User login
New studies suggest Omicron infections are less severe than Delta ones
People who get COVID-19 infections caused by the Omicron variant are less likely to need hospital care, compared with those infected by the Delta variant, according to two large new studies from the U.K. and South Africa.
The findings, which were released ahead of peer review, add to previous glimmers of evidence suggesting that Omicron – while extremely contagious -– may result in less severe symptoms than its predecessors.
“This is helping us quantify how much less severe Omicron is than Delta, and it appears to be between 40 to 75% reduced risk of hospitalizations, adjusted for many factors, which is very good,” said Eric Topol, MD, the editor-in-chief of Medscape and a cardiologist at Scripps Research Translational Institute in La Jolla, CA.
The first analysis, which was done by the World Health Organization Collaborating Centre for Infectious Disease Modelling and Imperial College London, found that overall, people infected by Omicron had about a 20% reduced risk of needing any hospital care for their infections and a 40% lower risk of an overnight hospital stay, compared to those infected with Delta.
Meanwhile, people who were re-infected – meaning they caught Omicron after recovering from a previous COVID-19 infection – had a 50%-60% lower risk of needing hospital care, likely reflecting the benefits of having some prior immunity against the same family of viruses.
The study included everyone with polymerase chain reaction-confirmed COVID-19 in the U.K. during the first 2 weeks of December – roughly 56,000 Omicron cases and 269,000 Delta infections.
The second study, from researchers at the National Institute for Communicable Diseases in South Africa, included more than 29,000 COVID-19 cases that had lab results highly suggestive of Omicron infections. Compared to people infected with the Delta variant, those with presumed Omicron infections were about 70% less likely to have severe disease.
While the news is hopeful for individuals, on a population level, health care systems may still be stressed, the study authors warned.
“Given the high transmissibility of the Omicron virus, there remains the potential for health services to face increasing demand if Omicron cases continue to grow at the rate that has been seen in recent weeks,” said study author Neil Ferguson, PhD, who studies how infectious diseases spread at Imperial College London.
The study authors say their findings are specific to the U.K. and South Africa, where substantial portions of the population have some immune protection from past infection. In other words, they may not apply to countries where fewer people have been vaccinated or recovered from a bout with COVID-19.
A version of this article first appeared on WebMD.com.
People who get COVID-19 infections caused by the Omicron variant are less likely to need hospital care, compared with those infected by the Delta variant, according to two large new studies from the U.K. and South Africa.
The findings, which were released ahead of peer review, add to previous glimmers of evidence suggesting that Omicron – while extremely contagious -– may result in less severe symptoms than its predecessors.
“This is helping us quantify how much less severe Omicron is than Delta, and it appears to be between 40 to 75% reduced risk of hospitalizations, adjusted for many factors, which is very good,” said Eric Topol, MD, the editor-in-chief of Medscape and a cardiologist at Scripps Research Translational Institute in La Jolla, CA.
The first analysis, which was done by the World Health Organization Collaborating Centre for Infectious Disease Modelling and Imperial College London, found that overall, people infected by Omicron had about a 20% reduced risk of needing any hospital care for their infections and a 40% lower risk of an overnight hospital stay, compared to those infected with Delta.
Meanwhile, people who were re-infected – meaning they caught Omicron after recovering from a previous COVID-19 infection – had a 50%-60% lower risk of needing hospital care, likely reflecting the benefits of having some prior immunity against the same family of viruses.
The study included everyone with polymerase chain reaction-confirmed COVID-19 in the U.K. during the first 2 weeks of December – roughly 56,000 Omicron cases and 269,000 Delta infections.
The second study, from researchers at the National Institute for Communicable Diseases in South Africa, included more than 29,000 COVID-19 cases that had lab results highly suggestive of Omicron infections. Compared to people infected with the Delta variant, those with presumed Omicron infections were about 70% less likely to have severe disease.
While the news is hopeful for individuals, on a population level, health care systems may still be stressed, the study authors warned.
“Given the high transmissibility of the Omicron virus, there remains the potential for health services to face increasing demand if Omicron cases continue to grow at the rate that has been seen in recent weeks,” said study author Neil Ferguson, PhD, who studies how infectious diseases spread at Imperial College London.
The study authors say their findings are specific to the U.K. and South Africa, where substantial portions of the population have some immune protection from past infection. In other words, they may not apply to countries where fewer people have been vaccinated or recovered from a bout with COVID-19.
A version of this article first appeared on WebMD.com.
People who get COVID-19 infections caused by the Omicron variant are less likely to need hospital care, compared with those infected by the Delta variant, according to two large new studies from the U.K. and South Africa.
The findings, which were released ahead of peer review, add to previous glimmers of evidence suggesting that Omicron – while extremely contagious -– may result in less severe symptoms than its predecessors.
“This is helping us quantify how much less severe Omicron is than Delta, and it appears to be between 40 to 75% reduced risk of hospitalizations, adjusted for many factors, which is very good,” said Eric Topol, MD, the editor-in-chief of Medscape and a cardiologist at Scripps Research Translational Institute in La Jolla, CA.
The first analysis, which was done by the World Health Organization Collaborating Centre for Infectious Disease Modelling and Imperial College London, found that overall, people infected by Omicron had about a 20% reduced risk of needing any hospital care for their infections and a 40% lower risk of an overnight hospital stay, compared to those infected with Delta.
Meanwhile, people who were re-infected – meaning they caught Omicron after recovering from a previous COVID-19 infection – had a 50%-60% lower risk of needing hospital care, likely reflecting the benefits of having some prior immunity against the same family of viruses.
The study included everyone with polymerase chain reaction-confirmed COVID-19 in the U.K. during the first 2 weeks of December – roughly 56,000 Omicron cases and 269,000 Delta infections.
The second study, from researchers at the National Institute for Communicable Diseases in South Africa, included more than 29,000 COVID-19 cases that had lab results highly suggestive of Omicron infections. Compared to people infected with the Delta variant, those with presumed Omicron infections were about 70% less likely to have severe disease.
While the news is hopeful for individuals, on a population level, health care systems may still be stressed, the study authors warned.
“Given the high transmissibility of the Omicron virus, there remains the potential for health services to face increasing demand if Omicron cases continue to grow at the rate that has been seen in recent weeks,” said study author Neil Ferguson, PhD, who studies how infectious diseases spread at Imperial College London.
The study authors say their findings are specific to the U.K. and South Africa, where substantial portions of the population have some immune protection from past infection. In other words, they may not apply to countries where fewer people have been vaccinated or recovered from a bout with COVID-19.
A version of this article first appeared on WebMD.com.
Could Fabkin hormonal complex spell the end of diabetes?
A hitherto unknown hormonal complex that regulates extracellular energy production in pancreatic islet (beta) cells could be a novel target to not only treat both type 1 and type 2 diabetes but also potentially to prevent their development in the first place, suggests basic science research led by U.S. investigators.
Fatty acid–binding protein 4 (FABP4), a recently identified hormone, was known to be elevated in type 2 diabetes, but the researchers now show that it is not only increased in type 1 diabetes but also that those increases predate its development.
They also show that antibodies against the hormone in mice models prevent type 1 diabetes and improve glycemic control in type 2 disease.
Moreover, it forms a complex with two other proteins that the researchers termed “Fabkin.”
The research, published in Nature, indicates that increased levels of the complex blunts beta cell function, while antibody treatment improves beta cell function.
“For many decades, we have been searching for the signal that communicates the status of energy reserves in adipocytes (fat cells) to generate appropriate endocrine responses, such as the insulin production from pancreatic beta cells,” said senior author Gökhan S. Hotamisligil, MD, PhD, in a press release. “We now have identified Fabkin as a novel hormone that controls this critical function through a very unusual molecular mechanism.”
Still a long way to go
Dr. Hotamisligil, who is director of the Sabri Ülker Center for Metabolic Research at the Harvard School of Public Health, Boston, explained in an interview that taking the findings to the clinic entails answering a number of questions.
“That will keep us busy for a long time, and there are also translational questions, which are extremely exciting,” but the team is very “optimistic” that the findings will transfer well into humans, he said.
One reason is that, in mice and humans with type 1 and type 2 diabetes, “we see exactly the same pattern of regulation” of Fabkin levels and that, “equally importantly,” sustained high levels of the hormone “correlate with poor diabetes control” in type 1 diabetes and disease severity in type 2 disease.
“This is the first strong indication that it will translate well, and the second is that, if we take human islets ... and then apply this hormone into those islets, we see the same suppression of insulin secretion and viability that we see in mice islets,” Dr. Hotamisligil said.
Moreover, blocking the hormone prevents the “negative effects” that we see on the islets, which is a “really critical” factor in suggesting that Fabkin could be a viable treatment target in humans, Dr. Hotamisligil explained.
He continued that, encouragingly, “nature has done some experiments in humans” with Fabkin, showing that “you can have a safe and healthy life with a mutation in the components of this complex ... that reduces levels of the hormone.
“These individuals have a greatly reduced risk for both diabetes and cardiovascular disease,” he said, “so this tells us that, if we can establish a safe agent that can be used in humans, this will be well tolerated for life, and it will have beneficial effects.”
Lastly, Dr. Hotamisligil said that such an agent already exists, “so it’s really just a matter of making it suitable for human use and taking it through the testing procedures.”
He cautioned, however, that “these are important pillars” for translational research “that we rarely, if ever, find in many of our projects,” and there is still a long way to go.
Study details: FABP4 levels associated with glycemic control
The team said the research was “inspired” by previous studies showing that FABP4 knockout mice had higher beta-cell mass in the pancreas and significantly increased glucose-stimulated insulin secretion.
While it is “well established” that FABP4 is increased in type 2 diabetes, they initially examined whether levels are also regulated in type 1 diabetes, independently of adiposity and insulin resistance.
Looking at serum samples from normoglycemic individuals and those with new-onset type 1 diabetes in the BABYDIAB and DiMELLI cohorts, they found that FABP4 was increased approximately 1.6-fold in the latter.
In another cohort of older patients with type 1 diabetes of variation durations, serum FABP4 levels were correlated with hemoglobin A1c levels (P = .005), “which suggests that FABP4 is associated with glycemic control.”
Mouse studies indicate that FABP4 levels are increased both shortly before and during new-onset type 1 diabetes, implying that the hormone “may have a role in beta-cell failure and pathogenesis” in both type 1 and type 2 diabetes.
Antibody targeting of FABP4 levels in mice also revealed that treatment from 10 weeks of age protected against the development of type 1 diabetes, while antibody-treated mice with diabetes had significantly reduced blood glucose and increased plasma insulin levels versus mice given control antibodies.
This, the team said, “suggests that these mice had a less severe diabetes phenotype” with the protection against type 1 diabetes similar to that seen in FABP4 knockout mice.
Mice with diet-induced obesity and nonobese mice with diabetes treated with anti-FABP4 antibodies had improved glucose tolerance tests and a significant increase in islet number and beta-cell mass versus controls.
Further work enabled the team to identify a complex formed by circulating FABP4, adenosine kinase, and nucleoside diphosphate kinase, which could be targeted by anti-FABP4 antibodies via both FABP4 and NPDK.
“We propose the name Fabkin for this new hormone complex formed by NDPK to indicate its unique constitution of a fatty acid–binding protein and kinases,” the researchers wrote.
The team then found that the Fabkin complex alters calcium homeostasis in the endoplasmic reticulum.
This, “results in [endoplasmic reticulum] dysfunction, increased sensitivity to environmental stress and potentiation of beta-cell death in vitro,” which are mechanisms “critical” to the pathogenesis of both type 1 and 2 diabetes.
Finally, they showed that targeting Fabkin with anti-FABP4 antibodies “preserves beta-cell mass and enhances beta-cell function to protect against diabetes in multiple models.”
Funding for this study came from National Institutes of Health and Juvenile Diabetes Research Foundation grants. The Hotamisligil Lab at the Sabri Ülker Center has generated intellectual property (assigned to Harvard University) related to hormonal FABP4 and its therapeutic targeting and receives funding for this project from Lab1636, an affiliate of Deerfield Management. Dr. Hotamisligil is on the scientific advisory board of Crescenta Pharmaceuticals and holds equity. The other authors have no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
A hitherto unknown hormonal complex that regulates extracellular energy production in pancreatic islet (beta) cells could be a novel target to not only treat both type 1 and type 2 diabetes but also potentially to prevent their development in the first place, suggests basic science research led by U.S. investigators.
Fatty acid–binding protein 4 (FABP4), a recently identified hormone, was known to be elevated in type 2 diabetes, but the researchers now show that it is not only increased in type 1 diabetes but also that those increases predate its development.
They also show that antibodies against the hormone in mice models prevent type 1 diabetes and improve glycemic control in type 2 disease.
Moreover, it forms a complex with two other proteins that the researchers termed “Fabkin.”
The research, published in Nature, indicates that increased levels of the complex blunts beta cell function, while antibody treatment improves beta cell function.
“For many decades, we have been searching for the signal that communicates the status of energy reserves in adipocytes (fat cells) to generate appropriate endocrine responses, such as the insulin production from pancreatic beta cells,” said senior author Gökhan S. Hotamisligil, MD, PhD, in a press release. “We now have identified Fabkin as a novel hormone that controls this critical function through a very unusual molecular mechanism.”
Still a long way to go
Dr. Hotamisligil, who is director of the Sabri Ülker Center for Metabolic Research at the Harvard School of Public Health, Boston, explained in an interview that taking the findings to the clinic entails answering a number of questions.
“That will keep us busy for a long time, and there are also translational questions, which are extremely exciting,” but the team is very “optimistic” that the findings will transfer well into humans, he said.
One reason is that, in mice and humans with type 1 and type 2 diabetes, “we see exactly the same pattern of regulation” of Fabkin levels and that, “equally importantly,” sustained high levels of the hormone “correlate with poor diabetes control” in type 1 diabetes and disease severity in type 2 disease.
“This is the first strong indication that it will translate well, and the second is that, if we take human islets ... and then apply this hormone into those islets, we see the same suppression of insulin secretion and viability that we see in mice islets,” Dr. Hotamisligil said.
Moreover, blocking the hormone prevents the “negative effects” that we see on the islets, which is a “really critical” factor in suggesting that Fabkin could be a viable treatment target in humans, Dr. Hotamisligil explained.
He continued that, encouragingly, “nature has done some experiments in humans” with Fabkin, showing that “you can have a safe and healthy life with a mutation in the components of this complex ... that reduces levels of the hormone.
“These individuals have a greatly reduced risk for both diabetes and cardiovascular disease,” he said, “so this tells us that, if we can establish a safe agent that can be used in humans, this will be well tolerated for life, and it will have beneficial effects.”
Lastly, Dr. Hotamisligil said that such an agent already exists, “so it’s really just a matter of making it suitable for human use and taking it through the testing procedures.”
He cautioned, however, that “these are important pillars” for translational research “that we rarely, if ever, find in many of our projects,” and there is still a long way to go.
Study details: FABP4 levels associated with glycemic control
The team said the research was “inspired” by previous studies showing that FABP4 knockout mice had higher beta-cell mass in the pancreas and significantly increased glucose-stimulated insulin secretion.
While it is “well established” that FABP4 is increased in type 2 diabetes, they initially examined whether levels are also regulated in type 1 diabetes, independently of adiposity and insulin resistance.
Looking at serum samples from normoglycemic individuals and those with new-onset type 1 diabetes in the BABYDIAB and DiMELLI cohorts, they found that FABP4 was increased approximately 1.6-fold in the latter.
In another cohort of older patients with type 1 diabetes of variation durations, serum FABP4 levels were correlated with hemoglobin A1c levels (P = .005), “which suggests that FABP4 is associated with glycemic control.”
Mouse studies indicate that FABP4 levels are increased both shortly before and during new-onset type 1 diabetes, implying that the hormone “may have a role in beta-cell failure and pathogenesis” in both type 1 and type 2 diabetes.
Antibody targeting of FABP4 levels in mice also revealed that treatment from 10 weeks of age protected against the development of type 1 diabetes, while antibody-treated mice with diabetes had significantly reduced blood glucose and increased plasma insulin levels versus mice given control antibodies.
This, the team said, “suggests that these mice had a less severe diabetes phenotype” with the protection against type 1 diabetes similar to that seen in FABP4 knockout mice.
Mice with diet-induced obesity and nonobese mice with diabetes treated with anti-FABP4 antibodies had improved glucose tolerance tests and a significant increase in islet number and beta-cell mass versus controls.
Further work enabled the team to identify a complex formed by circulating FABP4, adenosine kinase, and nucleoside diphosphate kinase, which could be targeted by anti-FABP4 antibodies via both FABP4 and NPDK.
“We propose the name Fabkin for this new hormone complex formed by NDPK to indicate its unique constitution of a fatty acid–binding protein and kinases,” the researchers wrote.
The team then found that the Fabkin complex alters calcium homeostasis in the endoplasmic reticulum.
This, “results in [endoplasmic reticulum] dysfunction, increased sensitivity to environmental stress and potentiation of beta-cell death in vitro,” which are mechanisms “critical” to the pathogenesis of both type 1 and 2 diabetes.
Finally, they showed that targeting Fabkin with anti-FABP4 antibodies “preserves beta-cell mass and enhances beta-cell function to protect against diabetes in multiple models.”
Funding for this study came from National Institutes of Health and Juvenile Diabetes Research Foundation grants. The Hotamisligil Lab at the Sabri Ülker Center has generated intellectual property (assigned to Harvard University) related to hormonal FABP4 and its therapeutic targeting and receives funding for this project from Lab1636, an affiliate of Deerfield Management. Dr. Hotamisligil is on the scientific advisory board of Crescenta Pharmaceuticals and holds equity. The other authors have no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
A hitherto unknown hormonal complex that regulates extracellular energy production in pancreatic islet (beta) cells could be a novel target to not only treat both type 1 and type 2 diabetes but also potentially to prevent their development in the first place, suggests basic science research led by U.S. investigators.
Fatty acid–binding protein 4 (FABP4), a recently identified hormone, was known to be elevated in type 2 diabetes, but the researchers now show that it is not only increased in type 1 diabetes but also that those increases predate its development.
They also show that antibodies against the hormone in mice models prevent type 1 diabetes and improve glycemic control in type 2 disease.
Moreover, it forms a complex with two other proteins that the researchers termed “Fabkin.”
The research, published in Nature, indicates that increased levels of the complex blunts beta cell function, while antibody treatment improves beta cell function.
“For many decades, we have been searching for the signal that communicates the status of energy reserves in adipocytes (fat cells) to generate appropriate endocrine responses, such as the insulin production from pancreatic beta cells,” said senior author Gökhan S. Hotamisligil, MD, PhD, in a press release. “We now have identified Fabkin as a novel hormone that controls this critical function through a very unusual molecular mechanism.”
Still a long way to go
Dr. Hotamisligil, who is director of the Sabri Ülker Center for Metabolic Research at the Harvard School of Public Health, Boston, explained in an interview that taking the findings to the clinic entails answering a number of questions.
“That will keep us busy for a long time, and there are also translational questions, which are extremely exciting,” but the team is very “optimistic” that the findings will transfer well into humans, he said.
One reason is that, in mice and humans with type 1 and type 2 diabetes, “we see exactly the same pattern of regulation” of Fabkin levels and that, “equally importantly,” sustained high levels of the hormone “correlate with poor diabetes control” in type 1 diabetes and disease severity in type 2 disease.
“This is the first strong indication that it will translate well, and the second is that, if we take human islets ... and then apply this hormone into those islets, we see the same suppression of insulin secretion and viability that we see in mice islets,” Dr. Hotamisligil said.
Moreover, blocking the hormone prevents the “negative effects” that we see on the islets, which is a “really critical” factor in suggesting that Fabkin could be a viable treatment target in humans, Dr. Hotamisligil explained.
He continued that, encouragingly, “nature has done some experiments in humans” with Fabkin, showing that “you can have a safe and healthy life with a mutation in the components of this complex ... that reduces levels of the hormone.
“These individuals have a greatly reduced risk for both diabetes and cardiovascular disease,” he said, “so this tells us that, if we can establish a safe agent that can be used in humans, this will be well tolerated for life, and it will have beneficial effects.”
Lastly, Dr. Hotamisligil said that such an agent already exists, “so it’s really just a matter of making it suitable for human use and taking it through the testing procedures.”
He cautioned, however, that “these are important pillars” for translational research “that we rarely, if ever, find in many of our projects,” and there is still a long way to go.
Study details: FABP4 levels associated with glycemic control
The team said the research was “inspired” by previous studies showing that FABP4 knockout mice had higher beta-cell mass in the pancreas and significantly increased glucose-stimulated insulin secretion.
While it is “well established” that FABP4 is increased in type 2 diabetes, they initially examined whether levels are also regulated in type 1 diabetes, independently of adiposity and insulin resistance.
Looking at serum samples from normoglycemic individuals and those with new-onset type 1 diabetes in the BABYDIAB and DiMELLI cohorts, they found that FABP4 was increased approximately 1.6-fold in the latter.
In another cohort of older patients with type 1 diabetes of variation durations, serum FABP4 levels were correlated with hemoglobin A1c levels (P = .005), “which suggests that FABP4 is associated with glycemic control.”
Mouse studies indicate that FABP4 levels are increased both shortly before and during new-onset type 1 diabetes, implying that the hormone “may have a role in beta-cell failure and pathogenesis” in both type 1 and type 2 diabetes.
Antibody targeting of FABP4 levels in mice also revealed that treatment from 10 weeks of age protected against the development of type 1 diabetes, while antibody-treated mice with diabetes had significantly reduced blood glucose and increased plasma insulin levels versus mice given control antibodies.
This, the team said, “suggests that these mice had a less severe diabetes phenotype” with the protection against type 1 diabetes similar to that seen in FABP4 knockout mice.
Mice with diet-induced obesity and nonobese mice with diabetes treated with anti-FABP4 antibodies had improved glucose tolerance tests and a significant increase in islet number and beta-cell mass versus controls.
Further work enabled the team to identify a complex formed by circulating FABP4, adenosine kinase, and nucleoside diphosphate kinase, which could be targeted by anti-FABP4 antibodies via both FABP4 and NPDK.
“We propose the name Fabkin for this new hormone complex formed by NDPK to indicate its unique constitution of a fatty acid–binding protein and kinases,” the researchers wrote.
The team then found that the Fabkin complex alters calcium homeostasis in the endoplasmic reticulum.
This, “results in [endoplasmic reticulum] dysfunction, increased sensitivity to environmental stress and potentiation of beta-cell death in vitro,” which are mechanisms “critical” to the pathogenesis of both type 1 and 2 diabetes.
Finally, they showed that targeting Fabkin with anti-FABP4 antibodies “preserves beta-cell mass and enhances beta-cell function to protect against diabetes in multiple models.”
Funding for this study came from National Institutes of Health and Juvenile Diabetes Research Foundation grants. The Hotamisligil Lab at the Sabri Ülker Center has generated intellectual property (assigned to Harvard University) related to hormonal FABP4 and its therapeutic targeting and receives funding for this project from Lab1636, an affiliate of Deerfield Management. Dr. Hotamisligil is on the scientific advisory board of Crescenta Pharmaceuticals and holds equity. The other authors have no conflicts of interest to declare.
A version of this article first appeared on Medscape.com.
FROM NATURE
Hyperprolactinemia – When, why, and how to evaluate prolactin
Because of the increasing popularity and success of in vitro fertilization, the field of reproductive endocrinology and infertility has steadily morphed toward the treatment of infertility. Nevertheless, a physician board certified in reproductive endocrinology and infertility is the referring physician of choice regarding prolactin disorders and gynecologists should be familiar with the symptoms and sequela of prolactin elevations. This month’s column will address when to obtain a serum prolactin and how to appropriately manage hyperprolactinemia.
Of all the anterior pituitary hormones (adrenocorticotropic hormone, follicle-stimulating hormone, growth hormone, luteinizing hormone, prolactin, thyroid-stimulating hormone ), prolactin is the only one under tonic inhibition by dopamine. Disturbances in this dopaminergic pathway result in elevated serum prolactin. The normal range for prolactin is approximately 5-20 ng/mL.
In the nonpregnant state, little is known regarding the purpose of prolactin, which is produced by the anterior pituitary cluster of cells called lactotrophs. To prepare the breast for postpartum lactation, increases in prolactin are necessary and sustained throughout pregnancy. Second to pregnancy, amenorrhea can occur in 10%-20% of cases of hyperprolactinemia. Outside of pregnancy, elevations in prolactin result in hypogonadism, through gonadotropin-releasing hormone suppression, resulting in infertility (48%), headache (39%), oligomenorrhea (29%) and galactorrhea (24%).1 Most hypogonadal symptoms are more likely to occur with prolactin levels greater than 100 ng/mL, whereas infertility and ovulation dysfunction can occur with mild to moderate hyperprolactinemia, respectively. Prolonged amenorrhea can risk bone mineral density loss.
While the focus of our discussion is the effect of prolactin on women, men with hyperprolactinemia can experience hypogonadotropic hypogonadism with resultant decreased libido, impotence, infertility, gynecomastia, or, rarely, galactorrhea.2
The three Ps – physiological, pharmacologic, pathological
Physiological causes of hyperprolactinemia include rising estradiol during the late follicular phase and into the secretory phase of the menstrual cycle or while taking combined oral contraception, nipple stimulation, pregnancy, lactation, meals, sleep, and stress.
Drugs can interrupt the dopaminergic pathway, thereby elevating serum prolactin but usually not above 100 ng/mL, except for the antipsychotic drug risperidone, which can cause marked elevation up to 300 or even 400 ng/mL. Medications that can cause hyperprolactinemia are estrogens, neuroleptic drugs such as risperidone, metoclopramide, antidepressant drugs, cimetidine, methyldopa, and verapamil.
A pituitary MRI can diagnose an adenoma, that is, a collection of cells in the pituitary that are responsible for hyperprolactinemia and is named based on its size. Microadenomas are less than 1 cm and are typically associated with serum prolactin values below 200 ng/mL. Macroadenomas can worsen while a patient is on combined oral contraception and during pregnancy; fortunately, this is not the case with a microadenoma.
Hypothyroidism can elevate serum prolactin since thyrotropin releasing hormone is known to stimulate prolactin secretion.3 Consequently, when a patient presents with both hypothyroidism and hyperprolactinemia, thyroid replacement should be initiated for thyroid regulation and potential restoration of prolactin levels. If hyperprolactinemia persists, then further evaluation is required. Chronic renal impairment can also elevate prolactin levels due to decreased clearance.
Management
The appropriate evaluation of hyperprolactinemia consists of a history to disclose medications, identify galactorrhea, and visual changes. Because of an adenoma compressing the optic chiasm, partial blindness may occur where vision is lost in the outer half of both the right and left visual field, called bitemporal hemianopsia. Mild elevations in prolactin should be tested at a time when physiological influences are at a minimum, that is, during menses, fasting, and in late morning.4 Persistent elevations should be appropriately evaluated rather than by using the empiric “shotgun” approach of prescribing a dopamine agonist. Laboratory testing for repeated elevations in prolactin includes a pituitary MRI looking for a mass in the hypothalamic-pituitary region that interrupts dopamine suppression.
Treatment of hyperprolactinemia begins with a dopamine agonist and is indicated when there is hypogonadism or intolerable galactorrhea. Cabergoline is the first choice because of effectiveness (reduced adenoma size in greater than 90% of patients) and lesser side effects, particularly nausea, than bromocriptine. Dopamine agonists, such as bromocriptine and cabergoline, belong to the category of ergot-derived dopamine agonists and have been used to treat Parkinson’s disease. At high doses used to treat Parkinson’s, cabergoline is associated with an increased risk of valvular heart disease. In the United States, pergolide was voluntarily withdrawn from the market in March 2007 because of this risk. At the lower doses generally used for the treatment of hyperprolactinemia, cabergoline is probably not associated with excess risk.5
Newer dopamine agonists are known as nonergot. These are pramipexole, ropinirole, rotigotine, and apomorphine. They have not been associated with a risk of heart damage and can be prescribed.
The initial prescribing dose of cabergoline should be 0.25 mg twice a week or 0.5 mg once a week. If bromocriptine is used, the starting dose is 1.25 mg after dinner or at bedtime for 1 week, then increasing to 1.25 mg twice a day (after breakfast and after dinner or at bedtime to reduce nausea and fatigue). After 1 month of a dopamine agonist, the patient should be evaluated for side effects and a serum prolactin level should be obtained. With a normal prolactin level, gonadal function will probably return within a few months. The dopamine agonist should typically be discontinued with pregnancy as pregnancy increases prolactin physiologically.
Treatment of a macroadenoma is essential when the tumor is large enough to cause neurologic symptoms, such as visual impairment or headache, and is preferable when it is invasive or when there are enlarging microadenomas since they are likely to continue to grow and become symptomatic. About 95% of microadenomas have not been shown to increase in size during 4-6 years of observation.6
Transsphenoidal surgery should be considered when there is:
- Persistent hyperprolactinemia and/or size of the adenoma, with associated symptoms or signs despite several months of dopamine agonist treatment at high doses.
- Presence of a giant lactotroph adenoma (e.g., >3 cm) with pregnancy desired including those whose adenoma responds to a dopamine agonist – to avoid significant growth during pregnancy while off medication.
Data from over 6,000 pregnancies suggest that the administration of bromocriptine during the first month of pregnancy does not harm the fetus.7
Discontinuing treatment
Three scenarios may allow for cessation of dopamine agonist therapy. The first is when a patient has had a normal serum prolactin test following 2 years of low-dose dopamine agonist. Another is the patient who had hyperprolactinemia and a microadenoma that responded to treatment with a normal prolactin level and no further evidence of an adenoma by MRI for at least 2 years. Lastly, the patient who had a macroadenoma prior to treatment and a subsequent normal serum prolactin level without an adenoma for at least 2 years.
Like the management of thyroid dysfunction, our field must be aware of prolactin disorders for early detection, prompt referral, and appropriate management to minimize long-term consequences.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Bayrak A et al. Fertil Steril. 2005 Jul;84(1):181-5.
2. Carter JN et al. N Engl J Med. 1978 Oct 19;299(16):847-52.
3. Sachson R et al. N Engl J Med. 1972;287:972.
4. Singh SP and Singh TP. Ann Endocrinol (Paris). 1984;45(2):137-41.
5. Valassi E et al. J Clin Endocrinol Metab. 2010 Mar;95(3):1025-33.
6. Sisam DA et al. Fertil Steril. 1987 Jul;48(1):67-71.
7. Molitch ME. Best Pract Res Clin Endocrinol Metab. 2011 Dec;25(6):885-96.
Because of the increasing popularity and success of in vitro fertilization, the field of reproductive endocrinology and infertility has steadily morphed toward the treatment of infertility. Nevertheless, a physician board certified in reproductive endocrinology and infertility is the referring physician of choice regarding prolactin disorders and gynecologists should be familiar with the symptoms and sequela of prolactin elevations. This month’s column will address when to obtain a serum prolactin and how to appropriately manage hyperprolactinemia.
Of all the anterior pituitary hormones (adrenocorticotropic hormone, follicle-stimulating hormone, growth hormone, luteinizing hormone, prolactin, thyroid-stimulating hormone ), prolactin is the only one under tonic inhibition by dopamine. Disturbances in this dopaminergic pathway result in elevated serum prolactin. The normal range for prolactin is approximately 5-20 ng/mL.
In the nonpregnant state, little is known regarding the purpose of prolactin, which is produced by the anterior pituitary cluster of cells called lactotrophs. To prepare the breast for postpartum lactation, increases in prolactin are necessary and sustained throughout pregnancy. Second to pregnancy, amenorrhea can occur in 10%-20% of cases of hyperprolactinemia. Outside of pregnancy, elevations in prolactin result in hypogonadism, through gonadotropin-releasing hormone suppression, resulting in infertility (48%), headache (39%), oligomenorrhea (29%) and galactorrhea (24%).1 Most hypogonadal symptoms are more likely to occur with prolactin levels greater than 100 ng/mL, whereas infertility and ovulation dysfunction can occur with mild to moderate hyperprolactinemia, respectively. Prolonged amenorrhea can risk bone mineral density loss.
While the focus of our discussion is the effect of prolactin on women, men with hyperprolactinemia can experience hypogonadotropic hypogonadism with resultant decreased libido, impotence, infertility, gynecomastia, or, rarely, galactorrhea.2
The three Ps – physiological, pharmacologic, pathological
Physiological causes of hyperprolactinemia include rising estradiol during the late follicular phase and into the secretory phase of the menstrual cycle or while taking combined oral contraception, nipple stimulation, pregnancy, lactation, meals, sleep, and stress.
Drugs can interrupt the dopaminergic pathway, thereby elevating serum prolactin but usually not above 100 ng/mL, except for the antipsychotic drug risperidone, which can cause marked elevation up to 300 or even 400 ng/mL. Medications that can cause hyperprolactinemia are estrogens, neuroleptic drugs such as risperidone, metoclopramide, antidepressant drugs, cimetidine, methyldopa, and verapamil.
A pituitary MRI can diagnose an adenoma, that is, a collection of cells in the pituitary that are responsible for hyperprolactinemia and is named based on its size. Microadenomas are less than 1 cm and are typically associated with serum prolactin values below 200 ng/mL. Macroadenomas can worsen while a patient is on combined oral contraception and during pregnancy; fortunately, this is not the case with a microadenoma.
Hypothyroidism can elevate serum prolactin since thyrotropin releasing hormone is known to stimulate prolactin secretion.3 Consequently, when a patient presents with both hypothyroidism and hyperprolactinemia, thyroid replacement should be initiated for thyroid regulation and potential restoration of prolactin levels. If hyperprolactinemia persists, then further evaluation is required. Chronic renal impairment can also elevate prolactin levels due to decreased clearance.
Management
The appropriate evaluation of hyperprolactinemia consists of a history to disclose medications, identify galactorrhea, and visual changes. Because of an adenoma compressing the optic chiasm, partial blindness may occur where vision is lost in the outer half of both the right and left visual field, called bitemporal hemianopsia. Mild elevations in prolactin should be tested at a time when physiological influences are at a minimum, that is, during menses, fasting, and in late morning.4 Persistent elevations should be appropriately evaluated rather than by using the empiric “shotgun” approach of prescribing a dopamine agonist. Laboratory testing for repeated elevations in prolactin includes a pituitary MRI looking for a mass in the hypothalamic-pituitary region that interrupts dopamine suppression.
Treatment of hyperprolactinemia begins with a dopamine agonist and is indicated when there is hypogonadism or intolerable galactorrhea. Cabergoline is the first choice because of effectiveness (reduced adenoma size in greater than 90% of patients) and lesser side effects, particularly nausea, than bromocriptine. Dopamine agonists, such as bromocriptine and cabergoline, belong to the category of ergot-derived dopamine agonists and have been used to treat Parkinson’s disease. At high doses used to treat Parkinson’s, cabergoline is associated with an increased risk of valvular heart disease. In the United States, pergolide was voluntarily withdrawn from the market in March 2007 because of this risk. At the lower doses generally used for the treatment of hyperprolactinemia, cabergoline is probably not associated with excess risk.5
Newer dopamine agonists are known as nonergot. These are pramipexole, ropinirole, rotigotine, and apomorphine. They have not been associated with a risk of heart damage and can be prescribed.
The initial prescribing dose of cabergoline should be 0.25 mg twice a week or 0.5 mg once a week. If bromocriptine is used, the starting dose is 1.25 mg after dinner or at bedtime for 1 week, then increasing to 1.25 mg twice a day (after breakfast and after dinner or at bedtime to reduce nausea and fatigue). After 1 month of a dopamine agonist, the patient should be evaluated for side effects and a serum prolactin level should be obtained. With a normal prolactin level, gonadal function will probably return within a few months. The dopamine agonist should typically be discontinued with pregnancy as pregnancy increases prolactin physiologically.
Treatment of a macroadenoma is essential when the tumor is large enough to cause neurologic symptoms, such as visual impairment or headache, and is preferable when it is invasive or when there are enlarging microadenomas since they are likely to continue to grow and become symptomatic. About 95% of microadenomas have not been shown to increase in size during 4-6 years of observation.6
Transsphenoidal surgery should be considered when there is:
- Persistent hyperprolactinemia and/or size of the adenoma, with associated symptoms or signs despite several months of dopamine agonist treatment at high doses.
- Presence of a giant lactotroph adenoma (e.g., >3 cm) with pregnancy desired including those whose adenoma responds to a dopamine agonist – to avoid significant growth during pregnancy while off medication.
Data from over 6,000 pregnancies suggest that the administration of bromocriptine during the first month of pregnancy does not harm the fetus.7
Discontinuing treatment
Three scenarios may allow for cessation of dopamine agonist therapy. The first is when a patient has had a normal serum prolactin test following 2 years of low-dose dopamine agonist. Another is the patient who had hyperprolactinemia and a microadenoma that responded to treatment with a normal prolactin level and no further evidence of an adenoma by MRI for at least 2 years. Lastly, the patient who had a macroadenoma prior to treatment and a subsequent normal serum prolactin level without an adenoma for at least 2 years.
Like the management of thyroid dysfunction, our field must be aware of prolactin disorders for early detection, prompt referral, and appropriate management to minimize long-term consequences.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Bayrak A et al. Fertil Steril. 2005 Jul;84(1):181-5.
2. Carter JN et al. N Engl J Med. 1978 Oct 19;299(16):847-52.
3. Sachson R et al. N Engl J Med. 1972;287:972.
4. Singh SP and Singh TP. Ann Endocrinol (Paris). 1984;45(2):137-41.
5. Valassi E et al. J Clin Endocrinol Metab. 2010 Mar;95(3):1025-33.
6. Sisam DA et al. Fertil Steril. 1987 Jul;48(1):67-71.
7. Molitch ME. Best Pract Res Clin Endocrinol Metab. 2011 Dec;25(6):885-96.
Because of the increasing popularity and success of in vitro fertilization, the field of reproductive endocrinology and infertility has steadily morphed toward the treatment of infertility. Nevertheless, a physician board certified in reproductive endocrinology and infertility is the referring physician of choice regarding prolactin disorders and gynecologists should be familiar with the symptoms and sequela of prolactin elevations. This month’s column will address when to obtain a serum prolactin and how to appropriately manage hyperprolactinemia.
Of all the anterior pituitary hormones (adrenocorticotropic hormone, follicle-stimulating hormone, growth hormone, luteinizing hormone, prolactin, thyroid-stimulating hormone ), prolactin is the only one under tonic inhibition by dopamine. Disturbances in this dopaminergic pathway result in elevated serum prolactin. The normal range for prolactin is approximately 5-20 ng/mL.
In the nonpregnant state, little is known regarding the purpose of prolactin, which is produced by the anterior pituitary cluster of cells called lactotrophs. To prepare the breast for postpartum lactation, increases in prolactin are necessary and sustained throughout pregnancy. Second to pregnancy, amenorrhea can occur in 10%-20% of cases of hyperprolactinemia. Outside of pregnancy, elevations in prolactin result in hypogonadism, through gonadotropin-releasing hormone suppression, resulting in infertility (48%), headache (39%), oligomenorrhea (29%) and galactorrhea (24%).1 Most hypogonadal symptoms are more likely to occur with prolactin levels greater than 100 ng/mL, whereas infertility and ovulation dysfunction can occur with mild to moderate hyperprolactinemia, respectively. Prolonged amenorrhea can risk bone mineral density loss.
While the focus of our discussion is the effect of prolactin on women, men with hyperprolactinemia can experience hypogonadotropic hypogonadism with resultant decreased libido, impotence, infertility, gynecomastia, or, rarely, galactorrhea.2
The three Ps – physiological, pharmacologic, pathological
Physiological causes of hyperprolactinemia include rising estradiol during the late follicular phase and into the secretory phase of the menstrual cycle or while taking combined oral contraception, nipple stimulation, pregnancy, lactation, meals, sleep, and stress.
Drugs can interrupt the dopaminergic pathway, thereby elevating serum prolactin but usually not above 100 ng/mL, except for the antipsychotic drug risperidone, which can cause marked elevation up to 300 or even 400 ng/mL. Medications that can cause hyperprolactinemia are estrogens, neuroleptic drugs such as risperidone, metoclopramide, antidepressant drugs, cimetidine, methyldopa, and verapamil.
A pituitary MRI can diagnose an adenoma, that is, a collection of cells in the pituitary that are responsible for hyperprolactinemia and is named based on its size. Microadenomas are less than 1 cm and are typically associated with serum prolactin values below 200 ng/mL. Macroadenomas can worsen while a patient is on combined oral contraception and during pregnancy; fortunately, this is not the case with a microadenoma.
Hypothyroidism can elevate serum prolactin since thyrotropin releasing hormone is known to stimulate prolactin secretion.3 Consequently, when a patient presents with both hypothyroidism and hyperprolactinemia, thyroid replacement should be initiated for thyroid regulation and potential restoration of prolactin levels. If hyperprolactinemia persists, then further evaluation is required. Chronic renal impairment can also elevate prolactin levels due to decreased clearance.
Management
The appropriate evaluation of hyperprolactinemia consists of a history to disclose medications, identify galactorrhea, and visual changes. Because of an adenoma compressing the optic chiasm, partial blindness may occur where vision is lost in the outer half of both the right and left visual field, called bitemporal hemianopsia. Mild elevations in prolactin should be tested at a time when physiological influences are at a minimum, that is, during menses, fasting, and in late morning.4 Persistent elevations should be appropriately evaluated rather than by using the empiric “shotgun” approach of prescribing a dopamine agonist. Laboratory testing for repeated elevations in prolactin includes a pituitary MRI looking for a mass in the hypothalamic-pituitary region that interrupts dopamine suppression.
Treatment of hyperprolactinemia begins with a dopamine agonist and is indicated when there is hypogonadism or intolerable galactorrhea. Cabergoline is the first choice because of effectiveness (reduced adenoma size in greater than 90% of patients) and lesser side effects, particularly nausea, than bromocriptine. Dopamine agonists, such as bromocriptine and cabergoline, belong to the category of ergot-derived dopamine agonists and have been used to treat Parkinson’s disease. At high doses used to treat Parkinson’s, cabergoline is associated with an increased risk of valvular heart disease. In the United States, pergolide was voluntarily withdrawn from the market in March 2007 because of this risk. At the lower doses generally used for the treatment of hyperprolactinemia, cabergoline is probably not associated with excess risk.5
Newer dopamine agonists are known as nonergot. These are pramipexole, ropinirole, rotigotine, and apomorphine. They have not been associated with a risk of heart damage and can be prescribed.
The initial prescribing dose of cabergoline should be 0.25 mg twice a week or 0.5 mg once a week. If bromocriptine is used, the starting dose is 1.25 mg after dinner or at bedtime for 1 week, then increasing to 1.25 mg twice a day (after breakfast and after dinner or at bedtime to reduce nausea and fatigue). After 1 month of a dopamine agonist, the patient should be evaluated for side effects and a serum prolactin level should be obtained. With a normal prolactin level, gonadal function will probably return within a few months. The dopamine agonist should typically be discontinued with pregnancy as pregnancy increases prolactin physiologically.
Treatment of a macroadenoma is essential when the tumor is large enough to cause neurologic symptoms, such as visual impairment or headache, and is preferable when it is invasive or when there are enlarging microadenomas since they are likely to continue to grow and become symptomatic. About 95% of microadenomas have not been shown to increase in size during 4-6 years of observation.6
Transsphenoidal surgery should be considered when there is:
- Persistent hyperprolactinemia and/or size of the adenoma, with associated symptoms or signs despite several months of dopamine agonist treatment at high doses.
- Presence of a giant lactotroph adenoma (e.g., >3 cm) with pregnancy desired including those whose adenoma responds to a dopamine agonist – to avoid significant growth during pregnancy while off medication.
Data from over 6,000 pregnancies suggest that the administration of bromocriptine during the first month of pregnancy does not harm the fetus.7
Discontinuing treatment
Three scenarios may allow for cessation of dopamine agonist therapy. The first is when a patient has had a normal serum prolactin test following 2 years of low-dose dopamine agonist. Another is the patient who had hyperprolactinemia and a microadenoma that responded to treatment with a normal prolactin level and no further evidence of an adenoma by MRI for at least 2 years. Lastly, the patient who had a macroadenoma prior to treatment and a subsequent normal serum prolactin level without an adenoma for at least 2 years.
Like the management of thyroid dysfunction, our field must be aware of prolactin disorders for early detection, prompt referral, and appropriate management to minimize long-term consequences.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
References
1. Bayrak A et al. Fertil Steril. 2005 Jul;84(1):181-5.
2. Carter JN et al. N Engl J Med. 1978 Oct 19;299(16):847-52.
3. Sachson R et al. N Engl J Med. 1972;287:972.
4. Singh SP and Singh TP. Ann Endocrinol (Paris). 1984;45(2):137-41.
5. Valassi E et al. J Clin Endocrinol Metab. 2010 Mar;95(3):1025-33.
6. Sisam DA et al. Fertil Steril. 1987 Jul;48(1):67-71.
7. Molitch ME. Best Pract Res Clin Endocrinol Metab. 2011 Dec;25(6):885-96.
Last call? Moderate alcohol’s health benefits look increasingly doubtful
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
When holiday shoppers recently went to their local liquor stores in search of some liquid spirit, many were instead greeted by the sight of increasingly barren shelves.
Although partly a result of global supply chain issues, this was also yet more evidence of the rising demand for alcohol among adults during these difficult COVID years. It’s a trend that has led to concerns of an echo pandemic of alcohol-related morbidity, which has begun to play out in the form of rising rates of gastrointestinal and liver disease, hospital admissions for alcoholic hepatitis, and alcohol-related incidents of domestic violence.
Those who imbibe alcohol in low to moderate levels may not see themselves reflected in such stories of drinking’s hefty tolls. They’re instead following established health guidance that a little bit of alcohol now and then actually has robust health benefits. Yet the past few of years have seen a notable fraying of this idea, as emerging data calls into question whether alcohol in moderation should really continue to be just what the doctor ordered.
Behind the curve: Alcohol’s diminishing cardioprotective value
Perhaps the most resonant argument for the benefits of light to moderate alcohol consumption – usually defined as between one to two drinks a day – has been its proposed cardioprotective value. In this way, alcohol differs from tobacco, which is unsafe at any level. Alcohol’s proposed cardioprotective effects are often represented as a J-shaped curve, with moderate drinking occupying the sweet spot between teetotaling and heavy/binge drinking when it comes to reduced mortality.
In reality, this association is more likely “a statistical artifact” largely derived from low-quality observational studies, according to Christopher Labos, MD, CM, MSc, an epidemiologist and cardiologist at the Queen Elizabeth Health Complex in Montreal.
“When you look at studies that correct for things like reverse causation, or the fact that some people who drink zero alcohol are former drinkers who used to drink alcohol, then you realize that the protective benefit of alcohol is either minimal or nonexistent and that alcohol does more harm than good to our society,” said Dr. Labos, who detailed the reasons underpinning alcohol’s unearned cardioprotective reputation in a 2020 Medscape commentary.
This statistical limitation was on display in July 2021 when BMC Medicine published results from meta-analyses suggesting that current drinkers need not stop consuming small amounts of alcohol for the secondary prevention of cardiovascular disease (CVD). The study’s own investigators noted that it likely overestimated the reduced risk of CVD by including former heavy drinkers as nondrinkers.
Even if the J-shaped curve exists, its simplicity is deceiving. CVD risk increases alongside alcohol consumption owning to a complicated array of genetic and lifestyle factors. The curve also presents something of a catch-22. If you like alcohol enough to drink it every day, staying at the nadir of the curve where you’d gain the most benefits may prove challenging.
Another factor dimming alcohol’s cardioprotective reputation came via recent data that atrial fibrillation episodes can be triggered by acute alcohol use. A randomized, controlled trial published in the New England Journal of Medicine concluded that abstinence reduced arrhythmia recurrences in regular drinkers with atrial fibrillation.
“If we can replicate that, I think we’ll find that reducing alcohol consumption might be a very effective way to prevent and treat atrial fibrillation,” said Dr. Labos.
However, J-curve proponents will note the publication of study data from the UK Biobank indicating that low levels of alcohol consumption confers the greatest reduction in atrial fibrillation risk.
An overlooked carcinogen no longer
Surveys indicate that less than half of Americans realize alcohol increases cancer risk. That might have changed just a bit this year. In early 2021, an epidemiological analysis estimated that alcohol contributed to 4.8% of cancer cases and 3.2% of cancer deaths in the United States. Then the Lancet Oncology published the results of a high-profile, population-based study on the global burden of cancer as a result of alcoho. Although the main takeaway message was that 4% of new cancer cases worldwide in 2020 were attributable to alcohol, it was also noteworthy that moderate drinking accounted for 103,100 out of 741,300 of these projected annual cases.
“The risk of cancer increases even with low or moderate levels of drinking,” said the study’s lead author Harriet Rumgay, BSc, from the International Agency for Research on Cancer in Lyon, France. “Drinking less means you’ll have a lower risk of cancer than if you drink heavily, but there is no safe limit of alcohol consumption.”
The study linked alcohol consumption with an increased risk of at least seven different cancer types, including cancers of the oral cavity, pharynx, larynx, esophagus, colon, rectum, liver, and breast.
Although in North America men represented about two-thirds of the burden of cancer caused by alcohol, Ms. Rumgay added that “low and moderate levels of drinking [one or two alcoholic drinks per day] contributed relatively more cancer cases among women than among men.”
Yet more negative news for moderate alcohol drinkers arrived in August 2021, when a team of South Korean researchers published data in JAMA Network Open showing that, when it came to the risk of developing gastrointestinal cancers, even binge drinking may be preferable to continuous but moderate consumption.
who in updating its guidelines in 2020 after an 8-year interim offered this succinct piece of advice: “It is best not to drink alcohol.”
Neurotoxic implications
There has similarly been a reconsideration of the effects of moderate alcohol consumption on brain health.
A recent report of multimodal MRI brain and cognitive testing data from over 25,000 participants in the UK Biobank study indicate that alcohol may have no safe dosage . Even moderate consumption reduced gray matter volume and functional connectivity, negative associations that were increased in those with higher blood pressure and body mass index.
Speaking with this news organization in May 2021, an investigator said: “The size of the effect is small, albeit greater than any other modifiable risk factor,” noting that the changes have been linked to decreased memory and dementia.
Louise Mewton, PhD, from the Center for Healthy Brain Aging at the University of New South Wales, Sydney, said that these results provide an interesting comparison with others into the association between alcohol and dementia.
“A recent study of over 1 million dementia cases in France indicated that problematic alcohol use (alcohol use disorders) were one of the strongest risk factors for dementia – even more so than things like high blood pressure and diabetes,” Dr. Mewton said in an interview. In comparison, “the most-recent reviews indicate that 4 drinks/week is associated with the lowest risk for dementia – so we’re talking about very low levels of alcohol use in terms of maintaining brain health.
“Understanding why very small amounts of alcohol appear to be protective in terms of dementia but damaging when we look at brain scans is something that would be really interesting to unpack.”
Dr. Mewton and colleagues recently published data suggesting that there are three periods when the brain might be particularly susceptible to alcohol’s neurotoxic effects: gestation (from conception to birth), later adolescence (15-19 years), and older adulthood (over 65 years). Directing behavioral interventions to patients in these stages may therefore be beneficial.
And there’s no time too soon to promote abstinence among those with alcohol use disorder, as brain damage is shown to still occur even in the immediate period after people cease drinking.
Although in one more argument for the J-shaped curve’s relevance, data from the Massachusetts General Brigham Biobank recently indicated that moderate alcohol use, unlike low and heavy use, lowered both stress-related neurobiological activity and major adverse cardiovascular events.
Getting patients to reconsider alcohol’s ‘benefits’
These new findings mean physicians will find themselves imparting a more nuanced message about the health impacts of moderate alcohol consumption than in prior years. To aid those efforts, Ms. Rumgay advised clinicians to consult a special issue of the journal Nutrients that features review articles of alcohol›s impact on various health outcomes.
Ms. Rumgay also supports broader policy changes.
“There is some evidence that adding cancer warnings to alcohol labels, similar to those used on cigarette packages, might deter people from purchasing alcohol products and increase awareness of the causal link with cancer,” she said. “But the most effective ways of reducing alcohol use in the population are through increasing the price of alcohol through higher taxes, limiting purchasing availability, and reducing marketing of alcohol brands to the public.”
Dr. Mewton recommended various interventions for patients who still find it difficult to curtail their drinking.
“For less severe, problematic use, things like cognitive-behavioral therapy and motivational therapy are very effective in reducing alcohol consumption,” she said in an interview.
For all the discussion about how the COVID-19 pandemic has exacerbated problematic drinking, it has also provided an opportunity for getting patients to reexamine their relationship to alcohol. And as Dr. Labos noted, emerging data on alcohol’s negative effects probably won’t be considered earth-shattering to most patients.
“Deep down, I think most people know that alcohol is not healthy, but it is part of our social culture and so we find ways to justify our own behavior,” he said in an interview.
Dr. Labos suggested that clinicians reframe alcohol in their patients’ minds for what it really is – “an indulgence that we shouldn’t have too much of very often.
“Just like junk food, that doesn’t mean we can’t enjoy small amounts occasionally, but we have to stop presenting that it is good for us, because it isn’t.”
A version of this article first appeared on Medscape.com.
Pruritic Eruption on the Trunk and Extremities
THE DIAGNOSIS:
Acquired Perforating Disorder of Renal Disease
A papule with the central plug removed left a pitlike depression, representing Kyrle disease (Figure 1). A punch biopsy of the left forearm revealed epidermal hyperplasia (Figure 2A) surrounding a keratin plug that contained degenerated basophilic material (Figure 2B), confirming the diagnosis of acquired perforating disorder of renal disease (APDRD), classically described as Kyrle disease.
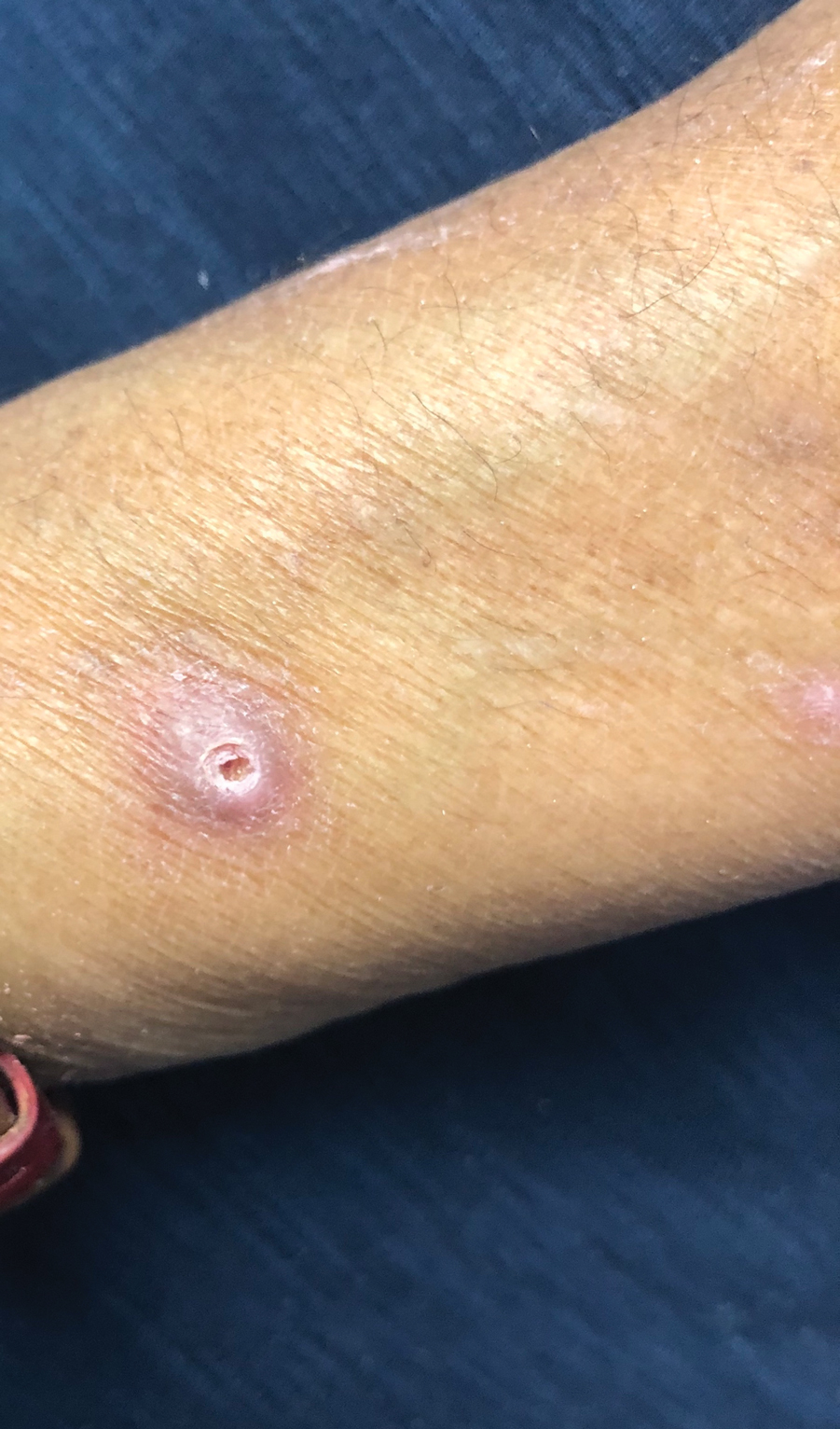
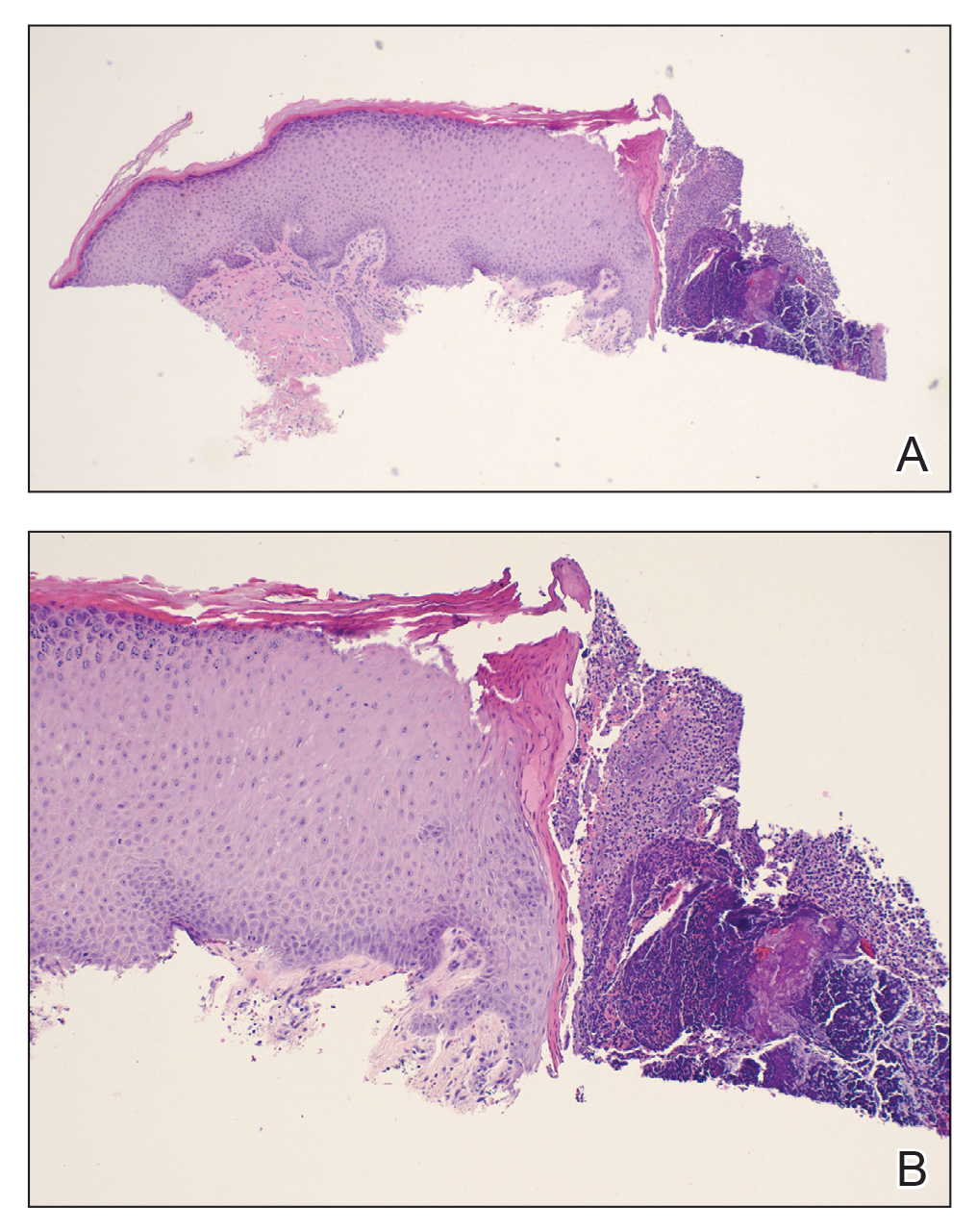
Acquired perforating disorder of renal disease is an uncommon condition in the general population. It is associated with systemic disease, commonly diabetes mellitus and chronic renal failure, and is seen in up to 10% patients receiving hemodialysis.1 The underlying etiology and pathogenesis of APDRD remains unknown. It has been proposed to be a variant of prurigo nodularis, representing end-stage excoriated folliculitis.1 Given that most cases appear in patients with systemic disease and metabolic abnormalities, APDRD also has been classified under the spectrum of acquired perforating dermatoses, a group of disorders defined by transepithelial elimination of dermal connective tissue. Elevated levels of serum and tissue fibronectin, uremia, and hyperphosphatemia have been observed in patients with APDRD.1,2 Fibronectin stimulates epithelial migration and proliferation and may lead to expulsion of keratin. Furthermore, dermal deposition of excess urea and/or phosphate could initiate transepithelial elimination of material. Alternative hypotheses implicate abnormal keratinization or an imbalance between the rates of epidermal proliferation/ differentiation and keratin production, whereby keratin production outpaces the former. Keratin deposited within the dermis subsequently elicits an inflammatory response along with alterations in the local dermis and connective tissue. These components become intermixed and are extruded through the plug opening.3 Lastly, immune dysregulation resulting from systemic disease could contribute to APDRD through increased expression of IL-31, a cytokine thought to play a role in several pruritic inflammatory skin diseases.4
Although standardized treatment guidelines for APDRD have not been established, the mainstay of therapy is control of the underlying systemic disorder. Intense pruritus and repeated scratching may contribute to microtrauma and subsequent koebnerization of new lesions.3 Thus, ameliorating pruritus can provide both symptomatic relief and prevent the development of new lesions. Retinoids, UV light, oral antibiotics, antihistamines, corticosteroids, keratolytic agents, and immunosuppressants (eg, allopurinol, tacrolimus) have shown some benefit.4
The differential diagnoses for APDRD include arthropod hypersensitivity reactions, eruptive keratoacanthomas, keratosis pilaris, and prurigo nodularis. Arthropod hypersensitivity reactions are seen in patients with a history of a bite or sting from arthropods such as bees, fleas, mites, ticks, and spiders. These reactions cause symptoms of pain, burning, or pruritus and present heterogeneously. They can be edematous and appear as single or multiple papules, pustules, plaques, vesicles, and/or bullae. A central punctum or crusting also may be present. Eruptive keratoacanthomas are seen in Grzybowski syndrome and Ferguson-Smith disease. Grzybowski syndrome arises in the fifth to seventh decades of life and is characterized by the eruptive onset of hundreds to thousands of pruritic, dome-shaped, follicular papules with or without central keratin plugs. Ectropion, mucosal lesions, and masklike facies are other clinical characteristics of Grzybowski syndrome. Ferguson-Smith disease begins in the second decade of life. The eruption of multiple keratoacanthomas and/or squamous cell carcinomas occurs in crops, rapidly growing over 2 to 4 weeks, and then self-resolves. This disease is inherited in an autosomal-dominant manner and is associated with chromosome 9q22. Keratosis pilaris is a benign condition of follicular hyperkeratosis that can appear in any age group and usually is absent of symptoms. It is not associated with any systemic disease. Clinically, the condition appears as folliculocentric keratotic papules with varying degrees of perifollicular erythema located along the extensor surfaces. Keratosis pilaris and APDRD share features of a follicular hyperkeratosis and dilated infundibulum; however, perforation is absent in keratosis pilaris. Lastly, prurigo nodularis is another intensely pruritic dermatosis associated with renal disease that presents as papulonodules on the extensor surfaces of the arms and legs. A biopsy can help to distinguish prurigo nodularis from APDRD.
- Rice AS, Zedek D. Kyrle disease. StatPearls [internet]. StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK532886/
- McKinley-Grant L, Peebles J. Renal disease. In: Kelly A, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill; 2016
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Forouzandeh M, Stratman S, Yosipovitch G. The treatment of Kyrle’s disease: a systematic review. J Eur Acad Dermatol Venereol. 2020;34:1457-1463. doi:10.1111/jdv.16182
THE DIAGNOSIS:
Acquired Perforating Disorder of Renal Disease
A papule with the central plug removed left a pitlike depression, representing Kyrle disease (Figure 1). A punch biopsy of the left forearm revealed epidermal hyperplasia (Figure 2A) surrounding a keratin plug that contained degenerated basophilic material (Figure 2B), confirming the diagnosis of acquired perforating disorder of renal disease (APDRD), classically described as Kyrle disease.


Acquired perforating disorder of renal disease is an uncommon condition in the general population. It is associated with systemic disease, commonly diabetes mellitus and chronic renal failure, and is seen in up to 10% patients receiving hemodialysis.1 The underlying etiology and pathogenesis of APDRD remains unknown. It has been proposed to be a variant of prurigo nodularis, representing end-stage excoriated folliculitis.1 Given that most cases appear in patients with systemic disease and metabolic abnormalities, APDRD also has been classified under the spectrum of acquired perforating dermatoses, a group of disorders defined by transepithelial elimination of dermal connective tissue. Elevated levels of serum and tissue fibronectin, uremia, and hyperphosphatemia have been observed in patients with APDRD.1,2 Fibronectin stimulates epithelial migration and proliferation and may lead to expulsion of keratin. Furthermore, dermal deposition of excess urea and/or phosphate could initiate transepithelial elimination of material. Alternative hypotheses implicate abnormal keratinization or an imbalance between the rates of epidermal proliferation/ differentiation and keratin production, whereby keratin production outpaces the former. Keratin deposited within the dermis subsequently elicits an inflammatory response along with alterations in the local dermis and connective tissue. These components become intermixed and are extruded through the plug opening.3 Lastly, immune dysregulation resulting from systemic disease could contribute to APDRD through increased expression of IL-31, a cytokine thought to play a role in several pruritic inflammatory skin diseases.4
Although standardized treatment guidelines for APDRD have not been established, the mainstay of therapy is control of the underlying systemic disorder. Intense pruritus and repeated scratching may contribute to microtrauma and subsequent koebnerization of new lesions.3 Thus, ameliorating pruritus can provide both symptomatic relief and prevent the development of new lesions. Retinoids, UV light, oral antibiotics, antihistamines, corticosteroids, keratolytic agents, and immunosuppressants (eg, allopurinol, tacrolimus) have shown some benefit.4
The differential diagnoses for APDRD include arthropod hypersensitivity reactions, eruptive keratoacanthomas, keratosis pilaris, and prurigo nodularis. Arthropod hypersensitivity reactions are seen in patients with a history of a bite or sting from arthropods such as bees, fleas, mites, ticks, and spiders. These reactions cause symptoms of pain, burning, or pruritus and present heterogeneously. They can be edematous and appear as single or multiple papules, pustules, plaques, vesicles, and/or bullae. A central punctum or crusting also may be present. Eruptive keratoacanthomas are seen in Grzybowski syndrome and Ferguson-Smith disease. Grzybowski syndrome arises in the fifth to seventh decades of life and is characterized by the eruptive onset of hundreds to thousands of pruritic, dome-shaped, follicular papules with or without central keratin plugs. Ectropion, mucosal lesions, and masklike facies are other clinical characteristics of Grzybowski syndrome. Ferguson-Smith disease begins in the second decade of life. The eruption of multiple keratoacanthomas and/or squamous cell carcinomas occurs in crops, rapidly growing over 2 to 4 weeks, and then self-resolves. This disease is inherited in an autosomal-dominant manner and is associated with chromosome 9q22. Keratosis pilaris is a benign condition of follicular hyperkeratosis that can appear in any age group and usually is absent of symptoms. It is not associated with any systemic disease. Clinically, the condition appears as folliculocentric keratotic papules with varying degrees of perifollicular erythema located along the extensor surfaces. Keratosis pilaris and APDRD share features of a follicular hyperkeratosis and dilated infundibulum; however, perforation is absent in keratosis pilaris. Lastly, prurigo nodularis is another intensely pruritic dermatosis associated with renal disease that presents as papulonodules on the extensor surfaces of the arms and legs. A biopsy can help to distinguish prurigo nodularis from APDRD.
THE DIAGNOSIS:
Acquired Perforating Disorder of Renal Disease
A papule with the central plug removed left a pitlike depression, representing Kyrle disease (Figure 1). A punch biopsy of the left forearm revealed epidermal hyperplasia (Figure 2A) surrounding a keratin plug that contained degenerated basophilic material (Figure 2B), confirming the diagnosis of acquired perforating disorder of renal disease (APDRD), classically described as Kyrle disease.


Acquired perforating disorder of renal disease is an uncommon condition in the general population. It is associated with systemic disease, commonly diabetes mellitus and chronic renal failure, and is seen in up to 10% patients receiving hemodialysis.1 The underlying etiology and pathogenesis of APDRD remains unknown. It has been proposed to be a variant of prurigo nodularis, representing end-stage excoriated folliculitis.1 Given that most cases appear in patients with systemic disease and metabolic abnormalities, APDRD also has been classified under the spectrum of acquired perforating dermatoses, a group of disorders defined by transepithelial elimination of dermal connective tissue. Elevated levels of serum and tissue fibronectin, uremia, and hyperphosphatemia have been observed in patients with APDRD.1,2 Fibronectin stimulates epithelial migration and proliferation and may lead to expulsion of keratin. Furthermore, dermal deposition of excess urea and/or phosphate could initiate transepithelial elimination of material. Alternative hypotheses implicate abnormal keratinization or an imbalance between the rates of epidermal proliferation/ differentiation and keratin production, whereby keratin production outpaces the former. Keratin deposited within the dermis subsequently elicits an inflammatory response along with alterations in the local dermis and connective tissue. These components become intermixed and are extruded through the plug opening.3 Lastly, immune dysregulation resulting from systemic disease could contribute to APDRD through increased expression of IL-31, a cytokine thought to play a role in several pruritic inflammatory skin diseases.4
Although standardized treatment guidelines for APDRD have not been established, the mainstay of therapy is control of the underlying systemic disorder. Intense pruritus and repeated scratching may contribute to microtrauma and subsequent koebnerization of new lesions.3 Thus, ameliorating pruritus can provide both symptomatic relief and prevent the development of new lesions. Retinoids, UV light, oral antibiotics, antihistamines, corticosteroids, keratolytic agents, and immunosuppressants (eg, allopurinol, tacrolimus) have shown some benefit.4
The differential diagnoses for APDRD include arthropod hypersensitivity reactions, eruptive keratoacanthomas, keratosis pilaris, and prurigo nodularis. Arthropod hypersensitivity reactions are seen in patients with a history of a bite or sting from arthropods such as bees, fleas, mites, ticks, and spiders. These reactions cause symptoms of pain, burning, or pruritus and present heterogeneously. They can be edematous and appear as single or multiple papules, pustules, plaques, vesicles, and/or bullae. A central punctum or crusting also may be present. Eruptive keratoacanthomas are seen in Grzybowski syndrome and Ferguson-Smith disease. Grzybowski syndrome arises in the fifth to seventh decades of life and is characterized by the eruptive onset of hundreds to thousands of pruritic, dome-shaped, follicular papules with or without central keratin plugs. Ectropion, mucosal lesions, and masklike facies are other clinical characteristics of Grzybowski syndrome. Ferguson-Smith disease begins in the second decade of life. The eruption of multiple keratoacanthomas and/or squamous cell carcinomas occurs in crops, rapidly growing over 2 to 4 weeks, and then self-resolves. This disease is inherited in an autosomal-dominant manner and is associated with chromosome 9q22. Keratosis pilaris is a benign condition of follicular hyperkeratosis that can appear in any age group and usually is absent of symptoms. It is not associated with any systemic disease. Clinically, the condition appears as folliculocentric keratotic papules with varying degrees of perifollicular erythema located along the extensor surfaces. Keratosis pilaris and APDRD share features of a follicular hyperkeratosis and dilated infundibulum; however, perforation is absent in keratosis pilaris. Lastly, prurigo nodularis is another intensely pruritic dermatosis associated with renal disease that presents as papulonodules on the extensor surfaces of the arms and legs. A biopsy can help to distinguish prurigo nodularis from APDRD.
- Rice AS, Zedek D. Kyrle disease. StatPearls [internet]. StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK532886/
- McKinley-Grant L, Peebles J. Renal disease. In: Kelly A, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill; 2016
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Forouzandeh M, Stratman S, Yosipovitch G. The treatment of Kyrle’s disease: a systematic review. J Eur Acad Dermatol Venereol. 2020;34:1457-1463. doi:10.1111/jdv.16182
- Rice AS, Zedek D. Kyrle disease. StatPearls [internet]. StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK532886/
- McKinley-Grant L, Peebles J. Renal disease. In: Kelly A, Taylor SC, Lim HW, et al, eds. Taylor and Kelly’s Dermatology for Skin of Color. 2nd ed. McGraw-Hill; 2016
- Patterson JW. The perforating disorders. J Am Acad Dermatol. 1984;10:561-581. doi:10.1016/s0190-9622(84)80259-5
- Forouzandeh M, Stratman S, Yosipovitch G. The treatment of Kyrle’s disease: a systematic review. J Eur Acad Dermatol Venereol. 2020;34:1457-1463. doi:10.1111/jdv.16182
A 74-year-old woman with a 30-year history of type 2 diabetes mellitus presented to our dermatology clinic with a pruritic eruption on the trunk, arms, and legs of 2 months’ duration. Several over-the-counter moisturizers had been used without improvement, and the pruritus was notably impacting her sleep. Physical examination revealed discrete, hyperkeratotic, predominantly follicular, eruptive papules with hyperkeratotic plugs diffusely distributed on the trunk, arms, and legs.
Wrist rash

The gradual development of a rash in an area of frequent direct contact between metal and skin is pathognomonic for allergic contact dermatitis (ACD). Contact dermatitis often results from exposure to metals. Stainless steel is a group of ferrous alloys composed of a variety of elements including nickel, which is added to increase corrosion resistance. Unfortunately, nickel is a metal commonly known to induce a delayed hypersensitivity response. In the upper left corner of the image shown here, one can see the metal plate of the watch band.
ACD is a T-cell mediated, delayed, type IV hypersensitivity response to foreign materials.1 These reactions typically occur around 48 to 72 hours following contact with the metal but can take weeks to appear, depending on the amount of T-cell activation. Symptoms may appear more rapidly on repeat exposures. Lesions manifest as erythematous, scaly plaques, which may include vesicles and bullae in severe cases.
The mainstay of treatment for allergic contact dermatitis is avoidance of the allergen once it has been identified. Nickel is commonly found in metal parts on clothing and in jewelry. One method of protection from nickel in these cases is to cover the metal that touches the skin with a clear nail polish or another clear barrier (commercial options are available). Duct tape or fabric can also be used to cover the metal.
Topical corticosteroids are the first-line therapy to treat lesions. Topical calcineurin inhibitors are an alternative. Systemic corticosteroids may be indicated if there is extensive body surface area involvement. Phototherapy or systemic immunosuppression may be considered in severe refractory cases.
Our patient was counseled on the nature of the disease process and educated on strategies to avoid future exposures. Treatment was initiated with topical triamcinolone 0.1% ointment with follow-up as needed.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Spenser Squire, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi: 10.1016/j.jaad.2015.02.1139

The gradual development of a rash in an area of frequent direct contact between metal and skin is pathognomonic for allergic contact dermatitis (ACD). Contact dermatitis often results from exposure to metals. Stainless steel is a group of ferrous alloys composed of a variety of elements including nickel, which is added to increase corrosion resistance. Unfortunately, nickel is a metal commonly known to induce a delayed hypersensitivity response. In the upper left corner of the image shown here, one can see the metal plate of the watch band.
ACD is a T-cell mediated, delayed, type IV hypersensitivity response to foreign materials.1 These reactions typically occur around 48 to 72 hours following contact with the metal but can take weeks to appear, depending on the amount of T-cell activation. Symptoms may appear more rapidly on repeat exposures. Lesions manifest as erythematous, scaly plaques, which may include vesicles and bullae in severe cases.
The mainstay of treatment for allergic contact dermatitis is avoidance of the allergen once it has been identified. Nickel is commonly found in metal parts on clothing and in jewelry. One method of protection from nickel in these cases is to cover the metal that touches the skin with a clear nail polish or another clear barrier (commercial options are available). Duct tape or fabric can also be used to cover the metal.
Topical corticosteroids are the first-line therapy to treat lesions. Topical calcineurin inhibitors are an alternative. Systemic corticosteroids may be indicated if there is extensive body surface area involvement. Phototherapy or systemic immunosuppression may be considered in severe refractory cases.
Our patient was counseled on the nature of the disease process and educated on strategies to avoid future exposures. Treatment was initiated with topical triamcinolone 0.1% ointment with follow-up as needed.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Spenser Squire, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.

The gradual development of a rash in an area of frequent direct contact between metal and skin is pathognomonic for allergic contact dermatitis (ACD). Contact dermatitis often results from exposure to metals. Stainless steel is a group of ferrous alloys composed of a variety of elements including nickel, which is added to increase corrosion resistance. Unfortunately, nickel is a metal commonly known to induce a delayed hypersensitivity response. In the upper left corner of the image shown here, one can see the metal plate of the watch band.
ACD is a T-cell mediated, delayed, type IV hypersensitivity response to foreign materials.1 These reactions typically occur around 48 to 72 hours following contact with the metal but can take weeks to appear, depending on the amount of T-cell activation. Symptoms may appear more rapidly on repeat exposures. Lesions manifest as erythematous, scaly plaques, which may include vesicles and bullae in severe cases.
The mainstay of treatment for allergic contact dermatitis is avoidance of the allergen once it has been identified. Nickel is commonly found in metal parts on clothing and in jewelry. One method of protection from nickel in these cases is to cover the metal that touches the skin with a clear nail polish or another clear barrier (commercial options are available). Duct tape or fabric can also be used to cover the metal.
Topical corticosteroids are the first-line therapy to treat lesions. Topical calcineurin inhibitors are an alternative. Systemic corticosteroids may be indicated if there is extensive body surface area involvement. Phototherapy or systemic immunosuppression may be considered in severe refractory cases.
Our patient was counseled on the nature of the disease process and educated on strategies to avoid future exposures. Treatment was initiated with topical triamcinolone 0.1% ointment with follow-up as needed.
Image courtesy of Daniel Stulberg, MD. Text courtesy of Spenser Squire, MD, and Daniel Stulberg, MD, FAAFP, Department of Family and Community Medicine, University of New Mexico School of Medicine, Albuquerque.
1. Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi: 10.1016/j.jaad.2015.02.1139
1. Mowad CM, Anderson B, Scheinman P, et al. Allergic contact dermatitis: patient diagnosis and evaluation. J Am Acad Dermatol. 2016;74:1029-1040. doi: 10.1016/j.jaad.2015.02.1139
Epilepsy in older adults: Misdiagnosis and case complexity are common
, a neurologist told an audience at the annual meeting of the American Epilepsy Society. She urged colleagues to focus on possible interactions with other neurological conditions, consider various complicating factors, and embrace a team strategy.
“There are lots of nuances,” said Rebecca O’Dwyer, MD, an adult epilepsy specialist with Rush Epilepsy Center in Chicago. “It takes a lot of time and requires a multidisciplinary approach. Taking care of older individuals with epilepsy truly is a team sport.”
According to a 2014 report highlighted by Dr. O’Dwyer, “nearly 25% of new-onset seizures occur after age 65. The incidence of epilepsy in this age group is almost twice the rate in children, and in people over age 80, it is triple the rate in children.”
Research suggests it can take up to 2 years to correctly diagnose epilepsy in older people, Dr. O’Dwyer said, and nearly two-thirds of cases may be misdiagnosed. “Some of it is just limited awareness. There’s this perception in the public that epilepsy is something that occurs in younger adults or young children, and that when you come to a certain age, you cannot have epilepsy. Also, there are differences in the clinical manifestations of their seizures, and many comorbid possibilities could also present in similar fashion to epilepsy. Some of our usual tools that we use to come to the diagnosis such as EEG are also known to be less sensitive in this age group.”
According to the 2014 report, research finds that the elderly are much more likely than young adults to have postictal sleepiness or unresponsiveness and seizures manifesting as brief moments of subtle confusion. They’re much less likely to have epileptic aura and generalized tonic seizures.
“An epileptic seizure in an older adult tends to be less dramatic with fewer motor manifestations, and they often tend to be monophasic. They may be so subtle that they’re missed by family members and other medical providers,” Dr. O’Dwyer said. “I had a patient whose seizure consisted of her tapping her left shoulder. She had been doing this for at least 6 months, and she came to my clinic after her daughter realized that she was a little confused afterward. She’d already seen a behavioral neurologist and been given the diagnosis of dementia. We were fortunate enough to catch one of these episodes while we were doing an EEG, and we diagnosed her with focal epilepsy. With one antiseizure medication, we stopped the seizures, and her memory came back.”
Make sure to take detailed histories and keep an eye out for descriptions of behaviors that are episodic but perhaps not typical of seizures, she said.
Epilepsy can be misdiagnosed as a variety of conditions, she said, such as syncope, Alzheimer’s disease, stroke, Parkinson’s disease, and atrial fibrillation. “When you do diagnose somebody older with new-onset epilepsy, you should work them up for a stroke. Because we know that within the first 4 weeks after their first seizure the likelihood that they could have a stroke is three times higher.”
It’s also possible that neurological conditions can be followed by new-onset epilepsy, she said, making dementia even worse. Low-dose antiepileptic drugs can be helpful in these patients.
But seniors are especially vulnerable to side effects of antiepileptic drugs such as sedation, dizziness, and cardiac-conduction abnormalities. “You must adhere to the mantra of going low and going slow because they are exquisitely susceptible,” Dr. O’Dwyer said.
She recommends lamotrigine, which is well tolerated with helpful mood-stabilizing effects, and levetiracetam, which attenuates cognitive decline in dementia but may cause side effects such as irritable mood. Zonisamide is showing promise in patients with parkinsonian syndromes, she said, and it may be helpful to maximize drugs that patients are already taking such as gabapentin or pregabalin.
Finally, Dr. O’Dwyer urged colleagues to work in teams that include caregivers, primary care doctors, social workers, and pharmacists. “Sometimes in all this,” she said, “my job is the easiest.”
Dr. O’Dwyer discloses research support from the Shapiro Foundation.
, a neurologist told an audience at the annual meeting of the American Epilepsy Society. She urged colleagues to focus on possible interactions with other neurological conditions, consider various complicating factors, and embrace a team strategy.
“There are lots of nuances,” said Rebecca O’Dwyer, MD, an adult epilepsy specialist with Rush Epilepsy Center in Chicago. “It takes a lot of time and requires a multidisciplinary approach. Taking care of older individuals with epilepsy truly is a team sport.”
According to a 2014 report highlighted by Dr. O’Dwyer, “nearly 25% of new-onset seizures occur after age 65. The incidence of epilepsy in this age group is almost twice the rate in children, and in people over age 80, it is triple the rate in children.”
Research suggests it can take up to 2 years to correctly diagnose epilepsy in older people, Dr. O’Dwyer said, and nearly two-thirds of cases may be misdiagnosed. “Some of it is just limited awareness. There’s this perception in the public that epilepsy is something that occurs in younger adults or young children, and that when you come to a certain age, you cannot have epilepsy. Also, there are differences in the clinical manifestations of their seizures, and many comorbid possibilities could also present in similar fashion to epilepsy. Some of our usual tools that we use to come to the diagnosis such as EEG are also known to be less sensitive in this age group.”
According to the 2014 report, research finds that the elderly are much more likely than young adults to have postictal sleepiness or unresponsiveness and seizures manifesting as brief moments of subtle confusion. They’re much less likely to have epileptic aura and generalized tonic seizures.
“An epileptic seizure in an older adult tends to be less dramatic with fewer motor manifestations, and they often tend to be monophasic. They may be so subtle that they’re missed by family members and other medical providers,” Dr. O’Dwyer said. “I had a patient whose seizure consisted of her tapping her left shoulder. She had been doing this for at least 6 months, and she came to my clinic after her daughter realized that she was a little confused afterward. She’d already seen a behavioral neurologist and been given the diagnosis of dementia. We were fortunate enough to catch one of these episodes while we were doing an EEG, and we diagnosed her with focal epilepsy. With one antiseizure medication, we stopped the seizures, and her memory came back.”
Make sure to take detailed histories and keep an eye out for descriptions of behaviors that are episodic but perhaps not typical of seizures, she said.
Epilepsy can be misdiagnosed as a variety of conditions, she said, such as syncope, Alzheimer’s disease, stroke, Parkinson’s disease, and atrial fibrillation. “When you do diagnose somebody older with new-onset epilepsy, you should work them up for a stroke. Because we know that within the first 4 weeks after their first seizure the likelihood that they could have a stroke is three times higher.”
It’s also possible that neurological conditions can be followed by new-onset epilepsy, she said, making dementia even worse. Low-dose antiepileptic drugs can be helpful in these patients.
But seniors are especially vulnerable to side effects of antiepileptic drugs such as sedation, dizziness, and cardiac-conduction abnormalities. “You must adhere to the mantra of going low and going slow because they are exquisitely susceptible,” Dr. O’Dwyer said.
She recommends lamotrigine, which is well tolerated with helpful mood-stabilizing effects, and levetiracetam, which attenuates cognitive decline in dementia but may cause side effects such as irritable mood. Zonisamide is showing promise in patients with parkinsonian syndromes, she said, and it may be helpful to maximize drugs that patients are already taking such as gabapentin or pregabalin.
Finally, Dr. O’Dwyer urged colleagues to work in teams that include caregivers, primary care doctors, social workers, and pharmacists. “Sometimes in all this,” she said, “my job is the easiest.”
Dr. O’Dwyer discloses research support from the Shapiro Foundation.
, a neurologist told an audience at the annual meeting of the American Epilepsy Society. She urged colleagues to focus on possible interactions with other neurological conditions, consider various complicating factors, and embrace a team strategy.
“There are lots of nuances,” said Rebecca O’Dwyer, MD, an adult epilepsy specialist with Rush Epilepsy Center in Chicago. “It takes a lot of time and requires a multidisciplinary approach. Taking care of older individuals with epilepsy truly is a team sport.”
According to a 2014 report highlighted by Dr. O’Dwyer, “nearly 25% of new-onset seizures occur after age 65. The incidence of epilepsy in this age group is almost twice the rate in children, and in people over age 80, it is triple the rate in children.”
Research suggests it can take up to 2 years to correctly diagnose epilepsy in older people, Dr. O’Dwyer said, and nearly two-thirds of cases may be misdiagnosed. “Some of it is just limited awareness. There’s this perception in the public that epilepsy is something that occurs in younger adults or young children, and that when you come to a certain age, you cannot have epilepsy. Also, there are differences in the clinical manifestations of their seizures, and many comorbid possibilities could also present in similar fashion to epilepsy. Some of our usual tools that we use to come to the diagnosis such as EEG are also known to be less sensitive in this age group.”
According to the 2014 report, research finds that the elderly are much more likely than young adults to have postictal sleepiness or unresponsiveness and seizures manifesting as brief moments of subtle confusion. They’re much less likely to have epileptic aura and generalized tonic seizures.
“An epileptic seizure in an older adult tends to be less dramatic with fewer motor manifestations, and they often tend to be monophasic. They may be so subtle that they’re missed by family members and other medical providers,” Dr. O’Dwyer said. “I had a patient whose seizure consisted of her tapping her left shoulder. She had been doing this for at least 6 months, and she came to my clinic after her daughter realized that she was a little confused afterward. She’d already seen a behavioral neurologist and been given the diagnosis of dementia. We were fortunate enough to catch one of these episodes while we were doing an EEG, and we diagnosed her with focal epilepsy. With one antiseizure medication, we stopped the seizures, and her memory came back.”
Make sure to take detailed histories and keep an eye out for descriptions of behaviors that are episodic but perhaps not typical of seizures, she said.
Epilepsy can be misdiagnosed as a variety of conditions, she said, such as syncope, Alzheimer’s disease, stroke, Parkinson’s disease, and atrial fibrillation. “When you do diagnose somebody older with new-onset epilepsy, you should work them up for a stroke. Because we know that within the first 4 weeks after their first seizure the likelihood that they could have a stroke is three times higher.”
It’s also possible that neurological conditions can be followed by new-onset epilepsy, she said, making dementia even worse. Low-dose antiepileptic drugs can be helpful in these patients.
But seniors are especially vulnerable to side effects of antiepileptic drugs such as sedation, dizziness, and cardiac-conduction abnormalities. “You must adhere to the mantra of going low and going slow because they are exquisitely susceptible,” Dr. O’Dwyer said.
She recommends lamotrigine, which is well tolerated with helpful mood-stabilizing effects, and levetiracetam, which attenuates cognitive decline in dementia but may cause side effects such as irritable mood. Zonisamide is showing promise in patients with parkinsonian syndromes, she said, and it may be helpful to maximize drugs that patients are already taking such as gabapentin or pregabalin.
Finally, Dr. O’Dwyer urged colleagues to work in teams that include caregivers, primary care doctors, social workers, and pharmacists. “Sometimes in all this,” she said, “my job is the easiest.”
Dr. O’Dwyer discloses research support from the Shapiro Foundation.
FROM AES 2021
FDA authorizes Pfizer antiviral pill for COVID-19
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
The Food and Drug Administration on Dec. 22, 2021, granted emergency use authorization (EUA) for a new antiviral pill to treat people with symptomatic COVID-19.
Pfizer’s ritonavir, name brand Paxlovid, can now be taken by patients ages 12 and up who weigh at least 88 pounds.
The antiviral is only for people who test positive for the coronavirus and who are at high risk for severe COVID-19, including hospitalization or death. It is available by prescription only and should be taken as soon as possible after diagnosis and within 5 days of the start of symptoms.
Paxlovid is taken as three tablets together orally twice a day for 5 days, for a total of 30 tablets.
Possible side effects include a reduced sense of taste, diarrhea, high blood pressure, and muscle aches.
The authorization arrives as U.S. cases of the Omicron variant are surging, some monoclonal antibody treatments are becoming less effective, and Americans struggle to maintain some sense of tradition and normalcy around the holidays.
Paxlovid joins remdesivir as an available antiviral to treat COVID-19. Remdesivir is fully approved by the FDA but is given only intravenously in a hospital.
The COVID-19 antiviral pills come with some obvious advantages, including greater convenience for consumers – such as home use – and the potential to expand treatment for people in low- and middle-income countries.
‘An exciting step forward’
The EUA for Pfizer’s new drug has been highly anticipated, and news of its impending authorization circulated on social media on Tuesday. Eric Topol, MD, called the development an “exciting step forward.” Dr. Topol is editor in chief of Medscape, the parent company of MDedge.
He and many others also expected the FDA to grant emergency use authorization for an antiviral from Merck. But there was no immediate word Wednesday if that was still going to happen.
An accelerated authorization?
The FDA’s authorization for Pfizer’s antiviral comes about 5 weeks after the company submitted an application to the agency. In its submission, the company said a study showed the pill reduced by 89% the rate of hospitalization and death for people with mild to moderate COVID-19 illness.
In April 2021, Pfizer announced its antiviral pill for COVID-19 could be available by year’s end. In September, an official at the National Institutes of Allergy and Infectious Diseases seconded the prediction.
Merck filed its EUA application with the FDA in October. The company included results of its phase 3 study showing the treatment was linked to a 50% reduction in COVID-19 hospitalizations.
Interestingly, in September, Merck announced the findings of laboratory studies suggesting that molnupiravir would work against variants of the coronavirus because the agent does not target the virus’s spike protein. At the time, Delta was the dominant variant in the United States.
Faith-based purchasing
The U.S. government has already recognized the potential of these oral therapies, at least in terms of preorders.
Last month, it announced intentions to purchase $1 billion worth of Merck’s molnupiravir, adding to the $1.2 billion worth of the pills the U.S. ordered in June 2021. Also in November, the government announced it would purchase 10 million courses of the Pfizer pill at an estimated cost of $5.3 billion.
The government preorders of the antiviral pills for COVID-19 are separate from the orders for COVID-19 vaccines. Most recently, the Biden administration announced it will make 500 million tests for coronavirus infection available to Americans for free in early 2022.
A version of this article first appeared on WebMD.com.
Margin marking of polyps before EMR cuts recurrence: Study
Margin marking before endoscopic mucosal resection (EMR) of large colorectal polyps cut the risk of recurrence by 80% when compared with traditional EMR, new data suggest.
A team of researchers, led by Dennis Yang, MD, with the Center for Interventional Endoscopy at AdventHealth, Orlando, compared polyp recurrence after patients received EMR with margin marking versus recurrence after conventional EMR in a historical control group. They conclude that the simple margin-marking strategy may offer an alternative to margin ablation.
The findings of the study were published online Nov. 29 in Gastrointestinal Endoscopy.
A single-center, historical control study
A total of 210 patients (average age, 66 years; 56.2% women) with 210 polyps (average size, 30 mm; interquartile range: 25-40 mm) had either EMR with margin marking (EMR-MM; n = 74) or conventional EMR (n = 136). The groups had similar patient and lesion characteristics.
For EMR-MM, cautery marks were drawn along the lateral margins of the polyp with the snare tip. EMR followed with resection of the healthy mucosa with the marks.
Physicians can confirm complete resection, including a healthy margin, when no cautery marks are visible after EMR, the authors write.
A follow-up colonoscopy was performed 3 to 6 months later, the results of which were compared against historical controls.
After 6 months, EMR-MM led to a lower recurrence rate compared with the historical control group with traditional EMR (8% vs. 29%, respectively; P < .001).
“This strategy allowed a more reliable wide-field EMR, which may account for why our preliminary results demonstrated an 80% reduction in the likelihood of recurrence even after controlling for other factors, including polyp size and histopathology,” the authors write.
Recurrence risk has been one of the main limitations of EMR compared with surgery, with rates from 10%-35%, the authors note, though it has fewer adverse reactions and offers better quality of life than surgery.
Dr. Yang told this news organization that multiple studies have looked at possible factors for recurrence, which is thought to primarily occur at the lateral resection margins of the polyp.
“That’s based on recent data that has shown that burning the resection margins after you actually take the lesion out reduces recurrence,” he said. “What that indirectly implies is that whenever we resect something, we may think we’ve got the entire lesion at the lateral margins, but we don’t.”
As Dr. Yang described, it was this implication that led to the premise of the study.
“If we were to somehow put visible marks outside the margins of the lesion, the marks would serve as visible cues to tell us how much more tissue we needed to resect and thereby help us get a more reliable way of ensuring clean resection margins.”
Dr. Yang and colleagues also found that EMR-MM was not linked with an increase in adverse events. On multivariable analysis, EMR-MM was the main predictor of recurrence (odds ratio, 0.20; 95% CI, 0.13-0.64; P = .003) aside from polyp size (OR, 2.81; 95% CI, 1.35-6.01; P = .008).
Expert: standard of care likely still better
Gastroenterologist Douglas Rex, MD, Distinguished Professor Emeritus of Medicine at Indiana University School of Medicine, Indianapolis, who was not involved in the study, told this news organization that he is not convinced that it is necessary or wise to use the margin-marking technique described in the paper over the current standard of care.
Dr. Rex explained that presently, physicians inject large lesions submucosally with fluid colored for contrast to delineate the margin of the polyp. This raises the question: if you can see the lesion well with that method, do you need to place the marks before you start around the border on the normal mucosa, as they did for the margin-marking group in this study?
Dr. Rex also noted that the researchers’ 29% control group recurrence rate is relatively high.
“Most of the evidence – if you look at the big meta-analyses – suggests that the recurrence rate with traditional methods is around 15%,” he said.
He added that even the recurrence rate in the current study’s active treatment arm is much higher than the 2%-5% rate seen in recent thermal ablation trials by Klein and colleagues and Sidhu and colleagues, both published in Gastroenterology.
“The methods described in those two papers should be considered the current standard of care,” Dr. Rex said. “Neither one of those involves this [margin-marking] method.”
Dr. Yang agrees that the Klein and Sidhu trials represent the standard of care, but he says it’s important to note that the 2% recurrence may not represent the actual practice of endoscopists of all skill levels.
“These are highly controlled studies coming from very experienced endoscopists,” he said.
“Our data are not trying to supplant what the high-quality studies on thermal ablation have shown. The point is to show that this is a concept that could potentially help,” he said.
“What I’m proposing is a potential alternative that could be better than that. Obviously, we won’t know until a comparative type of trial is performed.”
On that point, Dr. Yang and Dr. Rex agree.
Dr. Rex said that a randomized control trial would clarify some points and be useful to compare margin marking directly with the current standard of care, “which is to remove the whole thing and then burn up the margin.”
“Based on what we have seen so far, I would predict the current standard of care would have a very good chance of winning in terms of efficacy, because it’s hard to get lower than 2% [recurrence],” he said. “And it might well win with regard to safety, because burning the margin is at least theoretically safer than what they’re doing here.”
Dr. Rex said margin marking may be beneficial with the form of EMR that does not involve submucosal injection: underwater EMR. In underwater EMR, there’s no submucosal injection, and some people will mark the margin in those instances, he said.
“I do think it’s reasonable to do margin marking for underwater EMR,” Dr. Rex said.
Dr. Yang is a consultant for Boston Scientific, Olympus, Lumendi, and Steris. A coauthor is a consultant for Olympus, Boston Scientific, Cook Medical, Merit, Microtech, Steris, Lumendi, and Fujifilm. Another coauthor receives research grants from Steris and Cosmo/Aries Pharmaceuticals. Dr. Rex disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Margin marking before endoscopic mucosal resection (EMR) of large colorectal polyps cut the risk of recurrence by 80% when compared with traditional EMR, new data suggest.
A team of researchers, led by Dennis Yang, MD, with the Center for Interventional Endoscopy at AdventHealth, Orlando, compared polyp recurrence after patients received EMR with margin marking versus recurrence after conventional EMR in a historical control group. They conclude that the simple margin-marking strategy may offer an alternative to margin ablation.
The findings of the study were published online Nov. 29 in Gastrointestinal Endoscopy.
A single-center, historical control study
A total of 210 patients (average age, 66 years; 56.2% women) with 210 polyps (average size, 30 mm; interquartile range: 25-40 mm) had either EMR with margin marking (EMR-MM; n = 74) or conventional EMR (n = 136). The groups had similar patient and lesion characteristics.
For EMR-MM, cautery marks were drawn along the lateral margins of the polyp with the snare tip. EMR followed with resection of the healthy mucosa with the marks.
Physicians can confirm complete resection, including a healthy margin, when no cautery marks are visible after EMR, the authors write.
A follow-up colonoscopy was performed 3 to 6 months later, the results of which were compared against historical controls.
After 6 months, EMR-MM led to a lower recurrence rate compared with the historical control group with traditional EMR (8% vs. 29%, respectively; P < .001).
“This strategy allowed a more reliable wide-field EMR, which may account for why our preliminary results demonstrated an 80% reduction in the likelihood of recurrence even after controlling for other factors, including polyp size and histopathology,” the authors write.
Recurrence risk has been one of the main limitations of EMR compared with surgery, with rates from 10%-35%, the authors note, though it has fewer adverse reactions and offers better quality of life than surgery.
Dr. Yang told this news organization that multiple studies have looked at possible factors for recurrence, which is thought to primarily occur at the lateral resection margins of the polyp.
“That’s based on recent data that has shown that burning the resection margins after you actually take the lesion out reduces recurrence,” he said. “What that indirectly implies is that whenever we resect something, we may think we’ve got the entire lesion at the lateral margins, but we don’t.”
As Dr. Yang described, it was this implication that led to the premise of the study.
“If we were to somehow put visible marks outside the margins of the lesion, the marks would serve as visible cues to tell us how much more tissue we needed to resect and thereby help us get a more reliable way of ensuring clean resection margins.”
Dr. Yang and colleagues also found that EMR-MM was not linked with an increase in adverse events. On multivariable analysis, EMR-MM was the main predictor of recurrence (odds ratio, 0.20; 95% CI, 0.13-0.64; P = .003) aside from polyp size (OR, 2.81; 95% CI, 1.35-6.01; P = .008).
Expert: standard of care likely still better
Gastroenterologist Douglas Rex, MD, Distinguished Professor Emeritus of Medicine at Indiana University School of Medicine, Indianapolis, who was not involved in the study, told this news organization that he is not convinced that it is necessary or wise to use the margin-marking technique described in the paper over the current standard of care.
Dr. Rex explained that presently, physicians inject large lesions submucosally with fluid colored for contrast to delineate the margin of the polyp. This raises the question: if you can see the lesion well with that method, do you need to place the marks before you start around the border on the normal mucosa, as they did for the margin-marking group in this study?
Dr. Rex also noted that the researchers’ 29% control group recurrence rate is relatively high.
“Most of the evidence – if you look at the big meta-analyses – suggests that the recurrence rate with traditional methods is around 15%,” he said.
He added that even the recurrence rate in the current study’s active treatment arm is much higher than the 2%-5% rate seen in recent thermal ablation trials by Klein and colleagues and Sidhu and colleagues, both published in Gastroenterology.
“The methods described in those two papers should be considered the current standard of care,” Dr. Rex said. “Neither one of those involves this [margin-marking] method.”
Dr. Yang agrees that the Klein and Sidhu trials represent the standard of care, but he says it’s important to note that the 2% recurrence may not represent the actual practice of endoscopists of all skill levels.
“These are highly controlled studies coming from very experienced endoscopists,” he said.
“Our data are not trying to supplant what the high-quality studies on thermal ablation have shown. The point is to show that this is a concept that could potentially help,” he said.
“What I’m proposing is a potential alternative that could be better than that. Obviously, we won’t know until a comparative type of trial is performed.”
On that point, Dr. Yang and Dr. Rex agree.
Dr. Rex said that a randomized control trial would clarify some points and be useful to compare margin marking directly with the current standard of care, “which is to remove the whole thing and then burn up the margin.”
“Based on what we have seen so far, I would predict the current standard of care would have a very good chance of winning in terms of efficacy, because it’s hard to get lower than 2% [recurrence],” he said. “And it might well win with regard to safety, because burning the margin is at least theoretically safer than what they’re doing here.”
Dr. Rex said margin marking may be beneficial with the form of EMR that does not involve submucosal injection: underwater EMR. In underwater EMR, there’s no submucosal injection, and some people will mark the margin in those instances, he said.
“I do think it’s reasonable to do margin marking for underwater EMR,” Dr. Rex said.
Dr. Yang is a consultant for Boston Scientific, Olympus, Lumendi, and Steris. A coauthor is a consultant for Olympus, Boston Scientific, Cook Medical, Merit, Microtech, Steris, Lumendi, and Fujifilm. Another coauthor receives research grants from Steris and Cosmo/Aries Pharmaceuticals. Dr. Rex disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Margin marking before endoscopic mucosal resection (EMR) of large colorectal polyps cut the risk of recurrence by 80% when compared with traditional EMR, new data suggest.
A team of researchers, led by Dennis Yang, MD, with the Center for Interventional Endoscopy at AdventHealth, Orlando, compared polyp recurrence after patients received EMR with margin marking versus recurrence after conventional EMR in a historical control group. They conclude that the simple margin-marking strategy may offer an alternative to margin ablation.
The findings of the study were published online Nov. 29 in Gastrointestinal Endoscopy.
A single-center, historical control study
A total of 210 patients (average age, 66 years; 56.2% women) with 210 polyps (average size, 30 mm; interquartile range: 25-40 mm) had either EMR with margin marking (EMR-MM; n = 74) or conventional EMR (n = 136). The groups had similar patient and lesion characteristics.
For EMR-MM, cautery marks were drawn along the lateral margins of the polyp with the snare tip. EMR followed with resection of the healthy mucosa with the marks.
Physicians can confirm complete resection, including a healthy margin, when no cautery marks are visible after EMR, the authors write.
A follow-up colonoscopy was performed 3 to 6 months later, the results of which were compared against historical controls.
After 6 months, EMR-MM led to a lower recurrence rate compared with the historical control group with traditional EMR (8% vs. 29%, respectively; P < .001).
“This strategy allowed a more reliable wide-field EMR, which may account for why our preliminary results demonstrated an 80% reduction in the likelihood of recurrence even after controlling for other factors, including polyp size and histopathology,” the authors write.
Recurrence risk has been one of the main limitations of EMR compared with surgery, with rates from 10%-35%, the authors note, though it has fewer adverse reactions and offers better quality of life than surgery.
Dr. Yang told this news organization that multiple studies have looked at possible factors for recurrence, which is thought to primarily occur at the lateral resection margins of the polyp.
“That’s based on recent data that has shown that burning the resection margins after you actually take the lesion out reduces recurrence,” he said. “What that indirectly implies is that whenever we resect something, we may think we’ve got the entire lesion at the lateral margins, but we don’t.”
As Dr. Yang described, it was this implication that led to the premise of the study.
“If we were to somehow put visible marks outside the margins of the lesion, the marks would serve as visible cues to tell us how much more tissue we needed to resect and thereby help us get a more reliable way of ensuring clean resection margins.”
Dr. Yang and colleagues also found that EMR-MM was not linked with an increase in adverse events. On multivariable analysis, EMR-MM was the main predictor of recurrence (odds ratio, 0.20; 95% CI, 0.13-0.64; P = .003) aside from polyp size (OR, 2.81; 95% CI, 1.35-6.01; P = .008).
Expert: standard of care likely still better
Gastroenterologist Douglas Rex, MD, Distinguished Professor Emeritus of Medicine at Indiana University School of Medicine, Indianapolis, who was not involved in the study, told this news organization that he is not convinced that it is necessary or wise to use the margin-marking technique described in the paper over the current standard of care.
Dr. Rex explained that presently, physicians inject large lesions submucosally with fluid colored for contrast to delineate the margin of the polyp. This raises the question: if you can see the lesion well with that method, do you need to place the marks before you start around the border on the normal mucosa, as they did for the margin-marking group in this study?
Dr. Rex also noted that the researchers’ 29% control group recurrence rate is relatively high.
“Most of the evidence – if you look at the big meta-analyses – suggests that the recurrence rate with traditional methods is around 15%,” he said.
He added that even the recurrence rate in the current study’s active treatment arm is much higher than the 2%-5% rate seen in recent thermal ablation trials by Klein and colleagues and Sidhu and colleagues, both published in Gastroenterology.
“The methods described in those two papers should be considered the current standard of care,” Dr. Rex said. “Neither one of those involves this [margin-marking] method.”
Dr. Yang agrees that the Klein and Sidhu trials represent the standard of care, but he says it’s important to note that the 2% recurrence may not represent the actual practice of endoscopists of all skill levels.
“These are highly controlled studies coming from very experienced endoscopists,” he said.
“Our data are not trying to supplant what the high-quality studies on thermal ablation have shown. The point is to show that this is a concept that could potentially help,” he said.
“What I’m proposing is a potential alternative that could be better than that. Obviously, we won’t know until a comparative type of trial is performed.”
On that point, Dr. Yang and Dr. Rex agree.
Dr. Rex said that a randomized control trial would clarify some points and be useful to compare margin marking directly with the current standard of care, “which is to remove the whole thing and then burn up the margin.”
“Based on what we have seen so far, I would predict the current standard of care would have a very good chance of winning in terms of efficacy, because it’s hard to get lower than 2% [recurrence],” he said. “And it might well win with regard to safety, because burning the margin is at least theoretically safer than what they’re doing here.”
Dr. Rex said margin marking may be beneficial with the form of EMR that does not involve submucosal injection: underwater EMR. In underwater EMR, there’s no submucosal injection, and some people will mark the margin in those instances, he said.
“I do think it’s reasonable to do margin marking for underwater EMR,” Dr. Rex said.
Dr. Yang is a consultant for Boston Scientific, Olympus, Lumendi, and Steris. A coauthor is a consultant for Olympus, Boston Scientific, Cook Medical, Merit, Microtech, Steris, Lumendi, and Fujifilm. Another coauthor receives research grants from Steris and Cosmo/Aries Pharmaceuticals. Dr. Rex disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
FROM GASTROINTESTINAL ENDOSCOPY
High GI spending reveals research, public health need
GI, liver, and pancreatic diseases cost the U.S. health care system about $120B per year and account for approximately 250,000 annual deaths, according to a “conservative” estimate from a recent analysis.
These figures emphasize the need for more research funding in the area, along with additional clinical and public health initiatives, reported lead author Anne F. Peery, MD, of the University of North Carolina School of Medicine, Chapel Hill, and colleagues.
“Reports detailing the burden of GI diseases are necessary for clinical research, decision making, and priority setting,” the investigators wrote in Gastroenterology. “Our aim was to describe health care use, expenditures, and research funding across GI, liver, and pancreatic diseases in the United States.”
Dr. Peery and colleagues analyzed data from 14 sources, including the National Institutes of Health; the Centers for Disease Control and Prevention; the National Ambulatory Medical Care Survey; and others. GI-specific outcomes included mortality, readmissions, hospitalizations, office-based visits, and emergency department visits. The investigators also characterized trends in cancers, organ transplants, and GI endoscopy, as well as GI-specific health care costs and NIH research funding. Annual findings were presented for various periods.
Total GI health care spending was $119.6 billion in 2018, down from $135.9 billion in 2015. The top five most costly conditions were biliary tract diseases ($16.9 billion), esophageal disorders ($12.1 billion), abdominal pain ($9.5 billion), abdominal hernias ($9.0 billion), and diverticular disease ($9.0 billion). The investigators noted that medication costs were particularly high for two categories: inflammatory bowel diseases and esophageal disorders, which had prescription drug costs relative to total expenditures of 71% and 53%, respectively.
“This conservative estimate [of $119.6 billion] did not include most GI cancers and likely underestimated the costs associated with some GI conditions,” the investigators noted. “For example, the Medical Expenditure Panel Survey estimate associated with GI bleeding was $300 million. In comparison, the aggregate cost of GI bleeding was more realistically $3.7 billion, as estimated using inpatient data from the National Inpatient Sample.”
In 2016, the most common GI-related diagnosis in the U.S. was abdominal pain (15.7 million annual visits), followed by nausea and vomiting (5.0 million visits), gastroesophageal reflux disorder and reflux esophagitis (4.7 million visits), constipation (3.1 million visits), and abdominal wall/inguinal hernia (2.8 million visits).
The top three most common GI-related hospital admissions in 2018 were GI bleeding (1.3 million admissions), followed by cholelithiasis and cholecystitis (741,060 admissions), then pancreatitis (685,880 admissions). GI bleeding was also the leading cause of 30-day readmission in 2018 (84,533 readmissions).
“We found substantial numbers of GI conditions and symptoms listed in secondary positions on the discharge record,” the investigators wrote. “For example, liver disease accounted for 280,645 discharges with a primary diagnosis; however, there were 13-fold as many discharges (3.6 million in 2018) with liver disease as a secondary diagnosis. Including all diagnoses captures a burden of GI disease not previously reported.”
In 2018 and 2019, GI diseases and cancers caused 255,407 annual deaths. The most common noncancer deaths were caused by alcohol-associated liver disease (24,110 deaths), hepatic fibrosis/cirrhosis (20,184 deaths), and GI bleeding (9,548 deaths). Among GI-cancer related deaths, colorectal cancer (CRC) caused the most mortalities (52,163 deaths), followed by pancreatic cancer (44,914 deaths), and hepatic/biliary cancer (44,914 deaths). The investigators noted that CRC was disproportionately common among non-Hispanic Black individuals, whereas gastric cancer was relatively high among Hispanic individuals.
“GI cancers account for a large number of diagnoses and deaths annually, with persistent disparities in incidence and mortality rates by race/ethnicity,” the investigators wrote. “Racial, ethnic, and regional disparities in access to most GI endoscopy procedures exist, which suggests an unmet need for GI procedures across the United States.”
A total of 22.2 million endoscopies were performed in 2019, most commonly colonoscopy (13.8 million procedures), followed by upper endoscopy (7.5 million procedures), and flexible sigmoidoscopy (379,883 procedures).
In 2020, the NIH spent $3.1 billion, or approximately 7.5% of its budget, on GI disease research. Digestive diseases captured the bulk of this spending, with $2.3 billion. In the same year, the NIH spent 10.5% of its cancer research budget on GI cancers, with the greatest proportion ($325 million) awarded to CRC research.
“Carefully examining the data in this report can help generate areas for future investigation, prioritize research funding, identify areas of unmet need or disparities, and provide an important overview of the impact of digestive and liver conditions,” the investigators concluded. “We hope that others will use this report as motivation to take a deeper dive into individual diseases. There is much to learn from carefully studying existing data sources.”
The study was supported by the National Center for Advancing Translational Sciences, National Institutes of Health. The investigators disclosed no conflicts of interest.
GI, liver, and pancreatic diseases cost the U.S. health care system about $120B per year and account for approximately 250,000 annual deaths, according to a “conservative” estimate from a recent analysis.
These figures emphasize the need for more research funding in the area, along with additional clinical and public health initiatives, reported lead author Anne F. Peery, MD, of the University of North Carolina School of Medicine, Chapel Hill, and colleagues.
“Reports detailing the burden of GI diseases are necessary for clinical research, decision making, and priority setting,” the investigators wrote in Gastroenterology. “Our aim was to describe health care use, expenditures, and research funding across GI, liver, and pancreatic diseases in the United States.”
Dr. Peery and colleagues analyzed data from 14 sources, including the National Institutes of Health; the Centers for Disease Control and Prevention; the National Ambulatory Medical Care Survey; and others. GI-specific outcomes included mortality, readmissions, hospitalizations, office-based visits, and emergency department visits. The investigators also characterized trends in cancers, organ transplants, and GI endoscopy, as well as GI-specific health care costs and NIH research funding. Annual findings were presented for various periods.
Total GI health care spending was $119.6 billion in 2018, down from $135.9 billion in 2015. The top five most costly conditions were biliary tract diseases ($16.9 billion), esophageal disorders ($12.1 billion), abdominal pain ($9.5 billion), abdominal hernias ($9.0 billion), and diverticular disease ($9.0 billion). The investigators noted that medication costs were particularly high for two categories: inflammatory bowel diseases and esophageal disorders, which had prescription drug costs relative to total expenditures of 71% and 53%, respectively.
“This conservative estimate [of $119.6 billion] did not include most GI cancers and likely underestimated the costs associated with some GI conditions,” the investigators noted. “For example, the Medical Expenditure Panel Survey estimate associated with GI bleeding was $300 million. In comparison, the aggregate cost of GI bleeding was more realistically $3.7 billion, as estimated using inpatient data from the National Inpatient Sample.”
In 2016, the most common GI-related diagnosis in the U.S. was abdominal pain (15.7 million annual visits), followed by nausea and vomiting (5.0 million visits), gastroesophageal reflux disorder and reflux esophagitis (4.7 million visits), constipation (3.1 million visits), and abdominal wall/inguinal hernia (2.8 million visits).
The top three most common GI-related hospital admissions in 2018 were GI bleeding (1.3 million admissions), followed by cholelithiasis and cholecystitis (741,060 admissions), then pancreatitis (685,880 admissions). GI bleeding was also the leading cause of 30-day readmission in 2018 (84,533 readmissions).
“We found substantial numbers of GI conditions and symptoms listed in secondary positions on the discharge record,” the investigators wrote. “For example, liver disease accounted for 280,645 discharges with a primary diagnosis; however, there were 13-fold as many discharges (3.6 million in 2018) with liver disease as a secondary diagnosis. Including all diagnoses captures a burden of GI disease not previously reported.”
In 2018 and 2019, GI diseases and cancers caused 255,407 annual deaths. The most common noncancer deaths were caused by alcohol-associated liver disease (24,110 deaths), hepatic fibrosis/cirrhosis (20,184 deaths), and GI bleeding (9,548 deaths). Among GI-cancer related deaths, colorectal cancer (CRC) caused the most mortalities (52,163 deaths), followed by pancreatic cancer (44,914 deaths), and hepatic/biliary cancer (44,914 deaths). The investigators noted that CRC was disproportionately common among non-Hispanic Black individuals, whereas gastric cancer was relatively high among Hispanic individuals.
“GI cancers account for a large number of diagnoses and deaths annually, with persistent disparities in incidence and mortality rates by race/ethnicity,” the investigators wrote. “Racial, ethnic, and regional disparities in access to most GI endoscopy procedures exist, which suggests an unmet need for GI procedures across the United States.”
A total of 22.2 million endoscopies were performed in 2019, most commonly colonoscopy (13.8 million procedures), followed by upper endoscopy (7.5 million procedures), and flexible sigmoidoscopy (379,883 procedures).
In 2020, the NIH spent $3.1 billion, or approximately 7.5% of its budget, on GI disease research. Digestive diseases captured the bulk of this spending, with $2.3 billion. In the same year, the NIH spent 10.5% of its cancer research budget on GI cancers, with the greatest proportion ($325 million) awarded to CRC research.
“Carefully examining the data in this report can help generate areas for future investigation, prioritize research funding, identify areas of unmet need or disparities, and provide an important overview of the impact of digestive and liver conditions,” the investigators concluded. “We hope that others will use this report as motivation to take a deeper dive into individual diseases. There is much to learn from carefully studying existing data sources.”
The study was supported by the National Center for Advancing Translational Sciences, National Institutes of Health. The investigators disclosed no conflicts of interest.
GI, liver, and pancreatic diseases cost the U.S. health care system about $120B per year and account for approximately 250,000 annual deaths, according to a “conservative” estimate from a recent analysis.
These figures emphasize the need for more research funding in the area, along with additional clinical and public health initiatives, reported lead author Anne F. Peery, MD, of the University of North Carolina School of Medicine, Chapel Hill, and colleagues.
“Reports detailing the burden of GI diseases are necessary for clinical research, decision making, and priority setting,” the investigators wrote in Gastroenterology. “Our aim was to describe health care use, expenditures, and research funding across GI, liver, and pancreatic diseases in the United States.”
Dr. Peery and colleagues analyzed data from 14 sources, including the National Institutes of Health; the Centers for Disease Control and Prevention; the National Ambulatory Medical Care Survey; and others. GI-specific outcomes included mortality, readmissions, hospitalizations, office-based visits, and emergency department visits. The investigators also characterized trends in cancers, organ transplants, and GI endoscopy, as well as GI-specific health care costs and NIH research funding. Annual findings were presented for various periods.
Total GI health care spending was $119.6 billion in 2018, down from $135.9 billion in 2015. The top five most costly conditions were biliary tract diseases ($16.9 billion), esophageal disorders ($12.1 billion), abdominal pain ($9.5 billion), abdominal hernias ($9.0 billion), and diverticular disease ($9.0 billion). The investigators noted that medication costs were particularly high for two categories: inflammatory bowel diseases and esophageal disorders, which had prescription drug costs relative to total expenditures of 71% and 53%, respectively.
“This conservative estimate [of $119.6 billion] did not include most GI cancers and likely underestimated the costs associated with some GI conditions,” the investigators noted. “For example, the Medical Expenditure Panel Survey estimate associated with GI bleeding was $300 million. In comparison, the aggregate cost of GI bleeding was more realistically $3.7 billion, as estimated using inpatient data from the National Inpatient Sample.”
In 2016, the most common GI-related diagnosis in the U.S. was abdominal pain (15.7 million annual visits), followed by nausea and vomiting (5.0 million visits), gastroesophageal reflux disorder and reflux esophagitis (4.7 million visits), constipation (3.1 million visits), and abdominal wall/inguinal hernia (2.8 million visits).
The top three most common GI-related hospital admissions in 2018 were GI bleeding (1.3 million admissions), followed by cholelithiasis and cholecystitis (741,060 admissions), then pancreatitis (685,880 admissions). GI bleeding was also the leading cause of 30-day readmission in 2018 (84,533 readmissions).
“We found substantial numbers of GI conditions and symptoms listed in secondary positions on the discharge record,” the investigators wrote. “For example, liver disease accounted for 280,645 discharges with a primary diagnosis; however, there were 13-fold as many discharges (3.6 million in 2018) with liver disease as a secondary diagnosis. Including all diagnoses captures a burden of GI disease not previously reported.”
In 2018 and 2019, GI diseases and cancers caused 255,407 annual deaths. The most common noncancer deaths were caused by alcohol-associated liver disease (24,110 deaths), hepatic fibrosis/cirrhosis (20,184 deaths), and GI bleeding (9,548 deaths). Among GI-cancer related deaths, colorectal cancer (CRC) caused the most mortalities (52,163 deaths), followed by pancreatic cancer (44,914 deaths), and hepatic/biliary cancer (44,914 deaths). The investigators noted that CRC was disproportionately common among non-Hispanic Black individuals, whereas gastric cancer was relatively high among Hispanic individuals.
“GI cancers account for a large number of diagnoses and deaths annually, with persistent disparities in incidence and mortality rates by race/ethnicity,” the investigators wrote. “Racial, ethnic, and regional disparities in access to most GI endoscopy procedures exist, which suggests an unmet need for GI procedures across the United States.”
A total of 22.2 million endoscopies were performed in 2019, most commonly colonoscopy (13.8 million procedures), followed by upper endoscopy (7.5 million procedures), and flexible sigmoidoscopy (379,883 procedures).
In 2020, the NIH spent $3.1 billion, or approximately 7.5% of its budget, on GI disease research. Digestive diseases captured the bulk of this spending, with $2.3 billion. In the same year, the NIH spent 10.5% of its cancer research budget on GI cancers, with the greatest proportion ($325 million) awarded to CRC research.
“Carefully examining the data in this report can help generate areas for future investigation, prioritize research funding, identify areas of unmet need or disparities, and provide an important overview of the impact of digestive and liver conditions,” the investigators concluded. “We hope that others will use this report as motivation to take a deeper dive into individual diseases. There is much to learn from carefully studying existing data sources.”
The study was supported by the National Center for Advancing Translational Sciences, National Institutes of Health. The investigators disclosed no conflicts of interest.
FROM GASTROENTEROLOGY