User login
New data on worldwide mental health impact of COVID-19
A new survey that assessed the mental health impact of COVID-19 across the globe shows high rates of trauma and clinical mood disorders related to the pandemic.
The survey, carried out by Sapien Labs, was conducted in eight English-speaking countries and included 49,000 adults. It showed that 57% of respondents experienced some COVID-19–related adversity or trauma.
Roughly one-quarter showed clinical signs of or were at risk for a mood disorder, and 40% described themselves as “succeeding or thriving.”
Those who reported the poorest mental health were young adults and individuals who experienced financial adversity or were unable to receive care for other medical conditions. Nonbinary gender and not getting enough sleep, exercise, or face-to-face socialization also increased the risk for poorer mental well-being.
“The data suggest that there will be long-term fallout from the pandemic on the mental health front,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, said in a press release.
Novel initiative
Dr. Thiagarajan said in an interview that she was running a company that provided microloans to 30,000 villages in India. The company included a research group the goal of which was to understand what predicts success in an individual and in a particular ecosystem, she said – “Why did some villages succeed and others didn’t?”
Dr. Thiagarajan and associates thought that “something big is happening in our life circumstances that causes changes in our brain and felt that we need to understand what they are and how they affect humanity. This was the impetus for founding Sapien Labs. “
The survey, which is part of the company’s Mental Health Million project, is an ongoing research initiative that makes data freely available to other researchers.
The investigators developed a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability,” said Dr. Thiagarajan.
The MHQ consists of 47 “elements of mental well-being.” Respondents’ MHQ scores ranged from –100 to +200. Negative scores indicate poorer mental well-being. Respondents were categorized as clinical, at risk, enduring, managing, succeeding, and thriving.
MHQ scores were computed for six “broad dimensions” of mental health: Core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
Participants were recruited through advertising on Google and Facebook in eight English-speaking countries – Canada, the United States, the United Kingdom, South Africa, Singapore, Australia, New Zealand, and India. The researchers collected demographic information, including age, education, and gender.
First step
The assessment was completed by 48,808 respondents between April 8 and Dec. 31, 2020.
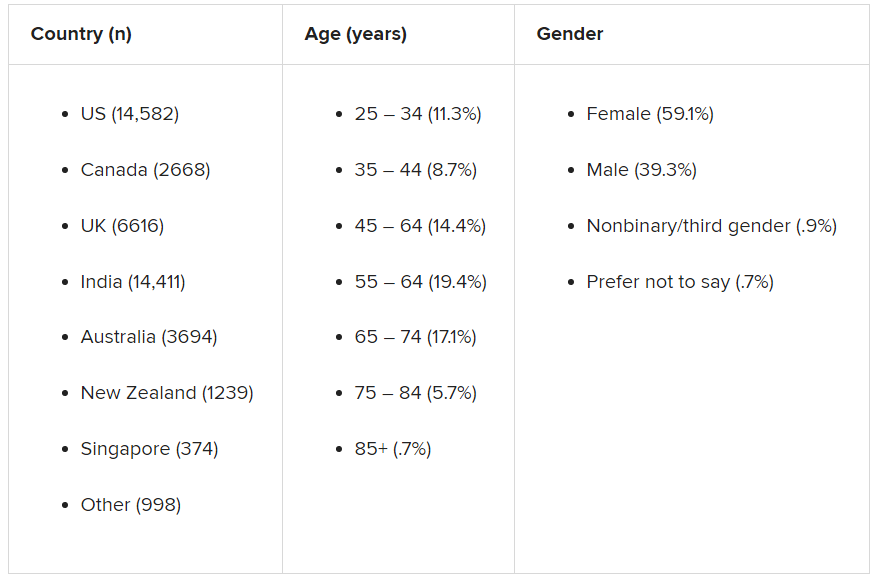
A smaller sample of 2,000 people from the same countries who were polled by the investigators in 2019 was used as a comparator.
Taken together, the overall mental well-being score for 2020 was 8% lower than the score obtained in 2019 from the same countries, and the percentage of respondents who fell into the “clinical” category increased from 14% in 2009 to 26% in 2020.
Residents of Singapore had the highest MHQ score, followed by residents of the United States. At the other extreme, respondents from the United Kingdom and South Africa had the poorest MHQ scores.
“It is important to keep in mind that the English-speaking, Internet-enabled populace is not necessarily representative of each country as a whole,” the authors noted.
Youth hardest hit
whose average MHQ score was 29% lower than those aged at least 65 years.
Worldwide, 70% of respondents aged at least 65 years fell into the categories of “succeeding” or “thriving,” compared with just 17% of those aged 18-24 years.
“We saw a massive trend of diminishing mental well-being in younger individuals, suggesting that some societal force is at play that we need to get to the bottom of,” said Dr. Thiagarajan.
“Young people are still learning how to calibrate themselves in the world, and with age comes maturity, leading to a difference in emotional resilience,” she said.
Highest risk group
Mental well-being was poorest among nonbinary/third-gender respondents. Among those persons, more than 50% were classified as being at clinical risk, in comparison with males and females combined, and their MHQ scores were about 47 points lower.
Nonbinary individuals “are universally doing very poorly, relative to males or females,” said Dr. Thiagarajan. “This is a demographic at very high risk with a lot of suicidal thoughts.”
Respondents who had insufficient sleep, who lacked social interaction, and whose level of exercise was insufficient had lower MHQ scores of an “unexpected magnitude,” compared with their counterparts who had sufficient sleep, more social interaction, and more exercise (a discrepancy of 82, 66, and 46 points, respectively).
Only 3.9% of respondents reported having had COVID-19; 0.7% reported having had a severe case. Yet 57% of respondents reported that the pandemic had had negative consequences with regard to their health or their finances or social situation.
Those who were unable to get care for their other health conditions because of the pandemic (2% of all respondents) reported the worst mental well-being, followed by those who struggled for basic necessities (1.4%).
Reduced household income was associated with a 4% lower score but affected a higher percentage of people (17%). Social isolation was associated with a score of about 20 less. Higher rates of lifetime traumas and adversities were likewise associated with lower scores for mental well-being.
Creative, generous approach
Commenting on the survey results, Ken Duckworth, MD, clinical professor at Harvard Medical School, Boston, and chief medical officer of the National Alliance of Mental Illness, noted that the findings were similar to findings from studies in the United States, which showed disproportionately higher rates of mental health problems in younger individuals. Dr. Duckworth was not involved with the survey.
“The idea that this is an international phenomenon and the broad-stroke finding that younger people are suffering across nations is compelling and important for policymakers to look at,” he said.
Dr. Duckworth noted that although the findings are not “representative” of entire populations in a given country, the report is a “first step in a long journey.”
He described the report as “extremely brilliant, creative, and generous, allowing any academician to get access to the data.”
He saw it “less as a definitive report and more as a directionally informative survey that will yield great fruit over time.”
In a comment, Joshua Morganstein, MD, chair of the American Psychiatric Association’s Committee on the Psychiatric Dimensions of Disaster, said: “One of the important things a document like this highlights is the importance of understanding more where risk [for mental health disorders] is concentrated and what things have occurred or might occur that can buffer against that risk or protect us from it. We see that each nation has similar but also different challenges.”
Dr. Thiagarajan is the founder and chief scientist of Sapien Labs. Her coauthors are employees of Sapien Labs. Dr. Duckworth and Dr. Morganstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new survey that assessed the mental health impact of COVID-19 across the globe shows high rates of trauma and clinical mood disorders related to the pandemic.
The survey, carried out by Sapien Labs, was conducted in eight English-speaking countries and included 49,000 adults. It showed that 57% of respondents experienced some COVID-19–related adversity or trauma.
Roughly one-quarter showed clinical signs of or were at risk for a mood disorder, and 40% described themselves as “succeeding or thriving.”
Those who reported the poorest mental health were young adults and individuals who experienced financial adversity or were unable to receive care for other medical conditions. Nonbinary gender and not getting enough sleep, exercise, or face-to-face socialization also increased the risk for poorer mental well-being.
“The data suggest that there will be long-term fallout from the pandemic on the mental health front,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, said in a press release.
Novel initiative
Dr. Thiagarajan said in an interview that she was running a company that provided microloans to 30,000 villages in India. The company included a research group the goal of which was to understand what predicts success in an individual and in a particular ecosystem, she said – “Why did some villages succeed and others didn’t?”
Dr. Thiagarajan and associates thought that “something big is happening in our life circumstances that causes changes in our brain and felt that we need to understand what they are and how they affect humanity. This was the impetus for founding Sapien Labs. “
The survey, which is part of the company’s Mental Health Million project, is an ongoing research initiative that makes data freely available to other researchers.
The investigators developed a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability,” said Dr. Thiagarajan.
The MHQ consists of 47 “elements of mental well-being.” Respondents’ MHQ scores ranged from –100 to +200. Negative scores indicate poorer mental well-being. Respondents were categorized as clinical, at risk, enduring, managing, succeeding, and thriving.
MHQ scores were computed for six “broad dimensions” of mental health: Core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
Participants were recruited through advertising on Google and Facebook in eight English-speaking countries – Canada, the United States, the United Kingdom, South Africa, Singapore, Australia, New Zealand, and India. The researchers collected demographic information, including age, education, and gender.
First step
The assessment was completed by 48,808 respondents between April 8 and Dec. 31, 2020.

A smaller sample of 2,000 people from the same countries who were polled by the investigators in 2019 was used as a comparator.
Taken together, the overall mental well-being score for 2020 was 8% lower than the score obtained in 2019 from the same countries, and the percentage of respondents who fell into the “clinical” category increased from 14% in 2009 to 26% in 2020.
Residents of Singapore had the highest MHQ score, followed by residents of the United States. At the other extreme, respondents from the United Kingdom and South Africa had the poorest MHQ scores.
“It is important to keep in mind that the English-speaking, Internet-enabled populace is not necessarily representative of each country as a whole,” the authors noted.
Youth hardest hit
whose average MHQ score was 29% lower than those aged at least 65 years.
Worldwide, 70% of respondents aged at least 65 years fell into the categories of “succeeding” or “thriving,” compared with just 17% of those aged 18-24 years.
“We saw a massive trend of diminishing mental well-being in younger individuals, suggesting that some societal force is at play that we need to get to the bottom of,” said Dr. Thiagarajan.
“Young people are still learning how to calibrate themselves in the world, and with age comes maturity, leading to a difference in emotional resilience,” she said.
Highest risk group
Mental well-being was poorest among nonbinary/third-gender respondents. Among those persons, more than 50% were classified as being at clinical risk, in comparison with males and females combined, and their MHQ scores were about 47 points lower.
Nonbinary individuals “are universally doing very poorly, relative to males or females,” said Dr. Thiagarajan. “This is a demographic at very high risk with a lot of suicidal thoughts.”
Respondents who had insufficient sleep, who lacked social interaction, and whose level of exercise was insufficient had lower MHQ scores of an “unexpected magnitude,” compared with their counterparts who had sufficient sleep, more social interaction, and more exercise (a discrepancy of 82, 66, and 46 points, respectively).
Only 3.9% of respondents reported having had COVID-19; 0.7% reported having had a severe case. Yet 57% of respondents reported that the pandemic had had negative consequences with regard to their health or their finances or social situation.
Those who were unable to get care for their other health conditions because of the pandemic (2% of all respondents) reported the worst mental well-being, followed by those who struggled for basic necessities (1.4%).
Reduced household income was associated with a 4% lower score but affected a higher percentage of people (17%). Social isolation was associated with a score of about 20 less. Higher rates of lifetime traumas and adversities were likewise associated with lower scores for mental well-being.
Creative, generous approach
Commenting on the survey results, Ken Duckworth, MD, clinical professor at Harvard Medical School, Boston, and chief medical officer of the National Alliance of Mental Illness, noted that the findings were similar to findings from studies in the United States, which showed disproportionately higher rates of mental health problems in younger individuals. Dr. Duckworth was not involved with the survey.
“The idea that this is an international phenomenon and the broad-stroke finding that younger people are suffering across nations is compelling and important for policymakers to look at,” he said.
Dr. Duckworth noted that although the findings are not “representative” of entire populations in a given country, the report is a “first step in a long journey.”
He described the report as “extremely brilliant, creative, and generous, allowing any academician to get access to the data.”
He saw it “less as a definitive report and more as a directionally informative survey that will yield great fruit over time.”
In a comment, Joshua Morganstein, MD, chair of the American Psychiatric Association’s Committee on the Psychiatric Dimensions of Disaster, said: “One of the important things a document like this highlights is the importance of understanding more where risk [for mental health disorders] is concentrated and what things have occurred or might occur that can buffer against that risk or protect us from it. We see that each nation has similar but also different challenges.”
Dr. Thiagarajan is the founder and chief scientist of Sapien Labs. Her coauthors are employees of Sapien Labs. Dr. Duckworth and Dr. Morganstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
A new survey that assessed the mental health impact of COVID-19 across the globe shows high rates of trauma and clinical mood disorders related to the pandemic.
The survey, carried out by Sapien Labs, was conducted in eight English-speaking countries and included 49,000 adults. It showed that 57% of respondents experienced some COVID-19–related adversity or trauma.
Roughly one-quarter showed clinical signs of or were at risk for a mood disorder, and 40% described themselves as “succeeding or thriving.”
Those who reported the poorest mental health were young adults and individuals who experienced financial adversity or were unable to receive care for other medical conditions. Nonbinary gender and not getting enough sleep, exercise, or face-to-face socialization also increased the risk for poorer mental well-being.
“The data suggest that there will be long-term fallout from the pandemic on the mental health front,” Tara Thiagarajan, PhD, Sapien Labs founder and chief scientist, said in a press release.
Novel initiative
Dr. Thiagarajan said in an interview that she was running a company that provided microloans to 30,000 villages in India. The company included a research group the goal of which was to understand what predicts success in an individual and in a particular ecosystem, she said – “Why did some villages succeed and others didn’t?”
Dr. Thiagarajan and associates thought that “something big is happening in our life circumstances that causes changes in our brain and felt that we need to understand what they are and how they affect humanity. This was the impetus for founding Sapien Labs. “
The survey, which is part of the company’s Mental Health Million project, is an ongoing research initiative that makes data freely available to other researchers.
The investigators developed a “free and anonymous assessment tool,” the Mental Health Quotient (MHQ), which “encompasses a comprehensive view of our emotional, social, and cognitive function and capability,” said Dr. Thiagarajan.
The MHQ consists of 47 “elements of mental well-being.” Respondents’ MHQ scores ranged from –100 to +200. Negative scores indicate poorer mental well-being. Respondents were categorized as clinical, at risk, enduring, managing, succeeding, and thriving.
MHQ scores were computed for six “broad dimensions” of mental health: Core cognition, complex cognition, mood and outlook, drive and motivation, social self, and mind-body connection.
Participants were recruited through advertising on Google and Facebook in eight English-speaking countries – Canada, the United States, the United Kingdom, South Africa, Singapore, Australia, New Zealand, and India. The researchers collected demographic information, including age, education, and gender.
First step
The assessment was completed by 48,808 respondents between April 8 and Dec. 31, 2020.

A smaller sample of 2,000 people from the same countries who were polled by the investigators in 2019 was used as a comparator.
Taken together, the overall mental well-being score for 2020 was 8% lower than the score obtained in 2019 from the same countries, and the percentage of respondents who fell into the “clinical” category increased from 14% in 2009 to 26% in 2020.
Residents of Singapore had the highest MHQ score, followed by residents of the United States. At the other extreme, respondents from the United Kingdom and South Africa had the poorest MHQ scores.
“It is important to keep in mind that the English-speaking, Internet-enabled populace is not necessarily representative of each country as a whole,” the authors noted.
Youth hardest hit
whose average MHQ score was 29% lower than those aged at least 65 years.
Worldwide, 70% of respondents aged at least 65 years fell into the categories of “succeeding” or “thriving,” compared with just 17% of those aged 18-24 years.
“We saw a massive trend of diminishing mental well-being in younger individuals, suggesting that some societal force is at play that we need to get to the bottom of,” said Dr. Thiagarajan.
“Young people are still learning how to calibrate themselves in the world, and with age comes maturity, leading to a difference in emotional resilience,” she said.
Highest risk group
Mental well-being was poorest among nonbinary/third-gender respondents. Among those persons, more than 50% were classified as being at clinical risk, in comparison with males and females combined, and their MHQ scores were about 47 points lower.
Nonbinary individuals “are universally doing very poorly, relative to males or females,” said Dr. Thiagarajan. “This is a demographic at very high risk with a lot of suicidal thoughts.”
Respondents who had insufficient sleep, who lacked social interaction, and whose level of exercise was insufficient had lower MHQ scores of an “unexpected magnitude,” compared with their counterparts who had sufficient sleep, more social interaction, and more exercise (a discrepancy of 82, 66, and 46 points, respectively).
Only 3.9% of respondents reported having had COVID-19; 0.7% reported having had a severe case. Yet 57% of respondents reported that the pandemic had had negative consequences with regard to their health or their finances or social situation.
Those who were unable to get care for their other health conditions because of the pandemic (2% of all respondents) reported the worst mental well-being, followed by those who struggled for basic necessities (1.4%).
Reduced household income was associated with a 4% lower score but affected a higher percentage of people (17%). Social isolation was associated with a score of about 20 less. Higher rates of lifetime traumas and adversities were likewise associated with lower scores for mental well-being.
Creative, generous approach
Commenting on the survey results, Ken Duckworth, MD, clinical professor at Harvard Medical School, Boston, and chief medical officer of the National Alliance of Mental Illness, noted that the findings were similar to findings from studies in the United States, which showed disproportionately higher rates of mental health problems in younger individuals. Dr. Duckworth was not involved with the survey.
“The idea that this is an international phenomenon and the broad-stroke finding that younger people are suffering across nations is compelling and important for policymakers to look at,” he said.
Dr. Duckworth noted that although the findings are not “representative” of entire populations in a given country, the report is a “first step in a long journey.”
He described the report as “extremely brilliant, creative, and generous, allowing any academician to get access to the data.”
He saw it “less as a definitive report and more as a directionally informative survey that will yield great fruit over time.”
In a comment, Joshua Morganstein, MD, chair of the American Psychiatric Association’s Committee on the Psychiatric Dimensions of Disaster, said: “One of the important things a document like this highlights is the importance of understanding more where risk [for mental health disorders] is concentrated and what things have occurred or might occur that can buffer against that risk or protect us from it. We see that each nation has similar but also different challenges.”
Dr. Thiagarajan is the founder and chief scientist of Sapien Labs. Her coauthors are employees of Sapien Labs. Dr. Duckworth and Dr. Morganstein disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Treatment of Generalized Pustular Psoriasis of Pregnancy With Infliximab
Generalized pustular psoriasis of pregnancy (GPPP), formerly known as impetigo herpetiformis, is a rare dermatosis that causes maternal and fetal morbidity and mortality. It is characterized by widespread, circular, erythematous plaques with pustules at the periphery.1 Conventional first-line treatment includes systemic corticosteroids and cyclosporine. The National Psoriasis Foundation Medical Board also has included infliximab among the first-line treatment options for GPPP.2 Herein, we report a case of GPPP treated with infliximab at 30 weeks’ gestation and during the postpartum period.
Case Report
A 22-year-old woman was admitted to our inpatient clinic at 20 weeks’ gestation in her second pregnancy for evaluation of cutaneous eruptions covering the entire body. The lesions first appeared 3 to 4 days prior to her admission and dramatically progressed. She had a history of psoriasis vulgaris diagnosed during her first pregnancy 2 years prior that was treated with topical steroids throughout the pregnancy and methotrexate during lactation for a total of 11 months. She then was started on cyclosporine, which she used for 6 months due to ineffectiveness of the methotrexate, but she stopped treatment 4 months before the second pregnancy.
At the current presentation, physical examination revealed erythroderma and widespread pustules on the chest, abdomen, arms, and legs, including the intertriginous regions, that tended to coalesce and form lakes of pus over an erythematous base (Figure 1). The mucosae were normal. She exhibited a low blood pressure (85/50 mmHg) and high body temperature (102 °F [38.9 °C]). Routine laboratory examination revealed anemia and a normal leukocyte count. Her erythrocyte sedimentation rate (57 mm/h [reference range, <20 mm/h]) and C-reactive protein level (102 mg/L [reference range, <6 mg/L]) were elevated, whereas total calcium (8.11 mg/dL [reference range, 8.2–10.6 mg/dL]) and albumin (3.15 g/dL [reference range, >4.0 g/dL]) levels were low.
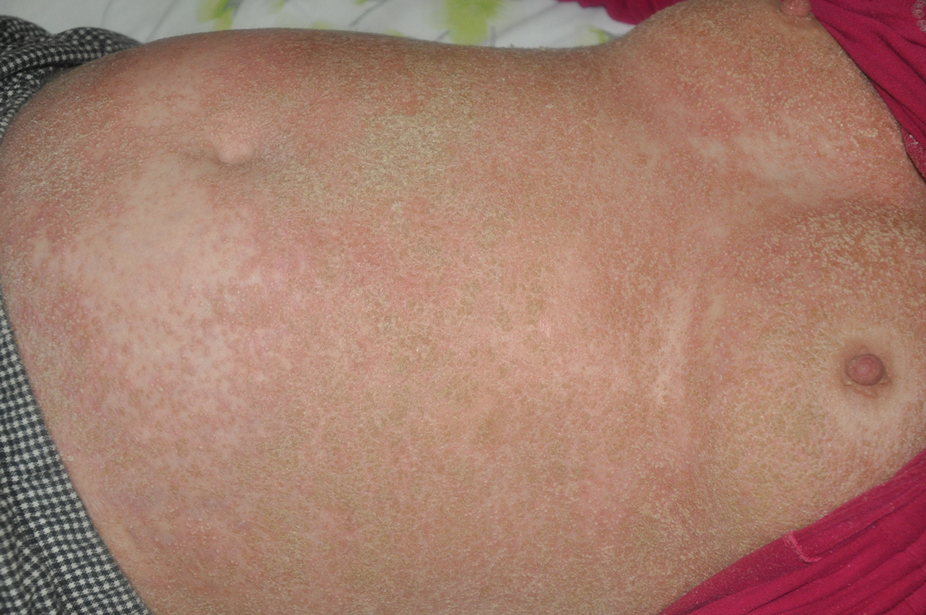
Empirical intravenous piperacillin/tazobactam was started due to hypotension, high fever, and elevated C-reactive protein levels; however, treatment was stopped after 4 days when microbiological cultures taken from blood and pustules revealed no bacterial growth, and therefore the fever was assumed to be caused by erythroderma. A skin biopsy before the start of topical and systemic treatment revealed changes consistent with GPPP.
Because her disease was extensive, systemic methylprednisolone 1.5 mg/kg once daily was started, and the dose was increased up to 2.5 mg/kg once daily on the tenth day of treatment to control new crops of eruptions. The dose was tapered to 2 mg/kg once daily when the lesions subsided 4 weeks into the treatment. The patient was discharged after 7 weeks at 27 weeks’ gestation.
Twelve days later, the patient was readmitted to the clinic in an erythrodermic state. The lesions were not controlled with increased doses of systemic corticosteroids. Treatment with cyclosporine was considered, but the patient refused; thus, infliximab treatment was planned. Isoniazid 300 mg once daily was started due to a risk of latent Mycobacterium tuberculosis infection revealed by a tuberculosis blood test. Other evaluations revealed no contraindications, and an infusion of infliximab 300 mg (5 mg/kg) was administered at 30 weeks’ gestation. There was visible improvement in the erythroderma and pustular lesions within the same day of treatment, and the lesions were completely cleared within 2 days of the infusion. The methylprednisolone dose was reduced to 1.5 mg/kg once daily.
Three days after treatment with infliximab, lesions with yellow encrustation appeared in the perioral region and on the oral mucosa and left ear. She was diagnosed with an oral herpes infection. Oral valacyclovir 1 g twice daily and topical mupirocin were started and the lesions subsided within 1 week. Twelve days after the infliximab infusion, new pustular lesions appeared, and a second infusion of infliximab was administered 13 days after the first, which cleared all lesions within 48 hours.
The patient’s methylprednisolone dose was tapered and stopped prior to delivery at 34 weeks’ gestation—2 weeks after the second dose of infliximab—as she did not have any new skin eruptions. A third infliximab infusion that normally would have occurred 4 weeks after the second treatment was postponed for a Cesarean section scheduled at 36 weeks’ gestation due to suspected intrauterine growth retardation. The patient stayed at the hospital until delivery without any new skin lesions. The gross and histopathologic examination of the placenta was normal. The neonate weighed 4.8 lb at birth and had neonatal jaundice that resolved spontaneously within 10 days but was otherwise healthy.
The patient returned to the clinic 3 weeks postpartum with a few pustules on erythematous plaques on the chest, abdomen, and back. At this time, she received a third infusion of infliximab 8 weeks after the second dose. For the past 5 years, the patient has been undergoing infliximab maintenance treatment, which she receives at the hospital every 8 weeks with excellent response. She has had no further pregnancies to date.
Comment
Generalized pustular psoriasis of pregnancy is a rare condition that typically occurs in the third trimester but also can start in the first and second trimesters. It may result in maternal and fetal morbidity by causing fluid and electrolyte imbalance and/or placental insufficiency, resulting in an increased risk for fetal abnormalities, stillbirth, and neonatal death.3 In subsequent pregnancies, GPPP has been observed to recur at an earlier gestational age with a more severe presentation.1,3
Generalized pustular psoriasis of pregnancy usually involves an eruption that begins symmetrically in the intertriginous areas and spreads to the rest of the body. The lesions present as erythematous annular plaques with pustules on the periphery and desquamation in the center due to older pustules.1,3 The mucous membranes also may be involved with erosive and exfoliative plaques, and there may be nail involvement. Patients often present with systemic symptoms such as fever, malaise, diarrhea, and vomiting.1 Laboratory investigations may reveal neutrophilic leukocytosis, high erythrocyte sedimentation rate, hypocalcemia, and hypoalbuminemia.4 Cultures from blood and pustules show no bacterial growth. A skin biopsy is helpful in diagnosis, with features similar to generalized pustular psoriasis, demonstrating spongiform pustules containing neutrophils, lymphocytic and neutrophilic infiltrates in the papillary dermis, and negative direct immunofluorescence.3
The differential diagnosis of GPPP includes subcorneal pustular dermatosis, dermatitis herpetiformis, herpes gestationis, impetigo, and acute generalized exanthematous pustulosis.1,3 Due to concerns of fetal implications, treatment options in GPPP are somewhat limited; however, the condition requires treatment because it may result in unfavorable pregnancy outcomes. Topical corticosteroids may be an option for limited disease.5,6 Systemic corticosteroids (eg, prednisone 60–80 mg/d) were previously considered as first-line agents, although they have shown limited efficacy in our case as well as in other case reports.7 Their ineffectiveness and risk for flare-up after dose tapering should be kept in mind when starting GPPP patients on systemic corticosteroids. Systemic cyclosporine (2–3 mg/kg/d) may be added to increase the efficacy of systemic steroids, which was done in several cases in literature.1,6,8 Although cyclosporine has been classified as a pregnancy category C drug, an analysis of pregnancy outcomes of 629 renal transplant patients revealed no association with adverse pregnancy outcomes compared to the general population and no increase in fetal malformations.9 Therefore, cyclosporine is a safe treatment option and was classified as a first-line drug for GPPP in a 2012 review by the National Psoriasis Foundation Medical Board.2 Narrowband UVB also has been reported to be used for the treatment of GPPP.10 Methotrexate and retinoids have been used in cases with lesions that persisted postpartum.1
Anti–tumor necrosis factor (TNF) α agents are another effective option for treatment of GPPP. Anti-TNF agents are classified as pregnancy category B due to results showing that anti-mouse TNF-α monoclonal antibodies did not cause embryotoxicity or teratogenicity in pregnant mice.11 Although Carter et al12 published a review of US Food and Drug Administration data on pregnant women receiving anti-TNF treatment and concluded that these agents were associated with the VACTERL group of malformations (vertebral defects, anal atresia, cardiac defect, tracheoesophageal fistula with esophageal atresia, cardiac defects, renal and limb anomalies), no such association was found in further studies. A 2014 study showed no difference in the rate of major malformations in infants born to women who were treated with anti-TNF drugs compared to the disease-matched group not treated with these agents and pregnant women counselled for nonteratogenic exposure.13 The same study detected an increase in preterm and low-birth-weight deliveries and suggested this might be caused by the increased severity of disease in patients requiring anti-TNF medication. The British Society of Rheumatology Biologics Register published data on pregnancy outcomes in 130 rheumatoid arthritis patients who had been exposed to anti-TNF agents.14 The results suggested an increased rate of spontaneous abortions in women exposed to anti-TNF treatment around the time of conception, especially in those taking these medications together with methotrexate or leflunomide; however, results also indicated that disease activity may have had an impact on the rate of spontaneous abortions in these patients. In a 2013 review of 462 women with inflammatory bowel disease who had been exposed to anti-TNF agents during pregnancy, the investigators concluded that pregnancy outcomes and the rate of congenital anomalies did not significantly differ from other inflammatory bowel disease patients not receiving anti-TNF drugs or the general population.15
In 2012, the National Board of the National Psoriasis Foundation put infliximab amongst the first-line treatment modalities for GPPP.2 In one case of GPPP in which the eruption persisted after delivery, the patient was treated with infliximab 7 weeks postpartum due to failure to control the disease with prednisolone 60 mg daily and cyclosporine 7.5 mg/kg daily. Unlike our patient, this patient was only started on an infliximab regimen after delivery.16 In another case reported in 2010, the patient was started on infliximab during the postpartum period of her first pregnancy following a pustular flare of previously diagnosed plaque psoriasis (not a generalized pustular psoriasis, as in our case).17 As a good response was obtained, infliximab treatment was continued in the patient throughout her second pregnancy.
Our case is unique in that infliximab was started during pregnancy because of intractable disease leading to systemic symptoms. Our patient showed an excellent response to infliximab after a 10-week disease course with repeated flare-ups and impairment to her overall condition. Delivery occurred at 36 weeks’ gestation due to suspected intrauterine growth retardation; however, the neonate was born with a 5-minute APGAR score of 10 and required no special medical care, which suggests that the low birth weight was constitutional due to the patient’s small frame (her height was 4 ft 11 in). The breast milk of patients with inflammatory bowel disease has been detected to contain very small amounts of infliximab (101 ng/mL, about 1/200 of the therapeutic blood level).18 Considering the large molecular weight of this agent and possible proteolysis in the stomach and intestines, infliximab is unlikely to affect the neonate.15 Thus, we encouraged our patient to breastfeed her baby. A case of fatal disseminated Bacille-Calmette-Guérin infection in an infant whose mother received infliximab treatment during pregnancy has been reported.19 It has been suggested that live vaccines should be avoided in neonates exposed to anti-TNF agents at least for the first 6 months of life or until the agent is no longer detectable in their blood.15 We therefore informed our patient’s family practitioner about this data.
Conclusion
We report a case of infliximab treatment for GPPP that was continued during the postpartum period. Infliximab was an effective treatment option in our patient with no detected serious adverse events and may be considered in other cases of GPPP that are not responsive to systemic steroids. However, further studies are warranted to evaluate the safety and efficacy of infliximab treatment for GPPP and psoriasis in pregnancy.
- Lerhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101-104.
- Gao QQ, Xi MR, Yao Q. Impetigo herpetiformis during pregnancy: a case report and literature review. Dermatology. 2013;226:35-40.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine [published May 12, 2011]. BMJ Case Rep. doi:10.1136/bcr.02.2011.3915
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Luan L, Han S, Zhang Z, et al. Personal treatment experience for severe generalized pustular psoriasis of pregnancy: two case reports. Dermatol Ther. 2014;27:174-177.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB. Cutan Ocul Toxicol. 2012;31:67-69.
- Treacy G. Using an analogous monoclonal antibody to evaluate the reproductive and chronic toxicity potential for a humanized anti-TNF alpha monoclonal antibody. Hum Exp Toxicol. 2000;19:226-228.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- Verstappen SM, King Y, Watson KD, et al. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:823-826.
- Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol. 2013;108:1426-1438.
- Sheth N, Greenblatt DT, Acland K, et al. Generalized pustular psoriasis of pregnancy treated with infliximab. Clin Exp Dermatol. 2009;34:521-522.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Ben-Horin S, Yavzori M, Kopylov U, et al. Detection of infliximab in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2011;5:555-558.
- Cheent K, Nolan J, Shariq S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
Generalized pustular psoriasis of pregnancy (GPPP), formerly known as impetigo herpetiformis, is a rare dermatosis that causes maternal and fetal morbidity and mortality. It is characterized by widespread, circular, erythematous plaques with pustules at the periphery.1 Conventional first-line treatment includes systemic corticosteroids and cyclosporine. The National Psoriasis Foundation Medical Board also has included infliximab among the first-line treatment options for GPPP.2 Herein, we report a case of GPPP treated with infliximab at 30 weeks’ gestation and during the postpartum period.
Case Report
A 22-year-old woman was admitted to our inpatient clinic at 20 weeks’ gestation in her second pregnancy for evaluation of cutaneous eruptions covering the entire body. The lesions first appeared 3 to 4 days prior to her admission and dramatically progressed. She had a history of psoriasis vulgaris diagnosed during her first pregnancy 2 years prior that was treated with topical steroids throughout the pregnancy and methotrexate during lactation for a total of 11 months. She then was started on cyclosporine, which she used for 6 months due to ineffectiveness of the methotrexate, but she stopped treatment 4 months before the second pregnancy.
At the current presentation, physical examination revealed erythroderma and widespread pustules on the chest, abdomen, arms, and legs, including the intertriginous regions, that tended to coalesce and form lakes of pus over an erythematous base (Figure 1). The mucosae were normal. She exhibited a low blood pressure (85/50 mmHg) and high body temperature (102 °F [38.9 °C]). Routine laboratory examination revealed anemia and a normal leukocyte count. Her erythrocyte sedimentation rate (57 mm/h [reference range, <20 mm/h]) and C-reactive protein level (102 mg/L [reference range, <6 mg/L]) were elevated, whereas total calcium (8.11 mg/dL [reference range, 8.2–10.6 mg/dL]) and albumin (3.15 g/dL [reference range, >4.0 g/dL]) levels were low.

Empirical intravenous piperacillin/tazobactam was started due to hypotension, high fever, and elevated C-reactive protein levels; however, treatment was stopped after 4 days when microbiological cultures taken from blood and pustules revealed no bacterial growth, and therefore the fever was assumed to be caused by erythroderma. A skin biopsy before the start of topical and systemic treatment revealed changes consistent with GPPP.
Because her disease was extensive, systemic methylprednisolone 1.5 mg/kg once daily was started, and the dose was increased up to 2.5 mg/kg once daily on the tenth day of treatment to control new crops of eruptions. The dose was tapered to 2 mg/kg once daily when the lesions subsided 4 weeks into the treatment. The patient was discharged after 7 weeks at 27 weeks’ gestation.
Twelve days later, the patient was readmitted to the clinic in an erythrodermic state. The lesions were not controlled with increased doses of systemic corticosteroids. Treatment with cyclosporine was considered, but the patient refused; thus, infliximab treatment was planned. Isoniazid 300 mg once daily was started due to a risk of latent Mycobacterium tuberculosis infection revealed by a tuberculosis blood test. Other evaluations revealed no contraindications, and an infusion of infliximab 300 mg (5 mg/kg) was administered at 30 weeks’ gestation. There was visible improvement in the erythroderma and pustular lesions within the same day of treatment, and the lesions were completely cleared within 2 days of the infusion. The methylprednisolone dose was reduced to 1.5 mg/kg once daily.
Three days after treatment with infliximab, lesions with yellow encrustation appeared in the perioral region and on the oral mucosa and left ear. She was diagnosed with an oral herpes infection. Oral valacyclovir 1 g twice daily and topical mupirocin were started and the lesions subsided within 1 week. Twelve days after the infliximab infusion, new pustular lesions appeared, and a second infusion of infliximab was administered 13 days after the first, which cleared all lesions within 48 hours.
The patient’s methylprednisolone dose was tapered and stopped prior to delivery at 34 weeks’ gestation—2 weeks after the second dose of infliximab—as she did not have any new skin eruptions. A third infliximab infusion that normally would have occurred 4 weeks after the second treatment was postponed for a Cesarean section scheduled at 36 weeks’ gestation due to suspected intrauterine growth retardation. The patient stayed at the hospital until delivery without any new skin lesions. The gross and histopathologic examination of the placenta was normal. The neonate weighed 4.8 lb at birth and had neonatal jaundice that resolved spontaneously within 10 days but was otherwise healthy.
The patient returned to the clinic 3 weeks postpartum with a few pustules on erythematous plaques on the chest, abdomen, and back. At this time, she received a third infusion of infliximab 8 weeks after the second dose. For the past 5 years, the patient has been undergoing infliximab maintenance treatment, which she receives at the hospital every 8 weeks with excellent response. She has had no further pregnancies to date.
Comment
Generalized pustular psoriasis of pregnancy is a rare condition that typically occurs in the third trimester but also can start in the first and second trimesters. It may result in maternal and fetal morbidity by causing fluid and electrolyte imbalance and/or placental insufficiency, resulting in an increased risk for fetal abnormalities, stillbirth, and neonatal death.3 In subsequent pregnancies, GPPP has been observed to recur at an earlier gestational age with a more severe presentation.1,3
Generalized pustular psoriasis of pregnancy usually involves an eruption that begins symmetrically in the intertriginous areas and spreads to the rest of the body. The lesions present as erythematous annular plaques with pustules on the periphery and desquamation in the center due to older pustules.1,3 The mucous membranes also may be involved with erosive and exfoliative plaques, and there may be nail involvement. Patients often present with systemic symptoms such as fever, malaise, diarrhea, and vomiting.1 Laboratory investigations may reveal neutrophilic leukocytosis, high erythrocyte sedimentation rate, hypocalcemia, and hypoalbuminemia.4 Cultures from blood and pustules show no bacterial growth. A skin biopsy is helpful in diagnosis, with features similar to generalized pustular psoriasis, demonstrating spongiform pustules containing neutrophils, lymphocytic and neutrophilic infiltrates in the papillary dermis, and negative direct immunofluorescence.3
The differential diagnosis of GPPP includes subcorneal pustular dermatosis, dermatitis herpetiformis, herpes gestationis, impetigo, and acute generalized exanthematous pustulosis.1,3 Due to concerns of fetal implications, treatment options in GPPP are somewhat limited; however, the condition requires treatment because it may result in unfavorable pregnancy outcomes. Topical corticosteroids may be an option for limited disease.5,6 Systemic corticosteroids (eg, prednisone 60–80 mg/d) were previously considered as first-line agents, although they have shown limited efficacy in our case as well as in other case reports.7 Their ineffectiveness and risk for flare-up after dose tapering should be kept in mind when starting GPPP patients on systemic corticosteroids. Systemic cyclosporine (2–3 mg/kg/d) may be added to increase the efficacy of systemic steroids, which was done in several cases in literature.1,6,8 Although cyclosporine has been classified as a pregnancy category C drug, an analysis of pregnancy outcomes of 629 renal transplant patients revealed no association with adverse pregnancy outcomes compared to the general population and no increase in fetal malformations.9 Therefore, cyclosporine is a safe treatment option and was classified as a first-line drug for GPPP in a 2012 review by the National Psoriasis Foundation Medical Board.2 Narrowband UVB also has been reported to be used for the treatment of GPPP.10 Methotrexate and retinoids have been used in cases with lesions that persisted postpartum.1
Anti–tumor necrosis factor (TNF) α agents are another effective option for treatment of GPPP. Anti-TNF agents are classified as pregnancy category B due to results showing that anti-mouse TNF-α monoclonal antibodies did not cause embryotoxicity or teratogenicity in pregnant mice.11 Although Carter et al12 published a review of US Food and Drug Administration data on pregnant women receiving anti-TNF treatment and concluded that these agents were associated with the VACTERL group of malformations (vertebral defects, anal atresia, cardiac defect, tracheoesophageal fistula with esophageal atresia, cardiac defects, renal and limb anomalies), no such association was found in further studies. A 2014 study showed no difference in the rate of major malformations in infants born to women who were treated with anti-TNF drugs compared to the disease-matched group not treated with these agents and pregnant women counselled for nonteratogenic exposure.13 The same study detected an increase in preterm and low-birth-weight deliveries and suggested this might be caused by the increased severity of disease in patients requiring anti-TNF medication. The British Society of Rheumatology Biologics Register published data on pregnancy outcomes in 130 rheumatoid arthritis patients who had been exposed to anti-TNF agents.14 The results suggested an increased rate of spontaneous abortions in women exposed to anti-TNF treatment around the time of conception, especially in those taking these medications together with methotrexate or leflunomide; however, results also indicated that disease activity may have had an impact on the rate of spontaneous abortions in these patients. In a 2013 review of 462 women with inflammatory bowel disease who had been exposed to anti-TNF agents during pregnancy, the investigators concluded that pregnancy outcomes and the rate of congenital anomalies did not significantly differ from other inflammatory bowel disease patients not receiving anti-TNF drugs or the general population.15
In 2012, the National Board of the National Psoriasis Foundation put infliximab amongst the first-line treatment modalities for GPPP.2 In one case of GPPP in which the eruption persisted after delivery, the patient was treated with infliximab 7 weeks postpartum due to failure to control the disease with prednisolone 60 mg daily and cyclosporine 7.5 mg/kg daily. Unlike our patient, this patient was only started on an infliximab regimen after delivery.16 In another case reported in 2010, the patient was started on infliximab during the postpartum period of her first pregnancy following a pustular flare of previously diagnosed plaque psoriasis (not a generalized pustular psoriasis, as in our case).17 As a good response was obtained, infliximab treatment was continued in the patient throughout her second pregnancy.
Our case is unique in that infliximab was started during pregnancy because of intractable disease leading to systemic symptoms. Our patient showed an excellent response to infliximab after a 10-week disease course with repeated flare-ups and impairment to her overall condition. Delivery occurred at 36 weeks’ gestation due to suspected intrauterine growth retardation; however, the neonate was born with a 5-minute APGAR score of 10 and required no special medical care, which suggests that the low birth weight was constitutional due to the patient’s small frame (her height was 4 ft 11 in). The breast milk of patients with inflammatory bowel disease has been detected to contain very small amounts of infliximab (101 ng/mL, about 1/200 of the therapeutic blood level).18 Considering the large molecular weight of this agent and possible proteolysis in the stomach and intestines, infliximab is unlikely to affect the neonate.15 Thus, we encouraged our patient to breastfeed her baby. A case of fatal disseminated Bacille-Calmette-Guérin infection in an infant whose mother received infliximab treatment during pregnancy has been reported.19 It has been suggested that live vaccines should be avoided in neonates exposed to anti-TNF agents at least for the first 6 months of life or until the agent is no longer detectable in their blood.15 We therefore informed our patient’s family practitioner about this data.
Conclusion
We report a case of infliximab treatment for GPPP that was continued during the postpartum period. Infliximab was an effective treatment option in our patient with no detected serious adverse events and may be considered in other cases of GPPP that are not responsive to systemic steroids. However, further studies are warranted to evaluate the safety and efficacy of infliximab treatment for GPPP and psoriasis in pregnancy.
Generalized pustular psoriasis of pregnancy (GPPP), formerly known as impetigo herpetiformis, is a rare dermatosis that causes maternal and fetal morbidity and mortality. It is characterized by widespread, circular, erythematous plaques with pustules at the periphery.1 Conventional first-line treatment includes systemic corticosteroids and cyclosporine. The National Psoriasis Foundation Medical Board also has included infliximab among the first-line treatment options for GPPP.2 Herein, we report a case of GPPP treated with infliximab at 30 weeks’ gestation and during the postpartum period.
Case Report
A 22-year-old woman was admitted to our inpatient clinic at 20 weeks’ gestation in her second pregnancy for evaluation of cutaneous eruptions covering the entire body. The lesions first appeared 3 to 4 days prior to her admission and dramatically progressed. She had a history of psoriasis vulgaris diagnosed during her first pregnancy 2 years prior that was treated with topical steroids throughout the pregnancy and methotrexate during lactation for a total of 11 months. She then was started on cyclosporine, which she used for 6 months due to ineffectiveness of the methotrexate, but she stopped treatment 4 months before the second pregnancy.
At the current presentation, physical examination revealed erythroderma and widespread pustules on the chest, abdomen, arms, and legs, including the intertriginous regions, that tended to coalesce and form lakes of pus over an erythematous base (Figure 1). The mucosae were normal. She exhibited a low blood pressure (85/50 mmHg) and high body temperature (102 °F [38.9 °C]). Routine laboratory examination revealed anemia and a normal leukocyte count. Her erythrocyte sedimentation rate (57 mm/h [reference range, <20 mm/h]) and C-reactive protein level (102 mg/L [reference range, <6 mg/L]) were elevated, whereas total calcium (8.11 mg/dL [reference range, 8.2–10.6 mg/dL]) and albumin (3.15 g/dL [reference range, >4.0 g/dL]) levels were low.

Empirical intravenous piperacillin/tazobactam was started due to hypotension, high fever, and elevated C-reactive protein levels; however, treatment was stopped after 4 days when microbiological cultures taken from blood and pustules revealed no bacterial growth, and therefore the fever was assumed to be caused by erythroderma. A skin biopsy before the start of topical and systemic treatment revealed changes consistent with GPPP.
Because her disease was extensive, systemic methylprednisolone 1.5 mg/kg once daily was started, and the dose was increased up to 2.5 mg/kg once daily on the tenth day of treatment to control new crops of eruptions. The dose was tapered to 2 mg/kg once daily when the lesions subsided 4 weeks into the treatment. The patient was discharged after 7 weeks at 27 weeks’ gestation.
Twelve days later, the patient was readmitted to the clinic in an erythrodermic state. The lesions were not controlled with increased doses of systemic corticosteroids. Treatment with cyclosporine was considered, but the patient refused; thus, infliximab treatment was planned. Isoniazid 300 mg once daily was started due to a risk of latent Mycobacterium tuberculosis infection revealed by a tuberculosis blood test. Other evaluations revealed no contraindications, and an infusion of infliximab 300 mg (5 mg/kg) was administered at 30 weeks’ gestation. There was visible improvement in the erythroderma and pustular lesions within the same day of treatment, and the lesions were completely cleared within 2 days of the infusion. The methylprednisolone dose was reduced to 1.5 mg/kg once daily.
Three days after treatment with infliximab, lesions with yellow encrustation appeared in the perioral region and on the oral mucosa and left ear. She was diagnosed with an oral herpes infection. Oral valacyclovir 1 g twice daily and topical mupirocin were started and the lesions subsided within 1 week. Twelve days after the infliximab infusion, new pustular lesions appeared, and a second infusion of infliximab was administered 13 days after the first, which cleared all lesions within 48 hours.
The patient’s methylprednisolone dose was tapered and stopped prior to delivery at 34 weeks’ gestation—2 weeks after the second dose of infliximab—as she did not have any new skin eruptions. A third infliximab infusion that normally would have occurred 4 weeks after the second treatment was postponed for a Cesarean section scheduled at 36 weeks’ gestation due to suspected intrauterine growth retardation. The patient stayed at the hospital until delivery without any new skin lesions. The gross and histopathologic examination of the placenta was normal. The neonate weighed 4.8 lb at birth and had neonatal jaundice that resolved spontaneously within 10 days but was otherwise healthy.
The patient returned to the clinic 3 weeks postpartum with a few pustules on erythematous plaques on the chest, abdomen, and back. At this time, she received a third infusion of infliximab 8 weeks after the second dose. For the past 5 years, the patient has been undergoing infliximab maintenance treatment, which she receives at the hospital every 8 weeks with excellent response. She has had no further pregnancies to date.
Comment
Generalized pustular psoriasis of pregnancy is a rare condition that typically occurs in the third trimester but also can start in the first and second trimesters. It may result in maternal and fetal morbidity by causing fluid and electrolyte imbalance and/or placental insufficiency, resulting in an increased risk for fetal abnormalities, stillbirth, and neonatal death.3 In subsequent pregnancies, GPPP has been observed to recur at an earlier gestational age with a more severe presentation.1,3
Generalized pustular psoriasis of pregnancy usually involves an eruption that begins symmetrically in the intertriginous areas and spreads to the rest of the body. The lesions present as erythematous annular plaques with pustules on the periphery and desquamation in the center due to older pustules.1,3 The mucous membranes also may be involved with erosive and exfoliative plaques, and there may be nail involvement. Patients often present with systemic symptoms such as fever, malaise, diarrhea, and vomiting.1 Laboratory investigations may reveal neutrophilic leukocytosis, high erythrocyte sedimentation rate, hypocalcemia, and hypoalbuminemia.4 Cultures from blood and pustules show no bacterial growth. A skin biopsy is helpful in diagnosis, with features similar to generalized pustular psoriasis, demonstrating spongiform pustules containing neutrophils, lymphocytic and neutrophilic infiltrates in the papillary dermis, and negative direct immunofluorescence.3
The differential diagnosis of GPPP includes subcorneal pustular dermatosis, dermatitis herpetiformis, herpes gestationis, impetigo, and acute generalized exanthematous pustulosis.1,3 Due to concerns of fetal implications, treatment options in GPPP are somewhat limited; however, the condition requires treatment because it may result in unfavorable pregnancy outcomes. Topical corticosteroids may be an option for limited disease.5,6 Systemic corticosteroids (eg, prednisone 60–80 mg/d) were previously considered as first-line agents, although they have shown limited efficacy in our case as well as in other case reports.7 Their ineffectiveness and risk for flare-up after dose tapering should be kept in mind when starting GPPP patients on systemic corticosteroids. Systemic cyclosporine (2–3 mg/kg/d) may be added to increase the efficacy of systemic steroids, which was done in several cases in literature.1,6,8 Although cyclosporine has been classified as a pregnancy category C drug, an analysis of pregnancy outcomes of 629 renal transplant patients revealed no association with adverse pregnancy outcomes compared to the general population and no increase in fetal malformations.9 Therefore, cyclosporine is a safe treatment option and was classified as a first-line drug for GPPP in a 2012 review by the National Psoriasis Foundation Medical Board.2 Narrowband UVB also has been reported to be used for the treatment of GPPP.10 Methotrexate and retinoids have been used in cases with lesions that persisted postpartum.1
Anti–tumor necrosis factor (TNF) α agents are another effective option for treatment of GPPP. Anti-TNF agents are classified as pregnancy category B due to results showing that anti-mouse TNF-α monoclonal antibodies did not cause embryotoxicity or teratogenicity in pregnant mice.11 Although Carter et al12 published a review of US Food and Drug Administration data on pregnant women receiving anti-TNF treatment and concluded that these agents were associated with the VACTERL group of malformations (vertebral defects, anal atresia, cardiac defect, tracheoesophageal fistula with esophageal atresia, cardiac defects, renal and limb anomalies), no such association was found in further studies. A 2014 study showed no difference in the rate of major malformations in infants born to women who were treated with anti-TNF drugs compared to the disease-matched group not treated with these agents and pregnant women counselled for nonteratogenic exposure.13 The same study detected an increase in preterm and low-birth-weight deliveries and suggested this might be caused by the increased severity of disease in patients requiring anti-TNF medication. The British Society of Rheumatology Biologics Register published data on pregnancy outcomes in 130 rheumatoid arthritis patients who had been exposed to anti-TNF agents.14 The results suggested an increased rate of spontaneous abortions in women exposed to anti-TNF treatment around the time of conception, especially in those taking these medications together with methotrexate or leflunomide; however, results also indicated that disease activity may have had an impact on the rate of spontaneous abortions in these patients. In a 2013 review of 462 women with inflammatory bowel disease who had been exposed to anti-TNF agents during pregnancy, the investigators concluded that pregnancy outcomes and the rate of congenital anomalies did not significantly differ from other inflammatory bowel disease patients not receiving anti-TNF drugs or the general population.15
In 2012, the National Board of the National Psoriasis Foundation put infliximab amongst the first-line treatment modalities for GPPP.2 In one case of GPPP in which the eruption persisted after delivery, the patient was treated with infliximab 7 weeks postpartum due to failure to control the disease with prednisolone 60 mg daily and cyclosporine 7.5 mg/kg daily. Unlike our patient, this patient was only started on an infliximab regimen after delivery.16 In another case reported in 2010, the patient was started on infliximab during the postpartum period of her first pregnancy following a pustular flare of previously diagnosed plaque psoriasis (not a generalized pustular psoriasis, as in our case).17 As a good response was obtained, infliximab treatment was continued in the patient throughout her second pregnancy.
Our case is unique in that infliximab was started during pregnancy because of intractable disease leading to systemic symptoms. Our patient showed an excellent response to infliximab after a 10-week disease course with repeated flare-ups and impairment to her overall condition. Delivery occurred at 36 weeks’ gestation due to suspected intrauterine growth retardation; however, the neonate was born with a 5-minute APGAR score of 10 and required no special medical care, which suggests that the low birth weight was constitutional due to the patient’s small frame (her height was 4 ft 11 in). The breast milk of patients with inflammatory bowel disease has been detected to contain very small amounts of infliximab (101 ng/mL, about 1/200 of the therapeutic blood level).18 Considering the large molecular weight of this agent and possible proteolysis in the stomach and intestines, infliximab is unlikely to affect the neonate.15 Thus, we encouraged our patient to breastfeed her baby. A case of fatal disseminated Bacille-Calmette-Guérin infection in an infant whose mother received infliximab treatment during pregnancy has been reported.19 It has been suggested that live vaccines should be avoided in neonates exposed to anti-TNF agents at least for the first 6 months of life or until the agent is no longer detectable in their blood.15 We therefore informed our patient’s family practitioner about this data.
Conclusion
We report a case of infliximab treatment for GPPP that was continued during the postpartum period. Infliximab was an effective treatment option in our patient with no detected serious adverse events and may be considered in other cases of GPPP that are not responsive to systemic steroids. However, further studies are warranted to evaluate the safety and efficacy of infliximab treatment for GPPP and psoriasis in pregnancy.
- Lerhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101-104.
- Gao QQ, Xi MR, Yao Q. Impetigo herpetiformis during pregnancy: a case report and literature review. Dermatology. 2013;226:35-40.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine [published May 12, 2011]. BMJ Case Rep. doi:10.1136/bcr.02.2011.3915
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Luan L, Han S, Zhang Z, et al. Personal treatment experience for severe generalized pustular psoriasis of pregnancy: two case reports. Dermatol Ther. 2014;27:174-177.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB. Cutan Ocul Toxicol. 2012;31:67-69.
- Treacy G. Using an analogous monoclonal antibody to evaluate the reproductive and chronic toxicity potential for a humanized anti-TNF alpha monoclonal antibody. Hum Exp Toxicol. 2000;19:226-228.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- Verstappen SM, King Y, Watson KD, et al. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:823-826.
- Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol. 2013;108:1426-1438.
- Sheth N, Greenblatt DT, Acland K, et al. Generalized pustular psoriasis of pregnancy treated with infliximab. Clin Exp Dermatol. 2009;34:521-522.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Ben-Horin S, Yavzori M, Kopylov U, et al. Detection of infliximab in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2011;5:555-558.
- Cheent K, Nolan J, Shariq S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
- Lerhoff S, Pomeranz MK. Specific dermatoses of pregnancy and their treatment. Dermatol Ther. 2013;26:274-284.
- Robinson A, Van Voorhees AS, Hsu S, et al. Treatment of pustular psoriasis: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:279-288.
- Oumeish OY, Parish JL. Impetigo herpetiformis. Clin Dermatol. 2006;24:101-104.
- Gao QQ, Xi MR, Yao Q. Impetigo herpetiformis during pregnancy: a case report and literature review. Dermatology. 2013;226:35-40.
- Bae YS, Van Voorhees AS, Hsu S, et al. Review of treatment options for psoriasis in pregnant or lactating women: from the Medical Board of the National Psoriasis Foundation. J Am Acad Dermatol. 2012;67:459-477.
- Shaw CJ, Wu P, Sriemevan A. First trimester impetigo herpetiformis in multiparous female successfully treated with oral cyclosporine [published May 12, 2011]. BMJ Case Rep. doi:10.1136/bcr.02.2011.3915
- Hazarika D. Generalized pustular psoriasis of pregnancy successfully treated with cyclosporine. Indian J Dermatol Venereol Leprol. 2009;75:638.
- Luan L, Han S, Zhang Z, et al. Personal treatment experience for severe generalized pustular psoriasis of pregnancy: two case reports. Dermatol Ther. 2014;27:174-177.
- Lamarque V, Leleu MF, Monka C, et al. Analysis of 629 pregnancy outcomes in transplant recipients treated with Sandimmun. Transplant Proc. 1997;29:2480.
- Bozdag K, Ozturk S, Ermete M. A case of recurrent impetigo herpetiformis treated with systemic corticosteroids and narrowband UVB. Cutan Ocul Toxicol. 2012;31:67-69.
- Treacy G. Using an analogous monoclonal antibody to evaluate the reproductive and chronic toxicity potential for a humanized anti-TNF alpha monoclonal antibody. Hum Exp Toxicol. 2000;19:226-228.
- Carter JD, Ladhani A, Ricca LR, et al. A safety assessment of tumor necrosis factor antagonists during pregnancy: a review of the Food and Drug Administration database. J Rheumatol. 2009;36:635-641.
- Diav-Citrin O, Otcheretianski-Volodarsky A, Shechtman S, et al. Pregnancy outcome following gestational exposure to TNF-alpha-inhibitors: a prospective, comparative, observational study. Reprod Toxicol. 2014;43:78-84.
- Verstappen SM, King Y, Watson KD, et al. Anti-TNF therapies and pregnancy: outcome of 130 pregnancies in the British Society for Rheumatology Biologics Register. Ann Rheum Dis. 2011;70:823-826.
- Gisbert JP, Chaparro M. Safety of anti-TNF agents during pregnancy and breastfeeding in women with inflammatory bowel disease. Am J Gastroenterol. 2013;108:1426-1438.
- Sheth N, Greenblatt DT, Acland K, et al. Generalized pustular psoriasis of pregnancy treated with infliximab. Clin Exp Dermatol. 2009;34:521-522.
- Puig L, Barco D, Alomar A. Treatment of psoriasis with anti-TNF drugs during pregnancy: case report and review of the literature. Dermatology. 2010;220:71-76.
- Ben-Horin S, Yavzori M, Kopylov U, et al. Detection of infliximab in breast milk of nursing mothers with inflammatory bowel disease. J Crohns Colitis. 2011;5:555-558.
- Cheent K, Nolan J, Shariq S, et al. Case report: fatal case of disseminated BCG infection in an infant born to a mother taking infliximab for Crohn’s disease. J Crohns Colitis. 2010;4:603-605.
Practice Points
- Generalized pustular psoriasis of pregnancy (GPPP) is a rare and severe condition that may lead to complications in both the mother and the fetus. Effective treatment with low impact on the fetus is essential.
- Infliximab, among other biologic agents, may be considered for the rapid clearing of skin lesions in GPPP.
New Approaches in Managing Cellulite: EXPERT INSIGHTS
Tips to share with patients feeling vaccine FOMO
COVID-19 has filled our lives with so many challenges, and now we are faced with a new one. For some of our patients, getting a vaccine appointment feels a lot like winning the lottery.
At first, it might have been easy to be joyful for others’ good fortune, but after weeks and now months of seeing others get vaccinated, patience can wear thin. It also creates an imbalance when one member of a “bubble” is vaccinated and others aren’t. It can be painful to be the one who continues to miss out on activities as those around resume pleasures such as seeing friends, dining out, shopping, and traveling.
So many of our patients are feeling worn down from the chronic stress and are not in the best shape to deal with another issue: the fear of missing out. Yet,
Here are some tips to share with patients who are feeling vaccine envy.
- Acknowledge your feelings. Sure, you want to be happy for those getting vaccinated but it does hurt to be left behind. These feelings are real and deserve space. Share them with a trusted friend or therapist. It is indeed quite upsetting to have to wait. In the United States, we are used to having speedy access to medical care. It is unfortunate that so many have to wait for such an important intervention. You have a right to be upset.
- Express your concern to the family member or friend who is vaccinated. Discuss how it could affect your relationship and activities.
- Focus on what you can control. Double down on efforts to not catch or spread COVID. Vaccines are only one very modern way out of the pandemic. Stick to the basics so you feel a sense of control over your health destiny.
- Take advantage of the remaining days or weeks of quarantine. What did you want to accomplish during your time of limited activity? Did you always want to play the piano? These last slower days or weeks might be a great time to try (over Zoom of course). Have you put off cleaning your closet and organizing your drawers? There is nothing like a deadline to kick us into gear.
- Take your best guess for when you will be vaccinated and start to plan. Start to make those plans for late summer and fall.
- Keep things in perspective. We are ALL so fortunate that several vaccines were developed so quickly. Even if the wait is a few more weeks, an end is in sight. One year ago, we had no idea what lay ahead and the uncertainty caused so much anxiety. Now we can feel hopeful that more “normal days” will be returning soon in a predictable time frame.
- Focus on the herd. By now we know that “we are all in this together.” Although we aren’t leaving at the exact same time, mere months will separate us. The more our friends and family get vaccinated, the safer we all are.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018).
COVID-19 has filled our lives with so many challenges, and now we are faced with a new one. For some of our patients, getting a vaccine appointment feels a lot like winning the lottery.
At first, it might have been easy to be joyful for others’ good fortune, but after weeks and now months of seeing others get vaccinated, patience can wear thin. It also creates an imbalance when one member of a “bubble” is vaccinated and others aren’t. It can be painful to be the one who continues to miss out on activities as those around resume pleasures such as seeing friends, dining out, shopping, and traveling.
So many of our patients are feeling worn down from the chronic stress and are not in the best shape to deal with another issue: the fear of missing out. Yet,
Here are some tips to share with patients who are feeling vaccine envy.
- Acknowledge your feelings. Sure, you want to be happy for those getting vaccinated but it does hurt to be left behind. These feelings are real and deserve space. Share them with a trusted friend or therapist. It is indeed quite upsetting to have to wait. In the United States, we are used to having speedy access to medical care. It is unfortunate that so many have to wait for such an important intervention. You have a right to be upset.
- Express your concern to the family member or friend who is vaccinated. Discuss how it could affect your relationship and activities.
- Focus on what you can control. Double down on efforts to not catch or spread COVID. Vaccines are only one very modern way out of the pandemic. Stick to the basics so you feel a sense of control over your health destiny.
- Take advantage of the remaining days or weeks of quarantine. What did you want to accomplish during your time of limited activity? Did you always want to play the piano? These last slower days or weeks might be a great time to try (over Zoom of course). Have you put off cleaning your closet and organizing your drawers? There is nothing like a deadline to kick us into gear.
- Take your best guess for when you will be vaccinated and start to plan. Start to make those plans for late summer and fall.
- Keep things in perspective. We are ALL so fortunate that several vaccines were developed so quickly. Even if the wait is a few more weeks, an end is in sight. One year ago, we had no idea what lay ahead and the uncertainty caused so much anxiety. Now we can feel hopeful that more “normal days” will be returning soon in a predictable time frame.
- Focus on the herd. By now we know that “we are all in this together.” Although we aren’t leaving at the exact same time, mere months will separate us. The more our friends and family get vaccinated, the safer we all are.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018).
COVID-19 has filled our lives with so many challenges, and now we are faced with a new one. For some of our patients, getting a vaccine appointment feels a lot like winning the lottery.
At first, it might have been easy to be joyful for others’ good fortune, but after weeks and now months of seeing others get vaccinated, patience can wear thin. It also creates an imbalance when one member of a “bubble” is vaccinated and others aren’t. It can be painful to be the one who continues to miss out on activities as those around resume pleasures such as seeing friends, dining out, shopping, and traveling.
So many of our patients are feeling worn down from the chronic stress and are not in the best shape to deal with another issue: the fear of missing out. Yet,
Here are some tips to share with patients who are feeling vaccine envy.
- Acknowledge your feelings. Sure, you want to be happy for those getting vaccinated but it does hurt to be left behind. These feelings are real and deserve space. Share them with a trusted friend or therapist. It is indeed quite upsetting to have to wait. In the United States, we are used to having speedy access to medical care. It is unfortunate that so many have to wait for such an important intervention. You have a right to be upset.
- Express your concern to the family member or friend who is vaccinated. Discuss how it could affect your relationship and activities.
- Focus on what you can control. Double down on efforts to not catch or spread COVID. Vaccines are only one very modern way out of the pandemic. Stick to the basics so you feel a sense of control over your health destiny.
- Take advantage of the remaining days or weeks of quarantine. What did you want to accomplish during your time of limited activity? Did you always want to play the piano? These last slower days or weeks might be a great time to try (over Zoom of course). Have you put off cleaning your closet and organizing your drawers? There is nothing like a deadline to kick us into gear.
- Take your best guess for when you will be vaccinated and start to plan. Start to make those plans for late summer and fall.
- Keep things in perspective. We are ALL so fortunate that several vaccines were developed so quickly. Even if the wait is a few more weeks, an end is in sight. One year ago, we had no idea what lay ahead and the uncertainty caused so much anxiety. Now we can feel hopeful that more “normal days” will be returning soon in a predictable time frame.
- Focus on the herd. By now we know that “we are all in this together.” Although we aren’t leaving at the exact same time, mere months will separate us. The more our friends and family get vaccinated, the safer we all are.
Dr. Ritvo, a psychiatrist with more than 25 years’ experience, practices in Miami Beach. She is the author of “Bekindr – The Transformative Power of Kindness” (Hellertown, Pa.: Momosa Publishing, 2018).
Dr. Richard W. Cohen joins CPN’s Editorial Advisory Board
Clinical Psychiatry News is pleased to announce the addition of Richard W. Cohen, MD, to its Editorial Advisory Board.
Dr. Cohen is a board-certified psychiatrist. For the last 25 years, he has been in full-time private practice in Center City Philadelphia, where he treats patients with depression, anxiety disorders, relationship problems using psychoanalytically oriented psychotherapy, cognitive-behavioral therapy, and medication management. Dr. Cohen has a special interest in sports psychology – improving the mental toughness and performance of junior, collegiate, and pro athletes.
He graduated from medical school at Temple University, Philadelphia, where he had a wonderful experience assisting the late behaviorist Joseph Wolpe, MD, in agoraphobia research.
Dr. Cohen was chief resident at Albert Einstein Medical Center in New York, and at one point held a trifaculty appointment at Thomas Jefferson University Hospital, Philadelphia, in psychiatry, family medicine, and otolaryngology. At Jefferson, Dr. Cohen codirector of the alcohol and substance abuse education program. He also edited a textbook entitled “What a Student Should Know,” which integrated issues of alcoholism into all subjects in the medical school curriculum.
He has lectured extensively both locally and nationwide at tennis academies helping players improve their overall accomplishments. In addition, Dr. Cohen has appeared on various television shows discussing addictions, relationship issues, and sports psychiatry. Furthermore, he has published numerous articles on these topics.
Dr. Cohen was the fifth-ranked high school tennis player in the United States and he has been ranked No. 1 in both the Middle States and the country in various junior and senior age divisions. He was the captain of the University of Pennsylvania Ivy League Championship tennis team and played No. 1 on Penn’s National Intercollegiate Championship squash team. Dr. Cohen has garnered 17 National Tennis Championship Gold Balls over the years. In 2012, Dr. Cohen was inducted into the Philadelphia Jewish Sports Hall of Fame.
He lives in Philadelphia with his wife, Nancy, and they have two adult children, Josh and Julia, who are world-class tennis players.
Clinical Psychiatry News is pleased to announce the addition of Richard W. Cohen, MD, to its Editorial Advisory Board.
Dr. Cohen is a board-certified psychiatrist. For the last 25 years, he has been in full-time private practice in Center City Philadelphia, where he treats patients with depression, anxiety disorders, relationship problems using psychoanalytically oriented psychotherapy, cognitive-behavioral therapy, and medication management. Dr. Cohen has a special interest in sports psychology – improving the mental toughness and performance of junior, collegiate, and pro athletes.
He graduated from medical school at Temple University, Philadelphia, where he had a wonderful experience assisting the late behaviorist Joseph Wolpe, MD, in agoraphobia research.
Dr. Cohen was chief resident at Albert Einstein Medical Center in New York, and at one point held a trifaculty appointment at Thomas Jefferson University Hospital, Philadelphia, in psychiatry, family medicine, and otolaryngology. At Jefferson, Dr. Cohen codirector of the alcohol and substance abuse education program. He also edited a textbook entitled “What a Student Should Know,” which integrated issues of alcoholism into all subjects in the medical school curriculum.
He has lectured extensively both locally and nationwide at tennis academies helping players improve their overall accomplishments. In addition, Dr. Cohen has appeared on various television shows discussing addictions, relationship issues, and sports psychiatry. Furthermore, he has published numerous articles on these topics.
Dr. Cohen was the fifth-ranked high school tennis player in the United States and he has been ranked No. 1 in both the Middle States and the country in various junior and senior age divisions. He was the captain of the University of Pennsylvania Ivy League Championship tennis team and played No. 1 on Penn’s National Intercollegiate Championship squash team. Dr. Cohen has garnered 17 National Tennis Championship Gold Balls over the years. In 2012, Dr. Cohen was inducted into the Philadelphia Jewish Sports Hall of Fame.
He lives in Philadelphia with his wife, Nancy, and they have two adult children, Josh and Julia, who are world-class tennis players.
Clinical Psychiatry News is pleased to announce the addition of Richard W. Cohen, MD, to its Editorial Advisory Board.
Dr. Cohen is a board-certified psychiatrist. For the last 25 years, he has been in full-time private practice in Center City Philadelphia, where he treats patients with depression, anxiety disorders, relationship problems using psychoanalytically oriented psychotherapy, cognitive-behavioral therapy, and medication management. Dr. Cohen has a special interest in sports psychology – improving the mental toughness and performance of junior, collegiate, and pro athletes.
He graduated from medical school at Temple University, Philadelphia, where he had a wonderful experience assisting the late behaviorist Joseph Wolpe, MD, in agoraphobia research.
Dr. Cohen was chief resident at Albert Einstein Medical Center in New York, and at one point held a trifaculty appointment at Thomas Jefferson University Hospital, Philadelphia, in psychiatry, family medicine, and otolaryngology. At Jefferson, Dr. Cohen codirector of the alcohol and substance abuse education program. He also edited a textbook entitled “What a Student Should Know,” which integrated issues of alcoholism into all subjects in the medical school curriculum.
He has lectured extensively both locally and nationwide at tennis academies helping players improve their overall accomplishments. In addition, Dr. Cohen has appeared on various television shows discussing addictions, relationship issues, and sports psychiatry. Furthermore, he has published numerous articles on these topics.
Dr. Cohen was the fifth-ranked high school tennis player in the United States and he has been ranked No. 1 in both the Middle States and the country in various junior and senior age divisions. He was the captain of the University of Pennsylvania Ivy League Championship tennis team and played No. 1 on Penn’s National Intercollegiate Championship squash team. Dr. Cohen has garnered 17 National Tennis Championship Gold Balls over the years. In 2012, Dr. Cohen was inducted into the Philadelphia Jewish Sports Hall of Fame.
He lives in Philadelphia with his wife, Nancy, and they have two adult children, Josh and Julia, who are world-class tennis players.
Managing Moderate to Severe Asthma in Adolescents
"The eye of the hurricane" is how adolescent asthma is often described, according to Dr Benjamin Gaston, of Indiana University School of Medicine, given the disease is more prevalent in children but more severe in patients whose asthma continues on into adulthood.
In adolescence, increasingly severe disease may find itself on a collision course with nonadherence, which remains a challenge at this age. Because adolescents adapt easily to telemedicine and mobile apps, pandemic-related disruptions to in-person appointments may not be a barrier for this group and indeed may be beneficial.
The 2019 GINA and 2020 NHLBI guidelines offer promising new approaches, including use of inhaled corticosteroids (ICS) with formoterol at the onset of symptoms. ICS with formoterol as rescue and long-acting monoclonal antibody injections have proven effective for some adolescents but are no substitute for daily prevention therapy, an important standard of care.
Obstacles remain, including the high cost of both ICS-formoterol and the new biologics, as well as the inconvenient packaging of formoterol, which is not included in most ICS-LABA combinations on the market.
Studies such as the NHLBI PrecISE trial are investigating predictive biomarkers that could lead to highly individualized treatments for adolescent patients with this heterogeneous disease.
--
Benjamin Gaston, MD, Department of Pediatrics, Indiana University School of Medicine; Vice Chair for Translational Research, Department of Pediatrics, Riley Hospital for Children, Indianapolis, Indiana.
Benjamin Gaston, MD, has disclosed no relevant financial relationships.
"The eye of the hurricane" is how adolescent asthma is often described, according to Dr Benjamin Gaston, of Indiana University School of Medicine, given the disease is more prevalent in children but more severe in patients whose asthma continues on into adulthood.
In adolescence, increasingly severe disease may find itself on a collision course with nonadherence, which remains a challenge at this age. Because adolescents adapt easily to telemedicine and mobile apps, pandemic-related disruptions to in-person appointments may not be a barrier for this group and indeed may be beneficial.
The 2019 GINA and 2020 NHLBI guidelines offer promising new approaches, including use of inhaled corticosteroids (ICS) with formoterol at the onset of symptoms. ICS with formoterol as rescue and long-acting monoclonal antibody injections have proven effective for some adolescents but are no substitute for daily prevention therapy, an important standard of care.
Obstacles remain, including the high cost of both ICS-formoterol and the new biologics, as well as the inconvenient packaging of formoterol, which is not included in most ICS-LABA combinations on the market.
Studies such as the NHLBI PrecISE trial are investigating predictive biomarkers that could lead to highly individualized treatments for adolescent patients with this heterogeneous disease.
--
Benjamin Gaston, MD, Department of Pediatrics, Indiana University School of Medicine; Vice Chair for Translational Research, Department of Pediatrics, Riley Hospital for Children, Indianapolis, Indiana.
Benjamin Gaston, MD, has disclosed no relevant financial relationships.
"The eye of the hurricane" is how adolescent asthma is often described, according to Dr Benjamin Gaston, of Indiana University School of Medicine, given the disease is more prevalent in children but more severe in patients whose asthma continues on into adulthood.
In adolescence, increasingly severe disease may find itself on a collision course with nonadherence, which remains a challenge at this age. Because adolescents adapt easily to telemedicine and mobile apps, pandemic-related disruptions to in-person appointments may not be a barrier for this group and indeed may be beneficial.
The 2019 GINA and 2020 NHLBI guidelines offer promising new approaches, including use of inhaled corticosteroids (ICS) with formoterol at the onset of symptoms. ICS with formoterol as rescue and long-acting monoclonal antibody injections have proven effective for some adolescents but are no substitute for daily prevention therapy, an important standard of care.
Obstacles remain, including the high cost of both ICS-formoterol and the new biologics, as well as the inconvenient packaging of formoterol, which is not included in most ICS-LABA combinations on the market.
Studies such as the NHLBI PrecISE trial are investigating predictive biomarkers that could lead to highly individualized treatments for adolescent patients with this heterogeneous disease.
--
Benjamin Gaston, MD, Department of Pediatrics, Indiana University School of Medicine; Vice Chair for Translational Research, Department of Pediatrics, Riley Hospital for Children, Indianapolis, Indiana.
Benjamin Gaston, MD, has disclosed no relevant financial relationships.

Intervention reduced racial disparities among patients in cancer trials
, according to researchers.
The team found that progression-free survival (PFS) was significantly worse for Black versus White women with endometrial cancer who were treated at the center between 2012 and 2018. However, PFS outcomes were similar for both races among patients from the center who were enrolled in clinical trials during the same period, after the center introduced a navigation program designed to reduce racial disparities.
The findings demonstrate that health care inequities can be overcome with specific interventions aimed at improving care for Black women, said Nathaniel L. Jones, MD of the Mitchell Cancer Institute at the University of South Alabama in Mobile.
Dr. Jones presented the findings at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 10910).
Rationale: Building trust, providing equitable care
Black women comprise 7% of endometrial cancer diagnoses across the United States but account for 15% of all deaths, Dr. Jones noted. Compared with White women, Black women are up to 80% more likely to die from endometrial cancer.
“Perhaps even more concerning is that endometrial cancer is one of few cancers with increasing incidence and mortality, which further exacerbates the disparities,” Dr. Jones said.
In the Deep South, which carries “an unequal burden” of endometrial cancer incidence and mortality compared with the rest of the United States, multiple additional barriers exist that further exacerbate health care inequities, Dr. Jones said.
The barriers include greater poverty, mortality, social and economic disadvantages, and mistrust in “the medical establishment” among Black patients.
“The onus is on us as providers to provide an approach to cancer care that allows our patients to trust us and provide equitable cancer care for the women we serve,” Dr. Jones said. “To that end, we sought to investigate clinical trial enrollment at our institution after the implementation of patient-based programs designed specifically to enhance minority enrollment in clinical trials. We then evaluated the impact of clinical trial enrollment and race on survival.”
A ‘multifaceted’ intervention
“An intentional, multifaceted intervention was created to address Black patient enrollment onto clinical trials,” Dr. Jones explained.
His center implemented a lay navigation program to increase trial awareness and participation among minorities, help patients understand the risks and benefits of clinical trial participation, and help patients and their families navigate the enrollment and participation processes.
Under the program, all new endometrial cancer patients were assigned a lay navigator. The program included an education component to inform patients of the risks and benefits of clinical trial participation.
Another aspect was hiring a “diverse lay navigation workforce ... that mirrored the demographics of our catchment area,” which has more than double the minority population, compared with the national average, Dr. Jones noted.
Results: Improved PFS
To evaluate the efficacy of their intervention, the researchers conducted a retrospective review of 1,021 patients with endometrial cancer treated at Mitchell Cancer Institute between 2012 and 2018. There were 277 Black women and 718 White women in the overall cohort, and 23 Black women and 61 White women were enrolled in clinical trials.
After accounting for age-adjusted endometrial cancer incidence in the United States, the observed trial enrollment of Black women was statistically similar to expected enrollment (1.03-fold lower than expected). Compared with regional “Deep South” data, however, enrollment was 1.15-fold higher than expected for Black patients, Dr. Jones said.
Among all women with endometrial cancer treated at the Mitchell Cancer Institute, the median PFS was 14 months in Black women and 20 months in White women (P = .002). Among patients enrolled in clinical trials, however, the median PFS was 13 months for Black women and 14 months for White women (P = .280).
In the entire cohort, Black women had more aggressive histology, more advanced-stage disease, and a higher proportion of Medicaid or self-pay status. Among those enrolled in clinical trials, there was no difference between races in stage, grade, histology, insurance, or performance status.
The findings show that inequities in clinical trial enrollment can be overcome, and patient-based interventions can be helpful in improving enrollment of minority women, Dr. Jones concluded.
Doing better, starting small
Invited discussant Kemi M. Doll, MD, commended Dr. Jones and his colleagues for their “incredible, intervention-focused work,” but she asked: “Is this good enough?”
Analyses are needed to understand what drove the differences among trial participants versus the overall population, said Dr. Doll, a gynecologic oncologist at the University of Washington in Seattle.
For example, determining whether outcomes in the trial participants were driven by better PFS among Black women or worse PFS among White women could “help to identify next steps,” Dr. Doll said.
She stressed that “everyone” can engage in local-level efforts to improve trial enrollment, equity, and outcomes.
“A powerful mantra I was exposed to several years ago regarding equity is, ‘Here. Now. Small. Doable.’ We are often paralyzed by the long-standing and deeply embedded inequities in our health care system, but we can choose to move into action by following ‘Here. Now. Small. Doable,’” Dr. Doll said. “It reminds us to start where we are..., to start now, and stop waiting for convenience because equity work is not convenient.”
The key is recognizing individual power to enact change and focusing on “what we can change and not what we can’t,” she said.
Tools are available on the national level to help facilitate clinical trial enrollment of historically excluded populations, Dr. Doll added.
She cited a report outlining strategies for accruing diverse populations in clinical trials at eight U.S. cancer centers. The report addresses development of community partnerships and community advisory boards, training in culturally competent and congruent trial design, use of lay navigation, the importance of balancing benefits of participation with patient time and risk, and invoking a sense of altruism for family and community, Dr. Doll said.
“Baking these into trial design and recruitment are known, evidence-based methods to improve enrollment of [minority] populations,” she said. “Deciding to make these design elements mandatory for trials to be approved and executed is the kind of paradigm-shifting action that is available to us now.”
Dr. Jones and Dr. Doll both reported having no disclosures.
, according to researchers.
The team found that progression-free survival (PFS) was significantly worse for Black versus White women with endometrial cancer who were treated at the center between 2012 and 2018. However, PFS outcomes were similar for both races among patients from the center who were enrolled in clinical trials during the same period, after the center introduced a navigation program designed to reduce racial disparities.
The findings demonstrate that health care inequities can be overcome with specific interventions aimed at improving care for Black women, said Nathaniel L. Jones, MD of the Mitchell Cancer Institute at the University of South Alabama in Mobile.
Dr. Jones presented the findings at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 10910).
Rationale: Building trust, providing equitable care
Black women comprise 7% of endometrial cancer diagnoses across the United States but account for 15% of all deaths, Dr. Jones noted. Compared with White women, Black women are up to 80% more likely to die from endometrial cancer.
“Perhaps even more concerning is that endometrial cancer is one of few cancers with increasing incidence and mortality, which further exacerbates the disparities,” Dr. Jones said.
In the Deep South, which carries “an unequal burden” of endometrial cancer incidence and mortality compared with the rest of the United States, multiple additional barriers exist that further exacerbate health care inequities, Dr. Jones said.
The barriers include greater poverty, mortality, social and economic disadvantages, and mistrust in “the medical establishment” among Black patients.
“The onus is on us as providers to provide an approach to cancer care that allows our patients to trust us and provide equitable cancer care for the women we serve,” Dr. Jones said. “To that end, we sought to investigate clinical trial enrollment at our institution after the implementation of patient-based programs designed specifically to enhance minority enrollment in clinical trials. We then evaluated the impact of clinical trial enrollment and race on survival.”
A ‘multifaceted’ intervention
“An intentional, multifaceted intervention was created to address Black patient enrollment onto clinical trials,” Dr. Jones explained.
His center implemented a lay navigation program to increase trial awareness and participation among minorities, help patients understand the risks and benefits of clinical trial participation, and help patients and their families navigate the enrollment and participation processes.
Under the program, all new endometrial cancer patients were assigned a lay navigator. The program included an education component to inform patients of the risks and benefits of clinical trial participation.
Another aspect was hiring a “diverse lay navigation workforce ... that mirrored the demographics of our catchment area,” which has more than double the minority population, compared with the national average, Dr. Jones noted.
Results: Improved PFS
To evaluate the efficacy of their intervention, the researchers conducted a retrospective review of 1,021 patients with endometrial cancer treated at Mitchell Cancer Institute between 2012 and 2018. There were 277 Black women and 718 White women in the overall cohort, and 23 Black women and 61 White women were enrolled in clinical trials.
After accounting for age-adjusted endometrial cancer incidence in the United States, the observed trial enrollment of Black women was statistically similar to expected enrollment (1.03-fold lower than expected). Compared with regional “Deep South” data, however, enrollment was 1.15-fold higher than expected for Black patients, Dr. Jones said.
Among all women with endometrial cancer treated at the Mitchell Cancer Institute, the median PFS was 14 months in Black women and 20 months in White women (P = .002). Among patients enrolled in clinical trials, however, the median PFS was 13 months for Black women and 14 months for White women (P = .280).
In the entire cohort, Black women had more aggressive histology, more advanced-stage disease, and a higher proportion of Medicaid or self-pay status. Among those enrolled in clinical trials, there was no difference between races in stage, grade, histology, insurance, or performance status.
The findings show that inequities in clinical trial enrollment can be overcome, and patient-based interventions can be helpful in improving enrollment of minority women, Dr. Jones concluded.
Doing better, starting small
Invited discussant Kemi M. Doll, MD, commended Dr. Jones and his colleagues for their “incredible, intervention-focused work,” but she asked: “Is this good enough?”
Analyses are needed to understand what drove the differences among trial participants versus the overall population, said Dr. Doll, a gynecologic oncologist at the University of Washington in Seattle.
For example, determining whether outcomes in the trial participants were driven by better PFS among Black women or worse PFS among White women could “help to identify next steps,” Dr. Doll said.
She stressed that “everyone” can engage in local-level efforts to improve trial enrollment, equity, and outcomes.
“A powerful mantra I was exposed to several years ago regarding equity is, ‘Here. Now. Small. Doable.’ We are often paralyzed by the long-standing and deeply embedded inequities in our health care system, but we can choose to move into action by following ‘Here. Now. Small. Doable,’” Dr. Doll said. “It reminds us to start where we are..., to start now, and stop waiting for convenience because equity work is not convenient.”
The key is recognizing individual power to enact change and focusing on “what we can change and not what we can’t,” she said.
Tools are available on the national level to help facilitate clinical trial enrollment of historically excluded populations, Dr. Doll added.
She cited a report outlining strategies for accruing diverse populations in clinical trials at eight U.S. cancer centers. The report addresses development of community partnerships and community advisory boards, training in culturally competent and congruent trial design, use of lay navigation, the importance of balancing benefits of participation with patient time and risk, and invoking a sense of altruism for family and community, Dr. Doll said.
“Baking these into trial design and recruitment are known, evidence-based methods to improve enrollment of [minority] populations,” she said. “Deciding to make these design elements mandatory for trials to be approved and executed is the kind of paradigm-shifting action that is available to us now.”
Dr. Jones and Dr. Doll both reported having no disclosures.
, according to researchers.
The team found that progression-free survival (PFS) was significantly worse for Black versus White women with endometrial cancer who were treated at the center between 2012 and 2018. However, PFS outcomes were similar for both races among patients from the center who were enrolled in clinical trials during the same period, after the center introduced a navigation program designed to reduce racial disparities.
The findings demonstrate that health care inequities can be overcome with specific interventions aimed at improving care for Black women, said Nathaniel L. Jones, MD of the Mitchell Cancer Institute at the University of South Alabama in Mobile.
Dr. Jones presented the findings at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 10910).
Rationale: Building trust, providing equitable care
Black women comprise 7% of endometrial cancer diagnoses across the United States but account for 15% of all deaths, Dr. Jones noted. Compared with White women, Black women are up to 80% more likely to die from endometrial cancer.
“Perhaps even more concerning is that endometrial cancer is one of few cancers with increasing incidence and mortality, which further exacerbates the disparities,” Dr. Jones said.
In the Deep South, which carries “an unequal burden” of endometrial cancer incidence and mortality compared with the rest of the United States, multiple additional barriers exist that further exacerbate health care inequities, Dr. Jones said.
The barriers include greater poverty, mortality, social and economic disadvantages, and mistrust in “the medical establishment” among Black patients.
“The onus is on us as providers to provide an approach to cancer care that allows our patients to trust us and provide equitable cancer care for the women we serve,” Dr. Jones said. “To that end, we sought to investigate clinical trial enrollment at our institution after the implementation of patient-based programs designed specifically to enhance minority enrollment in clinical trials. We then evaluated the impact of clinical trial enrollment and race on survival.”
A ‘multifaceted’ intervention
“An intentional, multifaceted intervention was created to address Black patient enrollment onto clinical trials,” Dr. Jones explained.
His center implemented a lay navigation program to increase trial awareness and participation among minorities, help patients understand the risks and benefits of clinical trial participation, and help patients and their families navigate the enrollment and participation processes.
Under the program, all new endometrial cancer patients were assigned a lay navigator. The program included an education component to inform patients of the risks and benefits of clinical trial participation.
Another aspect was hiring a “diverse lay navigation workforce ... that mirrored the demographics of our catchment area,” which has more than double the minority population, compared with the national average, Dr. Jones noted.
Results: Improved PFS
To evaluate the efficacy of their intervention, the researchers conducted a retrospective review of 1,021 patients with endometrial cancer treated at Mitchell Cancer Institute between 2012 and 2018. There were 277 Black women and 718 White women in the overall cohort, and 23 Black women and 61 White women were enrolled in clinical trials.
After accounting for age-adjusted endometrial cancer incidence in the United States, the observed trial enrollment of Black women was statistically similar to expected enrollment (1.03-fold lower than expected). Compared with regional “Deep South” data, however, enrollment was 1.15-fold higher than expected for Black patients, Dr. Jones said.
Among all women with endometrial cancer treated at the Mitchell Cancer Institute, the median PFS was 14 months in Black women and 20 months in White women (P = .002). Among patients enrolled in clinical trials, however, the median PFS was 13 months for Black women and 14 months for White women (P = .280).
In the entire cohort, Black women had more aggressive histology, more advanced-stage disease, and a higher proportion of Medicaid or self-pay status. Among those enrolled in clinical trials, there was no difference between races in stage, grade, histology, insurance, or performance status.
The findings show that inequities in clinical trial enrollment can be overcome, and patient-based interventions can be helpful in improving enrollment of minority women, Dr. Jones concluded.
Doing better, starting small
Invited discussant Kemi M. Doll, MD, commended Dr. Jones and his colleagues for their “incredible, intervention-focused work,” but she asked: “Is this good enough?”
Analyses are needed to understand what drove the differences among trial participants versus the overall population, said Dr. Doll, a gynecologic oncologist at the University of Washington in Seattle.
For example, determining whether outcomes in the trial participants were driven by better PFS among Black women or worse PFS among White women could “help to identify next steps,” Dr. Doll said.
She stressed that “everyone” can engage in local-level efforts to improve trial enrollment, equity, and outcomes.
“A powerful mantra I was exposed to several years ago regarding equity is, ‘Here. Now. Small. Doable.’ We are often paralyzed by the long-standing and deeply embedded inequities in our health care system, but we can choose to move into action by following ‘Here. Now. Small. Doable,’” Dr. Doll said. “It reminds us to start where we are..., to start now, and stop waiting for convenience because equity work is not convenient.”
The key is recognizing individual power to enact change and focusing on “what we can change and not what we can’t,” she said.
Tools are available on the national level to help facilitate clinical trial enrollment of historically excluded populations, Dr. Doll added.
She cited a report outlining strategies for accruing diverse populations in clinical trials at eight U.S. cancer centers. The report addresses development of community partnerships and community advisory boards, training in culturally competent and congruent trial design, use of lay navigation, the importance of balancing benefits of participation with patient time and risk, and invoking a sense of altruism for family and community, Dr. Doll said.
“Baking these into trial design and recruitment are known, evidence-based methods to improve enrollment of [minority] populations,” she said. “Deciding to make these design elements mandatory for trials to be approved and executed is the kind of paradigm-shifting action that is available to us now.”
Dr. Jones and Dr. Doll both reported having no disclosures.
FROM SGO 2021
1 in 3 on levothyroxine take meds that interfere with thyroid tests
, potentially compromising treatment decisions, new research shows.
“We know from previous studies that thyroid hormone use is common in older adults and that there are a multitude of medications that can interfere with thyroid function tests in different ways,” senior author Maria Papaleontiou, MD, told Medscape Medical News.
“However, to our knowledge, the extent of concurrent use of thyroid hormone and interfering medications in older adults, age 65 years and older, has not been previously explored,” added Dr. Papaleontiou, of the Division of Metabolism, Endocrinology and Diabetes, Department of Internal Medicine, University of Michigan, Ann Arbor.
The findings were presented as a poster during virtual ENDO 2021, the Endocrine Society’s annual meeting.
Commenting on the study, Thanh Duc Hoang, DO, an endocrinologist with the Walter Reed National Military Medical Center, in Bethesda, Md., said: “It is important for clinicians to be aware of various interactions and interferences of medications affecting the accuracy of thyroid function tests.”
“If patients are not able to discontinue the medications shortly before the bloodwork, the clinicians may consider ordering different thyroid tests or assays that avoid the interferences,” he told Medscape Medical News.
32% of patients taking meds that could interfere with tests
In evaluating data on 538,137 patients treated with thyroid hormones from the Corporate Data Warehouse of the Veterans Health Administration, spanning 2004-2017, first author Rachel Beeson, MD, and colleagues with the University of Michigan found most patients in the study were men (96.5%), White (77.1%), and had two or more comorbidities (62.6%).
Of this total, 170,261 (31.6%) patients treated with thyroid hormones, over a median follow-up of 56 months, were taking at least one drug that could potentially interfere with thyroid function tests.
Among the drugs with potential thyroid test interference, about 28% of patients were taking prednisone or prednisolone, 8% were taking amiodarone, and 1.42% were taking phenytoin. Other reported drugs that could potentially interfere included carbamazepine (0.91%), phenobarbital (0.15%), lithium (0.40%), and tamoxifen (0.11%).
Multivariate analysis showed that characteristics associated with those most likely to have concurrent medication use included non-Whites (OR, 1.18 vs Whites), Hispanic ethnicity (OR 1.11 vs non-Hispanic), female sex (OR 1.12 vs males), and presence of comorbidities (eg, Charlson-Deyo comorbidity score ≥ 2, OR, 2.47 vs score of 0).
Meanwhile, older patients age 85 years and over had a lower likelihood of concurrent medications interfering with thyroid tests (OR, 0.47 vs age 65-74 years).
The findings are concerning given the wide use of levothyroxine to treat hypothyroidism, which is the most widely prescribed drug in the United States.
“Our findings not only highlight the complexity of thyroid hormone management in older adults in the context of polypharmacy and multimorbidity, but they also draw attention to vulnerable groups for this practice, which included female patients, non-Whites, patients of Hispanic ethnicity, and patients with comorbidities,” Dr. Papaleontiou said.
Nature of interference possibilities varies
Medications or supplements can interfere with thyroid function tests in a variety of ways, she explained. “Some medications could lead to a decrease in the absorption of levothyroxine, others may affect how well the pill dissolves.”
In addition, certain medications can affect the circulation of thyroid hormone in the blood and how it binds with proteins, or they can lead to decreasing thyroid hormone levels due to a variety of interactions.
And in contrast, “What is even more challenging is that some medications or supplements may appear to affect thyroid function based on lab tests when in reality they don’t actually affect thyroid function and may lead to dose adjustments unnecessarily,” Dr. Papaleontiou noted.
Recommendations to counter interference
Current recommendations to try to counter the effects of polypharmacy on thyroid treatment include advising patients to take thyroid hormones on an empty stomach at least 30-60 minutes prior to eating for optimal absorption.
If the patient is taking medications known to interfere with absorption of thyroid hormones, the recommendation is to space those out by at least 4 hours.
“The big challenge in older adults is that many of them do experience polypharmacy, being at risk for multiple drug-drug interactions,” Dr. Papaleontiou said.
“Physicians and patients should be vigilant and communicate closely every time there is initiation of a new medication or supplement to consider whether there may be interference.”
The authors have reported no relevant financial relationships. Dr. Hoang has reported being a speaker for Acella Pharmaceuticals.
A version of this article first appeared on Medscape.com.
, potentially compromising treatment decisions, new research shows.
“We know from previous studies that thyroid hormone use is common in older adults and that there are a multitude of medications that can interfere with thyroid function tests in different ways,” senior author Maria Papaleontiou, MD, told Medscape Medical News.
“However, to our knowledge, the extent of concurrent use of thyroid hormone and interfering medications in older adults, age 65 years and older, has not been previously explored,” added Dr. Papaleontiou, of the Division of Metabolism, Endocrinology and Diabetes, Department of Internal Medicine, University of Michigan, Ann Arbor.
The findings were presented as a poster during virtual ENDO 2021, the Endocrine Society’s annual meeting.
Commenting on the study, Thanh Duc Hoang, DO, an endocrinologist with the Walter Reed National Military Medical Center, in Bethesda, Md., said: “It is important for clinicians to be aware of various interactions and interferences of medications affecting the accuracy of thyroid function tests.”
“If patients are not able to discontinue the medications shortly before the bloodwork, the clinicians may consider ordering different thyroid tests or assays that avoid the interferences,” he told Medscape Medical News.
32% of patients taking meds that could interfere with tests
In evaluating data on 538,137 patients treated with thyroid hormones from the Corporate Data Warehouse of the Veterans Health Administration, spanning 2004-2017, first author Rachel Beeson, MD, and colleagues with the University of Michigan found most patients in the study were men (96.5%), White (77.1%), and had two or more comorbidities (62.6%).
Of this total, 170,261 (31.6%) patients treated with thyroid hormones, over a median follow-up of 56 months, were taking at least one drug that could potentially interfere with thyroid function tests.
Among the drugs with potential thyroid test interference, about 28% of patients were taking prednisone or prednisolone, 8% were taking amiodarone, and 1.42% were taking phenytoin. Other reported drugs that could potentially interfere included carbamazepine (0.91%), phenobarbital (0.15%), lithium (0.40%), and tamoxifen (0.11%).
Multivariate analysis showed that characteristics associated with those most likely to have concurrent medication use included non-Whites (OR, 1.18 vs Whites), Hispanic ethnicity (OR 1.11 vs non-Hispanic), female sex (OR 1.12 vs males), and presence of comorbidities (eg, Charlson-Deyo comorbidity score ≥ 2, OR, 2.47 vs score of 0).
Meanwhile, older patients age 85 years and over had a lower likelihood of concurrent medications interfering with thyroid tests (OR, 0.47 vs age 65-74 years).
The findings are concerning given the wide use of levothyroxine to treat hypothyroidism, which is the most widely prescribed drug in the United States.
“Our findings not only highlight the complexity of thyroid hormone management in older adults in the context of polypharmacy and multimorbidity, but they also draw attention to vulnerable groups for this practice, which included female patients, non-Whites, patients of Hispanic ethnicity, and patients with comorbidities,” Dr. Papaleontiou said.
Nature of interference possibilities varies
Medications or supplements can interfere with thyroid function tests in a variety of ways, she explained. “Some medications could lead to a decrease in the absorption of levothyroxine, others may affect how well the pill dissolves.”
In addition, certain medications can affect the circulation of thyroid hormone in the blood and how it binds with proteins, or they can lead to decreasing thyroid hormone levels due to a variety of interactions.
And in contrast, “What is even more challenging is that some medications or supplements may appear to affect thyroid function based on lab tests when in reality they don’t actually affect thyroid function and may lead to dose adjustments unnecessarily,” Dr. Papaleontiou noted.
Recommendations to counter interference
Current recommendations to try to counter the effects of polypharmacy on thyroid treatment include advising patients to take thyroid hormones on an empty stomach at least 30-60 minutes prior to eating for optimal absorption.
If the patient is taking medications known to interfere with absorption of thyroid hormones, the recommendation is to space those out by at least 4 hours.
“The big challenge in older adults is that many of them do experience polypharmacy, being at risk for multiple drug-drug interactions,” Dr. Papaleontiou said.
“Physicians and patients should be vigilant and communicate closely every time there is initiation of a new medication or supplement to consider whether there may be interference.”
The authors have reported no relevant financial relationships. Dr. Hoang has reported being a speaker for Acella Pharmaceuticals.
A version of this article first appeared on Medscape.com.
, potentially compromising treatment decisions, new research shows.
“We know from previous studies that thyroid hormone use is common in older adults and that there are a multitude of medications that can interfere with thyroid function tests in different ways,” senior author Maria Papaleontiou, MD, told Medscape Medical News.
“However, to our knowledge, the extent of concurrent use of thyroid hormone and interfering medications in older adults, age 65 years and older, has not been previously explored,” added Dr. Papaleontiou, of the Division of Metabolism, Endocrinology and Diabetes, Department of Internal Medicine, University of Michigan, Ann Arbor.
The findings were presented as a poster during virtual ENDO 2021, the Endocrine Society’s annual meeting.
Commenting on the study, Thanh Duc Hoang, DO, an endocrinologist with the Walter Reed National Military Medical Center, in Bethesda, Md., said: “It is important for clinicians to be aware of various interactions and interferences of medications affecting the accuracy of thyroid function tests.”
“If patients are not able to discontinue the medications shortly before the bloodwork, the clinicians may consider ordering different thyroid tests or assays that avoid the interferences,” he told Medscape Medical News.
32% of patients taking meds that could interfere with tests
In evaluating data on 538,137 patients treated with thyroid hormones from the Corporate Data Warehouse of the Veterans Health Administration, spanning 2004-2017, first author Rachel Beeson, MD, and colleagues with the University of Michigan found most patients in the study were men (96.5%), White (77.1%), and had two or more comorbidities (62.6%).
Of this total, 170,261 (31.6%) patients treated with thyroid hormones, over a median follow-up of 56 months, were taking at least one drug that could potentially interfere with thyroid function tests.
Among the drugs with potential thyroid test interference, about 28% of patients were taking prednisone or prednisolone, 8% were taking amiodarone, and 1.42% were taking phenytoin. Other reported drugs that could potentially interfere included carbamazepine (0.91%), phenobarbital (0.15%), lithium (0.40%), and tamoxifen (0.11%).
Multivariate analysis showed that characteristics associated with those most likely to have concurrent medication use included non-Whites (OR, 1.18 vs Whites), Hispanic ethnicity (OR 1.11 vs non-Hispanic), female sex (OR 1.12 vs males), and presence of comorbidities (eg, Charlson-Deyo comorbidity score ≥ 2, OR, 2.47 vs score of 0).
Meanwhile, older patients age 85 years and over had a lower likelihood of concurrent medications interfering with thyroid tests (OR, 0.47 vs age 65-74 years).
The findings are concerning given the wide use of levothyroxine to treat hypothyroidism, which is the most widely prescribed drug in the United States.
“Our findings not only highlight the complexity of thyroid hormone management in older adults in the context of polypharmacy and multimorbidity, but they also draw attention to vulnerable groups for this practice, which included female patients, non-Whites, patients of Hispanic ethnicity, and patients with comorbidities,” Dr. Papaleontiou said.
Nature of interference possibilities varies
Medications or supplements can interfere with thyroid function tests in a variety of ways, she explained. “Some medications could lead to a decrease in the absorption of levothyroxine, others may affect how well the pill dissolves.”
In addition, certain medications can affect the circulation of thyroid hormone in the blood and how it binds with proteins, or they can lead to decreasing thyroid hormone levels due to a variety of interactions.
And in contrast, “What is even more challenging is that some medications or supplements may appear to affect thyroid function based on lab tests when in reality they don’t actually affect thyroid function and may lead to dose adjustments unnecessarily,” Dr. Papaleontiou noted.
Recommendations to counter interference
Current recommendations to try to counter the effects of polypharmacy on thyroid treatment include advising patients to take thyroid hormones on an empty stomach at least 30-60 minutes prior to eating for optimal absorption.
If the patient is taking medications known to interfere with absorption of thyroid hormones, the recommendation is to space those out by at least 4 hours.
“The big challenge in older adults is that many of them do experience polypharmacy, being at risk for multiple drug-drug interactions,” Dr. Papaleontiou said.
“Physicians and patients should be vigilant and communicate closely every time there is initiation of a new medication or supplement to consider whether there may be interference.”
The authors have reported no relevant financial relationships. Dr. Hoang has reported being a speaker for Acella Pharmaceuticals.
A version of this article first appeared on Medscape.com.
Success in achondroplasia spurs testing vosoritide in more growth disorders
On the basis of the quality of sustained bone growth achieved with vosoritide in dwarfism, studies are underway or being considered for more diseases that impair bone growth, according to discussion that followed the presentation of a phase 3 trial extension study at the annual meeting of the Endocrine Society.
After 1 year on active therapy in the randomized trial and another year in the extension study, patients in the vosoritide group had sustained growth velocity while placebo group patients who crossed over to active therapy caught up, reported Ravi Savarirayan, MD, Murdoch Children’s Research Institute, University of Melbourne, Australia.
Moreover, the quality and type of bone growth, such as the improvement in body segment ratios over the second year of the study, support a durable benefit. Dr. Savarirayan said that improvements in activities of daily living are expected from this improvement in upper-to-lower body segment ratios, as well as the growth seen in the limbs.
Currently there is no approved pharmacologic therapy for achondroplasia in the United States. Growth hormone has been approved in Japan, but Dr. Savarirayan said its effects have been limited. Surgery such as limb lengthening is another option, but this approach is not uniformly effective and carries risks.
The 52-week results from the multinational phase 3 trial with vosoritide, which stimulates bone growth, were published last year in The Lancet. In that trial, 121 patients between the ages of 5 and 18 years with achondroplasia were randomized to vosoritide at a dose of 15 μg/kg once daily or placebo.
Relative to those in the placebo arm, which did not experience any change in growth, the median growth at the end of 52 weeks was 1.75 cm/year greater (6.71 vs. 3.99 cm).
After crossover, placebo patients catch up
In the extension study, the placebo patients were crossed over to the active therapy and both groups were followed for an additional 52 weeks. Over this period, velocity declined modestly in those in the group initially randomized to vosoritide but climbed steeply in the placebo group so that rates after 1 year were nearly identical (5.57 vs. 5.65 cm, respectively).
“The results suggest this medication may well have a durable effect,” said Dr. Savarirayan, who believes that the benefit is derived from stimulation of the growth plates. Based on the very similar efficacy observed in the placebo group once switched to active therapy, the response to vosoritide appears to be predictable.
Of the 60 patients initially randomized to vosoritide, 58 entered the extension. Of the patients who did not remain in the study, two left due to discomfort from injection-site reactions. All 61 patients initially assigned to placebo crossed over.
“We did not see any evidence of tachyphylaxis in the randomized study or in the extension,” Dr. Savarirayan said.
Although two more patients initiated on vosoritide discontinued treatment before the end of 2 years, there were no new adverse events observed. Rather, injection-site pain, which self-resolved in all patients, appears to be the most significant side effect.
“In children, the daily subcutaneous injections can be an issue,” Dr. Savarirayan acknowledged.
Injection site reactions most common adverse event
In a detailed evaluation of safety in a previously published dose-finding phase 2 study, injection-site reactions were also the most common of treatment-related adverse events, but there were no episodes of anaphylaxis or other grade 3 or higher hypersensitivity reactions (N Engl J Med. 2019 Jul 4;381:25-35).
Prior to clinical trials, continuous infusion of endogenous C-type natriuretic peptide demonstrated an ability to stimulate long-bone growth in experimental studies. Vosoritide, a recombinant analogue of C-type natriuretic peptide, appears to provide the same activity but offers a longer half-life.
Based on the benefits observed in achondroplasia, other applications are now being explored.
“When you evaluate the quality of the bone growth associated with vosoritide, it is normal,” said Melita Irving, MD, a consultant in clinical genetics at the Guy’s and St .Thomas’ NHS Trust, London. Dr. Irving has been involved in other research initiatives with this therapy and she cited a variety of evidence that has supported healthy bone development, including favorable changes in markers of bone growth such as type 10 collagen.
As a result, vosoritide, which is now under review by the U.S. Food and Drug Administration for treatment of dwarfism, is being pursued for several other diseases that result in abnormal bone growth, such as hypochondroplasia. Not least, clinical studies in idiopathic short stature have reached “early stages,” Dr. Irving said.
Dr. Savarirayan and Dr. Irving report no relevant conflicts of interest.
On the basis of the quality of sustained bone growth achieved with vosoritide in dwarfism, studies are underway or being considered for more diseases that impair bone growth, according to discussion that followed the presentation of a phase 3 trial extension study at the annual meeting of the Endocrine Society.
After 1 year on active therapy in the randomized trial and another year in the extension study, patients in the vosoritide group had sustained growth velocity while placebo group patients who crossed over to active therapy caught up, reported Ravi Savarirayan, MD, Murdoch Children’s Research Institute, University of Melbourne, Australia.
Moreover, the quality and type of bone growth, such as the improvement in body segment ratios over the second year of the study, support a durable benefit. Dr. Savarirayan said that improvements in activities of daily living are expected from this improvement in upper-to-lower body segment ratios, as well as the growth seen in the limbs.
Currently there is no approved pharmacologic therapy for achondroplasia in the United States. Growth hormone has been approved in Japan, but Dr. Savarirayan said its effects have been limited. Surgery such as limb lengthening is another option, but this approach is not uniformly effective and carries risks.
The 52-week results from the multinational phase 3 trial with vosoritide, which stimulates bone growth, were published last year in The Lancet. In that trial, 121 patients between the ages of 5 and 18 years with achondroplasia were randomized to vosoritide at a dose of 15 μg/kg once daily or placebo.
Relative to those in the placebo arm, which did not experience any change in growth, the median growth at the end of 52 weeks was 1.75 cm/year greater (6.71 vs. 3.99 cm).
After crossover, placebo patients catch up
In the extension study, the placebo patients were crossed over to the active therapy and both groups were followed for an additional 52 weeks. Over this period, velocity declined modestly in those in the group initially randomized to vosoritide but climbed steeply in the placebo group so that rates after 1 year were nearly identical (5.57 vs. 5.65 cm, respectively).
“The results suggest this medication may well have a durable effect,” said Dr. Savarirayan, who believes that the benefit is derived from stimulation of the growth plates. Based on the very similar efficacy observed in the placebo group once switched to active therapy, the response to vosoritide appears to be predictable.
Of the 60 patients initially randomized to vosoritide, 58 entered the extension. Of the patients who did not remain in the study, two left due to discomfort from injection-site reactions. All 61 patients initially assigned to placebo crossed over.
“We did not see any evidence of tachyphylaxis in the randomized study or in the extension,” Dr. Savarirayan said.
Although two more patients initiated on vosoritide discontinued treatment before the end of 2 years, there were no new adverse events observed. Rather, injection-site pain, which self-resolved in all patients, appears to be the most significant side effect.
“In children, the daily subcutaneous injections can be an issue,” Dr. Savarirayan acknowledged.
Injection site reactions most common adverse event
In a detailed evaluation of safety in a previously published dose-finding phase 2 study, injection-site reactions were also the most common of treatment-related adverse events, but there were no episodes of anaphylaxis or other grade 3 or higher hypersensitivity reactions (N Engl J Med. 2019 Jul 4;381:25-35).
Prior to clinical trials, continuous infusion of endogenous C-type natriuretic peptide demonstrated an ability to stimulate long-bone growth in experimental studies. Vosoritide, a recombinant analogue of C-type natriuretic peptide, appears to provide the same activity but offers a longer half-life.
Based on the benefits observed in achondroplasia, other applications are now being explored.
“When you evaluate the quality of the bone growth associated with vosoritide, it is normal,” said Melita Irving, MD, a consultant in clinical genetics at the Guy’s and St .Thomas’ NHS Trust, London. Dr. Irving has been involved in other research initiatives with this therapy and she cited a variety of evidence that has supported healthy bone development, including favorable changes in markers of bone growth such as type 10 collagen.
As a result, vosoritide, which is now under review by the U.S. Food and Drug Administration for treatment of dwarfism, is being pursued for several other diseases that result in abnormal bone growth, such as hypochondroplasia. Not least, clinical studies in idiopathic short stature have reached “early stages,” Dr. Irving said.
Dr. Savarirayan and Dr. Irving report no relevant conflicts of interest.
On the basis of the quality of sustained bone growth achieved with vosoritide in dwarfism, studies are underway or being considered for more diseases that impair bone growth, according to discussion that followed the presentation of a phase 3 trial extension study at the annual meeting of the Endocrine Society.
After 1 year on active therapy in the randomized trial and another year in the extension study, patients in the vosoritide group had sustained growth velocity while placebo group patients who crossed over to active therapy caught up, reported Ravi Savarirayan, MD, Murdoch Children’s Research Institute, University of Melbourne, Australia.
Moreover, the quality and type of bone growth, such as the improvement in body segment ratios over the second year of the study, support a durable benefit. Dr. Savarirayan said that improvements in activities of daily living are expected from this improvement in upper-to-lower body segment ratios, as well as the growth seen in the limbs.
Currently there is no approved pharmacologic therapy for achondroplasia in the United States. Growth hormone has been approved in Japan, but Dr. Savarirayan said its effects have been limited. Surgery such as limb lengthening is another option, but this approach is not uniformly effective and carries risks.
The 52-week results from the multinational phase 3 trial with vosoritide, which stimulates bone growth, were published last year in The Lancet. In that trial, 121 patients between the ages of 5 and 18 years with achondroplasia were randomized to vosoritide at a dose of 15 μg/kg once daily or placebo.
Relative to those in the placebo arm, which did not experience any change in growth, the median growth at the end of 52 weeks was 1.75 cm/year greater (6.71 vs. 3.99 cm).
After crossover, placebo patients catch up
In the extension study, the placebo patients were crossed over to the active therapy and both groups were followed for an additional 52 weeks. Over this period, velocity declined modestly in those in the group initially randomized to vosoritide but climbed steeply in the placebo group so that rates after 1 year were nearly identical (5.57 vs. 5.65 cm, respectively).
“The results suggest this medication may well have a durable effect,” said Dr. Savarirayan, who believes that the benefit is derived from stimulation of the growth plates. Based on the very similar efficacy observed in the placebo group once switched to active therapy, the response to vosoritide appears to be predictable.
Of the 60 patients initially randomized to vosoritide, 58 entered the extension. Of the patients who did not remain in the study, two left due to discomfort from injection-site reactions. All 61 patients initially assigned to placebo crossed over.
“We did not see any evidence of tachyphylaxis in the randomized study or in the extension,” Dr. Savarirayan said.
Although two more patients initiated on vosoritide discontinued treatment before the end of 2 years, there were no new adverse events observed. Rather, injection-site pain, which self-resolved in all patients, appears to be the most significant side effect.
“In children, the daily subcutaneous injections can be an issue,” Dr. Savarirayan acknowledged.
Injection site reactions most common adverse event
In a detailed evaluation of safety in a previously published dose-finding phase 2 study, injection-site reactions were also the most common of treatment-related adverse events, but there were no episodes of anaphylaxis or other grade 3 or higher hypersensitivity reactions (N Engl J Med. 2019 Jul 4;381:25-35).
Prior to clinical trials, continuous infusion of endogenous C-type natriuretic peptide demonstrated an ability to stimulate long-bone growth in experimental studies. Vosoritide, a recombinant analogue of C-type natriuretic peptide, appears to provide the same activity but offers a longer half-life.
Based on the benefits observed in achondroplasia, other applications are now being explored.
“When you evaluate the quality of the bone growth associated with vosoritide, it is normal,” said Melita Irving, MD, a consultant in clinical genetics at the Guy’s and St .Thomas’ NHS Trust, London. Dr. Irving has been involved in other research initiatives with this therapy and she cited a variety of evidence that has supported healthy bone development, including favorable changes in markers of bone growth such as type 10 collagen.
As a result, vosoritide, which is now under review by the U.S. Food and Drug Administration for treatment of dwarfism, is being pursued for several other diseases that result in abnormal bone growth, such as hypochondroplasia. Not least, clinical studies in idiopathic short stature have reached “early stages,” Dr. Irving said.
Dr. Savarirayan and Dr. Irving report no relevant conflicts of interest.
FROM ENDO 2021
Imaging alternative to AVS could boost detection of primary aldosteronism
A noninvasive imaging method for identifying whether the source of a patient’s primary aldosteronism is from unilateral or bilateral adrenal adenomas worked as well as the standard method, invasive adrenal vein sampling, in a head-to-head comparison with 143 patients.
This noninvasive alternative, which also does not require the substantial technical expertise that AVS demands, should make assessment of adenoma laterality in patients with primary aldosteronism (PA) much more widely available and accessible, predicted Dr. Wu, a researcher at Queen Mary University of London.
“It will allow more places to do this, and I think it will definitely allow more patients to be diagnosed” with PA from a unilateral source. AVS “is a real bottleneck,” she said. “We hope metomidate, or molecular imaging using other selective radiotracers, will enable many more patients to be diagnosed and appropriately managed.” Creating new diagnostic options for patients with PA and potentially increasing the number of these patients who are surgical candidates “is the aim of this study.”
Patients with PA develop a curable form of hypertension if their excess aldosterone can be neutralized with a mineralocorticoid receptor antagonist (MRA), or even more definitively by surgical removal of the adrenal aldosteronoma generating the hormonal excess as long as the adenoma is unilateral. Conventional imaging of the adrenals with CT or MRI has proven unreliable for identifying adrenal nodules noninvasively, which has made the invasive and technically challenging standard option of AVS the only game in town.
But some endocrinologists caution that the results from this one study do not suffice to make 11C-metomidate-based PET-CT imaging a widely used alternative.
‘This is a first step.’
“This study is a first step. It will take lots more data for endocrinologists to buy into a scan over AVS,” commented David A. D’Alessio, MD, professor and chief of the division of endocrinology and metabolism at Duke University in Durham, N.C.
But Dr. D’Alessio also acknowledged the clear benefits from a safe and effective alternative to AVS.
“A reliable, less invasive, and less technical means of lateralizing excess aldosterone production would increase the number of people [with a unilateral PA source] going to surgery. The reality is that, if you are not a patient at the Mayo Clinic . . .or the National Institutes of Health, then AVS is a bit of crap shoot” that is very operator and institution dependent for its accuracy, Dr. D’Alessio said in an interview.
Metomidate specifically binds to key enzymes of the adrenal corticosteroid biosynthetic pathway, making it a precise targeting agent for a radioactive tag as documented almost a decade ago. One limitation is that this radiotracer labeling of metomidate has a 20-minute half life, which means it must be produced on site, thereby making the technology out of reach for locations that can’t set up this capability.
MATCHing imaging against AVS
To test the clinical utility of metomidate-based PET-CT directly against AVS, Dr. Wu and her associates enrolled 143 adults with confirmed PA and hypertension at two centers in London and one in Cambridge, England. The MATCH study cohort averaged 53 years of age; two-thirds were men, 58% were White, and 30% were Black. Their median blood pressure was 147/91 mm Hg, and they were maintained on a median of two antihypertensive drugs.
The researchers assessed every patient with both the imaging method and AVS, performed in random order and blindly scored. They then began each patient on a 1-month regimen with an MRA (usually spironolactone but eplerenone [Inspra] was also an option) to test the responsiveness of each patient’s hypertension to this drug class and to gauge their likely response to adrenalectomy. After the MRA test, the researchers assessed the lateralization tests and determined that 78 patients were appropriate candidates for unilateral adrenalectomy while the remaining 65 patients were not and continued on the MRA regimen. They recommended surgery if patients were clear positives by AVS, by PET-CT imaging, or both.
The study had four primary outcomes to assess the ability of the two diagnostic methods to predict the success of surgery based on four increasingly stringent postsurgical criteria calculated in hierarchical sequence: Partial or complete biochemical success, complete biochemical success, partial or complete clinical success (partial meaning any significant reduction in blood pressure), or complete clinical success (systolic pressure reduced to less than 135 mm Hg). Only one of the 78 patients treated with surgery failed to achieve at least a partial biochemical response.
For each of the four metrics, 11C-metomidate PET-CT produced point estimates of diagnostic accuracy that consistently edged out AVS. While these advantages were not large enough to meet the prespecified threshold for proving superiority, they comfortably showed the noninferiority of this imaging method compared with AVS.
For example, the PET-CT method had 43.6% accuracy for predicting a clinical cure, compared with 39.7% accuracy for AVS. For complete biochemical cure, imaging had 68.8% accuracy, compared with 62.3% for AVS, Dr. Wu reported.
Another notable finding from the study was how strongly a robust blood pressure response to spironolactone predicted the clinical outcome from surgery. Patients whose systolic blood pressure fell below 135 mm Hg on MRA treatment had a nearly 18-fold higher rate of achieving a complete clinical cure following surgery compared with patients who did not have as dramatic a blood pressure response to MRA treatment.
Woefully low rates of PA assessment
But regardless of the success that PET-CT imaging has for identifying surgical candidates, the first step is to identify patients with PA, a diagnosis that’s woefully underperformed worldwide. One example: A separate report at ENDO 2021 retrospectively reviewed nearly 12,000 patients with hypertension and an indication of PA, such as treatment-resistant hypertension or early-onset hypertension, and managed at either of two university outpatient clinics in Michigan during 2010-2019. The report documented that 3% underwent PA assessment.
Diagnosis of patients with PA “is a major problem,” noted Dr. D’Alessio. “I think of PA as an underdiagnosed and undertreated condition, with a huge impact on morbidity and mortality. Any advance in this area is likely to be useful.” But, he added, “I’m dubious whether this [new imaging approach] will increase diagnosis of PA.” What’s needed is “getting more primary care physicians to do more screening” for PA among their patients with hypertension and a suggestion of a PA cause.
“Surgical cures are glamorous, but medical management is also very effective, and we have good, inexpensive drugs to do this,” the MRAs, Dr. D’Alessio said.
The study received no commercial funding. Dr. Wu and her coauthors had no disclosures. Dr. D’Alessio has been a speaker on behalf of Novo Nordisk, a consultant to Intarcia and Lilly, and has received research funding from Lilly and Merck.
A noninvasive imaging method for identifying whether the source of a patient’s primary aldosteronism is from unilateral or bilateral adrenal adenomas worked as well as the standard method, invasive adrenal vein sampling, in a head-to-head comparison with 143 patients.
This noninvasive alternative, which also does not require the substantial technical expertise that AVS demands, should make assessment of adenoma laterality in patients with primary aldosteronism (PA) much more widely available and accessible, predicted Dr. Wu, a researcher at Queen Mary University of London.
“It will allow more places to do this, and I think it will definitely allow more patients to be diagnosed” with PA from a unilateral source. AVS “is a real bottleneck,” she said. “We hope metomidate, or molecular imaging using other selective radiotracers, will enable many more patients to be diagnosed and appropriately managed.” Creating new diagnostic options for patients with PA and potentially increasing the number of these patients who are surgical candidates “is the aim of this study.”
Patients with PA develop a curable form of hypertension if their excess aldosterone can be neutralized with a mineralocorticoid receptor antagonist (MRA), or even more definitively by surgical removal of the adrenal aldosteronoma generating the hormonal excess as long as the adenoma is unilateral. Conventional imaging of the adrenals with CT or MRI has proven unreliable for identifying adrenal nodules noninvasively, which has made the invasive and technically challenging standard option of AVS the only game in town.
But some endocrinologists caution that the results from this one study do not suffice to make 11C-metomidate-based PET-CT imaging a widely used alternative.
‘This is a first step.’
“This study is a first step. It will take lots more data for endocrinologists to buy into a scan over AVS,” commented David A. D’Alessio, MD, professor and chief of the division of endocrinology and metabolism at Duke University in Durham, N.C.
But Dr. D’Alessio also acknowledged the clear benefits from a safe and effective alternative to AVS.
“A reliable, less invasive, and less technical means of lateralizing excess aldosterone production would increase the number of people [with a unilateral PA source] going to surgery. The reality is that, if you are not a patient at the Mayo Clinic . . .or the National Institutes of Health, then AVS is a bit of crap shoot” that is very operator and institution dependent for its accuracy, Dr. D’Alessio said in an interview.
Metomidate specifically binds to key enzymes of the adrenal corticosteroid biosynthetic pathway, making it a precise targeting agent for a radioactive tag as documented almost a decade ago. One limitation is that this radiotracer labeling of metomidate has a 20-minute half life, which means it must be produced on site, thereby making the technology out of reach for locations that can’t set up this capability.
MATCHing imaging against AVS
To test the clinical utility of metomidate-based PET-CT directly against AVS, Dr. Wu and her associates enrolled 143 adults with confirmed PA and hypertension at two centers in London and one in Cambridge, England. The MATCH study cohort averaged 53 years of age; two-thirds were men, 58% were White, and 30% were Black. Their median blood pressure was 147/91 mm Hg, and they were maintained on a median of two antihypertensive drugs.
The researchers assessed every patient with both the imaging method and AVS, performed in random order and blindly scored. They then began each patient on a 1-month regimen with an MRA (usually spironolactone but eplerenone [Inspra] was also an option) to test the responsiveness of each patient’s hypertension to this drug class and to gauge their likely response to adrenalectomy. After the MRA test, the researchers assessed the lateralization tests and determined that 78 patients were appropriate candidates for unilateral adrenalectomy while the remaining 65 patients were not and continued on the MRA regimen. They recommended surgery if patients were clear positives by AVS, by PET-CT imaging, or both.
The study had four primary outcomes to assess the ability of the two diagnostic methods to predict the success of surgery based on four increasingly stringent postsurgical criteria calculated in hierarchical sequence: Partial or complete biochemical success, complete biochemical success, partial or complete clinical success (partial meaning any significant reduction in blood pressure), or complete clinical success (systolic pressure reduced to less than 135 mm Hg). Only one of the 78 patients treated with surgery failed to achieve at least a partial biochemical response.
For each of the four metrics, 11C-metomidate PET-CT produced point estimates of diagnostic accuracy that consistently edged out AVS. While these advantages were not large enough to meet the prespecified threshold for proving superiority, they comfortably showed the noninferiority of this imaging method compared with AVS.
For example, the PET-CT method had 43.6% accuracy for predicting a clinical cure, compared with 39.7% accuracy for AVS. For complete biochemical cure, imaging had 68.8% accuracy, compared with 62.3% for AVS, Dr. Wu reported.
Another notable finding from the study was how strongly a robust blood pressure response to spironolactone predicted the clinical outcome from surgery. Patients whose systolic blood pressure fell below 135 mm Hg on MRA treatment had a nearly 18-fold higher rate of achieving a complete clinical cure following surgery compared with patients who did not have as dramatic a blood pressure response to MRA treatment.
Woefully low rates of PA assessment
But regardless of the success that PET-CT imaging has for identifying surgical candidates, the first step is to identify patients with PA, a diagnosis that’s woefully underperformed worldwide. One example: A separate report at ENDO 2021 retrospectively reviewed nearly 12,000 patients with hypertension and an indication of PA, such as treatment-resistant hypertension or early-onset hypertension, and managed at either of two university outpatient clinics in Michigan during 2010-2019. The report documented that 3% underwent PA assessment.
Diagnosis of patients with PA “is a major problem,” noted Dr. D’Alessio. “I think of PA as an underdiagnosed and undertreated condition, with a huge impact on morbidity and mortality. Any advance in this area is likely to be useful.” But, he added, “I’m dubious whether this [new imaging approach] will increase diagnosis of PA.” What’s needed is “getting more primary care physicians to do more screening” for PA among their patients with hypertension and a suggestion of a PA cause.
“Surgical cures are glamorous, but medical management is also very effective, and we have good, inexpensive drugs to do this,” the MRAs, Dr. D’Alessio said.
The study received no commercial funding. Dr. Wu and her coauthors had no disclosures. Dr. D’Alessio has been a speaker on behalf of Novo Nordisk, a consultant to Intarcia and Lilly, and has received research funding from Lilly and Merck.
A noninvasive imaging method for identifying whether the source of a patient’s primary aldosteronism is from unilateral or bilateral adrenal adenomas worked as well as the standard method, invasive adrenal vein sampling, in a head-to-head comparison with 143 patients.
This noninvasive alternative, which also does not require the substantial technical expertise that AVS demands, should make assessment of adenoma laterality in patients with primary aldosteronism (PA) much more widely available and accessible, predicted Dr. Wu, a researcher at Queen Mary University of London.
“It will allow more places to do this, and I think it will definitely allow more patients to be diagnosed” with PA from a unilateral source. AVS “is a real bottleneck,” she said. “We hope metomidate, or molecular imaging using other selective radiotracers, will enable many more patients to be diagnosed and appropriately managed.” Creating new diagnostic options for patients with PA and potentially increasing the number of these patients who are surgical candidates “is the aim of this study.”
Patients with PA develop a curable form of hypertension if their excess aldosterone can be neutralized with a mineralocorticoid receptor antagonist (MRA), or even more definitively by surgical removal of the adrenal aldosteronoma generating the hormonal excess as long as the adenoma is unilateral. Conventional imaging of the adrenals with CT or MRI has proven unreliable for identifying adrenal nodules noninvasively, which has made the invasive and technically challenging standard option of AVS the only game in town.
But some endocrinologists caution that the results from this one study do not suffice to make 11C-metomidate-based PET-CT imaging a widely used alternative.
‘This is a first step.’
“This study is a first step. It will take lots more data for endocrinologists to buy into a scan over AVS,” commented David A. D’Alessio, MD, professor and chief of the division of endocrinology and metabolism at Duke University in Durham, N.C.
But Dr. D’Alessio also acknowledged the clear benefits from a safe and effective alternative to AVS.
“A reliable, less invasive, and less technical means of lateralizing excess aldosterone production would increase the number of people [with a unilateral PA source] going to surgery. The reality is that, if you are not a patient at the Mayo Clinic . . .or the National Institutes of Health, then AVS is a bit of crap shoot” that is very operator and institution dependent for its accuracy, Dr. D’Alessio said in an interview.
Metomidate specifically binds to key enzymes of the adrenal corticosteroid biosynthetic pathway, making it a precise targeting agent for a radioactive tag as documented almost a decade ago. One limitation is that this radiotracer labeling of metomidate has a 20-minute half life, which means it must be produced on site, thereby making the technology out of reach for locations that can’t set up this capability.
MATCHing imaging against AVS
To test the clinical utility of metomidate-based PET-CT directly against AVS, Dr. Wu and her associates enrolled 143 adults with confirmed PA and hypertension at two centers in London and one in Cambridge, England. The MATCH study cohort averaged 53 years of age; two-thirds were men, 58% were White, and 30% were Black. Their median blood pressure was 147/91 mm Hg, and they were maintained on a median of two antihypertensive drugs.
The researchers assessed every patient with both the imaging method and AVS, performed in random order and blindly scored. They then began each patient on a 1-month regimen with an MRA (usually spironolactone but eplerenone [Inspra] was also an option) to test the responsiveness of each patient’s hypertension to this drug class and to gauge their likely response to adrenalectomy. After the MRA test, the researchers assessed the lateralization tests and determined that 78 patients were appropriate candidates for unilateral adrenalectomy while the remaining 65 patients were not and continued on the MRA regimen. They recommended surgery if patients were clear positives by AVS, by PET-CT imaging, or both.
The study had four primary outcomes to assess the ability of the two diagnostic methods to predict the success of surgery based on four increasingly stringent postsurgical criteria calculated in hierarchical sequence: Partial or complete biochemical success, complete biochemical success, partial or complete clinical success (partial meaning any significant reduction in blood pressure), or complete clinical success (systolic pressure reduced to less than 135 mm Hg). Only one of the 78 patients treated with surgery failed to achieve at least a partial biochemical response.
For each of the four metrics, 11C-metomidate PET-CT produced point estimates of diagnostic accuracy that consistently edged out AVS. While these advantages were not large enough to meet the prespecified threshold for proving superiority, they comfortably showed the noninferiority of this imaging method compared with AVS.
For example, the PET-CT method had 43.6% accuracy for predicting a clinical cure, compared with 39.7% accuracy for AVS. For complete biochemical cure, imaging had 68.8% accuracy, compared with 62.3% for AVS, Dr. Wu reported.
Another notable finding from the study was how strongly a robust blood pressure response to spironolactone predicted the clinical outcome from surgery. Patients whose systolic blood pressure fell below 135 mm Hg on MRA treatment had a nearly 18-fold higher rate of achieving a complete clinical cure following surgery compared with patients who did not have as dramatic a blood pressure response to MRA treatment.
Woefully low rates of PA assessment
But regardless of the success that PET-CT imaging has for identifying surgical candidates, the first step is to identify patients with PA, a diagnosis that’s woefully underperformed worldwide. One example: A separate report at ENDO 2021 retrospectively reviewed nearly 12,000 patients with hypertension and an indication of PA, such as treatment-resistant hypertension or early-onset hypertension, and managed at either of two university outpatient clinics in Michigan during 2010-2019. The report documented that 3% underwent PA assessment.
Diagnosis of patients with PA “is a major problem,” noted Dr. D’Alessio. “I think of PA as an underdiagnosed and undertreated condition, with a huge impact on morbidity and mortality. Any advance in this area is likely to be useful.” But, he added, “I’m dubious whether this [new imaging approach] will increase diagnosis of PA.” What’s needed is “getting more primary care physicians to do more screening” for PA among their patients with hypertension and a suggestion of a PA cause.
“Surgical cures are glamorous, but medical management is also very effective, and we have good, inexpensive drugs to do this,” the MRAs, Dr. D’Alessio said.
The study received no commercial funding. Dr. Wu and her coauthors had no disclosures. Dr. D’Alessio has been a speaker on behalf of Novo Nordisk, a consultant to Intarcia and Lilly, and has received research funding from Lilly and Merck.
FROM ENDO 2021