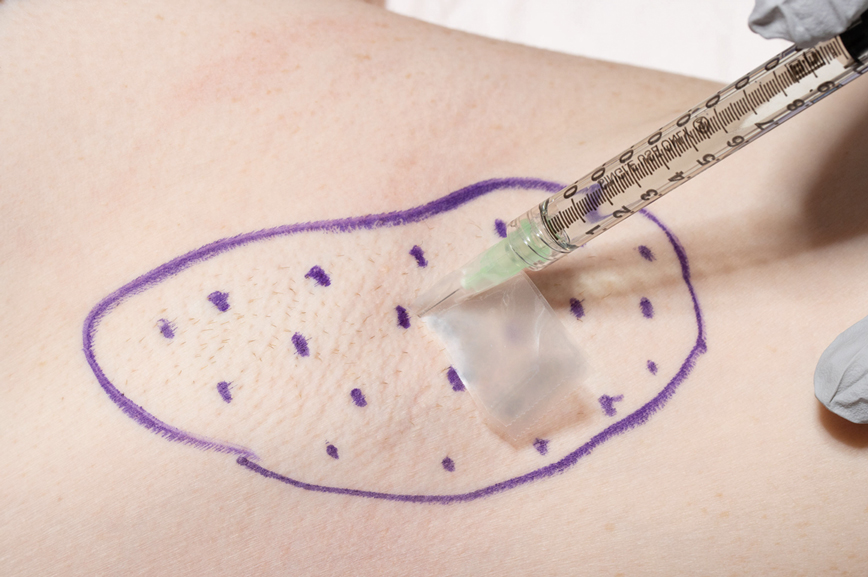
User login
‘Sit less, move more’ to reduce stroke risk
in a population-based study of middle aged and older adults.
The study also found relatively short periods of moderate to vigorous exercise were associated with reduced stroke risk.
“Our results suggest there are a number of ways to reduce stroke risk simply by moving about,” said lead author Steven P. Hooker, PhD, San Diego State University. “This could be with short periods of moderate to vigorous activity each day, longer periods of light activity, or just sedentary for shorter periods of time. All these things can make a difference.”
Dr. Hooker explained that, while it has been found previously that moderate to vigorous exercise reduces stroke risk, this study gives more information on light-intensity activities and sedentary behavior and the risk of stroke.
“Our results suggest that you don’t have to be a chronic exerciser to reduce stroke risk. Replacing sedentary time with light-intensity activity will be beneficial. Just go for a short walk, get up from your desk and move around the house at regular intervals. That can help to reduce stroke risk,” Dr. Hooker said.
“Our message is basically to sit less and move more,” he added.
The study was published online in JAMA Network Open.
The study involved 7,607 U.S. individuals without a history of stroke, with oversampling from the southeastern “Stroke Belt,” who were participating in the REGARDS cohort study.
The participants wore an accelerometer to measure physical activity and sedentary behavior for 7 consecutive days. The mean age of the individuals was 63 years; 54% were female, 32% were Black.
Over a mean follow-up of 7.4 years, 286 incident stroke cases occurred.
Results showed that increased levels of physical activity were associated with reduced risk of stroke.
For moderate to vigorous activity, compared with participants in the lowest tertile, those in the highest tertile of total daily time in moderate to vigorous activity had a 43% lower risk of stroke.
In the current study, the amount of moderate to vigorous activity associated with a significant reduction in stroke risk was approximately 25 minutes per day (3 hours per week).
Dr. Hooker noted that moderate to vigorous activity included things such as brisk walking, jogging, bike riding, swimming, or playing tennis or soccer. “Doing such activities for just 25 minutes per day reduced risk of stroke by 43%. This is very doable. Just commuting to work by bicycle would cover you here,” he said.
In terms of light-intensity activity, individuals who did 4-5 hours of light activities each day had a 26% reduced risk for first stroke, compared with those doing less than 3 hours of such light activities.
Dr. Hooker explained that examples of light activity included household chores, such as vacuuming, washing dishes, or going for a gentle stroll. “These activities do not require heaving breathing, increased heart rate or breaking into a sweat. They are activities of daily living and relatively easy to engage in.”
But he pointed out that the 4-5 hours of light activity every day linked to a reduction in stroke risk may be more difficult to achieve than the 25 minutes of moderate to vigorous activity, saying: “You have to have some intentionality here.”
Long bouts of sedentary time are harmful
The study also showed that sedentary time was associated with a higher risk for stroke.
The authors noted that time spent in sedentary behavior is of interest because most adults spend most of their awake time being physically inactive.
They report that participants in the highest tertile of sedentary time (more than 13 hours/day) exhibited a 44% increase in risk of stroke, compared with those in the lowest tertile (less than 11 hours/day), and the association remained significant when adjusted for several covariates, including moderate to vigorous activity.
“Even when controlling for the amount of other physical activity, sedentary behavior is still highly associated with risk of stroke. So even if you are active, long bouts of sedentary behavior are harmful,” Dr. Hooker commented.
They also found that longer bouts of sedentary time (more than 17 minutes at a time) were associated with a 54% higher risk of stroke than shorter bouts (less than 8 minutes).
“This suggests that breaking up periods of sedentary behavior into shorter bouts would be beneficial,” Dr. Hooker said.
“If you are going to spend the evening on the couch watching television, try to stand up and walk around every few minutes. Same for if you are sitting at a computer all day – try having a standing workstation, or at least take regular breaks to walk around,” he added.
This research was supported by grants from the National Institute of Neurological Disorders and Stroke and the National Institute on Aging. Additional funding was provided by an unrestricted grant from the Coca-Cola Company. The authors report no disclosures.
A version of this article first appeared on Medscape.com.
in a population-based study of middle aged and older adults.
The study also found relatively short periods of moderate to vigorous exercise were associated with reduced stroke risk.
“Our results suggest there are a number of ways to reduce stroke risk simply by moving about,” said lead author Steven P. Hooker, PhD, San Diego State University. “This could be with short periods of moderate to vigorous activity each day, longer periods of light activity, or just sedentary for shorter periods of time. All these things can make a difference.”
Dr. Hooker explained that, while it has been found previously that moderate to vigorous exercise reduces stroke risk, this study gives more information on light-intensity activities and sedentary behavior and the risk of stroke.
“Our results suggest that you don’t have to be a chronic exerciser to reduce stroke risk. Replacing sedentary time with light-intensity activity will be beneficial. Just go for a short walk, get up from your desk and move around the house at regular intervals. That can help to reduce stroke risk,” Dr. Hooker said.
“Our message is basically to sit less and move more,” he added.
The study was published online in JAMA Network Open.
The study involved 7,607 U.S. individuals without a history of stroke, with oversampling from the southeastern “Stroke Belt,” who were participating in the REGARDS cohort study.
The participants wore an accelerometer to measure physical activity and sedentary behavior for 7 consecutive days. The mean age of the individuals was 63 years; 54% were female, 32% were Black.
Over a mean follow-up of 7.4 years, 286 incident stroke cases occurred.
Results showed that increased levels of physical activity were associated with reduced risk of stroke.
For moderate to vigorous activity, compared with participants in the lowest tertile, those in the highest tertile of total daily time in moderate to vigorous activity had a 43% lower risk of stroke.
In the current study, the amount of moderate to vigorous activity associated with a significant reduction in stroke risk was approximately 25 minutes per day (3 hours per week).
Dr. Hooker noted that moderate to vigorous activity included things such as brisk walking, jogging, bike riding, swimming, or playing tennis or soccer. “Doing such activities for just 25 minutes per day reduced risk of stroke by 43%. This is very doable. Just commuting to work by bicycle would cover you here,” he said.
In terms of light-intensity activity, individuals who did 4-5 hours of light activities each day had a 26% reduced risk for first stroke, compared with those doing less than 3 hours of such light activities.
Dr. Hooker explained that examples of light activity included household chores, such as vacuuming, washing dishes, or going for a gentle stroll. “These activities do not require heaving breathing, increased heart rate or breaking into a sweat. They are activities of daily living and relatively easy to engage in.”
But he pointed out that the 4-5 hours of light activity every day linked to a reduction in stroke risk may be more difficult to achieve than the 25 minutes of moderate to vigorous activity, saying: “You have to have some intentionality here.”
Long bouts of sedentary time are harmful
The study also showed that sedentary time was associated with a higher risk for stroke.
The authors noted that time spent in sedentary behavior is of interest because most adults spend most of their awake time being physically inactive.
They report that participants in the highest tertile of sedentary time (more than 13 hours/day) exhibited a 44% increase in risk of stroke, compared with those in the lowest tertile (less than 11 hours/day), and the association remained significant when adjusted for several covariates, including moderate to vigorous activity.
“Even when controlling for the amount of other physical activity, sedentary behavior is still highly associated with risk of stroke. So even if you are active, long bouts of sedentary behavior are harmful,” Dr. Hooker commented.
They also found that longer bouts of sedentary time (more than 17 minutes at a time) were associated with a 54% higher risk of stroke than shorter bouts (less than 8 minutes).
“This suggests that breaking up periods of sedentary behavior into shorter bouts would be beneficial,” Dr. Hooker said.
“If you are going to spend the evening on the couch watching television, try to stand up and walk around every few minutes. Same for if you are sitting at a computer all day – try having a standing workstation, or at least take regular breaks to walk around,” he added.
This research was supported by grants from the National Institute of Neurological Disorders and Stroke and the National Institute on Aging. Additional funding was provided by an unrestricted grant from the Coca-Cola Company. The authors report no disclosures.
A version of this article first appeared on Medscape.com.
in a population-based study of middle aged and older adults.
The study also found relatively short periods of moderate to vigorous exercise were associated with reduced stroke risk.
“Our results suggest there are a number of ways to reduce stroke risk simply by moving about,” said lead author Steven P. Hooker, PhD, San Diego State University. “This could be with short periods of moderate to vigorous activity each day, longer periods of light activity, or just sedentary for shorter periods of time. All these things can make a difference.”
Dr. Hooker explained that, while it has been found previously that moderate to vigorous exercise reduces stroke risk, this study gives more information on light-intensity activities and sedentary behavior and the risk of stroke.
“Our results suggest that you don’t have to be a chronic exerciser to reduce stroke risk. Replacing sedentary time with light-intensity activity will be beneficial. Just go for a short walk, get up from your desk and move around the house at regular intervals. That can help to reduce stroke risk,” Dr. Hooker said.
“Our message is basically to sit less and move more,” he added.
The study was published online in JAMA Network Open.
The study involved 7,607 U.S. individuals without a history of stroke, with oversampling from the southeastern “Stroke Belt,” who were participating in the REGARDS cohort study.
The participants wore an accelerometer to measure physical activity and sedentary behavior for 7 consecutive days. The mean age of the individuals was 63 years; 54% were female, 32% were Black.
Over a mean follow-up of 7.4 years, 286 incident stroke cases occurred.
Results showed that increased levels of physical activity were associated with reduced risk of stroke.
For moderate to vigorous activity, compared with participants in the lowest tertile, those in the highest tertile of total daily time in moderate to vigorous activity had a 43% lower risk of stroke.
In the current study, the amount of moderate to vigorous activity associated with a significant reduction in stroke risk was approximately 25 minutes per day (3 hours per week).
Dr. Hooker noted that moderate to vigorous activity included things such as brisk walking, jogging, bike riding, swimming, or playing tennis or soccer. “Doing such activities for just 25 minutes per day reduced risk of stroke by 43%. This is very doable. Just commuting to work by bicycle would cover you here,” he said.
In terms of light-intensity activity, individuals who did 4-5 hours of light activities each day had a 26% reduced risk for first stroke, compared with those doing less than 3 hours of such light activities.
Dr. Hooker explained that examples of light activity included household chores, such as vacuuming, washing dishes, or going for a gentle stroll. “These activities do not require heaving breathing, increased heart rate or breaking into a sweat. They are activities of daily living and relatively easy to engage in.”
But he pointed out that the 4-5 hours of light activity every day linked to a reduction in stroke risk may be more difficult to achieve than the 25 minutes of moderate to vigorous activity, saying: “You have to have some intentionality here.”
Long bouts of sedentary time are harmful
The study also showed that sedentary time was associated with a higher risk for stroke.
The authors noted that time spent in sedentary behavior is of interest because most adults spend most of their awake time being physically inactive.
They report that participants in the highest tertile of sedentary time (more than 13 hours/day) exhibited a 44% increase in risk of stroke, compared with those in the lowest tertile (less than 11 hours/day), and the association remained significant when adjusted for several covariates, including moderate to vigorous activity.
“Even when controlling for the amount of other physical activity, sedentary behavior is still highly associated with risk of stroke. So even if you are active, long bouts of sedentary behavior are harmful,” Dr. Hooker commented.
They also found that longer bouts of sedentary time (more than 17 minutes at a time) were associated with a 54% higher risk of stroke than shorter bouts (less than 8 minutes).
“This suggests that breaking up periods of sedentary behavior into shorter bouts would be beneficial,” Dr. Hooker said.
“If you are going to spend the evening on the couch watching television, try to stand up and walk around every few minutes. Same for if you are sitting at a computer all day – try having a standing workstation, or at least take regular breaks to walk around,” he added.
This research was supported by grants from the National Institute of Neurological Disorders and Stroke and the National Institute on Aging. Additional funding was provided by an unrestricted grant from the Coca-Cola Company. The authors report no disclosures.
A version of this article first appeared on Medscape.com.
FROM JAMA NETWORK OPEN
At-home colorectal cancer testing and follow-up vary by ethnicity
Doctors were significantly less likely to order colorectal cancer screening with the at-home test Cologuard (Exact Sciences) for Black patients and were more likely to order the test for Asian patients, new evidence reveals.
Investigators retrospectively studied 557,156 patients in the Mayo Clinic health system from 2012 to 2022. They found that Cologuard was ordered for 8.7% of Black patients, compared to 11.9% of White patients and 13.1% of Asian patients.
Both minority groups were less likely than White patients to undergo a follow-up colonoscopy within 1 year of Cologuard testing. Cologuard tests the stool for blood and DNA markers associated with colorectal cancer.
Although the researchers did not examine the reasons driving the disparities, lead investigator Ahmed Ouni, MD, told this news organization that “it could be patient preferences ... or there could be some bias as providers ourselves in how we present the data to patients.”
Dr. Ouni presented the findings on May 22 at the annual Digestive Disease Week® (DDW), held in person in San Diego and virtually.
Breakdown by physician specialty
“We looked at the specialty of physicians ordering these because we wanted to see where the disparity was coming from, if there was a disparity,” said Dr. Ouni, a gastroenterologist at Mayo Clinic, Jacksonville, Florida.
Just over half (51%) of the patients received care from family medicine physicians, 27% received care from internists, and 22% were seen by gastroenterologists.
Family physicians ordered Cologuard testing for 8.7% of Black patients, compared with 16.1% of White patients, a significant difference (P < .001). Internists ordered the test for 10.5% of Black patients and 11.1% of White patients (P < .001). Gastroenterologists ordered Cologuard screening for 2.4% of Black patients and 3.2% of White patients (P = .009).
Gastroenterologists were 47% more likely to order Cologuard for Asian patients, and internists were 16% more likely to order it for this population than for White patients. However, the findings were not statistically significant for the overall cohort of Asian patients when the researchers adjusted for age and sex (P = 0.52).
Black patients were 25% less likely to have a follow-up colonoscopy within 1 year of undergoing a Cologuard test (odds ratio, 0.75; 95% confidence interval, 0.60-0.94), and Asian patients were 35% less likely (OR, 0.65; 95% CI, 0.52-0.82).
Ongoing and future research
Of the total study population, only 2.9% self-identified as Black; according to the 2020 U.S. Census, 12.4% of the population of the United States are Black persons.
When asked about the relatively low proportion of Black persons in the study, Dr. Ouni replied that the investigators are partnering with a Black physician group in the Jacksonville, Fla., area to expand the study to a more diverse population.
Additional plans include assessing how many positive Cologuard test results led to follow-up colonoscopies.
The investigators are also working with family physicians at the Mayo Clinic to examine how physicians explain colorectal cancer screening options to patients and are studying patient preferences regarding screening options, which include Cologuard, fecal immunochemical test (FIT)/fecal occult blood testing, CT colonography, and colonoscopy.
“We’re analyzing the data by ZIP code to see if this could be related to finances,” Dr. Ouni added. “So, if you’re Black or White and more financially impoverished, how does that affect how you view Cologuard and colorectal cancer screening?”
Some unanswered questions
“Overall this study supports other studies of a disparity in colorectal cancer screening for African Americans,” John M. Carethers, MD, told this news organization when asked to comment. “This is known for FIT and colonoscopy, and Cologuard, which is a genetic test in addition to FIT, appears to be in that same realm.”
“Noninvasive tests will have a role to reach populations who may not readily have access to colonoscopy,” said Dr. Carethers, John G. Searle Professor and chair of the department of internal medicine and professor of human genetics at the University of Michigan, Ann Arbor. “The key here is if the test is positive, it needs to be followed up with a colonoscopy.”
Dr. Carethers added that the study raises some unanswered questions; for example, does the cost difference between testing options make a difference?
“FIT is under $20, but Cologuard is generally $300 or more,” he said. What percentage of the study population were offered other options, such as FIT? How does insurance status affect screening in different populations?”
“The findings should be taken in context of what other screening options were offered to or elected by patients,” agreed Gregory S. Cooper, MD, professor of medicine and population and quantitative health sciences at Case Western Reserve University and a gastroenterologist at University Hospitals Cleveland Medical Center.
According to guidelines, patients can be offered a menu of options, including FIT, colonoscopy, and Cologuard, Dr. Cooper said in an interview.
“If more African Americans elected colonoscopy, for example, the findings may balance out,” said Dr. Cooper, who was not affiliated with the study. “It would also be of interest to know if the racial differences changed over time. With the pandemic, the use of noninvasive options, such as Cologuard, have increased.”
“I will note that specifically for colonoscopy in the United States, the disparity gap had been closing from about 15% to 18% 20 years ago to about 3% in 2020 pre-COVID,” Dr. Carethers added. “I am fearful that COVID may have led to a widening of that gap again as we get more data.”
“It is important that noninvasive tests for screening be a part of the portfolio of offerings to patients, as about 35% of eligible at-risk persons who need to be screened are not screened in the United States,” Dr. Carethers said.
The study was not industry sponsored. Dr. Ouni and Dr. Carethers report no relevant financial relationships. Dr. Cooper has received consulting fees from Exact Sciences.
A version of this article first appeared on Medscape.com.
Doctors were significantly less likely to order colorectal cancer screening with the at-home test Cologuard (Exact Sciences) for Black patients and were more likely to order the test for Asian patients, new evidence reveals.
Investigators retrospectively studied 557,156 patients in the Mayo Clinic health system from 2012 to 2022. They found that Cologuard was ordered for 8.7% of Black patients, compared to 11.9% of White patients and 13.1% of Asian patients.
Both minority groups were less likely than White patients to undergo a follow-up colonoscopy within 1 year of Cologuard testing. Cologuard tests the stool for blood and DNA markers associated with colorectal cancer.
Although the researchers did not examine the reasons driving the disparities, lead investigator Ahmed Ouni, MD, told this news organization that “it could be patient preferences ... or there could be some bias as providers ourselves in how we present the data to patients.”
Dr. Ouni presented the findings on May 22 at the annual Digestive Disease Week® (DDW), held in person in San Diego and virtually.
Breakdown by physician specialty
“We looked at the specialty of physicians ordering these because we wanted to see where the disparity was coming from, if there was a disparity,” said Dr. Ouni, a gastroenterologist at Mayo Clinic, Jacksonville, Florida.
Just over half (51%) of the patients received care from family medicine physicians, 27% received care from internists, and 22% were seen by gastroenterologists.
Family physicians ordered Cologuard testing for 8.7% of Black patients, compared with 16.1% of White patients, a significant difference (P < .001). Internists ordered the test for 10.5% of Black patients and 11.1% of White patients (P < .001). Gastroenterologists ordered Cologuard screening for 2.4% of Black patients and 3.2% of White patients (P = .009).
Gastroenterologists were 47% more likely to order Cologuard for Asian patients, and internists were 16% more likely to order it for this population than for White patients. However, the findings were not statistically significant for the overall cohort of Asian patients when the researchers adjusted for age and sex (P = 0.52).
Black patients were 25% less likely to have a follow-up colonoscopy within 1 year of undergoing a Cologuard test (odds ratio, 0.75; 95% confidence interval, 0.60-0.94), and Asian patients were 35% less likely (OR, 0.65; 95% CI, 0.52-0.82).
Ongoing and future research
Of the total study population, only 2.9% self-identified as Black; according to the 2020 U.S. Census, 12.4% of the population of the United States are Black persons.
When asked about the relatively low proportion of Black persons in the study, Dr. Ouni replied that the investigators are partnering with a Black physician group in the Jacksonville, Fla., area to expand the study to a more diverse population.
Additional plans include assessing how many positive Cologuard test results led to follow-up colonoscopies.
The investigators are also working with family physicians at the Mayo Clinic to examine how physicians explain colorectal cancer screening options to patients and are studying patient preferences regarding screening options, which include Cologuard, fecal immunochemical test (FIT)/fecal occult blood testing, CT colonography, and colonoscopy.
“We’re analyzing the data by ZIP code to see if this could be related to finances,” Dr. Ouni added. “So, if you’re Black or White and more financially impoverished, how does that affect how you view Cologuard and colorectal cancer screening?”
Some unanswered questions
“Overall this study supports other studies of a disparity in colorectal cancer screening for African Americans,” John M. Carethers, MD, told this news organization when asked to comment. “This is known for FIT and colonoscopy, and Cologuard, which is a genetic test in addition to FIT, appears to be in that same realm.”
“Noninvasive tests will have a role to reach populations who may not readily have access to colonoscopy,” said Dr. Carethers, John G. Searle Professor and chair of the department of internal medicine and professor of human genetics at the University of Michigan, Ann Arbor. “The key here is if the test is positive, it needs to be followed up with a colonoscopy.”
Dr. Carethers added that the study raises some unanswered questions; for example, does the cost difference between testing options make a difference?
“FIT is under $20, but Cologuard is generally $300 or more,” he said. What percentage of the study population were offered other options, such as FIT? How does insurance status affect screening in different populations?”
“The findings should be taken in context of what other screening options were offered to or elected by patients,” agreed Gregory S. Cooper, MD, professor of medicine and population and quantitative health sciences at Case Western Reserve University and a gastroenterologist at University Hospitals Cleveland Medical Center.
According to guidelines, patients can be offered a menu of options, including FIT, colonoscopy, and Cologuard, Dr. Cooper said in an interview.
“If more African Americans elected colonoscopy, for example, the findings may balance out,” said Dr. Cooper, who was not affiliated with the study. “It would also be of interest to know if the racial differences changed over time. With the pandemic, the use of noninvasive options, such as Cologuard, have increased.”
“I will note that specifically for colonoscopy in the United States, the disparity gap had been closing from about 15% to 18% 20 years ago to about 3% in 2020 pre-COVID,” Dr. Carethers added. “I am fearful that COVID may have led to a widening of that gap again as we get more data.”
“It is important that noninvasive tests for screening be a part of the portfolio of offerings to patients, as about 35% of eligible at-risk persons who need to be screened are not screened in the United States,” Dr. Carethers said.
The study was not industry sponsored. Dr. Ouni and Dr. Carethers report no relevant financial relationships. Dr. Cooper has received consulting fees from Exact Sciences.
A version of this article first appeared on Medscape.com.
Doctors were significantly less likely to order colorectal cancer screening with the at-home test Cologuard (Exact Sciences) for Black patients and were more likely to order the test for Asian patients, new evidence reveals.
Investigators retrospectively studied 557,156 patients in the Mayo Clinic health system from 2012 to 2022. They found that Cologuard was ordered for 8.7% of Black patients, compared to 11.9% of White patients and 13.1% of Asian patients.
Both minority groups were less likely than White patients to undergo a follow-up colonoscopy within 1 year of Cologuard testing. Cologuard tests the stool for blood and DNA markers associated with colorectal cancer.
Although the researchers did not examine the reasons driving the disparities, lead investigator Ahmed Ouni, MD, told this news organization that “it could be patient preferences ... or there could be some bias as providers ourselves in how we present the data to patients.”
Dr. Ouni presented the findings on May 22 at the annual Digestive Disease Week® (DDW), held in person in San Diego and virtually.
Breakdown by physician specialty
“We looked at the specialty of physicians ordering these because we wanted to see where the disparity was coming from, if there was a disparity,” said Dr. Ouni, a gastroenterologist at Mayo Clinic, Jacksonville, Florida.
Just over half (51%) of the patients received care from family medicine physicians, 27% received care from internists, and 22% were seen by gastroenterologists.
Family physicians ordered Cologuard testing for 8.7% of Black patients, compared with 16.1% of White patients, a significant difference (P < .001). Internists ordered the test for 10.5% of Black patients and 11.1% of White patients (P < .001). Gastroenterologists ordered Cologuard screening for 2.4% of Black patients and 3.2% of White patients (P = .009).
Gastroenterologists were 47% more likely to order Cologuard for Asian patients, and internists were 16% more likely to order it for this population than for White patients. However, the findings were not statistically significant for the overall cohort of Asian patients when the researchers adjusted for age and sex (P = 0.52).
Black patients were 25% less likely to have a follow-up colonoscopy within 1 year of undergoing a Cologuard test (odds ratio, 0.75; 95% confidence interval, 0.60-0.94), and Asian patients were 35% less likely (OR, 0.65; 95% CI, 0.52-0.82).
Ongoing and future research
Of the total study population, only 2.9% self-identified as Black; according to the 2020 U.S. Census, 12.4% of the population of the United States are Black persons.
When asked about the relatively low proportion of Black persons in the study, Dr. Ouni replied that the investigators are partnering with a Black physician group in the Jacksonville, Fla., area to expand the study to a more diverse population.
Additional plans include assessing how many positive Cologuard test results led to follow-up colonoscopies.
The investigators are also working with family physicians at the Mayo Clinic to examine how physicians explain colorectal cancer screening options to patients and are studying patient preferences regarding screening options, which include Cologuard, fecal immunochemical test (FIT)/fecal occult blood testing, CT colonography, and colonoscopy.
“We’re analyzing the data by ZIP code to see if this could be related to finances,” Dr. Ouni added. “So, if you’re Black or White and more financially impoverished, how does that affect how you view Cologuard and colorectal cancer screening?”
Some unanswered questions
“Overall this study supports other studies of a disparity in colorectal cancer screening for African Americans,” John M. Carethers, MD, told this news organization when asked to comment. “This is known for FIT and colonoscopy, and Cologuard, which is a genetic test in addition to FIT, appears to be in that same realm.”
“Noninvasive tests will have a role to reach populations who may not readily have access to colonoscopy,” said Dr. Carethers, John G. Searle Professor and chair of the department of internal medicine and professor of human genetics at the University of Michigan, Ann Arbor. “The key here is if the test is positive, it needs to be followed up with a colonoscopy.”
Dr. Carethers added that the study raises some unanswered questions; for example, does the cost difference between testing options make a difference?
“FIT is under $20, but Cologuard is generally $300 or more,” he said. What percentage of the study population were offered other options, such as FIT? How does insurance status affect screening in different populations?”
“The findings should be taken in context of what other screening options were offered to or elected by patients,” agreed Gregory S. Cooper, MD, professor of medicine and population and quantitative health sciences at Case Western Reserve University and a gastroenterologist at University Hospitals Cleveland Medical Center.
According to guidelines, patients can be offered a menu of options, including FIT, colonoscopy, and Cologuard, Dr. Cooper said in an interview.
“If more African Americans elected colonoscopy, for example, the findings may balance out,” said Dr. Cooper, who was not affiliated with the study. “It would also be of interest to know if the racial differences changed over time. With the pandemic, the use of noninvasive options, such as Cologuard, have increased.”
“I will note that specifically for colonoscopy in the United States, the disparity gap had been closing from about 15% to 18% 20 years ago to about 3% in 2020 pre-COVID,” Dr. Carethers added. “I am fearful that COVID may have led to a widening of that gap again as we get more data.”
“It is important that noninvasive tests for screening be a part of the portfolio of offerings to patients, as about 35% of eligible at-risk persons who need to be screened are not screened in the United States,” Dr. Carethers said.
The study was not industry sponsored. Dr. Ouni and Dr. Carethers report no relevant financial relationships. Dr. Cooper has received consulting fees from Exact Sciences.
A version of this article first appeared on Medscape.com.
Women are not being warned that anesthetic may reduce birth pill efficacy
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
The effectiveness of hormonal contraceptives, including the pill and mini-pill, may be compromised by sugammadex, a drug widely used in anesthesia for reversing neuromuscular blockade induced by rocuronium or vecuronium.
Yet women are not routinely informed that the drug may make their contraception less effective, delegates at Euroanaesthesia, the annual meeting of the European Society of Anaesthesiology and Intensive Care in Milan were told.
New research presented at the meeting supports the authors’ experience that “robust methods for identifying at-risk patients and informing them of the associated risk of contraceptive failures is not common practice across anesthetic departments within the United Kingdom, and likely further afield.”
This is according to a survey of almost 150 anesthetic professionals, including consultants, junior doctors, and physician assistants, working at University College London Hospitals NHS Foundation Trust.
Dr. Neha Passi, Dr. Matt Oliver, and colleagues at the trust’s department of anesthesiology sent out a seven-question survey to their 150 colleagues and received 82 responses, 94% of which claimed awareness of the risk of contraceptive failure with sugammadex. However, 70% of the respondents admitted that they do not routinely discuss this with patients who have received the drug.
Risk with all forms of hormonal contraceptive
Yet current guidance is to inform women of child-bearing age that they have received the drug and, because of increased risk of contraceptive failure, advise those taking oral hormonal contraceptives to follow the missed pill advice in the leaflet that comes with their contraceptives. It also counsels that clinicians should advise women using other types of hormonal contraceptive to use an additional nonhormonal means of contraception for 7 days.
The study authors also carried out a retrospective audit of sugammadex use in the trust and reported that during the 6 weeks covered by the audit, 234 patients were administered sugammadex of whom 65 (28%) were women of childbearing age. Of these, 17 had a medical history that meant they weren’t at risk of pregnancy, but the other 48 should have received advice on the risks of contraceptive failure – however there was no record in the medical notes of such advice having been given for any of the at-risk 48 women.
While sugammadex is the only anesthetic drug known to have this effect, it is recognized to interact with progesterone and so may reduce the effectiveness of hormonal contraceptives, including the progesterone-only pill, combined pill, vaginal rings, implants, and intrauterine devices.
Dr. Passi said: “It is concerning that we are so seldom informing patients of the risk of contraceptive failure following sugammadex use.
“Use of sugammadex is expected to rise as it becomes cheaper in the future, and ensuring that women receiving this medicine are aware it may increase their risk of unwanted pregnancy must be a priority.”
She added: “It is important to note, however, that most patients receiving an anesthetic do not need a muscle relaxant and that sugammadex is one of several drugs available to reverse muscle relaxation.”
Dr. Oliver said: “We only studied one hospital trust but we expect the results to be similar in elsewhere in the U.K.”
In response to their findings, the study’s authors have created patient information leaflets and letters and programmed the trust’s electronic patient record system to identify “at-risk” patients and deliver electronic prompts to the anesthetists caring for them in the perioperative period.
A version of this article first appeared on Medscape UK.
FROM EUROANAESTHESIA
Antipsychotic tied to dose-related weight gain, higher cholesterol
new research suggests.
Investigators analyzed 1-year data for more than 400 patients who were taking risperidone and/or its metabolite paliperidone (Invega). Results showed increments of 1 mg of risperidone-equivalent doses were associated with an increase of 0.25% of weight within a year of follow-up.
“Although our findings report a positive and statistically significant dose-dependence of weight gain and cholesterol, both total and LDL [cholesterol], the size of the predicted changes of metabolic effects is clinically nonrelevant,” lead author Marianna Piras, PharmD, Centre for Psychiatric Neuroscience, Lausanne (Switzerland) University Hospital, said in an interview.
“Therefore, dose lowering would not have a beneficial effect on attenuating weight gain or cholesterol increases and could lead to psychiatric decompensation,” said Ms. Piras, who is also a PhD candidate in the unit of pharmacogenetics and clinical psychopharmacology at the University of Lausanne.
However, she added that because dose increments could increase risk for significant weight gain in the first month of treatment – the dose can be increased typically in a range of 1-10 grams – and strong dose increments could contribute to metabolic worsening over time, “risperidone minimum effective doses should be preferred.”
The findings were published online in the Journal of Clinical Psychiatry.
‘Serious public health issue’
Compared with the general population, patients with mental illness present with a greater prevalence of metabolic disorders. In addition, several psychotropic medications, including antipsychotics, can induce metabolic alterations such as weight gain, the investigators noted.
Antipsychotic-induced metabolic adverse effects “constitute a serious public health issue” because they are risk factors for cardiovascular diseases such as obesity and/or dyslipidemia, “which have been associated with a 10-year reduced life expectancy in the psychiatric population,” Ms. Piras said.
“The dose-dependence of metabolic adverse effects is a debated subject that needs to be assessed for each psychotropic drug known to induce weight gain,” she added.
Several previous studies have examined whether there is a dose-related effect of antipsychotics on metabolic parameters, “with some results suggesting that [weight gain] seems to develop even when low off-label doses are prescribed,” Ms. Piras noted.
She and her colleagues had already studied dose-related metabolic effects of quetiapine (Seroquel) and olanzapine (Zyprexa).
Risperidone is an antipsychotic with a “medium to high metabolic risk profile,” the researchers note, and few studies have examined the impact of risperidone on metabolic parameters other than weight gain.
For the current analysis, they analyzed data from a longitudinal study that included 438 patients (mean age, 40.7 years; 50.7% men) who started treatment with risperidone and/or paliperidone between 2007 and 2018.
The participants had diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, depression, “other,” or “unknown.”
Clinical follow-up periods were up to a year, but were no shorter than 3 weeks. The investigators also assessed the data at different time intervals at 1, 3, 6, and 12 months “to appreciate the evolution of the metabolic parameters.”
In addition, they collected demographic and clinical information, such as comorbidities, and measured patients’ weight, height, waist circumference, blood pressure, plasma glucose, and lipids at baseline and at 1, 3, and 12 months and then annually. Weight, waist circumference, and BP were also assessed at 2 and 6 months.
Doses of paliperidone were converted into risperidone-equivalent doses.
Significant weight gain over time
The mean duration of follow-up for the participants, of whom 374 were being treated with risperidone and 64 with paliperidone, was 153 days. Close to half (48.2%) were taking other psychotropic medications known to be associated with some degree of metabolic risk.
Patients were divided into two cohorts based on their daily dose intake (DDI): less than 3 mg/day (n = 201) and at least 3 mg/day (n = 237).
In the overall cohort, a “significant effect of time on weight change was found for each time point,” the investigators reported.
When the researchers looked at the changes according to DDI, they found that each 1-mg dose increase was associated with incremental weight gain at each time point.
Patients who had 5% or greater weight gain in the first month continued to gain weight more than patients who did not reach that threshold, leading the researchers to call that early threshold a “strong predictor of important weight gain in the long term.” There was a weight gain of 6.68% at 3 months, of 7.36% at 6 months, and of 7.7% at 12 months.
After the patients were stratified by age, there were differences in the effect of DDI on various age groups at different time points.
Dose was shown to have a significant effect on weight gain for women at all four time points (P ≥ .001), but for men only at 3 months (P = .003).
For each additional 1-mg dose, there was a 0.05 mmol/L (1.93 mg/dL) increase in total cholesterol (P = .018) after 1 year and a 0.04 mmol/L (1.54 mg/dL) increase in LDL cholesterol (P = .011).
There were no significant effects of time or DDI on triglycerides, HDL cholesterol, glucose levels, and systolic BP, and there was a negative effect of DDI on diastolic BP (P = .001).
The findings “provide evidence for a small dose effect of risperidone” on weight gain and total and LDL cholesterol levels, the investigators note.
Ms. Piras added that because each antipsychotic differs in its metabolic risk profile, “further analyses on other antipsychotics are ongoing in our laboratory, so far confirming our findings.”
Small increases, big changes
Commenting on the study, Erika Nurmi, MD, PhD, associate professor in the department of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience, University of California, Los Angeles, said the study is “unique in the field.”
It “leverages real-world data from a large patient registry to ask a long-unanswered question: Are weight and metabolic adverse effects proportional to dose? Big data approaches like these are very powerful, given the large number of participants that can be included,” said Dr. Nurmi, who was not involved with the research.
However, she cautioned, the “biggest drawback [is that] these data are by nature much more complex and prone to confounding effects.”
In this case, a “critical confounder” for the study was that the majority of individuals taking higher risperidone doses were also taking other drugs known to cause weight gain, whereas the majority of those on lower risperidone doses were not. “This difference may explain the dose relationship observed,” she said.
Because real-world, big data are “valuable but also messy, conclusions drawn from them must be interpreted with caution,” Dr. Nurmi said.
She added that it is generally wise to use the lowest effective dose possible.
“Clinicians should appreciate that even small doses of antipsychotics can cause big changes in weight. Risks and benefits of medications must be carefully considered in clinical practice,” Dr. Nurmi said.
The research was funded in part by the Swiss National Research Foundation. Piras reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Nurmi reported no relevant financial relationships, but she is an unpaid member of the Tourette Association of America’s medical advisory board and of the Myriad Genetics scientific advisory board.
A version of this article first appeared on Medscape.com.
new research suggests.
Investigators analyzed 1-year data for more than 400 patients who were taking risperidone and/or its metabolite paliperidone (Invega). Results showed increments of 1 mg of risperidone-equivalent doses were associated with an increase of 0.25% of weight within a year of follow-up.
“Although our findings report a positive and statistically significant dose-dependence of weight gain and cholesterol, both total and LDL [cholesterol], the size of the predicted changes of metabolic effects is clinically nonrelevant,” lead author Marianna Piras, PharmD, Centre for Psychiatric Neuroscience, Lausanne (Switzerland) University Hospital, said in an interview.
“Therefore, dose lowering would not have a beneficial effect on attenuating weight gain or cholesterol increases and could lead to psychiatric decompensation,” said Ms. Piras, who is also a PhD candidate in the unit of pharmacogenetics and clinical psychopharmacology at the University of Lausanne.
However, she added that because dose increments could increase risk for significant weight gain in the first month of treatment – the dose can be increased typically in a range of 1-10 grams – and strong dose increments could contribute to metabolic worsening over time, “risperidone minimum effective doses should be preferred.”
The findings were published online in the Journal of Clinical Psychiatry.
‘Serious public health issue’
Compared with the general population, patients with mental illness present with a greater prevalence of metabolic disorders. In addition, several psychotropic medications, including antipsychotics, can induce metabolic alterations such as weight gain, the investigators noted.
Antipsychotic-induced metabolic adverse effects “constitute a serious public health issue” because they are risk factors for cardiovascular diseases such as obesity and/or dyslipidemia, “which have been associated with a 10-year reduced life expectancy in the psychiatric population,” Ms. Piras said.
“The dose-dependence of metabolic adverse effects is a debated subject that needs to be assessed for each psychotropic drug known to induce weight gain,” she added.
Several previous studies have examined whether there is a dose-related effect of antipsychotics on metabolic parameters, “with some results suggesting that [weight gain] seems to develop even when low off-label doses are prescribed,” Ms. Piras noted.
She and her colleagues had already studied dose-related metabolic effects of quetiapine (Seroquel) and olanzapine (Zyprexa).
Risperidone is an antipsychotic with a “medium to high metabolic risk profile,” the researchers note, and few studies have examined the impact of risperidone on metabolic parameters other than weight gain.
For the current analysis, they analyzed data from a longitudinal study that included 438 patients (mean age, 40.7 years; 50.7% men) who started treatment with risperidone and/or paliperidone between 2007 and 2018.
The participants had diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, depression, “other,” or “unknown.”
Clinical follow-up periods were up to a year, but were no shorter than 3 weeks. The investigators also assessed the data at different time intervals at 1, 3, 6, and 12 months “to appreciate the evolution of the metabolic parameters.”
In addition, they collected demographic and clinical information, such as comorbidities, and measured patients’ weight, height, waist circumference, blood pressure, plasma glucose, and lipids at baseline and at 1, 3, and 12 months and then annually. Weight, waist circumference, and BP were also assessed at 2 and 6 months.
Doses of paliperidone were converted into risperidone-equivalent doses.
Significant weight gain over time
The mean duration of follow-up for the participants, of whom 374 were being treated with risperidone and 64 with paliperidone, was 153 days. Close to half (48.2%) were taking other psychotropic medications known to be associated with some degree of metabolic risk.
Patients were divided into two cohorts based on their daily dose intake (DDI): less than 3 mg/day (n = 201) and at least 3 mg/day (n = 237).
In the overall cohort, a “significant effect of time on weight change was found for each time point,” the investigators reported.
When the researchers looked at the changes according to DDI, they found that each 1-mg dose increase was associated with incremental weight gain at each time point.
Patients who had 5% or greater weight gain in the first month continued to gain weight more than patients who did not reach that threshold, leading the researchers to call that early threshold a “strong predictor of important weight gain in the long term.” There was a weight gain of 6.68% at 3 months, of 7.36% at 6 months, and of 7.7% at 12 months.
After the patients were stratified by age, there were differences in the effect of DDI on various age groups at different time points.
Dose was shown to have a significant effect on weight gain for women at all four time points (P ≥ .001), but for men only at 3 months (P = .003).
For each additional 1-mg dose, there was a 0.05 mmol/L (1.93 mg/dL) increase in total cholesterol (P = .018) after 1 year and a 0.04 mmol/L (1.54 mg/dL) increase in LDL cholesterol (P = .011).
There were no significant effects of time or DDI on triglycerides, HDL cholesterol, glucose levels, and systolic BP, and there was a negative effect of DDI on diastolic BP (P = .001).
The findings “provide evidence for a small dose effect of risperidone” on weight gain and total and LDL cholesterol levels, the investigators note.
Ms. Piras added that because each antipsychotic differs in its metabolic risk profile, “further analyses on other antipsychotics are ongoing in our laboratory, so far confirming our findings.”
Small increases, big changes
Commenting on the study, Erika Nurmi, MD, PhD, associate professor in the department of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience, University of California, Los Angeles, said the study is “unique in the field.”
It “leverages real-world data from a large patient registry to ask a long-unanswered question: Are weight and metabolic adverse effects proportional to dose? Big data approaches like these are very powerful, given the large number of participants that can be included,” said Dr. Nurmi, who was not involved with the research.
However, she cautioned, the “biggest drawback [is that] these data are by nature much more complex and prone to confounding effects.”
In this case, a “critical confounder” for the study was that the majority of individuals taking higher risperidone doses were also taking other drugs known to cause weight gain, whereas the majority of those on lower risperidone doses were not. “This difference may explain the dose relationship observed,” she said.
Because real-world, big data are “valuable but also messy, conclusions drawn from them must be interpreted with caution,” Dr. Nurmi said.
She added that it is generally wise to use the lowest effective dose possible.
“Clinicians should appreciate that even small doses of antipsychotics can cause big changes in weight. Risks and benefits of medications must be carefully considered in clinical practice,” Dr. Nurmi said.
The research was funded in part by the Swiss National Research Foundation. Piras reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Nurmi reported no relevant financial relationships, but she is an unpaid member of the Tourette Association of America’s medical advisory board and of the Myriad Genetics scientific advisory board.
A version of this article first appeared on Medscape.com.
new research suggests.
Investigators analyzed 1-year data for more than 400 patients who were taking risperidone and/or its metabolite paliperidone (Invega). Results showed increments of 1 mg of risperidone-equivalent doses were associated with an increase of 0.25% of weight within a year of follow-up.
“Although our findings report a positive and statistically significant dose-dependence of weight gain and cholesterol, both total and LDL [cholesterol], the size of the predicted changes of metabolic effects is clinically nonrelevant,” lead author Marianna Piras, PharmD, Centre for Psychiatric Neuroscience, Lausanne (Switzerland) University Hospital, said in an interview.
“Therefore, dose lowering would not have a beneficial effect on attenuating weight gain or cholesterol increases and could lead to psychiatric decompensation,” said Ms. Piras, who is also a PhD candidate in the unit of pharmacogenetics and clinical psychopharmacology at the University of Lausanne.
However, she added that because dose increments could increase risk for significant weight gain in the first month of treatment – the dose can be increased typically in a range of 1-10 grams – and strong dose increments could contribute to metabolic worsening over time, “risperidone minimum effective doses should be preferred.”
The findings were published online in the Journal of Clinical Psychiatry.
‘Serious public health issue’
Compared with the general population, patients with mental illness present with a greater prevalence of metabolic disorders. In addition, several psychotropic medications, including antipsychotics, can induce metabolic alterations such as weight gain, the investigators noted.
Antipsychotic-induced metabolic adverse effects “constitute a serious public health issue” because they are risk factors for cardiovascular diseases such as obesity and/or dyslipidemia, “which have been associated with a 10-year reduced life expectancy in the psychiatric population,” Ms. Piras said.
“The dose-dependence of metabolic adverse effects is a debated subject that needs to be assessed for each psychotropic drug known to induce weight gain,” she added.
Several previous studies have examined whether there is a dose-related effect of antipsychotics on metabolic parameters, “with some results suggesting that [weight gain] seems to develop even when low off-label doses are prescribed,” Ms. Piras noted.
She and her colleagues had already studied dose-related metabolic effects of quetiapine (Seroquel) and olanzapine (Zyprexa).
Risperidone is an antipsychotic with a “medium to high metabolic risk profile,” the researchers note, and few studies have examined the impact of risperidone on metabolic parameters other than weight gain.
For the current analysis, they analyzed data from a longitudinal study that included 438 patients (mean age, 40.7 years; 50.7% men) who started treatment with risperidone and/or paliperidone between 2007 and 2018.
The participants had diagnoses of schizophrenia, schizoaffective disorder, bipolar disorder, depression, “other,” or “unknown.”
Clinical follow-up periods were up to a year, but were no shorter than 3 weeks. The investigators also assessed the data at different time intervals at 1, 3, 6, and 12 months “to appreciate the evolution of the metabolic parameters.”
In addition, they collected demographic and clinical information, such as comorbidities, and measured patients’ weight, height, waist circumference, blood pressure, plasma glucose, and lipids at baseline and at 1, 3, and 12 months and then annually. Weight, waist circumference, and BP were also assessed at 2 and 6 months.
Doses of paliperidone were converted into risperidone-equivalent doses.
Significant weight gain over time
The mean duration of follow-up for the participants, of whom 374 were being treated with risperidone and 64 with paliperidone, was 153 days. Close to half (48.2%) were taking other psychotropic medications known to be associated with some degree of metabolic risk.
Patients were divided into two cohorts based on their daily dose intake (DDI): less than 3 mg/day (n = 201) and at least 3 mg/day (n = 237).
In the overall cohort, a “significant effect of time on weight change was found for each time point,” the investigators reported.
When the researchers looked at the changes according to DDI, they found that each 1-mg dose increase was associated with incremental weight gain at each time point.
Patients who had 5% or greater weight gain in the first month continued to gain weight more than patients who did not reach that threshold, leading the researchers to call that early threshold a “strong predictor of important weight gain in the long term.” There was a weight gain of 6.68% at 3 months, of 7.36% at 6 months, and of 7.7% at 12 months.
After the patients were stratified by age, there were differences in the effect of DDI on various age groups at different time points.
Dose was shown to have a significant effect on weight gain for women at all four time points (P ≥ .001), but for men only at 3 months (P = .003).
For each additional 1-mg dose, there was a 0.05 mmol/L (1.93 mg/dL) increase in total cholesterol (P = .018) after 1 year and a 0.04 mmol/L (1.54 mg/dL) increase in LDL cholesterol (P = .011).
There were no significant effects of time or DDI on triglycerides, HDL cholesterol, glucose levels, and systolic BP, and there was a negative effect of DDI on diastolic BP (P = .001).
The findings “provide evidence for a small dose effect of risperidone” on weight gain and total and LDL cholesterol levels, the investigators note.
Ms. Piras added that because each antipsychotic differs in its metabolic risk profile, “further analyses on other antipsychotics are ongoing in our laboratory, so far confirming our findings.”
Small increases, big changes
Commenting on the study, Erika Nurmi, MD, PhD, associate professor in the department of psychiatry and biobehavioral sciences at the Semel Institute for Neuroscience, University of California, Los Angeles, said the study is “unique in the field.”
It “leverages real-world data from a large patient registry to ask a long-unanswered question: Are weight and metabolic adverse effects proportional to dose? Big data approaches like these are very powerful, given the large number of participants that can be included,” said Dr. Nurmi, who was not involved with the research.
However, she cautioned, the “biggest drawback [is that] these data are by nature much more complex and prone to confounding effects.”
In this case, a “critical confounder” for the study was that the majority of individuals taking higher risperidone doses were also taking other drugs known to cause weight gain, whereas the majority of those on lower risperidone doses were not. “This difference may explain the dose relationship observed,” she said.
Because real-world, big data are “valuable but also messy, conclusions drawn from them must be interpreted with caution,” Dr. Nurmi said.
She added that it is generally wise to use the lowest effective dose possible.
“Clinicians should appreciate that even small doses of antipsychotics can cause big changes in weight. Risks and benefits of medications must be carefully considered in clinical practice,” Dr. Nurmi said.
The research was funded in part by the Swiss National Research Foundation. Piras reports no relevant financial relationships. The other investigators’ disclosures are listed in the original article. Dr. Nurmi reported no relevant financial relationships, but she is an unpaid member of the Tourette Association of America’s medical advisory board and of the Myriad Genetics scientific advisory board.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL PSYCHIATRY
Is benzophenone safe in skin care? Part 2: Environmental effects
Although it has been . DiNardo and Downs point out that BP-3 has been linked to contact and photocontact allergies in humans and implicated as a potential endocrine disruptor. They add that it can yield deleterious by-products when reacting with chlorine in swimming pools and wastewater treatment plants and can cause additional side effects in humans who ingest fish.1 This column will focus on recent studies, mainly on the role of benzophenones in sunscreen agents that pose considerable risks to waterways and marine life, with concomitant effects on the food chain.
Environmental effects of BPs and legislative responses
Various UV filters, including BP-3, octinoxate, octocrylene, and ethylhexyl salicylate, are thought to pose considerable peril to the marine environment.2,3 In particular, BP-3 has been demonstrated to provoke coral reef bleaching in vitro, leading to ossification and deforming DNA in the larval stage.3,4
According to a 2018 report, BP-3 is believed to be present in approximately two thirds of organic sunscreens used in the United States.3 In addition, several studies have revealed that detectable levels of organic sunscreen ingredients, including BP-3, have been identified in coastal waters around the globe, including Hawaii and the U.S. Virgin Islands.4-8
A surfeit of tourists has been blamed in part, given that an estimated 25% of applied sunscreen is eliminated within 20 minutes of entering the water and thought to release about 4,000-6,000 tons/year into the surrounding coral reefs.9,10 In Hawaii in particular, sewage contamination of the waterways has resulted from wastewater treatment facilities ill-equipped to filter out organic substances such as BP-3 and octinoxate.10,11 In light of such circumstances, the use of sunscreens containing BP-3 and octinoxate have been restricted in Hawaii, particularly in proximity to beaches, since Jan. 1, 2021, because of their apparent environmental impact.10
The exposure of coral to these compounds is believed to result in bleaching because of impaired membrane integrity and photosynthetic pigment loss in the zooxanthellae that coral releases.9,10 Coral and the algae zooxanthellae have a symbiotic relationship, Siller et al. explain, with the coral delivering protection and components essential for photosynthesis and the algae ultimately serving as nutrients for the coral.10 Stress endured by coral is believed to cause algae to detach, rendering coral more vulnerable to disease and less viable overall.10
In 2016, Downs et al. showed that four out of five sampled locations had detectable levels of BP-3 (100 pp trillion) with a fifth tested site measured at 19.2 pp billion.4
In 2019, Sirois acknowledges the problem of coral bleaching around the world but speculates that banning sunscreen ingredients for this purpose will delude people that such a measure will reverse the decline of coral and may lead to the unintended consequence of lower use of sunscreens. Sirois adds that a more comprehensive investigation of the multiple causes of coral reef bleaching is warranted, as are deeper examinations of studies using higher concentrations of sunscreen ingredients in artificial conditions.12
In the same year, Raffa et al. discussed the impending ban in Hawaii of the two sunscreen ingredients (BP-3 and octinoxate) to help preserve coral reefs. In so doing, they detailed the natural and human-induced harm to coral reefs, including pollution, fishing practices, overall impact of global climate change, and alterations in ocean temperature and chemistry. The implication is that sunscreen ingredients, which help prevent sun damage in users, are not the only causes of harm to coral reefs. Nevertheless, they point out that concentration estimates and mechanism studies buttress the argument that sunscreen ingredients contribute to coral bleaching. Still, the ban in Hawaii is thought to be a trend. Opponents of the ban are concerned that human skin cancers will rise in such circumstances. Alternative chemical sunscreens are being investigated, and physical sunscreens have emerged as the go-to recommendation.13
Notably, oxybenzone has been virtually replaced in the European Union with other UV filters with broad-spectrum action, but the majority of such filters have not yet been approved for use in the United States by the Food and Drug Administration.3
Food chain implications
BP-3 and other UV filters have been investigated for their effects on fish and mammals. Schneider and Lim illustrate that BP-3 is among the frequently used organic UV filters (along with 4-methylbenzylidene camphor, octocrylene, and octinoxate [ethylhexyl methoxycinnamate]) found in most water sources in the world, as well as multiple fish species.2 Cod liver in Norway, for instance, was found to contain octocrylene in 80% of cod, with BP-3 identified in 50% of the sample. BP-3 and octinoxate were also found in white fish.2,14 In laboratory studies, BP-3 in particular has been found in high concentrations in rainbow trout and Japanese rice fish (medaka), causing reduced egg production and hatchlings in females and increased vitellogenin protein production in males, suggesting potential feminization.2,15
Schneider and Lim note that standard wastewater treatment approaches cannot address this issue and the presence of such contaminants in fish can pose dangerous ramifications in the food chain. They assert that, despite relatively low concentrations in the fish, bioaccumulation and biomagnification present the potential for chemicals accumulating over time and becoming more deleterious as such ingredients travel up the food chain. As higher-chain organisms absorb higher concentrations of the chemicals not broken down in the lower-chain organisms, though, there have not yet been reports of adverse effects of biomagnification in humans.2
BP-3 has been found by Brausch and Rand to have bioaccumulated in fish at higher levels than the ambient water, however.1,2,16 Schneider and Lim present these issues as relevant to the sun protection discussion, while advocating for dermatologists to continue to counsel wise sun-protective behaviors.2
Conclusion
While calls for additional research are necessary and encouraging, I think human, and likely environmental, health would be better protected by the use of inorganic sunscreens in general and near or in coastal waterways. In light of legislative actions, in particular, it is important for dermatologists to intervene to ensure that patients do not engage in riskier behaviors in the sun in areas facing imminent organic sunscreen bans.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. DiNardo JC and Downs CA. J Cosmet Dermatol. 2018 Feb;17(1):15-9.
2. Schneider SL and Lim HW. J Am Acad Dermatol. 2019 Jan;80(1):266-71.
3. Yeager DG and Lim HW. Dermatol Clin. 2019 Apr;37(2):149-57.
4. Downs CA et al. Arch Environ Contam Toxicol 2016 Feb;70(2):265-88.
5. Sánchez Rodríguez A et al. Chemosphere. 2015 Jul;131:85-90.
6. Tovar-Sánchez A et al. PLoS One. 2013 Jun 5;8(6):e65451.
7. Danovaro R and Corinaldesi C. Microb Ecol. 2003 Feb;45(2):109-18.
8. Daughton CG and Ternes TA. Environ Health Perspect. 1999 Dec;107 Suppl 6:907-38.
9. Danovaro R et al. Environ Health Perspect. 2008 Apr;116(4):441-7.
10. Siller A et al. Plast Surg Nur. 2019 Oct/Dec;39(4):157-60.
11. Ramos S et al. Sci Total Environ. 2015 Sep 1;526:278-311.
12. Sirois J. Sci Total Environ. 2019 Jul 15;674:211-2.
13. Raffa RB et al. J Clin Pharm Ther. 2019 Feb;44(1):134-9.
14. Langford KH et al. Environ Int. 2015 Jul;80:1-7.
15. Coronado M et al. Aquat Toxicol. 2008 Nov 21;90(3):182-7.
16. Brausch JM and Rand GM. Chemosphere. 2011 Mar;82(11):1518-32.
Although it has been . DiNardo and Downs point out that BP-3 has been linked to contact and photocontact allergies in humans and implicated as a potential endocrine disruptor. They add that it can yield deleterious by-products when reacting with chlorine in swimming pools and wastewater treatment plants and can cause additional side effects in humans who ingest fish.1 This column will focus on recent studies, mainly on the role of benzophenones in sunscreen agents that pose considerable risks to waterways and marine life, with concomitant effects on the food chain.
Environmental effects of BPs and legislative responses
Various UV filters, including BP-3, octinoxate, octocrylene, and ethylhexyl salicylate, are thought to pose considerable peril to the marine environment.2,3 In particular, BP-3 has been demonstrated to provoke coral reef bleaching in vitro, leading to ossification and deforming DNA in the larval stage.3,4
According to a 2018 report, BP-3 is believed to be present in approximately two thirds of organic sunscreens used in the United States.3 In addition, several studies have revealed that detectable levels of organic sunscreen ingredients, including BP-3, have been identified in coastal waters around the globe, including Hawaii and the U.S. Virgin Islands.4-8
A surfeit of tourists has been blamed in part, given that an estimated 25% of applied sunscreen is eliminated within 20 minutes of entering the water and thought to release about 4,000-6,000 tons/year into the surrounding coral reefs.9,10 In Hawaii in particular, sewage contamination of the waterways has resulted from wastewater treatment facilities ill-equipped to filter out organic substances such as BP-3 and octinoxate.10,11 In light of such circumstances, the use of sunscreens containing BP-3 and octinoxate have been restricted in Hawaii, particularly in proximity to beaches, since Jan. 1, 2021, because of their apparent environmental impact.10
The exposure of coral to these compounds is believed to result in bleaching because of impaired membrane integrity and photosynthetic pigment loss in the zooxanthellae that coral releases.9,10 Coral and the algae zooxanthellae have a symbiotic relationship, Siller et al. explain, with the coral delivering protection and components essential for photosynthesis and the algae ultimately serving as nutrients for the coral.10 Stress endured by coral is believed to cause algae to detach, rendering coral more vulnerable to disease and less viable overall.10
In 2016, Downs et al. showed that four out of five sampled locations had detectable levels of BP-3 (100 pp trillion) with a fifth tested site measured at 19.2 pp billion.4
In 2019, Sirois acknowledges the problem of coral bleaching around the world but speculates that banning sunscreen ingredients for this purpose will delude people that such a measure will reverse the decline of coral and may lead to the unintended consequence of lower use of sunscreens. Sirois adds that a more comprehensive investigation of the multiple causes of coral reef bleaching is warranted, as are deeper examinations of studies using higher concentrations of sunscreen ingredients in artificial conditions.12
In the same year, Raffa et al. discussed the impending ban in Hawaii of the two sunscreen ingredients (BP-3 and octinoxate) to help preserve coral reefs. In so doing, they detailed the natural and human-induced harm to coral reefs, including pollution, fishing practices, overall impact of global climate change, and alterations in ocean temperature and chemistry. The implication is that sunscreen ingredients, which help prevent sun damage in users, are not the only causes of harm to coral reefs. Nevertheless, they point out that concentration estimates and mechanism studies buttress the argument that sunscreen ingredients contribute to coral bleaching. Still, the ban in Hawaii is thought to be a trend. Opponents of the ban are concerned that human skin cancers will rise in such circumstances. Alternative chemical sunscreens are being investigated, and physical sunscreens have emerged as the go-to recommendation.13
Notably, oxybenzone has been virtually replaced in the European Union with other UV filters with broad-spectrum action, but the majority of such filters have not yet been approved for use in the United States by the Food and Drug Administration.3
Food chain implications
BP-3 and other UV filters have been investigated for their effects on fish and mammals. Schneider and Lim illustrate that BP-3 is among the frequently used organic UV filters (along with 4-methylbenzylidene camphor, octocrylene, and octinoxate [ethylhexyl methoxycinnamate]) found in most water sources in the world, as well as multiple fish species.2 Cod liver in Norway, for instance, was found to contain octocrylene in 80% of cod, with BP-3 identified in 50% of the sample. BP-3 and octinoxate were also found in white fish.2,14 In laboratory studies, BP-3 in particular has been found in high concentrations in rainbow trout and Japanese rice fish (medaka), causing reduced egg production and hatchlings in females and increased vitellogenin protein production in males, suggesting potential feminization.2,15
Schneider and Lim note that standard wastewater treatment approaches cannot address this issue and the presence of such contaminants in fish can pose dangerous ramifications in the food chain. They assert that, despite relatively low concentrations in the fish, bioaccumulation and biomagnification present the potential for chemicals accumulating over time and becoming more deleterious as such ingredients travel up the food chain. As higher-chain organisms absorb higher concentrations of the chemicals not broken down in the lower-chain organisms, though, there have not yet been reports of adverse effects of biomagnification in humans.2
BP-3 has been found by Brausch and Rand to have bioaccumulated in fish at higher levels than the ambient water, however.1,2,16 Schneider and Lim present these issues as relevant to the sun protection discussion, while advocating for dermatologists to continue to counsel wise sun-protective behaviors.2
Conclusion
While calls for additional research are necessary and encouraging, I think human, and likely environmental, health would be better protected by the use of inorganic sunscreens in general and near or in coastal waterways. In light of legislative actions, in particular, it is important for dermatologists to intervene to ensure that patients do not engage in riskier behaviors in the sun in areas facing imminent organic sunscreen bans.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. DiNardo JC and Downs CA. J Cosmet Dermatol. 2018 Feb;17(1):15-9.
2. Schneider SL and Lim HW. J Am Acad Dermatol. 2019 Jan;80(1):266-71.
3. Yeager DG and Lim HW. Dermatol Clin. 2019 Apr;37(2):149-57.
4. Downs CA et al. Arch Environ Contam Toxicol 2016 Feb;70(2):265-88.
5. Sánchez Rodríguez A et al. Chemosphere. 2015 Jul;131:85-90.
6. Tovar-Sánchez A et al. PLoS One. 2013 Jun 5;8(6):e65451.
7. Danovaro R and Corinaldesi C. Microb Ecol. 2003 Feb;45(2):109-18.
8. Daughton CG and Ternes TA. Environ Health Perspect. 1999 Dec;107 Suppl 6:907-38.
9. Danovaro R et al. Environ Health Perspect. 2008 Apr;116(4):441-7.
10. Siller A et al. Plast Surg Nur. 2019 Oct/Dec;39(4):157-60.
11. Ramos S et al. Sci Total Environ. 2015 Sep 1;526:278-311.
12. Sirois J. Sci Total Environ. 2019 Jul 15;674:211-2.
13. Raffa RB et al. J Clin Pharm Ther. 2019 Feb;44(1):134-9.
14. Langford KH et al. Environ Int. 2015 Jul;80:1-7.
15. Coronado M et al. Aquat Toxicol. 2008 Nov 21;90(3):182-7.
16. Brausch JM and Rand GM. Chemosphere. 2011 Mar;82(11):1518-32.
Although it has been . DiNardo and Downs point out that BP-3 has been linked to contact and photocontact allergies in humans and implicated as a potential endocrine disruptor. They add that it can yield deleterious by-products when reacting with chlorine in swimming pools and wastewater treatment plants and can cause additional side effects in humans who ingest fish.1 This column will focus on recent studies, mainly on the role of benzophenones in sunscreen agents that pose considerable risks to waterways and marine life, with concomitant effects on the food chain.
Environmental effects of BPs and legislative responses
Various UV filters, including BP-3, octinoxate, octocrylene, and ethylhexyl salicylate, are thought to pose considerable peril to the marine environment.2,3 In particular, BP-3 has been demonstrated to provoke coral reef bleaching in vitro, leading to ossification and deforming DNA in the larval stage.3,4
According to a 2018 report, BP-3 is believed to be present in approximately two thirds of organic sunscreens used in the United States.3 In addition, several studies have revealed that detectable levels of organic sunscreen ingredients, including BP-3, have been identified in coastal waters around the globe, including Hawaii and the U.S. Virgin Islands.4-8
A surfeit of tourists has been blamed in part, given that an estimated 25% of applied sunscreen is eliminated within 20 minutes of entering the water and thought to release about 4,000-6,000 tons/year into the surrounding coral reefs.9,10 In Hawaii in particular, sewage contamination of the waterways has resulted from wastewater treatment facilities ill-equipped to filter out organic substances such as BP-3 and octinoxate.10,11 In light of such circumstances, the use of sunscreens containing BP-3 and octinoxate have been restricted in Hawaii, particularly in proximity to beaches, since Jan. 1, 2021, because of their apparent environmental impact.10
The exposure of coral to these compounds is believed to result in bleaching because of impaired membrane integrity and photosynthetic pigment loss in the zooxanthellae that coral releases.9,10 Coral and the algae zooxanthellae have a symbiotic relationship, Siller et al. explain, with the coral delivering protection and components essential for photosynthesis and the algae ultimately serving as nutrients for the coral.10 Stress endured by coral is believed to cause algae to detach, rendering coral more vulnerable to disease and less viable overall.10
In 2016, Downs et al. showed that four out of five sampled locations had detectable levels of BP-3 (100 pp trillion) with a fifth tested site measured at 19.2 pp billion.4
In 2019, Sirois acknowledges the problem of coral bleaching around the world but speculates that banning sunscreen ingredients for this purpose will delude people that such a measure will reverse the decline of coral and may lead to the unintended consequence of lower use of sunscreens. Sirois adds that a more comprehensive investigation of the multiple causes of coral reef bleaching is warranted, as are deeper examinations of studies using higher concentrations of sunscreen ingredients in artificial conditions.12
In the same year, Raffa et al. discussed the impending ban in Hawaii of the two sunscreen ingredients (BP-3 and octinoxate) to help preserve coral reefs. In so doing, they detailed the natural and human-induced harm to coral reefs, including pollution, fishing practices, overall impact of global climate change, and alterations in ocean temperature and chemistry. The implication is that sunscreen ingredients, which help prevent sun damage in users, are not the only causes of harm to coral reefs. Nevertheless, they point out that concentration estimates and mechanism studies buttress the argument that sunscreen ingredients contribute to coral bleaching. Still, the ban in Hawaii is thought to be a trend. Opponents of the ban are concerned that human skin cancers will rise in such circumstances. Alternative chemical sunscreens are being investigated, and physical sunscreens have emerged as the go-to recommendation.13
Notably, oxybenzone has been virtually replaced in the European Union with other UV filters with broad-spectrum action, but the majority of such filters have not yet been approved for use in the United States by the Food and Drug Administration.3
Food chain implications
BP-3 and other UV filters have been investigated for their effects on fish and mammals. Schneider and Lim illustrate that BP-3 is among the frequently used organic UV filters (along with 4-methylbenzylidene camphor, octocrylene, and octinoxate [ethylhexyl methoxycinnamate]) found in most water sources in the world, as well as multiple fish species.2 Cod liver in Norway, for instance, was found to contain octocrylene in 80% of cod, with BP-3 identified in 50% of the sample. BP-3 and octinoxate were also found in white fish.2,14 In laboratory studies, BP-3 in particular has been found in high concentrations in rainbow trout and Japanese rice fish (medaka), causing reduced egg production and hatchlings in females and increased vitellogenin protein production in males, suggesting potential feminization.2,15
Schneider and Lim note that standard wastewater treatment approaches cannot address this issue and the presence of such contaminants in fish can pose dangerous ramifications in the food chain. They assert that, despite relatively low concentrations in the fish, bioaccumulation and biomagnification present the potential for chemicals accumulating over time and becoming more deleterious as such ingredients travel up the food chain. As higher-chain organisms absorb higher concentrations of the chemicals not broken down in the lower-chain organisms, though, there have not yet been reports of adverse effects of biomagnification in humans.2
BP-3 has been found by Brausch and Rand to have bioaccumulated in fish at higher levels than the ambient water, however.1,2,16 Schneider and Lim present these issues as relevant to the sun protection discussion, while advocating for dermatologists to continue to counsel wise sun-protective behaviors.2
Conclusion
While calls for additional research are necessary and encouraging, I think human, and likely environmental, health would be better protected by the use of inorganic sunscreens in general and near or in coastal waterways. In light of legislative actions, in particular, it is important for dermatologists to intervene to ensure that patients do not engage in riskier behaviors in the sun in areas facing imminent organic sunscreen bans.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann has written two textbooks and a New York Times Best Sellers book for consumers. Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Galderma, Revance, Evolus, and Burt’s Bees. She is the CEO of Skin Type Solutions Inc., a company that independently tests skin care products and makes recommendations to physicians on which skin care technologies are best. Write to her at [email protected].
References
1. DiNardo JC and Downs CA. J Cosmet Dermatol. 2018 Feb;17(1):15-9.
2. Schneider SL and Lim HW. J Am Acad Dermatol. 2019 Jan;80(1):266-71.
3. Yeager DG and Lim HW. Dermatol Clin. 2019 Apr;37(2):149-57.
4. Downs CA et al. Arch Environ Contam Toxicol 2016 Feb;70(2):265-88.
5. Sánchez Rodríguez A et al. Chemosphere. 2015 Jul;131:85-90.
6. Tovar-Sánchez A et al. PLoS One. 2013 Jun 5;8(6):e65451.
7. Danovaro R and Corinaldesi C. Microb Ecol. 2003 Feb;45(2):109-18.
8. Daughton CG and Ternes TA. Environ Health Perspect. 1999 Dec;107 Suppl 6:907-38.
9. Danovaro R et al. Environ Health Perspect. 2008 Apr;116(4):441-7.
10. Siller A et al. Plast Surg Nur. 2019 Oct/Dec;39(4):157-60.
11. Ramos S et al. Sci Total Environ. 2015 Sep 1;526:278-311.
12. Sirois J. Sci Total Environ. 2019 Jul 15;674:211-2.
13. Raffa RB et al. J Clin Pharm Ther. 2019 Feb;44(1):134-9.
14. Langford KH et al. Environ Int. 2015 Jul;80:1-7.
15. Coronado M et al. Aquat Toxicol. 2008 Nov 21;90(3):182-7.
16. Brausch JM and Rand GM. Chemosphere. 2011 Mar;82(11):1518-32.
Esophageal cancer screening isn’t for everyone: Study
Endoscopic screening for esophageal adenocarcinoma (EAC), may not be a cost-effective strategy for all populations, possibly even leading to net harm in some, according to a comparative cost-effectiveness analysis.
Several U.S. guidelines suggest the use of endoscopic screening for EAC, yet recommendations within these guidelines vary in terms of which population should receive screening, according study authors led by Joel H. Rubenstein, MD, of the Lieutenant Charles S. Kettles Veterans Affairs Medical Center, Ann Arbor, Mich. Their findings were published in Gastroenterology. In addition, there have been no randomized trials to date that have evaluated endoscopic screening outcomes among different populations. Population screening recommendations in the current guidelines have been informed mostly by observational data and expert opinion.
Existing cost-effectiveness analyses of EAC screening have mostly focused on screening older men with gastroesophageal reflux disease (GERD) at certain ages, and many of these analyses have limited data regarding diverse patient populations.
In their study, Dr. Rubenstein and colleagues performed a comparative cost-effectiveness analysis of endoscopic screening for EAC that was restricted to individuals with GERD symptoms in the general population. The analysis was stratified by race and sex. The primary objective of the analysis was to identify and establish the optimal age at which to offer endoscopic screening in the specific populations evaluated in the study.
The investigators conducted their comparative cost-effectiveness analyses using three independent simulation models. The independently developed models – which focused on EAC natural history, screening, surveillance, and treatment – are part of the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network. For each model, there were four cohorts, defined by race as either White or Black and sex, which were independently calibrated to targets to reproduce the EAC incidence in the United States. The three models were based on somewhat different structures and assumptions; for example, two of the models assumed stable prevalence of GERD symptoms of approximately 20% across ages, while the third assumed a near-linear increase across adulthood. All three assumed EAC develops only in individuals with Barrett’s esophagus.
In each base case, the researchers simulated cohorts of people in the United States who were born in 1950, and then stratified these individuals by race and sex and followed each individual from 40 years of age until 100 years of age. The researchers considered 42 strategies, such as no screening, a single endoscopic screening at six specified ages (between 40 and 65 years of age), and a single screening in individuals with GERD symptoms at the six specified ages.
Primary results were the averaged results across all three models. The optimal screening strategy, defined by the investigators, was the strategy with the highest effectiveness that had an incremental cost-effectiveness ratio of less than $100,000 per quality-adjusted life-year gained.
The most effective – yet the most costly – screening strategies for White men were those that screened all of them once between 40 and 55 years of age. The optimal screening strategy, however, was one that screened individuals with GERD twice, once at age 45 years and again at 60 years. The researchers determined that screening Black men with GERD once at 55 years of age was optimal.
By contrast, the optimal strategy for women, whether White or Black, was no screening at all. “In particular, among Black women, screening is, at best, very expensive with little benefit, and some strategies cause net harm,” the authors wrote.
The investigators wrote that there is a need for empiric, long-term studies “to confirm whether repeated screening has a substantial yield of incident” Barrett’s esophagus. The researchers also noted that their study was limited by the lack of inclusion of additional risk factors, such as smoking, obesity, and family history, which may have led to different conclusions on specific screening strategies.
“We certainly acknowledge the history of health care inequities, and that race is a social construct that, in the vast majority of medical contexts, has no biological basis. We are circumspect regarding making recommendations based on race or sex if environmental exposures or genetic factors on which to make to those recommendations were available,” they wrote.
The study was supported by National Institutes of Health/National Cancer Institute grants. Some authors disclosed relationships with Lucid Diagnostics, Value Analytics Labs, and Cernostics.
Over the past decades we have seen an alarming rise in the incidence of esophageal adenocarcinoma, mostly diagnosed at an advanced stage when curative treatment is no longer an option. Esophageal adenocarcinoma develops from Barrett’s esophagus that, if known to be present, can be surveilled to detect dysplasia and cancer at an early and curable stage.
Whereas currently screening for Barrett’s esophagus focused on White males with gastroesophageal reflux, little was known about screening in non-White and non-male populations. Identifying who and how to screen poses a challenge, and in real life such studies looking at varied populations would require many patients, years of follow-up, much effort and substantial costs. Rubenstein and colleagues used three independent simulation models to simulate many different screening scenarios, while taking gender and race into account. The outcomes of this study, which demonstrate that one size does not fit all, will be very relevant in guiding future strategies regarding screening for Barrett’s esophagus and early esophageal adenocarcinoma. Although the study is based around endoscopic screening, the insights gained from this study will also be relevant when considering the use of nonendoscopic screening tools.
R.E. Pouw, MD, PhD, is with Amsterdam University Medical Centers. She disclosed having been a consultant for MicroTech and Medtronic and having received speaker fees from Pentax.
Over the past decades we have seen an alarming rise in the incidence of esophageal adenocarcinoma, mostly diagnosed at an advanced stage when curative treatment is no longer an option. Esophageal adenocarcinoma develops from Barrett’s esophagus that, if known to be present, can be surveilled to detect dysplasia and cancer at an early and curable stage.
Whereas currently screening for Barrett’s esophagus focused on White males with gastroesophageal reflux, little was known about screening in non-White and non-male populations. Identifying who and how to screen poses a challenge, and in real life such studies looking at varied populations would require many patients, years of follow-up, much effort and substantial costs. Rubenstein and colleagues used three independent simulation models to simulate many different screening scenarios, while taking gender and race into account. The outcomes of this study, which demonstrate that one size does not fit all, will be very relevant in guiding future strategies regarding screening for Barrett’s esophagus and early esophageal adenocarcinoma. Although the study is based around endoscopic screening, the insights gained from this study will also be relevant when considering the use of nonendoscopic screening tools.
R.E. Pouw, MD, PhD, is with Amsterdam University Medical Centers. She disclosed having been a consultant for MicroTech and Medtronic and having received speaker fees from Pentax.
Over the past decades we have seen an alarming rise in the incidence of esophageal adenocarcinoma, mostly diagnosed at an advanced stage when curative treatment is no longer an option. Esophageal adenocarcinoma develops from Barrett’s esophagus that, if known to be present, can be surveilled to detect dysplasia and cancer at an early and curable stage.
Whereas currently screening for Barrett’s esophagus focused on White males with gastroesophageal reflux, little was known about screening in non-White and non-male populations. Identifying who and how to screen poses a challenge, and in real life such studies looking at varied populations would require many patients, years of follow-up, much effort and substantial costs. Rubenstein and colleagues used three independent simulation models to simulate many different screening scenarios, while taking gender and race into account. The outcomes of this study, which demonstrate that one size does not fit all, will be very relevant in guiding future strategies regarding screening for Barrett’s esophagus and early esophageal adenocarcinoma. Although the study is based around endoscopic screening, the insights gained from this study will also be relevant when considering the use of nonendoscopic screening tools.
R.E. Pouw, MD, PhD, is with Amsterdam University Medical Centers. She disclosed having been a consultant for MicroTech and Medtronic and having received speaker fees from Pentax.
Endoscopic screening for esophageal adenocarcinoma (EAC), may not be a cost-effective strategy for all populations, possibly even leading to net harm in some, according to a comparative cost-effectiveness analysis.
Several U.S. guidelines suggest the use of endoscopic screening for EAC, yet recommendations within these guidelines vary in terms of which population should receive screening, according study authors led by Joel H. Rubenstein, MD, of the Lieutenant Charles S. Kettles Veterans Affairs Medical Center, Ann Arbor, Mich. Their findings were published in Gastroenterology. In addition, there have been no randomized trials to date that have evaluated endoscopic screening outcomes among different populations. Population screening recommendations in the current guidelines have been informed mostly by observational data and expert opinion.
Existing cost-effectiveness analyses of EAC screening have mostly focused on screening older men with gastroesophageal reflux disease (GERD) at certain ages, and many of these analyses have limited data regarding diverse patient populations.
In their study, Dr. Rubenstein and colleagues performed a comparative cost-effectiveness analysis of endoscopic screening for EAC that was restricted to individuals with GERD symptoms in the general population. The analysis was stratified by race and sex. The primary objective of the analysis was to identify and establish the optimal age at which to offer endoscopic screening in the specific populations evaluated in the study.
The investigators conducted their comparative cost-effectiveness analyses using three independent simulation models. The independently developed models – which focused on EAC natural history, screening, surveillance, and treatment – are part of the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network. For each model, there were four cohorts, defined by race as either White or Black and sex, which were independently calibrated to targets to reproduce the EAC incidence in the United States. The three models were based on somewhat different structures and assumptions; for example, two of the models assumed stable prevalence of GERD symptoms of approximately 20% across ages, while the third assumed a near-linear increase across adulthood. All three assumed EAC develops only in individuals with Barrett’s esophagus.
In each base case, the researchers simulated cohorts of people in the United States who were born in 1950, and then stratified these individuals by race and sex and followed each individual from 40 years of age until 100 years of age. The researchers considered 42 strategies, such as no screening, a single endoscopic screening at six specified ages (between 40 and 65 years of age), and a single screening in individuals with GERD symptoms at the six specified ages.
Primary results were the averaged results across all three models. The optimal screening strategy, defined by the investigators, was the strategy with the highest effectiveness that had an incremental cost-effectiveness ratio of less than $100,000 per quality-adjusted life-year gained.
The most effective – yet the most costly – screening strategies for White men were those that screened all of them once between 40 and 55 years of age. The optimal screening strategy, however, was one that screened individuals with GERD twice, once at age 45 years and again at 60 years. The researchers determined that screening Black men with GERD once at 55 years of age was optimal.
By contrast, the optimal strategy for women, whether White or Black, was no screening at all. “In particular, among Black women, screening is, at best, very expensive with little benefit, and some strategies cause net harm,” the authors wrote.
The investigators wrote that there is a need for empiric, long-term studies “to confirm whether repeated screening has a substantial yield of incident” Barrett’s esophagus. The researchers also noted that their study was limited by the lack of inclusion of additional risk factors, such as smoking, obesity, and family history, which may have led to different conclusions on specific screening strategies.
“We certainly acknowledge the history of health care inequities, and that race is a social construct that, in the vast majority of medical contexts, has no biological basis. We are circumspect regarding making recommendations based on race or sex if environmental exposures or genetic factors on which to make to those recommendations were available,” they wrote.
The study was supported by National Institutes of Health/National Cancer Institute grants. Some authors disclosed relationships with Lucid Diagnostics, Value Analytics Labs, and Cernostics.
Endoscopic screening for esophageal adenocarcinoma (EAC), may not be a cost-effective strategy for all populations, possibly even leading to net harm in some, according to a comparative cost-effectiveness analysis.
Several U.S. guidelines suggest the use of endoscopic screening for EAC, yet recommendations within these guidelines vary in terms of which population should receive screening, according study authors led by Joel H. Rubenstein, MD, of the Lieutenant Charles S. Kettles Veterans Affairs Medical Center, Ann Arbor, Mich. Their findings were published in Gastroenterology. In addition, there have been no randomized trials to date that have evaluated endoscopic screening outcomes among different populations. Population screening recommendations in the current guidelines have been informed mostly by observational data and expert opinion.
Existing cost-effectiveness analyses of EAC screening have mostly focused on screening older men with gastroesophageal reflux disease (GERD) at certain ages, and many of these analyses have limited data regarding diverse patient populations.
In their study, Dr. Rubenstein and colleagues performed a comparative cost-effectiveness analysis of endoscopic screening for EAC that was restricted to individuals with GERD symptoms in the general population. The analysis was stratified by race and sex. The primary objective of the analysis was to identify and establish the optimal age at which to offer endoscopic screening in the specific populations evaluated in the study.
The investigators conducted their comparative cost-effectiveness analyses using three independent simulation models. The independently developed models – which focused on EAC natural history, screening, surveillance, and treatment – are part of the National Cancer Institute’s Cancer Intervention and Surveillance Modeling Network. For each model, there were four cohorts, defined by race as either White or Black and sex, which were independently calibrated to targets to reproduce the EAC incidence in the United States. The three models were based on somewhat different structures and assumptions; for example, two of the models assumed stable prevalence of GERD symptoms of approximately 20% across ages, while the third assumed a near-linear increase across adulthood. All three assumed EAC develops only in individuals with Barrett’s esophagus.
In each base case, the researchers simulated cohorts of people in the United States who were born in 1950, and then stratified these individuals by race and sex and followed each individual from 40 years of age until 100 years of age. The researchers considered 42 strategies, such as no screening, a single endoscopic screening at six specified ages (between 40 and 65 years of age), and a single screening in individuals with GERD symptoms at the six specified ages.
Primary results were the averaged results across all three models. The optimal screening strategy, defined by the investigators, was the strategy with the highest effectiveness that had an incremental cost-effectiveness ratio of less than $100,000 per quality-adjusted life-year gained.
The most effective – yet the most costly – screening strategies for White men were those that screened all of them once between 40 and 55 years of age. The optimal screening strategy, however, was one that screened individuals with GERD twice, once at age 45 years and again at 60 years. The researchers determined that screening Black men with GERD once at 55 years of age was optimal.
By contrast, the optimal strategy for women, whether White or Black, was no screening at all. “In particular, among Black women, screening is, at best, very expensive with little benefit, and some strategies cause net harm,” the authors wrote.
The investigators wrote that there is a need for empiric, long-term studies “to confirm whether repeated screening has a substantial yield of incident” Barrett’s esophagus. The researchers also noted that their study was limited by the lack of inclusion of additional risk factors, such as smoking, obesity, and family history, which may have led to different conclusions on specific screening strategies.
“We certainly acknowledge the history of health care inequities, and that race is a social construct that, in the vast majority of medical contexts, has no biological basis. We are circumspect regarding making recommendations based on race or sex if environmental exposures or genetic factors on which to make to those recommendations were available,” they wrote.
The study was supported by National Institutes of Health/National Cancer Institute grants. Some authors disclosed relationships with Lucid Diagnostics, Value Analytics Labs, and Cernostics.
FROM GASTROENTEROLOGY
Abbott baby formula plant in Michigan reopens
The Abbott baby formula factory in Sturgis, Mich., has reopened, a move that could ease the nationwide baby formula shortage.
“Abbott is restarting infant formula production at its Sturgis, Mich., facility today after meeting initial requirements agreed to with the U.S. Food and Drug Administration as part of the consent decree entered into on May 16,” according to a company statement issued June 4.
“Abbott is starting production of EleCare and other specialty and metabolic formulas, with initial EleCare product release to consumers beginning on or about June 20. We’re also working hard to fulfill the steps necessary to restart production of Similac and other formulas and will do so as soon as we can.”
The FDA began investigating when at least four infants became ill with Cronobacter sakazakii bacteria after consuming infant formula produced in the Sturgis plant. Two infants died. Several Abbott baby formula products were recalled and the Sturgis plant was shut down for months.
Abbott said an investigation found no evidence to link the formulas to the infant illnesses, though bacteria was found in parts of the factory that didn’t have contact with formula.
The FDA entered into a consent decree with Abbott in mid-May that allowed the plant to reopen if the company took corrective actions, including the implementation of a sanitation plan and an environmental monitoring plan and employee training programs. Abbott must also retain an independent expert to monitor operations.
The Abbott shutdown, along with supply chain problems, contributed to a nationwide shortage of formula. Reuters, citing the data firm Datasembly, reported that about 73% of baby products were out of stock nationwide as of May 22.
The shortage is so severe that the federal government authorized the importing of formula from overseas.
“We understand the urgent need for formula and our top priority is getting high-quality, safe formula into the hands of families across America. We will ramp production as quickly as we can while meeting all requirements,” the Abbott statement said.
A version of this article first appeared on Webmd.com.
The Abbott baby formula factory in Sturgis, Mich., has reopened, a move that could ease the nationwide baby formula shortage.
“Abbott is restarting infant formula production at its Sturgis, Mich., facility today after meeting initial requirements agreed to with the U.S. Food and Drug Administration as part of the consent decree entered into on May 16,” according to a company statement issued June 4.
“Abbott is starting production of EleCare and other specialty and metabolic formulas, with initial EleCare product release to consumers beginning on or about June 20. We’re also working hard to fulfill the steps necessary to restart production of Similac and other formulas and will do so as soon as we can.”
The FDA began investigating when at least four infants became ill with Cronobacter sakazakii bacteria after consuming infant formula produced in the Sturgis plant. Two infants died. Several Abbott baby formula products were recalled and the Sturgis plant was shut down for months.
Abbott said an investigation found no evidence to link the formulas to the infant illnesses, though bacteria was found in parts of the factory that didn’t have contact with formula.
The FDA entered into a consent decree with Abbott in mid-May that allowed the plant to reopen if the company took corrective actions, including the implementation of a sanitation plan and an environmental monitoring plan and employee training programs. Abbott must also retain an independent expert to monitor operations.
The Abbott shutdown, along with supply chain problems, contributed to a nationwide shortage of formula. Reuters, citing the data firm Datasembly, reported that about 73% of baby products were out of stock nationwide as of May 22.
The shortage is so severe that the federal government authorized the importing of formula from overseas.
“We understand the urgent need for formula and our top priority is getting high-quality, safe formula into the hands of families across America. We will ramp production as quickly as we can while meeting all requirements,” the Abbott statement said.
A version of this article first appeared on Webmd.com.
The Abbott baby formula factory in Sturgis, Mich., has reopened, a move that could ease the nationwide baby formula shortage.
“Abbott is restarting infant formula production at its Sturgis, Mich., facility today after meeting initial requirements agreed to with the U.S. Food and Drug Administration as part of the consent decree entered into on May 16,” according to a company statement issued June 4.
“Abbott is starting production of EleCare and other specialty and metabolic formulas, with initial EleCare product release to consumers beginning on or about June 20. We’re also working hard to fulfill the steps necessary to restart production of Similac and other formulas and will do so as soon as we can.”
The FDA began investigating when at least four infants became ill with Cronobacter sakazakii bacteria after consuming infant formula produced in the Sturgis plant. Two infants died. Several Abbott baby formula products were recalled and the Sturgis plant was shut down for months.
Abbott said an investigation found no evidence to link the formulas to the infant illnesses, though bacteria was found in parts of the factory that didn’t have contact with formula.
The FDA entered into a consent decree with Abbott in mid-May that allowed the plant to reopen if the company took corrective actions, including the implementation of a sanitation plan and an environmental monitoring plan and employee training programs. Abbott must also retain an independent expert to monitor operations.
The Abbott shutdown, along with supply chain problems, contributed to a nationwide shortage of formula. Reuters, citing the data firm Datasembly, reported that about 73% of baby products were out of stock nationwide as of May 22.
The shortage is so severe that the federal government authorized the importing of formula from overseas.
“We understand the urgent need for formula and our top priority is getting high-quality, safe formula into the hands of families across America. We will ramp production as quickly as we can while meeting all requirements,” the Abbott statement said.
A version of this article first appeared on Webmd.com.
Adhesive Tape to Guide Injection Depth of Botulinum Toxin for Axillary Hyperhidrosis
Practice Gap
OnabotulinumtoxinA is a US Food and Drug Administration–approved second-line treatment of axillary hyperhidrosis, with a long-term success rate greater than 80% and minimal adverse effects.1 The recommended depth and angle of injection of onabotulinumtoxinA for most cases of primary hyperhidrosis is 2 to 3 mm at a 45° angle to the skin surface.2 This small depth is difficult to accurately estimate once the needle tip is in the skin.
Injection Technique
We have found that measuring 2 to 3 mm on the needle tip and then wrapping a piece of adhesive tape at that point acts as a depth guide (Figure 1). The flag shape of the tape acts as a physical barrier to prevent the needle tip from penetrating too deeply (Figure 2). This barrier also allows the injector to inject quickly to reduce the amount of pain that the patient experiences.
Practice Implications
Applying adhesive tape to a needle tip at a premeasured distance is a fast, inexpensive, and effective tool to aid accurate depth of injection for both experienced clinicians and clinicians in-training. The tape is a common office supply and the amount of tape used for a patient costs a fraction of a cent. Additionally, applying the tape takes less than 1 minute. This technique is useful for axillary hyperhidrosis injection (Figures 1 and 2) but could be used in palmar and plantar hyperhidrosis injections as well as injections other than onabotulinumtoxinA that require a specific fixed depth.
- Naumann M, Lowe NJ, Kumar CR, et al; Hyperhidrosis Clinical Investigators Group. Botulinum toxin type A is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study. Arch Dermatol. 2003;139:731-736. doi:10.1001/archderm.139.6.731
- Botox. Prescribing information. Allergan Pharmaceuticals Ireland;2011. Accessed May 12, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5236lbl.pdf
Practice Gap
OnabotulinumtoxinA is a US Food and Drug Administration–approved second-line treatment of axillary hyperhidrosis, with a long-term success rate greater than 80% and minimal adverse effects.1 The recommended depth and angle of injection of onabotulinumtoxinA for most cases of primary hyperhidrosis is 2 to 3 mm at a 45° angle to the skin surface.2 This small depth is difficult to accurately estimate once the needle tip is in the skin.
Injection Technique
We have found that measuring 2 to 3 mm on the needle tip and then wrapping a piece of adhesive tape at that point acts as a depth guide (Figure 1). The flag shape of the tape acts as a physical barrier to prevent the needle tip from penetrating too deeply (Figure 2). This barrier also allows the injector to inject quickly to reduce the amount of pain that the patient experiences.
Practice Implications
Applying adhesive tape to a needle tip at a premeasured distance is a fast, inexpensive, and effective tool to aid accurate depth of injection for both experienced clinicians and clinicians in-training. The tape is a common office supply and the amount of tape used for a patient costs a fraction of a cent. Additionally, applying the tape takes less than 1 minute. This technique is useful for axillary hyperhidrosis injection (Figures 1 and 2) but could be used in palmar and plantar hyperhidrosis injections as well as injections other than onabotulinumtoxinA that require a specific fixed depth.
Practice Gap
OnabotulinumtoxinA is a US Food and Drug Administration–approved second-line treatment of axillary hyperhidrosis, with a long-term success rate greater than 80% and minimal adverse effects.1 The recommended depth and angle of injection of onabotulinumtoxinA for most cases of primary hyperhidrosis is 2 to 3 mm at a 45° angle to the skin surface.2 This small depth is difficult to accurately estimate once the needle tip is in the skin.
Injection Technique
We have found that measuring 2 to 3 mm on the needle tip and then wrapping a piece of adhesive tape at that point acts as a depth guide (Figure 1). The flag shape of the tape acts as a physical barrier to prevent the needle tip from penetrating too deeply (Figure 2). This barrier also allows the injector to inject quickly to reduce the amount of pain that the patient experiences.
Practice Implications
Applying adhesive tape to a needle tip at a premeasured distance is a fast, inexpensive, and effective tool to aid accurate depth of injection for both experienced clinicians and clinicians in-training. The tape is a common office supply and the amount of tape used for a patient costs a fraction of a cent. Additionally, applying the tape takes less than 1 minute. This technique is useful for axillary hyperhidrosis injection (Figures 1 and 2) but could be used in palmar and plantar hyperhidrosis injections as well as injections other than onabotulinumtoxinA that require a specific fixed depth.
- Naumann M, Lowe NJ, Kumar CR, et al; Hyperhidrosis Clinical Investigators Group. Botulinum toxin type A is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study. Arch Dermatol. 2003;139:731-736. doi:10.1001/archderm.139.6.731
- Botox. Prescribing information. Allergan Pharmaceuticals Ireland;2011. Accessed May 12, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5236lbl.pdf
- Naumann M, Lowe NJ, Kumar CR, et al; Hyperhidrosis Clinical Investigators Group. Botulinum toxin type A is a safe and effective treatment for axillary hyperhidrosis over 16 months: a prospective study. Arch Dermatol. 2003;139:731-736. doi:10.1001/archderm.139.6.731
- Botox. Prescribing information. Allergan Pharmaceuticals Ireland;2011. Accessed May 12, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/103000s5236lbl.pdf
Home-grown apps for ObGyn clerkship students
Technology has revolutionized how we access information. One example is the increased use of mobile applications (apps). On the surface, building a new app may seem a daunting and intimidating task. However, new software—such as Glide (glideapps.com)—make it easy for anyone to design, build, and launch a custom web app within hours. This software is free for basic apps but does offer an upgrade for those wanting more professional services (glide-apps.com/pro). Here, by way of example, we identify an area of need and walk the reader
through the process of making an app.
Although there are many apps aimed at educating users on different aspects of obstetrics and gynecology, few are focused on undergraduate medical education (UME). With the assistance of Glide app building software, we created an app focused on providing rapid access to resources aimed at fulfilling medical student objectives from the Association of Professors of Gynecology and Obstetrics (APGO).1 We included 16 of the APGO objectives. On clicking an objective, the user is taken to a screen with links to associated APGO Educational Topic Video and Basic Science Videos. Basic Science Video links were included in order to provide longitudinal learning between the pre-clinical and clinical years of UME. We also created a tab for additional educational resources (including excerpts from the APGO Basic Clinical Skills Curriculum).2 We eventually added two other tabs: one for clerkship schedules that allows students to organize their daily schedule and another that facilitates quick contact with members of the clerkship team. As expected, the app was well-received by our students.
The steps needed for you to make your own app are listed in the FIGURE along with accompanying images for easy navigation. ●
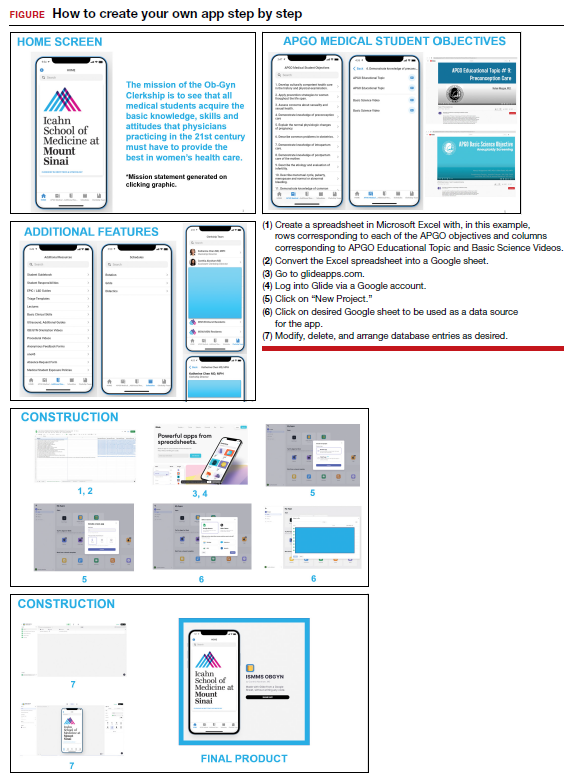
- Association of Professors of Gynecology and Obstetrics (APGO) Medical Student Educational Objectives, 11th ed;2019.
- Association of Professors of Gynecology and Obstetrics (APGO) Basic Clinical Skills Curriculum. Updated 2017.
Technology has revolutionized how we access information. One example is the increased use of mobile applications (apps). On the surface, building a new app may seem a daunting and intimidating task. However, new software—such as Glide (glideapps.com)—make it easy for anyone to design, build, and launch a custom web app within hours. This software is free for basic apps but does offer an upgrade for those wanting more professional services (glide-apps.com/pro). Here, by way of example, we identify an area of need and walk the reader
through the process of making an app.
Although there are many apps aimed at educating users on different aspects of obstetrics and gynecology, few are focused on undergraduate medical education (UME). With the assistance of Glide app building software, we created an app focused on providing rapid access to resources aimed at fulfilling medical student objectives from the Association of Professors of Gynecology and Obstetrics (APGO).1 We included 16 of the APGO objectives. On clicking an objective, the user is taken to a screen with links to associated APGO Educational Topic Video and Basic Science Videos. Basic Science Video links were included in order to provide longitudinal learning between the pre-clinical and clinical years of UME. We also created a tab for additional educational resources (including excerpts from the APGO Basic Clinical Skills Curriculum).2 We eventually added two other tabs: one for clerkship schedules that allows students to organize their daily schedule and another that facilitates quick contact with members of the clerkship team. As expected, the app was well-received by our students.
The steps needed for you to make your own app are listed in the FIGURE along with accompanying images for easy navigation. ●

Technology has revolutionized how we access information. One example is the increased use of mobile applications (apps). On the surface, building a new app may seem a daunting and intimidating task. However, new software—such as Glide (glideapps.com)—make it easy for anyone to design, build, and launch a custom web app within hours. This software is free for basic apps but does offer an upgrade for those wanting more professional services (glide-apps.com/pro). Here, by way of example, we identify an area of need and walk the reader
through the process of making an app.
Although there are many apps aimed at educating users on different aspects of obstetrics and gynecology, few are focused on undergraduate medical education (UME). With the assistance of Glide app building software, we created an app focused on providing rapid access to resources aimed at fulfilling medical student objectives from the Association of Professors of Gynecology and Obstetrics (APGO).1 We included 16 of the APGO objectives. On clicking an objective, the user is taken to a screen with links to associated APGO Educational Topic Video and Basic Science Videos. Basic Science Video links were included in order to provide longitudinal learning between the pre-clinical and clinical years of UME. We also created a tab for additional educational resources (including excerpts from the APGO Basic Clinical Skills Curriculum).2 We eventually added two other tabs: one for clerkship schedules that allows students to organize their daily schedule and another that facilitates quick contact with members of the clerkship team. As expected, the app was well-received by our students.
The steps needed for you to make your own app are listed in the FIGURE along with accompanying images for easy navigation. ●

- Association of Professors of Gynecology and Obstetrics (APGO) Medical Student Educational Objectives, 11th ed;2019.
- Association of Professors of Gynecology and Obstetrics (APGO) Basic Clinical Skills Curriculum. Updated 2017.
- Association of Professors of Gynecology and Obstetrics (APGO) Medical Student Educational Objectives, 11th ed;2019.
- Association of Professors of Gynecology and Obstetrics (APGO) Basic Clinical Skills Curriculum. Updated 2017.
Immunosuppressed rheumatic patients not at high risk of breakthrough COVID-19
COPENHAGEN – Most patients with immune-mediated inflammatory diseases (IMID) should not be considered at high risk for severe COVID-19 breakthrough infections, but those on anti-CD20 therapy are the exception, data from a large prospective, cohort study show.
“Overall, the data are reassuring, with conventional risk factors, such as age, and comorbidities seeming to be more important regarding risk of severe COVID-19 breakthrough infections than rheumatic disease or immunosuppressant medication,” said Laura Boekel, MD, from Amsterdam UMC, who presented the study at the annual European Congress of Rheumatology.
But, she added, there was an exception for anti-CD20 therapy. “This is especially relevant for patients with conventional risk factors that might accumulate, and rheumatologists might want to consider alternative treatment options if possible. It is important to inform patients about the risks of anti-CD20.”
Another study, presented during the same session at the congress by Rebecca Hasseli, MD, from the University of Giessen (Germany) saw no deaths and no COVID-19 related complications in a cohort of triple-vaccinated patients with inflammatory rheumatic diseases, despite a higher median age and a higher rate of comorbidities compared to double-vaccinated and unvaccinated cohorts.
Ingrid Jyssum, MD, from Diakonhjemmet Hospital, Oslo, who presented results of the Nor-vaC study investigating the impact of different DMARDs on the immunogenicity of a third COVID-19 vaccine dose, welcomed the research by Dr. Boekel and Dr. Hasseli.
“The findings of Hasseli are interesting in the light of our data on serological response after the third dose, with a lack of breakthrough infections after three doses corresponding well to the robust antibody response that we found in our cohort,” she remarked. “This is very reassuring for our patients. Our own work together with the findings of Hasseli and Boekel demonstrate that additional vaccine doses are important to keep this population well protected against severe COVID-19 infections.”
The Nor-vaC study was conducted with a cohort of 1,100 patients with inflammatory joint and bowel diseases. “These patients had attenuated antibody responses after two vaccine doses; however, we found that a third vaccine dose brought the humoral response in patients up to the antibody levels that healthy controls had after two doses,” said Dr. Jyssum. “In addition, we found that the decline in antibodies after the third dose was less than the decline seen after the second dose. Importantly, the third dose was safe in our patients, with no new safety issues.”
Breakthrough infections and immunosuppressants
“Like the rest of the world, we were wondering if our patients were at increased risk of COVID-19, and if the immunosuppressants used by these patients influenced their risk,” said Dr. Boekel.
The researchers compared both the incidence and severity of COVID-19 breakthrough infections with the SARS-CoV-2 Delta variant in a population of fully vaccinated IMID patients taking immunosuppressants and controls (IMID patients not taking immunosuppressants and healthy controls).
Two large ongoing, prospective, multicenter cohort studies provided pooled data collected between February and December 2021 using digital questionnaires, standardized electronic case record forms, and medical files.
Finger-prick tests were used to collect blood samples that were analyzed after vaccination against SARS-CoV-2 for anti–receptor-binding domain (RBD) antibodies, and antinucleocapsid antibodies to identify asymptomatic breakthrough infections. Any associations between antibodies and the incidence of breakthrough infections were generated, and results were adjusted for sex, cardiovascular disease, chronic pulmonary disease, obesity, and vaccine type.
The analysis included 3,207 IMID patients taking immunosuppressants, and 1,810 controls (985 IMID patients not on immunosuppressants and 825 healthy controls).
Initially, Dr. Boekel and her colleagues looked at incidence of infections and hospitalizations prior to vaccination, and then after vaccination, which was the main aim of the study.
Prior to vaccination, hospitalization risk for COVID-19 was somewhat higher for IMID patients overall compared with controls, reported Dr. Boekel. “But those treated with anti-CD20 therapy, demonstrated much greater risk for severe disease.”
After the SARS-CoV-2 vaccination campaign began, the researchers then looked at how immunosuppressants influenced humoral response to SARS-CoV-2 vaccination.
“Anti-CD20 therapy showed the greatest impact on humoral immune response after SARS-CoV-2 vaccination,” said Dr. Boekel. Other immunosuppressant drugs had variable effects on humoral and cellular immunity.
Once they had established that immunosuppressant drugs impaired immune responses to SARS-CoV-2 vaccination, the researchers wanted to determine if this affected clinical outcomes. Blood samples taken 28 days after the second vaccination enabled Dr. Boekel and her colleagues to see if antibody production was associated with breakthrough infections.
Breakthrough infections were seen in 5% of patients on immunosuppressants, 5% of patients not on immunosuppressants, and 4% of healthy controls. Also, asymptomatic COVID-19 breakthrough cases were comparable between IMID patients taking immunosuppressants and controls, at 10% in each group.
“We saw that the incidence [of getting COVID-19] was comparable between groups, independent of whether they were receiving immunosuppressants or not, or healthy controls. However, if they developed antibodies against the two vaccinations the chance of getting infected was lower,” reported Dr. Boekel.
Hospitalization (severe disease) rates were also comparable between groups. “Patients with rheumatic diseases, even when treated with immunosuppressants were not at increased risk of severe disease from Delta breakthrough infections,” added the researcher. “Cases that were hospitalized were mainly elderly and those with comorbidities, for example cardiovascular disease and cardiopulmonary disease.”
Hospital admissions were 5.4% in patients on immunosuppressants, 5.7% in those not on immunosuppressants, and 6% in health controls.
However, once again, there was one exception, Dr. Boekel stressed. “Patients treated with anti-CD20 therapy were at increased risk of severe disease and hospitalization.”
Omicron variant has a different transmissibility than Delta, so the researchers continued the study looking at the Omicron variant. The data “were mostly reassuring,” said Dr. Boekel. “As expected, hospitalization rates decreased overall, with the exception of patients on anti-CD20 therapy where, despite overall reduced pathogenicity, patients remain at increased risk.”
She said that they were awaiting long-term data so the data reflect only short-term immunity against Omicron. “However, we included many elderly and patients with comorbidities, so this made the analysis very sensitive to detect severe cases,” she added.
Breakthrough infection among double- and triple-vaccinated patients
A lower rate of COVID-19 related complications and deaths were seen in patients who were triple-vaccinated against SARS-CoV-2, than in double-vaccinated or unvaccinated patients, despite the former having more comorbidities and use of rituximab (Rituxan), said Dr. Hasseli.
“These data support the recommendation of booster vaccination to reduce COVID-19-related mortality in patients with inflammatory rheumatic diseases [IRDs],” she said.
“A small number of COVID-19 cases were seen in patients with IRD after vaccinations, and in a few cases, hospitalizations were required. Breakthrough infections were mostly seen in patients on B-cell depletion therapy,” she added.
Dr. Hasseli and her colleagues looked at the characteristics and outcomes of SARS-CoV-2 breakthrough infections among double- and triple-vaccinated patients with IRD.
“We wanted to understand if patients with IRD are protected in the same way as the general population following vaccination, given that these patients receive drugs that might impair the immune response,” she explained.
Data for analysis were drawn from the German COVID-19-IRD registry covering February 2021 and January 2022, and patients who were double- or triple- vaccinated against COVID-19 either 14 days or more prior to a SARS-CoV-2 infection were included. Type of IRD, vaccine, immunomodulation, comorbidities, and outcome of the infection were compared with 737 unvaccinated IRD patients with COVID-19. Those with prior COVID-19 were excluded.
Cases were stratified by vaccinations status: unvaccinated (1,388 patients, median age 57 years); double vaccinated (462, 56 years) and triple vaccinated (301, 53 years). Body mass index was similar across groups (25-26 kg/m2), and time between SARS-CoV-2 infection and last vaccination was 156 days in double-vaccinated patients, and 62 days in triple-vaccinated patients.
Patients had rheumatoid arthritis in 44.7% and 44.4% of unvaccinated and double-vaccinated patients respectively, but fewer triple-vaccinated patients had RA (37.2%). Triple vaccination was seen in 32.2% of patients with spondyloarthritis, 16.6% connective tissue diseases, 5.3% other vasculitis, and 3.3% ANCA-associated vasculitis. Of triple-vaccinated patients, 26.2% were treated with tumor necrosis factor-alpha (TNF-alpha) inhibitors, and 6.3% with rituximab, while 5.3% were not on immunomodulation. At least 25% were treated with glucocorticoids, reported Dr. Hasseli.
“Arterial hypertension and diabetes, that might be risk factors for COVID-19, were less frequently reported in triple-vaccinated patients. More patients in the double-vaccinated group [42.9%] than the triple-vaccinated [23.8%] reported absence of relevant comorbidities,” she said.
COVID-19 related complications were less often reported in double- and triple-vaccinated groups with hospitalizations at 9.5% and 4.3% in double and triple-vaccinated people respectively.
Dr. Boekel and Dr. Hasseli report no relevant conflicts of interest.
COPENHAGEN – Most patients with immune-mediated inflammatory diseases (IMID) should not be considered at high risk for severe COVID-19 breakthrough infections, but those on anti-CD20 therapy are the exception, data from a large prospective, cohort study show.
“Overall, the data are reassuring, with conventional risk factors, such as age, and comorbidities seeming to be more important regarding risk of severe COVID-19 breakthrough infections than rheumatic disease or immunosuppressant medication,” said Laura Boekel, MD, from Amsterdam UMC, who presented the study at the annual European Congress of Rheumatology.
But, she added, there was an exception for anti-CD20 therapy. “This is especially relevant for patients with conventional risk factors that might accumulate, and rheumatologists might want to consider alternative treatment options if possible. It is important to inform patients about the risks of anti-CD20.”
Another study, presented during the same session at the congress by Rebecca Hasseli, MD, from the University of Giessen (Germany) saw no deaths and no COVID-19 related complications in a cohort of triple-vaccinated patients with inflammatory rheumatic diseases, despite a higher median age and a higher rate of comorbidities compared to double-vaccinated and unvaccinated cohorts.
Ingrid Jyssum, MD, from Diakonhjemmet Hospital, Oslo, who presented results of the Nor-vaC study investigating the impact of different DMARDs on the immunogenicity of a third COVID-19 vaccine dose, welcomed the research by Dr. Boekel and Dr. Hasseli.
“The findings of Hasseli are interesting in the light of our data on serological response after the third dose, with a lack of breakthrough infections after three doses corresponding well to the robust antibody response that we found in our cohort,” she remarked. “This is very reassuring for our patients. Our own work together with the findings of Hasseli and Boekel demonstrate that additional vaccine doses are important to keep this population well protected against severe COVID-19 infections.”
The Nor-vaC study was conducted with a cohort of 1,100 patients with inflammatory joint and bowel diseases. “These patients had attenuated antibody responses after two vaccine doses; however, we found that a third vaccine dose brought the humoral response in patients up to the antibody levels that healthy controls had after two doses,” said Dr. Jyssum. “In addition, we found that the decline in antibodies after the third dose was less than the decline seen after the second dose. Importantly, the third dose was safe in our patients, with no new safety issues.”
Breakthrough infections and immunosuppressants
“Like the rest of the world, we were wondering if our patients were at increased risk of COVID-19, and if the immunosuppressants used by these patients influenced their risk,” said Dr. Boekel.
The researchers compared both the incidence and severity of COVID-19 breakthrough infections with the SARS-CoV-2 Delta variant in a population of fully vaccinated IMID patients taking immunosuppressants and controls (IMID patients not taking immunosuppressants and healthy controls).
Two large ongoing, prospective, multicenter cohort studies provided pooled data collected between February and December 2021 using digital questionnaires, standardized electronic case record forms, and medical files.
Finger-prick tests were used to collect blood samples that were analyzed after vaccination against SARS-CoV-2 for anti–receptor-binding domain (RBD) antibodies, and antinucleocapsid antibodies to identify asymptomatic breakthrough infections. Any associations between antibodies and the incidence of breakthrough infections were generated, and results were adjusted for sex, cardiovascular disease, chronic pulmonary disease, obesity, and vaccine type.
The analysis included 3,207 IMID patients taking immunosuppressants, and 1,810 controls (985 IMID patients not on immunosuppressants and 825 healthy controls).
Initially, Dr. Boekel and her colleagues looked at incidence of infections and hospitalizations prior to vaccination, and then after vaccination, which was the main aim of the study.
Prior to vaccination, hospitalization risk for COVID-19 was somewhat higher for IMID patients overall compared with controls, reported Dr. Boekel. “But those treated with anti-CD20 therapy, demonstrated much greater risk for severe disease.”
After the SARS-CoV-2 vaccination campaign began, the researchers then looked at how immunosuppressants influenced humoral response to SARS-CoV-2 vaccination.
“Anti-CD20 therapy showed the greatest impact on humoral immune response after SARS-CoV-2 vaccination,” said Dr. Boekel. Other immunosuppressant drugs had variable effects on humoral and cellular immunity.
Once they had established that immunosuppressant drugs impaired immune responses to SARS-CoV-2 vaccination, the researchers wanted to determine if this affected clinical outcomes. Blood samples taken 28 days after the second vaccination enabled Dr. Boekel and her colleagues to see if antibody production was associated with breakthrough infections.
Breakthrough infections were seen in 5% of patients on immunosuppressants, 5% of patients not on immunosuppressants, and 4% of healthy controls. Also, asymptomatic COVID-19 breakthrough cases were comparable between IMID patients taking immunosuppressants and controls, at 10% in each group.
“We saw that the incidence [of getting COVID-19] was comparable between groups, independent of whether they were receiving immunosuppressants or not, or healthy controls. However, if they developed antibodies against the two vaccinations the chance of getting infected was lower,” reported Dr. Boekel.
Hospitalization (severe disease) rates were also comparable between groups. “Patients with rheumatic diseases, even when treated with immunosuppressants were not at increased risk of severe disease from Delta breakthrough infections,” added the researcher. “Cases that were hospitalized were mainly elderly and those with comorbidities, for example cardiovascular disease and cardiopulmonary disease.”
Hospital admissions were 5.4% in patients on immunosuppressants, 5.7% in those not on immunosuppressants, and 6% in health controls.
However, once again, there was one exception, Dr. Boekel stressed. “Patients treated with anti-CD20 therapy were at increased risk of severe disease and hospitalization.”
Omicron variant has a different transmissibility than Delta, so the researchers continued the study looking at the Omicron variant. The data “were mostly reassuring,” said Dr. Boekel. “As expected, hospitalization rates decreased overall, with the exception of patients on anti-CD20 therapy where, despite overall reduced pathogenicity, patients remain at increased risk.”
She said that they were awaiting long-term data so the data reflect only short-term immunity against Omicron. “However, we included many elderly and patients with comorbidities, so this made the analysis very sensitive to detect severe cases,” she added.
Breakthrough infection among double- and triple-vaccinated patients
A lower rate of COVID-19 related complications and deaths were seen in patients who were triple-vaccinated against SARS-CoV-2, than in double-vaccinated or unvaccinated patients, despite the former having more comorbidities and use of rituximab (Rituxan), said Dr. Hasseli.
“These data support the recommendation of booster vaccination to reduce COVID-19-related mortality in patients with inflammatory rheumatic diseases [IRDs],” she said.
“A small number of COVID-19 cases were seen in patients with IRD after vaccinations, and in a few cases, hospitalizations were required. Breakthrough infections were mostly seen in patients on B-cell depletion therapy,” she added.
Dr. Hasseli and her colleagues looked at the characteristics and outcomes of SARS-CoV-2 breakthrough infections among double- and triple-vaccinated patients with IRD.
“We wanted to understand if patients with IRD are protected in the same way as the general population following vaccination, given that these patients receive drugs that might impair the immune response,” she explained.
Data for analysis were drawn from the German COVID-19-IRD registry covering February 2021 and January 2022, and patients who were double- or triple- vaccinated against COVID-19 either 14 days or more prior to a SARS-CoV-2 infection were included. Type of IRD, vaccine, immunomodulation, comorbidities, and outcome of the infection were compared with 737 unvaccinated IRD patients with COVID-19. Those with prior COVID-19 were excluded.
Cases were stratified by vaccinations status: unvaccinated (1,388 patients, median age 57 years); double vaccinated (462, 56 years) and triple vaccinated (301, 53 years). Body mass index was similar across groups (25-26 kg/m2), and time between SARS-CoV-2 infection and last vaccination was 156 days in double-vaccinated patients, and 62 days in triple-vaccinated patients.
Patients had rheumatoid arthritis in 44.7% and 44.4% of unvaccinated and double-vaccinated patients respectively, but fewer triple-vaccinated patients had RA (37.2%). Triple vaccination was seen in 32.2% of patients with spondyloarthritis, 16.6% connective tissue diseases, 5.3% other vasculitis, and 3.3% ANCA-associated vasculitis. Of triple-vaccinated patients, 26.2% were treated with tumor necrosis factor-alpha (TNF-alpha) inhibitors, and 6.3% with rituximab, while 5.3% were not on immunomodulation. At least 25% were treated with glucocorticoids, reported Dr. Hasseli.
“Arterial hypertension and diabetes, that might be risk factors for COVID-19, were less frequently reported in triple-vaccinated patients. More patients in the double-vaccinated group [42.9%] than the triple-vaccinated [23.8%] reported absence of relevant comorbidities,” she said.
COVID-19 related complications were less often reported in double- and triple-vaccinated groups with hospitalizations at 9.5% and 4.3% in double and triple-vaccinated people respectively.
Dr. Boekel and Dr. Hasseli report no relevant conflicts of interest.
COPENHAGEN – Most patients with immune-mediated inflammatory diseases (IMID) should not be considered at high risk for severe COVID-19 breakthrough infections, but those on anti-CD20 therapy are the exception, data from a large prospective, cohort study show.
“Overall, the data are reassuring, with conventional risk factors, such as age, and comorbidities seeming to be more important regarding risk of severe COVID-19 breakthrough infections than rheumatic disease or immunosuppressant medication,” said Laura Boekel, MD, from Amsterdam UMC, who presented the study at the annual European Congress of Rheumatology.
But, she added, there was an exception for anti-CD20 therapy. “This is especially relevant for patients with conventional risk factors that might accumulate, and rheumatologists might want to consider alternative treatment options if possible. It is important to inform patients about the risks of anti-CD20.”
Another study, presented during the same session at the congress by Rebecca Hasseli, MD, from the University of Giessen (Germany) saw no deaths and no COVID-19 related complications in a cohort of triple-vaccinated patients with inflammatory rheumatic diseases, despite a higher median age and a higher rate of comorbidities compared to double-vaccinated and unvaccinated cohorts.
Ingrid Jyssum, MD, from Diakonhjemmet Hospital, Oslo, who presented results of the Nor-vaC study investigating the impact of different DMARDs on the immunogenicity of a third COVID-19 vaccine dose, welcomed the research by Dr. Boekel and Dr. Hasseli.
“The findings of Hasseli are interesting in the light of our data on serological response after the third dose, with a lack of breakthrough infections after three doses corresponding well to the robust antibody response that we found in our cohort,” she remarked. “This is very reassuring for our patients. Our own work together with the findings of Hasseli and Boekel demonstrate that additional vaccine doses are important to keep this population well protected against severe COVID-19 infections.”
The Nor-vaC study was conducted with a cohort of 1,100 patients with inflammatory joint and bowel diseases. “These patients had attenuated antibody responses after two vaccine doses; however, we found that a third vaccine dose brought the humoral response in patients up to the antibody levels that healthy controls had after two doses,” said Dr. Jyssum. “In addition, we found that the decline in antibodies after the third dose was less than the decline seen after the second dose. Importantly, the third dose was safe in our patients, with no new safety issues.”
Breakthrough infections and immunosuppressants
“Like the rest of the world, we were wondering if our patients were at increased risk of COVID-19, and if the immunosuppressants used by these patients influenced their risk,” said Dr. Boekel.
The researchers compared both the incidence and severity of COVID-19 breakthrough infections with the SARS-CoV-2 Delta variant in a population of fully vaccinated IMID patients taking immunosuppressants and controls (IMID patients not taking immunosuppressants and healthy controls).
Two large ongoing, prospective, multicenter cohort studies provided pooled data collected between February and December 2021 using digital questionnaires, standardized electronic case record forms, and medical files.
Finger-prick tests were used to collect blood samples that were analyzed after vaccination against SARS-CoV-2 for anti–receptor-binding domain (RBD) antibodies, and antinucleocapsid antibodies to identify asymptomatic breakthrough infections. Any associations between antibodies and the incidence of breakthrough infections were generated, and results were adjusted for sex, cardiovascular disease, chronic pulmonary disease, obesity, and vaccine type.
The analysis included 3,207 IMID patients taking immunosuppressants, and 1,810 controls (985 IMID patients not on immunosuppressants and 825 healthy controls).
Initially, Dr. Boekel and her colleagues looked at incidence of infections and hospitalizations prior to vaccination, and then after vaccination, which was the main aim of the study.
Prior to vaccination, hospitalization risk for COVID-19 was somewhat higher for IMID patients overall compared with controls, reported Dr. Boekel. “But those treated with anti-CD20 therapy, demonstrated much greater risk for severe disease.”
After the SARS-CoV-2 vaccination campaign began, the researchers then looked at how immunosuppressants influenced humoral response to SARS-CoV-2 vaccination.
“Anti-CD20 therapy showed the greatest impact on humoral immune response after SARS-CoV-2 vaccination,” said Dr. Boekel. Other immunosuppressant drugs had variable effects on humoral and cellular immunity.
Once they had established that immunosuppressant drugs impaired immune responses to SARS-CoV-2 vaccination, the researchers wanted to determine if this affected clinical outcomes. Blood samples taken 28 days after the second vaccination enabled Dr. Boekel and her colleagues to see if antibody production was associated with breakthrough infections.
Breakthrough infections were seen in 5% of patients on immunosuppressants, 5% of patients not on immunosuppressants, and 4% of healthy controls. Also, asymptomatic COVID-19 breakthrough cases were comparable between IMID patients taking immunosuppressants and controls, at 10% in each group.
“We saw that the incidence [of getting COVID-19] was comparable between groups, independent of whether they were receiving immunosuppressants or not, or healthy controls. However, if they developed antibodies against the two vaccinations the chance of getting infected was lower,” reported Dr. Boekel.
Hospitalization (severe disease) rates were also comparable between groups. “Patients with rheumatic diseases, even when treated with immunosuppressants were not at increased risk of severe disease from Delta breakthrough infections,” added the researcher. “Cases that were hospitalized were mainly elderly and those with comorbidities, for example cardiovascular disease and cardiopulmonary disease.”
Hospital admissions were 5.4% in patients on immunosuppressants, 5.7% in those not on immunosuppressants, and 6% in health controls.
However, once again, there was one exception, Dr. Boekel stressed. “Patients treated with anti-CD20 therapy were at increased risk of severe disease and hospitalization.”
Omicron variant has a different transmissibility than Delta, so the researchers continued the study looking at the Omicron variant. The data “were mostly reassuring,” said Dr. Boekel. “As expected, hospitalization rates decreased overall, with the exception of patients on anti-CD20 therapy where, despite overall reduced pathogenicity, patients remain at increased risk.”
She said that they were awaiting long-term data so the data reflect only short-term immunity against Omicron. “However, we included many elderly and patients with comorbidities, so this made the analysis very sensitive to detect severe cases,” she added.
Breakthrough infection among double- and triple-vaccinated patients
A lower rate of COVID-19 related complications and deaths were seen in patients who were triple-vaccinated against SARS-CoV-2, than in double-vaccinated or unvaccinated patients, despite the former having more comorbidities and use of rituximab (Rituxan), said Dr. Hasseli.
“These data support the recommendation of booster vaccination to reduce COVID-19-related mortality in patients with inflammatory rheumatic diseases [IRDs],” she said.
“A small number of COVID-19 cases were seen in patients with IRD after vaccinations, and in a few cases, hospitalizations were required. Breakthrough infections were mostly seen in patients on B-cell depletion therapy,” she added.
Dr. Hasseli and her colleagues looked at the characteristics and outcomes of SARS-CoV-2 breakthrough infections among double- and triple-vaccinated patients with IRD.
“We wanted to understand if patients with IRD are protected in the same way as the general population following vaccination, given that these patients receive drugs that might impair the immune response,” she explained.
Data for analysis were drawn from the German COVID-19-IRD registry covering February 2021 and January 2022, and patients who were double- or triple- vaccinated against COVID-19 either 14 days or more prior to a SARS-CoV-2 infection were included. Type of IRD, vaccine, immunomodulation, comorbidities, and outcome of the infection were compared with 737 unvaccinated IRD patients with COVID-19. Those with prior COVID-19 were excluded.
Cases were stratified by vaccinations status: unvaccinated (1,388 patients, median age 57 years); double vaccinated (462, 56 years) and triple vaccinated (301, 53 years). Body mass index was similar across groups (25-26 kg/m2), and time between SARS-CoV-2 infection and last vaccination was 156 days in double-vaccinated patients, and 62 days in triple-vaccinated patients.
Patients had rheumatoid arthritis in 44.7% and 44.4% of unvaccinated and double-vaccinated patients respectively, but fewer triple-vaccinated patients had RA (37.2%). Triple vaccination was seen in 32.2% of patients with spondyloarthritis, 16.6% connective tissue diseases, 5.3% other vasculitis, and 3.3% ANCA-associated vasculitis. Of triple-vaccinated patients, 26.2% were treated with tumor necrosis factor-alpha (TNF-alpha) inhibitors, and 6.3% with rituximab, while 5.3% were not on immunomodulation. At least 25% were treated with glucocorticoids, reported Dr. Hasseli.
“Arterial hypertension and diabetes, that might be risk factors for COVID-19, were less frequently reported in triple-vaccinated patients. More patients in the double-vaccinated group [42.9%] than the triple-vaccinated [23.8%] reported absence of relevant comorbidities,” she said.
COVID-19 related complications were less often reported in double- and triple-vaccinated groups with hospitalizations at 9.5% and 4.3% in double and triple-vaccinated people respectively.
Dr. Boekel and Dr. Hasseli report no relevant conflicts of interest.
AT THE EULAR 2022 CONGRESS