User login
Heart benefits begin at well under 10,000 daily steps
– and the benefits accrue at well below the widely promoted threshold of 10,000 steps per day, new research shows.
Among adults aged 60 and older, those who took roughly 6,000 to 9,000 steps per day had a 40% to 50% lower risk of CVD, compared with peers logging just 2,000 steps per day.
“We hope this study will contribute evidence to future public health and clinical guidance on how many steps we need for health,” Amanda Paluch, PhD, with University of Massachusetts Amherst, told this news organization.
Getting in more steps per day can lower an individual’s risk for heart disease – but it’s not an “all or nothing” situation, Dr. Paluch said.
“The heart health benefits begin at lower than 10,000 steps per day. So, for the many adults that may find 10,000 steps a bit out of reach, it is important to promote that even small increases in steps can be beneficial for health,” Dr. Paluch said.
The study was published online in Circulation.
Attainable step goals
As part of the Steps for Health Collaborative, Dr. Paluch and colleagues examined the dose-response relationship between steps per day and CVD in a meta-analysis of eight prospective studies involving 20,152 adults (mean age 63, 52% women).
Steps were measured in each study using one of five different commercially available step-measuring devices. Adults aged 60 years and older took a median of 4,323 steps per day (interquartile range, 2,760-6,924), while younger adults walked a bit more (median 6,911 daily steps; IQR, 4,783-9,794).
During follow-up lasting an average of 6.2 years, a total of 1,523 CVD events were reported.
In the final adjusted model, for older adults, compared with those in quartile 1 who got the fewest steps per day (median 1,811), the risk of CVD was 20% lower in those in quartile 2, who got a median of 3,823 steps per day (hazard ratio, 0.80; 95% confidence interval, 0.69-0.93).
CVD risk was 38% lower in older adults in quartile 3 who got a median of 5,520 steps per day (HR, 0.62; 95% CI, 0.52-0.74) and 49% lower in those in quartile 4 who walked the most (a median of 9,259 steps per day; HR, 0.51; 95% CI, 0.41-0.63).
Restricting the analysis to individuals without known CVD at baseline showed similar results.
Among six studies that excluded adults with a history of CVD at baseline, compared with the lowest quartile, the HR for incident CVD events was 0.74 (95% CI, 0.60-0.91) in the second quartile, 0.60 (95% CI, 0.47-0.77) in the third quartile, and 0.55 (95% CI, 0.40-0.76) in the fourth quartile.
Despite the inverse association of steps with CVD in older adults, there was no association in younger adults. The researchers caution, however, that CVD is a disease of aging, and the follow-up period in these studies may not have been long enough to capture CVD incidence in younger adults.
Stepping rate (pace or cadence) was not associated with CVD risk beyond that of total steps per day. However, only four of the eight studies reported data on stepping rate, so this finding should be viewed as preliminary, Dr. Paluch and colleagues say.
Start small and go from there
Dr. Paluch said the take-home message from this study and numerous others is simple.
“Move more and sit less! Being physically active, by getting in your steps, is an important part of keeping your heart healthy,” she said in an interview.
For adults who are currently inactive, Dr. Paluch suggests finding small ways to get in a few more steps per day. “It does not need to be drastic changes. Consider a brief 5- to 10-minute walking break at lunch, taking the stairs, or playing a game of hide and seek with the grandchildren,” Dr. Paluch advised.
“For adults starting at 3,000 steps a day, set a goal of 4,000, and then 5,000. Each improvement can lead to better heart health,” Dr. Paluch said. “And for those who are already active, keep it up, as there are benefits with higher volumes of steps per day as well.”
Support for this research was provided by the Intergovernmental Personnel Act Agreement through the Centers for Disease Control and Prevention. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and the benefits accrue at well below the widely promoted threshold of 10,000 steps per day, new research shows.
Among adults aged 60 and older, those who took roughly 6,000 to 9,000 steps per day had a 40% to 50% lower risk of CVD, compared with peers logging just 2,000 steps per day.
“We hope this study will contribute evidence to future public health and clinical guidance on how many steps we need for health,” Amanda Paluch, PhD, with University of Massachusetts Amherst, told this news organization.
Getting in more steps per day can lower an individual’s risk for heart disease – but it’s not an “all or nothing” situation, Dr. Paluch said.
“The heart health benefits begin at lower than 10,000 steps per day. So, for the many adults that may find 10,000 steps a bit out of reach, it is important to promote that even small increases in steps can be beneficial for health,” Dr. Paluch said.
The study was published online in Circulation.
Attainable step goals
As part of the Steps for Health Collaborative, Dr. Paluch and colleagues examined the dose-response relationship between steps per day and CVD in a meta-analysis of eight prospective studies involving 20,152 adults (mean age 63, 52% women).
Steps were measured in each study using one of five different commercially available step-measuring devices. Adults aged 60 years and older took a median of 4,323 steps per day (interquartile range, 2,760-6,924), while younger adults walked a bit more (median 6,911 daily steps; IQR, 4,783-9,794).
During follow-up lasting an average of 6.2 years, a total of 1,523 CVD events were reported.
In the final adjusted model, for older adults, compared with those in quartile 1 who got the fewest steps per day (median 1,811), the risk of CVD was 20% lower in those in quartile 2, who got a median of 3,823 steps per day (hazard ratio, 0.80; 95% confidence interval, 0.69-0.93).
CVD risk was 38% lower in older adults in quartile 3 who got a median of 5,520 steps per day (HR, 0.62; 95% CI, 0.52-0.74) and 49% lower in those in quartile 4 who walked the most (a median of 9,259 steps per day; HR, 0.51; 95% CI, 0.41-0.63).
Restricting the analysis to individuals without known CVD at baseline showed similar results.
Among six studies that excluded adults with a history of CVD at baseline, compared with the lowest quartile, the HR for incident CVD events was 0.74 (95% CI, 0.60-0.91) in the second quartile, 0.60 (95% CI, 0.47-0.77) in the third quartile, and 0.55 (95% CI, 0.40-0.76) in the fourth quartile.
Despite the inverse association of steps with CVD in older adults, there was no association in younger adults. The researchers caution, however, that CVD is a disease of aging, and the follow-up period in these studies may not have been long enough to capture CVD incidence in younger adults.
Stepping rate (pace or cadence) was not associated with CVD risk beyond that of total steps per day. However, only four of the eight studies reported data on stepping rate, so this finding should be viewed as preliminary, Dr. Paluch and colleagues say.
Start small and go from there
Dr. Paluch said the take-home message from this study and numerous others is simple.
“Move more and sit less! Being physically active, by getting in your steps, is an important part of keeping your heart healthy,” she said in an interview.
For adults who are currently inactive, Dr. Paluch suggests finding small ways to get in a few more steps per day. “It does not need to be drastic changes. Consider a brief 5- to 10-minute walking break at lunch, taking the stairs, or playing a game of hide and seek with the grandchildren,” Dr. Paluch advised.
“For adults starting at 3,000 steps a day, set a goal of 4,000, and then 5,000. Each improvement can lead to better heart health,” Dr. Paluch said. “And for those who are already active, keep it up, as there are benefits with higher volumes of steps per day as well.”
Support for this research was provided by the Intergovernmental Personnel Act Agreement through the Centers for Disease Control and Prevention. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
– and the benefits accrue at well below the widely promoted threshold of 10,000 steps per day, new research shows.
Among adults aged 60 and older, those who took roughly 6,000 to 9,000 steps per day had a 40% to 50% lower risk of CVD, compared with peers logging just 2,000 steps per day.
“We hope this study will contribute evidence to future public health and clinical guidance on how many steps we need for health,” Amanda Paluch, PhD, with University of Massachusetts Amherst, told this news organization.
Getting in more steps per day can lower an individual’s risk for heart disease – but it’s not an “all or nothing” situation, Dr. Paluch said.
“The heart health benefits begin at lower than 10,000 steps per day. So, for the many adults that may find 10,000 steps a bit out of reach, it is important to promote that even small increases in steps can be beneficial for health,” Dr. Paluch said.
The study was published online in Circulation.
Attainable step goals
As part of the Steps for Health Collaborative, Dr. Paluch and colleagues examined the dose-response relationship between steps per day and CVD in a meta-analysis of eight prospective studies involving 20,152 adults (mean age 63, 52% women).
Steps were measured in each study using one of five different commercially available step-measuring devices. Adults aged 60 years and older took a median of 4,323 steps per day (interquartile range, 2,760-6,924), while younger adults walked a bit more (median 6,911 daily steps; IQR, 4,783-9,794).
During follow-up lasting an average of 6.2 years, a total of 1,523 CVD events were reported.
In the final adjusted model, for older adults, compared with those in quartile 1 who got the fewest steps per day (median 1,811), the risk of CVD was 20% lower in those in quartile 2, who got a median of 3,823 steps per day (hazard ratio, 0.80; 95% confidence interval, 0.69-0.93).
CVD risk was 38% lower in older adults in quartile 3 who got a median of 5,520 steps per day (HR, 0.62; 95% CI, 0.52-0.74) and 49% lower in those in quartile 4 who walked the most (a median of 9,259 steps per day; HR, 0.51; 95% CI, 0.41-0.63).
Restricting the analysis to individuals without known CVD at baseline showed similar results.
Among six studies that excluded adults with a history of CVD at baseline, compared with the lowest quartile, the HR for incident CVD events was 0.74 (95% CI, 0.60-0.91) in the second quartile, 0.60 (95% CI, 0.47-0.77) in the third quartile, and 0.55 (95% CI, 0.40-0.76) in the fourth quartile.
Despite the inverse association of steps with CVD in older adults, there was no association in younger adults. The researchers caution, however, that CVD is a disease of aging, and the follow-up period in these studies may not have been long enough to capture CVD incidence in younger adults.
Stepping rate (pace or cadence) was not associated with CVD risk beyond that of total steps per day. However, only four of the eight studies reported data on stepping rate, so this finding should be viewed as preliminary, Dr. Paluch and colleagues say.
Start small and go from there
Dr. Paluch said the take-home message from this study and numerous others is simple.
“Move more and sit less! Being physically active, by getting in your steps, is an important part of keeping your heart healthy,” she said in an interview.
For adults who are currently inactive, Dr. Paluch suggests finding small ways to get in a few more steps per day. “It does not need to be drastic changes. Consider a brief 5- to 10-minute walking break at lunch, taking the stairs, or playing a game of hide and seek with the grandchildren,” Dr. Paluch advised.
“For adults starting at 3,000 steps a day, set a goal of 4,000, and then 5,000. Each improvement can lead to better heart health,” Dr. Paluch said. “And for those who are already active, keep it up, as there are benefits with higher volumes of steps per day as well.”
Support for this research was provided by the Intergovernmental Personnel Act Agreement through the Centers for Disease Control and Prevention. The authors have reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CIRCULATION
Nearly 1,400% rise in young children ingesting cannabis edibles
according to a new analysis of data from poison control centers.
In 2017, centers received 207 reports of children aged 5 years and younger who ingested edible cannabis. In 2021, 3,054 such cases were reported, according to the study, which was published online in Pediatrics.
Many of the children experienced clinical effects, such as depression of the central nervous system, impaired coordination, confusion, agitation, an increase in heart rate, or dilated pupils. No deaths were reported.
“These exposures can cause significant toxicity and are responsible for an increasing number of hospitalizations,” study coauthor Marit S. Tweet, MD, of Southern Illinois University, Springfield, and colleagues wrote.
About 97% of the exposures occurred in residences – 90% at the child’s own home – and about half of the cases involved 2- and 3-year-olds, they noted.
Examining national trends
Twenty-one states have approved recreational cannabis for people aged 21 years and older.
Prior research has shown that calls to poison centers and visits to emergency departments for pediatric cannabis consumption increased in certain states after the drug became legal in those jurisdictions.
To assess national trends, Dr. Tweet’s group analyzed cases in the National Poison Data System, which tracks potentially toxic exposures reported to poison control centers in the United States.
During the 5-year period, they identified 7,043 exposures to edible cannabis by children younger than age 6. In 2.2% of the cases, the drug had a major effect, defined as being either life-threatening or causing residual disability. In 21.9% of cases, the effect was considered to be moderate, with symptoms that were more pronounced, prolonged, or systemic than minor effects.
About 8% of the children were admitted to critical care units; 14.6% were admitted to non–critical care units.
Of 4,827 cases for which there was information about the clinical effects of the exposure and therapies used, 70% involved CNS depression, including 1.9% with “more severe CNS effects, including major CNS depression or coma,” according to the report.
Patients also experienced ataxia (7.4%), agitation (7.1%), confusion (6.1%), tremor (2%), and seizures (1.6%). Other common symptoms included tachycardia (11.4%), vomiting (9.5%), mydriasis (5.9%), and respiratory depression (3.1%).
Treatments for the exposures included intravenous fluids (20.7%), food or snacks (10.3%), and oxygen therapy (4%). Some patients also received naloxone (1.4%) or charcoal (2.1%).
“The total number of children requiring intubation during the study period was 35, or approximately 1 in 140,” the researchers reported. “Although this was a relatively rare occurrence, it is important for clinicians to be aware that life-threatening sequelae can develop and may necessitate invasive supportive care measures.”
Tempting and toxic
For toddlers, edible cannabis may be especially tempting and toxic. Edibles can “resemble common treats such as candies, chocolates, cookies, or other baked goods,” the researchers wrote. Children would not recognize, for example, that one chocolate bar might contain multiple 10-mg servings of tetrahydrocannabinol intended for adults.
Poison centers have been fielding more calls about edible cannabis use by older children, as well.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health and Science University, Portland, recently found that many cases of intentional misuse and abuse by adolescents involve edible forms of cannabis.
“While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products,” Dr. Hughes said in an interview.
Measures to keep edibles away from children could include changing how the products are packaged, limiting the maximum dose of drug per package, and educating the public about the risks to children, Dr. Tweet’s group wrote. They highlighted a 2019 position statement from the American College of Medical Toxicology that includes recommendations for responsible storage habits.
Dr. Hughes echoed one suggestion that is mentioned in the position statement: Parents should consider keeping their cannabis products locked up.
The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new analysis of data from poison control centers.
In 2017, centers received 207 reports of children aged 5 years and younger who ingested edible cannabis. In 2021, 3,054 such cases were reported, according to the study, which was published online in Pediatrics.
Many of the children experienced clinical effects, such as depression of the central nervous system, impaired coordination, confusion, agitation, an increase in heart rate, or dilated pupils. No deaths were reported.
“These exposures can cause significant toxicity and are responsible for an increasing number of hospitalizations,” study coauthor Marit S. Tweet, MD, of Southern Illinois University, Springfield, and colleagues wrote.
About 97% of the exposures occurred in residences – 90% at the child’s own home – and about half of the cases involved 2- and 3-year-olds, they noted.
Examining national trends
Twenty-one states have approved recreational cannabis for people aged 21 years and older.
Prior research has shown that calls to poison centers and visits to emergency departments for pediatric cannabis consumption increased in certain states after the drug became legal in those jurisdictions.
To assess national trends, Dr. Tweet’s group analyzed cases in the National Poison Data System, which tracks potentially toxic exposures reported to poison control centers in the United States.
During the 5-year period, they identified 7,043 exposures to edible cannabis by children younger than age 6. In 2.2% of the cases, the drug had a major effect, defined as being either life-threatening or causing residual disability. In 21.9% of cases, the effect was considered to be moderate, with symptoms that were more pronounced, prolonged, or systemic than minor effects.
About 8% of the children were admitted to critical care units; 14.6% were admitted to non–critical care units.
Of 4,827 cases for which there was information about the clinical effects of the exposure and therapies used, 70% involved CNS depression, including 1.9% with “more severe CNS effects, including major CNS depression or coma,” according to the report.
Patients also experienced ataxia (7.4%), agitation (7.1%), confusion (6.1%), tremor (2%), and seizures (1.6%). Other common symptoms included tachycardia (11.4%), vomiting (9.5%), mydriasis (5.9%), and respiratory depression (3.1%).
Treatments for the exposures included intravenous fluids (20.7%), food or snacks (10.3%), and oxygen therapy (4%). Some patients also received naloxone (1.4%) or charcoal (2.1%).
“The total number of children requiring intubation during the study period was 35, or approximately 1 in 140,” the researchers reported. “Although this was a relatively rare occurrence, it is important for clinicians to be aware that life-threatening sequelae can develop and may necessitate invasive supportive care measures.”
Tempting and toxic
For toddlers, edible cannabis may be especially tempting and toxic. Edibles can “resemble common treats such as candies, chocolates, cookies, or other baked goods,” the researchers wrote. Children would not recognize, for example, that one chocolate bar might contain multiple 10-mg servings of tetrahydrocannabinol intended for adults.
Poison centers have been fielding more calls about edible cannabis use by older children, as well.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health and Science University, Portland, recently found that many cases of intentional misuse and abuse by adolescents involve edible forms of cannabis.
“While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products,” Dr. Hughes said in an interview.
Measures to keep edibles away from children could include changing how the products are packaged, limiting the maximum dose of drug per package, and educating the public about the risks to children, Dr. Tweet’s group wrote. They highlighted a 2019 position statement from the American College of Medical Toxicology that includes recommendations for responsible storage habits.
Dr. Hughes echoed one suggestion that is mentioned in the position statement: Parents should consider keeping their cannabis products locked up.
The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
according to a new analysis of data from poison control centers.
In 2017, centers received 207 reports of children aged 5 years and younger who ingested edible cannabis. In 2021, 3,054 such cases were reported, according to the study, which was published online in Pediatrics.
Many of the children experienced clinical effects, such as depression of the central nervous system, impaired coordination, confusion, agitation, an increase in heart rate, or dilated pupils. No deaths were reported.
“These exposures can cause significant toxicity and are responsible for an increasing number of hospitalizations,” study coauthor Marit S. Tweet, MD, of Southern Illinois University, Springfield, and colleagues wrote.
About 97% of the exposures occurred in residences – 90% at the child’s own home – and about half of the cases involved 2- and 3-year-olds, they noted.
Examining national trends
Twenty-one states have approved recreational cannabis for people aged 21 years and older.
Prior research has shown that calls to poison centers and visits to emergency departments for pediatric cannabis consumption increased in certain states after the drug became legal in those jurisdictions.
To assess national trends, Dr. Tweet’s group analyzed cases in the National Poison Data System, which tracks potentially toxic exposures reported to poison control centers in the United States.
During the 5-year period, they identified 7,043 exposures to edible cannabis by children younger than age 6. In 2.2% of the cases, the drug had a major effect, defined as being either life-threatening or causing residual disability. In 21.9% of cases, the effect was considered to be moderate, with symptoms that were more pronounced, prolonged, or systemic than minor effects.
About 8% of the children were admitted to critical care units; 14.6% were admitted to non–critical care units.
Of 4,827 cases for which there was information about the clinical effects of the exposure and therapies used, 70% involved CNS depression, including 1.9% with “more severe CNS effects, including major CNS depression or coma,” according to the report.
Patients also experienced ataxia (7.4%), agitation (7.1%), confusion (6.1%), tremor (2%), and seizures (1.6%). Other common symptoms included tachycardia (11.4%), vomiting (9.5%), mydriasis (5.9%), and respiratory depression (3.1%).
Treatments for the exposures included intravenous fluids (20.7%), food or snacks (10.3%), and oxygen therapy (4%). Some patients also received naloxone (1.4%) or charcoal (2.1%).
“The total number of children requiring intubation during the study period was 35, or approximately 1 in 140,” the researchers reported. “Although this was a relatively rare occurrence, it is important for clinicians to be aware that life-threatening sequelae can develop and may necessitate invasive supportive care measures.”
Tempting and toxic
For toddlers, edible cannabis may be especially tempting and toxic. Edibles can “resemble common treats such as candies, chocolates, cookies, or other baked goods,” the researchers wrote. Children would not recognize, for example, that one chocolate bar might contain multiple 10-mg servings of tetrahydrocannabinol intended for adults.
Poison centers have been fielding more calls about edible cannabis use by older children, as well.
Adrienne Hughes, MD, assistant professor of emergency medicine at Oregon Health and Science University, Portland, recently found that many cases of intentional misuse and abuse by adolescents involve edible forms of cannabis.
“While marijuana carries a low risk for severe toxicity, it can be inebriating to the point of poor judgment, risk of falls or other injury, and occasionally a panic reaction in the novice user and unsuspecting children who accidentally ingest these products,” Dr. Hughes said in an interview.
Measures to keep edibles away from children could include changing how the products are packaged, limiting the maximum dose of drug per package, and educating the public about the risks to children, Dr. Tweet’s group wrote. They highlighted a 2019 position statement from the American College of Medical Toxicology that includes recommendations for responsible storage habits.
Dr. Hughes echoed one suggestion that is mentioned in the position statement: Parents should consider keeping their cannabis products locked up.
The researchers disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PEDIATRICS
Women with cycle disorders across their life span may be at increased risk of cardiovascular disease
This finding is demonstrated in a new analysis of the Nurses’ Health Study II.
“To date, several studies have reported increased risks of cardiovascular risk factors or cardiovascular disease in connection with cycle disorders,” Yi-Xin Wang, MD, PhD, a research fellow in nutrition, and associates from the Harvard School of Public Health, Boston, wrote in an article published in JAMA Network Open.
Ute Seeland, MD, speaker of the Gender Medicine in Cardiology Working Group of the German Cardiology Society, said in an interview“We know that women who have indicated in their medical history that they have irregular menstrual cycles, invariably in connection with polycystic ovary syndrome (PCOS), more commonly develop diabetes and other metabolic disorders, as well as cardiovascular diseases.”
Cycle disorders’ role
However, the role that irregular or especially long cycles play at different points of a woman’s reproductive life span was unclear. Therefore, the research group investigated the associations in the Nurses’ Health Study II between cycle irregularity and cycle length in women of different age groups who later experienced cardiovascular events.
At the end of this study in 1989, the participants also provided information regarding the length and irregularity of their menstrual cycle from ages 14 to 17 years and again from ages 18 to 22 years. The information was updated in 1993 when the participants were aged 29-46 years. The data from 2019 to 2022 were analyzed.
“This kind of long-term cohort study is extremely rare and therefore something special,” said Dr. Seeland, who conducts research at the Institute for Social Medicine, Epidemiology, and Health Economics at the Charité – University Hospital Berlin.
The investigators used the following cycle classifications: very regular (no more than 3 or 4 days before or after the expected date), regular (within 5-7 days), usually irregular, always irregular, or no periods.
The cycle lengths were divided into the following categories: less than 21 days, 21-25 days, 26-31 days, 32-39 days, 40-50 days, more than 50 days, and too irregular to estimate the length.
The onset of cardiovascular diseases was determined using information from the participants and was confirmed by reviewing the medical files. Relevant to the study were lethal and nonlethal coronary heart diseases (such as myocardial infarction or coronary artery revascularization), as well as strokes.
Significant in adulthood
The data from 80,630 study participants were included in the analysis. At study inclusion, the average age of the participants was 37.7 years, and the average body mass index (BMI) was 25.1. “Since it was predominantly White nurses who took part in the study, the data are not transferable to other, more diverse populations,” said Dr. Seeland.
Over 24 years, 1,816 women (2.4%) had a cardiovascular event. “We observed an increased rate of cardiovascular events in women with an irregular cycle and longer cycle, both in early an in mid-adulthood,” wrote Dr. Wang and associates. “Similar trends were also observed for cycle disorders when younger, but this association was weaker than in adulthood.”
Compared with women with very regular cycles, women with irregular cycles or without periods who were aged 14-17 years, 18-22 years, or 29-49 years exhibited a 15%, 36%, and 40% higher risk of a cardiovascular event, respectively.
Similarly, women aged 18-22 years or 29-46 years with long cycles of 40 days or more had a 44% or 30% higher risk of cardiovascular disease, respectively, compared with women with cycle lengths of 26-31 days.
“The coronary heart diseases were decisive for the increase, and less so, the strokes,” wrote the researchers.
Classic risk factors?
Dr. Seeland praised the fact that the study authors tried to determine the role that classic cardiovascular risk factors played. “Compared with women with a regular cycle, women with an irregular cycle had a higher BMI, more frequently increased cholesterol levels, and an elevated blood pressure,” she said. Women with a long cycle displayed a similar pattern.
It can be assumed from this that over a woman’s life span, BMI affects the risk of cardiovascular disease. Therefore, Dr. Wang and coauthors adjusted the results on the basis of BMI, which varies over time.
Regarding other classic risk factors that may have played a role, “hypercholesterolemia, chronic high blood pressure, and type 2 diabetes were only responsible in 5.4%-13.5% of the associations,” wrote the researchers.
“Our results suggest that certain characteristics of the menstrual cycle across a woman’s reproductive lifespan may constitute additional risk markers for cardiovascular disease,” according to the authors.
The highest rates of cardiovascular disease were among women with permanently irregular or long cycles in early to mid adulthood, as well as women who had regular cycles when younger but had irregular cycles in mid adulthood. “This indicates that the change from one cycle phenotype to another could be a surrogate marker for metabolic changes, which in turn contribute to the formation of cardiovascular diseases,” wrote the authors.
The study was observational and so conclusions cannot be drawn regarding causal relationships. But Dr. Wang and associates indicate that the most common cause of an irregular menstrual cycle may be PCOS. “Roughly 90% of women with cycle disorders or oligomenorrhea have signs of PCOS. And it was shown that women with PCOS have an increased risk of cardiovascular disease.”
They concluded that “the associations observed between irregular and long cycles in early to mid-adulthood and cardiovascular diseases are likely attributable to underlying PCOS.”
For Dr. Seeland, however, this conclusion is “too monocausal. At no point in time did there seem to be any direct information regarding the frequency of PCOS during the data collection by the respondents.”
For now, we can only speculate about the mechanisms. “The association between a very irregular and long cycle and the increased risk of cardiovascular diseases has now only been described. More research should be done on the causes,” said Dr. Seeland.
This article was translated from the Medscape German edition. A version of this article first appeared on Medscape.com.
This finding is demonstrated in a new analysis of the Nurses’ Health Study II.
“To date, several studies have reported increased risks of cardiovascular risk factors or cardiovascular disease in connection with cycle disorders,” Yi-Xin Wang, MD, PhD, a research fellow in nutrition, and associates from the Harvard School of Public Health, Boston, wrote in an article published in JAMA Network Open.
Ute Seeland, MD, speaker of the Gender Medicine in Cardiology Working Group of the German Cardiology Society, said in an interview“We know that women who have indicated in their medical history that they have irregular menstrual cycles, invariably in connection with polycystic ovary syndrome (PCOS), more commonly develop diabetes and other metabolic disorders, as well as cardiovascular diseases.”
Cycle disorders’ role
However, the role that irregular or especially long cycles play at different points of a woman’s reproductive life span was unclear. Therefore, the research group investigated the associations in the Nurses’ Health Study II between cycle irregularity and cycle length in women of different age groups who later experienced cardiovascular events.
At the end of this study in 1989, the participants also provided information regarding the length and irregularity of their menstrual cycle from ages 14 to 17 years and again from ages 18 to 22 years. The information was updated in 1993 when the participants were aged 29-46 years. The data from 2019 to 2022 were analyzed.
“This kind of long-term cohort study is extremely rare and therefore something special,” said Dr. Seeland, who conducts research at the Institute for Social Medicine, Epidemiology, and Health Economics at the Charité – University Hospital Berlin.
The investigators used the following cycle classifications: very regular (no more than 3 or 4 days before or after the expected date), regular (within 5-7 days), usually irregular, always irregular, or no periods.
The cycle lengths were divided into the following categories: less than 21 days, 21-25 days, 26-31 days, 32-39 days, 40-50 days, more than 50 days, and too irregular to estimate the length.
The onset of cardiovascular diseases was determined using information from the participants and was confirmed by reviewing the medical files. Relevant to the study were lethal and nonlethal coronary heart diseases (such as myocardial infarction or coronary artery revascularization), as well as strokes.
Significant in adulthood
The data from 80,630 study participants were included in the analysis. At study inclusion, the average age of the participants was 37.7 years, and the average body mass index (BMI) was 25.1. “Since it was predominantly White nurses who took part in the study, the data are not transferable to other, more diverse populations,” said Dr. Seeland.
Over 24 years, 1,816 women (2.4%) had a cardiovascular event. “We observed an increased rate of cardiovascular events in women with an irregular cycle and longer cycle, both in early an in mid-adulthood,” wrote Dr. Wang and associates. “Similar trends were also observed for cycle disorders when younger, but this association was weaker than in adulthood.”
Compared with women with very regular cycles, women with irregular cycles or without periods who were aged 14-17 years, 18-22 years, or 29-49 years exhibited a 15%, 36%, and 40% higher risk of a cardiovascular event, respectively.
Similarly, women aged 18-22 years or 29-46 years with long cycles of 40 days or more had a 44% or 30% higher risk of cardiovascular disease, respectively, compared with women with cycle lengths of 26-31 days.
“The coronary heart diseases were decisive for the increase, and less so, the strokes,” wrote the researchers.
Classic risk factors?
Dr. Seeland praised the fact that the study authors tried to determine the role that classic cardiovascular risk factors played. “Compared with women with a regular cycle, women with an irregular cycle had a higher BMI, more frequently increased cholesterol levels, and an elevated blood pressure,” she said. Women with a long cycle displayed a similar pattern.
It can be assumed from this that over a woman’s life span, BMI affects the risk of cardiovascular disease. Therefore, Dr. Wang and coauthors adjusted the results on the basis of BMI, which varies over time.
Regarding other classic risk factors that may have played a role, “hypercholesterolemia, chronic high blood pressure, and type 2 diabetes were only responsible in 5.4%-13.5% of the associations,” wrote the researchers.
“Our results suggest that certain characteristics of the menstrual cycle across a woman’s reproductive lifespan may constitute additional risk markers for cardiovascular disease,” according to the authors.
The highest rates of cardiovascular disease were among women with permanently irregular or long cycles in early to mid adulthood, as well as women who had regular cycles when younger but had irregular cycles in mid adulthood. “This indicates that the change from one cycle phenotype to another could be a surrogate marker for metabolic changes, which in turn contribute to the formation of cardiovascular diseases,” wrote the authors.
The study was observational and so conclusions cannot be drawn regarding causal relationships. But Dr. Wang and associates indicate that the most common cause of an irregular menstrual cycle may be PCOS. “Roughly 90% of women with cycle disorders or oligomenorrhea have signs of PCOS. And it was shown that women with PCOS have an increased risk of cardiovascular disease.”
They concluded that “the associations observed between irregular and long cycles in early to mid-adulthood and cardiovascular diseases are likely attributable to underlying PCOS.”
For Dr. Seeland, however, this conclusion is “too monocausal. At no point in time did there seem to be any direct information regarding the frequency of PCOS during the data collection by the respondents.”
For now, we can only speculate about the mechanisms. “The association between a very irregular and long cycle and the increased risk of cardiovascular diseases has now only been described. More research should be done on the causes,” said Dr. Seeland.
This article was translated from the Medscape German edition. A version of this article first appeared on Medscape.com.
This finding is demonstrated in a new analysis of the Nurses’ Health Study II.
“To date, several studies have reported increased risks of cardiovascular risk factors or cardiovascular disease in connection with cycle disorders,” Yi-Xin Wang, MD, PhD, a research fellow in nutrition, and associates from the Harvard School of Public Health, Boston, wrote in an article published in JAMA Network Open.
Ute Seeland, MD, speaker of the Gender Medicine in Cardiology Working Group of the German Cardiology Society, said in an interview“We know that women who have indicated in their medical history that they have irregular menstrual cycles, invariably in connection with polycystic ovary syndrome (PCOS), more commonly develop diabetes and other metabolic disorders, as well as cardiovascular diseases.”
Cycle disorders’ role
However, the role that irregular or especially long cycles play at different points of a woman’s reproductive life span was unclear. Therefore, the research group investigated the associations in the Nurses’ Health Study II between cycle irregularity and cycle length in women of different age groups who later experienced cardiovascular events.
At the end of this study in 1989, the participants also provided information regarding the length and irregularity of their menstrual cycle from ages 14 to 17 years and again from ages 18 to 22 years. The information was updated in 1993 when the participants were aged 29-46 years. The data from 2019 to 2022 were analyzed.
“This kind of long-term cohort study is extremely rare and therefore something special,” said Dr. Seeland, who conducts research at the Institute for Social Medicine, Epidemiology, and Health Economics at the Charité – University Hospital Berlin.
The investigators used the following cycle classifications: very regular (no more than 3 or 4 days before or after the expected date), regular (within 5-7 days), usually irregular, always irregular, or no periods.
The cycle lengths were divided into the following categories: less than 21 days, 21-25 days, 26-31 days, 32-39 days, 40-50 days, more than 50 days, and too irregular to estimate the length.
The onset of cardiovascular diseases was determined using information from the participants and was confirmed by reviewing the medical files. Relevant to the study were lethal and nonlethal coronary heart diseases (such as myocardial infarction or coronary artery revascularization), as well as strokes.
Significant in adulthood
The data from 80,630 study participants were included in the analysis. At study inclusion, the average age of the participants was 37.7 years, and the average body mass index (BMI) was 25.1. “Since it was predominantly White nurses who took part in the study, the data are not transferable to other, more diverse populations,” said Dr. Seeland.
Over 24 years, 1,816 women (2.4%) had a cardiovascular event. “We observed an increased rate of cardiovascular events in women with an irregular cycle and longer cycle, both in early an in mid-adulthood,” wrote Dr. Wang and associates. “Similar trends were also observed for cycle disorders when younger, but this association was weaker than in adulthood.”
Compared with women with very regular cycles, women with irregular cycles or without periods who were aged 14-17 years, 18-22 years, or 29-49 years exhibited a 15%, 36%, and 40% higher risk of a cardiovascular event, respectively.
Similarly, women aged 18-22 years or 29-46 years with long cycles of 40 days or more had a 44% or 30% higher risk of cardiovascular disease, respectively, compared with women with cycle lengths of 26-31 days.
“The coronary heart diseases were decisive for the increase, and less so, the strokes,” wrote the researchers.
Classic risk factors?
Dr. Seeland praised the fact that the study authors tried to determine the role that classic cardiovascular risk factors played. “Compared with women with a regular cycle, women with an irregular cycle had a higher BMI, more frequently increased cholesterol levels, and an elevated blood pressure,” she said. Women with a long cycle displayed a similar pattern.
It can be assumed from this that over a woman’s life span, BMI affects the risk of cardiovascular disease. Therefore, Dr. Wang and coauthors adjusted the results on the basis of BMI, which varies over time.
Regarding other classic risk factors that may have played a role, “hypercholesterolemia, chronic high blood pressure, and type 2 diabetes were only responsible in 5.4%-13.5% of the associations,” wrote the researchers.
“Our results suggest that certain characteristics of the menstrual cycle across a woman’s reproductive lifespan may constitute additional risk markers for cardiovascular disease,” according to the authors.
The highest rates of cardiovascular disease were among women with permanently irregular or long cycles in early to mid adulthood, as well as women who had regular cycles when younger but had irregular cycles in mid adulthood. “This indicates that the change from one cycle phenotype to another could be a surrogate marker for metabolic changes, which in turn contribute to the formation of cardiovascular diseases,” wrote the authors.
The study was observational and so conclusions cannot be drawn regarding causal relationships. But Dr. Wang and associates indicate that the most common cause of an irregular menstrual cycle may be PCOS. “Roughly 90% of women with cycle disorders or oligomenorrhea have signs of PCOS. And it was shown that women with PCOS have an increased risk of cardiovascular disease.”
They concluded that “the associations observed between irregular and long cycles in early to mid-adulthood and cardiovascular diseases are likely attributable to underlying PCOS.”
For Dr. Seeland, however, this conclusion is “too monocausal. At no point in time did there seem to be any direct information regarding the frequency of PCOS during the data collection by the respondents.”
For now, we can only speculate about the mechanisms. “The association between a very irregular and long cycle and the increased risk of cardiovascular diseases has now only been described. More research should be done on the causes,” said Dr. Seeland.
This article was translated from the Medscape German edition. A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
FDA approves Wegovy (semaglutide) for obesity in teens 12 and up
The Food and Drug Administration has approved semaglutide 2.4 mg (Wegovy), a once-weekly subcutaneous injection, for the additional indication of treating obesity in adolescents aged 12 years and older.
This is defined as those with an initial body mass index at or above the 95th percentile for age and sex (based on CDC growth charts). Semaglutide must be administered along with lifestyle intervention of a reduced calorie meal plan and increased physical activity.
When Wegovy was approved for use in adults with obesity in June 2021, it was labeled a “game changer.”
The new approval is based on the results of the STEP TEENS phase 3 trial of once-weekly 2.4 mg of semaglutide in adolescents 12- to <18 years old with obesity, the drug’s manufacturer, Novo Nordisk, announced in a press release.
In STEP TEENS, reported at Obesity Week 2022 in November, and simultaneously published in the New England Journal of Medicine, adolescents with obesity treated with semaglutide for 68 weeks had a 16.1% reduction in BMI compared with a 0.6% increase in BMI in those receiving placebo. Both groups also received lifestyle intervention. Mean weight loss was 15.3 kg (33.7 pounds) among teens on semaglutide, while those on placebo gained 2.4 kg (5.3 pounds).
At the time, Claudia K. Fox, MD, MPH, codirector of the Center for Pediatric Obesity Medicine at the University of Minnesota – who was not involved with the research – told this news organization the results were “mind-blowing ... we are getting close to bariatric surgery results” in these adolescent patients with obesity.
Semaglutide is a GLP-1 agonist, as is a related agent, also from Novo Nordisk, liraglutide (Saxenda), a daily subcutaneous injection, which was approved for use in adolescents aged 12 and older in December 2020. Wegovy is the first weekly subcutaneous injection approved for use in adolescents.
Other agents approved for obesity in those older than 12 in the United States include the combination phentermine and topiramate extended-release capsules (Qsymia) in June 2022, and orlistat (Alli). Phentermine is approved for those aged 16 and older.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved semaglutide 2.4 mg (Wegovy), a once-weekly subcutaneous injection, for the additional indication of treating obesity in adolescents aged 12 years and older.
This is defined as those with an initial body mass index at or above the 95th percentile for age and sex (based on CDC growth charts). Semaglutide must be administered along with lifestyle intervention of a reduced calorie meal plan and increased physical activity.
When Wegovy was approved for use in adults with obesity in June 2021, it was labeled a “game changer.”
The new approval is based on the results of the STEP TEENS phase 3 trial of once-weekly 2.4 mg of semaglutide in adolescents 12- to <18 years old with obesity, the drug’s manufacturer, Novo Nordisk, announced in a press release.
In STEP TEENS, reported at Obesity Week 2022 in November, and simultaneously published in the New England Journal of Medicine, adolescents with obesity treated with semaglutide for 68 weeks had a 16.1% reduction in BMI compared with a 0.6% increase in BMI in those receiving placebo. Both groups also received lifestyle intervention. Mean weight loss was 15.3 kg (33.7 pounds) among teens on semaglutide, while those on placebo gained 2.4 kg (5.3 pounds).
At the time, Claudia K. Fox, MD, MPH, codirector of the Center for Pediatric Obesity Medicine at the University of Minnesota – who was not involved with the research – told this news organization the results were “mind-blowing ... we are getting close to bariatric surgery results” in these adolescent patients with obesity.
Semaglutide is a GLP-1 agonist, as is a related agent, also from Novo Nordisk, liraglutide (Saxenda), a daily subcutaneous injection, which was approved for use in adolescents aged 12 and older in December 2020. Wegovy is the first weekly subcutaneous injection approved for use in adolescents.
Other agents approved for obesity in those older than 12 in the United States include the combination phentermine and topiramate extended-release capsules (Qsymia) in June 2022, and orlistat (Alli). Phentermine is approved for those aged 16 and older.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved semaglutide 2.4 mg (Wegovy), a once-weekly subcutaneous injection, for the additional indication of treating obesity in adolescents aged 12 years and older.
This is defined as those with an initial body mass index at or above the 95th percentile for age and sex (based on CDC growth charts). Semaglutide must be administered along with lifestyle intervention of a reduced calorie meal plan and increased physical activity.
When Wegovy was approved for use in adults with obesity in June 2021, it was labeled a “game changer.”
The new approval is based on the results of the STEP TEENS phase 3 trial of once-weekly 2.4 mg of semaglutide in adolescents 12- to <18 years old with obesity, the drug’s manufacturer, Novo Nordisk, announced in a press release.
In STEP TEENS, reported at Obesity Week 2022 in November, and simultaneously published in the New England Journal of Medicine, adolescents with obesity treated with semaglutide for 68 weeks had a 16.1% reduction in BMI compared with a 0.6% increase in BMI in those receiving placebo. Both groups also received lifestyle intervention. Mean weight loss was 15.3 kg (33.7 pounds) among teens on semaglutide, while those on placebo gained 2.4 kg (5.3 pounds).
At the time, Claudia K. Fox, MD, MPH, codirector of the Center for Pediatric Obesity Medicine at the University of Minnesota – who was not involved with the research – told this news organization the results were “mind-blowing ... we are getting close to bariatric surgery results” in these adolescent patients with obesity.
Semaglutide is a GLP-1 agonist, as is a related agent, also from Novo Nordisk, liraglutide (Saxenda), a daily subcutaneous injection, which was approved for use in adolescents aged 12 and older in December 2020. Wegovy is the first weekly subcutaneous injection approved for use in adolescents.
Other agents approved for obesity in those older than 12 in the United States include the combination phentermine and topiramate extended-release capsules (Qsymia) in June 2022, and orlistat (Alli). Phentermine is approved for those aged 16 and older.
A version of this article first appeared on Medscape.com.
Study of beliefs about what causes cancer sparks debate
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
The study, entitled, “Everything Causes Cancer? Beliefs and Attitudes Towards Cancer Prevention Among Anti-Vaxxers, Flat Earthers, and Reptilian Conspiracists: Online Cross Sectional Survey,” was published in the Christmas 2022 issue of The British Medical Journal (BMJ).
The authors explain that they set out to evaluate “the patterns of beliefs about cancer among people who believed in conspiracies, rejected the COVID-19 vaccine, or preferred alternative medicine.”
They sought such people on social media and online chat platforms and asked them questions about real and mythical causes of cancer.
Almost half of survey participants agreed with the statement, “It seems like everything causes cancer.”
Overall, among all participants, awareness of the actual causes of cancer was greater than awareness of the mythical causes of cancer, the authors report. However, awareness of the actual causes of cancer was lower among the unvaccinated and members of conspiracy groups than among their counterparts.
The authors are concerned that their findings suggest “a direct connection between digital misinformation and consequent potential erroneous health decisions, which may represent a further preventable fraction of cancer.”
Backlash and criticism
The study “highlights the difficulty society encounters in distinguishing the actual causes of cancer from mythical causes,” The BMJ commented on Twitter.
However, both the study and the journal received some backlash.
This is a “horrible article seeking to smear people with concerns about COVID vaccines,” commented Clare Craig, a British consultant pathologist who specializes in cancer diagnostics.
The study and its methodology were also harshly criticized on Twitter by Normal Fenton, professor of risk information management at the Queen Mary University of London.
The senior author of the study, Laura Costas, a medical epidemiologist with the Catalan Institute of Oncology, Barcelona, told this news organization that the naysayers on social media, many of whom focused their comments on the COVID-19 vaccine, prove the purpose of the study – that misinformation spreads widely on the internet.
“Most comments focused on spreading COVID-19 myths, which were not the direct subject of the study, and questioned the motivations of BMJ authors and the scientific community, assuming they had a common malevolent hidden agenda,” Ms. Costas said.
“They stated the need of having critical thinking, a trait in common with the scientific method, but dogmatically dismissed any information that comes from official sources,” she added.
Ms. Costas commented that “society encounters difficulty in differentiating actual from mythical causes of cancer owing to mass information. We therefore planned this study with a certain satire, which is in line with the essence of The BMJ Christmas issue.”
The BMJ has a long history of publishing a lighthearted Christmas edition full of original, satirical, and nontraditional studies. Previous years have seen studies that explored potential harms from holly and ivy, survival time of chocolates on hospital wards, and the question, “Were James Bond’s drinks shaken because of alcohol induced tremor?”
Study details
Ms. Costas and colleagues sought participants for their survey from online forums that included 4chan and Reddit, which are known for their controversial content posted by anonymous users. Data were also collected from ForoCoches and HispaChan, well-known Spanish online forums. These online sites were intentionally chosen because researchers thought “conspiracy beliefs would be more prevalent,” according to Ms. Costas.
Across the multiple forums, there were 1,494 participants. Of these, 209 participants were unvaccinated against COVID-19, 112 preferred alternatives rather than conventional medicine, and 62 reported that they believed the earth was flat or believed that humanoids take reptilian forms to manipulate human societies.
The team then sought to assess beliefs about actual and mythical (nonestablished) causes of cancer by presenting the participants with the closed risk factor questions on two validated scales – the Cancer Awareness Measure (CAM) and CAM–Mythical Causes Scale (CAM-MYCS).
Responses to both were recorded on a five-point scale; answers ranged from “strongly disagree” to “strongly agree.”
The CAM assesses cancer risk perceptions of 11 established risk factors for cancer: smoking actively or passively, consuming alcohol, low levels of physical activity, consuming red or processed meat, getting sunburnt as a child, family history of cancer, human papillomavirus infection, being overweight, age greater than or equal to 70 years, and low vegetable and fruit consumption.
The CAM-MYCS measure includes 12 questions on risk perceptions of mythical causes of cancer – nonestablished causes that are commonly believed to cause cancer but for which there is no supporting scientific evidence, the authors explain. These items include drinking from plastic bottles; eating food containing artificial sweeteners or additives and genetically modified food; using microwave ovens, aerosol containers, mobile phones, and cleaning products; living near power lines; feeling stressed; experiencing physical trauma; and being exposed to electromagnetic frequencies/non-ionizing radiation, such as wi-fi networks, radio, and television.
The most endorsed mythical causes of cancer were eating food containing additives (63.9%) or sweeteners (50.7%), feeling stressed (59.7%), and eating genetically modified foods (38.4%).
A version of this article first appeared on Medscape.com.
Top cardiology societies call for revamp of clinical trials
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Leading cardiology societies have issued a “call for action” on a global scale to reinvent randomized clinical trials fit for the 21st century.
“Randomized trials are an essential tool for reliably assessing the effects of treatments, but they have become too costly and too burdensome,” first author Louise Bowman, University of Oxford, England, told this news organization. “We urgently need to modernize our approach to clinical trials in order to continue to improve patient care.”
The joint opinion is from the European Society of Cardiology, the American Heart Association, the American College of Cardiology, and the World Heart Federation. It was simultaneously published online in the European Heart Journal, Circulation, Journal of the American College of Cardiology, and Global Heart.
The authors note that the availability of large-scale “real-world” data is increasingly being touted as a way to bypass the challenges of conducting randomized trials. Yet, observational analyses of real-world data “are not a suitable alternative to randomization,” Prof. Bowman said.
Cardiology has historically led the way in transforming clinical practice with groundbreaking “mega-trials,” such as the International Study of Infarct Survival (ISIS), Gruppo Italiano per lo Studio della Streptochinasi nell’Infarto (GISSI), and Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries (GUSTO).
But over the past 25 years, there has been a huge increase in the rules and related bureaucracy governing clinical trials, which hinders the ability to conduct trials swiftly and affordably, the authors point out.
The COVID-19 pandemic has shown that important clinical trials can be performed quickly and efficiently in busy hospitals, they note.
“The RECOVERY trial in COVID-19 has been an excellent example of this, with results that are estimated to have saved around 1 million lives worldwide within just 1 year,” Prof. Bowman told this news organization.
A Good Clinical Trials Collaborative made up of key stakeholders recently developed new guidelines designed to promote better, more efficient randomized controlled trials.
“If widely adopted and used alongside valuable 21st century electronic health records, we could transform the clinical trials landscape and do many more high-quality trials very cost-effectively,” Prof. Bowman said.
“Widespread adoption and implementation of the revised guidelines will require collaboration with a wide range of national and international organizations, including patient, professional, academic, and industry groups, funders and government organizations, and ethics, health policy, and regulatory bodies,” Prof. Bowman acknowledged.
“This is work that the Good Clinical Trials Collaborative is leading. It is hoped that this endorsement by the joint cardiovascular societies will increase awareness and provide valuable support to his important work,” she added.
No commercial funding was received. The authors have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Pulling sensation in chest
The history and findings in this case are suggestive of breast cancer with metastatic spread to the chest wall and lungs.
Globally, breast cancer is the most frequently diagnosed life-threatening cancer and the leading cause of cancer death among women. In the United States, an estimated 287,850 new cases of invasive breast cancer will be diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur. Despite advances in adjuvant treatment strategies, such as tamoxifen for patients with ER-positive breast cancer, many patients with early breast cancer still experience disease recurrence after primary therapy. Because of its systemic nature and inevitable resistance to therapy, metastatic breast cancer is largely incurable.
Approximately 5%-35% of patients with breast cancer develop locoregional recurrence either alone or with distant metastases. The lung is a frequent site of breast cancer metastasis. In addition, approximately 11% of patients have persistent chest wall progression. Recurrent breast cancer in the chest wall is considered a marker of poor prognosis and is normally accompanied by or a precursor to distant metastases.
Risk factors for chest wall recurrence include primary tumor size, primary stage, and lymph node involvement; in addition, the risk is increased in patients aged 40 years or younger and in those with gross multifocal or multicentric disease. Histopathological risk factors include positive margin status, DCIS, extensive intraductal component, high grade, lymphovascular invasion, tumor oncogene, and tumor suppressor gene expression (eg, p53 and HER2), and ER status.
According to the National Comprehensive Cancer Network (NCCN) 2022 guidelines, the staging evaluation of patients who present with recurrent or stage IV breast cancer should include:
• History and physical exam
• Complete blood count and liver function tests
• Chest diagnostic CT
• Bone scan
• Radiographs of any long or weight-bearing bones that are painful or appear abnormal on bone scan
• Diagnostic CT of the abdomen (with or without diagnostic CT of the pelvis) or MRI of the abdomen
• Biopsy documentation of first recurrence, when possible
The use of sodium fluoride PET or PET-CT for the evaluation of patients with recurrent disease is largely discouraged.
Determination of hormone receptor status (ER and progesterone receptor [PR]) as well as HER2 status should be repeated because ER and PR assays may be falsely negative or falsely positive and there may be discordance between the primary and metastatic tumors.
In the metastatic setting, genetic testing results may have therapeutic implications; specifically, germline mutations in BRCA1/BRCA2 have demonstrated clinical utility and therapeutic impact. Thus, the NCCN panel recommends that germline BRCA1/BRCA2 mutations be evaluated in all patients with recurrent or metastatic breast cancer to identify candidates for appropriate targeted therapies (eg, poly adenosine diphosphate ribose polymerase–inhibitor therapy).
In patients with recurrence of breast cancer to the chest wall, complete chest wall resection and appropriate reconstruction may prolong overall survival, although appropriate patient selection is essential for optimal outcomes. Patients with tumors that display a more aggressive phenotype (eg, triple-negative or HER2-positive disease) may not benefit from this approach and supportive care may be more appropriate.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of breast cancer with metastatic spread to the chest wall and lungs.
Globally, breast cancer is the most frequently diagnosed life-threatening cancer and the leading cause of cancer death among women. In the United States, an estimated 287,850 new cases of invasive breast cancer will be diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur. Despite advances in adjuvant treatment strategies, such as tamoxifen for patients with ER-positive breast cancer, many patients with early breast cancer still experience disease recurrence after primary therapy. Because of its systemic nature and inevitable resistance to therapy, metastatic breast cancer is largely incurable.
Approximately 5%-35% of patients with breast cancer develop locoregional recurrence either alone or with distant metastases. The lung is a frequent site of breast cancer metastasis. In addition, approximately 11% of patients have persistent chest wall progression. Recurrent breast cancer in the chest wall is considered a marker of poor prognosis and is normally accompanied by or a precursor to distant metastases.
Risk factors for chest wall recurrence include primary tumor size, primary stage, and lymph node involvement; in addition, the risk is increased in patients aged 40 years or younger and in those with gross multifocal or multicentric disease. Histopathological risk factors include positive margin status, DCIS, extensive intraductal component, high grade, lymphovascular invasion, tumor oncogene, and tumor suppressor gene expression (eg, p53 and HER2), and ER status.
According to the National Comprehensive Cancer Network (NCCN) 2022 guidelines, the staging evaluation of patients who present with recurrent or stage IV breast cancer should include:
• History and physical exam
• Complete blood count and liver function tests
• Chest diagnostic CT
• Bone scan
• Radiographs of any long or weight-bearing bones that are painful or appear abnormal on bone scan
• Diagnostic CT of the abdomen (with or without diagnostic CT of the pelvis) or MRI of the abdomen
• Biopsy documentation of first recurrence, when possible
The use of sodium fluoride PET or PET-CT for the evaluation of patients with recurrent disease is largely discouraged.
Determination of hormone receptor status (ER and progesterone receptor [PR]) as well as HER2 status should be repeated because ER and PR assays may be falsely negative or falsely positive and there may be discordance between the primary and metastatic tumors.
In the metastatic setting, genetic testing results may have therapeutic implications; specifically, germline mutations in BRCA1/BRCA2 have demonstrated clinical utility and therapeutic impact. Thus, the NCCN panel recommends that germline BRCA1/BRCA2 mutations be evaluated in all patients with recurrent or metastatic breast cancer to identify candidates for appropriate targeted therapies (eg, poly adenosine diphosphate ribose polymerase–inhibitor therapy).
In patients with recurrence of breast cancer to the chest wall, complete chest wall resection and appropriate reconstruction may prolong overall survival, although appropriate patient selection is essential for optimal outcomes. Patients with tumors that display a more aggressive phenotype (eg, triple-negative or HER2-positive disease) may not benefit from this approach and supportive care may be more appropriate.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
The history and findings in this case are suggestive of breast cancer with metastatic spread to the chest wall and lungs.
Globally, breast cancer is the most frequently diagnosed life-threatening cancer and the leading cause of cancer death among women. In the United States, an estimated 287,850 new cases of invasive breast cancer will be diagnosed in 2022; in addition, 43,250 deaths because of breast cancer are expected to occur. Despite advances in adjuvant treatment strategies, such as tamoxifen for patients with ER-positive breast cancer, many patients with early breast cancer still experience disease recurrence after primary therapy. Because of its systemic nature and inevitable resistance to therapy, metastatic breast cancer is largely incurable.
Approximately 5%-35% of patients with breast cancer develop locoregional recurrence either alone or with distant metastases. The lung is a frequent site of breast cancer metastasis. In addition, approximately 11% of patients have persistent chest wall progression. Recurrent breast cancer in the chest wall is considered a marker of poor prognosis and is normally accompanied by or a precursor to distant metastases.
Risk factors for chest wall recurrence include primary tumor size, primary stage, and lymph node involvement; in addition, the risk is increased in patients aged 40 years or younger and in those with gross multifocal or multicentric disease. Histopathological risk factors include positive margin status, DCIS, extensive intraductal component, high grade, lymphovascular invasion, tumor oncogene, and tumor suppressor gene expression (eg, p53 and HER2), and ER status.
According to the National Comprehensive Cancer Network (NCCN) 2022 guidelines, the staging evaluation of patients who present with recurrent or stage IV breast cancer should include:
• History and physical exam
• Complete blood count and liver function tests
• Chest diagnostic CT
• Bone scan
• Radiographs of any long or weight-bearing bones that are painful or appear abnormal on bone scan
• Diagnostic CT of the abdomen (with or without diagnostic CT of the pelvis) or MRI of the abdomen
• Biopsy documentation of first recurrence, when possible
The use of sodium fluoride PET or PET-CT for the evaluation of patients with recurrent disease is largely discouraged.
Determination of hormone receptor status (ER and progesterone receptor [PR]) as well as HER2 status should be repeated because ER and PR assays may be falsely negative or falsely positive and there may be discordance between the primary and metastatic tumors.
In the metastatic setting, genetic testing results may have therapeutic implications; specifically, germline mutations in BRCA1/BRCA2 have demonstrated clinical utility and therapeutic impact. Thus, the NCCN panel recommends that germline BRCA1/BRCA2 mutations be evaluated in all patients with recurrent or metastatic breast cancer to identify candidates for appropriate targeted therapies (eg, poly adenosine diphosphate ribose polymerase–inhibitor therapy).
In patients with recurrence of breast cancer to the chest wall, complete chest wall resection and appropriate reconstruction may prolong overall survival, although appropriate patient selection is essential for optimal outcomes. Patients with tumors that display a more aggressive phenotype (eg, triple-negative or HER2-positive disease) may not benefit from this approach and supportive care may be more appropriate.
Avan J. Armaghani, MD, Assistant Member, Department of Breast Oncology, Moffitt Cancer Center, University of South Florida, Tampa, FL.
Avan J. Armaghani, MD, has disclosed no relevant financial relationships.
Image Quizzes are fictional or fictionalized clinical scenarios intended to provide evidence-based educational takeaways.
A 39-year-old nonsmoking woman in the United States presents with a history of a painful, pulling sensation in her chest that she originally attributed to a change in her exercise regimen. Six years earlier, the patient was diagnosed with estrogen receptor (ER)–positive ductal carcinoma in situ (DCIS) in her left breast. She opted for mastectomy and immediate reconstruction, followed by adjuvant therapy with tamoxifen (20 mg/d for 5 years). Physical examination reveals a palpable mass in the medial half of her left breast with several hard, painful nodules in the left axilla. Mild wheezing throughout the upper lungs is heard on auscultation. Abdominal examination does not reveal any abnormalities. Laboratory findings are all within normal range, apart from C-reactive protein, which is elevated. The patient is 5 ft 7 in and weighs 133 lb.
Rapidly Growing Nodule Within a Previously Radiated Area of the Scalp
The Diagnosis: Pseudoangiomatous Squamous Cell Carcinoma
Pseudoangiomatous squamous cell carcinoma (PSCC), a variant of acantholytic squamous cell carcinoma (SCC), is a rare epithelial neoplasm that can mimic angiosarcoma.1 Clinically, PSCC presents as a white-gray ulcer or nodular pink tumor on sun-exposed areas, typically on the head and neck. Due to its increased potential for metastasis, this variant of SCC is considered particularly aggressive. Histologically, PSCC shows nests of acantholytic atypical keratinocytes arranged in anastomosing arrays that form pseudovascular or pseudoglandular structures.2 Acantholytic spaces frequently are filled with erythrocytes. Immunohistochemically, PSCC tumor cells express classic squamous markers such as cytokeratin (CK) 5 and p63 but not vascular markers such as CD31, CD34, and von Willebrand factor.3 In our patient, histopathology of the lesion revealed invasive nests, lobules, and interconnected columns of well-differentiated squamous tumor cells that emanated from the base of the epidermis. The tumor exhibited acantholysis forming ectatic and slitlike spaces, some of which contained erythrocytes. The neoplastic cells, including those lining pseudovascular spaces, positively stained for CK5 (Figure 1A) and nuclear p63 but lacked reactivity to CD31 (Figure 1B) and CD34, corroborating squamous and not vascular differentiation. Current treatment guidelines include Mohs micrographic surgery, excisional surgery, or radiation.4 Our patient’s lesion was completely removed by Mohs micrographic surgery. Three months later, there was no evidence of recurrence.
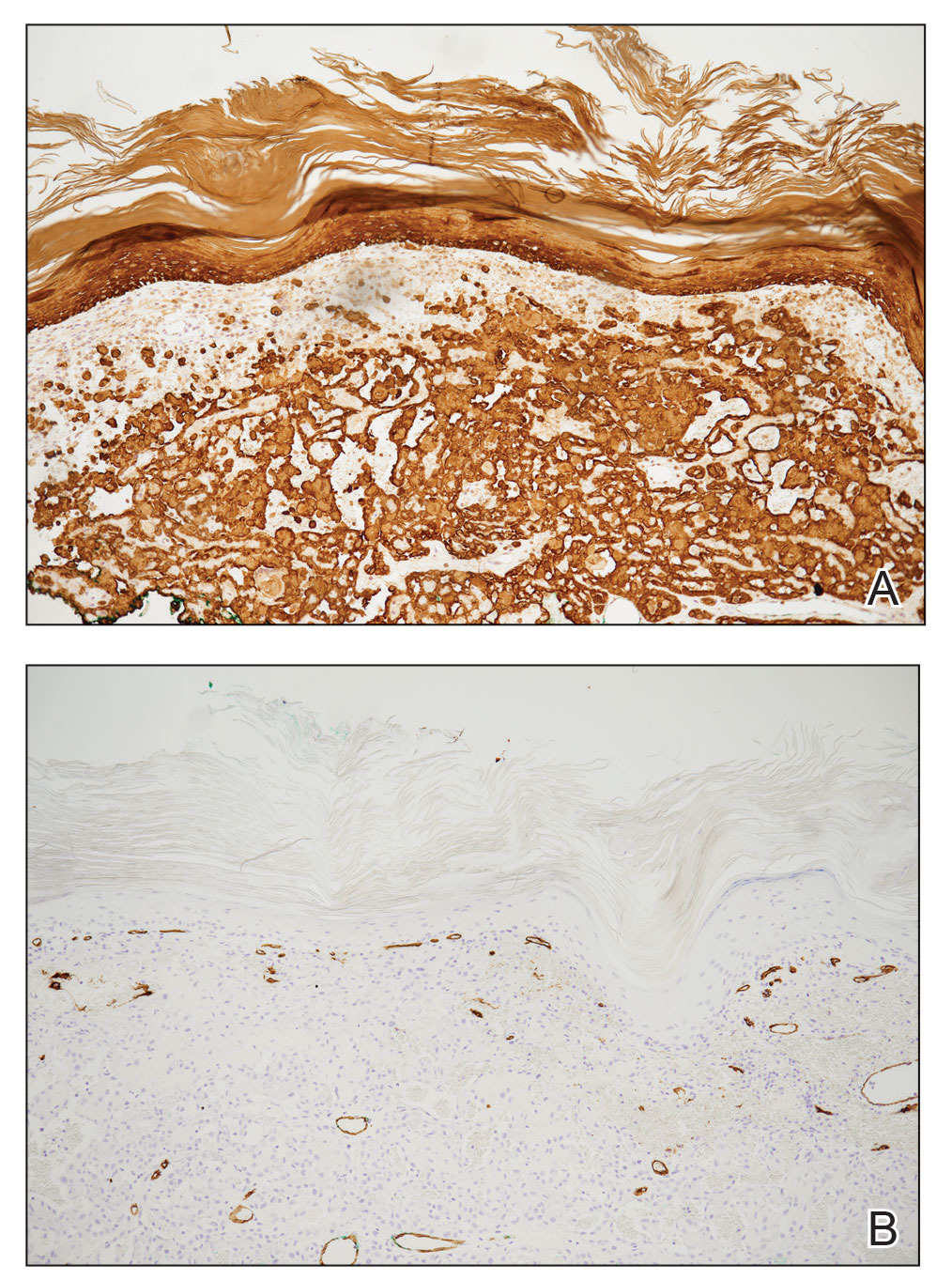
Angiosarcoma is an aggressive neoplasm associated with a poor prognosis and 5-year survival rate of 30% to 40%. The etiology of angiosarcoma still is unclear, but identified risk factors include prior radiation therapy, lymphedema (Stewart-Treves syndrome), and genetic predisposition.5 In the skin, angiosarcoma often occurs in the head and neck region, accounting for 60% of cutaneous cases.5,6 Early in the disease, most patients present with a bruiselike lesion on the scalp or forehead, often delaying the diagnosis.6 As the cancer progresses, tissue infiltration, edema, and hemorrhage contribute to the formation of violaceous nodules, which eventually prompt for biopsy. Angiosarcoma spans a broad histologic spectrum depending on the cytology of malignant cells (eg, spindle, small round, epithelioid) and their capacity for vasoformation. Welldifferentiated angiosarcoma shows retiform slitlike spaces in between collagen bundles that are lined by hyperchromatic hobnailing endothelial cells (Figure 2).7 Epithelioid angiosarcoma can be mistaken for SCC.8 Immunohistochemically, angiosarcoma stains positively for CD31, CD34, ETS-related gene 1, D2-40, and factor VIII.9 In our patient, the neoplasm was negative for vascular markers CD31 and CD34.
Bacillary angiomatosis (BA), caused by Bartonella henselae, is a rare disease that first was identified in HIV patients with diminished CD4+ T-cell counts. In the skin, BA often manifests as centrally ulcerated, single or clustered, reddish-purple nodules.10 Histologically, it is characterized by highly vascularized, histiocyterich infiltrates with admixed neutrophils and plasma cells (Figure 3). Capillaries often proliferate in a lobular fashion.11 Atypical cytology with areas of necrosis may mimic angiosarcoma.12 The pathognomonic feature of BA is the presence of enlarged histiocytes with pink-purplish cytoplasm corresponding to intracytoplasmic aggregates of bacteria, which can be revealed by Warthin-Starry or Grocott-Gomori methenamine-silver staining. Immunohistochemically, proliferative benign capillaries are highlighted by CD34 and CD31, and histiocytes are decorated by CD68.12 This diagnosis was excluded based on the patient’s history, clinical presentation, and positive staining for CK5 and p63.
Squamoid eccrine ductal carcinoma is an exceedingly rare subtype of eccrine carcinoma that mimics SCC both clinically and histologically.13 It most often occurs on the head and neck of elderly patients. This neoplasm can look similar to SCC and its variants, including PSCC. Histologically, squamoid eccrine ductal carcinoma exhibits a biphasic growth pattern.14 Well-differentiated squamous dysplasia transitions to carcinoma with eccrine duct formation as the tumor percolates deep into the dermis (Figure 4). As a result, superficial skin biopsies often lead to an incorrect diagnosis.15 Unlike SCC, the risk for locoregional and widespread metastasis is elevated. Identifying ducts in the deep aspect of the tumor is critical, thus immunohistochemical staining for carcinoembryonic antigen and epithelial membrane antigen is paramount for the diagnosis.15 Pseudoangiomatous SCC will stain negative for carcinoembryonic antigen, as was the case in our patient.
Pseudoepitheliomatous hyperplasia is a benign histologic reaction that can result from trauma, chronic inflammation (ie, pyoderma gangrenosum), tattoo placement, underlying neoplasia or fungal infection, or a spider bite reaction.14,15 It most commonly is seen as a well-demarcated nodule or plaque associated with scaling or crusting. Papules vary in size from less than 1 cm to several centimeters. Histologically, it is defined by an acanthotic proliferation of the adnexal epithelium and epidermis (Figure 5).16,17 Irregular strands, cords, and nests of squamoid cells can extend into the dermis.18 It can closely mimic SCC, but there are a few key differences. Pseudoepitheliomatous hyperplasia will not display atypical mitotic figures or atypical nuclei and will never invade lymphatics or vascular systems.19 Pseudoepitheliomatous hyperplasia shows identical histology to well-differentiated SCC, and thus clinicopathologic correlation and mindful histologic evaluation are crucial. The presence of an increased influx of neutrophils and histiocytes should prompt for microbial stains or deeper sectioning. A superficial biopsy should be followed by a deep biopsy. In our patient, microorganismal stains were negative.
- Kiyohara T, Miyamoto M, Shijimaya T, et al. Pseudovascular squamous cell carcinoma: a review of the published work and reassessment of prognosis. J Dermatol. 2018;45:1448-1451.
- Nagore E, Sánchez-Motilla JM, Pérez-Vallés A, et al. Pseudovascular squamous cell carcinoma of the skin. Clin Exp Dermatol. 2000;25:206-208.
- Han X, Lin X, Shao X. Pseudovascular adenoid squamous cell carcinoma of the tongue: a case report and literature review. Int J Clin Exp Pathol. 2020;13:1086-1089.
- Singh S, Bisht N, Purkayastha A, et al. Acantholytic squamous cell carcinoma of the scalp in an elderly patient treated with radical radiotherapy. J Cancer Res Pract. 2018;5:165-168.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post‐radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Shilpa K, Leelavathy B, Gorur D, et al. Early-onset epithelioid angiosarcoma: diagnostic enigma, a rare case report. Indian J Dermatopathol Diagn Dermatol. 2019;6:36-38.
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe [published online May 4, 2017]. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Hoffman CF, Papadopoulos D, Palmer DM, et al. A case report of bacillary angiomatosis in a patient infected with human immunodeficiency virus. Cutis. 2002;69:175-178.
- Biwer E, Uerlich M, Wimheuer R, et al. Bacillary angiomatosis: an important differential diagnosis in patients with HIV. Am J Dermatopathol. 1994;16:110.
- Medeiros LJ, Miranda RN. Bacillary angiomatosis. In: Medeiros LJ, Miranda RN, eds. Diagnostic Pathology: Lymph Nodes and Extranodal Lymphomas. 2nd ed. Elsevier; 2018:58-63.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Mckissack S, Wohltmann W, Dalton S, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Wollina U. Pyoderma gangrenosum—a review. Orphanet J Rare Dis. 2007;2:19
- Chow P, Goddard L, Greenway H, et al. Squamoid eccrine ductal carcinoma: the Scripps experience. Dermatol Surg. 2021;47:1115-1117.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126.
- Lynch JM. Understanding pseudoepitheliomatous hyperplasia. Pathol Case Rev. 2004;9:36-45.
- Goel R, Wallace ML. Pseudoepitheliomatous hyperplasia secondary to cutaneous aspergillus. Am J Dermatopathol. 2001;23:224-226.
The Diagnosis: Pseudoangiomatous Squamous Cell Carcinoma
Pseudoangiomatous squamous cell carcinoma (PSCC), a variant of acantholytic squamous cell carcinoma (SCC), is a rare epithelial neoplasm that can mimic angiosarcoma.1 Clinically, PSCC presents as a white-gray ulcer or nodular pink tumor on sun-exposed areas, typically on the head and neck. Due to its increased potential for metastasis, this variant of SCC is considered particularly aggressive. Histologically, PSCC shows nests of acantholytic atypical keratinocytes arranged in anastomosing arrays that form pseudovascular or pseudoglandular structures.2 Acantholytic spaces frequently are filled with erythrocytes. Immunohistochemically, PSCC tumor cells express classic squamous markers such as cytokeratin (CK) 5 and p63 but not vascular markers such as CD31, CD34, and von Willebrand factor.3 In our patient, histopathology of the lesion revealed invasive nests, lobules, and interconnected columns of well-differentiated squamous tumor cells that emanated from the base of the epidermis. The tumor exhibited acantholysis forming ectatic and slitlike spaces, some of which contained erythrocytes. The neoplastic cells, including those lining pseudovascular spaces, positively stained for CK5 (Figure 1A) and nuclear p63 but lacked reactivity to CD31 (Figure 1B) and CD34, corroborating squamous and not vascular differentiation. Current treatment guidelines include Mohs micrographic surgery, excisional surgery, or radiation.4 Our patient’s lesion was completely removed by Mohs micrographic surgery. Three months later, there was no evidence of recurrence.

Angiosarcoma is an aggressive neoplasm associated with a poor prognosis and 5-year survival rate of 30% to 40%. The etiology of angiosarcoma still is unclear, but identified risk factors include prior radiation therapy, lymphedema (Stewart-Treves syndrome), and genetic predisposition.5 In the skin, angiosarcoma often occurs in the head and neck region, accounting for 60% of cutaneous cases.5,6 Early in the disease, most patients present with a bruiselike lesion on the scalp or forehead, often delaying the diagnosis.6 As the cancer progresses, tissue infiltration, edema, and hemorrhage contribute to the formation of violaceous nodules, which eventually prompt for biopsy. Angiosarcoma spans a broad histologic spectrum depending on the cytology of malignant cells (eg, spindle, small round, epithelioid) and their capacity for vasoformation. Welldifferentiated angiosarcoma shows retiform slitlike spaces in between collagen bundles that are lined by hyperchromatic hobnailing endothelial cells (Figure 2).7 Epithelioid angiosarcoma can be mistaken for SCC.8 Immunohistochemically, angiosarcoma stains positively for CD31, CD34, ETS-related gene 1, D2-40, and factor VIII.9 In our patient, the neoplasm was negative for vascular markers CD31 and CD34.

Bacillary angiomatosis (BA), caused by Bartonella henselae, is a rare disease that first was identified in HIV patients with diminished CD4+ T-cell counts. In the skin, BA often manifests as centrally ulcerated, single or clustered, reddish-purple nodules.10 Histologically, it is characterized by highly vascularized, histiocyterich infiltrates with admixed neutrophils and plasma cells (Figure 3). Capillaries often proliferate in a lobular fashion.11 Atypical cytology with areas of necrosis may mimic angiosarcoma.12 The pathognomonic feature of BA is the presence of enlarged histiocytes with pink-purplish cytoplasm corresponding to intracytoplasmic aggregates of bacteria, which can be revealed by Warthin-Starry or Grocott-Gomori methenamine-silver staining. Immunohistochemically, proliferative benign capillaries are highlighted by CD34 and CD31, and histiocytes are decorated by CD68.12 This diagnosis was excluded based on the patient’s history, clinical presentation, and positive staining for CK5 and p63.

Squamoid eccrine ductal carcinoma is an exceedingly rare subtype of eccrine carcinoma that mimics SCC both clinically and histologically.13 It most often occurs on the head and neck of elderly patients. This neoplasm can look similar to SCC and its variants, including PSCC. Histologically, squamoid eccrine ductal carcinoma exhibits a biphasic growth pattern.14 Well-differentiated squamous dysplasia transitions to carcinoma with eccrine duct formation as the tumor percolates deep into the dermis (Figure 4). As a result, superficial skin biopsies often lead to an incorrect diagnosis.15 Unlike SCC, the risk for locoregional and widespread metastasis is elevated. Identifying ducts in the deep aspect of the tumor is critical, thus immunohistochemical staining for carcinoembryonic antigen and epithelial membrane antigen is paramount for the diagnosis.15 Pseudoangiomatous SCC will stain negative for carcinoembryonic antigen, as was the case in our patient.

Pseudoepitheliomatous hyperplasia is a benign histologic reaction that can result from trauma, chronic inflammation (ie, pyoderma gangrenosum), tattoo placement, underlying neoplasia or fungal infection, or a spider bite reaction.14,15 It most commonly is seen as a well-demarcated nodule or plaque associated with scaling or crusting. Papules vary in size from less than 1 cm to several centimeters. Histologically, it is defined by an acanthotic proliferation of the adnexal epithelium and epidermis (Figure 5).16,17 Irregular strands, cords, and nests of squamoid cells can extend into the dermis.18 It can closely mimic SCC, but there are a few key differences. Pseudoepitheliomatous hyperplasia will not display atypical mitotic figures or atypical nuclei and will never invade lymphatics or vascular systems.19 Pseudoepitheliomatous hyperplasia shows identical histology to well-differentiated SCC, and thus clinicopathologic correlation and mindful histologic evaluation are crucial. The presence of an increased influx of neutrophils and histiocytes should prompt for microbial stains or deeper sectioning. A superficial biopsy should be followed by a deep biopsy. In our patient, microorganismal stains were negative.

The Diagnosis: Pseudoangiomatous Squamous Cell Carcinoma
Pseudoangiomatous squamous cell carcinoma (PSCC), a variant of acantholytic squamous cell carcinoma (SCC), is a rare epithelial neoplasm that can mimic angiosarcoma.1 Clinically, PSCC presents as a white-gray ulcer or nodular pink tumor on sun-exposed areas, typically on the head and neck. Due to its increased potential for metastasis, this variant of SCC is considered particularly aggressive. Histologically, PSCC shows nests of acantholytic atypical keratinocytes arranged in anastomosing arrays that form pseudovascular or pseudoglandular structures.2 Acantholytic spaces frequently are filled with erythrocytes. Immunohistochemically, PSCC tumor cells express classic squamous markers such as cytokeratin (CK) 5 and p63 but not vascular markers such as CD31, CD34, and von Willebrand factor.3 In our patient, histopathology of the lesion revealed invasive nests, lobules, and interconnected columns of well-differentiated squamous tumor cells that emanated from the base of the epidermis. The tumor exhibited acantholysis forming ectatic and slitlike spaces, some of which contained erythrocytes. The neoplastic cells, including those lining pseudovascular spaces, positively stained for CK5 (Figure 1A) and nuclear p63 but lacked reactivity to CD31 (Figure 1B) and CD34, corroborating squamous and not vascular differentiation. Current treatment guidelines include Mohs micrographic surgery, excisional surgery, or radiation.4 Our patient’s lesion was completely removed by Mohs micrographic surgery. Three months later, there was no evidence of recurrence.

Angiosarcoma is an aggressive neoplasm associated with a poor prognosis and 5-year survival rate of 30% to 40%. The etiology of angiosarcoma still is unclear, but identified risk factors include prior radiation therapy, lymphedema (Stewart-Treves syndrome), and genetic predisposition.5 In the skin, angiosarcoma often occurs in the head and neck region, accounting for 60% of cutaneous cases.5,6 Early in the disease, most patients present with a bruiselike lesion on the scalp or forehead, often delaying the diagnosis.6 As the cancer progresses, tissue infiltration, edema, and hemorrhage contribute to the formation of violaceous nodules, which eventually prompt for biopsy. Angiosarcoma spans a broad histologic spectrum depending on the cytology of malignant cells (eg, spindle, small round, epithelioid) and their capacity for vasoformation. Welldifferentiated angiosarcoma shows retiform slitlike spaces in between collagen bundles that are lined by hyperchromatic hobnailing endothelial cells (Figure 2).7 Epithelioid angiosarcoma can be mistaken for SCC.8 Immunohistochemically, angiosarcoma stains positively for CD31, CD34, ETS-related gene 1, D2-40, and factor VIII.9 In our patient, the neoplasm was negative for vascular markers CD31 and CD34.

Bacillary angiomatosis (BA), caused by Bartonella henselae, is a rare disease that first was identified in HIV patients with diminished CD4+ T-cell counts. In the skin, BA often manifests as centrally ulcerated, single or clustered, reddish-purple nodules.10 Histologically, it is characterized by highly vascularized, histiocyterich infiltrates with admixed neutrophils and plasma cells (Figure 3). Capillaries often proliferate in a lobular fashion.11 Atypical cytology with areas of necrosis may mimic angiosarcoma.12 The pathognomonic feature of BA is the presence of enlarged histiocytes with pink-purplish cytoplasm corresponding to intracytoplasmic aggregates of bacteria, which can be revealed by Warthin-Starry or Grocott-Gomori methenamine-silver staining. Immunohistochemically, proliferative benign capillaries are highlighted by CD34 and CD31, and histiocytes are decorated by CD68.12 This diagnosis was excluded based on the patient’s history, clinical presentation, and positive staining for CK5 and p63.

Squamoid eccrine ductal carcinoma is an exceedingly rare subtype of eccrine carcinoma that mimics SCC both clinically and histologically.13 It most often occurs on the head and neck of elderly patients. This neoplasm can look similar to SCC and its variants, including PSCC. Histologically, squamoid eccrine ductal carcinoma exhibits a biphasic growth pattern.14 Well-differentiated squamous dysplasia transitions to carcinoma with eccrine duct formation as the tumor percolates deep into the dermis (Figure 4). As a result, superficial skin biopsies often lead to an incorrect diagnosis.15 Unlike SCC, the risk for locoregional and widespread metastasis is elevated. Identifying ducts in the deep aspect of the tumor is critical, thus immunohistochemical staining for carcinoembryonic antigen and epithelial membrane antigen is paramount for the diagnosis.15 Pseudoangiomatous SCC will stain negative for carcinoembryonic antigen, as was the case in our patient.

Pseudoepitheliomatous hyperplasia is a benign histologic reaction that can result from trauma, chronic inflammation (ie, pyoderma gangrenosum), tattoo placement, underlying neoplasia or fungal infection, or a spider bite reaction.14,15 It most commonly is seen as a well-demarcated nodule or plaque associated with scaling or crusting. Papules vary in size from less than 1 cm to several centimeters. Histologically, it is defined by an acanthotic proliferation of the adnexal epithelium and epidermis (Figure 5).16,17 Irregular strands, cords, and nests of squamoid cells can extend into the dermis.18 It can closely mimic SCC, but there are a few key differences. Pseudoepitheliomatous hyperplasia will not display atypical mitotic figures or atypical nuclei and will never invade lymphatics or vascular systems.19 Pseudoepitheliomatous hyperplasia shows identical histology to well-differentiated SCC, and thus clinicopathologic correlation and mindful histologic evaluation are crucial. The presence of an increased influx of neutrophils and histiocytes should prompt for microbial stains or deeper sectioning. A superficial biopsy should be followed by a deep biopsy. In our patient, microorganismal stains were negative.

- Kiyohara T, Miyamoto M, Shijimaya T, et al. Pseudovascular squamous cell carcinoma: a review of the published work and reassessment of prognosis. J Dermatol. 2018;45:1448-1451.
- Nagore E, Sánchez-Motilla JM, Pérez-Vallés A, et al. Pseudovascular squamous cell carcinoma of the skin. Clin Exp Dermatol. 2000;25:206-208.
- Han X, Lin X, Shao X. Pseudovascular adenoid squamous cell carcinoma of the tongue: a case report and literature review. Int J Clin Exp Pathol. 2020;13:1086-1089.
- Singh S, Bisht N, Purkayastha A, et al. Acantholytic squamous cell carcinoma of the scalp in an elderly patient treated with radical radiotherapy. J Cancer Res Pract. 2018;5:165-168.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post‐radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Shilpa K, Leelavathy B, Gorur D, et al. Early-onset epithelioid angiosarcoma: diagnostic enigma, a rare case report. Indian J Dermatopathol Diagn Dermatol. 2019;6:36-38.
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe [published online May 4, 2017]. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Hoffman CF, Papadopoulos D, Palmer DM, et al. A case report of bacillary angiomatosis in a patient infected with human immunodeficiency virus. Cutis. 2002;69:175-178.
- Biwer E, Uerlich M, Wimheuer R, et al. Bacillary angiomatosis: an important differential diagnosis in patients with HIV. Am J Dermatopathol. 1994;16:110.
- Medeiros LJ, Miranda RN. Bacillary angiomatosis. In: Medeiros LJ, Miranda RN, eds. Diagnostic Pathology: Lymph Nodes and Extranodal Lymphomas. 2nd ed. Elsevier; 2018:58-63.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Mckissack S, Wohltmann W, Dalton S, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Wollina U. Pyoderma gangrenosum—a review. Orphanet J Rare Dis. 2007;2:19
- Chow P, Goddard L, Greenway H, et al. Squamoid eccrine ductal carcinoma: the Scripps experience. Dermatol Surg. 2021;47:1115-1117.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126.
- Lynch JM. Understanding pseudoepitheliomatous hyperplasia. Pathol Case Rev. 2004;9:36-45.
- Goel R, Wallace ML. Pseudoepitheliomatous hyperplasia secondary to cutaneous aspergillus. Am J Dermatopathol. 2001;23:224-226.
- Kiyohara T, Miyamoto M, Shijimaya T, et al. Pseudovascular squamous cell carcinoma: a review of the published work and reassessment of prognosis. J Dermatol. 2018;45:1448-1451.
- Nagore E, Sánchez-Motilla JM, Pérez-Vallés A, et al. Pseudovascular squamous cell carcinoma of the skin. Clin Exp Dermatol. 2000;25:206-208.
- Han X, Lin X, Shao X. Pseudovascular adenoid squamous cell carcinoma of the tongue: a case report and literature review. Int J Clin Exp Pathol. 2020;13:1086-1089.
- Singh S, Bisht N, Purkayastha A, et al. Acantholytic squamous cell carcinoma of the scalp in an elderly patient treated with radical radiotherapy. J Cancer Res Pract. 2018;5:165-168.
- Cao J, Wang J, He C, et al. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res. 2019;9:2303-2313.
- Buehler D, Rice SR, Moody JS, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol. 2014;37:473-479.
- Ronen S, Ivan D, Torres-Cabala CA, et al. Post‐radiation vascular lesions of the breast. J Cutan Pathol. 2019;46:52-58.
- Shilpa K, Leelavathy B, Gorur D, et al. Early-onset epithelioid angiosarcoma: diagnostic enigma, a rare case report. Indian J Dermatopathol Diagn Dermatol. 2019;6:36-38.
- Gaballah AH, Jensen CT, Palmquist S, et al. Angiosarcoma: clinical and imaging features from head to toe [published online May 4, 2017]. Br J Radiol. 2017;90:20170039. doi:10.1259/bjr.20170039
- Hoffman CF, Papadopoulos D, Palmer DM, et al. A case report of bacillary angiomatosis in a patient infected with human immunodeficiency virus. Cutis. 2002;69:175-178.
- Biwer E, Uerlich M, Wimheuer R, et al. Bacillary angiomatosis: an important differential diagnosis in patients with HIV. Am J Dermatopathol. 1994;16:110.
- Medeiros LJ, Miranda RN. Bacillary angiomatosis. In: Medeiros LJ, Miranda RN, eds. Diagnostic Pathology: Lymph Nodes and Extranodal Lymphomas. 2nd ed. Elsevier; 2018:58-63.
- van der Horst MP, Garcia-Herrera A, Markiewicz D, et al. Squamoid eccrine ductal carcinoma: a clinicopathologic study of 30 cases. Am J Surg Pathol. 2016;40:755-760.
- Mckissack S, Wohltmann W, Dalton S, et al. Squamoid eccrine ductal carcinoma: an aggressive mimicker of squamous cell carcinoma. Am J Dermatopathol. 2019;41:140-143.
- Wollina U. Pyoderma gangrenosum—a review. Orphanet J Rare Dis. 2007;2:19
- Chow P, Goddard L, Greenway H, et al. Squamoid eccrine ductal carcinoma: the Scripps experience. Dermatol Surg. 2021;47:1115-1117.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126.
- Lynch JM. Understanding pseudoepitheliomatous hyperplasia. Pathol Case Rev. 2004;9:36-45.
- Goel R, Wallace ML. Pseudoepitheliomatous hyperplasia secondary to cutaneous aspergillus. Am J Dermatopathol. 2001;23:224-226.
An 84-year-old man with a history of nonmelanoma skin cancer presented to our clinic with a 1.6×1.5-cm exophytic lesion on the left posterior parietal scalp. The lesion nearly doubled in size over the last 4 months. The patient received radiation therapy in this area for the treatment of basal cell carcinoma 7 years prior to presentation. A shave biopsy was performed.
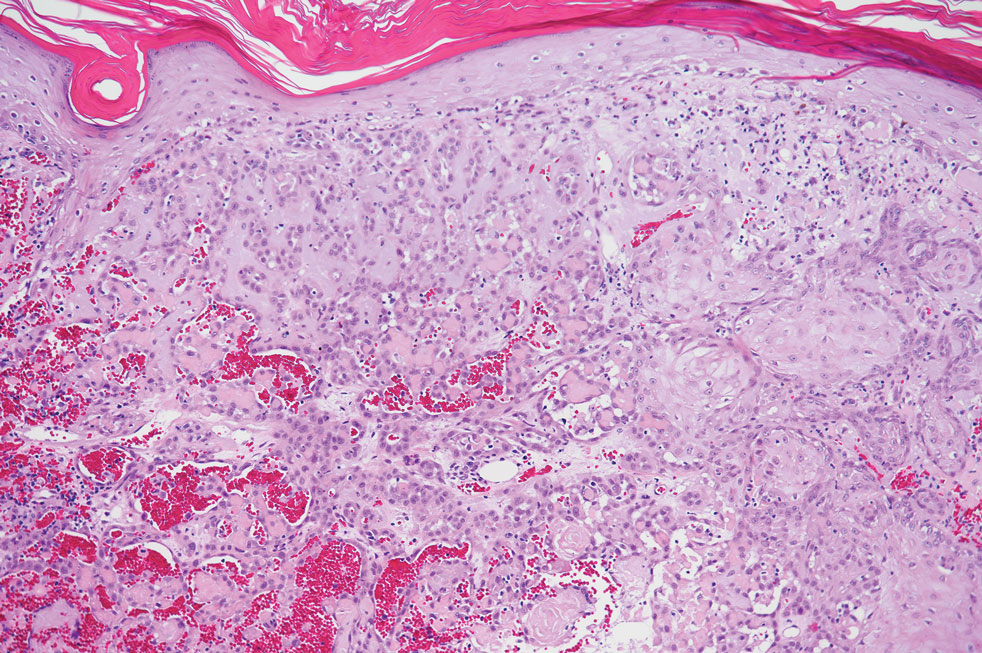
Rituximab Treatment and Improvement of Health-Related Quality of Life in Patients With Pemphigus
Pemphigus is a group of autoimmune blistering diseases characterized by the development of painful and flaccid blisters on the skin and/or mucous membranes. Pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are 2 major subtypes and can be distinguished by the location of blister formation or the specificity of autoantibodies directed against different desmogleins.1,2 Although rare, pemphigus is considered a serious and life-threatening condition with a great impact on quality of life (QOL) due to disease symptoms (eg, painful lesions, physical appearance of skin lesions) as well as treatment complications (eg, adverse drug effects, cost of treatment).3-6 Moreover, the physical and psychological effects can lead to marked functional morbidity and work-related disability during patients’ productive years.7 Therefore, affected individuals usually have a remarkably compromised health-related quality of life (HRQOL).8 Effective treatments may considerably improve the QOL of patients with pemphigus.6
Despite the available treatment options, finding the best regimen for pemphigus remains a challenge. Corticosteroids are assumed to be the main treatment, though they have considerable side effects.9,10 Adjuvant therapies are used to suppress or modulate immune responses, leading to remission with the least possible need for corticosteroids. Finding an optimal steroid-sparing agent has been the aim of research, and biologic agents seem to be the best option.8 Rituximab (RTX), an anti-CD20 monoclonal antibody, has shown great promise in several studies of its clinical efficacy and has become a first-line treatment in new guidelines.11-14 Rituximab treatment has been associated with notable improvement in physician-assessed outcome measures with a favorable safety profile in patients with pemphigus.11-15 However, it is important to assess response to treatment from a patient’s perspective through the use of outcome-assessment measures that encompass patient-reported outcomes to reflect the complete patient experience and establish the overall impact of RTX as well as its likelihood of acceptance by patients with pemphigus.
In our study, we compared clinical outcomes and HRQOL through the use of disease-specific measures as well as comprehensive generic health status measures among patients with PV and PF who received RTX treatment 3 months earlier and those who received RTX in the last 2 weeks. The clinical relevance of the patient-reported outcomes is discussed.
MATERIALS AND METHODS
Study Design
We conducted a single-center cross-sectional study of 96 patients with pemphigus aged 18 to 65 years of either sex who were willing to participate in this study. Patients with a confirmed diagnosis of PV or PF who received RTX 3 months earlier or in the last 2 weeks were enrolled in the study. Patients were identified using Dermatry.ir, an archiving software that contains patients’ medical data. Exclusion criteria included lack of sufficient knowledge of the concepts of the questionnaires as well as age younger than 16 years. The study was conducted from October 2019 to April 2020 by the Autoimmune Bullous Disease Research Center at Razi Hospital in Tehran, Iran, which is the main dermatology-specific center and teaching hospital of Iran. The study protocol was approved by the relevant ethics committee.
Patients were categorized into 2 groups: (1) those who received RTX 3 months earlier (3M group); and (2) those who received RTX in the last 2 weeks (R group).
After an explanation of the study to participants, informed written consent was signed by each patient, and their personal data (eg, age, sex, education, marital status, smoking status), as well as clinical data (eg, type of pemphigus, duration of disease, site of onset, prednisolone dosage, presence of Nikolsky sign, anti-DSG1 and anti-DSG3 values, Pemphigus Disease Area Index [PDAI] score, RTX treatment protocol); any known comorbidities such as hypertension, diabetes mellitus, or morbid obesity; and any chronic pulmonary, cardiac, endocrinologic, renal, or hepatic condition, were collected and recorded in a predefined Case Record.
Patient-Reported Outcome Measures
The effect of RTX on QOL in patients with pemphigus was assessed using 2 HRQOL instruments: (1) a general health status indicator, the 36-Item Short Form Survey (SF-36), and (2) a validated, Persian version of a dermatology-specific questionnaire, Dermatology Life Quality Index (DLQI). The questionnaires were completed by each patient or by an assistant if needed.
The SF-36 is a widely used 36-item questionnaire measuring functional health and well-being across 8 domains—mental health, pain, physical function, role emotional, role physical, social functioning, vitality, and general health perception—with scores for each ranging from 0 to 100. The physical component scores (PCSs) and mental component scores (MCSs) were derived from these 8 subscales, each ranging from 0 to 400, with higher scores indicating better health status.6
The DLQI, one of the most frequently used QOL measures in dermatology, contains 10 questions, each referring to the prior week and classified in the following 6 subscales: symptoms and feelings, daily activities, leisure, personal relationships, work and school, and treatment.16 The total score ranges from 0 (no impact) to 30 (very high impact), with a higher score indicating a lower QOL (eTable 1). The minimal clinically important difference (MCD) for the DLQI was considered to be 2- to 5-point changes in prior studies.17,18 In this study, we used an MCD of a 5-point change or more between study groups.
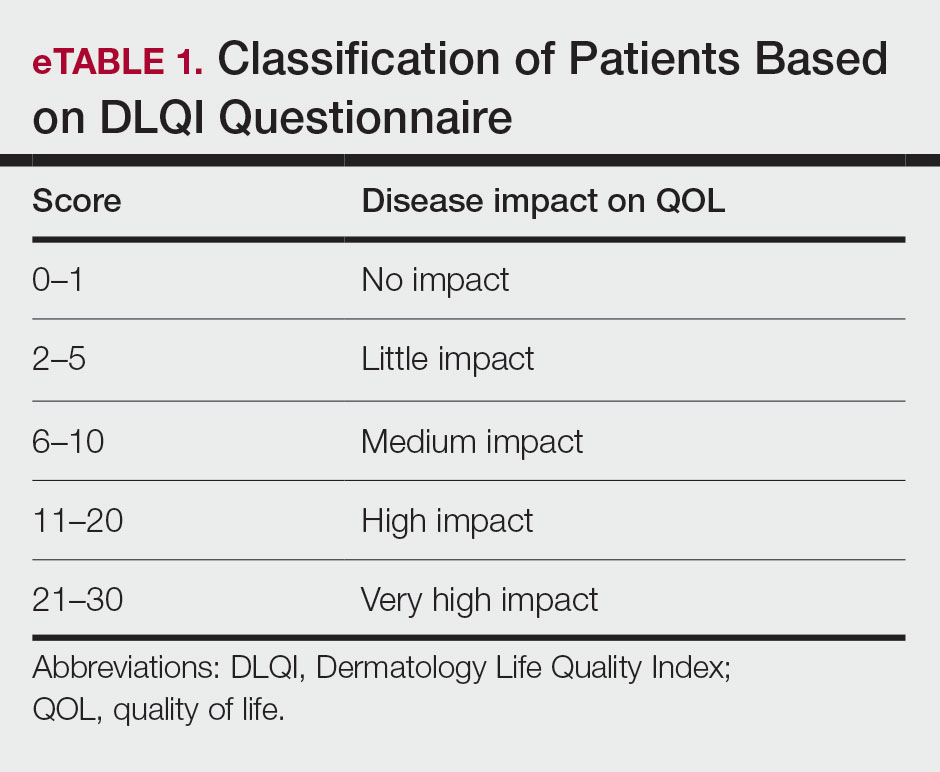
Moreover, the patient general assessment (PGA) of disease severity was identified using a 3-point scale (1=mild, 2=moderate, 3=severe).
Statistical Analysis
Data were analyzed using SPSS Statistics version 23. P≤.05 was considered significant. Mean and SD were calculated for descriptive data. The t test, Fisher exact test, analysis of variance, multiple regression analysis, and logistic regression analysis were used to identify the relationship between variables.
RESULTS
Patient Characteristics
A total of 96 patients were enrolled in this study. The mean (SD) age of participants was 41.42 (15.1) years (range, 18–58 years). Of 96 patients whose data were included, 55 (57.29%) patients had received RTX 3 months earlier (3M group) and 41 (42.71%) received RTX in the last 2 weeks (R group). A summary of study patient characteristics in each group is provided in eTable 2. There was no significant difference between the 2 groups in terms of age, sex, type of pemphigus, marital status, education, positive Nikolsky sign, smoking status, existence of comorbidities, site of lesions, and RTX treatment protocol. However, a significant difference was found for duration of disease (P=.0124) and mean prednisolone dosage (P=.001) as well as severity of disease measured by PDAI score (P=.003) and anti-DSG1 (P=.003) and anti-DSG3 (P=.021) values.
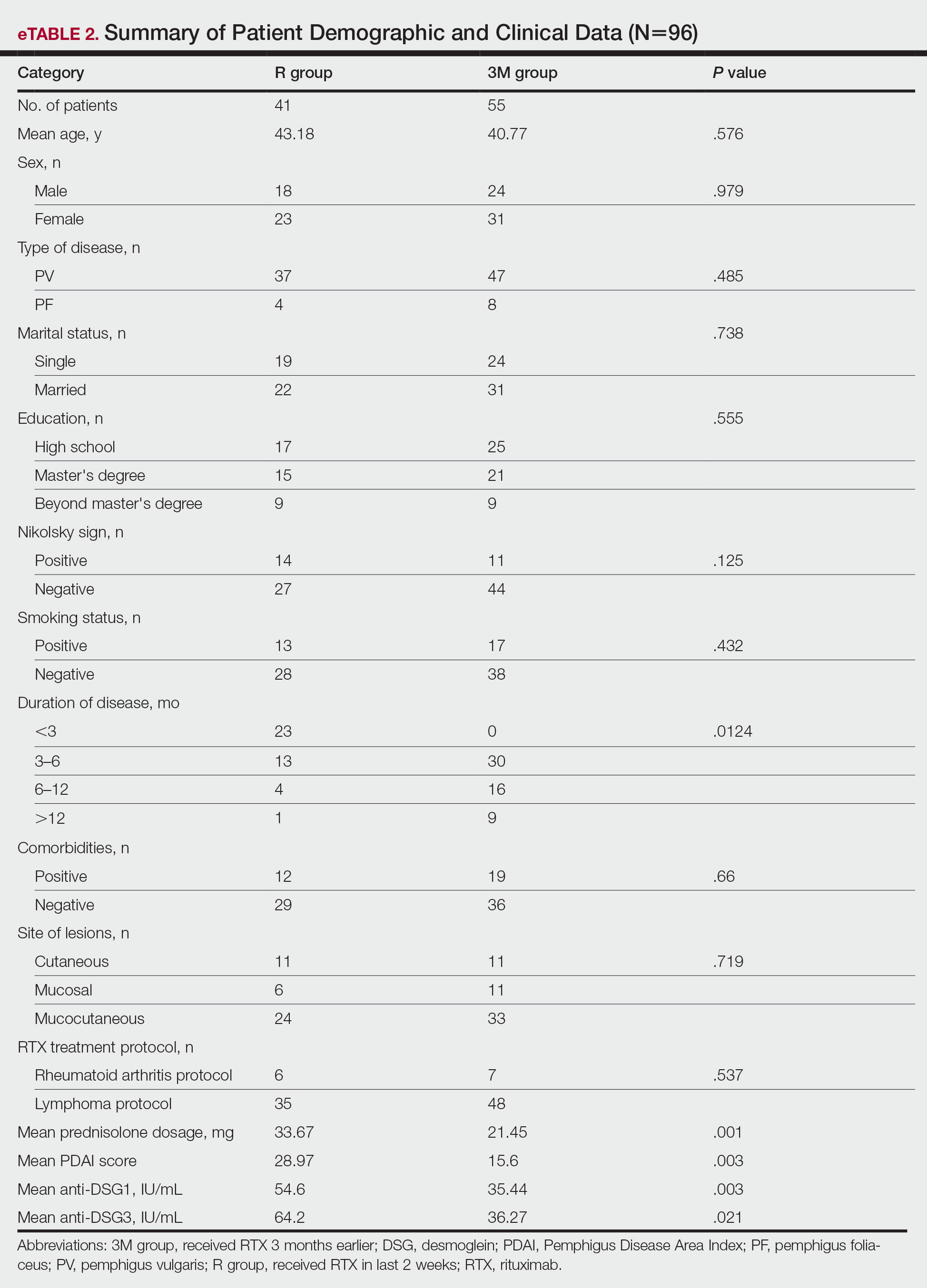
Patient-Reported Outcomes
Physical and mental component scores are summarized in eTable 3. Generally, SF-36 scores were improved with RTX treatment in all dimensions except for mental health, though these differences were not statistically significant, with the greatest mean improvement in the role physical index (75.45 in the 3M group vs 53.04 in the R group; P=.009). Mean SF-36 PCS and MCS scores were higher in the 3M group vs the R group, though the difference in MCS score did not reach the level of significance (eTable 3).
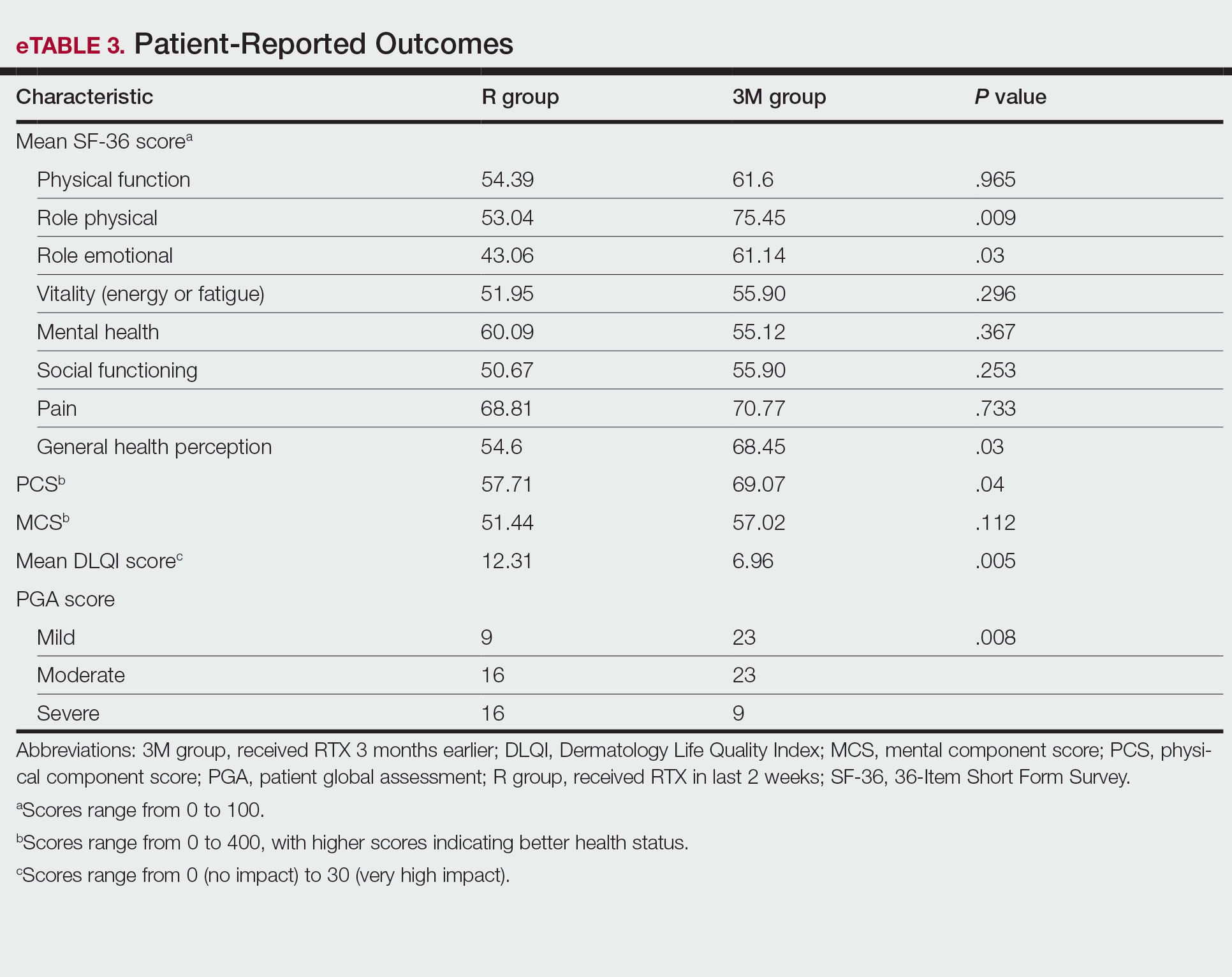
Mean DLQI scores in the R and 3M groups were 12.31 and 6.96, respectively, indicating a considerable burden on HRQOL in both groups. However, a statistically significant difference between these values was seen that also was clinically meaningful, indicating a significant improvement of QOL in patients receiving RTX 3 months earlier (P=.005)(eTable 3).
The PGA scores indicated that patients in the 3M group were significantly more likely to report less severe disease vs the R group (P=.008)(eTable 3).
Multivariate Analysis—Effect of the patient characteristics and some disease features on indices of QOL was evaluated using the multiple linear regression model. eTable 4 shows the P values of those analyses.
COMMENT
Pemphigus is a chronic disabling disease with notable QOL impairment due to disease burden as well as the need for long-term use of immunosuppressive agents during the disease course. To study the effect of RTX on QOL of patients with pemphigus, we compared 2 sets of patients. Prior studies have shown that clinically significant effects of RTX take 4 to 12 weeks to appear.19,20 Therefore, we selected patients who received RTX 3 months earlier to measure their HRQOL indices and compare them with patients who had received RTX in the last 2 weeks as a control group to investigate the effect of RTX intrinsically, as this was the focus of this study.
In our study, one of the research tools was the DLQI. Healthy patients typically have an average score of 0.5.21 The mean DLQI score of the patients in R group was 12.31, which was similar to prior analysis8 and reflects a substantial burden of disease comparable to atopic dermatitis and psoriasis.21,22 In patients in the 3M group, the mean DLQI score was lower than the R group (6.96 vs 12.31), indicating a significant (P=.005) and clinically meaningful improvement in QOL of patients due to the dramatic therapeutic effect of RTX. However, this score indicated a moderate effect on HRQOL, even in the context of clinical improvement due to RTX treatment, which may reflect that the short duration of treatment in the 3M group was a limitation of this study. Although the 12-week treatment duration was comparable with other studies19,20 and major differences in objective measures of treatment efficacy were found in PDAI as well as anti-DSG1 and anti-DSG3 values, longer treatment duration may be needed for a more comprehensive assessment of the benefit of RTX on HRQOL indices in patients with pemphigus.
Based on results of the SF-36 questionnaire, PCS and MCS scores were not substantially impaired in the R group considering the fact that a mean score of 50 has been articulated as a normative value for all scales.23 These data demonstrated the importance of using a dermatologic-specific instrument such as the DLQI instead of a general questionnaire to assess QOL in patients with pemphigus. However, better indices were reported with RTX treatment in the 3 SF-36 domains—role physical (P=.009), role emotional (P=.03), and general health perception (P=.03)—with the role physical showing the greatest magnitude of mean change (75.45 in the 3M group vs 53.04 in the R group). Notably, PCS was impaired to a greater extent than MCS in patients in the R group and showed a greater magnitude of improvement after 3 months of treatment. These results could be explained by the fact that MCS can be largely changed in diseases with a direct effect on the central nervous system.23
Our results also revealed that the dose of corticosteroid correlated to HRQOL of patients with pemphigus who recently received RTX therapy. Indeed, it is more likely that patients on lower-dose prednisolone have a higher QOL, especially on physical function and social function dimensions of SF-36. This finding is highly expectable by less severe disease due to RTX treatment and also lower potential dose-dependent adverse effects of long-term steroid therapy.
One of the most striking findings of this study was the correlation of location of lesions to QOL indices. We found that the mucocutaneous phenotype was significantly correlated to greater improvement in role emotional, role physical, and social functioning scores due to RTX treatment compared with cutaneous or mucosal types (P=.02, P=.025, and P=.017, respectively). Although mucosal involvement of the disease can be the most burdensome feature because of its large impact on essential activities such as eating and speaking, cutaneous lesions with unpleasant appearance and undesirable symptoms may have a similar impact on QOL. Therefore, having both mucosal and cutaneous lesions causes a worsened QOL and decreased treatment efficacy vs having only one area involved. This may explain the greater improvement in some QOL indices with RTX treatment.
Limitations—Given the cross-sectional design of this study in which patients were observed at a single time point during their treatment course, it is not possible to establish a clear cause-effect relationship between variables. Moreover, we did not evaluate the impact of RTX or prednisolone adverse effects on QOL. Therefore, further prospective studies with longer treatment durations may help to validate our findings. In addition, MCDs for DLQI and SF-36 in pemphigus need to be determined and validated in future studies.
CONCLUSION
The results of our study demonstrated that patients with pemphigus may benefit from taking RTX, not only in terms of clinical improvement of their disease measured by objective indices such as PDAI and anti-DSG1 and anti-DSG3 values but also in several domains that are important to patients, including physical and mental health status (SF-36), HRQOL (DLQI), and overall disease severity (PGA). Rituximab administration in patients with pemphigus can lead to rapid and significant improvement in HRQOL as well as patient- and physician-assessed measures. Its favorable safety profile along with its impact on patients’ daily lives and mental health makes RTX a suitable treatment option for patients with pemphigus. Moreover, we recommend taking QOL indices into account while evaluating the efficacy of new medications to improve our insight into the patient experience and provide better patient adherence to treatment, which is an important issue for optimal control of chronic disorders.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:175-197.
- Kasperkiewicz M, Ellebrecht CT, Takahashi H, et al. Pemphigus. Nat Rev Dis Primers. 2017;3:17026.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris, result from the German Bullous Skin Disease (BSD) Study Group. J Dtsch Dermatol Ges. 2005;3:431-435.
- Terrab Z, Benckikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034.
- Paradisi A, Sampogna F, Di Pietro, C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269.
- Heelan K, Hitzig SL, Knowles S, et al. Loss of work productivity and quality of life in patients with autoimmune bullous dermatoses. J Cutan Med Surg. 2015;19:546-554.
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and General Health Questionnaires. J Dermatol. 2012;39:141-144.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:2343.
- Mohammad-Javad N, Parvaneh H, Maryam G, et al. Randomized trial of tacrolimus 0.1% ointment versus triamcinolone acetonide 0.1% paste in the treatment of oral pemphigus vulgaris. Iranian J Dermatol. 2012;15:42-46.
- Lunardon L, Tsai KJ, Propert KJ, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148:1031-1036.
- Colliou N, Picard D, Caillot F, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30.
- Heelan K, Al-Mohammedi F, Smith MJ, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-708.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040
- Aryanian Z, Balighi K, Daneshpazhooh M, et al. Rituximab exhibits a better safety profile when used as a first line of treatment for pemphigus vulgaris: a retrospective study. Int Immunopharmacol. 2021;96:107755.
- Aghai S, Sodaifi M, Jafari P, et al. DLQI scores in vitiligo: reliability and validity of the Persian version. BMC Dermatol. 2004;4:8.
- Schünemann HJ, Akl EA, Guyatt GH. Interpreting the results of patient reported outcome measures in clinical trials: the clinician’s perspective. Health Qual Life Outcomes. 2006;4:62.
- Quality of life questionnaires. Cardiff University website. Accessed December 16, 2022. http://sites.cardiff.ac.uk/dermatology/quality-oflife/dermatology-quality-of-life-index-dlqi/dlqi-instructions-foruse-and-scoring/
- Kanwar AJ, Tsuruta D, Vinay K, et al. Efficacy and safety of rituximab treatment in Indian pemphigus patients. J Eur Acad Dermatol Venereol. 2013;27:E17-E23.
- Ingen-Housz-Oro S, Valeyrie-Allanore L, Cosnes A, et al. First-line treatment of pemphigus vulgaris with a combination of rituximab and high-potency topical corticosteroids. JAMA Dermatol. 2015;151:200-203.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Aghaei S, Moradi A, Ardekani GS. Impact of psoriasis on quality of life in Iran. Indian J Dermatol Venereol Leprol. 2009;75:220.
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). 1. conceptual framework and item selection. Med Care. 1992;30:473-483.
Pemphigus is a group of autoimmune blistering diseases characterized by the development of painful and flaccid blisters on the skin and/or mucous membranes. Pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are 2 major subtypes and can be distinguished by the location of blister formation or the specificity of autoantibodies directed against different desmogleins.1,2 Although rare, pemphigus is considered a serious and life-threatening condition with a great impact on quality of life (QOL) due to disease symptoms (eg, painful lesions, physical appearance of skin lesions) as well as treatment complications (eg, adverse drug effects, cost of treatment).3-6 Moreover, the physical and psychological effects can lead to marked functional morbidity and work-related disability during patients’ productive years.7 Therefore, affected individuals usually have a remarkably compromised health-related quality of life (HRQOL).8 Effective treatments may considerably improve the QOL of patients with pemphigus.6
Despite the available treatment options, finding the best regimen for pemphigus remains a challenge. Corticosteroids are assumed to be the main treatment, though they have considerable side effects.9,10 Adjuvant therapies are used to suppress or modulate immune responses, leading to remission with the least possible need for corticosteroids. Finding an optimal steroid-sparing agent has been the aim of research, and biologic agents seem to be the best option.8 Rituximab (RTX), an anti-CD20 monoclonal antibody, has shown great promise in several studies of its clinical efficacy and has become a first-line treatment in new guidelines.11-14 Rituximab treatment has been associated with notable improvement in physician-assessed outcome measures with a favorable safety profile in patients with pemphigus.11-15 However, it is important to assess response to treatment from a patient’s perspective through the use of outcome-assessment measures that encompass patient-reported outcomes to reflect the complete patient experience and establish the overall impact of RTX as well as its likelihood of acceptance by patients with pemphigus.
In our study, we compared clinical outcomes and HRQOL through the use of disease-specific measures as well as comprehensive generic health status measures among patients with PV and PF who received RTX treatment 3 months earlier and those who received RTX in the last 2 weeks. The clinical relevance of the patient-reported outcomes is discussed.
MATERIALS AND METHODS
Study Design
We conducted a single-center cross-sectional study of 96 patients with pemphigus aged 18 to 65 years of either sex who were willing to participate in this study. Patients with a confirmed diagnosis of PV or PF who received RTX 3 months earlier or in the last 2 weeks were enrolled in the study. Patients were identified using Dermatry.ir, an archiving software that contains patients’ medical data. Exclusion criteria included lack of sufficient knowledge of the concepts of the questionnaires as well as age younger than 16 years. The study was conducted from October 2019 to April 2020 by the Autoimmune Bullous Disease Research Center at Razi Hospital in Tehran, Iran, which is the main dermatology-specific center and teaching hospital of Iran. The study protocol was approved by the relevant ethics committee.
Patients were categorized into 2 groups: (1) those who received RTX 3 months earlier (3M group); and (2) those who received RTX in the last 2 weeks (R group).
After an explanation of the study to participants, informed written consent was signed by each patient, and their personal data (eg, age, sex, education, marital status, smoking status), as well as clinical data (eg, type of pemphigus, duration of disease, site of onset, prednisolone dosage, presence of Nikolsky sign, anti-DSG1 and anti-DSG3 values, Pemphigus Disease Area Index [PDAI] score, RTX treatment protocol); any known comorbidities such as hypertension, diabetes mellitus, or morbid obesity; and any chronic pulmonary, cardiac, endocrinologic, renal, or hepatic condition, were collected and recorded in a predefined Case Record.
Patient-Reported Outcome Measures
The effect of RTX on QOL in patients with pemphigus was assessed using 2 HRQOL instruments: (1) a general health status indicator, the 36-Item Short Form Survey (SF-36), and (2) a validated, Persian version of a dermatology-specific questionnaire, Dermatology Life Quality Index (DLQI). The questionnaires were completed by each patient or by an assistant if needed.
The SF-36 is a widely used 36-item questionnaire measuring functional health and well-being across 8 domains—mental health, pain, physical function, role emotional, role physical, social functioning, vitality, and general health perception—with scores for each ranging from 0 to 100. The physical component scores (PCSs) and mental component scores (MCSs) were derived from these 8 subscales, each ranging from 0 to 400, with higher scores indicating better health status.6
The DLQI, one of the most frequently used QOL measures in dermatology, contains 10 questions, each referring to the prior week and classified in the following 6 subscales: symptoms and feelings, daily activities, leisure, personal relationships, work and school, and treatment.16 The total score ranges from 0 (no impact) to 30 (very high impact), with a higher score indicating a lower QOL (eTable 1). The minimal clinically important difference (MCD) for the DLQI was considered to be 2- to 5-point changes in prior studies.17,18 In this study, we used an MCD of a 5-point change or more between study groups.

Moreover, the patient general assessment (PGA) of disease severity was identified using a 3-point scale (1=mild, 2=moderate, 3=severe).
Statistical Analysis
Data were analyzed using SPSS Statistics version 23. P≤.05 was considered significant. Mean and SD were calculated for descriptive data. The t test, Fisher exact test, analysis of variance, multiple regression analysis, and logistic regression analysis were used to identify the relationship between variables.
RESULTS
Patient Characteristics
A total of 96 patients were enrolled in this study. The mean (SD) age of participants was 41.42 (15.1) years (range, 18–58 years). Of 96 patients whose data were included, 55 (57.29%) patients had received RTX 3 months earlier (3M group) and 41 (42.71%) received RTX in the last 2 weeks (R group). A summary of study patient characteristics in each group is provided in eTable 2. There was no significant difference between the 2 groups in terms of age, sex, type of pemphigus, marital status, education, positive Nikolsky sign, smoking status, existence of comorbidities, site of lesions, and RTX treatment protocol. However, a significant difference was found for duration of disease (P=.0124) and mean prednisolone dosage (P=.001) as well as severity of disease measured by PDAI score (P=.003) and anti-DSG1 (P=.003) and anti-DSG3 (P=.021) values.

Patient-Reported Outcomes
Physical and mental component scores are summarized in eTable 3. Generally, SF-36 scores were improved with RTX treatment in all dimensions except for mental health, though these differences were not statistically significant, with the greatest mean improvement in the role physical index (75.45 in the 3M group vs 53.04 in the R group; P=.009). Mean SF-36 PCS and MCS scores were higher in the 3M group vs the R group, though the difference in MCS score did not reach the level of significance (eTable 3).

Mean DLQI scores in the R and 3M groups were 12.31 and 6.96, respectively, indicating a considerable burden on HRQOL in both groups. However, a statistically significant difference between these values was seen that also was clinically meaningful, indicating a significant improvement of QOL in patients receiving RTX 3 months earlier (P=.005)(eTable 3).
The PGA scores indicated that patients in the 3M group were significantly more likely to report less severe disease vs the R group (P=.008)(eTable 3).
Multivariate Analysis—Effect of the patient characteristics and some disease features on indices of QOL was evaluated using the multiple linear regression model. eTable 4 shows the P values of those analyses.

COMMENT
Pemphigus is a chronic disabling disease with notable QOL impairment due to disease burden as well as the need for long-term use of immunosuppressive agents during the disease course. To study the effect of RTX on QOL of patients with pemphigus, we compared 2 sets of patients. Prior studies have shown that clinically significant effects of RTX take 4 to 12 weeks to appear.19,20 Therefore, we selected patients who received RTX 3 months earlier to measure their HRQOL indices and compare them with patients who had received RTX in the last 2 weeks as a control group to investigate the effect of RTX intrinsically, as this was the focus of this study.
In our study, one of the research tools was the DLQI. Healthy patients typically have an average score of 0.5.21 The mean DLQI score of the patients in R group was 12.31, which was similar to prior analysis8 and reflects a substantial burden of disease comparable to atopic dermatitis and psoriasis.21,22 In patients in the 3M group, the mean DLQI score was lower than the R group (6.96 vs 12.31), indicating a significant (P=.005) and clinically meaningful improvement in QOL of patients due to the dramatic therapeutic effect of RTX. However, this score indicated a moderate effect on HRQOL, even in the context of clinical improvement due to RTX treatment, which may reflect that the short duration of treatment in the 3M group was a limitation of this study. Although the 12-week treatment duration was comparable with other studies19,20 and major differences in objective measures of treatment efficacy were found in PDAI as well as anti-DSG1 and anti-DSG3 values, longer treatment duration may be needed for a more comprehensive assessment of the benefit of RTX on HRQOL indices in patients with pemphigus.
Based on results of the SF-36 questionnaire, PCS and MCS scores were not substantially impaired in the R group considering the fact that a mean score of 50 has been articulated as a normative value for all scales.23 These data demonstrated the importance of using a dermatologic-specific instrument such as the DLQI instead of a general questionnaire to assess QOL in patients with pemphigus. However, better indices were reported with RTX treatment in the 3 SF-36 domains—role physical (P=.009), role emotional (P=.03), and general health perception (P=.03)—with the role physical showing the greatest magnitude of mean change (75.45 in the 3M group vs 53.04 in the R group). Notably, PCS was impaired to a greater extent than MCS in patients in the R group and showed a greater magnitude of improvement after 3 months of treatment. These results could be explained by the fact that MCS can be largely changed in diseases with a direct effect on the central nervous system.23
Our results also revealed that the dose of corticosteroid correlated to HRQOL of patients with pemphigus who recently received RTX therapy. Indeed, it is more likely that patients on lower-dose prednisolone have a higher QOL, especially on physical function and social function dimensions of SF-36. This finding is highly expectable by less severe disease due to RTX treatment and also lower potential dose-dependent adverse effects of long-term steroid therapy.
One of the most striking findings of this study was the correlation of location of lesions to QOL indices. We found that the mucocutaneous phenotype was significantly correlated to greater improvement in role emotional, role physical, and social functioning scores due to RTX treatment compared with cutaneous or mucosal types (P=.02, P=.025, and P=.017, respectively). Although mucosal involvement of the disease can be the most burdensome feature because of its large impact on essential activities such as eating and speaking, cutaneous lesions with unpleasant appearance and undesirable symptoms may have a similar impact on QOL. Therefore, having both mucosal and cutaneous lesions causes a worsened QOL and decreased treatment efficacy vs having only one area involved. This may explain the greater improvement in some QOL indices with RTX treatment.
Limitations—Given the cross-sectional design of this study in which patients were observed at a single time point during their treatment course, it is not possible to establish a clear cause-effect relationship between variables. Moreover, we did not evaluate the impact of RTX or prednisolone adverse effects on QOL. Therefore, further prospective studies with longer treatment durations may help to validate our findings. In addition, MCDs for DLQI and SF-36 in pemphigus need to be determined and validated in future studies.
CONCLUSION
The results of our study demonstrated that patients with pemphigus may benefit from taking RTX, not only in terms of clinical improvement of their disease measured by objective indices such as PDAI and anti-DSG1 and anti-DSG3 values but also in several domains that are important to patients, including physical and mental health status (SF-36), HRQOL (DLQI), and overall disease severity (PGA). Rituximab administration in patients with pemphigus can lead to rapid and significant improvement in HRQOL as well as patient- and physician-assessed measures. Its favorable safety profile along with its impact on patients’ daily lives and mental health makes RTX a suitable treatment option for patients with pemphigus. Moreover, we recommend taking QOL indices into account while evaluating the efficacy of new medications to improve our insight into the patient experience and provide better patient adherence to treatment, which is an important issue for optimal control of chronic disorders.
Pemphigus is a group of autoimmune blistering diseases characterized by the development of painful and flaccid blisters on the skin and/or mucous membranes. Pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are 2 major subtypes and can be distinguished by the location of blister formation or the specificity of autoantibodies directed against different desmogleins.1,2 Although rare, pemphigus is considered a serious and life-threatening condition with a great impact on quality of life (QOL) due to disease symptoms (eg, painful lesions, physical appearance of skin lesions) as well as treatment complications (eg, adverse drug effects, cost of treatment).3-6 Moreover, the physical and psychological effects can lead to marked functional morbidity and work-related disability during patients’ productive years.7 Therefore, affected individuals usually have a remarkably compromised health-related quality of life (HRQOL).8 Effective treatments may considerably improve the QOL of patients with pemphigus.6
Despite the available treatment options, finding the best regimen for pemphigus remains a challenge. Corticosteroids are assumed to be the main treatment, though they have considerable side effects.9,10 Adjuvant therapies are used to suppress or modulate immune responses, leading to remission with the least possible need for corticosteroids. Finding an optimal steroid-sparing agent has been the aim of research, and biologic agents seem to be the best option.8 Rituximab (RTX), an anti-CD20 monoclonal antibody, has shown great promise in several studies of its clinical efficacy and has become a first-line treatment in new guidelines.11-14 Rituximab treatment has been associated with notable improvement in physician-assessed outcome measures with a favorable safety profile in patients with pemphigus.11-15 However, it is important to assess response to treatment from a patient’s perspective through the use of outcome-assessment measures that encompass patient-reported outcomes to reflect the complete patient experience and establish the overall impact of RTX as well as its likelihood of acceptance by patients with pemphigus.
In our study, we compared clinical outcomes and HRQOL through the use of disease-specific measures as well as comprehensive generic health status measures among patients with PV and PF who received RTX treatment 3 months earlier and those who received RTX in the last 2 weeks. The clinical relevance of the patient-reported outcomes is discussed.
MATERIALS AND METHODS
Study Design
We conducted a single-center cross-sectional study of 96 patients with pemphigus aged 18 to 65 years of either sex who were willing to participate in this study. Patients with a confirmed diagnosis of PV or PF who received RTX 3 months earlier or in the last 2 weeks were enrolled in the study. Patients were identified using Dermatry.ir, an archiving software that contains patients’ medical data. Exclusion criteria included lack of sufficient knowledge of the concepts of the questionnaires as well as age younger than 16 years. The study was conducted from October 2019 to April 2020 by the Autoimmune Bullous Disease Research Center at Razi Hospital in Tehran, Iran, which is the main dermatology-specific center and teaching hospital of Iran. The study protocol was approved by the relevant ethics committee.
Patients were categorized into 2 groups: (1) those who received RTX 3 months earlier (3M group); and (2) those who received RTX in the last 2 weeks (R group).
After an explanation of the study to participants, informed written consent was signed by each patient, and their personal data (eg, age, sex, education, marital status, smoking status), as well as clinical data (eg, type of pemphigus, duration of disease, site of onset, prednisolone dosage, presence of Nikolsky sign, anti-DSG1 and anti-DSG3 values, Pemphigus Disease Area Index [PDAI] score, RTX treatment protocol); any known comorbidities such as hypertension, diabetes mellitus, or morbid obesity; and any chronic pulmonary, cardiac, endocrinologic, renal, or hepatic condition, were collected and recorded in a predefined Case Record.
Patient-Reported Outcome Measures
The effect of RTX on QOL in patients with pemphigus was assessed using 2 HRQOL instruments: (1) a general health status indicator, the 36-Item Short Form Survey (SF-36), and (2) a validated, Persian version of a dermatology-specific questionnaire, Dermatology Life Quality Index (DLQI). The questionnaires were completed by each patient or by an assistant if needed.
The SF-36 is a widely used 36-item questionnaire measuring functional health and well-being across 8 domains—mental health, pain, physical function, role emotional, role physical, social functioning, vitality, and general health perception—with scores for each ranging from 0 to 100. The physical component scores (PCSs) and mental component scores (MCSs) were derived from these 8 subscales, each ranging from 0 to 400, with higher scores indicating better health status.6
The DLQI, one of the most frequently used QOL measures in dermatology, contains 10 questions, each referring to the prior week and classified in the following 6 subscales: symptoms and feelings, daily activities, leisure, personal relationships, work and school, and treatment.16 The total score ranges from 0 (no impact) to 30 (very high impact), with a higher score indicating a lower QOL (eTable 1). The minimal clinically important difference (MCD) for the DLQI was considered to be 2- to 5-point changes in prior studies.17,18 In this study, we used an MCD of a 5-point change or more between study groups.

Moreover, the patient general assessment (PGA) of disease severity was identified using a 3-point scale (1=mild, 2=moderate, 3=severe).
Statistical Analysis
Data were analyzed using SPSS Statistics version 23. P≤.05 was considered significant. Mean and SD were calculated for descriptive data. The t test, Fisher exact test, analysis of variance, multiple regression analysis, and logistic regression analysis were used to identify the relationship between variables.
RESULTS
Patient Characteristics
A total of 96 patients were enrolled in this study. The mean (SD) age of participants was 41.42 (15.1) years (range, 18–58 years). Of 96 patients whose data were included, 55 (57.29%) patients had received RTX 3 months earlier (3M group) and 41 (42.71%) received RTX in the last 2 weeks (R group). A summary of study patient characteristics in each group is provided in eTable 2. There was no significant difference between the 2 groups in terms of age, sex, type of pemphigus, marital status, education, positive Nikolsky sign, smoking status, existence of comorbidities, site of lesions, and RTX treatment protocol. However, a significant difference was found for duration of disease (P=.0124) and mean prednisolone dosage (P=.001) as well as severity of disease measured by PDAI score (P=.003) and anti-DSG1 (P=.003) and anti-DSG3 (P=.021) values.

Patient-Reported Outcomes
Physical and mental component scores are summarized in eTable 3. Generally, SF-36 scores were improved with RTX treatment in all dimensions except for mental health, though these differences were not statistically significant, with the greatest mean improvement in the role physical index (75.45 in the 3M group vs 53.04 in the R group; P=.009). Mean SF-36 PCS and MCS scores were higher in the 3M group vs the R group, though the difference in MCS score did not reach the level of significance (eTable 3).

Mean DLQI scores in the R and 3M groups were 12.31 and 6.96, respectively, indicating a considerable burden on HRQOL in both groups. However, a statistically significant difference between these values was seen that also was clinically meaningful, indicating a significant improvement of QOL in patients receiving RTX 3 months earlier (P=.005)(eTable 3).
The PGA scores indicated that patients in the 3M group were significantly more likely to report less severe disease vs the R group (P=.008)(eTable 3).
Multivariate Analysis—Effect of the patient characteristics and some disease features on indices of QOL was evaluated using the multiple linear regression model. eTable 4 shows the P values of those analyses.

COMMENT
Pemphigus is a chronic disabling disease with notable QOL impairment due to disease burden as well as the need for long-term use of immunosuppressive agents during the disease course. To study the effect of RTX on QOL of patients with pemphigus, we compared 2 sets of patients. Prior studies have shown that clinically significant effects of RTX take 4 to 12 weeks to appear.19,20 Therefore, we selected patients who received RTX 3 months earlier to measure their HRQOL indices and compare them with patients who had received RTX in the last 2 weeks as a control group to investigate the effect of RTX intrinsically, as this was the focus of this study.
In our study, one of the research tools was the DLQI. Healthy patients typically have an average score of 0.5.21 The mean DLQI score of the patients in R group was 12.31, which was similar to prior analysis8 and reflects a substantial burden of disease comparable to atopic dermatitis and psoriasis.21,22 In patients in the 3M group, the mean DLQI score was lower than the R group (6.96 vs 12.31), indicating a significant (P=.005) and clinically meaningful improvement in QOL of patients due to the dramatic therapeutic effect of RTX. However, this score indicated a moderate effect on HRQOL, even in the context of clinical improvement due to RTX treatment, which may reflect that the short duration of treatment in the 3M group was a limitation of this study. Although the 12-week treatment duration was comparable with other studies19,20 and major differences in objective measures of treatment efficacy were found in PDAI as well as anti-DSG1 and anti-DSG3 values, longer treatment duration may be needed for a more comprehensive assessment of the benefit of RTX on HRQOL indices in patients with pemphigus.
Based on results of the SF-36 questionnaire, PCS and MCS scores were not substantially impaired in the R group considering the fact that a mean score of 50 has been articulated as a normative value for all scales.23 These data demonstrated the importance of using a dermatologic-specific instrument such as the DLQI instead of a general questionnaire to assess QOL in patients with pemphigus. However, better indices were reported with RTX treatment in the 3 SF-36 domains—role physical (P=.009), role emotional (P=.03), and general health perception (P=.03)—with the role physical showing the greatest magnitude of mean change (75.45 in the 3M group vs 53.04 in the R group). Notably, PCS was impaired to a greater extent than MCS in patients in the R group and showed a greater magnitude of improvement after 3 months of treatment. These results could be explained by the fact that MCS can be largely changed in diseases with a direct effect on the central nervous system.23
Our results also revealed that the dose of corticosteroid correlated to HRQOL of patients with pemphigus who recently received RTX therapy. Indeed, it is more likely that patients on lower-dose prednisolone have a higher QOL, especially on physical function and social function dimensions of SF-36. This finding is highly expectable by less severe disease due to RTX treatment and also lower potential dose-dependent adverse effects of long-term steroid therapy.
One of the most striking findings of this study was the correlation of location of lesions to QOL indices. We found that the mucocutaneous phenotype was significantly correlated to greater improvement in role emotional, role physical, and social functioning scores due to RTX treatment compared with cutaneous or mucosal types (P=.02, P=.025, and P=.017, respectively). Although mucosal involvement of the disease can be the most burdensome feature because of its large impact on essential activities such as eating and speaking, cutaneous lesions with unpleasant appearance and undesirable symptoms may have a similar impact on QOL. Therefore, having both mucosal and cutaneous lesions causes a worsened QOL and decreased treatment efficacy vs having only one area involved. This may explain the greater improvement in some QOL indices with RTX treatment.
Limitations—Given the cross-sectional design of this study in which patients were observed at a single time point during their treatment course, it is not possible to establish a clear cause-effect relationship between variables. Moreover, we did not evaluate the impact of RTX or prednisolone adverse effects on QOL. Therefore, further prospective studies with longer treatment durations may help to validate our findings. In addition, MCDs for DLQI and SF-36 in pemphigus need to be determined and validated in future studies.
CONCLUSION
The results of our study demonstrated that patients with pemphigus may benefit from taking RTX, not only in terms of clinical improvement of their disease measured by objective indices such as PDAI and anti-DSG1 and anti-DSG3 values but also in several domains that are important to patients, including physical and mental health status (SF-36), HRQOL (DLQI), and overall disease severity (PGA). Rituximab administration in patients with pemphigus can lead to rapid and significant improvement in HRQOL as well as patient- and physician-assessed measures. Its favorable safety profile along with its impact on patients’ daily lives and mental health makes RTX a suitable treatment option for patients with pemphigus. Moreover, we recommend taking QOL indices into account while evaluating the efficacy of new medications to improve our insight into the patient experience and provide better patient adherence to treatment, which is an important issue for optimal control of chronic disorders.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:175-197.
- Kasperkiewicz M, Ellebrecht CT, Takahashi H, et al. Pemphigus. Nat Rev Dis Primers. 2017;3:17026.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris, result from the German Bullous Skin Disease (BSD) Study Group. J Dtsch Dermatol Ges. 2005;3:431-435.
- Terrab Z, Benckikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034.
- Paradisi A, Sampogna F, Di Pietro, C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269.
- Heelan K, Hitzig SL, Knowles S, et al. Loss of work productivity and quality of life in patients with autoimmune bullous dermatoses. J Cutan Med Surg. 2015;19:546-554.
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and General Health Questionnaires. J Dermatol. 2012;39:141-144.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:2343.
- Mohammad-Javad N, Parvaneh H, Maryam G, et al. Randomized trial of tacrolimus 0.1% ointment versus triamcinolone acetonide 0.1% paste in the treatment of oral pemphigus vulgaris. Iranian J Dermatol. 2012;15:42-46.
- Lunardon L, Tsai KJ, Propert KJ, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148:1031-1036.
- Colliou N, Picard D, Caillot F, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30.
- Heelan K, Al-Mohammedi F, Smith MJ, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-708.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040
- Aryanian Z, Balighi K, Daneshpazhooh M, et al. Rituximab exhibits a better safety profile when used as a first line of treatment for pemphigus vulgaris: a retrospective study. Int Immunopharmacol. 2021;96:107755.
- Aghai S, Sodaifi M, Jafari P, et al. DLQI scores in vitiligo: reliability and validity of the Persian version. BMC Dermatol. 2004;4:8.
- Schünemann HJ, Akl EA, Guyatt GH. Interpreting the results of patient reported outcome measures in clinical trials: the clinician’s perspective. Health Qual Life Outcomes. 2006;4:62.
- Quality of life questionnaires. Cardiff University website. Accessed December 16, 2022. http://sites.cardiff.ac.uk/dermatology/quality-oflife/dermatology-quality-of-life-index-dlqi/dlqi-instructions-foruse-and-scoring/
- Kanwar AJ, Tsuruta D, Vinay K, et al. Efficacy and safety of rituximab treatment in Indian pemphigus patients. J Eur Acad Dermatol Venereol. 2013;27:E17-E23.
- Ingen-Housz-Oro S, Valeyrie-Allanore L, Cosnes A, et al. First-line treatment of pemphigus vulgaris with a combination of rituximab and high-potency topical corticosteroids. JAMA Dermatol. 2015;151:200-203.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Aghaei S, Moradi A, Ardekani GS. Impact of psoriasis on quality of life in Iran. Indian J Dermatol Venereol Leprol. 2009;75:220.
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). 1. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:175-197.
- Kasperkiewicz M, Ellebrecht CT, Takahashi H, et al. Pemphigus. Nat Rev Dis Primers. 2017;3:17026.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris, result from the German Bullous Skin Disease (BSD) Study Group. J Dtsch Dermatol Ges. 2005;3:431-435.
- Terrab Z, Benckikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034.
- Paradisi A, Sampogna F, Di Pietro, C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269.
- Heelan K, Hitzig SL, Knowles S, et al. Loss of work productivity and quality of life in patients with autoimmune bullous dermatoses. J Cutan Med Surg. 2015;19:546-554.
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and General Health Questionnaires. J Dermatol. 2012;39:141-144.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:2343.
- Mohammad-Javad N, Parvaneh H, Maryam G, et al. Randomized trial of tacrolimus 0.1% ointment versus triamcinolone acetonide 0.1% paste in the treatment of oral pemphigus vulgaris. Iranian J Dermatol. 2012;15:42-46.
- Lunardon L, Tsai KJ, Propert KJ, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148:1031-1036.
- Colliou N, Picard D, Caillot F, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30.
- Heelan K, Al-Mohammedi F, Smith MJ, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-708.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040
- Aryanian Z, Balighi K, Daneshpazhooh M, et al. Rituximab exhibits a better safety profile when used as a first line of treatment for pemphigus vulgaris: a retrospective study. Int Immunopharmacol. 2021;96:107755.
- Aghai S, Sodaifi M, Jafari P, et al. DLQI scores in vitiligo: reliability and validity of the Persian version. BMC Dermatol. 2004;4:8.
- Schünemann HJ, Akl EA, Guyatt GH. Interpreting the results of patient reported outcome measures in clinical trials: the clinician’s perspective. Health Qual Life Outcomes. 2006;4:62.
- Quality of life questionnaires. Cardiff University website. Accessed December 16, 2022. http://sites.cardiff.ac.uk/dermatology/quality-oflife/dermatology-quality-of-life-index-dlqi/dlqi-instructions-foruse-and-scoring/
- Kanwar AJ, Tsuruta D, Vinay K, et al. Efficacy and safety of rituximab treatment in Indian pemphigus patients. J Eur Acad Dermatol Venereol. 2013;27:E17-E23.
- Ingen-Housz-Oro S, Valeyrie-Allanore L, Cosnes A, et al. First-line treatment of pemphigus vulgaris with a combination of rituximab and high-potency topical corticosteroids. JAMA Dermatol. 2015;151:200-203.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Aghaei S, Moradi A, Ardekani GS. Impact of psoriasis on quality of life in Iran. Indian J Dermatol Venereol Leprol. 2009;75:220.
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). 1. conceptual framework and item selection. Med Care. 1992;30:473-483.
PRACTICE POINTS
- Pemphigus is an autoimmune blistering disease that can negatively affect patients’ lives.
- Assessing the impact of treatment from a patient’s perspective using outcome assessment measures is important and relevant in trials of new pemphigus treatments including rituximab.
- Rituximab administration in pemphigus patients led to rapid and notable improvement in health-related quality of life and patient-assessed measures.
Cutaneous Manifestations in Hereditary Alpha Tryptasemia
Hereditary alpha tryptasemia (HaT), an autosomal-dominant disorder of tryptase overproduction, was first described in 2014 by Lyons et al.1 It has been associated with multiple dermatologic, allergic, gastrointestinal (GI) tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities. These multisystem concerns may include cutaneous flushing, chronic pruritus, urticaria, GI tract symptoms, arthralgia, and autonomic dysfunction.2 The diverse symptoms and the recent discovery of HaT make recognition of this disorder challenging. Currently, it also is believed that HaT is associated with an elevated risk for anaphylaxis and is a biomarker for severe symptoms in disorders with increased mast cell burden such as mastocytosis.3-5
Given the potential cutaneous manifestations and the fact that dermatologic symptoms may be the initial presentation of HaT, awareness and recognition of this condition by dermatologists are essential for diagnosis and treatment. This review summarizes the cutaneous presentations consistent with HaT and discusses various conditions that share overlapping dermatologic symptoms with HaT.
Background on HaT
Mast cells are known to secrete several vasoactive mediators including tryptase and histamine when activated by foreign substances, similar to IgE-mediated hypersensitivity reactions. In their baseline state, mast cells continuously secrete immature forms of tryptases called protryptases.6 These protryptases come in 2 forms: α and β. Although mature tryptase is acutely elevatedin anaphylaxis, persistently elevated total serum tryptase levels frequently are regarded as indicative of a systemic mast cell disorder such as systemic mastocytosis (SM).3 Despite the wide-ranging phenotype of HaT, all individuals with the disorder have an elevated basal serum tryptase level (>8 ng/mL). Hereditary alpha tryptasemia has been identified as another possible cause of persistently elevated levels.2,6
Genetics and Epidemiology of HaT—The humantryptase locus at chromosome 16p13.3 is composed of 4 paralog genes: TPSG1, TPSB2, TPSAB1, and TPSD1.4 Only TPSAB1 encodes for α-tryptase, while both TPSB2 and TPSAB1 encode for β-tryptase.4 Hereditary alpha tryptasemia is an autosomal-dominant disorder resulting from a copy number increase in the α-tryptase encoding sequence within the TPSAB1 gene. Despite the wide-ranging phenotype of HaT, all individuals identified with the disorder have a basal serum tryptase level greater than 8 ng/mL, with mean (SD) levels of 15 (5) ng/mL and 24 (6) ng/mL with gene duplication and triplication, respectively (reference range, 0–11.4 ng/mL).2,6 Hereditary alpha tryptasemia likely is common and largely undiagnosed, with a recently estimated prevalence of 5% in the United Kingdom7 and 5.6% in a cohort of 125 individuals from Italy, Slovenia, and the United States.5
Implications of Increased α-tryptase Levels—After an inciting stimulus, the active portions of α-protryptase and β-protryptase are secreted as tetramers by activated mast cells via degranulation. In vitro, β-tryptase homotetramers have been found to play a role in anaphylaxis, while α-homotetramers are nearly inactive.8,9 Recently, however, it has been discovered that α2β2 tetramers also can form and do so in a higher ratio in individuals with increased α-tryptase–encoding gene copies, such as those with HaT.8 These heterotetramers exhibit unique properties compared with the homotetramers and may stimulate epidermal growth factor–like module-containing mucinlike hormone receptor 2 and protease-activated receptor 2 (PAR2). Epidermal growth factor–like module-containing mucinlike hormone receptor 2 activation likely contributes to vibratory urticaria in patients, while activation of PAR2 may have a range of clinical effects, including worsening asthma, inflammatory bowel disease, pruritus, and the exacerbation of dermal inflammation and hyperalgesia.8,10 Thus, α- and β-tryptase tetramers can be considered mediators that may influence the severity of disorders in which mast cells are naturally prevalent and likely contribute to the phenotype of those with HaT.7 Furthermore, these characteristics have been shown to potentially increase in severity with increasing tryptase levels and with increased TPSAB1 duplications.1,2 In contrast, more than 25% of the population is deficient in α-tryptase without known deleterious effects.5
Cutaneous Manifestations of HaT
A case series reported by Lyons et al1 in 2014 detailed persistent elevated basal serum tryptase levels in 9 families with an autosomal-dominant pattern of inheritance. In this cohort, 31 of 33 (94%) affected individuals had a history of atopic dermatitis (AD), and 26 of 33 (79%) affected individuals reported symptoms consistent with mast cell degranulation, including urticaria; flushing; and/or crampy abdominal pain unprovoked or triggered by heat, exercise, vibration, stress, certain foods, or minor physical stimulation.1 A later report by Lyons et al2 in 2016 identified the TPSAB1 α-tryptase–encoding sequence copy number increase as the causative entity for HaT by examining a group of 96 patients from 35 families with frequent recurrent cutaneous flushing and pruritus, sometimes associated with urticaria and sleep disruption. Flushing and pruritus were found in 45% (33/73) of those with a TPSAB1 duplication and 80% (12/15) of those with a triplication (P=.022), suggesting a gene dose effect regarding α-tryptase encoding sequence copy number and these symptoms.2
A 2019 study further explored the clinical finding of urticaria in patients with HaT by specifically examining if vibration-induced urticaria was affected by TPSAB1 gene dosage.8 A cohort of 56 volunteers—35 healthy and 21 with HaT—underwent tryptase genotyping and cutaneous vibratory challenge. The presence of TPSAB1 was significantly correlated with induction of vibration-induced urticaria (P<.01), as the severity and prevalence of the urticarial response increased along with α- and β-tryptase gene ratios.8
Urticaria and angioedema also were seen in 51% (36/70) of patients in a cohort of HaT patients in the United Kingdom, in which 41% (29/70) also had skin flushing. In contrast to prior studies, these manifestations were not more common in patients with gene triplications or quintuplications than those with duplications.7 In another recent retrospective evaluation conducted at Brigham and Women’s Hospital (Boston, Massachusetts)(N=101), 80% of patients aged 4 to 85 years with confirmed diagnoses of HaT had skin manifestations such as urticaria, flushing, and pruritus.4
HaT and Mast Cell Activation Syndrome—In 2019, a Mast Cell Disorders Committee Work Group Report outlined recommendations for diagnosing and treating primary mast cell activation syndrome (MCAS), a disorder in which mast cells seem to be more easily activated. Mast cell activation syndrome is defined as a primary clinical condition in which there are episodic signs and symptoms of systemic anaphylaxis (Table) concurrently affecting at least 2 organ systems, resulting from secreted mast cell mediators.9,11 The 2019 report also touched on clinical criteria that lack precision for diagnosing MCAS yet are in use, including dermographism and several types of rashes.9 Episode triggers frequent in MCAS include hot water, alcohol, stress, exercise, infection, hormonal changes, and physical stimuli.

Hereditary alpha tryptasemia has been suggested to be a risk factor for MCAS, which also can be associated with SM and clonal MCAS.9 Patients with MCAS should be tested for increased α-tryptase gene copy number given the overlap in symptoms, the likely predisposition of those with HaT to develop MCAS, and the fact that these patients could be at an increased risk for anaphylaxis.4,7,9,11 However, the clinical phenotype for HaT includes allergic disorders affecting the skin as well as neuropsychiatric and connective tissue abnormalities that are distinctive from MCAS. Although HaT may be considered a heritable risk factor for MCAS, MCAS is only 1 potential phenotype associated with HaT.9
Implications of HaT
Hereditary alpha tryptasemia should be considered in all patients with basal tryptase levels greater than 8 ng/mL. Cutaneous symptoms are among the most common presentations for individuals with HaT and can include AD, chronic or episodic urticaria, pruritus, flushing, and angioedema. However, HaT is unique because of the coupling of these common dermatologic findings with other abnormalities, including abdominal pain and diarrhea, hypermobile joints, and autonomic dysfunction. Patients with HaT also may manifest psychiatric concerns of anxiety, depression, and chronic pain, all of which have been linked to this disorder.
It is unclear in HaT if the presence of extra-allelic copies of tryptase in an individual is directly pathogenic. The effects of increased basal tryptase and α2β2 tetramers have been shown to likely be responsible for some of the clinical features in these individuals but also may magnify other individual underlying disease(s) or diathesis in which mast cells are naturally abundant.8 In the skin, this increased mast cell activation and subsequent histamine release frequently are visible as dermatographia and urticaria. However, mast cell numbers also are known to be increased in both psoriatic and AD skin lesions,12 thus severe presentation of these diseases in conjunction with the other symptoms associated with mast cell activation should prompt suspicion for HaT.
Effects of HaT on Other Cutaneous Disease—Given the increase of mast cells in AD skin lesions and fact that 94% of patients in the 2014 Lyons et al1 study cited a history of AD, HaT may be a risk factor in the development of AD. Interestingly, in addition to the increased mast cells in AD lesions, PAR2+ nerve fibers also are increased in AD lesions and have been implicated in the nonhistaminergic pruritus experienced by patients with AD.12 Thus, given the proposed propensity for α2β2 tetramers to activate PAR2, it is possible this mechanism may contribute to severe pruritus in individuals with AD and concurrent HaT, as those with HaT express increased α2β2 tetramers. However, no study to date has directly compared AD symptoms in patients with concurrent HaT vs patients without it. Further research is needed on how HaT impacts other allergic and inflammatory skin diseases such as AD and psoriasis, but one may reasonably consider HaT when treating chronic inflammatory skin diseases refractory to typical interventions and/or severe presentations. Although HaT is an autosomal-dominant disorder, it is not detected by standard whole exome sequencing or microarrays. A commercial test is available, utilizing a buccal swab to test for TPSAB1 copy number.
HaT and Mast Cell Disorders—When evaluating someone with suspected HaT, it is important to screen for other symptoms of mast cell activation. For instance, in the GI tract increased mast cell activation results in activation of motor neurons and nociceptors and increases secretion and peristalsis with consequent bloating, abdominal pain, and diarrhea.10 Likewise, tryptase also has neuromodulatory effects that amplify the perception of pain and are likely responsible for the feelings of hyperalgesia reported in patients with HaT.13
There is substantial overlap in the clinical pictures of HaT and MCAS, and HaT is considered a heritable risk factor for MCAS. Consequently, any patient undergoing workup for MCAS also should be tested for HaT. Although HaT is associated with consistently elevated tryptase, MCAS is episodic in nature, and an increase in tryptase levels of at least 20% plus 2 ng/mL from baseline only in the presence of other symptoms reflective of mast cell activation (Table) is a prerequisite for diagnosis.9 Chronic signs and symptoms of atopy, chronic urticaria, and severe asthma are not indicative of MCAS but are frequently seen in HaT.
Another cause of persistently elevated tryptase levels is SM. Systemic mastocytosis is defined by aberrant clonal mast cell expansion and systemic involvement11 and can cause persistent symptoms, unlike MCAS alone. However, SM also can be associated with MCAS.9 Notably, a baseline serum tryptase level greater than 20 ng/mL—much higher than the threshold of greater than 8 ng/mL for suspicion of HaT—is seen in 75% of SM cases and is part of the minor diagnostic criteria for the disease.9,11 However, the 2016 study identifying increased TPSAB1 α-tryptase–encoding sequences as the causative entity for HaT by Lyons et al2 found the average (SD) basal serum tryptase level in individuals with α-tryptase–encoding sequence duplications to be 15 (5) ng/mL and 24 (6) ng/mL in those with triplications. Thus, there likely is no threshold for elevated baseline tryptase levels that would indicate SM over HaT as a more likely diagnosis. However, SM will present with new persistently elevated tryptase levels, whereas the elevation in HaT is believed to be lifelong.5 Also in contrast to HaT, SM can present with liver, spleen, and lymph node involvement; bone sclerosis; and cytopenia.11,14
Mastocytosis is much rarer than HaT, with an estimated prevalence of 9 cases per 100,000 individuals in the United States.11 Although HaT diagnostic testing is noninvasive, SM requires a bone marrow biopsy for definitive diagnosis. Given the likely much higher prevalence of HaT than SM and the patient burden of a bone marrow biopsy, HaT should be considered before proceeding with a bone marrow biopsy to evaluate for SM when a patient presents with persistent systemic symptoms of mast cell activation and elevated baseline tryptase levels. Furthermore, it also would be prudent to test for HaT in patients with known SM, as a cohort study by Lyons et al5 indicated that HaT is likely more common in those with SM (12.2% [10/82] of cohort with known SM vs 5.3% of 398 controls), and patients with concurrent SM and HaT were at a higher risk for severe anaphylaxis (RR=9.5; P=.007).
Studies thus far surrounding HaT have not evaluated timing of initial symptom onset or age of initial presentation for HaT. Furthermore, there is no guarantee that those with increased TPSAB1 copy number will be symptomatic, as there have been reports of asymptomatic individuals with HaT who had basal serum levels greater than 8 ng/mL.7 As research into HaT continues and larger cohorts are evaluated, questions surrounding timing of symptom onset and various factors that may make someone more likely to display a particular phenotype will be answered.
Treatment—Long-term prognosis for individuals with HaT is largely unknown. Unfortunately, there are limited data to support a single effective treatment strategy for managing HaT, and treatment has varied based on predominant symptoms. For cutaneous and GI tract symptoms, trials of maximal H1 and H2 antihistamines twice daily have been recommended.4 Omalizumab was reported to improve chronic urticaria in 3 of 3 patients, showing potential promise as a treatment.4 Mast cell stabilizers, such as oral cromolyn, have been used for severe GI symptoms, while some patients also have reported improvement with oral ketotifen.6 Other medications, such as tricyclic antidepressants, clemastine fumarate, and gabapentin, have been beneficial anecdotally.6 Given the lack of harmful effects seen in individuals who are α-tryptase deficient, α-tryptase inhibition is an intriguing target for future therapies.
Conclusion
Patients who present with a constellation of dermatologic, allergic, GI tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities consistent with HaT may receive a prompt diagnosis if the association is recognized. The full relationship between HaT and other chronic dermatologic disorders is still unknown. Ultimately, heightened interest and research into HaT will lead to more treatment options available for affected patients.
1. Lyons JJ, Sun G, Stone KD, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474.
2. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
3. Schwartz L. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;6:451-463.
4. Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation–related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660.
5. Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;147:622-632.
6. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483-495.
7. Robey RC, Wilcock A, Bonin H, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556.
8. Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019;216:2348-2361.
9. Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144:883-896.
10. Ramsay DB, Stephen S, Borum M, et al. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2010;6:772-777.
11. Giannetti A, Filice E, Caffarelli C, et al. Mast cell activation disorders. Medicina (Kaunas). 2021;57:124.
12. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422.
13. Varrassi G, Fusco M, Skaper SD, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther. 2018;7:59-75.
14. Núñez E, Moreno-Borque R, García-Montero A, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:E76116.
Hereditary alpha tryptasemia (HaT), an autosomal-dominant disorder of tryptase overproduction, was first described in 2014 by Lyons et al.1 It has been associated with multiple dermatologic, allergic, gastrointestinal (GI) tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities. These multisystem concerns may include cutaneous flushing, chronic pruritus, urticaria, GI tract symptoms, arthralgia, and autonomic dysfunction.2 The diverse symptoms and the recent discovery of HaT make recognition of this disorder challenging. Currently, it also is believed that HaT is associated with an elevated risk for anaphylaxis and is a biomarker for severe symptoms in disorders with increased mast cell burden such as mastocytosis.3-5
Given the potential cutaneous manifestations and the fact that dermatologic symptoms may be the initial presentation of HaT, awareness and recognition of this condition by dermatologists are essential for diagnosis and treatment. This review summarizes the cutaneous presentations consistent with HaT and discusses various conditions that share overlapping dermatologic symptoms with HaT.
Background on HaT
Mast cells are known to secrete several vasoactive mediators including tryptase and histamine when activated by foreign substances, similar to IgE-mediated hypersensitivity reactions. In their baseline state, mast cells continuously secrete immature forms of tryptases called protryptases.6 These protryptases come in 2 forms: α and β. Although mature tryptase is acutely elevatedin anaphylaxis, persistently elevated total serum tryptase levels frequently are regarded as indicative of a systemic mast cell disorder such as systemic mastocytosis (SM).3 Despite the wide-ranging phenotype of HaT, all individuals with the disorder have an elevated basal serum tryptase level (>8 ng/mL). Hereditary alpha tryptasemia has been identified as another possible cause of persistently elevated levels.2,6
Genetics and Epidemiology of HaT—The humantryptase locus at chromosome 16p13.3 is composed of 4 paralog genes: TPSG1, TPSB2, TPSAB1, and TPSD1.4 Only TPSAB1 encodes for α-tryptase, while both TPSB2 and TPSAB1 encode for β-tryptase.4 Hereditary alpha tryptasemia is an autosomal-dominant disorder resulting from a copy number increase in the α-tryptase encoding sequence within the TPSAB1 gene. Despite the wide-ranging phenotype of HaT, all individuals identified with the disorder have a basal serum tryptase level greater than 8 ng/mL, with mean (SD) levels of 15 (5) ng/mL and 24 (6) ng/mL with gene duplication and triplication, respectively (reference range, 0–11.4 ng/mL).2,6 Hereditary alpha tryptasemia likely is common and largely undiagnosed, with a recently estimated prevalence of 5% in the United Kingdom7 and 5.6% in a cohort of 125 individuals from Italy, Slovenia, and the United States.5
Implications of Increased α-tryptase Levels—After an inciting stimulus, the active portions of α-protryptase and β-protryptase are secreted as tetramers by activated mast cells via degranulation. In vitro, β-tryptase homotetramers have been found to play a role in anaphylaxis, while α-homotetramers are nearly inactive.8,9 Recently, however, it has been discovered that α2β2 tetramers also can form and do so in a higher ratio in individuals with increased α-tryptase–encoding gene copies, such as those with HaT.8 These heterotetramers exhibit unique properties compared with the homotetramers and may stimulate epidermal growth factor–like module-containing mucinlike hormone receptor 2 and protease-activated receptor 2 (PAR2). Epidermal growth factor–like module-containing mucinlike hormone receptor 2 activation likely contributes to vibratory urticaria in patients, while activation of PAR2 may have a range of clinical effects, including worsening asthma, inflammatory bowel disease, pruritus, and the exacerbation of dermal inflammation and hyperalgesia.8,10 Thus, α- and β-tryptase tetramers can be considered mediators that may influence the severity of disorders in which mast cells are naturally prevalent and likely contribute to the phenotype of those with HaT.7 Furthermore, these characteristics have been shown to potentially increase in severity with increasing tryptase levels and with increased TPSAB1 duplications.1,2 In contrast, more than 25% of the population is deficient in α-tryptase without known deleterious effects.5
Cutaneous Manifestations of HaT
A case series reported by Lyons et al1 in 2014 detailed persistent elevated basal serum tryptase levels in 9 families with an autosomal-dominant pattern of inheritance. In this cohort, 31 of 33 (94%) affected individuals had a history of atopic dermatitis (AD), and 26 of 33 (79%) affected individuals reported symptoms consistent with mast cell degranulation, including urticaria; flushing; and/or crampy abdominal pain unprovoked or triggered by heat, exercise, vibration, stress, certain foods, or minor physical stimulation.1 A later report by Lyons et al2 in 2016 identified the TPSAB1 α-tryptase–encoding sequence copy number increase as the causative entity for HaT by examining a group of 96 patients from 35 families with frequent recurrent cutaneous flushing and pruritus, sometimes associated with urticaria and sleep disruption. Flushing and pruritus were found in 45% (33/73) of those with a TPSAB1 duplication and 80% (12/15) of those with a triplication (P=.022), suggesting a gene dose effect regarding α-tryptase encoding sequence copy number and these symptoms.2
A 2019 study further explored the clinical finding of urticaria in patients with HaT by specifically examining if vibration-induced urticaria was affected by TPSAB1 gene dosage.8 A cohort of 56 volunteers—35 healthy and 21 with HaT—underwent tryptase genotyping and cutaneous vibratory challenge. The presence of TPSAB1 was significantly correlated with induction of vibration-induced urticaria (P<.01), as the severity and prevalence of the urticarial response increased along with α- and β-tryptase gene ratios.8
Urticaria and angioedema also were seen in 51% (36/70) of patients in a cohort of HaT patients in the United Kingdom, in which 41% (29/70) also had skin flushing. In contrast to prior studies, these manifestations were not more common in patients with gene triplications or quintuplications than those with duplications.7 In another recent retrospective evaluation conducted at Brigham and Women’s Hospital (Boston, Massachusetts)(N=101), 80% of patients aged 4 to 85 years with confirmed diagnoses of HaT had skin manifestations such as urticaria, flushing, and pruritus.4
HaT and Mast Cell Activation Syndrome—In 2019, a Mast Cell Disorders Committee Work Group Report outlined recommendations for diagnosing and treating primary mast cell activation syndrome (MCAS), a disorder in which mast cells seem to be more easily activated. Mast cell activation syndrome is defined as a primary clinical condition in which there are episodic signs and symptoms of systemic anaphylaxis (Table) concurrently affecting at least 2 organ systems, resulting from secreted mast cell mediators.9,11 The 2019 report also touched on clinical criteria that lack precision for diagnosing MCAS yet are in use, including dermographism and several types of rashes.9 Episode triggers frequent in MCAS include hot water, alcohol, stress, exercise, infection, hormonal changes, and physical stimuli.

Hereditary alpha tryptasemia has been suggested to be a risk factor for MCAS, which also can be associated with SM and clonal MCAS.9 Patients with MCAS should be tested for increased α-tryptase gene copy number given the overlap in symptoms, the likely predisposition of those with HaT to develop MCAS, and the fact that these patients could be at an increased risk for anaphylaxis.4,7,9,11 However, the clinical phenotype for HaT includes allergic disorders affecting the skin as well as neuropsychiatric and connective tissue abnormalities that are distinctive from MCAS. Although HaT may be considered a heritable risk factor for MCAS, MCAS is only 1 potential phenotype associated with HaT.9
Implications of HaT
Hereditary alpha tryptasemia should be considered in all patients with basal tryptase levels greater than 8 ng/mL. Cutaneous symptoms are among the most common presentations for individuals with HaT and can include AD, chronic or episodic urticaria, pruritus, flushing, and angioedema. However, HaT is unique because of the coupling of these common dermatologic findings with other abnormalities, including abdominal pain and diarrhea, hypermobile joints, and autonomic dysfunction. Patients with HaT also may manifest psychiatric concerns of anxiety, depression, and chronic pain, all of which have been linked to this disorder.
It is unclear in HaT if the presence of extra-allelic copies of tryptase in an individual is directly pathogenic. The effects of increased basal tryptase and α2β2 tetramers have been shown to likely be responsible for some of the clinical features in these individuals but also may magnify other individual underlying disease(s) or diathesis in which mast cells are naturally abundant.8 In the skin, this increased mast cell activation and subsequent histamine release frequently are visible as dermatographia and urticaria. However, mast cell numbers also are known to be increased in both psoriatic and AD skin lesions,12 thus severe presentation of these diseases in conjunction with the other symptoms associated with mast cell activation should prompt suspicion for HaT.
Effects of HaT on Other Cutaneous Disease—Given the increase of mast cells in AD skin lesions and fact that 94% of patients in the 2014 Lyons et al1 study cited a history of AD, HaT may be a risk factor in the development of AD. Interestingly, in addition to the increased mast cells in AD lesions, PAR2+ nerve fibers also are increased in AD lesions and have been implicated in the nonhistaminergic pruritus experienced by patients with AD.12 Thus, given the proposed propensity for α2β2 tetramers to activate PAR2, it is possible this mechanism may contribute to severe pruritus in individuals with AD and concurrent HaT, as those with HaT express increased α2β2 tetramers. However, no study to date has directly compared AD symptoms in patients with concurrent HaT vs patients without it. Further research is needed on how HaT impacts other allergic and inflammatory skin diseases such as AD and psoriasis, but one may reasonably consider HaT when treating chronic inflammatory skin diseases refractory to typical interventions and/or severe presentations. Although HaT is an autosomal-dominant disorder, it is not detected by standard whole exome sequencing or microarrays. A commercial test is available, utilizing a buccal swab to test for TPSAB1 copy number.
HaT and Mast Cell Disorders—When evaluating someone with suspected HaT, it is important to screen for other symptoms of mast cell activation. For instance, in the GI tract increased mast cell activation results in activation of motor neurons and nociceptors and increases secretion and peristalsis with consequent bloating, abdominal pain, and diarrhea.10 Likewise, tryptase also has neuromodulatory effects that amplify the perception of pain and are likely responsible for the feelings of hyperalgesia reported in patients with HaT.13
There is substantial overlap in the clinical pictures of HaT and MCAS, and HaT is considered a heritable risk factor for MCAS. Consequently, any patient undergoing workup for MCAS also should be tested for HaT. Although HaT is associated with consistently elevated tryptase, MCAS is episodic in nature, and an increase in tryptase levels of at least 20% plus 2 ng/mL from baseline only in the presence of other symptoms reflective of mast cell activation (Table) is a prerequisite for diagnosis.9 Chronic signs and symptoms of atopy, chronic urticaria, and severe asthma are not indicative of MCAS but are frequently seen in HaT.
Another cause of persistently elevated tryptase levels is SM. Systemic mastocytosis is defined by aberrant clonal mast cell expansion and systemic involvement11 and can cause persistent symptoms, unlike MCAS alone. However, SM also can be associated with MCAS.9 Notably, a baseline serum tryptase level greater than 20 ng/mL—much higher than the threshold of greater than 8 ng/mL for suspicion of HaT—is seen in 75% of SM cases and is part of the minor diagnostic criteria for the disease.9,11 However, the 2016 study identifying increased TPSAB1 α-tryptase–encoding sequences as the causative entity for HaT by Lyons et al2 found the average (SD) basal serum tryptase level in individuals with α-tryptase–encoding sequence duplications to be 15 (5) ng/mL and 24 (6) ng/mL in those with triplications. Thus, there likely is no threshold for elevated baseline tryptase levels that would indicate SM over HaT as a more likely diagnosis. However, SM will present with new persistently elevated tryptase levels, whereas the elevation in HaT is believed to be lifelong.5 Also in contrast to HaT, SM can present with liver, spleen, and lymph node involvement; bone sclerosis; and cytopenia.11,14
Mastocytosis is much rarer than HaT, with an estimated prevalence of 9 cases per 100,000 individuals in the United States.11 Although HaT diagnostic testing is noninvasive, SM requires a bone marrow biopsy for definitive diagnosis. Given the likely much higher prevalence of HaT than SM and the patient burden of a bone marrow biopsy, HaT should be considered before proceeding with a bone marrow biopsy to evaluate for SM when a patient presents with persistent systemic symptoms of mast cell activation and elevated baseline tryptase levels. Furthermore, it also would be prudent to test for HaT in patients with known SM, as a cohort study by Lyons et al5 indicated that HaT is likely more common in those with SM (12.2% [10/82] of cohort with known SM vs 5.3% of 398 controls), and patients with concurrent SM and HaT were at a higher risk for severe anaphylaxis (RR=9.5; P=.007).
Studies thus far surrounding HaT have not evaluated timing of initial symptom onset or age of initial presentation for HaT. Furthermore, there is no guarantee that those with increased TPSAB1 copy number will be symptomatic, as there have been reports of asymptomatic individuals with HaT who had basal serum levels greater than 8 ng/mL.7 As research into HaT continues and larger cohorts are evaluated, questions surrounding timing of symptom onset and various factors that may make someone more likely to display a particular phenotype will be answered.
Treatment—Long-term prognosis for individuals with HaT is largely unknown. Unfortunately, there are limited data to support a single effective treatment strategy for managing HaT, and treatment has varied based on predominant symptoms. For cutaneous and GI tract symptoms, trials of maximal H1 and H2 antihistamines twice daily have been recommended.4 Omalizumab was reported to improve chronic urticaria in 3 of 3 patients, showing potential promise as a treatment.4 Mast cell stabilizers, such as oral cromolyn, have been used for severe GI symptoms, while some patients also have reported improvement with oral ketotifen.6 Other medications, such as tricyclic antidepressants, clemastine fumarate, and gabapentin, have been beneficial anecdotally.6 Given the lack of harmful effects seen in individuals who are α-tryptase deficient, α-tryptase inhibition is an intriguing target for future therapies.
Conclusion
Patients who present with a constellation of dermatologic, allergic, GI tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities consistent with HaT may receive a prompt diagnosis if the association is recognized. The full relationship between HaT and other chronic dermatologic disorders is still unknown. Ultimately, heightened interest and research into HaT will lead to more treatment options available for affected patients.
Hereditary alpha tryptasemia (HaT), an autosomal-dominant disorder of tryptase overproduction, was first described in 2014 by Lyons et al.1 It has been associated with multiple dermatologic, allergic, gastrointestinal (GI) tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities. These multisystem concerns may include cutaneous flushing, chronic pruritus, urticaria, GI tract symptoms, arthralgia, and autonomic dysfunction.2 The diverse symptoms and the recent discovery of HaT make recognition of this disorder challenging. Currently, it also is believed that HaT is associated with an elevated risk for anaphylaxis and is a biomarker for severe symptoms in disorders with increased mast cell burden such as mastocytosis.3-5
Given the potential cutaneous manifestations and the fact that dermatologic symptoms may be the initial presentation of HaT, awareness and recognition of this condition by dermatologists are essential for diagnosis and treatment. This review summarizes the cutaneous presentations consistent with HaT and discusses various conditions that share overlapping dermatologic symptoms with HaT.
Background on HaT
Mast cells are known to secrete several vasoactive mediators including tryptase and histamine when activated by foreign substances, similar to IgE-mediated hypersensitivity reactions. In their baseline state, mast cells continuously secrete immature forms of tryptases called protryptases.6 These protryptases come in 2 forms: α and β. Although mature tryptase is acutely elevatedin anaphylaxis, persistently elevated total serum tryptase levels frequently are regarded as indicative of a systemic mast cell disorder such as systemic mastocytosis (SM).3 Despite the wide-ranging phenotype of HaT, all individuals with the disorder have an elevated basal serum tryptase level (>8 ng/mL). Hereditary alpha tryptasemia has been identified as another possible cause of persistently elevated levels.2,6
Genetics and Epidemiology of HaT—The humantryptase locus at chromosome 16p13.3 is composed of 4 paralog genes: TPSG1, TPSB2, TPSAB1, and TPSD1.4 Only TPSAB1 encodes for α-tryptase, while both TPSB2 and TPSAB1 encode for β-tryptase.4 Hereditary alpha tryptasemia is an autosomal-dominant disorder resulting from a copy number increase in the α-tryptase encoding sequence within the TPSAB1 gene. Despite the wide-ranging phenotype of HaT, all individuals identified with the disorder have a basal serum tryptase level greater than 8 ng/mL, with mean (SD) levels of 15 (5) ng/mL and 24 (6) ng/mL with gene duplication and triplication, respectively (reference range, 0–11.4 ng/mL).2,6 Hereditary alpha tryptasemia likely is common and largely undiagnosed, with a recently estimated prevalence of 5% in the United Kingdom7 and 5.6% in a cohort of 125 individuals from Italy, Slovenia, and the United States.5
Implications of Increased α-tryptase Levels—After an inciting stimulus, the active portions of α-protryptase and β-protryptase are secreted as tetramers by activated mast cells via degranulation. In vitro, β-tryptase homotetramers have been found to play a role in anaphylaxis, while α-homotetramers are nearly inactive.8,9 Recently, however, it has been discovered that α2β2 tetramers also can form and do so in a higher ratio in individuals with increased α-tryptase–encoding gene copies, such as those with HaT.8 These heterotetramers exhibit unique properties compared with the homotetramers and may stimulate epidermal growth factor–like module-containing mucinlike hormone receptor 2 and protease-activated receptor 2 (PAR2). Epidermal growth factor–like module-containing mucinlike hormone receptor 2 activation likely contributes to vibratory urticaria in patients, while activation of PAR2 may have a range of clinical effects, including worsening asthma, inflammatory bowel disease, pruritus, and the exacerbation of dermal inflammation and hyperalgesia.8,10 Thus, α- and β-tryptase tetramers can be considered mediators that may influence the severity of disorders in which mast cells are naturally prevalent and likely contribute to the phenotype of those with HaT.7 Furthermore, these characteristics have been shown to potentially increase in severity with increasing tryptase levels and with increased TPSAB1 duplications.1,2 In contrast, more than 25% of the population is deficient in α-tryptase without known deleterious effects.5
Cutaneous Manifestations of HaT
A case series reported by Lyons et al1 in 2014 detailed persistent elevated basal serum tryptase levels in 9 families with an autosomal-dominant pattern of inheritance. In this cohort, 31 of 33 (94%) affected individuals had a history of atopic dermatitis (AD), and 26 of 33 (79%) affected individuals reported symptoms consistent with mast cell degranulation, including urticaria; flushing; and/or crampy abdominal pain unprovoked or triggered by heat, exercise, vibration, stress, certain foods, or minor physical stimulation.1 A later report by Lyons et al2 in 2016 identified the TPSAB1 α-tryptase–encoding sequence copy number increase as the causative entity for HaT by examining a group of 96 patients from 35 families with frequent recurrent cutaneous flushing and pruritus, sometimes associated with urticaria and sleep disruption. Flushing and pruritus were found in 45% (33/73) of those with a TPSAB1 duplication and 80% (12/15) of those with a triplication (P=.022), suggesting a gene dose effect regarding α-tryptase encoding sequence copy number and these symptoms.2
A 2019 study further explored the clinical finding of urticaria in patients with HaT by specifically examining if vibration-induced urticaria was affected by TPSAB1 gene dosage.8 A cohort of 56 volunteers—35 healthy and 21 with HaT—underwent tryptase genotyping and cutaneous vibratory challenge. The presence of TPSAB1 was significantly correlated with induction of vibration-induced urticaria (P<.01), as the severity and prevalence of the urticarial response increased along with α- and β-tryptase gene ratios.8
Urticaria and angioedema also were seen in 51% (36/70) of patients in a cohort of HaT patients in the United Kingdom, in which 41% (29/70) also had skin flushing. In contrast to prior studies, these manifestations were not more common in patients with gene triplications or quintuplications than those with duplications.7 In another recent retrospective evaluation conducted at Brigham and Women’s Hospital (Boston, Massachusetts)(N=101), 80% of patients aged 4 to 85 years with confirmed diagnoses of HaT had skin manifestations such as urticaria, flushing, and pruritus.4
HaT and Mast Cell Activation Syndrome—In 2019, a Mast Cell Disorders Committee Work Group Report outlined recommendations for diagnosing and treating primary mast cell activation syndrome (MCAS), a disorder in which mast cells seem to be more easily activated. Mast cell activation syndrome is defined as a primary clinical condition in which there are episodic signs and symptoms of systemic anaphylaxis (Table) concurrently affecting at least 2 organ systems, resulting from secreted mast cell mediators.9,11 The 2019 report also touched on clinical criteria that lack precision for diagnosing MCAS yet are in use, including dermographism and several types of rashes.9 Episode triggers frequent in MCAS include hot water, alcohol, stress, exercise, infection, hormonal changes, and physical stimuli.

Hereditary alpha tryptasemia has been suggested to be a risk factor for MCAS, which also can be associated with SM and clonal MCAS.9 Patients with MCAS should be tested for increased α-tryptase gene copy number given the overlap in symptoms, the likely predisposition of those with HaT to develop MCAS, and the fact that these patients could be at an increased risk for anaphylaxis.4,7,9,11 However, the clinical phenotype for HaT includes allergic disorders affecting the skin as well as neuropsychiatric and connective tissue abnormalities that are distinctive from MCAS. Although HaT may be considered a heritable risk factor for MCAS, MCAS is only 1 potential phenotype associated with HaT.9
Implications of HaT
Hereditary alpha tryptasemia should be considered in all patients with basal tryptase levels greater than 8 ng/mL. Cutaneous symptoms are among the most common presentations for individuals with HaT and can include AD, chronic or episodic urticaria, pruritus, flushing, and angioedema. However, HaT is unique because of the coupling of these common dermatologic findings with other abnormalities, including abdominal pain and diarrhea, hypermobile joints, and autonomic dysfunction. Patients with HaT also may manifest psychiatric concerns of anxiety, depression, and chronic pain, all of which have been linked to this disorder.
It is unclear in HaT if the presence of extra-allelic copies of tryptase in an individual is directly pathogenic. The effects of increased basal tryptase and α2β2 tetramers have been shown to likely be responsible for some of the clinical features in these individuals but also may magnify other individual underlying disease(s) or diathesis in which mast cells are naturally abundant.8 In the skin, this increased mast cell activation and subsequent histamine release frequently are visible as dermatographia and urticaria. However, mast cell numbers also are known to be increased in both psoriatic and AD skin lesions,12 thus severe presentation of these diseases in conjunction with the other symptoms associated with mast cell activation should prompt suspicion for HaT.
Effects of HaT on Other Cutaneous Disease—Given the increase of mast cells in AD skin lesions and fact that 94% of patients in the 2014 Lyons et al1 study cited a history of AD, HaT may be a risk factor in the development of AD. Interestingly, in addition to the increased mast cells in AD lesions, PAR2+ nerve fibers also are increased in AD lesions and have been implicated in the nonhistaminergic pruritus experienced by patients with AD.12 Thus, given the proposed propensity for α2β2 tetramers to activate PAR2, it is possible this mechanism may contribute to severe pruritus in individuals with AD and concurrent HaT, as those with HaT express increased α2β2 tetramers. However, no study to date has directly compared AD symptoms in patients with concurrent HaT vs patients without it. Further research is needed on how HaT impacts other allergic and inflammatory skin diseases such as AD and psoriasis, but one may reasonably consider HaT when treating chronic inflammatory skin diseases refractory to typical interventions and/or severe presentations. Although HaT is an autosomal-dominant disorder, it is not detected by standard whole exome sequencing or microarrays. A commercial test is available, utilizing a buccal swab to test for TPSAB1 copy number.
HaT and Mast Cell Disorders—When evaluating someone with suspected HaT, it is important to screen for other symptoms of mast cell activation. For instance, in the GI tract increased mast cell activation results in activation of motor neurons and nociceptors and increases secretion and peristalsis with consequent bloating, abdominal pain, and diarrhea.10 Likewise, tryptase also has neuromodulatory effects that amplify the perception of pain and are likely responsible for the feelings of hyperalgesia reported in patients with HaT.13
There is substantial overlap in the clinical pictures of HaT and MCAS, and HaT is considered a heritable risk factor for MCAS. Consequently, any patient undergoing workup for MCAS also should be tested for HaT. Although HaT is associated with consistently elevated tryptase, MCAS is episodic in nature, and an increase in tryptase levels of at least 20% plus 2 ng/mL from baseline only in the presence of other symptoms reflective of mast cell activation (Table) is a prerequisite for diagnosis.9 Chronic signs and symptoms of atopy, chronic urticaria, and severe asthma are not indicative of MCAS but are frequently seen in HaT.
Another cause of persistently elevated tryptase levels is SM. Systemic mastocytosis is defined by aberrant clonal mast cell expansion and systemic involvement11 and can cause persistent symptoms, unlike MCAS alone. However, SM also can be associated with MCAS.9 Notably, a baseline serum tryptase level greater than 20 ng/mL—much higher than the threshold of greater than 8 ng/mL for suspicion of HaT—is seen in 75% of SM cases and is part of the minor diagnostic criteria for the disease.9,11 However, the 2016 study identifying increased TPSAB1 α-tryptase–encoding sequences as the causative entity for HaT by Lyons et al2 found the average (SD) basal serum tryptase level in individuals with α-tryptase–encoding sequence duplications to be 15 (5) ng/mL and 24 (6) ng/mL in those with triplications. Thus, there likely is no threshold for elevated baseline tryptase levels that would indicate SM over HaT as a more likely diagnosis. However, SM will present with new persistently elevated tryptase levels, whereas the elevation in HaT is believed to be lifelong.5 Also in contrast to HaT, SM can present with liver, spleen, and lymph node involvement; bone sclerosis; and cytopenia.11,14
Mastocytosis is much rarer than HaT, with an estimated prevalence of 9 cases per 100,000 individuals in the United States.11 Although HaT diagnostic testing is noninvasive, SM requires a bone marrow biopsy for definitive diagnosis. Given the likely much higher prevalence of HaT than SM and the patient burden of a bone marrow biopsy, HaT should be considered before proceeding with a bone marrow biopsy to evaluate for SM when a patient presents with persistent systemic symptoms of mast cell activation and elevated baseline tryptase levels. Furthermore, it also would be prudent to test for HaT in patients with known SM, as a cohort study by Lyons et al5 indicated that HaT is likely more common in those with SM (12.2% [10/82] of cohort with known SM vs 5.3% of 398 controls), and patients with concurrent SM and HaT were at a higher risk for severe anaphylaxis (RR=9.5; P=.007).
Studies thus far surrounding HaT have not evaluated timing of initial symptom onset or age of initial presentation for HaT. Furthermore, there is no guarantee that those with increased TPSAB1 copy number will be symptomatic, as there have been reports of asymptomatic individuals with HaT who had basal serum levels greater than 8 ng/mL.7 As research into HaT continues and larger cohorts are evaluated, questions surrounding timing of symptom onset and various factors that may make someone more likely to display a particular phenotype will be answered.
Treatment—Long-term prognosis for individuals with HaT is largely unknown. Unfortunately, there are limited data to support a single effective treatment strategy for managing HaT, and treatment has varied based on predominant symptoms. For cutaneous and GI tract symptoms, trials of maximal H1 and H2 antihistamines twice daily have been recommended.4 Omalizumab was reported to improve chronic urticaria in 3 of 3 patients, showing potential promise as a treatment.4 Mast cell stabilizers, such as oral cromolyn, have been used for severe GI symptoms, while some patients also have reported improvement with oral ketotifen.6 Other medications, such as tricyclic antidepressants, clemastine fumarate, and gabapentin, have been beneficial anecdotally.6 Given the lack of harmful effects seen in individuals who are α-tryptase deficient, α-tryptase inhibition is an intriguing target for future therapies.
Conclusion
Patients who present with a constellation of dermatologic, allergic, GI tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities consistent with HaT may receive a prompt diagnosis if the association is recognized. The full relationship between HaT and other chronic dermatologic disorders is still unknown. Ultimately, heightened interest and research into HaT will lead to more treatment options available for affected patients.
1. Lyons JJ, Sun G, Stone KD, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474.
2. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
3. Schwartz L. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;6:451-463.
4. Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation–related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660.
5. Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;147:622-632.
6. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483-495.
7. Robey RC, Wilcock A, Bonin H, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556.
8. Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019;216:2348-2361.
9. Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144:883-896.
10. Ramsay DB, Stephen S, Borum M, et al. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2010;6:772-777.
11. Giannetti A, Filice E, Caffarelli C, et al. Mast cell activation disorders. Medicina (Kaunas). 2021;57:124.
12. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422.
13. Varrassi G, Fusco M, Skaper SD, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther. 2018;7:59-75.
14. Núñez E, Moreno-Borque R, García-Montero A, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:E76116.
1. Lyons JJ, Sun G, Stone KD, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474.
2. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
3. Schwartz L. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;6:451-463.
4. Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation–related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660.
5. Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;147:622-632.
6. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483-495.
7. Robey RC, Wilcock A, Bonin H, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556.
8. Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019;216:2348-2361.
9. Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144:883-896.
10. Ramsay DB, Stephen S, Borum M, et al. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2010;6:772-777.
11. Giannetti A, Filice E, Caffarelli C, et al. Mast cell activation disorders. Medicina (Kaunas). 2021;57:124.
12. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422.
13. Varrassi G, Fusco M, Skaper SD, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther. 2018;7:59-75.
14. Núñez E, Moreno-Borque R, García-Montero A, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:E76116.
Practice Points
- Chronic or episodic urticaria, flushing, and pruritus are the most consistent cutaneous abnormalities associated with hereditary alpha tryptasemia (HaT), but HaT also may augment symptoms of other underlying inflammatory skin disorders, such as atopic dermatitis and psoriasis.
- Individuals with episodic dermatologic manifestations indicative of mast cell activation accompanied by symptoms affecting 1 or more organ systems should be evaluated for mast cell activation syndrome as well as HaT.