User login
Youth with bipolar disorder at high risk of eating disorders
Investigators studied close to 200 youth with BD and found that more than 25% had a lifetime ED, which included anorexia nervosa (AN), bulimia nervosa (BN), and an ED not otherwise specified (NOS).
Those with comorbid EDs were more likely to be female and to have BD-II subtype. Their presentations were also more complicated and included a history of suicidality, additional psychiatric conditions, smoking, and a history of sexual abuse, as well as more severe depression and emotional instability.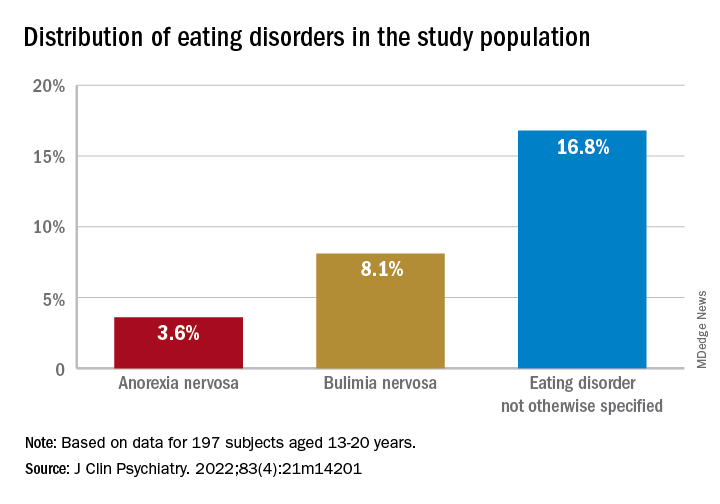
“We think the take-home message is that, in addition to other more recognized psychiatric comorbidities, youth with BD are also vulnerable to developing EDs. Thus, clinicians should be routinely monitoring for eating, appetite, and body image disturbances when working with this population,” lead author Diana Khoubaeva, research analyst at the Centre for Youth Bipolar Disorder, Centre for Addiction and Mental Health, Toronto, and senior author Benjamin Goldstein, MD, PhD, director of the Centre for Youth Bipolar Disorder, wrote in an e-mail to this news organization.
“Given the more complicated clinical picture of youth with co-occurring BD and EDs, this combination warrants careful attention,” the investigators note.
The study was published online May 11 in the Journal of Clinical Psychiatry.
Lack of research
“From the existing literature, we learned that EDs are not uncommon in individuals with BD, and that they are often associated with a more severe clinical profile,” say the researchers. “However, the majority of these studies have been limited to adult samples, and there was a real scarcity of studies that examined this co-occurrence in youth.”
This is “surprising” because EDs often have their onset in adolescence, so the researchers decided to explore the issue in their “fairly large sample of youth with BD.”
To investigate the issue, the researchers studied 197 youth (aged 13-20 years) with a diagnosis of BD (BD-I, BD-II, or BD-NOS) who were recruited between 2009 and 2017 (mean [standard deviation] age, 16.69 [1.50] years; 67.5% female).
ED diagnoses included both current and lifetime AN, BN, and ED-NOS. The researchers used the Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime Version (K-SADS-PL) to determine the diagnosis of BD.
They also collected information about comorbid psychiatric disorders, as well as substance use disorders and cigarette smoking. The Life Problems Inventory (LPI) was used to identify dimensional borderline personality traits.
Information about physical and sexual abuse, suicidal ideation, nonsuicidal self-injury (NSSI), and affect regulation were obtained from other measurement tools. Participants’ height and weight were measured to calculate body mass index.
Neurobiological and environmental factors
Of the total sample, 24.84% had received a diagnosis of ED in their lifetime.
Moreover, 28.9% had a lifetime history of binge eating. Of these, 17.7% also had been diagnosed with an ED.
Participants with BD-II were significantly more likely than those with BD-I to report both current and lifetime BN. There were no significant differences by BD subtype in AN, ED-NOS, or binge eating.
Higher correlates of clinical characteristics, psychiatric morbidity, treatment history, and dimensional traits in those with vs. those without an ED are detailed in the accompanying table.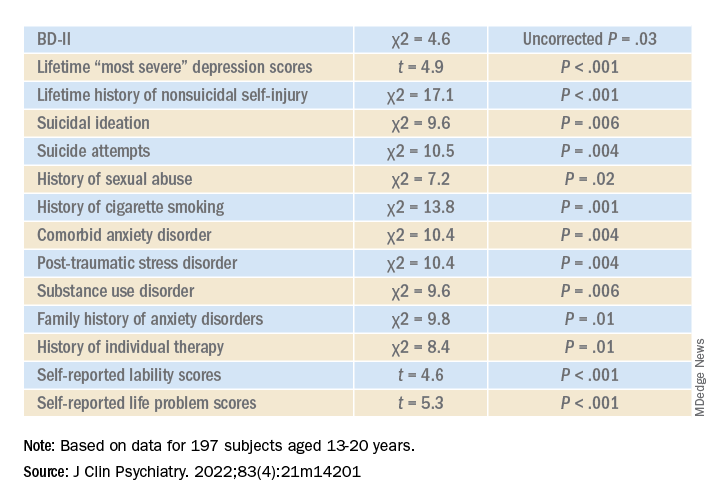
The ED group scored significantly higher on all LPI scores, including impulsivity, emotional dysregulation, identity confusion, and interpersonal problems, compared to those without an ED. They also were less likely to report lifetime lithium use (chi2 = 7.9, P = .01).
Multivariate analysis revealed that lifetime EDs were significantly associated with female sex, history of cigarette smoking, history of individual therapy, family history of anxiety, and LPI total score and were negatively associated with BD-I subtype.
“The comorbidity [between EDs and BD] could be driven by both neurobiological and environmental factors,” Dr. Khoubaeva and Dr. Goldstein noted. EDs and BD “are both illnesses that are fundamentally linked with dysfunction in reward systems – that is, there are imbalances in terms of too much or too little reward seeking.”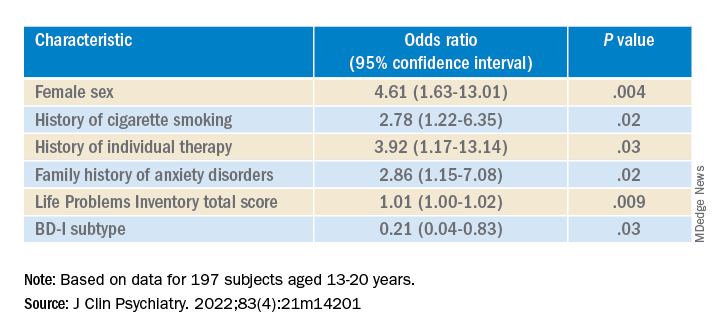
They added that individuals affected by these conditions have “ongoing challenges with instability of emotions and ability to manage emotions; and eating too much or too little can be a manifestation of coping with emotions.”
In addition, medications commonly used to treat BD “are known to have side effects such as weight/appetite/metabolic changes, which may make it harder to regulate eating, and which may exacerbate preexisting body image challenges.”
The researchers recommend implementing trauma-informed care, assessing and addressing suicidality and self-injury, and prioritizing therapies that target emotional dysregulation, such as dialectical behavioral therapy.
‘Clarion call’
Commenting on the study, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, said the study is “the first of its kind to comprehensively characterize the prevalence of ED in youth living with BD.
“It could be hypothesized that EDs have overlapping domain disturbances of cognitive dysfunction, such as executive function and impulse control, as well as cognitive reward processes,” said Dr. McIntyre, who is the chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, and was not involved with the study.
“The data are a clarion call for clinicians to routinely screen for EDs in youth with BD and, when present, to be aware of the greater complexity, severity, and risk in this patient subpopulation. The higher prevalence of ED in youth with BD-II is an additional reminder of the severity, morbidity, and complexity of BD-II,” Dr. McIntyre said.
The study received no direct funding. It was supported by philanthropic donations to the Centre for Youth Bipolar Disorder and the CAMH Discovery Fund. Dr. Goldstein reports grant support from Brain Canada, Canadian Institutes of Health Research, Heart and Stroke Foundation, National Institute of Mental Health, and the departments of psychiatry at the University of Toronto and Sunnybrook Health Sciences Centre. He also acknowledges his position as RBC investments chair in Children›s Mental Health and Developmental Psychopathology at CAMH, a joint Hospital-University chair among the University of Toronto, CAMH, and the CAMH Foundation. Ms. Khoubaeva reports no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC); speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
Investigators studied close to 200 youth with BD and found that more than 25% had a lifetime ED, which included anorexia nervosa (AN), bulimia nervosa (BN), and an ED not otherwise specified (NOS).
Those with comorbid EDs were more likely to be female and to have BD-II subtype. Their presentations were also more complicated and included a history of suicidality, additional psychiatric conditions, smoking, and a history of sexual abuse, as well as more severe depression and emotional instability.
“We think the take-home message is that, in addition to other more recognized psychiatric comorbidities, youth with BD are also vulnerable to developing EDs. Thus, clinicians should be routinely monitoring for eating, appetite, and body image disturbances when working with this population,” lead author Diana Khoubaeva, research analyst at the Centre for Youth Bipolar Disorder, Centre for Addiction and Mental Health, Toronto, and senior author Benjamin Goldstein, MD, PhD, director of the Centre for Youth Bipolar Disorder, wrote in an e-mail to this news organization.
“Given the more complicated clinical picture of youth with co-occurring BD and EDs, this combination warrants careful attention,” the investigators note.
The study was published online May 11 in the Journal of Clinical Psychiatry.
Lack of research
“From the existing literature, we learned that EDs are not uncommon in individuals with BD, and that they are often associated with a more severe clinical profile,” say the researchers. “However, the majority of these studies have been limited to adult samples, and there was a real scarcity of studies that examined this co-occurrence in youth.”
This is “surprising” because EDs often have their onset in adolescence, so the researchers decided to explore the issue in their “fairly large sample of youth with BD.”
To investigate the issue, the researchers studied 197 youth (aged 13-20 years) with a diagnosis of BD (BD-I, BD-II, or BD-NOS) who were recruited between 2009 and 2017 (mean [standard deviation] age, 16.69 [1.50] years; 67.5% female).
ED diagnoses included both current and lifetime AN, BN, and ED-NOS. The researchers used the Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime Version (K-SADS-PL) to determine the diagnosis of BD.
They also collected information about comorbid psychiatric disorders, as well as substance use disorders and cigarette smoking. The Life Problems Inventory (LPI) was used to identify dimensional borderline personality traits.
Information about physical and sexual abuse, suicidal ideation, nonsuicidal self-injury (NSSI), and affect regulation were obtained from other measurement tools. Participants’ height and weight were measured to calculate body mass index.
Neurobiological and environmental factors
Of the total sample, 24.84% had received a diagnosis of ED in their lifetime.
Moreover, 28.9% had a lifetime history of binge eating. Of these, 17.7% also had been diagnosed with an ED.
Participants with BD-II were significantly more likely than those with BD-I to report both current and lifetime BN. There were no significant differences by BD subtype in AN, ED-NOS, or binge eating.
Higher correlates of clinical characteristics, psychiatric morbidity, treatment history, and dimensional traits in those with vs. those without an ED are detailed in the accompanying table.
The ED group scored significantly higher on all LPI scores, including impulsivity, emotional dysregulation, identity confusion, and interpersonal problems, compared to those without an ED. They also were less likely to report lifetime lithium use (chi2 = 7.9, P = .01).
Multivariate analysis revealed that lifetime EDs were significantly associated with female sex, history of cigarette smoking, history of individual therapy, family history of anxiety, and LPI total score and were negatively associated with BD-I subtype.
“The comorbidity [between EDs and BD] could be driven by both neurobiological and environmental factors,” Dr. Khoubaeva and Dr. Goldstein noted. EDs and BD “are both illnesses that are fundamentally linked with dysfunction in reward systems – that is, there are imbalances in terms of too much or too little reward seeking.”
They added that individuals affected by these conditions have “ongoing challenges with instability of emotions and ability to manage emotions; and eating too much or too little can be a manifestation of coping with emotions.”
In addition, medications commonly used to treat BD “are known to have side effects such as weight/appetite/metabolic changes, which may make it harder to regulate eating, and which may exacerbate preexisting body image challenges.”
The researchers recommend implementing trauma-informed care, assessing and addressing suicidality and self-injury, and prioritizing therapies that target emotional dysregulation, such as dialectical behavioral therapy.
‘Clarion call’
Commenting on the study, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, said the study is “the first of its kind to comprehensively characterize the prevalence of ED in youth living with BD.
“It could be hypothesized that EDs have overlapping domain disturbances of cognitive dysfunction, such as executive function and impulse control, as well as cognitive reward processes,” said Dr. McIntyre, who is the chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, and was not involved with the study.
“The data are a clarion call for clinicians to routinely screen for EDs in youth with BD and, when present, to be aware of the greater complexity, severity, and risk in this patient subpopulation. The higher prevalence of ED in youth with BD-II is an additional reminder of the severity, morbidity, and complexity of BD-II,” Dr. McIntyre said.
The study received no direct funding. It was supported by philanthropic donations to the Centre for Youth Bipolar Disorder and the CAMH Discovery Fund. Dr. Goldstein reports grant support from Brain Canada, Canadian Institutes of Health Research, Heart and Stroke Foundation, National Institute of Mental Health, and the departments of psychiatry at the University of Toronto and Sunnybrook Health Sciences Centre. He also acknowledges his position as RBC investments chair in Children›s Mental Health and Developmental Psychopathology at CAMH, a joint Hospital-University chair among the University of Toronto, CAMH, and the CAMH Foundation. Ms. Khoubaeva reports no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC); speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
Investigators studied close to 200 youth with BD and found that more than 25% had a lifetime ED, which included anorexia nervosa (AN), bulimia nervosa (BN), and an ED not otherwise specified (NOS).
Those with comorbid EDs were more likely to be female and to have BD-II subtype. Their presentations were also more complicated and included a history of suicidality, additional psychiatric conditions, smoking, and a history of sexual abuse, as well as more severe depression and emotional instability.
“We think the take-home message is that, in addition to other more recognized psychiatric comorbidities, youth with BD are also vulnerable to developing EDs. Thus, clinicians should be routinely monitoring for eating, appetite, and body image disturbances when working with this population,” lead author Diana Khoubaeva, research analyst at the Centre for Youth Bipolar Disorder, Centre for Addiction and Mental Health, Toronto, and senior author Benjamin Goldstein, MD, PhD, director of the Centre for Youth Bipolar Disorder, wrote in an e-mail to this news organization.
“Given the more complicated clinical picture of youth with co-occurring BD and EDs, this combination warrants careful attention,” the investigators note.
The study was published online May 11 in the Journal of Clinical Psychiatry.
Lack of research
“From the existing literature, we learned that EDs are not uncommon in individuals with BD, and that they are often associated with a more severe clinical profile,” say the researchers. “However, the majority of these studies have been limited to adult samples, and there was a real scarcity of studies that examined this co-occurrence in youth.”
This is “surprising” because EDs often have their onset in adolescence, so the researchers decided to explore the issue in their “fairly large sample of youth with BD.”
To investigate the issue, the researchers studied 197 youth (aged 13-20 years) with a diagnosis of BD (BD-I, BD-II, or BD-NOS) who were recruited between 2009 and 2017 (mean [standard deviation] age, 16.69 [1.50] years; 67.5% female).
ED diagnoses included both current and lifetime AN, BN, and ED-NOS. The researchers used the Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime Version (K-SADS-PL) to determine the diagnosis of BD.
They also collected information about comorbid psychiatric disorders, as well as substance use disorders and cigarette smoking. The Life Problems Inventory (LPI) was used to identify dimensional borderline personality traits.
Information about physical and sexual abuse, suicidal ideation, nonsuicidal self-injury (NSSI), and affect regulation were obtained from other measurement tools. Participants’ height and weight were measured to calculate body mass index.
Neurobiological and environmental factors
Of the total sample, 24.84% had received a diagnosis of ED in their lifetime.
Moreover, 28.9% had a lifetime history of binge eating. Of these, 17.7% also had been diagnosed with an ED.
Participants with BD-II were significantly more likely than those with BD-I to report both current and lifetime BN. There were no significant differences by BD subtype in AN, ED-NOS, or binge eating.
Higher correlates of clinical characteristics, psychiatric morbidity, treatment history, and dimensional traits in those with vs. those without an ED are detailed in the accompanying table.
The ED group scored significantly higher on all LPI scores, including impulsivity, emotional dysregulation, identity confusion, and interpersonal problems, compared to those without an ED. They also were less likely to report lifetime lithium use (chi2 = 7.9, P = .01).
Multivariate analysis revealed that lifetime EDs were significantly associated with female sex, history of cigarette smoking, history of individual therapy, family history of anxiety, and LPI total score and were negatively associated with BD-I subtype.
“The comorbidity [between EDs and BD] could be driven by both neurobiological and environmental factors,” Dr. Khoubaeva and Dr. Goldstein noted. EDs and BD “are both illnesses that are fundamentally linked with dysfunction in reward systems – that is, there are imbalances in terms of too much or too little reward seeking.”
They added that individuals affected by these conditions have “ongoing challenges with instability of emotions and ability to manage emotions; and eating too much or too little can be a manifestation of coping with emotions.”
In addition, medications commonly used to treat BD “are known to have side effects such as weight/appetite/metabolic changes, which may make it harder to regulate eating, and which may exacerbate preexisting body image challenges.”
The researchers recommend implementing trauma-informed care, assessing and addressing suicidality and self-injury, and prioritizing therapies that target emotional dysregulation, such as dialectical behavioral therapy.
‘Clarion call’
Commenting on the study, Roger McIntyre, MD, professor of psychiatry and pharmacology, University of Toronto, and head of the Mood Disorders Psychopharmacology Unit, said the study is “the first of its kind to comprehensively characterize the prevalence of ED in youth living with BD.
“It could be hypothesized that EDs have overlapping domain disturbances of cognitive dysfunction, such as executive function and impulse control, as well as cognitive reward processes,” said Dr. McIntyre, who is the chairman and executive director of the Brain and Cognitive Discover Foundation, Toronto, and was not involved with the study.
“The data are a clarion call for clinicians to routinely screen for EDs in youth with BD and, when present, to be aware of the greater complexity, severity, and risk in this patient subpopulation. The higher prevalence of ED in youth with BD-II is an additional reminder of the severity, morbidity, and complexity of BD-II,” Dr. McIntyre said.
The study received no direct funding. It was supported by philanthropic donations to the Centre for Youth Bipolar Disorder and the CAMH Discovery Fund. Dr. Goldstein reports grant support from Brain Canada, Canadian Institutes of Health Research, Heart and Stroke Foundation, National Institute of Mental Health, and the departments of psychiatry at the University of Toronto and Sunnybrook Health Sciences Centre. He also acknowledges his position as RBC investments chair in Children›s Mental Health and Developmental Psychopathology at CAMH, a joint Hospital-University chair among the University of Toronto, CAMH, and the CAMH Foundation. Ms. Khoubaeva reports no relevant financial relationships. The other authors’ disclosures are listed in the original article. Dr. McIntyre has received research grant support from CIHR/GACD/National Natural Science Foundation of China (NSFC); speaker/consultation fees from Lundbeck, Janssen, Alkermes, Neumora Therapeutics, Mitsubishi Tanabe, Purdue, Pfizer, Otsuka, Takeda, Neurocrine, Sunovion, Bausch Health, Axsome, Novo Nordisk, Kris, Sanofi, Eisai, Intra-Cellular, NewBridge Pharmaceuticals, Abbvie, and Atai Life Sciences. Dr. McIntyre is a CEO of Braxia Scientific.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Genetic testing for best antidepressant accurate, cost effective
new research suggests.
CYP2D6 and CYP2C19, from the cytochrome P450 family, are involved in the metabolism and elimination of various molecules, including medications. Variants in the genes encoding these enzymes affect the speed at which drugs are metabolized, altering their pharmacokinetic profiles.
The researchers studied 125 patients with MDD and used CYP2D6 and CYP2C19 genotyping to determine the presence of actionable phenotypes in line with Food and Drug Administration labeling.
They found that, in many cases, pharmacogenetic testing could have predicted poor response to the initial treatment selection and could have helped guide subsequent choices to improve outcomes.
In addition, a pharmacoeconomic evaluation that combined direct and indirect costs resulting from MDD with the prevalence of CYP2D6 and CYP2C19 phenotypes showed that testing for functional variants in both genes would be cost effective at a national level.
Had psychiatrists who treated patients in the study known about their metabolizing profiles, it “might have contributed to switches in medication” and could have reduced “delays in response,” said lead researcher Alessio Squassina, PhD, associate professor of pharmacology at the University of Cagliari (Italy).
The findings were presented at the European Psychiatric Association 2022 Congress.
Highly variable response rates
Dr. Squassina noted that the response to antidepressants is a “highly variable trait,” and while it is known that genetics play a role, their contribution is “still not completely understood.”
He explained that the use of pharmacogenetics, which leverages genetic information to guide treatment decision-making, has increased significantly.
While regulatory bodies, including the FDA, have been “very active” in defining strict criteria for interpreting the information from pharmacogenetic tests, there remains some “discrepancy” in their clinical utility.
Dr. Squassina said the FDA provides guidance on use of genetic testing on the labels of 34 psychiatric medications. Of these, 79% relate to CYP2D6, 12% relate to CYP2C19, and 9% relate to other genes.
These labels provide guidance on when genetic testing is recommended or required, as well as potentially clinically actionable gene-drug associations in patients with certain functional alleles.
However, Dr. Squassina noted that the distribution of such alleles is not the same across Europe, so it’s possible that a psychiatrist in Italy may be less likely to treat a patient with a phenotype affecting response to treatment or risk of adverse events than one in Norway or Sweden.
For the study, the investigators examined the frequency of CYP2D6 and CYP2C19 phenotypes in psychiatric patients in Sardinia and their relationship with pharmacologic treatment and cost-effectiveness.
They set out to recruit 200 patients with MDD who had a documented 5-year medical and treatment history, including alterations in treatment, adverse events, hospitalizations, suicide, and symptom scores, as well as sociodemographic variables.
An interim analysis of the first 125 patients recruited to the study showed that the most common CYP2D6 phenotype was normal metabolizers (NM), at 60.5%, followed by intermediate metabolizers (IM), at 28.2%, ultrarapid metabolizers (UR), at 8.9%, and poor metabolizers (PM), at 2.4%.
For CYP2C19, the most common phenotype was NM (49%), followed by IM (29.0%), UR (25.0%), and PM (4.0%). While there were differences in the overall European averages, they were not significant.
To highlight the potential impact that pharmacogenetic testing could have had on patient care and outcome, Dr. Squassina highlighted two cases.
The first concerned a patient with a CYP2D6 IM and CYP2C19 UR phenotype, who did not respond to escitalopram. The FDA drug label indicates this phenotype is actionable and recommends an alternative drug.
The patient was subsequently switched to venlafaxine. The FDA drug label on venlafaxine notes that patients with this phenotype are likely to have a suboptimal response to this drug, and again, this patient did not respond to treatment.
Another patient with a CYP2D6 NM and CYP2C19 IM phenotype was also prescribed escitalopram. The FDA label on this drug notes that patients with this phenotype can try venlafaxine but may not respond. Indeed, this patient did not respond and was switched to venlafaxine and started responding.
“The psychiatrists [in these cases] may made have made different [drug] choices if they had known the genotypes in advance,” Dr. Squassina said.
Cost effective?
To determine the cost-effectiveness of screening for CYP2D6 and CYP2C19 phenotypes in patients with MDD, the researchers used real-world data to develop a Markov model with a hypothetical cohort of 2000 MDD patients, half of whom underwent pharmacogenetic testing, to determine the potential impact on outcomes over an 18-week period.
The model included the cost of medications and hospitalization, psychiatric counseling, loss of productivity, and the estimated probability of response and adverse events, adjusted for the patient’s likelihood of having a particular metabolizing phenotype.
Results showed that, for CYP2C19, compared to no testing, pharmacogenetic testing would be cost-effective at an incremental cost-effective ratio (ICER) of €60,000 ($64,000 USD) per quality-adjusted life-year (QALY).
This, Squassina said, is “below the willingness to pay threshold” for health authorities in developed countries.
For CYP2D6, pharmacogenetic testing would become cost-effective at an ICER of approximately €47,000 ($40,000 USD) per QALY.
The team plans to complete recruitment and perform a “detailed evaluation of all the variables, especially those relating to the medication history and changes in dosage, and adverse drug reactions.” The researchers would also like to study genetic phenotypes for other metabolizing enzymes and repeat the pharmacoeconomic analysis with the complete dataset.
A glimpse into the future
Approached for comment, Alessandro Serretti, MD, PhD, department of biomedical and neuromotor sciences, University of Bologna (Italy), who was not involved in the study, said the findings show there is a “small but evident benefit” from CYP profiling, “which makes sense.”
He added that in the Netherlands and other European countries, efforts are already underway to record the CYP status of patients at a national level. “Sooner or later, all Western countries will implement it as a routine,” he said in an interview.
He explained that, when such testing is widely available, electronic health record data will allow physicians to immediately select the optimal antidepressant for an individual patient. This will end the current trial-and-error process that leads to delayed treatment and will help avoid serious consequences, such as suicide.
While reducing a single patient’s treatment by a few weeks with the most appropriate antidepressant choice does not make a large difference in the cost per episode, at a population level, it has the potential to make a significant difference.
Dr. Serretti does not envisage genotyping all 333 million Europeans for the CYP phenotype at this point but imagines that in the future, individuals will undergo whole-genome sequencing to determine risks for cancer, dementia, and heart disease, at which point they will also undergo CYP functional allele profiling, and all these data will be recorded on individuals’ EHR.
“So, every doctor, a psychiatrist or cardiologist, can see everything, whenever they need it,” he said.
The study was funded by Fondazione di Sardegna and Regione Autonoma della Sardegna. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
CYP2D6 and CYP2C19, from the cytochrome P450 family, are involved in the metabolism and elimination of various molecules, including medications. Variants in the genes encoding these enzymes affect the speed at which drugs are metabolized, altering their pharmacokinetic profiles.
The researchers studied 125 patients with MDD and used CYP2D6 and CYP2C19 genotyping to determine the presence of actionable phenotypes in line with Food and Drug Administration labeling.
They found that, in many cases, pharmacogenetic testing could have predicted poor response to the initial treatment selection and could have helped guide subsequent choices to improve outcomes.
In addition, a pharmacoeconomic evaluation that combined direct and indirect costs resulting from MDD with the prevalence of CYP2D6 and CYP2C19 phenotypes showed that testing for functional variants in both genes would be cost effective at a national level.
Had psychiatrists who treated patients in the study known about their metabolizing profiles, it “might have contributed to switches in medication” and could have reduced “delays in response,” said lead researcher Alessio Squassina, PhD, associate professor of pharmacology at the University of Cagliari (Italy).
The findings were presented at the European Psychiatric Association 2022 Congress.
Highly variable response rates
Dr. Squassina noted that the response to antidepressants is a “highly variable trait,” and while it is known that genetics play a role, their contribution is “still not completely understood.”
He explained that the use of pharmacogenetics, which leverages genetic information to guide treatment decision-making, has increased significantly.
While regulatory bodies, including the FDA, have been “very active” in defining strict criteria for interpreting the information from pharmacogenetic tests, there remains some “discrepancy” in their clinical utility.
Dr. Squassina said the FDA provides guidance on use of genetic testing on the labels of 34 psychiatric medications. Of these, 79% relate to CYP2D6, 12% relate to CYP2C19, and 9% relate to other genes.
These labels provide guidance on when genetic testing is recommended or required, as well as potentially clinically actionable gene-drug associations in patients with certain functional alleles.
However, Dr. Squassina noted that the distribution of such alleles is not the same across Europe, so it’s possible that a psychiatrist in Italy may be less likely to treat a patient with a phenotype affecting response to treatment or risk of adverse events than one in Norway or Sweden.
For the study, the investigators examined the frequency of CYP2D6 and CYP2C19 phenotypes in psychiatric patients in Sardinia and their relationship with pharmacologic treatment and cost-effectiveness.
They set out to recruit 200 patients with MDD who had a documented 5-year medical and treatment history, including alterations in treatment, adverse events, hospitalizations, suicide, and symptom scores, as well as sociodemographic variables.
An interim analysis of the first 125 patients recruited to the study showed that the most common CYP2D6 phenotype was normal metabolizers (NM), at 60.5%, followed by intermediate metabolizers (IM), at 28.2%, ultrarapid metabolizers (UR), at 8.9%, and poor metabolizers (PM), at 2.4%.
For CYP2C19, the most common phenotype was NM (49%), followed by IM (29.0%), UR (25.0%), and PM (4.0%). While there were differences in the overall European averages, they were not significant.
To highlight the potential impact that pharmacogenetic testing could have had on patient care and outcome, Dr. Squassina highlighted two cases.
The first concerned a patient with a CYP2D6 IM and CYP2C19 UR phenotype, who did not respond to escitalopram. The FDA drug label indicates this phenotype is actionable and recommends an alternative drug.
The patient was subsequently switched to venlafaxine. The FDA drug label on venlafaxine notes that patients with this phenotype are likely to have a suboptimal response to this drug, and again, this patient did not respond to treatment.
Another patient with a CYP2D6 NM and CYP2C19 IM phenotype was also prescribed escitalopram. The FDA label on this drug notes that patients with this phenotype can try venlafaxine but may not respond. Indeed, this patient did not respond and was switched to venlafaxine and started responding.
“The psychiatrists [in these cases] may made have made different [drug] choices if they had known the genotypes in advance,” Dr. Squassina said.
Cost effective?
To determine the cost-effectiveness of screening for CYP2D6 and CYP2C19 phenotypes in patients with MDD, the researchers used real-world data to develop a Markov model with a hypothetical cohort of 2000 MDD patients, half of whom underwent pharmacogenetic testing, to determine the potential impact on outcomes over an 18-week period.
The model included the cost of medications and hospitalization, psychiatric counseling, loss of productivity, and the estimated probability of response and adverse events, adjusted for the patient’s likelihood of having a particular metabolizing phenotype.
Results showed that, for CYP2C19, compared to no testing, pharmacogenetic testing would be cost-effective at an incremental cost-effective ratio (ICER) of €60,000 ($64,000 USD) per quality-adjusted life-year (QALY).
This, Squassina said, is “below the willingness to pay threshold” for health authorities in developed countries.
For CYP2D6, pharmacogenetic testing would become cost-effective at an ICER of approximately €47,000 ($40,000 USD) per QALY.
The team plans to complete recruitment and perform a “detailed evaluation of all the variables, especially those relating to the medication history and changes in dosage, and adverse drug reactions.” The researchers would also like to study genetic phenotypes for other metabolizing enzymes and repeat the pharmacoeconomic analysis with the complete dataset.
A glimpse into the future
Approached for comment, Alessandro Serretti, MD, PhD, department of biomedical and neuromotor sciences, University of Bologna (Italy), who was not involved in the study, said the findings show there is a “small but evident benefit” from CYP profiling, “which makes sense.”
He added that in the Netherlands and other European countries, efforts are already underway to record the CYP status of patients at a national level. “Sooner or later, all Western countries will implement it as a routine,” he said in an interview.
He explained that, when such testing is widely available, electronic health record data will allow physicians to immediately select the optimal antidepressant for an individual patient. This will end the current trial-and-error process that leads to delayed treatment and will help avoid serious consequences, such as suicide.
While reducing a single patient’s treatment by a few weeks with the most appropriate antidepressant choice does not make a large difference in the cost per episode, at a population level, it has the potential to make a significant difference.
Dr. Serretti does not envisage genotyping all 333 million Europeans for the CYP phenotype at this point but imagines that in the future, individuals will undergo whole-genome sequencing to determine risks for cancer, dementia, and heart disease, at which point they will also undergo CYP functional allele profiling, and all these data will be recorded on individuals’ EHR.
“So, every doctor, a psychiatrist or cardiologist, can see everything, whenever they need it,” he said.
The study was funded by Fondazione di Sardegna and Regione Autonoma della Sardegna. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
new research suggests.
CYP2D6 and CYP2C19, from the cytochrome P450 family, are involved in the metabolism and elimination of various molecules, including medications. Variants in the genes encoding these enzymes affect the speed at which drugs are metabolized, altering their pharmacokinetic profiles.
The researchers studied 125 patients with MDD and used CYP2D6 and CYP2C19 genotyping to determine the presence of actionable phenotypes in line with Food and Drug Administration labeling.
They found that, in many cases, pharmacogenetic testing could have predicted poor response to the initial treatment selection and could have helped guide subsequent choices to improve outcomes.
In addition, a pharmacoeconomic evaluation that combined direct and indirect costs resulting from MDD with the prevalence of CYP2D6 and CYP2C19 phenotypes showed that testing for functional variants in both genes would be cost effective at a national level.
Had psychiatrists who treated patients in the study known about their metabolizing profiles, it “might have contributed to switches in medication” and could have reduced “delays in response,” said lead researcher Alessio Squassina, PhD, associate professor of pharmacology at the University of Cagliari (Italy).
The findings were presented at the European Psychiatric Association 2022 Congress.
Highly variable response rates
Dr. Squassina noted that the response to antidepressants is a “highly variable trait,” and while it is known that genetics play a role, their contribution is “still not completely understood.”
He explained that the use of pharmacogenetics, which leverages genetic information to guide treatment decision-making, has increased significantly.
While regulatory bodies, including the FDA, have been “very active” in defining strict criteria for interpreting the information from pharmacogenetic tests, there remains some “discrepancy” in their clinical utility.
Dr. Squassina said the FDA provides guidance on use of genetic testing on the labels of 34 psychiatric medications. Of these, 79% relate to CYP2D6, 12% relate to CYP2C19, and 9% relate to other genes.
These labels provide guidance on when genetic testing is recommended or required, as well as potentially clinically actionable gene-drug associations in patients with certain functional alleles.
However, Dr. Squassina noted that the distribution of such alleles is not the same across Europe, so it’s possible that a psychiatrist in Italy may be less likely to treat a patient with a phenotype affecting response to treatment or risk of adverse events than one in Norway or Sweden.
For the study, the investigators examined the frequency of CYP2D6 and CYP2C19 phenotypes in psychiatric patients in Sardinia and their relationship with pharmacologic treatment and cost-effectiveness.
They set out to recruit 200 patients with MDD who had a documented 5-year medical and treatment history, including alterations in treatment, adverse events, hospitalizations, suicide, and symptom scores, as well as sociodemographic variables.
An interim analysis of the first 125 patients recruited to the study showed that the most common CYP2D6 phenotype was normal metabolizers (NM), at 60.5%, followed by intermediate metabolizers (IM), at 28.2%, ultrarapid metabolizers (UR), at 8.9%, and poor metabolizers (PM), at 2.4%.
For CYP2C19, the most common phenotype was NM (49%), followed by IM (29.0%), UR (25.0%), and PM (4.0%). While there were differences in the overall European averages, they were not significant.
To highlight the potential impact that pharmacogenetic testing could have had on patient care and outcome, Dr. Squassina highlighted two cases.
The first concerned a patient with a CYP2D6 IM and CYP2C19 UR phenotype, who did not respond to escitalopram. The FDA drug label indicates this phenotype is actionable and recommends an alternative drug.
The patient was subsequently switched to venlafaxine. The FDA drug label on venlafaxine notes that patients with this phenotype are likely to have a suboptimal response to this drug, and again, this patient did not respond to treatment.
Another patient with a CYP2D6 NM and CYP2C19 IM phenotype was also prescribed escitalopram. The FDA label on this drug notes that patients with this phenotype can try venlafaxine but may not respond. Indeed, this patient did not respond and was switched to venlafaxine and started responding.
“The psychiatrists [in these cases] may made have made different [drug] choices if they had known the genotypes in advance,” Dr. Squassina said.
Cost effective?
To determine the cost-effectiveness of screening for CYP2D6 and CYP2C19 phenotypes in patients with MDD, the researchers used real-world data to develop a Markov model with a hypothetical cohort of 2000 MDD patients, half of whom underwent pharmacogenetic testing, to determine the potential impact on outcomes over an 18-week period.
The model included the cost of medications and hospitalization, psychiatric counseling, loss of productivity, and the estimated probability of response and adverse events, adjusted for the patient’s likelihood of having a particular metabolizing phenotype.
Results showed that, for CYP2C19, compared to no testing, pharmacogenetic testing would be cost-effective at an incremental cost-effective ratio (ICER) of €60,000 ($64,000 USD) per quality-adjusted life-year (QALY).
This, Squassina said, is “below the willingness to pay threshold” for health authorities in developed countries.
For CYP2D6, pharmacogenetic testing would become cost-effective at an ICER of approximately €47,000 ($40,000 USD) per QALY.
The team plans to complete recruitment and perform a “detailed evaluation of all the variables, especially those relating to the medication history and changes in dosage, and adverse drug reactions.” The researchers would also like to study genetic phenotypes for other metabolizing enzymes and repeat the pharmacoeconomic analysis with the complete dataset.
A glimpse into the future
Approached for comment, Alessandro Serretti, MD, PhD, department of biomedical and neuromotor sciences, University of Bologna (Italy), who was not involved in the study, said the findings show there is a “small but evident benefit” from CYP profiling, “which makes sense.”
He added that in the Netherlands and other European countries, efforts are already underway to record the CYP status of patients at a national level. “Sooner or later, all Western countries will implement it as a routine,” he said in an interview.
He explained that, when such testing is widely available, electronic health record data will allow physicians to immediately select the optimal antidepressant for an individual patient. This will end the current trial-and-error process that leads to delayed treatment and will help avoid serious consequences, such as suicide.
While reducing a single patient’s treatment by a few weeks with the most appropriate antidepressant choice does not make a large difference in the cost per episode, at a population level, it has the potential to make a significant difference.
Dr. Serretti does not envisage genotyping all 333 million Europeans for the CYP phenotype at this point but imagines that in the future, individuals will undergo whole-genome sequencing to determine risks for cancer, dementia, and heart disease, at which point they will also undergo CYP functional allele profiling, and all these data will be recorded on individuals’ EHR.
“So, every doctor, a psychiatrist or cardiologist, can see everything, whenever they need it,” he said.
The study was funded by Fondazione di Sardegna and Regione Autonoma della Sardegna. The authors disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM EPA 2022
Guidelines vary on what age to begin screening for cervical cancer. What age do you typically recommend for patients?
[polldaddy:11135441]
[polldaddy:11135441]
[polldaddy:11135441]
Alcohol, degraded sleep related in young adults
CHARLOTTE, N.C. – Sleep and alcohol consumption in young adults seems to follow a “vicious cycle,” as one observer called it. and those who went to bed earlier and slept longer tended to drink less the next day, a study of drinking and sleeping habits in 21- to 29-year-olds found.
“Sleep is a potential factor that we could intervene on to really identify how to improve drinking behaviors among young adults,” David Reichenberger, a graduate student at Penn State University, University Park, said in an interview after he presented his findings at the annual meeting of the Associated Professional Sleep Societies.
This is one of the few studies of alcohol consumption and sleep patterns that used an objective measure of alcohol consumption, Mr. Reichenberger said. The study evaluated sleep and alcohol consumption patterns in 222 regularly drinking young adults over 6 consecutive days. Study participants completed morning smartphone-based questionnaires, reporting their previous night’s bedtime, sleep duration, sleep quality, and number of drinks consumed. They also wore an alcohol monitor that continuously measured their transdermal alcohol consumption (TAC).
The study analyzed the data using two sets of multilevel models: A linear model that looked at how each drinking predictor was associated with each sleep variable and a Poisson model to determine how sleep predicted next-day alcohol use.
“We found that higher average peak TAC – that is, how intoxicated they got – was associated with a 19-minute later bedtime among young adults,” Mr. Reichenberger said. “Later bedtimes were then associated with a 26% greater TAC among those adults” (P < .02).
Patterns of alcohol consumption and sleep
On days when participants recorded a higher peak TAC, bedtime was delayed, sleep duration was shorter, and subjective sleep quality was worse, he said. However, none of the sleep variables predicted next-day peak TAC.
“We found an association between the duration of the drinking episode and later bedtimes among young adults,” he added. “And on days when the drinking episodes were longer, subsequent sleep was delayed and sleep quality was worse. But we also found that after nights when they had a later bedtime, next-day drinking episodes were about 7% longer.”
Conversely, young adults who had earlier bedtimes and longer sleep durations tended to consume fewer drinks and they achieved lower intoxication levels the next day, Mr. Reichenberger said.
Between-person results showed that young adults who tended to go to bed later drank on average 24% more the next day (P < .01). Also, each extra hour of sleep was associated with a 14% decrease in drinking the next day (P < .03).
Participants who drank more went to bed on average 12-19 minutes later (P < .01) and slept 5 fewer minutes (P < .01). Within-person results showed that on nights when participants drank more than usual they went to bed 8-13 minutes later (P < .01), slept 2-4 fewer minutes (P < .03), and had worse sleep quality (P < .01).
Mr. Reichenberger acknowledged one limitation of the study: Measuring sleep and alcohol consumption patterns over 6 days might not be long enough. Future studies should address that.
A ‘vicious cycle’
Hans P.A. Van Dongen, PhD, director of the Sleep and Performance Research Center at Washington State University, Spokane, said in an interview that the findings imply a “vicious cycle” between sleep and alcohol consumption. “You create a problem and then it perpetuates itself or reinforces itself.”
In older adults, alcohol tends to act as a “sleep aid,” Dr. Van Dongen noted. “Then it disrupts their sleep later on and then the next night they need to use the sleep aid again because they had a really poor night and they’re tired and they want to fall asleep.”
He added: “I think what is new here is that’s not very likely the mechanism that they’re using alcohol as a sleep aid in younger adults that we see in older adults, so I think there is a new element to it. Now does anybody know how that works exactly? No, that’s the next thing.”
The Penn State study identifies “a signal there that needs to be followed up on,” Dr. Van Dongen said. “There’s something nature’s trying to tell us but it’s not exactly clear what it’s trying to tell us.”
The National Institute on Drug Abuse provided funding for the study. Mr. Reichenberger has no relevant disclosures. Dr. Van Dongen has no disclosures to report.
CHARLOTTE, N.C. – Sleep and alcohol consumption in young adults seems to follow a “vicious cycle,” as one observer called it. and those who went to bed earlier and slept longer tended to drink less the next day, a study of drinking and sleeping habits in 21- to 29-year-olds found.
“Sleep is a potential factor that we could intervene on to really identify how to improve drinking behaviors among young adults,” David Reichenberger, a graduate student at Penn State University, University Park, said in an interview after he presented his findings at the annual meeting of the Associated Professional Sleep Societies.
This is one of the few studies of alcohol consumption and sleep patterns that used an objective measure of alcohol consumption, Mr. Reichenberger said. The study evaluated sleep and alcohol consumption patterns in 222 regularly drinking young adults over 6 consecutive days. Study participants completed morning smartphone-based questionnaires, reporting their previous night’s bedtime, sleep duration, sleep quality, and number of drinks consumed. They also wore an alcohol monitor that continuously measured their transdermal alcohol consumption (TAC).
The study analyzed the data using two sets of multilevel models: A linear model that looked at how each drinking predictor was associated with each sleep variable and a Poisson model to determine how sleep predicted next-day alcohol use.
“We found that higher average peak TAC – that is, how intoxicated they got – was associated with a 19-minute later bedtime among young adults,” Mr. Reichenberger said. “Later bedtimes were then associated with a 26% greater TAC among those adults” (P < .02).
Patterns of alcohol consumption and sleep
On days when participants recorded a higher peak TAC, bedtime was delayed, sleep duration was shorter, and subjective sleep quality was worse, he said. However, none of the sleep variables predicted next-day peak TAC.
“We found an association between the duration of the drinking episode and later bedtimes among young adults,” he added. “And on days when the drinking episodes were longer, subsequent sleep was delayed and sleep quality was worse. But we also found that after nights when they had a later bedtime, next-day drinking episodes were about 7% longer.”
Conversely, young adults who had earlier bedtimes and longer sleep durations tended to consume fewer drinks and they achieved lower intoxication levels the next day, Mr. Reichenberger said.
Between-person results showed that young adults who tended to go to bed later drank on average 24% more the next day (P < .01). Also, each extra hour of sleep was associated with a 14% decrease in drinking the next day (P < .03).
Participants who drank more went to bed on average 12-19 minutes later (P < .01) and slept 5 fewer minutes (P < .01). Within-person results showed that on nights when participants drank more than usual they went to bed 8-13 minutes later (P < .01), slept 2-4 fewer minutes (P < .03), and had worse sleep quality (P < .01).
Mr. Reichenberger acknowledged one limitation of the study: Measuring sleep and alcohol consumption patterns over 6 days might not be long enough. Future studies should address that.
A ‘vicious cycle’
Hans P.A. Van Dongen, PhD, director of the Sleep and Performance Research Center at Washington State University, Spokane, said in an interview that the findings imply a “vicious cycle” between sleep and alcohol consumption. “You create a problem and then it perpetuates itself or reinforces itself.”
In older adults, alcohol tends to act as a “sleep aid,” Dr. Van Dongen noted. “Then it disrupts their sleep later on and then the next night they need to use the sleep aid again because they had a really poor night and they’re tired and they want to fall asleep.”
He added: “I think what is new here is that’s not very likely the mechanism that they’re using alcohol as a sleep aid in younger adults that we see in older adults, so I think there is a new element to it. Now does anybody know how that works exactly? No, that’s the next thing.”
The Penn State study identifies “a signal there that needs to be followed up on,” Dr. Van Dongen said. “There’s something nature’s trying to tell us but it’s not exactly clear what it’s trying to tell us.”
The National Institute on Drug Abuse provided funding for the study. Mr. Reichenberger has no relevant disclosures. Dr. Van Dongen has no disclosures to report.
CHARLOTTE, N.C. – Sleep and alcohol consumption in young adults seems to follow a “vicious cycle,” as one observer called it. and those who went to bed earlier and slept longer tended to drink less the next day, a study of drinking and sleeping habits in 21- to 29-year-olds found.
“Sleep is a potential factor that we could intervene on to really identify how to improve drinking behaviors among young adults,” David Reichenberger, a graduate student at Penn State University, University Park, said in an interview after he presented his findings at the annual meeting of the Associated Professional Sleep Societies.
This is one of the few studies of alcohol consumption and sleep patterns that used an objective measure of alcohol consumption, Mr. Reichenberger said. The study evaluated sleep and alcohol consumption patterns in 222 regularly drinking young adults over 6 consecutive days. Study participants completed morning smartphone-based questionnaires, reporting their previous night’s bedtime, sleep duration, sleep quality, and number of drinks consumed. They also wore an alcohol monitor that continuously measured their transdermal alcohol consumption (TAC).
The study analyzed the data using two sets of multilevel models: A linear model that looked at how each drinking predictor was associated with each sleep variable and a Poisson model to determine how sleep predicted next-day alcohol use.
“We found that higher average peak TAC – that is, how intoxicated they got – was associated with a 19-minute later bedtime among young adults,” Mr. Reichenberger said. “Later bedtimes were then associated with a 26% greater TAC among those adults” (P < .02).
Patterns of alcohol consumption and sleep
On days when participants recorded a higher peak TAC, bedtime was delayed, sleep duration was shorter, and subjective sleep quality was worse, he said. However, none of the sleep variables predicted next-day peak TAC.
“We found an association between the duration of the drinking episode and later bedtimes among young adults,” he added. “And on days when the drinking episodes were longer, subsequent sleep was delayed and sleep quality was worse. But we also found that after nights when they had a later bedtime, next-day drinking episodes were about 7% longer.”
Conversely, young adults who had earlier bedtimes and longer sleep durations tended to consume fewer drinks and they achieved lower intoxication levels the next day, Mr. Reichenberger said.
Between-person results showed that young adults who tended to go to bed later drank on average 24% more the next day (P < .01). Also, each extra hour of sleep was associated with a 14% decrease in drinking the next day (P < .03).
Participants who drank more went to bed on average 12-19 minutes later (P < .01) and slept 5 fewer minutes (P < .01). Within-person results showed that on nights when participants drank more than usual they went to bed 8-13 minutes later (P < .01), slept 2-4 fewer minutes (P < .03), and had worse sleep quality (P < .01).
Mr. Reichenberger acknowledged one limitation of the study: Measuring sleep and alcohol consumption patterns over 6 days might not be long enough. Future studies should address that.
A ‘vicious cycle’
Hans P.A. Van Dongen, PhD, director of the Sleep and Performance Research Center at Washington State University, Spokane, said in an interview that the findings imply a “vicious cycle” between sleep and alcohol consumption. “You create a problem and then it perpetuates itself or reinforces itself.”
In older adults, alcohol tends to act as a “sleep aid,” Dr. Van Dongen noted. “Then it disrupts their sleep later on and then the next night they need to use the sleep aid again because they had a really poor night and they’re tired and they want to fall asleep.”
He added: “I think what is new here is that’s not very likely the mechanism that they’re using alcohol as a sleep aid in younger adults that we see in older adults, so I think there is a new element to it. Now does anybody know how that works exactly? No, that’s the next thing.”
The Penn State study identifies “a signal there that needs to be followed up on,” Dr. Van Dongen said. “There’s something nature’s trying to tell us but it’s not exactly clear what it’s trying to tell us.”
The National Institute on Drug Abuse provided funding for the study. Mr. Reichenberger has no relevant disclosures. Dr. Van Dongen has no disclosures to report.
At SLEEP 2022
Insurer told to pay $5.2 million to woman who caught STD in a car
A Missouri lawsuit adds a new twist to the kind of “bodily harm” in a car that’s covered by insurance.
On June 7,
The woman, identified in court documents as M.O., said she contracted human papillomavirus from her boyfriend. She said he knew he had the disease but didn’t tell her.
An arbitrator found in May 2021 that the in-car sex had “directly caused, or directly contributed to cause” the STD transmission. The man was found liable. The woman was awarded $5.2 million to be paid by GEICO, which insured the man’s vehicle.
GEICO filed for the award to be overturned, alleging it had been denied due process and that the arbitration deal was unenforceable.
Court documents show that GEICO claimed the man’s policy covered only injuries that came “out of the ownership, maintenance or use of the ... auto” and that the woman’s “injuries arose from an intervening cause – namely, her failure to prevent transmission of STDs by having unprotected sex.”
The state appellate panel ruled that the lower court made no mistake in the case and upheld the decision.
The Kansas City Star reported that one of the judges concurred but said GEICO was offered “no meaningful opportunity to participate” in the lawsuit and existing law “relegat(es) the insurer to the status of a bystander.”
“This case presents novel and potentially important issues about whether an insurance carrier can be held liable under such policies for the consequences of two adults voluntarily having unprotected sex in the insured’s automobile,” noted U.S. Magistrate Judge Angel D. Mitchell in court documents. “Interpretation of these policies could have far-reaching implications for other policies with similar terms.”
A version of this article first appeared on WebMD.com.
A Missouri lawsuit adds a new twist to the kind of “bodily harm” in a car that’s covered by insurance.
On June 7,
The woman, identified in court documents as M.O., said she contracted human papillomavirus from her boyfriend. She said he knew he had the disease but didn’t tell her.
An arbitrator found in May 2021 that the in-car sex had “directly caused, or directly contributed to cause” the STD transmission. The man was found liable. The woman was awarded $5.2 million to be paid by GEICO, which insured the man’s vehicle.
GEICO filed for the award to be overturned, alleging it had been denied due process and that the arbitration deal was unenforceable.
Court documents show that GEICO claimed the man’s policy covered only injuries that came “out of the ownership, maintenance or use of the ... auto” and that the woman’s “injuries arose from an intervening cause – namely, her failure to prevent transmission of STDs by having unprotected sex.”
The state appellate panel ruled that the lower court made no mistake in the case and upheld the decision.
The Kansas City Star reported that one of the judges concurred but said GEICO was offered “no meaningful opportunity to participate” in the lawsuit and existing law “relegat(es) the insurer to the status of a bystander.”
“This case presents novel and potentially important issues about whether an insurance carrier can be held liable under such policies for the consequences of two adults voluntarily having unprotected sex in the insured’s automobile,” noted U.S. Magistrate Judge Angel D. Mitchell in court documents. “Interpretation of these policies could have far-reaching implications for other policies with similar terms.”
A version of this article first appeared on WebMD.com.
A Missouri lawsuit adds a new twist to the kind of “bodily harm” in a car that’s covered by insurance.
On June 7,
The woman, identified in court documents as M.O., said she contracted human papillomavirus from her boyfriend. She said he knew he had the disease but didn’t tell her.
An arbitrator found in May 2021 that the in-car sex had “directly caused, or directly contributed to cause” the STD transmission. The man was found liable. The woman was awarded $5.2 million to be paid by GEICO, which insured the man’s vehicle.
GEICO filed for the award to be overturned, alleging it had been denied due process and that the arbitration deal was unenforceable.
Court documents show that GEICO claimed the man’s policy covered only injuries that came “out of the ownership, maintenance or use of the ... auto” and that the woman’s “injuries arose from an intervening cause – namely, her failure to prevent transmission of STDs by having unprotected sex.”
The state appellate panel ruled that the lower court made no mistake in the case and upheld the decision.
The Kansas City Star reported that one of the judges concurred but said GEICO was offered “no meaningful opportunity to participate” in the lawsuit and existing law “relegat(es) the insurer to the status of a bystander.”
“This case presents novel and potentially important issues about whether an insurance carrier can be held liable under such policies for the consequences of two adults voluntarily having unprotected sex in the insured’s automobile,” noted U.S. Magistrate Judge Angel D. Mitchell in court documents. “Interpretation of these policies could have far-reaching implications for other policies with similar terms.”
A version of this article first appeared on WebMD.com.
Harmony pulmonary valve update: Regurgitation resolved 1 year out
The 1-year results of the Harmony transcatheter pulmonary valve to treat severe pulmonary regurgitation have shown a high rate of eliminating or reducing the degree of symptoms as well as freedom from endocarditis, sustained ventricular tachycardia, and the need for further interventions.
“Simply put, the good news is no endocarditis,” said Daniel S. Levi, MD, in presenting results from three different studies with 108 patients who received three different iterations of the device at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“Endocarditis has been an issue for us in the pulmonary position; we have yet to have an endocarditis in these patients in 1 year,” he stressed.
The studies evaluated three different versions of the Harmony valve: TPV22 (42 patients), the first version with a 22-mm diameter; the Clinical TPV25 (17 patients), the first iteration of a 25 mm–wide device that has since been discontinued; and the modified TPV25 (45 patients), the second version of the 25-mm valve. The three studies are the early feasibility study of the TPV22, the continued-access study of the TPV22 and the mTPV25, and the pivotal study that included all three versions.
At baseline, 89% of patients had severe and 11% had moderate pulmonary regurgitation (PR). At 1 year, 92% had none or trace PR, 3% had mild PR, and 4% moderate disease.
Dr. Levi said the device “speaks for itself” in the results he presented. They include no deaths, no heart attacks, and no pulmonary thromboembolism. Other key outcomes include:
- One major stent fracture in one of the early feasibility study patients at 1-month follow-up.
- Four explants, with two in the discontinued cTPV25 and two with the TPV22 in the early-feasibility study.
- Four reinterventions, two with the discontinued cTPV25 and two valve-in-valve procedures with the mTPV25 in the continued-access study, one with stent placement in the right ventricular outflow tract.
Dr. Levi and coinvestigators also performed a breakdown of 1-year outcomes – freedom from PR, stenosis, and interventions – by device: 95.1% for TPV22; 89.7% for mTPV25; and 73.3% for the discontinued cTPV25.
Although the valve is indicated for adolescents and adults, most of the patients in the three studies were adults, with an average weight of 165 pounds (75 kg) who have had PR for decades, said Dr. Levi, an interventional pediatric cardiologist at the University of California, Los Angeles. “With a device like this we are hopefully shifting to treating that a little bit earlier, but fortunately we don’t usually need to treat it before puberty.” The 25-mm TPV gives “a really nice landing zone” for future valve placement. “The goal is to keep patients out of the operating room for at least a few decades if not their whole lives,” he said.
Dr. Levi said the Harmony investigators will follow outcomes with the 22- and modified 25-mm Harmony valves, both of which remain commercially available, out to 10 years.
The study represents the first collective cohort evaluating the Harmony device across the early feasibility, continued access and pivotal studies, said Brian Morray, MD. “It’s important that people understand that evolution and how that impacts the way we look at outcomes, because when you aggregate the data, particularly for the TPV25, some of the procedural outcomes and the adverse events are no longer really reflective in the current time frame.”
These Harmony results “represent another big step in the evolution of interventional cardiology and will be up there with development of the Melody valve and the utility and the use of the Sapien valve in the pulmonary position,” said Dr. Morray, an associate professor of pediatrics at the University of Washington, Seattle, and an interventional cardiologist at Seattle Children’s Hospital.
Dr. Levi disclosed he is a consultant to Medtronic and Edwards Lifesciences. Dr. Morray disclosed he is a clinical proctor for Abbott and a consultant to Medtronic, but not for the Harmony device.
The 1-year results of the Harmony transcatheter pulmonary valve to treat severe pulmonary regurgitation have shown a high rate of eliminating or reducing the degree of symptoms as well as freedom from endocarditis, sustained ventricular tachycardia, and the need for further interventions.
“Simply put, the good news is no endocarditis,” said Daniel S. Levi, MD, in presenting results from three different studies with 108 patients who received three different iterations of the device at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“Endocarditis has been an issue for us in the pulmonary position; we have yet to have an endocarditis in these patients in 1 year,” he stressed.
The studies evaluated three different versions of the Harmony valve: TPV22 (42 patients), the first version with a 22-mm diameter; the Clinical TPV25 (17 patients), the first iteration of a 25 mm–wide device that has since been discontinued; and the modified TPV25 (45 patients), the second version of the 25-mm valve. The three studies are the early feasibility study of the TPV22, the continued-access study of the TPV22 and the mTPV25, and the pivotal study that included all three versions.
At baseline, 89% of patients had severe and 11% had moderate pulmonary regurgitation (PR). At 1 year, 92% had none or trace PR, 3% had mild PR, and 4% moderate disease.
Dr. Levi said the device “speaks for itself” in the results he presented. They include no deaths, no heart attacks, and no pulmonary thromboembolism. Other key outcomes include:
- One major stent fracture in one of the early feasibility study patients at 1-month follow-up.
- Four explants, with two in the discontinued cTPV25 and two with the TPV22 in the early-feasibility study.
- Four reinterventions, two with the discontinued cTPV25 and two valve-in-valve procedures with the mTPV25 in the continued-access study, one with stent placement in the right ventricular outflow tract.
Dr. Levi and coinvestigators also performed a breakdown of 1-year outcomes – freedom from PR, stenosis, and interventions – by device: 95.1% for TPV22; 89.7% for mTPV25; and 73.3% for the discontinued cTPV25.
Although the valve is indicated for adolescents and adults, most of the patients in the three studies were adults, with an average weight of 165 pounds (75 kg) who have had PR for decades, said Dr. Levi, an interventional pediatric cardiologist at the University of California, Los Angeles. “With a device like this we are hopefully shifting to treating that a little bit earlier, but fortunately we don’t usually need to treat it before puberty.” The 25-mm TPV gives “a really nice landing zone” for future valve placement. “The goal is to keep patients out of the operating room for at least a few decades if not their whole lives,” he said.
Dr. Levi said the Harmony investigators will follow outcomes with the 22- and modified 25-mm Harmony valves, both of which remain commercially available, out to 10 years.
The study represents the first collective cohort evaluating the Harmony device across the early feasibility, continued access and pivotal studies, said Brian Morray, MD. “It’s important that people understand that evolution and how that impacts the way we look at outcomes, because when you aggregate the data, particularly for the TPV25, some of the procedural outcomes and the adverse events are no longer really reflective in the current time frame.”
These Harmony results “represent another big step in the evolution of interventional cardiology and will be up there with development of the Melody valve and the utility and the use of the Sapien valve in the pulmonary position,” said Dr. Morray, an associate professor of pediatrics at the University of Washington, Seattle, and an interventional cardiologist at Seattle Children’s Hospital.
Dr. Levi disclosed he is a consultant to Medtronic and Edwards Lifesciences. Dr. Morray disclosed he is a clinical proctor for Abbott and a consultant to Medtronic, but not for the Harmony device.
The 1-year results of the Harmony transcatheter pulmonary valve to treat severe pulmonary regurgitation have shown a high rate of eliminating or reducing the degree of symptoms as well as freedom from endocarditis, sustained ventricular tachycardia, and the need for further interventions.
“Simply put, the good news is no endocarditis,” said Daniel S. Levi, MD, in presenting results from three different studies with 108 patients who received three different iterations of the device at the Society for Cardiovascular Angiography & Interventions annual scientific sessions.
“Endocarditis has been an issue for us in the pulmonary position; we have yet to have an endocarditis in these patients in 1 year,” he stressed.
The studies evaluated three different versions of the Harmony valve: TPV22 (42 patients), the first version with a 22-mm diameter; the Clinical TPV25 (17 patients), the first iteration of a 25 mm–wide device that has since been discontinued; and the modified TPV25 (45 patients), the second version of the 25-mm valve. The three studies are the early feasibility study of the TPV22, the continued-access study of the TPV22 and the mTPV25, and the pivotal study that included all three versions.
At baseline, 89% of patients had severe and 11% had moderate pulmonary regurgitation (PR). At 1 year, 92% had none or trace PR, 3% had mild PR, and 4% moderate disease.
Dr. Levi said the device “speaks for itself” in the results he presented. They include no deaths, no heart attacks, and no pulmonary thromboembolism. Other key outcomes include:
- One major stent fracture in one of the early feasibility study patients at 1-month follow-up.
- Four explants, with two in the discontinued cTPV25 and two with the TPV22 in the early-feasibility study.
- Four reinterventions, two with the discontinued cTPV25 and two valve-in-valve procedures with the mTPV25 in the continued-access study, one with stent placement in the right ventricular outflow tract.
Dr. Levi and coinvestigators also performed a breakdown of 1-year outcomes – freedom from PR, stenosis, and interventions – by device: 95.1% for TPV22; 89.7% for mTPV25; and 73.3% for the discontinued cTPV25.
Although the valve is indicated for adolescents and adults, most of the patients in the three studies were adults, with an average weight of 165 pounds (75 kg) who have had PR for decades, said Dr. Levi, an interventional pediatric cardiologist at the University of California, Los Angeles. “With a device like this we are hopefully shifting to treating that a little bit earlier, but fortunately we don’t usually need to treat it before puberty.” The 25-mm TPV gives “a really nice landing zone” for future valve placement. “The goal is to keep patients out of the operating room for at least a few decades if not their whole lives,” he said.
Dr. Levi said the Harmony investigators will follow outcomes with the 22- and modified 25-mm Harmony valves, both of which remain commercially available, out to 10 years.
The study represents the first collective cohort evaluating the Harmony device across the early feasibility, continued access and pivotal studies, said Brian Morray, MD. “It’s important that people understand that evolution and how that impacts the way we look at outcomes, because when you aggregate the data, particularly for the TPV25, some of the procedural outcomes and the adverse events are no longer really reflective in the current time frame.”
These Harmony results “represent another big step in the evolution of interventional cardiology and will be up there with development of the Melody valve and the utility and the use of the Sapien valve in the pulmonary position,” said Dr. Morray, an associate professor of pediatrics at the University of Washington, Seattle, and an interventional cardiologist at Seattle Children’s Hospital.
Dr. Levi disclosed he is a consultant to Medtronic and Edwards Lifesciences. Dr. Morray disclosed he is a clinical proctor for Abbott and a consultant to Medtronic, but not for the Harmony device.
FROM SCAI 2022
Therapeutic patient education can help with adherence to treatment
, Andreas Wollenberg, MD, said at the Revolutionizing Atopic Dermatitis symposium.
A major goal of patient education is increasing medication adherence, noted Dr. Wollenberg, professor in the department of dermatology and allergy at Ludwig Maximilian University of Munich. Quoting former U.S. Surgeon General C. Everett Koop, MD, he said, “drugs don’t work in patients who don’t take them.”
While this is a simple message, it is important, Dr. Wollenberg said, noting that there can be a gap between a physician’s well-intentioned message and how it is interpreted by the patient. “Our messages may not be heard, not understood, not accepted, and even if they are put into place, how long will they last?” he asked. “We need to find a way [to] place sticky messages in the brains of our patients who are sitting and interacting with us.”
One way to improve treatment adherence is through patient education, such as using a written action plan or graphics; simplifying treatment regimens; minimizing treatment costs; setting up reminder programs, early follow-up visits, and short-term treatment goals; and minimizing nocebo effects. “This is more than providing just leaflets to patients. It is a complete program. It is a holistic approach. It should be structured and should be interdisciplinary, and it should contain a psychological component,” Dr. Wollenberg said.
Therapeutic patient education is recommended at baseline for children and adults with moderate to severe AD in the 2020 European Task Force on Atopic Dermatitis (ETFAD) and European Academy of Dermatology and Venereology (EADV) position paper on the diagnosis and treatment of AD in adults and children, alongside other interventions, such as emollients, bath oils, and avoidance of clinically relevant allergens, noted Dr. Wollenberg, the first author . “Therapeutic patient education is an extremely helpful tool to address patient beliefs and questions regarding disease and treatment,” he and his coauthors wrote in the paper.
When considering a therapeutic patient education program for AD, content is key, but just as important is consideration of legal and cultural conditions in the local area, Dr. Wollenberg explained. Every country will need some degree of standardization of content, he noted. Clinicians interested in adopting a patient education program need to consider who will pay for it – patients, foundations, or insurance companies – as well as the time commitment needed.
Dr. Wollenberg said that his team uses an evidence-based education program for AD in Germany that works across patients with different personalities, with a multidisciplinary team that includes a dermatologist, a specialist nurse, a nutrition expert, and a psychologist. “Sometimes we replace the specialized nurse with the dermatology resident because, in Germany, it’s difficult to find any type of specialized nurse,” although this is not an issue in many other countries, he said.
The model for children involves six 90-minute sessions, which cover topics that include emollients and basic care, food allergies and diet, medical treatment, and psychology of itch. The program for adults involves six 2-hour sessions, which cover topics that include psychology, skin care/nutrition, and medical treatment.
While this education program improves adherence in patients with AD, he acknowledged it is time consuming, and may not work for people who live far away from a clinic or who have other time commitments, making an alternative format necessary.
In terms of improving patient adherence to a doctor’s recommendations regarding chronic skin disease, “we cannot change our patients, we cannot change the disease, but we can strongly influence the treatment that we choose and how we interact as physicians with our patients,” said Dr. Wollenberg.
“Therapeutic patient education is virtually free of side effects, but evidence based. Have a look [at] it and adapt it to your own practice,” he added.
Dr. Wollenberg is a consultant, speaker and receives fees from numerous pharmaceutical companies.
, Andreas Wollenberg, MD, said at the Revolutionizing Atopic Dermatitis symposium.
A major goal of patient education is increasing medication adherence, noted Dr. Wollenberg, professor in the department of dermatology and allergy at Ludwig Maximilian University of Munich. Quoting former U.S. Surgeon General C. Everett Koop, MD, he said, “drugs don’t work in patients who don’t take them.”
While this is a simple message, it is important, Dr. Wollenberg said, noting that there can be a gap between a physician’s well-intentioned message and how it is interpreted by the patient. “Our messages may not be heard, not understood, not accepted, and even if they are put into place, how long will they last?” he asked. “We need to find a way [to] place sticky messages in the brains of our patients who are sitting and interacting with us.”
One way to improve treatment adherence is through patient education, such as using a written action plan or graphics; simplifying treatment regimens; minimizing treatment costs; setting up reminder programs, early follow-up visits, and short-term treatment goals; and minimizing nocebo effects. “This is more than providing just leaflets to patients. It is a complete program. It is a holistic approach. It should be structured and should be interdisciplinary, and it should contain a psychological component,” Dr. Wollenberg said.
Therapeutic patient education is recommended at baseline for children and adults with moderate to severe AD in the 2020 European Task Force on Atopic Dermatitis (ETFAD) and European Academy of Dermatology and Venereology (EADV) position paper on the diagnosis and treatment of AD in adults and children, alongside other interventions, such as emollients, bath oils, and avoidance of clinically relevant allergens, noted Dr. Wollenberg, the first author . “Therapeutic patient education is an extremely helpful tool to address patient beliefs and questions regarding disease and treatment,” he and his coauthors wrote in the paper.
When considering a therapeutic patient education program for AD, content is key, but just as important is consideration of legal and cultural conditions in the local area, Dr. Wollenberg explained. Every country will need some degree of standardization of content, he noted. Clinicians interested in adopting a patient education program need to consider who will pay for it – patients, foundations, or insurance companies – as well as the time commitment needed.
Dr. Wollenberg said that his team uses an evidence-based education program for AD in Germany that works across patients with different personalities, with a multidisciplinary team that includes a dermatologist, a specialist nurse, a nutrition expert, and a psychologist. “Sometimes we replace the specialized nurse with the dermatology resident because, in Germany, it’s difficult to find any type of specialized nurse,” although this is not an issue in many other countries, he said.
The model for children involves six 90-minute sessions, which cover topics that include emollients and basic care, food allergies and diet, medical treatment, and psychology of itch. The program for adults involves six 2-hour sessions, which cover topics that include psychology, skin care/nutrition, and medical treatment.
While this education program improves adherence in patients with AD, he acknowledged it is time consuming, and may not work for people who live far away from a clinic or who have other time commitments, making an alternative format necessary.
In terms of improving patient adherence to a doctor’s recommendations regarding chronic skin disease, “we cannot change our patients, we cannot change the disease, but we can strongly influence the treatment that we choose and how we interact as physicians with our patients,” said Dr. Wollenberg.
“Therapeutic patient education is virtually free of side effects, but evidence based. Have a look [at] it and adapt it to your own practice,” he added.
Dr. Wollenberg is a consultant, speaker and receives fees from numerous pharmaceutical companies.
, Andreas Wollenberg, MD, said at the Revolutionizing Atopic Dermatitis symposium.
A major goal of patient education is increasing medication adherence, noted Dr. Wollenberg, professor in the department of dermatology and allergy at Ludwig Maximilian University of Munich. Quoting former U.S. Surgeon General C. Everett Koop, MD, he said, “drugs don’t work in patients who don’t take them.”
While this is a simple message, it is important, Dr. Wollenberg said, noting that there can be a gap between a physician’s well-intentioned message and how it is interpreted by the patient. “Our messages may not be heard, not understood, not accepted, and even if they are put into place, how long will they last?” he asked. “We need to find a way [to] place sticky messages in the brains of our patients who are sitting and interacting with us.”
One way to improve treatment adherence is through patient education, such as using a written action plan or graphics; simplifying treatment regimens; minimizing treatment costs; setting up reminder programs, early follow-up visits, and short-term treatment goals; and minimizing nocebo effects. “This is more than providing just leaflets to patients. It is a complete program. It is a holistic approach. It should be structured and should be interdisciplinary, and it should contain a psychological component,” Dr. Wollenberg said.
Therapeutic patient education is recommended at baseline for children and adults with moderate to severe AD in the 2020 European Task Force on Atopic Dermatitis (ETFAD) and European Academy of Dermatology and Venereology (EADV) position paper on the diagnosis and treatment of AD in adults and children, alongside other interventions, such as emollients, bath oils, and avoidance of clinically relevant allergens, noted Dr. Wollenberg, the first author . “Therapeutic patient education is an extremely helpful tool to address patient beliefs and questions regarding disease and treatment,” he and his coauthors wrote in the paper.
When considering a therapeutic patient education program for AD, content is key, but just as important is consideration of legal and cultural conditions in the local area, Dr. Wollenberg explained. Every country will need some degree of standardization of content, he noted. Clinicians interested in adopting a patient education program need to consider who will pay for it – patients, foundations, or insurance companies – as well as the time commitment needed.
Dr. Wollenberg said that his team uses an evidence-based education program for AD in Germany that works across patients with different personalities, with a multidisciplinary team that includes a dermatologist, a specialist nurse, a nutrition expert, and a psychologist. “Sometimes we replace the specialized nurse with the dermatology resident because, in Germany, it’s difficult to find any type of specialized nurse,” although this is not an issue in many other countries, he said.
The model for children involves six 90-minute sessions, which cover topics that include emollients and basic care, food allergies and diet, medical treatment, and psychology of itch. The program for adults involves six 2-hour sessions, which cover topics that include psychology, skin care/nutrition, and medical treatment.
While this education program improves adherence in patients with AD, he acknowledged it is time consuming, and may not work for people who live far away from a clinic or who have other time commitments, making an alternative format necessary.
In terms of improving patient adherence to a doctor’s recommendations regarding chronic skin disease, “we cannot change our patients, we cannot change the disease, but we can strongly influence the treatment that we choose and how we interact as physicians with our patients,” said Dr. Wollenberg.
“Therapeutic patient education is virtually free of side effects, but evidence based. Have a look [at] it and adapt it to your own practice,” he added.
Dr. Wollenberg is a consultant, speaker and receives fees from numerous pharmaceutical companies.
FROM RAD 2022
2022 Update: Beyond prenatal exome sequencing
Last year, our Update focused on the expansion of sequencing in prenatal diagnosis. This year, we are taking a step sideways to remember the many diagnoses we may miss if we rely on exome sequencing alone. A recent case report in Prenatal Diagnosis describes a pregnancy affected by fetal akinesia sequence and polyhydramnios in which sequencing did not reveal a diagnosis. Expansion of the differential to include congenital myotonic dystrophy and subsequent triplet repeat testing led the clinicians to the diagnosis and identification of a triplet repeat expansion in the DMPK gene. This case serves as our first example of how complementary testing and technologies should continue to help us make critical diagnoses.
What is the yield of exome sequencing vs panels in nonimmune hydrops?
Rogers R, Moyer K, Moise KJ Jr. Congenital myotonic dystrophy: an overlooked diagnosis not amenable to detection by sequencing. Prenat Diagn. 2022;42:233-235. doi:10.1002/pd.6105.
Norton ME, Ziffle JV, Lianoglou BR, et al. Exome sequencing vs targeted gene panels for the evaluation of nonimmune hydrops fetalis. Am J Obstet Gynecol. 2021;28:S0002-9378(21)00828-0. doi:10.1016/j.ajog.2021.07.014.
We have had several illuminating discussions with our colleagues about the merits of exome sequencing (ES) versus panels and other modalities for fetal diagnosis. Many obstetricians practicing at the leading edge may feel like ES should be utilized uniformly for fetal anomalies with nondiagnostic karyotype or microarray. However, for well-defined phenotypes with clear and narrow lists of implicated genes (eg, skeletal dysplasias) or patients without insurance coverage, panel sequencing still has utility in prenatal diagnosis. The question of which phenotypes most benefit from ES versus panel sequencing is an area of interesting, ongoing research for several investigators.
Secondary analysis of nonimmune hydrops cohort
Norton and colleagues tackled one such cohort in a study presented in the American Journal of Obstetrics and Gynecology. They compared the proportion of diagnoses that would have been identified in commercial lab panels with their research of phenotype-driven ES in a cohort of 127 fetuses with features of nonimmune hydrops fetalis (NIHF). NIHF can be caused by a variety of single-gene disorders in addition to chromosomal disorders and copy number variants on chromosomal microarray. Patients were eligible for inclusion in the cohort if they had a nondiagnostic karyotype or microarray and any of the following features: nuchal translucency of 3.5 mm or greater, cystic hygroma, pleural effusion, pericardial effusion, ascites, or skin edema. Standard sequencing methods and variant analysis were performed. They assumed 100% analytical sensitivity and specificity of the panels for variant detection and collected cost information on the targeted gene panels.
Study outcomes
In the ES analysis of cases, 37 of 127 cases (29%) had a pathogenic or likely pathogenic variant in 1 of 29 genes, and another 12 of 127 cases (9%) had variants of uncertain significance that were strongly suspected to be the etiology during clinical analysis. The types of disorders that were identified are listed in the TABLE. In addition to a feature of NIHF, 50% of the cases had a structural anomaly.
There were 10 identified clinical panels from 7 clinical laboratories. These panels ranged in size from 11 to 128 genes. The highest simulated yield of any commercial panel was only 62% of the pathogenic variants identified by ES. The other commercial laboratory panels detection yield ranged from 11% to 62% of pathogenic variants detected by ES. For overall yield, the largest panel would have a diagnostic yield of 18% of diagnoses relative to the 29% diagnostic yield from ES.
The largest panel included 128 genes prior to the publication of the original cohort and was updated after publication to include 148 genes. The larger updated panel would have identified all of the patients in the ES cohort. However, many of the other panels listed would have identified a smaller fraction of the variants identified by ES (range, 11%-62%). At the time of publication, the cost of the panels ranged from $640 to $3,500, and the cost of prenatal ES ranged from $2,458 to $7,500.
Continue to: Strengths and limitations...
Strengths and limitations
Twenty-three percent of the patients who were sequenced had an increased fetal nuchal translucency or cystic hygroma, and another 17% had a single fetal effusion. This inclusivity makes this study more applicable to broader fetal anomaly populations. However, it is worth noting that only 61% of patients had NIHF by the definition of 2 or more fluid collections or skin thickening.
The authors assumed 100% sensitivity and specificity for the panel tests relative to diagnostic ES results, but this may not reflect real-life analysis. There is inherent subjectivity and subsequent differences in variant calling (deciding which genetic changes are pathogenic) between institutions and companies despite efforts to standardize this process. Due to the simulated nature of this study, these differences are not captured. Additionally, although the authors note that the research ES had at least 30 times the coverage (an adequate number of sequence reads for accurate testing) than did the commercial lab panels, some gene panels have additional sequencing of intronic regions, copy number analysis, and up to 10 times more coverage than ES, which could lead to more diagnoses.
This study illustrates that there is nuance involved in selecting which type of gene sequencing and which clinical laboratory to use for prenatal diagnosis. Labs with more updated literature searches and more inclusive gene panels may be excellent options for patients in whom ES is not covered by insurance or with phenotypes with a narrow range of suspected causative genes. However, there is a lag time in updating the genes offered on each panel, and new genedisease associations will not be captured by existing panels.
From a cost, speed-of-analysis, and depth-of-sequencing perspective, panel sequencing can have advantages that should be considered in some patients, particularly if the panels are large and regularly updated. However, the authors summarize our sentiments and their findings with the following:
“For disorders, such as NIHF with marked genetic heterogeneity and less clear in utero phenotypes of underlying genetic diseases, the broader coverage of exome sequencing makes it a superior option to targeted panel testing.”
We look forward to the publication of further anomaly-specific cohorts and secondary analyses of the utility of current panels and ES that may follow.
Frequency of Beckwith-Widemann syndrome in prenatally diagnosed omphaloceles
Abbasi N, Moore A, Chiu P, et al. Prenatally diagnosed omphaloceles: report of 92 cases and association with Beckwith-Wiedemann syndrome. Prenat Diagn. 2021;41:798-816. doi:10.1002/pd.5930.
An omphalocele is diagnosed prenatally on ultrasound when an anterior midline mass, often containing abdominal contents, is seen herniating into the base of the umbilical cord. Omphaloceles are often associated with additional structural abnormalities and underlying genetic syndromes, thus a thorough fetal assessment is required for accurate prenatal counseling and neonatal care.
Identification of Beckwith-Widemann syndrome (BWS) in the setting of a prenatally diagnosed omphalocele is difficult because of its wide range of clinical features and its unique genetic basis. Unlike many genetic disorders that are caused by specific genetic variants, or spelling changes in the genes, BWS results from a change in the expression of one or more of the genes in a specific region of chromosome 11. A high index of clinical suspicion as well as an understanding of the various genetic and epigenetics alterations that cause BWS is required for prenatal diagnosis.
Retrospective cohort at a single center
The authors in this study reviewed all pregnancies in which an omphalocele was diagnosed prenatally at a single center between 2010 and 2015. They describe a standard prenatal evaluation following identification of an omphalocele including echocardiogram, detailed anatomic survey, and availability of an amniocentesis to facilitate aneuploidy screening and testing for BWS. This review also includes an overview of perinatal and long-term outcomes for cases of BWS diagnosed at their center between 2000 and 2015.
Study outcomes
Results of prenatal genetic testing in this cohort were divided between cases of an isolated omphalocele (without other structural changes) and cases of nonisolated omphaloceles. In the group of pregnancies with an isolated omphalocele, 2 of 27 pregnancies (7.4%) were found to have an abnormal karyotype, and 6 of 16 of the remaining pregnancies (37.5%) were diagnosed with BWS. Among the group of pregnancies with a nonisolated omphalocele, 23 of 59 pregnancies (39%) were found to have an abnormal karyotype, and 1 of 20 pregnancies (5%) were diagnosed with BWS.
Prenatal sonographic features associated with cases of BWS included polyhydramnios in 12 of 19 cases (63%) and macrosomia in 8 of 19 cases (42%). Macroglossia is another characteristic feature of the disorder, which was identified in 4 of 19 cases (21%) prenatally and in an additional 10 of 19 cases (52.6%) postnatally. Interestingly, only 1 of the cases of BWS was caused by a microdeletion at 11p15.4—a change that was identified on microarray. The additional 6 cases of BWS were caused by imprinting changes in the region, which are only detectable with a specific methylation-analysis technique.
Among the 19 cases of BWS identified over a 15-year period, there was 1 intrauterine demise. Preterm birth occurred in 10 of 19 cases (52.6%), including 8 of 19 cases (42.1%) of spontaneous preterm labor. Respiratory distress (27.8%), hypoglycemia (61%), and gastrointestinal reflux (59%) were common neonatal complications. Embryonal tumors were diagnosed in 2 of 16 patients (12.5%). Although neurodevelopmental outcomes were incomplete, their data suggested normal development in 75% of children. There were 2 neonatal deaths in this cohort and 1 childhood death at age 2 years.
Study strengths and limitations
As with many studies investigating a rare disorder, this study is limited by its retrospective nature and small sample size. Nevertheless, it adds an important cohort of patients with a prenatally diagnosed omphalocele to the literature and illuminates the utility of a standardized approach to testing for BWS in this population.
In this cohort with prenatally diagnosed omphaloceles with standardized testing for BWS, the prevalence of the disorder was approximately 8% and more common in cases of an isolated omphalocele. The most common supporting sonographic features of BWS may not be detected until later in gestation, including polyhydramnios and macrosomia. This demonstrates the importance of both sonographic follow-up as well as universal testing for BWS in euploid cases of a prenatally diagnosed omphalocele. Almost all cases of BWS in this cohort required specialized molecular techniques for diagnosis, and the diagnosis would have been missed on karyotype, microarray, and ES.
Continue to: Genetic diagnoses that could have been identified by expanded carrier screening...
Genetic diagnoses that could have been identified by expanded carrier screening
Stevens BK, Nunley PB, Wagner C, et al. Utility of expanded carrier screening in pregnancies with ultrasound abnormalities. Prenat Diagn. 2022;42:60-78. doi:10.1002/pd.6069.
This series is a thorough retrospective review of patients evaluated in a pediatric genetics clinic from 2014 through 2017. Patients were included if they were evaluated in the first 6 months of life and had a structural abnormality that might be detected on prenatal ultrasonography. The genetic testing results were analyzed and categorized according to types of genetic disorders, with the goal of identifying how many patients might have been identified by expanded carrier screening (ECS) panels.
Study outcomes
A total of 931 charts were reviewed, and 85% (791 of 931) of patients evaluated in the first 6 months of life were determined to have a structural anomaly that might be detected on prenatal ultrasonography. Of those patients, 691 went on to have genetic testing and 32.1% (222 of 691) of them had a diagnostic (pathogenic) genetic testing result related to the phenotype. The types of diagnostic testing results are shown in the FIGURE. Notably, 42 single-gene disorders were detected.
FIGURE Diagnostic test result in pediatric patients evaluated under age 6 months
Of those 222 patients with diagnostic results, there were 8 patients with autosomal recessive and X-linked conditions that could be detected using a 500-gene ECS panel. Five patients could be detected with a 271-gene panel. After nondiagnostic microarray, 11.3% of patients had a condition that could be detected by using a 500-gene ECS panel. The identified conditions included cystic fibrosis, CYP21‐related congenital adrenal hyperplasia, autosomal recessive polycystic kidney disease, Antley‐Bixler syndrome, and Morquio syndrome type A.
Furthermore, the authors conducted a literature review of 271 conditions and found that 32% (88 of 271) of conditions may be associated with ultrasound findings.
Study strengths and limitations
When applying these data to prenatal populations, the authors acknowledge several notable limitations. There is a selection bias toward less-severe phenotypes for many patients choosing to continue rather than to interrupt a pregnancy. Additionally, only 23% of the patients in the study had a microarray and ES, which may lead to an underrepresentation of single-gene disorders and an underestimation of the utility of ECS. Finally, a retrospective classification of structural abnormalities that may be detectable by ultrasonography may not always reflect what is actually reported in prenatal imaging.
However, the work that the authors put forth to evaluate and categorize 931 participants by the results of genetic testing and structural anomalies is appreciated, and the level of detail is impressive for this retrospective chart review. Additionally, the tables itemizing the authors’ review of 271 ECS disorders that may have ultrasonography findings categorized by disorder and system are helpful and quick diagnostic references for clinicians providing prenatal care. ●
This study of potentially detectable prenatal findings from the lens of a pediatric genetics clinic lends an interesting perspective: Exome sequencing is not the primary route to establish a diagnosis; karyotype, microarray, methylation disorders, and triplet repeat disorders all have an established role in the diagnostic toolkit. Keeping in mind the contribution of these modalities to pediatric testing may shorten the diagnostic odyssey to continue pregnancies or help to fully counsel patients on expectations and decision-making after birth.
Carrier screening is not a substitute for diagnostic testing in pregnancy. However, in appropriately selected patients, a broad carrier screening panel may have added utility. ECS can be conducted while awaiting microarray results to help target testing and may be particularly useful for patients who decline diagnostic testing until the postnatal period. It is important to counsel patients that carrier screening is not a diagnostic test, and results will only report likely pathogenic or pathogenic variants, not variants of uncertain significance that may be of clinical relevance. However, our practice has had several insightful diagnoses reached through ECS, in conjunction with microarray testing that allowed for faster and more targeted sequencing and precise fetal diagnosis. This readily available molecular tool (often covered by insurance) deserves a spot in your fetal diagnosis tool belt based on available evidence.
Last year, our Update focused on the expansion of sequencing in prenatal diagnosis. This year, we are taking a step sideways to remember the many diagnoses we may miss if we rely on exome sequencing alone. A recent case report in Prenatal Diagnosis describes a pregnancy affected by fetal akinesia sequence and polyhydramnios in which sequencing did not reveal a diagnosis. Expansion of the differential to include congenital myotonic dystrophy and subsequent triplet repeat testing led the clinicians to the diagnosis and identification of a triplet repeat expansion in the DMPK gene. This case serves as our first example of how complementary testing and technologies should continue to help us make critical diagnoses.
What is the yield of exome sequencing vs panels in nonimmune hydrops?
Rogers R, Moyer K, Moise KJ Jr. Congenital myotonic dystrophy: an overlooked diagnosis not amenable to detection by sequencing. Prenat Diagn. 2022;42:233-235. doi:10.1002/pd.6105.
Norton ME, Ziffle JV, Lianoglou BR, et al. Exome sequencing vs targeted gene panels for the evaluation of nonimmune hydrops fetalis. Am J Obstet Gynecol. 2021;28:S0002-9378(21)00828-0. doi:10.1016/j.ajog.2021.07.014.
We have had several illuminating discussions with our colleagues about the merits of exome sequencing (ES) versus panels and other modalities for fetal diagnosis. Many obstetricians practicing at the leading edge may feel like ES should be utilized uniformly for fetal anomalies with nondiagnostic karyotype or microarray. However, for well-defined phenotypes with clear and narrow lists of implicated genes (eg, skeletal dysplasias) or patients without insurance coverage, panel sequencing still has utility in prenatal diagnosis. The question of which phenotypes most benefit from ES versus panel sequencing is an area of interesting, ongoing research for several investigators.
Secondary analysis of nonimmune hydrops cohort
Norton and colleagues tackled one such cohort in a study presented in the American Journal of Obstetrics and Gynecology. They compared the proportion of diagnoses that would have been identified in commercial lab panels with their research of phenotype-driven ES in a cohort of 127 fetuses with features of nonimmune hydrops fetalis (NIHF). NIHF can be caused by a variety of single-gene disorders in addition to chromosomal disorders and copy number variants on chromosomal microarray. Patients were eligible for inclusion in the cohort if they had a nondiagnostic karyotype or microarray and any of the following features: nuchal translucency of 3.5 mm or greater, cystic hygroma, pleural effusion, pericardial effusion, ascites, or skin edema. Standard sequencing methods and variant analysis were performed. They assumed 100% analytical sensitivity and specificity of the panels for variant detection and collected cost information on the targeted gene panels.
Study outcomes
In the ES analysis of cases, 37 of 127 cases (29%) had a pathogenic or likely pathogenic variant in 1 of 29 genes, and another 12 of 127 cases (9%) had variants of uncertain significance that were strongly suspected to be the etiology during clinical analysis. The types of disorders that were identified are listed in the TABLE. In addition to a feature of NIHF, 50% of the cases had a structural anomaly.
There were 10 identified clinical panels from 7 clinical laboratories. These panels ranged in size from 11 to 128 genes. The highest simulated yield of any commercial panel was only 62% of the pathogenic variants identified by ES. The other commercial laboratory panels detection yield ranged from 11% to 62% of pathogenic variants detected by ES. For overall yield, the largest panel would have a diagnostic yield of 18% of diagnoses relative to the 29% diagnostic yield from ES.
The largest panel included 128 genes prior to the publication of the original cohort and was updated after publication to include 148 genes. The larger updated panel would have identified all of the patients in the ES cohort. However, many of the other panels listed would have identified a smaller fraction of the variants identified by ES (range, 11%-62%). At the time of publication, the cost of the panels ranged from $640 to $3,500, and the cost of prenatal ES ranged from $2,458 to $7,500.
Continue to: Strengths and limitations...
Strengths and limitations
Twenty-three percent of the patients who were sequenced had an increased fetal nuchal translucency or cystic hygroma, and another 17% had a single fetal effusion. This inclusivity makes this study more applicable to broader fetal anomaly populations. However, it is worth noting that only 61% of patients had NIHF by the definition of 2 or more fluid collections or skin thickening.
The authors assumed 100% sensitivity and specificity for the panel tests relative to diagnostic ES results, but this may not reflect real-life analysis. There is inherent subjectivity and subsequent differences in variant calling (deciding which genetic changes are pathogenic) between institutions and companies despite efforts to standardize this process. Due to the simulated nature of this study, these differences are not captured. Additionally, although the authors note that the research ES had at least 30 times the coverage (an adequate number of sequence reads for accurate testing) than did the commercial lab panels, some gene panels have additional sequencing of intronic regions, copy number analysis, and up to 10 times more coverage than ES, which could lead to more diagnoses.
This study illustrates that there is nuance involved in selecting which type of gene sequencing and which clinical laboratory to use for prenatal diagnosis. Labs with more updated literature searches and more inclusive gene panels may be excellent options for patients in whom ES is not covered by insurance or with phenotypes with a narrow range of suspected causative genes. However, there is a lag time in updating the genes offered on each panel, and new genedisease associations will not be captured by existing panels.
From a cost, speed-of-analysis, and depth-of-sequencing perspective, panel sequencing can have advantages that should be considered in some patients, particularly if the panels are large and regularly updated. However, the authors summarize our sentiments and their findings with the following:
“For disorders, such as NIHF with marked genetic heterogeneity and less clear in utero phenotypes of underlying genetic diseases, the broader coverage of exome sequencing makes it a superior option to targeted panel testing.”
We look forward to the publication of further anomaly-specific cohorts and secondary analyses of the utility of current panels and ES that may follow.
Frequency of Beckwith-Widemann syndrome in prenatally diagnosed omphaloceles
Abbasi N, Moore A, Chiu P, et al. Prenatally diagnosed omphaloceles: report of 92 cases and association with Beckwith-Wiedemann syndrome. Prenat Diagn. 2021;41:798-816. doi:10.1002/pd.5930.
An omphalocele is diagnosed prenatally on ultrasound when an anterior midline mass, often containing abdominal contents, is seen herniating into the base of the umbilical cord. Omphaloceles are often associated with additional structural abnormalities and underlying genetic syndromes, thus a thorough fetal assessment is required for accurate prenatal counseling and neonatal care.
Identification of Beckwith-Widemann syndrome (BWS) in the setting of a prenatally diagnosed omphalocele is difficult because of its wide range of clinical features and its unique genetic basis. Unlike many genetic disorders that are caused by specific genetic variants, or spelling changes in the genes, BWS results from a change in the expression of one or more of the genes in a specific region of chromosome 11. A high index of clinical suspicion as well as an understanding of the various genetic and epigenetics alterations that cause BWS is required for prenatal diagnosis.
Retrospective cohort at a single center
The authors in this study reviewed all pregnancies in which an omphalocele was diagnosed prenatally at a single center between 2010 and 2015. They describe a standard prenatal evaluation following identification of an omphalocele including echocardiogram, detailed anatomic survey, and availability of an amniocentesis to facilitate aneuploidy screening and testing for BWS. This review also includes an overview of perinatal and long-term outcomes for cases of BWS diagnosed at their center between 2000 and 2015.
Study outcomes
Results of prenatal genetic testing in this cohort were divided between cases of an isolated omphalocele (without other structural changes) and cases of nonisolated omphaloceles. In the group of pregnancies with an isolated omphalocele, 2 of 27 pregnancies (7.4%) were found to have an abnormal karyotype, and 6 of 16 of the remaining pregnancies (37.5%) were diagnosed with BWS. Among the group of pregnancies with a nonisolated omphalocele, 23 of 59 pregnancies (39%) were found to have an abnormal karyotype, and 1 of 20 pregnancies (5%) were diagnosed with BWS.
Prenatal sonographic features associated with cases of BWS included polyhydramnios in 12 of 19 cases (63%) and macrosomia in 8 of 19 cases (42%). Macroglossia is another characteristic feature of the disorder, which was identified in 4 of 19 cases (21%) prenatally and in an additional 10 of 19 cases (52.6%) postnatally. Interestingly, only 1 of the cases of BWS was caused by a microdeletion at 11p15.4—a change that was identified on microarray. The additional 6 cases of BWS were caused by imprinting changes in the region, which are only detectable with a specific methylation-analysis technique.
Among the 19 cases of BWS identified over a 15-year period, there was 1 intrauterine demise. Preterm birth occurred in 10 of 19 cases (52.6%), including 8 of 19 cases (42.1%) of spontaneous preterm labor. Respiratory distress (27.8%), hypoglycemia (61%), and gastrointestinal reflux (59%) were common neonatal complications. Embryonal tumors were diagnosed in 2 of 16 patients (12.5%). Although neurodevelopmental outcomes were incomplete, their data suggested normal development in 75% of children. There were 2 neonatal deaths in this cohort and 1 childhood death at age 2 years.
Study strengths and limitations
As with many studies investigating a rare disorder, this study is limited by its retrospective nature and small sample size. Nevertheless, it adds an important cohort of patients with a prenatally diagnosed omphalocele to the literature and illuminates the utility of a standardized approach to testing for BWS in this population.
In this cohort with prenatally diagnosed omphaloceles with standardized testing for BWS, the prevalence of the disorder was approximately 8% and more common in cases of an isolated omphalocele. The most common supporting sonographic features of BWS may not be detected until later in gestation, including polyhydramnios and macrosomia. This demonstrates the importance of both sonographic follow-up as well as universal testing for BWS in euploid cases of a prenatally diagnosed omphalocele. Almost all cases of BWS in this cohort required specialized molecular techniques for diagnosis, and the diagnosis would have been missed on karyotype, microarray, and ES.
Continue to: Genetic diagnoses that could have been identified by expanded carrier screening...
Genetic diagnoses that could have been identified by expanded carrier screening
Stevens BK, Nunley PB, Wagner C, et al. Utility of expanded carrier screening in pregnancies with ultrasound abnormalities. Prenat Diagn. 2022;42:60-78. doi:10.1002/pd.6069.
This series is a thorough retrospective review of patients evaluated in a pediatric genetics clinic from 2014 through 2017. Patients were included if they were evaluated in the first 6 months of life and had a structural abnormality that might be detected on prenatal ultrasonography. The genetic testing results were analyzed and categorized according to types of genetic disorders, with the goal of identifying how many patients might have been identified by expanded carrier screening (ECS) panels.
Study outcomes
A total of 931 charts were reviewed, and 85% (791 of 931) of patients evaluated in the first 6 months of life were determined to have a structural anomaly that might be detected on prenatal ultrasonography. Of those patients, 691 went on to have genetic testing and 32.1% (222 of 691) of them had a diagnostic (pathogenic) genetic testing result related to the phenotype. The types of diagnostic testing results are shown in the FIGURE. Notably, 42 single-gene disorders were detected.
FIGURE Diagnostic test result in pediatric patients evaluated under age 6 months

Of those 222 patients with diagnostic results, there were 8 patients with autosomal recessive and X-linked conditions that could be detected using a 500-gene ECS panel. Five patients could be detected with a 271-gene panel. After nondiagnostic microarray, 11.3% of patients had a condition that could be detected by using a 500-gene ECS panel. The identified conditions included cystic fibrosis, CYP21‐related congenital adrenal hyperplasia, autosomal recessive polycystic kidney disease, Antley‐Bixler syndrome, and Morquio syndrome type A.
Furthermore, the authors conducted a literature review of 271 conditions and found that 32% (88 of 271) of conditions may be associated with ultrasound findings.
Study strengths and limitations
When applying these data to prenatal populations, the authors acknowledge several notable limitations. There is a selection bias toward less-severe phenotypes for many patients choosing to continue rather than to interrupt a pregnancy. Additionally, only 23% of the patients in the study had a microarray and ES, which may lead to an underrepresentation of single-gene disorders and an underestimation of the utility of ECS. Finally, a retrospective classification of structural abnormalities that may be detectable by ultrasonography may not always reflect what is actually reported in prenatal imaging.
However, the work that the authors put forth to evaluate and categorize 931 participants by the results of genetic testing and structural anomalies is appreciated, and the level of detail is impressive for this retrospective chart review. Additionally, the tables itemizing the authors’ review of 271 ECS disorders that may have ultrasonography findings categorized by disorder and system are helpful and quick diagnostic references for clinicians providing prenatal care. ●
This study of potentially detectable prenatal findings from the lens of a pediatric genetics clinic lends an interesting perspective: Exome sequencing is not the primary route to establish a diagnosis; karyotype, microarray, methylation disorders, and triplet repeat disorders all have an established role in the diagnostic toolkit. Keeping in mind the contribution of these modalities to pediatric testing may shorten the diagnostic odyssey to continue pregnancies or help to fully counsel patients on expectations and decision-making after birth.
Carrier screening is not a substitute for diagnostic testing in pregnancy. However, in appropriately selected patients, a broad carrier screening panel may have added utility. ECS can be conducted while awaiting microarray results to help target testing and may be particularly useful for patients who decline diagnostic testing until the postnatal period. It is important to counsel patients that carrier screening is not a diagnostic test, and results will only report likely pathogenic or pathogenic variants, not variants of uncertain significance that may be of clinical relevance. However, our practice has had several insightful diagnoses reached through ECS, in conjunction with microarray testing that allowed for faster and more targeted sequencing and precise fetal diagnosis. This readily available molecular tool (often covered by insurance) deserves a spot in your fetal diagnosis tool belt based on available evidence.
Last year, our Update focused on the expansion of sequencing in prenatal diagnosis. This year, we are taking a step sideways to remember the many diagnoses we may miss if we rely on exome sequencing alone. A recent case report in Prenatal Diagnosis describes a pregnancy affected by fetal akinesia sequence and polyhydramnios in which sequencing did not reveal a diagnosis. Expansion of the differential to include congenital myotonic dystrophy and subsequent triplet repeat testing led the clinicians to the diagnosis and identification of a triplet repeat expansion in the DMPK gene. This case serves as our first example of how complementary testing and technologies should continue to help us make critical diagnoses.
What is the yield of exome sequencing vs panels in nonimmune hydrops?
Rogers R, Moyer K, Moise KJ Jr. Congenital myotonic dystrophy: an overlooked diagnosis not amenable to detection by sequencing. Prenat Diagn. 2022;42:233-235. doi:10.1002/pd.6105.
Norton ME, Ziffle JV, Lianoglou BR, et al. Exome sequencing vs targeted gene panels for the evaluation of nonimmune hydrops fetalis. Am J Obstet Gynecol. 2021;28:S0002-9378(21)00828-0. doi:10.1016/j.ajog.2021.07.014.
We have had several illuminating discussions with our colleagues about the merits of exome sequencing (ES) versus panels and other modalities for fetal diagnosis. Many obstetricians practicing at the leading edge may feel like ES should be utilized uniformly for fetal anomalies with nondiagnostic karyotype or microarray. However, for well-defined phenotypes with clear and narrow lists of implicated genes (eg, skeletal dysplasias) or patients without insurance coverage, panel sequencing still has utility in prenatal diagnosis. The question of which phenotypes most benefit from ES versus panel sequencing is an area of interesting, ongoing research for several investigators.
Secondary analysis of nonimmune hydrops cohort
Norton and colleagues tackled one such cohort in a study presented in the American Journal of Obstetrics and Gynecology. They compared the proportion of diagnoses that would have been identified in commercial lab panels with their research of phenotype-driven ES in a cohort of 127 fetuses with features of nonimmune hydrops fetalis (NIHF). NIHF can be caused by a variety of single-gene disorders in addition to chromosomal disorders and copy number variants on chromosomal microarray. Patients were eligible for inclusion in the cohort if they had a nondiagnostic karyotype or microarray and any of the following features: nuchal translucency of 3.5 mm or greater, cystic hygroma, pleural effusion, pericardial effusion, ascites, or skin edema. Standard sequencing methods and variant analysis were performed. They assumed 100% analytical sensitivity and specificity of the panels for variant detection and collected cost information on the targeted gene panels.
Study outcomes
In the ES analysis of cases, 37 of 127 cases (29%) had a pathogenic or likely pathogenic variant in 1 of 29 genes, and another 12 of 127 cases (9%) had variants of uncertain significance that were strongly suspected to be the etiology during clinical analysis. The types of disorders that were identified are listed in the TABLE. In addition to a feature of NIHF, 50% of the cases had a structural anomaly.
There were 10 identified clinical panels from 7 clinical laboratories. These panels ranged in size from 11 to 128 genes. The highest simulated yield of any commercial panel was only 62% of the pathogenic variants identified by ES. The other commercial laboratory panels detection yield ranged from 11% to 62% of pathogenic variants detected by ES. For overall yield, the largest panel would have a diagnostic yield of 18% of diagnoses relative to the 29% diagnostic yield from ES.
The largest panel included 128 genes prior to the publication of the original cohort and was updated after publication to include 148 genes. The larger updated panel would have identified all of the patients in the ES cohort. However, many of the other panels listed would have identified a smaller fraction of the variants identified by ES (range, 11%-62%). At the time of publication, the cost of the panels ranged from $640 to $3,500, and the cost of prenatal ES ranged from $2,458 to $7,500.
Continue to: Strengths and limitations...
Strengths and limitations
Twenty-three percent of the patients who were sequenced had an increased fetal nuchal translucency or cystic hygroma, and another 17% had a single fetal effusion. This inclusivity makes this study more applicable to broader fetal anomaly populations. However, it is worth noting that only 61% of patients had NIHF by the definition of 2 or more fluid collections or skin thickening.
The authors assumed 100% sensitivity and specificity for the panel tests relative to diagnostic ES results, but this may not reflect real-life analysis. There is inherent subjectivity and subsequent differences in variant calling (deciding which genetic changes are pathogenic) between institutions and companies despite efforts to standardize this process. Due to the simulated nature of this study, these differences are not captured. Additionally, although the authors note that the research ES had at least 30 times the coverage (an adequate number of sequence reads for accurate testing) than did the commercial lab panels, some gene panels have additional sequencing of intronic regions, copy number analysis, and up to 10 times more coverage than ES, which could lead to more diagnoses.
This study illustrates that there is nuance involved in selecting which type of gene sequencing and which clinical laboratory to use for prenatal diagnosis. Labs with more updated literature searches and more inclusive gene panels may be excellent options for patients in whom ES is not covered by insurance or with phenotypes with a narrow range of suspected causative genes. However, there is a lag time in updating the genes offered on each panel, and new genedisease associations will not be captured by existing panels.
From a cost, speed-of-analysis, and depth-of-sequencing perspective, panel sequencing can have advantages that should be considered in some patients, particularly if the panels are large and regularly updated. However, the authors summarize our sentiments and their findings with the following:
“For disorders, such as NIHF with marked genetic heterogeneity and less clear in utero phenotypes of underlying genetic diseases, the broader coverage of exome sequencing makes it a superior option to targeted panel testing.”
We look forward to the publication of further anomaly-specific cohorts and secondary analyses of the utility of current panels and ES that may follow.
Frequency of Beckwith-Widemann syndrome in prenatally diagnosed omphaloceles
Abbasi N, Moore A, Chiu P, et al. Prenatally diagnosed omphaloceles: report of 92 cases and association with Beckwith-Wiedemann syndrome. Prenat Diagn. 2021;41:798-816. doi:10.1002/pd.5930.
An omphalocele is diagnosed prenatally on ultrasound when an anterior midline mass, often containing abdominal contents, is seen herniating into the base of the umbilical cord. Omphaloceles are often associated with additional structural abnormalities and underlying genetic syndromes, thus a thorough fetal assessment is required for accurate prenatal counseling and neonatal care.
Identification of Beckwith-Widemann syndrome (BWS) in the setting of a prenatally diagnosed omphalocele is difficult because of its wide range of clinical features and its unique genetic basis. Unlike many genetic disorders that are caused by specific genetic variants, or spelling changes in the genes, BWS results from a change in the expression of one or more of the genes in a specific region of chromosome 11. A high index of clinical suspicion as well as an understanding of the various genetic and epigenetics alterations that cause BWS is required for prenatal diagnosis.
Retrospective cohort at a single center
The authors in this study reviewed all pregnancies in which an omphalocele was diagnosed prenatally at a single center between 2010 and 2015. They describe a standard prenatal evaluation following identification of an omphalocele including echocardiogram, detailed anatomic survey, and availability of an amniocentesis to facilitate aneuploidy screening and testing for BWS. This review also includes an overview of perinatal and long-term outcomes for cases of BWS diagnosed at their center between 2000 and 2015.
Study outcomes
Results of prenatal genetic testing in this cohort were divided between cases of an isolated omphalocele (without other structural changes) and cases of nonisolated omphaloceles. In the group of pregnancies with an isolated omphalocele, 2 of 27 pregnancies (7.4%) were found to have an abnormal karyotype, and 6 of 16 of the remaining pregnancies (37.5%) were diagnosed with BWS. Among the group of pregnancies with a nonisolated omphalocele, 23 of 59 pregnancies (39%) were found to have an abnormal karyotype, and 1 of 20 pregnancies (5%) were diagnosed with BWS.
Prenatal sonographic features associated with cases of BWS included polyhydramnios in 12 of 19 cases (63%) and macrosomia in 8 of 19 cases (42%). Macroglossia is another characteristic feature of the disorder, which was identified in 4 of 19 cases (21%) prenatally and in an additional 10 of 19 cases (52.6%) postnatally. Interestingly, only 1 of the cases of BWS was caused by a microdeletion at 11p15.4—a change that was identified on microarray. The additional 6 cases of BWS were caused by imprinting changes in the region, which are only detectable with a specific methylation-analysis technique.
Among the 19 cases of BWS identified over a 15-year period, there was 1 intrauterine demise. Preterm birth occurred in 10 of 19 cases (52.6%), including 8 of 19 cases (42.1%) of spontaneous preterm labor. Respiratory distress (27.8%), hypoglycemia (61%), and gastrointestinal reflux (59%) were common neonatal complications. Embryonal tumors were diagnosed in 2 of 16 patients (12.5%). Although neurodevelopmental outcomes were incomplete, their data suggested normal development in 75% of children. There were 2 neonatal deaths in this cohort and 1 childhood death at age 2 years.
Study strengths and limitations
As with many studies investigating a rare disorder, this study is limited by its retrospective nature and small sample size. Nevertheless, it adds an important cohort of patients with a prenatally diagnosed omphalocele to the literature and illuminates the utility of a standardized approach to testing for BWS in this population.
In this cohort with prenatally diagnosed omphaloceles with standardized testing for BWS, the prevalence of the disorder was approximately 8% and more common in cases of an isolated omphalocele. The most common supporting sonographic features of BWS may not be detected until later in gestation, including polyhydramnios and macrosomia. This demonstrates the importance of both sonographic follow-up as well as universal testing for BWS in euploid cases of a prenatally diagnosed omphalocele. Almost all cases of BWS in this cohort required specialized molecular techniques for diagnosis, and the diagnosis would have been missed on karyotype, microarray, and ES.
Continue to: Genetic diagnoses that could have been identified by expanded carrier screening...
Genetic diagnoses that could have been identified by expanded carrier screening
Stevens BK, Nunley PB, Wagner C, et al. Utility of expanded carrier screening in pregnancies with ultrasound abnormalities. Prenat Diagn. 2022;42:60-78. doi:10.1002/pd.6069.
This series is a thorough retrospective review of patients evaluated in a pediatric genetics clinic from 2014 through 2017. Patients were included if they were evaluated in the first 6 months of life and had a structural abnormality that might be detected on prenatal ultrasonography. The genetic testing results were analyzed and categorized according to types of genetic disorders, with the goal of identifying how many patients might have been identified by expanded carrier screening (ECS) panels.
Study outcomes
A total of 931 charts were reviewed, and 85% (791 of 931) of patients evaluated in the first 6 months of life were determined to have a structural anomaly that might be detected on prenatal ultrasonography. Of those patients, 691 went on to have genetic testing and 32.1% (222 of 691) of them had a diagnostic (pathogenic) genetic testing result related to the phenotype. The types of diagnostic testing results are shown in the FIGURE. Notably, 42 single-gene disorders were detected.
FIGURE Diagnostic test result in pediatric patients evaluated under age 6 months

Of those 222 patients with diagnostic results, there were 8 patients with autosomal recessive and X-linked conditions that could be detected using a 500-gene ECS panel. Five patients could be detected with a 271-gene panel. After nondiagnostic microarray, 11.3% of patients had a condition that could be detected by using a 500-gene ECS panel. The identified conditions included cystic fibrosis, CYP21‐related congenital adrenal hyperplasia, autosomal recessive polycystic kidney disease, Antley‐Bixler syndrome, and Morquio syndrome type A.
Furthermore, the authors conducted a literature review of 271 conditions and found that 32% (88 of 271) of conditions may be associated with ultrasound findings.
Study strengths and limitations
When applying these data to prenatal populations, the authors acknowledge several notable limitations. There is a selection bias toward less-severe phenotypes for many patients choosing to continue rather than to interrupt a pregnancy. Additionally, only 23% of the patients in the study had a microarray and ES, which may lead to an underrepresentation of single-gene disorders and an underestimation of the utility of ECS. Finally, a retrospective classification of structural abnormalities that may be detectable by ultrasonography may not always reflect what is actually reported in prenatal imaging.
However, the work that the authors put forth to evaluate and categorize 931 participants by the results of genetic testing and structural anomalies is appreciated, and the level of detail is impressive for this retrospective chart review. Additionally, the tables itemizing the authors’ review of 271 ECS disorders that may have ultrasonography findings categorized by disorder and system are helpful and quick diagnostic references for clinicians providing prenatal care. ●
This study of potentially detectable prenatal findings from the lens of a pediatric genetics clinic lends an interesting perspective: Exome sequencing is not the primary route to establish a diagnosis; karyotype, microarray, methylation disorders, and triplet repeat disorders all have an established role in the diagnostic toolkit. Keeping in mind the contribution of these modalities to pediatric testing may shorten the diagnostic odyssey to continue pregnancies or help to fully counsel patients on expectations and decision-making after birth.
Carrier screening is not a substitute for diagnostic testing in pregnancy. However, in appropriately selected patients, a broad carrier screening panel may have added utility. ECS can be conducted while awaiting microarray results to help target testing and may be particularly useful for patients who decline diagnostic testing until the postnatal period. It is important to counsel patients that carrier screening is not a diagnostic test, and results will only report likely pathogenic or pathogenic variants, not variants of uncertain significance that may be of clinical relevance. However, our practice has had several insightful diagnoses reached through ECS, in conjunction with microarray testing that allowed for faster and more targeted sequencing and precise fetal diagnosis. This readily available molecular tool (often covered by insurance) deserves a spot in your fetal diagnosis tool belt based on available evidence.