User login
PHM20 Virtual: Impact of racism in medicine
Presenters
Michael Bryant, MD – Children’s Hospital of Los Angeles
Kimberly Manning, MD – Emory University, Atlanta
Kimberly Reynolds, MD – University of Miami
Samir Shah, MD, MSCE, MHM – Cincinnati Children’s Hospital
Ndidi Unaka, MD, MEd – Cincinnati Children’s Hospital
Moderator
Erin Shaughnessy, MD – Phoenix Children’s Hospital
Session summary
This session was devoted to a discussion about how pediatric hospital medicine (PHM) as a field can address racism in medicine. The structural inequity rooted in poverty, housing problems, and differential education represents the essential social determinant of health. No longer can pediatric hospitalists neglect or be in denial of the crucial role that race plays in propagating further inequalities in our society and at our workplace. Historically Black people were exploited in research and still are disproportionately affected when it comes to infant prematurity and mortality, asthma, pain treatments, and so on. The pediatric hospitalist must explore and understand the reasons behind nonadherence and noncompliance among Black patients and always seek to understand before criticizing.
Within learning environments, we must improve how to “autocorrect” and proactively work on our own biases. Dr. Bryant pointed out that each institution has the responsibility to build on the civil rights movement and seize the moment to create a robust response to the inequities manifested during the COVID-19 epidemic, as well as the events following the deaths of George Floyd, Breonna Taylor, Ahmoud Arbery, and many others. Dr. Shah called on the PHM community to take on that obligation by “stepping into the tension,” as Mark Shapiro, MD, has suggested in a conversation/podcast with Dr. Unaka.
As pediatric hospitalists, we will have to show up both individually and as constituents of institutions to address racism by specific projects looking at all data relevant for racism rather than race in quality and safety – thereby amplifying the voices of our Black patients and families, remarked Dr. Unaka. There was a brief reflection on the use of the word “allies” by Dr. Manning and Dr. Reynolds to remind the more than 200 session participants that a bidirectional framework of this process is crucial and that there is a clear need for a partnership to a common goal that should start by “a laydown of privilege of those who have it” to establish equal playing fields once and for all.
Dr. Bryant encouraged a deliberate and early thoughtful process to identify those with opportunities and help young Black people explore journeys in medicine and increase diversity among PHM faculty. Dr. Manning reminded the audience of the power that relationships have and hold in our lives, and not only those of mentors and mentees, but also relationships among all of us as humans. As with those simple situations in which we mess up and have to be able to admit it, apologize for it, and learn to move on, this requires also showing up as a mentee, articulating one’s needs, and learning to break the habits rooted in biases. Dr. Unaka warned against stereotypes and reminded us to look deeper and understand better all of our learners and their blind spots, as well as our own.
Key takeaways
- The field of PHM must recognize the role that race plays in propagating inequalities.
- Learning and mentorship environments have to be assessed for the safety of all learners and adjusted to correct (and autocorrect) as many biases as possible.
- Institutions must assume responsibilities to establish a conscious, robust response to injustice and racism in a timely and specific manner.
- Further research efforts must be made to address racism, rather than race.
- The PHM community must show up to create a new, healthy, and deliberate bidirectional framework to endorse and support diversity.
Dr. Giordano is assistant professor of pediatrics at Columbia University and a pediatric hospitalist at NewYork–Presbyterian Morgan Stanley Children’s Hospital, both in New York, with an interest in surgical comanagement. She serves on the Society of Hospital Medicine’s Pediatric Special Interest Group Executive Committee and is the chair of the Education Subcommittee. She is also an advisory board member for the New York/Westchester SHM Chapter.
Presenters
Michael Bryant, MD – Children’s Hospital of Los Angeles
Kimberly Manning, MD – Emory University, Atlanta
Kimberly Reynolds, MD – University of Miami
Samir Shah, MD, MSCE, MHM – Cincinnati Children’s Hospital
Ndidi Unaka, MD, MEd – Cincinnati Children’s Hospital
Moderator
Erin Shaughnessy, MD – Phoenix Children’s Hospital
Session summary
This session was devoted to a discussion about how pediatric hospital medicine (PHM) as a field can address racism in medicine. The structural inequity rooted in poverty, housing problems, and differential education represents the essential social determinant of health. No longer can pediatric hospitalists neglect or be in denial of the crucial role that race plays in propagating further inequalities in our society and at our workplace. Historically Black people were exploited in research and still are disproportionately affected when it comes to infant prematurity and mortality, asthma, pain treatments, and so on. The pediatric hospitalist must explore and understand the reasons behind nonadherence and noncompliance among Black patients and always seek to understand before criticizing.
Within learning environments, we must improve how to “autocorrect” and proactively work on our own biases. Dr. Bryant pointed out that each institution has the responsibility to build on the civil rights movement and seize the moment to create a robust response to the inequities manifested during the COVID-19 epidemic, as well as the events following the deaths of George Floyd, Breonna Taylor, Ahmoud Arbery, and many others. Dr. Shah called on the PHM community to take on that obligation by “stepping into the tension,” as Mark Shapiro, MD, has suggested in a conversation/podcast with Dr. Unaka.
As pediatric hospitalists, we will have to show up both individually and as constituents of institutions to address racism by specific projects looking at all data relevant for racism rather than race in quality and safety – thereby amplifying the voices of our Black patients and families, remarked Dr. Unaka. There was a brief reflection on the use of the word “allies” by Dr. Manning and Dr. Reynolds to remind the more than 200 session participants that a bidirectional framework of this process is crucial and that there is a clear need for a partnership to a common goal that should start by “a laydown of privilege of those who have it” to establish equal playing fields once and for all.
Dr. Bryant encouraged a deliberate and early thoughtful process to identify those with opportunities and help young Black people explore journeys in medicine and increase diversity among PHM faculty. Dr. Manning reminded the audience of the power that relationships have and hold in our lives, and not only those of mentors and mentees, but also relationships among all of us as humans. As with those simple situations in which we mess up and have to be able to admit it, apologize for it, and learn to move on, this requires also showing up as a mentee, articulating one’s needs, and learning to break the habits rooted in biases. Dr. Unaka warned against stereotypes and reminded us to look deeper and understand better all of our learners and their blind spots, as well as our own.
Key takeaways
- The field of PHM must recognize the role that race plays in propagating inequalities.
- Learning and mentorship environments have to be assessed for the safety of all learners and adjusted to correct (and autocorrect) as many biases as possible.
- Institutions must assume responsibilities to establish a conscious, robust response to injustice and racism in a timely and specific manner.
- Further research efforts must be made to address racism, rather than race.
- The PHM community must show up to create a new, healthy, and deliberate bidirectional framework to endorse and support diversity.
Dr. Giordano is assistant professor of pediatrics at Columbia University and a pediatric hospitalist at NewYork–Presbyterian Morgan Stanley Children’s Hospital, both in New York, with an interest in surgical comanagement. She serves on the Society of Hospital Medicine’s Pediatric Special Interest Group Executive Committee and is the chair of the Education Subcommittee. She is also an advisory board member for the New York/Westchester SHM Chapter.
Presenters
Michael Bryant, MD – Children’s Hospital of Los Angeles
Kimberly Manning, MD – Emory University, Atlanta
Kimberly Reynolds, MD – University of Miami
Samir Shah, MD, MSCE, MHM – Cincinnati Children’s Hospital
Ndidi Unaka, MD, MEd – Cincinnati Children’s Hospital
Moderator
Erin Shaughnessy, MD – Phoenix Children’s Hospital
Session summary
This session was devoted to a discussion about how pediatric hospital medicine (PHM) as a field can address racism in medicine. The structural inequity rooted in poverty, housing problems, and differential education represents the essential social determinant of health. No longer can pediatric hospitalists neglect or be in denial of the crucial role that race plays in propagating further inequalities in our society and at our workplace. Historically Black people were exploited in research and still are disproportionately affected when it comes to infant prematurity and mortality, asthma, pain treatments, and so on. The pediatric hospitalist must explore and understand the reasons behind nonadherence and noncompliance among Black patients and always seek to understand before criticizing.
Within learning environments, we must improve how to “autocorrect” and proactively work on our own biases. Dr. Bryant pointed out that each institution has the responsibility to build on the civil rights movement and seize the moment to create a robust response to the inequities manifested during the COVID-19 epidemic, as well as the events following the deaths of George Floyd, Breonna Taylor, Ahmoud Arbery, and many others. Dr. Shah called on the PHM community to take on that obligation by “stepping into the tension,” as Mark Shapiro, MD, has suggested in a conversation/podcast with Dr. Unaka.
As pediatric hospitalists, we will have to show up both individually and as constituents of institutions to address racism by specific projects looking at all data relevant for racism rather than race in quality and safety – thereby amplifying the voices of our Black patients and families, remarked Dr. Unaka. There was a brief reflection on the use of the word “allies” by Dr. Manning and Dr. Reynolds to remind the more than 200 session participants that a bidirectional framework of this process is crucial and that there is a clear need for a partnership to a common goal that should start by “a laydown of privilege of those who have it” to establish equal playing fields once and for all.
Dr. Bryant encouraged a deliberate and early thoughtful process to identify those with opportunities and help young Black people explore journeys in medicine and increase diversity among PHM faculty. Dr. Manning reminded the audience of the power that relationships have and hold in our lives, and not only those of mentors and mentees, but also relationships among all of us as humans. As with those simple situations in which we mess up and have to be able to admit it, apologize for it, and learn to move on, this requires also showing up as a mentee, articulating one’s needs, and learning to break the habits rooted in biases. Dr. Unaka warned against stereotypes and reminded us to look deeper and understand better all of our learners and their blind spots, as well as our own.
Key takeaways
- The field of PHM must recognize the role that race plays in propagating inequalities.
- Learning and mentorship environments have to be assessed for the safety of all learners and adjusted to correct (and autocorrect) as many biases as possible.
- Institutions must assume responsibilities to establish a conscious, robust response to injustice and racism in a timely and specific manner.
- Further research efforts must be made to address racism, rather than race.
- The PHM community must show up to create a new, healthy, and deliberate bidirectional framework to endorse and support diversity.
Dr. Giordano is assistant professor of pediatrics at Columbia University and a pediatric hospitalist at NewYork–Presbyterian Morgan Stanley Children’s Hospital, both in New York, with an interest in surgical comanagement. She serves on the Society of Hospital Medicine’s Pediatric Special Interest Group Executive Committee and is the chair of the Education Subcommittee. She is also an advisory board member for the New York/Westchester SHM Chapter.
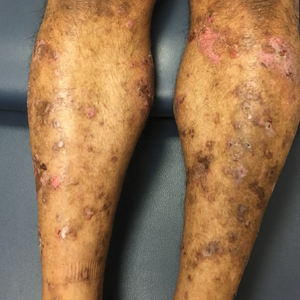
Bullae and Hyperpigmented Patches on the Legs
The Diagnosis: Lichen Planus Pemphigoides
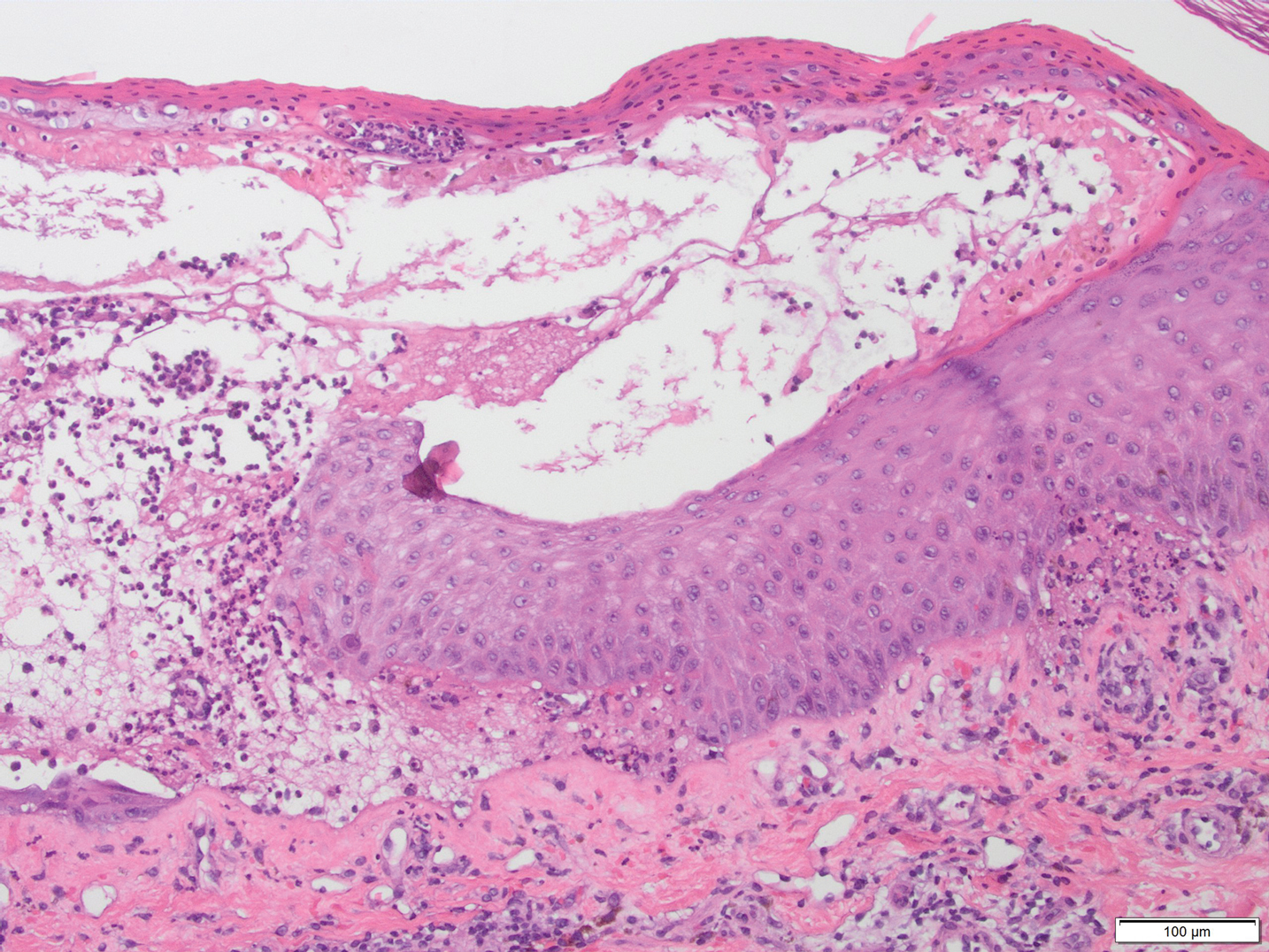
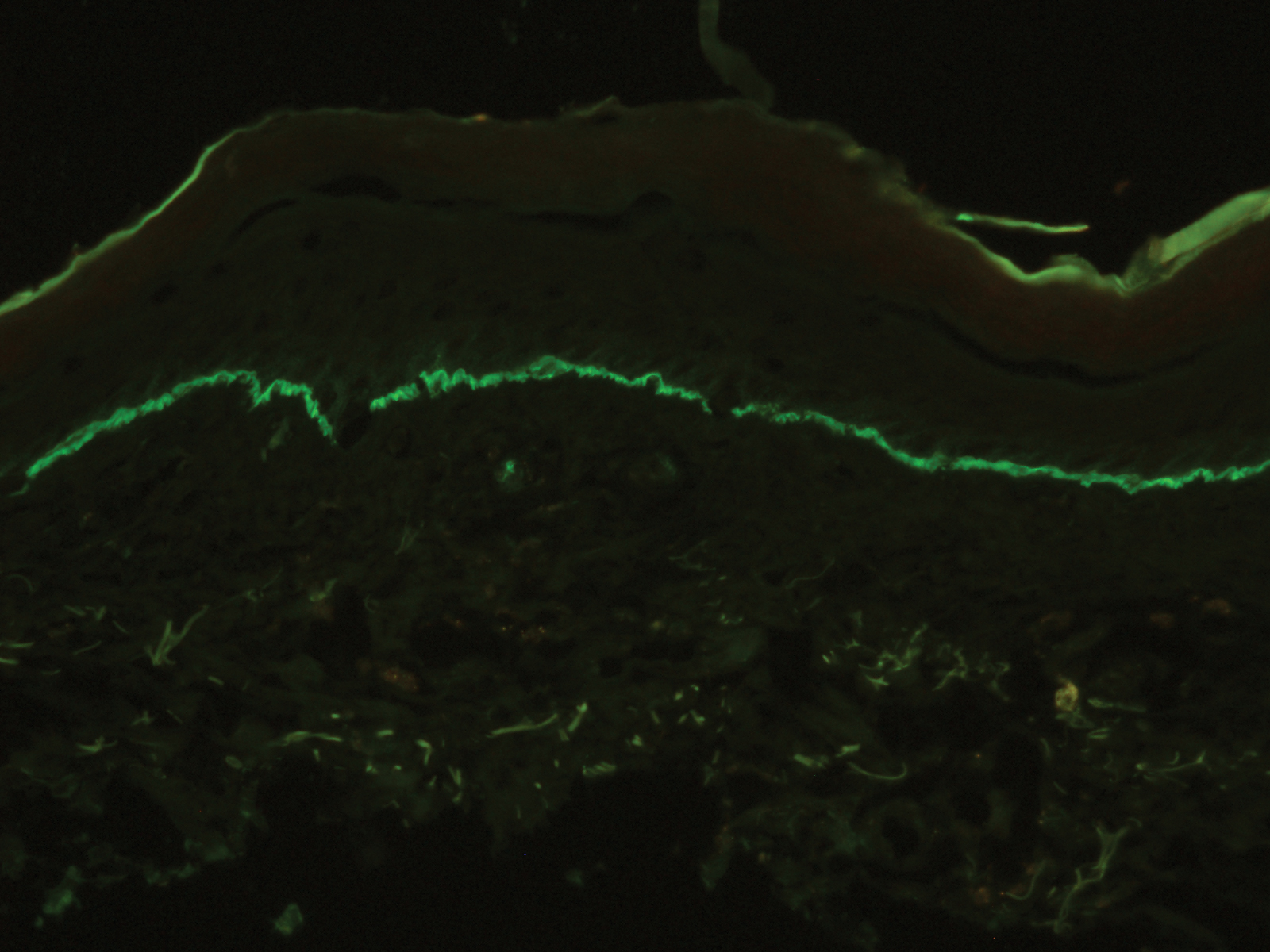
A skin biopsy from the right thigh demonstrated subepidermal blisters containing neutrophils (Figure 1). Direct immunofluorescence revealed linear basement membrane zone staining with C3 and trace staining with IgG (Figure 2), supporting a diagnosis of lichen planus pemphigoides (LPP). Oral prednisone—starting at 60 mg daily and tapered to 40 mg for a week, 20 mg for a week, then 10 mg for a month—along with triamcinolone ointment 0.1% to affected areas led to improvement. Hydrochlorothiazide and UV light therapy were discontinued. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily prescribed as adjunctive therapy also led to improvement. The patient achieved remission with doxycycline and was doing well without prednisone; however, he experienced a flare of his disease about 6 months later and was started on mycophenolate mofetil 1 g twice daily after clearance from his gastroenterologist, given his history of hepatitis B. He has been doing well since starting mycophenolate mofetil.
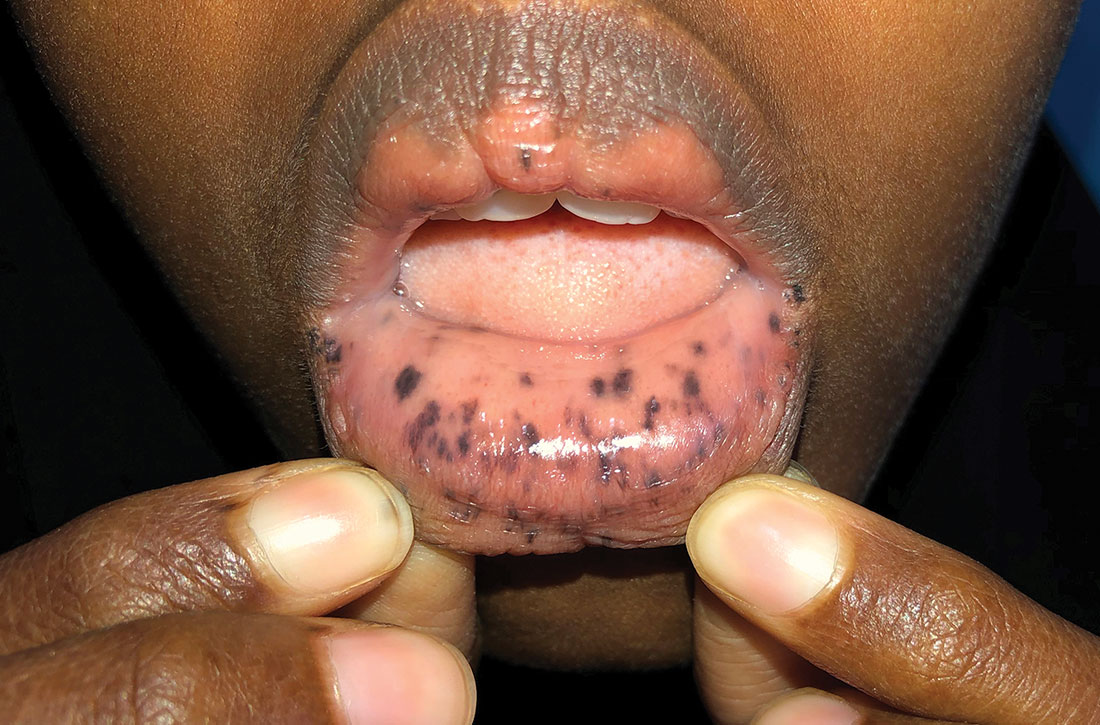
Lichen planus pemphigoides is a rare autoimmune bullous dermatosis with features of both lichen planus and bullous pemphigoid.1 Violaceous papules and tense bullae may be superimposed or arise independently. The chest, abdomen, back, and upper and lower extremities typically are involved.2 Oral mucosal involvement with white reticular streaks or erosions and nail involvement have been reported.2 Histopathologic and immunologic findings establish the diagnosis. Lichen planus pemphigoides is associated with subepidermal bullae and linear deposits of IgG and C3 on the basement membrane zone.1 Autoantibodies to bullous pemphigoid (BP) antigens BP180 and BP230 are associated with LPP.3 The pathogenesis of LPP remains unclear, but there are associations with chronic diseases, medications, and certain therapies.1,4-6 Several case reports have linked LPP to chronic viral hepatitis infections, as well as malignant tumors of the skin, mucosa, and gastrointestinal tract.2 Lichen planus pemphigoides has been reported in a patient on entecavir for hepatitis B as well as in a patient treated for hepatitis C with interferon and ribavirin.1 Lichen planus pemphigoides has been described in patients treated with the angiotensin-converting enzyme inhibitors enalapril, captopril, and ramipril.4,5,7 UV phototherapy also has been associated with the development of LPP.6 Hydrochlorothiazide previously has been reported as a cause of drug-induced lichen planus.8 A PubMed search of articles indexed for MEDLINE using the terms lichen planus pemphigoides and hydrochlorothiazide revealed no reports of hydrochlorothiazide-induced LPP.
Lichen planus pemphigoides demonstrates overlap with other blistering dermatoses, such as BP, bullous lupus erythematosus, and bullous lichen planus. Although histologically and immunologically similar to BP, LPP can be differentiated clinically by the presence of violaceous papules or plaques typical of lichen planus.9 Bullous pemphigoid is more common in individuals older than 70 years, whereas LPP tends to occur in middle-aged adults.2 Bullous lupus erythematosus usually is associated with manifestations of systemic lupus erythematosus and autoantibodies to collagen type VII.10 Salt-split skin studies demonstrate immunofluorescence on the dermal side of the split. Individuals affected by bullous lupus erythematosus typically have a history of photosensitivity.10 Blisters in LPP may form de novo from unaffected skin, whereas the bullae in bullous lichen planus are limited to existing lichenoid papules.9 The autoantibodies typical of LPP are absent in bullous lichen planus. Lichen planus actinicus is a variant of lichen planus that presents with annular, dyschromic, or violaceous plaques in a photodistributed pattern without bullous lesions.9
Lichen planus pemphigoides most commonly is treated with systemic corticosteroids. Topical steroids, dapsone, erythromycin, tetracycline and nicotinamide, azathioprine, and mycophenolate mofetil have been reported as adjuncts to systemic steroid therapy.2,11 Most reports describe treatment success with resolution of blistering lesions.
- Jang SH, Yun SJ, Lee SC, et al. Lichen planus pemphigoides associated with chronic hepatitis B virus infection. Clin Exp Dermatol. 2015;40:868-871.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol. 2017;9:217-224.
- Ben Salem C, Chengeul L, Ghariani N, et al. Captopril-induced lichen planus pemphigoides. Pharmacoepidemiol Drug Saf. 2008;17:722-724.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Zhu YI, Fitzpatrick JE, Kornfield BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Sin B, Miller M, Chew E. Hydrochlorothiazide induced lichen planus in the emergency department. J Pharm Pract. 2017;30:266-269.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Women Dermatol. 2015;1:140-149.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Fivenson DP, Kimbrough TL. Lichen planus pemphigoides: combination therapy with tetracycline and nicotinamide. J Am Acad Dermatol. 1997;36:638-640.
The Diagnosis: Lichen Planus Pemphigoides
A skin biopsy from the right thigh demonstrated subepidermal blisters containing neutrophils (Figure 1). Direct immunofluorescence revealed linear basement membrane zone staining with C3 and trace staining with IgG (Figure 2), supporting a diagnosis of lichen planus pemphigoides (LPP). Oral prednisone—starting at 60 mg daily and tapered to 40 mg for a week, 20 mg for a week, then 10 mg for a month—along with triamcinolone ointment 0.1% to affected areas led to improvement. Hydrochlorothiazide and UV light therapy were discontinued. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily prescribed as adjunctive therapy also led to improvement. The patient achieved remission with doxycycline and was doing well without prednisone; however, he experienced a flare of his disease about 6 months later and was started on mycophenolate mofetil 1 g twice daily after clearance from his gastroenterologist, given his history of hepatitis B. He has been doing well since starting mycophenolate mofetil.

Lichen planus pemphigoides is a rare autoimmune bullous dermatosis with features of both lichen planus and bullous pemphigoid.1 Violaceous papules and tense bullae may be superimposed or arise independently. The chest, abdomen, back, and upper and lower extremities typically are involved.2 Oral mucosal involvement with white reticular streaks or erosions and nail involvement have been reported.2 Histopathologic and immunologic findings establish the diagnosis. Lichen planus pemphigoides is associated with subepidermal bullae and linear deposits of IgG and C3 on the basement membrane zone.1 Autoantibodies to bullous pemphigoid (BP) antigens BP180 and BP230 are associated with LPP.3 The pathogenesis of LPP remains unclear, but there are associations with chronic diseases, medications, and certain therapies.1,4-6 Several case reports have linked LPP to chronic viral hepatitis infections, as well as malignant tumors of the skin, mucosa, and gastrointestinal tract.2 Lichen planus pemphigoides has been reported in a patient on entecavir for hepatitis B as well as in a patient treated for hepatitis C with interferon and ribavirin.1 Lichen planus pemphigoides has been described in patients treated with the angiotensin-converting enzyme inhibitors enalapril, captopril, and ramipril.4,5,7 UV phototherapy also has been associated with the development of LPP.6 Hydrochlorothiazide previously has been reported as a cause of drug-induced lichen planus.8 A PubMed search of articles indexed for MEDLINE using the terms lichen planus pemphigoides and hydrochlorothiazide revealed no reports of hydrochlorothiazide-induced LPP.

Lichen planus pemphigoides demonstrates overlap with other blistering dermatoses, such as BP, bullous lupus erythematosus, and bullous lichen planus. Although histologically and immunologically similar to BP, LPP can be differentiated clinically by the presence of violaceous papules or plaques typical of lichen planus.9 Bullous pemphigoid is more common in individuals older than 70 years, whereas LPP tends to occur in middle-aged adults.2 Bullous lupus erythematosus usually is associated with manifestations of systemic lupus erythematosus and autoantibodies to collagen type VII.10 Salt-split skin studies demonstrate immunofluorescence on the dermal side of the split. Individuals affected by bullous lupus erythematosus typically have a history of photosensitivity.10 Blisters in LPP may form de novo from unaffected skin, whereas the bullae in bullous lichen planus are limited to existing lichenoid papules.9 The autoantibodies typical of LPP are absent in bullous lichen planus. Lichen planus actinicus is a variant of lichen planus that presents with annular, dyschromic, or violaceous plaques in a photodistributed pattern without bullous lesions.9
Lichen planus pemphigoides most commonly is treated with systemic corticosteroids. Topical steroids, dapsone, erythromycin, tetracycline and nicotinamide, azathioprine, and mycophenolate mofetil have been reported as adjuncts to systemic steroid therapy.2,11 Most reports describe treatment success with resolution of blistering lesions.
The Diagnosis: Lichen Planus Pemphigoides
A skin biopsy from the right thigh demonstrated subepidermal blisters containing neutrophils (Figure 1). Direct immunofluorescence revealed linear basement membrane zone staining with C3 and trace staining with IgG (Figure 2), supporting a diagnosis of lichen planus pemphigoides (LPP). Oral prednisone—starting at 60 mg daily and tapered to 40 mg for a week, 20 mg for a week, then 10 mg for a month—along with triamcinolone ointment 0.1% to affected areas led to improvement. Hydrochlorothiazide and UV light therapy were discontinued. Doxycycline 100 mg twice daily and nicotinamide 500 mg twice daily prescribed as adjunctive therapy also led to improvement. The patient achieved remission with doxycycline and was doing well without prednisone; however, he experienced a flare of his disease about 6 months later and was started on mycophenolate mofetil 1 g twice daily after clearance from his gastroenterologist, given his history of hepatitis B. He has been doing well since starting mycophenolate mofetil.

Lichen planus pemphigoides is a rare autoimmune bullous dermatosis with features of both lichen planus and bullous pemphigoid.1 Violaceous papules and tense bullae may be superimposed or arise independently. The chest, abdomen, back, and upper and lower extremities typically are involved.2 Oral mucosal involvement with white reticular streaks or erosions and nail involvement have been reported.2 Histopathologic and immunologic findings establish the diagnosis. Lichen planus pemphigoides is associated with subepidermal bullae and linear deposits of IgG and C3 on the basement membrane zone.1 Autoantibodies to bullous pemphigoid (BP) antigens BP180 and BP230 are associated with LPP.3 The pathogenesis of LPP remains unclear, but there are associations with chronic diseases, medications, and certain therapies.1,4-6 Several case reports have linked LPP to chronic viral hepatitis infections, as well as malignant tumors of the skin, mucosa, and gastrointestinal tract.2 Lichen planus pemphigoides has been reported in a patient on entecavir for hepatitis B as well as in a patient treated for hepatitis C with interferon and ribavirin.1 Lichen planus pemphigoides has been described in patients treated with the angiotensin-converting enzyme inhibitors enalapril, captopril, and ramipril.4,5,7 UV phototherapy also has been associated with the development of LPP.6 Hydrochlorothiazide previously has been reported as a cause of drug-induced lichen planus.8 A PubMed search of articles indexed for MEDLINE using the terms lichen planus pemphigoides and hydrochlorothiazide revealed no reports of hydrochlorothiazide-induced LPP.

Lichen planus pemphigoides demonstrates overlap with other blistering dermatoses, such as BP, bullous lupus erythematosus, and bullous lichen planus. Although histologically and immunologically similar to BP, LPP can be differentiated clinically by the presence of violaceous papules or plaques typical of lichen planus.9 Bullous pemphigoid is more common in individuals older than 70 years, whereas LPP tends to occur in middle-aged adults.2 Bullous lupus erythematosus usually is associated with manifestations of systemic lupus erythematosus and autoantibodies to collagen type VII.10 Salt-split skin studies demonstrate immunofluorescence on the dermal side of the split. Individuals affected by bullous lupus erythematosus typically have a history of photosensitivity.10 Blisters in LPP may form de novo from unaffected skin, whereas the bullae in bullous lichen planus are limited to existing lichenoid papules.9 The autoantibodies typical of LPP are absent in bullous lichen planus. Lichen planus actinicus is a variant of lichen planus that presents with annular, dyschromic, or violaceous plaques in a photodistributed pattern without bullous lesions.9
Lichen planus pemphigoides most commonly is treated with systemic corticosteroids. Topical steroids, dapsone, erythromycin, tetracycline and nicotinamide, azathioprine, and mycophenolate mofetil have been reported as adjuncts to systemic steroid therapy.2,11 Most reports describe treatment success with resolution of blistering lesions.
- Jang SH, Yun SJ, Lee SC, et al. Lichen planus pemphigoides associated with chronic hepatitis B virus infection. Clin Exp Dermatol. 2015;40:868-871.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol. 2017;9:217-224.
- Ben Salem C, Chengeul L, Ghariani N, et al. Captopril-induced lichen planus pemphigoides. Pharmacoepidemiol Drug Saf. 2008;17:722-724.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Zhu YI, Fitzpatrick JE, Kornfield BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Sin B, Miller M, Chew E. Hydrochlorothiazide induced lichen planus in the emergency department. J Pharm Pract. 2017;30:266-269.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Women Dermatol. 2015;1:140-149.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Fivenson DP, Kimbrough TL. Lichen planus pemphigoides: combination therapy with tetracycline and nicotinamide. J Am Acad Dermatol. 1997;36:638-640.
- Jang SH, Yun SJ, Lee SC, et al. Lichen planus pemphigoides associated with chronic hepatitis B virus infection. Clin Exp Dermatol. 2015;40:868-871.
- Zaraa I, Mahfoudh A, Sellami MK, et al. Lichen planus pemphigoides: four new cases and a review of the literature. Int J Dermatol. 2013;52:406-412.
- Harting MS, Hsu S. Lichen planus pemphigoides: a case report and review of the literature. Dermatol Online J. 2006;12:10.
- Onprasert W, Chanprapaph K. Lichen planus pemphigoides induced by enalapril: a case report and a review of literature. Case Rep Dermatol. 2017;9:217-224.
- Ben Salem C, Chengeul L, Ghariani N, et al. Captopril-induced lichen planus pemphigoides. Pharmacoepidemiol Drug Saf. 2008;17:722-724.
- Kuramoto N, Kishimoto S, Shibagaki R, et al. PUVA-induced lichen planus pemphigoides. Br J Dermatol. 2000;142:509-512.
- Zhu YI, Fitzpatrick JE, Kornfield BW. Lichen planus pemphigoides associated with Ramipril. Int J Dermatol. 2006;45:1453-1455.
- Sin B, Miller M, Chew E. Hydrochlorothiazide induced lichen planus in the emergency department. J Pharm Pract. 2017;30:266-269.
- Weston G, Payette M. Update on lichen planus and its clinical variants. Int J Women Dermatol. 2015;1:140-149.
- Contestable JJ, Edhegard KD, Meyerle JH. Bullous systemic lupus erythematosus: a review and update to diagnosis and treatment. Am J Clin Dermatol. 2014;15:517-524.
- Fivenson DP, Kimbrough TL. Lichen planus pemphigoides: combination therapy with tetracycline and nicotinamide. J Am Acad Dermatol. 1997;36:638-640.
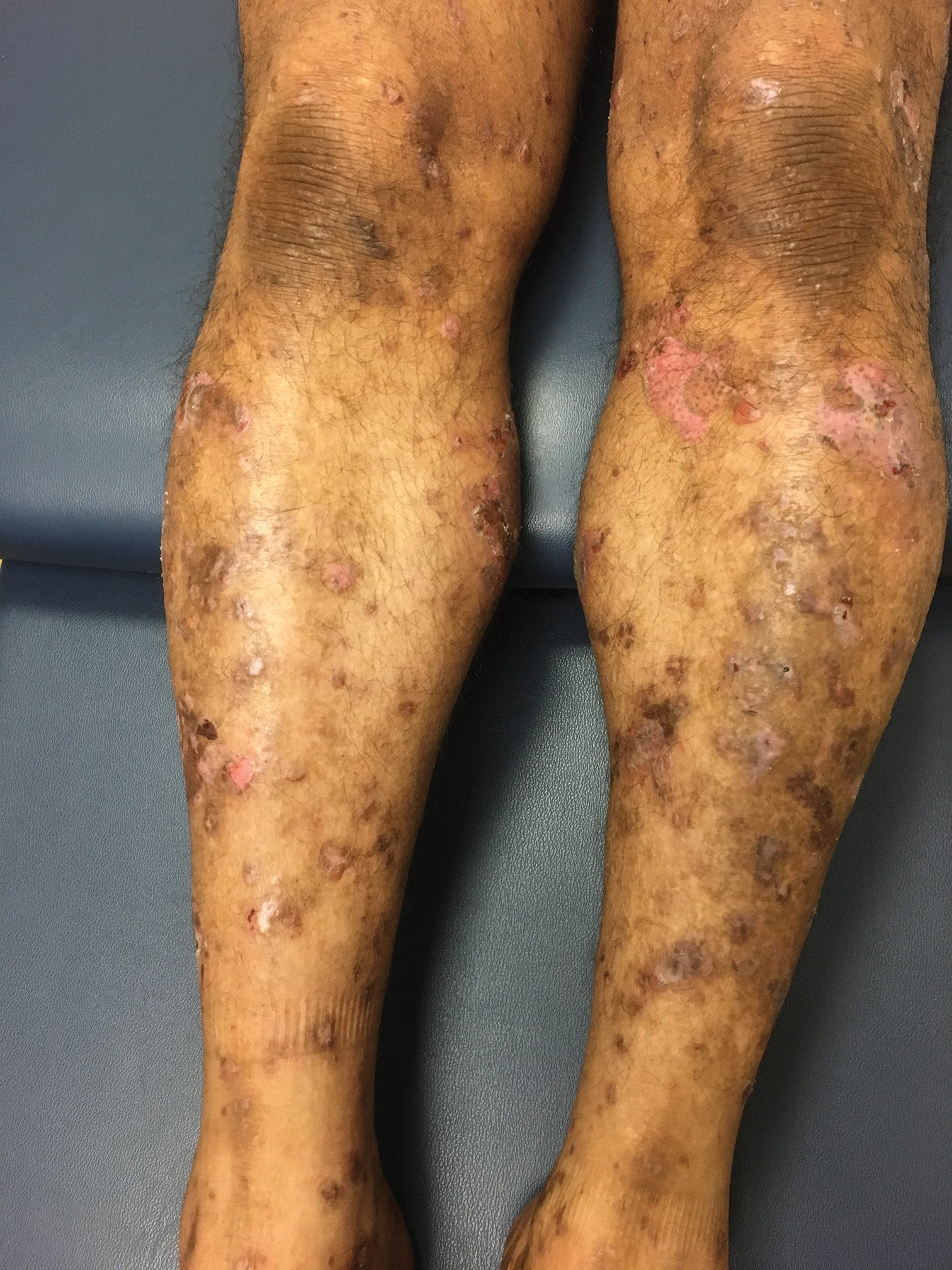
A 50-year-old man presented with a pruritic bullous dermatosis on the lower legs, arms, and back of 1 month’s duration. He had an 8-year history of lichen planus, and the lesions recently had worsened despite the addition of UVB phototherapy. His medical history was remarkable for hepatitis B treated with entecavir and the addition of hydrochlorothiazide for essential hypertension 2 weeks prior to the dramatic worsening of the rash. Physical examination revealed multiple bullae on the lower legs associated with violaceous and hyperpigmented papules and patches. He also had violaceous papules on the lower back and eroded lesions on the oral mucosa. Shave biopsies were obtained from the right thigh and mid back, and histopathologic analysis was performed for both routine histology and direct immunofluorescence.
Experts advocate for the elimination of daylight savings time
In the interest of public health and safety, – a recommendation that has garnered strong support from multiple medical and other high-profile organizations.
“Permanent, year-round standard time is the best choice to most closely match our circadian sleep-wake cycle,” M. Adeel Rishi, MD, lead author of the AASM position statement, said in a news release. “Daylight saving time results in more darkness in the morning and more light in the evening, disrupting the body’s natural rhythm,” said Dr. Rishi, of the department of pulmonology, critical care, and sleep medicine, Mayo Clinic, Eau Claire, Wis., and vice chair of the AASM Public Safety Committee.
The position statement was published Aug. 26 in the Journal of Clinical Sleep Medicine to coincide with the virtual annual meeting of the Associated Professional Sleep Societies .
Significant health risks
In the United States, the annual “spring forward” to daylight saving time and “fall back” to standard time is required by law, although under the statute some exceptions are permitted.
There has been intense debate over the last several years about transitioning between standard and daylight saving time. The AASM says there is “an abundance of evidence” to indicate that quick transition from standard time to daylight saving time incurs significant public health and safety risks, including increased risk of heart attack, stroke, mood disorders, and car crashes.
“Although chronic effects of remaining in daylight saving time year-round have not been well-studied, daylight saving time is less aligned with human circadian biology – which, because of the impacts of the delayed natural light/dark cycle on human activity, could result in circadian misalignment, which has been associated in some studies with increased cardiovascular disease risk, metabolic syndrome and other health risks,” the authors wrote.
A recent study also showed an increase in medical errors in the week after switching to daylight saving time.
“Because the adoption of permanent standard time would be beneficial for public health and safety, the AASM will be advocating at the federal level for this legislative change,” said AASM President Kannan Ramar, MBBS, MD, with the Mayo Clinic in Rochester, Minn.
It seems that many Americans are in favor of the change. In July, an AASM survey of roughly 2,000 U.S. adults showed that two-thirds support doing away with the seasonal time change. Only 11% opposed it. In addition, the academy’s 2019 survey showed more than half of adults feel extremely, or somewhat, tired after the springing ahead to daylight saving time.
Strong support
The position statement has been endorsed by 19 organizations, including the American Academy of Cardiovascular Sleep Medicine, American College of Chest Physicians (CHEST), American College of Occupational and Environmental Medicine, National PTA, National Safety Council, Society of Anesthesia and Sleep Medicine, and the Society of Behavioral Sleep Medicine.
Weighing in on the issue, Saul Rothenberg, PhD, from the Sleep Center at Greenwich Hospital, Conn., said the literature on daylight saving time has grown over the past 20 years. He said he was ”humbled” by the research that shows that a “relatively small” misalignment of biological and social clocks has a measurable impact on human health and behavior.
“Because misalignment is associated with negative health and performance outcomes, keeping one set of hours year-round is promoted to minimize misalignment and associated consequences,” he added.
In light of this research, the recommendation to dispense with daylight saving time seems “quite reasonable” from a public health perspective. “I am left with a strengthened view on the importance of regular adequate sleep as a way to enhance health, performance, and quality of life,” he added.
This research had no commercial funding. Dr. Rishi and Dr. Rothenberg have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the interest of public health and safety, – a recommendation that has garnered strong support from multiple medical and other high-profile organizations.
“Permanent, year-round standard time is the best choice to most closely match our circadian sleep-wake cycle,” M. Adeel Rishi, MD, lead author of the AASM position statement, said in a news release. “Daylight saving time results in more darkness in the morning and more light in the evening, disrupting the body’s natural rhythm,” said Dr. Rishi, of the department of pulmonology, critical care, and sleep medicine, Mayo Clinic, Eau Claire, Wis., and vice chair of the AASM Public Safety Committee.
The position statement was published Aug. 26 in the Journal of Clinical Sleep Medicine to coincide with the virtual annual meeting of the Associated Professional Sleep Societies .
Significant health risks
In the United States, the annual “spring forward” to daylight saving time and “fall back” to standard time is required by law, although under the statute some exceptions are permitted.
There has been intense debate over the last several years about transitioning between standard and daylight saving time. The AASM says there is “an abundance of evidence” to indicate that quick transition from standard time to daylight saving time incurs significant public health and safety risks, including increased risk of heart attack, stroke, mood disorders, and car crashes.
“Although chronic effects of remaining in daylight saving time year-round have not been well-studied, daylight saving time is less aligned with human circadian biology – which, because of the impacts of the delayed natural light/dark cycle on human activity, could result in circadian misalignment, which has been associated in some studies with increased cardiovascular disease risk, metabolic syndrome and other health risks,” the authors wrote.
A recent study also showed an increase in medical errors in the week after switching to daylight saving time.
“Because the adoption of permanent standard time would be beneficial for public health and safety, the AASM will be advocating at the federal level for this legislative change,” said AASM President Kannan Ramar, MBBS, MD, with the Mayo Clinic in Rochester, Minn.
It seems that many Americans are in favor of the change. In July, an AASM survey of roughly 2,000 U.S. adults showed that two-thirds support doing away with the seasonal time change. Only 11% opposed it. In addition, the academy’s 2019 survey showed more than half of adults feel extremely, or somewhat, tired after the springing ahead to daylight saving time.
Strong support
The position statement has been endorsed by 19 organizations, including the American Academy of Cardiovascular Sleep Medicine, American College of Chest Physicians (CHEST), American College of Occupational and Environmental Medicine, National PTA, National Safety Council, Society of Anesthesia and Sleep Medicine, and the Society of Behavioral Sleep Medicine.
Weighing in on the issue, Saul Rothenberg, PhD, from the Sleep Center at Greenwich Hospital, Conn., said the literature on daylight saving time has grown over the past 20 years. He said he was ”humbled” by the research that shows that a “relatively small” misalignment of biological and social clocks has a measurable impact on human health and behavior.
“Because misalignment is associated with negative health and performance outcomes, keeping one set of hours year-round is promoted to minimize misalignment and associated consequences,” he added.
In light of this research, the recommendation to dispense with daylight saving time seems “quite reasonable” from a public health perspective. “I am left with a strengthened view on the importance of regular adequate sleep as a way to enhance health, performance, and quality of life,” he added.
This research had no commercial funding. Dr. Rishi and Dr. Rothenberg have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
In the interest of public health and safety, – a recommendation that has garnered strong support from multiple medical and other high-profile organizations.
“Permanent, year-round standard time is the best choice to most closely match our circadian sleep-wake cycle,” M. Adeel Rishi, MD, lead author of the AASM position statement, said in a news release. “Daylight saving time results in more darkness in the morning and more light in the evening, disrupting the body’s natural rhythm,” said Dr. Rishi, of the department of pulmonology, critical care, and sleep medicine, Mayo Clinic, Eau Claire, Wis., and vice chair of the AASM Public Safety Committee.
The position statement was published Aug. 26 in the Journal of Clinical Sleep Medicine to coincide with the virtual annual meeting of the Associated Professional Sleep Societies .
Significant health risks
In the United States, the annual “spring forward” to daylight saving time and “fall back” to standard time is required by law, although under the statute some exceptions are permitted.
There has been intense debate over the last several years about transitioning between standard and daylight saving time. The AASM says there is “an abundance of evidence” to indicate that quick transition from standard time to daylight saving time incurs significant public health and safety risks, including increased risk of heart attack, stroke, mood disorders, and car crashes.
“Although chronic effects of remaining in daylight saving time year-round have not been well-studied, daylight saving time is less aligned with human circadian biology – which, because of the impacts of the delayed natural light/dark cycle on human activity, could result in circadian misalignment, which has been associated in some studies with increased cardiovascular disease risk, metabolic syndrome and other health risks,” the authors wrote.
A recent study also showed an increase in medical errors in the week after switching to daylight saving time.
“Because the adoption of permanent standard time would be beneficial for public health and safety, the AASM will be advocating at the federal level for this legislative change,” said AASM President Kannan Ramar, MBBS, MD, with the Mayo Clinic in Rochester, Minn.
It seems that many Americans are in favor of the change. In July, an AASM survey of roughly 2,000 U.S. adults showed that two-thirds support doing away with the seasonal time change. Only 11% opposed it. In addition, the academy’s 2019 survey showed more than half of adults feel extremely, or somewhat, tired after the springing ahead to daylight saving time.
Strong support
The position statement has been endorsed by 19 organizations, including the American Academy of Cardiovascular Sleep Medicine, American College of Chest Physicians (CHEST), American College of Occupational and Environmental Medicine, National PTA, National Safety Council, Society of Anesthesia and Sleep Medicine, and the Society of Behavioral Sleep Medicine.
Weighing in on the issue, Saul Rothenberg, PhD, from the Sleep Center at Greenwich Hospital, Conn., said the literature on daylight saving time has grown over the past 20 years. He said he was ”humbled” by the research that shows that a “relatively small” misalignment of biological and social clocks has a measurable impact on human health and behavior.
“Because misalignment is associated with negative health and performance outcomes, keeping one set of hours year-round is promoted to minimize misalignment and associated consequences,” he added.
In light of this research, the recommendation to dispense with daylight saving time seems “quite reasonable” from a public health perspective. “I am left with a strengthened view on the importance of regular adequate sleep as a way to enhance health, performance, and quality of life,” he added.
This research had no commercial funding. Dr. Rishi and Dr. Rothenberg have disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
FROM SLEEP 2020
Many advanced countries missing targets for HCV elimination
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
Help your patients better understand the risks and treatment for hepatitis C by sharing AGA GI Patient Center education at http://ow.ly/xV2S30r8L29.
A version of this article originally appeared on Medscape.com.
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
Help your patients better understand the risks and treatment for hepatitis C by sharing AGA GI Patient Center education at http://ow.ly/xV2S30r8L29.
A version of this article originally appeared on Medscape.com.
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
Help your patients better understand the risks and treatment for hepatitis C by sharing AGA GI Patient Center education at http://ow.ly/xV2S30r8L29.
A version of this article originally appeared on Medscape.com.
Final EVAPORATE results for Vascepa raise eyebrows
Final 18-month results of the EVAPORATE trial suggest icosapent ethyl (Vascepa) provides even greater slowing of coronary plaque progression when added to statins for patients with high triglyceride levels, but not all cardiologists are convinced.
The study was designed to explore a potential mechanism behind the cardiovascular event reduction in REDUCE-IT. Previously reported interim results showed that, after 9 months, the pharmaceutical-grade omega-3 fatty acid formation significantly slowed the progression of several plaque types but not the primary endpoint of change in low-attenuation plaque volume on multidetector CT.
From baseline to 18-month follow-up, however, the primary endpoint was significantly reduced by 17% in the icosapent ethyl group, whereas low-attenuation plaque volumes increased by 109% in the placebo group (P = .0061).
Significant declines were also seen with icosapent ethyl 4 g/day versus the mineral oil placebo for all other plaque types except dense calcium after adjustment for age, sex, diabetes, hypertension, and triglyceride levels at baseline:
- Dense calcium: –1% versus 15% (P = .0531).
- Fibro-fatty: –34% versus 32% (P = .0002).
- Fibrous: –20% versus 1% (P = .0028).
- Noncalcified: –19% versus 9% (P = .0005).
- Total plaque: –9% versus 11% (P = .0019).
The results parallel nicely with recent clinical data from REDUCE-IT REVASC, in which icosapent ethyl 4 g/day provided a very early benefit on first revascularization events that reached statistical significance after only 11 months (hazard ratio, 0.66), principal investigator Matthew Budoff, MD, director of cardiac CT at Harbor–University of California, Los Angeles, Medical Center in Torrance, Calif., said during the virtual European Society of Cardiology Congress 2020.
The findings were also published simultaneously in the European Heart Journal and quickly prompted a flurry of comments on social media.
Some were supportive. Christopher Cannon, MD, of Harvard Medical School, Boston; Dan Soffer, MD, a lipidologist at the University of Pennsylvania, Philadelphia; and Viet Le, MPAS, PA, a researcher at the Intermountain Heart Institute, Murray, Utah, took to Twitter to praise Dr. Budoff and the final results of the mechanistic study. Dr. Soffer called the study “elegant,” while Dr. Cannon said the results provide “important mechanistic data on plaque character.”
Others were highly critical, including a poll questioning whether the article should be retracted or revised.
Ibrahim H. Tanboga, MD, PhD, a cardiology professor and biostatistician at Hisar Intercontinental Hospital in Istanbul, questioned how the longitudinal change in low-attenuation plaque was possible clinically; his plot of the data showed these lesions getting worse in both arms before getting better in both arms.
A more volatile exchange concerned whether there were differences in the baseline characteristics between the two groups and whether the data might have been unblinded.
“I am sympathetic to the boss of a big laboratory [who] might not know how every step of the process was done and therefore might not be aware of opportunities for accidental bias. This can easily happen in a large and active department,” Darrel Francis, MD, professor of cardiology at the National Heart and Lung Institute, Imperial College, London, said in an interview.
An alternative explanation proffered on Twitter was that the interim analysis found no significant differences in baseline measures because it used nonparametric tests, whereas log transformation was applied to the final data. In any event, the tweets prompted a sharp rebuke from Dr. Budoff.
Dr. Francis raised another point of contention on Twitter regarding the degree of plaque progression in the placebo group.
In an interview, Dr. Francis pointed out that the final data represent the percentage change in the logarithm, not the actual percentage change in atheroma. So the increase in total atheroma volume in the placebo arm is not 11% but rather a scaling-up by 100.4 or 2.51, in other words, 151%.
He also offered a “less subtle feature of possible erroneous data,” in that the abstract reported low-attenuation plaque “more than doubles” in 18 months, which he described as a “ghastly supercharged version of Moore’s law for atheroma, instead of microchips.”
So “either it’s a mistake in the measurement or the placebo is harmful, because I can’t see how this is sustainable,” he said. “Why isn’t everyone dead from coronary disease?”
Concerns were raised previously over the possibility that the mineral oil placebo used in both EVAPORATE and REDUCE-IT could be having ill effects, notably, by increasing LDL cholesterol and C-reactive protein levels.
In an interview, Steven Nissen, MD, who is chair of cardiovascular medicine at the Cleveland Clinic and has been among the critics of the mineral oil placebo, also questioned the plaque progression over the 18 months.
“I’ve published more than dozen regression/progression trials, and we have never seen anything like this in a placebo group, ever,” he said. “If this was a clean placebo, why would this happen in a short amount of time?
“I’m concerned this is all about an increase, in the case of REDUCE-IT, in morbidity and mortality in the placebo group, and in the EVAPORATE trial, an increase in plaque in the placebo group,” Dr. Nissen said. “So this raises serious doubts about whether there is any benefit to icosapent ethyl.”
Asked about the 109% increase, Dr. Budoff said in an interview that low-attenuation plaque represents a much smaller quantity of overall plaque volume. “So the percentages might be exaggerated if you look at just percentage change because they;re small volumes.”
He also noted that previous trials that evaluated atherosclerosis progression used intravascular ultrasound (IVUS), whereas EVAPORATE is the first to make the transition to CT angiography-based analysis of plaque progression.
“I would point out that Dr. Nissen has only worked on intravascular ultrasound, which, while it’s parallel in its ability to measure plaque, measures different volumes and measures it in a totally different way,” said Dr. Budoff. “So I don’t think we can directly compare the results of CT angiography to Dr. Nissen’s examples of IVUS.”
During his presentation, Dr. Budoff highlighted their recent data showing a similar rate of plaque progression between the mineral oil placebo in EVAPORATE and a cellulose-based placebo in the Garlic5 study. “So we have high confidence that the benefits seen in this trial with icosapent ethyl represent icosapent ethyl’s beneficial effects on atherosclerosis and not harm of mineral oil,” he said.
Exactly how icosapent ethyl is slowing atherosclerosis, however, is not fully known, Dr. Budoff said in an interview. “It might be inflammation and oxidation; those have both been shown to be better with icosapent ethyl, but I don’t think we fully understand the implications of these results.”
Dr. Budoff dismissed tweets that suggest the data might have been unblinded as unprofessional and said they are requesting that Imperial College have Francis cease and desist.
“He doesn’t have the actual data, so there is no way to do statistics without the dataset. The whole thing is inappropriate,” Dr. Budoff said.
Amarin Pharma provided funding and drug for the trial. Dr. Budoff has received research funding from and has served as a speaker for Amarin Pharma, Amgen, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and Pfizer and has served as a speaker for Bristol-Myers Squibb. Dr. Francis has disclosed no relevant financial relationships..
A version of this article originally appeared on Medscape.com.
Final 18-month results of the EVAPORATE trial suggest icosapent ethyl (Vascepa) provides even greater slowing of coronary plaque progression when added to statins for patients with high triglyceride levels, but not all cardiologists are convinced.
The study was designed to explore a potential mechanism behind the cardiovascular event reduction in REDUCE-IT. Previously reported interim results showed that, after 9 months, the pharmaceutical-grade omega-3 fatty acid formation significantly slowed the progression of several plaque types but not the primary endpoint of change in low-attenuation plaque volume on multidetector CT.
From baseline to 18-month follow-up, however, the primary endpoint was significantly reduced by 17% in the icosapent ethyl group, whereas low-attenuation plaque volumes increased by 109% in the placebo group (P = .0061).
Significant declines were also seen with icosapent ethyl 4 g/day versus the mineral oil placebo for all other plaque types except dense calcium after adjustment for age, sex, diabetes, hypertension, and triglyceride levels at baseline:
- Dense calcium: –1% versus 15% (P = .0531).
- Fibro-fatty: –34% versus 32% (P = .0002).
- Fibrous: –20% versus 1% (P = .0028).
- Noncalcified: –19% versus 9% (P = .0005).
- Total plaque: –9% versus 11% (P = .0019).
The results parallel nicely with recent clinical data from REDUCE-IT REVASC, in which icosapent ethyl 4 g/day provided a very early benefit on first revascularization events that reached statistical significance after only 11 months (hazard ratio, 0.66), principal investigator Matthew Budoff, MD, director of cardiac CT at Harbor–University of California, Los Angeles, Medical Center in Torrance, Calif., said during the virtual European Society of Cardiology Congress 2020.
The findings were also published simultaneously in the European Heart Journal and quickly prompted a flurry of comments on social media.
Some were supportive. Christopher Cannon, MD, of Harvard Medical School, Boston; Dan Soffer, MD, a lipidologist at the University of Pennsylvania, Philadelphia; and Viet Le, MPAS, PA, a researcher at the Intermountain Heart Institute, Murray, Utah, took to Twitter to praise Dr. Budoff and the final results of the mechanistic study. Dr. Soffer called the study “elegant,” while Dr. Cannon said the results provide “important mechanistic data on plaque character.”
Others were highly critical, including a poll questioning whether the article should be retracted or revised.
Ibrahim H. Tanboga, MD, PhD, a cardiology professor and biostatistician at Hisar Intercontinental Hospital in Istanbul, questioned how the longitudinal change in low-attenuation plaque was possible clinically; his plot of the data showed these lesions getting worse in both arms before getting better in both arms.
A more volatile exchange concerned whether there were differences in the baseline characteristics between the two groups and whether the data might have been unblinded.
“I am sympathetic to the boss of a big laboratory [who] might not know how every step of the process was done and therefore might not be aware of opportunities for accidental bias. This can easily happen in a large and active department,” Darrel Francis, MD, professor of cardiology at the National Heart and Lung Institute, Imperial College, London, said in an interview.
An alternative explanation proffered on Twitter was that the interim analysis found no significant differences in baseline measures because it used nonparametric tests, whereas log transformation was applied to the final data. In any event, the tweets prompted a sharp rebuke from Dr. Budoff.
Dr. Francis raised another point of contention on Twitter regarding the degree of plaque progression in the placebo group.
In an interview, Dr. Francis pointed out that the final data represent the percentage change in the logarithm, not the actual percentage change in atheroma. So the increase in total atheroma volume in the placebo arm is not 11% but rather a scaling-up by 100.4 or 2.51, in other words, 151%.
He also offered a “less subtle feature of possible erroneous data,” in that the abstract reported low-attenuation plaque “more than doubles” in 18 months, which he described as a “ghastly supercharged version of Moore’s law for atheroma, instead of microchips.”
So “either it’s a mistake in the measurement or the placebo is harmful, because I can’t see how this is sustainable,” he said. “Why isn’t everyone dead from coronary disease?”
Concerns were raised previously over the possibility that the mineral oil placebo used in both EVAPORATE and REDUCE-IT could be having ill effects, notably, by increasing LDL cholesterol and C-reactive protein levels.
In an interview, Steven Nissen, MD, who is chair of cardiovascular medicine at the Cleveland Clinic and has been among the critics of the mineral oil placebo, also questioned the plaque progression over the 18 months.
“I’ve published more than dozen regression/progression trials, and we have never seen anything like this in a placebo group, ever,” he said. “If this was a clean placebo, why would this happen in a short amount of time?
“I’m concerned this is all about an increase, in the case of REDUCE-IT, in morbidity and mortality in the placebo group, and in the EVAPORATE trial, an increase in plaque in the placebo group,” Dr. Nissen said. “So this raises serious doubts about whether there is any benefit to icosapent ethyl.”
Asked about the 109% increase, Dr. Budoff said in an interview that low-attenuation plaque represents a much smaller quantity of overall plaque volume. “So the percentages might be exaggerated if you look at just percentage change because they;re small volumes.”
He also noted that previous trials that evaluated atherosclerosis progression used intravascular ultrasound (IVUS), whereas EVAPORATE is the first to make the transition to CT angiography-based analysis of plaque progression.
“I would point out that Dr. Nissen has only worked on intravascular ultrasound, which, while it’s parallel in its ability to measure plaque, measures different volumes and measures it in a totally different way,” said Dr. Budoff. “So I don’t think we can directly compare the results of CT angiography to Dr. Nissen’s examples of IVUS.”
During his presentation, Dr. Budoff highlighted their recent data showing a similar rate of plaque progression between the mineral oil placebo in EVAPORATE and a cellulose-based placebo in the Garlic5 study. “So we have high confidence that the benefits seen in this trial with icosapent ethyl represent icosapent ethyl’s beneficial effects on atherosclerosis and not harm of mineral oil,” he said.
Exactly how icosapent ethyl is slowing atherosclerosis, however, is not fully known, Dr. Budoff said in an interview. “It might be inflammation and oxidation; those have both been shown to be better with icosapent ethyl, but I don’t think we fully understand the implications of these results.”
Dr. Budoff dismissed tweets that suggest the data might have been unblinded as unprofessional and said they are requesting that Imperial College have Francis cease and desist.
“He doesn’t have the actual data, so there is no way to do statistics without the dataset. The whole thing is inappropriate,” Dr. Budoff said.
Amarin Pharma provided funding and drug for the trial. Dr. Budoff has received research funding from and has served as a speaker for Amarin Pharma, Amgen, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and Pfizer and has served as a speaker for Bristol-Myers Squibb. Dr. Francis has disclosed no relevant financial relationships..
A version of this article originally appeared on Medscape.com.
Final 18-month results of the EVAPORATE trial suggest icosapent ethyl (Vascepa) provides even greater slowing of coronary plaque progression when added to statins for patients with high triglyceride levels, but not all cardiologists are convinced.
The study was designed to explore a potential mechanism behind the cardiovascular event reduction in REDUCE-IT. Previously reported interim results showed that, after 9 months, the pharmaceutical-grade omega-3 fatty acid formation significantly slowed the progression of several plaque types but not the primary endpoint of change in low-attenuation plaque volume on multidetector CT.
From baseline to 18-month follow-up, however, the primary endpoint was significantly reduced by 17% in the icosapent ethyl group, whereas low-attenuation plaque volumes increased by 109% in the placebo group (P = .0061).
Significant declines were also seen with icosapent ethyl 4 g/day versus the mineral oil placebo for all other plaque types except dense calcium after adjustment for age, sex, diabetes, hypertension, and triglyceride levels at baseline:
- Dense calcium: –1% versus 15% (P = .0531).
- Fibro-fatty: –34% versus 32% (P = .0002).
- Fibrous: –20% versus 1% (P = .0028).
- Noncalcified: –19% versus 9% (P = .0005).
- Total plaque: –9% versus 11% (P = .0019).
The results parallel nicely with recent clinical data from REDUCE-IT REVASC, in which icosapent ethyl 4 g/day provided a very early benefit on first revascularization events that reached statistical significance after only 11 months (hazard ratio, 0.66), principal investigator Matthew Budoff, MD, director of cardiac CT at Harbor–University of California, Los Angeles, Medical Center in Torrance, Calif., said during the virtual European Society of Cardiology Congress 2020.
The findings were also published simultaneously in the European Heart Journal and quickly prompted a flurry of comments on social media.
Some were supportive. Christopher Cannon, MD, of Harvard Medical School, Boston; Dan Soffer, MD, a lipidologist at the University of Pennsylvania, Philadelphia; and Viet Le, MPAS, PA, a researcher at the Intermountain Heart Institute, Murray, Utah, took to Twitter to praise Dr. Budoff and the final results of the mechanistic study. Dr. Soffer called the study “elegant,” while Dr. Cannon said the results provide “important mechanistic data on plaque character.”
Others were highly critical, including a poll questioning whether the article should be retracted or revised.
Ibrahim H. Tanboga, MD, PhD, a cardiology professor and biostatistician at Hisar Intercontinental Hospital in Istanbul, questioned how the longitudinal change in low-attenuation plaque was possible clinically; his plot of the data showed these lesions getting worse in both arms before getting better in both arms.
A more volatile exchange concerned whether there were differences in the baseline characteristics between the two groups and whether the data might have been unblinded.
“I am sympathetic to the boss of a big laboratory [who] might not know how every step of the process was done and therefore might not be aware of opportunities for accidental bias. This can easily happen in a large and active department,” Darrel Francis, MD, professor of cardiology at the National Heart and Lung Institute, Imperial College, London, said in an interview.
An alternative explanation proffered on Twitter was that the interim analysis found no significant differences in baseline measures because it used nonparametric tests, whereas log transformation was applied to the final data. In any event, the tweets prompted a sharp rebuke from Dr. Budoff.
Dr. Francis raised another point of contention on Twitter regarding the degree of plaque progression in the placebo group.
In an interview, Dr. Francis pointed out that the final data represent the percentage change in the logarithm, not the actual percentage change in atheroma. So the increase in total atheroma volume in the placebo arm is not 11% but rather a scaling-up by 100.4 or 2.51, in other words, 151%.
He also offered a “less subtle feature of possible erroneous data,” in that the abstract reported low-attenuation plaque “more than doubles” in 18 months, which he described as a “ghastly supercharged version of Moore’s law for atheroma, instead of microchips.”
So “either it’s a mistake in the measurement or the placebo is harmful, because I can’t see how this is sustainable,” he said. “Why isn’t everyone dead from coronary disease?”
Concerns were raised previously over the possibility that the mineral oil placebo used in both EVAPORATE and REDUCE-IT could be having ill effects, notably, by increasing LDL cholesterol and C-reactive protein levels.
In an interview, Steven Nissen, MD, who is chair of cardiovascular medicine at the Cleveland Clinic and has been among the critics of the mineral oil placebo, also questioned the plaque progression over the 18 months.
“I’ve published more than dozen regression/progression trials, and we have never seen anything like this in a placebo group, ever,” he said. “If this was a clean placebo, why would this happen in a short amount of time?
“I’m concerned this is all about an increase, in the case of REDUCE-IT, in morbidity and mortality in the placebo group, and in the EVAPORATE trial, an increase in plaque in the placebo group,” Dr. Nissen said. “So this raises serious doubts about whether there is any benefit to icosapent ethyl.”
Asked about the 109% increase, Dr. Budoff said in an interview that low-attenuation plaque represents a much smaller quantity of overall plaque volume. “So the percentages might be exaggerated if you look at just percentage change because they;re small volumes.”
He also noted that previous trials that evaluated atherosclerosis progression used intravascular ultrasound (IVUS), whereas EVAPORATE is the first to make the transition to CT angiography-based analysis of plaque progression.
“I would point out that Dr. Nissen has only worked on intravascular ultrasound, which, while it’s parallel in its ability to measure plaque, measures different volumes and measures it in a totally different way,” said Dr. Budoff. “So I don’t think we can directly compare the results of CT angiography to Dr. Nissen’s examples of IVUS.”
During his presentation, Dr. Budoff highlighted their recent data showing a similar rate of plaque progression between the mineral oil placebo in EVAPORATE and a cellulose-based placebo in the Garlic5 study. “So we have high confidence that the benefits seen in this trial with icosapent ethyl represent icosapent ethyl’s beneficial effects on atherosclerosis and not harm of mineral oil,” he said.
Exactly how icosapent ethyl is slowing atherosclerosis, however, is not fully known, Dr. Budoff said in an interview. “It might be inflammation and oxidation; those have both been shown to be better with icosapent ethyl, but I don’t think we fully understand the implications of these results.”
Dr. Budoff dismissed tweets that suggest the data might have been unblinded as unprofessional and said they are requesting that Imperial College have Francis cease and desist.
“He doesn’t have the actual data, so there is no way to do statistics without the dataset. The whole thing is inappropriate,” Dr. Budoff said.
Amarin Pharma provided funding and drug for the trial. Dr. Budoff has received research funding from and has served as a speaker for Amarin Pharma, Amgen, AstraZeneca, Boehringer Ingelheim, Novo Nordisk, and Pfizer and has served as a speaker for Bristol-Myers Squibb. Dr. Francis has disclosed no relevant financial relationships..
A version of this article originally appeared on Medscape.com.
Petition seeks to oust AAD president-elect, citing private equity conflicts
A make him too conflicted for the job.
The petition, created anonymously, appeared in early August and is seeking 1,500 signatures – calculated as the number needed to initiate a vote to recall Mark Kaufmann, MD. Dr. Kaufmann was voted president-elect of the AAD and its sister organization, the AAD Association, in April. He takes the position in 2021 and would become president in 2022.
The petition objects to the fact that Dr. Kaufmann is the chief medical officer of Advanced Dermatology and Cosmetic Surgery (ADCS), the nation’s largest private equity–backed dermatology group. It claims that he took the position in July, after being elected, which it portrays as dishonest.
The petition said Dr. Kaufmann “will have concurrent fiduciary responsibilities to both the AAD and a corporation whose interests may or may not be aligned with the AAD.” Even if Dr. Kaufmann chose to recuse himself from some decisions, that would be a disservice to the AAD, said the petition, which added that he “cannot serve two masters simultaneously.”
Several efforts were made to reach Dr. Kaufmann for a comment, with no responses at press time. At press time, ACDS had not responded to a request for comment.
In response to questions, Bruce H. Thiers, MD, president of the AAD/A, said that the organization has no policy barring officers from holding capital in a private equity (PE)–backed corporation, but that any elected officer is subject to the organization’s “fiduciary duties and obligations.”
The AAD/A shared a 7-page administrative regulation on disclosure of outside interests and management of conflicts of interest. The policy notes that employment by a private equity group is considered a potential conflict of interest, but so is employment by a government agency, solo practice, academic medical center, or multiple other models. Key leaders, including the president, president-elect, and immediate past president, are required to divest themselves of any direct financial relationships with industry during their entire term.
Members who do not disclose potential conflicts each year and who fail to update their disclosure within 30 days of acquiring a new financial relationship “will lose the right to hold office, serve in the governance structure and, except in unusual circumstances approved in advance by the Board of Directors, to participate in Academy programs,” according to the policy.
Petitioners lament conflicts, private equity
At press time, the petition had more than 1,100 signatures. Most who signed indicated in their comments that they believed that Dr. Kaufmann’s association with private equity disqualifies him.
“I believe private equity should not have a place at the table of the governing body of the AAD,” commented Cynthia Abbott, MD, an Atlanta dermatologist. In his comment, Ron Birnbaum, MD a Los Angeles–based dermatologist, wrote, “I oppose the movement to PE-based purchase and consolidation of practices,” adding that he had not voted for Dr. Kaufmann in April. Dr. Kaufmann “might resolve the conflict of interest by resigning from the job of CMO of ADCS,” wrote Dr. Birnbaum.
“I have great respect for Dr. Kaufmann but am very troubled by the influence of PE infiltration into dermatology,” Mark Gaughan, MD, a dermatologist in Durango, Colo., wrote in the petition.
The petition seeks 1,500 signatures – which is 10% of the AAD membership. That’s key because, as per AAD administrative rules, members can initiate the removal of a board member through a petition that is signed by 10% of voting members or by a two-thirds vote of entire board of directors.
Dr. Thiers said the AAD/A’s “administrative regulations do not cover the removal of an elected officer prior to the start of his or her term.” Thus, it’s not even clear that Dr. Kaufmann could be recalled before he took office in 2021.
Dr. Kaufmann previously served on the AAD/A board of directors for 4 years and is currently deputy chair of the AAD/A’s Patient Access and Payer Relations Committee. He is chair of the AAD’s Workgroup on Innovation in Payment and Delivery Actinic Keratosis Alternative Payment Model Development and is a member of numerous other AAD/A committees, including: the AAD budget committee; the AAD appointment selection committee; the Resource-Based Relative Value Scale and Current Procedural Terminology committee; the work group on innovation in payment and delivery; the work group on ICD-10; and the work group on rapid response team Medicare Physician Fee Schedule.
Since March 2019, Dr. Kaufmann has also served as the chief medical officer of Florham Park, N.J.–based MoleSafe, a company that provides full-body skin cancer screening directly to patients.
In an interview, former AAD president Daniel Siegel, MD, said that he believes the petition is an unwarranted attack. “Mark is an honest upstanding individual who has, in the almost 2 decades I’ve known and worked with him, always put the interests of the specialty and the AAD ahead of any personal interests,” said Dr. Siegel of the department of dermatology at State University of New York, Brooklyn. Dr. Siegel also disclosed that he is chief compliance officer for the Florida-based Skin and Cancer Associates, a large group of practices owned by private equity.
Dr. Kaufmann is “an objective person who has no difficulty declaring a conflict where there is one,” Dr. Siegel said.
But Sailesh Konda, MD, a critic of private equity in dermatology, who signed the petition, said in an interview that he believed the “membership is rightfully concerned and responding to these new conflicts of interest with a valid petition.”
“This is readily apparent with a petition signed by more than 1,000 dermatologists,” added Dr. Konda of the department of dermatology at the University of Florida in Gainesville.
It might be hard to erase all potential conflicts at the AAD. Some members work for industry, some for academia, and some for government agencies – all of which come with their own biases.
But the AAD will not publicly release disclosures for members, board members, or officers. Those disclosures are compiled in an internal AAD database, but the organization leaves it up to each individual to decide what they will make public.
Second anonymous conflict petition
It is not clear what will happen with the Dr. Kaufmann recall effort, but it is not the first time that a petition has taken aim at an AAD official. An October 2019 petition – also submitted by an anonymous dermatologist – sought to remove Scott M. Dinehart, MD, from the AAD board.
The petition noted Dr. Dinehart’s involvement in creating the American Dermatology Board of Physician Assistants, a group that aims to offer board certification to PAs who work with dermatologists. Dr. Dinehart’s “concurrent relationships with both organizations is a major conflict of interest,” said the petition, which garnered some 4,200 signatures – far in excess of what is required to initiate a removal vote.
That led to a unanimous decision by the AAD/A board to present the membership with a resolution to remove Dr. Dinehart. Some 6,400 votes were cast, with 97% approving Dr. Dinehart’s removal.
He is no longer a member of the AAD/A board.
A make him too conflicted for the job.
The petition, created anonymously, appeared in early August and is seeking 1,500 signatures – calculated as the number needed to initiate a vote to recall Mark Kaufmann, MD. Dr. Kaufmann was voted president-elect of the AAD and its sister organization, the AAD Association, in April. He takes the position in 2021 and would become president in 2022.
The petition objects to the fact that Dr. Kaufmann is the chief medical officer of Advanced Dermatology and Cosmetic Surgery (ADCS), the nation’s largest private equity–backed dermatology group. It claims that he took the position in July, after being elected, which it portrays as dishonest.
The petition said Dr. Kaufmann “will have concurrent fiduciary responsibilities to both the AAD and a corporation whose interests may or may not be aligned with the AAD.” Even if Dr. Kaufmann chose to recuse himself from some decisions, that would be a disservice to the AAD, said the petition, which added that he “cannot serve two masters simultaneously.”
Several efforts were made to reach Dr. Kaufmann for a comment, with no responses at press time. At press time, ACDS had not responded to a request for comment.
In response to questions, Bruce H. Thiers, MD, president of the AAD/A, said that the organization has no policy barring officers from holding capital in a private equity (PE)–backed corporation, but that any elected officer is subject to the organization’s “fiduciary duties and obligations.”
The AAD/A shared a 7-page administrative regulation on disclosure of outside interests and management of conflicts of interest. The policy notes that employment by a private equity group is considered a potential conflict of interest, but so is employment by a government agency, solo practice, academic medical center, or multiple other models. Key leaders, including the president, president-elect, and immediate past president, are required to divest themselves of any direct financial relationships with industry during their entire term.
Members who do not disclose potential conflicts each year and who fail to update their disclosure within 30 days of acquiring a new financial relationship “will lose the right to hold office, serve in the governance structure and, except in unusual circumstances approved in advance by the Board of Directors, to participate in Academy programs,” according to the policy.
Petitioners lament conflicts, private equity
At press time, the petition had more than 1,100 signatures. Most who signed indicated in their comments that they believed that Dr. Kaufmann’s association with private equity disqualifies him.
“I believe private equity should not have a place at the table of the governing body of the AAD,” commented Cynthia Abbott, MD, an Atlanta dermatologist. In his comment, Ron Birnbaum, MD a Los Angeles–based dermatologist, wrote, “I oppose the movement to PE-based purchase and consolidation of practices,” adding that he had not voted for Dr. Kaufmann in April. Dr. Kaufmann “might resolve the conflict of interest by resigning from the job of CMO of ADCS,” wrote Dr. Birnbaum.
“I have great respect for Dr. Kaufmann but am very troubled by the influence of PE infiltration into dermatology,” Mark Gaughan, MD, a dermatologist in Durango, Colo., wrote in the petition.
The petition seeks 1,500 signatures – which is 10% of the AAD membership. That’s key because, as per AAD administrative rules, members can initiate the removal of a board member through a petition that is signed by 10% of voting members or by a two-thirds vote of entire board of directors.
Dr. Thiers said the AAD/A’s “administrative regulations do not cover the removal of an elected officer prior to the start of his or her term.” Thus, it’s not even clear that Dr. Kaufmann could be recalled before he took office in 2021.
Dr. Kaufmann previously served on the AAD/A board of directors for 4 years and is currently deputy chair of the AAD/A’s Patient Access and Payer Relations Committee. He is chair of the AAD’s Workgroup on Innovation in Payment and Delivery Actinic Keratosis Alternative Payment Model Development and is a member of numerous other AAD/A committees, including: the AAD budget committee; the AAD appointment selection committee; the Resource-Based Relative Value Scale and Current Procedural Terminology committee; the work group on innovation in payment and delivery; the work group on ICD-10; and the work group on rapid response team Medicare Physician Fee Schedule.
Since March 2019, Dr. Kaufmann has also served as the chief medical officer of Florham Park, N.J.–based MoleSafe, a company that provides full-body skin cancer screening directly to patients.
In an interview, former AAD president Daniel Siegel, MD, said that he believes the petition is an unwarranted attack. “Mark is an honest upstanding individual who has, in the almost 2 decades I’ve known and worked with him, always put the interests of the specialty and the AAD ahead of any personal interests,” said Dr. Siegel of the department of dermatology at State University of New York, Brooklyn. Dr. Siegel also disclosed that he is chief compliance officer for the Florida-based Skin and Cancer Associates, a large group of practices owned by private equity.
Dr. Kaufmann is “an objective person who has no difficulty declaring a conflict where there is one,” Dr. Siegel said.
But Sailesh Konda, MD, a critic of private equity in dermatology, who signed the petition, said in an interview that he believed the “membership is rightfully concerned and responding to these new conflicts of interest with a valid petition.”
“This is readily apparent with a petition signed by more than 1,000 dermatologists,” added Dr. Konda of the department of dermatology at the University of Florida in Gainesville.
It might be hard to erase all potential conflicts at the AAD. Some members work for industry, some for academia, and some for government agencies – all of which come with their own biases.
But the AAD will not publicly release disclosures for members, board members, or officers. Those disclosures are compiled in an internal AAD database, but the organization leaves it up to each individual to decide what they will make public.
Second anonymous conflict petition
It is not clear what will happen with the Dr. Kaufmann recall effort, but it is not the first time that a petition has taken aim at an AAD official. An October 2019 petition – also submitted by an anonymous dermatologist – sought to remove Scott M. Dinehart, MD, from the AAD board.
The petition noted Dr. Dinehart’s involvement in creating the American Dermatology Board of Physician Assistants, a group that aims to offer board certification to PAs who work with dermatologists. Dr. Dinehart’s “concurrent relationships with both organizations is a major conflict of interest,” said the petition, which garnered some 4,200 signatures – far in excess of what is required to initiate a removal vote.
That led to a unanimous decision by the AAD/A board to present the membership with a resolution to remove Dr. Dinehart. Some 6,400 votes were cast, with 97% approving Dr. Dinehart’s removal.
He is no longer a member of the AAD/A board.
A make him too conflicted for the job.
The petition, created anonymously, appeared in early August and is seeking 1,500 signatures – calculated as the number needed to initiate a vote to recall Mark Kaufmann, MD. Dr. Kaufmann was voted president-elect of the AAD and its sister organization, the AAD Association, in April. He takes the position in 2021 and would become president in 2022.
The petition objects to the fact that Dr. Kaufmann is the chief medical officer of Advanced Dermatology and Cosmetic Surgery (ADCS), the nation’s largest private equity–backed dermatology group. It claims that he took the position in July, after being elected, which it portrays as dishonest.
The petition said Dr. Kaufmann “will have concurrent fiduciary responsibilities to both the AAD and a corporation whose interests may or may not be aligned with the AAD.” Even if Dr. Kaufmann chose to recuse himself from some decisions, that would be a disservice to the AAD, said the petition, which added that he “cannot serve two masters simultaneously.”
Several efforts were made to reach Dr. Kaufmann for a comment, with no responses at press time. At press time, ACDS had not responded to a request for comment.
In response to questions, Bruce H. Thiers, MD, president of the AAD/A, said that the organization has no policy barring officers from holding capital in a private equity (PE)–backed corporation, but that any elected officer is subject to the organization’s “fiduciary duties and obligations.”
The AAD/A shared a 7-page administrative regulation on disclosure of outside interests and management of conflicts of interest. The policy notes that employment by a private equity group is considered a potential conflict of interest, but so is employment by a government agency, solo practice, academic medical center, or multiple other models. Key leaders, including the president, president-elect, and immediate past president, are required to divest themselves of any direct financial relationships with industry during their entire term.
Members who do not disclose potential conflicts each year and who fail to update their disclosure within 30 days of acquiring a new financial relationship “will lose the right to hold office, serve in the governance structure and, except in unusual circumstances approved in advance by the Board of Directors, to participate in Academy programs,” according to the policy.
Petitioners lament conflicts, private equity
At press time, the petition had more than 1,100 signatures. Most who signed indicated in their comments that they believed that Dr. Kaufmann’s association with private equity disqualifies him.
“I believe private equity should not have a place at the table of the governing body of the AAD,” commented Cynthia Abbott, MD, an Atlanta dermatologist. In his comment, Ron Birnbaum, MD a Los Angeles–based dermatologist, wrote, “I oppose the movement to PE-based purchase and consolidation of practices,” adding that he had not voted for Dr. Kaufmann in April. Dr. Kaufmann “might resolve the conflict of interest by resigning from the job of CMO of ADCS,” wrote Dr. Birnbaum.
“I have great respect for Dr. Kaufmann but am very troubled by the influence of PE infiltration into dermatology,” Mark Gaughan, MD, a dermatologist in Durango, Colo., wrote in the petition.
The petition seeks 1,500 signatures – which is 10% of the AAD membership. That’s key because, as per AAD administrative rules, members can initiate the removal of a board member through a petition that is signed by 10% of voting members or by a two-thirds vote of entire board of directors.
Dr. Thiers said the AAD/A’s “administrative regulations do not cover the removal of an elected officer prior to the start of his or her term.” Thus, it’s not even clear that Dr. Kaufmann could be recalled before he took office in 2021.
Dr. Kaufmann previously served on the AAD/A board of directors for 4 years and is currently deputy chair of the AAD/A’s Patient Access and Payer Relations Committee. He is chair of the AAD’s Workgroup on Innovation in Payment and Delivery Actinic Keratosis Alternative Payment Model Development and is a member of numerous other AAD/A committees, including: the AAD budget committee; the AAD appointment selection committee; the Resource-Based Relative Value Scale and Current Procedural Terminology committee; the work group on innovation in payment and delivery; the work group on ICD-10; and the work group on rapid response team Medicare Physician Fee Schedule.
Since March 2019, Dr. Kaufmann has also served as the chief medical officer of Florham Park, N.J.–based MoleSafe, a company that provides full-body skin cancer screening directly to patients.
In an interview, former AAD president Daniel Siegel, MD, said that he believes the petition is an unwarranted attack. “Mark is an honest upstanding individual who has, in the almost 2 decades I’ve known and worked with him, always put the interests of the specialty and the AAD ahead of any personal interests,” said Dr. Siegel of the department of dermatology at State University of New York, Brooklyn. Dr. Siegel also disclosed that he is chief compliance officer for the Florida-based Skin and Cancer Associates, a large group of practices owned by private equity.
Dr. Kaufmann is “an objective person who has no difficulty declaring a conflict where there is one,” Dr. Siegel said.
But Sailesh Konda, MD, a critic of private equity in dermatology, who signed the petition, said in an interview that he believed the “membership is rightfully concerned and responding to these new conflicts of interest with a valid petition.”
“This is readily apparent with a petition signed by more than 1,000 dermatologists,” added Dr. Konda of the department of dermatology at the University of Florida in Gainesville.
It might be hard to erase all potential conflicts at the AAD. Some members work for industry, some for academia, and some for government agencies – all of which come with their own biases.
But the AAD will not publicly release disclosures for members, board members, or officers. Those disclosures are compiled in an internal AAD database, but the organization leaves it up to each individual to decide what they will make public.
Second anonymous conflict petition
It is not clear what will happen with the Dr. Kaufmann recall effort, but it is not the first time that a petition has taken aim at an AAD official. An October 2019 petition – also submitted by an anonymous dermatologist – sought to remove Scott M. Dinehart, MD, from the AAD board.
The petition noted Dr. Dinehart’s involvement in creating the American Dermatology Board of Physician Assistants, a group that aims to offer board certification to PAs who work with dermatologists. Dr. Dinehart’s “concurrent relationships with both organizations is a major conflict of interest,” said the petition, which garnered some 4,200 signatures – far in excess of what is required to initiate a removal vote.
That led to a unanimous decision by the AAD/A board to present the membership with a resolution to remove Dr. Dinehart. Some 6,400 votes were cast, with 97% approving Dr. Dinehart’s removal.
He is no longer a member of the AAD/A board.
CSF metabolomic profile linked to cancer-related fatigue in children with ALL
Children and adolescents with cancer report significantly more fatigue than their counterparts without cancer, and cancer-related fatigue (CRF) is “one of the most prevalent and distressing symptoms reported during childhood cancer therapy,” according to Austin L. Brown, PhD, and his colleagues.
Cerebrospinal fluid (CSF) profiles suggest three metabolites are significantly associated with CRF in children with acute lymphoblastic leukemia (ALL), according to a report published in the Journal of Pain and Symptom Management.
The researchers assessed the clinical and demographic characteristics of 171 pediatric ALL patients, who were divided into discovery (n = 86) and replication (n = 85) cohorts.
The entire population had a mean age at diagnosis of 8.48 years; was 56.1% male; and 85.4% had B-lineage ALL. A total of 63.7% received high- or very-high-risk treatment.
CSF samples were obtained and subjected to metabolomic analysis, according to Dr. Brown, an assistant professor at the Baylor College of Medicine, Houston, and colleagues.
The researchers analyzed postinduction CSF from the aforementioned 171 patients as well as diagnostic CSF from 48 patients in an additional replication cohort.
Significant metabolites
Analysis of postinduction CSF showed that three metabolites were significantly associated with fatigue in both the discovery and replication cohorts, comprising gamma-glutamylglutamine, dimethylglycine, and asparagine (P < .05).
In diagnostic CSF samples, the abundance of gamma-glutamylglutamine was significantly associated with fatigue (P =.0062).
The metabolites have been implicated in neurotransmitter transportation and glutathione recycling, suggesting glutamatergic pathways or oxidative stress may contribute to ALL-associated CRF, according to the researchers.
“Ultimately, this line of investigation may aid in the development of new prevention and treatment approaches informed by an improved understanding of the etiology and risk factors for cancer-related fatigue,” the researchers concluded.
The study was sponsored by the National Cancer Institute and several nonprofit organizations. The authors reported that they had no conflicts of interest.
SOURCE: Brown AL et al. J Pain Symptom Manage. 2020 Sep 1. doi: 10.1016/j.jpainsymman.2020.08.030.
Children and adolescents with cancer report significantly more fatigue than their counterparts without cancer, and cancer-related fatigue (CRF) is “one of the most prevalent and distressing symptoms reported during childhood cancer therapy,” according to Austin L. Brown, PhD, and his colleagues.
Cerebrospinal fluid (CSF) profiles suggest three metabolites are significantly associated with CRF in children with acute lymphoblastic leukemia (ALL), according to a report published in the Journal of Pain and Symptom Management.
The researchers assessed the clinical and demographic characteristics of 171 pediatric ALL patients, who were divided into discovery (n = 86) and replication (n = 85) cohorts.
The entire population had a mean age at diagnosis of 8.48 years; was 56.1% male; and 85.4% had B-lineage ALL. A total of 63.7% received high- or very-high-risk treatment.
CSF samples were obtained and subjected to metabolomic analysis, according to Dr. Brown, an assistant professor at the Baylor College of Medicine, Houston, and colleagues.
The researchers analyzed postinduction CSF from the aforementioned 171 patients as well as diagnostic CSF from 48 patients in an additional replication cohort.
Significant metabolites
Analysis of postinduction CSF showed that three metabolites were significantly associated with fatigue in both the discovery and replication cohorts, comprising gamma-glutamylglutamine, dimethylglycine, and asparagine (P < .05).
In diagnostic CSF samples, the abundance of gamma-glutamylglutamine was significantly associated with fatigue (P =.0062).
The metabolites have been implicated in neurotransmitter transportation and glutathione recycling, suggesting glutamatergic pathways or oxidative stress may contribute to ALL-associated CRF, according to the researchers.
“Ultimately, this line of investigation may aid in the development of new prevention and treatment approaches informed by an improved understanding of the etiology and risk factors for cancer-related fatigue,” the researchers concluded.
The study was sponsored by the National Cancer Institute and several nonprofit organizations. The authors reported that they had no conflicts of interest.
SOURCE: Brown AL et al. J Pain Symptom Manage. 2020 Sep 1. doi: 10.1016/j.jpainsymman.2020.08.030.
Children and adolescents with cancer report significantly more fatigue than their counterparts without cancer, and cancer-related fatigue (CRF) is “one of the most prevalent and distressing symptoms reported during childhood cancer therapy,” according to Austin L. Brown, PhD, and his colleagues.
Cerebrospinal fluid (CSF) profiles suggest three metabolites are significantly associated with CRF in children with acute lymphoblastic leukemia (ALL), according to a report published in the Journal of Pain and Symptom Management.
The researchers assessed the clinical and demographic characteristics of 171 pediatric ALL patients, who were divided into discovery (n = 86) and replication (n = 85) cohorts.
The entire population had a mean age at diagnosis of 8.48 years; was 56.1% male; and 85.4% had B-lineage ALL. A total of 63.7% received high- or very-high-risk treatment.
CSF samples were obtained and subjected to metabolomic analysis, according to Dr. Brown, an assistant professor at the Baylor College of Medicine, Houston, and colleagues.
The researchers analyzed postinduction CSF from the aforementioned 171 patients as well as diagnostic CSF from 48 patients in an additional replication cohort.
Significant metabolites
Analysis of postinduction CSF showed that three metabolites were significantly associated with fatigue in both the discovery and replication cohorts, comprising gamma-glutamylglutamine, dimethylglycine, and asparagine (P < .05).
In diagnostic CSF samples, the abundance of gamma-glutamylglutamine was significantly associated with fatigue (P =.0062).
The metabolites have been implicated in neurotransmitter transportation and glutathione recycling, suggesting glutamatergic pathways or oxidative stress may contribute to ALL-associated CRF, according to the researchers.
“Ultimately, this line of investigation may aid in the development of new prevention and treatment approaches informed by an improved understanding of the etiology and risk factors for cancer-related fatigue,” the researchers concluded.
The study was sponsored by the National Cancer Institute and several nonprofit organizations. The authors reported that they had no conflicts of interest.
SOURCE: Brown AL et al. J Pain Symptom Manage. 2020 Sep 1. doi: 10.1016/j.jpainsymman.2020.08.030.
FROM THE JOURNAL OF PAIN AND SYMPTOM MANAGEMENT
Ten ways docs are cutting costs and saving money
“Some of our physician clients have seen their income decrease by as much as 50%,” says Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minnesota. “Many physicians had previously figured that whatever financial obligations they had wouldn’t be a problem because whatever amount they were making would continue, and if there were a decline it would be gradual.” However, assumption is now creating financial strain for many doctors.
Vikram Tarugu, MD, a gastroenterologist and CEO of Detox of South Florida in Okeechobee, Florida, says he has watched his budget for years, but has become even more careful with his spending in the past few months.
“It has helped me a lot to adjust to the new normal when it comes to the financial side of things,” Dr. Tarugu said. “Patients aren’t coming in as much as they used to, so my income has really been affected.”
Primary care physicians have seen a 55% decrease in revenue and a 20%-30% decrease in patient volume as a result of COVID-19. The impact has been even more severe for specialists. Even for physicians whose practices remain busy and whose family members haven’t lost their jobs or income, broader concerns about the economy may be reason enough for physicians to adopt cost-cutting measures.
In Medscape’s Physician Compensation Report 2020, we asked physicians to share their best cost-cutting tips. Many illustrate the lengths to which physicians are going to conserve money.
Here’s a look at some of the advice they shared, along with guidance from experts on how to make it work for you:
1. Create a written budget, even if you think it’s pointless.
Physicians said their most important piece of advice includes the following: “Use a formal budget to track progress,” “write out a budget,” “plan intermittent/large expenses in advance,” “Make sure all expenses are paid before you spend on leisure.”
Nearly 7 in 10 physicians say they have a budget for personal expenses, yet only one-quarter of those who do have a formal, written budget. Writing out a spending plan is key to being intentional about your spending, making sure that you’re living within your means, and identifying areas in which you may be able to cut back.
“Financial planning is all about cash flow, and everybody should know the amount of money coming in, how much is going out, and the difference between the two,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “That’s important in good times, but it’s even more important now when we see physicians taking pay cuts.”
Many physicians have found that budget apps or software programs are easier to work with than anticipated; some even walk you through the process of creating a budget. To get the most out of the apps, you’ll need to check them regularly and make changes based on their data.
“Sometimes there’s this false belief that just by signing up, you are automatically going to be better at budgeting,” says Scott Snider, CFP, a certified financial planner and partner with Mellen Money Management in Ponte Vedra Beach, Florida. “Basically, these apps are a great way to identify problem areas of spending. We have a tendency as humans to underestimate how much we spend on things like Starbucks, dining out, and Amazon shopping.”
One of the doctors’ tips that requires the most willpower is to “pay all expenses before spending on leisure.” That’s because we live in an instant gratification world, and want everything right away, Ms. Guerich said.
“I also think there’s an element of ‘keeping up with the Joneses’ and pressure associated with this profession,” she said. “The stereotype is that physicians are high-income earners so ‘We should be able to do that’ or ‘Mom and dad are doctors, so they can afford it.’ “
Creating and then revisiting your budget progress on a monthly or quarterly basis can give you a feeling of accomplishment and keep you motivated to stay with it.
Keep in mind that budgeting is a continual process rather than a singular event, and you’ll likely adjust it over time as your income and goals change.
2. Save more as you earn more.
Respondents to our Physician Compensation Report gave the following recommendations: “Pay yourself first,” “I put half of my bonus into an investment account no matter how much it is,” “I allocate extra money and put it into a savings account.”
Dr. Greenwald said, “I have a rule that every client needs to be saving 20% of their gross income toward retirement, including whatever the employer is putting in.”
Putting a portion of every paycheck into savings is key to making progress toward financial goals. Start by building an emergency fund with at least 3-6 months’ worth of expenses in it and making sure you’re saving at least enough for retirement to get any potential employer match.
Mr. Snider suggests increasing the percentage you save every time you get a raise.
“The thought behind that strategy is that when a doctor receives a pay raise – even if it’s just a cost-of-living raise of 3% – an extra 1% saved doesn’t reduce their take-home pay year-over-year,” he says. “In fact, they still take home more money, and they save more money. Win-win.”
3. Focus on paying down your debt.
Physicians told us how they were working to pay down debt with the following recommendations: “Accelerate debt reduction,” “I make additional principal payment to our home mortgage,” “We are aggressively attacking our remaining student loans.”
Reducing or eliminating debt is key to increasing cash flow, which can make it easier to meet all of your other financial goals. One-quarter of physicians have credit card debt, which typically carries interest rates higher than other types of debt, making it far more expensive. Focus on paying off such high-interest debt first, before moving on to other types of debt such as auto loans, student loans, or a mortgage.
“Credit card debt and any unsecured debt should be paid before anything else,” Mr. Snider says. “Getting rid of those high interest rates should be a priority. And that type of debt has less flexible terms than student debt.”
4. Great opportunity to take advantage of record-low interest rates.
Physicians said that, to save money, they are recommending the following: “Consolidating student debt into our mortgage,” “Accelerating payments of the principle on our mortgage,” “Making sure we have an affordable mortgage.”
With interest rates at an all-time low, even those who’ve recently refinanced might see significant savings by refinancing again. Given the associated fees, it typically makes sense to refinance if you can reduce your mortgage rate by at least a point, and you’re planning to stay in the home for at least 5 years.
“Depending on how much lower your rate is, refinancing can make a big difference in your monthly payments,” Ms. Guerich said. “For physicians who might need an emergency reserve but don’t have cash on hand, a HELOC [Home Equity Line of Credit] is a great way to accomplish that.”
5. Be wary of credit cards dangers; use cards wisely.
Physician respondents recommended the following: “Use 0% interest offers on credit cards,” “Only have one card and pay it off every month,” “Never carry over balance.”
Nearly 80% of physicians have three or more credit cards, with 18% reporting that they have seven or more. When used wisely, credit cards can be an important tool for managing finances. Many credit cards come with tools that can help with budgeting, and credit cards rewards and perks can offer real value to users. That said, rewards typically are not valuable enough to offset the cost of interest on balances (or the associated damage to your credit score) that aren’t paid off each month.
“If you’re paying a high rate on credit card balances that carry over every month, regardless of your income, that could be a symptom that you may be spending more than you should,” says Dan Keady, a CFP and chief financial planning strategist at financial services firm TIAA.
6. Give less to Uncle Sam: Keep it for yourself.
Physicians said that they do the following: “Maximize tax-free/deferred savings (401k, HSA, etc.),” “Give to charity to reduce tax,” “Use pre-tax dollars for childcare and healthcare.”
Not only does saving in workplace retirement accounts help you build your nest egg, but it also reduces the amount that you have to pay in taxes in a given year. Physicians should also take advantage of other ways to reduce their income for tax purposes, such as saving money in a health savings account or flexible savings account.
The 401(k) or 403(b) contribution limit for this year is $19,500 ($26,000) for those age 50 years and older. Self-employed physicians can save even more money via a Simplified Employee Pension (SEP) IRA, says Ms. Guerich said. They can save up to 25% of compensation, up to $57,000 in 2020.
7. Automate everything and spare yourself the headache.
Physicians said the following: “Designate money from your paycheck directly to tax deferred and taxable accounts automatically,” “Use automatic payment for credit card balance monthly,” “Automate your savings.”
You probably already automate your 401(k) contributions, but you can also automate bill payments, emergency savings contributions and other financial tasks. For busy physicians, this can make it easier to stick to your financial plan and achieve your goals.
“The older you get, the busier you get, said Mr. Snider says. “Automation can definitely help with that. But make sure you are checking in quarterly to make sure that everything is still in line with your plan. The problem with automation is when you forget about it completely and just let everything sit there.”
8. Save separately for big purchases.
Sometimes it’s the big major expenses that can start to derail a budget. Physicians told us the following tactics for large purchases: “We buy affordable cars and take budget vacations,” “I buy used cars,” “We save in advance for new cars and only buy cars with cash.”
The decision of which car to purchase or where to go on a family vacation is a personal one, and some physicians take great enjoyment and pride in driving a luxury vehicle or traveling to exotic locales. The key, experts say, is to factor the cost of that car into the rest of your budget, and make sure that it’s not preventing you from achieving other financial goals.
“I don’t like to judge or tell clients how they should spend their money,” said Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “Some people like cars, we have clients that have two planes, others want a second house or like to travel. Each person has their own interest where they may spend more relative to other people, but as long as they are meeting their savings targets, I encourage them to spend their money and enjoy what they enjoy most, guilt free.”
Mr. Snider suggests setting up a savings account separate from emergency or retirement accounts to set aside money if you have a goal for a large future purchase, such as a boat or a second home.
“That way, the funds don’t get commingled, and it’s explicitly clear whether or not the doctor is on target,” he says. “It also prevents them from treating their emergency savings account as an ATM machine.”
9. Start saving for college when the kids are little.
Respondents said the following: “We are buying less to save for the kids’ college education,” “We set up direct deposit into college and retirement savings plans,” “We have a 529 account for college savings.”
Helping pay for their children’s college education is an important financial goal for many physicians. The earlier that you start saving, the less you’ll have to save overall, thanks to compound interest. State 529 accounts are often a good place to start, especially if your state offers a tax incentive for doing so.
Mr. Snider recommends that physicians start small, with an initial investment of $1,000 per month and $100 per month contributions. Assuming a 7% rate of return and 17 years’ worth of savings, this would generate just over $42,000. (Note, current typical rates of return are less than 7%).
“Ideally, as other goals are accomplished and personal debt gets paid off, the doctor is ramping up their savings to have at least 50% of college expenses covered from their 529 college savings,” he says.
10. Watch out for the temptation of impulse purchases.
Physicians said the following: “Avoid impulse purchases,” “Avoid impulse shopping, make a list for the store and stick to it,” “Wait to buy things on sale.”
Nothing wrecks a budget like an impulse buy. More than half (54%) of U.S. shoppers have admitted to spending $100 or more on an impulse purchase. And 20% of shoppers have spent at least $1,000 on an impulse buy. Avoid buyers’ remorse by waiting a few days to make large purchase decisions or by limiting your unplanned spending to a certain dollar amount within your budget.
Online shopping may be a particular temptation. Dr. Tarugu, the Florida gastroenterologist, has focused on reducing those impulse buys as well, deleting all online shopping apps from his and his family’s phones.
“You won’t notice how much you have ordered online until it arrives at your doorstep,” he said. “It’s really important to keep it at bay.”
Mr. Keady, the TIAA chief planning strategist, recommended this tactic: Calculate the number of patients (or hours) you’d need to see in order to earn the cash required to make the purchase.
“Then, in a mindful way, figure out the amount of value derived from the purchase,” he said.
A version of this article originally appeared on Medscape.com.
“Some of our physician clients have seen their income decrease by as much as 50%,” says Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minnesota. “Many physicians had previously figured that whatever financial obligations they had wouldn’t be a problem because whatever amount they were making would continue, and if there were a decline it would be gradual.” However, assumption is now creating financial strain for many doctors.
Vikram Tarugu, MD, a gastroenterologist and CEO of Detox of South Florida in Okeechobee, Florida, says he has watched his budget for years, but has become even more careful with his spending in the past few months.
“It has helped me a lot to adjust to the new normal when it comes to the financial side of things,” Dr. Tarugu said. “Patients aren’t coming in as much as they used to, so my income has really been affected.”
Primary care physicians have seen a 55% decrease in revenue and a 20%-30% decrease in patient volume as a result of COVID-19. The impact has been even more severe for specialists. Even for physicians whose practices remain busy and whose family members haven’t lost their jobs or income, broader concerns about the economy may be reason enough for physicians to adopt cost-cutting measures.
In Medscape’s Physician Compensation Report 2020, we asked physicians to share their best cost-cutting tips. Many illustrate the lengths to which physicians are going to conserve money.
Here’s a look at some of the advice they shared, along with guidance from experts on how to make it work for you:
1. Create a written budget, even if you think it’s pointless.
Physicians said their most important piece of advice includes the following: “Use a formal budget to track progress,” “write out a budget,” “plan intermittent/large expenses in advance,” “Make sure all expenses are paid before you spend on leisure.”
Nearly 7 in 10 physicians say they have a budget for personal expenses, yet only one-quarter of those who do have a formal, written budget. Writing out a spending plan is key to being intentional about your spending, making sure that you’re living within your means, and identifying areas in which you may be able to cut back.
“Financial planning is all about cash flow, and everybody should know the amount of money coming in, how much is going out, and the difference between the two,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “That’s important in good times, but it’s even more important now when we see physicians taking pay cuts.”
Many physicians have found that budget apps or software programs are easier to work with than anticipated; some even walk you through the process of creating a budget. To get the most out of the apps, you’ll need to check them regularly and make changes based on their data.
“Sometimes there’s this false belief that just by signing up, you are automatically going to be better at budgeting,” says Scott Snider, CFP, a certified financial planner and partner with Mellen Money Management in Ponte Vedra Beach, Florida. “Basically, these apps are a great way to identify problem areas of spending. We have a tendency as humans to underestimate how much we spend on things like Starbucks, dining out, and Amazon shopping.”
One of the doctors’ tips that requires the most willpower is to “pay all expenses before spending on leisure.” That’s because we live in an instant gratification world, and want everything right away, Ms. Guerich said.
“I also think there’s an element of ‘keeping up with the Joneses’ and pressure associated with this profession,” she said. “The stereotype is that physicians are high-income earners so ‘We should be able to do that’ or ‘Mom and dad are doctors, so they can afford it.’ “
Creating and then revisiting your budget progress on a monthly or quarterly basis can give you a feeling of accomplishment and keep you motivated to stay with it.
Keep in mind that budgeting is a continual process rather than a singular event, and you’ll likely adjust it over time as your income and goals change.
2. Save more as you earn more.
Respondents to our Physician Compensation Report gave the following recommendations: “Pay yourself first,” “I put half of my bonus into an investment account no matter how much it is,” “I allocate extra money and put it into a savings account.”
Dr. Greenwald said, “I have a rule that every client needs to be saving 20% of their gross income toward retirement, including whatever the employer is putting in.”
Putting a portion of every paycheck into savings is key to making progress toward financial goals. Start by building an emergency fund with at least 3-6 months’ worth of expenses in it and making sure you’re saving at least enough for retirement to get any potential employer match.
Mr. Snider suggests increasing the percentage you save every time you get a raise.
“The thought behind that strategy is that when a doctor receives a pay raise – even if it’s just a cost-of-living raise of 3% – an extra 1% saved doesn’t reduce their take-home pay year-over-year,” he says. “In fact, they still take home more money, and they save more money. Win-win.”
3. Focus on paying down your debt.
Physicians told us how they were working to pay down debt with the following recommendations: “Accelerate debt reduction,” “I make additional principal payment to our home mortgage,” “We are aggressively attacking our remaining student loans.”
Reducing or eliminating debt is key to increasing cash flow, which can make it easier to meet all of your other financial goals. One-quarter of physicians have credit card debt, which typically carries interest rates higher than other types of debt, making it far more expensive. Focus on paying off such high-interest debt first, before moving on to other types of debt such as auto loans, student loans, or a mortgage.
“Credit card debt and any unsecured debt should be paid before anything else,” Mr. Snider says. “Getting rid of those high interest rates should be a priority. And that type of debt has less flexible terms than student debt.”
4. Great opportunity to take advantage of record-low interest rates.
Physicians said that, to save money, they are recommending the following: “Consolidating student debt into our mortgage,” “Accelerating payments of the principle on our mortgage,” “Making sure we have an affordable mortgage.”
With interest rates at an all-time low, even those who’ve recently refinanced might see significant savings by refinancing again. Given the associated fees, it typically makes sense to refinance if you can reduce your mortgage rate by at least a point, and you’re planning to stay in the home for at least 5 years.
“Depending on how much lower your rate is, refinancing can make a big difference in your monthly payments,” Ms. Guerich said. “For physicians who might need an emergency reserve but don’t have cash on hand, a HELOC [Home Equity Line of Credit] is a great way to accomplish that.”
5. Be wary of credit cards dangers; use cards wisely.
Physician respondents recommended the following: “Use 0% interest offers on credit cards,” “Only have one card and pay it off every month,” “Never carry over balance.”
Nearly 80% of physicians have three or more credit cards, with 18% reporting that they have seven or more. When used wisely, credit cards can be an important tool for managing finances. Many credit cards come with tools that can help with budgeting, and credit cards rewards and perks can offer real value to users. That said, rewards typically are not valuable enough to offset the cost of interest on balances (or the associated damage to your credit score) that aren’t paid off each month.
“If you’re paying a high rate on credit card balances that carry over every month, regardless of your income, that could be a symptom that you may be spending more than you should,” says Dan Keady, a CFP and chief financial planning strategist at financial services firm TIAA.
6. Give less to Uncle Sam: Keep it for yourself.
Physicians said that they do the following: “Maximize tax-free/deferred savings (401k, HSA, etc.),” “Give to charity to reduce tax,” “Use pre-tax dollars for childcare and healthcare.”
Not only does saving in workplace retirement accounts help you build your nest egg, but it also reduces the amount that you have to pay in taxes in a given year. Physicians should also take advantage of other ways to reduce their income for tax purposes, such as saving money in a health savings account or flexible savings account.
The 401(k) or 403(b) contribution limit for this year is $19,500 ($26,000) for those age 50 years and older. Self-employed physicians can save even more money via a Simplified Employee Pension (SEP) IRA, says Ms. Guerich said. They can save up to 25% of compensation, up to $57,000 in 2020.
7. Automate everything and spare yourself the headache.
Physicians said the following: “Designate money from your paycheck directly to tax deferred and taxable accounts automatically,” “Use automatic payment for credit card balance monthly,” “Automate your savings.”
You probably already automate your 401(k) contributions, but you can also automate bill payments, emergency savings contributions and other financial tasks. For busy physicians, this can make it easier to stick to your financial plan and achieve your goals.
“The older you get, the busier you get, said Mr. Snider says. “Automation can definitely help with that. But make sure you are checking in quarterly to make sure that everything is still in line with your plan. The problem with automation is when you forget about it completely and just let everything sit there.”
8. Save separately for big purchases.
Sometimes it’s the big major expenses that can start to derail a budget. Physicians told us the following tactics for large purchases: “We buy affordable cars and take budget vacations,” “I buy used cars,” “We save in advance for new cars and only buy cars with cash.”
The decision of which car to purchase or where to go on a family vacation is a personal one, and some physicians take great enjoyment and pride in driving a luxury vehicle or traveling to exotic locales. The key, experts say, is to factor the cost of that car into the rest of your budget, and make sure that it’s not preventing you from achieving other financial goals.
“I don’t like to judge or tell clients how they should spend their money,” said Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “Some people like cars, we have clients that have two planes, others want a second house or like to travel. Each person has their own interest where they may spend more relative to other people, but as long as they are meeting their savings targets, I encourage them to spend their money and enjoy what they enjoy most, guilt free.”
Mr. Snider suggests setting up a savings account separate from emergency or retirement accounts to set aside money if you have a goal for a large future purchase, such as a boat or a second home.
“That way, the funds don’t get commingled, and it’s explicitly clear whether or not the doctor is on target,” he says. “It also prevents them from treating their emergency savings account as an ATM machine.”
9. Start saving for college when the kids are little.
Respondents said the following: “We are buying less to save for the kids’ college education,” “We set up direct deposit into college and retirement savings plans,” “We have a 529 account for college savings.”
Helping pay for their children’s college education is an important financial goal for many physicians. The earlier that you start saving, the less you’ll have to save overall, thanks to compound interest. State 529 accounts are often a good place to start, especially if your state offers a tax incentive for doing so.
Mr. Snider recommends that physicians start small, with an initial investment of $1,000 per month and $100 per month contributions. Assuming a 7% rate of return and 17 years’ worth of savings, this would generate just over $42,000. (Note, current typical rates of return are less than 7%).
“Ideally, as other goals are accomplished and personal debt gets paid off, the doctor is ramping up their savings to have at least 50% of college expenses covered from their 529 college savings,” he says.
10. Watch out for the temptation of impulse purchases.
Physicians said the following: “Avoid impulse purchases,” “Avoid impulse shopping, make a list for the store and stick to it,” “Wait to buy things on sale.”
Nothing wrecks a budget like an impulse buy. More than half (54%) of U.S. shoppers have admitted to spending $100 or more on an impulse purchase. And 20% of shoppers have spent at least $1,000 on an impulse buy. Avoid buyers’ remorse by waiting a few days to make large purchase decisions or by limiting your unplanned spending to a certain dollar amount within your budget.
Online shopping may be a particular temptation. Dr. Tarugu, the Florida gastroenterologist, has focused on reducing those impulse buys as well, deleting all online shopping apps from his and his family’s phones.
“You won’t notice how much you have ordered online until it arrives at your doorstep,” he said. “It’s really important to keep it at bay.”
Mr. Keady, the TIAA chief planning strategist, recommended this tactic: Calculate the number of patients (or hours) you’d need to see in order to earn the cash required to make the purchase.
“Then, in a mindful way, figure out the amount of value derived from the purchase,” he said.
A version of this article originally appeared on Medscape.com.
“Some of our physician clients have seen their income decrease by as much as 50%,” says Joel Greenwald, MD, CEO of Greenwald Wealth Management in St. Louis Park, Minnesota. “Many physicians had previously figured that whatever financial obligations they had wouldn’t be a problem because whatever amount they were making would continue, and if there were a decline it would be gradual.” However, assumption is now creating financial strain for many doctors.
Vikram Tarugu, MD, a gastroenterologist and CEO of Detox of South Florida in Okeechobee, Florida, says he has watched his budget for years, but has become even more careful with his spending in the past few months.
“It has helped me a lot to adjust to the new normal when it comes to the financial side of things,” Dr. Tarugu said. “Patients aren’t coming in as much as they used to, so my income has really been affected.”
Primary care physicians have seen a 55% decrease in revenue and a 20%-30% decrease in patient volume as a result of COVID-19. The impact has been even more severe for specialists. Even for physicians whose practices remain busy and whose family members haven’t lost their jobs or income, broader concerns about the economy may be reason enough for physicians to adopt cost-cutting measures.
In Medscape’s Physician Compensation Report 2020, we asked physicians to share their best cost-cutting tips. Many illustrate the lengths to which physicians are going to conserve money.
Here’s a look at some of the advice they shared, along with guidance from experts on how to make it work for you:
1. Create a written budget, even if you think it’s pointless.
Physicians said their most important piece of advice includes the following: “Use a formal budget to track progress,” “write out a budget,” “plan intermittent/large expenses in advance,” “Make sure all expenses are paid before you spend on leisure.”
Nearly 7 in 10 physicians say they have a budget for personal expenses, yet only one-quarter of those who do have a formal, written budget. Writing out a spending plan is key to being intentional about your spending, making sure that you’re living within your means, and identifying areas in which you may be able to cut back.
“Financial planning is all about cash flow, and everybody should know the amount of money coming in, how much is going out, and the difference between the two,” says Amy Guerich, a partner with Stepp & Rothwell, a Kansas City–based financial planning firm. “That’s important in good times, but it’s even more important now when we see physicians taking pay cuts.”
Many physicians have found that budget apps or software programs are easier to work with than anticipated; some even walk you through the process of creating a budget. To get the most out of the apps, you’ll need to check them regularly and make changes based on their data.
“Sometimes there’s this false belief that just by signing up, you are automatically going to be better at budgeting,” says Scott Snider, CFP, a certified financial planner and partner with Mellen Money Management in Ponte Vedra Beach, Florida. “Basically, these apps are a great way to identify problem areas of spending. We have a tendency as humans to underestimate how much we spend on things like Starbucks, dining out, and Amazon shopping.”
One of the doctors’ tips that requires the most willpower is to “pay all expenses before spending on leisure.” That’s because we live in an instant gratification world, and want everything right away, Ms. Guerich said.
“I also think there’s an element of ‘keeping up with the Joneses’ and pressure associated with this profession,” she said. “The stereotype is that physicians are high-income earners so ‘We should be able to do that’ or ‘Mom and dad are doctors, so they can afford it.’ “
Creating and then revisiting your budget progress on a monthly or quarterly basis can give you a feeling of accomplishment and keep you motivated to stay with it.
Keep in mind that budgeting is a continual process rather than a singular event, and you’ll likely adjust it over time as your income and goals change.
2. Save more as you earn more.
Respondents to our Physician Compensation Report gave the following recommendations: “Pay yourself first,” “I put half of my bonus into an investment account no matter how much it is,” “I allocate extra money and put it into a savings account.”
Dr. Greenwald said, “I have a rule that every client needs to be saving 20% of their gross income toward retirement, including whatever the employer is putting in.”
Putting a portion of every paycheck into savings is key to making progress toward financial goals. Start by building an emergency fund with at least 3-6 months’ worth of expenses in it and making sure you’re saving at least enough for retirement to get any potential employer match.
Mr. Snider suggests increasing the percentage you save every time you get a raise.
“The thought behind that strategy is that when a doctor receives a pay raise – even if it’s just a cost-of-living raise of 3% – an extra 1% saved doesn’t reduce their take-home pay year-over-year,” he says. “In fact, they still take home more money, and they save more money. Win-win.”
3. Focus on paying down your debt.
Physicians told us how they were working to pay down debt with the following recommendations: “Accelerate debt reduction,” “I make additional principal payment to our home mortgage,” “We are aggressively attacking our remaining student loans.”
Reducing or eliminating debt is key to increasing cash flow, which can make it easier to meet all of your other financial goals. One-quarter of physicians have credit card debt, which typically carries interest rates higher than other types of debt, making it far more expensive. Focus on paying off such high-interest debt first, before moving on to other types of debt such as auto loans, student loans, or a mortgage.
“Credit card debt and any unsecured debt should be paid before anything else,” Mr. Snider says. “Getting rid of those high interest rates should be a priority. And that type of debt has less flexible terms than student debt.”
4. Great opportunity to take advantage of record-low interest rates.
Physicians said that, to save money, they are recommending the following: “Consolidating student debt into our mortgage,” “Accelerating payments of the principle on our mortgage,” “Making sure we have an affordable mortgage.”
With interest rates at an all-time low, even those who’ve recently refinanced might see significant savings by refinancing again. Given the associated fees, it typically makes sense to refinance if you can reduce your mortgage rate by at least a point, and you’re planning to stay in the home for at least 5 years.
“Depending on how much lower your rate is, refinancing can make a big difference in your monthly payments,” Ms. Guerich said. “For physicians who might need an emergency reserve but don’t have cash on hand, a HELOC [Home Equity Line of Credit] is a great way to accomplish that.”
5. Be wary of credit cards dangers; use cards wisely.
Physician respondents recommended the following: “Use 0% interest offers on credit cards,” “Only have one card and pay it off every month,” “Never carry over balance.”
Nearly 80% of physicians have three or more credit cards, with 18% reporting that they have seven or more. When used wisely, credit cards can be an important tool for managing finances. Many credit cards come with tools that can help with budgeting, and credit cards rewards and perks can offer real value to users. That said, rewards typically are not valuable enough to offset the cost of interest on balances (or the associated damage to your credit score) that aren’t paid off each month.
“If you’re paying a high rate on credit card balances that carry over every month, regardless of your income, that could be a symptom that you may be spending more than you should,” says Dan Keady, a CFP and chief financial planning strategist at financial services firm TIAA.
6. Give less to Uncle Sam: Keep it for yourself.
Physicians said that they do the following: “Maximize tax-free/deferred savings (401k, HSA, etc.),” “Give to charity to reduce tax,” “Use pre-tax dollars for childcare and healthcare.”
Not only does saving in workplace retirement accounts help you build your nest egg, but it also reduces the amount that you have to pay in taxes in a given year. Physicians should also take advantage of other ways to reduce their income for tax purposes, such as saving money in a health savings account or flexible savings account.
The 401(k) or 403(b) contribution limit for this year is $19,500 ($26,000) for those age 50 years and older. Self-employed physicians can save even more money via a Simplified Employee Pension (SEP) IRA, says Ms. Guerich said. They can save up to 25% of compensation, up to $57,000 in 2020.
7. Automate everything and spare yourself the headache.
Physicians said the following: “Designate money from your paycheck directly to tax deferred and taxable accounts automatically,” “Use automatic payment for credit card balance monthly,” “Automate your savings.”
You probably already automate your 401(k) contributions, but you can also automate bill payments, emergency savings contributions and other financial tasks. For busy physicians, this can make it easier to stick to your financial plan and achieve your goals.
“The older you get, the busier you get, said Mr. Snider says. “Automation can definitely help with that. But make sure you are checking in quarterly to make sure that everything is still in line with your plan. The problem with automation is when you forget about it completely and just let everything sit there.”
8. Save separately for big purchases.
Sometimes it’s the big major expenses that can start to derail a budget. Physicians told us the following tactics for large purchases: “We buy affordable cars and take budget vacations,” “I buy used cars,” “We save in advance for new cars and only buy cars with cash.”
The decision of which car to purchase or where to go on a family vacation is a personal one, and some physicians take great enjoyment and pride in driving a luxury vehicle or traveling to exotic locales. The key, experts say, is to factor the cost of that car into the rest of your budget, and make sure that it’s not preventing you from achieving other financial goals.
“I don’t like to judge or tell clients how they should spend their money,” said Andrew Musbach, a certified financial planner and cofounder of MD Wealth Management in Chelsea, Mich. “Some people like cars, we have clients that have two planes, others want a second house or like to travel. Each person has their own interest where they may spend more relative to other people, but as long as they are meeting their savings targets, I encourage them to spend their money and enjoy what they enjoy most, guilt free.”
Mr. Snider suggests setting up a savings account separate from emergency or retirement accounts to set aside money if you have a goal for a large future purchase, such as a boat or a second home.
“That way, the funds don’t get commingled, and it’s explicitly clear whether or not the doctor is on target,” he says. “It also prevents them from treating their emergency savings account as an ATM machine.”
9. Start saving for college when the kids are little.
Respondents said the following: “We are buying less to save for the kids’ college education,” “We set up direct deposit into college and retirement savings plans,” “We have a 529 account for college savings.”
Helping pay for their children’s college education is an important financial goal for many physicians. The earlier that you start saving, the less you’ll have to save overall, thanks to compound interest. State 529 accounts are often a good place to start, especially if your state offers a tax incentive for doing so.
Mr. Snider recommends that physicians start small, with an initial investment of $1,000 per month and $100 per month contributions. Assuming a 7% rate of return and 17 years’ worth of savings, this would generate just over $42,000. (Note, current typical rates of return are less than 7%).
“Ideally, as other goals are accomplished and personal debt gets paid off, the doctor is ramping up their savings to have at least 50% of college expenses covered from their 529 college savings,” he says.
10. Watch out for the temptation of impulse purchases.
Physicians said the following: “Avoid impulse purchases,” “Avoid impulse shopping, make a list for the store and stick to it,” “Wait to buy things on sale.”
Nothing wrecks a budget like an impulse buy. More than half (54%) of U.S. shoppers have admitted to spending $100 or more on an impulse purchase. And 20% of shoppers have spent at least $1,000 on an impulse buy. Avoid buyers’ remorse by waiting a few days to make large purchase decisions or by limiting your unplanned spending to a certain dollar amount within your budget.
Online shopping may be a particular temptation. Dr. Tarugu, the Florida gastroenterologist, has focused on reducing those impulse buys as well, deleting all online shopping apps from his and his family’s phones.
“You won’t notice how much you have ordered online until it arrives at your doorstep,” he said. “It’s really important to keep it at bay.”
Mr. Keady, the TIAA chief planning strategist, recommended this tactic: Calculate the number of patients (or hours) you’d need to see in order to earn the cash required to make the purchase.
“Then, in a mindful way, figure out the amount of value derived from the purchase,” he said.
A version of this article originally appeared on Medscape.com.
Many advanced countries missing targets for HCV elimination
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Eleven high-income countries are on track to meet World Health Organization targets to eliminate hepatitis C infection by 2030, compared with 9 countries 2 years ago, researchers reported. But 28 countries, including the United States, are not expected to eliminate HCV until 2050.
“In the countries making progress, the common elements are political will, a clear national plan, and easing of restrictions on the cascade of care and testing,” Yuri Sanchez Gonzalez, PhD, director of health economics and outcomes research for biopharmaceutical company AbbVie said in an interview. That would include offering hepatitis C treatment to individuals who have liver fibrosis and those struggling with sobriety, he said. “We can’t overstate how much this is a massive driver of the hepatitis C epidemic.”
His research, presented at the digital edition of the International Liver Congress this week, showed more countries on target than in a study published 2 years ago in Liver International . “But it’s not enough,” Dr. Sanchez Gonzalez said. “We know that more than 80% of infections are in people who inject drugs. Stigmatization of drug use is still a very major issue.” Despite data clearly showing that countries who have harm-reduction programs make progress, “in many countries these programs are still illegal.”
To evaluate which countries are on target to eliminate hepatitis C by 2030, researchers performed Markov disease progression models of HCV infection in 45 high-income countries. The results showed that Australia, Canada, France, Germany, Iceland, Italy, Japan, Spain, Sweden, Switzerland, and the United Kingdom are “in the green” (on target for 2030).
Austria, Malta, the Netherlands, New Zealand, and South Korea are “in the yellow” (on target for 2040), and 28 remaining countries, including the United States, are “in the red,” with targets estimated to be met by 2050.
Compared with an analysis performed 2 years ago, South Korea moved from green to yellow, while Canada, Germany, and Sweden moved from red to green.
Researchers say that the countries moving the needle are the ones addressing barriers to care.
EASL: Eliminate barriers to treatment
During this week’s Congress, the European Association for the Study of the Liver (EASL) launched a policy statement recommending breaking down all barriers that prevent people who inject drugs from getting access to hepatitis C treatment, including encouragement of laws and policies that “decriminalize drug use, drug possession and drug users themselves,” said statement coauthor Mojca Maticic, MD, PhD, University of Ljubljana, Slovenia.
“To reach the desired WHO goal, combining decriminalization of personal drug consumption and integrated interventions that include hepatitis C testing and treatment should be implemented,” she added. We need to adopt “an approach based on public health promotion, respect for human rights, and evidence.”
Although harm reduction is the top strategy for making 2030 targets, having precision data also helps a lot.
“High-quality data and harm-reduction innovation to curb the overdose crisis has moved us out of the red and into the green,” Canadian researcher Jordan Feld, MD, MPH, University of Toronto, said in an interview. He points to British Columbia, Canada’s third-most populous province, putting harm reduction programs in place as key to Canadian progress.
“Given the increasing opioid epidemic, you’re creating yourself a bigger problem if you don’t treat this population,” Dr. Feld said. When a person needs 6 months to get sober in order to be treated for HCV, that’s more potential time to pass the infection to others. His study, also presented at ILC this week, outlines anticipated timing of hepatitis C in Canada’s four most populous provinces (Ontario, Quebec, British Columbia, and Alberta), and shows British Columbia will reach targets by 2028.
Lifting all restrictions clearly helps, Dr. Sanchez Gonzalez reported. He pointed to Sweden as a good example, a country that recently lifted HCV treatment restrictions for individuals living with fibrosis. Sweden moved from a red to a green spot in this analysis and is now on target for 2030.
“As long as everyone who needs treatment gets treatment, you can make tremendous progress,” he said.
Keeping track is also essential to moving the needle. Since the WHO has no enforcement power, “these studies, which offer a report card of progress, really matter,” Dr. Sanchez Gonzalez explained. When a country knows where they stand, they are more likely to take action to change. “Nobody likes to be shown in the red.”
Still, “it’s not a shaming exercise,” he said. It’s about starting a conversation, showing who’s on track, and sharing how to get on track. “Knowing that there is something in your power to move the needle toward elimination by learning from your neighbors is powerful – often, it just takes political will.”
Dr. Feld has received consulting fees from AbbVie. Dr. Sanchez Gonzalez is on staff as the Director of Economics at AbbVie. Dr. Maticic has disclosed no relevant financial relationships.
A version of this article originally appeared on Medscape.com.
Chronic abdominal pain and diarrhea
A 15-year-old girl was brought to the Family Medicine Clinic in Somaliland, Africa, for evaluation of intermittent abdominal pain and watery diarrhea of 12 years’ duration. Over the previous 2 months, her symptoms had worsened and included vomiting and weight loss. She denied fever, melena, or hematemesis.
Physical examination revealed a thin female with a normal abdominal exam and numerous hyperpigmented macules on the lips, buccal mucosa, fingers, and toes (FIGURE 1). Her family reported that the black spots on her lips had been there since birth. There was no known family history of similar symptoms or black spots.

Her hemoglobin was 10 g/dL (reference range, 12–15 g/dL). A probable diagnosis was discussed with the family, and they elected to travel to India for further evaluation due to limited diagnostic resources in their location. In India, computed tomography (CT) and ultrasonography showed duodenojejunal intussusception. Upper gastrointestinal (GI) endoscopy revealed multiple polyps from the lower stomach to the jejunum of the small bowel; colonoscopy was normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Peutz-Jeghers syndrome
Our patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in the stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
What you’ll see. The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Intussusception is the most frequent cause of morbidity in childhood for PJS patients.3,4 Recurrent attacks of abdominal pain likely result from recurring transient episodes of incomplete intussusception. The polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1 Most cancers associated with PJS occur during adulthood.2
Other possible causes of hyperpigmentation
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing.
Laugier-Hunziker syndrome manifests with macular hyperpigmentation of the lips and buccal mucosa and pigmented bands on the nails in young or middle-aged adults. It is not associated with intestinal polyps.
Continue to: Cronkhite-Canada syndrome
Cronkhite-Canada syndrome consists of acral and oral pigmented macules and GI polyps as well as generalized darkening of the skin, extensive alopecia, loss of taste, and nail dystrophy.
Familial lentiginosis syndromes such as Noonan syndrome and NAME syndrome (nevi, atrial myxoma, myxoid neurofibroma, ephelides) have other systemic signs such as cardiac abnormalities, and the pigmentation is not as clearly perioral.
Albright syndrome manifests with oral pigmented macules but also is associated with precocious puberty and polyostotic fibrous dysplasia.
Addison disease may cause multiple hyperpigmented macules but has other systemic involvement; adrenocorticotropic hormone levels are elevated.
Juvenile polyposis syndrome manifests with GI polyps but is not associated with mucosal pigmentation.
Continue to: Use these 4 criteria to make the diagnosis
Use these 4 criteria to make the diagnosis
The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
When PJS is suspected, the entire GI tract should be investigated. The hamartomatous polyps may be found from the stomach to the anal canal, but the small bowel most commonly is involved. The polyps may occur in early childhood, with one study of 14 children reporting a median age of 4.5 years.5 Polyp biopsy will show smooth muscle arborization. When possible, those who meet clinical criteria for PJS should undergo genetic testing for a STK11 gene mutation. PJS may occur due to de novo mutations in patients with no family history.6
Long-term management involves surveillance for polyps and cancer
Screening guidelines for polyps vary. Some suggest starting screening at age 8 to 10 years with esophagogastroduodenoscopy or capsule endoscopy and if negative, colonoscopy at age 18. Others suggest starting screening at 4 to 5 years of age.5 The recommendation is to remove polyps if technically feasible.3 Surveillance for Sertoli cell tumors (sex cord stromal tumors) should be done before puberty, and evaluation of other organs at risk of malignancy should begin by the end of adolescence.
The pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
Our patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. We recommended follow-up in her home city with primary care and a GI specialist and explained the need for surveillance of her condition.
CORRESPONDENCE
Josette R. McMichael, MD, Department of Dermatology, Emory University, 1525 Clifton Road NE, 1st Floor, Atlanta, GA 30322; [email protected]
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
4. Vidal I, Podevin G, Piloquet H, et al. Follow-up and surgical management of Peutz-Jeghers syndrome in children. J Pediatr Gastroenterol Nutr. 2009;48:419-425.
5. Goldstein SA, Hoffenberg EJ. Peutz-Jegher syndrome in childhood: need for updated recommendations? J Pediatr Gastroenterol Nutr. 2013;56:191-195.
6. Hernan I, Roig I, Martin B, et al. De novo germline mutation in the serine-threonine kinase STK11/LKB1 gene associated with Peutz-Jeghers syndrome. Clin Genet. 2004;66:58-62.
A 15-year-old girl was brought to the Family Medicine Clinic in Somaliland, Africa, for evaluation of intermittent abdominal pain and watery diarrhea of 12 years’ duration. Over the previous 2 months, her symptoms had worsened and included vomiting and weight loss. She denied fever, melena, or hematemesis.
Physical examination revealed a thin female with a normal abdominal exam and numerous hyperpigmented macules on the lips, buccal mucosa, fingers, and toes (FIGURE 1). Her family reported that the black spots on her lips had been there since birth. There was no known family history of similar symptoms or black spots.

Her hemoglobin was 10 g/dL (reference range, 12–15 g/dL). A probable diagnosis was discussed with the family, and they elected to travel to India for further evaluation due to limited diagnostic resources in their location. In India, computed tomography (CT) and ultrasonography showed duodenojejunal intussusception. Upper gastrointestinal (GI) endoscopy revealed multiple polyps from the lower stomach to the jejunum of the small bowel; colonoscopy was normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Peutz-Jeghers syndrome
Our patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in the stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
What you’ll see. The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Intussusception is the most frequent cause of morbidity in childhood for PJS patients.3,4 Recurrent attacks of abdominal pain likely result from recurring transient episodes of incomplete intussusception. The polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1 Most cancers associated with PJS occur during adulthood.2
Other possible causes of hyperpigmentation
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing.
Laugier-Hunziker syndrome manifests with macular hyperpigmentation of the lips and buccal mucosa and pigmented bands on the nails in young or middle-aged adults. It is not associated with intestinal polyps.
Continue to: Cronkhite-Canada syndrome
Cronkhite-Canada syndrome consists of acral and oral pigmented macules and GI polyps as well as generalized darkening of the skin, extensive alopecia, loss of taste, and nail dystrophy.
Familial lentiginosis syndromes such as Noonan syndrome and NAME syndrome (nevi, atrial myxoma, myxoid neurofibroma, ephelides) have other systemic signs such as cardiac abnormalities, and the pigmentation is not as clearly perioral.
Albright syndrome manifests with oral pigmented macules but also is associated with precocious puberty and polyostotic fibrous dysplasia.
Addison disease may cause multiple hyperpigmented macules but has other systemic involvement; adrenocorticotropic hormone levels are elevated.
Juvenile polyposis syndrome manifests with GI polyps but is not associated with mucosal pigmentation.
Continue to: Use these 4 criteria to make the diagnosis
Use these 4 criteria to make the diagnosis
The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
When PJS is suspected, the entire GI tract should be investigated. The hamartomatous polyps may be found from the stomach to the anal canal, but the small bowel most commonly is involved. The polyps may occur in early childhood, with one study of 14 children reporting a median age of 4.5 years.5 Polyp biopsy will show smooth muscle arborization. When possible, those who meet clinical criteria for PJS should undergo genetic testing for a STK11 gene mutation. PJS may occur due to de novo mutations in patients with no family history.6
Long-term management involves surveillance for polyps and cancer
Screening guidelines for polyps vary. Some suggest starting screening at age 8 to 10 years with esophagogastroduodenoscopy or capsule endoscopy and if negative, colonoscopy at age 18. Others suggest starting screening at 4 to 5 years of age.5 The recommendation is to remove polyps if technically feasible.3 Surveillance for Sertoli cell tumors (sex cord stromal tumors) should be done before puberty, and evaluation of other organs at risk of malignancy should begin by the end of adolescence.
The pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
Our patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. We recommended follow-up in her home city with primary care and a GI specialist and explained the need for surveillance of her condition.
CORRESPONDENCE
Josette R. McMichael, MD, Department of Dermatology, Emory University, 1525 Clifton Road NE, 1st Floor, Atlanta, GA 30322; [email protected]
A 15-year-old girl was brought to the Family Medicine Clinic in Somaliland, Africa, for evaluation of intermittent abdominal pain and watery diarrhea of 12 years’ duration. Over the previous 2 months, her symptoms had worsened and included vomiting and weight loss. She denied fever, melena, or hematemesis.
Physical examination revealed a thin female with a normal abdominal exam and numerous hyperpigmented macules on the lips, buccal mucosa, fingers, and toes (FIGURE 1). Her family reported that the black spots on her lips had been there since birth. There was no known family history of similar symptoms or black spots.

Her hemoglobin was 10 g/dL (reference range, 12–15 g/dL). A probable diagnosis was discussed with the family, and they elected to travel to India for further evaluation due to limited diagnostic resources in their location. In India, computed tomography (CT) and ultrasonography showed duodenojejunal intussusception. Upper gastrointestinal (GI) endoscopy revealed multiple polyps from the lower stomach to the jejunum of the small bowel; colonoscopy was normal.
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Peutz-Jeghers syndrome
Our patient was given a diagnosis of Peutz-Jeghers syndrome (PJS) based on the characteristic pigmented mucocutaneous macules and numerous polyps in the stomach and small bowel. PJS is an autosomal dominant syndrome characterized by mucocutaneous pigmentation, polyposis of the GI tract, and increased cancer risk. The prevalence is approximately 1 in 100,000.1 Genetic testing for the STK11 gene mutation, which is found in 70% of familial cases and 30% to 67% of sporadic cases, is not required for diagnosis.1
What you’ll see. The bluish brown to black spots of PJS often are apparent at birth or in early infancy. They are most common on the lips, buccal mucosa, perioral region, palms, and soles.
The polyps may cause bleeding, anemia, and abdominal pain due to intussusception, obstruction, or infarction.2 Intussusception is the most frequent cause of morbidity in childhood for PJS patients.3,4 Recurrent attacks of abdominal pain likely result from recurring transient episodes of incomplete intussusception. The polyps usually are benign, but patients are at increased risk of GI and non-GI malignancies such as breast, pancreas, lung, and reproductive tract cancers.1 Most cancers associated with PJS occur during adulthood.2
Other possible causes of hyperpigmentation
PJS can be differentiated from other causes of hyperpigmentation by clinical presentation and/or genetic testing.
Laugier-Hunziker syndrome manifests with macular hyperpigmentation of the lips and buccal mucosa and pigmented bands on the nails in young or middle-aged adults. It is not associated with intestinal polyps.
Continue to: Cronkhite-Canada syndrome
Cronkhite-Canada syndrome consists of acral and oral pigmented macules and GI polyps as well as generalized darkening of the skin, extensive alopecia, loss of taste, and nail dystrophy.
Familial lentiginosis syndromes such as Noonan syndrome and NAME syndrome (nevi, atrial myxoma, myxoid neurofibroma, ephelides) have other systemic signs such as cardiac abnormalities, and the pigmentation is not as clearly perioral.
Albright syndrome manifests with oral pigmented macules but also is associated with precocious puberty and polyostotic fibrous dysplasia.
Addison disease may cause multiple hyperpigmented macules but has other systemic involvement; adrenocorticotropic hormone levels are elevated.
Juvenile polyposis syndrome manifests with GI polyps but is not associated with mucosal pigmentation.
Continue to: Use these 4 criteria to make the diagnosis
Use these 4 criteria to make the diagnosis
The diagnosis of PJS is made using the following criteria: (1) two or more histologically confirmed PJS polyps, (2) any number of PJS polyps and a family history of PJS, (3) characteristic mucocutaneous pigmentation and a family history of PJS, or (4) any number of PJS polyps and characteristic mucocutaneous pigmentation.2
When PJS is suspected, the entire GI tract should be investigated. The hamartomatous polyps may be found from the stomach to the anal canal, but the small bowel most commonly is involved. The polyps may occur in early childhood, with one study of 14 children reporting a median age of 4.5 years.5 Polyp biopsy will show smooth muscle arborization. When possible, those who meet clinical criteria for PJS should undergo genetic testing for a STK11 gene mutation. PJS may occur due to de novo mutations in patients with no family history.6
Long-term management involves surveillance for polyps and cancer
Screening guidelines for polyps vary. Some suggest starting screening at age 8 to 10 years with esophagogastroduodenoscopy or capsule endoscopy and if negative, colonoscopy at age 18. Others suggest starting screening at 4 to 5 years of age.5 The recommendation is to remove polyps if technically feasible.3 Surveillance for Sertoli cell tumors (sex cord stromal tumors) should be done before puberty, and evaluation of other organs at risk of malignancy should begin by the end of adolescence.
The pigmented macules do not require treatment. Macules on the lips may disappear with time, while those on the buccal mucosa persist. The lip lesions can be lightened with chemical peels or laser.
Our patient underwent laparotomy, which revealed a grossly dilated and gangrenous small bowel segment. Intussusception was not present and was thought to have spontaneously reduced. Resection and anastomosis of the affected small bowel was performed. The patient’s postoperative course was uneventful, and her diarrhea and abdominal pain resolved. We recommended follow-up in her home city with primary care and a GI specialist and explained the need for surveillance of her condition.
CORRESPONDENCE
Josette R. McMichael, MD, Department of Dermatology, Emory University, 1525 Clifton Road NE, 1st Floor, Atlanta, GA 30322; [email protected]
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
4. Vidal I, Podevin G, Piloquet H, et al. Follow-up and surgical management of Peutz-Jeghers syndrome in children. J Pediatr Gastroenterol Nutr. 2009;48:419-425.
5. Goldstein SA, Hoffenberg EJ. Peutz-Jegher syndrome in childhood: need for updated recommendations? J Pediatr Gastroenterol Nutr. 2013;56:191-195.
6. Hernan I, Roig I, Martin B, et al. De novo germline mutation in the serine-threonine kinase STK11/LKB1 gene associated with Peutz-Jeghers syndrome. Clin Genet. 2004;66:58-62.
1. Kopacova M, Tacheci I, Rejchrt S, et al. Peutz-Jeghers syndrome: diagnostic and therapeutic approach. World J Gastroenterol. 2009;15:5397-5408.
2. Beggs AD, Latchford AR, Vasen HF, et al. Peutz-Jeghers syndrome: a systematic review and recommendations for management. Gut. 2010;59:975-986.
3. van Lier MG, Mathus-Vliegen EM, Wagner A, et al. High cumulative risk of intussusception in patients with Peutz-Jeghers syndrome: time to update surveillance guidelines? Am J Gastroenterol. 2011;106:940-945.
4. Vidal I, Podevin G, Piloquet H, et al. Follow-up and surgical management of Peutz-Jeghers syndrome in children. J Pediatr Gastroenterol Nutr. 2009;48:419-425.
5. Goldstein SA, Hoffenberg EJ. Peutz-Jegher syndrome in childhood: need for updated recommendations? J Pediatr Gastroenterol Nutr. 2013;56:191-195.
6. Hernan I, Roig I, Martin B, et al. De novo germline mutation in the serine-threonine kinase STK11/LKB1 gene associated with Peutz-Jeghers syndrome. Clin Genet. 2004;66:58-62.