User login
Physician fired after slurs, including ‘cannibalism,’ against Israel
Fidaa Wishah, MD, a pediatric radiologist at Phoenix Children’s Hospital in Arizona, has been fired after the hospital reviewed evidence that included her anti-Israel comments on social media, according to the hospital’s statement.
On May 26, Dr. Wishah posted, “We will uncover your thirst to kill our Palestinian children. … We sense your fear. The fear of your collapse. A state based on atrocity, inhumanity, racism and cannibalism never last long! Hey #israel … your end is coming sooner than you think.”
Phoenix Children’s Hospital did not respond to this news organization’s request for comment but said in a statement to the Jewish News Syndicate : “After a thorough review of the facts related to this matter, this individual is no longer providing care at Phoenix Children’s. All children in the care of Phoenix Children’s receive hope, healing and the best possible health care, regardless of race, color, disability, religion, gender, gender identity, sexual orientation or national origin.”
Dr. Wishah’s profile has been removed from the hospital website. Her LinkedIn profile indicates she had been a pediatric radiology fellow at Stanford (Calif.) University, specializing in advanced magnetic resonance imaging and fetal imaging and had been a senior staff pediatric radiologist at Henry Ford Health System in Detroit.
It wasn’t the first time antisemitic comments have led to the firing of a physician. Last year, this news organization wrote about Lara Kollab, DO, a first-year resident fired for her antisemitic tweets. She was subsequently barred from medicine.
In the same post from May 26, Dr. Wishah also wrote: “We will not be #censored anymore! Bomb our media buildings and we have the phones[.] Bribe the mainstream media and we have our small #socialmedia platforms[.] From our windows ... from our streets ... next the rubble we will expose you to the world[.] We will expose the #massacre and #genocide you #zionists are proud of[.]”
Today, CAIR-AZ, a group whose mission is to “enhance understanding of Islam, protect civil rights, promote justice, and empower American Muslims,” according to its website, announced that it, along with three private law firms, will represent Dr. Wishah in what they referred to as “her wrongful termination case against Phoenix Children’s Hospital.”
The announcement, which mentions that Dr. Wishah was born and raised in Gaza, said, “Dr. Wishah has been a medical doctor since 2010 and has spent the vast majority of her career as a pediatric physician. Despite caring for thousands of children, many of whom are Jewish, she has never been accused of discriminating against any of her patients or colleagues.”
The statement added, “PCH’s decision to terminate Dr. Wishah is shameful and an attack on freedom of speech.”
A version of this article first appeared on Medscape.com.
Fidaa Wishah, MD, a pediatric radiologist at Phoenix Children’s Hospital in Arizona, has been fired after the hospital reviewed evidence that included her anti-Israel comments on social media, according to the hospital’s statement.
On May 26, Dr. Wishah posted, “We will uncover your thirst to kill our Palestinian children. … We sense your fear. The fear of your collapse. A state based on atrocity, inhumanity, racism and cannibalism never last long! Hey #israel … your end is coming sooner than you think.”
Phoenix Children’s Hospital did not respond to this news organization’s request for comment but said in a statement to the Jewish News Syndicate : “After a thorough review of the facts related to this matter, this individual is no longer providing care at Phoenix Children’s. All children in the care of Phoenix Children’s receive hope, healing and the best possible health care, regardless of race, color, disability, religion, gender, gender identity, sexual orientation or national origin.”
Dr. Wishah’s profile has been removed from the hospital website. Her LinkedIn profile indicates she had been a pediatric radiology fellow at Stanford (Calif.) University, specializing in advanced magnetic resonance imaging and fetal imaging and had been a senior staff pediatric radiologist at Henry Ford Health System in Detroit.
It wasn’t the first time antisemitic comments have led to the firing of a physician. Last year, this news organization wrote about Lara Kollab, DO, a first-year resident fired for her antisemitic tweets. She was subsequently barred from medicine.
In the same post from May 26, Dr. Wishah also wrote: “We will not be #censored anymore! Bomb our media buildings and we have the phones[.] Bribe the mainstream media and we have our small #socialmedia platforms[.] From our windows ... from our streets ... next the rubble we will expose you to the world[.] We will expose the #massacre and #genocide you #zionists are proud of[.]”
Today, CAIR-AZ, a group whose mission is to “enhance understanding of Islam, protect civil rights, promote justice, and empower American Muslims,” according to its website, announced that it, along with three private law firms, will represent Dr. Wishah in what they referred to as “her wrongful termination case against Phoenix Children’s Hospital.”
The announcement, which mentions that Dr. Wishah was born and raised in Gaza, said, “Dr. Wishah has been a medical doctor since 2010 and has spent the vast majority of her career as a pediatric physician. Despite caring for thousands of children, many of whom are Jewish, she has never been accused of discriminating against any of her patients or colleagues.”
The statement added, “PCH’s decision to terminate Dr. Wishah is shameful and an attack on freedom of speech.”
A version of this article first appeared on Medscape.com.
Fidaa Wishah, MD, a pediatric radiologist at Phoenix Children’s Hospital in Arizona, has been fired after the hospital reviewed evidence that included her anti-Israel comments on social media, according to the hospital’s statement.
On May 26, Dr. Wishah posted, “We will uncover your thirst to kill our Palestinian children. … We sense your fear. The fear of your collapse. A state based on atrocity, inhumanity, racism and cannibalism never last long! Hey #israel … your end is coming sooner than you think.”
Phoenix Children’s Hospital did not respond to this news organization’s request for comment but said in a statement to the Jewish News Syndicate : “After a thorough review of the facts related to this matter, this individual is no longer providing care at Phoenix Children’s. All children in the care of Phoenix Children’s receive hope, healing and the best possible health care, regardless of race, color, disability, religion, gender, gender identity, sexual orientation or national origin.”
Dr. Wishah’s profile has been removed from the hospital website. Her LinkedIn profile indicates she had been a pediatric radiology fellow at Stanford (Calif.) University, specializing in advanced magnetic resonance imaging and fetal imaging and had been a senior staff pediatric radiologist at Henry Ford Health System in Detroit.
It wasn’t the first time antisemitic comments have led to the firing of a physician. Last year, this news organization wrote about Lara Kollab, DO, a first-year resident fired for her antisemitic tweets. She was subsequently barred from medicine.
In the same post from May 26, Dr. Wishah also wrote: “We will not be #censored anymore! Bomb our media buildings and we have the phones[.] Bribe the mainstream media and we have our small #socialmedia platforms[.] From our windows ... from our streets ... next the rubble we will expose you to the world[.] We will expose the #massacre and #genocide you #zionists are proud of[.]”
Today, CAIR-AZ, a group whose mission is to “enhance understanding of Islam, protect civil rights, promote justice, and empower American Muslims,” according to its website, announced that it, along with three private law firms, will represent Dr. Wishah in what they referred to as “her wrongful termination case against Phoenix Children’s Hospital.”
The announcement, which mentions that Dr. Wishah was born and raised in Gaza, said, “Dr. Wishah has been a medical doctor since 2010 and has spent the vast majority of her career as a pediatric physician. Despite caring for thousands of children, many of whom are Jewish, she has never been accused of discriminating against any of her patients or colleagues.”
The statement added, “PCH’s decision to terminate Dr. Wishah is shameful and an attack on freedom of speech.”
A version of this article first appeared on Medscape.com.
Wiping Away Cellulitis: A Case of Factitious Disorder
To the Editor:
Patients with psychocutaneous disorders present unique challenges to physicians. We illustrate the critical role that dermoscopy may play to illuminate exogenous skin pathology.
A 50-year-old woman with a reported medical history of systemic lupus erythematosus, chronic pain, and nonhealing leg ulcers presented to the emergency department with severe pain of the left lower leg and redness that was concerning for cellulitis. She sought treatment at an outside hospital for cellulitis 2 weeks prior but left against medical advice. Symptomatic review revealed chest pain, shortness of breath, nausea, vomiting, and diarrhea. The primary team started her on intravenous clindamycin and vancomycin for the presumed infection and scheduled narcotic medications due to concerns of intractable pain in the left leg. The dermatology department was consulted after failure to improve with 1 week of systemic antibiotics.
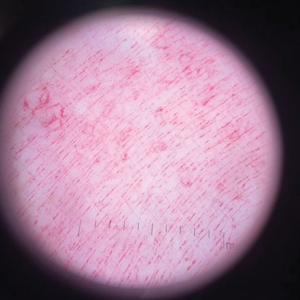
Physical examination revealed a geometric, atrophic, purple plaque on the left anterior shin from a prior leg ulcer as well as a diffuse red-pink patch extending from the knee to the ankle. Notably, the cellulitis spared the left posterior calf resting against the sheet and had a sharp line of demarcation at the distal shin. The leg was cool to the touch while the patient was distractible. She later reported that the leg was extremely tender to palpation. Dermoscopy revealed linear red pigments within skin furrows that accentuated skin lines (Figure). These findings raised suspicions of an external manipulation. The skin was wiped with an alcohol pad that removed a shimmering pink substance consistent in appearance to a cosmetic product. The skin beneath the cellulitis appeared normal.
On further review of the patient’s medical record, it was noted that she was admitted several months ago for ulcers of the left leg. She had been to multiple hospitals and had numerous rounds of antibiotics. Biopsy of an ulcer revealed dermal fibrosis consistent with scarring. Aerobic bacteria, atypical mycobacteria, and fungal cultures were all negative. The physicians suspected a self-induced etiology consistent with dermatitis artefacta. The patient emphasized multiple psychosocial stressors as well as having frequent lupus flares despite repeated negative workup. Given the exaggerated symptoms and unnecessary hospital visits, she was given the diagnosis of factitious disorder (malingering or Munchausen syndrome). After extensive discussion, the patient was amenable to outpatient mental health counseling.
Dermoscopy is not a standard method to diagnose cellulitis of the skin; however, when patients present with an atypical response to appropriate care, the presumed diagnosis must be challenged. This patient had dramatized symptoms, false medical history, and numerous hospitalizations that were suspicious for factitious disorder.1 Furthermore, the physical examination was inconsistent with the classic course of cellulitis. In this case, dermoscopy had advantages over biopsies because it was noninvasive, gave immediate feedback, and provided a macroscopic view of the morphology. Via dermoscopy, we had an objective lens to distinguish cellulitis from cosmetic product and to obtain the correct diagnosis.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
To the Editor:
Patients with psychocutaneous disorders present unique challenges to physicians. We illustrate the critical role that dermoscopy may play to illuminate exogenous skin pathology.
A 50-year-old woman with a reported medical history of systemic lupus erythematosus, chronic pain, and nonhealing leg ulcers presented to the emergency department with severe pain of the left lower leg and redness that was concerning for cellulitis. She sought treatment at an outside hospital for cellulitis 2 weeks prior but left against medical advice. Symptomatic review revealed chest pain, shortness of breath, nausea, vomiting, and diarrhea. The primary team started her on intravenous clindamycin and vancomycin for the presumed infection and scheduled narcotic medications due to concerns of intractable pain in the left leg. The dermatology department was consulted after failure to improve with 1 week of systemic antibiotics.
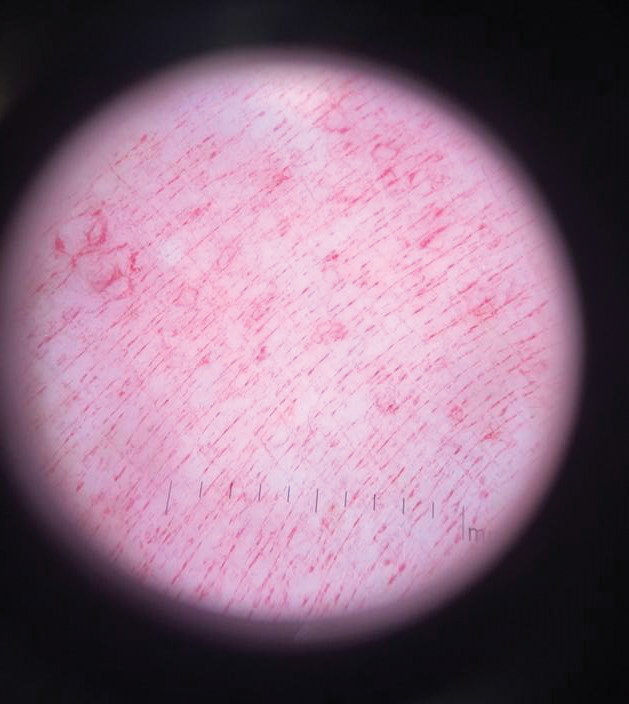
Physical examination revealed a geometric, atrophic, purple plaque on the left anterior shin from a prior leg ulcer as well as a diffuse red-pink patch extending from the knee to the ankle. Notably, the cellulitis spared the left posterior calf resting against the sheet and had a sharp line of demarcation at the distal shin. The leg was cool to the touch while the patient was distractible. She later reported that the leg was extremely tender to palpation. Dermoscopy revealed linear red pigments within skin furrows that accentuated skin lines (Figure). These findings raised suspicions of an external manipulation. The skin was wiped with an alcohol pad that removed a shimmering pink substance consistent in appearance to a cosmetic product. The skin beneath the cellulitis appeared normal.

On further review of the patient’s medical record, it was noted that she was admitted several months ago for ulcers of the left leg. She had been to multiple hospitals and had numerous rounds of antibiotics. Biopsy of an ulcer revealed dermal fibrosis consistent with scarring. Aerobic bacteria, atypical mycobacteria, and fungal cultures were all negative. The physicians suspected a self-induced etiology consistent with dermatitis artefacta. The patient emphasized multiple psychosocial stressors as well as having frequent lupus flares despite repeated negative workup. Given the exaggerated symptoms and unnecessary hospital visits, she was given the diagnosis of factitious disorder (malingering or Munchausen syndrome). After extensive discussion, the patient was amenable to outpatient mental health counseling.
Dermoscopy is not a standard method to diagnose cellulitis of the skin; however, when patients present with an atypical response to appropriate care, the presumed diagnosis must be challenged. This patient had dramatized symptoms, false medical history, and numerous hospitalizations that were suspicious for factitious disorder.1 Furthermore, the physical examination was inconsistent with the classic course of cellulitis. In this case, dermoscopy had advantages over biopsies because it was noninvasive, gave immediate feedback, and provided a macroscopic view of the morphology. Via dermoscopy, we had an objective lens to distinguish cellulitis from cosmetic product and to obtain the correct diagnosis.
To the Editor:
Patients with psychocutaneous disorders present unique challenges to physicians. We illustrate the critical role that dermoscopy may play to illuminate exogenous skin pathology.
A 50-year-old woman with a reported medical history of systemic lupus erythematosus, chronic pain, and nonhealing leg ulcers presented to the emergency department with severe pain of the left lower leg and redness that was concerning for cellulitis. She sought treatment at an outside hospital for cellulitis 2 weeks prior but left against medical advice. Symptomatic review revealed chest pain, shortness of breath, nausea, vomiting, and diarrhea. The primary team started her on intravenous clindamycin and vancomycin for the presumed infection and scheduled narcotic medications due to concerns of intractable pain in the left leg. The dermatology department was consulted after failure to improve with 1 week of systemic antibiotics.
Physical examination revealed a geometric, atrophic, purple plaque on the left anterior shin from a prior leg ulcer as well as a diffuse red-pink patch extending from the knee to the ankle. Notably, the cellulitis spared the left posterior calf resting against the sheet and had a sharp line of demarcation at the distal shin. The leg was cool to the touch while the patient was distractible. She later reported that the leg was extremely tender to palpation. Dermoscopy revealed linear red pigments within skin furrows that accentuated skin lines (Figure). These findings raised suspicions of an external manipulation. The skin was wiped with an alcohol pad that removed a shimmering pink substance consistent in appearance to a cosmetic product. The skin beneath the cellulitis appeared normal.

On further review of the patient’s medical record, it was noted that she was admitted several months ago for ulcers of the left leg. She had been to multiple hospitals and had numerous rounds of antibiotics. Biopsy of an ulcer revealed dermal fibrosis consistent with scarring. Aerobic bacteria, atypical mycobacteria, and fungal cultures were all negative. The physicians suspected a self-induced etiology consistent with dermatitis artefacta. The patient emphasized multiple psychosocial stressors as well as having frequent lupus flares despite repeated negative workup. Given the exaggerated symptoms and unnecessary hospital visits, she was given the diagnosis of factitious disorder (malingering or Munchausen syndrome). After extensive discussion, the patient was amenable to outpatient mental health counseling.
Dermoscopy is not a standard method to diagnose cellulitis of the skin; however, when patients present with an atypical response to appropriate care, the presumed diagnosis must be challenged. This patient had dramatized symptoms, false medical history, and numerous hospitalizations that were suspicious for factitious disorder.1 Furthermore, the physical examination was inconsistent with the classic course of cellulitis. In this case, dermoscopy had advantages over biopsies because it was noninvasive, gave immediate feedback, and provided a macroscopic view of the morphology. Via dermoscopy, we had an objective lens to distinguish cellulitis from cosmetic product and to obtain the correct diagnosis.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
- Harth W, Taube KM, Gieler U. Facticious disorders in dermatology. J Dtsch Dermatol Ges. 2010;8:361-372.
Practice Points
- Consider exogenous factors or alternative diagnoses when a patient does not respond to appropriate care.
- Although dermoscopy is not used to diagnose cellulitis, it could be helpful in distinguishing cosmetic products used in dermatitis artefacta.
Wrong-site surgery doc says he can’t be sued
A neurosurgeon who operated on the wrong side of his patient’s spine claims he can’t be sued because of a federal law that protects health care professionals during a public health emergency, according to a report by KSDK, an NBC-affiliated television station in St. Louis.
Natalie Avilez, who lives in Missouri with her husband and five children, had been suffering from intense back pain. At some point in the recent past (the story doesn’t identify precisely when), she was referred to Fangxiang Chen, MD, a neurosurgeon affiliated with Mercy Hospital and Mercy Hospital South, in St. Louis. Ms. Avilez reportedly claims that Dr. Chen told her that an “easy” surgery – a hemilaminectomy – could relieve her back pain.
Something went wrong during the procedure, however. Dr. Chen ended up operating on the left side of Avilez’s spine instead of the right side, where he had initially diagnosed disk-related pressure. Dr. Chen realized his mistake while his patient was under anesthesia but couldn’t remedy it.
As the patient awakened, Dr. Chen asked her to authorize an immediate right-side surgery, but, as Ms. Avilez told the TV station, her “charge nurse would not let him get authorization because I wasn’t fully awake.” In the recovery room afterward, Dr. Chen explained what had happened to his patient, who permitted him to redo the surgery the following day.
But the redo didn’t remedy Ms. Avilez’s pain; in fact, the second surgery made things worse. “I’m always in constant pain,” she said. “I kind of feel like I would have been better off not even doing it at all.”
In January of this year, Ms. Avilez filed a medical malpractice suit against Dr. Chen and Mercy. But the neurosurgeon made a surprising claim:
Initially passed in 2005, PREP was intended to shield doctors and other licensed health care professionals from liability during a public health emergency except in cases of willful misconduct. On March 17, 2020, then–Health and Human Services Secretary Alex Azar invoked the PREP Act “for activities related to medical countermeasures against COVID-19.”
But could this declaration – which has since been amended multiple times – shield a physician from a claim of wrong-site surgery?
Ms. Avilez’s attorney, Morgan Murphy, doesn’t think so. “Obviously, we are not claiming that COVID had anything to do with the fact that Dr. Chen operated on the incorrect side of Natalie’s spine. It is a fairly straightforward situation. A doctor should never perform the incorrect surgery, period.”
Other observers are less certain that the Chen defense won’t hold. It’s true the PREP Act doesn’t protect doctors against claims of willful or intentional misconduct, says Deidre Gilbert, who leads a national medical malpractice patient-advocacy group. But such claims are, she quickly adds, very difficult to prove, never more so than during a pandemic.
Several states, including Missouri, have passed or are considering additional measures to protect health care professionals against the expected wave of COVID-related claims. (One estimate places the number of those claims at almost 6,000 as of February 2021.) “We want to make sure that there is a heightened standard for holding somebody liable in ... COVID transmission cases,” said the sponsor of the proposed Show-Me State legislation.
As for Ms. Avilez, she feels lucky that she’s not even worse off than she is now. She worries, though, about other patients who are less fortunate and who are told that the pandemic protects their health care professionals from liability. “That’s just not fair,” she says.
Hidden beliefs about people of color raise liability risks
Clinicians’ “implicit bias” can exacerbate medical disparities and also malpractice claims, a story in the Dayton Daily News reports.
The story’s authors cite La Fleur Small, PhD, a medical sociologist at Wayne State University, in Detroit, who sees “implicit bias” as a set of “unconscious associations and judgments” that affect social behavior, causing people to act in ways that are often contrary to their perceived value system. In the medical profession, such thinking can have unintended consequences, especially for people of color.
Implicit bias can erode the physician-patient relationship, which in turn can make a malpractice suit more likely should an adverse event occur. Studies reported in recent years in the AMA Journal of Ethics, for instance, found that poor communication was a factor in almost three-quarters of closed claims. Other studies have revealed that, of patients seeking legal advice following a medical mishap, more than half cited a poor doctor-patient relationship as a contributing factor in their decision.
To remedy things, it would be helpful to boost the number of doctors of color, at least to the point that it more closely reflects the percentage in the general population, say experts. Currently, although Black and Hispanic persons constitute 13.4% and 18.5%, respectively, of the overall U.S. population, they make up only 5.0% and 5.8% of active physicians. (As of 2018, 56.2% of all physicians were White and 17.2% were Asian, according to data from the Association of American Medical Colleges.)
Father of impaired baby seeks mega damages
An Oregon man whose son sustained permanent neurologic injuries during childbirth has sued the hospital where the 2017 delivery took place, as reported in The Astorian.
In the suit on behalf of his son, Wesley Humphries claims that Columbia Memorial Hospital in Astoria, Oregon, failed to monitor the baby’s heart rate and other aspects of the labor and delivery. As a consequence, the baby needed to be transferred to Oregon Health and Science University Hospital in Portland, approximately 100 miles away, for emergency treatment. Doctors there diagnosed the child as having hypoxic ischemic encephalopathy, which his lawyers say resulted in cerebral palsy, among other neurologic conditions.
Because of his son’s permanent impairment, Mr. Humphries is seeking significant damages: more than $45 million in medical, custodial, and life-care expenses and $65 million in noneconomic damages. Should his claim prove successful, the payout would mark one of the largest awards – if not the largest award – in Oregon State history. The hospital has declined to comment.
At press time, a trial date hadn’t been set.
A version of this article first appeared on Medscape.com.
A neurosurgeon who operated on the wrong side of his patient’s spine claims he can’t be sued because of a federal law that protects health care professionals during a public health emergency, according to a report by KSDK, an NBC-affiliated television station in St. Louis.
Natalie Avilez, who lives in Missouri with her husband and five children, had been suffering from intense back pain. At some point in the recent past (the story doesn’t identify precisely when), she was referred to Fangxiang Chen, MD, a neurosurgeon affiliated with Mercy Hospital and Mercy Hospital South, in St. Louis. Ms. Avilez reportedly claims that Dr. Chen told her that an “easy” surgery – a hemilaminectomy – could relieve her back pain.
Something went wrong during the procedure, however. Dr. Chen ended up operating on the left side of Avilez’s spine instead of the right side, where he had initially diagnosed disk-related pressure. Dr. Chen realized his mistake while his patient was under anesthesia but couldn’t remedy it.
As the patient awakened, Dr. Chen asked her to authorize an immediate right-side surgery, but, as Ms. Avilez told the TV station, her “charge nurse would not let him get authorization because I wasn’t fully awake.” In the recovery room afterward, Dr. Chen explained what had happened to his patient, who permitted him to redo the surgery the following day.
But the redo didn’t remedy Ms. Avilez’s pain; in fact, the second surgery made things worse. “I’m always in constant pain,” she said. “I kind of feel like I would have been better off not even doing it at all.”
In January of this year, Ms. Avilez filed a medical malpractice suit against Dr. Chen and Mercy. But the neurosurgeon made a surprising claim:
Initially passed in 2005, PREP was intended to shield doctors and other licensed health care professionals from liability during a public health emergency except in cases of willful misconduct. On March 17, 2020, then–Health and Human Services Secretary Alex Azar invoked the PREP Act “for activities related to medical countermeasures against COVID-19.”
But could this declaration – which has since been amended multiple times – shield a physician from a claim of wrong-site surgery?
Ms. Avilez’s attorney, Morgan Murphy, doesn’t think so. “Obviously, we are not claiming that COVID had anything to do with the fact that Dr. Chen operated on the incorrect side of Natalie’s spine. It is a fairly straightforward situation. A doctor should never perform the incorrect surgery, period.”
Other observers are less certain that the Chen defense won’t hold. It’s true the PREP Act doesn’t protect doctors against claims of willful or intentional misconduct, says Deidre Gilbert, who leads a national medical malpractice patient-advocacy group. But such claims are, she quickly adds, very difficult to prove, never more so than during a pandemic.
Several states, including Missouri, have passed or are considering additional measures to protect health care professionals against the expected wave of COVID-related claims. (One estimate places the number of those claims at almost 6,000 as of February 2021.) “We want to make sure that there is a heightened standard for holding somebody liable in ... COVID transmission cases,” said the sponsor of the proposed Show-Me State legislation.
As for Ms. Avilez, she feels lucky that she’s not even worse off than she is now. She worries, though, about other patients who are less fortunate and who are told that the pandemic protects their health care professionals from liability. “That’s just not fair,” she says.
Hidden beliefs about people of color raise liability risks
Clinicians’ “implicit bias” can exacerbate medical disparities and also malpractice claims, a story in the Dayton Daily News reports.
The story’s authors cite La Fleur Small, PhD, a medical sociologist at Wayne State University, in Detroit, who sees “implicit bias” as a set of “unconscious associations and judgments” that affect social behavior, causing people to act in ways that are often contrary to their perceived value system. In the medical profession, such thinking can have unintended consequences, especially for people of color.
Implicit bias can erode the physician-patient relationship, which in turn can make a malpractice suit more likely should an adverse event occur. Studies reported in recent years in the AMA Journal of Ethics, for instance, found that poor communication was a factor in almost three-quarters of closed claims. Other studies have revealed that, of patients seeking legal advice following a medical mishap, more than half cited a poor doctor-patient relationship as a contributing factor in their decision.
To remedy things, it would be helpful to boost the number of doctors of color, at least to the point that it more closely reflects the percentage in the general population, say experts. Currently, although Black and Hispanic persons constitute 13.4% and 18.5%, respectively, of the overall U.S. population, they make up only 5.0% and 5.8% of active physicians. (As of 2018, 56.2% of all physicians were White and 17.2% were Asian, according to data from the Association of American Medical Colleges.)
Father of impaired baby seeks mega damages
An Oregon man whose son sustained permanent neurologic injuries during childbirth has sued the hospital where the 2017 delivery took place, as reported in The Astorian.
In the suit on behalf of his son, Wesley Humphries claims that Columbia Memorial Hospital in Astoria, Oregon, failed to monitor the baby’s heart rate and other aspects of the labor and delivery. As a consequence, the baby needed to be transferred to Oregon Health and Science University Hospital in Portland, approximately 100 miles away, for emergency treatment. Doctors there diagnosed the child as having hypoxic ischemic encephalopathy, which his lawyers say resulted in cerebral palsy, among other neurologic conditions.
Because of his son’s permanent impairment, Mr. Humphries is seeking significant damages: more than $45 million in medical, custodial, and life-care expenses and $65 million in noneconomic damages. Should his claim prove successful, the payout would mark one of the largest awards – if not the largest award – in Oregon State history. The hospital has declined to comment.
At press time, a trial date hadn’t been set.
A version of this article first appeared on Medscape.com.
A neurosurgeon who operated on the wrong side of his patient’s spine claims he can’t be sued because of a federal law that protects health care professionals during a public health emergency, according to a report by KSDK, an NBC-affiliated television station in St. Louis.
Natalie Avilez, who lives in Missouri with her husband and five children, had been suffering from intense back pain. At some point in the recent past (the story doesn’t identify precisely when), she was referred to Fangxiang Chen, MD, a neurosurgeon affiliated with Mercy Hospital and Mercy Hospital South, in St. Louis. Ms. Avilez reportedly claims that Dr. Chen told her that an “easy” surgery – a hemilaminectomy – could relieve her back pain.
Something went wrong during the procedure, however. Dr. Chen ended up operating on the left side of Avilez’s spine instead of the right side, where he had initially diagnosed disk-related pressure. Dr. Chen realized his mistake while his patient was under anesthesia but couldn’t remedy it.
As the patient awakened, Dr. Chen asked her to authorize an immediate right-side surgery, but, as Ms. Avilez told the TV station, her “charge nurse would not let him get authorization because I wasn’t fully awake.” In the recovery room afterward, Dr. Chen explained what had happened to his patient, who permitted him to redo the surgery the following day.
But the redo didn’t remedy Ms. Avilez’s pain; in fact, the second surgery made things worse. “I’m always in constant pain,” she said. “I kind of feel like I would have been better off not even doing it at all.”
In January of this year, Ms. Avilez filed a medical malpractice suit against Dr. Chen and Mercy. But the neurosurgeon made a surprising claim:
Initially passed in 2005, PREP was intended to shield doctors and other licensed health care professionals from liability during a public health emergency except in cases of willful misconduct. On March 17, 2020, then–Health and Human Services Secretary Alex Azar invoked the PREP Act “for activities related to medical countermeasures against COVID-19.”
But could this declaration – which has since been amended multiple times – shield a physician from a claim of wrong-site surgery?
Ms. Avilez’s attorney, Morgan Murphy, doesn’t think so. “Obviously, we are not claiming that COVID had anything to do with the fact that Dr. Chen operated on the incorrect side of Natalie’s spine. It is a fairly straightforward situation. A doctor should never perform the incorrect surgery, period.”
Other observers are less certain that the Chen defense won’t hold. It’s true the PREP Act doesn’t protect doctors against claims of willful or intentional misconduct, says Deidre Gilbert, who leads a national medical malpractice patient-advocacy group. But such claims are, she quickly adds, very difficult to prove, never more so than during a pandemic.
Several states, including Missouri, have passed or are considering additional measures to protect health care professionals against the expected wave of COVID-related claims. (One estimate places the number of those claims at almost 6,000 as of February 2021.) “We want to make sure that there is a heightened standard for holding somebody liable in ... COVID transmission cases,” said the sponsor of the proposed Show-Me State legislation.
As for Ms. Avilez, she feels lucky that she’s not even worse off than she is now. She worries, though, about other patients who are less fortunate and who are told that the pandemic protects their health care professionals from liability. “That’s just not fair,” she says.
Hidden beliefs about people of color raise liability risks
Clinicians’ “implicit bias” can exacerbate medical disparities and also malpractice claims, a story in the Dayton Daily News reports.
The story’s authors cite La Fleur Small, PhD, a medical sociologist at Wayne State University, in Detroit, who sees “implicit bias” as a set of “unconscious associations and judgments” that affect social behavior, causing people to act in ways that are often contrary to their perceived value system. In the medical profession, such thinking can have unintended consequences, especially for people of color.
Implicit bias can erode the physician-patient relationship, which in turn can make a malpractice suit more likely should an adverse event occur. Studies reported in recent years in the AMA Journal of Ethics, for instance, found that poor communication was a factor in almost three-quarters of closed claims. Other studies have revealed that, of patients seeking legal advice following a medical mishap, more than half cited a poor doctor-patient relationship as a contributing factor in their decision.
To remedy things, it would be helpful to boost the number of doctors of color, at least to the point that it more closely reflects the percentage in the general population, say experts. Currently, although Black and Hispanic persons constitute 13.4% and 18.5%, respectively, of the overall U.S. population, they make up only 5.0% and 5.8% of active physicians. (As of 2018, 56.2% of all physicians were White and 17.2% were Asian, according to data from the Association of American Medical Colleges.)
Father of impaired baby seeks mega damages
An Oregon man whose son sustained permanent neurologic injuries during childbirth has sued the hospital where the 2017 delivery took place, as reported in The Astorian.
In the suit on behalf of his son, Wesley Humphries claims that Columbia Memorial Hospital in Astoria, Oregon, failed to monitor the baby’s heart rate and other aspects of the labor and delivery. As a consequence, the baby needed to be transferred to Oregon Health and Science University Hospital in Portland, approximately 100 miles away, for emergency treatment. Doctors there diagnosed the child as having hypoxic ischemic encephalopathy, which his lawyers say resulted in cerebral palsy, among other neurologic conditions.
Because of his son’s permanent impairment, Mr. Humphries is seeking significant damages: more than $45 million in medical, custodial, and life-care expenses and $65 million in noneconomic damages. Should his claim prove successful, the payout would mark one of the largest awards – if not the largest award – in Oregon State history. The hospital has declined to comment.
At press time, a trial date hadn’t been set.
A version of this article first appeared on Medscape.com.
Lack of fever in ESRD with S. aureus bacteremia is common
Background: Fever is a common symptom in patients presenting to the ED. In patients with hemodialysis-dependent ESRD, the literature on febrile response during infection is scarce. In this study, authors compared ED triage temperatures of S. aureus bacteremic patients with and without hemodialysis-dependent ESRD.
Study design: Paired, retrospective cohort study.
Setting: Tertiary care referral center.
Synopsis: A total of 74 patients with methicillin-resistant or methicillin-susceptible S. aureus bacteremia were included in this study (37 patients with and 37 patients without hemodialysis-dependent ESRD). Upon triage, 54% (95% confidence interval, 38%-70%) and 82% (95% CI, 65%-91%) of hemodialysis and nonhemodialysis patients did not have a detectable fever (less than 100.4° F), respectively. The estimated mean ED triage temperatures were 100.5° F in the hemodialysis-dependent patients and 99.0° F in the non–hemodialysis-dependent patients (P < .001). The authors note the significant lack of fevers may be the result of insensitive methods for measuring body temperature, such as peripheral thermometers.
Bottom line: In this small retrospective cohort study, these data suggest a high incidence of afebrile bacteremia in patients with ESRD, especially those patients not dialysis dependent. This may lead to delays in obtaining blood cultures and initiating antibiotics. However, given the study design, the authors were unable to conclude a causal relationship between ESRD and febrile response.
Citation: Weatherall SL et al. Do bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg Med. 2020;20:2.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Fever is a common symptom in patients presenting to the ED. In patients with hemodialysis-dependent ESRD, the literature on febrile response during infection is scarce. In this study, authors compared ED triage temperatures of S. aureus bacteremic patients with and without hemodialysis-dependent ESRD.
Study design: Paired, retrospective cohort study.
Setting: Tertiary care referral center.
Synopsis: A total of 74 patients with methicillin-resistant or methicillin-susceptible S. aureus bacteremia were included in this study (37 patients with and 37 patients without hemodialysis-dependent ESRD). Upon triage, 54% (95% confidence interval, 38%-70%) and 82% (95% CI, 65%-91%) of hemodialysis and nonhemodialysis patients did not have a detectable fever (less than 100.4° F), respectively. The estimated mean ED triage temperatures were 100.5° F in the hemodialysis-dependent patients and 99.0° F in the non–hemodialysis-dependent patients (P < .001). The authors note the significant lack of fevers may be the result of insensitive methods for measuring body temperature, such as peripheral thermometers.
Bottom line: In this small retrospective cohort study, these data suggest a high incidence of afebrile bacteremia in patients with ESRD, especially those patients not dialysis dependent. This may lead to delays in obtaining blood cultures and initiating antibiotics. However, given the study design, the authors were unable to conclude a causal relationship between ESRD and febrile response.
Citation: Weatherall SL et al. Do bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg Med. 2020;20:2.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Background: Fever is a common symptom in patients presenting to the ED. In patients with hemodialysis-dependent ESRD, the literature on febrile response during infection is scarce. In this study, authors compared ED triage temperatures of S. aureus bacteremic patients with and without hemodialysis-dependent ESRD.
Study design: Paired, retrospective cohort study.
Setting: Tertiary care referral center.
Synopsis: A total of 74 patients with methicillin-resistant or methicillin-susceptible S. aureus bacteremia were included in this study (37 patients with and 37 patients without hemodialysis-dependent ESRD). Upon triage, 54% (95% confidence interval, 38%-70%) and 82% (95% CI, 65%-91%) of hemodialysis and nonhemodialysis patients did not have a detectable fever (less than 100.4° F), respectively. The estimated mean ED triage temperatures were 100.5° F in the hemodialysis-dependent patients and 99.0° F in the non–hemodialysis-dependent patients (P < .001). The authors note the significant lack of fevers may be the result of insensitive methods for measuring body temperature, such as peripheral thermometers.
Bottom line: In this small retrospective cohort study, these data suggest a high incidence of afebrile bacteremia in patients with ESRD, especially those patients not dialysis dependent. This may lead to delays in obtaining blood cultures and initiating antibiotics. However, given the study design, the authors were unable to conclude a causal relationship between ESRD and febrile response.
Citation: Weatherall SL et al. Do bacteremic patients with end-stage renal disease have a fever when presenting to the emergency department? A paired, retrospective cohort study. BMC Emerg Med. 2020;20:2.
Dr. Schmit is a hospitalist and associate professor of medicine at University of Texas Health, San Antonio.
Novel liver dialysis device may safely curb ACLF
An investigational liver dialysis device (DIALIVE) was associated with significantly greater survival of patients with acute-on-chronic liver failure (ACLF), compared with the standard of care in a multicenter randomized study.
Among 30 evaluable patients with ACLF from alcoholic cirrhosis randomized to treatment with the DIALIVE system or standard of care, two-thirds of patients assigned to DIALIVE had both survived and experienced resolution of ACLF by 28 days, compared with one-third of patients assigned to standard of care, reported Banwari Agarwal, MBBS, MD from the Royal Free Hospital in London at the meeting sponsored by the European Association for the Study of the Liver.
Different from MARS
The DIALIVE system differs from the Molecular Adsorbent Recirculating System (MARS) liver dialysis system in that DIALIVE removes and replaces albumin, including proinflammatory albumin, rather than filtering and recirculating it, he explained.
“It addresses systemic inflammation, which wasn’t quite the case with MARS,” he said in the question-and-answer portion of his presentation in a general session.
In patients with ACLF, the risk of 28-day mortality increases substantially as the grade of ACLF increases.
“ACLF, however, is potentially reversible, and the initial grade at presentation undergoes changes over time during the natural course of the illness, with some patients deteriorating, some improving, and some even achieving complete ACLF resolution. The final grade is reached by days 3-7, and it is this final grade which determines their future outcome trajectory. I therefore propose that ACLF resolution in itself is an important therapeutic target,” he said.
Study details
Dr. Agarwal and coinvestigators from eight centers in six European countries enrolled patients with a history indicative of alcohol-related cirrhosis, at least one acute decompensation event, and progression to ACLF grades 1, 2, or 3a.
Patients with an international normalized ratio above 3 were excluded, as were those with more than three organ failures, uncontrolled infections, patients with primary respiratory organ failure, and those with hemodynamic instability refractory to volume resuscitation and low-dose vasopressors.
A total of 32 patients, of whom 30 were evaluable, were randomized to receive liver dialysis in three to five DIALIVE sessions lasting 8-12 hours each (15 evaluable patients) or to standard of care at participating institutions (15 patients).
The investigators looked at safety of the device (the primary endpoint) in all patients who received at least one DIALIVE treatment (safety population), and a modified safety population of patients who received at least three DIALIVE treatments.
The median patient age in each arm was 49 years, and all patients had alcoholic cirrhosis, with alcoholic hepatitis accounting for at least one decompensation event. In addition, about 25% of patients in each arm had decompensation with infections and/or sepsis as precipitating factors.
Safety
Serious adverse events on days 1-10 occurred in 11 of 17 patients in the DIALIVE arm, and in 8 in the standard-of-care arm. In the DIALIVE arm, there were seven treatment-related serious device events, three unexpected serious device events (anemia, septic shock, and hypotension), and one patient discontinued dialysis after having unsafe levels of thrombocytopenia.
Four patients in the DIALIVE arm died on study. The first two died on day 1 one from hypotension, coagulopathy, and multiorgan failure, and this prompted a change in the protocol mandating that DIALIVE be conducted only in an ICU setting with more invasive monitoring and more frequent lab analysis of clotting and other biochemical parameters. Of the two other patients in the DIALIVE arm who on died on study, one died from non-MI cardiac arrest on day 8, and one patient with ACLF grade 3 and a European Foundation for the study of chronic liver failure (CLIF)–ACLF score of 68 died from multiorgan failure.
“I must emphasize that even this very sick patient tolerated the device very, very well,” Dr. Agarwal said.
In the standard-of-care arm, two patients died from progressive liver failure on days 17 and 27, respectively, and one died on day 17 from bacterial infections, bleeding, and progressive liver failure.
There were eight instances of filters clotting out of 64 filters used in total, and four episodes of device deficiency, including two instances where tubing could not be disconnected from an Oxiris filter during setup of the DIALIVE circuit, requiring use of new DIALIVE kits; one use of an incorrect dialysis fluid; and one incorrect setup of the DIALIVE circuit.
Significant improvements in many scores
In the DIALIVE group, there were significant improvements over baseline at day 10 in both liver scores (P < .05) and brain scores (P < .001). In contrast, in the standard-of-care group there were no improvements in individual organ scores, and respiration scores were significantly worse (P < .01).
DIALIVE was also associated with significant improvements in CLIF-C organ failure scores, compared with standard of care at day 5 and day 10 (P = .021 and .001, respectively); CLIF-C–ACLF scores at days 5 and 10 (P = .045 and .023); and Model for End-Stage Liver Disease scores at day 5 (P = .028).
In the DIALIVE group, 40% of patients had ACLF resolution by day 5, and 66.7% had resolution by day 10. In the standard-of-care arm, 15% had resolution on day 5, and 33.3% had resolution on day 10. DIALIVE was also associated with a significantly faster median time to resolution, compared with standard of care (10 days vs. not reached; P = .0307). At 28 days, 10 of 15 evaluable patients were alive and had resolution of ACLF with DIALIVE versus 5 of 15 with standard of care (P = .0281).
Dr. Agarwal said that the data justify the implementation of late-phase clinical trials of the liver dialysis device.
‘Hopeful’ findings
“It’s very early, but we’re really desperate in finding something to bridge to transplantation,” commented Tobias Boettler, MD, from the University of Freiburg (Germany), who was not involved in the study.
“I think this is very hopeful,” said Dr. Boettler, who moderated the briefing where Dr. Agarwal summarized the study findings.
In the question and answer following the talk in a general session, moderator Philip N. Newsome, MD, from University Hospitals Birmingham (England) asked whether patients who were not treated should have been included in the analysis.
Dr. Agarwal replied that “the whole idea behind this study was to understand what this device does to these patients, and how these patients react to this device, so really not looking at the efficacy.”
The study was supported by the European Union’s Horizon 2020 initiative. Dr. Agarwal received a study grant from the initiative, but had no other relevant disclosures. Dr. Boettler and Dr. Newsome had no disclosures relevant to the study.
An investigational liver dialysis device (DIALIVE) was associated with significantly greater survival of patients with acute-on-chronic liver failure (ACLF), compared with the standard of care in a multicenter randomized study.
Among 30 evaluable patients with ACLF from alcoholic cirrhosis randomized to treatment with the DIALIVE system or standard of care, two-thirds of patients assigned to DIALIVE had both survived and experienced resolution of ACLF by 28 days, compared with one-third of patients assigned to standard of care, reported Banwari Agarwal, MBBS, MD from the Royal Free Hospital in London at the meeting sponsored by the European Association for the Study of the Liver.
Different from MARS
The DIALIVE system differs from the Molecular Adsorbent Recirculating System (MARS) liver dialysis system in that DIALIVE removes and replaces albumin, including proinflammatory albumin, rather than filtering and recirculating it, he explained.
“It addresses systemic inflammation, which wasn’t quite the case with MARS,” he said in the question-and-answer portion of his presentation in a general session.
In patients with ACLF, the risk of 28-day mortality increases substantially as the grade of ACLF increases.
“ACLF, however, is potentially reversible, and the initial grade at presentation undergoes changes over time during the natural course of the illness, with some patients deteriorating, some improving, and some even achieving complete ACLF resolution. The final grade is reached by days 3-7, and it is this final grade which determines their future outcome trajectory. I therefore propose that ACLF resolution in itself is an important therapeutic target,” he said.
Study details
Dr. Agarwal and coinvestigators from eight centers in six European countries enrolled patients with a history indicative of alcohol-related cirrhosis, at least one acute decompensation event, and progression to ACLF grades 1, 2, or 3a.
Patients with an international normalized ratio above 3 were excluded, as were those with more than three organ failures, uncontrolled infections, patients with primary respiratory organ failure, and those with hemodynamic instability refractory to volume resuscitation and low-dose vasopressors.
A total of 32 patients, of whom 30 were evaluable, were randomized to receive liver dialysis in three to five DIALIVE sessions lasting 8-12 hours each (15 evaluable patients) or to standard of care at participating institutions (15 patients).
The investigators looked at safety of the device (the primary endpoint) in all patients who received at least one DIALIVE treatment (safety population), and a modified safety population of patients who received at least three DIALIVE treatments.
The median patient age in each arm was 49 years, and all patients had alcoholic cirrhosis, with alcoholic hepatitis accounting for at least one decompensation event. In addition, about 25% of patients in each arm had decompensation with infections and/or sepsis as precipitating factors.
Safety
Serious adverse events on days 1-10 occurred in 11 of 17 patients in the DIALIVE arm, and in 8 in the standard-of-care arm. In the DIALIVE arm, there were seven treatment-related serious device events, three unexpected serious device events (anemia, septic shock, and hypotension), and one patient discontinued dialysis after having unsafe levels of thrombocytopenia.
Four patients in the DIALIVE arm died on study. The first two died on day 1 one from hypotension, coagulopathy, and multiorgan failure, and this prompted a change in the protocol mandating that DIALIVE be conducted only in an ICU setting with more invasive monitoring and more frequent lab analysis of clotting and other biochemical parameters. Of the two other patients in the DIALIVE arm who on died on study, one died from non-MI cardiac arrest on day 8, and one patient with ACLF grade 3 and a European Foundation for the study of chronic liver failure (CLIF)–ACLF score of 68 died from multiorgan failure.
“I must emphasize that even this very sick patient tolerated the device very, very well,” Dr. Agarwal said.
In the standard-of-care arm, two patients died from progressive liver failure on days 17 and 27, respectively, and one died on day 17 from bacterial infections, bleeding, and progressive liver failure.
There were eight instances of filters clotting out of 64 filters used in total, and four episodes of device deficiency, including two instances where tubing could not be disconnected from an Oxiris filter during setup of the DIALIVE circuit, requiring use of new DIALIVE kits; one use of an incorrect dialysis fluid; and one incorrect setup of the DIALIVE circuit.
Significant improvements in many scores
In the DIALIVE group, there were significant improvements over baseline at day 10 in both liver scores (P < .05) and brain scores (P < .001). In contrast, in the standard-of-care group there were no improvements in individual organ scores, and respiration scores were significantly worse (P < .01).
DIALIVE was also associated with significant improvements in CLIF-C organ failure scores, compared with standard of care at day 5 and day 10 (P = .021 and .001, respectively); CLIF-C–ACLF scores at days 5 and 10 (P = .045 and .023); and Model for End-Stage Liver Disease scores at day 5 (P = .028).
In the DIALIVE group, 40% of patients had ACLF resolution by day 5, and 66.7% had resolution by day 10. In the standard-of-care arm, 15% had resolution on day 5, and 33.3% had resolution on day 10. DIALIVE was also associated with a significantly faster median time to resolution, compared with standard of care (10 days vs. not reached; P = .0307). At 28 days, 10 of 15 evaluable patients were alive and had resolution of ACLF with DIALIVE versus 5 of 15 with standard of care (P = .0281).
Dr. Agarwal said that the data justify the implementation of late-phase clinical trials of the liver dialysis device.
‘Hopeful’ findings
“It’s very early, but we’re really desperate in finding something to bridge to transplantation,” commented Tobias Boettler, MD, from the University of Freiburg (Germany), who was not involved in the study.
“I think this is very hopeful,” said Dr. Boettler, who moderated the briefing where Dr. Agarwal summarized the study findings.
In the question and answer following the talk in a general session, moderator Philip N. Newsome, MD, from University Hospitals Birmingham (England) asked whether patients who were not treated should have been included in the analysis.
Dr. Agarwal replied that “the whole idea behind this study was to understand what this device does to these patients, and how these patients react to this device, so really not looking at the efficacy.”
The study was supported by the European Union’s Horizon 2020 initiative. Dr. Agarwal received a study grant from the initiative, but had no other relevant disclosures. Dr. Boettler and Dr. Newsome had no disclosures relevant to the study.
An investigational liver dialysis device (DIALIVE) was associated with significantly greater survival of patients with acute-on-chronic liver failure (ACLF), compared with the standard of care in a multicenter randomized study.
Among 30 evaluable patients with ACLF from alcoholic cirrhosis randomized to treatment with the DIALIVE system or standard of care, two-thirds of patients assigned to DIALIVE had both survived and experienced resolution of ACLF by 28 days, compared with one-third of patients assigned to standard of care, reported Banwari Agarwal, MBBS, MD from the Royal Free Hospital in London at the meeting sponsored by the European Association for the Study of the Liver.
Different from MARS
The DIALIVE system differs from the Molecular Adsorbent Recirculating System (MARS) liver dialysis system in that DIALIVE removes and replaces albumin, including proinflammatory albumin, rather than filtering and recirculating it, he explained.
“It addresses systemic inflammation, which wasn’t quite the case with MARS,” he said in the question-and-answer portion of his presentation in a general session.
In patients with ACLF, the risk of 28-day mortality increases substantially as the grade of ACLF increases.
“ACLF, however, is potentially reversible, and the initial grade at presentation undergoes changes over time during the natural course of the illness, with some patients deteriorating, some improving, and some even achieving complete ACLF resolution. The final grade is reached by days 3-7, and it is this final grade which determines their future outcome trajectory. I therefore propose that ACLF resolution in itself is an important therapeutic target,” he said.
Study details
Dr. Agarwal and coinvestigators from eight centers in six European countries enrolled patients with a history indicative of alcohol-related cirrhosis, at least one acute decompensation event, and progression to ACLF grades 1, 2, or 3a.
Patients with an international normalized ratio above 3 were excluded, as were those with more than three organ failures, uncontrolled infections, patients with primary respiratory organ failure, and those with hemodynamic instability refractory to volume resuscitation and low-dose vasopressors.
A total of 32 patients, of whom 30 were evaluable, were randomized to receive liver dialysis in three to five DIALIVE sessions lasting 8-12 hours each (15 evaluable patients) or to standard of care at participating institutions (15 patients).
The investigators looked at safety of the device (the primary endpoint) in all patients who received at least one DIALIVE treatment (safety population), and a modified safety population of patients who received at least three DIALIVE treatments.
The median patient age in each arm was 49 years, and all patients had alcoholic cirrhosis, with alcoholic hepatitis accounting for at least one decompensation event. In addition, about 25% of patients in each arm had decompensation with infections and/or sepsis as precipitating factors.
Safety
Serious adverse events on days 1-10 occurred in 11 of 17 patients in the DIALIVE arm, and in 8 in the standard-of-care arm. In the DIALIVE arm, there were seven treatment-related serious device events, three unexpected serious device events (anemia, septic shock, and hypotension), and one patient discontinued dialysis after having unsafe levels of thrombocytopenia.
Four patients in the DIALIVE arm died on study. The first two died on day 1 one from hypotension, coagulopathy, and multiorgan failure, and this prompted a change in the protocol mandating that DIALIVE be conducted only in an ICU setting with more invasive monitoring and more frequent lab analysis of clotting and other biochemical parameters. Of the two other patients in the DIALIVE arm who on died on study, one died from non-MI cardiac arrest on day 8, and one patient with ACLF grade 3 and a European Foundation for the study of chronic liver failure (CLIF)–ACLF score of 68 died from multiorgan failure.
“I must emphasize that even this very sick patient tolerated the device very, very well,” Dr. Agarwal said.
In the standard-of-care arm, two patients died from progressive liver failure on days 17 and 27, respectively, and one died on day 17 from bacterial infections, bleeding, and progressive liver failure.
There were eight instances of filters clotting out of 64 filters used in total, and four episodes of device deficiency, including two instances where tubing could not be disconnected from an Oxiris filter during setup of the DIALIVE circuit, requiring use of new DIALIVE kits; one use of an incorrect dialysis fluid; and one incorrect setup of the DIALIVE circuit.
Significant improvements in many scores
In the DIALIVE group, there were significant improvements over baseline at day 10 in both liver scores (P < .05) and brain scores (P < .001). In contrast, in the standard-of-care group there were no improvements in individual organ scores, and respiration scores were significantly worse (P < .01).
DIALIVE was also associated with significant improvements in CLIF-C organ failure scores, compared with standard of care at day 5 and day 10 (P = .021 and .001, respectively); CLIF-C–ACLF scores at days 5 and 10 (P = .045 and .023); and Model for End-Stage Liver Disease scores at day 5 (P = .028).
In the DIALIVE group, 40% of patients had ACLF resolution by day 5, and 66.7% had resolution by day 10. In the standard-of-care arm, 15% had resolution on day 5, and 33.3% had resolution on day 10. DIALIVE was also associated with a significantly faster median time to resolution, compared with standard of care (10 days vs. not reached; P = .0307). At 28 days, 10 of 15 evaluable patients were alive and had resolution of ACLF with DIALIVE versus 5 of 15 with standard of care (P = .0281).
Dr. Agarwal said that the data justify the implementation of late-phase clinical trials of the liver dialysis device.
‘Hopeful’ findings
“It’s very early, but we’re really desperate in finding something to bridge to transplantation,” commented Tobias Boettler, MD, from the University of Freiburg (Germany), who was not involved in the study.
“I think this is very hopeful,” said Dr. Boettler, who moderated the briefing where Dr. Agarwal summarized the study findings.
In the question and answer following the talk in a general session, moderator Philip N. Newsome, MD, from University Hospitals Birmingham (England) asked whether patients who were not treated should have been included in the analysis.
Dr. Agarwal replied that “the whole idea behind this study was to understand what this device does to these patients, and how these patients react to this device, so really not looking at the efficacy.”
The study was supported by the European Union’s Horizon 2020 initiative. Dr. Agarwal received a study grant from the initiative, but had no other relevant disclosures. Dr. Boettler and Dr. Newsome had no disclosures relevant to the study.
FROM ILC 2021
Rate of cutaneous toxicities from ICIs may be lower than previously reported
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
A , according to research presented at the annual meeting of the Society for Investigative Dermatology, held virtually.
What’s more, many of the cutaneous immune-related adverse events (irAEs) from immune checkpoint inhibitors (ICIs) observed in the study may be unreported in clinical trial settings and by providers, according to one of the investigators, Yevgeniy Semenov, MD, MA, a dermatologist at Massachusetts General Hospital, Boston.
“Most cutaneous irAEs are low grade and might go unreported outside of clinical trial settings, as patients might not seek medical care, or when they do, providers might not report them in patient charts. As a result, the diagnoses identified in this study likely represent the most clinically relevant cutaneous events in the ICI population,” said Dr. Semenov, who presented the results at the meeting.
In the study, he said that one of the first issues he and his colleagues encountered was how to classify cutaneous irAEs, as they “can vary widely in morphology and severity.” Immune-related adverse events from ICIs are a “unique constellation of inflammatory toxicities,” affecting nearly every organ system, and may require treatment with immunosuppressive agents that can impact the effectiveness of the ICI. The matter is further complicated by a “lack of definitional standards of what constitutes a cutaneous immune-related adverse event, which greatly limits the research in this area,” Dr. Semenov said. There is also potential for misdiagnosis of irAEs as cutaneous eruptions occurring in patients receiving ICI therapy because of failure to account for the presence of skin disease at baseline, he pointed out.
Dr. Semenov noted that more than 40 cutaneous eruptions have been associated with ICI treatment. “Much of the observational data on cutaneous immune-related adverse events has been riddled with case reports and case series of cutaneous events that happen to be occurring in the setting of ICI therapy. These lack rigorous control groups and often associate events with little to no relationship to the actual ICI, which may have instead occurred in the setting of a competing medication,” he explained.
Real-world data
The researchers thus sought to identify the real-world incidence of cutaneous irAEs with population-level data. Using data from a national claims insurance database from January 2011 through 2019, they compared 8,637 of patients with cancer, treated with an ICI (who had not been treated with other cancer treatments within 6 months of starting an ICI) with 8,637 patients with cancer who were not treated with an ICI, matched for demographics, primary cancer type, and Charlson Comorbidity Index (CCI) score.
In both groups, the mean age of the patients was 67.5 years, 59.2% were men, and 93% had a severe CCI score. The most common cancer types were lung cancer (40%), melanoma (26.6%), and renal cell carcinoma (12.3%). The median follow-up time was 1.9 years, and the median treatment duration was 2.0 years.
Dr. Semenov and colleagues selected 42 dermatoses reported in the literature to evaluate and found an overall incidence of 25% within 2 years of starting ICI therapy. Of those 42 dermatoses, there were 10 with a significantly higher incidence among patients receiving ICIs, compared with controls: drug eruption or other nonspecific eruption (4.2%; incidence rate ratio, 5.00), bullous pemphigoid (0.3%; IRR, 4.91), maculopapular eruption (0.9%; IRR, 4.75), vitiligo (0.7%; IRR, 3.79), Grover’s disease (0.2%; IRR, 3.43), rash and other nonspecific eruption (9.0%; IRR, 2.34), mucositis (1.5%; IRR, 2.33), pruritus (4.8%; IRR, 1.92), lichen planus (0.5%; IRR, 1.75), and erythroderma (1.1%; IRR, 1.70).
After adjusting for a baseline history of squamous cell carcinoma and actinic keratosis, the researchers found that both were significantly less likely in patients receiving ICIs.
A delay in presentation of any cutaneous irAE after starting ICI therapy was also observed (a median of 16.1 weeks), which Dr. Semenov noted was longer than the 5 weeks reported in clinical trials. This delay in presentation increased to a median of 37.5 weeks for the 10 dermatoses with a significantly higher incidence among patients receiving ICIs, with 17.6% of patients presenting in the first month, 63.1% presenting by 6 months, and 84.6% presenting by 1 year.
Use of immunosuppressive treatment
The researchers also examined use of systemic immunosuppression for treating cutaneous toxicities, defined as “a new prescription for systemic glucocorticoids greater than 10 mg per day, prednisone equivalent, or nonsteroidal systemic immunosuppression,” administered within 7 days of the diagnosis of the cutaneous event. They found that 5% of patients overall received systemic immunosuppressive treatment within 7 days of a cutaneous event, which was “at the higher end of what was reported in clinical trials for the treatment of cutaneous toxicities,” Dr. Semenov noted.
“This is likely the result of the delays in diagnosis in nonclinical trial settings ... allowing more time for these events to progress to a higher grade. Also, there may be a greater willingness by providers to initiate systemic immunosuppression due to less stringent treatment protocols in real-world clinical settings,” he said.
Using a multivariable risk prediction model for cutaneous toxicities, the researchers identified use of ipilimumab, a CTLA-4-blocking antibody, as having a protective effect for not developing a cutaneous irAE, compared with the PD-1 blocker pembrolizumab (odds ratio, 0.78; 95% confidence interval, 0.62-0.98; P < .01). But combination ICI therapy (OR, 1.53; 95% CI, 1.25-1.88; P < .001), a melanoma diagnosis (OR, 2.47; 95% CI, 2.11-2.89; P < .001), and a renal cell carcinoma diagnosis (OR, 1.65; 95% CI, 1.36-2.00; P < .001) were found to be risk factors for developing cutaneous irAEs.
“The protective effect of ipilimumab identified in the study is interesting, as historically ipilimumab has been more likely to cause cutaneous toxicities,” Dr. Semenov said. “However, we believe that the majority of this association is mediated by the melanoma, for which ipilimumab was primarily used since its introduction. Independent of this relationship, it seems to be less likely to cause cutaneous toxicity than PD-1 inhibition, according to this data.”
Based on their findings, he said, “dermatologists can utilize this information to facilitate evaluations of high-risk patients so they can take steps to prevent progression to more severe toxicities and reduce reliance or systemic immunosuppression.”
The 25% real-world incidence of cutaneous irAEs observed in the study, Dr. Semenov said, is “somewhat lower than previous clinical trial estimates of over one-third of patients presenting with cutaneous toxicities” but he added that previous estimates were based primarily on studies of patients with melanoma.
That some patients delayed presentation with these conditions “should revise clinicians’ understanding of when to expect patients to present with these toxicities, and not to rule out a delayed onset of symptoms as being unrelated to immunotherapy,” Dr. Semenov said.
Most cutaneous irAEs are ‘manageable’
In an interview, Naiara Braghiroli, MD, PhD, a dermatologist at Baptist Health’s Miami Cancer Institute, Plantation, Fla., who was not an investigator in the study, noted that over the last decade, ICIs have “revolutionized the treatment of metastatic melanoma” and, more recently, the treatment of nonmelanoma skin cancers, with regard to survival rates and side effects.
She said that the results of the study show that “most of the cutaneous side effects are manageable with very few exceptions, like the cutaneous bullous disorders and rarely, more serious reactions [such as] Stevens-Johnson syndrome.”
The majority of the side effects are treatable “and when well controlled, the patient can have a good quality of life” during treatment, she added.
For future research, Dr. Braghiroli noted, it would be interesting to know more about whether the development of any specific cutaneous reaction associated with ICIs “is associated with a higher chance of good antitumor response,” as seen with other anticancer therapies such as epidermal growth factor receptor inhibitors.
Dr. Semenov and Dr. Braghiroli report having no relevant financial disclosures.
FROM SID 2021
EAS lipid guidance: Start high-risk patients on combo drug
Very-high-risk dyslipidemia patients unlikely to reach goal with a statin should be given combination statin–ezetimibe (Nustendi) therapy upfront, rather than wasting time and resources on trialing a statin alone, suggests a practical guidance document.
The document points out that, even with high-intensity statin therapy, patients achieve a reduction in low-density-lipoprotein (LDL) cholesterol levels of around 50%, which for many is not enough for them to achieve the stringent new guideline targets deemed necessary for risk reduction.
Instead, clinicians should determine at the first visit whether their patient, if they are not already on a statin, is likely to reach their goal with that drug alone, and if not, should immediately start them on the combination.
The guidance, which aims to offer a practical way to implement the 2019 European Society of Cardiology/EAS guidelines for the management of dyslipidemias, was published April 12 in Atherosclerosis .
Lead author Alberico L. Catapano, MD, PhD, discussed the new practical guidance at the recent European Atherosclerosis Society (EAS) 2021 Virtual Congress.
He explained that the motivation for creating the practical guidance was “very simple” and concerns something already embedded in the ESC/EAS guidelines; it’s just that “people didn’t notice” it.
Dr. Catapano, professor of pharmacology at the University of Milan and past president of the EAS, said the guidelines set out the average reduction in LDL-cholesterol levels “you can get by starting with high-intensity therapy and/or starting with a combination therapy.”
The guidelines, he said, suggest steps for achieving lipid control: Begin with a statin, add ezetimibe if the patient is still not at goal, and proceed to a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor if the patient is still not at target levels.
Dr. Catapano added that, “having said that, at the beginning, you can guess by knowing how far you are from the goal as to whether a statin by itself with help you get [there].”
If clinicians follow the new practical guidance of giving upfront combination statin–ezetimibe therapy in very-high-risk patients with high LDL-cholesterol levels, it will “save a lot of time, a lot of clinic visits, and will you get you to goal earlier.”
He gave the example of a patient who has an LDL-cholesterol level of 190 mg/dL, who would be classified as being at very high risk. With the target goal of 55 mg/dL, “you would never be able to get them to goal [with only] a high-intensity statin.”
The addition of ezetimibe to the regimen of this patient would have two advantages, Dr. Catapano said. The first is that “you get to goal more easily,” and the second is that, with the drugs available as a single-pill combination, it “makes it easier for the patient to be compliant.”
Consequently, there will be no “unnecessary back and forth,” he said. “Some of these are young people. They go to work; one less visit is less time lost at work.
“This is a practical issue,” he added. “It doesn’t contradict the guidelines,” it’s about “everyday clinical practice.”
Useful between updates
Responding to the guidance, Donald Lloyd-Jones, MD, president-elect of the American Heart Association (AHA), told this news organization that “this kind of document can be useful in periods between updates of the formal guidelines.”
New evidence comes out in between guidelines, and they “don’t often provide us with all of the practical solutions needed for everyday guidance when we’re dealing with individual patients with real-world problems.”
Dr. Lloyd-Jones, who is chair of the Department of Preventive Medicine at Northwestern University, Chicago, said the 2019 ESC/EAS guidelines set “quite aggressive targets, particularly for LDL cholesterol … but didn’t really provide much practical advice on how clinicians could get there for their patients.”
“While this document doesn’t completely address all patient groups, it does provide some good practical advice,” recognizing that “if you need to get to a certain LDL target, it’s unlikely you’re going to get there with just a statin in certain types of patients,” and “if you need a certain amount of LDL lowering, it’s certainly reasonable to start upfront with a statin and ezetimibe and see how you do.”
Crucially, Dr. Lloyd-Jones believes that the practical guidance does “flesh out some of the details the guidelines didn’t address.”
In terms of the aggressive LDL-cholesterol targets set out in the original guidelines, he said that “everyone agrees that lower is always better … and we’ve not get yet found a level that is too low.”
Further, “we’re certainly pushing patients lower and lower, especially with the use of PCSK9 inhibitors, so I think the general philosophy is consistent and correct,” although “it’s difficult to point to great evidence from clinical trials that specially says that 55 mg/dL or 40 mg/dL is the right target for a given group of patients.”
“There’s really very limited evidence for those specific numbers,” Dr. Lloyd-Jones added, “but I think everyone agrees, especially for patients at higher risk, the lower we can get them the better. What really matters, and what this document starts to address, is how we achieve as low as possible, and I think there are some important considerations that they take into account.”
Aside from how far patients need their LDL cholesterol lowered from baseline, there are issues like cost and patient preference for different types of medication, and these “weren’t particularly well addressed in the guidelines,” he added.
Scott D. Isaacs, MD, Secretary of the American Association of Clinical Endocrinologists (AACE), commented that the ESC/EAS recommendations echo the 2017 AACE/American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease.
He said that both guidelines “call for the need to lower LDL cholesterol as much as possible to prevent cardiovascular disease.”
He agreed, however, that high- or very-high-risk patients “have aggressive LDL targets that are often lower than what can be accomplished with high-dose, high-intensity statin monotherapy. Therefore, starting with combination therapy … will get more patients to goal more quickly and will prevent more cardiovascular events.”
Isaacs added: “It just makes sense that if you know a drug will not be strong enough, then you should start with two drugs.”
He noted that this approach is commonly used for conditions such as diabetes and hypertension, “when monotherapy is not expected to achieve the desired results.”
Dr. Isaacs also underlined that the combination of a statin plus ezetimibe “is appealing because of the price and ease of use.”
Although PCSK9 inhibitors are more potent and achieve even lower LDL levels, “the higher price and need to take an injection has limited their use,” he noted.
“One would expect that as the prices of PCSK9 inhibitors come down, their place in care pathways will move up since they are more effective and have proven cardiovascular benefit, but for now, statin plus ezetimibe is a potent and cost-effective way to achieve LDL targets in high- and very-high-risk patients,” Dr. Isaacs concluded.
One issue Dr. Lloyd-Jones raised with the ESC/EAS guidelines is that they seem to have put a lot of weight Mendelian randomization analysis.
“Those are useful in understanding whether having low LDL-cholesterol levels or triglycerides naturally are better for you – of course they are – but they actually provide no evidence about treatment effects, so I think what we need from that is actual data from the clinical trials to understand the treatment effects, both positive and negative.”
He added that that “really then helps us to drive to how and in whom we want to achieve the lowest levels possible.”
Dr. Lloyd-Jones said that Mendelian randomization analyses “continue to crop in a lot of these ESC and EAS documents,” and although they are “elegant and interesting,” they “don’t really inform treatment at all.”
No funding declared. Catapano declares relationships with Pfizer, Sanofi, Regeneron, Merck, Mediolanum, SigmaTau, Menarini, Kowa, Recordati, Eli Lilly, AstraZeneca, Merck, Aegerion and Amgen.
A version of this article first appeared on Medscape.com.
Very-high-risk dyslipidemia patients unlikely to reach goal with a statin should be given combination statin–ezetimibe (Nustendi) therapy upfront, rather than wasting time and resources on trialing a statin alone, suggests a practical guidance document.
The document points out that, even with high-intensity statin therapy, patients achieve a reduction in low-density-lipoprotein (LDL) cholesterol levels of around 50%, which for many is not enough for them to achieve the stringent new guideline targets deemed necessary for risk reduction.
Instead, clinicians should determine at the first visit whether their patient, if they are not already on a statin, is likely to reach their goal with that drug alone, and if not, should immediately start them on the combination.
The guidance, which aims to offer a practical way to implement the 2019 European Society of Cardiology/EAS guidelines for the management of dyslipidemias, was published April 12 in Atherosclerosis .
Lead author Alberico L. Catapano, MD, PhD, discussed the new practical guidance at the recent European Atherosclerosis Society (EAS) 2021 Virtual Congress.
He explained that the motivation for creating the practical guidance was “very simple” and concerns something already embedded in the ESC/EAS guidelines; it’s just that “people didn’t notice” it.
Dr. Catapano, professor of pharmacology at the University of Milan and past president of the EAS, said the guidelines set out the average reduction in LDL-cholesterol levels “you can get by starting with high-intensity therapy and/or starting with a combination therapy.”
The guidelines, he said, suggest steps for achieving lipid control: Begin with a statin, add ezetimibe if the patient is still not at goal, and proceed to a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor if the patient is still not at target levels.
Dr. Catapano added that, “having said that, at the beginning, you can guess by knowing how far you are from the goal as to whether a statin by itself with help you get [there].”
If clinicians follow the new practical guidance of giving upfront combination statin–ezetimibe therapy in very-high-risk patients with high LDL-cholesterol levels, it will “save a lot of time, a lot of clinic visits, and will you get you to goal earlier.”
He gave the example of a patient who has an LDL-cholesterol level of 190 mg/dL, who would be classified as being at very high risk. With the target goal of 55 mg/dL, “you would never be able to get them to goal [with only] a high-intensity statin.”
The addition of ezetimibe to the regimen of this patient would have two advantages, Dr. Catapano said. The first is that “you get to goal more easily,” and the second is that, with the drugs available as a single-pill combination, it “makes it easier for the patient to be compliant.”
Consequently, there will be no “unnecessary back and forth,” he said. “Some of these are young people. They go to work; one less visit is less time lost at work.
“This is a practical issue,” he added. “It doesn’t contradict the guidelines,” it’s about “everyday clinical practice.”
Useful between updates
Responding to the guidance, Donald Lloyd-Jones, MD, president-elect of the American Heart Association (AHA), told this news organization that “this kind of document can be useful in periods between updates of the formal guidelines.”
New evidence comes out in between guidelines, and they “don’t often provide us with all of the practical solutions needed for everyday guidance when we’re dealing with individual patients with real-world problems.”
Dr. Lloyd-Jones, who is chair of the Department of Preventive Medicine at Northwestern University, Chicago, said the 2019 ESC/EAS guidelines set “quite aggressive targets, particularly for LDL cholesterol … but didn’t really provide much practical advice on how clinicians could get there for their patients.”
“While this document doesn’t completely address all patient groups, it does provide some good practical advice,” recognizing that “if you need to get to a certain LDL target, it’s unlikely you’re going to get there with just a statin in certain types of patients,” and “if you need a certain amount of LDL lowering, it’s certainly reasonable to start upfront with a statin and ezetimibe and see how you do.”
Crucially, Dr. Lloyd-Jones believes that the practical guidance does “flesh out some of the details the guidelines didn’t address.”
In terms of the aggressive LDL-cholesterol targets set out in the original guidelines, he said that “everyone agrees that lower is always better … and we’ve not get yet found a level that is too low.”
Further, “we’re certainly pushing patients lower and lower, especially with the use of PCSK9 inhibitors, so I think the general philosophy is consistent and correct,” although “it’s difficult to point to great evidence from clinical trials that specially says that 55 mg/dL or 40 mg/dL is the right target for a given group of patients.”
“There’s really very limited evidence for those specific numbers,” Dr. Lloyd-Jones added, “but I think everyone agrees, especially for patients at higher risk, the lower we can get them the better. What really matters, and what this document starts to address, is how we achieve as low as possible, and I think there are some important considerations that they take into account.”
Aside from how far patients need their LDL cholesterol lowered from baseline, there are issues like cost and patient preference for different types of medication, and these “weren’t particularly well addressed in the guidelines,” he added.
Scott D. Isaacs, MD, Secretary of the American Association of Clinical Endocrinologists (AACE), commented that the ESC/EAS recommendations echo the 2017 AACE/American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease.
He said that both guidelines “call for the need to lower LDL cholesterol as much as possible to prevent cardiovascular disease.”
He agreed, however, that high- or very-high-risk patients “have aggressive LDL targets that are often lower than what can be accomplished with high-dose, high-intensity statin monotherapy. Therefore, starting with combination therapy … will get more patients to goal more quickly and will prevent more cardiovascular events.”
Isaacs added: “It just makes sense that if you know a drug will not be strong enough, then you should start with two drugs.”
He noted that this approach is commonly used for conditions such as diabetes and hypertension, “when monotherapy is not expected to achieve the desired results.”
Dr. Isaacs also underlined that the combination of a statin plus ezetimibe “is appealing because of the price and ease of use.”
Although PCSK9 inhibitors are more potent and achieve even lower LDL levels, “the higher price and need to take an injection has limited their use,” he noted.
“One would expect that as the prices of PCSK9 inhibitors come down, their place in care pathways will move up since they are more effective and have proven cardiovascular benefit, but for now, statin plus ezetimibe is a potent and cost-effective way to achieve LDL targets in high- and very-high-risk patients,” Dr. Isaacs concluded.
One issue Dr. Lloyd-Jones raised with the ESC/EAS guidelines is that they seem to have put a lot of weight Mendelian randomization analysis.
“Those are useful in understanding whether having low LDL-cholesterol levels or triglycerides naturally are better for you – of course they are – but they actually provide no evidence about treatment effects, so I think what we need from that is actual data from the clinical trials to understand the treatment effects, both positive and negative.”
He added that that “really then helps us to drive to how and in whom we want to achieve the lowest levels possible.”
Dr. Lloyd-Jones said that Mendelian randomization analyses “continue to crop in a lot of these ESC and EAS documents,” and although they are “elegant and interesting,” they “don’t really inform treatment at all.”
No funding declared. Catapano declares relationships with Pfizer, Sanofi, Regeneron, Merck, Mediolanum, SigmaTau, Menarini, Kowa, Recordati, Eli Lilly, AstraZeneca, Merck, Aegerion and Amgen.
A version of this article first appeared on Medscape.com.
Very-high-risk dyslipidemia patients unlikely to reach goal with a statin should be given combination statin–ezetimibe (Nustendi) therapy upfront, rather than wasting time and resources on trialing a statin alone, suggests a practical guidance document.
The document points out that, even with high-intensity statin therapy, patients achieve a reduction in low-density-lipoprotein (LDL) cholesterol levels of around 50%, which for many is not enough for them to achieve the stringent new guideline targets deemed necessary for risk reduction.
Instead, clinicians should determine at the first visit whether their patient, if they are not already on a statin, is likely to reach their goal with that drug alone, and if not, should immediately start them on the combination.
The guidance, which aims to offer a practical way to implement the 2019 European Society of Cardiology/EAS guidelines for the management of dyslipidemias, was published April 12 in Atherosclerosis .
Lead author Alberico L. Catapano, MD, PhD, discussed the new practical guidance at the recent European Atherosclerosis Society (EAS) 2021 Virtual Congress.
He explained that the motivation for creating the practical guidance was “very simple” and concerns something already embedded in the ESC/EAS guidelines; it’s just that “people didn’t notice” it.
Dr. Catapano, professor of pharmacology at the University of Milan and past president of the EAS, said the guidelines set out the average reduction in LDL-cholesterol levels “you can get by starting with high-intensity therapy and/or starting with a combination therapy.”
The guidelines, he said, suggest steps for achieving lipid control: Begin with a statin, add ezetimibe if the patient is still not at goal, and proceed to a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor if the patient is still not at target levels.
Dr. Catapano added that, “having said that, at the beginning, you can guess by knowing how far you are from the goal as to whether a statin by itself with help you get [there].”
If clinicians follow the new practical guidance of giving upfront combination statin–ezetimibe therapy in very-high-risk patients with high LDL-cholesterol levels, it will “save a lot of time, a lot of clinic visits, and will you get you to goal earlier.”
He gave the example of a patient who has an LDL-cholesterol level of 190 mg/dL, who would be classified as being at very high risk. With the target goal of 55 mg/dL, “you would never be able to get them to goal [with only] a high-intensity statin.”
The addition of ezetimibe to the regimen of this patient would have two advantages, Dr. Catapano said. The first is that “you get to goal more easily,” and the second is that, with the drugs available as a single-pill combination, it “makes it easier for the patient to be compliant.”
Consequently, there will be no “unnecessary back and forth,” he said. “Some of these are young people. They go to work; one less visit is less time lost at work.
“This is a practical issue,” he added. “It doesn’t contradict the guidelines,” it’s about “everyday clinical practice.”
Useful between updates
Responding to the guidance, Donald Lloyd-Jones, MD, president-elect of the American Heart Association (AHA), told this news organization that “this kind of document can be useful in periods between updates of the formal guidelines.”
New evidence comes out in between guidelines, and they “don’t often provide us with all of the practical solutions needed for everyday guidance when we’re dealing with individual patients with real-world problems.”
Dr. Lloyd-Jones, who is chair of the Department of Preventive Medicine at Northwestern University, Chicago, said the 2019 ESC/EAS guidelines set “quite aggressive targets, particularly for LDL cholesterol … but didn’t really provide much practical advice on how clinicians could get there for their patients.”
“While this document doesn’t completely address all patient groups, it does provide some good practical advice,” recognizing that “if you need to get to a certain LDL target, it’s unlikely you’re going to get there with just a statin in certain types of patients,” and “if you need a certain amount of LDL lowering, it’s certainly reasonable to start upfront with a statin and ezetimibe and see how you do.”
Crucially, Dr. Lloyd-Jones believes that the practical guidance does “flesh out some of the details the guidelines didn’t address.”
In terms of the aggressive LDL-cholesterol targets set out in the original guidelines, he said that “everyone agrees that lower is always better … and we’ve not get yet found a level that is too low.”
Further, “we’re certainly pushing patients lower and lower, especially with the use of PCSK9 inhibitors, so I think the general philosophy is consistent and correct,” although “it’s difficult to point to great evidence from clinical trials that specially says that 55 mg/dL or 40 mg/dL is the right target for a given group of patients.”
“There’s really very limited evidence for those specific numbers,” Dr. Lloyd-Jones added, “but I think everyone agrees, especially for patients at higher risk, the lower we can get them the better. What really matters, and what this document starts to address, is how we achieve as low as possible, and I think there are some important considerations that they take into account.”
Aside from how far patients need their LDL cholesterol lowered from baseline, there are issues like cost and patient preference for different types of medication, and these “weren’t particularly well addressed in the guidelines,” he added.
Scott D. Isaacs, MD, Secretary of the American Association of Clinical Endocrinologists (AACE), commented that the ESC/EAS recommendations echo the 2017 AACE/American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease.
He said that both guidelines “call for the need to lower LDL cholesterol as much as possible to prevent cardiovascular disease.”
He agreed, however, that high- or very-high-risk patients “have aggressive LDL targets that are often lower than what can be accomplished with high-dose, high-intensity statin monotherapy. Therefore, starting with combination therapy … will get more patients to goal more quickly and will prevent more cardiovascular events.”
Isaacs added: “It just makes sense that if you know a drug will not be strong enough, then you should start with two drugs.”
He noted that this approach is commonly used for conditions such as diabetes and hypertension, “when monotherapy is not expected to achieve the desired results.”
Dr. Isaacs also underlined that the combination of a statin plus ezetimibe “is appealing because of the price and ease of use.”
Although PCSK9 inhibitors are more potent and achieve even lower LDL levels, “the higher price and need to take an injection has limited their use,” he noted.
“One would expect that as the prices of PCSK9 inhibitors come down, their place in care pathways will move up since they are more effective and have proven cardiovascular benefit, but for now, statin plus ezetimibe is a potent and cost-effective way to achieve LDL targets in high- and very-high-risk patients,” Dr. Isaacs concluded.
One issue Dr. Lloyd-Jones raised with the ESC/EAS guidelines is that they seem to have put a lot of weight Mendelian randomization analysis.
“Those are useful in understanding whether having low LDL-cholesterol levels or triglycerides naturally are better for you – of course they are – but they actually provide no evidence about treatment effects, so I think what we need from that is actual data from the clinical trials to understand the treatment effects, both positive and negative.”
He added that that “really then helps us to drive to how and in whom we want to achieve the lowest levels possible.”
Dr. Lloyd-Jones said that Mendelian randomization analyses “continue to crop in a lot of these ESC and EAS documents,” and although they are “elegant and interesting,” they “don’t really inform treatment at all.”
No funding declared. Catapano declares relationships with Pfizer, Sanofi, Regeneron, Merck, Mediolanum, SigmaTau, Menarini, Kowa, Recordati, Eli Lilly, AstraZeneca, Merck, Aegerion and Amgen.
A version of this article first appeared on Medscape.com.
Worse survival with recurrent AIH after transplant
Autoimmune hepatitis that recurs following a liver transplant can impair both graft survival and overall survival, results of a large international study showed.
Among 736 patients with autoimmune hepatitis who underwent liver transplant and were followed for up to 20 years, those who had recurrent AIH had a more than 10-fold higher risk for graft failure and a more than twofold higher risk of death, compared with patients who did not have recurrences, reported Aldo J. Montano-Loza, MD, MSc, PhD, from the University of Alberta, Edmonton.
“Recurrent disease impacts graft and overall survival, highlighting the need for improved management strategies,” he said in an oral abstract presentation during the International Liver Congress sponsored by the European Association for the Study of the Liver.
AIH is characterized by the presence of high IgG levels, autoantibodies, and histologic evidence of interface hepatitis. Most patients with AIH respond to immunosuppressive therapy, but some have progression to end-stage liver disease; for these patients, a liver transplant can be lifesaving, with 1-year survival of approximately 90%, and 5-year survival of about 70%, he said.
AIH frequently recurs after transplant, and although previous studies have suggested that recurrent disease does not adversely affect either graft survival or long-term survival, those studies had limited patient numbers and inadequate follow-up, Dr. Montano-Loza said.
He cited two recent studies, one from UNOS, the United Network for Organ Sharing, and the other from ELTR, the European Liver Transplant Registry, that showed that overall survival after liver transplant was worse for patients with AIH, compared with those who underwent transplant for other autoimmune liver diseases.
Multicenter retrospective study
To get a better picture of long-term posttransplant outcomes in patients with AIH, investigators in 33 centers in North and South America, Europe, and Asia conducted a retrospective cohort study. Their goal was to establish the frequency of recurrent AIH, identify clinical factors and biomarkers for higher risk of recurrence, and evaluate the association between recurrent AIH and both patient and graft survival.
They accomplished this by performing chart reviews, including data on demographics, IgG levels before transplant, and Model for End-Stage Liver Disease scores.
They also collected data on serum liver function tests within the first year after transplant, posttransplant infections, rejection episodes, and immunosuppressive regimens, as well as variables such as donor age and sex, sex mismatch between donor and recipients, calendar year of transplant, and transplant volume for AIH at each center.
Of the 736 patients, 563 (76%) were female. The mean age at AIH diagnosis was 34 years, and the mean age at transplant was 42 years. About one-fifth of patients (21%) had concomitant autoimmune diseases.
Posttransplant immunosuppression regimens included the usual suspects: tacrolimus in 78% of patients, cyclosporine in 11%, prednisone in 76%, mycophenolate mofetil in 55%, and azathioprine in 10%.
In all, 147 of the 736 patients had a diagnosis of recurrent AIH. The investigators found that the cumulative probability of recurrent AIH was 49% after 20 years of follow-up.
Risk factors identified
In multivariate analysis controlling for age, concomitant disease, immunosuppressive regimens, organ-sex mismatch, acute rejection, liver function tests, bilirubin, and IgG, factors significantly associated with AIH recurrence included age 42 or younger at the time of transplant (hazard ratio, 3.15; P = .02), use of mycophenolate mofetil after transplant (HR, 3.06; P = .005), donor/recipient sex mismatch (HR, 2.57; P = .003), and high IgG levels pretransplant (HR, 1.04; P = .004).
Among 529 patients who had a liver biopsy after transplant, factors that remained as significant predictors of AIH recurrence were posttransplant mycophenolate mofetil (HR, 2.75; P = .003), donor/recipient sex mismatch (HR, 2.03; P = .02), and pretransplant IgG levels (HR, 1.04 per each g/L; P = .001).
An analysis of features associated with graft survival showed that recurrent AIH was associated with significantly increased risk for graft failure (HR, 10.79; P < .001). Patients with high bilirubin levels 1 year after transplantation were also at higher risk for failure (HR, 1.004 per micromol/L; P < .001).
Factors significantly associated with survival were recurrence of AIH (HR for death, 2.53; P = .001), elevated ALT at 12 months after transplant (HR, 1.002; P = .004), and elevated bilirubin at 12 months (HR, 1.003 per micromol/L; P < .001).
The investigators acknowledged that the study was limited by the retrospective design and by the fact that the diagnosis of recurrent AIH may have differed between centers that performed liver biopsy according to protocol and those that performed them only when clinically indicated, which may have resulted in differences in time to diagnosis.
Possible explanations for risk factors
In the question-and-answer session following his presentation, comoderator moderator Philip N. Newsome, PhD, from University Hospitals Birmingham (England), asked: “In terms of age, is that a reflection of worse disease, or is it adherence, or is it a combination, and should we be managing those patients more aggressively with immunosuppression?”
“We consider age is more a reflection of an aggressive disease,” Dr. Montano-Loza said. “Basically, in the univariate analysis we found that patients with a diagnosis at a younger age and even a transplant at a younger age were definitely associated with a higher risk of recurrence, so we think this is more related to an aggressive [disease] behavior in younger patient that translates into worse clinical outcomes.”
He added that patients younger than 40 who require transplants should be closely monitored for recurrence.
“Actually, we could make the argument that maybe these patients will benefit from protocol biopsies,” he said.
He noted that 15% of patients had significant fibrosis at the time of recurrent AIH diagnosis, and that the recurrences were not detected by laboratory monitoring alone.
Asked by an audience member why mycophenolate mofetil was associated with increased risk for recurrence, Dr. Montano-Loza replied that the retrospective nature of the data precludes the possibility of a definitive answer, but he noted that, for patients with other autoimmune liver diseases, the type of immunosuppression used has an impact on recurrence rates.
“For example, cyclosporine has a protective effect for patients transplanted for primary biliary cholangitis,” he said.
He said it may also be possible that there is a rebound effect leading to recurrence when patients are taken off mycophenolate or switched to another agent.
The study was supported by grants to individual researchers. Dr. Montano-Loza and Dr. Newsome reported having no relevant conflicts of interest.
Autoimmune hepatitis that recurs following a liver transplant can impair both graft survival and overall survival, results of a large international study showed.
Among 736 patients with autoimmune hepatitis who underwent liver transplant and were followed for up to 20 years, those who had recurrent AIH had a more than 10-fold higher risk for graft failure and a more than twofold higher risk of death, compared with patients who did not have recurrences, reported Aldo J. Montano-Loza, MD, MSc, PhD, from the University of Alberta, Edmonton.
“Recurrent disease impacts graft and overall survival, highlighting the need for improved management strategies,” he said in an oral abstract presentation during the International Liver Congress sponsored by the European Association for the Study of the Liver.
AIH is characterized by the presence of high IgG levels, autoantibodies, and histologic evidence of interface hepatitis. Most patients with AIH respond to immunosuppressive therapy, but some have progression to end-stage liver disease; for these patients, a liver transplant can be lifesaving, with 1-year survival of approximately 90%, and 5-year survival of about 70%, he said.
AIH frequently recurs after transplant, and although previous studies have suggested that recurrent disease does not adversely affect either graft survival or long-term survival, those studies had limited patient numbers and inadequate follow-up, Dr. Montano-Loza said.
He cited two recent studies, one from UNOS, the United Network for Organ Sharing, and the other from ELTR, the European Liver Transplant Registry, that showed that overall survival after liver transplant was worse for patients with AIH, compared with those who underwent transplant for other autoimmune liver diseases.
Multicenter retrospective study
To get a better picture of long-term posttransplant outcomes in patients with AIH, investigators in 33 centers in North and South America, Europe, and Asia conducted a retrospective cohort study. Their goal was to establish the frequency of recurrent AIH, identify clinical factors and biomarkers for higher risk of recurrence, and evaluate the association between recurrent AIH and both patient and graft survival.
They accomplished this by performing chart reviews, including data on demographics, IgG levels before transplant, and Model for End-Stage Liver Disease scores.
They also collected data on serum liver function tests within the first year after transplant, posttransplant infections, rejection episodes, and immunosuppressive regimens, as well as variables such as donor age and sex, sex mismatch between donor and recipients, calendar year of transplant, and transplant volume for AIH at each center.
Of the 736 patients, 563 (76%) were female. The mean age at AIH diagnosis was 34 years, and the mean age at transplant was 42 years. About one-fifth of patients (21%) had concomitant autoimmune diseases.
Posttransplant immunosuppression regimens included the usual suspects: tacrolimus in 78% of patients, cyclosporine in 11%, prednisone in 76%, mycophenolate mofetil in 55%, and azathioprine in 10%.
In all, 147 of the 736 patients had a diagnosis of recurrent AIH. The investigators found that the cumulative probability of recurrent AIH was 49% after 20 years of follow-up.
Risk factors identified
In multivariate analysis controlling for age, concomitant disease, immunosuppressive regimens, organ-sex mismatch, acute rejection, liver function tests, bilirubin, and IgG, factors significantly associated with AIH recurrence included age 42 or younger at the time of transplant (hazard ratio, 3.15; P = .02), use of mycophenolate mofetil after transplant (HR, 3.06; P = .005), donor/recipient sex mismatch (HR, 2.57; P = .003), and high IgG levels pretransplant (HR, 1.04; P = .004).
Among 529 patients who had a liver biopsy after transplant, factors that remained as significant predictors of AIH recurrence were posttransplant mycophenolate mofetil (HR, 2.75; P = .003), donor/recipient sex mismatch (HR, 2.03; P = .02), and pretransplant IgG levels (HR, 1.04 per each g/L; P = .001).
An analysis of features associated with graft survival showed that recurrent AIH was associated with significantly increased risk for graft failure (HR, 10.79; P < .001). Patients with high bilirubin levels 1 year after transplantation were also at higher risk for failure (HR, 1.004 per micromol/L; P < .001).
Factors significantly associated with survival were recurrence of AIH (HR for death, 2.53; P = .001), elevated ALT at 12 months after transplant (HR, 1.002; P = .004), and elevated bilirubin at 12 months (HR, 1.003 per micromol/L; P < .001).
The investigators acknowledged that the study was limited by the retrospective design and by the fact that the diagnosis of recurrent AIH may have differed between centers that performed liver biopsy according to protocol and those that performed them only when clinically indicated, which may have resulted in differences in time to diagnosis.
Possible explanations for risk factors
In the question-and-answer session following his presentation, comoderator moderator Philip N. Newsome, PhD, from University Hospitals Birmingham (England), asked: “In terms of age, is that a reflection of worse disease, or is it adherence, or is it a combination, and should we be managing those patients more aggressively with immunosuppression?”
“We consider age is more a reflection of an aggressive disease,” Dr. Montano-Loza said. “Basically, in the univariate analysis we found that patients with a diagnosis at a younger age and even a transplant at a younger age were definitely associated with a higher risk of recurrence, so we think this is more related to an aggressive [disease] behavior in younger patient that translates into worse clinical outcomes.”
He added that patients younger than 40 who require transplants should be closely monitored for recurrence.
“Actually, we could make the argument that maybe these patients will benefit from protocol biopsies,” he said.
He noted that 15% of patients had significant fibrosis at the time of recurrent AIH diagnosis, and that the recurrences were not detected by laboratory monitoring alone.
Asked by an audience member why mycophenolate mofetil was associated with increased risk for recurrence, Dr. Montano-Loza replied that the retrospective nature of the data precludes the possibility of a definitive answer, but he noted that, for patients with other autoimmune liver diseases, the type of immunosuppression used has an impact on recurrence rates.
“For example, cyclosporine has a protective effect for patients transplanted for primary biliary cholangitis,” he said.
He said it may also be possible that there is a rebound effect leading to recurrence when patients are taken off mycophenolate or switched to another agent.
The study was supported by grants to individual researchers. Dr. Montano-Loza and Dr. Newsome reported having no relevant conflicts of interest.
Autoimmune hepatitis that recurs following a liver transplant can impair both graft survival and overall survival, results of a large international study showed.
Among 736 patients with autoimmune hepatitis who underwent liver transplant and were followed for up to 20 years, those who had recurrent AIH had a more than 10-fold higher risk for graft failure and a more than twofold higher risk of death, compared with patients who did not have recurrences, reported Aldo J. Montano-Loza, MD, MSc, PhD, from the University of Alberta, Edmonton.
“Recurrent disease impacts graft and overall survival, highlighting the need for improved management strategies,” he said in an oral abstract presentation during the International Liver Congress sponsored by the European Association for the Study of the Liver.
AIH is characterized by the presence of high IgG levels, autoantibodies, and histologic evidence of interface hepatitis. Most patients with AIH respond to immunosuppressive therapy, but some have progression to end-stage liver disease; for these patients, a liver transplant can be lifesaving, with 1-year survival of approximately 90%, and 5-year survival of about 70%, he said.
AIH frequently recurs after transplant, and although previous studies have suggested that recurrent disease does not adversely affect either graft survival or long-term survival, those studies had limited patient numbers and inadequate follow-up, Dr. Montano-Loza said.
He cited two recent studies, one from UNOS, the United Network for Organ Sharing, and the other from ELTR, the European Liver Transplant Registry, that showed that overall survival after liver transplant was worse for patients with AIH, compared with those who underwent transplant for other autoimmune liver diseases.
Multicenter retrospective study
To get a better picture of long-term posttransplant outcomes in patients with AIH, investigators in 33 centers in North and South America, Europe, and Asia conducted a retrospective cohort study. Their goal was to establish the frequency of recurrent AIH, identify clinical factors and biomarkers for higher risk of recurrence, and evaluate the association between recurrent AIH and both patient and graft survival.
They accomplished this by performing chart reviews, including data on demographics, IgG levels before transplant, and Model for End-Stage Liver Disease scores.
They also collected data on serum liver function tests within the first year after transplant, posttransplant infections, rejection episodes, and immunosuppressive regimens, as well as variables such as donor age and sex, sex mismatch between donor and recipients, calendar year of transplant, and transplant volume for AIH at each center.
Of the 736 patients, 563 (76%) were female. The mean age at AIH diagnosis was 34 years, and the mean age at transplant was 42 years. About one-fifth of patients (21%) had concomitant autoimmune diseases.
Posttransplant immunosuppression regimens included the usual suspects: tacrolimus in 78% of patients, cyclosporine in 11%, prednisone in 76%, mycophenolate mofetil in 55%, and azathioprine in 10%.
In all, 147 of the 736 patients had a diagnosis of recurrent AIH. The investigators found that the cumulative probability of recurrent AIH was 49% after 20 years of follow-up.
Risk factors identified
In multivariate analysis controlling for age, concomitant disease, immunosuppressive regimens, organ-sex mismatch, acute rejection, liver function tests, bilirubin, and IgG, factors significantly associated with AIH recurrence included age 42 or younger at the time of transplant (hazard ratio, 3.15; P = .02), use of mycophenolate mofetil after transplant (HR, 3.06; P = .005), donor/recipient sex mismatch (HR, 2.57; P = .003), and high IgG levels pretransplant (HR, 1.04; P = .004).
Among 529 patients who had a liver biopsy after transplant, factors that remained as significant predictors of AIH recurrence were posttransplant mycophenolate mofetil (HR, 2.75; P = .003), donor/recipient sex mismatch (HR, 2.03; P = .02), and pretransplant IgG levels (HR, 1.04 per each g/L; P = .001).
An analysis of features associated with graft survival showed that recurrent AIH was associated with significantly increased risk for graft failure (HR, 10.79; P < .001). Patients with high bilirubin levels 1 year after transplantation were also at higher risk for failure (HR, 1.004 per micromol/L; P < .001).
Factors significantly associated with survival were recurrence of AIH (HR for death, 2.53; P = .001), elevated ALT at 12 months after transplant (HR, 1.002; P = .004), and elevated bilirubin at 12 months (HR, 1.003 per micromol/L; P < .001).
The investigators acknowledged that the study was limited by the retrospective design and by the fact that the diagnosis of recurrent AIH may have differed between centers that performed liver biopsy according to protocol and those that performed them only when clinically indicated, which may have resulted in differences in time to diagnosis.
Possible explanations for risk factors
In the question-and-answer session following his presentation, comoderator moderator Philip N. Newsome, PhD, from University Hospitals Birmingham (England), asked: “In terms of age, is that a reflection of worse disease, or is it adherence, or is it a combination, and should we be managing those patients more aggressively with immunosuppression?”
“We consider age is more a reflection of an aggressive disease,” Dr. Montano-Loza said. “Basically, in the univariate analysis we found that patients with a diagnosis at a younger age and even a transplant at a younger age were definitely associated with a higher risk of recurrence, so we think this is more related to an aggressive [disease] behavior in younger patient that translates into worse clinical outcomes.”
He added that patients younger than 40 who require transplants should be closely monitored for recurrence.
“Actually, we could make the argument that maybe these patients will benefit from protocol biopsies,” he said.
He noted that 15% of patients had significant fibrosis at the time of recurrent AIH diagnosis, and that the recurrences were not detected by laboratory monitoring alone.
Asked by an audience member why mycophenolate mofetil was associated with increased risk for recurrence, Dr. Montano-Loza replied that the retrospective nature of the data precludes the possibility of a definitive answer, but he noted that, for patients with other autoimmune liver diseases, the type of immunosuppression used has an impact on recurrence rates.
“For example, cyclosporine has a protective effect for patients transplanted for primary biliary cholangitis,” he said.
He said it may also be possible that there is a rebound effect leading to recurrence when patients are taken off mycophenolate or switched to another agent.
The study was supported by grants to individual researchers. Dr. Montano-Loza and Dr. Newsome reported having no relevant conflicts of interest.
FROM ILC 2021
New COVID-19 vaccinations decline again in 12- to 15-year-olds
Even though less than 21% of all children aged 12-15 years are fully vaccinated against COVID-19, the number seeking first vaccinations continues to decline, according to data from the Centers for Disease Control and Prevention.

and 462,000 during the week ending June 14. Collectively, 30.2% of 12- to 15-year-olds have gotten at least one dose of vaccine so far and 20.7% are now fully vaccinated, the CDC said on its COVID Data Tracker site.
Among children aged 16-17 years, who were able to start the vaccination process earlier, 42.9% have received at least one dose and 34.0% have completed the COVID-19 vaccine regimen. Vaccine initiation – measured as the proportion of all individuals getting a first shot over the previous 2 weeks – has been consistently around 4.8% during the month of June for this age group but has dropped from 17.9% on June 7 to 14.3% on June 28 for those aged 12-15, the CDC data show.
Looking at the same measure for vaccine completion, 16.7% of all those who reached full vaccination status in the 14 days ending June 28 were 12- to 15-years-olds, down from 21.5% on June 21 and 19.6% on June 14. The numbers for those aged 15-16 were, respectively, 4.6%, 4.5%, and 4.2%, the CDC reported.
Fortunately, in the wake of recent vaccination trends, new cases of COVID-19 in children were down to their lowest level – just 8,447 for the week ending June 24 – since May of 2020, according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
New cases had been well over 15,000 the previous week (June 17), following weeks of 14,000 (June 10) and 16,000 (June 3) new cases, so the latest drop down to just four digits represents a 1-week decline of over 46% in the 49 states (excluding New York) that are reporting age distribution, along with the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative number of child COVID-19 cases in those jurisdictions is about 4.03 million since the beginning of the pandemic, which represents 14.2% of all cases in the United States. At the state level, the cumulative rate of cases in children is highest in Vermont (22.7%) and lowest in Florida (8.9%), which uses an age range of 0-14 years for children, compared with 0-17 or 0-19 for most states, the AAP and CHA said.
Severe illness has been rare in children, which is reflected in the proportion of children among all hospitalizations, 2.2% in 24 jurisdictions, and the proportion of deaths, 0.06% in 46 jurisdictions, since the start of the pandemic, the AAP and CHA said, with a total of 336 COVID-19–related deaths reported.
Even though less than 21% of all children aged 12-15 years are fully vaccinated against COVID-19, the number seeking first vaccinations continues to decline, according to data from the Centers for Disease Control and Prevention.

and 462,000 during the week ending June 14. Collectively, 30.2% of 12- to 15-year-olds have gotten at least one dose of vaccine so far and 20.7% are now fully vaccinated, the CDC said on its COVID Data Tracker site.
Among children aged 16-17 years, who were able to start the vaccination process earlier, 42.9% have received at least one dose and 34.0% have completed the COVID-19 vaccine regimen. Vaccine initiation – measured as the proportion of all individuals getting a first shot over the previous 2 weeks – has been consistently around 4.8% during the month of June for this age group but has dropped from 17.9% on June 7 to 14.3% on June 28 for those aged 12-15, the CDC data show.
Looking at the same measure for vaccine completion, 16.7% of all those who reached full vaccination status in the 14 days ending June 28 were 12- to 15-years-olds, down from 21.5% on June 21 and 19.6% on June 14. The numbers for those aged 15-16 were, respectively, 4.6%, 4.5%, and 4.2%, the CDC reported.
Fortunately, in the wake of recent vaccination trends, new cases of COVID-19 in children were down to their lowest level – just 8,447 for the week ending June 24 – since May of 2020, according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
New cases had been well over 15,000 the previous week (June 17), following weeks of 14,000 (June 10) and 16,000 (June 3) new cases, so the latest drop down to just four digits represents a 1-week decline of over 46% in the 49 states (excluding New York) that are reporting age distribution, along with the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative number of child COVID-19 cases in those jurisdictions is about 4.03 million since the beginning of the pandemic, which represents 14.2% of all cases in the United States. At the state level, the cumulative rate of cases in children is highest in Vermont (22.7%) and lowest in Florida (8.9%), which uses an age range of 0-14 years for children, compared with 0-17 or 0-19 for most states, the AAP and CHA said.
Severe illness has been rare in children, which is reflected in the proportion of children among all hospitalizations, 2.2% in 24 jurisdictions, and the proportion of deaths, 0.06% in 46 jurisdictions, since the start of the pandemic, the AAP and CHA said, with a total of 336 COVID-19–related deaths reported.
Even though less than 21% of all children aged 12-15 years are fully vaccinated against COVID-19, the number seeking first vaccinations continues to decline, according to data from the Centers for Disease Control and Prevention.

and 462,000 during the week ending June 14. Collectively, 30.2% of 12- to 15-year-olds have gotten at least one dose of vaccine so far and 20.7% are now fully vaccinated, the CDC said on its COVID Data Tracker site.
Among children aged 16-17 years, who were able to start the vaccination process earlier, 42.9% have received at least one dose and 34.0% have completed the COVID-19 vaccine regimen. Vaccine initiation – measured as the proportion of all individuals getting a first shot over the previous 2 weeks – has been consistently around 4.8% during the month of June for this age group but has dropped from 17.9% on June 7 to 14.3% on June 28 for those aged 12-15, the CDC data show.
Looking at the same measure for vaccine completion, 16.7% of all those who reached full vaccination status in the 14 days ending June 28 were 12- to 15-years-olds, down from 21.5% on June 21 and 19.6% on June 14. The numbers for those aged 15-16 were, respectively, 4.6%, 4.5%, and 4.2%, the CDC reported.
Fortunately, in the wake of recent vaccination trends, new cases of COVID-19 in children were down to their lowest level – just 8,447 for the week ending June 24 – since May of 2020, according to a new report from the American Academy of Pediatrics and the Children’s Hospital Association.
New cases had been well over 15,000 the previous week (June 17), following weeks of 14,000 (June 10) and 16,000 (June 3) new cases, so the latest drop down to just four digits represents a 1-week decline of over 46% in the 49 states (excluding New York) that are reporting age distribution, along with the District of Columbia, New York City, Puerto Rico, and Guam.
The cumulative number of child COVID-19 cases in those jurisdictions is about 4.03 million since the beginning of the pandemic, which represents 14.2% of all cases in the United States. At the state level, the cumulative rate of cases in children is highest in Vermont (22.7%) and lowest in Florida (8.9%), which uses an age range of 0-14 years for children, compared with 0-17 or 0-19 for most states, the AAP and CHA said.
Severe illness has been rare in children, which is reflected in the proportion of children among all hospitalizations, 2.2% in 24 jurisdictions, and the proportion of deaths, 0.06% in 46 jurisdictions, since the start of the pandemic, the AAP and CHA said, with a total of 336 COVID-19–related deaths reported.
AMPLITUDE-O: Efpeglenatide benefits in high-risk diabetes
The AMPLITUDE-O phase 3 trial showed that investigational drug efpeglenatide (Sanofi/Hanmi Pharmaceutical) – an exendin-based glucagonlike peptide-1 receptor agonist – was safe and reduced the risk of worsening renal and cardiovascular outcomes in patients with type 2 diabetes at high cardiovascular risk.
That is, in patients with type 2 diabetes and a high prevalence of cardiovascular and kidney disease with a high hemoglobin A1c and moderate use of a sodium-glucose cotransporter 2 inhibitor, subcutaneous efpeglenatide (4 or 6 mg/week) significantly and safely reduced cardiovascular and renal outcomes, said study investigator Naveed Sattar, MD.
Dr. Sattar, of the University of Glasgow, summarized the results during a symposium at the annual scientific sessions of the American Diabetes Association. The study was simultaneously published online in the New England Journal of Medicine.
AMPLITUDE-O was a cardiovascular outcome trial (CVOT) in more than 4,000 high-risk patients with type 2 diabetes followed for a mean of 1.8 years.
Compared with patients who received placebo, those who received either dose of efpeglenatide had a 27% lower risk of a major adverse cardiovascular event, defined as nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular or undetermined causes; a 21% lower risk of expanded MACE (MACE, coronary revascularization, or hospitalization for unstable angina); a 32% lower risk of a composite renal outcome (decrease in kidney function or macroalbuminuria); and a 27% lower risk of MACE or noncardiovascular death.
And “these effects were independent of baseline SGLT2 inhibitors, estimated glomerular filtration rate (eGFR), or metformin use,” Dr. Sattar pointed out.
New and important findings, but Sanofi no longer developing drug
The trial’s primary investigator, Hertzel C. Gerstein, MD, pointed out several new and important findings of the drug and study, compared with CVOTs of seven other GLP-1 receptor agonists.
The trial included more patients (32%) with renal disease (eGFR, 25-60 mL/min) than the other trials.
There were enough patients taking SGLT2 inhibitors at baseline (15%) to show no difference in the effect of a GLP-1 receptor agonist in the presence/absence of an SGLT2 inhibitor.
So this is the first clearly positive GLP-1 receptor agonist CVOT with an exendin-4–based GLP-1 receptor agonist showing that the GLP-1 receptor agonist class is cardioprotective whether or not it is based on a human or animal GLP-1 structure.
And there was a significant reduction in MACE or noncardiovascular death.
“This would be good for people with type 2 diabetes and either cardiovascular or renal disease at high risk for cardiovascular and/or renal outcomes,” said Dr. Gerstein, professor of medicine at McMaster University, Hamilton, Ont.
However, the trial sponsor, Sanofi, is no longer developing the drug. The company returned the rights back to Hanmi, which had started this line of research. “Hopefully” Hanmi or another company will develop the drug further, said Dr. Gerstein.
Sicker patients than in 7 other GLP-1 agonist CVOTs
Efpeglenatide – like two other drugs in the class, exenatide and lixisenatide – is an exendin-based GLP-1 agonist. (Exendin-4 is a peptide found in the saliva of the Gila monster lizard.) In contrast, liraglutide, dulaglutide, albiglutide, and semaglutide are human-analog GLP-1 agonists.
A meta-analysis of the seven CVOTs of these other drugs in this class reported, among other things, that “overall, GLP-1 agonist treatment reduced MACE by 12%.”
Amanda I. Adler, MD, PhD, professor of diabetic medicine and health policy, University of Oxford, (England), and the assigned independent commenter at the symposium, cited many things “the investigators did well.”
Compared with the CVOTs of the other GLP-1 receptor agonists – ELIXA (lixisenatide), LEADER (liraglutide), SUSTAIN-6 (semaglutide), EXSCEL (exenatide), Harmony Outcomes (albiglutide), REWIND (dulaglutide), and PIONEER 6 (oral semaglutide) – patients in the AMPLITUDE-O trial were sicker, she noted.
AMPLITUDE-O participants had the longest duration of diabetes (15 years), lowest mean eGFR of 72 ml/min per 1.73 m2, highest A1c (8.9%), and highest percentage of insulin use (62%), she noted.
The study was primarily a safety and noninferiority trial, she pointed out, although a series of superiority analyses were prespecified that would be conducted if the drug was found to be noninferior to placebo for the primary outcome of 3-point MACE.
It was good that patients were stratified according to SGLT2 inhibitor use – into current user, likely future user, and not likely future user – although “likely future user” may have misclassified some patients.
The various stakeholders – patients, regulators, doctors, payers, statisticians, and the marketing department of any company providing the drug – would want to know more, such as quality of life, long-term effects, and cost, she observed.
Meta-analysis of 8 CVOTs shows stronger class benefit
Dr. Sattar presented an eight-trial meta-analysis (an update of the seven-trial meta-analysis that included data from AMPLITUDE-O), which showed patients with type 2 diabetes who received GLP-1 agonists had a decreased rate of the 3-component MACE and decreased individual components (stroke more so than MI) – regardless of the structure of these drugs (exenatide or human analogs).
The updated meta-analysis also showed that, overall, GLP-1 agonists decreased all-cause mortality and possibly reduced the risk of heart failure hospitalization (perhaps linked to atherosclerotic benefits) as well as renal dysfunction.
There was no increase in risk of severe hypoglycemia, retinopathy, or pancreatic adverse effects.
AMPLITUDE-O: Design and findings
AMPLITUDE-O included 4,076 adults with type 2 diabetes from 344 sites in 28 countries who were screened from May 2018 to April 2019. Participants also had cardiovascular disease or kidney disease (eGFR, 25-60 mL/min) plus at least one other cardiovascular risk factor. They were randomized 1:1:1 to receive subcutaneous efpeglenatide (4 or 6 mg/week) or placebo.
Patients were a mean age of 65, most (87%) were White, and 33% were female. They had a mean A1c of 8.9%. Most (90%) had a history of cardiovascular disease and 31% had current kidney disease.
MACE occurred in 189 participants (7.0%) assigned to efpeglenatide and 125 participants (9.2%) assigned to receive placebo (3.9 vs. 5.3 events/100 person-years) (hazard ratio, 0.73; 95% confidence interval, 0.58-0.92; P < .001 for noninferiority; P = .007 for superiority).
The composite renal outcome event (decreased kidney function or macroalbuminuria) occurred in 353 participants (13.0%) assigned to receive efpeglenatide and in 250 participants (18.4%) assigned to receive placebo (HR, 0.68; 95% CI, 0.57-0.79; P < .001).
Diarrhea, constipation, nausea, vomiting, or bloating occurred more frequently with efpeglenatide than placebo.
The study was funded by Sanofi. Dr. Sattar has reported being on advisory panels for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Novo Nordisk, Pfizer, and Sanofi, and receiving research support from Boehringer Ingelheim. Dr. Gerstein has reported being a member of advisory panels for Novo Nordisk, Pfizer, and Sanofi, and a consultant for Abbott, Covance, Eli Lilly, Kowa, and Sanofi. He reported receiving research support from AstraZeneca, Eli Lilly, Merck, Novo Nordisk, and Sanofi, and having other relationships with Boehringer Ingelheim, DKSH, Eli Lilly, Sanofi, and Zuellig Pharma. Dr. Adler has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The AMPLITUDE-O phase 3 trial showed that investigational drug efpeglenatide (Sanofi/Hanmi Pharmaceutical) – an exendin-based glucagonlike peptide-1 receptor agonist – was safe and reduced the risk of worsening renal and cardiovascular outcomes in patients with type 2 diabetes at high cardiovascular risk.
That is, in patients with type 2 diabetes and a high prevalence of cardiovascular and kidney disease with a high hemoglobin A1c and moderate use of a sodium-glucose cotransporter 2 inhibitor, subcutaneous efpeglenatide (4 or 6 mg/week) significantly and safely reduced cardiovascular and renal outcomes, said study investigator Naveed Sattar, MD.
Dr. Sattar, of the University of Glasgow, summarized the results during a symposium at the annual scientific sessions of the American Diabetes Association. The study was simultaneously published online in the New England Journal of Medicine.
AMPLITUDE-O was a cardiovascular outcome trial (CVOT) in more than 4,000 high-risk patients with type 2 diabetes followed for a mean of 1.8 years.
Compared with patients who received placebo, those who received either dose of efpeglenatide had a 27% lower risk of a major adverse cardiovascular event, defined as nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular or undetermined causes; a 21% lower risk of expanded MACE (MACE, coronary revascularization, or hospitalization for unstable angina); a 32% lower risk of a composite renal outcome (decrease in kidney function or macroalbuminuria); and a 27% lower risk of MACE or noncardiovascular death.
And “these effects were independent of baseline SGLT2 inhibitors, estimated glomerular filtration rate (eGFR), or metformin use,” Dr. Sattar pointed out.
New and important findings, but Sanofi no longer developing drug
The trial’s primary investigator, Hertzel C. Gerstein, MD, pointed out several new and important findings of the drug and study, compared with CVOTs of seven other GLP-1 receptor agonists.
The trial included more patients (32%) with renal disease (eGFR, 25-60 mL/min) than the other trials.
There were enough patients taking SGLT2 inhibitors at baseline (15%) to show no difference in the effect of a GLP-1 receptor agonist in the presence/absence of an SGLT2 inhibitor.
So this is the first clearly positive GLP-1 receptor agonist CVOT with an exendin-4–based GLP-1 receptor agonist showing that the GLP-1 receptor agonist class is cardioprotective whether or not it is based on a human or animal GLP-1 structure.
And there was a significant reduction in MACE or noncardiovascular death.
“This would be good for people with type 2 diabetes and either cardiovascular or renal disease at high risk for cardiovascular and/or renal outcomes,” said Dr. Gerstein, professor of medicine at McMaster University, Hamilton, Ont.
However, the trial sponsor, Sanofi, is no longer developing the drug. The company returned the rights back to Hanmi, which had started this line of research. “Hopefully” Hanmi or another company will develop the drug further, said Dr. Gerstein.
Sicker patients than in 7 other GLP-1 agonist CVOTs
Efpeglenatide – like two other drugs in the class, exenatide and lixisenatide – is an exendin-based GLP-1 agonist. (Exendin-4 is a peptide found in the saliva of the Gila monster lizard.) In contrast, liraglutide, dulaglutide, albiglutide, and semaglutide are human-analog GLP-1 agonists.
A meta-analysis of the seven CVOTs of these other drugs in this class reported, among other things, that “overall, GLP-1 agonist treatment reduced MACE by 12%.”
Amanda I. Adler, MD, PhD, professor of diabetic medicine and health policy, University of Oxford, (England), and the assigned independent commenter at the symposium, cited many things “the investigators did well.”
Compared with the CVOTs of the other GLP-1 receptor agonists – ELIXA (lixisenatide), LEADER (liraglutide), SUSTAIN-6 (semaglutide), EXSCEL (exenatide), Harmony Outcomes (albiglutide), REWIND (dulaglutide), and PIONEER 6 (oral semaglutide) – patients in the AMPLITUDE-O trial were sicker, she noted.
AMPLITUDE-O participants had the longest duration of diabetes (15 years), lowest mean eGFR of 72 ml/min per 1.73 m2, highest A1c (8.9%), and highest percentage of insulin use (62%), she noted.
The study was primarily a safety and noninferiority trial, she pointed out, although a series of superiority analyses were prespecified that would be conducted if the drug was found to be noninferior to placebo for the primary outcome of 3-point MACE.
It was good that patients were stratified according to SGLT2 inhibitor use – into current user, likely future user, and not likely future user – although “likely future user” may have misclassified some patients.
The various stakeholders – patients, regulators, doctors, payers, statisticians, and the marketing department of any company providing the drug – would want to know more, such as quality of life, long-term effects, and cost, she observed.
Meta-analysis of 8 CVOTs shows stronger class benefit
Dr. Sattar presented an eight-trial meta-analysis (an update of the seven-trial meta-analysis that included data from AMPLITUDE-O), which showed patients with type 2 diabetes who received GLP-1 agonists had a decreased rate of the 3-component MACE and decreased individual components (stroke more so than MI) – regardless of the structure of these drugs (exenatide or human analogs).
The updated meta-analysis also showed that, overall, GLP-1 agonists decreased all-cause mortality and possibly reduced the risk of heart failure hospitalization (perhaps linked to atherosclerotic benefits) as well as renal dysfunction.
There was no increase in risk of severe hypoglycemia, retinopathy, or pancreatic adverse effects.
AMPLITUDE-O: Design and findings
AMPLITUDE-O included 4,076 adults with type 2 diabetes from 344 sites in 28 countries who were screened from May 2018 to April 2019. Participants also had cardiovascular disease or kidney disease (eGFR, 25-60 mL/min) plus at least one other cardiovascular risk factor. They were randomized 1:1:1 to receive subcutaneous efpeglenatide (4 or 6 mg/week) or placebo.
Patients were a mean age of 65, most (87%) were White, and 33% were female. They had a mean A1c of 8.9%. Most (90%) had a history of cardiovascular disease and 31% had current kidney disease.
MACE occurred in 189 participants (7.0%) assigned to efpeglenatide and 125 participants (9.2%) assigned to receive placebo (3.9 vs. 5.3 events/100 person-years) (hazard ratio, 0.73; 95% confidence interval, 0.58-0.92; P < .001 for noninferiority; P = .007 for superiority).
The composite renal outcome event (decreased kidney function or macroalbuminuria) occurred in 353 participants (13.0%) assigned to receive efpeglenatide and in 250 participants (18.4%) assigned to receive placebo (HR, 0.68; 95% CI, 0.57-0.79; P < .001).
Diarrhea, constipation, nausea, vomiting, or bloating occurred more frequently with efpeglenatide than placebo.
The study was funded by Sanofi. Dr. Sattar has reported being on advisory panels for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Novo Nordisk, Pfizer, and Sanofi, and receiving research support from Boehringer Ingelheim. Dr. Gerstein has reported being a member of advisory panels for Novo Nordisk, Pfizer, and Sanofi, and a consultant for Abbott, Covance, Eli Lilly, Kowa, and Sanofi. He reported receiving research support from AstraZeneca, Eli Lilly, Merck, Novo Nordisk, and Sanofi, and having other relationships with Boehringer Ingelheim, DKSH, Eli Lilly, Sanofi, and Zuellig Pharma. Dr. Adler has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The AMPLITUDE-O phase 3 trial showed that investigational drug efpeglenatide (Sanofi/Hanmi Pharmaceutical) – an exendin-based glucagonlike peptide-1 receptor agonist – was safe and reduced the risk of worsening renal and cardiovascular outcomes in patients with type 2 diabetes at high cardiovascular risk.
That is, in patients with type 2 diabetes and a high prevalence of cardiovascular and kidney disease with a high hemoglobin A1c and moderate use of a sodium-glucose cotransporter 2 inhibitor, subcutaneous efpeglenatide (4 or 6 mg/week) significantly and safely reduced cardiovascular and renal outcomes, said study investigator Naveed Sattar, MD.
Dr. Sattar, of the University of Glasgow, summarized the results during a symposium at the annual scientific sessions of the American Diabetes Association. The study was simultaneously published online in the New England Journal of Medicine.
AMPLITUDE-O was a cardiovascular outcome trial (CVOT) in more than 4,000 high-risk patients with type 2 diabetes followed for a mean of 1.8 years.
Compared with patients who received placebo, those who received either dose of efpeglenatide had a 27% lower risk of a major adverse cardiovascular event, defined as nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular or undetermined causes; a 21% lower risk of expanded MACE (MACE, coronary revascularization, or hospitalization for unstable angina); a 32% lower risk of a composite renal outcome (decrease in kidney function or macroalbuminuria); and a 27% lower risk of MACE or noncardiovascular death.
And “these effects were independent of baseline SGLT2 inhibitors, estimated glomerular filtration rate (eGFR), or metformin use,” Dr. Sattar pointed out.
New and important findings, but Sanofi no longer developing drug
The trial’s primary investigator, Hertzel C. Gerstein, MD, pointed out several new and important findings of the drug and study, compared with CVOTs of seven other GLP-1 receptor agonists.
The trial included more patients (32%) with renal disease (eGFR, 25-60 mL/min) than the other trials.
There were enough patients taking SGLT2 inhibitors at baseline (15%) to show no difference in the effect of a GLP-1 receptor agonist in the presence/absence of an SGLT2 inhibitor.
So this is the first clearly positive GLP-1 receptor agonist CVOT with an exendin-4–based GLP-1 receptor agonist showing that the GLP-1 receptor agonist class is cardioprotective whether or not it is based on a human or animal GLP-1 structure.
And there was a significant reduction in MACE or noncardiovascular death.
“This would be good for people with type 2 diabetes and either cardiovascular or renal disease at high risk for cardiovascular and/or renal outcomes,” said Dr. Gerstein, professor of medicine at McMaster University, Hamilton, Ont.
However, the trial sponsor, Sanofi, is no longer developing the drug. The company returned the rights back to Hanmi, which had started this line of research. “Hopefully” Hanmi or another company will develop the drug further, said Dr. Gerstein.
Sicker patients than in 7 other GLP-1 agonist CVOTs
Efpeglenatide – like two other drugs in the class, exenatide and lixisenatide – is an exendin-based GLP-1 agonist. (Exendin-4 is a peptide found in the saliva of the Gila monster lizard.) In contrast, liraglutide, dulaglutide, albiglutide, and semaglutide are human-analog GLP-1 agonists.
A meta-analysis of the seven CVOTs of these other drugs in this class reported, among other things, that “overall, GLP-1 agonist treatment reduced MACE by 12%.”
Amanda I. Adler, MD, PhD, professor of diabetic medicine and health policy, University of Oxford, (England), and the assigned independent commenter at the symposium, cited many things “the investigators did well.”
Compared with the CVOTs of the other GLP-1 receptor agonists – ELIXA (lixisenatide), LEADER (liraglutide), SUSTAIN-6 (semaglutide), EXSCEL (exenatide), Harmony Outcomes (albiglutide), REWIND (dulaglutide), and PIONEER 6 (oral semaglutide) – patients in the AMPLITUDE-O trial were sicker, she noted.
AMPLITUDE-O participants had the longest duration of diabetes (15 years), lowest mean eGFR of 72 ml/min per 1.73 m2, highest A1c (8.9%), and highest percentage of insulin use (62%), she noted.
The study was primarily a safety and noninferiority trial, she pointed out, although a series of superiority analyses were prespecified that would be conducted if the drug was found to be noninferior to placebo for the primary outcome of 3-point MACE.
It was good that patients were stratified according to SGLT2 inhibitor use – into current user, likely future user, and not likely future user – although “likely future user” may have misclassified some patients.
The various stakeholders – patients, regulators, doctors, payers, statisticians, and the marketing department of any company providing the drug – would want to know more, such as quality of life, long-term effects, and cost, she observed.
Meta-analysis of 8 CVOTs shows stronger class benefit
Dr. Sattar presented an eight-trial meta-analysis (an update of the seven-trial meta-analysis that included data from AMPLITUDE-O), which showed patients with type 2 diabetes who received GLP-1 agonists had a decreased rate of the 3-component MACE and decreased individual components (stroke more so than MI) – regardless of the structure of these drugs (exenatide or human analogs).
The updated meta-analysis also showed that, overall, GLP-1 agonists decreased all-cause mortality and possibly reduced the risk of heart failure hospitalization (perhaps linked to atherosclerotic benefits) as well as renal dysfunction.
There was no increase in risk of severe hypoglycemia, retinopathy, or pancreatic adverse effects.
AMPLITUDE-O: Design and findings
AMPLITUDE-O included 4,076 adults with type 2 diabetes from 344 sites in 28 countries who were screened from May 2018 to April 2019. Participants also had cardiovascular disease or kidney disease (eGFR, 25-60 mL/min) plus at least one other cardiovascular risk factor. They were randomized 1:1:1 to receive subcutaneous efpeglenatide (4 or 6 mg/week) or placebo.
Patients were a mean age of 65, most (87%) were White, and 33% were female. They had a mean A1c of 8.9%. Most (90%) had a history of cardiovascular disease and 31% had current kidney disease.
MACE occurred in 189 participants (7.0%) assigned to efpeglenatide and 125 participants (9.2%) assigned to receive placebo (3.9 vs. 5.3 events/100 person-years) (hazard ratio, 0.73; 95% confidence interval, 0.58-0.92; P < .001 for noninferiority; P = .007 for superiority).
The composite renal outcome event (decreased kidney function or macroalbuminuria) occurred in 353 participants (13.0%) assigned to receive efpeglenatide and in 250 participants (18.4%) assigned to receive placebo (HR, 0.68; 95% CI, 0.57-0.79; P < .001).
Diarrhea, constipation, nausea, vomiting, or bloating occurred more frequently with efpeglenatide than placebo.
The study was funded by Sanofi. Dr. Sattar has reported being on advisory panels for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, Novo Nordisk, Pfizer, and Sanofi, and receiving research support from Boehringer Ingelheim. Dr. Gerstein has reported being a member of advisory panels for Novo Nordisk, Pfizer, and Sanofi, and a consultant for Abbott, Covance, Eli Lilly, Kowa, and Sanofi. He reported receiving research support from AstraZeneca, Eli Lilly, Merck, Novo Nordisk, and Sanofi, and having other relationships with Boehringer Ingelheim, DKSH, Eli Lilly, Sanofi, and Zuellig Pharma. Dr. Adler has reported no relevant financial relationships.
A version of this article first appeared on Medscape.com.