User login
Should ‘advanced maternal age’ be redefined? Study suggests benefits.
Pregnant women who were at or above the advanced maternal age (AMA) cutoff of 35 years on their due date received significantly more prenatal care, resulting in a slight decline in perinatal mortality, compared with women who were just a few months younger, according to a new study published in JAMA Health Forum. The findings “suggest that clinicians use the cutoff as a heuristic in their clinical recommendations and service provision,” noted lead author Caroline K. Geiger, PhD, who was a PhD student at Harvard University in Cambridge, Mass., during the course of the study, and now works as an associate health economist at Genentech in San Francisco. She and her coauthors suggest a slightly younger AMA cutoff might be beneficial. “Our results suggest that 3.9 perinatal deaths per 1,000 deliveries in this age range could be averted if patients just a few months younger than the AMA cutoff received similar care to those older than the cutoff,” they wrote. “Although the risk of adverse outcomes increases with maternal age, individuals 4 months older or younger than 35 years should not have different underlying risks.”
The cross-sectional study used a national sample of 51,290 commercially insured individuals who were pregnant between 2008 and 2019 and had delivery dates within 120 days of their 35th birthday. Just over half (50.9%) of the individuals were aged 34.7-34.9 years on their expected delivery date – just below the AMA cutoff – while 49.1% were just over the cutoff at age 35.0-35.3 years. A total of 4.7% had multiple gestation, 4.8% had pregestational diabetes, 4.4% had chronic hypertension, and 9.7% had obesity. There was also a subgroup analysis among individuals with low-risk pregnancy (defined as singleton, with no pregestational diabetes, chronic hypertension, or obesity) because they were less likely to have indications for additional prenatal care.
Although there was a slight, nonstatistically significant increase in the overall number of ob.gyn. visits at the AMA cutoff, compared with below it, the percentage of individuals with any maternal-fetal medicine visit increased by 4.27 percentage points (P < .001) at the cutoff. Additionally, while there was a “modest” increase in total ultrasounds (P = .006), there was a significant increase in detailed ultrasounds (P < .001) at the cutoff, and a “substantial” increase in antepartum surveillance (P < .001), the authors reported.
The AMA designation was associated with a 0.39 percentage-point decline in perinatal mortality (P = .04), “however, there were no significant changes in the proportion of individuals with severe maternal morbidity or with preterm birth or low birth weight at age 35 years,” they wrote.
In the subgroup analysis of low-risk pregnancies, “prenatal care services increased substantially at the 35-year cutoff, and in all cases, the increases at age 35 years for this group were larger than for the full sample,” they noted, adding that there was also a “substantially larger” decline in perinatal mortality at the AMA cutoff (P = .002), compared with the full sample.
The authors noted the need for more rigorous evidence on the value and effect of prenatal care guidelines on pregnancy outcomes. “Although pregnancy-related risks increase with maternal age, there is no known abrupt biological increase in underlying risk precisely at age 35 years,” they wrote, adding that “much of the content of prenatal care guidelines has persisted for decades without strong causal evidence to demonstrate its value.”
Their words echo those of Alex F. Peahl, MD, an ob.gyn. and assistant professor at the Institute for Healthcare Policy and Innovation, at the University of Michigan, in Ann Arbor, MI. In a recent review, Dr. Peahl and her colleague Joel D. Howell, MD, PhD, from the same university (Am J Obstet Gynecol. 2021 Apr;224[4]:339-47), note that the COVID-19 pandemic forced a much-needed rethink of prenatal care and its delivery. A look through the history of prenatal care shows “we have treated visit frequency and modality as fixed boxes, into which we must fit an ever-changing set of care recommendations,” they wrote. “We do not have data to support a specific prenatal visit schedule, recommended number of telemedicine visits, or specifications of additional services, and we never have. However, one thing is clear: we are long overdue for new prenatal care delivery guidelines in the United States.”
But when reached for comment on the new study Dr. Peahl cautioned that its conclusions are “limited and warrant future investigation. … While increased prenatal services may explain the improvement in outcomes, several other explanations should be considered,” she told this publication. “Perhaps, maternity care professional behavior differs for patients who are over the age of 35, resulting in increased caution in interpreting test results and symptoms; perhaps patients are more routinely induced at 39 weeks, limiting stillbirth rate; or perhaps patients are more hypervigilant when given the diagnosis of AMA.”
Priya Rajan, MD, agreed that while the paper showed an association between intensified antenatal interventions and decreased perinatal mortality, it did not show a causal relationship. “The study did not include information on other important factors that are also associated with perinatal risk,” noted Dr. Rajan, who is an associate professor in the department of ob.gyn. at Northwestern University in Chicago. Yet, she acknowledged that the findings “support what many clinicians know, which is that age 35 isn’t some tipping point; rather, obstetric risk is influenced by a range of factors, of which age may be one. This study, particularly when considered in the context of other studies and articles we have seen recently, confirms the need for us to rethink how we care for people during pregnancy and post partum. This includes delving further into understanding what aspects of the prenatal care that we provide have the biggest impact for both maternal and perinatal adverse outcomes.”
The study was supported by grant DGE1745303 from the National Science Foundation Graduate Research Fellowship Program. Dr. Geiger reported being a PhD student during the conduction of the study, but had no other disclosures. Dr. Peahl will soon be a consultant for Maven Clinic. Dr. Rajan had no relevant disclosures.
Pregnant women who were at or above the advanced maternal age (AMA) cutoff of 35 years on their due date received significantly more prenatal care, resulting in a slight decline in perinatal mortality, compared with women who were just a few months younger, according to a new study published in JAMA Health Forum. The findings “suggest that clinicians use the cutoff as a heuristic in their clinical recommendations and service provision,” noted lead author Caroline K. Geiger, PhD, who was a PhD student at Harvard University in Cambridge, Mass., during the course of the study, and now works as an associate health economist at Genentech in San Francisco. She and her coauthors suggest a slightly younger AMA cutoff might be beneficial. “Our results suggest that 3.9 perinatal deaths per 1,000 deliveries in this age range could be averted if patients just a few months younger than the AMA cutoff received similar care to those older than the cutoff,” they wrote. “Although the risk of adverse outcomes increases with maternal age, individuals 4 months older or younger than 35 years should not have different underlying risks.”
The cross-sectional study used a national sample of 51,290 commercially insured individuals who were pregnant between 2008 and 2019 and had delivery dates within 120 days of their 35th birthday. Just over half (50.9%) of the individuals were aged 34.7-34.9 years on their expected delivery date – just below the AMA cutoff – while 49.1% were just over the cutoff at age 35.0-35.3 years. A total of 4.7% had multiple gestation, 4.8% had pregestational diabetes, 4.4% had chronic hypertension, and 9.7% had obesity. There was also a subgroup analysis among individuals with low-risk pregnancy (defined as singleton, with no pregestational diabetes, chronic hypertension, or obesity) because they were less likely to have indications for additional prenatal care.
Although there was a slight, nonstatistically significant increase in the overall number of ob.gyn. visits at the AMA cutoff, compared with below it, the percentage of individuals with any maternal-fetal medicine visit increased by 4.27 percentage points (P < .001) at the cutoff. Additionally, while there was a “modest” increase in total ultrasounds (P = .006), there was a significant increase in detailed ultrasounds (P < .001) at the cutoff, and a “substantial” increase in antepartum surveillance (P < .001), the authors reported.
The AMA designation was associated with a 0.39 percentage-point decline in perinatal mortality (P = .04), “however, there were no significant changes in the proportion of individuals with severe maternal morbidity or with preterm birth or low birth weight at age 35 years,” they wrote.
In the subgroup analysis of low-risk pregnancies, “prenatal care services increased substantially at the 35-year cutoff, and in all cases, the increases at age 35 years for this group were larger than for the full sample,” they noted, adding that there was also a “substantially larger” decline in perinatal mortality at the AMA cutoff (P = .002), compared with the full sample.
The authors noted the need for more rigorous evidence on the value and effect of prenatal care guidelines on pregnancy outcomes. “Although pregnancy-related risks increase with maternal age, there is no known abrupt biological increase in underlying risk precisely at age 35 years,” they wrote, adding that “much of the content of prenatal care guidelines has persisted for decades without strong causal evidence to demonstrate its value.”
Their words echo those of Alex F. Peahl, MD, an ob.gyn. and assistant professor at the Institute for Healthcare Policy and Innovation, at the University of Michigan, in Ann Arbor, MI. In a recent review, Dr. Peahl and her colleague Joel D. Howell, MD, PhD, from the same university (Am J Obstet Gynecol. 2021 Apr;224[4]:339-47), note that the COVID-19 pandemic forced a much-needed rethink of prenatal care and its delivery. A look through the history of prenatal care shows “we have treated visit frequency and modality as fixed boxes, into which we must fit an ever-changing set of care recommendations,” they wrote. “We do not have data to support a specific prenatal visit schedule, recommended number of telemedicine visits, or specifications of additional services, and we never have. However, one thing is clear: we are long overdue for new prenatal care delivery guidelines in the United States.”
But when reached for comment on the new study Dr. Peahl cautioned that its conclusions are “limited and warrant future investigation. … While increased prenatal services may explain the improvement in outcomes, several other explanations should be considered,” she told this publication. “Perhaps, maternity care professional behavior differs for patients who are over the age of 35, resulting in increased caution in interpreting test results and symptoms; perhaps patients are more routinely induced at 39 weeks, limiting stillbirth rate; or perhaps patients are more hypervigilant when given the diagnosis of AMA.”
Priya Rajan, MD, agreed that while the paper showed an association between intensified antenatal interventions and decreased perinatal mortality, it did not show a causal relationship. “The study did not include information on other important factors that are also associated with perinatal risk,” noted Dr. Rajan, who is an associate professor in the department of ob.gyn. at Northwestern University in Chicago. Yet, she acknowledged that the findings “support what many clinicians know, which is that age 35 isn’t some tipping point; rather, obstetric risk is influenced by a range of factors, of which age may be one. This study, particularly when considered in the context of other studies and articles we have seen recently, confirms the need for us to rethink how we care for people during pregnancy and post partum. This includes delving further into understanding what aspects of the prenatal care that we provide have the biggest impact for both maternal and perinatal adverse outcomes.”
The study was supported by grant DGE1745303 from the National Science Foundation Graduate Research Fellowship Program. Dr. Geiger reported being a PhD student during the conduction of the study, but had no other disclosures. Dr. Peahl will soon be a consultant for Maven Clinic. Dr. Rajan had no relevant disclosures.
Pregnant women who were at or above the advanced maternal age (AMA) cutoff of 35 years on their due date received significantly more prenatal care, resulting in a slight decline in perinatal mortality, compared with women who were just a few months younger, according to a new study published in JAMA Health Forum. The findings “suggest that clinicians use the cutoff as a heuristic in their clinical recommendations and service provision,” noted lead author Caroline K. Geiger, PhD, who was a PhD student at Harvard University in Cambridge, Mass., during the course of the study, and now works as an associate health economist at Genentech in San Francisco. She and her coauthors suggest a slightly younger AMA cutoff might be beneficial. “Our results suggest that 3.9 perinatal deaths per 1,000 deliveries in this age range could be averted if patients just a few months younger than the AMA cutoff received similar care to those older than the cutoff,” they wrote. “Although the risk of adverse outcomes increases with maternal age, individuals 4 months older or younger than 35 years should not have different underlying risks.”
The cross-sectional study used a national sample of 51,290 commercially insured individuals who were pregnant between 2008 and 2019 and had delivery dates within 120 days of their 35th birthday. Just over half (50.9%) of the individuals were aged 34.7-34.9 years on their expected delivery date – just below the AMA cutoff – while 49.1% were just over the cutoff at age 35.0-35.3 years. A total of 4.7% had multiple gestation, 4.8% had pregestational diabetes, 4.4% had chronic hypertension, and 9.7% had obesity. There was also a subgroup analysis among individuals with low-risk pregnancy (defined as singleton, with no pregestational diabetes, chronic hypertension, or obesity) because they were less likely to have indications for additional prenatal care.
Although there was a slight, nonstatistically significant increase in the overall number of ob.gyn. visits at the AMA cutoff, compared with below it, the percentage of individuals with any maternal-fetal medicine visit increased by 4.27 percentage points (P < .001) at the cutoff. Additionally, while there was a “modest” increase in total ultrasounds (P = .006), there was a significant increase in detailed ultrasounds (P < .001) at the cutoff, and a “substantial” increase in antepartum surveillance (P < .001), the authors reported.
The AMA designation was associated with a 0.39 percentage-point decline in perinatal mortality (P = .04), “however, there were no significant changes in the proportion of individuals with severe maternal morbidity or with preterm birth or low birth weight at age 35 years,” they wrote.
In the subgroup analysis of low-risk pregnancies, “prenatal care services increased substantially at the 35-year cutoff, and in all cases, the increases at age 35 years for this group were larger than for the full sample,” they noted, adding that there was also a “substantially larger” decline in perinatal mortality at the AMA cutoff (P = .002), compared with the full sample.
The authors noted the need for more rigorous evidence on the value and effect of prenatal care guidelines on pregnancy outcomes. “Although pregnancy-related risks increase with maternal age, there is no known abrupt biological increase in underlying risk precisely at age 35 years,” they wrote, adding that “much of the content of prenatal care guidelines has persisted for decades without strong causal evidence to demonstrate its value.”
Their words echo those of Alex F. Peahl, MD, an ob.gyn. and assistant professor at the Institute for Healthcare Policy and Innovation, at the University of Michigan, in Ann Arbor, MI. In a recent review, Dr. Peahl and her colleague Joel D. Howell, MD, PhD, from the same university (Am J Obstet Gynecol. 2021 Apr;224[4]:339-47), note that the COVID-19 pandemic forced a much-needed rethink of prenatal care and its delivery. A look through the history of prenatal care shows “we have treated visit frequency and modality as fixed boxes, into which we must fit an ever-changing set of care recommendations,” they wrote. “We do not have data to support a specific prenatal visit schedule, recommended number of telemedicine visits, or specifications of additional services, and we never have. However, one thing is clear: we are long overdue for new prenatal care delivery guidelines in the United States.”
But when reached for comment on the new study Dr. Peahl cautioned that its conclusions are “limited and warrant future investigation. … While increased prenatal services may explain the improvement in outcomes, several other explanations should be considered,” she told this publication. “Perhaps, maternity care professional behavior differs for patients who are over the age of 35, resulting in increased caution in interpreting test results and symptoms; perhaps patients are more routinely induced at 39 weeks, limiting stillbirth rate; or perhaps patients are more hypervigilant when given the diagnosis of AMA.”
Priya Rajan, MD, agreed that while the paper showed an association between intensified antenatal interventions and decreased perinatal mortality, it did not show a causal relationship. “The study did not include information on other important factors that are also associated with perinatal risk,” noted Dr. Rajan, who is an associate professor in the department of ob.gyn. at Northwestern University in Chicago. Yet, she acknowledged that the findings “support what many clinicians know, which is that age 35 isn’t some tipping point; rather, obstetric risk is influenced by a range of factors, of which age may be one. This study, particularly when considered in the context of other studies and articles we have seen recently, confirms the need for us to rethink how we care for people during pregnancy and post partum. This includes delving further into understanding what aspects of the prenatal care that we provide have the biggest impact for both maternal and perinatal adverse outcomes.”
The study was supported by grant DGE1745303 from the National Science Foundation Graduate Research Fellowship Program. Dr. Geiger reported being a PhD student during the conduction of the study, but had no other disclosures. Dr. Peahl will soon be a consultant for Maven Clinic. Dr. Rajan had no relevant disclosures.
JAMA HEALTH FORUM
TikTok trends: Do or diet, plan ‘c,' garlic where?
The year is rapidly approaching an end, and with it, we can perhaps look forward to better and brighter days in 2022. With daylight savings, seasonal depression, and cold and flu season making a comeback as temperatures across the Northern Hemisphere drop,
The good: doctor reveals the truth about dieting
Chisom Ikeji, MD, is a critical care clinical fellow at the University of Pittsburgh Medical Center and on TikTok under the username @drchizmd. In a TikTok with almost 200,000 views and over 18,000 likes, Dr. Ikeji explains to all New Year’s resolution-makers that dieting isn’t all it’s cracked up to be. “Dieting leads to perpetually losing weight and gaining weight on a cycle,” she says, “so that your body never really settles at its set point weight.”
Celebrities and your friends who drink lemon water in the morning may tout all sorts of fad diets and some of them might even work. But Dr. Ikeji explains that diets with gimmicks don’t have lasting power and create cycles of losing and gaining weight. Additionally, associating “points” with foods, or separating them into “good” or “bad” categories, encourages habits that can lead to disordered eating.
The best way to keep weight off is to make actual lifestyle changes that stick. In contrast, going back to dieting every year (the rapid cycle of losing and gaining weight is called “yo-yo” dieting) can have a bad impact on your health, including increased risk of heart disease and metabolism issues.
“The best thing you can do for your body, and to help you lose weight, is to stop dieting,” Dr. Ikeji says. “Incorporate whole foods into your diet and make sure you move your body. If you don’t, you’ll be chasing that diet into your 80s and feeling guilty over a piece of cake forever. That’s no way to live life.”
The bad: vitamin C contraceptive
In this TikTok, user @itsdiosa reveals her contraception hack for anyone with a uterus who may be having unprotected sex. She claims that for those who forget to take the Plan B pill, vitamin C is a worthwhile substitute in preventing unwanted pregnancy. She recommends taking four or five vitamin C tablets a day for a few days for the return of a normal period.
Not surprisingly, Vitamin C isn’t safe or reliable and doesn’t have studies to back up @itsdiosa’s claim. If anything, all you’ll get from taking too much vitamin C is diarrhea and a stomachache.
Karan Rajan, MBBS, from Imperial College London and the University of Sunderland in the United Kingdom, responded to the TikTok to confirm that this claim isn’t backed up by science.
“Vitamin C doesn’t start or stop a period. Period,” he commented on the video.
The ugly: garlic sinus decongestant
Now this one went viral one went viral with over 5.2 million likes. In this TikTok, @hwannah5 and her boyfriend try out a trend that involves putting a clove of peeled garlic in each nostril in order to clear up congestion. The bubbles of ooey gooey snot coming out of her boyfriend’s nose certainly make it seem like it’s working, but what’s really going on?
This is hardly new; people have been putting strange things in nasal rinses for some time now and garlic is a tried-and-true favorite. Garlic does have some medically valid uses. These studies have shown that garlic taken orally may improve insulin in people with diabetes, slightly lower cholesterol, and reduce blood pressure in people with hypertension. When it comes to home remedies, people have historically used garlic as an antiseptic, antibacterial, and antifungal agent, though these claims are not widely supported by research. But taking a garlic supplement and sticking raw garlic up your nose are two very different things.
New York-based board-certified dermatologist Whitney Bowe, MD, weighed in on the viral trend.
In her own reaction video, she explained: “Guys, this is actually not safe. What’s happening is the garlic is actually triggering something called contact dermatitis and the mucosa is trying to protect itself by secreting tons of mucus. It’s creating swelling.”
For those tempted by this smelly “remedy,” a few drops of essential oil in a steamy shower is a much more pleasant (and significantly less gross) way to treat congestion.
A version of this article first appeared on Medscape.com.
The year is rapidly approaching an end, and with it, we can perhaps look forward to better and brighter days in 2022. With daylight savings, seasonal depression, and cold and flu season making a comeback as temperatures across the Northern Hemisphere drop,
The good: doctor reveals the truth about dieting
Chisom Ikeji, MD, is a critical care clinical fellow at the University of Pittsburgh Medical Center and on TikTok under the username @drchizmd. In a TikTok with almost 200,000 views and over 18,000 likes, Dr. Ikeji explains to all New Year’s resolution-makers that dieting isn’t all it’s cracked up to be. “Dieting leads to perpetually losing weight and gaining weight on a cycle,” she says, “so that your body never really settles at its set point weight.”
Celebrities and your friends who drink lemon water in the morning may tout all sorts of fad diets and some of them might even work. But Dr. Ikeji explains that diets with gimmicks don’t have lasting power and create cycles of losing and gaining weight. Additionally, associating “points” with foods, or separating them into “good” or “bad” categories, encourages habits that can lead to disordered eating.
The best way to keep weight off is to make actual lifestyle changes that stick. In contrast, going back to dieting every year (the rapid cycle of losing and gaining weight is called “yo-yo” dieting) can have a bad impact on your health, including increased risk of heart disease and metabolism issues.
“The best thing you can do for your body, and to help you lose weight, is to stop dieting,” Dr. Ikeji says. “Incorporate whole foods into your diet and make sure you move your body. If you don’t, you’ll be chasing that diet into your 80s and feeling guilty over a piece of cake forever. That’s no way to live life.”
The bad: vitamin C contraceptive
In this TikTok, user @itsdiosa reveals her contraception hack for anyone with a uterus who may be having unprotected sex. She claims that for those who forget to take the Plan B pill, vitamin C is a worthwhile substitute in preventing unwanted pregnancy. She recommends taking four or five vitamin C tablets a day for a few days for the return of a normal period.
Not surprisingly, Vitamin C isn’t safe or reliable and doesn’t have studies to back up @itsdiosa’s claim. If anything, all you’ll get from taking too much vitamin C is diarrhea and a stomachache.
Karan Rajan, MBBS, from Imperial College London and the University of Sunderland in the United Kingdom, responded to the TikTok to confirm that this claim isn’t backed up by science.
“Vitamin C doesn’t start or stop a period. Period,” he commented on the video.
The ugly: garlic sinus decongestant
Now this one went viral one went viral with over 5.2 million likes. In this TikTok, @hwannah5 and her boyfriend try out a trend that involves putting a clove of peeled garlic in each nostril in order to clear up congestion. The bubbles of ooey gooey snot coming out of her boyfriend’s nose certainly make it seem like it’s working, but what’s really going on?
This is hardly new; people have been putting strange things in nasal rinses for some time now and garlic is a tried-and-true favorite. Garlic does have some medically valid uses. These studies have shown that garlic taken orally may improve insulin in people with diabetes, slightly lower cholesterol, and reduce blood pressure in people with hypertension. When it comes to home remedies, people have historically used garlic as an antiseptic, antibacterial, and antifungal agent, though these claims are not widely supported by research. But taking a garlic supplement and sticking raw garlic up your nose are two very different things.
New York-based board-certified dermatologist Whitney Bowe, MD, weighed in on the viral trend.
In her own reaction video, she explained: “Guys, this is actually not safe. What’s happening is the garlic is actually triggering something called contact dermatitis and the mucosa is trying to protect itself by secreting tons of mucus. It’s creating swelling.”
For those tempted by this smelly “remedy,” a few drops of essential oil in a steamy shower is a much more pleasant (and significantly less gross) way to treat congestion.
A version of this article first appeared on Medscape.com.
The year is rapidly approaching an end, and with it, we can perhaps look forward to better and brighter days in 2022. With daylight savings, seasonal depression, and cold and flu season making a comeback as temperatures across the Northern Hemisphere drop,
The good: doctor reveals the truth about dieting
Chisom Ikeji, MD, is a critical care clinical fellow at the University of Pittsburgh Medical Center and on TikTok under the username @drchizmd. In a TikTok with almost 200,000 views and over 18,000 likes, Dr. Ikeji explains to all New Year’s resolution-makers that dieting isn’t all it’s cracked up to be. “Dieting leads to perpetually losing weight and gaining weight on a cycle,” she says, “so that your body never really settles at its set point weight.”
Celebrities and your friends who drink lemon water in the morning may tout all sorts of fad diets and some of them might even work. But Dr. Ikeji explains that diets with gimmicks don’t have lasting power and create cycles of losing and gaining weight. Additionally, associating “points” with foods, or separating them into “good” or “bad” categories, encourages habits that can lead to disordered eating.
The best way to keep weight off is to make actual lifestyle changes that stick. In contrast, going back to dieting every year (the rapid cycle of losing and gaining weight is called “yo-yo” dieting) can have a bad impact on your health, including increased risk of heart disease and metabolism issues.
“The best thing you can do for your body, and to help you lose weight, is to stop dieting,” Dr. Ikeji says. “Incorporate whole foods into your diet and make sure you move your body. If you don’t, you’ll be chasing that diet into your 80s and feeling guilty over a piece of cake forever. That’s no way to live life.”
The bad: vitamin C contraceptive
In this TikTok, user @itsdiosa reveals her contraception hack for anyone with a uterus who may be having unprotected sex. She claims that for those who forget to take the Plan B pill, vitamin C is a worthwhile substitute in preventing unwanted pregnancy. She recommends taking four or five vitamin C tablets a day for a few days for the return of a normal period.
Not surprisingly, Vitamin C isn’t safe or reliable and doesn’t have studies to back up @itsdiosa’s claim. If anything, all you’ll get from taking too much vitamin C is diarrhea and a stomachache.
Karan Rajan, MBBS, from Imperial College London and the University of Sunderland in the United Kingdom, responded to the TikTok to confirm that this claim isn’t backed up by science.
“Vitamin C doesn’t start or stop a period. Period,” he commented on the video.
The ugly: garlic sinus decongestant
Now this one went viral one went viral with over 5.2 million likes. In this TikTok, @hwannah5 and her boyfriend try out a trend that involves putting a clove of peeled garlic in each nostril in order to clear up congestion. The bubbles of ooey gooey snot coming out of her boyfriend’s nose certainly make it seem like it’s working, but what’s really going on?
This is hardly new; people have been putting strange things in nasal rinses for some time now and garlic is a tried-and-true favorite. Garlic does have some medically valid uses. These studies have shown that garlic taken orally may improve insulin in people with diabetes, slightly lower cholesterol, and reduce blood pressure in people with hypertension. When it comes to home remedies, people have historically used garlic as an antiseptic, antibacterial, and antifungal agent, though these claims are not widely supported by research. But taking a garlic supplement and sticking raw garlic up your nose are two very different things.
New York-based board-certified dermatologist Whitney Bowe, MD, weighed in on the viral trend.
In her own reaction video, she explained: “Guys, this is actually not safe. What’s happening is the garlic is actually triggering something called contact dermatitis and the mucosa is trying to protect itself by secreting tons of mucus. It’s creating swelling.”
For those tempted by this smelly “remedy,” a few drops of essential oil in a steamy shower is a much more pleasant (and significantly less gross) way to treat congestion.
A version of this article first appeared on Medscape.com.
New HIV PrEP guidelines call for clinicians to talk to patients about HIV prevention meds
Starting Dec. 8, the Centers for Disease Control and Prevention recommends all clinicians talk to their sexually active adolescent and adult patients about HIV pre-exposure prophylaxis (PrEP) at least once and prescribe the prevention pills to anyone who asks for them, whether or not you understand their need for it.
“PrEP is a part of good primary care,” Demetre Daskalakis, MD, CDC’s director of the division of HIV/AIDS prevention, said in an interview. “Listening to people and what they need, as opposed to assessing what you think they need, is a seismic shift in how PrEP should be offered.”
The expanded recommendation comes as part of the 2021 update to the U.S. Public Health Service’s PrEP prescribing guidelines. It’s the third iteration since the Food and Drug Administration approved the first HIV prevention pill in 2012, and the first to include guidance on how to prescribe and monitor an injectable version of PrEP, which the FDA may approve as early as December 2021.
There are currently two pills, Truvada (emtricitabine/tenofovir disoproxil fumarate, Gilead Sciences and generic) and Descovy (emtricitabine/tenofovir alafenamide, Gilead Sciences). The pills have been found to be up to 99% effective in preventing HIV acquisition. The new injectable cabotegravir appears to be even more effective.
The broadened guidance is part of an effort from the country’s top health officials to expand PrEP prescribing from infectious disease specialists and sexual health clinics to health care professionals, including gynecologists, internal medicine physicians, and family practice clinicians. It appears to be necessary. In 2020, just 25% of the 1.2 million Americans who could benefit from PrEP were taking it, according to CDC data.
But those rates belie stark disparities in PrEP use by race and gender. The vast majority of those using PrEP are White Americans and men. About 66% of White Americans who could benefit from PrEP used it in 2020, and more than a quarter of the men who could benefit used it. By contrast, just 16% of Latinx people who could benefit had a prescription. And fewer than 1 in 10 Black Americans, who make up nearly half of those with indications for PrEP, had a prescription. The same was true for the women who could benefit.
Researchers and data from early PrEP demonstration projects have documented that clinicians are less likely to refer or prescribe the HIV prevention pills to Black people, especially the Black cisgender and transgender women and same-gender-loving men who bear the disproportionate burden of new cases in the United States, as well as fail to prescribe the medication to people who inject drugs.
Normalizing PrEP in primary care
When Courtney Sherman, DNP, APRN, first heard about PrEP in the early 2010s, she joked that her reaction was: “You’re ridiculous. You’re making that up. That’s not real.”
Ms. Sherman is now launching a tele-PrEP program from CAN Community Health, a nonprofit network of community health centers in southern Florida. The tele-PrEP program is meant to serve people in Florida and beyond, to increase access to the pill in areas with few health care professionals, or clinicians unwilling to prescribe it.
“When I go other places, I can’t do what I do for a living without getting some sort of bizarre comment or look,” she said. But the looks don’t just come from family, friends, or her children’s teachers. They come from colleagues, too. “What I’ve learned is that anybody – anybody – can be impacted [by HIV] and the illusion that ‘those people who live over there do things that me and my kind don’t do’ is just garbage.”
That’s the PrEP stigma that the universal PrEP counseling in the guidelines is meant to override, said Dr. Daskalakis. Going forward, he said that informing people about PrEP should be treated as normally as counseling people about smoking.
“You can change the blank: You talk to all adolescents and adults about not smoking,” he said. “This is: ‘Tell adolescents and adults about ways you can prevent HIV, and PrEP is one of them.’ ”
The guidelines also simplify for monitoring lab levels for the current daily pills, checking creatinine clearance levels twice a year in people older than age 50 and once a year in those younger than 50 taking the oral pills. Dr. Daskalakis said that should ease the burden of monitoring PrEP patients for health care professionals with busy caseloads.
It’s a move that drew praise from Shawnika Hull, PhD, assistant professor of health communications at Rutgers University, New Brunswick, N.J.. Dr. Hull’s recent data showed that clinicians who espoused more biased racial views were also less likely to prescribe PrEP to Black women who asked for it.
“Public health practitioners and scientists have been advocating for this as a strategy, as one way to address several ongoing barriers to PrEP specifically but also equity in PrEP,” said Dr. Hull. “This sort of universal provision of information is really an important strategy to try to undo some of the deeply intertwined barriers to uptake.”
‘Don’t grill them’
The updated guidelines keep the number and proportion of Americans who could benefit from PrEP the same: 1.2 million Americans, with nearly half of those Black. And the reasons people would qualify for PrEP remain the same: inconsistent condom use, sharing injection drug equipment, and a STI diagnosis in the last 6 months. There are also 57 jurisdictions, including seven rural states, where dating and having sex carries an increased risk of acquiring HIV because of high rates of untreated HIV in the community.
That’s why the other big change in the update is guidance to prescribe PrEP to whoever asks for it, whether the patient divulges their risk or not. Or as Dr. Daskalakis puts it: “If someone asks for PrEP, don’t grill them.”
There are lots of reasons that someone might ask for PrEP without divulging their risk behaviors, said Dr. Daskalakis, who was an infectious disease doctor in New York back in 2012 (and a member of the FDA committee) when the first pill for PrEP was approved. He said he’s seen this particularly with women who ask about it. Asking for PrEP ends up being an “ice breaker” to discussing the woman’s sexual and injection drug use history, which can then improve the kinds of tests and vaccinations clinicians suggest for her.
“So many women will open the door and say, ‘I want to do this,’ and not necessarily want to go into the details,” he said. “Now, will they go into the details later? Absolutely. That’s how you create trust and connection.”
A mandate and a guideline
Leisha McKinley-Beach, MPH, a member of the U.S. Women and PrEP Working Group, has been urging greater funding and mandates to expand PrEP to women since the first pill was approved. And still, Ms. McKinley-Beach said she recently met a woman who worked for a community group scheduling PrEP appointments for gay men. But the woman didn’t know that she, too, could take it.
The American Academy of Family Physicians recommends health care professionals offer PrEP to those who can benefit. The American College of Obstetricians and Gynecologists have a 2014 committee opinion stating that PrEP “may be a useful tool for women at highest risk of HIV acquisition.”
But the ACOG opinion is not a recommendation, stating that it “should not be construed as dictating an exclusive course of treatment or procedure to be followed.” Ms. McKinley-Beach said she hopes that the new CDC guidelines will prompt ACOG and other professional organizations to issue statements to include PrEP education in all health assessments. A spokesperson for ACOG said that the organization had not seen the new CDC guidelines and had no statement on them, but pointed out that the 2014 committee opinion is one of the “highest level of documents we produce.
“We have failed for nearly a decade to raise awareness that PrEP is also a prevention strategy for women,” Ms. McKinley-Beach said in an interview. “In many ways, we’re still back in 2012 as it relates to women.”
Dr. Hull reported having done previous research funded by Gilead Sciences and having received consulting fees from Gilead Sciences in 2018. Ms. McKinley-Beach reported receiving honoraria from ViiV Healthcare. Ms. Sherman and Dr. Daskalakis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Starting Dec. 8, the Centers for Disease Control and Prevention recommends all clinicians talk to their sexually active adolescent and adult patients about HIV pre-exposure prophylaxis (PrEP) at least once and prescribe the prevention pills to anyone who asks for them, whether or not you understand their need for it.
“PrEP is a part of good primary care,” Demetre Daskalakis, MD, CDC’s director of the division of HIV/AIDS prevention, said in an interview. “Listening to people and what they need, as opposed to assessing what you think they need, is a seismic shift in how PrEP should be offered.”
The expanded recommendation comes as part of the 2021 update to the U.S. Public Health Service’s PrEP prescribing guidelines. It’s the third iteration since the Food and Drug Administration approved the first HIV prevention pill in 2012, and the first to include guidance on how to prescribe and monitor an injectable version of PrEP, which the FDA may approve as early as December 2021.
There are currently two pills, Truvada (emtricitabine/tenofovir disoproxil fumarate, Gilead Sciences and generic) and Descovy (emtricitabine/tenofovir alafenamide, Gilead Sciences). The pills have been found to be up to 99% effective in preventing HIV acquisition. The new injectable cabotegravir appears to be even more effective.
The broadened guidance is part of an effort from the country’s top health officials to expand PrEP prescribing from infectious disease specialists and sexual health clinics to health care professionals, including gynecologists, internal medicine physicians, and family practice clinicians. It appears to be necessary. In 2020, just 25% of the 1.2 million Americans who could benefit from PrEP were taking it, according to CDC data.
But those rates belie stark disparities in PrEP use by race and gender. The vast majority of those using PrEP are White Americans and men. About 66% of White Americans who could benefit from PrEP used it in 2020, and more than a quarter of the men who could benefit used it. By contrast, just 16% of Latinx people who could benefit had a prescription. And fewer than 1 in 10 Black Americans, who make up nearly half of those with indications for PrEP, had a prescription. The same was true for the women who could benefit.
Researchers and data from early PrEP demonstration projects have documented that clinicians are less likely to refer or prescribe the HIV prevention pills to Black people, especially the Black cisgender and transgender women and same-gender-loving men who bear the disproportionate burden of new cases in the United States, as well as fail to prescribe the medication to people who inject drugs.
Normalizing PrEP in primary care
When Courtney Sherman, DNP, APRN, first heard about PrEP in the early 2010s, she joked that her reaction was: “You’re ridiculous. You’re making that up. That’s not real.”
Ms. Sherman is now launching a tele-PrEP program from CAN Community Health, a nonprofit network of community health centers in southern Florida. The tele-PrEP program is meant to serve people in Florida and beyond, to increase access to the pill in areas with few health care professionals, or clinicians unwilling to prescribe it.
“When I go other places, I can’t do what I do for a living without getting some sort of bizarre comment or look,” she said. But the looks don’t just come from family, friends, or her children’s teachers. They come from colleagues, too. “What I’ve learned is that anybody – anybody – can be impacted [by HIV] and the illusion that ‘those people who live over there do things that me and my kind don’t do’ is just garbage.”
That’s the PrEP stigma that the universal PrEP counseling in the guidelines is meant to override, said Dr. Daskalakis. Going forward, he said that informing people about PrEP should be treated as normally as counseling people about smoking.
“You can change the blank: You talk to all adolescents and adults about not smoking,” he said. “This is: ‘Tell adolescents and adults about ways you can prevent HIV, and PrEP is one of them.’ ”
The guidelines also simplify for monitoring lab levels for the current daily pills, checking creatinine clearance levels twice a year in people older than age 50 and once a year in those younger than 50 taking the oral pills. Dr. Daskalakis said that should ease the burden of monitoring PrEP patients for health care professionals with busy caseloads.
It’s a move that drew praise from Shawnika Hull, PhD, assistant professor of health communications at Rutgers University, New Brunswick, N.J.. Dr. Hull’s recent data showed that clinicians who espoused more biased racial views were also less likely to prescribe PrEP to Black women who asked for it.
“Public health practitioners and scientists have been advocating for this as a strategy, as one way to address several ongoing barriers to PrEP specifically but also equity in PrEP,” said Dr. Hull. “This sort of universal provision of information is really an important strategy to try to undo some of the deeply intertwined barriers to uptake.”
‘Don’t grill them’
The updated guidelines keep the number and proportion of Americans who could benefit from PrEP the same: 1.2 million Americans, with nearly half of those Black. And the reasons people would qualify for PrEP remain the same: inconsistent condom use, sharing injection drug equipment, and a STI diagnosis in the last 6 months. There are also 57 jurisdictions, including seven rural states, where dating and having sex carries an increased risk of acquiring HIV because of high rates of untreated HIV in the community.
That’s why the other big change in the update is guidance to prescribe PrEP to whoever asks for it, whether the patient divulges their risk or not. Or as Dr. Daskalakis puts it: “If someone asks for PrEP, don’t grill them.”
There are lots of reasons that someone might ask for PrEP without divulging their risk behaviors, said Dr. Daskalakis, who was an infectious disease doctor in New York back in 2012 (and a member of the FDA committee) when the first pill for PrEP was approved. He said he’s seen this particularly with women who ask about it. Asking for PrEP ends up being an “ice breaker” to discussing the woman’s sexual and injection drug use history, which can then improve the kinds of tests and vaccinations clinicians suggest for her.
“So many women will open the door and say, ‘I want to do this,’ and not necessarily want to go into the details,” he said. “Now, will they go into the details later? Absolutely. That’s how you create trust and connection.”
A mandate and a guideline
Leisha McKinley-Beach, MPH, a member of the U.S. Women and PrEP Working Group, has been urging greater funding and mandates to expand PrEP to women since the first pill was approved. And still, Ms. McKinley-Beach said she recently met a woman who worked for a community group scheduling PrEP appointments for gay men. But the woman didn’t know that she, too, could take it.
The American Academy of Family Physicians recommends health care professionals offer PrEP to those who can benefit. The American College of Obstetricians and Gynecologists have a 2014 committee opinion stating that PrEP “may be a useful tool for women at highest risk of HIV acquisition.”
But the ACOG opinion is not a recommendation, stating that it “should not be construed as dictating an exclusive course of treatment or procedure to be followed.” Ms. McKinley-Beach said she hopes that the new CDC guidelines will prompt ACOG and other professional organizations to issue statements to include PrEP education in all health assessments. A spokesperson for ACOG said that the organization had not seen the new CDC guidelines and had no statement on them, but pointed out that the 2014 committee opinion is one of the “highest level of documents we produce.
“We have failed for nearly a decade to raise awareness that PrEP is also a prevention strategy for women,” Ms. McKinley-Beach said in an interview. “In many ways, we’re still back in 2012 as it relates to women.”
Dr. Hull reported having done previous research funded by Gilead Sciences and having received consulting fees from Gilead Sciences in 2018. Ms. McKinley-Beach reported receiving honoraria from ViiV Healthcare. Ms. Sherman and Dr. Daskalakis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Starting Dec. 8, the Centers for Disease Control and Prevention recommends all clinicians talk to their sexually active adolescent and adult patients about HIV pre-exposure prophylaxis (PrEP) at least once and prescribe the prevention pills to anyone who asks for them, whether or not you understand their need for it.
“PrEP is a part of good primary care,” Demetre Daskalakis, MD, CDC’s director of the division of HIV/AIDS prevention, said in an interview. “Listening to people and what they need, as opposed to assessing what you think they need, is a seismic shift in how PrEP should be offered.”
The expanded recommendation comes as part of the 2021 update to the U.S. Public Health Service’s PrEP prescribing guidelines. It’s the third iteration since the Food and Drug Administration approved the first HIV prevention pill in 2012, and the first to include guidance on how to prescribe and monitor an injectable version of PrEP, which the FDA may approve as early as December 2021.
There are currently two pills, Truvada (emtricitabine/tenofovir disoproxil fumarate, Gilead Sciences and generic) and Descovy (emtricitabine/tenofovir alafenamide, Gilead Sciences). The pills have been found to be up to 99% effective in preventing HIV acquisition. The new injectable cabotegravir appears to be even more effective.
The broadened guidance is part of an effort from the country’s top health officials to expand PrEP prescribing from infectious disease specialists and sexual health clinics to health care professionals, including gynecologists, internal medicine physicians, and family practice clinicians. It appears to be necessary. In 2020, just 25% of the 1.2 million Americans who could benefit from PrEP were taking it, according to CDC data.
But those rates belie stark disparities in PrEP use by race and gender. The vast majority of those using PrEP are White Americans and men. About 66% of White Americans who could benefit from PrEP used it in 2020, and more than a quarter of the men who could benefit used it. By contrast, just 16% of Latinx people who could benefit had a prescription. And fewer than 1 in 10 Black Americans, who make up nearly half of those with indications for PrEP, had a prescription. The same was true for the women who could benefit.
Researchers and data from early PrEP demonstration projects have documented that clinicians are less likely to refer or prescribe the HIV prevention pills to Black people, especially the Black cisgender and transgender women and same-gender-loving men who bear the disproportionate burden of new cases in the United States, as well as fail to prescribe the medication to people who inject drugs.
Normalizing PrEP in primary care
When Courtney Sherman, DNP, APRN, first heard about PrEP in the early 2010s, she joked that her reaction was: “You’re ridiculous. You’re making that up. That’s not real.”
Ms. Sherman is now launching a tele-PrEP program from CAN Community Health, a nonprofit network of community health centers in southern Florida. The tele-PrEP program is meant to serve people in Florida and beyond, to increase access to the pill in areas with few health care professionals, or clinicians unwilling to prescribe it.
“When I go other places, I can’t do what I do for a living without getting some sort of bizarre comment or look,” she said. But the looks don’t just come from family, friends, or her children’s teachers. They come from colleagues, too. “What I’ve learned is that anybody – anybody – can be impacted [by HIV] and the illusion that ‘those people who live over there do things that me and my kind don’t do’ is just garbage.”
That’s the PrEP stigma that the universal PrEP counseling in the guidelines is meant to override, said Dr. Daskalakis. Going forward, he said that informing people about PrEP should be treated as normally as counseling people about smoking.
“You can change the blank: You talk to all adolescents and adults about not smoking,” he said. “This is: ‘Tell adolescents and adults about ways you can prevent HIV, and PrEP is one of them.’ ”
The guidelines also simplify for monitoring lab levels for the current daily pills, checking creatinine clearance levels twice a year in people older than age 50 and once a year in those younger than 50 taking the oral pills. Dr. Daskalakis said that should ease the burden of monitoring PrEP patients for health care professionals with busy caseloads.
It’s a move that drew praise from Shawnika Hull, PhD, assistant professor of health communications at Rutgers University, New Brunswick, N.J.. Dr. Hull’s recent data showed that clinicians who espoused more biased racial views were also less likely to prescribe PrEP to Black women who asked for it.
“Public health practitioners and scientists have been advocating for this as a strategy, as one way to address several ongoing barriers to PrEP specifically but also equity in PrEP,” said Dr. Hull. “This sort of universal provision of information is really an important strategy to try to undo some of the deeply intertwined barriers to uptake.”
‘Don’t grill them’
The updated guidelines keep the number and proportion of Americans who could benefit from PrEP the same: 1.2 million Americans, with nearly half of those Black. And the reasons people would qualify for PrEP remain the same: inconsistent condom use, sharing injection drug equipment, and a STI diagnosis in the last 6 months. There are also 57 jurisdictions, including seven rural states, where dating and having sex carries an increased risk of acquiring HIV because of high rates of untreated HIV in the community.
That’s why the other big change in the update is guidance to prescribe PrEP to whoever asks for it, whether the patient divulges their risk or not. Or as Dr. Daskalakis puts it: “If someone asks for PrEP, don’t grill them.”
There are lots of reasons that someone might ask for PrEP without divulging their risk behaviors, said Dr. Daskalakis, who was an infectious disease doctor in New York back in 2012 (and a member of the FDA committee) when the first pill for PrEP was approved. He said he’s seen this particularly with women who ask about it. Asking for PrEP ends up being an “ice breaker” to discussing the woman’s sexual and injection drug use history, which can then improve the kinds of tests and vaccinations clinicians suggest for her.
“So many women will open the door and say, ‘I want to do this,’ and not necessarily want to go into the details,” he said. “Now, will they go into the details later? Absolutely. That’s how you create trust and connection.”
A mandate and a guideline
Leisha McKinley-Beach, MPH, a member of the U.S. Women and PrEP Working Group, has been urging greater funding and mandates to expand PrEP to women since the first pill was approved. And still, Ms. McKinley-Beach said she recently met a woman who worked for a community group scheduling PrEP appointments for gay men. But the woman didn’t know that she, too, could take it.
The American Academy of Family Physicians recommends health care professionals offer PrEP to those who can benefit. The American College of Obstetricians and Gynecologists have a 2014 committee opinion stating that PrEP “may be a useful tool for women at highest risk of HIV acquisition.”
But the ACOG opinion is not a recommendation, stating that it “should not be construed as dictating an exclusive course of treatment or procedure to be followed.” Ms. McKinley-Beach said she hopes that the new CDC guidelines will prompt ACOG and other professional organizations to issue statements to include PrEP education in all health assessments. A spokesperson for ACOG said that the organization had not seen the new CDC guidelines and had no statement on them, but pointed out that the 2014 committee opinion is one of the “highest level of documents we produce.
“We have failed for nearly a decade to raise awareness that PrEP is also a prevention strategy for women,” Ms. McKinley-Beach said in an interview. “In many ways, we’re still back in 2012 as it relates to women.”
Dr. Hull reported having done previous research funded by Gilead Sciences and having received consulting fees from Gilead Sciences in 2018. Ms. McKinley-Beach reported receiving honoraria from ViiV Healthcare. Ms. Sherman and Dr. Daskalakis disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Spam filter failure: Selling physician emails equals big $$
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.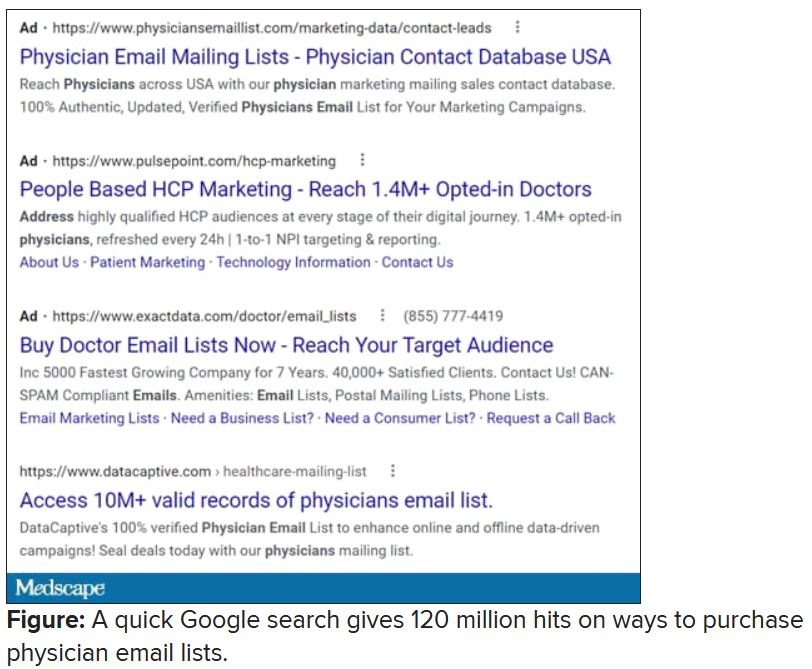
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Despite the best efforts of my institution’s spam filter, I’ve realized that I spend at least 4 minutes every day of the week removing junk email from my in basket: EMR vendors, predatory journals trying to lure me into paying their outrageous publication fees, people who want to help me with my billing software (evidently that .edu extension hasn’t clicked for them yet), headhunters trying to fill specialty positions in other states, market researchers offering a gift card for 40 minutes filling out a survey.
If you do the math, 4 minutes daily is 1,460 minutes per year. That’s an entire day of my life lost each year to this useless nonsense, which I never agreed to receive in the first place. Now multiply that by the 22 million health care workers in the United States, or even just by the 985,000 licensed physicians in this country. Then factor in the $638 per hour in gross revenue generated by the average primary care physician, as a conservative, well-documented value.
By my reckoning, these bozos owe the United States alone over $15 billion in lost GDP each year.
So why don’t we shut it down!? The CAN-SPAM Act of 2003 attempted to at least mitigate the problem. It applies only to commercial entities (I know, I’d love to report some political groups, too). To avoid violating the law and risking fines of up to $16,000 per individual email, senders must:
- Not use misleading header info (including domain name and email address)
- Not use deceptive subject lines
- Clearly label the email as an ad
- Give an actual physical address of the sender
- Tell recipients how to opt out of future emails
- Honor opt-out requests within 10 business days
- Monitor the activities of any subcontractor sending email on their behalf
I can say with certainty that much of the trash in my inbox violates at least one of these. But that doesn’t matter if there is not an efficient way to report the violators and ensure that they’ll be tracked down. Hard enough if they live here, impossible if the email is routed from overseas, as much of it clearly is.
If you receive email in violation of the act, experts recommend that you write down the email address and the business name of the sender, fill out a complaint form on the Federal Trade Commission website, or send an email to [email protected], then send an email to your Internet service provider’s abuse desk. If you’re not working within a big institution like mine that has hot and cold running IT personnel that operate their own abuse prevention office, the address you’ll need is likely abuse@domain_name or postmaster@domain_name. Just hitting the spam button at the top of your browser/email software may do the trick. There’s more good advice at the FTC’s consumer spam page.
The answer came, ironically, to my email inbox in the form of one of those emails that did indeed violate the law.
I rolled my eyes and started into my reporting subroutine but then stopped cold. Just 1 second. If this person is selling lists of email addresses of conference attendees, somebody within the conference structure must be providing them. How is that legal? I have never agreed, in registering for a medical conference, to allow them to share my email address with anyone. To think that they are making money from that is extremely galling.
Vermont, at least, has enacted a law requiring companies that traffic in such email lists to register with the state. Although it has been in effect for 2 years, the jury is out regarding its efficacy. Our European counterparts are protected by the General Data Protection Regulation, which specifies that commercial email can be sent only to individuals who have explicitly opted into such mailings, and that purchased email lists are not compliant with the requirement.
Anybody have the inside scoop on this? Can we demand that our professional societies safeguard their attendee databases so this won’t happen? If they won’t, why am I paying big money to attend their conferences, only for them to make even more money at my expense?
Dr. Hitchcock is assistant professor, department of radiation oncology, at the University of Florida, Gainesville. She reported receiving research grant money from Merck. A version of this article first appeared on Medscape.com.
Apixaban a reasonable alternative to warfarin in patients with severe renal impairment
Background: Over 6 million Americans are prescribed anticoagulation; however, available anticoagulation options for patients with concomitant renal impairment are limited. Until recently, warfarin was the only recommended option because of a lack of data to support the use of alternative agents in such patients. This study evaluates the safety and effectiveness of apixaban, compared with warfarin, in patients with severe renal dysfunction.
Study design: Multicenter retrospective cohort study.
Setting: Seven hospitals in Michigan between January 2013 and December 2015 and including adult patients with CrCl less than 25 cc/min who were newly initiated on apixaban or warfarin.
Synopsis: Patients in the apixaban group (n=128) had a higher rate of heart failure, atrial fibrillation, stent placement, and hyperlipidemia, while the warfarin group (n=733) had a higher rate of prior venous thromboembolism. The primary outcome was time to first bleeding or thrombotic event. Apixaban was associated with a lower risk of thrombotic or bleeding events, compared with warfarin (HR, 0.47). Post-hoc analysis controlling for patient differences showed similar results. There was no statistical difference in the severity of events or overall mortality. Further subgroup analysis showed that 5 mg B.I.D. dosing was not associated with higher risk of bleeding than 2.5 mg B.I.D.
The main limitation is the retrospective observational design, which may have introduced confounding variables that were not accounted for in the analyses. The study also did not account for patient nonadherence to medication.
Bottom line: Apixaban is a reasonable alternative to warfarin in patients with severe renal impairment.
Citation: Hanni C et al. Outcomes associated with apixaban vs. warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11): 2366-71. doi: 10.1182/bloodadvances.2019000972.
Dr. Narayan is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Over 6 million Americans are prescribed anticoagulation; however, available anticoagulation options for patients with concomitant renal impairment are limited. Until recently, warfarin was the only recommended option because of a lack of data to support the use of alternative agents in such patients. This study evaluates the safety and effectiveness of apixaban, compared with warfarin, in patients with severe renal dysfunction.
Study design: Multicenter retrospective cohort study.
Setting: Seven hospitals in Michigan between January 2013 and December 2015 and including adult patients with CrCl less than 25 cc/min who were newly initiated on apixaban or warfarin.
Synopsis: Patients in the apixaban group (n=128) had a higher rate of heart failure, atrial fibrillation, stent placement, and hyperlipidemia, while the warfarin group (n=733) had a higher rate of prior venous thromboembolism. The primary outcome was time to first bleeding or thrombotic event. Apixaban was associated with a lower risk of thrombotic or bleeding events, compared with warfarin (HR, 0.47). Post-hoc analysis controlling for patient differences showed similar results. There was no statistical difference in the severity of events or overall mortality. Further subgroup analysis showed that 5 mg B.I.D. dosing was not associated with higher risk of bleeding than 2.5 mg B.I.D.
The main limitation is the retrospective observational design, which may have introduced confounding variables that were not accounted for in the analyses. The study also did not account for patient nonadherence to medication.
Bottom line: Apixaban is a reasonable alternative to warfarin in patients with severe renal impairment.
Citation: Hanni C et al. Outcomes associated with apixaban vs. warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11): 2366-71. doi: 10.1182/bloodadvances.2019000972.
Dr. Narayan is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Background: Over 6 million Americans are prescribed anticoagulation; however, available anticoagulation options for patients with concomitant renal impairment are limited. Until recently, warfarin was the only recommended option because of a lack of data to support the use of alternative agents in such patients. This study evaluates the safety and effectiveness of apixaban, compared with warfarin, in patients with severe renal dysfunction.
Study design: Multicenter retrospective cohort study.
Setting: Seven hospitals in Michigan between January 2013 and December 2015 and including adult patients with CrCl less than 25 cc/min who were newly initiated on apixaban or warfarin.
Synopsis: Patients in the apixaban group (n=128) had a higher rate of heart failure, atrial fibrillation, stent placement, and hyperlipidemia, while the warfarin group (n=733) had a higher rate of prior venous thromboembolism. The primary outcome was time to first bleeding or thrombotic event. Apixaban was associated with a lower risk of thrombotic or bleeding events, compared with warfarin (HR, 0.47). Post-hoc analysis controlling for patient differences showed similar results. There was no statistical difference in the severity of events or overall mortality. Further subgroup analysis showed that 5 mg B.I.D. dosing was not associated with higher risk of bleeding than 2.5 mg B.I.D.
The main limitation is the retrospective observational design, which may have introduced confounding variables that were not accounted for in the analyses. The study also did not account for patient nonadherence to medication.
Bottom line: Apixaban is a reasonable alternative to warfarin in patients with severe renal impairment.
Citation: Hanni C et al. Outcomes associated with apixaban vs. warfarin in patients with renal dysfunction. Blood Adv. 2020;4(11): 2366-71. doi: 10.1182/bloodadvances.2019000972.
Dr. Narayan is a hospitalist in the Division of Hospital Medicine, Mount Sinai Health System, New York.
Dracunculiasis – guinea worm disease – is close to eradication. But will we ever reach the finish line?
When in 1988 former U.S. President Jimmy Carter toured Denchira and Elevanyo, two villages near Accra, Ghana, he noticed a young woman who appeared to be cradling a baby. Carter approached her for a chat, but was stopped in his tracks by a disquieting sight.
“It was not a baby. It was her right breast, which was about a foot long, and it had a guinea worm coming out of its nipple,” Mr. Carter later recalled. During his tour of Ghana that year, Mr. Carter saw hundreds of people affected by the guinea worm, an infection known as dracunculiasis – a disease caused by the nematode parasite Dracunculus medinensis. It’s a condition that can cause fever, severe pain, and even permanent damage to affected limbs.
In the late 1980s the country reported as many as 180,000 cases of guinea worm disease per year. Across the globe, that number was a staggering 3.5 million. However, by 2020, the world was down to just 27 cases, all of them in Africa.
This enormous reduction in prevalence is a direct effect of campaigns by endemic countries assisted by organizations such as the Centers for Disease Control and Prevention, the World Health Organization, and the Carter Center (a not-for-profit founded in 1982 by Jimmy Carter), which have strived since the 1980s to eradicate dracunculiasis, hoping to make it the second human disease purposefully wiped off the face of Earth. (Smallpox was the first.)
“That’s an extraordinary public health achievement,” David Molyneux, PhD, parasitologist at the Liverpool School of Tropical Medicine, said in an interview. Yet the eradication goal, currently set for 2030, seems unlikely to be met. What’s more, some experts argue that chasing eradication may be altogether a misguided idea.
Humanity has known dracunculiasis for millennia. Well-preserved specimens of Dracunculus medinensis were discovered in Egyptian mummies, while some researchers claim that the Old Testament’s “fiery serpents” that descended upon the Israelites near the Red Sea were in fact guinea worms, as the parasite was endemic to the area in the past. Even the serpent coiled around the staff of Asclepius, the god of medicine, might have been a guinea worm, according to some historians.
This would make sense considering how the disease is treated. When an adult worm emerges through the skin, a painful and crippling occurrence, it is wound up around a stick or a piece of gauze, a little at a time, to slowly draw it out of the skin. As the worm can be over 3 feet long, this procedure may take weeks. What you end up with is a stick with a long, snake-like animal coiled around it. Asclepius’s staff.
The first step in the infection is when a person drinks water contaminated with copepods, or water fleas, which contain the larvae of Dracunculus medinensis. Next, the larvae are freed in the stomach and start migrating through the body, looking to mate. The fertilized female worm is the one that causes the debilitating symptoms.
About a year after the initial infection, the pregnant female worm looks for exit points from the body, usually through legs or feet, ready to release new larvae. If the unlucky sufferer steps into a pond or a river, the immature larvae escape into the water, where they are eaten by water fleas. “People are fetching water to drink, and they walk into the water thinking they can get cleaner water not along the edge,” Adam Weiss, MPH, director of the Carter Center’s Guinea Worm Eradication Program, said in an interview. The vicious cycle begins anew.
Dracunculiasis may not be a killer disease, but it is painful and disabling. A study on school attendance in Nigeria showed that in 1995 when guinea worm infection prevalence among schoolchildren was as high as 27.7%, it was responsible for almost all school absences. As the result of the infection, children were seen wandering and sitting around the village helplessly. If it was the parents who got infected, children stayed out of school to help around the home. The dracunculiasis’ impact on work and earning capacity is so profound, in fact, that in Mali the infliction is known as “the disease of the empty granary.”
When in 1986 the Carter Center took the reins of the global dracunculiasis eradication campaign, India was the only country with a national program to get rid of the disease. Yet, once other nations joined the struggle, the results rapidly became visible. By 1993, the American Journal of Tropical Medicine and Hygiene published a paper titled, “Dracunculiasis Eradication: Beginning of the End.” The cases plummeted from 3.5 million in 1986 to 221,000 in 1993 and 32,000 in 2003, then to a mere 22 cases in 2015. What worked was a combination of surveillance, education campaigns, safe water provision, and treating potentially contaminated water with a chemical called Abate, a potent larvicide.
Today, many endemic countries, from Chad and Ethiopia to Mali and South Sudan, follow similar procedures. First and foremost is the supply of clean drinking water. However, Mr. Weiss said, this is not a “silver bullet, given how people live.” Those who are seminomadic or otherwise take care of livestock often fetch water outside of the village, from ponds or rivers. This is why dracunculiasis eradication programs include handing out portable water filters, which can be worn around the neck.
But if you don’t know why you should filter water, in all likelihood you won’t do it – cloth filters distributed for home water purification sometimes ended up as decorations or sewn into wedding dresses. That’s why education is key, too. Poster campaigns, comic books, radio broadcasts, instructions by volunteers, even t-shirts with health messages slowly but surely did change behaviors.
Cash rewards for reporting cases of dracunculiasis, which can be as high as $100, also work well to boost surveillance systems. Once a case is identified, patients may be moved to a containment center, both to treat the wound and to prevent patients from spreading the disease. Local water sources, meanwhile, may be sprayed with Abate.
1995 was the first year set as a target date for the eradication of dracunculiasis. Yet the goal wasn’t met – even though the total number of cases did decline by 97%. Next goals followed: 2009, 2020, and now, finally, 2030. For well over a decade now the world has been down to a trickle of cases per year, but the numbers don’t seem to want to budge lower. Mr. Weiss calls it a “limbo period” – we are almost there, but not quite. The final push, it seems, may be the one that’s the most difficult, especially now that we have two further complications: increasing conflicts in some endemic areas and zoonotic transmission.
According to WHO, in places like the Democratic Republic of the Congo, Mali, South Sudan, and Sudan, insecurity “hinders eradication efforts.” Not only does this insecurity make it difficult for health workers to reach endemic areas, but wars and violence also displace people, pushing those infected with guinea worm to walk far distances in search of safety, and spreading the disease during their travels. Case containment and contact tracing become challenging. A recent study by Dr. Molyneux and colleagues showed that, in the 3 years since 2018, conflicts in the endemic areas have increased dramatically.
And then there are the animals. Up until 2012, eradication of guinea worm seemed fairly simple, at least from a biological perspective: Stop infected humans from contaminating drinking water and the parasites won’t be able to continue their life cycle. But in 2012, news came from Chad that a significant number of local dogs were found infected with the Dracunculus medinensis parasite, the very same one that attacks humans. In 2020, close to 1,600 dogs were reported to be infected with guinea worm, most of them in Chad. This left scientists scratching their heads: Dracunculiasis was supposed to be a purely human infliction. How were the dogs getting infected? Did the parasite jump to a new species because we were so efficient at eliminating it from humans?
“I have first seen a guinea worm transmission in dogs back in 2003,” Teshome Gebre, PhD, said in an interview. Dr. Gebre is regional director for Africa at International Trachoma Initiative and has spent more than 40 years fighting to eradicate various diseases, including smallpox and guinea worm. Yet in 2003, Dr. Gebre’s report was dismissed: it couldn’t have been the same species of the parasite, the reasoning went, since Dracunculus medinensis was exclusive to humans.
“I think it’s fair to say that there were infections in dogs before 2012. I find it difficult to believe, logically, that it just came out of nowhere,” Mr. Weiss said. A 2018 genetic study showed that a novel host switch is an unlikely scenario – the parasites must have been infecting dogs in the past, we just haven’t been looking. By 2012, Chad had a very efficient guinea worm surveillance system, with generous cash rewards for human cases, and people started reporting the dogs, too. Soon money was also offered for news on infected animals, and the cases exploded. This was then followed by accounts of afflicted cats and baboons.
To announce the eradication of dracunculiasis in 2030, the requirement will be no more transmission of the parasite for at least 4 years prior anywhere in the world – not only zero human cases, but also no infections in dogs, cats, or baboons. Seven countries remain to be certified as guinea worm free, all of them in Africa. “We have to be a 100% sure that there is no transmission of the parasite in a country,” said Dr. Molyneux, who participated in country certification teams – a rigorous process to validate country reports. He believes that the presence of animal hosts as well as growing insecurities in the region make such certification extremely challenging over the next few years.
“Eradication as it is defined does not seem feasible by 2030 as things stand, [considering] political and resource constraints, the unknowns of the ecology of dogs, and the possible impact of climate change and geopolitical instability and with countries having other health priorities, including COVID,” Dr. Molyneux said.
For Mr. Weiss, dogs are not that much of a problem – since they can be tethered to prevent the spread of the disease. But you can’t tether baboons. “That does raise that more existential threat–related question of: Is this scientifically possible?” he said. Mr. Weiss and colleagues at the Centers for Disease Control and Prevention are currently working on a serologic assay to test whether baboons are important for human transmission.
For some experts, such as Dr. Gebre, the current struggles to bring cases down to zero put a spotlight on a bigger question: is it worthwhile to strive for eradication at all? That last stretch of the eradication campaign can appear a bit like a game of whack-a-mole. “There were times when we’ve achieved zero cases [in Ethiopia]. Zero. And then, it just reemerges,” Dr. Gebre said. Programs aimed at certification are costly, running up to $1.6 million per year in Nigeria. The funds often come from the same donor pockets that pay for the fight against malaria, HIV, polio, as well as other neglected tropical diseases. Dr. Gebre believed it would be more cost and time efficient to switch the effort from total eradication to elimination as a public health care problem.
Of course, there is the risk that the cases would go up again once we ease up on the pressure to eradicate dracunculiasis. “Do we want to be fighting guinea worm in perpetuity?” Mr. Weiss asked. However, Dr. Gebre believed the cases are unlikely to explode anymore.
“The situation in the countries is not the way it was 30 years ago,” Dr. Gebre said, pointing out increased awareness, higher education levels, and better community-based health facilities. “You can cap it around a trickle number of cases a year – 10, 15, 20 maybe.”
The keys, Dr. Gebre and Dr. Molyneux both said, include the provision of safe drinking water and strengthening the healthcare systems of endemic countries in general, so they can deal with whatever cases may come up. “Water, sanitation, surveillance, good public education – and the maintenance of the guinea worm–specific reward system to maintain awareness, as well as continuing research” are all needed, Dr. Molyneux said.
Getting out of the dracunculiasis limbo period won’t be easy. We certainly need more data on animal transmission to better understand what challenges we might be facing. The experts agree that what’s important is to follow the science and stay flexible. “We have made an incredible progress, our investment has been worthwhile,” Dr. Molyneux said. But “you have to adapt to the changing realities.”
Dr. Gebre received no financial support for the review article and has no other conflicts of interest to declare. Dr. Molyneux is a member of the WHO International Commission for the Certification of Dracunculus Eradication, an independent body appointed by the director general of WHO. He acts as a rapporteur for the ICCDE as a paid consultant. He declared he does not receive any financial support for other related activities. Mr. Weiss receives support from the nonprofit Carter Center.
A version of this article first appeared on Medscape.com.
When in 1988 former U.S. President Jimmy Carter toured Denchira and Elevanyo, two villages near Accra, Ghana, he noticed a young woman who appeared to be cradling a baby. Carter approached her for a chat, but was stopped in his tracks by a disquieting sight.
“It was not a baby. It was her right breast, which was about a foot long, and it had a guinea worm coming out of its nipple,” Mr. Carter later recalled. During his tour of Ghana that year, Mr. Carter saw hundreds of people affected by the guinea worm, an infection known as dracunculiasis – a disease caused by the nematode parasite Dracunculus medinensis. It’s a condition that can cause fever, severe pain, and even permanent damage to affected limbs.
In the late 1980s the country reported as many as 180,000 cases of guinea worm disease per year. Across the globe, that number was a staggering 3.5 million. However, by 2020, the world was down to just 27 cases, all of them in Africa.
This enormous reduction in prevalence is a direct effect of campaigns by endemic countries assisted by organizations such as the Centers for Disease Control and Prevention, the World Health Organization, and the Carter Center (a not-for-profit founded in 1982 by Jimmy Carter), which have strived since the 1980s to eradicate dracunculiasis, hoping to make it the second human disease purposefully wiped off the face of Earth. (Smallpox was the first.)
“That’s an extraordinary public health achievement,” David Molyneux, PhD, parasitologist at the Liverpool School of Tropical Medicine, said in an interview. Yet the eradication goal, currently set for 2030, seems unlikely to be met. What’s more, some experts argue that chasing eradication may be altogether a misguided idea.
Humanity has known dracunculiasis for millennia. Well-preserved specimens of Dracunculus medinensis were discovered in Egyptian mummies, while some researchers claim that the Old Testament’s “fiery serpents” that descended upon the Israelites near the Red Sea were in fact guinea worms, as the parasite was endemic to the area in the past. Even the serpent coiled around the staff of Asclepius, the god of medicine, might have been a guinea worm, according to some historians.
This would make sense considering how the disease is treated. When an adult worm emerges through the skin, a painful and crippling occurrence, it is wound up around a stick or a piece of gauze, a little at a time, to slowly draw it out of the skin. As the worm can be over 3 feet long, this procedure may take weeks. What you end up with is a stick with a long, snake-like animal coiled around it. Asclepius’s staff.
The first step in the infection is when a person drinks water contaminated with copepods, or water fleas, which contain the larvae of Dracunculus medinensis. Next, the larvae are freed in the stomach and start migrating through the body, looking to mate. The fertilized female worm is the one that causes the debilitating symptoms.
About a year after the initial infection, the pregnant female worm looks for exit points from the body, usually through legs or feet, ready to release new larvae. If the unlucky sufferer steps into a pond or a river, the immature larvae escape into the water, where they are eaten by water fleas. “People are fetching water to drink, and they walk into the water thinking they can get cleaner water not along the edge,” Adam Weiss, MPH, director of the Carter Center’s Guinea Worm Eradication Program, said in an interview. The vicious cycle begins anew.
Dracunculiasis may not be a killer disease, but it is painful and disabling. A study on school attendance in Nigeria showed that in 1995 when guinea worm infection prevalence among schoolchildren was as high as 27.7%, it was responsible for almost all school absences. As the result of the infection, children were seen wandering and sitting around the village helplessly. If it was the parents who got infected, children stayed out of school to help around the home. The dracunculiasis’ impact on work and earning capacity is so profound, in fact, that in Mali the infliction is known as “the disease of the empty granary.”
When in 1986 the Carter Center took the reins of the global dracunculiasis eradication campaign, India was the only country with a national program to get rid of the disease. Yet, once other nations joined the struggle, the results rapidly became visible. By 1993, the American Journal of Tropical Medicine and Hygiene published a paper titled, “Dracunculiasis Eradication: Beginning of the End.” The cases plummeted from 3.5 million in 1986 to 221,000 in 1993 and 32,000 in 2003, then to a mere 22 cases in 2015. What worked was a combination of surveillance, education campaigns, safe water provision, and treating potentially contaminated water with a chemical called Abate, a potent larvicide.
Today, many endemic countries, from Chad and Ethiopia to Mali and South Sudan, follow similar procedures. First and foremost is the supply of clean drinking water. However, Mr. Weiss said, this is not a “silver bullet, given how people live.” Those who are seminomadic or otherwise take care of livestock often fetch water outside of the village, from ponds or rivers. This is why dracunculiasis eradication programs include handing out portable water filters, which can be worn around the neck.
But if you don’t know why you should filter water, in all likelihood you won’t do it – cloth filters distributed for home water purification sometimes ended up as decorations or sewn into wedding dresses. That’s why education is key, too. Poster campaigns, comic books, radio broadcasts, instructions by volunteers, even t-shirts with health messages slowly but surely did change behaviors.
Cash rewards for reporting cases of dracunculiasis, which can be as high as $100, also work well to boost surveillance systems. Once a case is identified, patients may be moved to a containment center, both to treat the wound and to prevent patients from spreading the disease. Local water sources, meanwhile, may be sprayed with Abate.
1995 was the first year set as a target date for the eradication of dracunculiasis. Yet the goal wasn’t met – even though the total number of cases did decline by 97%. Next goals followed: 2009, 2020, and now, finally, 2030. For well over a decade now the world has been down to a trickle of cases per year, but the numbers don’t seem to want to budge lower. Mr. Weiss calls it a “limbo period” – we are almost there, but not quite. The final push, it seems, may be the one that’s the most difficult, especially now that we have two further complications: increasing conflicts in some endemic areas and zoonotic transmission.
According to WHO, in places like the Democratic Republic of the Congo, Mali, South Sudan, and Sudan, insecurity “hinders eradication efforts.” Not only does this insecurity make it difficult for health workers to reach endemic areas, but wars and violence also displace people, pushing those infected with guinea worm to walk far distances in search of safety, and spreading the disease during their travels. Case containment and contact tracing become challenging. A recent study by Dr. Molyneux and colleagues showed that, in the 3 years since 2018, conflicts in the endemic areas have increased dramatically.
And then there are the animals. Up until 2012, eradication of guinea worm seemed fairly simple, at least from a biological perspective: Stop infected humans from contaminating drinking water and the parasites won’t be able to continue their life cycle. But in 2012, news came from Chad that a significant number of local dogs were found infected with the Dracunculus medinensis parasite, the very same one that attacks humans. In 2020, close to 1,600 dogs were reported to be infected with guinea worm, most of them in Chad. This left scientists scratching their heads: Dracunculiasis was supposed to be a purely human infliction. How were the dogs getting infected? Did the parasite jump to a new species because we were so efficient at eliminating it from humans?
“I have first seen a guinea worm transmission in dogs back in 2003,” Teshome Gebre, PhD, said in an interview. Dr. Gebre is regional director for Africa at International Trachoma Initiative and has spent more than 40 years fighting to eradicate various diseases, including smallpox and guinea worm. Yet in 2003, Dr. Gebre’s report was dismissed: it couldn’t have been the same species of the parasite, the reasoning went, since Dracunculus medinensis was exclusive to humans.
“I think it’s fair to say that there were infections in dogs before 2012. I find it difficult to believe, logically, that it just came out of nowhere,” Mr. Weiss said. A 2018 genetic study showed that a novel host switch is an unlikely scenario – the parasites must have been infecting dogs in the past, we just haven’t been looking. By 2012, Chad had a very efficient guinea worm surveillance system, with generous cash rewards for human cases, and people started reporting the dogs, too. Soon money was also offered for news on infected animals, and the cases exploded. This was then followed by accounts of afflicted cats and baboons.
To announce the eradication of dracunculiasis in 2030, the requirement will be no more transmission of the parasite for at least 4 years prior anywhere in the world – not only zero human cases, but also no infections in dogs, cats, or baboons. Seven countries remain to be certified as guinea worm free, all of them in Africa. “We have to be a 100% sure that there is no transmission of the parasite in a country,” said Dr. Molyneux, who participated in country certification teams – a rigorous process to validate country reports. He believes that the presence of animal hosts as well as growing insecurities in the region make such certification extremely challenging over the next few years.
“Eradication as it is defined does not seem feasible by 2030 as things stand, [considering] political and resource constraints, the unknowns of the ecology of dogs, and the possible impact of climate change and geopolitical instability and with countries having other health priorities, including COVID,” Dr. Molyneux said.
For Mr. Weiss, dogs are not that much of a problem – since they can be tethered to prevent the spread of the disease. But you can’t tether baboons. “That does raise that more existential threat–related question of: Is this scientifically possible?” he said. Mr. Weiss and colleagues at the Centers for Disease Control and Prevention are currently working on a serologic assay to test whether baboons are important for human transmission.
For some experts, such as Dr. Gebre, the current struggles to bring cases down to zero put a spotlight on a bigger question: is it worthwhile to strive for eradication at all? That last stretch of the eradication campaign can appear a bit like a game of whack-a-mole. “There were times when we’ve achieved zero cases [in Ethiopia]. Zero. And then, it just reemerges,” Dr. Gebre said. Programs aimed at certification are costly, running up to $1.6 million per year in Nigeria. The funds often come from the same donor pockets that pay for the fight against malaria, HIV, polio, as well as other neglected tropical diseases. Dr. Gebre believed it would be more cost and time efficient to switch the effort from total eradication to elimination as a public health care problem.
Of course, there is the risk that the cases would go up again once we ease up on the pressure to eradicate dracunculiasis. “Do we want to be fighting guinea worm in perpetuity?” Mr. Weiss asked. However, Dr. Gebre believed the cases are unlikely to explode anymore.
“The situation in the countries is not the way it was 30 years ago,” Dr. Gebre said, pointing out increased awareness, higher education levels, and better community-based health facilities. “You can cap it around a trickle number of cases a year – 10, 15, 20 maybe.”
The keys, Dr. Gebre and Dr. Molyneux both said, include the provision of safe drinking water and strengthening the healthcare systems of endemic countries in general, so they can deal with whatever cases may come up. “Water, sanitation, surveillance, good public education – and the maintenance of the guinea worm–specific reward system to maintain awareness, as well as continuing research” are all needed, Dr. Molyneux said.
Getting out of the dracunculiasis limbo period won’t be easy. We certainly need more data on animal transmission to better understand what challenges we might be facing. The experts agree that what’s important is to follow the science and stay flexible. “We have made an incredible progress, our investment has been worthwhile,” Dr. Molyneux said. But “you have to adapt to the changing realities.”
Dr. Gebre received no financial support for the review article and has no other conflicts of interest to declare. Dr. Molyneux is a member of the WHO International Commission for the Certification of Dracunculus Eradication, an independent body appointed by the director general of WHO. He acts as a rapporteur for the ICCDE as a paid consultant. He declared he does not receive any financial support for other related activities. Mr. Weiss receives support from the nonprofit Carter Center.
A version of this article first appeared on Medscape.com.
When in 1988 former U.S. President Jimmy Carter toured Denchira and Elevanyo, two villages near Accra, Ghana, he noticed a young woman who appeared to be cradling a baby. Carter approached her for a chat, but was stopped in his tracks by a disquieting sight.
“It was not a baby. It was her right breast, which was about a foot long, and it had a guinea worm coming out of its nipple,” Mr. Carter later recalled. During his tour of Ghana that year, Mr. Carter saw hundreds of people affected by the guinea worm, an infection known as dracunculiasis – a disease caused by the nematode parasite Dracunculus medinensis. It’s a condition that can cause fever, severe pain, and even permanent damage to affected limbs.
In the late 1980s the country reported as many as 180,000 cases of guinea worm disease per year. Across the globe, that number was a staggering 3.5 million. However, by 2020, the world was down to just 27 cases, all of them in Africa.
This enormous reduction in prevalence is a direct effect of campaigns by endemic countries assisted by organizations such as the Centers for Disease Control and Prevention, the World Health Organization, and the Carter Center (a not-for-profit founded in 1982 by Jimmy Carter), which have strived since the 1980s to eradicate dracunculiasis, hoping to make it the second human disease purposefully wiped off the face of Earth. (Smallpox was the first.)
“That’s an extraordinary public health achievement,” David Molyneux, PhD, parasitologist at the Liverpool School of Tropical Medicine, said in an interview. Yet the eradication goal, currently set for 2030, seems unlikely to be met. What’s more, some experts argue that chasing eradication may be altogether a misguided idea.
Humanity has known dracunculiasis for millennia. Well-preserved specimens of Dracunculus medinensis were discovered in Egyptian mummies, while some researchers claim that the Old Testament’s “fiery serpents” that descended upon the Israelites near the Red Sea were in fact guinea worms, as the parasite was endemic to the area in the past. Even the serpent coiled around the staff of Asclepius, the god of medicine, might have been a guinea worm, according to some historians.
This would make sense considering how the disease is treated. When an adult worm emerges through the skin, a painful and crippling occurrence, it is wound up around a stick or a piece of gauze, a little at a time, to slowly draw it out of the skin. As the worm can be over 3 feet long, this procedure may take weeks. What you end up with is a stick with a long, snake-like animal coiled around it. Asclepius’s staff.
The first step in the infection is when a person drinks water contaminated with copepods, or water fleas, which contain the larvae of Dracunculus medinensis. Next, the larvae are freed in the stomach and start migrating through the body, looking to mate. The fertilized female worm is the one that causes the debilitating symptoms.
About a year after the initial infection, the pregnant female worm looks for exit points from the body, usually through legs or feet, ready to release new larvae. If the unlucky sufferer steps into a pond or a river, the immature larvae escape into the water, where they are eaten by water fleas. “People are fetching water to drink, and they walk into the water thinking they can get cleaner water not along the edge,” Adam Weiss, MPH, director of the Carter Center’s Guinea Worm Eradication Program, said in an interview. The vicious cycle begins anew.
Dracunculiasis may not be a killer disease, but it is painful and disabling. A study on school attendance in Nigeria showed that in 1995 when guinea worm infection prevalence among schoolchildren was as high as 27.7%, it was responsible for almost all school absences. As the result of the infection, children were seen wandering and sitting around the village helplessly. If it was the parents who got infected, children stayed out of school to help around the home. The dracunculiasis’ impact on work and earning capacity is so profound, in fact, that in Mali the infliction is known as “the disease of the empty granary.”
When in 1986 the Carter Center took the reins of the global dracunculiasis eradication campaign, India was the only country with a national program to get rid of the disease. Yet, once other nations joined the struggle, the results rapidly became visible. By 1993, the American Journal of Tropical Medicine and Hygiene published a paper titled, “Dracunculiasis Eradication: Beginning of the End.” The cases plummeted from 3.5 million in 1986 to 221,000 in 1993 and 32,000 in 2003, then to a mere 22 cases in 2015. What worked was a combination of surveillance, education campaigns, safe water provision, and treating potentially contaminated water with a chemical called Abate, a potent larvicide.
Today, many endemic countries, from Chad and Ethiopia to Mali and South Sudan, follow similar procedures. First and foremost is the supply of clean drinking water. However, Mr. Weiss said, this is not a “silver bullet, given how people live.” Those who are seminomadic or otherwise take care of livestock often fetch water outside of the village, from ponds or rivers. This is why dracunculiasis eradication programs include handing out portable water filters, which can be worn around the neck.
But if you don’t know why you should filter water, in all likelihood you won’t do it – cloth filters distributed for home water purification sometimes ended up as decorations or sewn into wedding dresses. That’s why education is key, too. Poster campaigns, comic books, radio broadcasts, instructions by volunteers, even t-shirts with health messages slowly but surely did change behaviors.
Cash rewards for reporting cases of dracunculiasis, which can be as high as $100, also work well to boost surveillance systems. Once a case is identified, patients may be moved to a containment center, both to treat the wound and to prevent patients from spreading the disease. Local water sources, meanwhile, may be sprayed with Abate.
1995 was the first year set as a target date for the eradication of dracunculiasis. Yet the goal wasn’t met – even though the total number of cases did decline by 97%. Next goals followed: 2009, 2020, and now, finally, 2030. For well over a decade now the world has been down to a trickle of cases per year, but the numbers don’t seem to want to budge lower. Mr. Weiss calls it a “limbo period” – we are almost there, but not quite. The final push, it seems, may be the one that’s the most difficult, especially now that we have two further complications: increasing conflicts in some endemic areas and zoonotic transmission.
According to WHO, in places like the Democratic Republic of the Congo, Mali, South Sudan, and Sudan, insecurity “hinders eradication efforts.” Not only does this insecurity make it difficult for health workers to reach endemic areas, but wars and violence also displace people, pushing those infected with guinea worm to walk far distances in search of safety, and spreading the disease during their travels. Case containment and contact tracing become challenging. A recent study by Dr. Molyneux and colleagues showed that, in the 3 years since 2018, conflicts in the endemic areas have increased dramatically.
And then there are the animals. Up until 2012, eradication of guinea worm seemed fairly simple, at least from a biological perspective: Stop infected humans from contaminating drinking water and the parasites won’t be able to continue their life cycle. But in 2012, news came from Chad that a significant number of local dogs were found infected with the Dracunculus medinensis parasite, the very same one that attacks humans. In 2020, close to 1,600 dogs were reported to be infected with guinea worm, most of them in Chad. This left scientists scratching their heads: Dracunculiasis was supposed to be a purely human infliction. How were the dogs getting infected? Did the parasite jump to a new species because we were so efficient at eliminating it from humans?
“I have first seen a guinea worm transmission in dogs back in 2003,” Teshome Gebre, PhD, said in an interview. Dr. Gebre is regional director for Africa at International Trachoma Initiative and has spent more than 40 years fighting to eradicate various diseases, including smallpox and guinea worm. Yet in 2003, Dr. Gebre’s report was dismissed: it couldn’t have been the same species of the parasite, the reasoning went, since Dracunculus medinensis was exclusive to humans.
“I think it’s fair to say that there were infections in dogs before 2012. I find it difficult to believe, logically, that it just came out of nowhere,” Mr. Weiss said. A 2018 genetic study showed that a novel host switch is an unlikely scenario – the parasites must have been infecting dogs in the past, we just haven’t been looking. By 2012, Chad had a very efficient guinea worm surveillance system, with generous cash rewards for human cases, and people started reporting the dogs, too. Soon money was also offered for news on infected animals, and the cases exploded. This was then followed by accounts of afflicted cats and baboons.
To announce the eradication of dracunculiasis in 2030, the requirement will be no more transmission of the parasite for at least 4 years prior anywhere in the world – not only zero human cases, but also no infections in dogs, cats, or baboons. Seven countries remain to be certified as guinea worm free, all of them in Africa. “We have to be a 100% sure that there is no transmission of the parasite in a country,” said Dr. Molyneux, who participated in country certification teams – a rigorous process to validate country reports. He believes that the presence of animal hosts as well as growing insecurities in the region make such certification extremely challenging over the next few years.
“Eradication as it is defined does not seem feasible by 2030 as things stand, [considering] political and resource constraints, the unknowns of the ecology of dogs, and the possible impact of climate change and geopolitical instability and with countries having other health priorities, including COVID,” Dr. Molyneux said.
For Mr. Weiss, dogs are not that much of a problem – since they can be tethered to prevent the spread of the disease. But you can’t tether baboons. “That does raise that more existential threat–related question of: Is this scientifically possible?” he said. Mr. Weiss and colleagues at the Centers for Disease Control and Prevention are currently working on a serologic assay to test whether baboons are important for human transmission.
For some experts, such as Dr. Gebre, the current struggles to bring cases down to zero put a spotlight on a bigger question: is it worthwhile to strive for eradication at all? That last stretch of the eradication campaign can appear a bit like a game of whack-a-mole. “There were times when we’ve achieved zero cases [in Ethiopia]. Zero. And then, it just reemerges,” Dr. Gebre said. Programs aimed at certification are costly, running up to $1.6 million per year in Nigeria. The funds often come from the same donor pockets that pay for the fight against malaria, HIV, polio, as well as other neglected tropical diseases. Dr. Gebre believed it would be more cost and time efficient to switch the effort from total eradication to elimination as a public health care problem.
Of course, there is the risk that the cases would go up again once we ease up on the pressure to eradicate dracunculiasis. “Do we want to be fighting guinea worm in perpetuity?” Mr. Weiss asked. However, Dr. Gebre believed the cases are unlikely to explode anymore.
“The situation in the countries is not the way it was 30 years ago,” Dr. Gebre said, pointing out increased awareness, higher education levels, and better community-based health facilities. “You can cap it around a trickle number of cases a year – 10, 15, 20 maybe.”
The keys, Dr. Gebre and Dr. Molyneux both said, include the provision of safe drinking water and strengthening the healthcare systems of endemic countries in general, so they can deal with whatever cases may come up. “Water, sanitation, surveillance, good public education – and the maintenance of the guinea worm–specific reward system to maintain awareness, as well as continuing research” are all needed, Dr. Molyneux said.
Getting out of the dracunculiasis limbo period won’t be easy. We certainly need more data on animal transmission to better understand what challenges we might be facing. The experts agree that what’s important is to follow the science and stay flexible. “We have made an incredible progress, our investment has been worthwhile,” Dr. Molyneux said. But “you have to adapt to the changing realities.”
Dr. Gebre received no financial support for the review article and has no other conflicts of interest to declare. Dr. Molyneux is a member of the WHO International Commission for the Certification of Dracunculus Eradication, an independent body appointed by the director general of WHO. He acts as a rapporteur for the ICCDE as a paid consultant. He declared he does not receive any financial support for other related activities. Mr. Weiss receives support from the nonprofit Carter Center.
A version of this article first appeared on Medscape.com.
New insights into psychogenic seizures in teens
, results of a small study suggest.
The school experience of teens with PNES is overwhelmingly negative, study investigator Andrea Tanner, PhD, a postdoctoral fellow at Indiana University School of Nursing, Indianapolis.
She hopes this research will spur a collaborative effort between students, schools, families, and health care providers “to develop an effective plan to help these adolescents cope, to manage this condition, and hopefully reach seizure freedom.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Anxiety, perfectionism
Although psychogenic seizures resemble epileptic seizures, they have a psychological basis and, unlike epilepsy, are not caused by abnormal electrical brain activity.
While the school experience has previously been identified as a source of predisposing, precipitating, and perpetuating factors for PNES, little is known about the school experience of adolescents with the disorder and the role it may play in PNES management, the investigators noted.
During her 20 years as a school nurse, Dr. Tanner saw firsthand how school staff struggled with responding appropriately to teens with PNES. “They wanted to call 911 every time; they wanted to respond as if it [were] an epileptic seizure.”
For the study, she interviewed 10 teens with PNES, aged 12 to 19 years, whom she found mostly through Facebook support groups but also through flyers. All participants had undergone video EEG and been diagnosed with PNES.
From the interviews, Dr. Tanner and colleagues conducted a qualitative content analysis and uncovered “overarching” themes.
A main theme was stress, some of which focused on bullying by peers or harassment by school personnel, much of which was related to accusations of the children “faking” seizures to get attention, said Dr. Tanner.
Some teens reported being banned from school events, such as field trips, out of concern they would be a “distraction,” which led to feelings of isolation and exclusion, said Dr. Tanner.
Research points to a growing incidence of PNES among adolescents. This may be because it is now better recognized, or it may stem from the unique stressors today’s teens face, said Dr. Tanner.
Adolescents discussed the pressures they feel to be the best at everything. “They wanted to be good in athletics; they wanted to be good in academics; they wanted to get into a good college,” said Dr. Tanner.
Some study participants had undergone psychotherapy, including cognitive-behavioral therapy, and others had investigated mindfulness-based therapy. However, not all were receiving treatment. For some, such care was inaccessible, while others had tried a mental health care intervention but had abandoned it.
Although all the study participants were female, Dr. Tanner has interviewed males outside this study and found their experiences are similar.
Her next research step is to try to quantify the findings. “I would like to begin to look at what would be the appropriate outcomes if I were to do an intervention to improve the school experience.”
Her message for doctors is to see school nurses as a “partner” or “liaison” who “can bridge the world of health care and education.”
Important, novel research
Commenting on the research, Barbara Dworetzky, MD, Chief, Epilepsy, Brigham and Women’s Hospital, and professor of neurology, Harvard Medical School, said it’s “important and novel.”
The study focuses on the main factors – or themes – that lead to increased stress, such as bullying, isolation, and “not being believed,” that are likely triggers for PNES, said Dr. Dworetzky.
The study is also important because it focuses on factors that help make the girls “feel supported and protected” – for example, having staff “take the episodes seriously,” she said.
The study’s qualitative measures “are a valid way of understanding these girls and giving them a voice,” said Dr. Dworetzky. She added the study provides “practical information” that could help target treatments to improve outcomes in this group.
A limitation of the study was that the very small cohort of teenage girls was selected only through families in Facebook support groups or flyers to school nurses, said Dr. Dworetzky.
“There are likely many other groups who don’t even have families trying to help them. Larger cohorts without this type of bias may be next steps.”
A version of this article first appeared on Medscape.com.
, results of a small study suggest.
The school experience of teens with PNES is overwhelmingly negative, study investigator Andrea Tanner, PhD, a postdoctoral fellow at Indiana University School of Nursing, Indianapolis.
She hopes this research will spur a collaborative effort between students, schools, families, and health care providers “to develop an effective plan to help these adolescents cope, to manage this condition, and hopefully reach seizure freedom.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Anxiety, perfectionism
Although psychogenic seizures resemble epileptic seizures, they have a psychological basis and, unlike epilepsy, are not caused by abnormal electrical brain activity.
While the school experience has previously been identified as a source of predisposing, precipitating, and perpetuating factors for PNES, little is known about the school experience of adolescents with the disorder and the role it may play in PNES management, the investigators noted.
During her 20 years as a school nurse, Dr. Tanner saw firsthand how school staff struggled with responding appropriately to teens with PNES. “They wanted to call 911 every time; they wanted to respond as if it [were] an epileptic seizure.”
For the study, she interviewed 10 teens with PNES, aged 12 to 19 years, whom she found mostly through Facebook support groups but also through flyers. All participants had undergone video EEG and been diagnosed with PNES.
From the interviews, Dr. Tanner and colleagues conducted a qualitative content analysis and uncovered “overarching” themes.
A main theme was stress, some of which focused on bullying by peers or harassment by school personnel, much of which was related to accusations of the children “faking” seizures to get attention, said Dr. Tanner.
Some teens reported being banned from school events, such as field trips, out of concern they would be a “distraction,” which led to feelings of isolation and exclusion, said Dr. Tanner.
Research points to a growing incidence of PNES among adolescents. This may be because it is now better recognized, or it may stem from the unique stressors today’s teens face, said Dr. Tanner.
Adolescents discussed the pressures they feel to be the best at everything. “They wanted to be good in athletics; they wanted to be good in academics; they wanted to get into a good college,” said Dr. Tanner.
Some study participants had undergone psychotherapy, including cognitive-behavioral therapy, and others had investigated mindfulness-based therapy. However, not all were receiving treatment. For some, such care was inaccessible, while others had tried a mental health care intervention but had abandoned it.
Although all the study participants were female, Dr. Tanner has interviewed males outside this study and found their experiences are similar.
Her next research step is to try to quantify the findings. “I would like to begin to look at what would be the appropriate outcomes if I were to do an intervention to improve the school experience.”
Her message for doctors is to see school nurses as a “partner” or “liaison” who “can bridge the world of health care and education.”
Important, novel research
Commenting on the research, Barbara Dworetzky, MD, Chief, Epilepsy, Brigham and Women’s Hospital, and professor of neurology, Harvard Medical School, said it’s “important and novel.”
The study focuses on the main factors – or themes – that lead to increased stress, such as bullying, isolation, and “not being believed,” that are likely triggers for PNES, said Dr. Dworetzky.
The study is also important because it focuses on factors that help make the girls “feel supported and protected” – for example, having staff “take the episodes seriously,” she said.
The study’s qualitative measures “are a valid way of understanding these girls and giving them a voice,” said Dr. Dworetzky. She added the study provides “practical information” that could help target treatments to improve outcomes in this group.
A limitation of the study was that the very small cohort of teenage girls was selected only through families in Facebook support groups or flyers to school nurses, said Dr. Dworetzky.
“There are likely many other groups who don’t even have families trying to help them. Larger cohorts without this type of bias may be next steps.”
A version of this article first appeared on Medscape.com.
, results of a small study suggest.
The school experience of teens with PNES is overwhelmingly negative, study investigator Andrea Tanner, PhD, a postdoctoral fellow at Indiana University School of Nursing, Indianapolis.
She hopes this research will spur a collaborative effort between students, schools, families, and health care providers “to develop an effective plan to help these adolescents cope, to manage this condition, and hopefully reach seizure freedom.”
The findings were presented at the annual meeting of the American Epilepsy Society.
Anxiety, perfectionism
Although psychogenic seizures resemble epileptic seizures, they have a psychological basis and, unlike epilepsy, are not caused by abnormal electrical brain activity.
While the school experience has previously been identified as a source of predisposing, precipitating, and perpetuating factors for PNES, little is known about the school experience of adolescents with the disorder and the role it may play in PNES management, the investigators noted.
During her 20 years as a school nurse, Dr. Tanner saw firsthand how school staff struggled with responding appropriately to teens with PNES. “They wanted to call 911 every time; they wanted to respond as if it [were] an epileptic seizure.”
For the study, she interviewed 10 teens with PNES, aged 12 to 19 years, whom she found mostly through Facebook support groups but also through flyers. All participants had undergone video EEG and been diagnosed with PNES.
From the interviews, Dr. Tanner and colleagues conducted a qualitative content analysis and uncovered “overarching” themes.
A main theme was stress, some of which focused on bullying by peers or harassment by school personnel, much of which was related to accusations of the children “faking” seizures to get attention, said Dr. Tanner.
Some teens reported being banned from school events, such as field trips, out of concern they would be a “distraction,” which led to feelings of isolation and exclusion, said Dr. Tanner.
Research points to a growing incidence of PNES among adolescents. This may be because it is now better recognized, or it may stem from the unique stressors today’s teens face, said Dr. Tanner.
Adolescents discussed the pressures they feel to be the best at everything. “They wanted to be good in athletics; they wanted to be good in academics; they wanted to get into a good college,” said Dr. Tanner.
Some study participants had undergone psychotherapy, including cognitive-behavioral therapy, and others had investigated mindfulness-based therapy. However, not all were receiving treatment. For some, such care was inaccessible, while others had tried a mental health care intervention but had abandoned it.
Although all the study participants were female, Dr. Tanner has interviewed males outside this study and found their experiences are similar.
Her next research step is to try to quantify the findings. “I would like to begin to look at what would be the appropriate outcomes if I were to do an intervention to improve the school experience.”
Her message for doctors is to see school nurses as a “partner” or “liaison” who “can bridge the world of health care and education.”
Important, novel research
Commenting on the research, Barbara Dworetzky, MD, Chief, Epilepsy, Brigham and Women’s Hospital, and professor of neurology, Harvard Medical School, said it’s “important and novel.”
The study focuses on the main factors – or themes – that lead to increased stress, such as bullying, isolation, and “not being believed,” that are likely triggers for PNES, said Dr. Dworetzky.
The study is also important because it focuses on factors that help make the girls “feel supported and protected” – for example, having staff “take the episodes seriously,” she said.
The study’s qualitative measures “are a valid way of understanding these girls and giving them a voice,” said Dr. Dworetzky. She added the study provides “practical information” that could help target treatments to improve outcomes in this group.
A limitation of the study was that the very small cohort of teenage girls was selected only through families in Facebook support groups or flyers to school nurses, said Dr. Dworetzky.
“There are likely many other groups who don’t even have families trying to help them. Larger cohorts without this type of bias may be next steps.”
A version of this article first appeared on Medscape.com.
From AES 2021
‘Alarming’ rate of abuse in pregnant women with epilepsy
, new research shows.
Study investigator Naveed Chaudhry, MD, a recent epilepsy fellow and assistant professor of neurology, University of Colorado School of Medicine, described the finding as “alarming” and called for more support for this patient population.
Investigators found that women with epilepsy are also more likely to report other stressors, including divorce, illness, lost pay, and partner discord, while expecting.
“As epilepsy physicians, it’s important that we ask the right questions and dive a little bit deeper with these patients, even if it’s uncomfortable and not something we’re used to,” said Dr. Chaudhry.
The findings were presented at the annual meeting of the American Epilepsy Society.
Cause for concern
Women with epilepsy may be under stress for a variety of social and economic reasons. In some women, stress can trigger seizures, and during pregnancy, this can lead to complications such as preterm labor and low birth weight.
For the study, researchers tapped into the Center for Disease Control and Prevention Pregnancy Risk Assessment and Monitoring System (PRAMS). This database includes information from surveys asking women across the U.S. about their pregnancy and postpartum period.
Thirteen states collected data on stresses in women with and without epilepsy. Respondents were asked about 14 economic and other worries in the year prior to their baby’s birth, including the pregnancy period.
The analysis included 64,951 women, 1,140 of whom had epilepsy, who were included in surveys from 2012-2020. There were no significant demographic differences between those with and those without the disorder.
After adjusting for maternal age, race, ethnicity, marital status, education, and socioeconomic status, the study found that women with epilepsy experienced an average of 2.41 of the stressors compared with 1.72 for women without epilepsy.
Women with epilepsy were more likely to have experienced family illness, divorce, homelessness, partner job loss, reduced work or pay, increased arguments, having a partner in jail, drug use, and the death of someone close to them.
The results showed that unmarried and younger women as well as those with lower incomes were particularly prone to experience stress during pregnancy.
It’s not clear why women with epilepsy report more stressors. “Looking at the literature, no one has really looked at the exact reason for this, but we postulate it could be a lack of supports and support systems,” said Dr. Chaudhry.
Women were asked about physical, sexual, and emotional abuse. Results showed that substantially more women with epilepsy than those without the disorder reported such abuse during pregnancy – 10.6% versus 4.1%. The adjusted odds ratio for women with epilepsy reporting abuse was 2.78 (95% CI, 2.07-3.74).
“That raises our concern and needs to be looked at in more detail,” said Dr. Chaudhry.
It is unclear whether some women might have had psychogenic non-epileptic seizures (PNES), which are linked to a higher rate of abuse, said Dr. Chaudhry. “But the prevalence of PNES in the general population is quite low, so we don’t think it’s contributing to a large extent to this finding.”
The findings highlight the importance of addressing stress in women with epilepsy during pregnancy, he said. “We need to have good support services and we need to counsel women to optimize good outcomes.”
This applies to all women of childbearing age. “We suspect abuse and stressors are going to be going on throughout that period,” said Dr. Chaudhry. “It’s important to ask about it and have appropriate support staff and social work and people available to help when an issue is identified.”
Stress a common seizure trigger
Commenting on the research, Kimford Meador, MD, professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, noted the study was well conducted and had a large sample size.
The findings are important, as stress is a common trigger for seizures in people with epilepsy and is associated with mood and anxiety, which can affect quality of life, said Dr. Meador.
Results of his analysis from the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study, also presented at this year’s AES meeting, showed that women with epilepsy had more depressive symptoms during the postpartum period and more anxiety symptoms during pregnancy and postpartum in comparison with those without epilepsy.
Dr. Meador’s group also recently conducted a study that was published in JAMA Neurology, showing that in women with epilepsy during the postpartum period, anxiety is associated with lower cognitive ability in their children at age 2 years.
“All these findings highlight the importance of assessing and managing stress, anxiety, and mood in women with epilepsy,” said Dr. Meador. “Interventions could impact seizures and quality of life in pregnant women with epilepsy and long-term outcomes in their children.”
A version of this article first appeared on Medscape.com.
, new research shows.
Study investigator Naveed Chaudhry, MD, a recent epilepsy fellow and assistant professor of neurology, University of Colorado School of Medicine, described the finding as “alarming” and called for more support for this patient population.
Investigators found that women with epilepsy are also more likely to report other stressors, including divorce, illness, lost pay, and partner discord, while expecting.
“As epilepsy physicians, it’s important that we ask the right questions and dive a little bit deeper with these patients, even if it’s uncomfortable and not something we’re used to,” said Dr. Chaudhry.
The findings were presented at the annual meeting of the American Epilepsy Society.
Cause for concern
Women with epilepsy may be under stress for a variety of social and economic reasons. In some women, stress can trigger seizures, and during pregnancy, this can lead to complications such as preterm labor and low birth weight.
For the study, researchers tapped into the Center for Disease Control and Prevention Pregnancy Risk Assessment and Monitoring System (PRAMS). This database includes information from surveys asking women across the U.S. about their pregnancy and postpartum period.
Thirteen states collected data on stresses in women with and without epilepsy. Respondents were asked about 14 economic and other worries in the year prior to their baby’s birth, including the pregnancy period.
The analysis included 64,951 women, 1,140 of whom had epilepsy, who were included in surveys from 2012-2020. There were no significant demographic differences between those with and those without the disorder.
After adjusting for maternal age, race, ethnicity, marital status, education, and socioeconomic status, the study found that women with epilepsy experienced an average of 2.41 of the stressors compared with 1.72 for women without epilepsy.
Women with epilepsy were more likely to have experienced family illness, divorce, homelessness, partner job loss, reduced work or pay, increased arguments, having a partner in jail, drug use, and the death of someone close to them.
The results showed that unmarried and younger women as well as those with lower incomes were particularly prone to experience stress during pregnancy.
It’s not clear why women with epilepsy report more stressors. “Looking at the literature, no one has really looked at the exact reason for this, but we postulate it could be a lack of supports and support systems,” said Dr. Chaudhry.
Women were asked about physical, sexual, and emotional abuse. Results showed that substantially more women with epilepsy than those without the disorder reported such abuse during pregnancy – 10.6% versus 4.1%. The adjusted odds ratio for women with epilepsy reporting abuse was 2.78 (95% CI, 2.07-3.74).
“That raises our concern and needs to be looked at in more detail,” said Dr. Chaudhry.
It is unclear whether some women might have had psychogenic non-epileptic seizures (PNES), which are linked to a higher rate of abuse, said Dr. Chaudhry. “But the prevalence of PNES in the general population is quite low, so we don’t think it’s contributing to a large extent to this finding.”
The findings highlight the importance of addressing stress in women with epilepsy during pregnancy, he said. “We need to have good support services and we need to counsel women to optimize good outcomes.”
This applies to all women of childbearing age. “We suspect abuse and stressors are going to be going on throughout that period,” said Dr. Chaudhry. “It’s important to ask about it and have appropriate support staff and social work and people available to help when an issue is identified.”
Stress a common seizure trigger
Commenting on the research, Kimford Meador, MD, professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, noted the study was well conducted and had a large sample size.
The findings are important, as stress is a common trigger for seizures in people with epilepsy and is associated with mood and anxiety, which can affect quality of life, said Dr. Meador.
Results of his analysis from the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study, also presented at this year’s AES meeting, showed that women with epilepsy had more depressive symptoms during the postpartum period and more anxiety symptoms during pregnancy and postpartum in comparison with those without epilepsy.
Dr. Meador’s group also recently conducted a study that was published in JAMA Neurology, showing that in women with epilepsy during the postpartum period, anxiety is associated with lower cognitive ability in their children at age 2 years.
“All these findings highlight the importance of assessing and managing stress, anxiety, and mood in women with epilepsy,” said Dr. Meador. “Interventions could impact seizures and quality of life in pregnant women with epilepsy and long-term outcomes in their children.”
A version of this article first appeared on Medscape.com.
, new research shows.
Study investigator Naveed Chaudhry, MD, a recent epilepsy fellow and assistant professor of neurology, University of Colorado School of Medicine, described the finding as “alarming” and called for more support for this patient population.
Investigators found that women with epilepsy are also more likely to report other stressors, including divorce, illness, lost pay, and partner discord, while expecting.
“As epilepsy physicians, it’s important that we ask the right questions and dive a little bit deeper with these patients, even if it’s uncomfortable and not something we’re used to,” said Dr. Chaudhry.
The findings were presented at the annual meeting of the American Epilepsy Society.
Cause for concern
Women with epilepsy may be under stress for a variety of social and economic reasons. In some women, stress can trigger seizures, and during pregnancy, this can lead to complications such as preterm labor and low birth weight.
For the study, researchers tapped into the Center for Disease Control and Prevention Pregnancy Risk Assessment and Monitoring System (PRAMS). This database includes information from surveys asking women across the U.S. about their pregnancy and postpartum period.
Thirteen states collected data on stresses in women with and without epilepsy. Respondents were asked about 14 economic and other worries in the year prior to their baby’s birth, including the pregnancy period.
The analysis included 64,951 women, 1,140 of whom had epilepsy, who were included in surveys from 2012-2020. There were no significant demographic differences between those with and those without the disorder.
After adjusting for maternal age, race, ethnicity, marital status, education, and socioeconomic status, the study found that women with epilepsy experienced an average of 2.41 of the stressors compared with 1.72 for women without epilepsy.
Women with epilepsy were more likely to have experienced family illness, divorce, homelessness, partner job loss, reduced work or pay, increased arguments, having a partner in jail, drug use, and the death of someone close to them.
The results showed that unmarried and younger women as well as those with lower incomes were particularly prone to experience stress during pregnancy.
It’s not clear why women with epilepsy report more stressors. “Looking at the literature, no one has really looked at the exact reason for this, but we postulate it could be a lack of supports and support systems,” said Dr. Chaudhry.
Women were asked about physical, sexual, and emotional abuse. Results showed that substantially more women with epilepsy than those without the disorder reported such abuse during pregnancy – 10.6% versus 4.1%. The adjusted odds ratio for women with epilepsy reporting abuse was 2.78 (95% CI, 2.07-3.74).
“That raises our concern and needs to be looked at in more detail,” said Dr. Chaudhry.
It is unclear whether some women might have had psychogenic non-epileptic seizures (PNES), which are linked to a higher rate of abuse, said Dr. Chaudhry. “But the prevalence of PNES in the general population is quite low, so we don’t think it’s contributing to a large extent to this finding.”
The findings highlight the importance of addressing stress in women with epilepsy during pregnancy, he said. “We need to have good support services and we need to counsel women to optimize good outcomes.”
This applies to all women of childbearing age. “We suspect abuse and stressors are going to be going on throughout that period,” said Dr. Chaudhry. “It’s important to ask about it and have appropriate support staff and social work and people available to help when an issue is identified.”
Stress a common seizure trigger
Commenting on the research, Kimford Meador, MD, professor, Department of Neurology and Neurological Sciences, Stanford University School of Medicine, noted the study was well conducted and had a large sample size.
The findings are important, as stress is a common trigger for seizures in people with epilepsy and is associated with mood and anxiety, which can affect quality of life, said Dr. Meador.
Results of his analysis from the Maternal Outcomes and Neurodevelopmental Effects of Antiepileptic Drugs (MONEAD) study, also presented at this year’s AES meeting, showed that women with epilepsy had more depressive symptoms during the postpartum period and more anxiety symptoms during pregnancy and postpartum in comparison with those without epilepsy.
Dr. Meador’s group also recently conducted a study that was published in JAMA Neurology, showing that in women with epilepsy during the postpartum period, anxiety is associated with lower cognitive ability in their children at age 2 years.
“All these findings highlight the importance of assessing and managing stress, anxiety, and mood in women with epilepsy,” said Dr. Meador. “Interventions could impact seizures and quality of life in pregnant women with epilepsy and long-term outcomes in their children.”
A version of this article first appeared on Medscape.com.
From AES 2021
Optimal epilepsy care extends well beyond managing seizures
, new research shows. Investigators also found racial and ethnic disparities in comorbidity prevalence.
“Our study identified that people with epilepsy have complex health care needs that extend well beyond their epilepsy,” said co-investigator Wyatt P. Bensken, a PhD candidate in the Department of Population and Quantitative Health Sciences at Case Western Reserve University, Cleveland.
The findings were presented at the annual meeting of the American Epilepsy Society.
A vulnerable population
Researchers identified individuals with epilepsy using Medicaid claims from 2010 to 2014. Mr. Bensken noted that the approximately one-third of patients with epilepsy covered by Medicaid represent “the most vulnerable” population with the disorder because they may not be working and often have other disabilities.
Based on an algorithm that puts diagnostic codes into clinically meaningful categories, the investigators focused on 190 conditions.
“A strength of the study was that we were able to cast such a broad net” to capture conditions, Mr. Bensken said.
Anxiety and mood disorders were originally in separate categories but were grouped together “after recognizing that those who had one pretty much had the other,” he added.
The researchers used a machine learning technique known as association rule mining (ARM) to uncover frequently occurring conditions and combinations of conditions. This same statistical technique is used by companies such as Amazon to determine future purchases based on articles people have bought.
Among 81,963 patients with epilepsy, the most common conditions were anxiety and mood disorders (46.5%). These were followed by hypertension (36.9%), back problems (35.2%), developmental disorders (31.6%), and headache including migraine (29.5%). Urinary tract infections (UTIs) were experienced by 22.8% of the sample.
The rate of anxiety and mood disorders was not unexpected, “but I was surprised to see hypertension so high on the list,” said Mr. Bensken. He noted there is also increasing evidence pointing to a cardiovascular-epilepsy connection.
What should neurologists do?
The study also highlights the relatively high rate of back problems, which are not usually considered a comorbidity in patients with epilepsy, Mr. Bensken said. “Back problems likely greatly impact a patient’s quality of life, and seeing them so high on the list makes me wonder if neurologists or epileptologists or primary care doctors are even asking about back pain and how that might impact the ability to function day to day,” he added.
How do these rates compare with the general population? From other studies, the estimated prevalence for anxiety and mood disorders is 20%-30%, compared with almost 50% of the current sample, said Mr. Bensken.
In addition, the rate of hypertension in the study’s epilepsy population was about 7% higher than the general population, and the rate of UTIs was about 12% higher, he reported.
When examining combinations of conditions, anxiety and mood disorders continued to have an “outsized” prevalence, appearing in nearly every combination, the investigators noted.
Almost a quarter (24.7%) of participants had back problems plus anxiety and a mood disorder, and about 15% had headaches and back problems as well as anxiety and a mood disorder.
“That’s a non-negligible amount of the population that have not just one or two things going on but three and four,” said Mr. Bensken.
These new results underscore how complex these patients can be and the need to integrate medical care among different specialties, he noted.
“We don’t believe it’s the neurologist’s job to also manage the hypertension, but being aware of how prevalent hypertension may be and working with the primary care doctor, or at least checking in with the patient and asking if they’re managing their hypertension, is a real priority,” he said.
Researchers also used the ARM system to identify racial disparities, “which have been largely understudied in the epilepsy context,” said Mr. Bensken.
American Indians and Alaskan Natives had a substantially higher prevalence of developmental disabilities, while Black participants had a higher prevalence of hypertension.
One of the study’s themes was that disparities were not uniform, Mr. Bensken noted. “It wasn’t that in every condition the prevalence was lowest for White individuals and highest for everybody else,” he said.
These results point to the need for a larger study to examine the cultural context of these subgroups and such things as structural racism that might drive disparities, he added.
When researchers examined combinations of comorbidities in individuals in the top quartile of hospitalizations and emergency department visits, they found high users had a much higher disease burden, with 75.8% having anxiety or a mood disorder.
The study highlights that patients with epilepsy on Medicaid are “a high priority population,” said Mr. Bensken.
‘Drift down hypothesis’
Commenting on the findings, Fred A. Lado, MD, PhD, director of epilepsy at Northwell Health Eastern and Central Regions, said the increased incidence of comorbidities in patients of low socioeconomic status was not surprising.
“The interesting data here is that we see an even higher incidence among people with epilepsy,” said Dr. Lado, who was not involved with the research.
The study shows how epilepsy exacerbates the effects of low socioeconomic status, he added.
“One of the determinants of socioeconomic status in this case may well be the fact they have seizures and have a limited ability to work and are often more dependent on state assistance and disability support,” Dr. Lado said.
He also referred to the “drift down hypothesis” of chronic disease. “If you have epilepsy and are born into a middle-class family, chances are you will be on disability and can’t work, so you probably have a lower socioeconomic status than your family did as you grew up.”
Dr. Lado noted how “extremely common” mood disorders are in this population and that certain pain syndromes “tracked with those mood disorders.”
“We know mood disorders are more prevalent in people with epilepsy, and now we see that pain-related problems – headache and back pain – are more prevalent in people with epilepsy,” he said.
The data showing “downstream effects of the mood disorders,” from epilepsy to mood disorders to pain disorders, was “very interesting,” Dr. Lado said.
The study was funded by the Centers for Disease Control and Prevention and the National Institute on Minority Health and Health Disparities of the National Institutes of Health. Mr. Bensken has reported receiving research support for this work from the NIH.
A version of this article first appeared on Medscape.com.
, new research shows. Investigators also found racial and ethnic disparities in comorbidity prevalence.
“Our study identified that people with epilepsy have complex health care needs that extend well beyond their epilepsy,” said co-investigator Wyatt P. Bensken, a PhD candidate in the Department of Population and Quantitative Health Sciences at Case Western Reserve University, Cleveland.
The findings were presented at the annual meeting of the American Epilepsy Society.
A vulnerable population
Researchers identified individuals with epilepsy using Medicaid claims from 2010 to 2014. Mr. Bensken noted that the approximately one-third of patients with epilepsy covered by Medicaid represent “the most vulnerable” population with the disorder because they may not be working and often have other disabilities.
Based on an algorithm that puts diagnostic codes into clinically meaningful categories, the investigators focused on 190 conditions.
“A strength of the study was that we were able to cast such a broad net” to capture conditions, Mr. Bensken said.
Anxiety and mood disorders were originally in separate categories but were grouped together “after recognizing that those who had one pretty much had the other,” he added.
The researchers used a machine learning technique known as association rule mining (ARM) to uncover frequently occurring conditions and combinations of conditions. This same statistical technique is used by companies such as Amazon to determine future purchases based on articles people have bought.
Among 81,963 patients with epilepsy, the most common conditions were anxiety and mood disorders (46.5%). These were followed by hypertension (36.9%), back problems (35.2%), developmental disorders (31.6%), and headache including migraine (29.5%). Urinary tract infections (UTIs) were experienced by 22.8% of the sample.
The rate of anxiety and mood disorders was not unexpected, “but I was surprised to see hypertension so high on the list,” said Mr. Bensken. He noted there is also increasing evidence pointing to a cardiovascular-epilepsy connection.
What should neurologists do?
The study also highlights the relatively high rate of back problems, which are not usually considered a comorbidity in patients with epilepsy, Mr. Bensken said. “Back problems likely greatly impact a patient’s quality of life, and seeing them so high on the list makes me wonder if neurologists or epileptologists or primary care doctors are even asking about back pain and how that might impact the ability to function day to day,” he added.
How do these rates compare with the general population? From other studies, the estimated prevalence for anxiety and mood disorders is 20%-30%, compared with almost 50% of the current sample, said Mr. Bensken.
In addition, the rate of hypertension in the study’s epilepsy population was about 7% higher than the general population, and the rate of UTIs was about 12% higher, he reported.
When examining combinations of conditions, anxiety and mood disorders continued to have an “outsized” prevalence, appearing in nearly every combination, the investigators noted.
Almost a quarter (24.7%) of participants had back problems plus anxiety and a mood disorder, and about 15% had headaches and back problems as well as anxiety and a mood disorder.
“That’s a non-negligible amount of the population that have not just one or two things going on but three and four,” said Mr. Bensken.
These new results underscore how complex these patients can be and the need to integrate medical care among different specialties, he noted.
“We don’t believe it’s the neurologist’s job to also manage the hypertension, but being aware of how prevalent hypertension may be and working with the primary care doctor, or at least checking in with the patient and asking if they’re managing their hypertension, is a real priority,” he said.
Researchers also used the ARM system to identify racial disparities, “which have been largely understudied in the epilepsy context,” said Mr. Bensken.
American Indians and Alaskan Natives had a substantially higher prevalence of developmental disabilities, while Black participants had a higher prevalence of hypertension.
One of the study’s themes was that disparities were not uniform, Mr. Bensken noted. “It wasn’t that in every condition the prevalence was lowest for White individuals and highest for everybody else,” he said.
These results point to the need for a larger study to examine the cultural context of these subgroups and such things as structural racism that might drive disparities, he added.
When researchers examined combinations of comorbidities in individuals in the top quartile of hospitalizations and emergency department visits, they found high users had a much higher disease burden, with 75.8% having anxiety or a mood disorder.
The study highlights that patients with epilepsy on Medicaid are “a high priority population,” said Mr. Bensken.
‘Drift down hypothesis’
Commenting on the findings, Fred A. Lado, MD, PhD, director of epilepsy at Northwell Health Eastern and Central Regions, said the increased incidence of comorbidities in patients of low socioeconomic status was not surprising.
“The interesting data here is that we see an even higher incidence among people with epilepsy,” said Dr. Lado, who was not involved with the research.
The study shows how epilepsy exacerbates the effects of low socioeconomic status, he added.
“One of the determinants of socioeconomic status in this case may well be the fact they have seizures and have a limited ability to work and are often more dependent on state assistance and disability support,” Dr. Lado said.
He also referred to the “drift down hypothesis” of chronic disease. “If you have epilepsy and are born into a middle-class family, chances are you will be on disability and can’t work, so you probably have a lower socioeconomic status than your family did as you grew up.”
Dr. Lado noted how “extremely common” mood disorders are in this population and that certain pain syndromes “tracked with those mood disorders.”
“We know mood disorders are more prevalent in people with epilepsy, and now we see that pain-related problems – headache and back pain – are more prevalent in people with epilepsy,” he said.
The data showing “downstream effects of the mood disorders,” from epilepsy to mood disorders to pain disorders, was “very interesting,” Dr. Lado said.
The study was funded by the Centers for Disease Control and Prevention and the National Institute on Minority Health and Health Disparities of the National Institutes of Health. Mr. Bensken has reported receiving research support for this work from the NIH.
A version of this article first appeared on Medscape.com.
, new research shows. Investigators also found racial and ethnic disparities in comorbidity prevalence.
“Our study identified that people with epilepsy have complex health care needs that extend well beyond their epilepsy,” said co-investigator Wyatt P. Bensken, a PhD candidate in the Department of Population and Quantitative Health Sciences at Case Western Reserve University, Cleveland.
The findings were presented at the annual meeting of the American Epilepsy Society.
A vulnerable population
Researchers identified individuals with epilepsy using Medicaid claims from 2010 to 2014. Mr. Bensken noted that the approximately one-third of patients with epilepsy covered by Medicaid represent “the most vulnerable” population with the disorder because they may not be working and often have other disabilities.
Based on an algorithm that puts diagnostic codes into clinically meaningful categories, the investigators focused on 190 conditions.
“A strength of the study was that we were able to cast such a broad net” to capture conditions, Mr. Bensken said.
Anxiety and mood disorders were originally in separate categories but were grouped together “after recognizing that those who had one pretty much had the other,” he added.
The researchers used a machine learning technique known as association rule mining (ARM) to uncover frequently occurring conditions and combinations of conditions. This same statistical technique is used by companies such as Amazon to determine future purchases based on articles people have bought.
Among 81,963 patients with epilepsy, the most common conditions were anxiety and mood disorders (46.5%). These were followed by hypertension (36.9%), back problems (35.2%), developmental disorders (31.6%), and headache including migraine (29.5%). Urinary tract infections (UTIs) were experienced by 22.8% of the sample.
The rate of anxiety and mood disorders was not unexpected, “but I was surprised to see hypertension so high on the list,” said Mr. Bensken. He noted there is also increasing evidence pointing to a cardiovascular-epilepsy connection.
What should neurologists do?
The study also highlights the relatively high rate of back problems, which are not usually considered a comorbidity in patients with epilepsy, Mr. Bensken said. “Back problems likely greatly impact a patient’s quality of life, and seeing them so high on the list makes me wonder if neurologists or epileptologists or primary care doctors are even asking about back pain and how that might impact the ability to function day to day,” he added.
How do these rates compare with the general population? From other studies, the estimated prevalence for anxiety and mood disorders is 20%-30%, compared with almost 50% of the current sample, said Mr. Bensken.
In addition, the rate of hypertension in the study’s epilepsy population was about 7% higher than the general population, and the rate of UTIs was about 12% higher, he reported.
When examining combinations of conditions, anxiety and mood disorders continued to have an “outsized” prevalence, appearing in nearly every combination, the investigators noted.
Almost a quarter (24.7%) of participants had back problems plus anxiety and a mood disorder, and about 15% had headaches and back problems as well as anxiety and a mood disorder.
“That’s a non-negligible amount of the population that have not just one or two things going on but three and four,” said Mr. Bensken.
These new results underscore how complex these patients can be and the need to integrate medical care among different specialties, he noted.
“We don’t believe it’s the neurologist’s job to also manage the hypertension, but being aware of how prevalent hypertension may be and working with the primary care doctor, or at least checking in with the patient and asking if they’re managing their hypertension, is a real priority,” he said.
Researchers also used the ARM system to identify racial disparities, “which have been largely understudied in the epilepsy context,” said Mr. Bensken.
American Indians and Alaskan Natives had a substantially higher prevalence of developmental disabilities, while Black participants had a higher prevalence of hypertension.
One of the study’s themes was that disparities were not uniform, Mr. Bensken noted. “It wasn’t that in every condition the prevalence was lowest for White individuals and highest for everybody else,” he said.
These results point to the need for a larger study to examine the cultural context of these subgroups and such things as structural racism that might drive disparities, he added.
When researchers examined combinations of comorbidities in individuals in the top quartile of hospitalizations and emergency department visits, they found high users had a much higher disease burden, with 75.8% having anxiety or a mood disorder.
The study highlights that patients with epilepsy on Medicaid are “a high priority population,” said Mr. Bensken.
‘Drift down hypothesis’
Commenting on the findings, Fred A. Lado, MD, PhD, director of epilepsy at Northwell Health Eastern and Central Regions, said the increased incidence of comorbidities in patients of low socioeconomic status was not surprising.
“The interesting data here is that we see an even higher incidence among people with epilepsy,” said Dr. Lado, who was not involved with the research.
The study shows how epilepsy exacerbates the effects of low socioeconomic status, he added.
“One of the determinants of socioeconomic status in this case may well be the fact they have seizures and have a limited ability to work and are often more dependent on state assistance and disability support,” Dr. Lado said.
He also referred to the “drift down hypothesis” of chronic disease. “If you have epilepsy and are born into a middle-class family, chances are you will be on disability and can’t work, so you probably have a lower socioeconomic status than your family did as you grew up.”
Dr. Lado noted how “extremely common” mood disorders are in this population and that certain pain syndromes “tracked with those mood disorders.”
“We know mood disorders are more prevalent in people with epilepsy, and now we see that pain-related problems – headache and back pain – are more prevalent in people with epilepsy,” he said.
The data showing “downstream effects of the mood disorders,” from epilepsy to mood disorders to pain disorders, was “very interesting,” Dr. Lado said.
The study was funded by the Centers for Disease Control and Prevention and the National Institute on Minority Health and Health Disparities of the National Institutes of Health. Mr. Bensken has reported receiving research support for this work from the NIH.
A version of this article first appeared on Medscape.com.
From AES 2021
Advocating for children’s health, one page at a time
Everyone can remember a book from their childhood that helped transform them, reinvent them, or turned the world on its head. Characters such as Harry Potter, Franklin the Turtle, Matilda, the Very Hungry Caterpillar, Corduroy, and Nancy Drew, among others, continue to exist in the cultural zeitgeist because they remind us of our childhood and the experience of discovering something innovative and exciting for the first time.
For many young children, introductions to these timeless characters first come from an adult reading to them. Those interactions, starting with watching mouths form words, to exploring pictures, to eventually reading along, leave a lasting impression. “Adults remember being read to,” says pediatrician Perri Klass, MD. “It’s a very powerful thing.”
Dr. Klass serves as national medical director of Reach Out and Read, a nonprofit organization that champions the positive effects of reading and other language-rich activities with young children.
And what better partners to involve in this mission than pediatricians? Before a child reaches the age of 4, the U.S. Department of Health and Human Services recommends that a child visit the pediatrician at least seven times. The Bright Futures/American Academy of Pediatrics suggests as many as 13 pediatrician visits before the child reaches that same milestone. Regardless of the exact number, almost all children are encountering a pediatrician multiple times during the most crucial years of their brain development.
In 1989, physicians Barry Zuckerman, MD, and Robert Needleman, MD, at Boston City Hospital (now Boston Medical Center) realized that they could reach a large population of children and parents, especially those coming from disadvantaged backgrounds, in the pediatrics ward of offices and hospitals all over the country.
The design of Reach Out and Read, the organization they founded, is to work with pediatricians in how they can best support parents in making reading to their children a part of their daily routine, advocating for the importance of books for children, and making sure that a child leaves the office with a book to take home.
Rather than just dumping books onto nervous or busy parents, the organization trains doctors on how to teach parents to read to their children: how to hold the books, how to make it as active as possible, how to point to the pictures and make them come to life, and how to make sure the child is grasping the language.
“You don’t just prop a baby up and read to them,” Dr. Klass told this news organization. “You have to make it interactive.”
Physician-driven success
Now an international organization, Dr. Klass has watched the nonprofit grow tremendously since it began during her fellowship in Boston over three decades ago. The initiative has over 6,100 sites in all 50 states and is able to get books into the hands of 4.2 million children every single year through government aid, individual contributions, and in-kind donations. On average, the organization is able to give parents 6.4 million books annually. Half of the children served every year by the program come from low-income backgrounds.
Dr. Klass ascribed some of the organization’s longevity and success to “practical realism,” its “mission-driven” approach, and its creation by people in primary care who understood the constraints, the upkeep, and the scaling.
“Our organization isn’t looking to pile 10 more things on to the hands of pediatricians who are already very busy,” she said. “We understand that conversation is important with our care providers. We always hear that watching children happily interacting with literature is one of the most rewarding parts of their job. So, we’re saying to them, ‘I want to help you do what you enjoy most.’”
Both Dr. Klass, who is also a presidential appointee to the Advisory Board of the National Institute For Literacy, and Brian Gallagher, MPA, the CEO of Reach Out and Read, said one of the most rewarding parts of their attachment to the organization is working with dedicated physicians all over the country.
“We hear all the time that physicians say working with these tools [from Reach Out and Read] is the most joyful part of their day,” said Mr. Gallagher. “Children are hope for the future and books help them grow.”
Amy Shriver, MD, an Iowa pediatrician and medical director of the Reach Out and Read Iowa Board, admitted that at first she just thought of the organization as a book drive. As Dr. Shriver got closer to the organization, though, she realized how she could utilize the book as a surveillance tool.
“At 6 months through 2 years, I hand the book to the patient, and I can always tell which children are familiar with books by their responses,” she said. After learning about and implementing Reach Out and Read’s ‘model, observe, coach’ methodology, Dr. Shriver said she was wowed by how much it helped families who weren’t reading to their child understand not only how to read with their children but why it’s so important.”
Dr. Shriver said that her clinic has purchased more diverse books to meet the needs of its patient population and has partnered with local libraries and a science center to promote early brain development. The biggest change is that Dr. Shriver finds herself spending more time observing and talking about parent/child relationships since starting with Reach Out and Read.
Mr. Gallagher attributed the organization’s success to the massive amounts of research that back up the practices of the organization. “Our model isn’t just a nice thing to do,” Mr. Gallagher said. “Our practice has been proven to be effective, and that’s why pediatricians continue to work with us. We’ve heard experts say that when they’re advocating for children’s health, they say ‘vaccines, sleep, and Reach Out and Read.’”
Nineteen independent studies have been done profiling the work of Reach Out and Read since its inception. The research has shown that exposure to the organization results in parents reading more often to their children, higher language scores, as well as an improvement in clinic culture and clinician well-being.
In 2014, the American Academy of Pediatrics quoted the research of Reach Out and Read in its policy statement “Literacy Promotion: An Essential Component of Primary Care Pediatric Practice,” which argued that pediatrics should advocate for literacy from birth. The abstract of the study suggests that practicing pediatricians “advise all parents that reading aloud with young children can enhance parent-child relationships and prepare young minds to learn language and early literacy skills ... provide developmentally appropriate books given at health supervision visits for all high-risk, low-income young children ... partnering with other child advocates to influence national messaging and policies that support and promote these key, early shared-reading experiences.”
Adapting to benefit children and parents
Reach Out and Read is not afraid to change with the times. When it began in 1989, there was no guidance for pediatricians on the importance of reading. Mr. Gallagher said that a common question Reach Out and Read received was, “Why not focus on physical health?” The organization was more interested in the shift in pediatric practice overtime.
“We used to advocate starting off kids with books at 6 months old, but we always listen to the research,” Mr. Gallagher said. Now, the organization as well as the American Academy of Pediatrics advocate for beginning at birth. Other publications such as Green Child Magazine and Psychology Today speak of the importance of reading to babies still in the womb. The Proceedings of the National Academy of Sciences published an article in 2013 that suggests that third-trimester babies can not only pick up on language patterns but also can identify words first heard in the womb.
“We aren’t afraid to adjust our practice if it will be more effective and beneficial for children,” Mr. Gallagher said, “We follow the research and share the work that we are doing. It’s important to stay as up to date as possible.”
Although the focus is largely on the health of children, the impact on parents is crucial as well. Mr. Gallagher described the books at the center of the mission as “a vehicle for bonding” between parents and their children. “The relationship-building we see between families is truly quite magical,” he said.
“Parents will say it’s a respite in their day,” Dr. Klass said of the daily practice of reading aloud. She recalled a memory of talking to a mother with two rowdy young boys, who would sit down and read to them, immediately calming them down.
“When parents sit down to read to their children they don’t have to make anything up. It’s a script, it’s a prompt. You have this story, a picture to show. And kids get preferences,” she said. “When they pick a book that they want you to read, they get to exercise some control. It’s a satisfying routine for parents. It helps open up the world to your child. And when kids come over and hand a book to you for you to read together, it’s them saying, ‘I like the way you look, sound, and interact with me when we do this together.’”
A study from Ambulatory Pediatrics demonstrated that families working with Reach Out and Read were more likely to report reading aloud at bedtime, to read aloud three or more days per week, to mention reading aloud as a favorite parenting activity, and to own 10 or more children’s books. The American Journal of Diseases for Children, in a 1991 article co-authored by Needleman and Zuckerman, noted that the effects of Reach Out and Read were greater for those families who were receiving Aid to Families with Dependent Children. In 2015, the Pew Research Center unveiled a report, “Parenting in America” on raising a child in the modern age, the first generation in American history expected, on average, to make less than their parents.
The report stated that “a broad, demographically-based look at the landscape of American families reveals stark parenting divides linked less to philosophies or values and more to economic circumstances and changing family structure.”
As questions of access and privilege loom over the growing world of education, Reach Out and Read is trying to shorten the gap one book at a time. They are hoping, in time, that their model will be able to reach 90% of children in the United States and foster a relationship with reading and protecting children from toxic stress.
“Every time I look at a newborn, I think about the power of relationships,” said Dr. Shriver, the Iowa-based pediatrician. “I think about how much love passes between infants and their parents, and how shared reading is such a powerful way to show our children we love them. I know from my own experiences how good it feels to snuggle every night and read together. Those moments when the world falls away, and it’s just you, your child, and a book are magical.”
“I want every parent and child to have that experience and create those loving memories. I want all children to feel safe, secure, and loved. I want every child to have the opportunity to use books as a mirror to see themselves and as a window to see the world.”
A version of this article first appeared on Medscape.com.
Everyone can remember a book from their childhood that helped transform them, reinvent them, or turned the world on its head. Characters such as Harry Potter, Franklin the Turtle, Matilda, the Very Hungry Caterpillar, Corduroy, and Nancy Drew, among others, continue to exist in the cultural zeitgeist because they remind us of our childhood and the experience of discovering something innovative and exciting for the first time.
For many young children, introductions to these timeless characters first come from an adult reading to them. Those interactions, starting with watching mouths form words, to exploring pictures, to eventually reading along, leave a lasting impression. “Adults remember being read to,” says pediatrician Perri Klass, MD. “It’s a very powerful thing.”
Dr. Klass serves as national medical director of Reach Out and Read, a nonprofit organization that champions the positive effects of reading and other language-rich activities with young children.
And what better partners to involve in this mission than pediatricians? Before a child reaches the age of 4, the U.S. Department of Health and Human Services recommends that a child visit the pediatrician at least seven times. The Bright Futures/American Academy of Pediatrics suggests as many as 13 pediatrician visits before the child reaches that same milestone. Regardless of the exact number, almost all children are encountering a pediatrician multiple times during the most crucial years of their brain development.
In 1989, physicians Barry Zuckerman, MD, and Robert Needleman, MD, at Boston City Hospital (now Boston Medical Center) realized that they could reach a large population of children and parents, especially those coming from disadvantaged backgrounds, in the pediatrics ward of offices and hospitals all over the country.
The design of Reach Out and Read, the organization they founded, is to work with pediatricians in how they can best support parents in making reading to their children a part of their daily routine, advocating for the importance of books for children, and making sure that a child leaves the office with a book to take home.
Rather than just dumping books onto nervous or busy parents, the organization trains doctors on how to teach parents to read to their children: how to hold the books, how to make it as active as possible, how to point to the pictures and make them come to life, and how to make sure the child is grasping the language.
“You don’t just prop a baby up and read to them,” Dr. Klass told this news organization. “You have to make it interactive.”
Physician-driven success
Now an international organization, Dr. Klass has watched the nonprofit grow tremendously since it began during her fellowship in Boston over three decades ago. The initiative has over 6,100 sites in all 50 states and is able to get books into the hands of 4.2 million children every single year through government aid, individual contributions, and in-kind donations. On average, the organization is able to give parents 6.4 million books annually. Half of the children served every year by the program come from low-income backgrounds.
Dr. Klass ascribed some of the organization’s longevity and success to “practical realism,” its “mission-driven” approach, and its creation by people in primary care who understood the constraints, the upkeep, and the scaling.
“Our organization isn’t looking to pile 10 more things on to the hands of pediatricians who are already very busy,” she said. “We understand that conversation is important with our care providers. We always hear that watching children happily interacting with literature is one of the most rewarding parts of their job. So, we’re saying to them, ‘I want to help you do what you enjoy most.’”
Both Dr. Klass, who is also a presidential appointee to the Advisory Board of the National Institute For Literacy, and Brian Gallagher, MPA, the CEO of Reach Out and Read, said one of the most rewarding parts of their attachment to the organization is working with dedicated physicians all over the country.
“We hear all the time that physicians say working with these tools [from Reach Out and Read] is the most joyful part of their day,” said Mr. Gallagher. “Children are hope for the future and books help them grow.”
Amy Shriver, MD, an Iowa pediatrician and medical director of the Reach Out and Read Iowa Board, admitted that at first she just thought of the organization as a book drive. As Dr. Shriver got closer to the organization, though, she realized how she could utilize the book as a surveillance tool.
“At 6 months through 2 years, I hand the book to the patient, and I can always tell which children are familiar with books by their responses,” she said. After learning about and implementing Reach Out and Read’s ‘model, observe, coach’ methodology, Dr. Shriver said she was wowed by how much it helped families who weren’t reading to their child understand not only how to read with their children but why it’s so important.”
Dr. Shriver said that her clinic has purchased more diverse books to meet the needs of its patient population and has partnered with local libraries and a science center to promote early brain development. The biggest change is that Dr. Shriver finds herself spending more time observing and talking about parent/child relationships since starting with Reach Out and Read.
Mr. Gallagher attributed the organization’s success to the massive amounts of research that back up the practices of the organization. “Our model isn’t just a nice thing to do,” Mr. Gallagher said. “Our practice has been proven to be effective, and that’s why pediatricians continue to work with us. We’ve heard experts say that when they’re advocating for children’s health, they say ‘vaccines, sleep, and Reach Out and Read.’”
Nineteen independent studies have been done profiling the work of Reach Out and Read since its inception. The research has shown that exposure to the organization results in parents reading more often to their children, higher language scores, as well as an improvement in clinic culture and clinician well-being.
In 2014, the American Academy of Pediatrics quoted the research of Reach Out and Read in its policy statement “Literacy Promotion: An Essential Component of Primary Care Pediatric Practice,” which argued that pediatrics should advocate for literacy from birth. The abstract of the study suggests that practicing pediatricians “advise all parents that reading aloud with young children can enhance parent-child relationships and prepare young minds to learn language and early literacy skills ... provide developmentally appropriate books given at health supervision visits for all high-risk, low-income young children ... partnering with other child advocates to influence national messaging and policies that support and promote these key, early shared-reading experiences.”
Adapting to benefit children and parents
Reach Out and Read is not afraid to change with the times. When it began in 1989, there was no guidance for pediatricians on the importance of reading. Mr. Gallagher said that a common question Reach Out and Read received was, “Why not focus on physical health?” The organization was more interested in the shift in pediatric practice overtime.
“We used to advocate starting off kids with books at 6 months old, but we always listen to the research,” Mr. Gallagher said. Now, the organization as well as the American Academy of Pediatrics advocate for beginning at birth. Other publications such as Green Child Magazine and Psychology Today speak of the importance of reading to babies still in the womb. The Proceedings of the National Academy of Sciences published an article in 2013 that suggests that third-trimester babies can not only pick up on language patterns but also can identify words first heard in the womb.
“We aren’t afraid to adjust our practice if it will be more effective and beneficial for children,” Mr. Gallagher said, “We follow the research and share the work that we are doing. It’s important to stay as up to date as possible.”
Although the focus is largely on the health of children, the impact on parents is crucial as well. Mr. Gallagher described the books at the center of the mission as “a vehicle for bonding” between parents and their children. “The relationship-building we see between families is truly quite magical,” he said.
“Parents will say it’s a respite in their day,” Dr. Klass said of the daily practice of reading aloud. She recalled a memory of talking to a mother with two rowdy young boys, who would sit down and read to them, immediately calming them down.
“When parents sit down to read to their children they don’t have to make anything up. It’s a script, it’s a prompt. You have this story, a picture to show. And kids get preferences,” she said. “When they pick a book that they want you to read, they get to exercise some control. It’s a satisfying routine for parents. It helps open up the world to your child. And when kids come over and hand a book to you for you to read together, it’s them saying, ‘I like the way you look, sound, and interact with me when we do this together.’”
A study from Ambulatory Pediatrics demonstrated that families working with Reach Out and Read were more likely to report reading aloud at bedtime, to read aloud three or more days per week, to mention reading aloud as a favorite parenting activity, and to own 10 or more children’s books. The American Journal of Diseases for Children, in a 1991 article co-authored by Needleman and Zuckerman, noted that the effects of Reach Out and Read were greater for those families who were receiving Aid to Families with Dependent Children. In 2015, the Pew Research Center unveiled a report, “Parenting in America” on raising a child in the modern age, the first generation in American history expected, on average, to make less than their parents.
The report stated that “a broad, demographically-based look at the landscape of American families reveals stark parenting divides linked less to philosophies or values and more to economic circumstances and changing family structure.”
As questions of access and privilege loom over the growing world of education, Reach Out and Read is trying to shorten the gap one book at a time. They are hoping, in time, that their model will be able to reach 90% of children in the United States and foster a relationship with reading and protecting children from toxic stress.
“Every time I look at a newborn, I think about the power of relationships,” said Dr. Shriver, the Iowa-based pediatrician. “I think about how much love passes between infants and their parents, and how shared reading is such a powerful way to show our children we love them. I know from my own experiences how good it feels to snuggle every night and read together. Those moments when the world falls away, and it’s just you, your child, and a book are magical.”
“I want every parent and child to have that experience and create those loving memories. I want all children to feel safe, secure, and loved. I want every child to have the opportunity to use books as a mirror to see themselves and as a window to see the world.”
A version of this article first appeared on Medscape.com.
Everyone can remember a book from their childhood that helped transform them, reinvent them, or turned the world on its head. Characters such as Harry Potter, Franklin the Turtle, Matilda, the Very Hungry Caterpillar, Corduroy, and Nancy Drew, among others, continue to exist in the cultural zeitgeist because they remind us of our childhood and the experience of discovering something innovative and exciting for the first time.
For many young children, introductions to these timeless characters first come from an adult reading to them. Those interactions, starting with watching mouths form words, to exploring pictures, to eventually reading along, leave a lasting impression. “Adults remember being read to,” says pediatrician Perri Klass, MD. “It’s a very powerful thing.”
Dr. Klass serves as national medical director of Reach Out and Read, a nonprofit organization that champions the positive effects of reading and other language-rich activities with young children.
And what better partners to involve in this mission than pediatricians? Before a child reaches the age of 4, the U.S. Department of Health and Human Services recommends that a child visit the pediatrician at least seven times. The Bright Futures/American Academy of Pediatrics suggests as many as 13 pediatrician visits before the child reaches that same milestone. Regardless of the exact number, almost all children are encountering a pediatrician multiple times during the most crucial years of their brain development.
In 1989, physicians Barry Zuckerman, MD, and Robert Needleman, MD, at Boston City Hospital (now Boston Medical Center) realized that they could reach a large population of children and parents, especially those coming from disadvantaged backgrounds, in the pediatrics ward of offices and hospitals all over the country.
The design of Reach Out and Read, the organization they founded, is to work with pediatricians in how they can best support parents in making reading to their children a part of their daily routine, advocating for the importance of books for children, and making sure that a child leaves the office with a book to take home.
Rather than just dumping books onto nervous or busy parents, the organization trains doctors on how to teach parents to read to their children: how to hold the books, how to make it as active as possible, how to point to the pictures and make them come to life, and how to make sure the child is grasping the language.
“You don’t just prop a baby up and read to them,” Dr. Klass told this news organization. “You have to make it interactive.”
Physician-driven success
Now an international organization, Dr. Klass has watched the nonprofit grow tremendously since it began during her fellowship in Boston over three decades ago. The initiative has over 6,100 sites in all 50 states and is able to get books into the hands of 4.2 million children every single year through government aid, individual contributions, and in-kind donations. On average, the organization is able to give parents 6.4 million books annually. Half of the children served every year by the program come from low-income backgrounds.
Dr. Klass ascribed some of the organization’s longevity and success to “practical realism,” its “mission-driven” approach, and its creation by people in primary care who understood the constraints, the upkeep, and the scaling.
“Our organization isn’t looking to pile 10 more things on to the hands of pediatricians who are already very busy,” she said. “We understand that conversation is important with our care providers. We always hear that watching children happily interacting with literature is one of the most rewarding parts of their job. So, we’re saying to them, ‘I want to help you do what you enjoy most.’”
Both Dr. Klass, who is also a presidential appointee to the Advisory Board of the National Institute For Literacy, and Brian Gallagher, MPA, the CEO of Reach Out and Read, said one of the most rewarding parts of their attachment to the organization is working with dedicated physicians all over the country.
“We hear all the time that physicians say working with these tools [from Reach Out and Read] is the most joyful part of their day,” said Mr. Gallagher. “Children are hope for the future and books help them grow.”
Amy Shriver, MD, an Iowa pediatrician and medical director of the Reach Out and Read Iowa Board, admitted that at first she just thought of the organization as a book drive. As Dr. Shriver got closer to the organization, though, she realized how she could utilize the book as a surveillance tool.
“At 6 months through 2 years, I hand the book to the patient, and I can always tell which children are familiar with books by their responses,” she said. After learning about and implementing Reach Out and Read’s ‘model, observe, coach’ methodology, Dr. Shriver said she was wowed by how much it helped families who weren’t reading to their child understand not only how to read with their children but why it’s so important.”
Dr. Shriver said that her clinic has purchased more diverse books to meet the needs of its patient population and has partnered with local libraries and a science center to promote early brain development. The biggest change is that Dr. Shriver finds herself spending more time observing and talking about parent/child relationships since starting with Reach Out and Read.
Mr. Gallagher attributed the organization’s success to the massive amounts of research that back up the practices of the organization. “Our model isn’t just a nice thing to do,” Mr. Gallagher said. “Our practice has been proven to be effective, and that’s why pediatricians continue to work with us. We’ve heard experts say that when they’re advocating for children’s health, they say ‘vaccines, sleep, and Reach Out and Read.’”
Nineteen independent studies have been done profiling the work of Reach Out and Read since its inception. The research has shown that exposure to the organization results in parents reading more often to their children, higher language scores, as well as an improvement in clinic culture and clinician well-being.
In 2014, the American Academy of Pediatrics quoted the research of Reach Out and Read in its policy statement “Literacy Promotion: An Essential Component of Primary Care Pediatric Practice,” which argued that pediatrics should advocate for literacy from birth. The abstract of the study suggests that practicing pediatricians “advise all parents that reading aloud with young children can enhance parent-child relationships and prepare young minds to learn language and early literacy skills ... provide developmentally appropriate books given at health supervision visits for all high-risk, low-income young children ... partnering with other child advocates to influence national messaging and policies that support and promote these key, early shared-reading experiences.”
Adapting to benefit children and parents
Reach Out and Read is not afraid to change with the times. When it began in 1989, there was no guidance for pediatricians on the importance of reading. Mr. Gallagher said that a common question Reach Out and Read received was, “Why not focus on physical health?” The organization was more interested in the shift in pediatric practice overtime.
“We used to advocate starting off kids with books at 6 months old, but we always listen to the research,” Mr. Gallagher said. Now, the organization as well as the American Academy of Pediatrics advocate for beginning at birth. Other publications such as Green Child Magazine and Psychology Today speak of the importance of reading to babies still in the womb. The Proceedings of the National Academy of Sciences published an article in 2013 that suggests that third-trimester babies can not only pick up on language patterns but also can identify words first heard in the womb.
“We aren’t afraid to adjust our practice if it will be more effective and beneficial for children,” Mr. Gallagher said, “We follow the research and share the work that we are doing. It’s important to stay as up to date as possible.”
Although the focus is largely on the health of children, the impact on parents is crucial as well. Mr. Gallagher described the books at the center of the mission as “a vehicle for bonding” between parents and their children. “The relationship-building we see between families is truly quite magical,” he said.
“Parents will say it’s a respite in their day,” Dr. Klass said of the daily practice of reading aloud. She recalled a memory of talking to a mother with two rowdy young boys, who would sit down and read to them, immediately calming them down.
“When parents sit down to read to their children they don’t have to make anything up. It’s a script, it’s a prompt. You have this story, a picture to show. And kids get preferences,” she said. “When they pick a book that they want you to read, they get to exercise some control. It’s a satisfying routine for parents. It helps open up the world to your child. And when kids come over and hand a book to you for you to read together, it’s them saying, ‘I like the way you look, sound, and interact with me when we do this together.’”
A study from Ambulatory Pediatrics demonstrated that families working with Reach Out and Read were more likely to report reading aloud at bedtime, to read aloud three or more days per week, to mention reading aloud as a favorite parenting activity, and to own 10 or more children’s books. The American Journal of Diseases for Children, in a 1991 article co-authored by Needleman and Zuckerman, noted that the effects of Reach Out and Read were greater for those families who were receiving Aid to Families with Dependent Children. In 2015, the Pew Research Center unveiled a report, “Parenting in America” on raising a child in the modern age, the first generation in American history expected, on average, to make less than their parents.
The report stated that “a broad, demographically-based look at the landscape of American families reveals stark parenting divides linked less to philosophies or values and more to economic circumstances and changing family structure.”
As questions of access and privilege loom over the growing world of education, Reach Out and Read is trying to shorten the gap one book at a time. They are hoping, in time, that their model will be able to reach 90% of children in the United States and foster a relationship with reading and protecting children from toxic stress.
“Every time I look at a newborn, I think about the power of relationships,” said Dr. Shriver, the Iowa-based pediatrician. “I think about how much love passes between infants and their parents, and how shared reading is such a powerful way to show our children we love them. I know from my own experiences how good it feels to snuggle every night and read together. Those moments when the world falls away, and it’s just you, your child, and a book are magical.”
“I want every parent and child to have that experience and create those loving memories. I want all children to feel safe, secure, and loved. I want every child to have the opportunity to use books as a mirror to see themselves and as a window to see the world.”
A version of this article first appeared on Medscape.com.

