User login
Similar prognosis in late- and younger-onset rheumatoid arthritis
Key clinical point: Patients with late-onset rheumatoid arthritis (LORA) and younger-onset rheumatoid arthritis (YORA) had similar prognosis, but patients with LORA who reached remission were more likely to be on a less intensive treatment regimen.
Major finding: Time to remission was similar among patients with LORA and YORA (P = .36). Patients with LORA vs YORA were less likely to be on biologics or Janus kinase inhibitors (16% vs 27%; P = .0039) and more likely to be on a single conventional synthetic disease-modifying antirheumatic drug (34% vs 27%; P = .0039) and oral glucocorticoids (27% vs 13%; P < .0001) at the time of remission.
Study details: This prospective cohort study included 872 patients diagnosed with RA at an age of ≥60 years (LORA; n = 354) or <60 years (YORA; n = 518).
Disclosures: This study was funded by peer-reviewed grants from the Canadian Arthritis Network and others and unrestricted grants from AbbVie, Novartis, and other sources. The authors declared no conflicts of interest.
Source: Li X et al. Late‑onset rheumatoid arthritis has a similar time to remission as younger‑onset rheumatoid arthritis: Results from the Ontario Best Practices Research Initiative. Arthritis Res Ther. 2022;24:255 (Nov 19). Doi: 10.1186/s13075-022-02952-1
Key clinical point: Patients with late-onset rheumatoid arthritis (LORA) and younger-onset rheumatoid arthritis (YORA) had similar prognosis, but patients with LORA who reached remission were more likely to be on a less intensive treatment regimen.
Major finding: Time to remission was similar among patients with LORA and YORA (P = .36). Patients with LORA vs YORA were less likely to be on biologics or Janus kinase inhibitors (16% vs 27%; P = .0039) and more likely to be on a single conventional synthetic disease-modifying antirheumatic drug (34% vs 27%; P = .0039) and oral glucocorticoids (27% vs 13%; P < .0001) at the time of remission.
Study details: This prospective cohort study included 872 patients diagnosed with RA at an age of ≥60 years (LORA; n = 354) or <60 years (YORA; n = 518).
Disclosures: This study was funded by peer-reviewed grants from the Canadian Arthritis Network and others and unrestricted grants from AbbVie, Novartis, and other sources. The authors declared no conflicts of interest.
Source: Li X et al. Late‑onset rheumatoid arthritis has a similar time to remission as younger‑onset rheumatoid arthritis: Results from the Ontario Best Practices Research Initiative. Arthritis Res Ther. 2022;24:255 (Nov 19). Doi: 10.1186/s13075-022-02952-1
Key clinical point: Patients with late-onset rheumatoid arthritis (LORA) and younger-onset rheumatoid arthritis (YORA) had similar prognosis, but patients with LORA who reached remission were more likely to be on a less intensive treatment regimen.
Major finding: Time to remission was similar among patients with LORA and YORA (P = .36). Patients with LORA vs YORA were less likely to be on biologics or Janus kinase inhibitors (16% vs 27%; P = .0039) and more likely to be on a single conventional synthetic disease-modifying antirheumatic drug (34% vs 27%; P = .0039) and oral glucocorticoids (27% vs 13%; P < .0001) at the time of remission.
Study details: This prospective cohort study included 872 patients diagnosed with RA at an age of ≥60 years (LORA; n = 354) or <60 years (YORA; n = 518).
Disclosures: This study was funded by peer-reviewed grants from the Canadian Arthritis Network and others and unrestricted grants from AbbVie, Novartis, and other sources. The authors declared no conflicts of interest.
Source: Li X et al. Late‑onset rheumatoid arthritis has a similar time to remission as younger‑onset rheumatoid arthritis: Results from the Ontario Best Practices Research Initiative. Arthritis Res Ther. 2022;24:255 (Nov 19). Doi: 10.1186/s13075-022-02952-1
Immunomodulators reduce cardiovascular risk in RA
Key clinical point: The addition of a tumor necrosis factor inhibitor (TNFi) or hydroxychloroquine+sulfasalazine (triple therapy) to weekly methotrexate significantly reduced atherosclerotic vascular inflammation in patients with rheumatoid arthritis (RA); however, TNFi was not associated with greater improvements than triple therapy.
Major finding: Over 24 weeks, the arterial target-to-background ratio (TBR) in carotid arteries or aorta reduced significantly with TNFi (Δ −0.24; P = .001) and triple therapy (Δ −0.19; P = .001), with no significant difference in TBR improvement between the two treatment groups (P = .79).
Study details: Findings are from the phase 4 TARGET trial including 115 patients with active RA despite being on weekly methotrexate who were randomly assigned to add TNFi (adalimumab or etanercept) or hydroxychloroquine+sulfasalazine.
Disclosures: This study was funded by the US National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH-NIAMS). The study drug was supplied by AbbVie and Amgen. Four authors declared receiving research support, unrestricted grants, or royalties, being co-inventors on patents, or consulting for various sources unrelated to current study.
Source: Solomon DH et al for the TARGET Trial Consortium. Reducing cardiovascular risk with immunomodulators: A randomised active comparator trial among patients with rheumatoid arthritis. Ann Rheum Dis. 2022 (Nov 30). Doi: 10.1136/ard-2022-223302
Key clinical point: The addition of a tumor necrosis factor inhibitor (TNFi) or hydroxychloroquine+sulfasalazine (triple therapy) to weekly methotrexate significantly reduced atherosclerotic vascular inflammation in patients with rheumatoid arthritis (RA); however, TNFi was not associated with greater improvements than triple therapy.
Major finding: Over 24 weeks, the arterial target-to-background ratio (TBR) in carotid arteries or aorta reduced significantly with TNFi (Δ −0.24; P = .001) and triple therapy (Δ −0.19; P = .001), with no significant difference in TBR improvement between the two treatment groups (P = .79).
Study details: Findings are from the phase 4 TARGET trial including 115 patients with active RA despite being on weekly methotrexate who were randomly assigned to add TNFi (adalimumab or etanercept) or hydroxychloroquine+sulfasalazine.
Disclosures: This study was funded by the US National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH-NIAMS). The study drug was supplied by AbbVie and Amgen. Four authors declared receiving research support, unrestricted grants, or royalties, being co-inventors on patents, or consulting for various sources unrelated to current study.
Source: Solomon DH et al for the TARGET Trial Consortium. Reducing cardiovascular risk with immunomodulators: A randomised active comparator trial among patients with rheumatoid arthritis. Ann Rheum Dis. 2022 (Nov 30). Doi: 10.1136/ard-2022-223302
Key clinical point: The addition of a tumor necrosis factor inhibitor (TNFi) or hydroxychloroquine+sulfasalazine (triple therapy) to weekly methotrexate significantly reduced atherosclerotic vascular inflammation in patients with rheumatoid arthritis (RA); however, TNFi was not associated with greater improvements than triple therapy.
Major finding: Over 24 weeks, the arterial target-to-background ratio (TBR) in carotid arteries or aorta reduced significantly with TNFi (Δ −0.24; P = .001) and triple therapy (Δ −0.19; P = .001), with no significant difference in TBR improvement between the two treatment groups (P = .79).
Study details: Findings are from the phase 4 TARGET trial including 115 patients with active RA despite being on weekly methotrexate who were randomly assigned to add TNFi (adalimumab or etanercept) or hydroxychloroquine+sulfasalazine.
Disclosures: This study was funded by the US National Institutes of Health National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIH-NIAMS). The study drug was supplied by AbbVie and Amgen. Four authors declared receiving research support, unrestricted grants, or royalties, being co-inventors on patents, or consulting for various sources unrelated to current study.
Source: Solomon DH et al for the TARGET Trial Consortium. Reducing cardiovascular risk with immunomodulators: A randomised active comparator trial among patients with rheumatoid arthritis. Ann Rheum Dis. 2022 (Nov 30). Doi: 10.1136/ard-2022-223302
Baricitinib monotherapy: An attractive option for RA patients with methotrexate intolerance
Key clinical point: Baricitinib monotherapy was as effective as baricitinib plus methotrexate therapy with high drug retention rates in patients with rheumatoid arthritis (RA), suggesting baricitinib monotherapy as a viable option in the case of methotrexate intolerance.
Major finding: The 28-joint Disease Activity Scores based on Erythrocyte Sedimentation Rate were not significantly different at all time points over 96 weeks (log-rank P = .44) and drug survival rates at 1 year (69% vs 67%) and 2 years (62% vs 56%) were comparable in the baricitinib alone vs baricitinib+methotrexate arm. No new safety signals were identified.
Study details: This prospective observational study included 139 patients with RA who switched to baricitinib monotherapy or baricitinib plus methotrexate therapy because of high disease activity or intolerance to previous conventional synthetic and biologic disease-modifying antirheumatic drugs.
Disclosures: This study was supported by the Deutsche Forschungsgemeinschaft and other sources. Several authors reported receiving speaker honoraria or travel support or serving as consultants for various sources.
Source: Bayat S et al. Efficacy and drug persistence of baricitinib monotherapy is similar to combination therapy in patients with active RA: A prospective observational study. RMD Open. 2022;8:e002674 (Nov 21). Doi: 10.1136/rmdopen-2022-002674
Key clinical point: Baricitinib monotherapy was as effective as baricitinib plus methotrexate therapy with high drug retention rates in patients with rheumatoid arthritis (RA), suggesting baricitinib monotherapy as a viable option in the case of methotrexate intolerance.
Major finding: The 28-joint Disease Activity Scores based on Erythrocyte Sedimentation Rate were not significantly different at all time points over 96 weeks (log-rank P = .44) and drug survival rates at 1 year (69% vs 67%) and 2 years (62% vs 56%) were comparable in the baricitinib alone vs baricitinib+methotrexate arm. No new safety signals were identified.
Study details: This prospective observational study included 139 patients with RA who switched to baricitinib monotherapy or baricitinib plus methotrexate therapy because of high disease activity or intolerance to previous conventional synthetic and biologic disease-modifying antirheumatic drugs.
Disclosures: This study was supported by the Deutsche Forschungsgemeinschaft and other sources. Several authors reported receiving speaker honoraria or travel support or serving as consultants for various sources.
Source: Bayat S et al. Efficacy and drug persistence of baricitinib monotherapy is similar to combination therapy in patients with active RA: A prospective observational study. RMD Open. 2022;8:e002674 (Nov 21). Doi: 10.1136/rmdopen-2022-002674
Key clinical point: Baricitinib monotherapy was as effective as baricitinib plus methotrexate therapy with high drug retention rates in patients with rheumatoid arthritis (RA), suggesting baricitinib monotherapy as a viable option in the case of methotrexate intolerance.
Major finding: The 28-joint Disease Activity Scores based on Erythrocyte Sedimentation Rate were not significantly different at all time points over 96 weeks (log-rank P = .44) and drug survival rates at 1 year (69% vs 67%) and 2 years (62% vs 56%) were comparable in the baricitinib alone vs baricitinib+methotrexate arm. No new safety signals were identified.
Study details: This prospective observational study included 139 patients with RA who switched to baricitinib monotherapy or baricitinib plus methotrexate therapy because of high disease activity or intolerance to previous conventional synthetic and biologic disease-modifying antirheumatic drugs.
Disclosures: This study was supported by the Deutsche Forschungsgemeinschaft and other sources. Several authors reported receiving speaker honoraria or travel support or serving as consultants for various sources.
Source: Bayat S et al. Efficacy and drug persistence of baricitinib monotherapy is similar to combination therapy in patients with active RA: A prospective observational study. RMD Open. 2022;8:e002674 (Nov 21). Doi: 10.1136/rmdopen-2022-002674
How to Optimize Wound Closure in Thin Skin
Practice Gap
Cutaneous surgery involves many areas where skin is quite thin and fragile, which often is encountered in elderly patients; the forearms and lower legs are the most frequent locations for thin skin.1 Dermatologic surgeons frequently encounter these situations, making this a highly practical arena for technical improvements.
For many of these patients, there is little meaningful dermis for placement of subcutaneous sutures. Therefore, a common approach following surgery, particularly following Mohs micrographic surgery in which tumors and defects typically are larger, is healing by secondary intention.2 Although healing by secondary intention often is a reasonable option, we have found that maximizing the use of epidermal skin for primary closure can be an effective means of closing many such defects. Antimicrobial reinforced skin closure strips have been incorporated in wound closure for thin skin. However, earlier efforts involving reinforcement perpendicular to the wound lacked critical details or used a different technique.3
The Technique
We developed a novel effective closure technique that minimizes these problems. Our technique has been used on the wounds of hundreds of patients with satisfying results. Early on, we used multiple variations to optimize outcomes, including different sizes of sutures and reinforced skin closure strips, application of medical liquid adhesive, liquid adhesive, and varying postoperative dressings. For 3 years, we tracked outcomes in-house and gradually narrowed down our successes into a single, user-friendly paradigm.
Supplies—To perform this technique, required supplies include:
• 2-0 Polypropylene suture with a PS-2 needle, or the equivalent. Polyglactin or silk suture can be utilized if a less-rigid suture is desired; however, we primarily have used polypropylene for repairs with good results. Each repair requires at least 2 sutures.
• Reinforced skin closure strips (1×5 inches). This width affords increased strength.
• Conforming stretch bandage and elastic self-adherent wrap.
• Polysporin (bacitracin zinc, polymyxin B sulfate, and petrolatum)(Johnson & Johnson).
• All usual surgical instruments and supplies, including paper tape and nonadherent gauze (surgeon dependent).
Step-by-step Technique—Close the wound using the following steps:
1. Once the defect is finalized following Mohs micrographic surgery or excision, excise the ellipse to be utilized for the closure and perform complete hemostasis.
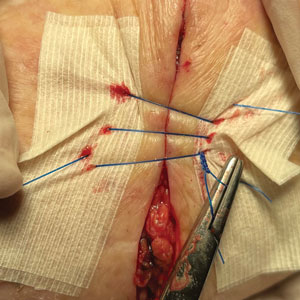
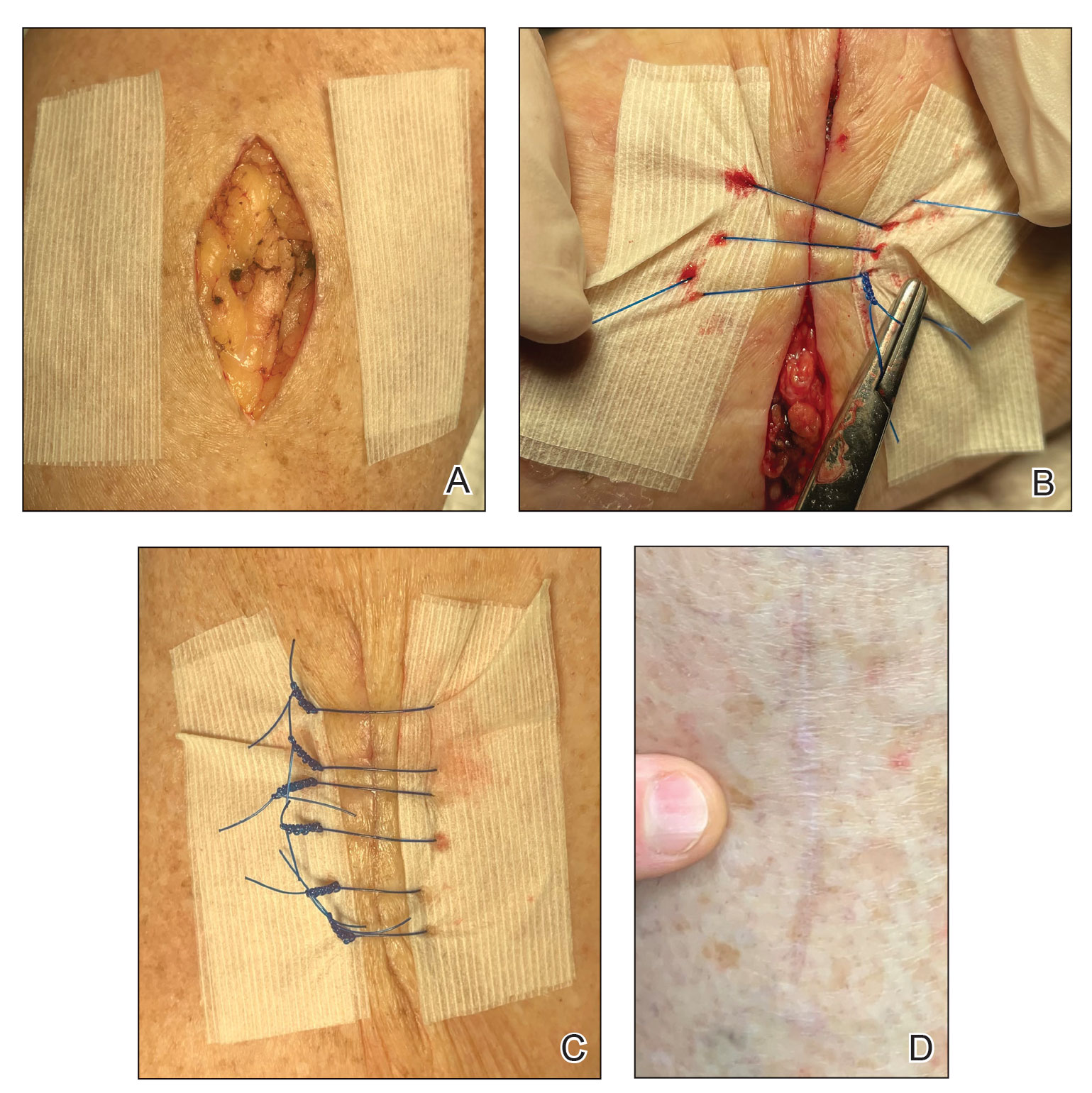
2. Place 2 layers of reinforced skin closure strips—one on top of the other—along each side of the defect, leaving approximately 1 cm of uncovered skin between the wound edges and the reinforced skin closure strips (Figure, A).
3. Take a big-bite pulley suture about one-third of the way from one end of the ellipse, with both punctures passing through the reinforced skin closure strips. Leave that in place or have the assistant hold it and wait. Place a second suture immediately adjacent to the pulley suture. Once that suture is placed but still untied, have the assistant carefully pull the pulley suture outward away from the wound edge while you carefully bring the suture together and tie it off gently (Figure, B). Doing this utilizes the pulley ability of the suture to protect the skin from tearing and releases sufficient pressure on the single suture so that it can be easily tightened without risk to the fragile skin.
4. Repeat step 3, this time placing a pulley suture near the midline of the ellipse and the subsequent single suture adjacent to it.
5. Take pulley sutures repeatedly as in steps 3 and 4 until multiple sutures are secured in place. Replace the pulley sutures with single sutures because the double-pulley sutures in areas of lower vascularity tend to have, in our experience, a slightly increased incidence of focal necrosis in comparison to single sutures.
6. Make a concerted attempt to keep as much blood as possible off the reinforced skin closure strips throughout the procedure; the less dried blood on the reinforced skin closure strips, the cleaner and better the final closure (Figure, C).
7. Most of these cases involve the forearms and the legs below the knees. Because any increase in pressure or swelling on the wound can result in skin breakdown, postoperative dressing is critical. We use a layered approach; the following sequence can be modified to the preference of the surgeon: Polysporin (bacitracin zinc, polymyxin B sulfate, and petrolatum), nonadherent gauze, paper tape, conforming stretch bandage, and elastic self-adherent wrap. Minimizing swelling and infection are the primary goals. The wrap is left on for 1 week and should be kept dry.
8. Have the patient return to the office in 1 week. Unwrap the entire wound; trim back the reinforced skin closure strips; and have the patient utilize typical wound care at home thereafter consisting of cleaning and application of Polysporin or plain petrolatum, nonadherent gauze, and a paper-tape bandage. Because liquid adhesive is not utilized in this technique, the reinforced skin closure strips can be carefully removed without tearing skin. Leave sutures in for 3 weeks for arm procedures and 4 weeks for leg procedures, unless irritation develops or rapid suture overgrowth occurs in either location.
Complications
Most outcomes after using this technique are typical of optimized linear surgeries, with reduced scarring and complete wound healing (Figure, D). We seldom see complications but the following are possible:
• Bleeding occurs but rarely; the weeklong wrap likely provides great benefit.
• Infection is rare but does occur occasionally, as in any surgical procedure.
• Breakdown of the entire wound is rare; however, we occasionally see focal necrosis near 1 stitch—or rarely 2 stitches—that does not require intervention, apart from longer use of topical Polysporin or petrolatum alone to maximize healing by secondary intention in those small areas.• Despite simple suture placement far from the edge of the wound, wound inversion is seldom a problem because these taut closures have a tendency to expand slightly due to postoperative swelling.
Practice Implications
Any experienced dermatologic surgeon can perfect this technique for closing a wound in thin skin. Because wound closure in areas of fragile skin frequently is encountered in cutaneous surgery, we hope that utilizing this technique results in an optimal outcome for many patients.
- Shuster S, Black MM, McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol. 1975;93:639-643. doi:10.1111/j.1365-2133.1975.tb05113.x
- Molina GE, Yu SH, Neel VA. Observations regarding infection risk in lower-extremity wound healing by second intention. Dermatol Surg. 2020;46:1342-1344. doi:10.1097/DSS.0000000000002094
- Davis M, Nakhdjevani A, Lidder S. Suture/Steri-Strip combination for the management of lacerations in thin-skinned individuals. J Emerg Med. 2011;40:322-323. doi:10.1016/j.jemermed.2010.05.077
Practice Gap
Cutaneous surgery involves many areas where skin is quite thin and fragile, which often is encountered in elderly patients; the forearms and lower legs are the most frequent locations for thin skin.1 Dermatologic surgeons frequently encounter these situations, making this a highly practical arena for technical improvements.
For many of these patients, there is little meaningful dermis for placement of subcutaneous sutures. Therefore, a common approach following surgery, particularly following Mohs micrographic surgery in which tumors and defects typically are larger, is healing by secondary intention.2 Although healing by secondary intention often is a reasonable option, we have found that maximizing the use of epidermal skin for primary closure can be an effective means of closing many such defects. Antimicrobial reinforced skin closure strips have been incorporated in wound closure for thin skin. However, earlier efforts involving reinforcement perpendicular to the wound lacked critical details or used a different technique.3
The Technique
We developed a novel effective closure technique that minimizes these problems. Our technique has been used on the wounds of hundreds of patients with satisfying results. Early on, we used multiple variations to optimize outcomes, including different sizes of sutures and reinforced skin closure strips, application of medical liquid adhesive, liquid adhesive, and varying postoperative dressings. For 3 years, we tracked outcomes in-house and gradually narrowed down our successes into a single, user-friendly paradigm.
Supplies—To perform this technique, required supplies include:
• 2-0 Polypropylene suture with a PS-2 needle, or the equivalent. Polyglactin or silk suture can be utilized if a less-rigid suture is desired; however, we primarily have used polypropylene for repairs with good results. Each repair requires at least 2 sutures.
• Reinforced skin closure strips (1×5 inches). This width affords increased strength.
• Conforming stretch bandage and elastic self-adherent wrap.
• Polysporin (bacitracin zinc, polymyxin B sulfate, and petrolatum)(Johnson & Johnson).
• All usual surgical instruments and supplies, including paper tape and nonadherent gauze (surgeon dependent).
Step-by-step Technique—Close the wound using the following steps:
1. Once the defect is finalized following Mohs micrographic surgery or excision, excise the ellipse to be utilized for the closure and perform complete hemostasis.
2. Place 2 layers of reinforced skin closure strips—one on top of the other—along each side of the defect, leaving approximately 1 cm of uncovered skin between the wound edges and the reinforced skin closure strips (Figure, A).
3. Take a big-bite pulley suture about one-third of the way from one end of the ellipse, with both punctures passing through the reinforced skin closure strips. Leave that in place or have the assistant hold it and wait. Place a second suture immediately adjacent to the pulley suture. Once that suture is placed but still untied, have the assistant carefully pull the pulley suture outward away from the wound edge while you carefully bring the suture together and tie it off gently (Figure, B). Doing this utilizes the pulley ability of the suture to protect the skin from tearing and releases sufficient pressure on the single suture so that it can be easily tightened without risk to the fragile skin.
4. Repeat step 3, this time placing a pulley suture near the midline of the ellipse and the subsequent single suture adjacent to it.
5. Take pulley sutures repeatedly as in steps 3 and 4 until multiple sutures are secured in place. Replace the pulley sutures with single sutures because the double-pulley sutures in areas of lower vascularity tend to have, in our experience, a slightly increased incidence of focal necrosis in comparison to single sutures.
6. Make a concerted attempt to keep as much blood as possible off the reinforced skin closure strips throughout the procedure; the less dried blood on the reinforced skin closure strips, the cleaner and better the final closure (Figure, C).
7. Most of these cases involve the forearms and the legs below the knees. Because any increase in pressure or swelling on the wound can result in skin breakdown, postoperative dressing is critical. We use a layered approach; the following sequence can be modified to the preference of the surgeon: Polysporin (bacitracin zinc, polymyxin B sulfate, and petrolatum), nonadherent gauze, paper tape, conforming stretch bandage, and elastic self-adherent wrap. Minimizing swelling and infection are the primary goals. The wrap is left on for 1 week and should be kept dry.
8. Have the patient return to the office in 1 week. Unwrap the entire wound; trim back the reinforced skin closure strips; and have the patient utilize typical wound care at home thereafter consisting of cleaning and application of Polysporin or plain petrolatum, nonadherent gauze, and a paper-tape bandage. Because liquid adhesive is not utilized in this technique, the reinforced skin closure strips can be carefully removed without tearing skin. Leave sutures in for 3 weeks for arm procedures and 4 weeks for leg procedures, unless irritation develops or rapid suture overgrowth occurs in either location.
Complications
Most outcomes after using this technique are typical of optimized linear surgeries, with reduced scarring and complete wound healing (Figure, D). We seldom see complications but the following are possible:
• Bleeding occurs but rarely; the weeklong wrap likely provides great benefit.
• Infection is rare but does occur occasionally, as in any surgical procedure.
• Breakdown of the entire wound is rare; however, we occasionally see focal necrosis near 1 stitch—or rarely 2 stitches—that does not require intervention, apart from longer use of topical Polysporin or petrolatum alone to maximize healing by secondary intention in those small areas.• Despite simple suture placement far from the edge of the wound, wound inversion is seldom a problem because these taut closures have a tendency to expand slightly due to postoperative swelling.

Practice Implications
Any experienced dermatologic surgeon can perfect this technique for closing a wound in thin skin. Because wound closure in areas of fragile skin frequently is encountered in cutaneous surgery, we hope that utilizing this technique results in an optimal outcome for many patients.
Practice Gap
Cutaneous surgery involves many areas where skin is quite thin and fragile, which often is encountered in elderly patients; the forearms and lower legs are the most frequent locations for thin skin.1 Dermatologic surgeons frequently encounter these situations, making this a highly practical arena for technical improvements.
For many of these patients, there is little meaningful dermis for placement of subcutaneous sutures. Therefore, a common approach following surgery, particularly following Mohs micrographic surgery in which tumors and defects typically are larger, is healing by secondary intention.2 Although healing by secondary intention often is a reasonable option, we have found that maximizing the use of epidermal skin for primary closure can be an effective means of closing many such defects. Antimicrobial reinforced skin closure strips have been incorporated in wound closure for thin skin. However, earlier efforts involving reinforcement perpendicular to the wound lacked critical details or used a different technique.3
The Technique
We developed a novel effective closure technique that minimizes these problems. Our technique has been used on the wounds of hundreds of patients with satisfying results. Early on, we used multiple variations to optimize outcomes, including different sizes of sutures and reinforced skin closure strips, application of medical liquid adhesive, liquid adhesive, and varying postoperative dressings. For 3 years, we tracked outcomes in-house and gradually narrowed down our successes into a single, user-friendly paradigm.
Supplies—To perform this technique, required supplies include:
• 2-0 Polypropylene suture with a PS-2 needle, or the equivalent. Polyglactin or silk suture can be utilized if a less-rigid suture is desired; however, we primarily have used polypropylene for repairs with good results. Each repair requires at least 2 sutures.
• Reinforced skin closure strips (1×5 inches). This width affords increased strength.
• Conforming stretch bandage and elastic self-adherent wrap.
• Polysporin (bacitracin zinc, polymyxin B sulfate, and petrolatum)(Johnson & Johnson).
• All usual surgical instruments and supplies, including paper tape and nonadherent gauze (surgeon dependent).
Step-by-step Technique—Close the wound using the following steps:
1. Once the defect is finalized following Mohs micrographic surgery or excision, excise the ellipse to be utilized for the closure and perform complete hemostasis.
2. Place 2 layers of reinforced skin closure strips—one on top of the other—along each side of the defect, leaving approximately 1 cm of uncovered skin between the wound edges and the reinforced skin closure strips (Figure, A).
3. Take a big-bite pulley suture about one-third of the way from one end of the ellipse, with both punctures passing through the reinforced skin closure strips. Leave that in place or have the assistant hold it and wait. Place a second suture immediately adjacent to the pulley suture. Once that suture is placed but still untied, have the assistant carefully pull the pulley suture outward away from the wound edge while you carefully bring the suture together and tie it off gently (Figure, B). Doing this utilizes the pulley ability of the suture to protect the skin from tearing and releases sufficient pressure on the single suture so that it can be easily tightened without risk to the fragile skin.
4. Repeat step 3, this time placing a pulley suture near the midline of the ellipse and the subsequent single suture adjacent to it.
5. Take pulley sutures repeatedly as in steps 3 and 4 until multiple sutures are secured in place. Replace the pulley sutures with single sutures because the double-pulley sutures in areas of lower vascularity tend to have, in our experience, a slightly increased incidence of focal necrosis in comparison to single sutures.
6. Make a concerted attempt to keep as much blood as possible off the reinforced skin closure strips throughout the procedure; the less dried blood on the reinforced skin closure strips, the cleaner and better the final closure (Figure, C).
7. Most of these cases involve the forearms and the legs below the knees. Because any increase in pressure or swelling on the wound can result in skin breakdown, postoperative dressing is critical. We use a layered approach; the following sequence can be modified to the preference of the surgeon: Polysporin (bacitracin zinc, polymyxin B sulfate, and petrolatum), nonadherent gauze, paper tape, conforming stretch bandage, and elastic self-adherent wrap. Minimizing swelling and infection are the primary goals. The wrap is left on for 1 week and should be kept dry.
8. Have the patient return to the office in 1 week. Unwrap the entire wound; trim back the reinforced skin closure strips; and have the patient utilize typical wound care at home thereafter consisting of cleaning and application of Polysporin or plain petrolatum, nonadherent gauze, and a paper-tape bandage. Because liquid adhesive is not utilized in this technique, the reinforced skin closure strips can be carefully removed without tearing skin. Leave sutures in for 3 weeks for arm procedures and 4 weeks for leg procedures, unless irritation develops or rapid suture overgrowth occurs in either location.
Complications
Most outcomes after using this technique are typical of optimized linear surgeries, with reduced scarring and complete wound healing (Figure, D). We seldom see complications but the following are possible:
• Bleeding occurs but rarely; the weeklong wrap likely provides great benefit.
• Infection is rare but does occur occasionally, as in any surgical procedure.
• Breakdown of the entire wound is rare; however, we occasionally see focal necrosis near 1 stitch—or rarely 2 stitches—that does not require intervention, apart from longer use of topical Polysporin or petrolatum alone to maximize healing by secondary intention in those small areas.• Despite simple suture placement far from the edge of the wound, wound inversion is seldom a problem because these taut closures have a tendency to expand slightly due to postoperative swelling.

Practice Implications
Any experienced dermatologic surgeon can perfect this technique for closing a wound in thin skin. Because wound closure in areas of fragile skin frequently is encountered in cutaneous surgery, we hope that utilizing this technique results in an optimal outcome for many patients.
- Shuster S, Black MM, McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol. 1975;93:639-643. doi:10.1111/j.1365-2133.1975.tb05113.x
- Molina GE, Yu SH, Neel VA. Observations regarding infection risk in lower-extremity wound healing by second intention. Dermatol Surg. 2020;46:1342-1344. doi:10.1097/DSS.0000000000002094
- Davis M, Nakhdjevani A, Lidder S. Suture/Steri-Strip combination for the management of lacerations in thin-skinned individuals. J Emerg Med. 2011;40:322-323. doi:10.1016/j.jemermed.2010.05.077
- Shuster S, Black MM, McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol. 1975;93:639-643. doi:10.1111/j.1365-2133.1975.tb05113.x
- Molina GE, Yu SH, Neel VA. Observations regarding infection risk in lower-extremity wound healing by second intention. Dermatol Surg. 2020;46:1342-1344. doi:10.1097/DSS.0000000000002094
- Davis M, Nakhdjevani A, Lidder S. Suture/Steri-Strip combination for the management of lacerations in thin-skinned individuals. J Emerg Med. 2011;40:322-323. doi:10.1016/j.jemermed.2010.05.077
Leflunomide ups RA-ILD progression risk in patients with severe ILD
Key clinical point: The use of conventional disease-modifying antirheumatic drugs did not increase the risk for rheumatoid arthritis-associated interstitial lung disease (RA-ILD) progression, except leflunomide, which increased the risk for ILD-progression in patients with severe ILD.
Major finding: Overall, the use vs no use of methotrexate, tacrolimus, or leflunomide was not associated with an increased risk for ILD progression; however, leflunomide significantly increased the risk for ILD progression in patients with reduced lung function (adjusted hazard ratio 8.42; 95% CI 2.61-27.15).
Study details: This prospective cohort study included 143 patients with RA-ILD who received methotrexate, leflunomide, or tacrolimus.
Disclosures: This study was supported by Seoul National University Hospital and Korea Health Technology R&D Project funded by the Ministry of Health and Welfare, Republic of Korea. The authors declared no conflicts of interest.
Source: Kim J-W et al. Methotrexate, leflunomide, and tacrolimus use and the progression of rheumatoid arthritis-associated interstitial lung disease. Rheumatology (Oxford). 2022 (Nov 17). Doi: 10.1093/rheumatology/keac651
Key clinical point: The use of conventional disease-modifying antirheumatic drugs did not increase the risk for rheumatoid arthritis-associated interstitial lung disease (RA-ILD) progression, except leflunomide, which increased the risk for ILD-progression in patients with severe ILD.
Major finding: Overall, the use vs no use of methotrexate, tacrolimus, or leflunomide was not associated with an increased risk for ILD progression; however, leflunomide significantly increased the risk for ILD progression in patients with reduced lung function (adjusted hazard ratio 8.42; 95% CI 2.61-27.15).
Study details: This prospective cohort study included 143 patients with RA-ILD who received methotrexate, leflunomide, or tacrolimus.
Disclosures: This study was supported by Seoul National University Hospital and Korea Health Technology R&D Project funded by the Ministry of Health and Welfare, Republic of Korea. The authors declared no conflicts of interest.
Source: Kim J-W et al. Methotrexate, leflunomide, and tacrolimus use and the progression of rheumatoid arthritis-associated interstitial lung disease. Rheumatology (Oxford). 2022 (Nov 17). Doi: 10.1093/rheumatology/keac651
Key clinical point: The use of conventional disease-modifying antirheumatic drugs did not increase the risk for rheumatoid arthritis-associated interstitial lung disease (RA-ILD) progression, except leflunomide, which increased the risk for ILD-progression in patients with severe ILD.
Major finding: Overall, the use vs no use of methotrexate, tacrolimus, or leflunomide was not associated with an increased risk for ILD progression; however, leflunomide significantly increased the risk for ILD progression in patients with reduced lung function (adjusted hazard ratio 8.42; 95% CI 2.61-27.15).
Study details: This prospective cohort study included 143 patients with RA-ILD who received methotrexate, leflunomide, or tacrolimus.
Disclosures: This study was supported by Seoul National University Hospital and Korea Health Technology R&D Project funded by the Ministry of Health and Welfare, Republic of Korea. The authors declared no conflicts of interest.
Source: Kim J-W et al. Methotrexate, leflunomide, and tacrolimus use and the progression of rheumatoid arthritis-associated interstitial lung disease. Rheumatology (Oxford). 2022 (Nov 17). Doi: 10.1093/rheumatology/keac651
Genetic test identifies parental source of cancer variant
A new hereditary test can determine whether a cancer-disposing gene was inherited from a patient’s father or mother without the need for parental DNA, potentially improving disease screening and management.
“The presence of parental imprints in regions of the genome has been known for a long time,” study author Peter Lansdorp, MD, PhD, of the BC Cancer Research Centre in Vancouver, said in an interview. In addition, the ability of a specific sequencing technology (Strand-seq) to generate a set of DNA variants that tend to be inherited together from a single parent has been documented in several studies.
“That these two pieces can be put together to assign alleles in a patient to one of the parents without studying the DNA of the parents is a major advance,” said Dr. Lansdorp.
Principal author Steven J.M. Jones, PhD, associate director of bioinformatics at BC Cancer Research Centre, explained, “for directing cascade genetic testing, the test could be used almost immediately, even as a research test. It just guides which side of the family to focus familial genetic testing efforts on and is internally validated by the patient’s variant and later confirmed by clinical testing in the family.”
Dr. Jones added, especially when parents are deceased or unavailable.
The study was published online in Cell Genomics.
Low error rate
Determining a parent of origin for hereditary variants “is essential to evaluate disease risk when a pathogenic variant has PofO effects, that is, when a patient’s risk of disease depends on from which parent it is inherited,” the authors wrote. An example is hereditary paraganglioma-pheochromocytoma syndrome as a result of pathogenic variants in SDHD or SDHAF2 genes. Individuals with the variants are at high risk of developing certain cancers, but only if a defective gene is inherited from their father. If inherited from their mother, there is no increased risk.
The new method relies on a technique called “phased DNA methylation” at maternally and paternally imprinted gene loci, as well as chromosome length phasing of DNA sequences.
The team used five human genome “trios” – two parents and the proband (the first person in a family to receive genetic testing or counseling for a suspected hereditary risk) – to pilot the approach. They showed that the method can correctly identify the PofO with an average mismatch error rate of 0.31% for single nucleotide variants and 1.89% for insertions or deletions (indels).
“We will need to validate this technology for different genes in real-world samples from individuals of diverse backgrounds,” said Dr. Jones. The first step is to validate the technology in scenarios with immediate clinical utility, like with SDHD, where lifelong medical management is affected by knowledge of whether the variant was inherited from the mother or father.
“We would also like to quickly validate this for common hereditary cancer genes, like BRCA1, BRCA2, and Lynch syndrome–associated genes, where prediction of PofO may improve low rates of genetic testing in family members by providing more accurate estimates of their risk to carry the familial variant.”
Challenges to moving the test to the clinic, Dr. Jones said, include scaling up the technology, demonstrating clinical and economic utility, compared with existing testing approaches, “and familiarizing clinicians with a new type of test that will routinely give this added dimension of information.”
‘Tremendously promising technology’
Pathologist Stephen Yip, MD, PhD, of the Vancouver Coastal Health Research Institute, who was not involved in the study but disclosed that he collaborates with the authors on other grant-funded projects, said in a comment that “this is a tremendously promising technology that has immediate practical implications in the investigation of PofO of a pathogenic locus, particularly when genetic material is available only from the proband.”
However, “rigorous validation against the current gold standard of short-reading, next-generation sequencing of trios is needed prior to clinical deployment,” he said. “This will take time and effort. However, the promise of this technology is worth the effort.
“Also, there is the possibility of uncovering novel genetics during testing, which could present an ethical dilemma,” he noted. “A robust consenting and ethical framework and early involvement of an ethicist would be helpful.”
Research in Dr. Lansdorp’s laboratory is funded by the Terry Fox Research Institute, the Canadian Institutes of Health Research, the Canadian Foundation for Innovation, and the government of British Columbia. Dr. Lansdorp, Dr. Jones, and Dr. Yip reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new hereditary test can determine whether a cancer-disposing gene was inherited from a patient’s father or mother without the need for parental DNA, potentially improving disease screening and management.
“The presence of parental imprints in regions of the genome has been known for a long time,” study author Peter Lansdorp, MD, PhD, of the BC Cancer Research Centre in Vancouver, said in an interview. In addition, the ability of a specific sequencing technology (Strand-seq) to generate a set of DNA variants that tend to be inherited together from a single parent has been documented in several studies.
“That these two pieces can be put together to assign alleles in a patient to one of the parents without studying the DNA of the parents is a major advance,” said Dr. Lansdorp.
Principal author Steven J.M. Jones, PhD, associate director of bioinformatics at BC Cancer Research Centre, explained, “for directing cascade genetic testing, the test could be used almost immediately, even as a research test. It just guides which side of the family to focus familial genetic testing efforts on and is internally validated by the patient’s variant and later confirmed by clinical testing in the family.”
Dr. Jones added, especially when parents are deceased or unavailable.
The study was published online in Cell Genomics.
Low error rate
Determining a parent of origin for hereditary variants “is essential to evaluate disease risk when a pathogenic variant has PofO effects, that is, when a patient’s risk of disease depends on from which parent it is inherited,” the authors wrote. An example is hereditary paraganglioma-pheochromocytoma syndrome as a result of pathogenic variants in SDHD or SDHAF2 genes. Individuals with the variants are at high risk of developing certain cancers, but only if a defective gene is inherited from their father. If inherited from their mother, there is no increased risk.
The new method relies on a technique called “phased DNA methylation” at maternally and paternally imprinted gene loci, as well as chromosome length phasing of DNA sequences.
The team used five human genome “trios” – two parents and the proband (the first person in a family to receive genetic testing or counseling for a suspected hereditary risk) – to pilot the approach. They showed that the method can correctly identify the PofO with an average mismatch error rate of 0.31% for single nucleotide variants and 1.89% for insertions or deletions (indels).
“We will need to validate this technology for different genes in real-world samples from individuals of diverse backgrounds,” said Dr. Jones. The first step is to validate the technology in scenarios with immediate clinical utility, like with SDHD, where lifelong medical management is affected by knowledge of whether the variant was inherited from the mother or father.
“We would also like to quickly validate this for common hereditary cancer genes, like BRCA1, BRCA2, and Lynch syndrome–associated genes, where prediction of PofO may improve low rates of genetic testing in family members by providing more accurate estimates of their risk to carry the familial variant.”
Challenges to moving the test to the clinic, Dr. Jones said, include scaling up the technology, demonstrating clinical and economic utility, compared with existing testing approaches, “and familiarizing clinicians with a new type of test that will routinely give this added dimension of information.”
‘Tremendously promising technology’
Pathologist Stephen Yip, MD, PhD, of the Vancouver Coastal Health Research Institute, who was not involved in the study but disclosed that he collaborates with the authors on other grant-funded projects, said in a comment that “this is a tremendously promising technology that has immediate practical implications in the investigation of PofO of a pathogenic locus, particularly when genetic material is available only from the proband.”
However, “rigorous validation against the current gold standard of short-reading, next-generation sequencing of trios is needed prior to clinical deployment,” he said. “This will take time and effort. However, the promise of this technology is worth the effort.
“Also, there is the possibility of uncovering novel genetics during testing, which could present an ethical dilemma,” he noted. “A robust consenting and ethical framework and early involvement of an ethicist would be helpful.”
Research in Dr. Lansdorp’s laboratory is funded by the Terry Fox Research Institute, the Canadian Institutes of Health Research, the Canadian Foundation for Innovation, and the government of British Columbia. Dr. Lansdorp, Dr. Jones, and Dr. Yip reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new hereditary test can determine whether a cancer-disposing gene was inherited from a patient’s father or mother without the need for parental DNA, potentially improving disease screening and management.
“The presence of parental imprints in regions of the genome has been known for a long time,” study author Peter Lansdorp, MD, PhD, of the BC Cancer Research Centre in Vancouver, said in an interview. In addition, the ability of a specific sequencing technology (Strand-seq) to generate a set of DNA variants that tend to be inherited together from a single parent has been documented in several studies.
“That these two pieces can be put together to assign alleles in a patient to one of the parents without studying the DNA of the parents is a major advance,” said Dr. Lansdorp.
Principal author Steven J.M. Jones, PhD, associate director of bioinformatics at BC Cancer Research Centre, explained, “for directing cascade genetic testing, the test could be used almost immediately, even as a research test. It just guides which side of the family to focus familial genetic testing efforts on and is internally validated by the patient’s variant and later confirmed by clinical testing in the family.”
Dr. Jones added, especially when parents are deceased or unavailable.
The study was published online in Cell Genomics.
Low error rate
Determining a parent of origin for hereditary variants “is essential to evaluate disease risk when a pathogenic variant has PofO effects, that is, when a patient’s risk of disease depends on from which parent it is inherited,” the authors wrote. An example is hereditary paraganglioma-pheochromocytoma syndrome as a result of pathogenic variants in SDHD or SDHAF2 genes. Individuals with the variants are at high risk of developing certain cancers, but only if a defective gene is inherited from their father. If inherited from their mother, there is no increased risk.
The new method relies on a technique called “phased DNA methylation” at maternally and paternally imprinted gene loci, as well as chromosome length phasing of DNA sequences.
The team used five human genome “trios” – two parents and the proband (the first person in a family to receive genetic testing or counseling for a suspected hereditary risk) – to pilot the approach. They showed that the method can correctly identify the PofO with an average mismatch error rate of 0.31% for single nucleotide variants and 1.89% for insertions or deletions (indels).
“We will need to validate this technology for different genes in real-world samples from individuals of diverse backgrounds,” said Dr. Jones. The first step is to validate the technology in scenarios with immediate clinical utility, like with SDHD, where lifelong medical management is affected by knowledge of whether the variant was inherited from the mother or father.
“We would also like to quickly validate this for common hereditary cancer genes, like BRCA1, BRCA2, and Lynch syndrome–associated genes, where prediction of PofO may improve low rates of genetic testing in family members by providing more accurate estimates of their risk to carry the familial variant.”
Challenges to moving the test to the clinic, Dr. Jones said, include scaling up the technology, demonstrating clinical and economic utility, compared with existing testing approaches, “and familiarizing clinicians with a new type of test that will routinely give this added dimension of information.”
‘Tremendously promising technology’
Pathologist Stephen Yip, MD, PhD, of the Vancouver Coastal Health Research Institute, who was not involved in the study but disclosed that he collaborates with the authors on other grant-funded projects, said in a comment that “this is a tremendously promising technology that has immediate practical implications in the investigation of PofO of a pathogenic locus, particularly when genetic material is available only from the proband.”
However, “rigorous validation against the current gold standard of short-reading, next-generation sequencing of trios is needed prior to clinical deployment,” he said. “This will take time and effort. However, the promise of this technology is worth the effort.
“Also, there is the possibility of uncovering novel genetics during testing, which could present an ethical dilemma,” he noted. “A robust consenting and ethical framework and early involvement of an ethicist would be helpful.”
Research in Dr. Lansdorp’s laboratory is funded by the Terry Fox Research Institute, the Canadian Institutes of Health Research, the Canadian Foundation for Innovation, and the government of British Columbia. Dr. Lansdorp, Dr. Jones, and Dr. Yip reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM CELL GENOMICS
Double CF combination safe, effective in 1- to 2-year-olds
In children with cystic fibrosis (CF) and homozygous for the F508 mutation, the combination of lumacaftor and ivacaftor appears generally safe, and biomarker data suggest its efficacy, according to results from a new open-label phase 3 trial.
In September, the Food and Drug Administration approved lumacaftor/ivacaftor for patients aged 1 years and above. Waiting in the wings is the triple combination of elexacaftor, tezacaftor, and ivacaftor (ETI), which is available for patients with at least one copy of the F508 mutation aged 6 and over. ETI is also being tested in younger patients.
One driving factor for early treatment is countering the malnutrition that can occur among CF patients because of poor pancreatic insufficiency and chronic inflammation. “We’ve known for many years that (being) at or above average body weight and height predict better lung function. And we’ve known for quite a while that the height-for-age percentile, in preschool years, actually predicts your lung function later, and how long you’re going to live, so nutrition is incredibly important,” said study author Susanna McColley, MD, in an interview. It’s also difficult to use lung function tests in young children, since even adults can find them challenging, she said.
“FEV1 [forced expiratory volume in 1 second] is the strongest predictor of survival, and then nutrition is the highest predictor of FEV1, so that’s kind of the construct. They had similar improvement in the functional measures of their pancreas and in the measures of inflammation in the gut. I think the story here is that starting a modulator early has a likelihood to have positive health effects that go forward. We can’t say that from the data in the paper. It’s a 24-week study, but looking at the pancreatic and intestinal functioning and also the fact that there was a decrease in sweat chloride is important,” said Dr. McColley, who is a professor of pediatrics in pulmonary and sleep medicine at Northwestern University, Chicago.
The study adds more evidence that earlier treatment in CF may lead to better outcomes, but the digestive improvements are an overlooked factor, according to Dr. McColley. “Even with early treatment, and even with pancreatic enzymes and supplements taken to digest food, it’s a huge burden. When they come in and say that digestion seems better, there is less bloating, things like that, these are the things that aren’t captured so much in the clinical trial data, but they’re meaningful to families,” she said.
Single-agent ivacaftor is available for children as young as 4 months, but is limited to patients with the G551D gating mutation. Most young children with CF can only be treated for symptoms.
The lack of new safety signals in the new study is reassuring, and the research presents some hope to young children who are not yet eligible to receive ETI, according to Carlos Milla, MD, who is a pediatric pulmonary physician at Stanford (Calif.) University. “We already know that the next version of this drug is much more efficacious, the triple-combination therapy. It’s a little bit like we’re falling behind when it comes to treating these young kids because we are offering right now what we know is a less effective drug as opposed to the ones that are available now down to age 6, and hopefully sometime soon down to age 2. It’s better [than] to have no treatment at all, so it’s a good start,” said Dr. Milla.
“I think this is a great bridge for babies while they’re waiting to grow up to be old enough to get [triple combination therapy] and will prevent some of the complications until they can get the even more highly effective therapy in the future,” said Jennifer Taylor-Cousar, MD, who was asked to comment on the study. Dr. Taylor-Cousar is codirector of the Adult CF Program at National Jewish Health, Denver.
She also noted that the therapy could rapidly become more important. Since the approval of elexacaftor/tezacaftor/ivacaftor in 2019, pregnancies in women with CF have increased markedly. There were 310 such pregnancies in 2019, and 675 in 2020 after the combination became generally available in November of 2019. Many of the resulting babies had false-negative CF diagnoses because the mother was taking the triple combination and the medication crossed the placenta and prevented disease progression. The drugs are present in breast milk, but when breastfeeding isn’t possible, newborns are left without a therapeutic option. “There was no approval for babies who had two copies of F508. This helps tremendously with that albeit small population, although I suspect it may grow larger over the upcoming years as we continue to see so many pregnancies in women with CF because they are so much healthier,” said Dr. Taylor-Cousar.
The study was a phase 3, open-label trial with a cohort aged 18-24 months (cohort 1, n = 14) and another aged 12-18 months (cohort 2, n = 46). Participants received a 15-day treatment with a dose based on weight at screening. Participants then underwent a 24-week treatment period with a dose determined by pharmacokinetic data collected during the initial treatment, the authors wrote in the American Journal of Respiratory and Critical Care Medicine.
A total of 95.7% of children experienced adverse events during the 24-week treatment period; 52.2% of events were mild, and 39.1% were moderate. The most frequent adverse events were cough (34.8%), infective exacerbation of CF (21.7%), pyrexia (21.7%), and vomiting (17.4%); 10.9% had elevations of alanine aminotransferase and/or aspartate aminotransferase higher than three times the upper limit of normal, and one (2.2%) had concentrations of both high enough that the study drug was discontinued.
There were significant reductions in sweat chloride concentration at week 24, suggesting strong efficacy (–29.1 mmol/L; 95% confidence interval, –34.8 to –23.4 mmol/L). Body mass, weight, and length remained normal during the 24-week treatment period, and there were trends towards improvement in biomarkers of pancreatic function and intestinal inflammation, including fecal elastase-1 (+73.1mcg/g; 95% CI, 29.40-116.80 mcg/g), serum immunoreactive trypsinogen (–295.50 mcg/g; 95% CI, –416.60 to –174.50 mcg/g), and fecal calprotectin (–106.63 mg/kg; 95% CI, –180.60 to –32.66 mg/kg)
Dr. McColley, Dr. Taylor-Cousar, and Dr. Milla have no relevant financial disclosures. The study was funded by Merck.
In children with cystic fibrosis (CF) and homozygous for the F508 mutation, the combination of lumacaftor and ivacaftor appears generally safe, and biomarker data suggest its efficacy, according to results from a new open-label phase 3 trial.
In September, the Food and Drug Administration approved lumacaftor/ivacaftor for patients aged 1 years and above. Waiting in the wings is the triple combination of elexacaftor, tezacaftor, and ivacaftor (ETI), which is available for patients with at least one copy of the F508 mutation aged 6 and over. ETI is also being tested in younger patients.
One driving factor for early treatment is countering the malnutrition that can occur among CF patients because of poor pancreatic insufficiency and chronic inflammation. “We’ve known for many years that (being) at or above average body weight and height predict better lung function. And we’ve known for quite a while that the height-for-age percentile, in preschool years, actually predicts your lung function later, and how long you’re going to live, so nutrition is incredibly important,” said study author Susanna McColley, MD, in an interview. It’s also difficult to use lung function tests in young children, since even adults can find them challenging, she said.
“FEV1 [forced expiratory volume in 1 second] is the strongest predictor of survival, and then nutrition is the highest predictor of FEV1, so that’s kind of the construct. They had similar improvement in the functional measures of their pancreas and in the measures of inflammation in the gut. I think the story here is that starting a modulator early has a likelihood to have positive health effects that go forward. We can’t say that from the data in the paper. It’s a 24-week study, but looking at the pancreatic and intestinal functioning and also the fact that there was a decrease in sweat chloride is important,” said Dr. McColley, who is a professor of pediatrics in pulmonary and sleep medicine at Northwestern University, Chicago.
The study adds more evidence that earlier treatment in CF may lead to better outcomes, but the digestive improvements are an overlooked factor, according to Dr. McColley. “Even with early treatment, and even with pancreatic enzymes and supplements taken to digest food, it’s a huge burden. When they come in and say that digestion seems better, there is less bloating, things like that, these are the things that aren’t captured so much in the clinical trial data, but they’re meaningful to families,” she said.
Single-agent ivacaftor is available for children as young as 4 months, but is limited to patients with the G551D gating mutation. Most young children with CF can only be treated for symptoms.
The lack of new safety signals in the new study is reassuring, and the research presents some hope to young children who are not yet eligible to receive ETI, according to Carlos Milla, MD, who is a pediatric pulmonary physician at Stanford (Calif.) University. “We already know that the next version of this drug is much more efficacious, the triple-combination therapy. It’s a little bit like we’re falling behind when it comes to treating these young kids because we are offering right now what we know is a less effective drug as opposed to the ones that are available now down to age 6, and hopefully sometime soon down to age 2. It’s better [than] to have no treatment at all, so it’s a good start,” said Dr. Milla.
“I think this is a great bridge for babies while they’re waiting to grow up to be old enough to get [triple combination therapy] and will prevent some of the complications until they can get the even more highly effective therapy in the future,” said Jennifer Taylor-Cousar, MD, who was asked to comment on the study. Dr. Taylor-Cousar is codirector of the Adult CF Program at National Jewish Health, Denver.
She also noted that the therapy could rapidly become more important. Since the approval of elexacaftor/tezacaftor/ivacaftor in 2019, pregnancies in women with CF have increased markedly. There were 310 such pregnancies in 2019, and 675 in 2020 after the combination became generally available in November of 2019. Many of the resulting babies had false-negative CF diagnoses because the mother was taking the triple combination and the medication crossed the placenta and prevented disease progression. The drugs are present in breast milk, but when breastfeeding isn’t possible, newborns are left without a therapeutic option. “There was no approval for babies who had two copies of F508. This helps tremendously with that albeit small population, although I suspect it may grow larger over the upcoming years as we continue to see so many pregnancies in women with CF because they are so much healthier,” said Dr. Taylor-Cousar.
The study was a phase 3, open-label trial with a cohort aged 18-24 months (cohort 1, n = 14) and another aged 12-18 months (cohort 2, n = 46). Participants received a 15-day treatment with a dose based on weight at screening. Participants then underwent a 24-week treatment period with a dose determined by pharmacokinetic data collected during the initial treatment, the authors wrote in the American Journal of Respiratory and Critical Care Medicine.
A total of 95.7% of children experienced adverse events during the 24-week treatment period; 52.2% of events were mild, and 39.1% were moderate. The most frequent adverse events were cough (34.8%), infective exacerbation of CF (21.7%), pyrexia (21.7%), and vomiting (17.4%); 10.9% had elevations of alanine aminotransferase and/or aspartate aminotransferase higher than three times the upper limit of normal, and one (2.2%) had concentrations of both high enough that the study drug was discontinued.
There were significant reductions in sweat chloride concentration at week 24, suggesting strong efficacy (–29.1 mmol/L; 95% confidence interval, –34.8 to –23.4 mmol/L). Body mass, weight, and length remained normal during the 24-week treatment period, and there were trends towards improvement in biomarkers of pancreatic function and intestinal inflammation, including fecal elastase-1 (+73.1mcg/g; 95% CI, 29.40-116.80 mcg/g), serum immunoreactive trypsinogen (–295.50 mcg/g; 95% CI, –416.60 to –174.50 mcg/g), and fecal calprotectin (–106.63 mg/kg; 95% CI, –180.60 to –32.66 mg/kg)
Dr. McColley, Dr. Taylor-Cousar, and Dr. Milla have no relevant financial disclosures. The study was funded by Merck.
In children with cystic fibrosis (CF) and homozygous for the F508 mutation, the combination of lumacaftor and ivacaftor appears generally safe, and biomarker data suggest its efficacy, according to results from a new open-label phase 3 trial.
In September, the Food and Drug Administration approved lumacaftor/ivacaftor for patients aged 1 years and above. Waiting in the wings is the triple combination of elexacaftor, tezacaftor, and ivacaftor (ETI), which is available for patients with at least one copy of the F508 mutation aged 6 and over. ETI is also being tested in younger patients.
One driving factor for early treatment is countering the malnutrition that can occur among CF patients because of poor pancreatic insufficiency and chronic inflammation. “We’ve known for many years that (being) at or above average body weight and height predict better lung function. And we’ve known for quite a while that the height-for-age percentile, in preschool years, actually predicts your lung function later, and how long you’re going to live, so nutrition is incredibly important,” said study author Susanna McColley, MD, in an interview. It’s also difficult to use lung function tests in young children, since even adults can find them challenging, she said.
“FEV1 [forced expiratory volume in 1 second] is the strongest predictor of survival, and then nutrition is the highest predictor of FEV1, so that’s kind of the construct. They had similar improvement in the functional measures of their pancreas and in the measures of inflammation in the gut. I think the story here is that starting a modulator early has a likelihood to have positive health effects that go forward. We can’t say that from the data in the paper. It’s a 24-week study, but looking at the pancreatic and intestinal functioning and also the fact that there was a decrease in sweat chloride is important,” said Dr. McColley, who is a professor of pediatrics in pulmonary and sleep medicine at Northwestern University, Chicago.
The study adds more evidence that earlier treatment in CF may lead to better outcomes, but the digestive improvements are an overlooked factor, according to Dr. McColley. “Even with early treatment, and even with pancreatic enzymes and supplements taken to digest food, it’s a huge burden. When they come in and say that digestion seems better, there is less bloating, things like that, these are the things that aren’t captured so much in the clinical trial data, but they’re meaningful to families,” she said.
Single-agent ivacaftor is available for children as young as 4 months, but is limited to patients with the G551D gating mutation. Most young children with CF can only be treated for symptoms.
The lack of new safety signals in the new study is reassuring, and the research presents some hope to young children who are not yet eligible to receive ETI, according to Carlos Milla, MD, who is a pediatric pulmonary physician at Stanford (Calif.) University. “We already know that the next version of this drug is much more efficacious, the triple-combination therapy. It’s a little bit like we’re falling behind when it comes to treating these young kids because we are offering right now what we know is a less effective drug as opposed to the ones that are available now down to age 6, and hopefully sometime soon down to age 2. It’s better [than] to have no treatment at all, so it’s a good start,” said Dr. Milla.
“I think this is a great bridge for babies while they’re waiting to grow up to be old enough to get [triple combination therapy] and will prevent some of the complications until they can get the even more highly effective therapy in the future,” said Jennifer Taylor-Cousar, MD, who was asked to comment on the study. Dr. Taylor-Cousar is codirector of the Adult CF Program at National Jewish Health, Denver.
She also noted that the therapy could rapidly become more important. Since the approval of elexacaftor/tezacaftor/ivacaftor in 2019, pregnancies in women with CF have increased markedly. There were 310 such pregnancies in 2019, and 675 in 2020 after the combination became generally available in November of 2019. Many of the resulting babies had false-negative CF diagnoses because the mother was taking the triple combination and the medication crossed the placenta and prevented disease progression. The drugs are present in breast milk, but when breastfeeding isn’t possible, newborns are left without a therapeutic option. “There was no approval for babies who had two copies of F508. This helps tremendously with that albeit small population, although I suspect it may grow larger over the upcoming years as we continue to see so many pregnancies in women with CF because they are so much healthier,” said Dr. Taylor-Cousar.
The study was a phase 3, open-label trial with a cohort aged 18-24 months (cohort 1, n = 14) and another aged 12-18 months (cohort 2, n = 46). Participants received a 15-day treatment with a dose based on weight at screening. Participants then underwent a 24-week treatment period with a dose determined by pharmacokinetic data collected during the initial treatment, the authors wrote in the American Journal of Respiratory and Critical Care Medicine.
A total of 95.7% of children experienced adverse events during the 24-week treatment period; 52.2% of events were mild, and 39.1% were moderate. The most frequent adverse events were cough (34.8%), infective exacerbation of CF (21.7%), pyrexia (21.7%), and vomiting (17.4%); 10.9% had elevations of alanine aminotransferase and/or aspartate aminotransferase higher than three times the upper limit of normal, and one (2.2%) had concentrations of both high enough that the study drug was discontinued.
There were significant reductions in sweat chloride concentration at week 24, suggesting strong efficacy (–29.1 mmol/L; 95% confidence interval, –34.8 to –23.4 mmol/L). Body mass, weight, and length remained normal during the 24-week treatment period, and there were trends towards improvement in biomarkers of pancreatic function and intestinal inflammation, including fecal elastase-1 (+73.1mcg/g; 95% CI, 29.40-116.80 mcg/g), serum immunoreactive trypsinogen (–295.50 mcg/g; 95% CI, –416.60 to –174.50 mcg/g), and fecal calprotectin (–106.63 mg/kg; 95% CI, –180.60 to –32.66 mg/kg)
Dr. McColley, Dr. Taylor-Cousar, and Dr. Milla have no relevant financial disclosures. The study was funded by Merck.
FROM THE AMERICAN JOURNAL OF RESPIRATORY AND CRITICAL CARE MEDICINE
ED docs need a clearer path to outpatient primary care
It was 2 p.m. on a sunny and humid day, 1 hour before my 8-hour shift was over in the emergency department at the community hospital that I was rotating through. It was part of my fourth-year emergency medicine (EM) clerkship. Not that I would have noticed the weather, save for the few seconds the sliding door to the ED would open periodically, as if on its own cadence, with the sounds of stomping boots and a rolling gurney making its way through.
We were busy. At this particular hospital, I was told, EM volume is already up 35% this year compared with the previous year, and bed holds had been hitting new highs each week.
One more hour until my shift is over and a poor soul will take over my computer, seat, and the chaos.
I took a glance at the electronic health record again, seeing whether there was anything I could do to discharge any of the patients to relieve some of the strain. Knee pain, toe pain, headache/migraine, shoulder pain, elevated blood pressure. Although it’s true that any of these listed complaints could have emergent etiologies, the truth was that all of these patients were here owing to exacerbations of chronic issues. And yet most, if not all, of these patients had been here for nearly 8 hours, some even longer, waiting for treatment and exacerbating an already busy ED.
“I don’t understand. Couldn’t these patients have sought care outpatient with their PCP [primary care physician]? It would have been a lot cheaper and faster.” I asked. A seasoned ED physician, bald, graying, and whom I had just met today and hadn’t spoken to much until this very moment, turned to me and said: “We have become the dumping ground for primary care complaints.”
‘Go to the ED’
“PCPs are already too busy,” the physician continued. “It’s just easier to say: ‘Go to the ED. They’ll take care of it.’ ”
He continued: “In my 30 years of practicing, emergency medicine has changed so much. When I first started in the 1980s, I was only seeing emergencies, and it was fun. Now, 80% of my patients are primary care complaints. These days, I am more of a primary care physician than an emergency physician.”
Hmmm, I thought. Was this physician burned out and jaded? Quite possibly. Was this change the physician experienced throughout his career more likely attributed to a capitalist-run, profit-driven health care system and its cohort of underinsured and noninsured citizens? Certainly. I’m only a fourth-year medical student, so my view of the situation is no doubt limited.
But something he said definitely rang my bell: I’m more of a primary care physician than an emergency physician. That is an argument I can consider. Whether it is caused by poorly designed reimbursement schedules or the state of America’s profit-driven health care system, which effectively makes these physicians double as PCPs on a daily basis.
I let this thought ruminate on my drive home, along with how there’s a such a huge demand for PCPs, resulting in it taking up to 3 months to get an appointment with one. That’s crazy, and I understand the need to come to the ED where you’ll (hopefully) be seen the same day.
I also ruminated on how emergency physicians have the highest rate of burnout among all the specialties, with no career recourse afterward. Either you’re part of the hospital machine complex, or you’re out. Practicing EM for nearly 30 years is apparently a rarity these days. Most emergency physicians last 5 years, 10 years tops, and then are so burned out that they retire to pursue a life outside of medicine (real estate seems to be popular). But this is a shame.
Emergency docs exiting medicine
Emergency physicians have seen a ton of wildly different pathologies and have treated a variety of different conditions, including conditions usually reserved for primary care. To let knowledgeable, experienced emergency physicians just exit medicine, with no recourse to further contribute to this country’s health system outside of the hospital machine, is a travesty in its own right.
I ruminated further on the 2021 American College of Emergency Physicians 2021 report on the EM physician workforce, which stated that there is projected to be an oversupply of emergency physicians by 2030, leaving thousands of them out of work. No doubt that report has left an impact on the volume of residency applications into EM in 2021. No one wants to go through residency and be unemployed at the end of it.
And finally, I ruminated on the sheer volume of patients visiting EDs across the country. Patient volumes are up, wait times in general are up, wait times in the ED are up, and bed holds to get admitted are hitting highs across the country each week. The deluge of patients visiting the ED is not getting better, and it’s only likely to get worse as the population ages.
It’s time to offer emergency physicians a path to outpatient primary care.
Now before I get hung for this suggestion (“I went into EM precisely not to do outpatient care!”), hear me out: Such a path should be offered via a 1-year accelerated fellowship and will allow emergency physicians to practice outpatient primary care medicine independently. And although working in urgent care centers is already an option, the opportunity to own and operate their own primary care practice should also exist.
In my humble opinion, by offering such a path, the following objectives could be accomplished:
Alleviate the pressure on primary care medicine in the United States. It’s no secret that the United States needs more primary care physicians. Allowing emergency physicians who got burnt out by hospital life an alternative way to serve their community and country via outpatient primary care would greatly alleviate the pressure on the need for PCPs today.
Provide an alternative career path for emergency physicians. We would be doing a disservice if we don’t offer emergency physicians a way to revive their burnout and utilize their skill set in a post-ED life. Outpatient primary care is the perfect way to do this, and it’s a win-win-win on several fronts: We need more outpatient physicians, they need an opportunity to flex their knowledge in an alternative setting.
Solve the “ACEP” problem. The ACEP report scared medical students away from applying to residency in EM. Who wants to go through 3 years of residency only to be unemployed at the end of it? By offering a path to outpatient primary care, we can offer an important and viable path for those emergency physicians who would be unemployed to continue to practice medicine and serve the community, thereby alleviating concern about an oversupply.
For better or for worse, because of the state of health care today, ED physicians have been exposed to a myriad of primary care concerns, all of which have prepared them for a career as an outpatient PCP. By offering such a path, we can provide more flexibility for an emergency physician’s career, help alleviate the primary care shortage affecting the United States, and serve our community and country in new and helpful ways.
Dr. Gogna is a fourth-year medical student at Philadelphia College of Osteopathic Medicine Georgia, Suwanee. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
It was 2 p.m. on a sunny and humid day, 1 hour before my 8-hour shift was over in the emergency department at the community hospital that I was rotating through. It was part of my fourth-year emergency medicine (EM) clerkship. Not that I would have noticed the weather, save for the few seconds the sliding door to the ED would open periodically, as if on its own cadence, with the sounds of stomping boots and a rolling gurney making its way through.
We were busy. At this particular hospital, I was told, EM volume is already up 35% this year compared with the previous year, and bed holds had been hitting new highs each week.
One more hour until my shift is over and a poor soul will take over my computer, seat, and the chaos.
I took a glance at the electronic health record again, seeing whether there was anything I could do to discharge any of the patients to relieve some of the strain. Knee pain, toe pain, headache/migraine, shoulder pain, elevated blood pressure. Although it’s true that any of these listed complaints could have emergent etiologies, the truth was that all of these patients were here owing to exacerbations of chronic issues. And yet most, if not all, of these patients had been here for nearly 8 hours, some even longer, waiting for treatment and exacerbating an already busy ED.
“I don’t understand. Couldn’t these patients have sought care outpatient with their PCP [primary care physician]? It would have been a lot cheaper and faster.” I asked. A seasoned ED physician, bald, graying, and whom I had just met today and hadn’t spoken to much until this very moment, turned to me and said: “We have become the dumping ground for primary care complaints.”
‘Go to the ED’
“PCPs are already too busy,” the physician continued. “It’s just easier to say: ‘Go to the ED. They’ll take care of it.’ ”
He continued: “In my 30 years of practicing, emergency medicine has changed so much. When I first started in the 1980s, I was only seeing emergencies, and it was fun. Now, 80% of my patients are primary care complaints. These days, I am more of a primary care physician than an emergency physician.”
Hmmm, I thought. Was this physician burned out and jaded? Quite possibly. Was this change the physician experienced throughout his career more likely attributed to a capitalist-run, profit-driven health care system and its cohort of underinsured and noninsured citizens? Certainly. I’m only a fourth-year medical student, so my view of the situation is no doubt limited.
But something he said definitely rang my bell: I’m more of a primary care physician than an emergency physician. That is an argument I can consider. Whether it is caused by poorly designed reimbursement schedules or the state of America’s profit-driven health care system, which effectively makes these physicians double as PCPs on a daily basis.
I let this thought ruminate on my drive home, along with how there’s a such a huge demand for PCPs, resulting in it taking up to 3 months to get an appointment with one. That’s crazy, and I understand the need to come to the ED where you’ll (hopefully) be seen the same day.
I also ruminated on how emergency physicians have the highest rate of burnout among all the specialties, with no career recourse afterward. Either you’re part of the hospital machine complex, or you’re out. Practicing EM for nearly 30 years is apparently a rarity these days. Most emergency physicians last 5 years, 10 years tops, and then are so burned out that they retire to pursue a life outside of medicine (real estate seems to be popular). But this is a shame.
Emergency docs exiting medicine
Emergency physicians have seen a ton of wildly different pathologies and have treated a variety of different conditions, including conditions usually reserved for primary care. To let knowledgeable, experienced emergency physicians just exit medicine, with no recourse to further contribute to this country’s health system outside of the hospital machine, is a travesty in its own right.
I ruminated further on the 2021 American College of Emergency Physicians 2021 report on the EM physician workforce, which stated that there is projected to be an oversupply of emergency physicians by 2030, leaving thousands of them out of work. No doubt that report has left an impact on the volume of residency applications into EM in 2021. No one wants to go through residency and be unemployed at the end of it.
And finally, I ruminated on the sheer volume of patients visiting EDs across the country. Patient volumes are up, wait times in general are up, wait times in the ED are up, and bed holds to get admitted are hitting highs across the country each week. The deluge of patients visiting the ED is not getting better, and it’s only likely to get worse as the population ages.
It’s time to offer emergency physicians a path to outpatient primary care.
Now before I get hung for this suggestion (“I went into EM precisely not to do outpatient care!”), hear me out: Such a path should be offered via a 1-year accelerated fellowship and will allow emergency physicians to practice outpatient primary care medicine independently. And although working in urgent care centers is already an option, the opportunity to own and operate their own primary care practice should also exist.
In my humble opinion, by offering such a path, the following objectives could be accomplished:
Alleviate the pressure on primary care medicine in the United States. It’s no secret that the United States needs more primary care physicians. Allowing emergency physicians who got burnt out by hospital life an alternative way to serve their community and country via outpatient primary care would greatly alleviate the pressure on the need for PCPs today.
Provide an alternative career path for emergency physicians. We would be doing a disservice if we don’t offer emergency physicians a way to revive their burnout and utilize their skill set in a post-ED life. Outpatient primary care is the perfect way to do this, and it’s a win-win-win on several fronts: We need more outpatient physicians, they need an opportunity to flex their knowledge in an alternative setting.
Solve the “ACEP” problem. The ACEP report scared medical students away from applying to residency in EM. Who wants to go through 3 years of residency only to be unemployed at the end of it? By offering a path to outpatient primary care, we can offer an important and viable path for those emergency physicians who would be unemployed to continue to practice medicine and serve the community, thereby alleviating concern about an oversupply.
For better or for worse, because of the state of health care today, ED physicians have been exposed to a myriad of primary care concerns, all of which have prepared them for a career as an outpatient PCP. By offering such a path, we can provide more flexibility for an emergency physician’s career, help alleviate the primary care shortage affecting the United States, and serve our community and country in new and helpful ways.
Dr. Gogna is a fourth-year medical student at Philadelphia College of Osteopathic Medicine Georgia, Suwanee. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
It was 2 p.m. on a sunny and humid day, 1 hour before my 8-hour shift was over in the emergency department at the community hospital that I was rotating through. It was part of my fourth-year emergency medicine (EM) clerkship. Not that I would have noticed the weather, save for the few seconds the sliding door to the ED would open periodically, as if on its own cadence, with the sounds of stomping boots and a rolling gurney making its way through.
We were busy. At this particular hospital, I was told, EM volume is already up 35% this year compared with the previous year, and bed holds had been hitting new highs each week.
One more hour until my shift is over and a poor soul will take over my computer, seat, and the chaos.
I took a glance at the electronic health record again, seeing whether there was anything I could do to discharge any of the patients to relieve some of the strain. Knee pain, toe pain, headache/migraine, shoulder pain, elevated blood pressure. Although it’s true that any of these listed complaints could have emergent etiologies, the truth was that all of these patients were here owing to exacerbations of chronic issues. And yet most, if not all, of these patients had been here for nearly 8 hours, some even longer, waiting for treatment and exacerbating an already busy ED.
“I don’t understand. Couldn’t these patients have sought care outpatient with their PCP [primary care physician]? It would have been a lot cheaper and faster.” I asked. A seasoned ED physician, bald, graying, and whom I had just met today and hadn’t spoken to much until this very moment, turned to me and said: “We have become the dumping ground for primary care complaints.”
‘Go to the ED’
“PCPs are already too busy,” the physician continued. “It’s just easier to say: ‘Go to the ED. They’ll take care of it.’ ”
He continued: “In my 30 years of practicing, emergency medicine has changed so much. When I first started in the 1980s, I was only seeing emergencies, and it was fun. Now, 80% of my patients are primary care complaints. These days, I am more of a primary care physician than an emergency physician.”
Hmmm, I thought. Was this physician burned out and jaded? Quite possibly. Was this change the physician experienced throughout his career more likely attributed to a capitalist-run, profit-driven health care system and its cohort of underinsured and noninsured citizens? Certainly. I’m only a fourth-year medical student, so my view of the situation is no doubt limited.
But something he said definitely rang my bell: I’m more of a primary care physician than an emergency physician. That is an argument I can consider. Whether it is caused by poorly designed reimbursement schedules or the state of America’s profit-driven health care system, which effectively makes these physicians double as PCPs on a daily basis.
I let this thought ruminate on my drive home, along with how there’s a such a huge demand for PCPs, resulting in it taking up to 3 months to get an appointment with one. That’s crazy, and I understand the need to come to the ED where you’ll (hopefully) be seen the same day.
I also ruminated on how emergency physicians have the highest rate of burnout among all the specialties, with no career recourse afterward. Either you’re part of the hospital machine complex, or you’re out. Practicing EM for nearly 30 years is apparently a rarity these days. Most emergency physicians last 5 years, 10 years tops, and then are so burned out that they retire to pursue a life outside of medicine (real estate seems to be popular). But this is a shame.
Emergency docs exiting medicine
Emergency physicians have seen a ton of wildly different pathologies and have treated a variety of different conditions, including conditions usually reserved for primary care. To let knowledgeable, experienced emergency physicians just exit medicine, with no recourse to further contribute to this country’s health system outside of the hospital machine, is a travesty in its own right.
I ruminated further on the 2021 American College of Emergency Physicians 2021 report on the EM physician workforce, which stated that there is projected to be an oversupply of emergency physicians by 2030, leaving thousands of them out of work. No doubt that report has left an impact on the volume of residency applications into EM in 2021. No one wants to go through residency and be unemployed at the end of it.
And finally, I ruminated on the sheer volume of patients visiting EDs across the country. Patient volumes are up, wait times in general are up, wait times in the ED are up, and bed holds to get admitted are hitting highs across the country each week. The deluge of patients visiting the ED is not getting better, and it’s only likely to get worse as the population ages.
It’s time to offer emergency physicians a path to outpatient primary care.
Now before I get hung for this suggestion (“I went into EM precisely not to do outpatient care!”), hear me out: Such a path should be offered via a 1-year accelerated fellowship and will allow emergency physicians to practice outpatient primary care medicine independently. And although working in urgent care centers is already an option, the opportunity to own and operate their own primary care practice should also exist.
In my humble opinion, by offering such a path, the following objectives could be accomplished:
Alleviate the pressure on primary care medicine in the United States. It’s no secret that the United States needs more primary care physicians. Allowing emergency physicians who got burnt out by hospital life an alternative way to serve their community and country via outpatient primary care would greatly alleviate the pressure on the need for PCPs today.
Provide an alternative career path for emergency physicians. We would be doing a disservice if we don’t offer emergency physicians a way to revive their burnout and utilize their skill set in a post-ED life. Outpatient primary care is the perfect way to do this, and it’s a win-win-win on several fronts: We need more outpatient physicians, they need an opportunity to flex their knowledge in an alternative setting.
Solve the “ACEP” problem. The ACEP report scared medical students away from applying to residency in EM. Who wants to go through 3 years of residency only to be unemployed at the end of it? By offering a path to outpatient primary care, we can offer an important and viable path for those emergency physicians who would be unemployed to continue to practice medicine and serve the community, thereby alleviating concern about an oversupply.
For better or for worse, because of the state of health care today, ED physicians have been exposed to a myriad of primary care concerns, all of which have prepared them for a career as an outpatient PCP. By offering such a path, we can provide more flexibility for an emergency physician’s career, help alleviate the primary care shortage affecting the United States, and serve our community and country in new and helpful ways.
Dr. Gogna is a fourth-year medical student at Philadelphia College of Osteopathic Medicine Georgia, Suwanee. He reported no relevant conflicts of interest.
A version of this article first appeared on Medscape.com.
Nursing exam failure rates spark review of test results
Nursing oversight groups in the United States and Canada are holding the line on testing standards as more would-be nurses fail entry exams. As a result, pressure is growing to make tests easier to pass given widespread nursing shortages, and some critics wonder whether the exams accurately assess students’ true abilities.
When it comes to training more nurses to keep up with growing demand, the U.S. organization that oversees the main licensing exams for nurses decided earlier this month not to change the passing standards for entry-level tests. Meanwhile,
A similar scenario also is unfolding in Quebec, where the agency overseeing nurse licensing exams announced last month it is holding the line on its passing rates despite an outcry from nurses after more than half of those taking the exam in September failed. Quebec’s commissioner for professional admissions is investigating dozens of complaints from nurses about the failure rate. Nurses who failed the test can sign up to retake it in March.
Joseph Oujeil, DESS, DEF, has been teaching in Canada for 4 years, now at two Quebec nursing schools. “This is surprising and very shocking to our students as well as nurses from outside Quebec who were [completing] an integration program to adjust their practice to Quebec guidelines,” Mr. Oujeil told this news organization. Students from outside the province failed the licensing exam at a higher rate than their Quebec-native peers, he explained.
Quebec’s professional Order of Nurses of Quebec (OIIQ) responded to the nurses’ outcry in a press release last month, saying that the pandemic may be partly to blame for the lower passing rate because it made it more difficult to access internships, labs, and face-to-face teaching. Some students weren’t able to demonstrate their ability to practice during the exam as in previous years, OIIQ reported.
Mr. Oujeil agreed. “I’m sure the pandemic has an impact on the situation as well as some students did less training in hospitals” because of restrictions caused by the pandemic, he said. But students also told Mr. Oujeil some questions seemed ambiguous.
OIIQ stated in its release that it doesn’t want to lower the standard. The goal is to protect the public “by and with nurses,” to “ensure the competence and integrity of nurses in Quebec,” and “promote quality nursing practice,” the release noted.
Similarly in the United States, the National Council of State Boards of Nursing (NCSBN) announced Dec. 8 that it would uphold the current passing standards for its entry-level NCLEX tests for registered nurses and practical nurses. NCSBN analyzes the passing standard every 3 years “to keep the test plan and passing standard current,” a press release explains.
NCLEX pass rates have dropped from about 73% for all candidates and 88% for first-time U.S.-educated candidates to 69% and 82% respectively in 2021, the last full year for which results are available, NCSBN spokesperson Dawn Kappel told this news organization.
Over the past 3 years, including during the pandemic, the board decided “that the current passing standard is appropriate as a measure of safe and effective entry-level nurse practice,” after reviewing national nurse surveys and the findings of panels of nurses representing NCSBN’s geographic areas in the United States and Canada, board president Jay Douglas, MSM, RN, CSAC, said in a press release.
Still, NCSBN is not blind to the larger issues facing nursing, Ms. Kappel told this news organization. “There is a huge nursing shortage in the U.S. and Canada. We want as many nurses in the workforce as possible, but we want to ensure safe practice,” she said.
“Everyone has access to the same test, regardless of which state, province, or country they take it in,” she said. Some international students may not perform as well as U.S.-educated students because of their command of English and the nursing education standards in their home countries, Ms. Kappel added.
“Obviously COVID and the challenge of education in general” affected the results, she said.
Mr. Oujeil, the nursing school professor, said he is frustrated by the test results because the majority of students who failed maintained good grades and passed all of their trainings. Yet they scored just below the passing rate of 55%. He said students are proposing the passing rate be lowered to 50%. The current test doesn’t reflect what students are learning in the classroom or during clinical trainings, Mr. Oujeil added. “I don’t know of any students who scored more than 60%.”
He said he understands that the mission of the OIIQ is protecting the population, but he doesn’t believe lowering the passing rate to 50% will put the population at risk – and it will help offset the staffing shortage.
“I’m especially frustrated by those who were doing integration programs – mothers and fathers with children with family lives and financial responsibilities. Many of them are good, hard workers and were shocked they should have to pass the exam another time.”
Since 2018, the pass rate on the first attempt at the Quebec test has generally been between 71% and 96%, compared with 51.4% during the exam in September, according to the OIIQ press release. Meanwhile, graduates from 30 of the 55 schools and universities in Quebec teaching nurses performed above the average on the recent professional exam, OIIQ reported.
The professional licensing organization pointed out that nursing candidates have three attempts to pass their exam. “In order to better prepare for their next attempt, all those who failed received an individual response detailing the difficulties encountered. The OIIQ offers all the tools necessary to pass the exam; a detailed guide and preparatory workshops are available online.
“In the run up to the next exams, we will continue to support students by working with educational institutions to provide the optimal conditions for passing the exam. This exam is usually successful and we are convinced that the return to face-to-face teaching, as well as support for students, will be factors of success,” OIIQ President Luc Mathieu said in a press release.
Nursing candidates “who have not passed the exam will be put to work in the network, with the possibility of practicing under the supervision of a nurse. ... In addition, we will contact health establishments in order to support them in their supervision activities” of the candidates, he said.
A version of this article first appeared on Medscape.com.
Nursing oversight groups in the United States and Canada are holding the line on testing standards as more would-be nurses fail entry exams. As a result, pressure is growing to make tests easier to pass given widespread nursing shortages, and some critics wonder whether the exams accurately assess students’ true abilities.
When it comes to training more nurses to keep up with growing demand, the U.S. organization that oversees the main licensing exams for nurses decided earlier this month not to change the passing standards for entry-level tests. Meanwhile,
A similar scenario also is unfolding in Quebec, where the agency overseeing nurse licensing exams announced last month it is holding the line on its passing rates despite an outcry from nurses after more than half of those taking the exam in September failed. Quebec’s commissioner for professional admissions is investigating dozens of complaints from nurses about the failure rate. Nurses who failed the test can sign up to retake it in March.
Joseph Oujeil, DESS, DEF, has been teaching in Canada for 4 years, now at two Quebec nursing schools. “This is surprising and very shocking to our students as well as nurses from outside Quebec who were [completing] an integration program to adjust their practice to Quebec guidelines,” Mr. Oujeil told this news organization. Students from outside the province failed the licensing exam at a higher rate than their Quebec-native peers, he explained.
Quebec’s professional Order of Nurses of Quebec (OIIQ) responded to the nurses’ outcry in a press release last month, saying that the pandemic may be partly to blame for the lower passing rate because it made it more difficult to access internships, labs, and face-to-face teaching. Some students weren’t able to demonstrate their ability to practice during the exam as in previous years, OIIQ reported.
Mr. Oujeil agreed. “I’m sure the pandemic has an impact on the situation as well as some students did less training in hospitals” because of restrictions caused by the pandemic, he said. But students also told Mr. Oujeil some questions seemed ambiguous.
OIIQ stated in its release that it doesn’t want to lower the standard. The goal is to protect the public “by and with nurses,” to “ensure the competence and integrity of nurses in Quebec,” and “promote quality nursing practice,” the release noted.
Similarly in the United States, the National Council of State Boards of Nursing (NCSBN) announced Dec. 8 that it would uphold the current passing standards for its entry-level NCLEX tests for registered nurses and practical nurses. NCSBN analyzes the passing standard every 3 years “to keep the test plan and passing standard current,” a press release explains.
NCLEX pass rates have dropped from about 73% for all candidates and 88% for first-time U.S.-educated candidates to 69% and 82% respectively in 2021, the last full year for which results are available, NCSBN spokesperson Dawn Kappel told this news organization.
Over the past 3 years, including during the pandemic, the board decided “that the current passing standard is appropriate as a measure of safe and effective entry-level nurse practice,” after reviewing national nurse surveys and the findings of panels of nurses representing NCSBN’s geographic areas in the United States and Canada, board president Jay Douglas, MSM, RN, CSAC, said in a press release.
Still, NCSBN is not blind to the larger issues facing nursing, Ms. Kappel told this news organization. “There is a huge nursing shortage in the U.S. and Canada. We want as many nurses in the workforce as possible, but we want to ensure safe practice,” she said.
“Everyone has access to the same test, regardless of which state, province, or country they take it in,” she said. Some international students may not perform as well as U.S.-educated students because of their command of English and the nursing education standards in their home countries, Ms. Kappel added.
“Obviously COVID and the challenge of education in general” affected the results, she said.
Mr. Oujeil, the nursing school professor, said he is frustrated by the test results because the majority of students who failed maintained good grades and passed all of their trainings. Yet they scored just below the passing rate of 55%. He said students are proposing the passing rate be lowered to 50%. The current test doesn’t reflect what students are learning in the classroom or during clinical trainings, Mr. Oujeil added. “I don’t know of any students who scored more than 60%.”
He said he understands that the mission of the OIIQ is protecting the population, but he doesn’t believe lowering the passing rate to 50% will put the population at risk – and it will help offset the staffing shortage.
“I’m especially frustrated by those who were doing integration programs – mothers and fathers with children with family lives and financial responsibilities. Many of them are good, hard workers and were shocked they should have to pass the exam another time.”
Since 2018, the pass rate on the first attempt at the Quebec test has generally been between 71% and 96%, compared with 51.4% during the exam in September, according to the OIIQ press release. Meanwhile, graduates from 30 of the 55 schools and universities in Quebec teaching nurses performed above the average on the recent professional exam, OIIQ reported.
The professional licensing organization pointed out that nursing candidates have three attempts to pass their exam. “In order to better prepare for their next attempt, all those who failed received an individual response detailing the difficulties encountered. The OIIQ offers all the tools necessary to pass the exam; a detailed guide and preparatory workshops are available online.
“In the run up to the next exams, we will continue to support students by working with educational institutions to provide the optimal conditions for passing the exam. This exam is usually successful and we are convinced that the return to face-to-face teaching, as well as support for students, will be factors of success,” OIIQ President Luc Mathieu said in a press release.
Nursing candidates “who have not passed the exam will be put to work in the network, with the possibility of practicing under the supervision of a nurse. ... In addition, we will contact health establishments in order to support them in their supervision activities” of the candidates, he said.
A version of this article first appeared on Medscape.com.
Nursing oversight groups in the United States and Canada are holding the line on testing standards as more would-be nurses fail entry exams. As a result, pressure is growing to make tests easier to pass given widespread nursing shortages, and some critics wonder whether the exams accurately assess students’ true abilities.
When it comes to training more nurses to keep up with growing demand, the U.S. organization that oversees the main licensing exams for nurses decided earlier this month not to change the passing standards for entry-level tests. Meanwhile,
A similar scenario also is unfolding in Quebec, where the agency overseeing nurse licensing exams announced last month it is holding the line on its passing rates despite an outcry from nurses after more than half of those taking the exam in September failed. Quebec’s commissioner for professional admissions is investigating dozens of complaints from nurses about the failure rate. Nurses who failed the test can sign up to retake it in March.
Joseph Oujeil, DESS, DEF, has been teaching in Canada for 4 years, now at two Quebec nursing schools. “This is surprising and very shocking to our students as well as nurses from outside Quebec who were [completing] an integration program to adjust their practice to Quebec guidelines,” Mr. Oujeil told this news organization. Students from outside the province failed the licensing exam at a higher rate than their Quebec-native peers, he explained.
Quebec’s professional Order of Nurses of Quebec (OIIQ) responded to the nurses’ outcry in a press release last month, saying that the pandemic may be partly to blame for the lower passing rate because it made it more difficult to access internships, labs, and face-to-face teaching. Some students weren’t able to demonstrate their ability to practice during the exam as in previous years, OIIQ reported.
Mr. Oujeil agreed. “I’m sure the pandemic has an impact on the situation as well as some students did less training in hospitals” because of restrictions caused by the pandemic, he said. But students also told Mr. Oujeil some questions seemed ambiguous.
OIIQ stated in its release that it doesn’t want to lower the standard. The goal is to protect the public “by and with nurses,” to “ensure the competence and integrity of nurses in Quebec,” and “promote quality nursing practice,” the release noted.
Similarly in the United States, the National Council of State Boards of Nursing (NCSBN) announced Dec. 8 that it would uphold the current passing standards for its entry-level NCLEX tests for registered nurses and practical nurses. NCSBN analyzes the passing standard every 3 years “to keep the test plan and passing standard current,” a press release explains.
NCLEX pass rates have dropped from about 73% for all candidates and 88% for first-time U.S.-educated candidates to 69% and 82% respectively in 2021, the last full year for which results are available, NCSBN spokesperson Dawn Kappel told this news organization.
Over the past 3 years, including during the pandemic, the board decided “that the current passing standard is appropriate as a measure of safe and effective entry-level nurse practice,” after reviewing national nurse surveys and the findings of panels of nurses representing NCSBN’s geographic areas in the United States and Canada, board president Jay Douglas, MSM, RN, CSAC, said in a press release.
Still, NCSBN is not blind to the larger issues facing nursing, Ms. Kappel told this news organization. “There is a huge nursing shortage in the U.S. and Canada. We want as many nurses in the workforce as possible, but we want to ensure safe practice,” she said.
“Everyone has access to the same test, regardless of which state, province, or country they take it in,” she said. Some international students may not perform as well as U.S.-educated students because of their command of English and the nursing education standards in their home countries, Ms. Kappel added.
“Obviously COVID and the challenge of education in general” affected the results, she said.
Mr. Oujeil, the nursing school professor, said he is frustrated by the test results because the majority of students who failed maintained good grades and passed all of their trainings. Yet they scored just below the passing rate of 55%. He said students are proposing the passing rate be lowered to 50%. The current test doesn’t reflect what students are learning in the classroom or during clinical trainings, Mr. Oujeil added. “I don’t know of any students who scored more than 60%.”
He said he understands that the mission of the OIIQ is protecting the population, but he doesn’t believe lowering the passing rate to 50% will put the population at risk – and it will help offset the staffing shortage.
“I’m especially frustrated by those who were doing integration programs – mothers and fathers with children with family lives and financial responsibilities. Many of them are good, hard workers and were shocked they should have to pass the exam another time.”
Since 2018, the pass rate on the first attempt at the Quebec test has generally been between 71% and 96%, compared with 51.4% during the exam in September, according to the OIIQ press release. Meanwhile, graduates from 30 of the 55 schools and universities in Quebec teaching nurses performed above the average on the recent professional exam, OIIQ reported.
The professional licensing organization pointed out that nursing candidates have three attempts to pass their exam. “In order to better prepare for their next attempt, all those who failed received an individual response detailing the difficulties encountered. The OIIQ offers all the tools necessary to pass the exam; a detailed guide and preparatory workshops are available online.
“In the run up to the next exams, we will continue to support students by working with educational institutions to provide the optimal conditions for passing the exam. This exam is usually successful and we are convinced that the return to face-to-face teaching, as well as support for students, will be factors of success,” OIIQ President Luc Mathieu said in a press release.
Nursing candidates “who have not passed the exam will be put to work in the network, with the possibility of practicing under the supervision of a nurse. ... In addition, we will contact health establishments in order to support them in their supervision activities” of the candidates, he said.
A version of this article first appeared on Medscape.com.
Vonoprazan triple therapy most cost-effective for H. pylori: Study
A new analysis finds that vonoprazan triple therapy (Voquezna) is the most cost-effective first-line regimen to eradicate Helicobacter pylori infection in the United States.
Rifabutin triple therapy (Talicia) is the second most cost-effective strategy for H. pylori eradication, followed in order of decreasing cost-effectiveness by vonoprazan dual therapy, bismuth quadruple therapy, and clarithromycin triple therapy.
The analysis is believed to be the first to report on the cost-effectiveness of vonoprazan- and rifabutin-based regimens as first-line treatments for H. pylori infection from the perspective of U.S. health care payers.
for U.S. payers, reported Ismaeel Yunusa, PharmD, PhD, of the University of South Carolina College of Pharmacy in Columbia, and colleagues.
The study was published online in the American Journal of Gastroenterology.
It’s estimated that more than 114 million people in the United States have H. pylori infection. Clinical practice guidelines recommend H. pylori eradication in all patients with a positive test of active infection.
Using a Markov model, Dr. Yunusa and colleagues estimated the cost-effectiveness of five prepackaged or co-formulated H. pylori eradication regimens: clarithromycin triple therapy, bismuth quadruple therapy, vonoprazan dual therapy, vonoprazan triple therapy, and rifabutin triple therapy.
The model estimated the expected costs in 2022 U.S. dollars, expected quality-adjusted life years (QALYs), incremental cost-effectiveness ratio (ICERs), and expected net monetary benefit over 20 years.
Among their key findings and conclusions:
- Bismuth quadruple therapy had the highest expected cost ($1,439) and rifabutin triple regimen had the lowest expected cost ($1,048).
- Because rifabutin triple therapy was predicted to cost less and was more effective than clarithromycin triple therapy, bismuth quadruple therapy, and vonoprazan dual therapy, it dominated all treatment strategies – except for vonoprazan triple therapy.
- Compared with rifabutin triple therapy, vonoprazan triple therapy had a higher expected cost ($1,172 vs. $1,048) and expected QALY (14.262 vs. 14.256), yielding an ICER of $22,573 per QALY.
- Vonoprazan triple therapy had the highest expected net monetary benefit and was the most cost-effective at willingness to pay thresholds between $50,000 and $150,000 per QALY, followed by rifabutin triple therapy.
- Vonoprazan triple therapy would result on average in an incremental net benefit of $1,655 per patient than clarithromycin triple therapy.
- Because the rifabutin-based regimen was more cost-effective than all but vonoprazan triple therapy, it has a potential role as an alternative first-line treatment.
- Rifabutin triple therapy and vonoprazan dual therapy would need to be considerably discounted (by 15%-43% and by 44%-85%, respectively), to be cost-effective at commonly used cost-effectiveness thresholds.
- Vonoprazan dual therapy demonstrated limited value relative to other available options; thus, its widespread adoption as a first-line strategy seems unlikely.
- Based on the results, it would be hard to justify the use of bismuth quadruple therapy or clarithromycin triple therapy since they provide the lowest net monetary benefit and have lower eradication rates.
The investigators noted that their analysis considered only direct costs of therapy, not other costs such as appointments, travel, and time away from work.
They also assumed medical costs, including endoscopy and H. pylori testing, would not change regardless of treatment regimen. Therefore, total health care costs may be underestimated.
The study did not receive any funding. The authors have declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis finds that vonoprazan triple therapy (Voquezna) is the most cost-effective first-line regimen to eradicate Helicobacter pylori infection in the United States.
Rifabutin triple therapy (Talicia) is the second most cost-effective strategy for H. pylori eradication, followed in order of decreasing cost-effectiveness by vonoprazan dual therapy, bismuth quadruple therapy, and clarithromycin triple therapy.
The analysis is believed to be the first to report on the cost-effectiveness of vonoprazan- and rifabutin-based regimens as first-line treatments for H. pylori infection from the perspective of U.S. health care payers.
for U.S. payers, reported Ismaeel Yunusa, PharmD, PhD, of the University of South Carolina College of Pharmacy in Columbia, and colleagues.
The study was published online in the American Journal of Gastroenterology.
It’s estimated that more than 114 million people in the United States have H. pylori infection. Clinical practice guidelines recommend H. pylori eradication in all patients with a positive test of active infection.
Using a Markov model, Dr. Yunusa and colleagues estimated the cost-effectiveness of five prepackaged or co-formulated H. pylori eradication regimens: clarithromycin triple therapy, bismuth quadruple therapy, vonoprazan dual therapy, vonoprazan triple therapy, and rifabutin triple therapy.
The model estimated the expected costs in 2022 U.S. dollars, expected quality-adjusted life years (QALYs), incremental cost-effectiveness ratio (ICERs), and expected net monetary benefit over 20 years.
Among their key findings and conclusions:
- Bismuth quadruple therapy had the highest expected cost ($1,439) and rifabutin triple regimen had the lowest expected cost ($1,048).
- Because rifabutin triple therapy was predicted to cost less and was more effective than clarithromycin triple therapy, bismuth quadruple therapy, and vonoprazan dual therapy, it dominated all treatment strategies – except for vonoprazan triple therapy.
- Compared with rifabutin triple therapy, vonoprazan triple therapy had a higher expected cost ($1,172 vs. $1,048) and expected QALY (14.262 vs. 14.256), yielding an ICER of $22,573 per QALY.
- Vonoprazan triple therapy had the highest expected net monetary benefit and was the most cost-effective at willingness to pay thresholds between $50,000 and $150,000 per QALY, followed by rifabutin triple therapy.
- Vonoprazan triple therapy would result on average in an incremental net benefit of $1,655 per patient than clarithromycin triple therapy.
- Because the rifabutin-based regimen was more cost-effective than all but vonoprazan triple therapy, it has a potential role as an alternative first-line treatment.
- Rifabutin triple therapy and vonoprazan dual therapy would need to be considerably discounted (by 15%-43% and by 44%-85%, respectively), to be cost-effective at commonly used cost-effectiveness thresholds.
- Vonoprazan dual therapy demonstrated limited value relative to other available options; thus, its widespread adoption as a first-line strategy seems unlikely.
- Based on the results, it would be hard to justify the use of bismuth quadruple therapy or clarithromycin triple therapy since they provide the lowest net monetary benefit and have lower eradication rates.
The investigators noted that their analysis considered only direct costs of therapy, not other costs such as appointments, travel, and time away from work.
They also assumed medical costs, including endoscopy and H. pylori testing, would not change regardless of treatment regimen. Therefore, total health care costs may be underestimated.
The study did not receive any funding. The authors have declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new analysis finds that vonoprazan triple therapy (Voquezna) is the most cost-effective first-line regimen to eradicate Helicobacter pylori infection in the United States.
Rifabutin triple therapy (Talicia) is the second most cost-effective strategy for H. pylori eradication, followed in order of decreasing cost-effectiveness by vonoprazan dual therapy, bismuth quadruple therapy, and clarithromycin triple therapy.
The analysis is believed to be the first to report on the cost-effectiveness of vonoprazan- and rifabutin-based regimens as first-line treatments for H. pylori infection from the perspective of U.S. health care payers.
for U.S. payers, reported Ismaeel Yunusa, PharmD, PhD, of the University of South Carolina College of Pharmacy in Columbia, and colleagues.
The study was published online in the American Journal of Gastroenterology.
It’s estimated that more than 114 million people in the United States have H. pylori infection. Clinical practice guidelines recommend H. pylori eradication in all patients with a positive test of active infection.
Using a Markov model, Dr. Yunusa and colleagues estimated the cost-effectiveness of five prepackaged or co-formulated H. pylori eradication regimens: clarithromycin triple therapy, bismuth quadruple therapy, vonoprazan dual therapy, vonoprazan triple therapy, and rifabutin triple therapy.
The model estimated the expected costs in 2022 U.S. dollars, expected quality-adjusted life years (QALYs), incremental cost-effectiveness ratio (ICERs), and expected net monetary benefit over 20 years.
Among their key findings and conclusions:
- Bismuth quadruple therapy had the highest expected cost ($1,439) and rifabutin triple regimen had the lowest expected cost ($1,048).
- Because rifabutin triple therapy was predicted to cost less and was more effective than clarithromycin triple therapy, bismuth quadruple therapy, and vonoprazan dual therapy, it dominated all treatment strategies – except for vonoprazan triple therapy.
- Compared with rifabutin triple therapy, vonoprazan triple therapy had a higher expected cost ($1,172 vs. $1,048) and expected QALY (14.262 vs. 14.256), yielding an ICER of $22,573 per QALY.
- Vonoprazan triple therapy had the highest expected net monetary benefit and was the most cost-effective at willingness to pay thresholds between $50,000 and $150,000 per QALY, followed by rifabutin triple therapy.
- Vonoprazan triple therapy would result on average in an incremental net benefit of $1,655 per patient than clarithromycin triple therapy.
- Because the rifabutin-based regimen was more cost-effective than all but vonoprazan triple therapy, it has a potential role as an alternative first-line treatment.
- Rifabutin triple therapy and vonoprazan dual therapy would need to be considerably discounted (by 15%-43% and by 44%-85%, respectively), to be cost-effective at commonly used cost-effectiveness thresholds.
- Vonoprazan dual therapy demonstrated limited value relative to other available options; thus, its widespread adoption as a first-line strategy seems unlikely.
- Based on the results, it would be hard to justify the use of bismuth quadruple therapy or clarithromycin triple therapy since they provide the lowest net monetary benefit and have lower eradication rates.
The investigators noted that their analysis considered only direct costs of therapy, not other costs such as appointments, travel, and time away from work.
They also assumed medical costs, including endoscopy and H. pylori testing, would not change regardless of treatment regimen. Therefore, total health care costs may be underestimated.
The study did not receive any funding. The authors have declared no relevant financial relationships.
A version of this article first appeared on Medscape.com.