitis among florists.1
Clinical Importance
Picking daffodils can start as early as October, when the flowers are still closed. The picker’s hand slides down the stem to snap the stalk at the base. This potentially traumatic maneuver to the web of the fingers leads to abrasions, which are irritated by the sap and cause granulomatous sores and paronychia. An experienced picker can pick 20,000 flowers a day, leading to extensive contact with sap.2
Eczematous or granulomatous rash on the arms also is seen as the sap irritates the wrist and forearm. The pickers often hold the flowers until a bunch of 10 has been collected. The 10 flowers are held together by a rubber band and stacked along the arm, the chin, and the axilla, causing the rash to extend to those areas. Sap also can be transferred by the hand to other parts of the body, such as the face. In men, sap can be transferred to the genitalia as the men urinate in the field.
Narcissus also can cause poisoning if ingested by humans or animals. Researchers who analyzed calls made to the New Zealand Natural Poisons Centre between 2003 and 2010 determined that daffodil was the 11th most common call for plant-related poisoning.3
Although the severity of plant poisoning often is low due to the small amount of plant material usually consumed, more severe poisoning can occur when the plant is eaten for medicinal purposes or mistaken for an edible plant.3 Vomiting, respiratory symptoms, abdominal pain, diarrhea, trembling, and convulsions can occur when daffodils are ingested. Death has been reported due to ingestion of the bulbs.4
In February 2010, 10 children aged 10 and 11 years and their 22-year-old guide presented to an emergency department in Israel after ingesting Narcissus bulbs, which were mistakenly believed to be the bulbs of onions.4 Eight children and the guide vomited. One child and the guide reported abdominal pain. All were discharged in stable condition after 4 hours of observation.4
Clinical Manifestations
Daffodil rash or lily rash was first described in 1910.5 The typical rash presents as dryness, fissures, scaling, and erythema of the fingertips, hands, and forearms, often with subungual hyperkeratosis. Vesicles and pustules may be seen. The rash may extend to other areas of the body, including the face.6
Prevention and Treatment
Use of protective gloves and clothing to avoid contact with the plant is recommended.2 Treatment includes stopping contact with the irritant, eye irrigation, and supportive measures (airway, breathing, and circulation). Activated charcoal can be helpful if used within 1 hour after ingestion but is contraindicated in vomiting patients.4
Identifying Features
The genus Narcissus is in the family Amaryllidaceae and contains ornamental plants, including daffodil (trumpet Narcissus, Narcissus pseudonarcissus), jonquil (Narcissus jonquilla), and poet’s narcissus (Narcissus poeticus). Most species are perennial; the plant emerges from a bulb in spring. Leaves originate from the base of the plant and range from 5-cm to 1.2-meters long, depending on the species. The flowers span a range of shapes and colors—from a trumpet (the daffodil) to a ringlike cup (poet’s Narcissus) and in yellow, white, and pink.7
Distribution and Plant Facts
Distribution—There are approximately 80 to 100 wild Narcissus species, which are found in southwestern Europe, North Africa, the Balkan Peninsula, Italy, and France. There are more than 27,000 Narcissus cultivars registered in the International Daffodil Register.8
Plant Facts—The daffodil is the national flower of Wales. It also is often used to depict hope and joy and is the symbol of cancer charities in many countries.9
The name Narcissus is believed to have originated from Greek mythology. A handsome youth, Narcissus, fell in love with his own reflection, for which the gods punished him by turning him into a flower.10
Another theory states that Narcissus is derived from the Greek word narkao (to benumb) due to its narcotic properties. When an open wound is subjected to an extract of the bulb, numbness of the entire nervous system is said to occur as well as paralysis of the heart. This narcotic effect led Socrates to refer to the Narcissus plant as the “chaplet of the infernal gods.”11
Narcissus is an important flower in various ethnic rituals. The Greeks often planted daffodils near tombs. In Muslim culture, white is believed to be the symbol of good and purity; Narcissus was one of the most common white-flowered plants found in Muslim graveyards.12
Medicinal Qualities and Uses—Narcissus species have been used as medicinal plants for a variety of ailments. For example, Narcissus tazetta contains flavonoids, alkaloids, saponins, tannins, cardiac glycosides, oil, steroids, terpenoids, and anthraquinones that contribute to its antibacterial, antifungal, antiviral, antimalarial, anticancer, antioxidant, dermatologic, cardiovascular, immunomodulatory, and acetylcholinesterase inhibitory effects.13 In a study, chloroform extracts from N tazetta bulbs were found to be more active than doxorubicin against hepatocellular and colon cancer cell lines.14
More than 500 alkaloids have been isolated from the Narcissus genus.15 In 2001, the US Food and Drug Administration approved one of the alkaloids, galantamine, for the treatment of mild to moderate stages of Alzheimer disease.16 Galantamine selectively and reversibly inhibits acetylcholinesterase, the enzyme believed responsible for neurodegeneration seen in Alzheimer disease. Plants are the main source of galantamine, despite the ability of pharmaceutical companies to synthesize the compound. Galantamine hydrobromide is sold by prescription (Razadyne [Janssen Pharmaceuticals, Inc]); generic formulations approved by the US Food and Drug Administration have been produced by more than 15 pharmaceutical companies.17,18
Irritant and Allergen
Sap found in the bulbs and hollow stems of Narcissus contains calcium oxalate crystals, or raphides. The minute, needle-shaped calcium oxalate crystals are believed to be a waste product of cellular metabolism.19 When the plant structure is compromised by pickers snapping the stalk, the sharp crystals penetrate the skin to cause an irritant contact dermatitis.
Relevant Research—A study used electron microscopy to characterize the structure of raphides from various plants,2 though not from Narcissus species; the structure of each raphide was then compared to the degree of irritation it produced. The researchers concluded that more elongated crystals (those containing barbs) produce a greater degree of irritation. Narcissus species are known to cause varying degrees of skin irritation: For example, N tazetta rarely causes skin irritation, whereas N pseudonarcissi (daffodil) tends to cause remarkably more skin irritation.2
Allergic reactions to and strong toxicity from Narcissus species are not well understood. In a study, only 2 alkaloids—homolycorine and masonin—produced a weakly positive reaction in patch tests on sensitized guinea pigs, which correlates with the finding of a different study, in which only 2 of 12 patients whose findings were examined over 14 years had a positive patch test for Narcissus.20,21
However, IgE-mediated allergies indicative of an allergic response to Narcissus have been reported. A study isolated an allergenic protein, narcin, from bulbs of N tazetta. Narcin is a 13-kDa protein with potent allergenic effects capable of inducing production of proinflammatory cytokines and increasing IgE levels in mononuclear cells in peripheral blood.22
More research is required to find and understand the compounds responsible for causing an allergic reaction to Narcissus.
- Modi GM, Doherty CB, Katta R, et al. Irritant contact dermatitis from plants. Dermatitis. 2009;20:63-78. doi:10.2310/6620.2009.08051
- Julian CG, Bowers PW. The nature and distribution of daffodil pickers’ rash. Contact Dermatitis. 1997;37:259-262. doi:10.1111/j.1600-0536.1997.tb02461.x
- Slaughter RJ, Beasley DMG, Lambie BS, et al. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012;125:87-118.
- Hussein A, Yassin A. Poisoning following ingestion of Narcissus tazetta bulbs by schoolchildren. Isr Med Assoc J. 2014;16:125-126.
- Hanks GR, ed. Narcissus and Daffodil: The Genus Narcissus. CRC Press; 2002. https://doi.org/10.1201/9780203219355
- McGovern TW. Botanical briefs: daffodils—Narcissus L. Cutis. 2000;65:130-132.
- The Editors of Encyclopaedia Britannica. Narcissus. Encyclopedia Britannica. Accessed December 13, 2022. https://www.britannica.com/plant/narcissus-plant
- M, A, D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018;41:208-218. doi:10.1007/s12272-017-1000-4
- Crampton L. Beautiful daffodils: plant facts, toxicity, and a symbol of hope. Owlcation. April 19, 2022. Accessed December 13, 2022. https://owlcation.com/stem/Daffodils-Beautiful-Flowers-and-a-Symbol-of-Hope
- Rademaker M. Daffodil. DermNet. Published 1999. Accessed December 13, 2022. https://dermnetnz.org/topics/daffodil
- Grieve M. Narcissus. Accessed December 13, 2022. https://botanical.com/botanical/mgmh/n/narcis01.html
- Dafni A, Lev E, Beckmann S, et al. Ritual plants of Muslim graveyards in northern Israel. J Ethnobiolog Ethnomed. 2006;2:38. doi:10.1186/1746-4269-2-38
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm. 2020;10:44-53.
- Shawky E, Abou-Donia AH, Darwish FA, et al. In vitro cytotoxicity of some Narcissus plants extracts. Nat Prod Res. 2015;29:363-365. doi:10.1080/14786419.2014.942302
- Havlasov J, M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014;9:1151-1155.
- Pigni NB, S, V, et al. Alkaloids from Narcissus serotinus. J Nat Prod. 2012;75:1643-1647. doi:10.1021/np3003595
- Razadyne. Prescribing information. Janssen Pharmaceuticals, Inc; 2013. Accessed December 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021169Orig1s032,021224Orig1s030,021615Orig1s023lbl.pdf
- Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci. 2013;14:11713-11741. doi:10.3390/ijms140611713
- Evans FJ, Schmidt RJ. Plants and plant products that induce contact dermatitis. Planta Med. 1980;38:289-316. doi:10.1055/s-2008-1074883
- Gude M, Hausen BM, Heitsch H, et al. An investigation of the irritant and allergenic properties of daffodils (Narcissus pseudonarcissus L., Amaryllidaceae). a review of daffodil dermatitis. Contact Dermatitis. 1988;19:1-10.
- Lamminpää A, Estlander T, Jolanki R, et al. Occupational allergic contact dermatitis caused by decorative plants. Contact Dermatitis. 1996;34:330-335.
- Sinha M, Singh A, Shokeen A, et al. Evidence of a novel allergenic protein Narcin in the bulbs of Narcissus tazetta. Int J Biochem Mol Biol. 2013;4:95-101.
Contact dermatitis is a common problem in the floral bulb industry and is considered an occupational disease. Daffodils (Narcissus species)(Figure) are thought to be the most common cause of irritant contact dermatitis among florists.1
Clinical Importance
Picking daffodils can start as early as October, when the flowers are still closed. The picker’s hand slides down the stem to snap the stalk at the base. This potentially traumatic maneuver to the web of the fingers leads to abrasions, which are irritated by the sap and cause granulomatous sores and paronychia. An experienced picker can pick 20,000 flowers a day, leading to extensive contact with sap.2
Eczematous or granulomatous rash on the arms also is seen as the sap irritates the wrist and forearm. The pickers often hold the flowers until a bunch of 10 has been collected. The 10 flowers are held together by a rubber band and stacked along the arm, the chin, and the axilla, causing the rash to extend to those areas. Sap also can be transferred by the hand to other parts of the body, such as the face. In men, sap can be transferred to the genitalia as the men urinate in the field.
Narcissus also can cause poisoning if ingested by humans or animals. Researchers who analyzed calls made to the New Zealand Natural Poisons Centre between 2003 and 2010 determined that daffodil was the 11th most common call for plant-related poisoning.3
Although the severity of plant poisoning often is low due to the small amount of plant material usually consumed, more severe poisoning can occur when the plant is eaten for medicinal purposes or mistaken for an edible plant.3 Vomiting, respiratory symptoms, abdominal pain, diarrhea, trembling, and convulsions can occur when daffodils are ingested. Death has been reported due to ingestion of the bulbs.4
In February 2010, 10 children aged 10 and 11 years and their 22-year-old guide presented to an emergency department in Israel after ingesting Narcissus bulbs, which were mistakenly believed to be the bulbs of onions.4 Eight children and the guide vomited. One child and the guide reported abdominal pain. All were discharged in stable condition after 4 hours of observation.4
Clinical Manifestations
Daffodil rash or lily rash was first described in 1910.5 The typical rash presents as dryness, fissures, scaling, and erythema of the fingertips, hands, and forearms, often with subungual hyperkeratosis. Vesicles and pustules may be seen. The rash may extend to other areas of the body, including the face.6
Prevention and Treatment
Use of protective gloves and clothing to avoid contact with the plant is recommended.2 Treatment includes stopping contact with the irritant, eye irrigation, and supportive measures (airway, breathing, and circulation). Activated charcoal can be helpful if used within 1 hour after ingestion but is contraindicated in vomiting patients.4
Identifying Features
The genus Narcissus is in the family Amaryllidaceae and contains ornamental plants, including daffodil (trumpet Narcissus, Narcissus pseudonarcissus), jonquil (Narcissus jonquilla), and poet’s narcissus (Narcissus poeticus). Most species are perennial; the plant emerges from a bulb in spring. Leaves originate from the base of the plant and range from 5-cm to 1.2-meters long, depending on the species. The flowers span a range of shapes and colors—from a trumpet (the daffodil) to a ringlike cup (poet’s Narcissus) and in yellow, white, and pink.7
Distribution and Plant Facts
Distribution—There are approximately 80 to 100 wild Narcissus species, which are found in southwestern Europe, North Africa, the Balkan Peninsula, Italy, and France. There are more than 27,000 Narcissus cultivars registered in the International Daffodil Register.8
Plant Facts—The daffodil is the national flower of Wales. It also is often used to depict hope and joy and is the symbol of cancer charities in many countries.9
The name Narcissus is believed to have originated from Greek mythology. A handsome youth, Narcissus, fell in love with his own reflection, for which the gods punished him by turning him into a flower.10
Another theory states that Narcissus is derived from the Greek word narkao (to benumb) due to its narcotic properties. When an open wound is subjected to an extract of the bulb, numbness of the entire nervous system is said to occur as well as paralysis of the heart. This narcotic effect led Socrates to refer to the Narcissus plant as the “chaplet of the infernal gods.”11
Narcissus is an important flower in various ethnic rituals. The Greeks often planted daffodils near tombs. In Muslim culture, white is believed to be the symbol of good and purity; Narcissus was one of the most common white-flowered plants found in Muslim graveyards.12
Medicinal Qualities and Uses—Narcissus species have been used as medicinal plants for a variety of ailments. For example, Narcissus tazetta contains flavonoids, alkaloids, saponins, tannins, cardiac glycosides, oil, steroids, terpenoids, and anthraquinones that contribute to its antibacterial, antifungal, antiviral, antimalarial, anticancer, antioxidant, dermatologic, cardiovascular, immunomodulatory, and acetylcholinesterase inhibitory effects.13 In a study, chloroform extracts from N tazetta bulbs were found to be more active than doxorubicin against hepatocellular and colon cancer cell lines.14
More than 500 alkaloids have been isolated from the Narcissus genus.15 In 2001, the US Food and Drug Administration approved one of the alkaloids, galantamine, for the treatment of mild to moderate stages of Alzheimer disease.16 Galantamine selectively and reversibly inhibits acetylcholinesterase, the enzyme believed responsible for neurodegeneration seen in Alzheimer disease. Plants are the main source of galantamine, despite the ability of pharmaceutical companies to synthesize the compound. Galantamine hydrobromide is sold by prescription (Razadyne [Janssen Pharmaceuticals, Inc]); generic formulations approved by the US Food and Drug Administration have been produced by more than 15 pharmaceutical companies.17,18
Irritant and Allergen
Sap found in the bulbs and hollow stems of Narcissus contains calcium oxalate crystals, or raphides. The minute, needle-shaped calcium oxalate crystals are believed to be a waste product of cellular metabolism.19 When the plant structure is compromised by pickers snapping the stalk, the sharp crystals penetrate the skin to cause an irritant contact dermatitis.
Relevant Research—A study used electron microscopy to characterize the structure of raphides from various plants,2 though not from Narcissus species; the structure of each raphide was then compared to the degree of irritation it produced. The researchers concluded that more elongated crystals (those containing barbs) produce a greater degree of irritation. Narcissus species are known to cause varying degrees of skin irritation: For example, N tazetta rarely causes skin irritation, whereas N pseudonarcissi (daffodil) tends to cause remarkably more skin irritation.2
Allergic reactions to and strong toxicity from Narcissus species are not well understood. In a study, only 2 alkaloids—homolycorine and masonin—produced a weakly positive reaction in patch tests on sensitized guinea pigs, which correlates with the finding of a different study, in which only 2 of 12 patients whose findings were examined over 14 years had a positive patch test for Narcissus.20,21
However, IgE-mediated allergies indicative of an allergic response to Narcissus have been reported. A study isolated an allergenic protein, narcin, from bulbs of N tazetta. Narcin is a 13-kDa protein with potent allergenic effects capable of inducing production of proinflammatory cytokines and increasing IgE levels in mononuclear cells in peripheral blood.22
More research is required to find and understand the compounds responsible for causing an allergic reaction to Narcissus.
Contact dermatitis is a common problem in the floral bulb industry and is considered an occupational disease. Daffodils (Narcissus species)(Figure) are thought to be the most common cause of irritant contact dermatitis among florists.1
Clinical Importance
Picking daffodils can start as early as October, when the flowers are still closed. The picker’s hand slides down the stem to snap the stalk at the base. This potentially traumatic maneuver to the web of the fingers leads to abrasions, which are irritated by the sap and cause granulomatous sores and paronychia. An experienced picker can pick 20,000 flowers a day, leading to extensive contact with sap.2
Eczematous or granulomatous rash on the arms also is seen as the sap irritates the wrist and forearm. The pickers often hold the flowers until a bunch of 10 has been collected. The 10 flowers are held together by a rubber band and stacked along the arm, the chin, and the axilla, causing the rash to extend to those areas. Sap also can be transferred by the hand to other parts of the body, such as the face. In men, sap can be transferred to the genitalia as the men urinate in the field.
Narcissus also can cause poisoning if ingested by humans or animals. Researchers who analyzed calls made to the New Zealand Natural Poisons Centre between 2003 and 2010 determined that daffodil was the 11th most common call for plant-related poisoning.3
Although the severity of plant poisoning often is low due to the small amount of plant material usually consumed, more severe poisoning can occur when the plant is eaten for medicinal purposes or mistaken for an edible plant.3 Vomiting, respiratory symptoms, abdominal pain, diarrhea, trembling, and convulsions can occur when daffodils are ingested. Death has been reported due to ingestion of the bulbs.4
In February 2010, 10 children aged 10 and 11 years and their 22-year-old guide presented to an emergency department in Israel after ingesting Narcissus bulbs, which were mistakenly believed to be the bulbs of onions.4 Eight children and the guide vomited. One child and the guide reported abdominal pain. All were discharged in stable condition after 4 hours of observation.4
Clinical Manifestations
Daffodil rash or lily rash was first described in 1910.5 The typical rash presents as dryness, fissures, scaling, and erythema of the fingertips, hands, and forearms, often with subungual hyperkeratosis. Vesicles and pustules may be seen. The rash may extend to other areas of the body, including the face.6
Prevention and Treatment
Use of protective gloves and clothing to avoid contact with the plant is recommended.2 Treatment includes stopping contact with the irritant, eye irrigation, and supportive measures (airway, breathing, and circulation). Activated charcoal can be helpful if used within 1 hour after ingestion but is contraindicated in vomiting patients.4
Identifying Features
The genus Narcissus is in the family Amaryllidaceae and contains ornamental plants, including daffodil (trumpet Narcissus, Narcissus pseudonarcissus), jonquil (Narcissus jonquilla), and poet’s narcissus (Narcissus poeticus). Most species are perennial; the plant emerges from a bulb in spring. Leaves originate from the base of the plant and range from 5-cm to 1.2-meters long, depending on the species. The flowers span a range of shapes and colors—from a trumpet (the daffodil) to a ringlike cup (poet’s Narcissus) and in yellow, white, and pink.7
Distribution and Plant Facts
Distribution—There are approximately 80 to 100 wild Narcissus species, which are found in southwestern Europe, North Africa, the Balkan Peninsula, Italy, and France. There are more than 27,000 Narcissus cultivars registered in the International Daffodil Register.8
Plant Facts—The daffodil is the national flower of Wales. It also is often used to depict hope and joy and is the symbol of cancer charities in many countries.9
The name Narcissus is believed to have originated from Greek mythology. A handsome youth, Narcissus, fell in love with his own reflection, for which the gods punished him by turning him into a flower.10
Another theory states that Narcissus is derived from the Greek word narkao (to benumb) due to its narcotic properties. When an open wound is subjected to an extract of the bulb, numbness of the entire nervous system is said to occur as well as paralysis of the heart. This narcotic effect led Socrates to refer to the Narcissus plant as the “chaplet of the infernal gods.”11
Narcissus is an important flower in various ethnic rituals. The Greeks often planted daffodils near tombs. In Muslim culture, white is believed to be the symbol of good and purity; Narcissus was one of the most common white-flowered plants found in Muslim graveyards.12
Medicinal Qualities and Uses—Narcissus species have been used as medicinal plants for a variety of ailments. For example, Narcissus tazetta contains flavonoids, alkaloids, saponins, tannins, cardiac glycosides, oil, steroids, terpenoids, and anthraquinones that contribute to its antibacterial, antifungal, antiviral, antimalarial, anticancer, antioxidant, dermatologic, cardiovascular, immunomodulatory, and acetylcholinesterase inhibitory effects.13 In a study, chloroform extracts from N tazetta bulbs were found to be more active than doxorubicin against hepatocellular and colon cancer cell lines.14
More than 500 alkaloids have been isolated from the Narcissus genus.15 In 2001, the US Food and Drug Administration approved one of the alkaloids, galantamine, for the treatment of mild to moderate stages of Alzheimer disease.16 Galantamine selectively and reversibly inhibits acetylcholinesterase, the enzyme believed responsible for neurodegeneration seen in Alzheimer disease. Plants are the main source of galantamine, despite the ability of pharmaceutical companies to synthesize the compound. Galantamine hydrobromide is sold by prescription (Razadyne [Janssen Pharmaceuticals, Inc]); generic formulations approved by the US Food and Drug Administration have been produced by more than 15 pharmaceutical companies.17,18
Irritant and Allergen
Sap found in the bulbs and hollow stems of Narcissus contains calcium oxalate crystals, or raphides. The minute, needle-shaped calcium oxalate crystals are believed to be a waste product of cellular metabolism.19 When the plant structure is compromised by pickers snapping the stalk, the sharp crystals penetrate the skin to cause an irritant contact dermatitis.
Relevant Research—A study used electron microscopy to characterize the structure of raphides from various plants,2 though not from Narcissus species; the structure of each raphide was then compared to the degree of irritation it produced. The researchers concluded that more elongated crystals (those containing barbs) produce a greater degree of irritation. Narcissus species are known to cause varying degrees of skin irritation: For example, N tazetta rarely causes skin irritation, whereas N pseudonarcissi (daffodil) tends to cause remarkably more skin irritation.2
Allergic reactions to and strong toxicity from Narcissus species are not well understood. In a study, only 2 alkaloids—homolycorine and masonin—produced a weakly positive reaction in patch tests on sensitized guinea pigs, which correlates with the finding of a different study, in which only 2 of 12 patients whose findings were examined over 14 years had a positive patch test for Narcissus.20,21
However, IgE-mediated allergies indicative of an allergic response to Narcissus have been reported. A study isolated an allergenic protein, narcin, from bulbs of N tazetta. Narcin is a 13-kDa protein with potent allergenic effects capable of inducing production of proinflammatory cytokines and increasing IgE levels in mononuclear cells in peripheral blood.22
More research is required to find and understand the compounds responsible for causing an allergic reaction to Narcissus.
- Modi GM, Doherty CB, Katta R, et al. Irritant contact dermatitis from plants. Dermatitis. 2009;20:63-78. doi:10.2310/6620.2009.08051
- Julian CG, Bowers PW. The nature and distribution of daffodil pickers’ rash. Contact Dermatitis. 1997;37:259-262. doi:10.1111/j.1600-0536.1997.tb02461.x
- Slaughter RJ, Beasley DMG, Lambie BS, et al. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012;125:87-118.
- Hussein A, Yassin A. Poisoning following ingestion of Narcissus tazetta bulbs by schoolchildren. Isr Med Assoc J. 2014;16:125-126.
- Hanks GR, ed. Narcissus and Daffodil: The Genus Narcissus. CRC Press; 2002. https://doi.org/10.1201/9780203219355
- McGovern TW. Botanical briefs: daffodils—Narcissus L. Cutis. 2000;65:130-132.
- The Editors of Encyclopaedia Britannica. Narcissus. Encyclopedia Britannica. Accessed December 13, 2022. https://www.britannica.com/plant/narcissus-plant
- M, A, D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018;41:208-218. doi:10.1007/s12272-017-1000-4
- Crampton L. Beautiful daffodils: plant facts, toxicity, and a symbol of hope. Owlcation. April 19, 2022. Accessed December 13, 2022. https://owlcation.com/stem/Daffodils-Beautiful-Flowers-and-a-Symbol-of-Hope
- Rademaker M. Daffodil. DermNet. Published 1999. Accessed December 13, 2022. https://dermnetnz.org/topics/daffodil
- Grieve M. Narcissus. Accessed December 13, 2022. https://botanical.com/botanical/mgmh/n/narcis01.html
- Dafni A, Lev E, Beckmann S, et al. Ritual plants of Muslim graveyards in northern Israel. J Ethnobiolog Ethnomed. 2006;2:38. doi:10.1186/1746-4269-2-38
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm. 2020;10:44-53.
- Shawky E, Abou-Donia AH, Darwish FA, et al. In vitro cytotoxicity of some Narcissus plants extracts. Nat Prod Res. 2015;29:363-365. doi:10.1080/14786419.2014.942302
- Havlasov J, M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014;9:1151-1155.
- Pigni NB, S, V, et al. Alkaloids from Narcissus serotinus. J Nat Prod. 2012;75:1643-1647. doi:10.1021/np3003595
- Razadyne. Prescribing information. Janssen Pharmaceuticals, Inc; 2013. Accessed December 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021169Orig1s032,021224Orig1s030,021615Orig1s023lbl.pdf
- Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci. 2013;14:11713-11741. doi:10.3390/ijms140611713
- Evans FJ, Schmidt RJ. Plants and plant products that induce contact dermatitis. Planta Med. 1980;38:289-316. doi:10.1055/s-2008-1074883
- Gude M, Hausen BM, Heitsch H, et al. An investigation of the irritant and allergenic properties of daffodils (Narcissus pseudonarcissus L., Amaryllidaceae). a review of daffodil dermatitis. Contact Dermatitis. 1988;19:1-10.
- Lamminpää A, Estlander T, Jolanki R, et al. Occupational allergic contact dermatitis caused by decorative plants. Contact Dermatitis. 1996;34:330-335.
- Sinha M, Singh A, Shokeen A, et al. Evidence of a novel allergenic protein Narcin in the bulbs of Narcissus tazetta. Int J Biochem Mol Biol. 2013;4:95-101.
- Modi GM, Doherty CB, Katta R, et al. Irritant contact dermatitis from plants. Dermatitis. 2009;20:63-78. doi:10.2310/6620.2009.08051
- Julian CG, Bowers PW. The nature and distribution of daffodil pickers’ rash. Contact Dermatitis. 1997;37:259-262. doi:10.1111/j.1600-0536.1997.tb02461.x
- Slaughter RJ, Beasley DMG, Lambie BS, et al. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012;125:87-118.
- Hussein A, Yassin A. Poisoning following ingestion of Narcissus tazetta bulbs by schoolchildren. Isr Med Assoc J. 2014;16:125-126.
- Hanks GR, ed. Narcissus and Daffodil: The Genus Narcissus. CRC Press; 2002. https://doi.org/10.1201/9780203219355
- McGovern TW. Botanical briefs: daffodils—Narcissus L. Cutis. 2000;65:130-132.
- The Editors of Encyclopaedia Britannica. Narcissus. Encyclopedia Britannica. Accessed December 13, 2022. https://www.britannica.com/plant/narcissus-plant
- M, A, D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018;41:208-218. doi:10.1007/s12272-017-1000-4
- Crampton L. Beautiful daffodils: plant facts, toxicity, and a symbol of hope. Owlcation. April 19, 2022. Accessed December 13, 2022. https://owlcation.com/stem/Daffodils-Beautiful-Flowers-and-a-Symbol-of-Hope
- Rademaker M. Daffodil. DermNet. Published 1999. Accessed December 13, 2022. https://dermnetnz.org/topics/daffodil
- Grieve M. Narcissus. Accessed December 13, 2022. https://botanical.com/botanical/mgmh/n/narcis01.html
- Dafni A, Lev E, Beckmann S, et al. Ritual plants of Muslim graveyards in northern Israel. J Ethnobiolog Ethnomed. 2006;2:38. doi:10.1186/1746-4269-2-38
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm. 2020;10:44-53.
- Shawky E, Abou-Donia AH, Darwish FA, et al. In vitro cytotoxicity of some Narcissus plants extracts. Nat Prod Res. 2015;29:363-365. doi:10.1080/14786419.2014.942302
- Havlasov J, M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014;9:1151-1155.
- Pigni NB, S, V, et al. Alkaloids from Narcissus serotinus. J Nat Prod. 2012;75:1643-1647. doi:10.1021/np3003595
- Razadyne. Prescribing information. Janssen Pharmaceuticals, Inc; 2013. Accessed December 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021169Orig1s032,021224Orig1s030,021615Orig1s023lbl.pdf
- Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci. 2013;14:11713-11741. doi:10.3390/ijms140611713
- Evans FJ, Schmidt RJ. Plants and plant products that induce contact dermatitis. Planta Med. 1980;38:289-316. doi:10.1055/s-2008-1074883
- Gude M, Hausen BM, Heitsch H, et al. An investigation of the irritant and allergenic properties of daffodils (Narcissus pseudonarcissus L., Amaryllidaceae). a review of daffodil dermatitis. Contact Dermatitis. 1988;19:1-10.
- Lamminpää A, Estlander T, Jolanki R, et al. Occupational allergic contact dermatitis caused by decorative plants. Contact Dermatitis. 1996;34:330-335.
- Sinha M, Singh A, Shokeen A, et al. Evidence of a novel allergenic protein Narcin in the bulbs of Narcissus tazetta. Int J Biochem Mol Biol. 2013;4:95-101.
Practice Points
- Narcissus species are thought to be the most common cause of irritant contact dermatitis among florists.
- Use of protective gloves and clothing to prevent Narcissus-induced contact dermatitis is recommended.