User login
Does evidence support the use of supplements to aid in BP control?
EVIDENCE SUMMARY
Cocoa. A 2017 Cochrane review evaluated data from more than 1800 patients (401 in hypertension studies) to determine the effect of cocoa on BP.1 Compared with placebo (in flavanol-free or low-flavanol controls), cocoa lowered systolic BP by 1.8 mm Hg (confidence interval [CI], –3.1 to –0.4) and diastolic BP by 1.8 mm Hg (CI, –2.6 to –0.9). Further analysis of patients with hypertension (only) showed a reduction in systolic BP of 4 mm Hg (CI, –6.7 to –1.3).
Omega-3 fatty acids. Similarly, a 2014 meta-analysis investigating omega-3 fatty acids (eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]) included data from 4489 patients (956 with hypertension) and showed reductions in systolic BP of 1.5 mm Hg (CI, –2.3 to –0.8) and diastolic BP of 1 mm Hg (CI, –1.5 to –0.4), compared with placebo.2 Again, subgroup analysis of patients with hypertension (only) at baseline revealed a greater decrease in systolic and diastolic BP: 4.5 mm Hg (CI, –6.1 to –2.8) and 3.1 mm Hg (CI, –4.4 to –1.8), respectively.
Garlic and potassium chloride. Separate meta-analyses that included only patients with hypertension found that both garlic and potassium significantly lowered BP.3,4 A 2015 meta-analysis comparing a variety of garlic preparations with placebo in patients with hypertension showed decreases in systolic BP of 9.1 mm Hg (CI, –12.7 to –5.4) and in diastolic BP of 3.8 mm Hg (CI, –6.7 to –1).3 Meanwhile, a meta-analysis in 2017 comparing different doses of potassium chloride with placebo demonstrated reductions in systolic BP of 4.3 mm Hg (CI, –6 to –2.5) and diastolic BP of 2.5 mm Hg (CI, –4.1 to –1).4
L-arginine. Another meta-analysis of randomized controlled trials reported evidence that oral L-arginine, compared with placebo, significantly reduced systolic BP by 5.4 mm Hg (CI, –8.5 to –2.3) and diastolic BP by 2.7 mm Hg (CI, –3.8 to –1.5).5 Close to one-third of patients had hypertension at baseline.
Beetroot juice. A double-blind, placebo-controlled study showed that consumption of beetroot juice (with nitrate) once daily reduced BP in 3 different settings (clinic, 24-hour ambulatory, and home readings) when compared with placebo (nitrate-free beetroot juice).6 Study participants were mostly British women, overweight, without significant cardiovascular or renal disease, and with uncontrolled ambulatory BP (> 135/85 mm Hg).
Flax seed. A prospective, double-blind trial of patients with peripheral artery disease compared the antihypertensive effects of flax seed with placebo in patients with and without hypertension and found marked decreases in systolic and diastolic BP.7 Study participants were all older than 40 years without other major cardiac or renal disease, and the majority of enrolled patients with hypertension were concurrently taking medications to treat hypertension during the study.
Olive leaf extract. A double-blind, parallel, and active-control clinical trial in Indonesia compared the BP-lowering effect of olive leaf extract (Olea europaea) to captopril as monotherapies in patients with stage 1 hypertension.8 After a 4-week period of dietary intervention, individuals who were still hypertensive (range, 140/90 to 159/99 mm Hg) were treated with either olive leaf extract or captopril. After 8 weeks of treatment, both groups saw comparable reductions in BP.
Continue to: Editor's takeaway
Editor’s takeaway
Many studies have demonstrated BP benefits from a variety of natural supplements. Although the studies’ durations are short, the effects sometimes modest, and the outcomes disease-oriented rather than patient-oriented, the findings can provide a useful complement to our efforts to manage this most common chronic disease.
1. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;(4):CD008893.
2. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885-896.
3. Rohner A, Ried K, Sobenin IA, et al. A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423.
4. Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0174967.
5. Dong JY, Qin LQ, Zhang Z, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965.
6. Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320-327.
7. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081-1089.
8. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251-258.
EVIDENCE SUMMARY
Cocoa. A 2017 Cochrane review evaluated data from more than 1800 patients (401 in hypertension studies) to determine the effect of cocoa on BP.1 Compared with placebo (in flavanol-free or low-flavanol controls), cocoa lowered systolic BP by 1.8 mm Hg (confidence interval [CI], –3.1 to –0.4) and diastolic BP by 1.8 mm Hg (CI, –2.6 to –0.9). Further analysis of patients with hypertension (only) showed a reduction in systolic BP of 4 mm Hg (CI, –6.7 to –1.3).

Omega-3 fatty acids. Similarly, a 2014 meta-analysis investigating omega-3 fatty acids (eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]) included data from 4489 patients (956 with hypertension) and showed reductions in systolic BP of 1.5 mm Hg (CI, –2.3 to –0.8) and diastolic BP of 1 mm Hg (CI, –1.5 to –0.4), compared with placebo.2 Again, subgroup analysis of patients with hypertension (only) at baseline revealed a greater decrease in systolic and diastolic BP: 4.5 mm Hg (CI, –6.1 to –2.8) and 3.1 mm Hg (CI, –4.4 to –1.8), respectively.

Garlic and potassium chloride. Separate meta-analyses that included only patients with hypertension found that both garlic and potassium significantly lowered BP.3,4 A 2015 meta-analysis comparing a variety of garlic preparations with placebo in patients with hypertension showed decreases in systolic BP of 9.1 mm Hg (CI, –12.7 to –5.4) and in diastolic BP of 3.8 mm Hg (CI, –6.7 to –1).3 Meanwhile, a meta-analysis in 2017 comparing different doses of potassium chloride with placebo demonstrated reductions in systolic BP of 4.3 mm Hg (CI, –6 to –2.5) and diastolic BP of 2.5 mm Hg (CI, –4.1 to –1).4
L-arginine. Another meta-analysis of randomized controlled trials reported evidence that oral L-arginine, compared with placebo, significantly reduced systolic BP by 5.4 mm Hg (CI, –8.5 to –2.3) and diastolic BP by 2.7 mm Hg (CI, –3.8 to –1.5).5 Close to one-third of patients had hypertension at baseline.
Beetroot juice. A double-blind, placebo-controlled study showed that consumption of beetroot juice (with nitrate) once daily reduced BP in 3 different settings (clinic, 24-hour ambulatory, and home readings) when compared with placebo (nitrate-free beetroot juice).6 Study participants were mostly British women, overweight, without significant cardiovascular or renal disease, and with uncontrolled ambulatory BP (> 135/85 mm Hg).
Flax seed. A prospective, double-blind trial of patients with peripheral artery disease compared the antihypertensive effects of flax seed with placebo in patients with and without hypertension and found marked decreases in systolic and diastolic BP.7 Study participants were all older than 40 years without other major cardiac or renal disease, and the majority of enrolled patients with hypertension were concurrently taking medications to treat hypertension during the study.
Olive leaf extract. A double-blind, parallel, and active-control clinical trial in Indonesia compared the BP-lowering effect of olive leaf extract (Olea europaea) to captopril as monotherapies in patients with stage 1 hypertension.8 After a 4-week period of dietary intervention, individuals who were still hypertensive (range, 140/90 to 159/99 mm Hg) were treated with either olive leaf extract or captopril. After 8 weeks of treatment, both groups saw comparable reductions in BP.
Continue to: Editor's takeaway
Editor’s takeaway
Many studies have demonstrated BP benefits from a variety of natural supplements. Although the studies’ durations are short, the effects sometimes modest, and the outcomes disease-oriented rather than patient-oriented, the findings can provide a useful complement to our efforts to manage this most common chronic disease.
EVIDENCE SUMMARY
Cocoa. A 2017 Cochrane review evaluated data from more than 1800 patients (401 in hypertension studies) to determine the effect of cocoa on BP.1 Compared with placebo (in flavanol-free or low-flavanol controls), cocoa lowered systolic BP by 1.8 mm Hg (confidence interval [CI], –3.1 to –0.4) and diastolic BP by 1.8 mm Hg (CI, –2.6 to –0.9). Further analysis of patients with hypertension (only) showed a reduction in systolic BP of 4 mm Hg (CI, –6.7 to –1.3).

Omega-3 fatty acids. Similarly, a 2014 meta-analysis investigating omega-3 fatty acids (eicosapentaenoic acid [EPA] + docosahexaenoic acid [DHA]) included data from 4489 patients (956 with hypertension) and showed reductions in systolic BP of 1.5 mm Hg (CI, –2.3 to –0.8) and diastolic BP of 1 mm Hg (CI, –1.5 to –0.4), compared with placebo.2 Again, subgroup analysis of patients with hypertension (only) at baseline revealed a greater decrease in systolic and diastolic BP: 4.5 mm Hg (CI, –6.1 to –2.8) and 3.1 mm Hg (CI, –4.4 to –1.8), respectively.

Garlic and potassium chloride. Separate meta-analyses that included only patients with hypertension found that both garlic and potassium significantly lowered BP.3,4 A 2015 meta-analysis comparing a variety of garlic preparations with placebo in patients with hypertension showed decreases in systolic BP of 9.1 mm Hg (CI, –12.7 to –5.4) and in diastolic BP of 3.8 mm Hg (CI, –6.7 to –1).3 Meanwhile, a meta-analysis in 2017 comparing different doses of potassium chloride with placebo demonstrated reductions in systolic BP of 4.3 mm Hg (CI, –6 to –2.5) and diastolic BP of 2.5 mm Hg (CI, –4.1 to –1).4
L-arginine. Another meta-analysis of randomized controlled trials reported evidence that oral L-arginine, compared with placebo, significantly reduced systolic BP by 5.4 mm Hg (CI, –8.5 to –2.3) and diastolic BP by 2.7 mm Hg (CI, –3.8 to –1.5).5 Close to one-third of patients had hypertension at baseline.
Beetroot juice. A double-blind, placebo-controlled study showed that consumption of beetroot juice (with nitrate) once daily reduced BP in 3 different settings (clinic, 24-hour ambulatory, and home readings) when compared with placebo (nitrate-free beetroot juice).6 Study participants were mostly British women, overweight, without significant cardiovascular or renal disease, and with uncontrolled ambulatory BP (> 135/85 mm Hg).
Flax seed. A prospective, double-blind trial of patients with peripheral artery disease compared the antihypertensive effects of flax seed with placebo in patients with and without hypertension and found marked decreases in systolic and diastolic BP.7 Study participants were all older than 40 years without other major cardiac or renal disease, and the majority of enrolled patients with hypertension were concurrently taking medications to treat hypertension during the study.
Olive leaf extract. A double-blind, parallel, and active-control clinical trial in Indonesia compared the BP-lowering effect of olive leaf extract (Olea europaea) to captopril as monotherapies in patients with stage 1 hypertension.8 After a 4-week period of dietary intervention, individuals who were still hypertensive (range, 140/90 to 159/99 mm Hg) were treated with either olive leaf extract or captopril. After 8 weeks of treatment, both groups saw comparable reductions in BP.
Continue to: Editor's takeaway
Editor’s takeaway
Many studies have demonstrated BP benefits from a variety of natural supplements. Although the studies’ durations are short, the effects sometimes modest, and the outcomes disease-oriented rather than patient-oriented, the findings can provide a useful complement to our efforts to manage this most common chronic disease.
1. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;(4):CD008893.
2. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885-896.
3. Rohner A, Ried K, Sobenin IA, et al. A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423.
4. Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0174967.
5. Dong JY, Qin LQ, Zhang Z, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965.
6. Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320-327.
7. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081-1089.
8. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251-258.
1. Ried K, Fakler P, Stocks NP. Effect of cocoa on blood pressure. Cochrane Database Syst Rev. 2017;(4):CD008893.
2. Miller PE, Van Elswyk M, Alexander DD. Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens. 2014;27:885-896.
3. Rohner A, Ried K, Sobenin IA, et al. A systematic review and meta-analysis on the effects of garlic preparations on blood pressure in individuals with hypertension. Am J Hypertens. 2015;28:414-423.
4. Poorolajal J, Zeraati F, Soltanian AR, et al. Oral potassium supplementation for management of essential hypertension: a meta-analysis of randomized controlled trials. PLoS One. 2017;12:e0174967.
5. Dong JY, Qin LQ, Zhang Z, et al. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959-965.
6. Kapil V, Khambata RS, Robertson A, et al. Dietary nitrate provides sustained blood pressure lowering in hypertensive patients: a randomized, phase 2, double-blind, placebo-controlled study. Hypertension. 2015;65:320-327.
7. Rodriguez-Leyva D, Weighell W, Edel AL, et al. Potent antihypertensive action of dietary flaxseed in hypertensive patients. Hypertension. 2013;62:1081-1089.
8. Susalit E, Agus N, Effendi I, et al. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: comparison with captopril. Phytomedicine. 2011;18:251-258.
EVIDENCE-BASED ANSWER:
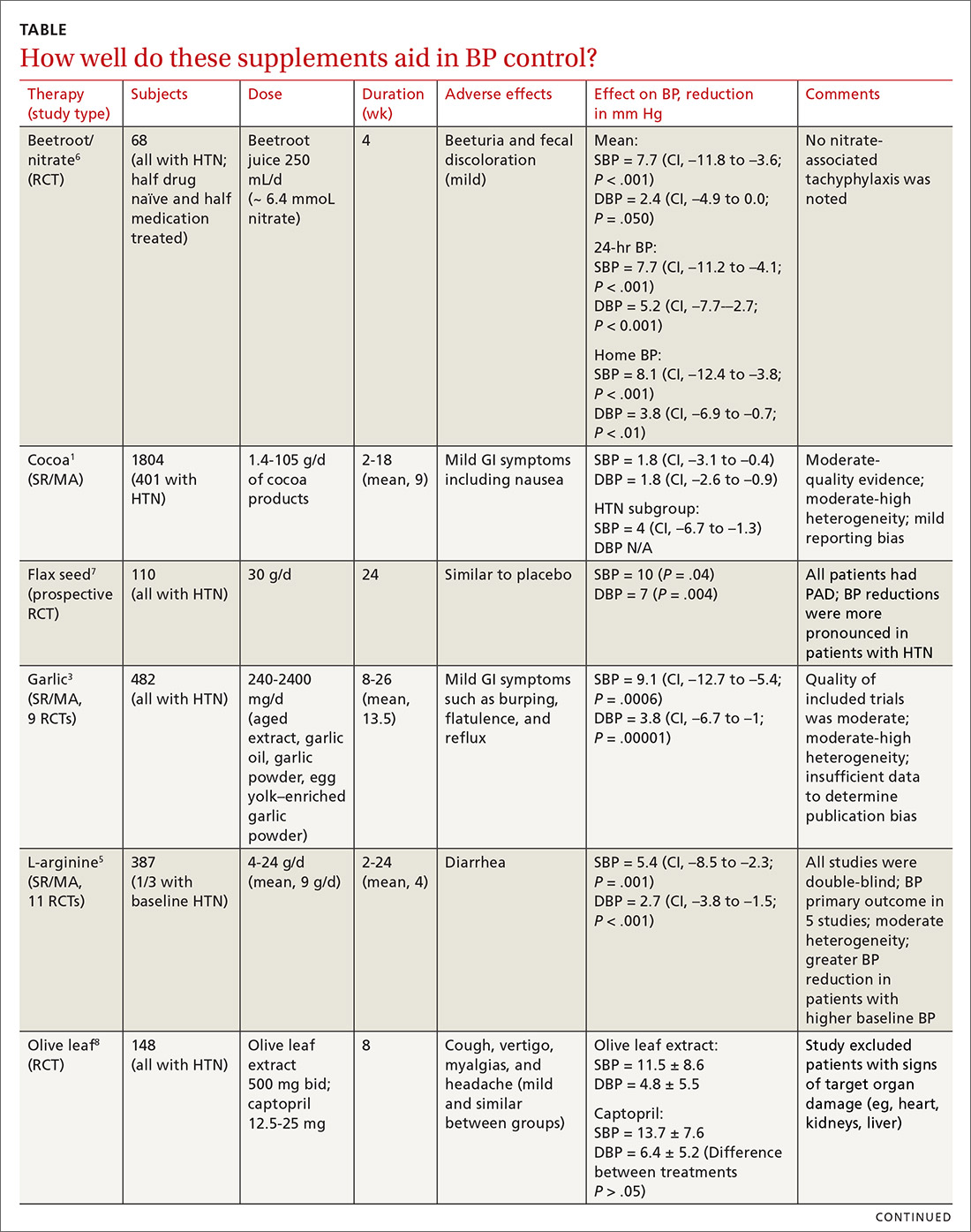
Yes. A number of well-tolerated natural therapies have been shown to reduce systolic and diastolic blood pressure (BP). (See Table1-8 for summary.) However, the studies don’t provide direct evidence of whether the decrease in BP is linked to patient-oriented outcomes. Nor do they allow definitive conclusions concerning the lasting nature of the reductions, because most studies were fewer than 6 months in duration (strength of recommendation: C, disease-oriented evidence).
AI can pinpoint COVID-19 from chest x-rays
Conventional chest x-rays combined with artificial intelligence (AI) can identify lung damage from COVID-19 and differentiate coronavirus patients from other patients, improving triage efforts, new research suggests.
The AI tool – developed by Jason Fleischer, PhD, and graduate student Mohammad Tariqul Islam, both from Princeton (N.J.) University – can distinguish COVID-19 patients from those with pneumonia or normal lung tissue with an accuracy of more than 95%.
“We were able to separate the COVID-19 patients with very high fidelity,” Dr. Fleischer said in an interview. “If you give me an x-ray now, I can say with very high confidence whether a patient has COVID-19.”
The diagnostic tool pinpoints patterns on x-ray images that are too subtle for even trained experts to notice. The precision of CT scanning is similar to that of the AI tool, but CT costs much more and has other disadvantages, said Dr. Fleischer, who presented his findings at the virtual European Respiratory Society International Congress 2020.
“CT is more expensive and uses higher doses of radiation,” he said. “Another big thing is that not everyone has tomography facilities – including a lot of rural places and developing countries – so you need something that’s on the spot.”
With machine learning, Dr. Fleischer analyzed 2,300 x-ray images: 1,018 “normal” images from patients who had neither pneumonia nor COVID-19, 1,011 from patients with pneumonia, and 271 from patients with COVID-19.
The AI tool uses a neural network to refine the number and type of lung features being tracked. A UMAP (Uniform Manifold Approximation and Projection) clustering algorithm then looks for similarities and differences in those images, he explained.
“We, as users, knew which type each x-ray was – normal, pneumonia positive, or COVID-19 positive – but the network did not,” he added.
Clinicians have observed two basic types of lung problems in COVID-19 patients: pneumonia that fills lung air sacs with fluid and dangerously low blood-oxygen levels despite nearly normal breathing patterns. Because treatment can vary according to type, it would be beneficial to quickly distinguish between them, Dr. Fleischer said.
The AI tool showed that there is a distinct difference in chest x-rays from pneumonia-positive patients and healthy people, he said. It also demonstrated two distinct clusters of COVID-19–positive chest x-rays: those that looked like pneumonia and those with a more normal presentation.
The fact that “the AI system recognizes something unique in chest x-rays from COVID-19–positive patients” indicates that the computer is able to identify visual markers for coronavirus, he explained. “We currently do not know what these markers are.”
Dr. Fleischer said his goal is not to replace physician decision-making, but to supplement it.
“I’m uncomfortable with having computers make the final decision,” he said. “They often have a narrow focus, whereas doctors have the big picture in mind.”
This AI tool is “very interesting,” especially in the context of expanding AI applications in various specialties, said Thierry Fumeaux, MD, from Nyon (Switzerland) Hospital. Some physicians currently disagree on whether a chest x-ray or CT scan is the better tool to help diagnose COVID-19.
“It seems better than the human eye and brain” to pinpoint COVID-19 lung damage, “so it’s very attractive as a technology,” Dr. Fumeaux said in an interview.
And AI can be used to supplement the efforts of busy and fatigued clinicians who might be stretched thin by large caseloads. “I cannot read 200 chest x-rays in a day, but a computer can do that in 2 minutes,” he said.
But Dr. Fumeaux offered a caveat: “Pattern recognition is promising, but at the moment I’m not aware of papers showing that, by using AI, you’re changing anything in the outcome of a patient.”
Ideally, Dr. Fleischer said he hopes that AI will soon be able to accurately indicate which treatments are most effective for individual COVID-19 patients. And the technology might eventually be used to help with treatment decisions for patients with asthma or chronic obstructive pulmonary disease, he noted.
But he needs more data before results indicate whether a COVID-19 patient would benefit from ventilator support, for example, and the tool can be used more widely. To contribute data or collaborate with Dr. Fleischer’s efforts, contact him.
“Machine learning is all about data, so you can find these correlations,” he said. “It would be nice to be able to use it to reassure a worried patient that their prognosis is good; to say that most of the people with symptoms like yours will be just fine.”
Dr. Fleischer and Dr. Fumeaux have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Conventional chest x-rays combined with artificial intelligence (AI) can identify lung damage from COVID-19 and differentiate coronavirus patients from other patients, improving triage efforts, new research suggests.
The AI tool – developed by Jason Fleischer, PhD, and graduate student Mohammad Tariqul Islam, both from Princeton (N.J.) University – can distinguish COVID-19 patients from those with pneumonia or normal lung tissue with an accuracy of more than 95%.
“We were able to separate the COVID-19 patients with very high fidelity,” Dr. Fleischer said in an interview. “If you give me an x-ray now, I can say with very high confidence whether a patient has COVID-19.”
The diagnostic tool pinpoints patterns on x-ray images that are too subtle for even trained experts to notice. The precision of CT scanning is similar to that of the AI tool, but CT costs much more and has other disadvantages, said Dr. Fleischer, who presented his findings at the virtual European Respiratory Society International Congress 2020.
“CT is more expensive and uses higher doses of radiation,” he said. “Another big thing is that not everyone has tomography facilities – including a lot of rural places and developing countries – so you need something that’s on the spot.”
With machine learning, Dr. Fleischer analyzed 2,300 x-ray images: 1,018 “normal” images from patients who had neither pneumonia nor COVID-19, 1,011 from patients with pneumonia, and 271 from patients with COVID-19.
The AI tool uses a neural network to refine the number and type of lung features being tracked. A UMAP (Uniform Manifold Approximation and Projection) clustering algorithm then looks for similarities and differences in those images, he explained.
“We, as users, knew which type each x-ray was – normal, pneumonia positive, or COVID-19 positive – but the network did not,” he added.
Clinicians have observed two basic types of lung problems in COVID-19 patients: pneumonia that fills lung air sacs with fluid and dangerously low blood-oxygen levels despite nearly normal breathing patterns. Because treatment can vary according to type, it would be beneficial to quickly distinguish between them, Dr. Fleischer said.
The AI tool showed that there is a distinct difference in chest x-rays from pneumonia-positive patients and healthy people, he said. It also demonstrated two distinct clusters of COVID-19–positive chest x-rays: those that looked like pneumonia and those with a more normal presentation.
The fact that “the AI system recognizes something unique in chest x-rays from COVID-19–positive patients” indicates that the computer is able to identify visual markers for coronavirus, he explained. “We currently do not know what these markers are.”
Dr. Fleischer said his goal is not to replace physician decision-making, but to supplement it.
“I’m uncomfortable with having computers make the final decision,” he said. “They often have a narrow focus, whereas doctors have the big picture in mind.”
This AI tool is “very interesting,” especially in the context of expanding AI applications in various specialties, said Thierry Fumeaux, MD, from Nyon (Switzerland) Hospital. Some physicians currently disagree on whether a chest x-ray or CT scan is the better tool to help diagnose COVID-19.
“It seems better than the human eye and brain” to pinpoint COVID-19 lung damage, “so it’s very attractive as a technology,” Dr. Fumeaux said in an interview.
And AI can be used to supplement the efforts of busy and fatigued clinicians who might be stretched thin by large caseloads. “I cannot read 200 chest x-rays in a day, but a computer can do that in 2 minutes,” he said.
But Dr. Fumeaux offered a caveat: “Pattern recognition is promising, but at the moment I’m not aware of papers showing that, by using AI, you’re changing anything in the outcome of a patient.”
Ideally, Dr. Fleischer said he hopes that AI will soon be able to accurately indicate which treatments are most effective for individual COVID-19 patients. And the technology might eventually be used to help with treatment decisions for patients with asthma or chronic obstructive pulmonary disease, he noted.
But he needs more data before results indicate whether a COVID-19 patient would benefit from ventilator support, for example, and the tool can be used more widely. To contribute data or collaborate with Dr. Fleischer’s efforts, contact him.
“Machine learning is all about data, so you can find these correlations,” he said. “It would be nice to be able to use it to reassure a worried patient that their prognosis is good; to say that most of the people with symptoms like yours will be just fine.”
Dr. Fleischer and Dr. Fumeaux have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Conventional chest x-rays combined with artificial intelligence (AI) can identify lung damage from COVID-19 and differentiate coronavirus patients from other patients, improving triage efforts, new research suggests.
The AI tool – developed by Jason Fleischer, PhD, and graduate student Mohammad Tariqul Islam, both from Princeton (N.J.) University – can distinguish COVID-19 patients from those with pneumonia or normal lung tissue with an accuracy of more than 95%.
“We were able to separate the COVID-19 patients with very high fidelity,” Dr. Fleischer said in an interview. “If you give me an x-ray now, I can say with very high confidence whether a patient has COVID-19.”
The diagnostic tool pinpoints patterns on x-ray images that are too subtle for even trained experts to notice. The precision of CT scanning is similar to that of the AI tool, but CT costs much more and has other disadvantages, said Dr. Fleischer, who presented his findings at the virtual European Respiratory Society International Congress 2020.
“CT is more expensive and uses higher doses of radiation,” he said. “Another big thing is that not everyone has tomography facilities – including a lot of rural places and developing countries – so you need something that’s on the spot.”
With machine learning, Dr. Fleischer analyzed 2,300 x-ray images: 1,018 “normal” images from patients who had neither pneumonia nor COVID-19, 1,011 from patients with pneumonia, and 271 from patients with COVID-19.
The AI tool uses a neural network to refine the number and type of lung features being tracked. A UMAP (Uniform Manifold Approximation and Projection) clustering algorithm then looks for similarities and differences in those images, he explained.
“We, as users, knew which type each x-ray was – normal, pneumonia positive, or COVID-19 positive – but the network did not,” he added.
Clinicians have observed two basic types of lung problems in COVID-19 patients: pneumonia that fills lung air sacs with fluid and dangerously low blood-oxygen levels despite nearly normal breathing patterns. Because treatment can vary according to type, it would be beneficial to quickly distinguish between them, Dr. Fleischer said.
The AI tool showed that there is a distinct difference in chest x-rays from pneumonia-positive patients and healthy people, he said. It also demonstrated two distinct clusters of COVID-19–positive chest x-rays: those that looked like pneumonia and those with a more normal presentation.
The fact that “the AI system recognizes something unique in chest x-rays from COVID-19–positive patients” indicates that the computer is able to identify visual markers for coronavirus, he explained. “We currently do not know what these markers are.”
Dr. Fleischer said his goal is not to replace physician decision-making, but to supplement it.
“I’m uncomfortable with having computers make the final decision,” he said. “They often have a narrow focus, whereas doctors have the big picture in mind.”
This AI tool is “very interesting,” especially in the context of expanding AI applications in various specialties, said Thierry Fumeaux, MD, from Nyon (Switzerland) Hospital. Some physicians currently disagree on whether a chest x-ray or CT scan is the better tool to help diagnose COVID-19.
“It seems better than the human eye and brain” to pinpoint COVID-19 lung damage, “so it’s very attractive as a technology,” Dr. Fumeaux said in an interview.
And AI can be used to supplement the efforts of busy and fatigued clinicians who might be stretched thin by large caseloads. “I cannot read 200 chest x-rays in a day, but a computer can do that in 2 minutes,” he said.
But Dr. Fumeaux offered a caveat: “Pattern recognition is promising, but at the moment I’m not aware of papers showing that, by using AI, you’re changing anything in the outcome of a patient.”
Ideally, Dr. Fleischer said he hopes that AI will soon be able to accurately indicate which treatments are most effective for individual COVID-19 patients. And the technology might eventually be used to help with treatment decisions for patients with asthma or chronic obstructive pulmonary disease, he noted.
But he needs more data before results indicate whether a COVID-19 patient would benefit from ventilator support, for example, and the tool can be used more widely. To contribute data or collaborate with Dr. Fleischer’s efforts, contact him.
“Machine learning is all about data, so you can find these correlations,” he said. “It would be nice to be able to use it to reassure a worried patient that their prognosis is good; to say that most of the people with symptoms like yours will be just fine.”
Dr. Fleischer and Dr. Fumeaux have declared no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Small weight loss produces impressive drop in type 2 diabetes risk
Intentional loss of a median of just 13% of body weight reduces the relative risk of developing type 2 diabetes by around 40% in people with obesity, among many other health benefits, shows a large real-world study in half a million adults.
Other findings associated with the same modest weight loss included a reduction in the risk of sleep apnea by 22%-27%, hypertension by 18%-25%, and dyslipidemia by 20%-22%.
Christiane Haase, PhD, of Novo Nordisk, led the work together with Nick Finer, MD, senior principal clinical scientist, Novo Nordisk.
“This is powerful evidence to say it is worthwhile to help people lose weight and that it is hugely beneficial. These are not small effects, and they show that weight loss has a huge impact on health. It’s extraordinary,” Dr. Finer asserted.
“These data show that if we treat obesity first, rather than the complications, we actually get big results in terms of health. This really should be a game-changer for those health care systems that are still prevaricating about treating obesity seriously,” he added.
The size of the study, of over 550,000 U.K. adults in primary care, makes it unique. In the real-world cohort, people who had lost 10%-25% of their body weight were followed for a mean 8 years to see how this affected their subsequent risk of obesity-related conditions. The results were presented during the virtual European and International Congress on Obesity.
“Weight loss was real-world without any artificial intervention and they experienced a real-life reduction in risk of various obesity-related conditions,” Dr. Haase said in an interview.
Carel Le Roux, MD, PhD, from the Diabetes Complications Research Centre, University College Dublin, welcomed the study because it showed those with obesity who maintained more than 10% weight loss experienced a significant reduction in the complications of obesity.
“In the study, intentional weight loss was achieved using mainly diets and exercise, but also some medications and surgical treatments. However, it did not matter how patients were able to maintain the 10% or more weight loss as regards the positive impact on complications of obesity,” he highlighted.
From a clinician standpoint, “it helps to consider all the weight-loss options available, but also for those who are not able to achieve weight-loss maintenance, to escalate treatment. This is now possible as we gain access to more effective treatments,” he added.
Also commenting on the findings, Matt Petersen, vice president of medical information and professional engagement at the American Diabetes Association, said: “It’s helpful to have further evidence that weight loss reduces risk for type 2 diabetes.”
However, “finding effective strategies to achieve and maintain long-term weight loss and maintenance remains a significant challenge,” he observed.
Large database of half a million people with obesity
For the research, anonymized data from over half a million patients documented in the Clinical Practice Research Datalink database, which holds information from 674 general practices in the United Kingdom, were linked to Hospital Episode Statistics and prescribing data to determine comorbidity outcomes.
At baseline, characteristics for the full study population included a median age of 54 years, around 50% of participants had hypertension, around 40% had dyslipidemia, and around 20% had type 2 diabetes. Less than 10% had sleep apnea, hip/knee osteoarthritis, or history of cardiovascular disease. All participants had a body mass index (BMI) of 25.0-50.0 kg/m2 at the start of the follow-up, between January 2001 and December 2010.
Patients may have been advised to lose weight, or take more exercise, or have been referred to a dietitian. Some had been prescribed antiobesity medications available between 2001 and 2010. (Novo Nordisk medications for obesity were unavailable during this period.) Less than 1% had been referred for bariatric surgery.
“This is typical of real-world management of obesity,” Dr. Haase pointed out.
Participants were divided into two categories based on their weight pattern during the 4-year period: one whose weight remained stable (492,380 individuals with BMI change within –5% to 5%) and one who lost weight (60,573 with BMI change –10% to –25%).
The median change in BMI in the weight-loss group was –13%. The researchers also extracted information on weight loss interventions and dietary advice to confirm intention to lose weight.
The benefits of losing 13% of body weight were then determined for three risk profiles: BMI reduction from 34.5 to 30 (obesity class I level); from 40.3 to 35 (obesity class II level), and from46 to 40 (obesity class III level).
Individuals with a baseline history of any particular outcome were excluded from the risk analysis for that same outcome. All analyses were adjusted for BMI, age, gender, smoking status, and baseline comorbidities.
Study strengths include the large number of participants and the relatively long follow-up period. But the observational nature of the study limits the ability to know the ways in which the participants who lost weight may have differed from those who maintained or gained weight, the authors said.
Type 2 diabetes, sleep apnea showed greatest risk reductions
The researchers looked at the risk reduction for various comorbidities after weight loss, compared with before weight loss. They also examined the risk reductions after weight loss, compared with someone who had always had a median 13% lower weight.
Effectively, the analysis provided a measure of the effect of risk reduction because of weight loss, compared with having that lower weight as a stable weight.
“The analysis asks if the person’s risk was reversed by the weight loss to the risk associated with that of the lower weight level,” explained Dr. Haase.
“We found that the risks of type 2 diabetes, dyslipidemia, and hypertension were reversed while the risk of sleep apnea and hip/knee osteoarthritis showed some residual risk,” she added.
With sleep apnea there was a risk reduction of up to 27%, compared with before weight loss.
“This is a condition that can’t be easily reversed except with mechanical sleeping devices and it is underrecognized and causes a lot of distress. There’s actually a link between sleep apnea, diabetes, and hypertension in a two-way connection,” noted Dr. Finer, who is also honorary professor of cardiovascular medicine at University College London.
“A reduction of this proportion is impressive,” he stressed.
Dyslipidemia, hypertension, and type 2 diabetes are well-known cardiovascular risk factors. “We did not see any impact on myocardial infarction,” which “might be due to length of follow-up,” noted Dr. Haase.
Response of type 2 diabetes to weight loss
Most patients in the study did not have type 2 diabetes at baseline, and Dr. Finer commented on how weight loss might affect type 2 diabetes risk.
“The complications of obesity resolve with weight loss at different speeds,” he said.
“Type 2 diabetes is very sensitive to weight loss and improvements are obvious in weeks to months.”
In contrast, reductions in risk of obstructive sleep apnea “take longer and might depend on the amount of weight lost.” And with osteoarthritis, “It’s hard to show improvement with weight loss because irreparable damage has [already] been done,” he explained.
The degree of improvement in diabetes because of weight loss is partly dependent on how long the person has had diabetes, Dr. Finer further explained. “If someone has less excess weight then the diabetes might have had a shorter duration and therefore response might be greater.”
Lucy Chambers, PhD, head of research communications at Diabetes UK, said: “We’ve known for a long time that carrying extra weight can increase your risk of developing type 2 diabetes, and this new study adds to the extensive body of evidence showing that losing some of this weight is associated with reduced risk.”
She acknowledged, however, that losing weight is difficult and that support is important: “We need government to urgently review provision of weight management services and take action to address the barriers to accessing them.”
Dr. Finer and Dr. Haase are both employees of Novo Nordisk. Dr. Le Roux reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Intentional loss of a median of just 13% of body weight reduces the relative risk of developing type 2 diabetes by around 40% in people with obesity, among many other health benefits, shows a large real-world study in half a million adults.
Other findings associated with the same modest weight loss included a reduction in the risk of sleep apnea by 22%-27%, hypertension by 18%-25%, and dyslipidemia by 20%-22%.
Christiane Haase, PhD, of Novo Nordisk, led the work together with Nick Finer, MD, senior principal clinical scientist, Novo Nordisk.
“This is powerful evidence to say it is worthwhile to help people lose weight and that it is hugely beneficial. These are not small effects, and they show that weight loss has a huge impact on health. It’s extraordinary,” Dr. Finer asserted.
“These data show that if we treat obesity first, rather than the complications, we actually get big results in terms of health. This really should be a game-changer for those health care systems that are still prevaricating about treating obesity seriously,” he added.
The size of the study, of over 550,000 U.K. adults in primary care, makes it unique. In the real-world cohort, people who had lost 10%-25% of their body weight were followed for a mean 8 years to see how this affected their subsequent risk of obesity-related conditions. The results were presented during the virtual European and International Congress on Obesity.
“Weight loss was real-world without any artificial intervention and they experienced a real-life reduction in risk of various obesity-related conditions,” Dr. Haase said in an interview.
Carel Le Roux, MD, PhD, from the Diabetes Complications Research Centre, University College Dublin, welcomed the study because it showed those with obesity who maintained more than 10% weight loss experienced a significant reduction in the complications of obesity.
“In the study, intentional weight loss was achieved using mainly diets and exercise, but also some medications and surgical treatments. However, it did not matter how patients were able to maintain the 10% or more weight loss as regards the positive impact on complications of obesity,” he highlighted.
From a clinician standpoint, “it helps to consider all the weight-loss options available, but also for those who are not able to achieve weight-loss maintenance, to escalate treatment. This is now possible as we gain access to more effective treatments,” he added.
Also commenting on the findings, Matt Petersen, vice president of medical information and professional engagement at the American Diabetes Association, said: “It’s helpful to have further evidence that weight loss reduces risk for type 2 diabetes.”
However, “finding effective strategies to achieve and maintain long-term weight loss and maintenance remains a significant challenge,” he observed.
Large database of half a million people with obesity
For the research, anonymized data from over half a million patients documented in the Clinical Practice Research Datalink database, which holds information from 674 general practices in the United Kingdom, were linked to Hospital Episode Statistics and prescribing data to determine comorbidity outcomes.
At baseline, characteristics for the full study population included a median age of 54 years, around 50% of participants had hypertension, around 40% had dyslipidemia, and around 20% had type 2 diabetes. Less than 10% had sleep apnea, hip/knee osteoarthritis, or history of cardiovascular disease. All participants had a body mass index (BMI) of 25.0-50.0 kg/m2 at the start of the follow-up, between January 2001 and December 2010.
Patients may have been advised to lose weight, or take more exercise, or have been referred to a dietitian. Some had been prescribed antiobesity medications available between 2001 and 2010. (Novo Nordisk medications for obesity were unavailable during this period.) Less than 1% had been referred for bariatric surgery.
“This is typical of real-world management of obesity,” Dr. Haase pointed out.
Participants were divided into two categories based on their weight pattern during the 4-year period: one whose weight remained stable (492,380 individuals with BMI change within –5% to 5%) and one who lost weight (60,573 with BMI change –10% to –25%).
The median change in BMI in the weight-loss group was –13%. The researchers also extracted information on weight loss interventions and dietary advice to confirm intention to lose weight.
The benefits of losing 13% of body weight were then determined for three risk profiles: BMI reduction from 34.5 to 30 (obesity class I level); from 40.3 to 35 (obesity class II level), and from46 to 40 (obesity class III level).
Individuals with a baseline history of any particular outcome were excluded from the risk analysis for that same outcome. All analyses were adjusted for BMI, age, gender, smoking status, and baseline comorbidities.
Study strengths include the large number of participants and the relatively long follow-up period. But the observational nature of the study limits the ability to know the ways in which the participants who lost weight may have differed from those who maintained or gained weight, the authors said.
Type 2 diabetes, sleep apnea showed greatest risk reductions
The researchers looked at the risk reduction for various comorbidities after weight loss, compared with before weight loss. They also examined the risk reductions after weight loss, compared with someone who had always had a median 13% lower weight.
Effectively, the analysis provided a measure of the effect of risk reduction because of weight loss, compared with having that lower weight as a stable weight.
“The analysis asks if the person’s risk was reversed by the weight loss to the risk associated with that of the lower weight level,” explained Dr. Haase.
“We found that the risks of type 2 diabetes, dyslipidemia, and hypertension were reversed while the risk of sleep apnea and hip/knee osteoarthritis showed some residual risk,” she added.
With sleep apnea there was a risk reduction of up to 27%, compared with before weight loss.
“This is a condition that can’t be easily reversed except with mechanical sleeping devices and it is underrecognized and causes a lot of distress. There’s actually a link between sleep apnea, diabetes, and hypertension in a two-way connection,” noted Dr. Finer, who is also honorary professor of cardiovascular medicine at University College London.
“A reduction of this proportion is impressive,” he stressed.
Dyslipidemia, hypertension, and type 2 diabetes are well-known cardiovascular risk factors. “We did not see any impact on myocardial infarction,” which “might be due to length of follow-up,” noted Dr. Haase.
Response of type 2 diabetes to weight loss
Most patients in the study did not have type 2 diabetes at baseline, and Dr. Finer commented on how weight loss might affect type 2 diabetes risk.
“The complications of obesity resolve with weight loss at different speeds,” he said.
“Type 2 diabetes is very sensitive to weight loss and improvements are obvious in weeks to months.”
In contrast, reductions in risk of obstructive sleep apnea “take longer and might depend on the amount of weight lost.” And with osteoarthritis, “It’s hard to show improvement with weight loss because irreparable damage has [already] been done,” he explained.
The degree of improvement in diabetes because of weight loss is partly dependent on how long the person has had diabetes, Dr. Finer further explained. “If someone has less excess weight then the diabetes might have had a shorter duration and therefore response might be greater.”
Lucy Chambers, PhD, head of research communications at Diabetes UK, said: “We’ve known for a long time that carrying extra weight can increase your risk of developing type 2 diabetes, and this new study adds to the extensive body of evidence showing that losing some of this weight is associated with reduced risk.”
She acknowledged, however, that losing weight is difficult and that support is important: “We need government to urgently review provision of weight management services and take action to address the barriers to accessing them.”
Dr. Finer and Dr. Haase are both employees of Novo Nordisk. Dr. Le Roux reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Intentional loss of a median of just 13% of body weight reduces the relative risk of developing type 2 diabetes by around 40% in people with obesity, among many other health benefits, shows a large real-world study in half a million adults.
Other findings associated with the same modest weight loss included a reduction in the risk of sleep apnea by 22%-27%, hypertension by 18%-25%, and dyslipidemia by 20%-22%.
Christiane Haase, PhD, of Novo Nordisk, led the work together with Nick Finer, MD, senior principal clinical scientist, Novo Nordisk.
“This is powerful evidence to say it is worthwhile to help people lose weight and that it is hugely beneficial. These are not small effects, and they show that weight loss has a huge impact on health. It’s extraordinary,” Dr. Finer asserted.
“These data show that if we treat obesity first, rather than the complications, we actually get big results in terms of health. This really should be a game-changer for those health care systems that are still prevaricating about treating obesity seriously,” he added.
The size of the study, of over 550,000 U.K. adults in primary care, makes it unique. In the real-world cohort, people who had lost 10%-25% of their body weight were followed for a mean 8 years to see how this affected their subsequent risk of obesity-related conditions. The results were presented during the virtual European and International Congress on Obesity.
“Weight loss was real-world without any artificial intervention and they experienced a real-life reduction in risk of various obesity-related conditions,” Dr. Haase said in an interview.
Carel Le Roux, MD, PhD, from the Diabetes Complications Research Centre, University College Dublin, welcomed the study because it showed those with obesity who maintained more than 10% weight loss experienced a significant reduction in the complications of obesity.
“In the study, intentional weight loss was achieved using mainly diets and exercise, but also some medications and surgical treatments. However, it did not matter how patients were able to maintain the 10% or more weight loss as regards the positive impact on complications of obesity,” he highlighted.
From a clinician standpoint, “it helps to consider all the weight-loss options available, but also for those who are not able to achieve weight-loss maintenance, to escalate treatment. This is now possible as we gain access to more effective treatments,” he added.
Also commenting on the findings, Matt Petersen, vice president of medical information and professional engagement at the American Diabetes Association, said: “It’s helpful to have further evidence that weight loss reduces risk for type 2 diabetes.”
However, “finding effective strategies to achieve and maintain long-term weight loss and maintenance remains a significant challenge,” he observed.
Large database of half a million people with obesity
For the research, anonymized data from over half a million patients documented in the Clinical Practice Research Datalink database, which holds information from 674 general practices in the United Kingdom, were linked to Hospital Episode Statistics and prescribing data to determine comorbidity outcomes.
At baseline, characteristics for the full study population included a median age of 54 years, around 50% of participants had hypertension, around 40% had dyslipidemia, and around 20% had type 2 diabetes. Less than 10% had sleep apnea, hip/knee osteoarthritis, or history of cardiovascular disease. All participants had a body mass index (BMI) of 25.0-50.0 kg/m2 at the start of the follow-up, between January 2001 and December 2010.
Patients may have been advised to lose weight, or take more exercise, or have been referred to a dietitian. Some had been prescribed antiobesity medications available between 2001 and 2010. (Novo Nordisk medications for obesity were unavailable during this period.) Less than 1% had been referred for bariatric surgery.
“This is typical of real-world management of obesity,” Dr. Haase pointed out.
Participants were divided into two categories based on their weight pattern during the 4-year period: one whose weight remained stable (492,380 individuals with BMI change within –5% to 5%) and one who lost weight (60,573 with BMI change –10% to –25%).
The median change in BMI in the weight-loss group was –13%. The researchers also extracted information on weight loss interventions and dietary advice to confirm intention to lose weight.
The benefits of losing 13% of body weight were then determined for three risk profiles: BMI reduction from 34.5 to 30 (obesity class I level); from 40.3 to 35 (obesity class II level), and from46 to 40 (obesity class III level).
Individuals with a baseline history of any particular outcome were excluded from the risk analysis for that same outcome. All analyses were adjusted for BMI, age, gender, smoking status, and baseline comorbidities.
Study strengths include the large number of participants and the relatively long follow-up period. But the observational nature of the study limits the ability to know the ways in which the participants who lost weight may have differed from those who maintained or gained weight, the authors said.
Type 2 diabetes, sleep apnea showed greatest risk reductions
The researchers looked at the risk reduction for various comorbidities after weight loss, compared with before weight loss. They also examined the risk reductions after weight loss, compared with someone who had always had a median 13% lower weight.
Effectively, the analysis provided a measure of the effect of risk reduction because of weight loss, compared with having that lower weight as a stable weight.
“The analysis asks if the person’s risk was reversed by the weight loss to the risk associated with that of the lower weight level,” explained Dr. Haase.
“We found that the risks of type 2 diabetes, dyslipidemia, and hypertension were reversed while the risk of sleep apnea and hip/knee osteoarthritis showed some residual risk,” she added.
With sleep apnea there was a risk reduction of up to 27%, compared with before weight loss.
“This is a condition that can’t be easily reversed except with mechanical sleeping devices and it is underrecognized and causes a lot of distress. There’s actually a link between sleep apnea, diabetes, and hypertension in a two-way connection,” noted Dr. Finer, who is also honorary professor of cardiovascular medicine at University College London.
“A reduction of this proportion is impressive,” he stressed.
Dyslipidemia, hypertension, and type 2 diabetes are well-known cardiovascular risk factors. “We did not see any impact on myocardial infarction,” which “might be due to length of follow-up,” noted Dr. Haase.
Response of type 2 diabetes to weight loss
Most patients in the study did not have type 2 diabetes at baseline, and Dr. Finer commented on how weight loss might affect type 2 diabetes risk.
“The complications of obesity resolve with weight loss at different speeds,” he said.
“Type 2 diabetes is very sensitive to weight loss and improvements are obvious in weeks to months.”
In contrast, reductions in risk of obstructive sleep apnea “take longer and might depend on the amount of weight lost.” And with osteoarthritis, “It’s hard to show improvement with weight loss because irreparable damage has [already] been done,” he explained.
The degree of improvement in diabetes because of weight loss is partly dependent on how long the person has had diabetes, Dr. Finer further explained. “If someone has less excess weight then the diabetes might have had a shorter duration and therefore response might be greater.”
Lucy Chambers, PhD, head of research communications at Diabetes UK, said: “We’ve known for a long time that carrying extra weight can increase your risk of developing type 2 diabetes, and this new study adds to the extensive body of evidence showing that losing some of this weight is associated with reduced risk.”
She acknowledged, however, that losing weight is difficult and that support is important: “We need government to urgently review provision of weight management services and take action to address the barriers to accessing them.”
Dr. Finer and Dr. Haase are both employees of Novo Nordisk. Dr. Le Roux reported no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
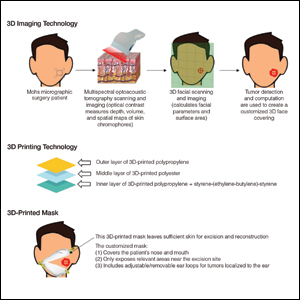
Use of 3D Technology to Support Dermatologists Returning to Practice Amid COVID-19
Coronavirus disease 2019 (COVID-19) has spread across all 7 continents, including 185 countries, and infected more than 21.9 million individuals worldwide as of August 18, 2020, according to the Johns Hopkins Coronavirus Resource Center. It has strained our health care system and affected all specialties, including dermatology. Dermatologists have taken important safety measures by canceling/deferring elective and nonemergency procedures and diagnosing/treating patients via telemedicine. Many residents and attending dermatologists have volunteered to care for COVID-19 inpatients and donated
N95 masks are necessary during the COVID-19 pandemic because they effectively filter at least 95% of 0.3-μm airborne particles and provide adequate face seals.1 3-Dimensional imaging integrated with 3D printers can be used to scan precise facial parameters (eg, jawline, nose) and account for facial hair density and length to produce comfortable tailored N95 masks and face seals.1,2 3-Dimensional printing utilizes robotics and
Face shields offer an additional layer of safety for the face and mucosae and also may provide longevity for N95 masks. Using synthetic polymers such as polycarbonate and polyethylene, 3D printers can be used to construct face shields via fused deposition modeling.1 These face shields may be worn over N95 masks and then can be sanitized and reused.
Mohs surgeons and staff may be at particularly high risk for COVID-19 infection due to their close proximity to the face during surgery, use of cautery, and prolonged time spent with patients while taking layers and suturing.

As dermatologists reopen and ramp up practice volume, there will be increased PPE requirements. Using 3D technology and imaging to produce N95 masks, face shields, and face coverings, we can offer effective diagnosis and treatment while optimizing safety for dermatologists, staff, and patients.
- Ishack S, Lipner SR. Applications of 3D printing technology to address COVID-19-related supply shortages [published online April 21, 2020]. Am J Med. 2020;133:771-773.
- Cai M, Li H, Shen S, et al. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J Occup Environ Hyg. 2018;3:226-234.
- Ishack S, Lipner SR. A review of 3-dimensional skin bioprinting techniques: applications, approaches, and trends [published online March 17, 2020]. Dermatol Surg. doi:10.1097/DSS.0000000000002378.
- Banerjee SS, Burbine S, Shivaprakash NK, et al. 3D-printable PP/SEBS thermoplastic elastomeric blends: preparation and properties [published online February 17, 2019]. Polymers (Basel). doi:10.3390/polym11020347.
- Chuah SY, Attia ABE, Long V. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography [published online November 2, 2016]. Skin Res Technol. 2017;23:221-226.
Coronavirus disease 2019 (COVID-19) has spread across all 7 continents, including 185 countries, and infected more than 21.9 million individuals worldwide as of August 18, 2020, according to the Johns Hopkins Coronavirus Resource Center. It has strained our health care system and affected all specialties, including dermatology. Dermatologists have taken important safety measures by canceling/deferring elective and nonemergency procedures and diagnosing/treating patients via telemedicine. Many residents and attending dermatologists have volunteered to care for COVID-19 inpatients and donated
N95 masks are necessary during the COVID-19 pandemic because they effectively filter at least 95% of 0.3-μm airborne particles and provide adequate face seals.1 3-Dimensional imaging integrated with 3D printers can be used to scan precise facial parameters (eg, jawline, nose) and account for facial hair density and length to produce comfortable tailored N95 masks and face seals.1,2 3-Dimensional printing utilizes robotics and
Face shields offer an additional layer of safety for the face and mucosae and also may provide longevity for N95 masks. Using synthetic polymers such as polycarbonate and polyethylene, 3D printers can be used to construct face shields via fused deposition modeling.1 These face shields may be worn over N95 masks and then can be sanitized and reused.
Mohs surgeons and staff may be at particularly high risk for COVID-19 infection due to their close proximity to the face during surgery, use of cautery, and prolonged time spent with patients while taking layers and suturing.

As dermatologists reopen and ramp up practice volume, there will be increased PPE requirements. Using 3D technology and imaging to produce N95 masks, face shields, and face coverings, we can offer effective diagnosis and treatment while optimizing safety for dermatologists, staff, and patients.
Coronavirus disease 2019 (COVID-19) has spread across all 7 continents, including 185 countries, and infected more than 21.9 million individuals worldwide as of August 18, 2020, according to the Johns Hopkins Coronavirus Resource Center. It has strained our health care system and affected all specialties, including dermatology. Dermatologists have taken important safety measures by canceling/deferring elective and nonemergency procedures and diagnosing/treating patients via telemedicine. Many residents and attending dermatologists have volunteered to care for COVID-19 inpatients and donated
N95 masks are necessary during the COVID-19 pandemic because they effectively filter at least 95% of 0.3-μm airborne particles and provide adequate face seals.1 3-Dimensional imaging integrated with 3D printers can be used to scan precise facial parameters (eg, jawline, nose) and account for facial hair density and length to produce comfortable tailored N95 masks and face seals.1,2 3-Dimensional printing utilizes robotics and
Face shields offer an additional layer of safety for the face and mucosae and also may provide longevity for N95 masks. Using synthetic polymers such as polycarbonate and polyethylene, 3D printers can be used to construct face shields via fused deposition modeling.1 These face shields may be worn over N95 masks and then can be sanitized and reused.
Mohs surgeons and staff may be at particularly high risk for COVID-19 infection due to their close proximity to the face during surgery, use of cautery, and prolonged time spent with patients while taking layers and suturing.

As dermatologists reopen and ramp up practice volume, there will be increased PPE requirements. Using 3D technology and imaging to produce N95 masks, face shields, and face coverings, we can offer effective diagnosis and treatment while optimizing safety for dermatologists, staff, and patients.
- Ishack S, Lipner SR. Applications of 3D printing technology to address COVID-19-related supply shortages [published online April 21, 2020]. Am J Med. 2020;133:771-773.
- Cai M, Li H, Shen S, et al. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J Occup Environ Hyg. 2018;3:226-234.
- Ishack S, Lipner SR. A review of 3-dimensional skin bioprinting techniques: applications, approaches, and trends [published online March 17, 2020]. Dermatol Surg. doi:10.1097/DSS.0000000000002378.
- Banerjee SS, Burbine S, Shivaprakash NK, et al. 3D-printable PP/SEBS thermoplastic elastomeric blends: preparation and properties [published online February 17, 2019]. Polymers (Basel). doi:10.3390/polym11020347.
- Chuah SY, Attia ABE, Long V. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography [published online November 2, 2016]. Skin Res Technol. 2017;23:221-226.
- Ishack S, Lipner SR. Applications of 3D printing technology to address COVID-19-related supply shortages [published online April 21, 2020]. Am J Med. 2020;133:771-773.
- Cai M, Li H, Shen S, et al. Customized design and 3D printing of face seal for an N95 filtering facepiece respirator. J Occup Environ Hyg. 2018;3:226-234.
- Ishack S, Lipner SR. A review of 3-dimensional skin bioprinting techniques: applications, approaches, and trends [published online March 17, 2020]. Dermatol Surg. doi:10.1097/DSS.0000000000002378.
- Banerjee SS, Burbine S, Shivaprakash NK, et al. 3D-printable PP/SEBS thermoplastic elastomeric blends: preparation and properties [published online February 17, 2019]. Polymers (Basel). doi:10.3390/polym11020347.
- Chuah SY, Attia ABE, Long V. Structural and functional 3D mapping of skin tumours with non-invasive multispectral optoacoustic tomography [published online November 2, 2016]. Skin Res Technol. 2017;23:221-226.
Practice Points
- Coronavirus disease 19 has overwhelmed our health care system and affected all specialties, including dermatology.
- There are concerns about shortages of personal protective equipment to safely care for patients.
- 3-Dimensional imaging and printing technologies can be harnessed to create face coverings and face shields for the dermatology outpatient setting.
What’s Eating You? Oriental Rat Flea (Xenopsylla cheopis)
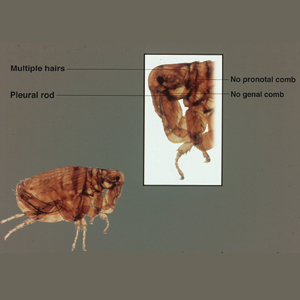
A dult Siphonaptera (fleas) are highly adapted to life on the surface of their hosts. Their small 2- to 10-mm bodies are laterally flattened and wingless. They utilize particularly strong hind legs for jumping up to 150 times their body length and backward-directed spines on their legs and bodies for moving forward through fur, hair, and feathers. Xenopsylla cheopis , the oriental rat flea, lacks pronotal and genal combs and has a mesopleuron divided by internal scleritinization (Figure). These features differentiate the species from its close relatives, Ctenocephalides (cat and dog fleas), which have both sets of combs, as well as Pulex irritans (human flea), which do not have a divided mesopleuron. 1,2
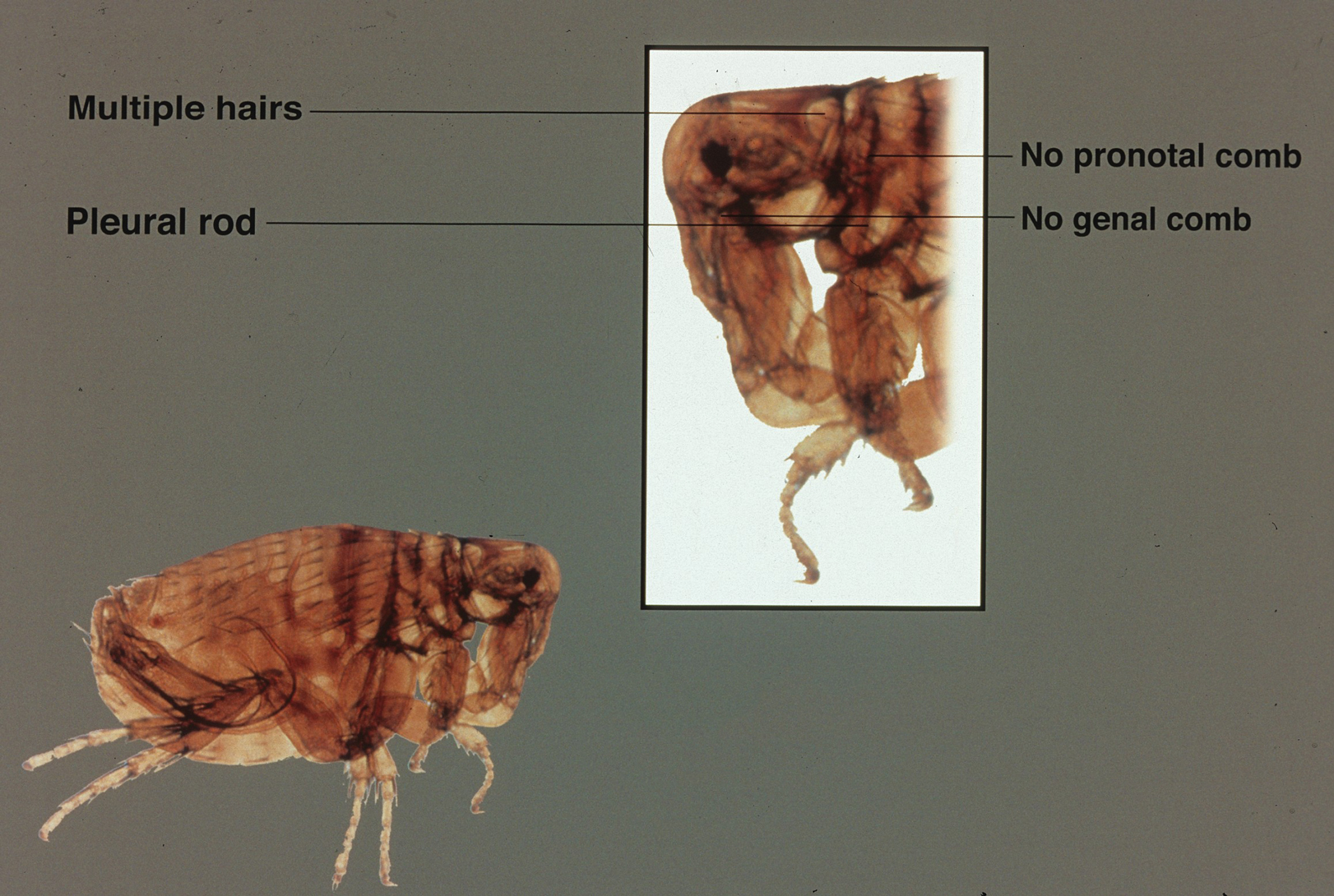
Flea-borne infections are extremely important to public health and are present throughout the world. Further, humidity and warmth are essential for the life cycle of many species of fleas. Predicted global warming likely will increase their distribution, allowing the spread of diseases they carry into previously untouched areas.1 Therefore, it is important to continue to examine species that carry particularly dangerous pathogens, such as X cheopis.
Disease Vector
Xenopsylla cheopis primarily is known for being a vector in the transmission of Yersinia pestis, the etiologic agent of the plague. Plague occurs in 3 forms: bubonic, pneumonic, and septicemic. It has caused major epidemics throughout history, the most widely known being the Black Death, which lasted for 130 years, beginning in the 1330s in China and spreading into Europe where it wiped out one-third of the population. However, bubonic plague is thought to have caused documented outbreaks as early as 320
Between January 2010 and December 2015, 3248 cases of plague in humans were reported, resulting in 584 deaths worldwide.5 It is thought that the plague originated in Central Asia, and this area still is a focus of disease. However, the at-risk population is reduced to breeders and hunters of gerbils and marmots, the main reservoirs in the area. In Africa, 4 countries still regularly report cases, with Madagascar being the most severely affected country in the world.5 The Democratic Republic of the Congo, Uganda, and Tanzania also are affected. The Americas also experience the plague. There are sporadic cases of bubonic plague in the northwest corner of Peru, mostly in rural areas. In the western United States, plague circulates among wild rodents, resulting in several reported cases each year, with the most recent confirmed case noted in California in August 2020.5,6 Further adding to its relevance, Y pestis is one of several organisms most likely to be used as a biologic weapon.3,4
Due to the historical and continued significance of Y pestis, many studies have been performed over the decades regarding its association with X cheopis. It has been discovered that fleas transmit the bacteria to the host in 2 ways. The most well-defined form of transmission occurs after an incubation period of Y pestis in the flea for 7 to 31 days. During this time, the bacteria form a dense biofilm on a valve in the flea foregut—the proventriculus—interfering with its function, which allows regurgitation of the blood and the bacteria it contains into the bite site and consequently disease transmission. The proventriculus can become completely blocked in some fleas, preventing any blood from reaching the midgut and causing starvation. In these scenarios, the flea will make continuous attempts to feed, increasing transmission.7 The hemin storage gene, hms, encoding the second messenger molecule cyclic di-GMP plays a critical role in biofilm formation and proventricular blockage.8 The phosphoheptose isomerase gene, GmhA, also has been elucidated as crucial in late transmission due to its role in biofilm formation.9 Early-phase transmission, or biofilm-independent transmission, has been documented to occur as early as 3 hours after infection of the flea but can occur for up to 4 days.10 Historically, the importance of early-phase transmission has been overlooked. Research has shown that it likely is crucial to the epizootic transmission of the plague.10 As a result, the search has begun for genes that contribute to the maintenance of Y pestis in the flea vector during the first 4 days of colonization. It is thought that a key evolutionary development was the selective loss-of-function mutation in a gene essential for the activity of urease, an enzyme that causes acute oral toxicity and mortality in fleas.11,12 The Yersinia murine toxin gene, Ymt, also allows for early survival of Y pestis in the flea midgut by producing a phospholipase D that protects the bacteria from toxic by-products produced during digestion of blood.11,13 In addition, gene products that function in lipid A modification are crucial for the ability of Y pestis to resist the action of cationic antimicrobial peptides it produces, such as cecropin A and polymyxin B.13
Murine typhus, an acute febrile illness caused by Rickettsia typhi, is another disease that can be spread by oriental rat fleas. It has a worldwide distribution. In the United States, R typhi–induced rickettsia mainly is concentrated in suburban areas of Texas and California where it is thought to be mainly spread by Ctenocephalides, but it also is found in Hawaii where spread by X cheopis has been documented.14,15 The most common symptoms of rickettsia include fever, headache, arthralgia, and a characteristic rash that is pruritic and maculopapular, starting on the trunk and spreading peripherally but sparing the palms and soles. This rash occurs about a week after the onset of fever.14Rickettsia felis also has been isolated in the oriental rat flea. However, only a handful of cases of human disease caused by this bacterium have been reported throughout the world, with clinical similarity to murine typhus likely leading to underestimation of disease prevalence.15Bartonella and other species of bacteria also have been documented to be spread by X cheopis.16 Unfortunately, the interactions of X cheopis with these other bacteria are not as well studied as its relationship with Y pestis.
Adverse Reactions
A flea bite itself can cause discomfort. It begins as a punctate hemorrhagic area that develops a surrounding wheal within 30 minutes. Over the course of 1 to 2 days, a delayed reaction occurs and there is a transition to an extremely pruritic, papular lesion. Bites often occur in clusters and can persist for weeks.1
Prevention and Treatment
Control of host animals via extermination and proper sanitation can secondarily reduce the population of X cheopis. Direct pesticide control of the flea population also has been suggested to reduce flea-borne disease. However, insecticides cause a selective pressure on the flea population, leading to populations that are resistant to them. For example, the flea population in Madagascar developed resistance to DDT (dichlorodiphenyltrichloroethane), dieldrin, deltamethrin, and cyfluthrin after their widespread use.17 Further, a recent study revealed resistance of X cheopis populations to alphacypermethrin, lambda-cyhalothrin, and etofenprox, none of which were used in mass vector control, indicating that some cross-resistance mechanism between these and the extensively used insecticides may exist. With the development of widespread resistance to most pesticides, flea control in endemic areas is difficult. Insecticide targeting to fleas on rodents (eg, rodent bait containing insecticide) can allow for more targeted insecticide treatment, limiting the development of resistance.17 Recent development of a maceration protocol used to detect zoonotic pathogens in fleas in the field also will allow management with pesticides to be targeted geographically and temporally where infected vectors are located.18 Research of the interaction between vector, pathogen, and insect microbiome also should continue, as it may allow for development of biopesticides, limiting the use of chemical pesticides all together. The strategy is based on the introduction of microorganisms that can reduce vector lifespan or their ability to transmit pathogens.17
When flea-transmitted diseases do occur, treatment with antibiotics is advised. Early treatment of the plague with effective antibiotics such as streptomycin, gentamicin, tetracycline, or chloramphenicol for a minimum of 10 days is critical for survival. Additionally, patients with bubonic plague should be isolated for at least 2 days after administration of antibiotics, while patients with the pneumonic form should be isolated for 4 days into therapy to prevent the spread of disease. Prophylactic therapy for individuals who come into contact with infected individuals also is advised.4 Patients with murine typhus typically respond to doxycycline, tetracycline, or fluoroquinolones. The few cases of R felis–induced disease have responded to doxycycline. Of note, short courses of treatment of doxycycline are appropriate and safe in young children. The short (3–7 day) nature of the course limits the chances of teeth staining.14
- Bitam I, Dittmar K, Parola P, et al. Flea and flea-borne diseases. Int J Infect Dis. 2010;14:E667-E676.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev. 2014;27:48-67.
- Ligon BL. Plague: a review of its history and potential as a biological weapon. Semin Pediatr Infect Dis. 2006;17:161-170.
- Josko D. Yersinia pestis: still a plague in the 21st century. Clin Lab Sci. 2004;17:25-29.
- Plague around the world, 2010–2015. Wkly Epidemiol Rec. 2016;91:89-93.
- Sullivan K. California confirms first human case of the plague in 5 years: what to know. NBC News website. https://www.nbcnews.com/health/health-news/california-confirms-first-human-case-bubonic-plague-5-years-what-n1237306. Published August 19, 2020. Accessed August 24, 2020.
- Hinnebusch BJ, Bland DM, Bosio CF, et al. Comparative ability of Oropsylla and Xenopsylla cheopis fleas to transmit Yersinia pestis by two different mechanisms. PLOS Negl Trop Dis. 2017;11:e0005276.
- Bobrov AG, Kirillina O, Vadyvaloo V, et al. The Yersinia pestis HmsCDE regulatory system is essential for blockage of the oriental rat flea (Xenopsylla cheopis), a classic plague vector. Environ Microbiol. 2015;17:947-959.
- Darby C, Ananth SL, Tan L, et al. Identification of gmhA, a Yersina pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect Immun. 2005;73:7236-7242.
- Eisen RJ, Dennis DT, Gage KL. The role of early-phase transmission in the spread of Yersinia pestis. J Med Entomol. 2015;52:1183-1192.
- Carniel E. Subtle genetic modifications transformed an enteropathogen into a flea-borne pathogen. Proc Natl Acad Sci U S A. 2014;111:18409-18410.
- Chouikha I, Hinnebusch BJ. Silencing urease: a key evolutionary step that facilitated the adaptation of Yersinia pestis to the flea-borne transmission route. Proc Natl Acad Sci U S A. 2014;111:18709-19714.
- Aoyagi KL, Brooks BD, Bearden SW, et al. LPS modification promotes maintenance of Yersinia pestis in fleas. Microbiology. 2015;161:628-638.
- Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918.
- Eremeeva ME, Warashina WR, Sturgeon MM, et al. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis. 2018;14:1613-1615.
- Billeter SA, Gundi VAKB, Rood MP, et al. Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvecus rats in Los Angeles, California. Appl Environ Microbiol. 2011;77:7850-7852.
- Miarinjara A, Boyer S. Current perspectives on plague vector control in Madagascar: susceptibility status of Xenopsylla cheopis to 12 insecticides. PLOS Negl Trop Dis. 2016;10:e0004414.
- Harrison GF, Scheirer JL, Melanson VR. Development and validation of an arthropod maceration protocol for zoonotic pathogen detection in mosquitoes and fleas. J Vector Ecol. 2014;40:83-89.
A dult Siphonaptera (fleas) are highly adapted to life on the surface of their hosts. Their small 2- to 10-mm bodies are laterally flattened and wingless. They utilize particularly strong hind legs for jumping up to 150 times their body length and backward-directed spines on their legs and bodies for moving forward through fur, hair, and feathers. Xenopsylla cheopis , the oriental rat flea, lacks pronotal and genal combs and has a mesopleuron divided by internal scleritinization (Figure). These features differentiate the species from its close relatives, Ctenocephalides (cat and dog fleas), which have both sets of combs, as well as Pulex irritans (human flea), which do not have a divided mesopleuron. 1,2

Flea-borne infections are extremely important to public health and are present throughout the world. Further, humidity and warmth are essential for the life cycle of many species of fleas. Predicted global warming likely will increase their distribution, allowing the spread of diseases they carry into previously untouched areas.1 Therefore, it is important to continue to examine species that carry particularly dangerous pathogens, such as X cheopis.
Disease Vector
Xenopsylla cheopis primarily is known for being a vector in the transmission of Yersinia pestis, the etiologic agent of the plague. Plague occurs in 3 forms: bubonic, pneumonic, and septicemic. It has caused major epidemics throughout history, the most widely known being the Black Death, which lasted for 130 years, beginning in the 1330s in China and spreading into Europe where it wiped out one-third of the population. However, bubonic plague is thought to have caused documented outbreaks as early as 320
Between January 2010 and December 2015, 3248 cases of plague in humans were reported, resulting in 584 deaths worldwide.5 It is thought that the plague originated in Central Asia, and this area still is a focus of disease. However, the at-risk population is reduced to breeders and hunters of gerbils and marmots, the main reservoirs in the area. In Africa, 4 countries still regularly report cases, with Madagascar being the most severely affected country in the world.5 The Democratic Republic of the Congo, Uganda, and Tanzania also are affected. The Americas also experience the plague. There are sporadic cases of bubonic plague in the northwest corner of Peru, mostly in rural areas. In the western United States, plague circulates among wild rodents, resulting in several reported cases each year, with the most recent confirmed case noted in California in August 2020.5,6 Further adding to its relevance, Y pestis is one of several organisms most likely to be used as a biologic weapon.3,4
Due to the historical and continued significance of Y pestis, many studies have been performed over the decades regarding its association with X cheopis. It has been discovered that fleas transmit the bacteria to the host in 2 ways. The most well-defined form of transmission occurs after an incubation period of Y pestis in the flea for 7 to 31 days. During this time, the bacteria form a dense biofilm on a valve in the flea foregut—the proventriculus—interfering with its function, which allows regurgitation of the blood and the bacteria it contains into the bite site and consequently disease transmission. The proventriculus can become completely blocked in some fleas, preventing any blood from reaching the midgut and causing starvation. In these scenarios, the flea will make continuous attempts to feed, increasing transmission.7 The hemin storage gene, hms, encoding the second messenger molecule cyclic di-GMP plays a critical role in biofilm formation and proventricular blockage.8 The phosphoheptose isomerase gene, GmhA, also has been elucidated as crucial in late transmission due to its role in biofilm formation.9 Early-phase transmission, or biofilm-independent transmission, has been documented to occur as early as 3 hours after infection of the flea but can occur for up to 4 days.10 Historically, the importance of early-phase transmission has been overlooked. Research has shown that it likely is crucial to the epizootic transmission of the plague.10 As a result, the search has begun for genes that contribute to the maintenance of Y pestis in the flea vector during the first 4 days of colonization. It is thought that a key evolutionary development was the selective loss-of-function mutation in a gene essential for the activity of urease, an enzyme that causes acute oral toxicity and mortality in fleas.11,12 The Yersinia murine toxin gene, Ymt, also allows for early survival of Y pestis in the flea midgut by producing a phospholipase D that protects the bacteria from toxic by-products produced during digestion of blood.11,13 In addition, gene products that function in lipid A modification are crucial for the ability of Y pestis to resist the action of cationic antimicrobial peptides it produces, such as cecropin A and polymyxin B.13
Murine typhus, an acute febrile illness caused by Rickettsia typhi, is another disease that can be spread by oriental rat fleas. It has a worldwide distribution. In the United States, R typhi–induced rickettsia mainly is concentrated in suburban areas of Texas and California where it is thought to be mainly spread by Ctenocephalides, but it also is found in Hawaii where spread by X cheopis has been documented.14,15 The most common symptoms of rickettsia include fever, headache, arthralgia, and a characteristic rash that is pruritic and maculopapular, starting on the trunk and spreading peripherally but sparing the palms and soles. This rash occurs about a week after the onset of fever.14Rickettsia felis also has been isolated in the oriental rat flea. However, only a handful of cases of human disease caused by this bacterium have been reported throughout the world, with clinical similarity to murine typhus likely leading to underestimation of disease prevalence.15Bartonella and other species of bacteria also have been documented to be spread by X cheopis.16 Unfortunately, the interactions of X cheopis with these other bacteria are not as well studied as its relationship with Y pestis.
Adverse Reactions
A flea bite itself can cause discomfort. It begins as a punctate hemorrhagic area that develops a surrounding wheal within 30 minutes. Over the course of 1 to 2 days, a delayed reaction occurs and there is a transition to an extremely pruritic, papular lesion. Bites often occur in clusters and can persist for weeks.1
Prevention and Treatment
Control of host animals via extermination and proper sanitation can secondarily reduce the population of X cheopis. Direct pesticide control of the flea population also has been suggested to reduce flea-borne disease. However, insecticides cause a selective pressure on the flea population, leading to populations that are resistant to them. For example, the flea population in Madagascar developed resistance to DDT (dichlorodiphenyltrichloroethane), dieldrin, deltamethrin, and cyfluthrin after their widespread use.17 Further, a recent study revealed resistance of X cheopis populations to alphacypermethrin, lambda-cyhalothrin, and etofenprox, none of which were used in mass vector control, indicating that some cross-resistance mechanism between these and the extensively used insecticides may exist. With the development of widespread resistance to most pesticides, flea control in endemic areas is difficult. Insecticide targeting to fleas on rodents (eg, rodent bait containing insecticide) can allow for more targeted insecticide treatment, limiting the development of resistance.17 Recent development of a maceration protocol used to detect zoonotic pathogens in fleas in the field also will allow management with pesticides to be targeted geographically and temporally where infected vectors are located.18 Research of the interaction between vector, pathogen, and insect microbiome also should continue, as it may allow for development of biopesticides, limiting the use of chemical pesticides all together. The strategy is based on the introduction of microorganisms that can reduce vector lifespan or their ability to transmit pathogens.17
When flea-transmitted diseases do occur, treatment with antibiotics is advised. Early treatment of the plague with effective antibiotics such as streptomycin, gentamicin, tetracycline, or chloramphenicol for a minimum of 10 days is critical for survival. Additionally, patients with bubonic plague should be isolated for at least 2 days after administration of antibiotics, while patients with the pneumonic form should be isolated for 4 days into therapy to prevent the spread of disease. Prophylactic therapy for individuals who come into contact with infected individuals also is advised.4 Patients with murine typhus typically respond to doxycycline, tetracycline, or fluoroquinolones. The few cases of R felis–induced disease have responded to doxycycline. Of note, short courses of treatment of doxycycline are appropriate and safe in young children. The short (3–7 day) nature of the course limits the chances of teeth staining.14
A dult Siphonaptera (fleas) are highly adapted to life on the surface of their hosts. Their small 2- to 10-mm bodies are laterally flattened and wingless. They utilize particularly strong hind legs for jumping up to 150 times their body length and backward-directed spines on their legs and bodies for moving forward through fur, hair, and feathers. Xenopsylla cheopis , the oriental rat flea, lacks pronotal and genal combs and has a mesopleuron divided by internal scleritinization (Figure). These features differentiate the species from its close relatives, Ctenocephalides (cat and dog fleas), which have both sets of combs, as well as Pulex irritans (human flea), which do not have a divided mesopleuron. 1,2

Flea-borne infections are extremely important to public health and are present throughout the world. Further, humidity and warmth are essential for the life cycle of many species of fleas. Predicted global warming likely will increase their distribution, allowing the spread of diseases they carry into previously untouched areas.1 Therefore, it is important to continue to examine species that carry particularly dangerous pathogens, such as X cheopis.
Disease Vector
Xenopsylla cheopis primarily is known for being a vector in the transmission of Yersinia pestis, the etiologic agent of the plague. Plague occurs in 3 forms: bubonic, pneumonic, and septicemic. It has caused major epidemics throughout history, the most widely known being the Black Death, which lasted for 130 years, beginning in the 1330s in China and spreading into Europe where it wiped out one-third of the population. However, bubonic plague is thought to have caused documented outbreaks as early as 320
Between January 2010 and December 2015, 3248 cases of plague in humans were reported, resulting in 584 deaths worldwide.5 It is thought that the plague originated in Central Asia, and this area still is a focus of disease. However, the at-risk population is reduced to breeders and hunters of gerbils and marmots, the main reservoirs in the area. In Africa, 4 countries still regularly report cases, with Madagascar being the most severely affected country in the world.5 The Democratic Republic of the Congo, Uganda, and Tanzania also are affected. The Americas also experience the plague. There are sporadic cases of bubonic plague in the northwest corner of Peru, mostly in rural areas. In the western United States, plague circulates among wild rodents, resulting in several reported cases each year, with the most recent confirmed case noted in California in August 2020.5,6 Further adding to its relevance, Y pestis is one of several organisms most likely to be used as a biologic weapon.3,4
Due to the historical and continued significance of Y pestis, many studies have been performed over the decades regarding its association with X cheopis. It has been discovered that fleas transmit the bacteria to the host in 2 ways. The most well-defined form of transmission occurs after an incubation period of Y pestis in the flea for 7 to 31 days. During this time, the bacteria form a dense biofilm on a valve in the flea foregut—the proventriculus—interfering with its function, which allows regurgitation of the blood and the bacteria it contains into the bite site and consequently disease transmission. The proventriculus can become completely blocked in some fleas, preventing any blood from reaching the midgut and causing starvation. In these scenarios, the flea will make continuous attempts to feed, increasing transmission.7 The hemin storage gene, hms, encoding the second messenger molecule cyclic di-GMP plays a critical role in biofilm formation and proventricular blockage.8 The phosphoheptose isomerase gene, GmhA, also has been elucidated as crucial in late transmission due to its role in biofilm formation.9 Early-phase transmission, or biofilm-independent transmission, has been documented to occur as early as 3 hours after infection of the flea but can occur for up to 4 days.10 Historically, the importance of early-phase transmission has been overlooked. Research has shown that it likely is crucial to the epizootic transmission of the plague.10 As a result, the search has begun for genes that contribute to the maintenance of Y pestis in the flea vector during the first 4 days of colonization. It is thought that a key evolutionary development was the selective loss-of-function mutation in a gene essential for the activity of urease, an enzyme that causes acute oral toxicity and mortality in fleas.11,12 The Yersinia murine toxin gene, Ymt, also allows for early survival of Y pestis in the flea midgut by producing a phospholipase D that protects the bacteria from toxic by-products produced during digestion of blood.11,13 In addition, gene products that function in lipid A modification are crucial for the ability of Y pestis to resist the action of cationic antimicrobial peptides it produces, such as cecropin A and polymyxin B.13
Murine typhus, an acute febrile illness caused by Rickettsia typhi, is another disease that can be spread by oriental rat fleas. It has a worldwide distribution. In the United States, R typhi–induced rickettsia mainly is concentrated in suburban areas of Texas and California where it is thought to be mainly spread by Ctenocephalides, but it also is found in Hawaii where spread by X cheopis has been documented.14,15 The most common symptoms of rickettsia include fever, headache, arthralgia, and a characteristic rash that is pruritic and maculopapular, starting on the trunk and spreading peripherally but sparing the palms and soles. This rash occurs about a week after the onset of fever.14Rickettsia felis also has been isolated in the oriental rat flea. However, only a handful of cases of human disease caused by this bacterium have been reported throughout the world, with clinical similarity to murine typhus likely leading to underestimation of disease prevalence.15Bartonella and other species of bacteria also have been documented to be spread by X cheopis.16 Unfortunately, the interactions of X cheopis with these other bacteria are not as well studied as its relationship with Y pestis.
Adverse Reactions
A flea bite itself can cause discomfort. It begins as a punctate hemorrhagic area that develops a surrounding wheal within 30 minutes. Over the course of 1 to 2 days, a delayed reaction occurs and there is a transition to an extremely pruritic, papular lesion. Bites often occur in clusters and can persist for weeks.1
Prevention and Treatment
Control of host animals via extermination and proper sanitation can secondarily reduce the population of X cheopis. Direct pesticide control of the flea population also has been suggested to reduce flea-borne disease. However, insecticides cause a selective pressure on the flea population, leading to populations that are resistant to them. For example, the flea population in Madagascar developed resistance to DDT (dichlorodiphenyltrichloroethane), dieldrin, deltamethrin, and cyfluthrin after their widespread use.17 Further, a recent study revealed resistance of X cheopis populations to alphacypermethrin, lambda-cyhalothrin, and etofenprox, none of which were used in mass vector control, indicating that some cross-resistance mechanism between these and the extensively used insecticides may exist. With the development of widespread resistance to most pesticides, flea control in endemic areas is difficult. Insecticide targeting to fleas on rodents (eg, rodent bait containing insecticide) can allow for more targeted insecticide treatment, limiting the development of resistance.17 Recent development of a maceration protocol used to detect zoonotic pathogens in fleas in the field also will allow management with pesticides to be targeted geographically and temporally where infected vectors are located.18 Research of the interaction between vector, pathogen, and insect microbiome also should continue, as it may allow for development of biopesticides, limiting the use of chemical pesticides all together. The strategy is based on the introduction of microorganisms that can reduce vector lifespan or their ability to transmit pathogens.17
When flea-transmitted diseases do occur, treatment with antibiotics is advised. Early treatment of the plague with effective antibiotics such as streptomycin, gentamicin, tetracycline, or chloramphenicol for a minimum of 10 days is critical for survival. Additionally, patients with bubonic plague should be isolated for at least 2 days after administration of antibiotics, while patients with the pneumonic form should be isolated for 4 days into therapy to prevent the spread of disease. Prophylactic therapy for individuals who come into contact with infected individuals also is advised.4 Patients with murine typhus typically respond to doxycycline, tetracycline, or fluoroquinolones. The few cases of R felis–induced disease have responded to doxycycline. Of note, short courses of treatment of doxycycline are appropriate and safe in young children. The short (3–7 day) nature of the course limits the chances of teeth staining.14
- Bitam I, Dittmar K, Parola P, et al. Flea and flea-borne diseases. Int J Infect Dis. 2010;14:E667-E676.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev. 2014;27:48-67.
- Ligon BL. Plague: a review of its history and potential as a biological weapon. Semin Pediatr Infect Dis. 2006;17:161-170.
- Josko D. Yersinia pestis: still a plague in the 21st century. Clin Lab Sci. 2004;17:25-29.
- Plague around the world, 2010–2015. Wkly Epidemiol Rec. 2016;91:89-93.
- Sullivan K. California confirms first human case of the plague in 5 years: what to know. NBC News website. https://www.nbcnews.com/health/health-news/california-confirms-first-human-case-bubonic-plague-5-years-what-n1237306. Published August 19, 2020. Accessed August 24, 2020.
- Hinnebusch BJ, Bland DM, Bosio CF, et al. Comparative ability of Oropsylla and Xenopsylla cheopis fleas to transmit Yersinia pestis by two different mechanisms. PLOS Negl Trop Dis. 2017;11:e0005276.
- Bobrov AG, Kirillina O, Vadyvaloo V, et al. The Yersinia pestis HmsCDE regulatory system is essential for blockage of the oriental rat flea (Xenopsylla cheopis), a classic plague vector. Environ Microbiol. 2015;17:947-959.
- Darby C, Ananth SL, Tan L, et al. Identification of gmhA, a Yersina pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect Immun. 2005;73:7236-7242.
- Eisen RJ, Dennis DT, Gage KL. The role of early-phase transmission in the spread of Yersinia pestis. J Med Entomol. 2015;52:1183-1192.
- Carniel E. Subtle genetic modifications transformed an enteropathogen into a flea-borne pathogen. Proc Natl Acad Sci U S A. 2014;111:18409-18410.
- Chouikha I, Hinnebusch BJ. Silencing urease: a key evolutionary step that facilitated the adaptation of Yersinia pestis to the flea-borne transmission route. Proc Natl Acad Sci U S A. 2014;111:18709-19714.
- Aoyagi KL, Brooks BD, Bearden SW, et al. LPS modification promotes maintenance of Yersinia pestis in fleas. Microbiology. 2015;161:628-638.
- Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918.
- Eremeeva ME, Warashina WR, Sturgeon MM, et al. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis. 2018;14:1613-1615.
- Billeter SA, Gundi VAKB, Rood MP, et al. Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvecus rats in Los Angeles, California. Appl Environ Microbiol. 2011;77:7850-7852.
- Miarinjara A, Boyer S. Current perspectives on plague vector control in Madagascar: susceptibility status of Xenopsylla cheopis to 12 insecticides. PLOS Negl Trop Dis. 2016;10:e0004414.
- Harrison GF, Scheirer JL, Melanson VR. Development and validation of an arthropod maceration protocol for zoonotic pathogen detection in mosquitoes and fleas. J Vector Ecol. 2014;40:83-89.
- Bitam I, Dittmar K, Parola P, et al. Flea and flea-borne diseases. Int J Infect Dis. 2010;14:E667-E676.
- Mathison BA, Pritt BS. Laboratory identification of arthropod ectoparasites. Clin Microbiol Rev. 2014;27:48-67.
- Ligon BL. Plague: a review of its history and potential as a biological weapon. Semin Pediatr Infect Dis. 2006;17:161-170.
- Josko D. Yersinia pestis: still a plague in the 21st century. Clin Lab Sci. 2004;17:25-29.
- Plague around the world, 2010–2015. Wkly Epidemiol Rec. 2016;91:89-93.
- Sullivan K. California confirms first human case of the plague in 5 years: what to know. NBC News website. https://www.nbcnews.com/health/health-news/california-confirms-first-human-case-bubonic-plague-5-years-what-n1237306. Published August 19, 2020. Accessed August 24, 2020.
- Hinnebusch BJ, Bland DM, Bosio CF, et al. Comparative ability of Oropsylla and Xenopsylla cheopis fleas to transmit Yersinia pestis by two different mechanisms. PLOS Negl Trop Dis. 2017;11:e0005276.
- Bobrov AG, Kirillina O, Vadyvaloo V, et al. The Yersinia pestis HmsCDE regulatory system is essential for blockage of the oriental rat flea (Xenopsylla cheopis), a classic plague vector. Environ Microbiol. 2015;17:947-959.
- Darby C, Ananth SL, Tan L, et al. Identification of gmhA, a Yersina pestis gene required for flea blockage, by using a Caenorhabditis elegans biofilm system. Infect Immun. 2005;73:7236-7242.
- Eisen RJ, Dennis DT, Gage KL. The role of early-phase transmission in the spread of Yersinia pestis. J Med Entomol. 2015;52:1183-1192.
- Carniel E. Subtle genetic modifications transformed an enteropathogen into a flea-borne pathogen. Proc Natl Acad Sci U S A. 2014;111:18409-18410.
- Chouikha I, Hinnebusch BJ. Silencing urease: a key evolutionary step that facilitated the adaptation of Yersinia pestis to the flea-borne transmission route. Proc Natl Acad Sci U S A. 2014;111:18709-19714.
- Aoyagi KL, Brooks BD, Bearden SW, et al. LPS modification promotes maintenance of Yersinia pestis in fleas. Microbiology. 2015;161:628-638.
- Civen R, Ngo V. Murine typhus: an unrecognized suburban vectorborne disease. Clin Infect Dis. 2008;46:913-918.
- Eremeeva ME, Warashina WR, Sturgeon MM, et al. Rickettsia typhi and R. felis in rat fleas (Xenopsylla cheopis), Oahu, Hawaii. Emerg Infect Dis. 2018;14:1613-1615.
- Billeter SA, Gundi VAKB, Rood MP, et al. Molecular detection and identification of Bartonella species in Xenopsylla cheopis fleas (Siphonaptera: Pulicidae) collected from Rattus norvecus rats in Los Angeles, California. Appl Environ Microbiol. 2011;77:7850-7852.
- Miarinjara A, Boyer S. Current perspectives on plague vector control in Madagascar: susceptibility status of Xenopsylla cheopis to 12 insecticides. PLOS Negl Trop Dis. 2016;10:e0004414.
- Harrison GF, Scheirer JL, Melanson VR. Development and validation of an arthropod maceration protocol for zoonotic pathogen detection in mosquitoes and fleas. J Vector Ecol. 2014;40:83-89.
Practice Points
- Xenopsylla cheopis, the oriental rat flea, is most known for carrying Yersinia pestis, the causative agent of the plague; however, it also is a vector for other bacteria, such as Rickettsia typhi, the species responsible for most cases of murine typhus.
- Despite the perception that it largely is a historical illness, modern outbreaks of plague occur in many parts of the world each year. Because fleas thrive in warm humid weather, global warming threatens the spread of the oriental rat flea and its diseases into previously unaffected parts of the world.
- There has been an effort to control oriental rat flea populations, which unfortunately has been complicated by pesticide resistance in many flea populations. It is important to continue to research the oriental rat flea and the bacterial species it carries in the hopes of finding better methods of controlling the pests and therefore decreasing illness in humans.
- Health care providers should be vigilant in identifying symptoms of flea-borne illnesses. If a patient is displaying symptoms, prompt recognition and antibiotic therapy is critical, particularly for the plague.
Which medications work best for menorrhagia?
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
EVIDENCE SUMMARY
A 2015 Cochrane review of the LNG-IUS for menorrhagia included 1 placebo-controlled RCT; most of the remaining 21 RCTs compared the LNG-IUS to invasive procedures such as endometrial ablation or hysterectomy.1 The placebo-controlled trial compared the LNG-IUS with placebo in 40 women on anticoagulation therapy and found a mean beneficial difference of 100 mL (95% confidence interval [CI], –116 to –83) using a subjective pictorial blood assessment chart.
Women are less likely to withdraw from LNG-IUS treatment
Four trials (379 patients) included in the Cochrane review compared LNG-IUS with combination or progesterone-only pills. All of the trials excluded women with palpable or large (> 5 cm) fibroids. In 3 trials (2 against OCPs and 1 against a 10-day course of oral progesterone), the LNG-IUS decreased MBL more than OCPs did. A fourth trial found LNG-IUS comparable to oral progesterone dosed 3 times a day from Day 5 to Day 26 of each menstrual cycle.
A recent large RCT (571 patients) that compared LNG-IUS with usual medical treatment (mefenamic acid [MFA], tranexamic acid, norethindrone, OCPs, progesterone-only pill, medroxyprogesterone acetate injection) found women significantly less likely to withdraw from LNG-IUS at 2 years (relative risk [RR] = 0.58; 95% CI, 0.49-0.70).2
Estrogen and progestin contraceptives significantly reduce bleeding
In addition to the trials in the 2015 Cochrane review comparing OCPs with LNG-IUS, a 2009 Cochrane review included a single 2-month crossover trial of 45 patients.3 This RCT compared OCPs with naproxen, MFA, and danazol to treat heavy menstrual bleeding (assessed using the alkaline haematin method).
Researchers didn’t analyze the data using intention-to-treat. No group was found to be superior. The OCP group (6 women) had a 43% reduction in MBL over baseline (no P value reported).
Tranexamic acid outperforms oral progesterone and NSAIDs but not ...
A 2018 Cochrane meta-analysis of 13 RCTs (1312 patients) of antifibrinolytics for reproductive-age women with regular heavy periods and no known underlying pathology included 4 RCTs (565 patients) that used placebo as a comparator.4 Therapy with tranexamic acid decreased blood loss by53 mL per cycle (95% CI, 44-63 mL), a 40% to 50% improvement compared with placebo. Three of the RCTs (271 patients) reported the percent of women improving on tranexamic acid as 43% to 63%, compared with 11% for placebo, resulting in an NNT of 2 to 3.
One trial (46 patients) found tranexamic acid superior to luteal phase oral progesterone, and another study (48 patients) demonstrated superiority to NSAIDs, with a mean decrease in MBL of 86 mL compared with 43 mL (P < .0027).
Continue to: On the other hand...
On the other hand, tranexamic acid compared unfavorably with LNG-IUS (1 RCT, 42 patients), showing a lower likelihood of improvement (RR = 0.43; 95% CI, 0.24-0.77). Whereas 85% of women improved with LNG-IUS, only 20% to 65% of women improved with tranexamic acid (NNT = 2 to 6).
No statistical difference was found in gastrointestinal adverse effects, headache, vaginal dryness, or dysmenorrhea.4 Only 1 thromboembolic event occurred in the 2 studies that reported this outcome, a known risk that prohibits its concomitant use with combination OCPs.
Different NSAIDs, equivalent efficacy
A 2013 Cochrane review of 18 RCTs included 8 (84 patients) that compared NSAIDs (5 MFA, 2 naproxen, 1 ibuprofen) with placebo.5 In 6 trials, NSAIDs produced a significant reduction in MBL compared with placebo, although most were crossover trials that couldn’t be compiled into the meta-analysis.
One trial (11 patients) showed a mean reduction of 124 mL (95% CI, 62-186 mL) in the MFA group. In another trial, women were less likely to report no improvement in the MFA group than in the placebo group (odds ratio [OR] = 0.08; 95% CI, 0.03-0.18). No NSAID had significantly higher efficacy than the others.
Danazol was superior to NSAIDs in a meta-analysis of 3 trials (79 patients) with a mean difference of 45 mL (95% CI, 19-71 mL), as was tranexamic acid in a single trial (48 patients) with a mean difference of 73 mL (95% CI, 22-124 mL).5 Comparisons with OCPs, oral progesterone, and an older model of LNG-IUS showed no significant differences. The most common adverse effects were gastrointestinal.
Continue to: Danazol linked to weight gain and other adverse effects
Danazol linked to weight gain and other adverse effects
A 2010 Cochrane review evaluated 9 RCTs, including 1 (66 patients) comparing danazol 200 mg with placebo that showed a significant decrease in subjectively assessed MBL in the danazol group.6 The study, which only 22 women finished, didn’t address intention-to-treat and used an unidentified scoring system. Patients also reported a significant 6.7-kg weight gain (95% CI, 1-12.4) after 3 months of treatment.
In addition to the 2013 meta-analysis showing danazol to be superior to NSAIDs, several studies6 compared danazol favorably with oral progesterone, although not all results reached significance. One study (37 patients) showed that women were more likely to rate the efficacy of danazol as moderate or high compared with progesterone (OR = 4.3; 95% CI, 1.1-17.0), but the mean difference in MBL (–36 mL; 95% CI, −102 to 31 mL) wasn’t statistically significant.
Of note, both a meta-analysis of 4 of the studies (117 patients) and another study comparing danazol with NSAIDs (20 patients) found significantly more adverse effects in the danazol group. Commonly reported adverse effects were acne, weight gain, headache, nausea, and tiredness.
RECOMMENDATIONS
A comparative effectiveness review by the Agency for Healthcare Research and Quality concluded that evidence showed efficacy for 4 primary care interventions for heavy cyclic bleeding: LNG-IUS, NSAIDs, tranexamic acid, and combination OCPs.7
The United Kingdom’s National Institute for Health Care and Excellence (NICE) recommends pharmaceutical treatment when no structural or histologic abnormality is present or when fibroids are < 3 cm in diameter.8 NICE advises considering pharmaceutical treatments in the following order: first, LNG-IUS if long-term use (at least 12 months) is anticipated; second, tranexamic acid or NSAIDs; and third, combination OCPs, norethisterone (15 mg) daily from Days 5 to 26 of the menstrual cycle, or injected long-acting progestogen.
Editor’s takeaway
I was taught to use combination OCPs as first-line treatment for menorrhagia, but better evidence supports using any of these 4: LNG-IUS, tranexamic acid, danazol, or NSAIDs. In the absence of clear evidence demonstrating differences in efficacy, I would use them in the reverse order for cost-effectiveness reasons.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
1. Lethaby A, Hussain M, Rishworth JR, et al. Progesterone or progesterone-releasing intrauterine systems for heavy menstrual bleeding. Cochrane Database Syst Rev. 2015;(4):CD002126.
2. Gupta J, Kai J, Middleton L, et al. Levonorgestrel intrauterine system versus medical therapy for menorrhagia N Engl J Med. 2013;368:128-137.
3. Farquhar C, Brown J. Oral contraceptive pill for heavy menstrual bleeding. Cochrane Database Syst Rev. 2009;(4):CD000154.
4. Bryant-Smith AC, Lethaby A, Farquhar C, et al. Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst Rev. 2018;(4):CD000249.
5. Lethaby A, Duckitt K, Farquhar C. Non-steroidal anti-inflammatory drugs for heavy menstrual bleeding. Cochrane Database Syst Rev. 2013;(1):CD000400.
6. Beaumont HH, Augood C, Duckitt K, et al. Danazol for heavy menstrual bleeding. Cochrane Database Syst Rev. 2010;(1):CD00107.
7. Hartmann KE, Jerome RN, Lindegren ML, et al. Primary Care Management of Abnormal Uterine Bleeding. Comparative Effectiveness Review No. 96 (AHRQ Publication No. 13-EHC025-EF). Rockville, MD: Agency for Healthcare Research and Quality; 2013. https://effectivehealthcare.ahrq.gov/topics/abnormal-uterine-bleeding. Accessed August 25, 2020.
8. National Institute for Health Care and Excellence (NICE). Heavy menstrual bleeding: assessment and management. NICE Guideline NG88; 2018. www.nice.org.uk/guidance/ng88. Accessed August 25, 2020.
EVIDENCE-BASED ANSWER:
Four medications have been shown to reduce menstrual blood loss (MBL) significantly in placebo-controlled randomized controlled trials (RCTs): the levonorgestrel-releasing intrauterine system (LNG-IUS), tranexamic acid, nonsteroidal anti-inflammatory drugs (NSAIDs), and danazol, a synthetic steroid (strength of recommendation: A, meta-analyses of RCTs).
A single trial showed that the LNG-IUS reduced MBL by about 100 mL, compared with placebo. In a meta-analysis of 4 placebo-controlled RCTs, tranexamic acid reduced MBL by about 53 mL, roughly a 40% to 50% decrease. The 8 NSAID trials (5 mefenamic acid, 2 naproxen, 1 ibuprofen) demonstrated effectiveness, but the effect size is difficult to quantify. The single danazol RCT used a subjective scoring system without reporting MBL.
No studies compared all effective medical therapies against one another. In head-to-head comparisons, women were more likely to experience improvement with the LNG-IUS than with tranexamic acid (number needed to treat [NNT] = 2 to 6). Both treatments are superior to NSAIDs. Danazol is also more efficacious than NSAIDs, but its use is limited by its adverse effects, including teratogenicity.
No placebo-controlled trials have studied oral contraceptive pills (OCPs) or oral progesterone to treat menorrhagia. However, multiple comparative RCTs have demonstrated that these commonly prescribed medications significantly decrease MBL. Trials have shown the reduction to be inferior to LNG-IUS and danazol and equivalent to NSAIDs.
Management of Classic Ulcerative Pyoderma Gangrenosum
Pyoderma gangrenosum (PG) is a rare, chronic, ulcerative, neutrophilic dermatosis of unclear etiology. Large, multicentered, randomized controlled trials (RCTs) are challenging due to the rarity of PG and the lack of a diagnostic confirmatory test; therefore, evidence-based guidelines for diagnosis and treatment are not well established. Current management of PG primarily is guided by case series, small clinical trials, and expert opinion.1-4 We conducted a survey of expert medical dermatologists to highlight best practices in diagnostic and therapeutic approaches to PG.
Methods
The Society of Dermatology Hospitalists (SDH) Scientific Task Force gathered expert opinions from members of the SDH and Rheumatologic Dermatology Society (RDS) regarding PG workup and treatment through an online survey of 15 items (eTable 1). Subscribers of the SDH and RDS LISTSERVs were invited via email to participate in the survey from January 2016 to February 2016. Anonymous survey responses were collected and collated using SurveyMonkey. The survey results identified expert recommendations for evaluation, diagnosis, and treatment of PG and are reported as the sum of the percentage of respondents who answered always (almost 100% of the time) or often (more than half the time) following a particular course of action. A subanalysis was performed defining 2 groups of respondents based on the number of cases of PG treated per year (≥10 vs <10). Survey responses between each group were compared using χ2 analysis with statistical significance set at P=.05.

Results
Fifty-one respondents completed the survey out of 140 surveyed (36% response rate). All respondents were dermatologists, and 96% (49/51) were affiliated with an academic institution. Among the respondents, the number of PG cases managed per year ranged from 2 to 35.
Respondents consistently ordered skin biopsies (92% [47/51]) and tissue cultures (90% [46/51]), as well as certain ancillary tests, including complete blood cell count (96% [49/51]), complete metabolic panel (86% [44/51]), serum protein electrophoresis (76% [39/51]), and hepatitis panel (71% [36/51]). Other frequently ordered studies were rheumatoid factor (69% [35/51]), antinuclear antibodies (67% [34/51]), and antineutrophilic antibodies (65% [33/51]). Respondents frequently ordered erythrocyte sedimentation rate (59% [30/51]), C-reactive protein (55% [28/51]), cryoglobulins (53% [27/51]), urine protein electrophoresis (53% [27/51]), hypercoagulability workup (49% [25/51]), and serum immunofixation test (49% [25/51]). Human immunodeficiency virus testing (43% [22/51]), chest radiograph (41% [21/51]), colonoscopy (41% [21/51]) and referral to other specialties for workup—gastroenterology (38% [19/51]), hematology/oncology (14% [7/51]), and rheumatology (10% [5/51])—were less frequently ordered (eTable 2).
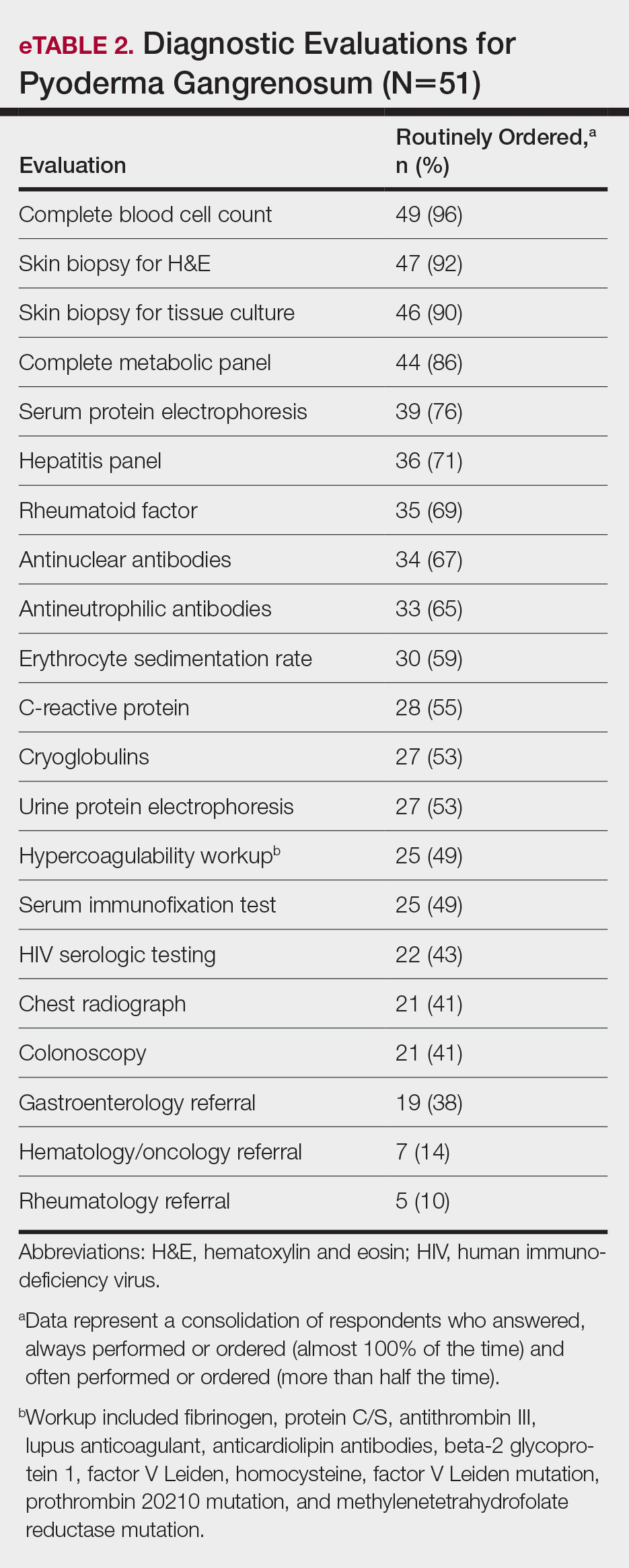
Systemic corticosteroids were reported as first-line therapy by most respondents (94% [48/51]), followed by topical immunomodulatory therapies (63% [32/51]). Topical corticosteroids (75% [38/51]) were the most common first-line topical agents. Thirty-nine percent of respondents (20/51) prescribed topical calcineurin inhibitors as first-line topical therapy. Additional therapies frequently used included systemic cyclosporine (47% [24/51]), antineutrophilic agents (41% [21/51]), and biologic agents (37% [19/51]). Fifty-seven percent of respondents (29/51) supported using combination topical and systemic therapy (Table).
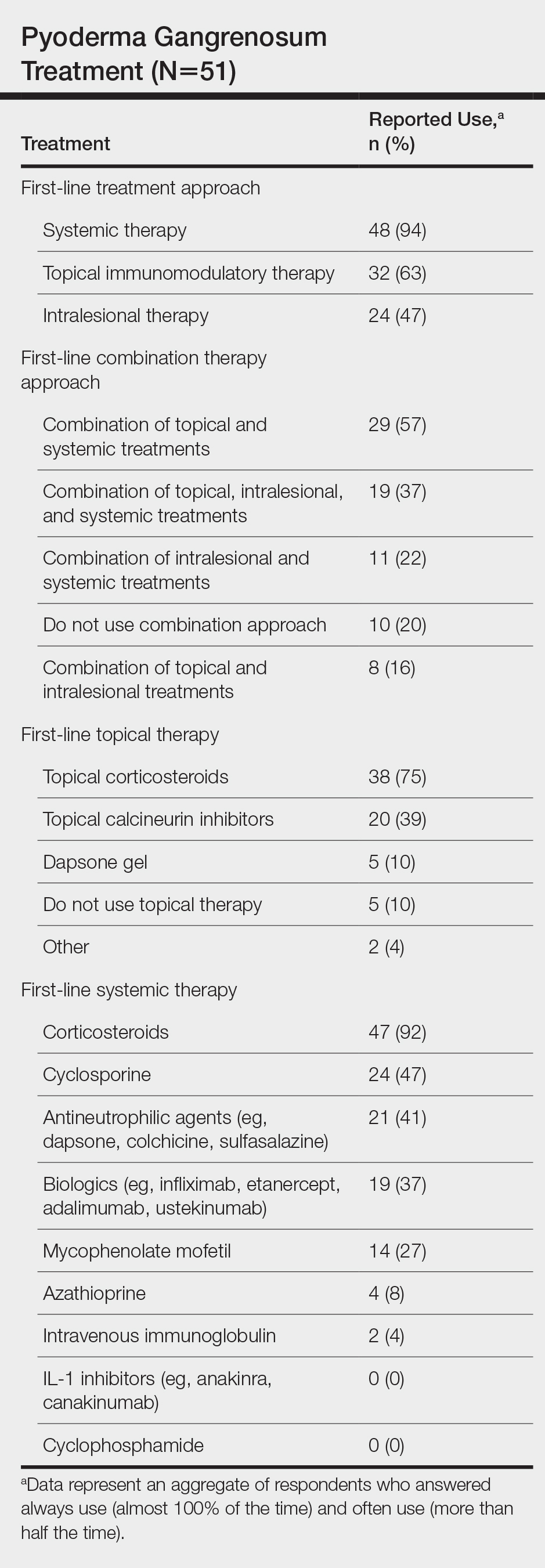
A wide variety of wound care practices were reported in the management of PG. Seventy-six percent of respondents (39/51) favored petroleum-impregnated gauze, 69% (35/51) used nonadhesive dressings, and 43% (22/51) added antimicrobial therapy for PG wound care (eTable 3). In the subanalysis, there were no significant differences in the majority of answer responses in patients treating 10 or more PG cases per year vs fewer than 10 PG cases, except with regard to the practice of combination therapy. Those treating more than 10 cases of PG per year more frequently reported use of combination therapies compared to respondents treating fewer than 10 cases (P=.04).
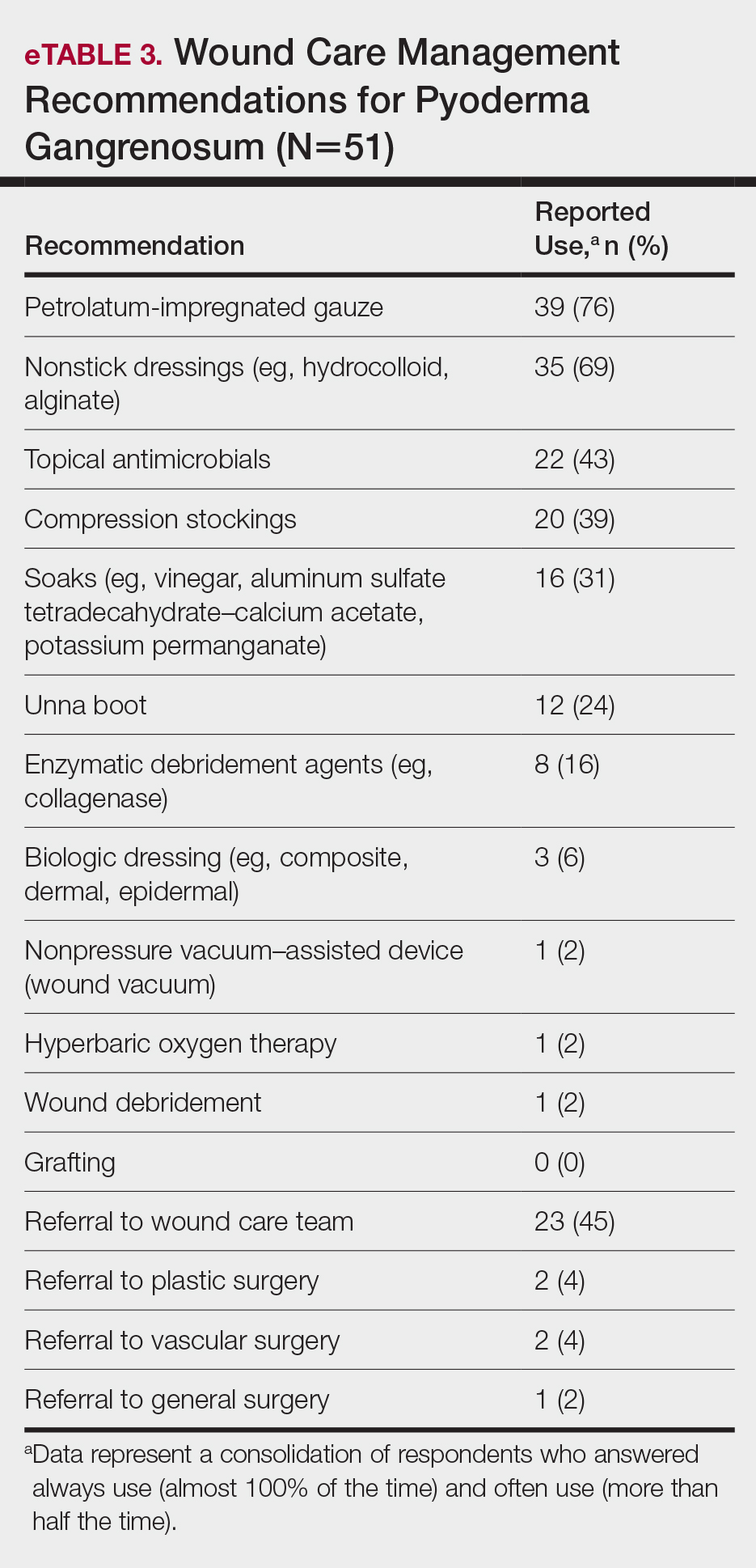
Comment
Skin biopsies and tissue cultures were strongly recommended (>90% survey respondents) for the initial evaluation of lesions suspected to be PG to evaluate for typical histopathologic changes that appear early in the disease, to rule out PG mimickers such as infectious or vascular causes, and to prevent the detrimental effects of inappropriate treatment and delayed diagnosis.5
Suspected PG warrants a reasonable search for related conditions because more than 50% of PG cases are associated with comorbidities such as rheumatoid arthritis, inflammatory bowel disease, and hematologic disease/malignancy.6,7 A complete blood cell count and comprehensive metabolic panel were recommended by most respondents, aiding in the preliminary screening for hematologic and infectious causes as well as detecting liver and kidney dysfunction associated with systemic conditions. Additionally, exclusion of infection or malignancy may be particularly important if the patient will undergo systemic immunosuppression. In challenging PG cases when initial findings are inconclusive and the clinical presentation does not direct workup (eg, colonoscopy to evaluate gastrointestinal tract symptoms), serum protein electrophoresis, hepatitis panel, rheumatoid factor, antinuclear antibodies, and antineutrophilic antibody tests also were frequently ordered by respondents to further evaluate for underlying or associated conditions.
This consensus regarding skin biopsies and certain ancillary tests is consistent with the proposed diagnostic criteria for classic ulcerative PG in which the absence or exclusion of other relevant causes of cutaneous ulcers is required based on the criteria.8 The importance of ensuring an accurate diagnosis is paramount, as a 10% misdiagnosis rate has been documented in the literature.5
Importantly, a stepwise diagnostic workup for PG is proposed based on survey results, which may limit unnecessary testing and the associated costs to the health care system (Figure 1). Selection of additional testing is guided by initial test results and features of the patient’s clinical presentation, including age, review of systems, and associated comorbidities. Available data suggest that underlying inflammatory bowel disease is more frequent in PG patients who are younger than 65 years, whereas those who are 65 years and older are more likely to have inflammatory arthritis, cancer, or an underlying hematologic disorder.9
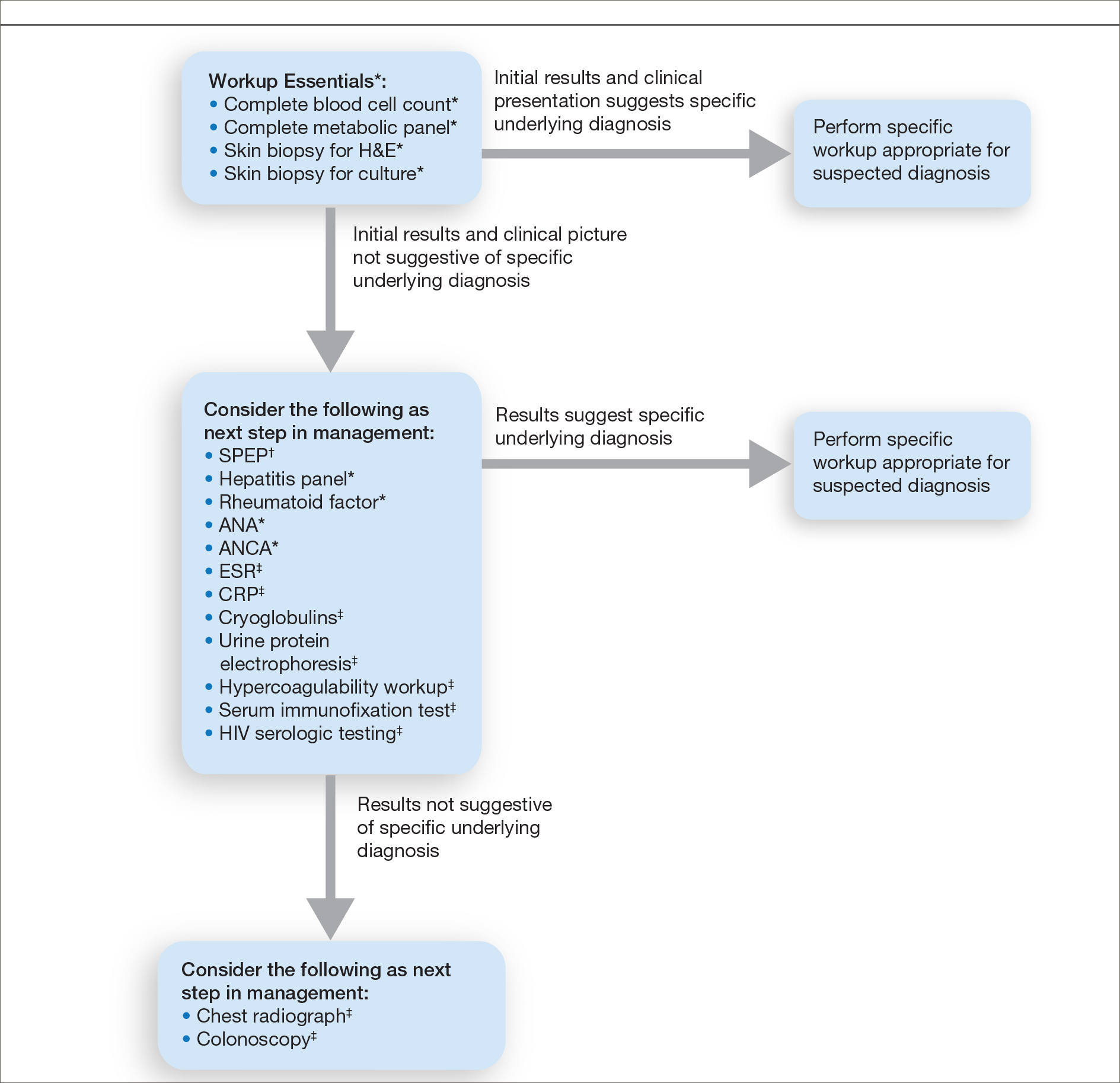
Treatment of PG should address both the inflammatory and wound components of the disease (Figure 2).7 In our survey results, systemic corticosteroids were identified as an important first-line therapy supported by reasonable evidence and were favored for their rapid response and minimal cost.1,10,11 Many respondents endorsed the use of systemic therapy in combination with topical steroids or calcineurin inhibitors. Combination therapy may provide more immediate control of rapidly progressing disease while minimizing adverse effects of long-term systemic corticosteroid use. A survey of German wound experts similarly endorsed frequent use of topical calcineurin inhibitors and combination systemic and topical glucocorticoid therapy as common therapeutic approaches.1
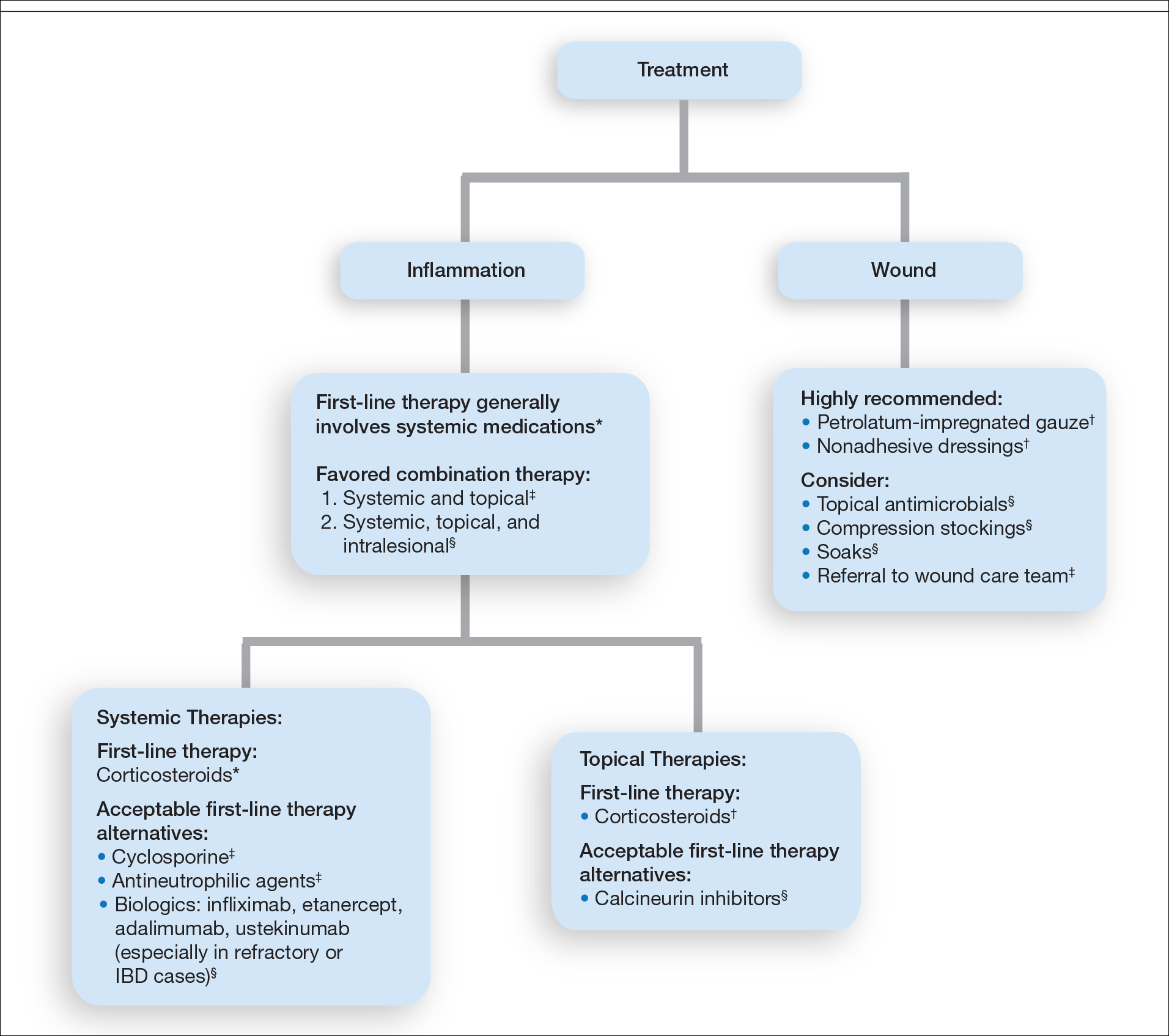
Importantly, treatments may vary depending on patient characteristics, comorbidities, and underlying disease, which underscores the need for individualized treatment approaches. Alternative first-line systemic treatments favored by respondents were cyclosporine, biologic medications, and antineutrophilic agents such as dapsone. Cyclosporine has demonstrated comparable efficacy to systemic glucocorticoids in one RCT and is considered an important steroid-sparing alternative for PG treatment.2 Biologic agents, especially tumor necrosis factor inhibitors, may be effective in treating cases of refractory PG or for concomitant inflammatory bowel disease management, as demonstrated by a small RCT documenting improvement of PG following infliximab infusion.3
Respondents strongly recommended petrolatum-impregnated gauze and other nonadhesive dressings, including alginate and hydrocolloid dressings, as part of PG wound care. Topical antimicrobials and compression stockings also were recommended by respondents. These practices aim to promote moist environments for healing, avoid maceration, prevent superinfection, optimize wound healing, and minimize damage from adhesive injury.12 Wound debridement and grafting generally were not recommended. However, pathergy is not a universal phenomenon in PG, and wounds that are no longer in the inflammatory phase may benefit from gentle debridement of necrotic tissue and/or grafting in select cases.10
Conclusion
An approach to modifying PG management based on clinical presentation and the practice of combination therapy with multiple systemic agents in refractory PG cases was not addressed in our survey. The low response rate is a limitation; however, the opinions of 51 medical dermatologist experts who regularly manage PG (in contrast to papers based on individualized clinical experience) can provide important clinical guidance until more scientific evidence is established.
Acknowledgments
We would like to thank the SDH and RDS membership for their participation in this survey. We especially acknowledge the other members of the SDH Scientific Task Force for their feedback: Misha Rosenbach, MD (Philadelphia, Pennsylvania); Robert G. Micheletti, MD (Philadelphia, Pennsylvania); Karolyn Wanat, MD (Milwaukee, Wisconsin); Amy Chen, MD (Cromwell, Connecticut); and A. Rambi Cardones, MD (Durham, North Carolina).
- Al Ghazal P, Dissemond J. Therapy of pyoderma gangrenosum in Germany: results of a survey among wound experts. J Dtsch Dermatol Ges . 2015;13:317-324.
- Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958.
- Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509.
- Al Ghazal P, Klode J, Dissemond J. Diagnostic criteria for pyoderma gangrenosum: results of a survey among dermatologic wound experts in Germany. J Dtsch Dermatol Ges. 2014;12:1129-1131.
- Weenig RH, Davis MD, Dahl PR, et al. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347:1412-1418.
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- Bennett ML, Jackson JM, Jorizzo JL, et al. Pyoderma gangrenosum: a comparison of typical and atypical forms with an emphasis on time to remission. case review of 86 patients from 2 institutions. Medicine. 2000;79:37-46.
- Su WP, Davis MD, Weening RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Aschyan H, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Binus AM, Qureshi AA, Li VW, et al. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244-1250.
- Reichrath J, Bens G, Bonowitz A, et al. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273-283.
- Miller J, Yentzer BA, Clark A, et al. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol. 2010;62:646-654.
Pyoderma gangrenosum (PG) is a rare, chronic, ulcerative, neutrophilic dermatosis of unclear etiology. Large, multicentered, randomized controlled trials (RCTs) are challenging due to the rarity of PG and the lack of a diagnostic confirmatory test; therefore, evidence-based guidelines for diagnosis and treatment are not well established. Current management of PG primarily is guided by case series, small clinical trials, and expert opinion.1-4 We conducted a survey of expert medical dermatologists to highlight best practices in diagnostic and therapeutic approaches to PG.
Methods
The Society of Dermatology Hospitalists (SDH) Scientific Task Force gathered expert opinions from members of the SDH and Rheumatologic Dermatology Society (RDS) regarding PG workup and treatment through an online survey of 15 items (eTable 1). Subscribers of the SDH and RDS LISTSERVs were invited via email to participate in the survey from January 2016 to February 2016. Anonymous survey responses were collected and collated using SurveyMonkey. The survey results identified expert recommendations for evaluation, diagnosis, and treatment of PG and are reported as the sum of the percentage of respondents who answered always (almost 100% of the time) or often (more than half the time) following a particular course of action. A subanalysis was performed defining 2 groups of respondents based on the number of cases of PG treated per year (≥10 vs <10). Survey responses between each group were compared using χ2 analysis with statistical significance set at P=.05.

Results
Fifty-one respondents completed the survey out of 140 surveyed (36% response rate). All respondents were dermatologists, and 96% (49/51) were affiliated with an academic institution. Among the respondents, the number of PG cases managed per year ranged from 2 to 35.
Respondents consistently ordered skin biopsies (92% [47/51]) and tissue cultures (90% [46/51]), as well as certain ancillary tests, including complete blood cell count (96% [49/51]), complete metabolic panel (86% [44/51]), serum protein electrophoresis (76% [39/51]), and hepatitis panel (71% [36/51]). Other frequently ordered studies were rheumatoid factor (69% [35/51]), antinuclear antibodies (67% [34/51]), and antineutrophilic antibodies (65% [33/51]). Respondents frequently ordered erythrocyte sedimentation rate (59% [30/51]), C-reactive protein (55% [28/51]), cryoglobulins (53% [27/51]), urine protein electrophoresis (53% [27/51]), hypercoagulability workup (49% [25/51]), and serum immunofixation test (49% [25/51]). Human immunodeficiency virus testing (43% [22/51]), chest radiograph (41% [21/51]), colonoscopy (41% [21/51]) and referral to other specialties for workup—gastroenterology (38% [19/51]), hematology/oncology (14% [7/51]), and rheumatology (10% [5/51])—were less frequently ordered (eTable 2).

Systemic corticosteroids were reported as first-line therapy by most respondents (94% [48/51]), followed by topical immunomodulatory therapies (63% [32/51]). Topical corticosteroids (75% [38/51]) were the most common first-line topical agents. Thirty-nine percent of respondents (20/51) prescribed topical calcineurin inhibitors as first-line topical therapy. Additional therapies frequently used included systemic cyclosporine (47% [24/51]), antineutrophilic agents (41% [21/51]), and biologic agents (37% [19/51]). Fifty-seven percent of respondents (29/51) supported using combination topical and systemic therapy (Table).

A wide variety of wound care practices were reported in the management of PG. Seventy-six percent of respondents (39/51) favored petroleum-impregnated gauze, 69% (35/51) used nonadhesive dressings, and 43% (22/51) added antimicrobial therapy for PG wound care (eTable 3). In the subanalysis, there were no significant differences in the majority of answer responses in patients treating 10 or more PG cases per year vs fewer than 10 PG cases, except with regard to the practice of combination therapy. Those treating more than 10 cases of PG per year more frequently reported use of combination therapies compared to respondents treating fewer than 10 cases (P=.04).

Comment
Skin biopsies and tissue cultures were strongly recommended (>90% survey respondents) for the initial evaluation of lesions suspected to be PG to evaluate for typical histopathologic changes that appear early in the disease, to rule out PG mimickers such as infectious or vascular causes, and to prevent the detrimental effects of inappropriate treatment and delayed diagnosis.5
Suspected PG warrants a reasonable search for related conditions because more than 50% of PG cases are associated with comorbidities such as rheumatoid arthritis, inflammatory bowel disease, and hematologic disease/malignancy.6,7 A complete blood cell count and comprehensive metabolic panel were recommended by most respondents, aiding in the preliminary screening for hematologic and infectious causes as well as detecting liver and kidney dysfunction associated with systemic conditions. Additionally, exclusion of infection or malignancy may be particularly important if the patient will undergo systemic immunosuppression. In challenging PG cases when initial findings are inconclusive and the clinical presentation does not direct workup (eg, colonoscopy to evaluate gastrointestinal tract symptoms), serum protein electrophoresis, hepatitis panel, rheumatoid factor, antinuclear antibodies, and antineutrophilic antibody tests also were frequently ordered by respondents to further evaluate for underlying or associated conditions.
This consensus regarding skin biopsies and certain ancillary tests is consistent with the proposed diagnostic criteria for classic ulcerative PG in which the absence or exclusion of other relevant causes of cutaneous ulcers is required based on the criteria.8 The importance of ensuring an accurate diagnosis is paramount, as a 10% misdiagnosis rate has been documented in the literature.5
Importantly, a stepwise diagnostic workup for PG is proposed based on survey results, which may limit unnecessary testing and the associated costs to the health care system (Figure 1). Selection of additional testing is guided by initial test results and features of the patient’s clinical presentation, including age, review of systems, and associated comorbidities. Available data suggest that underlying inflammatory bowel disease is more frequent in PG patients who are younger than 65 years, whereas those who are 65 years and older are more likely to have inflammatory arthritis, cancer, or an underlying hematologic disorder.9

Treatment of PG should address both the inflammatory and wound components of the disease (Figure 2).7 In our survey results, systemic corticosteroids were identified as an important first-line therapy supported by reasonable evidence and were favored for their rapid response and minimal cost.1,10,11 Many respondents endorsed the use of systemic therapy in combination with topical steroids or calcineurin inhibitors. Combination therapy may provide more immediate control of rapidly progressing disease while minimizing adverse effects of long-term systemic corticosteroid use. A survey of German wound experts similarly endorsed frequent use of topical calcineurin inhibitors and combination systemic and topical glucocorticoid therapy as common therapeutic approaches.1

Importantly, treatments may vary depending on patient characteristics, comorbidities, and underlying disease, which underscores the need for individualized treatment approaches. Alternative first-line systemic treatments favored by respondents were cyclosporine, biologic medications, and antineutrophilic agents such as dapsone. Cyclosporine has demonstrated comparable efficacy to systemic glucocorticoids in one RCT and is considered an important steroid-sparing alternative for PG treatment.2 Biologic agents, especially tumor necrosis factor inhibitors, may be effective in treating cases of refractory PG or for concomitant inflammatory bowel disease management, as demonstrated by a small RCT documenting improvement of PG following infliximab infusion.3
Respondents strongly recommended petrolatum-impregnated gauze and other nonadhesive dressings, including alginate and hydrocolloid dressings, as part of PG wound care. Topical antimicrobials and compression stockings also were recommended by respondents. These practices aim to promote moist environments for healing, avoid maceration, prevent superinfection, optimize wound healing, and minimize damage from adhesive injury.12 Wound debridement and grafting generally were not recommended. However, pathergy is not a universal phenomenon in PG, and wounds that are no longer in the inflammatory phase may benefit from gentle debridement of necrotic tissue and/or grafting in select cases.10
Conclusion
An approach to modifying PG management based on clinical presentation and the practice of combination therapy with multiple systemic agents in refractory PG cases was not addressed in our survey. The low response rate is a limitation; however, the opinions of 51 medical dermatologist experts who regularly manage PG (in contrast to papers based on individualized clinical experience) can provide important clinical guidance until more scientific evidence is established.
Acknowledgments
We would like to thank the SDH and RDS membership for their participation in this survey. We especially acknowledge the other members of the SDH Scientific Task Force for their feedback: Misha Rosenbach, MD (Philadelphia, Pennsylvania); Robert G. Micheletti, MD (Philadelphia, Pennsylvania); Karolyn Wanat, MD (Milwaukee, Wisconsin); Amy Chen, MD (Cromwell, Connecticut); and A. Rambi Cardones, MD (Durham, North Carolina).
Pyoderma gangrenosum (PG) is a rare, chronic, ulcerative, neutrophilic dermatosis of unclear etiology. Large, multicentered, randomized controlled trials (RCTs) are challenging due to the rarity of PG and the lack of a diagnostic confirmatory test; therefore, evidence-based guidelines for diagnosis and treatment are not well established. Current management of PG primarily is guided by case series, small clinical trials, and expert opinion.1-4 We conducted a survey of expert medical dermatologists to highlight best practices in diagnostic and therapeutic approaches to PG.
Methods
The Society of Dermatology Hospitalists (SDH) Scientific Task Force gathered expert opinions from members of the SDH and Rheumatologic Dermatology Society (RDS) regarding PG workup and treatment through an online survey of 15 items (eTable 1). Subscribers of the SDH and RDS LISTSERVs were invited via email to participate in the survey from January 2016 to February 2016. Anonymous survey responses were collected and collated using SurveyMonkey. The survey results identified expert recommendations for evaluation, diagnosis, and treatment of PG and are reported as the sum of the percentage of respondents who answered always (almost 100% of the time) or often (more than half the time) following a particular course of action. A subanalysis was performed defining 2 groups of respondents based on the number of cases of PG treated per year (≥10 vs <10). Survey responses between each group were compared using χ2 analysis with statistical significance set at P=.05.

Results
Fifty-one respondents completed the survey out of 140 surveyed (36% response rate). All respondents were dermatologists, and 96% (49/51) were affiliated with an academic institution. Among the respondents, the number of PG cases managed per year ranged from 2 to 35.
Respondents consistently ordered skin biopsies (92% [47/51]) and tissue cultures (90% [46/51]), as well as certain ancillary tests, including complete blood cell count (96% [49/51]), complete metabolic panel (86% [44/51]), serum protein electrophoresis (76% [39/51]), and hepatitis panel (71% [36/51]). Other frequently ordered studies were rheumatoid factor (69% [35/51]), antinuclear antibodies (67% [34/51]), and antineutrophilic antibodies (65% [33/51]). Respondents frequently ordered erythrocyte sedimentation rate (59% [30/51]), C-reactive protein (55% [28/51]), cryoglobulins (53% [27/51]), urine protein electrophoresis (53% [27/51]), hypercoagulability workup (49% [25/51]), and serum immunofixation test (49% [25/51]). Human immunodeficiency virus testing (43% [22/51]), chest radiograph (41% [21/51]), colonoscopy (41% [21/51]) and referral to other specialties for workup—gastroenterology (38% [19/51]), hematology/oncology (14% [7/51]), and rheumatology (10% [5/51])—were less frequently ordered (eTable 2).

Systemic corticosteroids were reported as first-line therapy by most respondents (94% [48/51]), followed by topical immunomodulatory therapies (63% [32/51]). Topical corticosteroids (75% [38/51]) were the most common first-line topical agents. Thirty-nine percent of respondents (20/51) prescribed topical calcineurin inhibitors as first-line topical therapy. Additional therapies frequently used included systemic cyclosporine (47% [24/51]), antineutrophilic agents (41% [21/51]), and biologic agents (37% [19/51]). Fifty-seven percent of respondents (29/51) supported using combination topical and systemic therapy (Table).

A wide variety of wound care practices were reported in the management of PG. Seventy-six percent of respondents (39/51) favored petroleum-impregnated gauze, 69% (35/51) used nonadhesive dressings, and 43% (22/51) added antimicrobial therapy for PG wound care (eTable 3). In the subanalysis, there were no significant differences in the majority of answer responses in patients treating 10 or more PG cases per year vs fewer than 10 PG cases, except with regard to the practice of combination therapy. Those treating more than 10 cases of PG per year more frequently reported use of combination therapies compared to respondents treating fewer than 10 cases (P=.04).

Comment
Skin biopsies and tissue cultures were strongly recommended (>90% survey respondents) for the initial evaluation of lesions suspected to be PG to evaluate for typical histopathologic changes that appear early in the disease, to rule out PG mimickers such as infectious or vascular causes, and to prevent the detrimental effects of inappropriate treatment and delayed diagnosis.5
Suspected PG warrants a reasonable search for related conditions because more than 50% of PG cases are associated with comorbidities such as rheumatoid arthritis, inflammatory bowel disease, and hematologic disease/malignancy.6,7 A complete blood cell count and comprehensive metabolic panel were recommended by most respondents, aiding in the preliminary screening for hematologic and infectious causes as well as detecting liver and kidney dysfunction associated with systemic conditions. Additionally, exclusion of infection or malignancy may be particularly important if the patient will undergo systemic immunosuppression. In challenging PG cases when initial findings are inconclusive and the clinical presentation does not direct workup (eg, colonoscopy to evaluate gastrointestinal tract symptoms), serum protein electrophoresis, hepatitis panel, rheumatoid factor, antinuclear antibodies, and antineutrophilic antibody tests also were frequently ordered by respondents to further evaluate for underlying or associated conditions.
This consensus regarding skin biopsies and certain ancillary tests is consistent with the proposed diagnostic criteria for classic ulcerative PG in which the absence or exclusion of other relevant causes of cutaneous ulcers is required based on the criteria.8 The importance of ensuring an accurate diagnosis is paramount, as a 10% misdiagnosis rate has been documented in the literature.5
Importantly, a stepwise diagnostic workup for PG is proposed based on survey results, which may limit unnecessary testing and the associated costs to the health care system (Figure 1). Selection of additional testing is guided by initial test results and features of the patient’s clinical presentation, including age, review of systems, and associated comorbidities. Available data suggest that underlying inflammatory bowel disease is more frequent in PG patients who are younger than 65 years, whereas those who are 65 years and older are more likely to have inflammatory arthritis, cancer, or an underlying hematologic disorder.9

Treatment of PG should address both the inflammatory and wound components of the disease (Figure 2).7 In our survey results, systemic corticosteroids were identified as an important first-line therapy supported by reasonable evidence and were favored for their rapid response and minimal cost.1,10,11 Many respondents endorsed the use of systemic therapy in combination with topical steroids or calcineurin inhibitors. Combination therapy may provide more immediate control of rapidly progressing disease while minimizing adverse effects of long-term systemic corticosteroid use. A survey of German wound experts similarly endorsed frequent use of topical calcineurin inhibitors and combination systemic and topical glucocorticoid therapy as common therapeutic approaches.1

Importantly, treatments may vary depending on patient characteristics, comorbidities, and underlying disease, which underscores the need for individualized treatment approaches. Alternative first-line systemic treatments favored by respondents were cyclosporine, biologic medications, and antineutrophilic agents such as dapsone. Cyclosporine has demonstrated comparable efficacy to systemic glucocorticoids in one RCT and is considered an important steroid-sparing alternative for PG treatment.2 Biologic agents, especially tumor necrosis factor inhibitors, may be effective in treating cases of refractory PG or for concomitant inflammatory bowel disease management, as demonstrated by a small RCT documenting improvement of PG following infliximab infusion.3
Respondents strongly recommended petrolatum-impregnated gauze and other nonadhesive dressings, including alginate and hydrocolloid dressings, as part of PG wound care. Topical antimicrobials and compression stockings also were recommended by respondents. These practices aim to promote moist environments for healing, avoid maceration, prevent superinfection, optimize wound healing, and minimize damage from adhesive injury.12 Wound debridement and grafting generally were not recommended. However, pathergy is not a universal phenomenon in PG, and wounds that are no longer in the inflammatory phase may benefit from gentle debridement of necrotic tissue and/or grafting in select cases.10
Conclusion
An approach to modifying PG management based on clinical presentation and the practice of combination therapy with multiple systemic agents in refractory PG cases was not addressed in our survey. The low response rate is a limitation; however, the opinions of 51 medical dermatologist experts who regularly manage PG (in contrast to papers based on individualized clinical experience) can provide important clinical guidance until more scientific evidence is established.
Acknowledgments
We would like to thank the SDH and RDS membership for their participation in this survey. We especially acknowledge the other members of the SDH Scientific Task Force for their feedback: Misha Rosenbach, MD (Philadelphia, Pennsylvania); Robert G. Micheletti, MD (Philadelphia, Pennsylvania); Karolyn Wanat, MD (Milwaukee, Wisconsin); Amy Chen, MD (Cromwell, Connecticut); and A. Rambi Cardones, MD (Durham, North Carolina).
- Al Ghazal P, Dissemond J. Therapy of pyoderma gangrenosum in Germany: results of a survey among wound experts. J Dtsch Dermatol Ges . 2015;13:317-324.
- Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958.
- Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509.
- Al Ghazal P, Klode J, Dissemond J. Diagnostic criteria for pyoderma gangrenosum: results of a survey among dermatologic wound experts in Germany. J Dtsch Dermatol Ges. 2014;12:1129-1131.
- Weenig RH, Davis MD, Dahl PR, et al. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347:1412-1418.
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- Bennett ML, Jackson JM, Jorizzo JL, et al. Pyoderma gangrenosum: a comparison of typical and atypical forms with an emphasis on time to remission. case review of 86 patients from 2 institutions. Medicine. 2000;79:37-46.
- Su WP, Davis MD, Weening RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Aschyan H, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Binus AM, Qureshi AA, Li VW, et al. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244-1250.
- Reichrath J, Bens G, Bonowitz A, et al. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273-283.
- Miller J, Yentzer BA, Clark A, et al. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol. 2010;62:646-654.
- Al Ghazal P, Dissemond J. Therapy of pyoderma gangrenosum in Germany: results of a survey among wound experts. J Dtsch Dermatol Ges . 2015;13:317-324.
- Ormerod AD, Thomas KS, Craig FE, et al. Comparison of the two most commonly used treatments for pyoderma gangrenosum: results of the STOP GAP randomised controlled trial. BMJ. 2015;350:h2958.
- Brooklyn TN, Dunnill MG, Shetty A, et al. Infliximab for the treatment of pyoderma gangrenosum: a randomised, double blind, placebo controlled trial. Gut. 2006;55:505-509.
- Al Ghazal P, Klode J, Dissemond J. Diagnostic criteria for pyoderma gangrenosum: results of a survey among dermatologic wound experts in Germany. J Dtsch Dermatol Ges. 2014;12:1129-1131.
- Weenig RH, Davis MD, Dahl PR, et al. Skin ulcers misdiagnosed as pyoderma gangrenosum. N Engl J Med. 2002;347:1412-1418.
- Powell FC, Su WP, Perry HO. Pyoderma gangrenosum: classification and management. J Am Acad Dermatol. 1996;34:395-409.
- Bennett ML, Jackson JM, Jorizzo JL, et al. Pyoderma gangrenosum: a comparison of typical and atypical forms with an emphasis on time to remission. case review of 86 patients from 2 institutions. Medicine. 2000;79:37-46.
- Su WP, Davis MD, Weening RH, et al. Pyoderma gangrenosum: clinicopathologic correlation and proposed diagnostic criteria. Int J Dermatol. 2004;43:790-800.
- Aschyan H, Butler DC, Nelson CA, et al. The association of age with clinical presentation and comorbidities of pyoderma gangrenosum. JAMA Dermatol. 2018;154:409-413.
- Binus AM, Qureshi AA, Li VW, et al. Pyoderma gangrenosum: a retrospective review of patient characteristics, comorbidities and therapy in 103 patients. Br J Dermatol. 2011;165:1244-1250.
- Reichrath J, Bens G, Bonowitz A, et al. Treatment recommendations for pyoderma gangrenosum: an evidence-based review of the literature based on more than 350 patients. J Am Acad Dermatol. 2005;53:273-283.
- Miller J, Yentzer BA, Clark A, et al. Pyoderma gangrenosum: a review and update on new therapies. J Am Acad Dermatol. 2010;62:646-654.
Practice Points
- The diagnosis of pyoderma gangrenosum (PG) poses a challenge in clinical practice that could be minimized by following a stepwise algorithm based on initial test results (including skin biopsies) and features of the patient’s clinical presentation.
- As there is no US Food and Drug Administration–approved treatment for PG, a stepwise algorithm approach in combination with the clinical experience addressing inflammation and wound care is essential to reach control and remission of PG.
Approximation of Alcohol-Based Hand Sanitizer Volume Using a Toothpaste Cap
Practice Gap
The Centers for Disease Control and Prevention recommends handwashing with soap and water or using alcohol-based hand sanitizers to prevent transmission of coronavirus disease 2019. Five steps are delineated for effective handwashing: wetting, lathering, scrubbing, rinsing, and drying. Although alcohol-based sanitizers may be perceived as more damaging to the skin, they are less likely to cause dermatitis than handwashing with soap and water.1 Instructions are precise for handwashing, while there are no recommendations for effective use of alcohol-based hand sanitizers. A common inquiry regarding alcohol-based hand sanitizers is the volume needed for efficacy without causing skin irritation.
The Technique
Approximately 1 mL of alcohol-based hand sanitizer is recommended by some manufacturers. However, abundant evidence refutes this recommendation, including a study that tested the microbial efficacy of alcohol-based sanitizers by volume. A volume of 2 mL was necessary to achieve the 2.0 log reduction of contaminants as required by the US Food and Drug Administration for antimicrobial efficacy.2 The precise measurement of hand sanitizer using a calibrated syringe before each use is impractical. Thus, we recommend using a screw-top toothpaste cap to assist in approximating the necessary volume (Figure). The cap holds approximately 1 mL of liquid as measured using a syringe; therefore, 2 caps filled with sanitizer should be used.

Practice Implications
The general public may be underutilizing hand sanitizer due to fear of excessive skin irritation or supply shortages, which will reduce efficacy. Patients and physicians can use this simple visual approximation to ensure adequate use of hand sanitizer volume.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Kampf G, Ruselack S, Eggerstedt S, et al. Less and less-influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 2013;13:472.
Practice Gap
The Centers for Disease Control and Prevention recommends handwashing with soap and water or using alcohol-based hand sanitizers to prevent transmission of coronavirus disease 2019. Five steps are delineated for effective handwashing: wetting, lathering, scrubbing, rinsing, and drying. Although alcohol-based sanitizers may be perceived as more damaging to the skin, they are less likely to cause dermatitis than handwashing with soap and water.1 Instructions are precise for handwashing, while there are no recommendations for effective use of alcohol-based hand sanitizers. A common inquiry regarding alcohol-based hand sanitizers is the volume needed for efficacy without causing skin irritation.
The Technique
Approximately 1 mL of alcohol-based hand sanitizer is recommended by some manufacturers. However, abundant evidence refutes this recommendation, including a study that tested the microbial efficacy of alcohol-based sanitizers by volume. A volume of 2 mL was necessary to achieve the 2.0 log reduction of contaminants as required by the US Food and Drug Administration for antimicrobial efficacy.2 The precise measurement of hand sanitizer using a calibrated syringe before each use is impractical. Thus, we recommend using a screw-top toothpaste cap to assist in approximating the necessary volume (Figure). The cap holds approximately 1 mL of liquid as measured using a syringe; therefore, 2 caps filled with sanitizer should be used.

Practice Implications
The general public may be underutilizing hand sanitizer due to fear of excessive skin irritation or supply shortages, which will reduce efficacy. Patients and physicians can use this simple visual approximation to ensure adequate use of hand sanitizer volume.
Practice Gap
The Centers for Disease Control and Prevention recommends handwashing with soap and water or using alcohol-based hand sanitizers to prevent transmission of coronavirus disease 2019. Five steps are delineated for effective handwashing: wetting, lathering, scrubbing, rinsing, and drying. Although alcohol-based sanitizers may be perceived as more damaging to the skin, they are less likely to cause dermatitis than handwashing with soap and water.1 Instructions are precise for handwashing, while there are no recommendations for effective use of alcohol-based hand sanitizers. A common inquiry regarding alcohol-based hand sanitizers is the volume needed for efficacy without causing skin irritation.
The Technique
Approximately 1 mL of alcohol-based hand sanitizer is recommended by some manufacturers. However, abundant evidence refutes this recommendation, including a study that tested the microbial efficacy of alcohol-based sanitizers by volume. A volume of 2 mL was necessary to achieve the 2.0 log reduction of contaminants as required by the US Food and Drug Administration for antimicrobial efficacy.2 The precise measurement of hand sanitizer using a calibrated syringe before each use is impractical. Thus, we recommend using a screw-top toothpaste cap to assist in approximating the necessary volume (Figure). The cap holds approximately 1 mL of liquid as measured using a syringe; therefore, 2 caps filled with sanitizer should be used.

Practice Implications
The general public may be underutilizing hand sanitizer due to fear of excessive skin irritation or supply shortages, which will reduce efficacy. Patients and physicians can use this simple visual approximation to ensure adequate use of hand sanitizer volume.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Kampf G, Ruselack S, Eggerstedt S, et al. Less and less-influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 2013;13:472.
- Stutz N, Becker D, Jappe U, et al. Nurses’ perceptions of the benefits and adverse effects of hand disinfection: alcohol-based hand rubs vs. hygienic handwashing: a multicentre questionnaire study with additional patch testing by the German Contact Dermatitis Research Group. Br J Dermatol. 2009;160:565-572.
- Kampf G, Ruselack S, Eggerstedt S, et al. Less and less-influence of volume on hand coverage and bactericidal efficacy in hand disinfection. BMC Infect Dis. 2013;13:472.
Skin Tightening
Minimally and noninvasive skin tightening has become one of the most requested cosmetic procedures. Skin laxity often is apparent in areas of the face, neck, jawline, hands, abdomen, and thighs, with features of fine lines, wrinkles, and cellulite. Intrinsic and extrinsic factors contribute to the development of skin laxity. Intrinsic aspects include chronological age, stress, and genetics, whereas extrinsic influences include exposure to solar radiation, environmental toxins, and smoking.1,2 These factors affect the production and maintenance of both collagen and elastic proteins, which are the main components that help the skin stay firm and smooth. With a goal of improving skin laxity, multiple skin tightening modalities have been developed.
Traditionally, skin laxity was treated by invasive surgical skin procedures (eg, rhytidectomy), which carry a high financial cost, require an operating room and general anesthesia, have a prolonged recovery time with notable postoperative care, and have possible risk of unwanted scars.3,4 The risks associated with invasive procedures have spurned a growing demand for minimally invasive and noninvasive methods, which have fostered the development of several skin laxity reversal modalities over the last decade. Although the achieved results of these technologies are less dramatic and require more treatments, they do not possess the associated risks and adverse effects seen in invasive surgical procedures. As such, demand for these techniques has been growing among cosmetic patients.
There are multiple technologies that currently are employed to achieve noninvasive skin tightening. Laser therapy, radiofrequency (RF), ultrasound, and intense pulsed light (IPL) are methods that focus targeted energy to elevate temperatures in the deeper layers of the skin. Elevated thermal energy causes denaturing of collagen with preservation of heat-stable intermolecular cross-links. Skin tightening is achieved through physical shortening of the collagen fibers with preservation of the heat-stable intermolecular hydrogen bonds, which leads to an increase in the rubber elastic properties of the collagen polymer and stimulation of new collagen formation.5,6 The temperature at which this process occurs has been frequently reported as approximately 65°C.7,8 Alternative noninvasive therapies that do not focus on elevated thermal energy for skin tightening include chemical peels and skin care products.
Given the multitude of treatment methods that have been developed to counteract skin laxity, this article seeks to provide an overview of some technologies, devices, and commonly used therapies to help dermatologists choose the appropriate modalities for their cosmetic patients.
Laser Therapy
Since its approval in the 1980s, laser therapy has become an alternative to invasive surgical skin tightening.9 Laser therapy utilized for treatment can be subcategorized into 2 types: ablative and nonablative.
Traditional ablative skin tightening utilized CO2 or erbium:YAG lasers. These lasers caused skin tightening by first ablating the epidermis cleanly off the dermis, with a partially coagulated area in the dermis, which triggered a wound-healing cascade followed by neocollagenesis and remodeling.10,11 Although this treatment displays notable retightening of the skin, traditional ablative lasers are not routinely used, likely because of lengthy recovery periods, risk for scar development, flares of acne and herpes simplex virus, hyperpigmentation, and delayed-onset hypopigmentation.9,12,13
Fractional ablative laser treatments soon emerged as an effective alternative to traditional ablative lasers. Various studies have noted better recovery times and side-effect profiles.14-18 This improvement is believed to be due to the method of wound healing in fractional ablative laser treatments. Ablative fractional photothermolysis works by generating deeply narrow focal ablations that involve the dermis and epidermis while leaving the surrounding skin unscathed, which allows for rapid re-epithelization, filling in of the dermal pockets, and stimulation of dermal remodeling.10,11,18,19 Studies have demonstrated a range of improvement in skin laxity from 56% to 65.3% at 6 months posttreatment.20,21 Although the incidence of reported side effects is better than with the traditional ablative laser, fractional ablative lasers have documented reports of similar types of side effects as traditional lasers due in part to ablation of the skin.22,23
Nonablative lasers were developed as alternatives to ablative laser treatments. This class of lasers produces a milder effect compared with its ablative counterpart. Studies show a quantitative improvement range of 8.9% to 11% in skin laxity 3 months posttreatment.24,25 Nonablative lasers induce controlled tissue injury in the dermis, which leads to stimulation of dermal remodeling and collagen production.11 Although the effects of nonablative lasers are milder compared with their ablative counterparts, they possess the superior benefit of minimal adverse events. Most studies reported transient erythema posttreatment, but no long-term adverse effects have been noted,26-31 in part due to preservation of the epidermal layer.
Radiofrequency
Radiofrequency technology was the first method marketed for noninvasive skin tightening. Radiofrequency devices work by generating heat through tissue resistance to an applied alternating electrical current, which leads to collagen contraction and remodeling along with neocollgenesis.32 The major electrode configurations used in these technologies are monopolar, bipolar, and multipolar, which differ by the electric field they produce. Reported side effects include erythema that arose 1 week following completion of treatment and resolved by 6-month follow-up, as well as hypertrophic scarring, transient postinflammatory hyperpigmentation, and pain.33,34
Monopolar systems were the first among these devices to be developed for use in skin tightening and remain the most extensively studied technology for treatment of skin laxity. Developed in 2001, the Thermage device (Solta Medical, Valent Pharmaceuticals) remains the most extensively studied technology for the treatment of skin laxity.35 In a trial performed by Fitzpatrick et al,36 treatment of skin laxity of the periorbital area with ThermaCool TC (Thermage, Inc) demonstrated an 83.2% improvement in at least 1 point treated and an overall 28.9% improvement of the entire treatment area at 6-month follow-up. Additionally, a survey study of 5700 patients who received monopolar RF skin tightening treatments demonstrated that 26% of patients experienced immediate tightening following treatment, and 54% observed tightening 6 months posttreatment.37
Bipolar and multipolar devices were developed following the success of monopolar devices in the treatment for skin laxity. In a study evaluating multipolar RF for the face and neck, all 11 patients were determined to have improvement of their skin laxity following weekly treatments for 8 weeks.38
Ultrasound
The use of ultrasound for skin tightening was first approved in 2009.39 The primary mechanism of skin tightening is through thermally induced contraction of collagen with subsequent collagen neogenesis achieved through absorption of the vibrational acoustic energy into target tissue.40 There are 2 types of ultrasound methods: microfocused and high-intensity focused. Microfocused ultrasound focuses on delivering lower-energy pulses to the deep reticular dermal and subdermal layers that lead to disruption of the underlying architecture of the skin, promoting increases in distensibility, elasticity, and viscoelasticity.41 To date, microfocused ultrasound is approved for treating skin laxity of the eyebrow and submental area and wrinkles of the décolleté. Currently, there are 2 devices approved by the US Food and Drug Administration for the treatment of skin laxity with ultrasound. These devices are the Ulthera System (Merz Pharmaceuticals) and the Sofwave system (Sofwave Medical Ltd).42 Oni et al43 evaluated 93 patients following treatment using Ulthera for skin laxity in the lower face. There was a noticeable improvement of 63.6% at 90 days following treatment. Brobst et al44 showed improvement in laxity at 6 months and 1.5 years following last treatment. The most commonly reported posttreatment side effects include transient purpura, transient edema, and transient postinflammatory pigmentation.42,45 Serious complications are rare and include development of palpable subcutaneous nodules and motor nerve paresis.42,46
High-intensity focused ultrasound has been more recently introduced as a modality for skin tightening and rejuvenation. This method focuses on applying heat to areas through acoustic energy to areas of the deep dermis, subdermal connective tissue, and fibromuscular layer in targeted microcoagulation zones without effect to the epidermis.47 The targeted thermal effects and microcoagulation are believed to cause skin tightening through collagen contraction and remodeling. Future studies are needed to determine the overall benefits in skin laxity to achieve approval by the US Food and Drug Administration for use as a treatment option.
IPL Therapy
Intense pulsed light therapy is different from lasers in that it utilizes a wider variety of wavelengths ranging from approximately 500 to 1200 nm.48 The process of skin tightening is achieved through selective photothermolysis in which thermal damage is focused solely on pigmented targets at the cellular or tissue levels in the epidermis and dermis.49 Intense pulsed light penetrates the tissues and is selectively absorbed by melanin and hemoglobin, thereby producing photothermal effects. The photothermal effects lead to reversible thermal damage to surrounding collagen and induction contraction of collagen fibers and fiber remodeling.50 Clinical studies on the effectiveness on skin tightening have shown incongruent results. Multiple studies have noted improvement in skin elasticity as well as increased deposits of collagen in treated areas. Other studies have shown no improvement of rhytides or wrinkle reduction. The side effects noted were transient pain, swelling, and erythema, along with rare instances of blisters and crusting.48,51-54 Due to the inhomogeneous results, the use of IPL is largely reserved for treatment of acne, hyperpigmentation, hypertrichosis, and superficial vascular malformations.
Chemical Peels
Chemical peels are used in the treatment of skin laxity through a process similar to ablative lasers. Unlike other methods described in this article, this type of treatment is only reserved for the facial areas. The peel must penetrate to the lower papillary dermis or deeper to allow for adequate collagen synthesis.55 As such, medium to deep peeling agents should be used.56 Peels cause coagulation of membrane proteins and necrosis of the epidermis and dermis, thereby stimulating collagen synthesis and keratinocyte regeneration. Additionally, there is an increase in the deposition of glycosaminoglycans, which play a major role in providing hydration for the skin because of their water-binding capacity.56 Deep peels have the added effect of restoring dermal architecture to its native state. Medium-depth peels work up to the layer of the epidermis and dermis.57 Trichloroacetic acid (TCA) 35% is the main ingredient used in these types of peels. Some examples include Monet combination (Jessner solution with 35% TCA), Brody combination (solid CO2 plus 35% TCA), and Coleman combination (70% glycolic acid and 35% TCA). Deep peels penetrate to the levels of the reticular dermis.58 The formulation of these peels contain croton oil and phenols in various concentrations.57,58 A study by Brody59 noted clinical improvement of skin laxity–attributed histologic depth achieved by medium-depth peels. The results of the study demonstrated that the depth of wounding from 3 consecutive applications of TCA led to greater epidermal hyperplasia and a more dense formation of dermal elastic fiber formation on histologic examination. Side effects noted in the study included transient erythema, edema, and erosions that resolved without scar formation at 30-day follow-up.59 Another study performed by Oresajo et al60 demonstrated that patients treated with either a chemical peel of 41% capryloyl salicylic acid or 30% glycolic acid led to notable reduction of fine lines/wrinkles vs baseline. Side effects noted included pruritus, erythema, increased skin sensitivity, epidermolysis, allergic and irritant contact dermatitis, and postinflammatory hyperpigmentation.60
Skin Care
Skin care products have been developed over the years and marketed to aid in the treatment of skin laxity. Some studied methods include photoprotection products, antioxidant-based products, and vitamin A products. Photoprotection plays a crucial role in the prevention of skin laxity. Unprotected sun exposure can induce damage to previously treated skin, leading to minimized or cancelled rejuvenation measures.61
Oxidation is a major contributor in the development of skin laxity. The skin naturally possesses endogenous antioxidant defense mechanisms that protect its cells from free radical damage. However, these mechanisms are reduced as skin ages and are further diminished with photodamage. Ascorbic acid is a collagen stimulator that is known to have antioxidant properties. In the appropriate formulations, topical vitamin C directly supplements the skin’s antioxidant reservoir.61
The use of vitamin A, a retinoic acid, for treatment of skin laxity is based on its ability to improve the production of procollagen and elastic fiber components, resulting in the restoration of dermal matrix proteins.61-65 Vitamin A in the skin plays a key role in the regulation and control of proliferation and differentiation of all major cell types found in the epidermis and dermis.61 Studies have shown that the long-term use of topical vitamin A improves fine and coarse wrinkling.65
Final Thoughts
Various technologies have been developed to provide clinically significant skin laxity reversal. Laser, RF, ultrasound, IPL, and topical therapies provide numerous options at our disposal. Although many devices are available, it is important to consider the desired outcome, cost, and adverse events when discussing therapeutic options for treating skin laxity (eTable). Patients should be advised that multiple treatment sessions over the course of months will likely be necessary. With the development of numerous technologies, we now have many options to offer our patients who desire minimally or noninvasive skin tightening.
- McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann NY Acad Sci. 2006;1067:323-331.
- Yaar M. Clinical and histological features of intrinsic versus extrinsic skin aging. In: Gilchrest BA, Krutmann J, eds. Skin Aging. Berlin, Germany: Springer, Heidelberg; 2006:9-21.
- Ramanadham SR, Costa CR, Narasimhan K, et al. Refining the anesthesia management of the face-lift patient: lessons learned from 1089 consecutive face lifts. Plast Reconstr Surg. 2015;135:723-730.
- Gupta V, Winocour J, Shi H, et al. Preoperative risk factors and complication rates in facelift: analysis of 11,300 patients. Aesthet Surg J. 2016;36:1-13.
- le Lous M, Flandin F, Herbage D, et al. Influence of collagen denaturation on the chemorheological properties of skin, assessed by differential scanning calorimetry and hydrothermal isometric tension measurement. Biochim Biophys Acta. 1982;717:295-300.
- Ross EV, Yashar SS, Naseef GS, et al. A pilot study of in vivo immediate tissue contraction with CO2 skin laser resurfacing in a live farm pig. Dermatol Surg. 1999;25:851-856.
- Arnoczky SP, Aksan A. Thermal modification of connective tissues: basic science considerations and clinical implications. J Am Acad Orthop Surg. 2000;8:305-313.
- Hsu TS, Kaminer MS. The use of nonablative radiofrequency technology to tighten the lower face and neck. Semin Cutan Med Surg. 2003;22:115-123.
- Alster TS. Cutaneous resurfacing with CO2 and erbium: YAG lasers: preoperative, intraoperative, and postoperative considerations. Plast Reconstr Surg. 1999;103:619-632; discussion 633-634.
- Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014;23:49-60.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Bernstein LJ, Kauvar AN, Grossman MC, et al. The short‐ and long‐term side effects of carbon dioxide laser resurfacing. Dermatol Surg. 1997;23:519-525.
- Nanni CA, Alster TS. Complications of carbon dioxide laser resurfacing. an evaluation of 500 patients. Dermatol Surg. 1998;24:315-320.
- Ortiz AE, Tremaine AM, Zachary CB. Long‐term efficacy of a fractional resurfacing device. Lasers Surg Med. 2010;42:168-170.
- Rahman Z, MacFalls H, Jiang K, et al. Fractional deep dermal ablation induces tissue tightening. Lasers Surg Med. 2009;41:78-86.
- Graber EM, Tanzi EL, Alster TS. Side effects and complications of fractional laser photothermolysis: experience with 961 treatments. Dermatol Surg. 2008;34:301-305; discussion 305-307.
- Fisher GH, Geronemus RG. Short‐term side effects of fractional photothermolysis. Dermatol Surg. 2005;31:1245-1249.
- Ortiz AE, Goldman MP, Fitzpatrick RE. Ablative CO2 lasers for skin tightening: traditional versus fractional. Dermatol Surg. 2014;40(suppl 12):S147-S151.
- Geronemus RG. Fractional photothermolysis: current and future applications. Lasers Surg Med. 2006;38:169-176.
- Tierney EP, Hanke CW, Petersen J. Ablative fractionated CO2 laser treatment of photoaging: a clinical and histologic study. Dermatol Surg. 2012;38:1777-1789.
- Tierney EP, Hanke CW, Watkins L. Treatment of lower eyelid rhytids and laxity with ablative fractionated carbon-dioxide laser resurfacing: case series and review of the literature. J Am Acad Dermatol. 2011;64:730-740.
- Fife DJ, Fitzpatrick RE, Zachary CB. Complications of fractional CO2 laser resurfacing: four cases. Lasers Surg Med. 2009;41:179-184.
- Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatol Surg. 2010;36:299-306.
- Miller L, Mishra V, Alsaad S, et al. Clinical evaluation of a non-ablative 1940 nm fractional laser. J Drugs Dermatol. 2014;13:1324-1329.
- Alexiades-Armenakas M. Nonablative skin tightening with a variable depth heating 1310-nm wavelength laser in combination with surface cooling. J Drugs Dermatol. 2007;6:1096-1103.
- Alster TS, Wanitphakdeedecha R. Improvement of postfractional laser erythema with light‐emitting diode photomodulation. Dermatol Surg. 2009;35:813-815.
- Fournier N, Lagarde JM, Turlier V, et al. A 35-month profilometric and clinical evaluation of non-ablative remodeling using a 1540-nm Er:glass laser. J Cosmet Laser Ther. 2004;6:126-130.
- Hædersdal M, Moreau KER, Beyer DM, et al. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers Surg Med. 2009;41:189-195.
- Lupton JR, Williams CM, Alster TS. Nonablative laser skin resurfacing using a 1540 nm erbium glass laser: a clinical and histologic analysis. Dermatol Surg. 2002;28:833-835.
- Moody BR, McCarthy JE, Hruza GJ. Collagen remodeling after 585‐nm pulsed dye laser irradiation: an ultrasonographic analysis. Dermatol Surg. 2003;29:997-999, discussion 999-1000.
- Pollock H, Pollock TA. NLite laser: nonablative wrinkle reduction.Aesthet Surg J. 2001;21:371-372.
- Burns JA. Thermage: monopolar radiofrequency. Aesthet Surg J. 2005;25:638-642.
- Weiss RA, Weiss MA, Munavelli G, et al. Monopolar radiofrequency facial tightening: a retrospective analysis of efficacy and safety in over 600 treatments. J Drugs Dermatol. 2006;5:707-712.
- Sadick NS, Makino Y. Selective electro‐thermolysis in aesthetic medicine: a review. Lasers Surg Med. 2004;34:91-97.
- Alster TS, Lupton JR. Nonablative cutaneous remodeling using radiofrequency devices. Clin Dermatol. 2007;25:487-491.
- Fitzpatrick R, Geronemus R, Goldberg D, et al. Multicenter study of noninvasive radiofrequency for periorbital tissue tightening. Lasers Surg Med. 2003;33:232-242.
- Dover JS, Zelickson B, 14-Physician Multispecialty Consensus Panel. Results of a survey of 5,700 patient monopolar radiofrequency facial skin tightening treatments: assessment of a low‐energy multiple‐pass technique leading to a clinical end point algorithm. Dermatol Surg. 2007;33:900-907.
- de Oliveira TC, Rocha SF, Ramos DG, et al. Effects of multipolar radiofrequency and pulsed electromagnetic field treatment for face and neck rejuvenation [published online March 8, 2017]. Dermatol Res Pract. doi:10.1155/2017/4146391.
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Van Leenders GJ, Beerlage HP, Ruijter ET, et al. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391-394.
- Wulkan AJ, Fabi SG, Green JB. Microfocused ultrasound for facial photorejuvenation: a review. Facial Plast Surg. 2016;32:269-275.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 201332:18-25.
- Oni G, Hoxworth R, Teotia S, et al. Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet Surg J. 2014;34:1099-1110.
- Brobst RW, Ferguson M, Perkins SW. Noninvasive treatment of the neck. Facial Plast Surg North Am. 2014;22:191-202.
- Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg. 2012;38:754-759.
- Missel L. Prevention of potential adverse events associated with use of Ulthera device. Tech Bull. 2011;32:18-25.
- Bove T, Zawada T, Serup J, et al. High‐frequency (20‐MHz) high‐intensity focused ultrasound (HIFU) system for dermal intervention: preclinical evaluation in skin equivalents. Skin Res Technol. 2019;25:217-228.
- Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med. 2003;32:78-87.
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Faucz LL, Will SE, Rodrigues CJ, et al. Quantitative evaluation of collagen and elastic fibers after intense pulsed light treatment of mouse skin. Lasers Surg Med. 2018;50:644-650.
- Goldberg DJ, Cutler KB. Nonablative treatment of rhytids with intense pulsed light. Lasers Surg Med. 2000;26:196-200.
- Li Y-H, Wu Y, Chen JZ, et al. Application of a new intense pulsed light device in the treatment of photoaging skin in Asian patients. Dermatol Surg. 2008;34:1459-1464.
- Shin J-W, Lee D-H, Choi S-Y, et al. Objective and non‐invasive evaluation of photorejuvenation effect with intense pulsed light treatment in Asian skin. J Eur Acad Dermatol Venereol. 2011;25:516-522.
- Weiss RA, Weiss MA, Beasley KL. Rejuvenation of photoaged skin: 5 years results with intense pulsed light of the face, neck, and chest. Dermatol Surg. 2002;28:1115-1119.
- Lee KC, Wambier CG, Soon SL, et al. Basic chemical peeling: superficial and medium-depth peels. J Am Acad Dermatol. 2019;81:313-324.
- Brody HJ. Do chemical peels tighten the skin? Dermatol Surg. 2014;40(suppl):S129-S133.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Brody HJ. Variations and comparisons in medium‐depth chemical peeling. J Dermatol Surg Oncol. 1989;15:953-963.
- Oresajo C, Yatskayer M, Hansenne I. Clinical tolerance and efficacy of capryloyl salicylic acid peel compared to a glycolic acid peel in subjects with fine lines/wrinkles and hyperpigmented skin. J Cosmet Dermatol. 2008;7:259-262.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- Griffiths C. The role of retinoids in the prevention and repair of aged and photoaged skin. Clin Exp Dermatol. 2001;26:613-618.
- Darlenski R, Surber C, Fluhr J. Topical retinoids in the management of photodamaged skin: from theory to evidence‐based practical approach. Br J Dermatol. 2010;163:1157-1165.
- Kang S, Bergfeld W, Gottlieb AB, et al. Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial. Am J Clin Dermatol. 2005;6:245-253.
- Riahi RR, Bush AE, Cohen PR. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am J Clin Dermatol. 2016;17:265-276.
- American Society of Plastic Surgeons. Average surgeon/physician fees. https://www.plasticsurgery.org/documents/News/Statistics/2019/cosmetic-procedures-average-cost-2019.pdf. Accessed August 24, 2020.
Minimally and noninvasive skin tightening has become one of the most requested cosmetic procedures. Skin laxity often is apparent in areas of the face, neck, jawline, hands, abdomen, and thighs, with features of fine lines, wrinkles, and cellulite. Intrinsic and extrinsic factors contribute to the development of skin laxity. Intrinsic aspects include chronological age, stress, and genetics, whereas extrinsic influences include exposure to solar radiation, environmental toxins, and smoking.1,2 These factors affect the production and maintenance of both collagen and elastic proteins, which are the main components that help the skin stay firm and smooth. With a goal of improving skin laxity, multiple skin tightening modalities have been developed.
Traditionally, skin laxity was treated by invasive surgical skin procedures (eg, rhytidectomy), which carry a high financial cost, require an operating room and general anesthesia, have a prolonged recovery time with notable postoperative care, and have possible risk of unwanted scars.3,4 The risks associated with invasive procedures have spurned a growing demand for minimally invasive and noninvasive methods, which have fostered the development of several skin laxity reversal modalities over the last decade. Although the achieved results of these technologies are less dramatic and require more treatments, they do not possess the associated risks and adverse effects seen in invasive surgical procedures. As such, demand for these techniques has been growing among cosmetic patients.
There are multiple technologies that currently are employed to achieve noninvasive skin tightening. Laser therapy, radiofrequency (RF), ultrasound, and intense pulsed light (IPL) are methods that focus targeted energy to elevate temperatures in the deeper layers of the skin. Elevated thermal energy causes denaturing of collagen with preservation of heat-stable intermolecular cross-links. Skin tightening is achieved through physical shortening of the collagen fibers with preservation of the heat-stable intermolecular hydrogen bonds, which leads to an increase in the rubber elastic properties of the collagen polymer and stimulation of new collagen formation.5,6 The temperature at which this process occurs has been frequently reported as approximately 65°C.7,8 Alternative noninvasive therapies that do not focus on elevated thermal energy for skin tightening include chemical peels and skin care products.
Given the multitude of treatment methods that have been developed to counteract skin laxity, this article seeks to provide an overview of some technologies, devices, and commonly used therapies to help dermatologists choose the appropriate modalities for their cosmetic patients.
Laser Therapy
Since its approval in the 1980s, laser therapy has become an alternative to invasive surgical skin tightening.9 Laser therapy utilized for treatment can be subcategorized into 2 types: ablative and nonablative.
Traditional ablative skin tightening utilized CO2 or erbium:YAG lasers. These lasers caused skin tightening by first ablating the epidermis cleanly off the dermis, with a partially coagulated area in the dermis, which triggered a wound-healing cascade followed by neocollagenesis and remodeling.10,11 Although this treatment displays notable retightening of the skin, traditional ablative lasers are not routinely used, likely because of lengthy recovery periods, risk for scar development, flares of acne and herpes simplex virus, hyperpigmentation, and delayed-onset hypopigmentation.9,12,13
Fractional ablative laser treatments soon emerged as an effective alternative to traditional ablative lasers. Various studies have noted better recovery times and side-effect profiles.14-18 This improvement is believed to be due to the method of wound healing in fractional ablative laser treatments. Ablative fractional photothermolysis works by generating deeply narrow focal ablations that involve the dermis and epidermis while leaving the surrounding skin unscathed, which allows for rapid re-epithelization, filling in of the dermal pockets, and stimulation of dermal remodeling.10,11,18,19 Studies have demonstrated a range of improvement in skin laxity from 56% to 65.3% at 6 months posttreatment.20,21 Although the incidence of reported side effects is better than with the traditional ablative laser, fractional ablative lasers have documented reports of similar types of side effects as traditional lasers due in part to ablation of the skin.22,23
Nonablative lasers were developed as alternatives to ablative laser treatments. This class of lasers produces a milder effect compared with its ablative counterpart. Studies show a quantitative improvement range of 8.9% to 11% in skin laxity 3 months posttreatment.24,25 Nonablative lasers induce controlled tissue injury in the dermis, which leads to stimulation of dermal remodeling and collagen production.11 Although the effects of nonablative lasers are milder compared with their ablative counterparts, they possess the superior benefit of minimal adverse events. Most studies reported transient erythema posttreatment, but no long-term adverse effects have been noted,26-31 in part due to preservation of the epidermal layer.
Radiofrequency
Radiofrequency technology was the first method marketed for noninvasive skin tightening. Radiofrequency devices work by generating heat through tissue resistance to an applied alternating electrical current, which leads to collagen contraction and remodeling along with neocollgenesis.32 The major electrode configurations used in these technologies are monopolar, bipolar, and multipolar, which differ by the electric field they produce. Reported side effects include erythema that arose 1 week following completion of treatment and resolved by 6-month follow-up, as well as hypertrophic scarring, transient postinflammatory hyperpigmentation, and pain.33,34
Monopolar systems were the first among these devices to be developed for use in skin tightening and remain the most extensively studied technology for treatment of skin laxity. Developed in 2001, the Thermage device (Solta Medical, Valent Pharmaceuticals) remains the most extensively studied technology for the treatment of skin laxity.35 In a trial performed by Fitzpatrick et al,36 treatment of skin laxity of the periorbital area with ThermaCool TC (Thermage, Inc) demonstrated an 83.2% improvement in at least 1 point treated and an overall 28.9% improvement of the entire treatment area at 6-month follow-up. Additionally, a survey study of 5700 patients who received monopolar RF skin tightening treatments demonstrated that 26% of patients experienced immediate tightening following treatment, and 54% observed tightening 6 months posttreatment.37
Bipolar and multipolar devices were developed following the success of monopolar devices in the treatment for skin laxity. In a study evaluating multipolar RF for the face and neck, all 11 patients were determined to have improvement of their skin laxity following weekly treatments for 8 weeks.38
Ultrasound
The use of ultrasound for skin tightening was first approved in 2009.39 The primary mechanism of skin tightening is through thermally induced contraction of collagen with subsequent collagen neogenesis achieved through absorption of the vibrational acoustic energy into target tissue.40 There are 2 types of ultrasound methods: microfocused and high-intensity focused. Microfocused ultrasound focuses on delivering lower-energy pulses to the deep reticular dermal and subdermal layers that lead to disruption of the underlying architecture of the skin, promoting increases in distensibility, elasticity, and viscoelasticity.41 To date, microfocused ultrasound is approved for treating skin laxity of the eyebrow and submental area and wrinkles of the décolleté. Currently, there are 2 devices approved by the US Food and Drug Administration for the treatment of skin laxity with ultrasound. These devices are the Ulthera System (Merz Pharmaceuticals) and the Sofwave system (Sofwave Medical Ltd).42 Oni et al43 evaluated 93 patients following treatment using Ulthera for skin laxity in the lower face. There was a noticeable improvement of 63.6% at 90 days following treatment. Brobst et al44 showed improvement in laxity at 6 months and 1.5 years following last treatment. The most commonly reported posttreatment side effects include transient purpura, transient edema, and transient postinflammatory pigmentation.42,45 Serious complications are rare and include development of palpable subcutaneous nodules and motor nerve paresis.42,46
High-intensity focused ultrasound has been more recently introduced as a modality for skin tightening and rejuvenation. This method focuses on applying heat to areas through acoustic energy to areas of the deep dermis, subdermal connective tissue, and fibromuscular layer in targeted microcoagulation zones without effect to the epidermis.47 The targeted thermal effects and microcoagulation are believed to cause skin tightening through collagen contraction and remodeling. Future studies are needed to determine the overall benefits in skin laxity to achieve approval by the US Food and Drug Administration for use as a treatment option.
IPL Therapy
Intense pulsed light therapy is different from lasers in that it utilizes a wider variety of wavelengths ranging from approximately 500 to 1200 nm.48 The process of skin tightening is achieved through selective photothermolysis in which thermal damage is focused solely on pigmented targets at the cellular or tissue levels in the epidermis and dermis.49 Intense pulsed light penetrates the tissues and is selectively absorbed by melanin and hemoglobin, thereby producing photothermal effects. The photothermal effects lead to reversible thermal damage to surrounding collagen and induction contraction of collagen fibers and fiber remodeling.50 Clinical studies on the effectiveness on skin tightening have shown incongruent results. Multiple studies have noted improvement in skin elasticity as well as increased deposits of collagen in treated areas. Other studies have shown no improvement of rhytides or wrinkle reduction. The side effects noted were transient pain, swelling, and erythema, along with rare instances of blisters and crusting.48,51-54 Due to the inhomogeneous results, the use of IPL is largely reserved for treatment of acne, hyperpigmentation, hypertrichosis, and superficial vascular malformations.
Chemical Peels
Chemical peels are used in the treatment of skin laxity through a process similar to ablative lasers. Unlike other methods described in this article, this type of treatment is only reserved for the facial areas. The peel must penetrate to the lower papillary dermis or deeper to allow for adequate collagen synthesis.55 As such, medium to deep peeling agents should be used.56 Peels cause coagulation of membrane proteins and necrosis of the epidermis and dermis, thereby stimulating collagen synthesis and keratinocyte regeneration. Additionally, there is an increase in the deposition of glycosaminoglycans, which play a major role in providing hydration for the skin because of their water-binding capacity.56 Deep peels have the added effect of restoring dermal architecture to its native state. Medium-depth peels work up to the layer of the epidermis and dermis.57 Trichloroacetic acid (TCA) 35% is the main ingredient used in these types of peels. Some examples include Monet combination (Jessner solution with 35% TCA), Brody combination (solid CO2 plus 35% TCA), and Coleman combination (70% glycolic acid and 35% TCA). Deep peels penetrate to the levels of the reticular dermis.58 The formulation of these peels contain croton oil and phenols in various concentrations.57,58 A study by Brody59 noted clinical improvement of skin laxity–attributed histologic depth achieved by medium-depth peels. The results of the study demonstrated that the depth of wounding from 3 consecutive applications of TCA led to greater epidermal hyperplasia and a more dense formation of dermal elastic fiber formation on histologic examination. Side effects noted in the study included transient erythema, edema, and erosions that resolved without scar formation at 30-day follow-up.59 Another study performed by Oresajo et al60 demonstrated that patients treated with either a chemical peel of 41% capryloyl salicylic acid or 30% glycolic acid led to notable reduction of fine lines/wrinkles vs baseline. Side effects noted included pruritus, erythema, increased skin sensitivity, epidermolysis, allergic and irritant contact dermatitis, and postinflammatory hyperpigmentation.60
Skin Care
Skin care products have been developed over the years and marketed to aid in the treatment of skin laxity. Some studied methods include photoprotection products, antioxidant-based products, and vitamin A products. Photoprotection plays a crucial role in the prevention of skin laxity. Unprotected sun exposure can induce damage to previously treated skin, leading to minimized or cancelled rejuvenation measures.61
Oxidation is a major contributor in the development of skin laxity. The skin naturally possesses endogenous antioxidant defense mechanisms that protect its cells from free radical damage. However, these mechanisms are reduced as skin ages and are further diminished with photodamage. Ascorbic acid is a collagen stimulator that is known to have antioxidant properties. In the appropriate formulations, topical vitamin C directly supplements the skin’s antioxidant reservoir.61
The use of vitamin A, a retinoic acid, for treatment of skin laxity is based on its ability to improve the production of procollagen and elastic fiber components, resulting in the restoration of dermal matrix proteins.61-65 Vitamin A in the skin plays a key role in the regulation and control of proliferation and differentiation of all major cell types found in the epidermis and dermis.61 Studies have shown that the long-term use of topical vitamin A improves fine and coarse wrinkling.65
Final Thoughts
Various technologies have been developed to provide clinically significant skin laxity reversal. Laser, RF, ultrasound, IPL, and topical therapies provide numerous options at our disposal. Although many devices are available, it is important to consider the desired outcome, cost, and adverse events when discussing therapeutic options for treating skin laxity (eTable). Patients should be advised that multiple treatment sessions over the course of months will likely be necessary. With the development of numerous technologies, we now have many options to offer our patients who desire minimally or noninvasive skin tightening.
Minimally and noninvasive skin tightening has become one of the most requested cosmetic procedures. Skin laxity often is apparent in areas of the face, neck, jawline, hands, abdomen, and thighs, with features of fine lines, wrinkles, and cellulite. Intrinsic and extrinsic factors contribute to the development of skin laxity. Intrinsic aspects include chronological age, stress, and genetics, whereas extrinsic influences include exposure to solar radiation, environmental toxins, and smoking.1,2 These factors affect the production and maintenance of both collagen and elastic proteins, which are the main components that help the skin stay firm and smooth. With a goal of improving skin laxity, multiple skin tightening modalities have been developed.
Traditionally, skin laxity was treated by invasive surgical skin procedures (eg, rhytidectomy), which carry a high financial cost, require an operating room and general anesthesia, have a prolonged recovery time with notable postoperative care, and have possible risk of unwanted scars.3,4 The risks associated with invasive procedures have spurned a growing demand for minimally invasive and noninvasive methods, which have fostered the development of several skin laxity reversal modalities over the last decade. Although the achieved results of these technologies are less dramatic and require more treatments, they do not possess the associated risks and adverse effects seen in invasive surgical procedures. As such, demand for these techniques has been growing among cosmetic patients.
There are multiple technologies that currently are employed to achieve noninvasive skin tightening. Laser therapy, radiofrequency (RF), ultrasound, and intense pulsed light (IPL) are methods that focus targeted energy to elevate temperatures in the deeper layers of the skin. Elevated thermal energy causes denaturing of collagen with preservation of heat-stable intermolecular cross-links. Skin tightening is achieved through physical shortening of the collagen fibers with preservation of the heat-stable intermolecular hydrogen bonds, which leads to an increase in the rubber elastic properties of the collagen polymer and stimulation of new collagen formation.5,6 The temperature at which this process occurs has been frequently reported as approximately 65°C.7,8 Alternative noninvasive therapies that do not focus on elevated thermal energy for skin tightening include chemical peels and skin care products.
Given the multitude of treatment methods that have been developed to counteract skin laxity, this article seeks to provide an overview of some technologies, devices, and commonly used therapies to help dermatologists choose the appropriate modalities for their cosmetic patients.
Laser Therapy
Since its approval in the 1980s, laser therapy has become an alternative to invasive surgical skin tightening.9 Laser therapy utilized for treatment can be subcategorized into 2 types: ablative and nonablative.
Traditional ablative skin tightening utilized CO2 or erbium:YAG lasers. These lasers caused skin tightening by first ablating the epidermis cleanly off the dermis, with a partially coagulated area in the dermis, which triggered a wound-healing cascade followed by neocollagenesis and remodeling.10,11 Although this treatment displays notable retightening of the skin, traditional ablative lasers are not routinely used, likely because of lengthy recovery periods, risk for scar development, flares of acne and herpes simplex virus, hyperpigmentation, and delayed-onset hypopigmentation.9,12,13
Fractional ablative laser treatments soon emerged as an effective alternative to traditional ablative lasers. Various studies have noted better recovery times and side-effect profiles.14-18 This improvement is believed to be due to the method of wound healing in fractional ablative laser treatments. Ablative fractional photothermolysis works by generating deeply narrow focal ablations that involve the dermis and epidermis while leaving the surrounding skin unscathed, which allows for rapid re-epithelization, filling in of the dermal pockets, and stimulation of dermal remodeling.10,11,18,19 Studies have demonstrated a range of improvement in skin laxity from 56% to 65.3% at 6 months posttreatment.20,21 Although the incidence of reported side effects is better than with the traditional ablative laser, fractional ablative lasers have documented reports of similar types of side effects as traditional lasers due in part to ablation of the skin.22,23
Nonablative lasers were developed as alternatives to ablative laser treatments. This class of lasers produces a milder effect compared with its ablative counterpart. Studies show a quantitative improvement range of 8.9% to 11% in skin laxity 3 months posttreatment.24,25 Nonablative lasers induce controlled tissue injury in the dermis, which leads to stimulation of dermal remodeling and collagen production.11 Although the effects of nonablative lasers are milder compared with their ablative counterparts, they possess the superior benefit of minimal adverse events. Most studies reported transient erythema posttreatment, but no long-term adverse effects have been noted,26-31 in part due to preservation of the epidermal layer.
Radiofrequency
Radiofrequency technology was the first method marketed for noninvasive skin tightening. Radiofrequency devices work by generating heat through tissue resistance to an applied alternating electrical current, which leads to collagen contraction and remodeling along with neocollgenesis.32 The major electrode configurations used in these technologies are monopolar, bipolar, and multipolar, which differ by the electric field they produce. Reported side effects include erythema that arose 1 week following completion of treatment and resolved by 6-month follow-up, as well as hypertrophic scarring, transient postinflammatory hyperpigmentation, and pain.33,34
Monopolar systems were the first among these devices to be developed for use in skin tightening and remain the most extensively studied technology for treatment of skin laxity. Developed in 2001, the Thermage device (Solta Medical, Valent Pharmaceuticals) remains the most extensively studied technology for the treatment of skin laxity.35 In a trial performed by Fitzpatrick et al,36 treatment of skin laxity of the periorbital area with ThermaCool TC (Thermage, Inc) demonstrated an 83.2% improvement in at least 1 point treated and an overall 28.9% improvement of the entire treatment area at 6-month follow-up. Additionally, a survey study of 5700 patients who received monopolar RF skin tightening treatments demonstrated that 26% of patients experienced immediate tightening following treatment, and 54% observed tightening 6 months posttreatment.37
Bipolar and multipolar devices were developed following the success of monopolar devices in the treatment for skin laxity. In a study evaluating multipolar RF for the face and neck, all 11 patients were determined to have improvement of their skin laxity following weekly treatments for 8 weeks.38
Ultrasound
The use of ultrasound for skin tightening was first approved in 2009.39 The primary mechanism of skin tightening is through thermally induced contraction of collagen with subsequent collagen neogenesis achieved through absorption of the vibrational acoustic energy into target tissue.40 There are 2 types of ultrasound methods: microfocused and high-intensity focused. Microfocused ultrasound focuses on delivering lower-energy pulses to the deep reticular dermal and subdermal layers that lead to disruption of the underlying architecture of the skin, promoting increases in distensibility, elasticity, and viscoelasticity.41 To date, microfocused ultrasound is approved for treating skin laxity of the eyebrow and submental area and wrinkles of the décolleté. Currently, there are 2 devices approved by the US Food and Drug Administration for the treatment of skin laxity with ultrasound. These devices are the Ulthera System (Merz Pharmaceuticals) and the Sofwave system (Sofwave Medical Ltd).42 Oni et al43 evaluated 93 patients following treatment using Ulthera for skin laxity in the lower face. There was a noticeable improvement of 63.6% at 90 days following treatment. Brobst et al44 showed improvement in laxity at 6 months and 1.5 years following last treatment. The most commonly reported posttreatment side effects include transient purpura, transient edema, and transient postinflammatory pigmentation.42,45 Serious complications are rare and include development of palpable subcutaneous nodules and motor nerve paresis.42,46
High-intensity focused ultrasound has been more recently introduced as a modality for skin tightening and rejuvenation. This method focuses on applying heat to areas through acoustic energy to areas of the deep dermis, subdermal connective tissue, and fibromuscular layer in targeted microcoagulation zones without effect to the epidermis.47 The targeted thermal effects and microcoagulation are believed to cause skin tightening through collagen contraction and remodeling. Future studies are needed to determine the overall benefits in skin laxity to achieve approval by the US Food and Drug Administration for use as a treatment option.
IPL Therapy
Intense pulsed light therapy is different from lasers in that it utilizes a wider variety of wavelengths ranging from approximately 500 to 1200 nm.48 The process of skin tightening is achieved through selective photothermolysis in which thermal damage is focused solely on pigmented targets at the cellular or tissue levels in the epidermis and dermis.49 Intense pulsed light penetrates the tissues and is selectively absorbed by melanin and hemoglobin, thereby producing photothermal effects. The photothermal effects lead to reversible thermal damage to surrounding collagen and induction contraction of collagen fibers and fiber remodeling.50 Clinical studies on the effectiveness on skin tightening have shown incongruent results. Multiple studies have noted improvement in skin elasticity as well as increased deposits of collagen in treated areas. Other studies have shown no improvement of rhytides or wrinkle reduction. The side effects noted were transient pain, swelling, and erythema, along with rare instances of blisters and crusting.48,51-54 Due to the inhomogeneous results, the use of IPL is largely reserved for treatment of acne, hyperpigmentation, hypertrichosis, and superficial vascular malformations.
Chemical Peels
Chemical peels are used in the treatment of skin laxity through a process similar to ablative lasers. Unlike other methods described in this article, this type of treatment is only reserved for the facial areas. The peel must penetrate to the lower papillary dermis or deeper to allow for adequate collagen synthesis.55 As such, medium to deep peeling agents should be used.56 Peels cause coagulation of membrane proteins and necrosis of the epidermis and dermis, thereby stimulating collagen synthesis and keratinocyte regeneration. Additionally, there is an increase in the deposition of glycosaminoglycans, which play a major role in providing hydration for the skin because of their water-binding capacity.56 Deep peels have the added effect of restoring dermal architecture to its native state. Medium-depth peels work up to the layer of the epidermis and dermis.57 Trichloroacetic acid (TCA) 35% is the main ingredient used in these types of peels. Some examples include Monet combination (Jessner solution with 35% TCA), Brody combination (solid CO2 plus 35% TCA), and Coleman combination (70% glycolic acid and 35% TCA). Deep peels penetrate to the levels of the reticular dermis.58 The formulation of these peels contain croton oil and phenols in various concentrations.57,58 A study by Brody59 noted clinical improvement of skin laxity–attributed histologic depth achieved by medium-depth peels. The results of the study demonstrated that the depth of wounding from 3 consecutive applications of TCA led to greater epidermal hyperplasia and a more dense formation of dermal elastic fiber formation on histologic examination. Side effects noted in the study included transient erythema, edema, and erosions that resolved without scar formation at 30-day follow-up.59 Another study performed by Oresajo et al60 demonstrated that patients treated with either a chemical peel of 41% capryloyl salicylic acid or 30% glycolic acid led to notable reduction of fine lines/wrinkles vs baseline. Side effects noted included pruritus, erythema, increased skin sensitivity, epidermolysis, allergic and irritant contact dermatitis, and postinflammatory hyperpigmentation.60
Skin Care
Skin care products have been developed over the years and marketed to aid in the treatment of skin laxity. Some studied methods include photoprotection products, antioxidant-based products, and vitamin A products. Photoprotection plays a crucial role in the prevention of skin laxity. Unprotected sun exposure can induce damage to previously treated skin, leading to minimized or cancelled rejuvenation measures.61
Oxidation is a major contributor in the development of skin laxity. The skin naturally possesses endogenous antioxidant defense mechanisms that protect its cells from free radical damage. However, these mechanisms are reduced as skin ages and are further diminished with photodamage. Ascorbic acid is a collagen stimulator that is known to have antioxidant properties. In the appropriate formulations, topical vitamin C directly supplements the skin’s antioxidant reservoir.61
The use of vitamin A, a retinoic acid, for treatment of skin laxity is based on its ability to improve the production of procollagen and elastic fiber components, resulting in the restoration of dermal matrix proteins.61-65 Vitamin A in the skin plays a key role in the regulation and control of proliferation and differentiation of all major cell types found in the epidermis and dermis.61 Studies have shown that the long-term use of topical vitamin A improves fine and coarse wrinkling.65
Final Thoughts
Various technologies have been developed to provide clinically significant skin laxity reversal. Laser, RF, ultrasound, IPL, and topical therapies provide numerous options at our disposal. Although many devices are available, it is important to consider the desired outcome, cost, and adverse events when discussing therapeutic options for treating skin laxity (eTable). Patients should be advised that multiple treatment sessions over the course of months will likely be necessary. With the development of numerous technologies, we now have many options to offer our patients who desire minimally or noninvasive skin tightening.
- McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann NY Acad Sci. 2006;1067:323-331.
- Yaar M. Clinical and histological features of intrinsic versus extrinsic skin aging. In: Gilchrest BA, Krutmann J, eds. Skin Aging. Berlin, Germany: Springer, Heidelberg; 2006:9-21.
- Ramanadham SR, Costa CR, Narasimhan K, et al. Refining the anesthesia management of the face-lift patient: lessons learned from 1089 consecutive face lifts. Plast Reconstr Surg. 2015;135:723-730.
- Gupta V, Winocour J, Shi H, et al. Preoperative risk factors and complication rates in facelift: analysis of 11,300 patients. Aesthet Surg J. 2016;36:1-13.
- le Lous M, Flandin F, Herbage D, et al. Influence of collagen denaturation on the chemorheological properties of skin, assessed by differential scanning calorimetry and hydrothermal isometric tension measurement. Biochim Biophys Acta. 1982;717:295-300.
- Ross EV, Yashar SS, Naseef GS, et al. A pilot study of in vivo immediate tissue contraction with CO2 skin laser resurfacing in a live farm pig. Dermatol Surg. 1999;25:851-856.
- Arnoczky SP, Aksan A. Thermal modification of connective tissues: basic science considerations and clinical implications. J Am Acad Orthop Surg. 2000;8:305-313.
- Hsu TS, Kaminer MS. The use of nonablative radiofrequency technology to tighten the lower face and neck. Semin Cutan Med Surg. 2003;22:115-123.
- Alster TS. Cutaneous resurfacing with CO2 and erbium: YAG lasers: preoperative, intraoperative, and postoperative considerations. Plast Reconstr Surg. 1999;103:619-632; discussion 633-634.
- Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014;23:49-60.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Bernstein LJ, Kauvar AN, Grossman MC, et al. The short‐ and long‐term side effects of carbon dioxide laser resurfacing. Dermatol Surg. 1997;23:519-525.
- Nanni CA, Alster TS. Complications of carbon dioxide laser resurfacing. an evaluation of 500 patients. Dermatol Surg. 1998;24:315-320.
- Ortiz AE, Tremaine AM, Zachary CB. Long‐term efficacy of a fractional resurfacing device. Lasers Surg Med. 2010;42:168-170.
- Rahman Z, MacFalls H, Jiang K, et al. Fractional deep dermal ablation induces tissue tightening. Lasers Surg Med. 2009;41:78-86.
- Graber EM, Tanzi EL, Alster TS. Side effects and complications of fractional laser photothermolysis: experience with 961 treatments. Dermatol Surg. 2008;34:301-305; discussion 305-307.
- Fisher GH, Geronemus RG. Short‐term side effects of fractional photothermolysis. Dermatol Surg. 2005;31:1245-1249.
- Ortiz AE, Goldman MP, Fitzpatrick RE. Ablative CO2 lasers for skin tightening: traditional versus fractional. Dermatol Surg. 2014;40(suppl 12):S147-S151.
- Geronemus RG. Fractional photothermolysis: current and future applications. Lasers Surg Med. 2006;38:169-176.
- Tierney EP, Hanke CW, Petersen J. Ablative fractionated CO2 laser treatment of photoaging: a clinical and histologic study. Dermatol Surg. 2012;38:1777-1789.
- Tierney EP, Hanke CW, Watkins L. Treatment of lower eyelid rhytids and laxity with ablative fractionated carbon-dioxide laser resurfacing: case series and review of the literature. J Am Acad Dermatol. 2011;64:730-740.
- Fife DJ, Fitzpatrick RE, Zachary CB. Complications of fractional CO2 laser resurfacing: four cases. Lasers Surg Med. 2009;41:179-184.
- Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatol Surg. 2010;36:299-306.
- Miller L, Mishra V, Alsaad S, et al. Clinical evaluation of a non-ablative 1940 nm fractional laser. J Drugs Dermatol. 2014;13:1324-1329.
- Alexiades-Armenakas M. Nonablative skin tightening with a variable depth heating 1310-nm wavelength laser in combination with surface cooling. J Drugs Dermatol. 2007;6:1096-1103.
- Alster TS, Wanitphakdeedecha R. Improvement of postfractional laser erythema with light‐emitting diode photomodulation. Dermatol Surg. 2009;35:813-815.
- Fournier N, Lagarde JM, Turlier V, et al. A 35-month profilometric and clinical evaluation of non-ablative remodeling using a 1540-nm Er:glass laser. J Cosmet Laser Ther. 2004;6:126-130.
- Hædersdal M, Moreau KER, Beyer DM, et al. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers Surg Med. 2009;41:189-195.
- Lupton JR, Williams CM, Alster TS. Nonablative laser skin resurfacing using a 1540 nm erbium glass laser: a clinical and histologic analysis. Dermatol Surg. 2002;28:833-835.
- Moody BR, McCarthy JE, Hruza GJ. Collagen remodeling after 585‐nm pulsed dye laser irradiation: an ultrasonographic analysis. Dermatol Surg. 2003;29:997-999, discussion 999-1000.
- Pollock H, Pollock TA. NLite laser: nonablative wrinkle reduction.Aesthet Surg J. 2001;21:371-372.
- Burns JA. Thermage: monopolar radiofrequency. Aesthet Surg J. 2005;25:638-642.
- Weiss RA, Weiss MA, Munavelli G, et al. Monopolar radiofrequency facial tightening: a retrospective analysis of efficacy and safety in over 600 treatments. J Drugs Dermatol. 2006;5:707-712.
- Sadick NS, Makino Y. Selective electro‐thermolysis in aesthetic medicine: a review. Lasers Surg Med. 2004;34:91-97.
- Alster TS, Lupton JR. Nonablative cutaneous remodeling using radiofrequency devices. Clin Dermatol. 2007;25:487-491.
- Fitzpatrick R, Geronemus R, Goldberg D, et al. Multicenter study of noninvasive radiofrequency for periorbital tissue tightening. Lasers Surg Med. 2003;33:232-242.
- Dover JS, Zelickson B, 14-Physician Multispecialty Consensus Panel. Results of a survey of 5,700 patient monopolar radiofrequency facial skin tightening treatments: assessment of a low‐energy multiple‐pass technique leading to a clinical end point algorithm. Dermatol Surg. 2007;33:900-907.
- de Oliveira TC, Rocha SF, Ramos DG, et al. Effects of multipolar radiofrequency and pulsed electromagnetic field treatment for face and neck rejuvenation [published online March 8, 2017]. Dermatol Res Pract. doi:10.1155/2017/4146391.
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Van Leenders GJ, Beerlage HP, Ruijter ET, et al. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391-394.
- Wulkan AJ, Fabi SG, Green JB. Microfocused ultrasound for facial photorejuvenation: a review. Facial Plast Surg. 2016;32:269-275.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 201332:18-25.
- Oni G, Hoxworth R, Teotia S, et al. Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet Surg J. 2014;34:1099-1110.
- Brobst RW, Ferguson M, Perkins SW. Noninvasive treatment of the neck. Facial Plast Surg North Am. 2014;22:191-202.
- Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg. 2012;38:754-759.
- Missel L. Prevention of potential adverse events associated with use of Ulthera device. Tech Bull. 2011;32:18-25.
- Bove T, Zawada T, Serup J, et al. High‐frequency (20‐MHz) high‐intensity focused ultrasound (HIFU) system for dermal intervention: preclinical evaluation in skin equivalents. Skin Res Technol. 2019;25:217-228.
- Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med. 2003;32:78-87.
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Faucz LL, Will SE, Rodrigues CJ, et al. Quantitative evaluation of collagen and elastic fibers after intense pulsed light treatment of mouse skin. Lasers Surg Med. 2018;50:644-650.
- Goldberg DJ, Cutler KB. Nonablative treatment of rhytids with intense pulsed light. Lasers Surg Med. 2000;26:196-200.
- Li Y-H, Wu Y, Chen JZ, et al. Application of a new intense pulsed light device in the treatment of photoaging skin in Asian patients. Dermatol Surg. 2008;34:1459-1464.
- Shin J-W, Lee D-H, Choi S-Y, et al. Objective and non‐invasive evaluation of photorejuvenation effect with intense pulsed light treatment in Asian skin. J Eur Acad Dermatol Venereol. 2011;25:516-522.
- Weiss RA, Weiss MA, Beasley KL. Rejuvenation of photoaged skin: 5 years results with intense pulsed light of the face, neck, and chest. Dermatol Surg. 2002;28:1115-1119.
- Lee KC, Wambier CG, Soon SL, et al. Basic chemical peeling: superficial and medium-depth peels. J Am Acad Dermatol. 2019;81:313-324.
- Brody HJ. Do chemical peels tighten the skin? Dermatol Surg. 2014;40(suppl):S129-S133.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Brody HJ. Variations and comparisons in medium‐depth chemical peeling. J Dermatol Surg Oncol. 1989;15:953-963.
- Oresajo C, Yatskayer M, Hansenne I. Clinical tolerance and efficacy of capryloyl salicylic acid peel compared to a glycolic acid peel in subjects with fine lines/wrinkles and hyperpigmented skin. J Cosmet Dermatol. 2008;7:259-262.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- Griffiths C. The role of retinoids in the prevention and repair of aged and photoaged skin. Clin Exp Dermatol. 2001;26:613-618.
- Darlenski R, Surber C, Fluhr J. Topical retinoids in the management of photodamaged skin: from theory to evidence‐based practical approach. Br J Dermatol. 2010;163:1157-1165.
- Kang S, Bergfeld W, Gottlieb AB, et al. Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial. Am J Clin Dermatol. 2005;6:245-253.
- Riahi RR, Bush AE, Cohen PR. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am J Clin Dermatol. 2016;17:265-276.
- American Society of Plastic Surgeons. Average surgeon/physician fees. https://www.plasticsurgery.org/documents/News/Statistics/2019/cosmetic-procedures-average-cost-2019.pdf. Accessed August 24, 2020.
- McCullough JL, Kelly KM. Prevention and treatment of skin aging. Ann NY Acad Sci. 2006;1067:323-331.
- Yaar M. Clinical and histological features of intrinsic versus extrinsic skin aging. In: Gilchrest BA, Krutmann J, eds. Skin Aging. Berlin, Germany: Springer, Heidelberg; 2006:9-21.
- Ramanadham SR, Costa CR, Narasimhan K, et al. Refining the anesthesia management of the face-lift patient: lessons learned from 1089 consecutive face lifts. Plast Reconstr Surg. 2015;135:723-730.
- Gupta V, Winocour J, Shi H, et al. Preoperative risk factors and complication rates in facelift: analysis of 11,300 patients. Aesthet Surg J. 2016;36:1-13.
- le Lous M, Flandin F, Herbage D, et al. Influence of collagen denaturation on the chemorheological properties of skin, assessed by differential scanning calorimetry and hydrothermal isometric tension measurement. Biochim Biophys Acta. 1982;717:295-300.
- Ross EV, Yashar SS, Naseef GS, et al. A pilot study of in vivo immediate tissue contraction with CO2 skin laser resurfacing in a live farm pig. Dermatol Surg. 1999;25:851-856.
- Arnoczky SP, Aksan A. Thermal modification of connective tissues: basic science considerations and clinical implications. J Am Acad Orthop Surg. 2000;8:305-313.
- Hsu TS, Kaminer MS. The use of nonablative radiofrequency technology to tighten the lower face and neck. Semin Cutan Med Surg. 2003;22:115-123.
- Alster TS. Cutaneous resurfacing with CO2 and erbium: YAG lasers: preoperative, intraoperative, and postoperative considerations. Plast Reconstr Surg. 1999;103:619-632; discussion 633-634.
- Omi T, Numano K. The role of the CO2 laser and fractional CO2 laser in dermatology. Laser Ther. 2014;23:49-60.
- Preissig J, Hamilton K, Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin Plast Surg. 2012;26:109-116.
- Bernstein LJ, Kauvar AN, Grossman MC, et al. The short‐ and long‐term side effects of carbon dioxide laser resurfacing. Dermatol Surg. 1997;23:519-525.
- Nanni CA, Alster TS. Complications of carbon dioxide laser resurfacing. an evaluation of 500 patients. Dermatol Surg. 1998;24:315-320.
- Ortiz AE, Tremaine AM, Zachary CB. Long‐term efficacy of a fractional resurfacing device. Lasers Surg Med. 2010;42:168-170.
- Rahman Z, MacFalls H, Jiang K, et al. Fractional deep dermal ablation induces tissue tightening. Lasers Surg Med. 2009;41:78-86.
- Graber EM, Tanzi EL, Alster TS. Side effects and complications of fractional laser photothermolysis: experience with 961 treatments. Dermatol Surg. 2008;34:301-305; discussion 305-307.
- Fisher GH, Geronemus RG. Short‐term side effects of fractional photothermolysis. Dermatol Surg. 2005;31:1245-1249.
- Ortiz AE, Goldman MP, Fitzpatrick RE. Ablative CO2 lasers for skin tightening: traditional versus fractional. Dermatol Surg. 2014;40(suppl 12):S147-S151.
- Geronemus RG. Fractional photothermolysis: current and future applications. Lasers Surg Med. 2006;38:169-176.
- Tierney EP, Hanke CW, Petersen J. Ablative fractionated CO2 laser treatment of photoaging: a clinical and histologic study. Dermatol Surg. 2012;38:1777-1789.
- Tierney EP, Hanke CW, Watkins L. Treatment of lower eyelid rhytids and laxity with ablative fractionated carbon-dioxide laser resurfacing: case series and review of the literature. J Am Acad Dermatol. 2011;64:730-740.
- Fife DJ, Fitzpatrick RE, Zachary CB. Complications of fractional CO2 laser resurfacing: four cases. Lasers Surg Med. 2009;41:179-184.
- Metelitsa AI, Alster TS. Fractionated laser skin resurfacing treatment complications: a review. Dermatol Surg. 2010;36:299-306.
- Miller L, Mishra V, Alsaad S, et al. Clinical evaluation of a non-ablative 1940 nm fractional laser. J Drugs Dermatol. 2014;13:1324-1329.
- Alexiades-Armenakas M. Nonablative skin tightening with a variable depth heating 1310-nm wavelength laser in combination with surface cooling. J Drugs Dermatol. 2007;6:1096-1103.
- Alster TS, Wanitphakdeedecha R. Improvement of postfractional laser erythema with light‐emitting diode photomodulation. Dermatol Surg. 2009;35:813-815.
- Fournier N, Lagarde JM, Turlier V, et al. A 35-month profilometric and clinical evaluation of non-ablative remodeling using a 1540-nm Er:glass laser. J Cosmet Laser Ther. 2004;6:126-130.
- Hædersdal M, Moreau KER, Beyer DM, et al. Fractional nonablative 1540 nm laser resurfacing for thermal burn scars: a randomized controlled trial. Lasers Surg Med. 2009;41:189-195.
- Lupton JR, Williams CM, Alster TS. Nonablative laser skin resurfacing using a 1540 nm erbium glass laser: a clinical and histologic analysis. Dermatol Surg. 2002;28:833-835.
- Moody BR, McCarthy JE, Hruza GJ. Collagen remodeling after 585‐nm pulsed dye laser irradiation: an ultrasonographic analysis. Dermatol Surg. 2003;29:997-999, discussion 999-1000.
- Pollock H, Pollock TA. NLite laser: nonablative wrinkle reduction.Aesthet Surg J. 2001;21:371-372.
- Burns JA. Thermage: monopolar radiofrequency. Aesthet Surg J. 2005;25:638-642.
- Weiss RA, Weiss MA, Munavelli G, et al. Monopolar radiofrequency facial tightening: a retrospective analysis of efficacy and safety in over 600 treatments. J Drugs Dermatol. 2006;5:707-712.
- Sadick NS, Makino Y. Selective electro‐thermolysis in aesthetic medicine: a review. Lasers Surg Med. 2004;34:91-97.
- Alster TS, Lupton JR. Nonablative cutaneous remodeling using radiofrequency devices. Clin Dermatol. 2007;25:487-491.
- Fitzpatrick R, Geronemus R, Goldberg D, et al. Multicenter study of noninvasive radiofrequency for periorbital tissue tightening. Lasers Surg Med. 2003;33:232-242.
- Dover JS, Zelickson B, 14-Physician Multispecialty Consensus Panel. Results of a survey of 5,700 patient monopolar radiofrequency facial skin tightening treatments: assessment of a low‐energy multiple‐pass technique leading to a clinical end point algorithm. Dermatol Surg. 2007;33:900-907.
- de Oliveira TC, Rocha SF, Ramos DG, et al. Effects of multipolar radiofrequency and pulsed electromagnetic field treatment for face and neck rejuvenation [published online March 8, 2017]. Dermatol Res Pract. doi:10.1155/2017/4146391.
- Fabi SG. Noninvasive skin tightening: focus on new ultrasound techniques. Clin Cosmet Investig Dermatol. 2015;8:47-52.
- Van Leenders GJ, Beerlage HP, Ruijter ET, et al. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391-394.
- Wulkan AJ, Fabi SG, Green JB. Microfocused ultrasound for facial photorejuvenation: a review. Facial Plast Surg. 2016;32:269-275.
- MacGregor JL, Tanzi EL. Microfocused ultrasound for skin tightening. Semin Cutan Med Surg. 201332:18-25.
- Oni G, Hoxworth R, Teotia S, et al. Evaluation of a microfocused ultrasound system for improving skin laxity and tightening in the lower face. Aesthet Surg J. 2014;34:1099-1110.
- Brobst RW, Ferguson M, Perkins SW. Noninvasive treatment of the neck. Facial Plast Surg North Am. 2014;22:191-202.
- Alster TS, Tanzi EL. Noninvasive lifting of arm, thigh, and knee skin with transcutaneous intense focused ultrasound. Dermatol Surg. 2012;38:754-759.
- Missel L. Prevention of potential adverse events associated with use of Ulthera device. Tech Bull. 2011;32:18-25.
- Bove T, Zawada T, Serup J, et al. High‐frequency (20‐MHz) high‐intensity focused ultrasound (HIFU) system for dermal intervention: preclinical evaluation in skin equivalents. Skin Res Technol. 2019;25:217-228.
- Raulin C, Greve B, Grema H. IPL technology: a review. Lasers Surg Med. 2003;32:78-87.
- Anderson RR, Parrish JA. Selective photothermolysis: precise microsurgery by selective absorption of pulsed radiation. Science. 1983;220:524-527.
- Faucz LL, Will SE, Rodrigues CJ, et al. Quantitative evaluation of collagen and elastic fibers after intense pulsed light treatment of mouse skin. Lasers Surg Med. 2018;50:644-650.
- Goldberg DJ, Cutler KB. Nonablative treatment of rhytids with intense pulsed light. Lasers Surg Med. 2000;26:196-200.
- Li Y-H, Wu Y, Chen JZ, et al. Application of a new intense pulsed light device in the treatment of photoaging skin in Asian patients. Dermatol Surg. 2008;34:1459-1464.
- Shin J-W, Lee D-H, Choi S-Y, et al. Objective and non‐invasive evaluation of photorejuvenation effect with intense pulsed light treatment in Asian skin. J Eur Acad Dermatol Venereol. 2011;25:516-522.
- Weiss RA, Weiss MA, Beasley KL. Rejuvenation of photoaged skin: 5 years results with intense pulsed light of the face, neck, and chest. Dermatol Surg. 2002;28:1115-1119.
- Lee KC, Wambier CG, Soon SL, et al. Basic chemical peeling: superficial and medium-depth peels. J Am Acad Dermatol. 2019;81:313-324.
- Brody HJ. Do chemical peels tighten the skin? Dermatol Surg. 2014;40(suppl):S129-S133.
- Landau M. Chemical peels. Clin Dermatol. 2008;26:200-208.
- Meaike JD, Agrawal N, Chang D, et al. Noninvasive facial rejuvenation. part 3: physician-directed-lasers, chemical peels, and other noninvasive modalities. Semin Plast Surg. 2016;30:143-150.
- Brody HJ. Variations and comparisons in medium‐depth chemical peeling. J Dermatol Surg Oncol. 1989;15:953-963.
- Oresajo C, Yatskayer M, Hansenne I. Clinical tolerance and efficacy of capryloyl salicylic acid peel compared to a glycolic acid peel in subjects with fine lines/wrinkles and hyperpigmented skin. J Cosmet Dermatol. 2008;7:259-262.
- Aust MC, Fernandes D, Kolokythas P, et al. Percutaneous collagen induction therapy: an alternative treatment for scars, wrinkles, and skin laxity. Plast Reconstr Surg. 2008;121:1421-1429.
- Griffiths C. The role of retinoids in the prevention and repair of aged and photoaged skin. Clin Exp Dermatol. 2001;26:613-618.
- Darlenski R, Surber C, Fluhr J. Topical retinoids in the management of photodamaged skin: from theory to evidence‐based practical approach. Br J Dermatol. 2010;163:1157-1165.
- Kang S, Bergfeld W, Gottlieb AB, et al. Long-term efficacy and safety of tretinoin emollient cream 0.05% in the treatment of photodamaged facial skin: a two-year, randomized, placebo-controlled trial. Am J Clin Dermatol. 2005;6:245-253.
- Riahi RR, Bush AE, Cohen PR. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am J Clin Dermatol. 2016;17:265-276.
- American Society of Plastic Surgeons. Average surgeon/physician fees. https://www.plasticsurgery.org/documents/News/Statistics/2019/cosmetic-procedures-average-cost-2019.pdf. Accessed August 24, 2020.
Practice Points
- There are a multitude of noninvasive modalities available to treat skin laxity.
- Understanding the mechanisms of each modality is crucial to selecting the appropriate treatment for your patients.
- Treatments should be tailored to the individual patient based on desired outcome, possible adverse events, patient preferences, and cost.
Filling Gaps: Moving Toward Better Treatment of Children With Atopic Dermatitis
It is a brand-new day for the treatment of children with severe inflammatory skin diseases. Not coincidentally, it also is a new day for the treatment of atopic dermatitis (AD). Why?
Historically, children have largely been ignored by pharmaceutical companies and the US Food and Drug Administration (FDA). Drug trials of new medications have been the exclusive province of adults; therefore, information they have generated has had only derivative relevance to the pediatric population. Pediatricians and providers who care for children, aware that they are not simply “little adults,” have been forced to extrapolate best practices.
My institution is poised to enroll a 3-year-old child with severe AD into a biologic trial (ClinicalTrials.gov identifier NCT03346434). The age range for this study is 6 months to 6 years. This extraordinary democratization of clinical trials is no accident. The Best Pharmaceuticals for Children Act, which was passed in 2002, was a first step. This legislation incentivized pharmaceutical companies to include children, who are notoriously more costly to study for myriad reasons, by extending patent protection for approved medications. Subsequent efforts spearheaded by advocacy groups such as the National Eczema Association included the production of guidance documents for industry1 and presentations directly to the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee meeting punctuated by powerful patient testimonials.2
Serendipitously, AD, a disease that presents by kindergarten in up to 90% of affected individuals, also has caught the eye of the pharmaceutical industry. Remarkable advances in the understanding of AD inflammation have led to an explosion of new therapeutic targets of interest. By way of context, between the introduction of topical calcineurin inhibitors in 2001 and the FDA approval of dupilumab and crisaborole in 2017, there were precisely zero new molecules approved for the treatment of AD. Viewed through another lens, prior to 2017, the only FDA-approved systemic medication for AD was prednisone, a drug most AD experts would list as the least appropriate choice for treatment of this condition.
Fast-forward to 2020 and we have a plethora of new possibilities. The National Eczema Association’s research web page
This confluence of better science, powerful advocacy, and enlightened self-interest has been revolutionary. It is most evident when parents/guardians—many of whom had long ago given up on new therapies for themselves—are gobsmacked by the new therapeutic landscape outlined for their children. Parents/guardians realize their children need not struggle as they may have themselves. The impact on quality of life has long been known, but several recent publications have brought it into finer relief. Drucker et al5 highlighted the overall burden of disease, and several subsequent papers have focused specifically on affective impacts including increased risk for depression, suicidal ideation, and suicide.6,7 In this issue of Cutis, Tracy et al8 provide an update on pediatric AD with an emphasis on comorbidities, quality of life, and evolving practices and therapies.
Better science, better drugs, better advocacy, better outcomes—it has not been a straight line, but it has indisputably been a forward-marching one. It is a new day, indeed.
- Siegfried EC, Jaworski JC, Eichenfield LF, et al. Developing drugs for the treatment of atopic dermatitis in children (≥3 months to <18 years of age): draft guidance for industry [published online March 30, 2018]. Pediatr Dermatol. 2018; May 35:303-322.
- Pediatric trials for AD systemic treatments. Dermatology Times. May 21, 2015. https://www.dermatologytimes.com/view/pediatric-trials-ad-systemic-treatments. Accessed August 11, 2020.
- Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized controlled trial. JAMA Pediatr. 2019;174:29-37.
- Drucker AM, Wang AR, Li W-Q, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association [published online September 8, 2016]. J Invest Dermatol. 2017;137:26-30.
- Sandhu JK, Wu KK, Bui T-L, et al. Association between atopic dermatitis and suicidality: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:178-187.
- Patel KR, Immaneni S, Singam V, et al. Association between atopic dermatitis, depression, and suicidal ideation: a systematic review and meta-analysis [published online October 23, 2018]. J Am Acad Dermatol. 2019;80:402-410.
- Tracy A, Bhatti S, Eichenfield LF. Update on pediatric atopic dermatitis. Cutis. 2020;106:143-146.
It is a brand-new day for the treatment of children with severe inflammatory skin diseases. Not coincidentally, it also is a new day for the treatment of atopic dermatitis (AD). Why?
Historically, children have largely been ignored by pharmaceutical companies and the US Food and Drug Administration (FDA). Drug trials of new medications have been the exclusive province of adults; therefore, information they have generated has had only derivative relevance to the pediatric population. Pediatricians and providers who care for children, aware that they are not simply “little adults,” have been forced to extrapolate best practices.
My institution is poised to enroll a 3-year-old child with severe AD into a biologic trial (ClinicalTrials.gov identifier NCT03346434). The age range for this study is 6 months to 6 years. This extraordinary democratization of clinical trials is no accident. The Best Pharmaceuticals for Children Act, which was passed in 2002, was a first step. This legislation incentivized pharmaceutical companies to include children, who are notoriously more costly to study for myriad reasons, by extending patent protection for approved medications. Subsequent efforts spearheaded by advocacy groups such as the National Eczema Association included the production of guidance documents for industry1 and presentations directly to the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee meeting punctuated by powerful patient testimonials.2
Serendipitously, AD, a disease that presents by kindergarten in up to 90% of affected individuals, also has caught the eye of the pharmaceutical industry. Remarkable advances in the understanding of AD inflammation have led to an explosion of new therapeutic targets of interest. By way of context, between the introduction of topical calcineurin inhibitors in 2001 and the FDA approval of dupilumab and crisaborole in 2017, there were precisely zero new molecules approved for the treatment of AD. Viewed through another lens, prior to 2017, the only FDA-approved systemic medication for AD was prednisone, a drug most AD experts would list as the least appropriate choice for treatment of this condition.
Fast-forward to 2020 and we have a plethora of new possibilities. The National Eczema Association’s research web page
This confluence of better science, powerful advocacy, and enlightened self-interest has been revolutionary. It is most evident when parents/guardians—many of whom had long ago given up on new therapies for themselves—are gobsmacked by the new therapeutic landscape outlined for their children. Parents/guardians realize their children need not struggle as they may have themselves. The impact on quality of life has long been known, but several recent publications have brought it into finer relief. Drucker et al5 highlighted the overall burden of disease, and several subsequent papers have focused specifically on affective impacts including increased risk for depression, suicidal ideation, and suicide.6,7 In this issue of Cutis, Tracy et al8 provide an update on pediatric AD with an emphasis on comorbidities, quality of life, and evolving practices and therapies.
Better science, better drugs, better advocacy, better outcomes—it has not been a straight line, but it has indisputably been a forward-marching one. It is a new day, indeed.
It is a brand-new day for the treatment of children with severe inflammatory skin diseases. Not coincidentally, it also is a new day for the treatment of atopic dermatitis (AD). Why?
Historically, children have largely been ignored by pharmaceutical companies and the US Food and Drug Administration (FDA). Drug trials of new medications have been the exclusive province of adults; therefore, information they have generated has had only derivative relevance to the pediatric population. Pediatricians and providers who care for children, aware that they are not simply “little adults,” have been forced to extrapolate best practices.
My institution is poised to enroll a 3-year-old child with severe AD into a biologic trial (ClinicalTrials.gov identifier NCT03346434). The age range for this study is 6 months to 6 years. This extraordinary democratization of clinical trials is no accident. The Best Pharmaceuticals for Children Act, which was passed in 2002, was a first step. This legislation incentivized pharmaceutical companies to include children, who are notoriously more costly to study for myriad reasons, by extending patent protection for approved medications. Subsequent efforts spearheaded by advocacy groups such as the National Eczema Association included the production of guidance documents for industry1 and presentations directly to the FDA’s Dermatologic and Ophthalmic Drugs Advisory Committee meeting punctuated by powerful patient testimonials.2
Serendipitously, AD, a disease that presents by kindergarten in up to 90% of affected individuals, also has caught the eye of the pharmaceutical industry. Remarkable advances in the understanding of AD inflammation have led to an explosion of new therapeutic targets of interest. By way of context, between the introduction of topical calcineurin inhibitors in 2001 and the FDA approval of dupilumab and crisaborole in 2017, there were precisely zero new molecules approved for the treatment of AD. Viewed through another lens, prior to 2017, the only FDA-approved systemic medication for AD was prednisone, a drug most AD experts would list as the least appropriate choice for treatment of this condition.
Fast-forward to 2020 and we have a plethora of new possibilities. The National Eczema Association’s research web page
This confluence of better science, powerful advocacy, and enlightened self-interest has been revolutionary. It is most evident when parents/guardians—many of whom had long ago given up on new therapies for themselves—are gobsmacked by the new therapeutic landscape outlined for their children. Parents/guardians realize their children need not struggle as they may have themselves. The impact on quality of life has long been known, but several recent publications have brought it into finer relief. Drucker et al5 highlighted the overall burden of disease, and several subsequent papers have focused specifically on affective impacts including increased risk for depression, suicidal ideation, and suicide.6,7 In this issue of Cutis, Tracy et al8 provide an update on pediatric AD with an emphasis on comorbidities, quality of life, and evolving practices and therapies.
Better science, better drugs, better advocacy, better outcomes—it has not been a straight line, but it has indisputably been a forward-marching one. It is a new day, indeed.
- Siegfried EC, Jaworski JC, Eichenfield LF, et al. Developing drugs for the treatment of atopic dermatitis in children (≥3 months to <18 years of age): draft guidance for industry [published online March 30, 2018]. Pediatr Dermatol. 2018; May 35:303-322.
- Pediatric trials for AD systemic treatments. Dermatology Times. May 21, 2015. https://www.dermatologytimes.com/view/pediatric-trials-ad-systemic-treatments. Accessed August 11, 2020.
- Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized controlled trial. JAMA Pediatr. 2019;174:29-37.
- Drucker AM, Wang AR, Li W-Q, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association [published online September 8, 2016]. J Invest Dermatol. 2017;137:26-30.
- Sandhu JK, Wu KK, Bui T-L, et al. Association between atopic dermatitis and suicidality: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:178-187.
- Patel KR, Immaneni S, Singam V, et al. Association between atopic dermatitis, depression, and suicidal ideation: a systematic review and meta-analysis [published online October 23, 2018]. J Am Acad Dermatol. 2019;80:402-410.
- Tracy A, Bhatti S, Eichenfield LF. Update on pediatric atopic dermatitis. Cutis. 2020;106:143-146.
- Siegfried EC, Jaworski JC, Eichenfield LF, et al. Developing drugs for the treatment of atopic dermatitis in children (≥3 months to <18 years of age): draft guidance for industry [published online March 30, 2018]. Pediatr Dermatol. 2018; May 35:303-322.
- Pediatric trials for AD systemic treatments. Dermatology Times. May 21, 2015. https://www.dermatologytimes.com/view/pediatric-trials-ad-systemic-treatments. Accessed August 11, 2020.
- Solimani F, Meier K, Ghoreschi K. Emerging topical and systemic JAK inhibitors in dermatology. Front Immunol. 2019;10:2847.
- Chan S, Cornelius V, Cro S, et al. Treatment effect of omalizumab on severe pediatric atopic dermatitis: the ADAPT randomized controlled trial. JAMA Pediatr. 2019;174:29-37.
- Drucker AM, Wang AR, Li W-Q, et al. The burden of atopic dermatitis: summary of a report for the National Eczema Association [published online September 8, 2016]. J Invest Dermatol. 2017;137:26-30.
- Sandhu JK, Wu KK, Bui T-L, et al. Association between atopic dermatitis and suicidality: a systematic review and meta-analysis. JAMA Dermatol. 2019;155:178-187.
- Patel KR, Immaneni S, Singam V, et al. Association between atopic dermatitis, depression, and suicidal ideation: a systematic review and meta-analysis [published online October 23, 2018]. J Am Acad Dermatol. 2019;80:402-410.
- Tracy A, Bhatti S, Eichenfield LF. Update on pediatric atopic dermatitis. Cutis. 2020;106:143-146.