User login
Health care–associated infections spiked in 2020 in U.S. hospitals
Several health care-associated infections in U.S. hospitals spiked in 2020 compared to the previous year, according to a Centers for Disease Control and Prevention analysis published Sept. 2 in Infection Control and Hospital Epidemiology. Soaring hospitalization rates, sicker patients who required more frequent and intense care, and staffing and supply shortages caused by the COVID-19 pandemic are thought to have contributed to this increase.
This is the first increase in health care–associated infections since 2015.
These findings “are a reflection of the enormous stress that COVID has placed on our health care system,” Arjun Srinivasan, MD (Capt, USPHS), the associate director of the CDC’s Health care-Associated Infection Prevention Programs, Atlanta, told this news organization. He was not an author of the article, but he supervised the research. “We don’t want anyone to read this report and think that it represents a failure of the individual provider or a failure of health care providers in this country in their care of COVID patients,” he said. He noted that health care professionals have provided “tremendously good care to patients under extremely difficult circumstances.”
“People don’t fail – systems fail – and that’s what happened here,” he said. “Our systems that we need to have in place to prevent health care–associated infection simply were not as strong as they needed to be to survive this challenge.”
In the study, researchers used data reported to the National Healthcare Safety Network, the CDC’s tracking system for health care–associated infections. The team compared national standard infection ratios – calculated by dividing the number of reported infections by the number of predicted infections – between 2019 and 2020 for six routinely tracked events:
- Central line–associated bloodstream infections.
- Catheter-associated urinary tract infections (CAUTIs).
- Ventilator-associated events (VAEs).
- Infections associated with colon surgery and abdominal hysterectomy.
- Clostridioides difficile infections.
- Methicillin-resistant Staphylococcus aureus (MRSA) infections.
Infections were estimated using regression models created with baseline data from 2015.
“The new report highlights the need for health care facilities to strengthen their infection prevention programs and support them with adequate resources so that they can handle emerging threats to public health, while at the same time ensuring that gains made in combating HAIs [health care–associated infections] are not lost,” said the Association for Professionals in Infection Control and Epidemiology in a statement.
The analysis revealed significant national increases in central line–associated bloodstream infections, CAUTIs, VAEs, and MRSA infections in 2020 compared to 2019. Among all infection types, the greatest increase was in central-line infections, which were 46% to 47% higher in the third quarter and fourth quarter (Q4) of 2020 relative to the same periods the previous year. VAEs rose by 45%, MRSA infections increased by 34%, and CAUTIs increased by 19% in Q4 of 2020 compared to 2019.
The influx of sicker patients in hospitals throughout 2020 led to more frequent and longer use of medical devices such as catheters and ventilators. The use of these devices increases risk for infection, David P. Calfee, MD, chief medical epidemiologist at the New York–Presbyterian/Weill Cornell Medical Center, said in an interview. He is an editor of Infection Control and Hospital Epidemiology and was not involved with the study. Shortages in personal protective equipment and crowded intensive care units could also have affected how care was delivered, he said. These factors could have led to “reductions in the ability to provide some of the types of care that are needed to optimally reduce the risk of infection.”
There was either no change or decreases in infections associated with colon surgery or abdominal hysterectomy, likely because there were fewer elective surgeries performed, said Dr. Srinivasan. C. difficile–associated infections also decreased throughout 2020 compared to the previous year. Common practices to prevent the spread of COVID-19 in hospitals, such as environmental cleaning, use of personal protective equipment, and patient isolation, likely helped to curb the spread of C. difficile. Although these mitigating procedures do help protect against MRSA infection, many other factors, notably, the use of medical devices such as ventilators and catheters, can increase the risk for MRSA infection, Dr. Srinivasan added.
Although more research is needed to identify the reasons for these spikes in infection, the findings help quantify the scope of these increases across the United States, Dr. Calfee said. The data allow hospitals and health care professionals to “look back at what we did and then think forward in terms of what we can do different in the future,” he added, “so that these stresses to the system have less of an impact on how we are able to provide care.”
Dr. Srinivasan and Dr. Calfee report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Several health care-associated infections in U.S. hospitals spiked in 2020 compared to the previous year, according to a Centers for Disease Control and Prevention analysis published Sept. 2 in Infection Control and Hospital Epidemiology. Soaring hospitalization rates, sicker patients who required more frequent and intense care, and staffing and supply shortages caused by the COVID-19 pandemic are thought to have contributed to this increase.
This is the first increase in health care–associated infections since 2015.
These findings “are a reflection of the enormous stress that COVID has placed on our health care system,” Arjun Srinivasan, MD (Capt, USPHS), the associate director of the CDC’s Health care-Associated Infection Prevention Programs, Atlanta, told this news organization. He was not an author of the article, but he supervised the research. “We don’t want anyone to read this report and think that it represents a failure of the individual provider or a failure of health care providers in this country in their care of COVID patients,” he said. He noted that health care professionals have provided “tremendously good care to patients under extremely difficult circumstances.”
“People don’t fail – systems fail – and that’s what happened here,” he said. “Our systems that we need to have in place to prevent health care–associated infection simply were not as strong as they needed to be to survive this challenge.”
In the study, researchers used data reported to the National Healthcare Safety Network, the CDC’s tracking system for health care–associated infections. The team compared national standard infection ratios – calculated by dividing the number of reported infections by the number of predicted infections – between 2019 and 2020 for six routinely tracked events:
- Central line–associated bloodstream infections.
- Catheter-associated urinary tract infections (CAUTIs).
- Ventilator-associated events (VAEs).
- Infections associated with colon surgery and abdominal hysterectomy.
- Clostridioides difficile infections.
- Methicillin-resistant Staphylococcus aureus (MRSA) infections.
Infections were estimated using regression models created with baseline data from 2015.
“The new report highlights the need for health care facilities to strengthen their infection prevention programs and support them with adequate resources so that they can handle emerging threats to public health, while at the same time ensuring that gains made in combating HAIs [health care–associated infections] are not lost,” said the Association for Professionals in Infection Control and Epidemiology in a statement.
The analysis revealed significant national increases in central line–associated bloodstream infections, CAUTIs, VAEs, and MRSA infections in 2020 compared to 2019. Among all infection types, the greatest increase was in central-line infections, which were 46% to 47% higher in the third quarter and fourth quarter (Q4) of 2020 relative to the same periods the previous year. VAEs rose by 45%, MRSA infections increased by 34%, and CAUTIs increased by 19% in Q4 of 2020 compared to 2019.
The influx of sicker patients in hospitals throughout 2020 led to more frequent and longer use of medical devices such as catheters and ventilators. The use of these devices increases risk for infection, David P. Calfee, MD, chief medical epidemiologist at the New York–Presbyterian/Weill Cornell Medical Center, said in an interview. He is an editor of Infection Control and Hospital Epidemiology and was not involved with the study. Shortages in personal protective equipment and crowded intensive care units could also have affected how care was delivered, he said. These factors could have led to “reductions in the ability to provide some of the types of care that are needed to optimally reduce the risk of infection.”
There was either no change or decreases in infections associated with colon surgery or abdominal hysterectomy, likely because there were fewer elective surgeries performed, said Dr. Srinivasan. C. difficile–associated infections also decreased throughout 2020 compared to the previous year. Common practices to prevent the spread of COVID-19 in hospitals, such as environmental cleaning, use of personal protective equipment, and patient isolation, likely helped to curb the spread of C. difficile. Although these mitigating procedures do help protect against MRSA infection, many other factors, notably, the use of medical devices such as ventilators and catheters, can increase the risk for MRSA infection, Dr. Srinivasan added.
Although more research is needed to identify the reasons for these spikes in infection, the findings help quantify the scope of these increases across the United States, Dr. Calfee said. The data allow hospitals and health care professionals to “look back at what we did and then think forward in terms of what we can do different in the future,” he added, “so that these stresses to the system have less of an impact on how we are able to provide care.”
Dr. Srinivasan and Dr. Calfee report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Several health care-associated infections in U.S. hospitals spiked in 2020 compared to the previous year, according to a Centers for Disease Control and Prevention analysis published Sept. 2 in Infection Control and Hospital Epidemiology. Soaring hospitalization rates, sicker patients who required more frequent and intense care, and staffing and supply shortages caused by the COVID-19 pandemic are thought to have contributed to this increase.
This is the first increase in health care–associated infections since 2015.
These findings “are a reflection of the enormous stress that COVID has placed on our health care system,” Arjun Srinivasan, MD (Capt, USPHS), the associate director of the CDC’s Health care-Associated Infection Prevention Programs, Atlanta, told this news organization. He was not an author of the article, but he supervised the research. “We don’t want anyone to read this report and think that it represents a failure of the individual provider or a failure of health care providers in this country in their care of COVID patients,” he said. He noted that health care professionals have provided “tremendously good care to patients under extremely difficult circumstances.”
“People don’t fail – systems fail – and that’s what happened here,” he said. “Our systems that we need to have in place to prevent health care–associated infection simply were not as strong as they needed to be to survive this challenge.”
In the study, researchers used data reported to the National Healthcare Safety Network, the CDC’s tracking system for health care–associated infections. The team compared national standard infection ratios – calculated by dividing the number of reported infections by the number of predicted infections – between 2019 and 2020 for six routinely tracked events:
- Central line–associated bloodstream infections.
- Catheter-associated urinary tract infections (CAUTIs).
- Ventilator-associated events (VAEs).
- Infections associated with colon surgery and abdominal hysterectomy.
- Clostridioides difficile infections.
- Methicillin-resistant Staphylococcus aureus (MRSA) infections.
Infections were estimated using regression models created with baseline data from 2015.
“The new report highlights the need for health care facilities to strengthen their infection prevention programs and support them with adequate resources so that they can handle emerging threats to public health, while at the same time ensuring that gains made in combating HAIs [health care–associated infections] are not lost,” said the Association for Professionals in Infection Control and Epidemiology in a statement.
The analysis revealed significant national increases in central line–associated bloodstream infections, CAUTIs, VAEs, and MRSA infections in 2020 compared to 2019. Among all infection types, the greatest increase was in central-line infections, which were 46% to 47% higher in the third quarter and fourth quarter (Q4) of 2020 relative to the same periods the previous year. VAEs rose by 45%, MRSA infections increased by 34%, and CAUTIs increased by 19% in Q4 of 2020 compared to 2019.
The influx of sicker patients in hospitals throughout 2020 led to more frequent and longer use of medical devices such as catheters and ventilators. The use of these devices increases risk for infection, David P. Calfee, MD, chief medical epidemiologist at the New York–Presbyterian/Weill Cornell Medical Center, said in an interview. He is an editor of Infection Control and Hospital Epidemiology and was not involved with the study. Shortages in personal protective equipment and crowded intensive care units could also have affected how care was delivered, he said. These factors could have led to “reductions in the ability to provide some of the types of care that are needed to optimally reduce the risk of infection.”
There was either no change or decreases in infections associated with colon surgery or abdominal hysterectomy, likely because there were fewer elective surgeries performed, said Dr. Srinivasan. C. difficile–associated infections also decreased throughout 2020 compared to the previous year. Common practices to prevent the spread of COVID-19 in hospitals, such as environmental cleaning, use of personal protective equipment, and patient isolation, likely helped to curb the spread of C. difficile. Although these mitigating procedures do help protect against MRSA infection, many other factors, notably, the use of medical devices such as ventilators and catheters, can increase the risk for MRSA infection, Dr. Srinivasan added.
Although more research is needed to identify the reasons for these spikes in infection, the findings help quantify the scope of these increases across the United States, Dr. Calfee said. The data allow hospitals and health care professionals to “look back at what we did and then think forward in terms of what we can do different in the future,” he added, “so that these stresses to the system have less of an impact on how we are able to provide care.”
Dr. Srinivasan and Dr. Calfee report no relevant financial relationships.
A version of this article first appeared on Medscape.com.
NIH on HIV vaccine failure: ‘Get your HIV-negative, at-risk patients on PrEP tomorrow’
Last year, Katherine Gill, MBChB, an HIV prevention researcher in Cape Town, South Africa, realized how jaded she’d become to vaccine research when the Pfizer COVID-19 vaccine came back as 95% effective. In her career conducting HIV clinical trials, she had never seen anything like it. Even HIV prevention methods she had studied that had worked, such as the dapivirine ring, had an overall efficacy of 30%.
The COVID-19 success story started to soften her views toward another vaccine trial she was helping to conduct, a trial in HIV that used the same platform as Johnson & Johnson’s successful COVID-19 vaccine.
“When the COVID vaccine was cracked so quickly and seemingly quite easily, I did start to think, ‘Well, maybe … maybe this will work for HIV,’ “ she said in an interview.
That turned out to be false hope. The National Institutes of Health (NIH) announced that the trial Dr. Gill was helping to conduct, HVTN 705, was stopping early because it hadn’t generated enough of an immune response in participants to justify continuing. It was the second HIV vaccine to fail in the last year. It’s also the latest in what has been a litany of disappointments in the attempt to boost the human immune system to fight HIV without the need for ongoing HIV treatment.
In HVTN 705, known as the Imbokodo study (imbokodo is a Zulu word that’s part of a saying about women being strong as rocks), researchers used the platform made up of a common cold virus, adenovirus 26, to deliver a computer-generated mosaic of HIV antigens to participants’ immune systems. That mosaic of antigens is meant to goose the immune system into recognizing HIV if it were exposed to it.
When HIV enters the body, it infiltrates immune cells and replicates within them. To the rest of the immune system, those cells still register as just typical T-cells. The rest of the immune system can’t see that the virus is spreading through the very cells meant to protect the body from illness. That, plus the armor of sugary glycoproteins encasing the virus, has made HIV nearly impervious to vaccination.
Then, those so-called “prime” shots were followed by a second shot that targets glycoprotein 140, on the most common HIV subtype (or clade) in Africa, clade C. In the Imbokodo trial, a total of 2,637 women from five sub-Saharan African countries received shots at baseline, 3 months, 6 months, and 1 year. Then researchers followed the women from month 7 to 2 years after their third dose, testing their blood to see if their immune systems had generated the immune response the vaccine was meant to induce – and whether such immune response was associated with lower rates of HIV.
When researchers looked at the first 2 years of data, they learned that the vaccine was safe. And they found a total of 114 new cases of HIV – 51 among women who received the vaccine and 63 among those who received a placebo. That’s a 25.2% efficacy rate – but it wasn’t statistically significant.
The results are frustrating, said Carl Dieffenbach, PhD, director of the AIDS division at the National Institute of Allergy and Infectious Diseases (NIAID). NIAID is one of the funders of the study.
“This [trial] is a little more confounding, in that there is this low level of statistically insignificant difference between vaccine and placebo that starts somewhere around month 9 and then just kind of indolently is maintained over the next 15 months,” he said in an interview. “That’s kind of frustrating. Does it mean there’s a signal or is this just chance? Because that’s what statistics tell us, not to believe your last data point.”
What this means for the future direction of vaccine research is unclear. A sister study to Imbokodo, called Mosaico, recently finished enrolling participants. Mosaico uses the same adenovirus 26 platform, but it’s loaded with different antigens and targets a different glycoprotein for a different HIV subtype. If that trial shows success, it could mean that the platform is right, but the targets in the Imbokodo vaccine were wrong.
Dr. Dieffenbach said that before NIAID decides what to do with Mosaico they’ve asked researchers to analyze the data on the people who did respond, to see if those people have some specific variant of HIV or some other biomarker that could be used to form the next iteration of an HIV vaccine candidate. Only after that will they make a decision about Mosaico.
But he added that it does make him wonder if vaccine approaches that rely on nonneutralizing antibodies like this one have a ceiling of effectiveness that’s just too low to alter the course of the epidemic.
“I think we’ve discovered that there’s not a floor to [these nonneutralizing approaches], but there probably is a ceiling,” he said. “I don’t know if we’re going to get better than” a 25%-29% efficacy rate with those approaches.
The Imbokodo findings reminded Mitchell Warren, executive director of the global HIV prevention nonprofit, AVAC, of the data released in January from the Antibody Mediated Prevention (AMP) trial. That trial pitted the broadly neutralizing antibody (bNAb) VCR01 against HIV – and mostly, it lost.
VCR01 worked only on HIV variants that 30% of participants had. But in those 30%, it was 75% effective at preventing HIV. Now you have Imbokodo, with its potential 25% activity against HIV, something that may have been a fluke. This, to Mr. Warren, requires a rethinking of the whole HIV vaccine enterprise while “doubling, tripling, quadrupling down” on the HIV prevention methods we know work, such as preexposure prophylaxis (PrEP).
Dr. Dieffenbach agreed. To clinicians, Dr. Dieffenbach said the message of this HIV vaccine trial is flush with urgency: “Get your HIV-negative, at-risk people on PrEP tomorrow.”
There are now two pills approved for HIV prevention, both of which have been found to be up to 99% effective when taken consistently. A third option, injectable cabotegravir (Vocabria), has been submitted to the Food and Drug Administration for approval. The federal Ready, Set, PrEP program makes the pill available for free for those who qualify, and recently the Biden administration reaffirmed that, under the Affordable Care Act, insurance companies should cover all costs associated with PrEP, including lab work and exam visits.
But for the 157 women who participated in the trial at Dr. Gill’s site in Masiphumelele, on the southwestern tip of South Africa, the trial was personal, said Jason Naidoo, community liaison officer at the Desmond Tutu HIV Foundation, which conducted a portion of the study. These were women whose parents, siblings, or children were living with HIV or had died from AIDS-defining illnesses, he said. Their lives were chaotic, traveling at a moment’s notice to hometowns on the Eastern Cape, an 11-hour car ride away – longer by bus – for traditional prayers, funerals, and other important events.
Mr. Naidoo remembers arranging buses for the women to return for scheduled clinic visits, leaving the Eastern Cape in the afternoon and arriving in Masiphumelele in the early morning hours, just to keep the clinic appointment. Then, they’d turn around and return east.
They did this for 3 years, he said.
“The fact that these participants have stuck to this and been dedicated amidst all of the chaos talks about their commitment to actually having a vaccine for HIV,” he said. “They know their own risk profile as young Black women in South Africa, and they understand the need for an intervention for the future generations.
“So you can understand the emotion and the sense of sadness, the disappointment – the incredible [dis]belief that this [the failure of the vaccine] could have happened, because the expectations are so, so high.”
For Dr. Gill, who is lead investigator for Imbokodo in Masiphumelele, the weariness toward vaccines is back. Another trial is underway for an HIV vaccine with a platform that was successful in COVID-19 – using messenger RNA (mRNA), like the Pfizer and Moderna COVID-19 vaccines did.
“I think we need to be careful,” she said, “thinking that the mRNA vaccines are going to crack it.”
Dr. Dieffenbach, Dr. Gill, and Mr. Naidoo have disclosed no relevant financial relationships. The study was funded by Janssen, a Johnson & Johnson company, with NIAID and the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape.com.
Last year, Katherine Gill, MBChB, an HIV prevention researcher in Cape Town, South Africa, realized how jaded she’d become to vaccine research when the Pfizer COVID-19 vaccine came back as 95% effective. In her career conducting HIV clinical trials, she had never seen anything like it. Even HIV prevention methods she had studied that had worked, such as the dapivirine ring, had an overall efficacy of 30%.
The COVID-19 success story started to soften her views toward another vaccine trial she was helping to conduct, a trial in HIV that used the same platform as Johnson & Johnson’s successful COVID-19 vaccine.
“When the COVID vaccine was cracked so quickly and seemingly quite easily, I did start to think, ‘Well, maybe … maybe this will work for HIV,’ “ she said in an interview.
That turned out to be false hope. The National Institutes of Health (NIH) announced that the trial Dr. Gill was helping to conduct, HVTN 705, was stopping early because it hadn’t generated enough of an immune response in participants to justify continuing. It was the second HIV vaccine to fail in the last year. It’s also the latest in what has been a litany of disappointments in the attempt to boost the human immune system to fight HIV without the need for ongoing HIV treatment.
In HVTN 705, known as the Imbokodo study (imbokodo is a Zulu word that’s part of a saying about women being strong as rocks), researchers used the platform made up of a common cold virus, adenovirus 26, to deliver a computer-generated mosaic of HIV antigens to participants’ immune systems. That mosaic of antigens is meant to goose the immune system into recognizing HIV if it were exposed to it.
When HIV enters the body, it infiltrates immune cells and replicates within them. To the rest of the immune system, those cells still register as just typical T-cells. The rest of the immune system can’t see that the virus is spreading through the very cells meant to protect the body from illness. That, plus the armor of sugary glycoproteins encasing the virus, has made HIV nearly impervious to vaccination.
Then, those so-called “prime” shots were followed by a second shot that targets glycoprotein 140, on the most common HIV subtype (or clade) in Africa, clade C. In the Imbokodo trial, a total of 2,637 women from five sub-Saharan African countries received shots at baseline, 3 months, 6 months, and 1 year. Then researchers followed the women from month 7 to 2 years after their third dose, testing their blood to see if their immune systems had generated the immune response the vaccine was meant to induce – and whether such immune response was associated with lower rates of HIV.
When researchers looked at the first 2 years of data, they learned that the vaccine was safe. And they found a total of 114 new cases of HIV – 51 among women who received the vaccine and 63 among those who received a placebo. That’s a 25.2% efficacy rate – but it wasn’t statistically significant.
The results are frustrating, said Carl Dieffenbach, PhD, director of the AIDS division at the National Institute of Allergy and Infectious Diseases (NIAID). NIAID is one of the funders of the study.
“This [trial] is a little more confounding, in that there is this low level of statistically insignificant difference between vaccine and placebo that starts somewhere around month 9 and then just kind of indolently is maintained over the next 15 months,” he said in an interview. “That’s kind of frustrating. Does it mean there’s a signal or is this just chance? Because that’s what statistics tell us, not to believe your last data point.”
What this means for the future direction of vaccine research is unclear. A sister study to Imbokodo, called Mosaico, recently finished enrolling participants. Mosaico uses the same adenovirus 26 platform, but it’s loaded with different antigens and targets a different glycoprotein for a different HIV subtype. If that trial shows success, it could mean that the platform is right, but the targets in the Imbokodo vaccine were wrong.
Dr. Dieffenbach said that before NIAID decides what to do with Mosaico they’ve asked researchers to analyze the data on the people who did respond, to see if those people have some specific variant of HIV or some other biomarker that could be used to form the next iteration of an HIV vaccine candidate. Only after that will they make a decision about Mosaico.
But he added that it does make him wonder if vaccine approaches that rely on nonneutralizing antibodies like this one have a ceiling of effectiveness that’s just too low to alter the course of the epidemic.
“I think we’ve discovered that there’s not a floor to [these nonneutralizing approaches], but there probably is a ceiling,” he said. “I don’t know if we’re going to get better than” a 25%-29% efficacy rate with those approaches.
The Imbokodo findings reminded Mitchell Warren, executive director of the global HIV prevention nonprofit, AVAC, of the data released in January from the Antibody Mediated Prevention (AMP) trial. That trial pitted the broadly neutralizing antibody (bNAb) VCR01 against HIV – and mostly, it lost.
VCR01 worked only on HIV variants that 30% of participants had. But in those 30%, it was 75% effective at preventing HIV. Now you have Imbokodo, with its potential 25% activity against HIV, something that may have been a fluke. This, to Mr. Warren, requires a rethinking of the whole HIV vaccine enterprise while “doubling, tripling, quadrupling down” on the HIV prevention methods we know work, such as preexposure prophylaxis (PrEP).
Dr. Dieffenbach agreed. To clinicians, Dr. Dieffenbach said the message of this HIV vaccine trial is flush with urgency: “Get your HIV-negative, at-risk people on PrEP tomorrow.”
There are now two pills approved for HIV prevention, both of which have been found to be up to 99% effective when taken consistently. A third option, injectable cabotegravir (Vocabria), has been submitted to the Food and Drug Administration for approval. The federal Ready, Set, PrEP program makes the pill available for free for those who qualify, and recently the Biden administration reaffirmed that, under the Affordable Care Act, insurance companies should cover all costs associated with PrEP, including lab work and exam visits.
But for the 157 women who participated in the trial at Dr. Gill’s site in Masiphumelele, on the southwestern tip of South Africa, the trial was personal, said Jason Naidoo, community liaison officer at the Desmond Tutu HIV Foundation, which conducted a portion of the study. These were women whose parents, siblings, or children were living with HIV or had died from AIDS-defining illnesses, he said. Their lives were chaotic, traveling at a moment’s notice to hometowns on the Eastern Cape, an 11-hour car ride away – longer by bus – for traditional prayers, funerals, and other important events.
Mr. Naidoo remembers arranging buses for the women to return for scheduled clinic visits, leaving the Eastern Cape in the afternoon and arriving in Masiphumelele in the early morning hours, just to keep the clinic appointment. Then, they’d turn around and return east.
They did this for 3 years, he said.
“The fact that these participants have stuck to this and been dedicated amidst all of the chaos talks about their commitment to actually having a vaccine for HIV,” he said. “They know their own risk profile as young Black women in South Africa, and they understand the need for an intervention for the future generations.
“So you can understand the emotion and the sense of sadness, the disappointment – the incredible [dis]belief that this [the failure of the vaccine] could have happened, because the expectations are so, so high.”
For Dr. Gill, who is lead investigator for Imbokodo in Masiphumelele, the weariness toward vaccines is back. Another trial is underway for an HIV vaccine with a platform that was successful in COVID-19 – using messenger RNA (mRNA), like the Pfizer and Moderna COVID-19 vaccines did.
“I think we need to be careful,” she said, “thinking that the mRNA vaccines are going to crack it.”
Dr. Dieffenbach, Dr. Gill, and Mr. Naidoo have disclosed no relevant financial relationships. The study was funded by Janssen, a Johnson & Johnson company, with NIAID and the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape.com.
Last year, Katherine Gill, MBChB, an HIV prevention researcher in Cape Town, South Africa, realized how jaded she’d become to vaccine research when the Pfizer COVID-19 vaccine came back as 95% effective. In her career conducting HIV clinical trials, she had never seen anything like it. Even HIV prevention methods she had studied that had worked, such as the dapivirine ring, had an overall efficacy of 30%.
The COVID-19 success story started to soften her views toward another vaccine trial she was helping to conduct, a trial in HIV that used the same platform as Johnson & Johnson’s successful COVID-19 vaccine.
“When the COVID vaccine was cracked so quickly and seemingly quite easily, I did start to think, ‘Well, maybe … maybe this will work for HIV,’ “ she said in an interview.
That turned out to be false hope. The National Institutes of Health (NIH) announced that the trial Dr. Gill was helping to conduct, HVTN 705, was stopping early because it hadn’t generated enough of an immune response in participants to justify continuing. It was the second HIV vaccine to fail in the last year. It’s also the latest in what has been a litany of disappointments in the attempt to boost the human immune system to fight HIV without the need for ongoing HIV treatment.
In HVTN 705, known as the Imbokodo study (imbokodo is a Zulu word that’s part of a saying about women being strong as rocks), researchers used the platform made up of a common cold virus, adenovirus 26, to deliver a computer-generated mosaic of HIV antigens to participants’ immune systems. That mosaic of antigens is meant to goose the immune system into recognizing HIV if it were exposed to it.
When HIV enters the body, it infiltrates immune cells and replicates within them. To the rest of the immune system, those cells still register as just typical T-cells. The rest of the immune system can’t see that the virus is spreading through the very cells meant to protect the body from illness. That, plus the armor of sugary glycoproteins encasing the virus, has made HIV nearly impervious to vaccination.
Then, those so-called “prime” shots were followed by a second shot that targets glycoprotein 140, on the most common HIV subtype (or clade) in Africa, clade C. In the Imbokodo trial, a total of 2,637 women from five sub-Saharan African countries received shots at baseline, 3 months, 6 months, and 1 year. Then researchers followed the women from month 7 to 2 years after their third dose, testing their blood to see if their immune systems had generated the immune response the vaccine was meant to induce – and whether such immune response was associated with lower rates of HIV.
When researchers looked at the first 2 years of data, they learned that the vaccine was safe. And they found a total of 114 new cases of HIV – 51 among women who received the vaccine and 63 among those who received a placebo. That’s a 25.2% efficacy rate – but it wasn’t statistically significant.
The results are frustrating, said Carl Dieffenbach, PhD, director of the AIDS division at the National Institute of Allergy and Infectious Diseases (NIAID). NIAID is one of the funders of the study.
“This [trial] is a little more confounding, in that there is this low level of statistically insignificant difference between vaccine and placebo that starts somewhere around month 9 and then just kind of indolently is maintained over the next 15 months,” he said in an interview. “That’s kind of frustrating. Does it mean there’s a signal or is this just chance? Because that’s what statistics tell us, not to believe your last data point.”
What this means for the future direction of vaccine research is unclear. A sister study to Imbokodo, called Mosaico, recently finished enrolling participants. Mosaico uses the same adenovirus 26 platform, but it’s loaded with different antigens and targets a different glycoprotein for a different HIV subtype. If that trial shows success, it could mean that the platform is right, but the targets in the Imbokodo vaccine were wrong.
Dr. Dieffenbach said that before NIAID decides what to do with Mosaico they’ve asked researchers to analyze the data on the people who did respond, to see if those people have some specific variant of HIV or some other biomarker that could be used to form the next iteration of an HIV vaccine candidate. Only after that will they make a decision about Mosaico.
But he added that it does make him wonder if vaccine approaches that rely on nonneutralizing antibodies like this one have a ceiling of effectiveness that’s just too low to alter the course of the epidemic.
“I think we’ve discovered that there’s not a floor to [these nonneutralizing approaches], but there probably is a ceiling,” he said. “I don’t know if we’re going to get better than” a 25%-29% efficacy rate with those approaches.
The Imbokodo findings reminded Mitchell Warren, executive director of the global HIV prevention nonprofit, AVAC, of the data released in January from the Antibody Mediated Prevention (AMP) trial. That trial pitted the broadly neutralizing antibody (bNAb) VCR01 against HIV – and mostly, it lost.
VCR01 worked only on HIV variants that 30% of participants had. But in those 30%, it was 75% effective at preventing HIV. Now you have Imbokodo, with its potential 25% activity against HIV, something that may have been a fluke. This, to Mr. Warren, requires a rethinking of the whole HIV vaccine enterprise while “doubling, tripling, quadrupling down” on the HIV prevention methods we know work, such as preexposure prophylaxis (PrEP).
Dr. Dieffenbach agreed. To clinicians, Dr. Dieffenbach said the message of this HIV vaccine trial is flush with urgency: “Get your HIV-negative, at-risk people on PrEP tomorrow.”
There are now two pills approved for HIV prevention, both of which have been found to be up to 99% effective when taken consistently. A third option, injectable cabotegravir (Vocabria), has been submitted to the Food and Drug Administration for approval. The federal Ready, Set, PrEP program makes the pill available for free for those who qualify, and recently the Biden administration reaffirmed that, under the Affordable Care Act, insurance companies should cover all costs associated with PrEP, including lab work and exam visits.
But for the 157 women who participated in the trial at Dr. Gill’s site in Masiphumelele, on the southwestern tip of South Africa, the trial was personal, said Jason Naidoo, community liaison officer at the Desmond Tutu HIV Foundation, which conducted a portion of the study. These were women whose parents, siblings, or children were living with HIV or had died from AIDS-defining illnesses, he said. Their lives were chaotic, traveling at a moment’s notice to hometowns on the Eastern Cape, an 11-hour car ride away – longer by bus – for traditional prayers, funerals, and other important events.
Mr. Naidoo remembers arranging buses for the women to return for scheduled clinic visits, leaving the Eastern Cape in the afternoon and arriving in Masiphumelele in the early morning hours, just to keep the clinic appointment. Then, they’d turn around and return east.
They did this for 3 years, he said.
“The fact that these participants have stuck to this and been dedicated amidst all of the chaos talks about their commitment to actually having a vaccine for HIV,” he said. “They know their own risk profile as young Black women in South Africa, and they understand the need for an intervention for the future generations.
“So you can understand the emotion and the sense of sadness, the disappointment – the incredible [dis]belief that this [the failure of the vaccine] could have happened, because the expectations are so, so high.”
For Dr. Gill, who is lead investigator for Imbokodo in Masiphumelele, the weariness toward vaccines is back. Another trial is underway for an HIV vaccine with a platform that was successful in COVID-19 – using messenger RNA (mRNA), like the Pfizer and Moderna COVID-19 vaccines did.
“I think we need to be careful,” she said, “thinking that the mRNA vaccines are going to crack it.”
Dr. Dieffenbach, Dr. Gill, and Mr. Naidoo have disclosed no relevant financial relationships. The study was funded by Janssen, a Johnson & Johnson company, with NIAID and the Bill and Melinda Gates Foundation.
A version of this article first appeared on Medscape.com.
Beta-blocker reduces lung inflammation in critical COVID-19
In a small study, intravenous administration of the beta-blocker metoprolol to critically ill COVID-19 patients with acute respiratory distress syndrome (ARDS) safely blunted lung inflammation associated with the disease.
Metoprolol administration also resulted in better oxygenation and fewer days on intensive mechanical ventilation and in the ICU, compared with no treatment.
These data suggest that metoprolol repurposing for the treatment of ARDS in COVID-19 patients is a safe and inexpensive strategy with the potential to improve outcomes, the researchers said.
“Metoprolol repurposing for the treatment of ARDS associated with COVID-19 is a safe and cheap intervention that can help to alleviate the massive personal and health care burden associated with the pandemic,” they concluded.
The results, from the MADRID-COVID pilot trial from Agustin Clemente-Moragon, BSc, Centro National de Investigaciones Cardiovasculares, Madrid, and colleagues, were published online Aug. 30, 2021, in the Journal of the American College of Cardiology.
In previous work, the researchers showed that metoprolol, but not other clinically available intravenous beta-blockers, abrogates neutrophil-driven exacerbated inflammation, neutrophil-platelet interaction, and formation of neutrophil extracellular traps in a mouse model of acute lung injury.
These results prompted the current pilot trial in 20 patients, ages 18-80 years, with COVID-19–associated ARDS.
Randomization was stratified by age (59 and younger vs. 60 and older), history of hypertension (yes or no), and circulating neutrophil counts (<6,000 vs. ≥6,000). Bronchoalveolar lavage (BAL) fluid and blood samples were obtained from patients at randomization and 24 hours after the third metoprolol dose in the treatment group, and on day 4 in controls.
Because of the cardiovascular effects of metoprolol, patients were monitored invasively and by echocardiography, the authors noted.
As expected, metoprolol significantly reduced heart rate (P < .01) and systolic blood pressure (P < .05), although both remained within the physiological range. Echocardiography showed no deterioration of cardiac function after metoprolol treatment.
To assess the ability of metoprolol to address neutrophil-mediated exacerbated lung inflammation, the researchers analyzed leukocyte populations in BAL samples by flow cytometry at baseline and on day 4.
At baseline, the metoprolol and control groups showed no differences in BAL neutrophil content. But on day 4, after 3 days of treatment with metoprolol, neutrophil content was significantly lower in the metoprolol group (median, 14.3 neutrophils/mcL) than in the control group (median, 397 neutrophils/mcL).
Metoprolol-treated patients also had lower total inflammatory-cell content and lower monocyte/macrophage content. Lymphocytes did not differ between the groups.
The investigators also explored the impact of metoprolol on the chemokine, monocyte chemoattractant protein–1 (MCP-1), as it has been shown to promote pulmonary fibrosis in late-stage ARDS.
They found that MCP-1 was significantly attenuated after 3 days of metoprolol treatment. At baseline, the median MCP-1 level was 298 pg/mL; on day 4 after metoprolol, it was 203 pg/mL (P = .009).
MCP-1 levels remained unchanged in control patients.
An elegant study
In an accompanying editorial, Mourad H. Senussi, MD, assistant professor at Baylor College of Medicine, Houston, wrote: “Although the study has a small sample size, we commend the authors, who attempt to shed light on the important pathophysiological underpinnings that help establish biological plausibility for this inexpensive, safe, and widely available medication.”
In an interview with this news organization, Dr. Senussi added that metoprolol is not itself something primarily used to treat COVID-19 per se. “Rather, the drug blunts the sympathetic-host response. There is a fine balance between that sympathetic surge that is helpful to the body, and then a sympathetic surge that if left unchecked, can lead to significant damage. And so, I think this study really shows that medications like metoprolol can help blunt that initial sympathetic effect.”
A larger study is “absolutely” warranted, he added, “this is a drug that is readily available, safe, and inexpensive. The study design here was simple and most importantly, showed biological plausibility.”
Dr. Senussi also noted that, although the benefit was noted in COVID-19 patients, the study sets the groundwork for further research in the use of beta-blockade in the critically ill. “Further studies are needed to elucidate and identify where along the inflammatory spectrum these critically ill patients lie, which patients would benefit from beta-blockers, and at what time point during their hospital stay.”
The MADRID-COVID authors and Dr. Senussi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a small study, intravenous administration of the beta-blocker metoprolol to critically ill COVID-19 patients with acute respiratory distress syndrome (ARDS) safely blunted lung inflammation associated with the disease.
Metoprolol administration also resulted in better oxygenation and fewer days on intensive mechanical ventilation and in the ICU, compared with no treatment.
These data suggest that metoprolol repurposing for the treatment of ARDS in COVID-19 patients is a safe and inexpensive strategy with the potential to improve outcomes, the researchers said.
“Metoprolol repurposing for the treatment of ARDS associated with COVID-19 is a safe and cheap intervention that can help to alleviate the massive personal and health care burden associated with the pandemic,” they concluded.
The results, from the MADRID-COVID pilot trial from Agustin Clemente-Moragon, BSc, Centro National de Investigaciones Cardiovasculares, Madrid, and colleagues, were published online Aug. 30, 2021, in the Journal of the American College of Cardiology.
In previous work, the researchers showed that metoprolol, but not other clinically available intravenous beta-blockers, abrogates neutrophil-driven exacerbated inflammation, neutrophil-platelet interaction, and formation of neutrophil extracellular traps in a mouse model of acute lung injury.
These results prompted the current pilot trial in 20 patients, ages 18-80 years, with COVID-19–associated ARDS.
Randomization was stratified by age (59 and younger vs. 60 and older), history of hypertension (yes or no), and circulating neutrophil counts (<6,000 vs. ≥6,000). Bronchoalveolar lavage (BAL) fluid and blood samples were obtained from patients at randomization and 24 hours after the third metoprolol dose in the treatment group, and on day 4 in controls.
Because of the cardiovascular effects of metoprolol, patients were monitored invasively and by echocardiography, the authors noted.
As expected, metoprolol significantly reduced heart rate (P < .01) and systolic blood pressure (P < .05), although both remained within the physiological range. Echocardiography showed no deterioration of cardiac function after metoprolol treatment.
To assess the ability of metoprolol to address neutrophil-mediated exacerbated lung inflammation, the researchers analyzed leukocyte populations in BAL samples by flow cytometry at baseline and on day 4.
At baseline, the metoprolol and control groups showed no differences in BAL neutrophil content. But on day 4, after 3 days of treatment with metoprolol, neutrophil content was significantly lower in the metoprolol group (median, 14.3 neutrophils/mcL) than in the control group (median, 397 neutrophils/mcL).
Metoprolol-treated patients also had lower total inflammatory-cell content and lower monocyte/macrophage content. Lymphocytes did not differ between the groups.
The investigators also explored the impact of metoprolol on the chemokine, monocyte chemoattractant protein–1 (MCP-1), as it has been shown to promote pulmonary fibrosis in late-stage ARDS.
They found that MCP-1 was significantly attenuated after 3 days of metoprolol treatment. At baseline, the median MCP-1 level was 298 pg/mL; on day 4 after metoprolol, it was 203 pg/mL (P = .009).
MCP-1 levels remained unchanged in control patients.
An elegant study
In an accompanying editorial, Mourad H. Senussi, MD, assistant professor at Baylor College of Medicine, Houston, wrote: “Although the study has a small sample size, we commend the authors, who attempt to shed light on the important pathophysiological underpinnings that help establish biological plausibility for this inexpensive, safe, and widely available medication.”
In an interview with this news organization, Dr. Senussi added that metoprolol is not itself something primarily used to treat COVID-19 per se. “Rather, the drug blunts the sympathetic-host response. There is a fine balance between that sympathetic surge that is helpful to the body, and then a sympathetic surge that if left unchecked, can lead to significant damage. And so, I think this study really shows that medications like metoprolol can help blunt that initial sympathetic effect.”
A larger study is “absolutely” warranted, he added, “this is a drug that is readily available, safe, and inexpensive. The study design here was simple and most importantly, showed biological plausibility.”
Dr. Senussi also noted that, although the benefit was noted in COVID-19 patients, the study sets the groundwork for further research in the use of beta-blockade in the critically ill. “Further studies are needed to elucidate and identify where along the inflammatory spectrum these critically ill patients lie, which patients would benefit from beta-blockers, and at what time point during their hospital stay.”
The MADRID-COVID authors and Dr. Senussi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
In a small study, intravenous administration of the beta-blocker metoprolol to critically ill COVID-19 patients with acute respiratory distress syndrome (ARDS) safely blunted lung inflammation associated with the disease.
Metoprolol administration also resulted in better oxygenation and fewer days on intensive mechanical ventilation and in the ICU, compared with no treatment.
These data suggest that metoprolol repurposing for the treatment of ARDS in COVID-19 patients is a safe and inexpensive strategy with the potential to improve outcomes, the researchers said.
“Metoprolol repurposing for the treatment of ARDS associated with COVID-19 is a safe and cheap intervention that can help to alleviate the massive personal and health care burden associated with the pandemic,” they concluded.
The results, from the MADRID-COVID pilot trial from Agustin Clemente-Moragon, BSc, Centro National de Investigaciones Cardiovasculares, Madrid, and colleagues, were published online Aug. 30, 2021, in the Journal of the American College of Cardiology.
In previous work, the researchers showed that metoprolol, but not other clinically available intravenous beta-blockers, abrogates neutrophil-driven exacerbated inflammation, neutrophil-platelet interaction, and formation of neutrophil extracellular traps in a mouse model of acute lung injury.
These results prompted the current pilot trial in 20 patients, ages 18-80 years, with COVID-19–associated ARDS.
Randomization was stratified by age (59 and younger vs. 60 and older), history of hypertension (yes or no), and circulating neutrophil counts (<6,000 vs. ≥6,000). Bronchoalveolar lavage (BAL) fluid and blood samples were obtained from patients at randomization and 24 hours after the third metoprolol dose in the treatment group, and on day 4 in controls.
Because of the cardiovascular effects of metoprolol, patients were monitored invasively and by echocardiography, the authors noted.
As expected, metoprolol significantly reduced heart rate (P < .01) and systolic blood pressure (P < .05), although both remained within the physiological range. Echocardiography showed no deterioration of cardiac function after metoprolol treatment.
To assess the ability of metoprolol to address neutrophil-mediated exacerbated lung inflammation, the researchers analyzed leukocyte populations in BAL samples by flow cytometry at baseline and on day 4.
At baseline, the metoprolol and control groups showed no differences in BAL neutrophil content. But on day 4, after 3 days of treatment with metoprolol, neutrophil content was significantly lower in the metoprolol group (median, 14.3 neutrophils/mcL) than in the control group (median, 397 neutrophils/mcL).
Metoprolol-treated patients also had lower total inflammatory-cell content and lower monocyte/macrophage content. Lymphocytes did not differ between the groups.
The investigators also explored the impact of metoprolol on the chemokine, monocyte chemoattractant protein–1 (MCP-1), as it has been shown to promote pulmonary fibrosis in late-stage ARDS.
They found that MCP-1 was significantly attenuated after 3 days of metoprolol treatment. At baseline, the median MCP-1 level was 298 pg/mL; on day 4 after metoprolol, it was 203 pg/mL (P = .009).
MCP-1 levels remained unchanged in control patients.
An elegant study
In an accompanying editorial, Mourad H. Senussi, MD, assistant professor at Baylor College of Medicine, Houston, wrote: “Although the study has a small sample size, we commend the authors, who attempt to shed light on the important pathophysiological underpinnings that help establish biological plausibility for this inexpensive, safe, and widely available medication.”
In an interview with this news organization, Dr. Senussi added that metoprolol is not itself something primarily used to treat COVID-19 per se. “Rather, the drug blunts the sympathetic-host response. There is a fine balance between that sympathetic surge that is helpful to the body, and then a sympathetic surge that if left unchecked, can lead to significant damage. And so, I think this study really shows that medications like metoprolol can help blunt that initial sympathetic effect.”
A larger study is “absolutely” warranted, he added, “this is a drug that is readily available, safe, and inexpensive. The study design here was simple and most importantly, showed biological plausibility.”
Dr. Senussi also noted that, although the benefit was noted in COVID-19 patients, the study sets the groundwork for further research in the use of beta-blockade in the critically ill. “Further studies are needed to elucidate and identify where along the inflammatory spectrum these critically ill patients lie, which patients would benefit from beta-blockers, and at what time point during their hospital stay.”
The MADRID-COVID authors and Dr. Senussi disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Clinical Edge Journal Scan Commentary: CML September 2021
Latin American countries have a high rate of SARS-CoV-2 infection and some of the highest COVID-19 deaths worldwide. Brazil, Colombia, Argentina, and Mexico have reported the highest number of confirmed cases. More recently has been reported that in series form US and Europe the mortality of COVID-19 has not been as high as reported in other hematological conditions and the response to vaccination also has bene described as high. In a recent report, Pagnano et al. (Leuk Lymphoma. 2021) has recently reported the clinical evolution and outcome of COVID-19 in patients with chronic myeloid leukemia in Latin America. In an observational multicenter study with a total of 92 patients with COVID-19 between March and December 2020 with 26% of whom were severe or critical. Eighty-one patients recovered (88%), and 11 (11.9%) died from COVID-19. Almost half of them had at least one comorbidity. Patients with a major molecular response presented superior overall survival compared to patients with no major molecular response (91 vs. 61%, respectively; P = 0.004). Patients in treatment-free remission and receiving tyrosine kinase inhibitors showed higher survival rates (100 and 89%) than patients who underwent hematopoietic stem cell transplantation and those who did not receive tyrosine kinase inhibitors (50 and 33%).
Currently the most common reason for TKI discontinuation is intolerance. Ma et al (Leuk Res. 2021) reports the long-term outcomes associated with switch to an alternative TKI after first-line therapy with a 2GTKI. Of 232 patients who initiated a 2GTKI during the study period, 76 (33 %) switched to an alternative TKI. Reasons for switching included intolerance (79 %) and resistance (21 %). Among the 60 patients who switched due to intolerance, 53 (88 %) were able to achieve or maintain a major molecular response (MMR) with 5-year progression-free survival (PFS) 90.5 % (95 % CI 90.4–90.6 %). However, amongst the 16 patients who switched due to resistance, 8 patients (50 %) were able to achieve MMR with 5-year PFS 80.4 % (95 % CI 80.2–80.6 %). Most patients who switched due to intolerance remained on their second-line TKI. Patients who switch for intolerance continue to enjoy excellent long term clinical outcomes.
Latin American countries have a high rate of SARS-CoV-2 infection and some of the highest COVID-19 deaths worldwide. Brazil, Colombia, Argentina, and Mexico have reported the highest number of confirmed cases. More recently has been reported that in series form US and Europe the mortality of COVID-19 has not been as high as reported in other hematological conditions and the response to vaccination also has bene described as high. In a recent report, Pagnano et al. (Leuk Lymphoma. 2021) has recently reported the clinical evolution and outcome of COVID-19 in patients with chronic myeloid leukemia in Latin America. In an observational multicenter study with a total of 92 patients with COVID-19 between March and December 2020 with 26% of whom were severe or critical. Eighty-one patients recovered (88%), and 11 (11.9%) died from COVID-19. Almost half of them had at least one comorbidity. Patients with a major molecular response presented superior overall survival compared to patients with no major molecular response (91 vs. 61%, respectively; P = 0.004). Patients in treatment-free remission and receiving tyrosine kinase inhibitors showed higher survival rates (100 and 89%) than patients who underwent hematopoietic stem cell transplantation and those who did not receive tyrosine kinase inhibitors (50 and 33%).
Currently the most common reason for TKI discontinuation is intolerance. Ma et al (Leuk Res. 2021) reports the long-term outcomes associated with switch to an alternative TKI after first-line therapy with a 2GTKI. Of 232 patients who initiated a 2GTKI during the study period, 76 (33 %) switched to an alternative TKI. Reasons for switching included intolerance (79 %) and resistance (21 %). Among the 60 patients who switched due to intolerance, 53 (88 %) were able to achieve or maintain a major molecular response (MMR) with 5-year progression-free survival (PFS) 90.5 % (95 % CI 90.4–90.6 %). However, amongst the 16 patients who switched due to resistance, 8 patients (50 %) were able to achieve MMR with 5-year PFS 80.4 % (95 % CI 80.2–80.6 %). Most patients who switched due to intolerance remained on their second-line TKI. Patients who switch for intolerance continue to enjoy excellent long term clinical outcomes.
Latin American countries have a high rate of SARS-CoV-2 infection and some of the highest COVID-19 deaths worldwide. Brazil, Colombia, Argentina, and Mexico have reported the highest number of confirmed cases. More recently has been reported that in series form US and Europe the mortality of COVID-19 has not been as high as reported in other hematological conditions and the response to vaccination also has bene described as high. In a recent report, Pagnano et al. (Leuk Lymphoma. 2021) has recently reported the clinical evolution and outcome of COVID-19 in patients with chronic myeloid leukemia in Latin America. In an observational multicenter study with a total of 92 patients with COVID-19 between March and December 2020 with 26% of whom were severe or critical. Eighty-one patients recovered (88%), and 11 (11.9%) died from COVID-19. Almost half of them had at least one comorbidity. Patients with a major molecular response presented superior overall survival compared to patients with no major molecular response (91 vs. 61%, respectively; P = 0.004). Patients in treatment-free remission and receiving tyrosine kinase inhibitors showed higher survival rates (100 and 89%) than patients who underwent hematopoietic stem cell transplantation and those who did not receive tyrosine kinase inhibitors (50 and 33%).
Currently the most common reason for TKI discontinuation is intolerance. Ma et al (Leuk Res. 2021) reports the long-term outcomes associated with switch to an alternative TKI after first-line therapy with a 2GTKI. Of 232 patients who initiated a 2GTKI during the study period, 76 (33 %) switched to an alternative TKI. Reasons for switching included intolerance (79 %) and resistance (21 %). Among the 60 patients who switched due to intolerance, 53 (88 %) were able to achieve or maintain a major molecular response (MMR) with 5-year progression-free survival (PFS) 90.5 % (95 % CI 90.4–90.6 %). However, amongst the 16 patients who switched due to resistance, 8 patients (50 %) were able to achieve MMR with 5-year PFS 80.4 % (95 % CI 80.2–80.6 %). Most patients who switched due to intolerance remained on their second-line TKI. Patients who switch for intolerance continue to enjoy excellent long term clinical outcomes.
FDA approves zanubrutinib in Waldenström’s macroglobulinemia
The Food and Drug Administration has approved zanubrutinib (Brukinsa) capsules for use in the treatment of adult patients with Waldenström’s macroglobulinemia (WM), a rare non-Hodgkin lymphoma, according to an approval letter from the agency to BeiGene, the drug’s maker.
The FDA stipulated that the company conduct an additional clinical trial (rather than an observational study) to assess the “known serious risk of second primary malignancies” associated with use of zanubrutinib. The study should further characterize the clinical benefit and safety of zanubrutinib for the treatment of patients with newly diagnosed WM with MYD88 mutation, the agency said.
The drug, which is a small-molecule inhibitor of Bruton’s tyrosine kinase (BTK), previously received accelerated approval for use in patients with mantle cell lymphoma who have received one prior therapy. It is also being studied for the treatment of chronic lymphocytic leukemia.
The new approval is primarily based on results from ASPEN, a randomized, active control, open-label trial that compared zanubrutinib and ibrutinib.
The ASPEN trial provided “compelling evidence” that zanubrutinib is a highly active BTK inhibitor in WM and that it showed improved tolerability across a number of clinically important side effects in comparison with the first-generation BTK inhibitor ibrutinib, said study investigator Steven Treon, MD, PhD, director of the Bing Center for Waldenström’s Macroglobulinemia Research at the Dana-Farber Cancer Institute, Boston. “The approval of [zanubrutinib] provides an important new option for targeted therapy in Waldenström’s macroglobulinemia,” he added in a company press statement.
The recommended dosage is 160 mg orally twice daily or 320 mg orally once; the drug should be swallowed whole with water with or without food.
In ASPEN, all patients had MYD88 mutation WM. Patients in cohort 1 (n = 201) were randomly assigned in a 1:1 ratio to receive zanubrutinib 160 mg twice daily or ibrutinib 420 mg once daily until disease progression or unacceptable toxicity. A total of 82% of patients had relapsed/refractory disease.
The major efficacy outcome was the response rate, defined as partial response or better (i.e., partial response, very good partial response, and complete response), as determined on the basis of standard consensus response criteria from the International Workshop on Waldenström’s Macroglobulinemia (IWWM-6) criteria.
The drugs had nearly identical response rates (roughly 77%). There were no complete responses with either drug. However, zanubrutinib had twice the rate of very good partial responses compared with ibrutinib (15.7% vs. 7.1%). In addition, on the basis of modified IWWM-6 criteria, the very good partial response rate was 28% with zanubrutinib, compared to 19% with ibrutinib.
An additional efficacy outcome measure was duration of response, which was measured by the percentage of patients who were event free at 12 months. Zanubrutinib bested ibrutinib in this measure (94.4% vs. 87.9%).
The safety of zanubrutinib was also investigated in the ASPEN trial. Among patients who received zanubrutinib, 93% were exposed for 6 months or longer, and 89% were exposed for longer than 1 year. In cohort 1, serious adverse reactions occurred in 44% of patients who received zanubrutinib. Serious adverse reactions that occurred in > 2% of patients included influenza (3%), pneumonia (4%), neutropenia and decreased neutrophil count (3%), hemorrhage (4%), pyrexia (3%), and febrile neutropenia (3%).
In the FDA’s prescribing information for the drug, which includes approved indications and pooled safety data, the most common adverse reactions for zanubrutinib (≥ 20%) are listed as decreased neutrophil count, upper respiratory tract infection, decreased platelet count, rash, hemorrhage, musculoskeletal pain, decreased hemoglobin, bruising, diarrhea, pneumonia, and cough.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved zanubrutinib (Brukinsa) capsules for use in the treatment of adult patients with Waldenström’s macroglobulinemia (WM), a rare non-Hodgkin lymphoma, according to an approval letter from the agency to BeiGene, the drug’s maker.
The FDA stipulated that the company conduct an additional clinical trial (rather than an observational study) to assess the “known serious risk of second primary malignancies” associated with use of zanubrutinib. The study should further characterize the clinical benefit and safety of zanubrutinib for the treatment of patients with newly diagnosed WM with MYD88 mutation, the agency said.
The drug, which is a small-molecule inhibitor of Bruton’s tyrosine kinase (BTK), previously received accelerated approval for use in patients with mantle cell lymphoma who have received one prior therapy. It is also being studied for the treatment of chronic lymphocytic leukemia.
The new approval is primarily based on results from ASPEN, a randomized, active control, open-label trial that compared zanubrutinib and ibrutinib.
The ASPEN trial provided “compelling evidence” that zanubrutinib is a highly active BTK inhibitor in WM and that it showed improved tolerability across a number of clinically important side effects in comparison with the first-generation BTK inhibitor ibrutinib, said study investigator Steven Treon, MD, PhD, director of the Bing Center for Waldenström’s Macroglobulinemia Research at the Dana-Farber Cancer Institute, Boston. “The approval of [zanubrutinib] provides an important new option for targeted therapy in Waldenström’s macroglobulinemia,” he added in a company press statement.
The recommended dosage is 160 mg orally twice daily or 320 mg orally once; the drug should be swallowed whole with water with or without food.
In ASPEN, all patients had MYD88 mutation WM. Patients in cohort 1 (n = 201) were randomly assigned in a 1:1 ratio to receive zanubrutinib 160 mg twice daily or ibrutinib 420 mg once daily until disease progression or unacceptable toxicity. A total of 82% of patients had relapsed/refractory disease.
The major efficacy outcome was the response rate, defined as partial response or better (i.e., partial response, very good partial response, and complete response), as determined on the basis of standard consensus response criteria from the International Workshop on Waldenström’s Macroglobulinemia (IWWM-6) criteria.
The drugs had nearly identical response rates (roughly 77%). There were no complete responses with either drug. However, zanubrutinib had twice the rate of very good partial responses compared with ibrutinib (15.7% vs. 7.1%). In addition, on the basis of modified IWWM-6 criteria, the very good partial response rate was 28% with zanubrutinib, compared to 19% with ibrutinib.
An additional efficacy outcome measure was duration of response, which was measured by the percentage of patients who were event free at 12 months. Zanubrutinib bested ibrutinib in this measure (94.4% vs. 87.9%).
The safety of zanubrutinib was also investigated in the ASPEN trial. Among patients who received zanubrutinib, 93% were exposed for 6 months or longer, and 89% were exposed for longer than 1 year. In cohort 1, serious adverse reactions occurred in 44% of patients who received zanubrutinib. Serious adverse reactions that occurred in > 2% of patients included influenza (3%), pneumonia (4%), neutropenia and decreased neutrophil count (3%), hemorrhage (4%), pyrexia (3%), and febrile neutropenia (3%).
In the FDA’s prescribing information for the drug, which includes approved indications and pooled safety data, the most common adverse reactions for zanubrutinib (≥ 20%) are listed as decreased neutrophil count, upper respiratory tract infection, decreased platelet count, rash, hemorrhage, musculoskeletal pain, decreased hemoglobin, bruising, diarrhea, pneumonia, and cough.
A version of this article first appeared on Medscape.com.
The Food and Drug Administration has approved zanubrutinib (Brukinsa) capsules for use in the treatment of adult patients with Waldenström’s macroglobulinemia (WM), a rare non-Hodgkin lymphoma, according to an approval letter from the agency to BeiGene, the drug’s maker.
The FDA stipulated that the company conduct an additional clinical trial (rather than an observational study) to assess the “known serious risk of second primary malignancies” associated with use of zanubrutinib. The study should further characterize the clinical benefit and safety of zanubrutinib for the treatment of patients with newly diagnosed WM with MYD88 mutation, the agency said.
The drug, which is a small-molecule inhibitor of Bruton’s tyrosine kinase (BTK), previously received accelerated approval for use in patients with mantle cell lymphoma who have received one prior therapy. It is also being studied for the treatment of chronic lymphocytic leukemia.
The new approval is primarily based on results from ASPEN, a randomized, active control, open-label trial that compared zanubrutinib and ibrutinib.
The ASPEN trial provided “compelling evidence” that zanubrutinib is a highly active BTK inhibitor in WM and that it showed improved tolerability across a number of clinically important side effects in comparison with the first-generation BTK inhibitor ibrutinib, said study investigator Steven Treon, MD, PhD, director of the Bing Center for Waldenström’s Macroglobulinemia Research at the Dana-Farber Cancer Institute, Boston. “The approval of [zanubrutinib] provides an important new option for targeted therapy in Waldenström’s macroglobulinemia,” he added in a company press statement.
The recommended dosage is 160 mg orally twice daily or 320 mg orally once; the drug should be swallowed whole with water with or without food.
In ASPEN, all patients had MYD88 mutation WM. Patients in cohort 1 (n = 201) were randomly assigned in a 1:1 ratio to receive zanubrutinib 160 mg twice daily or ibrutinib 420 mg once daily until disease progression or unacceptable toxicity. A total of 82% of patients had relapsed/refractory disease.
The major efficacy outcome was the response rate, defined as partial response or better (i.e., partial response, very good partial response, and complete response), as determined on the basis of standard consensus response criteria from the International Workshop on Waldenström’s Macroglobulinemia (IWWM-6) criteria.
The drugs had nearly identical response rates (roughly 77%). There were no complete responses with either drug. However, zanubrutinib had twice the rate of very good partial responses compared with ibrutinib (15.7% vs. 7.1%). In addition, on the basis of modified IWWM-6 criteria, the very good partial response rate was 28% with zanubrutinib, compared to 19% with ibrutinib.
An additional efficacy outcome measure was duration of response, which was measured by the percentage of patients who were event free at 12 months. Zanubrutinib bested ibrutinib in this measure (94.4% vs. 87.9%).
The safety of zanubrutinib was also investigated in the ASPEN trial. Among patients who received zanubrutinib, 93% were exposed for 6 months or longer, and 89% were exposed for longer than 1 year. In cohort 1, serious adverse reactions occurred in 44% of patients who received zanubrutinib. Serious adverse reactions that occurred in > 2% of patients included influenza (3%), pneumonia (4%), neutropenia and decreased neutrophil count (3%), hemorrhage (4%), pyrexia (3%), and febrile neutropenia (3%).
In the FDA’s prescribing information for the drug, which includes approved indications and pooled safety data, the most common adverse reactions for zanubrutinib (≥ 20%) are listed as decreased neutrophil count, upper respiratory tract infection, decreased platelet count, rash, hemorrhage, musculoskeletal pain, decreased hemoglobin, bruising, diarrhea, pneumonia, and cough.
A version of this article first appeared on Medscape.com.
NCCN recommends third COVID-19 dose for patients with cancer
Experts at the National Comprehensive Cancer Network have now issued an updated recommendation for COVID-19 vaccination in people with cancer. The panel calls for these patients to be among the highest-priority group to be vaccinated against COVID-19 and to receive the newly approved third dose of vaccine.
The NCCN has recommended in February that all patients receiving active cancer treatment should receive a COVID-19 vaccine and should be prioritized for vaccination. In August, the FDA authorized a third dose of either the Pfizer or Moderna COVID-19 vaccines for people with compromised immune systems. Those eligible for a third dose include solid organ transplant recipients, those undergoing cancer treatments, and people with autoimmune diseases that suppress their immune systems
The new NCCN recommendations state that the following groups should be considered eligible for a third dose of the mRNA COVID-19 vaccine immediately, based on the latest decisions from the Food and Drug Administration and the Centers for Disease Control and Prevention:
- Patients with solid tumors (either new or recurring) receiving treatment within 1 year of their initial vaccine dose, regardless of their type of cancer therapy.
- Patients with active hematologic malignancies regardless of whether they are currently receiving cancer therapy.
- Anyone who received a stem cell transplant (SCT) or engineered cellular therapy (for example, chimeric antigen receptor T cells), especially within the past 2 years.
- Any recipients of allogeneic SCT on immunosuppressive therapy or with a history of graft-versus-host disease regardless of the time of transplant.
- Anyone with an additional immunosuppressive condition (for example, HIV) or being treated with immunosuppressive agents unrelated to their cancer therapy.
Cancer patients at high risk of complications
As previously reported by this news organization, infection with COVID-19 in people with cancer can severely impact survival. One study published in 2020 found that patients with both COVID-19 infection and progressing cancer had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer.
Another study found that cancer type, stage, and recent treatment could affect outcomes of COVID-19 in patients with cancer. Patients with hematologic malignancies and metastatic cancers had higher risks of developing severe or critical COVID-19 symptoms, being admitted to the ICU, requiring ventilation, and dying. Conversely, those with nonmetastatic disease had outcomes that were comparable with persons without cancer and a COVID-19 infection. This study also found that having undergone recent surgery or receiving immunotherapy also put patients at a higher risk of poor outcomes, although patients with cancer who were treated with radiotherapy had outcomes similar to those of noncancer COVID-19 patients.
“COVID-19 can be very dangerous, especially for people living with cancer, which is why we’re so grateful for safe and effective vaccines that are saving lives,” Robert W. Carlson, MD, CEO of NCCN, said in a statement.
Right timing and location
The current NCCN update also recommends that individuals wait at least 4 weeks between the second and third doses, and those who are infected with COVID-19 after being vaccinated should wait until they have documented clearance of the virus before receiving a third dose.
It also recommends that people who live in the same household with immunocompromised individuals should also get a third dose once it becomes available, and that it is best to have a third dose of the same type of vaccine as the first two doses. However, a different mRNA vaccine is also acceptable.
Immunocompromised individuals should try to receive their third dose in a health care delivery setting, as opposed to a pharmacy or public vaccination clinic if possible, as it would limit their risk of exposure to the general population.
Steve Pergam, MD, MPH, associate professor, vaccine and infectious disease division, Fred Hutchinson Cancer Research Center, Seattle, commented that it is still necessary to take precautions, even after getting the booster dose.
“That means, even after a third dose of vaccine, we still recommend immunocompromised people, such as those undergoing cancer treatment, continue to be cautious, wear masks, and avoid large group gatherings, particularly around those who are unvaccinated,” said Dr. Pergam, who is also coleader of the NCCN COVID-19 Vaccination Advisory Committee. “All of us should do our part to reduce the spread of COVID-19 and get vaccinated to protect those around us from preventable suffering.”
A version of this article first appeared on Medscape.com.
Experts at the National Comprehensive Cancer Network have now issued an updated recommendation for COVID-19 vaccination in people with cancer. The panel calls for these patients to be among the highest-priority group to be vaccinated against COVID-19 and to receive the newly approved third dose of vaccine.
The NCCN has recommended in February that all patients receiving active cancer treatment should receive a COVID-19 vaccine and should be prioritized for vaccination. In August, the FDA authorized a third dose of either the Pfizer or Moderna COVID-19 vaccines for people with compromised immune systems. Those eligible for a third dose include solid organ transplant recipients, those undergoing cancer treatments, and people with autoimmune diseases that suppress their immune systems
The new NCCN recommendations state that the following groups should be considered eligible for a third dose of the mRNA COVID-19 vaccine immediately, based on the latest decisions from the Food and Drug Administration and the Centers for Disease Control and Prevention:
- Patients with solid tumors (either new or recurring) receiving treatment within 1 year of their initial vaccine dose, regardless of their type of cancer therapy.
- Patients with active hematologic malignancies regardless of whether they are currently receiving cancer therapy.
- Anyone who received a stem cell transplant (SCT) or engineered cellular therapy (for example, chimeric antigen receptor T cells), especially within the past 2 years.
- Any recipients of allogeneic SCT on immunosuppressive therapy or with a history of graft-versus-host disease regardless of the time of transplant.
- Anyone with an additional immunosuppressive condition (for example, HIV) or being treated with immunosuppressive agents unrelated to their cancer therapy.
Cancer patients at high risk of complications
As previously reported by this news organization, infection with COVID-19 in people with cancer can severely impact survival. One study published in 2020 found that patients with both COVID-19 infection and progressing cancer had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer.
Another study found that cancer type, stage, and recent treatment could affect outcomes of COVID-19 in patients with cancer. Patients with hematologic malignancies and metastatic cancers had higher risks of developing severe or critical COVID-19 symptoms, being admitted to the ICU, requiring ventilation, and dying. Conversely, those with nonmetastatic disease had outcomes that were comparable with persons without cancer and a COVID-19 infection. This study also found that having undergone recent surgery or receiving immunotherapy also put patients at a higher risk of poor outcomes, although patients with cancer who were treated with radiotherapy had outcomes similar to those of noncancer COVID-19 patients.
“COVID-19 can be very dangerous, especially for people living with cancer, which is why we’re so grateful for safe and effective vaccines that are saving lives,” Robert W. Carlson, MD, CEO of NCCN, said in a statement.
Right timing and location
The current NCCN update also recommends that individuals wait at least 4 weeks between the second and third doses, and those who are infected with COVID-19 after being vaccinated should wait until they have documented clearance of the virus before receiving a third dose.
It also recommends that people who live in the same household with immunocompromised individuals should also get a third dose once it becomes available, and that it is best to have a third dose of the same type of vaccine as the first two doses. However, a different mRNA vaccine is also acceptable.
Immunocompromised individuals should try to receive their third dose in a health care delivery setting, as opposed to a pharmacy or public vaccination clinic if possible, as it would limit their risk of exposure to the general population.
Steve Pergam, MD, MPH, associate professor, vaccine and infectious disease division, Fred Hutchinson Cancer Research Center, Seattle, commented that it is still necessary to take precautions, even after getting the booster dose.
“That means, even after a third dose of vaccine, we still recommend immunocompromised people, such as those undergoing cancer treatment, continue to be cautious, wear masks, and avoid large group gatherings, particularly around those who are unvaccinated,” said Dr. Pergam, who is also coleader of the NCCN COVID-19 Vaccination Advisory Committee. “All of us should do our part to reduce the spread of COVID-19 and get vaccinated to protect those around us from preventable suffering.”
A version of this article first appeared on Medscape.com.
Experts at the National Comprehensive Cancer Network have now issued an updated recommendation for COVID-19 vaccination in people with cancer. The panel calls for these patients to be among the highest-priority group to be vaccinated against COVID-19 and to receive the newly approved third dose of vaccine.
The NCCN has recommended in February that all patients receiving active cancer treatment should receive a COVID-19 vaccine and should be prioritized for vaccination. In August, the FDA authorized a third dose of either the Pfizer or Moderna COVID-19 vaccines for people with compromised immune systems. Those eligible for a third dose include solid organ transplant recipients, those undergoing cancer treatments, and people with autoimmune diseases that suppress their immune systems
The new NCCN recommendations state that the following groups should be considered eligible for a third dose of the mRNA COVID-19 vaccine immediately, based on the latest decisions from the Food and Drug Administration and the Centers for Disease Control and Prevention:
- Patients with solid tumors (either new or recurring) receiving treatment within 1 year of their initial vaccine dose, regardless of their type of cancer therapy.
- Patients with active hematologic malignancies regardless of whether they are currently receiving cancer therapy.
- Anyone who received a stem cell transplant (SCT) or engineered cellular therapy (for example, chimeric antigen receptor T cells), especially within the past 2 years.
- Any recipients of allogeneic SCT on immunosuppressive therapy or with a history of graft-versus-host disease regardless of the time of transplant.
- Anyone with an additional immunosuppressive condition (for example, HIV) or being treated with immunosuppressive agents unrelated to their cancer therapy.
Cancer patients at high risk of complications
As previously reported by this news organization, infection with COVID-19 in people with cancer can severely impact survival. One study published in 2020 found that patients with both COVID-19 infection and progressing cancer had a fivefold increase in the risk of 30-day mortality, compared with COVID-19–positive cancer patients who were in remission or had no evidence of cancer.
Another study found that cancer type, stage, and recent treatment could affect outcomes of COVID-19 in patients with cancer. Patients with hematologic malignancies and metastatic cancers had higher risks of developing severe or critical COVID-19 symptoms, being admitted to the ICU, requiring ventilation, and dying. Conversely, those with nonmetastatic disease had outcomes that were comparable with persons without cancer and a COVID-19 infection. This study also found that having undergone recent surgery or receiving immunotherapy also put patients at a higher risk of poor outcomes, although patients with cancer who were treated with radiotherapy had outcomes similar to those of noncancer COVID-19 patients.
“COVID-19 can be very dangerous, especially for people living with cancer, which is why we’re so grateful for safe and effective vaccines that are saving lives,” Robert W. Carlson, MD, CEO of NCCN, said in a statement.
Right timing and location
The current NCCN update also recommends that individuals wait at least 4 weeks between the second and third doses, and those who are infected with COVID-19 after being vaccinated should wait until they have documented clearance of the virus before receiving a third dose.
It also recommends that people who live in the same household with immunocompromised individuals should also get a third dose once it becomes available, and that it is best to have a third dose of the same type of vaccine as the first two doses. However, a different mRNA vaccine is also acceptable.
Immunocompromised individuals should try to receive their third dose in a health care delivery setting, as opposed to a pharmacy or public vaccination clinic if possible, as it would limit their risk of exposure to the general population.
Steve Pergam, MD, MPH, associate professor, vaccine and infectious disease division, Fred Hutchinson Cancer Research Center, Seattle, commented that it is still necessary to take precautions, even after getting the booster dose.
“That means, even after a third dose of vaccine, we still recommend immunocompromised people, such as those undergoing cancer treatment, continue to be cautious, wear masks, and avoid large group gatherings, particularly around those who are unvaccinated,” said Dr. Pergam, who is also coleader of the NCCN COVID-19 Vaccination Advisory Committee. “All of us should do our part to reduce the spread of COVID-19 and get vaccinated to protect those around us from preventable suffering.”
A version of this article first appeared on Medscape.com.
Lesions on the Thigh After an Organ Transplant
The Diagnosis: Microcystic Lymphatic Malformation
The shave biopsy demonstrated numerous thin-walled vascular spaces filled with lymphatic fluid within the dermis (Figure), consistent with a diagnosis of microcystic lymphatic malformation (LM). Lymphatic malformations represent a class of benign vascular lesions consisting of anomalous or dilated lymphatic vessels, which can be broadly categorized as macrocystic (formerly cavernous lymphangioma or cystic hygroma), microcystic (formerly lymphangioma circumscriptum), or mixed.1 Patients often will present with pruritus, crusting, secondary infection, edema, or oozing.2 The superficial blebs of microcystic LMs resemble frog spawn and range in color from clear to pink, brawny, or deep maroon.3 Although the lymphatic vessels involved in microcystic LMs appear disconnected from the major lymphatic circulation,3 systemic fluid overload could plausibly promote lesional swelling and tenderness; we attributed our patient's worsening symptoms to the cumulative 7.8 L of intravenous fluid he received intraoperatively during his cardiac transplant. The excess fluid allowed communication between lymphatic cisterns and thin-walled vesicles on the skin surface through dilated channels. Overall, LMs represent roughly 26% of pediatric benign vascular tumors and approximately 4% of all vascular tumors.4
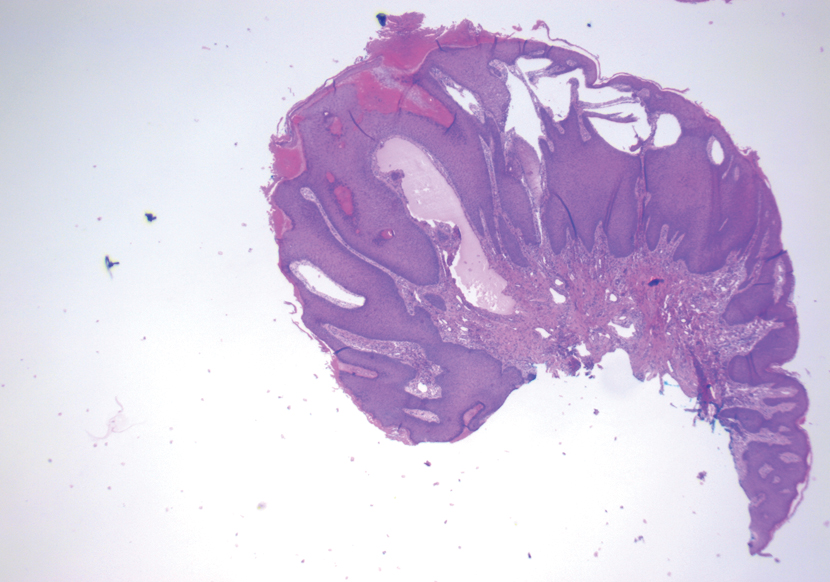
Although microcystic LMs may appear especially vascular or verrucous, the differential diagnosis for our patient's LM included condyloma acuminatum,5,6 condyloma lata,7 epidermal nevus, and lymphangiosarcoma. Epidermal nevi are congenital lesions, varying in appearance from velvety to verrucous patches and plaques that often evolve during puberty and become thicker, more verrucous, and hyperpigmented. Keratinocytic epidermal nevus syndromes and other entities such as nevus sebaceous have been associated with somatic mutations affecting proteins in the fibroblast growth factor receptor signaling pathway (eg, FGFR3, HRAS).8 Although the clinical appearance alone may be similar, lymphangiosarcoma can be distinguished from LM via biopsy.
There are several methods to diagnose LM. Duplex sonography is possibly the best noninvasive method to identify the flow between venous valves. Magnetic resonance imaging can detect larger occurrences of LM, and lymphangiography can be utilized to confirm a normal or abnormal lymphatic network.4 Treatment options are broad, including surgical excision, laser ablation, and topical sirolimus. Hypertonic saline sclerotherapy can be injected into the afflicted lymphatic channels to decrease inflammation, erythema, and hyperpigmentation without further treatment or major side effects.4
However, the benefits of sclerotherapy alone in the treatment of LM often come gradually, and radiofrequency ablation may need to be utilized to achieve more immediate results.2 Overall, outcomes are highly variable, but favorable outcomes often can be difficult to obtain due to a high recurrence rate.2,8 Our patient's symptoms improved during his postoperative recovery, and he declined further intervention.
- Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23:178-185. doi:10.1053/j.sempedsurg.2014.07.002
- Niti K, Manish P. Microcystic lymphatic malformation (lymphangioma circumscriptum) treated using a minimally invasive technique of radiofrequency ablation and sclerotherapy. Dermatol Surg. 2010;36:1711-1717. doi:10.1111/j.1524-4725.2010.01723.x
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111 /j.1365-4632.2009.04226.x
- Bikowski JB, Dumont AM. Lymphangioma circumscriptum: treatment with hypertonic saline sclerotherapy. J Am Acad Dermatol. 2005;53:442-444. doi:10.1016/j.jaad.2005.04.086
- Costa-Silva M, Fernandes I, Rodrigues AG, et al. Anogenital warts in pediatric population. An Bras Dermatol. 2017;92:675-681. doi:10.1590 /abd1806-4841.201756411
- Darmstadt GL. Perianal lymphangioma circumscriptum mistaken for genital warts. Pediatrics 1996;98;461.
- Bruins FG, van Deudekom FJA, de Vries HJC. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259. doi:10.1136 /bmj.h1259
- Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi:10.1111 /pde.13273
The Diagnosis: Microcystic Lymphatic Malformation
The shave biopsy demonstrated numerous thin-walled vascular spaces filled with lymphatic fluid within the dermis (Figure), consistent with a diagnosis of microcystic lymphatic malformation (LM). Lymphatic malformations represent a class of benign vascular lesions consisting of anomalous or dilated lymphatic vessels, which can be broadly categorized as macrocystic (formerly cavernous lymphangioma or cystic hygroma), microcystic (formerly lymphangioma circumscriptum), or mixed.1 Patients often will present with pruritus, crusting, secondary infection, edema, or oozing.2 The superficial blebs of microcystic LMs resemble frog spawn and range in color from clear to pink, brawny, or deep maroon.3 Although the lymphatic vessels involved in microcystic LMs appear disconnected from the major lymphatic circulation,3 systemic fluid overload could plausibly promote lesional swelling and tenderness; we attributed our patient's worsening symptoms to the cumulative 7.8 L of intravenous fluid he received intraoperatively during his cardiac transplant. The excess fluid allowed communication between lymphatic cisterns and thin-walled vesicles on the skin surface through dilated channels. Overall, LMs represent roughly 26% of pediatric benign vascular tumors and approximately 4% of all vascular tumors.4

Although microcystic LMs may appear especially vascular or verrucous, the differential diagnosis for our patient's LM included condyloma acuminatum,5,6 condyloma lata,7 epidermal nevus, and lymphangiosarcoma. Epidermal nevi are congenital lesions, varying in appearance from velvety to verrucous patches and plaques that often evolve during puberty and become thicker, more verrucous, and hyperpigmented. Keratinocytic epidermal nevus syndromes and other entities such as nevus sebaceous have been associated with somatic mutations affecting proteins in the fibroblast growth factor receptor signaling pathway (eg, FGFR3, HRAS).8 Although the clinical appearance alone may be similar, lymphangiosarcoma can be distinguished from LM via biopsy.
There are several methods to diagnose LM. Duplex sonography is possibly the best noninvasive method to identify the flow between venous valves. Magnetic resonance imaging can detect larger occurrences of LM, and lymphangiography can be utilized to confirm a normal or abnormal lymphatic network.4 Treatment options are broad, including surgical excision, laser ablation, and topical sirolimus. Hypertonic saline sclerotherapy can be injected into the afflicted lymphatic channels to decrease inflammation, erythema, and hyperpigmentation without further treatment or major side effects.4
However, the benefits of sclerotherapy alone in the treatment of LM often come gradually, and radiofrequency ablation may need to be utilized to achieve more immediate results.2 Overall, outcomes are highly variable, but favorable outcomes often can be difficult to obtain due to a high recurrence rate.2,8 Our patient's symptoms improved during his postoperative recovery, and he declined further intervention.
The Diagnosis: Microcystic Lymphatic Malformation
The shave biopsy demonstrated numerous thin-walled vascular spaces filled with lymphatic fluid within the dermis (Figure), consistent with a diagnosis of microcystic lymphatic malformation (LM). Lymphatic malformations represent a class of benign vascular lesions consisting of anomalous or dilated lymphatic vessels, which can be broadly categorized as macrocystic (formerly cavernous lymphangioma or cystic hygroma), microcystic (formerly lymphangioma circumscriptum), or mixed.1 Patients often will present with pruritus, crusting, secondary infection, edema, or oozing.2 The superficial blebs of microcystic LMs resemble frog spawn and range in color from clear to pink, brawny, or deep maroon.3 Although the lymphatic vessels involved in microcystic LMs appear disconnected from the major lymphatic circulation,3 systemic fluid overload could plausibly promote lesional swelling and tenderness; we attributed our patient's worsening symptoms to the cumulative 7.8 L of intravenous fluid he received intraoperatively during his cardiac transplant. The excess fluid allowed communication between lymphatic cisterns and thin-walled vesicles on the skin surface through dilated channels. Overall, LMs represent roughly 26% of pediatric benign vascular tumors and approximately 4% of all vascular tumors.4

Although microcystic LMs may appear especially vascular or verrucous, the differential diagnosis for our patient's LM included condyloma acuminatum,5,6 condyloma lata,7 epidermal nevus, and lymphangiosarcoma. Epidermal nevi are congenital lesions, varying in appearance from velvety to verrucous patches and plaques that often evolve during puberty and become thicker, more verrucous, and hyperpigmented. Keratinocytic epidermal nevus syndromes and other entities such as nevus sebaceous have been associated with somatic mutations affecting proteins in the fibroblast growth factor receptor signaling pathway (eg, FGFR3, HRAS).8 Although the clinical appearance alone may be similar, lymphangiosarcoma can be distinguished from LM via biopsy.
There are several methods to diagnose LM. Duplex sonography is possibly the best noninvasive method to identify the flow between venous valves. Magnetic resonance imaging can detect larger occurrences of LM, and lymphangiography can be utilized to confirm a normal or abnormal lymphatic network.4 Treatment options are broad, including surgical excision, laser ablation, and topical sirolimus. Hypertonic saline sclerotherapy can be injected into the afflicted lymphatic channels to decrease inflammation, erythema, and hyperpigmentation without further treatment or major side effects.4
However, the benefits of sclerotherapy alone in the treatment of LM often come gradually, and radiofrequency ablation may need to be utilized to achieve more immediate results.2 Overall, outcomes are highly variable, but favorable outcomes often can be difficult to obtain due to a high recurrence rate.2,8 Our patient's symptoms improved during his postoperative recovery, and he declined further intervention.
- Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23:178-185. doi:10.1053/j.sempedsurg.2014.07.002
- Niti K, Manish P. Microcystic lymphatic malformation (lymphangioma circumscriptum) treated using a minimally invasive technique of radiofrequency ablation and sclerotherapy. Dermatol Surg. 2010;36:1711-1717. doi:10.1111/j.1524-4725.2010.01723.x
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111 /j.1365-4632.2009.04226.x
- Bikowski JB, Dumont AM. Lymphangioma circumscriptum: treatment with hypertonic saline sclerotherapy. J Am Acad Dermatol. 2005;53:442-444. doi:10.1016/j.jaad.2005.04.086
- Costa-Silva M, Fernandes I, Rodrigues AG, et al. Anogenital warts in pediatric population. An Bras Dermatol. 2017;92:675-681. doi:10.1590 /abd1806-4841.201756411
- Darmstadt GL. Perianal lymphangioma circumscriptum mistaken for genital warts. Pediatrics 1996;98;461.
- Bruins FG, van Deudekom FJA, de Vries HJC. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259. doi:10.1136 /bmj.h1259
- Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi:10.1111 /pde.13273
- Elluru RG, Balakrishnan K, Padua HM. Lymphatic malformations: diagnosis and management. Semin Pediatr Surg. 2014;23:178-185. doi:10.1053/j.sempedsurg.2014.07.002
- Niti K, Manish P. Microcystic lymphatic malformation (lymphangioma circumscriptum) treated using a minimally invasive technique of radiofrequency ablation and sclerotherapy. Dermatol Surg. 2010;36:1711-1717. doi:10.1111/j.1524-4725.2010.01723.x
- Patel GA, Schwartz RA. Cutaneous lymphangioma circumscriptum: frog spawn on the skin. Int J Dermatol. 2009;48:1290-1295. doi:10.1111 /j.1365-4632.2009.04226.x
- Bikowski JB, Dumont AM. Lymphangioma circumscriptum: treatment with hypertonic saline sclerotherapy. J Am Acad Dermatol. 2005;53:442-444. doi:10.1016/j.jaad.2005.04.086
- Costa-Silva M, Fernandes I, Rodrigues AG, et al. Anogenital warts in pediatric population. An Bras Dermatol. 2017;92:675-681. doi:10.1590 /abd1806-4841.201756411
- Darmstadt GL. Perianal lymphangioma circumscriptum mistaken for genital warts. Pediatrics 1996;98;461.
- Bruins FG, van Deudekom FJA, de Vries HJC. Syphilitic condylomata lata mimicking anogenital warts. BMJ. 2015;350:h1259. doi:10.1136 /bmj.h1259
- Asch S, Sugarman JL. Epidermal nevus syndromes: new insights into whorls and swirls. Pediatr Dermatol. 2018;35:21-29. doi:10.1111 /pde.13273
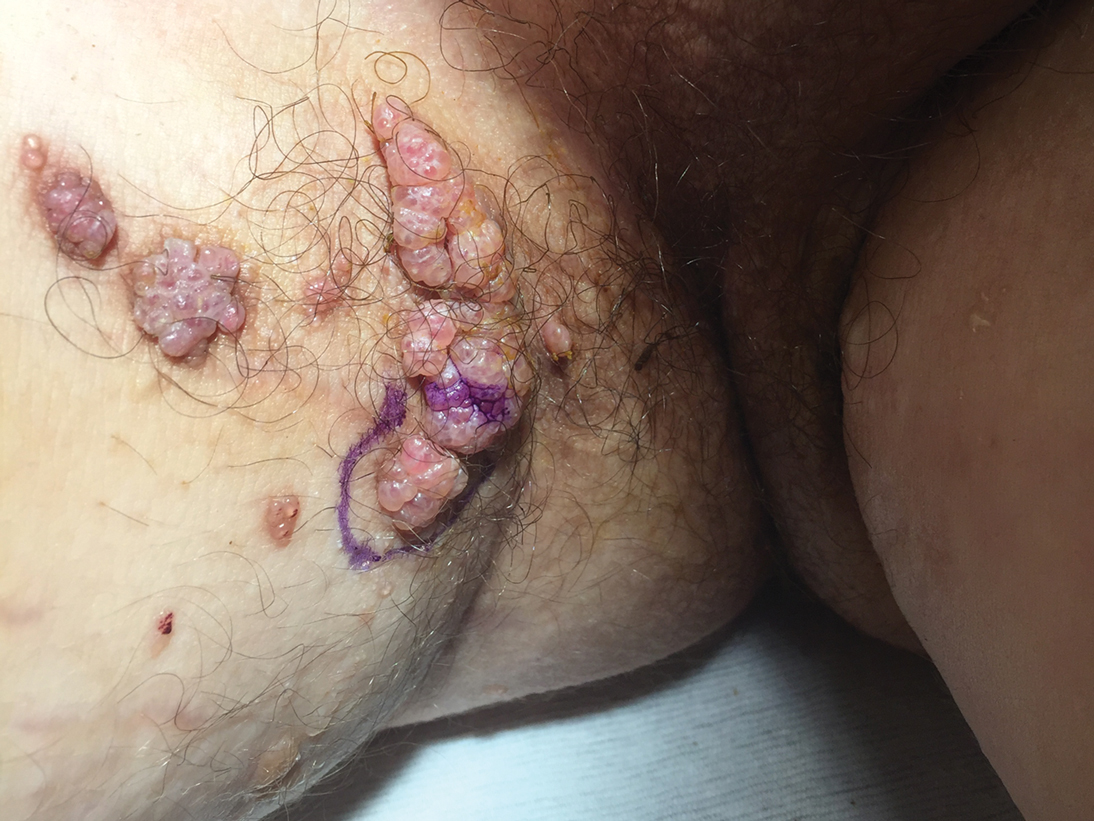
A 17-year-old adolescent boy presented with increasingly painful genital warts on the right thigh, groin, and scrotum that had been present since birth. The patient had a medical history of cardiac transplantation in the months prior to presentation and was on immunosuppressive therapy. The lesions had become more swollen and bothersome in the weeks following the transplantation and now prevented him from ambulating due to discomfort. He denied any history of sexual contact or oral lesions. Physical examination revealed numerous translucent and hemorrhagic vesicles clustered and linearly distributed on the right medial thigh. A shave biopsy of a vesicle was performed.
Clinical Edge Journal Scan Commentary: AML September 2021
Of the 50 patients enrolled, 90%, 8% and 2% had newly diagnosed AML, myelodysplastic syndrome and mixed phenotype acute leukemia respectively. Overall, 94% (95% confidence interval [CI], 83%-98%) had a composite complete response and 82% (95% CI, 68%-92%) achieved measurable residual disease negativity. At 12 months, the rates of duration of response, overall survival, and event-free survival were 74% (95% CI, 60%-92%), 85% (95% CI, 75%-97%), and 68% (95% CI, 54%-85%), respectively. The most common grade 3 or worse adverse events were febrile neutropenia (84%), infection (12%), and alanine aminotransferase elevations (12%). Only one patient had a p53 mutation and that patient did not respond. Another study by Alwash et al, demonstrated that 15% of patients acquire a TP53 mutation during AML therapy.
The poor prognosis of patients with a p53 ,mutation was also seen in a retrospective study of patients with newly diagnosed or relapsed/refractory AML treated with 10-day decitabine + venetoclax (DEC10-VEN). Of the 118 patients, 35 had a TP53 mutation. Complete remission/complete remission with incomplete count recovery (57% vs 775), and overall response was better for patients without vs with a TP53 mutation. In addition, overall survival was dismal (5.2 months) for patients with TP53 mutation vs those without (19.4 months).
This study reiterates the need for newer therapies for this group of patients with a TP53 mutation (Kim K et al). The overall outcome of patients treated with DEC10-VEN was better compared to intensive chemotherapy (IC) in patients with relapsed refractory AML. This was evaluated in a retrospective study assessing the outcomes of adult patients with R/R AML treated with DEC10-VEN (n=65) a vs IC-based regimen (n=130) using propensity score-matched analysis. Patients receiving DEC10-VEN vs IC had superior overall response rate (odds ratio [OR], 3.28; P < .001), minimal residual disease negativity (OR, 2.48; P = .017), event-free survival (hazard ratio [HR], 0.46; P < .001), and overall survival (HR, 0.56; P = .008). Rates of refractory disease (OR, 0.46; P = .011) and 60-day mortality (OR, 0.40; P = .029) were significantly lower in patients receiving DEC10-VEN vs IC.
Of the 50 patients enrolled, 90%, 8% and 2% had newly diagnosed AML, myelodysplastic syndrome and mixed phenotype acute leukemia respectively. Overall, 94% (95% confidence interval [CI], 83%-98%) had a composite complete response and 82% (95% CI, 68%-92%) achieved measurable residual disease negativity. At 12 months, the rates of duration of response, overall survival, and event-free survival were 74% (95% CI, 60%-92%), 85% (95% CI, 75%-97%), and 68% (95% CI, 54%-85%), respectively. The most common grade 3 or worse adverse events were febrile neutropenia (84%), infection (12%), and alanine aminotransferase elevations (12%). Only one patient had a p53 mutation and that patient did not respond. Another study by Alwash et al, demonstrated that 15% of patients acquire a TP53 mutation during AML therapy.
The poor prognosis of patients with a p53 ,mutation was also seen in a retrospective study of patients with newly diagnosed or relapsed/refractory AML treated with 10-day decitabine + venetoclax (DEC10-VEN). Of the 118 patients, 35 had a TP53 mutation. Complete remission/complete remission with incomplete count recovery (57% vs 775), and overall response was better for patients without vs with a TP53 mutation. In addition, overall survival was dismal (5.2 months) for patients with TP53 mutation vs those without (19.4 months).
This study reiterates the need for newer therapies for this group of patients with a TP53 mutation (Kim K et al). The overall outcome of patients treated with DEC10-VEN was better compared to intensive chemotherapy (IC) in patients with relapsed refractory AML. This was evaluated in a retrospective study assessing the outcomes of adult patients with R/R AML treated with DEC10-VEN (n=65) a vs IC-based regimen (n=130) using propensity score-matched analysis. Patients receiving DEC10-VEN vs IC had superior overall response rate (odds ratio [OR], 3.28; P < .001), minimal residual disease negativity (OR, 2.48; P = .017), event-free survival (hazard ratio [HR], 0.46; P < .001), and overall survival (HR, 0.56; P = .008). Rates of refractory disease (OR, 0.46; P = .011) and 60-day mortality (OR, 0.40; P = .029) were significantly lower in patients receiving DEC10-VEN vs IC.
Of the 50 patients enrolled, 90%, 8% and 2% had newly diagnosed AML, myelodysplastic syndrome and mixed phenotype acute leukemia respectively. Overall, 94% (95% confidence interval [CI], 83%-98%) had a composite complete response and 82% (95% CI, 68%-92%) achieved measurable residual disease negativity. At 12 months, the rates of duration of response, overall survival, and event-free survival were 74% (95% CI, 60%-92%), 85% (95% CI, 75%-97%), and 68% (95% CI, 54%-85%), respectively. The most common grade 3 or worse adverse events were febrile neutropenia (84%), infection (12%), and alanine aminotransferase elevations (12%). Only one patient had a p53 mutation and that patient did not respond. Another study by Alwash et al, demonstrated that 15% of patients acquire a TP53 mutation during AML therapy.
The poor prognosis of patients with a p53 ,mutation was also seen in a retrospective study of patients with newly diagnosed or relapsed/refractory AML treated with 10-day decitabine + venetoclax (DEC10-VEN). Of the 118 patients, 35 had a TP53 mutation. Complete remission/complete remission with incomplete count recovery (57% vs 775), and overall response was better for patients without vs with a TP53 mutation. In addition, overall survival was dismal (5.2 months) for patients with TP53 mutation vs those without (19.4 months).
This study reiterates the need for newer therapies for this group of patients with a TP53 mutation (Kim K et al). The overall outcome of patients treated with DEC10-VEN was better compared to intensive chemotherapy (IC) in patients with relapsed refractory AML. This was evaluated in a retrospective study assessing the outcomes of adult patients with R/R AML treated with DEC10-VEN (n=65) a vs IC-based regimen (n=130) using propensity score-matched analysis. Patients receiving DEC10-VEN vs IC had superior overall response rate (odds ratio [OR], 3.28; P < .001), minimal residual disease negativity (OR, 2.48; P = .017), event-free survival (hazard ratio [HR], 0.46; P < .001), and overall survival (HR, 0.56; P = .008). Rates of refractory disease (OR, 0.46; P = .011) and 60-day mortality (OR, 0.40; P = .029) were significantly lower in patients receiving DEC10-VEN vs IC.
Working without a net
My first hospital consult was also on my first day of practice, in July, 1998.
I was in a small room, subleased from an oncology group. My schedule, as first day schedules are, was sparse.
Around noon one of the oncology docs asked me to come to his exam room, so I went across the hall. There he had a lady in her late 50s, with known metastatic cancer. He’d brought her in for a few days of worsening headaches and diplopia, and my 10-second neurological exam showed dysconjugate gaze and dysarthria. He said he was admitting her to the hospital, and asked if I’d consult on her.
I hung out in the hospital’s MRI control room later that day, waiting for her images to come up. I was nervous, maybe even a little scared. In spite of having survived medical school, residency, and fellowship, I was worried I’d screwed up the case, somehow. If the MRI was normal, I’d look like an idiot. My career would be over, on day one. No one would ever consult me again.
Of course, the MRI showed a brainstem metastasis (in addition to other places), and my initial differential was correct. Good for me, terrible for the patient. I ordered Decadron, called the oncologist, spoke to the patient and her family, and went home. I followed her for maybe a another few days, mainly because I didn’t know what the protocol was for signing off.
Self-doubt is common in all fields, especially when starting out, but probably strongest in medicine. A lot depends on us getting the right answer – quickly – in cases like that one. In my case this was compounded by its being my first day of practice. There was no attending I could call for help. I was working without a net.
But the years of training paid off, I got the case right, and moved on. Twenty-three years later it seems silly that I was so worried. Nowadays I order the MRI, move to the next patient, and try not to think about it until the results come back or a nurse calls with a status change. If my initial impression is wrong, I move down the differential list.
But
It’s what makes us better doctors.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My first hospital consult was also on my first day of practice, in July, 1998.
I was in a small room, subleased from an oncology group. My schedule, as first day schedules are, was sparse.
Around noon one of the oncology docs asked me to come to his exam room, so I went across the hall. There he had a lady in her late 50s, with known metastatic cancer. He’d brought her in for a few days of worsening headaches and diplopia, and my 10-second neurological exam showed dysconjugate gaze and dysarthria. He said he was admitting her to the hospital, and asked if I’d consult on her.
I hung out in the hospital’s MRI control room later that day, waiting for her images to come up. I was nervous, maybe even a little scared. In spite of having survived medical school, residency, and fellowship, I was worried I’d screwed up the case, somehow. If the MRI was normal, I’d look like an idiot. My career would be over, on day one. No one would ever consult me again.
Of course, the MRI showed a brainstem metastasis (in addition to other places), and my initial differential was correct. Good for me, terrible for the patient. I ordered Decadron, called the oncologist, spoke to the patient and her family, and went home. I followed her for maybe a another few days, mainly because I didn’t know what the protocol was for signing off.
Self-doubt is common in all fields, especially when starting out, but probably strongest in medicine. A lot depends on us getting the right answer – quickly – in cases like that one. In my case this was compounded by its being my first day of practice. There was no attending I could call for help. I was working without a net.
But the years of training paid off, I got the case right, and moved on. Twenty-three years later it seems silly that I was so worried. Nowadays I order the MRI, move to the next patient, and try not to think about it until the results come back or a nurse calls with a status change. If my initial impression is wrong, I move down the differential list.
But
It’s what makes us better doctors.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
My first hospital consult was also on my first day of practice, in July, 1998.
I was in a small room, subleased from an oncology group. My schedule, as first day schedules are, was sparse.
Around noon one of the oncology docs asked me to come to his exam room, so I went across the hall. There he had a lady in her late 50s, with known metastatic cancer. He’d brought her in for a few days of worsening headaches and diplopia, and my 10-second neurological exam showed dysconjugate gaze and dysarthria. He said he was admitting her to the hospital, and asked if I’d consult on her.
I hung out in the hospital’s MRI control room later that day, waiting for her images to come up. I was nervous, maybe even a little scared. In spite of having survived medical school, residency, and fellowship, I was worried I’d screwed up the case, somehow. If the MRI was normal, I’d look like an idiot. My career would be over, on day one. No one would ever consult me again.
Of course, the MRI showed a brainstem metastasis (in addition to other places), and my initial differential was correct. Good for me, terrible for the patient. I ordered Decadron, called the oncologist, spoke to the patient and her family, and went home. I followed her for maybe a another few days, mainly because I didn’t know what the protocol was for signing off.
Self-doubt is common in all fields, especially when starting out, but probably strongest in medicine. A lot depends on us getting the right answer – quickly – in cases like that one. In my case this was compounded by its being my first day of practice. There was no attending I could call for help. I was working without a net.
But the years of training paid off, I got the case right, and moved on. Twenty-three years later it seems silly that I was so worried. Nowadays I order the MRI, move to the next patient, and try not to think about it until the results come back or a nurse calls with a status change. If my initial impression is wrong, I move down the differential list.
But
It’s what makes us better doctors.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The trauma and healing of 9/11 echo in COVID-19
The scope and magnitude of the Sept. 11, 2001, attacks on the World Trade Center and the Pentagon were unprecedented in U.S. history. It was arguably the most serious trauma to beset Americans on U.S. soil. The 20th anniversary of 9/11 will take place during another crisis, not only in American history but also in world history – the COVID-19 pandemic.

“As different as these two events are, there are obvious points of comparison,” Jonathan DePierro, PhD, assistant professor of psychiatry, Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Both were unprecedented life-threatening situations, presenting threats to individuals’ lives and profoundly traumatizing not only society as a whole but also first responders.”
Dr. DePierro, who is also the clinical director of the Center for Stress, Resilience, and Personal Growth at Mount Sinai, thinks there are many lessons to be learned from the mental health response to 9/11 that can inform our understanding of and response to the mental health needs of today’s first responders in the COVID-19 crisis, particularly health care professionals.
“Every one of our hospitals became a ‘ground zero’ early during the pandemic, and we see the numbers rising again and hospitals again overwhelmed, so he said.
Placing trauma within a new framework
According to Priscilla Dass-Brailsford, EdD, MPH, professor of psychology, department of psychiatry, Georgetown University, Washington, Sept. 11, 2001, “placed trauma within a new framework.”
“Prior to 9/11, crisis protocols and how to manage stress in the aftermath of violent events were uncommon,” Dr. Dass-Brailsford, a clinical psychologist with expertise in trauma who also chairs a clinical psychology program for the Chicago School of Professional Psychology, said in an interview.
As a first responder, she was involved in early interventions for survivors of 9/11. On Sept. 11, 2001, she had just resigned her position as coordinator of the community crisis response team – the first of its kind in the United States – through the Victims of Violence Program in Cambridge, in Cambridge, Mass.
The program responded to communities in which there were high rates of drive-by shootings and similar acts of violence. Because of her crisis experience, Dr. Dass-Brailsford was asked to conduct debriefings in Boston in the area where the 9/11 terrorists had stayed prior to boarding the planes that were used in the terrorist attacks. She subsequently went to New York City to conduct similar psychological debriefings with affected communities.
“What we’ve learned is that we had no crisis protocol on how to manage the stress in the aftermath of such a violent event, no standard operating procedures. There were very few people trained in crisis and trauma response at that time. Partially spurred by 9/11, trauma training programs became more prolific,” she said. Dr. Dass-Brailsford developed a trauma certification program at Lesley University in Cambridge, Mass., where she began to teach after 9/11. “I saw the importance of having clinicians trained to respond in a crisis, because responding to a crisis is very different from conducting regular mental health interventions.”
Short- and long-term interventions
Dr. DePierro said that Mount Sinai has a 20-year history of responding to the physical and mental health needs of 9/11 responders.
“We saw a number of first responders experiencing clinical depression, anxiety, a lot of worry, symptoms of posttraumatic stress disorder, and an increase in alcohol and/or substance use,” he recounted. In some, these responses were immediate; in others, the onset of symptoms was more gradual. Some responders had acute reactions that lasted for several months to a year, whereas for others, the reactions were prolonged, and they remained “chronically distressed long after the immediate exposure to the event,” he said.
Recent studies have shown that, during the COVID-19 pandemic, health care professionals and many essential workers have experienced similar symptoms, Dr. DePierro noted.
Mental health care professionals who provided interventions for workers involved in recovery and cleanup at the World Trade Center have highlighted the need for long-term monitoring of people on the front lines during the COVID-19 pandemic – especially health care workers, other essential personnel (for example, delivery, postal, and grocery store workers) and surviving family members. “Health monitoring and treatment efforts for 9/11 survivors and responders were put into place soon after the attacks and continue to this day,” using funding provided through the James Zadroga Act, Dr. DePierro said.
“Without similarly unified health registry and treatment services, many individuals – especially from underserved groups – will likely experience chronic mental health consequences and will be unable to access high-quality health care services,” he stated.
‘Psychological first aid’
“Although many people who go through a crisis – whether as a result of terrorism, such as 9/11, or a medical crisis, such as the current pandemic, or a natural disaster, such as Hurricane Katrina – experience PTSD, it’s important to note that not everyone who goes through a crisis and is traumatized will go on to develop PTSD,” Dr. Dass-Brailsford emphasized.
“To me, 9/11 placed psychological first aid on the map. Even if you are not a clinician, you can be trained to provide psychological first aid by becoming familiar with people’s reactions to trauma and how you can support them through it,” she continued.
For example, if a coworker is agitated or “seems to be having a meltdown, you can be there by offering support and getting them the appropriate help.” Research has suggested that having social support before and after a traumatic event can be helpful in determining vulnerability to the development of PTSD and in modulating the impact of the trauma.
Psychological first aid is helpful as an interim measure. “If you see a coworker holding their head in their hands all day and staring at the screen, identifying whether the person might be having a dissociative episode is critical. Providing some support is important, but if more intensive professional support is needed, determining that and making a referral becomes key,” Dr. Dass-Brailsford stated.
Dr. DePierro added: “One of the most important messages that I want health care workers to know from my years of working with 9/11 survivors is that feeling distressed after a traumatic event is very common, but with effective care, one doesn’t necessarily need to be in treatment for years.”
Danielle Ofri, MD, PhD, clinical professor, department of medicine, New York University, agreed. “It is important to continue keeping tabs on each other and remaining sensitive to the collateral struggles of our colleagues. Some have children who are struggling in school, others have parents who have lost a job. Continuing to check in on others and offer support is critical going forward,” she said in an interview.
Cohesiveness and volunteerism
One of the most powerful antidotes to long-term traumatization is a sense of community cohesiveness. This was the case following 9/11, and it is the case during the COVID-19 pandemic, according to Dr. Ofri, an internist at Bellevue Hospital in New York.
“There was an enormous mobilization. Bellevue is a city hospital with a level 1 trauma center, and we expected to be swamped, so the whole hospital shifted into gear,” said Dr. Ofri. “What would have been terrifying seemed tolerable because we felt that we were in it together. We discharged the inpatients to make beds available. Within hours, we had converted clinics into emergency departments and ICUs. We worked seamlessly, and the crisis brought us together ... but then, of course, no patients showed up.”
She described her relationship with her colleagues as “feeling almost like a family, especially during the pandemic, when so many others were in lockdown and feeling isolated and useless.”
She and her colleagues saw each other daily. Although the content of their tasks and responsibilities changed and people were redeployed to other areas, “our workday didn’t really change. It would have been overwhelming if we hadn’t had our daily meetings to regroup and assess where we were. Each day, everything we had learned or implemented the day before – treatment protocols, testing protocols, our understanding of how the virus was communicated – would change and need to be reevaluated. Those morning meetings were critical to staying centered. It felt as though we were building a plane and flying it at the same time, which felt both scary and heady. Luckily, it took place within the fraternity of a committed and caring group.”
Dr. Ofri recounted that, after 9/11, as well as during the pandemic, “professionals kept jumping in from the sidelines to volunteer. Within hours of the collapse of the towers, the ED had filled with staff. People came out of retirement and out from vacation and out of the woodwork. It was very heartening.”
Even more inspiring, “all the departmental barriers seemed to break down. People were willing to step out of their ordinary roles and check their egos at the door. Seasoned physicians were willing to function as medical interns.”
This generosity of time and spirit “helped keep us going,” she said.
Dr. DePierro agreed. “One of the things I’ve seen on medical floors is that COVID actually brought some units together, increasing their cohesion and mutual support and increasing the bonds between people.” These intensified bonds “increased the resilience of everyone involved.”
Commitment to the community
Dr. Ofri recalls families gathering at the hospital after 9/11, watching posters of missing people going up all over the hospital as well as on mailboxes and lampposts. Because the center for missing people was located right next door to Bellevue, there were long lines of families coming in to register. The chief medical office was there, and a huge tent was built to accommodate the families. The tent took up the entire block. “We felt a lot of ownership, because families were coming here,” she said.
The street remained closed even as the days, weeks, and years stretched on, and the tent remained. It was used as a reflection area for families. During the pandemic, that area was used for refrigerated trucks that served as temporary morgues.
“Both logistically and emotionally, we had a feeling during the pandemic of, ‘We’ve been here before, we’ll do it again and be there for the community,’ ” Dr. Ofri said.
She noted that the sense of commitment to the community carried her and fellow clinicians through the toughest parts of 9/11 and of the COVID-19 pandemic.
“People look to the medical system as a lodestar. ‘Where’s my family member? What should I do? Should I be tested? Vaccinated?’ We were there to be a steady presence for the community physically, psychologically, emotionally, and medically, which helped center us as well,” Dr. Ofri said. “If we didn’t have that, we might have all given in to existential panic.”
She added: “Although we had to work twice as hard, often amid great personal risk, we had the good fortune of having a sense of purpose, something to contribute, plus the community of colleagues we cared about and trusted with our lives.”
Crisis and personal growth
Dr. DePierro said that participants who went through 9/11 have been coming to Mount Sinai’s World Trade Center Health Program for care for nearly two decades. “Many are doing quite well, despite the emotional trauma and the dust and toxin exposure, which has given us a window into what makes people resilient.”
Social and community support are key factors in resilience. Another is recognizing opportunities for personal or professional growth during the crisis, according to Dr. DePierro.
During the pandemic, hospital staff were redeployed to departments where they didn’t typically work. They worked with new colleagues and used skills in patient care that they hadn’t needed for years or even decades. “Although this was stressful and distressing, quite a number said they came through with more medical knowledge than before and that they had forged relationships in the trenches that have been lasting and have become important to them,” he reported.
He noted that, during both crises, for first responders and health care practitioners, religious or spiritual faith was a source of resilience. “During the peak of the pandemic, chaplains provided an exorbitant amount of staff support as clinicians turned to the chaplain to help make sense of what they were going through and connect to something greater than themselves.” Similarly, during 9/11, police and fire department chaplains “played a huge role in supporting the first responders,” Dr. DePierro said.
He said that Mount Sinai holds resilience workshops “where we focus on these topics and teach health care workers how to build resilience in their lives, heal day-to-day stressors, and even grow from the experience.”
Dr. Ofri, who is the founder and editor-in-chief of the Bellevue Literary Review, added that the arts played an important role in bolstering resilience and providing a creative outlet for clinicians after 9/11 and again during the pandemic.
The publication is celebrating its twentieth anniversary – its first issue went to press in September 2001. The cover contained an acknowledgment of 9/11.
Dr. Ofri said that a gala event had been planned for Oct. 7, 2001, to celebrate the inaugural issue of the publication. She assumed no one would show up, given that the United States had invaded Afghanistan only hours earlier. To her surprise, over a hundred people attended, “which made me realize the role of the arts during trauma. People were seeking to come together and hear poetry, fiction, and creative nonfiction.”
Dr. Ofri has been “impressed by the amount of incredible creative writing of all sorts that has been submitted [to the publication] during the pandemic, an unexpected flowering of the arts.”
Unique challenges, unique opportunities
All three experts pointed to several noteworthy differences between the experiences of first responders following 9/11 and those of today’s health care professionals during the pandemic.
“What happened on Sept. 11 was one discrete event, and although it obviously led to years of recovering body parts and cleaning up Ground Zero, and on a national level it led to a war, it nevertheless was a single event,” Dr. DePierro observed. By contrast, the COVID-19 pandemic is ongoing, and for health care practitioners, “it’s by no means over. Again and again, they are being thrown back into battle, dealing with fatigue, weariness, and loss of life.”
Moreover, “it is my understanding that immediately following 9/11, there was a general coming together in our country, but it’s obvious that today, there’s a great deal of fractiousness, contention, disagreement, and disunity in our country when it comes to COVID-19,” Dr. DePierro continued.
“This takes a great toll, particularly on health care workers who are dealing with COVID-19 on a daily basis and experience a disconnect between what they see on their floors and ICUs of the hospital, experiencing loss of life they’ve likely never encountered in their careers, and what people are saying when they downplay the seriousness of COVID-19,” he said.
Dr. Ofri agreed. “The fragmentation of our country and the failure of leadership at the highest level to provide even the basics, such as PPE [personal protective equipment] for health care professionals, left us baffled, profoundly hurt, and angry.”
A positive difference between the COVID-19 pandemic and the aftermath of 9/11 is the development of sophisticated technology that allows interventions for traumatized individuals – both health care professionals and the general public – through telehealth, Dr. DePierro pointed out.
“I would say that these resources and technologies are a silver lining and should continue to be expanded on,” he said. “Now, busy health care workers can access all manner of supportive services, including teletherapy, right from home or between shifts.”
Another “silver lining” is that the pandemic has shone a spotlight on an issue that predated the pandemic – the mental health of health care professionals. Opening a discussion about this has reduced stigma and hopefully has paved the way for improved treatments and for providing resources.
Dr. Dass-Brailsford added that “it is important, going forward, for all of us to be trauma informed, to know how trauma and trauma-related stress unfolds in both other people and yourself, and to know what coping skills can be used to avoid crises from developing – a task that extends across all types of disasters.”
A version of this article first appeared on Medscape.com.
The scope and magnitude of the Sept. 11, 2001, attacks on the World Trade Center and the Pentagon were unprecedented in U.S. history. It was arguably the most serious trauma to beset Americans on U.S. soil. The 20th anniversary of 9/11 will take place during another crisis, not only in American history but also in world history – the COVID-19 pandemic.

“As different as these two events are, there are obvious points of comparison,” Jonathan DePierro, PhD, assistant professor of psychiatry, Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Both were unprecedented life-threatening situations, presenting threats to individuals’ lives and profoundly traumatizing not only society as a whole but also first responders.”
Dr. DePierro, who is also the clinical director of the Center for Stress, Resilience, and Personal Growth at Mount Sinai, thinks there are many lessons to be learned from the mental health response to 9/11 that can inform our understanding of and response to the mental health needs of today’s first responders in the COVID-19 crisis, particularly health care professionals.
“Every one of our hospitals became a ‘ground zero’ early during the pandemic, and we see the numbers rising again and hospitals again overwhelmed, so he said.
Placing trauma within a new framework
According to Priscilla Dass-Brailsford, EdD, MPH, professor of psychology, department of psychiatry, Georgetown University, Washington, Sept. 11, 2001, “placed trauma within a new framework.”
“Prior to 9/11, crisis protocols and how to manage stress in the aftermath of violent events were uncommon,” Dr. Dass-Brailsford, a clinical psychologist with expertise in trauma who also chairs a clinical psychology program for the Chicago School of Professional Psychology, said in an interview.
As a first responder, she was involved in early interventions for survivors of 9/11. On Sept. 11, 2001, she had just resigned her position as coordinator of the community crisis response team – the first of its kind in the United States – through the Victims of Violence Program in Cambridge, in Cambridge, Mass.
The program responded to communities in which there were high rates of drive-by shootings and similar acts of violence. Because of her crisis experience, Dr. Dass-Brailsford was asked to conduct debriefings in Boston in the area where the 9/11 terrorists had stayed prior to boarding the planes that were used in the terrorist attacks. She subsequently went to New York City to conduct similar psychological debriefings with affected communities.
“What we’ve learned is that we had no crisis protocol on how to manage the stress in the aftermath of such a violent event, no standard operating procedures. There were very few people trained in crisis and trauma response at that time. Partially spurred by 9/11, trauma training programs became more prolific,” she said. Dr. Dass-Brailsford developed a trauma certification program at Lesley University in Cambridge, Mass., where she began to teach after 9/11. “I saw the importance of having clinicians trained to respond in a crisis, because responding to a crisis is very different from conducting regular mental health interventions.”
Short- and long-term interventions
Dr. DePierro said that Mount Sinai has a 20-year history of responding to the physical and mental health needs of 9/11 responders.
“We saw a number of first responders experiencing clinical depression, anxiety, a lot of worry, symptoms of posttraumatic stress disorder, and an increase in alcohol and/or substance use,” he recounted. In some, these responses were immediate; in others, the onset of symptoms was more gradual. Some responders had acute reactions that lasted for several months to a year, whereas for others, the reactions were prolonged, and they remained “chronically distressed long after the immediate exposure to the event,” he said.
Recent studies have shown that, during the COVID-19 pandemic, health care professionals and many essential workers have experienced similar symptoms, Dr. DePierro noted.
Mental health care professionals who provided interventions for workers involved in recovery and cleanup at the World Trade Center have highlighted the need for long-term monitoring of people on the front lines during the COVID-19 pandemic – especially health care workers, other essential personnel (for example, delivery, postal, and grocery store workers) and surviving family members. “Health monitoring and treatment efforts for 9/11 survivors and responders were put into place soon after the attacks and continue to this day,” using funding provided through the James Zadroga Act, Dr. DePierro said.
“Without similarly unified health registry and treatment services, many individuals – especially from underserved groups – will likely experience chronic mental health consequences and will be unable to access high-quality health care services,” he stated.
‘Psychological first aid’
“Although many people who go through a crisis – whether as a result of terrorism, such as 9/11, or a medical crisis, such as the current pandemic, or a natural disaster, such as Hurricane Katrina – experience PTSD, it’s important to note that not everyone who goes through a crisis and is traumatized will go on to develop PTSD,” Dr. Dass-Brailsford emphasized.
“To me, 9/11 placed psychological first aid on the map. Even if you are not a clinician, you can be trained to provide psychological first aid by becoming familiar with people’s reactions to trauma and how you can support them through it,” she continued.
For example, if a coworker is agitated or “seems to be having a meltdown, you can be there by offering support and getting them the appropriate help.” Research has suggested that having social support before and after a traumatic event can be helpful in determining vulnerability to the development of PTSD and in modulating the impact of the trauma.
Psychological first aid is helpful as an interim measure. “If you see a coworker holding their head in their hands all day and staring at the screen, identifying whether the person might be having a dissociative episode is critical. Providing some support is important, but if more intensive professional support is needed, determining that and making a referral becomes key,” Dr. Dass-Brailsford stated.
Dr. DePierro added: “One of the most important messages that I want health care workers to know from my years of working with 9/11 survivors is that feeling distressed after a traumatic event is very common, but with effective care, one doesn’t necessarily need to be in treatment for years.”
Danielle Ofri, MD, PhD, clinical professor, department of medicine, New York University, agreed. “It is important to continue keeping tabs on each other and remaining sensitive to the collateral struggles of our colleagues. Some have children who are struggling in school, others have parents who have lost a job. Continuing to check in on others and offer support is critical going forward,” she said in an interview.
Cohesiveness and volunteerism
One of the most powerful antidotes to long-term traumatization is a sense of community cohesiveness. This was the case following 9/11, and it is the case during the COVID-19 pandemic, according to Dr. Ofri, an internist at Bellevue Hospital in New York.
“There was an enormous mobilization. Bellevue is a city hospital with a level 1 trauma center, and we expected to be swamped, so the whole hospital shifted into gear,” said Dr. Ofri. “What would have been terrifying seemed tolerable because we felt that we were in it together. We discharged the inpatients to make beds available. Within hours, we had converted clinics into emergency departments and ICUs. We worked seamlessly, and the crisis brought us together ... but then, of course, no patients showed up.”
She described her relationship with her colleagues as “feeling almost like a family, especially during the pandemic, when so many others were in lockdown and feeling isolated and useless.”
She and her colleagues saw each other daily. Although the content of their tasks and responsibilities changed and people were redeployed to other areas, “our workday didn’t really change. It would have been overwhelming if we hadn’t had our daily meetings to regroup and assess where we were. Each day, everything we had learned or implemented the day before – treatment protocols, testing protocols, our understanding of how the virus was communicated – would change and need to be reevaluated. Those morning meetings were critical to staying centered. It felt as though we were building a plane and flying it at the same time, which felt both scary and heady. Luckily, it took place within the fraternity of a committed and caring group.”
Dr. Ofri recounted that, after 9/11, as well as during the pandemic, “professionals kept jumping in from the sidelines to volunteer. Within hours of the collapse of the towers, the ED had filled with staff. People came out of retirement and out from vacation and out of the woodwork. It was very heartening.”
Even more inspiring, “all the departmental barriers seemed to break down. People were willing to step out of their ordinary roles and check their egos at the door. Seasoned physicians were willing to function as medical interns.”
This generosity of time and spirit “helped keep us going,” she said.
Dr. DePierro agreed. “One of the things I’ve seen on medical floors is that COVID actually brought some units together, increasing their cohesion and mutual support and increasing the bonds between people.” These intensified bonds “increased the resilience of everyone involved.”
Commitment to the community
Dr. Ofri recalls families gathering at the hospital after 9/11, watching posters of missing people going up all over the hospital as well as on mailboxes and lampposts. Because the center for missing people was located right next door to Bellevue, there were long lines of families coming in to register. The chief medical office was there, and a huge tent was built to accommodate the families. The tent took up the entire block. “We felt a lot of ownership, because families were coming here,” she said.
The street remained closed even as the days, weeks, and years stretched on, and the tent remained. It was used as a reflection area for families. During the pandemic, that area was used for refrigerated trucks that served as temporary morgues.
“Both logistically and emotionally, we had a feeling during the pandemic of, ‘We’ve been here before, we’ll do it again and be there for the community,’ ” Dr. Ofri said.
She noted that the sense of commitment to the community carried her and fellow clinicians through the toughest parts of 9/11 and of the COVID-19 pandemic.
“People look to the medical system as a lodestar. ‘Where’s my family member? What should I do? Should I be tested? Vaccinated?’ We were there to be a steady presence for the community physically, psychologically, emotionally, and medically, which helped center us as well,” Dr. Ofri said. “If we didn’t have that, we might have all given in to existential panic.”
She added: “Although we had to work twice as hard, often amid great personal risk, we had the good fortune of having a sense of purpose, something to contribute, plus the community of colleagues we cared about and trusted with our lives.”
Crisis and personal growth
Dr. DePierro said that participants who went through 9/11 have been coming to Mount Sinai’s World Trade Center Health Program for care for nearly two decades. “Many are doing quite well, despite the emotional trauma and the dust and toxin exposure, which has given us a window into what makes people resilient.”
Social and community support are key factors in resilience. Another is recognizing opportunities for personal or professional growth during the crisis, according to Dr. DePierro.
During the pandemic, hospital staff were redeployed to departments where they didn’t typically work. They worked with new colleagues and used skills in patient care that they hadn’t needed for years or even decades. “Although this was stressful and distressing, quite a number said they came through with more medical knowledge than before and that they had forged relationships in the trenches that have been lasting and have become important to them,” he reported.
He noted that, during both crises, for first responders and health care practitioners, religious or spiritual faith was a source of resilience. “During the peak of the pandemic, chaplains provided an exorbitant amount of staff support as clinicians turned to the chaplain to help make sense of what they were going through and connect to something greater than themselves.” Similarly, during 9/11, police and fire department chaplains “played a huge role in supporting the first responders,” Dr. DePierro said.
He said that Mount Sinai holds resilience workshops “where we focus on these topics and teach health care workers how to build resilience in their lives, heal day-to-day stressors, and even grow from the experience.”
Dr. Ofri, who is the founder and editor-in-chief of the Bellevue Literary Review, added that the arts played an important role in bolstering resilience and providing a creative outlet for clinicians after 9/11 and again during the pandemic.
The publication is celebrating its twentieth anniversary – its first issue went to press in September 2001. The cover contained an acknowledgment of 9/11.
Dr. Ofri said that a gala event had been planned for Oct. 7, 2001, to celebrate the inaugural issue of the publication. She assumed no one would show up, given that the United States had invaded Afghanistan only hours earlier. To her surprise, over a hundred people attended, “which made me realize the role of the arts during trauma. People were seeking to come together and hear poetry, fiction, and creative nonfiction.”
Dr. Ofri has been “impressed by the amount of incredible creative writing of all sorts that has been submitted [to the publication] during the pandemic, an unexpected flowering of the arts.”
Unique challenges, unique opportunities
All three experts pointed to several noteworthy differences between the experiences of first responders following 9/11 and those of today’s health care professionals during the pandemic.
“What happened on Sept. 11 was one discrete event, and although it obviously led to years of recovering body parts and cleaning up Ground Zero, and on a national level it led to a war, it nevertheless was a single event,” Dr. DePierro observed. By contrast, the COVID-19 pandemic is ongoing, and for health care practitioners, “it’s by no means over. Again and again, they are being thrown back into battle, dealing with fatigue, weariness, and loss of life.”
Moreover, “it is my understanding that immediately following 9/11, there was a general coming together in our country, but it’s obvious that today, there’s a great deal of fractiousness, contention, disagreement, and disunity in our country when it comes to COVID-19,” Dr. DePierro continued.
“This takes a great toll, particularly on health care workers who are dealing with COVID-19 on a daily basis and experience a disconnect between what they see on their floors and ICUs of the hospital, experiencing loss of life they’ve likely never encountered in their careers, and what people are saying when they downplay the seriousness of COVID-19,” he said.
Dr. Ofri agreed. “The fragmentation of our country and the failure of leadership at the highest level to provide even the basics, such as PPE [personal protective equipment] for health care professionals, left us baffled, profoundly hurt, and angry.”
A positive difference between the COVID-19 pandemic and the aftermath of 9/11 is the development of sophisticated technology that allows interventions for traumatized individuals – both health care professionals and the general public – through telehealth, Dr. DePierro pointed out.
“I would say that these resources and technologies are a silver lining and should continue to be expanded on,” he said. “Now, busy health care workers can access all manner of supportive services, including teletherapy, right from home or between shifts.”
Another “silver lining” is that the pandemic has shone a spotlight on an issue that predated the pandemic – the mental health of health care professionals. Opening a discussion about this has reduced stigma and hopefully has paved the way for improved treatments and for providing resources.
Dr. Dass-Brailsford added that “it is important, going forward, for all of us to be trauma informed, to know how trauma and trauma-related stress unfolds in both other people and yourself, and to know what coping skills can be used to avoid crises from developing – a task that extends across all types of disasters.”
A version of this article first appeared on Medscape.com.
The scope and magnitude of the Sept. 11, 2001, attacks on the World Trade Center and the Pentagon were unprecedented in U.S. history. It was arguably the most serious trauma to beset Americans on U.S. soil. The 20th anniversary of 9/11 will take place during another crisis, not only in American history but also in world history – the COVID-19 pandemic.

“As different as these two events are, there are obvious points of comparison,” Jonathan DePierro, PhD, assistant professor of psychiatry, Icahn School of Medicine at Mount Sinai, New York, said in an interview. “Both were unprecedented life-threatening situations, presenting threats to individuals’ lives and profoundly traumatizing not only society as a whole but also first responders.”
Dr. DePierro, who is also the clinical director of the Center for Stress, Resilience, and Personal Growth at Mount Sinai, thinks there are many lessons to be learned from the mental health response to 9/11 that can inform our understanding of and response to the mental health needs of today’s first responders in the COVID-19 crisis, particularly health care professionals.
“Every one of our hospitals became a ‘ground zero’ early during the pandemic, and we see the numbers rising again and hospitals again overwhelmed, so he said.
Placing trauma within a new framework
According to Priscilla Dass-Brailsford, EdD, MPH, professor of psychology, department of psychiatry, Georgetown University, Washington, Sept. 11, 2001, “placed trauma within a new framework.”
“Prior to 9/11, crisis protocols and how to manage stress in the aftermath of violent events were uncommon,” Dr. Dass-Brailsford, a clinical psychologist with expertise in trauma who also chairs a clinical psychology program for the Chicago School of Professional Psychology, said in an interview.
As a first responder, she was involved in early interventions for survivors of 9/11. On Sept. 11, 2001, she had just resigned her position as coordinator of the community crisis response team – the first of its kind in the United States – through the Victims of Violence Program in Cambridge, in Cambridge, Mass.
The program responded to communities in which there were high rates of drive-by shootings and similar acts of violence. Because of her crisis experience, Dr. Dass-Brailsford was asked to conduct debriefings in Boston in the area where the 9/11 terrorists had stayed prior to boarding the planes that were used in the terrorist attacks. She subsequently went to New York City to conduct similar psychological debriefings with affected communities.
“What we’ve learned is that we had no crisis protocol on how to manage the stress in the aftermath of such a violent event, no standard operating procedures. There were very few people trained in crisis and trauma response at that time. Partially spurred by 9/11, trauma training programs became more prolific,” she said. Dr. Dass-Brailsford developed a trauma certification program at Lesley University in Cambridge, Mass., where she began to teach after 9/11. “I saw the importance of having clinicians trained to respond in a crisis, because responding to a crisis is very different from conducting regular mental health interventions.”
Short- and long-term interventions
Dr. DePierro said that Mount Sinai has a 20-year history of responding to the physical and mental health needs of 9/11 responders.
“We saw a number of first responders experiencing clinical depression, anxiety, a lot of worry, symptoms of posttraumatic stress disorder, and an increase in alcohol and/or substance use,” he recounted. In some, these responses were immediate; in others, the onset of symptoms was more gradual. Some responders had acute reactions that lasted for several months to a year, whereas for others, the reactions were prolonged, and they remained “chronically distressed long after the immediate exposure to the event,” he said.
Recent studies have shown that, during the COVID-19 pandemic, health care professionals and many essential workers have experienced similar symptoms, Dr. DePierro noted.
Mental health care professionals who provided interventions for workers involved in recovery and cleanup at the World Trade Center have highlighted the need for long-term monitoring of people on the front lines during the COVID-19 pandemic – especially health care workers, other essential personnel (for example, delivery, postal, and grocery store workers) and surviving family members. “Health monitoring and treatment efforts for 9/11 survivors and responders were put into place soon after the attacks and continue to this day,” using funding provided through the James Zadroga Act, Dr. DePierro said.
“Without similarly unified health registry and treatment services, many individuals – especially from underserved groups – will likely experience chronic mental health consequences and will be unable to access high-quality health care services,” he stated.
‘Psychological first aid’
“Although many people who go through a crisis – whether as a result of terrorism, such as 9/11, or a medical crisis, such as the current pandemic, or a natural disaster, such as Hurricane Katrina – experience PTSD, it’s important to note that not everyone who goes through a crisis and is traumatized will go on to develop PTSD,” Dr. Dass-Brailsford emphasized.
“To me, 9/11 placed psychological first aid on the map. Even if you are not a clinician, you can be trained to provide psychological first aid by becoming familiar with people’s reactions to trauma and how you can support them through it,” she continued.
For example, if a coworker is agitated or “seems to be having a meltdown, you can be there by offering support and getting them the appropriate help.” Research has suggested that having social support before and after a traumatic event can be helpful in determining vulnerability to the development of PTSD and in modulating the impact of the trauma.
Psychological first aid is helpful as an interim measure. “If you see a coworker holding their head in their hands all day and staring at the screen, identifying whether the person might be having a dissociative episode is critical. Providing some support is important, but if more intensive professional support is needed, determining that and making a referral becomes key,” Dr. Dass-Brailsford stated.
Dr. DePierro added: “One of the most important messages that I want health care workers to know from my years of working with 9/11 survivors is that feeling distressed after a traumatic event is very common, but with effective care, one doesn’t necessarily need to be in treatment for years.”
Danielle Ofri, MD, PhD, clinical professor, department of medicine, New York University, agreed. “It is important to continue keeping tabs on each other and remaining sensitive to the collateral struggles of our colleagues. Some have children who are struggling in school, others have parents who have lost a job. Continuing to check in on others and offer support is critical going forward,” she said in an interview.
Cohesiveness and volunteerism
One of the most powerful antidotes to long-term traumatization is a sense of community cohesiveness. This was the case following 9/11, and it is the case during the COVID-19 pandemic, according to Dr. Ofri, an internist at Bellevue Hospital in New York.
“There was an enormous mobilization. Bellevue is a city hospital with a level 1 trauma center, and we expected to be swamped, so the whole hospital shifted into gear,” said Dr. Ofri. “What would have been terrifying seemed tolerable because we felt that we were in it together. We discharged the inpatients to make beds available. Within hours, we had converted clinics into emergency departments and ICUs. We worked seamlessly, and the crisis brought us together ... but then, of course, no patients showed up.”
She described her relationship with her colleagues as “feeling almost like a family, especially during the pandemic, when so many others were in lockdown and feeling isolated and useless.”
She and her colleagues saw each other daily. Although the content of their tasks and responsibilities changed and people were redeployed to other areas, “our workday didn’t really change. It would have been overwhelming if we hadn’t had our daily meetings to regroup and assess where we were. Each day, everything we had learned or implemented the day before – treatment protocols, testing protocols, our understanding of how the virus was communicated – would change and need to be reevaluated. Those morning meetings were critical to staying centered. It felt as though we were building a plane and flying it at the same time, which felt both scary and heady. Luckily, it took place within the fraternity of a committed and caring group.”
Dr. Ofri recounted that, after 9/11, as well as during the pandemic, “professionals kept jumping in from the sidelines to volunteer. Within hours of the collapse of the towers, the ED had filled with staff. People came out of retirement and out from vacation and out of the woodwork. It was very heartening.”
Even more inspiring, “all the departmental barriers seemed to break down. People were willing to step out of their ordinary roles and check their egos at the door. Seasoned physicians were willing to function as medical interns.”
This generosity of time and spirit “helped keep us going,” she said.
Dr. DePierro agreed. “One of the things I’ve seen on medical floors is that COVID actually brought some units together, increasing their cohesion and mutual support and increasing the bonds between people.” These intensified bonds “increased the resilience of everyone involved.”
Commitment to the community
Dr. Ofri recalls families gathering at the hospital after 9/11, watching posters of missing people going up all over the hospital as well as on mailboxes and lampposts. Because the center for missing people was located right next door to Bellevue, there were long lines of families coming in to register. The chief medical office was there, and a huge tent was built to accommodate the families. The tent took up the entire block. “We felt a lot of ownership, because families were coming here,” she said.
The street remained closed even as the days, weeks, and years stretched on, and the tent remained. It was used as a reflection area for families. During the pandemic, that area was used for refrigerated trucks that served as temporary morgues.
“Both logistically and emotionally, we had a feeling during the pandemic of, ‘We’ve been here before, we’ll do it again and be there for the community,’ ” Dr. Ofri said.
She noted that the sense of commitment to the community carried her and fellow clinicians through the toughest parts of 9/11 and of the COVID-19 pandemic.
“People look to the medical system as a lodestar. ‘Where’s my family member? What should I do? Should I be tested? Vaccinated?’ We were there to be a steady presence for the community physically, psychologically, emotionally, and medically, which helped center us as well,” Dr. Ofri said. “If we didn’t have that, we might have all given in to existential panic.”
She added: “Although we had to work twice as hard, often amid great personal risk, we had the good fortune of having a sense of purpose, something to contribute, plus the community of colleagues we cared about and trusted with our lives.”
Crisis and personal growth
Dr. DePierro said that participants who went through 9/11 have been coming to Mount Sinai’s World Trade Center Health Program for care for nearly two decades. “Many are doing quite well, despite the emotional trauma and the dust and toxin exposure, which has given us a window into what makes people resilient.”
Social and community support are key factors in resilience. Another is recognizing opportunities for personal or professional growth during the crisis, according to Dr. DePierro.
During the pandemic, hospital staff were redeployed to departments where they didn’t typically work. They worked with new colleagues and used skills in patient care that they hadn’t needed for years or even decades. “Although this was stressful and distressing, quite a number said they came through with more medical knowledge than before and that they had forged relationships in the trenches that have been lasting and have become important to them,” he reported.
He noted that, during both crises, for first responders and health care practitioners, religious or spiritual faith was a source of resilience. “During the peak of the pandemic, chaplains provided an exorbitant amount of staff support as clinicians turned to the chaplain to help make sense of what they were going through and connect to something greater than themselves.” Similarly, during 9/11, police and fire department chaplains “played a huge role in supporting the first responders,” Dr. DePierro said.
He said that Mount Sinai holds resilience workshops “where we focus on these topics and teach health care workers how to build resilience in their lives, heal day-to-day stressors, and even grow from the experience.”
Dr. Ofri, who is the founder and editor-in-chief of the Bellevue Literary Review, added that the arts played an important role in bolstering resilience and providing a creative outlet for clinicians after 9/11 and again during the pandemic.
The publication is celebrating its twentieth anniversary – its first issue went to press in September 2001. The cover contained an acknowledgment of 9/11.
Dr. Ofri said that a gala event had been planned for Oct. 7, 2001, to celebrate the inaugural issue of the publication. She assumed no one would show up, given that the United States had invaded Afghanistan only hours earlier. To her surprise, over a hundred people attended, “which made me realize the role of the arts during trauma. People were seeking to come together and hear poetry, fiction, and creative nonfiction.”
Dr. Ofri has been “impressed by the amount of incredible creative writing of all sorts that has been submitted [to the publication] during the pandemic, an unexpected flowering of the arts.”
Unique challenges, unique opportunities
All three experts pointed to several noteworthy differences between the experiences of first responders following 9/11 and those of today’s health care professionals during the pandemic.
“What happened on Sept. 11 was one discrete event, and although it obviously led to years of recovering body parts and cleaning up Ground Zero, and on a national level it led to a war, it nevertheless was a single event,” Dr. DePierro observed. By contrast, the COVID-19 pandemic is ongoing, and for health care practitioners, “it’s by no means over. Again and again, they are being thrown back into battle, dealing with fatigue, weariness, and loss of life.”
Moreover, “it is my understanding that immediately following 9/11, there was a general coming together in our country, but it’s obvious that today, there’s a great deal of fractiousness, contention, disagreement, and disunity in our country when it comes to COVID-19,” Dr. DePierro continued.
“This takes a great toll, particularly on health care workers who are dealing with COVID-19 on a daily basis and experience a disconnect between what they see on their floors and ICUs of the hospital, experiencing loss of life they’ve likely never encountered in their careers, and what people are saying when they downplay the seriousness of COVID-19,” he said.
Dr. Ofri agreed. “The fragmentation of our country and the failure of leadership at the highest level to provide even the basics, such as PPE [personal protective equipment] for health care professionals, left us baffled, profoundly hurt, and angry.”
A positive difference between the COVID-19 pandemic and the aftermath of 9/11 is the development of sophisticated technology that allows interventions for traumatized individuals – both health care professionals and the general public – through telehealth, Dr. DePierro pointed out.
“I would say that these resources and technologies are a silver lining and should continue to be expanded on,” he said. “Now, busy health care workers can access all manner of supportive services, including teletherapy, right from home or between shifts.”
Another “silver lining” is that the pandemic has shone a spotlight on an issue that predated the pandemic – the mental health of health care professionals. Opening a discussion about this has reduced stigma and hopefully has paved the way for improved treatments and for providing resources.
Dr. Dass-Brailsford added that “it is important, going forward, for all of us to be trauma informed, to know how trauma and trauma-related stress unfolds in both other people and yourself, and to know what coping skills can be used to avoid crises from developing – a task that extends across all types of disasters.”
A version of this article first appeared on Medscape.com.