User login
Clinical Edge Journal Scan Commentary: AML August 2021
CPX-351 is a liposomal cytarabine and daunorubicin. It was FDA approved in 2017 for the treatment of patients with newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes (AML-MRC). The approval was based on the results from a randomized trial comparing CPX-351 vs standard 7 +3 chemotherapy in patients older that 65 with t-AML or AML-MRC. The 5-year results from that study were recently published by Lancet et al. At 5 years of follow-up, CPX-351 continued to show benefit in older patients with t-AML or AML-MRC vs standard chemotherapy with cytarabine for 7 days and daunorubicin for 3 days (7+3) . The median OS in favor of CPX-351 vs 7+3 group was maintained (hazard ratio, 0.70; 95% confidence interval [CI], 0.55-0.91). At 5 years, survival estimates were higher for CPX-351 vs 7+3 (18% [95% CI, 12%-25%] vs 8% [95% CI, 4%-13%]). Overall, 5% of deaths in both groups were considered related to the study treatment. Overall, more patients treated with CPX-351 were able to proceed to stem cell transplantation (SCT) compared to those treated with 7 + 3 (35% vs 25%).
The 3-year overall survival from SCT was 56% vs 23% for patients treated with CPX-351 vs 7 +3. Of the responding patients who did not proceed to SCT, only 3 patients were alive at 5 years. Although this data is encouraging, it demonstrates that we have a long way to go to improve the outcome of these patients. In addition, more patients who achieve remission should proceed to SCT in order to improve the survival of this patient population (Lancet JE et al). In terms of supportive care during induction chemotherapy, two published studies this month evaluated two approaches to aiming to decrease the morbidity from infections: prospective monitoring for fungal infections and the use of romyelocel with G-CSF. Prospectively monitoring for fungal infections was performed as an observation study imbedded within a phase 3 Children’s Oncology Group trial (ACCL0933). The study included 471 patients with AML (age, 3 months-30 years) receiving fluconazole (n=235) or caspofungin (n=236).
Twice-weekly surveillance with galactomannan enzyme immunoassay (GM-EIA) and b-D-glucan (BDG) assay were performed in all patients. The negative predictive value was greater than 99% for an individual or combination of GM-EIA and BDG assays. However, true positive results were not observed in any sample collected within 7 days of an invasive aspergillosis/candidiasis diagnosis, resulting in sensitivity and positive predictive value for each test of 0%. This approach was ineffective at detecting invasive fungal diseases (IFDs) in children, adolescents, and young adults with acute myeloid leukemia (AML) receiving antifungal prophylaxis (Fisher BT et al).
A different approach to reduce infection morbidity and mortality during induction chemotherapy is the administration of romyelocel. Myeloid progenitor cells are cells that can produce granulocytes but have no long-term reconstitution capability. Romyelocel is a cryopreserved product, of MPC manufactured by ex vivo expansion of CD34+ hematopoietic stem cells. Romyelocel is capable of producing granulocytes, and thereby may reduce the severity or duration of neutropenic fevers. This phase 2 study included 163 patients with de novo AML receiving induction chemotherapy. Evaluable patients (n=120) were randomly assigned to receive either romyelocel-L plus G-CSF (n=59) or G-CSF monotherapy (n=61). From days 15 to 28, romyelocel-L plus G-CSF vs G-CSF monotherapy significantly reduced the mean duration of febrile episodes (2.36 days vs 3.90 days; P = .02) and incidence of infections (6.8% vs 27.9%; P = .0013). Length of hospitalization was significantly shorter in the romyelocel-L plus G-CSF vs G-CSF monotherapy group (25.5 days vs 28.7 days; P = .002). These results are encouraging, and a phase III trial is suggested by the authors. (Desai PM et al).
Finally, a study by MDACC reported disappointing results with the use of venetoclax in patients with tp53 mutation. Findings are from a retrospective analysis of 238 patients with newly diagnosed TP53-mutated AML treated with either VEN-based (n=58) or non-VEN-based (n=180) therapies. The addition of venetoclax to standard treatment regimens (VEN-based) did not improve clinical outcomes in patients with TP53-mutated acute myeloid leukemia (AML), highlighting the need for novel therapies in this patient population. Overall, there was no significant differences in overall survival (median, 5.7 months vs 6.6 months; P = .4), relapse-free survival (median, 3.5 months vs 4.7 months; P = .43), 4-week mortality (7% vs 10%; P = .5), and 8-week mortality (22% vs 17%; P = .4) in patients receiving VEN-based vs non-VEN-based therapies (Venugopal S et al). Clearly, better therapies are needed for this patient population.
CPX-351 is a liposomal cytarabine and daunorubicin. It was FDA approved in 2017 for the treatment of patients with newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes (AML-MRC). The approval was based on the results from a randomized trial comparing CPX-351 vs standard 7 +3 chemotherapy in patients older that 65 with t-AML or AML-MRC. The 5-year results from that study were recently published by Lancet et al. At 5 years of follow-up, CPX-351 continued to show benefit in older patients with t-AML or AML-MRC vs standard chemotherapy with cytarabine for 7 days and daunorubicin for 3 days (7+3) . The median OS in favor of CPX-351 vs 7+3 group was maintained (hazard ratio, 0.70; 95% confidence interval [CI], 0.55-0.91). At 5 years, survival estimates were higher for CPX-351 vs 7+3 (18% [95% CI, 12%-25%] vs 8% [95% CI, 4%-13%]). Overall, 5% of deaths in both groups were considered related to the study treatment. Overall, more patients treated with CPX-351 were able to proceed to stem cell transplantation (SCT) compared to those treated with 7 + 3 (35% vs 25%).
The 3-year overall survival from SCT was 56% vs 23% for patients treated with CPX-351 vs 7 +3. Of the responding patients who did not proceed to SCT, only 3 patients were alive at 5 years. Although this data is encouraging, it demonstrates that we have a long way to go to improve the outcome of these patients. In addition, more patients who achieve remission should proceed to SCT in order to improve the survival of this patient population (Lancet JE et al). In terms of supportive care during induction chemotherapy, two published studies this month evaluated two approaches to aiming to decrease the morbidity from infections: prospective monitoring for fungal infections and the use of romyelocel with G-CSF. Prospectively monitoring for fungal infections was performed as an observation study imbedded within a phase 3 Children’s Oncology Group trial (ACCL0933). The study included 471 patients with AML (age, 3 months-30 years) receiving fluconazole (n=235) or caspofungin (n=236).
Twice-weekly surveillance with galactomannan enzyme immunoassay (GM-EIA) and b-D-glucan (BDG) assay were performed in all patients. The negative predictive value was greater than 99% for an individual or combination of GM-EIA and BDG assays. However, true positive results were not observed in any sample collected within 7 days of an invasive aspergillosis/candidiasis diagnosis, resulting in sensitivity and positive predictive value for each test of 0%. This approach was ineffective at detecting invasive fungal diseases (IFDs) in children, adolescents, and young adults with acute myeloid leukemia (AML) receiving antifungal prophylaxis (Fisher BT et al).
A different approach to reduce infection morbidity and mortality during induction chemotherapy is the administration of romyelocel. Myeloid progenitor cells are cells that can produce granulocytes but have no long-term reconstitution capability. Romyelocel is a cryopreserved product, of MPC manufactured by ex vivo expansion of CD34+ hematopoietic stem cells. Romyelocel is capable of producing granulocytes, and thereby may reduce the severity or duration of neutropenic fevers. This phase 2 study included 163 patients with de novo AML receiving induction chemotherapy. Evaluable patients (n=120) were randomly assigned to receive either romyelocel-L plus G-CSF (n=59) or G-CSF monotherapy (n=61). From days 15 to 28, romyelocel-L plus G-CSF vs G-CSF monotherapy significantly reduced the mean duration of febrile episodes (2.36 days vs 3.90 days; P = .02) and incidence of infections (6.8% vs 27.9%; P = .0013). Length of hospitalization was significantly shorter in the romyelocel-L plus G-CSF vs G-CSF monotherapy group (25.5 days vs 28.7 days; P = .002). These results are encouraging, and a phase III trial is suggested by the authors. (Desai PM et al).
Finally, a study by MDACC reported disappointing results with the use of venetoclax in patients with tp53 mutation. Findings are from a retrospective analysis of 238 patients with newly diagnosed TP53-mutated AML treated with either VEN-based (n=58) or non-VEN-based (n=180) therapies. The addition of venetoclax to standard treatment regimens (VEN-based) did not improve clinical outcomes in patients with TP53-mutated acute myeloid leukemia (AML), highlighting the need for novel therapies in this patient population. Overall, there was no significant differences in overall survival (median, 5.7 months vs 6.6 months; P = .4), relapse-free survival (median, 3.5 months vs 4.7 months; P = .43), 4-week mortality (7% vs 10%; P = .5), and 8-week mortality (22% vs 17%; P = .4) in patients receiving VEN-based vs non-VEN-based therapies (Venugopal S et al). Clearly, better therapies are needed for this patient population.
CPX-351 is a liposomal cytarabine and daunorubicin. It was FDA approved in 2017 for the treatment of patients with newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes (AML-MRC). The approval was based on the results from a randomized trial comparing CPX-351 vs standard 7 +3 chemotherapy in patients older that 65 with t-AML or AML-MRC. The 5-year results from that study were recently published by Lancet et al. At 5 years of follow-up, CPX-351 continued to show benefit in older patients with t-AML or AML-MRC vs standard chemotherapy with cytarabine for 7 days and daunorubicin for 3 days (7+3) . The median OS in favor of CPX-351 vs 7+3 group was maintained (hazard ratio, 0.70; 95% confidence interval [CI], 0.55-0.91). At 5 years, survival estimates were higher for CPX-351 vs 7+3 (18% [95% CI, 12%-25%] vs 8% [95% CI, 4%-13%]). Overall, 5% of deaths in both groups were considered related to the study treatment. Overall, more patients treated with CPX-351 were able to proceed to stem cell transplantation (SCT) compared to those treated with 7 + 3 (35% vs 25%).
The 3-year overall survival from SCT was 56% vs 23% for patients treated with CPX-351 vs 7 +3. Of the responding patients who did not proceed to SCT, only 3 patients were alive at 5 years. Although this data is encouraging, it demonstrates that we have a long way to go to improve the outcome of these patients. In addition, more patients who achieve remission should proceed to SCT in order to improve the survival of this patient population (Lancet JE et al). In terms of supportive care during induction chemotherapy, two published studies this month evaluated two approaches to aiming to decrease the morbidity from infections: prospective monitoring for fungal infections and the use of romyelocel with G-CSF. Prospectively monitoring for fungal infections was performed as an observation study imbedded within a phase 3 Children’s Oncology Group trial (ACCL0933). The study included 471 patients with AML (age, 3 months-30 years) receiving fluconazole (n=235) or caspofungin (n=236).
Twice-weekly surveillance with galactomannan enzyme immunoassay (GM-EIA) and b-D-glucan (BDG) assay were performed in all patients. The negative predictive value was greater than 99% for an individual or combination of GM-EIA and BDG assays. However, true positive results were not observed in any sample collected within 7 days of an invasive aspergillosis/candidiasis diagnosis, resulting in sensitivity and positive predictive value for each test of 0%. This approach was ineffective at detecting invasive fungal diseases (IFDs) in children, adolescents, and young adults with acute myeloid leukemia (AML) receiving antifungal prophylaxis (Fisher BT et al).
A different approach to reduce infection morbidity and mortality during induction chemotherapy is the administration of romyelocel. Myeloid progenitor cells are cells that can produce granulocytes but have no long-term reconstitution capability. Romyelocel is a cryopreserved product, of MPC manufactured by ex vivo expansion of CD34+ hematopoietic stem cells. Romyelocel is capable of producing granulocytes, and thereby may reduce the severity or duration of neutropenic fevers. This phase 2 study included 163 patients with de novo AML receiving induction chemotherapy. Evaluable patients (n=120) were randomly assigned to receive either romyelocel-L plus G-CSF (n=59) or G-CSF monotherapy (n=61). From days 15 to 28, romyelocel-L plus G-CSF vs G-CSF monotherapy significantly reduced the mean duration of febrile episodes (2.36 days vs 3.90 days; P = .02) and incidence of infections (6.8% vs 27.9%; P = .0013). Length of hospitalization was significantly shorter in the romyelocel-L plus G-CSF vs G-CSF monotherapy group (25.5 days vs 28.7 days; P = .002). These results are encouraging, and a phase III trial is suggested by the authors. (Desai PM et al).
Finally, a study by MDACC reported disappointing results with the use of venetoclax in patients with tp53 mutation. Findings are from a retrospective analysis of 238 patients with newly diagnosed TP53-mutated AML treated with either VEN-based (n=58) or non-VEN-based (n=180) therapies. The addition of venetoclax to standard treatment regimens (VEN-based) did not improve clinical outcomes in patients with TP53-mutated acute myeloid leukemia (AML), highlighting the need for novel therapies in this patient population. Overall, there was no significant differences in overall survival (median, 5.7 months vs 6.6 months; P = .4), relapse-free survival (median, 3.5 months vs 4.7 months; P = .43), 4-week mortality (7% vs 10%; P = .5), and 8-week mortality (22% vs 17%; P = .4) in patients receiving VEN-based vs non-VEN-based therapies (Venugopal S et al). Clearly, better therapies are needed for this patient population.
Short sleep is linked to future dementia
, according to a new analysis of data from the Whitehall II cohort study.
Previous work had identified links between short sleep duration and dementia risk, but few studies examined sleep habits long before onset of dementia. Those that did produced inconsistent results, according to Séverine Sabia, PhD, who is a research associate at Inserm (France) and the University College London.
“One potential reason for these inconstancies is the large range of ages of the study populations, and the small number of participants within each sleep duration group. The novelty of our study is to examine this association among almost 8,000 participants with a follow-up of 30 years, using repeated measures of sleep duration starting in midlife to consider sleep duration at specific ages,” Dr. Sabia said in an interview. She presented the research at the 2021 Alzheimer’s Association International Conference.
Those previous studies found a U-shaped association between sleep duration and dementia risk, with lowest risk associated with 7-8 hours of sleep, but greater risk for shorter and longer durations. However, because the studies had follow-up periods shorter than 10 years, they are at greater risk of reverse causation bias. Longer follow-up studies tended to have small sample sizes or to focus on older adults.
The longer follow-up in the current study makes for a more compelling case, said Claire Sexton, DPhil, director of Scientific Programs & Outreach for the Alzheimer’s Association. Observations of short or long sleep closer to the onset of symptoms could just be a warning sign of dementia. “But looking at age 50, age 60 ... if you’re seeing those relationships, then it’s less likely that it is just purely prodromal,” said Dr. Sexton. But it still doesn’t necessarily confirm causation. “It could also be a risk factor,” Dr. Sexton added.
Multifactorial risk
Dr. Sabia also noted that the magnitude of risk was similar to that seen with smoking or obesity, and many factors play a role in dementia risk. “Even if the risk of dementia was 30% higher in those with persistent short sleep duration, in absolute terms, the percentage of those with persistent short duration who developed dementia was 8%, and 6% in those with persistent sleep duration of 7 hours. Dementia is a multifactorial disease, which means that several factors are likely to influence its onset. Sleep duration is one of them, but if a person has poor sleep and does not manage to increase it, there are other important prevention measures. It is important to keep a healthy lifestyle and cardiometabolic measures in the normal range. All together it is likely to be beneficial for brain health in later life,” she said.
Dr. Sexton agreed. “With sleep we’re still trying to tease apart what aspect of sleep is important. Is it the sleep duration? Is it the quality of sleep? Is it certain sleep stages?” she said.
Regardless of sleep’s potential influence on dementia risk, both Dr. Sexton and Dr. Sabia noted the importance of sleep for general health. “These types of problems are very prevalent, so it’s good for people to be aware of them. And then if they notice any problems with their sleep, or any changes, to go and see their health care provider, and to be discussing them, and then to be investigating the cause, and to see whether changes in sleep hygiene and treatments for insomnia could address these sleep problems,” said Dr. Sexton.
Decades of data
During the Whitehall II study, researchers assessed average sleep duration (“How many hours of sleep do you have on an average weeknight?”) six times over 30 years of follow-up. Dr. Sabia’s group extracted self-reported sleep duration data at ages 50, 60, and 70. Short sleep duration was defined as fewer than 5 hours, or 6 hours. Normal sleep duration was defined as 7 hours. Long duration was defined as 8 hours or more.
A questioner during the Q&A period noted that this grouping is a little unusual. Many studies define 7-8 hours as normal. Dr. Sabia answered that they were unable to examine periods of 9 hours or more due to the nature of the data, and the lowest associated risk was found at 7 hours.
The researchers analyzed data from 7,959 participants (33.0% women). At age 50, compared with 7 hours of sleep, 6 or few hours of sleep was associated with a higher risk of dementia over the ensuing 25 years of follow-up (hazard ratio [HR], 1.22; 95% confidence interval [CI], 1.01-1.48). The same was true at age 60 (15 years of follow-up HR, 1.37; 95% CI, 1.10-1.72). There was a trend at age 70 (8 years follow-up; HR, 1.24; 95% CI, 0.98-1.57). For 8 or more hours of sleep, there were trends toward increased risk at age 50 (HR, 1.25; 95% CI, 0.98-1.60). Long sleep at age 60 and 70 was associated with heightened risk, but the confidence intervals were well outside statistical significance.
Twenty percent of participants had persistent short sleep over the course of follow-up, 37% had persistent normal sleep, and 7% had persistent long sleep. Seven percent of participants experienced a change from normal sleep to short sleep, 16% had a change from short sleep to normal sleep, and 13% had a change from normal sleep to long sleep.
Persistent short sleep between age 50 and 70 was associated with a 30% increased risk of dementia (HR, 1.30; 95% CI, 1.00-1.69). There were no statistically significant associations between dementia risk and any of the changing sleep pattern groups.
Dr. Sabia and Dr. Sexton have no relevant financial disclosures.
, according to a new analysis of data from the Whitehall II cohort study.
Previous work had identified links between short sleep duration and dementia risk, but few studies examined sleep habits long before onset of dementia. Those that did produced inconsistent results, according to Séverine Sabia, PhD, who is a research associate at Inserm (France) and the University College London.
“One potential reason for these inconstancies is the large range of ages of the study populations, and the small number of participants within each sleep duration group. The novelty of our study is to examine this association among almost 8,000 participants with a follow-up of 30 years, using repeated measures of sleep duration starting in midlife to consider sleep duration at specific ages,” Dr. Sabia said in an interview. She presented the research at the 2021 Alzheimer’s Association International Conference.
Those previous studies found a U-shaped association between sleep duration and dementia risk, with lowest risk associated with 7-8 hours of sleep, but greater risk for shorter and longer durations. However, because the studies had follow-up periods shorter than 10 years, they are at greater risk of reverse causation bias. Longer follow-up studies tended to have small sample sizes or to focus on older adults.
The longer follow-up in the current study makes for a more compelling case, said Claire Sexton, DPhil, director of Scientific Programs & Outreach for the Alzheimer’s Association. Observations of short or long sleep closer to the onset of symptoms could just be a warning sign of dementia. “But looking at age 50, age 60 ... if you’re seeing those relationships, then it’s less likely that it is just purely prodromal,” said Dr. Sexton. But it still doesn’t necessarily confirm causation. “It could also be a risk factor,” Dr. Sexton added.
Multifactorial risk
Dr. Sabia also noted that the magnitude of risk was similar to that seen with smoking or obesity, and many factors play a role in dementia risk. “Even if the risk of dementia was 30% higher in those with persistent short sleep duration, in absolute terms, the percentage of those with persistent short duration who developed dementia was 8%, and 6% in those with persistent sleep duration of 7 hours. Dementia is a multifactorial disease, which means that several factors are likely to influence its onset. Sleep duration is one of them, but if a person has poor sleep and does not manage to increase it, there are other important prevention measures. It is important to keep a healthy lifestyle and cardiometabolic measures in the normal range. All together it is likely to be beneficial for brain health in later life,” she said.
Dr. Sexton agreed. “With sleep we’re still trying to tease apart what aspect of sleep is important. Is it the sleep duration? Is it the quality of sleep? Is it certain sleep stages?” she said.
Regardless of sleep’s potential influence on dementia risk, both Dr. Sexton and Dr. Sabia noted the importance of sleep for general health. “These types of problems are very prevalent, so it’s good for people to be aware of them. And then if they notice any problems with their sleep, or any changes, to go and see their health care provider, and to be discussing them, and then to be investigating the cause, and to see whether changes in sleep hygiene and treatments for insomnia could address these sleep problems,” said Dr. Sexton.
Decades of data
During the Whitehall II study, researchers assessed average sleep duration (“How many hours of sleep do you have on an average weeknight?”) six times over 30 years of follow-up. Dr. Sabia’s group extracted self-reported sleep duration data at ages 50, 60, and 70. Short sleep duration was defined as fewer than 5 hours, or 6 hours. Normal sleep duration was defined as 7 hours. Long duration was defined as 8 hours or more.
A questioner during the Q&A period noted that this grouping is a little unusual. Many studies define 7-8 hours as normal. Dr. Sabia answered that they were unable to examine periods of 9 hours or more due to the nature of the data, and the lowest associated risk was found at 7 hours.
The researchers analyzed data from 7,959 participants (33.0% women). At age 50, compared with 7 hours of sleep, 6 or few hours of sleep was associated with a higher risk of dementia over the ensuing 25 years of follow-up (hazard ratio [HR], 1.22; 95% confidence interval [CI], 1.01-1.48). The same was true at age 60 (15 years of follow-up HR, 1.37; 95% CI, 1.10-1.72). There was a trend at age 70 (8 years follow-up; HR, 1.24; 95% CI, 0.98-1.57). For 8 or more hours of sleep, there were trends toward increased risk at age 50 (HR, 1.25; 95% CI, 0.98-1.60). Long sleep at age 60 and 70 was associated with heightened risk, but the confidence intervals were well outside statistical significance.
Twenty percent of participants had persistent short sleep over the course of follow-up, 37% had persistent normal sleep, and 7% had persistent long sleep. Seven percent of participants experienced a change from normal sleep to short sleep, 16% had a change from short sleep to normal sleep, and 13% had a change from normal sleep to long sleep.
Persistent short sleep between age 50 and 70 was associated with a 30% increased risk of dementia (HR, 1.30; 95% CI, 1.00-1.69). There were no statistically significant associations between dementia risk and any of the changing sleep pattern groups.
Dr. Sabia and Dr. Sexton have no relevant financial disclosures.
, according to a new analysis of data from the Whitehall II cohort study.
Previous work had identified links between short sleep duration and dementia risk, but few studies examined sleep habits long before onset of dementia. Those that did produced inconsistent results, according to Séverine Sabia, PhD, who is a research associate at Inserm (France) and the University College London.
“One potential reason for these inconstancies is the large range of ages of the study populations, and the small number of participants within each sleep duration group. The novelty of our study is to examine this association among almost 8,000 participants with a follow-up of 30 years, using repeated measures of sleep duration starting in midlife to consider sleep duration at specific ages,” Dr. Sabia said in an interview. She presented the research at the 2021 Alzheimer’s Association International Conference.
Those previous studies found a U-shaped association between sleep duration and dementia risk, with lowest risk associated with 7-8 hours of sleep, but greater risk for shorter and longer durations. However, because the studies had follow-up periods shorter than 10 years, they are at greater risk of reverse causation bias. Longer follow-up studies tended to have small sample sizes or to focus on older adults.
The longer follow-up in the current study makes for a more compelling case, said Claire Sexton, DPhil, director of Scientific Programs & Outreach for the Alzheimer’s Association. Observations of short or long sleep closer to the onset of symptoms could just be a warning sign of dementia. “But looking at age 50, age 60 ... if you’re seeing those relationships, then it’s less likely that it is just purely prodromal,” said Dr. Sexton. But it still doesn’t necessarily confirm causation. “It could also be a risk factor,” Dr. Sexton added.
Multifactorial risk
Dr. Sabia also noted that the magnitude of risk was similar to that seen with smoking or obesity, and many factors play a role in dementia risk. “Even if the risk of dementia was 30% higher in those with persistent short sleep duration, in absolute terms, the percentage of those with persistent short duration who developed dementia was 8%, and 6% in those with persistent sleep duration of 7 hours. Dementia is a multifactorial disease, which means that several factors are likely to influence its onset. Sleep duration is one of them, but if a person has poor sleep and does not manage to increase it, there are other important prevention measures. It is important to keep a healthy lifestyle and cardiometabolic measures in the normal range. All together it is likely to be beneficial for brain health in later life,” she said.
Dr. Sexton agreed. “With sleep we’re still trying to tease apart what aspect of sleep is important. Is it the sleep duration? Is it the quality of sleep? Is it certain sleep stages?” she said.
Regardless of sleep’s potential influence on dementia risk, both Dr. Sexton and Dr. Sabia noted the importance of sleep for general health. “These types of problems are very prevalent, so it’s good for people to be aware of them. And then if they notice any problems with their sleep, or any changes, to go and see their health care provider, and to be discussing them, and then to be investigating the cause, and to see whether changes in sleep hygiene and treatments for insomnia could address these sleep problems,” said Dr. Sexton.
Decades of data
During the Whitehall II study, researchers assessed average sleep duration (“How many hours of sleep do you have on an average weeknight?”) six times over 30 years of follow-up. Dr. Sabia’s group extracted self-reported sleep duration data at ages 50, 60, and 70. Short sleep duration was defined as fewer than 5 hours, or 6 hours. Normal sleep duration was defined as 7 hours. Long duration was defined as 8 hours or more.
A questioner during the Q&A period noted that this grouping is a little unusual. Many studies define 7-8 hours as normal. Dr. Sabia answered that they were unable to examine periods of 9 hours or more due to the nature of the data, and the lowest associated risk was found at 7 hours.
The researchers analyzed data from 7,959 participants (33.0% women). At age 50, compared with 7 hours of sleep, 6 or few hours of sleep was associated with a higher risk of dementia over the ensuing 25 years of follow-up (hazard ratio [HR], 1.22; 95% confidence interval [CI], 1.01-1.48). The same was true at age 60 (15 years of follow-up HR, 1.37; 95% CI, 1.10-1.72). There was a trend at age 70 (8 years follow-up; HR, 1.24; 95% CI, 0.98-1.57). For 8 or more hours of sleep, there were trends toward increased risk at age 50 (HR, 1.25; 95% CI, 0.98-1.60). Long sleep at age 60 and 70 was associated with heightened risk, but the confidence intervals were well outside statistical significance.
Twenty percent of participants had persistent short sleep over the course of follow-up, 37% had persistent normal sleep, and 7% had persistent long sleep. Seven percent of participants experienced a change from normal sleep to short sleep, 16% had a change from short sleep to normal sleep, and 13% had a change from normal sleep to long sleep.
Persistent short sleep between age 50 and 70 was associated with a 30% increased risk of dementia (HR, 1.30; 95% CI, 1.00-1.69). There were no statistically significant associations between dementia risk and any of the changing sleep pattern groups.
Dr. Sabia and Dr. Sexton have no relevant financial disclosures.
FROM AAIC 2021
DOACs best aspirin after ventricular ablation: STROKE-VT
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Catheter ablation has been around a lot longer for ventricular arrhythmia than for atrial fibrillation, but far less is settled about what antithrombotic therapy should follow ventricular ablations, as there have been no big, randomized trials for guidance.
But the evidence base grew stronger this week, and it favors postprocedure treatment with a direct oral anticoagulant (DOAC) over antiplatelet therapy with aspirin for patients undergoing radiofrequency (RF) ablation to treat left ventricular (LV) arrhythmias.
The 30-day risk for ischemic stroke or transient ischemia attack (TIA) was sharply higher for patients who took daily aspirin after RF ablation for ventricular tachycardia (VT) or premature ventricular contractions (PVC) in a multicenter randomized trial.
Those of its 246 patients who received aspirin were also far more likely to show asymptomatic lesions on cerebral MRI scans performed both 24 hours and 30 days after the procedure.
The findings show the importance of DOAC therapy after ventricular ablation procedures, a setting for which there are no evidence-based guidelines, “to mitigate the risk of systemic thromboembolic events,” said Dhanunjaya Lakkireddy, MD, Kansas City Heart Rhythm Institute, Overland Park. He spoke at a media presentation on the trial, called STROKE-VT, during the Heart Rhythm Society 2021 Scientific Sessions, held virtually and on-site in Boston.
The risk for stroke and TIA went up in association with several procedural issues, including some that operators might be able to change in order to reach for better outcomes, Dr. Lakkireddy observed.
“Prolonged radiofrequency ablation times, especially in those with low left ventricle ejection fractions, are definitely higher risk,” as are procedures that involved the retrograde transaortic approach for advancing the ablation catheter, rather than a trans-septal approach.
The retrograde transaortic approach should be avoided in such procedures, “whenever it can be avoided,” said Dr. Lakkireddy, who formally presented STROKE-VT at the HRS sessions and is lead author on its report published about the same time in JACC: Clinical Electrophysiology.
The trial has limitations, but “it’s a very important study, and I think that this could become our standard of care for managing anticoagulation after VT and PVC left-sided ablations,” Mina K. Chung, MD, Cleveland Clinic, said as an invited discussant after Dr. Lakkireddy’s presentation.
How patients are treated with antithrombotics after ventricular ablations can vary widely, sometimes based on the operator’s “subjective feeling of how extensive the ablation is,” Christine M. Albert, MD, MPH, Cedars-Sinai Medical Center, Los Angeles, not involved in the study, said during the STROKE-VT media briefing.
That’s consistent with the guidelines, which propose oral anticoagulation therapy after more extensive ventricular ablations and antiplatelets when the ablation is more limited – based more on consensus than firm evidence – as described by Jeffrey R. Winterfield, MD, Medical University of South Carolina, Charleston, and Usha Tedrow, MD, MSc, Brigham and Women’s Hospital, Boston, in an accompanying editorial.
“This is really the first randomized trial data, that I know of, that we have on this. So I do think it will be guideline-influencing,” Dr. Albert said.
“This should change practice,” agreed Jonathan P. Piccini, MD, MHS, Duke University, Durham, N.C., also not part of STROKE-VT. “A lot of evidence in the trial is consistent and provides a compelling story, not to mention that, in my opinion, the study probably underestimates the value of DOACs,” he told this news organization.
That’s because patients assigned to DOACs had far longer ablation times, “so their risk was even greater than in the aspirin arm,” Dr. Piccini said. Ablation times averaged 2,095 seconds in the DOAC group, compared with only 1,708 seconds in the aspirin group, probably because the preponderance of VT over PVC ablations for those getting a DOAC was even greater in the aspirin group.
Of the 246 patients assigned to either aspirin or a DOAC, usually a factor Xa inhibitor, 75% had undergone VT ablation and the remainder ablation for PVCs. Their mean age was 60 years and only 18% were women. None had experienced a cerebrovascular event in the previous 3 months.
The 30-day odds ratio for TIA or ischemic stroke in patients who received aspirin, compared with a DOAC, was 12.6 (95% confidence interval, 4.10-39.11; P < .001).
The corresponding OR for asymptomatic cerebral lesions by MRI at 24 hours was 2.15 (95% CI, 1.02-4.54; P = .04) and at 30 days was 3.48 (95% CI, 1.38-8.80; P = .008).
The rate of stroke or TIA was similar in patients who underwent ablation for VT and for PVCs (14% vs. 16%, respectively; P = .70). There were fewer asymptomatic cerebrovascular events by MRI at 24 hours for those undergoing VT ablations (14.7% and 25.8%, respectively; P = .046); but difference between rates attenuated by 30 days (11.4% and 14.5%, respectively; P = .52).
The OR for TIA or stroke associated with the retrograde transaortic approach, performed in about 40% of the patients, compared with the trans-septal approach in the remainder was 2.60 (95% CI, 1.06-6.37; P = .04).
“The study tells us it’s safe and indeed preferable to anticoagulate after an ablation procedure. But the more important finding, perhaps, wasn’t the one related to the core hypothesis. And that was the effect of retrograde access,” Paul A. Friedman, MD, Mayo Clinic, Rochester, Minn., said as an invited discussant after Dr. Lakkireddy’s formal presentation of the trial.
Whether a ventricular ablation is performed using the retrograde transaortic or trans-septal approach often depends on the location of the ablation targets in the left ventricle. But in some cases it’s a matter of operator preference, Dr. Piccini observed.
“There are some situations where, really, it is better to do retrograde aortic, and there are some cases that are better to do trans-septal. But now there’s going to be a higher burden of proof,” he said. Given the findings of STROKE-VT, operators may need to consider that a ventricular ablation procedure that can be done by the trans-septal route perhaps ought to be consistently done that way.
Dr. Lakkireddy discloses financial relationships with Boston Scientific, Biosense Webster, Janssen Pharmaceuticals, and more. Dr. Chung had “nothing relevant to disclose.” Dr. Piccini discloses receiving honoraria or speaking or consulting fees from Sanofi, Abbott, ARCA Biopharma, Medtronic, Philips, Biotronik, Allergan, LivaNova, and Myokardia; and research in conjunction with Bayer Healthcare, Abbott, Boston Scientific, and Philips. Dr. Friedman discloses conducting research in conjunction with Medtronic and Abbott; holding intellectual property rights with AliveCor, Inference, Medicool, Eko, and Anumana; and receiving honoraria or speaking or consulting fees from Boston Scientific. Dr. Winterfield and Dr. Tedrow had no disclosures.
A version of this article first appeared on Medscape.com.
Bronchitis the leader at putting children in the hospital
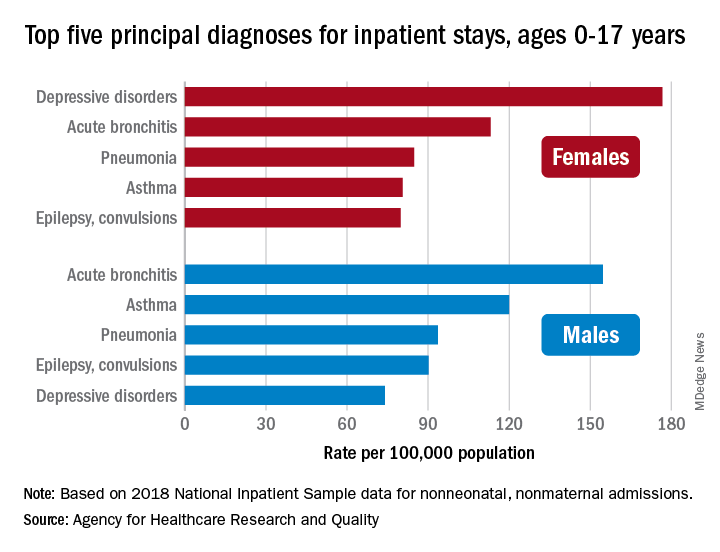
About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.

About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.

About 7% (99,000) of the 1.47 million nonmaternal, nonneonatal hospital stays in children aged 0-17 years involved a primary diagnosis of acute bronchitis in 2018, representing the leading cause of admissions in boys (154.7 stays per 100,000 population) and the second-leading diagnosis in girls (113.1 stays per 100,000), Kimberly W. McDermott, PhD, and Marc Roemer, MS, said in a statistical brief.
Depressive disorders were the most common primary diagnosis in girls, with a rate of 176.7 stays per 100,000, and the second-leading diagnosis overall, although the rate was less than half that (74.0 per 100,000) in boys. Two other respiratory conditions, asthma and pneumonia, were among the top five for both girls and boys, as was epilepsy, they reported.
The combined rate for all diagnoses was slightly higher for boys, 2,051 per 100,000, compared with 1,922 for girls, they said based on data from the National Inpatient Sample.
“Identifying the most frequent primary conditions for which patients are admitted to the hospital is important to the implementation and improvement of health care delivery, quality initiatives, and health policy,” said Dr. McDermott of IBM Watson Health and Mr. Roemer of the AHRQ.
Physicians wearing white coats rated more experienced
Physicians wearing white coats were rated as significantly more experienced and professional than peers wearing casual attire. Regardless of their attire, however, female physicians were more likely to be judged as appearing less professional and were more likely to be misidentified as medical technicians, physician assistants, or nurses, found research published in JAMA Network Open.
“A white coat with scrubs attire was most preferred for surgeons (mean preference index, 1.3), whereas a white coat with business attire was preferred for family physicians and dermatologists (mean preference indexes, 1.6 and 1.2, respectively; P < .001),” Helen Xun, MD, Johns Hopkins University, Baltimore, and colleagues wrote. “A male model wearing business inner wear with a white coat, fleece jacket, or softshell jacket was perceived as significantly more professional than a female model wearing the same attire (mean professionalism score: male, 65.8; female, 56.2; mean difference in professionalism score: white coat, 12.06; fleece, 7.89; softshell, 8.82; P < .001). ... A male model wearing hospital scrubs or fashion scrubs alone was also perceived as more professional than a female model in the same attire.”
While casual attire, such as fleece or softshell jackets emblazoned with the names of the institution and wearer, has become more popular attire for physicians in recent years, the researchers noted theirs is the first published research to identify associations between gender, attire, and how people distinguish between various health care roles. The study authors launched their web-based survey from May to June 2020 and asked people aged 18 years and older to rate a series of photographs of deidentified models wearing health care attire. Inner wear choices were business attire versus scrubs with and without outer wear options of a long white coat, gray fleece jacket, or black softshell jackets. Survey respondents ranked the images on a 6-point Likert scale with 1 being the least experienced, professional, and friendly and 6 being the most experienced, professional, and friendly. Survey respondents also viewed individual images of male or female models and were asked to rate their professionalism on a scale of 0-100 – with 100 as the “most professional” as well as to identify their profession as either physician, surgeon, nurse, medical technician, or physician assistant.
The study team included 487 (93.3%) of 522 completed surveys in their analyses. Respondents’ mean age was 36.2 years; 260 (53.4%) were female; 372 (76.4%) were White; 33 (6.8%) were Black or African American. Younger respondents and those living in the Western United States who had more exposure to physician casual attire appeared more accepting of it, the authors wrote.
“I remember attending my white-coat ceremony as a medical student, and the symbolism of it all representing me entering the profession. It felt very emotional and heavy and I felt very proud to be there. I also remember taking a ‘selfie’ in my long white coat as a doctor for the first time before my first shift as a resident. But, I’ve also been wearing that same white coat, and a large badge with a ‘DOCTOR’ label on it, and been mistaken by a patient or parent for something other than the physician,” Alexandra M. Sims, a pediatrician and health equity researcher in Cincinnati, said in an interview. “So, I’d really hope that the take-home here is not simply that we must wear our white coats to be considered more professional. I think we have to unpack and dismantle how we’ve even built this notion of ‘professionalism’ in the first place. Women, people of color, and other marginalized groups were certainly not a part of the defining, but we must be a part of the reimagining of an equitable health care profession in this new era.”
As sartorial trends usher in more casual attire, clinicians should redouble efforts to build rapport and enhance communication with patients, such as clarifying team members’ roles when introducing themselves. Dr. Xun and coauthors noted that addressing gender bias is important for all clinicians – not just women – and point to the need for institutional and organizational support for disciplines where gender bias is “especially prevalent,” like surgery. “This responsibility should not be undertaken only by the individuals that experience the biases, which may result in additional cumulative career disadvantages. The promotion of equality and diversity begins with recognition, characterization, and evidence-supported interventions and is a community operation,” Dr. Xun and colleagues concluded.
“I do not equate attire to professionalism or experience, nor is it connected to my satisfaction with the physician. For myself and my daughter, it is the experience of care that ultimately influences our perceptions regarding the professionalism of the physician,” Hala H. Durrah, MTA, parent to a chronically ill child with special health care needs and a Patient and Family Engagement Consultant, said in an interview. “My respect for a physician will ultimately be determined by how my daughter and I were treated, not just from a clinical perspective, but how we felt during those interactions.”
Physicians wearing white coats were rated as significantly more experienced and professional than peers wearing casual attire. Regardless of their attire, however, female physicians were more likely to be judged as appearing less professional and were more likely to be misidentified as medical technicians, physician assistants, or nurses, found research published in JAMA Network Open.
“A white coat with scrubs attire was most preferred for surgeons (mean preference index, 1.3), whereas a white coat with business attire was preferred for family physicians and dermatologists (mean preference indexes, 1.6 and 1.2, respectively; P < .001),” Helen Xun, MD, Johns Hopkins University, Baltimore, and colleagues wrote. “A male model wearing business inner wear with a white coat, fleece jacket, or softshell jacket was perceived as significantly more professional than a female model wearing the same attire (mean professionalism score: male, 65.8; female, 56.2; mean difference in professionalism score: white coat, 12.06; fleece, 7.89; softshell, 8.82; P < .001). ... A male model wearing hospital scrubs or fashion scrubs alone was also perceived as more professional than a female model in the same attire.”
While casual attire, such as fleece or softshell jackets emblazoned with the names of the institution and wearer, has become more popular attire for physicians in recent years, the researchers noted theirs is the first published research to identify associations between gender, attire, and how people distinguish between various health care roles. The study authors launched their web-based survey from May to June 2020 and asked people aged 18 years and older to rate a series of photographs of deidentified models wearing health care attire. Inner wear choices were business attire versus scrubs with and without outer wear options of a long white coat, gray fleece jacket, or black softshell jackets. Survey respondents ranked the images on a 6-point Likert scale with 1 being the least experienced, professional, and friendly and 6 being the most experienced, professional, and friendly. Survey respondents also viewed individual images of male or female models and were asked to rate their professionalism on a scale of 0-100 – with 100 as the “most professional” as well as to identify their profession as either physician, surgeon, nurse, medical technician, or physician assistant.
The study team included 487 (93.3%) of 522 completed surveys in their analyses. Respondents’ mean age was 36.2 years; 260 (53.4%) were female; 372 (76.4%) were White; 33 (6.8%) were Black or African American. Younger respondents and those living in the Western United States who had more exposure to physician casual attire appeared more accepting of it, the authors wrote.
“I remember attending my white-coat ceremony as a medical student, and the symbolism of it all representing me entering the profession. It felt very emotional and heavy and I felt very proud to be there. I also remember taking a ‘selfie’ in my long white coat as a doctor for the first time before my first shift as a resident. But, I’ve also been wearing that same white coat, and a large badge with a ‘DOCTOR’ label on it, and been mistaken by a patient or parent for something other than the physician,” Alexandra M. Sims, a pediatrician and health equity researcher in Cincinnati, said in an interview. “So, I’d really hope that the take-home here is not simply that we must wear our white coats to be considered more professional. I think we have to unpack and dismantle how we’ve even built this notion of ‘professionalism’ in the first place. Women, people of color, and other marginalized groups were certainly not a part of the defining, but we must be a part of the reimagining of an equitable health care profession in this new era.”
As sartorial trends usher in more casual attire, clinicians should redouble efforts to build rapport and enhance communication with patients, such as clarifying team members’ roles when introducing themselves. Dr. Xun and coauthors noted that addressing gender bias is important for all clinicians – not just women – and point to the need for institutional and organizational support for disciplines where gender bias is “especially prevalent,” like surgery. “This responsibility should not be undertaken only by the individuals that experience the biases, which may result in additional cumulative career disadvantages. The promotion of equality and diversity begins with recognition, characterization, and evidence-supported interventions and is a community operation,” Dr. Xun and colleagues concluded.
“I do not equate attire to professionalism or experience, nor is it connected to my satisfaction with the physician. For myself and my daughter, it is the experience of care that ultimately influences our perceptions regarding the professionalism of the physician,” Hala H. Durrah, MTA, parent to a chronically ill child with special health care needs and a Patient and Family Engagement Consultant, said in an interview. “My respect for a physician will ultimately be determined by how my daughter and I were treated, not just from a clinical perspective, but how we felt during those interactions.”
Physicians wearing white coats were rated as significantly more experienced and professional than peers wearing casual attire. Regardless of their attire, however, female physicians were more likely to be judged as appearing less professional and were more likely to be misidentified as medical technicians, physician assistants, or nurses, found research published in JAMA Network Open.
“A white coat with scrubs attire was most preferred for surgeons (mean preference index, 1.3), whereas a white coat with business attire was preferred for family physicians and dermatologists (mean preference indexes, 1.6 and 1.2, respectively; P < .001),” Helen Xun, MD, Johns Hopkins University, Baltimore, and colleagues wrote. “A male model wearing business inner wear with a white coat, fleece jacket, or softshell jacket was perceived as significantly more professional than a female model wearing the same attire (mean professionalism score: male, 65.8; female, 56.2; mean difference in professionalism score: white coat, 12.06; fleece, 7.89; softshell, 8.82; P < .001). ... A male model wearing hospital scrubs or fashion scrubs alone was also perceived as more professional than a female model in the same attire.”
While casual attire, such as fleece or softshell jackets emblazoned with the names of the institution and wearer, has become more popular attire for physicians in recent years, the researchers noted theirs is the first published research to identify associations between gender, attire, and how people distinguish between various health care roles. The study authors launched their web-based survey from May to June 2020 and asked people aged 18 years and older to rate a series of photographs of deidentified models wearing health care attire. Inner wear choices were business attire versus scrubs with and without outer wear options of a long white coat, gray fleece jacket, or black softshell jackets. Survey respondents ranked the images on a 6-point Likert scale with 1 being the least experienced, professional, and friendly and 6 being the most experienced, professional, and friendly. Survey respondents also viewed individual images of male or female models and were asked to rate their professionalism on a scale of 0-100 – with 100 as the “most professional” as well as to identify their profession as either physician, surgeon, nurse, medical technician, or physician assistant.
The study team included 487 (93.3%) of 522 completed surveys in their analyses. Respondents’ mean age was 36.2 years; 260 (53.4%) were female; 372 (76.4%) were White; 33 (6.8%) were Black or African American. Younger respondents and those living in the Western United States who had more exposure to physician casual attire appeared more accepting of it, the authors wrote.
“I remember attending my white-coat ceremony as a medical student, and the symbolism of it all representing me entering the profession. It felt very emotional and heavy and I felt very proud to be there. I also remember taking a ‘selfie’ in my long white coat as a doctor for the first time before my first shift as a resident. But, I’ve also been wearing that same white coat, and a large badge with a ‘DOCTOR’ label on it, and been mistaken by a patient or parent for something other than the physician,” Alexandra M. Sims, a pediatrician and health equity researcher in Cincinnati, said in an interview. “So, I’d really hope that the take-home here is not simply that we must wear our white coats to be considered more professional. I think we have to unpack and dismantle how we’ve even built this notion of ‘professionalism’ in the first place. Women, people of color, and other marginalized groups were certainly not a part of the defining, but we must be a part of the reimagining of an equitable health care profession in this new era.”
As sartorial trends usher in more casual attire, clinicians should redouble efforts to build rapport and enhance communication with patients, such as clarifying team members’ roles when introducing themselves. Dr. Xun and coauthors noted that addressing gender bias is important for all clinicians – not just women – and point to the need for institutional and organizational support for disciplines where gender bias is “especially prevalent,” like surgery. “This responsibility should not be undertaken only by the individuals that experience the biases, which may result in additional cumulative career disadvantages. The promotion of equality and diversity begins with recognition, characterization, and evidence-supported interventions and is a community operation,” Dr. Xun and colleagues concluded.
“I do not equate attire to professionalism or experience, nor is it connected to my satisfaction with the physician. For myself and my daughter, it is the experience of care that ultimately influences our perceptions regarding the professionalism of the physician,” Hala H. Durrah, MTA, parent to a chronically ill child with special health care needs and a Patient and Family Engagement Consultant, said in an interview. “My respect for a physician will ultimately be determined by how my daughter and I were treated, not just from a clinical perspective, but how we felt during those interactions.”
FROM JAMA NETWORK OPEN
How diet affects NASH-to-HCC progression
A new study sought to establish a new, clinically relevant mouse model of nonalcoholic steatohepatitis (NASH) that closely reflects human disease as well as the multitissue dynamics involved in the progression and regression of the condition, according to the researchers. This study focused on the association between progression of NASH and consumption of a Western diet, as well as the development of HCC.
The study used a model consisting of hyperphagic mice that lacked a functional ALMS1 gene (Foz/Foz), in addition to wild-type littermates. The model ultimately defined “the key signaling and cytokine pathways that are critical for disease development and resolution” associated with NASH, wrote Souradipta Ganguly, PhD, of the University of California, San Diego, and colleagues. The report was published in Cellular and Molecular Gastroenterology and Hepatology.
According to the researchers, this study is unique given “current rodent models of NASH do not reproduce the complete spectrum of metabolic and histologic” nonalcoholic fatty liver disease (NAFLD) phenotypes. Likewise, the lack of “systemic studies in a single rodent model of NASH that closely recapitulates the human pathology” reinforces the importance of the new model, the researchers added.
Over time, NASH can progress to cirrhosis and hepatocellular carcinoma (HCC). Studies that fed wild-type mice a Western diet have largely failed to mimic the full pathology of NASH to fibrosis to HCC. In addition, the models in these studies fail to reflect the multitissue injuries frequently observed in NASH.
To circumvent these challenges, Dr. Ganguly and colleagues used ALMS1-mutated mice to develop a rodent model of metabolic syndrome that included NASH with fibrosis, chronic kidney disease, and cardiovascular disease. The ALMS1 mutation also resulted in the mice becoming hyperphagic, which increases hunger and leads to early-onset obesity, among other conditions characteristic of metabolic syndrome.
Researchers fed the hyperphagic Foz/Foz mice and wild-type littermates a Western diet or standard diet during a 12-week period for NASH/fibrosis and a 24-week period for HCC. After NASH was established, mice were switched back to normal chow to see if the condition regressed.
Macronutrient distribution of the study’s Western diet included 40% fat, 15% protein, and 44% carbohydrates, based on total caloric content. In contrast, the standard chow included 12% fat, 23% protein, and 65% carbohydrates from total calories.
Within 1-2 weeks, Foz mice fed the Western diet were considered steatotic. These mice subsequently developed NASH by 4 weeks of the study and grade 3 fibrosis by 12 weeks. The researchers concurrently observed the development of chronic kidney injury in the animals. Mice continuing to the 24 weeks ultimately progressed to cirrhosis and HCC; these mice demonstrated reduced survival due to cardiac dysfunction.
Mice that developed NASH were then switched to a diet consisting of normal chow. Following this switch, NASH began to regress, and survival improved. These mice did not appear to develop HCC, and total liver weight was significantly reduced compared with the mice that didn’t enter the regression phase of the study. The researchers wrote that the resolution of hepatic steatosis was also consistent with improved glucose tolerance.
In transcriptomic and histologic analyses, the researchers found strong concordance between Foz/Foz mice NASH liver and human NASH.
The study also found that early disruption of gut barrier, microbial dysbiosis, lipopolysaccharide leakage, and intestinal inflammation preceded NASH in the Foz/Foz mice fed the Western diet, resulting in acute-phase liver inflammation. The early inflammation was reflected by an increase in several chemokines and cytokines by 1-2 weeks. As NASH progressed, the liver cytokine/chemokine profile continued to evolve, leading to monocyte recruitment predominance. “Further studies will elaborate the roles of these NASH-specific microbiomial features in the development and progression of NASH fibrosis,” wrote the researchers.
The study received financial support Janssen, in addition to funding from an ALF Liver Scholar award, ACTRI/National Institutes of Health, the SDDRC, and the NIAAA/National Institutes of Health. The authors disclosed no conflicts.
A new study sought to establish a new, clinically relevant mouse model of nonalcoholic steatohepatitis (NASH) that closely reflects human disease as well as the multitissue dynamics involved in the progression and regression of the condition, according to the researchers. This study focused on the association between progression of NASH and consumption of a Western diet, as well as the development of HCC.
The study used a model consisting of hyperphagic mice that lacked a functional ALMS1 gene (Foz/Foz), in addition to wild-type littermates. The model ultimately defined “the key signaling and cytokine pathways that are critical for disease development and resolution” associated with NASH, wrote Souradipta Ganguly, PhD, of the University of California, San Diego, and colleagues. The report was published in Cellular and Molecular Gastroenterology and Hepatology.
According to the researchers, this study is unique given “current rodent models of NASH do not reproduce the complete spectrum of metabolic and histologic” nonalcoholic fatty liver disease (NAFLD) phenotypes. Likewise, the lack of “systemic studies in a single rodent model of NASH that closely recapitulates the human pathology” reinforces the importance of the new model, the researchers added.
Over time, NASH can progress to cirrhosis and hepatocellular carcinoma (HCC). Studies that fed wild-type mice a Western diet have largely failed to mimic the full pathology of NASH to fibrosis to HCC. In addition, the models in these studies fail to reflect the multitissue injuries frequently observed in NASH.
To circumvent these challenges, Dr. Ganguly and colleagues used ALMS1-mutated mice to develop a rodent model of metabolic syndrome that included NASH with fibrosis, chronic kidney disease, and cardiovascular disease. The ALMS1 mutation also resulted in the mice becoming hyperphagic, which increases hunger and leads to early-onset obesity, among other conditions characteristic of metabolic syndrome.
Researchers fed the hyperphagic Foz/Foz mice and wild-type littermates a Western diet or standard diet during a 12-week period for NASH/fibrosis and a 24-week period for HCC. After NASH was established, mice were switched back to normal chow to see if the condition regressed.
Macronutrient distribution of the study’s Western diet included 40% fat, 15% protein, and 44% carbohydrates, based on total caloric content. In contrast, the standard chow included 12% fat, 23% protein, and 65% carbohydrates from total calories.
Within 1-2 weeks, Foz mice fed the Western diet were considered steatotic. These mice subsequently developed NASH by 4 weeks of the study and grade 3 fibrosis by 12 weeks. The researchers concurrently observed the development of chronic kidney injury in the animals. Mice continuing to the 24 weeks ultimately progressed to cirrhosis and HCC; these mice demonstrated reduced survival due to cardiac dysfunction.
Mice that developed NASH were then switched to a diet consisting of normal chow. Following this switch, NASH began to regress, and survival improved. These mice did not appear to develop HCC, and total liver weight was significantly reduced compared with the mice that didn’t enter the regression phase of the study. The researchers wrote that the resolution of hepatic steatosis was also consistent with improved glucose tolerance.
In transcriptomic and histologic analyses, the researchers found strong concordance between Foz/Foz mice NASH liver and human NASH.
The study also found that early disruption of gut barrier, microbial dysbiosis, lipopolysaccharide leakage, and intestinal inflammation preceded NASH in the Foz/Foz mice fed the Western diet, resulting in acute-phase liver inflammation. The early inflammation was reflected by an increase in several chemokines and cytokines by 1-2 weeks. As NASH progressed, the liver cytokine/chemokine profile continued to evolve, leading to monocyte recruitment predominance. “Further studies will elaborate the roles of these NASH-specific microbiomial features in the development and progression of NASH fibrosis,” wrote the researchers.
The study received financial support Janssen, in addition to funding from an ALF Liver Scholar award, ACTRI/National Institutes of Health, the SDDRC, and the NIAAA/National Institutes of Health. The authors disclosed no conflicts.
A new study sought to establish a new, clinically relevant mouse model of nonalcoholic steatohepatitis (NASH) that closely reflects human disease as well as the multitissue dynamics involved in the progression and regression of the condition, according to the researchers. This study focused on the association between progression of NASH and consumption of a Western diet, as well as the development of HCC.
The study used a model consisting of hyperphagic mice that lacked a functional ALMS1 gene (Foz/Foz), in addition to wild-type littermates. The model ultimately defined “the key signaling and cytokine pathways that are critical for disease development and resolution” associated with NASH, wrote Souradipta Ganguly, PhD, of the University of California, San Diego, and colleagues. The report was published in Cellular and Molecular Gastroenterology and Hepatology.
According to the researchers, this study is unique given “current rodent models of NASH do not reproduce the complete spectrum of metabolic and histologic” nonalcoholic fatty liver disease (NAFLD) phenotypes. Likewise, the lack of “systemic studies in a single rodent model of NASH that closely recapitulates the human pathology” reinforces the importance of the new model, the researchers added.
Over time, NASH can progress to cirrhosis and hepatocellular carcinoma (HCC). Studies that fed wild-type mice a Western diet have largely failed to mimic the full pathology of NASH to fibrosis to HCC. In addition, the models in these studies fail to reflect the multitissue injuries frequently observed in NASH.
To circumvent these challenges, Dr. Ganguly and colleagues used ALMS1-mutated mice to develop a rodent model of metabolic syndrome that included NASH with fibrosis, chronic kidney disease, and cardiovascular disease. The ALMS1 mutation also resulted in the mice becoming hyperphagic, which increases hunger and leads to early-onset obesity, among other conditions characteristic of metabolic syndrome.
Researchers fed the hyperphagic Foz/Foz mice and wild-type littermates a Western diet or standard diet during a 12-week period for NASH/fibrosis and a 24-week period for HCC. After NASH was established, mice were switched back to normal chow to see if the condition regressed.
Macronutrient distribution of the study’s Western diet included 40% fat, 15% protein, and 44% carbohydrates, based on total caloric content. In contrast, the standard chow included 12% fat, 23% protein, and 65% carbohydrates from total calories.
Within 1-2 weeks, Foz mice fed the Western diet were considered steatotic. These mice subsequently developed NASH by 4 weeks of the study and grade 3 fibrosis by 12 weeks. The researchers concurrently observed the development of chronic kidney injury in the animals. Mice continuing to the 24 weeks ultimately progressed to cirrhosis and HCC; these mice demonstrated reduced survival due to cardiac dysfunction.
Mice that developed NASH were then switched to a diet consisting of normal chow. Following this switch, NASH began to regress, and survival improved. These mice did not appear to develop HCC, and total liver weight was significantly reduced compared with the mice that didn’t enter the regression phase of the study. The researchers wrote that the resolution of hepatic steatosis was also consistent with improved glucose tolerance.
In transcriptomic and histologic analyses, the researchers found strong concordance between Foz/Foz mice NASH liver and human NASH.
The study also found that early disruption of gut barrier, microbial dysbiosis, lipopolysaccharide leakage, and intestinal inflammation preceded NASH in the Foz/Foz mice fed the Western diet, resulting in acute-phase liver inflammation. The early inflammation was reflected by an increase in several chemokines and cytokines by 1-2 weeks. As NASH progressed, the liver cytokine/chemokine profile continued to evolve, leading to monocyte recruitment predominance. “Further studies will elaborate the roles of these NASH-specific microbiomial features in the development and progression of NASH fibrosis,” wrote the researchers.
The study received financial support Janssen, in addition to funding from an ALF Liver Scholar award, ACTRI/National Institutes of Health, the SDDRC, and the NIAAA/National Institutes of Health. The authors disclosed no conflicts.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Treating the unvaccinated
The following is not anything I’m doing. It’s written solely as a thought exercise.
I don’t think it’s illegal, any more than if I refused to see smokers, or gum chewers. I mean, it’s my practice. I’m the only one here.
It’s certainly unethical, though. Part of being a doctor is caring for those who need our help. I’m vaccinated, so hopefully I’m at lower risk of getting sick if exposed. But that’s not a guarantee.
The vaccine is 95% effective. But that still means 1 in 20 vaccinated people can still contract the disease. Of course, people who aren’t vaccinated have no protection at all, aside from their immune system.
If the decision to not vaccinate, or not wear a mask, only affected themselves, I wouldn’t have as much of an issue with it. Like bungee jumping, the consequences of something going wrong affect only the person who made the choice (not including costs to the health care system or loved ones, now caretakers).
But with an easily spread infectious disease, a better analogy is probably that of drunk drivers. Their actions affect not only themselves, but everyone else on (or near) the road: other drivers, their passengers, pedestrians. ...
In a neurology practice not all of my patients have great immune systems. Sure, there are healthy migraine patients, but I also see patients with multiple sclerosis (on drugs like Ocrevus), patients with myasthenia gravis (on steroids or Imuran), and other folks whose survival depends on keeping their immune systems working at a suboptimal level. Not to mention those with malignancies, leukemias, and lymphomas.
These people have no real defense against the virus, and many of them can’t even get the vaccine. They depend on precautions, herd immunity, and luck. So, to protect them, maybe I should keep the unvaccinated out. Granted, this isn’t a guarantee, either, and doesn’t protect them during more mundane activities, such as grocery shopping or filling up their car.
Besides, the unvaccinated have their own, unrelated, neurological issues. Migraines, seizures, neuropathy, and so they need to see me. My job is to help anyone who needs me. Isn’t that what being a doctor is all about?
It’s an interesting question. Like most things in medicine, there is no black or white, just different shades of gray.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The following is not anything I’m doing. It’s written solely as a thought exercise.
I don’t think it’s illegal, any more than if I refused to see smokers, or gum chewers. I mean, it’s my practice. I’m the only one here.
It’s certainly unethical, though. Part of being a doctor is caring for those who need our help. I’m vaccinated, so hopefully I’m at lower risk of getting sick if exposed. But that’s not a guarantee.
The vaccine is 95% effective. But that still means 1 in 20 vaccinated people can still contract the disease. Of course, people who aren’t vaccinated have no protection at all, aside from their immune system.
If the decision to not vaccinate, or not wear a mask, only affected themselves, I wouldn’t have as much of an issue with it. Like bungee jumping, the consequences of something going wrong affect only the person who made the choice (not including costs to the health care system or loved ones, now caretakers).
But with an easily spread infectious disease, a better analogy is probably that of drunk drivers. Their actions affect not only themselves, but everyone else on (or near) the road: other drivers, their passengers, pedestrians. ...
In a neurology practice not all of my patients have great immune systems. Sure, there are healthy migraine patients, but I also see patients with multiple sclerosis (on drugs like Ocrevus), patients with myasthenia gravis (on steroids or Imuran), and other folks whose survival depends on keeping their immune systems working at a suboptimal level. Not to mention those with malignancies, leukemias, and lymphomas.
These people have no real defense against the virus, and many of them can’t even get the vaccine. They depend on precautions, herd immunity, and luck. So, to protect them, maybe I should keep the unvaccinated out. Granted, this isn’t a guarantee, either, and doesn’t protect them during more mundane activities, such as grocery shopping or filling up their car.
Besides, the unvaccinated have their own, unrelated, neurological issues. Migraines, seizures, neuropathy, and so they need to see me. My job is to help anyone who needs me. Isn’t that what being a doctor is all about?
It’s an interesting question. Like most things in medicine, there is no black or white, just different shades of gray.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
The following is not anything I’m doing. It’s written solely as a thought exercise.
I don’t think it’s illegal, any more than if I refused to see smokers, or gum chewers. I mean, it’s my practice. I’m the only one here.
It’s certainly unethical, though. Part of being a doctor is caring for those who need our help. I’m vaccinated, so hopefully I’m at lower risk of getting sick if exposed. But that’s not a guarantee.
The vaccine is 95% effective. But that still means 1 in 20 vaccinated people can still contract the disease. Of course, people who aren’t vaccinated have no protection at all, aside from their immune system.
If the decision to not vaccinate, or not wear a mask, only affected themselves, I wouldn’t have as much of an issue with it. Like bungee jumping, the consequences of something going wrong affect only the person who made the choice (not including costs to the health care system or loved ones, now caretakers).
But with an easily spread infectious disease, a better analogy is probably that of drunk drivers. Their actions affect not only themselves, but everyone else on (or near) the road: other drivers, their passengers, pedestrians. ...
In a neurology practice not all of my patients have great immune systems. Sure, there are healthy migraine patients, but I also see patients with multiple sclerosis (on drugs like Ocrevus), patients with myasthenia gravis (on steroids or Imuran), and other folks whose survival depends on keeping their immune systems working at a suboptimal level. Not to mention those with malignancies, leukemias, and lymphomas.
These people have no real defense against the virus, and many of them can’t even get the vaccine. They depend on precautions, herd immunity, and luck. So, to protect them, maybe I should keep the unvaccinated out. Granted, this isn’t a guarantee, either, and doesn’t protect them during more mundane activities, such as grocery shopping or filling up their car.
Besides, the unvaccinated have their own, unrelated, neurological issues. Migraines, seizures, neuropathy, and so they need to see me. My job is to help anyone who needs me. Isn’t that what being a doctor is all about?
It’s an interesting question. Like most things in medicine, there is no black or white, just different shades of gray.
Dr. Block has a solo neurology practice in Scottsdale, Ariz.
MR elastography could predict cirrhosis in NAFLD
Liver stiffness measurement with magnetic resonance elastography (MRE) may prove predictive of future cirrhosis risk in patients with nonalcoholic fatty liver disease (NAFLD), according to researchers from the Mayo Clinic in Rochester, Minn.
“These data expand the role of MRE from an accurate diagnostic method to a prognostic noninvasive imaging biomarker that can risk-stratify patients with NAFLD and guide the timing of surveillance and further refine their clinical management,” wrote Tolga Gidener, MD, and colleagues. The study authors added that the research further expands “the role of MRE beyond liver fibrosis estimation by adding a predictive feature to improve individualized disease monitoring and patient counseling.” Their study was published in Clinical Gastroenterology and Hepatology.
Currently, there are no established noninvasive strategies that can effectively identify patients with NAFLD who are at high risk of progression to cirrhosis and liver-related complications. While fibrosis stage on histology may predict liver-associated outcomes in these patients, this approach is invasive, time consuming, and is generally not well tolerated by patients.
Although the technique has been noted for its high success rate and excellent levels of reliability and reproducibility, a possible limitation of MRE is its cost. That said, standalone MRE is reimbursed under Medicare Category I Current Procedural Terminology code 76391 with a cost of $240.02. However, there is also a lack of data on whether baseline liver stiffness measurement by MRE can predict progression of NAFLD to cirrhosis.
To gauge the role of baseline liver stiffness measurement by MRE, Dr. Gidener and colleagues performed a retrospective cohort study that evaluated hard liver–related outcomes in 829 adult patients with NAFLD with or without cirrhosis (median age, 58 years; 54% female) who underwent MRE during 2007-2019.
Patients in the study were followed from the first MRE until death, last clinical encounter, or the end of the study. Clinical outcomes assessed in individual chart review included cirrhosis, hepatic decompensation, and death.
At baseline, the median liver stiffness measurement was 2.8 kPa in 639 patients with NAFLD but without cirrhosis. Over a median 4-year follow-up period, a total of 20 patients developed cirrhosis, with an overall annual incidence rate of 1%.
Baseline liver stiffness measurement by MRE was significantly predictive of subsequent cirrhosis (hazard ratio, 2.93; 95% confidence interval, 1.86-4.62; P < .0001) per 1-kPa difference in liver stiffness measurement at baseline.
According to the researchers, the probability of future cirrhosis development can be ascertained using current liver stiffness measurement. As such, a greater than 1% probability threshold can be reached in 5 years in patients with a measurement of 2 kPa, 3 years in patients with a measurement of 3 kPA, and 1 year in patients with 4-5 kPa. “These time frames inform about estimated time to progression to hard outcomes and provide guidance for subsequent noninvasive monitoring for disease progression,” wrote the researchers.
The baseline liver stiffness measurement by MRE was also significantly predictive of future hepatic decompensation or death (HR, 1.32; 95% CI, 1.13-1.56; P = .0007) per 1-kPa increment in the liver stiffness measurement. Likewise, the 1-year probability of subsequent hepatic decompensation or death in patients with cirrhosis and baseline liver stiffness measurement of 5 kPa versus 8 kPa was 9% versus 20%, respectively. In terms of covariates, age was the only factor that increased the risk of hepatic decompensation or death.
While the current study offers a glimpse into the potential clinical implications of liver stiffness measurement by MRE in NAFLD, the researchers suggest the applicability of the findings are limited by the study’s small sample size, relatively short follow-up duration, and the small number of cirrhosis events.
The researchers received study funding from the National Institute of Diabetes and Digestive and Kidney Diseases, American College of Gastroenterology, National Institutes of Health, and the Department of Defense. The researchers disclosed no other relevant conflicts of interest.
Liver stiffness measurement with magnetic resonance elastography (MRE) may prove predictive of future cirrhosis risk in patients with nonalcoholic fatty liver disease (NAFLD), according to researchers from the Mayo Clinic in Rochester, Minn.
“These data expand the role of MRE from an accurate diagnostic method to a prognostic noninvasive imaging biomarker that can risk-stratify patients with NAFLD and guide the timing of surveillance and further refine their clinical management,” wrote Tolga Gidener, MD, and colleagues. The study authors added that the research further expands “the role of MRE beyond liver fibrosis estimation by adding a predictive feature to improve individualized disease monitoring and patient counseling.” Their study was published in Clinical Gastroenterology and Hepatology.
Currently, there are no established noninvasive strategies that can effectively identify patients with NAFLD who are at high risk of progression to cirrhosis and liver-related complications. While fibrosis stage on histology may predict liver-associated outcomes in these patients, this approach is invasive, time consuming, and is generally not well tolerated by patients.
Although the technique has been noted for its high success rate and excellent levels of reliability and reproducibility, a possible limitation of MRE is its cost. That said, standalone MRE is reimbursed under Medicare Category I Current Procedural Terminology code 76391 with a cost of $240.02. However, there is also a lack of data on whether baseline liver stiffness measurement by MRE can predict progression of NAFLD to cirrhosis.
To gauge the role of baseline liver stiffness measurement by MRE, Dr. Gidener and colleagues performed a retrospective cohort study that evaluated hard liver–related outcomes in 829 adult patients with NAFLD with or without cirrhosis (median age, 58 years; 54% female) who underwent MRE during 2007-2019.
Patients in the study were followed from the first MRE until death, last clinical encounter, or the end of the study. Clinical outcomes assessed in individual chart review included cirrhosis, hepatic decompensation, and death.
At baseline, the median liver stiffness measurement was 2.8 kPa in 639 patients with NAFLD but without cirrhosis. Over a median 4-year follow-up period, a total of 20 patients developed cirrhosis, with an overall annual incidence rate of 1%.
Baseline liver stiffness measurement by MRE was significantly predictive of subsequent cirrhosis (hazard ratio, 2.93; 95% confidence interval, 1.86-4.62; P < .0001) per 1-kPa difference in liver stiffness measurement at baseline.
According to the researchers, the probability of future cirrhosis development can be ascertained using current liver stiffness measurement. As such, a greater than 1% probability threshold can be reached in 5 years in patients with a measurement of 2 kPa, 3 years in patients with a measurement of 3 kPA, and 1 year in patients with 4-5 kPa. “These time frames inform about estimated time to progression to hard outcomes and provide guidance for subsequent noninvasive monitoring for disease progression,” wrote the researchers.
The baseline liver stiffness measurement by MRE was also significantly predictive of future hepatic decompensation or death (HR, 1.32; 95% CI, 1.13-1.56; P = .0007) per 1-kPa increment in the liver stiffness measurement. Likewise, the 1-year probability of subsequent hepatic decompensation or death in patients with cirrhosis and baseline liver stiffness measurement of 5 kPa versus 8 kPa was 9% versus 20%, respectively. In terms of covariates, age was the only factor that increased the risk of hepatic decompensation or death.
While the current study offers a glimpse into the potential clinical implications of liver stiffness measurement by MRE in NAFLD, the researchers suggest the applicability of the findings are limited by the study’s small sample size, relatively short follow-up duration, and the small number of cirrhosis events.
The researchers received study funding from the National Institute of Diabetes and Digestive and Kidney Diseases, American College of Gastroenterology, National Institutes of Health, and the Department of Defense. The researchers disclosed no other relevant conflicts of interest.
Liver stiffness measurement with magnetic resonance elastography (MRE) may prove predictive of future cirrhosis risk in patients with nonalcoholic fatty liver disease (NAFLD), according to researchers from the Mayo Clinic in Rochester, Minn.
“These data expand the role of MRE from an accurate diagnostic method to a prognostic noninvasive imaging biomarker that can risk-stratify patients with NAFLD and guide the timing of surveillance and further refine their clinical management,” wrote Tolga Gidener, MD, and colleagues. The study authors added that the research further expands “the role of MRE beyond liver fibrosis estimation by adding a predictive feature to improve individualized disease monitoring and patient counseling.” Their study was published in Clinical Gastroenterology and Hepatology.
Currently, there are no established noninvasive strategies that can effectively identify patients with NAFLD who are at high risk of progression to cirrhosis and liver-related complications. While fibrosis stage on histology may predict liver-associated outcomes in these patients, this approach is invasive, time consuming, and is generally not well tolerated by patients.
Although the technique has been noted for its high success rate and excellent levels of reliability and reproducibility, a possible limitation of MRE is its cost. That said, standalone MRE is reimbursed under Medicare Category I Current Procedural Terminology code 76391 with a cost of $240.02. However, there is also a lack of data on whether baseline liver stiffness measurement by MRE can predict progression of NAFLD to cirrhosis.
To gauge the role of baseline liver stiffness measurement by MRE, Dr. Gidener and colleagues performed a retrospective cohort study that evaluated hard liver–related outcomes in 829 adult patients with NAFLD with or without cirrhosis (median age, 58 years; 54% female) who underwent MRE during 2007-2019.
Patients in the study were followed from the first MRE until death, last clinical encounter, or the end of the study. Clinical outcomes assessed in individual chart review included cirrhosis, hepatic decompensation, and death.
At baseline, the median liver stiffness measurement was 2.8 kPa in 639 patients with NAFLD but without cirrhosis. Over a median 4-year follow-up period, a total of 20 patients developed cirrhosis, with an overall annual incidence rate of 1%.
Baseline liver stiffness measurement by MRE was significantly predictive of subsequent cirrhosis (hazard ratio, 2.93; 95% confidence interval, 1.86-4.62; P < .0001) per 1-kPa difference in liver stiffness measurement at baseline.
According to the researchers, the probability of future cirrhosis development can be ascertained using current liver stiffness measurement. As such, a greater than 1% probability threshold can be reached in 5 years in patients with a measurement of 2 kPa, 3 years in patients with a measurement of 3 kPA, and 1 year in patients with 4-5 kPa. “These time frames inform about estimated time to progression to hard outcomes and provide guidance for subsequent noninvasive monitoring for disease progression,” wrote the researchers.
The baseline liver stiffness measurement by MRE was also significantly predictive of future hepatic decompensation or death (HR, 1.32; 95% CI, 1.13-1.56; P = .0007) per 1-kPa increment in the liver stiffness measurement. Likewise, the 1-year probability of subsequent hepatic decompensation or death in patients with cirrhosis and baseline liver stiffness measurement of 5 kPa versus 8 kPa was 9% versus 20%, respectively. In terms of covariates, age was the only factor that increased the risk of hepatic decompensation or death.
While the current study offers a glimpse into the potential clinical implications of liver stiffness measurement by MRE in NAFLD, the researchers suggest the applicability of the findings are limited by the study’s small sample size, relatively short follow-up duration, and the small number of cirrhosis events.
The researchers received study funding from the National Institute of Diabetes and Digestive and Kidney Diseases, American College of Gastroenterology, National Institutes of Health, and the Department of Defense. The researchers disclosed no other relevant conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Modest calorie reduction plus exercise linked with improved vascular health
Finding applies to seniors with obesity, who were part of a new study
The authors of the paper, published in Circulation, found a link between greater vascular benefits and exercise with modest – rather than intense – calorie restriction (CR) in elderly individuals with obesity.
“The finding that higher-intensity calorie restriction may not be necessary or advised has important implications for weight loss recommendations,” noted Tina E. Brinkley, Ph.D., lead author of the study and associate professor of gerontology and geriatric medicine at the Sticht Center for Healthy Aging and Alzheimer’s Prevention at Wake Forest University in Winston-Salem, N.C.
It’s “not entirely clear” why greater calorie restriction did not translate to greater vascular benefit, but it “could be related in part to potentially adverse effects of severe CR on vascular function,” she noted. “These findings have important implications for reducing cardiovascular risk with nonpharmacological interventions in high-risk populations.”
Methods and findings
The study included 160 men and women aged 65-79 years, with a body mass index (BMI) of 30 to 45 kg/m2. The subjects were randomized to one of three groups for 20 weeks of aerobic exercise only, aerobic exercise plus moderate CR, or aerobic exercise plus more intensive CR. Their exercise regimen involved 30 minutes of supervised treadmill walking for 4 days per week at 65%-70% of heart rate reserve.
Subjects in the moderate CR group decreased caloric intake by 250 kcals a day, while the intense calorie reduction group cut 600 kcals per day. Their meals contained less than 30% of calories from fat and at least 0.8 g of protein per kg of ideal body weight. They were also provided with supplemental calcium (1,200 mg/day) and vitamin D (800 IU/day).
Cardiovascular magnetic resonance imaging was used to assess various aspects of aortic structure and function, including aortic arch pulse wave velocity, aortic distensibility and dimensions, and periaortic fat.
Weight loss was greater among subjects with CR plus exercise, compared with that of patients in the exercise-only group. The degree of weight loss was not significantly different between those with moderate versus intense CR ( 8.02 kg vs. 8.98 kg).
Among the exercise-only group, researchers observed no changes in aortic stiffness. However, adding moderate CR significantly improved this measure, while intense CR did not.
Specifically, subjects in the moderate-CR group had a “robust” 21% increase in distensibility in the descending aorta (DA), and an 8% decrease in aortic arch pulse wave velocity, whereas there were no significant vascular changes in the intense-CR group.
Bests results seen in exercise plus modest CR group
“Collectively, these data suggest that combining exercise with modest CR (as opposed to more intensive CR or no CR) provides the greatest benefit for proximal aortic stiffness, while also optimizing weight loss and improvements in body composition and body fat distribution,” noted the authors in their paper.
“Our data support the growing number of studies indicating that intentional weight loss can be safe for older adults with obesity and extend our previous findings, suggesting that obesity may blunt the beneficial effects of exercise for not only cardiorespiratory fitness, but likely vascular health as well.”
William E. Kraus, MD, professor in the Department of Medicine, Division of Cardiology at Duke University Medical Center, in Durham, NC, described the study as important and interesting for several reasons.
“First, it demonstrates one can change aortic vascular function with a combined diet and exercise program, even in older, obese Americans. This implies it is never too late to make meaningful lifestyle changes that will benefit cardiovascular health,” he said. “Second, it is among an increasing number of studies demonstrating that more is not always better than less in exercise and diet lifestyle changes - and in fact the converse is true.”
“This gives hope that more people can benefit from modest lifestyle changes - in this case following guidelines for physical activity and only a modest reduction of 250 kilocalories per day resulted in benefit,” Dr. Kraus added.
The authors of the paper and Dr. Kraus disclosed no conflicts of interest.
Finding applies to seniors with obesity, who were part of a new study
Finding applies to seniors with obesity, who were part of a new study
The authors of the paper, published in Circulation, found a link between greater vascular benefits and exercise with modest – rather than intense – calorie restriction (CR) in elderly individuals with obesity.
“The finding that higher-intensity calorie restriction may not be necessary or advised has important implications for weight loss recommendations,” noted Tina E. Brinkley, Ph.D., lead author of the study and associate professor of gerontology and geriatric medicine at the Sticht Center for Healthy Aging and Alzheimer’s Prevention at Wake Forest University in Winston-Salem, N.C.
It’s “not entirely clear” why greater calorie restriction did not translate to greater vascular benefit, but it “could be related in part to potentially adverse effects of severe CR on vascular function,” she noted. “These findings have important implications for reducing cardiovascular risk with nonpharmacological interventions in high-risk populations.”
Methods and findings
The study included 160 men and women aged 65-79 years, with a body mass index (BMI) of 30 to 45 kg/m2. The subjects were randomized to one of three groups for 20 weeks of aerobic exercise only, aerobic exercise plus moderate CR, or aerobic exercise plus more intensive CR. Their exercise regimen involved 30 minutes of supervised treadmill walking for 4 days per week at 65%-70% of heart rate reserve.
Subjects in the moderate CR group decreased caloric intake by 250 kcals a day, while the intense calorie reduction group cut 600 kcals per day. Their meals contained less than 30% of calories from fat and at least 0.8 g of protein per kg of ideal body weight. They were also provided with supplemental calcium (1,200 mg/day) and vitamin D (800 IU/day).
Cardiovascular magnetic resonance imaging was used to assess various aspects of aortic structure and function, including aortic arch pulse wave velocity, aortic distensibility and dimensions, and periaortic fat.
Weight loss was greater among subjects with CR plus exercise, compared with that of patients in the exercise-only group. The degree of weight loss was not significantly different between those with moderate versus intense CR ( 8.02 kg vs. 8.98 kg).
Among the exercise-only group, researchers observed no changes in aortic stiffness. However, adding moderate CR significantly improved this measure, while intense CR did not.
Specifically, subjects in the moderate-CR group had a “robust” 21% increase in distensibility in the descending aorta (DA), and an 8% decrease in aortic arch pulse wave velocity, whereas there were no significant vascular changes in the intense-CR group.
Bests results seen in exercise plus modest CR group
“Collectively, these data suggest that combining exercise with modest CR (as opposed to more intensive CR or no CR) provides the greatest benefit for proximal aortic stiffness, while also optimizing weight loss and improvements in body composition and body fat distribution,” noted the authors in their paper.
“Our data support the growing number of studies indicating that intentional weight loss can be safe for older adults with obesity and extend our previous findings, suggesting that obesity may blunt the beneficial effects of exercise for not only cardiorespiratory fitness, but likely vascular health as well.”
William E. Kraus, MD, professor in the Department of Medicine, Division of Cardiology at Duke University Medical Center, in Durham, NC, described the study as important and interesting for several reasons.
“First, it demonstrates one can change aortic vascular function with a combined diet and exercise program, even in older, obese Americans. This implies it is never too late to make meaningful lifestyle changes that will benefit cardiovascular health,” he said. “Second, it is among an increasing number of studies demonstrating that more is not always better than less in exercise and diet lifestyle changes - and in fact the converse is true.”
“This gives hope that more people can benefit from modest lifestyle changes - in this case following guidelines for physical activity and only a modest reduction of 250 kilocalories per day resulted in benefit,” Dr. Kraus added.
The authors of the paper and Dr. Kraus disclosed no conflicts of interest.
The authors of the paper, published in Circulation, found a link between greater vascular benefits and exercise with modest – rather than intense – calorie restriction (CR) in elderly individuals with obesity.
“The finding that higher-intensity calorie restriction may not be necessary or advised has important implications for weight loss recommendations,” noted Tina E. Brinkley, Ph.D., lead author of the study and associate professor of gerontology and geriatric medicine at the Sticht Center for Healthy Aging and Alzheimer’s Prevention at Wake Forest University in Winston-Salem, N.C.
It’s “not entirely clear” why greater calorie restriction did not translate to greater vascular benefit, but it “could be related in part to potentially adverse effects of severe CR on vascular function,” she noted. “These findings have important implications for reducing cardiovascular risk with nonpharmacological interventions in high-risk populations.”
Methods and findings
The study included 160 men and women aged 65-79 years, with a body mass index (BMI) of 30 to 45 kg/m2. The subjects were randomized to one of three groups for 20 weeks of aerobic exercise only, aerobic exercise plus moderate CR, or aerobic exercise plus more intensive CR. Their exercise regimen involved 30 minutes of supervised treadmill walking for 4 days per week at 65%-70% of heart rate reserve.
Subjects in the moderate CR group decreased caloric intake by 250 kcals a day, while the intense calorie reduction group cut 600 kcals per day. Their meals contained less than 30% of calories from fat and at least 0.8 g of protein per kg of ideal body weight. They were also provided with supplemental calcium (1,200 mg/day) and vitamin D (800 IU/day).
Cardiovascular magnetic resonance imaging was used to assess various aspects of aortic structure and function, including aortic arch pulse wave velocity, aortic distensibility and dimensions, and periaortic fat.
Weight loss was greater among subjects with CR plus exercise, compared with that of patients in the exercise-only group. The degree of weight loss was not significantly different between those with moderate versus intense CR ( 8.02 kg vs. 8.98 kg).
Among the exercise-only group, researchers observed no changes in aortic stiffness. However, adding moderate CR significantly improved this measure, while intense CR did not.
Specifically, subjects in the moderate-CR group had a “robust” 21% increase in distensibility in the descending aorta (DA), and an 8% decrease in aortic arch pulse wave velocity, whereas there were no significant vascular changes in the intense-CR group.
Bests results seen in exercise plus modest CR group
“Collectively, these data suggest that combining exercise with modest CR (as opposed to more intensive CR or no CR) provides the greatest benefit for proximal aortic stiffness, while also optimizing weight loss and improvements in body composition and body fat distribution,” noted the authors in their paper.
“Our data support the growing number of studies indicating that intentional weight loss can be safe for older adults with obesity and extend our previous findings, suggesting that obesity may blunt the beneficial effects of exercise for not only cardiorespiratory fitness, but likely vascular health as well.”
William E. Kraus, MD, professor in the Department of Medicine, Division of Cardiology at Duke University Medical Center, in Durham, NC, described the study as important and interesting for several reasons.
“First, it demonstrates one can change aortic vascular function with a combined diet and exercise program, even in older, obese Americans. This implies it is never too late to make meaningful lifestyle changes that will benefit cardiovascular health,” he said. “Second, it is among an increasing number of studies demonstrating that more is not always better than less in exercise and diet lifestyle changes - and in fact the converse is true.”
“This gives hope that more people can benefit from modest lifestyle changes - in this case following guidelines for physical activity and only a modest reduction of 250 kilocalories per day resulted in benefit,” Dr. Kraus added.
The authors of the paper and Dr. Kraus disclosed no conflicts of interest.
FROM CIRCULATION
The Precision Oncology Program for Cancer of the Prostate (POPCaP) Network: A Veterans Affairs/Prostate Cancer Foundation Collaboration(FULL)
The US Department of Veterans Affairs (VA) is home to the Veterans Health Administration (VHA), which delivers care at 1,255 health care facilities, including 170 medical centers. The VA serves 6 million veterans each year and is the largest integrated provider of cancer care in the US. The system uses a single, enterprise-wide electronic health record. The detailed curation of clinical outcomes, laboratory results, and radiology is used in VA efforts to improve oncology outcomes for veterans. The VA also has a National Precision Oncology Program (NPOP), which offers system-wide DNA sequencing for veterans with cancer. Given its size, integration, and capabilities, the VA is an ideal setting for rapid learning cycles of testing and implementing best practices at scale.
Prostate cancer is the most common malignancy affecting men in the US. It is the most commonly-diagnosed solid tumor in the VA, and in 2014, there were 11,376 prostate cancer diagnoses in the VA.1 The clinical characteristics and treatment of veterans with prostate cancer largely parallel the broader population of men in the US.1 Although the majority of men diagnosed with prostate cancer have disease localized to the prostate, an important minority develop metastatic disease, which represents a risk for substantial morbidity and is the lethal form of the disease. Research has yielded transformative advances in the care of men with metastatic prostate cancer, including drugs targeting the testosterone/androgen signaling axis, taxane chemotherapy, the radionuclide radium-223, and a dendritic cell vaccine. Unfortunately, the magnitude and duration of response to these therapies varies widely, and determining the biology relevant to an individual patient that would better inform their treatment decisions is a critical next step. As the ability to interrogate the cancer genome has improved, relevant drivers of tumorigenesis and predictive biomarkers are being identified rapidly, and oncology care has evolved from a one-size-fits-all approach to a precision approach, which uses these biomarkers to assist in therapeutic decision making.
Precision Oncology for Prostate Cancer
A series of studies interrogating the genomics of metastatic prostate cancer have been critical to defining the relevance of precision oncology for prostate cancer. Most of what is known about the genomics of prostate cancer has been derived from analysis of samples from the prostate itself. These samples may not reflect the biology of metastasis and genetic evolution in response to treatment pressure, so the genomic alterations in metastatic disease remained incompletely characterized. Two large research teams supported by grants from the American Association for Cancer Research, Stand Up 2 Cancer, and Prostate Cancer Foundation (PCF) focused their efforts on sampling and analyzing metastatic tissue to define the most relevant genomic alterations in advanced prostate cancer.
These efforts defined a broad range of relatively common alterations in the androgen receptor, as well as the tumor suppressors TP53 and PTEN.2,3 Important subsets of less common alterations in pathways that were potentially targetable were also found, including new alterations in PIK3CA/B, BRAF/RAF1, and β-catenin. Most surprisingly, alterations of DNA repair pathways, including mismatch repair and homologous recombination were found in 20% of tumors, and half of these tumors contained germline alterations. The same groups performed a follow up analysis of germline DNA from men with metastatic prostate cancer, which confirmed that 12% of these patients carry a pathogenic germline alteration in a DNA repair pathway gene.4 These efforts immediately invigorated precision oncology clinical trials for prostate cancer and spurred an effort to find the molecular alterations that could be leveraged to improve care for men with advanced prostate cancer.
Targetable Alterations
Currently a number of genomic alterations are immediately actionable. There are several agents approved by the US Food and Drug Administration (FDA) that exploit these Achilles heels of prostate cancer. Mismatch repair deficiency occurs when any of a group of genes responsible for proofreading the fidelity of DNA replication is compromised by mutation or deletion. Imperfect reading and correction subsequently lead to many DNA mutations in a tissue (hypermutation), which then increases the risk of developing malignancy. If a defective gene in the mismatch repair pathway is inherited, a patient has a genetic predisposition to specific malignancies that are part of the Lynch syndrome.5 Prostate cancer is a relatively rare manifestation of Lynch syndrome, although it is considered one of the malignancies in the Lynch syndrome spectrum.6
Alteration of one of the mismatch repair genes also can occur spontaneously in a tumor, resulting in the same high frequency of spontaneous DNA mutations. Overall, between 3% and 5% of metastatic prostate cancers contain mismatch repair deficiency. The majority of these cases are a result of spontaneous loss or mutation of the relevant gene, but 1 in 5 of these tumors occurs as a component of Lynch syndrome.7 Identification of mismatch repair deficiency is critical because the resulting hypermutation makes these tumors particularly susceptible to intervention with immunotherapy. Up to half of patients with metastatic prostate cancer can have durable responses. This finding is consistent with the experience treating other malignancies with mismatch repair deficiency.8 Although screening for mismatch repair deficiency is standard of care for patients with malignancies such as colorectal cancer, few patients with prostate cancer may receive the mismatch repair deficiency screening (based on unpublished data). In contrast, screening is routine for patients with adenocarcinoma of the lung because their proportion of ROS1 and ALK alterations is similar to the frequency of mismatch repair deficiency when compared with patients with prostate cancer.9
Homologous recombination is another mechanism by which cells repair DNA damage and is responsible for repairing double strand breaks, the type of DNA damage most likely to lead to carcinogenesis. In advanced prostate cancer, BRCA2, ATM, BRCA1 and other members of the Fanconi Anemia/BRCA gene family are altered 20% of the time. These genes also are the most common germline alterations implicated in the development of prostate cancer.2,10 Prostate cancer is considered a BRCA-related cancer much like breast, ovarian, and pancreatic cancers. Defects in homologous recombination repair make BRCA-altered prostate cancers susceptible to DNA damaging chemotherapy, such as platinum and to the use of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors because cancer cells then accumulate cytotoxic and apoptotic levels of DNA.11
In May 2020, the FDA approved the use of PARP inhibitors for the treatment of prostate cancers that contain BRCA and other DNA repair alterations. Rucaparib received accelerated approval for the treatment of prostate cancers containing BRCA alterations and olaparib received full approval for treatment of prostate cancers containing an array of alterations in DNA repair genes.12,13 Both approvals were the direct result of the cited landmark studies that demonstrated the frequency of these alterations in advanced prostate cancer.2,3
Beyond mismatch and homologous recombination repair, there are a large number of potentially targetable alterations found in advanced prostate cancer. It is thus critical that we put systems into place both to find germline and somatic alterations that will inform a veteran’s clinical care and to provide veterans access to precision oncology clinical trials.
The POPCaP Network
Because prostate cancer is such a significant issue in the VA and best practices for precision oncology can be implemented broadly once defined as successful, the PCF and the VA formed a collaboration to support a network of centers that would focus on implementing a comprehensive strategy for precision oncology in prostate cancer. There are currently 11 centers in the Precision Oncology Program for Cancer of the Prostate (POPCaP) network (Figure). These centers are tasked with comprehensively sequencing germline and somatic tissue from veterans with metastatic prostate cancer to find alterations, which could provide access to treatments that would otherwise not be available or appropriate.
The network is collaborating with NPOP, which provides clinical grade tumor gene panel sequencing for veterans with prostate cancer from > 90% of VA medical centers. POPCaP also partners with the University of Washington to use its OncoPlex gene panel and University of Michigan to use the Oncomine panel to define the best platform for defining targetable alterations for veterans with prostate cancer. Investigators participate in a monthly molecular oncology tumor board continuing medical education-accredited program, which provides guidance and education across the VA about the evidence available to assist in decision making for veterans sequenced through NPOP and the academic platforms. These efforts leverage VA’s partnership with IBM Watson for Genomics to annotate DNA sequencing results to provide clinicians with potential therapeutic options for veterans.
A clinical trials mechanism is embedded in POPCaP to broaden treatment options, improve care for men with prostate cancer, and leverage the sequencing efforts in the network. The Prostate Cancer Analysis for Therapy Choice (PATCH) clinical trials network employs an umbrella study approach whereby alterations are identified through sequencing and veterans are given access to studies embedded at sites across the network. Graff and Huang provide a detailed description of the PATCH network and its potential as a multisite clinical trials mechanism.14 For studies within the network, funds can be provided to support travel to participate in clinical trials for veterans who would be eligible for study but do not live in a catchment for a network site. POPCaP also leverages both the resources of the National Cancer Institute (NCI)-designated cancer centers that are VA academic affiliates, as well as a VA/NCI partnership (NAVIGATE) to increase veteran access to NCI cutting-edge clinical trials.
The network has regular teleconference meetings of the investigators, coordinators, and stakeholders and face-to-face meetings, which are coordinated around other national meetings. These meetings enable investigators to work collaboratively to advance current knowledge in prostate cancer through the application of complementary and synergistic research approaches. Since research plays a critical role within the learning health care system, POPCaP investigators are working to optimize the transfer of knowledge from the clinic to the bench and back to the clinic. In this regard, investigators from network sites have organized themselves into working groups to focus on multiple critical aspects of research and care within the network, including sequencing, phenotyping, health services, health disparities, and a network biorepository.
VA Office of Research and Development
With support from the VA Office of Research and Development, there are research efforts focused on the development of data analytics to identify veterans with metastatic prostate cancer within the electronic health record to ensure access to appropriate testing, treatment, and clinical trials. This will optimize tracking and continuous quality improvement in precision oncology. The Office of Research and Development also supports the use of artificial intelligence to identify predictive markers for diagnosis, prognosis, therapeutic response and patient stratification. POPCaP investigators, along with other investigators from across the VA, conduct research that continually improves the care of veterans with prostate cancer. POPCaP has a special focus on prostate cancer among African Americans, who are disproportionately affected by the disease and well represented in VA. The efforts of the working groups, the research studies and the network as a whole also serve to recruit both junior and senior investigators to the VA in order to support the VA research enterprise.
Active collaborations between the network and other elements of VA include efforts to optimize germline testing and genetic counseling in prostate cancer through the Genomic Medicine Service, which provides telehealth genetic counseling throughout the VA. POPCaP pilots innovative approaches to increase access to clinical genetics and genetic counseling services to support the volume of genetic testing of veterans with cancer. Current National Comprehensive Cancer Network (NCCN) guidelines recommend germline testing for all men with metastatic prostate cancer, which can efficiently identify the roughly 10% of veterans with metastatic disease who carry a germline alteration and provide them with access to studies, FDA-approved treatments, while also offering critical health care information to family members who may also carry a pathogenic germline alteration.
Million Veteran Program
The Million Veteran Program (MVP) has collected > 825,000 germline DNA samples from an anticipated enrollment of > 1 million veterans in one of the most ambitious genetic research efforts to correlate how germline DNA interacts with lifestyle, medications and military exposure to affect health and illness (www.research.va.gov/mvp). MVP is a racially and ethnically diverse veteran cohort that is roughly 20% African American and 7% Hispanic. More than 40,000 of the participants have had prostate cancer, one third of whom are African Americans, giving researchers unprecedented ability to discover factors that impact the development and treatment of the disease in this population. In particular, MVP will provide unique insights into the genetic mutations that drive the development of aggressive prostate cancer in all male veterans, including African Americans. These discoveries will undoubtedly lead to improved screening of and treatment for prostate cancer.
In order to demonstrate clinical utility as well as the infrastructure needs to scale up within the VHA, MVP has launched a pilot project that offers to return clinically actionable genetic results to MVP participants with metastatic prostate cancer, opening the door to new therapies to improve the length and quality of these veterans’ lives. Importantly, the pilot includes cascade testing in family members of enrolled veterans. Given that the original MVP consent did not allow for return of results, and MVP genetic testing is research grade, veterans who volunteer will provide a second consent and undergo clinical genetic testing to confirm the variants. Results from this pilot study also will inform expansion of VA precision oncology efforts for patients with other cancers such as breast cancer or ovarian cancer, where the specific genetic mutations are known to play a role, (eg, BRCA2). In addition, through an interagency agreement with the US Department of Energy (DOE), MVP is leveraging DOE expertise and high-performance computing capabilities to identify clinical and genetic risk factors for prostate cancer that will progress to metastatic disease.
This active research collaboration between POPCaP, MVP, and the Genomic Medicine Service will identify germline BRCA alterations from MVP participants with metastatic prostate cancer and give them access to therapies that may provide better outcomes and access to genetic testing for their family members.
Future Directions
The POPCaP network and its partnership with VA clinical and research efforts is anticipated to provide important insights into barriers and solutions to the implementation of precision oncology for prostate cancer across the VA. These lessons learned may also be relevant for precision oncology care in other settings. As an example, the role of germline testing and genetic counseling is growing more relevant in precision oncology, yet it is clear that the number of men and women dealing with malignancy who actually receive counseling and testing is suboptimal in most health care systems.14 Optimizing the quality and efficiency of oncogenetics within the VA system in a manner that gives access to these services for every veteran in urban or rural environments is an important goal.
The VA has done extensive work in teleoncology and the Genomic Medicine Service provides telehealth genetic counseling service to 90 VA medical facilities nationwide. Expanding on this model to create a distributed network system across the country is an opportunity that will continue to raise VA profile as a leader in this area while providing increased access to genetic services.
Finally, the clinical trials network within POPCaP already has provided valuable insights into how research efforts that originate within the VA can leverage the VA’s strengths. The use of the NPOP centralized sequencing platform to identify potentially targetable alterations across medical centers provides the potential to bring critical access to research to veterans where they live through virtual clinical trials. The VA has a centralized institutional review board that can service large multisite study participation efficiently across the VA. The promise of virtual clinical trials to interrogate relatively rare biomarkers would benefit from institution of a virtual clinical trials workflow. In theory patients with a potentially targetable biomarker could be identified through the centralized DNA sequencing platform and a clinical trial team of virtual investigators and research coordinators would work with health care providers at sites for study startup and performance. Efforts to design and implement this approach are actively being pursued.
The goal of the VA/PCF POPCaP network is to make certain that every veteran has access to appropriate genetic and genomic testing and that the results are utilized so that veterans with targetable alterations receive the best clinical care and have access to clinical trials that could benefit them individually while advancing knowledge that benefits all.
1. Montgomery B, Williams C. Prostate cancer federal health care data trends. https://www.mdedge.com/fedprac/article/208077/oncology/prostate-cancer-federal-health-care-data-trends. Published September 1, 2019. Accessed July 16, 2020.
2. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
3. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer [published correction appears in Cell. 2018 Oct 18;175(3):889]. Cell. 2018;174(3):758-769.e9. doi:10.1016/j.cell.2018.06.039
4. Pritchard CC, Offit K, Nelson PS. DNA-repair gene mutations in metastatic prostate cancer. N Engl J Med. 2016;375(18):1804-1805. doi:10.1056/NEJMc1611137
5. Guillem JG. Molecular diagnosis of hereditary nonpolyposis colon cancer. N Engl J Med. 1998;339(13):924-925. doi:10.1056/nejm199809243391316
6. Ryan S, Jenkins MA, Win AK. Risk of prostate cancer in Lynch syndrome: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(3):437-449. doi:10.1158/1055-9965.EPI-13-1165
7. Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471-478. doi:10.1001/jamaoncol.2018.5801
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. Published 2020 May 26. doi:10.1371/journal.pone.0233260
9. Yu HA, Planchard D, Lovly CM. Sequencing therapy for genetically defined subgroups of non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2018;38:726-739. doi:10.1200/EDBK_201331
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917-921. doi:10.1038/nature03445
12. Abida W, Campbell D, Patnaik A, et al. Preliminary results from the TRITON2 study of rucaparib in patients with DNA damage repair deficiency metastatic, castration resistant prostate cancer: updated analyses. Ann Oncol. 2019;30(suppl 5): v325-v355. doi:10.1093/annonc/mdz248
13. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
14. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(suppl 4):S62-S67. doi: 10.12788/fp.0028
The US Department of Veterans Affairs (VA) is home to the Veterans Health Administration (VHA), which delivers care at 1,255 health care facilities, including 170 medical centers. The VA serves 6 million veterans each year and is the largest integrated provider of cancer care in the US. The system uses a single, enterprise-wide electronic health record. The detailed curation of clinical outcomes, laboratory results, and radiology is used in VA efforts to improve oncology outcomes for veterans. The VA also has a National Precision Oncology Program (NPOP), which offers system-wide DNA sequencing for veterans with cancer. Given its size, integration, and capabilities, the VA is an ideal setting for rapid learning cycles of testing and implementing best practices at scale.
Prostate cancer is the most common malignancy affecting men in the US. It is the most commonly-diagnosed solid tumor in the VA, and in 2014, there were 11,376 prostate cancer diagnoses in the VA.1 The clinical characteristics and treatment of veterans with prostate cancer largely parallel the broader population of men in the US.1 Although the majority of men diagnosed with prostate cancer have disease localized to the prostate, an important minority develop metastatic disease, which represents a risk for substantial morbidity and is the lethal form of the disease. Research has yielded transformative advances in the care of men with metastatic prostate cancer, including drugs targeting the testosterone/androgen signaling axis, taxane chemotherapy, the radionuclide radium-223, and a dendritic cell vaccine. Unfortunately, the magnitude and duration of response to these therapies varies widely, and determining the biology relevant to an individual patient that would better inform their treatment decisions is a critical next step. As the ability to interrogate the cancer genome has improved, relevant drivers of tumorigenesis and predictive biomarkers are being identified rapidly, and oncology care has evolved from a one-size-fits-all approach to a precision approach, which uses these biomarkers to assist in therapeutic decision making.
Precision Oncology for Prostate Cancer
A series of studies interrogating the genomics of metastatic prostate cancer have been critical to defining the relevance of precision oncology for prostate cancer. Most of what is known about the genomics of prostate cancer has been derived from analysis of samples from the prostate itself. These samples may not reflect the biology of metastasis and genetic evolution in response to treatment pressure, so the genomic alterations in metastatic disease remained incompletely characterized. Two large research teams supported by grants from the American Association for Cancer Research, Stand Up 2 Cancer, and Prostate Cancer Foundation (PCF) focused their efforts on sampling and analyzing metastatic tissue to define the most relevant genomic alterations in advanced prostate cancer.
These efforts defined a broad range of relatively common alterations in the androgen receptor, as well as the tumor suppressors TP53 and PTEN.2,3 Important subsets of less common alterations in pathways that were potentially targetable were also found, including new alterations in PIK3CA/B, BRAF/RAF1, and β-catenin. Most surprisingly, alterations of DNA repair pathways, including mismatch repair and homologous recombination were found in 20% of tumors, and half of these tumors contained germline alterations. The same groups performed a follow up analysis of germline DNA from men with metastatic prostate cancer, which confirmed that 12% of these patients carry a pathogenic germline alteration in a DNA repair pathway gene.4 These efforts immediately invigorated precision oncology clinical trials for prostate cancer and spurred an effort to find the molecular alterations that could be leveraged to improve care for men with advanced prostate cancer.
Targetable Alterations
Currently a number of genomic alterations are immediately actionable. There are several agents approved by the US Food and Drug Administration (FDA) that exploit these Achilles heels of prostate cancer. Mismatch repair deficiency occurs when any of a group of genes responsible for proofreading the fidelity of DNA replication is compromised by mutation or deletion. Imperfect reading and correction subsequently lead to many DNA mutations in a tissue (hypermutation), which then increases the risk of developing malignancy. If a defective gene in the mismatch repair pathway is inherited, a patient has a genetic predisposition to specific malignancies that are part of the Lynch syndrome.5 Prostate cancer is a relatively rare manifestation of Lynch syndrome, although it is considered one of the malignancies in the Lynch syndrome spectrum.6
Alteration of one of the mismatch repair genes also can occur spontaneously in a tumor, resulting in the same high frequency of spontaneous DNA mutations. Overall, between 3% and 5% of metastatic prostate cancers contain mismatch repair deficiency. The majority of these cases are a result of spontaneous loss or mutation of the relevant gene, but 1 in 5 of these tumors occurs as a component of Lynch syndrome.7 Identification of mismatch repair deficiency is critical because the resulting hypermutation makes these tumors particularly susceptible to intervention with immunotherapy. Up to half of patients with metastatic prostate cancer can have durable responses. This finding is consistent with the experience treating other malignancies with mismatch repair deficiency.8 Although screening for mismatch repair deficiency is standard of care for patients with malignancies such as colorectal cancer, few patients with prostate cancer may receive the mismatch repair deficiency screening (based on unpublished data). In contrast, screening is routine for patients with adenocarcinoma of the lung because their proportion of ROS1 and ALK alterations is similar to the frequency of mismatch repair deficiency when compared with patients with prostate cancer.9
Homologous recombination is another mechanism by which cells repair DNA damage and is responsible for repairing double strand breaks, the type of DNA damage most likely to lead to carcinogenesis. In advanced prostate cancer, BRCA2, ATM, BRCA1 and other members of the Fanconi Anemia/BRCA gene family are altered 20% of the time. These genes also are the most common germline alterations implicated in the development of prostate cancer.2,10 Prostate cancer is considered a BRCA-related cancer much like breast, ovarian, and pancreatic cancers. Defects in homologous recombination repair make BRCA-altered prostate cancers susceptible to DNA damaging chemotherapy, such as platinum and to the use of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors because cancer cells then accumulate cytotoxic and apoptotic levels of DNA.11
In May 2020, the FDA approved the use of PARP inhibitors for the treatment of prostate cancers that contain BRCA and other DNA repair alterations. Rucaparib received accelerated approval for the treatment of prostate cancers containing BRCA alterations and olaparib received full approval for treatment of prostate cancers containing an array of alterations in DNA repair genes.12,13 Both approvals were the direct result of the cited landmark studies that demonstrated the frequency of these alterations in advanced prostate cancer.2,3
Beyond mismatch and homologous recombination repair, there are a large number of potentially targetable alterations found in advanced prostate cancer. It is thus critical that we put systems into place both to find germline and somatic alterations that will inform a veteran’s clinical care and to provide veterans access to precision oncology clinical trials.
The POPCaP Network
Because prostate cancer is such a significant issue in the VA and best practices for precision oncology can be implemented broadly once defined as successful, the PCF and the VA formed a collaboration to support a network of centers that would focus on implementing a comprehensive strategy for precision oncology in prostate cancer. There are currently 11 centers in the Precision Oncology Program for Cancer of the Prostate (POPCaP) network (Figure). These centers are tasked with comprehensively sequencing germline and somatic tissue from veterans with metastatic prostate cancer to find alterations, which could provide access to treatments that would otherwise not be available or appropriate.
The network is collaborating with NPOP, which provides clinical grade tumor gene panel sequencing for veterans with prostate cancer from > 90% of VA medical centers. POPCaP also partners with the University of Washington to use its OncoPlex gene panel and University of Michigan to use the Oncomine panel to define the best platform for defining targetable alterations for veterans with prostate cancer. Investigators participate in a monthly molecular oncology tumor board continuing medical education-accredited program, which provides guidance and education across the VA about the evidence available to assist in decision making for veterans sequenced through NPOP and the academic platforms. These efforts leverage VA’s partnership with IBM Watson for Genomics to annotate DNA sequencing results to provide clinicians with potential therapeutic options for veterans.
A clinical trials mechanism is embedded in POPCaP to broaden treatment options, improve care for men with prostate cancer, and leverage the sequencing efforts in the network. The Prostate Cancer Analysis for Therapy Choice (PATCH) clinical trials network employs an umbrella study approach whereby alterations are identified through sequencing and veterans are given access to studies embedded at sites across the network. Graff and Huang provide a detailed description of the PATCH network and its potential as a multisite clinical trials mechanism.14 For studies within the network, funds can be provided to support travel to participate in clinical trials for veterans who would be eligible for study but do not live in a catchment for a network site. POPCaP also leverages both the resources of the National Cancer Institute (NCI)-designated cancer centers that are VA academic affiliates, as well as a VA/NCI partnership (NAVIGATE) to increase veteran access to NCI cutting-edge clinical trials.
The network has regular teleconference meetings of the investigators, coordinators, and stakeholders and face-to-face meetings, which are coordinated around other national meetings. These meetings enable investigators to work collaboratively to advance current knowledge in prostate cancer through the application of complementary and synergistic research approaches. Since research plays a critical role within the learning health care system, POPCaP investigators are working to optimize the transfer of knowledge from the clinic to the bench and back to the clinic. In this regard, investigators from network sites have organized themselves into working groups to focus on multiple critical aspects of research and care within the network, including sequencing, phenotyping, health services, health disparities, and a network biorepository.
VA Office of Research and Development
With support from the VA Office of Research and Development, there are research efforts focused on the development of data analytics to identify veterans with metastatic prostate cancer within the electronic health record to ensure access to appropriate testing, treatment, and clinical trials. This will optimize tracking and continuous quality improvement in precision oncology. The Office of Research and Development also supports the use of artificial intelligence to identify predictive markers for diagnosis, prognosis, therapeutic response and patient stratification. POPCaP investigators, along with other investigators from across the VA, conduct research that continually improves the care of veterans with prostate cancer. POPCaP has a special focus on prostate cancer among African Americans, who are disproportionately affected by the disease and well represented in VA. The efforts of the working groups, the research studies and the network as a whole also serve to recruit both junior and senior investigators to the VA in order to support the VA research enterprise.
Active collaborations between the network and other elements of VA include efforts to optimize germline testing and genetic counseling in prostate cancer through the Genomic Medicine Service, which provides telehealth genetic counseling throughout the VA. POPCaP pilots innovative approaches to increase access to clinical genetics and genetic counseling services to support the volume of genetic testing of veterans with cancer. Current National Comprehensive Cancer Network (NCCN) guidelines recommend germline testing for all men with metastatic prostate cancer, which can efficiently identify the roughly 10% of veterans with metastatic disease who carry a germline alteration and provide them with access to studies, FDA-approved treatments, while also offering critical health care information to family members who may also carry a pathogenic germline alteration.
Million Veteran Program
The Million Veteran Program (MVP) has collected > 825,000 germline DNA samples from an anticipated enrollment of > 1 million veterans in one of the most ambitious genetic research efforts to correlate how germline DNA interacts with lifestyle, medications and military exposure to affect health and illness (www.research.va.gov/mvp). MVP is a racially and ethnically diverse veteran cohort that is roughly 20% African American and 7% Hispanic. More than 40,000 of the participants have had prostate cancer, one third of whom are African Americans, giving researchers unprecedented ability to discover factors that impact the development and treatment of the disease in this population. In particular, MVP will provide unique insights into the genetic mutations that drive the development of aggressive prostate cancer in all male veterans, including African Americans. These discoveries will undoubtedly lead to improved screening of and treatment for prostate cancer.
In order to demonstrate clinical utility as well as the infrastructure needs to scale up within the VHA, MVP has launched a pilot project that offers to return clinically actionable genetic results to MVP participants with metastatic prostate cancer, opening the door to new therapies to improve the length and quality of these veterans’ lives. Importantly, the pilot includes cascade testing in family members of enrolled veterans. Given that the original MVP consent did not allow for return of results, and MVP genetic testing is research grade, veterans who volunteer will provide a second consent and undergo clinical genetic testing to confirm the variants. Results from this pilot study also will inform expansion of VA precision oncology efforts for patients with other cancers such as breast cancer or ovarian cancer, where the specific genetic mutations are known to play a role, (eg, BRCA2). In addition, through an interagency agreement with the US Department of Energy (DOE), MVP is leveraging DOE expertise and high-performance computing capabilities to identify clinical and genetic risk factors for prostate cancer that will progress to metastatic disease.
This active research collaboration between POPCaP, MVP, and the Genomic Medicine Service will identify germline BRCA alterations from MVP participants with metastatic prostate cancer and give them access to therapies that may provide better outcomes and access to genetic testing for their family members.
Future Directions
The POPCaP network and its partnership with VA clinical and research efforts is anticipated to provide important insights into barriers and solutions to the implementation of precision oncology for prostate cancer across the VA. These lessons learned may also be relevant for precision oncology care in other settings. As an example, the role of germline testing and genetic counseling is growing more relevant in precision oncology, yet it is clear that the number of men and women dealing with malignancy who actually receive counseling and testing is suboptimal in most health care systems.14 Optimizing the quality and efficiency of oncogenetics within the VA system in a manner that gives access to these services for every veteran in urban or rural environments is an important goal.
The VA has done extensive work in teleoncology and the Genomic Medicine Service provides telehealth genetic counseling service to 90 VA medical facilities nationwide. Expanding on this model to create a distributed network system across the country is an opportunity that will continue to raise VA profile as a leader in this area while providing increased access to genetic services.
Finally, the clinical trials network within POPCaP already has provided valuable insights into how research efforts that originate within the VA can leverage the VA’s strengths. The use of the NPOP centralized sequencing platform to identify potentially targetable alterations across medical centers provides the potential to bring critical access to research to veterans where they live through virtual clinical trials. The VA has a centralized institutional review board that can service large multisite study participation efficiently across the VA. The promise of virtual clinical trials to interrogate relatively rare biomarkers would benefit from institution of a virtual clinical trials workflow. In theory patients with a potentially targetable biomarker could be identified through the centralized DNA sequencing platform and a clinical trial team of virtual investigators and research coordinators would work with health care providers at sites for study startup and performance. Efforts to design and implement this approach are actively being pursued.
The goal of the VA/PCF POPCaP network is to make certain that every veteran has access to appropriate genetic and genomic testing and that the results are utilized so that veterans with targetable alterations receive the best clinical care and have access to clinical trials that could benefit them individually while advancing knowledge that benefits all.
The US Department of Veterans Affairs (VA) is home to the Veterans Health Administration (VHA), which delivers care at 1,255 health care facilities, including 170 medical centers. The VA serves 6 million veterans each year and is the largest integrated provider of cancer care in the US. The system uses a single, enterprise-wide electronic health record. The detailed curation of clinical outcomes, laboratory results, and radiology is used in VA efforts to improve oncology outcomes for veterans. The VA also has a National Precision Oncology Program (NPOP), which offers system-wide DNA sequencing for veterans with cancer. Given its size, integration, and capabilities, the VA is an ideal setting for rapid learning cycles of testing and implementing best practices at scale.
Prostate cancer is the most common malignancy affecting men in the US. It is the most commonly-diagnosed solid tumor in the VA, and in 2014, there were 11,376 prostate cancer diagnoses in the VA.1 The clinical characteristics and treatment of veterans with prostate cancer largely parallel the broader population of men in the US.1 Although the majority of men diagnosed with prostate cancer have disease localized to the prostate, an important minority develop metastatic disease, which represents a risk for substantial morbidity and is the lethal form of the disease. Research has yielded transformative advances in the care of men with metastatic prostate cancer, including drugs targeting the testosterone/androgen signaling axis, taxane chemotherapy, the radionuclide radium-223, and a dendritic cell vaccine. Unfortunately, the magnitude and duration of response to these therapies varies widely, and determining the biology relevant to an individual patient that would better inform their treatment decisions is a critical next step. As the ability to interrogate the cancer genome has improved, relevant drivers of tumorigenesis and predictive biomarkers are being identified rapidly, and oncology care has evolved from a one-size-fits-all approach to a precision approach, which uses these biomarkers to assist in therapeutic decision making.
Precision Oncology for Prostate Cancer
A series of studies interrogating the genomics of metastatic prostate cancer have been critical to defining the relevance of precision oncology for prostate cancer. Most of what is known about the genomics of prostate cancer has been derived from analysis of samples from the prostate itself. These samples may not reflect the biology of metastasis and genetic evolution in response to treatment pressure, so the genomic alterations in metastatic disease remained incompletely characterized. Two large research teams supported by grants from the American Association for Cancer Research, Stand Up 2 Cancer, and Prostate Cancer Foundation (PCF) focused their efforts on sampling and analyzing metastatic tissue to define the most relevant genomic alterations in advanced prostate cancer.
These efforts defined a broad range of relatively common alterations in the androgen receptor, as well as the tumor suppressors TP53 and PTEN.2,3 Important subsets of less common alterations in pathways that were potentially targetable were also found, including new alterations in PIK3CA/B, BRAF/RAF1, and β-catenin. Most surprisingly, alterations of DNA repair pathways, including mismatch repair and homologous recombination were found in 20% of tumors, and half of these tumors contained germline alterations. The same groups performed a follow up analysis of germline DNA from men with metastatic prostate cancer, which confirmed that 12% of these patients carry a pathogenic germline alteration in a DNA repair pathway gene.4 These efforts immediately invigorated precision oncology clinical trials for prostate cancer and spurred an effort to find the molecular alterations that could be leveraged to improve care for men with advanced prostate cancer.
Targetable Alterations
Currently a number of genomic alterations are immediately actionable. There are several agents approved by the US Food and Drug Administration (FDA) that exploit these Achilles heels of prostate cancer. Mismatch repair deficiency occurs when any of a group of genes responsible for proofreading the fidelity of DNA replication is compromised by mutation or deletion. Imperfect reading and correction subsequently lead to many DNA mutations in a tissue (hypermutation), which then increases the risk of developing malignancy. If a defective gene in the mismatch repair pathway is inherited, a patient has a genetic predisposition to specific malignancies that are part of the Lynch syndrome.5 Prostate cancer is a relatively rare manifestation of Lynch syndrome, although it is considered one of the malignancies in the Lynch syndrome spectrum.6
Alteration of one of the mismatch repair genes also can occur spontaneously in a tumor, resulting in the same high frequency of spontaneous DNA mutations. Overall, between 3% and 5% of metastatic prostate cancers contain mismatch repair deficiency. The majority of these cases are a result of spontaneous loss or mutation of the relevant gene, but 1 in 5 of these tumors occurs as a component of Lynch syndrome.7 Identification of mismatch repair deficiency is critical because the resulting hypermutation makes these tumors particularly susceptible to intervention with immunotherapy. Up to half of patients with metastatic prostate cancer can have durable responses. This finding is consistent with the experience treating other malignancies with mismatch repair deficiency.8 Although screening for mismatch repair deficiency is standard of care for patients with malignancies such as colorectal cancer, few patients with prostate cancer may receive the mismatch repair deficiency screening (based on unpublished data). In contrast, screening is routine for patients with adenocarcinoma of the lung because their proportion of ROS1 and ALK alterations is similar to the frequency of mismatch repair deficiency when compared with patients with prostate cancer.9
Homologous recombination is another mechanism by which cells repair DNA damage and is responsible for repairing double strand breaks, the type of DNA damage most likely to lead to carcinogenesis. In advanced prostate cancer, BRCA2, ATM, BRCA1 and other members of the Fanconi Anemia/BRCA gene family are altered 20% of the time. These genes also are the most common germline alterations implicated in the development of prostate cancer.2,10 Prostate cancer is considered a BRCA-related cancer much like breast, ovarian, and pancreatic cancers. Defects in homologous recombination repair make BRCA-altered prostate cancers susceptible to DNA damaging chemotherapy, such as platinum and to the use of poly–(adenosine diphosphate–ribose) polymerase (PARP) inhibitors because cancer cells then accumulate cytotoxic and apoptotic levels of DNA.11
In May 2020, the FDA approved the use of PARP inhibitors for the treatment of prostate cancers that contain BRCA and other DNA repair alterations. Rucaparib received accelerated approval for the treatment of prostate cancers containing BRCA alterations and olaparib received full approval for treatment of prostate cancers containing an array of alterations in DNA repair genes.12,13 Both approvals were the direct result of the cited landmark studies that demonstrated the frequency of these alterations in advanced prostate cancer.2,3
Beyond mismatch and homologous recombination repair, there are a large number of potentially targetable alterations found in advanced prostate cancer. It is thus critical that we put systems into place both to find germline and somatic alterations that will inform a veteran’s clinical care and to provide veterans access to precision oncology clinical trials.
The POPCaP Network
Because prostate cancer is such a significant issue in the VA and best practices for precision oncology can be implemented broadly once defined as successful, the PCF and the VA formed a collaboration to support a network of centers that would focus on implementing a comprehensive strategy for precision oncology in prostate cancer. There are currently 11 centers in the Precision Oncology Program for Cancer of the Prostate (POPCaP) network (Figure). These centers are tasked with comprehensively sequencing germline and somatic tissue from veterans with metastatic prostate cancer to find alterations, which could provide access to treatments that would otherwise not be available or appropriate.
The network is collaborating with NPOP, which provides clinical grade tumor gene panel sequencing for veterans with prostate cancer from > 90% of VA medical centers. POPCaP also partners with the University of Washington to use its OncoPlex gene panel and University of Michigan to use the Oncomine panel to define the best platform for defining targetable alterations for veterans with prostate cancer. Investigators participate in a monthly molecular oncology tumor board continuing medical education-accredited program, which provides guidance and education across the VA about the evidence available to assist in decision making for veterans sequenced through NPOP and the academic platforms. These efforts leverage VA’s partnership with IBM Watson for Genomics to annotate DNA sequencing results to provide clinicians with potential therapeutic options for veterans.
A clinical trials mechanism is embedded in POPCaP to broaden treatment options, improve care for men with prostate cancer, and leverage the sequencing efforts in the network. The Prostate Cancer Analysis for Therapy Choice (PATCH) clinical trials network employs an umbrella study approach whereby alterations are identified through sequencing and veterans are given access to studies embedded at sites across the network. Graff and Huang provide a detailed description of the PATCH network and its potential as a multisite clinical trials mechanism.14 For studies within the network, funds can be provided to support travel to participate in clinical trials for veterans who would be eligible for study but do not live in a catchment for a network site. POPCaP also leverages both the resources of the National Cancer Institute (NCI)-designated cancer centers that are VA academic affiliates, as well as a VA/NCI partnership (NAVIGATE) to increase veteran access to NCI cutting-edge clinical trials.
The network has regular teleconference meetings of the investigators, coordinators, and stakeholders and face-to-face meetings, which are coordinated around other national meetings. These meetings enable investigators to work collaboratively to advance current knowledge in prostate cancer through the application of complementary and synergistic research approaches. Since research plays a critical role within the learning health care system, POPCaP investigators are working to optimize the transfer of knowledge from the clinic to the bench and back to the clinic. In this regard, investigators from network sites have organized themselves into working groups to focus on multiple critical aspects of research and care within the network, including sequencing, phenotyping, health services, health disparities, and a network biorepository.
VA Office of Research and Development
With support from the VA Office of Research and Development, there are research efforts focused on the development of data analytics to identify veterans with metastatic prostate cancer within the electronic health record to ensure access to appropriate testing, treatment, and clinical trials. This will optimize tracking and continuous quality improvement in precision oncology. The Office of Research and Development also supports the use of artificial intelligence to identify predictive markers for diagnosis, prognosis, therapeutic response and patient stratification. POPCaP investigators, along with other investigators from across the VA, conduct research that continually improves the care of veterans with prostate cancer. POPCaP has a special focus on prostate cancer among African Americans, who are disproportionately affected by the disease and well represented in VA. The efforts of the working groups, the research studies and the network as a whole also serve to recruit both junior and senior investigators to the VA in order to support the VA research enterprise.
Active collaborations between the network and other elements of VA include efforts to optimize germline testing and genetic counseling in prostate cancer through the Genomic Medicine Service, which provides telehealth genetic counseling throughout the VA. POPCaP pilots innovative approaches to increase access to clinical genetics and genetic counseling services to support the volume of genetic testing of veterans with cancer. Current National Comprehensive Cancer Network (NCCN) guidelines recommend germline testing for all men with metastatic prostate cancer, which can efficiently identify the roughly 10% of veterans with metastatic disease who carry a germline alteration and provide them with access to studies, FDA-approved treatments, while also offering critical health care information to family members who may also carry a pathogenic germline alteration.
Million Veteran Program
The Million Veteran Program (MVP) has collected > 825,000 germline DNA samples from an anticipated enrollment of > 1 million veterans in one of the most ambitious genetic research efforts to correlate how germline DNA interacts with lifestyle, medications and military exposure to affect health and illness (www.research.va.gov/mvp). MVP is a racially and ethnically diverse veteran cohort that is roughly 20% African American and 7% Hispanic. More than 40,000 of the participants have had prostate cancer, one third of whom are African Americans, giving researchers unprecedented ability to discover factors that impact the development and treatment of the disease in this population. In particular, MVP will provide unique insights into the genetic mutations that drive the development of aggressive prostate cancer in all male veterans, including African Americans. These discoveries will undoubtedly lead to improved screening of and treatment for prostate cancer.
In order to demonstrate clinical utility as well as the infrastructure needs to scale up within the VHA, MVP has launched a pilot project that offers to return clinically actionable genetic results to MVP participants with metastatic prostate cancer, opening the door to new therapies to improve the length and quality of these veterans’ lives. Importantly, the pilot includes cascade testing in family members of enrolled veterans. Given that the original MVP consent did not allow for return of results, and MVP genetic testing is research grade, veterans who volunteer will provide a second consent and undergo clinical genetic testing to confirm the variants. Results from this pilot study also will inform expansion of VA precision oncology efforts for patients with other cancers such as breast cancer or ovarian cancer, where the specific genetic mutations are known to play a role, (eg, BRCA2). In addition, through an interagency agreement with the US Department of Energy (DOE), MVP is leveraging DOE expertise and high-performance computing capabilities to identify clinical and genetic risk factors for prostate cancer that will progress to metastatic disease.
This active research collaboration between POPCaP, MVP, and the Genomic Medicine Service will identify germline BRCA alterations from MVP participants with metastatic prostate cancer and give them access to therapies that may provide better outcomes and access to genetic testing for their family members.
Future Directions
The POPCaP network and its partnership with VA clinical and research efforts is anticipated to provide important insights into barriers and solutions to the implementation of precision oncology for prostate cancer across the VA. These lessons learned may also be relevant for precision oncology care in other settings. As an example, the role of germline testing and genetic counseling is growing more relevant in precision oncology, yet it is clear that the number of men and women dealing with malignancy who actually receive counseling and testing is suboptimal in most health care systems.14 Optimizing the quality and efficiency of oncogenetics within the VA system in a manner that gives access to these services for every veteran in urban or rural environments is an important goal.
The VA has done extensive work in teleoncology and the Genomic Medicine Service provides telehealth genetic counseling service to 90 VA medical facilities nationwide. Expanding on this model to create a distributed network system across the country is an opportunity that will continue to raise VA profile as a leader in this area while providing increased access to genetic services.
Finally, the clinical trials network within POPCaP already has provided valuable insights into how research efforts that originate within the VA can leverage the VA’s strengths. The use of the NPOP centralized sequencing platform to identify potentially targetable alterations across medical centers provides the potential to bring critical access to research to veterans where they live through virtual clinical trials. The VA has a centralized institutional review board that can service large multisite study participation efficiently across the VA. The promise of virtual clinical trials to interrogate relatively rare biomarkers would benefit from institution of a virtual clinical trials workflow. In theory patients with a potentially targetable biomarker could be identified through the centralized DNA sequencing platform and a clinical trial team of virtual investigators and research coordinators would work with health care providers at sites for study startup and performance. Efforts to design and implement this approach are actively being pursued.
The goal of the VA/PCF POPCaP network is to make certain that every veteran has access to appropriate genetic and genomic testing and that the results are utilized so that veterans with targetable alterations receive the best clinical care and have access to clinical trials that could benefit them individually while advancing knowledge that benefits all.
1. Montgomery B, Williams C. Prostate cancer federal health care data trends. https://www.mdedge.com/fedprac/article/208077/oncology/prostate-cancer-federal-health-care-data-trends. Published September 1, 2019. Accessed July 16, 2020.
2. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
3. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer [published correction appears in Cell. 2018 Oct 18;175(3):889]. Cell. 2018;174(3):758-769.e9. doi:10.1016/j.cell.2018.06.039
4. Pritchard CC, Offit K, Nelson PS. DNA-repair gene mutations in metastatic prostate cancer. N Engl J Med. 2016;375(18):1804-1805. doi:10.1056/NEJMc1611137
5. Guillem JG. Molecular diagnosis of hereditary nonpolyposis colon cancer. N Engl J Med. 1998;339(13):924-925. doi:10.1056/nejm199809243391316
6. Ryan S, Jenkins MA, Win AK. Risk of prostate cancer in Lynch syndrome: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(3):437-449. doi:10.1158/1055-9965.EPI-13-1165
7. Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471-478. doi:10.1001/jamaoncol.2018.5801
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. Published 2020 May 26. doi:10.1371/journal.pone.0233260
9. Yu HA, Planchard D, Lovly CM. Sequencing therapy for genetically defined subgroups of non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2018;38:726-739. doi:10.1200/EDBK_201331
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917-921. doi:10.1038/nature03445
12. Abida W, Campbell D, Patnaik A, et al. Preliminary results from the TRITON2 study of rucaparib in patients with DNA damage repair deficiency metastatic, castration resistant prostate cancer: updated analyses. Ann Oncol. 2019;30(suppl 5): v325-v355. doi:10.1093/annonc/mdz248
13. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
14. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(suppl 4):S62-S67. doi: 10.12788/fp.0028
1. Montgomery B, Williams C. Prostate cancer federal health care data trends. https://www.mdedge.com/fedprac/article/208077/oncology/prostate-cancer-federal-health-care-data-trends. Published September 1, 2019. Accessed July 16, 2020.
2. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015 Jul 16;162(2):454]. Cell. 2015;161(5):1215-1228. doi:10.1016/j.cell.2015.05.001
3. Quigley DA, Dang HX, Zhao SG, et al. Genomic hallmarks and structural variation in metastatic prostate cancer [published correction appears in Cell. 2018 Oct 18;175(3):889]. Cell. 2018;174(3):758-769.e9. doi:10.1016/j.cell.2018.06.039
4. Pritchard CC, Offit K, Nelson PS. DNA-repair gene mutations in metastatic prostate cancer. N Engl J Med. 2016;375(18):1804-1805. doi:10.1056/NEJMc1611137
5. Guillem JG. Molecular diagnosis of hereditary nonpolyposis colon cancer. N Engl J Med. 1998;339(13):924-925. doi:10.1056/nejm199809243391316
6. Ryan S, Jenkins MA, Win AK. Risk of prostate cancer in Lynch syndrome: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev. 2014;23(3):437-449. doi:10.1158/1055-9965.EPI-13-1165
7. Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471-478. doi:10.1001/jamaoncol.2018.5801
8. Graham LS, Montgomery B, Cheng HH, et al. Mismatch repair deficiency in metastatic prostate cancer: Response to PD-1 blockade and standard therapies. PLoS One. 2020;15(5):e0233260. Published 2020 May 26. doi:10.1371/journal.pone.0233260
9. Yu HA, Planchard D, Lovly CM. Sequencing therapy for genetically defined subgroups of non-small cell lung cancer. Am Soc Clin Oncol Educ Book. 2018;38:726-739. doi:10.1200/EDBK_201331
10. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453. doi:10.1056/NEJMoa1603144
11. Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917-921. doi:10.1038/nature03445
12. Abida W, Campbell D, Patnaik A, et al. Preliminary results from the TRITON2 study of rucaparib in patients with DNA damage repair deficiency metastatic, castration resistant prostate cancer: updated analyses. Ann Oncol. 2019;30(suppl 5): v325-v355. doi:10.1093/annonc/mdz248
13. de Bono J, Mateo J, Fizazi K, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med. 2020;382(22):2091-2102. doi:10.1056/NEJMoa1911440
14. Graff JN, Huang GD. Leveraging Veterans Health Administration clinical and research resources to accelerate discovery and testing in precision oncology. Fed Pract. 2020;37(suppl 4):S62-S67. doi: 10.12788/fp.0028