User login
Study finds most adverse events from microneedling are minimal
according to the results of a systematic review of nearly 3,000 patients.
Microneedling involves the use of instruments including dermarollers and microneedling pens to cause controlled microtraumas at various skin depths and induce a wounding cascade that ultimately improves the visual appearance of the skin, Sherman Chu, DO, of the department of dermatology at the University of California, Irvine, and colleagues wrote.
Microneedling has increased in popularity because of its relatively low cost, effectiveness, and ease of use, and is often promoted as “a safe alternative treatment, particularly in skin of color, but the safety of microneedling and its complications are not often discussed,” the researchers noted.
In the study, published in Dermatologic Surgery, Dr. Chu and coauthors identified 85 articles for the systematic review of safety data on microneedling. The studies included 30 randomized, controlled trials; 24 prospective studies; 16 case series; 12 case reports; and 3 retrospective cohort studies, with a total of 2,805 patients treated with microneedling.
The devices used in the studies were primarily dermarollers (1,758 procedures), but 425 procedures involved dermapens, and 176 involved unidentified microneedling devices.
The most common adverse effect after microneedling with any device was any of anticipated transient procedural side effects including transient erythema or edema, pain, burning, bruising, pruritus, stinging, bleeding, crusting, and desquamation. Overall, these effects resolved within a week with little or no treatment, the researchers said.
The most commonly reported postprocedure side effects of microneedling were postinflammatory hyperpigmentation (46 incidents), followed by dry skin and exfoliation (41 incidents). Fewer than 15 incidents were reported of each the following: acne flare, pruritus, persistent erythema, herpetic infection, flushing, seborrheic dermatitis, burning, headache, stinging, milia, tram-track scarring, facial allergic granulomatous reaction and systematic hypersensitivity, and tender cervical lymphadenopathy. In addition, one incident each was reported of periorbital dermatitis, phototoxic reaction, pressure urticaria, irritant contact dermatitis, widespread facial inoculation of varicella, pustular folliculitis, and tinea corporis.
The studies suggest that microneedling is generally well tolerated, the researchers wrote. Factors that increased the risk of adverse events included the presence of active infections, darker skin types, metal allergies, and the use of combination therapies. For example, they noted, one randomized, controlled trial showed greater skin irritation in patients treated with both microneedling and tranexamic acid compared with those treated with tranexamic acid alone.
Other studies described increased risk of postinflammatory hyperpigmentation in patients treated with both microneedling and platelet-rich plasma, and with microneedling and topical 5-FU or tacrolimus. Also, in one of the studies in the review, “the development of a delayed granulomatous hypersensitivity reaction in 2 patients was attributed to a reaction to vitamin C serum, whereas another study attributes vitamin A and vitamin C oil to be the cause of a patient’s prolonged erythema and pruritus,” the researchers said.
The study findings were limited to adverse events reported by clinicians in published literature, and did not account for adverse events that occur when microneedling is performed at home or in medical spas. Although the results suggest that microneedling is relatively safe for patients of most skin types, “great caution should be taken when performing microneedling with products not approved to be used intradermally,” they emphasized.
“Further studies are needed to determine which patients are at a higher chance of developing scarring because depth of the needle and skin type do not directly correlate as initially believed,” they concluded.
Microneedling offers safe alternative to lasers
“Microneedling is a popular procedure that can be used as an alternative to laser treatments to provide low down time, and lower-cost treatments for similar indications in which lasers are used, such as rhytides and scars,” Catherine M. DiGiorgio, MD, a laser and cosmetic dermatologist at the Boston Center for Facial Rejuvenation, said in an interview.
“Many clinicians and/or providers utilize microneedling in their practice also because they may not have the ability to perform laser and energy-based device treatments,” noted Dr. DiGiorgio, who was asked to comment on the study findings. “Microneedling is safer than energy-based devices in darker skin types due to the lack of energy or heat being delivered to the epidermis. However, as shown in this study, darker skin types remain at risk for [postinflammatory hyperpigmentation], particularly in the hands of an unskilled, inexperienced operator.”
Dr. DiGiorgio said she was not surprised by the study findings. “Microneedling creates microwounds in the skin, which contributes to the risk of all of the side effects listed in the study. Further, the proper use of microneedling devices by the providers performing the procedure is variable and depths of penetration can vary based on which device or roller pen is used and the experience of the person performing the procedures. Depth, after a certain point, can be inaccurate and can superficially abrade the epidermis rather than the intended individual microneedle punctures.”
Laser and energy-based device treatments can be performed safely in patients with darker skin types in the hands of skilled and experienced laser surgeons, said Dr. DiGiorgio. However, “more studies are needed to determine the effectiveness of microneedling alone compared to other treatment modalities. Patients tend to select microneedling due to affordability and less down time; however, sometimes it may not be the best treatment option for their skin condition.
“Patient education is an important factor because one treatment that worked for one of their friends, for example, may not be the best treatment option for their skin complaints.”
Dr. DiGiorgio added that there are few randomized, controlled trials comparing microneedling to laser treatment. “More studies of this nature would benefit the scientific literature and the addition of histological analysis would help us better understand how these treatments compare on a microscopic level.”
The study received no outside funding and the author has no disclosures. Dr. DiGiorgio has served as a consultant for Allergan Aesthetics.
according to the results of a systematic review of nearly 3,000 patients.
Microneedling involves the use of instruments including dermarollers and microneedling pens to cause controlled microtraumas at various skin depths and induce a wounding cascade that ultimately improves the visual appearance of the skin, Sherman Chu, DO, of the department of dermatology at the University of California, Irvine, and colleagues wrote.
Microneedling has increased in popularity because of its relatively low cost, effectiveness, and ease of use, and is often promoted as “a safe alternative treatment, particularly in skin of color, but the safety of microneedling and its complications are not often discussed,” the researchers noted.
In the study, published in Dermatologic Surgery, Dr. Chu and coauthors identified 85 articles for the systematic review of safety data on microneedling. The studies included 30 randomized, controlled trials; 24 prospective studies; 16 case series; 12 case reports; and 3 retrospective cohort studies, with a total of 2,805 patients treated with microneedling.
The devices used in the studies were primarily dermarollers (1,758 procedures), but 425 procedures involved dermapens, and 176 involved unidentified microneedling devices.
The most common adverse effect after microneedling with any device was any of anticipated transient procedural side effects including transient erythema or edema, pain, burning, bruising, pruritus, stinging, bleeding, crusting, and desquamation. Overall, these effects resolved within a week with little or no treatment, the researchers said.
The most commonly reported postprocedure side effects of microneedling were postinflammatory hyperpigmentation (46 incidents), followed by dry skin and exfoliation (41 incidents). Fewer than 15 incidents were reported of each the following: acne flare, pruritus, persistent erythema, herpetic infection, flushing, seborrheic dermatitis, burning, headache, stinging, milia, tram-track scarring, facial allergic granulomatous reaction and systematic hypersensitivity, and tender cervical lymphadenopathy. In addition, one incident each was reported of periorbital dermatitis, phototoxic reaction, pressure urticaria, irritant contact dermatitis, widespread facial inoculation of varicella, pustular folliculitis, and tinea corporis.
The studies suggest that microneedling is generally well tolerated, the researchers wrote. Factors that increased the risk of adverse events included the presence of active infections, darker skin types, metal allergies, and the use of combination therapies. For example, they noted, one randomized, controlled trial showed greater skin irritation in patients treated with both microneedling and tranexamic acid compared with those treated with tranexamic acid alone.
Other studies described increased risk of postinflammatory hyperpigmentation in patients treated with both microneedling and platelet-rich plasma, and with microneedling and topical 5-FU or tacrolimus. Also, in one of the studies in the review, “the development of a delayed granulomatous hypersensitivity reaction in 2 patients was attributed to a reaction to vitamin C serum, whereas another study attributes vitamin A and vitamin C oil to be the cause of a patient’s prolonged erythema and pruritus,” the researchers said.
The study findings were limited to adverse events reported by clinicians in published literature, and did not account for adverse events that occur when microneedling is performed at home or in medical spas. Although the results suggest that microneedling is relatively safe for patients of most skin types, “great caution should be taken when performing microneedling with products not approved to be used intradermally,” they emphasized.
“Further studies are needed to determine which patients are at a higher chance of developing scarring because depth of the needle and skin type do not directly correlate as initially believed,” they concluded.
Microneedling offers safe alternative to lasers
“Microneedling is a popular procedure that can be used as an alternative to laser treatments to provide low down time, and lower-cost treatments for similar indications in which lasers are used, such as rhytides and scars,” Catherine M. DiGiorgio, MD, a laser and cosmetic dermatologist at the Boston Center for Facial Rejuvenation, said in an interview.
“Many clinicians and/or providers utilize microneedling in their practice also because they may not have the ability to perform laser and energy-based device treatments,” noted Dr. DiGiorgio, who was asked to comment on the study findings. “Microneedling is safer than energy-based devices in darker skin types due to the lack of energy or heat being delivered to the epidermis. However, as shown in this study, darker skin types remain at risk for [postinflammatory hyperpigmentation], particularly in the hands of an unskilled, inexperienced operator.”
Dr. DiGiorgio said she was not surprised by the study findings. “Microneedling creates microwounds in the skin, which contributes to the risk of all of the side effects listed in the study. Further, the proper use of microneedling devices by the providers performing the procedure is variable and depths of penetration can vary based on which device or roller pen is used and the experience of the person performing the procedures. Depth, after a certain point, can be inaccurate and can superficially abrade the epidermis rather than the intended individual microneedle punctures.”
Laser and energy-based device treatments can be performed safely in patients with darker skin types in the hands of skilled and experienced laser surgeons, said Dr. DiGiorgio. However, “more studies are needed to determine the effectiveness of microneedling alone compared to other treatment modalities. Patients tend to select microneedling due to affordability and less down time; however, sometimes it may not be the best treatment option for their skin condition.
“Patient education is an important factor because one treatment that worked for one of their friends, for example, may not be the best treatment option for their skin complaints.”
Dr. DiGiorgio added that there are few randomized, controlled trials comparing microneedling to laser treatment. “More studies of this nature would benefit the scientific literature and the addition of histological analysis would help us better understand how these treatments compare on a microscopic level.”
The study received no outside funding and the author has no disclosures. Dr. DiGiorgio has served as a consultant for Allergan Aesthetics.
according to the results of a systematic review of nearly 3,000 patients.
Microneedling involves the use of instruments including dermarollers and microneedling pens to cause controlled microtraumas at various skin depths and induce a wounding cascade that ultimately improves the visual appearance of the skin, Sherman Chu, DO, of the department of dermatology at the University of California, Irvine, and colleagues wrote.
Microneedling has increased in popularity because of its relatively low cost, effectiveness, and ease of use, and is often promoted as “a safe alternative treatment, particularly in skin of color, but the safety of microneedling and its complications are not often discussed,” the researchers noted.
In the study, published in Dermatologic Surgery, Dr. Chu and coauthors identified 85 articles for the systematic review of safety data on microneedling. The studies included 30 randomized, controlled trials; 24 prospective studies; 16 case series; 12 case reports; and 3 retrospective cohort studies, with a total of 2,805 patients treated with microneedling.
The devices used in the studies were primarily dermarollers (1,758 procedures), but 425 procedures involved dermapens, and 176 involved unidentified microneedling devices.
The most common adverse effect after microneedling with any device was any of anticipated transient procedural side effects including transient erythema or edema, pain, burning, bruising, pruritus, stinging, bleeding, crusting, and desquamation. Overall, these effects resolved within a week with little or no treatment, the researchers said.
The most commonly reported postprocedure side effects of microneedling were postinflammatory hyperpigmentation (46 incidents), followed by dry skin and exfoliation (41 incidents). Fewer than 15 incidents were reported of each the following: acne flare, pruritus, persistent erythema, herpetic infection, flushing, seborrheic dermatitis, burning, headache, stinging, milia, tram-track scarring, facial allergic granulomatous reaction and systematic hypersensitivity, and tender cervical lymphadenopathy. In addition, one incident each was reported of periorbital dermatitis, phototoxic reaction, pressure urticaria, irritant contact dermatitis, widespread facial inoculation of varicella, pustular folliculitis, and tinea corporis.
The studies suggest that microneedling is generally well tolerated, the researchers wrote. Factors that increased the risk of adverse events included the presence of active infections, darker skin types, metal allergies, and the use of combination therapies. For example, they noted, one randomized, controlled trial showed greater skin irritation in patients treated with both microneedling and tranexamic acid compared with those treated with tranexamic acid alone.
Other studies described increased risk of postinflammatory hyperpigmentation in patients treated with both microneedling and platelet-rich plasma, and with microneedling and topical 5-FU or tacrolimus. Also, in one of the studies in the review, “the development of a delayed granulomatous hypersensitivity reaction in 2 patients was attributed to a reaction to vitamin C serum, whereas another study attributes vitamin A and vitamin C oil to be the cause of a patient’s prolonged erythema and pruritus,” the researchers said.
The study findings were limited to adverse events reported by clinicians in published literature, and did not account for adverse events that occur when microneedling is performed at home or in medical spas. Although the results suggest that microneedling is relatively safe for patients of most skin types, “great caution should be taken when performing microneedling with products not approved to be used intradermally,” they emphasized.
“Further studies are needed to determine which patients are at a higher chance of developing scarring because depth of the needle and skin type do not directly correlate as initially believed,” they concluded.
Microneedling offers safe alternative to lasers
“Microneedling is a popular procedure that can be used as an alternative to laser treatments to provide low down time, and lower-cost treatments for similar indications in which lasers are used, such as rhytides and scars,” Catherine M. DiGiorgio, MD, a laser and cosmetic dermatologist at the Boston Center for Facial Rejuvenation, said in an interview.
“Many clinicians and/or providers utilize microneedling in their practice also because they may not have the ability to perform laser and energy-based device treatments,” noted Dr. DiGiorgio, who was asked to comment on the study findings. “Microneedling is safer than energy-based devices in darker skin types due to the lack of energy or heat being delivered to the epidermis. However, as shown in this study, darker skin types remain at risk for [postinflammatory hyperpigmentation], particularly in the hands of an unskilled, inexperienced operator.”
Dr. DiGiorgio said she was not surprised by the study findings. “Microneedling creates microwounds in the skin, which contributes to the risk of all of the side effects listed in the study. Further, the proper use of microneedling devices by the providers performing the procedure is variable and depths of penetration can vary based on which device or roller pen is used and the experience of the person performing the procedures. Depth, after a certain point, can be inaccurate and can superficially abrade the epidermis rather than the intended individual microneedle punctures.”
Laser and energy-based device treatments can be performed safely in patients with darker skin types in the hands of skilled and experienced laser surgeons, said Dr. DiGiorgio. However, “more studies are needed to determine the effectiveness of microneedling alone compared to other treatment modalities. Patients tend to select microneedling due to affordability and less down time; however, sometimes it may not be the best treatment option for their skin condition.
“Patient education is an important factor because one treatment that worked for one of their friends, for example, may not be the best treatment option for their skin complaints.”
Dr. DiGiorgio added that there are few randomized, controlled trials comparing microneedling to laser treatment. “More studies of this nature would benefit the scientific literature and the addition of histological analysis would help us better understand how these treatments compare on a microscopic level.”
The study received no outside funding and the author has no disclosures. Dr. DiGiorgio has served as a consultant for Allergan Aesthetics.
FROM DERMATOLOGIC SURGERY
Hemophagocytic Lymphohistiocytosis: Early Treatment Leading to an Excellent Outcome
HLH is a rare and deadly disease increasingly more present in adults, but following treatment protocol may yield favorable results.
Hemophagocytic lymphohistiocytosis (HLH) is a rare and deadly disease in which unregulated proliferation of histiocytes and T-cell infiltration takes place. It is known as a pediatric disease in which gene defects result in impaired cytotoxic NK- and T-cell function. It has been associated with autosomal recessive inheritance pattern. Without therapy, survival for these patients with active familial HLH is approximately 2 months.
Recognition of the disease has increased over the years, and as a result the diagnosis of HLH in adults also has increased. An acquired form can be triggered by viruses like Epstein-Barr virus, influenza, HIV, lymphoid malignancies, rheumatologic disorders, or immunodeficiency disorders. Survival rates for untreated HLH have been reported at < 5%.1 Despite early recognition and adequate treatment, HLH carries an overall mortality of 50% in the initial presentation, 90% die in the first 8 weeks of treatment due to uncontrolled disease.2
Case Presentation
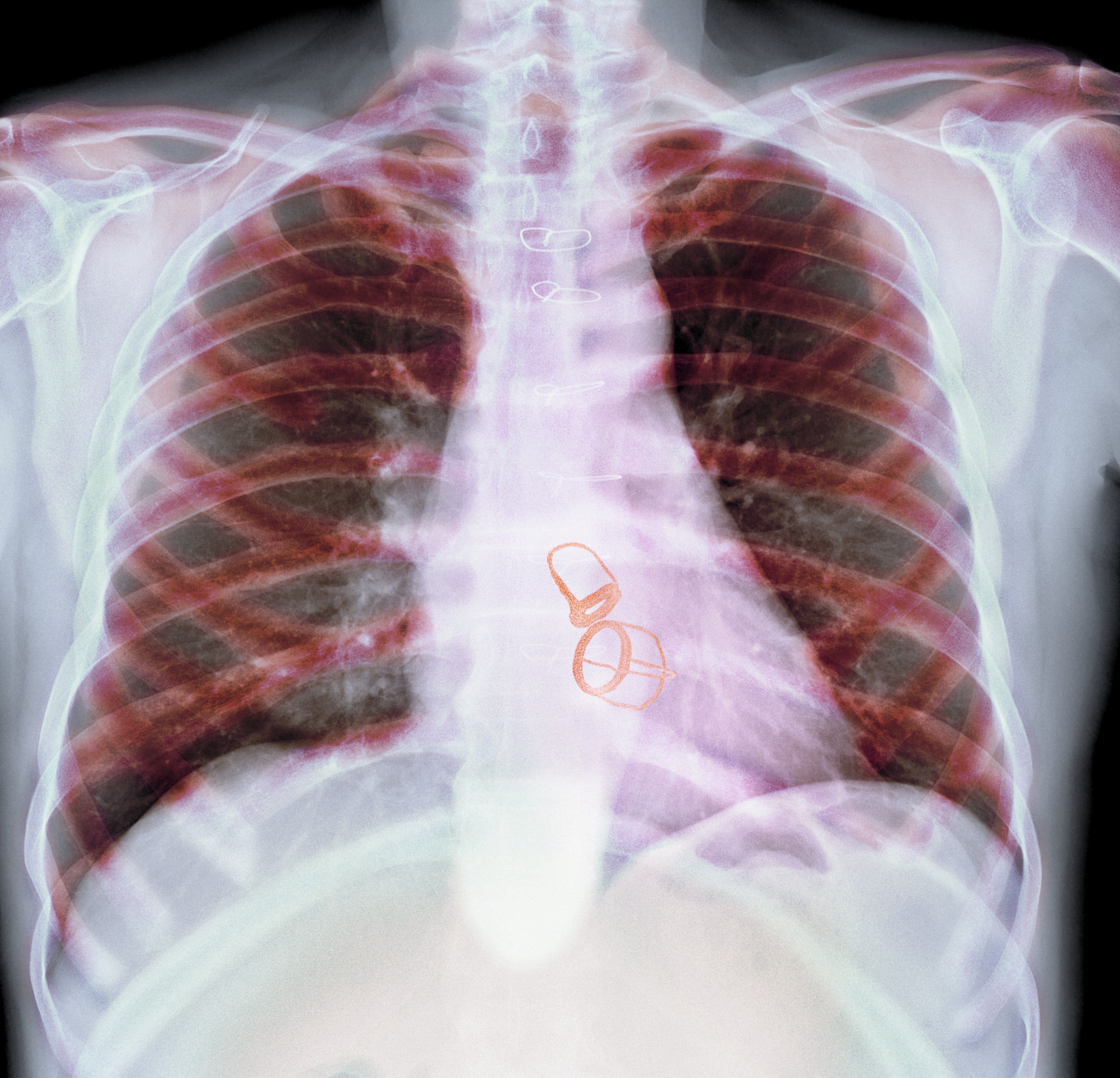
A 56-year-old man with no active medical issues except for a remote history of non-Hodgkin lymphoma treated with chemotherapy and splenectomy in 1990 presented to the Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico. He was admitted to the medicine ward due to community acquired pneumonia. Three days into admission his clinical status deteriorated, and the patient was transferred to the intensive care unit (ICU) due to acute respiratory failure and sepsis secondary to worsening pneumonia. Chest imaging demonstrated rapidly progressing diffuse bilateral infiltrates. Due to the severity of the chest imaging, a diagnostic bronchoscopy was performed.
The patient’s antibiotics regimen was empirically escalated to vancomycin 1500 mg IV every 12 hours and meropenem 2 g IV every 8 hours. Despite optimization of therapy, the patient did not show clinical signs of improvement. Febrile episodes persisted, pulmonary infiltrates and hypoxemia worsened, and the patient required a neuromuscular blockade. Since the bronchoscopy was nondiagnostic and deterioration persistent, the differential diagnosis was broadened. This led to the ordering of inflammatory markers. Laboratory testing showed ferritin levels > 16,000 ng/mL, pointing to HLH as a possible diagnosis. Further workup was remarkable for triglycerides of 1234 mg/dL and a fibrinogen of 0.77 g/L. In the setting of bicytopenia and persistent fever, HLH-94 regimen was started with dexamethasone 40 mg daily and etoposide 100 mg/m2. CD25 levels of 154,701 pg/mL were demonstrated as well as a decreased immunoglobulin (Ig) G levels with absent IgM and IgA. Bone marrow biopsy was consistent with hemophagocytosis. The patient eventually was extubated and sent to the oncology ward to continue chemotherapy.
Discussion
A high clinical suspicion is warranted for rapid diagnosis and treatment as HLH evolves in most cases to multiorgan failure and death. The diagnostic criteria for HLH was developed by the Histiocyte Society in 1991 and then restructured in 2004.3,4 In the first diagnostic tool developed in 1991, diagnosis was based on 5 criteria (fever, splenomegaly, bicytopenia, hypertriglyceridemia and/or hypofibrinogenemia, and hemophagocytosis). Three additional laboratory findings were also described as part of HLH diagnosis since 2004: low or absent NK-cell-activity, hyperferritinemia of > 500 ng/dL, and high-soluble interleukin-2-receptor levels (CD25) > 2400 U/mL. Overall, 5 of 8 criteria are needed for the HLH diagnosis.
Despite the common use of these diagnostic criteria, they were developed for the pediatric population but have not been validated for adult patients.5 For adult patients, the HScore was developed in 2014. It has 9 variables: 3 are based on clinical findings (known underlying immunosuppression, high temperature, and organomegaly; 5 are based on laboratory values (ferritin, serum glutamic oxaloacetic transaminase, cytopenia, triglycerides, and fibrinogen levels); the last variable uses cytologic findings in the bone marrow. In the initial study, probability of having HLH ranged from < 1% with an HScore of ≤ 90% to > 99% with an HScore of ≥ 250 in noncritically ill adults.5 A recently published retrospective study demonstrated the diagnostic reliability of both the HLH-2004 criteria and HScore in critically ill adult patients. This study concluded that the best prediction accuracy of HLH diagnosis for a cutoff of 4 fulfilled HLH-2004 criteria had a 95.0% sensitivity and 93.6% specificity and HScore cutoff of 168 reached a 100% sensitivity and 94.1% specificity.6
The early negative bronchoscopy lowered the possibility of an infection as the etiology of the clinical presentation and narrowed the hyperferritinemia differential diagnosis. Hyperferritinemia has a sensitivity and specificity of > 90% for diagnosis when above 10,000 ng/dL in the pediatric population.7 This is not the case in adults. Hyperferritinemia is a marker of different inflammatory responses, such as histoplasmosis infection, malignancy, or iron overload rather than an isolated diagnostic tool for HLH.8 It has been reported that CD25 levels less than the diagnostic threshold of 2400 U/mL have a 100% sensitivity for the diagnosis and therefore can rule out the diagnosis. When this is taken into consideration, it can be concluded that CD25 level is a better diagnostic tool when compared with ferritin, but its main limitation is its lack of widespread availability.9 Still, there is a limited number of pathologies that are associated with marked hyperferritinemia, specifically using thresholds of more than 6000 ng/dL.10 Taking into consideration the high mortality of untreated HLH, isolated hyperferritinemia still warrants HLH workup to aggressively pursue the diagnosis and improve outcomes.
The goal of therapy in HLH is prompt inactivation of the dysregulated inflammation with aggressive immunosuppression. In our deteriorating patient, the treatment was started with only 4 of the 8 HLH-2004 diagnostic criteria being met. As per the 2018 Histiocyte Society consensus statement, the decision to start the HLH-94 treatment relies on not only the HLH-2004 diagnostic criteria, but also the patient’s clinical evolution.11 In 1994 the Histiocyte Society also published a treatment protocol termed HLH-94. A Korean retrospective study demonstrated that this protocol led to a 5-year survival rate of 60 to 80% depending on the HLH trigger and response to initial treatment.12 The protocol consists of etoposide at 150 mg/m2, 2 weekly doses in the first 2 weeks and then 1 dose weekly for the next 6 weeks. Dexamethasone is the steroid of choice as it readily crosses the blood-brain barrier. Its dosage consists of 10 mg/m2 for the first 2 weeks and then it is halved every 2 weeks until the eighth week of treatment. A slow taper follows to avoid adrenal insufficiency. Once 8 weeks of treatment have been completed, cyclosporine is added to a goal trough of 200 mcg/dL. If there is central nervous system (CNS) involvement, early aggressive treatment with intrathecal methotrexate is indicated if no improvement is noted during initial therapy.11
In 2004 the Histiocyte Society restructured the HLH-94 treatment protocol with the aim of presenting a more aggressive treatment strategy. The protocol added cyclosporine to the initial induction therapy, rather than later in the ninth week as HLH-94. Neither the use of cyclosporine nor the HLH-2004 have been demonstrated to be superior to the use of etoposide and dexamethasone alone or in the HLH-94 protocol, respectively.13 Cyclosporine is associated with adverse effects (AEs) and may have many contraindications in the acute phase of the disease. Therefore, the HLH-94 protocol is still the recommended regimen.11
To assess adequate clinical response, several clinical and laboratory parameters are followed. Clinically, resolution of fever, improvement in hepatosplenomegaly, lymphadenopathy, and mental status can be useful. Laboratories can be used to assess improvement from organ specific damage such as hepatic involvement or cytopenia. The limitation of these diagnostic studies is that they could falsely suggest an inadequate response to treatment due to concomitant infection or medication AEs. Other markers such as ferritin levels, CD25, and NK cell activity levels are more specific to HLH. Out of them, a decreasing ferritin level has the needed specificity and widespread availability for repeated assessment. On the other hand, both CD25 and NK cell activity are readily available only in specialized centers. An initial high ferritin level is a marker for a poor prognosis, and the rate of decline correlates with mortality. Studies have demonstrated that persistently elevated ferritin levels after treatment initiation are associated with worse outcomes.14,15
Several salvage treatments have been identified in recalcitrant or relapsing disease. In general, chemotherapy needs to be intensified, either by returning to the initial high dosage if recurrence occurs in the weaning phase of treatment or adding other agents if no response was initially achieved. Emapalumab, an interferon γ antibody, was approved by the US Food and Drug Administration for the treatment of intractable HLH after it demonstrated that when added to dexamethasone, it lead to treatment response in 17 out of 27 pediatric patients, with a relatively safe AE profile.16 The goal of intensifying chemotherapy is to have the patient tolerate allogenic stem cell transplant, which is clinically indicated in familial HLH, malignancy induced HLH, and recalcitrant cases. In patients who undergo hematopoietic cell transplantation (HCT) there is a tendency to increase survival to 66% at 5 years.12
Conclusions
HLH is a rare and deadly disease increasingly more present in adults. Our patient who initially presented with a sepsis diagnosis was suspected of having a hematologic etiology for his clinical findings due to markedly elevated ferritin levels. In our patient, the HLH-94 treatment protocol was used, yielding favorable results. Given the lack of specific scientific data backing updated protocols such as HLH-2004 and a comparatively favorable safety profile, current guidelines still recommend using the HLH-94 treatment protocol. Decreasing ferritin levels may be used in conjunction with clinical improvement to demonstrate therapeutic response. Persistence of disease despite standard treatment may warrant novel therapies, such as emapalumab or HCT. Physicians need to be wary of an HLH diagnosis as early identification and treatment may improve its otherwise grim prognosis.
1. Chen TY, Hsu MH, Kuo HC, Sheen JM, Cheng MC, Lin YJ. Outcome analysis of pediatric hemophagocytic lymphohistiocytosis. J Formos Med Assoc. 2021;120(1, pt 1):172-179. doi:10.1016/j.jfma.2020.03.025
2. Henter JI, Samuelsson-Horne A, Aricò M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367-2373. doi:10.1182/blood-2002-01-0172
3. Henter JI, Elinder G, Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FHL Study Group of the Histiocyte Society. Semin Oncol. 1991;18(1):29-33.
4. Henter JI, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. doi:10.1002/pbc.21039
5. Knaak C, Nyvlt P, Schuster FS, et al. Hemophagocytic lymphohistiocytosis in critically ill patients: diagnostic reliability of HLH-2004 criteria and HScore. Crit Care. 2020;24(1):244. Published 2020 May 24. doi:10.1186/s13054-020-02941-3
6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613-2620. doi:10.1002/art.38690
7. La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465-2477. doi:10.1182/blood.2018894618
8. Schaffner M, Rosenstein L, Ballas Z, Suneja M. Significance of Hyperferritinemia in Hospitalized Adults. Am J Med Sci. 2017;354(2):152-158. doi:10.1016/j.amjms.2017.04.016
9. Hayden A, Lin M, Park S, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv. 2017;1(26):2529-2534. Published 2017 Dec 6. doi:10.1182/bloodadvances.2017012310
10. Belfeki N, Strazzulla A, Picque M, Diamantis S. Extreme hyperferritinemia: etiological spectrum and impact on prognosis. Reumatismo. 2020;71(4):199-202. Published 2020 Jan 28. doi:10.4081/reumatismo.2019.1221
11. Ehl S, Astigarraga I, von Bahr Greenwood T, et al. Recommendations for the use of etoposide-based therapy and bone marrow transplantation for the treatment of HLH: consensus statements by the HLH Steering Committee of the Histiocyte Society. J Allergy Clin Immunol Pract. 2018;6(5):1508-1517. doi:10.1016/j.jaip.2018.05.031
12. Yoon JH, Park SS, Jeon YW, et al. Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy. Haematologica. 2019;104(2):269-276. doi:10.3324/haematol.2018.198655
13. Bergsten E, Horne A, Aricó M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728-2738. doi:10.1182/blood-2017-06-788349
14. Lin TF, Ferlic-Stark LL, Allen CE, Kozinetz CA, McClain KL. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56(1):154-155. doi:10.1002/pbc.22774
15. Zhou J, Zhou J, Shen DT, Goyal H, Wu ZQ, Xu HG. Development and validation of the prognostic value of ferritin in adult patients with Hemophagocytic Lymphohistiocytosis. Orphanet J Rare Dis. 2020;15(1):71. Published 2020 Mar 12. doi:10.1186/s13023-020-1336-616. Locatelli F, Jordan MB, Allen CE, et al. Safety and efficacy of emapalumab in pediatric patients with primary hemophagocytic lymphohistiocytosis. Presented at: American Society of Hematology Annual Meeting, November 29, 2018. Blood. 2018;132(suppl 1):LBA-6. doi:10.1182/blood-2018-120810
HLH is a rare and deadly disease increasingly more present in adults, but following treatment protocol may yield favorable results.
HLH is a rare and deadly disease increasingly more present in adults, but following treatment protocol may yield favorable results.
Hemophagocytic lymphohistiocytosis (HLH) is a rare and deadly disease in which unregulated proliferation of histiocytes and T-cell infiltration takes place. It is known as a pediatric disease in which gene defects result in impaired cytotoxic NK- and T-cell function. It has been associated with autosomal recessive inheritance pattern. Without therapy, survival for these patients with active familial HLH is approximately 2 months.
Recognition of the disease has increased over the years, and as a result the diagnosis of HLH in adults also has increased. An acquired form can be triggered by viruses like Epstein-Barr virus, influenza, HIV, lymphoid malignancies, rheumatologic disorders, or immunodeficiency disorders. Survival rates for untreated HLH have been reported at < 5%.1 Despite early recognition and adequate treatment, HLH carries an overall mortality of 50% in the initial presentation, 90% die in the first 8 weeks of treatment due to uncontrolled disease.2
Case Presentation
A 56-year-old man with no active medical issues except for a remote history of non-Hodgkin lymphoma treated with chemotherapy and splenectomy in 1990 presented to the Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico. He was admitted to the medicine ward due to community acquired pneumonia. Three days into admission his clinical status deteriorated, and the patient was transferred to the intensive care unit (ICU) due to acute respiratory failure and sepsis secondary to worsening pneumonia. Chest imaging demonstrated rapidly progressing diffuse bilateral infiltrates. Due to the severity of the chest imaging, a diagnostic bronchoscopy was performed.
The patient’s antibiotics regimen was empirically escalated to vancomycin 1500 mg IV every 12 hours and meropenem 2 g IV every 8 hours. Despite optimization of therapy, the patient did not show clinical signs of improvement. Febrile episodes persisted, pulmonary infiltrates and hypoxemia worsened, and the patient required a neuromuscular blockade. Since the bronchoscopy was nondiagnostic and deterioration persistent, the differential diagnosis was broadened. This led to the ordering of inflammatory markers. Laboratory testing showed ferritin levels > 16,000 ng/mL, pointing to HLH as a possible diagnosis. Further workup was remarkable for triglycerides of 1234 mg/dL and a fibrinogen of 0.77 g/L. In the setting of bicytopenia and persistent fever, HLH-94 regimen was started with dexamethasone 40 mg daily and etoposide 100 mg/m2. CD25 levels of 154,701 pg/mL were demonstrated as well as a decreased immunoglobulin (Ig) G levels with absent IgM and IgA. Bone marrow biopsy was consistent with hemophagocytosis. The patient eventually was extubated and sent to the oncology ward to continue chemotherapy.
Discussion
A high clinical suspicion is warranted for rapid diagnosis and treatment as HLH evolves in most cases to multiorgan failure and death. The diagnostic criteria for HLH was developed by the Histiocyte Society in 1991 and then restructured in 2004.3,4 In the first diagnostic tool developed in 1991, diagnosis was based on 5 criteria (fever, splenomegaly, bicytopenia, hypertriglyceridemia and/or hypofibrinogenemia, and hemophagocytosis). Three additional laboratory findings were also described as part of HLH diagnosis since 2004: low or absent NK-cell-activity, hyperferritinemia of > 500 ng/dL, and high-soluble interleukin-2-receptor levels (CD25) > 2400 U/mL. Overall, 5 of 8 criteria are needed for the HLH diagnosis.
Despite the common use of these diagnostic criteria, they were developed for the pediatric population but have not been validated for adult patients.5 For adult patients, the HScore was developed in 2014. It has 9 variables: 3 are based on clinical findings (known underlying immunosuppression, high temperature, and organomegaly; 5 are based on laboratory values (ferritin, serum glutamic oxaloacetic transaminase, cytopenia, triglycerides, and fibrinogen levels); the last variable uses cytologic findings in the bone marrow. In the initial study, probability of having HLH ranged from < 1% with an HScore of ≤ 90% to > 99% with an HScore of ≥ 250 in noncritically ill adults.5 A recently published retrospective study demonstrated the diagnostic reliability of both the HLH-2004 criteria and HScore in critically ill adult patients. This study concluded that the best prediction accuracy of HLH diagnosis for a cutoff of 4 fulfilled HLH-2004 criteria had a 95.0% sensitivity and 93.6% specificity and HScore cutoff of 168 reached a 100% sensitivity and 94.1% specificity.6
The early negative bronchoscopy lowered the possibility of an infection as the etiology of the clinical presentation and narrowed the hyperferritinemia differential diagnosis. Hyperferritinemia has a sensitivity and specificity of > 90% for diagnosis when above 10,000 ng/dL in the pediatric population.7 This is not the case in adults. Hyperferritinemia is a marker of different inflammatory responses, such as histoplasmosis infection, malignancy, or iron overload rather than an isolated diagnostic tool for HLH.8 It has been reported that CD25 levels less than the diagnostic threshold of 2400 U/mL have a 100% sensitivity for the diagnosis and therefore can rule out the diagnosis. When this is taken into consideration, it can be concluded that CD25 level is a better diagnostic tool when compared with ferritin, but its main limitation is its lack of widespread availability.9 Still, there is a limited number of pathologies that are associated with marked hyperferritinemia, specifically using thresholds of more than 6000 ng/dL.10 Taking into consideration the high mortality of untreated HLH, isolated hyperferritinemia still warrants HLH workup to aggressively pursue the diagnosis and improve outcomes.
The goal of therapy in HLH is prompt inactivation of the dysregulated inflammation with aggressive immunosuppression. In our deteriorating patient, the treatment was started with only 4 of the 8 HLH-2004 diagnostic criteria being met. As per the 2018 Histiocyte Society consensus statement, the decision to start the HLH-94 treatment relies on not only the HLH-2004 diagnostic criteria, but also the patient’s clinical evolution.11 In 1994 the Histiocyte Society also published a treatment protocol termed HLH-94. A Korean retrospective study demonstrated that this protocol led to a 5-year survival rate of 60 to 80% depending on the HLH trigger and response to initial treatment.12 The protocol consists of etoposide at 150 mg/m2, 2 weekly doses in the first 2 weeks and then 1 dose weekly for the next 6 weeks. Dexamethasone is the steroid of choice as it readily crosses the blood-brain barrier. Its dosage consists of 10 mg/m2 for the first 2 weeks and then it is halved every 2 weeks until the eighth week of treatment. A slow taper follows to avoid adrenal insufficiency. Once 8 weeks of treatment have been completed, cyclosporine is added to a goal trough of 200 mcg/dL. If there is central nervous system (CNS) involvement, early aggressive treatment with intrathecal methotrexate is indicated if no improvement is noted during initial therapy.11
In 2004 the Histiocyte Society restructured the HLH-94 treatment protocol with the aim of presenting a more aggressive treatment strategy. The protocol added cyclosporine to the initial induction therapy, rather than later in the ninth week as HLH-94. Neither the use of cyclosporine nor the HLH-2004 have been demonstrated to be superior to the use of etoposide and dexamethasone alone or in the HLH-94 protocol, respectively.13 Cyclosporine is associated with adverse effects (AEs) and may have many contraindications in the acute phase of the disease. Therefore, the HLH-94 protocol is still the recommended regimen.11
To assess adequate clinical response, several clinical and laboratory parameters are followed. Clinically, resolution of fever, improvement in hepatosplenomegaly, lymphadenopathy, and mental status can be useful. Laboratories can be used to assess improvement from organ specific damage such as hepatic involvement or cytopenia. The limitation of these diagnostic studies is that they could falsely suggest an inadequate response to treatment due to concomitant infection or medication AEs. Other markers such as ferritin levels, CD25, and NK cell activity levels are more specific to HLH. Out of them, a decreasing ferritin level has the needed specificity and widespread availability for repeated assessment. On the other hand, both CD25 and NK cell activity are readily available only in specialized centers. An initial high ferritin level is a marker for a poor prognosis, and the rate of decline correlates with mortality. Studies have demonstrated that persistently elevated ferritin levels after treatment initiation are associated with worse outcomes.14,15
Several salvage treatments have been identified in recalcitrant or relapsing disease. In general, chemotherapy needs to be intensified, either by returning to the initial high dosage if recurrence occurs in the weaning phase of treatment or adding other agents if no response was initially achieved. Emapalumab, an interferon γ antibody, was approved by the US Food and Drug Administration for the treatment of intractable HLH after it demonstrated that when added to dexamethasone, it lead to treatment response in 17 out of 27 pediatric patients, with a relatively safe AE profile.16 The goal of intensifying chemotherapy is to have the patient tolerate allogenic stem cell transplant, which is clinically indicated in familial HLH, malignancy induced HLH, and recalcitrant cases. In patients who undergo hematopoietic cell transplantation (HCT) there is a tendency to increase survival to 66% at 5 years.12
Conclusions
HLH is a rare and deadly disease increasingly more present in adults. Our patient who initially presented with a sepsis diagnosis was suspected of having a hematologic etiology for his clinical findings due to markedly elevated ferritin levels. In our patient, the HLH-94 treatment protocol was used, yielding favorable results. Given the lack of specific scientific data backing updated protocols such as HLH-2004 and a comparatively favorable safety profile, current guidelines still recommend using the HLH-94 treatment protocol. Decreasing ferritin levels may be used in conjunction with clinical improvement to demonstrate therapeutic response. Persistence of disease despite standard treatment may warrant novel therapies, such as emapalumab or HCT. Physicians need to be wary of an HLH diagnosis as early identification and treatment may improve its otherwise grim prognosis.
Hemophagocytic lymphohistiocytosis (HLH) is a rare and deadly disease in which unregulated proliferation of histiocytes and T-cell infiltration takes place. It is known as a pediatric disease in which gene defects result in impaired cytotoxic NK- and T-cell function. It has been associated with autosomal recessive inheritance pattern. Without therapy, survival for these patients with active familial HLH is approximately 2 months.
Recognition of the disease has increased over the years, and as a result the diagnosis of HLH in adults also has increased. An acquired form can be triggered by viruses like Epstein-Barr virus, influenza, HIV, lymphoid malignancies, rheumatologic disorders, or immunodeficiency disorders. Survival rates for untreated HLH have been reported at < 5%.1 Despite early recognition and adequate treatment, HLH carries an overall mortality of 50% in the initial presentation, 90% die in the first 8 weeks of treatment due to uncontrolled disease.2
Case Presentation
A 56-year-old man with no active medical issues except for a remote history of non-Hodgkin lymphoma treated with chemotherapy and splenectomy in 1990 presented to the Veterans Affairs Caribbean Healthcare System in San Juan, Puerto Rico. He was admitted to the medicine ward due to community acquired pneumonia. Three days into admission his clinical status deteriorated, and the patient was transferred to the intensive care unit (ICU) due to acute respiratory failure and sepsis secondary to worsening pneumonia. Chest imaging demonstrated rapidly progressing diffuse bilateral infiltrates. Due to the severity of the chest imaging, a diagnostic bronchoscopy was performed.
The patient’s antibiotics regimen was empirically escalated to vancomycin 1500 mg IV every 12 hours and meropenem 2 g IV every 8 hours. Despite optimization of therapy, the patient did not show clinical signs of improvement. Febrile episodes persisted, pulmonary infiltrates and hypoxemia worsened, and the patient required a neuromuscular blockade. Since the bronchoscopy was nondiagnostic and deterioration persistent, the differential diagnosis was broadened. This led to the ordering of inflammatory markers. Laboratory testing showed ferritin levels > 16,000 ng/mL, pointing to HLH as a possible diagnosis. Further workup was remarkable for triglycerides of 1234 mg/dL and a fibrinogen of 0.77 g/L. In the setting of bicytopenia and persistent fever, HLH-94 regimen was started with dexamethasone 40 mg daily and etoposide 100 mg/m2. CD25 levels of 154,701 pg/mL were demonstrated as well as a decreased immunoglobulin (Ig) G levels with absent IgM and IgA. Bone marrow biopsy was consistent with hemophagocytosis. The patient eventually was extubated and sent to the oncology ward to continue chemotherapy.
Discussion
A high clinical suspicion is warranted for rapid diagnosis and treatment as HLH evolves in most cases to multiorgan failure and death. The diagnostic criteria for HLH was developed by the Histiocyte Society in 1991 and then restructured in 2004.3,4 In the first diagnostic tool developed in 1991, diagnosis was based on 5 criteria (fever, splenomegaly, bicytopenia, hypertriglyceridemia and/or hypofibrinogenemia, and hemophagocytosis). Three additional laboratory findings were also described as part of HLH diagnosis since 2004: low or absent NK-cell-activity, hyperferritinemia of > 500 ng/dL, and high-soluble interleukin-2-receptor levels (CD25) > 2400 U/mL. Overall, 5 of 8 criteria are needed for the HLH diagnosis.
Despite the common use of these diagnostic criteria, they were developed for the pediatric population but have not been validated for adult patients.5 For adult patients, the HScore was developed in 2014. It has 9 variables: 3 are based on clinical findings (known underlying immunosuppression, high temperature, and organomegaly; 5 are based on laboratory values (ferritin, serum glutamic oxaloacetic transaminase, cytopenia, triglycerides, and fibrinogen levels); the last variable uses cytologic findings in the bone marrow. In the initial study, probability of having HLH ranged from < 1% with an HScore of ≤ 90% to > 99% with an HScore of ≥ 250 in noncritically ill adults.5 A recently published retrospective study demonstrated the diagnostic reliability of both the HLH-2004 criteria and HScore in critically ill adult patients. This study concluded that the best prediction accuracy of HLH diagnosis for a cutoff of 4 fulfilled HLH-2004 criteria had a 95.0% sensitivity and 93.6% specificity and HScore cutoff of 168 reached a 100% sensitivity and 94.1% specificity.6
The early negative bronchoscopy lowered the possibility of an infection as the etiology of the clinical presentation and narrowed the hyperferritinemia differential diagnosis. Hyperferritinemia has a sensitivity and specificity of > 90% for diagnosis when above 10,000 ng/dL in the pediatric population.7 This is not the case in adults. Hyperferritinemia is a marker of different inflammatory responses, such as histoplasmosis infection, malignancy, or iron overload rather than an isolated diagnostic tool for HLH.8 It has been reported that CD25 levels less than the diagnostic threshold of 2400 U/mL have a 100% sensitivity for the diagnosis and therefore can rule out the diagnosis. When this is taken into consideration, it can be concluded that CD25 level is a better diagnostic tool when compared with ferritin, but its main limitation is its lack of widespread availability.9 Still, there is a limited number of pathologies that are associated with marked hyperferritinemia, specifically using thresholds of more than 6000 ng/dL.10 Taking into consideration the high mortality of untreated HLH, isolated hyperferritinemia still warrants HLH workup to aggressively pursue the diagnosis and improve outcomes.
The goal of therapy in HLH is prompt inactivation of the dysregulated inflammation with aggressive immunosuppression. In our deteriorating patient, the treatment was started with only 4 of the 8 HLH-2004 diagnostic criteria being met. As per the 2018 Histiocyte Society consensus statement, the decision to start the HLH-94 treatment relies on not only the HLH-2004 diagnostic criteria, but also the patient’s clinical evolution.11 In 1994 the Histiocyte Society also published a treatment protocol termed HLH-94. A Korean retrospective study demonstrated that this protocol led to a 5-year survival rate of 60 to 80% depending on the HLH trigger and response to initial treatment.12 The protocol consists of etoposide at 150 mg/m2, 2 weekly doses in the first 2 weeks and then 1 dose weekly for the next 6 weeks. Dexamethasone is the steroid of choice as it readily crosses the blood-brain barrier. Its dosage consists of 10 mg/m2 for the first 2 weeks and then it is halved every 2 weeks until the eighth week of treatment. A slow taper follows to avoid adrenal insufficiency. Once 8 weeks of treatment have been completed, cyclosporine is added to a goal trough of 200 mcg/dL. If there is central nervous system (CNS) involvement, early aggressive treatment with intrathecal methotrexate is indicated if no improvement is noted during initial therapy.11
In 2004 the Histiocyte Society restructured the HLH-94 treatment protocol with the aim of presenting a more aggressive treatment strategy. The protocol added cyclosporine to the initial induction therapy, rather than later in the ninth week as HLH-94. Neither the use of cyclosporine nor the HLH-2004 have been demonstrated to be superior to the use of etoposide and dexamethasone alone or in the HLH-94 protocol, respectively.13 Cyclosporine is associated with adverse effects (AEs) and may have many contraindications in the acute phase of the disease. Therefore, the HLH-94 protocol is still the recommended regimen.11
To assess adequate clinical response, several clinical and laboratory parameters are followed. Clinically, resolution of fever, improvement in hepatosplenomegaly, lymphadenopathy, and mental status can be useful. Laboratories can be used to assess improvement from organ specific damage such as hepatic involvement or cytopenia. The limitation of these diagnostic studies is that they could falsely suggest an inadequate response to treatment due to concomitant infection or medication AEs. Other markers such as ferritin levels, CD25, and NK cell activity levels are more specific to HLH. Out of them, a decreasing ferritin level has the needed specificity and widespread availability for repeated assessment. On the other hand, both CD25 and NK cell activity are readily available only in specialized centers. An initial high ferritin level is a marker for a poor prognosis, and the rate of decline correlates with mortality. Studies have demonstrated that persistently elevated ferritin levels after treatment initiation are associated with worse outcomes.14,15
Several salvage treatments have been identified in recalcitrant or relapsing disease. In general, chemotherapy needs to be intensified, either by returning to the initial high dosage if recurrence occurs in the weaning phase of treatment or adding other agents if no response was initially achieved. Emapalumab, an interferon γ antibody, was approved by the US Food and Drug Administration for the treatment of intractable HLH after it demonstrated that when added to dexamethasone, it lead to treatment response in 17 out of 27 pediatric patients, with a relatively safe AE profile.16 The goal of intensifying chemotherapy is to have the patient tolerate allogenic stem cell transplant, which is clinically indicated in familial HLH, malignancy induced HLH, and recalcitrant cases. In patients who undergo hematopoietic cell transplantation (HCT) there is a tendency to increase survival to 66% at 5 years.12
Conclusions
HLH is a rare and deadly disease increasingly more present in adults. Our patient who initially presented with a sepsis diagnosis was suspected of having a hematologic etiology for his clinical findings due to markedly elevated ferritin levels. In our patient, the HLH-94 treatment protocol was used, yielding favorable results. Given the lack of specific scientific data backing updated protocols such as HLH-2004 and a comparatively favorable safety profile, current guidelines still recommend using the HLH-94 treatment protocol. Decreasing ferritin levels may be used in conjunction with clinical improvement to demonstrate therapeutic response. Persistence of disease despite standard treatment may warrant novel therapies, such as emapalumab or HCT. Physicians need to be wary of an HLH diagnosis as early identification and treatment may improve its otherwise grim prognosis.
1. Chen TY, Hsu MH, Kuo HC, Sheen JM, Cheng MC, Lin YJ. Outcome analysis of pediatric hemophagocytic lymphohistiocytosis. J Formos Med Assoc. 2021;120(1, pt 1):172-179. doi:10.1016/j.jfma.2020.03.025
2. Henter JI, Samuelsson-Horne A, Aricò M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367-2373. doi:10.1182/blood-2002-01-0172
3. Henter JI, Elinder G, Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FHL Study Group of the Histiocyte Society. Semin Oncol. 1991;18(1):29-33.
4. Henter JI, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. doi:10.1002/pbc.21039
5. Knaak C, Nyvlt P, Schuster FS, et al. Hemophagocytic lymphohistiocytosis in critically ill patients: diagnostic reliability of HLH-2004 criteria and HScore. Crit Care. 2020;24(1):244. Published 2020 May 24. doi:10.1186/s13054-020-02941-3
6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613-2620. doi:10.1002/art.38690
7. La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465-2477. doi:10.1182/blood.2018894618
8. Schaffner M, Rosenstein L, Ballas Z, Suneja M. Significance of Hyperferritinemia in Hospitalized Adults. Am J Med Sci. 2017;354(2):152-158. doi:10.1016/j.amjms.2017.04.016
9. Hayden A, Lin M, Park S, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv. 2017;1(26):2529-2534. Published 2017 Dec 6. doi:10.1182/bloodadvances.2017012310
10. Belfeki N, Strazzulla A, Picque M, Diamantis S. Extreme hyperferritinemia: etiological spectrum and impact on prognosis. Reumatismo. 2020;71(4):199-202. Published 2020 Jan 28. doi:10.4081/reumatismo.2019.1221
11. Ehl S, Astigarraga I, von Bahr Greenwood T, et al. Recommendations for the use of etoposide-based therapy and bone marrow transplantation for the treatment of HLH: consensus statements by the HLH Steering Committee of the Histiocyte Society. J Allergy Clin Immunol Pract. 2018;6(5):1508-1517. doi:10.1016/j.jaip.2018.05.031
12. Yoon JH, Park SS, Jeon YW, et al. Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy. Haematologica. 2019;104(2):269-276. doi:10.3324/haematol.2018.198655
13. Bergsten E, Horne A, Aricó M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728-2738. doi:10.1182/blood-2017-06-788349
14. Lin TF, Ferlic-Stark LL, Allen CE, Kozinetz CA, McClain KL. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56(1):154-155. doi:10.1002/pbc.22774
15. Zhou J, Zhou J, Shen DT, Goyal H, Wu ZQ, Xu HG. Development and validation of the prognostic value of ferritin in adult patients with Hemophagocytic Lymphohistiocytosis. Orphanet J Rare Dis. 2020;15(1):71. Published 2020 Mar 12. doi:10.1186/s13023-020-1336-616. Locatelli F, Jordan MB, Allen CE, et al. Safety and efficacy of emapalumab in pediatric patients with primary hemophagocytic lymphohistiocytosis. Presented at: American Society of Hematology Annual Meeting, November 29, 2018. Blood. 2018;132(suppl 1):LBA-6. doi:10.1182/blood-2018-120810
1. Chen TY, Hsu MH, Kuo HC, Sheen JM, Cheng MC, Lin YJ. Outcome analysis of pediatric hemophagocytic lymphohistiocytosis. J Formos Med Assoc. 2021;120(1, pt 1):172-179. doi:10.1016/j.jfma.2020.03.025
2. Henter JI, Samuelsson-Horne A, Aricò M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100(7):2367-2373. doi:10.1182/blood-2002-01-0172
3. Henter JI, Elinder G, Ost A. Diagnostic guidelines for hemophagocytic lymphohistiocytosis. The FHL Study Group of the Histiocyte Society. Semin Oncol. 1991;18(1):29-33.
4. Henter JI, Horne A, Aricó M, et al. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48(2):124-131. doi:10.1002/pbc.21039
5. Knaak C, Nyvlt P, Schuster FS, et al. Hemophagocytic lymphohistiocytosis in critically ill patients: diagnostic reliability of HLH-2004 criteria and HScore. Crit Care. 2020;24(1):244. Published 2020 May 24. doi:10.1186/s13054-020-02941-3
6. Fardet L, Galicier L, Lambotte O, et al. Development and validation of the HScore, a score for the diagnosis of reactive hemophagocytic syndrome. Arthritis Rheumatol. 2014;66(9):2613-2620. doi:10.1002/art.38690
7. La Rosée P, Horne A, Hines M, et al. Recommendations for the management of hemophagocytic lymphohistiocytosis in adults. Blood. 2019;133(23):2465-2477. doi:10.1182/blood.2018894618
8. Schaffner M, Rosenstein L, Ballas Z, Suneja M. Significance of Hyperferritinemia in Hospitalized Adults. Am J Med Sci. 2017;354(2):152-158. doi:10.1016/j.amjms.2017.04.016
9. Hayden A, Lin M, Park S, et al. Soluble interleukin-2 receptor is a sensitive diagnostic test in adult HLH. Blood Adv. 2017;1(26):2529-2534. Published 2017 Dec 6. doi:10.1182/bloodadvances.2017012310
10. Belfeki N, Strazzulla A, Picque M, Diamantis S. Extreme hyperferritinemia: etiological spectrum and impact on prognosis. Reumatismo. 2020;71(4):199-202. Published 2020 Jan 28. doi:10.4081/reumatismo.2019.1221
11. Ehl S, Astigarraga I, von Bahr Greenwood T, et al. Recommendations for the use of etoposide-based therapy and bone marrow transplantation for the treatment of HLH: consensus statements by the HLH Steering Committee of the Histiocyte Society. J Allergy Clin Immunol Pract. 2018;6(5):1508-1517. doi:10.1016/j.jaip.2018.05.031
12. Yoon JH, Park SS, Jeon YW, et al. Treatment outcomes and prognostic factors in adult patients with secondary hemophagocytic lymphohistiocytosis not associated with malignancy. Haematologica. 2019;104(2):269-276. doi:10.3324/haematol.2018.198655
13. Bergsten E, Horne A, Aricó M, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728-2738. doi:10.1182/blood-2017-06-788349
14. Lin TF, Ferlic-Stark LL, Allen CE, Kozinetz CA, McClain KL. Rate of decline of ferritin in patients with hemophagocytic lymphohistiocytosis as a prognostic variable for mortality. Pediatr Blood Cancer. 2011;56(1):154-155. doi:10.1002/pbc.22774
15. Zhou J, Zhou J, Shen DT, Goyal H, Wu ZQ, Xu HG. Development and validation of the prognostic value of ferritin in adult patients with Hemophagocytic Lymphohistiocytosis. Orphanet J Rare Dis. 2020;15(1):71. Published 2020 Mar 12. doi:10.1186/s13023-020-1336-616. Locatelli F, Jordan MB, Allen CE, et al. Safety and efficacy of emapalumab in pediatric patients with primary hemophagocytic lymphohistiocytosis. Presented at: American Society of Hematology Annual Meeting, November 29, 2018. Blood. 2018;132(suppl 1):LBA-6. doi:10.1182/blood-2018-120810
Genetic shift increases susceptibility to childhood ALL
A genetically induced shift toward higher lymphocyte counts was found to increase susceptibility to childhood acute lymphoblastic leukemia, according to the results of a large genome-wide association study of 2,666 childhood patients with ALL as compared with 60,272 control individuals.
The development of ALL is thought to follow a two-hit model of leukemogenesis; in utero formation of a preleukemic clone and subsequent postnatal acquisition of secondary somatic mutations that leads to overt leukemia, according to Linda Kachuri, PhD, of the department of epidemiology and biostatistics, University of California, San Francisco, and colleagues.
The development of ALL is thought to follow a two-hit model of leukemogenesis; in utero formation of a preleukemic clone and subsequent postnatal acquisition of secondary somatic mutations that leads to overt leukemia, according to Linda Kachuri, PhD, of the Department of Epidemiology and Biostatistics, University of California San Francisco, and colleagues.
Previous research has shown that several childhood-ALL–risk regions have also been associated with variation in blood-cell traits and a recent phenome-wide association study of childhood ALL identified platelet count as the most enriched trait among known ALL-risk loci. To further explore this issue, the researchers conducted their comprehensive study of the role of blood-cell-trait variation in the etiology of childhood ALL.
The researchers identified 3,000 blood-cell-trait–associated variants, which accounted for 4.0% to 23.9% of trait variation and included 115 loci associated with blood-cell ratios: lymphocyte-to-monocyte ratio (LMR); neutrophil-to-lymphocyte ratio (NLR); and platelet-to-lymphocyte ratio (PLR), according to a report published online in The American Journal of Human Genetics.
Lymphocyte risk
The researchers found that ALL susceptibility was genetically correlated with lymphocyte counts (rg = 0.088, P = .0004) and PLR (rg = 0.072, P = .0017).
Using Mendelian randomization analyses, a genetically predicted increase in lymphocyte counts was found to be associated with increased ALL risk (odds ratio [OR] = 1.16, P = .031). This correlation was strengthened after the researchers accounted for other cell types (OR = 1.43, P = .0009).
The researchers observed positive associations with increasing LMR (OR = 1.22, P = .0017) as well as inverse effects for NLR (OR = 0.67, P = .0003) and PLR (OR = 0.80, P = .002).
“We identified the cell-type ratios LMR, NLR, and PLR as independent risk factors for ALL and found evidence that these ratios have distinct genetic mechanisms that are not captured by their component traits. In multivariable MR analyses that concurrently modeled the effects of lymphocyte, monocyte, neutrophil, and platelet counts on ALL, lymphocytes remained as the only independent risk factor and this association with ALL strengthened compared to univariate analyses,” the researchers stated.
They reported that they had no competing interests.
A genetically induced shift toward higher lymphocyte counts was found to increase susceptibility to childhood acute lymphoblastic leukemia, according to the results of a large genome-wide association study of 2,666 childhood patients with ALL as compared with 60,272 control individuals.
The development of ALL is thought to follow a two-hit model of leukemogenesis; in utero formation of a preleukemic clone and subsequent postnatal acquisition of secondary somatic mutations that leads to overt leukemia, according to Linda Kachuri, PhD, of the department of epidemiology and biostatistics, University of California, San Francisco, and colleagues.
The development of ALL is thought to follow a two-hit model of leukemogenesis; in utero formation of a preleukemic clone and subsequent postnatal acquisition of secondary somatic mutations that leads to overt leukemia, according to Linda Kachuri, PhD, of the Department of Epidemiology and Biostatistics, University of California San Francisco, and colleagues.
Previous research has shown that several childhood-ALL–risk regions have also been associated with variation in blood-cell traits and a recent phenome-wide association study of childhood ALL identified platelet count as the most enriched trait among known ALL-risk loci. To further explore this issue, the researchers conducted their comprehensive study of the role of blood-cell-trait variation in the etiology of childhood ALL.
The researchers identified 3,000 blood-cell-trait–associated variants, which accounted for 4.0% to 23.9% of trait variation and included 115 loci associated with blood-cell ratios: lymphocyte-to-monocyte ratio (LMR); neutrophil-to-lymphocyte ratio (NLR); and platelet-to-lymphocyte ratio (PLR), according to a report published online in The American Journal of Human Genetics.
Lymphocyte risk
The researchers found that ALL susceptibility was genetically correlated with lymphocyte counts (rg = 0.088, P = .0004) and PLR (rg = 0.072, P = .0017).
Using Mendelian randomization analyses, a genetically predicted increase in lymphocyte counts was found to be associated with increased ALL risk (odds ratio [OR] = 1.16, P = .031). This correlation was strengthened after the researchers accounted for other cell types (OR = 1.43, P = .0009).
The researchers observed positive associations with increasing LMR (OR = 1.22, P = .0017) as well as inverse effects for NLR (OR = 0.67, P = .0003) and PLR (OR = 0.80, P = .002).
“We identified the cell-type ratios LMR, NLR, and PLR as independent risk factors for ALL and found evidence that these ratios have distinct genetic mechanisms that are not captured by their component traits. In multivariable MR analyses that concurrently modeled the effects of lymphocyte, monocyte, neutrophil, and platelet counts on ALL, lymphocytes remained as the only independent risk factor and this association with ALL strengthened compared to univariate analyses,” the researchers stated.
They reported that they had no competing interests.
A genetically induced shift toward higher lymphocyte counts was found to increase susceptibility to childhood acute lymphoblastic leukemia, according to the results of a large genome-wide association study of 2,666 childhood patients with ALL as compared with 60,272 control individuals.
The development of ALL is thought to follow a two-hit model of leukemogenesis; in utero formation of a preleukemic clone and subsequent postnatal acquisition of secondary somatic mutations that leads to overt leukemia, according to Linda Kachuri, PhD, of the department of epidemiology and biostatistics, University of California, San Francisco, and colleagues.
The development of ALL is thought to follow a two-hit model of leukemogenesis; in utero formation of a preleukemic clone and subsequent postnatal acquisition of secondary somatic mutations that leads to overt leukemia, according to Linda Kachuri, PhD, of the Department of Epidemiology and Biostatistics, University of California San Francisco, and colleagues.
Previous research has shown that several childhood-ALL–risk regions have also been associated with variation in blood-cell traits and a recent phenome-wide association study of childhood ALL identified platelet count as the most enriched trait among known ALL-risk loci. To further explore this issue, the researchers conducted their comprehensive study of the role of blood-cell-trait variation in the etiology of childhood ALL.
The researchers identified 3,000 blood-cell-trait–associated variants, which accounted for 4.0% to 23.9% of trait variation and included 115 loci associated with blood-cell ratios: lymphocyte-to-monocyte ratio (LMR); neutrophil-to-lymphocyte ratio (NLR); and platelet-to-lymphocyte ratio (PLR), according to a report published online in The American Journal of Human Genetics.
Lymphocyte risk
The researchers found that ALL susceptibility was genetically correlated with lymphocyte counts (rg = 0.088, P = .0004) and PLR (rg = 0.072, P = .0017).
Using Mendelian randomization analyses, a genetically predicted increase in lymphocyte counts was found to be associated with increased ALL risk (odds ratio [OR] = 1.16, P = .031). This correlation was strengthened after the researchers accounted for other cell types (OR = 1.43, P = .0009).
The researchers observed positive associations with increasing LMR (OR = 1.22, P = .0017) as well as inverse effects for NLR (OR = 0.67, P = .0003) and PLR (OR = 0.80, P = .002).
“We identified the cell-type ratios LMR, NLR, and PLR as independent risk factors for ALL and found evidence that these ratios have distinct genetic mechanisms that are not captured by their component traits. In multivariable MR analyses that concurrently modeled the effects of lymphocyte, monocyte, neutrophil, and platelet counts on ALL, lymphocytes remained as the only independent risk factor and this association with ALL strengthened compared to univariate analyses,” the researchers stated.
They reported that they had no competing interests.
FROM THE AMERICAN JOURNAL OF HUMAN GENETICS
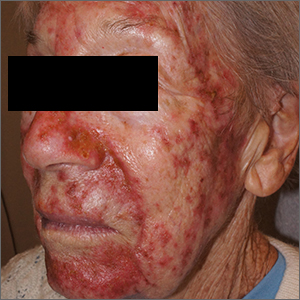
Facial eruptions
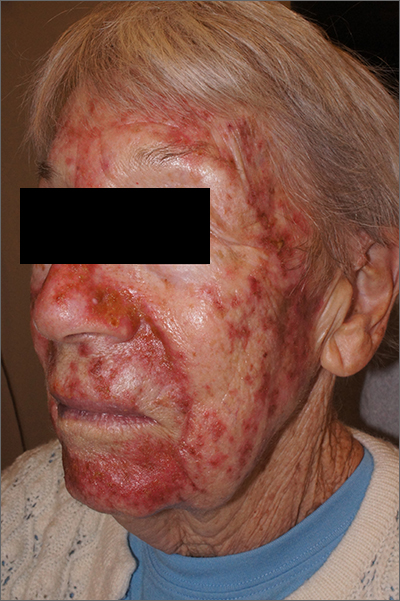
This was a vigorous response to the 5-FU treatment and was actually within the range of expected outcomes for a patient with a heavy burden of AKs. The erythema and superficial skin flaking spared areas unaffected by pre-cancers.
AKs manifest as rough, pink to brown macules or papules on sun-damaged skin and represent a precancerous change in keratinocytes that can lead to invasive squamous cell carcinoma. For this reason, AKs are often treated when they are observed. When targeting an entire “field” of AKs, a gold standard therapy is topical 5-FU. Prescribing 5-FU is safe and effective, but requires patient education, therapy customization, and anticipatory guidance.
Compared with other field treatments (eg, photodynamic therapy, topical diclofenac, imiquimod), 5-FU is the most successful and cost effective; it is first-line therapy and has the longest track record.1,2 5-FU represses DNA synthesis. It’s helpful to describe 5-FU to patients as “fake DNA” that targets precancerous cells that are dividing rapidly. But a word of caution: Patients should be advised, in advance, to avoid significant sun exposure while using 5-FU, as the drug will lose its targeted effect and cause more generalized skin damage.
Physicians can modulate the severity of the response to 5-FU by decreasing the frequency or length of therapy by using a weaker (and more expensive) once daily 0.5% long-acting formulation. Additionally, to improve comfort, low-potency topical steroids such as hydrocortisone ointment 0.5% to 2.5% can be applied after completion of therapy to speed up the healing process. These adjustments improve tolerance of therapy, but the precise effect on efficacy is unknown.
Because of the degree of redness and erythema that developed in this patient, treatment was stopped a week early. There was also concern about possible bacterial involvement in the heavy skin sloughing, so the patient was given topical mupirocin ointment to apply TID for 7 days. Her skin cleared after 3 weeks and all previous AKs were clinically eliminated.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Gupta AK, Paquet M. Network meta-analysis of the outcome 'participant complete clearance' in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
2. Jansen MHE, Kessels JPHM, Merks I, et al. A trial-based cost-effectiveness analysis of topical 5-fluorouracil vs. imiquimod vs. ingenol mebutate vs. methyl aminolaevulinate conventional photodynamic therapy for the treatment of actinic keratosis in the head and neck area performed in the Netherlands. Br J Dermatol. 2020;183:738-744. doi: 10.1111/bjd.18884

This was a vigorous response to the 5-FU treatment and was actually within the range of expected outcomes for a patient with a heavy burden of AKs. The erythema and superficial skin flaking spared areas unaffected by pre-cancers.
AKs manifest as rough, pink to brown macules or papules on sun-damaged skin and represent a precancerous change in keratinocytes that can lead to invasive squamous cell carcinoma. For this reason, AKs are often treated when they are observed. When targeting an entire “field” of AKs, a gold standard therapy is topical 5-FU. Prescribing 5-FU is safe and effective, but requires patient education, therapy customization, and anticipatory guidance.
Compared with other field treatments (eg, photodynamic therapy, topical diclofenac, imiquimod), 5-FU is the most successful and cost effective; it is first-line therapy and has the longest track record.1,2 5-FU represses DNA synthesis. It’s helpful to describe 5-FU to patients as “fake DNA” that targets precancerous cells that are dividing rapidly. But a word of caution: Patients should be advised, in advance, to avoid significant sun exposure while using 5-FU, as the drug will lose its targeted effect and cause more generalized skin damage.
Physicians can modulate the severity of the response to 5-FU by decreasing the frequency or length of therapy by using a weaker (and more expensive) once daily 0.5% long-acting formulation. Additionally, to improve comfort, low-potency topical steroids such as hydrocortisone ointment 0.5% to 2.5% can be applied after completion of therapy to speed up the healing process. These adjustments improve tolerance of therapy, but the precise effect on efficacy is unknown.
Because of the degree of redness and erythema that developed in this patient, treatment was stopped a week early. There was also concern about possible bacterial involvement in the heavy skin sloughing, so the patient was given topical mupirocin ointment to apply TID for 7 days. Her skin cleared after 3 weeks and all previous AKs were clinically eliminated.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).

This was a vigorous response to the 5-FU treatment and was actually within the range of expected outcomes for a patient with a heavy burden of AKs. The erythema and superficial skin flaking spared areas unaffected by pre-cancers.
AKs manifest as rough, pink to brown macules or papules on sun-damaged skin and represent a precancerous change in keratinocytes that can lead to invasive squamous cell carcinoma. For this reason, AKs are often treated when they are observed. When targeting an entire “field” of AKs, a gold standard therapy is topical 5-FU. Prescribing 5-FU is safe and effective, but requires patient education, therapy customization, and anticipatory guidance.
Compared with other field treatments (eg, photodynamic therapy, topical diclofenac, imiquimod), 5-FU is the most successful and cost effective; it is first-line therapy and has the longest track record.1,2 5-FU represses DNA synthesis. It’s helpful to describe 5-FU to patients as “fake DNA” that targets precancerous cells that are dividing rapidly. But a word of caution: Patients should be advised, in advance, to avoid significant sun exposure while using 5-FU, as the drug will lose its targeted effect and cause more generalized skin damage.
Physicians can modulate the severity of the response to 5-FU by decreasing the frequency or length of therapy by using a weaker (and more expensive) once daily 0.5% long-acting formulation. Additionally, to improve comfort, low-potency topical steroids such as hydrocortisone ointment 0.5% to 2.5% can be applied after completion of therapy to speed up the healing process. These adjustments improve tolerance of therapy, but the precise effect on efficacy is unknown.
Because of the degree of redness and erythema that developed in this patient, treatment was stopped a week early. There was also concern about possible bacterial involvement in the heavy skin sloughing, so the patient was given topical mupirocin ointment to apply TID for 7 days. Her skin cleared after 3 weeks and all previous AKs were clinically eliminated.
Text courtesy of Jonathan Karnes, MD, medical director, MDFMR Dermatology Services, Augusta, ME. Photos courtesy of Jonathan Karnes, MD (copyright retained).
1. Gupta AK, Paquet M. Network meta-analysis of the outcome 'participant complete clearance' in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
2. Jansen MHE, Kessels JPHM, Merks I, et al. A trial-based cost-effectiveness analysis of topical 5-fluorouracil vs. imiquimod vs. ingenol mebutate vs. methyl aminolaevulinate conventional photodynamic therapy for the treatment of actinic keratosis in the head and neck area performed in the Netherlands. Br J Dermatol. 2020;183:738-744. doi: 10.1111/bjd.18884
1. Gupta AK, Paquet M. Network meta-analysis of the outcome 'participant complete clearance' in nonimmunosuppressed participants of eight interventions for actinic keratosis: a follow-up on a Cochrane review. Br J Dermatol. 2013;169:250-259. doi: 10.1111/bjd.12343
2. Jansen MHE, Kessels JPHM, Merks I, et al. A trial-based cost-effectiveness analysis of topical 5-fluorouracil vs. imiquimod vs. ingenol mebutate vs. methyl aminolaevulinate conventional photodynamic therapy for the treatment of actinic keratosis in the head and neck area performed in the Netherlands. Br J Dermatol. 2020;183:738-744. doi: 10.1111/bjd.18884
Atopic dermatitis doubles risk of mental health issues in children
, according to a newly published cohort study of more than 11,000 children between the ages of 3 and 18 years.
Along with previous studies that have also linked AD to depression and other mental health issues in children, these data highlight the need for “clinical awareness of the psychosocial needs of children and adolescents with AD,” reported a multicenter team of investigators from the University of California, San Francisco, the University of Pennsylvania, and the London School of Hygiene and Tropical Medicine.
Unlike some previous studies, in this study, published online in JAMA Dermatology on Sept. 1, children were evaluated longitudinally, rather than at a single point in time, with a mean follow-up of 10 years. For those with active AD, compared with children without AD, the odds ratio for depression overall in any child with AD relative to those without AD was not significant after adjustment for variables such socioeconomic factors.
However, among children with severe AD, the risk was more than twofold greater even after adjustment (adjusted OR, 2.38; 95% confidence interval, 1.21- 4.72), reported the investigators, led by senior author Katrina Abuabara, MD, associate professor of dermatology and epidemiology at UCSF.
Internalizing symptoms seen with mild to severe AD
Internalizing behavior, which is closely linked to depression and describes a spectrum of inward-focusing activities, such as social withdrawal, was significantly more common in children with any degree of AD relative to those without AD: After adjustment, the risk climbed from a 29% increased risk in those with mild AD (aOR, 1.29; 95% CI, 1.06-1.57) to a more than 80% increased risk in children with moderate AD (aOR, 1.84; 95% CI, 1.40-2.41) and in children with severe AD (aOR, 1.90; 95% CI, 1.14-3.16).
In the study, depression was measured with the Short Moods and Feelings Questionnaire (SMFQ). Parental response to the Emotional Symptoms subscale of the Strength and Difficulties Questionnaire (SDQ) was used to measure internalizing behaviors.
The data were drawn from the Avon Longitudinal Study for Parents and Children (ALSPAC), a cohort that enrolled pregnant women in a defined area in southwest England and then followed children born from these pregnancies. Of the 14,062 children enrolled in ALSPAC, data from 11,181 children were available for this study.
In a previous meta-analysis of studies that have documented a link between AD and adverse effects on mood and mental health, an impact was identified in both children and adults. In children, AD was associated with a 27% increase in risk of depression (OR, 1.27; 95% CI, 1.12 -1.45). In adults, the risk was more than doubled (OR, 2.19; 95% CI, 1.87-2.57). The same meta-analysis found that the risk of suicidal ideation among adolescents and adults with AD was increased more than fourfold (OR, 4.32; 95% CI, 1.93-9.66).
In the ALSPAC data, the investigators were unable to find compelling evidence that sleep disturbances or concomitant asthma contributed to the increased risk of depression, which is a mechanism proposed by past investigators.
In an interview, Dr. Abuabara said that these and other data provide the basis for encouraging clinical awareness of the psychological needs of children with AD, but she suggested there is a gap in understanding what this means clinically. “We need more data on how dermatologists can effectively screen and manage these patients before we try to set expectations for clinical practice,” she said.
In addition, these data along with previously published studies suggest that change in mental health outcomes should be included in the evaluation of new therapies, according to Dr. Abuabara. She noted that there are several tools for evaluating mental health in children that might be appropriate, each with their own advantages and disadvantages.
“Ideally, recommendations would be issued through a group consensus process with patients, clinicians, researchers, and industry representatives working together as has been done for other outcomes through the Harmonizing Measures for Eczema (HOME) group,” Dr. Abuabara said.
Mental health assessments recommended
Others who have looked at the relationship between AD and depression have also recommended adding mental health outcomes to an assessment of efficacy for AD therapies.
Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, is one such investigator. He is already monitoring depression systematically with the Hospital Anxiety and Depression Scale (HADS).
“HADS has been validated in AD and provides very important information about the emotional burden of AD,” explained Dr. Silverberg, whose most recent article on this topic appeared earlier this year. In that study, the relationship between AD and depression was found to be more pronounced in White children from families with lower incomes.
“Just a few hours ago, one of my patients thanked me for asking about their mental health and recognizing the holistic effects of AD,” Dr. Silverberg said.
The recent study based on ALSPAC data add to the evidence that AD, particularly severe AD, produces deleterious effects on mental health in children, and Dr. Silverberg believes clinicians should be acting on this evidence.
“I strongly encourage clinicians to routinely assess mental health. It will elevate the quality of care they provide, and their patients will appreciate them more for it,” he said.
Dr. Abuabara and another author report receiving research funding from Pfizer to their universities for unrelated work; there were no other disclosures. Dr. Silverberg reports financial relationships with more than 15 pharmaceutical companies.
Commentary by Lawrence F. Eichenfield, MD
More severe atopic dermatitis (AD) carries with it significant mental health concerns in children, as well as adults. Multiple studies have shown significantly higher rates of depression, anxiety, and “internalizing behaviors” (discussed as social withdrawal and other inward-focused activities) as well as attention-deficit/hyperactivity disorder. The study by Dr. Abuabara and colleagues is important as it followed children over time (an average of 10 years) and adjusted the data for socioeconomic factors, showing a rate of depression in children with severe AD twice that of those without. It appears that we are in the midst of a mental health crisis in children and teens, with markedly higher rates of pediatric and adolescent depression and anxiety, certainly influenced by COVID-19 societal changes. As the literature has developed on depression and AD, we have appreciated the importance of addressing this as part of our assessment of the disease effect on the individual and family, and it is one factor we consider in selections of systemic vs. topical therapies.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
, according to a newly published cohort study of more than 11,000 children between the ages of 3 and 18 years.
Along with previous studies that have also linked AD to depression and other mental health issues in children, these data highlight the need for “clinical awareness of the psychosocial needs of children and adolescents with AD,” reported a multicenter team of investigators from the University of California, San Francisco, the University of Pennsylvania, and the London School of Hygiene and Tropical Medicine.
Unlike some previous studies, in this study, published online in JAMA Dermatology on Sept. 1, children were evaluated longitudinally, rather than at a single point in time, with a mean follow-up of 10 years. For those with active AD, compared with children without AD, the odds ratio for depression overall in any child with AD relative to those without AD was not significant after adjustment for variables such socioeconomic factors.
However, among children with severe AD, the risk was more than twofold greater even after adjustment (adjusted OR, 2.38; 95% confidence interval, 1.21- 4.72), reported the investigators, led by senior author Katrina Abuabara, MD, associate professor of dermatology and epidemiology at UCSF.
Internalizing symptoms seen with mild to severe AD
Internalizing behavior, which is closely linked to depression and describes a spectrum of inward-focusing activities, such as social withdrawal, was significantly more common in children with any degree of AD relative to those without AD: After adjustment, the risk climbed from a 29% increased risk in those with mild AD (aOR, 1.29; 95% CI, 1.06-1.57) to a more than 80% increased risk in children with moderate AD (aOR, 1.84; 95% CI, 1.40-2.41) and in children with severe AD (aOR, 1.90; 95% CI, 1.14-3.16).
In the study, depression was measured with the Short Moods and Feelings Questionnaire (SMFQ). Parental response to the Emotional Symptoms subscale of the Strength and Difficulties Questionnaire (SDQ) was used to measure internalizing behaviors.
The data were drawn from the Avon Longitudinal Study for Parents and Children (ALSPAC), a cohort that enrolled pregnant women in a defined area in southwest England and then followed children born from these pregnancies. Of the 14,062 children enrolled in ALSPAC, data from 11,181 children were available for this study.
In a previous meta-analysis of studies that have documented a link between AD and adverse effects on mood and mental health, an impact was identified in both children and adults. In children, AD was associated with a 27% increase in risk of depression (OR, 1.27; 95% CI, 1.12 -1.45). In adults, the risk was more than doubled (OR, 2.19; 95% CI, 1.87-2.57). The same meta-analysis found that the risk of suicidal ideation among adolescents and adults with AD was increased more than fourfold (OR, 4.32; 95% CI, 1.93-9.66).
In the ALSPAC data, the investigators were unable to find compelling evidence that sleep disturbances or concomitant asthma contributed to the increased risk of depression, which is a mechanism proposed by past investigators.
In an interview, Dr. Abuabara said that these and other data provide the basis for encouraging clinical awareness of the psychological needs of children with AD, but she suggested there is a gap in understanding what this means clinically. “We need more data on how dermatologists can effectively screen and manage these patients before we try to set expectations for clinical practice,” she said.
In addition, these data along with previously published studies suggest that change in mental health outcomes should be included in the evaluation of new therapies, according to Dr. Abuabara. She noted that there are several tools for evaluating mental health in children that might be appropriate, each with their own advantages and disadvantages.
“Ideally, recommendations would be issued through a group consensus process with patients, clinicians, researchers, and industry representatives working together as has been done for other outcomes through the Harmonizing Measures for Eczema (HOME) group,” Dr. Abuabara said.
Mental health assessments recommended
Others who have looked at the relationship between AD and depression have also recommended adding mental health outcomes to an assessment of efficacy for AD therapies.
Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, is one such investigator. He is already monitoring depression systematically with the Hospital Anxiety and Depression Scale (HADS).
“HADS has been validated in AD and provides very important information about the emotional burden of AD,” explained Dr. Silverberg, whose most recent article on this topic appeared earlier this year. In that study, the relationship between AD and depression was found to be more pronounced in White children from families with lower incomes.
“Just a few hours ago, one of my patients thanked me for asking about their mental health and recognizing the holistic effects of AD,” Dr. Silverberg said.
The recent study based on ALSPAC data add to the evidence that AD, particularly severe AD, produces deleterious effects on mental health in children, and Dr. Silverberg believes clinicians should be acting on this evidence.
“I strongly encourage clinicians to routinely assess mental health. It will elevate the quality of care they provide, and their patients will appreciate them more for it,” he said.
Dr. Abuabara and another author report receiving research funding from Pfizer to their universities for unrelated work; there were no other disclosures. Dr. Silverberg reports financial relationships with more than 15 pharmaceutical companies.
Commentary by Lawrence F. Eichenfield, MD
More severe atopic dermatitis (AD) carries with it significant mental health concerns in children, as well as adults. Multiple studies have shown significantly higher rates of depression, anxiety, and “internalizing behaviors” (discussed as social withdrawal and other inward-focused activities) as well as attention-deficit/hyperactivity disorder. The study by Dr. Abuabara and colleagues is important as it followed children over time (an average of 10 years) and adjusted the data for socioeconomic factors, showing a rate of depression in children with severe AD twice that of those without. It appears that we are in the midst of a mental health crisis in children and teens, with markedly higher rates of pediatric and adolescent depression and anxiety, certainly influenced by COVID-19 societal changes. As the literature has developed on depression and AD, we have appreciated the importance of addressing this as part of our assessment of the disease effect on the individual and family, and it is one factor we consider in selections of systemic vs. topical therapies.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
, according to a newly published cohort study of more than 11,000 children between the ages of 3 and 18 years.
Along with previous studies that have also linked AD to depression and other mental health issues in children, these data highlight the need for “clinical awareness of the psychosocial needs of children and adolescents with AD,” reported a multicenter team of investigators from the University of California, San Francisco, the University of Pennsylvania, and the London School of Hygiene and Tropical Medicine.
Unlike some previous studies, in this study, published online in JAMA Dermatology on Sept. 1, children were evaluated longitudinally, rather than at a single point in time, with a mean follow-up of 10 years. For those with active AD, compared with children without AD, the odds ratio for depression overall in any child with AD relative to those without AD was not significant after adjustment for variables such socioeconomic factors.
However, among children with severe AD, the risk was more than twofold greater even after adjustment (adjusted OR, 2.38; 95% confidence interval, 1.21- 4.72), reported the investigators, led by senior author Katrina Abuabara, MD, associate professor of dermatology and epidemiology at UCSF.
Internalizing symptoms seen with mild to severe AD
Internalizing behavior, which is closely linked to depression and describes a spectrum of inward-focusing activities, such as social withdrawal, was significantly more common in children with any degree of AD relative to those without AD: After adjustment, the risk climbed from a 29% increased risk in those with mild AD (aOR, 1.29; 95% CI, 1.06-1.57) to a more than 80% increased risk in children with moderate AD (aOR, 1.84; 95% CI, 1.40-2.41) and in children with severe AD (aOR, 1.90; 95% CI, 1.14-3.16).
In the study, depression was measured with the Short Moods and Feelings Questionnaire (SMFQ). Parental response to the Emotional Symptoms subscale of the Strength and Difficulties Questionnaire (SDQ) was used to measure internalizing behaviors.
The data were drawn from the Avon Longitudinal Study for Parents and Children (ALSPAC), a cohort that enrolled pregnant women in a defined area in southwest England and then followed children born from these pregnancies. Of the 14,062 children enrolled in ALSPAC, data from 11,181 children were available for this study.
In a previous meta-analysis of studies that have documented a link between AD and adverse effects on mood and mental health, an impact was identified in both children and adults. In children, AD was associated with a 27% increase in risk of depression (OR, 1.27; 95% CI, 1.12 -1.45). In adults, the risk was more than doubled (OR, 2.19; 95% CI, 1.87-2.57). The same meta-analysis found that the risk of suicidal ideation among adolescents and adults with AD was increased more than fourfold (OR, 4.32; 95% CI, 1.93-9.66).
In the ALSPAC data, the investigators were unable to find compelling evidence that sleep disturbances or concomitant asthma contributed to the increased risk of depression, which is a mechanism proposed by past investigators.
In an interview, Dr. Abuabara said that these and other data provide the basis for encouraging clinical awareness of the psychological needs of children with AD, but she suggested there is a gap in understanding what this means clinically. “We need more data on how dermatologists can effectively screen and manage these patients before we try to set expectations for clinical practice,” she said.
In addition, these data along with previously published studies suggest that change in mental health outcomes should be included in the evaluation of new therapies, according to Dr. Abuabara. She noted that there are several tools for evaluating mental health in children that might be appropriate, each with their own advantages and disadvantages.
“Ideally, recommendations would be issued through a group consensus process with patients, clinicians, researchers, and industry representatives working together as has been done for other outcomes through the Harmonizing Measures for Eczema (HOME) group,” Dr. Abuabara said.
Mental health assessments recommended
Others who have looked at the relationship between AD and depression have also recommended adding mental health outcomes to an assessment of efficacy for AD therapies.
Jonathan I. Silverberg, MD, PhD, MPH, associate professor of dermatology, George Washington University, Washington, is one such investigator. He is already monitoring depression systematically with the Hospital Anxiety and Depression Scale (HADS).
“HADS has been validated in AD and provides very important information about the emotional burden of AD,” explained Dr. Silverberg, whose most recent article on this topic appeared earlier this year. In that study, the relationship between AD and depression was found to be more pronounced in White children from families with lower incomes.
“Just a few hours ago, one of my patients thanked me for asking about their mental health and recognizing the holistic effects of AD,” Dr. Silverberg said.
The recent study based on ALSPAC data add to the evidence that AD, particularly severe AD, produces deleterious effects on mental health in children, and Dr. Silverberg believes clinicians should be acting on this evidence.
“I strongly encourage clinicians to routinely assess mental health. It will elevate the quality of care they provide, and their patients will appreciate them more for it,” he said.
Dr. Abuabara and another author report receiving research funding from Pfizer to their universities for unrelated work; there were no other disclosures. Dr. Silverberg reports financial relationships with more than 15 pharmaceutical companies.
Commentary by Lawrence F. Eichenfield, MD
More severe atopic dermatitis (AD) carries with it significant mental health concerns in children, as well as adults. Multiple studies have shown significantly higher rates of depression, anxiety, and “internalizing behaviors” (discussed as social withdrawal and other inward-focused activities) as well as attention-deficit/hyperactivity disorder. The study by Dr. Abuabara and colleagues is important as it followed children over time (an average of 10 years) and adjusted the data for socioeconomic factors, showing a rate of depression in children with severe AD twice that of those without. It appears that we are in the midst of a mental health crisis in children and teens, with markedly higher rates of pediatric and adolescent depression and anxiety, certainly influenced by COVID-19 societal changes. As the literature has developed on depression and AD, we have appreciated the importance of addressing this as part of our assessment of the disease effect on the individual and family, and it is one factor we consider in selections of systemic vs. topical therapies.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego. He is vice chair of the department of dermatology and professor of dermatology and pediatrics at the University of California, San Diego. He disclosed that he has served as an investigator and/or consultant to AbbVie, Lilly, Pfizer, Regeneron, Sanofi-Genzyme, and Verrica.
A version of this article first appeared on Medscape.com.
This article was updated 6/18/22.
FROM JAMA DERMATOLOGY
New valvular heart disease guidelines change several repair indications
Antithrombotic recommendations also altered
New European guidelines for the management of valvular heart disease offer more than 45 revised or completely new recommendations relative to the previous version published in 2017, according to members of the writing committee who presented the changes during the annual congress of the European Society of Cardiology.
With their emphasis on early diagnosis and expansion of indications, these 2021 ESC/European Association for Cardio-Thoracic Surgery guidelines are likely to further accelerate the already steep growth in this area of interventional cardiology, according to Alec Vahanian, MD, a professor of cardiology at the University of Paris.
“Valvular heart disease is too often undetected, and these guidelines stress the importance of clinical examination and the best strategies for diagnosis as well as treatment,” he said.
Of the multiple sections and subsections, which follow the same format of the previous guidelines, the greatest number of revisions and new additions involve perioperative antithrombotic therapy, according to the document, which was published in conjunction with the ESC Congress.
Eleven new guidelines for anticoagulants
On the basis of evidence published since the previous guidelines, there are 11 completely new recommendations regarding the use of anticoagulants or antiplatelet therapies. The majority of these have received a grade I indication, which signifies “recommended” or “indicated.” These include indications for stopping or starting anticoagulants and which anticoagulants or antiplatelet drugs to consider in specific patient populations.
The next most common focus of new or revised recommendations involves when to consider surgical aortic valve repair (SAVR) relative to transcatheter aortic valve implantation (TAVI) in severe aortic stenosis. Most of these represent revisions from the previous guidelines, but almost all are also grade I recommendations.
“SAVR and TAVI are both excellent options in appropriate patients, but they are not interchangeable,” explained Bernard D. Prendergast, BMedSci, MD, director of the cardiac structural intervention program, Guy’s and St Thomas’ Hospital, London.
While the previous guidelines generally reserved TAVI for those not suitable for SAVR, the new guidelines are more nuanced.
As a rule, SAVR is generally preferred for younger patients. The reason, according to Dr. Prendergast, is concern that younger patients might outlive the expected lifespan of the prosthetic TAVI device.
No single criterion for selecting SAVR over TAVI
However, there are many exceptions and additional considerations beyond age. When both SAVR and TAVI are otherwise suitable options, but TAVI cannot be performed with a transfemoral access, Dr. Prendergast pointed out that SAVR might be a better choice.
Transfemoral access is the preferred strategy in TAVI, but Dr. Prendergast emphasized that a collaborative “heart team” should help patients select the most appropriate option. In fact, there is a grade IIb recommendation (“usefulness or efficacy is less well established”) to consider other access sites in patients at high surgical risk with contraindications for transfemoral TAVI.
Of new recommendations in the area of severe aortic stenosis, valvular repair may now be considered in asymptomatic patients with a left ventricular ejection fraction of less than 55%, according to a grade IIb recommendation. The 2017 guidelines did not address this issue.
Grade I recommendation for bioprostheses
A substantial number of the revisions involve minor clarifications without a change in the grade, but a revision regarding bioprosthetics is substantial. In the 2017 guidelines, there was a grade IIa recommendation (“weight of evidence is in favor”) to consider bioprosthetics in patients with a life expectancy less than the expected durability of the device. The 2021 guidelines have changed this to a grade I indication, adding an indication for bioprostheses in patients contraindicated for or unlikely to achieve good-quality anticoagulation.
On this same topic, there is a new grade IIb recommendation to consider bioprosthesis over alternative devices in patients already on a long-term non–vitamin K oral anticoagulant because of the high risk of thromboembolism.
There are two new recommendations in the realm of severe secondary mitral regurgitation, reported Fabien Praz, MD, an interventional cardiologist at the University of Bern (Switzerland). The first, which is perhaps the most significant, is a grade I recommendation for valve surgery in patients with severe secondary mitral regurgitation who remain symptomatic despite optimized medical therapy.
The second is a grade IIa recommendation for invasive procedures, such as a percutaneous coronary intervention, for patients with symptomatic secondary mitral regurgitation and coexisting coronary artery disease. In both cases, however, Dr. Praz emphasized language in the guidelines that calls for a collaborative heart team to agree on the suitability of these treatments.
Ultimately, none of these recommendations can be divorced from patient expectations and values, according to Friedhelm Beyersdorf, MD, chairman of the department of cardiovascular surgery at the Heart Center of the University of Freiburg (Germany).
Even if treatment is not expected to prolong life, “symptom relief on its own may justify intervention,” said Dr. Beyersdorf. However, he emphasized that “thoroughly informed” patients are an essential part of the process in selecting a treatment strategy most likely to satisfy patient goals.
Dr. Vahanian and Dr. Prendergast reported no conflicts of interest relevant to these guidelines. Dr. Praz reported a financial relationship with Edwards Lifesciences. Dr. Beyersdorf reported a financial relationship with Resuscitec.
Antithrombotic recommendations also altered
Antithrombotic recommendations also altered
New European guidelines for the management of valvular heart disease offer more than 45 revised or completely new recommendations relative to the previous version published in 2017, according to members of the writing committee who presented the changes during the annual congress of the European Society of Cardiology.
With their emphasis on early diagnosis and expansion of indications, these 2021 ESC/European Association for Cardio-Thoracic Surgery guidelines are likely to further accelerate the already steep growth in this area of interventional cardiology, according to Alec Vahanian, MD, a professor of cardiology at the University of Paris.
“Valvular heart disease is too often undetected, and these guidelines stress the importance of clinical examination and the best strategies for diagnosis as well as treatment,” he said.
Of the multiple sections and subsections, which follow the same format of the previous guidelines, the greatest number of revisions and new additions involve perioperative antithrombotic therapy, according to the document, which was published in conjunction with the ESC Congress.
Eleven new guidelines for anticoagulants
On the basis of evidence published since the previous guidelines, there are 11 completely new recommendations regarding the use of anticoagulants or antiplatelet therapies. The majority of these have received a grade I indication, which signifies “recommended” or “indicated.” These include indications for stopping or starting anticoagulants and which anticoagulants or antiplatelet drugs to consider in specific patient populations.
The next most common focus of new or revised recommendations involves when to consider surgical aortic valve repair (SAVR) relative to transcatheter aortic valve implantation (TAVI) in severe aortic stenosis. Most of these represent revisions from the previous guidelines, but almost all are also grade I recommendations.
“SAVR and TAVI are both excellent options in appropriate patients, but they are not interchangeable,” explained Bernard D. Prendergast, BMedSci, MD, director of the cardiac structural intervention program, Guy’s and St Thomas’ Hospital, London.
While the previous guidelines generally reserved TAVI for those not suitable for SAVR, the new guidelines are more nuanced.
As a rule, SAVR is generally preferred for younger patients. The reason, according to Dr. Prendergast, is concern that younger patients might outlive the expected lifespan of the prosthetic TAVI device.
No single criterion for selecting SAVR over TAVI
However, there are many exceptions and additional considerations beyond age. When both SAVR and TAVI are otherwise suitable options, but TAVI cannot be performed with a transfemoral access, Dr. Prendergast pointed out that SAVR might be a better choice.
Transfemoral access is the preferred strategy in TAVI, but Dr. Prendergast emphasized that a collaborative “heart team” should help patients select the most appropriate option. In fact, there is a grade IIb recommendation (“usefulness or efficacy is less well established”) to consider other access sites in patients at high surgical risk with contraindications for transfemoral TAVI.
Of new recommendations in the area of severe aortic stenosis, valvular repair may now be considered in asymptomatic patients with a left ventricular ejection fraction of less than 55%, according to a grade IIb recommendation. The 2017 guidelines did not address this issue.
Grade I recommendation for bioprostheses
A substantial number of the revisions involve minor clarifications without a change in the grade, but a revision regarding bioprosthetics is substantial. In the 2017 guidelines, there was a grade IIa recommendation (“weight of evidence is in favor”) to consider bioprosthetics in patients with a life expectancy less than the expected durability of the device. The 2021 guidelines have changed this to a grade I indication, adding an indication for bioprostheses in patients contraindicated for or unlikely to achieve good-quality anticoagulation.
On this same topic, there is a new grade IIb recommendation to consider bioprosthesis over alternative devices in patients already on a long-term non–vitamin K oral anticoagulant because of the high risk of thromboembolism.
There are two new recommendations in the realm of severe secondary mitral regurgitation, reported Fabien Praz, MD, an interventional cardiologist at the University of Bern (Switzerland). The first, which is perhaps the most significant, is a grade I recommendation for valve surgery in patients with severe secondary mitral regurgitation who remain symptomatic despite optimized medical therapy.
The second is a grade IIa recommendation for invasive procedures, such as a percutaneous coronary intervention, for patients with symptomatic secondary mitral regurgitation and coexisting coronary artery disease. In both cases, however, Dr. Praz emphasized language in the guidelines that calls for a collaborative heart team to agree on the suitability of these treatments.
Ultimately, none of these recommendations can be divorced from patient expectations and values, according to Friedhelm Beyersdorf, MD, chairman of the department of cardiovascular surgery at the Heart Center of the University of Freiburg (Germany).
Even if treatment is not expected to prolong life, “symptom relief on its own may justify intervention,” said Dr. Beyersdorf. However, he emphasized that “thoroughly informed” patients are an essential part of the process in selecting a treatment strategy most likely to satisfy patient goals.
Dr. Vahanian and Dr. Prendergast reported no conflicts of interest relevant to these guidelines. Dr. Praz reported a financial relationship with Edwards Lifesciences. Dr. Beyersdorf reported a financial relationship with Resuscitec.
New European guidelines for the management of valvular heart disease offer more than 45 revised or completely new recommendations relative to the previous version published in 2017, according to members of the writing committee who presented the changes during the annual congress of the European Society of Cardiology.
With their emphasis on early diagnosis and expansion of indications, these 2021 ESC/European Association for Cardio-Thoracic Surgery guidelines are likely to further accelerate the already steep growth in this area of interventional cardiology, according to Alec Vahanian, MD, a professor of cardiology at the University of Paris.
“Valvular heart disease is too often undetected, and these guidelines stress the importance of clinical examination and the best strategies for diagnosis as well as treatment,” he said.
Of the multiple sections and subsections, which follow the same format of the previous guidelines, the greatest number of revisions and new additions involve perioperative antithrombotic therapy, according to the document, which was published in conjunction with the ESC Congress.
Eleven new guidelines for anticoagulants
On the basis of evidence published since the previous guidelines, there are 11 completely new recommendations regarding the use of anticoagulants or antiplatelet therapies. The majority of these have received a grade I indication, which signifies “recommended” or “indicated.” These include indications for stopping or starting anticoagulants and which anticoagulants or antiplatelet drugs to consider in specific patient populations.
The next most common focus of new or revised recommendations involves when to consider surgical aortic valve repair (SAVR) relative to transcatheter aortic valve implantation (TAVI) in severe aortic stenosis. Most of these represent revisions from the previous guidelines, but almost all are also grade I recommendations.
“SAVR and TAVI are both excellent options in appropriate patients, but they are not interchangeable,” explained Bernard D. Prendergast, BMedSci, MD, director of the cardiac structural intervention program, Guy’s and St Thomas’ Hospital, London.
While the previous guidelines generally reserved TAVI for those not suitable for SAVR, the new guidelines are more nuanced.
As a rule, SAVR is generally preferred for younger patients. The reason, according to Dr. Prendergast, is concern that younger patients might outlive the expected lifespan of the prosthetic TAVI device.
No single criterion for selecting SAVR over TAVI
However, there are many exceptions and additional considerations beyond age. When both SAVR and TAVI are otherwise suitable options, but TAVI cannot be performed with a transfemoral access, Dr. Prendergast pointed out that SAVR might be a better choice.
Transfemoral access is the preferred strategy in TAVI, but Dr. Prendergast emphasized that a collaborative “heart team” should help patients select the most appropriate option. In fact, there is a grade IIb recommendation (“usefulness or efficacy is less well established”) to consider other access sites in patients at high surgical risk with contraindications for transfemoral TAVI.
Of new recommendations in the area of severe aortic stenosis, valvular repair may now be considered in asymptomatic patients with a left ventricular ejection fraction of less than 55%, according to a grade IIb recommendation. The 2017 guidelines did not address this issue.
Grade I recommendation for bioprostheses
A substantial number of the revisions involve minor clarifications without a change in the grade, but a revision regarding bioprosthetics is substantial. In the 2017 guidelines, there was a grade IIa recommendation (“weight of evidence is in favor”) to consider bioprosthetics in patients with a life expectancy less than the expected durability of the device. The 2021 guidelines have changed this to a grade I indication, adding an indication for bioprostheses in patients contraindicated for or unlikely to achieve good-quality anticoagulation.
On this same topic, there is a new grade IIb recommendation to consider bioprosthesis over alternative devices in patients already on a long-term non–vitamin K oral anticoagulant because of the high risk of thromboembolism.
There are two new recommendations in the realm of severe secondary mitral regurgitation, reported Fabien Praz, MD, an interventional cardiologist at the University of Bern (Switzerland). The first, which is perhaps the most significant, is a grade I recommendation for valve surgery in patients with severe secondary mitral regurgitation who remain symptomatic despite optimized medical therapy.
The second is a grade IIa recommendation for invasive procedures, such as a percutaneous coronary intervention, for patients with symptomatic secondary mitral regurgitation and coexisting coronary artery disease. In both cases, however, Dr. Praz emphasized language in the guidelines that calls for a collaborative heart team to agree on the suitability of these treatments.
Ultimately, none of these recommendations can be divorced from patient expectations and values, according to Friedhelm Beyersdorf, MD, chairman of the department of cardiovascular surgery at the Heart Center of the University of Freiburg (Germany).
Even if treatment is not expected to prolong life, “symptom relief on its own may justify intervention,” said Dr. Beyersdorf. However, he emphasized that “thoroughly informed” patients are an essential part of the process in selecting a treatment strategy most likely to satisfy patient goals.
Dr. Vahanian and Dr. Prendergast reported no conflicts of interest relevant to these guidelines. Dr. Praz reported a financial relationship with Edwards Lifesciences. Dr. Beyersdorf reported a financial relationship with Resuscitec.
FROM ESC 2021
A Rapidly Progressive Thoracic Tumor
Introduction
SMARCA4-deficient thoracic sarcomas are a rare entity, first described in 2015 in a study of 19 patients with a median age of 41 years who presented with large compressive masses with frequent infiltration into surrounding tissues [1]. This malignancy is more frequent in younger males (median 41-59 years) with an extensive smoking history and has an aggressive course with a median overall survival of 4-7 months [1-3]. There is currently no established treatment, but case reports show promise for immunotherapy and immuno- chemotherapy [4-8].
Case Report
We present the case of a 62 year old male with a 44 pack year smoking history who first presented to the emergency department (ED) with left shoulder pain in December 2020. He was initially treated with muscle relaxers but returned to the ED ten days later with hemoptysis and rapid weight loss. X-ray showed a 14.2 X 11.7 cm mass with rightward deviation of the trachea. PET scan showed extensive central necrosis with a surrounding pleural effusion and local pleural and nodal metastasis but no distant disease. He underwent thoracentesis which was negative for malignant cells. He underwent CT-guided biopsy in 1/2021, which showed predominantly discohesive small blue cells with pleomorphic cell contour and slightly plasmacytoid features. Extensive pathology review led to a diagnosis of SMARCA4 deficient thoracic sarcoma. On presentation to oncology clinic in 2/2021 his functional status had markedly deteriorated. He was started on ipilimumab/ nivolumab (ipi/nivo) and 1 week after his first cycle was admitted for severe left arm swelling and pain. Imaging showed significant progression of disease and new adrenal metastasis. He received cycle two of ipi/ nivo and was able to be discharged home on oxygen. By his follow-up appointment for cycle three of ipi/nivo in 3/2021, the patient was wheelchair bound with severe dyspnea. X-ray showed the mass now occupied the majority of the left hemi-thorax with worsening tracheal deviation. After discussion, the patient went home on hospice and died 8 days later. As demonstrated by this case, SMARCA4-deficient sarcoma requires high clinical suspicion with prompt diagnosis and treatment given its remarkably rapid progression and poor outcomes.
Introduction
SMARCA4-deficient thoracic sarcomas are a rare entity, first described in 2015 in a study of 19 patients with a median age of 41 years who presented with large compressive masses with frequent infiltration into surrounding tissues [1]. This malignancy is more frequent in younger males (median 41-59 years) with an extensive smoking history and has an aggressive course with a median overall survival of 4-7 months [1-3]. There is currently no established treatment, but case reports show promise for immunotherapy and immuno- chemotherapy [4-8].
Case Report
We present the case of a 62 year old male with a 44 pack year smoking history who first presented to the emergency department (ED) with left shoulder pain in December 2020. He was initially treated with muscle relaxers but returned to the ED ten days later with hemoptysis and rapid weight loss. X-ray showed a 14.2 X 11.7 cm mass with rightward deviation of the trachea. PET scan showed extensive central necrosis with a surrounding pleural effusion and local pleural and nodal metastasis but no distant disease. He underwent thoracentesis which was negative for malignant cells. He underwent CT-guided biopsy in 1/2021, which showed predominantly discohesive small blue cells with pleomorphic cell contour and slightly plasmacytoid features. Extensive pathology review led to a diagnosis of SMARCA4 deficient thoracic sarcoma. On presentation to oncology clinic in 2/2021 his functional status had markedly deteriorated. He was started on ipilimumab/ nivolumab (ipi/nivo) and 1 week after his first cycle was admitted for severe left arm swelling and pain. Imaging showed significant progression of disease and new adrenal metastasis. He received cycle two of ipi/ nivo and was able to be discharged home on oxygen. By his follow-up appointment for cycle three of ipi/nivo in 3/2021, the patient was wheelchair bound with severe dyspnea. X-ray showed the mass now occupied the majority of the left hemi-thorax with worsening tracheal deviation. After discussion, the patient went home on hospice and died 8 days later. As demonstrated by this case, SMARCA4-deficient sarcoma requires high clinical suspicion with prompt diagnosis and treatment given its remarkably rapid progression and poor outcomes.
Introduction
SMARCA4-deficient thoracic sarcomas are a rare entity, first described in 2015 in a study of 19 patients with a median age of 41 years who presented with large compressive masses with frequent infiltration into surrounding tissues [1]. This malignancy is more frequent in younger males (median 41-59 years) with an extensive smoking history and has an aggressive course with a median overall survival of 4-7 months [1-3]. There is currently no established treatment, but case reports show promise for immunotherapy and immuno- chemotherapy [4-8].
Case Report
We present the case of a 62 year old male with a 44 pack year smoking history who first presented to the emergency department (ED) with left shoulder pain in December 2020. He was initially treated with muscle relaxers but returned to the ED ten days later with hemoptysis and rapid weight loss. X-ray showed a 14.2 X 11.7 cm mass with rightward deviation of the trachea. PET scan showed extensive central necrosis with a surrounding pleural effusion and local pleural and nodal metastasis but no distant disease. He underwent thoracentesis which was negative for malignant cells. He underwent CT-guided biopsy in 1/2021, which showed predominantly discohesive small blue cells with pleomorphic cell contour and slightly plasmacytoid features. Extensive pathology review led to a diagnosis of SMARCA4 deficient thoracic sarcoma. On presentation to oncology clinic in 2/2021 his functional status had markedly deteriorated. He was started on ipilimumab/ nivolumab (ipi/nivo) and 1 week after his first cycle was admitted for severe left arm swelling and pain. Imaging showed significant progression of disease and new adrenal metastasis. He received cycle two of ipi/ nivo and was able to be discharged home on oxygen. By his follow-up appointment for cycle three of ipi/nivo in 3/2021, the patient was wheelchair bound with severe dyspnea. X-ray showed the mass now occupied the majority of the left hemi-thorax with worsening tracheal deviation. After discussion, the patient went home on hospice and died 8 days later. As demonstrated by this case, SMARCA4-deficient sarcoma requires high clinical suspicion with prompt diagnosis and treatment given its remarkably rapid progression and poor outcomes.
Choosing Wisely campaign targets waste and overuse in hospital pediatrics
“Health care spending and health care waste is a huge problem in the U.S., including for children,” Vivian Lee, MD, of Children’s Hospital, Los Angeles, said in a presentation at the 2021 virtual Pediatric Hospital Medicine conference.
Data from a 2019 study suggested that approximately 25% of health care spending in the United States qualifies as “wasteful spending,” in categories such as overtesting, and unnecessary hospitalization, Dr. Lee said. “It is essential for physicians in hospitals to be stewards of high-value care,” she emphasized.
To combat wasteful spending and control health care costs, the Choosing Wisely campaign was created in 2012 as an initiative from the American Board of Internal Medicine Foundation. An ongoing goal of the campaign is to raise awareness among physicians and patients about potential areas of low-value services and overuse. The overall campaign includes clinician-driven recommendations from multiple medical organizations.
The PHM produced its first set of five recommendations in 2012, Dr. Lee said. These recommendations, titled “Five Things Physicians and Patients Should Question,” have been updated for 2021. The updated recommendations were created as a partnership among the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine. A joint committee reviewed the latest evidence, and the updates were approved by the societies and published by the ABIM in January 2021.
“We think these recommendations truly reflect an exciting and evolving landscape for pediatric hospitalists,” Dr. Lee said. “There is a greater focus on opportunities to transition out of the hospital sooner, or avoid hospitalization altogether. There is an emphasis on antibiotic stewardship and a growing recognition of the impact that overuse may have on our vulnerable neonatal population,” she said. Several members of the Choosing Wisely panel presented the recommendations during the virtual presentation.
Revised recommendations
The new “Five Things Physicians and Patients Should Question” are as follows:
1. Do not prescribe IV antibiotics for predetermined durations for patients hospitalized with infections such as pyelonephritis, osteomyelitis, and complicated pneumonia. Consider early transition to oral antibiotics.
Many antibiotic doses used in clinical practice are preset durations that are not based on high-quality evidence, said Mike Tchou, MD, of Children’s Hospital of Colorado in Aurora. However, studies now show that earlier transition to enteral antibiotics can improve a range of outcomes including neonatal UTIs, osteomyelitis, and complicated pneumonia, he said. Considering early transition based on a patient’s response can decrease adverse events, pain, length of stay, and health care costs, he explained.
2. Do not continue hospitalization in well-appearing febrile infants once bacterial cultures (i.e., blood, cerebrospinal, and/or urine) have been confirmed negative for 24-36 hours, if adequate outpatient follow-up can be assured.
Recent data indicate that continuing hospitalization beyond 24-36 hours of confirmed negative bacterial cultures does not improve clinical outcomes for well-appearing infants admitted for concern of serious bacterial infection, said Paula Soung, MD, of Children’s Wisconsin in Milwaukee. In fact, “blood culture yield is highest in the first 12-36 hours after incubation with multiple studies demonstrating > 90% of pathogen cultures being positive by 24 hours,” Dr. Soung said. “If adequate outpatient follow-up can be assured, discharging well-appearing febrile infants at 24-36 hours after confirming cultures are negative has many positive outcomes,” she said.
3. Do not initiate phototherapy in term or late preterm well-appearing infants with neonatal hyperbilirubinemia if their bilirubin is below levels at which the AAP guidelines recommend treatment.
In making this recommendation, “we considered that the risk of kernicterus and cerebral palsy is extremely low in otherwise healthy term and late preterm newborns,” said Allison Holmes, MD, of Children’s Hospital at Dartmouth-Hitchcock, Manchester, N.H. “Subthreshold phototherapy leads to unnecessary hospitalization and its associated costs and harms,” and data show that kernicterus generally occurs close to 40 mg/dL and occurs most often in infants with hemolysis, she added.
The evidence for the recommendations included data showing that, among other factors, 8.6 of 100,000 babies have a bilirubin greater than 30 mg/dL, said Dr. Holmes. Risks of using subthreshold phototherapy include increased length of stay, increased readmissions, and increased costs, as well as decreased breastfeeding, bonding with parents, and increased parental anxiety. “Adding prolonged hospitalization for an intervention that might not be necessary can be stressful for parents,” she said.
4. Do not use broad-spectrum antibiotics such as ceftriaxone for children hospitalized with uncomplicated community-acquired pneumonia. Use narrow-spectrum antibiotics such as penicillin, ampicillin, or amoxicillin.
Michelle Lossius, MD, of the Shands Hospital for Children at the University of Florida, Gainesville, noted that the recommendations reflect IDSA guidelines from 2011 advising the use of ampicillin or penicillin for this population of children. More recent studies with large populations support the ability of narrow-spectrum antibiotics to limit the development of resistant organisms while achieving the same or better outcomes for children hospitalized with CAP, she said.
5. Do not start IV antibiotic therapy on well-appearing newborn infants with isolated risk factors for sepsis such as maternal chorioamnionitis, prolonged rupture of membranes, or untreated group-B streptococcal colonization. Use clinical tools such as an evidence-based sepsis risk calculator to guide management.
“This recommendation combines other recommendations,” said Prabi Rajbhandari, MD, of Akron (Ohio) Children’s Hospital. The evidence is ample, as the Centers for Disease Control and Prevention recommends the use of sepsis calculators to guide clinical management in sepsis patients, she said.
Data comparing periods before and after the adoption of a sepsis risk calculator showed a significant reduction in the use of blood cultures and antibiotics, she noted. Other risks of jumping to IV antibiotics include increased hospital stay, increased parental anxiety, and decreased parental bonding, Dr. Rajbhandari added.
Next steps include how to prioritize implementation, as well as deimplementation of outdated practices, said Francisco Alvarez, MD, of Lucile Packard Children’s Hospital, Palo Alto, Calif. “A lot of our practices were started without good evidence for why they should be done,” he said. Other steps include value improvement research; use of dashboards and benchmarking; involving other stakeholders including patients, families, and other health care providers; and addressing racial disparities, he concluded.
The presenters had no financial conflicts to disclose. The conference was sponsored by the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine.
“Health care spending and health care waste is a huge problem in the U.S., including for children,” Vivian Lee, MD, of Children’s Hospital, Los Angeles, said in a presentation at the 2021 virtual Pediatric Hospital Medicine conference.
Data from a 2019 study suggested that approximately 25% of health care spending in the United States qualifies as “wasteful spending,” in categories such as overtesting, and unnecessary hospitalization, Dr. Lee said. “It is essential for physicians in hospitals to be stewards of high-value care,” she emphasized.
To combat wasteful spending and control health care costs, the Choosing Wisely campaign was created in 2012 as an initiative from the American Board of Internal Medicine Foundation. An ongoing goal of the campaign is to raise awareness among physicians and patients about potential areas of low-value services and overuse. The overall campaign includes clinician-driven recommendations from multiple medical organizations.
The PHM produced its first set of five recommendations in 2012, Dr. Lee said. These recommendations, titled “Five Things Physicians and Patients Should Question,” have been updated for 2021. The updated recommendations were created as a partnership among the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine. A joint committee reviewed the latest evidence, and the updates were approved by the societies and published by the ABIM in January 2021.
“We think these recommendations truly reflect an exciting and evolving landscape for pediatric hospitalists,” Dr. Lee said. “There is a greater focus on opportunities to transition out of the hospital sooner, or avoid hospitalization altogether. There is an emphasis on antibiotic stewardship and a growing recognition of the impact that overuse may have on our vulnerable neonatal population,” she said. Several members of the Choosing Wisely panel presented the recommendations during the virtual presentation.
Revised recommendations
The new “Five Things Physicians and Patients Should Question” are as follows:
1. Do not prescribe IV antibiotics for predetermined durations for patients hospitalized with infections such as pyelonephritis, osteomyelitis, and complicated pneumonia. Consider early transition to oral antibiotics.
Many antibiotic doses used in clinical practice are preset durations that are not based on high-quality evidence, said Mike Tchou, MD, of Children’s Hospital of Colorado in Aurora. However, studies now show that earlier transition to enteral antibiotics can improve a range of outcomes including neonatal UTIs, osteomyelitis, and complicated pneumonia, he said. Considering early transition based on a patient’s response can decrease adverse events, pain, length of stay, and health care costs, he explained.
2. Do not continue hospitalization in well-appearing febrile infants once bacterial cultures (i.e., blood, cerebrospinal, and/or urine) have been confirmed negative for 24-36 hours, if adequate outpatient follow-up can be assured.
Recent data indicate that continuing hospitalization beyond 24-36 hours of confirmed negative bacterial cultures does not improve clinical outcomes for well-appearing infants admitted for concern of serious bacterial infection, said Paula Soung, MD, of Children’s Wisconsin in Milwaukee. In fact, “blood culture yield is highest in the first 12-36 hours after incubation with multiple studies demonstrating > 90% of pathogen cultures being positive by 24 hours,” Dr. Soung said. “If adequate outpatient follow-up can be assured, discharging well-appearing febrile infants at 24-36 hours after confirming cultures are negative has many positive outcomes,” she said.
3. Do not initiate phototherapy in term or late preterm well-appearing infants with neonatal hyperbilirubinemia if their bilirubin is below levels at which the AAP guidelines recommend treatment.
In making this recommendation, “we considered that the risk of kernicterus and cerebral palsy is extremely low in otherwise healthy term and late preterm newborns,” said Allison Holmes, MD, of Children’s Hospital at Dartmouth-Hitchcock, Manchester, N.H. “Subthreshold phototherapy leads to unnecessary hospitalization and its associated costs and harms,” and data show that kernicterus generally occurs close to 40 mg/dL and occurs most often in infants with hemolysis, she added.
The evidence for the recommendations included data showing that, among other factors, 8.6 of 100,000 babies have a bilirubin greater than 30 mg/dL, said Dr. Holmes. Risks of using subthreshold phototherapy include increased length of stay, increased readmissions, and increased costs, as well as decreased breastfeeding, bonding with parents, and increased parental anxiety. “Adding prolonged hospitalization for an intervention that might not be necessary can be stressful for parents,” she said.
4. Do not use broad-spectrum antibiotics such as ceftriaxone for children hospitalized with uncomplicated community-acquired pneumonia. Use narrow-spectrum antibiotics such as penicillin, ampicillin, or amoxicillin.
Michelle Lossius, MD, of the Shands Hospital for Children at the University of Florida, Gainesville, noted that the recommendations reflect IDSA guidelines from 2011 advising the use of ampicillin or penicillin for this population of children. More recent studies with large populations support the ability of narrow-spectrum antibiotics to limit the development of resistant organisms while achieving the same or better outcomes for children hospitalized with CAP, she said.
5. Do not start IV antibiotic therapy on well-appearing newborn infants with isolated risk factors for sepsis such as maternal chorioamnionitis, prolonged rupture of membranes, or untreated group-B streptococcal colonization. Use clinical tools such as an evidence-based sepsis risk calculator to guide management.
“This recommendation combines other recommendations,” said Prabi Rajbhandari, MD, of Akron (Ohio) Children’s Hospital. The evidence is ample, as the Centers for Disease Control and Prevention recommends the use of sepsis calculators to guide clinical management in sepsis patients, she said.
Data comparing periods before and after the adoption of a sepsis risk calculator showed a significant reduction in the use of blood cultures and antibiotics, she noted. Other risks of jumping to IV antibiotics include increased hospital stay, increased parental anxiety, and decreased parental bonding, Dr. Rajbhandari added.
Next steps include how to prioritize implementation, as well as deimplementation of outdated practices, said Francisco Alvarez, MD, of Lucile Packard Children’s Hospital, Palo Alto, Calif. “A lot of our practices were started without good evidence for why they should be done,” he said. Other steps include value improvement research; use of dashboards and benchmarking; involving other stakeholders including patients, families, and other health care providers; and addressing racial disparities, he concluded.
The presenters had no financial conflicts to disclose. The conference was sponsored by the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine.
“Health care spending and health care waste is a huge problem in the U.S., including for children,” Vivian Lee, MD, of Children’s Hospital, Los Angeles, said in a presentation at the 2021 virtual Pediatric Hospital Medicine conference.
Data from a 2019 study suggested that approximately 25% of health care spending in the United States qualifies as “wasteful spending,” in categories such as overtesting, and unnecessary hospitalization, Dr. Lee said. “It is essential for physicians in hospitals to be stewards of high-value care,” she emphasized.
To combat wasteful spending and control health care costs, the Choosing Wisely campaign was created in 2012 as an initiative from the American Board of Internal Medicine Foundation. An ongoing goal of the campaign is to raise awareness among physicians and patients about potential areas of low-value services and overuse. The overall campaign includes clinician-driven recommendations from multiple medical organizations.
The PHM produced its first set of five recommendations in 2012, Dr. Lee said. These recommendations, titled “Five Things Physicians and Patients Should Question,” have been updated for 2021. The updated recommendations were created as a partnership among the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine. A joint committee reviewed the latest evidence, and the updates were approved by the societies and published by the ABIM in January 2021.
“We think these recommendations truly reflect an exciting and evolving landscape for pediatric hospitalists,” Dr. Lee said. “There is a greater focus on opportunities to transition out of the hospital sooner, or avoid hospitalization altogether. There is an emphasis on antibiotic stewardship and a growing recognition of the impact that overuse may have on our vulnerable neonatal population,” she said. Several members of the Choosing Wisely panel presented the recommendations during the virtual presentation.
Revised recommendations
The new “Five Things Physicians and Patients Should Question” are as follows:
1. Do not prescribe IV antibiotics for predetermined durations for patients hospitalized with infections such as pyelonephritis, osteomyelitis, and complicated pneumonia. Consider early transition to oral antibiotics.
Many antibiotic doses used in clinical practice are preset durations that are not based on high-quality evidence, said Mike Tchou, MD, of Children’s Hospital of Colorado in Aurora. However, studies now show that earlier transition to enteral antibiotics can improve a range of outcomes including neonatal UTIs, osteomyelitis, and complicated pneumonia, he said. Considering early transition based on a patient’s response can decrease adverse events, pain, length of stay, and health care costs, he explained.
2. Do not continue hospitalization in well-appearing febrile infants once bacterial cultures (i.e., blood, cerebrospinal, and/or urine) have been confirmed negative for 24-36 hours, if adequate outpatient follow-up can be assured.
Recent data indicate that continuing hospitalization beyond 24-36 hours of confirmed negative bacterial cultures does not improve clinical outcomes for well-appearing infants admitted for concern of serious bacterial infection, said Paula Soung, MD, of Children’s Wisconsin in Milwaukee. In fact, “blood culture yield is highest in the first 12-36 hours after incubation with multiple studies demonstrating > 90% of pathogen cultures being positive by 24 hours,” Dr. Soung said. “If adequate outpatient follow-up can be assured, discharging well-appearing febrile infants at 24-36 hours after confirming cultures are negative has many positive outcomes,” she said.
3. Do not initiate phototherapy in term or late preterm well-appearing infants with neonatal hyperbilirubinemia if their bilirubin is below levels at which the AAP guidelines recommend treatment.
In making this recommendation, “we considered that the risk of kernicterus and cerebral palsy is extremely low in otherwise healthy term and late preterm newborns,” said Allison Holmes, MD, of Children’s Hospital at Dartmouth-Hitchcock, Manchester, N.H. “Subthreshold phototherapy leads to unnecessary hospitalization and its associated costs and harms,” and data show that kernicterus generally occurs close to 40 mg/dL and occurs most often in infants with hemolysis, she added.
The evidence for the recommendations included data showing that, among other factors, 8.6 of 100,000 babies have a bilirubin greater than 30 mg/dL, said Dr. Holmes. Risks of using subthreshold phototherapy include increased length of stay, increased readmissions, and increased costs, as well as decreased breastfeeding, bonding with parents, and increased parental anxiety. “Adding prolonged hospitalization for an intervention that might not be necessary can be stressful for parents,” she said.
4. Do not use broad-spectrum antibiotics such as ceftriaxone for children hospitalized with uncomplicated community-acquired pneumonia. Use narrow-spectrum antibiotics such as penicillin, ampicillin, or amoxicillin.
Michelle Lossius, MD, of the Shands Hospital for Children at the University of Florida, Gainesville, noted that the recommendations reflect IDSA guidelines from 2011 advising the use of ampicillin or penicillin for this population of children. More recent studies with large populations support the ability of narrow-spectrum antibiotics to limit the development of resistant organisms while achieving the same or better outcomes for children hospitalized with CAP, she said.
5. Do not start IV antibiotic therapy on well-appearing newborn infants with isolated risk factors for sepsis such as maternal chorioamnionitis, prolonged rupture of membranes, or untreated group-B streptococcal colonization. Use clinical tools such as an evidence-based sepsis risk calculator to guide management.
“This recommendation combines other recommendations,” said Prabi Rajbhandari, MD, of Akron (Ohio) Children’s Hospital. The evidence is ample, as the Centers for Disease Control and Prevention recommends the use of sepsis calculators to guide clinical management in sepsis patients, she said.
Data comparing periods before and after the adoption of a sepsis risk calculator showed a significant reduction in the use of blood cultures and antibiotics, she noted. Other risks of jumping to IV antibiotics include increased hospital stay, increased parental anxiety, and decreased parental bonding, Dr. Rajbhandari added.
Next steps include how to prioritize implementation, as well as deimplementation of outdated practices, said Francisco Alvarez, MD, of Lucile Packard Children’s Hospital, Palo Alto, Calif. “A lot of our practices were started without good evidence for why they should be done,” he said. Other steps include value improvement research; use of dashboards and benchmarking; involving other stakeholders including patients, families, and other health care providers; and addressing racial disparities, he concluded.
The presenters had no financial conflicts to disclose. The conference was sponsored by the Academic Pediatric Association, the American Academy of Pediatrics, and the Society of Hospital Medicine.
FROM PHM 2021
Walking 7,000 steps per day may be enough to reduce mortality risk
based on prospective data from more than 2,000 people.
Findings were consistent regardless of race or sex, and step intensity had no impact on mortality risk, reported lead author Amanda E. Paluch, PhD, of the University of Massachusetts Amherst, and colleagues.
“In response to the need for empirical data on the associations of step volume and intensity with mortality in younger and diverse populations, we conducted a prospective study in middle-aged Black and White adults followed up for mortality for approximately 11 years,” the investigators wrote in JAMA Network Open. “The objectives of our study were to examine the associations of step volume and intensity with mortality overall and by race and sex.”
Steps per day is easy to communicate
Dr. Paluch noted that steps per day is a “very appealing metric to quantify activity,” for both researchers and laypeople.
“Steps per day is simple and easy to communicate in public health and clinical settings,” Dr. Paluch said in an interview. “Additionally, the dramatic growth of wearable devices measuring steps makes it appealing and broadens the reach of promoting physical activity to many individuals. Walking is an activity that most of the general population can pursue. It can also be accumulated throughout daily living and may seem more achievable to fit into busy lives than a structured exercise session.”
The present investigation was conducted as part of the Coronary Artery Risk Development in Young Adults (CARDIA) Study. The dataset included 2,110 participants ranging from 38-50 years of age, with a mean age of 45.2 years. A slightly higher proportion of the subjects were women (57.1%) and White (57.9%).
All participants wore an ActiGraph 7164 accelerometer for 1 week and were then followed for death of any cause, with a mean follow-up of 10.8 years. Multivariable-adjusted Cox proportional hazards models included a range of covariates, such as smoking history, body weight, alcohol intake, blood pressure, total cholesterol, and others. Step counts were grouped into low (less than 7,000 steps per day), moderate (7,000-9,999), and high (at least 10,000 steps per day) categories.
Compared with individuals who took less than 7,000 steps per day, those who took 7,000-9,000 steps per day had a 72% reduced risk of mortality (hazard ratio, 0.28; 95% confidence interval, 0.15-0.54). Going beyond 10,000 steps appeared to add no benefit, based on a 55% lower risk of all-cause mortality in the highly active group, compared with those taking less than 7,000 steps per day (HR, 0.45; 95% CI, 0.25-0.81).
Walking faster didn’t appear to help either, as stepping intensity was not associated with mortality risk; however, Dr. Paluch urged a cautious interpretation of this finding, calling it “inconclusive,” and suggesting that more research is needed.
“It is also important to note that this study only looked at premature all-cause mortality, and therefore the results may be different for other health outcomes, such as the risk of cardiovascular disease, or diabetes, cancer, or mental health outcomes,” Dr. Paluch said.
“The results from our study demonstrated that those who are least active have the most to gain,” Dr. Paluch said. “Even small incremental increases in steps per day are associated with a lower mortality risk during middle age. A walking plan that gradually works up toward 7,000-10,000 steps per day in middle-aged adults may have health benefits and lower the risk of premature mortality.”
Causality cannot be confirmed
According to Raed A. Joundi, MD, DPhil, of the University of Calgary (Alta.), the study size, diverse population, and length of follow-up should increase confidence in the findings, although a causal relationship remains elusive.
“As this study is observational, causality between step count and mortality cannot be confirmed; however, the authors accounted for many factors, and the association was consistent in different analyses and with prior literature,” Dr. Joundi said in an interview. “The authors did not assess the risk of other important events like stroke and heart attack, and these could be addressed in a future study.”
Dr. Joundi, who recently published a study linking exercise with a 50% reduction in mortality after stroke, noted that “physical activity has innumerable benefits, and it’s important that people engage in activity that can be regular and consistent, regardless of the type or intensity.”
To this end, he highlighted the use of “devices capable of monitoring step count, which can be an important motivational tool,” and suggested that these findings may bring a sigh of relief to step counters who come up a little short on a common daily goal.
“A target of 10,000 steps is often used for public health promotion, and this study now provides convincing observational evidence that it may be an optimal step count target for mortality reduction,” Dr. Joundi said. “However, if 10,000 steps per day is not feasible, 7,000 steps seems to be a very reasonable target given its association with markedly lower mortality in this study.”
Not all step counters are equal
Unfortunately, such recommendations are complicated by uncertainty in measurement, as widely used step counting devices, like smart watches, may not yield the same results as research-grade accelerometers, according to Nicole L. Spartano, PhD, of Boston University.
“Many comparison studies have been conducted in laboratory settings among young healthy adults, but these do not necessarily reflect real-life wear experiences that will be generalizable to the population as a whole,” Dr. Spartano wrote in an accompanying editorial.
She called for large-scale comparison studies to compare research-grade and consumer devices.
“The reason for conducting comparison studies is not to develop distinct guidelines for different devices or subgroups of the population, but rather to understand the variability so that we can develop one clear message that is most appropriate to the public,” Dr. Spartano wrote. “Some devices may have bias in terms of step measurement at different activity intensity and may not record steps as accurately in older adults or individuals with obesity or mobility disorders. For example, when adults who were obese wore an ActiGraph monitor in a laboratory setting, the device only recorded 80% of steps walked at a moderate pace, while other devices recorded close to 100% of steps walked. If we in the public health community are to move toward using these devices more for physical activity prescription, these details will need to be explored in more depth.”
CARDIA was conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham, Northwestern University, the University of Minnesota, and the Kaiser Foundation Research Institute. Some study authors received grants from the National Institutes of Health and the Kaiser Foundation Research Institute. Dr Spartano disclosed relationships with Novo Nordisk, the American Heart Association, the Alzheimer’s Association, and the National Institutes of Health. Dr. Joundi and Dr. Paluch disclosed no relevant financial relationships.
based on prospective data from more than 2,000 people.
Findings were consistent regardless of race or sex, and step intensity had no impact on mortality risk, reported lead author Amanda E. Paluch, PhD, of the University of Massachusetts Amherst, and colleagues.
“In response to the need for empirical data on the associations of step volume and intensity with mortality in younger and diverse populations, we conducted a prospective study in middle-aged Black and White adults followed up for mortality for approximately 11 years,” the investigators wrote in JAMA Network Open. “The objectives of our study were to examine the associations of step volume and intensity with mortality overall and by race and sex.”
Steps per day is easy to communicate
Dr. Paluch noted that steps per day is a “very appealing metric to quantify activity,” for both researchers and laypeople.
“Steps per day is simple and easy to communicate in public health and clinical settings,” Dr. Paluch said in an interview. “Additionally, the dramatic growth of wearable devices measuring steps makes it appealing and broadens the reach of promoting physical activity to many individuals. Walking is an activity that most of the general population can pursue. It can also be accumulated throughout daily living and may seem more achievable to fit into busy lives than a structured exercise session.”
The present investigation was conducted as part of the Coronary Artery Risk Development in Young Adults (CARDIA) Study. The dataset included 2,110 participants ranging from 38-50 years of age, with a mean age of 45.2 years. A slightly higher proportion of the subjects were women (57.1%) and White (57.9%).
All participants wore an ActiGraph 7164 accelerometer for 1 week and were then followed for death of any cause, with a mean follow-up of 10.8 years. Multivariable-adjusted Cox proportional hazards models included a range of covariates, such as smoking history, body weight, alcohol intake, blood pressure, total cholesterol, and others. Step counts were grouped into low (less than 7,000 steps per day), moderate (7,000-9,999), and high (at least 10,000 steps per day) categories.
Compared with individuals who took less than 7,000 steps per day, those who took 7,000-9,000 steps per day had a 72% reduced risk of mortality (hazard ratio, 0.28; 95% confidence interval, 0.15-0.54). Going beyond 10,000 steps appeared to add no benefit, based on a 55% lower risk of all-cause mortality in the highly active group, compared with those taking less than 7,000 steps per day (HR, 0.45; 95% CI, 0.25-0.81).
Walking faster didn’t appear to help either, as stepping intensity was not associated with mortality risk; however, Dr. Paluch urged a cautious interpretation of this finding, calling it “inconclusive,” and suggesting that more research is needed.
“It is also important to note that this study only looked at premature all-cause mortality, and therefore the results may be different for other health outcomes, such as the risk of cardiovascular disease, or diabetes, cancer, or mental health outcomes,” Dr. Paluch said.
“The results from our study demonstrated that those who are least active have the most to gain,” Dr. Paluch said. “Even small incremental increases in steps per day are associated with a lower mortality risk during middle age. A walking plan that gradually works up toward 7,000-10,000 steps per day in middle-aged adults may have health benefits and lower the risk of premature mortality.”
Causality cannot be confirmed
According to Raed A. Joundi, MD, DPhil, of the University of Calgary (Alta.), the study size, diverse population, and length of follow-up should increase confidence in the findings, although a causal relationship remains elusive.
“As this study is observational, causality between step count and mortality cannot be confirmed; however, the authors accounted for many factors, and the association was consistent in different analyses and with prior literature,” Dr. Joundi said in an interview. “The authors did not assess the risk of other important events like stroke and heart attack, and these could be addressed in a future study.”
Dr. Joundi, who recently published a study linking exercise with a 50% reduction in mortality after stroke, noted that “physical activity has innumerable benefits, and it’s important that people engage in activity that can be regular and consistent, regardless of the type or intensity.”
To this end, he highlighted the use of “devices capable of monitoring step count, which can be an important motivational tool,” and suggested that these findings may bring a sigh of relief to step counters who come up a little short on a common daily goal.
“A target of 10,000 steps is often used for public health promotion, and this study now provides convincing observational evidence that it may be an optimal step count target for mortality reduction,” Dr. Joundi said. “However, if 10,000 steps per day is not feasible, 7,000 steps seems to be a very reasonable target given its association with markedly lower mortality in this study.”
Not all step counters are equal
Unfortunately, such recommendations are complicated by uncertainty in measurement, as widely used step counting devices, like smart watches, may not yield the same results as research-grade accelerometers, according to Nicole L. Spartano, PhD, of Boston University.
“Many comparison studies have been conducted in laboratory settings among young healthy adults, but these do not necessarily reflect real-life wear experiences that will be generalizable to the population as a whole,” Dr. Spartano wrote in an accompanying editorial.
She called for large-scale comparison studies to compare research-grade and consumer devices.
“The reason for conducting comparison studies is not to develop distinct guidelines for different devices or subgroups of the population, but rather to understand the variability so that we can develop one clear message that is most appropriate to the public,” Dr. Spartano wrote. “Some devices may have bias in terms of step measurement at different activity intensity and may not record steps as accurately in older adults or individuals with obesity or mobility disorders. For example, when adults who were obese wore an ActiGraph monitor in a laboratory setting, the device only recorded 80% of steps walked at a moderate pace, while other devices recorded close to 100% of steps walked. If we in the public health community are to move toward using these devices more for physical activity prescription, these details will need to be explored in more depth.”
CARDIA was conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham, Northwestern University, the University of Minnesota, and the Kaiser Foundation Research Institute. Some study authors received grants from the National Institutes of Health and the Kaiser Foundation Research Institute. Dr Spartano disclosed relationships with Novo Nordisk, the American Heart Association, the Alzheimer’s Association, and the National Institutes of Health. Dr. Joundi and Dr. Paluch disclosed no relevant financial relationships.
based on prospective data from more than 2,000 people.
Findings were consistent regardless of race or sex, and step intensity had no impact on mortality risk, reported lead author Amanda E. Paluch, PhD, of the University of Massachusetts Amherst, and colleagues.
“In response to the need for empirical data on the associations of step volume and intensity with mortality in younger and diverse populations, we conducted a prospective study in middle-aged Black and White adults followed up for mortality for approximately 11 years,” the investigators wrote in JAMA Network Open. “The objectives of our study were to examine the associations of step volume and intensity with mortality overall and by race and sex.”
Steps per day is easy to communicate
Dr. Paluch noted that steps per day is a “very appealing metric to quantify activity,” for both researchers and laypeople.
“Steps per day is simple and easy to communicate in public health and clinical settings,” Dr. Paluch said in an interview. “Additionally, the dramatic growth of wearable devices measuring steps makes it appealing and broadens the reach of promoting physical activity to many individuals. Walking is an activity that most of the general population can pursue. It can also be accumulated throughout daily living and may seem more achievable to fit into busy lives than a structured exercise session.”
The present investigation was conducted as part of the Coronary Artery Risk Development in Young Adults (CARDIA) Study. The dataset included 2,110 participants ranging from 38-50 years of age, with a mean age of 45.2 years. A slightly higher proportion of the subjects were women (57.1%) and White (57.9%).
All participants wore an ActiGraph 7164 accelerometer for 1 week and were then followed for death of any cause, with a mean follow-up of 10.8 years. Multivariable-adjusted Cox proportional hazards models included a range of covariates, such as smoking history, body weight, alcohol intake, blood pressure, total cholesterol, and others. Step counts were grouped into low (less than 7,000 steps per day), moderate (7,000-9,999), and high (at least 10,000 steps per day) categories.
Compared with individuals who took less than 7,000 steps per day, those who took 7,000-9,000 steps per day had a 72% reduced risk of mortality (hazard ratio, 0.28; 95% confidence interval, 0.15-0.54). Going beyond 10,000 steps appeared to add no benefit, based on a 55% lower risk of all-cause mortality in the highly active group, compared with those taking less than 7,000 steps per day (HR, 0.45; 95% CI, 0.25-0.81).
Walking faster didn’t appear to help either, as stepping intensity was not associated with mortality risk; however, Dr. Paluch urged a cautious interpretation of this finding, calling it “inconclusive,” and suggesting that more research is needed.
“It is also important to note that this study only looked at premature all-cause mortality, and therefore the results may be different for other health outcomes, such as the risk of cardiovascular disease, or diabetes, cancer, or mental health outcomes,” Dr. Paluch said.
“The results from our study demonstrated that those who are least active have the most to gain,” Dr. Paluch said. “Even small incremental increases in steps per day are associated with a lower mortality risk during middle age. A walking plan that gradually works up toward 7,000-10,000 steps per day in middle-aged adults may have health benefits and lower the risk of premature mortality.”
Causality cannot be confirmed
According to Raed A. Joundi, MD, DPhil, of the University of Calgary (Alta.), the study size, diverse population, and length of follow-up should increase confidence in the findings, although a causal relationship remains elusive.
“As this study is observational, causality between step count and mortality cannot be confirmed; however, the authors accounted for many factors, and the association was consistent in different analyses and with prior literature,” Dr. Joundi said in an interview. “The authors did not assess the risk of other important events like stroke and heart attack, and these could be addressed in a future study.”
Dr. Joundi, who recently published a study linking exercise with a 50% reduction in mortality after stroke, noted that “physical activity has innumerable benefits, and it’s important that people engage in activity that can be regular and consistent, regardless of the type or intensity.”
To this end, he highlighted the use of “devices capable of monitoring step count, which can be an important motivational tool,” and suggested that these findings may bring a sigh of relief to step counters who come up a little short on a common daily goal.
“A target of 10,000 steps is often used for public health promotion, and this study now provides convincing observational evidence that it may be an optimal step count target for mortality reduction,” Dr. Joundi said. “However, if 10,000 steps per day is not feasible, 7,000 steps seems to be a very reasonable target given its association with markedly lower mortality in this study.”
Not all step counters are equal
Unfortunately, such recommendations are complicated by uncertainty in measurement, as widely used step counting devices, like smart watches, may not yield the same results as research-grade accelerometers, according to Nicole L. Spartano, PhD, of Boston University.
“Many comparison studies have been conducted in laboratory settings among young healthy adults, but these do not necessarily reflect real-life wear experiences that will be generalizable to the population as a whole,” Dr. Spartano wrote in an accompanying editorial.
She called for large-scale comparison studies to compare research-grade and consumer devices.
“The reason for conducting comparison studies is not to develop distinct guidelines for different devices or subgroups of the population, but rather to understand the variability so that we can develop one clear message that is most appropriate to the public,” Dr. Spartano wrote. “Some devices may have bias in terms of step measurement at different activity intensity and may not record steps as accurately in older adults or individuals with obesity or mobility disorders. For example, when adults who were obese wore an ActiGraph monitor in a laboratory setting, the device only recorded 80% of steps walked at a moderate pace, while other devices recorded close to 100% of steps walked. If we in the public health community are to move toward using these devices more for physical activity prescription, these details will need to be explored in more depth.”
CARDIA was conducted and supported by the National Heart, Lung, and Blood Institute in collaboration with the University of Alabama at Birmingham, Northwestern University, the University of Minnesota, and the Kaiser Foundation Research Institute. Some study authors received grants from the National Institutes of Health and the Kaiser Foundation Research Institute. Dr Spartano disclosed relationships with Novo Nordisk, the American Heart Association, the Alzheimer’s Association, and the National Institutes of Health. Dr. Joundi and Dr. Paluch disclosed no relevant financial relationships.
FROM JAMA NETWORK OPEN
Repeat gastroscopy shows real-world value for detecting malignant ulcers
Most malignancies with new ulcer were identified on initial gastroscopy in a retrospective cohort study, but it’s still worth performing follow-up procedures, according to investigators.
“Although the additional yield of malignancy at follow-up gastroscopy is low at 2%, our data supports the current strategy of repeat endoscopic assessment given variables in obtaining adequate ulcer histology and the lack of reliable endoscopic predictors of a malignant ulcer,” the study’s authors Linda Yang, MBBS, of the University of Melbourne, and colleagues wrote in the Journal of Clinical Gastroenterology.
Recommendations from the British Society of Gastroenterology emphasize the importance of repeat gastroscopy and biopsy of gastric ulcers within an 8-week period of the index gastroscopy. Additionally, the American Society of Gastrointestinal Endoscopy similarly recommends repeat gastroscopy in high-risk patients (ulcers >2 cm) within a 12-week period of the initial endoscopy. The authors noted that, despite these recommendations, there is a lack of consensus regarding timing of repeat gastroscopy, and no established ulcer biopsy protocols exist. Additionally, there is a lack of data on real-world repeat gastroscopy practices and follow-up outcomes.
To understand the current practice in gastric ulcer follow-up, Dr. Yang and researchers retrospectively examined new gastric ulcers diagnosed on gastroscopy between 2013 and 2017 at two separate Australian institutions.
Out of 795 patients (median age, 69 years; 59% male), approximately 55% (n = 440) underwent repeat gastroscopy at a median of 8 weeks after the initial endoscopy procedure. Overall, 52 patients (7%) received a malignancy diagnosis, with 83% (n = 43) of these diagnoses detected at the index gastroscopy; 2% overall received the diagnosis based on follow-up gastroscopy.
“I think these numbers would support the assumptions of most endoscopists that a small but still significant portion of new gastric ulcers will turn out to be malignant,” explained Michael DeSimone, MD, gastroenterologist at Emerson Hospital in Concord, Mass. Dr. DeSimone, who wasn’t involved in the study, said the data support the importance of “biopsy in the initial exam and bringing these patients back for a repeat endoscopy to check healing and biopsy unless malignancy was confirmed on the initial exam.”
In the study, a multivariate analysis revealed several predictors of benign ulcers, including lack of endoscopic suspicion at the index gastroscopy (odds ratio, 0.1; 95% confidence interval, 0.03-0.13; P ≤ .005), complete healing on repeat gastroscopy (OR, 0.5; 95% CI, 0.34-0.70; P = .036), and benign histology on initial biopsy (OR, 0.12; 95% CI, 0.43-0.90; P ≤ .005). However, no patient-related factors – such as H. pylori status and ethnicity – were associated with an increased likelihood of malignancy.
“Knowing that low suspicion for malignancy on initial exam and benign histology on initial biopsies predict benign ulcers ... reasonable endoscopists could feel more comfortable not repeating an exam where procedure safety is a significant concern if their suspicion was low on the index exam, especially if they had the opportunity to take initial biopsies and those ultimately show benign histology,” said Dr. DeSimone.
The investigators noted that the main reason behind 45% receiving no follow-up gastroscopy is that the ulcers had nonsuspicious appearance.
“Although not recommended, this is widely accepted clinical practice, especially in comorbid or elderly patients where the decision to undergo repeat gastroscopy requires consideration of their comorbidities, frailty, and life expectancy,” they wrote. They suggested that this, combined with high nonattendence rate in the cohort, emphasize the importance of ulcer biopsy at index gastroscopy, even in the absence of suspicious features.
Clinicians in the current study performed random gastric biopsies in 27% (n = 218) of patients. Helicobacter pylori, a component frequently described in high-risk populations, was detected in 22% of patients who had an ulcer or gastric biopsy performed.
The relatively low frequency with which random gastric biopsies were performed during the index endoscopy to look for H. pylori is a bit surprising, said Linda Lee, MD, medical director of endoscopy at Brigham and Women’s Hospital in Boston, given the bacterium remains a common and readily treatable etiology of gastric ulcers. “While it is known that yield of biopsy can be lower for H. pylori in the setting of acute upper gastrointestinal bleeding, it is still important to evaluate for this especially since biopsies carry low risk for bleeding,” explained Dr. Lee, who also wasn’t part of the study.
She added said that the study’s high negative predictive value for endoscopic suspicion of malignancy (96%) is reassuring. “This, combined with benign histology on initial biopsies, could serve to identify which patients should return for repeat endoscopy.”
“We need to ensure that more biopsies are obtained during the index endoscopy from gastric ulcers as well as randomly and that, during follow-up endoscopy, biopsies are obtained from all [partially or fully] nonhealed ulcers,” added Dr. Lee. She suggested it could be helpful to develop an evidence-based, prospectively validated algorithm and/or identify risk factors that reliably help endoscopists decide who would benefit from repeat endoscopy, “especially since there is a relatively high rate of noncompliance with a low rate of malignancy.”
A primary limitation of the study included its retrospective nature; however, the authors pointed out that the study currently represents the largest multicenter, retrospective cohort analysis of endoscopic follow-up for gastric ulcers.
“Before any change can be recommended to current clinical practice, prospective and potentially randomized studies are required to validate our findings and elucidate any high-risk features associated with malignant gastric ulcer,” the investigators wrote. Doing so could lead to reductions in health care cost and patient burden.
Some of the study authors received funding from the National Health and Medical Research Council of Australia, but the remaining authors declared having nothing to disclose. Dr. DeSimone and Dr Lee reported having no relevant conflicts.
Most malignancies with new ulcer were identified on initial gastroscopy in a retrospective cohort study, but it’s still worth performing follow-up procedures, according to investigators.
“Although the additional yield of malignancy at follow-up gastroscopy is low at 2%, our data supports the current strategy of repeat endoscopic assessment given variables in obtaining adequate ulcer histology and the lack of reliable endoscopic predictors of a malignant ulcer,” the study’s authors Linda Yang, MBBS, of the University of Melbourne, and colleagues wrote in the Journal of Clinical Gastroenterology.
Recommendations from the British Society of Gastroenterology emphasize the importance of repeat gastroscopy and biopsy of gastric ulcers within an 8-week period of the index gastroscopy. Additionally, the American Society of Gastrointestinal Endoscopy similarly recommends repeat gastroscopy in high-risk patients (ulcers >2 cm) within a 12-week period of the initial endoscopy. The authors noted that, despite these recommendations, there is a lack of consensus regarding timing of repeat gastroscopy, and no established ulcer biopsy protocols exist. Additionally, there is a lack of data on real-world repeat gastroscopy practices and follow-up outcomes.
To understand the current practice in gastric ulcer follow-up, Dr. Yang and researchers retrospectively examined new gastric ulcers diagnosed on gastroscopy between 2013 and 2017 at two separate Australian institutions.
Out of 795 patients (median age, 69 years; 59% male), approximately 55% (n = 440) underwent repeat gastroscopy at a median of 8 weeks after the initial endoscopy procedure. Overall, 52 patients (7%) received a malignancy diagnosis, with 83% (n = 43) of these diagnoses detected at the index gastroscopy; 2% overall received the diagnosis based on follow-up gastroscopy.
“I think these numbers would support the assumptions of most endoscopists that a small but still significant portion of new gastric ulcers will turn out to be malignant,” explained Michael DeSimone, MD, gastroenterologist at Emerson Hospital in Concord, Mass. Dr. DeSimone, who wasn’t involved in the study, said the data support the importance of “biopsy in the initial exam and bringing these patients back for a repeat endoscopy to check healing and biopsy unless malignancy was confirmed on the initial exam.”
In the study, a multivariate analysis revealed several predictors of benign ulcers, including lack of endoscopic suspicion at the index gastroscopy (odds ratio, 0.1; 95% confidence interval, 0.03-0.13; P ≤ .005), complete healing on repeat gastroscopy (OR, 0.5; 95% CI, 0.34-0.70; P = .036), and benign histology on initial biopsy (OR, 0.12; 95% CI, 0.43-0.90; P ≤ .005). However, no patient-related factors – such as H. pylori status and ethnicity – were associated with an increased likelihood of malignancy.
“Knowing that low suspicion for malignancy on initial exam and benign histology on initial biopsies predict benign ulcers ... reasonable endoscopists could feel more comfortable not repeating an exam where procedure safety is a significant concern if their suspicion was low on the index exam, especially if they had the opportunity to take initial biopsies and those ultimately show benign histology,” said Dr. DeSimone.
The investigators noted that the main reason behind 45% receiving no follow-up gastroscopy is that the ulcers had nonsuspicious appearance.
“Although not recommended, this is widely accepted clinical practice, especially in comorbid or elderly patients where the decision to undergo repeat gastroscopy requires consideration of their comorbidities, frailty, and life expectancy,” they wrote. They suggested that this, combined with high nonattendence rate in the cohort, emphasize the importance of ulcer biopsy at index gastroscopy, even in the absence of suspicious features.
Clinicians in the current study performed random gastric biopsies in 27% (n = 218) of patients. Helicobacter pylori, a component frequently described in high-risk populations, was detected in 22% of patients who had an ulcer or gastric biopsy performed.
The relatively low frequency with which random gastric biopsies were performed during the index endoscopy to look for H. pylori is a bit surprising, said Linda Lee, MD, medical director of endoscopy at Brigham and Women’s Hospital in Boston, given the bacterium remains a common and readily treatable etiology of gastric ulcers. “While it is known that yield of biopsy can be lower for H. pylori in the setting of acute upper gastrointestinal bleeding, it is still important to evaluate for this especially since biopsies carry low risk for bleeding,” explained Dr. Lee, who also wasn’t part of the study.
She added said that the study’s high negative predictive value for endoscopic suspicion of malignancy (96%) is reassuring. “This, combined with benign histology on initial biopsies, could serve to identify which patients should return for repeat endoscopy.”
“We need to ensure that more biopsies are obtained during the index endoscopy from gastric ulcers as well as randomly and that, during follow-up endoscopy, biopsies are obtained from all [partially or fully] nonhealed ulcers,” added Dr. Lee. She suggested it could be helpful to develop an evidence-based, prospectively validated algorithm and/or identify risk factors that reliably help endoscopists decide who would benefit from repeat endoscopy, “especially since there is a relatively high rate of noncompliance with a low rate of malignancy.”
A primary limitation of the study included its retrospective nature; however, the authors pointed out that the study currently represents the largest multicenter, retrospective cohort analysis of endoscopic follow-up for gastric ulcers.
“Before any change can be recommended to current clinical practice, prospective and potentially randomized studies are required to validate our findings and elucidate any high-risk features associated with malignant gastric ulcer,” the investigators wrote. Doing so could lead to reductions in health care cost and patient burden.
Some of the study authors received funding from the National Health and Medical Research Council of Australia, but the remaining authors declared having nothing to disclose. Dr. DeSimone and Dr Lee reported having no relevant conflicts.
Most malignancies with new ulcer were identified on initial gastroscopy in a retrospective cohort study, but it’s still worth performing follow-up procedures, according to investigators.
“Although the additional yield of malignancy at follow-up gastroscopy is low at 2%, our data supports the current strategy of repeat endoscopic assessment given variables in obtaining adequate ulcer histology and the lack of reliable endoscopic predictors of a malignant ulcer,” the study’s authors Linda Yang, MBBS, of the University of Melbourne, and colleagues wrote in the Journal of Clinical Gastroenterology.
Recommendations from the British Society of Gastroenterology emphasize the importance of repeat gastroscopy and biopsy of gastric ulcers within an 8-week period of the index gastroscopy. Additionally, the American Society of Gastrointestinal Endoscopy similarly recommends repeat gastroscopy in high-risk patients (ulcers >2 cm) within a 12-week period of the initial endoscopy. The authors noted that, despite these recommendations, there is a lack of consensus regarding timing of repeat gastroscopy, and no established ulcer biopsy protocols exist. Additionally, there is a lack of data on real-world repeat gastroscopy practices and follow-up outcomes.
To understand the current practice in gastric ulcer follow-up, Dr. Yang and researchers retrospectively examined new gastric ulcers diagnosed on gastroscopy between 2013 and 2017 at two separate Australian institutions.
Out of 795 patients (median age, 69 years; 59% male), approximately 55% (n = 440) underwent repeat gastroscopy at a median of 8 weeks after the initial endoscopy procedure. Overall, 52 patients (7%) received a malignancy diagnosis, with 83% (n = 43) of these diagnoses detected at the index gastroscopy; 2% overall received the diagnosis based on follow-up gastroscopy.
“I think these numbers would support the assumptions of most endoscopists that a small but still significant portion of new gastric ulcers will turn out to be malignant,” explained Michael DeSimone, MD, gastroenterologist at Emerson Hospital in Concord, Mass. Dr. DeSimone, who wasn’t involved in the study, said the data support the importance of “biopsy in the initial exam and bringing these patients back for a repeat endoscopy to check healing and biopsy unless malignancy was confirmed on the initial exam.”
In the study, a multivariate analysis revealed several predictors of benign ulcers, including lack of endoscopic suspicion at the index gastroscopy (odds ratio, 0.1; 95% confidence interval, 0.03-0.13; P ≤ .005), complete healing on repeat gastroscopy (OR, 0.5; 95% CI, 0.34-0.70; P = .036), and benign histology on initial biopsy (OR, 0.12; 95% CI, 0.43-0.90; P ≤ .005). However, no patient-related factors – such as H. pylori status and ethnicity – were associated with an increased likelihood of malignancy.
“Knowing that low suspicion for malignancy on initial exam and benign histology on initial biopsies predict benign ulcers ... reasonable endoscopists could feel more comfortable not repeating an exam where procedure safety is a significant concern if their suspicion was low on the index exam, especially if they had the opportunity to take initial biopsies and those ultimately show benign histology,” said Dr. DeSimone.
The investigators noted that the main reason behind 45% receiving no follow-up gastroscopy is that the ulcers had nonsuspicious appearance.
“Although not recommended, this is widely accepted clinical practice, especially in comorbid or elderly patients where the decision to undergo repeat gastroscopy requires consideration of their comorbidities, frailty, and life expectancy,” they wrote. They suggested that this, combined with high nonattendence rate in the cohort, emphasize the importance of ulcer biopsy at index gastroscopy, even in the absence of suspicious features.
Clinicians in the current study performed random gastric biopsies in 27% (n = 218) of patients. Helicobacter pylori, a component frequently described in high-risk populations, was detected in 22% of patients who had an ulcer or gastric biopsy performed.
The relatively low frequency with which random gastric biopsies were performed during the index endoscopy to look for H. pylori is a bit surprising, said Linda Lee, MD, medical director of endoscopy at Brigham and Women’s Hospital in Boston, given the bacterium remains a common and readily treatable etiology of gastric ulcers. “While it is known that yield of biopsy can be lower for H. pylori in the setting of acute upper gastrointestinal bleeding, it is still important to evaluate for this especially since biopsies carry low risk for bleeding,” explained Dr. Lee, who also wasn’t part of the study.
She added said that the study’s high negative predictive value for endoscopic suspicion of malignancy (96%) is reassuring. “This, combined with benign histology on initial biopsies, could serve to identify which patients should return for repeat endoscopy.”
“We need to ensure that more biopsies are obtained during the index endoscopy from gastric ulcers as well as randomly and that, during follow-up endoscopy, biopsies are obtained from all [partially or fully] nonhealed ulcers,” added Dr. Lee. She suggested it could be helpful to develop an evidence-based, prospectively validated algorithm and/or identify risk factors that reliably help endoscopists decide who would benefit from repeat endoscopy, “especially since there is a relatively high rate of noncompliance with a low rate of malignancy.”
A primary limitation of the study included its retrospective nature; however, the authors pointed out that the study currently represents the largest multicenter, retrospective cohort analysis of endoscopic follow-up for gastric ulcers.
“Before any change can be recommended to current clinical practice, prospective and potentially randomized studies are required to validate our findings and elucidate any high-risk features associated with malignant gastric ulcer,” the investigators wrote. Doing so could lead to reductions in health care cost and patient burden.
Some of the study authors received funding from the National Health and Medical Research Council of Australia, but the remaining authors declared having nothing to disclose. Dr. DeSimone and Dr Lee reported having no relevant conflicts.
FROM THE JOURNAL OF CLINICAL GASTROENTEROLOGY