User login
Novel oral inhibitor may block intestinal damage in celiac disease
A novel oral inhibitor of transglutaminase 2 appears to block gluten-induced mucosal damage in patients with celiac disease at three different doses, based on proof-of-concept trial data from 132 patients.
“Currently, no drug therapy reliably prevents the effects of dietary gluten or has been approved by regulators to treat celiac disease,” which remains an unmet need in these patients, many of whom struggle with symptoms even when they adhere to a gluten-free diet, wrote Detlef Schuppan, MD, of Johannes Gutenberg University of Mainz (Germany) and colleagues.
Celiac disease is driven in part by the enzyme transglutaminase 2, and a transglutaminase 2 inhibitor known as ZED1227 has been tested safely in phase 1 trials, they reported.
“ZED1227 targets the intestinal mucosa predominantly and thereby mediates protection; thus, it is unaffected by the complexity of the food matrix and is less dependent on the timing of ingestion of gluten-containing food,” the researchers explained.
In a study published in the New England Journal of Medicine, the researchers assessed the safety and efficacy of three dose levels of ZED1227. Adults with controlled celiac disease were randomized to doses of 10 mg (41 patients), 50 mg (41 patients), and 100 mg (41 patients), and 40 patients received a placebo. Of these, 35, 39, 28, and 30 patients, respectively, had sufficient duodenal biopsy samples for analysis.
Patients underwent a daily gluten challenge of 3 g for 6 weeks. At the end of 6 weeks, the primary study endpoint of attenuation of gluten-induced mucosal damage was measured by the ratio of villus height to crypt depth.
Patients in all three treatment groups showed significant attenuation of mucosal damage. The change in the average ratio of villus height to crypt depth compared to placebo in the 10-mg, 50-mg, and 100-mg groups was 0.44, 0.49, and 0.48, respectively, with P values equal to .001 in the 10-mg group and less than .001 in the 50-mg and 100-mg groups.
Adverse events were similar across all treatment groups and the placebo group, with the exception of a rash in three patients in the 100-mg group. A total of 74 patients reported adverse events, and the most common were headache, nausea, diarrhea, vomiting, and abdominal pain. The investigators determined that from 34% to 55% of the adverse events across groups were related to the study drug or placebo.
Two patients developed serious adverse events that were deemed related to the study drug or placebo; one patient in the 50-mg group developed migraine with aura, and one placebo patient developed ventricular extrasystoles. The patients recovered after discontinuing the drug or placebo.
Secondary endpoints included intraepithelial lymphocyte density, the Celiac Symptom Index score, and the Celiac Disease Questionnaire score. Estimated changes in intraepithelial lymphocyte density, compared with placebo, were –2.7 cells per 100 epithelial cells in the 10-mg group, −4.2 cells per 100 epithelial cells in the 50-mg group, and −9.6 cells per 100 epithelial cells in the 100-mg group. Compared with those of patients taking placebo, the 6-week changes in Celiac Symptom Index scores and Celiac Disease Questionnaire scores suggested slight improvements in symptoms and quality of life for the 100-mg dose.
The study findings were limited by several factors including missing data and loss of several patients to follow-up, as well as the short trial duration and use of controlled gluten ingestion, the researchers noted. Larger studies involving real-world conditions of minor gluten ingestion are needed to support the preliminary signs of safety and efficacy, they said.
Study strengths include high levels of patient adherence to the treatment and the gluten challenge, they said. “Future studies of ZED1227 in more patients are needed to provide additional evidence of the safety and efficacy of the drug, potentially in real-life conditions with minor gluten ingestion,” they concluded.
Translating potential into practice
“An absence of mucosal damage is a critical criterion to ensure the long-term health of a patient, and this clinical trial in celiac disease meets this important endpoint,” Bana Jabri, MD, of the University of Chicago, wrote in an accompanying editorial.
The primary endpoint of no mucosal damage is “especially notable because it was achieved under a controlled gluten challenge, albeit with a relatively moderate amount of gluten (a regular diet contains 12 g of gluten daily, whereas the challenge involved 3 g daily) and for a short period of time,” Dr. Jabri said. The reduction of disease-associated symptoms and apparent improvement in quality of life with 100-mg dose added value to the findings, she said.
Future research areas include whether cross-reactive T cells, which were not analyzed in the current study, might “expand and become pathogenic after a long-term gluten challenge,” Dr. Jabri noted.
However, “ZED1227 is the first nondietary treatment that has preliminarily shown the capacity to prevent mucosal damage in persons with celiac disease,” she said.
“Although this trial is very encouraging, whether treatment with ZED1227, and more generally transglutaminase 2 inhibition, in patients with celiac disease will be efficient in real life and during long-term gluten exposure remains to be determined,” Dr. Jabri concluded.
Need for data on dosing consistency
“Celiac disease affects up to 2% of the population in many countries, and the main therapy of celiac disease is avoidance of gluten,” Kim Isaacs, MD, of the University of North Carolina, Chapel Hill, said in an interview. “This is challenging due to the ubiquitous nature of gluten in many food products,” she said. “Restrictive eating also affects social interaction which is often focused around food,” she added. “Availability of an oral therapy that is effective to treat celiac in the face of gluten exposure will have a profound impact on patients in terms of liberalization of dietary intake.”
Overall, “the changes in the villus height to crypt depth was similar between all the active treatment groups, whereas there was a dose-dependent reduction in transepithelial lymphocyte density,” Dr. Isaacs noted. “The symptom improvement was greatest in the 100-mg group, suggesting that symptoms may be related to a greater extent to the lymphocyte density than the minimal differences in villus height to crypt depth ratios seen in the active treatment groups,” she said.
Potential barriers to the use of the treatment include cost because “this will need to be a daily long-term therapy,” said Dr. Isaacs. “Compliance is a potential barrier as well,” she said. “This study looks at daily administration of the transglutaminase 2 inhibitor and shows a benefit, but it is not clear whether missing doses of the medication will have a prolonged impact on efficacy,” she emphasized. Consequently, long-term efficacy studies are needed, Dr. Isaacs said. Other research questions to answer include whether patients will become refractory to the beneficial effects over time, the effect of missing doses, and whether patients would lose all the benefits of the therapy if dosing is not consistent, she emphasized.
The study was funded by Dr. Falk Pharma. The researchers, as well as Dr. Jabri and Dr. Isaacs, had no financial conflicts to disclose. Dr. Isaacs is on the editorial advisory board of GI & Hepatology News.
A novel oral inhibitor of transglutaminase 2 appears to block gluten-induced mucosal damage in patients with celiac disease at three different doses, based on proof-of-concept trial data from 132 patients.
“Currently, no drug therapy reliably prevents the effects of dietary gluten or has been approved by regulators to treat celiac disease,” which remains an unmet need in these patients, many of whom struggle with symptoms even when they adhere to a gluten-free diet, wrote Detlef Schuppan, MD, of Johannes Gutenberg University of Mainz (Germany) and colleagues.
Celiac disease is driven in part by the enzyme transglutaminase 2, and a transglutaminase 2 inhibitor known as ZED1227 has been tested safely in phase 1 trials, they reported.
“ZED1227 targets the intestinal mucosa predominantly and thereby mediates protection; thus, it is unaffected by the complexity of the food matrix and is less dependent on the timing of ingestion of gluten-containing food,” the researchers explained.
In a study published in the New England Journal of Medicine, the researchers assessed the safety and efficacy of three dose levels of ZED1227. Adults with controlled celiac disease were randomized to doses of 10 mg (41 patients), 50 mg (41 patients), and 100 mg (41 patients), and 40 patients received a placebo. Of these, 35, 39, 28, and 30 patients, respectively, had sufficient duodenal biopsy samples for analysis.
Patients underwent a daily gluten challenge of 3 g for 6 weeks. At the end of 6 weeks, the primary study endpoint of attenuation of gluten-induced mucosal damage was measured by the ratio of villus height to crypt depth.
Patients in all three treatment groups showed significant attenuation of mucosal damage. The change in the average ratio of villus height to crypt depth compared to placebo in the 10-mg, 50-mg, and 100-mg groups was 0.44, 0.49, and 0.48, respectively, with P values equal to .001 in the 10-mg group and less than .001 in the 50-mg and 100-mg groups.
Adverse events were similar across all treatment groups and the placebo group, with the exception of a rash in three patients in the 100-mg group. A total of 74 patients reported adverse events, and the most common were headache, nausea, diarrhea, vomiting, and abdominal pain. The investigators determined that from 34% to 55% of the adverse events across groups were related to the study drug or placebo.
Two patients developed serious adverse events that were deemed related to the study drug or placebo; one patient in the 50-mg group developed migraine with aura, and one placebo patient developed ventricular extrasystoles. The patients recovered after discontinuing the drug or placebo.
Secondary endpoints included intraepithelial lymphocyte density, the Celiac Symptom Index score, and the Celiac Disease Questionnaire score. Estimated changes in intraepithelial lymphocyte density, compared with placebo, were –2.7 cells per 100 epithelial cells in the 10-mg group, −4.2 cells per 100 epithelial cells in the 50-mg group, and −9.6 cells per 100 epithelial cells in the 100-mg group. Compared with those of patients taking placebo, the 6-week changes in Celiac Symptom Index scores and Celiac Disease Questionnaire scores suggested slight improvements in symptoms and quality of life for the 100-mg dose.
The study findings were limited by several factors including missing data and loss of several patients to follow-up, as well as the short trial duration and use of controlled gluten ingestion, the researchers noted. Larger studies involving real-world conditions of minor gluten ingestion are needed to support the preliminary signs of safety and efficacy, they said.
Study strengths include high levels of patient adherence to the treatment and the gluten challenge, they said. “Future studies of ZED1227 in more patients are needed to provide additional evidence of the safety and efficacy of the drug, potentially in real-life conditions with minor gluten ingestion,” they concluded.
Translating potential into practice
“An absence of mucosal damage is a critical criterion to ensure the long-term health of a patient, and this clinical trial in celiac disease meets this important endpoint,” Bana Jabri, MD, of the University of Chicago, wrote in an accompanying editorial.
The primary endpoint of no mucosal damage is “especially notable because it was achieved under a controlled gluten challenge, albeit with a relatively moderate amount of gluten (a regular diet contains 12 g of gluten daily, whereas the challenge involved 3 g daily) and for a short period of time,” Dr. Jabri said. The reduction of disease-associated symptoms and apparent improvement in quality of life with 100-mg dose added value to the findings, she said.
Future research areas include whether cross-reactive T cells, which were not analyzed in the current study, might “expand and become pathogenic after a long-term gluten challenge,” Dr. Jabri noted.
However, “ZED1227 is the first nondietary treatment that has preliminarily shown the capacity to prevent mucosal damage in persons with celiac disease,” she said.
“Although this trial is very encouraging, whether treatment with ZED1227, and more generally transglutaminase 2 inhibition, in patients with celiac disease will be efficient in real life and during long-term gluten exposure remains to be determined,” Dr. Jabri concluded.
Need for data on dosing consistency
“Celiac disease affects up to 2% of the population in many countries, and the main therapy of celiac disease is avoidance of gluten,” Kim Isaacs, MD, of the University of North Carolina, Chapel Hill, said in an interview. “This is challenging due to the ubiquitous nature of gluten in many food products,” she said. “Restrictive eating also affects social interaction which is often focused around food,” she added. “Availability of an oral therapy that is effective to treat celiac in the face of gluten exposure will have a profound impact on patients in terms of liberalization of dietary intake.”
Overall, “the changes in the villus height to crypt depth was similar between all the active treatment groups, whereas there was a dose-dependent reduction in transepithelial lymphocyte density,” Dr. Isaacs noted. “The symptom improvement was greatest in the 100-mg group, suggesting that symptoms may be related to a greater extent to the lymphocyte density than the minimal differences in villus height to crypt depth ratios seen in the active treatment groups,” she said.
Potential barriers to the use of the treatment include cost because “this will need to be a daily long-term therapy,” said Dr. Isaacs. “Compliance is a potential barrier as well,” she said. “This study looks at daily administration of the transglutaminase 2 inhibitor and shows a benefit, but it is not clear whether missing doses of the medication will have a prolonged impact on efficacy,” she emphasized. Consequently, long-term efficacy studies are needed, Dr. Isaacs said. Other research questions to answer include whether patients will become refractory to the beneficial effects over time, the effect of missing doses, and whether patients would lose all the benefits of the therapy if dosing is not consistent, she emphasized.
The study was funded by Dr. Falk Pharma. The researchers, as well as Dr. Jabri and Dr. Isaacs, had no financial conflicts to disclose. Dr. Isaacs is on the editorial advisory board of GI & Hepatology News.
A novel oral inhibitor of transglutaminase 2 appears to block gluten-induced mucosal damage in patients with celiac disease at three different doses, based on proof-of-concept trial data from 132 patients.
“Currently, no drug therapy reliably prevents the effects of dietary gluten or has been approved by regulators to treat celiac disease,” which remains an unmet need in these patients, many of whom struggle with symptoms even when they adhere to a gluten-free diet, wrote Detlef Schuppan, MD, of Johannes Gutenberg University of Mainz (Germany) and colleagues.
Celiac disease is driven in part by the enzyme transglutaminase 2, and a transglutaminase 2 inhibitor known as ZED1227 has been tested safely in phase 1 trials, they reported.
“ZED1227 targets the intestinal mucosa predominantly and thereby mediates protection; thus, it is unaffected by the complexity of the food matrix and is less dependent on the timing of ingestion of gluten-containing food,” the researchers explained.
In a study published in the New England Journal of Medicine, the researchers assessed the safety and efficacy of three dose levels of ZED1227. Adults with controlled celiac disease were randomized to doses of 10 mg (41 patients), 50 mg (41 patients), and 100 mg (41 patients), and 40 patients received a placebo. Of these, 35, 39, 28, and 30 patients, respectively, had sufficient duodenal biopsy samples for analysis.
Patients underwent a daily gluten challenge of 3 g for 6 weeks. At the end of 6 weeks, the primary study endpoint of attenuation of gluten-induced mucosal damage was measured by the ratio of villus height to crypt depth.
Patients in all three treatment groups showed significant attenuation of mucosal damage. The change in the average ratio of villus height to crypt depth compared to placebo in the 10-mg, 50-mg, and 100-mg groups was 0.44, 0.49, and 0.48, respectively, with P values equal to .001 in the 10-mg group and less than .001 in the 50-mg and 100-mg groups.
Adverse events were similar across all treatment groups and the placebo group, with the exception of a rash in three patients in the 100-mg group. A total of 74 patients reported adverse events, and the most common were headache, nausea, diarrhea, vomiting, and abdominal pain. The investigators determined that from 34% to 55% of the adverse events across groups were related to the study drug or placebo.
Two patients developed serious adverse events that were deemed related to the study drug or placebo; one patient in the 50-mg group developed migraine with aura, and one placebo patient developed ventricular extrasystoles. The patients recovered after discontinuing the drug or placebo.
Secondary endpoints included intraepithelial lymphocyte density, the Celiac Symptom Index score, and the Celiac Disease Questionnaire score. Estimated changes in intraepithelial lymphocyte density, compared with placebo, were –2.7 cells per 100 epithelial cells in the 10-mg group, −4.2 cells per 100 epithelial cells in the 50-mg group, and −9.6 cells per 100 epithelial cells in the 100-mg group. Compared with those of patients taking placebo, the 6-week changes in Celiac Symptom Index scores and Celiac Disease Questionnaire scores suggested slight improvements in symptoms and quality of life for the 100-mg dose.
The study findings were limited by several factors including missing data and loss of several patients to follow-up, as well as the short trial duration and use of controlled gluten ingestion, the researchers noted. Larger studies involving real-world conditions of minor gluten ingestion are needed to support the preliminary signs of safety and efficacy, they said.
Study strengths include high levels of patient adherence to the treatment and the gluten challenge, they said. “Future studies of ZED1227 in more patients are needed to provide additional evidence of the safety and efficacy of the drug, potentially in real-life conditions with minor gluten ingestion,” they concluded.
Translating potential into practice
“An absence of mucosal damage is a critical criterion to ensure the long-term health of a patient, and this clinical trial in celiac disease meets this important endpoint,” Bana Jabri, MD, of the University of Chicago, wrote in an accompanying editorial.
The primary endpoint of no mucosal damage is “especially notable because it was achieved under a controlled gluten challenge, albeit with a relatively moderate amount of gluten (a regular diet contains 12 g of gluten daily, whereas the challenge involved 3 g daily) and for a short period of time,” Dr. Jabri said. The reduction of disease-associated symptoms and apparent improvement in quality of life with 100-mg dose added value to the findings, she said.
Future research areas include whether cross-reactive T cells, which were not analyzed in the current study, might “expand and become pathogenic after a long-term gluten challenge,” Dr. Jabri noted.
However, “ZED1227 is the first nondietary treatment that has preliminarily shown the capacity to prevent mucosal damage in persons with celiac disease,” she said.
“Although this trial is very encouraging, whether treatment with ZED1227, and more generally transglutaminase 2 inhibition, in patients with celiac disease will be efficient in real life and during long-term gluten exposure remains to be determined,” Dr. Jabri concluded.
Need for data on dosing consistency
“Celiac disease affects up to 2% of the population in many countries, and the main therapy of celiac disease is avoidance of gluten,” Kim Isaacs, MD, of the University of North Carolina, Chapel Hill, said in an interview. “This is challenging due to the ubiquitous nature of gluten in many food products,” she said. “Restrictive eating also affects social interaction which is often focused around food,” she added. “Availability of an oral therapy that is effective to treat celiac in the face of gluten exposure will have a profound impact on patients in terms of liberalization of dietary intake.”
Overall, “the changes in the villus height to crypt depth was similar between all the active treatment groups, whereas there was a dose-dependent reduction in transepithelial lymphocyte density,” Dr. Isaacs noted. “The symptom improvement was greatest in the 100-mg group, suggesting that symptoms may be related to a greater extent to the lymphocyte density than the minimal differences in villus height to crypt depth ratios seen in the active treatment groups,” she said.
Potential barriers to the use of the treatment include cost because “this will need to be a daily long-term therapy,” said Dr. Isaacs. “Compliance is a potential barrier as well,” she said. “This study looks at daily administration of the transglutaminase 2 inhibitor and shows a benefit, but it is not clear whether missing doses of the medication will have a prolonged impact on efficacy,” she emphasized. Consequently, long-term efficacy studies are needed, Dr. Isaacs said. Other research questions to answer include whether patients will become refractory to the beneficial effects over time, the effect of missing doses, and whether patients would lose all the benefits of the therapy if dosing is not consistent, she emphasized.
The study was funded by Dr. Falk Pharma. The researchers, as well as Dr. Jabri and Dr. Isaacs, had no financial conflicts to disclose. Dr. Isaacs is on the editorial advisory board of GI & Hepatology News.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Opioid prescriptions decrease in young kids, long dosages increase
The opioid prescription rates have significantly decreased for children, teens, and younger adults between 2006 and 2018, according to new research.
“What’s important about this new study is that it documented that these improvements were also occurring for children and young adults specifically,” said Kao-Ping Chua, MD, PhD, primary care physician and assistant professor of pediatrics at the University of Michigan, Ann Arbor, who was not involved in the study. “The reason that’s important is that changes in medical practice for adults aren’t always reflected in pediatrics.”
The study, published in JAMA Pediatrics, found that dispensed opioid prescriptions for this population have decreased by 15% annually since 2013. However, the study also examined specific prescribing variables, such as duration of opioid prescription and high-dosage prescriptions. Researchers found reduced rates of high-dosage and long-duration prescriptions for adolescents and younger adults. However, these types of prescription practices increased in children aged 0-5 years.
“I think [the findings are] promising, suggesting that opiate prescribing practices may be improving,” study author Madeline Renny, MD, pediatric emergency medicine doctor at New York University Langone Health, said in an interview. “But we did find that there were increases in the young children for the practice variables, which we didn’t expect. I think that was kind of one of the findings that we were a bit surprised about and want to explore further.”
Previous studies have linked prescription opioid use in children and teens to an increased risk of future opioid misuse. A 2015 study published in Pediatrics found that using prescribed opioids before the 12th grade is associated with a 33% increase in the risk of future opioid misuse by the age of 23. The study also found that for those with a low predicted risk of future opioid misuse, an opioid prescription increases the risk for misuse after high school threefold.
Furthermore, a 2018 study published in JAMA Network Open found that, between 1999 and 2016, the annual estimated mortality rate for all children and adolescents from prescription and illicit opioid use rose 268.2%.
In the new study, Dr. Renny and colleagues examined data from 2006 to 2018 from IQVIA Longitudinal Prescription Data, which captured 74%-92% of U.S. retail outpatient opioid prescriptions dispensed to people up to the age of 24. Researchers also examined prescribing practice variables, which included opioid dispensing rates, average amount of opioid dispensed per prescription, duration of opioid prescription, high-dosage opioid prescription for individuals, and the rate in which extended-release or long-acting opioids are prescribed.
Researchers found that between 2006 and 2018, the total U.S. annual opioid prescriptions dispensed to patients younger than 25 years was highest in 2007 at 15,689,779 prescriptions, and since 2012 has steadily decreased to 6,705,478 in 2018.
“Our study did show that there were declines, but opioids remain readily dispensed,” Dr. Renny said. “And I think it’s good that rates have gone down, but I think opioids are still commonly dispensed to children and adolescents and young adults and all of our age groups.”
Dr. Chua said that the study was important, but when it came to younger children, it didn’t account for the fact that “the underlying population of patients who were getting opioids changed because it’s not the same group of children.”
“Maybe at the beginning there were more surgical patients who are getting shorter duration, lower dosage opioids,” he added. “Now some of those surgical exceptions kind of went away and who’s left in the population of people who get opioids is a sicker population.”
“Who are the 0 to 5-year-olds who are getting opioids now?” Dr. Chua asked. “Well, some of them are going to be cancer or surgical patients. If you think about it, over time their surgeons may be more judicious and they stop prescribing opioids for some things like circumcision or something like that. So that means that who’s left in the population of children who get opiate prescriptions are the cancer patients. Cancer patients’ opioid dosages are going to be higher because they have chronic pain.”
Dr. Chua said it is important to remember that the number of children who are affected by those high-risk prescriptions are lower because the overall number of opioid prescriptions has gone down. He added that the key piece of missing information is the absolute number of prescriptions that were high risk.
Researchers of the current study suggested that, because of the differences between pediatric and adult pain and indications for opioid prescribing, there should be national guidelines on general opioid prescribing for children and adolescents.
Experts did not disclose relevant financial relationships.
The opioid prescription rates have significantly decreased for children, teens, and younger adults between 2006 and 2018, according to new research.
“What’s important about this new study is that it documented that these improvements were also occurring for children and young adults specifically,” said Kao-Ping Chua, MD, PhD, primary care physician and assistant professor of pediatrics at the University of Michigan, Ann Arbor, who was not involved in the study. “The reason that’s important is that changes in medical practice for adults aren’t always reflected in pediatrics.”
The study, published in JAMA Pediatrics, found that dispensed opioid prescriptions for this population have decreased by 15% annually since 2013. However, the study also examined specific prescribing variables, such as duration of opioid prescription and high-dosage prescriptions. Researchers found reduced rates of high-dosage and long-duration prescriptions for adolescents and younger adults. However, these types of prescription practices increased in children aged 0-5 years.
“I think [the findings are] promising, suggesting that opiate prescribing practices may be improving,” study author Madeline Renny, MD, pediatric emergency medicine doctor at New York University Langone Health, said in an interview. “But we did find that there were increases in the young children for the practice variables, which we didn’t expect. I think that was kind of one of the findings that we were a bit surprised about and want to explore further.”
Previous studies have linked prescription opioid use in children and teens to an increased risk of future opioid misuse. A 2015 study published in Pediatrics found that using prescribed opioids before the 12th grade is associated with a 33% increase in the risk of future opioid misuse by the age of 23. The study also found that for those with a low predicted risk of future opioid misuse, an opioid prescription increases the risk for misuse after high school threefold.
Furthermore, a 2018 study published in JAMA Network Open found that, between 1999 and 2016, the annual estimated mortality rate for all children and adolescents from prescription and illicit opioid use rose 268.2%.
In the new study, Dr. Renny and colleagues examined data from 2006 to 2018 from IQVIA Longitudinal Prescription Data, which captured 74%-92% of U.S. retail outpatient opioid prescriptions dispensed to people up to the age of 24. Researchers also examined prescribing practice variables, which included opioid dispensing rates, average amount of opioid dispensed per prescription, duration of opioid prescription, high-dosage opioid prescription for individuals, and the rate in which extended-release or long-acting opioids are prescribed.
Researchers found that between 2006 and 2018, the total U.S. annual opioid prescriptions dispensed to patients younger than 25 years was highest in 2007 at 15,689,779 prescriptions, and since 2012 has steadily decreased to 6,705,478 in 2018.
“Our study did show that there were declines, but opioids remain readily dispensed,” Dr. Renny said. “And I think it’s good that rates have gone down, but I think opioids are still commonly dispensed to children and adolescents and young adults and all of our age groups.”
Dr. Chua said that the study was important, but when it came to younger children, it didn’t account for the fact that “the underlying population of patients who were getting opioids changed because it’s not the same group of children.”
“Maybe at the beginning there were more surgical patients who are getting shorter duration, lower dosage opioids,” he added. “Now some of those surgical exceptions kind of went away and who’s left in the population of people who get opioids is a sicker population.”
“Who are the 0 to 5-year-olds who are getting opioids now?” Dr. Chua asked. “Well, some of them are going to be cancer or surgical patients. If you think about it, over time their surgeons may be more judicious and they stop prescribing opioids for some things like circumcision or something like that. So that means that who’s left in the population of children who get opiate prescriptions are the cancer patients. Cancer patients’ opioid dosages are going to be higher because they have chronic pain.”
Dr. Chua said it is important to remember that the number of children who are affected by those high-risk prescriptions are lower because the overall number of opioid prescriptions has gone down. He added that the key piece of missing information is the absolute number of prescriptions that were high risk.
Researchers of the current study suggested that, because of the differences between pediatric and adult pain and indications for opioid prescribing, there should be national guidelines on general opioid prescribing for children and adolescents.
Experts did not disclose relevant financial relationships.
The opioid prescription rates have significantly decreased for children, teens, and younger adults between 2006 and 2018, according to new research.
“What’s important about this new study is that it documented that these improvements were also occurring for children and young adults specifically,” said Kao-Ping Chua, MD, PhD, primary care physician and assistant professor of pediatrics at the University of Michigan, Ann Arbor, who was not involved in the study. “The reason that’s important is that changes in medical practice for adults aren’t always reflected in pediatrics.”
The study, published in JAMA Pediatrics, found that dispensed opioid prescriptions for this population have decreased by 15% annually since 2013. However, the study also examined specific prescribing variables, such as duration of opioid prescription and high-dosage prescriptions. Researchers found reduced rates of high-dosage and long-duration prescriptions for adolescents and younger adults. However, these types of prescription practices increased in children aged 0-5 years.
“I think [the findings are] promising, suggesting that opiate prescribing practices may be improving,” study author Madeline Renny, MD, pediatric emergency medicine doctor at New York University Langone Health, said in an interview. “But we did find that there were increases in the young children for the practice variables, which we didn’t expect. I think that was kind of one of the findings that we were a bit surprised about and want to explore further.”
Previous studies have linked prescription opioid use in children and teens to an increased risk of future opioid misuse. A 2015 study published in Pediatrics found that using prescribed opioids before the 12th grade is associated with a 33% increase in the risk of future opioid misuse by the age of 23. The study also found that for those with a low predicted risk of future opioid misuse, an opioid prescription increases the risk for misuse after high school threefold.
Furthermore, a 2018 study published in JAMA Network Open found that, between 1999 and 2016, the annual estimated mortality rate for all children and adolescents from prescription and illicit opioid use rose 268.2%.
In the new study, Dr. Renny and colleagues examined data from 2006 to 2018 from IQVIA Longitudinal Prescription Data, which captured 74%-92% of U.S. retail outpatient opioid prescriptions dispensed to people up to the age of 24. Researchers also examined prescribing practice variables, which included opioid dispensing rates, average amount of opioid dispensed per prescription, duration of opioid prescription, high-dosage opioid prescription for individuals, and the rate in which extended-release or long-acting opioids are prescribed.
Researchers found that between 2006 and 2018, the total U.S. annual opioid prescriptions dispensed to patients younger than 25 years was highest in 2007 at 15,689,779 prescriptions, and since 2012 has steadily decreased to 6,705,478 in 2018.
“Our study did show that there were declines, but opioids remain readily dispensed,” Dr. Renny said. “And I think it’s good that rates have gone down, but I think opioids are still commonly dispensed to children and adolescents and young adults and all of our age groups.”
Dr. Chua said that the study was important, but when it came to younger children, it didn’t account for the fact that “the underlying population of patients who were getting opioids changed because it’s not the same group of children.”
“Maybe at the beginning there were more surgical patients who are getting shorter duration, lower dosage opioids,” he added. “Now some of those surgical exceptions kind of went away and who’s left in the population of people who get opioids is a sicker population.”
“Who are the 0 to 5-year-olds who are getting opioids now?” Dr. Chua asked. “Well, some of them are going to be cancer or surgical patients. If you think about it, over time their surgeons may be more judicious and they stop prescribing opioids for some things like circumcision or something like that. So that means that who’s left in the population of children who get opiate prescriptions are the cancer patients. Cancer patients’ opioid dosages are going to be higher because they have chronic pain.”
Dr. Chua said it is important to remember that the number of children who are affected by those high-risk prescriptions are lower because the overall number of opioid prescriptions has gone down. He added that the key piece of missing information is the absolute number of prescriptions that were high risk.
Researchers of the current study suggested that, because of the differences between pediatric and adult pain and indications for opioid prescribing, there should be national guidelines on general opioid prescribing for children and adolescents.
Experts did not disclose relevant financial relationships.
FROM JAMA PEDIATRICS
A pacemaker that 'just disappears' and a magnetic diet device
Ignore this pacemaker and it will go away
At some point – and now seems to be that point – we have to say enough is enough. The throwaway culture that produces phones, TVs, and computers that get tossed in the trash because they can’t be repaired has gone too far. That’s right, we’re looking at you, medical science!
This time, it’s a pacemaker that just disappears when it’s no longer needed. Some lazy heart surgeon decided that it was way too much trouble to do another surgery to remove the leads when a temporary pacemaker was no longer needed. You know the type: “It sure would be nice if the pacemaker components were biocompatible and were naturally absorbed by the body over the course of a few weeks and wouldn’t need to be surgically extracted.” Slacker.
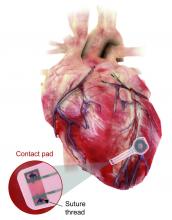
Well, get a load of this. Researchers at Northwestern and George Washington universities say that they have come up with a transient pacemaker that “harvests energy from an external, remote antenna using near-field communication protocols – the same technology used in smartphones for electronic payments and in RFID tags.”
That means no batteries and no wires that have to be removed and can cause infections. Because the infectious disease docs also are too lazy to do their jobs, apparently.
The lack of onboard infrastructure means that the device can be very small – it weighs less than half a gram and is only 250 microns thick. And yes, it is bioresorbable and completely harmless. It fully degrades and disappears in 5-7 weeks through the body’s natural biologic processes, “thereby avoiding the need for physical removal of the pacemaker electrodes. This is potentially a major victory for postoperative patients,” said Dr. Rishi Arora, one of the investigators.
A victory for patients, he says. Not a word about the time and effort saved by the surgeons. Typical.
It’s a mask! No, it’s a COVID-19 test!
Mask wearing has gotten more lax as people get vaccinated for COVID-19, but as wearing masks for virus prevention is becoming more normalized in western society, some saw an opportunity to make them work for diagnosis.
Researchers from the Massachusetts Institute of Technology and the Wyss Institute for Biologically Inspired Engineering at Harvard University have found a way to do just that with their wearable freeze-dried cell-free (wFDCF) technology. A single push of a button releases water from a reservoir in the mask that sequentially activates three different freeze-dried biological reactions, which detect the SARS-CoV-2 virus in the wearer’s breath.
Initially meant as a tool for the Zika outbreak in 2015, the team made a quick pivot in May 2020. But this isn’t just some run-of-the-mill, at-home test. The data prove that the wFDCF mask is comparable to polymerase chain reactions tests, the standard in COVID-19 detection. Plus there aren’t any extra factors to deal with, like room or instrument temperature to ensure accuracy. In just 90 minutes, the mask gives results on a readout in a way similar to that of a pregnancy test. Voilà! To have COVID-19 or not to have COVID-19 is an easily answered question.
At LOTME, we think this is a big improvement from having dogs, or even three-foot rats, sniffing out coronavirus.
But wait, there’s more. “In addition to face masks, our programmable biosensors can be integrated into other garments to provide on-the-go detection of dangerous substances including viruses, bacteria, toxins, and chemical agents,” said Peter Nguyen, PhD, study coauthor and research scientist at the Wyss Institute. The technology can be used on lab coats, scrubs, military uniforms, and uniforms of first responders who may come in contact with hazardous pathogens and toxins. Think of all the lives saved and possible avoidances.
If only it could diagnose bad breath.
Finally, an excuse for the all-beer diet
Weight loss is hard work. Extremely hard work, and, as evidenced by the constant inundation and advertisement of quick fixes, crash diets, and expensive gym memberships, there’s not really a solid, 100% solution to the issue. Until now, thanks to a team of doctors from New Zealand, who’ve decided that the best way to combat obesity is to leave you in constant agony.
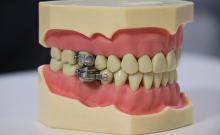
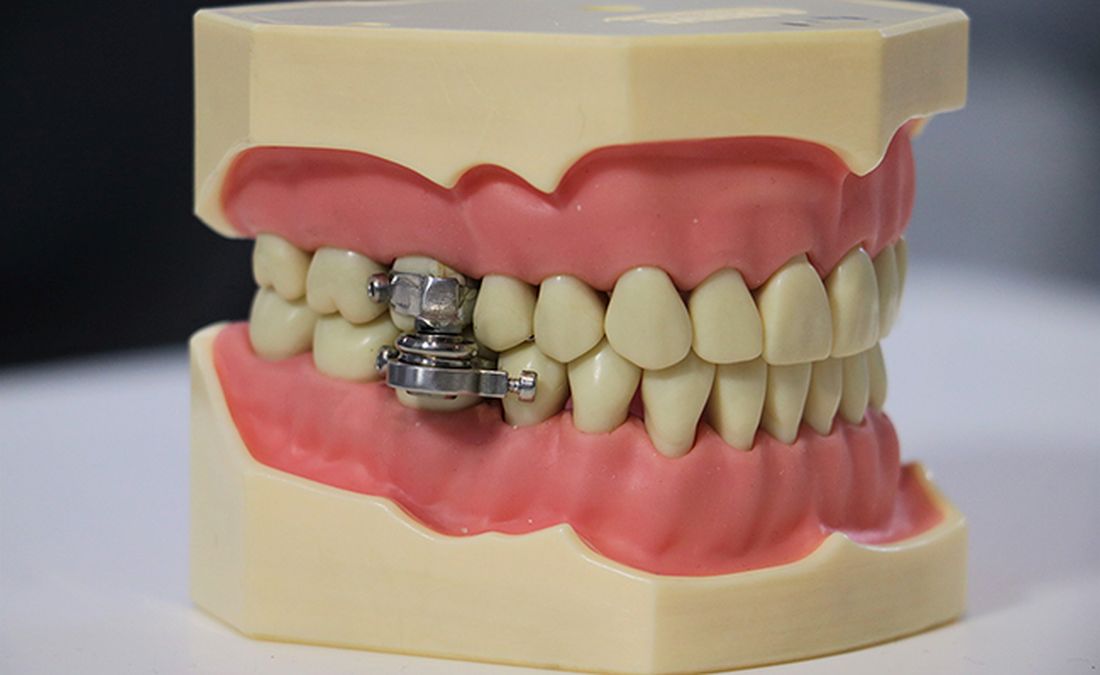
The DentalSlim Diet Control device is certainly a radical yet comically logical attempt to combat obesity. The creators say that the biggest problem with dieting is compliance, and, well, it’s difficult to eat too much if you can’t actually open your mouth. The metal contraption is mounted onto your teeth and uses magnetic locks to prevent the user from opening their mouths more than 2 mm. That’s less than a tenth of an inch. Which is not a lot. So not a lot that essentially all you can consume is liquid.
Oh, and they’ve got results to back up their madness. In a small study, seven otherwise healthy obese women lost an average of 5.1% of their body weight after using the DentalSlim for 2 weeks, though they did complain that the device was difficult to use, caused discomfort and difficulty speaking, made them more tense, and in general made life “less satisfying.” And one participant was able to cheat the system and consume nonhealthy food like chocolate by melting it.
So, there you are, if you want a weight-loss solution that tortures you and has far bigger holes than the one it leaves for your mouth, try the DentalSlim. Or, you know, don’t eat that eighth slice of pizza and maybe go for a walk later. Your choice.
Ignore this pacemaker and it will go away
At some point – and now seems to be that point – we have to say enough is enough. The throwaway culture that produces phones, TVs, and computers that get tossed in the trash because they can’t be repaired has gone too far. That’s right, we’re looking at you, medical science!
This time, it’s a pacemaker that just disappears when it’s no longer needed. Some lazy heart surgeon decided that it was way too much trouble to do another surgery to remove the leads when a temporary pacemaker was no longer needed. You know the type: “It sure would be nice if the pacemaker components were biocompatible and were naturally absorbed by the body over the course of a few weeks and wouldn’t need to be surgically extracted.” Slacker.
Well, get a load of this. Researchers at Northwestern and George Washington universities say that they have come up with a transient pacemaker that “harvests energy from an external, remote antenna using near-field communication protocols – the same technology used in smartphones for electronic payments and in RFID tags.”
That means no batteries and no wires that have to be removed and can cause infections. Because the infectious disease docs also are too lazy to do their jobs, apparently.
The lack of onboard infrastructure means that the device can be very small – it weighs less than half a gram and is only 250 microns thick. And yes, it is bioresorbable and completely harmless. It fully degrades and disappears in 5-7 weeks through the body’s natural biologic processes, “thereby avoiding the need for physical removal of the pacemaker electrodes. This is potentially a major victory for postoperative patients,” said Dr. Rishi Arora, one of the investigators.
A victory for patients, he says. Not a word about the time and effort saved by the surgeons. Typical.
It’s a mask! No, it’s a COVID-19 test!
Mask wearing has gotten more lax as people get vaccinated for COVID-19, but as wearing masks for virus prevention is becoming more normalized in western society, some saw an opportunity to make them work for diagnosis.
Researchers from the Massachusetts Institute of Technology and the Wyss Institute for Biologically Inspired Engineering at Harvard University have found a way to do just that with their wearable freeze-dried cell-free (wFDCF) technology. A single push of a button releases water from a reservoir in the mask that sequentially activates three different freeze-dried biological reactions, which detect the SARS-CoV-2 virus in the wearer’s breath.
Initially meant as a tool for the Zika outbreak in 2015, the team made a quick pivot in May 2020. But this isn’t just some run-of-the-mill, at-home test. The data prove that the wFDCF mask is comparable to polymerase chain reactions tests, the standard in COVID-19 detection. Plus there aren’t any extra factors to deal with, like room or instrument temperature to ensure accuracy. In just 90 minutes, the mask gives results on a readout in a way similar to that of a pregnancy test. Voilà! To have COVID-19 or not to have COVID-19 is an easily answered question.
At LOTME, we think this is a big improvement from having dogs, or even three-foot rats, sniffing out coronavirus.
But wait, there’s more. “In addition to face masks, our programmable biosensors can be integrated into other garments to provide on-the-go detection of dangerous substances including viruses, bacteria, toxins, and chemical agents,” said Peter Nguyen, PhD, study coauthor and research scientist at the Wyss Institute. The technology can be used on lab coats, scrubs, military uniforms, and uniforms of first responders who may come in contact with hazardous pathogens and toxins. Think of all the lives saved and possible avoidances.
If only it could diagnose bad breath.
Finally, an excuse for the all-beer diet
Weight loss is hard work. Extremely hard work, and, as evidenced by the constant inundation and advertisement of quick fixes, crash diets, and expensive gym memberships, there’s not really a solid, 100% solution to the issue. Until now, thanks to a team of doctors from New Zealand, who’ve decided that the best way to combat obesity is to leave you in constant agony.
The DentalSlim Diet Control device is certainly a radical yet comically logical attempt to combat obesity. The creators say that the biggest problem with dieting is compliance, and, well, it’s difficult to eat too much if you can’t actually open your mouth. The metal contraption is mounted onto your teeth and uses magnetic locks to prevent the user from opening their mouths more than 2 mm. That’s less than a tenth of an inch. Which is not a lot. So not a lot that essentially all you can consume is liquid.
Oh, and they’ve got results to back up their madness. In a small study, seven otherwise healthy obese women lost an average of 5.1% of their body weight after using the DentalSlim for 2 weeks, though they did complain that the device was difficult to use, caused discomfort and difficulty speaking, made them more tense, and in general made life “less satisfying.” And one participant was able to cheat the system and consume nonhealthy food like chocolate by melting it.
So, there you are, if you want a weight-loss solution that tortures you and has far bigger holes than the one it leaves for your mouth, try the DentalSlim. Or, you know, don’t eat that eighth slice of pizza and maybe go for a walk later. Your choice.
Ignore this pacemaker and it will go away
At some point – and now seems to be that point – we have to say enough is enough. The throwaway culture that produces phones, TVs, and computers that get tossed in the trash because they can’t be repaired has gone too far. That’s right, we’re looking at you, medical science!
This time, it’s a pacemaker that just disappears when it’s no longer needed. Some lazy heart surgeon decided that it was way too much trouble to do another surgery to remove the leads when a temporary pacemaker was no longer needed. You know the type: “It sure would be nice if the pacemaker components were biocompatible and were naturally absorbed by the body over the course of a few weeks and wouldn’t need to be surgically extracted.” Slacker.
Well, get a load of this. Researchers at Northwestern and George Washington universities say that they have come up with a transient pacemaker that “harvests energy from an external, remote antenna using near-field communication protocols – the same technology used in smartphones for electronic payments and in RFID tags.”
That means no batteries and no wires that have to be removed and can cause infections. Because the infectious disease docs also are too lazy to do their jobs, apparently.
The lack of onboard infrastructure means that the device can be very small – it weighs less than half a gram and is only 250 microns thick. And yes, it is bioresorbable and completely harmless. It fully degrades and disappears in 5-7 weeks through the body’s natural biologic processes, “thereby avoiding the need for physical removal of the pacemaker electrodes. This is potentially a major victory for postoperative patients,” said Dr. Rishi Arora, one of the investigators.
A victory for patients, he says. Not a word about the time and effort saved by the surgeons. Typical.
It’s a mask! No, it’s a COVID-19 test!
Mask wearing has gotten more lax as people get vaccinated for COVID-19, but as wearing masks for virus prevention is becoming more normalized in western society, some saw an opportunity to make them work for diagnosis.
Researchers from the Massachusetts Institute of Technology and the Wyss Institute for Biologically Inspired Engineering at Harvard University have found a way to do just that with their wearable freeze-dried cell-free (wFDCF) technology. A single push of a button releases water from a reservoir in the mask that sequentially activates three different freeze-dried biological reactions, which detect the SARS-CoV-2 virus in the wearer’s breath.
Initially meant as a tool for the Zika outbreak in 2015, the team made a quick pivot in May 2020. But this isn’t just some run-of-the-mill, at-home test. The data prove that the wFDCF mask is comparable to polymerase chain reactions tests, the standard in COVID-19 detection. Plus there aren’t any extra factors to deal with, like room or instrument temperature to ensure accuracy. In just 90 minutes, the mask gives results on a readout in a way similar to that of a pregnancy test. Voilà! To have COVID-19 or not to have COVID-19 is an easily answered question.
At LOTME, we think this is a big improvement from having dogs, or even three-foot rats, sniffing out coronavirus.
But wait, there’s more. “In addition to face masks, our programmable biosensors can be integrated into other garments to provide on-the-go detection of dangerous substances including viruses, bacteria, toxins, and chemical agents,” said Peter Nguyen, PhD, study coauthor and research scientist at the Wyss Institute. The technology can be used on lab coats, scrubs, military uniforms, and uniforms of first responders who may come in contact with hazardous pathogens and toxins. Think of all the lives saved and possible avoidances.
If only it could diagnose bad breath.
Finally, an excuse for the all-beer diet
Weight loss is hard work. Extremely hard work, and, as evidenced by the constant inundation and advertisement of quick fixes, crash diets, and expensive gym memberships, there’s not really a solid, 100% solution to the issue. Until now, thanks to a team of doctors from New Zealand, who’ve decided that the best way to combat obesity is to leave you in constant agony.
The DentalSlim Diet Control device is certainly a radical yet comically logical attempt to combat obesity. The creators say that the biggest problem with dieting is compliance, and, well, it’s difficult to eat too much if you can’t actually open your mouth. The metal contraption is mounted onto your teeth and uses magnetic locks to prevent the user from opening their mouths more than 2 mm. That’s less than a tenth of an inch. Which is not a lot. So not a lot that essentially all you can consume is liquid.
Oh, and they’ve got results to back up their madness. In a small study, seven otherwise healthy obese women lost an average of 5.1% of their body weight after using the DentalSlim for 2 weeks, though they did complain that the device was difficult to use, caused discomfort and difficulty speaking, made them more tense, and in general made life “less satisfying.” And one participant was able to cheat the system and consume nonhealthy food like chocolate by melting it.
So, there you are, if you want a weight-loss solution that tortures you and has far bigger holes than the one it leaves for your mouth, try the DentalSlim. Or, you know, don’t eat that eighth slice of pizza and maybe go for a walk later. Your choice.
Two case reports identify Guillain-Barré variants after SARS-CoV-2 vaccination
Guillain-Barré syndrome, a rare peripheral nerve disorder that can occur after certain types of viral and bacterial infections, has not to date been definitively linked to infection by SARS-CoV-2 or with vaccination against the virus, despite surveillance searching for such associations.
Spikes in Guillain-Barré syndrome incidence have previously, but rarely, been associated with outbreaks of other viral diseases, including Zika, but not with vaccination, except for a 1976-1977 swine influenza vaccine campaign in the United States that was seen associated with a slight elevation in risk, and was halted when that risk became known. Since then, all sorts of vaccines in the European Union and United States have come with warnings about Guillain-Barré syndrome in their package inserts – a fact that some Guillain-Barré syndrome experts lament as perpetuating the notion that vaccines cause Guillain-Barré syndrome.
Epidemiologic studies in the United Kingdom and Singapore did not detect increases in Guillain-Barré syndrome incidence during the COVID-19 pandemic. And as mass vaccination against COVID-19 got underway early this year, experts cautioned against the temptation to attribute incident Guillain-Barré syndrome cases following vaccination to SARS-CoV-2 without careful statistical and epidemiological analysis. Until now reports of Guillain-Barré syndrome have been scant: clinical trials of a viral vector vaccine developed by Johnson & Johnson saw one in the placebo arm and another in the intervention arm, while another case was reported following administration of a Pfizer mRNA SARS-Cov-2 vaccine.
Recent case reports
None of the patients had evidence of current SARS-CoV-2 infection.
From India, Boby V. Maramattom, MD, of Aster Medcity in Kochi, India, and colleagues reported on seven severe cases of Guillain-Barré syndrome occurring between 10 and 14 days after a first dose of the AstraZeneca vaccine. All but one of the patients were women, all had bilateral facial paresis, all progressed to areflexic quadriplegia, and six required respiratory support. Patients’ ages ranged from 43 to 70. Four developed other cranial neuropathies, including abducens palsy and trigeminal sensory nerve involvement, which are rare in reports of Guillain-Barré syndrome from India, Dr. Maramattom and colleagues noted.
The authors argued that their findings “should prompt all physicians to be vigilant in recognizing Guillain-Barré syndrome in patients who have received the AstraZeneca vaccine. While the risk per patient (5.8 per million) may be relatively low, our observations suggest that this clinically distinct [Guillain-Barré syndrome] variant is more severe than usual and may require mechanical ventilation.”
The U.K. cases, reported by Christopher Martin Allen, MD, and colleagues at Nottingham (England) University Hospitals NHS Trust, describe bifacial weakness and normal facial sensation in four men between 11 and 22 days after their first doses of the Astra-Zeneca vaccine. This type of facial palsy, the authors wrote, was unusual Guillain-Barré syndrome variant that one rapid review found in 3 of 42 European patients diagnosed with Guillain-Barré syndrome following SARS-CoV-2 infection.
Dr. Allen and colleagues acknowledged that causality could not be assumed from the temporal relationship of immunization to onset of bifacial weakness in their report, but argued that their findings argued for “robust postvaccination surveillance” and that “the report of a similar syndrome in the setting of SARS-CoV-2 infection suggests an immunologic response to the spike protein.” If the link is casual, they wrote, “it could be due to a cross-reactive immune response to the SARS-CoV-2 spike protein and components of the peripheral immune system.”
‘The jury is still out’
Asked for comment, neurologist Anthony Amato, MD, of Brigham and Women’s Hospital, Boston, said that he did not see what the two new studies add to what is already known. “Guillain-Barré syndrome has already been reported temporally following COVID-19 along with accompanying editorials that such temporal occurrences do not imply causation and there is a need for surveillance and epidemiological studies.”
Robert Lisak, MD, of Wayne State University, Detroit, and a longtime adviser to the GBS-CIDP Foundation International, commented that “the relationship between vaccines and association with Guillain-Barré syndrome continues to be controversial in part because Guillain-Barré syndrome, a rare disorder, has many reported associated illnesses including infections. Many vaccines have been implicated but with the probable exception of the ‘swine flu’ vaccine in the 1970s, most have not stood up to scrutiny.”
With SARS-Cov-2 infection and vaccines, “the jury is still out,” Dr. Lisak said. “The report from the U.K. is intriguing since they report several cases of an uncommon variant, but the cases from India seem to be more of the usual forms of Guillain-Barré syndrome.”
Dr. Lisak noted that, even if an association turns out to be valid, “we are talking about a very low incidence of Guillain-Barré syndrome associated with COVID-19 vaccines,” one that would not justify avoiding them because of a possible association with Guillain-Barré syndrome.
The GBS-CIDP Foundation, which supports research into Guillain-Barré syndrome and related diseases, has likewise stressed the low risk presented by SARS-CoV-2 vaccines, noting on its website that “the risk of death or long-term complications from COVID in adults still far exceeds the risk of any possible risk of Guillain-Barré syndrome by several orders of magnitude.”
None of the study authors reported financial conflicts of interest related to their research. Dr. Amato is an adviser to the pharmaceutical firms Alexion and Argenx, while Dr. Lisak has received research support or honoraria from Alexion, Novartis, Hoffmann–La Roche, and others.
Guillain-Barré syndrome, a rare peripheral nerve disorder that can occur after certain types of viral and bacterial infections, has not to date been definitively linked to infection by SARS-CoV-2 or with vaccination against the virus, despite surveillance searching for such associations.
Spikes in Guillain-Barré syndrome incidence have previously, but rarely, been associated with outbreaks of other viral diseases, including Zika, but not with vaccination, except for a 1976-1977 swine influenza vaccine campaign in the United States that was seen associated with a slight elevation in risk, and was halted when that risk became known. Since then, all sorts of vaccines in the European Union and United States have come with warnings about Guillain-Barré syndrome in their package inserts – a fact that some Guillain-Barré syndrome experts lament as perpetuating the notion that vaccines cause Guillain-Barré syndrome.
Epidemiologic studies in the United Kingdom and Singapore did not detect increases in Guillain-Barré syndrome incidence during the COVID-19 pandemic. And as mass vaccination against COVID-19 got underway early this year, experts cautioned against the temptation to attribute incident Guillain-Barré syndrome cases following vaccination to SARS-CoV-2 without careful statistical and epidemiological analysis. Until now reports of Guillain-Barré syndrome have been scant: clinical trials of a viral vector vaccine developed by Johnson & Johnson saw one in the placebo arm and another in the intervention arm, while another case was reported following administration of a Pfizer mRNA SARS-Cov-2 vaccine.
Recent case reports
None of the patients had evidence of current SARS-CoV-2 infection.
From India, Boby V. Maramattom, MD, of Aster Medcity in Kochi, India, and colleagues reported on seven severe cases of Guillain-Barré syndrome occurring between 10 and 14 days after a first dose of the AstraZeneca vaccine. All but one of the patients were women, all had bilateral facial paresis, all progressed to areflexic quadriplegia, and six required respiratory support. Patients’ ages ranged from 43 to 70. Four developed other cranial neuropathies, including abducens palsy and trigeminal sensory nerve involvement, which are rare in reports of Guillain-Barré syndrome from India, Dr. Maramattom and colleagues noted.
The authors argued that their findings “should prompt all physicians to be vigilant in recognizing Guillain-Barré syndrome in patients who have received the AstraZeneca vaccine. While the risk per patient (5.8 per million) may be relatively low, our observations suggest that this clinically distinct [Guillain-Barré syndrome] variant is more severe than usual and may require mechanical ventilation.”
The U.K. cases, reported by Christopher Martin Allen, MD, and colleagues at Nottingham (England) University Hospitals NHS Trust, describe bifacial weakness and normal facial sensation in four men between 11 and 22 days after their first doses of the Astra-Zeneca vaccine. This type of facial palsy, the authors wrote, was unusual Guillain-Barré syndrome variant that one rapid review found in 3 of 42 European patients diagnosed with Guillain-Barré syndrome following SARS-CoV-2 infection.
Dr. Allen and colleagues acknowledged that causality could not be assumed from the temporal relationship of immunization to onset of bifacial weakness in their report, but argued that their findings argued for “robust postvaccination surveillance” and that “the report of a similar syndrome in the setting of SARS-CoV-2 infection suggests an immunologic response to the spike protein.” If the link is casual, they wrote, “it could be due to a cross-reactive immune response to the SARS-CoV-2 spike protein and components of the peripheral immune system.”
‘The jury is still out’
Asked for comment, neurologist Anthony Amato, MD, of Brigham and Women’s Hospital, Boston, said that he did not see what the two new studies add to what is already known. “Guillain-Barré syndrome has already been reported temporally following COVID-19 along with accompanying editorials that such temporal occurrences do not imply causation and there is a need for surveillance and epidemiological studies.”
Robert Lisak, MD, of Wayne State University, Detroit, and a longtime adviser to the GBS-CIDP Foundation International, commented that “the relationship between vaccines and association with Guillain-Barré syndrome continues to be controversial in part because Guillain-Barré syndrome, a rare disorder, has many reported associated illnesses including infections. Many vaccines have been implicated but with the probable exception of the ‘swine flu’ vaccine in the 1970s, most have not stood up to scrutiny.”
With SARS-Cov-2 infection and vaccines, “the jury is still out,” Dr. Lisak said. “The report from the U.K. is intriguing since they report several cases of an uncommon variant, but the cases from India seem to be more of the usual forms of Guillain-Barré syndrome.”
Dr. Lisak noted that, even if an association turns out to be valid, “we are talking about a very low incidence of Guillain-Barré syndrome associated with COVID-19 vaccines,” one that would not justify avoiding them because of a possible association with Guillain-Barré syndrome.
The GBS-CIDP Foundation, which supports research into Guillain-Barré syndrome and related diseases, has likewise stressed the low risk presented by SARS-CoV-2 vaccines, noting on its website that “the risk of death or long-term complications from COVID in adults still far exceeds the risk of any possible risk of Guillain-Barré syndrome by several orders of magnitude.”
None of the study authors reported financial conflicts of interest related to their research. Dr. Amato is an adviser to the pharmaceutical firms Alexion and Argenx, while Dr. Lisak has received research support or honoraria from Alexion, Novartis, Hoffmann–La Roche, and others.
Guillain-Barré syndrome, a rare peripheral nerve disorder that can occur after certain types of viral and bacterial infections, has not to date been definitively linked to infection by SARS-CoV-2 or with vaccination against the virus, despite surveillance searching for such associations.
Spikes in Guillain-Barré syndrome incidence have previously, but rarely, been associated with outbreaks of other viral diseases, including Zika, but not with vaccination, except for a 1976-1977 swine influenza vaccine campaign in the United States that was seen associated with a slight elevation in risk, and was halted when that risk became known. Since then, all sorts of vaccines in the European Union and United States have come with warnings about Guillain-Barré syndrome in their package inserts – a fact that some Guillain-Barré syndrome experts lament as perpetuating the notion that vaccines cause Guillain-Barré syndrome.
Epidemiologic studies in the United Kingdom and Singapore did not detect increases in Guillain-Barré syndrome incidence during the COVID-19 pandemic. And as mass vaccination against COVID-19 got underway early this year, experts cautioned against the temptation to attribute incident Guillain-Barré syndrome cases following vaccination to SARS-CoV-2 without careful statistical and epidemiological analysis. Until now reports of Guillain-Barré syndrome have been scant: clinical trials of a viral vector vaccine developed by Johnson & Johnson saw one in the placebo arm and another in the intervention arm, while another case was reported following administration of a Pfizer mRNA SARS-Cov-2 vaccine.
Recent case reports
None of the patients had evidence of current SARS-CoV-2 infection.
From India, Boby V. Maramattom, MD, of Aster Medcity in Kochi, India, and colleagues reported on seven severe cases of Guillain-Barré syndrome occurring between 10 and 14 days after a first dose of the AstraZeneca vaccine. All but one of the patients were women, all had bilateral facial paresis, all progressed to areflexic quadriplegia, and six required respiratory support. Patients’ ages ranged from 43 to 70. Four developed other cranial neuropathies, including abducens palsy and trigeminal sensory nerve involvement, which are rare in reports of Guillain-Barré syndrome from India, Dr. Maramattom and colleagues noted.
The authors argued that their findings “should prompt all physicians to be vigilant in recognizing Guillain-Barré syndrome in patients who have received the AstraZeneca vaccine. While the risk per patient (5.8 per million) may be relatively low, our observations suggest that this clinically distinct [Guillain-Barré syndrome] variant is more severe than usual and may require mechanical ventilation.”
The U.K. cases, reported by Christopher Martin Allen, MD, and colleagues at Nottingham (England) University Hospitals NHS Trust, describe bifacial weakness and normal facial sensation in four men between 11 and 22 days after their first doses of the Astra-Zeneca vaccine. This type of facial palsy, the authors wrote, was unusual Guillain-Barré syndrome variant that one rapid review found in 3 of 42 European patients diagnosed with Guillain-Barré syndrome following SARS-CoV-2 infection.
Dr. Allen and colleagues acknowledged that causality could not be assumed from the temporal relationship of immunization to onset of bifacial weakness in their report, but argued that their findings argued for “robust postvaccination surveillance” and that “the report of a similar syndrome in the setting of SARS-CoV-2 infection suggests an immunologic response to the spike protein.” If the link is casual, they wrote, “it could be due to a cross-reactive immune response to the SARS-CoV-2 spike protein and components of the peripheral immune system.”
‘The jury is still out’
Asked for comment, neurologist Anthony Amato, MD, of Brigham and Women’s Hospital, Boston, said that he did not see what the two new studies add to what is already known. “Guillain-Barré syndrome has already been reported temporally following COVID-19 along with accompanying editorials that such temporal occurrences do not imply causation and there is a need for surveillance and epidemiological studies.”
Robert Lisak, MD, of Wayne State University, Detroit, and a longtime adviser to the GBS-CIDP Foundation International, commented that “the relationship between vaccines and association with Guillain-Barré syndrome continues to be controversial in part because Guillain-Barré syndrome, a rare disorder, has many reported associated illnesses including infections. Many vaccines have been implicated but with the probable exception of the ‘swine flu’ vaccine in the 1970s, most have not stood up to scrutiny.”
With SARS-Cov-2 infection and vaccines, “the jury is still out,” Dr. Lisak said. “The report from the U.K. is intriguing since they report several cases of an uncommon variant, but the cases from India seem to be more of the usual forms of Guillain-Barré syndrome.”
Dr. Lisak noted that, even if an association turns out to be valid, “we are talking about a very low incidence of Guillain-Barré syndrome associated with COVID-19 vaccines,” one that would not justify avoiding them because of a possible association with Guillain-Barré syndrome.
The GBS-CIDP Foundation, which supports research into Guillain-Barré syndrome and related diseases, has likewise stressed the low risk presented by SARS-CoV-2 vaccines, noting on its website that “the risk of death or long-term complications from COVID in adults still far exceeds the risk of any possible risk of Guillain-Barré syndrome by several orders of magnitude.”
None of the study authors reported financial conflicts of interest related to their research. Dr. Amato is an adviser to the pharmaceutical firms Alexion and Argenx, while Dr. Lisak has received research support or honoraria from Alexion, Novartis, Hoffmann–La Roche, and others.
FROM ANNALS OF NEUROLOGY
Rapid core antigen HCV tests could expand accessibility
A proposed rapid diagnostic test for hepatitis C viral infections that combines an inexpensive but lower-sensitivity core-antigen test with lab RNA confirmation of negative tests could expand testing and same-day initiation of antiviral therapy in places where resources are limited, investigators said.
Applying the proposed method to the Republic of Georgia, with a hepatitis C virus (HCV) prevalence of 5.4% as reported by the World Health Organization, would result in a 95.4% diagnosis rate, compared with 78.8% for lab-based RNA testing, which is the standard of care. Applied to Malaysia, the proposed method would boost diagnosis rates from 57.0% to 91.2%, reported Madeline Adee, MPH, from Massachusetts General Hospital’s Institute for Technology Assessment in Boston and colleagues.
“We found that a novel core antigen rapid diagnostic test for HCV could improve the diagnosis rate and result in cost savings. Although not yet developed, such a test could be a game changer and have a substantial impact on the feasibility and cost of HCV elimination, especially in low and middle-income countries,” they wrote in a poster presented at the meeting sponsored by the European Association for the Study of the Liver.
Although rapid diagnostic tests for HCV can improve diagnosis and treatment rates, currently available molecular tests are expensive and require a solid clinical laboratory infrastructure, which can put such tests out of the reach for clinicians in low- or middle-income countries. Rapid immunoassays based on HCV core antigens are comparative bargains, but their sensitivity ranges from 70% to 90%; in contrast, the third-generation HCV enzyme immunoassay has about a 98% sensitivity.
Could it work?
The proposed testing method would be likely to improve diagnosis, but whether that would translate into increased treatment is uncertain, commented Lesley Miller, MD, who specializes in HCV screening and treatment in underserved populations at Emory University, Atlanta.
“When we’re talking about hepatitis C, it’s all about the care cascade, the drop-off at each step from those who have the disease and aren’t diagnosed, to those who are tested and only partially diagnosed because they don’t have a confirmed infection, to those that get into care, get treated, and get cured,” she said in an interview.
“It’s all about closing the gaps in the care cascade in order to achieve elimination of the virus, which is what we’re all trying to do,” she added.
She pointed that there are certain at-risk populations in the United States, such as injectable-drug users, who might be able to benefit from such a system.
“These folks often have less access to traditional care, so bringing rapid testing and care to where those folks are is really important, so if we can deploy mobile units to areas where there is high prevalence and do it at the point of care, it simplifies the entire process,” she said.
Thomas J. Hoerger, PhD, a senior fellow in health economics and financing at the nonprofit research group RTI International in Research Triangle Park, N.C., said in an interview that the proposed model could eliminate the step in testing in which patients are required to return for confirmation.
“People don’t always come back for further testing, so if you can do it immediately and have the results of a screening test, you might be able to get people to come back more quickly. You still have the problem of the high cost of treatment, but this would at least make it a little more convenient,” he said.
He noted that the success of the strategy would be dependent on the sensitivity of the rapid core antigen test, it’s cost relative to HCV RNA testing, and whether the availability of the rapid test would translate into an improvement in follow-up.
Neither Dr. Miller nor Dr. Hoerger were involved in the study.
Evaluating the approach
To determine whether a lower-cost rapid test could be cost effective, the researchers created a microsimulation model of the natural history of HCV to compare potential outcomes from either core antigen rapid diagnostic testing with a base case sensitivity for HCV viremia of 80% with lab-based RNA confirmation for negative results or the current standard of care with lab-based RNA confirmation only.
The model incorporated METAVIR stage F0-F4, decompensated cirrhosis, hepatocellular carcinoma, and liver-related death. The investigators determined the baseline characteristics of HCV patients in each country based on different distributions of sex, HCV genotype, and METAVIR fibrosis stage.
They simulated outcomes for 10,000 adults in the Republic of Georgia, with an HCV prevalence of 5.4%, and Malaysia, with an HCV prevalence of 1.5%.
The model considers costs from a health care payer’s perspective, and the investigations performed deterministic and probabilistic sensitivity analyses to evaluate how the cost-effectiveness of testing pathways might change when various factors were plugged into the model.
As noted before, the investigators determined that the core antigen rapid test algorithm would improve diagnosis rates in Georgia from 78.8% to 95.4% and in Malaysia from 57.9% to 91.2%.
The use of the rapid test would also increase quality-adjusted life-years in Georgia by 207 per 10,000 and in Malaysia by 146 per 10,000.
Cost savings, primarily from averting the costs of care for patients with HCV, begin within the first year of the model. Over 50 years, the lifetime horizon cost savings in Georgia would be $232,000 per 10,000 people, and the corresponding savings in Malaysia would be $504,000 per 10,000 people.
Even when allowing for variations in parameters, the core antigen rapid diagnostic test approach remained the preferred model, the investigators reported.
The study was supported by the global health agency Unitaid. The researchers, Dr. Miller, and Dr. Hoerger reported no conflicts of interest relevant to the study.
A proposed rapid diagnostic test for hepatitis C viral infections that combines an inexpensive but lower-sensitivity core-antigen test with lab RNA confirmation of negative tests could expand testing and same-day initiation of antiviral therapy in places where resources are limited, investigators said.
Applying the proposed method to the Republic of Georgia, with a hepatitis C virus (HCV) prevalence of 5.4% as reported by the World Health Organization, would result in a 95.4% diagnosis rate, compared with 78.8% for lab-based RNA testing, which is the standard of care. Applied to Malaysia, the proposed method would boost diagnosis rates from 57.0% to 91.2%, reported Madeline Adee, MPH, from Massachusetts General Hospital’s Institute for Technology Assessment in Boston and colleagues.
“We found that a novel core antigen rapid diagnostic test for HCV could improve the diagnosis rate and result in cost savings. Although not yet developed, such a test could be a game changer and have a substantial impact on the feasibility and cost of HCV elimination, especially in low and middle-income countries,” they wrote in a poster presented at the meeting sponsored by the European Association for the Study of the Liver.
Although rapid diagnostic tests for HCV can improve diagnosis and treatment rates, currently available molecular tests are expensive and require a solid clinical laboratory infrastructure, which can put such tests out of the reach for clinicians in low- or middle-income countries. Rapid immunoassays based on HCV core antigens are comparative bargains, but their sensitivity ranges from 70% to 90%; in contrast, the third-generation HCV enzyme immunoassay has about a 98% sensitivity.
Could it work?
The proposed testing method would be likely to improve diagnosis, but whether that would translate into increased treatment is uncertain, commented Lesley Miller, MD, who specializes in HCV screening and treatment in underserved populations at Emory University, Atlanta.
“When we’re talking about hepatitis C, it’s all about the care cascade, the drop-off at each step from those who have the disease and aren’t diagnosed, to those who are tested and only partially diagnosed because they don’t have a confirmed infection, to those that get into care, get treated, and get cured,” she said in an interview.
“It’s all about closing the gaps in the care cascade in order to achieve elimination of the virus, which is what we’re all trying to do,” she added.
She pointed that there are certain at-risk populations in the United States, such as injectable-drug users, who might be able to benefit from such a system.
“These folks often have less access to traditional care, so bringing rapid testing and care to where those folks are is really important, so if we can deploy mobile units to areas where there is high prevalence and do it at the point of care, it simplifies the entire process,” she said.
Thomas J. Hoerger, PhD, a senior fellow in health economics and financing at the nonprofit research group RTI International in Research Triangle Park, N.C., said in an interview that the proposed model could eliminate the step in testing in which patients are required to return for confirmation.
“People don’t always come back for further testing, so if you can do it immediately and have the results of a screening test, you might be able to get people to come back more quickly. You still have the problem of the high cost of treatment, but this would at least make it a little more convenient,” he said.
He noted that the success of the strategy would be dependent on the sensitivity of the rapid core antigen test, it’s cost relative to HCV RNA testing, and whether the availability of the rapid test would translate into an improvement in follow-up.
Neither Dr. Miller nor Dr. Hoerger were involved in the study.
Evaluating the approach
To determine whether a lower-cost rapid test could be cost effective, the researchers created a microsimulation model of the natural history of HCV to compare potential outcomes from either core antigen rapid diagnostic testing with a base case sensitivity for HCV viremia of 80% with lab-based RNA confirmation for negative results or the current standard of care with lab-based RNA confirmation only.
The model incorporated METAVIR stage F0-F4, decompensated cirrhosis, hepatocellular carcinoma, and liver-related death. The investigators determined the baseline characteristics of HCV patients in each country based on different distributions of sex, HCV genotype, and METAVIR fibrosis stage.
They simulated outcomes for 10,000 adults in the Republic of Georgia, with an HCV prevalence of 5.4%, and Malaysia, with an HCV prevalence of 1.5%.
The model considers costs from a health care payer’s perspective, and the investigations performed deterministic and probabilistic sensitivity analyses to evaluate how the cost-effectiveness of testing pathways might change when various factors were plugged into the model.
As noted before, the investigators determined that the core antigen rapid test algorithm would improve diagnosis rates in Georgia from 78.8% to 95.4% and in Malaysia from 57.9% to 91.2%.
The use of the rapid test would also increase quality-adjusted life-years in Georgia by 207 per 10,000 and in Malaysia by 146 per 10,000.
Cost savings, primarily from averting the costs of care for patients with HCV, begin within the first year of the model. Over 50 years, the lifetime horizon cost savings in Georgia would be $232,000 per 10,000 people, and the corresponding savings in Malaysia would be $504,000 per 10,000 people.
Even when allowing for variations in parameters, the core antigen rapid diagnostic test approach remained the preferred model, the investigators reported.
The study was supported by the global health agency Unitaid. The researchers, Dr. Miller, and Dr. Hoerger reported no conflicts of interest relevant to the study.
A proposed rapid diagnostic test for hepatitis C viral infections that combines an inexpensive but lower-sensitivity core-antigen test with lab RNA confirmation of negative tests could expand testing and same-day initiation of antiviral therapy in places where resources are limited, investigators said.
Applying the proposed method to the Republic of Georgia, with a hepatitis C virus (HCV) prevalence of 5.4% as reported by the World Health Organization, would result in a 95.4% diagnosis rate, compared with 78.8% for lab-based RNA testing, which is the standard of care. Applied to Malaysia, the proposed method would boost diagnosis rates from 57.0% to 91.2%, reported Madeline Adee, MPH, from Massachusetts General Hospital’s Institute for Technology Assessment in Boston and colleagues.
“We found that a novel core antigen rapid diagnostic test for HCV could improve the diagnosis rate and result in cost savings. Although not yet developed, such a test could be a game changer and have a substantial impact on the feasibility and cost of HCV elimination, especially in low and middle-income countries,” they wrote in a poster presented at the meeting sponsored by the European Association for the Study of the Liver.
Although rapid diagnostic tests for HCV can improve diagnosis and treatment rates, currently available molecular tests are expensive and require a solid clinical laboratory infrastructure, which can put such tests out of the reach for clinicians in low- or middle-income countries. Rapid immunoassays based on HCV core antigens are comparative bargains, but their sensitivity ranges from 70% to 90%; in contrast, the third-generation HCV enzyme immunoassay has about a 98% sensitivity.
Could it work?
The proposed testing method would be likely to improve diagnosis, but whether that would translate into increased treatment is uncertain, commented Lesley Miller, MD, who specializes in HCV screening and treatment in underserved populations at Emory University, Atlanta.
“When we’re talking about hepatitis C, it’s all about the care cascade, the drop-off at each step from those who have the disease and aren’t diagnosed, to those who are tested and only partially diagnosed because they don’t have a confirmed infection, to those that get into care, get treated, and get cured,” she said in an interview.
“It’s all about closing the gaps in the care cascade in order to achieve elimination of the virus, which is what we’re all trying to do,” she added.
She pointed that there are certain at-risk populations in the United States, such as injectable-drug users, who might be able to benefit from such a system.
“These folks often have less access to traditional care, so bringing rapid testing and care to where those folks are is really important, so if we can deploy mobile units to areas where there is high prevalence and do it at the point of care, it simplifies the entire process,” she said.
Thomas J. Hoerger, PhD, a senior fellow in health economics and financing at the nonprofit research group RTI International in Research Triangle Park, N.C., said in an interview that the proposed model could eliminate the step in testing in which patients are required to return for confirmation.
“People don’t always come back for further testing, so if you can do it immediately and have the results of a screening test, you might be able to get people to come back more quickly. You still have the problem of the high cost of treatment, but this would at least make it a little more convenient,” he said.
He noted that the success of the strategy would be dependent on the sensitivity of the rapid core antigen test, it’s cost relative to HCV RNA testing, and whether the availability of the rapid test would translate into an improvement in follow-up.
Neither Dr. Miller nor Dr. Hoerger were involved in the study.
Evaluating the approach
To determine whether a lower-cost rapid test could be cost effective, the researchers created a microsimulation model of the natural history of HCV to compare potential outcomes from either core antigen rapid diagnostic testing with a base case sensitivity for HCV viremia of 80% with lab-based RNA confirmation for negative results or the current standard of care with lab-based RNA confirmation only.
The model incorporated METAVIR stage F0-F4, decompensated cirrhosis, hepatocellular carcinoma, and liver-related death. The investigators determined the baseline characteristics of HCV patients in each country based on different distributions of sex, HCV genotype, and METAVIR fibrosis stage.
They simulated outcomes for 10,000 adults in the Republic of Georgia, with an HCV prevalence of 5.4%, and Malaysia, with an HCV prevalence of 1.5%.
The model considers costs from a health care payer’s perspective, and the investigations performed deterministic and probabilistic sensitivity analyses to evaluate how the cost-effectiveness of testing pathways might change when various factors were plugged into the model.
As noted before, the investigators determined that the core antigen rapid test algorithm would improve diagnosis rates in Georgia from 78.8% to 95.4% and in Malaysia from 57.9% to 91.2%.
The use of the rapid test would also increase quality-adjusted life-years in Georgia by 207 per 10,000 and in Malaysia by 146 per 10,000.
Cost savings, primarily from averting the costs of care for patients with HCV, begin within the first year of the model. Over 50 years, the lifetime horizon cost savings in Georgia would be $232,000 per 10,000 people, and the corresponding savings in Malaysia would be $504,000 per 10,000 people.
Even when allowing for variations in parameters, the core antigen rapid diagnostic test approach remained the preferred model, the investigators reported.
The study was supported by the global health agency Unitaid. The researchers, Dr. Miller, and Dr. Hoerger reported no conflicts of interest relevant to the study.
FROM ILC 2021
Efficacy of Etanercept in the Treatment of Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis
Regarded as dermatologic emergencies, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) represent a spectrum of blistering skin diseases that have a high mortality rate. Because of a misguided immune response to medications or infections, CD8+ T lymphocytes release proinflammatory cytokines, giving rise to the extensive epidermal destruction seen in SJS and TEN. The exact pathogenesis of SJS and TEN is still poorly defined, but studies have proposed that T cells mediate keratinocyte (KC) apoptosis through perforin and granzyme release and activation of the Fas/Fas ligand (FasL). Functioning as a transmembrane death receptor in the tumor necrosis factor (TNF) superfamily, Fas (CD95) activates Fas-associated death domain protein, caspases, and nucleases, resulting in organized cell destruction. Likewise, perforin and granzymes also have been shown to play a similar role in apoptosis via activation of caspases.1
Evidence for the role of TNF-α in SJS and TEN has been supported by findings of elevated levels of TNF-α within the blister fluid, serum, and KC cell surface. Additionally, TNF-α has been shown to upregulate inducible nitric oxide synthase in KCs, causing an accumulation of nitric oxide and subsequent FasL-mediated cell death.1-3 Notably, studies have demonstrated a relative lack of lymphocytes in the tissue of TEN patients despite the extensive destruction that is observed, thus emphasizing the importance of amplification and cell signaling via inflammatory mediators such as TNF-α.1 In this proposed model, T cells release IFN-γ, causing KCs to release TNF-α that subsequently promotes the upregulation of the aforementioned FasL.1 Tumor necrosis factor α also may promote increased MHC class I complex deposition on KC surfaces that may play a role in perforin and granzyme-mediated apoptosis of KCs.1
There is still debate on the standard of care for the treatment of SJS and TEN, attributed to the absence of randomized controlled trials and the rarity of the disease as well as the numerous conflicting studies evaluating potential treatments.1,4 Despite conflicting data to support their use, supportive care and intravenous immunoglobulin (IVIG) continue to be common treatments for SJS and TEN in hospitals worldwide. Elucidation of the role of TNF-α has prompted the use of infliximab and etanercept. In a case series of Italian patients with TEN (average SCORTEN, 3.6) treated with the TNF-α antagonist etanercept, no mortality was observed, which was well below the calculated expected mortality of 46.9%.2 Our retrospective study compared the use of a TNF antagonist to other therapies in the treatment of SJS/TEN. Our data suggest that etanercept is a lifesaving and disease-modifying therapy.
Methods
Twenty-two patients with SJS/TEN were included in this analysis. This included all patients who carried a clinical diagnosis of SJS/TEN with a confirmatory biopsy at our 2 university centers—University of California, Los Angeles, and Keck-LA County-Norris Hospital at the University of Southern California, Los Angeles—from 2013 to 2016. The diagnosis was rendered when a clinical diagnosis of SJS/TEN was given by a dermatologist and a confirmatory biopsy was performed. Every patient given a diagnosis of SJS/TEN at either university system from 2015 onward received an injection of etanercept given the positive results reported by Paradisi et al.2
The 9 patients who presented from 2013 to 2014 to our 2 hospital systems and were given a diagnosis of SJS/TEN received either IVIG or supportive care alone and had an average body surface area (BSA) affected of 23%. The 13 patients who presented from 2015 to 2016 were treated with etanercept in the form of a 50-mg subcutaneous injection given once to the right upper arm. Of this group, 4 patients received dual therapy with both IVIG and etanercept. In the etanercept-treated group (etanercept alone and etanercept plus IVIG), the average BSA affected was 30%. At the time of preliminary diagnosis, all patient medications were evaluated for a possible temporal relationship to the onset of rash and were discontinued if felt to be causative. The causative agent and treatment course for each patient is summarized in Table 1.
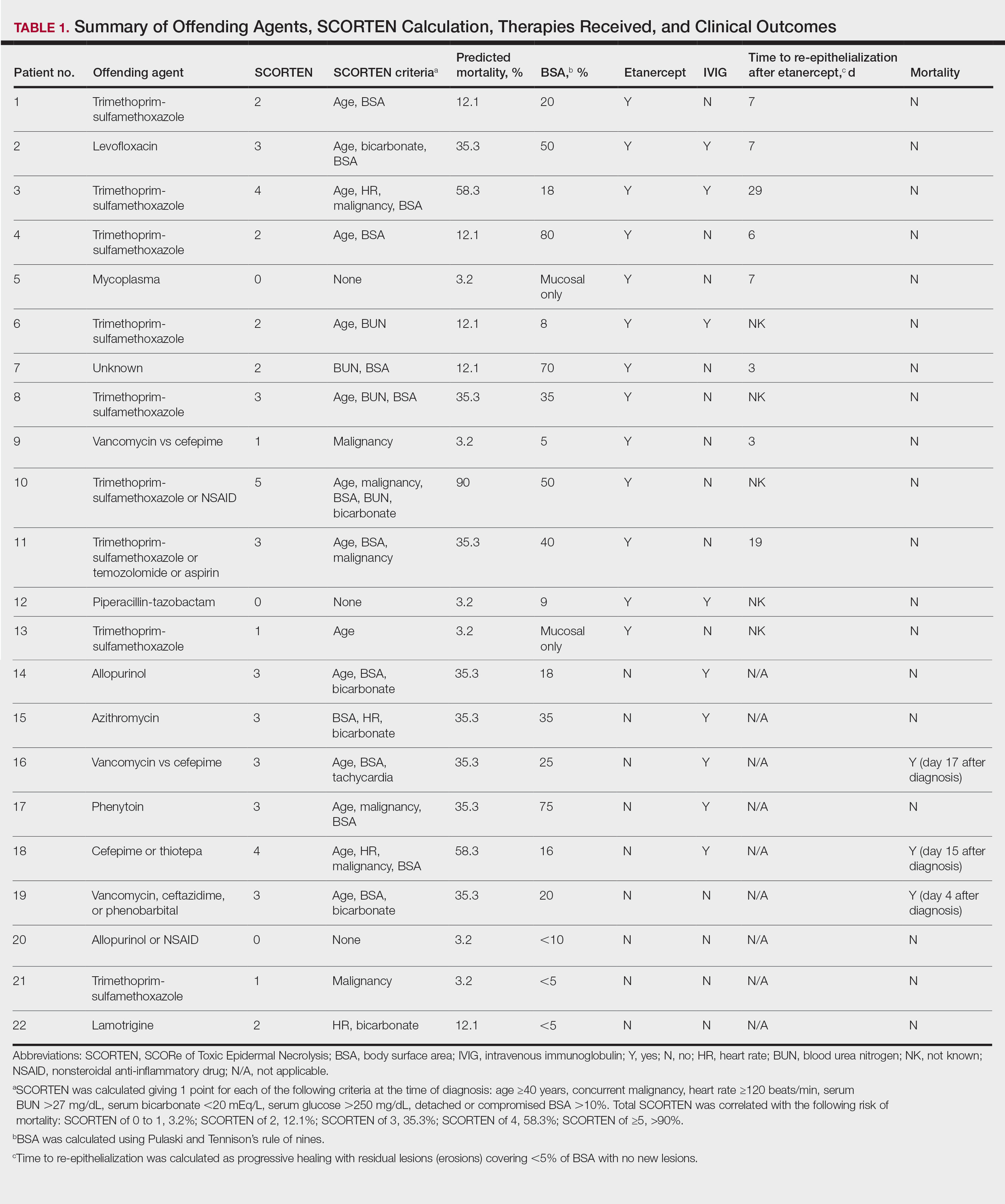
Patients were monitored daily in the hospital for improvement, and time to re-epithelialization was measured. Re-epithelialization was defined as progressive healing with residual lesions (erosions, ulcers, or bullae) covering no more than 5% BSA and was contingent on the patient having no new lesions within 24 hours.5 SCORe of Toxic Epidermal Necrosis (SCORTEN), a validated severity-of-illness score,6 was calculated by giving 1 point for each of the following criteria at the time of diagnosis: age ≥40 years, concurrent malignancy, heart rate ≥120 beats/min, serum blood urea nitrogen >27 mg/dL, serum bicarbonate <20 mEq/L, serum glucose >250 mg/dL, and detached or compromised BSA >10%. The total SCORTEN was correlated with the following risk of mortality as supported by prior validation studies: SCORTEN of 0 to 1, 3.2%; SCORTEN of 2, 12.1%; SCORTEN of 3, 35.3%; SCORTEN of 4, 58.3%; SCORTEN of ≥5, >90%.
Results
A total of 13 patients received etanercept. The mean SCORTEN was 2.2. The observed mortality was 0%, which was markedly lower than the predicted mortality of 24.3% (as determined by linear interpolation). Of this cohort, 9 patients received etanercept alone (mean SCORTEN of 2.1, predicted mortality of 22.9%), whereas 4 patients received a combination of etanercept and IVIG (mean SCORTEN of 2.3, predicted mortality of 27.2%).
The 4 patients who received both etanercept and IVIG received dual therapy for varying reasons. In patient 2 (Table 1), the perceived severity of this case ultimately led to the decision to start IVIG in addition to etanercept, resulting in rapid recovery and discharge after only 1 week of hospitalization. Intravenous immunoglobulin also was given in patient 3 (SCORTEN of 4) and patient 6 (SCORTEN of 2) for progression of disease despite administration of etanercept, with subsequent cessation of progression after the addition of the second agent (IVIG). Patient 12 might have done well on etanercept monotherapy but was administered IVIG as a precautionary measure because of hospital treatment algorithms.
Nine patients did not receive etanercept. Of this group, 5 received IVIG and 4 were managed with supportive care alone. The average SCORTEN for this group was 2.4, only slightly higher than the group that received etanercept (Table 2). The mortality rate in this group was 33%, which was higher than the predicted mortality of 28.1%.
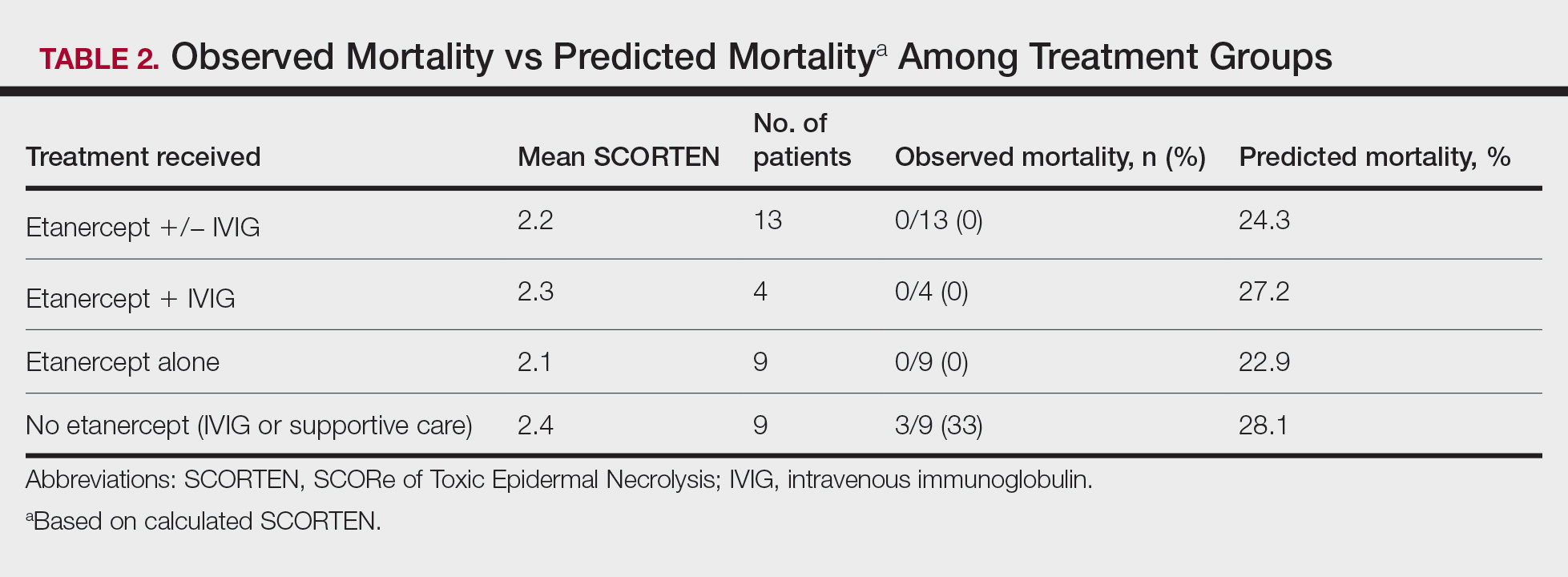
Re-epithelialization data were available for 8 patients who received etanercept. The average time to re-epithelialization for these patients was 8.9 days and ranged from 3 to 19 days. Of these patients, 2 received both IVIG and etanercept, with an average time to re-epithelialization of 13 days. For the 6 patients who received etanercept alone, the average time to re-epithelialization was 7.5 days. Re-epithelialization data were not available for any of the patients who received only IVIG or supportive care but to our recollection ranged from 14 to 21 days.
The clinical course of the 13 patients after the administration of a single dose of etanercept was remarkable, as there was complete absence of mortality and an increase in speed of recovery in most patients receiving this intervention (time to re-epithelialization, 3–19 days). We also observed another interesting trend from our patients treated with etanercept, which was the suggestion that treatment with etanercept may be less effective if IVIG and/or steroids are given prior to etanercept; likewise, treatment is more effective when etanercept is given quickly. For patients 1, 4, 5, 7, 9, and 11 (as shown in Table 1), no prior IVIG therapy or other immunosuppressive therapy had been given before etanercept was administered. In these 6 patients, the average time to re-epithelialization after etanercept administration was 7.5 days; average time to re-epithelialization, unfortunately, is not available for the patients who were not treated with etanercept. In addition, as shown in the Figure, it was noted in some patients that the depth of denudation was markedly more superficial than what would typically be clinically observed with TEN after administration of other immunomodulatory therapies such as IVIG or prednisone or with supportive care alone. In these 2 patients with superficial desquamation—patients 7 and 9—etanercept notably was given within 6 hours of onset of skin pain.
Comment
There is no definitive gold standard treatment of SJS, SJS/TEN overlap, or TEN. However, generally agreed upon management includes immediate discontinuation of the offending medication and supportive therapy with aggressive electrolyte replacement and wound care. Management in a burn unit or intensive care unit is recommended in severe cases. Contention over the efficacy of various medications in the treatment of SJS and TEN continues and largely is due to the rarity of SJS and TEN; studies are small and almost all lack randomization. Therapies that have been used include high-dose steroids, IVIG, plasmapheresis, cyclophosphamide, cyclosporine A, and TNF inhibitors (eg, etanercept, infliximab).1
Evidence for the use of anti–TNF-α antibodies has been limited thus far, with most of the literature focusing on infliximab and etanercept. Adalimumab, a fully humanized clonal antibody, has no reported cases in the dermatologic literature for use in patients with SJS/TEN. Two case reports of adalimumab paradoxically causing SJS have been documented. In both cases, adalimumab was stopped and patients responded to intravenous corticosteroids and infliximab.7,8 Similarly, thalidomide has not proven to be a promising anti–TNF-α agent for the treatment of SJS/TEN. In the only attempted randomized controlled trial for SJS and TEN, thalidomide appeared to increase mortality, eventuating in this trial being terminated prior to the planned end date.9Infliximab and etanercept have several case reports and a few case series highlighting potentially efficacious application of TNF-α inhibitors for the treatment of SJS/TEN.10-13 In 2002, Fischer et al10 reported the first case of TEN treated successfully with a single dose of infliximab 5 mg/kg. Kreft et al14 reported on etoricoxib-induced TEN that was treated with infliximab 5 mg/kg, which led to re-epithelialization within 5 weeks (notably a 5-week re-epithelialization time is not necessarily an improvement).
In 2005, Hunger et al3 demonstrated TNF-α’s release by KCs in the epidermis and by inflammatory cells in the dermis of a TEN patient. Twenty-four hours after the administration of infliximab 5 mg/kg in these patients, TNF-α was found to be below normal and epidermal detachment ceased.3 Wojtkietwicz et al13 demonstrated benefit following an infusion of infliximab 5 mg/kg in a patient whose disease continued to progress despite treatment with dexamethasone and 1.8 g/kg of IVIG.
Then 2 subsequent case series added further support for the efficacy of infliximab in the treatment of TEN. Patmanidis et al15 and Gaitanis et al16 reported similar results in 4 patients, each treated with infliximab 5 mg/kg immediately followed by initiation of high-dose IVIG (2 g/kg over 5 days). Zárate-Correa et al17 reported a 0% mortality rate and near-complete re-epithelialization after 5 to 14 days in 4 patients treated with a single 300-mg dose of infliximab.
However, the success of infliximab in the treatment of TEN has been countered by the pilot study by Paquet et al,18 which compared the efficacy of 150 mg/kg of N-acetylcysteine alone vs adding infliximab 5 mg/kg to treat 10 TEN patients. The study demonstrated no benefit at 48 hours in the group given infliximab, the time frame in which prior case reports touting infliximab’s benefit claimed the benefit was observed. Similarly, there was no effect on mortality for either treatment modality as assessed by illness auxiliary score.18
Evidence in support of the use of etanercept in the treatment of SJS/TEN is mounting, and some centers have begun to use it as the first-choice therapy for SJS/TEN. The first case was reported by Famularo et al,19 in which a patient with TEN was given 2 doses of etanercept 25 mg after failure to improve with prednisolone 1 mg/kg. The patient showed near-complete and rapid re-epithelization in 6 days before death due to disseminated intravascular coagulation 10 days after admission.19 Gubinelli et al20 and Sadighha21 independently reported cases of TEN and TEN/acute generalized exanthematous pustulosis overlap treated with a total of 50 mg of etanercept, demonstrating rapid cessation of lesion progression. Didona et al22 found similar benefit using etanercept 50 mg to treat TEN secondary to rituximab after failure to improve with prednisone and cyclophosphamide. Treatment of TEN with etanercept in an HIV-positive patient also has been reported. Lee et al23 described a patient who was administered 50-mg and 25-mg injections on days 3 and 5 of hospitalization, respectively, with re-epithelialization occurring by day 8. Finally, Owczarczyk-Saczonek et al24 reported a case of SJS in a patient with a 4-year history of etanercept and sulfasalazine treatment of rheumatoid arthritis; sulfasalazine was stopped, but this patient was continued on etanercept until resolution of skin and mucosal symptoms. However, it is important to consider the possibility of publication bias among these cases selected for their positive outcomes.
Perhaps the most compelling literature regarding the use of etanercept for TEN was described in a case series by Paradisi et al.2 This study included 10 patients with TEN, all of whom demonstrated complete re-epithelialization shortly after receiving etanercept 50 mg. Average SCORTEN was 3.6 with a range of 2 to 6. Eight patients in this study had severe comorbidities and all 10 patients survived, with a time to re-epithelialization ranging from 7 to 20 days.2 Additionally, a randomized controlled trial showed that 38 etanercept-treated patients had improved mortality (P=.266) and re-epithelialization time (P=.01) compared to patients treated with intravenous methylprednisolone.25Limitations to our study are similar to other reports of SJS/TEN and included the small number of cases and lack of randomization. Additionally, we do not have data available for all patients for time between onset of disease and treatment initiation. Because of these challenges, data presented in this case series is observational only. Additionally, the patients treated with etanercept alone had a slightly lower SCORTEN compared to the group that received IVIG or supportive care alone (2.1 and 2.4 respectively). However, the etanercept-only group actually had higher involvement of epidermal detachment (33%) compared to the non-etanercept group (23%).
Conclusion
Although treatment with etanercept lacks the support of a randomized controlled trial, similar to all other treatments currently used for SJS and TEN, preliminary reports highlight a benefit in disease progression and improvement in time to re-epithelialization. In particular, if etanercept 50 mg subcutaneously is given as monotherapy or is given early in the disease course (prior to other therapies being attempted and ideally within 6 hours of presentation), our data suggest an even greater trend toward improved mortality and decreased time to re-epithelialization. Additionally, our findings may suggest that in some patients, etanercept monotherapy is not an adequate intervention but the addition of IVIG may be helpful; however, the senior author (S.W.) notes anecdotally that in his experience with the patients treated at the University of California Los Angeles, the order of administration of combination therapies—etanercept followed by IVIG—was important in addition to the choice of therapy. These findings are promising enough to warrant a multicenter randomized controlled trial comparing the efficacy of etanercept to other more commonly used treatments for this spectrum of disease, including IVIG and/or cyclosporine. Based on the data presented in this case series, including the 13 patients who received etanercept and had a 0% mortality rate, etanercept may be viewed as a targeted therapeutic intervention for patients with SJS and TEN.
- Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56:181-200.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Hunger RE, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-α treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Worswick S, Cotliar J. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of treatment options. Dermatol Ther. 2011;24:207-218.
- Wallace AB. The exposure treatment of burns. Lancet Lond Engl. 1951;1:501-504.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Mounach A, Rezqi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
- Salama M, Lawrance I-C. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet Lond Engl. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch WC, et al Antitumour necrosis factor-α antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-709.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumour necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Al-Shouli S, Abouchala N, Bogusz MJ, et al. Toxic epidermal necrolysis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-535.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulin. Dermatol Basel Switz. 2012;224:134-139.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paquet P, Jennes S, Rousseau AF, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. a proof-of-concept study. Burns J Int Soc Burn Inj. 2014;40:1707-1712.
- Famularo G, Dona BD, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Didona D, Paolino G, Garcovich S, et al. Successful use of etanercept in a case of toxic epidermal necrolysis induced by rituximab. J Eur Acad Dermatol Venereol. 2016;30:E83-E84.
- Lee Y-Y, Ko J-H, Wei C-H, et al. Use of etanercept to treat toxic epidermal necrolysis in a human immunodeficiency virus-positive patient. Dermatol Sin. 2013;31:78-81.
- Owczarczyk-Saczonek A, Zdanowska N, Znajewska-Pander A, et al. Stevens-Johnson syndrome in a patient with rheumatoid arthritis during long-term etanercept therapy. J Dermatol Case Rep. 2016;10:14-16.
- Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF-α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
Regarded as dermatologic emergencies, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) represent a spectrum of blistering skin diseases that have a high mortality rate. Because of a misguided immune response to medications or infections, CD8+ T lymphocytes release proinflammatory cytokines, giving rise to the extensive epidermal destruction seen in SJS and TEN. The exact pathogenesis of SJS and TEN is still poorly defined, but studies have proposed that T cells mediate keratinocyte (KC) apoptosis through perforin and granzyme release and activation of the Fas/Fas ligand (FasL). Functioning as a transmembrane death receptor in the tumor necrosis factor (TNF) superfamily, Fas (CD95) activates Fas-associated death domain protein, caspases, and nucleases, resulting in organized cell destruction. Likewise, perforin and granzymes also have been shown to play a similar role in apoptosis via activation of caspases.1
Evidence for the role of TNF-α in SJS and TEN has been supported by findings of elevated levels of TNF-α within the blister fluid, serum, and KC cell surface. Additionally, TNF-α has been shown to upregulate inducible nitric oxide synthase in KCs, causing an accumulation of nitric oxide and subsequent FasL-mediated cell death.1-3 Notably, studies have demonstrated a relative lack of lymphocytes in the tissue of TEN patients despite the extensive destruction that is observed, thus emphasizing the importance of amplification and cell signaling via inflammatory mediators such as TNF-α.1 In this proposed model, T cells release IFN-γ, causing KCs to release TNF-α that subsequently promotes the upregulation of the aforementioned FasL.1 Tumor necrosis factor α also may promote increased MHC class I complex deposition on KC surfaces that may play a role in perforin and granzyme-mediated apoptosis of KCs.1
There is still debate on the standard of care for the treatment of SJS and TEN, attributed to the absence of randomized controlled trials and the rarity of the disease as well as the numerous conflicting studies evaluating potential treatments.1,4 Despite conflicting data to support their use, supportive care and intravenous immunoglobulin (IVIG) continue to be common treatments for SJS and TEN in hospitals worldwide. Elucidation of the role of TNF-α has prompted the use of infliximab and etanercept. In a case series of Italian patients with TEN (average SCORTEN, 3.6) treated with the TNF-α antagonist etanercept, no mortality was observed, which was well below the calculated expected mortality of 46.9%.2 Our retrospective study compared the use of a TNF antagonist to other therapies in the treatment of SJS/TEN. Our data suggest that etanercept is a lifesaving and disease-modifying therapy.
Methods
Twenty-two patients with SJS/TEN were included in this analysis. This included all patients who carried a clinical diagnosis of SJS/TEN with a confirmatory biopsy at our 2 university centers—University of California, Los Angeles, and Keck-LA County-Norris Hospital at the University of Southern California, Los Angeles—from 2013 to 2016. The diagnosis was rendered when a clinical diagnosis of SJS/TEN was given by a dermatologist and a confirmatory biopsy was performed. Every patient given a diagnosis of SJS/TEN at either university system from 2015 onward received an injection of etanercept given the positive results reported by Paradisi et al.2
The 9 patients who presented from 2013 to 2014 to our 2 hospital systems and were given a diagnosis of SJS/TEN received either IVIG or supportive care alone and had an average body surface area (BSA) affected of 23%. The 13 patients who presented from 2015 to 2016 were treated with etanercept in the form of a 50-mg subcutaneous injection given once to the right upper arm. Of this group, 4 patients received dual therapy with both IVIG and etanercept. In the etanercept-treated group (etanercept alone and etanercept plus IVIG), the average BSA affected was 30%. At the time of preliminary diagnosis, all patient medications were evaluated for a possible temporal relationship to the onset of rash and were discontinued if felt to be causative. The causative agent and treatment course for each patient is summarized in Table 1.

Patients were monitored daily in the hospital for improvement, and time to re-epithelialization was measured. Re-epithelialization was defined as progressive healing with residual lesions (erosions, ulcers, or bullae) covering no more than 5% BSA and was contingent on the patient having no new lesions within 24 hours.5 SCORe of Toxic Epidermal Necrosis (SCORTEN), a validated severity-of-illness score,6 was calculated by giving 1 point for each of the following criteria at the time of diagnosis: age ≥40 years, concurrent malignancy, heart rate ≥120 beats/min, serum blood urea nitrogen >27 mg/dL, serum bicarbonate <20 mEq/L, serum glucose >250 mg/dL, and detached or compromised BSA >10%. The total SCORTEN was correlated with the following risk of mortality as supported by prior validation studies: SCORTEN of 0 to 1, 3.2%; SCORTEN of 2, 12.1%; SCORTEN of 3, 35.3%; SCORTEN of 4, 58.3%; SCORTEN of ≥5, >90%.
Results
A total of 13 patients received etanercept. The mean SCORTEN was 2.2. The observed mortality was 0%, which was markedly lower than the predicted mortality of 24.3% (as determined by linear interpolation). Of this cohort, 9 patients received etanercept alone (mean SCORTEN of 2.1, predicted mortality of 22.9%), whereas 4 patients received a combination of etanercept and IVIG (mean SCORTEN of 2.3, predicted mortality of 27.2%).
The 4 patients who received both etanercept and IVIG received dual therapy for varying reasons. In patient 2 (Table 1), the perceived severity of this case ultimately led to the decision to start IVIG in addition to etanercept, resulting in rapid recovery and discharge after only 1 week of hospitalization. Intravenous immunoglobulin also was given in patient 3 (SCORTEN of 4) and patient 6 (SCORTEN of 2) for progression of disease despite administration of etanercept, with subsequent cessation of progression after the addition of the second agent (IVIG). Patient 12 might have done well on etanercept monotherapy but was administered IVIG as a precautionary measure because of hospital treatment algorithms.
Nine patients did not receive etanercept. Of this group, 5 received IVIG and 4 were managed with supportive care alone. The average SCORTEN for this group was 2.4, only slightly higher than the group that received etanercept (Table 2). The mortality rate in this group was 33%, which was higher than the predicted mortality of 28.1%.

Re-epithelialization data were available for 8 patients who received etanercept. The average time to re-epithelialization for these patients was 8.9 days and ranged from 3 to 19 days. Of these patients, 2 received both IVIG and etanercept, with an average time to re-epithelialization of 13 days. For the 6 patients who received etanercept alone, the average time to re-epithelialization was 7.5 days. Re-epithelialization data were not available for any of the patients who received only IVIG or supportive care but to our recollection ranged from 14 to 21 days.
The clinical course of the 13 patients after the administration of a single dose of etanercept was remarkable, as there was complete absence of mortality and an increase in speed of recovery in most patients receiving this intervention (time to re-epithelialization, 3–19 days). We also observed another interesting trend from our patients treated with etanercept, which was the suggestion that treatment with etanercept may be less effective if IVIG and/or steroids are given prior to etanercept; likewise, treatment is more effective when etanercept is given quickly. For patients 1, 4, 5, 7, 9, and 11 (as shown in Table 1), no prior IVIG therapy or other immunosuppressive therapy had been given before etanercept was administered. In these 6 patients, the average time to re-epithelialization after etanercept administration was 7.5 days; average time to re-epithelialization, unfortunately, is not available for the patients who were not treated with etanercept. In addition, as shown in the Figure, it was noted in some patients that the depth of denudation was markedly more superficial than what would typically be clinically observed with TEN after administration of other immunomodulatory therapies such as IVIG or prednisone or with supportive care alone. In these 2 patients with superficial desquamation—patients 7 and 9—etanercept notably was given within 6 hours of onset of skin pain.

Comment
There is no definitive gold standard treatment of SJS, SJS/TEN overlap, or TEN. However, generally agreed upon management includes immediate discontinuation of the offending medication and supportive therapy with aggressive electrolyte replacement and wound care. Management in a burn unit or intensive care unit is recommended in severe cases. Contention over the efficacy of various medications in the treatment of SJS and TEN continues and largely is due to the rarity of SJS and TEN; studies are small and almost all lack randomization. Therapies that have been used include high-dose steroids, IVIG, plasmapheresis, cyclophosphamide, cyclosporine A, and TNF inhibitors (eg, etanercept, infliximab).1
Evidence for the use of anti–TNF-α antibodies has been limited thus far, with most of the literature focusing on infliximab and etanercept. Adalimumab, a fully humanized clonal antibody, has no reported cases in the dermatologic literature for use in patients with SJS/TEN. Two case reports of adalimumab paradoxically causing SJS have been documented. In both cases, adalimumab was stopped and patients responded to intravenous corticosteroids and infliximab.7,8 Similarly, thalidomide has not proven to be a promising anti–TNF-α agent for the treatment of SJS/TEN. In the only attempted randomized controlled trial for SJS and TEN, thalidomide appeared to increase mortality, eventuating in this trial being terminated prior to the planned end date.9Infliximab and etanercept have several case reports and a few case series highlighting potentially efficacious application of TNF-α inhibitors for the treatment of SJS/TEN.10-13 In 2002, Fischer et al10 reported the first case of TEN treated successfully with a single dose of infliximab 5 mg/kg. Kreft et al14 reported on etoricoxib-induced TEN that was treated with infliximab 5 mg/kg, which led to re-epithelialization within 5 weeks (notably a 5-week re-epithelialization time is not necessarily an improvement).
In 2005, Hunger et al3 demonstrated TNF-α’s release by KCs in the epidermis and by inflammatory cells in the dermis of a TEN patient. Twenty-four hours after the administration of infliximab 5 mg/kg in these patients, TNF-α was found to be below normal and epidermal detachment ceased.3 Wojtkietwicz et al13 demonstrated benefit following an infusion of infliximab 5 mg/kg in a patient whose disease continued to progress despite treatment with dexamethasone and 1.8 g/kg of IVIG.
Then 2 subsequent case series added further support for the efficacy of infliximab in the treatment of TEN. Patmanidis et al15 and Gaitanis et al16 reported similar results in 4 patients, each treated with infliximab 5 mg/kg immediately followed by initiation of high-dose IVIG (2 g/kg over 5 days). Zárate-Correa et al17 reported a 0% mortality rate and near-complete re-epithelialization after 5 to 14 days in 4 patients treated with a single 300-mg dose of infliximab.
However, the success of infliximab in the treatment of TEN has been countered by the pilot study by Paquet et al,18 which compared the efficacy of 150 mg/kg of N-acetylcysteine alone vs adding infliximab 5 mg/kg to treat 10 TEN patients. The study demonstrated no benefit at 48 hours in the group given infliximab, the time frame in which prior case reports touting infliximab’s benefit claimed the benefit was observed. Similarly, there was no effect on mortality for either treatment modality as assessed by illness auxiliary score.18
Evidence in support of the use of etanercept in the treatment of SJS/TEN is mounting, and some centers have begun to use it as the first-choice therapy for SJS/TEN. The first case was reported by Famularo et al,19 in which a patient with TEN was given 2 doses of etanercept 25 mg after failure to improve with prednisolone 1 mg/kg. The patient showed near-complete and rapid re-epithelization in 6 days before death due to disseminated intravascular coagulation 10 days after admission.19 Gubinelli et al20 and Sadighha21 independently reported cases of TEN and TEN/acute generalized exanthematous pustulosis overlap treated with a total of 50 mg of etanercept, demonstrating rapid cessation of lesion progression. Didona et al22 found similar benefit using etanercept 50 mg to treat TEN secondary to rituximab after failure to improve with prednisone and cyclophosphamide. Treatment of TEN with etanercept in an HIV-positive patient also has been reported. Lee et al23 described a patient who was administered 50-mg and 25-mg injections on days 3 and 5 of hospitalization, respectively, with re-epithelialization occurring by day 8. Finally, Owczarczyk-Saczonek et al24 reported a case of SJS in a patient with a 4-year history of etanercept and sulfasalazine treatment of rheumatoid arthritis; sulfasalazine was stopped, but this patient was continued on etanercept until resolution of skin and mucosal symptoms. However, it is important to consider the possibility of publication bias among these cases selected for their positive outcomes.
Perhaps the most compelling literature regarding the use of etanercept for TEN was described in a case series by Paradisi et al.2 This study included 10 patients with TEN, all of whom demonstrated complete re-epithelialization shortly after receiving etanercept 50 mg. Average SCORTEN was 3.6 with a range of 2 to 6. Eight patients in this study had severe comorbidities and all 10 patients survived, with a time to re-epithelialization ranging from 7 to 20 days.2 Additionally, a randomized controlled trial showed that 38 etanercept-treated patients had improved mortality (P=.266) and re-epithelialization time (P=.01) compared to patients treated with intravenous methylprednisolone.25Limitations to our study are similar to other reports of SJS/TEN and included the small number of cases and lack of randomization. Additionally, we do not have data available for all patients for time between onset of disease and treatment initiation. Because of these challenges, data presented in this case series is observational only. Additionally, the patients treated with etanercept alone had a slightly lower SCORTEN compared to the group that received IVIG or supportive care alone (2.1 and 2.4 respectively). However, the etanercept-only group actually had higher involvement of epidermal detachment (33%) compared to the non-etanercept group (23%).
Conclusion
Although treatment with etanercept lacks the support of a randomized controlled trial, similar to all other treatments currently used for SJS and TEN, preliminary reports highlight a benefit in disease progression and improvement in time to re-epithelialization. In particular, if etanercept 50 mg subcutaneously is given as monotherapy or is given early in the disease course (prior to other therapies being attempted and ideally within 6 hours of presentation), our data suggest an even greater trend toward improved mortality and decreased time to re-epithelialization. Additionally, our findings may suggest that in some patients, etanercept monotherapy is not an adequate intervention but the addition of IVIG may be helpful; however, the senior author (S.W.) notes anecdotally that in his experience with the patients treated at the University of California Los Angeles, the order of administration of combination therapies—etanercept followed by IVIG—was important in addition to the choice of therapy. These findings are promising enough to warrant a multicenter randomized controlled trial comparing the efficacy of etanercept to other more commonly used treatments for this spectrum of disease, including IVIG and/or cyclosporine. Based on the data presented in this case series, including the 13 patients who received etanercept and had a 0% mortality rate, etanercept may be viewed as a targeted therapeutic intervention for patients with SJS and TEN.
Regarded as dermatologic emergencies, Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) represent a spectrum of blistering skin diseases that have a high mortality rate. Because of a misguided immune response to medications or infections, CD8+ T lymphocytes release proinflammatory cytokines, giving rise to the extensive epidermal destruction seen in SJS and TEN. The exact pathogenesis of SJS and TEN is still poorly defined, but studies have proposed that T cells mediate keratinocyte (KC) apoptosis through perforin and granzyme release and activation of the Fas/Fas ligand (FasL). Functioning as a transmembrane death receptor in the tumor necrosis factor (TNF) superfamily, Fas (CD95) activates Fas-associated death domain protein, caspases, and nucleases, resulting in organized cell destruction. Likewise, perforin and granzymes also have been shown to play a similar role in apoptosis via activation of caspases.1
Evidence for the role of TNF-α in SJS and TEN has been supported by findings of elevated levels of TNF-α within the blister fluid, serum, and KC cell surface. Additionally, TNF-α has been shown to upregulate inducible nitric oxide synthase in KCs, causing an accumulation of nitric oxide and subsequent FasL-mediated cell death.1-3 Notably, studies have demonstrated a relative lack of lymphocytes in the tissue of TEN patients despite the extensive destruction that is observed, thus emphasizing the importance of amplification and cell signaling via inflammatory mediators such as TNF-α.1 In this proposed model, T cells release IFN-γ, causing KCs to release TNF-α that subsequently promotes the upregulation of the aforementioned FasL.1 Tumor necrosis factor α also may promote increased MHC class I complex deposition on KC surfaces that may play a role in perforin and granzyme-mediated apoptosis of KCs.1
There is still debate on the standard of care for the treatment of SJS and TEN, attributed to the absence of randomized controlled trials and the rarity of the disease as well as the numerous conflicting studies evaluating potential treatments.1,4 Despite conflicting data to support their use, supportive care and intravenous immunoglobulin (IVIG) continue to be common treatments for SJS and TEN in hospitals worldwide. Elucidation of the role of TNF-α has prompted the use of infliximab and etanercept. In a case series of Italian patients with TEN (average SCORTEN, 3.6) treated with the TNF-α antagonist etanercept, no mortality was observed, which was well below the calculated expected mortality of 46.9%.2 Our retrospective study compared the use of a TNF antagonist to other therapies in the treatment of SJS/TEN. Our data suggest that etanercept is a lifesaving and disease-modifying therapy.
Methods
Twenty-two patients with SJS/TEN were included in this analysis. This included all patients who carried a clinical diagnosis of SJS/TEN with a confirmatory biopsy at our 2 university centers—University of California, Los Angeles, and Keck-LA County-Norris Hospital at the University of Southern California, Los Angeles—from 2013 to 2016. The diagnosis was rendered when a clinical diagnosis of SJS/TEN was given by a dermatologist and a confirmatory biopsy was performed. Every patient given a diagnosis of SJS/TEN at either university system from 2015 onward received an injection of etanercept given the positive results reported by Paradisi et al.2
The 9 patients who presented from 2013 to 2014 to our 2 hospital systems and were given a diagnosis of SJS/TEN received either IVIG or supportive care alone and had an average body surface area (BSA) affected of 23%. The 13 patients who presented from 2015 to 2016 were treated with etanercept in the form of a 50-mg subcutaneous injection given once to the right upper arm. Of this group, 4 patients received dual therapy with both IVIG and etanercept. In the etanercept-treated group (etanercept alone and etanercept plus IVIG), the average BSA affected was 30%. At the time of preliminary diagnosis, all patient medications were evaluated for a possible temporal relationship to the onset of rash and were discontinued if felt to be causative. The causative agent and treatment course for each patient is summarized in Table 1.

Patients were monitored daily in the hospital for improvement, and time to re-epithelialization was measured. Re-epithelialization was defined as progressive healing with residual lesions (erosions, ulcers, or bullae) covering no more than 5% BSA and was contingent on the patient having no new lesions within 24 hours.5 SCORe of Toxic Epidermal Necrosis (SCORTEN), a validated severity-of-illness score,6 was calculated by giving 1 point for each of the following criteria at the time of diagnosis: age ≥40 years, concurrent malignancy, heart rate ≥120 beats/min, serum blood urea nitrogen >27 mg/dL, serum bicarbonate <20 mEq/L, serum glucose >250 mg/dL, and detached or compromised BSA >10%. The total SCORTEN was correlated with the following risk of mortality as supported by prior validation studies: SCORTEN of 0 to 1, 3.2%; SCORTEN of 2, 12.1%; SCORTEN of 3, 35.3%; SCORTEN of 4, 58.3%; SCORTEN of ≥5, >90%.
Results
A total of 13 patients received etanercept. The mean SCORTEN was 2.2. The observed mortality was 0%, which was markedly lower than the predicted mortality of 24.3% (as determined by linear interpolation). Of this cohort, 9 patients received etanercept alone (mean SCORTEN of 2.1, predicted mortality of 22.9%), whereas 4 patients received a combination of etanercept and IVIG (mean SCORTEN of 2.3, predicted mortality of 27.2%).
The 4 patients who received both etanercept and IVIG received dual therapy for varying reasons. In patient 2 (Table 1), the perceived severity of this case ultimately led to the decision to start IVIG in addition to etanercept, resulting in rapid recovery and discharge after only 1 week of hospitalization. Intravenous immunoglobulin also was given in patient 3 (SCORTEN of 4) and patient 6 (SCORTEN of 2) for progression of disease despite administration of etanercept, with subsequent cessation of progression after the addition of the second agent (IVIG). Patient 12 might have done well on etanercept monotherapy but was administered IVIG as a precautionary measure because of hospital treatment algorithms.
Nine patients did not receive etanercept. Of this group, 5 received IVIG and 4 were managed with supportive care alone. The average SCORTEN for this group was 2.4, only slightly higher than the group that received etanercept (Table 2). The mortality rate in this group was 33%, which was higher than the predicted mortality of 28.1%.

Re-epithelialization data were available for 8 patients who received etanercept. The average time to re-epithelialization for these patients was 8.9 days and ranged from 3 to 19 days. Of these patients, 2 received both IVIG and etanercept, with an average time to re-epithelialization of 13 days. For the 6 patients who received etanercept alone, the average time to re-epithelialization was 7.5 days. Re-epithelialization data were not available for any of the patients who received only IVIG or supportive care but to our recollection ranged from 14 to 21 days.
The clinical course of the 13 patients after the administration of a single dose of etanercept was remarkable, as there was complete absence of mortality and an increase in speed of recovery in most patients receiving this intervention (time to re-epithelialization, 3–19 days). We also observed another interesting trend from our patients treated with etanercept, which was the suggestion that treatment with etanercept may be less effective if IVIG and/or steroids are given prior to etanercept; likewise, treatment is more effective when etanercept is given quickly. For patients 1, 4, 5, 7, 9, and 11 (as shown in Table 1), no prior IVIG therapy or other immunosuppressive therapy had been given before etanercept was administered. In these 6 patients, the average time to re-epithelialization after etanercept administration was 7.5 days; average time to re-epithelialization, unfortunately, is not available for the patients who were not treated with etanercept. In addition, as shown in the Figure, it was noted in some patients that the depth of denudation was markedly more superficial than what would typically be clinically observed with TEN after administration of other immunomodulatory therapies such as IVIG or prednisone or with supportive care alone. In these 2 patients with superficial desquamation—patients 7 and 9—etanercept notably was given within 6 hours of onset of skin pain.

Comment
There is no definitive gold standard treatment of SJS, SJS/TEN overlap, or TEN. However, generally agreed upon management includes immediate discontinuation of the offending medication and supportive therapy with aggressive electrolyte replacement and wound care. Management in a burn unit or intensive care unit is recommended in severe cases. Contention over the efficacy of various medications in the treatment of SJS and TEN continues and largely is due to the rarity of SJS and TEN; studies are small and almost all lack randomization. Therapies that have been used include high-dose steroids, IVIG, plasmapheresis, cyclophosphamide, cyclosporine A, and TNF inhibitors (eg, etanercept, infliximab).1
Evidence for the use of anti–TNF-α antibodies has been limited thus far, with most of the literature focusing on infliximab and etanercept. Adalimumab, a fully humanized clonal antibody, has no reported cases in the dermatologic literature for use in patients with SJS/TEN. Two case reports of adalimumab paradoxically causing SJS have been documented. In both cases, adalimumab was stopped and patients responded to intravenous corticosteroids and infliximab.7,8 Similarly, thalidomide has not proven to be a promising anti–TNF-α agent for the treatment of SJS/TEN. In the only attempted randomized controlled trial for SJS and TEN, thalidomide appeared to increase mortality, eventuating in this trial being terminated prior to the planned end date.9Infliximab and etanercept have several case reports and a few case series highlighting potentially efficacious application of TNF-α inhibitors for the treatment of SJS/TEN.10-13 In 2002, Fischer et al10 reported the first case of TEN treated successfully with a single dose of infliximab 5 mg/kg. Kreft et al14 reported on etoricoxib-induced TEN that was treated with infliximab 5 mg/kg, which led to re-epithelialization within 5 weeks (notably a 5-week re-epithelialization time is not necessarily an improvement).
In 2005, Hunger et al3 demonstrated TNF-α’s release by KCs in the epidermis and by inflammatory cells in the dermis of a TEN patient. Twenty-four hours after the administration of infliximab 5 mg/kg in these patients, TNF-α was found to be below normal and epidermal detachment ceased.3 Wojtkietwicz et al13 demonstrated benefit following an infusion of infliximab 5 mg/kg in a patient whose disease continued to progress despite treatment with dexamethasone and 1.8 g/kg of IVIG.
Then 2 subsequent case series added further support for the efficacy of infliximab in the treatment of TEN. Patmanidis et al15 and Gaitanis et al16 reported similar results in 4 patients, each treated with infliximab 5 mg/kg immediately followed by initiation of high-dose IVIG (2 g/kg over 5 days). Zárate-Correa et al17 reported a 0% mortality rate and near-complete re-epithelialization after 5 to 14 days in 4 patients treated with a single 300-mg dose of infliximab.
However, the success of infliximab in the treatment of TEN has been countered by the pilot study by Paquet et al,18 which compared the efficacy of 150 mg/kg of N-acetylcysteine alone vs adding infliximab 5 mg/kg to treat 10 TEN patients. The study demonstrated no benefit at 48 hours in the group given infliximab, the time frame in which prior case reports touting infliximab’s benefit claimed the benefit was observed. Similarly, there was no effect on mortality for either treatment modality as assessed by illness auxiliary score.18
Evidence in support of the use of etanercept in the treatment of SJS/TEN is mounting, and some centers have begun to use it as the first-choice therapy for SJS/TEN. The first case was reported by Famularo et al,19 in which a patient with TEN was given 2 doses of etanercept 25 mg after failure to improve with prednisolone 1 mg/kg. The patient showed near-complete and rapid re-epithelization in 6 days before death due to disseminated intravascular coagulation 10 days after admission.19 Gubinelli et al20 and Sadighha21 independently reported cases of TEN and TEN/acute generalized exanthematous pustulosis overlap treated with a total of 50 mg of etanercept, demonstrating rapid cessation of lesion progression. Didona et al22 found similar benefit using etanercept 50 mg to treat TEN secondary to rituximab after failure to improve with prednisone and cyclophosphamide. Treatment of TEN with etanercept in an HIV-positive patient also has been reported. Lee et al23 described a patient who was administered 50-mg and 25-mg injections on days 3 and 5 of hospitalization, respectively, with re-epithelialization occurring by day 8. Finally, Owczarczyk-Saczonek et al24 reported a case of SJS in a patient with a 4-year history of etanercept and sulfasalazine treatment of rheumatoid arthritis; sulfasalazine was stopped, but this patient was continued on etanercept until resolution of skin and mucosal symptoms. However, it is important to consider the possibility of publication bias among these cases selected for their positive outcomes.
Perhaps the most compelling literature regarding the use of etanercept for TEN was described in a case series by Paradisi et al.2 This study included 10 patients with TEN, all of whom demonstrated complete re-epithelialization shortly after receiving etanercept 50 mg. Average SCORTEN was 3.6 with a range of 2 to 6. Eight patients in this study had severe comorbidities and all 10 patients survived, with a time to re-epithelialization ranging from 7 to 20 days.2 Additionally, a randomized controlled trial showed that 38 etanercept-treated patients had improved mortality (P=.266) and re-epithelialization time (P=.01) compared to patients treated with intravenous methylprednisolone.25Limitations to our study are similar to other reports of SJS/TEN and included the small number of cases and lack of randomization. Additionally, we do not have data available for all patients for time between onset of disease and treatment initiation. Because of these challenges, data presented in this case series is observational only. Additionally, the patients treated with etanercept alone had a slightly lower SCORTEN compared to the group that received IVIG or supportive care alone (2.1 and 2.4 respectively). However, the etanercept-only group actually had higher involvement of epidermal detachment (33%) compared to the non-etanercept group (23%).
Conclusion
Although treatment with etanercept lacks the support of a randomized controlled trial, similar to all other treatments currently used for SJS and TEN, preliminary reports highlight a benefit in disease progression and improvement in time to re-epithelialization. In particular, if etanercept 50 mg subcutaneously is given as monotherapy or is given early in the disease course (prior to other therapies being attempted and ideally within 6 hours of presentation), our data suggest an even greater trend toward improved mortality and decreased time to re-epithelialization. Additionally, our findings may suggest that in some patients, etanercept monotherapy is not an adequate intervention but the addition of IVIG may be helpful; however, the senior author (S.W.) notes anecdotally that in his experience with the patients treated at the University of California Los Angeles, the order of administration of combination therapies—etanercept followed by IVIG—was important in addition to the choice of therapy. These findings are promising enough to warrant a multicenter randomized controlled trial comparing the efficacy of etanercept to other more commonly used treatments for this spectrum of disease, including IVIG and/or cyclosporine. Based on the data presented in this case series, including the 13 patients who received etanercept and had a 0% mortality rate, etanercept may be viewed as a targeted therapeutic intervention for patients with SJS and TEN.
- Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56:181-200.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Hunger RE, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-α treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Worswick S, Cotliar J. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of treatment options. Dermatol Ther. 2011;24:207-218.
- Wallace AB. The exposure treatment of burns. Lancet Lond Engl. 1951;1:501-504.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Mounach A, Rezqi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
- Salama M, Lawrance I-C. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet Lond Engl. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch WC, et al Antitumour necrosis factor-α antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-709.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumour necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Al-Shouli S, Abouchala N, Bogusz MJ, et al. Toxic epidermal necrolysis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-535.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulin. Dermatol Basel Switz. 2012;224:134-139.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paquet P, Jennes S, Rousseau AF, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. a proof-of-concept study. Burns J Int Soc Burn Inj. 2014;40:1707-1712.
- Famularo G, Dona BD, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Didona D, Paolino G, Garcovich S, et al. Successful use of etanercept in a case of toxic epidermal necrolysis induced by rituximab. J Eur Acad Dermatol Venereol. 2016;30:E83-E84.
- Lee Y-Y, Ko J-H, Wei C-H, et al. Use of etanercept to treat toxic epidermal necrolysis in a human immunodeficiency virus-positive patient. Dermatol Sin. 2013;31:78-81.
- Owczarczyk-Saczonek A, Zdanowska N, Znajewska-Pander A, et al. Stevens-Johnson syndrome in a patient with rheumatoid arthritis during long-term etanercept therapy. J Dermatol Case Rep. 2016;10:14-16.
- Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF-α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
- Pereira FA, Mudgil AV, Rosmarin DM. Toxic epidermal necrolysis. J Am Acad Dermatol. 2007;56:181-200.
- Paradisi A, Abeni D, Bergamo F, et al. Etanercept therapy for toxic epidermal necrolysis. J Am Acad Dermatol. 2014;71:278-283.
- Hunger RE, Hunziker T, Buettiker U, et al. Rapid resolution of toxic epidermal necrolysis with anti-TNF-α treatment. J Allergy Clin Immunol. 2005;116:923-924.
- Worswick S, Cotliar J. Stevens-Johnson syndrome and toxic epidermal necrolysis: a review of treatment options. Dermatol Ther. 2011;24:207-218.
- Wallace AB. The exposure treatment of burns. Lancet Lond Engl. 1951;1:501-504.
- Bastuji-Garin S, Fouchard N, Bertocchi M, et al. SCORTEN: a severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149-153.
- Mounach A, Rezqi A, Nouijai A, et al. Stevens-Johnson syndrome complicating adalimumab therapy in rheumatoid arthritis disease. Rheumatol Int. 2013;33:1351-1353.
- Salama M, Lawrance I-C. Stevens-Johnson syndrome complicating adalimumab therapy in Crohn’s disease. World J Gastroenterol. 2009;15:4449-4452.
- Wolkenstein P, Latarjet J, Roujeau JC, et al. Randomised comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet Lond Engl. 1998;352:1586-1589.
- Fischer M, Fiedler E, Marsch WC, et al Antitumour necrosis factor-α antibodies (infliximab) in the treatment of a patient with toxic epidermal necrolysis. Br J Dermatol. 2002;146:707-709.
- Meiss F, Helmbold P, Meykadeh N, et al. Overlap of acute generalized exanthematous pustulosis and toxic epidermal necrolysis: response to antitumour necrosis factor-alpha antibody infliximab: report of three cases. J Eur Acad Dermatol Venereol. 2007;21:717-719.
- Al-Shouli S, Abouchala N, Bogusz MJ, et al. Toxic epidermal necrolysis associated with high intake of sildenafil and its response to infliximab. Acta Derm Venereol. 2005;85:534-535.
- Wojtkiewicz A, Wysocki M, Fortuna J, et al. Beneficial and rapid effect of infliximab on the course of toxic epidermal necrolysis. Acta Derm Venereol. 2008;88:420-421.
- Kreft B, Wohlrab J, Bramsiepe I, et al. Etoricoxib-induced toxic epidermal necrolysis: successful treatment with infliximab. J Dermatol. 2010;37:904-906.
- Patmanidis K, Sidiras A, Dolianitis K, et al. Combination of infliximab and high-dose intravenous immunoglobulin for toxic epidermal necrolysis: successful treatment of an elderly patient. Case Rep Dermatol Med. 2012;2012:915314.
- Gaitanis G, Spyridonos P, Patmanidis K, et al. Treatment of toxic epidermal necrolysis with the combination of infliximab and high-dose intravenous immunoglobulin. Dermatol Basel Switz. 2012;224:134-139.
- Zárate-Correa LC, Carrillo-Gómez DC, Ramírez-Escobar AF, et al. Toxic epidermal necrolysis successfully treated with infliximab. J Investig Allergol Clin Immunol. 2013;23:61-63.
- Paquet P, Jennes S, Rousseau AF, et al. Effect of N-acetylcysteine combined with infliximab on toxic epidermal necrolysis. a proof-of-concept study. Burns J Int Soc Burn Inj. 2014;40:1707-1712.
- Famularo G, Dona BD, Canzona F, et al. Etanercept for toxic epidermal necrolysis. Ann Pharmacother. 2007;41:1083-1084.
- Gubinelli E, Canzona F, Tonanzi T, et al. Toxic epidermal necrolysis successfully treated with etanercept. J Dermatol. 2009;36:150-153.
- Sadighha A. Etanercept in the treatment of a patient with acute generalized exanthematous pustulosis/toxic epidermal necrolysis: definition of a new model based on translational research. Int J Dermatol. 2009;48:913-914.
- Didona D, Paolino G, Garcovich S, et al. Successful use of etanercept in a case of toxic epidermal necrolysis induced by rituximab. J Eur Acad Dermatol Venereol. 2016;30:E83-E84.
- Lee Y-Y, Ko J-H, Wei C-H, et al. Use of etanercept to treat toxic epidermal necrolysis in a human immunodeficiency virus-positive patient. Dermatol Sin. 2013;31:78-81.
- Owczarczyk-Saczonek A, Zdanowska N, Znajewska-Pander A, et al. Stevens-Johnson syndrome in a patient with rheumatoid arthritis during long-term etanercept therapy. J Dermatol Case Rep. 2016;10:14-16.
- Wang CW, Yang LY, Chen CB, et al. Randomized, controlled trial of TNF-α antagonist in CTL mediated severe cutaneous adverse reactions. J Clin Invest. 2018;128:985-996.
Practice Points
- Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) are life-threatening dermatologic emergencies without a universally accepted treatment.
- Results of this study support the use of single-dose subcutaneous etanercept 50 mg as a potentially lifesaving therapy for patients with SJS/TEN.
Dermatopathology Etiquette 101
The Accreditation Council for Graduate Medical Education has established core competencies to serve as a foundation for the training received in a dermatology residency program.1 Although programs are required to have the same concentrations—patient care, medical knowledge, practice-based learning and improvement, interpersonal and communication skills, professionalism, and systems-based practice—no specific guidelines are in place regarding how each of these competencies should be reached within a training period.2 Instead, it remains the responsibility of each program to formulate an individualized curriculum to facilitate proficiency in the multiple areas encompassed by a residency.
In many dermatology residency programs, dermatopathology is a substantial component of educational objectives and the curriculum.1 Residents may spend as much as 25% of their training on dermatopathology. However, there is great variability among programs in methods of teaching dermatopathology. When Hinshaw3 surveyed 52 of 109 dermatology residency programs, they identified differences in dermatopathology teaching that included, but was not limited to, utilization of problem-based learning (in 40.4% of programs), integration of journal reviews (53.8%), and computer-based learning (19.2%). In addition, differences were identified in the recommended primary textbook and the makeup of faculty who taught dermatopathology.3
Although residency programs vary in their methods of teaching this important component of dermatology, most use a multiheaded microscope in some capacity for didactics or sign-out. For most trainees, the dermatopathology laboratory is a new environment compared to the clinical space that medical students and residents become accustomed to throughout their education, thus creating a knowledge gap for trainees on proper dermatopathology etiquette and universal guidelines.
With medical students, residents, and fellows in mind, we have prepared a basic “dermatopathology etiquette” reference for trainees. Just as there are universal rules in the operating room for surgery (eg, sterile technique), we want to establish a code of conduct at the microscope. We hope that these 10 tips will, first, be useful to those who are unsure how to approach their first experience with dermatopathology and, second, serve as a guideline to aid development of appropriate communication skills and functioning within this novel setting. This list also can serve as a resource for dermatopathology attendings to provide to rotating residents and students.
1. New to pathology? It’s okay to ask. Do not hesitate to ask upper-year residents, fellows, and attendings for instructions on such matters as how to adjust your eyepiece to get the best resolution.
2. If a slide drops on the floor, do not move! Your first instinct might be to move your chair to look for the dropped slide, but you might roll over it and break it.
3. When the attending is looking through the scope, you look through the scope. Dermatopathology is a visual exercise. Getting in your “optic mileage” is best done under the guidance of an experienced dermatopathologist.
4. Rules regarding food and drink at the microscope vary by pathologist. It’s best to ask what each attending prefers. Safe advice is to avoid foods that make noise, such as chewing gum and chips, and food that has a strong odor, such as microwaved leftovers.
5. Limit use of a laptop, cell phone, and smartwatch. If you think that using any of these is necessary, it generally is best to announce that you are looking up something related to the case and then share your findings (but not the most recent post on your Facebook News Feed).
6. If you notice that something needs correcting on the report, speak up! We are all human; we all make typos. Do not hesitate to mention this as soon as possible, especially before the case is signed out. You will likely be thanked by your attending because it is harder to rectify once the report has been signed out.
7. Small talk often is welcome during large excisions. This is a great time to ask what others are doing next weekend or what happened in clinic earlier that day, or just to tell a good (clean) joke that is making the rounds. Conversely, if the case is complex, it often is best to wait until it is completed before asking questions.
8. When participating in a roundtable diagnosis, you are welcome to directly state the diagnosis for bread-and-butter cases, such as basal cell carcinomas and seborrheic keratoses. It is appropriate to be more descriptive and methodical in more complex cases. When evaluating a rash, give the general inflammatory pattern first. For example, is it spongiotic? Psoriasiform? Interface? Or a mixed pattern?
9. Extra points for identifying special sites! These include mucosal, genital, and acral sites. You might even get bonus points if you can determine something about the patient (child or adult) based on the pathologic features, such as variation in collagen patterns.
10. Whenever you are in doubt, just describe what you see. You can use the traditional top-down approach or start with stating the most evident finding, then proceed to a top-down description. If it is a neoplasm, describe the overall architecture; then, what you see at a cellular level will get you some points as well.
We acknowledge that this list of 10 tips is not comprehensive and might vary by attending and each institution’s distinctive training format. We are hopeful, however, that these 10 points of etiquette can serve as a guideline.
- Hinshaw M, Hsu P, Lee L-Y, et al. The current state of dermatopathology education: a survey of the Association of Professors of Dermatology. J Cutan Pathol. 2009;36:620-628. doi:10.1111/j.1600-0560.2008.01128.x
- Hinshaw MA, Stratman EJ. Core competencies in dermatopathology. J Cutan Pathol. 2006;33:160-165. doi:10.1111/j.0303-6987.2006.00442.x
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826. doi:10.1016/j.det.2012.06.003
The Accreditation Council for Graduate Medical Education has established core competencies to serve as a foundation for the training received in a dermatology residency program.1 Although programs are required to have the same concentrations—patient care, medical knowledge, practice-based learning and improvement, interpersonal and communication skills, professionalism, and systems-based practice—no specific guidelines are in place regarding how each of these competencies should be reached within a training period.2 Instead, it remains the responsibility of each program to formulate an individualized curriculum to facilitate proficiency in the multiple areas encompassed by a residency.
In many dermatology residency programs, dermatopathology is a substantial component of educational objectives and the curriculum.1 Residents may spend as much as 25% of their training on dermatopathology. However, there is great variability among programs in methods of teaching dermatopathology. When Hinshaw3 surveyed 52 of 109 dermatology residency programs, they identified differences in dermatopathology teaching that included, but was not limited to, utilization of problem-based learning (in 40.4% of programs), integration of journal reviews (53.8%), and computer-based learning (19.2%). In addition, differences were identified in the recommended primary textbook and the makeup of faculty who taught dermatopathology.3
Although residency programs vary in their methods of teaching this important component of dermatology, most use a multiheaded microscope in some capacity for didactics or sign-out. For most trainees, the dermatopathology laboratory is a new environment compared to the clinical space that medical students and residents become accustomed to throughout their education, thus creating a knowledge gap for trainees on proper dermatopathology etiquette and universal guidelines.
With medical students, residents, and fellows in mind, we have prepared a basic “dermatopathology etiquette” reference for trainees. Just as there are universal rules in the operating room for surgery (eg, sterile technique), we want to establish a code of conduct at the microscope. We hope that these 10 tips will, first, be useful to those who are unsure how to approach their first experience with dermatopathology and, second, serve as a guideline to aid development of appropriate communication skills and functioning within this novel setting. This list also can serve as a resource for dermatopathology attendings to provide to rotating residents and students.
1. New to pathology? It’s okay to ask. Do not hesitate to ask upper-year residents, fellows, and attendings for instructions on such matters as how to adjust your eyepiece to get the best resolution.
2. If a slide drops on the floor, do not move! Your first instinct might be to move your chair to look for the dropped slide, but you might roll over it and break it.
3. When the attending is looking through the scope, you look through the scope. Dermatopathology is a visual exercise. Getting in your “optic mileage” is best done under the guidance of an experienced dermatopathologist.
4. Rules regarding food and drink at the microscope vary by pathologist. It’s best to ask what each attending prefers. Safe advice is to avoid foods that make noise, such as chewing gum and chips, and food that has a strong odor, such as microwaved leftovers.
5. Limit use of a laptop, cell phone, and smartwatch. If you think that using any of these is necessary, it generally is best to announce that you are looking up something related to the case and then share your findings (but not the most recent post on your Facebook News Feed).
6. If you notice that something needs correcting on the report, speak up! We are all human; we all make typos. Do not hesitate to mention this as soon as possible, especially before the case is signed out. You will likely be thanked by your attending because it is harder to rectify once the report has been signed out.
7. Small talk often is welcome during large excisions. This is a great time to ask what others are doing next weekend or what happened in clinic earlier that day, or just to tell a good (clean) joke that is making the rounds. Conversely, if the case is complex, it often is best to wait until it is completed before asking questions.
8. When participating in a roundtable diagnosis, you are welcome to directly state the diagnosis for bread-and-butter cases, such as basal cell carcinomas and seborrheic keratoses. It is appropriate to be more descriptive and methodical in more complex cases. When evaluating a rash, give the general inflammatory pattern first. For example, is it spongiotic? Psoriasiform? Interface? Or a mixed pattern?
9. Extra points for identifying special sites! These include mucosal, genital, and acral sites. You might even get bonus points if you can determine something about the patient (child or adult) based on the pathologic features, such as variation in collagen patterns.
10. Whenever you are in doubt, just describe what you see. You can use the traditional top-down approach or start with stating the most evident finding, then proceed to a top-down description. If it is a neoplasm, describe the overall architecture; then, what you see at a cellular level will get you some points as well.
We acknowledge that this list of 10 tips is not comprehensive and might vary by attending and each institution’s distinctive training format. We are hopeful, however, that these 10 points of etiquette can serve as a guideline.
The Accreditation Council for Graduate Medical Education has established core competencies to serve as a foundation for the training received in a dermatology residency program.1 Although programs are required to have the same concentrations—patient care, medical knowledge, practice-based learning and improvement, interpersonal and communication skills, professionalism, and systems-based practice—no specific guidelines are in place regarding how each of these competencies should be reached within a training period.2 Instead, it remains the responsibility of each program to formulate an individualized curriculum to facilitate proficiency in the multiple areas encompassed by a residency.
In many dermatology residency programs, dermatopathology is a substantial component of educational objectives and the curriculum.1 Residents may spend as much as 25% of their training on dermatopathology. However, there is great variability among programs in methods of teaching dermatopathology. When Hinshaw3 surveyed 52 of 109 dermatology residency programs, they identified differences in dermatopathology teaching that included, but was not limited to, utilization of problem-based learning (in 40.4% of programs), integration of journal reviews (53.8%), and computer-based learning (19.2%). In addition, differences were identified in the recommended primary textbook and the makeup of faculty who taught dermatopathology.3
Although residency programs vary in their methods of teaching this important component of dermatology, most use a multiheaded microscope in some capacity for didactics or sign-out. For most trainees, the dermatopathology laboratory is a new environment compared to the clinical space that medical students and residents become accustomed to throughout their education, thus creating a knowledge gap for trainees on proper dermatopathology etiquette and universal guidelines.
With medical students, residents, and fellows in mind, we have prepared a basic “dermatopathology etiquette” reference for trainees. Just as there are universal rules in the operating room for surgery (eg, sterile technique), we want to establish a code of conduct at the microscope. We hope that these 10 tips will, first, be useful to those who are unsure how to approach their first experience with dermatopathology and, second, serve as a guideline to aid development of appropriate communication skills and functioning within this novel setting. This list also can serve as a resource for dermatopathology attendings to provide to rotating residents and students.
1. New to pathology? It’s okay to ask. Do not hesitate to ask upper-year residents, fellows, and attendings for instructions on such matters as how to adjust your eyepiece to get the best resolution.
2. If a slide drops on the floor, do not move! Your first instinct might be to move your chair to look for the dropped slide, but you might roll over it and break it.
3. When the attending is looking through the scope, you look through the scope. Dermatopathology is a visual exercise. Getting in your “optic mileage” is best done under the guidance of an experienced dermatopathologist.
4. Rules regarding food and drink at the microscope vary by pathologist. It’s best to ask what each attending prefers. Safe advice is to avoid foods that make noise, such as chewing gum and chips, and food that has a strong odor, such as microwaved leftovers.
5. Limit use of a laptop, cell phone, and smartwatch. If you think that using any of these is necessary, it generally is best to announce that you are looking up something related to the case and then share your findings (but not the most recent post on your Facebook News Feed).
6. If you notice that something needs correcting on the report, speak up! We are all human; we all make typos. Do not hesitate to mention this as soon as possible, especially before the case is signed out. You will likely be thanked by your attending because it is harder to rectify once the report has been signed out.
7. Small talk often is welcome during large excisions. This is a great time to ask what others are doing next weekend or what happened in clinic earlier that day, or just to tell a good (clean) joke that is making the rounds. Conversely, if the case is complex, it often is best to wait until it is completed before asking questions.
8. When participating in a roundtable diagnosis, you are welcome to directly state the diagnosis for bread-and-butter cases, such as basal cell carcinomas and seborrheic keratoses. It is appropriate to be more descriptive and methodical in more complex cases. When evaluating a rash, give the general inflammatory pattern first. For example, is it spongiotic? Psoriasiform? Interface? Or a mixed pattern?
9. Extra points for identifying special sites! These include mucosal, genital, and acral sites. You might even get bonus points if you can determine something about the patient (child or adult) based on the pathologic features, such as variation in collagen patterns.
10. Whenever you are in doubt, just describe what you see. You can use the traditional top-down approach or start with stating the most evident finding, then proceed to a top-down description. If it is a neoplasm, describe the overall architecture; then, what you see at a cellular level will get you some points as well.
We acknowledge that this list of 10 tips is not comprehensive and might vary by attending and each institution’s distinctive training format. We are hopeful, however, that these 10 points of etiquette can serve as a guideline.
- Hinshaw M, Hsu P, Lee L-Y, et al. The current state of dermatopathology education: a survey of the Association of Professors of Dermatology. J Cutan Pathol. 2009;36:620-628. doi:10.1111/j.1600-0560.2008.01128.x
- Hinshaw MA, Stratman EJ. Core competencies in dermatopathology. J Cutan Pathol. 2006;33:160-165. doi:10.1111/j.0303-6987.2006.00442.x
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826. doi:10.1016/j.det.2012.06.003
- Hinshaw M, Hsu P, Lee L-Y, et al. The current state of dermatopathology education: a survey of the Association of Professors of Dermatology. J Cutan Pathol. 2009;36:620-628. doi:10.1111/j.1600-0560.2008.01128.x
- Hinshaw MA, Stratman EJ. Core competencies in dermatopathology. J Cutan Pathol. 2006;33:160-165. doi:10.1111/j.0303-6987.2006.00442.x
- Hinshaw MA. Dermatopathology education: an update. Dermatol Clin. 2012;30:815-826. doi:10.1016/j.det.2012.06.003
The pandemic hurt patients with liver disease in many ways
The COVID-19 pandemic has worsened the health of patients with liver disease worldwide, researchers say.
Not only does liver disease make people more vulnerable to the virus that causes COVID-19, precautions to prevent its spread have delayed health care and worsened alcohol abuse.
At this year’s virtual International Liver Congress (ILC) 2021, experts from around the world documented this toll on their patients.
Surgeons have seen a surge in patients needing transplants because of alcoholic liver disease, the campaign to snuff out hepatitis C slowed down, and procedures such as endoscopy and ultrasound exams postponed, said Mario Mondelli, MD, PhD, a professor and consultant physician of infectious diseases at the University of Pavia, Italy.
“We were able to ensure only emergency treatments, not routine surveillance,” he said in an interview.
Of 1,994 people with chronic liver disease who responded to a survey through the Global Liver Registry, 11% reported that the pandemic had affected their liver health.
This proportion was not statistically different for the 165 patients (8.2%) who had been diagnosed with COVID-19 compared with those who had not. But many of those who had been diagnosed with COVID-19 reported that it severely affected them. They reported worse overall heath, more mental illness, and greater need for supportive service than those who evaded the virus. Thirty-three respondents (20.8%) were hospitalized.
The global effort to eradicate hepatitis C slowed as a result of the pandemic. Already in 2019, the United States was behind the World Health Organization schedule for eliminating this virus. In 2020, it slipped further, with 25% fewer patients starting treatment for hepatitis C than in 2019, according to researchers at the U.S. Centers for Disease Control and Prevention.
Similar delays in eliminating hepatitis C occurred around the world, Dr. Mondelli said, noting that the majority of countries will not be able to reach the WHO objectives.
One striking result of the pandemic was an uptick of patients needing liver transplants as a result of alcoholic liver disease, said George Cholankeril, MD, a liver transplant surgeon at Baylor College of Medicine, Houston.
Before the pandemic, he and his colleagues had noted an increase in the number of people needing liver transplants because of alcohol abuse. During the pandemic, that trend accelerated.
They defined the pre-COVID era as June 1, 2019, to Feb. 29, 2020, and the COVID era as after April 1, 2020. In the COVID era, alcoholic liver disease accounted for 40% of patients whom the hospital put on its list for liver transplant. Hepatitis C and nonalcoholic fatty liver disease combined accounted for only 36%.
The change has resulted in part from the effectiveness of hepatitis C treatments, which have reduced the number of patients with livers damaged by that virus. But the change also resulted from the increased severity of illness in the patients with alcoholic liver disease, Dr. Cholankeril said in an interview. Overall, Model for End-Stage Liver Disease scores – which are used to predict survival – worsened for patients with alcoholic liver disease but remained the same for patients with nonalcoholic liver disease.
In the pre-COVID era, patients with alcoholic liver disease had a 10% higher chance for undergoing transplant, compared with patients with nonalcoholic liver disease. In the COVID era, they had a 50% higher chance, a statistically significant change (P < .001).
The finding parallels those of other studies that have shown a spike in consults for alcohol-related gastrointestinal and liver diseases, as reported by this news organization.
“We feel that the increase in alcoholic hepatitis is possibly from binge drinking and alcoholic consumption during the pandemic,” said Dr. Cholankeril. “Anecdotally, I can’t tell you how many patients say that the video meetings for Alcoholic Anonymous just don’t work. It’s not the same as in person. They don’t feel that they’re getting the support that they need.”
In Europe, fewer of the people who need liver transplants may be receiving them, said Dr. Mondelli.
“There are several papers indicating, particularly in Italy, in France, and in the United Kingdom, that during the pandemic, the offer for organs significantly declined,” he said.
Other studies have shown increases in mortality from liver disease during the pandemic, Dr. Mondelli said. The same is true of myocardial infarction, cancer, and most other life-threatening illnesses, he pointed out.
“Because of the enormous tsunami that has affected hospital services during the peaks of the pandemic, there has been an increase in deceased patients from a variety of other diseases, not only liver disease,” he said.
But Dr. Mondelli also added that physicians had improved in their ability to safely care for their patients while protecting themselves over the course of the pandemic.
Dr. Mondelli and Dr. Cholankeril have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic has worsened the health of patients with liver disease worldwide, researchers say.
Not only does liver disease make people more vulnerable to the virus that causes COVID-19, precautions to prevent its spread have delayed health care and worsened alcohol abuse.
At this year’s virtual International Liver Congress (ILC) 2021, experts from around the world documented this toll on their patients.
Surgeons have seen a surge in patients needing transplants because of alcoholic liver disease, the campaign to snuff out hepatitis C slowed down, and procedures such as endoscopy and ultrasound exams postponed, said Mario Mondelli, MD, PhD, a professor and consultant physician of infectious diseases at the University of Pavia, Italy.
“We were able to ensure only emergency treatments, not routine surveillance,” he said in an interview.
Of 1,994 people with chronic liver disease who responded to a survey through the Global Liver Registry, 11% reported that the pandemic had affected their liver health.
This proportion was not statistically different for the 165 patients (8.2%) who had been diagnosed with COVID-19 compared with those who had not. But many of those who had been diagnosed with COVID-19 reported that it severely affected them. They reported worse overall heath, more mental illness, and greater need for supportive service than those who evaded the virus. Thirty-three respondents (20.8%) were hospitalized.
The global effort to eradicate hepatitis C slowed as a result of the pandemic. Already in 2019, the United States was behind the World Health Organization schedule for eliminating this virus. In 2020, it slipped further, with 25% fewer patients starting treatment for hepatitis C than in 2019, according to researchers at the U.S. Centers for Disease Control and Prevention.
Similar delays in eliminating hepatitis C occurred around the world, Dr. Mondelli said, noting that the majority of countries will not be able to reach the WHO objectives.
One striking result of the pandemic was an uptick of patients needing liver transplants as a result of alcoholic liver disease, said George Cholankeril, MD, a liver transplant surgeon at Baylor College of Medicine, Houston.
Before the pandemic, he and his colleagues had noted an increase in the number of people needing liver transplants because of alcohol abuse. During the pandemic, that trend accelerated.
They defined the pre-COVID era as June 1, 2019, to Feb. 29, 2020, and the COVID era as after April 1, 2020. In the COVID era, alcoholic liver disease accounted for 40% of patients whom the hospital put on its list for liver transplant. Hepatitis C and nonalcoholic fatty liver disease combined accounted for only 36%.
The change has resulted in part from the effectiveness of hepatitis C treatments, which have reduced the number of patients with livers damaged by that virus. But the change also resulted from the increased severity of illness in the patients with alcoholic liver disease, Dr. Cholankeril said in an interview. Overall, Model for End-Stage Liver Disease scores – which are used to predict survival – worsened for patients with alcoholic liver disease but remained the same for patients with nonalcoholic liver disease.
In the pre-COVID era, patients with alcoholic liver disease had a 10% higher chance for undergoing transplant, compared with patients with nonalcoholic liver disease. In the COVID era, they had a 50% higher chance, a statistically significant change (P < .001).
The finding parallels those of other studies that have shown a spike in consults for alcohol-related gastrointestinal and liver diseases, as reported by this news organization.
“We feel that the increase in alcoholic hepatitis is possibly from binge drinking and alcoholic consumption during the pandemic,” said Dr. Cholankeril. “Anecdotally, I can’t tell you how many patients say that the video meetings for Alcoholic Anonymous just don’t work. It’s not the same as in person. They don’t feel that they’re getting the support that they need.”
In Europe, fewer of the people who need liver transplants may be receiving them, said Dr. Mondelli.
“There are several papers indicating, particularly in Italy, in France, and in the United Kingdom, that during the pandemic, the offer for organs significantly declined,” he said.
Other studies have shown increases in mortality from liver disease during the pandemic, Dr. Mondelli said. The same is true of myocardial infarction, cancer, and most other life-threatening illnesses, he pointed out.
“Because of the enormous tsunami that has affected hospital services during the peaks of the pandemic, there has been an increase in deceased patients from a variety of other diseases, not only liver disease,” he said.
But Dr. Mondelli also added that physicians had improved in their ability to safely care for their patients while protecting themselves over the course of the pandemic.
Dr. Mondelli and Dr. Cholankeril have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The COVID-19 pandemic has worsened the health of patients with liver disease worldwide, researchers say.
Not only does liver disease make people more vulnerable to the virus that causes COVID-19, precautions to prevent its spread have delayed health care and worsened alcohol abuse.
At this year’s virtual International Liver Congress (ILC) 2021, experts from around the world documented this toll on their patients.
Surgeons have seen a surge in patients needing transplants because of alcoholic liver disease, the campaign to snuff out hepatitis C slowed down, and procedures such as endoscopy and ultrasound exams postponed, said Mario Mondelli, MD, PhD, a professor and consultant physician of infectious diseases at the University of Pavia, Italy.
“We were able to ensure only emergency treatments, not routine surveillance,” he said in an interview.
Of 1,994 people with chronic liver disease who responded to a survey through the Global Liver Registry, 11% reported that the pandemic had affected their liver health.
This proportion was not statistically different for the 165 patients (8.2%) who had been diagnosed with COVID-19 compared with those who had not. But many of those who had been diagnosed with COVID-19 reported that it severely affected them. They reported worse overall heath, more mental illness, and greater need for supportive service than those who evaded the virus. Thirty-three respondents (20.8%) were hospitalized.
The global effort to eradicate hepatitis C slowed as a result of the pandemic. Already in 2019, the United States was behind the World Health Organization schedule for eliminating this virus. In 2020, it slipped further, with 25% fewer patients starting treatment for hepatitis C than in 2019, according to researchers at the U.S. Centers for Disease Control and Prevention.
Similar delays in eliminating hepatitis C occurred around the world, Dr. Mondelli said, noting that the majority of countries will not be able to reach the WHO objectives.
One striking result of the pandemic was an uptick of patients needing liver transplants as a result of alcoholic liver disease, said George Cholankeril, MD, a liver transplant surgeon at Baylor College of Medicine, Houston.
Before the pandemic, he and his colleagues had noted an increase in the number of people needing liver transplants because of alcohol abuse. During the pandemic, that trend accelerated.
They defined the pre-COVID era as June 1, 2019, to Feb. 29, 2020, and the COVID era as after April 1, 2020. In the COVID era, alcoholic liver disease accounted for 40% of patients whom the hospital put on its list for liver transplant. Hepatitis C and nonalcoholic fatty liver disease combined accounted for only 36%.
The change has resulted in part from the effectiveness of hepatitis C treatments, which have reduced the number of patients with livers damaged by that virus. But the change also resulted from the increased severity of illness in the patients with alcoholic liver disease, Dr. Cholankeril said in an interview. Overall, Model for End-Stage Liver Disease scores – which are used to predict survival – worsened for patients with alcoholic liver disease but remained the same for patients with nonalcoholic liver disease.
In the pre-COVID era, patients with alcoholic liver disease had a 10% higher chance for undergoing transplant, compared with patients with nonalcoholic liver disease. In the COVID era, they had a 50% higher chance, a statistically significant change (P < .001).
The finding parallels those of other studies that have shown a spike in consults for alcohol-related gastrointestinal and liver diseases, as reported by this news organization.
“We feel that the increase in alcoholic hepatitis is possibly from binge drinking and alcoholic consumption during the pandemic,” said Dr. Cholankeril. “Anecdotally, I can’t tell you how many patients say that the video meetings for Alcoholic Anonymous just don’t work. It’s not the same as in person. They don’t feel that they’re getting the support that they need.”
In Europe, fewer of the people who need liver transplants may be receiving them, said Dr. Mondelli.
“There are several papers indicating, particularly in Italy, in France, and in the United Kingdom, that during the pandemic, the offer for organs significantly declined,” he said.
Other studies have shown increases in mortality from liver disease during the pandemic, Dr. Mondelli said. The same is true of myocardial infarction, cancer, and most other life-threatening illnesses, he pointed out.
“Because of the enormous tsunami that has affected hospital services during the peaks of the pandemic, there has been an increase in deceased patients from a variety of other diseases, not only liver disease,” he said.
But Dr. Mondelli also added that physicians had improved in their ability to safely care for their patients while protecting themselves over the course of the pandemic.
Dr. Mondelli and Dr. Cholankeril have disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Doxycycline trumps azithromycin for asymptomatic rectal chlamydia in men who have sex with men
A 1-week course of doxycycline is more effective than single-dose azithromycin to treat rectal chlamydia in men who have sex with men (MSM), according to newly published results in the New England Journal of Medicine.
Chlamydia is the most commonly reported bacterial STI in the United States, with 4 million cases reported in 2018, and 127 million globally. Most infections are asymptomatic.
Rates of rectal chlamydia among MSM screened for infection range from 3% to 10.5%.
The most recent Centers for Disease Control and Prevention chlamydia guidelines recommend either a single dose of azithromycin (1 g) or doxycycline 100 mg twice daily for 7 days. These 2015 guidelines were based on a meta-analysis of urogenital chlamydia infections, which showed comparable efficacy of 97% or 98%, respectively.
Study coauthor Jane S. Hocking, PhD, head of the sexual health unit at the University of Melbourne, told this news organization that “observational studies had suggested that azithromycin was about 20% less effective than doxycycline,” prompting this clinical trial.
The study, conducted at five sexual health clinics in Australia, was a double-blind, randomized, controlled trial of doxycycline (100 mg twice daily for 7 days) or azithromycin (1-g single dose).
Because 85% of infected men are asymptomatic, the study’s primary outcome was a negative nucleic acid amplification test at 4 weeks, confirming a microbiologic cure.
Using a modified intention-to-treat population, the study showed a microbiologic cure in 281 of 290 men (96.9%) in the doxycycline group and 227 of 297 (76.4%) in the azithromycin group (P < .001).
Adverse events were more common in the azithromycin group. Nausea, diarrhea, and vomiting occurred in 134 (45.1%) men in that group versus 98 men (33.8%) in those receiving doxycycline (P = .006).
A similar study was reported in Clinical Infectious Diseases in February 2021 by Dombrowski and colleagues. It was also randomized, double blinded, and placebo controlled but was smaller and conducted in Seattle and Boston. A 20% difference was found, with 80/88 (91%) in the doxycycline group and 63/89 (71%) in the azithromycin group having a microbiologic cure at 4 weeks of follow-up.
Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, said in an interview that the researchers focused solely on asymptomatic proctitis because “other symptoms might indicate need for broader presumptive antibiotics” for coinfections. Similarly, symptomatic proctitis “could indicate LGV [lymphogranuloma venereum] chlamydia, which ... automatically mandates that 3-weeks of doxycycline be used.” Dr. Marrazzo concluded: “The fact that this was a blinded study obviously strengthens the conclusions/findings, which is great. It’s very reassuring that results overall are so consistent with the CID paper.” Dr. Marrazzo was not involved in either the New England Journal of Medicine investigation or CID study.
Ina Park, MD, associate professor in the department of family and community medicine at the University of California, San Francisco, and author of “Strange Bedfellows: Adventures in the Science, History, and Surprising Secrets of STDs,” (New York: Flatiron Books, 2021) was not involved in either study but has a long history of working with adolescents in clinics for STDs. Based on that experience, she told this news organization that, while doxycycline now clearly appears to be the drug of choice, “if compliance is an issue and rectal chlamydia is not likely, then I think azithromycin is still something we need to consider, particularly for younger patients, and folks for whom compliance is going to be an issue.” She added: “with adolescent patients, there are issues of parents possibly discovering the antibiotic and asking lots of questions. So, it’s very nice for folks to be able to get therapy, sort of a one and done approach in the clinic.”
The 2020 CDC Guidelines for Gonococcal Infections says: “CDC recommends a single 500 mg intramuscular dose of ceftriaxone for uncomplicated gonorrhea. Treatment for coinfection with Chlamydia trachomatis with oral doxycycline (100 mg twice daily for 7 days) should be administered when chlamydial infection has not been excluded.”
Hocking concluded – and Dr. Marrazzo and Dr. Park concur – that this study “provides conclusive evidence that doxycycline should be the first-line treatment for rectal chlamydia, but probably for just any chlamydia infection,” with specific exceptions.
The University of Melbourne researchers also noted that the doxycycline course requires more compliant patients, as adherence isn’t assured. The issue of compliance and need for directly observed therapy, allergy to doxycycline, and pregnancy (where doxycycline is contraindicated) will remain the primary indications for continued use of azithromycin.
A version of this article first appeared on Medscape.com.
A 1-week course of doxycycline is more effective than single-dose azithromycin to treat rectal chlamydia in men who have sex with men (MSM), according to newly published results in the New England Journal of Medicine.
Chlamydia is the most commonly reported bacterial STI in the United States, with 4 million cases reported in 2018, and 127 million globally. Most infections are asymptomatic.
Rates of rectal chlamydia among MSM screened for infection range from 3% to 10.5%.
The most recent Centers for Disease Control and Prevention chlamydia guidelines recommend either a single dose of azithromycin (1 g) or doxycycline 100 mg twice daily for 7 days. These 2015 guidelines were based on a meta-analysis of urogenital chlamydia infections, which showed comparable efficacy of 97% or 98%, respectively.
Study coauthor Jane S. Hocking, PhD, head of the sexual health unit at the University of Melbourne, told this news organization that “observational studies had suggested that azithromycin was about 20% less effective than doxycycline,” prompting this clinical trial.
The study, conducted at five sexual health clinics in Australia, was a double-blind, randomized, controlled trial of doxycycline (100 mg twice daily for 7 days) or azithromycin (1-g single dose).
Because 85% of infected men are asymptomatic, the study’s primary outcome was a negative nucleic acid amplification test at 4 weeks, confirming a microbiologic cure.
Using a modified intention-to-treat population, the study showed a microbiologic cure in 281 of 290 men (96.9%) in the doxycycline group and 227 of 297 (76.4%) in the azithromycin group (P < .001).
Adverse events were more common in the azithromycin group. Nausea, diarrhea, and vomiting occurred in 134 (45.1%) men in that group versus 98 men (33.8%) in those receiving doxycycline (P = .006).
A similar study was reported in Clinical Infectious Diseases in February 2021 by Dombrowski and colleagues. It was also randomized, double blinded, and placebo controlled but was smaller and conducted in Seattle and Boston. A 20% difference was found, with 80/88 (91%) in the doxycycline group and 63/89 (71%) in the azithromycin group having a microbiologic cure at 4 weeks of follow-up.
Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, said in an interview that the researchers focused solely on asymptomatic proctitis because “other symptoms might indicate need for broader presumptive antibiotics” for coinfections. Similarly, symptomatic proctitis “could indicate LGV [lymphogranuloma venereum] chlamydia, which ... automatically mandates that 3-weeks of doxycycline be used.” Dr. Marrazzo concluded: “The fact that this was a blinded study obviously strengthens the conclusions/findings, which is great. It’s very reassuring that results overall are so consistent with the CID paper.” Dr. Marrazzo was not involved in either the New England Journal of Medicine investigation or CID study.
Ina Park, MD, associate professor in the department of family and community medicine at the University of California, San Francisco, and author of “Strange Bedfellows: Adventures in the Science, History, and Surprising Secrets of STDs,” (New York: Flatiron Books, 2021) was not involved in either study but has a long history of working with adolescents in clinics for STDs. Based on that experience, she told this news organization that, while doxycycline now clearly appears to be the drug of choice, “if compliance is an issue and rectal chlamydia is not likely, then I think azithromycin is still something we need to consider, particularly for younger patients, and folks for whom compliance is going to be an issue.” She added: “with adolescent patients, there are issues of parents possibly discovering the antibiotic and asking lots of questions. So, it’s very nice for folks to be able to get therapy, sort of a one and done approach in the clinic.”
The 2020 CDC Guidelines for Gonococcal Infections says: “CDC recommends a single 500 mg intramuscular dose of ceftriaxone for uncomplicated gonorrhea. Treatment for coinfection with Chlamydia trachomatis with oral doxycycline (100 mg twice daily for 7 days) should be administered when chlamydial infection has not been excluded.”
Hocking concluded – and Dr. Marrazzo and Dr. Park concur – that this study “provides conclusive evidence that doxycycline should be the first-line treatment for rectal chlamydia, but probably for just any chlamydia infection,” with specific exceptions.
The University of Melbourne researchers also noted that the doxycycline course requires more compliant patients, as adherence isn’t assured. The issue of compliance and need for directly observed therapy, allergy to doxycycline, and pregnancy (where doxycycline is contraindicated) will remain the primary indications for continued use of azithromycin.
A version of this article first appeared on Medscape.com.
A 1-week course of doxycycline is more effective than single-dose azithromycin to treat rectal chlamydia in men who have sex with men (MSM), according to newly published results in the New England Journal of Medicine.
Chlamydia is the most commonly reported bacterial STI in the United States, with 4 million cases reported in 2018, and 127 million globally. Most infections are asymptomatic.
Rates of rectal chlamydia among MSM screened for infection range from 3% to 10.5%.
The most recent Centers for Disease Control and Prevention chlamydia guidelines recommend either a single dose of azithromycin (1 g) or doxycycline 100 mg twice daily for 7 days. These 2015 guidelines were based on a meta-analysis of urogenital chlamydia infections, which showed comparable efficacy of 97% or 98%, respectively.
Study coauthor Jane S. Hocking, PhD, head of the sexual health unit at the University of Melbourne, told this news organization that “observational studies had suggested that azithromycin was about 20% less effective than doxycycline,” prompting this clinical trial.
The study, conducted at five sexual health clinics in Australia, was a double-blind, randomized, controlled trial of doxycycline (100 mg twice daily for 7 days) or azithromycin (1-g single dose).
Because 85% of infected men are asymptomatic, the study’s primary outcome was a negative nucleic acid amplification test at 4 weeks, confirming a microbiologic cure.
Using a modified intention-to-treat population, the study showed a microbiologic cure in 281 of 290 men (96.9%) in the doxycycline group and 227 of 297 (76.4%) in the azithromycin group (P < .001).
Adverse events were more common in the azithromycin group. Nausea, diarrhea, and vomiting occurred in 134 (45.1%) men in that group versus 98 men (33.8%) in those receiving doxycycline (P = .006).
A similar study was reported in Clinical Infectious Diseases in February 2021 by Dombrowski and colleagues. It was also randomized, double blinded, and placebo controlled but was smaller and conducted in Seattle and Boston. A 20% difference was found, with 80/88 (91%) in the doxycycline group and 63/89 (71%) in the azithromycin group having a microbiologic cure at 4 weeks of follow-up.
Jeanne Marrazzo, MD, director of the division of infectious diseases at the University of Alabama at Birmingham, said in an interview that the researchers focused solely on asymptomatic proctitis because “other symptoms might indicate need for broader presumptive antibiotics” for coinfections. Similarly, symptomatic proctitis “could indicate LGV [lymphogranuloma venereum] chlamydia, which ... automatically mandates that 3-weeks of doxycycline be used.” Dr. Marrazzo concluded: “The fact that this was a blinded study obviously strengthens the conclusions/findings, which is great. It’s very reassuring that results overall are so consistent with the CID paper.” Dr. Marrazzo was not involved in either the New England Journal of Medicine investigation or CID study.
Ina Park, MD, associate professor in the department of family and community medicine at the University of California, San Francisco, and author of “Strange Bedfellows: Adventures in the Science, History, and Surprising Secrets of STDs,” (New York: Flatiron Books, 2021) was not involved in either study but has a long history of working with adolescents in clinics for STDs. Based on that experience, she told this news organization that, while doxycycline now clearly appears to be the drug of choice, “if compliance is an issue and rectal chlamydia is not likely, then I think azithromycin is still something we need to consider, particularly for younger patients, and folks for whom compliance is going to be an issue.” She added: “with adolescent patients, there are issues of parents possibly discovering the antibiotic and asking lots of questions. So, it’s very nice for folks to be able to get therapy, sort of a one and done approach in the clinic.”
The 2020 CDC Guidelines for Gonococcal Infections says: “CDC recommends a single 500 mg intramuscular dose of ceftriaxone for uncomplicated gonorrhea. Treatment for coinfection with Chlamydia trachomatis with oral doxycycline (100 mg twice daily for 7 days) should be administered when chlamydial infection has not been excluded.”
Hocking concluded – and Dr. Marrazzo and Dr. Park concur – that this study “provides conclusive evidence that doxycycline should be the first-line treatment for rectal chlamydia, but probably for just any chlamydia infection,” with specific exceptions.
The University of Melbourne researchers also noted that the doxycycline course requires more compliant patients, as adherence isn’t assured. The issue of compliance and need for directly observed therapy, allergy to doxycycline, and pregnancy (where doxycycline is contraindicated) will remain the primary indications for continued use of azithromycin.
A version of this article first appeared on Medscape.com.
Almost all U.S. COVID-19 deaths now in the unvaccinated
If you, a friend, or a loved one remain unvaccinated against COVID-19 at this point – for whatever reason – you are at higher risk of dying if you become infected.
That’s the conclusion of a new report released by the Associated Press looking at COVID-19 deaths during May 2021.
Of more than 18,000 people who died from COVID-19, for example, only about 150 were fully vaccinated. That’s less than 1%.
“Recently, I was working in the emergency room [and] I saw a 21-year-old African American who came in with shortness of breath,” said Vino K. Palli, MD, MPH, a physician specializing in emergency medicine, internal medicine, and urgent care.
The patient rapidly deteriorated and required intubation and ventilation. She was transferred to a specialized hospital for possible extracorporeal membrane oxygenation (ECMO) treatment.
“This patient was unvaccinated, along with her entire family. This would have been easily preventable,” added Dr. Palli, who is also founder and CEO of MiDoctor Urgent Care in New York City.
“Vaccine misinformation, compounded with vaccine inertia and vaccine access, have contributed to this,” he added. “Even though we have a surplus amount of vaccines at this time, we are only seeing 50% to 55% of completely vaccinated patients.”
Authors of the Associated Press report also acknowledge that some people who are fully vaccinated can get a breakthrough infection. These occurred in fewer than 1,200 of more than 853,000 people hospitalized for COVID-19 in May, or about 0.1%.
The Associated Press came up with these numbers using data from the Centers for Disease Control and Prevention. The CDC tracks the numbers of cases, hospitalizations, and deaths but does not breakdown rates by vaccination status.
Stronger argument for vaccination?
“The fact that only 0.8% of COVID-19 deaths are in the fully vaccinated should persuade those people still hesitant about vaccination,” said Hugh Cassiere, MD, medical director of Respiratory Therapy Services at North Shore University Hospital in Manhasset, New York.
Stuart C. Ray, MD, professor of medicine and oncology in the Division of Infectious Diseases at Johns Hopkins University, Baltimore, agreed. “It seems compelling, even for skeptics, that unvaccinated people represent 99% of those now dying from COVID-19 when they represent less than 50% of the adult population in the United States.”
The findings from the study could be more persuasive than previous arguments made in favor of immunization, Dr. Ray said. “These recent findings of striking reductions in risk of death in the vaccinated are more directly attributable and harder to ignore or dismiss.”
Brian Labus, PhD, MPH, of the University of Nevada Las Vegas (UNLV) is less convinced. “While this might change some peoples’ minds, it probably won’t make a major difference. People have many different reasons for not getting vaccinated, and this is only one of the things they consider.”
The study adds information that was not available before, said Dr. Labus, assistant professor in the Department of Epidemiology and Biostatistics at the UNLV School of Public Health. “We study the vaccine under tightly controlled, ideal conditions. This is the evidence that it works as well in the real world as it did in the trials, and that is what is most important in implementing a vaccination program,” added Dr. Labus.
“The scientific data has honed in on one thing: Vaccines are effective in preventing hospitalizations, ICU admissions, ventilations, and deaths,” agreed Dr. Palli.
“We now know that almost all deaths occurred in patients who were not vaccinated. We also know that all vaccines are effective against various strains that are in circulation right now, including the Delta variant, which is rapidly spreading,” Dr. Palli said.
Dr. Cassiere pointed out that the unvaccinated are not only at higher risk of developing COVID-19 but also of spreading, being hospitalized for, and dying from the infection. Avoiding “long hauler” symptoms is another argument in favor of immunization, he added.
As of June 28, the CDC reports that 63% of Americans 12 years and older have received at least one dose of a COVID-19 vaccine, and 54% are fully vaccinated.
Worldwide worry?
Although overall rates of U.S. COVID-19 hospitalizations and deaths are down, the outlook may not remain as encouraging. “I hope I’m wrong about this, but I anticipate that the coming fall and winter will bring increasingly localized versions of similar findings – severe disease and death due to SARS-CoV-2 infection in regions or groups with lower vaccination rates,” Dr. Ray said.
There could be a silver lining, he added: “If this unfortunate surge occurs, the health and economic consequences seem likely to erode much of the remaining hesitancy regarding vaccination.”
The rise of more infectious SARS-CoV-2 variants, such as the Delta variant, could also throw a wrench in controlling COVID-19. “This isn’t just a domestic issue,” Dr. Ray said. “We have learned that the world is a small place in pandemic times.”
The Associated Press investigators state that their findings support the high efficacy of the vaccine. Also, given the current widespread availability of COVID-19 vaccines in the United States, they believe many of the COVID-19 deaths now occurring are preventable.
Public health measures should have continued longer to protect unvaccinated individuals, especially Black Americans, Hispanic Americans, and other minorities, Dr. Palli said. “Only time will tell if re-opening and abandoning all public health measures by the CDC was premature.”
A version of this article first appeared on Medscape.com.
If you, a friend, or a loved one remain unvaccinated against COVID-19 at this point – for whatever reason – you are at higher risk of dying if you become infected.
That’s the conclusion of a new report released by the Associated Press looking at COVID-19 deaths during May 2021.
Of more than 18,000 people who died from COVID-19, for example, only about 150 were fully vaccinated. That’s less than 1%.
“Recently, I was working in the emergency room [and] I saw a 21-year-old African American who came in with shortness of breath,” said Vino K. Palli, MD, MPH, a physician specializing in emergency medicine, internal medicine, and urgent care.
The patient rapidly deteriorated and required intubation and ventilation. She was transferred to a specialized hospital for possible extracorporeal membrane oxygenation (ECMO) treatment.
“This patient was unvaccinated, along with her entire family. This would have been easily preventable,” added Dr. Palli, who is also founder and CEO of MiDoctor Urgent Care in New York City.
“Vaccine misinformation, compounded with vaccine inertia and vaccine access, have contributed to this,” he added. “Even though we have a surplus amount of vaccines at this time, we are only seeing 50% to 55% of completely vaccinated patients.”
Authors of the Associated Press report also acknowledge that some people who are fully vaccinated can get a breakthrough infection. These occurred in fewer than 1,200 of more than 853,000 people hospitalized for COVID-19 in May, or about 0.1%.
The Associated Press came up with these numbers using data from the Centers for Disease Control and Prevention. The CDC tracks the numbers of cases, hospitalizations, and deaths but does not breakdown rates by vaccination status.
Stronger argument for vaccination?
“The fact that only 0.8% of COVID-19 deaths are in the fully vaccinated should persuade those people still hesitant about vaccination,” said Hugh Cassiere, MD, medical director of Respiratory Therapy Services at North Shore University Hospital in Manhasset, New York.
Stuart C. Ray, MD, professor of medicine and oncology in the Division of Infectious Diseases at Johns Hopkins University, Baltimore, agreed. “It seems compelling, even for skeptics, that unvaccinated people represent 99% of those now dying from COVID-19 when they represent less than 50% of the adult population in the United States.”
The findings from the study could be more persuasive than previous arguments made in favor of immunization, Dr. Ray said. “These recent findings of striking reductions in risk of death in the vaccinated are more directly attributable and harder to ignore or dismiss.”
Brian Labus, PhD, MPH, of the University of Nevada Las Vegas (UNLV) is less convinced. “While this might change some peoples’ minds, it probably won’t make a major difference. People have many different reasons for not getting vaccinated, and this is only one of the things they consider.”
The study adds information that was not available before, said Dr. Labus, assistant professor in the Department of Epidemiology and Biostatistics at the UNLV School of Public Health. “We study the vaccine under tightly controlled, ideal conditions. This is the evidence that it works as well in the real world as it did in the trials, and that is what is most important in implementing a vaccination program,” added Dr. Labus.
“The scientific data has honed in on one thing: Vaccines are effective in preventing hospitalizations, ICU admissions, ventilations, and deaths,” agreed Dr. Palli.
“We now know that almost all deaths occurred in patients who were not vaccinated. We also know that all vaccines are effective against various strains that are in circulation right now, including the Delta variant, which is rapidly spreading,” Dr. Palli said.
Dr. Cassiere pointed out that the unvaccinated are not only at higher risk of developing COVID-19 but also of spreading, being hospitalized for, and dying from the infection. Avoiding “long hauler” symptoms is another argument in favor of immunization, he added.
As of June 28, the CDC reports that 63% of Americans 12 years and older have received at least one dose of a COVID-19 vaccine, and 54% are fully vaccinated.
Worldwide worry?
Although overall rates of U.S. COVID-19 hospitalizations and deaths are down, the outlook may not remain as encouraging. “I hope I’m wrong about this, but I anticipate that the coming fall and winter will bring increasingly localized versions of similar findings – severe disease and death due to SARS-CoV-2 infection in regions or groups with lower vaccination rates,” Dr. Ray said.
There could be a silver lining, he added: “If this unfortunate surge occurs, the health and economic consequences seem likely to erode much of the remaining hesitancy regarding vaccination.”
The rise of more infectious SARS-CoV-2 variants, such as the Delta variant, could also throw a wrench in controlling COVID-19. “This isn’t just a domestic issue,” Dr. Ray said. “We have learned that the world is a small place in pandemic times.”
The Associated Press investigators state that their findings support the high efficacy of the vaccine. Also, given the current widespread availability of COVID-19 vaccines in the United States, they believe many of the COVID-19 deaths now occurring are preventable.
Public health measures should have continued longer to protect unvaccinated individuals, especially Black Americans, Hispanic Americans, and other minorities, Dr. Palli said. “Only time will tell if re-opening and abandoning all public health measures by the CDC was premature.”
A version of this article first appeared on Medscape.com.
If you, a friend, or a loved one remain unvaccinated against COVID-19 at this point – for whatever reason – you are at higher risk of dying if you become infected.
That’s the conclusion of a new report released by the Associated Press looking at COVID-19 deaths during May 2021.
Of more than 18,000 people who died from COVID-19, for example, only about 150 were fully vaccinated. That’s less than 1%.
“Recently, I was working in the emergency room [and] I saw a 21-year-old African American who came in with shortness of breath,” said Vino K. Palli, MD, MPH, a physician specializing in emergency medicine, internal medicine, and urgent care.
The patient rapidly deteriorated and required intubation and ventilation. She was transferred to a specialized hospital for possible extracorporeal membrane oxygenation (ECMO) treatment.
“This patient was unvaccinated, along with her entire family. This would have been easily preventable,” added Dr. Palli, who is also founder and CEO of MiDoctor Urgent Care in New York City.
“Vaccine misinformation, compounded with vaccine inertia and vaccine access, have contributed to this,” he added. “Even though we have a surplus amount of vaccines at this time, we are only seeing 50% to 55% of completely vaccinated patients.”
Authors of the Associated Press report also acknowledge that some people who are fully vaccinated can get a breakthrough infection. These occurred in fewer than 1,200 of more than 853,000 people hospitalized for COVID-19 in May, or about 0.1%.
The Associated Press came up with these numbers using data from the Centers for Disease Control and Prevention. The CDC tracks the numbers of cases, hospitalizations, and deaths but does not breakdown rates by vaccination status.
Stronger argument for vaccination?
“The fact that only 0.8% of COVID-19 deaths are in the fully vaccinated should persuade those people still hesitant about vaccination,” said Hugh Cassiere, MD, medical director of Respiratory Therapy Services at North Shore University Hospital in Manhasset, New York.
Stuart C. Ray, MD, professor of medicine and oncology in the Division of Infectious Diseases at Johns Hopkins University, Baltimore, agreed. “It seems compelling, even for skeptics, that unvaccinated people represent 99% of those now dying from COVID-19 when they represent less than 50% of the adult population in the United States.”
The findings from the study could be more persuasive than previous arguments made in favor of immunization, Dr. Ray said. “These recent findings of striking reductions in risk of death in the vaccinated are more directly attributable and harder to ignore or dismiss.”
Brian Labus, PhD, MPH, of the University of Nevada Las Vegas (UNLV) is less convinced. “While this might change some peoples’ minds, it probably won’t make a major difference. People have many different reasons for not getting vaccinated, and this is only one of the things they consider.”
The study adds information that was not available before, said Dr. Labus, assistant professor in the Department of Epidemiology and Biostatistics at the UNLV School of Public Health. “We study the vaccine under tightly controlled, ideal conditions. This is the evidence that it works as well in the real world as it did in the trials, and that is what is most important in implementing a vaccination program,” added Dr. Labus.
“The scientific data has honed in on one thing: Vaccines are effective in preventing hospitalizations, ICU admissions, ventilations, and deaths,” agreed Dr. Palli.
“We now know that almost all deaths occurred in patients who were not vaccinated. We also know that all vaccines are effective against various strains that are in circulation right now, including the Delta variant, which is rapidly spreading,” Dr. Palli said.
Dr. Cassiere pointed out that the unvaccinated are not only at higher risk of developing COVID-19 but also of spreading, being hospitalized for, and dying from the infection. Avoiding “long hauler” symptoms is another argument in favor of immunization, he added.
As of June 28, the CDC reports that 63% of Americans 12 years and older have received at least one dose of a COVID-19 vaccine, and 54% are fully vaccinated.
Worldwide worry?
Although overall rates of U.S. COVID-19 hospitalizations and deaths are down, the outlook may not remain as encouraging. “I hope I’m wrong about this, but I anticipate that the coming fall and winter will bring increasingly localized versions of similar findings – severe disease and death due to SARS-CoV-2 infection in regions or groups with lower vaccination rates,” Dr. Ray said.
There could be a silver lining, he added: “If this unfortunate surge occurs, the health and economic consequences seem likely to erode much of the remaining hesitancy regarding vaccination.”
The rise of more infectious SARS-CoV-2 variants, such as the Delta variant, could also throw a wrench in controlling COVID-19. “This isn’t just a domestic issue,” Dr. Ray said. “We have learned that the world is a small place in pandemic times.”
The Associated Press investigators state that their findings support the high efficacy of the vaccine. Also, given the current widespread availability of COVID-19 vaccines in the United States, they believe many of the COVID-19 deaths now occurring are preventable.
Public health measures should have continued longer to protect unvaccinated individuals, especially Black Americans, Hispanic Americans, and other minorities, Dr. Palli said. “Only time will tell if re-opening and abandoning all public health measures by the CDC was premature.”
A version of this article first appeared on Medscape.com.