User login
2021 Update on bone health
Recently, the National Osteoporosis Foundation (NOF) changed its name to the Bone Health and Osteoporosis Foundation (BHOF). Several years ago, in 2016 at my urging, this column was renamed from “Update on osteoporosis” to “Update on bone health.” I believe we were on the leading edge of this movement. As expressed in last year’s Update, our patients’ bone health must be emphasized more than it has been in the past.1
Consider that localized breast cancer carries a 5-year survival rate of 99%.2 Most of my patients are keenly aware that periodic competent breast imaging is the key to the earliest possible diagnosis. By contrast, in this country a hip fracture carries a mortality in the first year of 21%!3 Furthermore, approximately one-third of women who fracture their hip do not have osteoporosis.4 While the risk of hip fracture is greatest in women with osteoporosis, it is not absent in those without the condition. Finally, the role of muscle mass, strength, and performance in bone health is a rapidly emerging topic and one that constitutes the core of this year’s Update.
Muscle mass and strength play key role in bone health
de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504. doi:10.1080/13697137.2021.1950967.
Recently, de Villiers and Goldstein offered an overview of osteoporosis.5 What is worthy of reporting here is the role of muscle in bone health.
The bone-muscle relationship
Most clinicians know that osteoporosis and osteopenia are well-defined conditions with known risks associated with fracture. According to a review of PubMed, the first article with the keyword “osteoporosis” was published in 1894; through May 2020, 93,335 articles used that keyword. “Osteoporosis” is derived from the Greek osteon (bone) and poros (little hole). Thus, osteoporosis means “porous bone.”
Sarcopenia is characterized by progressive and generalized loss of skeletal muscle mass, strength, and function, and the condition is associated with a risk of adverse outcomes that include physical disabilities, poor quality of life, and death.6,7 “Sarcopenia” has its roots in the Greek words sarx (flesh) and penia (loss), and the term was coined in 1989.8 A PubMed review that included “sarcopenia” as the keyword revealed that the first article was published in 1993, with 12,068 articles published through May 2020.
Notably, muscle accounts for about 60% of the body’s protein. Muscle mass decreases with age, but younger patients with malnutrition, cachexia, or inflammatory diseases are also prone to decreased muscle mass. While osteoporosis has a well-accepted definition based on dual-energy x-ray absorptiometry (DXA) measurements, sarcopenia has no universally accepted definition, consensus diagnostic criteria, or treatment guidelines. In 2016, however, the International Classification of Diseases, Tenth Revision, Clinical Modification (CD-10-CM) finally recognized sarcopenia as a disease entity.
Currently, the most widely accepted definition comes from the European Working Group on Sarcopenia in Older People, which labeled presarcopenia as low muscle mass without impact on muscle strength or performance; sarcopenia as low muscle mass with either low muscle strength or low physical performance; and severe sarcopenia has all 3 criteria being present.9
When osteosarcopenia (osteoporosis or osteopenia combined with sarcopenia) exists, it can result in a threefold increase in risk of falls and a fourfold increase in fracture risk compared with women who have osteopenia or osteoporosis alone.10
The morbidity and mortality from fragility fractures are well known. Initially, diagnosis of risk seemed to be mainly T-scores on bone mineral density (BMD) testing (normal, osteopenic, osteoporosis). The FRAX fracture risk assessment tool, which includes a number of variables, further refined risk assessment. Increasingly, there is evidence of crosstalk between muscle and bone. Sarcopenia, the loss of skeletal muscle mass, strength, and performance, appears to play an important role as well for fracture risk. Simple tools to evaluate a patient’s muscle status exist. At the very least, resistance and balance exercises should be part of all clinicians’ patient counseling for bone health.
Continue to: Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives...
Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives
El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232. doi: 10.1007/s10067-021 -05757-w.
Osteosarcopenia, the combination of osteoporosis or osteopenia with sarcopenia, has been shown to increase the overall rate of falls and fracture when compared with fall and fracture rates in women with osteopenia or osteoporosis alone.10 A study by El Miedany and colleagues examined whether denosumab treatment had a possible dual therapeutic effect on osteoporosis and sarcopenia.11
Study details
The investigators looked at 135 patients diagnosed with postmenopausal osteoporosis and who were prescribed denosumab and compared them with a control group of 272 patients stratified into 2 subgroups: 136 were prescribed alendronate and 136 were prescribed zoledronate.
Assessments were performed for all participants for BMD (DXA), fall risk (falls risk assessment score [FRAS]), fracture risk (FRAX assessment tool), and sarcopenia measures. Reassessments were conducted after 5 years of denosumab or alendronate therapy, 3 years of zoledronate therapy, and 1 year after stopping the osteoporosis therapy.
The FRAS uses the clinical variables of history of falls in the last 12 months, impaired sight, weak hand grip, history of loss of balance in the last 12 months, and slowing of the walking speed/change in gait to yield a percent chance of sustaining a fall.12 Sarcopenic measures include grip strength, timed up and go (TUG) mobility test, and gait speed. There were no significant demographic differences between the 3 groups.
Denosumab reduced risk of falls and positively affected muscle strength
On completion of the 5-year denosumab therapy, falls risk was significantly decreased (P = .001) and significant improvements were seen in all sarcopenia measures (P = .01). One year after denosumab was discontinued, a significant worsening of both falls risk and sarcopenia measures (P = .01) occurred. This was in contrast to results in both control groups (alendronate and zoledronate), in which there was an improvement, although less robust in gait speed and the TUG test (P = .05) but no improvement in risk of falls. Thus, the results of this study showed that denosumab not only improved bone mass but also reduced falls risk.
Compared with bisphosphonates, denosumab showed the highest significant positive effect on both physical performance and skeletal muscle strength. This is evidenced by improvement of the gait speed, TUG test, and 4-m walk test (P<.001) in the denosumab group versus in the alendronate and zoledronate group (P<.05).
These results agree with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis 6 months) trial, which revealed that not only did denosumab treatment reduce the risk of vertebral, nonvertebral, and hip fracture over 36 months, but also that the denosumab-treated group had fewer falls (4.5%) compared with the other groups (5.7%) (P = .02).13
These data highlight that osteoporosis and sarcopenia may share similar underlying risk factors and that muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. While all 3 antiresorptives (denosumab, alendronate, zoledronate) improved measures of BMD and sarcopenia, only denosumab resulted in a reduction in the FRAS risk of falls score.
Continue to: Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia...
Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia
Mandelli A, Tacconi E, Levinger I, et al. The role of estrogens in osteosarcopenia: from biology to potential dual therapeutic effects. Climacteric. 2021;1-7. doi: 10.1080/13697137.2021.1965118.
Osteosarcopenia is a particular term used to describe the coexistence of 2 pathologies, osteopenia/ osteoporosis and sarcopenia.14 Sarcopenia is characterized by a loss of muscle mass, strength, and performance. Numerous studies indicate that higher lean body mass is related to increased BMD and reduced fracture risk, especially in postmenopausal women.15
Menopause, muscle, and estrogen’s physiologic effects
Estrogens play a critical role in maintaining bone and muscle mass in women. Women experience a decline in musculoskeletal quantity and quality at the onset of menopause.16 Muscle mass and strength decrease rapidly after menopause, which suggests that degradation of muscle protein begins to exert a more significant effect due to a decrease in protein synthesis. Indeed, a reduced response to anabolic stimuli has been shown in postmenopausal women.17 Normalization of the protein synthesis response after restoring estrogen levels with estrogen therapy supports this hypothesis.18
In a meta-analysis to identify the role of estrogen therapy on muscle strength, the authors concluded that estrogens benefit muscle strength not by increasing the skeletal mass but by improving muscle quality and its ability to generate force.19 In addition, however, it has been demonstrated that exercise prevents and delays the onset of osteosarcopenia.20
Estrogens play a crucial role in maintaining bone and skeletal muscle health in women. Estrogen therapy is an accepted treatment for osteoporosis, whereas its effects on sarcopenia, although promising, indicate that additional studies are required before it can be recommended solely for that purpose. Given the well-described benefits of exercise on muscle and bone health, postmenopausal women should be encouraged to engage in regular physical exercise as a preventive or disease-modifying treatment for osteosarcopenia.
When should bone mass be measured in premenopausal women?
Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14. doi: 10.1080/13697137 .2021.1926974.
Most women’s clinicians are somewhat well acquainted with the increasing importance of preventing, diagnosing, and treating postmenopausal osteoporosis, which predisposes to fragility fracture and the morbidity and even mortality that brings. Increasingly, some younger women are asking for and receiving both bone mass measurements that may be inappropriately ordered and/or wrongly interpreted. Conradie and de Villiers provided an overview of premenopausal osteoporosis, containing important facts that all clinicians who care for women should be aware of.21
Indications for testing
BMD testing is only indicated in younger women in settings in which the result may influence management decisions, such as:
- a history of fragility fracture
- diseases associated with low bone mass, such as anorexia nervosa, hypogonadism, hyperparathyroidism, hyperthyroidism, celiac disease, irritable bowel disease, rheumatoid arthritis, lupus, renal disease, Marfan syndrome
- medications, such as glucocorticoids, aromatase inhibitors, premenopausal tamoxifen, excess thyroid hormone replacement, progesterone contraception
- excessive alcohol consumption, heavy smoking, vitamin D deficiency, calcium deficiency, occasionally veganism or vegetarianism.
BMD interpretation in premenopausal women does not use the T-scores developed for postmenopausal women in which standard deviations (SD) from the mean for a young reference population are employed. In that population, the normal range is up to -1.0 SD; osteopenia > -1.0 < -2.5 SD; and osteoporosis > -2.5 SD. Instead, in premenopausal patients, Z-scores, which compare the measured bone mass to an age- and gender-matched cohort, are employed. Z-scores > 2 SD below the matched population should be used rather than the T-scores that are already familiar to most clinicians.
Up to 90% of these premenopausal women with such skeletal fragility will display the secondary causes described above. ●
Very specific indications are required to consider bone mass measurements in premenopausal women. When measurements are indicated, the values are evaluated by Z-scores that compare them to those of matched-aged women and not by T-scores meant for postmenopausal women. When fragility or low-trauma fractures or Z-scores more than 2 SD below their peers are present, secondary causes of premenopausal osteoporosis include a variety of disease states, medications, and lifestyle situations. When such factors are present, many general women’s health clinicians may want to refer patients for consultation to a metabolic bone specialist for workup and management.
- Goldstein SR. Update on bone health. OBG Manag. 2020;32:16-20, 22-23.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 11, 2021.
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202.
- de Villiers, TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504.
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059-1064.
- Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177-180.
- Rosenberg I. Epidemiological and methodological problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1989. Am J Clin Nutr. 1989;50:1231-1233.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis—report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423.
- Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- El Miedany Y, El Gaafary M, Toth M, et al. Falls risk assessment score (FRAS): time to rethink. J Clin Gerontol Geriatr. 2011;21-26.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Inoue T, Maeda K, Nagano A, et al. Related factors and clinical outcomes of osteosarcopenia: a narrative review. Nutrients. 2021;13:291.
- Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16:272-277.
- Sipilä S, Törmäkangas T, Sillanpää E, et al. Muscle and bone mass in middle‐aged women: role of menopausal status and physical activity. J Cachexia Sarcopenia Muscle. 2020;11: 698-709.
- Bamman MM, Hill VJ, Adams GR, et al. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci. 2003;58:108-116.
- Hansen M, Skovgaard D, Reitelseder S, et al. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2012;67:1005-1013.
- Greising SM, Baltgalvis KA, Lowe DA, et al. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071-1081.
- Cariati I, Bonanni R, Onorato F, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol. 2021;6:55.
- Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14.
Recently, the National Osteoporosis Foundation (NOF) changed its name to the Bone Health and Osteoporosis Foundation (BHOF). Several years ago, in 2016 at my urging, this column was renamed from “Update on osteoporosis” to “Update on bone health.” I believe we were on the leading edge of this movement. As expressed in last year’s Update, our patients’ bone health must be emphasized more than it has been in the past.1
Consider that localized breast cancer carries a 5-year survival rate of 99%.2 Most of my patients are keenly aware that periodic competent breast imaging is the key to the earliest possible diagnosis. By contrast, in this country a hip fracture carries a mortality in the first year of 21%!3 Furthermore, approximately one-third of women who fracture their hip do not have osteoporosis.4 While the risk of hip fracture is greatest in women with osteoporosis, it is not absent in those without the condition. Finally, the role of muscle mass, strength, and performance in bone health is a rapidly emerging topic and one that constitutes the core of this year’s Update.
Muscle mass and strength play key role in bone health
de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504. doi:10.1080/13697137.2021.1950967.
Recently, de Villiers and Goldstein offered an overview of osteoporosis.5 What is worthy of reporting here is the role of muscle in bone health.
The bone-muscle relationship
Most clinicians know that osteoporosis and osteopenia are well-defined conditions with known risks associated with fracture. According to a review of PubMed, the first article with the keyword “osteoporosis” was published in 1894; through May 2020, 93,335 articles used that keyword. “Osteoporosis” is derived from the Greek osteon (bone) and poros (little hole). Thus, osteoporosis means “porous bone.”
Sarcopenia is characterized by progressive and generalized loss of skeletal muscle mass, strength, and function, and the condition is associated with a risk of adverse outcomes that include physical disabilities, poor quality of life, and death.6,7 “Sarcopenia” has its roots in the Greek words sarx (flesh) and penia (loss), and the term was coined in 1989.8 A PubMed review that included “sarcopenia” as the keyword revealed that the first article was published in 1993, with 12,068 articles published through May 2020.
Notably, muscle accounts for about 60% of the body’s protein. Muscle mass decreases with age, but younger patients with malnutrition, cachexia, or inflammatory diseases are also prone to decreased muscle mass. While osteoporosis has a well-accepted definition based on dual-energy x-ray absorptiometry (DXA) measurements, sarcopenia has no universally accepted definition, consensus diagnostic criteria, or treatment guidelines. In 2016, however, the International Classification of Diseases, Tenth Revision, Clinical Modification (CD-10-CM) finally recognized sarcopenia as a disease entity.
Currently, the most widely accepted definition comes from the European Working Group on Sarcopenia in Older People, which labeled presarcopenia as low muscle mass without impact on muscle strength or performance; sarcopenia as low muscle mass with either low muscle strength or low physical performance; and severe sarcopenia has all 3 criteria being present.9
When osteosarcopenia (osteoporosis or osteopenia combined with sarcopenia) exists, it can result in a threefold increase in risk of falls and a fourfold increase in fracture risk compared with women who have osteopenia or osteoporosis alone.10
The morbidity and mortality from fragility fractures are well known. Initially, diagnosis of risk seemed to be mainly T-scores on bone mineral density (BMD) testing (normal, osteopenic, osteoporosis). The FRAX fracture risk assessment tool, which includes a number of variables, further refined risk assessment. Increasingly, there is evidence of crosstalk between muscle and bone. Sarcopenia, the loss of skeletal muscle mass, strength, and performance, appears to play an important role as well for fracture risk. Simple tools to evaluate a patient’s muscle status exist. At the very least, resistance and balance exercises should be part of all clinicians’ patient counseling for bone health.
Continue to: Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives...
Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives
El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232. doi: 10.1007/s10067-021 -05757-w.
Osteosarcopenia, the combination of osteoporosis or osteopenia with sarcopenia, has been shown to increase the overall rate of falls and fracture when compared with fall and fracture rates in women with osteopenia or osteoporosis alone.10 A study by El Miedany and colleagues examined whether denosumab treatment had a possible dual therapeutic effect on osteoporosis and sarcopenia.11
Study details
The investigators looked at 135 patients diagnosed with postmenopausal osteoporosis and who were prescribed denosumab and compared them with a control group of 272 patients stratified into 2 subgroups: 136 were prescribed alendronate and 136 were prescribed zoledronate.
Assessments were performed for all participants for BMD (DXA), fall risk (falls risk assessment score [FRAS]), fracture risk (FRAX assessment tool), and sarcopenia measures. Reassessments were conducted after 5 years of denosumab or alendronate therapy, 3 years of zoledronate therapy, and 1 year after stopping the osteoporosis therapy.
The FRAS uses the clinical variables of history of falls in the last 12 months, impaired sight, weak hand grip, history of loss of balance in the last 12 months, and slowing of the walking speed/change in gait to yield a percent chance of sustaining a fall.12 Sarcopenic measures include grip strength, timed up and go (TUG) mobility test, and gait speed. There were no significant demographic differences between the 3 groups.
Denosumab reduced risk of falls and positively affected muscle strength
On completion of the 5-year denosumab therapy, falls risk was significantly decreased (P = .001) and significant improvements were seen in all sarcopenia measures (P = .01). One year after denosumab was discontinued, a significant worsening of both falls risk and sarcopenia measures (P = .01) occurred. This was in contrast to results in both control groups (alendronate and zoledronate), in which there was an improvement, although less robust in gait speed and the TUG test (P = .05) but no improvement in risk of falls. Thus, the results of this study showed that denosumab not only improved bone mass but also reduced falls risk.
Compared with bisphosphonates, denosumab showed the highest significant positive effect on both physical performance and skeletal muscle strength. This is evidenced by improvement of the gait speed, TUG test, and 4-m walk test (P<.001) in the denosumab group versus in the alendronate and zoledronate group (P<.05).
These results agree with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis 6 months) trial, which revealed that not only did denosumab treatment reduce the risk of vertebral, nonvertebral, and hip fracture over 36 months, but also that the denosumab-treated group had fewer falls (4.5%) compared with the other groups (5.7%) (P = .02).13
These data highlight that osteoporosis and sarcopenia may share similar underlying risk factors and that muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. While all 3 antiresorptives (denosumab, alendronate, zoledronate) improved measures of BMD and sarcopenia, only denosumab resulted in a reduction in the FRAS risk of falls score.
Continue to: Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia...
Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia
Mandelli A, Tacconi E, Levinger I, et al. The role of estrogens in osteosarcopenia: from biology to potential dual therapeutic effects. Climacteric. 2021;1-7. doi: 10.1080/13697137.2021.1965118.
Osteosarcopenia is a particular term used to describe the coexistence of 2 pathologies, osteopenia/ osteoporosis and sarcopenia.14 Sarcopenia is characterized by a loss of muscle mass, strength, and performance. Numerous studies indicate that higher lean body mass is related to increased BMD and reduced fracture risk, especially in postmenopausal women.15
Menopause, muscle, and estrogen’s physiologic effects
Estrogens play a critical role in maintaining bone and muscle mass in women. Women experience a decline in musculoskeletal quantity and quality at the onset of menopause.16 Muscle mass and strength decrease rapidly after menopause, which suggests that degradation of muscle protein begins to exert a more significant effect due to a decrease in protein synthesis. Indeed, a reduced response to anabolic stimuli has been shown in postmenopausal women.17 Normalization of the protein synthesis response after restoring estrogen levels with estrogen therapy supports this hypothesis.18
In a meta-analysis to identify the role of estrogen therapy on muscle strength, the authors concluded that estrogens benefit muscle strength not by increasing the skeletal mass but by improving muscle quality and its ability to generate force.19 In addition, however, it has been demonstrated that exercise prevents and delays the onset of osteosarcopenia.20
Estrogens play a crucial role in maintaining bone and skeletal muscle health in women. Estrogen therapy is an accepted treatment for osteoporosis, whereas its effects on sarcopenia, although promising, indicate that additional studies are required before it can be recommended solely for that purpose. Given the well-described benefits of exercise on muscle and bone health, postmenopausal women should be encouraged to engage in regular physical exercise as a preventive or disease-modifying treatment for osteosarcopenia.
When should bone mass be measured in premenopausal women?
Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14. doi: 10.1080/13697137 .2021.1926974.
Most women’s clinicians are somewhat well acquainted with the increasing importance of preventing, diagnosing, and treating postmenopausal osteoporosis, which predisposes to fragility fracture and the morbidity and even mortality that brings. Increasingly, some younger women are asking for and receiving both bone mass measurements that may be inappropriately ordered and/or wrongly interpreted. Conradie and de Villiers provided an overview of premenopausal osteoporosis, containing important facts that all clinicians who care for women should be aware of.21
Indications for testing
BMD testing is only indicated in younger women in settings in which the result may influence management decisions, such as:
- a history of fragility fracture
- diseases associated with low bone mass, such as anorexia nervosa, hypogonadism, hyperparathyroidism, hyperthyroidism, celiac disease, irritable bowel disease, rheumatoid arthritis, lupus, renal disease, Marfan syndrome
- medications, such as glucocorticoids, aromatase inhibitors, premenopausal tamoxifen, excess thyroid hormone replacement, progesterone contraception
- excessive alcohol consumption, heavy smoking, vitamin D deficiency, calcium deficiency, occasionally veganism or vegetarianism.
BMD interpretation in premenopausal women does not use the T-scores developed for postmenopausal women in which standard deviations (SD) from the mean for a young reference population are employed. In that population, the normal range is up to -1.0 SD; osteopenia > -1.0 < -2.5 SD; and osteoporosis > -2.5 SD. Instead, in premenopausal patients, Z-scores, which compare the measured bone mass to an age- and gender-matched cohort, are employed. Z-scores > 2 SD below the matched population should be used rather than the T-scores that are already familiar to most clinicians.
Up to 90% of these premenopausal women with such skeletal fragility will display the secondary causes described above. ●
Very specific indications are required to consider bone mass measurements in premenopausal women. When measurements are indicated, the values are evaluated by Z-scores that compare them to those of matched-aged women and not by T-scores meant for postmenopausal women. When fragility or low-trauma fractures or Z-scores more than 2 SD below their peers are present, secondary causes of premenopausal osteoporosis include a variety of disease states, medications, and lifestyle situations. When such factors are present, many general women’s health clinicians may want to refer patients for consultation to a metabolic bone specialist for workup and management.
Recently, the National Osteoporosis Foundation (NOF) changed its name to the Bone Health and Osteoporosis Foundation (BHOF). Several years ago, in 2016 at my urging, this column was renamed from “Update on osteoporosis” to “Update on bone health.” I believe we were on the leading edge of this movement. As expressed in last year’s Update, our patients’ bone health must be emphasized more than it has been in the past.1
Consider that localized breast cancer carries a 5-year survival rate of 99%.2 Most of my patients are keenly aware that periodic competent breast imaging is the key to the earliest possible diagnosis. By contrast, in this country a hip fracture carries a mortality in the first year of 21%!3 Furthermore, approximately one-third of women who fracture their hip do not have osteoporosis.4 While the risk of hip fracture is greatest in women with osteoporosis, it is not absent in those without the condition. Finally, the role of muscle mass, strength, and performance in bone health is a rapidly emerging topic and one that constitutes the core of this year’s Update.
Muscle mass and strength play key role in bone health
de Villiers TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504. doi:10.1080/13697137.2021.1950967.
Recently, de Villiers and Goldstein offered an overview of osteoporosis.5 What is worthy of reporting here is the role of muscle in bone health.
The bone-muscle relationship
Most clinicians know that osteoporosis and osteopenia are well-defined conditions with known risks associated with fracture. According to a review of PubMed, the first article with the keyword “osteoporosis” was published in 1894; through May 2020, 93,335 articles used that keyword. “Osteoporosis” is derived from the Greek osteon (bone) and poros (little hole). Thus, osteoporosis means “porous bone.”
Sarcopenia is characterized by progressive and generalized loss of skeletal muscle mass, strength, and function, and the condition is associated with a risk of adverse outcomes that include physical disabilities, poor quality of life, and death.6,7 “Sarcopenia” has its roots in the Greek words sarx (flesh) and penia (loss), and the term was coined in 1989.8 A PubMed review that included “sarcopenia” as the keyword revealed that the first article was published in 1993, with 12,068 articles published through May 2020.
Notably, muscle accounts for about 60% of the body’s protein. Muscle mass decreases with age, but younger patients with malnutrition, cachexia, or inflammatory diseases are also prone to decreased muscle mass. While osteoporosis has a well-accepted definition based on dual-energy x-ray absorptiometry (DXA) measurements, sarcopenia has no universally accepted definition, consensus diagnostic criteria, or treatment guidelines. In 2016, however, the International Classification of Diseases, Tenth Revision, Clinical Modification (CD-10-CM) finally recognized sarcopenia as a disease entity.
Currently, the most widely accepted definition comes from the European Working Group on Sarcopenia in Older People, which labeled presarcopenia as low muscle mass without impact on muscle strength or performance; sarcopenia as low muscle mass with either low muscle strength or low physical performance; and severe sarcopenia has all 3 criteria being present.9
When osteosarcopenia (osteoporosis or osteopenia combined with sarcopenia) exists, it can result in a threefold increase in risk of falls and a fourfold increase in fracture risk compared with women who have osteopenia or osteoporosis alone.10
The morbidity and mortality from fragility fractures are well known. Initially, diagnosis of risk seemed to be mainly T-scores on bone mineral density (BMD) testing (normal, osteopenic, osteoporosis). The FRAX fracture risk assessment tool, which includes a number of variables, further refined risk assessment. Increasingly, there is evidence of crosstalk between muscle and bone. Sarcopenia, the loss of skeletal muscle mass, strength, and performance, appears to play an important role as well for fracture risk. Simple tools to evaluate a patient’s muscle status exist. At the very least, resistance and balance exercises should be part of all clinicians’ patient counseling for bone health.
Continue to: Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives...
Denosumab decreased falls risk, improved sarcopenia measures vs comparator antiresorptives
El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232. doi: 10.1007/s10067-021 -05757-w.
Osteosarcopenia, the combination of osteoporosis or osteopenia with sarcopenia, has been shown to increase the overall rate of falls and fracture when compared with fall and fracture rates in women with osteopenia or osteoporosis alone.10 A study by El Miedany and colleagues examined whether denosumab treatment had a possible dual therapeutic effect on osteoporosis and sarcopenia.11
Study details
The investigators looked at 135 patients diagnosed with postmenopausal osteoporosis and who were prescribed denosumab and compared them with a control group of 272 patients stratified into 2 subgroups: 136 were prescribed alendronate and 136 were prescribed zoledronate.
Assessments were performed for all participants for BMD (DXA), fall risk (falls risk assessment score [FRAS]), fracture risk (FRAX assessment tool), and sarcopenia measures. Reassessments were conducted after 5 years of denosumab or alendronate therapy, 3 years of zoledronate therapy, and 1 year after stopping the osteoporosis therapy.
The FRAS uses the clinical variables of history of falls in the last 12 months, impaired sight, weak hand grip, history of loss of balance in the last 12 months, and slowing of the walking speed/change in gait to yield a percent chance of sustaining a fall.12 Sarcopenic measures include grip strength, timed up and go (TUG) mobility test, and gait speed. There were no significant demographic differences between the 3 groups.
Denosumab reduced risk of falls and positively affected muscle strength
On completion of the 5-year denosumab therapy, falls risk was significantly decreased (P = .001) and significant improvements were seen in all sarcopenia measures (P = .01). One year after denosumab was discontinued, a significant worsening of both falls risk and sarcopenia measures (P = .01) occurred. This was in contrast to results in both control groups (alendronate and zoledronate), in which there was an improvement, although less robust in gait speed and the TUG test (P = .05) but no improvement in risk of falls. Thus, the results of this study showed that denosumab not only improved bone mass but also reduced falls risk.
Compared with bisphosphonates, denosumab showed the highest significant positive effect on both physical performance and skeletal muscle strength. This is evidenced by improvement of the gait speed, TUG test, and 4-m walk test (P<.001) in the denosumab group versus in the alendronate and zoledronate group (P<.05).
These results agree with the outcomes of the FREEDOM (Fracture Reduction Evaluation of Denosumab in Osteoporosis 6 months) trial, which revealed that not only did denosumab treatment reduce the risk of vertebral, nonvertebral, and hip fracture over 36 months, but also that the denosumab-treated group had fewer falls (4.5%) compared with the other groups (5.7%) (P = .02).13
These data highlight that osteoporosis and sarcopenia may share similar underlying risk factors and that muscle-bone interactions are important to minimize the risk of falls, fractures, and hospitalizations. While all 3 antiresorptives (denosumab, alendronate, zoledronate) improved measures of BMD and sarcopenia, only denosumab resulted in a reduction in the FRAS risk of falls score.
Continue to: Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia...
Estrogen’s role in bone health and its therapeutic potential in osteosarcopenia
Mandelli A, Tacconi E, Levinger I, et al. The role of estrogens in osteosarcopenia: from biology to potential dual therapeutic effects. Climacteric. 2021;1-7. doi: 10.1080/13697137.2021.1965118.
Osteosarcopenia is a particular term used to describe the coexistence of 2 pathologies, osteopenia/ osteoporosis and sarcopenia.14 Sarcopenia is characterized by a loss of muscle mass, strength, and performance. Numerous studies indicate that higher lean body mass is related to increased BMD and reduced fracture risk, especially in postmenopausal women.15
Menopause, muscle, and estrogen’s physiologic effects
Estrogens play a critical role in maintaining bone and muscle mass in women. Women experience a decline in musculoskeletal quantity and quality at the onset of menopause.16 Muscle mass and strength decrease rapidly after menopause, which suggests that degradation of muscle protein begins to exert a more significant effect due to a decrease in protein synthesis. Indeed, a reduced response to anabolic stimuli has been shown in postmenopausal women.17 Normalization of the protein synthesis response after restoring estrogen levels with estrogen therapy supports this hypothesis.18
In a meta-analysis to identify the role of estrogen therapy on muscle strength, the authors concluded that estrogens benefit muscle strength not by increasing the skeletal mass but by improving muscle quality and its ability to generate force.19 In addition, however, it has been demonstrated that exercise prevents and delays the onset of osteosarcopenia.20
Estrogens play a crucial role in maintaining bone and skeletal muscle health in women. Estrogen therapy is an accepted treatment for osteoporosis, whereas its effects on sarcopenia, although promising, indicate that additional studies are required before it can be recommended solely for that purpose. Given the well-described benefits of exercise on muscle and bone health, postmenopausal women should be encouraged to engage in regular physical exercise as a preventive or disease-modifying treatment for osteosarcopenia.
When should bone mass be measured in premenopausal women?
Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14. doi: 10.1080/13697137 .2021.1926974.
Most women’s clinicians are somewhat well acquainted with the increasing importance of preventing, diagnosing, and treating postmenopausal osteoporosis, which predisposes to fragility fracture and the morbidity and even mortality that brings. Increasingly, some younger women are asking for and receiving both bone mass measurements that may be inappropriately ordered and/or wrongly interpreted. Conradie and de Villiers provided an overview of premenopausal osteoporosis, containing important facts that all clinicians who care for women should be aware of.21
Indications for testing
BMD testing is only indicated in younger women in settings in which the result may influence management decisions, such as:
- a history of fragility fracture
- diseases associated with low bone mass, such as anorexia nervosa, hypogonadism, hyperparathyroidism, hyperthyroidism, celiac disease, irritable bowel disease, rheumatoid arthritis, lupus, renal disease, Marfan syndrome
- medications, such as glucocorticoids, aromatase inhibitors, premenopausal tamoxifen, excess thyroid hormone replacement, progesterone contraception
- excessive alcohol consumption, heavy smoking, vitamin D deficiency, calcium deficiency, occasionally veganism or vegetarianism.
BMD interpretation in premenopausal women does not use the T-scores developed for postmenopausal women in which standard deviations (SD) from the mean for a young reference population are employed. In that population, the normal range is up to -1.0 SD; osteopenia > -1.0 < -2.5 SD; and osteoporosis > -2.5 SD. Instead, in premenopausal patients, Z-scores, which compare the measured bone mass to an age- and gender-matched cohort, are employed. Z-scores > 2 SD below the matched population should be used rather than the T-scores that are already familiar to most clinicians.
Up to 90% of these premenopausal women with such skeletal fragility will display the secondary causes described above. ●
Very specific indications are required to consider bone mass measurements in premenopausal women. When measurements are indicated, the values are evaluated by Z-scores that compare them to those of matched-aged women and not by T-scores meant for postmenopausal women. When fragility or low-trauma fractures or Z-scores more than 2 SD below their peers are present, secondary causes of premenopausal osteoporosis include a variety of disease states, medications, and lifestyle situations. When such factors are present, many general women’s health clinicians may want to refer patients for consultation to a metabolic bone specialist for workup and management.
- Goldstein SR. Update on bone health. OBG Manag. 2020;32:16-20, 22-23.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 11, 2021.
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202.
- de Villiers, TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504.
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059-1064.
- Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177-180.
- Rosenberg I. Epidemiological and methodological problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1989. Am J Clin Nutr. 1989;50:1231-1233.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis—report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423.
- Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- El Miedany Y, El Gaafary M, Toth M, et al. Falls risk assessment score (FRAS): time to rethink. J Clin Gerontol Geriatr. 2011;21-26.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Inoue T, Maeda K, Nagano A, et al. Related factors and clinical outcomes of osteosarcopenia: a narrative review. Nutrients. 2021;13:291.
- Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16:272-277.
- Sipilä S, Törmäkangas T, Sillanpää E, et al. Muscle and bone mass in middle‐aged women: role of menopausal status and physical activity. J Cachexia Sarcopenia Muscle. 2020;11: 698-709.
- Bamman MM, Hill VJ, Adams GR, et al. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci. 2003;58:108-116.
- Hansen M, Skovgaard D, Reitelseder S, et al. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2012;67:1005-1013.
- Greising SM, Baltgalvis KA, Lowe DA, et al. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071-1081.
- Cariati I, Bonanni R, Onorato F, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol. 2021;6:55.
- Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14.
- Goldstein SR. Update on bone health. OBG Manag. 2020;32:16-20, 22-23.
- American Cancer Society. Cancer Facts & Figures 2020. Atlanta, GA: American Cancer Society; 2020. https://www .cancer.org/content/dam/cancer-org/research/cancer-facts -and-statistics/annual-cancer-facts-and-figures/2020/cancer -facts-and-figures-2020.pdf. Accessed November 11, 2021.
- Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture—a systematic review. World J Orthop. 2019;10:166-175.
- Schuit SC, van der Klift M, Weel AE, et al. Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone. 2004;34:195-202.
- de Villiers, TJ, Goldstein SR. Update on bone health: the International Menopause Society white paper 2021. Climacteric. 2021;24:498-504.
- Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059-1064.
- Santilli V, Bernetti A, Mangone M, et al. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11:177-180.
- Rosenberg I. Epidemiological and methodological problems in determining nutritional status of older persons. Proceedings of a conference. Albuquerque, New Mexico, October 19-21, 1989. Am J Clin Nutr. 1989;50:1231-1233.
- Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al; European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis—report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412-423.
- Sepúlveda-Loyola W, Phu S, Bani Hassan E, et al. The joint occurrence of osteoporosis and sarcopenia (osteosarcopenia): definitions and characteristics. J Am Med Dir Assoc. 2020;21:220-225.
- El Miedany Y, El Gaafary M, Toth M, et al; Egyptian Academy of Bone Health, Metabolic Bone Diseases. Is there a potential dual effect of denosumab for treatment of osteoporosis and sarcopenia? Clin Rheumatol. 2021;40:4225-4232.
- El Miedany Y, El Gaafary M, Toth M, et al. Falls risk assessment score (FRAS): time to rethink. J Clin Gerontol Geriatr. 2011;21-26.
- Cummings SR, Martin JS, McClung MR, et al; FREEDOM Trial. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361: 756-765.
- Inoue T, Maeda K, Nagano A, et al. Related factors and clinical outcomes of osteosarcopenia: a narrative review. Nutrients. 2021;13:291.
- Kaji H. Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care. 2013;16:272-277.
- Sipilä S, Törmäkangas T, Sillanpää E, et al. Muscle and bone mass in middle‐aged women: role of menopausal status and physical activity. J Cachexia Sarcopenia Muscle. 2020;11: 698-709.
- Bamman MM, Hill VJ, Adams GR, et al. Gender differences in resistance-training-induced myofiber hypertrophy among older adults. J Gerontol A Biol Sci Med Sci. 2003;58:108-116.
- Hansen M, Skovgaard D, Reitelseder S, et al. Effects of estrogen replacement and lower androgen status on skeletal muscle collagen and myofibrillar protein synthesis in postmenopausal women. J Gerontol A Biol Sci Med Sci. 2012;67:1005-1013.
- Greising SM, Baltgalvis KA, Lowe DA, et al. Hormone therapy and skeletal muscle strength: a meta-analysis. J Gerontol A Biol Sci Med Sci. 2009;64:1071-1081.
- Cariati I, Bonanni R, Onorato F, et al. Role of physical activity in bone-muscle crosstalk: biological aspects and clinical implications. J Funct Morphol Kinesiol. 2021;6:55.
- Conradie M, de Villiers T. Premenopausal osteoporosis. Climacteric. 2021:1-14.
Cancer risk-reducing strategies: Focus on chemoprevention
In her presentation at The North American Menopause Society (NAMS) 2021 annual meeting (September 22–25, 2021, in Washington, DC), Dr. Holly J. Pederson offered her expert perspectives on breast cancer prevention in at-risk women in “Chemoprevention for risk reduction: Women’s health clinicians have a role.”
Which patients would benefit from chemoprevention?
Holly J. Pederson, MD: Obviously, women with significant family history are at risk. And approximately 10% of biopsies that are done for other reasons incidentally show atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS)—which are not precancers or cancers but are markers for the development of the disease—and they markedly increase risk. Atypical hyperplasia confers a 30% risk for developing breast cancer over the next 25 years, and LCIS is associated with up to a 2% per year risk. In this setting, preventive medication has been shown to cut risk by 56% to 86%; this is a targeted population that is often overlooked.
Mathematical risk models can be used to assess risk by assessing women’s risk factors. The United States Preventive Services Task Force (USPSTF) has set forth a threshold at which they believe the benefits outweigh the risks of preventive medications. That threshold is 3% or greater over the next 5 years using the Gail breast cancer risk assessment tool.1 The American Society of Clinical Oncology (ASCO) uses the Tyrer-Cuzick breast cancer risk evaluation model with a threshold of 5% over the next 10 years.2 In general, those are the situations in which chemoprevention is a no-brainer.
Certain genetic mutations also predispose to estrogen-sensitive breast cancer. While preventive medications specifically have not been studied in large groups of gene carriers, chemoprevention makes sense because these medications prevent estrogen-sensitive breast cancers that those patients are prone to. Examples would be patients with ATM and CHEK2 gene mutations, which are very common, and patients with BRCA2 and even BRCA1 variants in the postmenopausal years. Those are the big targets.
Risk assessment models
Dr. Pederson: Yes, I almost exclusively use the Tyrer-Cuzick risk model, version 8, which incorporates breast density. This model is intimidating to some practitioners initially, but once you get used to it, you can complete it very quickly.
The Gail model is very limited. It assesses only first-degree relatives, so you don’t get the paternal information at all, and you don’t use age at diagnosis, family structure, genetic testing, results of breast density, or body mass index (BMI). There are many limitations of the Gail model, but most people use it because it is so easy and they are familiar with it.
Possibly the best model is the CanRisk tool, which incorporates the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), but it takes too much time to use in clinic; it’s too complicated. The Tyrer-Cuzick model is easy to use once you get used to it.
Dr. Pederson: Risk doesn’t always need to be formally calculated, which can be time-consuming. It’s one of those situations where most practitioners know it when they see it. Benign atypical biopsies, a strong family history, or, obviously, the presence of a genetic mutation are huge red flags.
If a practitioner has a nearby high-risk center where they can refer patients, that can be so useful, even for a one-time consultation to guide management. For example, with the virtual world now, I do a lot of consultations for patients and outline a plan, and then the referring practitioner can carry out the plan with confidence and then send the patient back periodically. There are so many more options now that previously did not exist for the busy ObGyn or primary care provider to rely on.
Continue to: Chemoprevention uptake in at-risk women...
Chemoprevention uptake in at-risk women
Dr. Pederson: We really never practice medicine using numbers. We use clinical judgment, and we use relationships with patients in terms of developing confidence and trust. I think that the uptake that we exhibit in our center probably is more based on the patients’ perception that we are confident in our recommendations. I think that many practitioners simply are not comfortable with explaining medications, explaining and managing adverse effects, and using alternative medications. While the modeling helps, I think the personal expertise really makes the difference.
Going forward, the addition of the polygenic risk score to the mathematical risk models is going to make a big difference. Right now, the mathematical risk model is simply that: it takes the traditional risk factors that a patient has and spits out a number. But adding the patient’s genomic data—that is, a weighted summation of SNPs, or single nucleotide polymorphisms, now numbering over 300 for breast cancer—can explain more about their personalized risk, which is going to be more powerful in influencing a woman to take medication or not to take medication, in my opinion. Knowing their actual genomic risk will be a big step forward in individualized risk stratification and increased medication uptake as well as vigilance with high risk screening and attention to diet, exercise, and drinking alcohol in moderation.
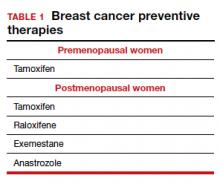
Dr. Pederson: The only drug that can be used in the premenopausal setting is tamoxifen (TABLE 1). Women can’t take it if they are pregnant, planning to become pregnant, or if they don’t use a reliable form of birth control because it is teratogenic. Women also cannot take tamoxifen if they have had a history of blood clots, stroke, or transient ischemic attack; if they are on warfarin or estrogen preparations; or if they have had atypical endometrial biopsies or endometrial cancer. Those are the absolute contraindications for tamoxifen use.
Tamoxifen is generally very well tolerated in most women; some women experience hot flashes and night sweats that often will subside (or become tolerable) over the first 90 days. In addition, some women experience vaginal discharge rather than dryness, but it is not as bothersome to patients as dryness can be.
Tamoxifen can be used in the pre- or postmenopausal setting. In healthy premenopausal women, there’s no increased risk of the serious adverse effects that are seen with tamoxifen use in postmenopausal women, such as the 1% risk of blood clots and the 1% risk of endometrial cancer.
In postmenopausal women who still have their uterus, I’ll preferentially use raloxifene over tamoxifen. If they don’t have their uterus, tamoxifen is slightly more effective than the raloxifene, and I’ll use that.
Tamoxifen and raloxifene are both selective estrogen receptor modulators, or SERMs, which means that they stimulate receptors in some tissues, like bone, keeping bones strong, and block the receptors in other tissues, like the breast, reducing risk. And so you get kind of a two-for-one in terms of breast cancer risk reduction and osteoporosis prevention.
Another class of preventive drugs is the aromatase inhibitors (AIs). They block the enzyme aromatase, which converts androgens to estrogens peripherally; that is, the androgens that are produced primarily in the adrenal gland, but in part in postmenopausal ovaries.
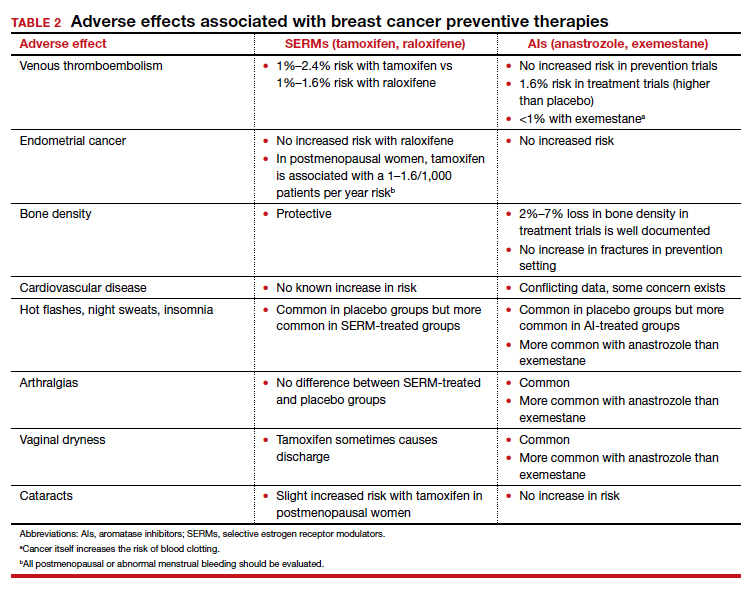
In general, AIs are less well tolerated. There are generally more hot flashes and night sweats, and more vaginal dryness than with the SERMs. Anastrozole use is associated with arthralgias; and with exemestane use, there can be some hair loss (TABLE 2). Relative contraindications to SERMs become more important in the postmenopausal setting because of the increased frequency of both blood clots and uterine cancer in the postmenopausal years. I won’t give it to smokers. I won’t give tamoxifen to smokers in the premenopausal period either. With obese women, care must be taken because of the risk of blood clots with the SERMS, so then I’ll resort to the AIs. In the postmenopausal setting, you have to think a lot harder about the choices you use for preventive medication. Preferentially, I’ll use the SERMS if possible as they have fewer adverse effects.
Dr. Pederson: All of them are recommended to be given for 5 years, but the MAP.3 trial, which studied exemestane compared with placebo, showed a 65% risk reduction with 3 years of therapy.3 So occasionally, we’ll use 3 years of therapy. Why the treatment recommendation is universally 5 years is unclear, given that the trial with that particular drug was done in 3 years. And with low-dose tamoxifen, the recommended duration is 3 years. That study was done in Italy with 5 mg daily for 3 years.4 In the United States we use 10 mg every other day for 3 years because the 5-mg tablet is not available here.
Continue to: Counseling points...
Counseling points
Dr. Pederson: Patients’ fears about adverse effects are often worse than the adverse effects themselves. Women will fester over, Should I take it? Should I take it possibly for years? And then they take the medication and they tell me, “I don’t even notice that I’m taking it, and I know I’m being proactive.” The majority of patients who take these medications don’t have a lot of significant adverse effects.
Severe hot flashes can be managed in a number of ways, primarily and most effectively with certain antidepressants. Oxybutynin use is another good way to manage vasomotor symptoms. Sometimes we use local vaginal estrogen if a patient has vaginal dryness. In general, however, I would say at least 80% of my patients who take preventive medications do not require management of adverse side effects, that they are tolerable.
I counsel women this way, “Don’t think of this as a 5-year course of medication. Think of it as a 90-day trial, and let’s see how you do. If you hate it, then we don’t do it.” They often are pleasantly surprised that the medication is much easier to tolerate than they thought it would be.
Dr. Pederson: It would be neat if a trial would directly compare lifestyle interventions with medications, because probably lifestyle change is as effective as medication is—but we don’t know that and probably will never have that data. We do know that alcohol consumption, every drink per day, increases risk by 10%. We know that obesity is responsible for 30% of breast cancers in this country, and that hormone replacement probably is overrated as a significant risk factor. Updated data from the Women’s Health Initiative study suggest that hormone replacement may actually reduce both breast cancer and cardiovascular risk in women in their 50s, but that’s in average-risk women and not in high-risk women, so we can’t generalize. We do recommend lifestyle measures including weight loss, exercise, and limiting alcohol consumption for all of our patients and certainly for our high-risk patients.
The only 2 things a woman can do to reduce the risk of triple negative breast cancer are to achieve and maintain ideal body weight and to breastfeed. The medications that I have mentioned don’t reduce the risk of triple negative breast cancer. Staying thin and breastfeeding do. It’s a problem in this country because at least 35% of all women and 58% of Black women are obese in America, and Black women tend to be prone to triple-negative breast cancer. That’s a real public health issue that we need to address. If we were going to focus on one thing, it would be focusing on obesity in terms of risk reduction.
Final thoughts
Dr. Pederson: I would like to direct attention to the American Heart Association scientific statement published at the end of 2020 that reported that hormone replacement in average-risk women reduced both cardiovascular events and overall mortality in women in their 50s by 30%.5 While that’s not directly related to what we are talking about, we need to weigh the pros and cons of estrogen versus estrogen blockade in women in terms of breast cancer risk management discussions. Part of shared decision making now needs to include cardiovascular risk factors and how estrogen is going to play into that.
In women with atypical hyperplasia or LCIS, they may benefit from the preventive medications we discussed. But in women with family history or in women with genetic mutations who have not had benign atypical biopsies, they may choose to consider estrogen during their 50s and perhaps take tamoxifen either beforehand or raloxifene afterward.
We need to look at patients holistically and consider all their risk factors together. We can’t look at one dimension alone.
- US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867.
- Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152-3165.
- Goss PE, Ingle JN, Alex-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37:1629-1637.
- El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532.
In her presentation at The North American Menopause Society (NAMS) 2021 annual meeting (September 22–25, 2021, in Washington, DC), Dr. Holly J. Pederson offered her expert perspectives on breast cancer prevention in at-risk women in “Chemoprevention for risk reduction: Women’s health clinicians have a role.”
Which patients would benefit from chemoprevention?
Holly J. Pederson, MD: Obviously, women with significant family history are at risk. And approximately 10% of biopsies that are done for other reasons incidentally show atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS)—which are not precancers or cancers but are markers for the development of the disease—and they markedly increase risk. Atypical hyperplasia confers a 30% risk for developing breast cancer over the next 25 years, and LCIS is associated with up to a 2% per year risk. In this setting, preventive medication has been shown to cut risk by 56% to 86%; this is a targeted population that is often overlooked.
Mathematical risk models can be used to assess risk by assessing women’s risk factors. The United States Preventive Services Task Force (USPSTF) has set forth a threshold at which they believe the benefits outweigh the risks of preventive medications. That threshold is 3% or greater over the next 5 years using the Gail breast cancer risk assessment tool.1 The American Society of Clinical Oncology (ASCO) uses the Tyrer-Cuzick breast cancer risk evaluation model with a threshold of 5% over the next 10 years.2 In general, those are the situations in which chemoprevention is a no-brainer.
Certain genetic mutations also predispose to estrogen-sensitive breast cancer. While preventive medications specifically have not been studied in large groups of gene carriers, chemoprevention makes sense because these medications prevent estrogen-sensitive breast cancers that those patients are prone to. Examples would be patients with ATM and CHEK2 gene mutations, which are very common, and patients with BRCA2 and even BRCA1 variants in the postmenopausal years. Those are the big targets.
Risk assessment models
Dr. Pederson: Yes, I almost exclusively use the Tyrer-Cuzick risk model, version 8, which incorporates breast density. This model is intimidating to some practitioners initially, but once you get used to it, you can complete it very quickly.
The Gail model is very limited. It assesses only first-degree relatives, so you don’t get the paternal information at all, and you don’t use age at diagnosis, family structure, genetic testing, results of breast density, or body mass index (BMI). There are many limitations of the Gail model, but most people use it because it is so easy and they are familiar with it.
Possibly the best model is the CanRisk tool, which incorporates the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), but it takes too much time to use in clinic; it’s too complicated. The Tyrer-Cuzick model is easy to use once you get used to it.
Dr. Pederson: Risk doesn’t always need to be formally calculated, which can be time-consuming. It’s one of those situations where most practitioners know it when they see it. Benign atypical biopsies, a strong family history, or, obviously, the presence of a genetic mutation are huge red flags.
If a practitioner has a nearby high-risk center where they can refer patients, that can be so useful, even for a one-time consultation to guide management. For example, with the virtual world now, I do a lot of consultations for patients and outline a plan, and then the referring practitioner can carry out the plan with confidence and then send the patient back periodically. There are so many more options now that previously did not exist for the busy ObGyn or primary care provider to rely on.
Continue to: Chemoprevention uptake in at-risk women...
Chemoprevention uptake in at-risk women
Dr. Pederson: We really never practice medicine using numbers. We use clinical judgment, and we use relationships with patients in terms of developing confidence and trust. I think that the uptake that we exhibit in our center probably is more based on the patients’ perception that we are confident in our recommendations. I think that many practitioners simply are not comfortable with explaining medications, explaining and managing adverse effects, and using alternative medications. While the modeling helps, I think the personal expertise really makes the difference.
Going forward, the addition of the polygenic risk score to the mathematical risk models is going to make a big difference. Right now, the mathematical risk model is simply that: it takes the traditional risk factors that a patient has and spits out a number. But adding the patient’s genomic data—that is, a weighted summation of SNPs, or single nucleotide polymorphisms, now numbering over 300 for breast cancer—can explain more about their personalized risk, which is going to be more powerful in influencing a woman to take medication or not to take medication, in my opinion. Knowing their actual genomic risk will be a big step forward in individualized risk stratification and increased medication uptake as well as vigilance with high risk screening and attention to diet, exercise, and drinking alcohol in moderation.
Dr. Pederson: The only drug that can be used in the premenopausal setting is tamoxifen (TABLE 1). Women can’t take it if they are pregnant, planning to become pregnant, or if they don’t use a reliable form of birth control because it is teratogenic. Women also cannot take tamoxifen if they have had a history of blood clots, stroke, or transient ischemic attack; if they are on warfarin or estrogen preparations; or if they have had atypical endometrial biopsies or endometrial cancer. Those are the absolute contraindications for tamoxifen use.
Tamoxifen is generally very well tolerated in most women; some women experience hot flashes and night sweats that often will subside (or become tolerable) over the first 90 days. In addition, some women experience vaginal discharge rather than dryness, but it is not as bothersome to patients as dryness can be.
Tamoxifen can be used in the pre- or postmenopausal setting. In healthy premenopausal women, there’s no increased risk of the serious adverse effects that are seen with tamoxifen use in postmenopausal women, such as the 1% risk of blood clots and the 1% risk of endometrial cancer.
In postmenopausal women who still have their uterus, I’ll preferentially use raloxifene over tamoxifen. If they don’t have their uterus, tamoxifen is slightly more effective than the raloxifene, and I’ll use that.
Tamoxifen and raloxifene are both selective estrogen receptor modulators, or SERMs, which means that they stimulate receptors in some tissues, like bone, keeping bones strong, and block the receptors in other tissues, like the breast, reducing risk. And so you get kind of a two-for-one in terms of breast cancer risk reduction and osteoporosis prevention.
Another class of preventive drugs is the aromatase inhibitors (AIs). They block the enzyme aromatase, which converts androgens to estrogens peripherally; that is, the androgens that are produced primarily in the adrenal gland, but in part in postmenopausal ovaries.
In general, AIs are less well tolerated. There are generally more hot flashes and night sweats, and more vaginal dryness than with the SERMs. Anastrozole use is associated with arthralgias; and with exemestane use, there can be some hair loss (TABLE 2). Relative contraindications to SERMs become more important in the postmenopausal setting because of the increased frequency of both blood clots and uterine cancer in the postmenopausal years. I won’t give it to smokers. I won’t give tamoxifen to smokers in the premenopausal period either. With obese women, care must be taken because of the risk of blood clots with the SERMS, so then I’ll resort to the AIs. In the postmenopausal setting, you have to think a lot harder about the choices you use for preventive medication. Preferentially, I’ll use the SERMS if possible as they have fewer adverse effects.

Dr. Pederson: All of them are recommended to be given for 5 years, but the MAP.3 trial, which studied exemestane compared with placebo, showed a 65% risk reduction with 3 years of therapy.3 So occasionally, we’ll use 3 years of therapy. Why the treatment recommendation is universally 5 years is unclear, given that the trial with that particular drug was done in 3 years. And with low-dose tamoxifen, the recommended duration is 3 years. That study was done in Italy with 5 mg daily for 3 years.4 In the United States we use 10 mg every other day for 3 years because the 5-mg tablet is not available here.
Continue to: Counseling points...
Counseling points
Dr. Pederson: Patients’ fears about adverse effects are often worse than the adverse effects themselves. Women will fester over, Should I take it? Should I take it possibly for years? And then they take the medication and they tell me, “I don’t even notice that I’m taking it, and I know I’m being proactive.” The majority of patients who take these medications don’t have a lot of significant adverse effects.
Severe hot flashes can be managed in a number of ways, primarily and most effectively with certain antidepressants. Oxybutynin use is another good way to manage vasomotor symptoms. Sometimes we use local vaginal estrogen if a patient has vaginal dryness. In general, however, I would say at least 80% of my patients who take preventive medications do not require management of adverse side effects, that they are tolerable.
I counsel women this way, “Don’t think of this as a 5-year course of medication. Think of it as a 90-day trial, and let’s see how you do. If you hate it, then we don’t do it.” They often are pleasantly surprised that the medication is much easier to tolerate than they thought it would be.
Dr. Pederson: It would be neat if a trial would directly compare lifestyle interventions with medications, because probably lifestyle change is as effective as medication is—but we don’t know that and probably will never have that data. We do know that alcohol consumption, every drink per day, increases risk by 10%. We know that obesity is responsible for 30% of breast cancers in this country, and that hormone replacement probably is overrated as a significant risk factor. Updated data from the Women’s Health Initiative study suggest that hormone replacement may actually reduce both breast cancer and cardiovascular risk in women in their 50s, but that’s in average-risk women and not in high-risk women, so we can’t generalize. We do recommend lifestyle measures including weight loss, exercise, and limiting alcohol consumption for all of our patients and certainly for our high-risk patients.
The only 2 things a woman can do to reduce the risk of triple negative breast cancer are to achieve and maintain ideal body weight and to breastfeed. The medications that I have mentioned don’t reduce the risk of triple negative breast cancer. Staying thin and breastfeeding do. It’s a problem in this country because at least 35% of all women and 58% of Black women are obese in America, and Black women tend to be prone to triple-negative breast cancer. That’s a real public health issue that we need to address. If we were going to focus on one thing, it would be focusing on obesity in terms of risk reduction.
Final thoughts
Dr. Pederson: I would like to direct attention to the American Heart Association scientific statement published at the end of 2020 that reported that hormone replacement in average-risk women reduced both cardiovascular events and overall mortality in women in their 50s by 30%.5 While that’s not directly related to what we are talking about, we need to weigh the pros and cons of estrogen versus estrogen blockade in women in terms of breast cancer risk management discussions. Part of shared decision making now needs to include cardiovascular risk factors and how estrogen is going to play into that.
In women with atypical hyperplasia or LCIS, they may benefit from the preventive medications we discussed. But in women with family history or in women with genetic mutations who have not had benign atypical biopsies, they may choose to consider estrogen during their 50s and perhaps take tamoxifen either beforehand or raloxifene afterward.
We need to look at patients holistically and consider all their risk factors together. We can’t look at one dimension alone.
In her presentation at The North American Menopause Society (NAMS) 2021 annual meeting (September 22–25, 2021, in Washington, DC), Dr. Holly J. Pederson offered her expert perspectives on breast cancer prevention in at-risk women in “Chemoprevention for risk reduction: Women’s health clinicians have a role.”
Which patients would benefit from chemoprevention?
Holly J. Pederson, MD: Obviously, women with significant family history are at risk. And approximately 10% of biopsies that are done for other reasons incidentally show atypical hyperplasia (AH) or lobular carcinoma in situ (LCIS)—which are not precancers or cancers but are markers for the development of the disease—and they markedly increase risk. Atypical hyperplasia confers a 30% risk for developing breast cancer over the next 25 years, and LCIS is associated with up to a 2% per year risk. In this setting, preventive medication has been shown to cut risk by 56% to 86%; this is a targeted population that is often overlooked.
Mathematical risk models can be used to assess risk by assessing women’s risk factors. The United States Preventive Services Task Force (USPSTF) has set forth a threshold at which they believe the benefits outweigh the risks of preventive medications. That threshold is 3% or greater over the next 5 years using the Gail breast cancer risk assessment tool.1 The American Society of Clinical Oncology (ASCO) uses the Tyrer-Cuzick breast cancer risk evaluation model with a threshold of 5% over the next 10 years.2 In general, those are the situations in which chemoprevention is a no-brainer.
Certain genetic mutations also predispose to estrogen-sensitive breast cancer. While preventive medications specifically have not been studied in large groups of gene carriers, chemoprevention makes sense because these medications prevent estrogen-sensitive breast cancers that those patients are prone to. Examples would be patients with ATM and CHEK2 gene mutations, which are very common, and patients with BRCA2 and even BRCA1 variants in the postmenopausal years. Those are the big targets.
Risk assessment models
Dr. Pederson: Yes, I almost exclusively use the Tyrer-Cuzick risk model, version 8, which incorporates breast density. This model is intimidating to some practitioners initially, but once you get used to it, you can complete it very quickly.
The Gail model is very limited. It assesses only first-degree relatives, so you don’t get the paternal information at all, and you don’t use age at diagnosis, family structure, genetic testing, results of breast density, or body mass index (BMI). There are many limitations of the Gail model, but most people use it because it is so easy and they are familiar with it.
Possibly the best model is the CanRisk tool, which incorporates the Breast and Ovarian Analysis of Disease Incidence and Carrier Estimation Algorithm (BOADICEA), but it takes too much time to use in clinic; it’s too complicated. The Tyrer-Cuzick model is easy to use once you get used to it.
Dr. Pederson: Risk doesn’t always need to be formally calculated, which can be time-consuming. It’s one of those situations where most practitioners know it when they see it. Benign atypical biopsies, a strong family history, or, obviously, the presence of a genetic mutation are huge red flags.
If a practitioner has a nearby high-risk center where they can refer patients, that can be so useful, even for a one-time consultation to guide management. For example, with the virtual world now, I do a lot of consultations for patients and outline a plan, and then the referring practitioner can carry out the plan with confidence and then send the patient back periodically. There are so many more options now that previously did not exist for the busy ObGyn or primary care provider to rely on.
Continue to: Chemoprevention uptake in at-risk women...
Chemoprevention uptake in at-risk women
Dr. Pederson: We really never practice medicine using numbers. We use clinical judgment, and we use relationships with patients in terms of developing confidence and trust. I think that the uptake that we exhibit in our center probably is more based on the patients’ perception that we are confident in our recommendations. I think that many practitioners simply are not comfortable with explaining medications, explaining and managing adverse effects, and using alternative medications. While the modeling helps, I think the personal expertise really makes the difference.
Going forward, the addition of the polygenic risk score to the mathematical risk models is going to make a big difference. Right now, the mathematical risk model is simply that: it takes the traditional risk factors that a patient has and spits out a number. But adding the patient’s genomic data—that is, a weighted summation of SNPs, or single nucleotide polymorphisms, now numbering over 300 for breast cancer—can explain more about their personalized risk, which is going to be more powerful in influencing a woman to take medication or not to take medication, in my opinion. Knowing their actual genomic risk will be a big step forward in individualized risk stratification and increased medication uptake as well as vigilance with high risk screening and attention to diet, exercise, and drinking alcohol in moderation.
Dr. Pederson: The only drug that can be used in the premenopausal setting is tamoxifen (TABLE 1). Women can’t take it if they are pregnant, planning to become pregnant, or if they don’t use a reliable form of birth control because it is teratogenic. Women also cannot take tamoxifen if they have had a history of blood clots, stroke, or transient ischemic attack; if they are on warfarin or estrogen preparations; or if they have had atypical endometrial biopsies or endometrial cancer. Those are the absolute contraindications for tamoxifen use.
Tamoxifen is generally very well tolerated in most women; some women experience hot flashes and night sweats that often will subside (or become tolerable) over the first 90 days. In addition, some women experience vaginal discharge rather than dryness, but it is not as bothersome to patients as dryness can be.
Tamoxifen can be used in the pre- or postmenopausal setting. In healthy premenopausal women, there’s no increased risk of the serious adverse effects that are seen with tamoxifen use in postmenopausal women, such as the 1% risk of blood clots and the 1% risk of endometrial cancer.
In postmenopausal women who still have their uterus, I’ll preferentially use raloxifene over tamoxifen. If they don’t have their uterus, tamoxifen is slightly more effective than the raloxifene, and I’ll use that.
Tamoxifen and raloxifene are both selective estrogen receptor modulators, or SERMs, which means that they stimulate receptors in some tissues, like bone, keeping bones strong, and block the receptors in other tissues, like the breast, reducing risk. And so you get kind of a two-for-one in terms of breast cancer risk reduction and osteoporosis prevention.
Another class of preventive drugs is the aromatase inhibitors (AIs). They block the enzyme aromatase, which converts androgens to estrogens peripherally; that is, the androgens that are produced primarily in the adrenal gland, but in part in postmenopausal ovaries.
In general, AIs are less well tolerated. There are generally more hot flashes and night sweats, and more vaginal dryness than with the SERMs. Anastrozole use is associated with arthralgias; and with exemestane use, there can be some hair loss (TABLE 2). Relative contraindications to SERMs become more important in the postmenopausal setting because of the increased frequency of both blood clots and uterine cancer in the postmenopausal years. I won’t give it to smokers. I won’t give tamoxifen to smokers in the premenopausal period either. With obese women, care must be taken because of the risk of blood clots with the SERMS, so then I’ll resort to the AIs. In the postmenopausal setting, you have to think a lot harder about the choices you use for preventive medication. Preferentially, I’ll use the SERMS if possible as they have fewer adverse effects.

Dr. Pederson: All of them are recommended to be given for 5 years, but the MAP.3 trial, which studied exemestane compared with placebo, showed a 65% risk reduction with 3 years of therapy.3 So occasionally, we’ll use 3 years of therapy. Why the treatment recommendation is universally 5 years is unclear, given that the trial with that particular drug was done in 3 years. And with low-dose tamoxifen, the recommended duration is 3 years. That study was done in Italy with 5 mg daily for 3 years.4 In the United States we use 10 mg every other day for 3 years because the 5-mg tablet is not available here.
Continue to: Counseling points...
Counseling points
Dr. Pederson: Patients’ fears about adverse effects are often worse than the adverse effects themselves. Women will fester over, Should I take it? Should I take it possibly for years? And then they take the medication and they tell me, “I don’t even notice that I’m taking it, and I know I’m being proactive.” The majority of patients who take these medications don’t have a lot of significant adverse effects.
Severe hot flashes can be managed in a number of ways, primarily and most effectively with certain antidepressants. Oxybutynin use is another good way to manage vasomotor symptoms. Sometimes we use local vaginal estrogen if a patient has vaginal dryness. In general, however, I would say at least 80% of my patients who take preventive medications do not require management of adverse side effects, that they are tolerable.
I counsel women this way, “Don’t think of this as a 5-year course of medication. Think of it as a 90-day trial, and let’s see how you do. If you hate it, then we don’t do it.” They often are pleasantly surprised that the medication is much easier to tolerate than they thought it would be.
Dr. Pederson: It would be neat if a trial would directly compare lifestyle interventions with medications, because probably lifestyle change is as effective as medication is—but we don’t know that and probably will never have that data. We do know that alcohol consumption, every drink per day, increases risk by 10%. We know that obesity is responsible for 30% of breast cancers in this country, and that hormone replacement probably is overrated as a significant risk factor. Updated data from the Women’s Health Initiative study suggest that hormone replacement may actually reduce both breast cancer and cardiovascular risk in women in their 50s, but that’s in average-risk women and not in high-risk women, so we can’t generalize. We do recommend lifestyle measures including weight loss, exercise, and limiting alcohol consumption for all of our patients and certainly for our high-risk patients.
The only 2 things a woman can do to reduce the risk of triple negative breast cancer are to achieve and maintain ideal body weight and to breastfeed. The medications that I have mentioned don’t reduce the risk of triple negative breast cancer. Staying thin and breastfeeding do. It’s a problem in this country because at least 35% of all women and 58% of Black women are obese in America, and Black women tend to be prone to triple-negative breast cancer. That’s a real public health issue that we need to address. If we were going to focus on one thing, it would be focusing on obesity in terms of risk reduction.
Final thoughts
Dr. Pederson: I would like to direct attention to the American Heart Association scientific statement published at the end of 2020 that reported that hormone replacement in average-risk women reduced both cardiovascular events and overall mortality in women in their 50s by 30%.5 While that’s not directly related to what we are talking about, we need to weigh the pros and cons of estrogen versus estrogen blockade in women in terms of breast cancer risk management discussions. Part of shared decision making now needs to include cardiovascular risk factors and how estrogen is going to play into that.
In women with atypical hyperplasia or LCIS, they may benefit from the preventive medications we discussed. But in women with family history or in women with genetic mutations who have not had benign atypical biopsies, they may choose to consider estrogen during their 50s and perhaps take tamoxifen either beforehand or raloxifene afterward.
We need to look at patients holistically and consider all their risk factors together. We can’t look at one dimension alone.
- US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867.
- Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152-3165.
- Goss PE, Ingle JN, Alex-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37:1629-1637.
- El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532.
- US Preventive Services Task Force. Medication use to reduce risk of breast cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;322:857-867.
- Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO clinical practice guideline update. J Clin Oncol. 2019;37:3152-3165.
- Goss PE, Ingle JN, Alex-Martinez JE, et al. Exemestane for breast-cancer prevention in postmenopausal women. N Engl J Med. 2011;364:2381-2391.
- DeCensi A, Puntoni M, Guerrieri-Gonzaga A, et al. Randomized placebo controlled trial of low-dose tamoxifen to prevent local and contralateral recurrence in breast intraepithelial neoplasia. J Clin Oncol. 2019;37:1629-1637.
- El Khoudary SR, Aggarwal B, Beckie TM, et al; American Heart Association Prevention Science Committee of the Council on Epidemiology and Prevention, and Council on Cardiovascular and Stroke Nursing. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142:e506-e532.
Cancer prevention through cascade genetic testing: A review of the current practice guidelines, barriers to testing and proposed solutions

CASE Woman with BRCA2 mutation
An 80-year-old woman presents for evaluation of newly diagnosed metastatic pancreatic adenocarcinoma. Her medical history is notable for breast cancer. Genetic testing of pancreatic tumor tissue detected a pathogenic variant in BRCA2. Family history revealed a history of melanoma as well as bladder, prostate, breast, and colon cancer. The patient subsequently underwent germline genetic testing with an 86-gene panel and a pathogenic mutation in BRCA2 was identified.
Watch a video of this patient and her clinician, Dr. Andrea Hagemann: https://www.youtube.com/watch?
Methods of genetic testing
It is estimated that 1 in 300 to 1 in 500 women in the United States carry a deleterious mutation in BRCA1 or BRCA2. This equates to between 250,000 and 415,000 women who are at high risk for breast and ovarian cancer.1 Looking at all women with cancer, 20% with ovarian,2 10% with breast,3 2% to 3% with endometrial,4 and 5% with colon cancer5 will have a germline mutation predisposing them to cancer. Identification of germline or somatic (tumor) mutations now inform treatment for patients with cancer. An equally important goal of germline genetic testing is cancer prevention. Cancer prevention strategies include risk-based screening for breast, colon, melanoma, and pancreatic cancer and prophylactic surgeries to reduce the risk of breast and ovarian cancer based on mutation type. Evidence-based screening guidelines by mutation type and absolute risk of associated cancers can be found on the National Comprehensive Cancer Network (NCCN).6,7
Multiple strategies have been proposed to identify patients for germline genetic testing. Patients can be identified based on a detailed multigenerational family history. This strategy requires clinicians or genetic counselors to take and update family histories, to recognize when a patient requires referral for testing, and for such testing to be completed. Even then the generation of a detailed pedigree is not very sensitive or specific. Population-based screening for high-penetrance breast and ovarian cancer susceptibility genes, regardless of family history, also has been proposed.8 Such a strategy has become increasingly realistic with decreasing cost and increasing availability of genetic testing. However, it would require increased genetic counseling resources to feasibly and equitably reach the target population and to explain the results to those patients and their relatives.
An alternative is to test the enriched population of family members of a patient with cancer who has been found to carry a pathogenic variant in a clinically relevant cancer susceptibility gene. This type of testing is termed cascade genetic testing. Cascade testing in first-degree family members carries a 50% probability of detecting the same pathogenic mutation. A related testing model is traceback testing where genetic testing is performed on pathology or tumor registry specimens from deceased patients with cancer.9 This genetic testing information is then provided to the family. Traceback models of genetic testing are an active area of research but can introduce ethical dilemmas. The more widely accepted cascade testing starts with the testing of a living patient affected with cancer. A recent article demonstrated the feasibility of a cascade testing model. Using a multiple linear regression model, the authors determined that all carriers of pathogenic mutations in 18 clinically relevant cancer susceptibility genes in the United States could be identified in 9.9 years if there was a 70% cascade testing rate of first-, second- and third-degree relatives, compared to 59.5 years with no cascade testing.10
Gaps in practice
Identification of mutation carriers, either through screening triggered by family history or through testing of patients affected with cancer, represents a gap between guidelines and clinical practice. Current NCCN guidelines outline genetic testing criteria for hereditary breast and ovarian cancer syndrome and for hereditary colorectal cancer. Despite well-established criteria, a survey in the United States revealed that only 19% of primary care providers were able to accurately assess family history for BRCA1 and 2 testing.11 Looking at patients who meet criteria for testing for Lynch syndrome, only 1 in 4 individuals have undergone genetic testing.12 Among patients diagnosed with breast and ovarian cancer, current NCCN guidelines recommend germline genetic testing for all patients with epithelial ovarian cancer; emerging evidence suggests all patients with breast cancer should be offered germline genetic testing.7,13 Large population-based studies have repeatedly demonstrated that testing rates fall short of this goal, with only 10% to 30% of patients undergoing genetic testing.9,14
Among families with a known hereditary mutation, rates of cascade genetic testing are also low, ranging from 17% to 50%.15-18 Evidence-based management guidelines, for both hereditary breast and ovarian cancer as well as Lynch syndrome, have been shown to reduce mortality.19,20 Failure to identify patients who carry these genetic mutations equates to increased mortality for our patients.
Barriers to cascade genetic testing
Cascade genetic testing ideally would be performed on entire families. Actual practice is far from ideal, and barriers to cascade testing exist. Barriers encompass resistance on the part of the family and provider as well as environmental or system factors.
Family factors
Because of privacy laws, the responsibility of disclosure of genetic testing results to family members falls primarily to the patient. Proband education is critical to ensure disclosure amongst family members. Family dynamics and geographic distribution of family members can further complicate disclosure. Following disclosure, family member gender, education, and demographics as well as personal views, attitudes, and emotions affect whether a family member decides to undergo testing.21 Furthermore, insurance status and awareness of and access to specialty-specific care for the proband’s family members may influence cascade genetic testing rates.
Provider factors
Provider factors that affect cascade genetic testing include awareness of testing guidelines, interpretation of genetic testing results, and education and knowledge of specific mutations. For instance, providers must recognize that cascade testing is not appropriate for variants of uncertain significance. This can lead to unnecessary surveillance testing and prophylactic surgeries. Providers, however, must continue to follow patients and periodically update testing results as variants may be reclassified over time. Additionally, providers must be knowledgeable about the complex and nuanced nature of the screening guidelines for each mutation. The NCCN provides detailed recommendations by mutation.7 Patients may benefit from care with cancer specialists who are aware of the guidelines, particularly for moderate-penetrance genes like BRIP1 and PALB2, as discussions about the timing of risk-reducing surgery are more nuanced in this population. Finally, which providers are responsible for facilitating cascade testing may be unclear; oncologists and genetic counselors not primarily treating probands’ relatives may assume the proper information has been passed along to family members without a practical means to follow up, and primary care providers may assume it is being taken care of by the oncology provider.
Continue to: Environmental or system factors...
Environmental or system factors
Accessibility of genetic counseling and testing is a common barrier to cascade testing. Family members may be geographically remote and connecting them to counseling and testing can be challenging. Working with local genetic counselors can facilitate this process. Insurance coverage of testing is a common perceived barrier; however, many testing companies now provide cascade testing free of charge if within a certain window from the initial test. Despite this, patients often site cost as a barrier to undergoing testing. Concerns about insurance coverage are common after a positive result. The Genetic Information Nondiscrimination Act of 2008 prohibits discrimination against employees or insurance applicants because of genetic information. Life insurance or long-term care policies, however, can incorporate genetic testing information into policy rates, so patients should be recommended to consider purchasing life insurance prior to undergoing genetic testing. This is especially important if the person considering testing has not yet been diagnosed with cancer.
Implications of a positive result
Family members who receive a positive test result should be referred for genetic counseling and to the appropriate specialists for evidence-based screening and discussion for risk-reducing surgery (FIGURE).7 For mutations associated with hereditary breast and ovarian cancer, referral to breast and gynecologic surgeons with expertise in risk reducing surgery is critical as the risk of diagnosing an occult malignancy is approximately 1%.22 Surgical technique with a 2-cm margin on the
Patient resources: decision aids, websites
As genetic testing becomes more accessible and people are tested at younger ages, studies examining the balance of risk reduction and quality of life (QOL) are increasingly important. Fertility concerns, effects of early menopause, and the interrelatedness between decisions for breast and gynecologic risk reduction should all be considered in the counseling for surgical risk reduction. Patient decision aids can help mutation carriers navigate the complex information and decisions.25 Websites specifically designed by advocacy groups can be useful adjuncts to in-office counseling (Facing Our Risk Empowered, FORCE; Facingourrisk.org).
Family letters
The American College of Obstetricians and Gynecologists recommends an ObGyn have a letter or documentation stating that the patient’s relative has a specific mutation before initiating cascade testing for an at-risk family member. The indicated test (such as BRCA1) should be ordered only after the patient has been counseled about potential outcomes and has expressly decided to be tested.26 Letters, such as the example given in the American College of Obstetricians and Gynecologists practice bulletin,26 are a key component of communication between oncology providers, probands, family members, and their primary care providers. ObGyn providers should work together with genetic counselors and gynecologic oncologists to determine the most efficient strategies in their communities.
Technology
Access to genetic testing and genetic counseling has been improved with the rise in telemedicine. Geographically remote patients can now access genetic counseling through medical center–based counselors as well as company-provided genetic counseling over the phone. Patients also can submit samples remotely without needing to be tested in a doctor’s office.
Databases from cancer centers that detail cascade genetic testing rates. As the preventive impact of cascade genetic testing becomes clearer, strategies to have recurrent discussions with cancer patients regarding their family members’ risk should be implemented. It is still unclear which providers—genetic counselors, gynecologic oncologists, medical oncologists, breast surgeons, ObGyns, to name a few—are primarily responsible for remembering to have these follow-up discussions, and despite advances, the burden still rests on the cancer patient themselves. Databases with automated follow-up surveys done every 6 to 12 months could provide some aid to busy providers in this regard.
Emerging research
If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged. The Women Choosing Surgical Prevention (NCT02760849) in the United States and the TUBA study (NCT02321228) in the Netherlands were designed to compare menopause-related QOL between standard risk-reducing salpingo-oophorectomy (RRSO) and the innovative risk-reducing salpingectomy with delayed oophorectomy for mutation carriers. Results from the nonrandomized controlled TUBA trial suggest that patients have better menopause-related QOL after risk-reducing salpingectomy than after RRSO, regardless of hormone replacement therapy.27 International collaboration is continuing to better understand oncologic safety. In the United States, the SOROCk trial (NCT04251052) is a noninferiority surgical choice study underway for BRCA1 mutation carriers aged 35 to 50, powered to determine oncologic outcome differences in addition to QOL outcomes between RRSO and delayed oophorectomy arms.
Returning to the case
The patient and her family underwent genetic counseling. The patient’s 2 daughters, each in their 50s, underwent cascade genetic testing and were found to carry the same pathogenic mutation in BRCA2. After counseling from both breast and gynecologic surgeons, they both elected to undergo risk reducing bilateral salpingo-oophorectomy with hysterectomy. Both now complete regular screening for breast cancer and melanoma with plans to start screening for pancreatic cancer. Both are currently cancer free.
Summary
Cascade genetic testing is an efficient strategy to identify mutation carriers for hereditary breast and ovarian cancer syndrome. Implementation of the best patient-centric care will require continued collaboration and communication across and within disciplines. ●
Cascade, or targeted, genetic testing within families known to carry a hereditary mutation in a cancer susceptibility gene should be performed on all living first-degree family members over the age of 18. All mutation carriers should be connected to a multidisciplinary care team (FIGURE) to ensure implementation of evidence-based screening and risk-reducing surgery for cancer prevention. If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged.
- Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111:14205-14210.
- Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482-490.
- Yamauchi H, Takei J. Management of hereditary breast and ovarian cancer. Int J Clin Oncol. 2018;23:45-51.
- Kahn RM, Gordhandas S, Maddy BP, et al. Universal endometrial cancer tumor typing: how much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125:3172-3183.
- Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
- Gupta S, Provenzale D, Llor X, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 2.2019. J Natl Compr Canc Netw. 2019;17:1032-1041.
- Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:77-102.
- King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091-1092.
- Samimi G, et al. Traceback: a proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J Clin Oncol. 2017;35:2329-2337.
- Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: an alternative to population-based screening. J Clin Oncol. 2020;38:1398-1408.
- Bellcross CA, Kolor K, Goddard KAB, et al. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40:61-66.
- Cross DS, Rahm AK, Kauffman TL, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933-940.
- Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453-460.
- Childers CP, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7:230-237.
- Sharaf RN, Myer P, Stave CD, et al. Uptake of genetic testing by relatives of Lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013;11:1093-1100.
- Menko FH, Ter Stege JA, van der Kolk LE, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18:127-135.
- Griffin NE, Buchanan TR, Smith SH, et al. Low rates of cascade genetic testing among families with hereditary gynecologic cancer: an opportunity to improve cancer prevention. Gynecol Oncol. 2020;156:140-146.
- Roberts MC, Dotson WD, DeVore CS, et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff (Millwood). 2018;37:801-808.
- Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Srinivasan S, Won NY, Dotson WD, et al. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28:1631-1644.
- Piedimonte S, Frank C, Laprise C, et al. Occult tubal carcinoma after risk-reducing salpingo-oophorectomy: a systematic review. Obstet Gynecol. 2020;135:498-508.
- Shu CA, Pike MC, Jotwani AR, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2016;2:1434-1440.
- Gordhandas S, Norquist BM, Pennington KP, et al. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol. 2019;153:192-200.
- Steenbeek MP, van Bommel MHD, Harmsen MG, et al. Evaluation of a patient decision aid for BRCA1/2 pathogenic variant carriers choosing an ovarian cancer prevention strategy. Gynecol Oncol. 2021;163:371-377.
- Committee on Gynecologic Practice. ACOG committee opinion No. 727: Cascade testing: testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol. 2018;131:E31-E34.
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA1/2 pathogenic variant carriers: a nonrandomized controlled trial. JAMA Oncol. 2021;7:1203-1212.

CASE Woman with BRCA2 mutation
An 80-year-old woman presents for evaluation of newly diagnosed metastatic pancreatic adenocarcinoma. Her medical history is notable for breast cancer. Genetic testing of pancreatic tumor tissue detected a pathogenic variant in BRCA2. Family history revealed a history of melanoma as well as bladder, prostate, breast, and colon cancer. The patient subsequently underwent germline genetic testing with an 86-gene panel and a pathogenic mutation in BRCA2 was identified.
Watch a video of this patient and her clinician, Dr. Andrea Hagemann: https://www.youtube.com/watch?
Methods of genetic testing
It is estimated that 1 in 300 to 1 in 500 women in the United States carry a deleterious mutation in BRCA1 or BRCA2. This equates to between 250,000 and 415,000 women who are at high risk for breast and ovarian cancer.1 Looking at all women with cancer, 20% with ovarian,2 10% with breast,3 2% to 3% with endometrial,4 and 5% with colon cancer5 will have a germline mutation predisposing them to cancer. Identification of germline or somatic (tumor) mutations now inform treatment for patients with cancer. An equally important goal of germline genetic testing is cancer prevention. Cancer prevention strategies include risk-based screening for breast, colon, melanoma, and pancreatic cancer and prophylactic surgeries to reduce the risk of breast and ovarian cancer based on mutation type. Evidence-based screening guidelines by mutation type and absolute risk of associated cancers can be found on the National Comprehensive Cancer Network (NCCN).6,7
Multiple strategies have been proposed to identify patients for germline genetic testing. Patients can be identified based on a detailed multigenerational family history. This strategy requires clinicians or genetic counselors to take and update family histories, to recognize when a patient requires referral for testing, and for such testing to be completed. Even then the generation of a detailed pedigree is not very sensitive or specific. Population-based screening for high-penetrance breast and ovarian cancer susceptibility genes, regardless of family history, also has been proposed.8 Such a strategy has become increasingly realistic with decreasing cost and increasing availability of genetic testing. However, it would require increased genetic counseling resources to feasibly and equitably reach the target population and to explain the results to those patients and their relatives.
An alternative is to test the enriched population of family members of a patient with cancer who has been found to carry a pathogenic variant in a clinically relevant cancer susceptibility gene. This type of testing is termed cascade genetic testing. Cascade testing in first-degree family members carries a 50% probability of detecting the same pathogenic mutation. A related testing model is traceback testing where genetic testing is performed on pathology or tumor registry specimens from deceased patients with cancer.9 This genetic testing information is then provided to the family. Traceback models of genetic testing are an active area of research but can introduce ethical dilemmas. The more widely accepted cascade testing starts with the testing of a living patient affected with cancer. A recent article demonstrated the feasibility of a cascade testing model. Using a multiple linear regression model, the authors determined that all carriers of pathogenic mutations in 18 clinically relevant cancer susceptibility genes in the United States could be identified in 9.9 years if there was a 70% cascade testing rate of first-, second- and third-degree relatives, compared to 59.5 years with no cascade testing.10
Gaps in practice
Identification of mutation carriers, either through screening triggered by family history or through testing of patients affected with cancer, represents a gap between guidelines and clinical practice. Current NCCN guidelines outline genetic testing criteria for hereditary breast and ovarian cancer syndrome and for hereditary colorectal cancer. Despite well-established criteria, a survey in the United States revealed that only 19% of primary care providers were able to accurately assess family history for BRCA1 and 2 testing.11 Looking at patients who meet criteria for testing for Lynch syndrome, only 1 in 4 individuals have undergone genetic testing.12 Among patients diagnosed with breast and ovarian cancer, current NCCN guidelines recommend germline genetic testing for all patients with epithelial ovarian cancer; emerging evidence suggests all patients with breast cancer should be offered germline genetic testing.7,13 Large population-based studies have repeatedly demonstrated that testing rates fall short of this goal, with only 10% to 30% of patients undergoing genetic testing.9,14
Among families with a known hereditary mutation, rates of cascade genetic testing are also low, ranging from 17% to 50%.15-18 Evidence-based management guidelines, for both hereditary breast and ovarian cancer as well as Lynch syndrome, have been shown to reduce mortality.19,20 Failure to identify patients who carry these genetic mutations equates to increased mortality for our patients.
Barriers to cascade genetic testing
Cascade genetic testing ideally would be performed on entire families. Actual practice is far from ideal, and barriers to cascade testing exist. Barriers encompass resistance on the part of the family and provider as well as environmental or system factors.
Family factors
Because of privacy laws, the responsibility of disclosure of genetic testing results to family members falls primarily to the patient. Proband education is critical to ensure disclosure amongst family members. Family dynamics and geographic distribution of family members can further complicate disclosure. Following disclosure, family member gender, education, and demographics as well as personal views, attitudes, and emotions affect whether a family member decides to undergo testing.21 Furthermore, insurance status and awareness of and access to specialty-specific care for the proband’s family members may influence cascade genetic testing rates.
Provider factors
Provider factors that affect cascade genetic testing include awareness of testing guidelines, interpretation of genetic testing results, and education and knowledge of specific mutations. For instance, providers must recognize that cascade testing is not appropriate for variants of uncertain significance. This can lead to unnecessary surveillance testing and prophylactic surgeries. Providers, however, must continue to follow patients and periodically update testing results as variants may be reclassified over time. Additionally, providers must be knowledgeable about the complex and nuanced nature of the screening guidelines for each mutation. The NCCN provides detailed recommendations by mutation.7 Patients may benefit from care with cancer specialists who are aware of the guidelines, particularly for moderate-penetrance genes like BRIP1 and PALB2, as discussions about the timing of risk-reducing surgery are more nuanced in this population. Finally, which providers are responsible for facilitating cascade testing may be unclear; oncologists and genetic counselors not primarily treating probands’ relatives may assume the proper information has been passed along to family members without a practical means to follow up, and primary care providers may assume it is being taken care of by the oncology provider.
Continue to: Environmental or system factors...
Environmental or system factors
Accessibility of genetic counseling and testing is a common barrier to cascade testing. Family members may be geographically remote and connecting them to counseling and testing can be challenging. Working with local genetic counselors can facilitate this process. Insurance coverage of testing is a common perceived barrier; however, many testing companies now provide cascade testing free of charge if within a certain window from the initial test. Despite this, patients often site cost as a barrier to undergoing testing. Concerns about insurance coverage are common after a positive result. The Genetic Information Nondiscrimination Act of 2008 prohibits discrimination against employees or insurance applicants because of genetic information. Life insurance or long-term care policies, however, can incorporate genetic testing information into policy rates, so patients should be recommended to consider purchasing life insurance prior to undergoing genetic testing. This is especially important if the person considering testing has not yet been diagnosed with cancer.
Implications of a positive result
Family members who receive a positive test result should be referred for genetic counseling and to the appropriate specialists for evidence-based screening and discussion for risk-reducing surgery (FIGURE).7 For mutations associated with hereditary breast and ovarian cancer, referral to breast and gynecologic surgeons with expertise in risk reducing surgery is critical as the risk of diagnosing an occult malignancy is approximately 1%.22 Surgical technique with a 2-cm margin on the

Patient resources: decision aids, websites
As genetic testing becomes more accessible and people are tested at younger ages, studies examining the balance of risk reduction and quality of life (QOL) are increasingly important. Fertility concerns, effects of early menopause, and the interrelatedness between decisions for breast and gynecologic risk reduction should all be considered in the counseling for surgical risk reduction. Patient decision aids can help mutation carriers navigate the complex information and decisions.25 Websites specifically designed by advocacy groups can be useful adjuncts to in-office counseling (Facing Our Risk Empowered, FORCE; Facingourrisk.org).
Family letters
The American College of Obstetricians and Gynecologists recommends an ObGyn have a letter or documentation stating that the patient’s relative has a specific mutation before initiating cascade testing for an at-risk family member. The indicated test (such as BRCA1) should be ordered only after the patient has been counseled about potential outcomes and has expressly decided to be tested.26 Letters, such as the example given in the American College of Obstetricians and Gynecologists practice bulletin,26 are a key component of communication between oncology providers, probands, family members, and their primary care providers. ObGyn providers should work together with genetic counselors and gynecologic oncologists to determine the most efficient strategies in their communities.
Technology
Access to genetic testing and genetic counseling has been improved with the rise in telemedicine. Geographically remote patients can now access genetic counseling through medical center–based counselors as well as company-provided genetic counseling over the phone. Patients also can submit samples remotely without needing to be tested in a doctor’s office.
Databases from cancer centers that detail cascade genetic testing rates. As the preventive impact of cascade genetic testing becomes clearer, strategies to have recurrent discussions with cancer patients regarding their family members’ risk should be implemented. It is still unclear which providers—genetic counselors, gynecologic oncologists, medical oncologists, breast surgeons, ObGyns, to name a few—are primarily responsible for remembering to have these follow-up discussions, and despite advances, the burden still rests on the cancer patient themselves. Databases with automated follow-up surveys done every 6 to 12 months could provide some aid to busy providers in this regard.
Emerging research
If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged. The Women Choosing Surgical Prevention (NCT02760849) in the United States and the TUBA study (NCT02321228) in the Netherlands were designed to compare menopause-related QOL between standard risk-reducing salpingo-oophorectomy (RRSO) and the innovative risk-reducing salpingectomy with delayed oophorectomy for mutation carriers. Results from the nonrandomized controlled TUBA trial suggest that patients have better menopause-related QOL after risk-reducing salpingectomy than after RRSO, regardless of hormone replacement therapy.27 International collaboration is continuing to better understand oncologic safety. In the United States, the SOROCk trial (NCT04251052) is a noninferiority surgical choice study underway for BRCA1 mutation carriers aged 35 to 50, powered to determine oncologic outcome differences in addition to QOL outcomes between RRSO and delayed oophorectomy arms.
Returning to the case
The patient and her family underwent genetic counseling. The patient’s 2 daughters, each in their 50s, underwent cascade genetic testing and were found to carry the same pathogenic mutation in BRCA2. After counseling from both breast and gynecologic surgeons, they both elected to undergo risk reducing bilateral salpingo-oophorectomy with hysterectomy. Both now complete regular screening for breast cancer and melanoma with plans to start screening for pancreatic cancer. Both are currently cancer free.
Summary
Cascade genetic testing is an efficient strategy to identify mutation carriers for hereditary breast and ovarian cancer syndrome. Implementation of the best patient-centric care will require continued collaboration and communication across and within disciplines. ●
Cascade, or targeted, genetic testing within families known to carry a hereditary mutation in a cancer susceptibility gene should be performed on all living first-degree family members over the age of 18. All mutation carriers should be connected to a multidisciplinary care team (FIGURE) to ensure implementation of evidence-based screening and risk-reducing surgery for cancer prevention. If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged.

CASE Woman with BRCA2 mutation
An 80-year-old woman presents for evaluation of newly diagnosed metastatic pancreatic adenocarcinoma. Her medical history is notable for breast cancer. Genetic testing of pancreatic tumor tissue detected a pathogenic variant in BRCA2. Family history revealed a history of melanoma as well as bladder, prostate, breast, and colon cancer. The patient subsequently underwent germline genetic testing with an 86-gene panel and a pathogenic mutation in BRCA2 was identified.
Watch a video of this patient and her clinician, Dr. Andrea Hagemann: https://www.youtube.com/watch?
Methods of genetic testing
It is estimated that 1 in 300 to 1 in 500 women in the United States carry a deleterious mutation in BRCA1 or BRCA2. This equates to between 250,000 and 415,000 women who are at high risk for breast and ovarian cancer.1 Looking at all women with cancer, 20% with ovarian,2 10% with breast,3 2% to 3% with endometrial,4 and 5% with colon cancer5 will have a germline mutation predisposing them to cancer. Identification of germline or somatic (tumor) mutations now inform treatment for patients with cancer. An equally important goal of germline genetic testing is cancer prevention. Cancer prevention strategies include risk-based screening for breast, colon, melanoma, and pancreatic cancer and prophylactic surgeries to reduce the risk of breast and ovarian cancer based on mutation type. Evidence-based screening guidelines by mutation type and absolute risk of associated cancers can be found on the National Comprehensive Cancer Network (NCCN).6,7
Multiple strategies have been proposed to identify patients for germline genetic testing. Patients can be identified based on a detailed multigenerational family history. This strategy requires clinicians or genetic counselors to take and update family histories, to recognize when a patient requires referral for testing, and for such testing to be completed. Even then the generation of a detailed pedigree is not very sensitive or specific. Population-based screening for high-penetrance breast and ovarian cancer susceptibility genes, regardless of family history, also has been proposed.8 Such a strategy has become increasingly realistic with decreasing cost and increasing availability of genetic testing. However, it would require increased genetic counseling resources to feasibly and equitably reach the target population and to explain the results to those patients and their relatives.
An alternative is to test the enriched population of family members of a patient with cancer who has been found to carry a pathogenic variant in a clinically relevant cancer susceptibility gene. This type of testing is termed cascade genetic testing. Cascade testing in first-degree family members carries a 50% probability of detecting the same pathogenic mutation. A related testing model is traceback testing where genetic testing is performed on pathology or tumor registry specimens from deceased patients with cancer.9 This genetic testing information is then provided to the family. Traceback models of genetic testing are an active area of research but can introduce ethical dilemmas. The more widely accepted cascade testing starts with the testing of a living patient affected with cancer. A recent article demonstrated the feasibility of a cascade testing model. Using a multiple linear regression model, the authors determined that all carriers of pathogenic mutations in 18 clinically relevant cancer susceptibility genes in the United States could be identified in 9.9 years if there was a 70% cascade testing rate of first-, second- and third-degree relatives, compared to 59.5 years with no cascade testing.10
Gaps in practice
Identification of mutation carriers, either through screening triggered by family history or through testing of patients affected with cancer, represents a gap between guidelines and clinical practice. Current NCCN guidelines outline genetic testing criteria for hereditary breast and ovarian cancer syndrome and for hereditary colorectal cancer. Despite well-established criteria, a survey in the United States revealed that only 19% of primary care providers were able to accurately assess family history for BRCA1 and 2 testing.11 Looking at patients who meet criteria for testing for Lynch syndrome, only 1 in 4 individuals have undergone genetic testing.12 Among patients diagnosed with breast and ovarian cancer, current NCCN guidelines recommend germline genetic testing for all patients with epithelial ovarian cancer; emerging evidence suggests all patients with breast cancer should be offered germline genetic testing.7,13 Large population-based studies have repeatedly demonstrated that testing rates fall short of this goal, with only 10% to 30% of patients undergoing genetic testing.9,14
Among families with a known hereditary mutation, rates of cascade genetic testing are also low, ranging from 17% to 50%.15-18 Evidence-based management guidelines, for both hereditary breast and ovarian cancer as well as Lynch syndrome, have been shown to reduce mortality.19,20 Failure to identify patients who carry these genetic mutations equates to increased mortality for our patients.
Barriers to cascade genetic testing
Cascade genetic testing ideally would be performed on entire families. Actual practice is far from ideal, and barriers to cascade testing exist. Barriers encompass resistance on the part of the family and provider as well as environmental or system factors.
Family factors
Because of privacy laws, the responsibility of disclosure of genetic testing results to family members falls primarily to the patient. Proband education is critical to ensure disclosure amongst family members. Family dynamics and geographic distribution of family members can further complicate disclosure. Following disclosure, family member gender, education, and demographics as well as personal views, attitudes, and emotions affect whether a family member decides to undergo testing.21 Furthermore, insurance status and awareness of and access to specialty-specific care for the proband’s family members may influence cascade genetic testing rates.
Provider factors
Provider factors that affect cascade genetic testing include awareness of testing guidelines, interpretation of genetic testing results, and education and knowledge of specific mutations. For instance, providers must recognize that cascade testing is not appropriate for variants of uncertain significance. This can lead to unnecessary surveillance testing and prophylactic surgeries. Providers, however, must continue to follow patients and periodically update testing results as variants may be reclassified over time. Additionally, providers must be knowledgeable about the complex and nuanced nature of the screening guidelines for each mutation. The NCCN provides detailed recommendations by mutation.7 Patients may benefit from care with cancer specialists who are aware of the guidelines, particularly for moderate-penetrance genes like BRIP1 and PALB2, as discussions about the timing of risk-reducing surgery are more nuanced in this population. Finally, which providers are responsible for facilitating cascade testing may be unclear; oncologists and genetic counselors not primarily treating probands’ relatives may assume the proper information has been passed along to family members without a practical means to follow up, and primary care providers may assume it is being taken care of by the oncology provider.
Continue to: Environmental or system factors...
Environmental or system factors
Accessibility of genetic counseling and testing is a common barrier to cascade testing. Family members may be geographically remote and connecting them to counseling and testing can be challenging. Working with local genetic counselors can facilitate this process. Insurance coverage of testing is a common perceived barrier; however, many testing companies now provide cascade testing free of charge if within a certain window from the initial test. Despite this, patients often site cost as a barrier to undergoing testing. Concerns about insurance coverage are common after a positive result. The Genetic Information Nondiscrimination Act of 2008 prohibits discrimination against employees or insurance applicants because of genetic information. Life insurance or long-term care policies, however, can incorporate genetic testing information into policy rates, so patients should be recommended to consider purchasing life insurance prior to undergoing genetic testing. This is especially important if the person considering testing has not yet been diagnosed with cancer.
Implications of a positive result
Family members who receive a positive test result should be referred for genetic counseling and to the appropriate specialists for evidence-based screening and discussion for risk-reducing surgery (FIGURE).7 For mutations associated with hereditary breast and ovarian cancer, referral to breast and gynecologic surgeons with expertise in risk reducing surgery is critical as the risk of diagnosing an occult malignancy is approximately 1%.22 Surgical technique with a 2-cm margin on the

Patient resources: decision aids, websites
As genetic testing becomes more accessible and people are tested at younger ages, studies examining the balance of risk reduction and quality of life (QOL) are increasingly important. Fertility concerns, effects of early menopause, and the interrelatedness between decisions for breast and gynecologic risk reduction should all be considered in the counseling for surgical risk reduction. Patient decision aids can help mutation carriers navigate the complex information and decisions.25 Websites specifically designed by advocacy groups can be useful adjuncts to in-office counseling (Facing Our Risk Empowered, FORCE; Facingourrisk.org).
Family letters
The American College of Obstetricians and Gynecologists recommends an ObGyn have a letter or documentation stating that the patient’s relative has a specific mutation before initiating cascade testing for an at-risk family member. The indicated test (such as BRCA1) should be ordered only after the patient has been counseled about potential outcomes and has expressly decided to be tested.26 Letters, such as the example given in the American College of Obstetricians and Gynecologists practice bulletin,26 are a key component of communication between oncology providers, probands, family members, and their primary care providers. ObGyn providers should work together with genetic counselors and gynecologic oncologists to determine the most efficient strategies in their communities.
Technology
Access to genetic testing and genetic counseling has been improved with the rise in telemedicine. Geographically remote patients can now access genetic counseling through medical center–based counselors as well as company-provided genetic counseling over the phone. Patients also can submit samples remotely without needing to be tested in a doctor’s office.
Databases from cancer centers that detail cascade genetic testing rates. As the preventive impact of cascade genetic testing becomes clearer, strategies to have recurrent discussions with cancer patients regarding their family members’ risk should be implemented. It is still unclear which providers—genetic counselors, gynecologic oncologists, medical oncologists, breast surgeons, ObGyns, to name a few—are primarily responsible for remembering to have these follow-up discussions, and despite advances, the burden still rests on the cancer patient themselves. Databases with automated follow-up surveys done every 6 to 12 months could provide some aid to busy providers in this regard.
Emerging research
If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged. The Women Choosing Surgical Prevention (NCT02760849) in the United States and the TUBA study (NCT02321228) in the Netherlands were designed to compare menopause-related QOL between standard risk-reducing salpingo-oophorectomy (RRSO) and the innovative risk-reducing salpingectomy with delayed oophorectomy for mutation carriers. Results from the nonrandomized controlled TUBA trial suggest that patients have better menopause-related QOL after risk-reducing salpingectomy than after RRSO, regardless of hormone replacement therapy.27 International collaboration is continuing to better understand oncologic safety. In the United States, the SOROCk trial (NCT04251052) is a noninferiority surgical choice study underway for BRCA1 mutation carriers aged 35 to 50, powered to determine oncologic outcome differences in addition to QOL outcomes between RRSO and delayed oophorectomy arms.
Returning to the case
The patient and her family underwent genetic counseling. The patient’s 2 daughters, each in their 50s, underwent cascade genetic testing and were found to carry the same pathogenic mutation in BRCA2. After counseling from both breast and gynecologic surgeons, they both elected to undergo risk reducing bilateral salpingo-oophorectomy with hysterectomy. Both now complete regular screening for breast cancer and melanoma with plans to start screening for pancreatic cancer. Both are currently cancer free.
Summary
Cascade genetic testing is an efficient strategy to identify mutation carriers for hereditary breast and ovarian cancer syndrome. Implementation of the best patient-centric care will require continued collaboration and communication across and within disciplines. ●
Cascade, or targeted, genetic testing within families known to carry a hereditary mutation in a cancer susceptibility gene should be performed on all living first-degree family members over the age of 18. All mutation carriers should be connected to a multidisciplinary care team (FIGURE) to ensure implementation of evidence-based screening and risk-reducing surgery for cancer prevention. If gynecologic risk-reducing surgery is chosen, clinical trial involvement should be encouraged.
- Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111:14205-14210.
- Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482-490.
- Yamauchi H, Takei J. Management of hereditary breast and ovarian cancer. Int J Clin Oncol. 2018;23:45-51.
- Kahn RM, Gordhandas S, Maddy BP, et al. Universal endometrial cancer tumor typing: how much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125:3172-3183.
- Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
- Gupta S, Provenzale D, Llor X, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 2.2019. J Natl Compr Canc Netw. 2019;17:1032-1041.
- Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:77-102.
- King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091-1092.
- Samimi G, et al. Traceback: a proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J Clin Oncol. 2017;35:2329-2337.
- Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: an alternative to population-based screening. J Clin Oncol. 2020;38:1398-1408.
- Bellcross CA, Kolor K, Goddard KAB, et al. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40:61-66.
- Cross DS, Rahm AK, Kauffman TL, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933-940.
- Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453-460.
- Childers CP, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7:230-237.
- Sharaf RN, Myer P, Stave CD, et al. Uptake of genetic testing by relatives of Lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013;11:1093-1100.
- Menko FH, Ter Stege JA, van der Kolk LE, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18:127-135.
- Griffin NE, Buchanan TR, Smith SH, et al. Low rates of cascade genetic testing among families with hereditary gynecologic cancer: an opportunity to improve cancer prevention. Gynecol Oncol. 2020;156:140-146.
- Roberts MC, Dotson WD, DeVore CS, et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff (Millwood). 2018;37:801-808.
- Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Srinivasan S, Won NY, Dotson WD, et al. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28:1631-1644.
- Piedimonte S, Frank C, Laprise C, et al. Occult tubal carcinoma after risk-reducing salpingo-oophorectomy: a systematic review. Obstet Gynecol. 2020;135:498-508.
- Shu CA, Pike MC, Jotwani AR, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2016;2:1434-1440.
- Gordhandas S, Norquist BM, Pennington KP, et al. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol. 2019;153:192-200.
- Steenbeek MP, van Bommel MHD, Harmsen MG, et al. Evaluation of a patient decision aid for BRCA1/2 pathogenic variant carriers choosing an ovarian cancer prevention strategy. Gynecol Oncol. 2021;163:371-377.
- Committee on Gynecologic Practice. ACOG committee opinion No. 727: Cascade testing: testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol. 2018;131:E31-E34.
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA1/2 pathogenic variant carriers: a nonrandomized controlled trial. JAMA Oncol. 2021;7:1203-1212.
- Gabai-Kapara E, Lahad A, Kaufman B, et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111:14205-14210.
- Norquist BM, Harrell MI, Brady MF, et al. Inherited mutations in women with ovarian carcinoma. JAMA Oncol. 2016;2:482-490.
- Yamauchi H, Takei J. Management of hereditary breast and ovarian cancer. Int J Clin Oncol. 2018;23:45-51.
- Kahn RM, Gordhandas S, Maddy BP, et al. Universal endometrial cancer tumor typing: how much has immunohistochemistry, microsatellite instability, and MLH1 methylation improved the diagnosis of Lynch syndrome across the population? Cancer. 2019;125:3172-3183.
- Jasperson KW, Tuohy TM, Neklason DW, et al. Hereditary and familial colon cancer. Gastroenterology. 2010;138:2044-2058.
- Gupta S, Provenzale D, Llor X, et al. NCCN guidelines insights: genetic/familial high-risk assessment: colorectal, version 2.2019. J Natl Compr Canc Netw. 2019;17:1032-1041.
- Daly MB, Pal T, Berry MP, et al. Genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2021;19:77-102.
- King MC, Levy-Lahad E, Lahad A. Population-based screening for BRCA1 and BRCA2: 2014 Lasker Award. JAMA. 2014;312:1091-1092.
- Samimi G, et al. Traceback: a proposed framework to increase identification and genetic counseling of BRCA1 and BRCA2 mutation carriers through family-based outreach. J Clin Oncol. 2017;35:2329-2337.
- Offit K, Tkachuk KA, Stadler ZK, et al. Cascading after peridiagnostic cancer genetic testing: an alternative to population-based screening. J Clin Oncol. 2020;38:1398-1408.
- Bellcross CA, Kolor K, Goddard KAB, et al. Awareness and utilization of BRCA1/2 testing among U.S. primary care physicians. Am J Prev Med. 2011;40:61-66.
- Cross DS, Rahm AK, Kauffman TL, et al. Underutilization of Lynch syndrome screening in a multisite study of patients with colorectal cancer. Genet Med. 2013;15:933-940.
- Beitsch PD, Whitworth PW, Hughes K, et al. Underdiagnosis of hereditary breast cancer: are genetic testing guidelines a tool or an obstacle? J Clin Oncol. 2019;37:453-460.
- Childers CP, Childers KK, Maggard-Gibbons M, et al. National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol. 2017;35:3800-3806.
- Samadder NJ, Riegert-Johnson D, Boardman L, et al. Comparison of universal genetic testing vs guideline-directed targeted testing for patients with hereditary cancer syndrome. JAMA Oncol. 2021;7:230-237.
- Sharaf RN, Myer P, Stave CD, et al. Uptake of genetic testing by relatives of Lynch syndrome probands: a systematic review. Clin Gastroenterol Hepatol. 2013;11:1093-1100.
- Menko FH, Ter Stege JA, van der Kolk LE, et al. The uptake of presymptomatic genetic testing in hereditary breast-ovarian cancer and Lynch syndrome: a systematic review of the literature and implications for clinical practice. Fam Cancer. 2019;18:127-135.
- Griffin NE, Buchanan TR, Smith SH, et al. Low rates of cascade genetic testing among families with hereditary gynecologic cancer: an opportunity to improve cancer prevention. Gynecol Oncol. 2020;156:140-146.
- Roberts MC, Dotson WD, DeVore CS, et al. Delivery of cascade screening for hereditary conditions: a scoping review of the literature. Health Aff (Millwood). 2018;37:801-808.
- Finch AP, Lubinski J, Møller P, et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2014;32:1547-1553.
- Srinivasan S, Won NY, Dotson WD, et al. Barriers and facilitators for cascade testing in genetic conditions: a systematic review. Eur J Hum Genet. 2020;28:1631-1644.
- Piedimonte S, Frank C, Laprise C, et al. Occult tubal carcinoma after risk-reducing salpingo-oophorectomy: a systematic review. Obstet Gynecol. 2020;135:498-508.
- Shu CA, Pike MC, Jotwani AR, et al. Uterine cancer after risk-reducing salpingo-oophorectomy without hysterectomy in women with BRCA mutations. JAMA Oncol. 2016;2:1434-1440.
- Gordhandas S, Norquist BM, Pennington KP, et al. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol Oncol. 2019;153:192-200.
- Steenbeek MP, van Bommel MHD, Harmsen MG, et al. Evaluation of a patient decision aid for BRCA1/2 pathogenic variant carriers choosing an ovarian cancer prevention strategy. Gynecol Oncol. 2021;163:371-377.
- Committee on Gynecologic Practice. ACOG committee opinion No. 727: Cascade testing: testing women for known hereditary genetic mutations associated with cancer. Obstet Gynecol. 2018;131:E31-E34.
- Steenbeek MP, Harmsen MG, Hoogerbrugge N, et al. Association of salpingectomy with delayed oophorectomy versus salpingo-oophorectomy with quality of life in BRCA1/2 pathogenic variant carriers: a nonrandomized controlled trial. JAMA Oncol. 2021;7:1203-1212.
Fever following cesarean delivery: What are your steps for management?
CASE Woman who has undergone recent cesarean delivery
A 23-year-old woman had a primary cesarean delivery 72 hours ago due to an arrest of dilation at 6 cm. She was in labor for 22 hours, and her membranes were ruptured for 18 hours. She had 10 internal vaginal examinations, and the duration of internal fetal monitoring was 12 hours; 24 hours after delivery, she developed a fever of 39°C, in association with lower abdominal pain and tenderness. She was presumptively treated for endometritis with cefepime; 48 hours after the initiation of antibiotics, she remains febrile and symptomatic.
- What are the most likely causes of her persistent fever?
- What should be the next steps in her evaluation?
Cesarean delivery background
Cesarean delivery is now the most common major operation performed in US hospitals. Cesarean delivery rates hover between 25% and 30% in most medical centers in the United States.1 The most common postoperative complication of cesarean delivery is infection. Infection typically takes 1 of 3 forms: endometritis (organ space infection), wound infection (surgical site infection), and urinary tract infection (UTI).1 This article will review the initial differential diagnosis, evaluation, and management of the patient with a postoperative fever and also will describe the appropriate assessment and treatment of the patient who has a persistent postoperative fever despite therapy. The article will also highlight key interventions that help to prevent postoperative infections.
Initial evaluation of the febrile patient
In the first 24 to 48 hours after cesarean delivery, the most common cause of fever is endometritis (organ space infection). This condition is a polymicrobial, mixed aerobic-anaerobic infection (FIGURE). The principal pathogens include anaerobic gram-positive cocci (
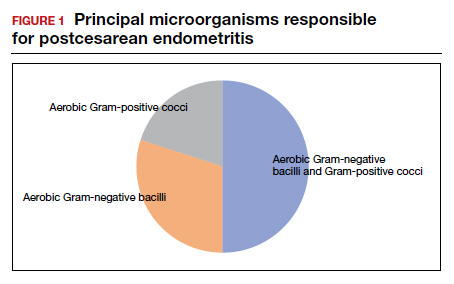
The major risk factors for postcesarean endometritis are extended duration of labor and ruptured membranes, multiple internal vaginal examinations, invasive fetal monitoring, and pre-existing colonization with group B Streptococcus and/or the organisms that cause bacterial vaginosis. Affected patients typically have a fever in the range of 38 to 39°C, tachycardia, mild tachypnea, lower abdominal pain and tenderness, and purulent lochia in some individuals.1
Differential for postoperative fever
The initial differential diagnosis of postoperative fever is relatively limited (TABLE 1). In addition to endometritis, it includes extensive atelectasis, perhaps resulting from general anesthesia; lower respiratory tract infection, either viral influenza or bacterial pneumonia; and acute pyelonephritis. A simple infection of the bladder (cystitis or asymptomatic bacteriuria) should not cause a substantial temperature elevation and systemic symptoms.1
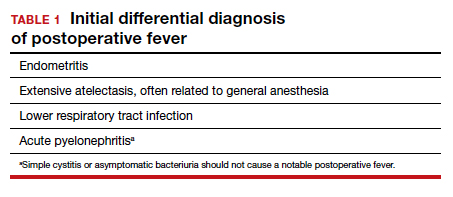
Differentiation between these entities usually is possible based on physical examination and a few laboratory tests. The peripheral white blood cell count usually is elevated, and a left shift may be evident. If a respiratory tract infection is suspected, chest radiography is indicated. A urine culture should be obtained if acute pyelonephritis strongly is considered. Lower genital tract cultures are rarely of value, and uncontaminated upper tract cultures are difficult to obtain. I do not believe that blood cultures should be performed as a matter of routine. They are expensive, and the results are often not available until after the patient has cleared her infection and left the hospital. However, I would obtain blood cultures in patients who meet one of these criteria1,2:
- They are immunocompromised (eg, HIV infection).
- They have a cardiac or vascular prosthesis and, thus, are at increased risk of complications related to bacteremia.
- They seem critically ill at the onset of evaluation.
- They fail to respond appropriately to initial therapy.
The cornerstone of therapy is broad spectrum antibiotics that target the multiple organisms responsible for endometritis.3 There are several single agents and several combination antibiotic regimens that provide excellent coverage against the usual pelvic pathogens (TABLE 2). I personally favor the generic combination regimen (clindamycin plus gentamicin) because it is relatively inexpensive and has been very well validated in multiple studies. In patients who have underlying renal dysfunction, aztreonam can be substituted for gentamicin.
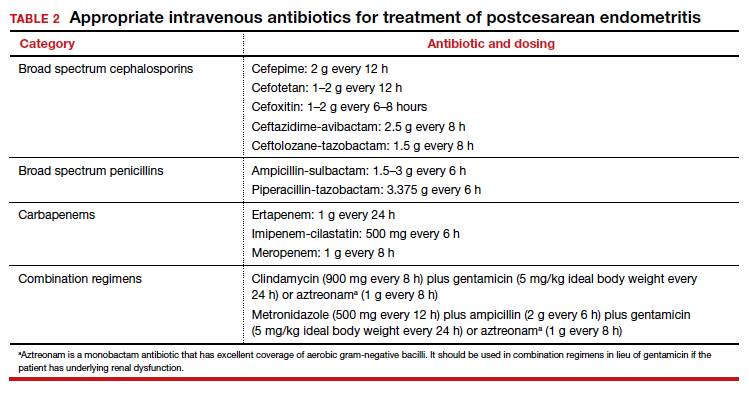
Approximately 90% of patients will show clear evidence of clinical improvement (ie, decrease in temperature and resolution of abdominopelvic pain) within 48 hours of starting antibiotics. Patients should then continue therapy until they have been afebrile and asymptomatic for approximately 24 hours. At that point, antibiotics should be discontinued, and the patient can be discharged. With rare exceptions, there is no indication for administration of oral antibiotics on an outpatient basis.1,4
Continue to: Persistent postoperative fever...
Persistent postoperative fever
Resistant microorganism
The most common cause of a persistent fever after initiating antibiotic therapy is a resistant microorganism. There are potential gaps in coverage for the antibiotic regimens commonly used to treat postcesarean endometritis (TABLE 3).1,4 Assuming there is no other obvious cause for treatment failure, I recommend that therapy be changed to the triple combination of metronidazole plus ampicillin plus gentamicin (or aztreonam). The first drug provides superb coverage against anaerobes; the second covers enterococci. Gentamicin or aztreonam cover virtually all aerobic Gram-negative bacilli likely to cause postcesarean infection. I prefer metronidazole rather than clindamycin in this regimen because, unlike clindamycin, it is less likely to trigger diarrhea when used in combination with ampicillin. The 3-drug regimen should be continued until the patient has been afebrile and asymptomatic for approximately 24 hours.1,3,4
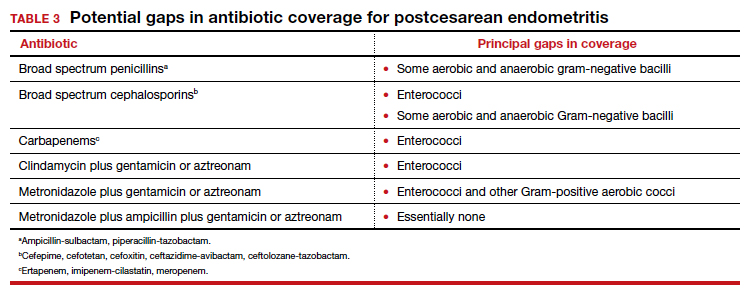
Wound infection
The second most common reason for a poor response to initial antibiotic therapy is a wound (surgical site) infection. Wound infections are caused by many of the same pelvic pathogens responsible for endometritis combined with skin flora, notably Streptococcus and Staphylococcus species, including methicillin-resistant Staphylococcus aureus (MRSA).1,4
Wound infections typically take one of two forms. The first is an actual incisional abscess. The patient is febrile; the margins of the wound are warm, indurated, erythematous, and tender; and purulent material drains from the incision. In this situation, the wound should be opened widely to drain the purulent collection. The fascia should then be probed to be certain that dehiscence has not occurred. In addition, intravenous vancomycin (1 g every 12 h) should be included in the antibiotic regimen to ensure adequate coverage of hospital-acquired MRSA.1,4
The second common presentation of a wound infection is cellulitis. The patient is febrile, and there is a spreading area of erythema, warmth, and exquisite tenderness extending from the edges of the incision; however, no purulent drainage is apparent. In this second scenario, the wound should not be opened, but intravenous vancomycin should be added to the treatment regimen.1,3,4
A third and very rare form of wound infection is necrotizing fasciitis. In affected patients, the margins of the wound are darkened and necrotic rather than erythematous and indurated. Two other key physical findings are crepitance and loss of sensation along the margins of the wound. Necrotizing fasciitis is truly a life-threatening emergency and requires immediate and extensive debridement of the devitalized tissue, combined with broad spectrum therapy with antibiotics that provide excellent coverage against anaerobes, aerobic streptococci (particularly group A streptococci), and staphylococci. The requirement for debridement may be so extensive that a skin graft subsequently is necessary to close the defect.1,4
Continue to: Unusual causes of persistent postoperative fever...
Unusual causes of persistent postoperative fever
If a resistant microorganism and wound infection can be excluded, the clinician then must begin a diligent search for “zebras” (ie, uncommon but potentially serious causes of persistent fever).1,4 One possible cause is a pelvic abscess. These purulent collections typically form in the retrovesicle space as a result of infection of a hematoma that formed between the posterior bladder wall and the lower uterine segment, in the leaves of the broad ligament, or in the posterior cul-de-sac. The abscess may or may not be palpable. The patient’s peripheral white blood cell count usually is elevated, with a preponderance of neutrophils. The best imaging test for an abscess is a computed tomography (CT) scan. Abscesses require drainage, which usually can be accomplished by insertion of a percutaneous drain under ultrasonographic or CT guidance.
A second unusual cause of persistent fever is septic pelvic vein thrombophlebitis. The infected venous emboli usually are present in the ovarian veins, with the right side predominant. The patient’s peripheral white blood cell count usually is elevated, and the infected clots are best imaged by CT scan with contrast or magnetic resonance angiography. The appropriate treatment is continuation of broad-spectrum antibiotics and administration of therapeutic doses of parenteral anticoagulants such as enoxaparin or unfractionated heparin.
A third explanation for persistent fever is retained products of conception. This diagnosis is best made by ultrasonography. The placental fragments should be removed by sharp curettage.
A fourth consideration when evaluating the patient with persistent fever is an allergic drug reaction. In most instances, the increase in the patient’s temperature will correspond with administration of the offending antibiotic(s). Affected patients typically have an increased number of eosinophils in their peripheral white blood cell count. The appropriate management of drug fever is discontinuation of antibiotics.
A final and distinctly unusual consideration is recrudescence of a connective tissue disorder such as systemic lupus erythematosus. The best test to confirm this diagnosis is the serum complement assay, which will demonstrate a decreased serum concentration of complement, reflecting consumption of this serum protein during the inflammatory process. The correct management for this condition is administration of a short course of systemic glucocorticoids. TABLE 4 summarizes a simple, systematic plan for evaluation of the patient with a persistent postoperative fever.
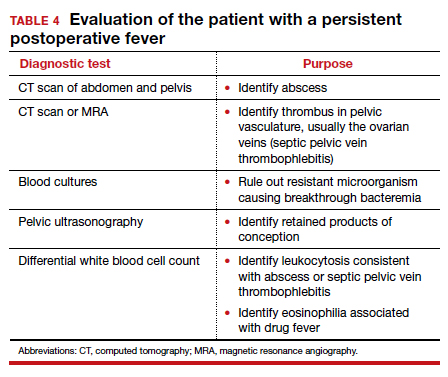
Preventive measures
We all remember the simple but profound statement by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” That folksy adage rings true with respect to postoperative infection because this complication extends hospital stay, increases hospital expense, and causes considerable discomfort and inconvenience for the patient. Therefore, we would do well to prevent as many instances of postoperative infection as possible.
Endometritis
On the basis of well-designed, prospective, randomized trials (Level 1 evidence), 3 interventions have proven effective in reducing the frequency of postcesarean endometritis. The first is irrigation of the vaginal canal preoperatively with an iodophor solution.5,6 The second is preoperative administration of systemic antibiotics.7-9 The combination of cefazolin (2 g IV within 30 minutes of incision) plus azithromycin (500 mg IV over 1 hour prior to incision) is superior to cefazolin alone.10,11 The third important preventive measure is removing the placenta by traction on the umbilical cord rather than by manual extraction.12,13
Wound infection
Several interventions are of proven effectiveness in reducing the frequency of postcesarean wound (surgical site) infection. The first is removal of hair at the incision site by clipping rather than by shaving (Level 2 evidence).14 The second is cleansing of the skin with chlorhexidine rather than iodophor (Level 1 evidence).15 The third is closing of the deep subcutaneous layer of the incision if it exceeds 2 cm in depth (Level 1 evidence).16,17 The fourth is closure of the skin with subcutaneous sutures rather than staples (Level 1 evidence).18 The monofilament suture poliglecaprone 25 is superior to the multifilament suture polyglactin 910 for this purpose (Level 1 evidence).19 Finally, in obese patients (body mass index >30 kg/m2), application of a negative pressure wound vacuum dressing may offer additional protection against infection (Level 1 evidence).20 Such dressings are too expensive, however, to be used routinely in all patients.
Urinary tract infection
The most important measures for preventing postoperative UTIs are identifying and clearing asymptomatic bacteriuria prior to delivery, inserting the urinary catheter prior to surgery using strict sterile technique, and removing the catheter as soon as possible after surgery, ideally within 12 hours.1,4
CASE Resolved
The 2 most likely causes for this patient’s poor response to initial therapy are resistant microorganism and wound infection. If a wound infection can be excluded by physical examination, the patient’s antibiotic regimen should be changed to metronidazole plus ampicillin plus gentamicin (or aztreonam). If an incisional abscess is identified, the incision should be opened and drained, and vancomycin should be added to the treatment regimen. If a wound cellulitis is evident, the incision should not be opened, but vancomycin should be added to the treatment regimen to enhance coverage against aerobic Streptococcus and Staphylococcus species. ●
- Duff WP. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2020:1124-1146.
- Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111-114.
- Duff P. Antibiotic selection in obstetric patients. Infect Dis Clin N Am. 1997;11:1-12.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams, JD, et al, eds. Creasy & Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2017;130:527-538.
- Sullivan SA, Smith T, Change E, et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity; a randomized controlled trial. Am J Obstet Gynecol. 2007;196:455.e1-455.e5.
- Tita ATN, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
- Tita ATN, Owen J, Stamm AM, et al. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199: 303.e1-303.e3.
- Tita ATN, Szchowski JM, Boggess K, et al. Two antibiotics before cesarean delivery reduce infection rates further than one agent. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Lasley DS, Eblen A, Yancey MK, et al. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176:1250-1254.
- Anorlu RI, Maholwana B, Hofmeyr GJ. Methods of delivering the placenta at cesarean section. Cochrane Database Syst Rev. 2008;3:CD004737.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-209.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:657-665.
- Del Valle GO, Combs P, Qualls C, et al. Does closure of camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
- Chelmow D. Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103:974-980.
- Tuuli MG, Rampersod RM, Carbone JF, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2011;117:682-690.
- Buresch AM, Arsdale AV, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery. a randomized controlled trial. Obstet Gynecol. 2017;130:521-526.
- Yu L, Kronen RJ, Simon LE, et al. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:200-210.
CASE Woman who has undergone recent cesarean delivery
A 23-year-old woman had a primary cesarean delivery 72 hours ago due to an arrest of dilation at 6 cm. She was in labor for 22 hours, and her membranes were ruptured for 18 hours. She had 10 internal vaginal examinations, and the duration of internal fetal monitoring was 12 hours; 24 hours after delivery, she developed a fever of 39°C, in association with lower abdominal pain and tenderness. She was presumptively treated for endometritis with cefepime; 48 hours after the initiation of antibiotics, she remains febrile and symptomatic.
- What are the most likely causes of her persistent fever?
- What should be the next steps in her evaluation?
Cesarean delivery background
Cesarean delivery is now the most common major operation performed in US hospitals. Cesarean delivery rates hover between 25% and 30% in most medical centers in the United States.1 The most common postoperative complication of cesarean delivery is infection. Infection typically takes 1 of 3 forms: endometritis (organ space infection), wound infection (surgical site infection), and urinary tract infection (UTI).1 This article will review the initial differential diagnosis, evaluation, and management of the patient with a postoperative fever and also will describe the appropriate assessment and treatment of the patient who has a persistent postoperative fever despite therapy. The article will also highlight key interventions that help to prevent postoperative infections.
Initial evaluation of the febrile patient
In the first 24 to 48 hours after cesarean delivery, the most common cause of fever is endometritis (organ space infection). This condition is a polymicrobial, mixed aerobic-anaerobic infection (FIGURE). The principal pathogens include anaerobic gram-positive cocci (

The major risk factors for postcesarean endometritis are extended duration of labor and ruptured membranes, multiple internal vaginal examinations, invasive fetal monitoring, and pre-existing colonization with group B Streptococcus and/or the organisms that cause bacterial vaginosis. Affected patients typically have a fever in the range of 38 to 39°C, tachycardia, mild tachypnea, lower abdominal pain and tenderness, and purulent lochia in some individuals.1
Differential for postoperative fever
The initial differential diagnosis of postoperative fever is relatively limited (TABLE 1). In addition to endometritis, it includes extensive atelectasis, perhaps resulting from general anesthesia; lower respiratory tract infection, either viral influenza or bacterial pneumonia; and acute pyelonephritis. A simple infection of the bladder (cystitis or asymptomatic bacteriuria) should not cause a substantial temperature elevation and systemic symptoms.1

Differentiation between these entities usually is possible based on physical examination and a few laboratory tests. The peripheral white blood cell count usually is elevated, and a left shift may be evident. If a respiratory tract infection is suspected, chest radiography is indicated. A urine culture should be obtained if acute pyelonephritis strongly is considered. Lower genital tract cultures are rarely of value, and uncontaminated upper tract cultures are difficult to obtain. I do not believe that blood cultures should be performed as a matter of routine. They are expensive, and the results are often not available until after the patient has cleared her infection and left the hospital. However, I would obtain blood cultures in patients who meet one of these criteria1,2:
- They are immunocompromised (eg, HIV infection).
- They have a cardiac or vascular prosthesis and, thus, are at increased risk of complications related to bacteremia.
- They seem critically ill at the onset of evaluation.
- They fail to respond appropriately to initial therapy.
The cornerstone of therapy is broad spectrum antibiotics that target the multiple organisms responsible for endometritis.3 There are several single agents and several combination antibiotic regimens that provide excellent coverage against the usual pelvic pathogens (TABLE 2). I personally favor the generic combination regimen (clindamycin plus gentamicin) because it is relatively inexpensive and has been very well validated in multiple studies. In patients who have underlying renal dysfunction, aztreonam can be substituted for gentamicin.

Approximately 90% of patients will show clear evidence of clinical improvement (ie, decrease in temperature and resolution of abdominopelvic pain) within 48 hours of starting antibiotics. Patients should then continue therapy until they have been afebrile and asymptomatic for approximately 24 hours. At that point, antibiotics should be discontinued, and the patient can be discharged. With rare exceptions, there is no indication for administration of oral antibiotics on an outpatient basis.1,4
Continue to: Persistent postoperative fever...
Persistent postoperative fever
Resistant microorganism
The most common cause of a persistent fever after initiating antibiotic therapy is a resistant microorganism. There are potential gaps in coverage for the antibiotic regimens commonly used to treat postcesarean endometritis (TABLE 3).1,4 Assuming there is no other obvious cause for treatment failure, I recommend that therapy be changed to the triple combination of metronidazole plus ampicillin plus gentamicin (or aztreonam). The first drug provides superb coverage against anaerobes; the second covers enterococci. Gentamicin or aztreonam cover virtually all aerobic Gram-negative bacilli likely to cause postcesarean infection. I prefer metronidazole rather than clindamycin in this regimen because, unlike clindamycin, it is less likely to trigger diarrhea when used in combination with ampicillin. The 3-drug regimen should be continued until the patient has been afebrile and asymptomatic for approximately 24 hours.1,3,4

Wound infection
The second most common reason for a poor response to initial antibiotic therapy is a wound (surgical site) infection. Wound infections are caused by many of the same pelvic pathogens responsible for endometritis combined with skin flora, notably Streptococcus and Staphylococcus species, including methicillin-resistant Staphylococcus aureus (MRSA).1,4
Wound infections typically take one of two forms. The first is an actual incisional abscess. The patient is febrile; the margins of the wound are warm, indurated, erythematous, and tender; and purulent material drains from the incision. In this situation, the wound should be opened widely to drain the purulent collection. The fascia should then be probed to be certain that dehiscence has not occurred. In addition, intravenous vancomycin (1 g every 12 h) should be included in the antibiotic regimen to ensure adequate coverage of hospital-acquired MRSA.1,4
The second common presentation of a wound infection is cellulitis. The patient is febrile, and there is a spreading area of erythema, warmth, and exquisite tenderness extending from the edges of the incision; however, no purulent drainage is apparent. In this second scenario, the wound should not be opened, but intravenous vancomycin should be added to the treatment regimen.1,3,4
A third and very rare form of wound infection is necrotizing fasciitis. In affected patients, the margins of the wound are darkened and necrotic rather than erythematous and indurated. Two other key physical findings are crepitance and loss of sensation along the margins of the wound. Necrotizing fasciitis is truly a life-threatening emergency and requires immediate and extensive debridement of the devitalized tissue, combined with broad spectrum therapy with antibiotics that provide excellent coverage against anaerobes, aerobic streptococci (particularly group A streptococci), and staphylococci. The requirement for debridement may be so extensive that a skin graft subsequently is necessary to close the defect.1,4
Continue to: Unusual causes of persistent postoperative fever...
Unusual causes of persistent postoperative fever
If a resistant microorganism and wound infection can be excluded, the clinician then must begin a diligent search for “zebras” (ie, uncommon but potentially serious causes of persistent fever).1,4 One possible cause is a pelvic abscess. These purulent collections typically form in the retrovesicle space as a result of infection of a hematoma that formed between the posterior bladder wall and the lower uterine segment, in the leaves of the broad ligament, or in the posterior cul-de-sac. The abscess may or may not be palpable. The patient’s peripheral white blood cell count usually is elevated, with a preponderance of neutrophils. The best imaging test for an abscess is a computed tomography (CT) scan. Abscesses require drainage, which usually can be accomplished by insertion of a percutaneous drain under ultrasonographic or CT guidance.
A second unusual cause of persistent fever is septic pelvic vein thrombophlebitis. The infected venous emboli usually are present in the ovarian veins, with the right side predominant. The patient’s peripheral white blood cell count usually is elevated, and the infected clots are best imaged by CT scan with contrast or magnetic resonance angiography. The appropriate treatment is continuation of broad-spectrum antibiotics and administration of therapeutic doses of parenteral anticoagulants such as enoxaparin or unfractionated heparin.
A third explanation for persistent fever is retained products of conception. This diagnosis is best made by ultrasonography. The placental fragments should be removed by sharp curettage.
A fourth consideration when evaluating the patient with persistent fever is an allergic drug reaction. In most instances, the increase in the patient’s temperature will correspond with administration of the offending antibiotic(s). Affected patients typically have an increased number of eosinophils in their peripheral white blood cell count. The appropriate management of drug fever is discontinuation of antibiotics.
A final and distinctly unusual consideration is recrudescence of a connective tissue disorder such as systemic lupus erythematosus. The best test to confirm this diagnosis is the serum complement assay, which will demonstrate a decreased serum concentration of complement, reflecting consumption of this serum protein during the inflammatory process. The correct management for this condition is administration of a short course of systemic glucocorticoids. TABLE 4 summarizes a simple, systematic plan for evaluation of the patient with a persistent postoperative fever.

Preventive measures
We all remember the simple but profound statement by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” That folksy adage rings true with respect to postoperative infection because this complication extends hospital stay, increases hospital expense, and causes considerable discomfort and inconvenience for the patient. Therefore, we would do well to prevent as many instances of postoperative infection as possible.
Endometritis
On the basis of well-designed, prospective, randomized trials (Level 1 evidence), 3 interventions have proven effective in reducing the frequency of postcesarean endometritis. The first is irrigation of the vaginal canal preoperatively with an iodophor solution.5,6 The second is preoperative administration of systemic antibiotics.7-9 The combination of cefazolin (2 g IV within 30 minutes of incision) plus azithromycin (500 mg IV over 1 hour prior to incision) is superior to cefazolin alone.10,11 The third important preventive measure is removing the placenta by traction on the umbilical cord rather than by manual extraction.12,13
Wound infection
Several interventions are of proven effectiveness in reducing the frequency of postcesarean wound (surgical site) infection. The first is removal of hair at the incision site by clipping rather than by shaving (Level 2 evidence).14 The second is cleansing of the skin with chlorhexidine rather than iodophor (Level 1 evidence).15 The third is closing of the deep subcutaneous layer of the incision if it exceeds 2 cm in depth (Level 1 evidence).16,17 The fourth is closure of the skin with subcutaneous sutures rather than staples (Level 1 evidence).18 The monofilament suture poliglecaprone 25 is superior to the multifilament suture polyglactin 910 for this purpose (Level 1 evidence).19 Finally, in obese patients (body mass index >30 kg/m2), application of a negative pressure wound vacuum dressing may offer additional protection against infection (Level 1 evidence).20 Such dressings are too expensive, however, to be used routinely in all patients.
Urinary tract infection
The most important measures for preventing postoperative UTIs are identifying and clearing asymptomatic bacteriuria prior to delivery, inserting the urinary catheter prior to surgery using strict sterile technique, and removing the catheter as soon as possible after surgery, ideally within 12 hours.1,4
CASE Resolved
The 2 most likely causes for this patient’s poor response to initial therapy are resistant microorganism and wound infection. If a wound infection can be excluded by physical examination, the patient’s antibiotic regimen should be changed to metronidazole plus ampicillin plus gentamicin (or aztreonam). If an incisional abscess is identified, the incision should be opened and drained, and vancomycin should be added to the treatment regimen. If a wound cellulitis is evident, the incision should not be opened, but vancomycin should be added to the treatment regimen to enhance coverage against aerobic Streptococcus and Staphylococcus species. ●
CASE Woman who has undergone recent cesarean delivery
A 23-year-old woman had a primary cesarean delivery 72 hours ago due to an arrest of dilation at 6 cm. She was in labor for 22 hours, and her membranes were ruptured for 18 hours. She had 10 internal vaginal examinations, and the duration of internal fetal monitoring was 12 hours; 24 hours after delivery, she developed a fever of 39°C, in association with lower abdominal pain and tenderness. She was presumptively treated for endometritis with cefepime; 48 hours after the initiation of antibiotics, she remains febrile and symptomatic.
- What are the most likely causes of her persistent fever?
- What should be the next steps in her evaluation?
Cesarean delivery background
Cesarean delivery is now the most common major operation performed in US hospitals. Cesarean delivery rates hover between 25% and 30% in most medical centers in the United States.1 The most common postoperative complication of cesarean delivery is infection. Infection typically takes 1 of 3 forms: endometritis (organ space infection), wound infection (surgical site infection), and urinary tract infection (UTI).1 This article will review the initial differential diagnosis, evaluation, and management of the patient with a postoperative fever and also will describe the appropriate assessment and treatment of the patient who has a persistent postoperative fever despite therapy. The article will also highlight key interventions that help to prevent postoperative infections.
Initial evaluation of the febrile patient
In the first 24 to 48 hours after cesarean delivery, the most common cause of fever is endometritis (organ space infection). This condition is a polymicrobial, mixed aerobic-anaerobic infection (FIGURE). The principal pathogens include anaerobic gram-positive cocci (

The major risk factors for postcesarean endometritis are extended duration of labor and ruptured membranes, multiple internal vaginal examinations, invasive fetal monitoring, and pre-existing colonization with group B Streptococcus and/or the organisms that cause bacterial vaginosis. Affected patients typically have a fever in the range of 38 to 39°C, tachycardia, mild tachypnea, lower abdominal pain and tenderness, and purulent lochia in some individuals.1
Differential for postoperative fever
The initial differential diagnosis of postoperative fever is relatively limited (TABLE 1). In addition to endometritis, it includes extensive atelectasis, perhaps resulting from general anesthesia; lower respiratory tract infection, either viral influenza or bacterial pneumonia; and acute pyelonephritis. A simple infection of the bladder (cystitis or asymptomatic bacteriuria) should not cause a substantial temperature elevation and systemic symptoms.1

Differentiation between these entities usually is possible based on physical examination and a few laboratory tests. The peripheral white blood cell count usually is elevated, and a left shift may be evident. If a respiratory tract infection is suspected, chest radiography is indicated. A urine culture should be obtained if acute pyelonephritis strongly is considered. Lower genital tract cultures are rarely of value, and uncontaminated upper tract cultures are difficult to obtain. I do not believe that blood cultures should be performed as a matter of routine. They are expensive, and the results are often not available until after the patient has cleared her infection and left the hospital. However, I would obtain blood cultures in patients who meet one of these criteria1,2:
- They are immunocompromised (eg, HIV infection).
- They have a cardiac or vascular prosthesis and, thus, are at increased risk of complications related to bacteremia.
- They seem critically ill at the onset of evaluation.
- They fail to respond appropriately to initial therapy.
The cornerstone of therapy is broad spectrum antibiotics that target the multiple organisms responsible for endometritis.3 There are several single agents and several combination antibiotic regimens that provide excellent coverage against the usual pelvic pathogens (TABLE 2). I personally favor the generic combination regimen (clindamycin plus gentamicin) because it is relatively inexpensive and has been very well validated in multiple studies. In patients who have underlying renal dysfunction, aztreonam can be substituted for gentamicin.

Approximately 90% of patients will show clear evidence of clinical improvement (ie, decrease in temperature and resolution of abdominopelvic pain) within 48 hours of starting antibiotics. Patients should then continue therapy until they have been afebrile and asymptomatic for approximately 24 hours. At that point, antibiotics should be discontinued, and the patient can be discharged. With rare exceptions, there is no indication for administration of oral antibiotics on an outpatient basis.1,4
Continue to: Persistent postoperative fever...
Persistent postoperative fever
Resistant microorganism
The most common cause of a persistent fever after initiating antibiotic therapy is a resistant microorganism. There are potential gaps in coverage for the antibiotic regimens commonly used to treat postcesarean endometritis (TABLE 3).1,4 Assuming there is no other obvious cause for treatment failure, I recommend that therapy be changed to the triple combination of metronidazole plus ampicillin plus gentamicin (or aztreonam). The first drug provides superb coverage against anaerobes; the second covers enterococci. Gentamicin or aztreonam cover virtually all aerobic Gram-negative bacilli likely to cause postcesarean infection. I prefer metronidazole rather than clindamycin in this regimen because, unlike clindamycin, it is less likely to trigger diarrhea when used in combination with ampicillin. The 3-drug regimen should be continued until the patient has been afebrile and asymptomatic for approximately 24 hours.1,3,4

Wound infection
The second most common reason for a poor response to initial antibiotic therapy is a wound (surgical site) infection. Wound infections are caused by many of the same pelvic pathogens responsible for endometritis combined with skin flora, notably Streptococcus and Staphylococcus species, including methicillin-resistant Staphylococcus aureus (MRSA).1,4
Wound infections typically take one of two forms. The first is an actual incisional abscess. The patient is febrile; the margins of the wound are warm, indurated, erythematous, and tender; and purulent material drains from the incision. In this situation, the wound should be opened widely to drain the purulent collection. The fascia should then be probed to be certain that dehiscence has not occurred. In addition, intravenous vancomycin (1 g every 12 h) should be included in the antibiotic regimen to ensure adequate coverage of hospital-acquired MRSA.1,4
The second common presentation of a wound infection is cellulitis. The patient is febrile, and there is a spreading area of erythema, warmth, and exquisite tenderness extending from the edges of the incision; however, no purulent drainage is apparent. In this second scenario, the wound should not be opened, but intravenous vancomycin should be added to the treatment regimen.1,3,4
A third and very rare form of wound infection is necrotizing fasciitis. In affected patients, the margins of the wound are darkened and necrotic rather than erythematous and indurated. Two other key physical findings are crepitance and loss of sensation along the margins of the wound. Necrotizing fasciitis is truly a life-threatening emergency and requires immediate and extensive debridement of the devitalized tissue, combined with broad spectrum therapy with antibiotics that provide excellent coverage against anaerobes, aerobic streptococci (particularly group A streptococci), and staphylococci. The requirement for debridement may be so extensive that a skin graft subsequently is necessary to close the defect.1,4
Continue to: Unusual causes of persistent postoperative fever...
Unusual causes of persistent postoperative fever
If a resistant microorganism and wound infection can be excluded, the clinician then must begin a diligent search for “zebras” (ie, uncommon but potentially serious causes of persistent fever).1,4 One possible cause is a pelvic abscess. These purulent collections typically form in the retrovesicle space as a result of infection of a hematoma that formed between the posterior bladder wall and the lower uterine segment, in the leaves of the broad ligament, or in the posterior cul-de-sac. The abscess may or may not be palpable. The patient’s peripheral white blood cell count usually is elevated, with a preponderance of neutrophils. The best imaging test for an abscess is a computed tomography (CT) scan. Abscesses require drainage, which usually can be accomplished by insertion of a percutaneous drain under ultrasonographic or CT guidance.
A second unusual cause of persistent fever is septic pelvic vein thrombophlebitis. The infected venous emboli usually are present in the ovarian veins, with the right side predominant. The patient’s peripheral white blood cell count usually is elevated, and the infected clots are best imaged by CT scan with contrast or magnetic resonance angiography. The appropriate treatment is continuation of broad-spectrum antibiotics and administration of therapeutic doses of parenteral anticoagulants such as enoxaparin or unfractionated heparin.
A third explanation for persistent fever is retained products of conception. This diagnosis is best made by ultrasonography. The placental fragments should be removed by sharp curettage.
A fourth consideration when evaluating the patient with persistent fever is an allergic drug reaction. In most instances, the increase in the patient’s temperature will correspond with administration of the offending antibiotic(s). Affected patients typically have an increased number of eosinophils in their peripheral white blood cell count. The appropriate management of drug fever is discontinuation of antibiotics.
A final and distinctly unusual consideration is recrudescence of a connective tissue disorder such as systemic lupus erythematosus. The best test to confirm this diagnosis is the serum complement assay, which will demonstrate a decreased serum concentration of complement, reflecting consumption of this serum protein during the inflammatory process. The correct management for this condition is administration of a short course of systemic glucocorticoids. TABLE 4 summarizes a simple, systematic plan for evaluation of the patient with a persistent postoperative fever.

Preventive measures
We all remember the simple but profound statement by Benjamin Franklin, “An ounce of prevention is worth a pound of cure.” That folksy adage rings true with respect to postoperative infection because this complication extends hospital stay, increases hospital expense, and causes considerable discomfort and inconvenience for the patient. Therefore, we would do well to prevent as many instances of postoperative infection as possible.
Endometritis
On the basis of well-designed, prospective, randomized trials (Level 1 evidence), 3 interventions have proven effective in reducing the frequency of postcesarean endometritis. The first is irrigation of the vaginal canal preoperatively with an iodophor solution.5,6 The second is preoperative administration of systemic antibiotics.7-9 The combination of cefazolin (2 g IV within 30 minutes of incision) plus azithromycin (500 mg IV over 1 hour prior to incision) is superior to cefazolin alone.10,11 The third important preventive measure is removing the placenta by traction on the umbilical cord rather than by manual extraction.12,13
Wound infection
Several interventions are of proven effectiveness in reducing the frequency of postcesarean wound (surgical site) infection. The first is removal of hair at the incision site by clipping rather than by shaving (Level 2 evidence).14 The second is cleansing of the skin with chlorhexidine rather than iodophor (Level 1 evidence).15 The third is closing of the deep subcutaneous layer of the incision if it exceeds 2 cm in depth (Level 1 evidence).16,17 The fourth is closure of the skin with subcutaneous sutures rather than staples (Level 1 evidence).18 The monofilament suture poliglecaprone 25 is superior to the multifilament suture polyglactin 910 for this purpose (Level 1 evidence).19 Finally, in obese patients (body mass index >30 kg/m2), application of a negative pressure wound vacuum dressing may offer additional protection against infection (Level 1 evidence).20 Such dressings are too expensive, however, to be used routinely in all patients.
Urinary tract infection
The most important measures for preventing postoperative UTIs are identifying and clearing asymptomatic bacteriuria prior to delivery, inserting the urinary catheter prior to surgery using strict sterile technique, and removing the catheter as soon as possible after surgery, ideally within 12 hours.1,4
CASE Resolved
The 2 most likely causes for this patient’s poor response to initial therapy are resistant microorganism and wound infection. If a wound infection can be excluded by physical examination, the patient’s antibiotic regimen should be changed to metronidazole plus ampicillin plus gentamicin (or aztreonam). If an incisional abscess is identified, the incision should be opened and drained, and vancomycin should be added to the treatment regimen. If a wound cellulitis is evident, the incision should not be opened, but vancomycin should be added to the treatment regimen to enhance coverage against aerobic Streptococcus and Staphylococcus species. ●
- Duff WP. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2020:1124-1146.
- Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111-114.
- Duff P. Antibiotic selection in obstetric patients. Infect Dis Clin N Am. 1997;11:1-12.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams, JD, et al, eds. Creasy & Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2017;130:527-538.
- Sullivan SA, Smith T, Change E, et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity; a randomized controlled trial. Am J Obstet Gynecol. 2007;196:455.e1-455.e5.
- Tita ATN, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
- Tita ATN, Owen J, Stamm AM, et al. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199: 303.e1-303.e3.
- Tita ATN, Szchowski JM, Boggess K, et al. Two antibiotics before cesarean delivery reduce infection rates further than one agent. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Lasley DS, Eblen A, Yancey MK, et al. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176:1250-1254.
- Anorlu RI, Maholwana B, Hofmeyr GJ. Methods of delivering the placenta at cesarean section. Cochrane Database Syst Rev. 2008;3:CD004737.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-209.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:657-665.
- Del Valle GO, Combs P, Qualls C, et al. Does closure of camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
- Chelmow D. Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103:974-980.
- Tuuli MG, Rampersod RM, Carbone JF, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2011;117:682-690.
- Buresch AM, Arsdale AV, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery. a randomized controlled trial. Obstet Gynecol. 2017;130:521-526.
- Yu L, Kronen RJ, Simon LE, et al. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:200-210.
- Duff WP. Maternal and perinatal infection in pregnancy: bacterial. In: Landon MB, Galan HL, Jauniaux ERM, et al, eds. Gabbe’s Obstetrics: Normal and Problem Pregnancies. 8th ed. Elsevier; 2020:1124-1146.
- Locksmith GJ, Duff P. Assessment of the value of routine blood cultures in the evaluation and treatment of patients with chorioamnionitis. Infect Dis Obstet Gynecol. 1994;2:111-114.
- Duff P. Antibiotic selection in obstetric patients. Infect Dis Clin N Am. 1997;11:1-12.
- Duff P. Maternal and fetal infections. In: Creasy RK, Resnik R, Iams, JD, et al, eds. Creasy & Resnik’s Maternal Fetal Medicine: Principles and Practice. 8th ed. Elsevier; 2019:862-919.
- Haas DM, Morgan S, Contreras K. Vaginal preparation with antiseptic solution before cesarean section for preventing postoperative infections. Cochrane Database Syst Rev. 2014;12:CD007892.
- Caissutti C, Saccone G, Zullo F, et al. Vaginal cleansing before cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2017;130:527-538.
- Sullivan SA, Smith T, Change E, et al. Administration of cefazolin prior to skin incision is superior to cefazolin at cord clamping in preventing postcesarean infectious morbidity; a randomized controlled trial. Am J Obstet Gynecol. 2007;196:455.e1-455.e5.
- Tita ATN, Hauth JC, Grimes A, et al. Decreasing incidence of postcesarean endometritis with extended-spectrum antibiotic prophylaxis. Obstet Gynecol. 2008;111:51-56.
- Tita ATN, Owen J, Stamm AM, et al. Impact of extended-spectrum antibiotic prophylaxis on incidence of postcesarean surgical wound infection. Am J Obstet Gynecol. 2008;199: 303.e1-303.e3.
- Tita ATN, Szchowski JM, Boggess K, et al. Two antibiotics before cesarean delivery reduce infection rates further than one agent. N Engl J Med. 2016;375:1231-1241.
- Harper LM, Kilgore M, Szychowski JM, et al. Economic evaluation of adjunctive azithromycin prophylaxis for cesarean delivery. Obstet Gynecol. 2017;130:328-334.
- Lasley DS, Eblen A, Yancey MK, et al. The effect of placental removal method on the incidence of postcesarean infections. Am J Obstet Gynecol. 1997;176:1250-1254.
- Anorlu RI, Maholwana B, Hofmeyr GJ. Methods of delivering the placenta at cesarean section. Cochrane Database Syst Rev. 2008;3:CD004737.
- Cruse PJ, Foord R. A five-year prospective study of 23,649 surgical wounds. Arch Surg. 1973;107:206-209.
- Tuuli MG, Liu J, Stout MJ, et al. A randomized trial comparing skin antiseptic agents at cesarean delivery. N Engl J Med. 2016;374:657-665.
- Del Valle GO, Combs P, Qualls C, et al. Does closure of camper fascia reduce the incidence of post-cesarean superficial wound disruption? Obstet Gynecol. 1992;80:1013-1016.
- Chelmow D. Rodriguez EJ, Sabatini MM. Suture closure of subcutaneous fat and wound disruption after cesarean delivery: a meta-analysis. Obstet Gynecol. 2004;103:974-980.
- Tuuli MG, Rampersod RM, Carbone JF, et al. Staples compared with subcuticular suture for skin closure after cesarean delivery. a systematic review and meta-analysis. Obstet Gynecol. 2011;117:682-690.
- Buresch AM, Arsdale AV, Ferzli M, et al. Comparison of subcuticular suture type for skin closure after cesarean delivery. a randomized controlled trial. Obstet Gynecol. 2017;130:521-526.
- Yu L, Kronen RJ, Simon LE, et al. Prophylactic negative-pressure wound therapy after cesarean is associated with reduced risk of surgical site infection: a systematic review and meta-analysis. Am J Obstet Gynecol. 2018;218:200-210.
Does prophylactic manual rotation of OP and OT positions in early second stage of labor decrease operative vaginal and/or CDs?
Blanc J, Castel P, Mauviel F, et al. Prophylactic manual rotation of occiput posterior and transverse positions to decrease operative delivery: the PROPOP randomized clinical trial. Am J Obstet Gynecol. 2021;225:444.e1-444.e8. doi: 10.1016/j.ajog.2021.05.020.
EXPERT COMMENTARY
Occiput posterior or occiput transverse positions are reported at a rate of 20% in labor, with 5% persistent at the time of delivery. These lead to a higher risk of maternal complications, such as cesarean delivery (CD), prolonged second stage, severe perineal lacerations, postpartum hemorrhage, chorioamnionitis, and operative vaginal delivery.
Several options are available for rotation to occiput anterior (OA) to increase the likelihood of spontaneous delivery. These include instrument (which requires forceps or vacuum experience in rotation), maternal positioning changes, or manual rotation. Timing of manual rotation can be at full dilation (“prophylactic”) or at failure to progress (“therapeutic”), with the latter less likely to succeed.
Although the existing literature is somewhat limited, both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommend consideration of manual rotation to reduce the rate of operative delivery. A recent study by Blanc and colleagues sought to add to the evidence for the effectiveness of manual rotation in reducing operative delivery.
Details of the study
The multicenter, open-label, randomized clinical trial included 257 patients at 4 French hospitals (2 academic, 2 community). The 126 patients in the intervention group underwent a trial of prophylactic manual rotation, while the 131 in the standard group had no trial of prophylactic manual rotation. The study’s primary objective was to determine the effect of prophylactic manual rotation on operative delivery (vaginal or cesarean). The hypothesis was that manual rotation would decrease the risk of operative delivery.
The inclusion criteria were patients with a singleton pregnancy at more than 37 weeks, epidural anesthesia, and OP or OT presentation (confirmed by ultrasonography) in the early second stage of labor at diagnosis of full dilation. Manual rotation was attempted using the previously described Tarnier and Chantreiul technique, and all investigators were trained in this technique at the beginning of the study using a mannequin.
The primary outcome was vaginal or cesarean operative delivery. Secondary outcomes included length of the second stage of labor as well as maternal and neonatal complications.
Results. The intervention group had a significantly lower rate of operative delivery (29.4%) compared with the standard group (41.2%). Length of the second stage was also lower in the intervention group (146.7 minutes) compared with that of the standard group (164.4 minutes). The 5-minute Apgar score was reported as significantly higher in the intervention group as well (9.8 vs 9.6). There were no other differences between the groups in either maternal or neonatal complications.
Study strengths and limitations
The strengths of this study included randomization and no loss to follow-up. The 4 different study sites with different levels of care and acuity added to the generalizability of the results. Given the potential for inaccuracy of digital exam for fetal head positioning, the use of ultrasonography for confirmation of the OP or OT position is a study strength. Additional strengths are the prestudy training in the maneuver using simulation and the high level of success in the rotations (89.7%).
The study’s main limitation is that it was not double blinded; therefore, bias in management was a possibility. Additionally, the study looked only at short-term outcomes for the delivery itself and not at the potential long-term pelvic floor outcomes. The authors reported that the study was underpowered for operative vaginal delivery and cesarean delivery separately, as well as the secondary outcomes. Other limitations were the high frequency of operative vaginal delivery, low rate of consent for the study, and lack of patient satisfaction data. ●
In this study, a trial of prophylactic manual rotation of the occiput posterior or occiput transverse presentation decreased the rate of operative delivery and reduced the length of the second stage of labor without differences in maternal or neonatal complications. Obstetrical providers should consider this strategy to resolve the OP or OT presentation prior to performing an operative vaginal delivery or cesarean delivery. Simulation training in this maneuver may be a useful adjunct for both trainees and providers unfamiliar with the procedure.
JAIMEY M. PAULI, MD
Blanc J, Castel P, Mauviel F, et al. Prophylactic manual rotation of occiput posterior and transverse positions to decrease operative delivery: the PROPOP randomized clinical trial. Am J Obstet Gynecol. 2021;225:444.e1-444.e8. doi: 10.1016/j.ajog.2021.05.020.
EXPERT COMMENTARY
Occiput posterior or occiput transverse positions are reported at a rate of 20% in labor, with 5% persistent at the time of delivery. These lead to a higher risk of maternal complications, such as cesarean delivery (CD), prolonged second stage, severe perineal lacerations, postpartum hemorrhage, chorioamnionitis, and operative vaginal delivery.
Several options are available for rotation to occiput anterior (OA) to increase the likelihood of spontaneous delivery. These include instrument (which requires forceps or vacuum experience in rotation), maternal positioning changes, or manual rotation. Timing of manual rotation can be at full dilation (“prophylactic”) or at failure to progress (“therapeutic”), with the latter less likely to succeed.
Although the existing literature is somewhat limited, both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommend consideration of manual rotation to reduce the rate of operative delivery. A recent study by Blanc and colleagues sought to add to the evidence for the effectiveness of manual rotation in reducing operative delivery.
Details of the study
The multicenter, open-label, randomized clinical trial included 257 patients at 4 French hospitals (2 academic, 2 community). The 126 patients in the intervention group underwent a trial of prophylactic manual rotation, while the 131 in the standard group had no trial of prophylactic manual rotation. The study’s primary objective was to determine the effect of prophylactic manual rotation on operative delivery (vaginal or cesarean). The hypothesis was that manual rotation would decrease the risk of operative delivery.
The inclusion criteria were patients with a singleton pregnancy at more than 37 weeks, epidural anesthesia, and OP or OT presentation (confirmed by ultrasonography) in the early second stage of labor at diagnosis of full dilation. Manual rotation was attempted using the previously described Tarnier and Chantreiul technique, and all investigators were trained in this technique at the beginning of the study using a mannequin.
The primary outcome was vaginal or cesarean operative delivery. Secondary outcomes included length of the second stage of labor as well as maternal and neonatal complications.
Results. The intervention group had a significantly lower rate of operative delivery (29.4%) compared with the standard group (41.2%). Length of the second stage was also lower in the intervention group (146.7 minutes) compared with that of the standard group (164.4 minutes). The 5-minute Apgar score was reported as significantly higher in the intervention group as well (9.8 vs 9.6). There were no other differences between the groups in either maternal or neonatal complications.
Study strengths and limitations
The strengths of this study included randomization and no loss to follow-up. The 4 different study sites with different levels of care and acuity added to the generalizability of the results. Given the potential for inaccuracy of digital exam for fetal head positioning, the use of ultrasonography for confirmation of the OP or OT position is a study strength. Additional strengths are the prestudy training in the maneuver using simulation and the high level of success in the rotations (89.7%).
The study’s main limitation is that it was not double blinded; therefore, bias in management was a possibility. Additionally, the study looked only at short-term outcomes for the delivery itself and not at the potential long-term pelvic floor outcomes. The authors reported that the study was underpowered for operative vaginal delivery and cesarean delivery separately, as well as the secondary outcomes. Other limitations were the high frequency of operative vaginal delivery, low rate of consent for the study, and lack of patient satisfaction data. ●
In this study, a trial of prophylactic manual rotation of the occiput posterior or occiput transverse presentation decreased the rate of operative delivery and reduced the length of the second stage of labor without differences in maternal or neonatal complications. Obstetrical providers should consider this strategy to resolve the OP or OT presentation prior to performing an operative vaginal delivery or cesarean delivery. Simulation training in this maneuver may be a useful adjunct for both trainees and providers unfamiliar with the procedure.
JAIMEY M. PAULI, MD
Blanc J, Castel P, Mauviel F, et al. Prophylactic manual rotation of occiput posterior and transverse positions to decrease operative delivery: the PROPOP randomized clinical trial. Am J Obstet Gynecol. 2021;225:444.e1-444.e8. doi: 10.1016/j.ajog.2021.05.020.
EXPERT COMMENTARY
Occiput posterior or occiput transverse positions are reported at a rate of 20% in labor, with 5% persistent at the time of delivery. These lead to a higher risk of maternal complications, such as cesarean delivery (CD), prolonged second stage, severe perineal lacerations, postpartum hemorrhage, chorioamnionitis, and operative vaginal delivery.
Several options are available for rotation to occiput anterior (OA) to increase the likelihood of spontaneous delivery. These include instrument (which requires forceps or vacuum experience in rotation), maternal positioning changes, or manual rotation. Timing of manual rotation can be at full dilation (“prophylactic”) or at failure to progress (“therapeutic”), with the latter less likely to succeed.
Although the existing literature is somewhat limited, both the American College of Obstetricians and Gynecologists and the Society for Maternal-Fetal Medicine recommend consideration of manual rotation to reduce the rate of operative delivery. A recent study by Blanc and colleagues sought to add to the evidence for the effectiveness of manual rotation in reducing operative delivery.
Details of the study
The multicenter, open-label, randomized clinical trial included 257 patients at 4 French hospitals (2 academic, 2 community). The 126 patients in the intervention group underwent a trial of prophylactic manual rotation, while the 131 in the standard group had no trial of prophylactic manual rotation. The study’s primary objective was to determine the effect of prophylactic manual rotation on operative delivery (vaginal or cesarean). The hypothesis was that manual rotation would decrease the risk of operative delivery.
The inclusion criteria were patients with a singleton pregnancy at more than 37 weeks, epidural anesthesia, and OP or OT presentation (confirmed by ultrasonography) in the early second stage of labor at diagnosis of full dilation. Manual rotation was attempted using the previously described Tarnier and Chantreiul technique, and all investigators were trained in this technique at the beginning of the study using a mannequin.
The primary outcome was vaginal or cesarean operative delivery. Secondary outcomes included length of the second stage of labor as well as maternal and neonatal complications.
Results. The intervention group had a significantly lower rate of operative delivery (29.4%) compared with the standard group (41.2%). Length of the second stage was also lower in the intervention group (146.7 minutes) compared with that of the standard group (164.4 minutes). The 5-minute Apgar score was reported as significantly higher in the intervention group as well (9.8 vs 9.6). There were no other differences between the groups in either maternal or neonatal complications.
Study strengths and limitations
The strengths of this study included randomization and no loss to follow-up. The 4 different study sites with different levels of care and acuity added to the generalizability of the results. Given the potential for inaccuracy of digital exam for fetal head positioning, the use of ultrasonography for confirmation of the OP or OT position is a study strength. Additional strengths are the prestudy training in the maneuver using simulation and the high level of success in the rotations (89.7%).
The study’s main limitation is that it was not double blinded; therefore, bias in management was a possibility. Additionally, the study looked only at short-term outcomes for the delivery itself and not at the potential long-term pelvic floor outcomes. The authors reported that the study was underpowered for operative vaginal delivery and cesarean delivery separately, as well as the secondary outcomes. Other limitations were the high frequency of operative vaginal delivery, low rate of consent for the study, and lack of patient satisfaction data. ●
In this study, a trial of prophylactic manual rotation of the occiput posterior or occiput transverse presentation decreased the rate of operative delivery and reduced the length of the second stage of labor without differences in maternal or neonatal complications. Obstetrical providers should consider this strategy to resolve the OP or OT presentation prior to performing an operative vaginal delivery or cesarean delivery. Simulation training in this maneuver may be a useful adjunct for both trainees and providers unfamiliar with the procedure.
JAIMEY M. PAULI, MD
Reduce the use of perioperative opioids with a multimodal pain management strategy
Opioid-related deaths are a major cause of mortality in the United States. The Centers for Disease Control and Prevention (CDC) reported 72,151 and 93,331 drug overdose deaths in 2019 and 2020, respectively, and drug overdose deaths have continued to increase in 2021.1 The majority of drug overdose deaths are due to opioids. There are many factors contributing to this rise, including an incredibly high rate of opioid prescriptions in this country.2 The CDC reported that in 3.6% of US counties, there are more opioid prescriptions filled each year than number of residents in the county.3 The consumption of opioids per person in the US is approximately four times greater than countries with excellent health outcomes, including Sweden, Netherlands, Norway, and the United Kingdom.4 Some US physicians have opioid prescribing practices that are inconsistent with good medical practice in other countries, prescribing powerful opioids and an excessive number of pills per opioid prescription.2 We must continue to evolve our clinical practices to reduce opioid use while continually improving patient outcomes.
Cesarean birth is one of the most common major surgical procedures performed in the United States. The National Center for Health Statistics reported that in 2020 there were approximately 1,150,000 US cesarean births.5 Following cesarean birth, patients who were previously naïve to opioid medications were reported to have a 0.33% to 2.2% probability of transitioning to the persistent use of opioid prescriptions.6-8 Predictors of persistent opioid use after cesarean birth included a history of tobacco use, back pain, migraine headaches, and antidepressant or benzodiazepine use.6 The use of cesarean birth pain management protocols that prioritize multimodal analgesia and opioid sparing is warranted.
Multimodal pain management protocols for cesarean birth have been shown to reduce the use of opioid medications in the hospital and at discharge without a clinically significant increase in pain scores or a reduction in patient satisfaction (TABLE).9-13 For example, Holland and colleagues9 reported that the implementation of a multimodal pain management protocol reduced the percent of patients using oral opioids during hospitalization for cesarean birth from 68% to 45%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients using opioids during hospitalization for cesarean birth was reduced from 45% preintervention to 18% postintervention. In addition, these studies showed that multimodal pain management protocols for cesarean birth also reduced opioid prescribing at discharge. Holland and colleagues9 reported that the percent of patients provided an opioid prescription at discharge was reduced from 91% to 40%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients who took opioids after discharge was reduced from 24% preintervention to 9% postintervention. These studies were not randomized controlled clinical trials, but they do provide strong evidence that a focused intervention to reduce opioid medications in the management of pain after cesarean surgery can be successful without decreasing patient satisfaction or increasing reported pain scores. In these studies, it is likely that the influence, enthusiasm, and commitment of the study leaders to the change process contributed to the success of these opioid-sparing pain management programs.
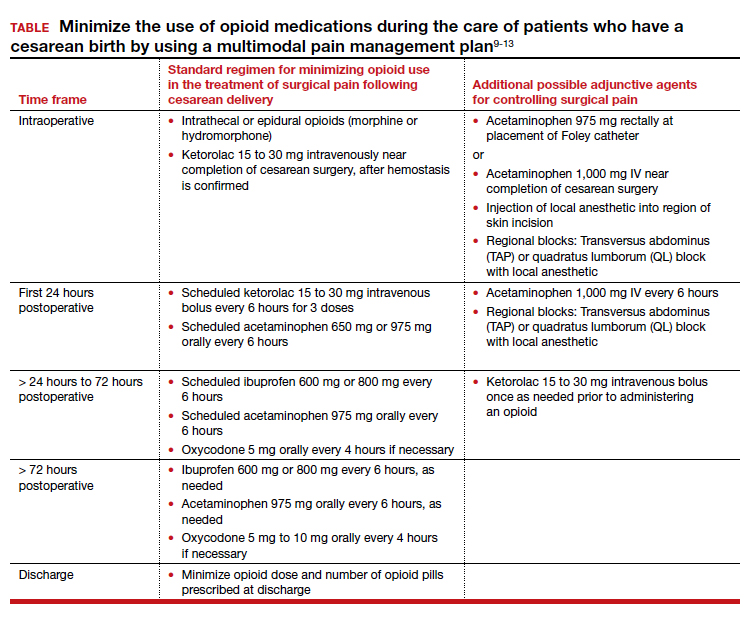
Continue to: Key features of a multimodal analgesia intervention for cesarean surgery...
Key features of a multimodal analgesia intervention for cesarean surgery
Fundamental inclusions of multimodal analgesia for cesarean surgery include:
- exquisite attention to pain control during the surgical procedure by both the anesthesiologist and surgeon, with prioritization of spinal anesthesia that includes morphine and fentanyl
- regularly scheduled administration of intravenous ketorolac during the first 24 hours postcesarean
- regularly scheduled administration of both acetaminophen and ibuprofen, rather than “as needed” dosing
- using analgesics that work through different molecular pathways (ibuprofen and acetaminophen) (See Table.).
The significance of neuraxial and truncal nerve blockade for post-cesarean delivery pain control
Administration of a long-acting intrathecal opioid such as morphine lengthens time to first analgesic request after surgery and lowers 24-hour post‒cesarean delivery opioid requirement.14 If a patient requires general anesthesia and receives no spinal opioid, a transversus abdominis plane (TAP) block or quadratus lumborum (QL) block for postpartum pain control can lower associated postpartum opioid consumption. However, TAP or QL blocks confer no additional benefit to patients who receive spinal morphine,15 nor do they confer added benefit when combined with a multimodal pain management regimen postdelivery vs the multimodal regimen alone.16). TAP blocks administered to patients with severe breakthrough pain after spinal anesthesia help to lower opioid consumption.17 Further research is warranted on the use of TAP, QL, or other truncal blocks to spare opioid requirement after cesarean delivery in women with chronic pain, opioid use disorder, or those undergoing higher-complexity surgery such as cesarean hysterectomy for placenta accreta spectrum.
NSAIDs: Potential adverse effects
As we decrease the use of opioid medications and increase the use of nonsteroidal anti-inflammatory drugs (NSAIDs), we should reflect on the potential adverse effects of NSAID treatment in some patients. Specifically, the impact of ketorolac on hypertension, platelet function, and breastfeeding warrant consideration.
In the past, some studies reported that NSAID treatment is associated with a modest increase in blood pressure (BP), with a mean increase of 5 mm Hg.18 However, multiple recent studies report that in women with preeclampsia with and without severe features, postpartum administration of ibuprofen and ketorolac did not increase BP or delay resolution of hypertension.19-22 In a meta-analysis of randomized controlled studies comparing the effects of ibuprofen and acetaminophen on BP, neither medication was associated with an increase in BP.19 The American College of Obstetricians and Gynecologists supports the use of NSAIDs as one component of multimodal analgesia to help reduce the use of opioids.23
NSAIDs can inhibit platelet function and this effect is of clinical concern for people with platelet defects. However, a meta-analysis of clinical trials reported no difference in bleeding between surgical patients administered ketorolac or control participants.24 Alternative opioid-sparing adjuncts (TAP or QL blocks) may be considered for patients who cannot receive ketorolac based on a history of platelet deficiency. Furthermore, patients with ongoing coagulation defects after surgery from severe postpartum hemorrhage, hyperfibrinolysis, disseminated intravascular coagulation, or dilutional coagulopathy may have both limited platelet reserves and acute kidney injury. The need to postpone the initiation of NSAIDs in such patients should prompt alternate options such as TAP or QL blocks or dosing of an indwelling epidural when possible, in conjunction with acetaminophen. Patients who have a contraindication to ketorolac due to peptic ulcer disease or renal insufficiency may also benefit from TAP and QL blocks after cesarean delivery, although more studies are needed in these patients.
Both ketorolac and ibuprofen transfer to breast milk. The relative infant dose for ketorolac and ibuprofen is very low—0.2% and 0.9%, respectively.25,26 The World Health Organization advises that ibuprofen is compatible with breastfeeding.27 Of interest, in an enhanced recovery after cesarean clinical trial, scheduled ketorolac administration resulted in more mothers exclusively breastfeeding at discharge compared with “as needed” ketorolac treatment, 67% versus 48%, respectively; P = .046.28
Conclusion
Many factors influence a person’s experience of their surgery, including their pain symptoms. Factors that modulate a person’s perception of pain following surgery include their personality, social supports, and genetic factors. The technical skill of the anesthesiologist, surgeon, and nurses, and the confidence of the patient in the surgical care team are important factors influencing a person’s global experience of their surgery, including their experience of pain. Patients’ expectations regarding postoperative pain and psychological distress surrounding surgery may also influence their pain experience. Assuring patients that their pain will be addressed adequately, and helping them manage peripartum anxiety, also may favorably impact their pain experience.
Following a surgical procedure, a surgeon’s top goal is the full recovery of the patient to normal activity as soon as possible with as few complications as possible. Persistent opioid dependence is a serious long-term complication of surgery. Decades ago, most heroin users reported that heroin was the first opioid they used. However, the gateway drug to heroin use has evolved. In a recent study, 75% of heroin users reported that the first opioid they used was a prescription opioid.29 In managing surgical pain we want to minimize the use of opioids and reduce the risk of persistent opioid use following discharge. We believe that implementing a multimodal approach to the management of pain with additional targeted therapy for patients at risk for higher opioid requirement will reduce the perioperative and postdischarge use of opioid analgesics. ●
- Drug overdose deaths in the U.S. up 30% in 2020. Centers for Disease Control and Prevention web- site. July 14, 2020. https://www.cdc.gov/nchs /pressroom/nchs_press_releases/2021/20210714 .htm. Last reviewed July 14, 2021
- Jani M, Girard N, Bates DW, et al. Opioid prescribing among new users for non-cancer pain in the USA, Canada, UK, and Taiwan: a population-based cohort study. PLoS Med. 2021;18:e1003829.
- U.S. opioid dispensing rate maps. Centers for Disease Control and Prevention website. https://www. cdc.gov/drugoverdose/rxrate-maps/index.html. Last reviewed November 10, 2021.
- Richards GC, Aronson JK, Mahtani KR, et al. Global, regional, and national consumption of controlled opioids: a cross-sectional study of 214 countries and non-metropolitan areas. British J Pain. 2021. https://doi .org/10.1177/20494637211013052.
- Hamilton BE, Martin JA, Osterman MJK. Births: Provisional data for 2020. Vital Statistics Rapid Release; no 12. Hyattsville MD: National Center for Health Statistics. May 2021.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1-e8. doi: 10.1016/j.ajog.2016.03.016.
- Osmundson SS, Wiese AD, Min JY, et al. Delivery type, opioid prescribing and the risk of persistent opioid use after delivery. Am J Obstet Gynecol. 2019;220:405-407. doi: 10.1016/j.ajog.2018.10.026.
- Peahl AF, Dalton VK, Montgomery JR, et al. Rates of new persistent opioid use after vaginal or cesarean birth among U.S. women. JAMA Netw Open. 2019;e197863. doi: 10.1001/jamanetworkopen.2019.7863.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97. doi: 10.1097/AOG.0000000000003010.
- Smith AM, Young P, Blosser CC, et al. Multimodal stepwise approach to reducing in-hospital opioid use after cesarean delivery. Obstet Gynecol. 2019;133:700-706. doi: 10.1097/AOG.0000000000003156.
- Herbert KA, Yuraschevich M, Fuller M, et al. Impact of multimodeal analgesic protocol modification on opioid consumption after cesarean delivery: a retrospective cohort study. J Matern Fetal Neonatal Med. 2021;3:1-7. doi: 10.1080/14767058.2020.1863364.
- Mehraban SS, Suddle R, Mehraban S, et al. Opioid-free multimodal analgesia pathway to decrease opioid utilization after cesarean delivery. J Obstet Gynaecol Res. 2021;47:873-881. doi: 10.1111/jog.14582.
- Meyer MF, Broman AT, Gnadt SE, et al. A standardized post-cesarean analgesia regimen reduces postpartum opioid use. J Matern Fetal Neonatal Med. 2021;26:1-8. doi: 10.1080/14767058.2021.1970132.
- Seki H, Shiga T, Mihara T, et al. Effects of intrathecal opioids on cesarean section: a systematic review and Bayesian network meta-analysis of randomized controlled trials. J Anesth. 2021;35:911-927. doi: 10.1007/s00540-021-02980-2.
- Yang TR, He XM, Li XH, et al. Intrathecal morphine versus transversus abdominis plane block for cesarean delivery: a systematic review and meta-analysis. BMC Anesthesiol. 2021;21:174. doi: 10.1186/s12871-021-01392-9.
- Yu Y, Gao S, Yuen VMY, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane (TAP) block combined with oral multimodal analgesia in comparison with oral multimodal analgesia after cesarean delivery: a randomized controlled trial. BMC Anesthesiol. 2021;21:7. doi: 10.1186/s12871-020-01223-3.
- Mirza F, Carvalho B. Transversus abdominis plane blocks for rescue analgesia following cesarean delivery: a case series. Can J Anesth. 2013;60:299-303.
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Int Med. 1994;121:289-300.
- Wang B, Yang X, Yu H, et al. The comparison of ibuprofen versus acetaminophen for blood pressure in preeclampsia: a meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2020:1-6. doi: 10.1080/14767058.2020.1720641.
- Viteri OA, England JA, Alrais MA, et al. Association of nonsteroidal anti-inflammatory drugs and postpartum hypertension in women with preeclampsia with severe features. Obstet Gynecol. 2017;130:830. doi: 10.1097/AOG.0000000000002247.
- Blue NR, Murray-Krezan C, Drake-Lavelle S, et al. Effect of ibuprofen vs acetaminophen on postpartum hypertension in preeclampsia with severe features: a double-masked, randomized controlled trial. Am J Obstet Gynecol. 2018;218:616.e1. doi: 10.1016/j.ajog.2018.02.016.
- Penfield CA, McNulty JA, Oakes MC, et al. Ibuprofen and postpartum blood pressure in women with hypertensive disorders of pregnancy: a randomized controlled trial. Obstet Gynecol. 2019;134:1219. doi: 10.1097/AOG.0000000000003553.
- American College of Obstetricians and Gynecologists. Pharmacologic stepwise multimodal approach for postpartum pain management. Obstet Gynecol. 2021;138:507-517. doi: 10.1097/AOG.0000000000004517.
- Gobble RM, Hoang HLT, Kachniarz B, et al. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2014;133:741. doi: 10.1097/01.prs.0000438459.60474.b5.
- Wischik A, Manth SM, Lloyd J, et al. The excretion of ketorolac tromethamine into breast milk after multiple oral dosing. Eur J Clin Pharmacol. 1989;36:521-524. doi: 10.1007/BF00558080.
- Rigourd V, de Villepin B, Amirouche A, et al. Ibuprofen concentrations in human mature milk-first data about pharmacokinetics study in breast milk with AOR-10127 “Antalait” study. The Drug Monit. 2014;36:590-596. doi: 10.1097/FTD.0000000000000058.
- World Health Organization. Breastfeeding and maternal medication, recommendations for drugs in the eleventh WHO model list of essential drugs. 2002. http://www.who.int/maternal _child_adolescent/documents/55732/en/.
- Teigen NC, Sahasrabudhe N, Doulaveris G. Enhanced recovery after surgery at cesarean delivery to reduce postoperative length of stay: a randomized controlled trial. Am J Obstet Gynecol. 2020;222:372.e1-e10. doi: 10.1016/j.ajog.2019.10.009.
- Cicero T, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826. doi: 10.1001 /jamapsychiatry.2014.366.
Opioid-related deaths are a major cause of mortality in the United States. The Centers for Disease Control and Prevention (CDC) reported 72,151 and 93,331 drug overdose deaths in 2019 and 2020, respectively, and drug overdose deaths have continued to increase in 2021.1 The majority of drug overdose deaths are due to opioids. There are many factors contributing to this rise, including an incredibly high rate of opioid prescriptions in this country.2 The CDC reported that in 3.6% of US counties, there are more opioid prescriptions filled each year than number of residents in the county.3 The consumption of opioids per person in the US is approximately four times greater than countries with excellent health outcomes, including Sweden, Netherlands, Norway, and the United Kingdom.4 Some US physicians have opioid prescribing practices that are inconsistent with good medical practice in other countries, prescribing powerful opioids and an excessive number of pills per opioid prescription.2 We must continue to evolve our clinical practices to reduce opioid use while continually improving patient outcomes.
Cesarean birth is one of the most common major surgical procedures performed in the United States. The National Center for Health Statistics reported that in 2020 there were approximately 1,150,000 US cesarean births.5 Following cesarean birth, patients who were previously naïve to opioid medications were reported to have a 0.33% to 2.2% probability of transitioning to the persistent use of opioid prescriptions.6-8 Predictors of persistent opioid use after cesarean birth included a history of tobacco use, back pain, migraine headaches, and antidepressant or benzodiazepine use.6 The use of cesarean birth pain management protocols that prioritize multimodal analgesia and opioid sparing is warranted.
Multimodal pain management protocols for cesarean birth have been shown to reduce the use of opioid medications in the hospital and at discharge without a clinically significant increase in pain scores or a reduction in patient satisfaction (TABLE).9-13 For example, Holland and colleagues9 reported that the implementation of a multimodal pain management protocol reduced the percent of patients using oral opioids during hospitalization for cesarean birth from 68% to 45%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients using opioids during hospitalization for cesarean birth was reduced from 45% preintervention to 18% postintervention. In addition, these studies showed that multimodal pain management protocols for cesarean birth also reduced opioid prescribing at discharge. Holland and colleagues9 reported that the percent of patients provided an opioid prescription at discharge was reduced from 91% to 40%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients who took opioids after discharge was reduced from 24% preintervention to 9% postintervention. These studies were not randomized controlled clinical trials, but they do provide strong evidence that a focused intervention to reduce opioid medications in the management of pain after cesarean surgery can be successful without decreasing patient satisfaction or increasing reported pain scores. In these studies, it is likely that the influence, enthusiasm, and commitment of the study leaders to the change process contributed to the success of these opioid-sparing pain management programs.

Continue to: Key features of a multimodal analgesia intervention for cesarean surgery...
Key features of a multimodal analgesia intervention for cesarean surgery
Fundamental inclusions of multimodal analgesia for cesarean surgery include:
- exquisite attention to pain control during the surgical procedure by both the anesthesiologist and surgeon, with prioritization of spinal anesthesia that includes morphine and fentanyl
- regularly scheduled administration of intravenous ketorolac during the first 24 hours postcesarean
- regularly scheduled administration of both acetaminophen and ibuprofen, rather than “as needed” dosing
- using analgesics that work through different molecular pathways (ibuprofen and acetaminophen) (See Table.).
The significance of neuraxial and truncal nerve blockade for post-cesarean delivery pain control
Administration of a long-acting intrathecal opioid such as morphine lengthens time to first analgesic request after surgery and lowers 24-hour post‒cesarean delivery opioid requirement.14 If a patient requires general anesthesia and receives no spinal opioid, a transversus abdominis plane (TAP) block or quadratus lumborum (QL) block for postpartum pain control can lower associated postpartum opioid consumption. However, TAP or QL blocks confer no additional benefit to patients who receive spinal morphine,15 nor do they confer added benefit when combined with a multimodal pain management regimen postdelivery vs the multimodal regimen alone.16). TAP blocks administered to patients with severe breakthrough pain after spinal anesthesia help to lower opioid consumption.17 Further research is warranted on the use of TAP, QL, or other truncal blocks to spare opioid requirement after cesarean delivery in women with chronic pain, opioid use disorder, or those undergoing higher-complexity surgery such as cesarean hysterectomy for placenta accreta spectrum.
NSAIDs: Potential adverse effects
As we decrease the use of opioid medications and increase the use of nonsteroidal anti-inflammatory drugs (NSAIDs), we should reflect on the potential adverse effects of NSAID treatment in some patients. Specifically, the impact of ketorolac on hypertension, platelet function, and breastfeeding warrant consideration.
In the past, some studies reported that NSAID treatment is associated with a modest increase in blood pressure (BP), with a mean increase of 5 mm Hg.18 However, multiple recent studies report that in women with preeclampsia with and without severe features, postpartum administration of ibuprofen and ketorolac did not increase BP or delay resolution of hypertension.19-22 In a meta-analysis of randomized controlled studies comparing the effects of ibuprofen and acetaminophen on BP, neither medication was associated with an increase in BP.19 The American College of Obstetricians and Gynecologists supports the use of NSAIDs as one component of multimodal analgesia to help reduce the use of opioids.23
NSAIDs can inhibit platelet function and this effect is of clinical concern for people with platelet defects. However, a meta-analysis of clinical trials reported no difference in bleeding between surgical patients administered ketorolac or control participants.24 Alternative opioid-sparing adjuncts (TAP or QL blocks) may be considered for patients who cannot receive ketorolac based on a history of platelet deficiency. Furthermore, patients with ongoing coagulation defects after surgery from severe postpartum hemorrhage, hyperfibrinolysis, disseminated intravascular coagulation, or dilutional coagulopathy may have both limited platelet reserves and acute kidney injury. The need to postpone the initiation of NSAIDs in such patients should prompt alternate options such as TAP or QL blocks or dosing of an indwelling epidural when possible, in conjunction with acetaminophen. Patients who have a contraindication to ketorolac due to peptic ulcer disease or renal insufficiency may also benefit from TAP and QL blocks after cesarean delivery, although more studies are needed in these patients.
Both ketorolac and ibuprofen transfer to breast milk. The relative infant dose for ketorolac and ibuprofen is very low—0.2% and 0.9%, respectively.25,26 The World Health Organization advises that ibuprofen is compatible with breastfeeding.27 Of interest, in an enhanced recovery after cesarean clinical trial, scheduled ketorolac administration resulted in more mothers exclusively breastfeeding at discharge compared with “as needed” ketorolac treatment, 67% versus 48%, respectively; P = .046.28
Conclusion
Many factors influence a person’s experience of their surgery, including their pain symptoms. Factors that modulate a person’s perception of pain following surgery include their personality, social supports, and genetic factors. The technical skill of the anesthesiologist, surgeon, and nurses, and the confidence of the patient in the surgical care team are important factors influencing a person’s global experience of their surgery, including their experience of pain. Patients’ expectations regarding postoperative pain and psychological distress surrounding surgery may also influence their pain experience. Assuring patients that their pain will be addressed adequately, and helping them manage peripartum anxiety, also may favorably impact their pain experience.
Following a surgical procedure, a surgeon’s top goal is the full recovery of the patient to normal activity as soon as possible with as few complications as possible. Persistent opioid dependence is a serious long-term complication of surgery. Decades ago, most heroin users reported that heroin was the first opioid they used. However, the gateway drug to heroin use has evolved. In a recent study, 75% of heroin users reported that the first opioid they used was a prescription opioid.29 In managing surgical pain we want to minimize the use of opioids and reduce the risk of persistent opioid use following discharge. We believe that implementing a multimodal approach to the management of pain with additional targeted therapy for patients at risk for higher opioid requirement will reduce the perioperative and postdischarge use of opioid analgesics. ●
Opioid-related deaths are a major cause of mortality in the United States. The Centers for Disease Control and Prevention (CDC) reported 72,151 and 93,331 drug overdose deaths in 2019 and 2020, respectively, and drug overdose deaths have continued to increase in 2021.1 The majority of drug overdose deaths are due to opioids. There are many factors contributing to this rise, including an incredibly high rate of opioid prescriptions in this country.2 The CDC reported that in 3.6% of US counties, there are more opioid prescriptions filled each year than number of residents in the county.3 The consumption of opioids per person in the US is approximately four times greater than countries with excellent health outcomes, including Sweden, Netherlands, Norway, and the United Kingdom.4 Some US physicians have opioid prescribing practices that are inconsistent with good medical practice in other countries, prescribing powerful opioids and an excessive number of pills per opioid prescription.2 We must continue to evolve our clinical practices to reduce opioid use while continually improving patient outcomes.
Cesarean birth is one of the most common major surgical procedures performed in the United States. The National Center for Health Statistics reported that in 2020 there were approximately 1,150,000 US cesarean births.5 Following cesarean birth, patients who were previously naïve to opioid medications were reported to have a 0.33% to 2.2% probability of transitioning to the persistent use of opioid prescriptions.6-8 Predictors of persistent opioid use after cesarean birth included a history of tobacco use, back pain, migraine headaches, and antidepressant or benzodiazepine use.6 The use of cesarean birth pain management protocols that prioritize multimodal analgesia and opioid sparing is warranted.
Multimodal pain management protocols for cesarean birth have been shown to reduce the use of opioid medications in the hospital and at discharge without a clinically significant increase in pain scores or a reduction in patient satisfaction (TABLE).9-13 For example, Holland and colleagues9 reported that the implementation of a multimodal pain management protocol reduced the percent of patients using oral opioids during hospitalization for cesarean birth from 68% to 45%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients using opioids during hospitalization for cesarean birth was reduced from 45% preintervention to 18% postintervention. In addition, these studies showed that multimodal pain management protocols for cesarean birth also reduced opioid prescribing at discharge. Holland and colleagues9 reported that the percent of patients provided an opioid prescription at discharge was reduced from 91% to 40%, pre- and post-intervention, respectively. Mehraban and colleagues12 reported that the percent of patients who took opioids after discharge was reduced from 24% preintervention to 9% postintervention. These studies were not randomized controlled clinical trials, but they do provide strong evidence that a focused intervention to reduce opioid medications in the management of pain after cesarean surgery can be successful without decreasing patient satisfaction or increasing reported pain scores. In these studies, it is likely that the influence, enthusiasm, and commitment of the study leaders to the change process contributed to the success of these opioid-sparing pain management programs.

Continue to: Key features of a multimodal analgesia intervention for cesarean surgery...
Key features of a multimodal analgesia intervention for cesarean surgery
Fundamental inclusions of multimodal analgesia for cesarean surgery include:
- exquisite attention to pain control during the surgical procedure by both the anesthesiologist and surgeon, with prioritization of spinal anesthesia that includes morphine and fentanyl
- regularly scheduled administration of intravenous ketorolac during the first 24 hours postcesarean
- regularly scheduled administration of both acetaminophen and ibuprofen, rather than “as needed” dosing
- using analgesics that work through different molecular pathways (ibuprofen and acetaminophen) (See Table.).
The significance of neuraxial and truncal nerve blockade for post-cesarean delivery pain control
Administration of a long-acting intrathecal opioid such as morphine lengthens time to first analgesic request after surgery and lowers 24-hour post‒cesarean delivery opioid requirement.14 If a patient requires general anesthesia and receives no spinal opioid, a transversus abdominis plane (TAP) block or quadratus lumborum (QL) block for postpartum pain control can lower associated postpartum opioid consumption. However, TAP or QL blocks confer no additional benefit to patients who receive spinal morphine,15 nor do they confer added benefit when combined with a multimodal pain management regimen postdelivery vs the multimodal regimen alone.16). TAP blocks administered to patients with severe breakthrough pain after spinal anesthesia help to lower opioid consumption.17 Further research is warranted on the use of TAP, QL, or other truncal blocks to spare opioid requirement after cesarean delivery in women with chronic pain, opioid use disorder, or those undergoing higher-complexity surgery such as cesarean hysterectomy for placenta accreta spectrum.
NSAIDs: Potential adverse effects
As we decrease the use of opioid medications and increase the use of nonsteroidal anti-inflammatory drugs (NSAIDs), we should reflect on the potential adverse effects of NSAID treatment in some patients. Specifically, the impact of ketorolac on hypertension, platelet function, and breastfeeding warrant consideration.
In the past, some studies reported that NSAID treatment is associated with a modest increase in blood pressure (BP), with a mean increase of 5 mm Hg.18 However, multiple recent studies report that in women with preeclampsia with and without severe features, postpartum administration of ibuprofen and ketorolac did not increase BP or delay resolution of hypertension.19-22 In a meta-analysis of randomized controlled studies comparing the effects of ibuprofen and acetaminophen on BP, neither medication was associated with an increase in BP.19 The American College of Obstetricians and Gynecologists supports the use of NSAIDs as one component of multimodal analgesia to help reduce the use of opioids.23
NSAIDs can inhibit platelet function and this effect is of clinical concern for people with platelet defects. However, a meta-analysis of clinical trials reported no difference in bleeding between surgical patients administered ketorolac or control participants.24 Alternative opioid-sparing adjuncts (TAP or QL blocks) may be considered for patients who cannot receive ketorolac based on a history of platelet deficiency. Furthermore, patients with ongoing coagulation defects after surgery from severe postpartum hemorrhage, hyperfibrinolysis, disseminated intravascular coagulation, or dilutional coagulopathy may have both limited platelet reserves and acute kidney injury. The need to postpone the initiation of NSAIDs in such patients should prompt alternate options such as TAP or QL blocks or dosing of an indwelling epidural when possible, in conjunction with acetaminophen. Patients who have a contraindication to ketorolac due to peptic ulcer disease or renal insufficiency may also benefit from TAP and QL blocks after cesarean delivery, although more studies are needed in these patients.
Both ketorolac and ibuprofen transfer to breast milk. The relative infant dose for ketorolac and ibuprofen is very low—0.2% and 0.9%, respectively.25,26 The World Health Organization advises that ibuprofen is compatible with breastfeeding.27 Of interest, in an enhanced recovery after cesarean clinical trial, scheduled ketorolac administration resulted in more mothers exclusively breastfeeding at discharge compared with “as needed” ketorolac treatment, 67% versus 48%, respectively; P = .046.28
Conclusion
Many factors influence a person’s experience of their surgery, including their pain symptoms. Factors that modulate a person’s perception of pain following surgery include their personality, social supports, and genetic factors. The technical skill of the anesthesiologist, surgeon, and nurses, and the confidence of the patient in the surgical care team are important factors influencing a person’s global experience of their surgery, including their experience of pain. Patients’ expectations regarding postoperative pain and psychological distress surrounding surgery may also influence their pain experience. Assuring patients that their pain will be addressed adequately, and helping them manage peripartum anxiety, also may favorably impact their pain experience.
Following a surgical procedure, a surgeon’s top goal is the full recovery of the patient to normal activity as soon as possible with as few complications as possible. Persistent opioid dependence is a serious long-term complication of surgery. Decades ago, most heroin users reported that heroin was the first opioid they used. However, the gateway drug to heroin use has evolved. In a recent study, 75% of heroin users reported that the first opioid they used was a prescription opioid.29 In managing surgical pain we want to minimize the use of opioids and reduce the risk of persistent opioid use following discharge. We believe that implementing a multimodal approach to the management of pain with additional targeted therapy for patients at risk for higher opioid requirement will reduce the perioperative and postdischarge use of opioid analgesics. ●
- Drug overdose deaths in the U.S. up 30% in 2020. Centers for Disease Control and Prevention web- site. July 14, 2020. https://www.cdc.gov/nchs /pressroom/nchs_press_releases/2021/20210714 .htm. Last reviewed July 14, 2021
- Jani M, Girard N, Bates DW, et al. Opioid prescribing among new users for non-cancer pain in the USA, Canada, UK, and Taiwan: a population-based cohort study. PLoS Med. 2021;18:e1003829.
- U.S. opioid dispensing rate maps. Centers for Disease Control and Prevention website. https://www. cdc.gov/drugoverdose/rxrate-maps/index.html. Last reviewed November 10, 2021.
- Richards GC, Aronson JK, Mahtani KR, et al. Global, regional, and national consumption of controlled opioids: a cross-sectional study of 214 countries and non-metropolitan areas. British J Pain. 2021. https://doi .org/10.1177/20494637211013052.
- Hamilton BE, Martin JA, Osterman MJK. Births: Provisional data for 2020. Vital Statistics Rapid Release; no 12. Hyattsville MD: National Center for Health Statistics. May 2021.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1-e8. doi: 10.1016/j.ajog.2016.03.016.
- Osmundson SS, Wiese AD, Min JY, et al. Delivery type, opioid prescribing and the risk of persistent opioid use after delivery. Am J Obstet Gynecol. 2019;220:405-407. doi: 10.1016/j.ajog.2018.10.026.
- Peahl AF, Dalton VK, Montgomery JR, et al. Rates of new persistent opioid use after vaginal or cesarean birth among U.S. women. JAMA Netw Open. 2019;e197863. doi: 10.1001/jamanetworkopen.2019.7863.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97. doi: 10.1097/AOG.0000000000003010.
- Smith AM, Young P, Blosser CC, et al. Multimodal stepwise approach to reducing in-hospital opioid use after cesarean delivery. Obstet Gynecol. 2019;133:700-706. doi: 10.1097/AOG.0000000000003156.
- Herbert KA, Yuraschevich M, Fuller M, et al. Impact of multimodeal analgesic protocol modification on opioid consumption after cesarean delivery: a retrospective cohort study. J Matern Fetal Neonatal Med. 2021;3:1-7. doi: 10.1080/14767058.2020.1863364.
- Mehraban SS, Suddle R, Mehraban S, et al. Opioid-free multimodal analgesia pathway to decrease opioid utilization after cesarean delivery. J Obstet Gynaecol Res. 2021;47:873-881. doi: 10.1111/jog.14582.
- Meyer MF, Broman AT, Gnadt SE, et al. A standardized post-cesarean analgesia regimen reduces postpartum opioid use. J Matern Fetal Neonatal Med. 2021;26:1-8. doi: 10.1080/14767058.2021.1970132.
- Seki H, Shiga T, Mihara T, et al. Effects of intrathecal opioids on cesarean section: a systematic review and Bayesian network meta-analysis of randomized controlled trials. J Anesth. 2021;35:911-927. doi: 10.1007/s00540-021-02980-2.
- Yang TR, He XM, Li XH, et al. Intrathecal morphine versus transversus abdominis plane block for cesarean delivery: a systematic review and meta-analysis. BMC Anesthesiol. 2021;21:174. doi: 10.1186/s12871-021-01392-9.
- Yu Y, Gao S, Yuen VMY, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane (TAP) block combined with oral multimodal analgesia in comparison with oral multimodal analgesia after cesarean delivery: a randomized controlled trial. BMC Anesthesiol. 2021;21:7. doi: 10.1186/s12871-020-01223-3.
- Mirza F, Carvalho B. Transversus abdominis plane blocks for rescue analgesia following cesarean delivery: a case series. Can J Anesth. 2013;60:299-303.
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Int Med. 1994;121:289-300.
- Wang B, Yang X, Yu H, et al. The comparison of ibuprofen versus acetaminophen for blood pressure in preeclampsia: a meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2020:1-6. doi: 10.1080/14767058.2020.1720641.
- Viteri OA, England JA, Alrais MA, et al. Association of nonsteroidal anti-inflammatory drugs and postpartum hypertension in women with preeclampsia with severe features. Obstet Gynecol. 2017;130:830. doi: 10.1097/AOG.0000000000002247.
- Blue NR, Murray-Krezan C, Drake-Lavelle S, et al. Effect of ibuprofen vs acetaminophen on postpartum hypertension in preeclampsia with severe features: a double-masked, randomized controlled trial. Am J Obstet Gynecol. 2018;218:616.e1. doi: 10.1016/j.ajog.2018.02.016.
- Penfield CA, McNulty JA, Oakes MC, et al. Ibuprofen and postpartum blood pressure in women with hypertensive disorders of pregnancy: a randomized controlled trial. Obstet Gynecol. 2019;134:1219. doi: 10.1097/AOG.0000000000003553.
- American College of Obstetricians and Gynecologists. Pharmacologic stepwise multimodal approach for postpartum pain management. Obstet Gynecol. 2021;138:507-517. doi: 10.1097/AOG.0000000000004517.
- Gobble RM, Hoang HLT, Kachniarz B, et al. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2014;133:741. doi: 10.1097/01.prs.0000438459.60474.b5.
- Wischik A, Manth SM, Lloyd J, et al. The excretion of ketorolac tromethamine into breast milk after multiple oral dosing. Eur J Clin Pharmacol. 1989;36:521-524. doi: 10.1007/BF00558080.
- Rigourd V, de Villepin B, Amirouche A, et al. Ibuprofen concentrations in human mature milk-first data about pharmacokinetics study in breast milk with AOR-10127 “Antalait” study. The Drug Monit. 2014;36:590-596. doi: 10.1097/FTD.0000000000000058.
- World Health Organization. Breastfeeding and maternal medication, recommendations for drugs in the eleventh WHO model list of essential drugs. 2002. http://www.who.int/maternal _child_adolescent/documents/55732/en/.
- Teigen NC, Sahasrabudhe N, Doulaveris G. Enhanced recovery after surgery at cesarean delivery to reduce postoperative length of stay: a randomized controlled trial. Am J Obstet Gynecol. 2020;222:372.e1-e10. doi: 10.1016/j.ajog.2019.10.009.
- Cicero T, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826. doi: 10.1001 /jamapsychiatry.2014.366.
- Drug overdose deaths in the U.S. up 30% in 2020. Centers for Disease Control and Prevention web- site. July 14, 2020. https://www.cdc.gov/nchs /pressroom/nchs_press_releases/2021/20210714 .htm. Last reviewed July 14, 2021
- Jani M, Girard N, Bates DW, et al. Opioid prescribing among new users for non-cancer pain in the USA, Canada, UK, and Taiwan: a population-based cohort study. PLoS Med. 2021;18:e1003829.
- U.S. opioid dispensing rate maps. Centers for Disease Control and Prevention website. https://www. cdc.gov/drugoverdose/rxrate-maps/index.html. Last reviewed November 10, 2021.
- Richards GC, Aronson JK, Mahtani KR, et al. Global, regional, and national consumption of controlled opioids: a cross-sectional study of 214 countries and non-metropolitan areas. British J Pain. 2021. https://doi .org/10.1177/20494637211013052.
- Hamilton BE, Martin JA, Osterman MJK. Births: Provisional data for 2020. Vital Statistics Rapid Release; no 12. Hyattsville MD: National Center for Health Statistics. May 2021.
- Bateman BT, Franklin JM, Bykov K, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am J Obstet Gynecol. 2016;215:353.e1-e8. doi: 10.1016/j.ajog.2016.03.016.
- Osmundson SS, Wiese AD, Min JY, et al. Delivery type, opioid prescribing and the risk of persistent opioid use after delivery. Am J Obstet Gynecol. 2019;220:405-407. doi: 10.1016/j.ajog.2018.10.026.
- Peahl AF, Dalton VK, Montgomery JR, et al. Rates of new persistent opioid use after vaginal or cesarean birth among U.S. women. JAMA Netw Open. 2019;e197863. doi: 10.1001/jamanetworkopen.2019.7863.
- Holland E, Bateman BT, Cole N, et al. Evaluation of a quality improvement intervention that eliminated routine use of opioids after cesarean delivery. Obstet Gynecol. 2019;133:91-97. doi: 10.1097/AOG.0000000000003010.
- Smith AM, Young P, Blosser CC, et al. Multimodal stepwise approach to reducing in-hospital opioid use after cesarean delivery. Obstet Gynecol. 2019;133:700-706. doi: 10.1097/AOG.0000000000003156.
- Herbert KA, Yuraschevich M, Fuller M, et al. Impact of multimodeal analgesic protocol modification on opioid consumption after cesarean delivery: a retrospective cohort study. J Matern Fetal Neonatal Med. 2021;3:1-7. doi: 10.1080/14767058.2020.1863364.
- Mehraban SS, Suddle R, Mehraban S, et al. Opioid-free multimodal analgesia pathway to decrease opioid utilization after cesarean delivery. J Obstet Gynaecol Res. 2021;47:873-881. doi: 10.1111/jog.14582.
- Meyer MF, Broman AT, Gnadt SE, et al. A standardized post-cesarean analgesia regimen reduces postpartum opioid use. J Matern Fetal Neonatal Med. 2021;26:1-8. doi: 10.1080/14767058.2021.1970132.
- Seki H, Shiga T, Mihara T, et al. Effects of intrathecal opioids on cesarean section: a systematic review and Bayesian network meta-analysis of randomized controlled trials. J Anesth. 2021;35:911-927. doi: 10.1007/s00540-021-02980-2.
- Yang TR, He XM, Li XH, et al. Intrathecal morphine versus transversus abdominis plane block for cesarean delivery: a systematic review and meta-analysis. BMC Anesthesiol. 2021;21:174. doi: 10.1186/s12871-021-01392-9.
- Yu Y, Gao S, Yuen VMY, et al. The analgesic efficacy of ultrasound-guided transversus abdominis plane (TAP) block combined with oral multimodal analgesia in comparison with oral multimodal analgesia after cesarean delivery: a randomized controlled trial. BMC Anesthesiol. 2021;21:7. doi: 10.1186/s12871-020-01223-3.
- Mirza F, Carvalho B. Transversus abdominis plane blocks for rescue analgesia following cesarean delivery: a case series. Can J Anesth. 2013;60:299-303.
- Johnson AG, Nguyen TV, Day RO. Do nonsteroidal anti-inflammatory drugs affect blood pressure? A meta-analysis. Ann Int Med. 1994;121:289-300.
- Wang B, Yang X, Yu H, et al. The comparison of ibuprofen versus acetaminophen for blood pressure in preeclampsia: a meta-analysis of randomized controlled studies. J Matern Fetal Neonatal Med. 2020:1-6. doi: 10.1080/14767058.2020.1720641.
- Viteri OA, England JA, Alrais MA, et al. Association of nonsteroidal anti-inflammatory drugs and postpartum hypertension in women with preeclampsia with severe features. Obstet Gynecol. 2017;130:830. doi: 10.1097/AOG.0000000000002247.
- Blue NR, Murray-Krezan C, Drake-Lavelle S, et al. Effect of ibuprofen vs acetaminophen on postpartum hypertension in preeclampsia with severe features: a double-masked, randomized controlled trial. Am J Obstet Gynecol. 2018;218:616.e1. doi: 10.1016/j.ajog.2018.02.016.
- Penfield CA, McNulty JA, Oakes MC, et al. Ibuprofen and postpartum blood pressure in women with hypertensive disorders of pregnancy: a randomized controlled trial. Obstet Gynecol. 2019;134:1219. doi: 10.1097/AOG.0000000000003553.
- American College of Obstetricians and Gynecologists. Pharmacologic stepwise multimodal approach for postpartum pain management. Obstet Gynecol. 2021;138:507-517. doi: 10.1097/AOG.0000000000004517.
- Gobble RM, Hoang HLT, Kachniarz B, et al. Ketorolac does not increase perioperative bleeding: a meta-analysis of randomized controlled trials. Plast Reconstr Surg. 2014;133:741. doi: 10.1097/01.prs.0000438459.60474.b5.
- Wischik A, Manth SM, Lloyd J, et al. The excretion of ketorolac tromethamine into breast milk after multiple oral dosing. Eur J Clin Pharmacol. 1989;36:521-524. doi: 10.1007/BF00558080.
- Rigourd V, de Villepin B, Amirouche A, et al. Ibuprofen concentrations in human mature milk-first data about pharmacokinetics study in breast milk with AOR-10127 “Antalait” study. The Drug Monit. 2014;36:590-596. doi: 10.1097/FTD.0000000000000058.
- World Health Organization. Breastfeeding and maternal medication, recommendations for drugs in the eleventh WHO model list of essential drugs. 2002. http://www.who.int/maternal _child_adolescent/documents/55732/en/.
- Teigen NC, Sahasrabudhe N, Doulaveris G. Enhanced recovery after surgery at cesarean delivery to reduce postoperative length of stay: a randomized controlled trial. Am J Obstet Gynecol. 2020;222:372.e1-e10. doi: 10.1016/j.ajog.2019.10.009.
- Cicero T, Ellis MS, Surratt HL, et al. The changing face of heroin use in the United States: a retrospective analysis of the past 50 years. JAMA Psychiatry. 2014;71:821-826. doi: 10.1001 /jamapsychiatry.2014.366.
Giant cell arteritis fast-track clinics pave way for greater ultrasound use in U.S.
Temporal artery biopsy has been the standard for diagnosing giant cell arteritis (GCA), but vascular ultrasound, a procedure that’s less invasive, less time-intensive, less expensive, and more convenient, has gained widespread use in Europe, and now clinics in the United States are adopting this approach and moving toward having rheumatologists take on the role of ultrasonographer.
However, directors at these clinics – known as GCA fast-track clinics – caution that the bar can be high for adopting vascular ultrasound (VUS) as a tool to diagnose GCA. Ultrasonographers need specialized training to perform the task and an adequate caseload to maintain their skills. Clinics also need to be outfitted with high-definition ultrasound machines.
“It definitely takes adequate training and learning of how to adjust the settings on the ultrasound machine to be able to visualize the findings appropriately,” said Minna Kohler, MD, director of the rheumatology and musculoskeletal ultrasound program at Massachusetts General Hospital in Boston, which has its own GCA fast-track clinic.
“And the clinical context is very important,” she said. “If you have a high suspicion for someone with temporal arteritis, or GCA, and the patient has been on steroids for weeks before you see them, the ultrasound findings may not show signs of the disease. In those cases in which the imaging is equivocal, we would still pursue a biopsy.”
The idea of the fast-track clinic is as the name implies: to quickly confirm the presence of GCA in a matter of hours, or days at the most, in an outpatient setting without the hassles of a biopsy. Temporal artery biopsy (TAB), by comparison, “is more costly because it requires operating room time, a surgical consultation, and surgery time, whereas ultrasound is a very inexpensive exam since it’s done in the clinic by the rheumatologist,” Dr. Kohler said.
European experience
Use of VUS to diagnose GCA is supplanting TAB in Europe and other countries. In Denmark alone – with a population of 6 million – three outpatient fast-track clinics are operating. The United States, with a population more than 50 times larger than Denmark’s, has six.
Stavros Chrysidis, MD, PhD, chief of rheumatology at the Hospital South West Jutland, one of the fast-track clinic sites in Denmark, led a recent multicenter study, known as EUREKA, of VUS in patients with suspected GCA. He and his colleagues reported in The Lancet Rheumatology that the sensitivity and specificity of VUS was superior to TAB in confirming a diagnosis of GCA. Dr. Chrysidis has instructed U.S. rheumatologists and ultrasonographers in performing and interpreting VUS for GCA.
The study emphasizes the importance of training for ultrasonographers, said Dr. Chrysidis, who regularly performs VUS at his institution. “The most important finding is that, when we apply VUS by systematically trained ultrasonographers using appropriate equipment in appropriate settings, it has excellent diagnostic accuracy on GCA,” he told this news organization.
He noted that The Lancet Rheumatology report is the first multicenter study of VUS for diagnosing GCA in which all the ultrasonographers participated in a standardized training program, which his group developed. “Ultrasound is very operator dependent,” he said. “That’s why the training is very important.”
The training occurred over a year and included two workshops consisting of 5 days of theoretical training on VUS; supervised hands-on evaluation of healthy individuals and patients with known GCA; and evaluation of ultrasound images. Over the course of the year, trainees performed at least 50 VUS evaluations, half of which were in patients with confirmed GCA. During the training period, an external rheumatologist with broad experience in VUS made the final diagnosis.
“The equipment and settings are very important because ultrasound can be very time consuming if you are not educated well and if your equipment is not adjusted well,” Dr. Chrysidis said. The equipment must be calibrated beforehand “so you don’t spend time on adjustments.”
For diagnosing temporal artery anomalies, the ultrasound equipment must have a resolution of 0.3-0.4 mm, he said. “When you have a transducer of 10 MHz, you cannot visualize changes smaller than 5 mm.”
The EUREKA study stated that VUS could replace TAB as a first-line diagnostic tool for GCA – provided the ultrasonographers are systematically trained and the equipment and settings are appropriate. In the Jutland clinic, VUS already has replaced TAB, Dr. Chrysidis said.
“In my department since 2017, when we started the fast-track clinic after the EUREKA study was terminated, we have performed three temporal artery biopsies in the last 4 years, and we screen 60-70 patients per year because we use ultrasound as the primary imaging,” he said. In cases when the results are inconclusive, they order a PET scan. “We don’t perform biopsies anymore,” he said.
U.S. fast-track clinic models
The fast-track clinic models in the United States vary. Results of a survey of the U.S. clinics were presented as an abstract at the 2021 American College of Rheumatology annual meeting. Some centers have a vasculitis specialist obtain and interpret the imaging. At others, a vasculitis specialist refers patients to a VUS-trained rheumatologist to perform and interpret the test. Another approach is to have vasculitis specialists refer patients to ultrasound technicians trained in VUS, with a vascular surgeon interpreting the images and either a VUS-trained rheumatologist or vascular medicine specialist verifying the images.
The take-home message of that survey is that “ultrasound evaluation should be considered in the hands of experts, realizing that not everyone has that skill set, but if it is available, it’s a way to expedite diagnosis and it can be helpful in managing the GCA patient in an appropriate way, quicker than trying to schedule cross-sectional imaging,” said Massachusetts General’s Dr. Kohler, who is a coauthor of the abstract. “Certainly, cross-sectional imaging also plays an important role, but when it comes to confirming whether to continue with treatment or not for a very serious condition, ultrasound is a quick way to get the answer.”
In addition to the fast-track clinic at Massachusetts General, the survey included fast-track clinics at the University of Washington, Seattle; Brigham and Women’s Hospital, Boston; Loma Linda (Calif.) University; University of California, Los Angeles; and at a private practice, Arthritis and Rheumatism Associates in the Washington area.
Advantages of VUS vs. TAB
At Massachusetts General, some of the rheumatologists are trained to perform VUS. The rheumatologists also perform the clinical evaluation of suspected cases of GCA. The advantage of VUS, Dr. Kohler said, is that the answer is “right there”; that is, the imaging yields a diagnosis almost instantaneously whereas a biopsy must be sent to a lab for analysis.
“Since a lot of patients with suspected vasculitis may already come to us on steroid therapy, and if there’s a low probability or low suspicion for vasculitis, [VUS] actually confirms that, and we’re able to taper prednisone or steroids quickly rather than keep them on a prolonged course.”
Alison Bays, MD, MPH, of the department of rheumatology at the University of Washington, said that the advantages of avoiding biopsy aren’t to be overlooked. “Temporal artery biopsies are invasive and carry surgical risks, especially as many of our patients are elderly,” she told this news organization.
“These patients occasionally refuse biopsy, but the acceptance of ultrasound is high,” Dr. Bays said. “Scheduling surgery can be more complicated, resulting in delays to biopsy and potentially higher rates of false negatives. Additionally, ultrasound has resulted in a higher rate of diagnosis with GCA as TAB misses large-vessel involvement.” The fast-track clinic at the university has evaluated 250 patients since it opened in 2017.
Dr. Bays and colleagues published the first United States study of a fast-track protocol using vascular sonographers. “Our group has demonstrated that fast-track clinics can rapidly and effectively evaluate patients, and we demonstrated a different method of evaluation using vascular sonographers rather than rheumatologists to do the vascular ultrasound,” she said. “It utilizes the familiarity vascular sonographers already have with imaging blood vessels.”
She added that the TABUL study in the United Kingdom in 2016 demonstrated that VUS yielded a savings of $686 (£484 as reported in the study), compared with TAB. “Further studies need to be done in the United States,” she said. “Beyond direct comparison of costs, reduction in steroid burden due to quick evaluation and diagnosis may carry additional benefits.”
At Brigham and Women’s Hospital, the division of rheumatology and the vascular section of the cardiology division collaborate on the GCA evaluation, said Sara Tedeschi, MD, MPH, codirector of the fast-track clinic there. Trained vascular technologists credentialed in the procedure and specifically trained in using VUS for evaluating arteritis perform the VUS. Cardiologists with a subspecialty in vascular medicine interpret the studies.
VUS patients have a rheumatology evaluation just before they have the ultrasound. “The rheumatology evaluation is then able to incorporate information from the VUS together with laboratory data, the patient’s history, and physical examination,” Dr. Tedeschi said.
“If the rheumatologist recommends a temporal artery biopsy as a next step, we arrange this with vascular surgery,” she said. “If the rheumatologist recommends other imaging such as MRA [magnetic resonance angiography] or PET-CT, we frequently review the images together with our colleagues in cardiovascular radiology and/or nuclear medicine.”
But in the United States, it will take some time for GCA fast-track clinics to become the standard, Dr. Tedeschi said. “Temporal artery biopsy may be faster to arrange in certain practice settings if VUS is not already being employed for giant cell arteritis evaluation,” she said.
Dr. Bays recognized this limitation, saying, “We are hoping that in the future, the American College of Rheumatology will consider vascular ultrasound as a first-line diagnostic test in diagnosis as rheumatologists and vascular sonographers gain familiarity over time.”
But that would mean that centers performing VUS for GCA would have to meet rigorous standards for the procedure. “With that, standardization of a protocol, high-quality equipment, and adequate training are necessary to ensure quality and reduce the chance of false-positive or false-negative results,” she said.
Dr. Chrysidis, Dr. Bays, and Dr. Tedeschi have disclosed no relevant financial relationships. Dr. Kohler is a board member of Ultrasound School of North American Rheumatologists.
A version of this article first appeared on Medscape.com.
Temporal artery biopsy has been the standard for diagnosing giant cell arteritis (GCA), but vascular ultrasound, a procedure that’s less invasive, less time-intensive, less expensive, and more convenient, has gained widespread use in Europe, and now clinics in the United States are adopting this approach and moving toward having rheumatologists take on the role of ultrasonographer.
However, directors at these clinics – known as GCA fast-track clinics – caution that the bar can be high for adopting vascular ultrasound (VUS) as a tool to diagnose GCA. Ultrasonographers need specialized training to perform the task and an adequate caseload to maintain their skills. Clinics also need to be outfitted with high-definition ultrasound machines.
“It definitely takes adequate training and learning of how to adjust the settings on the ultrasound machine to be able to visualize the findings appropriately,” said Minna Kohler, MD, director of the rheumatology and musculoskeletal ultrasound program at Massachusetts General Hospital in Boston, which has its own GCA fast-track clinic.
“And the clinical context is very important,” she said. “If you have a high suspicion for someone with temporal arteritis, or GCA, and the patient has been on steroids for weeks before you see them, the ultrasound findings may not show signs of the disease. In those cases in which the imaging is equivocal, we would still pursue a biopsy.”
The idea of the fast-track clinic is as the name implies: to quickly confirm the presence of GCA in a matter of hours, or days at the most, in an outpatient setting without the hassles of a biopsy. Temporal artery biopsy (TAB), by comparison, “is more costly because it requires operating room time, a surgical consultation, and surgery time, whereas ultrasound is a very inexpensive exam since it’s done in the clinic by the rheumatologist,” Dr. Kohler said.
European experience
Use of VUS to diagnose GCA is supplanting TAB in Europe and other countries. In Denmark alone – with a population of 6 million – three outpatient fast-track clinics are operating. The United States, with a population more than 50 times larger than Denmark’s, has six.
Stavros Chrysidis, MD, PhD, chief of rheumatology at the Hospital South West Jutland, one of the fast-track clinic sites in Denmark, led a recent multicenter study, known as EUREKA, of VUS in patients with suspected GCA. He and his colleagues reported in The Lancet Rheumatology that the sensitivity and specificity of VUS was superior to TAB in confirming a diagnosis of GCA. Dr. Chrysidis has instructed U.S. rheumatologists and ultrasonographers in performing and interpreting VUS for GCA.
The study emphasizes the importance of training for ultrasonographers, said Dr. Chrysidis, who regularly performs VUS at his institution. “The most important finding is that, when we apply VUS by systematically trained ultrasonographers using appropriate equipment in appropriate settings, it has excellent diagnostic accuracy on GCA,” he told this news organization.
He noted that The Lancet Rheumatology report is the first multicenter study of VUS for diagnosing GCA in which all the ultrasonographers participated in a standardized training program, which his group developed. “Ultrasound is very operator dependent,” he said. “That’s why the training is very important.”
The training occurred over a year and included two workshops consisting of 5 days of theoretical training on VUS; supervised hands-on evaluation of healthy individuals and patients with known GCA; and evaluation of ultrasound images. Over the course of the year, trainees performed at least 50 VUS evaluations, half of which were in patients with confirmed GCA. During the training period, an external rheumatologist with broad experience in VUS made the final diagnosis.
“The equipment and settings are very important because ultrasound can be very time consuming if you are not educated well and if your equipment is not adjusted well,” Dr. Chrysidis said. The equipment must be calibrated beforehand “so you don’t spend time on adjustments.”
For diagnosing temporal artery anomalies, the ultrasound equipment must have a resolution of 0.3-0.4 mm, he said. “When you have a transducer of 10 MHz, you cannot visualize changes smaller than 5 mm.”
The EUREKA study stated that VUS could replace TAB as a first-line diagnostic tool for GCA – provided the ultrasonographers are systematically trained and the equipment and settings are appropriate. In the Jutland clinic, VUS already has replaced TAB, Dr. Chrysidis said.
“In my department since 2017, when we started the fast-track clinic after the EUREKA study was terminated, we have performed three temporal artery biopsies in the last 4 years, and we screen 60-70 patients per year because we use ultrasound as the primary imaging,” he said. In cases when the results are inconclusive, they order a PET scan. “We don’t perform biopsies anymore,” he said.
U.S. fast-track clinic models
The fast-track clinic models in the United States vary. Results of a survey of the U.S. clinics were presented as an abstract at the 2021 American College of Rheumatology annual meeting. Some centers have a vasculitis specialist obtain and interpret the imaging. At others, a vasculitis specialist refers patients to a VUS-trained rheumatologist to perform and interpret the test. Another approach is to have vasculitis specialists refer patients to ultrasound technicians trained in VUS, with a vascular surgeon interpreting the images and either a VUS-trained rheumatologist or vascular medicine specialist verifying the images.
The take-home message of that survey is that “ultrasound evaluation should be considered in the hands of experts, realizing that not everyone has that skill set, but if it is available, it’s a way to expedite diagnosis and it can be helpful in managing the GCA patient in an appropriate way, quicker than trying to schedule cross-sectional imaging,” said Massachusetts General’s Dr. Kohler, who is a coauthor of the abstract. “Certainly, cross-sectional imaging also plays an important role, but when it comes to confirming whether to continue with treatment or not for a very serious condition, ultrasound is a quick way to get the answer.”
In addition to the fast-track clinic at Massachusetts General, the survey included fast-track clinics at the University of Washington, Seattle; Brigham and Women’s Hospital, Boston; Loma Linda (Calif.) University; University of California, Los Angeles; and at a private practice, Arthritis and Rheumatism Associates in the Washington area.
Advantages of VUS vs. TAB
At Massachusetts General, some of the rheumatologists are trained to perform VUS. The rheumatologists also perform the clinical evaluation of suspected cases of GCA. The advantage of VUS, Dr. Kohler said, is that the answer is “right there”; that is, the imaging yields a diagnosis almost instantaneously whereas a biopsy must be sent to a lab for analysis.
“Since a lot of patients with suspected vasculitis may already come to us on steroid therapy, and if there’s a low probability or low suspicion for vasculitis, [VUS] actually confirms that, and we’re able to taper prednisone or steroids quickly rather than keep them on a prolonged course.”
Alison Bays, MD, MPH, of the department of rheumatology at the University of Washington, said that the advantages of avoiding biopsy aren’t to be overlooked. “Temporal artery biopsies are invasive and carry surgical risks, especially as many of our patients are elderly,” she told this news organization.
“These patients occasionally refuse biopsy, but the acceptance of ultrasound is high,” Dr. Bays said. “Scheduling surgery can be more complicated, resulting in delays to biopsy and potentially higher rates of false negatives. Additionally, ultrasound has resulted in a higher rate of diagnosis with GCA as TAB misses large-vessel involvement.” The fast-track clinic at the university has evaluated 250 patients since it opened in 2017.
Dr. Bays and colleagues published the first United States study of a fast-track protocol using vascular sonographers. “Our group has demonstrated that fast-track clinics can rapidly and effectively evaluate patients, and we demonstrated a different method of evaluation using vascular sonographers rather than rheumatologists to do the vascular ultrasound,” she said. “It utilizes the familiarity vascular sonographers already have with imaging blood vessels.”
She added that the TABUL study in the United Kingdom in 2016 demonstrated that VUS yielded a savings of $686 (£484 as reported in the study), compared with TAB. “Further studies need to be done in the United States,” she said. “Beyond direct comparison of costs, reduction in steroid burden due to quick evaluation and diagnosis may carry additional benefits.”
At Brigham and Women’s Hospital, the division of rheumatology and the vascular section of the cardiology division collaborate on the GCA evaluation, said Sara Tedeschi, MD, MPH, codirector of the fast-track clinic there. Trained vascular technologists credentialed in the procedure and specifically trained in using VUS for evaluating arteritis perform the VUS. Cardiologists with a subspecialty in vascular medicine interpret the studies.
VUS patients have a rheumatology evaluation just before they have the ultrasound. “The rheumatology evaluation is then able to incorporate information from the VUS together with laboratory data, the patient’s history, and physical examination,” Dr. Tedeschi said.
“If the rheumatologist recommends a temporal artery biopsy as a next step, we arrange this with vascular surgery,” she said. “If the rheumatologist recommends other imaging such as MRA [magnetic resonance angiography] or PET-CT, we frequently review the images together with our colleagues in cardiovascular radiology and/or nuclear medicine.”
But in the United States, it will take some time for GCA fast-track clinics to become the standard, Dr. Tedeschi said. “Temporal artery biopsy may be faster to arrange in certain practice settings if VUS is not already being employed for giant cell arteritis evaluation,” she said.
Dr. Bays recognized this limitation, saying, “We are hoping that in the future, the American College of Rheumatology will consider vascular ultrasound as a first-line diagnostic test in diagnosis as rheumatologists and vascular sonographers gain familiarity over time.”
But that would mean that centers performing VUS for GCA would have to meet rigorous standards for the procedure. “With that, standardization of a protocol, high-quality equipment, and adequate training are necessary to ensure quality and reduce the chance of false-positive or false-negative results,” she said.
Dr. Chrysidis, Dr. Bays, and Dr. Tedeschi have disclosed no relevant financial relationships. Dr. Kohler is a board member of Ultrasound School of North American Rheumatologists.
A version of this article first appeared on Medscape.com.
Temporal artery biopsy has been the standard for diagnosing giant cell arteritis (GCA), but vascular ultrasound, a procedure that’s less invasive, less time-intensive, less expensive, and more convenient, has gained widespread use in Europe, and now clinics in the United States are adopting this approach and moving toward having rheumatologists take on the role of ultrasonographer.
However, directors at these clinics – known as GCA fast-track clinics – caution that the bar can be high for adopting vascular ultrasound (VUS) as a tool to diagnose GCA. Ultrasonographers need specialized training to perform the task and an adequate caseload to maintain their skills. Clinics also need to be outfitted with high-definition ultrasound machines.
“It definitely takes adequate training and learning of how to adjust the settings on the ultrasound machine to be able to visualize the findings appropriately,” said Minna Kohler, MD, director of the rheumatology and musculoskeletal ultrasound program at Massachusetts General Hospital in Boston, which has its own GCA fast-track clinic.
“And the clinical context is very important,” she said. “If you have a high suspicion for someone with temporal arteritis, or GCA, and the patient has been on steroids for weeks before you see them, the ultrasound findings may not show signs of the disease. In those cases in which the imaging is equivocal, we would still pursue a biopsy.”
The idea of the fast-track clinic is as the name implies: to quickly confirm the presence of GCA in a matter of hours, or days at the most, in an outpatient setting without the hassles of a biopsy. Temporal artery biopsy (TAB), by comparison, “is more costly because it requires operating room time, a surgical consultation, and surgery time, whereas ultrasound is a very inexpensive exam since it’s done in the clinic by the rheumatologist,” Dr. Kohler said.
European experience
Use of VUS to diagnose GCA is supplanting TAB in Europe and other countries. In Denmark alone – with a population of 6 million – three outpatient fast-track clinics are operating. The United States, with a population more than 50 times larger than Denmark’s, has six.
Stavros Chrysidis, MD, PhD, chief of rheumatology at the Hospital South West Jutland, one of the fast-track clinic sites in Denmark, led a recent multicenter study, known as EUREKA, of VUS in patients with suspected GCA. He and his colleagues reported in The Lancet Rheumatology that the sensitivity and specificity of VUS was superior to TAB in confirming a diagnosis of GCA. Dr. Chrysidis has instructed U.S. rheumatologists and ultrasonographers in performing and interpreting VUS for GCA.
The study emphasizes the importance of training for ultrasonographers, said Dr. Chrysidis, who regularly performs VUS at his institution. “The most important finding is that, when we apply VUS by systematically trained ultrasonographers using appropriate equipment in appropriate settings, it has excellent diagnostic accuracy on GCA,” he told this news organization.
He noted that The Lancet Rheumatology report is the first multicenter study of VUS for diagnosing GCA in which all the ultrasonographers participated in a standardized training program, which his group developed. “Ultrasound is very operator dependent,” he said. “That’s why the training is very important.”
The training occurred over a year and included two workshops consisting of 5 days of theoretical training on VUS; supervised hands-on evaluation of healthy individuals and patients with known GCA; and evaluation of ultrasound images. Over the course of the year, trainees performed at least 50 VUS evaluations, half of which were in patients with confirmed GCA. During the training period, an external rheumatologist with broad experience in VUS made the final diagnosis.
“The equipment and settings are very important because ultrasound can be very time consuming if you are not educated well and if your equipment is not adjusted well,” Dr. Chrysidis said. The equipment must be calibrated beforehand “so you don’t spend time on adjustments.”
For diagnosing temporal artery anomalies, the ultrasound equipment must have a resolution of 0.3-0.4 mm, he said. “When you have a transducer of 10 MHz, you cannot visualize changes smaller than 5 mm.”
The EUREKA study stated that VUS could replace TAB as a first-line diagnostic tool for GCA – provided the ultrasonographers are systematically trained and the equipment and settings are appropriate. In the Jutland clinic, VUS already has replaced TAB, Dr. Chrysidis said.
“In my department since 2017, when we started the fast-track clinic after the EUREKA study was terminated, we have performed three temporal artery biopsies in the last 4 years, and we screen 60-70 patients per year because we use ultrasound as the primary imaging,” he said. In cases when the results are inconclusive, they order a PET scan. “We don’t perform biopsies anymore,” he said.
U.S. fast-track clinic models
The fast-track clinic models in the United States vary. Results of a survey of the U.S. clinics were presented as an abstract at the 2021 American College of Rheumatology annual meeting. Some centers have a vasculitis specialist obtain and interpret the imaging. At others, a vasculitis specialist refers patients to a VUS-trained rheumatologist to perform and interpret the test. Another approach is to have vasculitis specialists refer patients to ultrasound technicians trained in VUS, with a vascular surgeon interpreting the images and either a VUS-trained rheumatologist or vascular medicine specialist verifying the images.
The take-home message of that survey is that “ultrasound evaluation should be considered in the hands of experts, realizing that not everyone has that skill set, but if it is available, it’s a way to expedite diagnosis and it can be helpful in managing the GCA patient in an appropriate way, quicker than trying to schedule cross-sectional imaging,” said Massachusetts General’s Dr. Kohler, who is a coauthor of the abstract. “Certainly, cross-sectional imaging also plays an important role, but when it comes to confirming whether to continue with treatment or not for a very serious condition, ultrasound is a quick way to get the answer.”
In addition to the fast-track clinic at Massachusetts General, the survey included fast-track clinics at the University of Washington, Seattle; Brigham and Women’s Hospital, Boston; Loma Linda (Calif.) University; University of California, Los Angeles; and at a private practice, Arthritis and Rheumatism Associates in the Washington area.
Advantages of VUS vs. TAB
At Massachusetts General, some of the rheumatologists are trained to perform VUS. The rheumatologists also perform the clinical evaluation of suspected cases of GCA. The advantage of VUS, Dr. Kohler said, is that the answer is “right there”; that is, the imaging yields a diagnosis almost instantaneously whereas a biopsy must be sent to a lab for analysis.
“Since a lot of patients with suspected vasculitis may already come to us on steroid therapy, and if there’s a low probability or low suspicion for vasculitis, [VUS] actually confirms that, and we’re able to taper prednisone or steroids quickly rather than keep them on a prolonged course.”
Alison Bays, MD, MPH, of the department of rheumatology at the University of Washington, said that the advantages of avoiding biopsy aren’t to be overlooked. “Temporal artery biopsies are invasive and carry surgical risks, especially as many of our patients are elderly,” she told this news organization.
“These patients occasionally refuse biopsy, but the acceptance of ultrasound is high,” Dr. Bays said. “Scheduling surgery can be more complicated, resulting in delays to biopsy and potentially higher rates of false negatives. Additionally, ultrasound has resulted in a higher rate of diagnosis with GCA as TAB misses large-vessel involvement.” The fast-track clinic at the university has evaluated 250 patients since it opened in 2017.
Dr. Bays and colleagues published the first United States study of a fast-track protocol using vascular sonographers. “Our group has demonstrated that fast-track clinics can rapidly and effectively evaluate patients, and we demonstrated a different method of evaluation using vascular sonographers rather than rheumatologists to do the vascular ultrasound,” she said. “It utilizes the familiarity vascular sonographers already have with imaging blood vessels.”
She added that the TABUL study in the United Kingdom in 2016 demonstrated that VUS yielded a savings of $686 (£484 as reported in the study), compared with TAB. “Further studies need to be done in the United States,” she said. “Beyond direct comparison of costs, reduction in steroid burden due to quick evaluation and diagnosis may carry additional benefits.”
At Brigham and Women’s Hospital, the division of rheumatology and the vascular section of the cardiology division collaborate on the GCA evaluation, said Sara Tedeschi, MD, MPH, codirector of the fast-track clinic there. Trained vascular technologists credentialed in the procedure and specifically trained in using VUS for evaluating arteritis perform the VUS. Cardiologists with a subspecialty in vascular medicine interpret the studies.
VUS patients have a rheumatology evaluation just before they have the ultrasound. “The rheumatology evaluation is then able to incorporate information from the VUS together with laboratory data, the patient’s history, and physical examination,” Dr. Tedeschi said.
“If the rheumatologist recommends a temporal artery biopsy as a next step, we arrange this with vascular surgery,” she said. “If the rheumatologist recommends other imaging such as MRA [magnetic resonance angiography] or PET-CT, we frequently review the images together with our colleagues in cardiovascular radiology and/or nuclear medicine.”
But in the United States, it will take some time for GCA fast-track clinics to become the standard, Dr. Tedeschi said. “Temporal artery biopsy may be faster to arrange in certain practice settings if VUS is not already being employed for giant cell arteritis evaluation,” she said.
Dr. Bays recognized this limitation, saying, “We are hoping that in the future, the American College of Rheumatology will consider vascular ultrasound as a first-line diagnostic test in diagnosis as rheumatologists and vascular sonographers gain familiarity over time.”
But that would mean that centers performing VUS for GCA would have to meet rigorous standards for the procedure. “With that, standardization of a protocol, high-quality equipment, and adequate training are necessary to ensure quality and reduce the chance of false-positive or false-negative results,” she said.
Dr. Chrysidis, Dr. Bays, and Dr. Tedeschi have disclosed no relevant financial relationships. Dr. Kohler is a board member of Ultrasound School of North American Rheumatologists.
A version of this article first appeared on Medscape.com.
Don’t give up on relentless youth depression
As pediatricians, we are acutely aware of the increase in depression in our teen patients. Lifetime prevalence is now approaching 20%, and we are doing our best to help.
The Guidelines for Adolescent Depression in Primary Care (GLAD-PC, 2018) has advice on screening and primary care provider (PCP) management, verifying our role in care. But GLAD-PC also advises “referral to a mental health specialist” in patient scenarios we see multiple times per week. Even when patients are willing and able to go, mental health specialists are in short supply or have months-long waiting lists. What should we do to help the more severely depressed adolescent when immediate referral is not possible? What should we expect of specialist care for what is called treatment-resistant or treatment-refractory depression (TRD)?
To know what to do for a youth with TRD, first you need to know what constitutes an adequate trial of treatment. After diagnosis of major depressive disorder (MDD) from a validated screening tool or an interview based on DSM-5 criteria and an appropriate assessment (as described in GLAD-PC), patients and parents need education on symptoms, course, prognosis including suicide risk, and treatment options. Known TRD risk factors, besides longer or greater depression severity, anhedonia, and poor global functioning, can benefit from being specifically addressed: trauma, bullying, comorbid anxiety or substance use, subsyndromal mania, insomnia, hypothyroidism, nutritional deficiencies from eating disorders, certain genetic variants, LGBTQ identification, family conflict, and parental depression. Screening and assessment for suicidal ideation/attempts is needed initially and in follow-up as MDD increases risk of suicide 30 times.
PCPs can manage mild depression with regular visits every 1-2 weeks for active support for 6-8 weeks. Advise all depressed youth on healthy eating, adequate sleep and exercise, pleasurable activities, and refraining from substance use. With a full response (50%+ reduction in symptom score from baseline), monthly monitoring for symptoms, suicidality, and stressors (phone/televisits suffice) should continue for 6-24 months as half recur. Monitoring with ratings by both youth and parent are recommended and may be required by insurers. Scores below cutoff suggest “remission,” although functioning must be considered. Youth report symptoms best but parents may better report improved functioning and affect that can precede symptom reduction.
If there is no initial response (< 25% decrease in symptom score) or a partial response (25%-49% decrease), PCPs should begin treatment as for moderate depression with either a selective serotonin reuptake inhibitor (SSRI) or psychotherapy. Use of both has the best evidence; cognitive behavior therapy (CBT) and interpersonal psychotherapy for adolescents are equally effective.
Side effects from SSRIs are almost universal with GI upset, headaches, and sexual dysfunction most common, but activation (increased agitation or irritability) may occur. Educate patients about these and encourage tolerating them as they tend to subside in weeks, allowing continuation of these most effective medicines. Activation rarely indicates true mania, which would require stopping and referral.
Moderate depression with only comorbid anxiety may be addressed by PCPs with problem-focused supportive counseling and SSRIs, but mental health consultation or referral also are appropriate. Fluoxetine starting at 5-10 mg/day has best evidence and Food and Drug Administration approval for MDD from age 8. Starting at a higher dose may increase risk of suicidal ideation. Alternatively, escitalopram is FDA approved for MDD at age 12 starting at 10 mg/day, although meta-analyses do not distinguish effectiveness within the SSRI class. Although benefit usually appears within 2 weeks, a trial of at least 4 weeks should be used to assess effect.
If after 4 weeks, the SSRI is tolerated but has little or no response, reassess the diagnosis, try a different SSRI, e.g. sertraline, and add CBT (combined SSRI+CBT has an advantage). To switch SSRIs, reduce the first every 1-2 weeks (by 10-20 mg for fluoxetine; 5-10 for escitalopram) to reduce side effects. If overlapping, the replacement SSRI may start midway in the wean at low dose with patients educated about serotonin syndrome. If instead there was a partial response to the initial SSRI, progressively increase the dose (by 10 mg for fluoxetine or 5 mg for escitalopram monthly) as indicated by symptom change up to the maximum (60-80 mg fluoxetine or 20 mg escitalopram), if needed, and maintain for another 4 weeks. Alternatively, or in addition, start psychotherapy or ask to change current therapy, as therapy focus makes a difference in effect. Initial CBT focus on anxiety acts fastest when anxiety is comorbid.
Once a regimen produces a response, maintain it for 16-20 weeks, the longer for more severe depression. Although three-fourths of mildly to moderately depressed youth are late responders, emerging near 6 weeks, a rapid initial response is associated with better outcome. The recommended 8 weeks on a final tolerated dose constituting an adequate trial before changing may be shortened to 6 weeks in severe unremitting cases. Youth not remitting by 12 weeks should be offered alternative treatment. Referral is recommended for moderately severe depression with comorbidity or severe depression but also for unresponsive moderate depression or by family or clinician preference.
Treatment-resistant depression is defined as “clinically impairing depression symptoms despite an adequate trial of an evidence-based psychotherapy and an antidepressant with grade A evidence (fluoxetine, escitalopram, or sertraline),” sequentially or together; treatment-refractory depression comprises the above with failure on at least two antidepressants, with at least one being grade A. Unfortunately, TRD occurs in 30%-40% of children and remission is only 30%. Low adherence based on pill counts (> 30% missed) or with therapy (fewer than nine visits) should be considered in treatment failures.
With manageable factors addressed, the next step for TRD is treatment augmentation. The best evidence-based augmentation for TRD is CBT; 55% of those receiving CBT responded within 12 weeks. TRD augmentations and interventions with evidence in adults have either no evidence of effect in children (SNRIs, lithium), no randomized controlled trials, or support only from small suggestive studies, e.g., antipsychotics, 16 g/day omega-3 fatty acid supplementation, folic acid supplementation, repetitive transcranial magnetic stimulation, electroconvulsive therapy, or ketamine. Prompt referral to a child psychiatrist is essential for youth classified as TRD as earlier more aggressive treatment may avoid the long-term morbidity of chronic depression.
Fortunately, a meta-analysis of studies showed that PCP medication management visits with monitoring could improve outcomes, even for TRD.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Reference
Dwyer J et al. Annual research review: Defining and treating pediatric treatment-resistant depression. J Child Psychol Psychiatry. 2020 March;61(3):312-32.
As pediatricians, we are acutely aware of the increase in depression in our teen patients. Lifetime prevalence is now approaching 20%, and we are doing our best to help.
The Guidelines for Adolescent Depression in Primary Care (GLAD-PC, 2018) has advice on screening and primary care provider (PCP) management, verifying our role in care. But GLAD-PC also advises “referral to a mental health specialist” in patient scenarios we see multiple times per week. Even when patients are willing and able to go, mental health specialists are in short supply or have months-long waiting lists. What should we do to help the more severely depressed adolescent when immediate referral is not possible? What should we expect of specialist care for what is called treatment-resistant or treatment-refractory depression (TRD)?
To know what to do for a youth with TRD, first you need to know what constitutes an adequate trial of treatment. After diagnosis of major depressive disorder (MDD) from a validated screening tool or an interview based on DSM-5 criteria and an appropriate assessment (as described in GLAD-PC), patients and parents need education on symptoms, course, prognosis including suicide risk, and treatment options. Known TRD risk factors, besides longer or greater depression severity, anhedonia, and poor global functioning, can benefit from being specifically addressed: trauma, bullying, comorbid anxiety or substance use, subsyndromal mania, insomnia, hypothyroidism, nutritional deficiencies from eating disorders, certain genetic variants, LGBTQ identification, family conflict, and parental depression. Screening and assessment for suicidal ideation/attempts is needed initially and in follow-up as MDD increases risk of suicide 30 times.
PCPs can manage mild depression with regular visits every 1-2 weeks for active support for 6-8 weeks. Advise all depressed youth on healthy eating, adequate sleep and exercise, pleasurable activities, and refraining from substance use. With a full response (50%+ reduction in symptom score from baseline), monthly monitoring for symptoms, suicidality, and stressors (phone/televisits suffice) should continue for 6-24 months as half recur. Monitoring with ratings by both youth and parent are recommended and may be required by insurers. Scores below cutoff suggest “remission,” although functioning must be considered. Youth report symptoms best but parents may better report improved functioning and affect that can precede symptom reduction.
If there is no initial response (< 25% decrease in symptom score) or a partial response (25%-49% decrease), PCPs should begin treatment as for moderate depression with either a selective serotonin reuptake inhibitor (SSRI) or psychotherapy. Use of both has the best evidence; cognitive behavior therapy (CBT) and interpersonal psychotherapy for adolescents are equally effective.
Side effects from SSRIs are almost universal with GI upset, headaches, and sexual dysfunction most common, but activation (increased agitation or irritability) may occur. Educate patients about these and encourage tolerating them as they tend to subside in weeks, allowing continuation of these most effective medicines. Activation rarely indicates true mania, which would require stopping and referral.
Moderate depression with only comorbid anxiety may be addressed by PCPs with problem-focused supportive counseling and SSRIs, but mental health consultation or referral also are appropriate. Fluoxetine starting at 5-10 mg/day has best evidence and Food and Drug Administration approval for MDD from age 8. Starting at a higher dose may increase risk of suicidal ideation. Alternatively, escitalopram is FDA approved for MDD at age 12 starting at 10 mg/day, although meta-analyses do not distinguish effectiveness within the SSRI class. Although benefit usually appears within 2 weeks, a trial of at least 4 weeks should be used to assess effect.
If after 4 weeks, the SSRI is tolerated but has little or no response, reassess the diagnosis, try a different SSRI, e.g. sertraline, and add CBT (combined SSRI+CBT has an advantage). To switch SSRIs, reduce the first every 1-2 weeks (by 10-20 mg for fluoxetine; 5-10 for escitalopram) to reduce side effects. If overlapping, the replacement SSRI may start midway in the wean at low dose with patients educated about serotonin syndrome. If instead there was a partial response to the initial SSRI, progressively increase the dose (by 10 mg for fluoxetine or 5 mg for escitalopram monthly) as indicated by symptom change up to the maximum (60-80 mg fluoxetine or 20 mg escitalopram), if needed, and maintain for another 4 weeks. Alternatively, or in addition, start psychotherapy or ask to change current therapy, as therapy focus makes a difference in effect. Initial CBT focus on anxiety acts fastest when anxiety is comorbid.
Once a regimen produces a response, maintain it for 16-20 weeks, the longer for more severe depression. Although three-fourths of mildly to moderately depressed youth are late responders, emerging near 6 weeks, a rapid initial response is associated with better outcome. The recommended 8 weeks on a final tolerated dose constituting an adequate trial before changing may be shortened to 6 weeks in severe unremitting cases. Youth not remitting by 12 weeks should be offered alternative treatment. Referral is recommended for moderately severe depression with comorbidity or severe depression but also for unresponsive moderate depression or by family or clinician preference.
Treatment-resistant depression is defined as “clinically impairing depression symptoms despite an adequate trial of an evidence-based psychotherapy and an antidepressant with grade A evidence (fluoxetine, escitalopram, or sertraline),” sequentially or together; treatment-refractory depression comprises the above with failure on at least two antidepressants, with at least one being grade A. Unfortunately, TRD occurs in 30%-40% of children and remission is only 30%. Low adherence based on pill counts (> 30% missed) or with therapy (fewer than nine visits) should be considered in treatment failures.
With manageable factors addressed, the next step for TRD is treatment augmentation. The best evidence-based augmentation for TRD is CBT; 55% of those receiving CBT responded within 12 weeks. TRD augmentations and interventions with evidence in adults have either no evidence of effect in children (SNRIs, lithium), no randomized controlled trials, or support only from small suggestive studies, e.g., antipsychotics, 16 g/day omega-3 fatty acid supplementation, folic acid supplementation, repetitive transcranial magnetic stimulation, electroconvulsive therapy, or ketamine. Prompt referral to a child psychiatrist is essential for youth classified as TRD as earlier more aggressive treatment may avoid the long-term morbidity of chronic depression.
Fortunately, a meta-analysis of studies showed that PCP medication management visits with monitoring could improve outcomes, even for TRD.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Reference
Dwyer J et al. Annual research review: Defining and treating pediatric treatment-resistant depression. J Child Psychol Psychiatry. 2020 March;61(3):312-32.
As pediatricians, we are acutely aware of the increase in depression in our teen patients. Lifetime prevalence is now approaching 20%, and we are doing our best to help.
The Guidelines for Adolescent Depression in Primary Care (GLAD-PC, 2018) has advice on screening and primary care provider (PCP) management, verifying our role in care. But GLAD-PC also advises “referral to a mental health specialist” in patient scenarios we see multiple times per week. Even when patients are willing and able to go, mental health specialists are in short supply or have months-long waiting lists. What should we do to help the more severely depressed adolescent when immediate referral is not possible? What should we expect of specialist care for what is called treatment-resistant or treatment-refractory depression (TRD)?
To know what to do for a youth with TRD, first you need to know what constitutes an adequate trial of treatment. After diagnosis of major depressive disorder (MDD) from a validated screening tool or an interview based on DSM-5 criteria and an appropriate assessment (as described in GLAD-PC), patients and parents need education on symptoms, course, prognosis including suicide risk, and treatment options. Known TRD risk factors, besides longer or greater depression severity, anhedonia, and poor global functioning, can benefit from being specifically addressed: trauma, bullying, comorbid anxiety or substance use, subsyndromal mania, insomnia, hypothyroidism, nutritional deficiencies from eating disorders, certain genetic variants, LGBTQ identification, family conflict, and parental depression. Screening and assessment for suicidal ideation/attempts is needed initially and in follow-up as MDD increases risk of suicide 30 times.
PCPs can manage mild depression with regular visits every 1-2 weeks for active support for 6-8 weeks. Advise all depressed youth on healthy eating, adequate sleep and exercise, pleasurable activities, and refraining from substance use. With a full response (50%+ reduction in symptom score from baseline), monthly monitoring for symptoms, suicidality, and stressors (phone/televisits suffice) should continue for 6-24 months as half recur. Monitoring with ratings by both youth and parent are recommended and may be required by insurers. Scores below cutoff suggest “remission,” although functioning must be considered. Youth report symptoms best but parents may better report improved functioning and affect that can precede symptom reduction.
If there is no initial response (< 25% decrease in symptom score) or a partial response (25%-49% decrease), PCPs should begin treatment as for moderate depression with either a selective serotonin reuptake inhibitor (SSRI) or psychotherapy. Use of both has the best evidence; cognitive behavior therapy (CBT) and interpersonal psychotherapy for adolescents are equally effective.
Side effects from SSRIs are almost universal with GI upset, headaches, and sexual dysfunction most common, but activation (increased agitation or irritability) may occur. Educate patients about these and encourage tolerating them as they tend to subside in weeks, allowing continuation of these most effective medicines. Activation rarely indicates true mania, which would require stopping and referral.
Moderate depression with only comorbid anxiety may be addressed by PCPs with problem-focused supportive counseling and SSRIs, but mental health consultation or referral also are appropriate. Fluoxetine starting at 5-10 mg/day has best evidence and Food and Drug Administration approval for MDD from age 8. Starting at a higher dose may increase risk of suicidal ideation. Alternatively, escitalopram is FDA approved for MDD at age 12 starting at 10 mg/day, although meta-analyses do not distinguish effectiveness within the SSRI class. Although benefit usually appears within 2 weeks, a trial of at least 4 weeks should be used to assess effect.
If after 4 weeks, the SSRI is tolerated but has little or no response, reassess the diagnosis, try a different SSRI, e.g. sertraline, and add CBT (combined SSRI+CBT has an advantage). To switch SSRIs, reduce the first every 1-2 weeks (by 10-20 mg for fluoxetine; 5-10 for escitalopram) to reduce side effects. If overlapping, the replacement SSRI may start midway in the wean at low dose with patients educated about serotonin syndrome. If instead there was a partial response to the initial SSRI, progressively increase the dose (by 10 mg for fluoxetine or 5 mg for escitalopram monthly) as indicated by symptom change up to the maximum (60-80 mg fluoxetine or 20 mg escitalopram), if needed, and maintain for another 4 weeks. Alternatively, or in addition, start psychotherapy or ask to change current therapy, as therapy focus makes a difference in effect. Initial CBT focus on anxiety acts fastest when anxiety is comorbid.
Once a regimen produces a response, maintain it for 16-20 weeks, the longer for more severe depression. Although three-fourths of mildly to moderately depressed youth are late responders, emerging near 6 weeks, a rapid initial response is associated with better outcome. The recommended 8 weeks on a final tolerated dose constituting an adequate trial before changing may be shortened to 6 weeks in severe unremitting cases. Youth not remitting by 12 weeks should be offered alternative treatment. Referral is recommended for moderately severe depression with comorbidity or severe depression but also for unresponsive moderate depression or by family or clinician preference.
Treatment-resistant depression is defined as “clinically impairing depression symptoms despite an adequate trial of an evidence-based psychotherapy and an antidepressant with grade A evidence (fluoxetine, escitalopram, or sertraline),” sequentially or together; treatment-refractory depression comprises the above with failure on at least two antidepressants, with at least one being grade A. Unfortunately, TRD occurs in 30%-40% of children and remission is only 30%. Low adherence based on pill counts (> 30% missed) or with therapy (fewer than nine visits) should be considered in treatment failures.
With manageable factors addressed, the next step for TRD is treatment augmentation. The best evidence-based augmentation for TRD is CBT; 55% of those receiving CBT responded within 12 weeks. TRD augmentations and interventions with evidence in adults have either no evidence of effect in children (SNRIs, lithium), no randomized controlled trials, or support only from small suggestive studies, e.g., antipsychotics, 16 g/day omega-3 fatty acid supplementation, folic acid supplementation, repetitive transcranial magnetic stimulation, electroconvulsive therapy, or ketamine. Prompt referral to a child psychiatrist is essential for youth classified as TRD as earlier more aggressive treatment may avoid the long-term morbidity of chronic depression.
Fortunately, a meta-analysis of studies showed that PCP medication management visits with monitoring could improve outcomes, even for TRD.
Dr. Howard is assistant professor of pediatrics at Johns Hopkins University, Baltimore, and creator of CHADIS (www.CHADIS.com). She had no other relevant disclosures. Dr. Howard’s contribution to this publication was as a paid expert to MDedge News. E-mail her at [email protected].
Reference
Dwyer J et al. Annual research review: Defining and treating pediatric treatment-resistant depression. J Child Psychol Psychiatry. 2020 March;61(3):312-32.
‘Highest survival’ with combo immunotherapy in advanced melanoma
An researchers say.
Nearly half the patients treated with nivolumab (Opdivo) and ipilimumab (Yervoy) were alive at 6½ years. Within this group, 77% had not received further systemic treatment after coming off the study drugs.
After a minimum follow-up of 77 months, median overall survival was 72.1 months in patients on the combination, which was more than three times longer than the 19.9 months with ipilimumab alone (hazard ratio, 0.52; 95% confidence interval, 0.43-0.64) and twice as long as the 36.9 months with nivolumab alone (HR, 0.84; 95% CI, 0.67-1.04).
The results represent the longest median overall survival seen in a phase 3 trial of advanced melanoma and are evidence of “a substantial development in the melanoma treatment landscape versus the standard median survival of 8 months a decade ago,” researchers wrote in a study published online in the Journal of Clinical Oncology.
However, lead author Jedd D. Wolchok, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, noted that the study was not designed to compare nivolumab alone with the combination. “It wasn’t powered for that. [But] what we can say is that the highest survival was in the combination group,” Dr. Wolchok told this news organization.
Dr. Wolchok cautioned that the combination therapy is not currently standard of care. “PD-1 blockade – either nivolumab or the combination – are both excellent options for care,” he added. “I can’t tell you that one of them is the standard of care because that’s too complex of a decision.”
For example, he explained, “for a patient who only has lung metastases, a single-agent PD-1 blockade might be sufficient. But if it has spread to other organs, such as the liver or bones, which are more difficult to treat, that’s when we often reach for the combination.”
Other factors that weigh into the therapeutic decision are the patient’s performance status and their so-called clinical reserve for tolerating side effects. “The likelihood of having a high-grade side effect with the combination is more than twice that of the single agent,” Dr. Wolchok said.
Until 2011, only two therapies were approved for metastatic melanoma: Chemotherapy with dacarbazine and immunotherapy with high-dose interleukin-2, neither of which was very effective at prolonging life. But patient survival changed with the advent of targeted therapies and immunotherapy. Some patients are now living for years, and as the current study shows, many have surpassed the 5-year mark and are treatment free.
The updated CheckMate 067 analysis included patients with previously untreated, unresectable stage III/IV melanoma who were randomly assigned to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (four doses) followed by nivolumab 3 mg/kg every 2 weeks (n = 314), nivolumab 3 mg/kg every 2 weeks (n = 316), or ipilimumab 3 mg/ kg every 3 weeks (four doses; n = 315).
The authors reported the 5-year overall survival rates from the trial, published in the New England Journal of Medicine in 2019 – 52% with the combination, 44% with nivolumab alone, and 26% with ipilimumab alone.
In the updated study, overall survival at 6½ years had dropped slightly to 49%, 42%, and 23%, respectively. Patients with BRAF-mutant tumors had overall survival rates of 57%, 43%, and 25% versus 46%, 42%, and 22% in those with BRAF wild-type tumors.
Overall, median investigator-assessed progression-free survival was 11.5 months with the combination, 6.9 months with nivolumab alone, and 2.9 months with ipilimumab.
The new analysis also evaluated melanoma-specific survival (MSS), which removes competing causes of deaths from the long-term follow-up. The MSS was not reached in the combination group, and was 58.7 months in the nivolumab group and 21.9 months for ipilimumab, with MSS rates at 6.5 years of 56%, 48%, and 27%, respectively.
No new safety signals were detected, but there was more immune-mediated toxicity in the combination group, the researchers reported.
“The patients will continue to be followed,” said Dr. Wolchok, “And data are still being collected.”
The trial was supported by Bristol-Myers Squibb, the National Cancer Institute, and the National Institute for Health Research Royal Marsden–Institute of Cancer Research Biomedical Research Centre. Dr. Wolchok and coauthors reported relationships with Bristol-Myers Squibb and other drugmakers.
A version of this article first appeared on Medscape.com.
An researchers say.
Nearly half the patients treated with nivolumab (Opdivo) and ipilimumab (Yervoy) were alive at 6½ years. Within this group, 77% had not received further systemic treatment after coming off the study drugs.
After a minimum follow-up of 77 months, median overall survival was 72.1 months in patients on the combination, which was more than three times longer than the 19.9 months with ipilimumab alone (hazard ratio, 0.52; 95% confidence interval, 0.43-0.64) and twice as long as the 36.9 months with nivolumab alone (HR, 0.84; 95% CI, 0.67-1.04).
The results represent the longest median overall survival seen in a phase 3 trial of advanced melanoma and are evidence of “a substantial development in the melanoma treatment landscape versus the standard median survival of 8 months a decade ago,” researchers wrote in a study published online in the Journal of Clinical Oncology.
However, lead author Jedd D. Wolchok, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, noted that the study was not designed to compare nivolumab alone with the combination. “It wasn’t powered for that. [But] what we can say is that the highest survival was in the combination group,” Dr. Wolchok told this news organization.
Dr. Wolchok cautioned that the combination therapy is not currently standard of care. “PD-1 blockade – either nivolumab or the combination – are both excellent options for care,” he added. “I can’t tell you that one of them is the standard of care because that’s too complex of a decision.”
For example, he explained, “for a patient who only has lung metastases, a single-agent PD-1 blockade might be sufficient. But if it has spread to other organs, such as the liver or bones, which are more difficult to treat, that’s when we often reach for the combination.”
Other factors that weigh into the therapeutic decision are the patient’s performance status and their so-called clinical reserve for tolerating side effects. “The likelihood of having a high-grade side effect with the combination is more than twice that of the single agent,” Dr. Wolchok said.
Until 2011, only two therapies were approved for metastatic melanoma: Chemotherapy with dacarbazine and immunotherapy with high-dose interleukin-2, neither of which was very effective at prolonging life. But patient survival changed with the advent of targeted therapies and immunotherapy. Some patients are now living for years, and as the current study shows, many have surpassed the 5-year mark and are treatment free.
The updated CheckMate 067 analysis included patients with previously untreated, unresectable stage III/IV melanoma who were randomly assigned to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (four doses) followed by nivolumab 3 mg/kg every 2 weeks (n = 314), nivolumab 3 mg/kg every 2 weeks (n = 316), or ipilimumab 3 mg/ kg every 3 weeks (four doses; n = 315).
The authors reported the 5-year overall survival rates from the trial, published in the New England Journal of Medicine in 2019 – 52% with the combination, 44% with nivolumab alone, and 26% with ipilimumab alone.
In the updated study, overall survival at 6½ years had dropped slightly to 49%, 42%, and 23%, respectively. Patients with BRAF-mutant tumors had overall survival rates of 57%, 43%, and 25% versus 46%, 42%, and 22% in those with BRAF wild-type tumors.
Overall, median investigator-assessed progression-free survival was 11.5 months with the combination, 6.9 months with nivolumab alone, and 2.9 months with ipilimumab.
The new analysis also evaluated melanoma-specific survival (MSS), which removes competing causes of deaths from the long-term follow-up. The MSS was not reached in the combination group, and was 58.7 months in the nivolumab group and 21.9 months for ipilimumab, with MSS rates at 6.5 years of 56%, 48%, and 27%, respectively.
No new safety signals were detected, but there was more immune-mediated toxicity in the combination group, the researchers reported.
“The patients will continue to be followed,” said Dr. Wolchok, “And data are still being collected.”
The trial was supported by Bristol-Myers Squibb, the National Cancer Institute, and the National Institute for Health Research Royal Marsden–Institute of Cancer Research Biomedical Research Centre. Dr. Wolchok and coauthors reported relationships with Bristol-Myers Squibb and other drugmakers.
A version of this article first appeared on Medscape.com.
An researchers say.
Nearly half the patients treated with nivolumab (Opdivo) and ipilimumab (Yervoy) were alive at 6½ years. Within this group, 77% had not received further systemic treatment after coming off the study drugs.
After a minimum follow-up of 77 months, median overall survival was 72.1 months in patients on the combination, which was more than three times longer than the 19.9 months with ipilimumab alone (hazard ratio, 0.52; 95% confidence interval, 0.43-0.64) and twice as long as the 36.9 months with nivolumab alone (HR, 0.84; 95% CI, 0.67-1.04).
The results represent the longest median overall survival seen in a phase 3 trial of advanced melanoma and are evidence of “a substantial development in the melanoma treatment landscape versus the standard median survival of 8 months a decade ago,” researchers wrote in a study published online in the Journal of Clinical Oncology.
However, lead author Jedd D. Wolchok, MD, PhD, of Memorial Sloan Kettering Cancer Center in New York, noted that the study was not designed to compare nivolumab alone with the combination. “It wasn’t powered for that. [But] what we can say is that the highest survival was in the combination group,” Dr. Wolchok told this news organization.
Dr. Wolchok cautioned that the combination therapy is not currently standard of care. “PD-1 blockade – either nivolumab or the combination – are both excellent options for care,” he added. “I can’t tell you that one of them is the standard of care because that’s too complex of a decision.”
For example, he explained, “for a patient who only has lung metastases, a single-agent PD-1 blockade might be sufficient. But if it has spread to other organs, such as the liver or bones, which are more difficult to treat, that’s when we often reach for the combination.”
Other factors that weigh into the therapeutic decision are the patient’s performance status and their so-called clinical reserve for tolerating side effects. “The likelihood of having a high-grade side effect with the combination is more than twice that of the single agent,” Dr. Wolchok said.
Until 2011, only two therapies were approved for metastatic melanoma: Chemotherapy with dacarbazine and immunotherapy with high-dose interleukin-2, neither of which was very effective at prolonging life. But patient survival changed with the advent of targeted therapies and immunotherapy. Some patients are now living for years, and as the current study shows, many have surpassed the 5-year mark and are treatment free.
The updated CheckMate 067 analysis included patients with previously untreated, unresectable stage III/IV melanoma who were randomly assigned to receive nivolumab 1 mg/kg plus ipilimumab 3 mg/kg every 3 weeks (four doses) followed by nivolumab 3 mg/kg every 2 weeks (n = 314), nivolumab 3 mg/kg every 2 weeks (n = 316), or ipilimumab 3 mg/ kg every 3 weeks (four doses; n = 315).
The authors reported the 5-year overall survival rates from the trial, published in the New England Journal of Medicine in 2019 – 52% with the combination, 44% with nivolumab alone, and 26% with ipilimumab alone.
In the updated study, overall survival at 6½ years had dropped slightly to 49%, 42%, and 23%, respectively. Patients with BRAF-mutant tumors had overall survival rates of 57%, 43%, and 25% versus 46%, 42%, and 22% in those with BRAF wild-type tumors.
Overall, median investigator-assessed progression-free survival was 11.5 months with the combination, 6.9 months with nivolumab alone, and 2.9 months with ipilimumab.
The new analysis also evaluated melanoma-specific survival (MSS), which removes competing causes of deaths from the long-term follow-up. The MSS was not reached in the combination group, and was 58.7 months in the nivolumab group and 21.9 months for ipilimumab, with MSS rates at 6.5 years of 56%, 48%, and 27%, respectively.
No new safety signals were detected, but there was more immune-mediated toxicity in the combination group, the researchers reported.
“The patients will continue to be followed,” said Dr. Wolchok, “And data are still being collected.”
The trial was supported by Bristol-Myers Squibb, the National Cancer Institute, and the National Institute for Health Research Royal Marsden–Institute of Cancer Research Biomedical Research Centre. Dr. Wolchok and coauthors reported relationships with Bristol-Myers Squibb and other drugmakers.
A version of this article first appeared on Medscape.com.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
AMA president calls on Congress to stabilize Medicare payments to physicians
Physician practices around the country took an unprecedented financial hit with the arrival of the COVID-19 pandemic in March 2020. Recent research from the American Medical Association reveals an estimated pandemic-related shortfall in Medicare physician fee spending of $13.9 billion, or a 14% reduction, across all states and all major specialties in 2020.
While the report pointed to a “strong recovery” in May and June, that recovery stalled in the second half of 2020, and spending never returned to pre–COVID-19 levels.
“Physicians experienced a significant and sustained drop in Medicare revenue during the first 10 months of the pandemic,” said AMA President Gerald Harmon, MD, in a statement. “Medical practices that have not buckled under financial strain continue to be stretched clinically, emotionally, and fiscally as the pandemic persists. Yet physicians face an array of planned cuts that would reduce Medicare physician payments by nearly 10% for 2022.”
The reduction in the Medicare physician fee schedule payments means providers may face payment cuts of more than 9% starting Jan. 1, 2022, when the cuts take effect. That is, unless Congress makes changes.
Medicare physician fee schedule spending on telehealth stood at $4.1 billion, or 5% of the total Medicare spent in 2020. From March 16 to June 30, $1.8 billion of this amount was on telehealth, while $1.1 billion came in during third and fourth quarters of 2020, respectively, per the report.
According to AMA’s research:
- Medicare physician fee schedule spending for 2020, relative to expected 2020 spending, dipped 32% between March 16 and June 30; spending was down during the last 6 months of the year by between 9% and 10%.
- The care settings hit the worst were ambulatory surgical centers, outpatient hospitals, and physician offices; the next worst off were hospital emergency departments, inpatient hospitals, and skilled nursing facilities.
- The specialties that fared worst included physical therapists (-28%), opthamologists (-19%), podiatrists (-18%), and dermatologists (-18%).
- Cumulative spending was down the most in Minnesota (-22%), Maine (-19%), and New York (-19%); less affected states included Idaho (-9%), Oklahoma (-9%), and South Carolina (9%).
AMA: Budget neutrality hurting physicians’ financial stability
Dr. Harmon is calling for financial stability in Medicare spending. In particular, the AMA is “strongly urging Congress to avert the planned payment cuts,” he said in a statement.
The challenge: The Medicare physician fee schedule is currently “budget neutral,” meaning that the budget is fixed, Dr. Harmon, a family medicine specialist in South Carolina, told this news organization.
“If you rob from Peter to pay Paul, Paul is going to be less efficient or less rewarded. It continues to be that there’s always a ‘pay for’ in these things. So budget neutrality is probably one of the first things we need to address,” he said.
Lack of routine care expected to affect health outcomes
The result of reduced screening and treatment during the pandemic could be as many as 10,000 excess deaths due to cancers of the breast and colon during the next 10 years, wrote Norman Sharpless, MD, director of the National Cancer Institute, in Science in June. Combined, breast cancer and colon cancer account for one-sixth of all cancers in the U.S., he wrote.
In addition, blood pressure control has gotten worse since the start of the pandemic, said Michael Rakotz, MD, FAHA, FAAFP, vice president of improving health outcomes at the AMA, in an AMA blog post.
Dr. Harmon’s advice for physician practices on getting patients in for routine care:
- Educate the area’s largest employers to encourage their employees.
- Engage with hospital employees, since hospitals are often the largest employers in many communities.
- Partner with health insurers.
- Show up at athletic events, which is a particularly good fit for “small town America,” said Dr. Harmon.
The AMA’s research doesn’t consider reimbursement from other public and private payers. It also doesn’t account for funding sources such as Provider Relief Fund grants, Paycheck Protection Program loans, and the temporary suspension of the Medicare sequester, per the report.
A version of this article first appeared on Medscape.com.
Physician practices around the country took an unprecedented financial hit with the arrival of the COVID-19 pandemic in March 2020. Recent research from the American Medical Association reveals an estimated pandemic-related shortfall in Medicare physician fee spending of $13.9 billion, or a 14% reduction, across all states and all major specialties in 2020.
While the report pointed to a “strong recovery” in May and June, that recovery stalled in the second half of 2020, and spending never returned to pre–COVID-19 levels.
“Physicians experienced a significant and sustained drop in Medicare revenue during the first 10 months of the pandemic,” said AMA President Gerald Harmon, MD, in a statement. “Medical practices that have not buckled under financial strain continue to be stretched clinically, emotionally, and fiscally as the pandemic persists. Yet physicians face an array of planned cuts that would reduce Medicare physician payments by nearly 10% for 2022.”
The reduction in the Medicare physician fee schedule payments means providers may face payment cuts of more than 9% starting Jan. 1, 2022, when the cuts take effect. That is, unless Congress makes changes.
Medicare physician fee schedule spending on telehealth stood at $4.1 billion, or 5% of the total Medicare spent in 2020. From March 16 to June 30, $1.8 billion of this amount was on telehealth, while $1.1 billion came in during third and fourth quarters of 2020, respectively, per the report.
According to AMA’s research:
- Medicare physician fee schedule spending for 2020, relative to expected 2020 spending, dipped 32% between March 16 and June 30; spending was down during the last 6 months of the year by between 9% and 10%.
- The care settings hit the worst were ambulatory surgical centers, outpatient hospitals, and physician offices; the next worst off were hospital emergency departments, inpatient hospitals, and skilled nursing facilities.
- The specialties that fared worst included physical therapists (-28%), opthamologists (-19%), podiatrists (-18%), and dermatologists (-18%).
- Cumulative spending was down the most in Minnesota (-22%), Maine (-19%), and New York (-19%); less affected states included Idaho (-9%), Oklahoma (-9%), and South Carolina (9%).
AMA: Budget neutrality hurting physicians’ financial stability
Dr. Harmon is calling for financial stability in Medicare spending. In particular, the AMA is “strongly urging Congress to avert the planned payment cuts,” he said in a statement.
The challenge: The Medicare physician fee schedule is currently “budget neutral,” meaning that the budget is fixed, Dr. Harmon, a family medicine specialist in South Carolina, told this news organization.
“If you rob from Peter to pay Paul, Paul is going to be less efficient or less rewarded. It continues to be that there’s always a ‘pay for’ in these things. So budget neutrality is probably one of the first things we need to address,” he said.
Lack of routine care expected to affect health outcomes
The result of reduced screening and treatment during the pandemic could be as many as 10,000 excess deaths due to cancers of the breast and colon during the next 10 years, wrote Norman Sharpless, MD, director of the National Cancer Institute, in Science in June. Combined, breast cancer and colon cancer account for one-sixth of all cancers in the U.S., he wrote.
In addition, blood pressure control has gotten worse since the start of the pandemic, said Michael Rakotz, MD, FAHA, FAAFP, vice president of improving health outcomes at the AMA, in an AMA blog post.
Dr. Harmon’s advice for physician practices on getting patients in for routine care:
- Educate the area’s largest employers to encourage their employees.
- Engage with hospital employees, since hospitals are often the largest employers in many communities.
- Partner with health insurers.
- Show up at athletic events, which is a particularly good fit for “small town America,” said Dr. Harmon.
The AMA’s research doesn’t consider reimbursement from other public and private payers. It also doesn’t account for funding sources such as Provider Relief Fund grants, Paycheck Protection Program loans, and the temporary suspension of the Medicare sequester, per the report.
A version of this article first appeared on Medscape.com.
Physician practices around the country took an unprecedented financial hit with the arrival of the COVID-19 pandemic in March 2020. Recent research from the American Medical Association reveals an estimated pandemic-related shortfall in Medicare physician fee spending of $13.9 billion, or a 14% reduction, across all states and all major specialties in 2020.
While the report pointed to a “strong recovery” in May and June, that recovery stalled in the second half of 2020, and spending never returned to pre–COVID-19 levels.
“Physicians experienced a significant and sustained drop in Medicare revenue during the first 10 months of the pandemic,” said AMA President Gerald Harmon, MD, in a statement. “Medical practices that have not buckled under financial strain continue to be stretched clinically, emotionally, and fiscally as the pandemic persists. Yet physicians face an array of planned cuts that would reduce Medicare physician payments by nearly 10% for 2022.”
The reduction in the Medicare physician fee schedule payments means providers may face payment cuts of more than 9% starting Jan. 1, 2022, when the cuts take effect. That is, unless Congress makes changes.
Medicare physician fee schedule spending on telehealth stood at $4.1 billion, or 5% of the total Medicare spent in 2020. From March 16 to June 30, $1.8 billion of this amount was on telehealth, while $1.1 billion came in during third and fourth quarters of 2020, respectively, per the report.
According to AMA’s research:
- Medicare physician fee schedule spending for 2020, relative to expected 2020 spending, dipped 32% between March 16 and June 30; spending was down during the last 6 months of the year by between 9% and 10%.
- The care settings hit the worst were ambulatory surgical centers, outpatient hospitals, and physician offices; the next worst off were hospital emergency departments, inpatient hospitals, and skilled nursing facilities.
- The specialties that fared worst included physical therapists (-28%), opthamologists (-19%), podiatrists (-18%), and dermatologists (-18%).
- Cumulative spending was down the most in Minnesota (-22%), Maine (-19%), and New York (-19%); less affected states included Idaho (-9%), Oklahoma (-9%), and South Carolina (9%).
AMA: Budget neutrality hurting physicians’ financial stability
Dr. Harmon is calling for financial stability in Medicare spending. In particular, the AMA is “strongly urging Congress to avert the planned payment cuts,” he said in a statement.
The challenge: The Medicare physician fee schedule is currently “budget neutral,” meaning that the budget is fixed, Dr. Harmon, a family medicine specialist in South Carolina, told this news organization.
“If you rob from Peter to pay Paul, Paul is going to be less efficient or less rewarded. It continues to be that there’s always a ‘pay for’ in these things. So budget neutrality is probably one of the first things we need to address,” he said.
Lack of routine care expected to affect health outcomes
The result of reduced screening and treatment during the pandemic could be as many as 10,000 excess deaths due to cancers of the breast and colon during the next 10 years, wrote Norman Sharpless, MD, director of the National Cancer Institute, in Science in June. Combined, breast cancer and colon cancer account for one-sixth of all cancers in the U.S., he wrote.
In addition, blood pressure control has gotten worse since the start of the pandemic, said Michael Rakotz, MD, FAHA, FAAFP, vice president of improving health outcomes at the AMA, in an AMA blog post.
Dr. Harmon’s advice for physician practices on getting patients in for routine care:
- Educate the area’s largest employers to encourage their employees.
- Engage with hospital employees, since hospitals are often the largest employers in many communities.
- Partner with health insurers.
- Show up at athletic events, which is a particularly good fit for “small town America,” said Dr. Harmon.
The AMA’s research doesn’t consider reimbursement from other public and private payers. It also doesn’t account for funding sources such as Provider Relief Fund grants, Paycheck Protection Program loans, and the temporary suspension of the Medicare sequester, per the report.
A version of this article first appeared on Medscape.com.