User login
Here we go again? Rate of COVID-19 in children takes a turn for the worse
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
After declining for 8 consecutive weeks, new cases of COVID-19 rose among children in the United States, according to the American Academy of Pediatrics and the Children’s Hospital Association.

, ending a streak of declines going back to mid-January, the AAP and CHA said in their weekly COVID-19 report.
Also up for the week was the proportion of all cases occurring in children. The 57,000-plus cases represented 18.7% of the total (304,610) for all ages, and that is the largest share of the new-case burden for the entire pandemic. The previous high, 18.0%, came just 2 weeks earlier, based on data collected from 49 states (excluding New York), the District of Columbia, New York City, Puerto Rico, and Guam.
Speaking of the entire pandemic, the total number of COVID-19 cases in children is over 3.34 million, and that represents 13.3% of cases among all ages in the United States. The cumulative rate of infection as of March 18 was 4,440 cases per 100,000 children, up from 4,364 per 100,000 a week earlier, the AAP and CHA said.
At the state level, Vermont has now passed the 20% mark (20.1%, to be exact) for children’s proportion of cases and is higher in that measure than any other state. The highest rate of infection (8,763 cases per 100,000) can be found in North Dakota, the AAP/CHA data show.
There were only two new coronavirus-related deaths during the week of March 12-18 after Kansas revised its mortality data, bringing the total to 268 in the 46 jurisdictions (43 states, New York City, Puerto Rico, and Guam) that are reporting deaths by age, the AAP and CHA said.
Match Day 2021: Pediatrics experiences slow, steady growth
Match Day 2021 was another record breaker, despite the pandemic, and pediatrics played its part, adding nearly 40 more slots than 2020 and filling nearly 50 more, according to the National Resident Matching Program (NRMP).
“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a press release. Overall, 35,194 first-year (PGY-1) slots were offered and 33,353 were filled, both more than ever before, for a fill rate of 94.8%, a slight increase from the 94.6% fill rate last year.
Pediatrics offered 2,901 slots in 2021, up from 2,864 in 2020, though the proportion of pediatrics slots in the overall total fell slightly to 8.2% from 8.4% in 2020. Of those 2,901 slots, 2,860 were filled, for a fill rate of 98.6%, up from 98.2% last year. Of those filled positions, 60.3% were filled by MD seniors, and 78.2% were filled by U.S. graduates.
Since 2017, pediatrics has offered more slots every year, rising from 2,738 in 2017 up to the 2,901 in 2021, an overall growth rate of just under 6%.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized, [as] growth in registration was seen in every applicant group,” the NRMP noted. Rank-order lists submissions in 2021 were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen international medical graduates, and 15.0% for non–U.S.-citizen IMGs, compared with 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. ... The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in the press release.
Match Day 2021 was another record breaker, despite the pandemic, and pediatrics played its part, adding nearly 40 more slots than 2020 and filling nearly 50 more, according to the National Resident Matching Program (NRMP).

“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a press release. Overall, 35,194 first-year (PGY-1) slots were offered and 33,353 were filled, both more than ever before, for a fill rate of 94.8%, a slight increase from the 94.6% fill rate last year.
Pediatrics offered 2,901 slots in 2021, up from 2,864 in 2020, though the proportion of pediatrics slots in the overall total fell slightly to 8.2% from 8.4% in 2020. Of those 2,901 slots, 2,860 were filled, for a fill rate of 98.6%, up from 98.2% last year. Of those filled positions, 60.3% were filled by MD seniors, and 78.2% were filled by U.S. graduates.
Since 2017, pediatrics has offered more slots every year, rising from 2,738 in 2017 up to the 2,901 in 2021, an overall growth rate of just under 6%.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized, [as] growth in registration was seen in every applicant group,” the NRMP noted. Rank-order lists submissions in 2021 were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen international medical graduates, and 15.0% for non–U.S.-citizen IMGs, compared with 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. ... The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in the press release.
Match Day 2021 was another record breaker, despite the pandemic, and pediatrics played its part, adding nearly 40 more slots than 2020 and filling nearly 50 more, according to the National Resident Matching Program (NRMP).

“Rather than faltering in these uncertain times, program fill rates increased across the board,” the NRMP said in a press release. Overall, 35,194 first-year (PGY-1) slots were offered and 33,353 were filled, both more than ever before, for a fill rate of 94.8%, a slight increase from the 94.6% fill rate last year.
Pediatrics offered 2,901 slots in 2021, up from 2,864 in 2020, though the proportion of pediatrics slots in the overall total fell slightly to 8.2% from 8.4% in 2020. Of those 2,901 slots, 2,860 were filled, for a fill rate of 98.6%, up from 98.2% last year. Of those filled positions, 60.3% were filled by MD seniors, and 78.2% were filled by U.S. graduates.
Since 2017, pediatrics has offered more slots every year, rising from 2,738 in 2017 up to the 2,901 in 2021, an overall growth rate of just under 6%.
“Concerns about the impact of virtual recruitment on applicants’ matching into PGY-1 positions were not realized, [as] growth in registration was seen in every applicant group,” the NRMP noted. Rank-order lists submissions in 2021 were up by 2.8% for U.S. MD seniors, 7.9% for U.S. DO seniors, 2.5% among U.S.-citizen international medical graduates, and 15.0% for non–U.S.-citizen IMGs, compared with 2020.
“The NRMP is honored to have delivered a strong Match to the many applicants pursuing their dreams of medicine. ... The application and recruitment cycle was upended as a result of the pandemic, yet the results of the Match continue to demonstrate strong and consistent outcomes for participants,” Donna L. Lamb, DHSc, MBA, BSN, NRMP President and CEO, said in the press release.
Metyrapone for Cushing’s syndrome: Safe, effective in first test
Metyrapone, an inhibitor of endogenous adrenal corticosteroid synthesis currently used in U.S. practice to test adrenocorticotropic hormone (ACTH) function, was safe and effective for treating endogenous Cushing’s syndrome in a multicenter, open-label, single-arm study of 50 patients, the first prospective test of metyrapone (Metopirone) as a therapeutic agent.
Treatment with metyrapone for 12 weeks normalized mean levels of urinary free cortisol (UFC) in 23 of the 49 patients (47%) in the efficacy analysis, and cut pretreatment mean UFC levels by at least 50% in another 16 patients (33%). Treatment also improved clinical signs of hypercortisolism, associated comorbidities, and quality of life, and was well tolerated, Lynnette K. Nieman, MD, said at the annual meeting of the Endocrine Society.
“This prospective study confirms that metyrapone is effective, has a rapid onset of action, and is a safe medical treatment for endogenous Cushing’s syndrome,” declared Dr. Nieman, chief of the endocrinology consultation service of the National Institutes of Health Clinical Center in Bethesda, Md.
The study included a 24-week extension phase of continued metyrapone treatment in patients whose mean UFC level fell to less than two times the upper limit of normal (ULN), but Dr. Nieman did not report results from this extension.
Confirmation of off-label and European experience
“This was the first prospective study of metyrapone, albeit a small study and with only short-term data presented. It confirms what we have known from its off label use in the U.S. and retrospective studies in the U.K. and Europe: Metyrapone normalizes mean UFC in approximately half of patients. Probably with more aggressive up titration efficacy will have been even higher, but of course with the trade-off of adrenal insufficiency,” said Maria Fleseriu, MD, professor and director of the pituitary center at Oregon Health & Science University in Portland, who was not involved with the study.
“Longer-term data from this prospective study is clearly needed to evaluate for possible loss of response, as well as adverse events related to precursors accumulation. We also need data on tumor size with long-term use in patients with Cushing’s disease, “Dr. Fleseriu added in an interview.
“Metyrapone, an 11-hydroxylase inhibitor, is not [Food and Drug Administration–approved for therapy] and thus it will be hard for it to become a first-line medical therapy,” she continued. “Furthermore, multiple times a day administration is not ideal for most patients; however if metyrapone is readily available and cheaper than other drugs, its use might increase over time. Hirsutism in women (though not all women develop this) and hypertension could be issues with long-term use,” she cautioned.
“We have used metyrapone off label for many years. It has a rapid onset of action, and we also have experience using it in combination therapy with ketoconazole, especially in patients with severe Cushing’s, although ketoconazole is not [FDA] approved for Cushing’s syndrome, and all combination therapies are off-label, too,” Dr. Fleseriu noted.
Metyrapone is approved by the European Medicines Agency for treating Cushing’s syndrome based “on observational, retrospective studies published over more than 50 years,” according to Dr. Nieman. The drug has FDA approval only for diagnostic purposes.
The PROMPT (Effects of Metyrapone in Patients With Endogenous Cushing’s Syndrome) study enrolled patients in eight European countries who were newly diagnosed with endogenous Cushing’s syndrome of any etiology. The study excluded patients with an advanced adrenal carcinoma, as well as patients with recurrent or persistent Cushing’s disease following transsphenoidal surgery. Patients also needed three 24-hour measures of UFC that were at least 50% above the ULN (165 nmol/24 hours).
The average age of the patients was 46 years; 69% were women, 90% had Cushing’s disease, and 8% had ectopic ACTH secretion. The average time from initial symptom onset was 4 years. Sixty-one percent had a history of pituitary surgery, 69% were hypertensive, 43% had diabetes or glucose intolerance, and 41% had osteoporosis. The median mean UFC at entry was 570 nmol/24 hours, which is 3.5 times the ULN, and ranged from 291 to 8,476 nmol/24 hours.
Patients began on a metyrapone dosage of 750 mg/day unless their mean UFC exceeded 5 times the ULN, in which case the dosage doubled to 1,500 mg/day. During the 12-week period, clinicians up- or down-titrated the dosage to ideally achieve a UFC less than the ULN while maintaining serum cortisol levels of 7-12 mcg/dL to preclude adrenal insufficiency effects. The median dosage at the end of 12 weeks was 1,500 mg/day, and ranged from 250 to 5,500 mg/day. One of the 50 patients dropped out because of an unrelated acute medical condition, and two patients underwent pituitary surgery despite a response to metyrapone and were included in the efficacy analysis.
After the first week on treatment, patients had a median 49% cut from their baseline UFC level, and after 12 weeks this rose to a median 74% cut from baseline. The study’s primary endpoint was normalization of UFC after 12 weeks, which occurred in 47% of patients, while 22% had a normal level in a late-night salivary cortisol measurement.
Two-thirds of patients had an improvement or resolution of their signs and symptoms, on average quality of life scores improved, median systolic and diastolic blood pressures decreased by 4-5 mm Hg, and average A1c levels were stable, but the mean cholesterol level decreased significantly, and testosterone levels rose significantly in women.
Proper dose titration makes a difference
Adverse events occurred in 26 of the 50 patients (52%) who received any treatment; 1 patient had a serious adverse event, 7 patients required a dosage adjustment because of adverse events, and 6 patients stopped treatment. The most common adverse events were gastrointestinal – nausea in 24% and decreased appetite in 18% – as well as mild symptoms consistent with adrenal insufficiency such as fatigue and headache. Six patients (12%) were identified with reversible adrenal insufficiency, and no patients complained of worsening acne or hirsutism.
“I think the adverse events are a function of [less than optimal] dose titration and variability in UFC levels,” said Dr. Nieman.
This test of metyrapone’s efficacy comes a year after the FDA approved osilodrostat (Isturisa) for treating Cushing’s disease (but not Cushing’s syndrome). Like metyrapone, osilodrostat controls cortisol overproduction by blocking the enzyme 11-beta-hydroxylase and preventing cortisol synthesis, and osilodrostat was the first agent with these properties to receive an FDA label for therapy.
Osilodrostat “is the only adrenal steroidogenesis inhibitor assessed in randomized controlled long-term trials – over 200 patients with Cushing’s disease – and it has been shown to be highly effective at maintaining normal urinary free cortisol in large majority of patients with Cushing’s disease, as well as Cushing’s syndrome in a study in Japan. Adrenal insufficiency was high [with osilodrostat], especially with the high dose in the trial with forced uptitration. In my clinical practice I have noticed less adrenal insufficiency, but I use much slower drug titration,” said Dr. Fleseriu.
“I think these drugs [metyrapone and osilodrostat] are relatively equivalent,” Dr. Nieman said during discussion of her report. “One nonmedical judgment will be cost,” she added. “Everyone is looking forward to what the pricing structure will be for the new drugs.”
Dr. Fleseriu noted that “for most patients, surgery is first-line therapy, and rarely medication is an alternative first option, especially for Cushing’s disease. However, medical therapy is essential in the management of patients with Cushing’s syndrome when curative surgery fails, surgery is not feasible, when a patient is awaiting radiation’s effect, and for recurrent cases of Cushing’s syndrome.”
PROMPT was sponsored by HRA Pharma, the company that markets metyrapone. Dr. Nieman had no disclosures, but several of her associates on the study are HRA employees. Dr. Fleseriu has been a consultant to Novartis, Recordati, Sparrow, and Strongbridge.
Metyrapone, an inhibitor of endogenous adrenal corticosteroid synthesis currently used in U.S. practice to test adrenocorticotropic hormone (ACTH) function, was safe and effective for treating endogenous Cushing’s syndrome in a multicenter, open-label, single-arm study of 50 patients, the first prospective test of metyrapone (Metopirone) as a therapeutic agent.
Treatment with metyrapone for 12 weeks normalized mean levels of urinary free cortisol (UFC) in 23 of the 49 patients (47%) in the efficacy analysis, and cut pretreatment mean UFC levels by at least 50% in another 16 patients (33%). Treatment also improved clinical signs of hypercortisolism, associated comorbidities, and quality of life, and was well tolerated, Lynnette K. Nieman, MD, said at the annual meeting of the Endocrine Society.
“This prospective study confirms that metyrapone is effective, has a rapid onset of action, and is a safe medical treatment for endogenous Cushing’s syndrome,” declared Dr. Nieman, chief of the endocrinology consultation service of the National Institutes of Health Clinical Center in Bethesda, Md.
The study included a 24-week extension phase of continued metyrapone treatment in patients whose mean UFC level fell to less than two times the upper limit of normal (ULN), but Dr. Nieman did not report results from this extension.
Confirmation of off-label and European experience
“This was the first prospective study of metyrapone, albeit a small study and with only short-term data presented. It confirms what we have known from its off label use in the U.S. and retrospective studies in the U.K. and Europe: Metyrapone normalizes mean UFC in approximately half of patients. Probably with more aggressive up titration efficacy will have been even higher, but of course with the trade-off of adrenal insufficiency,” said Maria Fleseriu, MD, professor and director of the pituitary center at Oregon Health & Science University in Portland, who was not involved with the study.
“Longer-term data from this prospective study is clearly needed to evaluate for possible loss of response, as well as adverse events related to precursors accumulation. We also need data on tumor size with long-term use in patients with Cushing’s disease, “Dr. Fleseriu added in an interview.
“Metyrapone, an 11-hydroxylase inhibitor, is not [Food and Drug Administration–approved for therapy] and thus it will be hard for it to become a first-line medical therapy,” she continued. “Furthermore, multiple times a day administration is not ideal for most patients; however if metyrapone is readily available and cheaper than other drugs, its use might increase over time. Hirsutism in women (though not all women develop this) and hypertension could be issues with long-term use,” she cautioned.
“We have used metyrapone off label for many years. It has a rapid onset of action, and we also have experience using it in combination therapy with ketoconazole, especially in patients with severe Cushing’s, although ketoconazole is not [FDA] approved for Cushing’s syndrome, and all combination therapies are off-label, too,” Dr. Fleseriu noted.
Metyrapone is approved by the European Medicines Agency for treating Cushing’s syndrome based “on observational, retrospective studies published over more than 50 years,” according to Dr. Nieman. The drug has FDA approval only for diagnostic purposes.
The PROMPT (Effects of Metyrapone in Patients With Endogenous Cushing’s Syndrome) study enrolled patients in eight European countries who were newly diagnosed with endogenous Cushing’s syndrome of any etiology. The study excluded patients with an advanced adrenal carcinoma, as well as patients with recurrent or persistent Cushing’s disease following transsphenoidal surgery. Patients also needed three 24-hour measures of UFC that were at least 50% above the ULN (165 nmol/24 hours).
The average age of the patients was 46 years; 69% were women, 90% had Cushing’s disease, and 8% had ectopic ACTH secretion. The average time from initial symptom onset was 4 years. Sixty-one percent had a history of pituitary surgery, 69% were hypertensive, 43% had diabetes or glucose intolerance, and 41% had osteoporosis. The median mean UFC at entry was 570 nmol/24 hours, which is 3.5 times the ULN, and ranged from 291 to 8,476 nmol/24 hours.
Patients began on a metyrapone dosage of 750 mg/day unless their mean UFC exceeded 5 times the ULN, in which case the dosage doubled to 1,500 mg/day. During the 12-week period, clinicians up- or down-titrated the dosage to ideally achieve a UFC less than the ULN while maintaining serum cortisol levels of 7-12 mcg/dL to preclude adrenal insufficiency effects. The median dosage at the end of 12 weeks was 1,500 mg/day, and ranged from 250 to 5,500 mg/day. One of the 50 patients dropped out because of an unrelated acute medical condition, and two patients underwent pituitary surgery despite a response to metyrapone and were included in the efficacy analysis.
After the first week on treatment, patients had a median 49% cut from their baseline UFC level, and after 12 weeks this rose to a median 74% cut from baseline. The study’s primary endpoint was normalization of UFC after 12 weeks, which occurred in 47% of patients, while 22% had a normal level in a late-night salivary cortisol measurement.
Two-thirds of patients had an improvement or resolution of their signs and symptoms, on average quality of life scores improved, median systolic and diastolic blood pressures decreased by 4-5 mm Hg, and average A1c levels were stable, but the mean cholesterol level decreased significantly, and testosterone levels rose significantly in women.
Proper dose titration makes a difference
Adverse events occurred in 26 of the 50 patients (52%) who received any treatment; 1 patient had a serious adverse event, 7 patients required a dosage adjustment because of adverse events, and 6 patients stopped treatment. The most common adverse events were gastrointestinal – nausea in 24% and decreased appetite in 18% – as well as mild symptoms consistent with adrenal insufficiency such as fatigue and headache. Six patients (12%) were identified with reversible adrenal insufficiency, and no patients complained of worsening acne or hirsutism.
“I think the adverse events are a function of [less than optimal] dose titration and variability in UFC levels,” said Dr. Nieman.
This test of metyrapone’s efficacy comes a year after the FDA approved osilodrostat (Isturisa) for treating Cushing’s disease (but not Cushing’s syndrome). Like metyrapone, osilodrostat controls cortisol overproduction by blocking the enzyme 11-beta-hydroxylase and preventing cortisol synthesis, and osilodrostat was the first agent with these properties to receive an FDA label for therapy.
Osilodrostat “is the only adrenal steroidogenesis inhibitor assessed in randomized controlled long-term trials – over 200 patients with Cushing’s disease – and it has been shown to be highly effective at maintaining normal urinary free cortisol in large majority of patients with Cushing’s disease, as well as Cushing’s syndrome in a study in Japan. Adrenal insufficiency was high [with osilodrostat], especially with the high dose in the trial with forced uptitration. In my clinical practice I have noticed less adrenal insufficiency, but I use much slower drug titration,” said Dr. Fleseriu.
“I think these drugs [metyrapone and osilodrostat] are relatively equivalent,” Dr. Nieman said during discussion of her report. “One nonmedical judgment will be cost,” she added. “Everyone is looking forward to what the pricing structure will be for the new drugs.”
Dr. Fleseriu noted that “for most patients, surgery is first-line therapy, and rarely medication is an alternative first option, especially for Cushing’s disease. However, medical therapy is essential in the management of patients with Cushing’s syndrome when curative surgery fails, surgery is not feasible, when a patient is awaiting radiation’s effect, and for recurrent cases of Cushing’s syndrome.”
PROMPT was sponsored by HRA Pharma, the company that markets metyrapone. Dr. Nieman had no disclosures, but several of her associates on the study are HRA employees. Dr. Fleseriu has been a consultant to Novartis, Recordati, Sparrow, and Strongbridge.
Metyrapone, an inhibitor of endogenous adrenal corticosteroid synthesis currently used in U.S. practice to test adrenocorticotropic hormone (ACTH) function, was safe and effective for treating endogenous Cushing’s syndrome in a multicenter, open-label, single-arm study of 50 patients, the first prospective test of metyrapone (Metopirone) as a therapeutic agent.
Treatment with metyrapone for 12 weeks normalized mean levels of urinary free cortisol (UFC) in 23 of the 49 patients (47%) in the efficacy analysis, and cut pretreatment mean UFC levels by at least 50% in another 16 patients (33%). Treatment also improved clinical signs of hypercortisolism, associated comorbidities, and quality of life, and was well tolerated, Lynnette K. Nieman, MD, said at the annual meeting of the Endocrine Society.
“This prospective study confirms that metyrapone is effective, has a rapid onset of action, and is a safe medical treatment for endogenous Cushing’s syndrome,” declared Dr. Nieman, chief of the endocrinology consultation service of the National Institutes of Health Clinical Center in Bethesda, Md.
The study included a 24-week extension phase of continued metyrapone treatment in patients whose mean UFC level fell to less than two times the upper limit of normal (ULN), but Dr. Nieman did not report results from this extension.
Confirmation of off-label and European experience
“This was the first prospective study of metyrapone, albeit a small study and with only short-term data presented. It confirms what we have known from its off label use in the U.S. and retrospective studies in the U.K. and Europe: Metyrapone normalizes mean UFC in approximately half of patients. Probably with more aggressive up titration efficacy will have been even higher, but of course with the trade-off of adrenal insufficiency,” said Maria Fleseriu, MD, professor and director of the pituitary center at Oregon Health & Science University in Portland, who was not involved with the study.
“Longer-term data from this prospective study is clearly needed to evaluate for possible loss of response, as well as adverse events related to precursors accumulation. We also need data on tumor size with long-term use in patients with Cushing’s disease, “Dr. Fleseriu added in an interview.
“Metyrapone, an 11-hydroxylase inhibitor, is not [Food and Drug Administration–approved for therapy] and thus it will be hard for it to become a first-line medical therapy,” she continued. “Furthermore, multiple times a day administration is not ideal for most patients; however if metyrapone is readily available and cheaper than other drugs, its use might increase over time. Hirsutism in women (though not all women develop this) and hypertension could be issues with long-term use,” she cautioned.
“We have used metyrapone off label for many years. It has a rapid onset of action, and we also have experience using it in combination therapy with ketoconazole, especially in patients with severe Cushing’s, although ketoconazole is not [FDA] approved for Cushing’s syndrome, and all combination therapies are off-label, too,” Dr. Fleseriu noted.
Metyrapone is approved by the European Medicines Agency for treating Cushing’s syndrome based “on observational, retrospective studies published over more than 50 years,” according to Dr. Nieman. The drug has FDA approval only for diagnostic purposes.
The PROMPT (Effects of Metyrapone in Patients With Endogenous Cushing’s Syndrome) study enrolled patients in eight European countries who were newly diagnosed with endogenous Cushing’s syndrome of any etiology. The study excluded patients with an advanced adrenal carcinoma, as well as patients with recurrent or persistent Cushing’s disease following transsphenoidal surgery. Patients also needed three 24-hour measures of UFC that were at least 50% above the ULN (165 nmol/24 hours).
The average age of the patients was 46 years; 69% were women, 90% had Cushing’s disease, and 8% had ectopic ACTH secretion. The average time from initial symptom onset was 4 years. Sixty-one percent had a history of pituitary surgery, 69% were hypertensive, 43% had diabetes or glucose intolerance, and 41% had osteoporosis. The median mean UFC at entry was 570 nmol/24 hours, which is 3.5 times the ULN, and ranged from 291 to 8,476 nmol/24 hours.
Patients began on a metyrapone dosage of 750 mg/day unless their mean UFC exceeded 5 times the ULN, in which case the dosage doubled to 1,500 mg/day. During the 12-week period, clinicians up- or down-titrated the dosage to ideally achieve a UFC less than the ULN while maintaining serum cortisol levels of 7-12 mcg/dL to preclude adrenal insufficiency effects. The median dosage at the end of 12 weeks was 1,500 mg/day, and ranged from 250 to 5,500 mg/day. One of the 50 patients dropped out because of an unrelated acute medical condition, and two patients underwent pituitary surgery despite a response to metyrapone and were included in the efficacy analysis.
After the first week on treatment, patients had a median 49% cut from their baseline UFC level, and after 12 weeks this rose to a median 74% cut from baseline. The study’s primary endpoint was normalization of UFC after 12 weeks, which occurred in 47% of patients, while 22% had a normal level in a late-night salivary cortisol measurement.
Two-thirds of patients had an improvement or resolution of their signs and symptoms, on average quality of life scores improved, median systolic and diastolic blood pressures decreased by 4-5 mm Hg, and average A1c levels were stable, but the mean cholesterol level decreased significantly, and testosterone levels rose significantly in women.
Proper dose titration makes a difference
Adverse events occurred in 26 of the 50 patients (52%) who received any treatment; 1 patient had a serious adverse event, 7 patients required a dosage adjustment because of adverse events, and 6 patients stopped treatment. The most common adverse events were gastrointestinal – nausea in 24% and decreased appetite in 18% – as well as mild symptoms consistent with adrenal insufficiency such as fatigue and headache. Six patients (12%) were identified with reversible adrenal insufficiency, and no patients complained of worsening acne or hirsutism.
“I think the adverse events are a function of [less than optimal] dose titration and variability in UFC levels,” said Dr. Nieman.
This test of metyrapone’s efficacy comes a year after the FDA approved osilodrostat (Isturisa) for treating Cushing’s disease (but not Cushing’s syndrome). Like metyrapone, osilodrostat controls cortisol overproduction by blocking the enzyme 11-beta-hydroxylase and preventing cortisol synthesis, and osilodrostat was the first agent with these properties to receive an FDA label for therapy.
Osilodrostat “is the only adrenal steroidogenesis inhibitor assessed in randomized controlled long-term trials – over 200 patients with Cushing’s disease – and it has been shown to be highly effective at maintaining normal urinary free cortisol in large majority of patients with Cushing’s disease, as well as Cushing’s syndrome in a study in Japan. Adrenal insufficiency was high [with osilodrostat], especially with the high dose in the trial with forced uptitration. In my clinical practice I have noticed less adrenal insufficiency, but I use much slower drug titration,” said Dr. Fleseriu.
“I think these drugs [metyrapone and osilodrostat] are relatively equivalent,” Dr. Nieman said during discussion of her report. “One nonmedical judgment will be cost,” she added. “Everyone is looking forward to what the pricing structure will be for the new drugs.”
Dr. Fleseriu noted that “for most patients, surgery is first-line therapy, and rarely medication is an alternative first option, especially for Cushing’s disease. However, medical therapy is essential in the management of patients with Cushing’s syndrome when curative surgery fails, surgery is not feasible, when a patient is awaiting radiation’s effect, and for recurrent cases of Cushing’s syndrome.”
PROMPT was sponsored by HRA Pharma, the company that markets metyrapone. Dr. Nieman had no disclosures, but several of her associates on the study are HRA employees. Dr. Fleseriu has been a consultant to Novartis, Recordati, Sparrow, and Strongbridge.
FROM ENDO 2021
Permanent Alopecia in Breast Cancer Patients: Role of Taxanes and Endocrine Therapies
Anagen effluvium during chemotherapy is common, typically beginning within 1 month of treatment onset and resolving by 6 months after the final course.1 Permanent chemotherapy-induced alopecia (PCIA), in which hair loss persists beyond 6 months after chemotherapy without recovery to original density, was first reported in patients following high-dose chemotherapy regimens for allogeneic bone marrow transplantation.2 There are now increasing reports of PCIA in patients with breast cancer; at least 400 such cases have been documented.3-16 In addition to chemotherapy, patients often receive adjuvant endocrine therapy with selective estrogen receptor modulators, aromatase inhibitors, or gonadotropin-releasing hormone agonists.5-16 Endocrine therapies also can lead to alopecia, but their role in PCIA has not been well defined.15,16 We describe 3 patients with breast cancer who experienced PCIA following chemotherapy with taxanes with or without endocrine therapies. We also review the literature on non–bone marrow transplantation PCIA to better characterize this entity and explore the role of endocrine therapies in PCIA.
Case Reports
Patient 1
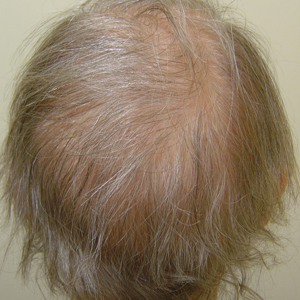
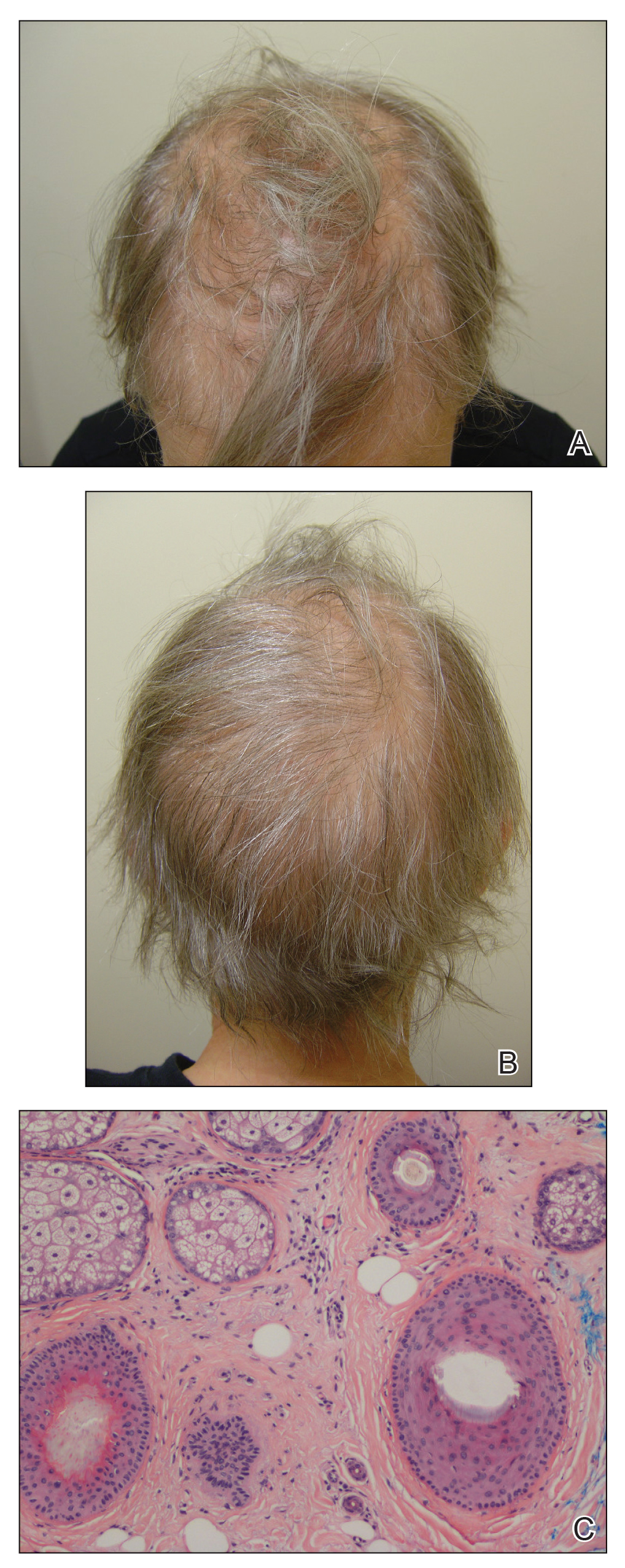
A 62-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 5 years after completing chemotherapy. She underwent 6 cycles of docetaxel and carboplatin along with radiation therapy as well as 1 year of trastuzumab and did not receive endocrine therapy. At the current presentation, she reported patchy hair regrowth that gradually filled in but failed to return to full density. Physical examination revealed the hair was diffusely thin, especially bitemporally (Figures 1A and 1B), and she did not experience any loss of body hair. She had no family history of hair loss. Her medical history was notable for hypertension, chronic obstructive bronchitis, osteopenia, and depression. Her thyroid stimulating hormone (TSH) level was within reference range. Medications included lisinopril, metoprolol, escitalopram, and trazodone. A biopsy from the occipital scalp showed nonscarring alopecia with variation of hair follicle size, a decreased number of hair follicles, and a decreased anagen to telogen ratio (Figure 1C). She was treated with clobetasol solution and minoxidil solution 5% for 1 year with mild improvement. She experienced no further hair loss but did not regain original hair density.
Patient 2
A 35-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 10 months after chemotherapy. She underwent 4 cycles of doxorubicin and cyclophosphamide followed by 4 cycles of paclitaxel and was started on trastuzumab. Tamoxifen was initiated 1 month after completing chemotherapy. She received radiation therapy the following month and continued trastuzumab for 1 year. At the current presentation, the patient noted that hair regrowth had started 1 month after the last course of chemotherapy but had progressed slowly. She denied body hair loss. Physical examination revealed diffuse thinning, especially over the crown, with scattered broken hairs throughout the scalp and several miniaturized hairs over the crown. She was evaluated as grade 3 on the Sinclair clinical grading scale used to evaluate female pattern hair loss (FPHL).17 Her family history was remarkable for FPHL in her maternal grandmother. She had no notable medical history, her TSH was normal, and she was taking tamoxifen and trastuzumab. Biopsy was not performed. The patient was started on minoxidil solution 2% and had mild improvement with no further broken-off hairs after 10 months. At that point, she was evaluated as grade 2 to 3 on the Sinclair scale.17
Patient 3
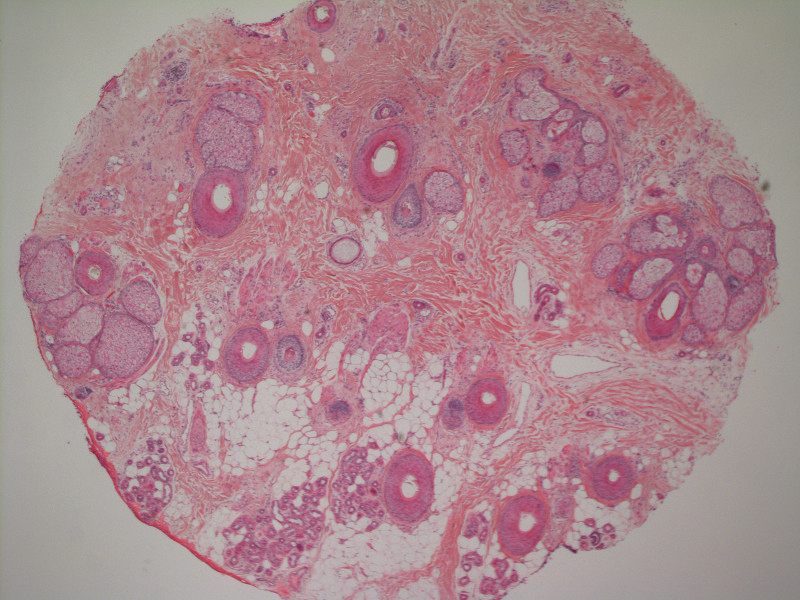
A 51-year-old woman with a history of papillary carcinoma and extensive ductal carcinoma in situ presented with persistent hair loss for 3.5 years following chemotherapy for recurrent breast cancer. After her initial diagnosis in the left breast, she received cyclophosphamide, methotrexate, and 5-fluorouracil but did not receive endocrine therapy. Her hair thinned during chemotherapy but returned to normal density within 1 year. She had a recurrence of the cancer in the right breast 14 years later and received 6 cycles of chemotherapy with cyclophosphamide and docetaxel followed by radiation therapy. After this course, her hair loss incompletely recovered. One year after chemotherapy, she underwent bilateral salpingo-oophorectomy and started anastrozole. Three months later, she noticed increased shedding and progressive thinning of the hair. Physical examination revealed diffuse thinning that was most pronounced over the crown. She also experienced lateral thinning of the eyebrows, decreased eyelashes, and dystrophic fingernails. Fluocinonide solution was discontinued by the patient due to scalp burning. She had a brother with bitemporal recession. Her medical history was notable for Hashimoto thyroiditis, vitamin D deficiency, and peripheral neuropathy. Her TSH occasionally was elevated, and she was intermittently on levothyroxine; however, her free T4 was maintained within reference range on all records. Her medications at the time of evaluation were anastrozole and gabapentin. Biopsies taken from the right and left temporal scalp revealed decreased follicle density with a majority of follicles in anagen, scattered miniaturized follicles, and a mild perivascular and perifollicular lymphoid infiltrate. Mild dermal fibrosis was present without evidence of frank scarring (Figure 2). She declined treatment, and there was no change in her condition over 3 years of follow-up.
Comment
Classification of Chemotherapy-Induced Hair Loss
Chemotherapy-induced alopecia is typically an anagen effluvium that is reversed within 6 months following the final course of chemotherapy. When incomplete regrowth persists, the patient is considered to have PCIA.1 The pathophysiology of PCIA is unclear.
Traditional grading for chemotherapy-induced alopecia does not account for the patterns of loss seen in PCIA, of which the most common appears to be a female pattern with accentuated hair loss in androgen-dependent regions of the scalp.18 Other patterns include a diffuse type with body hair loss, patchy alopecia, and complete alopecia with or without body hair loss (Table).3-8 Whether these patterns all can be attributed to chemotherapy remains to be explored.
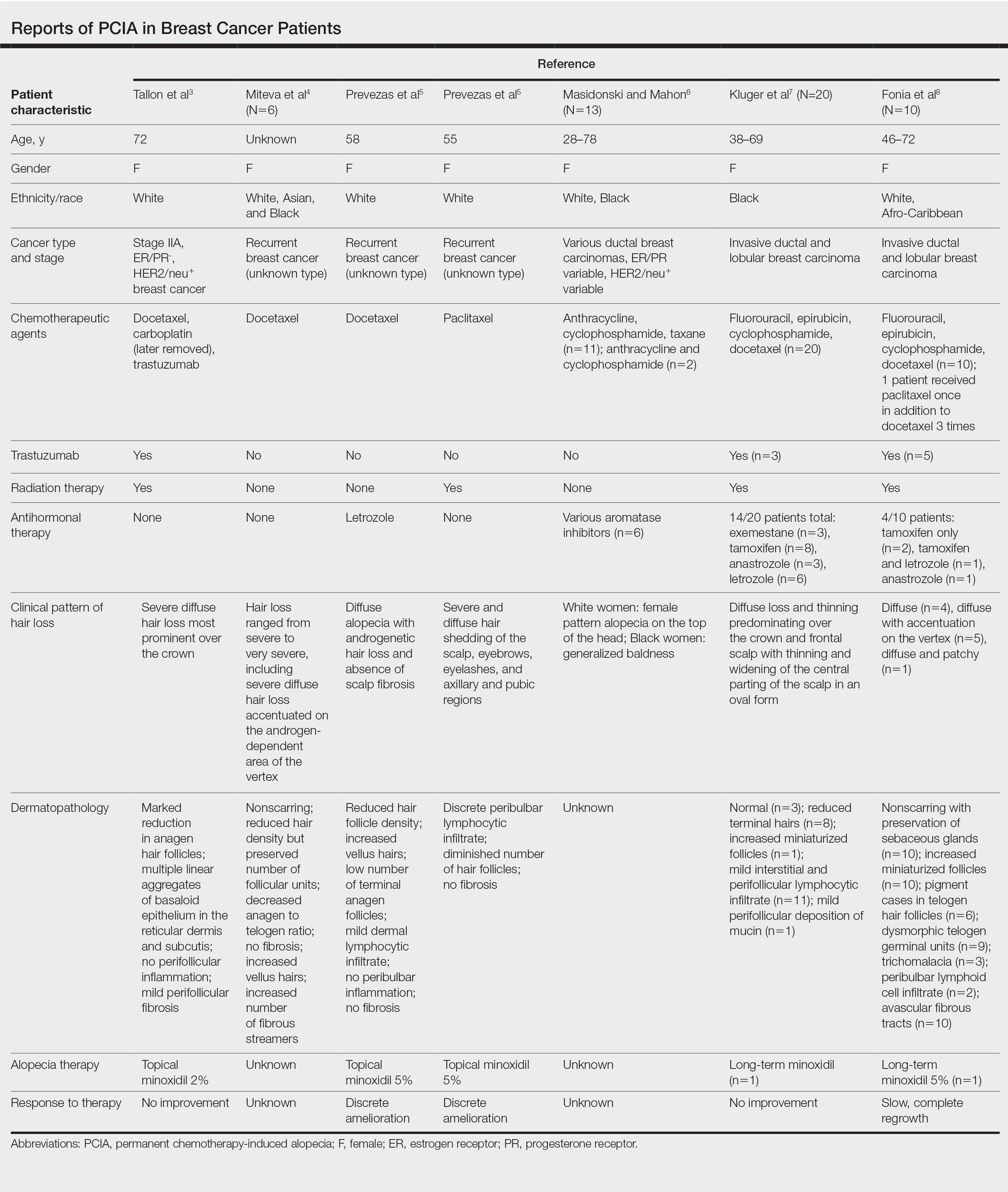
Breast Cancer Therapies Causing PCIA
The main agents thought to be responsible for PCIA in breast cancer patients are taxanes. The role of endocrine therapies has not been well explored. Trastuzumab lacks several of the common side effects of chemotherapy due to its specificity for the HER2/neu receptor and has not been found to increase the rate of hair loss when combined with standard chemotherapy.19,20 Although radiation therapy has the potential to damage hair follicles, and a dose-dependent relationship has been described for temporary and permanent alopecia at irradiated sites, permanent alopecia predominantly has been reported with cranial radiation used in the treatment of intracranial malignancies.21 The role of radiation therapy of the breasts in PCIA is unclear, as its inclusion in therapy has not been consistently reported in the literature.
Docetaxel is known to cause chemotherapy-induced alopecia, with an 83.4% incidence in phase 2 trials; however, it also appears to be related to PCIA.20 A PubMed search of articles indexed for MEDLINE was performed using the terms permanent chemotherapy induced alopecia, chemotherapy, docetaxel, endocrine therapies, hair loss, alopecia, and breast cancer. More than 400 cases of PCIA related to chemotherapy in breast cancer patients have been reported in the literature from a combination of case reports/series, retrospective surveys, and at least one prospective study. Data from some of the more detailed reports (n=52) are summarized in the Table. In the single-center, 3-year prospective study of women given adjuvant taxane-based or non–taxane-based chemotherapy, those who received taxane therapy were more likely to develop PCIA (odds ratio, 8.01).9
All 3 of our patients received taxanes. Interestingly, patient 3 underwent 2 rounds of chemotherapy 14 years apart and experienced full regrowth of the hair after the first course of taxane-free chemotherapy but experienced persistent hair loss following docetaxel treatment. Adjuvant endocrine therapies also may contribute to PCIA. A review of the side effects of endocrine therapies revealed an incidence of alopecia that was higher than expected; tamoxifen was the greatest offender. Additionally, using endocrine treatments in combination was found to have a synergistic effect on alopecia.18 Adjuvant endocrine therapy was used in patients 2 and 3. Although endocrine therapies appear to have a milder effect on hair loss compared to chemotherapy, these medications are continued for a longer duration, potentially contributing to the severity of hair loss and prolonging the time to regrowth.
Furthermore, endocrine therapies used in breast cancer treatment decrease estrogen levels or antagonize estrogen receptors, creating an environment of relative hyperandrogenism that may contribute to FPHL in genetically susceptible women.18 Although taxanes may cause irreversible hair loss in these patients, the action of endocrine therapies on the remaining hair follicles may affect the typical female pattern seen clinically. Patients 2 and 3 who presented with FPHL received adjuvant endocrine therapies and had positive family history, while patient 1 did not. Of note, patient 3 experienced worsening hair loss following the addition of anastrozole, which suggests a contribution of endocrine therapy to her PCIA. Our limited cases do not allow for evaluation of a worsened outcome with the combination of taxanes and endocrine therapies; however, we suggest further evaluation for a synergistic effect that may be contributing to PCIA.
Conclusion
Permanent alopecia in breast cancer patients appears to be a true potential adverse effect of taxanes and endocrine therapies, and it is important to characterize it appropriately so that its mechanism can be understood and appropriate treatment and counseling can take place. Although it may not influence clinical decision-making, patients should be informed that hair loss with chemotherapy can be permanent. Treatment with scalp cooling can reduce the risk for severe chemotherapy-induced alopecia, but it is unclear if it reduces risk for PCIA.12,15 Topical or oral minoxidil may be helpful in the treatment of PCIA once it has developed.7,8,15,22 Better characterization of these cases may elucidate risk factors for developing permanent alopecia, allowing for more appropriate risk stratification, counseling, and treatment.
- Dorr VJ. A practitioner’s guide to cancer-related alopecia. Semin Oncol. 1998;25:562-570.
- Machado M, Moreb JS, Khan SA. Six cases of permanent alopecia after various conditioning regimens commonly used in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40:979-982.
- Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol. 2010;63:333-336.
- Miteva M, Misciali C, Fanti PA, et al. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. Am J Dermatopathol. 2011;33:345-350.
- Prevezas C, Matard B, Pinquier L, et al. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br J Dermatol. 2009;160:883-885.
- Masidonski P, Mahon SM. Permanent alopecia in women being treated for breast cancer. Clin J Oncol Nurs. 2009;13:13-14.
- Kluger N, Jacot W, Frouin E, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol. 2012;23:2879-2884.
- Fonia A, Cota C, Setterfield JF, et al. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: clinicopathologic findings in a cohort of 10 patients. J Am Acad Dermatol. 2017;76:948-957.
- Kang D, Kim IR, Choi EK, et al. Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study [published online August 17, 2018]. Oncologist. 2019;24:414-420.
- Chan J, Adderley H, Alameddine M, et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: a retrospective survey at two tertiary UK cancer centres [published online December 22, 2020]. Eur J Cancer Care (Engl). doi:10.1111/ecc.13395
- Bourgeois H, Denis F, Kerbrat P, et al. Long term persistent alopecia and suboptimal hair regrowth after adjuvant chemotherapy for breast cancer: alert for an emerging side effect: ALOPERS Observatory. Cancer Res. 2009;69(24 suppl). doi:10.1158/0008-5472.SABCS-09-3174
- Bertrand M, Mailliez A, Vercambre S, et al. Permanent chemotherapy induced alopecia in early breast cancer patients after (neo)adjuvant chemotherapy: long term follow up. Cancer Res. 2013;73(24 suppl). doi:10.1158/0008-5472.SABCS13-P3-09-15
- Kim S, Park HS, Kim JY, et al. Irreversible chemotherapy-induced alopecia in breast cancer patient. Cancer Res. 2016;76(4 suppl). doi:10.1158/1538-7445.SABCS15-P1-15-04
- Thorp NJ, Swift F, Arundell D, et al. Long term hair loss in patients with early breast cancer receiving docetaxel chemotherapy. Cancer Res. 2015;75(9 suppl). doi:10.1158/1538-7445.SABCS14-P5-17-04
- Freites-Martinez A, Shapiro J, van den Hurk C, et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80:1199-1213.
- Freites-Martinez A, Chan D, Sibaud V, et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol. 2019;155:724-728.
- Sinclair R, Jolley D, Mallari R, et al. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51:189-199.
- Saggar V, Wu S, Dickler MN, et al. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18:1126-1134.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Baselga J. Clinical trials of single-agent trastuzumab (Herceptin). Semin Oncol. 2000;27(5 suppl 9):20-26.
- Lawenda BD, Gagne HM, Gierga DP, et al. Permanent alopecia after cranial irradiation: dose-response relationship. Int J Radiat Oncol Biol Phys. 2004;60:879-887.
- Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil [published online May 13, 2015]. Australas J Dermatol. 2016;57:E130-E132.
Anagen effluvium during chemotherapy is common, typically beginning within 1 month of treatment onset and resolving by 6 months after the final course.1 Permanent chemotherapy-induced alopecia (PCIA), in which hair loss persists beyond 6 months after chemotherapy without recovery to original density, was first reported in patients following high-dose chemotherapy regimens for allogeneic bone marrow transplantation.2 There are now increasing reports of PCIA in patients with breast cancer; at least 400 such cases have been documented.3-16 In addition to chemotherapy, patients often receive adjuvant endocrine therapy with selective estrogen receptor modulators, aromatase inhibitors, or gonadotropin-releasing hormone agonists.5-16 Endocrine therapies also can lead to alopecia, but their role in PCIA has not been well defined.15,16 We describe 3 patients with breast cancer who experienced PCIA following chemotherapy with taxanes with or without endocrine therapies. We also review the literature on non–bone marrow transplantation PCIA to better characterize this entity and explore the role of endocrine therapies in PCIA.
Case Reports
Patient 1
A 62-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 5 years after completing chemotherapy. She underwent 6 cycles of docetaxel and carboplatin along with radiation therapy as well as 1 year of trastuzumab and did not receive endocrine therapy. At the current presentation, she reported patchy hair regrowth that gradually filled in but failed to return to full density. Physical examination revealed the hair was diffusely thin, especially bitemporally (Figures 1A and 1B), and she did not experience any loss of body hair. She had no family history of hair loss. Her medical history was notable for hypertension, chronic obstructive bronchitis, osteopenia, and depression. Her thyroid stimulating hormone (TSH) level was within reference range. Medications included lisinopril, metoprolol, escitalopram, and trazodone. A biopsy from the occipital scalp showed nonscarring alopecia with variation of hair follicle size, a decreased number of hair follicles, and a decreased anagen to telogen ratio (Figure 1C). She was treated with clobetasol solution and minoxidil solution 5% for 1 year with mild improvement. She experienced no further hair loss but did not regain original hair density.

Patient 2
A 35-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 10 months after chemotherapy. She underwent 4 cycles of doxorubicin and cyclophosphamide followed by 4 cycles of paclitaxel and was started on trastuzumab. Tamoxifen was initiated 1 month after completing chemotherapy. She received radiation therapy the following month and continued trastuzumab for 1 year. At the current presentation, the patient noted that hair regrowth had started 1 month after the last course of chemotherapy but had progressed slowly. She denied body hair loss. Physical examination revealed diffuse thinning, especially over the crown, with scattered broken hairs throughout the scalp and several miniaturized hairs over the crown. She was evaluated as grade 3 on the Sinclair clinical grading scale used to evaluate female pattern hair loss (FPHL).17 Her family history was remarkable for FPHL in her maternal grandmother. She had no notable medical history, her TSH was normal, and she was taking tamoxifen and trastuzumab. Biopsy was not performed. The patient was started on minoxidil solution 2% and had mild improvement with no further broken-off hairs after 10 months. At that point, she was evaluated as grade 2 to 3 on the Sinclair scale.17
Patient 3
A 51-year-old woman with a history of papillary carcinoma and extensive ductal carcinoma in situ presented with persistent hair loss for 3.5 years following chemotherapy for recurrent breast cancer. After her initial diagnosis in the left breast, she received cyclophosphamide, methotrexate, and 5-fluorouracil but did not receive endocrine therapy. Her hair thinned during chemotherapy but returned to normal density within 1 year. She had a recurrence of the cancer in the right breast 14 years later and received 6 cycles of chemotherapy with cyclophosphamide and docetaxel followed by radiation therapy. After this course, her hair loss incompletely recovered. One year after chemotherapy, she underwent bilateral salpingo-oophorectomy and started anastrozole. Three months later, she noticed increased shedding and progressive thinning of the hair. Physical examination revealed diffuse thinning that was most pronounced over the crown. She also experienced lateral thinning of the eyebrows, decreased eyelashes, and dystrophic fingernails. Fluocinonide solution was discontinued by the patient due to scalp burning. She had a brother with bitemporal recession. Her medical history was notable for Hashimoto thyroiditis, vitamin D deficiency, and peripheral neuropathy. Her TSH occasionally was elevated, and she was intermittently on levothyroxine; however, her free T4 was maintained within reference range on all records. Her medications at the time of evaluation were anastrozole and gabapentin. Biopsies taken from the right and left temporal scalp revealed decreased follicle density with a majority of follicles in anagen, scattered miniaturized follicles, and a mild perivascular and perifollicular lymphoid infiltrate. Mild dermal fibrosis was present without evidence of frank scarring (Figure 2). She declined treatment, and there was no change in her condition over 3 years of follow-up.

Comment
Classification of Chemotherapy-Induced Hair Loss
Chemotherapy-induced alopecia is typically an anagen effluvium that is reversed within 6 months following the final course of chemotherapy. When incomplete regrowth persists, the patient is considered to have PCIA.1 The pathophysiology of PCIA is unclear.
Traditional grading for chemotherapy-induced alopecia does not account for the patterns of loss seen in PCIA, of which the most common appears to be a female pattern with accentuated hair loss in androgen-dependent regions of the scalp.18 Other patterns include a diffuse type with body hair loss, patchy alopecia, and complete alopecia with or without body hair loss (Table).3-8 Whether these patterns all can be attributed to chemotherapy remains to be explored.

Breast Cancer Therapies Causing PCIA
The main agents thought to be responsible for PCIA in breast cancer patients are taxanes. The role of endocrine therapies has not been well explored. Trastuzumab lacks several of the common side effects of chemotherapy due to its specificity for the HER2/neu receptor and has not been found to increase the rate of hair loss when combined with standard chemotherapy.19,20 Although radiation therapy has the potential to damage hair follicles, and a dose-dependent relationship has been described for temporary and permanent alopecia at irradiated sites, permanent alopecia predominantly has been reported with cranial radiation used in the treatment of intracranial malignancies.21 The role of radiation therapy of the breasts in PCIA is unclear, as its inclusion in therapy has not been consistently reported in the literature.
Docetaxel is known to cause chemotherapy-induced alopecia, with an 83.4% incidence in phase 2 trials; however, it also appears to be related to PCIA.20 A PubMed search of articles indexed for MEDLINE was performed using the terms permanent chemotherapy induced alopecia, chemotherapy, docetaxel, endocrine therapies, hair loss, alopecia, and breast cancer. More than 400 cases of PCIA related to chemotherapy in breast cancer patients have been reported in the literature from a combination of case reports/series, retrospective surveys, and at least one prospective study. Data from some of the more detailed reports (n=52) are summarized in the Table. In the single-center, 3-year prospective study of women given adjuvant taxane-based or non–taxane-based chemotherapy, those who received taxane therapy were more likely to develop PCIA (odds ratio, 8.01).9
All 3 of our patients received taxanes. Interestingly, patient 3 underwent 2 rounds of chemotherapy 14 years apart and experienced full regrowth of the hair after the first course of taxane-free chemotherapy but experienced persistent hair loss following docetaxel treatment. Adjuvant endocrine therapies also may contribute to PCIA. A review of the side effects of endocrine therapies revealed an incidence of alopecia that was higher than expected; tamoxifen was the greatest offender. Additionally, using endocrine treatments in combination was found to have a synergistic effect on alopecia.18 Adjuvant endocrine therapy was used in patients 2 and 3. Although endocrine therapies appear to have a milder effect on hair loss compared to chemotherapy, these medications are continued for a longer duration, potentially contributing to the severity of hair loss and prolonging the time to regrowth.
Furthermore, endocrine therapies used in breast cancer treatment decrease estrogen levels or antagonize estrogen receptors, creating an environment of relative hyperandrogenism that may contribute to FPHL in genetically susceptible women.18 Although taxanes may cause irreversible hair loss in these patients, the action of endocrine therapies on the remaining hair follicles may affect the typical female pattern seen clinically. Patients 2 and 3 who presented with FPHL received adjuvant endocrine therapies and had positive family history, while patient 1 did not. Of note, patient 3 experienced worsening hair loss following the addition of anastrozole, which suggests a contribution of endocrine therapy to her PCIA. Our limited cases do not allow for evaluation of a worsened outcome with the combination of taxanes and endocrine therapies; however, we suggest further evaluation for a synergistic effect that may be contributing to PCIA.
Conclusion
Permanent alopecia in breast cancer patients appears to be a true potential adverse effect of taxanes and endocrine therapies, and it is important to characterize it appropriately so that its mechanism can be understood and appropriate treatment and counseling can take place. Although it may not influence clinical decision-making, patients should be informed that hair loss with chemotherapy can be permanent. Treatment with scalp cooling can reduce the risk for severe chemotherapy-induced alopecia, but it is unclear if it reduces risk for PCIA.12,15 Topical or oral minoxidil may be helpful in the treatment of PCIA once it has developed.7,8,15,22 Better characterization of these cases may elucidate risk factors for developing permanent alopecia, allowing for more appropriate risk stratification, counseling, and treatment.
Anagen effluvium during chemotherapy is common, typically beginning within 1 month of treatment onset and resolving by 6 months after the final course.1 Permanent chemotherapy-induced alopecia (PCIA), in which hair loss persists beyond 6 months after chemotherapy without recovery to original density, was first reported in patients following high-dose chemotherapy regimens for allogeneic bone marrow transplantation.2 There are now increasing reports of PCIA in patients with breast cancer; at least 400 such cases have been documented.3-16 In addition to chemotherapy, patients often receive adjuvant endocrine therapy with selective estrogen receptor modulators, aromatase inhibitors, or gonadotropin-releasing hormone agonists.5-16 Endocrine therapies also can lead to alopecia, but their role in PCIA has not been well defined.15,16 We describe 3 patients with breast cancer who experienced PCIA following chemotherapy with taxanes with or without endocrine therapies. We also review the literature on non–bone marrow transplantation PCIA to better characterize this entity and explore the role of endocrine therapies in PCIA.
Case Reports
Patient 1
A 62-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 5 years after completing chemotherapy. She underwent 6 cycles of docetaxel and carboplatin along with radiation therapy as well as 1 year of trastuzumab and did not receive endocrine therapy. At the current presentation, she reported patchy hair regrowth that gradually filled in but failed to return to full density. Physical examination revealed the hair was diffusely thin, especially bitemporally (Figures 1A and 1B), and she did not experience any loss of body hair. She had no family history of hair loss. Her medical history was notable for hypertension, chronic obstructive bronchitis, osteopenia, and depression. Her thyroid stimulating hormone (TSH) level was within reference range. Medications included lisinopril, metoprolol, escitalopram, and trazodone. A biopsy from the occipital scalp showed nonscarring alopecia with variation of hair follicle size, a decreased number of hair follicles, and a decreased anagen to telogen ratio (Figure 1C). She was treated with clobetasol solution and minoxidil solution 5% for 1 year with mild improvement. She experienced no further hair loss but did not regain original hair density.

Patient 2
A 35-year-old woman with a history of stage II invasive ductal carcinoma presented with persistent hair loss 10 months after chemotherapy. She underwent 4 cycles of doxorubicin and cyclophosphamide followed by 4 cycles of paclitaxel and was started on trastuzumab. Tamoxifen was initiated 1 month after completing chemotherapy. She received radiation therapy the following month and continued trastuzumab for 1 year. At the current presentation, the patient noted that hair regrowth had started 1 month after the last course of chemotherapy but had progressed slowly. She denied body hair loss. Physical examination revealed diffuse thinning, especially over the crown, with scattered broken hairs throughout the scalp and several miniaturized hairs over the crown. She was evaluated as grade 3 on the Sinclair clinical grading scale used to evaluate female pattern hair loss (FPHL).17 Her family history was remarkable for FPHL in her maternal grandmother. She had no notable medical history, her TSH was normal, and she was taking tamoxifen and trastuzumab. Biopsy was not performed. The patient was started on minoxidil solution 2% and had mild improvement with no further broken-off hairs after 10 months. At that point, she was evaluated as grade 2 to 3 on the Sinclair scale.17
Patient 3
A 51-year-old woman with a history of papillary carcinoma and extensive ductal carcinoma in situ presented with persistent hair loss for 3.5 years following chemotherapy for recurrent breast cancer. After her initial diagnosis in the left breast, she received cyclophosphamide, methotrexate, and 5-fluorouracil but did not receive endocrine therapy. Her hair thinned during chemotherapy but returned to normal density within 1 year. She had a recurrence of the cancer in the right breast 14 years later and received 6 cycles of chemotherapy with cyclophosphamide and docetaxel followed by radiation therapy. After this course, her hair loss incompletely recovered. One year after chemotherapy, she underwent bilateral salpingo-oophorectomy and started anastrozole. Three months later, she noticed increased shedding and progressive thinning of the hair. Physical examination revealed diffuse thinning that was most pronounced over the crown. She also experienced lateral thinning of the eyebrows, decreased eyelashes, and dystrophic fingernails. Fluocinonide solution was discontinued by the patient due to scalp burning. She had a brother with bitemporal recession. Her medical history was notable for Hashimoto thyroiditis, vitamin D deficiency, and peripheral neuropathy. Her TSH occasionally was elevated, and she was intermittently on levothyroxine; however, her free T4 was maintained within reference range on all records. Her medications at the time of evaluation were anastrozole and gabapentin. Biopsies taken from the right and left temporal scalp revealed decreased follicle density with a majority of follicles in anagen, scattered miniaturized follicles, and a mild perivascular and perifollicular lymphoid infiltrate. Mild dermal fibrosis was present without evidence of frank scarring (Figure 2). She declined treatment, and there was no change in her condition over 3 years of follow-up.

Comment
Classification of Chemotherapy-Induced Hair Loss
Chemotherapy-induced alopecia is typically an anagen effluvium that is reversed within 6 months following the final course of chemotherapy. When incomplete regrowth persists, the patient is considered to have PCIA.1 The pathophysiology of PCIA is unclear.
Traditional grading for chemotherapy-induced alopecia does not account for the patterns of loss seen in PCIA, of which the most common appears to be a female pattern with accentuated hair loss in androgen-dependent regions of the scalp.18 Other patterns include a diffuse type with body hair loss, patchy alopecia, and complete alopecia with or without body hair loss (Table).3-8 Whether these patterns all can be attributed to chemotherapy remains to be explored.

Breast Cancer Therapies Causing PCIA
The main agents thought to be responsible for PCIA in breast cancer patients are taxanes. The role of endocrine therapies has not been well explored. Trastuzumab lacks several of the common side effects of chemotherapy due to its specificity for the HER2/neu receptor and has not been found to increase the rate of hair loss when combined with standard chemotherapy.19,20 Although radiation therapy has the potential to damage hair follicles, and a dose-dependent relationship has been described for temporary and permanent alopecia at irradiated sites, permanent alopecia predominantly has been reported with cranial radiation used in the treatment of intracranial malignancies.21 The role of radiation therapy of the breasts in PCIA is unclear, as its inclusion in therapy has not been consistently reported in the literature.
Docetaxel is known to cause chemotherapy-induced alopecia, with an 83.4% incidence in phase 2 trials; however, it also appears to be related to PCIA.20 A PubMed search of articles indexed for MEDLINE was performed using the terms permanent chemotherapy induced alopecia, chemotherapy, docetaxel, endocrine therapies, hair loss, alopecia, and breast cancer. More than 400 cases of PCIA related to chemotherapy in breast cancer patients have been reported in the literature from a combination of case reports/series, retrospective surveys, and at least one prospective study. Data from some of the more detailed reports (n=52) are summarized in the Table. In the single-center, 3-year prospective study of women given adjuvant taxane-based or non–taxane-based chemotherapy, those who received taxane therapy were more likely to develop PCIA (odds ratio, 8.01).9
All 3 of our patients received taxanes. Interestingly, patient 3 underwent 2 rounds of chemotherapy 14 years apart and experienced full regrowth of the hair after the first course of taxane-free chemotherapy but experienced persistent hair loss following docetaxel treatment. Adjuvant endocrine therapies also may contribute to PCIA. A review of the side effects of endocrine therapies revealed an incidence of alopecia that was higher than expected; tamoxifen was the greatest offender. Additionally, using endocrine treatments in combination was found to have a synergistic effect on alopecia.18 Adjuvant endocrine therapy was used in patients 2 and 3. Although endocrine therapies appear to have a milder effect on hair loss compared to chemotherapy, these medications are continued for a longer duration, potentially contributing to the severity of hair loss and prolonging the time to regrowth.
Furthermore, endocrine therapies used in breast cancer treatment decrease estrogen levels or antagonize estrogen receptors, creating an environment of relative hyperandrogenism that may contribute to FPHL in genetically susceptible women.18 Although taxanes may cause irreversible hair loss in these patients, the action of endocrine therapies on the remaining hair follicles may affect the typical female pattern seen clinically. Patients 2 and 3 who presented with FPHL received adjuvant endocrine therapies and had positive family history, while patient 1 did not. Of note, patient 3 experienced worsening hair loss following the addition of anastrozole, which suggests a contribution of endocrine therapy to her PCIA. Our limited cases do not allow for evaluation of a worsened outcome with the combination of taxanes and endocrine therapies; however, we suggest further evaluation for a synergistic effect that may be contributing to PCIA.
Conclusion
Permanent alopecia in breast cancer patients appears to be a true potential adverse effect of taxanes and endocrine therapies, and it is important to characterize it appropriately so that its mechanism can be understood and appropriate treatment and counseling can take place. Although it may not influence clinical decision-making, patients should be informed that hair loss with chemotherapy can be permanent. Treatment with scalp cooling can reduce the risk for severe chemotherapy-induced alopecia, but it is unclear if it reduces risk for PCIA.12,15 Topical or oral minoxidil may be helpful in the treatment of PCIA once it has developed.7,8,15,22 Better characterization of these cases may elucidate risk factors for developing permanent alopecia, allowing for more appropriate risk stratification, counseling, and treatment.
- Dorr VJ. A practitioner’s guide to cancer-related alopecia. Semin Oncol. 1998;25:562-570.
- Machado M, Moreb JS, Khan SA. Six cases of permanent alopecia after various conditioning regimens commonly used in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40:979-982.
- Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol. 2010;63:333-336.
- Miteva M, Misciali C, Fanti PA, et al. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. Am J Dermatopathol. 2011;33:345-350.
- Prevezas C, Matard B, Pinquier L, et al. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br J Dermatol. 2009;160:883-885.
- Masidonski P, Mahon SM. Permanent alopecia in women being treated for breast cancer. Clin J Oncol Nurs. 2009;13:13-14.
- Kluger N, Jacot W, Frouin E, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol. 2012;23:2879-2884.
- Fonia A, Cota C, Setterfield JF, et al. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: clinicopathologic findings in a cohort of 10 patients. J Am Acad Dermatol. 2017;76:948-957.
- Kang D, Kim IR, Choi EK, et al. Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study [published online August 17, 2018]. Oncologist. 2019;24:414-420.
- Chan J, Adderley H, Alameddine M, et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: a retrospective survey at two tertiary UK cancer centres [published online December 22, 2020]. Eur J Cancer Care (Engl). doi:10.1111/ecc.13395
- Bourgeois H, Denis F, Kerbrat P, et al. Long term persistent alopecia and suboptimal hair regrowth after adjuvant chemotherapy for breast cancer: alert for an emerging side effect: ALOPERS Observatory. Cancer Res. 2009;69(24 suppl). doi:10.1158/0008-5472.SABCS-09-3174
- Bertrand M, Mailliez A, Vercambre S, et al. Permanent chemotherapy induced alopecia in early breast cancer patients after (neo)adjuvant chemotherapy: long term follow up. Cancer Res. 2013;73(24 suppl). doi:10.1158/0008-5472.SABCS13-P3-09-15
- Kim S, Park HS, Kim JY, et al. Irreversible chemotherapy-induced alopecia in breast cancer patient. Cancer Res. 2016;76(4 suppl). doi:10.1158/1538-7445.SABCS15-P1-15-04
- Thorp NJ, Swift F, Arundell D, et al. Long term hair loss in patients with early breast cancer receiving docetaxel chemotherapy. Cancer Res. 2015;75(9 suppl). doi:10.1158/1538-7445.SABCS14-P5-17-04
- Freites-Martinez A, Shapiro J, van den Hurk C, et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80:1199-1213.
- Freites-Martinez A, Chan D, Sibaud V, et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol. 2019;155:724-728.
- Sinclair R, Jolley D, Mallari R, et al. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51:189-199.
- Saggar V, Wu S, Dickler MN, et al. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18:1126-1134.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Baselga J. Clinical trials of single-agent trastuzumab (Herceptin). Semin Oncol. 2000;27(5 suppl 9):20-26.
- Lawenda BD, Gagne HM, Gierga DP, et al. Permanent alopecia after cranial irradiation: dose-response relationship. Int J Radiat Oncol Biol Phys. 2004;60:879-887.
- Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil [published online May 13, 2015]. Australas J Dermatol. 2016;57:E130-E132.
- Dorr VJ. A practitioner’s guide to cancer-related alopecia. Semin Oncol. 1998;25:562-570.
- Machado M, Moreb JS, Khan SA. Six cases of permanent alopecia after various conditioning regimens commonly used in hematopoietic stem cell transplantation. Bone Marrow Transplant. 2007;40:979-982.
- Tallon B, Blanchard E, Goldberg LJ. Permanent chemotherapy-induced alopecia: case report and review of the literature. J Am Acad Dermatol. 2010;63:333-336.
- Miteva M, Misciali C, Fanti PA, et al. Permanent alopecia after systemic chemotherapy: a clinicopathological study of 10 cases. Am J Dermatopathol. 2011;33:345-350.
- Prevezas C, Matard B, Pinquier L, et al. Irreversible and severe alopecia following docetaxel or paclitaxel cytotoxic therapy for breast cancer. Br J Dermatol. 2009;160:883-885.
- Masidonski P, Mahon SM. Permanent alopecia in women being treated for breast cancer. Clin J Oncol Nurs. 2009;13:13-14.
- Kluger N, Jacot W, Frouin E, et al. Permanent scalp alopecia related to breast cancer chemotherapy by sequential fluorouracil/epirubicin/cyclophosphamide (FEC) and docetaxel: a prospective study of 20 patients. Ann Oncol. 2012;23:2879-2884.
- Fonia A, Cota C, Setterfield JF, et al. Permanent alopecia in patients with breast cancer after taxane chemotherapy and adjuvant hormonal therapy: clinicopathologic findings in a cohort of 10 patients. J Am Acad Dermatol. 2017;76:948-957.
- Kang D, Kim IR, Choi EK, et al. Permanent chemotherapy-induced alopecia in patients with breast cancer: a 3-year prospective cohort study [published online August 17, 2018]. Oncologist. 2019;24:414-420.
- Chan J, Adderley H, Alameddine M, et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: a retrospective survey at two tertiary UK cancer centres [published online December 22, 2020]. Eur J Cancer Care (Engl). doi:10.1111/ecc.13395
- Bourgeois H, Denis F, Kerbrat P, et al. Long term persistent alopecia and suboptimal hair regrowth after adjuvant chemotherapy for breast cancer: alert for an emerging side effect: ALOPERS Observatory. Cancer Res. 2009;69(24 suppl). doi:10.1158/0008-5472.SABCS-09-3174
- Bertrand M, Mailliez A, Vercambre S, et al. Permanent chemotherapy induced alopecia in early breast cancer patients after (neo)adjuvant chemotherapy: long term follow up. Cancer Res. 2013;73(24 suppl). doi:10.1158/0008-5472.SABCS13-P3-09-15
- Kim S, Park HS, Kim JY, et al. Irreversible chemotherapy-induced alopecia in breast cancer patient. Cancer Res. 2016;76(4 suppl). doi:10.1158/1538-7445.SABCS15-P1-15-04
- Thorp NJ, Swift F, Arundell D, et al. Long term hair loss in patients with early breast cancer receiving docetaxel chemotherapy. Cancer Res. 2015;75(9 suppl). doi:10.1158/1538-7445.SABCS14-P5-17-04
- Freites-Martinez A, Shapiro J, van den Hurk C, et al. Hair disorders in cancer survivors. J Am Acad Dermatol. 2019;80:1199-1213.
- Freites-Martinez A, Chan D, Sibaud V, et al. Assessment of quality of life and treatment outcomes of patients with persistent postchemotherapy alopecia. JAMA Dermatol. 2019;155:724-728.
- Sinclair R, Jolley D, Mallari R, et al. The reliability of horizontally sectioned scalp biopsies in the diagnosis of chronic diffuse telogen hair loss in women. J Am Acad Dermatol. 2004;51:189-199.
- Saggar V, Wu S, Dickler MN, et al. Alopecia with endocrine therapies in patients with cancer. Oncologist. 2013;18:1126-1134.
- Yeager CE, Olsen EA. Treatment of chemotherapy-induced alopecia. Dermatol Ther. 2011;24:432-442.
- Baselga J. Clinical trials of single-agent trastuzumab (Herceptin). Semin Oncol. 2000;27(5 suppl 9):20-26.
- Lawenda BD, Gagne HM, Gierga DP, et al. Permanent alopecia after cranial irradiation: dose-response relationship. Int J Radiat Oncol Biol Phys. 2004;60:879-887.
- Yang X, Thai KE. Treatment of permanent chemotherapy-induced alopecia with low dose oral minoxidil [published online May 13, 2015]. Australas J Dermatol. 2016;57:E130-E132.
Practice Points
- Permanent chemotherapy-induced alopecia (PCIA) is defined as hair loss that persists beyond 6 months after treatment with chemotherapy. It may be complicated by the addition of endocrine therapies.
- Patients and clinicians should be aware that PCIA can occur and appears to be a higher risk with taxane therapy.
Wax Stripping and Isotretinoin Treatment: A Warning Not to Be Missed
To the Editor:
Oral isotretinoin is a widely used treatment modality in dermatologic practice that is highly effective for severe and recalcitrant acne vulgaris in addition to other conditions. Its use is accompanied by a variety of side effects that are mainly mucocutaneous. These dose-dependent side effects are experienced by almost all patients treated with this medication.1
A generally healthy 14-year-old adolescent girl presented with severe widespread erosions located in a linear pattern corresponding to areas of wax depilation on the shins and thighs (Figure). Approximately 5 months prior, the patient started oral isotretinoin 40 mg daily for severe and recalcitrant acne vulgaris. She was not taking other medications. After 4 months of treatment, during which the acne lesions improved and the patient experienced only mild xerosis and cheilitis, the dosage was increased to 60 mg daily. Three weeks later, the patient underwent wax depilation, which resulted in the erosions.
Oral isotretinoin treatment leads to structural and functional changes to the skin, related to epidermal dyscohesion and sebo-suppression. Although these changes may not be clinically evident in all patients, they still make the skin much more sensitive to external mechanical stimuli.1 Wax depilation commonly is used for treating excess hair on the body. Because it exerts remarkable mechanical stress on the epidermis, it may lead to epidermal stripping in patients taking isotretinoin, manifesting as widespread erosions and resulting in notable patient distress.
Dermatologists typically advise patients to avoid wax epilation while being treated with isotretinoin; however, some patients do not adhere to this recommendation. Also, there are dermatologists who are not aware of this potential side effect. In one survey (N=54), only 4% of consulting dermatologists were aware of this complication.2 A PubMed search of articles indexed for MEDLINE using the terms isotretinoin and wax revealed that this severe side effect with isotretinoin has been reported only 4 times in the medical literature.2-5 The fact that wax epilation should be avoided during isotretinoin treatment previously was not included in the prescribing information. It currently is included in the isotretinoin prescribing information6 with an indication not to perform wax depilation for 6 months after stopping treatment. This case should serve as a reminder to avoid wax depilation during isotretinoin treatment.
- Del Rosso JQ. Clinical relevance of skin barrier changes associated with the use of oral isotretinoin: the importance of barrier repair therapy in patient management. J Drugs Dermatol. 2013;12:626-631.
Woollons A, Price ML. Roaccutane and wax epilation: a cautionary tale. Br J Dermatol. 1997;137:839-840. - Egido Romo M. Isotretinoin and wax epilation. Br J Dermatol. 1991;124:393.
- Holmes SC, Thomson J. Isotretinoin and skin fragility. Br J Dermatol. 1995;132:165.
- Turel-Ermertcan A, Sahin MT, Yurtman D, et al. Inappropriate treatments at beauty centers: a case report of burns caused by hot wax stripping. J Dermatol. 2004;31:854-855.
- Accutane. Package insert. Roche; 2008.
To the Editor:
Oral isotretinoin is a widely used treatment modality in dermatologic practice that is highly effective for severe and recalcitrant acne vulgaris in addition to other conditions. Its use is accompanied by a variety of side effects that are mainly mucocutaneous. These dose-dependent side effects are experienced by almost all patients treated with this medication.1
A generally healthy 14-year-old adolescent girl presented with severe widespread erosions located in a linear pattern corresponding to areas of wax depilation on the shins and thighs (Figure). Approximately 5 months prior, the patient started oral isotretinoin 40 mg daily for severe and recalcitrant acne vulgaris. She was not taking other medications. After 4 months of treatment, during which the acne lesions improved and the patient experienced only mild xerosis and cheilitis, the dosage was increased to 60 mg daily. Three weeks later, the patient underwent wax depilation, which resulted in the erosions.

Oral isotretinoin treatment leads to structural and functional changes to the skin, related to epidermal dyscohesion and sebo-suppression. Although these changes may not be clinically evident in all patients, they still make the skin much more sensitive to external mechanical stimuli.1 Wax depilation commonly is used for treating excess hair on the body. Because it exerts remarkable mechanical stress on the epidermis, it may lead to epidermal stripping in patients taking isotretinoin, manifesting as widespread erosions and resulting in notable patient distress.
Dermatologists typically advise patients to avoid wax epilation while being treated with isotretinoin; however, some patients do not adhere to this recommendation. Also, there are dermatologists who are not aware of this potential side effect. In one survey (N=54), only 4% of consulting dermatologists were aware of this complication.2 A PubMed search of articles indexed for MEDLINE using the terms isotretinoin and wax revealed that this severe side effect with isotretinoin has been reported only 4 times in the medical literature.2-5 The fact that wax epilation should be avoided during isotretinoin treatment previously was not included in the prescribing information. It currently is included in the isotretinoin prescribing information6 with an indication not to perform wax depilation for 6 months after stopping treatment. This case should serve as a reminder to avoid wax depilation during isotretinoin treatment.
To the Editor:
Oral isotretinoin is a widely used treatment modality in dermatologic practice that is highly effective for severe and recalcitrant acne vulgaris in addition to other conditions. Its use is accompanied by a variety of side effects that are mainly mucocutaneous. These dose-dependent side effects are experienced by almost all patients treated with this medication.1
A generally healthy 14-year-old adolescent girl presented with severe widespread erosions located in a linear pattern corresponding to areas of wax depilation on the shins and thighs (Figure). Approximately 5 months prior, the patient started oral isotretinoin 40 mg daily for severe and recalcitrant acne vulgaris. She was not taking other medications. After 4 months of treatment, during which the acne lesions improved and the patient experienced only mild xerosis and cheilitis, the dosage was increased to 60 mg daily. Three weeks later, the patient underwent wax depilation, which resulted in the erosions.

Oral isotretinoin treatment leads to structural and functional changes to the skin, related to epidermal dyscohesion and sebo-suppression. Although these changes may not be clinically evident in all patients, they still make the skin much more sensitive to external mechanical stimuli.1 Wax depilation commonly is used for treating excess hair on the body. Because it exerts remarkable mechanical stress on the epidermis, it may lead to epidermal stripping in patients taking isotretinoin, manifesting as widespread erosions and resulting in notable patient distress.
Dermatologists typically advise patients to avoid wax epilation while being treated with isotretinoin; however, some patients do not adhere to this recommendation. Also, there are dermatologists who are not aware of this potential side effect. In one survey (N=54), only 4% of consulting dermatologists were aware of this complication.2 A PubMed search of articles indexed for MEDLINE using the terms isotretinoin and wax revealed that this severe side effect with isotretinoin has been reported only 4 times in the medical literature.2-5 The fact that wax epilation should be avoided during isotretinoin treatment previously was not included in the prescribing information. It currently is included in the isotretinoin prescribing information6 with an indication not to perform wax depilation for 6 months after stopping treatment. This case should serve as a reminder to avoid wax depilation during isotretinoin treatment.
- Del Rosso JQ. Clinical relevance of skin barrier changes associated with the use of oral isotretinoin: the importance of barrier repair therapy in patient management. J Drugs Dermatol. 2013;12:626-631.
Woollons A, Price ML. Roaccutane and wax epilation: a cautionary tale. Br J Dermatol. 1997;137:839-840. - Egido Romo M. Isotretinoin and wax epilation. Br J Dermatol. 1991;124:393.
- Holmes SC, Thomson J. Isotretinoin and skin fragility. Br J Dermatol. 1995;132:165.
- Turel-Ermertcan A, Sahin MT, Yurtman D, et al. Inappropriate treatments at beauty centers: a case report of burns caused by hot wax stripping. J Dermatol. 2004;31:854-855.
- Accutane. Package insert. Roche; 2008.
- Del Rosso JQ. Clinical relevance of skin barrier changes associated with the use of oral isotretinoin: the importance of barrier repair therapy in patient management. J Drugs Dermatol. 2013;12:626-631.
Woollons A, Price ML. Roaccutane and wax epilation: a cautionary tale. Br J Dermatol. 1997;137:839-840. - Egido Romo M. Isotretinoin and wax epilation. Br J Dermatol. 1991;124:393.
- Holmes SC, Thomson J. Isotretinoin and skin fragility. Br J Dermatol. 1995;132:165.
- Turel-Ermertcan A, Sahin MT, Yurtman D, et al. Inappropriate treatments at beauty centers: a case report of burns caused by hot wax stripping. J Dermatol. 2004;31:854-855.
- Accutane. Package insert. Roche; 2008.
Practice Points
- Oral isotretinoin treatment leads to structural and functional changes to the skin, making it much more sensitive to external mechanical stimuli.
- Wax depilation may lead to epidermal stripping in patients taking isotretinoin and therefore should be avoided in these patients.
Black nonsmokers still at high risk for secondhand smoke exposure
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.
While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.

While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
Despite 30+ years of antismoking public policies and dramatic overall decline in secondhand smoke (SHS) exposure, .
No risk-free SHS exposure
Surendranath S. Shastri, MD, of MD Anderson Cancer Center, Houston, and colleagues underscored the U.S. Surgeon General’s determination that there is no risk-free level of SHS exposure in a recent JAMA Internal Medicine Research Letter.
“With the outbreak of the coronavirus disease 2019, which affects lung function, improving smoke-free policies to enhance air quality should be a growing priority,”they wrote.
Dr. Shastri and colleagues looked at 2011-2018 data from the National Health and Nutrition Examination Survey (NHANES), which detailed prevalence of SHS exposure in the U.S. population aged 3 years and older using interviews and biological specimens to test for cotinine levels. For the survey, nonsmokers having serum cotinine levels of 0.05 to 10 ng/mL were considered to have SHS exposure.

While the prevalence of SHS exposure among nonsmokers declined from 87.5% to 25.3% between 1988 and 2012, levels have stagnated since 2012 and racial and economic disparities are evident. Higher smoking rates, less knowledge about health risks, higher workplace exposure, greater likelihood of living in low-income, multi-unit housing, plus having their communities targeted by tobacco companies, may all help explain higher serum levels of cotinine in populations with lower socioeconomic status.
“Multivariable logistic regression identified younger age (odds ratio [OR], 1.88, for 12-19 years, and OR, 2.29, for 3-11 years), non-Hispanic Black race/ethnicity (OR, 2.75), less than high school education (OR, 1.59), and living below the poverty level (OR, 2.61) as risk factors for SHSe in the 2017-2018 cycle, with little change across all data cycles,” the researchers wrote.
Disparities in SHS exposure
A second report from NHANES data for 2015-2018, published in a National Center for Health Statistics Data Brief (No. 396, February 2021) showed that 20.8% of nonsmoking U.S. adults had SHS exposure, again with greater prevalence among non-Hispanic Black adults (39.7%), than for non-Hispanic White (18.4%), non-Hispanic Asian (20.9%), and Hispanic (17.2%) adults. Exposure was also greater in the younger age groups, with SHS rates for adults aged 18-39 years, 40-59 years, and ≥60 years at 25.6%, 19.1%, and 17.6%, respectively. Lower education (high school or less vs. some college education) and lower income levels were also associated with higher levels of SHS exposure. The investigators noted that among households with smokers, non-Hispanic Black adults are less likely to have complete smoking bans in homes, and among Medicaid or uninsured parents of any race or ethnicity, bans on smoking in family vehicles are less likely.
Overall, the prevalence of SHS exposure declined from 27.7% to 20.7% from 2009 to 2018, but the decreases were mediated by race and income.
SHS exposure in private spaces
A research brief from the Centers for Disease Control and Prevention on SHS exposure in homes and vehicles in the U.S. among middle and high school students also found a general decline in SHS exposure over 2011-2018 in homes (26.8%-20.9%; P < .001) and vehicles (30.2%-19.8%; P < .001). The findings, derived from the National Youth Tobacco Survey for 2011-2019, showed that no reduction occurred in homes among non-Hispanic Black students. Overall, a significant difference in home SHS exposure was observed by race/ethnicity: non-Hispanic Black (28.4%) and non-Hispanic White (27.4%) students both had a higher prevalence compared with Hispanic (20.0%) and non-Hispanic other (20.2%) students (P < .001).
Progress in reducing SHS exposure in public spaces has been made over the last 2 decades, with 27 states and more than 1,000 municipalities implementing comprehensive smoke-free laws that prohibit smoking in indoor public places, including workplaces, restaurants, and bars. While the prevalence of voluntary smoke-free home (83.7%) and vehicle (78.1%) rules has increased over time, private settings remain major sources of SHS exposure for many people, including youths. “Although SHS exposures have declined,” the authors wrote, “more than 6 million young people remain exposed to SHS in these private settings.”
In reviewing the data, Mary Cataletto, MD, FCCP, clinical professor of pediatrics at NYU Long Island School of Medicine, stated that these studies “highlight the need for implementation of smoke-free policies to reduce exposure to secondhand smoke, especially in homes and cars and with focused advocacy efforts in highly affected communities.”
Panagis Galiatsatos, MD, MHS, assistant professor of medicine at Johns Hopkins University, Baltimore, emphasized implementation of smoke-free policies but also treatment for smokers. “I’m not at all surprised by these statistics,” he noted in an interview. “Public health policies have helped us to get to where we are now, but there’s a reason that we have plateaued over the last decade. It’s hard to mitigate secondhand smoke exposure because the ones who are smoking now are the most refractory, challenging cases. ... You need good clinical interventions with counseling supported by pharmacological agents to help them if you want to stop secondhand smoke exposure.” He added, “You have to look at current smokers no differently than you look at patients with stage IV cancer – a group that requires a lot of resources to help them get through. Remember, all of them want to quit, but the promise of well-designed, precision-medicine strategies to help them quit has not been kept. Public health policy isn’t going to do it. We need to manage these patients clinically.”
The investigators had no conflict disclosures.
PCOS equivalent in men: No ovaries required
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
The concept that there is a male equivalent of polycystic ovary syndrome (PCOS) was first described more than 15 years ago; a new study has further validated the principle using a polygenic risk score.
By demonstrating a high rates of cardiometabolic dysfunction and androgenic conditions in men with a high PCOS risk score, “we have shown that these genetic risk factors can act independently of ovarian function,” reported Jia Zhu, MD, a clinical endocrinology fellow at Boston Children’s Hospital.
The characterization of a male equivalent of PCOS has implications for both men and women, according to Dr. Zhu. For men, better definition of a phenotype has potential to accelerate the recognition and treatment of an inherited metabolic disorder. For women, this direction of study might help to unravel the relationship between the metabolic pathology and symptoms involving the reproductive system.
Affecting up to 10% of women, PCOS is characterized by ovulatory dysfunction and hyperandrogenism commonly associated with insulin resistance, obesity, and elevation in cardiovascular risk factors. Familial clustering implies an important genetic component, but the relationship between metabolic and ovulatory dysfunction remains incompletely understood.
“Both ovarian-related and ovarian-independent factors have been implicated in the pathogenesis of PCOS, but it remains to be determined which are the inciting events and which are the secondary consequences,” Dr. Zhu explained during his presentation of the study at the annual meeting of the Endocrine Society.
Polygenic risk score applied to men
In this study, a polygenic risk score algorithm developed to predict PCOS in women was applied to men. The risk score was developed through genetic testing in 206,851 unrelated women in the UK Biobank. This algorithm was then applied to stratify risk in 176,360 men from the same biobank. For males, several adjustments were made, including those for age and genetic components relevant to ancestry.
When stratified into quintiles, those at highest risk, relative to those at lower risk, had numerically modest but highly significant increased odds ratio for obesity defined by a body mass index (BMI) of at least 30 kg/m2 (OR, 1.17; P < .13 x 10–29) and type 2 diabetes (OR, 1.15; P = .53 x 10–7). Those in the highest risk group were also more likely to have coronary artery disease (HR, 1.05; P = .01) as well as androgenic alopecia (OR, 1.05; P = .03).
When stratified into deciles of risk, a stepwise increase was observed for the prevalence of several cardiovascular risk factors. These included hemoglobin A1c, triglycerides, BMI, and free androgen, reported Dr. Zhu.
The relationship between the risk score and both coronary artery disease and several dyslipidemias appeared to be mediated by BMI, but the relationship between the PCOS polygenic risk score and type 2 diabetes persisted after adjusting for BMI.
For women, the implication of this analysis is that the reproductive dysfunction associated with PCOS might arise in at least some cases “secondarily from the genetically determined disruption of biological pathways common to both men and women,” Dr. Zhu said. She suggested that efforts to dissect these biological pathways might provide a path to under-standing the underlying mechanism of the ovarian complications, such as irregular menstrual periods, infertility, and ovarian cysts.
Family history of PCOS central to male risk
For men, a family history of PCOS might be relevant to predicting increased risk of type 2 diabetes, obesity and cardiovascular disease, Dr. Zhu indicated. In addition, this syndrome is also likely relevant to such signs of hyperandrogenism as hair loss and low testosterone levels in males with the PCOS-equivalent syndrome.
Other investigators have also suggested that male-equivalent PCOS exists and might be clinically relevant. According to Frederica Di Guardio, MD, a gynecologist in the department of medical surgical specialties, University of Catania (Italy), there is enough evidence for a PCOS-equivalent syndrome in men to consider asking males with obesity or other evidence of the metabolic abnormalities about a family history of PCOS.
“These patients have a high risk of developing cardiovascular disease, metabolic syndrome, and carotid atherosclerotic plaques,” she advised on the basis of her own and previous studies. By asking about a family history of PCOS in males, it can raise clinical suspicion and permit early intervention.
Not least important, identifying males at risk can allow them “to adopt a healthy lifestyle, preventing the risk of metabolic and cardiovascular events,” Dr. Di Guardio said.
In a recent review article on the male PCOS syndrome, Dr. Di Guardio traced the male PCOS-equivalent syndrome to a 2004 article. She reported that more than 30 articles have been published subsequently.
There is no formal clinical definition of male equivalent PCOS. According to her review of published studies, Dr. Di Guardio acknowledged that there has been considerable heterogeneity in the prevalence of the associated features, but the unifying factor is the presence of a set of genes associated with PCOS. In men, as well as in women, these appear to drive an increased risk of metabolic abnormalities and cardiovascular disease.
Dr. Zhu and Dr. Di Guardio reported no relevant conflicts of interest.
FROM ENDO 2021
Cytoreduction in advanced ovarian cancer: ‘Keep the status quo’
A retrospective, case-control study showed that optimal cytoreductive surgery is an independent predictor of overall survival, even when controlling for response to chemotherapy.
The findings were presented at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 10243).
Response to platinum-based chemotherapy is the strongest predictor of overall survival in advanced ovarian cancer, noted Nicholas Cardillo, MD, a gynecologic oncology fellow at the University of Iowa, Iowa City, who presented the findings at the meeting.
In recent years, a poor response to chemotherapy has sometimes been used as justification to forgo cytoreduction, Dr. Cardillo added.
He and his colleagues looked into this issue because evidence to support the practice is lacking. With their study, the researchers found that optimal cytoreduction – removing all disease of 1 cm or more – improved survival regardless of the response to chemotherapy.
“My advice right now is that debulking surgery should still be attempted in all patients with ovarian cancer because, as far as we know right now, optimal cytoreduction will improve survival,” Dr. Cardillo said in an interview. “Basically, this study argues to keep the status quo, which is to perform surgery.”
The status quo might change with future research, Dr. Cardillo acknowledged, “but as of right now, we have no evidence to support not pursuing cytoreduction in these patients.”
Study details and results
The researchers analyzed data on 234 patients who responded to platinum-based chemotherapy – meaning they had no evidence of disease for at least 6 months afterward – and 98 patients who did not respond – meaning they progressed during therapy, had stable disease, did not respond completely, or had a progression-free survival duration of less than 6 months. Subjects had stage III or IV high-grade serous ovarian cancer.
About three-quarters of responders and 57% of nonresponders had optimal surgery. Only seven patients in each group had fewer than six cycles of chemotherapy.
The mean age was 59 years in the responder group and 62 years among nonresponders. Stage IV disease, including upper-abdominal and chest involvement, was more common in the nonresponder group.
The median overall survival was 44.8 months in the responder group and 18.1 months among nonresponders (P < .001). The median overall survival was 34.2 months among patients who underwent optimal surgery and 24.8 months among those who did not (P < .001).
Predictors of survival
A multivariate analysis showed that response to chemotherapy had the greatest effect on survival, with a hazard ratio of 0.27 (P < .001).
“The second most significant predictor of overall survival was receipt of neoadjuvant chemotherapy [HR, 2.84; P < .001], which is intuitive as that is typically a marker for worse disease burden,” Dr. Cardillo said.
“But most importantly for our question is that optimal surgery is an independent significant factor in overall survival, even when controlling for other significant risk factors, including whether or not a patient responds to chemotherapy. The hazard ratio is 0.73 [P = .023], indicating a 25%-30% improvement in overall survival,” he added.
Based on these results, “surgical debulking should still be considered a component of the treatment algorithm in ovarian cancer patients who have a poor response to chemotherapy, if an optimal surgery is deemed feasible,” Dr. Cardillo concluded.
There was no funding for this study, and the investigators had no relevant disclosures.
A retrospective, case-control study showed that optimal cytoreductive surgery is an independent predictor of overall survival, even when controlling for response to chemotherapy.
The findings were presented at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 10243).
Response to platinum-based chemotherapy is the strongest predictor of overall survival in advanced ovarian cancer, noted Nicholas Cardillo, MD, a gynecologic oncology fellow at the University of Iowa, Iowa City, who presented the findings at the meeting.
In recent years, a poor response to chemotherapy has sometimes been used as justification to forgo cytoreduction, Dr. Cardillo added.
He and his colleagues looked into this issue because evidence to support the practice is lacking. With their study, the researchers found that optimal cytoreduction – removing all disease of 1 cm or more – improved survival regardless of the response to chemotherapy.
“My advice right now is that debulking surgery should still be attempted in all patients with ovarian cancer because, as far as we know right now, optimal cytoreduction will improve survival,” Dr. Cardillo said in an interview. “Basically, this study argues to keep the status quo, which is to perform surgery.”
The status quo might change with future research, Dr. Cardillo acknowledged, “but as of right now, we have no evidence to support not pursuing cytoreduction in these patients.”
Study details and results
The researchers analyzed data on 234 patients who responded to platinum-based chemotherapy – meaning they had no evidence of disease for at least 6 months afterward – and 98 patients who did not respond – meaning they progressed during therapy, had stable disease, did not respond completely, or had a progression-free survival duration of less than 6 months. Subjects had stage III or IV high-grade serous ovarian cancer.
About three-quarters of responders and 57% of nonresponders had optimal surgery. Only seven patients in each group had fewer than six cycles of chemotherapy.
The mean age was 59 years in the responder group and 62 years among nonresponders. Stage IV disease, including upper-abdominal and chest involvement, was more common in the nonresponder group.
The median overall survival was 44.8 months in the responder group and 18.1 months among nonresponders (P < .001). The median overall survival was 34.2 months among patients who underwent optimal surgery and 24.8 months among those who did not (P < .001).
Predictors of survival
A multivariate analysis showed that response to chemotherapy had the greatest effect on survival, with a hazard ratio of 0.27 (P < .001).
“The second most significant predictor of overall survival was receipt of neoadjuvant chemotherapy [HR, 2.84; P < .001], which is intuitive as that is typically a marker for worse disease burden,” Dr. Cardillo said.
“But most importantly for our question is that optimal surgery is an independent significant factor in overall survival, even when controlling for other significant risk factors, including whether or not a patient responds to chemotherapy. The hazard ratio is 0.73 [P = .023], indicating a 25%-30% improvement in overall survival,” he added.
Based on these results, “surgical debulking should still be considered a component of the treatment algorithm in ovarian cancer patients who have a poor response to chemotherapy, if an optimal surgery is deemed feasible,” Dr. Cardillo concluded.
There was no funding for this study, and the investigators had no relevant disclosures.
A retrospective, case-control study showed that optimal cytoreductive surgery is an independent predictor of overall survival, even when controlling for response to chemotherapy.
The findings were presented at the Society of Gynecologic Oncology’s Virtual Annual Meeting on Women’s Cancer (Abstract 10243).
Response to platinum-based chemotherapy is the strongest predictor of overall survival in advanced ovarian cancer, noted Nicholas Cardillo, MD, a gynecologic oncology fellow at the University of Iowa, Iowa City, who presented the findings at the meeting.
In recent years, a poor response to chemotherapy has sometimes been used as justification to forgo cytoreduction, Dr. Cardillo added.
He and his colleagues looked into this issue because evidence to support the practice is lacking. With their study, the researchers found that optimal cytoreduction – removing all disease of 1 cm or more – improved survival regardless of the response to chemotherapy.
“My advice right now is that debulking surgery should still be attempted in all patients with ovarian cancer because, as far as we know right now, optimal cytoreduction will improve survival,” Dr. Cardillo said in an interview. “Basically, this study argues to keep the status quo, which is to perform surgery.”
The status quo might change with future research, Dr. Cardillo acknowledged, “but as of right now, we have no evidence to support not pursuing cytoreduction in these patients.”
Study details and results
The researchers analyzed data on 234 patients who responded to platinum-based chemotherapy – meaning they had no evidence of disease for at least 6 months afterward – and 98 patients who did not respond – meaning they progressed during therapy, had stable disease, did not respond completely, or had a progression-free survival duration of less than 6 months. Subjects had stage III or IV high-grade serous ovarian cancer.
About three-quarters of responders and 57% of nonresponders had optimal surgery. Only seven patients in each group had fewer than six cycles of chemotherapy.
The mean age was 59 years in the responder group and 62 years among nonresponders. Stage IV disease, including upper-abdominal and chest involvement, was more common in the nonresponder group.
The median overall survival was 44.8 months in the responder group and 18.1 months among nonresponders (P < .001). The median overall survival was 34.2 months among patients who underwent optimal surgery and 24.8 months among those who did not (P < .001).
Predictors of survival
A multivariate analysis showed that response to chemotherapy had the greatest effect on survival, with a hazard ratio of 0.27 (P < .001).
“The second most significant predictor of overall survival was receipt of neoadjuvant chemotherapy [HR, 2.84; P < .001], which is intuitive as that is typically a marker for worse disease burden,” Dr. Cardillo said.
“But most importantly for our question is that optimal surgery is an independent significant factor in overall survival, even when controlling for other significant risk factors, including whether or not a patient responds to chemotherapy. The hazard ratio is 0.73 [P = .023], indicating a 25%-30% improvement in overall survival,” he added.
Based on these results, “surgical debulking should still be considered a component of the treatment algorithm in ovarian cancer patients who have a poor response to chemotherapy, if an optimal surgery is deemed feasible,” Dr. Cardillo concluded.
There was no funding for this study, and the investigators had no relevant disclosures.
FROM SGO 2021
How has the pandemic changed your personal/professional priorities?
COVID-19: Remaining flexible amid the uncertainty
Editor’s Note: With 1 year of the COVID-19 pandemic in the rearview mirror, we decided to check in with the Editorial Advisory Board members of Clinical Psychiatry News about the impact it has had on their practices and lives.
Redefining how to engage
The COVID-19 pandemic has triggered a wave of mental health problems in our population, such as general stress, addiction, weight gain, depression, and social isolation, and these symptoms are exacerbated in mental health patients who are already struggling to cope with personal issues.
When the pandemic lockdown was announced in March 2020, many of my patients became overwhelmed and panicked at the idea of not being able to come to my office for in-person therapy. As an alternative, I started phone call sessions with my clients. These calls forced me to listen extra carefully to patient voice intonations to ascertain their true feelings, since I was unable to view the clients.
Soon thereafter, I transitioned to telemedicine over the Internet, and this visual helped me assess each patient. In addition, my patients became accustomed to telemedicine and embraced it once they saw me and were able to interact with me on the screen.
Although the pandemic disrupted my medical practice, it has redefined the way I can do therapy, as I can practice medicine from a distance. Telemedicine is time efficient for both my patients and me and it provides extreme social distancing, eliminating COVID-19 exposure between doctor and patient.
The pandemic has forced me to be adaptable and to recognize that, if you are open to changing habits, you can find a solution to any situation, including a pandemic.
Richard W. Cohen, MD
Private Practice
Philadelphia
Adjusting to fate
As it became clear in January 2020 that a pandemic was upon us, I made plans and prepared. I needed to remain healthy for my patients and my 102-year-old best friend, Doc.
I purchased PPE and 6 months’ of nonperishable groceries and toilet tissue from a commercial vendor. I made certain that Doc’s caregivers had what they needed to care for him and their families and preached to them, family, patients, and friends the public health guidelines of the day. Also, I needed to remain healthy for my patients who live in a dementia care facility, and I joined other workers there in being careful and proud that our facility remained COVID free.
By March 2020, I left my office, because it was in a building where both residents and staff were becoming ill with COVID. I started audio and video telemedicine as well as standing outside the windows of patients who only read lips and do not use digital technology. Under these new circumstances, patients (and Doc) revealed things about themselves that had remained hidden for decades. There was a sense of urgency and uncertainty.
I also started weekly COVID testing, at first at CVS and then in a public park. Doc, who had had congestive heart failure for 2 years, had celebrated his 103rd birthday in February, and continued to be a source of encouragement and support. We weathered through the spring and summer with him on lockdown in his senior residence. The dementia care facility remained free of COVID.
My plan had been to return to my office in July, however, the facility manager determined that they were not ready to receive my outpatients. I took on a short-term lease for August and was told I could return to my regular office Sept. 1, which I did.
On Aug. 31, 2020, Doc had a middle cerebral artery stroke. He received the clot buster within 40 minutes and was in surgery within 90 minutes. He regained consciousness and lucidity but would always have a left-sided disability. During his third postoperative day he was told that he would never again swallow properly, and he yanked out his nasogastric tube. He had always told me that he would not accept artificial feeding. M. Leslie Felmly, MD, a psychiatrist, died on Sept. 12, 2020, and I buried him beside his family in New Jersey, on Sept. 22, 2020.
After that, I needed routine and normalcy, and therefore, stayed out of work only on the day of Doc’s burial. I took on new patients and continued with my old patients. As the holidays neared I braced myself; for 26 years I had spent Thanksgiving and New Year’s with Doc and Christmas with my family in Texas. None of that was going to happen in 2020. My best female friend and her husband invited me to a socially distanced Thanksgiving meal with the two of them, and I accepted. Christmas and New Year’s I spent alone (I live alone and enjoy my company). Both of those holidays were made special because I spent the eve days at the dementia care facility.
I received my first Pfizer injection on Jan. 6, 2021. One day later, I went to a park to get COVID testing before I returned to the dementia care facility. There, I learned that I was COVID positive, and when I called into the dementia facility, I learned that one resident and several staff members had also tested positive. As I stood in the sunshine outside the testing facility I thought: “So, now what will I do with the rest of my life?”
I began to feel profoundly tired, and over time, developed what felt like a very bad head cold. I had no high temperature or difficulty breathing. Truly, the worst of it was the profound fatigue and the terrorizing fear that I would develop problems breathing. By Jan. 21, I had only symptoms of fatigue, and on the 23rd, I had a negative COVID test. I attributed my course and recovery to my whole-food plant-based diet and routine high-dose vitamin D3 – in spite of my being an overweight, older African American woman. Through it all, I learned to ask for help, and one of my colleagues brought me a thermometer and 2 days of vegan Pho. I learned to be resourceful and ordered myself a fruit basket from Edible Arrangements when I was too fatigued to arrange deliveries by computer. I told Edible Arrangements that I was too weak to cut up a pineapple, and the manager included a cut-up pineapple in the box. I am grateful for the kindness of others.
I returned to work Jan. 25, and for most of each day, I feel better than I have ever felt in my adult life. It is amazing what 2 solid weeks can do for 50 years of arrears of sleep. The overwhelming fatigue was such that I could not not sleep. Thankfully, my remaining fatigue is less and less each day.
On Jan. 27, 2021, I received my second COVID vaccine injection and had no adverse reaction. Then on the 28th, I learned that my male cousin, who was just 6 months younger than I am, died of complications of COVID. Later, I learned that a resident of the dementia care facility had died from the same outbreak that had sickened me.
Since the beginning of the pandemic, I had tried so hard to remain healthy and COVID free and have my family, friends, and patients do the same. I planned, prepared, and executed but fate had other plans in store. Doc and my cousin are gone; I was exposed to COVID in my dementia care facility; and I know what matters for the rest of my life. I will continue to pursue and espouse health for me, my family, my friends, and my patients, and I will endeavor to be the best family member, friend, and physician that I can. To help with this, I remember the wise words of Dr. Felmly, “Your level of frustration will rise directly with your level of expectation” and “Above all else, remain flexible.” Going forward, I am reminded that I am not in charge; I am grateful for so many things; and I will continue to be as flexible as I can be.
Thelissa A. Harris, MD
Private Practice
Hartford, Conn.
Taking time for reflection
One year into the pandemic, I continue to learn to expect the unexpected, anticipate that things may not go as planned, accept that it is not business as usual, appreciate what I have, and focus on what is most important in my life – my family and most especially my children.
Despite the disruptions in our daily lives from the lockdowns, quarantines, and social distancing, my Catholic faith has grown stronger. I am not overly religious, but since the pandemic my children and I have attended online Mass regularly, sometimes in far away churches with different languages. It seems like we listen better now, reflect more on the homilies, and are really more in touch with our spirituality.
Professionally, I have seen the pandemic bring together geriatric psychiatrists from around the globe to tackle issues relevant to the mental health care of older adults. Within the International Psychogeriatric Association, we were spurred into collaborative actions with international colleagues in advocating for human rights of older adults in the context of the pandemic, creating online educational activities, and contributing to the special COVID-19 issue of the International Psychogeriatrics journal.
Maria I. Lapid, MD
Mayo Clinic
Rochester, Minn.
Concentrating on safety
The first year of the pandemic is over. How have my personal and professional goals changed? How has my life changed? Let us start with the latter.
I have been very lucky. I have continued to go into work at my hospital every day, which provides structure and socializing. My hospital has supplied PPE, although, like everywhere else, the rules keep changing.
Masks, face shield, goggles, etc.: I try to loop the mask around my earrings just right so it does not catch and pull the hooks off. I think the goggles make me look cool, like an ant man.
My world has narrowed to work and home. Like all of us, I no longer go to conferences. I do outpatient treatment from my office desk. I see inpatients from 6 feet away, in mask and goggles. The cookies I pass out are now individually wrapped. Takeout instead of restaurants. A new home gym.
I have learned a lot. I now know how to manage psychiatric wards where COVID clusters occur. How to transfer psychiatric patients who convert to COVID positive over to the medicine ward. I faithfully swab my own nose twice a week.
I am constantly saying (very nicely): “Please pull your mask up over your nose.” “Six feet apart, please.”
I saved my surgical masks in case I needed to reuse them. Fortunately, I did not. Now I have three overflowing drawers of masks. Plus, the heavy homemade cloth ones that friends and family sent.
Back to how have my goals changed? Basically they have not. I fix my eyes straight ahead and concentrate on safety. Safety of patients, staff, my family, myself.
And daily add another yellow, or blue, or white, surgical mask to the drawers.
Elspeth Cameron Ritchie, MD, MPH
Washington
Awaiting project’s return
I had been actively involved in community service as the cofounder of The Bold Beauty Project since 2015. We are a Miami-based nonprofit, and we pair women with disabilities with volunteer photographers and create art shows. Our motto: Disability becomes Beauty becomes Art becomes Change.
I have dedicated about half of my time to the project, and it has been incredibly rewarding. It all came to a halt in March 2020, and it has left a real void in my daily schedule and my psyche. I am eagerly awaiting the return of the Bold Beauty Project with a renewed appreciation and enthusiasm. I hope you will check us out boldbeautyproject.com. If you are inspired to get involved, please contact me at [email protected].
Eva Ritvo, MD
Private Practice
Miami Beach, Fla.
COVID-19: Remaining flexible amid the uncertainty
COVID-19: Remaining flexible amid the uncertainty
Editor’s Note: With 1 year of the COVID-19 pandemic in the rearview mirror, we decided to check in with the Editorial Advisory Board members of Clinical Psychiatry News about the impact it has had on their practices and lives.
Redefining how to engage
The COVID-19 pandemic has triggered a wave of mental health problems in our population, such as general stress, addiction, weight gain, depression, and social isolation, and these symptoms are exacerbated in mental health patients who are already struggling to cope with personal issues.
When the pandemic lockdown was announced in March 2020, many of my patients became overwhelmed and panicked at the idea of not being able to come to my office for in-person therapy. As an alternative, I started phone call sessions with my clients. These calls forced me to listen extra carefully to patient voice intonations to ascertain their true feelings, since I was unable to view the clients.
Soon thereafter, I transitioned to telemedicine over the Internet, and this visual helped me assess each patient. In addition, my patients became accustomed to telemedicine and embraced it once they saw me and were able to interact with me on the screen.
Although the pandemic disrupted my medical practice, it has redefined the way I can do therapy, as I can practice medicine from a distance. Telemedicine is time efficient for both my patients and me and it provides extreme social distancing, eliminating COVID-19 exposure between doctor and patient.
The pandemic has forced me to be adaptable and to recognize that, if you are open to changing habits, you can find a solution to any situation, including a pandemic.
Richard W. Cohen, MD
Private Practice
Philadelphia
Adjusting to fate
As it became clear in January 2020 that a pandemic was upon us, I made plans and prepared. I needed to remain healthy for my patients and my 102-year-old best friend, Doc.
I purchased PPE and 6 months’ of nonperishable groceries and toilet tissue from a commercial vendor. I made certain that Doc’s caregivers had what they needed to care for him and their families and preached to them, family, patients, and friends the public health guidelines of the day. Also, I needed to remain healthy for my patients who live in a dementia care facility, and I joined other workers there in being careful and proud that our facility remained COVID free.
By March 2020, I left my office, because it was in a building where both residents and staff were becoming ill with COVID. I started audio and video telemedicine as well as standing outside the windows of patients who only read lips and do not use digital technology. Under these new circumstances, patients (and Doc) revealed things about themselves that had remained hidden for decades. There was a sense of urgency and uncertainty.
I also started weekly COVID testing, at first at CVS and then in a public park. Doc, who had had congestive heart failure for 2 years, had celebrated his 103rd birthday in February, and continued to be a source of encouragement and support. We weathered through the spring and summer with him on lockdown in his senior residence. The dementia care facility remained free of COVID.
My plan had been to return to my office in July, however, the facility manager determined that they were not ready to receive my outpatients. I took on a short-term lease for August and was told I could return to my regular office Sept. 1, which I did.
On Aug. 31, 2020, Doc had a middle cerebral artery stroke. He received the clot buster within 40 minutes and was in surgery within 90 minutes. He regained consciousness and lucidity but would always have a left-sided disability. During his third postoperative day he was told that he would never again swallow properly, and he yanked out his nasogastric tube. He had always told me that he would not accept artificial feeding. M. Leslie Felmly, MD, a psychiatrist, died on Sept. 12, 2020, and I buried him beside his family in New Jersey, on Sept. 22, 2020.
After that, I needed routine and normalcy, and therefore, stayed out of work only on the day of Doc’s burial. I took on new patients and continued with my old patients. As the holidays neared I braced myself; for 26 years I had spent Thanksgiving and New Year’s with Doc and Christmas with my family in Texas. None of that was going to happen in 2020. My best female friend and her husband invited me to a socially distanced Thanksgiving meal with the two of them, and I accepted. Christmas and New Year’s I spent alone (I live alone and enjoy my company). Both of those holidays were made special because I spent the eve days at the dementia care facility.
I received my first Pfizer injection on Jan. 6, 2021. One day later, I went to a park to get COVID testing before I returned to the dementia care facility. There, I learned that I was COVID positive, and when I called into the dementia facility, I learned that one resident and several staff members had also tested positive. As I stood in the sunshine outside the testing facility I thought: “So, now what will I do with the rest of my life?”
I began to feel profoundly tired, and over time, developed what felt like a very bad head cold. I had no high temperature or difficulty breathing. Truly, the worst of it was the profound fatigue and the terrorizing fear that I would develop problems breathing. By Jan. 21, I had only symptoms of fatigue, and on the 23rd, I had a negative COVID test. I attributed my course and recovery to my whole-food plant-based diet and routine high-dose vitamin D3 – in spite of my being an overweight, older African American woman. Through it all, I learned to ask for help, and one of my colleagues brought me a thermometer and 2 days of vegan Pho. I learned to be resourceful and ordered myself a fruit basket from Edible Arrangements when I was too fatigued to arrange deliveries by computer. I told Edible Arrangements that I was too weak to cut up a pineapple, and the manager included a cut-up pineapple in the box. I am grateful for the kindness of others.
I returned to work Jan. 25, and for most of each day, I feel better than I have ever felt in my adult life. It is amazing what 2 solid weeks can do for 50 years of arrears of sleep. The overwhelming fatigue was such that I could not not sleep. Thankfully, my remaining fatigue is less and less each day.
On Jan. 27, 2021, I received my second COVID vaccine injection and had no adverse reaction. Then on the 28th, I learned that my male cousin, who was just 6 months younger than I am, died of complications of COVID. Later, I learned that a resident of the dementia care facility had died from the same outbreak that had sickened me.
Since the beginning of the pandemic, I had tried so hard to remain healthy and COVID free and have my family, friends, and patients do the same. I planned, prepared, and executed but fate had other plans in store. Doc and my cousin are gone; I was exposed to COVID in my dementia care facility; and I know what matters for the rest of my life. I will continue to pursue and espouse health for me, my family, my friends, and my patients, and I will endeavor to be the best family member, friend, and physician that I can. To help with this, I remember the wise words of Dr. Felmly, “Your level of frustration will rise directly with your level of expectation” and “Above all else, remain flexible.” Going forward, I am reminded that I am not in charge; I am grateful for so many things; and I will continue to be as flexible as I can be.
Thelissa A. Harris, MD
Private Practice
Hartford, Conn.
Taking time for reflection
One year into the pandemic, I continue to learn to expect the unexpected, anticipate that things may not go as planned, accept that it is not business as usual, appreciate what I have, and focus on what is most important in my life – my family and most especially my children.
Despite the disruptions in our daily lives from the lockdowns, quarantines, and social distancing, my Catholic faith has grown stronger. I am not overly religious, but since the pandemic my children and I have attended online Mass regularly, sometimes in far away churches with different languages. It seems like we listen better now, reflect more on the homilies, and are really more in touch with our spirituality.
Professionally, I have seen the pandemic bring together geriatric psychiatrists from around the globe to tackle issues relevant to the mental health care of older adults. Within the International Psychogeriatric Association, we were spurred into collaborative actions with international colleagues in advocating for human rights of older adults in the context of the pandemic, creating online educational activities, and contributing to the special COVID-19 issue of the International Psychogeriatrics journal.
Maria I. Lapid, MD
Mayo Clinic
Rochester, Minn.
Concentrating on safety
The first year of the pandemic is over. How have my personal and professional goals changed? How has my life changed? Let us start with the latter.
I have been very lucky. I have continued to go into work at my hospital every day, which provides structure and socializing. My hospital has supplied PPE, although, like everywhere else, the rules keep changing.
Masks, face shield, goggles, etc.: I try to loop the mask around my earrings just right so it does not catch and pull the hooks off. I think the goggles make me look cool, like an ant man.
My world has narrowed to work and home. Like all of us, I no longer go to conferences. I do outpatient treatment from my office desk. I see inpatients from 6 feet away, in mask and goggles. The cookies I pass out are now individually wrapped. Takeout instead of restaurants. A new home gym.
I have learned a lot. I now know how to manage psychiatric wards where COVID clusters occur. How to transfer psychiatric patients who convert to COVID positive over to the medicine ward. I faithfully swab my own nose twice a week.
I am constantly saying (very nicely): “Please pull your mask up over your nose.” “Six feet apart, please.”
I saved my surgical masks in case I needed to reuse them. Fortunately, I did not. Now I have three overflowing drawers of masks. Plus, the heavy homemade cloth ones that friends and family sent.
Back to how have my goals changed? Basically they have not. I fix my eyes straight ahead and concentrate on safety. Safety of patients, staff, my family, myself.
And daily add another yellow, or blue, or white, surgical mask to the drawers.
Elspeth Cameron Ritchie, MD, MPH
Washington
Awaiting project’s return
I had been actively involved in community service as the cofounder of The Bold Beauty Project since 2015. We are a Miami-based nonprofit, and we pair women with disabilities with volunteer photographers and create art shows. Our motto: Disability becomes Beauty becomes Art becomes Change.
I have dedicated about half of my time to the project, and it has been incredibly rewarding. It all came to a halt in March 2020, and it has left a real void in my daily schedule and my psyche. I am eagerly awaiting the return of the Bold Beauty Project with a renewed appreciation and enthusiasm. I hope you will check us out boldbeautyproject.com. If you are inspired to get involved, please contact me at [email protected].
Eva Ritvo, MD
Private Practice
Miami Beach, Fla.
Editor’s Note: With 1 year of the COVID-19 pandemic in the rearview mirror, we decided to check in with the Editorial Advisory Board members of Clinical Psychiatry News about the impact it has had on their practices and lives.
Redefining how to engage
The COVID-19 pandemic has triggered a wave of mental health problems in our population, such as general stress, addiction, weight gain, depression, and social isolation, and these symptoms are exacerbated in mental health patients who are already struggling to cope with personal issues.
When the pandemic lockdown was announced in March 2020, many of my patients became overwhelmed and panicked at the idea of not being able to come to my office for in-person therapy. As an alternative, I started phone call sessions with my clients. These calls forced me to listen extra carefully to patient voice intonations to ascertain their true feelings, since I was unable to view the clients.
Soon thereafter, I transitioned to telemedicine over the Internet, and this visual helped me assess each patient. In addition, my patients became accustomed to telemedicine and embraced it once they saw me and were able to interact with me on the screen.
Although the pandemic disrupted my medical practice, it has redefined the way I can do therapy, as I can practice medicine from a distance. Telemedicine is time efficient for both my patients and me and it provides extreme social distancing, eliminating COVID-19 exposure between doctor and patient.
The pandemic has forced me to be adaptable and to recognize that, if you are open to changing habits, you can find a solution to any situation, including a pandemic.
Richard W. Cohen, MD
Private Practice
Philadelphia
Adjusting to fate
As it became clear in January 2020 that a pandemic was upon us, I made plans and prepared. I needed to remain healthy for my patients and my 102-year-old best friend, Doc.
I purchased PPE and 6 months’ of nonperishable groceries and toilet tissue from a commercial vendor. I made certain that Doc’s caregivers had what they needed to care for him and their families and preached to them, family, patients, and friends the public health guidelines of the day. Also, I needed to remain healthy for my patients who live in a dementia care facility, and I joined other workers there in being careful and proud that our facility remained COVID free.
By March 2020, I left my office, because it was in a building where both residents and staff were becoming ill with COVID. I started audio and video telemedicine as well as standing outside the windows of patients who only read lips and do not use digital technology. Under these new circumstances, patients (and Doc) revealed things about themselves that had remained hidden for decades. There was a sense of urgency and uncertainty.
I also started weekly COVID testing, at first at CVS and then in a public park. Doc, who had had congestive heart failure for 2 years, had celebrated his 103rd birthday in February, and continued to be a source of encouragement and support. We weathered through the spring and summer with him on lockdown in his senior residence. The dementia care facility remained free of COVID.
My plan had been to return to my office in July, however, the facility manager determined that they were not ready to receive my outpatients. I took on a short-term lease for August and was told I could return to my regular office Sept. 1, which I did.
On Aug. 31, 2020, Doc had a middle cerebral artery stroke. He received the clot buster within 40 minutes and was in surgery within 90 minutes. He regained consciousness and lucidity but would always have a left-sided disability. During his third postoperative day he was told that he would never again swallow properly, and he yanked out his nasogastric tube. He had always told me that he would not accept artificial feeding. M. Leslie Felmly, MD, a psychiatrist, died on Sept. 12, 2020, and I buried him beside his family in New Jersey, on Sept. 22, 2020.
After that, I needed routine and normalcy, and therefore, stayed out of work only on the day of Doc’s burial. I took on new patients and continued with my old patients. As the holidays neared I braced myself; for 26 years I had spent Thanksgiving and New Year’s with Doc and Christmas with my family in Texas. None of that was going to happen in 2020. My best female friend and her husband invited me to a socially distanced Thanksgiving meal with the two of them, and I accepted. Christmas and New Year’s I spent alone (I live alone and enjoy my company). Both of those holidays were made special because I spent the eve days at the dementia care facility.
I received my first Pfizer injection on Jan. 6, 2021. One day later, I went to a park to get COVID testing before I returned to the dementia care facility. There, I learned that I was COVID positive, and when I called into the dementia facility, I learned that one resident and several staff members had also tested positive. As I stood in the sunshine outside the testing facility I thought: “So, now what will I do with the rest of my life?”
I began to feel profoundly tired, and over time, developed what felt like a very bad head cold. I had no high temperature or difficulty breathing. Truly, the worst of it was the profound fatigue and the terrorizing fear that I would develop problems breathing. By Jan. 21, I had only symptoms of fatigue, and on the 23rd, I had a negative COVID test. I attributed my course and recovery to my whole-food plant-based diet and routine high-dose vitamin D3 – in spite of my being an overweight, older African American woman. Through it all, I learned to ask for help, and one of my colleagues brought me a thermometer and 2 days of vegan Pho. I learned to be resourceful and ordered myself a fruit basket from Edible Arrangements when I was too fatigued to arrange deliveries by computer. I told Edible Arrangements that I was too weak to cut up a pineapple, and the manager included a cut-up pineapple in the box. I am grateful for the kindness of others.
I returned to work Jan. 25, and for most of each day, I feel better than I have ever felt in my adult life. It is amazing what 2 solid weeks can do for 50 years of arrears of sleep. The overwhelming fatigue was such that I could not not sleep. Thankfully, my remaining fatigue is less and less each day.
On Jan. 27, 2021, I received my second COVID vaccine injection and had no adverse reaction. Then on the 28th, I learned that my male cousin, who was just 6 months younger than I am, died of complications of COVID. Later, I learned that a resident of the dementia care facility had died from the same outbreak that had sickened me.
Since the beginning of the pandemic, I had tried so hard to remain healthy and COVID free and have my family, friends, and patients do the same. I planned, prepared, and executed but fate had other plans in store. Doc and my cousin are gone; I was exposed to COVID in my dementia care facility; and I know what matters for the rest of my life. I will continue to pursue and espouse health for me, my family, my friends, and my patients, and I will endeavor to be the best family member, friend, and physician that I can. To help with this, I remember the wise words of Dr. Felmly, “Your level of frustration will rise directly with your level of expectation” and “Above all else, remain flexible.” Going forward, I am reminded that I am not in charge; I am grateful for so many things; and I will continue to be as flexible as I can be.
Thelissa A. Harris, MD
Private Practice
Hartford, Conn.
Taking time for reflection
One year into the pandemic, I continue to learn to expect the unexpected, anticipate that things may not go as planned, accept that it is not business as usual, appreciate what I have, and focus on what is most important in my life – my family and most especially my children.
Despite the disruptions in our daily lives from the lockdowns, quarantines, and social distancing, my Catholic faith has grown stronger. I am not overly religious, but since the pandemic my children and I have attended online Mass regularly, sometimes in far away churches with different languages. It seems like we listen better now, reflect more on the homilies, and are really more in touch with our spirituality.
Professionally, I have seen the pandemic bring together geriatric psychiatrists from around the globe to tackle issues relevant to the mental health care of older adults. Within the International Psychogeriatric Association, we were spurred into collaborative actions with international colleagues in advocating for human rights of older adults in the context of the pandemic, creating online educational activities, and contributing to the special COVID-19 issue of the International Psychogeriatrics journal.
Maria I. Lapid, MD
Mayo Clinic
Rochester, Minn.
Concentrating on safety
The first year of the pandemic is over. How have my personal and professional goals changed? How has my life changed? Let us start with the latter.
I have been very lucky. I have continued to go into work at my hospital every day, which provides structure and socializing. My hospital has supplied PPE, although, like everywhere else, the rules keep changing.
Masks, face shield, goggles, etc.: I try to loop the mask around my earrings just right so it does not catch and pull the hooks off. I think the goggles make me look cool, like an ant man.
My world has narrowed to work and home. Like all of us, I no longer go to conferences. I do outpatient treatment from my office desk. I see inpatients from 6 feet away, in mask and goggles. The cookies I pass out are now individually wrapped. Takeout instead of restaurants. A new home gym.
I have learned a lot. I now know how to manage psychiatric wards where COVID clusters occur. How to transfer psychiatric patients who convert to COVID positive over to the medicine ward. I faithfully swab my own nose twice a week.
I am constantly saying (very nicely): “Please pull your mask up over your nose.” “Six feet apart, please.”
I saved my surgical masks in case I needed to reuse them. Fortunately, I did not. Now I have three overflowing drawers of masks. Plus, the heavy homemade cloth ones that friends and family sent.
Back to how have my goals changed? Basically they have not. I fix my eyes straight ahead and concentrate on safety. Safety of patients, staff, my family, myself.
And daily add another yellow, or blue, or white, surgical mask to the drawers.
Elspeth Cameron Ritchie, MD, MPH
Washington
Awaiting project’s return
I had been actively involved in community service as the cofounder of The Bold Beauty Project since 2015. We are a Miami-based nonprofit, and we pair women with disabilities with volunteer photographers and create art shows. Our motto: Disability becomes Beauty becomes Art becomes Change.
I have dedicated about half of my time to the project, and it has been incredibly rewarding. It all came to a halt in March 2020, and it has left a real void in my daily schedule and my psyche. I am eagerly awaiting the return of the Bold Beauty Project with a renewed appreciation and enthusiasm. I hope you will check us out boldbeautyproject.com. If you are inspired to get involved, please contact me at [email protected].
Eva Ritvo, MD
Private Practice
Miami Beach, Fla.
Our role in colorectal cancer prevention education
Each year in the month of March, advocates, physicians, and health care educators come together to promote the importance of colorectal cancer screening during Colorectal Cancer Awareness Month. As independent GI physicians, we work within our communities to promote colorectal screening year-round.
We also understand that our education efforts do not end with the people in our community who need to be screened. Independent GI practices also engage with primary care physicians who often initiate conversations about available screening tests and when people should be screened.
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States.1 It is expected to kill more than 50,000 Americans this year alone.2 This is why screening for colorectal cancer is so important. The American Cancer Society recommends screening for all average-risk patients aged 45-75 years.3
The good news? If caught early, the survival rate is very high. In fact, when caught early, the five-year survival rate is 90 percent. Unfortunately, one in three Americans who are eligible for screenings do not get screened. For certain groups, there are larger numbers of people who are not getting screened. And there are groups for whom the death rates from colorectal cancer are much higher.
Disparities in colorectal cancer screenings
According to the American Cancer Society, Blacks and Hispanics are less likely to receive prompt follow up after an abnormal CRC screening result and are more likely to be diagnosed with late-stage cancer.4 African Americans have the highest death rate when compared with all other racial groups in the United States. American Indians and Alaska Natives are the only groups for which CRC death rates are not declining.
There are many factors that drive disparities, but the main factors seem to be socioeconomic status and differences in access to early detection and treatment. While some of these issues are complex and difficult to change, increasing awareness and providing education can be easier than you might think.
Working with your community as a private GI practitioner
To address economic factors, Atlanta Gastroenterology Associates has a program that provides resources on a sliding fee scale to people in our community who do not have insurance and are concerned about having to pay for CRC screening out of pocket. This includes the costs for anesthesia, colonoscopy, and pathology services.
We also have a Direct Access Program, which allows people to self-schedule a screening and fill out a survey that assesses their candidacy for screening colonoscopy. This allows our patients to bypass an initial prescreening office visit and associated copays. Patients are provided instructions for colonoscopy prep and show up for the colonoscopy on the day of their procedure. When the colonoscopy is completed, we give them a patient education card on CRC screening to share with friends and family members who need to be screened.
Atlanta is a very diverse city, and representation is important. But, fortunately, the size of Atlanta Gastroenterology Associates allows us to have representation within many communities. We attend a significant number of health fairs and community events, many of which are sourced internally. Our physicians and staff are members of churches and social groups that we work with to provide screening materials and conduct informational events.
Word of mouth is the best advertising, and it works the same way with health education. There are a lot of myths that we must debunk. And in many of our communities, people are worrying about paying the bills to keep the lights on – they are not thinking about getting screened. But, if they hear from a friend or family member that their screening colonoscopy was a good experience and that resources were provided to help pay for the procedure, it really does make a difference.
You do not need to join a large practice to have an impact. All over the country, there are community groups working to increase screening rates, and engaging with those groups is a good start. During the COVID-19 pandemic, we are all using social media and other platforms to connect. You do not need a lot of resources to set up a Zoom meeting with people in your community to discuss CRC screening.
Engaging with referring physicians
As a private practice practitioner, part of growing your practice is engaging with the primary care physicians in your area to ensure that they are up to date on the latest research in CRC screening and that they are discussing available screening options with their patients.
Preventing cancer should always be our first goal. Most CRCs begin as a polyp. Finding, quantifying, localizing, and removing polyps through screening colonoscopy is the most effective strategy for preventing this cancer. That is why colonoscopy remains the preferred method for colon cancer screening.
The Multi-Society Task Force on Colorectal Cancer recommends that, in sequential approaches, physicians should offer colonoscopy first.5 For patients who decline to have a colonoscopy, the FIT test should be offered next, followed by second-tier tests such as Cologuard and CT colonography for patients who decline both first-tier options.
Beyond the science of colorectal screening, we want to make sure that our primary care partners are aware of the disparities that exist – and which patients are at higher risk – so that they can engage with their patients to encourage screening.
For example, in our practice, we work with local Asian American community groups to help make sure that the “model minority” myth – that Asian Americans are healthier, wealthier, and better educated than the average American – does not become a barrier to screening. While Asian Americans may have lower overall rates of some types of cancer, there are some cancers that disproportionately affect certain Asian American groups. Rates of CRC in Japanese men, for instance, are 23% higher than in non-Hispanic Whites.
Additionally, we work with our primary care colleagues to help them understand that patients may have insurance considerations when choosing a test. While insurance typically covers 100% of a preventive screening test, a follow-up colonoscopy for a positive stool test is considered a diagnostic or therapeutic service and may not be fully covered. Medicare patients may face a coinsurance bill after their follow-up colonoscopy for a positive stool test. Legislation was passed last year to remove this barrier, but Medicare beneficiaries may have some out-of-pocket costs until it is completely removed in 2030.
Are you joining a practice that supports CRC education? Just ask!
We all want to work for an organization that aligns with our core values, and for GI physicians like us, CRC screening is a core component of our everyday work.
If you are considering joining a private practice, ask how the practice is doing with their CRC awareness programs and if it leads to increases in screenings. Inquire about the groups that are being engaged with and why. Is the practice focused on communities that have disparities in screening and treatment, and is it able to complete the entire screening process for individuals in communities that are more adversely affected by colorectal cancer?
We have found that candidates who have the most success in our practice are people who want to work at Atlanta Gastroenterology Associates but are also active in their communities and have a sense of how they want to be of service in their community. It is a sign of leadership in people – the idea that they are really going to get out and network and build a practice that serves everyone in their community. These actions make a difference in getting more people screened and in decreasing the disparities that exist.
Dr. Aja McCutchen is the chair of the quality committee at Atlanta Gastroenterology Associates and serves as chair of the Digestive Health Physicians Association’s Diversity, Equity and Inclusion Committee. She reports having nothing to disclose.
References
1. Siegel RL et al. CA Cancer J Clin. 2018 Jan;68(1):7-30.
2. Key Statistics for Colorectal Cancer. Cancer.org.
3. Wolf AMD et al. CA Cancer J Clin. 2018 Jul;68(4):250-281.
4. American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022.
5. Rex DK et al. Am J Gastroenterol. 2017;112(7):1016-30.
Each year in the month of March, advocates, physicians, and health care educators come together to promote the importance of colorectal cancer screening during Colorectal Cancer Awareness Month. As independent GI physicians, we work within our communities to promote colorectal screening year-round.
We also understand that our education efforts do not end with the people in our community who need to be screened. Independent GI practices also engage with primary care physicians who often initiate conversations about available screening tests and when people should be screened.
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States.1 It is expected to kill more than 50,000 Americans this year alone.2 This is why screening for colorectal cancer is so important. The American Cancer Society recommends screening for all average-risk patients aged 45-75 years.3
The good news? If caught early, the survival rate is very high. In fact, when caught early, the five-year survival rate is 90 percent. Unfortunately, one in three Americans who are eligible for screenings do not get screened. For certain groups, there are larger numbers of people who are not getting screened. And there are groups for whom the death rates from colorectal cancer are much higher.
Disparities in colorectal cancer screenings
According to the American Cancer Society, Blacks and Hispanics are less likely to receive prompt follow up after an abnormal CRC screening result and are more likely to be diagnosed with late-stage cancer.4 African Americans have the highest death rate when compared with all other racial groups in the United States. American Indians and Alaska Natives are the only groups for which CRC death rates are not declining.
There are many factors that drive disparities, but the main factors seem to be socioeconomic status and differences in access to early detection and treatment. While some of these issues are complex and difficult to change, increasing awareness and providing education can be easier than you might think.
Working with your community as a private GI practitioner
To address economic factors, Atlanta Gastroenterology Associates has a program that provides resources on a sliding fee scale to people in our community who do not have insurance and are concerned about having to pay for CRC screening out of pocket. This includes the costs for anesthesia, colonoscopy, and pathology services.
We also have a Direct Access Program, which allows people to self-schedule a screening and fill out a survey that assesses their candidacy for screening colonoscopy. This allows our patients to bypass an initial prescreening office visit and associated copays. Patients are provided instructions for colonoscopy prep and show up for the colonoscopy on the day of their procedure. When the colonoscopy is completed, we give them a patient education card on CRC screening to share with friends and family members who need to be screened.
Atlanta is a very diverse city, and representation is important. But, fortunately, the size of Atlanta Gastroenterology Associates allows us to have representation within many communities. We attend a significant number of health fairs and community events, many of which are sourced internally. Our physicians and staff are members of churches and social groups that we work with to provide screening materials and conduct informational events.
Word of mouth is the best advertising, and it works the same way with health education. There are a lot of myths that we must debunk. And in many of our communities, people are worrying about paying the bills to keep the lights on – they are not thinking about getting screened. But, if they hear from a friend or family member that their screening colonoscopy was a good experience and that resources were provided to help pay for the procedure, it really does make a difference.
You do not need to join a large practice to have an impact. All over the country, there are community groups working to increase screening rates, and engaging with those groups is a good start. During the COVID-19 pandemic, we are all using social media and other platforms to connect. You do not need a lot of resources to set up a Zoom meeting with people in your community to discuss CRC screening.
Engaging with referring physicians
As a private practice practitioner, part of growing your practice is engaging with the primary care physicians in your area to ensure that they are up to date on the latest research in CRC screening and that they are discussing available screening options with their patients.
Preventing cancer should always be our first goal. Most CRCs begin as a polyp. Finding, quantifying, localizing, and removing polyps through screening colonoscopy is the most effective strategy for preventing this cancer. That is why colonoscopy remains the preferred method for colon cancer screening.
The Multi-Society Task Force on Colorectal Cancer recommends that, in sequential approaches, physicians should offer colonoscopy first.5 For patients who decline to have a colonoscopy, the FIT test should be offered next, followed by second-tier tests such as Cologuard and CT colonography for patients who decline both first-tier options.
Beyond the science of colorectal screening, we want to make sure that our primary care partners are aware of the disparities that exist – and which patients are at higher risk – so that they can engage with their patients to encourage screening.
For example, in our practice, we work with local Asian American community groups to help make sure that the “model minority” myth – that Asian Americans are healthier, wealthier, and better educated than the average American – does not become a barrier to screening. While Asian Americans may have lower overall rates of some types of cancer, there are some cancers that disproportionately affect certain Asian American groups. Rates of CRC in Japanese men, for instance, are 23% higher than in non-Hispanic Whites.
Additionally, we work with our primary care colleagues to help them understand that patients may have insurance considerations when choosing a test. While insurance typically covers 100% of a preventive screening test, a follow-up colonoscopy for a positive stool test is considered a diagnostic or therapeutic service and may not be fully covered. Medicare patients may face a coinsurance bill after their follow-up colonoscopy for a positive stool test. Legislation was passed last year to remove this barrier, but Medicare beneficiaries may have some out-of-pocket costs until it is completely removed in 2030.
Are you joining a practice that supports CRC education? Just ask!
We all want to work for an organization that aligns with our core values, and for GI physicians like us, CRC screening is a core component of our everyday work.
If you are considering joining a private practice, ask how the practice is doing with their CRC awareness programs and if it leads to increases in screenings. Inquire about the groups that are being engaged with and why. Is the practice focused on communities that have disparities in screening and treatment, and is it able to complete the entire screening process for individuals in communities that are more adversely affected by colorectal cancer?
We have found that candidates who have the most success in our practice are people who want to work at Atlanta Gastroenterology Associates but are also active in their communities and have a sense of how they want to be of service in their community. It is a sign of leadership in people – the idea that they are really going to get out and network and build a practice that serves everyone in their community. These actions make a difference in getting more people screened and in decreasing the disparities that exist.
Dr. Aja McCutchen is the chair of the quality committee at Atlanta Gastroenterology Associates and serves as chair of the Digestive Health Physicians Association’s Diversity, Equity and Inclusion Committee. She reports having nothing to disclose.
References
1. Siegel RL et al. CA Cancer J Clin. 2018 Jan;68(1):7-30.
2. Key Statistics for Colorectal Cancer. Cancer.org.
3. Wolf AMD et al. CA Cancer J Clin. 2018 Jul;68(4):250-281.
4. American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022.
5. Rex DK et al. Am J Gastroenterol. 2017;112(7):1016-30.
Each year in the month of March, advocates, physicians, and health care educators come together to promote the importance of colorectal cancer screening during Colorectal Cancer Awareness Month. As independent GI physicians, we work within our communities to promote colorectal screening year-round.
We also understand that our education efforts do not end with the people in our community who need to be screened. Independent GI practices also engage with primary care physicians who often initiate conversations about available screening tests and when people should be screened.
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States.1 It is expected to kill more than 50,000 Americans this year alone.2 This is why screening for colorectal cancer is so important. The American Cancer Society recommends screening for all average-risk patients aged 45-75 years.3
The good news? If caught early, the survival rate is very high. In fact, when caught early, the five-year survival rate is 90 percent. Unfortunately, one in three Americans who are eligible for screenings do not get screened. For certain groups, there are larger numbers of people who are not getting screened. And there are groups for whom the death rates from colorectal cancer are much higher.
Disparities in colorectal cancer screenings
According to the American Cancer Society, Blacks and Hispanics are less likely to receive prompt follow up after an abnormal CRC screening result and are more likely to be diagnosed with late-stage cancer.4 African Americans have the highest death rate when compared with all other racial groups in the United States. American Indians and Alaska Natives are the only groups for which CRC death rates are not declining.
There are many factors that drive disparities, but the main factors seem to be socioeconomic status and differences in access to early detection and treatment. While some of these issues are complex and difficult to change, increasing awareness and providing education can be easier than you might think.
Working with your community as a private GI practitioner
To address economic factors, Atlanta Gastroenterology Associates has a program that provides resources on a sliding fee scale to people in our community who do not have insurance and are concerned about having to pay for CRC screening out of pocket. This includes the costs for anesthesia, colonoscopy, and pathology services.
We also have a Direct Access Program, which allows people to self-schedule a screening and fill out a survey that assesses their candidacy for screening colonoscopy. This allows our patients to bypass an initial prescreening office visit and associated copays. Patients are provided instructions for colonoscopy prep and show up for the colonoscopy on the day of their procedure. When the colonoscopy is completed, we give them a patient education card on CRC screening to share with friends and family members who need to be screened.
Atlanta is a very diverse city, and representation is important. But, fortunately, the size of Atlanta Gastroenterology Associates allows us to have representation within many communities. We attend a significant number of health fairs and community events, many of which are sourced internally. Our physicians and staff are members of churches and social groups that we work with to provide screening materials and conduct informational events.
Word of mouth is the best advertising, and it works the same way with health education. There are a lot of myths that we must debunk. And in many of our communities, people are worrying about paying the bills to keep the lights on – they are not thinking about getting screened. But, if they hear from a friend or family member that their screening colonoscopy was a good experience and that resources were provided to help pay for the procedure, it really does make a difference.
You do not need to join a large practice to have an impact. All over the country, there are community groups working to increase screening rates, and engaging with those groups is a good start. During the COVID-19 pandemic, we are all using social media and other platforms to connect. You do not need a lot of resources to set up a Zoom meeting with people in your community to discuss CRC screening.
Engaging with referring physicians
As a private practice practitioner, part of growing your practice is engaging with the primary care physicians in your area to ensure that they are up to date on the latest research in CRC screening and that they are discussing available screening options with their patients.
Preventing cancer should always be our first goal. Most CRCs begin as a polyp. Finding, quantifying, localizing, and removing polyps through screening colonoscopy is the most effective strategy for preventing this cancer. That is why colonoscopy remains the preferred method for colon cancer screening.
The Multi-Society Task Force on Colorectal Cancer recommends that, in sequential approaches, physicians should offer colonoscopy first.5 For patients who decline to have a colonoscopy, the FIT test should be offered next, followed by second-tier tests such as Cologuard and CT colonography for patients who decline both first-tier options.
Beyond the science of colorectal screening, we want to make sure that our primary care partners are aware of the disparities that exist – and which patients are at higher risk – so that they can engage with their patients to encourage screening.
For example, in our practice, we work with local Asian American community groups to help make sure that the “model minority” myth – that Asian Americans are healthier, wealthier, and better educated than the average American – does not become a barrier to screening. While Asian Americans may have lower overall rates of some types of cancer, there are some cancers that disproportionately affect certain Asian American groups. Rates of CRC in Japanese men, for instance, are 23% higher than in non-Hispanic Whites.
Additionally, we work with our primary care colleagues to help them understand that patients may have insurance considerations when choosing a test. While insurance typically covers 100% of a preventive screening test, a follow-up colonoscopy for a positive stool test is considered a diagnostic or therapeutic service and may not be fully covered. Medicare patients may face a coinsurance bill after their follow-up colonoscopy for a positive stool test. Legislation was passed last year to remove this barrier, but Medicare beneficiaries may have some out-of-pocket costs until it is completely removed in 2030.
Are you joining a practice that supports CRC education? Just ask!
We all want to work for an organization that aligns with our core values, and for GI physicians like us, CRC screening is a core component of our everyday work.
If you are considering joining a private practice, ask how the practice is doing with their CRC awareness programs and if it leads to increases in screenings. Inquire about the groups that are being engaged with and why. Is the practice focused on communities that have disparities in screening and treatment, and is it able to complete the entire screening process for individuals in communities that are more adversely affected by colorectal cancer?
We have found that candidates who have the most success in our practice are people who want to work at Atlanta Gastroenterology Associates but are also active in their communities and have a sense of how they want to be of service in their community. It is a sign of leadership in people – the idea that they are really going to get out and network and build a practice that serves everyone in their community. These actions make a difference in getting more people screened and in decreasing the disparities that exist.
Dr. Aja McCutchen is the chair of the quality committee at Atlanta Gastroenterology Associates and serves as chair of the Digestive Health Physicians Association’s Diversity, Equity and Inclusion Committee. She reports having nothing to disclose.
References
1. Siegel RL et al. CA Cancer J Clin. 2018 Jan;68(1):7-30.
2. Key Statistics for Colorectal Cancer. Cancer.org.
3. Wolf AMD et al. CA Cancer J Clin. 2018 Jul;68(4):250-281.
4. American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022.
5. Rex DK et al. Am J Gastroenterol. 2017;112(7):1016-30.