User login
USPSTF updates recommendations on aspirin and CVD
In April 2022, the US Preventive Services Task Force (USPSTF) issued new recommendations for the use of aspirin to prevent cardiovascular disease (CVD).1 These recommendations differ markedly from those issued in 2016.
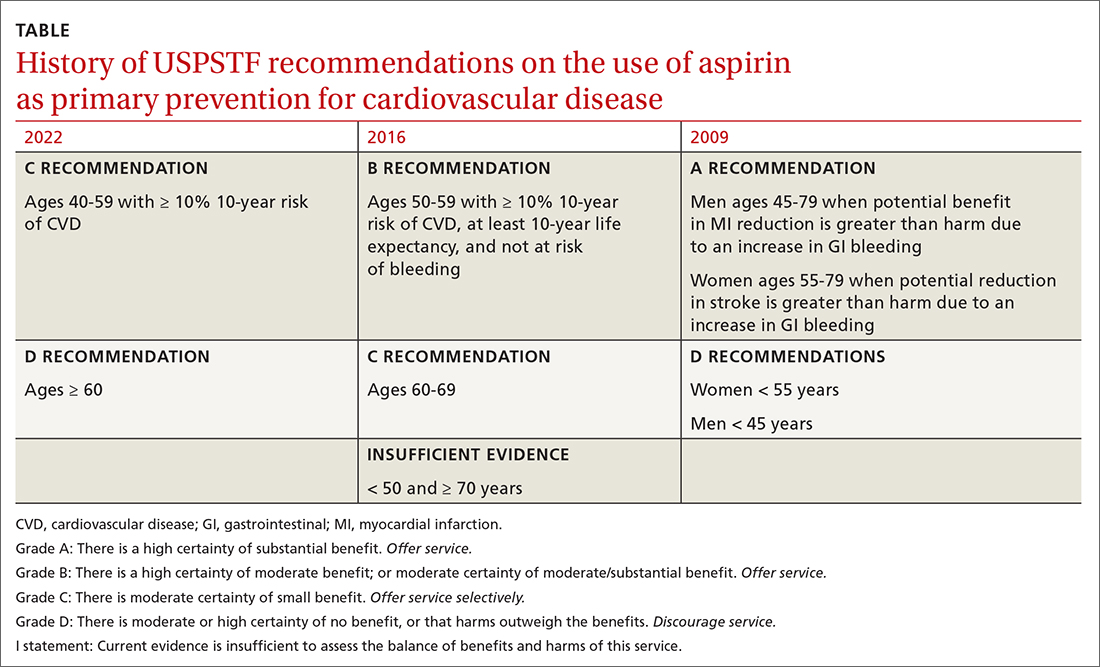
First, for individuals ages 40 through 59 years who have a ≥ 10% 10-year risk of CVD, the decision to initiate low-dose aspirin to prevent CVD is selective. This is in contrast to the 2016 recommendation that advised offering aspirin to any individual ages 50 to 59 whose 10-year risk of CVD was ≥ 10% and whose life expectancy was at least 10 years (TABLE).
Second, according to the new recommendations, individuals who are ages 60 years and older should not initiate low-dose aspirin for the primary prevention of CVD. Previously, selected individuals ages 60 to 69 could be advised to take low-dose aspirin.
The 2016 recommendations also considered the potential benefit of aspirin for preventing colorectal cancer. The 2022 recommendations are silent on this topic, because the USPSTF now concludes that the evidence is insufficient to form an opinion about it.
Important details to keep in mind
These new recommendations pertain to those without signs or symptoms of CVD or known CVD. They do not apply to the use of aspirin for harm reduction or tertiary prevention in those with known CVD. Moreover, the recommendations address the initiation of aspirin at the suggested dose of 81 mg/d, not the continuation of it by those already using it (more on this later). The tool recommended for calculating 10-year CVD risk is the one developed by the American College of Cardiology (ACC) and the American Heart Association (AHA) (www.cvriskcalculator.com).
An ongoing controversy. Daily low-dose aspirin for the prevention of CVD has been controversial for decades. The TABLE shows how USPSTF recommendations on this topic have changed from 2009 to the present. In 2009, the recommendations were primarily based on 2 studies; today, they are based on 13 studies and a microsimulation to estimate the benefits and harms of aspirin prophylaxis at different patient ages.2 This increase in the quantity of the evidence, as well as the elevation in quality, has led to much more nuanced and conservative recommendations. These new recommendations from the USPSTF align much more closely with those of the ACC and the AHA, differing only on the upper age limit at which aspirin initiation should be discouraged (60 years for the USPSTF, 70 for ACC/AHA).
Advise aspirin use selectively per the USPSTF recommendations
Several issues must be addressed when considering daily aspirin use for those ages 40 through 59 years (C recommendation; see TABLE for grade definitions):
- Risk of bleeding is elevated with past or current peptic ulcer disease, diabetes, smoking, high blood pressure, and the use of anti-inflammatory medications, steroids, and anticoagulants.
- The harms from bleeding complications tend to occur early in the use of aspirin and can include gastrointestinal bleeding, intracranial bleeding, and hemorrhagic stroke.
- The higher the 10-year CVD risk, the greater the benefit from low-dose aspirin.
- Benefits of aspirin for the prevention of CVD increase with the number of years of use.
- If an individual has been taking low-dose aspirin without complications, a reasonable age to discontinue its use is 75 years because little incremental benefit occurs with use after that age.
Continue to: More on low-dose aspirin benefits and harms
More on low-dose aspirin benefits and harms. What exactly is the absolute benefit and harm from daily low-dose aspirin use for primary prevention of CVD? As one might expect, it varies by age. Researchers used a microsimulation model to examine updated clinical data from systematic reviews. Looking at life years gained, the largest benefit was in men with a 10-year CVD risk of 20% and aspirin initiated between the ages of 40 and 49.3 This resulted in 52.4 lifetime years gained per 1000 people.3 The results from a meta-analysis of 11 studies, published in the evidence report, found an absolute reduction in major CVD events of 0.4% (number needed to treat = 250) and an absolute increase in major bleeds of 0.5% (number needed to harm = 200).2 There was no reduction found for CVD-related or all-cause deaths.
One reason for the increased caution on using aspirin as primary prevention for CVD is the role that statins now play in reducing CVD risk, a factor not accounted for in the studies assessed. It is unknown if the addition of aspirin to statins is beneficial. Remember that the USPSTF recommends the use of a low- to moderate-dose statin in those ages 40 to 75 years if they have one or more CVD risk factors and a 10-year CVD risk ≥ 10%.4
How aspirin use might change. The use of aspirin for CVD prevention is widespread. One analysis estimates that one-third of those ages 50 years and older are using aspirin for CVD prevention, including 45% of those older than 75.5 If the recommendations from the USPSTF are widely adopted, there could be a gradual decrease in aspirin use for primary prevention with little or no effect on overall population health. Other interventions such as smoking prevention, weight reduction, high blood pressure control, and targeted use of statins—if more widely used—would contribute to the downward trend in CVD deaths that has occurred over the past several decades, with fewer complications caused by regular aspirin use.
Take-home message
Follow these steps when caring for adults ages 40 years and older who do not have known CVD:
1. Assess their 10-year CVD risk using the ACC/AHA tool. If the risk is ≥ 10%:
- Discuss the use of a low- or moderate-dose statin if they are age 75 years or younger.
- Discuss the potential for benefit and harm of low-dose aspirin if they are between the ages of 40 and 59 years.
- Mention to those taking daily low-dose aspirin that it has low benefit if continued after age 75.
2. Perform these interventions:
- Screen for hypertension and high cholesterol.
- Screen for type 2 diabetes and pre-diabetes in patients up to age 70 years who are overweight or obese.
- Ask about smoking.
- Measure body mass index.
- Offer preventive interventions when any of these CVD risks are found.
1. Davidson KW, Barry MJ, Mangione CM, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:1577-1584. doi: 10.1001/jama.2022.4983
2. Guirguis-Blake JM, Evans CV, Perdue LA, et al. Aspirin use to prevent cardiovascular disease and colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;327:1585-1597. doi: 10.1001/jama.2022.3337
3. Dehmer SP, O’Keefe LR, Evans CV, et al. Aspirin use to prevent cardiovascular disease and colorectal cancer: updated modeling study for the US Preventive Services Task Force. JAMA. 2022;327:1598-1607. doi:10.1001/jama.2022.3385
4. USPSTF. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. Accessed June 24, 2022. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/statin-use-primary-prevention-cardiovascular-disease-adults#:~:text=The%20USPSTF%20recommends%20that%20clinicians,event%20of%2010%25%20or%20greater
5. Rhee TG, Kumar M, Ross JS, et al. Age-related trajectories of cardiovascular risk and use of aspirin and statin among U.S. adults aged 50 or older, 2011-2018. J Am Geriatr Soc. 2021;69:1272-1282. doi: 10.1111/jgs.17038
In April 2022, the US Preventive Services Task Force (USPSTF) issued new recommendations for the use of aspirin to prevent cardiovascular disease (CVD).1 These recommendations differ markedly from those issued in 2016.
First, for individuals ages 40 through 59 years who have a ≥ 10% 10-year risk of CVD, the decision to initiate low-dose aspirin to prevent CVD is selective. This is in contrast to the 2016 recommendation that advised offering aspirin to any individual ages 50 to 59 whose 10-year risk of CVD was ≥ 10% and whose life expectancy was at least 10 years (TABLE).

Second, according to the new recommendations, individuals who are ages 60 years and older should not initiate low-dose aspirin for the primary prevention of CVD. Previously, selected individuals ages 60 to 69 could be advised to take low-dose aspirin.
The 2016 recommendations also considered the potential benefit of aspirin for preventing colorectal cancer. The 2022 recommendations are silent on this topic, because the USPSTF now concludes that the evidence is insufficient to form an opinion about it.
Important details to keep in mind
These new recommendations pertain to those without signs or symptoms of CVD or known CVD. They do not apply to the use of aspirin for harm reduction or tertiary prevention in those with known CVD. Moreover, the recommendations address the initiation of aspirin at the suggested dose of 81 mg/d, not the continuation of it by those already using it (more on this later). The tool recommended for calculating 10-year CVD risk is the one developed by the American College of Cardiology (ACC) and the American Heart Association (AHA) (www.cvriskcalculator.com).
An ongoing controversy. Daily low-dose aspirin for the prevention of CVD has been controversial for decades. The TABLE shows how USPSTF recommendations on this topic have changed from 2009 to the present. In 2009, the recommendations were primarily based on 2 studies; today, they are based on 13 studies and a microsimulation to estimate the benefits and harms of aspirin prophylaxis at different patient ages.2 This increase in the quantity of the evidence, as well as the elevation in quality, has led to much more nuanced and conservative recommendations. These new recommendations from the USPSTF align much more closely with those of the ACC and the AHA, differing only on the upper age limit at which aspirin initiation should be discouraged (60 years for the USPSTF, 70 for ACC/AHA).
Advise aspirin use selectively per the USPSTF recommendations
Several issues must be addressed when considering daily aspirin use for those ages 40 through 59 years (C recommendation; see TABLE for grade definitions):
- Risk of bleeding is elevated with past or current peptic ulcer disease, diabetes, smoking, high blood pressure, and the use of anti-inflammatory medications, steroids, and anticoagulants.
- The harms from bleeding complications tend to occur early in the use of aspirin and can include gastrointestinal bleeding, intracranial bleeding, and hemorrhagic stroke.
- The higher the 10-year CVD risk, the greater the benefit from low-dose aspirin.
- Benefits of aspirin for the prevention of CVD increase with the number of years of use.
- If an individual has been taking low-dose aspirin without complications, a reasonable age to discontinue its use is 75 years because little incremental benefit occurs with use after that age.
Continue to: More on low-dose aspirin benefits and harms
More on low-dose aspirin benefits and harms. What exactly is the absolute benefit and harm from daily low-dose aspirin use for primary prevention of CVD? As one might expect, it varies by age. Researchers used a microsimulation model to examine updated clinical data from systematic reviews. Looking at life years gained, the largest benefit was in men with a 10-year CVD risk of 20% and aspirin initiated between the ages of 40 and 49.3 This resulted in 52.4 lifetime years gained per 1000 people.3 The results from a meta-analysis of 11 studies, published in the evidence report, found an absolute reduction in major CVD events of 0.4% (number needed to treat = 250) and an absolute increase in major bleeds of 0.5% (number needed to harm = 200).2 There was no reduction found for CVD-related or all-cause deaths.
One reason for the increased caution on using aspirin as primary prevention for CVD is the role that statins now play in reducing CVD risk, a factor not accounted for in the studies assessed. It is unknown if the addition of aspirin to statins is beneficial. Remember that the USPSTF recommends the use of a low- to moderate-dose statin in those ages 40 to 75 years if they have one or more CVD risk factors and a 10-year CVD risk ≥ 10%.4
How aspirin use might change. The use of aspirin for CVD prevention is widespread. One analysis estimates that one-third of those ages 50 years and older are using aspirin for CVD prevention, including 45% of those older than 75.5 If the recommendations from the USPSTF are widely adopted, there could be a gradual decrease in aspirin use for primary prevention with little or no effect on overall population health. Other interventions such as smoking prevention, weight reduction, high blood pressure control, and targeted use of statins—if more widely used—would contribute to the downward trend in CVD deaths that has occurred over the past several decades, with fewer complications caused by regular aspirin use.
Take-home message
Follow these steps when caring for adults ages 40 years and older who do not have known CVD:
1. Assess their 10-year CVD risk using the ACC/AHA tool. If the risk is ≥ 10%:
- Discuss the use of a low- or moderate-dose statin if they are age 75 years or younger.
- Discuss the potential for benefit and harm of low-dose aspirin if they are between the ages of 40 and 59 years.
- Mention to those taking daily low-dose aspirin that it has low benefit if continued after age 75.
2. Perform these interventions:
- Screen for hypertension and high cholesterol.
- Screen for type 2 diabetes and pre-diabetes in patients up to age 70 years who are overweight or obese.
- Ask about smoking.
- Measure body mass index.
- Offer preventive interventions when any of these CVD risks are found.
In April 2022, the US Preventive Services Task Force (USPSTF) issued new recommendations for the use of aspirin to prevent cardiovascular disease (CVD).1 These recommendations differ markedly from those issued in 2016.
First, for individuals ages 40 through 59 years who have a ≥ 10% 10-year risk of CVD, the decision to initiate low-dose aspirin to prevent CVD is selective. This is in contrast to the 2016 recommendation that advised offering aspirin to any individual ages 50 to 59 whose 10-year risk of CVD was ≥ 10% and whose life expectancy was at least 10 years (TABLE).

Second, according to the new recommendations, individuals who are ages 60 years and older should not initiate low-dose aspirin for the primary prevention of CVD. Previously, selected individuals ages 60 to 69 could be advised to take low-dose aspirin.
The 2016 recommendations also considered the potential benefit of aspirin for preventing colorectal cancer. The 2022 recommendations are silent on this topic, because the USPSTF now concludes that the evidence is insufficient to form an opinion about it.
Important details to keep in mind
These new recommendations pertain to those without signs or symptoms of CVD or known CVD. They do not apply to the use of aspirin for harm reduction or tertiary prevention in those with known CVD. Moreover, the recommendations address the initiation of aspirin at the suggested dose of 81 mg/d, not the continuation of it by those already using it (more on this later). The tool recommended for calculating 10-year CVD risk is the one developed by the American College of Cardiology (ACC) and the American Heart Association (AHA) (www.cvriskcalculator.com).
An ongoing controversy. Daily low-dose aspirin for the prevention of CVD has been controversial for decades. The TABLE shows how USPSTF recommendations on this topic have changed from 2009 to the present. In 2009, the recommendations were primarily based on 2 studies; today, they are based on 13 studies and a microsimulation to estimate the benefits and harms of aspirin prophylaxis at different patient ages.2 This increase in the quantity of the evidence, as well as the elevation in quality, has led to much more nuanced and conservative recommendations. These new recommendations from the USPSTF align much more closely with those of the ACC and the AHA, differing only on the upper age limit at which aspirin initiation should be discouraged (60 years for the USPSTF, 70 for ACC/AHA).
Advise aspirin use selectively per the USPSTF recommendations
Several issues must be addressed when considering daily aspirin use for those ages 40 through 59 years (C recommendation; see TABLE for grade definitions):
- Risk of bleeding is elevated with past or current peptic ulcer disease, diabetes, smoking, high blood pressure, and the use of anti-inflammatory medications, steroids, and anticoagulants.
- The harms from bleeding complications tend to occur early in the use of aspirin and can include gastrointestinal bleeding, intracranial bleeding, and hemorrhagic stroke.
- The higher the 10-year CVD risk, the greater the benefit from low-dose aspirin.
- Benefits of aspirin for the prevention of CVD increase with the number of years of use.
- If an individual has been taking low-dose aspirin without complications, a reasonable age to discontinue its use is 75 years because little incremental benefit occurs with use after that age.
Continue to: More on low-dose aspirin benefits and harms
More on low-dose aspirin benefits and harms. What exactly is the absolute benefit and harm from daily low-dose aspirin use for primary prevention of CVD? As one might expect, it varies by age. Researchers used a microsimulation model to examine updated clinical data from systematic reviews. Looking at life years gained, the largest benefit was in men with a 10-year CVD risk of 20% and aspirin initiated between the ages of 40 and 49.3 This resulted in 52.4 lifetime years gained per 1000 people.3 The results from a meta-analysis of 11 studies, published in the evidence report, found an absolute reduction in major CVD events of 0.4% (number needed to treat = 250) and an absolute increase in major bleeds of 0.5% (number needed to harm = 200).2 There was no reduction found for CVD-related or all-cause deaths.
One reason for the increased caution on using aspirin as primary prevention for CVD is the role that statins now play in reducing CVD risk, a factor not accounted for in the studies assessed. It is unknown if the addition of aspirin to statins is beneficial. Remember that the USPSTF recommends the use of a low- to moderate-dose statin in those ages 40 to 75 years if they have one or more CVD risk factors and a 10-year CVD risk ≥ 10%.4
How aspirin use might change. The use of aspirin for CVD prevention is widespread. One analysis estimates that one-third of those ages 50 years and older are using aspirin for CVD prevention, including 45% of those older than 75.5 If the recommendations from the USPSTF are widely adopted, there could be a gradual decrease in aspirin use for primary prevention with little or no effect on overall population health. Other interventions such as smoking prevention, weight reduction, high blood pressure control, and targeted use of statins—if more widely used—would contribute to the downward trend in CVD deaths that has occurred over the past several decades, with fewer complications caused by regular aspirin use.
Take-home message
Follow these steps when caring for adults ages 40 years and older who do not have known CVD:
1. Assess their 10-year CVD risk using the ACC/AHA tool. If the risk is ≥ 10%:
- Discuss the use of a low- or moderate-dose statin if they are age 75 years or younger.
- Discuss the potential for benefit and harm of low-dose aspirin if they are between the ages of 40 and 59 years.
- Mention to those taking daily low-dose aspirin that it has low benefit if continued after age 75.
2. Perform these interventions:
- Screen for hypertension and high cholesterol.
- Screen for type 2 diabetes and pre-diabetes in patients up to age 70 years who are overweight or obese.
- Ask about smoking.
- Measure body mass index.
- Offer preventive interventions when any of these CVD risks are found.
1. Davidson KW, Barry MJ, Mangione CM, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:1577-1584. doi: 10.1001/jama.2022.4983
2. Guirguis-Blake JM, Evans CV, Perdue LA, et al. Aspirin use to prevent cardiovascular disease and colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;327:1585-1597. doi: 10.1001/jama.2022.3337
3. Dehmer SP, O’Keefe LR, Evans CV, et al. Aspirin use to prevent cardiovascular disease and colorectal cancer: updated modeling study for the US Preventive Services Task Force. JAMA. 2022;327:1598-1607. doi:10.1001/jama.2022.3385
4. USPSTF. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. Accessed June 24, 2022. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/statin-use-primary-prevention-cardiovascular-disease-adults#:~:text=The%20USPSTF%20recommends%20that%20clinicians,event%20of%2010%25%20or%20greater
5. Rhee TG, Kumar M, Ross JS, et al. Age-related trajectories of cardiovascular risk and use of aspirin and statin among U.S. adults aged 50 or older, 2011-2018. J Am Geriatr Soc. 2021;69:1272-1282. doi: 10.1111/jgs.17038
1. Davidson KW, Barry MJ, Mangione CM, et al. Aspirin use to prevent cardiovascular disease: US Preventive Services Task Force recommendation statement. JAMA. 2022;327:1577-1584. doi: 10.1001/jama.2022.4983
2. Guirguis-Blake JM, Evans CV, Perdue LA, et al. Aspirin use to prevent cardiovascular disease and colorectal cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;327:1585-1597. doi: 10.1001/jama.2022.3337
3. Dehmer SP, O’Keefe LR, Evans CV, et al. Aspirin use to prevent cardiovascular disease and colorectal cancer: updated modeling study for the US Preventive Services Task Force. JAMA. 2022;327:1598-1607. doi:10.1001/jama.2022.3385
4. USPSTF. Statin use for the primary prevention of cardiovascular disease in adults: preventive medication. Accessed June 24, 2022. https://uspreventiveservicestaskforce.org/uspstf/draft-recommendation/statin-use-primary-prevention-cardiovascular-disease-adults#:~:text=The%20USPSTF%20recommends%20that%20clinicians,event%20of%2010%25%20or%20greater
5. Rhee TG, Kumar M, Ross JS, et al. Age-related trajectories of cardiovascular risk and use of aspirin and statin among U.S. adults aged 50 or older, 2011-2018. J Am Geriatr Soc. 2021;69:1272-1282. doi: 10.1111/jgs.17038
Essential strategies and tactics for managing sickle cell disease
The group of disorders known as sickle cell disease (SCD) is one of the more common genetic hemoglobinopathies. Homozygous production of the S variant of hemoglobin (Hb) in red blood cells (RBCs) results in profound sickling under conditions of physiologic stress, a condition known as Hb SS disease. People with Hb SS disease are at risk of chronic hemolytic anemia, tissue ischemia that causes vaso-occlusive pain syndrome, and other vaso-occlusive complications.1 They also experience a > 20-year reduction in life expectancy, compared to age-matched controls; onset of risk of early death is usually after age 25 years.
People with heterozygous expression of the Hb S variant—that is, from one parent, and expression of Hb A from the other parent—are said to have sickle cell trait (SCT). They typically do not have symptoms of SCD, although they can experience vaso-occlusive pain under severe physiologic stress and suffer sudden death more often than age-matched controls. People who are heterozygous for Hb S but have another hemoglobinopathy (eg,
Alleviating the harsh burden of illness. All patients with SCD are more likely than age-matched counterparts to experience income loss because of their disability; the same loss is true for their caregivers. Such loss, when combined with time spent in the health care system, can be catastrophic.2,3 But this loss can be mitigated with access to regular, comprehensive health care that includes the steps described here to detect SCD early and reduce the likelihood of complications.4,5
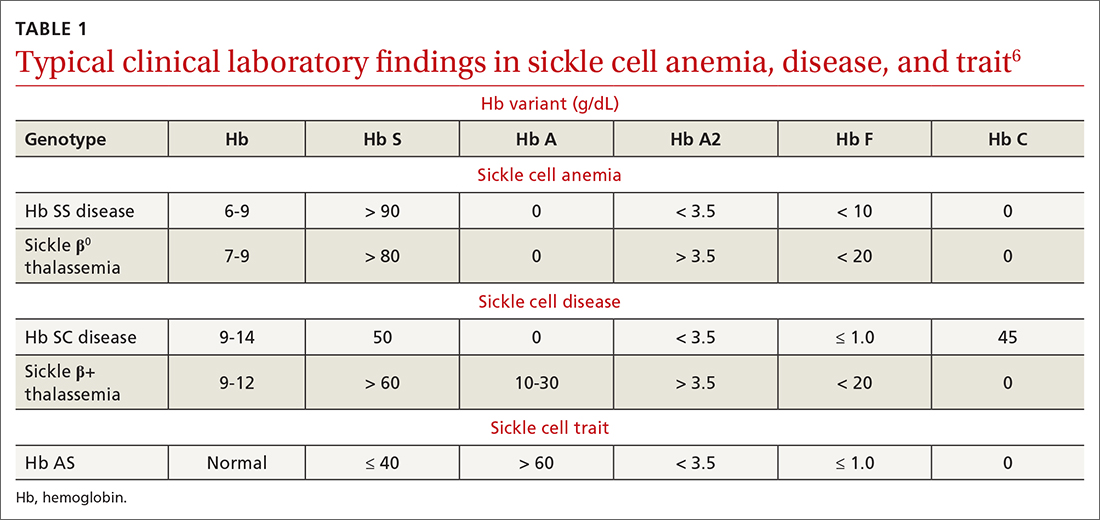
To begin, TABLE 16 lists typical laboratory findings and classifications in patients who are homozygous or heterozygous for Hb S, and therefore experience more severe Hb SS disease or milder SCD, respectively.
Who should be screened for hemoglobinopathy?
Because of the presence of the fetal Hb (Hb F) in newborns and infants, clinical signs of Hb SS before age 2 months are uncommon. Neonatal clinical laboratory testing is necessary for prompt identification of Hb SS; universal screening is now required by all states (although parents can opt out by claiming a religious exemption). A positive test result requires confirmatory testing: most often, Hb electrophoresis or DNA testing.
A confirmed positive homozygous (Hb SS) or heterozygous (Hb S) result is reported to the patient’s identified medical home for subsequent management. Thus, pediatric patients with SCD can be identified, and prophylactic treatment initiated, as early as possible. Later in the patient’s life, repeat screening for SCD and SCT is recommended at the initiation of pregnancy care7 and prior to the start of high-intensity physical training, as occurs in college and professional athletics and in certain branches of the military.8
Putting prevention into practice
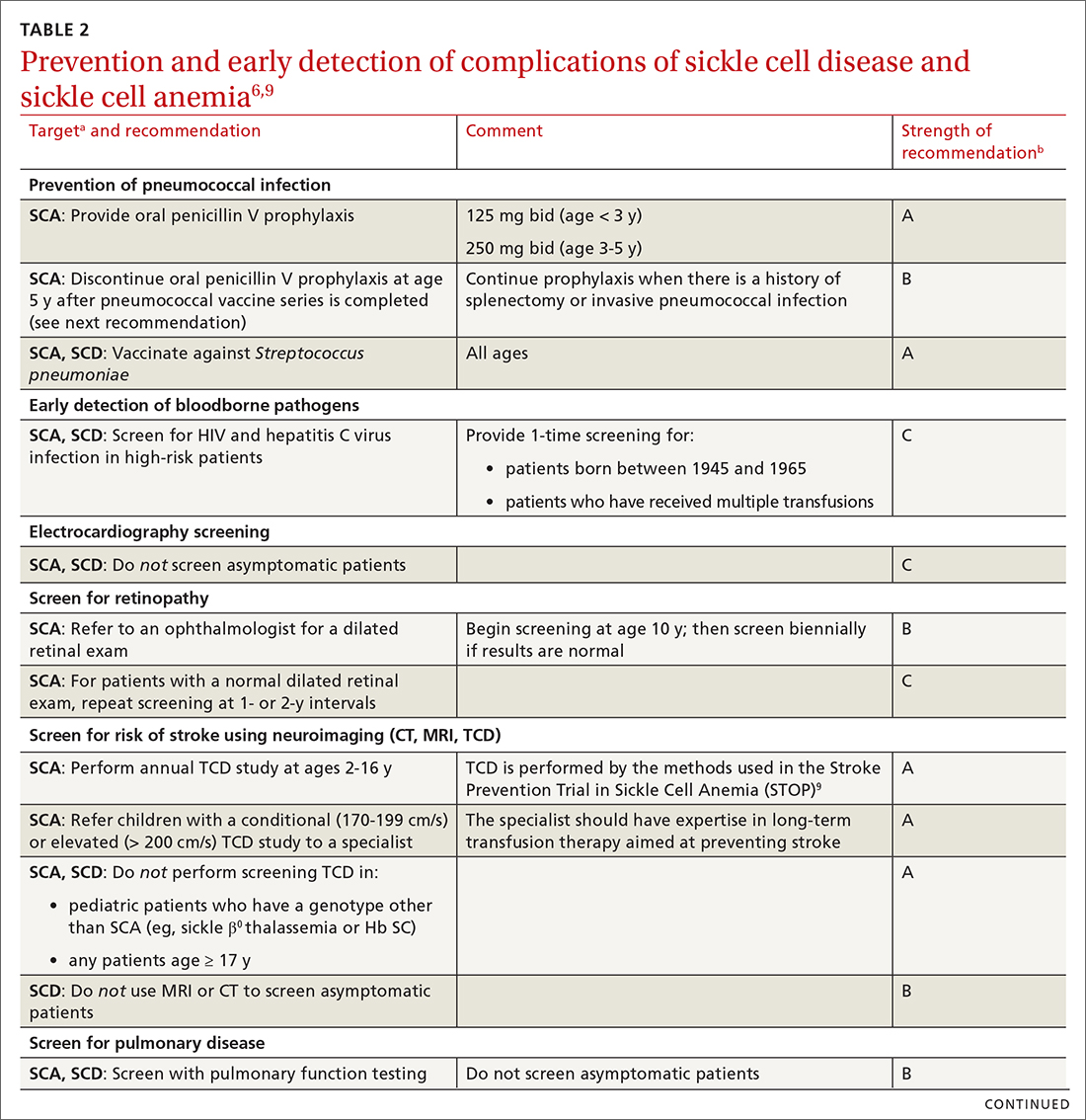
Some of the recommendations we make to prevent complications of SCD are directed only at patients with severe disease—ie, those who have Hb SS SCD or sickle β0 thalassemia (SCA); the rest apply to all patients with SCD (TABLE 26,9). (For patients with SCT, follow guidelines as you would for patients who do not have SCD, unless otherwise noted.)
Continue to: In addition, keep in mind...
In addition, keep in mind that preventive recommendations made by the US Preventive Services Task Force (Exhibit 5 in the Expert Panel Report)6 apply to all patients with SCD and SCT.
Prevention of invasive pneumococcal disease
All patients with SCD are assumed to have lifelong splenic dysfunction that begins in childhood. This is particularly true for those with SCA. In the absence of vaccination, the lifetime incidence of pneumococcal bacteremia resulting in serious complications is as high as 16% in SCD.10 In multiple randomized clinical trials, prophylactic penicillin dosing has proved beneficial in these patients, demonstrating a decrease in the risk of (1) pneumococcal infection and (2) early death during the study period, with minimal adverse effects.5
Prophylactic penicillin dosing should be initiated during infancy in patients with SCA. From ages 3 months to 3 years, the dosage of penicillin V is 125 mg twice daily; from 3 to 5 years, 250 mg twice daily. After age 5 years, the decision to continue penicillin is individualized, with consideration of prior severe pneumococcal infection and general preventive health maintenance. Penicillin-allergic patients can be given erythromycin. All patients with SCD who have had surgical splenectomy should be placed on antibiotic prophylaxis (ie, penicillin as dosed above).5
The polyvalent pneumococcal vaccine has resulted in significant protection against invasive pneumococcal disease; mortality from pneumococcal disease among patients with SCD who are younger than 14 years has decreased dramatically since the vaccine was introduced.6 For all patients with SCD, the standard PCV13 series should be administered beginning at age 6 weeks. A 2-dose series of the PPSV23 vaccine, which includes more Streptococcus pneumoniae serotypes than the PCV13 vaccine, should be administered beginning at age 2 years or 8 weeks after completion of the PCV13 series, whichever comes first.
Prevention of flu, COVID-19, and other vaccine-preventable illness
Influenza. Beginning at age 6 months, all patients with SCD should receive inactivated influenza vaccine annually at the beginning of the influenza season. Avoid using the live attenuated vaccine (Flumist) because of an associated increased risk of severe or complicated infection.11
Continue to: COVID-19, caused by severe...
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is especially problematic in patients with SCD12; infection causes mortality at a rate as high as 7%.5 The SARS-CoV-2 mRNA vaccine series is potentially lifesaving for these patients.12 In addition, at times of high community prevalence, make an effort to minimize patients’ exposure to SARS-CoV-2, by providing telemedicine visits.
Follow the immunization schedule. Patients with SCD should receive all standard recommended vaccinations (ie, those recommended by the Advisory Committee on Immunization Practices.a) Inactivated virus vaccines are preferred in SCD, when available. For patients who are behind on vaccinations, use a standard vaccine catch-up schedule.
Screening and prevention of complications such as stroke
Determining the risk of stroke. Patients with SCA who are not monitored have a 10% to 11% lifetime prevalence of stroke.5,6,10 An abnormal transcranial Doppler (TCD) study (defined as a time-averaged mean maximum velocity ≥ 200 cm/s in the distal internal carotid artery or proximal middle cerebral artery) is predictive of a 40% risk of stroke in patients with SCA. With chronic transfusion therapy, a 92% reduction in the risk of stroke is achievable.10
All patients with SCA should undergo annual screening with TCD ultrasonography from ages 2 to 16 years.6 Those who have an abnormal TCD study should receive chronic transfusion therapy. Screening is not recommended for patients with SCD or SCT.
Other complications. Screen and manage as follows:
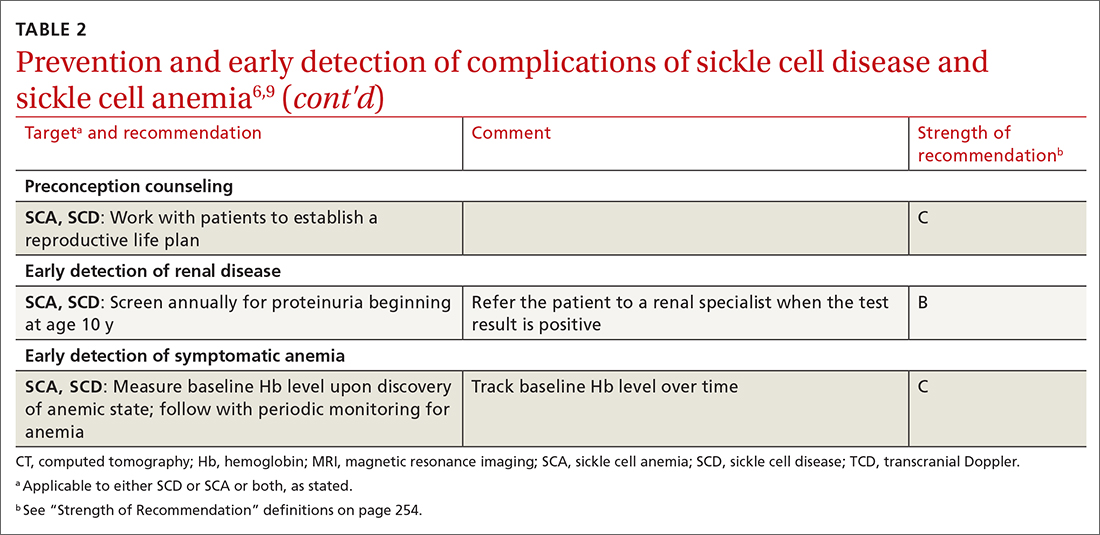
- Proteinuria. Left untreated, SCD can affect the kidneys and lead to renal failure. Annual screening for proteinuria is recommended beginning at age 10 years, with referral when the test is positive and reproducible.
- Lung disease and cardiovascular disease. Screening for progression of lung disease and for cardiovascular disease is not recommended in asymptomatic patients with SCD, except through the history.
- Blood pressure screening and management of hypertension are based on Joint National Committee (JNC 8) guidelines.13
- Screening for ocular complications by an eye care provider is recommended beginning at age 10 years.
TABLE 26,9 summarizes recommendations on the prevention and early detection of complications of SCD.
Continue to: Pregnancy planning
Pregnancy planning
The Centers for Disease Control and Prevention recommends that a “reproductive life plan” be part of every person’s health journey (TABLE 2).6,9 The plan is especially relevant for female patients who have a known heritable concern, such as SCD, in which a pregnancy is more likely to be complicated by growth restriction, preterm delivery, and fetal demise. These risks are reduced—but not eliminated—with intensive surveillance of the pregnancy. Pregnancy in patients with SCD is also more likely to be complicated by preeclampsia, venous thromboembolism, infection, and maternal death.
Other recommendations:
- Every patient with SCD should receive genetic counselling before conceiving, when possible.
- Pregnancy should be considered high risk in women who have SCD, and monitored as such.
- Women with SCD can use any method of contraception—none of which put them at increased risk of complications, compared to the general population. Rather, it is pregnancy that puts them at greater risk of morbidity and mortality in every age group.
Ambulatory management of acute complications
Vaso-occlusive pain crisis. The hallmark of SCD is the acute pain crisis. Almost all patients with SCD (and the occasional patient with SCT) will experience a pain crisis. In more affluent countries, management of an acute pain crisis almost always includes opioid analgesia.6
For the most part, pain crises manifest in a predictable pattern. Although patients with SCD might have acute pain, other causes of acute pain, such as an acute intra-abdominal process or (in older patients) a cardiac process, should be considered as well.
For patients having a vaso-occlusive pain crisis, achieving rapid analgesia is key to management. Ready availability of narcotics, at home or under observation, prevents subsequent hospitalization; nonsteroidal anti-inflammatory drugs can be used as adjuvant treatment in patients without contraindications.5,6 An individualized treatment plan, including access to analgesia at an appropriate dosage, should be negotiated, and adhered to, by the patient and the care team.
Continue to: Rapid access to higher-level care...
Rapid access to higher-level care, including parenteral analgesia, is important if outpatient management is desired. In addition, escalation to a higher level of care should occur if there is hypoxia (or another reason to suspect acute chest syndrome [ACS; discussed in a bit]) or dehydration that requires parenteral therapy. Use of nondrug therapy, such as heat, should be encouraged. The care team should work with the patient’s school or employer to negotiate time away through the federal Family Medical Leave Act of 1993, or other means, because a pain crisis is not a planned event.
Fever. Because of the risk of serious infection as a consequence of functional asplenia, fever is particularly worrisome in patients with SCA and problematic in patients with SCD. The increased risk begins as the physiologic level of Hb F declines beginning at age 2 months.
ACS, characterized by a combination of respiratory symptoms or new infiltrates, often manifests initially with fever, and can progress rapidly to death if treatment is delayed. The initial signs and symptoms may be subtle; suspicion should remain high in any patient with respiratory symptoms who is newly hypoxic, even those who do not have fever. Osteomyelitis, another febrile illness, is also potentially life threatening if not treated promptly.
All patients with SCD whose body temperature is > 101.3 °F should be evaluated with appropriate clinical laboratory testing (complete blood count; inflammatory markers, such as C-reactive protein; basic chemistry parameters; and other tests as indicated, including serum lactate and urine culture), blood culture, and chest radiography. Empiric parenteral antibiotics are required until the patient is known to be nonbacteremic, regardless of vaccination status. Outpatient follow-up and even outpatient management with ceftriaxone can be offered in select circumstances (eg, if the patient so desires or is nontoxic, and if close follow-up can be assured).14 If ACS is a possibility based on symptoms or radiographic findings, outpatient management is not an option.
Anemia. Patients with SCA, and some with SCD, have an Hb level that is chronically, sometimes critically, low. A baseline Hb level should be established for a patient with SCD and then monitored periodically. A drop in the Hb level > 2 g/dL from baseline (or an initial Hb level of 6 g/dL if the baseline is unknown) constitutes acute anemia. Patients in whom anemia has been diagnosed should be emergently evaluated for acute splenic sequestration, an aplastic episode, a delayed hemolytic transfusion reaction, ACS, or infection, and should be treated appropriately.
Continue to: Simple transfusion can be used...
Simple transfusion can be used in an acute setting to restore and maintain Hb at a safe level. Iron overload and formation of RBC alloantigen are associated with multiple transfusions; once either of these conditions is established, subsequent transfusion therapy can be harmful. Care must be taken to prescribe transfusion appropriately; leukocyte-depleted RBCs should be used when available.
It is important to define specific goals of transfusion to optimize its use. Patients who have received multiple transfusions should have enhanced monitoring for bloodborne infection, such as hepatitis C virus. Acute aplastic crises are caused by parvovirus B19; when other members of the household who have SCD are present, they should be monitored for this viral infection with serial measurement of Hb and white blood cell count.6
Other acute problems. Should stroke, acute renal failure, priapism, or hepatobiliary complications develop, evaluate the patient rapidly and refer them to the appropriate care team for management.
Management of chronic complications
Chronic pain is a problem for many patients with SCD. The etiology of this symptom should be investigated fully because a vaso-occlusive crisis is characterized by acute pain. Avascular necrosis or ulcers due to chronic vaso-occlusion should be managed definitively when possible. Adjuvant therapy for chronic pain, such as heat or massage, should be encouraged.
In some patients, chronic pain without objective findings develops over time and becomes unresponsive to nonopioid pharmacotherapy. Such patients might require chronic opioid therapy, the need for which is dictated by the ability of the patient to perform their activities of daily living. For patients who require long-term daily narcotic drugs, best practices—obtaining informed consent, using registries and contracts, random drug testing, and providing naloxone [Narcan] for overdose emergency use—should be employed.15
Continue to: Chronic anemia
Chronic anemia can be managed with transfusion when elevating the Hb level is required (eg, preoperatively, to prevent stroke, to manage priapism). For some patients, ongoing transfusion is required; care should be taken to avoid iron overload and hemolysis due to antibody formation. Ongoing surveillance for these complications is required.6
Other chronic problems. Patients with SCD who develop avascular necrosis, vaso-occlusive ulcers, pulmonary hypertension, renal disease, recurrent priapism, or ophthalmologic complications should be co-managed with a care team.6
Pharmacotherapy and SCA
A principal goal in the management of patients with SCA is prevention of vaso-occlusive events, including ACS and acute pain crises.
Hydroxyurea, a key component of SCA treatment, is a ribonucleotide reductase inhibitor that increases the level of Hb F, thus reducing the absolute number of symptomatic vaso-occlusive events and increasing arterial blood flow. It is most useful for patients who have multiple crises. The drug prolongs survival and reduces the need for transfusion and hospitalization.4,5
Hydroxyurea can be started in patients at age 9 months; blood testing should be performed at the start of treatment and the dosage titrated based on blood counts. Initial blood work includes:
- Hb level;
- Hb electrophoresis with the quantitative percentage of Hb F;
- complete blood count with differential and reticulocyte counts;
- chemistry profile (electrolytes, lactate dehydrogenase, total protein, albumin, total bilirubin);
- liver function tests (aspartate aminotransferase, alanine aminotransferase);
- measurement of renal function (blood urea nitrogen, creatinine);
- serum vitamin B12 and folate;
- serum iron, total iron-binding capacity, and ferritin;
- hepatitis B, hepatitis C, and parvovirus B19 antigen; and
- serologic testing for HIV.
Continue to: Testing should also...
Testing should also include a pregnancy test for postmenarchal females because hydroxyurea is in US Food and Drug Administration pregnancy risk category X.
Avoid hydroxyurea in lactating women; dose the drug renally in the setting of renal disease. Because hydroxyurea has a high rate of serious adverse effects and drug-drug interactions, it should be offered in conjunction with an individualized care plan.
Hydroxyurea can also be offered to patients with other forms of SCD who have recurrent vaso-occlusive symptoms.
Two newer medications improve oxygen delivery in patients with SCD. Voxelotor, approved in 2019, works to reduce Hb S polymerization by binding to the alpha chain of Hb S and, subsequently, increasing its oxygen affinity. The drug is generally well tolerated and can be used in patients with SCD who are ≥ 12 years.16 Crizanlizumab is a monoclonal antibody directed against P-selectin, an adhesion molecule located on endothelial cells and activated platelets. The efficacy of crizanlizumab was demonstrated in the SUSTAIN trial, in which it reduced vaso-occlusive pain in patients ≥ 16 years.17
All of these medications have a narrow toxic–therapeutic window. They should therefore be administered with the participation of a multidisciplinary care team.
Continue to: The need to coordinated, comprehensive care
The need for coordinated, comprehensive care
Patients with SCD report how challenging their disease is. All patients with SCD are more likely than age-matched counterparts to experience loss, including workdays for disability, educational potential, workdays for caregivers of affected children, and time spent in the hospital or the emergency department.4,5 These losses, with the concomitant stress associated with chronic illness and the struggle to manage recurrent pain crises and chronic complications, are often overwhelming.
Comprehensive care can, as we have illustrated in this discussion, mitigate these losses. Such care should include extensive education, genetic counseling, infection prevention, pain management, and implementation of evidence-based management guidelines.3,4,6 Patients with SCD report that their illness outlook would be better with
- greater provider knowledge of SCD,
- destigmatization of narcotics for SCD vaso-occlusive pain management,
- optimal coordination among members of the health care team, and
- improved transportation for appointments.
Patients also report that barriers associated with the unique US health care financing system are often insurmountable. As patients with SCD live longer, improved care management should focus on reducing these barriers and enhancing their quality of life.2,18,19
CORRESPONDENCE
Robert Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama College of Medicine, 1601 Center Street, 2N, Mobile, AL 36604; perkins@health. southalabama.edu
1. Lubeck D, Agodoa I, Bhakta N, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2:e1915374. doi:10.1001/jamanetworkopen.2019.15374
2. Swanson ME, Grosse SD, Kulkarni R. Disability among individuals with sickle cell disease: literature review from a public health perspective. Am J Prev Med. 2011;41(6 suppl 4):S390-S397. doi: 10.1016/j.amepre.2011.09.006
3. Moskowitz JT, Butensky E, Harmatz P, et al. Caregiving time in sickle cell disease: psychological effects in maternal caregivers. Pediatr Blood Cancer. 2007;48:64-71. doi:10.1002/pbc.20792
4. Mehta SR, Afenyi-Annan A, Lottenberg R. Opportunities to improve outcomes in sickle cell disease. Am Fam Phys. 2006;74:303-310.
5. Yawn BP, John-Sowah J. Management of sickle cell disease: recommendations from the 2014 Expert Panel Report. Am Fam Phys. 2015;92:1069-1077.
6. National Heart, Lung, and Blood Institute, National Institutes of Health. Evidence-based management of sickle cell disease. Expert Panel Report, 2014. Accessed June 9, 2022. www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines
7. Committee Opinion No. 690: Carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40. doi: 10.1097/AOG.0000000000001951
8. Jordan LB, Smith-Whitley K, Treadwell MJ, et al. Screening U.S. college athletes for their sickle cell disease carrier status. Am J Prev Med. 2011;41:S406-S412. doi: 10.1016/j.amepre.2011.09.014
9. Adams RJ, McKie VC, Brambilla D, et al. Stroke prevention trial in sickle cell anemia. Control Clin Trials. 1998;19:110-129. doi: 10.1016/s0197-2456(97)00099-8
10. Alzahrani F, Alaidarous K, Alqarni S, et al. Incidence and predictors of bacterial infections in febrile children with sickle cell disease. Int J Pediatr Adolesc Med. 2021;8:236-238 doi: 10.1016/j.ijpam.2020.12.005
11. Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2021-2022. Pediatrics. 2021;148: e2021053745. doi: 10.1542/peds.2021-053745
12. Centers for Disease Control and Prevention. Study finds people with sickle cell disease who developed coronavirus disease have high rates of hospitalization, intensive care unit admission, and death. October 20, 2020. Accessed June 9, 2022. www.cdc.gov/ncbddd/sicklecell/features/scd-and-covid-19.html
13. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
14. Baskin MN, Goh XL, Heeney MM, et al. Bacteremia risk and outpatient management of febrile patients with sickle cell disease. Pediatrics. 2013;131:1035-1041. doi: 10.1542/peds.2012-2139
15. Osunkwo I, Veeramreddy P, Arnall J, et al. Use of buprenorphine/naloxone in amelio rating acute care utilization and chronic opioid use in adults with sickle cell disease. Blood. 2019;134 (suppl 1):790. doi: 10.1182/blood-2019-126589
16. Vichinsky E, Hoppe CC, Ataga KI, et al; HOPE Trial Investigators. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381:509-519. doi: 10.1056/NEJMoa1903212
17. Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376:429-439. doi: 10.1056/NEJMoa1611770
18. Dixit R, Nettem S, Madan SS, et al. Folate supplementation in people with sickle cell disease. Cochrane Database Syst Rev. 2016;2:CD011130. doi: 10.1002/14651858.CD011130.pub2
19. Brennan-Cook J, Bonnabeau E, Aponte R, et al. Barriers to care for persons with sickle cell disease: the case manager’s opportunity to improve patient outcomes. Prof Case Manag. 2018;23:213-219. doi: 10.1097/NCM.0000000000000260
The group of disorders known as sickle cell disease (SCD) is one of the more common genetic hemoglobinopathies. Homozygous production of the S variant of hemoglobin (Hb) in red blood cells (RBCs) results in profound sickling under conditions of physiologic stress, a condition known as Hb SS disease. People with Hb SS disease are at risk of chronic hemolytic anemia, tissue ischemia that causes vaso-occlusive pain syndrome, and other vaso-occlusive complications.1 They also experience a > 20-year reduction in life expectancy, compared to age-matched controls; onset of risk of early death is usually after age 25 years.
People with heterozygous expression of the Hb S variant—that is, from one parent, and expression of Hb A from the other parent—are said to have sickle cell trait (SCT). They typically do not have symptoms of SCD, although they can experience vaso-occlusive pain under severe physiologic stress and suffer sudden death more often than age-matched controls. People who are heterozygous for Hb S but have another hemoglobinopathy (eg,
Alleviating the harsh burden of illness. All patients with SCD are more likely than age-matched counterparts to experience income loss because of their disability; the same loss is true for their caregivers. Such loss, when combined with time spent in the health care system, can be catastrophic.2,3 But this loss can be mitigated with access to regular, comprehensive health care that includes the steps described here to detect SCD early and reduce the likelihood of complications.4,5
To begin, TABLE 16 lists typical laboratory findings and classifications in patients who are homozygous or heterozygous for Hb S, and therefore experience more severe Hb SS disease or milder SCD, respectively.

Who should be screened for hemoglobinopathy?
Because of the presence of the fetal Hb (Hb F) in newborns and infants, clinical signs of Hb SS before age 2 months are uncommon. Neonatal clinical laboratory testing is necessary for prompt identification of Hb SS; universal screening is now required by all states (although parents can opt out by claiming a religious exemption). A positive test result requires confirmatory testing: most often, Hb electrophoresis or DNA testing.
A confirmed positive homozygous (Hb SS) or heterozygous (Hb S) result is reported to the patient’s identified medical home for subsequent management. Thus, pediatric patients with SCD can be identified, and prophylactic treatment initiated, as early as possible. Later in the patient’s life, repeat screening for SCD and SCT is recommended at the initiation of pregnancy care7 and prior to the start of high-intensity physical training, as occurs in college and professional athletics and in certain branches of the military.8

Putting prevention into practice
Some of the recommendations we make to prevent complications of SCD are directed only at patients with severe disease—ie, those who have Hb SS SCD or sickle β0 thalassemia (SCA); the rest apply to all patients with SCD (TABLE 26,9). (For patients with SCT, follow guidelines as you would for patients who do not have SCD, unless otherwise noted.)

Continue to: In addition, keep in mind...
In addition, keep in mind that preventive recommendations made by the US Preventive Services Task Force (Exhibit 5 in the Expert Panel Report)6 apply to all patients with SCD and SCT.
Prevention of invasive pneumococcal disease
All patients with SCD are assumed to have lifelong splenic dysfunction that begins in childhood. This is particularly true for those with SCA. In the absence of vaccination, the lifetime incidence of pneumococcal bacteremia resulting in serious complications is as high as 16% in SCD.10 In multiple randomized clinical trials, prophylactic penicillin dosing has proved beneficial in these patients, demonstrating a decrease in the risk of (1) pneumococcal infection and (2) early death during the study period, with minimal adverse effects.5
Prophylactic penicillin dosing should be initiated during infancy in patients with SCA. From ages 3 months to 3 years, the dosage of penicillin V is 125 mg twice daily; from 3 to 5 years, 250 mg twice daily. After age 5 years, the decision to continue penicillin is individualized, with consideration of prior severe pneumococcal infection and general preventive health maintenance. Penicillin-allergic patients can be given erythromycin. All patients with SCD who have had surgical splenectomy should be placed on antibiotic prophylaxis (ie, penicillin as dosed above).5
The polyvalent pneumococcal vaccine has resulted in significant protection against invasive pneumococcal disease; mortality from pneumococcal disease among patients with SCD who are younger than 14 years has decreased dramatically since the vaccine was introduced.6 For all patients with SCD, the standard PCV13 series should be administered beginning at age 6 weeks. A 2-dose series of the PPSV23 vaccine, which includes more Streptococcus pneumoniae serotypes than the PCV13 vaccine, should be administered beginning at age 2 years or 8 weeks after completion of the PCV13 series, whichever comes first.
Prevention of flu, COVID-19, and other vaccine-preventable illness
Influenza. Beginning at age 6 months, all patients with SCD should receive inactivated influenza vaccine annually at the beginning of the influenza season. Avoid using the live attenuated vaccine (Flumist) because of an associated increased risk of severe or complicated infection.11
Continue to: COVID-19, caused by severe...
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is especially problematic in patients with SCD12; infection causes mortality at a rate as high as 7%.5 The SARS-CoV-2 mRNA vaccine series is potentially lifesaving for these patients.12 In addition, at times of high community prevalence, make an effort to minimize patients’ exposure to SARS-CoV-2, by providing telemedicine visits.
Follow the immunization schedule. Patients with SCD should receive all standard recommended vaccinations (ie, those recommended by the Advisory Committee on Immunization Practices.a) Inactivated virus vaccines are preferred in SCD, when available. For patients who are behind on vaccinations, use a standard vaccine catch-up schedule.
Screening and prevention of complications such as stroke
Determining the risk of stroke. Patients with SCA who are not monitored have a 10% to 11% lifetime prevalence of stroke.5,6,10 An abnormal transcranial Doppler (TCD) study (defined as a time-averaged mean maximum velocity ≥ 200 cm/s in the distal internal carotid artery or proximal middle cerebral artery) is predictive of a 40% risk of stroke in patients with SCA. With chronic transfusion therapy, a 92% reduction in the risk of stroke is achievable.10
All patients with SCA should undergo annual screening with TCD ultrasonography from ages 2 to 16 years.6 Those who have an abnormal TCD study should receive chronic transfusion therapy. Screening is not recommended for patients with SCD or SCT.
Other complications. Screen and manage as follows:
- Proteinuria. Left untreated, SCD can affect the kidneys and lead to renal failure. Annual screening for proteinuria is recommended beginning at age 10 years, with referral when the test is positive and reproducible.
- Lung disease and cardiovascular disease. Screening for progression of lung disease and for cardiovascular disease is not recommended in asymptomatic patients with SCD, except through the history.
- Blood pressure screening and management of hypertension are based on Joint National Committee (JNC 8) guidelines.13
- Screening for ocular complications by an eye care provider is recommended beginning at age 10 years.
TABLE 26,9 summarizes recommendations on the prevention and early detection of complications of SCD.
Continue to: Pregnancy planning
Pregnancy planning
The Centers for Disease Control and Prevention recommends that a “reproductive life plan” be part of every person’s health journey (TABLE 2).6,9 The plan is especially relevant for female patients who have a known heritable concern, such as SCD, in which a pregnancy is more likely to be complicated by growth restriction, preterm delivery, and fetal demise. These risks are reduced—but not eliminated—with intensive surveillance of the pregnancy. Pregnancy in patients with SCD is also more likely to be complicated by preeclampsia, venous thromboembolism, infection, and maternal death.
Other recommendations:
- Every patient with SCD should receive genetic counselling before conceiving, when possible.
- Pregnancy should be considered high risk in women who have SCD, and monitored as such.
- Women with SCD can use any method of contraception—none of which put them at increased risk of complications, compared to the general population. Rather, it is pregnancy that puts them at greater risk of morbidity and mortality in every age group.
Ambulatory management of acute complications
Vaso-occlusive pain crisis. The hallmark of SCD is the acute pain crisis. Almost all patients with SCD (and the occasional patient with SCT) will experience a pain crisis. In more affluent countries, management of an acute pain crisis almost always includes opioid analgesia.6
For the most part, pain crises manifest in a predictable pattern. Although patients with SCD might have acute pain, other causes of acute pain, such as an acute intra-abdominal process or (in older patients) a cardiac process, should be considered as well.
For patients having a vaso-occlusive pain crisis, achieving rapid analgesia is key to management. Ready availability of narcotics, at home or under observation, prevents subsequent hospitalization; nonsteroidal anti-inflammatory drugs can be used as adjuvant treatment in patients without contraindications.5,6 An individualized treatment plan, including access to analgesia at an appropriate dosage, should be negotiated, and adhered to, by the patient and the care team.
Continue to: Rapid access to higher-level care...
Rapid access to higher-level care, including parenteral analgesia, is important if outpatient management is desired. In addition, escalation to a higher level of care should occur if there is hypoxia (or another reason to suspect acute chest syndrome [ACS; discussed in a bit]) or dehydration that requires parenteral therapy. Use of nondrug therapy, such as heat, should be encouraged. The care team should work with the patient’s school or employer to negotiate time away through the federal Family Medical Leave Act of 1993, or other means, because a pain crisis is not a planned event.
Fever. Because of the risk of serious infection as a consequence of functional asplenia, fever is particularly worrisome in patients with SCA and problematic in patients with SCD. The increased risk begins as the physiologic level of Hb F declines beginning at age 2 months.
ACS, characterized by a combination of respiratory symptoms or new infiltrates, often manifests initially with fever, and can progress rapidly to death if treatment is delayed. The initial signs and symptoms may be subtle; suspicion should remain high in any patient with respiratory symptoms who is newly hypoxic, even those who do not have fever. Osteomyelitis, another febrile illness, is also potentially life threatening if not treated promptly.
All patients with SCD whose body temperature is > 101.3 °F should be evaluated with appropriate clinical laboratory testing (complete blood count; inflammatory markers, such as C-reactive protein; basic chemistry parameters; and other tests as indicated, including serum lactate and urine culture), blood culture, and chest radiography. Empiric parenteral antibiotics are required until the patient is known to be nonbacteremic, regardless of vaccination status. Outpatient follow-up and even outpatient management with ceftriaxone can be offered in select circumstances (eg, if the patient so desires or is nontoxic, and if close follow-up can be assured).14 If ACS is a possibility based on symptoms or radiographic findings, outpatient management is not an option.
Anemia. Patients with SCA, and some with SCD, have an Hb level that is chronically, sometimes critically, low. A baseline Hb level should be established for a patient with SCD and then monitored periodically. A drop in the Hb level > 2 g/dL from baseline (or an initial Hb level of 6 g/dL if the baseline is unknown) constitutes acute anemia. Patients in whom anemia has been diagnosed should be emergently evaluated for acute splenic sequestration, an aplastic episode, a delayed hemolytic transfusion reaction, ACS, or infection, and should be treated appropriately.
Continue to: Simple transfusion can be used...
Simple transfusion can be used in an acute setting to restore and maintain Hb at a safe level. Iron overload and formation of RBC alloantigen are associated with multiple transfusions; once either of these conditions is established, subsequent transfusion therapy can be harmful. Care must be taken to prescribe transfusion appropriately; leukocyte-depleted RBCs should be used when available.
It is important to define specific goals of transfusion to optimize its use. Patients who have received multiple transfusions should have enhanced monitoring for bloodborne infection, such as hepatitis C virus. Acute aplastic crises are caused by parvovirus B19; when other members of the household who have SCD are present, they should be monitored for this viral infection with serial measurement of Hb and white blood cell count.6
Other acute problems. Should stroke, acute renal failure, priapism, or hepatobiliary complications develop, evaluate the patient rapidly and refer them to the appropriate care team for management.
Management of chronic complications
Chronic pain is a problem for many patients with SCD. The etiology of this symptom should be investigated fully because a vaso-occlusive crisis is characterized by acute pain. Avascular necrosis or ulcers due to chronic vaso-occlusion should be managed definitively when possible. Adjuvant therapy for chronic pain, such as heat or massage, should be encouraged.
In some patients, chronic pain without objective findings develops over time and becomes unresponsive to nonopioid pharmacotherapy. Such patients might require chronic opioid therapy, the need for which is dictated by the ability of the patient to perform their activities of daily living. For patients who require long-term daily narcotic drugs, best practices—obtaining informed consent, using registries and contracts, random drug testing, and providing naloxone [Narcan] for overdose emergency use—should be employed.15
Continue to: Chronic anemia
Chronic anemia can be managed with transfusion when elevating the Hb level is required (eg, preoperatively, to prevent stroke, to manage priapism). For some patients, ongoing transfusion is required; care should be taken to avoid iron overload and hemolysis due to antibody formation. Ongoing surveillance for these complications is required.6
Other chronic problems. Patients with SCD who develop avascular necrosis, vaso-occlusive ulcers, pulmonary hypertension, renal disease, recurrent priapism, or ophthalmologic complications should be co-managed with a care team.6
Pharmacotherapy and SCA
A principal goal in the management of patients with SCA is prevention of vaso-occlusive events, including ACS and acute pain crises.
Hydroxyurea, a key component of SCA treatment, is a ribonucleotide reductase inhibitor that increases the level of Hb F, thus reducing the absolute number of symptomatic vaso-occlusive events and increasing arterial blood flow. It is most useful for patients who have multiple crises. The drug prolongs survival and reduces the need for transfusion and hospitalization.4,5
Hydroxyurea can be started in patients at age 9 months; blood testing should be performed at the start of treatment and the dosage titrated based on blood counts. Initial blood work includes:
- Hb level;
- Hb electrophoresis with the quantitative percentage of Hb F;
- complete blood count with differential and reticulocyte counts;
- chemistry profile (electrolytes, lactate dehydrogenase, total protein, albumin, total bilirubin);
- liver function tests (aspartate aminotransferase, alanine aminotransferase);
- measurement of renal function (blood urea nitrogen, creatinine);
- serum vitamin B12 and folate;
- serum iron, total iron-binding capacity, and ferritin;
- hepatitis B, hepatitis C, and parvovirus B19 antigen; and
- serologic testing for HIV.
Continue to: Testing should also...
Testing should also include a pregnancy test for postmenarchal females because hydroxyurea is in US Food and Drug Administration pregnancy risk category X.
Avoid hydroxyurea in lactating women; dose the drug renally in the setting of renal disease. Because hydroxyurea has a high rate of serious adverse effects and drug-drug interactions, it should be offered in conjunction with an individualized care plan.
Hydroxyurea can also be offered to patients with other forms of SCD who have recurrent vaso-occlusive symptoms.
Two newer medications improve oxygen delivery in patients with SCD. Voxelotor, approved in 2019, works to reduce Hb S polymerization by binding to the alpha chain of Hb S and, subsequently, increasing its oxygen affinity. The drug is generally well tolerated and can be used in patients with SCD who are ≥ 12 years.16 Crizanlizumab is a monoclonal antibody directed against P-selectin, an adhesion molecule located on endothelial cells and activated platelets. The efficacy of crizanlizumab was demonstrated in the SUSTAIN trial, in which it reduced vaso-occlusive pain in patients ≥ 16 years.17
All of these medications have a narrow toxic–therapeutic window. They should therefore be administered with the participation of a multidisciplinary care team.
Continue to: The need to coordinated, comprehensive care
The need for coordinated, comprehensive care
Patients with SCD report how challenging their disease is. All patients with SCD are more likely than age-matched counterparts to experience loss, including workdays for disability, educational potential, workdays for caregivers of affected children, and time spent in the hospital or the emergency department.4,5 These losses, with the concomitant stress associated with chronic illness and the struggle to manage recurrent pain crises and chronic complications, are often overwhelming.
Comprehensive care can, as we have illustrated in this discussion, mitigate these losses. Such care should include extensive education, genetic counseling, infection prevention, pain management, and implementation of evidence-based management guidelines.3,4,6 Patients with SCD report that their illness outlook would be better with
- greater provider knowledge of SCD,
- destigmatization of narcotics for SCD vaso-occlusive pain management,
- optimal coordination among members of the health care team, and
- improved transportation for appointments.
Patients also report that barriers associated with the unique US health care financing system are often insurmountable. As patients with SCD live longer, improved care management should focus on reducing these barriers and enhancing their quality of life.2,18,19
CORRESPONDENCE
Robert Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama College of Medicine, 1601 Center Street, 2N, Mobile, AL 36604; perkins@health. southalabama.edu
The group of disorders known as sickle cell disease (SCD) is one of the more common genetic hemoglobinopathies. Homozygous production of the S variant of hemoglobin (Hb) in red blood cells (RBCs) results in profound sickling under conditions of physiologic stress, a condition known as Hb SS disease. People with Hb SS disease are at risk of chronic hemolytic anemia, tissue ischemia that causes vaso-occlusive pain syndrome, and other vaso-occlusive complications.1 They also experience a > 20-year reduction in life expectancy, compared to age-matched controls; onset of risk of early death is usually after age 25 years.
People with heterozygous expression of the Hb S variant—that is, from one parent, and expression of Hb A from the other parent—are said to have sickle cell trait (SCT). They typically do not have symptoms of SCD, although they can experience vaso-occlusive pain under severe physiologic stress and suffer sudden death more often than age-matched controls. People who are heterozygous for Hb S but have another hemoglobinopathy (eg,
Alleviating the harsh burden of illness. All patients with SCD are more likely than age-matched counterparts to experience income loss because of their disability; the same loss is true for their caregivers. Such loss, when combined with time spent in the health care system, can be catastrophic.2,3 But this loss can be mitigated with access to regular, comprehensive health care that includes the steps described here to detect SCD early and reduce the likelihood of complications.4,5
To begin, TABLE 16 lists typical laboratory findings and classifications in patients who are homozygous or heterozygous for Hb S, and therefore experience more severe Hb SS disease or milder SCD, respectively.

Who should be screened for hemoglobinopathy?
Because of the presence of the fetal Hb (Hb F) in newborns and infants, clinical signs of Hb SS before age 2 months are uncommon. Neonatal clinical laboratory testing is necessary for prompt identification of Hb SS; universal screening is now required by all states (although parents can opt out by claiming a religious exemption). A positive test result requires confirmatory testing: most often, Hb electrophoresis or DNA testing.
A confirmed positive homozygous (Hb SS) or heterozygous (Hb S) result is reported to the patient’s identified medical home for subsequent management. Thus, pediatric patients with SCD can be identified, and prophylactic treatment initiated, as early as possible. Later in the patient’s life, repeat screening for SCD and SCT is recommended at the initiation of pregnancy care7 and prior to the start of high-intensity physical training, as occurs in college and professional athletics and in certain branches of the military.8

Putting prevention into practice
Some of the recommendations we make to prevent complications of SCD are directed only at patients with severe disease—ie, those who have Hb SS SCD or sickle β0 thalassemia (SCA); the rest apply to all patients with SCD (TABLE 26,9). (For patients with SCT, follow guidelines as you would for patients who do not have SCD, unless otherwise noted.)

Continue to: In addition, keep in mind...
In addition, keep in mind that preventive recommendations made by the US Preventive Services Task Force (Exhibit 5 in the Expert Panel Report)6 apply to all patients with SCD and SCT.
Prevention of invasive pneumococcal disease
All patients with SCD are assumed to have lifelong splenic dysfunction that begins in childhood. This is particularly true for those with SCA. In the absence of vaccination, the lifetime incidence of pneumococcal bacteremia resulting in serious complications is as high as 16% in SCD.10 In multiple randomized clinical trials, prophylactic penicillin dosing has proved beneficial in these patients, demonstrating a decrease in the risk of (1) pneumococcal infection and (2) early death during the study period, with minimal adverse effects.5
Prophylactic penicillin dosing should be initiated during infancy in patients with SCA. From ages 3 months to 3 years, the dosage of penicillin V is 125 mg twice daily; from 3 to 5 years, 250 mg twice daily. After age 5 years, the decision to continue penicillin is individualized, with consideration of prior severe pneumococcal infection and general preventive health maintenance. Penicillin-allergic patients can be given erythromycin. All patients with SCD who have had surgical splenectomy should be placed on antibiotic prophylaxis (ie, penicillin as dosed above).5
The polyvalent pneumococcal vaccine has resulted in significant protection against invasive pneumococcal disease; mortality from pneumococcal disease among patients with SCD who are younger than 14 years has decreased dramatically since the vaccine was introduced.6 For all patients with SCD, the standard PCV13 series should be administered beginning at age 6 weeks. A 2-dose series of the PPSV23 vaccine, which includes more Streptococcus pneumoniae serotypes than the PCV13 vaccine, should be administered beginning at age 2 years or 8 weeks after completion of the PCV13 series, whichever comes first.
Prevention of flu, COVID-19, and other vaccine-preventable illness
Influenza. Beginning at age 6 months, all patients with SCD should receive inactivated influenza vaccine annually at the beginning of the influenza season. Avoid using the live attenuated vaccine (Flumist) because of an associated increased risk of severe or complicated infection.11
Continue to: COVID-19, caused by severe...
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is especially problematic in patients with SCD12; infection causes mortality at a rate as high as 7%.5 The SARS-CoV-2 mRNA vaccine series is potentially lifesaving for these patients.12 In addition, at times of high community prevalence, make an effort to minimize patients’ exposure to SARS-CoV-2, by providing telemedicine visits.
Follow the immunization schedule. Patients with SCD should receive all standard recommended vaccinations (ie, those recommended by the Advisory Committee on Immunization Practices.a) Inactivated virus vaccines are preferred in SCD, when available. For patients who are behind on vaccinations, use a standard vaccine catch-up schedule.
Screening and prevention of complications such as stroke
Determining the risk of stroke. Patients with SCA who are not monitored have a 10% to 11% lifetime prevalence of stroke.5,6,10 An abnormal transcranial Doppler (TCD) study (defined as a time-averaged mean maximum velocity ≥ 200 cm/s in the distal internal carotid artery or proximal middle cerebral artery) is predictive of a 40% risk of stroke in patients with SCA. With chronic transfusion therapy, a 92% reduction in the risk of stroke is achievable.10
All patients with SCA should undergo annual screening with TCD ultrasonography from ages 2 to 16 years.6 Those who have an abnormal TCD study should receive chronic transfusion therapy. Screening is not recommended for patients with SCD or SCT.
Other complications. Screen and manage as follows:
- Proteinuria. Left untreated, SCD can affect the kidneys and lead to renal failure. Annual screening for proteinuria is recommended beginning at age 10 years, with referral when the test is positive and reproducible.
- Lung disease and cardiovascular disease. Screening for progression of lung disease and for cardiovascular disease is not recommended in asymptomatic patients with SCD, except through the history.
- Blood pressure screening and management of hypertension are based on Joint National Committee (JNC 8) guidelines.13
- Screening for ocular complications by an eye care provider is recommended beginning at age 10 years.
TABLE 26,9 summarizes recommendations on the prevention and early detection of complications of SCD.
Continue to: Pregnancy planning
Pregnancy planning
The Centers for Disease Control and Prevention recommends that a “reproductive life plan” be part of every person’s health journey (TABLE 2).6,9 The plan is especially relevant for female patients who have a known heritable concern, such as SCD, in which a pregnancy is more likely to be complicated by growth restriction, preterm delivery, and fetal demise. These risks are reduced—but not eliminated—with intensive surveillance of the pregnancy. Pregnancy in patients with SCD is also more likely to be complicated by preeclampsia, venous thromboembolism, infection, and maternal death.
Other recommendations:
- Every patient with SCD should receive genetic counselling before conceiving, when possible.
- Pregnancy should be considered high risk in women who have SCD, and monitored as such.
- Women with SCD can use any method of contraception—none of which put them at increased risk of complications, compared to the general population. Rather, it is pregnancy that puts them at greater risk of morbidity and mortality in every age group.
Ambulatory management of acute complications
Vaso-occlusive pain crisis. The hallmark of SCD is the acute pain crisis. Almost all patients with SCD (and the occasional patient with SCT) will experience a pain crisis. In more affluent countries, management of an acute pain crisis almost always includes opioid analgesia.6
For the most part, pain crises manifest in a predictable pattern. Although patients with SCD might have acute pain, other causes of acute pain, such as an acute intra-abdominal process or (in older patients) a cardiac process, should be considered as well.
For patients having a vaso-occlusive pain crisis, achieving rapid analgesia is key to management. Ready availability of narcotics, at home or under observation, prevents subsequent hospitalization; nonsteroidal anti-inflammatory drugs can be used as adjuvant treatment in patients without contraindications.5,6 An individualized treatment plan, including access to analgesia at an appropriate dosage, should be negotiated, and adhered to, by the patient and the care team.
Continue to: Rapid access to higher-level care...
Rapid access to higher-level care, including parenteral analgesia, is important if outpatient management is desired. In addition, escalation to a higher level of care should occur if there is hypoxia (or another reason to suspect acute chest syndrome [ACS; discussed in a bit]) or dehydration that requires parenteral therapy. Use of nondrug therapy, such as heat, should be encouraged. The care team should work with the patient’s school or employer to negotiate time away through the federal Family Medical Leave Act of 1993, or other means, because a pain crisis is not a planned event.
Fever. Because of the risk of serious infection as a consequence of functional asplenia, fever is particularly worrisome in patients with SCA and problematic in patients with SCD. The increased risk begins as the physiologic level of Hb F declines beginning at age 2 months.
ACS, characterized by a combination of respiratory symptoms or new infiltrates, often manifests initially with fever, and can progress rapidly to death if treatment is delayed. The initial signs and symptoms may be subtle; suspicion should remain high in any patient with respiratory symptoms who is newly hypoxic, even those who do not have fever. Osteomyelitis, another febrile illness, is also potentially life threatening if not treated promptly.
All patients with SCD whose body temperature is > 101.3 °F should be evaluated with appropriate clinical laboratory testing (complete blood count; inflammatory markers, such as C-reactive protein; basic chemistry parameters; and other tests as indicated, including serum lactate and urine culture), blood culture, and chest radiography. Empiric parenteral antibiotics are required until the patient is known to be nonbacteremic, regardless of vaccination status. Outpatient follow-up and even outpatient management with ceftriaxone can be offered in select circumstances (eg, if the patient so desires or is nontoxic, and if close follow-up can be assured).14 If ACS is a possibility based on symptoms or radiographic findings, outpatient management is not an option.
Anemia. Patients with SCA, and some with SCD, have an Hb level that is chronically, sometimes critically, low. A baseline Hb level should be established for a patient with SCD and then monitored periodically. A drop in the Hb level > 2 g/dL from baseline (or an initial Hb level of 6 g/dL if the baseline is unknown) constitutes acute anemia. Patients in whom anemia has been diagnosed should be emergently evaluated for acute splenic sequestration, an aplastic episode, a delayed hemolytic transfusion reaction, ACS, or infection, and should be treated appropriately.
Continue to: Simple transfusion can be used...
Simple transfusion can be used in an acute setting to restore and maintain Hb at a safe level. Iron overload and formation of RBC alloantigen are associated with multiple transfusions; once either of these conditions is established, subsequent transfusion therapy can be harmful. Care must be taken to prescribe transfusion appropriately; leukocyte-depleted RBCs should be used when available.
It is important to define specific goals of transfusion to optimize its use. Patients who have received multiple transfusions should have enhanced monitoring for bloodborne infection, such as hepatitis C virus. Acute aplastic crises are caused by parvovirus B19; when other members of the household who have SCD are present, they should be monitored for this viral infection with serial measurement of Hb and white blood cell count.6
Other acute problems. Should stroke, acute renal failure, priapism, or hepatobiliary complications develop, evaluate the patient rapidly and refer them to the appropriate care team for management.
Management of chronic complications
Chronic pain is a problem for many patients with SCD. The etiology of this symptom should be investigated fully because a vaso-occlusive crisis is characterized by acute pain. Avascular necrosis or ulcers due to chronic vaso-occlusion should be managed definitively when possible. Adjuvant therapy for chronic pain, such as heat or massage, should be encouraged.
In some patients, chronic pain without objective findings develops over time and becomes unresponsive to nonopioid pharmacotherapy. Such patients might require chronic opioid therapy, the need for which is dictated by the ability of the patient to perform their activities of daily living. For patients who require long-term daily narcotic drugs, best practices—obtaining informed consent, using registries and contracts, random drug testing, and providing naloxone [Narcan] for overdose emergency use—should be employed.15
Continue to: Chronic anemia
Chronic anemia can be managed with transfusion when elevating the Hb level is required (eg, preoperatively, to prevent stroke, to manage priapism). For some patients, ongoing transfusion is required; care should be taken to avoid iron overload and hemolysis due to antibody formation. Ongoing surveillance for these complications is required.6
Other chronic problems. Patients with SCD who develop avascular necrosis, vaso-occlusive ulcers, pulmonary hypertension, renal disease, recurrent priapism, or ophthalmologic complications should be co-managed with a care team.6
Pharmacotherapy and SCA
A principal goal in the management of patients with SCA is prevention of vaso-occlusive events, including ACS and acute pain crises.
Hydroxyurea, a key component of SCA treatment, is a ribonucleotide reductase inhibitor that increases the level of Hb F, thus reducing the absolute number of symptomatic vaso-occlusive events and increasing arterial blood flow. It is most useful for patients who have multiple crises. The drug prolongs survival and reduces the need for transfusion and hospitalization.4,5
Hydroxyurea can be started in patients at age 9 months; blood testing should be performed at the start of treatment and the dosage titrated based on blood counts. Initial blood work includes:
- Hb level;
- Hb electrophoresis with the quantitative percentage of Hb F;
- complete blood count with differential and reticulocyte counts;
- chemistry profile (electrolytes, lactate dehydrogenase, total protein, albumin, total bilirubin);
- liver function tests (aspartate aminotransferase, alanine aminotransferase);
- measurement of renal function (blood urea nitrogen, creatinine);
- serum vitamin B12 and folate;
- serum iron, total iron-binding capacity, and ferritin;
- hepatitis B, hepatitis C, and parvovirus B19 antigen; and
- serologic testing for HIV.
Continue to: Testing should also...
Testing should also include a pregnancy test for postmenarchal females because hydroxyurea is in US Food and Drug Administration pregnancy risk category X.
Avoid hydroxyurea in lactating women; dose the drug renally in the setting of renal disease. Because hydroxyurea has a high rate of serious adverse effects and drug-drug interactions, it should be offered in conjunction with an individualized care plan.
Hydroxyurea can also be offered to patients with other forms of SCD who have recurrent vaso-occlusive symptoms.
Two newer medications improve oxygen delivery in patients with SCD. Voxelotor, approved in 2019, works to reduce Hb S polymerization by binding to the alpha chain of Hb S and, subsequently, increasing its oxygen affinity. The drug is generally well tolerated and can be used in patients with SCD who are ≥ 12 years.16 Crizanlizumab is a monoclonal antibody directed against P-selectin, an adhesion molecule located on endothelial cells and activated platelets. The efficacy of crizanlizumab was demonstrated in the SUSTAIN trial, in which it reduced vaso-occlusive pain in patients ≥ 16 years.17
All of these medications have a narrow toxic–therapeutic window. They should therefore be administered with the participation of a multidisciplinary care team.
Continue to: The need to coordinated, comprehensive care
The need for coordinated, comprehensive care
Patients with SCD report how challenging their disease is. All patients with SCD are more likely than age-matched counterparts to experience loss, including workdays for disability, educational potential, workdays for caregivers of affected children, and time spent in the hospital or the emergency department.4,5 These losses, with the concomitant stress associated with chronic illness and the struggle to manage recurrent pain crises and chronic complications, are often overwhelming.
Comprehensive care can, as we have illustrated in this discussion, mitigate these losses. Such care should include extensive education, genetic counseling, infection prevention, pain management, and implementation of evidence-based management guidelines.3,4,6 Patients with SCD report that their illness outlook would be better with
- greater provider knowledge of SCD,
- destigmatization of narcotics for SCD vaso-occlusive pain management,
- optimal coordination among members of the health care team, and
- improved transportation for appointments.
Patients also report that barriers associated with the unique US health care financing system are often insurmountable. As patients with SCD live longer, improved care management should focus on reducing these barriers and enhancing their quality of life.2,18,19
CORRESPONDENCE
Robert Allen Perkins, MD, MPH, Department of Family Medicine, University of South Alabama College of Medicine, 1601 Center Street, 2N, Mobile, AL 36604; perkins@health. southalabama.edu
1. Lubeck D, Agodoa I, Bhakta N, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2:e1915374. doi:10.1001/jamanetworkopen.2019.15374
2. Swanson ME, Grosse SD, Kulkarni R. Disability among individuals with sickle cell disease: literature review from a public health perspective. Am J Prev Med. 2011;41(6 suppl 4):S390-S397. doi: 10.1016/j.amepre.2011.09.006
3. Moskowitz JT, Butensky E, Harmatz P, et al. Caregiving time in sickle cell disease: psychological effects in maternal caregivers. Pediatr Blood Cancer. 2007;48:64-71. doi:10.1002/pbc.20792
4. Mehta SR, Afenyi-Annan A, Lottenberg R. Opportunities to improve outcomes in sickle cell disease. Am Fam Phys. 2006;74:303-310.
5. Yawn BP, John-Sowah J. Management of sickle cell disease: recommendations from the 2014 Expert Panel Report. Am Fam Phys. 2015;92:1069-1077.
6. National Heart, Lung, and Blood Institute, National Institutes of Health. Evidence-based management of sickle cell disease. Expert Panel Report, 2014. Accessed June 9, 2022. www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines
7. Committee Opinion No. 690: Carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40. doi: 10.1097/AOG.0000000000001951
8. Jordan LB, Smith-Whitley K, Treadwell MJ, et al. Screening U.S. college athletes for their sickle cell disease carrier status. Am J Prev Med. 2011;41:S406-S412. doi: 10.1016/j.amepre.2011.09.014
9. Adams RJ, McKie VC, Brambilla D, et al. Stroke prevention trial in sickle cell anemia. Control Clin Trials. 1998;19:110-129. doi: 10.1016/s0197-2456(97)00099-8
10. Alzahrani F, Alaidarous K, Alqarni S, et al. Incidence and predictors of bacterial infections in febrile children with sickle cell disease. Int J Pediatr Adolesc Med. 2021;8:236-238 doi: 10.1016/j.ijpam.2020.12.005
11. Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2021-2022. Pediatrics. 2021;148: e2021053745. doi: 10.1542/peds.2021-053745
12. Centers for Disease Control and Prevention. Study finds people with sickle cell disease who developed coronavirus disease have high rates of hospitalization, intensive care unit admission, and death. October 20, 2020. Accessed June 9, 2022. www.cdc.gov/ncbddd/sicklecell/features/scd-and-covid-19.html
13. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
14. Baskin MN, Goh XL, Heeney MM, et al. Bacteremia risk and outpatient management of febrile patients with sickle cell disease. Pediatrics. 2013;131:1035-1041. doi: 10.1542/peds.2012-2139
15. Osunkwo I, Veeramreddy P, Arnall J, et al. Use of buprenorphine/naloxone in amelio rating acute care utilization and chronic opioid use in adults with sickle cell disease. Blood. 2019;134 (suppl 1):790. doi: 10.1182/blood-2019-126589
16. Vichinsky E, Hoppe CC, Ataga KI, et al; HOPE Trial Investigators. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381:509-519. doi: 10.1056/NEJMoa1903212
17. Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376:429-439. doi: 10.1056/NEJMoa1611770
18. Dixit R, Nettem S, Madan SS, et al. Folate supplementation in people with sickle cell disease. Cochrane Database Syst Rev. 2016;2:CD011130. doi: 10.1002/14651858.CD011130.pub2
19. Brennan-Cook J, Bonnabeau E, Aponte R, et al. Barriers to care for persons with sickle cell disease: the case manager’s opportunity to improve patient outcomes. Prof Case Manag. 2018;23:213-219. doi: 10.1097/NCM.0000000000000260
1. Lubeck D, Agodoa I, Bhakta N, et al. Estimated life expectancy and income of patients with sickle cell disease compared with those without sickle cell disease. JAMA Netw Open. 2019;2:e1915374. doi:10.1001/jamanetworkopen.2019.15374
2. Swanson ME, Grosse SD, Kulkarni R. Disability among individuals with sickle cell disease: literature review from a public health perspective. Am J Prev Med. 2011;41(6 suppl 4):S390-S397. doi: 10.1016/j.amepre.2011.09.006
3. Moskowitz JT, Butensky E, Harmatz P, et al. Caregiving time in sickle cell disease: psychological effects in maternal caregivers. Pediatr Blood Cancer. 2007;48:64-71. doi:10.1002/pbc.20792
4. Mehta SR, Afenyi-Annan A, Lottenberg R. Opportunities to improve outcomes in sickle cell disease. Am Fam Phys. 2006;74:303-310.
5. Yawn BP, John-Sowah J. Management of sickle cell disease: recommendations from the 2014 Expert Panel Report. Am Fam Phys. 2015;92:1069-1077.
6. National Heart, Lung, and Blood Institute, National Institutes of Health. Evidence-based management of sickle cell disease. Expert Panel Report, 2014. Accessed June 9, 2022. www.nhlbi.nih.gov/health-pro/guidelines/sickle-cell-disease-guidelines
7. Committee Opinion No. 690: Carrier screening in the age of genomic medicine. Obstet Gynecol. 2017;129:e35-e40. doi: 10.1097/AOG.0000000000001951
8. Jordan LB, Smith-Whitley K, Treadwell MJ, et al. Screening U.S. college athletes for their sickle cell disease carrier status. Am J Prev Med. 2011;41:S406-S412. doi: 10.1016/j.amepre.2011.09.014
9. Adams RJ, McKie VC, Brambilla D, et al. Stroke prevention trial in sickle cell anemia. Control Clin Trials. 1998;19:110-129. doi: 10.1016/s0197-2456(97)00099-8
10. Alzahrani F, Alaidarous K, Alqarni S, et al. Incidence and predictors of bacterial infections in febrile children with sickle cell disease. Int J Pediatr Adolesc Med. 2021;8:236-238 doi: 10.1016/j.ijpam.2020.12.005
11. Committee on Infectious Diseases. Recommendations for prevention and control of influenza in children, 2021-2022. Pediatrics. 2021;148: e2021053745. doi: 10.1542/peds.2021-053745
12. Centers for Disease Control and Prevention. Study finds people with sickle cell disease who developed coronavirus disease have high rates of hospitalization, intensive care unit admission, and death. October 20, 2020. Accessed June 9, 2022. www.cdc.gov/ncbddd/sicklecell/features/scd-and-covid-19.html
13. James PA, Oparil S, Carter BL, et al. 2014 Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507-520.
14. Baskin MN, Goh XL, Heeney MM, et al. Bacteremia risk and outpatient management of febrile patients with sickle cell disease. Pediatrics. 2013;131:1035-1041. doi: 10.1542/peds.2012-2139
15. Osunkwo I, Veeramreddy P, Arnall J, et al. Use of buprenorphine/naloxone in amelio rating acute care utilization and chronic opioid use in adults with sickle cell disease. Blood. 2019;134 (suppl 1):790. doi: 10.1182/blood-2019-126589
16. Vichinsky E, Hoppe CC, Ataga KI, et al; HOPE Trial Investigators. A phase 3 randomized trial of voxelotor in sickle cell disease. N Engl J Med. 2019;381:509-519. doi: 10.1056/NEJMoa1903212
17. Ataga KI, Kutlar A, Kanter J, et al. Crizanlizumab for the prevention of pain crises in sickle cell disease. N Engl J Med. 2017;376:429-439. doi: 10.1056/NEJMoa1611770
18. Dixit R, Nettem S, Madan SS, et al. Folate supplementation in people with sickle cell disease. Cochrane Database Syst Rev. 2016;2:CD011130. doi: 10.1002/14651858.CD011130.pub2
19. Brennan-Cook J, Bonnabeau E, Aponte R, et al. Barriers to care for persons with sickle cell disease: the case manager’s opportunity to improve patient outcomes. Prof Case Manag. 2018;23:213-219. doi: 10.1097/NCM.0000000000000260
PRACTICE RECOMMENDATIONS
› Offer rapid access to narcotic analgesia for patients with sickle cell disease (SCD) who have recurrent vaso-occlusive crises, to prevent unnecessary hospitalization. A
› Provide oral penicillin prophylaxis against pneumococcal disease in patients < 5 years of age who have sickle cell anemia (SCA), but not in children whose SCD is less severe. A
› Screen all patients with SCA annually, beginning at age 2 years until age 16 years, for their risk of stroke, using a transcranial Doppler study. A
› Administer the COVID-19 mRNA vaccine series to all patients with SCD, unless contraindicated. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Immune checkpoint and VEGF inhibitors superior in renal cell carcinoma treatment
Treatment with immune checkpoint inhibitors and vascular endothelial growth factor (VEGF) inhibitors has made a notable dent in survival statistics for patients with metastatic renal cell carcinoma, but up to 20% of patients will only ever achieve progressive disease status and at 1 year, 20% of patients will have died from the condition.
The treatment is still superior to doublet immune checkpoint blockade therapy, shows a cohort study among patients with metastatic renal cell carcinoma published in JAMA Network Open.
The investigation also found that objective imaging responses were associated with improvement in overall survival among patients receiving either type of therapy. Led by Vishal Navani, MBBS, University of Calgary (Alta.), this was a multicenter international cohort study of 899 patients (median age 62.8 years; 74.2% male) with a histologically confirmed diagnosis of metastatic renal cell carcinoma (mRCC) nested in routine clinical practice (International Metastatic Renal Cell Carcinoma Database Consortium [IMDC]). Complete or partial responses were independently more likely with first-line VEGF inhibitor therapy (IOVE) (including axitinib-avelumab, axitinib-pembrolizumab, cabozantinib-nivolumab, and lenvatinib-pembrolizumab therapies) than with the first-line immuno-oncology doublet (IOIO) of ipilimumab-nivolumab (odds ratio [OR], 1.89; 95% confidence interval [CI], 1.26-2.81; P = .002). Analysis of factors affecting responses showed they were more likely to occur in the presence of lung metastases (OR, 1.49: 95% CI, 1.01-2.20), receipt of cytoreductive nephrectomy (OR, 1.59; 95% CI, 1.04-2.43), and favorable IMDC risk.
Beyond imaging response, Dr. Navani and colleagues tested the association between objective imaging response and overall survival as a secondary endpoint. Among responders versus nonresponders, median overall survival was not estimable (95%CI, 48.2 months to not estimable) versus 31.6 months (95%CI, 24.2-41.4 months; log rank P < .001). The overall survival advantage for objective imaging response versus nonresponse persisted in both the IOIO and IOVE groups taken separately (log rank P < .001 and log rank P = .02, respectively).
A large proportion of patients (27.5%) in the IOIO group experienced progressive disease as the best overall response, with significantly reduced median overall survival of 8.4 months (95%CI, 7.2-13.0 months). In the IOVE group, by contrast, 12.2% experienced progressive disease as the best overall response, with improved median overall survival of 18.5 months (95%CI, 4.9-22.4 months).
While first-line combination therapies have brought meaningful overall survival benefits in this population, up to 20% of patients, the researchers wrote, have progressive disease as their best overall response, and all-cause mortality in clinical trials is as high as 20% at 1 year. Improving the survival curve has been hampered by the lack of biomarkers to predict objective imaging response or survival benefit with first-line therapies. Also, the association between treatment with first-line immuno-oncology combination therapies and physician-assessed objective imaging response among patients with metastatic renal cell carcinoma has remained uncharacterized. particularly if tumor reduction is needed to inhibit life-limiting disease progression and to palliate tumor-induced symptoms.
The authors pointed out the study’s strength, citing the inclusion of a large data set from a 90% nonclinical trial population, and its limitations, which include a lack of independent blinded centralized imaging review.
They declared no relevant disclosures.
Treatment with immune checkpoint inhibitors and vascular endothelial growth factor (VEGF) inhibitors has made a notable dent in survival statistics for patients with metastatic renal cell carcinoma, but up to 20% of patients will only ever achieve progressive disease status and at 1 year, 20% of patients will have died from the condition.
The treatment is still superior to doublet immune checkpoint blockade therapy, shows a cohort study among patients with metastatic renal cell carcinoma published in JAMA Network Open.
The investigation also found that objective imaging responses were associated with improvement in overall survival among patients receiving either type of therapy. Led by Vishal Navani, MBBS, University of Calgary (Alta.), this was a multicenter international cohort study of 899 patients (median age 62.8 years; 74.2% male) with a histologically confirmed diagnosis of metastatic renal cell carcinoma (mRCC) nested in routine clinical practice (International Metastatic Renal Cell Carcinoma Database Consortium [IMDC]). Complete or partial responses were independently more likely with first-line VEGF inhibitor therapy (IOVE) (including axitinib-avelumab, axitinib-pembrolizumab, cabozantinib-nivolumab, and lenvatinib-pembrolizumab therapies) than with the first-line immuno-oncology doublet (IOIO) of ipilimumab-nivolumab (odds ratio [OR], 1.89; 95% confidence interval [CI], 1.26-2.81; P = .002). Analysis of factors affecting responses showed they were more likely to occur in the presence of lung metastases (OR, 1.49: 95% CI, 1.01-2.20), receipt of cytoreductive nephrectomy (OR, 1.59; 95% CI, 1.04-2.43), and favorable IMDC risk.
Beyond imaging response, Dr. Navani and colleagues tested the association between objective imaging response and overall survival as a secondary endpoint. Among responders versus nonresponders, median overall survival was not estimable (95%CI, 48.2 months to not estimable) versus 31.6 months (95%CI, 24.2-41.4 months; log rank P < .001). The overall survival advantage for objective imaging response versus nonresponse persisted in both the IOIO and IOVE groups taken separately (log rank P < .001 and log rank P = .02, respectively).
A large proportion of patients (27.5%) in the IOIO group experienced progressive disease as the best overall response, with significantly reduced median overall survival of 8.4 months (95%CI, 7.2-13.0 months). In the IOVE group, by contrast, 12.2% experienced progressive disease as the best overall response, with improved median overall survival of 18.5 months (95%CI, 4.9-22.4 months).
While first-line combination therapies have brought meaningful overall survival benefits in this population, up to 20% of patients, the researchers wrote, have progressive disease as their best overall response, and all-cause mortality in clinical trials is as high as 20% at 1 year. Improving the survival curve has been hampered by the lack of biomarkers to predict objective imaging response or survival benefit with first-line therapies. Also, the association between treatment with first-line immuno-oncology combination therapies and physician-assessed objective imaging response among patients with metastatic renal cell carcinoma has remained uncharacterized. particularly if tumor reduction is needed to inhibit life-limiting disease progression and to palliate tumor-induced symptoms.
The authors pointed out the study’s strength, citing the inclusion of a large data set from a 90% nonclinical trial population, and its limitations, which include a lack of independent blinded centralized imaging review.
They declared no relevant disclosures.
Treatment with immune checkpoint inhibitors and vascular endothelial growth factor (VEGF) inhibitors has made a notable dent in survival statistics for patients with metastatic renal cell carcinoma, but up to 20% of patients will only ever achieve progressive disease status and at 1 year, 20% of patients will have died from the condition.
The treatment is still superior to doublet immune checkpoint blockade therapy, shows a cohort study among patients with metastatic renal cell carcinoma published in JAMA Network Open.
The investigation also found that objective imaging responses were associated with improvement in overall survival among patients receiving either type of therapy. Led by Vishal Navani, MBBS, University of Calgary (Alta.), this was a multicenter international cohort study of 899 patients (median age 62.8 years; 74.2% male) with a histologically confirmed diagnosis of metastatic renal cell carcinoma (mRCC) nested in routine clinical practice (International Metastatic Renal Cell Carcinoma Database Consortium [IMDC]). Complete or partial responses were independently more likely with first-line VEGF inhibitor therapy (IOVE) (including axitinib-avelumab, axitinib-pembrolizumab, cabozantinib-nivolumab, and lenvatinib-pembrolizumab therapies) than with the first-line immuno-oncology doublet (IOIO) of ipilimumab-nivolumab (odds ratio [OR], 1.89; 95% confidence interval [CI], 1.26-2.81; P = .002). Analysis of factors affecting responses showed they were more likely to occur in the presence of lung metastases (OR, 1.49: 95% CI, 1.01-2.20), receipt of cytoreductive nephrectomy (OR, 1.59; 95% CI, 1.04-2.43), and favorable IMDC risk.
Beyond imaging response, Dr. Navani and colleagues tested the association between objective imaging response and overall survival as a secondary endpoint. Among responders versus nonresponders, median overall survival was not estimable (95%CI, 48.2 months to not estimable) versus 31.6 months (95%CI, 24.2-41.4 months; log rank P < .001). The overall survival advantage for objective imaging response versus nonresponse persisted in both the IOIO and IOVE groups taken separately (log rank P < .001 and log rank P = .02, respectively).
A large proportion of patients (27.5%) in the IOIO group experienced progressive disease as the best overall response, with significantly reduced median overall survival of 8.4 months (95%CI, 7.2-13.0 months). In the IOVE group, by contrast, 12.2% experienced progressive disease as the best overall response, with improved median overall survival of 18.5 months (95%CI, 4.9-22.4 months).
While first-line combination therapies have brought meaningful overall survival benefits in this population, up to 20% of patients, the researchers wrote, have progressive disease as their best overall response, and all-cause mortality in clinical trials is as high as 20% at 1 year. Improving the survival curve has been hampered by the lack of biomarkers to predict objective imaging response or survival benefit with first-line therapies. Also, the association between treatment with first-line immuno-oncology combination therapies and physician-assessed objective imaging response among patients with metastatic renal cell carcinoma has remained uncharacterized. particularly if tumor reduction is needed to inhibit life-limiting disease progression and to palliate tumor-induced symptoms.
The authors pointed out the study’s strength, citing the inclusion of a large data set from a 90% nonclinical trial population, and its limitations, which include a lack of independent blinded centralized imaging review.
They declared no relevant disclosures.
FROM JAMA NETWORK OPEN
Analysis shows no benefit for cytoreductive nephrectomy
Researchers writing in JAMA Network Open report that .
The finding, which was derived using instrumental variable analysis to adjust for bias due to unmeasured variables, is in contradiction to findings from prior observational data sets.
“These observational studies did not account for selection bias related to unmeasured confounding by surgical indication, and as such, their results may not accurately reflect the effectiveness of the intervention,” wrote the authors, led by Nicholas H. Chakiryan, MD, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Fla.
The primary outcome analysis using conventional adjustments for selection bias in the final study population of 12,766 patients (median age 63 years, 68% male, 88% White) found cytoreductive nephrectomy performed in 5,005 patients (39%) to be associated with a significant overall survival benefit (multivariable Cox proportional hazards regression: hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.47-0.51; propensity score matching: HR, 0.48; 95%CI, 0.46-0.50). Analysis using instrumental variable estimates, however, did not demonstrate an association between cytoreductive nephrectomy and overall survival (HR, 0.92; 95%CI, 0.78-1.09). “This discrepancy likely reflects the fact that surgical indication for cytoreductive nephrectomy is primarily driven by factors that are not commonly measured or available in observational data sets,” wrote Dr. Chakiryan and colleagues.
For metastatic clear cell renal cell carcinoma (ccRCC) surgical candidates lacking poor-risk disease, cytoreductive nephrectomy has been a clinical standard for decades. Several large observational studies conducted during the current postcytokine, tyrosine kinase–inhibiting targeted therapy era have demonstrated that cytoreductive nephrectomy continues to offer substantial overall survival benefit. These studies did not, however, account for selection bias related to unmeasured confounding by surgical indication.
The researchers identified 12,766 cases of ccRCC from the National Cancer Database, which includes more than 70% of incident cancer cases diagnosed in the United States, from Jan. 1, 2006, to Dec. 31, 2016. Their primary objective was to assess the effect of cytoreductive nephrectomy on overall survival for patients with metastatic ccRCC using instrumental variable analysis to adjust for unmeasured confounding and to compare these results with those generated by conventional adjustments for selection bias.
Instrumental variables are used to control for confounding and measurement error in observational studies. In this study, increasing distance to the treating facility was a significant instrumental variable (P < .001), with an increasing proportion of patients undergoing cytoreductive nephrectomy as distance to a facility increased. “It is worth reinforcing that instrumental variable estimates reflect the outcomes of marginal patients in the sample,” the researchers noted. “In this instance, marginal patients are those whose cytoreductive nephrectomy status was primarily associated with their distance to the treating facility. Increasing distance to the treating facility was significantly associated with receipt of a cytoreductive nephrectomy, presumably because patients are more willing to travel to referral centers for complex surgical care with a limited number of visits, as opposed to receipt of systemic therapy that requires frequent visits for an indefinite period and can be effectively administered locally.”
“Consistent with contemporary level 1 evidence, instrumental variable analysis demonstrated that cytoreductive nephrectomy was not associated with improved overall survival for patients with metastatic clear cell renal cell carcinoma,” the authors concluded.
Among limitations of the analysis, they noted that instrumental variable analyses functionally compare marginal patient populations within the overall cohort, potentially limiting the generalizability of the results.
Researchers writing in JAMA Network Open report that .
The finding, which was derived using instrumental variable analysis to adjust for bias due to unmeasured variables, is in contradiction to findings from prior observational data sets.
“These observational studies did not account for selection bias related to unmeasured confounding by surgical indication, and as such, their results may not accurately reflect the effectiveness of the intervention,” wrote the authors, led by Nicholas H. Chakiryan, MD, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Fla.
The primary outcome analysis using conventional adjustments for selection bias in the final study population of 12,766 patients (median age 63 years, 68% male, 88% White) found cytoreductive nephrectomy performed in 5,005 patients (39%) to be associated with a significant overall survival benefit (multivariable Cox proportional hazards regression: hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.47-0.51; propensity score matching: HR, 0.48; 95%CI, 0.46-0.50). Analysis using instrumental variable estimates, however, did not demonstrate an association between cytoreductive nephrectomy and overall survival (HR, 0.92; 95%CI, 0.78-1.09). “This discrepancy likely reflects the fact that surgical indication for cytoreductive nephrectomy is primarily driven by factors that are not commonly measured or available in observational data sets,” wrote Dr. Chakiryan and colleagues.
For metastatic clear cell renal cell carcinoma (ccRCC) surgical candidates lacking poor-risk disease, cytoreductive nephrectomy has been a clinical standard for decades. Several large observational studies conducted during the current postcytokine, tyrosine kinase–inhibiting targeted therapy era have demonstrated that cytoreductive nephrectomy continues to offer substantial overall survival benefit. These studies did not, however, account for selection bias related to unmeasured confounding by surgical indication.
The researchers identified 12,766 cases of ccRCC from the National Cancer Database, which includes more than 70% of incident cancer cases diagnosed in the United States, from Jan. 1, 2006, to Dec. 31, 2016. Their primary objective was to assess the effect of cytoreductive nephrectomy on overall survival for patients with metastatic ccRCC using instrumental variable analysis to adjust for unmeasured confounding and to compare these results with those generated by conventional adjustments for selection bias.
Instrumental variables are used to control for confounding and measurement error in observational studies. In this study, increasing distance to the treating facility was a significant instrumental variable (P < .001), with an increasing proportion of patients undergoing cytoreductive nephrectomy as distance to a facility increased. “It is worth reinforcing that instrumental variable estimates reflect the outcomes of marginal patients in the sample,” the researchers noted. “In this instance, marginal patients are those whose cytoreductive nephrectomy status was primarily associated with their distance to the treating facility. Increasing distance to the treating facility was significantly associated with receipt of a cytoreductive nephrectomy, presumably because patients are more willing to travel to referral centers for complex surgical care with a limited number of visits, as opposed to receipt of systemic therapy that requires frequent visits for an indefinite period and can be effectively administered locally.”
“Consistent with contemporary level 1 evidence, instrumental variable analysis demonstrated that cytoreductive nephrectomy was not associated with improved overall survival for patients with metastatic clear cell renal cell carcinoma,” the authors concluded.
Among limitations of the analysis, they noted that instrumental variable analyses functionally compare marginal patient populations within the overall cohort, potentially limiting the generalizability of the results.
Researchers writing in JAMA Network Open report that .
The finding, which was derived using instrumental variable analysis to adjust for bias due to unmeasured variables, is in contradiction to findings from prior observational data sets.
“These observational studies did not account for selection bias related to unmeasured confounding by surgical indication, and as such, their results may not accurately reflect the effectiveness of the intervention,” wrote the authors, led by Nicholas H. Chakiryan, MD, H. Lee Moffitt Cancer Center and Research Institute, Tampa, Fla.
The primary outcome analysis using conventional adjustments for selection bias in the final study population of 12,766 patients (median age 63 years, 68% male, 88% White) found cytoreductive nephrectomy performed in 5,005 patients (39%) to be associated with a significant overall survival benefit (multivariable Cox proportional hazards regression: hazard ratio [HR], 0.49; 95% confidence interval [CI], 0.47-0.51; propensity score matching: HR, 0.48; 95%CI, 0.46-0.50). Analysis using instrumental variable estimates, however, did not demonstrate an association between cytoreductive nephrectomy and overall survival (HR, 0.92; 95%CI, 0.78-1.09). “This discrepancy likely reflects the fact that surgical indication for cytoreductive nephrectomy is primarily driven by factors that are not commonly measured or available in observational data sets,” wrote Dr. Chakiryan and colleagues.
For metastatic clear cell renal cell carcinoma (ccRCC) surgical candidates lacking poor-risk disease, cytoreductive nephrectomy has been a clinical standard for decades. Several large observational studies conducted during the current postcytokine, tyrosine kinase–inhibiting targeted therapy era have demonstrated that cytoreductive nephrectomy continues to offer substantial overall survival benefit. These studies did not, however, account for selection bias related to unmeasured confounding by surgical indication.
The researchers identified 12,766 cases of ccRCC from the National Cancer Database, which includes more than 70% of incident cancer cases diagnosed in the United States, from Jan. 1, 2006, to Dec. 31, 2016. Their primary objective was to assess the effect of cytoreductive nephrectomy on overall survival for patients with metastatic ccRCC using instrumental variable analysis to adjust for unmeasured confounding and to compare these results with those generated by conventional adjustments for selection bias.
Instrumental variables are used to control for confounding and measurement error in observational studies. In this study, increasing distance to the treating facility was a significant instrumental variable (P < .001), with an increasing proportion of patients undergoing cytoreductive nephrectomy as distance to a facility increased. “It is worth reinforcing that instrumental variable estimates reflect the outcomes of marginal patients in the sample,” the researchers noted. “In this instance, marginal patients are those whose cytoreductive nephrectomy status was primarily associated with their distance to the treating facility. Increasing distance to the treating facility was significantly associated with receipt of a cytoreductive nephrectomy, presumably because patients are more willing to travel to referral centers for complex surgical care with a limited number of visits, as opposed to receipt of systemic therapy that requires frequent visits for an indefinite period and can be effectively administered locally.”
“Consistent with contemporary level 1 evidence, instrumental variable analysis demonstrated that cytoreductive nephrectomy was not associated with improved overall survival for patients with metastatic clear cell renal cell carcinoma,” the authors concluded.
Among limitations of the analysis, they noted that instrumental variable analyses functionally compare marginal patient populations within the overall cohort, potentially limiting the generalizability of the results.
FROM JAMA NETWORK OPEN
The shifting sands of lung cancer screening
An analysis of trends in lung cancer screening since March 2021 when the U.S. Preventive Services Task Force (USPSTF) expanded the eligibility criteria for lung cancer screening, shows that significantly more Black men have been screened for lung cancer, but not women or undereducated people.
The eligibility for lung cancer screening was expanded in 2021 to include men and women under 50 years old and people who smoke at least one pack of cigarettes a day for the last 20 years. “
“Expansion of screening criteria is a critical first step to achieving equity in lung cancer screening for all high-risk populations, but myriad challenges remain before individuals enter the door for screening,” wrote the authors, led by Julie A. Barta, MD, Thomas Jefferson University, Philadelphia. “Health policy changes must occur simultaneously with efforts to expand community outreach, overcome logistical barriers, and facilitate screening adherence. Only after comprehensive strategies to dismantle screening barriers are identified, validated, and implemented can there be a truly equitable landscape for lung cancer screening.”
For the study, published in JAMA Open Network, researchers examined rates of centralized lung cancer screening in the Baltimore area. In addition to expanding lung cancer screening generally, there was hope that the expanded criteria might increase uptake of screening in populations that are traditionally underserved, such as African American, Hispanic, and female patients. Of 815 people screened during the study period (March-December 2021), 161 were newly eligible for screening under the 2021 criteria.
“There’s been quite a bit of work in the field demonstrating that Black men and women develop lung cancer at more advanced stages of disease, and they often are diagnosed at younger ages and have fewer pack-years of smoking. So the hypothesis was that this would reduce some of the disparities seen in lung cancer screening by making more people eligible,” Dr. Barta said in an interview.
The researchers categorized participants as those who would have been eligible for screening under the USPSTF 2013 guideline (age 55 or older, 30 or more pack-years, quit within the past 15 years), and those who would be eligible under the 2021 guideline (age 50 or older, 20 or more pack-years, quit within the past 15 years). Of the 2021 cohort, 54.5% were African American, versus 39.5% of the 2013 cohort (P = .002). There were no differences between the cohorts with respect to education level or gender.
“Although we’ve seen some encouraging improvement in terms of getting more eligible patients into our screening program, there’s still a lot of work to be done in the field,” Dr. Barta said. “Diagnosing lung cancer at earlier stages of disease is more cost effective in general for the health care system than fighting lung cancer at advanced stages, which requires more complex and multimodal and prolonged therapies.”
New evidence: Chest CTs for lung cancer screening reduces incidence of advanced lung cancer
In an analysis of the SEER database presented in June at the annual meeting of the American Society of Clinical Oncology, the adoption of low-dose chest computed tomography (LDCT) led to fewer diagnoses of advanced lung cancer, although these declines varied significantly by race and ethnicity. Non-Hispanic Blacks seemed to benefit the most with a 55% decline (P < .01), while Hispanics had the lowest rate of decline at 41% (P < .01). The change was recommended by USPSTF in 2013 after the National Lung Screening Trial revealed a 20% relative reduction in mortality when CT scans were used instead of chest radiography. The Centers for Medicare and Medicaid Services approved coverage of the screen in 2015.
The SEER study looked at data from 400,343 individuals from 2004-2014 (preintervention) and 2015-2018 (postintervention). The age-adjusted incidence of advanced lung cancer declined during both periods, but the decline was sharper between 2015 and 2018, with three fewer cases per 100,000 people than 2004-2014 (P < .01). Similar patterns were seen in subanalyses of males and females, non-Hispanic Whites, non-Hispanic Blacks, and Hispanics. The relative declines were largest in women, non-Hispanic Blacks, and people who lived outside of Metropolitan areas.
During a Q&A session that followed the presentation, Robert Smith, PhD, pointed out that the bar for eligibility of lung cancer risk has been set quite high, following the eligibility criteria for clinical trials. He noted that . “We are missing opportunities to prevent avertable lung cancer deaths,” said Dr. Smith, senior vice president of cancer screening at the American Cancer Society.
On the other hand, screening-prompted biopsies have the potential to cause harm, particularly in patients who already have lung disease, said Douglas Allen Arenberg, MD, professor at the University of Michigan, Ann Arbor. “I think that’s what scares most people is the potential downside, which is very hard to measure outside of a clinical trial,” said Dr. Arenberg, who served as a discussant for the presentation.
One way to reduce that risk is to identify biomarkers, either for screens or for incidentally-detected nodules, that have good negative predictive value. “If I had a blood test that is as good as a negative PET scan, I’m going to be much more likely to say, ‘Yeah, you’re 40 and your grandfather had lung cancer. Maybe you should get a CT. If we had that, we could screen a lot more people. Right now, I would discourage anybody who is at low risk from getting screened because when they come to me, the biggest opportunity I have to do harm is when I do a biopsy, and you always remember the ones that go wrong,” he said.
Dr. Arenberg also called for improvements in electronic medical records to better flag at-risk patients. “I think we as physicians have to demand more of the software developers that create these EMRs for us,” he said.
Another study in the same session used data from 1,391,088 patients drawn from the National Cancer Database between 2010 and 2017 to examine trends in diagnosis of stage I cancer. In 2010, 23.5% of patients were diagnosed as stage I, versus 29.1% in 2017. Stage I incidence increased from 25.8% to 31.7% in non–small cell lung cancer, but there was no statistically significant change in small cell lung cancer. As with the SEER database study, the researchers noted that the shift toward stage I diagnoses predated the recommendation of LDCT.
Dr. Arenberg suggested that the trend may come down to increased frequency of CT scans, which often collect incidental images of the lungs. He added that better access to care may also be helping to drive the change. “How much of that might have had something to do with the introduction 5 or 10 years earlier of the Affordable Care Act and people just simply having access to care and taking advantage of that?” Dr. Arenberg said.
But Dr. Arenberg said that not even screening can explain all the data. He referenced a stage shift in patients of all age groups in the National Cancer Database study, even those too young to be eligible for screening. “There’s something else going on here. It would be nice for us to understand what caused these trends, so perhaps we could accentuate that trend even more, but stage shifts are clearly occurring in lung cancer,” Dr. Arenberg said.
Dr. Barta has received grants from Genentech Health Equity Innovations Fund. Dr. Arenberg has no relevant financial disclosures. Dr. Smith’s potential disclosures could not be ascertained.
An analysis of trends in lung cancer screening since March 2021 when the U.S. Preventive Services Task Force (USPSTF) expanded the eligibility criteria for lung cancer screening, shows that significantly more Black men have been screened for lung cancer, but not women or undereducated people.
The eligibility for lung cancer screening was expanded in 2021 to include men and women under 50 years old and people who smoke at least one pack of cigarettes a day for the last 20 years. “
“Expansion of screening criteria is a critical first step to achieving equity in lung cancer screening for all high-risk populations, but myriad challenges remain before individuals enter the door for screening,” wrote the authors, led by Julie A. Barta, MD, Thomas Jefferson University, Philadelphia. “Health policy changes must occur simultaneously with efforts to expand community outreach, overcome logistical barriers, and facilitate screening adherence. Only after comprehensive strategies to dismantle screening barriers are identified, validated, and implemented can there be a truly equitable landscape for lung cancer screening.”
For the study, published in JAMA Open Network, researchers examined rates of centralized lung cancer screening in the Baltimore area. In addition to expanding lung cancer screening generally, there was hope that the expanded criteria might increase uptake of screening in populations that are traditionally underserved, such as African American, Hispanic, and female patients. Of 815 people screened during the study period (March-December 2021), 161 were newly eligible for screening under the 2021 criteria.
“There’s been quite a bit of work in the field demonstrating that Black men and women develop lung cancer at more advanced stages of disease, and they often are diagnosed at younger ages and have fewer pack-years of smoking. So the hypothesis was that this would reduce some of the disparities seen in lung cancer screening by making more people eligible,” Dr. Barta said in an interview.
The researchers categorized participants as those who would have been eligible for screening under the USPSTF 2013 guideline (age 55 or older, 30 or more pack-years, quit within the past 15 years), and those who would be eligible under the 2021 guideline (age 50 or older, 20 or more pack-years, quit within the past 15 years). Of the 2021 cohort, 54.5% were African American, versus 39.5% of the 2013 cohort (P = .002). There were no differences between the cohorts with respect to education level or gender.
“Although we’ve seen some encouraging improvement in terms of getting more eligible patients into our screening program, there’s still a lot of work to be done in the field,” Dr. Barta said. “Diagnosing lung cancer at earlier stages of disease is more cost effective in general for the health care system than fighting lung cancer at advanced stages, which requires more complex and multimodal and prolonged therapies.”
New evidence: Chest CTs for lung cancer screening reduces incidence of advanced lung cancer
In an analysis of the SEER database presented in June at the annual meeting of the American Society of Clinical Oncology, the adoption of low-dose chest computed tomography (LDCT) led to fewer diagnoses of advanced lung cancer, although these declines varied significantly by race and ethnicity. Non-Hispanic Blacks seemed to benefit the most with a 55% decline (P < .01), while Hispanics had the lowest rate of decline at 41% (P < .01). The change was recommended by USPSTF in 2013 after the National Lung Screening Trial revealed a 20% relative reduction in mortality when CT scans were used instead of chest radiography. The Centers for Medicare and Medicaid Services approved coverage of the screen in 2015.
The SEER study looked at data from 400,343 individuals from 2004-2014 (preintervention) and 2015-2018 (postintervention). The age-adjusted incidence of advanced lung cancer declined during both periods, but the decline was sharper between 2015 and 2018, with three fewer cases per 100,000 people than 2004-2014 (P < .01). Similar patterns were seen in subanalyses of males and females, non-Hispanic Whites, non-Hispanic Blacks, and Hispanics. The relative declines were largest in women, non-Hispanic Blacks, and people who lived outside of Metropolitan areas.
During a Q&A session that followed the presentation, Robert Smith, PhD, pointed out that the bar for eligibility of lung cancer risk has been set quite high, following the eligibility criteria for clinical trials. He noted that . “We are missing opportunities to prevent avertable lung cancer deaths,” said Dr. Smith, senior vice president of cancer screening at the American Cancer Society.
On the other hand, screening-prompted biopsies have the potential to cause harm, particularly in patients who already have lung disease, said Douglas Allen Arenberg, MD, professor at the University of Michigan, Ann Arbor. “I think that’s what scares most people is the potential downside, which is very hard to measure outside of a clinical trial,” said Dr. Arenberg, who served as a discussant for the presentation.
One way to reduce that risk is to identify biomarkers, either for screens or for incidentally-detected nodules, that have good negative predictive value. “If I had a blood test that is as good as a negative PET scan, I’m going to be much more likely to say, ‘Yeah, you’re 40 and your grandfather had lung cancer. Maybe you should get a CT. If we had that, we could screen a lot more people. Right now, I would discourage anybody who is at low risk from getting screened because when they come to me, the biggest opportunity I have to do harm is when I do a biopsy, and you always remember the ones that go wrong,” he said.
Dr. Arenberg also called for improvements in electronic medical records to better flag at-risk patients. “I think we as physicians have to demand more of the software developers that create these EMRs for us,” he said.
Another study in the same session used data from 1,391,088 patients drawn from the National Cancer Database between 2010 and 2017 to examine trends in diagnosis of stage I cancer. In 2010, 23.5% of patients were diagnosed as stage I, versus 29.1% in 2017. Stage I incidence increased from 25.8% to 31.7% in non–small cell lung cancer, but there was no statistically significant change in small cell lung cancer. As with the SEER database study, the researchers noted that the shift toward stage I diagnoses predated the recommendation of LDCT.
Dr. Arenberg suggested that the trend may come down to increased frequency of CT scans, which often collect incidental images of the lungs. He added that better access to care may also be helping to drive the change. “How much of that might have had something to do with the introduction 5 or 10 years earlier of the Affordable Care Act and people just simply having access to care and taking advantage of that?” Dr. Arenberg said.
But Dr. Arenberg said that not even screening can explain all the data. He referenced a stage shift in patients of all age groups in the National Cancer Database study, even those too young to be eligible for screening. “There’s something else going on here. It would be nice for us to understand what caused these trends, so perhaps we could accentuate that trend even more, but stage shifts are clearly occurring in lung cancer,” Dr. Arenberg said.
Dr. Barta has received grants from Genentech Health Equity Innovations Fund. Dr. Arenberg has no relevant financial disclosures. Dr. Smith’s potential disclosures could not be ascertained.
An analysis of trends in lung cancer screening since March 2021 when the U.S. Preventive Services Task Force (USPSTF) expanded the eligibility criteria for lung cancer screening, shows that significantly more Black men have been screened for lung cancer, but not women or undereducated people.
The eligibility for lung cancer screening was expanded in 2021 to include men and women under 50 years old and people who smoke at least one pack of cigarettes a day for the last 20 years. “
“Expansion of screening criteria is a critical first step to achieving equity in lung cancer screening for all high-risk populations, but myriad challenges remain before individuals enter the door for screening,” wrote the authors, led by Julie A. Barta, MD, Thomas Jefferson University, Philadelphia. “Health policy changes must occur simultaneously with efforts to expand community outreach, overcome logistical barriers, and facilitate screening adherence. Only after comprehensive strategies to dismantle screening barriers are identified, validated, and implemented can there be a truly equitable landscape for lung cancer screening.”
For the study, published in JAMA Open Network, researchers examined rates of centralized lung cancer screening in the Baltimore area. In addition to expanding lung cancer screening generally, there was hope that the expanded criteria might increase uptake of screening in populations that are traditionally underserved, such as African American, Hispanic, and female patients. Of 815 people screened during the study period (March-December 2021), 161 were newly eligible for screening under the 2021 criteria.
“There’s been quite a bit of work in the field demonstrating that Black men and women develop lung cancer at more advanced stages of disease, and they often are diagnosed at younger ages and have fewer pack-years of smoking. So the hypothesis was that this would reduce some of the disparities seen in lung cancer screening by making more people eligible,” Dr. Barta said in an interview.
The researchers categorized participants as those who would have been eligible for screening under the USPSTF 2013 guideline (age 55 or older, 30 or more pack-years, quit within the past 15 years), and those who would be eligible under the 2021 guideline (age 50 or older, 20 or more pack-years, quit within the past 15 years). Of the 2021 cohort, 54.5% were African American, versus 39.5% of the 2013 cohort (P = .002). There were no differences between the cohorts with respect to education level or gender.
“Although we’ve seen some encouraging improvement in terms of getting more eligible patients into our screening program, there’s still a lot of work to be done in the field,” Dr. Barta said. “Diagnosing lung cancer at earlier stages of disease is more cost effective in general for the health care system than fighting lung cancer at advanced stages, which requires more complex and multimodal and prolonged therapies.”
New evidence: Chest CTs for lung cancer screening reduces incidence of advanced lung cancer
In an analysis of the SEER database presented in June at the annual meeting of the American Society of Clinical Oncology, the adoption of low-dose chest computed tomography (LDCT) led to fewer diagnoses of advanced lung cancer, although these declines varied significantly by race and ethnicity. Non-Hispanic Blacks seemed to benefit the most with a 55% decline (P < .01), while Hispanics had the lowest rate of decline at 41% (P < .01). The change was recommended by USPSTF in 2013 after the National Lung Screening Trial revealed a 20% relative reduction in mortality when CT scans were used instead of chest radiography. The Centers for Medicare and Medicaid Services approved coverage of the screen in 2015.
The SEER study looked at data from 400,343 individuals from 2004-2014 (preintervention) and 2015-2018 (postintervention). The age-adjusted incidence of advanced lung cancer declined during both periods, but the decline was sharper between 2015 and 2018, with three fewer cases per 100,000 people than 2004-2014 (P < .01). Similar patterns were seen in subanalyses of males and females, non-Hispanic Whites, non-Hispanic Blacks, and Hispanics. The relative declines were largest in women, non-Hispanic Blacks, and people who lived outside of Metropolitan areas.
During a Q&A session that followed the presentation, Robert Smith, PhD, pointed out that the bar for eligibility of lung cancer risk has been set quite high, following the eligibility criteria for clinical trials. He noted that . “We are missing opportunities to prevent avertable lung cancer deaths,” said Dr. Smith, senior vice president of cancer screening at the American Cancer Society.
On the other hand, screening-prompted biopsies have the potential to cause harm, particularly in patients who already have lung disease, said Douglas Allen Arenberg, MD, professor at the University of Michigan, Ann Arbor. “I think that’s what scares most people is the potential downside, which is very hard to measure outside of a clinical trial,” said Dr. Arenberg, who served as a discussant for the presentation.
One way to reduce that risk is to identify biomarkers, either for screens or for incidentally-detected nodules, that have good negative predictive value. “If I had a blood test that is as good as a negative PET scan, I’m going to be much more likely to say, ‘Yeah, you’re 40 and your grandfather had lung cancer. Maybe you should get a CT. If we had that, we could screen a lot more people. Right now, I would discourage anybody who is at low risk from getting screened because when they come to me, the biggest opportunity I have to do harm is when I do a biopsy, and you always remember the ones that go wrong,” he said.
Dr. Arenberg also called for improvements in electronic medical records to better flag at-risk patients. “I think we as physicians have to demand more of the software developers that create these EMRs for us,” he said.
Another study in the same session used data from 1,391,088 patients drawn from the National Cancer Database between 2010 and 2017 to examine trends in diagnosis of stage I cancer. In 2010, 23.5% of patients were diagnosed as stage I, versus 29.1% in 2017. Stage I incidence increased from 25.8% to 31.7% in non–small cell lung cancer, but there was no statistically significant change in small cell lung cancer. As with the SEER database study, the researchers noted that the shift toward stage I diagnoses predated the recommendation of LDCT.
Dr. Arenberg suggested that the trend may come down to increased frequency of CT scans, which often collect incidental images of the lungs. He added that better access to care may also be helping to drive the change. “How much of that might have had something to do with the introduction 5 or 10 years earlier of the Affordable Care Act and people just simply having access to care and taking advantage of that?” Dr. Arenberg said.
But Dr. Arenberg said that not even screening can explain all the data. He referenced a stage shift in patients of all age groups in the National Cancer Database study, even those too young to be eligible for screening. “There’s something else going on here. It would be nice for us to understand what caused these trends, so perhaps we could accentuate that trend even more, but stage shifts are clearly occurring in lung cancer,” Dr. Arenberg said.
Dr. Barta has received grants from Genentech Health Equity Innovations Fund. Dr. Arenberg has no relevant financial disclosures. Dr. Smith’s potential disclosures could not be ascertained.
FROM JAMA NETWORK OPEN
Berdazimer gel beats vehicle for molluscum contagiosum in phase 3 study
Treatment with .
Molluscum contagiosum (MC) remains a common infection that, despite being self-limiting, may persist for months or years, and is associated with quality of life concerns and the need for ongoing therapy, wrote John C. Browning, MD, of Texas Dermatology and Laser Specialists, San Antonio, and colleagues, who conducted the phase 3 randomized study.
The infection is most common in children aged 1-14 years, and treatment may be needed in part to avoid infecting peers and family members, they said. No treatments for molluscum are currently approved by the Food and Drug Administration.
In the study, which was published in JAMA Dermatology, the researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years (range was 0.9-49 years), and about 85% were White. Participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment. At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
Most adverse events were mild or moderate, and rates of adverse events resulting in treatment discontinuation were low overall for both groups; the most common adverse events were application-site pain and erythema, which were mostly mild. Overall, 4.1% of berdazimer-treated patients and 0.7% of placebo patients discontinued the study because of adverse events.
The study findings were limited by several factors, including the small number of patients in subgroups for race, ethnicity, and age; and the lack of data on patients with sexually transmitted MC and on concomitant use with other topical MC therapies, the researchers noted.
However, the results represent the largest randomized clinical trial of berdazimer 10.3% to date, and support its potential as a first-line therapy for MC patients aged 6 months and older, according to the authors. “Berdazimer is under consideration as a first in-class therapeutic agent for MC and may provide a topical prescription alternative to other therapies used for this highly contagious and psychosocially challenging skin condition,” they said.
Having a reliable, steroid-free, safe, and efficacious medication to treat molluscum in the pediatric population, as early as age 6 months, that can be used at home would “change the whole therapeutic paradigm,” one of the study authors, Adelaide Hebert, MD, said in an interview at the Society for Pediatric Dermatology annual meeting in July, where she presented phase 2 data on berdazimer gel. “This is a common problem and the rate of infections among siblings if it goes untreated is 41%. Affected kids have a sense of isolation; they don’t get invited to swimming parties.”
The lack of a safe and effective topical therapy “has been challenging,” added Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. She noted that treatments that have been used but have not been successful include imiquimod. “I’m not impressed with tretinoin,” although it is prescribed for MC, and the most common treatment prescribed by pediatricians for molluscum – mupirocin – is “usually not effective,” she said.
Another MC treatment in trials
Another investigative treatment for molluscum contagiosum, VP-102, a drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, has been evaluated in phase 3 studies of patients with MC aged 2 years and older. The results of two phase 3 studies were published in 2020.
In May 2022, Verrica Pharmaceuticals, which is developing VP-102, announced that Food and Drug Administration approval had been delayed because of deficiencies identified at a contract manufacturing organization, and that the company was working with the agency to bring VP-102 to the market as soon as possible.
A step in the right direction
Although MC is self-resolving, cases last an average of 13.5 months, and “many families look to fast-forward their child’s experience with the infection,” Vikash S. Oza, MD, a pediatric dermatologist at New York University, New York, wrote in an editorial that accompanied the berdazimer study.
“To truly create a paradigm shift in the decision to treat MC, a therapeutic treatment would need to be developed that would lead to resolution of the infection over a short time frame (ideally, weeks) with minimal discomfort,” Dr. Oza noted. “Both VP-102 and berdazimer gel, 10.3%, have the potential to be the first-ever MC therapies approved by the U.S. Food and Drug Administration,” and families seeking to reduce MC in visible areas would welcome this option for a home therapy, he said.
However, Dr. Oza emphasized that potential barriers to widespread use of these therapies include whether the efficacy can be maintained in patients who fail to comply with daily application, and the ongoing need for office-based therapy to manage sexually transmitted MC in adults and periocular and perianal MC in children. The study was funded by Novan. Lead author Dr. Browning disclosed grants from Novan during the conduct of the study; Dr. Hebert reported grants from the University of Texas Health Science Center McGovern Medical School-Houston during the conduct of the study. Disclosures of other authors included having reported equity in Novan during the conduct of the study and receiving a grant from Novan. Dr. Oza had no financial conflicts to disclose.
Treatment with .
Molluscum contagiosum (MC) remains a common infection that, despite being self-limiting, may persist for months or years, and is associated with quality of life concerns and the need for ongoing therapy, wrote John C. Browning, MD, of Texas Dermatology and Laser Specialists, San Antonio, and colleagues, who conducted the phase 3 randomized study.
The infection is most common in children aged 1-14 years, and treatment may be needed in part to avoid infecting peers and family members, they said. No treatments for molluscum are currently approved by the Food and Drug Administration.
In the study, which was published in JAMA Dermatology, the researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years (range was 0.9-49 years), and about 85% were White. Participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment. At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
Most adverse events were mild or moderate, and rates of adverse events resulting in treatment discontinuation were low overall for both groups; the most common adverse events were application-site pain and erythema, which were mostly mild. Overall, 4.1% of berdazimer-treated patients and 0.7% of placebo patients discontinued the study because of adverse events.
The study findings were limited by several factors, including the small number of patients in subgroups for race, ethnicity, and age; and the lack of data on patients with sexually transmitted MC and on concomitant use with other topical MC therapies, the researchers noted.
However, the results represent the largest randomized clinical trial of berdazimer 10.3% to date, and support its potential as a first-line therapy for MC patients aged 6 months and older, according to the authors. “Berdazimer is under consideration as a first in-class therapeutic agent for MC and may provide a topical prescription alternative to other therapies used for this highly contagious and psychosocially challenging skin condition,” they said.
Having a reliable, steroid-free, safe, and efficacious medication to treat molluscum in the pediatric population, as early as age 6 months, that can be used at home would “change the whole therapeutic paradigm,” one of the study authors, Adelaide Hebert, MD, said in an interview at the Society for Pediatric Dermatology annual meeting in July, where she presented phase 2 data on berdazimer gel. “This is a common problem and the rate of infections among siblings if it goes untreated is 41%. Affected kids have a sense of isolation; they don’t get invited to swimming parties.”
The lack of a safe and effective topical therapy “has been challenging,” added Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. She noted that treatments that have been used but have not been successful include imiquimod. “I’m not impressed with tretinoin,” although it is prescribed for MC, and the most common treatment prescribed by pediatricians for molluscum – mupirocin – is “usually not effective,” she said.
Another MC treatment in trials
Another investigative treatment for molluscum contagiosum, VP-102, a drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, has been evaluated in phase 3 studies of patients with MC aged 2 years and older. The results of two phase 3 studies were published in 2020.
In May 2022, Verrica Pharmaceuticals, which is developing VP-102, announced that Food and Drug Administration approval had been delayed because of deficiencies identified at a contract manufacturing organization, and that the company was working with the agency to bring VP-102 to the market as soon as possible.
A step in the right direction
Although MC is self-resolving, cases last an average of 13.5 months, and “many families look to fast-forward their child’s experience with the infection,” Vikash S. Oza, MD, a pediatric dermatologist at New York University, New York, wrote in an editorial that accompanied the berdazimer study.
“To truly create a paradigm shift in the decision to treat MC, a therapeutic treatment would need to be developed that would lead to resolution of the infection over a short time frame (ideally, weeks) with minimal discomfort,” Dr. Oza noted. “Both VP-102 and berdazimer gel, 10.3%, have the potential to be the first-ever MC therapies approved by the U.S. Food and Drug Administration,” and families seeking to reduce MC in visible areas would welcome this option for a home therapy, he said.
However, Dr. Oza emphasized that potential barriers to widespread use of these therapies include whether the efficacy can be maintained in patients who fail to comply with daily application, and the ongoing need for office-based therapy to manage sexually transmitted MC in adults and periocular and perianal MC in children. The study was funded by Novan. Lead author Dr. Browning disclosed grants from Novan during the conduct of the study; Dr. Hebert reported grants from the University of Texas Health Science Center McGovern Medical School-Houston during the conduct of the study. Disclosures of other authors included having reported equity in Novan during the conduct of the study and receiving a grant from Novan. Dr. Oza had no financial conflicts to disclose.
Treatment with .
Molluscum contagiosum (MC) remains a common infection that, despite being self-limiting, may persist for months or years, and is associated with quality of life concerns and the need for ongoing therapy, wrote John C. Browning, MD, of Texas Dermatology and Laser Specialists, San Antonio, and colleagues, who conducted the phase 3 randomized study.
The infection is most common in children aged 1-14 years, and treatment may be needed in part to avoid infecting peers and family members, they said. No treatments for molluscum are currently approved by the Food and Drug Administration.
In the study, which was published in JAMA Dermatology, the researchers randomized 444 patients to berdazimer gel 10.3% and 447 to a placebo gel, applied once daily in a thin layer on all MC lesions for 12 weeks. The study was conducted at 55 clinics across the United States between Sept. 1, 2020, and July 21, 2021. The mean age of the patients was about 6.5 years (range was 0.9-49 years), and about 85% were White. Participants had 3-70 raised MC lesions; those with sexually transmitted MC or MC in the periocular area were excluded. The primary endpoint was complete clearance of MC lesions after 12 weeks of treatment. At 12 weeks, significantly more patients treated with berdazimer gel achieved complete clearance than those on vehicle (32.4% vs. 19.7%; P < .001). A total of 64 (14.4%) patients in the berdazimer group discontinued treatment because of MC clearance, compared with 40 patients (8.9%) in the vehicle group.
Most adverse events were mild or moderate, and rates of adverse events resulting in treatment discontinuation were low overall for both groups; the most common adverse events were application-site pain and erythema, which were mostly mild. Overall, 4.1% of berdazimer-treated patients and 0.7% of placebo patients discontinued the study because of adverse events.
The study findings were limited by several factors, including the small number of patients in subgroups for race, ethnicity, and age; and the lack of data on patients with sexually transmitted MC and on concomitant use with other topical MC therapies, the researchers noted.
However, the results represent the largest randomized clinical trial of berdazimer 10.3% to date, and support its potential as a first-line therapy for MC patients aged 6 months and older, according to the authors. “Berdazimer is under consideration as a first in-class therapeutic agent for MC and may provide a topical prescription alternative to other therapies used for this highly contagious and psychosocially challenging skin condition,” they said.
Having a reliable, steroid-free, safe, and efficacious medication to treat molluscum in the pediatric population, as early as age 6 months, that can be used at home would “change the whole therapeutic paradigm,” one of the study authors, Adelaide Hebert, MD, said in an interview at the Society for Pediatric Dermatology annual meeting in July, where she presented phase 2 data on berdazimer gel. “This is a common problem and the rate of infections among siblings if it goes untreated is 41%. Affected kids have a sense of isolation; they don’t get invited to swimming parties.”
The lack of a safe and effective topical therapy “has been challenging,” added Dr. Hebert, professor of dermatology and pediatrics, and chief of pediatric dermatology at the University of Texas, Houston. She noted that treatments that have been used but have not been successful include imiquimod. “I’m not impressed with tretinoin,” although it is prescribed for MC, and the most common treatment prescribed by pediatricians for molluscum – mupirocin – is “usually not effective,” she said.
Another MC treatment in trials
Another investigative treatment for molluscum contagiosum, VP-102, a drug-device combination of cantharidin 0.7% administered through a single-use precision applicator, has been evaluated in phase 3 studies of patients with MC aged 2 years and older. The results of two phase 3 studies were published in 2020.
In May 2022, Verrica Pharmaceuticals, which is developing VP-102, announced that Food and Drug Administration approval had been delayed because of deficiencies identified at a contract manufacturing organization, and that the company was working with the agency to bring VP-102 to the market as soon as possible.
A step in the right direction
Although MC is self-resolving, cases last an average of 13.5 months, and “many families look to fast-forward their child’s experience with the infection,” Vikash S. Oza, MD, a pediatric dermatologist at New York University, New York, wrote in an editorial that accompanied the berdazimer study.
“To truly create a paradigm shift in the decision to treat MC, a therapeutic treatment would need to be developed that would lead to resolution of the infection over a short time frame (ideally, weeks) with minimal discomfort,” Dr. Oza noted. “Both VP-102 and berdazimer gel, 10.3%, have the potential to be the first-ever MC therapies approved by the U.S. Food and Drug Administration,” and families seeking to reduce MC in visible areas would welcome this option for a home therapy, he said.
However, Dr. Oza emphasized that potential barriers to widespread use of these therapies include whether the efficacy can be maintained in patients who fail to comply with daily application, and the ongoing need for office-based therapy to manage sexually transmitted MC in adults and periocular and perianal MC in children. The study was funded by Novan. Lead author Dr. Browning disclosed grants from Novan during the conduct of the study; Dr. Hebert reported grants from the University of Texas Health Science Center McGovern Medical School-Houston during the conduct of the study. Disclosures of other authors included having reported equity in Novan during the conduct of the study and receiving a grant from Novan. Dr. Oza had no financial conflicts to disclose.
FROM JAMA DERMATOLOGY
Moderate drinking shows more benefit for older vs. younger adults
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
The health risks and benefits of moderate alcohol consumption are complex and remain a hot topic of debate. The data suggest that small amounts of alcohol may reduce the risk of certain health outcomes over time, but increase the risk of others, wrote Dana Bryazka, MS, a researcher at the Institute for Health Metrics and Evaluation (IHME) at the University of Washington, Seattle, and colleagues, in a paper published in the Lancet.
“The amount of alcohol that minimizes health loss is likely to depend on the distribution of underlying causes of disease burden in a given population. Since this distribution varies widely by geography, age, sex, and time, the level of alcohol consumption associated with the lowest risk to health would depend on the age structure and disease composition of that population,” the researchers wrote.
“We estimate that 1.78 million people worldwide died due to alcohol use in 2020,” Ms. Bryazka said in an interview. “It is important that alcohol consumption guidelines and policies are updated to minimize this harm, particularly in the populations at greatest risk,” she said.
“Existing alcohol consumption guidelines frequently vary by sex, with higher consumption thresholds set for males compared to females. Interestingly, with the currently available data we do not see evidence that risk of alcohol use varies by sex,” she noted.
Methods and results
In the study, the researchers conducted a systematic analysis of burden-weighted dose-response relative risk curves across 22 health outcomes. They used disease rates from the Global Burden of Diseases, Injuries, and Risk Factors Study (GBD) 2020 for the years 1990-2020 for 21 regions, including 204 countries and territories. The data were analyzed by 5-year age group, sex, and year for individuals aged 15-95 years and older. The researchers estimated the theoretical minimum risk exposure level (TMREL) and nondrinker equivalent (NDE), meaning the amount of alcohol at which the health risk equals that of a nondrinker.
One standard drink was defined as 10 g of pure alcohol, equivalent to a small glass of red wine (100 mL or 3.4 fluid ounces) at 13% alcohol by volume, a can or bottle of beer (375 mL or 12 fluid ounces) at 3.5% alcohol by volume, or a shot of whiskey or other spirits (30 mL or 1.0 fluid ounces) at 40% alcohol by volume.
Overall, the TMREL was low regardless of age, sex, time, or geography, and varied from 0 to 1.87 standard drinks per day. However, it was lowest for males aged 15-39 years (0.136 drinks per day) and only slightly higher for females aged 15-39 (0.273), representing 1-2 tenths of a standard drink.
For adults aged 40 and older without any underlying health conditions, drinking a small amount of alcohol may provide some benefits, such as reducing the risk of ischemic heart disease, stroke, and diabetes, the researchers noted. In general, for individuals aged 40-64 years, TMRELs ranged from about half a standard drink per day (0.527 drinks for males and 0.562 standard drinks per day for females) to almost two standard drinks (1.69 standard drinks per day for males and 1.82 for females). For those older than 65 years, the TMRELs represented just over 3 standard drinks per day (3.19 for males and 3.51 for females). For individuals aged 40 years and older, the distribution of disease burden varied by region, but was J-shaped across all regions, the researchers noted.
The researchers also found that those individuals consuming harmful amounts of alcohol were most likely to be aged 15-39 (59.1%) and male (76.9%).
The study findings were limited by several factors including the observational design and lack of data on drinking patterns, such as binge drinking, the researchers noted. Other limitations include the lack of data reflecting patterns of alcohol consumption during the COVID-19 pandemic, and exclusion of outcomes often associated with alcohol use, such as depression, anxiety, and dementia, that might reduce estimates of TMREL and NDE.
However, the results add to the ongoing discussion of the relationship between moderate alcohol consumption and health, the researchers said.
“The findings of this study support the development of tailored guidelines and recommendations on alcohol consumption by age and across regions and highlight that existing low consumption thresholds are too high for younger populations in all regions,” they concluded.
Consider individual factors when counseling patients
The takeaway message for primary care is that alcohol consumed in moderation can reduce the risk of ischemic heart disease, stroke, and diabetes, Ms. Bryazka noted. “However, it also increases the risk of many cancers, intentional and unintentional injuries, and infectious diseases like tuberculosis,” she said. “Of these health outcomes, young people are most likely to experience injuries, and as a result, we find that there are significant health risks associated with consuming alcohol for young people. Among older individuals, the relative proportions of these outcomes vary by geography, and so do the risks associated with consuming alcohol,” she explained.
“Importantly, our analysis was conducted at the population level; when evaluating risk at the individual level, it is also important to consider other factors such as the presence of comorbidities and interactions between alcohol and medications,” she emphasized.
Health and alcohol interaction is complicated
“These findings seemingly contradict a previous [Global Burden of Diseases, Injuries, and Risk Factors Study] estimate published in The Lancet, which emphasized that any alcohol use, regardless of amount, leads to health loss across populations,” wrote Robyn Burton, PhD, and Nick Sheron, MD, both of King’s College, London, in an accompanying comment.
However, the novel methods of weighting relative risk curves according to levels of underlying disease drive the difference in results, along with disaggregated estimates by age, sex, and region, they said.
“Across most geographical regions in this latest analysis, injuries accounted for most alcohol-related harm in younger age groups. This led to a minimum risk level of zero, or very close to zero, among individuals aged 15-39 years across all geographical regions,” which is lower than the level for older adults because of the shift in alcohol-related disease burden towards cardiovascular disease and cancers, they said. “This highlights the need to consider existing rates of disease in a population when trying to determine the total harm posed by alcohol,” the commentators wrote.
In an additional commentary, Tony Rao, MD, a visiting clinical research fellow in psychiatry at King’s College, London, noted that “the elephant in the room with this study is the interpretation of risk based on outcomes for cardiovascular disease – particularly in older people. We know that any purported health benefits from alcohol on the heart and circulation are balanced out by the increased risk from other conditions such as cancer, liver disease, and mental disorders such as depression and dementia,” Dr. Rao said. “If we are to simply draw the conclusion that older people should continue or start drinking small amounts because it protects against diseases affecting heart and circulation – which still remains controversial – other lifestyle changes or the use of drugs targeted at individual cardiovascular disorders seem like a less harmful way of improving health and wellbeing.”
Data can guide clinical practice
No previous study has examined the effect of the theoretical minimum risk of alcohol consumption by geography, age, sex, and time in the context of background disease rates, said Noel Deep, MD, in an interview.
“This study enabled the researchers to quantify the proportion of the population that consumed alcohol in amounts that exceeded the thresholds by location, age, sex, and year, and this can serve as a guide in our efforts to target the control of alcohol intake by individuals,” said Dr. Deep, a general internist in private practice in Antigo, Wisc. He also serves as chief medical officer and a staff physician at Aspirus Langlade Hospital in Antigo.
The first take-home message for clinicians is that even low levels of alcohol consumption can have deleterious effects on the health of patients, and patients should be advised accordingly based on the prevalence of diseases in that community and geographic area, Dr. Deep said. “Secondly, clinicians should also consider the risk of alcohol consumption on all forms of health impacts in a given population rather than just focusing on alcohol-related health conditions,” he added.
“This study provides us with the data to tailor our efforts in educating the clinicians and the public about the relationship between alcohol consumption and disease outcomes based on the observed disease rates in each population,” Dr. Deep explained. “The data should provide another reason for physicians to advise their younger patients, especially the younger males, to avoid or minimize alcohol use,” he said. The data also can help clinicians formulate public health messaging and community education to reduce harmful alcohol use, he added.
As for additional research, Dr. Deep said he would like to see data on the difference in the health-related effects of alcohol in binge-drinkers vs. those who regularly consume alcohol on a daily basis. “It would probably also be helpful to figure out what type of alcohol is being studied and the quality of the alcohol,” he said.
The study was supported by the Bill and Melinda Gates Foundation. Ms. Bryazka and colleagues had no financial conflicts to disclose. Dr. Burton disclosed serving as a consultant to the World Health Organization European Office for the Prevention and Control of Noncommunicable Diseases. Dr. Sheron had no financial conflicts to disclose. Dr. Deep had no financial conflicts to disclose, but serves on the Editorial Advisory Board of Internal Medicine News.
The study was supported by the Bill and Melinda Gates Foundation.
FROM THE LANCET
Eight must-read GI studies for the primary care physician
This transcript has been edited for clarity.
Hi. I’m Dr. Vivek Kaul, and I’m professor of medicine in the division of gastroenterology and hepatology at the University of Rochester (N.Y.) Medical Center. It gives me great pleasure to do this presentation in collaboration with Medscape. I have just returned from Digestive Disease Week 2022, which is the largest international GI conference, and it was held in person for the first time in 3 years because of the pandemic.
Whereas there are a lot of “Best of DDW” presentations for gastroenterologists, there are not that many for primary care providers, so I thought it would be a good idea to do this. , talk a little about the middle-future range where things will become available in the next few months to years, and then, of course, reflect upon concepts that might become more standard paradigms of care in the distant future.
My collection of papers is divided into the esophagus and the colon, and then finishes up with the liver. [Editor’s note: Some of the abstracts that Dr Kaul refers to are available here.]
The first paper in the esophagus realm is a multicenter study called “The Association of Proton Pump Inhibitor Use and Cognitive Decline and Incident Dementia in Older Adults.” The researchers looked at about 19,000 patients, 65 years and older, who were on PPI therapy and had no significant disability or prior dementia. They followed them for about 5 years and found that a total of about 566 of them developed dementia in this time frame and 235 or so had Alzheimer’s. What they concluded based on that analysis was that PPI use was not associated with dementia or changes in the overall cognitive score.
This is important information. As the American Gastroenterological Association guidelines and others have recommended, those patients who do require a PPI on clinical grounds should definitely receive it, and the side-effect profiles are quite acceptable.
The second paper, “Double-Blind Randomized Trial of the Potassium-Competitive Acid Blocker Vonoprazan vs. the Proton Pump Inhibitor Lansoprazole in U.S. and European Patients with Erosive Esophagitis,” is also related to GERD [gastroesophageal reflux disease] therapy but introduces a new paradigm known as potassium-competitive channel blockers. This is a new drug that has now become available, called vonoprazan.
This was a double-blinded, randomized trial of the potassium-competitive acid blocker vonoprazan in comparison with lansoprazole, which is a well-established medical agent available for the treatment of esophagitis. These are patients with erosive esophagitis in a multicenter U.S. and European cohort of about 1,000 patients who were prospectively treated. The crux of this study was to say that vonoprazan is quicker to provide healing and symptom relief, and that these results are maintained in both the initial phase, which is the treatment phase, and the maintenance phase.
So, there might be some advantages in terms of how quickly we can treat these patients and get them symptom free. I thought that study was worth mentioning because it reflects, after a long period of time, a new class of acid-suppression therapy, which we should all be familiar with, and certainly at the primary care level.
The next paper relates to Barrett’s esophagus and esophageal cancer. This paper came out of the OneFlorida+ Clinical Research Network and was titled “Alarming Rise Found in Esophageal Cancer and Barrett’s Esophagus in Middle-Aged Adults: Findings From a Statewide Database of Over 5 Million Patients.” This paper talks about the increasing prevalence of esophageal cancer in Barrett’s in middle-aged patients – those in the 45- to 64-years age group; the prevalence of esophageal cancer is rising in this cohort. So, as is shown in the first graph, the orange line is depicting the 45-64 age group patients whose esophageal cancer prevalence has gone up. And in the second graph, it’s actually the gray line which looks at the Barrett’s esophagus prevalence, which is also increasing. And all the other cohorts have either plateaued or are declining.
This is important information because these patients who are at risk in these age groups with these demographic profiles should be referred on for endoscopic screening to rule out Barrett’s at least once in their lifetime. And most certainly a percentage of them will be found to have dysplasia and or early esophageal cancer that might be amenable to endoscopic therapy.
The next section that we’ll talk about is the colon section. We have a few very good, high-quality papers with some provocative information in this realm. The first paper (“Multi-modal Blood-based Colorectal Cancer Screening Is a Viable Colorectal Cancer Screening Option – a Prospective Study”) in the colonoscopy section involves the concept of colorectal cancer screening. While we have multiple modalities available for colorectal cancer screening today, a third of eligible patients are not getting screened.
This study looks at a blood test for colorectal cancer screening. We have colonoscopy, we have stool DNA and other tests, but now we have a blood test looking at circulating tumor DNA. For this prospective, multicenter study, researchers from Madrid enrolled about 550 patients between 45 and 84 years of age. The blood test was completed prior to the complete colonoscopy. The prevalence of colorectal cancer screening in this study was about 2%; the sensitivity ranged from about 90% to 95%, and the specificity ranged from about 100% to 88%, depending on what confidence levels you were looking at.
In this prospective study, a blood-based colorectal cancer screening test was able to perform very similarly to stool-based options. Therefore, it may further increase the probability that patients might come in for screening.
The message from this paper is that there’s yet another modality for colorectal cancer screening, and now we have a blood test potentially, but obviously we look forward to more data on how the test itself performs. And there probably will be other candidates in the same realm.
The second paper (“Real-World Comparative Effectiveness of Fidaxomicin vs. Vancomycin Among Medicare Beneficiaries with Clostridioides difficile Infection”) in the colon section is also about a very important topic in clinical practice for all of us, and that is C. difficile infection. As you may know, current guidelines have recommended the use of fidaxomicin over vancomycin as the initial treatment for C. diff infection. This paper looked retrospectively at a cohort of patients in the real world and compared the efficacy of fidaxomicin vs. vancomycin among Medicare beneficiaries with C. diff infection.
The initial results of this multicenter study suggest that treatment with fidaxomicin had higher sustained response compared with vancomycin at both weeks 4 and 8, as well as decreased recurrence of C. difficile.
This retrospective study further confirms that C. diff infection remains a problem and that we might have better solutions now with fidaxomicin compared with vancomycin. That’s important information and is already endorsed by the guidelines.
The next paper in the colon realm, “A Randomized Controlled Trial on the Effectiveness of Cognitive-Behavioral and Mindfulness Intervention on Pain, Fatigue and Impairments at Work and Daily Activity in Patients With Crohn’s Disease,” is also an important paradigm that has entered our medical practice. When we are treating patients with GI symptoms, the role for cognitive-behavioral therapy and mindfulness interventions has now come of age.
This paper was a randomized controlled trial on the effectiveness of cognitive-behavioral therapy and mindfulness intervention on the aspects of pain and fatigue, as well as impairments at work and daily activity in patients with Crohn’s disease.
This is a difficult population with chronic illness, and this study comes out of Israel. About 120 patients were randomized to seven 1-hour sessions of psychological training over 12 weeks. The placebo group was the control group that did not get this treatment.
These interventions reduced both fatigue as well as pain levels, and also reduced work and home impairment, and so overall led to a better quality of life.
This paper is important because it shows us in a randomized trial design fashion that a difficult clinical population with Crohn’s disease, with a multitude of systemic symptoms and psychological, psychosomatic issues as well, can be positively impacted by these newer strategies related to cognitive-behavioral therapy and mindfulness interventions. We’ll likely be seeing more of these types of papers coming out, not just for Crohn’s and inflammatory bowel disease, but also for functional disease, which is where this started.
Our final paper in the colon section relates to an interesting concept, which is the treatment of chronic idiopathic constipation – not irritable bowel syndrome with constipation, but chronic idiopathic constipation – being managed with a novel device known as the Vibrant capsule. The Vibrant capsule is exactly that: It’s a capsule that the patient ingests, and it vibrates and therefore creates a mechanical movement.
“Efficacy and Safety of Vibrant Capsule vs. Placebo for the Treatment of Chronic Idiopathic Constipation (Vibrant)” was a U.S. multicenter study. The device is an orally ingestible, programmable vibrating capsule developed in Israel. It basically mimics the biological clock and increases the stool frequency by augmenting the circadian rhythm. This was a prospective trial of around 350 patients, and there was significant improvement in the complete spontaneous bowel movement pattern, both for one and two bowel movements per week. This significant improvement persisted at week 3, peaked at about week 6, and then remained sustained through 8 weeks.
The Vibrant capsule also was able to improve stool consistency and the overall quality of life. So this is a novel treatment intervention over and above all the medical therapies in the bowel regimens, which of course our patients find somewhat difficult, understandably. But this might be a complementary direction to go in, and we’ll probably hear more of these novel interventions for chronic constipation, which is a huge problem both at the primary care level as well as in subspecialty practice.
In the final section, which is the liver section, I found one paper very interesting, which refers to the concept of lean nonalcoholic fatty liver disease (NAFLD) (“Lean NAFLD in the United States is Characterized by Increased Central and Visceral Adiposity That Is Comparable to Overweight and Obese Persons”). NAFLD is an epidemic throughout the country, with obvious implications both for the metabolic syndrome as well as chronic liver disease. This paper from Wisconsin looks at lean NALFD in the United States, characterizes the central and visceral adiposity, and compares it with that of overweight and obese patients. Lean NAFLD occurs in about 10%-20% of patients with a normal body mass index; 1,800 patients were evaluated in this particular study, and they underwent cross-sectional analysis and the so-called gap score, which looks at the measurement of fat in the liver, DEXA measurements, and so forth.
What they found was that patients with lean NAFLD are more likely to have hypertension, diabetes, high triglycerides, and are more likely to smoke, compared with lean patients without NAFLD, despite having similar BMIs.
A couple of additional observations from the study were that central adiposity was similar in lean NAFLD compared with the obese non-NAFLD population, and the visceral abdominal fat in patients who have lean NAFLD was slightly higher, actually, than in the obese NAFLD patients, but the P values were not significant.
The overall summary from this paper was that NAFLD should be considered in lean patients with risk factors of the metabolic syndrome. This is an important paper because it highlights the fact that we don’t necessarily have to be externally obese or have a high BMI to be at risk for the metabolic syndrome. I think the importance of evaluating for the metabolic syndrome, even in those patients who have a relatively lower BMI, is underscored by this paper, which has significant implications given the larger denominator of this population in this country.
So, with that, we come to the conclusion of these top papers from Digestive Disease Week 2022. We covered the gamut of conditions, from the esophagus to the colon and to the liver. And these represent some of the best science that was presented at this very large international meeting. I hope you will find value in this information for the care of your patients, and I look forward to presenting again when the next opportunity arises.
Vivek Kaul, MD, is Segal-Watson Professor of Medicine in the gastroenterology & hepatology division at the University of Rochester Medical Center in Rochester, N.Y.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Dr. Vivek Kaul, and I’m professor of medicine in the division of gastroenterology and hepatology at the University of Rochester (N.Y.) Medical Center. It gives me great pleasure to do this presentation in collaboration with Medscape. I have just returned from Digestive Disease Week 2022, which is the largest international GI conference, and it was held in person for the first time in 3 years because of the pandemic.
Whereas there are a lot of “Best of DDW” presentations for gastroenterologists, there are not that many for primary care providers, so I thought it would be a good idea to do this. , talk a little about the middle-future range where things will become available in the next few months to years, and then, of course, reflect upon concepts that might become more standard paradigms of care in the distant future.
My collection of papers is divided into the esophagus and the colon, and then finishes up with the liver. [Editor’s note: Some of the abstracts that Dr Kaul refers to are available here.]
The first paper in the esophagus realm is a multicenter study called “The Association of Proton Pump Inhibitor Use and Cognitive Decline and Incident Dementia in Older Adults.” The researchers looked at about 19,000 patients, 65 years and older, who were on PPI therapy and had no significant disability or prior dementia. They followed them for about 5 years and found that a total of about 566 of them developed dementia in this time frame and 235 or so had Alzheimer’s. What they concluded based on that analysis was that PPI use was not associated with dementia or changes in the overall cognitive score.
This is important information. As the American Gastroenterological Association guidelines and others have recommended, those patients who do require a PPI on clinical grounds should definitely receive it, and the side-effect profiles are quite acceptable.
The second paper, “Double-Blind Randomized Trial of the Potassium-Competitive Acid Blocker Vonoprazan vs. the Proton Pump Inhibitor Lansoprazole in U.S. and European Patients with Erosive Esophagitis,” is also related to GERD [gastroesophageal reflux disease] therapy but introduces a new paradigm known as potassium-competitive channel blockers. This is a new drug that has now become available, called vonoprazan.
This was a double-blinded, randomized trial of the potassium-competitive acid blocker vonoprazan in comparison with lansoprazole, which is a well-established medical agent available for the treatment of esophagitis. These are patients with erosive esophagitis in a multicenter U.S. and European cohort of about 1,000 patients who were prospectively treated. The crux of this study was to say that vonoprazan is quicker to provide healing and symptom relief, and that these results are maintained in both the initial phase, which is the treatment phase, and the maintenance phase.
So, there might be some advantages in terms of how quickly we can treat these patients and get them symptom free. I thought that study was worth mentioning because it reflects, after a long period of time, a new class of acid-suppression therapy, which we should all be familiar with, and certainly at the primary care level.
The next paper relates to Barrett’s esophagus and esophageal cancer. This paper came out of the OneFlorida+ Clinical Research Network and was titled “Alarming Rise Found in Esophageal Cancer and Barrett’s Esophagus in Middle-Aged Adults: Findings From a Statewide Database of Over 5 Million Patients.” This paper talks about the increasing prevalence of esophageal cancer in Barrett’s in middle-aged patients – those in the 45- to 64-years age group; the prevalence of esophageal cancer is rising in this cohort. So, as is shown in the first graph, the orange line is depicting the 45-64 age group patients whose esophageal cancer prevalence has gone up. And in the second graph, it’s actually the gray line which looks at the Barrett’s esophagus prevalence, which is also increasing. And all the other cohorts have either plateaued or are declining.
This is important information because these patients who are at risk in these age groups with these demographic profiles should be referred on for endoscopic screening to rule out Barrett’s at least once in their lifetime. And most certainly a percentage of them will be found to have dysplasia and or early esophageal cancer that might be amenable to endoscopic therapy.
The next section that we’ll talk about is the colon section. We have a few very good, high-quality papers with some provocative information in this realm. The first paper (“Multi-modal Blood-based Colorectal Cancer Screening Is a Viable Colorectal Cancer Screening Option – a Prospective Study”) in the colonoscopy section involves the concept of colorectal cancer screening. While we have multiple modalities available for colorectal cancer screening today, a third of eligible patients are not getting screened.
This study looks at a blood test for colorectal cancer screening. We have colonoscopy, we have stool DNA and other tests, but now we have a blood test looking at circulating tumor DNA. For this prospective, multicenter study, researchers from Madrid enrolled about 550 patients between 45 and 84 years of age. The blood test was completed prior to the complete colonoscopy. The prevalence of colorectal cancer screening in this study was about 2%; the sensitivity ranged from about 90% to 95%, and the specificity ranged from about 100% to 88%, depending on what confidence levels you were looking at.
In this prospective study, a blood-based colorectal cancer screening test was able to perform very similarly to stool-based options. Therefore, it may further increase the probability that patients might come in for screening.
The message from this paper is that there’s yet another modality for colorectal cancer screening, and now we have a blood test potentially, but obviously we look forward to more data on how the test itself performs. And there probably will be other candidates in the same realm.
The second paper (“Real-World Comparative Effectiveness of Fidaxomicin vs. Vancomycin Among Medicare Beneficiaries with Clostridioides difficile Infection”) in the colon section is also about a very important topic in clinical practice for all of us, and that is C. difficile infection. As you may know, current guidelines have recommended the use of fidaxomicin over vancomycin as the initial treatment for C. diff infection. This paper looked retrospectively at a cohort of patients in the real world and compared the efficacy of fidaxomicin vs. vancomycin among Medicare beneficiaries with C. diff infection.
The initial results of this multicenter study suggest that treatment with fidaxomicin had higher sustained response compared with vancomycin at both weeks 4 and 8, as well as decreased recurrence of C. difficile.
This retrospective study further confirms that C. diff infection remains a problem and that we might have better solutions now with fidaxomicin compared with vancomycin. That’s important information and is already endorsed by the guidelines.
The next paper in the colon realm, “A Randomized Controlled Trial on the Effectiveness of Cognitive-Behavioral and Mindfulness Intervention on Pain, Fatigue and Impairments at Work and Daily Activity in Patients With Crohn’s Disease,” is also an important paradigm that has entered our medical practice. When we are treating patients with GI symptoms, the role for cognitive-behavioral therapy and mindfulness interventions has now come of age.
This paper was a randomized controlled trial on the effectiveness of cognitive-behavioral therapy and mindfulness intervention on the aspects of pain and fatigue, as well as impairments at work and daily activity in patients with Crohn’s disease.
This is a difficult population with chronic illness, and this study comes out of Israel. About 120 patients were randomized to seven 1-hour sessions of psychological training over 12 weeks. The placebo group was the control group that did not get this treatment.
These interventions reduced both fatigue as well as pain levels, and also reduced work and home impairment, and so overall led to a better quality of life.
This paper is important because it shows us in a randomized trial design fashion that a difficult clinical population with Crohn’s disease, with a multitude of systemic symptoms and psychological, psychosomatic issues as well, can be positively impacted by these newer strategies related to cognitive-behavioral therapy and mindfulness interventions. We’ll likely be seeing more of these types of papers coming out, not just for Crohn’s and inflammatory bowel disease, but also for functional disease, which is where this started.
Our final paper in the colon section relates to an interesting concept, which is the treatment of chronic idiopathic constipation – not irritable bowel syndrome with constipation, but chronic idiopathic constipation – being managed with a novel device known as the Vibrant capsule. The Vibrant capsule is exactly that: It’s a capsule that the patient ingests, and it vibrates and therefore creates a mechanical movement.
“Efficacy and Safety of Vibrant Capsule vs. Placebo for the Treatment of Chronic Idiopathic Constipation (Vibrant)” was a U.S. multicenter study. The device is an orally ingestible, programmable vibrating capsule developed in Israel. It basically mimics the biological clock and increases the stool frequency by augmenting the circadian rhythm. This was a prospective trial of around 350 patients, and there was significant improvement in the complete spontaneous bowel movement pattern, both for one and two bowel movements per week. This significant improvement persisted at week 3, peaked at about week 6, and then remained sustained through 8 weeks.
The Vibrant capsule also was able to improve stool consistency and the overall quality of life. So this is a novel treatment intervention over and above all the medical therapies in the bowel regimens, which of course our patients find somewhat difficult, understandably. But this might be a complementary direction to go in, and we’ll probably hear more of these novel interventions for chronic constipation, which is a huge problem both at the primary care level as well as in subspecialty practice.
In the final section, which is the liver section, I found one paper very interesting, which refers to the concept of lean nonalcoholic fatty liver disease (NAFLD) (“Lean NAFLD in the United States is Characterized by Increased Central and Visceral Adiposity That Is Comparable to Overweight and Obese Persons”). NAFLD is an epidemic throughout the country, with obvious implications both for the metabolic syndrome as well as chronic liver disease. This paper from Wisconsin looks at lean NALFD in the United States, characterizes the central and visceral adiposity, and compares it with that of overweight and obese patients. Lean NAFLD occurs in about 10%-20% of patients with a normal body mass index; 1,800 patients were evaluated in this particular study, and they underwent cross-sectional analysis and the so-called gap score, which looks at the measurement of fat in the liver, DEXA measurements, and so forth.
What they found was that patients with lean NAFLD are more likely to have hypertension, diabetes, high triglycerides, and are more likely to smoke, compared with lean patients without NAFLD, despite having similar BMIs.
A couple of additional observations from the study were that central adiposity was similar in lean NAFLD compared with the obese non-NAFLD population, and the visceral abdominal fat in patients who have lean NAFLD was slightly higher, actually, than in the obese NAFLD patients, but the P values were not significant.
The overall summary from this paper was that NAFLD should be considered in lean patients with risk factors of the metabolic syndrome. This is an important paper because it highlights the fact that we don’t necessarily have to be externally obese or have a high BMI to be at risk for the metabolic syndrome. I think the importance of evaluating for the metabolic syndrome, even in those patients who have a relatively lower BMI, is underscored by this paper, which has significant implications given the larger denominator of this population in this country.
So, with that, we come to the conclusion of these top papers from Digestive Disease Week 2022. We covered the gamut of conditions, from the esophagus to the colon and to the liver. And these represent some of the best science that was presented at this very large international meeting. I hope you will find value in this information for the care of your patients, and I look forward to presenting again when the next opportunity arises.
Vivek Kaul, MD, is Segal-Watson Professor of Medicine in the gastroenterology & hepatology division at the University of Rochester Medical Center in Rochester, N.Y.
A version of this article first appeared on Medscape.com.
This transcript has been edited for clarity.
Hi. I’m Dr. Vivek Kaul, and I’m professor of medicine in the division of gastroenterology and hepatology at the University of Rochester (N.Y.) Medical Center. It gives me great pleasure to do this presentation in collaboration with Medscape. I have just returned from Digestive Disease Week 2022, which is the largest international GI conference, and it was held in person for the first time in 3 years because of the pandemic.
Whereas there are a lot of “Best of DDW” presentations for gastroenterologists, there are not that many for primary care providers, so I thought it would be a good idea to do this. , talk a little about the middle-future range where things will become available in the next few months to years, and then, of course, reflect upon concepts that might become more standard paradigms of care in the distant future.
My collection of papers is divided into the esophagus and the colon, and then finishes up with the liver. [Editor’s note: Some of the abstracts that Dr Kaul refers to are available here.]
The first paper in the esophagus realm is a multicenter study called “The Association of Proton Pump Inhibitor Use and Cognitive Decline and Incident Dementia in Older Adults.” The researchers looked at about 19,000 patients, 65 years and older, who were on PPI therapy and had no significant disability or prior dementia. They followed them for about 5 years and found that a total of about 566 of them developed dementia in this time frame and 235 or so had Alzheimer’s. What they concluded based on that analysis was that PPI use was not associated with dementia or changes in the overall cognitive score.
This is important information. As the American Gastroenterological Association guidelines and others have recommended, those patients who do require a PPI on clinical grounds should definitely receive it, and the side-effect profiles are quite acceptable.
The second paper, “Double-Blind Randomized Trial of the Potassium-Competitive Acid Blocker Vonoprazan vs. the Proton Pump Inhibitor Lansoprazole in U.S. and European Patients with Erosive Esophagitis,” is also related to GERD [gastroesophageal reflux disease] therapy but introduces a new paradigm known as potassium-competitive channel blockers. This is a new drug that has now become available, called vonoprazan.
This was a double-blinded, randomized trial of the potassium-competitive acid blocker vonoprazan in comparison with lansoprazole, which is a well-established medical agent available for the treatment of esophagitis. These are patients with erosive esophagitis in a multicenter U.S. and European cohort of about 1,000 patients who were prospectively treated. The crux of this study was to say that vonoprazan is quicker to provide healing and symptom relief, and that these results are maintained in both the initial phase, which is the treatment phase, and the maintenance phase.
So, there might be some advantages in terms of how quickly we can treat these patients and get them symptom free. I thought that study was worth mentioning because it reflects, after a long period of time, a new class of acid-suppression therapy, which we should all be familiar with, and certainly at the primary care level.
The next paper relates to Barrett’s esophagus and esophageal cancer. This paper came out of the OneFlorida+ Clinical Research Network and was titled “Alarming Rise Found in Esophageal Cancer and Barrett’s Esophagus in Middle-Aged Adults: Findings From a Statewide Database of Over 5 Million Patients.” This paper talks about the increasing prevalence of esophageal cancer in Barrett’s in middle-aged patients – those in the 45- to 64-years age group; the prevalence of esophageal cancer is rising in this cohort. So, as is shown in the first graph, the orange line is depicting the 45-64 age group patients whose esophageal cancer prevalence has gone up. And in the second graph, it’s actually the gray line which looks at the Barrett’s esophagus prevalence, which is also increasing. And all the other cohorts have either plateaued or are declining.
This is important information because these patients who are at risk in these age groups with these demographic profiles should be referred on for endoscopic screening to rule out Barrett’s at least once in their lifetime. And most certainly a percentage of them will be found to have dysplasia and or early esophageal cancer that might be amenable to endoscopic therapy.
The next section that we’ll talk about is the colon section. We have a few very good, high-quality papers with some provocative information in this realm. The first paper (“Multi-modal Blood-based Colorectal Cancer Screening Is a Viable Colorectal Cancer Screening Option – a Prospective Study”) in the colonoscopy section involves the concept of colorectal cancer screening. While we have multiple modalities available for colorectal cancer screening today, a third of eligible patients are not getting screened.
This study looks at a blood test for colorectal cancer screening. We have colonoscopy, we have stool DNA and other tests, but now we have a blood test looking at circulating tumor DNA. For this prospective, multicenter study, researchers from Madrid enrolled about 550 patients between 45 and 84 years of age. The blood test was completed prior to the complete colonoscopy. The prevalence of colorectal cancer screening in this study was about 2%; the sensitivity ranged from about 90% to 95%, and the specificity ranged from about 100% to 88%, depending on what confidence levels you were looking at.
In this prospective study, a blood-based colorectal cancer screening test was able to perform very similarly to stool-based options. Therefore, it may further increase the probability that patients might come in for screening.
The message from this paper is that there’s yet another modality for colorectal cancer screening, and now we have a blood test potentially, but obviously we look forward to more data on how the test itself performs. And there probably will be other candidates in the same realm.
The second paper (“Real-World Comparative Effectiveness of Fidaxomicin vs. Vancomycin Among Medicare Beneficiaries with Clostridioides difficile Infection”) in the colon section is also about a very important topic in clinical practice for all of us, and that is C. difficile infection. As you may know, current guidelines have recommended the use of fidaxomicin over vancomycin as the initial treatment for C. diff infection. This paper looked retrospectively at a cohort of patients in the real world and compared the efficacy of fidaxomicin vs. vancomycin among Medicare beneficiaries with C. diff infection.
The initial results of this multicenter study suggest that treatment with fidaxomicin had higher sustained response compared with vancomycin at both weeks 4 and 8, as well as decreased recurrence of C. difficile.
This retrospective study further confirms that C. diff infection remains a problem and that we might have better solutions now with fidaxomicin compared with vancomycin. That’s important information and is already endorsed by the guidelines.
The next paper in the colon realm, “A Randomized Controlled Trial on the Effectiveness of Cognitive-Behavioral and Mindfulness Intervention on Pain, Fatigue and Impairments at Work and Daily Activity in Patients With Crohn’s Disease,” is also an important paradigm that has entered our medical practice. When we are treating patients with GI symptoms, the role for cognitive-behavioral therapy and mindfulness interventions has now come of age.
This paper was a randomized controlled trial on the effectiveness of cognitive-behavioral therapy and mindfulness intervention on the aspects of pain and fatigue, as well as impairments at work and daily activity in patients with Crohn’s disease.
This is a difficult population with chronic illness, and this study comes out of Israel. About 120 patients were randomized to seven 1-hour sessions of psychological training over 12 weeks. The placebo group was the control group that did not get this treatment.
These interventions reduced both fatigue as well as pain levels, and also reduced work and home impairment, and so overall led to a better quality of life.
This paper is important because it shows us in a randomized trial design fashion that a difficult clinical population with Crohn’s disease, with a multitude of systemic symptoms and psychological, psychosomatic issues as well, can be positively impacted by these newer strategies related to cognitive-behavioral therapy and mindfulness interventions. We’ll likely be seeing more of these types of papers coming out, not just for Crohn’s and inflammatory bowel disease, but also for functional disease, which is where this started.
Our final paper in the colon section relates to an interesting concept, which is the treatment of chronic idiopathic constipation – not irritable bowel syndrome with constipation, but chronic idiopathic constipation – being managed with a novel device known as the Vibrant capsule. The Vibrant capsule is exactly that: It’s a capsule that the patient ingests, and it vibrates and therefore creates a mechanical movement.
“Efficacy and Safety of Vibrant Capsule vs. Placebo for the Treatment of Chronic Idiopathic Constipation (Vibrant)” was a U.S. multicenter study. The device is an orally ingestible, programmable vibrating capsule developed in Israel. It basically mimics the biological clock and increases the stool frequency by augmenting the circadian rhythm. This was a prospective trial of around 350 patients, and there was significant improvement in the complete spontaneous bowel movement pattern, both for one and two bowel movements per week. This significant improvement persisted at week 3, peaked at about week 6, and then remained sustained through 8 weeks.
The Vibrant capsule also was able to improve stool consistency and the overall quality of life. So this is a novel treatment intervention over and above all the medical therapies in the bowel regimens, which of course our patients find somewhat difficult, understandably. But this might be a complementary direction to go in, and we’ll probably hear more of these novel interventions for chronic constipation, which is a huge problem both at the primary care level as well as in subspecialty practice.
In the final section, which is the liver section, I found one paper very interesting, which refers to the concept of lean nonalcoholic fatty liver disease (NAFLD) (“Lean NAFLD in the United States is Characterized by Increased Central and Visceral Adiposity That Is Comparable to Overweight and Obese Persons”). NAFLD is an epidemic throughout the country, with obvious implications both for the metabolic syndrome as well as chronic liver disease. This paper from Wisconsin looks at lean NALFD in the United States, characterizes the central and visceral adiposity, and compares it with that of overweight and obese patients. Lean NAFLD occurs in about 10%-20% of patients with a normal body mass index; 1,800 patients were evaluated in this particular study, and they underwent cross-sectional analysis and the so-called gap score, which looks at the measurement of fat in the liver, DEXA measurements, and so forth.
What they found was that patients with lean NAFLD are more likely to have hypertension, diabetes, high triglycerides, and are more likely to smoke, compared with lean patients without NAFLD, despite having similar BMIs.
A couple of additional observations from the study were that central adiposity was similar in lean NAFLD compared with the obese non-NAFLD population, and the visceral abdominal fat in patients who have lean NAFLD was slightly higher, actually, than in the obese NAFLD patients, but the P values were not significant.
The overall summary from this paper was that NAFLD should be considered in lean patients with risk factors of the metabolic syndrome. This is an important paper because it highlights the fact that we don’t necessarily have to be externally obese or have a high BMI to be at risk for the metabolic syndrome. I think the importance of evaluating for the metabolic syndrome, even in those patients who have a relatively lower BMI, is underscored by this paper, which has significant implications given the larger denominator of this population in this country.
So, with that, we come to the conclusion of these top papers from Digestive Disease Week 2022. We covered the gamut of conditions, from the esophagus to the colon and to the liver. And these represent some of the best science that was presented at this very large international meeting. I hope you will find value in this information for the care of your patients, and I look forward to presenting again when the next opportunity arises.
Vivek Kaul, MD, is Segal-Watson Professor of Medicine in the gastroenterology & hepatology division at the University of Rochester Medical Center in Rochester, N.Y.
A version of this article first appeared on Medscape.com.
August 2022 - ICYMI
Gastroenterology
May 2022
Predicting Pancreatic Cancer in the UK Biobank Cohort Using Polygenic Risk Scores and Diabetes Mellitus
Sharma S et al. Gastroenterology. 2022 May;162(6):1665-1674.e2. doi: 10.1053/j.gastro.2022.01.016.
Evaluating Eosinophilic Colitis as a Unique Disease Using Colonic Molecular Profiles: A Multi-Site Study
Shoda T et al. Gastroenterology. 2022 May;162(6):1635-1649. doi: 10.1053/j.gastro.2022.01.022.
June 2022
How to Incorporate Advanced Tissue Resection Techniques in Your Institution
Repici A et al. Gastroenterology. 2022 Jun; 162(7):1825-1830. doi: 10.1053/j.gastro.2022.03.034.
July 2022
Pregnancy-Associated Liver Diseases
Terrault NA, Williamson C. Gastroenterology. 2022 Jul;163(1):97-117.e1. doi: 10.1053/j.gastro.2022.01.060.
Databases for Gastrointestinal Clinical and Public Health Research: Have Database, Will Research
Rustgi SD et al. Gastroenterology. 2022 Jul;163(1):31-34. doi: 10.1053/j.gastro.2022.04.024.
Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia
Wallace MB et al. Gastroenterology. 2022 Jul;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
The Association Between Proton Pump Inhibitor Exposure and Key Liver-Related Outcomes in Patients With Cirrhosis: A Veterans Affairs Cohort Study
Mahmud N et al. Gastroenterology. 2022 Jul;163(1):257-269.e6. doi: 10.1053/j.gastro.2022.03.052.
Clinical Gastroenterology and Hepatology
May 2022
Practice Patterns and Predictors of Stopping Colonoscopy in Older Adults With Colorectal Polyps
Rege S et al. Clin Gastroenterol Hepatol. 2022 May;20(5):e1050-e1060. doi: 10.1016/j.cgh.2021.06.041.
Duodenal Mucosal Barrier in Functional Dyspepsia
Narayanan SP et al. Clin Gastroenterol Hepatol. 2022 May;20(5):1019-1028.e3. doi: 10.1016/j.cgh.2021.09.029.
June 2022
Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: A Prospective Cohort Study
Lo CH et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1323-e1337. doi: 10.1016/j.cgh.2021.08.031.
Changes in Lifestyle Factors After Endoscopic Screening: A Prospective Study in the United States
Knudsen MD et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1240-e1249. doi: 10.1016/j.cgh.2021.07.014.
Trends in Early-onset vs Late-onset Colorectal Cancer Incidence by Race/Ethnicity in the United States Cancer Statistics Database
Chang SH et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1365-e1377. doi: 10.1016/j.cgh.2021.07.035.
July 2022
Updates in Telemedicine for Gastroenterology Practices in the United States
Serper M, Volk ML. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1432-1435. doi: 10.1016/j.cgh.2022.03.024.
Prevalence and Financial Burden of Digestive Diseases in a Commercially Insured Population
Mathews SC et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1480-1487.e7. doi: 10.1016/j.cgh.2021.06.047.
Prevalence of Forceps Polypectomy of Nondiminutive Polyps Is Substantial But Modifiable
Fudman DI et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1508-1515. doi: 10.1016/j.cgh.2021.11.031.
Long-Term Treatment of Eosinophilic Esophagitis With Budesonide Oral Suspension
Dellon ES et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1488-1498.e11. doi: 10.1016/j.cgh.2021.06.020.
Techniques and Innovations in Gastrointestinal Endoscopy
Intervention Versus Observation in Patients Presenting With Lower Gastrointestinal Bleeding
Lipcsey MS et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(2):145-152. doi: 10.1016/j.tige.2021.12.001.
Physician Reimbursement for Endoscopic Submucosal Dissection: A Single Center Analysis
Gajula P et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(2):153-158. doi: 10.1016/j.tige.2021.12.003.
Gastroenterology
May 2022
Predicting Pancreatic Cancer in the UK Biobank Cohort Using Polygenic Risk Scores and Diabetes Mellitus
Sharma S et al. Gastroenterology. 2022 May;162(6):1665-1674.e2. doi: 10.1053/j.gastro.2022.01.016.
Evaluating Eosinophilic Colitis as a Unique Disease Using Colonic Molecular Profiles: A Multi-Site Study
Shoda T et al. Gastroenterology. 2022 May;162(6):1635-1649. doi: 10.1053/j.gastro.2022.01.022.
June 2022
How to Incorporate Advanced Tissue Resection Techniques in Your Institution
Repici A et al. Gastroenterology. 2022 Jun; 162(7):1825-1830. doi: 10.1053/j.gastro.2022.03.034.
July 2022
Pregnancy-Associated Liver Diseases
Terrault NA, Williamson C. Gastroenterology. 2022 Jul;163(1):97-117.e1. doi: 10.1053/j.gastro.2022.01.060.
Databases for Gastrointestinal Clinical and Public Health Research: Have Database, Will Research
Rustgi SD et al. Gastroenterology. 2022 Jul;163(1):31-34. doi: 10.1053/j.gastro.2022.04.024.
Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia
Wallace MB et al. Gastroenterology. 2022 Jul;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
The Association Between Proton Pump Inhibitor Exposure and Key Liver-Related Outcomes in Patients With Cirrhosis: A Veterans Affairs Cohort Study
Mahmud N et al. Gastroenterology. 2022 Jul;163(1):257-269.e6. doi: 10.1053/j.gastro.2022.03.052.
Clinical Gastroenterology and Hepatology
May 2022
Practice Patterns and Predictors of Stopping Colonoscopy in Older Adults With Colorectal Polyps
Rege S et al. Clin Gastroenterol Hepatol. 2022 May;20(5):e1050-e1060. doi: 10.1016/j.cgh.2021.06.041.
Duodenal Mucosal Barrier in Functional Dyspepsia
Narayanan SP et al. Clin Gastroenterol Hepatol. 2022 May;20(5):1019-1028.e3. doi: 10.1016/j.cgh.2021.09.029.
June 2022
Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: A Prospective Cohort Study
Lo CH et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1323-e1337. doi: 10.1016/j.cgh.2021.08.031.
Changes in Lifestyle Factors After Endoscopic Screening: A Prospective Study in the United States
Knudsen MD et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1240-e1249. doi: 10.1016/j.cgh.2021.07.014.
Trends in Early-onset vs Late-onset Colorectal Cancer Incidence by Race/Ethnicity in the United States Cancer Statistics Database
Chang SH et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1365-e1377. doi: 10.1016/j.cgh.2021.07.035.
July 2022
Updates in Telemedicine for Gastroenterology Practices in the United States
Serper M, Volk ML. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1432-1435. doi: 10.1016/j.cgh.2022.03.024.
Prevalence and Financial Burden of Digestive Diseases in a Commercially Insured Population
Mathews SC et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1480-1487.e7. doi: 10.1016/j.cgh.2021.06.047.
Prevalence of Forceps Polypectomy of Nondiminutive Polyps Is Substantial But Modifiable
Fudman DI et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1508-1515. doi: 10.1016/j.cgh.2021.11.031.
Long-Term Treatment of Eosinophilic Esophagitis With Budesonide Oral Suspension
Dellon ES et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1488-1498.e11. doi: 10.1016/j.cgh.2021.06.020.
Techniques and Innovations in Gastrointestinal Endoscopy
Intervention Versus Observation in Patients Presenting With Lower Gastrointestinal Bleeding
Lipcsey MS et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(2):145-152. doi: 10.1016/j.tige.2021.12.001.
Physician Reimbursement for Endoscopic Submucosal Dissection: A Single Center Analysis
Gajula P et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(2):153-158. doi: 10.1016/j.tige.2021.12.003.
Gastroenterology
May 2022
Predicting Pancreatic Cancer in the UK Biobank Cohort Using Polygenic Risk Scores and Diabetes Mellitus
Sharma S et al. Gastroenterology. 2022 May;162(6):1665-1674.e2. doi: 10.1053/j.gastro.2022.01.016.
Evaluating Eosinophilic Colitis as a Unique Disease Using Colonic Molecular Profiles: A Multi-Site Study
Shoda T et al. Gastroenterology. 2022 May;162(6):1635-1649. doi: 10.1053/j.gastro.2022.01.022.
June 2022
How to Incorporate Advanced Tissue Resection Techniques in Your Institution
Repici A et al. Gastroenterology. 2022 Jun; 162(7):1825-1830. doi: 10.1053/j.gastro.2022.03.034.
July 2022
Pregnancy-Associated Liver Diseases
Terrault NA, Williamson C. Gastroenterology. 2022 Jul;163(1):97-117.e1. doi: 10.1053/j.gastro.2022.01.060.
Databases for Gastrointestinal Clinical and Public Health Research: Have Database, Will Research
Rustgi SD et al. Gastroenterology. 2022 Jul;163(1):31-34. doi: 10.1053/j.gastro.2022.04.024.
Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia
Wallace MB et al. Gastroenterology. 2022 Jul;163(1):295-304.e5. doi: 10.1053/j.gastro.2022.03.007.
The Association Between Proton Pump Inhibitor Exposure and Key Liver-Related Outcomes in Patients With Cirrhosis: A Veterans Affairs Cohort Study
Mahmud N et al. Gastroenterology. 2022 Jul;163(1):257-269.e6. doi: 10.1053/j.gastro.2022.03.052.
Clinical Gastroenterology and Hepatology
May 2022
Practice Patterns and Predictors of Stopping Colonoscopy in Older Adults With Colorectal Polyps
Rege S et al. Clin Gastroenterol Hepatol. 2022 May;20(5):e1050-e1060. doi: 10.1016/j.cgh.2021.06.041.
Duodenal Mucosal Barrier in Functional Dyspepsia
Narayanan SP et al. Clin Gastroenterol Hepatol. 2022 May;20(5):1019-1028.e3. doi: 10.1016/j.cgh.2021.09.029.
June 2022
Ultra-processed Foods and Risk of Crohn’s Disease and Ulcerative Colitis: A Prospective Cohort Study
Lo CH et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1323-e1337. doi: 10.1016/j.cgh.2021.08.031.
Changes in Lifestyle Factors After Endoscopic Screening: A Prospective Study in the United States
Knudsen MD et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1240-e1249. doi: 10.1016/j.cgh.2021.07.014.
Trends in Early-onset vs Late-onset Colorectal Cancer Incidence by Race/Ethnicity in the United States Cancer Statistics Database
Chang SH et al. Clin Gastroenterol Hepatol. 2022 Jun;20(6):e1365-e1377. doi: 10.1016/j.cgh.2021.07.035.
July 2022
Updates in Telemedicine for Gastroenterology Practices in the United States
Serper M, Volk ML. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1432-1435. doi: 10.1016/j.cgh.2022.03.024.
Prevalence and Financial Burden of Digestive Diseases in a Commercially Insured Population
Mathews SC et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1480-1487.e7. doi: 10.1016/j.cgh.2021.06.047.
Prevalence of Forceps Polypectomy of Nondiminutive Polyps Is Substantial But Modifiable
Fudman DI et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1508-1515. doi: 10.1016/j.cgh.2021.11.031.
Long-Term Treatment of Eosinophilic Esophagitis With Budesonide Oral Suspension
Dellon ES et al. Clin Gastroenterol Hepatol. 2022 Jul;20(7):1488-1498.e11. doi: 10.1016/j.cgh.2021.06.020.
Techniques and Innovations in Gastrointestinal Endoscopy
Intervention Versus Observation in Patients Presenting With Lower Gastrointestinal Bleeding
Lipcsey MS et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(2):145-152. doi: 10.1016/j.tige.2021.12.001.
Physician Reimbursement for Endoscopic Submucosal Dissection: A Single Center Analysis
Gajula P et al. Tech Innov Gastrointest Endosc. 2022 Jan 01;24(2):153-158. doi: 10.1016/j.tige.2021.12.003.
Antidepressants may curb opioid overdose
Investigators analyzed insurance claims for more than 200,000 adults with a history of depression. Of these, 8,200 experienced adverse events (AEs) during the year after initiation of opioid therapy.
However, the risk for an AE such as overdose and other forms of self-harm was reduced among patients who had been treated with antidepressants for at least 6 weeks.
The take-home message is that clinicians and health systems need to be more aware that individuals in pain are more likely to be depressed and at higher risk for AEs – so the depression should be treated “more liberally,” corresponding author Bradley Stein, MD, PhD, a practicing psychiatrist in Pittsburgh and director of the Rand Corporation Opioid Policy Center, told this news organization.
“If you are treating someone with pain, particularly chronic pain, it’s critically important to better assess their depression and not to attribute depressive symptoms only to pain,” Dr. Stein said.
The findings were published online in Psychiatric Services.
Promising approach?
Opioid treatment for pain “complicates the interactions among pain, depression, and self-harm,” the investigators write. Individuals with depression receiving long-term opioid therapy are two to three times more likely to misuse opioids, compared with individuals who do not have depression.
Although comorbid depression “substantially increases overdose and suicide risk, it remains underdiagnosed and undertreated among individuals with chronic pain,” the researchers note. They add that increasing access to depression treatment may be a “potentially promising approach to preventing overdoses and suicide” in these patients.
“We know that individuals using opioids who have a history of depression are more likely to have negative outcomes, such as overdoses and self-harm events,” Dr. Stein said. “We wanted to see whether antidepressants, which would treat depression in these individuals, would help with that.”
The researchers assessed a database of commercial insurance claims of adults with a history of depression who received opioids between 2007 and 2017 (n = 283,374). The data included 336,599 opioid treatment episodes.
To be included in the study, patients had to have been diagnosed with depression before they filled their first opioid prescription.
The “outcome of interest” was time from the beginning of an opioid episode until an adverse event, such as opioid poisoning, overdose of nonopioid controlled or illicit substances, or self-harm unrelated to overdose.
Participants were followed from the onset of the opioid episode until an AE occurred, loss to follow-up, or week 52, whichever came first.
The “key independent variable” was filling an antidepressant prescription. The patient’s sex and age were considered to be independent variables as well.
Teasing out antidepressant effect
Of participants with a history of depression treatment, 8,203 experienced at least one AE during the 12 months after treatment initiation (n = 47,486 AEs). Approximately half (50.8%) filled an antidepressant prescription at least once during the 12 months after the opioid episode began.
AEs were more likely among men than among women. The highest risk was in patients aged 18-24 years.
After adjusting for age and sex, participants who had received antidepressants had a greater risk for all adverse outcomes during the first 6 weeks of antidepressant treatment. However, those who had received antidepressants for 6 weeks or longer were at reduced risk for all adverse outcomes.

“We took advantage of the fact that, for most people, antidepressants take a while to work and aren’t immediately effective, so we were able to use that difference in our research,” Dr. Stein said.
“We wouldn’t expect to see an immediate effect of antidepressants, so the difference between what we saw immediately after the person had started treatment and the time it took for the antidepressant to be effective enabled us to tease out the effect of the antidepressant,” he added.
Consider CBT?
Andrew Saxon, MD, professor, department of psychiatry and behavioral sciences, University of Washington School of Medicine, Seattle, said clinicians “tend to think categorically and give people diagnoses that are clear-cut.” But neurobiologically, “it may be hard to distinguish where chronic pain ends and depression begins, or whether there’s some commonality.”
For patients with chronic pain and those taking opioids, “we need to be very attuned to the possibility or likelihood that they have major depression and other psychiatric diagnoses, like PTSD and anxiety disorders, which are very common,” said Dr. Saxon, who is also the director of the Center of Excellence in Substance Abuse Treatment and Education at the VA Puget Sound Health Care System. He was not involved with the current research.
He noted that treating those disorders “is a very important component of managing chronic pain.” However, “patients just starting antidepressants need to be carefully monitored when they’re getting stabilized on their antidepressants because they can have side effects, particularly early on, that can destabilize them.”
Dr. Saxon added that beyond pharmacotherapy, cognitive-behavioral therapy (CBT) for pain might be an even better intervention for addressing both pain and depression.
Also commenting for this article, Brian Hurley, MD, an addiction medicine specialist and the medical director of the Division of Substance Abuse Prevention and Control for the Los Angeles County Department of Public Health, said: “In the context of the largest wave of overdose mortality in U.S. history, we know comparatively little about the impact of mental health interventions that mitigate overdose risks.”
This study “contributes important new information that treating depression with antidepressant medications reduces overdose and self-harm risks for people who are prescribed opioids,” said Dr. Hurley, who is also the president-elect of the American Society of Addiction Medicine.
It also “underscores the general importance of integrated mental health and substance use disorder treatment in both primary care and in mental health settings,” added Dr. Hurley, who was not involved with the study.
The study was funded by the National Institute on Drug Abuse. The investigators and commenters reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed insurance claims for more than 200,000 adults with a history of depression. Of these, 8,200 experienced adverse events (AEs) during the year after initiation of opioid therapy.
However, the risk for an AE such as overdose and other forms of self-harm was reduced among patients who had been treated with antidepressants for at least 6 weeks.
The take-home message is that clinicians and health systems need to be more aware that individuals in pain are more likely to be depressed and at higher risk for AEs – so the depression should be treated “more liberally,” corresponding author Bradley Stein, MD, PhD, a practicing psychiatrist in Pittsburgh and director of the Rand Corporation Opioid Policy Center, told this news organization.
“If you are treating someone with pain, particularly chronic pain, it’s critically important to better assess their depression and not to attribute depressive symptoms only to pain,” Dr. Stein said.
The findings were published online in Psychiatric Services.
Promising approach?
Opioid treatment for pain “complicates the interactions among pain, depression, and self-harm,” the investigators write. Individuals with depression receiving long-term opioid therapy are two to three times more likely to misuse opioids, compared with individuals who do not have depression.
Although comorbid depression “substantially increases overdose and suicide risk, it remains underdiagnosed and undertreated among individuals with chronic pain,” the researchers note. They add that increasing access to depression treatment may be a “potentially promising approach to preventing overdoses and suicide” in these patients.
“We know that individuals using opioids who have a history of depression are more likely to have negative outcomes, such as overdoses and self-harm events,” Dr. Stein said. “We wanted to see whether antidepressants, which would treat depression in these individuals, would help with that.”
The researchers assessed a database of commercial insurance claims of adults with a history of depression who received opioids between 2007 and 2017 (n = 283,374). The data included 336,599 opioid treatment episodes.
To be included in the study, patients had to have been diagnosed with depression before they filled their first opioid prescription.
The “outcome of interest” was time from the beginning of an opioid episode until an adverse event, such as opioid poisoning, overdose of nonopioid controlled or illicit substances, or self-harm unrelated to overdose.
Participants were followed from the onset of the opioid episode until an AE occurred, loss to follow-up, or week 52, whichever came first.
The “key independent variable” was filling an antidepressant prescription. The patient’s sex and age were considered to be independent variables as well.
Teasing out antidepressant effect
Of participants with a history of depression treatment, 8,203 experienced at least one AE during the 12 months after treatment initiation (n = 47,486 AEs). Approximately half (50.8%) filled an antidepressant prescription at least once during the 12 months after the opioid episode began.
AEs were more likely among men than among women. The highest risk was in patients aged 18-24 years.
After adjusting for age and sex, participants who had received antidepressants had a greater risk for all adverse outcomes during the first 6 weeks of antidepressant treatment. However, those who had received antidepressants for 6 weeks or longer were at reduced risk for all adverse outcomes.

“We took advantage of the fact that, for most people, antidepressants take a while to work and aren’t immediately effective, so we were able to use that difference in our research,” Dr. Stein said.
“We wouldn’t expect to see an immediate effect of antidepressants, so the difference between what we saw immediately after the person had started treatment and the time it took for the antidepressant to be effective enabled us to tease out the effect of the antidepressant,” he added.
Consider CBT?
Andrew Saxon, MD, professor, department of psychiatry and behavioral sciences, University of Washington School of Medicine, Seattle, said clinicians “tend to think categorically and give people diagnoses that are clear-cut.” But neurobiologically, “it may be hard to distinguish where chronic pain ends and depression begins, or whether there’s some commonality.”
For patients with chronic pain and those taking opioids, “we need to be very attuned to the possibility or likelihood that they have major depression and other psychiatric diagnoses, like PTSD and anxiety disorders, which are very common,” said Dr. Saxon, who is also the director of the Center of Excellence in Substance Abuse Treatment and Education at the VA Puget Sound Health Care System. He was not involved with the current research.
He noted that treating those disorders “is a very important component of managing chronic pain.” However, “patients just starting antidepressants need to be carefully monitored when they’re getting stabilized on their antidepressants because they can have side effects, particularly early on, that can destabilize them.”
Dr. Saxon added that beyond pharmacotherapy, cognitive-behavioral therapy (CBT) for pain might be an even better intervention for addressing both pain and depression.
Also commenting for this article, Brian Hurley, MD, an addiction medicine specialist and the medical director of the Division of Substance Abuse Prevention and Control for the Los Angeles County Department of Public Health, said: “In the context of the largest wave of overdose mortality in U.S. history, we know comparatively little about the impact of mental health interventions that mitigate overdose risks.”
This study “contributes important new information that treating depression with antidepressant medications reduces overdose and self-harm risks for people who are prescribed opioids,” said Dr. Hurley, who is also the president-elect of the American Society of Addiction Medicine.
It also “underscores the general importance of integrated mental health and substance use disorder treatment in both primary care and in mental health settings,” added Dr. Hurley, who was not involved with the study.
The study was funded by the National Institute on Drug Abuse. The investigators and commenters reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Investigators analyzed insurance claims for more than 200,000 adults with a history of depression. Of these, 8,200 experienced adverse events (AEs) during the year after initiation of opioid therapy.
However, the risk for an AE such as overdose and other forms of self-harm was reduced among patients who had been treated with antidepressants for at least 6 weeks.
The take-home message is that clinicians and health systems need to be more aware that individuals in pain are more likely to be depressed and at higher risk for AEs – so the depression should be treated “more liberally,” corresponding author Bradley Stein, MD, PhD, a practicing psychiatrist in Pittsburgh and director of the Rand Corporation Opioid Policy Center, told this news organization.
“If you are treating someone with pain, particularly chronic pain, it’s critically important to better assess their depression and not to attribute depressive symptoms only to pain,” Dr. Stein said.
The findings were published online in Psychiatric Services.
Promising approach?
Opioid treatment for pain “complicates the interactions among pain, depression, and self-harm,” the investigators write. Individuals with depression receiving long-term opioid therapy are two to three times more likely to misuse opioids, compared with individuals who do not have depression.
Although comorbid depression “substantially increases overdose and suicide risk, it remains underdiagnosed and undertreated among individuals with chronic pain,” the researchers note. They add that increasing access to depression treatment may be a “potentially promising approach to preventing overdoses and suicide” in these patients.
“We know that individuals using opioids who have a history of depression are more likely to have negative outcomes, such as overdoses and self-harm events,” Dr. Stein said. “We wanted to see whether antidepressants, which would treat depression in these individuals, would help with that.”
The researchers assessed a database of commercial insurance claims of adults with a history of depression who received opioids between 2007 and 2017 (n = 283,374). The data included 336,599 opioid treatment episodes.
To be included in the study, patients had to have been diagnosed with depression before they filled their first opioid prescription.
The “outcome of interest” was time from the beginning of an opioid episode until an adverse event, such as opioid poisoning, overdose of nonopioid controlled or illicit substances, or self-harm unrelated to overdose.
Participants were followed from the onset of the opioid episode until an AE occurred, loss to follow-up, or week 52, whichever came first.
The “key independent variable” was filling an antidepressant prescription. The patient’s sex and age were considered to be independent variables as well.
Teasing out antidepressant effect
Of participants with a history of depression treatment, 8,203 experienced at least one AE during the 12 months after treatment initiation (n = 47,486 AEs). Approximately half (50.8%) filled an antidepressant prescription at least once during the 12 months after the opioid episode began.
AEs were more likely among men than among women. The highest risk was in patients aged 18-24 years.
After adjusting for age and sex, participants who had received antidepressants had a greater risk for all adverse outcomes during the first 6 weeks of antidepressant treatment. However, those who had received antidepressants for 6 weeks or longer were at reduced risk for all adverse outcomes.

“We took advantage of the fact that, for most people, antidepressants take a while to work and aren’t immediately effective, so we were able to use that difference in our research,” Dr. Stein said.
“We wouldn’t expect to see an immediate effect of antidepressants, so the difference between what we saw immediately after the person had started treatment and the time it took for the antidepressant to be effective enabled us to tease out the effect of the antidepressant,” he added.
Consider CBT?
Andrew Saxon, MD, professor, department of psychiatry and behavioral sciences, University of Washington School of Medicine, Seattle, said clinicians “tend to think categorically and give people diagnoses that are clear-cut.” But neurobiologically, “it may be hard to distinguish where chronic pain ends and depression begins, or whether there’s some commonality.”
For patients with chronic pain and those taking opioids, “we need to be very attuned to the possibility or likelihood that they have major depression and other psychiatric diagnoses, like PTSD and anxiety disorders, which are very common,” said Dr. Saxon, who is also the director of the Center of Excellence in Substance Abuse Treatment and Education at the VA Puget Sound Health Care System. He was not involved with the current research.
He noted that treating those disorders “is a very important component of managing chronic pain.” However, “patients just starting antidepressants need to be carefully monitored when they’re getting stabilized on their antidepressants because they can have side effects, particularly early on, that can destabilize them.”
Dr. Saxon added that beyond pharmacotherapy, cognitive-behavioral therapy (CBT) for pain might be an even better intervention for addressing both pain and depression.
Also commenting for this article, Brian Hurley, MD, an addiction medicine specialist and the medical director of the Division of Substance Abuse Prevention and Control for the Los Angeles County Department of Public Health, said: “In the context of the largest wave of overdose mortality in U.S. history, we know comparatively little about the impact of mental health interventions that mitigate overdose risks.”
This study “contributes important new information that treating depression with antidepressant medications reduces overdose and self-harm risks for people who are prescribed opioids,” said Dr. Hurley, who is also the president-elect of the American Society of Addiction Medicine.
It also “underscores the general importance of integrated mental health and substance use disorder treatment in both primary care and in mental health settings,” added Dr. Hurley, who was not involved with the study.
The study was funded by the National Institute on Drug Abuse. The investigators and commenters reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM PSYCHIATRIC SERVICES